- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board

What Is Gene Therapy?
A New Type of Therapy for Genetic Diseases
- Why It's Done
- What to Expect
Frequently Asked Questions
Gene therapy is a type of treatment being developed to fight diseases that are caused by genetic defects. This is a relatively new medical intervention that is mainly in the experimental phase, including human trials and animal trials, for the treatment of some conditions, such as cystic fibrosis .
Gene therapy aims to change the unhealthy proteins that are produced as a result of disease-causing genes.
KATERYNA KON / SCIENCE PHOTO LIBRARY / Getty Images
What Is Gene Therapy?
Some diseases are caused by a known genetic defect or a gene mutation. This means that there is a hereditary or acquired error in the DNA molecule that codes for producing a specific protein in the body. The altered protein doesn’t function as it should, resulting in a disease.
The idea behind gene therapy is to direct the body to produce healthy proteins that do not cause disease.
This therapy involves the delivery of DNA or RNA. The RNA molecule is an intermediate molecule that is formed in the process of protein production. The genetic defect for some diseases has been identified, but many genetic mutations haven’t been identified (they might be in the future).
Research is ongoing into ways to correct genetic defects that have been associated with certain diseases. There are different types and methods of gene therapy that are being investigated.
Types of Gene Therapy
Genetic mutations can be hereditary, which means that they are inherited from parents. Genetic defects can also be acquired, sometimes due to environmental factors, like smoking.
Gene therapy is being evaluated as a potential treatment for both types of mutations. There are several ways of delivering the corrected DNA or RNA into a person’s body.
Most cells in your body are somatic cells. The only cells that are not somatic cells are the germline cells, which create the egg and sperm cells that can produce offspring.
Somatic gene therapy : Somatic gene therapy aims to correct a defect in the DNA of a somatic cell or to provide an RNA molecule to treat or prevent a genetic disease in the person who is undergoing the therapy. This treatment may be used if you have an inherited mutation or if the mutation developed due to environmental factors.
Germline gene therapy : Germline gene therapy aims to correct a defect in an egg or a sperm cell to prevent a hereditary disease from eventually affecting future offspring.
Bone Marrow
Sometimes a person’s own cells can be removed from the bone marrow , genetically modified in a laboratory, and then reinserted into the body.
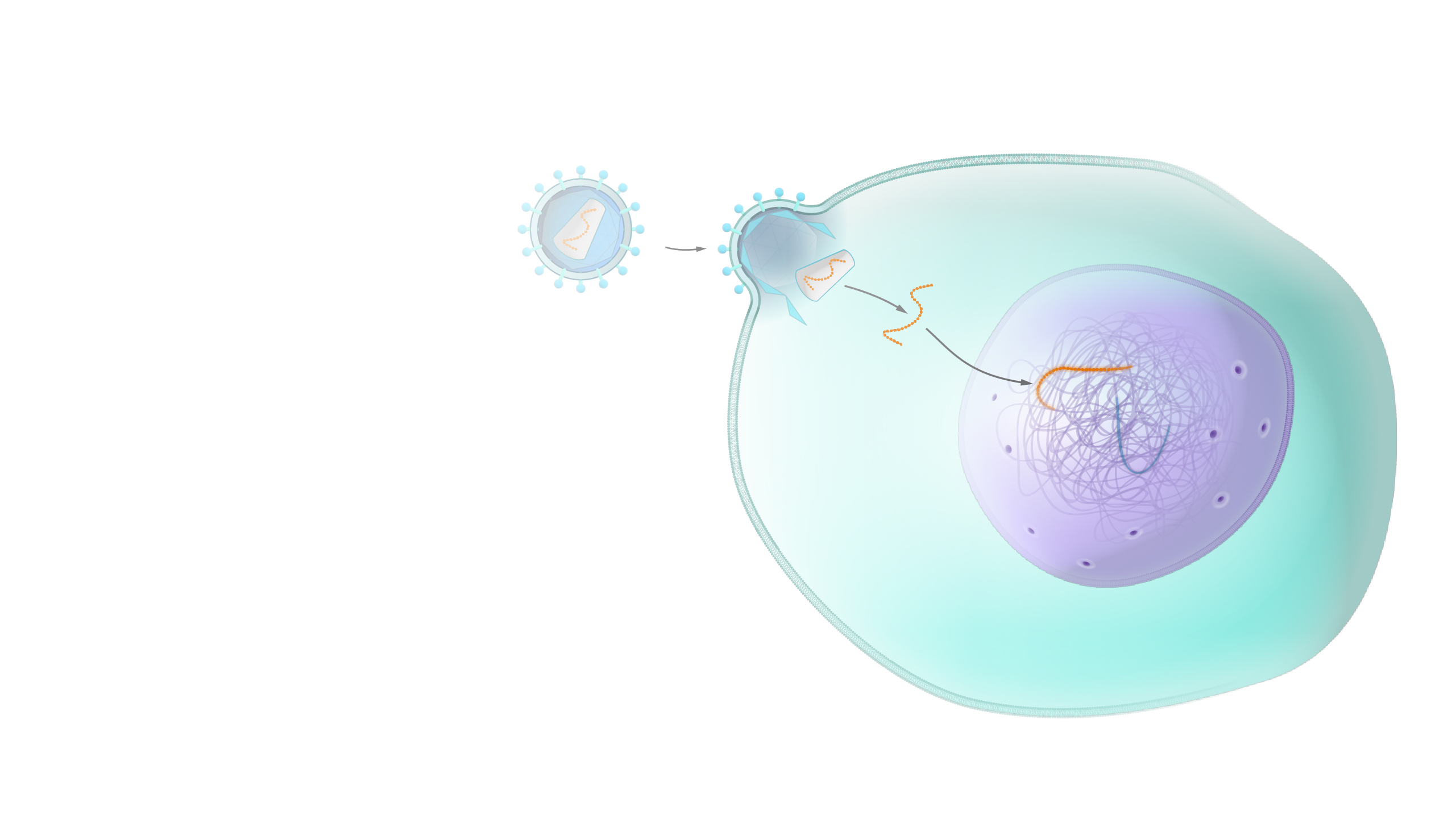
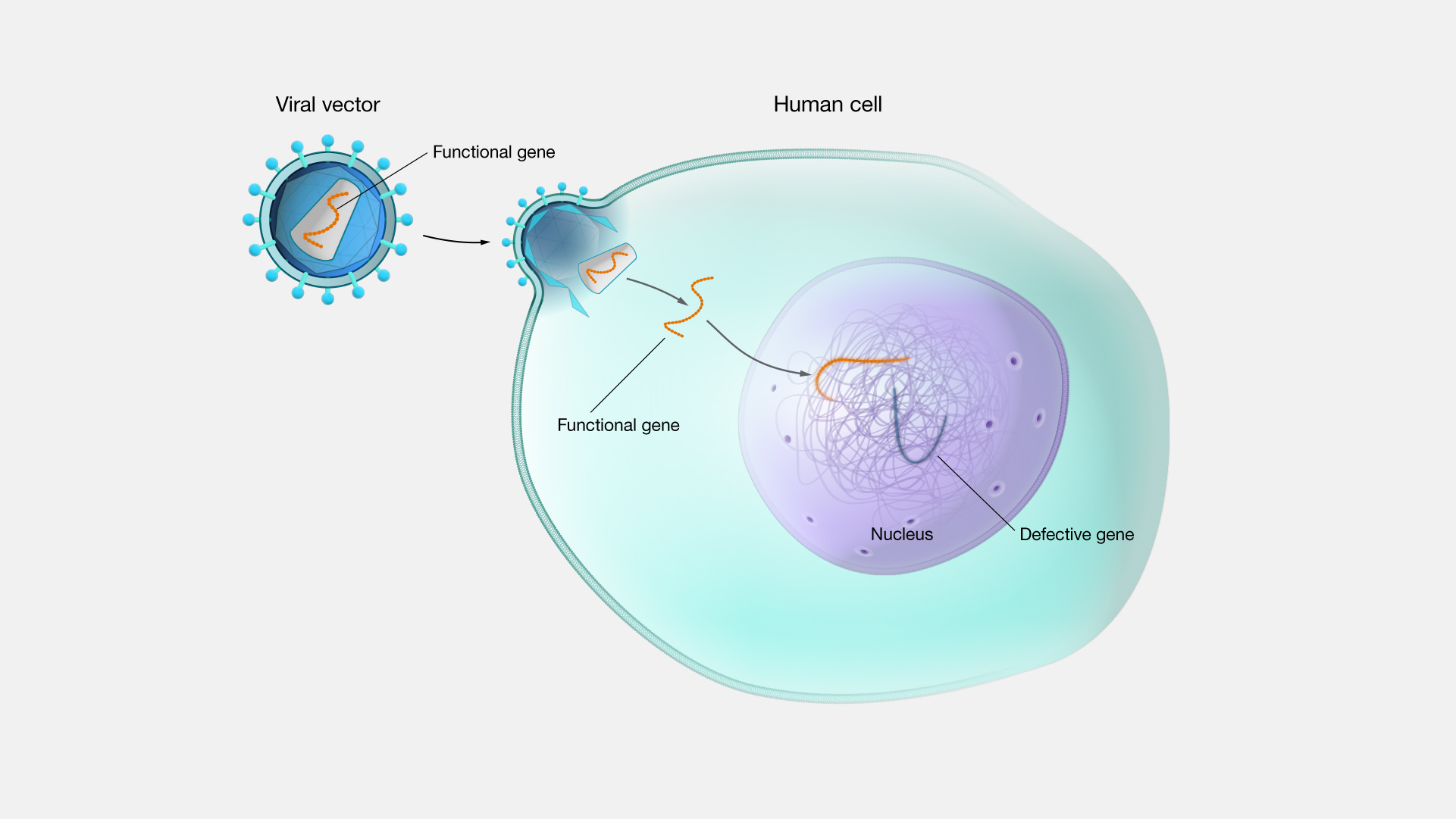
Viral Vector
A viral vector is a virus that has been altered so that it will not cause a viral infection. It is then infused with the correct DNA or RNA sequence. The viral vector containing the correct gene may be injected into a person for delivery of gene therapy.
Stem Cells
Stem cells are immature cells that have the potential to develop into different types of cells. Sometimes stem cells that have been genetically modified are transplanted into a person’s body to replace the defective cells as a way to treat disease.
This technique uses a lipid (fat) to deliver the genetic DNA or RNA material.
Why Is Gene Therapy Done?
Some gene mutations direct the body to make disease-causing proteins. And some genetic mutations are not functional—they cause disease because the body lacks the healthy proteins that should be normally produced by the gene.
Gene therapy aims to direct the body to produce healthy proteins or to inhibit the production of defective proteins. This depends on the type of mutation that is causing the disease.
Gene Augmentation Therapy: Replacing Mutated Genes
With gene augmentation, the goal is to help the body make a healthy protein.
Sometimes the DNA molecule can have a gene inserted into it. This is intended to permanently alter the DNA so that the body can make new cells with the correct DNA code. The new cells will then also make healthy products.
Some research using gene augmentation therapy involves the insertion of a healthy DNA molecule or an RNA sequence into a cell, but not into the DNA of the recipient. This has been shown in experimental studies to trigger the production of healthy proteins, but future copies of the cell are not expected to contain the healthy gene.
Gene Inhibition Therapy: Inactivating Mutated Genes
Sometimes gene therapy aims to cancel out the activity of a mutated gene to prevent the production of a disease-causing protein. This is done by the insertion of a non-mutated gene DNA sequence into a DNA molecule.
Making Diseases Cells Apparent to the Immune System
Another type of gene therapy involves the body’s immune system . An example of this therapy is the use of checkpoint inhibitors. With this therapy, the immune system is modified to recognize material in the body that is produced by the mutated genes in order to destroy them and to prevent the illnesses they cause.
Risks of Gene Therapy
There are some known risks of gene therapy. So far, the most common problem associated with gene therapy is a lack of effectiveness. However, there are also adverse effects that may occur.
Unwanted Immune System Reaction
Gene therapy that involves the immune system may cause excess immune reactivity to healthy cells that resemble the disease cells, potentially causing damage to healthy cells.
Wrong Target Cell
Potentially, the immune reaction that is mediated by gene therapy can affect the wrong cell type, instead of the intended target cells.
Infection Caused by Viral Vector
When a viral vector is used, there may be a risk that the virus could cause an infection. Depending on the primary disease that is being treated, a person receiving gene therapy may have a weak immune system and, therefore, could have difficulty fighting the virus.
Possible Tumor
A new DNA sequence that’s inserted into a person’s genes could potentially lead to a mutation that could cause cancer to form.
What to Expect From Gene Therapy
If you are considering gene therapy, you will go through a process of diagnosis, treatment, and medical surveillance to assess the effects.
This step will determine whether you have a medical condition that can be treated with gene therapy. This means that you would have a blood sample sent to a laboratory to identify treatable gene mutations that are associated with your medical condition.
Examples of conditions that may be treatable with gene therapy include:
- Cystic fibrosis : An inherited disorder in which thick mucus is produced, clogging the airways and blocking the secretion of digestive enzymes
- Sickle cell disease : An inherited disorder that results in abnormal hemoglobin production (the protein that carries oxygen in the red blood cells)
- Leber's hereditary optic neuropathy (LHON) : An inherited disorder that causes the death of cells in the optic nerve , resulting in damage to central vision
- Inherited or acquired retinal disease : Conditions that damage to the retina , the light-sensing layer in the back of the eye
- WW domain-containing oxidoreductase (WWOX) epileptic encephalopathy syndrome : A genetic condition resulting in severe epilepsy, developmental delays, and early death
- Spinocerebellar ataxia and autosomal recessive 12 (SCAR12) : An inherited disorder resulting in seizures in infancy, developmental delays, and inability to coordinate movement
- Cancer : Many types of cancer
Your treatment may involve collection of your cells and delivery of the genes into your cells with a viral vector or liposome. The modified cells will be restored to your body after the treatment.
Surveillance
The effects of your treatment will be assessed, and you will be monitored for adverse events (side effects). If this occurs, you may be treated again.
Clinical Trials
You can find clinical trials for gene therapy by talking to your doctor or by searching for organizations that support your medical condition, such as the Cystic Fibrosis Foundation.
Gene therapy is a relatively new treatment designed to alleviate disease by modifying defective genes or altering the production of proteins by faulty genes. There are several ways that healthy genes can be inserted into the body, such as inside a deactivated virus or inside a fat particle.
Sometimes immature and healthy cells are transplanted to replace cells that have a disease-causing mutation. This type of therapy can cause side effects, and there is also a risk that it might not work.
A Word From Verywell
If you have a genetic disease with a known and identified gene mutation, you might be a candidate for gene therapy treatment in a clinical trial. This type of treatment is not a standard therapy, and you would need to be monitored closely so that you and your doctors would know if the therapy is working and whether you are having any side effects.
You can talk to your doctor about gene therapy. This treatment is not widespread, so there is a possibility that you may need to travel to be able to participate in a clinical trial if there is not a research study near you.
This therapy is considered safe, but there are risks and side effects. You may have an opportunity to participate in a clinical trial, and side effects and adverse effects would be monitored.
One example of this therapy is the use of a deactivated virus to insert a portion of a DNA molecule into the body’s cells so that the healthy DNA sequence can provide a blueprint for healthy proteins.
The main goal of gene therapy is to provide DNA or RNA to code for healthy proteins so the body will not be affected by a genetic disease.
Cystic Fibrosis Foundation. Gene therapy for cystic fibrosis .
Food and Drug Administration. What is gene therapy?
Jiang J, Sun G, Miao Q, Li B, Wang D, Yuan J, Chen C. Observation of peripapillary choroidal vascularity in natural disease course and after gene therapy for Leber's hereditary optic neuropathy. Front Med (Lausanne) . 2021;8:770069. doi:10.3389/fmed.2021.770069
Repudi S, Kustanovich I, Abu-Swai S, Stern S, Aqeilan RI. Neonatal neuronal WWOX gene therapy rescues Wwox null phenotypes . EMBO Mol Med. 2021;13(12):e14599. doi:10.15252/emmm.202114599
Tan TE, Fenner BJ, Barathi VA, Tun SBB, Wey YS, Tsai ASH, Su X, Lee SY, Cheung CMG, Wong TY, Mehta JS, Teo KYC. Gene-based therapeutics for acquired retinal disease: Opportunities and progress . Front Genet. 2021;12:795010. doi:10.3389/fgene.2021.795010
Lu Z, Chen H, Jiao X, et. al. Germline HLA-B evolutionary divergence influences the efficacy of immune checkpoint blockade therapy in gastrointestinal cancer . Genome Med . 2021;13(1):175. doi:10.1186/s13073-021-00997-6
By Heidi Moawad, MD Dr. Moawad is a neurologist and expert in brain health. She regularly writes and edits health content for medical books and publications.
- Introduction to Genomics
- Educational Resources
- Policy Issues in Genomics
- The Human Genome Project
- Funding Opportunities
- Funded Programs & Projects
- Division and Program Directors
- Scientific Program Analysts
- Contact by Research Area
- News & Events
- Research Areas
- Research investigators
- Research Projects
- Clinical Research
- Data Tools & Resources
- Genomics & Medicine
- Family Health History
- For Patients & Families
- For Health Professionals
- Jobs at NHGRI
- Training at NHGRI
- Funding for Research Training
- Professional Development Programs
- NHGRI Culture
- Social Media
- Broadcast Media
- Image Gallery
- Press Resources
- Organization
- NHGRI Director
- Mission & Vision
- Policies & Guidance
- Institute Advisors
- Strategic Vision
- Leadership Initiatives
- Diversity, Equity, and Inclusion
- Partner with NHGRI
- Staff Search
Gene Therapy
Gene therapy is a technique that uses a gene(s) to treat, prevent or cure a disease or medical disorder. Often, gene therapy works by adding new copies of a gene that is broken, or by replacing a defective or missing gene in a patient’s cells with a healthy version of that gene. Both inherited genetic diseases (e.g., hemophilia and sickle cell disease) and acquired disorders (e.g., leukemia) have been treated with gene therapy.
Gene therapy. Gene therapy is a direct way to treat genetic conditions as well as other conditions. There are also other related approaches like gene editing. There are many different versions and approaches to gene therapy and gene editing. It all rests on understanding how genes work and how changes in genes can affect our health. Researchers all over the world are studying many different facets of gene therapy and gene editing.
Clinical Director
Office of the Clinical Director
- Share full article
Advertisement
Supported by
Guest Essay
The Future of Medicine Is Unfolding Before Us. Are We Nurturing It?

By Elizabeth Currid-Halkett
Dr. Currid-Halkett is a Guggenheim fellow and professor of public policy at the University of Southern California.
On Jan. 8, 2020, as I was parking my car, I got a long-awaited phone call from one of my son’s doctors. She informed me that our 7-month-old son, Eliot, had Duchenne muscular dystrophy, a fatal neuromuscular disease.
I can still remember the way the Los Angeles winter sunlight hit the dashboard. I can see my neighbor walking up her steps with groceries, a leaf falling, oblivious to the devastation below. “Life changes in an instant,” Joan Didion wrote. “The ordinary instant.” Our son had a fatal illness. He would die before us.
D.M.D. prevents the production of dystrophin, a protein needed to protect and repair muscle cells. It is caused by a genetic mutation on the X chromosome, thus the disease almost exclusively affects boys (one in 3,300). Over time, children with D.M.D. lose muscle mass and thus the ability to do basic things like run and walk. Eventually they lose their ability to breathe, and they experience heart failure. There is no known cure. While existing treatments have helped extend the life span of sufferers, they mainly focus on managing symptoms.
In my search for answers for how to save my son, I contacted Dr. Jerry Mendell, a now-retired neurologist at Nationwide Children’s Hospital in Columbus, Ohio, who was running clinical trials for an experimental gene therapy he developed to enable dystrophin production in boys with D.M.D. The treatment, now known as Elevidys, offered the prospect of not merely managing symptoms, but slowing the disease’s progression or even stopping it in its tracks — and potentially, for the first time in the history of this terrible disease, allowing boys with D.M.D. a chance to thrive.
Since I had that first conversation with Dr. Mendell (also a senior adviser for Sarepta, the maker of Elevidys), clinical trials for the gene therapy have had their ups and downs , and some adverse effects have been reported. But in June 2023, based on a two-part clinical trial, the Food and Drug Administration granted accelerated approval for the treatment for 4- and 5-year-olds who do not have other disqualifying conditions. The F.D.A.’s approval was contingent on continuing trials showing evidence of improved motor function, which had not yet been established.
Before Eliot received his treatment, he had difficulty going up stairs. He complained about being tired after walking only a block or two, even on Halloween, when candy ought to have motivated him. Hopping on one foot, a milestone for a 4-year-old, was impossible.
On Aug. 29, he finally received the one-time infusion. Three weeks later, he was marching upstairs and able to jump over and over. After four weeks, he could hop on one foot. Six weeks after treatment, Eliot’s neurologist decided to re-administer the North Star Ambulatory Assessment , used to test boys with D.M.D. on skills like balance, jumping and getting up from the floor unassisted. In June, Eliot’s score was 22 out of 34. In the second week of October, it was a perfect 34 — that of a typically developing , healthy 4-year-old boy. Head in my hands, I wept with joy. This was science at its very best, close to a miracle.
But the goal to offer this possible future to more patients with D.M.D. is in jeopardy. Sarepta is seeking F.D.A. approval to treat boys over 5 . Disagreements over the latest clinical trial’s results threaten to derail that outcome.
Moreover, what the F.D.A. decides to do next with Elevidys could set the tone for how it handles other emerging gene therapies for rare diseases. We can already see roadblocks that prevent more families from gaining access to these new treatments — from high costs and insurance challenges to dissent over how flexible regulators should be in interpreting clinical trial results and taking qualitative improvements into account. What is at stake with the debate around Elevidys is more than just the chance to give other boys with D.M.D. a more normal life. The challenges that we are witnessing with Elevidys are a harbinger of the fights we may see with gene therapies developed for other rare diseases.
There’s an opportunity to reduce those barriers now, while these treatments are still in their early phases. Every child afflicted with a life-threatening disease deserves the chance Eliot has been given.
The biggest obstacle to getting these treatments is cost. Gene therapies cost, on average, $1 million to $2 million . At $3.2 million per patient, Elevidys is the second-most-expensive drug in the world . Insurance companies would probably prefer not to foot the bill, and without full F.D.A. approval, insurance companies can refuse to cover these treatments by claiming they are medically unnecessary or experimental . Before Eliot’s treatment began, my insurance company initially said it would cover the cost but then started stalling on coverage and questioning the urgency of Eliot’s treatment. I was able to call Dana Goldman, the dean of the Sol Price School of Public Policy at the University of Southern California, where I work, to help me navigate the process. I was in the rare position to marshal resources and assistance to pressure my insurance company into covering Elevidys. Across the country, physicians are fighting denials and seeking appeals for their young patients.
Dr. Goldman has argued that one way to incentivize insurance companies to cover the high costs of treatments like gene therapies is to amortize how much the companies pay over time if the effectiveness of such treatments does not last (analogous to a pay-for-performance model). Another option is for pharmaceutical companies to offer a warranty that gives a prorated refund to the insurance company if a patient needs to return to prophylaxis treatment within a certain number of years. Costs are an especially frustrating problem for rare diseases like D.M.D., for which the extremely small patient population deters companies from investing money and resources to develop new treatments. Some experts believe the federal government ought to do more to directly complement research funding for rare diseases , as it has through the Orphan Drug Act for over four decades. The government could also defray the cost to consumers by offering subsidies directly to patients.
There’s another big role the government can play to accelerate gene therapies besides intervening in costs, and that’s to make the wheels of regulatory approval for these drugs less onerous. Flexibility doesn’t have to come at the cost of safety. The F.D.A. acted swiftly to approve an antiretroviral drug for H.I.V. in the 1980s and the Covid vaccines in December 2020, saving millions of lives without putting people in harm’s way.
But Elevidys is a case study in how the F.D.A. can get in its own way. D.M.D. patients 4 or 5 years old received access to the drug under fast-tracked approval, the first time a drug was approved under this new framework. But this was reportedly only because Peter Marks , the director of the F.D.A.’s Center for Biologics Evaluation and Research, disagreed with his own staff’s rejection . Current concern over Elevidys’s approval for boys over 5 focuses on the most recent clinical trial results , which showed older boys, whose muscular decline is further along, did not improve on motor function as measured by the North Star Ambulatory Assessment after treatment. However, as Sarepta has noted, they still saw gains in their ability to rise from the floor and walk 10 meters, indicating possible slowing of the disease that could significantly improve and extend their lives.
Detractors suggest this improvement is not enough to meet the bar for approval. This is a common problem for rare disease trials because they often consist of very few participants. In such cases, a narrow focus on numbers ignores the real quality-of-life benefits doctors, patients and their families see from these treatments. During the advisory committee meeting for Elevidys in May 2023, I listened to F.D.A. analysts express skepticism about the drug after they watched videos of boys treated with Elevidys swimming and riding bikes. These experts — given the highest responsibility to evaluate treatments on behalf of others’ lives — seemed unable to see the forest for the trees as they focused on statistics versus real-life examples.
The F.D.A. can have a more flexible view of treatment efficacy without losing focus on safety. As with any drug, whether for migraines or asthma, there will be a spectrum of effectiveness. The same will be true of all gene therapies, and the F.D.A. should reconsider the metrics it uses to green-light these treatments now, before it potentially leaves thousands of patients in the lurch, out of access to something lifesaving.
Gene therapy is the future of medicine. Our bureaucracy and insurance companies should not hinder patients from receiving pioneering treatments that could transform their lives. As parents, we are not asking for the moon. We just want our children to live.
Elizabeth Currid-Halkett is a Guggenheim fellow and professor of public policy at the University of Southern California.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , X and Threads .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Welcome to Gene Therapy
A key title for the exploration of new genetic and stem cell therapy techniques
Targeted shock-and-kill HIV-1 gene therapy approach combining CRISPR activation, suicide gene tBid and retargeted adenovirus delivery
- Sarah Klinnert
- Corinne D. Schenkel
- Karin J. Metzner
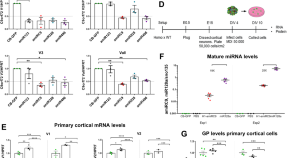
Artificial microRNA suppresses C9ORF72 variants and decreases toxic dipeptide repeat proteins in vivo
- Gabriela Toro Cabrera
- Katharina E. Meijboom
- Christian Mueller
Safety and biodistribution of XC001 (encoberminogene rezmadenovec) gene therapy in rats: a potential therapy for cardiovascular diseases
- Duncan J. Stewart
- Albert Gianchetti
- Rickey R. Reinhardt
A practical approach for adoption of a hub and spoke model for cell and gene therapies in low- and middle-income countries: framework and case studies
- Shadi Saleh
- Omar Dabbous
Current issue
Implications of maternal-fetal health on perinatal stem cell banking.
- Mehri Barabadi
- Rebecca Lim
Acoustically targeted noninvasive gene therapy in large brain volumes
- Shirin Nouraein
- Sangsin Lee
- Jerzy O. Szablowski
Long-term effects of a fat-directed FGF21 gene therapy in aged female mice
- Jacqueline M. Anderson
- W. David Arnold
p53 dry gene powder enhances anti-cancer effects of chemotherapy against malignant pleural mesothelioma
- Naomi Muramatsu
- Misa Ichikawa
- Hirokazu Okamoto
Announcements
Peer review terminology.
Gene Therapy is participating in a pilot of NISO/STM's Working Group on Peer Review Terminology. to identify and standardize definitions and terminology in peer review practices. Please see the section entitled Peer Review Terminology under the Guide for Authors, Editorial Process or For Referees for more information and to locate the link to complete the short survey.
Meet the Editors
Every month or so we will be featuring one of our editorial team, allowing them the opportunity to impart some of their wisdom and knowledge with you, our authors and readers, and you the opportunity to get to know them a little better. To kick it off we have our Editor-in-Chief, Janine Scholefield
Gene Editing
This web collection showcases some of the recent research in this exciting field.
Reader's Choice 2021
Read the journal's latest collection highlighting those papers from 2021 most read, cited and shared by our readers. We hope you enjoy.
Research Data

Guide to Authors

Gene Therapy Twitter

Advertisement
Browse articles

Long-term benefits of hematopoietic stem cell-based macrophage/microglia delivery of GDNF to the CNS in a mouse model of Parkinson’s disease
- Barath P. Sivasubramanian
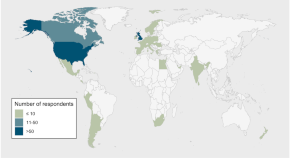
A multinational survey of potential participant perspectives on ocular gene therapy
- Alexis Ceecee Britten-Jones
- Myra B. McGuinness
- Lauren N. Ayton
Adeno-associated virus genome quantification with amplification-free CRISPR-Cas12a
- Zach Hetzler
- Stella M. Marinakos
- Qingshan Wei
Precision ophthalmology: a call for Africa not to be left in the dark
- Lisa Roberts
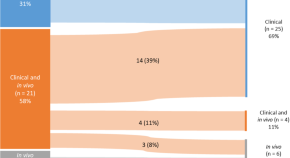
Advancing rare disease treatment: EMA’s decade-long insights into engineered adoptive cell therapy for rare cancers and orphan designation
- Maria Elisabeth Kalland
- Tomas Pose-Boirazian
- Segundo Mariz
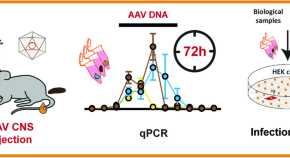
A shedding analysis after AAV8 CNS injection revealed fragmented viral DNA without evidence of functional AAV particles in mice
- Felix Krause
- Katja Schmidtke
- Max O. Rybarski
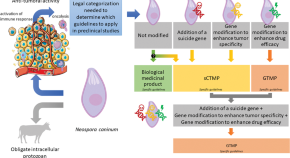
Are genetically modified protozoa eligible for ATMP status? Concerning the legal categorization of an oncolytic protozoan drug candidate
- Mathieu Guerriaud
- Cyril Poupet
- Nathalie Moiré
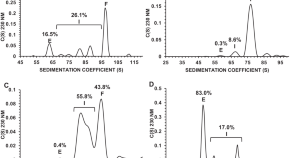
Analytical characterization of full, intermediate, and empty AAV capsids
- Aisleen McColl-Carboni
- Serena Dollive
- James B. McGivney IV
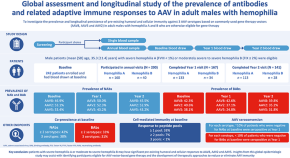
Multicenter assessment and longitudinal study of the prevalence of antibodies and related adaptive immune responses to AAV in adult males with hemophilia
- Ingrid Pabinger
- Mila Ayash-Rashkovsky
- John Chapin
Collections
Gene Therapy and Cancer
Trending - Altmetric
Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature
Gene Therapy Progress and Prospects: Electroporation and other physical methods
Lentiviral-mediated delivery of siRNAs for antiviral therapy
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Gene Therapy: Risks and Benefits Research Paper
Introduction, summary of the mechanisms of gene therapy, discussion of paper and critique of the methods used, argument and future experiments proposed, works cited.
Gene therapy is the “insertion or removal of genes which can also be alternated within the cell or tissues of an organism for purposes of treating diseases” (Cross & Burmester). All over the world, “the technique is best known for the correction of defective genes so as to treat diseases; the most common procedural form of gene therapy involves the insertion of the functional gene in order to replace the mutated gene within an organism” (Cross & Burmester).
Over the recent past use of gene therapy has revolutionized the treatment approaches, and research on this subject is justified because of the great potential that this technique offers. Similarly, further research will also shed light on possible risk factors that are associated with this method of treatment. The purpose of this paper is to undertake a cost-benefit analysis of gene therapy as a treatment option.
Germ-line gene therapy
Under normal circumstances, germ-line gene therapy procedures involve alternating and replacing all defective genes within the body of an organism (Walesgenepark.co.uk). In this case, functional genes are isolated and inserted into the interior lobes of the reproductive tissues or cells of the relevant organism. Considerably, this therapy operates on the basis that fresh and healthy genes get inoculated into the gametes of an organism in order to reduce the risks of later transfer of the defective genes to the next generation of the organism (Walesgenepark.co.uk).
Moreover, there is the involvement of complete alteration of the genetic makeup of early-stage blastomere so as to enhance changes of the genetic codes which can be passed on from generation to generation. Secondly, germ-line therapy also involves alteration of the gametes before fertilization since, after fertilization, the characteristics are passed on to the offspring’s (Walesgenepark.co.uk).
Somatic gene therapy
Generally, “somatic gene therapy involves the process of alteration of the genetic makeup of somatic cells (i.e., all body cells except sex cells) of an individual” (Rothchild, Laura & Lauren). Contrary to germ-line therapy, there is no transfer of these cellular changes to the next generation of the organism, simply because they are neither sex cells nor gametes which fuse during fertilization (Rothchild, Laura & Lauren). The main focus on the process involves changing the arrangements of the genetic codes of the specific cells using either an in-vitro or ex-vivo DNA delivery system. This technique in today’s life can be employed medically in treatments of a variety of diseases, including; hemophilia, muscular dystrophy, among many others; currently, it is used for cancer treatments (Rothchild et al.).
Telomerase inhibition strategies
In humans, it’s evident that the telomerase RNA abbreviated (hTR) plays an important role in anticancer therapy. This hTR can be used as an anticancer either individually or in combination with another human telomerase reverse transcriptase (hTERT) (Li, Li, Yao, Geng, Xie, Feng, Zhang, Kong, Xue, Cheng, Zhou & Xiao 4). Current findings indicate that when these two agents combine with the recombinant adenovirus, another nucleotide called small-interfering RNA (siRNA) is formed (Li et al. 4).
Furthermore, it has been established that the levels of telomerase activities together with mRNA, hTR are greatly reduced by the activities of the recombinant adenovirus resulting in inhibition of Xenograft tumor growth (Li et al. 4). This implies that siRNA, which is specifically expressed in recombinant adenoviruses, is the best tool to be used as an anticancer and also in the treatment of oral squamous cell carcinoma (OSCC) (Li et al. 4). The major advantage of this technique is that the anticancer effect on OSCC is virtually accomplished by cellular proliferation in addition to cellular apoptosis (inhibition of tumor angiogenesis) (Li et al. 4).
Monoclonal antibodies in target therapies of breast cancer
Globally, breast cancer is known to be a killer disease that largely affects females; the major causative agent in about 10% of world breast cancer cases is the mutation of the gene, which is inherited from any of the parents (Grammatikakis, Zervoudis & Kassanos 640). Furthermore, the most effective known therapeutic alternative diagnosis or treatment of breast cancer globally is the use of gene therapy. Some of the procedures used in treatment include molecular chemotherapy, antiangiogenic gene therapy, among many other therapies currently being used (Grammatikakis et al. 640).
bacteria-mediated anti-angiogenesis therapy
Most of the recent studies have revealed that there are some bacterial species that are capable of colonizing solid tumors. This inherited characteristic, however, can further be enhanced via genetic engineering, developing a natural anti-tumor activity that always enables the specific bacteria to transfer its therapeutic molecules directly into the target cells (Gardlik, Behuliak, Palffy, Celec & Li 7). There are few completed studies that have completely documented the anti-angiogenesis process, which is basically a bacterial mediated therapy for cancer (Gardlik et al. 7).
There are four recognized approaches that scientists use when utilizing the use of bacteria in cancer treatments; these include anticancer therapeutic-autofiction, DNA vaccination, transkingdom RNA interference, and alternative gene therapy (Gardlik et al. 7). Notable to mention is that the major primary goal of all these approaches is that they all focus on stimulation of angiogenesis suppression.
Based on the evidence of this paper, the discussion focuses on the argument of whether gene therapy is effective when the cost-benefit analysis is undertaken. Personally, I totally agree with the essay topic that gene therapy has more benefits than costs since it has been successful in the treatment of most chronic cancerous diseases. Gene therapy usually works by relying on immunotherapy and the use of vector organisms like viral particles to modify the genome of the host cell that triggers an immune response, which finally destroys the cancer cells present in the body (Cross & Burmester).
By using gene therapy, many cancerous diseases, i.e., lung, prostate, pancreatic cancers, have a higher chance of being completely treated. As such, gene therapy is now emerging to be the most common preferred treatment of choice because of its efficacy over other treatment methods (Cross & Burmester). The gene treatment also allows the use of a single vector or a combination of several vectors aimed at achieving optimal results (Cross & Burmester).
Currently, further research on gene therapy is still ongoing, and various clinical trials have so far been completed, which now has led to the evolution of vaccine productions (Cross & Burmester). The vaccines are promising to be the most effective technique since they only require autologous cells to have them manufactured, although this has many cost implications; this is one of the disadvantages of this technique. The second disadvantage is that very few hospitals globally are capable of having such vaccines manufactured because of the costly technology associated with the production of the vaccine. All these factors ultimately limit the availability of the vaccine as a viable treatment option.
A recent research study provides findings that show evidence of secondary gliomas stem cell, which originates from an astrocytic tumor that contains a genetic mutation that possesses the tumor suppressor gene recognized as p53 (Cross & Burmester). As part of the procedure, it was proposed in the experiments that in order to induce apoptosis of tumor cells, one must incorporate the use of an integrated suicide factor together with the adenovirus-mediated transfer of p53 (Cross & Burmester). Considerably, the main strength of this approach is supported by the fact that p53 mediate apoptosis follows two distinct and separate pathways reducing pathogenicity (Cross & Burmester).
Gene therapy technology has brought a great 21st-century revolution to the modern system of disease treatments, precisely when it comes to cancer treatments. It is remarkable to note that the development of anticancer treatments by modified immunotherapy and gene therapy, among others, has helped to cure many cases of cancers and saved many others from death. However, through gene therapy, many victims of cancer have attained a prolonged lifespan after being subjected to this treatment.
Scientifically, the principle behind gene therapy when it comes to cancer treatment is that a successful cancer treatment involving therapeutic modality always aims at real activation of death pathways within the cancer cells.
Therefore, there is no doubt that for cancerous diseases, the development of genetic engineering and specific cancer vaccines are proving to be the most effective treatment approaches. There are hope and confidence that in the near future, all obstacles encountered during the first generation cancerous and precancerous treatments will be eliminated by the development of second-generation modern therapeutic intervention as far as disease (cancer) treatments is concerned (Cross & Burmester).
Cross, Deanna., & James, Burmester. “ Gene Therapy for Cancer Treatment: Past, Present and Future .” Clinical Medicine and Research . 2006. Web.
Gardlik, R., Behuliak, M., Palffy, R., Celec, P., & Li, C. “Gene therapy for cancer: bacteria- mediated anti-angiogenesis therapy.” Clinigene Current Gene Therapy Weekly (2011): 7.
Grammatikakis, I., Zervoudis, S., & Kassanos, D. “Synopsis of new antiangiogenetic factors, mutation compensation agents, and monoclonal antibodies in target therapies of breast cancer.” Clinigene Current Gene Therapy Weekly (2011): 7.
Li, Y., Li, M., Yao, G., Geng, N., Xie, Y., Feng, Y., Zhang, P., et al. “Telomerase inhibition strategies by siRNAs against either hTR or hTERT in oral squamous cell carcinoma.” Clinigene Current Gene Therapy Weekly (2011): 4.
Rothchild, Allisa., Laura, Martin., & Lauren, Lubrano. “Gene Therapy and the Gametes.” Somatic Gene Therapy . 2010. Web.
Walesgenepark.co.uk . “What is Germline Gene Therapy.” Wales Gene Park . 2011. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 17). Gene Therapy: Risks and Benefits. https://ivypanda.com/essays/gene-therapy-risks-and-benefits/
"Gene Therapy: Risks and Benefits." IvyPanda , 17 Feb. 2024, ivypanda.com/essays/gene-therapy-risks-and-benefits/.
IvyPanda . (2024) 'Gene Therapy: Risks and Benefits'. 17 February.
IvyPanda . 2024. "Gene Therapy: Risks and Benefits." February 17, 2024. https://ivypanda.com/essays/gene-therapy-risks-and-benefits/.
1. IvyPanda . "Gene Therapy: Risks and Benefits." February 17, 2024. https://ivypanda.com/essays/gene-therapy-risks-and-benefits/.
Bibliography
IvyPanda . "Gene Therapy: Risks and Benefits." February 17, 2024. https://ivypanda.com/essays/gene-therapy-risks-and-benefits/.
- Role of Telomerase Reactivation in Slowing Senescence
- Cancer: Angiogenesis, Recent Research, Ethical Concerns
- Angiostatic Approaches to Cancer Therapy
- Meiosis and Splitting of the Dna Into Gametes
- Cancer and Tumor Suppressor Genes
- Protein Diet, Telomere Length, and Cancer
- The Gene Therapy Development and Purpose
- Cancer: Gene Mutation’s Influence, Treatments
- Epithelial Ovarian Cancer Investigation
- Genes Cause Breast Cancer
- Genetics: the Eugenics Movement
- Longevity in "Live Long, Pass It On" by Tina Saey
- Debate on Human Reproductive Cloning
- Michael Smith: Nobel Prize-Winning Biochemist
- Genetics: "Bad Blood" Educational Series by BBC
Home — Essay Samples — Science — DNA — Gene Therapy: Concept and Types
Gene Therapy: Concept and Types
- Categories: DNA Gene
About this sample

Words: 492 |
Published: Feb 12, 2019
Words: 492 | Page: 1 | 3 min read

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr Jacklynne
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 1071 words
6 pages / 2721 words
1 pages / 497 words
3 pages / 1596 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on DNA
Bitter. (n.d.). Strategic family therapy.McGoldrick. (n.d.). Developing a genogram to track family patterns.Nichols, W., & Everett, C. (1986). Systemic family therapy : an integrative approach. New York: Guilford Press.
It is joining together of DNA molecules from two different species that are inserted into a host organism to produce new genetic combinations that are of value to science, medicine, agriculture, and industry. Since the focus of [...]
Polymerase chain reaction or PCR is the in vitro technique for the synthesis of the DNA or we can say that it is an in vitro DNA replication procedure. This technique is generally used in case of molecular biology for the [...]
In the 1980’s, DNA analysis were found and had became a great advance crime-solving tool for investigators. DNA analysis were created to be used in crime cases and it can help clear suspects and identify criminals. Also, [...]
Heredity is the transmission of genetic characteristics from ancestor to descendant through the genes. As a subject, it is tied closely to genetics, the area of biological study concerned with hereditary traits. The study of [...]
Most of The tumors have markers in blood and serum which can be monitored . An electrochemical immunosensor is special device applied for tracking of some diseases, in which a sandwich immunoreaction is commonly applied on an [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.29(8); 2021 Aug 4
Updating the ethical guidance for gene and cell therapy research participation
Stephanie solomon cargill.
1 Center for Health Care Ethics, Saint Louis University, St. Louis, MO, USA
4 Chair of Castle IRB, Wildwood, MO, USA
Andrea Eidsvik
2 Center for Health Care Ethics, Saint Louis University, St. Louis, MO, USA
Marilyn Lamm
3 Institutional Biosafety Committee (IBC), Clinical Biosafety Services, Wildwood, MO, USA
Since the US Food and Drug Administration (FDA) approved the first gene therapy in 2017, five additional modalities of gene and cell therapy (GCT) have been approved 1 and 3,032 clinial trials worldwide employ GCT technologies targeting a broad array of diseases and conditions ( https://asgct.careboxhealth.com ). Many of these studies target diseases or conditions that have currently approved clinical treatments and are also being offered to patients before they have tried and failed these alternative treatment options, thus potentially conflicting with existing ethical standards. We argue here that the current trend toward enrolling patients into early-phase GCT trials as a front-line approach is not unethical, but nevertheless problematic, unless it is approached with caution and consistency. Guidance is needed to assist researchers, sponsors, and review bodies in determining whether to offer GCT trial participation as a front-line option to patients and the key information to disclose to them if or when they do.
Historically, enrollment of participants in early-phase GCT trials proceeded with extreme caution. 2 , 3 , 4 Conditions for ethical enrollment required that participants have a very serious unmet medical need and only when patients have no other options (i.e., the “only line” approach) or when patients are treatment-refractory (i.e., the “last line” approach). Moreover, it has been considered unethical to offer participation to treatment-naive patients or patients who are on other treatments but who might desire to enroll in GCT trials instead (i.e., a front-line approach).
The absence of “viable and reasonable” alternatives is a part of nearly all historic and current ethical criteria for early-phase GCT clinical trial participation. While this threshold is clear for diseases with no treatment options, it becomes more complex when applied to diseases or disorders that do have clinical alternatives. In these cases, the ethical standard is frequently assumed to require attempting and failing any clinically approved treatment options available. While equating any approved standard of care with a viable and reasonable alternative is understandable both for ease of application and intuitive appeal, this equation is problematic for several reasons.
First, this interpretation is not in line with existing guidance on the issue, including from the National Academies of Science, 5 the FDA, 6 and bioethicists, 7 , 8 who emphasize that the standard is meant to be based on judgments about alternatives in particular circumstances, not applied to any alternatives. While standard of care treatments are inherently more predictable than experimental ones because of a history of evidence and clinical usage, they should not thereby be assumed to be less risky or more beneficial than clinical trial alternatives. Many approved interventions, especially in the treatment of rare diseases, cancer, and chronic diseases, are suboptimal in many ways and carry with them significant risks as well as limited benefits. Patient lifestyle, stage of disease progression, and disease manifestation, as well as socioeconomic and psychological circumstances, also impact the success rate and acceptability of various existing treatments.
Further, in the decades since the tragic deaths of Jesse Gelsinger and Jolee Mohr, the landscape of GCT has changed significantly. More is known about GCT and it “is increasingly becoming an important tool in the medical armamentarium for medical treatment.” 9 While GCT technology is still relatively new and evolving, it is a mistake to consider all GCT clinical trials as characterized by the global uncertainties of decades ago. Vector designs and packaging systems have evolved to minimize risks of insertional mutagenesis, immune responses, cytotoxicity, and recombination with wild-type viruses, leading to improved safety. This increase in knowledge provides a good reason to revisit the standard ethical approach as it undermines the assumption of global uncertainty about the risks and benefits of GCT clinical trials in comparison to existing treatments. In 1997, bioethicist Leroy Walters consequently proposed that, as the field of gene therapy matured, the requirement that there be no effective alternative therapy may need to be relaxed. 7
While there are good reasons to challenge the standard approach, we must be wary of abandoning it completely. If we uncritically enroll participants in novel GCT trials as a front-line approach, we risk overromanticizing the anticipated (but as yet unproven) benefits and underplaying their risks and uncertainties. Without close consideration of the available standard alternatives in each case, optimism among researchers and patients about GCT is likely to overshadow the standard care even in cases when the approved alternatives may be the more beneficial treatment options for patients. The potential and expectation for GCT has driven and pressured researchers to advance the field for new clinical treatments as quickly as possible. We agree with critics who urge that optimism should be balanced with caution. 7 Thus, an evaluation will need to be made that takes into consideration the specific factors of a GCT study to determine whether front-line enrollment is ethical. For this more nuanced judgement, a framework of considerations is needed to guide enrollment decisions, ensuring that the choices made are consistent and justified.
We suggest four considerations that must be applied by researchers and review bodies to determine when enrollment in GCT trials can ethically be offered as a front-line approach to participants.
1. Evidence for safety and efficacy of GCT
While first-in-human phase I GCT studies that only have previous evidence of safety and efficacy from animal models should remain on the precautionary end of the spectrum, often early phase trials are assessing well-studied components with only slight modifications that classify them as experimental. While any novelty introduced into a clinical trial design has the possibility to alter its safety or efficacy profile, studies that use well-studied investigational products should require a lower threshold for comparison to existing treatments as those that involve more novel components. Further, dose escalation studies, where it is likely that participants may receive a subclinical dose, should be considered more cautiously than dose expansion trials where the range between the minimum effective dose and the maximum tolerated dose has been established and some efficacy can be expected for all participants.
2. Evidence for safety and efficacy of clinical alternatives
The safety and efficacy of existing clinical alternatives should be compared with the safety/efficacy profile produced from consideration number one. This should include considerations of the side effects, toxicities, clinical effectiveness, length of effectiveness, etc. of the standard alternatives. Comparisons with interventions approved through alternative mechanisms (such as accelerated approvals by the FDA 6 ) should acknowledge that the safety and efficacy thresholds required for approval may have been lower than in standard approval mechanisms.
3. Impact of therapeutic windows or interactions on timing of research and clinical alternatives
Many GCT trials have therapeutic windows for eligibility. Patients with degenerative diseases can only participate in these trials prior to a certain point of disease progression or a certain age. 10 When there are clinical alternatives, an important consideration is whether these alternatives can be delayed, paused, or continued and with what drawbacks, in light of the potential benefits, of participating in a trial within its therapeutic window.
4. Value-based and practical patient considerations concerning clinical alternatives
The risk-benefit comparison between GCT trials and the clinically approved alternatives involves not only information regarding safety and efficacy, but also subjective or context-based considerations of interest to patients. Echoing Kimmelman, 8 we suggest involving patient and disease advocacy organizations to provide input about the contextual risks and burdens of clinical alternatives. 8 It is crucial for researchers and isponsors to be aware of these concerns and refer to them explicitly in their justification for offering front-line GCT, especially when consideration number two may indicate that standard of care is preferable. We recognize that these stakeholders may embrace the romanticization of GCT that could lead to studies that pose an unacceptable risk to participants. On the other hand, with well-supported guidance from disease groups and stakeholders, both the permission to enroll in these studies as well as strategies to explain the alternatives transparently (see below) can prove these enrollment decisions are ethically justified based on the autonomy of the participants.
Ultimately the ethical balance for GCT enrollment is struck in two ways. First, the historical ethical standard of excluding front-line participants should remain only as a default, with the burden of justification placed on enrolling front-line participants. If the four considerations above demonstrate that the clinical alternatives are not clearly superior to the GCT approach under study, then early-phase GCT trials can be justified as a front-line option. Second, when GCT research is being offered as a front-line approach, the ethical onus shifts from the enrollment to the consent process. To ethically enroll patients into early GCT studies who have other clinical alternatives, these alternatives must be laid out clearly, honestly, and specifically in the consent process. This responsibility lies with both those who design and deliver the consent process, as well as those who review it. In this way, caution is preserved while acknowledging the evolving state of the field of GCT.

IMAGES
VIDEO
COMMENTS
In germline gene therapy, the stem cells, e.g., with the sperm and egg, are modified by the introduction of functional genes, which are integrated into the genome. The modifications are hereditary and pass on to subsequent generations. In theory, this approach should be highly effective in the fight against genetic and hereditary diseases.
Nature Communications (2023) Gene therapy is at an inflection point. Recent successes in genetic medicine have paved the path for a broader second wave of therapies and laid the foundation for ...
INTRODUCTION. Gene therapy is the correction of defective genes that are responsible for disease development. It is a medical field that focuses on the genetic modification of cells to produce a therapeutic effect[] or the treatment of disease by repairing or reconstructing defective genetic material.[] In gene therapy, scientists can do one of several things depending on the problem that is ...
Gene therapy is a type of treatment being developed to fight diseases that are caused by genetic defects. This is a relatively new medical intervention that is mainly in the experimental phase, including human trials and animal trials, for the treatment of some conditions, such as cystic fibrosis. Gene therapy aims to change the unhealthy ...
Gene therapy, introduction of a normal gene into an individual's genome in order to repair a mutation that causes a genetic disease. Human gene therapy has been attempted on somatic (body) cells for diseases such as cystic fibrosis and cancer. Learn about approaches to and issues surrounding gene therapy.
Twenty-four of these papers related specifically to cell therapy while 11 pertained to gene therapy. Five of the included studies were conducted in the UK 12 - 16 , eight in the USA 17 - 24 , six in Canada 25 - 30 , two were conducted in Australia 31 , 32 and one each from Belgium 33 , China 34 , Germany 35 , Hungary 36 , Ireland 37 ...
Gene therapy has made inroads against cancer, too. An approach known as chimeric antigen receptor (CAR) T cell therapy works by programming a patient's immune cells to recognize and target cells ...
GENE THERAPY. Gene Therapy: A Comprehensive Review. Santosh R Patil 1), Ibrahim A. Al-Zoubi2), Raghuram PH 3), Neeta Misra 4), Nidhi Y adav 5), Mohammad Khursheed Alam6) ABSTRACT. Background: As ...
Gene therapy is a technique that uses a gene(s) to treat, prevent or cure a disease or medical disorder. Often, gene therapy works by adding new copies of a gene that is broken, or by replacing a defective or missing gene in a patient's cells with a healthy version of that gene. Both inherited genetic diseases (e.g., hemophilia and sickle ...
Gene therapy enjoyed an initial phase of excitement, until the recognition of immediate and delayed adverse effects resulted in death and caused a major setback. More recently, the discovery and development of CRISPR/Cas9 has re-opened a door for gene therapy and changed the way scientists can approach a genetic aberration—by fixing a non ...
Gene therapy is the future of medicine. Our bureaucracy and insurance companies should not hinder patients from receiving pioneering treatments that could transform their lives. As parents, we are ...
Better Essays. 4691 Words. 19 Pages. 12 Works Cited. Open Document. Gene Therapy. Gene therapy is a powerful new technology that has the ability to change the way medicine is practiced in the future. The potential of gene therapy offers great hope for cure and alleviation of suffering from genetic disorders that now plague numerous people.
Essay on the History of Gene Therapy: Gene therapy was conceived in 1960, the breakthrough was the synthesis of recombinant DNA molecule (rDNA) in 1972. The rDNA molecules were first duplicated and grown in bacteria in 1973. Later split gene was discovered through the use of recombinant DNA technologies in 1977.
High quality studies exploring patient and public opinions and experiences of cell and gene therapy are required. ... Twenty-four of these papers related specifically to cell therapy while 11 ...
Gene therapy's rise to prominence has come with an extraordinarily high price tag. Novartis's newly approved gene therapy, a one-time treatment for spinal muscular atrophy, is now the world ...
Gene Therapy is participating in a pilot of NISO/STM's Working Group on Peer Review Terminology. to identify and standardize definitions and terminology in peer review practices. ... The papers in ...
Personally, I totally agree with the essay topic that gene therapy has more benefits than costs since it has been successful in the treatment of most chronic cancerous diseases. Gene therapy usually works by relying on immunotherapy and the use of vector organisms like viral particles to modify the genome of the host cell that triggers an ...
Gene therapy is a medical field that shows promise in correcting genetic disorders by targeting the genes responsible for the disease. It involves introducing genetic material into cells to ...
Gene therapy was first discovered in the mid 1970's where researchers were able to isolate certain type of genes from DNA. The term gene therapy came about in the 1980's and pushed research further. When we're born each individual are born with a set of chromosomes that the gene that codes for our appearances, personality, and long term ...
Design of Integrating Vectors Used for HSC Gene Therapy. Vectors engineered from gamma retroviruses, 28 long under study as the cause of a variety of cancers in mice, had the desired property of efficient insertion into the genome of target cells. Murine gamma retroviruses and their derivatives were the first of the genome integrating vectors to be applied to T lymphocytes and HSC in the ...
Gene therapy is the process through which healthy genetic code is included in cells to replace abnormal genes or create a desirable protein. Researchers explore various gene therapy strategies to resolve problems such as mutated gene replacement with a healthy gene code. This deactivates any mutated genes and adds a healthy gene into the body ...
Argumentative Essay On Gene Therapy. 1113 Words5 Pages. According to Oxford dictionaries, gene therapy is defined as the introduction of normal genes into cells in place of missing or defective ones in order to correct genetic disorders. The first gene therapy trial occurred in 1990 where a young girl suffering from adenosine deaminase (ADA ...
Persuasive Essay On Gene Therapy. 881 Words4 Pages. Another huge part of medicine is expected to involve genes sequencing, or in other words: genetic screening. Originally, it cost several million dollars to sequence all the genes in a single human body. Today, because of improved technologies, it would cost $50,000.
Main text. Since the US Food and Drug Administration (FDA) approved the first gene therapy in 2017, five additional modalities of gene and cell therapy (GCT) have been approved 1 and 3,032 clinial trials worldwide employ GCT technologies targeting a broad array of diseases and conditions (https://asgct.careboxhealth.com).Many of these studies target diseases or conditions that have currently ...