- Patient Care & Health Information
- Diseases & Conditions
- What is lymphoma? An expert explains
Learn more from hematologist Stephen Ansell, M.D.
Hi. I'm Dr. Stephen Ansell. I'm a hematologist at Mayo Clinic. In this video, we're going to cover the basics of lymphoma. What is it? Who gets it? The symptoms. Diagnosis and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. Lymphoma is an overarching term for a kind of cancer that starts in the lymphatic system. Cancer diagnosis of any kind can be very difficult to deal with. But recent advances in how we treat lymphoma and ongoing research means there's a lot of hope. Treatment is highly successful for the majority of patients. First, what exactly is the lymphatic system? Well, it's actually a crucial part of the immune system. The lymphatic system produces lymphocytes, or white blood cells, which fight off pathogens, bacteria and the like. There are two types of lymphocytes, T cells and B cells. And lymphoma occurs when one of these types of lymphocytes grow and multiply uncontrollably. Often, these abnormal cells will build up and cause tumors in the lymph nodes, which are actually present throughout your body. And so lymphoma cells can collect anywhere.
There are different types of lymphoma, but really two main categories. Firstly, Hodgkin lymphoma. This is an uncommon form of lymphoma identified by the presence of rare large cells, which are called Reed- Sternberg cells. And it usually begins in lymph nodes of the neck, the chest, under the arms, and progresses in an orderly fashion and predictable fashion to other lymph node sites. This often means that it can be detected and treated early. And it's actually considered one of the most treatable forms of cancer. Non-Hodgkin lymphoma, while more common than Hodgkin lymphoma, is still very uncommon and a relatively rare disease overall. This category includes any cancer of lymphocytes that doesn't involve Reed- Sternberg cells.
Like all cancers, lymphoma is the result of mutations in DNA that instruct the cells on how to grow, and the cells often grow out of control and live longer than they should. These disease cells then continue to multiply at a rapid rate, producing more disease cells. These particular DNA mutations affect lymphocytes, which accumulate in the lymph nodes and other parts of the lymphatic system to form tumors, crowding out healthy tissue and limiting its ability to function. We don't always know exactly what caused that initial mutation, but we know the effects that it has downstream. There are, however, a variety of things that can increase your risk. Although both Hodgkin and non-Hodgkin lymphoma can occur at any age, they do have a pattern. For non-Hodgkin lymphoma, the risk increases as you get older, with about half the people diagnosed over the age of 65. In Hodgkin lymphoma, cases are predominantly seen in two peaks, often in young adults 20 to 40, and again in older people over 55. Whether because of an immune disease or immune suppressive drugs, lymphoma is more common in people with an impaired immune system. Certain infections can be connected with higher rates of lymphoma. These infections include Epstein-Barr virus infections and helicobacter pylori infections.
Common symptoms of having lymphoma include swelling of lymph nodes in your neck, in your armpits or your groin. This is often but not always painless and often could be associated with fevers, or unexplained weight loss, or drenching night sweats, sometimes chills, persistent fatigue. Shortness of breath can often be found. And patients with Hodgkin lymphoma may develop an itchy skin. Just because you're experiencing these types of things doesn't mean you have lymphoma, but it is important to see your doctor if you're experiencing recurring symptoms.
Firstly, they're likely to give you a physical exam to check for swollen lymph nodes and see whether your spleen or liver feel swollen. A lymph node may in fact be removed for a biopsy. This can show not only if lymphoma cells are present but will actually help to identify the type of lymphoma. The bone marrow is where the cells are made, and so a sample of the bone marrow may also be taken. This is usually performed both on the liquid of the bone marrow, the so-called aspirate, and then a biopsy is taken from the solid portion of the bone marrow. This is done using a needle, and the sample is usually extracted from the hipbone and sent for analysis. Additionally, your doctor may recommend other kinds of tests including imaging studies. This could include a PET scan, a CT scan, or an MRI scan. All of them are being done to look for signs of lymphoma in other areas of your body.
A specialized team of doctors can work with you to develop a strategy for treating your lymphoma. And the strategy is based on the type of lymphoma, the stage of the lymphoma, the aggressiveness of the cancer, as well as your overall health. Some lymphomas grow very slowly, and it may not be necessary to start treatment right away. Active surveillance is often your best option. You and your doctor may decide not to treat the lymphoma until it interferes with your lifestyle. We call this watchful waiting. However, until then, you would need to have periodic tests to monitor your disease. Now, you may be given chemotherapy. These are usually powerful drugs that will kill lymphoma. Additional treatments are coming out that allow for targeted therapy. Targeted drug treatment focuses just on specific abnormalities in cancer cells and is highly effective. A further strategy is immunotherapy. And immunotherapy drugs use your own immune system to fight your cancer.
Finding out you have lymphoma and going through treatment can be overwhelming, but there are things you can do to help you cope. Learn about your lymphoma. Don't be afraid to ask your doctor questions and have them recommend resources for more information. Knowing more about your particular case and the options you have will help you feel confident and empower you to make decisions regarding treatment. Keep your friends and family close, rely on them for practical and emotional support. It may also be important to find a support group to talk with people who are going through the same thing. Although cancer and going through treatment is never easy, with lymphoma, there is plenty of hope and long-term success. A lot of progress has been made in fighting lymphoma, getting patients into remission, getting them back to a normal life. And as the research continues, ever-improving and more effective ways to combat lymphoma are being developed. If you want to learn more about your lymphoma, please go ahead and watch the related videos or visit mayoclinic.org. We wish you well.
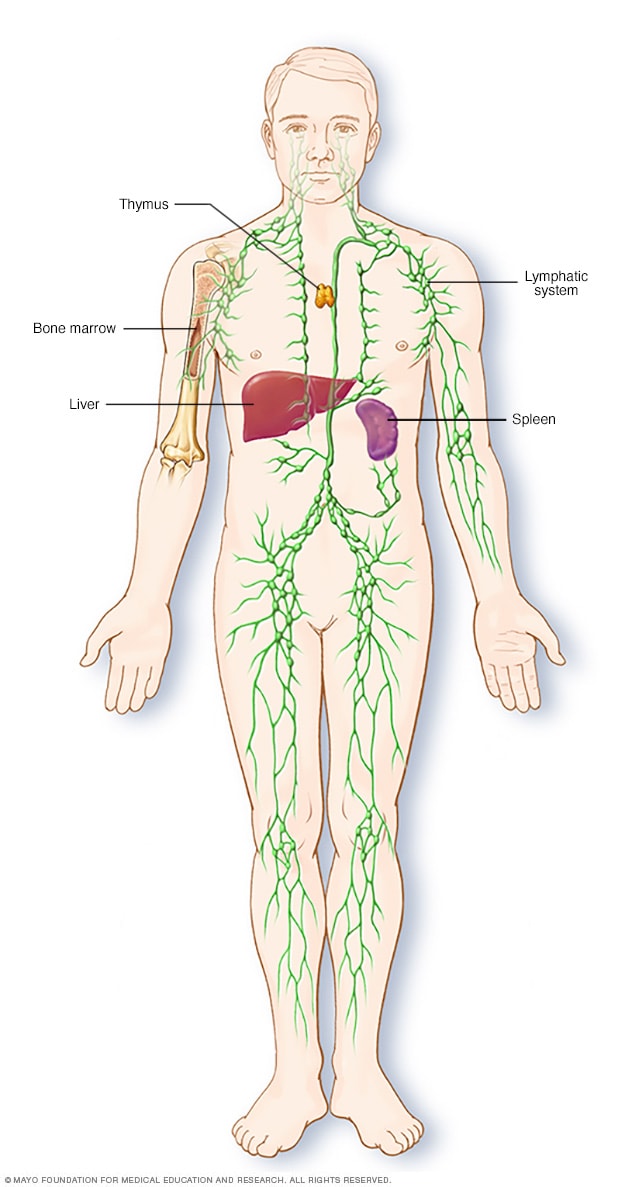

Parts of the immune system
The lymphatic system is part of the body's immune system, which protects against infection and disease. The lymphatic system includes the spleen, thymus, lymph nodes and lymph channels, as well as the tonsils and adenoids.
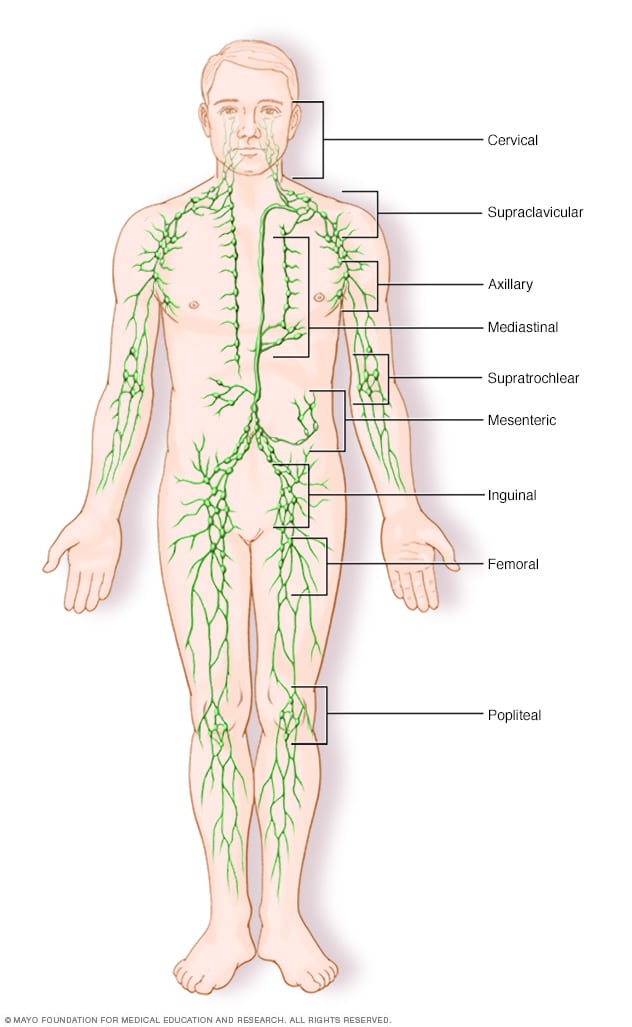
- Lymph node clusters
Lymph nodes are bean-sized collections of cells called lymphocytes. Hundreds of these nodes cluster throughout the lymphatic system, for example, near the knee, groin, neck and armpits. The nodes are connected by a network of lymphatic vessels.
Lymphoma is a cancer of the lymphatic system, which is part of the body's germ-fighting network.
The lymphatic system includes the lymph nodes (lymph glands), spleen, thymus gland and bone marrow. Lymphoma can affect all those areas as well as other organs throughout the body.
Many types of lymphoma exist. The main subtypes are:
- Hodgkin's lymphoma (formerly called Hodgkin's disease)
- Non-Hodgkin's lymphoma
What lymphoma treatment is best for you depends on your lymphoma type and its severity. Lymphoma treatment may involve chemotherapy, immunotherapy medications, radiation therapy, a bone marrow transplant or some combination of these.
Products & Services
- A Book: Living Medicine
- Chronic lymphocytic leukemia
- Cutaneous B-cell lymphoma
- Cutaneous T-cell lymphoma
- Hodgkin's lymphoma (Hodgkin's disease)
- Waldenstrom macroglobulinemia
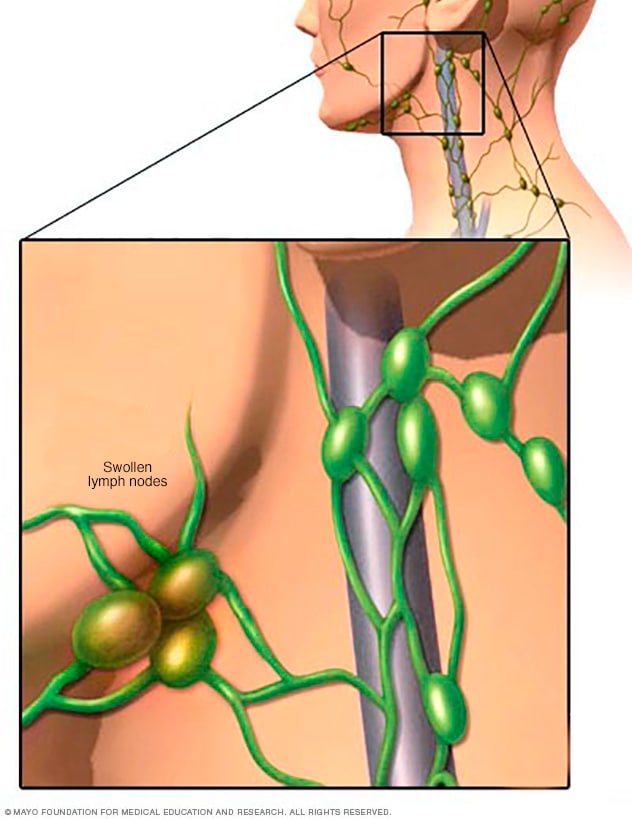
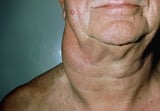
Swollen lymph nodes
One of the most common places to find swollen lymph nodes is in the neck. The inset shows three swollen lymph nodes below the lower jaw.
Signs and symptoms of lymphoma may include:
- Painless swelling of lymph nodes in your neck, armpits or groin
- Persistent fatigue
- Night sweats
- Shortness of breath
- Unexplained weight loss
When to see a doctor
Make an appointment with your doctor if you have any persistent signs or symptoms that worry you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Doctors aren't sure what causes lymphoma. But it begins when a disease-fighting white blood cell called a lymphocyte develops a genetic mutation. The mutation tells the cell to multiply rapidly, causing many diseased lymphocytes that continue multiplying.
The mutation also allows the cells to go on living when other normal cells would die. This causes too many diseased and ineffective lymphocytes in your lymph nodes and causes the lymph nodes, spleen and liver to swell.
Risk factors
Factors that can increase the risk of lymphoma include:
- Your age. Some types of lymphoma are more common in young adults, while others are most often diagnosed in people over 55.
- Being male. Males are slightly more likely to develop lymphoma than are females.
- Having an impaired immune system. Lymphoma is more common in people with immune system diseases or in people who take drugs that suppress their immune system.
- Developing certain infections. Some infections are associated with an increased risk of lymphoma, including the Epstein-Barr virus and Helicobacter pylori infection.
Lymphoma care at Mayo Clinic
Living with lymphoma?
Connect with others like you for support and answers to your questions in the CAR-T Cell Therapy support group on Mayo Clinic Connect, a patient community.
CAR-T Cell Therapy Discussions

204 Replies Thu, Apr 11, 2024

24 Replies Sun, Apr 07, 2024

7 Replies Fri, Mar 15, 2024
- Lymphoma — Hodgkin. Cancer.net. https://www.cancer.net/cancer-types/lymphoma-hodgkin/view-all. Accessed Sept. 1, 2019.
- Lymphoma — Non-Hodgkin. Cancer.net. https://www.cancer.net/cancer-types/lymphoma-non-hodgkin/view-all. Accessed Sept. 1, 2019.
- Adult Hodgkin lymphoma treatment (PDQ) — Health professional version. National Cancer Institute. https://www.cancer.gov/types/lymphoma/hp/adult-hodgkin-treatment-pdq. Accessed Sept. 1, 2019.
- Adult non-Hodgkin lymphoma treatment (PDQ) — Health professional version. National Cancer Institute. https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq. Accessed Sept. 1, 2019.
- Warner KJ. Allscripts EPSi. Mayo Clinic. July 2, 2019.
- Lymphoma SPOREs. National Cancer Institute. https://trp.cancer.gov/spores/lymphoma.htm. Accessed Sept. 1, 2019.
- Hoffman R, et al. Hematology: Basic Principles and Practice. 7th ed. Elsevier; 2018. https://www.clinicalkey.com. Accessed June 13, 2019.
- Laurent C, et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French Lymphopath Network. Journal of Clinical Oncology. 2017; doi: 10.1200/JCO.2016.71.2083.
- Mayo Clinic first in the U.S. to offer genetic test for lymphoma. Forefront. 2017;6. https://www.mayo.edu/research/forefront/mayo-clinic-first-us-offer-genetic-test-lymphoma. Accessed Sept. 1, 2019.
- Distress management. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed Sept. 1, 2019.
- Lymphoma FAQs
News from Mayo Clinic
- Mayo Clinic Minute: How precise diagnosis of lymphoma offers patients best treatment options Jan. 26, 2024, 05:00 p.m. CDT
- Mayo Clinic Q and A: What is lymphoma? Nov. 03, 2022, 01:04 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

WILLIAM D. LEWIS, MD, SETH LILLY, PharmD, BCPS, AND KRISTIN L. JONES, PA-C
Am Fam Physician. 2020;101(1):34-41
Related editorial: Breast Implant-Associated Anaplastic Large Cell Lymphoma .
Patient information: See related handout on lymphoma , written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Lymphoma is a group of malignant neoplasms of lymphocytes with more than 90 subtypes. It is traditionally classified broadly as non-Hodgkin or Hodgkin lymphoma. Approximately 82,000 new U.S. patients are diagnosed with lymphoma annually. Any tobacco use and obesity are major modifiable risk factors, with genetic, infectious, and inflammatory etiologies also contributing. Lymphoma typically presents as painless adenopathy, with systemic symptoms of fever, unexplained weight loss, and night sweats occurring in more advanced stages of the disease. An open lymph node biopsy is preferred for diagnosis. The Lugano classification system incorporates symptoms and the extent of the disease as shown on positron emission tomography/computed tomography to stage lymphoma, which is then used to determine treatment. Chemotherapy treatment plans differ between the main subtypes of lymphoma. Non-Hodgkin lymphoma is treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) with or without rituximab (R-CHOP), bendamustine, and lenalidomide. Hodgkin lymphoma is treated with combined chemotherapy with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), Stanford V (a chemotherapy regimen consisting of mechlorethamine, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, and prednisone), or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) with radiotherapy. Subsequent chemotherapy toxicities include neuropathy, cardiotoxicity, and secondary cancers such as lung and breast, and should be considered in the shared decision-making process to select a treatment regimen. Once remission is achieved, patients need routine surveillance to monitor for complications and relapse, in addition to age-appropriate screenings recommended by the U.S. Preventive Services Task Force. Patients should receive a 13-valent pneumococcal conjugate vaccine followed by a 23-valent pneumococcal polysaccharide vaccine at least eight weeks later with additional age-appropriate vaccinations because lymphoma is an immunosuppressive condition. Household contacts should also be current with their immunizations.
Lymphoma represents a heterogeneous group of malignant neoplasms of lymphocytes, which can involve lymphatic tissue, bone marrow, or extranodal sites. The World Health Organization’s classification system identifies more than 90 different subtypes ( Table 1 ) . 1 , 2 The initial stratification is derived from B-cell, T-cell, or natural killer cell origin. Further classification of distinct lymphoma subtypes is beyond the scope of this article; however, they are ultimately each defined by morphology, immunopheno-type, genetic, molecular, and clinical features. 1 , 3 This article will focus on the types of lymphoma traditionally classified as non-Hodgkin or Hodgkin.
Epidemiology
More than 82,000 new patients are projected to be diagnosed with lymphoma in 2019, representing 4.7% of all new cancer cases in the United States. The current five-year survival rate for non-Hodgkin lymphoma is 72.0%, and for Hodgkin lymphoma it is 86.6%. Almost 21,000 people are projected to die from lymphoma in 2019, representing 3.5% of all cancer deaths. Incidence of non-Hodgkin lymphoma is higher in men and whites, and it increases with age. The median age of patients at diagnosis of non-Hodgkin lymphoma is 67 years, and the median age at death is 76. Hodgkin lymphoma is most commonly diagnosed at 20 to 34 years of age; however, the median age at death is 68 because of the higher survival rate among younger patients. 2 , 4
Risk Factors
Genetic, infectious, and inflammatory etiologies increase the risk of lymphoma. First-degree relatives of patients with non-Hodgkin lymphoma and Hodgkin lymphoma have a respective 1.7-fold and 3.1-fold increased risk of developing lymphoma. A family history of a specific subtype of lymphoma is associated with developing that same subtype. 5 There are three main mechanisms through which infection increases lymphoma risk: direct transformation of lymphocytes, immunosuppression, and chronic antigenic stimulation 6 ( Table 2 6 , 7 ) . Rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, dermatomyositis, and celiac disease are inflammatory conditions that increase the risk of lymphoma through disease-specific causes and the chronic use of immunosuppressive medications. 8
Modifiable risk factors include current or former tobacco use 9 and obesity (body mass index of 30 kg per m 2 or higher). 10 Breast implants and long-term pesticide exposure have also been associated with non-Hodgkin lymphoma. 11 – 13
Clinical Presentation
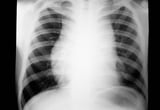
Lymphoma commonly presents as painless adenopathy. Adenopathy can wax and wane over years in indolent presentations or involve rapidly progressive adenopathy in more aggressive subtypes. Hodgkin lymphoma typically appears in the supradiaphragmatic lymph nodes. Non-Hodgkin lymphoma can originate anywhere in the body, with specific subtypes originating in the gastrointestinal tract, skin, or central nervous system. Systemic symptoms of fever, unexplained weight loss, and night sweats occur in a subset of patients with more advanced disease. Lymphoma spreads to extranodal sites by direct invasion or by hematogenous spread to the spleen, liver, lungs, or bone marrow. 14 , 15 High-grade lymphomas can present as oncologic emergencies because of the structural compression from the enlarging tumor, including superior vena cava syndrome, malignant epidural spinal cord compression, or malignant pericardial effusion. 16 Paraneoplastic syndromes are rare with lymphoma, occurring as paraneoplastic cerebellar degeneration in Hodgkin lymphoma and as dermatomyositis and polymyositis in Hodgkin and non-Hodgkin lymphomas. 17
The diagnosis of lymphoma is made using an open lymph node biopsy, based off morphology, immunohistochemistry, and flow cytometry. 3 Although fine-needle aspiration and core needle biopsy are often part of the initial evaluation of any adenopathy, neither will provide adequate tissue for the diagnosis of lymphoma because of the need to verify Hodgkin lymphoma via the presence of Reed-Sternberg cells. 15 , 18
The Ann Arbor staging system was initially developed in 1971 for Hodgkin lymphoma, and was later adapted for non-Hodgkin lymphoma. The Lugano classification system further modified staging by incorporating positron emission tomography/computed tomography (PET-CT) results to determine the staging of the lymphoma ( Table 3 19 ) . PET-CT is used for fluorodeoxyglucose-avid lymphoma subtypes, with symptoms alone being used for staging the remaining subtypes. The new staging system incorporates two symptom-based classifications: A (absence of symptoms) and B (presence of fever, weight loss, and night sweats) for Hodgkin lymphoma. A bone marrow biopsy is now recommended only for diffuse large B-cell lymphoma with a negative PET-CT result. 19
The International Prognostic Index is used broadly for all subtypes of non-Hodgkin lymphoma, and the International Prognostic Score is used for Hodgkin lymphoma 20 , 21 ( Table 4 22 , 23 ) .
Treatment of lymphoma consists of chemotherapy alone or in combination with radiotherapy. 24 Radiotherapy alone is not recommended. 25 Toxicity from radiotherapy can lead to serious long-term complications such as secondary cancers in the irradiated area, including breast or lung cancers. 25 Additionally, patients receiving chemotherapy can subsequently develop breast or lung cancers, melanoma, or acute myeloid leukemia. 26 , 27 Patients who are older than 60 years at diagnosis have worse outcomes, regardless of the staging. The National Comprehensive Cancer Network (NCCN) recommends avoiding certain chemotherapeutic agents in patients older than 60 years. The physician should focus on shared decision-making when discussing treatment options with all patients, but particularly for those older than 60 years, including whether the patient should pursue treatment. 25
The standard treatment for Hodgkin lymphoma is ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine [Velban], and dacarbazine), but other regimens such as the Stanford V (doxorubicin, vinblastine, mechlorethamine, etoposide [Toposar], vincristine, bleomycin, and prednisone) and escalated-BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine [Matulane], and prednisone) can be used. 24 – 28 Treatment for non-Hodgkin lymphoma varies depending on the histology, but often uses treatments such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) with or without rituximab (Rituxan; R-CHOP), a monoclonal antibody specific for CD20-positive B lymphocytes. 29 Other medications such as bendamustine (Bendeka), an alkylating agent, and lenalidomide (Revlimid) are also used in many non-Hodgkin lymphoma treatments. 30 , 31 Common complications of these therapies are listed in Table 5 . 25 – 27 , 29 – 36
A Cochrane review that examined seven trials consisting of more than 2,500 adult patients with early Hodgkin lymphoma concluded that the use of combined therapy could increase progression-free survival with little difference between the overall survival rates. 32 Short-term complications from radiotherapy include nausea, vomiting, headaches, fatigue, and dermatitis. Radiotherapy can also lead to long-term complications, including cardiac and pulmonary toxicity, hypothyroidism, or breast or lung cancers. 24 – 32 Radiotherapy can be avoided in patients with stage IA or IIA lymphoma without bulky disease 25 ( Table 3 19 ) .
Interim Reassessment
PET-CT scans, and subsequent Deauville scoring ( Table 6 21 ) , should be used to assess the response to chemotherapy in non-Hodgkin and Hodgkin lymphoma. 25 , 30 , 31 , 33 A score of 3 or less is considered complete remission in non-Hodgkin lymphoma and should conclude the current treatment course. A score of 4 or 5 is an indicator to consider escalating therapy. 25 Patients with Hodgkin lymphoma with a Deauville score of 1 or 2 have been shown to have similar progression and mortality outcomes between radiotherapy and no further treatment. 32 Patients who receive a score of 3 or 4 should receive additional chemotherapy and/or radiotherapy, and a score of 5 indicates the need for a biopsy (excisional or core needle) in addition to chemotherapy and radiotherapy. 25 A positive biopsy should be considered refractory disease. 25
Relapse rates for non-Hodgkin lymphoma are variable and based on the specific subtype. The most common subtype, diffuse large B-cell lymphoma, has a 40% lifetime relapse rate. 37 Lifetime relapse in Hodgkin lymphoma occurs in 10% to 15% of patients with early stage disease and 40% of patients with advanced stage disease. 38
Surveillance
Patients who have achieved remission need routine surveillance to monitor for complications and relapse, as well as age-appropriate screenings recommended by the U.S. Preventive Services Task Force. 39 Complications of lymphoma treatment include secondary malignancies (e.g., breast, lung, skin, colon), cardiac disease, infertility, and endocrine, neurologic, and psychiatric dysfunctions. Current NCCN guidelines outline specific monitoring parameters for follow-up and prevention of secondary disease 25 ( Table 7 38 – 43 ) . The extent and frequency of follow-up specifically depend on the histologic subtype of lymphoma. Patients should follow up with an oncologist every three to six months for the first two years , every six to 12 months until year 3, then annually thereafter. After five years of being cancer free, the patient can be transitioned to a primary care physician. 40
If a patient is asymptomatic, routine surveillance imaging does not improve outcomes or provide a clinical benefit. 40 , 41 Surveillance imaging should be used in patients who have reported symptoms or who are at high risk of relapse in a place that would not be easily examined, and who would be candidates for treatment. However, NCCN imaging guidelines for lymphoma surveillance state that it is acceptable to perform chest radiography or CT of the chest every six to 12 months for the first two years and then yearly for the next three to five years posttreatment. 41 Surveillance imaging with PET-CT scans following complete remission is not recommended. 40 , 41 Disease marker research is ongoing, examining minimal residual disease measurements, a polymerase chain reaction–based method that looks at identifying tumor-specific DNA sequences. 41
Immunizations
All patients with lymphoma should receive pneumococcal vaccination initially with a 13-valent pneumococcal conjugate vaccine (Prevnar 13), followed at least eight weeks later by a 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23) and then another PPSV23 at least five years later. 44 Patients receiving anti–B-cell antibodies should not receive annual influenza vaccination, and administration of live vaccines is contraindicated during chemotherapy. Routine vaccinations recommended by the Centers for Disease Control and Prevention (CDC) should resume, including any recommended inactivated or live vaccines three months after chemotherapy or six months after anti–B-cell antibody therapy. 43 , 45 Patients receiving a hematopoietic stem cell transplant should receive a series of three doses of Haemophilus influenzae type b vaccine starting six to 12 months after a successful transplant. Household contacts should receive appropriate CDC-recommended immunizations. 43
This article updates a previous article on this topic by Glass . 46
Data Sources: A PubMed search was completed using combinations of the key terms lymphoma, non-Hodgkin, Hodgkin, presentation, diagnosis, staging, treatment, and follow up. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: April 18, May 17, and May 31, 2018, and August 30, 2019. We also searched the Agency for Healthcare Research and Quality evidence reports, UpToDate, the Cochrane database, Essential Evidence Plus, the National Comprehensive Cancer Network, and the Surveillance, Epidemiology, and End Results database. Search dates: April 18, 2018, and August 30, 2019.
Research reported in this article was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 5U54GM104942-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
National Institutes of Health, National Cancer Institute. Surveillance, epidemiology, and end results program cancer stat facts: non-Hodgkin lymphoma. Accessed September 30, 2019. https://seer.cancer.gov/csr/1975_2016/results_merged/sect_19_nhl.pdf
Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019-5032.
National Institutes of Health, National Cancer Institute. Surveillance, epidemiology, and end results program cancer stat facts: Hodgkin lymphoma. Accessed September 30, 2019. https://seer.cancer.gov/statfacts/html/hodg.html
Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood. 2015;126(20):2265-2273.
Suarez F, Lecuit M. Infection-associated non-Hodgkin lymphomas. Clin Microbiol Infect. 2015;21(11):991-997.
Coghill AE, Hildesheim A. Epstein-Barr virus antibodies and the risk of associated malignancies: review of the literature. Am J Epidemiol. 2014;180(7):687-695.
Yadlapati S, Efthimiou P. Autoimmune/inflammatory arthritis associated lymphomas: who is at risk?. Biomed Res Int. 2016:8631061.
Sergentanis TN, Kanavidis P, Michelakos T, et al. Cigarette smoking and risk of lymphoma in adults: a comprehensive meta-analysis on Hodgkin and non-Hodgkin disease. Eur J Cancer Prev. 2013;22(2):131-150.
Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15(10):1083-1101.
Gidengil CA, Predmore Z, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg. 2015;135(3):713-720.
Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(4):4449-4527.
U.S. Food & Drug Administration. Questions and answers about breast implant-associated anaplastic large cell lymphoma. Accessed September 14, 2019. https://www.fda.gov/medical-devices/breast-implants/questions-and-answers-about-breast-implant-associated-anaplastic-large-cell-lymphoma-bia-alcl
Ansell SM. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(8):1152-1163.
Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(11):1574-1583.
Higdon ML, Atkinson CJ, Lawrence KV. Oncologic emergencies: recognition and initial management. Am Fam Physician. 2018;97(11):741-748. https://www.aafp.org/afp/2018/0601/p741.html
Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238.
Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94(11):896-903. https://www.aafp.org/afp/2016/1201/p896.html
Cheson BD, Fisher RI, Barrington SF, et al.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Corsortium. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
Townsend W, Linch D. Hodgkin’s lymphoma in adults. Lancet. 2012;380(9844):836-847.
Armitage JO, Gascoyne RD, Lunning MA, et al. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298-310.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl JMed. 1993;329(14):987-994.
Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506-1514.
Torok JA, Wu Y, Chino J, et al. Chemotherapy or combined modality therapy for early-stage Hodgkin lymphoma. Anticancer Res. 2018;38(5):2875-2881.
National Comprehensive Cancer Network. NCCN guidelines & clinical resources. Hodgkin lymphoma guideline. Accessed May 15, 2018. https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf
Edwards-Bennett SM, Jacks LM, Moskowitz CH, et al. Stanford V program for locally extensive and advanced Hodgkin lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Ann Oncol. 2010;21(3):574-581.
Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096-4104.
Filippi AR, Levis M, Parikh R, et al. Optimal therapy for early-stage Hodgkin’s lymphoma: risk adapting, response adapting, and role of radiotherapy. Curr Oncol Rep. 2017;19(5):34.
Pfreundschuh M, Trümper L, Osterborg A, et al.; MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379-391.
National Comprehensive Cancer Network. NCCN guidelines & clinical resources. B-cell lymphomas guideline. Accessed June 16, 2018. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
National Comprehensive Cancer Network. NCCN guidelines & clinical resources. T-cell lymphoma. Accessed June 16, 2018. https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf
Blank O, von Tresckow B, Monsef I, et al. Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017(4):CD007110.
Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther. 2017;11:1719-1728.
Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598-1607.
van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129(16):2257-2265.
Conway JL, Connors JM, Tyldesley S, et al. Secondary breast cancer risk by radiation volume in women with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2017;97(1):35-41.
Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31(3):209-216.
Bröckelmann PJ, Goergen H, Kohnhorst C, et al. Late relapse of classical Hodgkin lymphoma: an analysis of the German Hodgkin study group HD7 to HD12 trials. J Clin Oncol. 2017;35(13):1444-1450.
U.S. Preventive Services Task Force. Published recommendations. Accessed February 13, 2019. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index
Hiniker SM, Hoppe RT. Post-treatment surveillance imaging in lymphoma. Semin Oncol. 2017;44(5):310-322.
Cohen JB, Kurtz DM, Staton AD, et al. Next-generation surveillance strategies for patients with lymphoma. Future Oncol. 2015;11(13):1977-1991.
El-Galaly TC, Jakobsen LH, Hutchings M, et al. Routine imaging for diffuse large B-cell lymphoma in first complete remission does not improve post-treatment survival: a Danish-Swedish population-based study. J Clin Oncol. 2015;33(34):3993-3998.
Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best practices guidance of the Advisory Committee on Immunization Practices. Accessed February 2, 2019. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf
Centers for Disease Control and Prevention. Adult immunization schedule: pneumococcal vaccine. Accessed February 2, 2019. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html#note-pneumo
Rubin LG, Levin MJ, Ljungman P, et al.; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host [published correction appears in Clin Infect Dis . 2014;59(1):144]. Clin Infect Dis. 2014;58(3):e44-e100.
Glass C. Role of the primary care physician in Hodgkin lymphoma. Am Fam Physician. 2008;78(5):615-622. https://www.aafp.org/afp/2008/0901/p615.html
Continue Reading
More in AFP
More in pubmed.
Copyright © 2020 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Non-Hodgkin Lymphomas
, MD, Weill Cornell Medicine;
, MD, Weill Cornell Medicine
- Pathophysiology
- Classification
- Symptoms and Signs
More Information
- 3D Models (0)
- Calculators (0)

Non-Hodgkin lymphomas are a heterogeneous group of disorders involving malignant, monoclonal proliferation of lymphoid cells in lymphoreticular sites, including lymph nodes, bone marrow, the spleen, the liver, and the gastrointestinal tract. Presenting symptoms usually include peripheral lymphadenopathy. However, some patients present without lymphadenopathy but with abnormal lymphocytes in circulation. Disease is likely to be disseminated at the time of presentation, and diagnosis is usually based on lymph node or bone marrow biopsy or both. Management strategies may include watch and wait, chemotherapy, targeted drugs (eg, kinase inhibitors), and immunotherapies (eg, monoclonal antibodies, chimeric antigen receptor T cells); occasionally, radiation therapy is added. With few exceptions, stem cell transplantation is usually reserved for patients with aggressive lymphomas after incomplete remission or relapse.
(See also Overview of Lymphomas Overview of Lymphomas Lymphomas are a heterogeneous group of tumors arising in the reticuloendothelial and lymphatic systems. The major types are Hodgkin lymphoma Non-Hodgkin lymphoma See table . Lymphomas were once... read more .)
General reference
1. Siegel RL, Miller KD, Wagle NS, Jemal A . Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17-48. doi:10.3322/caac.21763
Etiology of Non-Hodgkin Lymphomas
Patients at increased risk of non-Hodgkin lymphoma include those with
Primary immunodeficiency Primary Immunodeficiencies Immunodeficiency disorders are associated with or predispose patients to various complications, including infections, autoimmune disorders, and lymphomas and other cancers. Primary immunodeficiencies... read more
Secondary immunodeficiency Secondary Immunodeficiencies Immunodeficiency disorders are associated with or predispose patients to various complications, including infections, autoimmune disorders, and lymphomas and other cancers. Primary immunodeficiencies... read more (eg, when induced by immunosuppressants, such as those used in systemic rheumatic diseases and after solid organ transplant)

Chronic inflammation and reactive lymph node hyperplasia
Possibly exposure to certain chemicals (eg, some herbicides and insecticides)
Non-Hodgkin lymphoma is one of the most common cancer in patients with HIV infection Non-Hodgkin lymphoma AIDS-defining cancers in patients infected with HIV are Kaposi sarcoma Lymphoma, Burkitt (or equivalent term) Lymphoma, immunoblastic (or equivalent term) Lymphoma, primary, of central nervous system read more , and some patients with HIV present with lymphoma. Patients with non-Hodgkin lymphoma should generally be screened for HIV and hepatitis viruses.
Genetic factors appear to play a role. Certain single nucleotide polymorphisms increase the risk of lymphoma. Patients with a first-degree relative with Hodgkin or non-Hodgkin lymphoma have an increased risk of non-Hodgkin lymphoma.
Pathophysiology of Non-Hodgkin Lymphomas
Most non-Hodgkin lymphomas arise from B lymphocytes; the remainder arise from T lymphocytes or natural killer cells. The stage of lymphocyte differentiation at which the oncogenic event occurs determines the disease presentation and outcome.
Most lymphomas are nodal with variable involvement of the bone marrow and peripheral blood, although some lymphomas arise in or involve extranodal sites (eg, skin, gastrointestinal tract, lung, central nervous system). A leukemia-like picture with peripheral lymphocytosis and bone marrow involvement may be present in up to 50% of children and about 20% of adults with some types of non-Hodgkin lymphoma.
Hypogammaglobulinemia caused by a progressive decrease in immunoglobulin production is present in 15% of patients at diagnosis. Hypogammaglobulinemia increases the risk of serious bacterial infection, and patients may require IV immune globulin to replace deficient immunoglobulins.
Pearls & Pitfalls
Classification of non-hodgkin lymphomas.
Non-Hodgkin lymphomas are commonly also categorized as indolent or aggressive:
Indolent: Slowly progressive and responsive to therapy but not typically curable with standard approaches
Aggressive: Rapidly progressive but responsive to chemotherapy and often curable

Classification references
1. Alaggio R, Amador C, Anagnostopoulos I, et al . The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms [published correction appears in Leukemia 2023 Sep;37(9):1944-1951]. Leukemia 2022;36(7):1720-1748. doi:10.1038/s41375-022-01620-2
2. Campo E, Jaffe ES, Cook JR, et al . The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee [published correction appears in Blood 2023 Jan 26;141(4):437]. Blood 2022;140(11):1229-1253. doi:10.1182/blood.2022015851
Symptoms and Signs of Non-Hodgkin Lymphomas
Most patients present with
Asymptomatic peripheral lymphadenopathy
Enlarged lymph nodes can be rubbery and discrete and later coalesce into masses. Affected nodes are usually not painful, unlike the tender nodes that often occur with viral infections. Nodal involvement is localized in some patients, but most patients have several areas affected. The initial physical examination should carefully look for nodes in the cervical, axillary, inguinal, and femoral regions.
In some patients, enlarged mediastinal and retroperitoneal nodes press on nearby structures, leading to symptoms. The most important of these are
Compression of the external biliary tree: Jaundice
Compression of the ureters: Hydronephrosis
Bowel obstruction: Vomiting and obstipation
Interference with lymph drainage: Chylous pleural or peritoneal fluid or lymphedema of a lower extremity
The skin is involved in some non-Hodgkin lymphomas. B-cell non-Hodgkin lymphoma can affect the scalp (follicular non-Hodgkin lymphoma) or the legs (large cell non-Hodgkin lymphoma), typically causing slightly raised, erythematous nodules. In cutaneous T-cell non-Hodgkin lymphoma, skin lesions can be diffuse, nonpalpable erythema or discrete papules, plaques, or tumors. In patients with dark skin, erythema may be subtle.
Systemic symptoms (eg, fatigue Fatigue Fatigue is difficulty initiating and sustaining activity due to a lack of energy and accompanied by a desire to rest. Fatigue is normal after physical exertion, prolonged stress, and sleep deprivation... read more , fevers, night sweats, weight loss Involuntary Weight Loss Involuntary weight loss generally develops over weeks or months. It can be a sign of a significant physical or mental disorder and is associated with an increased risk for mortality. The causative... read more ) can be the first manifestations in some patients, most commonly in aggressive lymphomas. These patients may not have noticed lymphadenopathy or not have external, palpable disease; these patients require CT or positron emission tomography (PET) imaging to discover the lesion(s).
Anemia is initially present in some patients and eventually develops in many. It may be caused by
Bleeding due to gastrointestinal lymphoma, with or without low platelet levels
Hemolysis due to hypersplenism or Coombs’-positive hemolytic anemia
Bone marrow infiltration due to lymphoma
Bone marrow suppression due to chemotherapy or radiation therapy
Suppressed bone marrow function related to chronic inflammation
Manifestations of some specific lymphomas
Adult T-cell leukemia-lymphoma, which is associated with human T-lymphotropic virus 1 (HTLV-1), has a fulminating clinical course with skin infiltrates, lymphadenopathy, hepatosplenomegaly, and leukemia Overview of Leukemia Leukemia is a malignant condition involving the excess production of immature or abnormal leukocytes, which eventually suppresses the production of normal blood cells and results in symptoms... read more . The leukemic cells are malignant T cells, many with convoluted nuclei. Hypercalcemia Hypercalcemia Hypercalcemia is a total serum calcium concentration > 10.4 mg/dL (> 2.60 mmol/L) or ionized serum calcium > 5.2 mg/dL (> 1.30 mmol/L). Principal causes include hyperparathyroidism... read more often develops, related to humoral factors rather than to direct bone invasion.
Diagnosis of Non-Hodgkin Lymphomas
Lymph node biopsy
Often unilateral bone marrow aspiration and biopsy
FDG-PET/CT of chest, abdomen, and pelvis for staging
MRI of brain and/or spinal cord if neurologic symptoms are present
As with Hodgkin lymphoma, non-Hodgkin lymphoma is usually suspected in patients with
Painless lymphadenopathy
Adenopathy detected on a chest radiograph or CT done for other reasons

Diagnostic tests
Enlarged lymph nodes are biopsied. If a node is palpable, no imaging is required initially, although CT or ultrasonography may be needed to properly plan subsequent tests.
If the lesion is easily palpable, an open biopsy is preferred. If the lesion is in the lung or abdomen, a core needle biopsy (18- to 20-gauge needle) done using CT or ultrasound guidance can often obtain an adequate specimen for diagnosis. A fine needle biopsy (percutaneous or bronchoscopic) frequently will not produce adequate tissue, especially for initial diagnosis; core biopsy is preferred if deemed safe.
Biopsy samples should be reviewed by a pathologist with expertise in lymphoma diagnosis so that the lymphoma can be correctly classified. If this review is not available locally, the slides should be sent to a reference laboratory with hematopathology expertise. The proper classification of non-Hodgkin lymphoma is critical for treatment planning. Non-Hodgkin lymphomas are potentially curable, but without a precise diagnosis, optimal therapy may not be chosen.
Histologic criteria on biopsy include destruction of normal lymph node architecture and invasion of the capsule and adjacent fat by characteristic neoplastic cells.
Immunophenotyping studies (using immunohistochemistry or flow cytometry) to determine the cell of origin are of great value in identifying specific subtypes and helping define prognosis and management; these studies also can be done on peripheral cells if they are present, but typically these stains are applied to formalin-fixed, paraffin-embedded tissue.
Demonstration of the leukocyte common antigen CD45 by immunoperoxidase rules out metastatic cancer, which is often in the differential diagnosis of “undifferentiated” cancers. The test for leukocyte common antigen, most surface marker studies, and gene rearrangement (to document B-cell or T-cell clonality) can be done on fixed tissues. Cytogenetics and flow cytometry require fresh tissue.
Next generation sequencing may hold diagnostic or prognostic significance in cases of non-Hodgkin lymphoma and can be performed on fresh or fixed tissues (assay dependent).
Staging tests
Once the diagnosis of lymphoma is made, staging tests are done.
A combined fluorodeoxyglucose (FDG)-PET/CT scan of the chest, abdomen, and pelvis is recommended. PET/CT provides accurate location of lesions, their size (from CT) and tumor metabolism (from FDG-PET). If combined FDG-PET/CT is not available, a contrast-enhanced CT scan of the chest, abdomen, and pelvis is done.
Unilateral bone marrow aspiration and biopsy is often done in patients with non-Hodgkin lymphoma. While marrow evaluation may be of diagnostic value, its utility in staging and prognosis in most lymphomas is less clear. Bone marrow assessment may be of limited value in settings where marrow involvement is unlikely (eg, early-stage diffuse large B-cell lymphoma) or in settings where results would not likely influence management (eg, advanced-stage disease).
Testing for complications and prognosis
Blood tests typically include complete blood count with white blood cell differential, kidney function and liver tests (including serum creatinine, bilirubin, calcium, aspartate aminotransferase, albumin , alkaline phosphatase, and lactate dehydrogenase), uric acid, beta-2 microglobulin, and vitamin D levels. Serum protein electrophoresis with IgG, IgA, and IgM immunoglobulin levels are also done.
Testing for etiology
After diagnosis, stage is determined to guide therapy. The commonly used Lugano staging system (see table ) incorporates
Physical examination findings
Results of imaging tests, including CT of the chest, abdomen, and pelvis, and functional imaging with FDG-PET
Bone marrow biopsy (in selected cases)
Although stage I non-Hodgkin lymphoma does occur, the disease is typically disseminated when first recognized.
Diagnosis reference
1. Cheson BD, Fisher RI, Barrington SF, et al : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32(27):3059–3068, 2014.
Treatment of Non-Hodgkin Lymphomas
Watch and wait (for indolent, largely asymptomatic lymphomas)
Chemotherapy
Radiation therapy (most common in patients with limited-stage disease and sometimes in those with advanced-stage disease)
Immunotherapy (eg, monoclonal antibodies or antibody-drug conjugates targeting CD20, CD19, or CD79; bispecific antibodies targeting CD20 and CD3; or chimeric antigen receptor T cells [CAR T cells])
Targeted drugs (eg, BTK [Bruton tyrosine kinase] inhibitors, PI3K [phosphoinositide 3-kinase] inhibitors, cereblon inhibitors, EZH2 [enhancer of zeste homolog 2] inhibitors, XPO1 [exportin 1] inhibitors)
Sometimes hematopoietic stem cell transplantation Hematopoietic Stem Cell Transplantation Hematopoietic stem cell (HSC) transplantation is a rapidly evolving technique that offers a potential cure for hematologic cancers ( leukemias, lymphomas, myeloma) and other hematologic disorders... read more (autologous or allogeneic)
Limited disease (stages I-II)

Limited-stage aggressive non-Hodgkin lymphomas can be managed with a combination of chemotherapy plus radiation therapy or with chemotherapy alone (plus anti-CD20 monoclonal antibodies for B-cell lymphomas).
Advanced disease (stages II-IV)
Stage II non-Hodgkin lymphoma is managed as advanced stage disease in many circumstances. Most patients with all types of non-Hodgkin lymphoma who have stage II to IV disease are candidates for chemoimmunotherapy. In these cases, radiation therapy may be used to limit the number of cycles of chemoimmunotherapy or provide localized treatment for residual sites of bulk disease.
For indolent lymphomas, treatment varies considerably. Because indolent lymphomas are highly treatable but not reliably curable, treatment may not be recommended initially for patients without symptoms. Some patients who do not have symptoms are given anti-CD20 immunotherapy using rituximab alone. This strategy can delay the need for myelosuppressive chemotherapy, but early immunotherapy alone has not been shown to impact overall survival. Patients with symptoms or bulky disease that puts vital organs at risk are treated with chemoimmunotherapy. In selected cases (eg, chemo-refractory with limited bone marrow involvement), radiolabeled anti-CD20 antibody can be used to target radiation to the tumor cell with potentially fewer effects on nearby normal organs.

The approach in peripheral T-cell non-Hodgkin lymphoma and primary central nervous system lymphoma is different. In these patients, autologous stem cell transplantation Hematopoietic Stem Cell Transplantation Hematopoietic stem cell (HSC) transplantation is a rapidly evolving technique that offers a potential cure for hematologic cancers ( leukemias, lymphomas, myeloma) and other hematologic disorders... read more may be offered to initial responders before relapse occurs with the intention of improving the likelihood of cure. In autologous stem cell transplantation, stem cells are obtained from the patient by peripheral blood leukopheresis and are transfused back into the patient after high-dose chemotherapy. Similarly, in some younger patients with mantle cell lymphoma who have responded to initial therapy, autologous stem cell transplantation may be done to prolong remission.
Lymphoma relapse
Patients with aggressive non-Hodgkin lymphoma who are not in remission at end of therapy or who relapse are treated with second-line chemotherapy regimens followed by autologous stem cell transplantation Hematopoietic Stem Cell Transplantation Hematopoietic stem cell (HSC) transplantation is a rapidly evolving technique that offers a potential cure for hematologic cancers ( leukemias, lymphomas, myeloma) and other hematologic disorders... read more if they are relatively young and in good health. In some patients at very high risk of relapse as well as in those for whom autologous transplant is not feasible or has already failed, stem cells from a matched sibling or unrelated donor (allogeneic transplants) can be effective. In general, the older the patient, the less likely an allogeneic transplantation will be offered because older patients have higher rates of transplantation complications.
Patients not eligible for either stem cell transplantation or CAR T cells, or for whom these treatments have failed, may receive treatment with various therapies, mostly for palliation. These therapies vary widely and are constantly changing as new treatments are developed.
In indolent lymphomas, patients may be managed using a wide variety of strategies depending on
Lymphoma-related factors (eg, histopathology, stage, molecular characteristics, immunologic characteristics)
Patient-related factors (eg, age, comorbidities)
The type of and response to prior therapy.
Many of the same agents used for first-line treatment may be given to patients in relapse. In some cases, the same treatment may be repeated if it was previously effective and well tolerated. High-dose chemotherapy combined with autologous stem cell transplantation Hematopoietic Stem Cell Transplantation Hematopoietic stem cell (HSC) transplantation is a rapidly evolving technique that offers a potential cure for hematologic cancers ( leukemias, lymphomas, myeloma) and other hematologic disorders... read more is used occasionally in patients who have high-risk lymphoma biology (including a poor response to chemotherapy), and although cure remains unlikely, remission may be superior to that with secondary palliative therapy alone. Reduced intensity allogeneic transplantation is a potentially curative option in some patients with indolent lymphoma. The mortality rate of patients undergoing myeloablative transplantation has decreased dramatically.
Complications of treatment
An immediate complication of most therapies is infection that occurs during periods of neutropenia Neutropenia Neutropenia is a reduction in the blood neutrophil count. If it is severe, the risk and severity of bacterial and fungal infections increase. Focal symptoms of infection may be muted, but fever... read more . Although use of growth factors that stimulate white blood cell production has helped, infection continues to pose a problem.
The gastrointestinal adverse effects of chemotherapy can be largely relieved or prevented by antiemetics and bowel programs.
After successful treatment, patients should be referred to a cancer survivorship clinic for a care plan that can be implemented by the patient's primary care team. This plan is tailored to the patient's comorbidities and risks specific to the treatment they received.
Chemotherapy and radiation therapy have late complications. In the first 10 years after treatment, there is a risk of myelodysplasia Myelodysplasia and Iron-Transport Deficiency Anemia In myelodysplastic syndrome, anemia is commonly prominent. The anemia is usually normocytic or macrocytic, and a dimorphic (large and small) population of circulating cells can be present. ... read more or acute leukemia Acute leukemias Leukemia is a malignant condition involving the excess production of immature or abnormal leukocytes, which eventually suppresses the production of normal blood cells and results in symptoms... read more due to bone marrow damage from certain chemotherapy agents. After 10 years, the risk of secondary cancers increases, especially in patients who received radiation to the chest.
Treatment references
1. Lo AC, Campbell BA, Pickles T, et al . Long-term outcomes for patients with limited-stage follicular lymphoma: update of a population-based study. Blood 2020;136(8):1006-1010. doi:10.1182/blood.2019004588
2. Wilder RB, Jones D, Tucker SL, et al . Long-term results with radiotherapy for Stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys 2001;51(5):1219-1227. doi:10.1016/s0360-3016(01)01747-3
3. Tilly H, Morschhauser F, Sehn LH, et al . Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med 2022;386(4):351-363. doi:10.1056/NEJMoa2115304
4. Abramson JS, Palomba ML, Gordon LI, et al . Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396(10254):839-852. doi:10.1016/S0140-6736(20)31366-0
5. Abramson JS, Solomon SR, Arnason J, et al . Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood 2023;141(14):1675-1684. doi:10.1182/blood.2022018730
5. Locke FL, Miklos DB, Jacobson CA, et al . Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 2022;386(7):640-654. doi:10.1056/NEJMoa2116133
7. Neelapu SS, Locke FL, Bartlett NL, et al . Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377(26):2531-2544. doi:10.1056/NEJMoa1707447
8. Schuster SJ, Bishop MR, Tam CS, et al . Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380(1):45-56. doi:10.1056/NEJMoa1804980
Prognosis for Non-Hodgkin Lymphomas
Prognosis varies by the type and stage of lymphoma and individual patient factors. In general, patients with peripheral T-cell or natural killer (NK)/T-cell lymphomas typically have a worse prognosis than those with B-cell non-Hodgkin lymphoma. Within each non-Hodgkin lymphoma variant, prognosis is related to differences in tumor cell biology.
The most commonly used prognostic scoring system is the International Prognostic Index (IPI) for diffuse large B-cell lymphoma . However, the IPI score is used only for diffuse large B-cell lymphoma (DLBCL). There are also scoring systems for follicular lymphoma (FLIPI) and mantle cell lymphoma (MIPI). Online calculators are available to estimate prognosis in other types of non-Hodgkin lymphoma as well.
The IPI considers 5 risk factors:
Age > 60 years
Poor performance status (can be measured using the Eastern Cooperative Oncology Group tool )
Elevated lactate dehydrogenase (LDH) level
> 1 extranodal site
Stage III or IV disease
Outcome is worse with an increasing number of risk factors. Patients without any of the risk factors have a very high cure rate. The original IPI score uses the 5 factors as discrete variables (eg, either age over 60 years or under 60 years). A modification, the Diffuse Large B-cell Lymphoma Prognosis (IPI24) , which calculates the chance of being disease free at 24 months from diagnosis, includes the above factors as continuous variables and also includes absolute lymphocyte count.
Non-Hodgkin lymphomas are a group of related cancers involving lymphocytes; they vary significantly in their rate of growth and response to treatment.
The disease is usually already disseminated at the time of diagnosis.
Molecular and genetic tests are essential for diagnosis and management.
Limited indolent disease may be treated with radiation therapy.
Treat more advanced disease (indolent or aggressive) with immunotherapy, chemotherapy, hematopoietic stem cell transplantation, or a combination depending on the type and stage of non-Hodgkin lymphoma.
The following English language resource provides information for clinicians and support and information for patients. THE MANUAL is not responsible for the content of this resource.
Leukemia & Lymphoma Society: Resources for Healthcare Professionals : provides educational resources for health care practitioners as well as information for patient referrals

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Manual
- IN THIS TOPIC
Lymphomas: pathogenesis, clinical features and diagnosis
Lymphomas are some of the most common cancers in the UK, with a wide variation in disease progression and prognosis between subtypes.
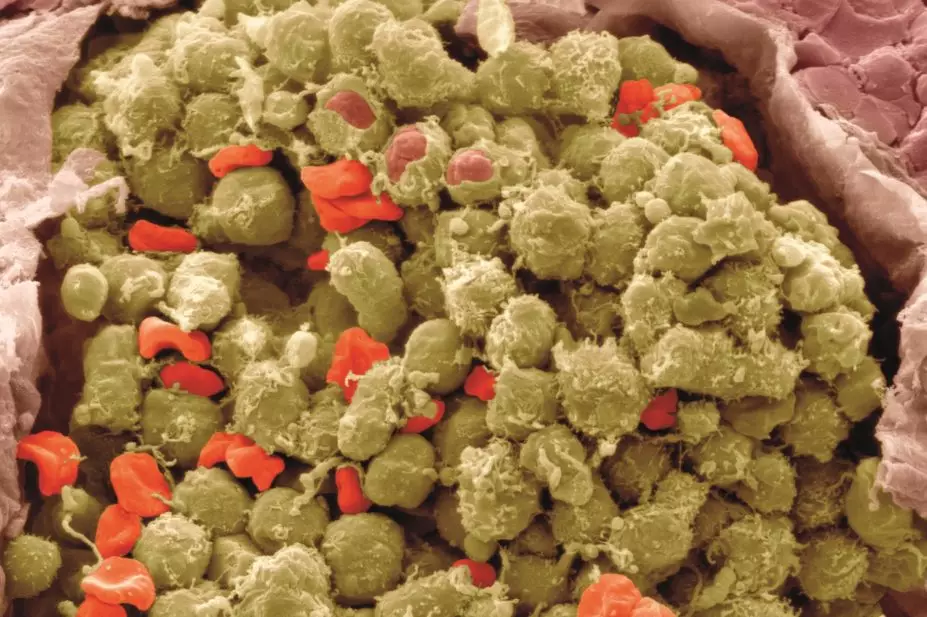
Steve Gschmeissner / Science Photo Library
The lymphomas are a large group of blood cancers with many subtypes. Hodgkin lymphoma has an incidence of 2.8 per 100,000 people per year in the UK, while non-Hodgkin lymphoma has an incidence of 15.5 per 100,000 people per year.
Both forms typically present with painless enlarged lymph nodes. Around a quarter of patients with Hodgkin lymphoma will also have night sweats, unexplained fever and weight loss. Diagnosis is made following a lymph node biopsy, a CT scan, and – in non-Hodgkin lymphoma – a bone marrow biopsy.
The lymphomas are a heterogenous group of blood cancers caused by the clonal proliferation of B or T lymphocytes. There are a large number of recognised subtypes of lymphoma, and it is beyond the scope of these articles to discuss each of them individually. Instead, the main focus will be on Hodgkin lymphoma and the most common forms of non-Hodgkin lymphoma.
Incidence and risk factors
Hodgkin lymphoma has an incidence in the UK of 2.8 per 100,000 people per year, which translates into just over 1,800 new cases per year [1] . The incidence of both Hodgkin lymphoma and non-Hodgkin lymphoma has increased by 11-15% in the past decade. This is most likely because of a combination of better diagnosis and reporting, the ageing population and an increase in the number of patients with a compromised immune system, such as those with HIV and AIDS.
Hodgkin lymphoma has a bimodal distribution, with an initial peak in young adults aged 20–24 years and a second peak between the ages of 70 years and 80 years, although it can occur at any age [1] .
The disease is slightly more common in men, with an incidence ratio of 1.2:1. The cause of Hodgkin lymphoma is not known, but it does have a strong association with being infected with Epstein-Barr virus, which is implicated in 45% of cases. It also occurs more commonly in patients who are immunocompromised; HIV infection is associated with an 11-fold increase in risk of Hodgkin lymphoma [1] , and patients who are receiving immunosuppressant therapy following an organ transplant or with autoimmune conditions such as rheumatoid arthritis and systemic lupus erythematosus are also at increased risk.
A small increase in risk of Hodgkin lymphoma has also been associated with tobacco exposure, having a first degree relative with the disease, and obesity. Rates of Hodgkin lymphoma in younger patients are lower for those with three or more siblings, suggesting that exposure to common childhood infections may somehow reduce the risk of developing the disease [2] .
Non-Hodgkin lymphoma has an incidence in the UK of 15.5 per 100,000 people per year, with almost 14,000 new cases reported in 2011 [1] . It is the sixth most common type of cancer in the UK, and accounts for about 4% of all cancers. It has a relatively good prognosis and, despite its high incidence, is the tenth most common cause of cancer death in the UK.
The incidence of non-Hodgkin lymphoma correlates closely with increasing age, and the majority of cases occur in patients aged 65 years or older. One exception to this rule is the relatively uncommon Burkitt’s lymphoma, in which almost 50% of cases occur in patients younger than 45 years.
There is a strong association between immunodeficiency, such as HIV infection, and risk of developing non-Hodgkin lymphoma. Recipients of organ transplantation who are receiving immunosuppressants such as ciclosporin or tacrolimus are at risk of developing post-transplant lymphoproliferative disease (PTLD), a proliferation of B cells caused by the Epstein-Barr virus that, if untreated, can progress to non-Hodgkin lymphoma.
Burkitt’s lymphoma is a highly aggressive form of non-Hodgkin lymphoma that, in its endemic form, is associated with malarial regions of equatorial Africa. In the UK, it accounts for about 2% of cases of lymphoma. It is more common in children and young adults.
The Epstein-Barr virus is implicated in the development of Burkitt’s lymphoma, although its overall importance as a risk factor is much less than for Hodgkin lymphoma.
Helicobacter pylori infection is strongly associated with mucosa-associated lymphoid tissue (MALT) lymphoma, a form of non-Hodgkin lymphoma that occurs in the stomach. H.pylori eradication regimens are the mainstay of treatment for this relatively rare subtype.
Other risk factors for non-Hodgkin lymphoma include hepatitis B and hepatitis C, working in rubber production and exposure to chemicals such as benzene and ethylene oxide. There is no proven association between smoking and an increased risk of non-Hodgkin lymphoma.
Classification
A variety of classification systems has been developed for lymphomas. The World Health Organization (WHO) classification, last updated in 2008, is currently the most widely used and recognises more than 50 different subtypes [3] .
Hodgkin lymphoma can itself be subdivided into two forms: classic Hodgkin lymphoma, which accounts for 95% of cases, and nodular lymphocyte predominant Hodgkin lymphoma.
The simplest way of classifying non-Hodgkin lymphomas is by the cell of origin. More than 90% originate in B-lymphocytes, with less than 10% being T-cell or NK cell lymphomas.
Clinically, it is often useful to separate non-Hodgkin lymphoma into aggressive (high grade) and indolent (low grade) forms (see ‘Aggressive and indolent lymphomas’).
Presentation
Hodgkin lymphoma commonly presents with painless swollen lymph nodes (lymphadenopathy), often affecting the cervical or supraclavicular nodes in the neck. About 25% of patients present with the three ‘B symptoms’: night sweats, unexplained fever and weight loss of more than 10% over six months. These symptoms are associated with a poorer prognosis [4] . Other presenting features include fatigue, itching and alcohol-induced pain.
Non-Hodgkin lymphoma also classically presents with painless enlarged lymph nodes. These are usually widespread in indolent lymphomas (such as follicular lymphoma), whereas progression is more rapid and often accompanied by B symptoms in aggressive lymphomas, such as diffuse large B-cell lymphoma.
In both Hodgkin lymphoma and non-Hodgkin lymphoma, patients are more likely to be unwell due to chemotherapy side effects than their cancer. A minority of patients will have lymphoma present in the bone marrow, which can lead to symptoms related to myelosuppression. These include fatigue, breathlessness, increased susceptibility to infections and unexpected bruising or bleeding.
Rarely, the location of the lymphoma mass may cause life-threatening complications such as spinal cord compression or obstruction of the superior vena cava. These are medical emergencies that require urgent treatment with chemotherapy or radiotherapy.
A lymph node or extranodal tissue biopsy is used to diagnose lymphoma, and immunohistochemistry is used to guide classification.
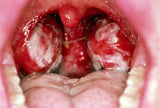
For example, classic Hodgkin lymphoma is defined by the presence of Reed-Sternberg (RS) cells, which stain positive for the antigens CD30 and CD15 located on the cell’s membranes. In contrast, lymphocyte predominant cells, which characteristically stain positive for the antigens CD20 and CD45, are expressed by cells in lymphocyte-predominant Hodgkin lymphoma [5] . These differences in surface antigen expression have important implications for treatment selection.
Determining the stage of the disease generally involves a contrast-enhanced computed tomography (CT) scan of the neck, chest, abdomen and pelvis and (in non-Hodgkin lymphoma) a bone marrow biopsy. Recently positron emission tomography (PET) scanning has become more widely used, both as part of diagnosis and as a means of accurately assessing response to treatment.
In addition, full blood count, lactic dehydrogenase, erythrocyte sedimentation rate, liver enzymes and urea and creatinine should be checked, and patients should be screened for hepatitis B, hepatitis C and HIV. Baseline cardiac function should be checked in patients who are going to receive anthracycline-based chemotherapy (see accompanying article).
Patients with either Hodgkin lymphoma or non-Hodgkin lymphoma are classified according to the Ann Arbor staging system (see ‘Ann Arbor staging system’).
For Hodgkin lymphoma, the following categories are then used to guide treatment [5] :
- Limited stage – Ann Arbor stage 1 or 2 disease without risk factors
- Intermediate stage – Ann Arbor stage 1 or 2 disease with risk factors (e.g., age >50 years, large mediastinal mass, elevated erythrocyte sedimentation rate)
- Advanced stage – Ann Arbor stage 3 or 4 disease
Around one third of patients with Hodgkin lymphoma will have advanced stage disease at diagnosis, and their prognosis can be calculated further (see ‘Hasenclever International Prognostic Score’) [6] .
Similar scoring systems can be used to guide treatment for some non-Hodgkin lymphomas, such as the international prognostic index (IPI) [7] for diffuse large B cell lymphoma, and the follicular lymphoma international prognostic index (FLIPI) [8] for follicular lymphoma.
The prognosis for patients diagnosed with non-Hodgkin lymphoma in the UK has improved markedly in the past 30 years. The five-year survival rate is 63%, and half of all patients survive for at least ten years after diagnosis [1] .
However, there are marked variations in survival rates between subtypes of the disease. The most recent UK statistics indicate that 87% of patients with follicular lymphoma survive for at least five years, compared with 27% of patients with mantle cell lymphoma [1] . In general, patients with the rarer T-cell lymphomas have a poorer prognosis than patients with B-cell lymphomas.
Hodgkin lymphoma has a cure rate in the region of 80-90% [5] . The prognosis after intensive chemotherapy (with or without radiotherapy) has improved so much in recent decades that the focus has begun to move towards potentially less intensive approaches, with the aim of reducing long-term complications of therapy such as cardiac and pulmonary toxicity.
Nick Duncan MRPharmS MSc is principal pharmacist in haematology and oncology at University Hospitals Birmingham NHS Foundation Trust.
[1] Cancer Research UK. Cancer stats (Online). http://publications.cancerresearchuk.org/cancerstats (accessed 4 September 2014).
[2] Chang ET, Montgomery SM, Richiardi L et al. Number of siblings and risk of Hodgkin’s lymphoma. Cancer Epidemiology Biomarkers Prevention 2004;13:1236–1243.
[3] Swerdlow, Steven H. International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumours 2 (4th ed.). Geneva: World Health Organization, 2008.
[4] Follows GA, Ardeshna KM, Barrington SF et al. Guidelines for the first line management of classical Hodgkin lymphoma. Br J Haem 2014;166:34–49.
[5] Eichenauer DA, Engert A, André M et al. Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(suppl 3):iii70–iii75.
[6] Hasenclever D, Diehl V, Armitage JO et al . A prognostic score for advanced Hodgkin’s disease. New Engl J Med 1998;339:1506–1514.
[7] The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. New Engl J Med 1993;329:987–994.
[8] Buske C, Hoster E, Dreyling M et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) with respect to treatment outcome. Blood 2006;108:1504–1508.
You might also be interested in…

Community pharmacy pilot to focus on spotting patients with early signs of lung cancer

First referrals for ‘red flag’ cancer symptoms made under community pharmacy pilot

Our drive to increase diversity on the stem cell register
Demystifying the diagnosis and classification of lymphoma: a guide to the hematopathologist’s galaxy
Gabriel Caponetti, MD, and Adam Bagg, MD
Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia

JCSO 2017;15(1):43-48. ©2017 Frontline Medical Communications. doi: https://doi.org/10.12788/jcso.0328.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient. 1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
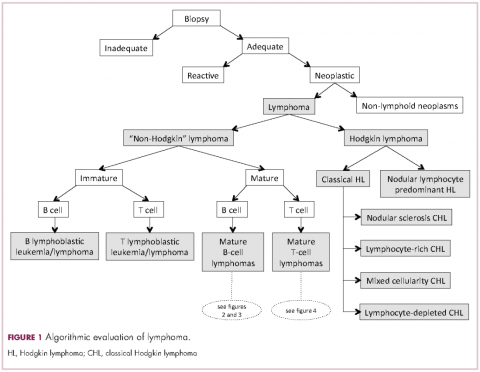
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms. 2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated 3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide. 1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable. 1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis. 2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
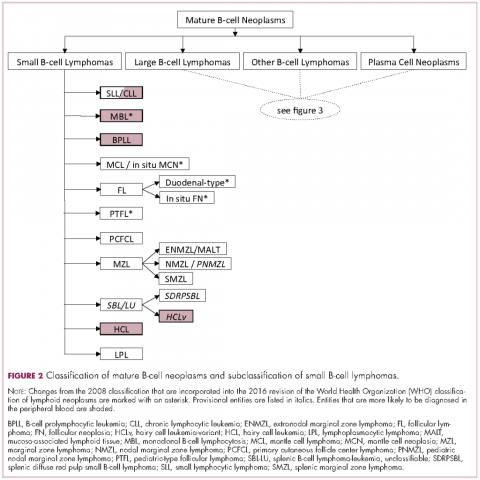
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt ) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/ IGH - BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
Recommended Reading
- Lymphoma & Plasma Cell Disorders
- Patient & Survivor Care
- Follicular Lymphoma
- MDedge Home
- Clinician Reviews
- Dermatology
- Emergency Medicine
- Endocrinology/Diabetes
- Family Medicine
- Federal Practitioner
- Gastroenterology
- Hematology/Oncology
- Infectious Disease
- Internal Medicine
- Obstetrics And Gynecology
- Pulmonology
- Rheumatology
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The definition of primary GI lymphoma has differed among authors, but typically refers to a lymphoma that predominantly involves any section of the GI tract from the oropharynx to the rectum [ 1,2 ]. While the disease typically involves a single primary site, multiple sites within the GI tract may be involved, as can local and distant lymph nodes. The vast majority are non-Hodgkin lymphomas (NHLs), although Hodgkin lymphoma has been reported [ 3,4 ].
GI lymphomas typically present with nonspecific signs and symptoms attributable to the site of involvement. This topic review will discuss the salient clinical features and diagnostic evaluation of GI lymphomas. The management of GI lymphoma is presented separately. (See "Treatment of extranodal marginal zone lymphoma of mucosa associated lymphoid tissue (MALT lymphoma)" .)
Distribution — The GI tract is the predominant site of extranodal non-Hodgkin lymphomas (NHLs) [ 5 ]. Primary NHLs of the GI tract are rare, accounting for only 1 to 4 percent of malignancies arising in the stomach, small intestine, or colon [ 6 ]. In contrast, secondary GI involvement is relatively common, occurring in approximately 10 percent of patients with limited stage NHL at the time of diagnosis, and up to 60 percent of those dying from advanced NHL [ 5,7 ]. (See "Epidemiology and clinical features of small bowel neoplasms" .)
The two largest studies of GI lymphoma reported the following sites of involvement in Greek and German populations [ 8,9 ]:
- Request A New Patient Appointment
- Doctor Directory
- Other Provider Directory
- Find a Location
- Your First Appointment With OHC
- New Patient Forms
- Who to Call For Symptoms
- List of Clinical Trials
- Clinical Trial Locations
- Frequently Asked Questions About Clinical Trials
- OHC Locations, Phone, Fax
- Frequently Asked Questions
- Financial Help and Support
- Pharmacy Services
- Community Support Resources
- Patient Guide to Cancer Treatment
- Patient Guide to Radiation Treatment
- Patient Guide for Nutrition
- What Differentiates OHC?
- Patient Referral Form
- OHC Forms For Your Patient
- OHC Medical Records Forms
- OHC Patient Genetic Counseling Referral Form
- Oncology Liaison Visits & Support
- Treatments & Services
- Stories of Hope
- OHC Newsletters
- DAISY Award
- News Releases
- Our Clinical Team
- Our Leaders
- Certifications, Recognitions & Innovations
- Public Notice
- Refer a Patient
- Pay Your Bill
The Clinical Presentation of Lymphomas

From Miguel Islas-Ohlmayer, M.D.
April 20, 2015

Miguel Islas-Ohlmayer, M.D.
Lymphomas, blood cell tumors that usually originate in lymphatic tissues (and can spread to other organs), are among the most diverse and most curable of all malignancies. There are two major groups of lymphomas: About 90 percent are non-Hodgkin lymphomas (NHLs) and about 10 percent are Hodgkin lymphomas (HLs).
NON-HODGKIN LYMPHOMAS (NHLs) Let’s start with NHLs, malignant neoplasms derived from B cell progenitors, T cell progenitors, mature B cells, mature T cells, or (rarely) natural killer cells. NHLs are the seventh most common type of cancer in American men and the sixth most common in American women. The American Cancer Society estimates nearly 72,000 cases and 20,000 deaths in 2015.
Clinical Presentation
Most NHL patients are first seen by their PCPs because they are showing signs of lymphadenopathy, although the enlarged nodes are usually painless. Here’s what your patients might present with:
- Aggressive NHLs usually present acutely or subacutely with a rapidly growing mass, systemic B symptoms (fever, night sweats, weight loss), and/or elevated levels of serum lactate. Treatment in these cases should be treated promptly to manage symptoms, slow or revert progression, and optimize survival.
- Indolent lymphomas often present only with slow-growing lymphadenopathy, hepatomegaly, splenomegaly, or cytopenias.
- Less commons presentations include skin rash, pruritus, hypersensitivity to insect stings and bites, generalized fatigue, malaise, fever of unknown origins, ascites, and effusions.
- Patients with primary GI track lymphoma may present with anorexia, weight loss, nausea and vomiting, chronic pain, abdominal fullness, early satiety, symptoms of visceral obstruction, or even acute perforation and GI hemorrhage.
- Patients with primary CNS lymphoma may have headache, lethargy, facial neurologic symptoms, seizures, paralysis, spinal cord compression or lymphomatous meningitis.
Risk Factors:
- Getting older is a strong risk factor for lymphoma overall, with most cases occurring in people in their 60s or older
- The risk of NHL is higher in men than in women, but there are certain types of non-Hodgkin lymphoma that are more common in women
- NHL in the United States is more common in Caucasians than African Americans or Asians
- Inordinate contact with chemicals such as benzene, and certain herbicides and insecticides
- Inordinate radiation exposure
- History of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, Sjogren disease, and celiac sprue disease
- History of infections that directly transform lymphocytes such as T-cell leukemia/lymphoma virus (HTLV-1) and the Epstein-Barr virus (EBV)
- History of therapy with immunosuppressant drugs used in organ transplants, autoimmune disease, and HIV
- Inherited immune disorders such as hypogammaglobulinemia and Wiskott-Aldrich syndrome
HODGKIN LYMPHOMAS (HLs)
There are two major types of Hodgkin lymphomas: classical Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma. Both are marked by the presence of HLs of the Reed-Sternberg cell. Note that this type of cell also can be found in reactive lymphadenopathy (such as infectious mononucleosis) and more rarely in other types of non-Hodgkin lymphomas. Both are marked by the presence of Reed-Sternberg cells.
According to the National Cancer Institute, the five-year survival rate (2004-2010) is 85.3 percent. And the number of deaths has been declining. Clinical Presentation:
Because there are no widely recommended screening tests for Hodgkin lymphomas, you will need to pay attention to your patient’s risk factors in combination with these clinical presentations:
- Asymptomatic lymphadenopathy, above the diaphragm 80% of time but also found under the arm or in the groin
- Intermittent fever is observed in approximately 35% of cases; infrequently, the classic Pel-Ebstein fever is observed (high fever for 1-2 weeks, followed by an afebrile period of 1-2 weeks)
- Chest pain, cough, shortness of breath, or a combination of those may be present due to a large mediastinal mass or lung involvement
- Patients may present with pruritus
- Pain at sites of nodal disease, precipitated by drinking alcohol, occurs in fewer than 10% of patients but is specific for Hodgkin lymphoma
- Back or bone pain may rarely occur
- A family history is also helpful; in particular, nodular sclerosis Hodgkin lymphoma (NSHL) has a strong genetic component and has often previously been diagnosed in the family
- History of infectious mononucleosis caused by Epstein Barr virus (EBV)
- Occurs slightly more often in men than women
- Most common with young adults (peaking at about 20) and older adults (peaking at about 65); and those in the United States, Canada, and Northern Europe
- History of younger siblings with the disease (but only by 5% per the American Cancer Society), and especially so with identical twins
- People from higher socioeconomic backgrounds (though the correlation is still unclear)
- People infected with HIV
- Lymphoma is widely diverse group of diseases that originate in lymphatic tissues
- Because the symptoms caused by lymphomas are often the same as those from infections, patient history, risk factors, and clinical presentation need to be viewed in total
- Presentation most often includes asymptomatic lymphadenopathy, usually found in the neck (above the diaphragm)
- Precise diagnosis and classification requires evaluation of lymph node tissue obtained via adequately sized biopsy
- Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs) are curable in the majority of patients
Miguel Islas-Ohlmayer, M.D. , is a hematologist and blood and marrow transplant physician. Board certified in internal medicine and hematology, his main interests include hematologic malignancies as well as blood and marrow stem cell transplantation. Dr. Islas-Ohlmayer practices at our OHC Kenwood office and is a clinician at the Blood Cancer Center at The Jewish Hospital-Mercy Health. The Blood Cancer Center, powered by OHC physicians, is a recent recipient of The Joint Commission’s Gold Seal of Approval for Blood & Marrow Transplants .
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Colorectal Cancer: Risk Factors, Symptoms & Treatment Options
March 22, 2024
March is National Colorectal Cancer Month. According to the American Cancer Society, colorectal cancer is the third most
Help Reduce Your Family’s Risk of Colon Cancer With These Foods
March 19, 2024
Colorectal cancer is the third most common cancer diagnosed in both women and men in the U.S., excluding skin cancers.
- Find a Doctor
- Clinical Trials
- Patient Resources
- For Medical Professionals
- Request an Appointment
Oncology Hematology Care, Inc.
Corporate Office
5053 Wooster Road
Cincinnati, OH 45226
1-800-710-4674 toll free
513-762-2483 fax
© 2015-2024 Oncology Hematology Care, Inc.
All Rights Reserved.
Privacy Policy
You are using an outdated browser. Please upgrade your browser to improve your experience.
Skip to Site Navigation Skip to Page Content
- Physician Referrals
- Patient Resources
- Why UT Southwestern
Refine your search: Find a Doctor Search Conditions & Treatments Find a Location

Appointment New Patient Appointment or Call 214-645-4673
Lymphoma Awareness and Prevention
Appointment New Patient Appointment or 214-645-4673
Explore Lymphoma
While the risk factors and causes of lymphoma aren’t yet fully understood, it’s important to be aware of the suspected risk factors, especially for people who have a family history of the disease.
Risk Factors
For non-Hodgkin lymphoma (NHL), suspected risk factors include:
- Age, most common in people age 60 or older
- Ethnicity, with Caucasians having a higher risk of developing NHL than African-Americans and Asian-Americans
- Exposure to radiation and certain chemicals
- Immune system deficiency
- Some autoimmune diseases
- Infections that weaken the immune system, such as HIV, Hepatitis B, and Hepatitis C
Suspected risk factors for Hodgkin lymphoma (HL) include:
- Epstein-Barr virus infection/mononucleosis
- Age, most common in people between ages 15 and 40, particularly those in their 20s
- Family history
- HIV infection
Symptoms of Lymphoma
Some people experience no symptoms during early-stage lymphoma, and the disease is discovered during unrelated medical care or testing. Others do experience symptoms, such as:
- Painless swelling in one or more lymph nodes, often in the neck, armpit, upper chest, or groin
- Unexplained or persistent fever, fatigue, or weight loss
- Drenching sweats, particularly at night
- Persistent cough or chest pain
- Loss of appetite
- Abdominal bloating or tenderness due to an enlarged spleen
- Shortness of breath with normal activity
- Rashes or lumps on the skin
- Lymph node pain after drinking alcohol
Lymphoma doesn’t always start in the lymph nodes; it sometimes originates in sites such as the skin, bones, lungs, or gastrointestinal tract. In those cases, patients might experience symptoms related to those specific areas.
Patients should speak with their doctor if they experience any symptoms of lymphoma.
Presentation, staging and diagnosis of lymphoma: a clinical perspective
Affiliation.
- 1 Department of Physiology, Fatima Jinnah Dental College, Karachi, Pakistan. [email protected]
- PMID: 19999217
Background: Due to lack of awareness among health professionals, lymphoma is often misdiagnosed. This study was done to evaluate the clinical features and histopathologic subtypes of lymphoma.
Methods: Sixty diagnosed cases of lymphoma were selected (aged 12-65 years) from medical units of Civil Hospital Karachi, during 1993 to 1998. Clinical history, physical examination and basic laboratory investigations including imaging procedures were done in all the patients. The diagnosis of lymphoma was based on histology, following the International Working Formulation classification system. This included lymph node biopsy and in some cases, biopsy of the bone marrow. The Ann Arbor Staging Classification was used to classify the extent of disease.
Results: Out of 60 cases of lymphoma, 81.6% (49 cases) were diagnosed as non-Hodgkin's lymphoma and 18.3% (11 cases) as Hodgkin's disease, with an overall male predominance. Both categories exhibited a bimodal age distribution. Lymphadenopathy was the commonest presenting features in both the types of lymphomas; however, patients with Hodgkin's disease had a prominence of 'B' symptoms, whereas abdominal signs and symptoms were more common in non-Hodgkin's lymphoma. On histopathology, majority of non-Hodgkin's lymphomas (91.8%) showed a diffuse pattern, while mixed cellularity was the commonest type seen in Hodgkin's disease (81.8%).
Conclusion: Non-Hodgkin's lymphoma was 4 times more common than Hodgkin's disease. The vast clinical spectrum of lymphoma sometimes delays its diagnosis, leading to its eventual presentation in late stages. A general awareness is hence required among the health professionals regarding its varied clinical presentations.
- Cross-Sectional Studies
- Hospitals / statistics & numerical data
- Lymphoma / diagnosis*
- Lymphoma / epidemiology
- Lymphoma / pathology
- Middle Aged
- Neoplasm Staging
- Pakistan / epidemiology
- Young Adult
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 11 April 2024
Lymphatic vessels in the age of cancer immunotherapy
- Triantafyllia Karakousi 1 na1 ,
- Tenny Mudianto 1 na1 &
- Amanda W. Lund ORCID: orcid.org/0000-0001-7389-9983 1 , 2 , 3
Nature Reviews Cancer ( 2024 ) Cite this article
22 Altmetric
Metrics details
- Cancer immunotherapy
- Immunosurveillance
- Tumour immunology
Lymphatic transport maintains homeostatic health and is necessary for immune surveillance, and yet lymphatic growth is often associated with solid tumour development and dissemination. Although tumour-associated lymphatic remodelling and growth were initially presumed to simply expand a passive route for regional metastasis, emerging research puts lymphatic vessels and their active transport at the interface of metastasis, tumour-associated inflammation and systemic immune surveillance. Here, we discuss active mechanisms through which lymphatic vessels shape their transport function to influence peripheral tissue immunity and the current understanding of how tumour-associated lymphatic vessels may both augment and disrupt antitumour immune surveillance. We end by looking forward to emerging areas of interest in the field of cancer immunotherapy in which lymphatic vessels and their transport function are likely key players: the formation of tertiary lymphoid structures, immune surveillance in the central nervous system, the microbiome, obesity and ageing. The lessons learnt support a working framework that defines the lymphatic system as a key determinant of both local and systemic inflammatory networks and thereby a crucial player in the response to cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Morton, D. L. et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 370 , 599–609 (2014).
Article CAS PubMed PubMed Central Google Scholar
Zahoor, S. et al. Sentinel lymph node biopsy in breast cancer: a clinical review and update. J. Breast Cancer 20 , 217 (2017).
Article PubMed PubMed Central Google Scholar
Stacker, S. A. et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14 , 159–172 (2014).
Article CAS PubMed Google Scholar
Leiter, U. et al. Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J. Clin. Oncol. 37 , 3000–3008 (2019).
Faries, M. B. et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N. Engl. J. Med. 376 , 2211–2222 (2017).
Patel, S. P. et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N. Engl. J. Med. 388 , 813–823 (2023).
Rozeman, E. A. et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 27 , 256–263 (2021).
Provencio, M. et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 389 , 504–513 (2023).
Delclaux, I., Ventre, K. S., Jones, D. & Lund, A. W. The tumor-draining lymph node as a reservoir for systemic immune surveillance. Trends Cancer 10 , 28–37 (2023).
Article PubMed Google Scholar
Reticker-Flynn, N. E. et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185 , 1924–1942.e23 (2022). First preclinical demonstration that LN metastasis suppresses systemic immune surveillance through the local induction of T reg cells and thereby indirectly enables distant tumour progression.
Lei, P.-J. et al. Cancer cell plasticity and MHC-II-mediated immune tolerance promote breast cancer metastasis to lymph nodes. J. Exp. Med. 220 , e20221847 (2023).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science 369 , eaax4063 (2020).
Chary, S. R. & Jain, R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA 86 , 5385–5389 (1989).
Fleury, M. E., Boardman, K. C. & Swartz, M. A. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 91 , 113–121 (2006).
Charman, S. A., McLennan, D. N., Edwards, G. A. & Porter, C. J. H. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm. Res. 18 , 1620–1626 (2001).
Martel, C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123 , 1571–1579 (2013).
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11 , 526–538 (2007).
Hansen, K. C., D’Alessandro, A., Clement, C. C. & Santambrogio, L. Lymph formation, composition and circulation: a proteomics perspective. Int. Immunol. 27 , 219–227 (2015).
Clement, C. C. & Santambrogio, L. The lymph self-antigen repertoire. Front. Immunol. 4 , 424 (2013).
Yao, L.-C., Baluk, P., Srinivasan, R. S., Oliver, G. & McDonald, D. M. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am. J. Pathol. 180 , 2561–2575 (2012).
Zhang, F. et al. Lacteal junction zippering protects against diet-induced obesity. Science 361 , 599–603 (2018).
Churchill, M. J. et al. Infection-induced lymphatic zippering restricts fluid transport and viral dissemination from skin. J. Exp. Med. 219 , e20211830 (2022).
Jannaway, M. et al. VEGFR3 is required for button junction formation in lymphatic vessels. Cell Rep. 42 , 112777 (2023).
Triacca, V., Güç, E., Kilarski, W. W., Pisano, M. & Swartz, M. A. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ. Res. 120 , 1440–1452 (2017).
Prevo, R., Banerji, S., Ferguson, D. J. P., Clasper, S. & Jackson, D. G. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276 , 19420–19430 (2001).
Proulx, S. T. et al. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 70 , 7053–7062 (2010).
Harrell, M. I., Iritani, B. M. & Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 170 , 774–786 (2007).
Gogineni, A. et al. Inhibition of VEGF-C modulates distal lymphatic remodeling and secondary metastasis. PLoS ONE 8 , e68755 (2013).
Broggi, M. A. S. et al. Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J. Exp. Med. 216 , 1091–1107 (2019).
García-Silva, S. et al. Use of extracellular vesicles from lymphatic drainage as surrogate markers of melanoma progression and BRAF V600E mutation. J. Exp. Med. 216 , 1061–1070 (2019).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22 , 19–29 (2005).
Rantakari, P. et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 16 , 386–396 (2015).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30 , 264–276 (2009).
Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. I. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node. Cortex. J. Exp. Med. 192 , 1425–1440 (2000).
Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27 , 160–171 (2007).
Pape, K. A., Catron, D. M., Itano, A. A. & Jenkins, M. K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26 , 491–502 (2007).
Steele, M. M. & Lund, A. W. Afferent lymphatic transport and peripheral tissue immunity. J. Immunol. 206 , 264–272 (2021).
Loo, C. P. et al. Lymphatic vessels balance viral dissemination and immune activation following cutaneous viral infection. Cell Rep. 20 , 3176–3187 (2017).
Lund, A. W. et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest. 126 , 3389–3402 (2016).
Ferris, S. T. et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 584 , 624–629 (2020).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α + dendritic cells in cytotoxic T cell immunity. Science 322 , 1097–1100 (2008).
Roberts, E. W. et al. Critical role for CD103 + /CD141 + dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30 , 324–336 (2016).
Salmon, H. et al. Expansion and activation of CD103 + dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44 , 924–938 (2016).
Vigl, B. et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 118 , 205–215 (2011).
Saeki, H., Moore, A. M., Brown, M. J. & Hwang, S. T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162 , 2472–2475 (1999).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99 , 23–33 (1999).
Davalos-Misslitz, A. C. M. et al. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur. J. Immunol. 37 , 613–622 (2007).
Thomas, S. N. et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 189 , 2181–2190 (2012).
Pflicke, H. & Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206 , 2925–2935 (2009).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204 , 2349–2362 (2007).
Lämmermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453 , 51–55 (2008).
Nitschké, M. et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood 120 , 2249–2258 (2012).
Miteva, D. O. et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 106 , 920–931 (2010).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339 , 328–332 (2013).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32 , 703–713 (2010).
Russo, E. et al. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep. 14 , 1723–1734 (2016).
Bastow, C. R. et al. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc. Natl Acad. Sci. USA 118 , e2025763118 (2021).
Friess, M. C. et al. Mechanosensitive ACKR4 scavenges CCR7 chemokines to facilitate T cell de-adhesion and passive transport by flow in inflamed afferent lymphatics. Cell Rep. 38 , 110334 (2022).
Ulvmar, M. H. et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 15 , 623–630 (2014).
Druzd, D. et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46 , 120–132 (2017).
Holtkamp, S. J. et al. Circadian clocks guide dendritic cells into skin lymphatics. Nat. Immunol. 22 , 1375–1381 (2021). This study enhanced our understanding of oscillatory DC migration through lymphatic vessels, demonstrating rhythmic expression of key transcripts in LECs whose protein products mediate DC migration and which are dependent on the clock gene Bmal1 .
Qian, D. C. et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 22 , 1777–1786 (2021).
Johnson, L. A. et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 203 , 2763–2777 (2006).
Johnson, L. A., Prevo, R., Clasper, S. & Jackson, D. G. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J. Biol. Chem. 282 , 33671–33680 (2007).
Johnson, L. A. et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 18 , 762–770 (2017).
Maddaluno, L. et al. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J. Exp. Med. 206 , 623–635 (2009).
Debes, G. F. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6 , 889–894 (2005).
Bromley, S. K., Thomas, S. Y. & Luster, A. D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6 , 895–901 (2005).
Brown, M. N. et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J. Immunol. 185 , 4873–4882 (2010).
Torcellan, T. et al. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc. Natl Acad. Sci. USA 114 , 5677–5682 (2017).
Pham, T. H. M. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207 , 17–27 (2010).
Skon, C. N. et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14 , 1285–1293 (2013).
Evrard, M. et al. Sphingosine 1-phosphate receptor 5 (S1PR5) regulates the peripheral retention of tissue-resident lymphocytes. J. Exp. Med. 219 , e20210116 (2022).
Mackay, L. K. et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194 , 2059–2063 (2015).
Locati, M. et al. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 16 , 679–686 (2005).
Weber, M. et al. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell 15 , 2492–2508 (2004).
De La Torre, Y. M. et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur. J. Immunol. 35 , 1342–1346 (2005).
Article Google Scholar
Vetrano, S. et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut 59 , 197–206 (2010).
Lee, K. M. et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 118 , 6220–6229 (2011).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201 , 1089–1099 (2005).
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109 , 1010–1017 (2007).
Angeli, V. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24 , 203–215 (2006).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7 , 192–198 (2001).
Padera, T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296 , 1883–1886 (2002).
Stritt, S., Koltowska, K. & Mäkinen, T. Homeostatic maintenance of the lymphatic vasculature. Trends Mol. Med. 27 , 955–970 (2021).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276 , 1423–1425 (1997).
Stacker, S. A. et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 7 , 186–191 (2001).
Karnezis, T. et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21 , 181–195 (2012).
Skobe, M. et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 159 , 893–903 (2001).
Schoppmann, S. F. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161 , 947–956 (2002).
Kubota, Y. et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 206 , 1089–1102 (2009).
Petkova, M. et al. Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation. J. Exp. Med. 220 , e20220741 (2023). This study defined a novel immune interacting subtype of LECs residing at the end of capillaries in oncogenic Pik3ca -dependent lymphatic malformations that activated a positive feedback loop with local macrophages to promote lymphatic growth.
Weichand, B. et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 214 , 2695–2713 (2017).
Lyons, T. R. et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J. Clin. Invest. 124 , 3901–3912 (2014).
Farnsworth, R. H., Karnezis, T., Maciburko, S. J., Mueller, S. N. & Stacker, S. A. The interplay between lymphatic vessels and chemokines. Front. Immunol. 10 , 518 (2019).
Hong, H. et al. TNF-alpha promotes lymphangiogenesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway. BMC Cancer 16 , 240 (2016).
Ji, H. et al. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun. 5 , 4944 (2014).
Zhuo, W. et al. The CXCL12–CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin. Cancer Res. 18 , 5387–5398 (2012).
Savetsky, I. L. et al. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 10 , e0126908 (2015).
Kataru, R. P. et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34 , 96–107 (2011).
Garnier, L. et al. IFN-γ-dependent tumor-antigen cross-presentation by lymphatic endothelial cells promotes their killing by T cells and inhibits metastasis. Sci. Adv. 8 , eabl5162 (2022). Building off previous work that demonstrated the antigen presentation capabilities of LECs, here the authors of this study demonstrate that IFNγ -induced antigen presentation in tumour-associated LECs renders them susceptible to cytotoxic T cell killing, which in turn reduces LN metastasis.
Xu, W., Harris, N. R. & Caron, K. M. Lymphatic vasculature: an emerging therapeutic target and drug delivery route. Annu. Rev. Med. 72 , 167–182 (2021).
Lohela, M., Heloterä, H., Haiko, P., Dumont, D. J. & Alitalo, K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am. J. Pathol. 173 , 1891–1901 (2008).
Gengenbacher, N. et al. Timed Ang2-targeted therapy identifies the angiopoietin–tie pathway as key regulator of fatal lymphogenous metastasis. Cancer Discov. 11 , 424–445 (2021). Using a preclinical model of grafted melanoma fragments, the authors of this study establish that targeting the lymphangiogenic molecule, angiopoietin-2, in the adjuvant setting is sufficient to reduce regional metastasis and improve survival.
Cao, R. et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl Acad. Sci. USA 109 , 15894–15899 (2012).
Björndahl, M. et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc. Natl Acad. Sci. USA 102 , 15593–15598 (2005).
Wong, B. W. et al. The role of fatty acid β-oxidation in lymphangiogenesis. Nature 542 , 49–54 (2017).
Yu, P. et al. FGF-dependent metabolic control of vascular development. Nature 545 , 224–228 (2017).
Lund, A. W. et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1 , 191–199 (2012). The first study to specifically investigate the impact of VEGFC on antitumor immune surveillance, identifying a pro-inflammatory but immune suppressive effect in the melanoma TME.
Kataru, R. P. et al. Critical role of CD11b + macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113 , 5650–5659 (2009).
Weinkopff, T. et al. Leishmania major infection-induced VEGF-A/VEGFR-2 signaling promotes lymphangiogenesis that controls disease. J. Immunol. Baltim. MD 1950 197 , 1823–1831 (2016).
CAS Google Scholar
D’Alessio, S. et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124 , 3863–3878 (2014).
Alitalo, A. K. et al. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 73 , 4212–4221 (2013).
Lane, R. S. et al. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 215 , 3057–3074 (2018). Direct in vivo demonstration that lymphatic intrinsic sensing of IFNγ in tumours regulates the persistence of effector immune responses, establishing a mechanism of lymphatic adaptation that promotes immune suppression. Together with Garnier et al. (2022), these two studies highlight the impact of IFNγ on lymphatic remodelling and the impact of this lymphatic remodelling on both immune surveillance and metastasis.
Dieterich, L. C. et al. Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Front. Immunol. 8 , 66 (2017).
Mueller, S. N. et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Invest. 120 , 2508–2515 (2010).
Cousin, N. et al. Lymphatic PD-L1 expression restricts tumor-specific CD8+ T-cell responses. Cancer Res. 81 , 4133–4144 (2021).
Gkountidi, A. O. et al. MHC class II antigen presentation by lymphatic endothelial cells in tumors promotes intratumoral regulatory T cell-suppressive functions. Cancer Immunol. Res. 9 , 748–764 (2021). This study uses a genetic model to specifically delete MHCII in LECs and demonstrates a direct effect on the accumulation and suppressive function of T reg cells within the TME. These data provide the most recent evidence that LECs have antigen-presenting capabilities that functionally shape peripheral T cell responses.
Fankhauser, M. et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 9 , eaal4712 (2017).
Song, E. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577 , 689–694 (2020). This study demonstrates that VEGFC-driven lymphangiogenesis in the meninges enhances immunosurveillance and response to immunotherapy in brain tumours.
Issa, A., Le, T. X., Shoushtari, A. N., Shields, J. D. & Swartz, M. A. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 69 , 349–357 (2009).
Sasso, M. S. et al. Lymphangiogenesis-inducing vaccines elicit potent and long-lasting T cell immunity against melanomas. Sci. Adv. 7 , eabe4362 (2021). This study demonstrates that VEGFC expression in the context of topical adjuvants promotes antigen-specific T cell priming providing superior tumour protection and long-term immunological memory.
Fear, V. S. et al. Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol. Immunother. 70 , 3249–3258 (2021).
Huang, Q. et al. The primordial differentiation of tumor-specific memory CD8+ T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 185 , 4049–4066.e25 (2022).
Fransen, M. F. et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3 , e124507 (2018).
Connolly, K. A. et al. A reservoir of stem-like CD8 + T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6 , eabg7836 (2021).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168 , 487–502.e15 (2017).
Tewalt, E. F. et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120 , 4772–4782 (2012).
Cohen, J. N. et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 207 , 681–688 (2010).
Hirosue, S. et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J. Immunol. 192 , 5002–5011 (2014).
Vokali, E. et al. Lymphatic endothelial cells prime naïve CD8+ T cells into memory cells under steady-state conditions. Nat. Commun. 11 , 538 (2020).
Tamburini, B. A., Burchill, M. A. & Kedl, R. M. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat. Commun. 5 , 3989 (2014).
Lukacs-Kornek, V. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12 , 1096–1104 (2011).
Dubrot, J. et al. Lymph node stromal cells acquire peptide–MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 211 , 1153–1166 (2014).
Baptista, A. P. et al. Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. eLife 3 , e04433 (2014).
Nadafi, R. et al. Lymph node stromal cells generate antigen-specific regulatory T cells and control autoreactive T and B cell responses. Cell Rep. 30 , 4110–4123.e4 (2020).
Dubrot, J. et al. Absence of MHC-II expression by lymph node stromal cells results in autoimmunity. Life Sci. Alliance 1 , e201800164 (2018).
Horton, B. L. et al. Lack of CD8 + T cell effector differentiation during priming mediates checkpoint blockade resistance in non-small cell lung cancer. Sci. Immunol. 6 , eabi8800 (2021).
Zagorulya, M. et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity 56 , 386–405.e10 (2023).
Esterházy, D. et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569 , 126–130 (2019).
Houston, S. A. et al. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9 , 468–478 (2016).
Brown, H., Komnick, M. R., Brigleb, P. H., Dermody, T. S. & Esterházy, D. Lymph node sharing between pancreas, gut, and liver leads to immune crosstalk and regulation of pancreatic autoimmunity. Immunity 56 , 2070–2085.e11 (2023). This study demonstrates LN sharing among the gut, liver and pancreas where tissue-specific characteristics of migratory DC populations impact antigen presentation within the shared LNs.
Pöysti, S. et al. Infection with the enteric pathogen C. rodentium promotes islet-specific autoimmunity by activating a lymphatic route from the gut to pancreatic lymph node. Mucosal Immunol. 15 , 471–479 (2022).
Laidlaw, B. J., Gray, E. E., Zhang, Y., Ramírez-Valle, F. & Cyster, J. G. Sphingosine-1-phosphate receptor 2 restrains egress of γδ T cells from the skin. J. Exp. Med. 216 , 1487–1496 (2019).
Ataide, M. A. et al. Lymphatic migration of unconventional T cells promotes site-specific immunity in distinct lymph nodes. Immunity 55 , 1813–1828.e9 (2022). This study importantly demonstrates that site-specific LN immunity is installed in part by the lymphatic migration of unconventional T cells.
Steele, M. M. et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat. Immunol. 24 , 664–675 (2023). This study demonstrates that CD8 + T cell egress out of tumours depends on local antigen encounter and lymphatic-derived chemokines and provides proof of principle that targeting egress could shape the intratumoural T cell repertoire and response to immunotherapy.
Li, Z. et al. In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J. Exp. Med. 219 , e20210749 (2022). Together with Steele et al. (2023), this paper indicates that antigen-experienced TCF1 + cells recirculate out of the TME and that at least a subset of these may have stem-like properties.
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45 , 415–427 (2016).
Gearty, S. V. et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602 , 156–161 (2022).
Prokhnevska, N. et al. CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56 , 107–124.e5 (2023).
Salmi, M., Karikoski, M., Elima, K., Rantakari, P. & Jalkanen, S. CD44 binds to macrophage mannose receptor on lymphatic endothelium and supports lymphocyte migration via afferent lymphatics. Circ. Res. 112 , 1577–1582 (2013).
Teijeira, A. et al. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM-1/LFA-1 interactions. Cell Rep. 18 , 857–865 (2017).
Karikoski, M. et al. Clever-1/stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation: innate immunity. Eur. J. Immunol. 39 , 3477–3487 (2009).
Li, G. et al. TGF-β-dependent lymphoid tissue residency of stem-like T cells limits response to tumor vaccine. Nat. Commun. 13 , 6043 (2022).
Molodtsov, A. K. et al. Resident memory CD8+ T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity 54 , 2117–2132.e7 (2021). This study establishes the presence of T RM cells in mouse and human tumour-draining LNs, indicating that tumour-draining LNs may possess mechanisms that protect from regional metastasis.
Siddiqui, I. et al. Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50 , 195–211.e10 (2019).
Heim, T. A. et al. Lymphatic vessel transit seeds precursors to cytotoxic resident memory T cells in skin draining lymph nodes. Preprint at bioRxiv https://doi.org/10.1101/2023.08.29.555369 (2023).
Stolley, J. M. et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 217 , e20192197 (2020).
Pai, J. A. et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell 41 , 776–790.e7 (2023).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25 , 1251–1259 (2019).
Beura, L. K. et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48 , 327–338.e5 (2018).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184 , 4512–4530.e22 (2021).
Lesch, S. et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat. Biomed. Eng. 5 , 1246–1260 (2021).
Heim, T. A., Lin, Z., Steele, M. M., Mudianto, T. & Lund, A. W. CXCR6 promotes dermal CD8+ T cell survival and transition to long-term tissue residence. Preprint at bioRxiv https://doi.org/10.1101/2023.02.14.528487 (2023).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372 , eaba1786 (2021).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25 , 477–486 (2019).
Schudel, A., Francis, D. M. & Thomas, S. N. Material design for lymph node drug delivery. Nat. Rev. Mater. 4 , 415–428 (2019).
Van Pul, K. M. et al. Local delivery of low-dose anti-CTLA-4 to the melanoma lymphatic basin leads to systemic T reg reduction and effector T cell activation. Sci. Immunol. 7 , eabn8097 (2022). The results of a recent phase I clinical trial leveraging lymphatic transport for local delivery of CTLA4 antibodies that led to boosted melanoma-specific T cell responses, suggesting that the tumour-draining LN may be a functional target for ICB.
Koster, B. D. et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I–II melanoma: data from two randomized phase II trials. Clin. Cancer Res. 23 , 5679–5686 (2017).
Gur-Cohen, S. et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366 , 1218–1225 (2019).
Niec, R. E. et al. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell 29 , 1067–1082.e18 (2022).
Palikuqi, B. et al. Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. Cell Stem Cell 29 , 1262–1272.e5 (2022).
Liu, X. et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 588 , 705–711 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577 , 561–565 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577 , 549–555 (2020).
Ruddle, N. H. Lymphatic vessels and tertiary lymphoid organs. J. Clin. Invest. 124 , 953–959 (2014).
Femel, J. et al. Quantitative multiplex immunohistochemistry reveals inter-patient lymphovascular and immune heterogeneity in primary cutaneous melanoma. Front. Immunol. 15 , 1328602 (2021).
Overacre-Delgoffe, A. E. et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 54 , 2812–2824.e4 (2021).
Mounzer, R. H. et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood 116 , 2173–2182 (2010).
Cao, E. et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat. Metab. 3 , 1175–1188 (2021).
Shields, J. D., Kourtis, I. C., Tomei, A. A., Roberts, J. M. & Swartz, M. A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328 , 749–752 (2010).
Czepielewski, R. S. et al. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity 54 , 2795–2811.e9 (2021). This study demonstrates that TLS formation at lymphatic valves in a TNF-dependent mouse model of intestinal inflammation leads to impaired lymphatic drainage and leukocyte trafficking that may further exacerbate local inflammation.
Reed, H. O. et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Invest. 129 , 2514–2526 (2019).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523 , 337–341 (2015).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21 , 1380–1391 (2018).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212 , 991–999 (2015).
Jacob, L. et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med. 219 , e20220035 (2022).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572 , 62–66 (2019).
Smyth, L. C. D. et al. Identification of direct connections between the dura and the brain. Nature https://doi.org/10.1038/s41586-023-06993-7 (2024).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8 , 1434 (2017).
Ma, Q., Decker, Y., Müller, A., Ineichen, B. V. & Proulx, S. T. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 216 , 2492–2502 (2019).
Spera, I. et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. eBioMedicine 91 , 104558 (2023).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4 , 147ra111 (2012).
Kovacs, M. A. et al. Meningeal lymphatic drainage promotes T cell responses against Toxoplasma gondii but is dispensable for parasite control in the brain. eLife 11 , e80775 (2022).
Li, X. et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat. Neurosci. 25 , 577–587 (2022).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10 , 229 (2019).
Li, Z. et al. Blockade of VEGFR3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci. Immunol. 8 , eabq0375 (2023).
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30 , 229–243 (2020).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359 , 97–103 (2018).
Bernier-Latmani, J. et al. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J. Clin. Invest. 125 , 4572–4586 (2015).
Suh, S. H. et al. Gut microbiota regulates lacteal integrity by inducing VEGF‐C in intestinal villus macrophages. EMBO Rep. 20 , e46927 (2019).
Becker, F. et al. Dynamic gut microbiome changes following regional intestinal lymphatic obstruction in primates. Pathophysiology 26 , 253–261 (2019).
Yu, Y. et al. Mesenteric lymph system constitutes the second route in gut–liver axis and transports metabolism-modulating gut microbial metabolites. J. Genet. Genomics 49 , 612–623 (2022).
Choi, Y. et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci. Immunol. 8 , eabo2003 (2023).
Eun, Y.-G. et al. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci. Rep. 11 , 23176 (2021).
Luu, K. et al. Fecal and tissue microbiota are associated with tumor T-cell infiltration and mesenteric lymph node involvement in colorectal cancer. Nutrients 15 , 316 (2023).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15 , 139–154 (2019).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25 , 141–151 (2019).
Cortellini, A. et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J. Immunother. Cancer 7 , 57 (2019).
McQuade, J. L. et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19 , 310–322 (2018).
Harvey, N. L. et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37 , 1072–1081 (2005).
Cifarelli, V. et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat. Commun. 12 , 3350 (2021).
Weitman, E. S. et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS ONE 8 , e70703 (2013).
García Nores, G. D. et al. Obesity but not high-fat diet impairs lymphatic function. Int. J. Obes. 40 , 1582–1590 (2016).
Escobedo, N. et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1 , e85096 (2016).
Sawane, M. et al. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62 , 1970–1980 (2013).
Chakraborty, A. et al. Vascular endothelial growth factor-D (VEGF-D) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am. J. Pathol. 189 , 924–939 (2019).
Karaman, S. et al. Decline of lymphatic vessel density and function in murine skin during aging. Angiogenesis 18 , 489–498 (2015).
Kataru, R. P. et al. Structural and functional changes in aged skin lymphatic vessels. Front. Aging 3 , 864860 (2022).
Nagai, T., Bridenbaugh, E. A. & Gashev, A. A. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels: aging of mesenteric lymphatic vessels. Microcirculation 18 , 463–473 (2011).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560 , 185–191 (2018).
Rustenhoven, J. et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J. Exp. Med. 220 , e20221929 (2023). This study demonstrates that ageing is associated with an increase in IFNγ in the meninges that drives age-related impairment in meningeal-lymphatic function.
Ecker, B. L. et al. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 9 , 82–95 (2019). This study reports clinical data indicating low retention of radiotracer during SLN biopsy in patients who are aged, which is associated with reduced rates of LN metastasis but counterintuitively reduced survival, indicating that impaired lymphatic integrity with ageing may influence disease outcomes.
Xie, X. et al. Young age increases the risk for lymph node metastasis in patients with early colon cancer. BMC Cancer 19 , 803 (2019).
Behring, M. et al. Age-dependent heterogeneity of lymph node metastases and survival identified by analysis of a national breast cancer registry. J. Pharm. Pharmacol. Res. 6 , 147–157 (2022).
Tian, Z. et al. Young age increases the risk of lymph-node metastasis in patients with muscle-invasive bladder urothelial carcinoma. BMC Cancer 20 , 851 (2020).
Jain, V. et al. Association of age with efficacy of immunotherapy in metastatic melanoma. Oncologist 25 , e381–e385 (2020).
Erbe, R. et al. Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Rep. 36 , 109599 (2021).
Takeda, A. et al. Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity 51 , 561–572.e5 (2019).
Abe, Y. et al. A single-cell atlas of non-haematopoietic cells in human lymph nodes and lymphoma reveals a landscape of stromal remodelling. Nat. Cell Biol. 24 , 565–578 (2022).
Qian, J. et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 30 , 745–762 (2020).
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37 , 21–36.e13 (2020).
Geldhof, V. et al. Single cell atlas identifies lipid-processing and immunomodulatory endothelial cells in healthy and malignant breast. Nat. Commun. 13 , 5511 (2022).
Xiang, M. et al. A single-cell transcriptional roadmap of the mouse and human lymph node lymphatic vasculature. Front. Cardiovasc. Med. 7 , 52 (2020).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180 , 764–779.e20 (2020).
González-Loyola, A. et al. FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Sci. Adv. 7 , eabf4335 (2021).
Fujimoto, N. et al. Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLOS Biol. 18 , e3000704 (2020).
Sibler, E. et al. Immunomodulatory responses of subcapsular sinus floor lymphatic endothelial cells in tumor-draining lymph nodes. Cancers 14 , 3602 (2022).
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214 , 3645–3667 (2017).
Nurmi, H. et al. VEGF‐C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 7 , 1418–1425 (2015).
Brouillard, P. et al. Primary lymphoedema. Nat. Rev. Dis. Prim. 7 , 77 (2021).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98 , 769–778 (1999).
Srinivasan, R. S. et al. The Prox1–Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 28 , 2175–2187 (2014).
Hong, S. P. et al. Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi. Nat. Commun. 11 , 4102 (2020).
Wiig, H. et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Invest. 123 , 2803–2815 (2013).
Li, J. et al. Neurotensin is an anti-thermogenic peptide produced by lymphatic endothelial cells. Cell Metab. 33 , 1449–1465.e6 (2021).
Jakus, Z. et al. Lymphatic function is required prenatally for lung inflation at birth. J. Exp. Med. 211 , 815–826 (2014).
Biswas, L. et al. Lymphatic vessels in bone support regeneration after injury. Cell 186 , 382–397.e24 (2023).
Polomska, A. K. & Proulx, S. T. Imaging technology of the lymphatic system. Adv. Drug Deliv. Rev. 170 , 294–311 (2021).
Download references
Acknowledgements
T.K. and T.M. contributed equally and are listed alphabetically. The authors thank L.H.M. Geraldo, S. Naik and C. Nowosad for critical reading of the drafted manuscript. T.K. received support from the A.G. Leventis Foundation. A.W.L. is supported by the National Institutes of Health (R01 CA238163, R01 AR080068, U54 CA263001, P50 CA225450), the Department of Defense (ME200052), the Cancer Research Institute (Lloyd J. Old STAR Award), the Mark Foundation for Cancer Research (19-047-ELA), the American Cancer Society (RSG-18-169-01-LIB) and the American Association for Cancer Research (AACR-BMS Midcareer Female Investigator Grant).

Author information
These authors contributed equally: Triantafyllia Karakousi, Tenny Mudianto.
Authors and Affiliations
Ronald O. Perelman Department of Dermatology, NYU Grossman School of Medicine, New York, NY, USA
Triantafyllia Karakousi, Tenny Mudianto & Amanda W. Lund
Department of Pathology, NYU Grossman School of Medicine, New York, NY, USA
Amanda W. Lund
Laura and Isaac Perlmutter Cancer Center, NYU Langone Health, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Amanda W. Lund .
Ethics declarations
Competing interests.
A.W.L. reports consulting services for AGS Therapeutics. T.K. and T.M. declare no competing interests.
Peer review
Peer review information.
Nature Reviews Cancer thanks Sirpa Jalkanen, Steven Stacker, who co-reviewed with Rae Farnsworth, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A medical condition in which lymph formed in the digestive system (chyle) leaks and accumulates into body cavities owing to a blockage in lymphatic drainage.
The directional and active migration of leukocytes out of tissues, lymphoid and non-lymphoid, through the efferent lymphatic vasculature.
A waste clearance system in the brain of vertebrates that uses perivascular channels formed by astroglial cells to promote the transport of macromolecules from the CNS.
Specialized blood vessels found in lymphoid organs that are crucial to facilitate lymphocyte migration to lymphoid tissues.
The slow movements of fluid through the extracellular matrix in connective tissues between blood and lymphatic vessels. Increases with inflammation as a function of vascular leakiness.
Specialized lymphatic vessels found in the small intestine that are crucial in the absorption of dietary fats and fat-soluble molecules.
The inner area of the LN cortex between the outer cortex, which contains the B cell follicles, and the medulla. This area is rich in T lymphocytes and antigen-presenting cells (APCs).
A surgical procedure to remove one or more lymph nodes. Often used as an important part of cancer staging and treatment.
Molecules produced by lymphatic endothelial cells that can stimulate organ-specific repair activities in damaged or diseased organs.
A process of lymphatic growth in which new vessels form from the proliferation of pre-existing vessels.
An imaging technique that leverages the uptake of interstitial dyes to image lymphatic structures and their functional transport to lymph nodes.
The reorganization, dilation, activation or growth of the lymphatic vasculature.
A chronic condition characterized by swelling and interstitial fluid accumulation owing to impaired lymphatic drainage.
(LN). Secondary lymphoid organs that are linked by lymphatic vessels, provide an anatomical structure for the accumulation of T and B cells and perform important immune surveillance functions. Lymph node structure is maintained by a network of specialized non-haematopoietic cells, including lymphatic endothelial cells, that compartmentalize and distribute cells, antigens and activating signals for proper immune function. Lymph nodes are also often first sites of metastasis.
In the small intestine, a fan-shaped membrane that attaches the jejunum and ileum to the posterior abdominal wall. The mesentery helps store fat and facilitates blood and lymphatic connections to the intestines.
The therapeutic administration of a drug or treatment given before definitive surgical therapy.
(SLN). A clinical term to define the first lymph node draining the tumour as determined by the accumulation of injected radiotracers at the time of surgery (sentinel lymph node biopsy).
A mechanical force that acts in parallel with a surface, in this case referred to as the stresses formed by luminal fluid flow along an endothelial monolayer.
Antigen-expanded, TCF1 + T cells with the capacity to maintain persistent T cell responses in the context of chronic antigen, for example, chronic infection, autoimmunity and cancer. Also known as progenitor-exhausted T cells.
A memory T cell state that resides long term in peripheral non-lymphoid and lymphoid tissue and presumably surveys locally for re-encounter with pathogens or neoplastic cells. T RM cells do not recirculate in the absence of additional stimulation and are found in both mouse and human models.
A functional term to define any lymph nodes that are connected to and receive direct lymph output from the tumour.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Karakousi, T., Mudianto, T. & Lund, A.W. Lymphatic vessels in the age of cancer immunotherapy. Nat Rev Cancer (2024). https://doi.org/10.1038/s41568-024-00681-y
Download citation
Accepted : 27 February 2024
Published : 11 April 2024
DOI : https://doi.org/10.1038/s41568-024-00681-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Recent advances in liquid biopsy of central nervous system lymphomas: case presentations and review of the literature
- Rapid Short communication
- Published: 10 April 2024
Cite this article
- Manabu Natsumeda ORCID: orcid.org/0000-0003-3098-8323 1 , 2 ,
- Satoshi Shibuma 1 ,
- Haruhiko Takahashi 1 ,
- Jotaro On 1 ,
- Yoshihiro Mouri 3 ,
- Kaoru Tomikawa 1 ,
- Hidemoto Fujiwara 1 ,
- Jun Watanabe 1 ,
- Yoshihiro Tsukamoto 1 ,
- Masayasu Okada 1 ,
- Rui Takeda 4 ,
- Hiroshi Shimizu 5 ,
- Jun Takizawa 4 ,
- Akiyoshi Kakita 5 &
- Makoto Oishi 1
Explore all metrics
Surgical biopsy is the gold standard for diagnosing central nervous system (CNS) lymphomas. However, reliable liquid biopsy methods for diagnosing CNS lymphomas have quickly developed and have been implicated in clinical decision-making. In the current report, we introduce two patients for whom liquid biopsy was essential for diagnosing CNS lymphomas and discuss the rapidly growing applications of this technology.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
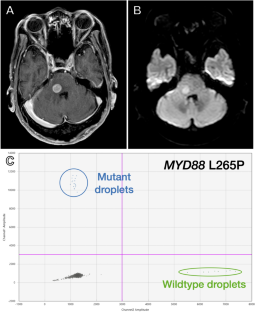
Data availability
Data are available upon reasonable request to the corresponding author.
Abbreviations
Central nervous system
Cerebrospinal fluid
Cell free DNA
Circulating tumor DNA
Diffuse large-B-cell lymphoma
- Droplet digital PCR
Ethylenediaminetetraacetic acid
Intravascular large-B-cell lymphoma
Lactate dehydrogenase
Primary central nervous system lymphoma
Rituximab–cyclophosphamide, doxorubicin hydrochloride, vincristine (oncovin), prednisolone
Secondary central nervous system lymphoma
Soluble IL-2 receptor sIL-2R
Variant allele frequency
Nakamura T, Tateishi K, Niwa T, Matsushita Y, Tamura K, Kinoshita M, Tanaka K, Fukushima S, Takami H, Arita H et al (2016) Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 42:279–290
Article CAS PubMed Google Scholar
Yamaguchi J, Ohka F, Lushun C, Motomura K, Aoki K, Takeuchi K, Nagata Y, Ito S, Mizutani N, Ohno M et al (2023) CD79B Y196 mutation is a potent predictive marker for favorable response to R-MPV in primary central nervous system lymphoma. Cancer Med 12:7116–7126
Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, Lawrence MS, Roemer MGM, Li AJ, Ziepert M et al (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679–690
Article CAS PubMed PubMed Central Google Scholar
Raja H, Salomão DR, Viswanatha DS, Pulido JS (2016) Prevalence of MYD 88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina 36:624–628
Bonzheim I, Giese S, Deuter C, Süsskind D, Zierhut M, Waizel M, Szurman P, Federmann B, Schmidt J, Quintanilla-Martinez L et al (2015) High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood 126:76–79
Yonese I, Takase H, Yoshimori M, Onozawa E, Tsuzura A, Miki T, Mochizuki M, Miura O, Arai A (2019) CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential. Eur J Haematol 102:191–196
Watanabe J, Natsumeda M, Okada M, Kobayashi D, Kanemaru Y, Tsukamoto Y, Oishi M, Kakita A, Fujii Y (2019) High detection rate of MYD88 mutations in cerebrospinal fluid from patients with central nervous system lymphomas. JCO Precis Oncol 3:1–13
Watanabe J, Natsumeda M, Kanemaru Y, Okada M, Oishi M, Kakita A, Fujii Y (2019) Comparison of circulating tumor DNA between body fluids in patients with primary central nervous system lymphoma. Leuk Lymphoma 60:3587–3589
Article PubMed Google Scholar
Baraniskin A, Schroers R (2021) Liquid biopsy and other non-invasive diagnostic measures in PCNSL. Cancers 13:2665
Morell AA, Shah AA-O, Cavallo C, Eichberg DG, Sarkiss CA, Benveniste R, Ivan ME, Komotar RJ (2019) Diagnosis of primary central nervous system lymphoma: a systematic review of the utility of csf screening and the role of early brain biopsy. Neurooncol Pract 6:415–423
PubMed PubMed Central Google Scholar
On J, Natsumeda M, Watanabe J, Saito S, Kanemaru Y, Abe H, Tsukamoto Y, Okada M, Oishi M, Yoshimura J et al (2021) Low detection rate of h3k27m mutations in cerebrospinal fluid obtained from lumbar puncture in newly diagnosed diffuse midline gliomas. Diagnostics (Basel) 11:681
Natsumeda M, On J, Watanabe J, Takahashi H, Tsukamoto Y, Okada M, Hiraishi T, Yoshimura J, Oishi M, Fujii Y (2022) Characteristics of diffuse mildline gliomas with positive detection of H3K27M mutations in cerebrospinal fluid. Nerv Syst Child 44:358–364
Google Scholar
Natsumeda M, On J, Watanabe J, Tsukamoto Y, Okada M, Fujii Y, Adachi J, Nishikawa R (2021) The present and future of less-invasive liquid biopsy for the diagnosis of gliomas and brain tumors. No Shinkei Geka 49:527–534
CAS PubMed Google Scholar
Mutter JA, Alig SK, Esfahani MS, Lauer EM, Mitschke J, Kurtz DM, Kuhn J, Bleul S, Olsen M, Liu CL et al (2023) Circulating tumor DNA profiling for detection, risk stratification, and classification of brain lymphomas. J Clin Oncol 41:1684–1694
Takahashi H, Natsumeda M, On J, Watanabe J, Tada M, Shimizu H, Tsukamoto Y, Okada M, Oishi M, Takizawa J et al (2023) Administration of glucocorticoids prior to liquid biopsy dramatically reduces the detection rate of MYD88 L265P mutation in cerebrospinal fluid of primary CNS lymphoma patients. Leuk Lymphoma 64:1219–1222
Yamagishi Y, Sasaki N, Nakano Y, Matushita Y, Omura T, Shimizu S, Saito K, Kobayashi K, Narita Y, Kondo A et al (2021) Liquid biopsy of cerebrospinal fluid for MYD88 L265P mutation is useful for diagnosis of central nervous system lymphoma. Cancer Sci 112:4702–4710
Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, Jiwa NM, de Boer M, Leguit RJ, de Weger RA, Huibers MMH (2018) The use of droplet digital PCR in liquid biopsies: a highly sensitive technique for MYD88 p. (L265P) detection in cerebrospinal fluid. Hematol Oncol 36:429–435
Rimelen V, Ahle G, Pencreach E, Zinniger N, Debliquis A, Zalmai L, Harzallah I, Hurstel R, Alamome I, Lamy F et al (2019) Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol Commun 7:43
Heger J-M, Mattlener J, Schneider J, Gödel P, Sieg N, Ullrich F, Lewis RI, Bucaciuc-Mracica T, Schwarz RF, Rueß D et al (2023) Entirely noninvasive outcome prediction in central nervous system lymphomas using circulating tumor DNA. Blood 143:522–534
Article Google Scholar
Zheng X, Li P, Dong Q, Duan Y, Yang S, Cai Z, Chen F, Li W (2021) MicroRNAs as diagnostic biomarkers in primary central nervous system lymphoma: a systematic review and meta-analysis. Front Oncol 11:743542
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA et al (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 14:985–990
Gonzolez-Aguilar A, Ibdaih A, Boisselier B, Habbita N, Rosetto M, Laurenge A, Bruno A, Jouvet A, Polivka M, Adam C et al (2012) Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res 18:5203–5211
Bruno A, Boisselier B, Labreche K, Marie Y, Polivka M, Jouvet A, Adam C, Figarella-Branger D, Miquel C, Eimer S et al (2014) Mutational analysis of primary central nervous system lymphoma. Oncotarget 5:5065–5075
Article PubMed PubMed Central Google Scholar
Braggio E, Van Wier S, Ojha J, McPhail E, Asmann YW, Egan J, da Silva JA, Schiff D, Lopes MB, Decker PA et al (2015) Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res 21:3986–3994
Yamada S, Ishida Y, Masuno A, Yamazaki K (2015) Primary diffuse large b-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma 56:2141–2145
Hattori K, Sakata-Yanagimoto M, Okoshi Y, Goshima Y, Yanagimoto S, Nakamoto-Matsubara R, Sato T, Noguchi M, Takano S, Ishikawa E et al (2016) MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br J Haematol 177:481–497
Nayyar N, White MD, Gill CM, Lastrapes M, Bertalan M, Kaplan A, D’Andrea MR, Bihun I, Kaneb A, Dietrich J et al (2019) MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas. Blood Adv 3:375–383
Bravetti C, Degaud M, Armand M, Sourdeau E, Mokhtari K, Maloum K, Osman J, Verrier P, Houillier C, Roos-Weil D et al (2023) Combining MYD88 L265P mutation detection and clonality determination on CSF cellular and cell-free DNA improves diagnosis of primary CNS lymphoma. Br J Haematol 201:1088–1096
Ferreri AJM, Calimeri T, Lopodote P, Francaviglia I, Daverio R, Iacona C, Belloni C, Steffanoni S, Gulino A, Anghileri E et al (2021) MYD88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary central nervous system lymphoma: results from a prospective study. Br J Haematol 193:497–505
Sasayama T, Nakamizo S, Nishihara M, Kawamura A, Tanaka H, Mizukawa K, Miyake S, Taniguchi M, Hosoda K, Kohmura E (2012) Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol 14:368–380
Gupta M, Burns EJ, Georgantas NZ, Theirauf J, Nayyar N, Gordon A, Jones SS, Pisapia M, Sun Y, Burns RP et al (2021) A rapid genotyping panel for detection of primary central nervous system lymphoma. Blood 138:382–386
Hayashi T, Tateishi K, Matsuyama S, Iwashita H, Miyake Y, Oshima A, Honma H, Sasame J, Takabayashi K, Sugino K et al (2023) Intraoperative integrated diagnostic system for malignant central nervous system tumors. Clin Cancer Res 30:116–126
Haider Z, Wasterlid T, Spangberg LD, Rabbani L, Jylha C, Thorvaldsdottir B, Skaftason A, Awier HN, Krstic A, Gellerbring A et al (2023) Whole-genome informed circulating tumor DNA analysis by multiplex digital pcr for disease monitoring in B-cell lymphomas: a proof-of-concept study. Front Oncol 13:1176698
Yamaguchi J, Ohka F, Kitano Y, Maeda S, Motomura K, Aoki K, Takeuchi K, Nagata Y, Hattori H, Tsujiuchi T et al (2023) Rapid detection of the MYD88 L265P mutation for pre- and intra-operative diagnosis of primary central nervous system lymphoma. Cancer Sci 114:2544–2551
Yu X, Li W, Deng Q, Li L, Hsi ED, Young KH, Zhang M, Li Y (2018) MYD88 L265P mutation in lymphoid malignancies. Cancer Res 78:2457–2462
Fernando MR, Chen K, Norton S, Krzyzanowski G, Bourne D, Hunsley B, Ryan WL, Bassett C (2010) A new methodology to preserve the original proportion and integrity of cell-free fetal DNA in maternal plasma during sample processing and storage. Prenat Diagn 30:418–424
Alidousty C, Brandes D, Heydt C, Wagener S, Wittersheim M, Schafer SC, Holz B, Merkelbach-Bruse S, Buttner R, Fassunke J, Schultheis AM (2017) Comparison of blood collection tubes from three different manufacturers for the collection of cell-free DNA for liquid biopsy mutation testing. J Mol Diag 19:801–804
Article CAS Google Scholar
Rajyaguru DJ, Bhaskar C, Borgert AJ, Smith A, Parsons B (2016) Intravascular large B cell lymphoma in the United States (US): a population- based study using surveillance, epidemiology, and end results program and national cancer data base. Blood 128:1114
Ferreri AJM, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, Zucca E, Rossi G, López-Guillermo A, Pavlovsky MA et al (2004) Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol 127:173–183
Hirami Y, Nishimura MF, Urata T, Morimoto M, Maekawa Y, Yoshino T, Nishimura Y, Sato Y (2022) Comparison of serum sIL-2R and LDH levels in patients with intravascular large B-cell lymphoma and patients with advanced stage diffuse large B-cell lymphoma. J Clin Exp Hematop 63:25–31
Kitahara S, Kanazawa M, Natsumeda M, Sato A, Ishikawa M, Hara K, Tabe H, Makino K, Okamoto K, Fujita N et al (2023) Progressive conus medullaris lesions are suggestive of intravascular large B-cell lymphoma. Eur J Neurol 30:3236–3243
Matsue K, Abe Y, Kitadate A, Miura D, Narita K, Kobayashi H, Takeuchi M, Enzan N, Tanaka A, Takeuchi K (2019) Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood 133:1257–1259
Matsue K, Asada N, Odawara J, Aoki T, Kimura S, Iwama K, Fujiwara H, Yamakura M, Takeuchi M (2011) Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol 90:417–421
Shimada K, Yoshida K, Suzuki Y, Iriyama C, Inoue Y, Sanada M, Kataoka K, Yuge M, Takagi Y, Kusumoto S et al (2021) Frequent genetic alterations in immune checkpoint–related genes in intravascular large B-cell lymphoma. Blood 137:1491–1502
Meriranta L, Alkodsi A, Pasanen A, Lepistö M, Mapar P, Blaker YN, Jørgensen J, Karjalainen-Lindsberg M-L, Fiskvik I, Mikalsen LTG et al (2022) Molecular features encoded in the ctDNA reveal heterogeneity and predict outcome in high-risk aggressive B-cell lymphoma. Blood 139:1863–1877
Tateishi K, Miyake Y, Kawazu M, Sasaki N, Nakamura T, Sasame J, Yoshii Y, Ueno T, Miyake A, Watanabe J et al (2020) A hyperactive rela/p65-hexokinase 2 signaling axis drives primary central nervous system lymphoma. Cancer Res 80:5330–5343
Download references
Acknowledgements
The authors thank Takeyoshi Eda, Akiko Yoshii, Tamami Murakami, and Shingo Nigorikawa for their technical support.
This study was partially funded by a Japanese Society for Promotion of Science (JSPS) KAKENHI grant to MN (21KK0156). This research was also supported in part by the New Sustainable Growth (NSG) group.
Author information
Authors and affiliations.
Department of Neurosurgery, Brain Research Institute, Niigata University, Niigata, Japan
Manabu Natsumeda, Satoshi Shibuma, Haruhiko Takahashi, Jotaro On, Kaoru Tomikawa, Hidemoto Fujiwara, Jun Watanabe, Yoshihiro Tsukamoto, Masayasu Okada & Makoto Oishi
Advanced Treatment of Neurological Diseases Branch, Brain Research Institute, Niigata University, Niigata, Japan
Manabu Natsumeda
Department of Neurosurgery, Niigata Prefectural Central Hospital, Joetsu, Japan
Yoshihiro Mouri
Department of Hematology, Endocrinology and Metabolism, Faculty of Medicine, Niigata University, Niigata, Japan
Rui Takeda & Jun Takizawa
Department of Pathology, Brain Research Institute, Niigata University, Niigata, Japan
Hiroshi Shimizu & Akiyoshi Kakita
You can also search for this author in PubMed Google Scholar
Contributions
MN designed the project and wrote the manuscript. SS, HT, JO, JW, and MOk analyzed data. MN, YM, HF, YT, and RT treated the patients and provided samples. HS and AK provided pathologic diagnosis. JT and MOi supervised the study. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Manabu Natsumeda .
Ethics declarations
Conflict of interest.
The authors have no conflicts of interest to declare.
Ethical approval
This study was conducted per the Declaration of Helsinki, and the study approval was obtained from the institutional review boards of Niigata University (#G2018-008). Written consent was obtained from all patients.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Natsumeda, M., Shibuma, S., Takahashi, H. et al. Recent advances in liquid biopsy of central nervous system lymphomas: case presentations and review of the literature. Brain Tumor Pathol (2024). https://doi.org/10.1007/s10014-024-00483-y
Download citation
Received : 18 February 2024
Accepted : 01 April 2024
Published : 10 April 2024
DOI : https://doi.org/10.1007/s10014-024-00483-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liquid biopsy
- Central nervous system lymphomas
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 03 January 2019
Clinical presentation and characteristics of lymphoma in the head and neck region
- Katharina Storck ORCID: orcid.org/0000-0001-8953-0785 1 ,
- Markus Brandstetter 1 ,
- Ulrich Keller 2 &
- Andreas Knopf 1
Head & Face Medicine volume 15 , Article number: 1 ( 2019 ) Cite this article
25k Accesses
49 Citations
1 Altmetric
Metrics details
The study analyses clinical characteristics of histologically defined head and neck (H&N) lymphoma to raise the awareness of ENT specialists to the leading symptoms.
From 2003 to 2011, all patients with histologically defined H&N lymphoma from our clinic were evaluated.
This study identified 221 patients with H&N lymphoma comprising 193 non-Hodgkin lymphomas (NHL) and 28 Hodgkin lymphomas (HL). Among NHL there were 77 indolent (iNHL), 110 aggressive (aNHL), six highly aggressive NHL and further 28 HL. Patients with highly aggressive NHL and HL were significantly younger ( p < 0.0001). Corresponding to the leading symptoms, we found nodal and extranodal involvement. NHL demonstrated manifestation in neck lymph nodes, tonsils, major salivary glands, sinonasal-system and hypopharynx/larynx. HL showed exclusive manifestation in lymph nodes of the neck and the tonsils ( p < 0.0001). The mean time from first symptoms to diagnosis ranged from 1.5 ± 0.7 months in highly aggressive lymphoma to 7.5 ± 11.5 months in iNHL.
Conclusions
The variable clinical presentation of lymphoma is a challenge for the ENT specialist. Fast diagnosis is crucial for rapid treatment, especially in highly aggressive NHL like the Burkitt-lymphoma and HL. A standardized medical history, clinical examination and imaging evaluations paired with patient’s signs, symptoms and demographic knowledge might indicate lymphoma. Biopsies in the H&N region should always be immediately performed in suspicious findings.
Peer Review reports
Lymphomas are a heterogeneous group of malignant tumours of the haematopoietic system and are characterized by the aberrant proliferation of mature lymphoid cells or their precursors [ 1 ]. Lymphomas can be divided into two major entities: Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). Over 20 different subtypes of NHL have been classified according to the specific subtype of lymphoid cells involved.
Several classifications have been developed over the years for lymphomas. The currently used classification is that of the World Health Organization (WHO) and is based on the principles of the Revised European-American Classification of Lymphoid Neoplasm (REAL) from 1994 [ 2 ]. The latest update of the classification was published in two reviews in Blood in 2016 [ 3 , 4 , 5 ]. The subtype of the lymphomas is defined based on the cell of origin: B-cell lymphomas, T-cell and natural killer-cell lymphomas (T/NK-NHL) and HL [ 6 , 7 ]. The two recent WHO classifications from 2008 and 2016 include and encompass (as previously stated in previous classifications) morphology, immunophenotype, genetic and clinical features in order to define “real” diseases [ 3 , 4 ]. HLs frequently involve lymph nodes of the neck and mediastinum, whereas extranodal sites account for only 5% of HLs for example in the tonsils. In contrast, approximately 30% of NHLs show heterogeneous extranodal manifestations, such as in the major salivary glands, paranasal sinuses, mandible, maxilla and Waldeyer’s ring (largely depending and often characteristic for the specific NHL subtype) [ 3 , 8 ]. Other than the gastrointestinal tract, the head and neck region is frequently involved as an extranodal site in NHL, affecting 11–33% of patients [ 9 ]. The clinical behaviour and manifestations of lymphomas in the head and neck region usually lack specific characteristics that would enable attribution to a specific lymphoma entity without biopsy and histological evidence. In particular, with regard to lymphomas having an aggressive course, immediate histological evidence is crucial for patient management, early treatment initiation and often for the outcome [ 10 , 11 ]. Available imaging techniques (ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET)) fail to distinguish HL from NHL and cannot differentiate their various subtypes, necessitating pathological diagnosis [ 8 ]. Sometimes, clinical parameters and the various sites within the head and neck can help to distinguish between the two categories as they each have predilections as mentioned above [ 3 ]. Typical symptoms can include an indolent lymphadenopathy (here, we concentrate on the cervical lymph nodes), fatigue, occasionally B-symptoms such as fever > 38 °C, night sweats and weight loss (> 10% within 6 month), susceptibility to infections and changes in the haemogram. Especially with respect to the differential blood count, iNHL presents cytopenia more often than aNHL but it does not lead to the diagnosis as a single parameter. In chronic lymphatic leukemia (CLL), for example, the frequency of lymphocytes in the differential blood count is elevated as a characteristic sign. Further symptoms of lymphoma might include anaemia, leucopenia/leucocytosis and thrombopenia, although specific serum and blood parameters might sometimes also suggest indolent vs aggressive lymphoma, e.g. elevated lactate dehydrogenase (LDH) in cases of highly proliferative disease or increased β2-microglobulin. The most important differential diagnosis for head and neck lymphadenopathy is infection or lymph-node metastasis from regional or distant primaries being affected by solid cancer.
Our retrospective study includes 221 patients who were suffering from NHL and HL and who were consecutively diagnosed in the Department of Otorhinolaryngology, Head and Neck surgery. Histologically confirmed lymphomas were classified according to the clinical system defined below. Thorough analysis of epidemiological data, leading symptomatology, clinical disease presentation and laboratory testing were carried out to identify clinical parameters in order to expedite diagnostic regimes/work-up.
Patients and methods
All patients presenting with head and neck symptoms that resulted in the histologically established diagnosis of lymphoma from January 2003 to December 2011 ( n = 221) were included in this retrospective study. The study has been approved by the ethic committee of the Technical University of Munich (Permit Number: 493/17).
A standardized medical history was obtained from all patients: clinical examination, age at diagnosis, gender, location in the head and neck region, imaging evaluations (especially ultrasound), leading symptoms (B-symptoms; fever, night sweats, weight loss), time to diagnosis, known risk factors (HIV, EBV), histological findings and survival outcome (Munich cancer centre). All patients underwent clinical examination and a high-resolution B-mode ultrasound of the neck (S2000, tissue harmonic imaging, 9 MHz linear array, Siemens, Germany). Blood chemistry (including LDH and C-reactive protein (CRP)) and a complete blood count with a leucocyte differential count were undertaken. During diagnostic work-up (staging), all patients also underwent contrast CT-scans of the neck, chest, abdomen and pelvic cavity and bone marrow biopsy. If central nervous system involvement was suspected, staging also included contrast MR imaging of the brain and/or the spine.
Lymphomas were classified according to the lymphoma classification effective at the time: up until 2008, we used the Revised European American Lymphoma Classification (REAL) [ 2 ], and starting from 2008, we employed the WHO classification [ 3 , 4 ]. For practical purposes and because the observation period spanned two classifications, we refer here to lymphomas in two main categories, namely Hodgkin lymphomas (HL) and non-Hodgkin lymphomas (NHL). NHL were further clinically subgrouped into 1. indolent lymphoma (iNHL) (including follicular lymphomas and margional zone lymphomas), 2. aggressive lymphoma (aNHL) (e.g. DLBCL) and 3. highly aggressive lymphoma (Burkitt lymphoma and lymphoblastic lymphomas) (Fig. 1 ).
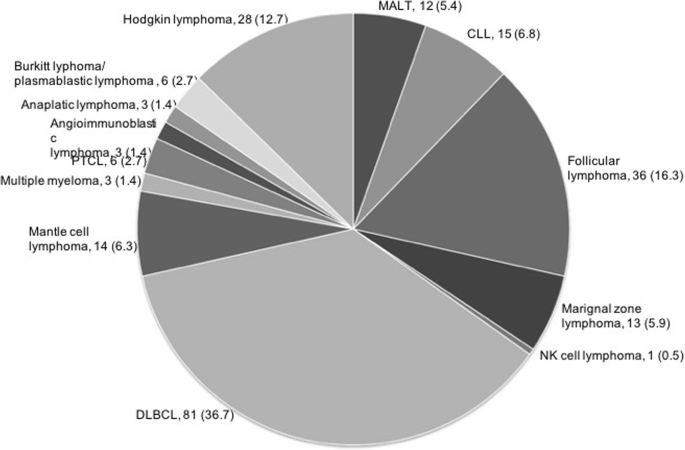
Frequency of histologic subtypes within the iNHL, aNHL and highly aggressive lymphoma and HL
Statistical analyses were performed by using unpaired t-tests and one-way ANOVA testing (SPSS Inc., Chicago, IL). Post-hoc analysis was carried out with Tukey’s test. A p value of < 0.05 was considered statistically significant and a p value < 0.001 was defined as highly significant.
Epidemiology and characteristics of the head and neck cohort
A total of 221 patients were included in this study: 193 with NHL and 28 with HL. With respect to the clinical classification system, we included 77 indolent NHL (iNHL), 110 aggressive lymphomas (aNHL), 6 highly aggressive lymphomas and 28 HL (Fig. 1 ). The median age for indolent and for aggressive lymphoma was 70 years, for highly aggressive lymphoma 34 years and for HL 33 years. Patients with highly aggressive lymphoma and HL were significantly younger than their counterparts with less aggressive types ( p < 0.0001; Table 1 ). The study comprised 114 [52%] males and 107 [48%] females without differences between the groups (Table 1 ).
The mean time from first symptoms to diagnosis ranged from 1.5 ± 0.7 months in highly aggressive lymphoma to 7.5 ± 11.5 months in indolent lymphoma. This difference was not statistically significant (Table 1 ).
Independent from the classification the vast majority of lymphoma patients ( n = 168) suffered from cervical masses as the leading symptom. Fifty-nine patients complained of odyno−/dysphagia. Globus pharyngis, dysphonia and dyspnea occurred infrequently. Occult lymphoma without clinical symptoms was diagnosed in five patients during sonographic procedure for another disease (Table 1 ). The distribution of leading symptoms differed significantly between the groups ( p < 0.05, Table 1 ). Whereas patients with highly aggressive lymphoma and HL usually presented with a cervical mass and/or odyno/dysphagia, patients with indolent and aggressive lymphoma demonstrated a broad variety of leading symptoms (Table 1 ). B-symptoms occurred in 28 (13%) patients (NHL, n = 25; HL, n = 3).
Disease manifestation
Corresponding to the diverse leading symptoms, we found a nodal and an extranodal involvement of the head and neck organs. NHL demonstrated manifestation in neck lymph nodes ( n = 83), tonsils ( n = 60), major salivary glands ( n = 32), the sinonasal system ( n = 6) and the hypopharynx/larynx ( n = 7) whereas HL showed exclusive manifestation in neck lymph nodes ( n = 27) and the tonsils ( n = 1). Although highly aggressive NHL and HL exclusively originated in indolent neck lymph nodes and the tonsils, indolent and aggressive lymphomas showed a distinct disease heterotopia ( p < 0.0001; Table 1 ). In our study, extranodal head and neck manifestation occurred in 57% NHL and in 4% HL. NHL presented a unilateral localization in 84% of cases, and HLs were unilateral in 79% of cases. We found n = 12 cases (20%) of NHL with bilaterally affected tonsils. Systemic involvement was seen in n = 43 [56%] patients with iNHL, in n = 75 [68%] patients with aNHL, in n = 6 [100%] patients with highly aggressive lymphoma, and in n = 22 [79%] patients with HL.
Laboratory findings
Basic laboratory testing included blood counts, C-reactive protein (CRP) and lactate dehydrogenase (LDH). The level of leucocytes (normal range: 4.0–9.0 [G/l]) differed significantly between the groups ( p < 0.05; Table 1 ). However, all levels ranged within the norm. Patients with iNHL exhibited a leucocyte level of 11.8 ± 12.0 (mean ± SD), patients with aNHL 7.7 ± 3.6 and patients with HL 8.0 ± 3.0. In patients with highly aggressive lymphoma, the leucocyte level was significantly decreased at 4.5 ± 3.2. Similar results were seen in haemoglobin levels, which showed a normal level in all the groups, with the lowest level in highly aggressive lymphomas at 10.5 ± 6.1 (mean ± SD). Differences between haemoglobin levels were not statistically significant. CRP (normal range: < 0.5 [mg/dl]) was slightly elevated in all groups, with the highest level in HL at 3.30 ± 4.30 (mean ± SD). LDH levels (normal range: < 244 [U/l]) were elevated in aNHL at 278 ± 291[U/l] (mean ± SD) and in HL at 271 ± 121[U/l] (Table 1 ).
Survival outcome
Available overall survival data for the previously defined subgroups are shown in Fig. 2 .
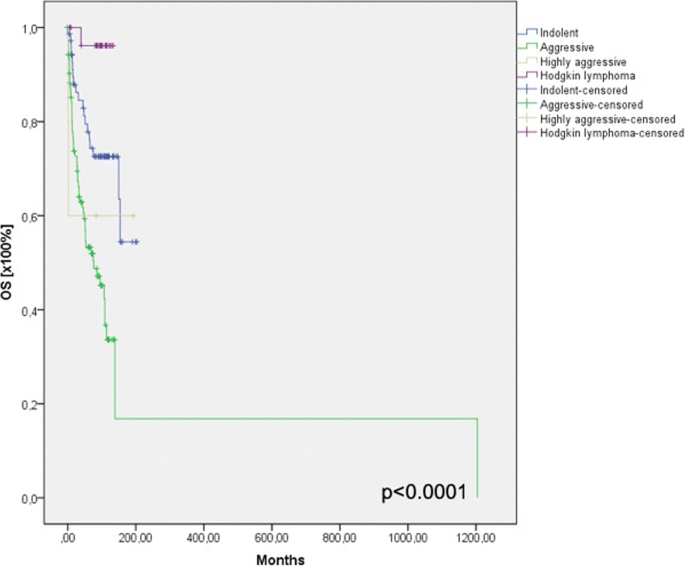
Overall survival data for the previously defined subgroups
Using the cox’s regression for forward selection, we also evaluated the survival rate depending on the laboratory findings. We could not find any significant differences in the survival rate depending on (pathological) laboratory findings (Fig. 3 ).
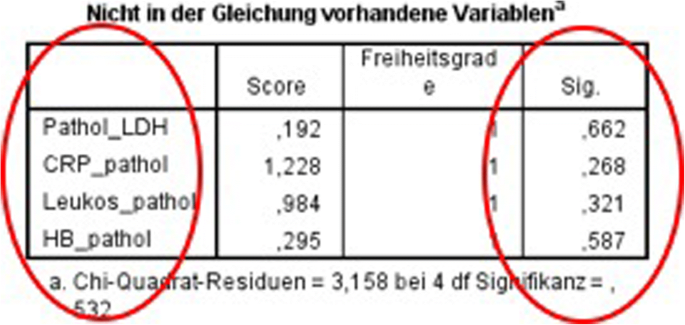
Survival rate depending on the laboratory findings
Lymphoma is the third most common malignancy worldwide representing 3% of all malignant tumours. With 12% of all malignant tumours of the head and neck region, lymphomas are the third most frequent malignancy after squamous cell carcinoma (46%) and thyroid carcinoma (33%) [ 12 , 13 ] and should thus always be taken into consideration in cases of unknown cervical or oral masses. Misinterpretation of the clinical appearance and of the radiological findings (ultrasound, CT-scan, MRI) can lead to delay in diagnosis, delayed treatment initiation and impairment of the patient’s prognosis. In the current study, we have analysed clinical and epidemiological data of the entire lymphoma cohort diagnosed within an eight-year time frame at our ENT Department in order to bring to the attention of ENT specialists the specific clinical symptoms that allow the early diagnosis of lymphomas. In agreement with recent publications, we saw no differences in the gender distribution. Patients with HL (33 years) and highly aggressive lymphomas (34 years) were significantly younger than patients having other lymphoma subtypes (70 years) [ 14 , 15 ]. In our study, we found 193 NHL and 28 HL. Concordant with the present literature, diffuse large B-cell NHL (DLBCL) comprised the largest percentage of NHL in the head and neck region with 36.7% of cases [ 16 , 17 ]. Cervical lymphadenopathy (syn. nodal) is the most common site for both NHL and HL in the head and neck region. Differentiation from other causes of pathological lymph node enlargement caused by infectious diseases (CMV, EBV) or metastatic squamous cell carcinoma is crucial and often difficult and requires histopathological assessment. Certain differences, including the history of alcohol and / or tobacco use, the age of the patient, abnormalities in the clinical ENT examination, constitutional symptoms and systemic lymphadenopathy, may increase the probability of one versus the other. Associated mediastinal adenopathy is more common in HL and abdominal adenopathy in NHL [ 15 ]. In our series, we found n = 110 (50%) patients presenting with cervical lymphadenopathy. Of these, 90 were unilateral and only 20 presented with a bilateral cervical lymphadenopathy. In HL, 22/27 were unilateral. An important aspect for ENT and maxillofacial specialists is the variety of extranodal sites. Taking all lymphomas together, we found 111 (50%) lymphomas in extranodal sites. In particular, NHL contributed to this high proportion. With n = 110 of 193 NHL (57%), NHL presented an extranodal site in a surprisingly large number of cases. In the literature, 25–30% NHL occur in extranodal sites [ 18 ]. In HL, we found an extranodal site in just one case (palatine tonsils), whereas all other patients presented with cervical lymph nodes (96%). The literature also describes > 90% manifestations of HL occurring in the lymph nodes and only 1–4% involving extranodal areas [ 8 , 14 , 19 ]. The extranodal sites included in this study were the major salivary glands ( n = 33), sinonasal system ( n = 6), palatinal tonsils/nasopharynx ( n = 60) and hypopharynx/larynx ( n = 7). The literature also describes extranodal sites such as the palate, buccal mucosa, maxilla and mandible [ 8 , 20 ]. In our clinic, the patients with lymphomas in bone regions usually attend the Department of Maxillofacial Surgery; thus, patients with extranodal sites are partially preselected. The leading symptoms were correlated with the localization of the tumour mass, the majority of patients presenting with a cervical mass (76%) followed by odyno−/dysphagia, globus pharyngis, dysphonia and dyspnea.
Taking all patients together ( n = 221), we found that only n = 8 (13%) of the patients presented with constitutional symptoms or specific B-symptoms. Only n = 3 patients with HL suffered from B-symptoms. This low percentage agrees with the data in the literature [ 21 , 22 ]. Concentration on the presence or absence of B-symptoms might thus mislead the physician, as the rate of patients without such symptoms is high. The same also applies to results from blood and serum testing, as the majority of all of our patients had normal haemoglobin and leucocyte counts and only a few had slightly elevated levels of LDH and CRP. According to the WHO classification, two major subtypes of NHL (DLBCL 70–80% and Burkitt 7–20%) are related to HIV [ 23 ]. In our cohort, we found two HIV-positive patients. One of them was diagnosed with a nodal DLBCL and the other with nodal plasmablastic lymphoma (PBL), an aggressive and rare DLBCL subtype that is commonly found in patients with HIV [ 24 ].
Burkitt lymphoma (BL) is listed in the WHO’s classification of lymphoid tumours as an “aggressive B-cell non-Hodgkin’s lymphoma” characterized by a high degree of proliferation of malignant cells and deregulation of the MYC gene [ 25 ]. In our study, we found 5 cases of BL and all were male, as reported in the literature [ 20 ]; none of them were associated with HIV or EBV [ 26 ]. With a median age of 34 years, these patients were significantly younger ( p < 0.0001) than patients suffering from iNHL or aNHL. Only 1.2% of BL are of extranodal origin in the head and neck [ 27 ]. We found three to be extranodal in the tonsils. The median time to diagnosis was 1.5 months. Despite its highly aggressive nature, BL is a curable lymphoma. Patients have a better prognosis when the diagnosis is established rapidly and if they present with a limited stage [ 28 ]. BL patients exhibited the lowest leucocyte and haemoglobin levels but the levels still ranged within the norm and the number of patients was too low to make a statistically valid statement. Patients with highly aggressive lymphomas and HL all presented with either a cervical mass as a sign of a nodal lymphoma or odynophagia / dysphagia, with the tonsils as the extranodal site in all cases, reflecting their admission to the ENT Department. These findings were highly significant compared with iNHL and aNHL aggressive lymphomas with a larger variety of localizations and leading symptoms. All six highly aggressive lymphomas showed a unilateral cervical mass but a systemic dissemination.
All Patients received standard therapies through the hematology department or associated hematologists/oncologist.
The focus of this report is to describe the different clinical presentations of lymphomas in the head and neck region and to raise awareness on the wide variety of symptoms. As we included all types of lymphoma that may manifest in the head and neck region, there is a large variety standard therapy approaches which sometimes were also adapted to comorbidity. Thus, the intend of this report was not to focus on this clearly important issue which is covered by numerous publications and results from prospective studies. Furthermore, treatment standards have evolved during the observation time of the patient groups described herein.
Concerning the survival outcome, we could see significant differences between the groups as expected. In the blood and serum testings we did not see any differences concerning the survival rate, which strengthens our assumption that we can not identify lymphomas in the head and neck region only by laboratory findings.
Lymphomas comprise 12% of all head and neck malignancies. The variable clinical presentation of lymphoma, in addition to the nodal involvement, is sometimes a challenge for the ENT specialist. A rapid diagnosis is crucial for early treatment initiation, especially in cases of BL and HL, which mostly affect younger patients. A standardized medical history, clinical examination and imaging evaluations (especially ultrasound) paired with patient’s signs, symptoms and demographic knowledge (e.g. age, gender, HIV, EBV) may lead to a correct diagnosis and accelerated the decision for a biopsy.
Tumours in the head and neck are easily accessible and a biopsy should immediately be performed following suspicious findings. In particular, for NHL with extranodal involvement in the head and neck occurring at a frequency of 20–30%, biopsy should always be part of the diagnosis in any head and neck lesion, including those in the oral cavity, major salivary glands, oropharynx, nasopharynx, paranasal sinus and larynx. Unilaterality, the absence of EBV or other acute viruses, the absence of an obvious tumour and a systemic involvement (if previously noted at the first presentation) should alert the ENT specialist to lymphomas, even in the absence of B-symptoms or blood disturbances.
Mawardi H, Cutler C, Treister N. Medical management update: non-Hodgkin lymphoma. Oral Surg Oral med Oral Pathol Oral Radiol Oral. J Endod. 2009;107(1):19–33.
Google Scholar
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary M, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood. 1994;84:1361–92.
CAS PubMed Google Scholar
Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, editors. World Health Organization classification of Tumours. Lyon. France: IARC; 2008.
Swerdlow ST, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
Article CAS Google Scholar
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Harris NL, Jaffe ES, Stein H, et al. A revised European American classification of lymphoid neoplasm: a pro- posal from the international lymphoma study group. Blood. 1994;84:1361–92.
Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol. 1999;111(Suppl):8–12.
Weber AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am. 2003;13:371–92.
Article Google Scholar
Wulfrank D, Pauwels C, Roels H, De Schryver A. Extranodal non-Hodgkin’s lymphoma of the head and neck. Radiother Oncol. 1987;8(3):199–207.
Sohani AR, Hasserjian RP. Diagnosis of Burkitt lymphoma and related high-grad B-cell neoplasms. Surg Pathol Clin. 2010;3(4):1035–59.
Toader C, Toader M, Stoica A, Pop G, Oprea A, Constantin AS, Niculescu L, Vivisenco IC, Drăghici MS, Osman A, Mogoantă CA. Tonsillar lymphoma masquerading as obstructive sleep apnea - pediatric case report. Rom J Morphol Embryol. 2016;57(2 Suppl):885–91.
PubMed Google Scholar
Hiddemann W, Longo DL, Coiffier B, Fisher RI, Cabanillas F, Cavalli F, Nadler LM, De Vita VT, Lister TA, Armitage JO. Lymphoma classification--the gap between biology and clinical management is closing. Blood. 1996;88(11):4085–9.
Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, Gay EG, Langer CJ. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–58.
Iyengar P, Mazloom A, Shihadeh F, Berjawi G, Dabaja B. Hodgkin lymphoma involving extranodal and nodal head and neck sites: characteristics and outcomes. Cancer. 2010;116(16):3825–9. https://doi.org/10.1002/cncr.2513 .
Article PubMed Google Scholar
Urquhart A, Berg R. Hodgkin’s and non-Hodgkin’s lymphoma of the head and neck. Laryngoscope. 2001;111:1565–9.
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The non-Hodgkin's lymphoma classification project. Blood 1997; 89(11):3909–18.
Simonitsch-Klupp I, Hauser I, Ott G, Drach J, Ackermann J, Kaufmann J, Weltermann A, Greinix HT, Skrabs C, Dittrich C, Lutz D, Pötter R, Mannhalter C, Lechner K, Chott A, Jaeger U. Diffuse large B-cell lymphomas with plasmablastic/plasmacytoid features are associated with TP53 deletions and poor clinical outcome. Leukemia. 2004;18(1):146–55.
Wolvius EB, van der Valk P, van der Wal JE, et al. Primary non-Hodgkin’s lymphoma of the salivary glands. An analysis of 22 cases. J Oral Pathol Med. 1996;25:177–81.
Kemp S, Gallagher G, Kabani S, Noonan V, O'Hara C. Oral non-Hodgkin's lymphoma: review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):194–201 Epub 2007 Jun 29.
Silva TD, Ferreira CB, Leite GB, de Menezes Pontes JR, Antunes HS. Oral manifestations of lymphoma: a systematic review. Ecancermedicalscience. 2016;17(10):665. https://doi.org/10.3332/ecancer.2016.665 eCollection 2016.
Picard A, Cardinne C, Denoux Y, Wagner I, Chabolle F, Bach CA. Extranodal lymphoma of the head and neck: a 67-case series. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(2):71–5. https://doi.org/10.1016/j.anorl.2014.07.005 Epub 2014 Dec 29.
Article CAS PubMed Google Scholar
Hart S, Horsman JM, Radstone CR, Hancock H, Goepel JR, Hancock BW. Localised extranodal lymphoma of the head and neck: the Sheffield lymphoma group experience (1971-2000). Clin Oncol (R Coll Radiol). 2004;16(3):186–92.
Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151–9.
Corti M, Minué G, Campitelli A, Narbaitz M, Gilardi L. An aggressive Plasmablastic lymphoma of the Oral cavity as primary manifestation of acquired immunodeficiency syndrome: case report and literature review. Int Arch Otorhinolaryngol. 2015;19(4):354–8. https://doi.org/10.1055/s-0034-1397335 Epub 2015 Jan 8.
Article PubMed PubMed Central Google Scholar
Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumours IARC press. France: Lyon; 2008.
Brady G, MacArthur JG, Farrell PJ. Epstein-Barr virus and Burkitt lymphoms. J Clin Pathol. 2007;60:1397–402.
CAS PubMed PubMed Central Google Scholar
Castillo JJ, Winer ES, Olszewski AJ. Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the surveillance, epidemiology, and End Results database. Cancer. 2013;119(20):3672–9. https://doi.org/10.1002/cncr.28264 Epub 2013 Jul 30.
Hoelzer D, Walewski J, Döhner H, Viardot A, Hiddemann W, Spiekermann K, Serve H, Dührsen U, Hüttmann A, Thiel E, Dengler J, Kneba M, Schaich M, Schmidt-Wolf IG, Beck J, Hertenstein B, Reichle A, Domanska-Czyz K, Fietkau R, Horst HA, Rieder H, Schwartz S, Burmeister T, Gökbuget N. German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–9. https://doi.org/10.1182/blood-2014-03-563627 Epub 2014 Oct 30.
Article CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgments
This work was supported by the German Research Foundation (DFG) and the Technical University of Munich within the funding programme Open Access Publishing.
The corresponding author states no financial or other relationships with other people or organizations, which may lead to a conflict of interest.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
Department of ENT, Head and Neck Surgery, Klinikum Rechts der Isar, Technical University of Munich, Ismaninger Strasse 22, 81675, Munich, Germany
Katharina Storck, Markus Brandstetter & Andreas Knopf
Third Department of Internal Medicine, Haematology and Oncology, Klinikum rechts der Isar, Technische Universität München, Ismaningerstr. 22, 81675, Munich, Germany
Ulrich Keller
You can also search for this author in PubMed Google Scholar
Contributions
Conceived and designed the study: KS, AK, MB. Performed the study and analysed the data: KS, MB, UK, AK. Wrote the paper: KS. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Katharina Storck .
Ethics declarations
Ethics approval and consent to participate.
The study has been approved by the ethic committee of the Technical University of Munich (Permit Number: 493/17).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Storck, K., Brandstetter, M., Keller, U. et al. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med 15 , 1 (2019). https://doi.org/10.1186/s13005-018-0186-0
Download citation
Received : 12 April 2018
Accepted : 12 December 2018
Published : 03 January 2019
DOI : https://doi.org/10.1186/s13005-018-0186-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Head and neck
- Tonsillitis
Head & Face Medicine
ISSN: 1746-160X
- General enquiries: [email protected]
Investigators develop novel treatment for T-cell leukemias and lymphomas
A novel treatment for leukemias and lymphomas that arise from immune system T cells, developed by investigators at the Johns Hopkins Kimmel Cancer Center and its Ludwig Center and Lustgarten Laboratory, was found to be effective at killing these cancers in mice bearing human T-cell tumors.
The therapy, an antibody-drug conjugate (ADC), combines an antibody that targets a protein called TRBC1 expressed on the surface of T-cell cancers with an anti-cancer drug, called SG3249. The ADC works by using the antibody to seek out the cancer cells that express TRBC1. Then, those cancer cells ingest the ADC, where SG3249 is released and kills the cancer cells. A description of the work was published March 27 in Nature .
Each year, about 100,000 patients worldwide are affected by T-cell leukemias and lymphomas. Adults with relapsed T-cell cancers have limited therapeutic options and five-year survival rates of 7-38%.
"Developing treatments for T-cell leukemias and lymphomas is much more difficult than for leukemias and lymphomas arising from immune system B cells," explains senior study author Suman Paul, M.B.B.S., Ph.D., an assistant professor of oncology at the Johns Hopkins University School of Medicine. Effective therapies for B-cell cancers wipe out both cancerous and noncancerous B cells, but patients still do well even without the immune system B cells that help fight infections. However, if similar approaches are used and a treatment wipes out both normal and cancerous T cells, it would leave patients without a functioning immune system and at high risk of dying from infections.
"Not much drug development has happened in this space of T-cell leukemias and lymphomas," Paul says. "We need new therapies for these cancers, but whatever therapies we develop in the space have to be cancer-specific. We have to preserve some of the normal T cells and wipe out cancerous T cells at the same time."
T-cell cancers express either TRBC1 or TRBC2, while normal T cells express a mix of TRBC1 and TRBC2. Therefore, selective targeting of TRBC1 can potentially eradicate the normal and cancerous T cells expressing TRBC1 while preserving normal T cells expressing TRBC2. A recent clinical trial conducted elsewhere attempted to target TRBC1 cancers using chimeric antigen receptor (CAR) T-cell therapy. These CAR T cells are genetically engineered T cells that bind to and kill TRBC1 cells. CAR T-cell therapies are FDA-approved treatment options used in several B-cell cancers. However, after administering the TRBC1-targeting CAR T cell therapy in human patients, trial investigators reported that the CAR T cells were not persisting inside the patients. Such persistence is required for effective cancer cell-killing. Interested to understand why, Paul and colleagues found that the CAR T cells targeting TRBC1 could be killed by normal T cells, limiting their persistence.
This lack of CAR T-cell persistence led the team to try TRBC1 targeting with the use of antibody-drug conjugates. Paul and colleagues tried two different formulations of ADCs in mouse models of T-cell cancers. After a single injection of one formulation of the treatment, the cancers initially regressed but then recurred. After a single treatment with the anti-TRBC1-SG3249 ADC combination, investigators observed signs of cancer elimination within seven days and the cancers were eventually undetectable, with no recurrences. "The tumors didn't come back, and we followed the mice for more than 200 days," Paul explains.
The treatment was able to eliminate the cancer while preserving half of the remaining normal T cells. "The residual normal T cells should be sufficient to maintain some immune system protection against infectious diseases," Paul says.
"Witnessing the successful elimination of T-cell cancers while sparing normal T cells in preclinical models was truly gratifying," adds Jiaxin Ge, a co-author of the study and third-year Ph.D. student in the Ludwig Center. "We believe this approach has the potential to address a critical unmet need in oncology, and we're committed to advancing it through further research."
Tushar Nichakawade, first author on the study and a fourth-year Ph.D. student at the Ludwig Center, says, "There are so many lessons to learn from the clinic and it has been exciting to be a part of the iterative process of drug discovery. Every therapy has its pros and cons, but the preclinical efficacy of our ADC gives me hope that it can make a difference for patients suffering from these terrible cancers."
Investigators are now working with an industry partner to conduct early-phase safety and efficacy trials in human patients.
The study's co-authors were Brian J. Mog, Bum Seok Lee, Alexander H. Pearlman, Michael S. Hwang, Sarah R. DiNapoli, Nicholas Wyhs, Nikita Marcou, Stephanie Glavaris, Maximilian F. Konig, Sandra B. Gabelli, Evangeline Watson, Cole Sterling, Nina Wagner-Johnston, Sima Rozati, Lode Swinnen, Ephraim Fuchs, Drew M. Pardoll, Kathy Gabrielson, Nickolas Papadopoulos, Chetan Bettegowda, Kenneth W. Kinzler, Shibin Zhou, Surojit Sur and Bert Vogelstein of Johns Hopkins.
The work was supported in part by The Virginia and D.K. Ludwig Fund for Cancer Research, Lustgarten Foundation for Pancreatic Cancer Research, Commonwealth Fund, Bloomberg~Kimmel Institute for Cancer Immunotherapy, Bloomberg Philanthropies and the National Institutes of Health Cancer Center Support Grant P30 CA006973. Paul was supported by the National Cancer Institute (grant K08CA270403), the Leukemia Lymphoma Society Translation Research Program Award, the American Society of Hematology Scholar Award and the Swim Across America Translational Cancer Research Award.
- Brain Tumor
- Skin Cancer
- Lung Cancer
- Immune System
- Breast Cancer
- Bone marrow
- Natural killer cell
- Chemotherapy
- Monoclonal antibody therapy
Story Source:
Materials provided by Johns Hopkins Medicine . Note: Content may be edited for style and length.
Journal Reference :
- Tushar D. Nichakawade, Jiaxin Ge, Brian J. Mog, Bum Seok Lee, Alexander H. Pearlman, Michael S. Hwang, Sarah R. DiNapoli, Nicolas Wyhs, Nikita Marcou, Stephanie Glavaris, Maximilian F. Konig, Sandra B. Gabelli, Evangeline Watson, Cole Sterling, Nina Wagner-Johnston, Sima Rozati, Lode Swinnen, Ephraim Fuchs, Drew M. Pardoll, Kathy Gabrielson, Nickolas Papadopoulos, Chetan Bettegowda, Kenneth W. Kinzler, Shibin Zhou, Surojit Sur, Bert Vogelstein, Suman Paul. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers . Nature , 2024; DOI: 10.1038/s41586-024-07233-2
Cite This Page :
Explore More
- Genes for Strong Muscles: Healthy Long Life
- Brightest Gamma-Ray Burst
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
- Clash of Stars Solves Stellar Mystery
- Secure Quantum Computing at Home
- Ocean Currents: Collapse of Antarctic Ice ...
- Pacific Cities Much Older Than Previously ...
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Med Paediatr Oncol
- v.34(4); Oct-Dec 2013
Histopathological pattern of lymphomas and clinical presentation and outcomes of diffuse large B cell lymphoma: A multicenter registry based study from India
Ramesh b. v. nimmagadda.
Apollo Specialty Hospital, Chennai, Tamil Nadu, India
Raghunadharao Digumarti
1 Nizam's Institute of Medical Sciences, Hyderabad, Andhra Pradesh, India
2 Tata Memorial Hospital, Mumbai, Maharashtra, India
Dinesh Bhurani
3 Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India
Vinod Raina
4 All India Institute of Medical Sciences, New Delhi, India
Shyam Aggarwal
5 Sir Ganga Ram Hospital, New Delhi, India
Shekhar Patil
6 HCG Enterprises, Bengaluru, Karnataka, India
Pabitra K. Gogoi
7 Guwahati Medical College Hospital, Guwahati, Assam, India
Subramanian Sundaram
8 V.S. Hospital, Chennai, Tamil Nadu, India
Chanchal Goswami
9 B. P. Poddar Cancer Hospital and Research Centre, Kolkata, West Bengal, India
Shashikant Apte
10 Sahyadri Speciality Hospital, Pune, Maharashtra, India
Srinivas Chakravarthy
11 Apollo Cancer Hospital, Hyderabad, Andhra Pradesh, India
Anand Pathak
12 Cancer Care Clinic, Nagpur, Maharashtra, India
The distribution of various subtypes of lymphomas in India is different from other parts of the world. There is scarce multicentric data on the pattern and outcomes of lymphomas in India.
The aim of this study is to evaluate the histopathological and the clinical pattern and treatment outcomes of lymphomas in India based on the retrospective data collected from a multicenter registry.
Materials and Methods:
Retrospective data was collected at 13 public and private hospitals in India for patients diagnosed with lymphoma between January 2005 and December 2009. The data collection was performed in the setting of a multicenter lymphoma registry Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test.
Non-Hodgkin's lymphoma (NHL) constituted 83.17% and Hodgkin's lymphoma (HL) for 16.83% of the 1733 registered and analyzed cases. Diffuse large B cell lymphoma (DLBCL) was the most common NHL (55%) followed by follicular lymphoma (11%). CHOP was the most common chemotherapy regimen administered (84%) while rituximab was used in 42.7% of those with DLBCL. Survival analysis of treatment naïve DLBCL patients ( n = 791) was performed. Of these, 29% were lost to follow-up, 20% with active disease. The median follow-up in surviving patients is 31 (range: 1-88) months. Median progression-free survival (PFS) and overall survival (OS) in DLBCL patients has not reached. There was no significant difference in median PFS (69 months vs. 61 months, P = 0.1341), but OS was significant not reached (NR) vs. NR, P = 0.0012) within international prognostic index high or intermediate subgroups. Rituximab use was associated with significantly prolonged PFS (NR vs. 82 months, P = 0.0123), but not OS (NR vs. NR, P = 0.2214). Cox regression analysis in treatment naïve DLBCL patients showed a performatnce status, stage and receipt of six or more cycles of chemotherapy to be significantly associated with OS and all of the preceding plus rituximab use significantly associated with PFS.
Conclusions:
Our analysis confirms previous reports of distribution of lymphoma subtypes in India and suggests that patients who are able to receive the full course of chemotherapy achieve a better outcome. This indicates the importance of ensuring compliance to treatment utilizing various measures including patient and family counseling. Prospective studies are required to confirm these findings.
INTRODUCTION
World-wide, the incidence of non-Hodgkin's lymphoma (NHL) is increasing faster than any non-cutaneous malignancy.[ 1 ] Globally, NHL incidence rates vary as much as five fold and lowest rates are reported from Asia;[ 2 ] the highest incidence rates are reported from United States and Europe. The frequency of various subtypes of NHL also differs in various regions.
National Cancer Registry Program by Indian Council of Medical Research has reported age adjusted rate (AAR) of NHL from 21 cities and towns. Highest AAR is from Imphal (6.8/100000) and lowest from Barshi (1.8/100000).[ 3 ] The pattern of NHL in India was first reported from AIIMS, New Delhi, India and showed that high grade lymphoma was the most common subtype (44.2%) followed by intermediate (39%) and low grade lymphomas (12.2%).[ 4 ] A single center study reported the distribution and clinicopathological characteristics of adult NHL presenting over a 1 year period.[ 5 ] B-and T-cell NHL constituted 79.3% and 18.8% of cases respectively and diffuse large B cell lymphoma (DLBCL) was the most common subtype (50.2%). Lower frequencies of follicular lymphoma (FL), marginal zone lymphoma, mantle cell lymphoma (MCL), peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and extranodal NK/T-cell lymphoma were seen compared with other Asian countries while higher frequencies of DLBCL and precursor T-lymphoblastic leukemia/lymphoma were noted. Extranodal and bone marrow involvement in MCL and PTCL-NOS were less frequent. Although these and other Indian studies[ 5 , 6 , 7 ] indicate differences in clinical and histopathological patterns of lymphomas in India, the data is limited and not nationally representative, especially with respect to the outcomes of treatment.
A Lymphoma Registry was established to understand the clinical, pathological and treatment patterns in lymphoma patients presenting to collaborating centers and their long-term outcomes. This is the first report of this registry that especially emphasizes the results in patients with DLBCL.
MATERIALS AND METHODS
The Lymphoma Registry was formed in August 2010 by 13 oncology institutions\hospitals in India [ Figure 1 ] belonging both to public and private sectors. This enabled data to be collected on a continuum of socio-economic strata from low to high. The registry centers were distributed across various regions of the country and collectively likely to be representative of the Indian population. The objectives of establishing the registry were to study the clinical and pathological patterns and treatment outcomes of lymphoma patients in India. The data for this registry was collected using a proprietary Access Based Software (R Lymph 20) Priya Venkatadesikan, The Registrar of Trade Mark. This software was developed and managed by the lead author and Principal Investigator of the Registry. Data elements were included in the software in order to logically collect information on demographic characteristics, histopathological subtype, stage, treatment regimen, survival and important adverse effects in lymphoma patients. The collaborating centers/investigators provided important feedback prior to finalization of the software.

Lymphoma registry centers
Data collection
Retrospective data collection was undertaken by assessment of medical case records of lymphoma patients treated at each registry center from January 2005 to December 2009. The data of all patients who had received at least one cycle of chemotherapy for newly diagnosed lymphoma was collected and that of patients who had received prior chemotherapy was not included.
Study design and analysis
This is a retrospective analysis of patients diagnosed and treated with lymphomas at the collaborating centers of the registry from January 2005 to December 2009. The primary analyses are descriptive reports of the clinical and pathological characteristics of the patients and their tumors and survival outcomes in those with DLBCL. Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. Cox regression analysis was used to compare the effect of various factors on survival in a multivariate model. Patients were censored at the time of their last follow-up date. Progression free survival (PFS) was defined as the time interval from diagnosis until disease progression or relapse and overall survival (OS) was defined as the time interval from diagnosis until death. MedCalc, Medcalc Software, bvba, Belgium, and NCSS statistical packages were used for analysis.
The study was conducted after approval by respective institutional\independent ethics committees as applicable.
Patterns of lymphomas
From October 2010 to June 2012, data was collected on 1723 lymphoma patients who were treated between January 2005 and December 2009 at 13 centers across India. There were 290 (16.83%) Hodgkin's lymphomas (HL) and 1433 (83.16%) NHL. The most common pathological subtypes of NHL were DLBCL (55%) and FL (11%). The other noteworthy subtypes of NHL were anaplastic lymphoma (3%), PTCL (2.7%), Burkitt's lymphoma (2.5%) and MCL (1.8%) [ Table 1 ].
Distribution of subtypes of lymphoma

The data of DLBCL patients in the registry is separately reported here. Other lymphoma subtype data will be reported in subsequent publications.
The patient characteristics are shown in Table 2 . The median age was 52 years. We are only publishing here the results of patients more than 16 years of age (range: 16-92). Male to female ratio was 2:1, a majority of patients (70%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, 31.1% were diagnosed with stage IV disease, 44.3% had low international prognostic index (IPI) score, 37.8% reported B symptoms at presentation, 18.3% patients had bulky disease at initial presentation and bone marrow was involved in 15.3%. Immunohistochemistry was done in 83% patients.
Patient characteristics of DLBCL patients

Treatment pattern
The standard regimen of cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) was the most commonly prescribed treatment (84%), while 42.7% of patients received rituximab in addition to chemotherapy. The median number of chemotherapy cycles was six (range: 1-12). Data on chemotherapy dose modification was available in 88% of patients and of these dose reduction was done in 9% of patients and 12% of patients discontinued chemotherapy. Radiation therapy was given to 36% of patients.
Outcome of DLBCL patients
At a median follow-up of 31 months (range: 1-88 months), 47% of patients were alive with no evidence of disease and 8% were alive with disease. Deaths were reported in 14% patients. Nearly 9% patients were lost to follow-up in remission and 20% were lost to follow-up with evidence of active disease at the time of the last evaluation.
Response rates
After completion of the first line therapy, 431 (55%) patients achieved a complete response (CR), 88 (11%) achieved a partial response (PR) and 203 (25%) reported not evaluated.
The progression-free and OS in all DLBCL patients is shown in Figures Figures2 2 and and3 3 respectively. The median PFS and OS are not reached and the 2-year PFS and OS are 75% and 79% respectively.

Progression-free survival in all diffuse large B cell lymphoma patients

Overall survival in all diffuse large B cell lymphoma patients
Prognostic factors
In univariate analyses of PFS, age >60 years, presence of B symptoms, bone marrow involvement, poor performance status (3-4) and advanced stage (≥III) were significant adverse factors [ Table 3 ]. It is noteworthy that PFS in low risk IPI group patients was significantly superior to intermediate ( P = 0.0005) and high ( P < 0.0001) risk patients, but there was no difference between intermediate and high risk IPI patients [ Figure 4 ]. Median PFS has not been reached in patients with Stage I and II, whereas it was 57 months in patients with Stage III and IV disease [ Figure 5 ].
Univariate analysis of prognostic factors

Progression-free survival in diffuse large B cell lymphoma patients in international prognostic index risk groups

Progression-free survival in diffuse large B cell lymphoma patients as per modified Ann Arbor stages
Impact of Rituximab and number of chemotherapy cycles
The median PFS has not been reached in patients who received rituximab, whereas it was 82 months in those who didn’t receive this drug ( P = 0.0123). However, this PFS benefit did not convert into prolongation of OS ( P = 0.22). the median PFS has not been reached in patients receiving more than or equal to six chemotherapy cycles, whereas it was 42 months in those who received less than six cycles (1-5) ( P < 0.0001).
In multivariate analysis of treatment naïve DLBCL patients, significant variables associated with adverse PFS were poor performance status, higher stage, use of less than six cycles of chemotherapy and non-use of rituximab whereas adverse prognostic factors for OS were all the preceding except rituximab non-use [ Table 4 ].
Multivariate analysis for OS and PFS

Geographic variations in lymphoid malignancies are well-known, but data from India is limited. Individual centers have published their experience,[ 6 , 7 ] but no nationwide registry data has been published until now. Our lymphoma registry is the first multicentric effort in collecting, analyzing and publishing the patterns of care and outcome data of lymphoma patients in India. Such coordinated activities are challenging in a country like India where oncologists and cancer centers are burdened with huge patient load in diverse conditions. The current effort is proof of concept of the feasibility and reliability of a nationwide, multicenter, patterns of care and outcome type of registry for cancers.
The distribution of pathological subtypes of lymphoma (NHL vs. HL) in our registry is consistent with other reports from India[ 7 , 8 ] and somewhat higher for NHL compared to a rural registry.[ 8 ] Within NHL the preponderance of DLBCL followed by FL in our report is also consistent with other reports from India.[ 5 , 6 , 7 , 8 ] Median age of our DLBCL population was 52 years, which is younger compared with that seen in Western populations,[ 9 , 10 ] but is consistent with most other reports from India.[ 6 , 11 ] The younger average age of our patients is also consistent with the pattern seen in most other malignancies in India and is likely due to the effect of a younger population pyramid in this country. B symptoms were present in 37% DLBCL patients and have been variably reported from other reports from this country.[ 6 , 11 ] ECOG performance status of 0-I are 70% in our study, which is higher when compared to published data from a tertiary center.[ 12 ] This could be due to the data from a mix of private and public institutions.
Our survival data appears to be high possibly due to a significant proportion of patients lost to follow-up and censored at that point as a non-event. Our results show that low IPI score is a reliable prognostic indicator in DLBCL, but there was no difference between intermediate and high risk patients. This is in contrast to most reports from Western countries, which show a robust prognostic significance between the three groups. The reasons for this discrepancy are unclear.
As with other expensive medications, the use of rituximab was low in our population (42.7%), but is increasing compared to reports from earlier years.[ 13 ] The addition of rituximab to chemotherapy resulted in superior PFS, but not OS in our analysis, which is in contrast to reports from randomized trials.[ 14 , 15 ] Although the reasons for this result are unclear it is possible that features of retrospective analyses like considerable fraction being lost to follow-up, imbalance in numbers in the two groups and other systematic biases could be explanatory. It is also possible that with increasing access to rituximab over the years in India, a considerable fraction of the first line rituximab naïve patients received it after relapse, blurring the OS difference. Whatever be the reason, our analysis cannot negate the proven benefit of rituximab as a component of the first line treatment regimen in DLBCL.
In our study, 25% of patients received <6 cycles of chemotherapy. Similarly, a high percentage of patients are reported who have taken <6 cycles in Prakash et al .[ 12 ] study from a tertiary cancer center in India. Our results are also suggest that receipt of an adequate regimen of at least six cycles of frontline chemotherapy was associated with a superior outcome. The four main reasons for patients receiving less than six cycles of treatment could be low stage disease, severe adverse effects of chemotherapy and non-compliance to treatment and resistance to the first line (mainly CHOP) regimen, the last two of which could be responsible for the poor outcome in these patients. In the absence of detailed data on the reasons for receipt of less number of cycles, it is not possible to be definitive about the contribution of non-compliance to this result. However, it is likely that it contributed in at least some measure to the poor outcome in this subgroup, because of a variety of factors such as financial and logistic constraints in our population. It will be important to study this factor in prospective studies because it is a potentially correctable cause of inferior outcomes of treatment.
Our study has several weaknesses mainly related to its retrospective nature. It is possible that data collected from the case charts was neither complete nor entirely accurate. Moreover, there is likely to have been some pathological misclassification because of the variable expertise and infrastructure available at different centers. There were a relatively large number of patients who were lost to follow-up.
Despite these caveats, we believe that our analysis is a valuable contribution to the lymphoma literature from this part of the world. A valuable outcome has been the establishment of feasibility of systematic data collection by a large group of oncologist, spread across the country, together. We hope this will encourage others to form groups and collect data prospectively in various malignancies.
ACKNOWLEDGMENT
The authors would like to thank the funding for the establishment and running of lymphoma registry was provided by unrestricted educational grant from Roche Products India Pvt. Ltd. The authors are grateful acknowledge RPIPL for their support and special thanks to Dr. Anil Kukreja and Dr. Rupesh Pophale for assisting in manuscript writing.
Source of Support: Lymphoma registry established with unrestricted educational grant from Roche Products (India) Pvt. Ltd.
Conflict of Interest: Registry members received honorarium from Roche as advisory board members.

IMAGES
VIDEO
COMMENTS
Lymphoma is a type of cancer that affects the lymphatic system, which is part of the immune system. It can cause symptoms such as swollen lymph nodes, fever, weight loss and fatigue. Mayo Clinic offers comprehensive diagnosis and treatment options for lymphoma, including chemotherapy, radiation, stem cell transplant and targeted therapy. Learn more about the causes, types and outlook of ...
Lymphomas are a heterogeneous group of malignancies that arise from the clonal proliferation of B- cell, T- cell and natural killer (NK) cell subsets of lymphocytes at different stages of maturation.[1][2] Lymphoma comprises heterogeneous malignancies that arise from the clonal proliferation of lymphocytes. It represents approximately 5% of malignancies. Overall survival is estimated to be 72%.
Diagnosis and classification of NHL requires an adequate biopsy specimen and expert pathologic review because the clinical manifestations, prognosis, and management of lymphomas vary widely according to the type of lymphoma. This topic will review the clinical presentation and initial evaluation of a patient with suspected NHL.
Lymphoma commonly presents as painless adenopathy. Adenopathy can wax and wane over years in indolent presentations or involve rapidly progressive adenopathy in more aggressive subtypes.
Hodgkin Lymphoma. Characterized by abnormal cells called Reed Sternberg Cells. Reed Sternberg Cells only make up make up 2% of the tumor tissue. Remainder of the tumor is other cells of inflammation. Hodgkin lymphoma was the first to be distinguished from other lymphomas. First to be cured with radiation.
Lymphoma can start almost anywhere you have lymphocytes. These are infection-fighting white blood cells that are found throughout your lymphatic system. You have lymphocytes in your lymph nodes, spleen, bone marrow and other areas of your body. The most common early symptom is swollen lymph nodes in your neck, upper chest, armpit, belly or groin.
Non-Hodgkin lymphoma (NHL) is a neoplasm of the lymphoid tissues originating from B cell precursors, mature B cells, T cell precursors, and mature T cells. ... The patients with immunodeficiency-related Burkitt Lymphoma have presentation according to the signs or symptoms related to the underlying immunodeficiency (e.g., AIDS, congenital ...
Non-Hodgkin lymphoma is more common than Hodgkin lymphoma Hodgkin Lymphoma Hodgkin lymphoma is a localized or disseminated malignant proliferation of cells of the lymphoreticular system, primarily involving lymph node tissue, spleen, liver, and bone marrow. Symptoms... read more .It is the sixth most common cancer in the United States and represents 4% of all new cancers in the United States ...
Hodgkin lymphoma (HL), formerly called Hodgkin disease, is a rare monoclonal lymphoid neoplasm with high cure rates. Biological and clinical studies have divided this disease entity into two distinct categories: classical Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma (NLP-HL). These two disease entities show differences in the clinical picture and pathology.
The term lymphoma describes a heterogeneous group of malignancies with different biology and prognosis. In general, lymphomas are divided into 2 large groups of neoplasms, namely non-Hodgkin lymphoma (NHL) and Hodgkin disease. ... Peripheral adenopathy that is painless and slowly progressive is the most common clinical presentation in these ...
Hodgkin lymphoma has an incidence in the UK of 2.8 per 100,000 people per year, ... Presentation. Hodgkin lymphoma commonly presents with painless swollen lymph nodes (lymphadenopathy), often affecting the cervical or supraclavicular nodes in the neck. About 25% of patients present with the three 'B symptoms': night sweats, unexplained ...
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient. 1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the ...
The definition of primary GI lymphoma has differed among authors, but typically refers to a lymphoma that predominantly involves any section of the GI tract from the oropharynx to the rectum [ 1,2 ]. While the disease typically involves a single primary site, multiple sites within the GI tract may be involved, as can local and distant lymph nodes.
The Clinical Presentation of Lymphomas. From Miguel Islas-Ohlmayer, M.D. Lymphomas, blood cell tumors that usually originate in lymphatic tissues (and can spread to other organs), are among the most diverse and most curable of all malignancies. There are two major groups of lymphomas: About 90 percent are non-Hodgkin lymphomas (NHLs) and about ...
Symptoms of Lymphoma. Some people experience no symptoms during early-stage lymphoma, and the disease is discovered during unrelated medical care or testing. Others do experience symptoms, such as: Painless swelling in one or more lymph nodes, often in the neck, armpit, upper chest, or groin; Unexplained or persistent fever, fatigue, or weight loss
The presentation of patients with non-Hodgkin lymphoma is acute or subacute, in contrast to the indolent course that characterizes most lymphomas in adults. The duration of symptoms before diagnosis is generally 1 month or less, with specific complaints varying according to the predominant sites of involvement.
Non-Hodgkin's lymphoma was 4 times more common than Hodgkin's disease. The vast clinical spectrum of lymphoma sometimes delays its diagnosis, leading to its eventual presentation in late stages. A general awareness is hence required among the health professionals regarding its varied clinical presen …
B-cell lymphomas occur with an incidence of 20 new cases per 100 000 people per year in high-income countries. They can affect any organ and are characterised by heterogeneous clinical presentations and courses, varying from asymptomatic, to indolent, to very aggressive cases. Since the topic of B-cell non-Hodgkin lymphomas was last reviewed in The Lancet in 2017, a deeper understanding of the ...
A novel treatment for leukemias and lymphomas that arise from immune system T cells, developed by investigators at the Johns Hopkins Kimmel Cancer Center and its Ludwig Center and Lustgarten Laboratory, was found to be effective at killing these cancers in mice bearing human T-cell tumors.. The therapy, an antibody-drug conjugate (ADC), combines an antibody that targets a protein called TRBC1 ...
Perhaps counter to the idea that new lymphatic growth is necessary for robust antigen presentation, the potent adaptive immune responses to vaccinia virus infection in the skin do not require ...
The variable clinical presentation of lymphoma is a challenge for the ENT specialist. Fast diagnosis is crucial for rapid treatment, especially in highly aggressive NHL like the Burkitt-lymphoma and HL. A standardized medical history, clinical examination and imaging evaluations paired with patient's signs, symptoms and demographic knowledge ...
A 49-year-old male was admitted to the intensive care unit in June 2023 for symptoms of severe refractory idiopathic anaphylaxis. His symptoms began with acute diffuse urticaria, which progressed to lip angioedema, wheezing, dyspnea, dizziness, and pre-syncope. At initial evaluation in the emergency department he was hypotensive to 72/52 mm Hg requiring two rounds of intramuscular epinephrine ...
Surgical biopsy is the gold standard for diagnosing central nervous system (CNS) lymphomas. However, reliable liquid biopsy methods for diagnosing CNS lymphomas have quickly developed and have been implicated in clinical decision-making. In the current report, we introduce two patients for whom liquid biopsy was essential for diagnosing CNS lymphomas and discuss the rapidly growing ...
The variable clinical presentation of lymphoma is a challenge for the ENT specialist. Fast diagnosis is crucial for rapid treatment, especially in highly aggressive NHL like the Burkitt-lymphoma and HL. A standardized medical history, clinical examination and imaging evaluations paired with patient's signs, symptoms and demographic knowledge ...
A novel treatment for leukemias and lymphomas that arise from immune system T cells was found to be effective at killing these cancers in mice bearing human T-cell tumors. A novel treatment for ...
A Lymphoma Registry was established to understand the clinical, pathological and treatment patterns in lymphoma patients presenting to collaborating centers and their long-term outcomes. ... 37.8% reported B symptoms at presentation, 18.3% patients had bulky disease at initial presentation and bone marrow was involved in 15.3% ...
1 INTRODUCTION. Primary cardiac tumors are exceptionally rare, with autopsy findings indicating an incidence of about 0.02%. 1 Among these, about 75% are benign, primarily myxomas, whereas the remaining 25% consist of malignant entities, various types of sarcomas, and, less frequently, lymphomas. 2 Myxofibrosarcoma predominantly occurs in the extremities of elderlies 3 and is one of the rarest ...
24th Annual Fellows' Research Presentations Ritz-Carlton, Amelia Island, Florida July 25-27, 2024 . Program schedule is subject to change without notice. Thursday, July 25, 2024 7:00 a.m. Registration and Continental Breakfast 8:00 Welcome and Review of Course with Pre-Test Gerardo Colon-Otero, M.D. (MCF) and Rami Manochakian, M.D. (MCF)