Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 August 2017
- Margaret A. Phillips 1 ,
- Jeremy N. Burrows 2 ,
- Christine Manyando 3 ,
- Rob Hooft van Huijsduijnen 2 ,
- Wesley C. Van Voorhis 4 &
- Timothy N. C. Wells 2
Nature Reviews Disease Primers volume 3 , Article number: 17050 ( 2017 ) Cite this article
74k Accesses
380 Citations
202 Altmetric
Metrics details
- Antimicrobial resistance
- Public health
Malaria is caused in humans by five species of single-celled eukaryotic Plasmodium parasites (mainly Plasmodium falciparum and Plasmodium vivax ) that are transmitted by the bite of Anopheles spp. mosquitoes. Malaria remains one of the most serious infectious diseases; it threatens nearly half of the world's population and led to hundreds of thousands of deaths in 2015, predominantly among children in Africa. Malaria is managed through a combination of vector control approaches (such as insecticide spraying and the use of insecticide-treated bed nets) and drugs for both treatment and prevention. The widespread use of artemisinin-based combination therapies has contributed to substantial declines in the number of malaria-related deaths; however, the emergence of drug resistance threatens to reverse this progress. Advances in our understanding of the underlying molecular basis of pathogenesis have fuelled the development of new diagnostics, drugs and insecticides. Several new combination therapies are in clinical development that have efficacy against drug-resistant parasites and the potential to be used in single-dose regimens to improve compliance. This ambitious programme to eliminate malaria also includes new approaches that could yield malaria vaccines or novel vector control strategies. However, despite these achievements, a well-coordinated global effort on multiple fronts is needed if malaria elimination is to be achieved.

Similar content being viewed by others

Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa
Tadele Emiru, Dejene Getachew, … Fitsum G. Tadesse
The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density
Hannah C. Slater, Amanda Ross, … Lucy C Okell
Emergence, transmission dynamics and mechanisms of artemisinin partial resistance in malaria parasites in Africa
Philip J. Rosenthal, Victor Asua & Melissa D. Conrad
Introduction
Malaria has had a profound effect on human lives for thousands of years and remains one of the most serious, life-threatening infectious diseases 1 – 3 . The disease is caused by protozoan pathogens of the Plasmodium spp.; Plasmodium falciparum and Plasmodium vivax , for which humans are the exclusive mammalian hosts, are the most common species and are responsible for the largest public health burden. Malaria is transmitted by the bite of Plasmodium spp.-infected female mosquitoes of the Anopheles genus 1 – 3 . During a blood meal, infected mosquitoes inject — along with their anticoagulating saliva — sporozoites, which are the infective, motile stage of Plasmodium spp. Sporozoites journey through the skin to the lymphatics and into hepatocytes in the liver ( Fig. 1 ). Inside the hepatocyte, a single sporozoite can generate tens of thousands of merozoites (the stage that results from multiple asexual fissions (schizogony) of a sporozoite within the body of the host), which are released into the bloodstream where they enter red blood cells to replicate (erythrocytic schizogony). A fraction of merozoites (those that are sexually committed) also differentiate and mature into male and female gametocytes, which is the stage that infects the mosquito host when it takes a blood meal 4 , 5 . The onset of clinical symptoms generally occurs 7–10 days after the initial mosquito bite. P. vivax and Plasmodium ovale also have dormant forms, called hypnozoites, which can emerge from the liver years after the initial infection 6 , leading to relapse if not treated properly.
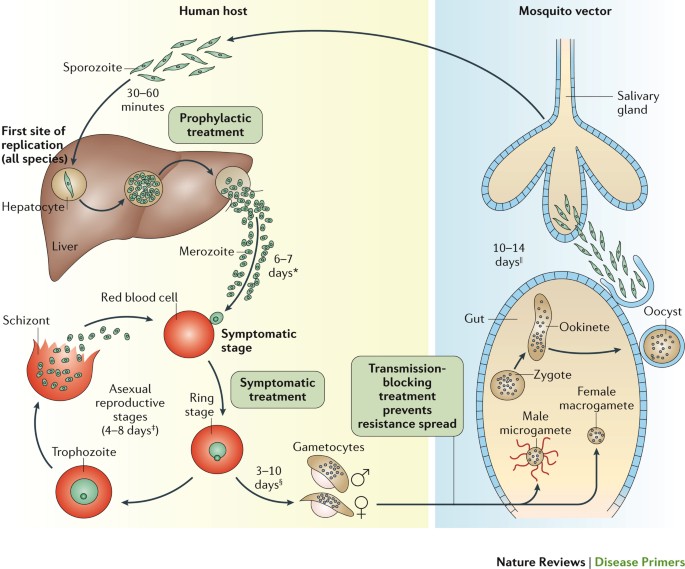
The mosquito vector transmits the Plasmodium spp. parasite in the sporozoite stage to the host during a blood meal. Within 30–60 minutes, sporozoites invade liver cells, where they replicate and divide as merozoites. The infected liver cell ruptures, releasing the merozoites into the bloodstream, where they invade red blood cells and begin the asexual reproductive stage, which is the symptomatic stage of the disease. Symptoms develop 4–8 days after the initial red blood cell invasion. The replication cycle of the merozoites within the red blood cells lasts 36–72 hours (from red blood cell invasion to haemolysis). Thus, in synchronous infections (infections that originate from a single infectious bite), fever occurs every 36–72 hours, when the infected red blood cells lyse and release endotoxins en masse 70 – 72 . Plasmodium vivax and Plasmodium ovale can also enter a dormant state in the liver, the hypnozoite. Merozoites released from red blood cells can invade other red blood cells and continue to replicate, or in some cases, they differentiate into male or female gametocytes 4 , 5 . The transcription factor AP2-G (not shown) has been shown to regulate the commitment to gametocytogenesis. Gametocytes concentrate in skin capillaries and are then taken up by the mosquito vector in another blood meal. In the gut of the mosquito, each male gametocyte produces eight microgametes after three rounds of mitosis; the female gametocyte matures into a macrogamete. Male microgametes are motile forms with flagellae and seek the female macrogamete. The male and female gametocytes fuse, forming a diploid zygote, which elongates into an ookinete; this motile form exits from the lumen of the gut across the epithelium 254 as an oocyst. Oocysts undergo cycles of replication and form sporozoites, which move from the abdomen of the mosquito to the salivary glands. Thus, 7–10 days after the mosquito feeds on blood containing gametocytes, it may be ‘armed’ and able to infect another human with Plasmodium spp. with her bite. Drugs that prevent Plasmodium spp. invasion or proliferation in the liver have prophylactic activity, drugs that block the red blood cell stage are required for the treatment of the symptomatic phase of the disease, and compounds that inhibit the formation of gametocytes or their development in the mosquito (including drugs that kill mosquitoes feeding on blood) are transmission-blocking agents. *Merozoite invasion of red blood cells can be delayed by months or years in case of hypnozoites. ‡ The number of days until symptoms are evident. § The duration of gametogenesis differs by species. || The maturation of sporozoites in the gut of the mosquito is highly temperature-dependent. Adapted with permission from Ref. 255 , Macmillan Publishers Ltd.
PowerPoint slide
The consequences of Plasmodium spp. infection vary in severity depending on the species and on host factors, including the level of host immunity, which is linked to the past extent of parasite exposure 7 , 8 . Malaria is usually classified as asymptomatic, uncomplicated or severe (complicated) 9 ( Box 1 ). Typical initial symptoms are low-grade fever, shaking chills, muscle aches and, in children, digestive symptoms. These symptoms can present suddenly (paroxysms), and then progress to drenching sweats, high fever and exhaustion. Malaria paroxysmal symptoms manifest after the haemolysis of Plasmodium spp.-invaded red blood cells. Severe malaria is often fatal, and presents with severe anaemia and various manifestations of multi-organ damage, which can include cerebral malaria 8 ( Box 1 ). Severe malaria complications are due to microvascular obstruction caused by the presence of red blood cell-stage parasites in capillaries 8 , 10 , 11 . This Primer focuses on our understanding of malaria pathology in the context of parasite and vector biology, progress in diagnostics and new treatments (drugs and vaccines), chemoprotection and chemoprevention.
Box 1: Malaria key terms
Asymptomatic malaria: can be caused by all Plasmodium spp.; the patient has circulating parasites but no symptoms.
Uncomplicated malaria: can be caused by all Plasmodium spp. Symptoms are nonspecific and can include fever, moderate-to-severe shaking chills, profuse sweating, headache, nausea, vomiting, diarrhoea and anaemia, with no clinical or laboratory findings of severe organ dysfunction.
Severe (complicated) malaria: usually caused by infection with Plasmodium falciparum , although less frequently it can also be caused by Plasmodium vivax or Plasmodium knowlesi . Complications include severe anaemia and end-organ damage, including coma (cerebral malaria), pulmonary complications (for example, oedema and hyperpnoeic syndrome 228 ), and hypoglycaemia or acute kidney injury. Severe malaria is often associated with hyperparasitaemia and is associated with increased mortality.
Placental malaria: parasites are present in the placenta, leading to poor outcomes for the fetus and possibly for the mother.
Epidemiology
Human malaria parasites are transmitted exclusively by ∼ 40 species of the mosquito genus Anopheles 12 . During Anopheles spp. mating, males transfer high levels of the steroid hormone 20-hydroxyecdysone to the females, and the presence of this hormone has been associated with favourable conditions for Plasmodium spp. development 13 . Malaria-competent Anopheles spp. are abundant and distributed all over the globe, including the Arctic. However, the efficacy of malaria transmission depends on the vector species and, therefore, varies considerably worldwide; for example, in tropical Africa, Anopheles gambiae is a major and highly efficient vector 14 . The first WHO Global Malaria Eradication Programme (1955–1972) involved, in addition to chloroquine-based treatments, large-scale insecticide campaigns using dichlorodiphenyltrichloroethane (DDT) 15 . This strategy was quite effective against P. falciparum ; although the mosquitoes gradually repopulated DDT-treated areas (because they developed resistance to the insecticide, and the use of DDT itself waned owing to its costs and increasing environmental concerns), these areas have often remained malaria-free and in some cases still are. More-selective vector control approaches, such as the use of insecticide-treated bed nets and indoor residual spraying, have eliminated malaria from several areas (see Diagnosis, screening and prevention, below). However, mosquito resistance to insecticides is a growing concern. Of the 78 countries that monitor mosquito resistance to insecticides, 60 have reported resistance to one or more insecticides since 2010 (Ref. 16 ).
The parasite
Plasmodium spp. are single-celled eukaryotic organisms 17 – 19 that belong to the phylum Apicomplexa, which is named for the apical complex that is involved in host cell invasion. A discussion of the parasite genome and the genetic approaches used to study parasite biology is provided in Box 2 . Of the five human-infective Plasmodium spp., P. falciparum causes the bulk of malaria-associated morbidity and mortality in sub-Saharan Africa, with mortality peaking in the late 1990s at over 1 million deaths annually in the continent 20 ( Fig. 2 ). P. falciparum is associated with severe malaria and complications in pregnancy ( Box 3 ); most malaria-related deaths are associated with this species, which kills ∼ 1,200 African children <5 years of age each day 21 . However, P. falciparum is also found in malarious tropical areas around the world. P. vivax is found in malarious tropical and temperate areas, primarily Southeast Asia, Ethopia and South America, and generally accounts for the majority of malaria cases in Central and South America and in temperate climates. This distribution can be explained by the fact that P. vivax can survive in climatically unfavourable regions and can stay dormant in a hypnozoite form in its human host's liver for many years. Furthermore, many Africans are negative for the Duffy antigen (also known as atypical chemokine receptor 1) on the surface of red blood cells, and this genotype provides protection from P. vivax malaria, as it makes it more difficult for P. vivax to bind to and penetrate red blood cells 22 . However, some cases of P. vivax transmission to Duffy antigen-negative individuals have been reported, which suggests that alternative mechanisms of invasion might be present in some strains, and this might portend the escalation of P. vivax malaria to Africa 23 , 24 . P. ovale is also found in Africa and Asia, but is especially prevalent in West Africa. Two sympatric species exist: P.o. curtisi and P.o. wallikeri 25 . Plasmodium malariae — which can be found worldwide but is especially prevalent in West Africa — causes the mildest infections, although it has been associated with splenomegaly or renal damage upon chronic infection. Plasmodium knowlesi — which was initially considered as a parasite of non-human primates — can not only cause malaria in humans but can also lead to severe and even fatal malaria complications 26 , 27 . The reasons for the emergence of P. knowlesi in humans are not yet fully understood but are possibly linked to land-use changes that have brought humans into close contact with P. knowlesi -infected mosquitoes 28 . Regardless, the possible recent emergence of a form of malaria as a zoonosis poses obvious complications for elimination. In addition, co-infections between P. falciparum and P. vivax have been well-documented and have been reported to occur in up to 10–30% of patients living in areas where both parasites are prevalent 29 , 30 . Mixed infections can also include other species such as P. ovale and P. malariae , and newer diagnostic methods are being developed that will enable better assessment of the frequency and distribution of these types of co-infection (for example, Ref. 31 ).
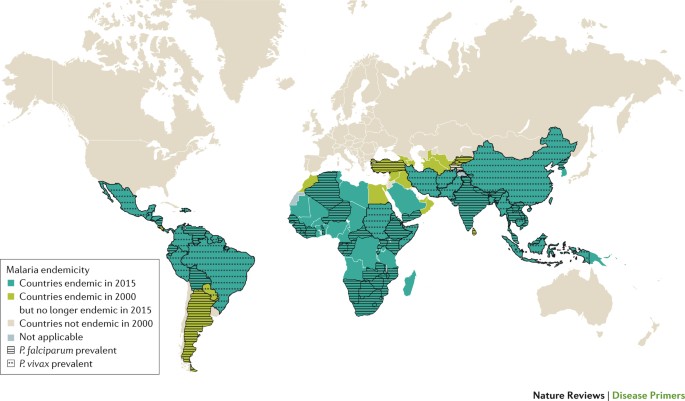
The most-deadly malaria parasite, Plasmodium falciparum , is only found in tropical areas because its gametocytes require 10–18 days at a temperature of >21°C to mate and mature into infectious sporozoites inside the vector 256 . This development timeline is only possible in hot, tropical conditions; where the ambient temperature is lower, mosquitoes can still propagate, but sporozoite maturation is slowed down and, therefore, incomplete, and parasites perish without progeny when the mosquitoes die. Thus, P. falciparum is quite temperature-sensitive; a global temperature rise of 2–3 °C might result in an additional 5% of the world population (that is, several hundred million people) being exposed to malaria 257 . Of note, Plasmodium vivax and Plasmodium ovale can develop in mosquitoes at ambient temperatures as low as 16 °C. The abilities of these parasites to propagate at subtropical temperatures and to remain in the hypnozoite state in the liver are likely to explain their ability to survive dry or cold seasons, and the broader global distribution of these parasites 258 . Countries coded ‘not applicable’ in the Figure were not separately surveyed. Figure based on data from Ref. 16 , WHO.
Box 2: The Plasmodium spp. genome and genomic tools for understanding gene function
Characteristics of the Plasmodium spp. genome
Each haploid genome comprises 23 Mb, which encode the programme for the complex life cycle of the parasite within ∼ 5,500 genes 17 – 19 .
Many genes encode proteins that have similarities to host proteins, many are novel, and many (approximately half) remain annotated as genes with hypothetical or of unknown function.
The Plasmodium spp. genome includes an essential plastid, the apicoplast, which is derived from two sequential endosymbiotic events, and encodes genes from both plant (red algal) and bacterial (cyanobacterium) origin 229 . The bacterial origin of some enzymes encoded by the plastid makes Plasmodium spp. sensitive to some antibacterial agents, whereas the plant-like pathways can be targeted by some herbicides. This plastid is one source of genes that differ from the host and that have been considered as potential drug targets.
Gene transcription across the Plasmodium spp. intra-red blood cell life cycle follows a preprogrammed cyclic cascade during which most genes are expressed at peak levels only once per life cycle 230 – 232 . Genes that encode cell surface proteins involved in host–parasite interactions are the exception.
Gene expression patterns have been reported to lack responses to perturbations. Minimal changes were observed after treatment with antifolates and chloroquine; however, larger changes have been observed for other drug classes 233 , 234 . Species-specific differences in transcription have been observed that seem to be linked to the mammalian host 235 .
Ribosome profiling has demonstrated that transcription and translation are tightly coupled for 90% of genes 236 . Exceptions of translationally upregulated genes are typically found for proteins involved in merozoite egress and invasion.
Epigenetic mechanisms to control gene expression include post-translational histone modifications (methylation and acetylation of the amino terminus are the best-characterized). Many of these modifications have been linked to parasite development 63 , 237 .
Genomic tools
Gene knockouts are possible, but RNA interference-mediated knockdown mechanisms do not function in Plasmodium spp. 238 , 239 .
Regulated RNA aptamer-based approaches have led to methods that enable gene knockouts to be functionally rescued; these methods are key for studying essential genes 238 , 239 .
CRISPR–Cas9-directed genome editing has greatly facilitated the genetic manipulation of Plasmodium falciparum 238 , 239 .
Barcoded mutant Plasmodium berghei libraries have been developed to screen for competitive fitness across tens of mutants in a single mouse 240 .
The in vitro selection of drug-resistant mutant parasites followed by whole-genome sequencing has also become a well-established method for revealing candidate drug targets 241 .
Metabolomics approaches facilitate the understanding of Plasmodium spp. biology, and have been used to profile several antimalarial compounds that have both known and unknown mechanisms of action 242 .
Box 3: Malaria and pregnancy
Pregnant women are more susceptible to Plasmodium spp. infection, particularly in their first pregnancy, as the mother-to-be has not yet acquired immunity to parasites that express the protein variant surface antigen 2-CSA (VAR2CSA) 35 . VAR2CSA on the surface of infected red blood cells facilitates adhesion to chondroitin sulfate A (which is part of placental proteoglycans), leading to red blood cell sequestration in the placenta 7 , 64 . The risk of placental malaria is reduced in multigravid women from endemic areas, who generally have antibodies against VAR2CSA 65 – 67 .
Malaria during pregnancy leads to increased risks to the mother and fetus 36 , 243 . Most studies have focused on sub-Saharan Africa; however, pregnancy-related risks are a problem throughout the world, including in Latin America, where Plasmodium vivax is the dominant causative agent 244 .
Placental malaria might be asymptomatic or clinically mild, but it also leads to an increased risk of death for both the fetus and the mother. It predisposes to miscarriage, stillbirth, preterm delivery and babies with low birth weight whose quality of life will probably be poor because of cognitive, mobility, self-care and sensation limitations; such babies also have a high mortality rate 36 , 243 .
Intermittent preventive treatment with sulfadoxine–pyrimethamine in endemic regions is recommended and is generally administered at each antenatal visit following quickening 108 , although the emergence of resistance is threatening its efficacy 245 .
Treatments for pregnant women must take into account the availability of safety data for the fetus. As a consequence, newer treatments require time to obtain sufficient confirmation of their tolerability in the different trimesters. The WHO recommends quinine sulfate and clindamycin in the first trimester. One study has shown that artemisinin derivatives provide comparable safety to quinine 246 , but, at the time of publication, the results of this study have not yet been incorporated into the WHO guidelines. In the second or third trimester, the WHO recommends artemisinin-based combination therapies 108 .
The treatment of pregnant women with P. vivax , Plasmodium ovale or Plasmodium malariae infection can also include chloroquine, unless resistance is suspected 108 . Women who are at a high risk of relapse can be given weekly chloroquine chemoprophylaxis until after delivery. Follow-up therapy with primaquine against P. vivax and P. ovale hypnozoites is not thought to be safe during pregnancy.
The disease
Malaria remains a major burden to people residing in resource-limited areas in Africa, Asia and Central and South America ( Fig. 2 ). An estimated 214 million cases of malaria occurred in 2015 (Ref. 16 ). Africa bears the brunt of the burden, with 88% of the cases, followed by Southeast Asia (10%), the eastern Mediterranean region (2%) and Central and South America (<1%). Malaria continues to kill over three-times as many people as all armed conflicts; in 2015, there were an estimated 438,000 (Ref. 16 ) — 631,000 (Ref. 20 ) deaths resulting from malaria, compared with an estimated 167,000 deaths due to armed conflicts 32 , 33 . In areas of continuous transmission of malaria, children <5 years of age and the fetuses of infected pregnant women experience the most morbidity and mortality from the disease. Children >6 months of age are particularly susceptible because they have lost their maternal antibodies but have not yet developed protective immunity. In fact, adults and children >5 years of age who live in regions of year-round P. falciparum transmission develop a partial protective immunity owing to repeated exposure to the parasite. There is evidence that immunity against P. vivax is acquired more quickly 34 . Individuals with low protective immunity against P. falciparum are particularly vulnerable to severe malaria. Severe malaria occurs in only 1% of infections in African children and is more common in patients who lack strong immune protection (for example, individuals who live in low-transmission settings, children <5 years of age and naive hosts). Severe malaria is deadly in 10% of children and 20% of adults 7 . Pregnant women are more susceptible to Plasmodium spp. infection because the placenta itself selects for the emergence of parasites that express receptors that recognize the placental vasculature; these receptors are antigens to which pregnant women have not yet become partially immune 7 ( Box 3 ). This vulnerability increases the risk of miscarriage; parasitaemia in the placenta can have adverse effects on the fetus 35 – 37 ( Box 3 ).
Co-infection of Plasmodium spp. with other pathogens — including HIV, Mycobacterium tuberculosis and helminths — is common. HIV-infected adults are at an increased risk of severe malaria and death 38 . The overall prevalence of helminth infection is very high (>50% of the population) in malaria-endemic regions and is associated with increased malaria parasitaemia 39 . Surprisingly, naturally occurring iron deficiency and anaemia protect against severe malaria, which was an unexpected finding 40 , as numerous clinical studies have aimed to fortify children and prevent anaemia by distributing iron supplements 41 .
From 2000 to 2015, the incidence of malaria fell by 37% and the malaria mortality rate fell by 60% globally 16 . The WHO attributes much of this reduction of malaria-associated morbidity and mortality to the scale-up of three interventions: insecticide-treated bed nets (69% of the reduction), artemisinin-based combination therapies (ACTs; 21%) and indoor residual insecticide spraying (10%) 16 (see Diagnosis, screening and prevention, below). Until ACT was introduced, progress in malaria control in most malarious countries was threatened or reversed by the nearly worldwide emergence of chloroquine-resistant and sulfadoxine–pyrimethamine-resistant P. falciparum strains and, more recently, of other resistant Plasmodium spp. ACT has become the antimalarial medicine of choice in most malarious areas, and demonstrates rapid parasite clearance, superior efficacy (compared with other clinically approved drugs) and >98% cure rates (typically defined as the percentage of patients who remain malaria-free for 28 days; re-infection events do not count as a recurrence). ACTs achieve these results even in strains that are resistant to older antimalarials, effectively turning the tide against antimalarial drug resistance. However, the emergence of artemisinin-resistant strains in Southeast Asia threatens the usefulness of ACTs 42 – 45 (see Drug resistance, below).
Mechanisms/pathophysiology
The red blood cell stage.
As previously mentioned, the red blood cell stage of Plasmodium spp. infection is the cause of symptomatic malaria, as red blood cells are the site of abundant parasite replication.
Invasion . Plasmodium spp. parasites gain entry into the red blood cell through specific ligand–receptor interactions mediated by proteins on the surface of the parasite that interact with receptors on the host erythrocyte (mature red blood cell) or reticulocyte (immature red blood cell) 46 ( Fig. 3 ). Whereas P. falciparum can invade and replicate in erythrocytes and reticulocytes, P. vivax and other species predominantly invade reticulocytes, which are less abundant than erythrocytes 47 . Most of the parasite erythrocyte-binding proteins or reticulocyte-binding proteins that have been associated with invasion are redundant or are expressed as a family of variant forms; however, for P. falciparum , two essential red blood cell receptors (basigin and complement decay-accelerating factor (also known as CD55)) have been identified ( Fig. 3 ).
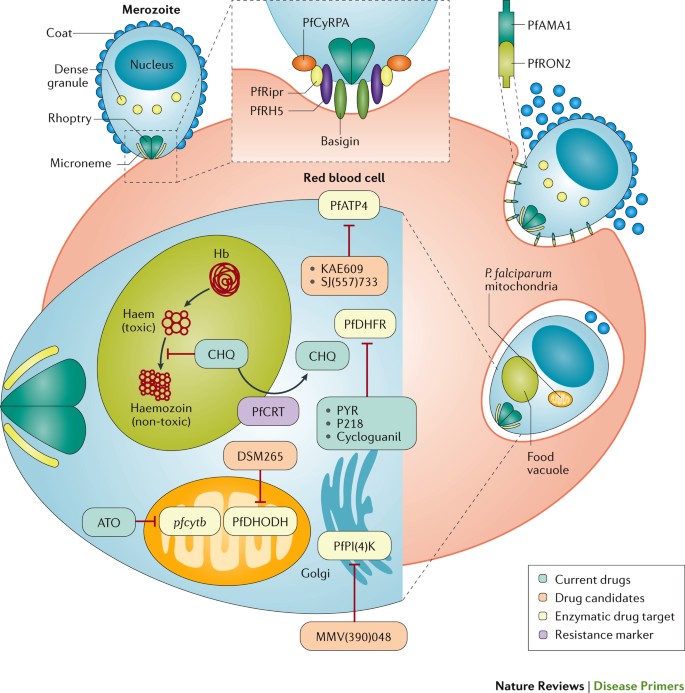
Invasion occurs through a multistep process 259 . During pre-invasion, low-affinity contacts are formed with the red blood cell membrane. Reorientation of the merozoite is necessary to enable close contact between parasite ligands and host cell receptors, and this is then followed by tight junction formation. In Plasmodium falciparum , a forward genetic screen has shown that complement decay-accelerating factor (not shown) on the host red blood cell is essential for the invasion of all P. falciparum strains 260 . The interaction of a complex of P. falciparum proteins (reticulocyte-binding protein homologue 5 (PfRH5), PfRH5-interacting protein (PfRipr) and cysteine-rich protective antigen (PfCyRPA)) with basigin on the red blood cell surface is also essential for the invasion in all strains 261 , 262 . PfRH5 has been studied as a potential vaccine candidate 46 , and antibodies against basigin have been considered as a potential therapeutic strategy 263 . During the PfRH5–PfRipr–PfCyRPA–basigin binding step, an opening forms between the parasite and the red blood cell, and this triggers Ca 2+ release and enables parasite-released proteins to be inserted into the red blood cell membrane. These proteins are secreted from the micronemes (the small secretory organelles that cluster at the apical end of the merozoite) and from the neck of the rhoptries, and include rhoptry neck protein 2 (PfRON2). Binding between PfRON2 and apical membrane antigen 1 (PfAMA1) on the merozoite surface is required to mediate tight junction formation before the internalization process 264 , and PfAMA1 is also being evaluated as a vaccine candidate 265 . Parasite replication within the red blood cell requires the synthesis of DNA, which can be blocked by several antimalarials: pyrimethamine (PYR), P218 and cycloguanil target P. falciparum dihydrofolate reductase (PfDHFR) 266 , and atovaquone (ATO) blocks pyrimidine biosynthesis by inhibiting the expression of the mitochondrial gene pfcytb (which encodes P. falciparum cytochrome b ) and by preventing the formation of oxidized coenzyme Q, which is needed to enable the pyrimidine biosynthetic enzyme dihydroorotate dehydrogenase (PfDHODH) to perform its reaction within the mitochondria 50 . The phase II clinical candidate DSM265 also blocks pyrimidine biosynthesis by directly inhibiting PfDHODH 186 . In addition to DNA synthesis, other processes can be targeted by antimalarial drugs. Chloroquine (CHQ) inhibits haem polymerization in the food vacuole 52 but can be expelled from this compartment by the P. falciparum chloroquine-resistance transporter (PfCRT) 267 . The phase II clinical candidate KAE609 and the preclinical candidate SJ(557)733 both inhibit P. falciparum p-type ATPase 4 (PfATP4), which is required for Na + homeostasis during nutrient acquisition 57 , 183 , 184 . The phase I clinical candidate MMV(390)048 (Ref. 191 ) inhibits P. falciparum phosphatidylinositol 4-kinase (PfPI(4)K), which is required for the generation of transport vesicles that are needed to promote membrane alterations during ingression 58 . Hb, haemoglobin.
Replication . Once Plasmodium spp. gain entry into the red blood cell, they export hundreds of proteins into the host cell cytoplasm and cell surface that modulate the acquisition of nutrients, cell adhesion and sequestration in tissues, and pathogenesis 3 , 48 , 49 . Molecular and cell biology approaches are expanding our understanding of the molecular machinery that is required for the export, as well as the identification and function of the exported proteins.
In the red blood cell, Plasmodium spp. replicate rapidly, and during symptomatic disease the parasites may replicate exponentially to >10 12 parasites per patient. This rapid growth requires sustained pools of nucleotides for the synthesis of DNA and RNA, and as a consequence, many antimalarials target pyrimidine biosynthesis 50 ( Fig. 3 ). Plasmodium spp. are auxotrophic for all of the amino acids they need (that is, they must acquire all of these from food because they cannot synthesize them from precursors). Haemoglobin digestion (in a specialized food vacuole) supplies all amino acids except isoleucine, which must be obtained from other host cell components 51 . Haemoglobin digestion also releases haem, which is toxic to the parasite and, therefore, is polymerized into haemozoin (often called malaria pigment, which is visible as a blue pigment under light microscopy), which is an insoluble crystal that sequesters the toxic metabolite 52 . How haem polymerization is facilitated by the parasite remains unclear. A complex of several proteases and haem detoxification protein (HDP) have been identified in the food vacuole; follow-up in vitro studies have shown that components of this complex (for example, falcipain 2, HDP and lipids) were able to catalyse the conversion of haem into haemozoin 53 . The importance of understanding this mechanism is highlighted by the finding that chloroquine and other antimalarials act by inhibiting haem polymerization 54 ( Fig. 3 ). There is also evidence that the iron (haem-bound or free) liberated in the food vacuole during haemoglobin digestion plays a part in activating the toxicity to the parasite of artemisinin derivatives 42 .
Nutrient uptake by the parasite is coupled to the detrimental accumulation of Na + ; however, the parasite expresses an essential plasma membrane Na + export pump (the cation ATPase P. falciparum p-type ATPase 4 (PfATP4)) that can maintain Na + homeostasis 55 – 57 ( Fig. 3 ). The remodelling of the plasma membrane (membrane ingression) to generate daughter merozoites in the late schizont stage requires P. falciparum phosphatidylinositol 4-kinase (PfPI(4)K) 58 . Both PfPI(4)K and PfATP4 are targets of new drugs that are under development ( Fig. 3 ).
Immune evasion and host immunity
Malaria parasites first encounter the host immune system when sporozoites are injected in the skin (measured to be ∼ 15 per mosquito bite in one study 59 ), where they may be phagocytosed by dendritic cells for antigen presentation in the lymph node draining the skin inoculation site 60 . The chances of transmission are increased when the host is bitten by mosquitoes that carry a larger number of sporozoites, despite the fact that the number of sporozoites that can simultaneously pass through the mosquito's proximal duct is limited by the duct diameter 61 . Sporozoites encounter several other effectors of the immune system, and how a minority of them can reach the liver and infect the hepatocytes is not well understood. Immune evasion in the liver could be in part explained by the ability of sporozoites to suppress the function of Kupffer cells (also known as stellate macrophages, which are the resident macrophages of the liver) and repress the expression of genes that encode MHC class I molecules 62 . Our understanding of the host immunity associated with the red blood cell stage is more complete. Virulence genes in Plasmodium spp. are part of large expanded multigene families that are found in specialized (for example, subtelomeric) regions of the chromosomes 7 , 63 , 64 . These gene families (for example, var genes in P. falciparum ) encode variants of cell surface proteins that function in immune evasion through antigenic variation and also are involved in mediating cytoadherence of infected red blood cells to endothelial cells, which leads to red blood cell sequestration in tissues.
Malaria disease severity — in terms of both parasite burden and the risk of complicated malaria — is dependent on the levels of protective immunity acquired by the human host 65 – 67 , which can help to decrease the severity of symptoms and reduce the risk of severe malaria. Immunity is thought to result from circulating IgG antibodies against surface proteins on sporozoites (thereby blocking hepatocyte invasion) and merozoites (thereby blocking red blood cell invasion). In high-transmission areas where malaria is prevalent year-round, adults develop partially protective immunity. Young infants (<6 months of age) are also afforded some protection, probably from the antibodies acquired from their mother, whereas children from 6 months to 5 years of age have the lowest levels of protective immunity and are the most susceptible to developing high parasitaemia with risks for complications and death (for example, see the study conducted in Kilifi, Kenya 68 ). In low-transmission areas or areas that have seasonal malaria, individuals develop lower levels of protective immunity and typically have worse symptomatic malaria upon infection. This correlation between protective immunity and malaria severity poses a challenge for successful malaria treatment programmes; as the number of infections and the transmission rates decrease, increasing numbers of patients will lose protective immunity and become susceptible to severe disease. The re-introduction of malaria in areas that had been malaria-free for many years could be devastating in the short term and, therefore, well-organized surveillance is required.
Pathogenesis
The predominant pathogenic mechanism is the haemolysis of Plasmodium spp.-infected red blood cells, which release parasites and malaria endotoxin — understood to be a complex of haemozoin and parasite DNA, which trigger Toll-like receptor 9 (TLR9), a nucleotide-sensing receptor involved in the host immune response against pathogens 69 — that leads to high levels of tumour necrosis factor (TNF) production and to clinical symptoms such as fever 70 – 72 . In addition, the membrane of infected red blood cells stiffens, and this loss of deformability contributes to the obstruction of capillaries, which has life-threatening consequences in severe malaria when vital organs are affected 73 .
Parasite factors that influence disease severity . Disease severity and pathogenesis are linked to surface proteins that are expressed by the parasite. In P. falciparum , a major surface antigen is encoded by the var gene family, which contains ∼ 60 members 7 , 11 , 63 , 64 . The majority of the var genes are classified into three subfamilies — A, B and C — on the basis of their genomic location and sequence: the B and C groups mediate binding to host cells via CD36 (also known as platelet glycoprotein 4), whereas the A group genes mediate non-CD36 binding interactions that have been linked to severe malaria, including cerebral malaria 7 , 64 . The var genes encode P. falciparum erythrocyte membrane protein 1 (PfEMP1), with the B and C groups accounting for >80% of PfEMP1 variants. PfEMP1 is the major protein involved in cytoadherence and mediates the binding of infected erythrocytes to the endothelial vasculature. In cerebral malaria, A group PfEMP1 variants mediate the binding of infected erythrocytes to endothelial protein C receptor (EPCR) and intercellular adhesion molecule 1 (ICAM1) in the brain, causing pathology 8 , 11 , 74 , 75 . However, our knowledge of the host cell receptors that are involved in interactions with the infected erythrocytes is probably incomplete. For example, thrombin — which regulates blood coagulation via vitamin K-dependent protein C — can cleave PfEMP1, thereby reversing and preventing the endothelial binding of infected erythrocytes 74 . In pregnancy, the expression of a specific PfEMP1 variant, variant surface antigen 2-CSA (VAR2CSA) — which is not encoded by one of the three main subfamilies — leads to an increased risk of placental malaria 7 , 64 ( Box 3 ).
High parasitaemia levels also seem to correlate with poor outcomes 7 , 75 , and the circulating levels of P. falciparum histidine-rich protein 2 (which is encoded by pfhrp2 ) have been used as a biomarker of parasitaemia that predicts the risks for microvascular obstruction and severe disease 76 . The brain pathology in children with severe malaria has recently been described in detail 77 .
Additionally, P. vivax does not express the same family of var genes that have been found to be strongly associated with endothelium binding and tissue sequestration, which drives severe disease in P. falciparum , and the ability of P. vivax to only invade reticulocytes leads to lower parasite levels 7 .
Host traits that influence disease severity . Malaria has exerted a strong selection pressure on the evolution of the human genome 78 , 79 . Some haemoglobin-encoding alleles that in homozygous genotypes cause severe blood disorders (such as thalassaemia, the earliest described example, and sickle cell disease) have been positively selected in populations living in malaria-endemic areas because heterozygous genotypes protect against malaria 80 . Other inherited haemoglobin abnormalities (for example, mutations affecting haemoglobin C and haemoglobin E) can also provide protection against malaria 81 .
In addition, genetic polymorphisms that affect proteins expressed by red blood cells or that lead to enzyme deficiencies can also be protective against severe disease. The red blood cell Duffy antigen is a key receptor that mediates the invasion of P. vivax through interaction with the Duffy antigen-binding protein on the parasite surface 46 . The genetic inheritance of mutations in ACKR1 (which encodes the Duffy antigen) in Africa is credited with reducing the spread of P. vivax in this continent, although the finding of Duffy antigen-negative individuals who can be infected with P. vivax suggests that we still have an incomplete understanding of the factors involved in P. vivax invasion 82 , 83 . Glucose-6-phosphate dehydrogenase (G6PD) deficiency 78 , 79 provides protection against severe malaria through an unknown mechanism, at least in hemizygous males 84 , but unfortunately also leads to haemolytic anaemia in patients treated with primaquine, which is an 8-aminoquinoline antimalarial and the only agent currently approved for the treatment of latent (liver-stage) P. vivax malaria. The mode of action of primaquine, which is a prodrug, remains unknown.
The mechanisms of malaria protection in these varied genetic disorders have been widely studied 81 . Common findings include increased phagocytosis and elimination by the spleen of infected mutant erythrocytes, which reduces parasitaemia; reduced parasite invasion of mutant red blood cells; reduced intracellular growth rates; and reduced cytoadherence of infected mutant red blood cells. All of these effects increase protection against severe malaria, which is the main driver of human evolution in this case. Some point mutations in the gene that encodes haemoglobin alter the display of PfEMP1 on the surface of infected red blood cells, thereby diminishing cytoadherence to endothelial cells 85 , 86 . This finding highlights the crucial role of cytoadherence in promoting severe disease.
Finally, variability in the response to TNF, which is secreted from almost all tissues in response to malaria endotoxins, has also been proposed as a factor that mediates differential host responses and contributes to severe malaria when levels are high 7 .
Diagnosis, screening and prevention
The WHO criteria for the diagnosis of malaria consider two key aspects of the disease pathology: fever and the presence of parasites 87 . Parasites can be detected upon light microscopic examination of a blood smear ( Fig. 4 ) or by a rapid diagnostic test (RDT) 87 . The patient's risk of exposure (for example, whether the patient lives in an endemic region or their travel history) can assist in making the diagnosis. Furthermore, the clinical expression of Plasmodium spp. infection correlates with the species’ level of transmission in the area. The symptoms of uncomplicated malaria include sustained episodes of high fever ( Box 1 ); when high levels of parasitaemia are reached, several life-threatening complications might occur (severe malaria) ( Box 1 ).
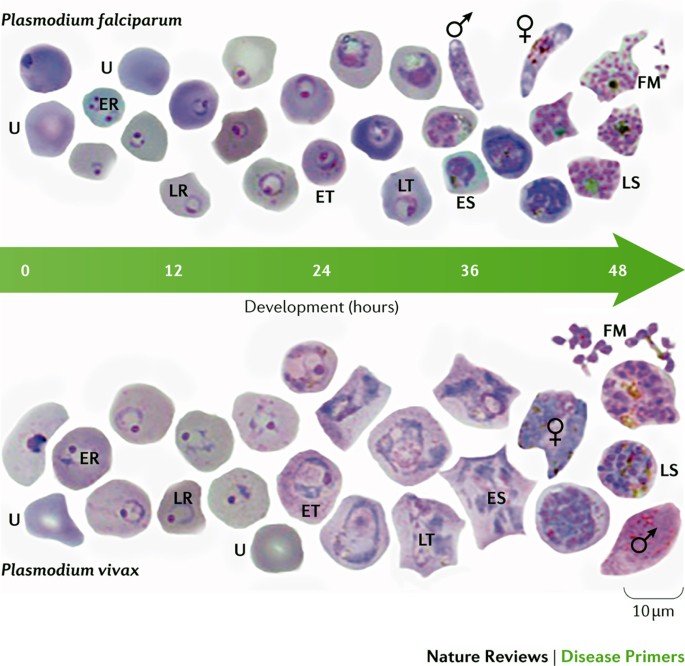
Thin blood films showing Plasmodium falciparum (upper panel) and Plasmodium vivax (lower panel) at different stages of blood-stage development. The images are from methanol-fixed thin films that were stained for 30 minutes in 5% Giemsa. The samples were taken from Thai and Korean patients with malaria: Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand (reference no. 4/2549, 6 February 2006). The sex symbols represent microgametes (male symbol) and macrogametes (female symbol). ER, early ring stage; ES, early schizont stage; ET, early trophozoite stage; FM, free merozoites; LR, late ring stage; LS, late schizont stage; LT, late trophozoite stage; U, uninfected red blood cell. The slides used were from a previously published study 268 but the images shown have not been previously published. Images courtesy of A.-R. Eruera and B. Russell, University of Otago, New Zealand.
The complications of severe malaria mostly relate to the blocking of blood vessels by infected red blood cells, with the severity and symptoms depending on what organ is affected ( Box 1 ) and to what extent, and differ by age; lung and kidney disease are unusual in children in Africa but are common in non-immune adults.
Parasitaemia . Patients with uncomplicated malaria typically have parasitaemia in the range of 1,000–50,000 parasites per microlitre of blood (however, non-immune travellers and young children who have parasite numbers <1,000 can also present with symptoms). The higher numbers tend to be associated with severe malaria, but the correlation is imprecise and there is no cut-off density. In a pooled analysis of patient data from 61 studies that were designed to measure the efficacy of ACTs (throughout 1998–2012), parasitaemia averaged ∼ 4,000 parasites per microlitre in South America, ∼ 10,000 parasites per microlitre in Asia and ∼ 20,000 parasites per microlitre in Africa 88 . The limit of detection by thick-smear microscopy is ∼ 50 parasites per microlitre 89 . WHO-validated RDTs can detect 50–1,000 parasites per microlitre with high specificity, but many lack sensitivity, especially when compared with PCR-based methods 90 . The ability to detect low levels of parasitaemia is important for predicting clinical relapses, as parasitaemia can increase 20-fold over a 48-hour cycle period. These data are based on measurements in healthy volunteers (controlled human infection models) who were infected at a defined time point with a known number or parasites, and in whom the asymptomatic parasite reproduction was monitored by quantitative PCR up to the point at which the individual received rescue treatment 91 .
In hyperendemic areas (with year-round disease transmission), often many children and adults are asymptomatic carriers of the parasite. In these individuals, the immune system maintains parasites at equilibrium levels in a ‘tug-of-war’. However, parasitaemia in asymptomatic carriers can be extremely high, with reports of levels as high as 50,000 parasites per microlitre in a study of asymptomatic pregnant women (range: 80–55,400 parasites per microlitre) 92 . In addition to the obvious risks for such people, they represent a reservoir for infecting mosquitoes, leading to continued transmission. In clinical studies, the parasitaemia of asymptomatic carriers can be monitored using PCR-based methods, which can detect as few as 22 parasites per millilitre 93 . However, the detection of low-level parasitaemia in low-resource settings requires advanced technology. Loop-mediated isothermal amplification (LAMP) 94 is one promising approach. This type of PCR is fast (10 9 -fold amplification in 1 hour) and does not require thermal cycling, which reduces the requirement for expensive hardware. Versions of this method that do not require electricity are being developed 95 . Nucleic acid-based techniques such as LAMP and PCR-based methods also have the advantage that they can be used to detect multiple pathogens simultaneously and, in theory, identify drug-resistant strains 96 . This approach enables the accurate diagnosis of which Plasmodium spp. is involved, and in the future could lead to the development of multiplexed diagnostics that enable differential diagnosis of the causative pathogens (including bacteria and viruses) in patients who present with fever 97 .
RDTs . RDTs are based on the immunological detection of parasite antigens (such as lactate dehydrogenase (LDH) or histidine-rich protein 2) in the blood, have sensitivities comparable to that of light microscopy examination and have the advantage that they do not require extensive training of the user. These tests provide rapid diagnosis at a point-of-care level in resource-limited settings and can, therefore, substantially improve malaria control. However, occasionally, false-positive results from RDTs can be problematic because they could lead to the wrong perception that antimalarial medicines are ineffective. False-negative test results have been reportedly caused by pfhrp2 gene deletions in P. falciparum strains in South America 98 – 103 . Current data indicate that LDH-targeting RDTs are less sensitive for P. vivax than for P. falciparum 104 , and limited information on the sensitivity of these tests for the rarer species, such as P. ovale or P. malariae , is available. RDTs also offer a great opportunity to track malaria epidemiology; photos taken with mobile phones of the results of the tests can be uploaded to databases (even using cloud-based data architecture 105 ) and provide an automated collection of surveillance data 106 .
Prevention in vulnerable populations
The prevention of Plasmodium spp. infection can be accomplished by different means: vector control, chemoprevention and vaccines. Mosquito (vector) control methods include the following (from the broadest to the most targeted): the widespread use of insecticides, such as DDT campaigns; the use of larvicides; the destruction of breeding grounds (that is, draining marshes and other breeding reservoirs); indoor residual spraying with insecticides (that is, the application of residual insecticide inside dwellings, on walls, curtains or other surfaces); and the use of insecticide-treated bed nets. The use of endectocides has also been proposed; these drugs, such as ivermectin, kill or reduce the lifespan of mosquitoes that feed on individuals who have taken them 107 . However, this approach is still experimental; individuals would be taking drugs that have no direct benefit to themselves (as they do not directly prevent human illness), and so the level of safety data required for the registration of endectocides for this purpose will need to be substantial. Vector control approaches differ in terms of their efficacy, costs and the extent of their effect on the environment. Targeted approaches such as insecticide-treated bed nets have had a strong effect. Chemoprevention is an effective strategy that has been used to reduce malaria incidence in campaigns of seasonal malaria chemoprevention, in intermittent preventive treatment for children and pregnant women, and for mass drug administration 108 . Such antimalarials need to have an excellent safety profile as they are given to large numbers of healthy people. Vaccines excel in eradicating disease, but effective malaria vaccines are challenging because — unlike viruses and bacteria, against which effective vaccines have been developed — protist pathogens (such as Plasmodium spp.) are large-genome microorganisms that have evolved highly effective immune evasion strategies (such as encoding dozens or hundreds of cell surface protein variants). Nevertheless, the improved biotechnological arsenal to generate antigens and improved adjuvants could help to overcome these issues.
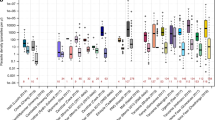
Vector control measures . The eradication of mosquitoes is no longer considered an option to eliminate malaria; however, changing the capacity of the vector reservoir has substantial effects on malaria incidence. Long-lasting insecticide-treated bed nets and indoor residual spraying have been calculated to be responsible for two-thirds of the malaria cases averted in Africa between 2000 and 2015 (Ref. 12 ). Today's favoured and more-focused vector control approach involves the use of fine-mazed, sturdy, long-lasting and wash-proof insecticide-treated bed nets 109 . The fabric of these nets is impregnated with an insecticide that maintains its efficacy after ≥20 standardized laboratory washes, and these nets have a 3-year recommended use. Insects are attracted by the person below the net but are killed as they touch the net. However, the efficacy of bed nets is threatened by several factors, including their inappropriate use (for example, for fishing purposes) and behavioural changes in the mosquitoes, which have also begun to bite during the day 110 . The main problem, however, is the increasing emergence of vector resistance to insecticides, especially pyrethroids 110 and, therefore, new insecticides with different modes of action are urgently needed. New insecticides have been identified by screening millions of compounds from the libraries of agrochemical companies, but even those at the most advanced stages of development are still 5–7 years from deployment (see the International Vector Control Consortium website ( http://www.ivcc.com ) and Ref. 111 ) ( Fig. 5 ). Few of these new insecticides are suitable for application in bed nets (because of high costs or unfavourable chemical properties), but some can be used for indoor residual spraying. New ways of deploying these molecules are also being developed, such as improved spraying technologies 112 , timed release to coincide with seasonal transmission and slow-release polymer-based wall linings 113 , 114 .
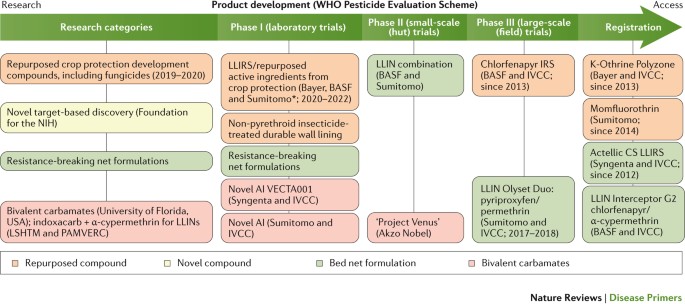
The categories of compounds that are currently under study are defined in the first column on the left; compounds belonging to these categories have advanced to phase I trials or later stages. New screening hits (developed by Syngenta, Bayer, Sumitomo and the Innovative Vector Control Consortium (IVCC)) are at early research stages and are not expected to be deployed until 2020–2022. Similarly, species-specific approaches to the biological control of mosquitoes are not expected to move forward before 2025. The main data source for this Figure was the IVCC; for the latest updates visit the IVCC website ( www.ivcc.com ). Note that not all compounds listed on the IVCC website are shown in this Figure. The dates reflect the expected deployment dates. AI, active ingredient; CS, capsule suspension; IRS, indoor residual spray; LLIN, long-lasting insecticidal mosquito net; LLIRS, long-lasting indoor residual spray; LSHTM, London School of Hygiene and Tropical Medicine (UK); PAMVERC, Pan-African Malaria Vector Research Consortium. *Clothianidin and chlorfenapyr.
Genetic approaches, fuelled by advances in the CRISPR–Cas9 gene editing technology, represent an exciting area of development for novel insect control strategies. There are currently two main approaches: population suppression, whereby mosquitoes are modified so that any progeny are sterile; and population alteration, whereby mosquitoes are modified so that the progeny are refractory to Plasmodium spp. infection 115 , 116 . Initial approaches to population suppression involved releasing sterile male insects 117 . These strategies have now been developed further, with the release of male insects carrying a dominant lethal gene that kills their progeny 118 , 119 . Gene drive systems can be used for both population suppression and population alteration. These systems use homing endonucleases, which are microbial enzymes that induce the lateral transfer of an intervening DNA sequence and can, therefore, convert a heterozygote individual into a homozygote. Homing endonucleases have been re-engineered to recognize mosquito genes 120 and can rapidly increase the frequency of desirable traits in a mosquito population 121 . Gene drive systems have now been used in feasibility studies to reduce the size of mosquito populations 122 or to make mosquitoes less able to transmit malaria-causing parasites 123 . Another approach is inspired by the finding that Aedes aegypti mosquitoes (the vector for Dengue, yellow fever and Zika viruses) infected with bacteria of the Wolbachia spp. (a parasite that naturally colonizes numerous species of insects) cannot transmit the Dengue virus to human hosts 124 . Symbiont Wolbachia spp. can be modified to make them deleterious to other parasites in the same host, and progress has been made in finding symbionts that can colonize Anopheles spp. mosquitoes 125 , 126 . Although all of the above approaches are very promising, they are still at a very early stage, and the environmental uncertainties associated with the widespread distribution of such technologies, as well as the complex regulatory requirements, provide additional hurdles that will need to be overcome.
Chemoprotection and chemoprevention . Chemoprotection describes the use of medicines (given at prophylactic doses) to temporarily protect subjects entering an area of high endemicity — historically, tourists and military personnel — and populations at risk from emergent epidemics, but is also being increasingly considered for individuals visiting areas that have recently become malaria-free. Chemoprevention, which is often used in the context of seasonal malaria, describes the use of medicines with demonstrated efficacy that are given regularly to large populations at full treatment doses (as some of the individuals treated will be asymptomatic carriers).
Currently, there are three ‘gold-standard’ alternatives for chemoprotection: daily atovaquone–proguanil, daily doxycycline and weekly mefloquine. Mefloquine is the current mainstay drug used to prevent the spread of multidrug-resistant Plasmodium spp. in the Greater Mekong subregion of Southeast Asia, despite having a ‘black box warning’ for psychiatric adverse events; however, an analysis of pooled data from 20,000 well-studied patients found that this risk was small (<12 cases per 10,000 treatments) 127 . An active search to find new medicines that could be useful in chemoprotection, in particular medicines that can be given weekly or even less frequently, is underway. One interesting possibility is the use of long-acting injectable intramuscular combination chemoprotectants, which, if effective, could easily compete with vaccination, if they provided protection with 3–4 injections per year. Such an approach (called pre-exposure prophylaxis) is being studied for HIV infection (which also poses major challenges to the development of an effective vaccine) 128 and may lead to the development of long-acting injectable drug formulations 129 produced as crystalline nanoparticles (to enhance water solubility) using the milling technique.
Chemoprevention generally refers to seasonal malaria chemoprevention campaigns, which target children <5 years of age 130 . In the Sahel region (the area just south of the Sahara Desert, where there are seasonal rains and a recurrent threat of malaria), seasonal malaria chemoprevention with a combination of sulfadoxine–pyrimethamine plus amodiaquine had a strong effect 131 – 135 , with a >80% reduction in the number of malaria cases among children and a >50% reduction in mortality 136 . Although these campaigns are operationally complex — as the treatment has to be given monthly — >20 million children have been protected between 2015 and 2016, at a cost of ∼ US$1 per treatment. A concern about seasonal malaria chemoprevention is the potential for a rebound effect of the disease. Rebound could occur if children lose immunity to malaria while receiving treatment that is later stopped because they reached the age limit, if campaigns are interrupted because of economic difficulties or social unrest (war), or if drug resistance develops. Owing to the presence of resistant strains, a different approach is needed in African areas south of the Equator 137 , and this led to trials of monthly 3-day courses of ACTs in seasonal chemoprevention 135 ; there is an increasing amount of literature on the impressive efficacy of dihydroartemisinin (DHA)–piperaquine to prevent malaria in high-risk groups 138 . To reduce the potential for the emergence of drug resistance, the WHO good practice standards state that, when possible, drugs used for chemoprevention should differ from the front-line treatment that is used in the same country or region 108 , which emphasizes the need for the development of multiple, new and diverse treatments to provide a wider range of options.
Finally, intermittent preventive treatment is also recommended to protect pregnant women in all malaria-endemic areas 108 ( Box 3 ).
Vaccines . Malaria, along with tuberculosis and HIV infection, is a disease in which all components of the immune response (both cellular, in particular, during the liver stage, and humoral, during the blood stage) are involved yet provide only partial protection, which means that developing an effective vaccine will be a challenge. The fact that adults living in high-transmission malarious areas acquire partial protective immunity indicates that vaccination is a possibility. As a consequence, parasite proteins targeted by natural immunity, such as the circumsporozoite protein (the most prominent surface antigen expressed by sporozoites), proteins expressed by merozoites and parasite antigens exposed on the surface of infected red blood cells 139 have been studied for their potential to be used in vaccine programmes 140 . However, experimental malaria vaccines tend to target specific parasite species and surface proteins, an approach that both restricts their use and provides scope for the emergence of resistance. Sustained exposure to malaria is needed to maintain natural protective immunity, which is otherwise lost within 3–5 years 141 , perhaps as a result of the clearance of circulating antibodies and the failure of memory B cells to develop into long-lived plasma B cells. Controlled human infection models 142 – 144 have started to provide a more precise understanding of the early cytokine and T cell responses in naive subjects, emphasizing the role of regulatory T cells in dampening the response against the parasite, which results in the exhaustion of T cells 145 . Vaccine development is currently focusing on using multiple antigens from different stages of the parasite life cycle. Future work will also need to focus on the nature of the immune response in humans and specifically the factors that lead to diminished T cell responses. New generations of adjuvants are needed, possibly compounds that produce the desired specific response rather than inducing general immune stimulation. This is a challenging area of research, as adjuvants often have a completely different efficacy in humans compared with in preclinical animal models.
Currently, there is no vaccine deployed against malaria. The ideal vaccine should protect against both P. falciparum and P. vivax , with a protective, lasting efficacy of at least 75%. The most advanced candidate is RTS,S (trade name: Mosquirix; developed by GlaxoSmithKline and the Program for Appropriate Technology in Health Malaria Vaccine Initiative), which contains a recombinant protein with parts of the P. falciparum circumsporozoite protein combined with the hepatitis B virus surface antigen and a proprietary adjuvant. RTS,S reduced the number of malaria cases by half in 4,358 children 5–17 months of age during the first year following vaccination 146 , preventing 1,774 cases for every 1,000 children also owing to herd immunity, and had an efficacy of 40% over the entire 48 months of follow-up in children who received four vaccine doses over a 4-year period 147 . The efficacy of RTS,S during the entire follow-up period dropped to 26% when children only received three vaccine doses. The efficacy during the first year in 6–12-week-old children was limited to 33%. Thus, the RTS,S vaccine failed to provide long-term protection. Further studies, as requested by the WHO, will be done in pilot implementations of 720,000 children in Ghana, Kenya and Malawi (240,000 in each country, half of whom will receive the vaccine) before a final policy recommendation is made. However, a vaccine with only partial and short-term efficacy could still be used in the fight against malaria. RTS,S could be combined with chemoprevention to interrupt malaria transmission in low-endemic areas 148 . Thus, vaccines that are unable to prevent Plasmodium spp. infection could be used to prevent transmission (for example, by targeting gametocytes) or used as an additional protective measure in pregnant women.
A large pipeline of vaccine candidates is under evaluation ( Fig. 6 ). These include irradiated sporozoites — an approach that maximizes the variety of antigens exposed 149 — and subunit vaccines, which could be developed into multicomponent, multistage and multi-antigen formulations 150 . Although vaccines are typically designed for children, as the malaria map shrinks, both paediatric and adult populations living in newly malaria-free zones will need protection because they would probably lose any naturally acquired immunity and would, therefore, be more susceptible. Indeed, in recent years, there has been a focus on developing transmission-blocking vaccines to drive malaria elimination. This approach has been labelled altruistic, as vaccination would have no direct benefit for the person receiving it, but it would benefit the community; a regulatory pathway for such a novel approach has been proposed 151 , 152 . The most clinically advanced vaccine candidate that is based on this approach is a conjugate vaccine that targets the female gametocyte marker Pfs25 (Ref. 153 ), and other antigens are being tested preclinically. Monoclonal antibodies are another potential tool to provide protection. Improvements in manufacturing and high-expressing cell lines are helping to overcome the major barrier to the use of monoclonal antibodies (high costs) 154 , and improvements in potency and pharmacokinetics are reducing the volume and frequency of administration 155 . Monoclonal antibodies could be particularly useful to safely provide the relatively short-term protection needed in pregnancy. The molecular basis of the interaction between parasites and the placenta is quite well understood; two phase I trials of vaccines that are based on the VAR2CSA antigen are under way 156 , 157 .
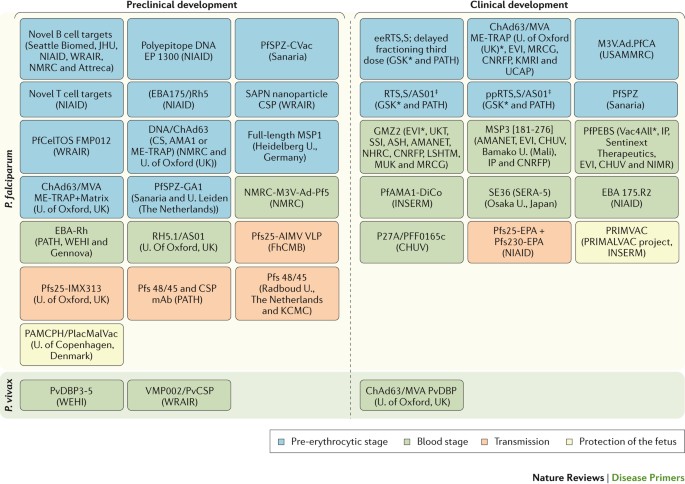
The main data source for this Figure was Ref. 269 . Not all vaccines under development are shown in the Figure. AIMV VLP, Alfalfa mosaic virus virus-like particle; AMA1, apical membrane antigen 1; AMANET, African Malaria Network Trust; ASH, Albert Schweitzer Hospital (Gabon); ChAd63, chimpanzee adenovirus 63; CHUV, Centre Hospitalier Universitaire Vaudois (Switzerland); CNRFP, Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso); CS, circumsporozoite protein; CSP, circumsporozoite protein; EBA, erythrocyte-binding antigen; ee, elimination eradication; EP, electroporation; EPA, Pseudomonas aeruginosa exoprotein A; EVI, European Vaccine Initiative; CVac, chemoprophylaxis vaccine; FhCMB, Fraunhofer Center for Molecular Biotechnology (USA); GSK, GlaxoSmithKline; IP, Institut Pasteur (France); INSERM, Institut National de la Santé et de la Recherche Médicale (France); JHU, Johns Hopkins University (USA); KCMC, Kilimanjaro Christian Medical College (Tanzania); KMRI, Kenyan Medical Research Institute; LSHTM, London School of Hygiene and Tropical Medicine (UK); M3V.Ad.PfCA, multi-antigen, multistage, adenovirus-vectored vaccine expressing Plasmodium falciparum CSP and AMA1 antigens; mAb, monoclonal antibody; ME-TRAP multiple epitope thrombospondin-related adhesion protein; MRCG, Medical Research Council (The Gambia); MSP, merozoite surface protein; MVA, modified vaccinia virus Ankara; MUK, Makerere University Kampala (Uganda); NHRC, Navrongo Health Research Centre (Ghana); NIAID, National Institute of Allergy and Infectious Diseases (USA); NIMR, National Institute for Medical Research (UK); NMRC, Naval Medical Research Center (USA); PAMCPH, pregnancy-associated malaria Copenhagen; PATH, Program for Appropriate Technology in Health; PfAMA1-DiCo, diversity-covering Plasmodium falciparum AMA1; PfCelTOS, Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites; PfPEBS, Plasmodium falciparum pre-erythrocytic and blood stage; PfSPZ, Plasmodium falciparum sporozoite; PfSPZ-GA1, genetically attenuated PfSPZ; pp, paediatric prevention; PRIMALVAC, PRIMVAC project (INSERM); PRIMVAC, recombinant var2CSA protein as vaccine candidate for placental malaria; Pfs25, Plasmodium falciparum 25 kDa ookinete surface antigen; PvCSP, Plasmodium vivax circumsporozoite protein; PvDBP, Plasmodium vivax Duffy-binding protein; Rh or RH, reticulocyte-binding protein homologue; SAPN, self-assembling protein nanoparticle; SSI, Statens Serum Institut (Denmark); U., University; UCAP, Université Cheikh Anta Diop (Senegal); UKT, Institute of Tropical Medicine, University of Tübingen (Germany); USAMMRC, US Army Medical Research and Materiel Command; WEHI, Walter and Eliza Hall Institute of Medical Research (Australia); WRAIR, Walter Reed Army Institute of Research (USA). *Sponsors of late-stage clinical trials. ‡ Pending review or approval by WHO prequalification, or by regulatory bodies who are members or observers of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
No single drug is ideal against all Plasmodium spp. or all of the manifestations of the disease that occur in different patient populations. Thus, treatment must be tailored to each situation appropriately 108 , 158 . First, the treatment of uncomplicated malaria and that of severe malaria are distinct. In uncomplicated malaria, the treatment of choice is an oral medicine with high efficacy and a low adverse-effect profile. However, the preferred initial therapy in severe malaria requires rapid onset and includes the parenteral administration of an artemisinin derivative, which can rapidly clear the parasites from the blood, and it is also suitable for those patients who have changes in mental status (such as coma) that make swallowing oral medications impossible. For the treatment of malaria during pregnancy, the options are limited to the drugs that are known to be safe for both the expectant mother and the fetus, and different regimens are needed ( Box 2 ). Different drugs are used for different Plasmodium spp., and the choice is usually driven more by drug resistance frequencies (which are lower in P. vivax , P. ovale , P. malariae and P. knowlesi than in P. falciparum ) than by species differences as such. Thus, chloroquine, with its low cost and excellent safety, is used in most cases of non- P. falciparum malaria, where it remains effective, whereas P. falciparum malaria requires newer medicines that overcome resistance issues. The persistence of P. vivax and P. ovale hypnozoites, even after clearance of the stages that cause symptoms, necessitates additional treatments. Only primaquine targets hypnozoites.
P. falciparum malaria
The mainstay treatments for uncomplicated P. falciparum malaria are ACTs: fixed-dose combinations of two drugs, an artemisinin derivative and a quinine derivative 108 ( Box 4 ; Table 1 ).
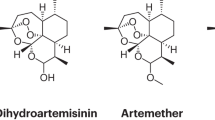
Owing to its high lipophilicity, artemisinin itself is not the molecule of choice in any stringent regulatory authority-approved combination. Instead, semisynthetic derivatives are used: namely, DHA (the reduced hemiacetal of the major active metabolite of many artemisinin derivatives), artesunate (a succinate prodrug of DHA that is highly water-soluble) or artemether (a methylether prodrug of DHA).
Quinine has been used in medicine for centuries 159 , but it was only in the mid-20th century that a synthetic form was made and the emerging pharmaceutical and government research sectors delivered the next-generation medicines that built on it. The combination partners of choice are 4-aminoquinolines (for example, amodiaquine, piperaquine and pyronaridine) and amino-alcohols (such as mefloquine or lumefantrine); these molecules are believed to interfere with haemozoin formation. There are now five ACTs that have been approved or are close to approval by the FDA, the European Medicines Agency (EMA) or WHO prequalification ( Figs 7 , 8 ; Table 1 ). In pivotal clinical studies, these combinations have proven extremely effective (achieving an adequate clinical and parasitological response (that is, the absence of parasitaemia at day 28 in >94% of patients; for example, see Ref. 160 ), are well-tolerated (as they have been given to >300 million paediatric patients), are affordable (typically under US$1 per dose) and, thanks to ingenious formulations and packaging, are stable in tropical climate conditions.
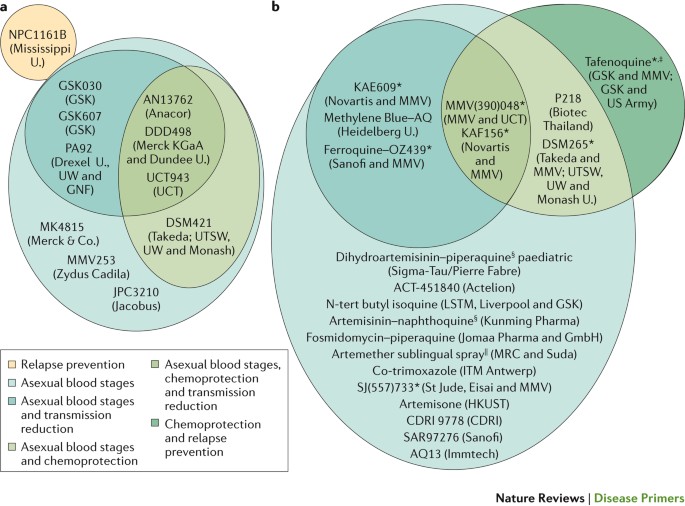
a | Preclinical candidates. b | Compounds or compound combinations that are in clinical development. The multitude of molecules that target only the asexual blood stages reflects the fact that many of these compounds are at an early stage of development, and further assessment of their Target Candidate Profile is still ongoing. KAF156 and KAE609 were discovered in a multiparty collaboration between the Novartis Institute for Tropical Disease (Singapore), the Genomics Institute of the Novartis Research Foundation (GNF; USA), the Swiss Tropical and Public Health Institute, the Biomedical Primate Research Centre (The Netherlands), the Wellcome Trust (UK) and Medicines for Malaria Venture (MMV). DSM265 was discovered through a collaboration involving the University of Texas Southwestern (UTSW; USA), the University of Washington (UW; USA), Monash University (Australia), GlaxoSmithKline (GSK) and MMV. MMV(390)048 was discovered through a collaboration involving the University of Cape Town (UCT; South Africa), the Swiss Tropical and Public Health Institute, Monash University, Syngene and MMV. SJ(557)733 was discovered in a collaboration involving St Jude Children's Research Hospital (USA), Rutgers University (USA), Monash University and MMV. Note that not all compounds are shown in this Figure, and updates can be found on the MMV website ( www.mmv.org) . CDRI, Central Drug Research Institute (India); ITM, Institute of Tropical Medicine; MRC, Medical Research Council; HKUST, Hong Kong University of Science and Technology; U., University. *Part of a combination that aims to be a new single-exposure radical cure (Target Product Profile 1). ‡ Product that targets the prevention of relapse in Plasmodium vivax malaria. § 3-day cure, artemisinin-based combination therapy. || Severe malaria and pre-referral treatment.
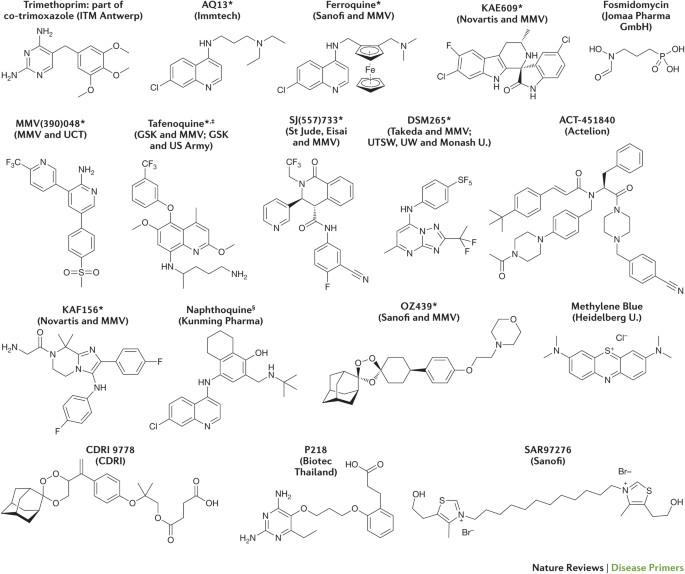
See the Medicines for Malaria Venture (MMV) website ( www.mmv.org ) for updates. CDRI, Central Drug Research Institute (India); GSK, GlaxoSmithKline; ITM, Institute of Tropical Medicine; U., University; UCT, University of Cape Town (South Africa); UTSW, University of Texas Southwestern (USA); UW, University of Washington (USA). *Part of a combination that aims to be a new single-exposure radical cure (Target Product Profile 1). ‡ Product that targets the prevention of relapse in Plasmodium vivax malaria. § 3-day cure, artemisinin-based combination therapy.
Following the results of comprehensive studies in Africa and Asia, the injectable treatment of choice for severe P. falciparum malaria is artesunate 161 – 163 . In the United States, artesunate for intravenous use is available as an Investigational New Drug (IND) through the Centers for Disease Control and Prevention (CDC) malaria hotline and shows efficacies of >90% even in patients who are already unconscious 161 . Sometimes, however, in low-income countries, it is necessary to administer intravenous quinine or quinine while awaiting an artesunate supply. Suppositories of artesunate are in late-stage product development 164 and are already available in Africa as a pre-referral treatment to keep patients alive while they reach a health clinic.
Box 4: Artemisinin
Artemisinin (also known as qinghaosu in China; see the structure) is extracted from the leaves of the Artemisia annua plant.
Youyou Tu was recognized by the 2015 Nobel Prize committee for her contribution to medicine for the discovery of artemisinin, which she achieved by retrieving and following instructions from ancient Chinese texts 247 . Owing to the ability of artemisinin to rapidly reduce parasitaemia and fever, the effect that artemisinin and its derivatives has had on the management of malaria cannot be overstated; since their introduction in the 1970s and their subsequent wider implementation — which was possible particularly owing to the work of Nicholas White and colleagues 248 – 251 — millions of lives have been saved. These drugs seem to be activated by haem-derived iron, and their toxicity is probably mediated through the formation of reactive oxidative radicals 42 . Data indicate that they interfere with phosphatidylinositol-3-phosphate metabolism (which is thought to be involved in the trafficking of haemoglobin to the digestive vacuole 252 ) and provide possible mechanistic insights into the nature of clinically observed artemisinin resistance 253 .
P. vivax malaria
Chloroquine or ACTs are WHO-recommended for uncomplicated P. vivax malaria 108 (although chloroquine is no longer used in several countries, such as Indonesia). As chloroquine-resistant P. vivax is becoming increasingly widespread, particularly in Asia, the use of ACTs is increasing; although only artesunate–pyronaridine is approved for the treatment of blood-stage P. vivax malaria, the other ACTs are also effective and are used off-label. Relapses of P. vivax malaria present a problem in malaria control. Relapse frequencies differ among P. vivax strains; they are high (typically within 3 weeks) in all-year transmission areas, such as Papua New Guinea, but relapse occurs on average after 7 months in areas with a dry or winter season. Some P. vivax strains, such as the Moscow and North Korea strains, are not, in most cases, symptomatic at the time of first infection but become symptomatic only following reactivation of the hypnozoites 165 . Primaquine needs to be administered in addition to the primary treatment to prevent relapse and transmission, which can occur even years after the primary infection. Primaquine treatment, however, requires 14 days of treatment, has gastrointestinal adverse effects in some patients, and is contraindicated in pregnant women and in patients who are deficient in or express low levels of G6PD (as it can cause haemolysis). Tafenoquine 166 , a next-generation 8-aminoquinoline, is currently completing phase III clinical studies. As with patients receiving primaquine, patients receiving tafenoquine will still require an assessment of their G6PD enzyme activity to ensure safe use of the drug and to determine the optimal dose. In phase II studies, tafenoquine was shown to have an efficacy similar to that of primaquine but with a single dose only compared with the 7–14-day treatment with primaquine; higher patient compliance is expected to be a major benefit of a single-dose regimen. The ultimate elimination of P. vivax malaria will be dependent on the availability of safe and effective anti-relapse agents, and is, therefore, a major focus of the drug discovery community.
Drug resistance
The two drugs in ACTs have very different pharmacokinetic profiles in patients. The artemisinin components have a plasma half-life of only a few hours yet can reduce parasitaemia by three-to-four orders of magnitude. By contrast, the 4-aminoquinolines and amino-alcohols have long terminal half-lives (>4 days), providing cure (defined as an adequate clinical and parasitological response) and varying levels of post-treatment prophylaxis. The prolonged half-life of the non-artemisinin component of ACTs has raised concerns in the research community owing to the risk of drug resistance development. However, the effectiveness of the ACTs in rapidly reducing parasitaemia suggests that any emerging resistance has arisen largely as a result of poor clinical practice, including the use of artemisinin derivatives as monotherapy, a lack of patient compliance and substandard medicine quality (including counterfeits); these are all situations in which large numbers of parasites are exposed to a single active molecule 167 . However, resistance to piperaquine 168 and partial resistance to artemisinin 169 (which manifests as a reduced rate of parasite clearance rate rather than a shift in the half-maximal inhibitory concentration (IC 50 )) has been confirmed in the Greater Mekong subregion, as well as resistance to mefloquine and amodiaquine in various parts of the world 170 . Africa has so far been spared, but reports of treatment failure for either artemisinin 171 or ACT 172 in African isolates of P. falciparum have raised concerns. Thus, artemisinin-resistant Plasmodium spp. and insecticide-resistant mosquitoes are major threats to the progress that has been made in reducing the number of malaria-related deaths through current control programmes. It is important to emphasize that progress against malaria has historically been volatile; in many areas, the disease has re-emerged as the efficacy of old drugs has been lost in strains that developed resistance.
Many advances have been made in identifying genetic markers in Plasmodium spp. that correlate with resistance to clinically used drugs ( Table 2 ). These markers enable the research and medical communities to proactively survey parasite populations to make informed treatment choices. Cross-resistance profiles reveal reciprocity between 4-aminoquinolines and amino-alcohols (that is, parasites resistant to one class are also less sensitive to the other). In addition, a drug can exert two opposite selective pressures: one towards the selection of resistant mutants and the other towards the selection of strains that have increased sensitivity to a different drug, a phenomenon known as ‘inverse selective pressure’ (Refs 173 , 174 ). These findings support the introduction of treatment rotation or triple combination therapies as potential future options. Finally, the drug discovery and development pipeline is delivering not only new compounds that have novel modes of action and overcome known resistant strains but also chemicals that have the potential to be effective in a single dose, which could overcome compliance issues. Nevertheless, policymakers need to be on high alert to prevent or rapidly eliminate outbreaks of resistant strains, and to prioritize the development of new treatments.
The drug discovery and development pipeline
The most comprehensive antimalarial discovery portfolio has been developed by the not-for-profit product development partnership Medicines for Malaria Venture (MMV) in collaboration with its partners in both academia and the pharmaceutical industry, with support from donors (mainly government agencies and philanthropic foundations) ( Fig. 7 ). Promising compound series have been identified from three approaches: hypothesis-driven design to develop alternatives to marketed compounds (for example, synthetic peroxides such as ozonides); target-based screening and rational design (for example, screening of inhibitors of P. falciparum dihydroorotate dehydrogenase (PfDHODH)); and phenotypic screening 175 . Phenotypic screening has been the most successful approach to date, in terms of delivering preclinical candidates and identifying — through the sequencing of resistant mutants — novel molecular targets. However, with advances in the understanding of parasite biology and in molecular biology technology, target-based approaches will probably have a substantial role in coming years.
Two combinations — OZ439 (also known as artefenomel) with ferroquine (Sanofi and MMV) and KAF156 with lumefantrine (Novartis and MMV) — are about to begin phase IIb development to test the efficacy of single-dose cure and, in the case of KAF156–lumefantrine, also 2-day or 3-day cures. OZ439 is a fully synthetic peroxide for which sustained plasma exposure is achieved by a single oral dose in humans 176 , 177 ; the hope is that it could replace the three independent doses required for artemisinin derivatives. Ferroquine is a next-generation 4-aminoquinoline without cross-resistance to chloroquine, amodiaquine or piperaquine 178 , 179 . KAF156 is a novel imidazolopiperazine that has an unknown mechanism of action 180 – 182 , but its resistance marker — P. falciparum cyclic amine resistance locus ( pfcarl ) — seems to encode a transporter on the endoplasmic reticulum membrane of the parasite. Interestingly, whereas OZ439 and ferroquine principally affect the asexual blood stages, KAF156 also targets both the asexual liver stage and the sexual gametocyte stage and, therefore, could have an effect on transmission.
Two other compounds, KAE609 (also known as cipargamin 183 , 184 ) and DSM265 (Refs 185 – 188 ), are poised to begin phase IIb and are awaiting decisions on combination partners. KAE609 is a highly potent spiroindolone that provides parasite clearance in patients even more rapidly than peroxides; its assumed mode of action is the inhibition of PfATP4 ( Fig. 3 ), which is encoded by its resistance marker and is a transporter on the parasite plasma membrane that regulates Na + and H + homeostasis. Inhibition of this channel, which was identified through the sequencing of resistant mutants, increases Na + concentrations and pH, resulting in parasite swelling, rigidity and fragility, thereby contributing to host parasite clearance in the spleen in addition to intrinsic parasite killing. In addition, effects on cholesterol levels in the parasite plasma membrane have been noted that are also likely to contribute to parasite killing by leading to an increased rigidity that results in more rapid clearance in vivo 189 . DSM265 is a novel triazolopyrimidine that has both blood-stage and liver-stage activity, and that selectively inhibits PfDHODH ( Fig. 3 ). It was optimized for drug-like qualities from a compound that was identified from a high-throughput screen of a small-molecule library 186 , 190 . DSM265 maintains a serum concentration that is above its minimum parasiticidal concentration in humans for 8 days, and has shown efficacy in both treatment and chemoprotection models in human volunteers in phase Ib trials 185 , 188 .
Within phase I, new compounds are first assessed for safety and pharmacokinetics, and then for efficacy against the asexual blood or liver stages of Plasmodium spp. using a controlled human malaria infection model in healthy volunteers 144 . This model provides a rapid and cost-effective early proof of principle and, by modelling the concentration–response correlation, increases the accuracy of dose predictions for further clinical studies. The 2-aminopyridine MMV(390)048 (also known as MMV048 (Refs 191 , 192 )), SJ(557)733 (also known as (+)-SJ733 (Refs 57 , 193 )) and P218 (Ref. 194 ) are currently progressing through phase I. MMV(390)048 inhibits PfPI(4)K ( Fig. 3 ), and this inhibition affects the asexual liver and blood stages, as well as the sexual gametocyte stage. MMV(390)048 has good exposure in animal models 192 , suggesting that it could potentially be used in a single dose in combination with another drug. SJ(557)733, which is a dihydroisoquinoline, inhibits PfATP4 and is an alternative partner that has a completely different structure from that of KAE609, and it has excellent preclinical safety and development potential. P218 is currently being evaluated for testing in controlled human malaria infection cohorts.
A further eight compounds are undergoing active preclinical development 195 . Of these compounds, four are alternatives to the leading compounds that target established mechanisms: the aminopyrazole PA92 (also known as PA-21A092 (Ref. 196 )) and the thiotriazole GSK030 (also known as GSK3212030A) both target PfATP4; DSM421 (Ref. 197 ) is a triazolopyrimidine alternative to DSM265; and UCT943 (also known as MMV642943) 198 is an alternative to MMV(390)048. Three compounds show novel mechanisms of action or resistance markers: M5717 (also known as DDD498 or DDD107498 (Ref. 199 )) inhibits P. falciparum elongation factor 2 (and, therefore, protein synthesis) and has outstanding efficacy against all parasite life-cycle stages; MMV253 (also known as AZ13721412) 200 is a fast-acting triaminopyrimidine with a V-type ATPase as resistance marker; and AN13762 (also known as AN762) is a novel oxaborole 201 with a novel resistance marker. All of these compounds have been developed through collaborations with MMV.
The eighth compound in active preclinical development, led by Jacobus Pharmaceuticals, is JPC3210 (Ref. 202 ), which is a novel aminocresol that improves upon the historical candidate (WR194965) that was developed by the Walter Reed Army Institute of Research and tested in patients at the time of the development of mefloquine in the 1970s. JPC3210 has an unknown mechanism of action and has potent, long-lasting efficacy in preclinical models, suggesting its potential to be used in a single dose for both treatment and prophylaxis 202 .
Quality of life
Malaria is one among the diseases of poverty. The WHO website states the following: “There is general agreement that poverty not only increases the risk of ill health and vulnerability of people, it also has serious implications for the delivery of effective health-care such as reduced demand for services, lack of continuity or compliance in medical treatment, and increased transmission of infectious diseases” (Ref. 203 ). The socioeconomic burden of malaria is enormous, and although the disease predominantly affects children, it is a serious obstacle to a country's development and economy 204 . Malaria is responsible for annual expenses of billions of euros in some African countries 205 . In many endemic areas, each individual suffers multiple episodes of malaria per year, with each episode causing a loss of school time for children and work time for their parents and guardians. Despite the declining trends in malaria morbidity and mortality, the figures are still disconcertingly high for a disease that is entirely preventable and treatable 16 .
Malaria also has long-term detrimental effects on the non-health-related quality of life of the affected population; it intensifies poverty by limiting education opportunities, as it leads to absenteeism in schools and reduced productivity at work 16 . The effects of acute illness normally drive families to seek urgent attention, which may consist of self-medication, if the disease is familiar to the household. Yet, even an episode of uncomplicated malaria can be potentially fatal, owing to a delay in promptly accessing efficacious antimalarial drugs. As malaria is so familiar to many households, patients — especially children — may be presented late for early diagnosis and treatment in health facilities. Late presentation prolongs morbidity, increases the risk of severe malaria, and deprives the families of income through direct expenses and reduced productivity. Frequent disease episodes experienced in the endemic areas as well as their possible complications can negatively affect child growth and nutrition, shortening the lives of children and family members. The neurological consequences can affect a child's ability to learn and become a self-reliant adult 206 – 208 , as they often occur during an important brain growth phase, when brain areas involved in higher learning (such as planning, decision-making, self-awareness and social sensitivity) mature. Cognitive deficits occurring during the early education years affect the entire family, as they impair the ability of the child to contribute to the well-being of the family as they grow and put additional strain on the parents, who may sometimes have to care for a substantially disabled child and, later, a disabled adult 209 .
The agenda set by the WHO aims for malaria incidence and mortality to decrease by 90% over the next 15 years, with increasing numbers of countries that eliminate the disease 210 . Even if we achieve the ambitious goals set by the WHO, there will still be a child dying of malaria every 10 minutes in 2030. The ACTs are extraordinarily effective, and much of the disease burden could be reduced by the complete deployment and availability of these medicines. There are now two approved ACTs that are specifically designed (taste-masked and sweetened) for paediatric use.
However, the emergence of drug-resistant Plasmodium spp. and insecticide-resistant mosquitoes is a major concern. The first clinical reports of artemisinin resistance came from the Thai–Cambodian border region in the mid-2000s 211 . So far, resistant strains have not spread to Africa, and the severity of the malaria caused by artemisinin-resistant parasites is not different from that of disease caused by wild-type strains. However, if artemisinin derivatives became ineffective, no alternative first-line treatments would be available, as new therapies are still only in phase II clinical trials, and their safety and efficacy will need to be effectively assessed in the field before they can be deployed for widespread clinical use.
Diagnostics
Future diagnostics should address two main issues. First, new diagnostic tests would ideally be non-invasive and not require a blood sample. Many approaches have been piloted, including parasite antigen detection in saliva 212 or urine 213 , the detection of specific volatile chemicals in breath 214 , and direct non-invasive measurements of iron-rich haemozoin in skin blood vessels 215 . Second, diagnostic tests should be able to detect drug-resistant strains directly in the point-of-care setting, rather than in sentinel sites, to provide better treatment and generate more-detailed epidemiological maps 216 . A next-generation amplicon-sequencing method suitable for use in endemic countries would enable the high-throughput detection of genetic mutations in six P. falciparum genes that are associated with resistance to antimalarial drugs, including ACTs, chloroquine and sulfadoxine–pyrimethamine 217 .
Malaria challenges
In addition to the length of the process of discovering and developing new drugs, insecticides and vaccines, in malaria there is the hurdle of the delivery of these new compounds, which first need to obtain approval from all local regulatory authorities. There is a trend for harmonization of the approval requirements among different authorities, with an initiative involving several regional African organizations, for example, to review data on behalf of many countries, similarly to the EMA reviewing files on behalf of all of the European Union countries. These events are paving the way to shorten the time from the end of clinical studies to the day of large-scale deployment, when affected populations will start to reap the benefits.
The move towards elimination and eradication
High-content cellular assays have become available to test inhibitors of transmission and compounds that target hypnozoites 218 , 219 . Discovery efforts for treatment and chemoprotection combinations conform to the malaria Target Product Profiles — a planning tool for therapeutic candidates that is based on FDA guidelines — to ensure that what is delivered has clinical relevance. The MMV has defined 220 and updated 221 Target Candidate Profiles (TCPs), which define the attributes that are required for the ideal medicines and have proven invaluable in guiding single-molecule optimization and decision-making.
The current focus is moving beyond TCP1 (which includes molecules that clear asexual blood-stage parasitaemia); the goal is to deliver compounds that do not simply treat patients and control symptoms but that also have biological activity that disrupts the life cycle of the parasite and hence break the transmission cycle, a step that is necessary in the move towards elimination. Particular areas of interest are anti-relapse agents for P. vivax malaria (TCP3; compounds that target hypnozoites), compounds that kill hepatic schizonts (TCP4) and protect against the onset of symptoms, and gametocytocidal compounds to block transmission (TCP5). Future projects include work on long-lasting endectocides (TCP6), such as ivermectin 107 . The MMV Discovery Portfolio also includes alternative compounds to the clinical frontrunners, molecules with new mechanisms of action (which target, for example, N -myristoyltransferase 222 , coenzyme A biosynthesis 223 , phenylalanyl tRNA synthetase 224 , prolyl 225 tRNA synthetase, plasmepsin V 226 and the Q i site of cytochrome bc 1 (Ref. 227 )) and compounds that seem to be resistance-proof (at least in vitro ).
In conclusion, while much progress has been made towards reducing the burden of malaria, much work remains to be done if these gains are to bring lasting relief to those living under the threat of infection. Without a continued focus on developing new antimalarials and new approaches for diagnosis and vector control, malaria will continue to exert an unacceptable toll on people living in disease endemic areas.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
How to cite this article
Phillips, M. A. et al . Malaria. Nat. Rev. Dis. Primers 3 , 17050 (2017).
Miller, L. H., Ackerman, H. C., Su, X. Z. & Wellems, T. E. Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19 , 156–167 (2013).
CAS PubMed PubMed Central Google Scholar
White, N. J. et al . Malaria. Lancet 383 , 723–735 (2014).
PubMed Google Scholar
Cowman, A. F., Healer, J., Marapana, D. & Marsh, K. Malaria: biology and disease. Cell 167 , 610–624 (2016). References 1–3 comprehensively review malaria biology and the disease.
CAS PubMed Google Scholar
Baker, D. A. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 172 , 57–65 (2010).
Waters, A. P. Epigenetic roulette in blood stream Plasmodium : gambling on sex. PLoS Pathog. 12 , e1005353 (2016).
PubMed PubMed Central Google Scholar
White, N. J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 10 , 297 (2011).
Wassmer, S. C. et al . Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am. J. Trop. Med. Hyg. 93 , 42–56 (2015). Comprehensively reviews the causes of severe malaria and ongoing research efforts to understand malaria pathophysiology.
Wassmer, S. C. & Grau, G. E. Severe malaria: what's new on the pathogenesis front? Int. J. Parasitol. 47 , 145–152 (2017).
World Health Organization. Severe malaria. Trop. Med. Int. Health 19 (Suppl. 1), 7–131 (2014).
Google Scholar
Dondorp, A. M. & Day, N. P. The treatment of severe malaria. Trans. R. Soc. Trop. Med. Hyg. 101 , 633–634 (2007).
Bernabeu, M. & Smith, J. D. EPCR and malaria severity: the center of a perfect storm. Trends Parasitol. 33 , 295–308 (2017). Reviews the molecular basis of parasite sequestration in the tissues, which leads to the obstruction of the microvasculature and severe disease, and discusses the key role of EPCR in these processes.
Bhatt, S. et al . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 , 207–211 (2015). Attempts to identify the relative contribution of different antimalaria measures in reducing the number of malaria cases over the 2000–2015 period.
Mitchell, S. N. et al . Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347 , 985–988 (2015).
Sinka, M. E. et al . The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit. Vectors 3 , 117 (2010).
World Health Organization. Eliminating malaria: learning from the past, looking ahead. WHO http://www.path.org/publications/files/MCP_rbm_pi_rpt_8.pdf (2011).
World Health Organization. World malaria report 2015. WHO http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (2015).
Florens, L. et al . A proteomic view of the Plasmodium falciparum life cycle. Nature 419 , 520–526 (2002).
Gardner, M. J. et al . Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419 , 498–511 (2002). Reports for the first time the P. falciparum genome, which has formed the basis for research into the molecular basis of pathogenesis and parasite biology; a modern-day understanding of the disease would not be possible without this groundbreaking work.
Winzeler, E. A. Advances in parasite genomics: from sequences to regulatory networks. PLoS Pathog. 5 , e1000649 (2009).
Gething, P. W. et al . Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N. Engl. J. Med. 375 , 2435–2445 (2016).
Maitland, K. Severe malaria in African children — the need for continuing investment. N. Engl. J. Med. 375 , 2416–2417 (2016).
Miller, L. H., Mason, S. J., Clyde, D. F. & McGinniss, M. H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy . N. Engl. J. Med. 295 , 302–304 (1976). Describes the discovery that led to a molecular understanding of why most Africans are resistant to infection by P. vivax , thereby explaining the limited penetration of P. vivax in Africa.
Mercereau-Puijalon, O. & Menard, D. Plasmodium vivax and the Duffy antigen: a paradigm revisited. Transfus. Clin. Biol. 17 , 176–183 (2010).
Howes, R. E. et al . Plasmodium vivax transmission in Africa. PLoS Negl. Trop. Dis. 9 , e0004222 (2015).
Sutherland, C. J. et al . Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 201 , 1544–1550 (2010).
Cox-Singh, J. et al . Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46 , 165–171 (2008).
Singh, B. et al . A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363 , 1017–1024 (2004). Describes the discovery that P. knowlesi , which was previously thought to primarily infect macaques, accounted for over half of the cases of malaria in their study in the Kapit district (Malaysia). Demonstrates for the first time that P. knowlesi should be considered as an emerging infectious disease in humans. Whether transmission via mosquitoes was occurring from monkey to man or from man to man remained an open question.
Brock, P. M. et al . Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology 143 , 389–400 (2016).
Imwong, M., Nakeesathit, S., Day, N. P. & White, N. J. A review of mixed malaria species infections in anopheline mosquitoes. Malar. J. 10 , 253 (2011).
Ginouves, M. et al . Frequency and distribution of mixed Plasmodium falciparum – vivax infections in French Guiana between 2000 and 2008. Malar. J. 14 , 446 (2015).
Srisutham, S. et al . Four human Plasmodium species quantification using droplet digital PCR. PLoS ONE 12 , e0175771 (2017).
Armed Forces Health Surveillance Branch. Update: malaria, U. S. Armed Forces, 2016. MSMR 24 , 2–7 (2017).
IISS: International Institute for Strategic Studies. Armed Conflict Survey 2016 (IISS, 2016).
Mueller, I. et al . Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv. Parasitol. 81 , 77–131 (2013).
Ataide, R., Mayor, A. & Rogerson, S. J. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol. 30 , 85–94 (2014).
Desai, M. et al . Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7 , 93–104 (2007).
McGready, R. et al . Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect. Dis. 12 , 388–396 (2012).
Cohen, C. et al . Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin. Infect. Dis. 41 , 1631–1637 (2005).
Mulu, A. et al . Epidemiological and clinical correlates of malaria–helminth co-infections in southern Ethiopia. Malar. J. 12 , 227 (2013).
Gwamaka, M. et al . Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin. Infect. Dis. 54 , 1137–1144 (2012).
Neuberger, A., Okebe, J., Yahav, D. & Paul, M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev. 2 , CD006589 (2016).
Tilley, L., Straimer, J., Gnadig, N. F., Ralph, S. A. & Fidock, D. A. Artemisinin action and resistance in Plasmodium falciparum . Trends Parasitol. 32 , 682–696 (2016). Comprehensively reviews the emerging threat of artemisinin resistance, covering what is known about the mechanism of action of the drug and the molecular basis of artemisinin resistance.
Woodrow, C. J. & White, N. J. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol. Rev. 41 , 34–48 (2017).
Menard, D. et al . A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N. Engl. J. Med. 374 , 2453–2464 (2016).
Imwong, M. et al . The spread of artemisinin-resistant Plasmodium falciparum in the greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect. Dis. 17 , 491–497 (2017).
Paul, A. S., Egan, E. S. & Duraisingh, M. T. Host–parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 22 , 220–226 (2015). Reviews the molecular basis of parasite invasion.
Lim, C. et al . Reticulocyte preference and stage development of Plasmodium vivax isolates. J. Infect. Dis. 214 , 1081–1084 (2016).
Boddey, J. A. & Cowman, A. F. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 67 , 243–269 (2013).
Spillman, N. J., Beck, J. R. & Goldberg, D. E. Protein export into malaria parasite-infected erythrocytes: mechanisms and functional consequences. Annu. Rev. Biochem. 84 , 813–841 (2015). Reviews the biology associated with red blood cell remodelling upon parasite invasion.
Phillips, M. A. in Neglected Diseases and Drug Discovery (eds Palmer, M. & Wells, T. N. C. ) 65–87 (RCS Publishing, 2011).
Istvan, E. S. et al . Validation of isoleucine utilization targets in Plasmodium falciparum . Proc. Natl Acad. Sci. USA 108 , 1627–1632 (2011).
Wunderlich, J., Rohrbach, P. & Dalton, J. P. The malaria digestive vacuole. Front. Biosci. (Schol. Ed.) 4 , 1424–1448 (2012).
Chugh, M. et al . Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum . Proc. Natl Acad. Sci. USA 110 , 5392–5397 (2013).
Sigala, P. A. & Goldberg, D. E. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 68 , 259–278 (2014).
Spillman, N. J. et al . Na + regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13 , 227–237 (2013). Identifies the protein target of one of the key new antimalarials that is in clinical development.
Spillman, N. J. & Kirk, K. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int. J. Parasitol. Drugs Drug Resist. 5 , 149–162 (2015).
Jimenez-Diaz, M. B. et al . (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium . Proc. Natl Acad. Sci. USA 111 , E5455–E5462 (2014).
McNamara, C. W. et al . Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504 , 248–253 (2013). Identifies an important new target for drug discovery.
Rosenberg, R., Wirtz, R. A., Schneider, I. & Burge, R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans. R. Soc. Trop. Med. Hyg. 84 , 209–212 (1990).
Sinnis, P. & Zavala, F. The skin: where malaria infection and the host immune response begin. Semin. Immunopathol. 34 , 787–792 (2012).
Frischknecht, F. et al . Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell. Microbiol. 6 , 687–694 (2004).
Zheng, H., Tan, Z. & Xu, W. Immune evasion strategies of pre-erythrocytic malaria parasites. Mediators Inflamm. 2014 , 362605 (2014).
Duraisingh, M. T. & Horn, D. Epigenetic regulation of virulence gene expression in parasitic protozoa. Cell Host Microbe 19 , 629–640 (2016).
Smith, J. D. The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research. Mol. Biochem. Parasitol. 195 , 82–87 (2014).
Dobbs, K. R. & Dent, A. E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 143 , 129–138 (2016).
Fowkes, F. J., Boeuf, P. & Beeson, J. G. Immunity to malaria in an era of declining malaria transmission. Parasitology 143 , 139–153 (2016).
Teo, A., Feng, G., Brown, G. V., Beeson, J. G. & Rogerson, S. J. Functional antibodies and protection against blood-stage malaria. Trends Parasitol. 32 , 887–898 (2016).
Marsh, K. & Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 28 , 51–60 (2006).
Parroche, P. et al . Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl Acad. Sci. USA 104 , 1919–1924 (2007).
Karunaweera, N. D., Grau, G. E., Gamage, P., Carter, R. & Mendis, K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl Acad. Sci. USA 89 , 3200–3203 (1992).
Vijaykumar, M., Naik, R. S. & Gowda, D. C. Plasmodium falciparum glycosylphosphatidylinositol-induced TNF-α secretion by macrophages is mediated without membrane insertion or endocytosis. J. Biol. Chem. 276 , 6909–6912 (2001).
Wijesekera, S. K., Carter, R., Rathnayaka, L. & Mendis, K. N. A malaria parasite toxin associated with Plasmodium vivax paroxysms. Clin. Exp. Immunol. 104 , 221–227 (1996).
Hosseini, S. M. & Feng, J. J. How malaria parasites reduce the deformability of infected red blood cells. Biophys. J. 103 , 1–10 (2012).
Gillrie M. R. et al . Thrombin cleavage of Plasmodium falciparum erythrocyte membrane protein 1 inhibits cytoadherence. mBio 7 e01120-16 (2016).
Bernabeu, M. et al . Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc. Natl Acad. Sci. USA 113 , E3270–E3279 (2016).
Fox, L. L. et al . Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J. Infect. Dis. 208 , 500–503 (2013).
Seydel, K. B. et al . Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 372 , 1126–1137 (2015).
Lopez, C., Saravia, C., Gomez, A., Hoebeke, J. & Patarroyo, M. A. Mechanisms of genetically-based resistance to malaria. Gene 467 , 1–12 (2010).
Piel, F. B. The present and future global burden of the inherited disorders of hemoglobin. Hematol. Oncol. Clin. North Am. 30 , 327–341 (2016).
Elguero, E. et al . Malaria continues to select for sickle cell trait in Central Africa. Proc. Natl Acad. Sci. USA 112 , 7051–7054 (2015).
Kwiatkowski, D. P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77 , 171–192 (2005).
Cheng, Y. et al . Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA) binds human erythrocytes independent of Duffy antigen status. Sci. Rep. 6 , 35581 (2016).
Gunalan, K. et al . Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc. Natl Acad. Sci. USA 113 , 6271–6276 (2016).
Guindo, A., Fairhurst, R. M., Doumbo, O. K., Wellems, T. E. & Diallo, D. A. X-Linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 4 , e66 (2007).
Cholera, R. et al . Impaired cytoadherence of Plasmodium falciparum -infected erythrocytes containing sickle hemoglobin. Proc. Natl Acad. Sci. USA 105 , 991–996 (2008).
Fairhurst, R. M. et al . Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 435 , 1117–1121 (2005). References 84 and 86 identify key genetic traits that are associated with protection against P. falciparum malaria.
World Health Organization. Malaria: diagnostic testing. WHO http://www.who.int/malaria/areas/diagnosis/en/ (2016).
Worldwide Antimalarial Resistance Network (WWARN) AL Dose Impact Study Group. The effect of dose on the antimalarial efficacy of artemether–lumefantrine: a systematic review and pooled analysis of individual patient data. Lancet Infect. Dis. 15 , 692–702 (2015). Describes data from the Worldwide Antimalarial Resistance Network (WWARN), which has set up computational tools that measure parasite reduction from human data; the WWARN also collects information on emerging resistance.
Joanny, F., Lohr, S. J., Engleitner, T., Lell, B. & Mordmuller, B. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar. J. 13 , 234 (2014).
Azikiwe, C. C. et al . A comparative laboratory diagnosis of malaria: microscopy versus rapid diagnostic test kits. Asian Pac. J. Trop. Biomed. 2 , 307–310 (2012).
McCarthy, J. S. et al . A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS ONE 6 , e21914 (2011). Describes the use of the human blood-stage challenge model for drug testing. This model has gone on to be an important tool for the early evaluation of the clinical efficacy of new antimalarial compounds.
Phiri, K. et al . Parasitological clearance rates and drug concentrations of a fixed dose combination of azithromycin–chloroquine in asymptomatic pregnant women with Plasmodium Falciparum parasitemia: an open-label, non-comparative study in sub-Saharan Africa. PLoS ONE 11 , e0165692 (2016).
Imwong, M. et al . Numerical distributions of parasite densities during asymptomatic malaria. J. Infect. Dis. 213 , 1322–1329 (2016).
Notomi, T. et al . Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28 , E63 (2000).
Sema, M. et al . Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in northwest Ethiopia. Malar. J. 14 , 44 (2015).
Pholwat, S. et al . The malaria TaqMan array card includes 87 assays for Plasmodium falciparum drug resistance, identification of species, and genotyping in a single reaction. Antimicrob. Agents Chemother. 61 e00110-17 (2017).
Foundation for Innovative New Diagnostics. Acute febrile syndrome strategy. FiND http://r4d.dfid.gov.uk/PDF/Outputs/FIND/0031-FIND-NMFI-document-print-inhouse.pdf (2012).
Gamboa, D. et al . A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3 : implications for malaria rapid diagnostic tests. PLoS ONE 5 , e8091 (2010).
Akinyi, S. et al . Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci. Rep. 3 , 2797 (2013).
Cheng, Q. et al . Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar. J. 13 , 283 (2014).
Bharti, P. K. et al . Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS ONE 11 , e0157949 (2016).
Deme, A. B. et al . Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malar. J. 13 , 34 (2014).
Parr, J. B. et al . Estimation of Plasmodium falciparum transmission intensity in Lilongwe, Malawi, by microscopy, rapid diagnostic testing, and nucleic acid detection. Am. J. Trop. Med. Hyg. 95 , 373–377 (2016).
Mouatcho, J. C. & Goldring, J. P. Malaria rapid diagnostic tests: challenges and prospects. J. Med. Microbiol. 62 , 1491–1505 (2013).
Soti, D. O. et al . Feasibility of an innovative electronic mobile system to assist health workers to collect accurate, complete and timely data in a malaria control programme in a remote setting in Kenya. Malar. J. 14 , 430 (2015).
Scherr, T. F., Gupta, S., Wright, D. W. & Haselton, F. R. Mobile phone imaging and cloud-based analysis for standardized malaria detection and reporting. Sci. Rep. 6 , 28645 (2016).
Ouédraogo, A. L. et al . Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin. Infect. Dis. 60 , 357–365 (2015).
World Health Organization. Guidelines for the treatment of malaria, 3rd edn. WHO http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf (2015).
Chanda, E., Remijo, C. D., Pasquale, H., Baba, S. P. & Lako, R. L. Scale-up of a programme for malaria vector control using long-lasting insecticide-treated nets: lessons from South Sudan. Bull. World Health Organ. 92 , 290–296 (2014).
Ojuka, P. et al . Early biting and insecticide resistance in the malaria vector Anopheles might compromise the effectiveness of vector control intervention in southwestern Uganda. Malar. J. 14 , 148 (2015).
Hemingway, J. et al . Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 387 , 1785–1788 (2016). Discusses key issues relating to the continued control of the mosquito that transmits malaria.
Knapp, J., Macdonald, M., Malone, D., Hamon, N. & Richardson, J. H. Disruptive technology for vector control: the Innovative Vector Control Consortium and the US Military join forces to explore transformative insecticide application technology for mosquito control programmes. Malar. J. 14 , 371 (2015).
Sibanda, M. & Focke, W. Development of an insecticide impregnated polymer wall lining for malaria vector control. Malar. J. 13 (Suppl. 1), 80 (2014).
Kruger, T., Sibanda, M. M., Focke, W. W., Bornman, M. S. & de Jager, C. Acceptability and effectiveness of a monofilament, polyethylene insecticide-treated wall lining for malaria control after six months in dwellings in Vhembe District, Limpopo Province, South Africa. Malar. J. 14 , 485 (2015).
Burt, A. Heritable strategies for controlling insect vectors of disease. Phil. Trans. R. Soc. B 369 , 20130432 (2014).
Committee on Gene Drive Research in Non-Human Organisms. Recommendations for Responsible Conduct. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty and Aligning Research with Public Values (National Academies Press, 2016).
Oliva, C. F. et al . Current status and future challenges for controlling malaria with the sterile insect technique: technical and social perspectives. Acta Trop. 132 , S130–S139 (2014).
Bourtzis, K., Lees, R. S., Hendrichs, J. & Vreysen, M. J. More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 157 , 115–130 (2016).
Black, W. C. 4th, Alphey, L. & James, A. A. Why RIDL is not SIT. Trends Parasitol. 27 , 362–370 (2011).
Windbichler, N. et al . A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473 , 212–215 (2011).
Esvelt, K. M., Smidler, A. L., Catteruccia, F. & Church, G. M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3 , e03401 (2014).
Hammond, A. et al . A CRISPR–Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae . Nat. Biotechnol. 34 , 78–83 (2016).
Gantz, V. M. et al . Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi . Proc. Natl Acad. Sci. USA 112 , E6736–E6743 (2015).
Bull, J. J. & Turelli, M. Wolbachia versus dengue: evolutionary forecasts. Evol. Med. Public Health 2013 , 197–207 (2013).
Wilke, A. B. & Marrelli, M. T. Paratransgenesis: a promising new strategy for mosquito vector control. Parasit. Vectors 8 , 342 (2015).
Wang, S. et al . Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl Acad. Sci. USA 109 , 12734–12739 (2012).
Lee, S. J., Ter Kuile, F. O., Price, R. N., Luxemburger, C. & Nosten, F. Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: a pooled analysis of 19, 850 individual patients. PLoS ONE 12 , e0168780 (2017).
Spinner, C. D. et al . HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection 44 , 151–158 (2016).
Margolis, D. A. & Boffito, M. Long-acting antiviral agents for HIV treatment. Curr. Opin. HIV AIDS 10 , 246–252 (2015).
World Health Organization. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. WHO http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendation/en/ (2012).
Cairns, M. et al . Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat. Commun. 3 , 881 (2012).
Noor, A. M. et al . Sub-national targeting of seasonal malaria chemoprevention in the Sahelian countries of the Nouakchott initiative. PLoS ONE 10 , e0136919 (2015).
Tagbor, H. et al . Seasonal malaria chemoprevention in an area of extended seasonal transmission in Ashanti, Ghana: an individually randomised clinical trial. Trop. Med. Int. Health 21 , 224–235 (2016).
Tine, R. C. et al . Feasibility, safety and effectiveness of combining home based malaria management and seasonal malaria chemoprevention in children less than 10 years in Senegal: a cluster-randomised trial. Trans. R. Soc. Trop. Med. Hyg. 108 , 13–21 (2014).
Zongo, I. et al . Randomized noninferiority trial of dihydroartemisinin–piperaquine compared with sulfadoxine–pyrimethamine plus amodiaquine for seasonal malaria chemoprevention in Burkina Faso. Antimicrob. Agents Chemother. 59 , 4387–4396 (2015).
Wilson, A. L. & IPTc Taskforce. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS ONE 6 , e16976 (2011).
Matondo, S. I. et al . High levels of sulphadoxine–pyrimethamine resistance Pfdhfr – Pfdhps quintuple mutations: a cross sectional survey of six regions in Tanzania. Malar. J. 13 , 152 (2014).
Gutman, J., Kovacs, S., Dorsey, G., Stergachis, A. & Ter Kuile, F. O. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin–piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 17 , 184–193 (2017).
Doolan, D. L., Dobano, C. & Baird, J. K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22 , 13–36 (2009).
Hoffman, S. L., Vekemans, J., Richie, T. L. & Duffy, P. E. The march toward malaria vaccines. Am. J. Prev. Med. 49 , S319–S333 (2015).
Keegan, L. T. & Dushoff, J. Population-level effects of clinical immunity to malaria. BMC Infect. Dis. 13 , 428 (2013).
McCarthy, J. S. et al . Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J. Infect. Dis. 208 , 1688–1694 (2013).
Roestenberg, M. et al . Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377 , 1770–1776 (2011).
Engwerda, C. R., Minigo, G., Amante, F. H. & McCarthy, J. S. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 28 , 515–521 (2012).
Wykes, M. N., Horne-Debets, J. M., Leow, C. Y. & Karunarathne, D. S. Malaria drives T cells to exhaustion. Front. Microbiol. 5 , 249 (2014).
RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386 , 31–45 (2015). Describes a key clinical study that evaluates the efficacy of the only malaria vaccine that is currently at an advanced stage of clinical development.
Penny, M. A. et al . Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet 387 , 367–375 (2015).
Gosling, R. & von Seidlein, L. The future of the RTS, S/AS01 malaria vaccine: an alternative development plan. PLoS Med. 13 , e1001994 (2016). Describes strategies for effectively integrating the RTS,S/AS01 vaccine into malaria control strategies.
Hoffman, S. L. et al . Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185 , 1155–1164 (2002).
Draper, S. J. et al . Recent advances in recombinant protein-based malaria vaccines. Vaccine 33 , 7433–7443 (2015).
World Health Organization. WHO Product Development for Vaccines Advisory Committee (PD-VAC) meeting — 2015. WHO http://www.who.int/immunization/research/meetings_workshops/pdvac/en/ (2015).
Nunes, J. K. et al . Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine 32 , 5531–5539 (2014).
Shimp, R. L. Jr et al . Development of a Pfs25–EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31 , 2954–2962 (2013).
Kelley, B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs 1 , 443–452 (2009).
Robbie, G. J. et al . A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 57 , 6147–6153 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01939899 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02647489 (2016).
Daily, J. P. Malaria 2017: update on the clinical literature and management. Curr. Infect. Dis. Rep. http://dx.doi.org/ 10.1007/s11908-017-0583-8 (2017).
Harrison, N. In celebration of the Jesuit's powder: a history of malaria treatment. Lancet Infect. Dis. 15 , 1143 (2015).
Kinfu, G., Gebre-Selassie, S. & Fikrie, N. Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in northern Ethiopia. Malar. Res. Treat. 2012 , 548710 (2012).
Sinclair, D., Donegan, S., Isba, R. & Lalloo, D. G. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst. Rev. 6 , CD005967 (2012).
Dondorp, A. et al . Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366 , 717–725 (2005). Describes a key study that demonstrates the efficacy of artesunate for the treatment of malaria and, therefore, supports the clinical use of artesunate.
Dondorp, A. M. et al . Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376 , 1647–1657 (2010).
Okebe, J. & Eisenhut, M. Pre-referral rectal artesunate for severe malaria. Cochrane Database Syst. Rev. 5 , CD009964 (2014).
Verhave, J. P. Experimental, therapeutic and natural transmission of Plasmodium vivax tertian malaria: scientific and anecdotal data on the history of Dutch malaria studies. Parasit. Vectors 6 , 19 (2013).
Llanos-Cuentas, A. et al . Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 383 , 1049–1058 (2014). Describes a key clinical study that demonstrates the efficacy of the only new compound that can prevent P. vivax relapse; tafenoquine and primaquine are the only compounds that have such activity.
White, N. J. Does antimalarial mass drug administration increase or decrease the risk of resistance? Lancet Infect. Dis. 17 , e15–e20 (2017).
Amato, R. et al . Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect. Dis. 17 , 164–173 (2017).
Ariey, F. et al . A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505 , 50–55 (2014).
World Health Organization. World malaria report 2016. WHO http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (2016).
Lu, F. et al . Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N. Engl. J. Med. 376 , 991–993 (2017).
Sutherland, C. J. et al . Pfk13 -independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether–lumefantrine in the United Kingdom. Antimicrob. Agents Chemother. 61 e02382-16 (2017).
Lukens, A. K. et al . Harnessing evolutionary fitness in Plasmodium falciparum for drug discovery and suppressing resistance. Proc. Natl Acad. Sci. USA 111 , 799–804 (2014).
Taylor, A. R. et al . Artemether–lumefantrine and dihydroartemisinin–piperaquine exert inverse selective pressure on Plasmodium falciparum drug sensitivity-associated haplotypes in Uganda. Open Forum Infect. Dis. 4 , ofw229 (2017).
Gamo, F. J. et al . Thousands of chemical starting points for antimalarial lead identification. Nature 465 , 305–310 (2010).
Möhrle, J. J. et al . First-in-man safety and pharmacokinetics of synthetic ozonide OZ439 demonstrates an improved exposure profile relative to other peroxide antimalarials. Br. J. Clin. Pharmacol. 75 , 524–537 (2013).
Phyo, A. P. et al . Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect. Dis. 16 , 61–69 (2016). Describes the key phase II clinical study of one of only two new clinical candidates that have reached phase IIb clinical development.
McCarthy, J. S. et al . A phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar. J. 15 469 (2016).
Held, J. et al . Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study. Lancet Infect. Dis. 15 , 1409–1419 (2015).
Leong, F. J. et al . A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel imidazolopiperazine KAF156 to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob. Agents Chemother. 58 , 6437–6443 (2014).
White, N. J. et al . Antimalarial activity of KAF156 in falciparum and vivax malaria. N. Engl. J. Med. 375 , 1152–1160 (2016).
Kuhen, K. L. et al . KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment and prevention of disease transmission. Antimicrob. Agents Chemother. 58 , 5060–5067 (2014).
Huskey, S. E. et al . KAE609 (Cipargamin), a new spiroindolone agent for the treatment of malaria: evaluation of the absorption, distribution, metabolism, and excretion of a single oral 300- mg dose of [ 14 C]KAE609 in healthy male subjects. Drug Metab. Dispos. 44 , 672–682 (2016).
White, N. J. et al . Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 371 , 403–410 (2014).
McCarthy, J. S. et al . Safety, tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. Lancet Infect. Dis. 17 , 626–635 (2017). References 184 and 185 describe key clinical studies that support the efficacy of new antimalarials in the clinical development portfolio.
Phillips, M. A. et al . A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl Med . 7 , 296ra111 (2015). Describes the biological activity and product profile of the first inhibitor of PfDHODH to reach clinical development.
Rüeckle, T. et al . A phase IIa proof-of-concept study to assess the efficacy, safety, tolerability and pharmacokinetics of single doses of DSM265 in adult patients with acute, uncomplicated Plasmodium falciparum or vivax malaria mono-infection over a 28-day-extended observation period in Iquitos, Peru. ASTMH http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=2e5199c4-aceb-4d61-8769-342586917c5a&cKey=d7fda50e-36c6-4a28-a76b-96bad2ec308b&mKey=%7bAB652FDF-0111-45C7-A5E5-0BA9D4AF5E12%7d (2015).
Sulyok, M. et al . DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection. Lancet Infect. Dis. 17 , 636–644 (2017). Describes the first use of a sporozoite human challenge study to demonstrate the chemopreventive activity of a compound that is under clinical development.
Das, S. et al . Na + influx induced by new antimalarials causes rapid alterations in the cholesterol content and morphology of Plasmodium falciparum . PLoS Pathog. 12 , e1005647 (2016).
Coteron, J. M. et al . Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J. Med. Chem. 54 , 5540–5561 (2011).
Ghidelli-Disse, S. et al . Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malar. J. 13 , S21 (2014).
Paquet, T. et al . Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl Med. 9 eaad9735 (2017).
Ge, J. F. et al . Discovery of novel benzo[ a ]phenoxazine SSJ-183 as a drug candidate for malaria. ACS Med. Chem. Lett. 1 , 360–364 (2010).
Abbat, S., Jain, V. & Bharatam, P. V. Origins of the specificity of inhibitor P218 toward wild-type and mutant PfDHFR: a molecular dynamics analysis. J. Biomol. Struct. Dyn. 33 , 1913–1928 (2015).
Wells, T. N. C., Hooft van Huijsduijnen, R. & Van Voorhis, W. C. Malaria medicines: a glass half full? Nat. Rev. Drug Discov. 14 , 424–442 (2015). Comprehensively reviews the malaria drug discovery pipeline.
Das, S. et al . Rapid reorganization of the parasite plasma membrane in response to a new class of antimalarial drugs. ISMMID http://immid-is.drexelmed.edu/2014_ISMMID_Abstract.pdf (2014).
Phillips, M. A. et al . A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with improved drug-like properties for treatment and prevention of malaria. ACS Infect. Dis. 2 , 945–957 (2016).
Le Manach, C. et al . Identification of a potential antimalarial drug candidate from a series of 2-aminopyrazines by optimization of aqueous solubility and potency across the parasite life cycle. J. Med. Chem. 59 , 9890–9905 (2016).
Baragaña, B. et al . A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522 , 315–320 (2015).
Hameed, P. S. et al . Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate. Nat. Commun. 6 , 6715 (2015).
Zhang, Y. K. et al . Synthesis and structure–activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 21 , 644–651 (2011).
Chavchich, M. et al . Lead selection of the new aminomethylphenol, JPC-3210 for malaria treatment and prevention. Antimicrob. Agents Chemother. 60 , 3115–3118 (2016).
Basch, P. Textbook of International Health 2nd edn (Oxford Univ. Press, 1999).
Ouattara, A. F. et al . Malaria knowledge and long-lasting insecticidal net use in rural communities of central Cote d’Ivoire. Malar. J. 10 , 288 (2011).
Adetokunbo, O. & Gilles, H. Short Textbook of Public Health, Medicine for the Tropics (BookPower, 2006).
Carter, J. A. et al . Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J. Neurol. Neurosurg. Psychiatry 76 , 476–481 (2005).
Fernando, S. D., Rodrigo, C. & Rajapakse, S. The ‘hidden’ burden of malaria: cognitive impairment following infection. Malar. J. 9 , 366 (2010).
Kihara, M., Carter, J. A. & Newton, C. R. The effect of Plasmodium falciparum on cognition: a systematic review. Trop. Med. Int. Health 11 , 386–397 (2006).
Holding, P. A. & Snow, R. W. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am. J. Trop. Med. Hyg. 64 , 68–75 (2001).
World Health Organization. Global technical strategy for Malaria 2016–2030. WHO http://who.int/malaria/areas/global_technical_strategy/en/ (2016).
Noedl, H. et al . Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359 , 2619–2620 (2008).
Singh, R. et al . Comparison of three PCR-based assays for the non-invasive diagnosis of malaria: detection of Plasmodium parasites in blood and saliva. Eur. J. Clin. Microbiol. Infect. Dis. 33 , 1631–1639 (2014).
Oguonu, T. et al . The performance evaluation of a urine malaria test (UMT) kit for the diagnosis of malaria in individuals with fever in South-east Nigeria: cross-sectional analytical study. Malar. J. 13 , 403 (2014).
Berna, A. Z. et al . Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J. Infect. Dis. 212 , 1120–1128 (2015).
Lukianova-Hleb, E. et al . Transdermal diagnosis of malaria using vapor nanobubbles. Emerg. Infect. Dis. 21 , 1122–1127 (2015).
World Health Organization. Emergency response to artemisinin resistance in the greater Mekong subregion. Regional framework for action 2013–2015. WHO http://www.who.int/malaria/publications/atoz/9789241505321/en/ (2013).
Rao, P. N. et al . A method for amplicon deep sequencing of drug resistance genes in Plasmodium falciparum clinical isolates from India. J. Clin. Microbiol. 54 , 1500–1511 (2016).
Wells, T. N., Burrows, J. N. & Baird, J. K. Targeting the hypnozoite reservoir of Plasmodium vivax : the hidden obstacle to malaria elimination. Trends Parasitol. 26 , 145–151 (2010). Discusses issues related to targeting the latent stages of P. vivax to address the lack of drug options that are safe in all patients to treat these stages of the parasite.
Chattopadhyay, R. et al . Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS ONE 5 , e14275 (2010).
Burrows, J. N., Hooft van Huijsduijnen, R., Möhrle, J. J., Oeuvray, C. & Wells, T. N. C. Designing the next generation of medicines for malaria control and eradication. Malar. J. 12 , 187 (2013). Defines TCPs and Target Product Profiles for malaria.
Burrows, J. N. et al . New developments in anti-malarial target candidate and product profiles. Malar. J. 16 , 26 (2017). Comprehensively analyses the compound properties that are required for the development of effective antimalarials that will cover all species and stages of the disease.
Tate, E. W., Bell, A. S., Rackham, M. D. & Wright, M. H. N -Myristoyltransferase as a potential drug target in malaria and leishmaniasis. Parasitology 141 , 37–49 (2014).
Pett, H. E. et al . Novel pantothenate derivatives for anti-malarial chemotherapy. Malar. J. 14 , 169 (2015).
Kato, N. et al . Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538 , 344–349 (2016).
Herman, J. D. et al . The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs. Sci. Transl Med. 7 , 288ra277 (2015).
Russo, I. et al . Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 463 , 632–636 (2010).
Nilsen, A. et al . Quinolone-3-diarylethers: a new class of antimalarial drug. Sci. Transl Med. 5 , 177ra137 (2013).
Newton, C. R., Taylor, T. E. & Whitten, R. O. Pathophysiology of fatal falciparum malaria in African children. Am. J. Trop. Med. Hyg. 58 , 673–683 (1998).
Kalanon, M. & McFadden, G. I. Malaria. Plasmodium falciparum and its apicoplast. Biochem. Soc. Trans. 38 , 775–782 (2010). Describes the importance of the apicoplast genome to the malaria parasite and opportunities to target this unique organelle for drug discovery.
Bozdech, Z. et al . The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum . PLoS Biol. 1 , E5 (2003).
Hughes, K. R., Philip, N., Starnes, G. L., Taylor, S. & Waters, A. P. From cradle to grave: RNA biology in malaria parasites. Wiley Interdiscip. Rev. RNA 1 , 287–303 (2010).
Llinas, M., Bozdech, Z., Wong, E. D., Adai, A. T. & DeRisi, J. L. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 34 , 1166–1173 (2006).
Ganesan, K. et al . A genetically hard-wired metabolic transcriptome in Plasmodium falciparum fails to mount protective responses to lethal antifolates. PLoS Pathog. 4 , e1000214 (2008).
Hu, G. et al . Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum . Nat. Biotechnol. 28 , 91–98 (2010).
Hoo, R. et al . Integrated analysis of the Plasmodium species transcriptome. EBioMedicine 7 , 255–266 (2016).
Caro, F., Ahyong, V., Betegon, M. & DeRisi, J. L. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife 3 , e04106 (2014). Describes a comprehensive analysis of the P. falciparum transcriptome over the red blood cell cycle.
PubMed Central Google Scholar
Kirchner, S., Power, B. J. & Waters, A. P. Recent advances in malaria genomics and epigenomics. Genome Med. 8 , 92 (2016). Comprehensively reviews of the role of epigenomics in gene expression in Plasmodium spp.
Lee, M. C. & Fidock, D. A. CRISPR-mediated genome editing of Plasmodium falciparum malaria parasites. Genome Med. 6 , 63 (2014). Reviews genetic approaches to manipulating the P. falciparum genome.
de Koning-Ward, T. F., Gilson, P. R. & Crabb, B. S. Advances in molecular genetic systems in malaria. Nat. Rev. Microbiol. 13 , 373–387 (2015). Reviews genetic approaches to manipulating the Plasmodium spp. genome.
Gomes, A. R. et al . A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe 17 , 404–413 (2015).
Corey, V. C. et al . A broad analysis of resistance development in the malaria parasite. Nat. Commun. 7 , 11901 (2016).
Allman, E. L., Painter, H. J., Samra, J., Carrasquilla, M. & Llinas, M. Metabolomic profiling of the malaria box reveals antimalarial target pathways. Antimicrob. Agents Chemother. 60 , 6635–6649 (2016).
Manyando, C. et al . Safety of artemether–lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar. J. 9 , 249 (2010).
Yanow, S. K., Gavina, K., Gnidehou, S. & Maestre, A. Impact of malaria in pregnancy as Latin America approaches elimination. Trends Parasitol. 32 , 416–427 (2016).
Harrington, W. E., Morrison, R., Fried, M. & Duffy, P. E. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring. PLoS ONE 8 , e56183 (2013).
Dellicour, S. et al . First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: a meta-analysis of observational studies. PLoS Med. 14 , e1002290 (2017).
Van Voorhis, W. C., Hooft van Huijsduijnen, R. & Wells, T. N. C. Profile of William C. Campbell, Satoshi O®mura, and Youyou Tu, 2015 Nobel Laureates in Physiology or Medicine. Proc. Natl Acad. Sci. USA 112 , 15773–15776 (2015).
White, N. J., Hien, T. T. & Nosten, F. H. A. Brief history of qinghaosu. Trends Parasitol. 31 , 607–610 (2015). Reviews the history of the discovery of artemisinin derivatives.
White, N. J. et al . Averting a malaria disaster. Lancet 353 , 1965–1967 (1999).
White, N. J. & Olliaro, P. L. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol. Today 12 , 399–401 (1996).
Hien, T. T. & White, N. J. Qinghaosu. Lancet 341 , 603–608 (1993).
Vaid, A., Ranjan, R., Smythe, W. A., Hoppe, H. C. & Sharma, P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum , is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 115 , 2500–2507 (2010).
Mbengue, A. et al . A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520 , 683–687 (2015).
Annan, Z. et al . Population genetic structure of Plasmodium falciparum in the two main African vectors. Anopheles gambiae and Anopheles funestus . Proc. Natl Acad. Sci. USA 104 , 7987–7992 (2007).
Josling, G. A. & Llinas, M. Sexual development in Plasmodium parasites: knowing when it's time to commit. Nat. Rev. Microbiol. 13 , 573–587 (2015).
Aly, A. S., Vaughan, A. M. & Kappe, S. H. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63 , 195–221 (2009).
World Health Organization. Climate change and human health. WHO http://www.who.int/globalchange/summary/en/index5.html (2017).
Gething, P. W. et al . Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax . Parasit. Vectors 4 , 92 (2011).
Weiss, G. E. et al . Revealing the sequence and resulting cellular morphology of receptor–ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 11 , e1004670 (2015).
Egan, E. S. et al . Malaria. A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion. Science 348 , 711–714 (2015).
Crosnier, C. et al . Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum . Nature 480 , 534–537 (2011). References 260 and 261 identify key receptors involved in P. falciparum invasion.
Volz, J. C. et al . Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe 20 , 60–71 (2016). Provides a comprehensive molecular understanding of the role of the receptor basigin in P. falciparum invasion and provides a comprehensive molecular model for the invasion process that highlights the three key steps.
Zenonos, Z. A. et al . Basigin is a druggable target for host-oriented antimalarial interventions. J. Exp. Med. 212 , 1145–1151 (2015).
Srinivasan, P. et al . Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc. Natl Acad. Sci. USA 108 , 13275–13280 (2011).
Remarque, E. J., Faber, B. W., Kocken, C. H. & Thomas, A. W. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 24 , 74–84 (2008).
Yuthavong, Y. et al . Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl Acad. Sci. USA 109 , 16823–16828 (2012).
Sidhu, A. B., Verdier-Pinard, D. & Fidock, D. A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298 , 210–213 (2002). Describes the identification of the molecular basis for chloroquine resistance.
Lek-Uthai, U. et al . Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum . Antimicrob. Agents Chemother. 52 , 2435–2441 (2008).
World Health Organization. Rainbow tables. WHO http://www.who.int/immunization/research/development/Rainbow_tables/en/ (2017).
Mishra, N. et al . Emerging polymorphisms in falciparum Kelch 13 gene in northeastern region of India. Malar. J. 15 , 583 (2016).
Spring, M. D. et al . Dihydroartemisinin–piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect. Dis. 15 , 683–691 (2015).
Thuy-Nhien, N. et al . K13-propeller mutations in Plasmodium falciparum populations in malaria endemic regions of Vietnam from 2009 to 2016. Antimicrob. Agents Chemother. 61 , e01578-16 (2017).
Thanh, N. V. et al . Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin–piperaquine in the south of Vietnam. Malar. J. 16 , 27 (2017).
Sanchez, C. P. et al . Evidence for a pfcrt -associated chloroquine efflux system in the human malarial parasite Plasmodium falciparum . Biochemistry 44 , 9862–9870 (2005).
Valderramos, S. G. & Fidock, D. A. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 27 , 594–601 (2006). Comprehensively reviews transporter mutants that are involved in resistance to the aminoquinoline series of antimalarial drugs (for example, chloroquine).
Borges, S. et al . Genomewide scan reveals amplification of mdr1 as a common denominator of resistance to mefloquine, lumefantrine, and artemisinin in Plasmodium chabaudi malaria parasites. Antimicrob. Agents Chemother. 55 , 4858–4865 (2011).
Humphreys, G. S. et al . Amodiaquine and artemether–lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 51 , 991–997 (2007).
Baliraine, F. N. & Rosenthal, P. J. Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether–lumefantrine in Uganda. J. Infect. Dis. 204 , 1120–1124 (2011).
Martin, R. E. & Kirk, K. The malaria parasite's chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 21 , 1938–1949 (2004).
Ehrhardt, S. et al . Large-scale surveillance of Plasmodium falciparum crt(K76T) in northern Ghana. Antimicrob. Agents Chemother. 51 , 3407–3409 (2007).
Figueiredo, P. et al . Prevalence of pfmdr1 , pfcrt , pfdhfr and pfdhps mutations associated with drug resistance, in Luanda, Angola. Malar. J. 7 , 236 (2008).
Mens, P. F. Ambiguous role of pfcrt K76 in Plasmodium falciparum : a marker of resistance or increased susceptibility. Expert Rev. Anti Infect. Ther. 7 , 409–412 (2009).
Restrepo-Pineda, E., Arango, E., Maestre, A., Rosário, V. E. D. & Cravo, P. Studies on antimalarial drug susceptibility in Colombia, in relation to Pfmdr1 and Pfcrt . Parasitology 135 , 547–553 (2008).
Drew, M. E. et al . Plasmodium food vacuole plasmepsins are activated by falcipains. J. Biol. Chem. 283 , 12870–12876 (2008).
Witkowski, B. et al . A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study. Lancet Infect. Dis. 17 , 174–183 (2017).
Download references
Acknowledgements
The authors thank R. Bryant, A. Hill, S. Rees and S. L. Hoffman for their help with the content of Figure 4 and Figure 6 , and S. Duparc for critical reading of the clinical sections of the manuscript.
Author information
Authors and affiliations.
Department of Biochemistry, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Boulevard, Dallas, 75390–9038, Texas, USA
Margaret A. Phillips
Medicines for Malaria Venture, Geneva, Switzerland
Jeremy N. Burrows, Rob Hooft van Huijsduijnen & Timothy N. C. Wells
Tropical Diseases Research Centre, Ndola, Zambia
Christine Manyando
Department of Medicine, Division of Allergy and Infectious Diseases, University of Washington, Center for Emerging and Re-emerging Infectious Diseases, Seattle, Washington, USA
Wesley C. Van Voorhis
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (M.A.P., J.N.B. and W.C.V.V.); Epidemiology (M.A.P. and W.C.V.V.); Mechanisms/pathophysiology (M.A.P.); Diagnosis, screening and prevention (M.A.P., J.N.B., R.H.v.H. and T.N.C.W.); Management (J.N.B., R.H.v.H. and T.N.C.W.); Quality of life (C.M.); Outlook (R.H.v.H. and T.N.C.W.); Overview of Primer (M.A.P.).
Corresponding author
Correspondence to Margaret A. Phillips .
Ethics declarations
Competing interests.
T.N.C.W. is a non-executive director of Kymab in the United Kingdom. Kymab has programmes in malaria that are funded by the Bill & Melinda Gates Foundation. All other authors declare no competing interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Phillips, M., Burrows, J., Manyando, C. et al. Malaria. Nat Rev Dis Primers 3 , 17050 (2017). https://doi.org/10.1038/nrdp.2017.50
Download citation
Published : 03 August 2017
DOI : https://doi.org/10.1038/nrdp.2017.50
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Gene expression analyses reveal differences in children’s response to malaria according to their age.
- Kieran Tebben
- Salif Yirampo
- David Serre
Nature Communications (2024)
Histone modification analysis reveals common regulators of gene expression in liver and blood stage merozoites of Plasmodium parasites
- Ashley B. Reers
- Rodriel Bautista
- Evelien M. Bunnik
Epigenetics & Chromatin (2023)
Impact of insecticide resistance on malaria vector competence: a literature review
- Pierre Fongho Suh
- Emmanuel Elanga-Ndille
- Cyrille Ndo
Malaria Journal (2023)
High-throughput analysis of the transcriptional patterns of sexual genes in malaria
- Abel Cruz Camacho
- Neta Regev-Rudzki
Parasites & Vectors (2023)
Development of a human malaria-on-a-chip disease model for drug efficacy and off-target toxicity evaluation
- Michael J. Rupar
- Trevor Sasserath
- James J. Hickman
Scientific Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
On this page
When to see a doctor, risk factors, complications.
Malaria is a disease caused by a parasite. The parasite is spread to humans through the bites of infected mosquitoes. People who have malaria usually feel very sick with a high fever and shaking chills.
While the disease is uncommon in temperate climates, malaria is still common in tropical and subtropical countries. Each year nearly 290 million people are infected with malaria, and more than 400,000 people die of the disease.
To reduce malaria infections, world health programs distribute preventive drugs and insecticide-treated bed nets to protect people from mosquito bites. The World Health Organization has recommended a malaria vaccine for use in children who live in countries with high numbers of malaria cases.
Protective clothing, bed nets and insecticides can protect you while traveling. You also can take preventive medicine before, during and after a trip to a high-risk area. Many malaria parasites have developed resistance to common drugs used to treat the disease.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of malaria may include:
- General feeling of discomfort
- Nausea and vomiting
- Abdominal pain
- Muscle or joint pain
- Rapid breathing
- Rapid heart rate
Some people who have malaria experience cycles of malaria "attacks." An attack usually starts with shivering and chills, followed by a high fever, followed by sweating and a return to normal temperature.
Malaria signs and symptoms typically begin within a few weeks after being bitten by an infected mosquito. However, some types of malaria parasites can lie dormant in your body for up to a year.
Talk to your doctor if you experience a fever while living in or after traveling to a high-risk malaria region. If you have severe symptoms, seek emergency medical attention.
From Mayo Clinic to your inbox
Malaria is caused by a single-celled parasite of the genus plasmodium. The parasite is transmitted to humans most commonly through mosquito bites.
Mosquito transmission cycle
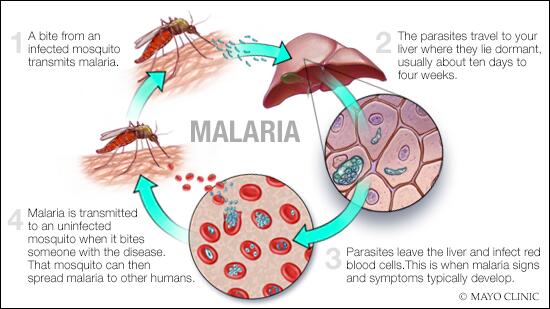
- Malaria transmission cycle
Malaria spreads when a mosquito becomes infected with the disease after biting an infected person, and the infected mosquito then bites a noninfected person. The malaria parasites enter that person's bloodstream and travel to the liver. When the parasites mature, they leave the liver and infect red blood cells.
- Uninfected mosquito. A mosquito becomes infected by feeding on a person who has malaria.
- Transmission of parasite. If this mosquito bites you in the future, it can transmit malaria parasites to you.
- In the liver. Once the parasites enter your body, they travel to your liver — where some types can lie dormant for as long as a year.
- Into the bloodstream. When the parasites mature, they leave the liver and infect your red blood cells. This is when people typically develop malaria symptoms.
- On to the next person. If an uninfected mosquito bites you at this point in the cycle, it will become infected with your malaria parasites and can spread them to the other people it bites.
Other modes of transmission
Because the parasites that cause malaria affect red blood cells, people can also catch malaria from exposure to infected blood, including:
- From mother to unborn child
- Through blood transfusions
- By sharing needles used to inject drugs
The greatest risk factor for developing malaria is to live in or to visit areas where the disease is common. These include the tropical and subtropical regions of:
- Sub-Saharan Africa
- South and Southeast Asia
- Pacific Islands
- Central America and northern South America
The degree of risk depends on local malaria control, seasonal changes in malaria rates and the precautions you take to prevent mosquito bites.
Risks of more-severe disease
People at increased risk of serious disease include:
- Young children and infants
- Older adults
- Travelers coming from areas with no malaria
- Pregnant women and their unborn children
In many countries with high malaria rates, the problem is worsened by lack of access to preventive measures, medical care and information.
Immunity can wane
Residents of a malaria region may be exposed to the disease enough to acquire a partial immunity, which can lessen the severity of malaria symptoms. However, this partial immunity can disappear if you move to a place where you're no longer frequently exposed to the parasite.
Malaria can be fatal, particularly when caused by the plasmodium species common in Africa. The World Health Organization estimates that about 94% of all malaria deaths occur in Africa — most commonly in children under the age of 5.
Malaria deaths are usually related to one or more serious complications, including:
- Cerebral malaria. If parasite-filled blood cells block small blood vessels to your brain (cerebral malaria), swelling of your brain or brain damage may occur. Cerebral malaria may cause seizures and coma.
- Breathing problems. Accumulated fluid in your lungs (pulmonary edema) can make it difficult to breathe.
- Organ failure. Malaria can damage the kidneys or liver or cause the spleen to rupture. Any of these conditions can be life-threatening.
- Anemia. Malaria may result in not having enough red blood cells for an adequate supply of oxygen to your body's tissues (anemia).
- Low blood sugar. Severe forms of malaria can cause low blood sugar (hypoglycemia), as can quinine — a common medication used to combat malaria. Very low blood sugar can result in coma or death.
Malaria may recur
Some varieties of the malaria parasite, which typically cause milder forms of the disease, can persist for years and cause relapses.
If you live in or are traveling to an area where malaria is common, take steps to avoid mosquito bites. Mosquitoes are most active between dusk and dawn. To protect yourself from mosquito bites, you should:
- Cover your skin. Wear pants and long-sleeved shirts. Tuck in your shirt, and tuck pant legs into socks.
- Apply insect repellent to skin. Use an insect repellent registered with the Environmental Protection Agency on any exposed skin. These include repellents that contain DEET, picaridin, IR3535, oil of lemon eucalyptus (OLE), para-menthane-3,8-diol (PMD) or 2-undecanone. Do not use a spray directly on your face. Do not use products with oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) on children under age 3.
- Apply repellent to clothing. Sprays containing permethrin are safe to apply to clothing.
- Sleep under a net. Bed nets, particularly those treated with insecticides, such as permethrin, help prevent mosquito bites while you are sleeping.
Preventive medicine
If you'll be traveling to a location where malaria is common, talk to your doctor a few months ahead of time about whether you should take drugs before, during and after your trip to help protect you from malaria parasites.
In general, the drugs taken to prevent malaria are the same drugs used to treat the disease. What drug you take depends on where and how long you are traveling and your own health.
The World Health Organization has recommended a malaria vaccine for use in children who live in countries with high numbers of malaria cases.
Researchers are continuing to develop and study malaria vaccines to prevent infection.
Feb 09, 2023
- AskMayoExpert. Malaria. Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2018.
- Jameson JL, et al., eds. Malaria. In: Harrison's Principles of Internal Medicine. 20th ed. New York, N.Y.: The McGraw-Hill Companies; 2018. https://accessmedicine.mhmedical.com. Accessed Oct. 9, 2018.
- Tintinalli JE, et al., eds. Malaria. In: Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, N.Y.: McGraw-Hill Education; 2016. http://www.accessmedicine.mhmedical.com. Accessed Oct. 9, 2018.
- Malaria. Merck Manual Professional Version. http://www.merckmanuals.com/professional/infectious-diseases/extraintestinal-protozoa/malaria. Accessed Oct. 9, 2018.
- Malaria. Centers for Disease Control and Prevention. http://wwwnc.cdc.gov/travel/diseases/malaria. Accessed Nov. 6, 2015.
- Breman JG. Clinical manifestations of malaria in nonpregnant adults and children. https://www.uptodate.com/contents/search. Accessed Oct. 9, 2018.
- Daily J. Treatment of uncomplicated falciparum malaria in nonpregnant adults and children. https://www.uptodate.com/contents/search. Accessed Oct. 9, 2018.
- Key points: World malaria report 2017. World Health Organization. https://www.who.int/malaria/media/world-malaria-report-2017/en/. Accessed Oct. 9, 2018.
- Malaria. World Health Organization. https://www.who.int/malaria/en/. Accessed Oct. 9, 2018.
- Mutebi JP, et al. Protection against mosquitoes, ticks, & other arthropods. In: CDC Yellow Book 2018: Health Information for International Travelers. https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods. Accessed Oct. 27, 2018.
- Diseases & Conditions
- Malaria symptoms & causes
News from Mayo Clinic
More Information
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Search by keyword
- Search by citation
Page 1 of 162
Microsatellites reveal high polymorphism and high potential for use in anti-malarial efficacy studies in areas with different transmission intensities in mainland Tanzania
Tanzania is currently implementing therapeutic efficacy studies (TES) in areas of varying malaria transmission intensities as per the World Health Organization (WHO) recommendations. In TES, distinguishing rei...
- View Full Text
Application of advanced very high-resolution radiometer (AVHRR)-based vegetation health indices for modelling and predicting malaria in Northern Benin, West Africa
Vegetation health (VH) is a powerful characteristic for forecasting malaria incidence in regions where the disease is prevalent. This study aims to determine how vegetation health affects the prevalence of mal...
Insecticide susceptibility status of Anopheles gambiae mosquitoes and the effect of pre-exposure to a piperonyl butoxide (PBO) synergist on resistance to deltamethrin in northern Namibia
Pyrethroid-based indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) have been employed as key vector control measures against malaria in Namibia. However, pyrethroid resistance in Anopheles
Epidemiology of Plasmodium vivax in Duffy negatives and Duffy positives from community and health centre collections in Ethiopia
Malaria remains a significant cause of morbidity and mortality in Ethiopia with an estimated 3.8 million cases in 2021 and 61% of the population living in areas at risk of malaria transmission. Throughout the ...
Comparing malaria risk exposure in rural Cambodia population using GPS tracking and questionnaires
The Great Mekong Subregion has attained a major decline in malaria cases and fatalities over the last years, but residual transmission hotspots remain, supposedly fueled by forest workers and migrant populatio...
Malaria transmission heterogeneity in different eco-epidemiological areas of western Kenya: a region-wide observational and risk classification study for adaptive intervention planning
Understanding of malaria ecology is a prerequisite for designing locally adapted control strategies in resource-limited settings. The aim of this study was to utilize the spatial heterogeneity in malaria trans...
Factors affecting delays in seeking treatment among malaria patients during the pre-certification phase in China
Delays in malaria treatment can not only lead to severe and even life-threatening complications, but also foster transmission, putting more people at risk of infection. This study aimed to investigate the fact...
First report of natural infection of Anopheles gambiae s.s. and Anopheles coluzzii by Wolbachia and Microsporidia in Benin: a cross-sectional study
Recently, bacterial endosymbiont, including Wolbachia and Microsporidia were found to limit the infection of Anopheles mosquitoes with Plasmodium falciparum . This study aimed to investigate the natural presence o...
Trends of Plasmodium falciparum molecular markers associated with resistance to artemisinins and reduced susceptibility to lumefantrine in Mainland Tanzania from 2016 to 2021
Therapeutic efficacy studies (TESs) and detection of molecular markers of drug resistance are recommended by the World Health Organization (WHO) to monitor the efficacy of artemisinin-based combination therapy...
RNAscope in situ hybridization reveals microvascular sequestration of Plasmodium relictum pSGS1 blood stages but absence of exo-erythrocytic dormant stages during latent infection of Serinus canaria
Birds chronically infected with avian malaria parasites often show relapses of parasitaemia after latent stages marked by absence of parasites in the peripheral circulation. These relapses are assumed to resul...
The impact of agrochemical pollutant mixtures on the selection of insecticide resistance in the malaria vector Anopheles gambiae : insights from experimental evolution and transcriptomics
There are several indications that pesticides used in agriculture contribute to the emergence and spread of resistance of mosquitoes to vector control insecticides. However, the impact of such an indirect sele...
Evaluating the performance of Plasmodium falciparum genetic metrics for inferring National Malaria Control Programme reported incidence in Senegal
Genetic surveillance of the Plasmodium falciparum parasite shows great promise for helping National Malaria Control Programmes (NMCPs) assess parasite transmission. Genetic metrics such as the frequency of polyge...
Insecticide-treated bed nets and residual indoor spraying reduce malaria in areas with low transmission: a reanalysis of the Maltrials study
The malaria incidence data from a malaria prevention study from the Rift Valley, Central Ethiopia, were reanalysed. The objective was to investigate whether including an administrative structure within the soc...
Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya
Insecticide-treated nets (ITNs) contributed significantly to the decline in malaria since 2000. Their protective efficacy depends not only on access, use, and net integrity, but also location of people within ...
Adult mosquitoes of the sibling species Anopheles gambiae and Anopheles coluzzii exhibit contrasting patterns of susceptibility to four neonicotinoid insecticides along an urban-to-rural gradient in Yaoundé, Cameroon
Neonicotinoids are potential alternatives for controlling pyrethroid-resistant mosquitoes, but their efficacy against malaria vector populations of sub-Saharan Africa has yet to be investigated. The aim of the...
Progress towards malaria elimination in the Greater Mekong Subregion: perspectives from the World Health Organization
Malaria remains a global health challenge, disproportionately affecting vulnerable communities. Despite substantial progress, the emergence of anti-malarial drug resistance poses a constant threat. The Greater...
Correction: Testing and treatment for malaria elimination: a systematic review
The original article was published in Malaria Journal 2023 22 :254
Malaria elimination and the need for intensive inter-country cooperation. a critical evaluation of regional technical co-operation in Southern Africa
Malaria elimination requires closely co-ordinated action between neighbouring countries. In Southern Africa several countries have reduced malaria to low levels, but the goal of elimination has eluded them thu...
Safety and efficacy of pyronaridine–artesunate paediatric granules in the treatment of uncomplicated malaria in children: insights from randomized clinical trials and a real-world study
Children are particularly at risk of malaria. This analysis consolidates the clinical data for pyronaridine–artesunate (PA) paediatric granules in children from three randomized clinical trials and a real-worl...
Sibling species of the major malaria vector Anopheles gambiae display divergent preferences for aquatic breeding sites in southern Nigeria
When integrated with insecticide-treated bed nets, larval control of Anopheles mosquitoes could fast-track reductions in the incidence of human malaria. However, larval control interventions may deliver suboptim...
How using light touch immersion research revealed important insights into the lack of progress in malaria elimination in Eastern Indonesia
By 2022, the Government of Indonesia had successfully eliminated malaria in 389 out of 514 districts but continues to face a challenge in Eastern Indonesia where 95% of the total 2021 malaria cases were report...
Competency of malaria laboratory diagnosis at national and provincial levels at the beginning of malaria post-elimination phase, China
Qualified malaria diagnosis competency has contributed to the great achievement of malaria elimination in China. After eliminating malaria, it is still critical to the prevention of re-establishment of malaria...
Bayesian spatio-temporal analysis of malaria prevalence in children between 2 and 10 years of age in Gabon
Gabon still bears significant malaria burden despite numerous efforts. To reduce this burden, policy-makers need strategies to design effective interventions. Besides, malaria distribution is well known to be ...
Radical cure for Plasmodium vivax malaria after G6PD qualitative testing in four provinces in Cambodia, results from Phase I implementation
Cambodia aims to eliminate all forms of malaria by 2025. In 2020, 90% of all malaria cases were Plasmodium vivax. Thus, preventing P . vivax and relapse malaria is a top priority for elimination. 14-day primaquine...
Genetic differentiation of Plasmodium vivax duffy binding protein in Ethiopia and comparison with other geographical isolates
Plasmodium vivax Duffy binding protein (PvDBP) is a merozoite surface protein located in the micronemes of P. vivax . The invasion of human reticulocytes by P. vivax merozoites depends on the parasite DBP binding ...
Risk factors for non-participation in ivermectin and dihydroartemisinin-piperaquine mass drug administration for malaria control in the MASSIV trial
Mass Drug Administration (MDA) has become a mainstay for the control of several diseases over the last two decades. Successful implementation of MDA programmes requires community participation and can be threa...
Correlative light-electron microscopy methods to characterize the ultrastructural features of the replicative and dormant liver stages of Plasmodium parasites
The infection of the liver by Plasmodium parasites is an obligatory step leading to malaria disease. Following hepatocyte invasion, parasites differentiate into replicative liver stage schizonts and, in the case ...

Trend analysis of malaria surveillance data in West Wallaga, West Oromia, Ethiopia: a framework for planning and elimination
Although Ethiopia has made a remarkable progress towards malaria prevention and control, malaria remains one of the most devastating parasitic diseases affecting humans. However, the distribution and transmiss...
Making the most of malaria chemoprevention
Against a backdrop of stalled progress in malaria control, it is surprising that the various forms of malaria chemoprevention are not more widely used. The World Health Organization (WHO) has recommended sever...
Time series analysis of malaria cases to assess the impact of various interventions over the last three decades and forecasting malaria in India towards the 2030 elimination goals
Despite the progress made in this decade towards malaria elimination, it remains a significant public health concern in India and many other countries in South Asia and Asia Pacific region. Understanding the h...
Using serological diagnostics to characterize remaining high-incidence pockets of malaria in forest-fringe Cambodia
Over the last decades, the number of malaria cases has drastically reduced in Cambodia. As the overall prevalence of malaria in Cambodia declines, residual malaria transmission becomes increasingly fragmented ...
Classification and clinical significance of immunogenic cell death-related genes in Plasmodium falciparum infection determined by integrated bioinformatics analysis and machine learning
Immunogenic cell death (ICD) is a type of regulated cell death that plays a crucial role in activating the immune system in response to various stressors, including cancer cells and pathogens. However, the inv...
Evaluation of the malaria case surveillance system in KwaZulu-Natal Province, South Africa, 2022: a focus on DHIS2
South Africa set a target to eliminate malaria by 2023, with KwaZulu-Natal (KZN) Province the malaria-endemic province closest to achieving this goal. Objective two of the National Malaria Elimination Strategi...
A machine learning approach for early identification of patients with severe imported malaria
The aim of this study is to design ad hoc malaria learning (ML) approaches to predict clinical outcome in all patients with imported malaria and, therefore, to identify the best clinical setting.
Two promising candidates for paratransgenesis, Elizabethkingia and Asaia , increase in both sexes of Anopheles gambiae mosquitoes after feeding
The male mosquito microbiome may be important for identifying ideal candidates for disease control. Among other criteria, mosquito-associated symbionts that have high localization in both male and female mosqu...
Epigenetic regulation as a therapeutic target in the malaria parasite Plasmodium falciparum
Over the past thirty years, epigenetic regulation of gene expression has gained increasing interest as it was shown to be implicated in illnesses ranging from cancers to parasitic diseases. In the malaria para...
Evaluation of the malaria elimination programme in Muara Enim Regency: a qualitative study from Indonesia
Malaria remains an enduring public health concern in Indonesia, exacerbated by its equatorial climate that fosters the proliferation of Anopheles mosquitoes. This study seeks to assess the performance of the mala...
Artificial nighttime lighting impacts Plasmodium falciparum mature stage V gametocytes infectivity in Anopheles stephensi
Malaria is one of the most important vector-borne diseases of humans with an estimated 241 million cases worldwide in 2020. As an urban and periurban mosquito species, Anopheles stephensi is exposed to artificial...
Assessing availability, prices, and market share of quality-assured malaria ACT and RDT in the private retail sector in Nigeria and Uganda
An estimated 50% of suspected malaria cases in sub-Saharan Africa first seek care in the private sector, especially in private medicine retail outlets. Quality of care in these outlets is generally unknown but...
Effectiveness of artemether–lumefantrine for treating uncomplicated malaria in low- and high-transmission areas of Ghana
Artemisinin-based combination therapy (ACT) has been effective in the supervised treatment of uncomplicated malaria in Ghana. Since ACT usage is primarily unsupervised, this study aimed to determine the effect...
From efficacy to effectiveness: a comprehensive framework for monitoring, evaluating and optimizing seasonal malaria chemoprevention programmes
Seasonal Malaria Chemoprevention (SMC) is a highly effective intervention for preventing malaria, particularly in areas with highly seasonal transmission. Monitoring and evaluating (M&E) SMC programmes are com...
Genetic analysis and molecular basis of G6PD deficiency among malaria patients in Thailand: implications for safe use of 8-aminoquinolines
It was hypothesized that glucose-6-phosphate dehydrogenase (G6PD) deficiency confers a protective effect against malaria infection, however, safety concerns have been raised regarding haemolytic toxicity cause...
Forest-goers as a heterogeneous population at high-risk for malaria: a case–control study in Aceh Province, Indonesia
A major challenge to malaria elimination is identifying and targeting populations that are harbouring residual infections and contributing to persistent transmission. In many near-elimination settings in South...
Molecular surveillance of Kelch 13 polymorphisms in Plasmodium falciparum isolates from Kenya and Ethiopia
Timely molecular surveillance of Plasmodium falciparum kelch 13 ( k13 ) gene mutations is essential for monitoring the emergence and stemming the spread of artemisinin resistance. Widespread artemisinin resistance,...
msp1, msp2, and glurp genotyping to differentiate Plasmodium falciparum recrudescence from reinfections during prevention of reestablishment phase, Sri Lanka, 2014–2019
Sri Lanka after eliminating malaria in 2012, is in the prevention of re-establishment (POR) phase. Being a tropical country with high malariogenic potential, maintaining vigilance is important. All malaria cas...
Emergence of Plasmodium falciparum strains with artemisinin partial resistance in East Africa and the Horn of Africa: is there a need to panic?
The emergence and spread of artemisinin partial resistance in East and Horn of Africa is alarming. However, artemisinin-based combination therapy (ACT) generally remains efficacious for the treatment of falcip...
A quasi-experimental study to estimate effectiveness of seasonal malaria chemoprevention in Aweil South County in Northern Bahr El Ghazal, South Sudan
Seasonal malaria chemoprevention (SMC) is an effective intervention to prevent malaria in children in locations where the burden of malaria is high and transmission is seasonal. There is growing evidence sugge...
Analysing the six-year malaria trends at Metehara Health Centre in Central Ethiopia: the impact of resurgence on the 2030 elimination goals
Despite Ethiopia’s concerted efforts to eliminate malaria by 2030, the disease continues to pose a significant public health and socioeconomic challenge in the country. The year 2021 witnessed 2.78 million mal...
Impact of a spatial repellent intervention on Anopheles kdr insecticide resistance allele in Sumba, Indonesia
The emergence of insecticide resistance and outdoor transmission in malaria-endemic areas underlines the urgent need to develop innovative tools, such as spatial repellents (SR), that may circumvent this resid...
Post hospital admission blood lactate measurements are associated with mortality but not neurologic morbidity in children with cerebral malaria
In children with cerebral malaria (CM) admission blood lactate has previously guided intravenous fluid therapy and been validated as a prognostic biomarker associated with death. The usefulness of post-admissi...

- Editorial Board
- Manuscript editing services
- SNAPP Editor link
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 3.0 - 2-year Impact Factor 3.2 - 5-year Impact Factor 1.148 - SNIP (Source Normalized Impact per Paper) 1.237 - SJR (SCImago Journal Rank)
2023 Speed 7 days submission to first editorial decision for all manuscripts (Median) 131 days submission to accept (Median)
2023 Usage 4,093,320 downloads 5,053 Altmetric mentions
- More about our metrics
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Loading metrics
Open Access
Peer-reviewed
Research Article
Rethinking malaria: Governance lessons from other disease programs
Contributed equally to this work with: Kelechi Ohiri, Ifeyinwa Aniebo, Oluwafunmilayo Akinlade
Roles Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – review & editing
* E-mail: [email protected]
Affiliations Health Strategy and Delivery Foundation, Abuja, Nigeria, Harvard T. H Chan School of Public Health, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing
Roles Data curation, Investigation, Validation, Writing – review & editing
Affiliations Health Strategy and Delivery Foundation, Abuja, Nigeria, Department of Emergency Medicine, University of Virginia, Charlottesville, Virginia, United States of America
- Kelechi Ohiri,
- Ifeyinwa Aniebo,
- Oluwafunmilayo Akinlade

- Published: September 27, 2022
- https://doi.org/10.1371/journal.pgph.0000966
- Reader Comments
The global disruptions brought about by the COVID-19 pandemic as well as the stagnation of progress of global malaria elimination efforts have provided an opportunity to rethink several aspects of the global malaria program, including its governance at all levels, from the community to the nation and to the world. Approaching this issue requires an examination of the critical governance factors that affect malaria elimination as well as lessons that could be learned from the governance of other global health programs. The paper, therefore, first reviews malaria program governance challenges at the global, national, and sub-national levels. We then conducted a literature review of governance factors that affected four major global disease elimination programs; (1) the global smallpox eradication program; (2) polio eradication efforts (focus on Latin America); (3) the onchocerciasis eradication program; and (4) global COVID-19 pandemic control efforts. Based on this review, we identified eight comment governance themes that impact disease elimination programs. These include 1) International support and coordination; 2) Financing; 3) Data use for engagement and decision making, 4) Country ownership; 5) National program structure and management, 6) Community support/engagement; 7) Multisectoral engagement; and 8) Technology and innovation The paper then illustrates how these eight governance themes were factored in the four disease control programs, draws lessons and insights about the role of governance from these programs and outlines the implications for governance of malaria elimination efforts. The paper concludes by making recommendations for improving governance of malaria elimination programs and how the analyses of other global disease control programs can provide new ideas and inspiration for a more robust push towards malaria eradication.
Citation: Ohiri K, Aniebo I, Akinlade O (2022) Rethinking malaria: Governance lessons from other disease programs. PLOS Glob Public Health 2(9): e0000966. https://doi.org/10.1371/journal.pgph.0000966
Editor: Srinivasa Rao Mutheneni, CSIR-Indian Institute of Chemcial Technology, INDIA
Received: March 7, 2022; Accepted: August 3, 2022; Published: September 27, 2022
Copyright: © 2022 Ohiri et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are in the manuscript and/or Supporting information files.
Funding: Support for this global engagement was provided by Harvard University’s Defeating Malaria: From the Genes to the Globe Initiative and the Takemi Program in International Health at the Harvard T.H. Chan School of Public Health. Additional grant support was received from the Bill & Melinda Gates Foundation (INV-032429) and the JC Flowers Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In May 2015, the World Health Organization (WHO), through its Global Technical Strategy for Malaria 2016–2030 [ 1 ], provided a comprehensive framework to countries and development partners to scale up malaria responses and move towards elimination. This document set the target of reducing global malaria incidence and mortality rates by at least 90% by 2030. However, since 2015, the reduction in the global burden of malaria appears to have stagnated with only marginal annual reductions in the case burden. There has also been a slowing of the rate of decline of malaria case incidence (cases per 1000 population at risk) [ 2 ]. As progress stalled, the global community began recognizing the need to rethink the approach to malaria elimination, culminating in the WHO calling for an ‘aggressive new approach’ in the 10+1 countries with the highest malaria burden: the “High burden to high impact” country-led approach [ 3 ].
The Covid-19 pandemic also created another major obstacle to progress in reducing the global malaria burden, particularly in its diversion of human and financial resources essential for malaria services and interventions towards combating the pandemic. On the other hand, the pandemic also provides an opportunity to rethink the approach to malaria and learn from other programs that have successfully put in place, governance structures and processes in the control, eradication, or elimination of infectious diseases such as smallpox, polio, and onchocerciasis. Although these programs have different disease dynamics and interventions, there may be relevant and useful governance lessons that could be applied to the global malaria elimination program, since these programs have financial, political, administrative, and operational similarities. It is worth mentioning that whilst not all the disease programs this paper considers, successfully achieved global elimination/eradication (e.g., onchocerciasis, Covid-19), we believe there are lessons about the governance of these disease programs that the malaria elimination program would benefit from.
In this paper, we investigate governance issues that affect malaria elimination efforts by reviewing and identifying factors that can strengthen malaria program governance at the global, national and sub-national levels. The paper then presents the literature review and methods for learning key lessons from four other global disease control programs, and how eight common governance themes were identified. We then illustrated the impacts of eight themes in the four disease control programs and the implications for malaria elimination efforts. The paper concludes with a few recommendations about how these governance lessons can be used to strengthen malaria elimination globally.
Malaria governance challenges
Governance in the health sector commonly refers to the use of formal and informal institutions, processes and rules by states, nonstate actors and intergovernmental organizations to manage challenges to improving health conditions [ 4 ]. The governance of malaria control and elimination typically involves many different players, and can result in competition for leadership, influence, and resources at the global, national and community levels. We briefly review some of the challenges at these three levels.
At the global level, the number and variety of global health problems on foreign policy agendas has increased and continues to expand [ 5 ]. This creates two main issues for global health governance. First, global health problems generate different levels of interest from countries and development partners. Countries tend to be more interested in problems that directly threaten their interests. This pattern can be seen in the level of attention given to direct, cross-border transmission of dangerous communicable diseases such as Ebola. On the other hand, diseases that do not involve such transmission (including noncommunicable diseases) are perceived to get less attention. Secondly, the need to prioritize resources and responses may create a zero-sum scenario, often resulting in disagreements about how priorities are established [ 6 ] and complaints about some disease programs getting a disproportionate share of attention and resources. It is not surprising that this paper is being written against a background of perceived diversion of attention and resources to combating the COVID-19 pandemic. Whilst malaria gets more attention on the global agenda than neglected tropical diseases, it does not get as much attention as HIV/AIDS or COVID-19. In fact, in West Africa, for example, donor support for malaria is seen to be waning [ 7 ].
At the country level, the governance of malaria can have a direct impact on elimination of the disease. In malaria endemic countries, the National Malaria Control/Elimination Program (NMCP/NMEP) is responsible for developing malaria policies and strategies and provides technical leadership for the Ministry of Health (MOH) with respect to malaria prevention and control [ 8 ]. Organizational structure (administrative location), the effectiveness of administrative processes (earmarking and financial control), and strong leadership (assertion of state ownership and resourcefulness of leaders in overcoming bottlenecks) appear to influence the performance of malaria programs [ 9 ]. In addition, the financing dynamics, particularly the balance (or lack thereof) between donor and domestic funding, may have an impact on the level of alignment of such funds with country’s needs and priorities. Recipient countries often have restricted autonomy over donor resource allocation (which could be quite significant and influential), hence limited power to make decisions on how best to use donor resources to implement malaria programs in their own countries [ 7 ].
At the community level, the main challenge is the level of ownership the community has over malaria programs. This affects how communities respond to the implementation of policies. When the views of the community, who are the primary participants of policy implementation, are not fully considered during policy development, they are less likely to take ownership of the interventions during implementation [ 10 ]. For example, communities may accept free Long-Lasting Insecticidal Nets (LLINs) but not use them correctly. Most successful public health programs have involved significant community engagement in co-creation and involvement in implementation.
To better identify and analyze governance challenges in malaria elimination programs, this paper examined the governance experiences of other global disease control programs, and then sought to identify lessons for malaria governance, globally and within countries For the purpose of this paper, we examined four disease programs: 1) the global smallpox eradication program; (2) polio eradication efforts (with a focus on Latin America); (3) the onchocerciasis eradication program; and (4) global COVID-19 pandemic responses.
For the analysis, we first conducted a literature search on each disease control program and identified pivotal papers and publications that discuss governance. The search was conducted on Pubmed for the four diseases of interest. The original search utilized terms synonymous with all four diseases. Using smallpox for example, the search utilized Smallpox OR “small-pox” OR “variola major” “variola minor” OR “orthopoxvirus” OR “pox” ( Table 1 ). This was repeated for polio, Onchocerciasis and Covid-19 ( Table 2 ). The search also utilized the following terms: Eradication OR elimination OR success OR program OR lessons learnt OR case study OR disease control OR disease eradication OR disease elimination OR leadership OR programmatic OR governance, and interview OR “focus group” OR qualitative for all disease programs.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0000966.t001
https://doi.org/10.1371/journal.pgph.0000966.t002
The search terms could appear anywhere in the full text of the paper (including title, abstract, text, keywords), and limits were applied by date (1980-August 2021 for smallpox, 1999- August 2021 for Polio, 1958- August 2021 for onchocerciasis, and January 2020- August 2021 for Covid-19). Only papers reporting research on human subjects in English language were included. The search was carried out by two independent reviewers.
Coupling the search terms for all four diseases ( Fig 1 ) identified 612 articles. After review of titles and abstracts, 522 articles were considered potentially relevant and retrieved for full text review. 90 articles were considered relevant to the objectives of this review. 14 additional relevant articles were identified either from a simple desk search or reference lists of retrieved articles. These searches collectively provide information that assist in the understanding of governance themes for all four diseases, and highlights lessons learnt from these disease programs which the malaria elimination program could learn from.
https://doi.org/10.1371/journal.pgph.0000966.g001
We then examined the papers to identify common governance themes throughout the papers for the four disease program areas of interest. Eight governance themes that were common across the disease programs. ( Table 1 ) presents the eight themes and illustrates each theme with examples from the four disease control programs.
Our analysis of these four disease programs recognizes that they differ in many ways. For instance, they involve different pathogens (some are viruses, whilst some bacteria), they affect different geographic regions/populations, and some are yet to be fully eradicated (such as onchocerciasis and COVID-19) Nonetheless, we believe that there are still governance lessons to draw from these programs that could be applicable to the malaria elimination efforts. The next section presents key lessons for each of the eight governance themes, with illustrations from the four disease control programs and proposed implications for malaria efforts.
Results: Key lessons for the eight governance themes
1. international support and coordination.
One of the main features of these programs was the high level of international collaboration, advocacy and support that galvanized the world to prioritize and tackle these issues. International coordination was considered important to avoid “ping-pong smallpox” [ 12 ] in which infections would be continually reintroduced from country to country. The smallpox program survived and was successful in part because it had international support and strong backing from the major powers of the era, the United States, and the Soviet Union [ 13 ]. There was no common pattern to the origin of such international support, beyond the presence of an influential global leader who made the programs part of their legacy. In the case of smallpox, the eradication effort coincided with the reemergence of the Soviet Union on the global scene and the opportunity to exercise some soft power, through the then Deputy Minister of Health. It helped that the United States was also fully engaged, and its presidents emerged as champions for these causes. (For example, President Roosevelt created the March of Dimes to support polio eradication and President Johnson sought to lead efforts by the UN and provided support to the smallpox eradication.) In other instances, it was technocrats, such as World Bank President Robert McNamara, who supported the onchocerciasis program after a visit to Burkina Faso in 1972.
With regards to COVID-19, we have seen both some degree of global solidarity–through the establishment of the COVAX facility–but also a lack of global cooperation through increasing vaccine nationalism. This suggests three lessons about international support and collaboration: Firstly, the global champion or influencer plays a critical role by promoting and pushing international cooperation as a legacy. Secondly, global efforts need to be anchored within a multilateral organization (such as WHO or the World Bank) or regional organizations such as the Africa CDC [ 14 ] to convene the best minds and to organize operations to achieve this goal in a short to medium term. Thirdly, global collaboration is critical for success (over public health nationalism).
Implications for malaria.
The global malaria program needs to identify a global champion (perhaps a world leader or head of an influential global organization) who can accelerate and promote elimination as a global priority. Questions that the global malaria community need to reflect on include: Would malaria benefit from a global political champion? Would the focus on elimination or eradication resonate better politically than more nuanced approaches e.g., control? These should be considered in the next global strategy.
2. Financing
Closely linked to global advocacy is international and domestic resource mobilization to support the global efforts at disease control and elimination. There was international financial support for smallpox, polio, and onchocerciasis from a combination of players in global health, ranging from multilateral institutions to the private sector. For example, in the case of smallpox, in addition to the countries committing resources to the eradication effort, there was significant resource mobilization by the international community. The WHO provided a dedicated smallpox funding in 1967 which incentivized countries to scale up their national programs [ 15 ]. The World Health Assembly (WHA) committed to a minimum annual spend over 10 years, and the US committed 5-year financing. However, domestic resources from countries with smallpox also played a large role, as more than two-thirds of the financing between 1967 and 1978 came from endemic countries. A similar situation occurred in Latin America’s polio eradication program, where endemic countries contributed $74 million of the $120 million spent in the first five years of the program. The program received financial and logistical support from partners such as the WHO, UNICEF, CDC, the Task Force for Global Health, Rotary International, and Gavi [ 16 ], which facilitated advocacy and social mobilization. With the COVID-19 pandemic, the world has witnessed unprecedented resource mobilization for the health response, as well as for financing to cushion the impact on the economy (micro and macro). Most of this is at the national level, but internationally, a lot of financing has also been mobilized [ 16 ].
Key questions for malaria (for both donors as well as national governments of endemic countries) are: whether current funding is enough, given the global burden; whether current funding levels can be sustained, given other demands; and if current funding is being effectively utilized? These questions require coherent and persuasive responses from the global malaria community. For example, malaria programs today frequently experience challenges with expenditure, including delays. Grants from the Global Fund to Fight AIDS, Tuberculosis, and Malaria are not spent on schedule in many countries due to various reasons, such as weak data systems, delays in procurement, and lack of human resources. The smallpox eradication program created a flexible fund to address implementation bottlenecks in endemic countries as they arose. This method could be applied to malaria elimination programs, provided there is sufficient transparency and accountability to ensure that funds are spent for their intended function. Investment in local manufacturing as a means of reducing dependence on donor-funded commodities (such as bed nets) may also need to be considered.
Efforts must also be made to reduce the cost of eradicating malaria and make it more affordable. One reason for the pivot away from earlier efforts (in the 1960s) at malaria eradication to smallpox was the cost of the program per person. According to a reported interview with D.A. Henderson, the malaria program accounted for over 20% of all funds available to WHO in the 1960s [ 17 ]. This was perceived as unsustainable as it resulted in less funding being available for other programs, coupled with the realization that eradication would be more costly and take longer than planned. The onchocerciasis eradication program on the other hand, cost $1 per person protected, and the smallpox vaccine cost 1–2 cents per dose.
3. Country ownership
Independent actions by countries to test many approaches simultaneously across different sociocultural and epidemiological contexts was an important success factor for other disease control programs. For example, the global smallpox eradication effort was built on leadership and support from WHO, but in practice was a collection of individual national programs attempting to solve their own problems through their own systems and in their own ways [ 18 ]. Experimental learning rather than formalized programming was encouraged, and this facilitated the identification of local solutions. This is somewhat different from the way donor financing for several malaria programs currently operate. Smallpox’s profile within the WHO was maintained, and countries were encouraged to contribute funding and resources. The annual meeting of the WHO assembly was an important opportunity to keep eradication on the minds of health ministers [ 19 ] and surveillance reports with summaries of progress and problems was used to maintain the public profile of the disease. The smallpox eradication effort was successful also because it was a collection of individual national programs, each contextualizing solutions to their own [ 20 ], rather than a top-down, centrally managed approach [ 21 ].
Malaria endemic countries need to be encouraged to test various context-appropriate strategies while encouraging adoption of proven best practices. Although current malaria guidance embraces the belief that adapting and tailoring interventions to the local context is important for elimination success [ 22 ], the reality often does not match the rhetoric. Resources are deployed in ways that result in the recipient countries not having full autonomy over malaria policy and resource allocation; therefore, they cannot make decisions on how best to implement malaria prevention, diagnosis, and treatment in their own countries [ 7 ]. The existence of multiple players in malaria at the global level also contributes to competition for leadership, influence, and resources at the national level [ 23 ]. Country ownership is important, for example, Zambia takes ownership, makes decisions, and provides evidence to the global entity to change policy. One of the reasons for this is the maturity and strength of Zambia’s NMEP, which enables its staff to make decisions. This is emphasized in the country’s creation of a technical working group formed to avoid clashes in governance that may occur between partners at the global and national level. In situations where the technical working group’s decisions are challenged or pushed back by partners at the global level, the malaria manager makes the final decisions.
4. National program structure and management
Successful disease programs have strong management, integration in the national health system, and buy-in by top political decision makers. These programs also integrated their control structures within the country’s health systems in ways that strengthened national systems. Successful execution of the smallpox program, for example, was said to consist of 10% technical skill and 90% organization and leadership skills [ 24 ], with its approach to certain interventions such as contact tracing often described as ‘military-like’. Smallpox eradication had problem-solving staff with reputations for adaptability, imagination, and hard work; they served as catalysts, rather than controllers, and strong managers and operations officers were hired to ensure execution. Some factors were important for elimination of smallpox. First, smallpox programs were integrated with basic health systems, which allowed case management and surveillance to occur on a routine basis [ 13 ]. Second, smallpox programs had staff who were creative problem-solvers [ 21 , 25 ], and who could figure out how to overcome any obstacle that arose, thereby adapting solutions challenges faced [ 15 ]. Third, the smallpox program highlighted the importance of strong management in all aspects of the program [ 26 ]. The polio eradication initiative was also used to strengthen national immunization programs in Latin America. Some successful disease control programs (including COVID-19 responses) have leveraged proximity to top political leaders effectively, for instance in Nigeria, there was a Presidential task force on Polio. Most National Malaria programs are currently housed within departments in the MoH, which constrains their ability to galvanize political support and multisectoral action.
The management and leadership skills of National Program Managers need to be strengthened for successful program implementation. NMCP/NMEP managers need to have the right level of skills and visibility to be effective, including engaging with communities, problem solving, and creating context-appropriate solutions to problems that may arise. Managers usually don’t have enough training on leadership/management, and most are put in new positions based on their technical expertise and experience.
5. Community engagement
Community engagement and participation were critical for these global disease programs. Top-down approaches alone, have limited effectiveness. For the polio eradication program, health worker mobilization played an impactful role in providing human resources that went house-to-house in communities with existing polio cases or had low coverage [ 27 ] . Community participation with the smallpox program was considered to be strong [ 6 ]. Gaining the support of the community leaders was an important step towards community acceptance. Polio and smallpox efforts in Nigeria, for example, were successful because community/religious leaders trusted by communities were enlisted and engaged as part of the program [ 28 ]. For the APOC program, extensive community engagement and involvement in the implementation of Community-Directed Treatment with Mectizan (ComDT) contributed to its success [ 29 ]. Engaging the community should not be limited to a specific disease program but involve building capabilities to provide broader health services. In the smallpox eradication program, there were combined mobilization efforts with other community initiatives (e.g., neonatal care). For the polio eradication program, the training the community volunteers received included training on disease surveillance and cold chain management.
Malaria programs should engage communities and community leaders in ways that complement existing top-down approaches such as campaigns to distribute nets. Communities need to understand and own the issues and the interventions. For instance, do communities understand and own vector control mechanisms to destroy breeding sites in their environment? Do communities also understand and own the goal of malaria elimination? There should also be continuous communication and collaboration with communities as real partners in the conceptualization, design, and implementation of malaria elimination programs.
6. Data use for engagement and decision-making
The availability of real-time, high-quality data for surveillance and monitoring was a critical success factor for the disease eradication programs. In the polio eradication program, over 20,000 facilities were included in the surveillance network and an emphasis was placed on surveillance to track outbreaks, facilitated by the surveillance system’s computerization [ 27 ]. In the APOC program, epidemiological mapping techniques were used to map 12,000 miles of rivers for the program [ 29 ]. The COVID-19 response also effectively leveraged technology and data. Real time epidemiological data was used to efficiently align program strategy and deploy interventions in many countries. The smallpox program used surveillance data to seek out cases and then vaccination efforts were concentrated to those in their proximity and their contacts [ 30 ]. The surveillance strategy helped focus vaccination on the places where it was most likely needed, rather than laboring to achieve implausibly perfect coverage everywhere. This contributed to eradication’s ultimate success [ 31 , 32 ]. Data were also used effectively to engage the population and various stakeholder groups in a simple and compelling manner. For instance, the COVID response programs in different countries used simple dashboards that were updated daily, to inform and engage citizens on the evolution of the pandemic, the progress made, and risks, for instance, epidemiological assessments informed the control measures that were implemented and the epidemic in Wuhan was under control within 100 days [ 33 ].
Malaria programs need to provide more frequent high quality malaria data at the national, state and community levels, and to use data to engage stakeholders and target interventions. Malaria programs should focus more on impacts and outcomes, including more frequent measurements of prevalence and incidence (which are directly linked to eradication) and perhaps less on outputs and activities conducted. Such data can be used in better engagement with stakeholders and communities on the status of eradication efforts. Unfortunately, the malaria indicator survey (MIS) is carried out every five years, which is not frequent enough. Performance indicators from programs could also be better targeted, for instance not just on number of nets delivered, but on whether nets are delivered to those most at risk, or if the nets achieve the desired outcome of reductions in malaria prevalence/incidence in the target communities. Questions the global malaria community may need to reflect on include: Can malaria data be used and presented in more engaging ways? To what extent should malaria programs rely heavily on modelling estimates to make decisions? Can we improve surveillance to include genomic data and other high-quality data in real time or with greater frequency?
7. Multisectoral collaboration
Lessons learned from diseases like Covid-19 show multisectoral collaboration for instance between governments and the biopharmaceutical industry via fast tracked regulatory approval process that led to the rapid development and deployment of vaccines was critical to control the spread of infectious diseases as well as mitigate its impact on populations [ 33 , 34 ]. The relevant sectors span healthcare, education, research & development, tourism, and others. Most national COVID-19 responses have been multisectoral in nature, involving coordination of several public sector line ministries as well as the private sector. Pharmaceutical companies have been in public-private collaboration with governments, regulatory agencies, research institutions and international organizations. Other successful programs also involved the private sector, for example, Merck was highly involved in both the APOC and onchocerciasis control programs (OCP) [ 29 ].
Successful malaria elimination programs also involved multisectoral collaboration in their malaria strategic plan. For example, Zambia works with multiple sectors for malaria elimination, such as the mining industry and civil society. In fact, Zambia created a multisectoral ‘end malaria council’ to deepen its multisectoral approach. This involved representatives from various sectors including the private sector and development partners. Other countries might find this multisectoral approach coordination and governance approach useful.
8. Technology and innovation
Innovation played a crucial role in the success of some global programs by transforming the options available for interventions and thereby accelerating disease eradication. In the smallpox program, two innovations were pivotal. One was an inexpensive bifurcated needle that was easy to use and required only a quarter of the vaccine dose normally required [ 29 ]. The second innovation was freeze-dried vaccines that provided fully potent heat-stable vaccines that could be stored for months [ 35 ]. The innovation of the discovery of the drug Mectizan was at the heart of the APOC program [ 29 ]. Successfully eradicating Polio in Latin America and the Caribbean was a global, collaborative feat. Some critical factors for success were international support, the development of the inactivated polio vaccine (IPV), and massive community health worker mobilization [ 16 , 27 ]. In addition, the Smallpox programs relied upon having a stable, reliable, effective vaccine [ 36 ]. In the fight against COVID-19, the rapid, unprecedented development and deployment of vaccines has been the game-changer in the global fight against the pandemic.
Innovations in the available interventions may accelerate attainment of malaria eradication goals. For example, an effective vaccine could be a game-changer–a new malaria vaccine showed about 77 percent efficacy in a small clinical trial among children in Burkina Faso, shows some promise in this regard [ 37 ]. A single-dose antimalarial drug could also radically improve treatment options.
There is no ‘ideal program’ that can be directly compared to the malaria elimination program, as each has contextual issues, success factors and challenges. However, some governance lessons from other programs could provide new ideas and inspiration for a more robust push towards malaria elimination.
Some of these learnings are as follows: Firstly, the role of the sponsor or global champion is important; although the malaria program has many champions, it would benefit from having a global leader who makes this his/her priority and legacy. Secondly, national programs (and the international institutions that support them) must embrace flexibility and efficiency in execution and must be adaptive in their approach at all levels including the way stakeholders such as political leaders, other sectors, and the community are engaged. Thirdly, successful programs highlight extensive community engagement and involvement in the implementation of interventions, including behavioral change modifications. Fourthly, there is an opportunity to rethink the type of data being collected, its frequency, and its use in engaging stakeholders. Lastly, whereas other programs have clear mandates to eradicate the diseases, resulting in a focused, almost binary approach to measuring success–eradicated or not–success for the malaria program seems to be more complex, with eradication, elimination, and control as parallel, simultaneous goals. This may be pragmatic at a national level but may not have the same political resonance as a clear, single focus on global eradication.
Supporting information
S1 text. "rethinking malaria in the context of covid–19," a global engagement organized by harvard university..
https://doi.org/10.1371/journal.pgph.0000966.s001
S2 Text. Synopsis case studies from other programs.
https://doi.org/10.1371/journal.pgph.0000966.s002
S1 Table. Expert panel table comprising list of stakeholders and key experts.
https://doi.org/10.1371/journal.pgph.0000966.s003
S1 Checklist.
https://doi.org/10.1371/journal.pgph.0000966.s004
Acknowledgments
The Working Group on Malaria Governance appreciates the comments from the “Rethinking Malaria in the Context of COVID-19” global engagement governance bodies who met with the Working Group and provided useful suggestions on how to improve this paper. For a list of individuals comprising the Steering Committee, Working Group Co-Chairs and contributing authors, and an External Advisory Committee see the Supporting information section of this paper.
- 1. World Health Organization. A framework to malaria elimination. Geneva: World Health Organization; 2017.
- 2. World Health Organization. World malaria report 2020. Geneva: Global Malaria Programme. World Health Organization; 2020.
- 3. World Health Organization. High burden to high impact: a targeted malaria response. Geneva: World Health Organization; 2018.
- 4. Fidler DP. The challenges of global health governance. New York: Council on Foreign Relations; 2010.
- View Article
- Google Scholar
- PubMed/NCBI
- 8. Novartis. Malaria for Futures for Africa (MalaFA) report. New York: Novartis; 2018.
- 9. Steketee R, Macheso A, Heymann D, Campbell C, McDermott J, McFarland D, et al. A decade of progress in malaria policy and program development in Malawi: 1984–1993. Washington: United States Agency for International Development; 1995.
- 12. Henderson DA. Smallpox: the death of a disease. Amherst: Prometheus Books; 2009.
- 13. Fenner F. Smallpox and its eradication. Geneva: World Health Organization; 1981.
- 14. africa-union.org [Internet]. Addis Ababa; c2021 [cited 2021 September 1]. Kenya: Africa Center for Disease Control. https://africacdc.org/covid-19/ .
- 16. Pedreira C. Efforts and progress towards polio elimination in the Americas and the world. Washington, D.C.: Sabin Vaccine Institute; 2018.
- 17. World Health Organization. The Tashkent Declaration: the move from malaria control to elimination in the WHO European Region–a commitment to action. Copenhagen: World Health Organization Regional Office for Europe; 2006.
- 21. Foege WH. House on fire: the fight to eradicate smallpox. Berkeley: University of California Press; 2011.
- 24. The World Bank. Implementation completion report (IDA-23 170) on a credit in the amount of SDR 95.9 million to the People’s Republic of China for an infectious and endemic disease control project. Washington, D.C.: The World Bank; 2002.
- 25. Dowdle WR, Hopkins DR. The eradication of infectious diseases. Hoboken: Wiley; 1998.
- 27. Center for Global Development. Eliminating polio in Latin America and the Caribbean–Case 5. Washington, D.C.: Center for Global Development; 2020.
- 29. Center for Global Development. Controlling onchocerciasis in sub-Saharan Africa–Case 7. Washington, D.C.: Center for Global Development; 2020.
- 32. Stepan N. Eradication: ridding the world of diseases forever? Ithaca: Cornell University Press; 2011
- 33. who.int [Internet]. Geneva: The World Health Organization; c2021 [cited 2021 September 1]. https://www.who.int/news/item/29-06-2020-covidtimeline .
- 34. cell.com [Internet]. Philadelphia: Elsevier Inc.; c2021 [cited 2021 September 1]. https://www.cell.com/med/pdf/S2666-6340(20)30031-3.pdf .
- 37. Datoo, Mehreen S. et al (2021), High Efficacy of a Low Dose Candidate Malaria Vaccine, R21 in 1 Adjuvant Matrix-M™, with Seasonal Administration to Children in Burkina Faso (April 20, 2021). SSRN: http://dx.doi.org/10.2139/ssrn.3830681
March 15, 2024
Ancient Malaria Genome from Roman Skeleton Hints at Disease’s History
Genetic information from ancient Roman remains is helping to reveal how malaria has moved and evolved alongside people
By Tosin Thompson & Nature magazine
Malaria, an endemic disease caused by hematozoic parasites (Plasmodium falciparum) transmitted by the blood to humans through the bite of the female anophele mosquito.
BSIP SA/Alamy Stock Photo
Researchers have sequenced the mitochondrial genome of the deadliest form of malaria from an ancient Roman skeleton. They say the results could help to untangle the history of the disease in Europe.
It’s difficult to find signs of malaria in ancient human remains , and DNA from the malaria-causing parasite Plasmodium rarely shows up in them. As a result, there had never been a complete genomic sequence of the deadliest species, Plasmodium falciparum , from before the twentieth century — until now. “ P. falciparum was eliminated in Europe a half century ago, and genetic data from European parasites — ancient or recent — has been an elusive piece in the puzzle of understanding how humans have moved parasites around the globe,” says Daniel Neafsey, who studies the genomics of malaria parasites and mosquito vectors at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts.
Malaria has long been a leading cause of human deaths . “With the development of treatments such as quinine in the last hundreds of years, it seems clear [humans and malaria] are co-evolving,” says Carles Lalueza Fox, a palaeogenomicist at the Institute of Evolutionary Biology in Barcelona, Spain. “Discovering the genomes of the ancient, pre-quinine plasmodia will likely reveal information about how they have adapted to the different anti-malarial drugs.”
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Ancient pathogen
There are five malaria-causing species of Plasmodium , which are thought to have arisen in Africa between 50,000 and 60,000 years ago, and then spread worldwide. Most researchers agree that they reached Europe at least 2,000 years ago, by the time of the Roman Empire.
Plasmodium falciparum “has significantly impacted human history and evolution”, says Neafsey. “So, that makes it particularly important to discover how long different societies have had to deal with [it], and how human migration and trade activities spread it.”
Researchers can glean valuable information about the origin, evolution and virulence of the parasite from DNA extracted from the ancient remains of infected people. But it is difficult to know where to look: it is not always obvious whether a person was infected with Plasmodium , and whether DNA can be recovered depends on how well it has been preserved.
In a preprint posted on the server bioRxiv, a team of researchers led by a group at the University of Vienna identified the first complete mitochondrial genome sequence of P. falciparum from the bones of a Roman who lived in Italy in the second century AD, known as Velia-186.
Plasmodium falciparum had been detected in Velia-186 in a previous study. The authors of the latest preprint extracted the parasite’s DNA from the body’s teeth, and were able to identify 5,458 pieces of unique genetic information that they combined to get a sequence covering 99.1% of the mitochondrial genome. They also used software to compare the genome with modern samples, and found that the Velia-186 sequence is closely related to a group of present-day strains found in India.
Carried by migration
The researchers say their findings support a hypothesis that P. falciparum spread to Europe from Asia around at least 2,000 years ago. The Indian strains “were already present in Europe [then]; thus, a potential arrival with globalization episodes such as the Hellenistic period — when it is first described by Greeks — seems plausible”, says Lalueza Fox.
Neafsey says the work is a “technical tour de force” and an interesting addition to the limited field of ancient malaria genomics. But he adds that the results should be interpreted with caution because there are only a few samples, and points out that a genome sequence from DNA in the parasite’s cell nuclei, rather than its mitochondria, “might indicate a more complex story of parasite movement among ancient human populations”.
Lalueza Fox suggests exploring other potential sources of Plasmodium DNA, such as old bones, antique medical equipment and even mosquito specimens in museums. “The integration of genetic data from these heterogeneous sources will provide a nuanced view of this disease,” he says. “It would be interesting to see what lessons we can learn from the past on the strains and dispersals of this pathogen.”
This article is reproduced with permission and was first published on March 13, 2024 .
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Identification and...
Identification and management of co-infections in people with malaria
- Related content
- Peer review
- Aubrey J Cunnington , professor of paediatric infectious disease 1 2 ,
- Aula Abbara , consultant in infectious diseases , infectious diseases and malaria adviser 2 3 ,
- Flavia Kaduni Bawa , PhD candidate 4 ,
- Jane Achan , principal adviser 5
- 1 Section of Paediatric Infectious Disease and Centre for Paediatrics and Child Health, Imperial College London, UK
- 2 Imperial College Healthcare NHS Trust, London
- 3 Médecins sans Frontières OCA, Amsterdam, Netherlands
- 4 West African Centre for Cell Biology of Infectious Pathogens, Department of Biochemistry, Cell and Molecular Biology, University of Ghana, Accra, Ghana
- 5 Global Technical Team, Malaria Consortium, London
- Correspondence to A J Cunnington a.cunnington{at}imperial.ac.uk
What you need to know
Co-infections with malaria affect up to half of children in endemic countries and around one in seven travellers with malaria
A positive diagnostic test does not mean malaria is the only, or even a contributing, cause of current illness
In settings where resources are constrained, limited diagnostic capacity can influence the diagnosis of co-infections, so vigilance is required for clinical features atypical for malaria
A 16 year old Ugandan girl is brought to the emergency department with a three day history of fever, headache, cough, and myalgia. She has had several episodes of malaria in the past. On admission, she is febrile, tachycardic, tachypnoeic, and has oxygen saturations of 90% in air. A malaria rapid antigen test is positive for Plasmodium falciparum and a chest radiograph shows left sided pneumonia. She is admitted and treated with antimalarials, antibiotics, and oxygen. She makes a full recovery over five days. At discharge, the cause of the pneumonia and the contribution of malaria to the illness remain unresolved.
Introduction
Malaria is the symptomatic illness caused by the mosquito transmitted parasites Plasmodium falciparum , P vivax , P ovale , P malariae , and P knowlesi . It is one of the most common causes of fever in many malaria endemic countries and in travellers returning from those countries. 1 The World Health Organization estimated 249 million malaria cases in 2022 worldwide, 94% attributable to P falciparum infections in Africa, where children have the greatest burden of severe disease. 2
In patients who have evidence of acute or recent malaria infection, co-infections with other pathogens occur commonly. 3 In this article, we consider the challenges of diagnosing bacterial, viral, and parasitic co-infection in patients who have malaria, and the related challenge of attributing illness to malaria in endemic countries. We focus on how to assess and manage co-infection in children with severe P falciparum malaria in sub-Saharan Africa (who account for most deaths from malaria globally) and in travellers of all ages with imported malaria who present in non-endemic countries (where all age groups are at risk of severe illness). We do not focus on malaria endemic countries outside Africa, or non-falciparum malaria.

Does detection of malaria parasites always indicate a diagnosis of malaria?
Individuals living in malaria endemic areas can acquire “clinical immunity” to malaria through repeated infections, enabling persistent asymptomatic parasitaemia. 4 The age at which this tolerance is acquired depends on the frequency of exposure. In some African countries with high malaria transmission, asymptomatic P falciparum parasitaemia can be found in up to 80% of school age children 2 and symptomatic malaria is uncommon in adults. It is likely, therefore, that co-infection with non-malarial illnesses in these populations will be accompanied by incidental malaria parasitaemia.
How common are co-infections?
Although comprehensive data are lacking, co-infections are probably very common. 3 Prevalence is higher in populations living in malaria endemic countries than in those where malaria is imported, but estimates depend on how intensively co-infections are sought and availability of diagnostics. In one large observational study of outpatient children in Tanzania undergoing extensive diagnostic evaluation for a spectrum of causes of fever, half of patients with malaria had at least one co-infection. 5 A postmortem study of Malawian children who met diagnostic criteria for cerebral malaria at time of death found an alternative infectious cause of death in at least 19% (6/31). 6 Among imported malaria patients at specialist university hospitals in Italy and Germany, co-infection rates were 13% (9/70) and 16% (41/264), respectively. 7 8
Bacteraemia
Risk of bacteraemic co-infection has been studied extensively. Malaria is thought to increase susceptibility to bacteraemia by impairment of gastrointestinal barrier defences and impairment of immune responses. 9 10 The most commonly reported bacterial co-infections are enteric Gram negative organisms (eg, Salmonella species, particularly non-typhoidal salmonella in African children) and Staphylococcus aureus . 11 12
A large epidemiological study that used mendelian randomisation with malaria protective sickle cell trait to establish causality, provided strong evidence that malaria increases the risk of bacteraemia in Kenyan children, explaining 62% of cases when malaria prevalence was highest. 13 Systematic reviews report a pooled prevalence of bacteraemia in 7.6% (95% confidence interval (CI) 6.7% to 8.7%) of patients with malaria who were tested for bacteraemia, 12 and 6.4% (95% CI 5.8% to 7.0%) in African children with severe malaria, 11 but noted substantial heterogeneity in prevalence between studies.
Large observational studies suggest the prevalence of bacteraemic co-infection is lower in those who do not reside in high malaria transmission settings. Bacteraemia was present in 1% (95% CI 0.4% to 1.8%) of Vietnamese adults with severe malaria, 14 1.4% (3/219) of adult patients with imported malaria at a German university hospital, 8 and 0.3% (2/417) of imported malaria cases in Sweden. 15 Overall rates of bacterial co-infection (including non-bacteraemic infections) were 4.3% (12/291) in Sweden 15 and 11% (29/264) in adults in Germany. 8 Higher rates of bacterial co-infection have been reported in patients with imported severe malaria: 20% (10/49) in German adults 8 and 14% (13/91) in a French intensive care unit. 16
Acute viral co-infections are likely more common than bacterial co-infections, but they are frequently undocumented because of limited diagnostic testing capacity in malaria endemic countries. In outpatient children in Tanzania with malaria, about one third had concomitant viral upper respiratory tract infections or a systemic viral illness. 5 In Malawian children with a clinical diagnosis of cerebral malaria, 35% (27/78) also had a central nervous system viral infection. 17 Conversely, only 5% (14/264) of adults with imported malaria at a German university hospital were found to have a viral co-infection. 8
The overlapping epidemiology of malaria transmission with areas of high prevalence of HIV and chronic hepatitis viruses means that these will also be common viral co-infections. A large cross sectional study in Mozambique, a country with high HIV prevalence and high malaria transmission, found malaria parasites in 33% of adult patients with HIV. 18 Viral haemorrhagic fevers are rare co-infections compared with respiratory viruses and bacteraemia, but can be more common in endemic areas and outbreaks. In an area of Nigeria where Lassa fever is endemic, Lassa virus was identified in 4.6% (4/87) of febrile children with malaria parasitaemia. 19
Mixed infections of P falciparum and non- P falciparum malaria parasites are a common finding in sub-Saharan Africa, particularly when sensitive molecular techniques are used for the detection of non- P falciparum species. One recent study reported mixed infection in 25.8% (523/2027) of outpatients with malaria in Kenya. 20 Helminths (eg, hookworm, roundworm, Schistosoma ) are widely distributed and also common co-infections in many malaria endemic regions, with a pooled prevalence of 17.7% (95% CI 12.7% to 23.2%) in a recent systematic review. 21 Many countries where malaria is endemic are also endemic for systemic parasitic diseases, with clinical features overlapping those of malaria (eg, visceral leishmaniasis, human African trypanosomiasis), and co-infections are well documented in populations with a high overlapping incidence. 22 23
Few data are available on malaria and fungal co-infections, but several case reports documented disseminated aspergillosis following malaria in individuals who were previously healthy, possibly as a result of immune dysfunction related to malaria. 24
Do co-infections influence severity of illness?
The implication of assuming the diagnosis is only malaria can range from insignificant, usually for self-resolving viral co-infections, to severe and life threatening, for treatable invasive bacterial co-infections or viral haemorrhagic fevers.
A systematic review of studies in African children reported a higher pooled case fatality rate (24.1%) in severe malaria with invasive bacterial co-infection than in severe malaria alone (10.2%). 11 Another systematic review reported a mortality rate of 15% (95% CI 8.0% to 23.0%) across all patients with malaria and bacteraemic co-infection. 12 Bacterial co-infection was more common in fatal cases (40%, 4/10) of imported severe malaria than non-fatal cases (11%, 9/83) in a European intensive care unit. 16 Recent estimates suggest up to a third of the malaria deaths in African children may be the result of bacterial co-infection rather than the malaria parasites. 25
Most acute viral co-infections are self-limiting, but incorrect diagnosis can result in missed opportunities to detect, treat, and prevent transmission of more significant viral diseases such as dengue, viral haemorrhagic fevers, or covid-19. The clinical consequences of viral co-infections in individuals with severe malaria and, conversely of malaria co-infection in individuals with severe viral diseases, are less well established. In Malawian children with suspected central nervous system infection, mortality was higher (38%, odds ratio 3.6 (95% CI 1.6 to 8.0)) for children with P falciparum parasitaemia and central nervous system viral infection than in those with parasitaemia alone (14%). 17 Conclusive data for the most common or severe viral infections, including Ebola virus 26 and SARS-CoV-2, are lacking. 27 28
Data on the impact of malaria and co-infections with Leishmania or Trypanosoma on severity of illness and survival are inconclusive. 22 23 Helminths transmitted in soil may contribute to the severity of anaemia associated with malaria. 21
What are the challenges for diagnosing malaria and co-infections?
Presentation.
Malaria usually presents as an acute febrile illness with systemic symptoms such as chills, headache, and body aches. 29 Most clinical features of the disease are indistinguishable from many other systemic febrile illnesses ( table 1 ), including some non-infectious causes. Only one clinical finding, malarial retinopathy, is highly specific for malaria (up to 100% specificity for diagnosis of cerebral malaria 30 ), but it does not exclude co-infection with other pathogens. 17
Clinical features of malaria, severe malaria, and their overlap with other causes of fever in children and adults
- View inline
In box 1 and figure 1 , we outline features of other infections (and selected non-infectious febrile illnesses) which do not usually occur in malaria. Focal symptoms and signs, such as lymphadenitis or unilateral lung crepitations, are not typical of malaria and should prompt consideration of an additional cause. In a setting with low resource healthcare, WHO’s Integrated Management of Childhood Illness guidelines recommend assessing for stiff neck, runny nose, localised tenderness, oral sores, refusal to use a limb, hot tender swelling, red tender skin or boils, lower abdominal pain, pain on passing urine, and signs of measles, which may suggest a diagnosis other than malaria. 31 At the end of this article we highlight additional sources of guidance for evaluation of travellers from malaria endemic countries (box ‘Guidelines’), which should include a detailed history, considering risk factors for other infections and the chronology of illness.
Features in history that may suggest co-infection with other pathogens
Insidious onset, gradual weight loss
Prolonged fever (>7 days)
Profuse vomiting, diarrhoea (including presence of blood or mucus)
Coryza, conjunctivitis, sore throat, stridor, prominent/productive/whooping cough
Focal musculoskeletal symptoms
Rash, skin or mucosal lesions
Strong or foul smelling urine, dysuria
Risk factors
Recent exposure to others with transmissible infections
Presence of HIV, other immunodeficiencies, or immunosuppression
Malnourished state
Presence of sickle cell disease
Congenital or acquired heart disease
Close contact with animals
Positive travel history (including within malaria endemic countries)
Presence of indwelling medical devices (eg, catheters, ventriculoperitoneal shunts) or recent surgery
Drug history (which may modify risk or influence diagnostic test results)
Recent use of antibiotics and antimalarials
Vaccinations
Immunosuppressive medication

Features on physical examination that may suggest malaria is not the only cause of illness. Example non-malarial causes are provided under respective clinical features (in red text). Those in bold are most common. Those in italics are specific to children. MIS-C=multisystem inflammatory syndrome in children
- Download figure
- Open in new tab
- Download powerpoint
Malaria must be confirmed by diagnostic testing, most commonly microscopy for parasites within red blood cells and/or the detection of one or more parasite antigens in blood using lateral flow rapid diagnostic tests (RDTs). 29 A full blood count is also helpful, with thrombocytopenia being a typical finding in malaria. In Africa, common RDTs based on the detection of the parasite antigen PfHRP2 are around 95% sensitive and 95% specific for symptomatic P falciparum malaria, 32 but with caveats:
Sensitivity is diminished in low parasitaemia asymptomatic infections 33
Results from PfHRP2 RDTs can remain positive for several weeks after successful treatment of malaria 29 33 :
They can detect malaria even if treatment was given before testing in the current illness
A false positive test may arise from a previous malaria infection, especially in settings with high transmission rates
Increasingly, false negative PfHRP2 RDT results occur because of deletions of the parasite PfHRP2/3 genes. 33
Current RDTs for malaria have lower sensitivity for non-falciparum parasite species, and their detection by microscopy may be challenging because parasitaemia is often lower than that of P falciparum . 34
Rapid multiplex molecular assays for efficient syndromic testing ( table 2 ) are increasingly available in resource rich settings, 35 but diagnostics for infections other than malaria can be scarce in resource limited settings. 36 Diagnostics for bacterial co-infection usually require the culture of bacteria from sterile site samples before starting antimicrobial therapy. Presenting features and patient age determine appropriate microbiological samples, which can generally be performed in line with context appropriate guidelines for management of fever or sepsis (eg, guidance from WHO 37 or the National Institute for Health and Care Excellence 38 ). Diagnostics for rarer pathogens are often available only in reference laboratories and should be requested only after expert consultation, in parallel with any necessary infection prevention and control processes (box ‘Guidelines’).
Examples of diagnostic tests for co-infections with clinical features overlapping those of malaria
Assessing risk of clinically significant co-infection
To our knowledge, there are no validated prediction rules or prospective studies of risk stratification for clinically significant co-infection in patients with malaria. In a retrospective study of adult patients with imported malaria in Germany, multivariate analysis showed that clinical evidence of an alternative focus of infection was associated with an odds ratio of 3.9 (1.5 to 11.5) for bacterial co-infection, while C reactive protein was not significantly different in those with and without bacterial co-infection. 8
Some risk stratification may be possible based on patient and clinical factors. One large systematic review identified bacteraemia as most common in high transmission settings, in younger children, and in those with severe malarial anaemia. 11 However, retrospective observational studies indicate that laboratory measurements can help to identify two groups of patients who appear to have severe malaria and are at highest risk of bacterial co-infection ( fig 2 ).

Malaria and bacterial co-infection. Bacterial co-infection can occur in individuals with incidental (asymptomatic) parasitaemia, or individuals with symptomatic malaria. In malaria endemic countries it is common for individuals to have asymptomatic parasitaemia. In those sick enough to require admission to hospital, bacterial co-infection is most common among those with the lowest and highest parasite loads. Those with the lowest parasite load are likely to have been asymptomatically infected with malaria parasites and the cause of their illness is more likely to be a bacterial infection. Those with the highest parasite load are most likely to have severe malaria and are at highest risk of bacterial co-infection. *Parasite load is correlated with percentage parasitaemia and parasite density, but these can underestimate the total number of parasites in severe malaria when many parasites are sequestered in the microvasculature. In research settings, P falciparum parasite load is often estimated by quantification of the plasma concentration of the parasite antigen PfHRP2
These include:
Individuals who have incidental parasitaemia and another cause of severe illness, characterised by low parasite load and absence of polymorphonuclear leucocytes containing malaria pigment (determined by microscopy), high white cell count for age, and normal platelet count 39 40 41
Individuals who have true severe malaria with very high parasite load, 42 low platelet count, lower white cell counts, 40 and often >5% of polymorphonuclear leucocytes contain malaria pigment, 39 at increased risk of bacterial co-infection as a direct consequence of their malaria infection.
Malaria parasitaemia is quantified as the percentage of infected red blood cells. Parasitaemia is lowest in asymptomatic infections, intermediate in uncomplicated malaria, and highest in severe malaria, but the groups overlap considerably. 2 In severe P falciparum malaria, many parasites are sequestered in the microvasculature and not visible on blood film. Research studies quantify the total parasite load of circulating and sequestered parasites by using plasma or serum PfHRP2 concentration, which discriminates better between asymptomatic, uncomplicated, and severe groups, 41 42 but these are not available in routine clinical practice. Parasitaemia and PfHRP2 concentrations are only moderately correlated, and their relations with symptomatic or severe disease can vary with age and endemicity, making it challenging to set generalisable risk thresholds. Nevertheless, very high parasitaemia indicates a high parasite load, and in a study of 845 adults with severe malaria in Vietnam, bacteraemia was 8.1 (95% CI 2.2 to 29.5) times more common in those with >20% parasitaemia than in those with lower parasitaemia. 14
Prolonged fever, recurrence of fever, or deterioration after starting antimalarial treatment, all warrant evaluation for acquisition of bacterial infection and antibiotic treatment, as well as consideration of antimalarial resistance.
Consider the potential for viral haemorrhagic fever co-infection in patients from areas where such diseases are endemic (eg, Lassa fever in West Africa) or when outbreaks occur. Test patients with suspected viral haemorrhagic fever for malaria to rule out a treatable co-infection, and consider viral haemorrhagic fever co-infection in patients with malaria to enable appropriate measures of infection control. Risk of viral haemorrhagic fever can be stratified by a detailed travel history, including dates of travel to endemic areas (most have an incubation period under 21 days), exposures, and contacts ( box ‘Guidelines’ ). Risk factors for other significant viral infections may be identified through careful history taking and attention to current epidemiology. In areas with a high prevalence of HIV, it may be appropriate to screen all individuals with severe malaria for HIV.
Parasitic and fungal
Consider significant parasitic or fungal co-infections when the patient has a high risk of exposure or clinical features that are atypical for malaria ( fig 1 ) or which fail to respond fully to antimalarial treatment.
How to manage possible co-infection
Figure 3 shows an algorithm for assessment and management of possible co-infection, based on our experience and in line with international guidelines. 43 44 45 46 47 48 49 50 Our recommendations apply to the management of possible co-infection in children with malaria in sub-Saharan Africa and in travellers with malaria in non-endemic settings. Antimalarial treatment is always indicated in a patient with a positive test for malaria and compatible symptoms, even if a co-infection is suspected or there is uncertainty about whether malaria is causing illness. If the patient has obvious focal infection, empirical treatment is indicated after taking appropriate diagnostic samples.

Suggested algorithm for assessment and management of possible co-infection in patients with malaria. The algorithm is for care of children with malaria in sub-Saharan Africa and travellers of all ages presenting with malaria in non-endemic countries. VHF=viral haemorrhagic fever; IPC=infection prevention and control
In children with malaria in an endemic country:
Initiate antimalarial treatment
Examine and investigate, if possible, for focal bacterial co-infection
Commence broad spectrum antibiotic treatment in all children with severe malaria.
In returning travellers:
Examine and investigate for focal bacterial co-infection
Commence broad spectrum antibiotic treatment in all severely ill children and in adults with signs of shock or respiratory failure.
Consider empirical treatment also for patients with severe illness who have inconsistent clinical or laboratory findings, and those with very high parasitaemia (>20%). Some national guidelines recommend more restrictive approaches to empirical antibiotic treatment, focusing on patients with circulatory shock, respiratory failure, very high lactate. 46 47 48
Treatment with a third generation cephalosporin (eg, ceftriaxone) is likely to be effective against the most common bacterial co-infections, non-typhoidal Salmonella and S aureus, but this may not be feasible for every child with severe malaria in endemic countries because of cost and limited availability. Alternative empirical treatment regimens using gentamicin plus narrower spectrum β lactam antibiotics may not provide adequate cover. Even third generation cephalosporins may sometimes be inadequate because of increasing prevalence of resistant organisms. 43 Some guidelines for imported malaria recommend broader spectrum treatment with piperacillin/tazobactam or carbapenems, plus an aminoglycoside. 47 48
Diagnostics and specific treatments for many viral infections are rarely available outside advanced healthcare facilities. If viral co-infections of public health importance are suspected, such as measles or a viral haemorrhagic fever, take available infection control precautions, and notify appropriate authorities according to local and national procedures. Post-exposure vaccination or immunoglobulin may protect and prevent further spread for specific infections. 51
After stratifying risk for viral haemorrhagic fevers and other transmissible infections, follow standard local infection control policies for patients at low risk. Isolate patients at high risk immediately, and use enhanced personal protective equipment while urgently seeking specialist guidance ( box ‘Guidelines’ ).
Treatment of specific viral co-infections with antiviral agents and/or adjunctive treatments depends on the virus detected.
Treatment of specific parasitic and fungal co-infections depend on the organism. Empirical treatment with albendazole or mebendazole may be given to anaemic children with malaria, if not received in the last six months, to treat soil transmitted helminths.
Areas of uncertainty
What is the burden of different clinically significant co-infections with malaria in different settings and different age groups?
What are their prevalences in patients with a positive malaria test?
Are they more common in patients with malaria than in the general population?
What are their impacts on morbidity and mortality in different settings?
How can we identify patients with a positive malaria test who are at greatest risk of having clinically significant co-infections?
Which additional diagnostic tests for co-infection should be performed in different geographical and healthcare settings?
Which patients with malaria should receive empirical antibiotics?
Which empirical antibiotics are most appropriate in which settings?
What is the impact of giving empirical antibiotics on antimicrobial resistance?
Patient perspective
There are times when I am convinced that I have malaria. During those times, I am okay when I visit the hospital and get tested for malaria and start my antimalarial drugs. There are other times I am convinced it is something else, but then, I still must test for malaria. On these occasions, I become worried because I might be receiving treatment for just a part of my symptoms and risk infecting my family members if the second cause is infectious. Being able to test for different pathogens puts my mind at ease and makes me trust the doctor’s final diagnosis. Although this is more expensive, it saves me from multiple trips to the hospital, and makes me confident in the healthcare system.
Kambe, university student, Ghana
How patients were involved in the creation of this article
Patients were not directly involved in in the writing of this article, but a representative patient story has been included.
How this article was made
We searched PubMed using combinations of the terms: “Malaria”, “Plasmodium”, “Co-infection”, “Coinfection”, and names of specific infections. We supplemented this with personal archives of references, and references within identified articles.
Management of malaria
WHO Malaria management guideline. https://app.magicapp.org/#/guideline/7661
UK Malaria treatment guideline. https://doi.org/10.1016/j.jinf.2016.02.001
Canadian malaria treatment guideline. https://www.canada.ca/en/public-health/services/catmat/canadian-recommendations-prevention-treatment-malaria/chapter-7-treatment.html#a7
French guidelines for management of imported malaria in children. https://doi.org/10.1016/j.medmal.2019.02.005 , and severe imported malaria in adults https://doi.org/10.1016/j.medmal.2018.08.003
Assessment and management of infections acquired in malaria endemic countries
Assessment and initial management of acute undifferentiated fever in tropical and subtropical regions. https://www.bmj.com/content/363/bmj.k4766.long
Fever in the returning traveller. https://www.bmj.com/content/360/bmj.j5773.full
Management of suspected severe infection in children in resource limited settings
WHO pocket book of hospital care for children: Second edition. https://www.who.int/publications/i/item/978-92-4-154837-3
Integrated management of childhood illness for primary health care services. https://cdn.who.int/media/docs/default-source/mca-documents/child/imci-integrated-management-of-childhood-illness/imci-in-service-training/imci-chart-booklet.pdf
Risk assessment and approach to viral haemorrhagic fever
World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers. https://www.who.int/publications/i/item/9789241549608
Assessment of fever in the returning traveller. https://www.bmj.com/content/360/bmj.j5773
UK Advisory Committee on Dangerous Pathogens (ACDP) viral haemorrhagic fever guidance. https://www.gov.uk/government/publications/viral-haemorrhagic-fever-algorithm-and-guidance-on-management-of-patients
United States CDC TOUR (Treat patient, Obtain history, Urine/ blood work, Rule out malaria) approach to risk-assessment for VHF. https://www.cdc.gov/vhf/abroad/assessing-fever-returning-traveler-no-risk-viral-hemorrhagic-fever.html
United States CDC CALM (Consider, Act, Laboratory, Monitor) approach to VHF. https://www.cdc.gov/vhf/abroad/assessing-vhf-returning-traveler.html
Specialist advice on diagnostic testing and management
UK Imported Fever Service. https://www.gov.uk/guidance/imported-fever-service-ifs
Information about outbreaks of infectious diseases around the world
The International Society for Infectious Diseases’ ProMed and HealthMap are useful and up to date resources for current outbreaks. https://www.healthmap.org/en/
Education into practice
How do you assess for additional or alternative infection diagnoses in patients with a positive malaria test?
Of your patients with severe malaria, what proportion had blood cultures taken and received empirical broad spectrum antibiotic treatment?
Competing interests: The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: AC, FKB, and JA are supported by a grant (NIHR134694) from the National Institute for Health and Care Research using UK aid from the UK government to support global health research to study novel diagnostics for malaria and other infections in Africa. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK government. AC is supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 848196 to study novel diagnostics for infectious diseases.
All four authors made substantial contributions to the conception and design of the article, drafting and revising it critically for important intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AC is responsible for the overall content as guarantor.
Consent: The case in this article is fictitious and therefore no consent was needed. The patient story in this article is real and written consent has been obtained.
Provenance and peer review: commissioned; externally peer reviewed.
- D’Acremont V
- ↵ World Health Organization. World Malaria Report, 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 .
- McArdle AJ ,
- Turkova A ,
- Cunnington AJ
- Phillips MA ,
- Burrows JN ,
- Manyando C ,
- van Huijsduijnen RH ,
- Van Voorhis WC ,
- D’Acremont V ,
- Kilowoko M ,
- Taylor TE ,
- Antinori S ,
- Galimberti L ,
- Gianelli E ,
- Küpper-Tetzel CP ,
- Nyirenda TS ,
- Mandala WL ,
- Gordon MA ,
- Mastroeni P
- Wilairatana P ,
- Masangkay FR ,
- Kotepui KU ,
- Berkley JA ,
- Sandlund J ,
- Naucler P ,
- Bruneel F ,
- Hocqueloux L ,
- Alberti C ,
- Mallewa M ,
- Vallely P ,
- Faragher B ,
- Di Gennaro F ,
- Marotta C ,
- Akhuemokhan OC ,
- Ewah-Odiase RO ,
- Watson OJ ,
- Afolabi MO ,
- Dabira ED ,
- Ornellas-Garcia U ,
- Ribeiro-Gomes FL
- De Jesus Milanez G ,
- Eckerle I ,
- Ebinger D ,
- Gotthardt D ,
- Watson JA ,
- Williams TN ,
- Maitland KM
- Edwards HM ,
- Counihan H ,
- Bonnington C ,
- Tibenderana JK
- Serwanga A ,
- Wanzira H ,
- Hussein R ,
- Ibraheim N ,
- Ashley EA ,
- Pyae Phyo A ,
- World Health Organization
- Olliaro P ,
- Martiáñez-Vendrell X ,
- Skjefte M ,
- Gimenez AM ,
- Marques RF ,
- Regiart M ,
- Bargieri DY
- Dumkow LE ,
- Worden LJ ,
- Fleming KA ,
- Wilson ML ,
- ↵ National Institute for Health and Care Excellence. NICE guideline NG143. Fever in under 5s: assessment and initial management 2021. https://www.nice.org.uk/guidance/ng143 .
- Srinamon K ,
- Silamut K ,
- Wanjiku P ,
- Hendriksen IC ,
- Veenemans J ,
- Vanaenrode J ,
- Verbakel JY ,
- Ministry of Health (MOH), Ghana Health Service (GHS) and National Malaria Control Programme (NMCP)
- ↵ Ministry of Health national malaria control program. National guidelines for diagnosis, treatment, and prevention of malaria in Kenya. 5th ed. 2016.
- ↵ Committee to advise on tropical medicine and travel. Chapter 7—Treatment of malaria: Canadian recommendations for the prevention and treatment of malaria. 2019.
- Raffetin A ,
- Leblanc C ,
- Minodier P ,
- Lalloo DG ,
- Shingadia D ,
- Beeching NJ ,
- Whitty CJM ,
- Chiodini PL ,
- PHE Advisory Committee on Malaria Prevention in UK Travellers
- Department of Health Republic of South Africa
- Gallagher T ,
Essay on Malaria Awareness
Introduction.
Malaria is a common disease in tropical countries where children and pregnant women are the main victims. It is a parasitic infection caused by plasmodium, which can be deadly to these vulnerable sections. As children are more prone to this disease, it is important to create awareness in them. So, this essay on malaria awareness will be beneficial for them to know more about it.
The main symptom of malaria is high fever with chills. So, it is possible that people confuse it with a viral fever, and malaria gets untreated, leading to other serious consequences. Since it is the life of our children that is at stake, we must take necessary measures to prevent and treat malaria. This short essay on malaria awareness will alert both children and elders on how to tackle this life-threatening disease.

Importance of Malaria Awareness
Malaria is spread from one individual to another by female Anopheles mosquitoes, and the symptoms in affected individuals resemble that of any viral fever. This is why knowledge about the disease is given due importance in this essay on malaria awareness. While high temperature and headache are the most common signs of malaria, nausea and drowsiness are also found in sick people. By detecting the disease early, we will be able to start the treatment soon, thus reducing the risk in children.
As we mentioned earlier in the short essay on malaria awareness that malaria is more prevalent in tropical countries, travelling to such places with little awareness about the disease could be dangerous. Children could fall ill at the end of the trip, which will ruin all the fun they had. So, this malaria awareness essay will be useful to know that it is risky to travel in the wet season to places that have humid climates.
All these points emphasise that it is important to have knowledge about malaria to prevent infections in children as well as make their journeys memorable. They will also be able to write about a memorable day of my life .
Ways to Raise Malaria Awareness
Just like how crucial it is to bring attention to the spread of malaria, it is equally important to understand that prevention is better than cure. Since we know that malaria is transmitted through mosquitoes, the first and foremost step in raising awareness of the disease is by spreading messages about mosquito breeding and destroying its breeding places. In this section of the essay on malaria awareness, we will see effective methods to create awareness among children.
While elders can understand the gravity of the disease, it is a struggle to teach our children about the same. Malaria is a disease that must be feared but let us not induce this threat in our kids. Instead, let us focus on imparting knowledge about malaria, its symptoms, and treatment to them in a fun way. By asking them to do simple tasks like cleaning their houses and surroundings and emptying the stagnant water from broken cups and bottles, we can build their awareness about the disease.
This short essay on malaria awareness concludes that however much of the threat is malaria, we can control it with proper awareness. So, let us nurture our children to grow in health and happiness with BYJU’S amazing essays on various topics.
Frequently Asked Questions on Essay on Malaria Awareness
Why should we raise awareness about malaria.
Malaria is a dangerous disease that has the potential to threaten one’s life. So, it is essential to create awareness about it to understand the symptoms and thereby treat it without any delay.
What is the importance of the malaria awareness essay?
The essay on malaria awareness can be useful for children to know more about the disease and the ways to prevent it.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

The Transformation Agenda »

- Regional Director
- Speeches & Messages
High-level Ministerial Meeting on Tackling Malaria in Countries Hardest Hit by the Disease
Opening Remarks by WHO Regional Director for Africa, Dr Matshidiso Moeti
Your Excellency Chief Dr Joseph Dion Ngute, Prime Minister of the Republic of Cameroon,
Honourable Ministers of Health,
Your Excellency Aminata Samaté Cessouma, the Commissioner for Health Humanitarian Affairs and Social Development at the African Union,
Dr Jean Kaseya, the Director-General of Africa CDC,
Mr Peter Sands, the Executive Director of the Global Fund to Fight AIDs, Tuberculosis and Malaria,
Dr David Walton, the US Global Malaria Coordinator,
Ms Joy Phumaphi, the Executive Secretary of the African Leaders Malaria Alliance,
Esteemed scientists, members of the Malaria Policy Advisory Group,
Partners and colleagues,
Ladies and gentlemen,
I am delighted to welcome you to this historic event.
We’ve gathered to celebrate a transformative moment in the history of public health.
Introducing two effective malaria vaccines, complementing other malaria control interventions, will be a game-changer!
It is the moment we’ve been waiting for in malaria control efforts—for millions of children, their mothers, families, and communities.
We’ve brought together countries most affected by malaria. Our global partners join us to revitalize our commitment to reducing malaria deaths in the most affected countries.
We have two approved vaccines - RTS,S and R21, which will be rolled out in 20 African countries this year, providing new hope for millions of children at risk of dying from malaria.
We piloted the groundbreaking Malaria Vaccine Implementation Programme (MVIP), during which we delivered RTS,S vaccines to more than 2 million children in Ghana, Malawi, and Kenya.
I thank the governments of these countries for their political will and collaboration. During the trials, it became clear that the vaccine reduced child deaths by a phenomenal 13% and severe illness by 22%.
We are showing that eliminating and controlling diseases are possible in Africa.
Algeria and Cabo Verde have eliminated malaria;
Togo eliminated four neglected tropical diseases;
And Botswana is on the road to eliminating mother-to-child transmission of HIV.
The development and rollout of malaria vaccines, along with the evidence of controlling and eliminating disease in Africa, speaks to the power of partnerships.
I would like to thank all our partners including the Global Fund, the US President’s Malaria Initiative, BMGF, and the African Leaders Malaria Alliance.
I also acknowledge the critical role played by GAVI to ensure access to life-saving vaccines and UNITAID, which provide catalytic funding for innovative strategies.
As science, research, and innovation change our approach to diseases, our office plays a central role in the regulatory space in our region through the African Vaccine Regulatory Forum (AVAREF), which we established two decades ago.
AVAREF - a network of national regulatory authorities and ethics committees from 55 member states, has been building capacity in these committees to oversee clinical trials and vaccine registration. This was highlighted recently by the accelerated introduction and rollout of COVID-19 vaccines, and now, malaria vaccines.
I applaud GAVI’s recent commitment to the African Manufacturing Vaccine Accelerator and the launch of the BioNTech facility in Kigali last year. Both are excellent moves towards localizing critically needed manufacturing capacity in our continent.
I commend Dr Jean Kaseya, the DG of Africa CDC, for his leadership in setting up the African Manufacturing Vaccine Accelerator.
Investment in strong regulatory systems is essential, highlighting the critical role of AVAREF and the operationalization of the African Medicines Agency across the African continent.
Excellencies, ladies and gentlemen,
We have started the rollout of RTS,S in Cameroon and Burkina Faso.
I commend these countries as the first two expected to introduce malaria vaccines this year. I particularly congratulate the Prime Ministers of Cameroon and Burkina Faso and their Ministers of Health.
Twins were the first children in Cameroon to receive the vaccine. Their mother, Helene, knows firsthand just how devastating malaria can be. No mother should watch her child die of a preventable disease like malaria.
Our regional team is strongly committed to convening and coordinating the actions of governments and partners in malaria vaccine introduction through our strategic initiative, the Accelerated Malaria Vaccine Introduction and Rollout in Africa (AMVIRA).
Additionally, our country presence enables us to support all our Member States’ work, in conducting disease programme reviews, developing national plans, and estimating the budget required for disease control and elimination.
We have also enhanced the support to countries in developing quality applications submitted to the Global Health Initiatives, while ensuring that grants are implemented in line with existing WHO guidelines on malaria, HIV and other diseases.
This effort has resulted in the resource mobilization required to address the malaria burden in countries, and other health concerns, such as immunization, HIV, and TB.
Despite all our efforts, global gains in malaria have levelled off, and we are greatly concerned that the number of deaths remained higher in 2022 than in 2019; and that cases have continued to increase since 2015, with our region recording over 95% of cases and deaths.
I will not cease to emphasize the devastating effect of malaria in our region. Around 80% of these deaths are in children under the age of five years.
We cannot continue to allow our children to die.
We cannot continue to experience the costs of this disease, in the burden on mothers caring for sick children, in people unable to work while doing this, in the economic and social consequences of millions of children failing to grow to adulthood.
In response to this and given the flat-lining of funding for malaria and competing health priorities regionally, we have redefined priorities for investments to ensure that interventions have an impact:
We have developed the Regional Framework for controlling, eliminating, and eradicating tropical and vector-borne diseases (2022-2030), adopted by all Ministers of Health at the 72nd session of the Regional Committee for Africa.
We have introduced an innovative Strategy for ending disease in Africa. It is grounded in deploying data analytics and evidence-based policy; it will drive the delivery of relevant and tailored public health interventions to prioritized populations and communities.
We are documenting and disseminating best practices to illustrate the importance of country-driven and country-led solutions.
Allow me to share two examples:
Nigeria, with the highest burden of malaria globally, has conducted a deep dive to understand the distribution of the disease, leading to a subnational malaria report with tailored state-level interventions.
Ghana, another high-burden country, registered a 33% decrease in malaria cases and a 7% decrease in deaths between 2015 and 2021. It has just launched the first National Strategy for Malaria Elimination, to end malaria deaths by 2028.
Malaria is one of many priorities for our countries. Global Health Initiatives now recognize the importance of more integrated approaches, particularly within health systems, to addressing priority diseases.
In a person-centred approach, we need to accelerate the implementation of disease interventions in alignment with national priorities. We must involve communities, civil society, healthcare workers, and all stakeholders.
I am going to leave you with these key priorities.
First, we must act urgently to accelerate the groundbreaking rollout of malaria vaccines across eligible countries. We also need to encourage other innovative tools and strategies.
Second, Honourable Ministers, we must now scale up the malaria vaccine rollout and the full package of malaria interventions to achieve the 2030 targets.
Your decision to sign the Yaoundé declaration clearly demonstrates your commitment to changing the malaria experience in Africa.
Third, we need high-level regional and country-level leadership to strengthen health systems. It will ensure the effective delivery of the interventions needed at all levels, from primary health care to tertiary facilities.
I remain committed to providing a convening and collaborating role, supporting our countries as they work with all stakeholders to accelerate our response to malaria and other diseases in Africa.
Thank you for your kind attention.
- Open access
- Published: 15 March 2024
Impact of climate change on temperature variations and extrinsic incubation period of malaria parasites in Chennai, India: implications for its disease transmission potential
- P. K. Kripa 1 , 2 na1 ,
- P. S. Thanzeen 1 , 2 na1 ,
- Nagaraj Jaganathasamy 3 ,
- Sangamithra Ravishankaran 1 ,
- Anupkumar R. Anvikar 4 &
- Alex Eapen 1 , 2
Parasites & Vectors volume 17 , Article number: 134 ( 2024 ) Cite this article
Metrics details
The global temperature has significantly risen in the past century. Studies have indicated that higher temperature intensifies malaria transmission in tropical and temperate countries. Temperature fluctuations will have a potential impact on parasite development in the vector Anopheles mosquito.
Year-long microclimate temperatures were recorded from a malaria-endemic area, Chennai, India, from September 2021 to August 2022. HOBO data loggers were placed in different vector resting sites including indoor and outdoor roof types. Downloaded temperatures were categorised by season, and the mean temperature was compared with data from the same study area recorded from November 2012 to October 2013. The extrinsic incubation period for Plasmodium falciparum and P. vivax was calculated from longitudinal temperatures recorded during both periods. Vector surveillance was also carried out in the area during the summer season.
In general, temperature and daily temperature range (DTR) have increased significantly compared to the 2012–2013 data, especially the DTR of indoor asbestos structures, from 4.30 ℃ to 12.62 ℃ in 2021–2022, unlike the marginal increase observed in thatched and concrete structures. Likewise, the average DTR of outdoor asbestos structures increased from 5.02 ℃ (2012–2013) to 8.76 ℃ (2021–2022) although the increase was marginal in thatched structures and, surprisingly, showed no such changes in concrete structures. The key finding of the extrinsic incubation period (EIP) is that a decreasing trend was observed in 2021–2022 compared to 2012–2013, mainly in indoor asbestos structures from 7.01 to 6.35 days, which negatively correlated with the current observation of an increase in temperature. Vector surveillance undertaken in the summer season revealed the presence of Anopheles breeding in various habitats. Anopheles stephensi could be collected using CDC light traps along with other mosquito species.
The microclimate temperature has increased significantly over the years, and mosquitoes are gradually adapting to this rising temperature. Temperature negatively correlates with the extrinsic incubation period of the parasite. As the temperature increases, the development of the parasite in An. stephensi will be faster because of a decrease in EIP, thus requiring relatively fewer days, posing a risk for disease transmission and a hindrance to malaria elimination efforts.
Graphical Abstract
Despite the global efforts towards malaria elimination, around 63,000 deaths were reported globally between 2019 and 2021, mainly due to disruption to essential, malaria-related services during the COVID-19 pandemic, increasing the need to accelerate efforts to eliminate the disease [ 1 ]. A new challenge arising in this scenario is the spread of Anopheles stephensi , native to South Asia and parts of the Arabian Peninsula, to Djibouti (2012), Ethiopia and Sudan (2016), Somalia (2019), Nigeria (2020), Yemen (2021) and Ghana, and Kenya (2022) [ 1 ]. This invasion of An. stephensi in sub-Saharan Africa, where the burden of malaria is the highest and > 40% of the population lives in urban environments, is a matter of grave concern. Anopheles stephensi is notorious as an urban malaria vector, and global urbanization adds to the threat of the spread of the disease [ 1 ]. Moreover, shifts in climate conditions within these regions may alter habitats that are typically unfavourable for malaria-transmitting mosquitoes or temporarily lengthen the period during which people are vulnerable to malaria [ 2 ]. WHO has launched an initiative to halt the continued expansion of An. stephensi in Africa. To bolster an efficient regional reaction, WHO has put forth a comprehensive five-part strategy. This includes fostering greater collaboration, enhancing surveillance efforts, improving the exchange of information, creating guidance, and prioritising research [ 3 ].
The transmission of malaria is highly influenced by the dynamic environmental temperature and parasites and mosquitoes that are exposed to the variations in daily temperature. Earlier studies confirmed that the mean temperature is highly influenced by these variations [ 4 ]. Empirical evidence is available showing that along with mean temperatures, daily fluctuations in temperature also affect parasite infection, the rate of parasite development, and the essential elements of mosquito biology. These factors play a huge role in disease transmission intensity [ 5 ], dispersion, and distribution of the vectors and patterns of disease transmission that are known to be highly influenced by the changing climatic conditions [ 6 , 7 ]. The temperature variations affect the length of the gonotrophic cycle, fecundity, biting rate, longevity, and development of immature mosquitoes [ 8 ]. Sporogonic development of the parasite in the vector is also affected by variables such as temperature, relative humidity, and rainfall [ 9 ]. Even negligible alterations in mean or diurnal temperature can result in significant variations in the life cycle of both vector and parasite, which eventually determine transmission intensity [ 10 ].
It has been hypothesised that a rise in temperature above the average range would not only aid in the selection of temperature-tolerant mosquitoes in a population but also affect both intrinsic and extrinsic factors that have direct implications for disease transmission, survival rates, and vectorial capacity [ 11 ]. Considering the global rise in temperature and the rapid spread of An. stephensi to African countries, the impact of temperature on the vectors is a matter of concern and needs in-depth investigations to understand its disease transmission potential. The extrinsic incubation period (EIP) of the parasite (time required for development within a mosquito and becoming infectious) is one such factor that determines the transmission potential of the disease [ 12 ]. As temperature plays a role in the EIP, the alarming increase in temperature due to global warming will have a significant impact on the EIP of malaria parasites.
The current study was conducted in the city of Chennai in Tamil Nadu, Southeast India, where the major vector of malaria is An. stephensi . It has been reported that this species rests in both indoor and outdoor environments [ 10 ]. The study aims to analyse the impact of changes in temperature, both indoor and outdoor resting sites of the vector, and the effect of temperature on the EIP of parasites by recording year-long temperature data using HOBO data loggers (U10-003). The study also analysed the variations in temperature and EIP over a gap of 10 years, comparing them with our previously published study data from 2012–2013 [ 13 ].
Study site and sampling method
Since the study was focused on the variations in temperature over 10 years, the same study area of 2012–2013 was selected to avoid bias due to site/area-based fluctuations [ 13 ]. The region covered by Besant Nagar clinic (13.0002°N, 80.2668°E) was selected previously based on the malaria prevalence during the 2006–2012 period obtained from the Regional Office for Health and Family Welfare (ROH & FW) at Besant Nagar, Chennai. Suitable human dwellings were selected after obtaining the necessary consent for year-long environmental monitoring of the micro-climatic temperature of various roof types in indoor and outdoor environments.
Recording the microclimatic temperature and relative humidity (RH)
Year-long microclimate data of ambient, atmospheric temperature from the preferred resting sites of adult An. stephensi were recorded from Besant Nagar, Chennai, using temperature and relative humidity data loggers (Onset HOBO U10-003) from September 2021 to August 2022. The data loggers were launched using HOBOWARE Lite (version 1.2.3) software [ 13 ] and were placed in three different resting sites, which include various indoor and outdoor roof types: thatched, asbestos, and concrete structures. A total of 18 HOBO data loggers were placed with three replicates for each structure type (indoor as well as outdoor). Both indoors and outdoors, HOBO data loggers were attached to the wall or horizontal flat surface 1–2 feet down from the roof after obtaining consent from household members. Data loggers were carefully placed away from places such as the kitchen, ventilators, bathrooms, etc., to avoid errors/discrepancies in temperature data reading. The launching date and time of the HOBO data logger, data collection site with address, geo-coordinates, habitat type (household roof characteristics), and other relevant information were recorded. Field visits were undertaken fortnightly, and temperature and relative humidity readings were downloaded onto a laptop using the software. During these visits, the data loggers with low battery levels were replaced to ensure continuous data recording. After the readings were downloaded, they were fixed in the same place to continue recording to obtain year-long data. The geo-coordinates of the resting habitats along with altitude data were recorded using Garmin GPS (version 2.40).
Vector surveillance
The immature surveillance was undertaken in malaria-endemic areas focusing on the anopheline breeding habitats (both intra- and peri-domestic) and natural aquatic habitats where Anopheles mosquitoes preferably breed in houses/apartments and their premises during the summer season. Since we were focusing on the impact of high temperatures on vectors, mainly An. stephensi , the surveillance was conducted during this season. Immature sampling was undertaken following standard/appropriate sampling techniques such as dipping, bucketing, and well net sampling methods [ 14 ]. The larval sampling was done twice a month. The collected samples were transferred to properly labelled plastic containers and then carefully transported to the laboratory to avoid mortality. The collected immatures were reared in the laboratory in standard conditions (27 °C and 80% RH). The mosquito species that emerged from the collected samples were identified morphologically using standard mosquito identification keys [ 15 ].
Adult surveillance was conducted using mechanical/oral aspirators and flashlights to estimate the density in the study sites by undertaking resting collections, pyrethrum spray sheet (PSC) and light trap collections from March to May 2022 (summer season). Indoor resting collections were undertaken during dawn in the appropriate houses and cattle sheds in the area. Pyrethrum spray sheet catches were done to estimate the number of mosquitoes resting indoors where people had slept the previous night during the morning hours before the households started cooking. Thatched/tiled or asbestos houses with separate bedrooms were selected, depending on the availability of such houses in the area for PSCs to collect the maximum number of indoor resting mosquitoes. Light traps were placed indoors near the host by hanging them ~ 1.8 m from the ground to collect anophelines [ 16 ].
A total of 10 resting (eight human dwellings, one cattle shed, and one outdoor), three pyrethrum spray sheet, and three light trap collections (from two households and one cattle shed) were carried out in both areas. The collected adult mosquitoes were kept in test tubes/plastic containers depending on the density and labelled with the date of collection. All the mosquito samples were brought to the laboratory in temperature-controlled conditions and the live mosquitoes were kept in thermocol/styrofoam boxes to prevent mortality during transportation. The mosquito species collected were identified following standard identification keys [ 15 ].
Data analysis
The downloaded data points were arranged and categorised into four seasons, namely winter (December–February), summer (March–May), pre-monsoon (June- August), and monsoon (September–November), as experienced in the study area. The monthly mean temperature and DTR were calculated for all the months. Microenvironmental data were statistically analysed in IBM SPSS Statistics, version 23. All the data points were checked for normality using the Shapiro-Wilk test. Differences in temperature and DTR of different structure types for all seasons during the 2021–2022 period were statistically analysed by one-way ANOVA. Since the ANOVA results were significant, a post hoc test was performed to identify the data set that contributed to the significant results. The data obtained during 2021–2022 were then compared with the data from the same study area from 2012 to 2013 of our previously published study [ 13 ] using paired t-tests. The microenvironmental temperature was then compared with the macroenvironmental temperature obtained from https://power.larc.nasa.gov [ 17 ]. The monthly average precipitation data were obtained from https://power.larc.nasa.gov [ 17 ] to analyse the recorded relative humidity.
Extrinsic incubation period for Plasmodium vivax and P. falciparum
The season-wise EIP for the development of Plasmodium in mosquitoes was calculated using Detinova’s degree-day model [ 18 ]. In the model, the sum of heat in degree-days required for completing a sporogonic cycle is 105 °C and 111 °C for Plasmodium vivax and P. falciparum , respectively. The sum of heat is the total number of degree-days in the given period. A degree-day (the degree-24 h) is the number of degrees by which the mean temperature of the day concerned exceeds the lower threshold temperature for the development of the organism of the given species, i.e. the temperature below which development does not occur [ 19 , 20 ]. The EIP based on this method was calculated using the formula EIP = 111/(T-16) for P. falciparum , where 111 indicates the degree-days and Tmin = 16, and for P. vivax the EIP = 105/(T-14.5), where 105 indicates the degree-days for P. vivax and Tmin = 14.5 [ 18 ]. Pearson correlation analysis was performed to investigate the relationship between average temperature and EIP for P. vivax and P. falciparum.
Diversity of seasonal temperature profiles in indoor and outdoor environments of different roof types during 2021–2022
The indoor and outdoor temperatures of concrete and thatched roof structures did not show any significant difference ( p = 0.96) during the pre-monsoon season. Similarly, the outdoor temperature for concrete and asbestos roof types did not vary ( p = 1.00) during the monsoon season. All other roof types showed significant differences in temperature for all other seasons (Fig. 1 ).

Season-wise mean temperature and relative humidity recorded from different roof types in 2012–2013 and 2021–2022. a Mean indoor temperature. b Mean outdoor temperature. c Mean indoor RH. d Mean outdoor RH
For the indoor temperature of the roof types, the highest temperature was observed in asbestos (34.92 ± 1.78) in summer and the lowest in thatched (27.90 ± 0.97) during winter (Fig. 1 ). For the outdoor temperature of all roof types, asbestos had the highest temperature in all seasons except winter. However, during winter, outdoor temperatures for concrete roof types were higher (29.30 ± 1.08). However, the outdoor temperature for thatched roof types remained lower throughout all the seasons.
Seasonal variations in temperature and relative humidity across various roof types between 2012–2013 and 2021–2022
Comparing the 2021–2022 temperature data of the winter season with those of 2012–2013, there was a significant increase in the temperature during 2021–2022, for both indoor and outdoor temperatures of all roof types, except the indoor temperature of thatched structures (Fig. 1 ) (Table 1 ). For thatched roofs, indoors showed a significant decrease in temperature ( p = 0.00) during 2021–2022 (27.50 °C ± 0.97) compared to 2012–2013 (27.90 °C ± 0.51).
In the summer season, all roof types were warmer in 2021–2022 except for the indoor temperature of the concrete roofs (31.36 °C ± 0.47). Concrete structures were warmer in 2012–2013 (32.06 °C ± 1.01). The outdoor temperature of the thatched structures did not show any significant difference in temperature compared to the temperature profile between 2012–2013 and 2021–2022 (Fig. 1 ) (Table 1 ). Similarly, in the pre-monsoon season, all the roof types were warmer during 2021–2022 except the indoor temperature of concrete and thatched structures. There was no significant difference between the means in 2012–2013 and 2021–2022 for the indoor concrete ( p = 0.68) and thatched structures ( p = 0.23).
In the monsoon season, the outdoor temperatures for the thatched roof type did not show any significant change in temperature in 2012–2013 and 2021–2022. Nevertheless, the other two roof types were warmer in 2021–2022 during the monsoon season. Comparing the indoor temperatures in the monsoon season, all the structures were warmer in 2021–2022 except the thatched roof type. The indoors of the thatched roof structures were warmer in 2012–2013.
In general, there was a significant decrease in relative humidity during 2021–2022 compared to 2012–2013 for indoors of all roof types across all seasons except the indoors of thatched structure wherein the RH increased in the monsoon season. For outdoors, the RH increased in thatched roof type across all seasons in 2021–2022. Furthermore, the RH recorded in the outdoors of concrete structures increased for monsoon and pre-monsoon seasons during 2021–2022. Similarly, the outdoors of asbestos roofs also showed increased RH during the monsoon season in 2021–2022. (Fig. 1 ).
The minimum humidity for the asbestos roof type (60.09 ± 15%) was recorded indoors during 2021–2022 in the pre-monsoon season, unlike in 2012–2013, when it was observed indoors for the concrete roof type (68.82 ± 8.48%) during the same season. Nonetheless, the relative humidity observed outdoors was different. The minimum humidity was recorded in the summer season for the outdoor asbestos (64.70 ± 12.46%) roof type during 2021–2022, while in 2012–2013 it was observed outdoors for the concrete (68.81 ± 8.48%) roof type in the pre-monsoon season (Fig. 1 ).
Vector surveillance (2021–2022)
Mosquito breeding was observed in plastic overhead tanks (pOHT), cemented overhead tanks (cOHT), wells, cement tanks, curing pits, barrels, discarded mud pots, discarded aluminium vessels, etc. Of 518 breeding habitats surveyed, 79 (15.2%) were positive for anophelines. Among the surveyed habitats, the highest water temperature recorded was 36.2 \(^\circ {\text{C}}\) , which was observed in stagnant water on a terrace. The adults that emerged from the immature collections were An. stephensi . In adult collections, only one An. stephensi was collected in the resting collection along with other species such as Culex quinquefasciatus, Stegomyia aegypti, Cx. gelidus , and Cx. tritaeniorhynchus . Surprisingly, no anophelines were collected in the pyrethrum spray sheet collections. However, in light trap collections, An. stephensi (8), An. pallidus (3), and An. subpictus (1) were collected besides, Cx. tritaeniorhynchus and St. aegypti .
Difference between macro- and microenvironmental temperature profiles
The average daily temperatures recorded from data loggers within the local transmission sites were significantly warmer than the data obtained from https://power.larc.nasa.gov for both periods, 2012–2013 and 2021–2022, indicating the importance of microclimatic variables in vector resting habitats.
Comparison of the daily temperature range in 2012–2013 and 2021–2022
The daily temperature range showed a significant increase during 2021–2022 compared to 2012–2013 data for all the structure types in all seasons except for the indoor concrete structure in the winter season. The average DTR for indoor asbestos structures increased drastically from 4.30 °C in 2012–2013 to 12.62 °C in 2021–2022. However, for the indoor thatched structures, the increase in DTR was marginal from 4.08 °C in 2012–2013 to 5.55 °C in 2021–2022. Similarly, for indoor concrete structures, the average DTR increased from 1.93 °C in 2012–2013 to 2.95 °C in 2021–2022. The DTR was lower in 2021–2022 in the winter season for concrete structures (Fig. 2 ). The DTR showed a steady pattern for the indoors of all the roof types in 2012–2013, but it fluctuated throughout the seasons when in the 2021–2022 period. Furthermore, a broader spectrum of DTR could be noted within the interior of asbestos buildings, and this variation was conspicuous in 2021–2022 (Fig. 2 ).
Daily temperature range (DTR) observed in various roof types in 2012–2013 and 2021–2022. a Indoor, b outdoor
The DTR of all roof types for outdoors showed an increase in general for 2021–2022 except for concrete structures. The average DTR for outdoor asbestos structures increased from 5.02 °C in 2012–2013 to 8.76 °C in 2021–2022. However, in the outdoors of thatched structures, the DTR showed a marginal increase from 5.37 °C in 2012–2013 to 5.64 °C in 2021–2022. Nevertheless, for the outdoor concrete structures, the average DTR did not show any change over the years (4.46 °C).
Variations in the extrinsic incubation period of Plasmodium vivax and P. falciparum across various roof types
In general, when we compared the EIP for P. vivax and P. falciparum for 2012–2013 and 2021–2022, the EIP showed a decreasing trend, which negatively correlated with our observation of an increase in temperature from 2012–2013 to 2021–2022 (Fig. 3 ) (Table 2 ). The average EIP for indoor asbestos structures decreased from 7.01 days in 2012–2013 to 6.35 days in 2021–2022. However, for indoor thatched structures, EIP showed a slight increase from 7.57 days in 2012–2013 to 7.74 days in 2021–2022. Likewise, the average EIP decreased from 7.10 days in 2012–2013 to 6.96 in 2021–2022 inside concrete structures
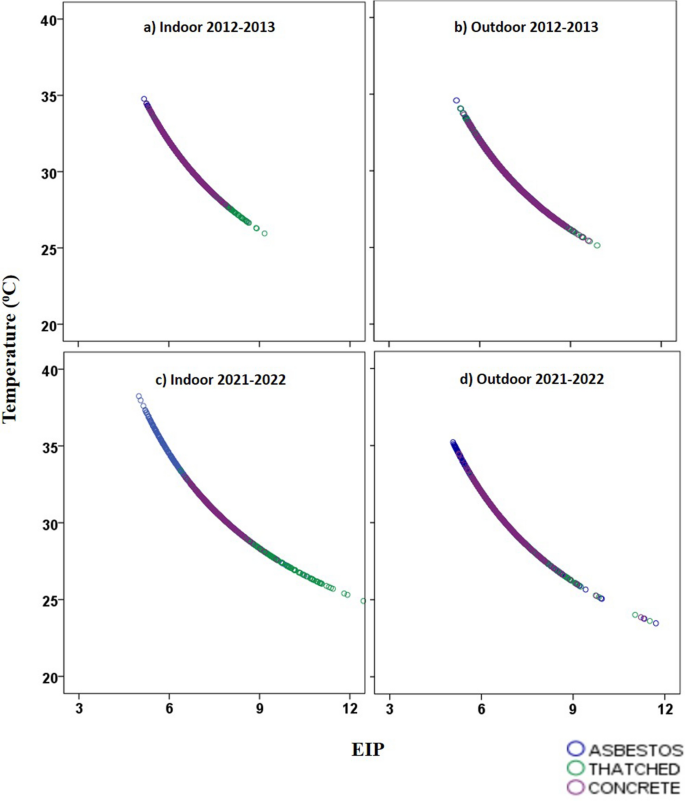
Correlation between mean temperature and extrinsic incubation of Plasmodium in a indoor structure type 2012–2013, b outdoor structure type 2012–2013, c indoor structure type 2021–2012, d outdoor structure type 2021–2022
During the pre-monsoon season, both indoor and outdoor EIPs of all the structure types showed a decrease in 2021–2022, indicating that parasite development now occurred much faster than in 2012–2013. In winter, summer, and monsoon seasons, the indoor EIP for thatched structures showed an increase which correlated with the decrease in temperature during this period. In monsoon season, the outdoor EIP increased for thatched structures, indicating that in thatched structures the parasite development rate was generally slower owing to the decrease in temperature. During summer, the indoor EIP for concrete structures showed an increase in 2021–2022, which was again negatively correlated with the decreased indoor temperature of concrete roof types. An increase in the DTR in 2021–2022 was observed compared to the 2012–2013 data, and this observation correlated with the increased range for EIP. However, in concrete structures, except for winter, the DTR decreased, and so the EIP range for this structure was narrow.
The current study focuses on data recording and analysis of microclimatic variables and temperature for 2021–2022 across three different structural roof types, namely, asbestos, thatched, and concrete, where the mosquitoes preferred to rest indoors. The data obtained were then compared to the microclimate data recorded in 2012–2013 from our previously published study [ 13 ] to determine the variation between microenvironment temperature variables over these years. Multiple studies have already shown that microclimatic variables are significant factors in disease transmission as opposed to overall weather station data recorded far from the study site [ 10 , 13 ]. This study showed that the local transmission site is warmer by at least 3–4 °C compared to the weather station data recorded and analysed. A similar observation was noted in our previously published study [ 13 ].
Generally, it has been observed that, over the years, there has been a significant increase in both indoor and outdoor temperatures across all roof types indicating the impact of global warming. Studies have already reported that, in tropical countries, an increase in temperature carries an increased risk of malaria burden due to global warming [ 10 ]. Since Chennai features a tropical dry and wet climate, the current study with a warmer environment is a matter of concern when it comes to disease transmission potential. With the increased temperature, a question may arise whether the vector will survive in higher temperatures or not. In vector surveillance undertaken in the same area, there were a few habitats with immature anophelines during summer indicating that vectors do survive in higher temperatures. However, in adult collections, An. stephensi was rarely collected, but other mosquito species such as Cx. quinquefasciatus, Cx. gelidus , and St. aegypti were collected. A few studies reported that, over the years, the density of adult An. stephensi has declined drastically with fewer breeding habitats and relatively low breeding in habitats/sources due to habitat manipulation and vector interventions unlike in earlier years. Furthermore, ethological studies on mosquitoes have shown that in such cases mosquitoes prefer cooler areas and avoid hotter temperatures [ 21 , 22 ].
Previous studies indicated that mosquito abundance and relative humidity have a weak negative correlation, that is, when RH decreases there are chances that the abundance of mosquitoes may increase (21). In the monsoon season, the average precipitation (mm/day) was observed to be higher during 2021–2022 (11.82 mm/day) than in 2012–2013 (3.51 mm/day), which was positively correlated with recorded relative humidity, that is, all roof types showed higher RH in 2021–2022 during the season. Thatched outdoor roof type showed a significant increase in RH during 2021–2022 throughout the seasons. In 2021–2022, our study sites experienced precipitation almost every month. Therefore, thatched roofs were always moist, hence the increased humidity.
DTR was relatively narrow and more stable previously, but currently, it is showing a wider range and is fluctuating more (Fig. 2 ). An increase in temperature and DTR has a significant impact on parasite prevalence, parasite intensity, and mortality of mosquitoes, and this has decreased overall vectorial capacity for both mosquito species, An. stephensi and An. gambiae [ 23 ].
Regarding EIP of parasites calculated based on Detinova’s degree-day model, the main observation was that when there is an increase in temperature, the EIP decreases steadily for both P. falciparum and P. vivax . In the current study, the area is experiencing a warmer climate pattern; hence, the EIP is decreasing, indicating a strong negative correlation in the study (Fig. 3 ). A mechanistic mathematical model aligns with our observations. According to the model, it is predicted that an increase in temperature from 21˚C to 34 °C decreased the EIP 50 from 16.1 to 8.8 days [ 22 ]. According to the thermodynamic model, parasite development rate (PDR) is directly proportional to temperature and EIP is reciprocal to PDR [ 13 ]. Hence, when temperature increases, PDR increases, while EIP tends to decrease, which is similar to the case observed from our findings with Detinova’s degree-day model [ 18 ].
The study reiterates the importance of the ambient environmental temperature to which the vector is exposed in the resting site and the factors that influence parasite development. Previous studies have also shown that temperature affects the sporogonic development of P. falciparum in anophelines and the ookinete maturation rate. At lower temperatures (21–27 °C), infection rates of both ookinetes and oocysts were unaffected, but at higher temperatures (30 and 32 °C), there was a significant impact on parasite densities and infection rates because this changes the developmental processes between fertilization and ookinete production [ 24 ]. Another notable observation was a wide range of DTR with fluctuation in 2021–2022, unlike the 2012–2013 data (Table 1 ). For the current dataset, EIP shows a similar pattern with a wide range of days (Table 2 ). There is ample evidence from previous studies that even a small change in EIP for minimal days can have a drastic impact on the transmission risk. Therefore, with increased temperature and the resultant decrease in EIP, it is obvious that transmission of the disease might quickly get faster.
Global warming has increased the atmospheric temperature; as a result, the same has been observed in the DTR. The EIP of parasites has a strong negative correlation with the temperature. Currently, the EIP of P. falciparum and P. vivax is decreasing. Consequently, the development of the parasite will be faster and require relatively fewer days. Current models predicting the relationship between temperature and PDR of a parasite have an upper thermal limit for temperature. However, with increasing temperature, this upper limit has to be reconsidered. In general, the impact of global warming and increasing temperatures will thereby pose a risk for disease transmission and also may foil the efforts made to eliminate malaria.
Limitations of the study
Analysing the data showed that the DTR increased in 2021–2022 compared to 2012–2013, as did the EIP. EIP had a wide range in 2021–2022, unlike in 2012–2013. However, the implications of these observations are difficult to derive given the lack of a real-time experiment exposing infected mosquitoes to varying daily temperatures, which was not conducted in the present study.
The prevailing models explaining the role of temperature in parasite development, such as Paaijman’s model, could not be followed in this study as our higher temperature exceeds the critical higher temperature of the model. Hence, we used the degree-day model of Detinova for this study.
The data comparison showed that in 2021–2022 the EIP of the parasite decreased significantly, indicating an increased transmission potential given that parasites will take less time to develop compared to the 2012–2013 period due to an increase in temperature as temperature and EIP are negatively correlated. Due to the unforeseen COVID-19 pandemic-related circumstances, few malaria cases were reported during the study period in the same area, mainly because of the disruption of technical services.
Availability of data and materials
The dataset generated during and/or analysed during the current study is available from the corresponding author upon reasonable request.
Abbreviations
- Daily temperature range
- Extrinsic incubation period
Global positioning system
Pyrethrum spray sheet collection
Parasite development rate
Regional Office for Health and Family Welfare
References
Organization. World Malaria Report. Geneva: World Health Organization; 2022.
Google Scholar
Ortiz-Prado E, Camacho-Vasconez A, Izquierdo-Condoy JS, Bambaren C, Hernández-Galindo L, Sanchez JC. El Niño-Southern Oscillation: a call to action for public health emergency preparedness and response. Lancet Reg Health—Am The. 2023;27:100601.
World Health Organization. WHO Initiative to Stop the Spread of Anopheles stephensi in Africa (No. WHO/UCN/GMP/2022.06). 2022.
Zhao X, Chen F, Feng Z, Li X, Zhou X-H. Characterizing the effect of temperature fluctuation on the incidence of malaria: an epidemiological study in south-west China using the varying coefficient distributed lag non-linear model. Malar J. 2014;13(1):1–10. https://doi.org/10.1186/1475-2875-13-192 .
Article PubMed PubMed Central Google Scholar
Blanford JI, Blanford S, Crane RG, et al. Implications of temperature variation for malaria parasite development across Africa. Sci Rep. 2013;3(1):1300. https://doi.org/10.1038/srep01300 .
Elbers ARW, Koenraadt C, Meiswinkel R. Mosquitoes and Culicoides biting midges: vector range and the influence of climate change. Rev Sci Tech OIE. 2015;34:123–37.
Article CAS Google Scholar
McIntyre KM, Setzkorn C, Hepworth PJ, Morand S, Morse AP, Baylis M. Systematic assessment of the climate sensitivity of important human and domestic animals pathogens in Europe. Sci Rep. 2017;7(1):7134. https://doi.org/10.1038/s41598-017-06948-9 .
Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol. 2014;51:55–62.
Article PubMed Google Scholar
Afrane YA, Zhou G, Githeko AK, Yan G. Clinical malaria case definition and malaria attributable fraction in the highlands of western Kenya. Malar J. 2014;13(1):1–7. https://doi.org/10.1186/1475-2875-13-405 .
Cator LJ, Thomas S, Paaijmans KP, Ravishankaran S, Justin JA, Mathai MT, et al. Characterizing microclimate in urban malaria transmission settings: a case study from Chennai. India Malar J. 2013;12(1):1–10. https://doi.org/10.1186/1475-2875-12-84 .
Raghavendra K, Barik TK, Adak T. Development of larval thermotolerance and its impact on adult susceptibility to malathion insecticide and Plasmodium vivax infection in Anopheles stephensi . Parasitol Res. 2010;107:1291–7.
Ohm JR, Baldini F, Barreaux P, Lefevre T, Lynch PA, Suh E, et al. Rethinking the extrinsic incubation period of malaria parasites. Parasites Vectors. 2018;11(1): 1–9. https://doi.org/10.1186/s13071-018-2761-4 .
Thomas S, Ravishankaran S, Justin NAJA, Asokan A, Kalsingh TMJ, Mathai MT, et al. Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of Plasmodium vivax and P lasmodium falciparum : a study from a malaria-endemic urban setting Chennai in India. Malar J. 2018;17(1):1–17. https://doi.org/10.1186/s12936-018-2342-1 .
Silver JB. Mosquito ecology: field sampling methods. Berlin: Springer science & business media; 2007.
Nagpal BN. Indian Anophelines. Lebanon, NH; 1995.
Pratt HD, Barnes RC, Littig KS. Mosquitoes of Public Health Importance and Their Control. Centers for Disease Control (CDC). 55.
Stackhouse P. NASA POWER | Data Access Viewer. https://power.larc.nasa.gov/data-access-viewer/ .
Detinova TS. Age-grouping methods in diptera of medical importance: with special reference to some vectors of Malaria. J Parasitol. 1962;48:456.
Article Google Scholar
Moshkovsky SD, Rashina MG. Epidemiology and medical parasitology for entomologists. Moscow (in Russian, unknown publisher, after Detinova, TS Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Geneva: WHO; 1962.
Organization. WH. Manual of Practical Entomology in Malaria: Vector Bionomics and Organization of Anti-Malaria Activities. 1975.
Ngarakana-Gwasira ET, Bhunu CP, Masocha M, Mashonjowa E. Assessing the role of climate change in Malaria transmission in Africa. Malar Res Treat. 2016;2016:1–7.
Kim Y-M, Park J-W, Cheong H-K. Estimated effect of climatic variables on the transmission of Plasmodium vivax Malaria in the Republic of Korea. Environ Health Perspect. 2012;120:1314–9.
Stopard IJ, Churcher TS, Lambert B. Estimating the extrinsic incubation period of malaria using a mechanistic model of sporogony. PLoS Comput Biol. 2021;17:e1008658.
Article CAS PubMed PubMed Central Google Scholar
Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi . J Parasitol. 1995;111:539–45.
Download references
Acknowledgements
We thank AcSIR, NIMR, and ICMR for providing the necessary facilities and support. We gratefully acknowledge E. Elumalai and other staff of the NIMR field unit, Chennai, and the communities of Besant Nagar for permitting us to place the temperature/RH data loggers year long. We gratefully acknowledge the Regional Office for Health and Family Welfare (ROH & FW), Besant Nagar, Chennai, for providing us with the malaria prevalence data and https://power.larc.nasa.gov/data-access-viewer/ for macroenvironmental temperature. We also thank A. Elangovan, Scientist G, ICMR-National Institute of Epidemiology, Chennai, for his valuable suggestions during the study period. The financial assistance of UGC (Junior Research Fellowship), New Delhi, to Kripa P.K. and Thanzeen P.S. for this study is thankfully noted.
The work was supported by the intramural grant received from the Indian Council of Medical Research. The content of this manuscript is solely the responsibility of the authors.
Author information
P. K.Kripa and P.S. Thanzeen have contributed equally to the study.
Authors and Affiliations
Field Unit, ICMR-National Institute of Malaria Research, Chennai, India
P. K. Kripa, P. S. Thanzeen, Sangamithra Ravishankaran & Alex Eapen
Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India
P. K. Kripa, P. S. Thanzeen & Alex Eapen
ICMR-National Institute of Immunohaematology, Chandrapur Unit, Chandrapur, Maharashtra, India
Nagaraj Jaganathasamy
ICMR-National Institute of Malaria Research, Sector 8, Dwarka, New Delhi, India
Anupkumar R. Anvikar
You can also search for this author in PubMed Google Scholar
Contributions
KPK: Carried out the placement and downloading of temperature data loggers (HOBO) in the field and performed the data analysis, contributed to the conceptualization of the manuscript. TPS: Drafted the original manuscript with KPK, contributed to conceptualization of the manuscript. NJ: Contributed to data analysis. SR: Conceptualization of the manuscript and review. AA: Contributed to the conceptualization of the manuscript and also reviewed the manuscript. AE: Design and conceptualization of the experiment, oversaw its implementation, edited and reviewed the final manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Alex Eapen .
Ethics declarations
Ethics approval and consent to participate.
Informed consent was obtained from the households to place HOBO's and download the microclimate data (Temperature and Relative Humidity). Furthurmore, informed consent was also obtained for immature collection and adult surveillance (resting collections, pyrethrum spray sheet collections and light trap collections) from the respective households.
Consenting for publication
Not applicable.
Competing interests
The authors have no competing interests to disclose.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kripa, P.K., Thanzeen, P.S., Jaganathasamy, N. et al. Impact of climate change on temperature variations and extrinsic incubation period of malaria parasites in Chennai, India: implications for its disease transmission potential. Parasites Vectors 17 , 134 (2024). https://doi.org/10.1186/s13071-024-06165-0
Download citation
Received : 20 October 2023
Accepted : 25 January 2024
Published : 15 March 2024
DOI : https://doi.org/10.1186/s13071-024-06165-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Degree-day model
- Anopheles stephensi
Parasites & Vectors
ISSN: 1756-3305
- Submission enquiries: [email protected]
Celebrating 75 Years of CDC and 70 Years of Malaria Elimination from the United States: A Photo Essay
This year, we commemorate the 75th anniversary of the establishment of CDC and recognize the 70th anniversary of the elimination of malaria from the United States.
In 1942, CDC’s predecessor—the Office of Malaria Control in War Areas (MCWA)—was created to control malaria around the military bases through the Southeastern United States. MCWA worked to eliminate mosquitoes that fed on malaria-infected soldiers and trained state and local health department officials in malaria control techniques and strategies.
Then, four years later on July 1, 1946, the U.S. government established the Communicable Disease Center in Atlanta to continue the work of MCWA and to serve as the nation’s public health agency.
Early malaria control work consisted primarily of insecticide spraying and draining water from mosquito breeding sites. In 1951, malaria was declared eliminated from the United States.
Now, 70 years later, our name may have changed, but our mission to stamp out malaria—both internationally and domestically—remains very much intact. To read more about how CDC’s Division of Parasitic Diseases and Malaria fights malaria and other parasitic diseases around the world and in the United States, see our Strategic Priorities 2021–2025 .

This historic image from the 1920s shows three malaria control workers in the process of using dynamite to remove tree stumps in Virginia to create a proper drainage ditch. In early malaria elimination efforts, these ditches would drain standing water where mosquitoes that carried malaria bred. Credit: CDC-PHIL

A Stearman biplane sprays an insecticide during a malaria control operation in Savannah, Georgia. Date unknown. Credit: CDC-PHIL

Three public health personnel carry out tasks involved in Office of Malaria Control in War Areas (MCWA) projects. MCWA (1942–1945) was established to control malaria around military training bases in the southern United States and its territories, where malaria was still a problem. Many of the bases were established in areas where mosquitoes that carried malaria thrived. MCWA aimed to prevent reintroduction of malaria into the civilian population, which was transmitted by mosquitoes that fed on malaria-infected soldiers in training, or soldiers who returned from areas where malaria was present. Credit: CDC-PHIL

Photographed between 1942 and 1945 in an unknown location, this image shows a small United States Public Health Service (USPHS) Office of Malaria Control in War Areas (MCWA) office. During MCWA’s mosquito control efforts to help eliminate malaria from the United States, MCWA trained state and local health department staff in malaria control techniques and strategies from offices like this. Credit: CDC-PHIL

This historic image depicts one of the buildings that housed members of the Office of Malaria Control in War Areas (MCWA) program, established in 1942, which was the precursor to the current U.S. Centers for Disease Control and Prevention (CDC). Credit: CDC-PHIL

In 1945, Office of Malaria Control in War Areas (MCWA) personnel gathered for a meeting to analyze data received during investigations in the field. These data were used to formulate an epidemiologic plan of action in order to solve an unidentified epidemiologic outbreak and ultimately eliminate malaria from the United States. The MCWA was the forerunner organization for what is now known as CDC. Included in this image are malaria elimination pioneers Drs. Mark F. Boyd and Melvin H. Goodwin. Credit: CDC-PHIL

In 1946, the Communicable Disease Center, or CDC, opened in the old Office of Malaria Control in War Areas (MCWA) in downtown Atlanta, Georgia. The agency was located in Atlanta (rather than Washington, DC) because the southern United States was the area of the country with the most malaria transmission. Credit: CDC-PHIL
To receive email updates about this page, enter your email address:
New! Locally Acquired Cases of Malaria in Florida, Texas, Maryland, and Arkansas
New! Update to Guidance for use of Artemether-Lumefantrine (Coartem®) in Pregnancy for Uncomplicated Malaria New! Discontinuation of CDC’s Distribution of Intravenous Artesunate as Commercial Drug Guidance for Malaria Diagnosis in Patients Suspected of Ebola Infection in the United States -->
See all Malaria Notices
- New! Malaria is a Serious Disease
- New! La malaria (paludismo) es una enfermedad grave
- How to Report a Case of Malaria
- CDC Yellow Book
- Red Pages: Malaria-endemic areas by country
- Drugs for Prevention
- Choosing a Drug to Prevent Malaria
- Drugs for Treatment in the U.S.
- Frequently Asked Questions (FAQs)
- Blood Banks
Click here for contact information
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

From malaria, to smallpox, to polio – here’s how we know life in ancient Egypt was ravaged by disease
Senior Lecturer in Microbiology, Western Sydney University
Disclosure statement
Thomas Jeffries does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Western Sydney University provides funding as a member of The Conversation AU.
View all partners
The mention of ancient Egypt usually conjures images of colossal pyramids and precious, golden tombs.
But as with most civilisations, the invisible world of infectious disease underpinned life and death along the Nile. In fact, fear of disease was so pervasive it influenced social and religious customs. It even featured in the statues, monuments and graves of the Kingdom of the Pharaohs.
By studying ancient specimens and artefacts, scientists are uncovering how disease rocked this ancient culture.
Tutankhamun’s malaria, and other examples
The most direct evidence of epidemics in ancient Egypt comes from skeletal and DNA evidence obtained from the mummies themselves.
For instance, DNA recovered from the mummy of the boy pharaoh Tutankhamun (1332–1323 BC) led to the discovery he suffered from malaria , along with several other New Kingdom mummies (circa 1800 BC).
In other examples:
- skeletal and DNA evidence found in the city of Abydos suggests one in four people may have had tuberculosis
- the mummy of Ramesses V (circa 1149–1145 BC) has scars indicating smallpox
- the wives of Mentuhotep II (circa 2000 BC) were buried hastily in a “mass grave”, suggesting a pandemic had occurred
- and the mummies of two pharaohs, Siptah (1197–1191 BC) and Khnum-Nekht (circa 1800 BC), were found with the deformed equinus foot which is characteristic of the viral disease polio.
Signs of a disease-ravaged people
Amenhotep III was the ninth pharaoh of the 18th dynasty, and ruled from about 1388–1351 BC.
There are several reasons experts think his reign was marked by a devastating disease outbreak. For instance, two separate carvings from this time depict a priest and a royal couple with the polio dropped-foot.

Statues of the lion-headed goddess of disease and health, Sekhmet, also increased significantly , suggesting a reliance on divine protection.
Another sign of a potential major disease outbreak comes in the form of what may be an early case of quarantine, wherein Amenhotep III moved his palace to the more isolated site of Malqata. This is further supported by the burning of a workers’ cemetery near Thebes.
Grave goods also became less extravagant and tombs less complex during this period, which suggests more burials were needed in a shorter time frame. These burials can’t be explained by war since this was an unusually peaceful period.
Did disease trigger early monotheism?
Amenohotep’s son – “the heretic King” Akhenaten (who was also Tutankhamun’s father) – abandoned the old gods of Egypt. In one of the earliest cases of monotheism, Akhenaten made worship of the Sun the official state religion.

Some researchers think Akhenaten’s dramatic loss of faith may have been due to the devastating disease he witnessed during his childhood and into his reign, with several of his children and wives having died from disease. But we’ve yet to find clear evidence for the role of disease in shaping his theology.
There’s also no direct DNA evidence of an outbreak under his father, Amenhotep III. There are only descriptions of one in letters Amenhotep III and Akhenaten exchanged with the Babylonians.

To confirm an outbreak under Amenhotep III, we’d need to first recover pathogen DNA in human remains from this time, has been found in other Egyptian burial sites and for other pandemics .
Also, while many ancient epidemics are referred to as “plagues”, we can’t confirm whether any outbreaks in ancient Egypt were indeed caused by Yersinia pestis , the bacteria responsible for bubonic plague pandemics such as the Black Death in Europe (1347-1351).
That said, researchers have confirmed the Nile rat, which was widespread during the time of the Pharaohs, would have been able to carry the Yersinia infection.

How were outbreaks managed?
Much like modern pandemics, factors such as population growth, sanitation, population density and mobilisation for war would have influenced the spread of disease in ancient Egypt.
In the case of war, it’s thought the Hittite army was weakened by disease spread when it was famously defeated by Egyptian Pharaoh Ramses the Great in the battle of Kadesh (1274 BC).
In some ways, Egyptian medicine was advanced for its time. While these outbreaks occurred long before the development of antibiotics or vaccines, there is some evidence of public health measures such as the burning of towns and quarantining people. This suggests a basic understanding of how disease spreads.
Diseases caused by microorganisms would have been viewed as supernatural, or as a corruption of the air . This is similar to other explanations held in different parts of the world, before germ theory was popularised in the 19th century.
New world, old problems

Many of the most widespread diseases that afflicted the ancient world are still with us.
Along with Tutankhamun, it’s thought up to 70% of the Egyptian population was infected with malaria caused by the Plasmodium falciparum parasite – spread by swarms of mosquitoes occupying the stagnant pools of the Nile delta.
Today, malaria affects about 250 million people, mostly in developing nations. Tuberculosis kills more than a million people each year. And smallpox and polio have only recently been eradicated or controlled through vaccination programs.
More work is yet to be done to detect individual pathogens in Egyptian mummies. This knowledge could shed light on how, throughout history, people much like us have grappled with these unseen organisms.
Read more: Did everyone in Bridgerton have syphilis? Just how sexy would it really have been in Regency era London?
- Disease control
- Medical Histories
- Ancient Egypt
- Tutankhamun
- Ancient DNA
- The Pharaohs
- Egyptian history
- Ancient world

Research Assistant (AIM Clinic)

Visiting Professor - 2024-25 Australia-Korea Chair in Australian Studies at Seoul National University

Dean, School of Computer, Data and Mathematical Sciences

School of Social Sciences – Academic appointment opportunities

Union Organiser (part-time 0.8)
share this!
March 7, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New study finds the malaria parasite generates genetic diversity using an evolutionary 'copy-paste' tactic
by European Molecular Biology Laboratory
By dissecting the genetic diversity of the most deadly human malaria parasite—Plasmodium falciparum—researchers at EMBL's European Bioinformatics Institute (EMBL-EBI) have identified a mechanism of 'copy-paste' genetics that increases the genetic diversity of the parasite at accelerated time scales. This helps solve a long-standing mystery regarding why the parasite displays hotspots of genetic diversity in an otherwise unremarkable genetic landscape.
Malaria is most commonly transmitted through the bites of female Anopheles mosquitoes infected with P. falciparum. The latest world malaria report states that in 2022, there were an estimated 249 million malaria cases and over 600,000 malaria deaths across the globe. 94% of malaria cases and 95% of malaria deaths are found in Africa, with infants, pregnant women, travelers, and people with HIV/AIDS being at higher risk.
The new study, published in the journal PLOS Biology , provides key insights into the evolutionary history of P. falciparum through the analysis of two genes that encode surface proteins critical to immune evasion. The genes in question are DBLMSP and DBLMSP2.
These findings deepen our understanding of how the malaria parasite has evolved and could help to inform new approaches to vaccine development, offering hope for more effective prevention methods against a disease that continues to impact millions globally.
Copy-paste genetics
Usually, the sequence of an individual's gene is inherited from their parents, but in some circumstances, part of a gene sequence can be copied between different genes on the same DNA molecule—this is known as non-allelic gene conversion. This process has been linked to the evolution of important gene families, including those involved in the functioning of the human immune system.
One of this study's key discoveries is that gene conversion takes place between the P. falciparum DBLMSP and DBLMSP2 genes and results in increased genetic diversity within the surface proteins of the parasite. Since these proteins are exposed to, and interact with our immune system, they are potential vaccine targets, and a fuller understanding of their genetic diversity could be very valuable for vaccine design.
"The discovery of 'copy-paste' genetics within malaria's DNA reveals the impact of an underestimated evolutionary mechanism," said Brice Letcher, Postdoctoral Researcher at the Laboratory for Biology and Modeling of the Cell (LBMC, France) and former Ph.D. student at EMBL-EBI.
"Here we show that gene conversion was a potentially important strategy behind malaria's ability to adapt and thrive in humans, including possibly to evade the human immune system. Understanding this genetic flexibility offers new perspectives on malaria's persistence in and adaptation to the human host."
Mapping hidden genetic diversity in malaria parasites
Any immune-interacting protein is potentially a vaccine target, but knowledge of global genetic diversity is an important requirement for vaccine development . For example, influenza and SARS-CoV-2 vaccines are developed based on the knowledge of how their genomes have evolved.
However, the very unusual hotspots of genetic diversity in the P. falciparum DBLMSP and DBLMSP2 genes are so extreme that current algorithms for mapping genetic variants failed to capture them, leaving researchers unaware of a large proportion of the variation in these genes.
To address this, the researchers developed new bioinformatics software that uses genome graphs and analyzed a broad sample of parasites from 29 countries. This new approach revealed a wide range of previously hidden variants, and with these, they were able to demonstrate that multiple gene conversion events had occurred. These new variants, available for download from the website linked to the study , provide a valuable resource for the malaria research community.
"Genome graphs are a great bioinformatics method to help us decode the complex genetic landscapes arising from the interplay between pathogens and human hosts," said Sorina Maciuca, co-author and former Ph.D. student in the Iqbal group and Genomics Data Scientist at Genomics England. "They allow us to take into account a broader spectrum of genetic diversity and obtain new insights into how pathogens like P. falciparum evolve and evade our immune defenses."
What are genome graphs?
The traditional approach in genomics is to define one reference genome and describe any other genome as a set of small differences from this reference. This does not work well when genomes differ too much. Genome graphs take a population of genomes and build an ensemble reference that is aware of all of the genetic variation in the species.
"This research provides a comprehensive map of genetic diversity of these two fascinating genes in P. falciparum," said Zamin Iqbal, Group Leader at EMBL-EBI and Professor of Algorithmic and Microbial Genomics at the University of Bath.
"We have been trying to understand the unusual patterns in these genes for almost a decade now, and our best hypothesis had been that the really different 'versions' of the gene were being preserved by natural selection, for unknown reasons.
"We have shown here that, in fact, this copying mechanism—gene conversion—has been repeatedly creating these anomalous different 'versions' of the genes. This data not only enhances our grasp of malaria's biology, but also will be valuable to researchers across the world studying these genes and their interaction with our immune system."
Journal information: PLoS Biology
Provided by European Molecular Biology Laboratory
Explore further
Feedback to editors

Einasto Supercluster: The new heavyweight contender in the universe
23 minutes ago

Protein fragments ID two new 'extremophile' microbes—and may help find alien life
56 minutes ago

Bridging the gap: Computer scientists develop model to enhance water data from satellites

Researchers develop a new strategy to enhance blue perovskite LED performance

Brighter, cheaper blue light could revolutionize screen technology

Gender and racial discrimination uncovered in leadership positions at Australia's leading universities

The 'baritone' of red giants refines cosmic distance measurements

Even inactive deep-sea 'smokers' are densely colonized by microbial communities, study shows
Meteorology research: Weak polar vortex makes weather more predictable

Scientists develop new system to record 2D crystal synthesis in real time
2 hours ago
Relevant PhysicsForums posts
Potentially fatal dog parasite found in the colorado river.
3 hours ago
Biological culture and cultural biology
15 hours ago
Electrical potential difference and charge separation
18 hours ago
Are all biological catabolic reactions exergonic?
Mar 13, 2024
Nick Lanes on Sean Carroll's podcast
Mar 11, 2024
A First of Its Kind: A Calcium-based signal in the Human Brain
Mar 9, 2024
More from Biology and Medical
Related Stories
Discovery of key molecules involved in severe malaria – new target for malaria vaccine
Dec 4, 2017
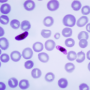
Researchers map druggable genomic targets in evolving malaria parasite
Jan 11, 2018
Unlocking the genetic secrets of the malaria parasite
Oct 7, 2019

Genome structure of malaria parasites linked to virulence
Feb 4, 2019

For a new approach to fighting malaria, study focuses on special RNA molecules in human malaria parasite
Aug 28, 2023

New model may explain rarity of certain malaria-blocking mutations
Oct 8, 2020
Recommended for you

Scientists generate new targeted protein degradation system that tunes a cell's own proteins
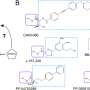
Study shows that antibiotics targeting the same enzyme elicit varied responses
Research team identifies three bacteria species in the human gut that can break down cellulose
Research uncovers specific protein interactions needed for cells to break down and remove damaged mitochondria
21 hours ago
New bioengineered protein design shows promise in fighting COVID-19
22 hours ago

Dragonflies with waxy coating better able to resist a warming climate, research suggests
Mar 14, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Med Sci (Basel)

An Overview of Malaria Transmission Mechanisms, Control, and Modeling
Merveille koissi savi.
1 Center for Development Research ZEF, University of Bonn, Genscherallee 3, 53113 Bonn, Germany; [email protected] or ude.dravrah.hpsh@ivaskm
2 T.H. Chan Harvard School of Public Health, Boston, MA 02115, USA
Associated Data
All data are generated from the content of publications mentioned in this manuscript and they are equally available from the corresponding authors on reasonable request.
In sub-Saharan Africa, malaria is a leading cause of mortality and morbidity. As a result of the interplay between many factors, the control of this disease can be challenging. However, few studies have demonstrated malaria’s complexity, control, and modeling although this perspective could lead to effective policy recommendations. This paper aims to be a didactic material providing the reader with an overview of malaria. More importantly, using a system approach lens, we intend to highlight the debated topics and the multifaceted thematic aspects of malaria transmission mechanisms, while showing the control approaches used as well as the model supporting the dynamics of malaria. As there is a large amount of information on each subject, we have attempted to provide a basic understanding of malaria that needs to be further developed. Nevertheless, this study illustrates the importance of using a multidisciplinary approach to designing next-generation malaria control policies.
1. Introduction
Approximately 2000 children die daily from malaria, with 90% of the victims located in sub-Saharan Africa (SSA) [ 1 , 2 ]. Transmission and control of malaria are mediated by complex interactions and feedback loops among humans, mosquitoes, parasites, their environments, healthcare systems, and policy implementation at a given period of time [ 3 ]. Therefore, malaria transmission can be described as complex, nonlinear, and dynamic. The endemism of malaria in West Africa, for example, has huge economic impacts [ 2 , 4 ]. In Ghana, the cost of treating a single episode of malaria can reach 34% of a household’s yearly income [ 5 ]. As a result of infected people being unable to produce wealth, and as wealth is low, the risk of exposure to malaria increases. There is a negative relationship between national economic growth and malaria control expenditures [ 2 , 6 ]. As a result, malaria can be considered both as a cause of poverty and as a consequence of it [ 7 ].
Furthermore, past interventions have been ineffective as they were unable to account for the nonlinear nature of malaria infection. Particularly, the unexpected consequences of interventions such as mosquito resistance to chemical compounds and parasite resistance to drugs led to their abandonment. This was the case with the eradication control program that was abandoned in 1969 and the Global Malaria Control Strategy in 1992 [ 8 ]. Scientists have widely acknowledged the complexity of malaria, but a depiction of this complexity is weakly documented, and further limits the scope of malaria control.
This short review embracing several topics or thematic areas provides the reader with a piece of basic knowledge of malaria transmission, control, and modeling while illustrating its complexity. Therefore, the manuscript intends to be a didactical material, providing a brief overview of the disease, reducing information asymmetry, while arousing the curiosity of readers who can refer to more specific literature.
2. Materials and Methods
We conducted an unsystematic narrative review of online literature to gain a deeper insight into malaria transmission mechanism, control strategy, and modeling approaches. Thus, we adopted the following approach ( Figure 1 ).

Synthesis of the method. In gray, the final objective of the review is shown, in green, the investigated databases are indicated, in yellow, the exclusion process is indicated, and in red, the clustering of the selected papers is shown.
2.1. Inclusion and Exclusion Criteria for Paper Eligibility
Our search for peer-reviewed papers on malaria in the online scientific database was restricted to the period from 1950 to 2020. The rationale for this time window lies in the fact that the first malaria model was developed in the 1950s and since then, most of the recommendations for malaria control are supported by quantitative analysis. Besides, we excluded all duplicated letters, narrative reviews, case reports, and commentary. Given the tremendous research done on malaria, we limited the scope of the study to only peer-reviewed published papers in either French or English. This was to avoid evidence that may be challenging to replicate and biased translation. Within the topic of interest, we exclude all case reports which happen in non-endemic areas.
2.2. Search Strategy
We searched three databases PUBMED, Google Scholar, and MEDLINE for relevant literature on malaria from 1950 to 2020.
2.3. Screening of Studies and Evidence Extraction
Our screening process focuses on the title of the study published in peer-reviewed journals. Our next step was to review the papers’ abstracts and remove the studies that did not fit under the umbrella of the keywords.
2.4. Analysis and Gaps in the Analysis
To get a comprehensive understanding of malaria, we categorized the list of 77 eligible peer-reviewed papers into topics of interest, such as mechanisms, prevention and treatment, mathematical modeling, and perspectives on modeling. The topical organization of the collected papers allowed us to get a broad perspective of the state of the art.
We found 2,150,000 studies after the search. A total of 150,000 studies were obtained after removing duplicates. We included only 300 studies after screening both titles and abstracts. Furthermore, we removed 50 case reports, 146 studies in malaria non-endemic areas, and 16 reports including 10 thesis and 6 NGO reports.
Using the remaining 77 eligible studies, we provided an overview of each topic and complemented our narrative with WHO, CDC reports, and 10 complimentary readings. There were 23 studies supporting malaria transmission, 19 on prevention and treatment, 23 on mathematical modeling, and 12 on future research on malaria modeling ( Figure 2 ).

Overview of the thematic distribution of the selected papers.
3.1. Malaria Transmission Mechanism
Malaria infection is caused by interactions between humans, mosquitoes, and the pathogen ( Figure 3 ). Female mosquitoes change during the egg-laying period when they experience a deficiency in protein and plant sucrose necessary for egg maturation. Consequently, they blood-feed on humans and can infect them after effective biting where infectious protozoans (of the genus Plasmodium ) are injected into the human bloodstream [ 9 , 10 ].

Complex interactions between humans, mosquitos, and parasites explaining the persistence of malaria. Plasmodium parasites, mosquitoes, and humans constitute three subsystems that interact with one another. The green cycle represents the human sub-system (SS). In addition to the micro-environment, which includes housing conditions, network connectivity, socioeconomic conditions, and the environment within the host (i.e., the individual’s genetic makeup), this SS is characterized by the social environment (social interaction, culture, and behavior regarding malaria risk-taking). The red indicates the SS of mosquitoes defined by the growth conditions favored by humans using or not either ITNs or IRS, and their adaptation to their living environment, including the harsh environmental conditions of cities, such as dirty water and weather conditions. In purple, Plasmodium parasite SS is characterized by interactions with the environment inside the host that may drive resistance to malaria drugs.
Female mosquitoes most found in West Africa belong to the Anopheles Giles genus [ 11 ]. Anopheles gambiae ( sensu stricto ) prefers to use human blood (anthropophilic) to complete its gonotrophic cycle, bites primarily indoors (endophagic), and rests indoors (endophilic). As far as its feeding habits are concerned, An . gambiae is the major malaria vector in West Africa [ 12 ]. In addition, there are other vectors, such as An. arabiensis , An. funestus and An. melas able to carry P. falciparum . Similar to An. gambiae , An. funestus is anthropophilic, endophagic, and endophilic [ 4 , 13 ]. On the other hand, An. arabiensis is zoophilic (prefers blood-feeding animals), exophilic (bites outdoors), and exophagic (rests outdoors) [ 11 ]. An. melas is also anthropophagic, exophagic, and exophilic [ 12 ]. Therefore, P. falciparum adapts well to Anopheles species, increasing malarial vulnerability in the host. The wide range of vector conditions, feeding habits, and geographical heterogeneity in malaria transmission make malaria modeling even more challenging [ 14 ].
A mosquito’s ability to adapt to environmental conditions such as humidity and temperature, is influenced by its sensitivity to those conditions. This adaptation can take the form of a change in the life cycle length, or the biting efficiency [ 15 , 16 , 17 , 18 , 19 ]. As a long-term study carried out in Kenya (1999–2010) has shown, vectors’ feeding habits and effectiveness can change over time [ 20 ].
The transmission intensity may differ significantly across host populations because vectors’ attraction to the host varies [ 21 ]. This variation is elicited by the difference in human bodies’ microflora and their reaction to mosquito bites [ 22 ]. For instance, an infected host is more attractive to other mosquitoes than a non-infected one [ 23 ]. Likewise, mosquitoes prefer the scent of children to that of the adults [ 21 , 24 ].
In SSA, infectious protozoans are, Plasmodium vivax, P. ovale, P. malariae, P. knowlesi, and P. falciparum where P. falciparum accounts for 95% of malaria cases [ 25 , 26 ]. P. falciparum ’s complete life cycle consists of an incubation period of 10 days in the vector and a period of 7 to 20 days in the human host [ 27 ]. When P. falciparum protozoans are injected into a host’s bloodstream via the vector saliva, the protozoan completes its life cycle. After entering the host bloodstream, the sporozoites (asexual protozoans) move into the liver, where they penetrate the hepatocytes (liver cells). In the host hepatocytes, the parasite divides asexually several times, a process known as schizogony [ 28 ]. Eventually, the hepatocytes break, releasing merozoites which infest red blood cells in the host bloodstream. The parasites can diverge into gametocytes after repeated divisions of the red blood cells [ 29 ]. Malaria can cause symptoms such as fever, chills, and sweat before and during the differentiation of P. falciparum into sexual stages. Even so, some hosts may not exhibit clinical symptoms and are considered asymptomatic [ 30 ]. Whenever mosquitoes bite infected people (symptomatic and asymptomatic), they primarily suck out the gametocytes [ 29 ]. Detecting asymptomatic individuals in endemic areas can be challenging, and mass testing may not always be cost-effective in detecting such individuals [ 31 ].
3.2. Malaria Prevention and Treatment
Due to recursive interactions between humans, mosquitoes, and parasites that cause malaria transmission, existing control methods might not be effective in the sense that they target either mosquitoes or parasites. Mosquitoes have been controlled mainly using the obliteration of larvae and adult stages while reducing the entomological inoculation rate. These controls include physical, biological, or chemical changes to the vector’s environment. As part of the physical modification, breeding sites are removed, and the sources of larvae are managed using drainage and weeding [ 32 ]. However, this method requires a constant working force needed for weeding and drain cleaning which led SSA governments to abandon it although it is among the most effective [ 33 ]. Chemical modification of larval environments includes the application of larvicides and insecticides. The downside of this method is that it is also costly, and mosquitoes often become resistant to insecticides [ 34 ]. Natural enemies of mosquito larvae, such as larvivorous fish and bacteria (e.g., Bacillus thuringiensis ), are used in the biological method. Nevertheless, this process is hindered by the temporary persistence of natural enemies [ 32 ].
The Global Malaria Action Plan (GMAP) and the World Health Organization (WHO) recommended the use of indoor residual spray (IRS) and insecticide-treated bed nets (ITNs) for controlling mosquito adult populations in the 2010s across Africa [ 35 ]. The main chemical component of ITNs and IRS is synthetic pyrethroid, a lethal compound, that repels mosquitoes, remains in the environment, and is harmless to mammals. From 2005 to 2010, malaria cases in Ghana declined by 41% due to the successful use of ITNs and IRS [ 36 ]. The residual effects of synthetic pyrethroids in the environment resulting from IRS, ITNs, and agricultural pest management force mosquitoes to develop coping mechanisms [ 37 ]. These include indoor and outdoor spray avoidance mechanisms, earlier biting, and insecticide resistance [ 38 ].
The control of malaria parasites is achieved through three lines of defense: antimalarial drugs, seasonal prevention, and vaccination. The first line of the curative measures adopted by GMAP is the Artemisinin-based Combination Therapies (ACT). ACTs are highly potent against P. falciparum with a higher clearance rate and symptoms resorption than other curative therapies [ 39 ]. Nevertheless, P. falciparum has progressively developed ACT resistance [ 40 ]. Malaria parasite resistance in SSA is exacerbated by using counterfeit malaria drugs [ 41 , 42 ]. Another preventive measure is intermittent prevention, which involves administering single-dose malaria therapy to pregnant women [ 43 ]. The treatment includes the combination of primaquine, sulfadoxine-pyrimethamine, amodiaquine, methylene blue, and dihydroartemisinin-piperaquine which have been proven to be able to prevent the transmission of P. falciparum [ 44 ]. A seasonal vaccine “RTS, S/AS01” is proven effective for children under 5 years old for a short period with an efficacy rate of 82% [ 45 , 46 ]. The authors demonstrated that RTS, S/AS01 induces an immune response to the proteins of infected sporozoites. Vaccine components protect against clinical malaria. The effectiveness of its protective action can wane over time and with advancing age. Particularly, children between the ages of 6 to 12 weeks benefit from the vaccine more than children between the ages of 5 to 17 months.
A less explored line of malaria treatment consists of phytotherapy. For example, a study on phytochemicals identified more than 20 local plants and herbs in central Africa that can heal and prevent malaria transmission with almost zero risks of P. falciparum developing resistance against these natural compounds [ 47 , 48 , 49 ]. According to the authors, the synergistic interplay between a wide range of chemical substances in the plants enables treating malaria holistically while preventing the development of resistance [ 48 , 50 ].
3.3. Mathematical Modeling of Malaria
Mathematical models developed to gain insight into disease transmission have influenced past and present interventions to prevent or treat diseases. Mathematical modeling of malaria transmission began in 1911 with the Susceptible-Infectious-Removed model (SIR), which compartmentalized the population of hosts and vectors into three groups [ 51 , 52 ]. The compartments were denoted susceptible (S), referring to the population likely to become infected, infectious (I), composed of the fraction of the infected population, and removed/ recovered (R), accounting for the fraction of the population that either died or healed from the disease. Several assumptions were assumed in the SIR model, including a closed and finite population, a homogeneous mosquito bite rate, and a well-mixed population. Although the Ross model (SIR) was unable to adjust to the new incidence data due to its limited predictability, SIR provides insights into the intricate relationship between infected hosts and mosquito densities. Because Ross’s model was theoretical and simulation-based, it failed to document proof for campaigns for mosquito eradication.
Subsequently, George Macdonald complemented the SIR model [ 51 ] by feeding the model with real data and embedding an additional compartment for the latency period between the mosquito bite and the onset of symptoms denoted Exposed (E). Additionally, Macdonald’s study argued for eradicating mosquitoes through a massive campaign based on the theory of superinfection [ 53 ]. Consequently, between 1955 and 1969, WHO implemented widespread and rigorous mosquito eradication campaigns as part of the Global Malaria Eradication Program (GMEP). In these campaigns, the pesticide Dichlorodiphenyltrichloroethane (DDT) was successfully used to control malaria in major European and American countries [ 54 ]. The GMEP, however, failed to eradicate malaria worldwide due to mosquito resistance [ 55 ]. Apart from mosquito insecticide resistance, the absence of basic healthcare services, high malaria transmission intensity, and other socio-ecological factors hindered the success of eradication campaigns in the SSA [ 56 ].
The mathematical epidemiology of malaria has evolved steadily in recent decades from “toy models” (which were not realistic but captured the key features of the disease) to “high-level models” (which are precise and sacrifice generality) [ 57 ]. As such, complex models display common features such as the interaction between many components [ 58 ]. For example, an earlier partial differential model split the infected population into age-dependent infections in the SIR framework [ 59 ].
An important metric that summarizes the transmission of a disease is the reproductive number (R 0 ). R 0 represents the expected number of infected human hosts after effective mosquito bites in a fully susceptible population [ 60 , 61 , 62 ]. Thus, R 0 provides a measurement for the intensity of transmission and contributes to the definition of disease-endemic areas when it is greater than one (R 0 > 1) [ 63 ]. Heterogeneity was therefore incorporated in populations for the computation of R 0 . These include, for example, the age structure of the host [ 64 ], migration of the vectors and hosts [ 65 ], host beliefs and practices [ 66 ], and host income classes [ 67 , 68 ]. R 0 also varies with the degree of complexity introduced into the compartmentalization of the population [ 66 ]. The more the modeling framework embodies the heterogeneity in the host population, the closer to real-life transmission dynamics the value obtained for R 0 becomes [ 63 , 68 , 69 , 70 ]. The pioneering models that provided insight into malaria dynamics and its controls were unrealistic for various reasons, and maximum control has hardly been achieved in [ 56 ]. Most specifically, a large R 0 indicates a higher density of mosquitoes that reduces the effectiveness of ITN in a large and likely heterogeneous host population [ 63 ].
Many mathematical models were developed combining the compartments S, E, I, and R (e.g., SIR, SIS, SI, SIRS, SEIR, SEIRS, SEI) and included the heterogeneity of the population (meta-population modeling approaches) [ 69 ]. Most of these models do not capture the dynamics of subpopulations because they tend to assume large and homogeneous populations that do not take into account the existence and specific characteristics of subpopulations [ 71 , 72 ]. Another issue is that they fail to account for host behavior, which influences the disease’s dynamics and control [ 73 , 74 ]. Besides, compartmental models are either knowledge or data-driven (e.g., Ross model, Macdonald models). The models cannot address the complex interactions between determinants that guide the disease dynamics in both cases. For example, socioeconomic determinants interfere with the parameters generally considered in the calculation of R 0 such as mortality, mobility, and birth rate [ 66 ]. Thus, models that capture, realistically, the transmission process of malaria are still missing.
3.4. Malaria Modeling: Present and Future
The ability to track epidemics became more accurate with the advancement in computing power and the availability of data. The deterministic framework uses in this context provides insight into disease dynamics, but usually fails to take uncertainties into account. Stochastic models, on the other hand, incorporate randomness that may occur during the epidemic and, most importantly, accounts for a large range of uncertainty that occurs during epidemics. For example, a stochastic model was used to predict the complex transmission pattern concerning environmental changes [ 75 ]. Like deterministic models, the stochastic models help to disclose interesting features of the transmission such as the impact of the network structure, and the properties of an outbreak most specifically get insights into the rate of the surge of vector-borne diseases in a new region in a context of climate change [ 76 , 77 ]. Furthermore, both stochastic and deterministic models are population-based and often fail to consider the feature of individuals which alternatively the individual-based models (IBMs) do [ 78 ]. IBMs can account for heterogeneity across individual agents and spatial gradients [ 79 , 80 ]. However, parametrizing them remains a daunting task, especially due to the lack and accuracy of data [ 81 ]. Beyond meta-population modeling (Ross-based models), within-host modeling opened new possibilities to address malaria infectiousness while examining the interplay between hosts’ immune systems and the dynamics of the parasite. However, modeling the superinfection of malaria while tracking each parasite strain and incorporating the diverse genetic makeup of the parasites remains extremely challenging, especially in terms of the model parameterization [ 82 , 83 ]. Most recently, the introduction of a new type of data such as human mobility collected from data providers (Facebook, Twitter, Google, etc.) refined the field of epidemiological surveillance. These data have been used to pinpoint malaria hotspots and predict their spread [ 84 , 85 , 86 ]. The use of fine-grained mobility data opens a new prospect of modeling and documenting evidence for policy recommendations; however, privacy concerns need to be addressed [ 87 ].
4. Discussion
Malaria remains a major health challenge in Sub-Saharan Africa, despite extensive research. Several factors contribute to malaria transmission, including socio-ecological factors and changes in the human host and vector behavior, which may be channeled by parasite genetic changes. Considering malaria’s complex nature, modeling it presents numerous challenges. As an example, it can be difficult to accurately parameterize a model with a compartment of asymptomatic individuals as there is a lack of reliable historical data. However, evidence suggests that malaria symptoms could progress rapidly from none to life-threatening while leading to a lifetime burden of cognitive impairments for some patients. It is thus necessary to develop a strategy that allows for the production at a low cost of reliable primary data on asymptomatic people with the capability of identifying infectious Plasmodium [ 88 ]. Additionally, the review highlighted that for each of the thematic areas discussed, there is a web of determinants that can be studied, using different perspectives including the use of a multidisciplinary lens to examine clinically relevant malaria issues.
A historical approach was used to illustrate the evolution of the malaria model from simplistic to more elaborate models. Moreover, this review outlines the challenges of modeling, where, for instance, introducing mobile data to a mathematical framework may pose a problem of re-identification. The number and scale of parameters involved in a complex modeling approach make model parameterization challenging.
Finally, this review demonstrates that malaria can efficiently be controlled by combining several strategies. Among them are constant awareness and education campaigns in communities at risk and among individuals whose resistance to non-pharmaceutical interventions (such as ITNs) can hinder malaria control efforts.
Given the wide range of thematic areas discussed, we acknowledge that we have only scratched the surface of the various cross-cutting topics. Consequently, we did not elaborate on the host immune response to malaria nor on technological advances in diagnosing and treating malaria since they are widely covered elsewhere [ 89 , 90 , 91 , 92 , 93 , 94 ]. Additionally, this paper faces two challenges, namely (1) a self-selection bias that might lead to a more prominent emphasis on some cross-cutting topics, and (2) not considering recently published papers [ 95 ]. Nonetheless, this review provides readers with an overview of malaria from a global perspective.
Acknowledgments
The author wants to thank Christian Borgemeister, Daniel Callo-Concha, Lauren Childs, Deguenon Jean Marcel, Henri Tonnang, and Joshua Ntajal for their edits, comments, and suggestions. In addition, we would like to extend our gratitude to the Center for Development Research (ZEF), Germany, for providing us with the logistics required for this review.
Abbreviations
Funding statement.
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Center for Development Research (ZEF) ethics committees, University of Bonn.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The author declares no competing interests.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
In the early 21st century the incidence of malaria, and the number of deaths caused by the disease, appeared to be declining. For example, the World Health Organization (WHO) estimated that in 2000 there were 233 million cases of malaria worldwide, which resulted in roughly 985,000 deaths, mostly of young children in Africa. In 2010 there were ...
Malaria is caused by the parasites, not by a virus or by a type of bacterium. If it isn't treated, malaria can cause severe health problems such as seizures, brain damage, trouble breathing, organ failure and death. The disease is rare in the U.S., with about 2,000 cases per year.
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening.
Unlike other dangerous creatures, mosquitoes do their deadly work by spreading diseases - one of the worst of these is malaria. Malaria is an infectious disease caused by a parasite, called Plasmodium that invades red blood cells and liver cells. The parasites are transferred to humans by the bite of an infected Anopheles mosquito.
Malaria disease can be categorized as uncomplicated or severe (complicated). In general, malaria is a curable disease if diagnosed and treated promptly and correctly. All the clinical symptoms associated with malaria are caused by the asexual erythrocytic or blood stage parasites. When the parasite develops in the erythrocyte, numerous known ...
Malaria is a climate-sensitive disease, influenced by changes in temperature, rainfall, and humidity. There is growing concern that such changes might alter malaria's geographical distribution and transmission, expanding it into areas in which the disease has been controlled or in non-endemic areas. Patterns of distribution of vector-borne ...
Malaria — Epidemiology, Treatment, ... The narrative takes a deep dive into prevention of the disease, including strategies to control mosquito vectors, new vaccines, and monoclonal antibodies. ...
Sub-Saharan Africa carried the brunt of the disease, where a child probably died of malaria every 45 seconds and efforts to control the disease were very limited. Yet this desperate situation had ...
Malaria has had a profound effect on human lives for thousands of years and remains one of the most serious, life-threatening infectious diseases 1-3.The disease is caused by protozoan pathogens ...
Malaria is a disease that typically features a fever, chills, and headaches. It can progress to cause severe or life threatening complications. How it affects people can vary widely.
Malaria. Malaria is a mosquito-borne disease caused by a parasite. People with malaria often experience fever, chills, and flu-like illness. Left untreated, they may develop severe complications and die. In 2020 an estimated 241 million cases of malaria occurred worldwide and 627,000 people died, mostly children in sub-Saharan Africa.
Malaria is a disease caused by a parasite. The parasite is spread to humans through the bites of infected mosquitoes. People who have malaria usually feel very sick with a high fever and shaking chills. While the disease is uncommon in temperate climates, malaria is still common in tropical and subtropical countries. Each year nearly 290 ...
Global Malaria Epidemiology. The number of total malaria cases globally increased in 2021 (from 245 million in 2020 to 247 million in 2021), with most of the increase occurring in Africa. However, case incidence remained stable from 2020 to 2021 (59 cases per 1000 population at risk) following an increase from 2019 (57 cases/1000 population ...
690 Words. 3 Pages. Open Document. It is one of the ten deadliest diseases of all time. It effects men, women, children, and animals. It is in full force in Africa, India, Asia, China, South America, and the Caribbean. This disease is malaria. Nearly 40 percent of the world's population lives in areas that are effected by the disease.
View Full Text ; View PDF ; Epidemiology of Plasmodium vivax in Duffy negatives and Duffy positives from community and health centre collections in Ethiopia. Malaria remains a significant cause of morbidity and mortality in Ethiopia with an estimated 3.8 million cases in 2021 and 61% of the population living in areas at risk of malaria transmission.
At the country level, the governance of malaria can have a direct impact on elimination of the disease. In malaria endemic countries, the National Malaria Control/Elimination Program ... For the analysis, we first conducted a literature search on each disease control program and identified pivotal papers and publications that discuss governance ...
Study: Malaria vaccination: hurdles to reach high-risk children.Image Credit: Media Lens King/Shutterstock.com. Background. Despite decades of efforts, malaria remains a significant health ...
Malaria, an endemic disease caused by hematozoic parasites (Plasmodium falciparum) transmitted by the blood to humans through the bite of the female anophele mosquito. Researchers have sequenced ...
Introduction. Malaria is the symptomatic illness caused by the mosquito transmitted parasites Plasmodium falciparum, P vivax, P ovale, P malariae, and P knowlesi.It is one of the most common causes of fever in many malaria endemic countries and in travellers returning from those countries. 1 The World Health Organization estimated 249 million malaria cases in 2022 worldwide, 94% attributable ...
This is why knowledge about the disease is given due importance in this essay on malaria awareness. While high temperature and headache are the most common signs of malaria, nausea and drowsiness are also found in sick people. By detecting the disease early, we will be able to start the treatment soon, thus reducing the risk in children.
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a ...
Nigeria, with the highest burden of malaria globally, has conducted a deep dive to understand the distribution of the disease, leading to a subnational malaria report with tailored state-level interventions. Ghana, another high-burden country, registered a 33% decrease in malaria cases and a 7% decrease in deaths between 2015 and 2021.
Despite the global efforts towards malaria elimination, around 63,000 deaths were reported globally between 2019 and 2021, mainly due to disruption to essential, malaria-related services during the COVID-19 pandemic, increasing the need to accelerate efforts to eliminate the disease [].A new challenge arising in this scenario is the spread of Anopheles stephensi, native to South Asia and parts ...
Early malaria control work consisted primarily of insecticide spraying and draining water from mosquito breeding sites. In 1951, malaria was declared eliminated from the United States. Now, 70 years later, our name may have changed, but our mission to stamp out malaria—both internationally and domestically—remains very much intact.
Signs of a disease-ravaged people. Amenhotep III was the ninth pharaoh of the 18th dynasty, and ruled from about 1388-1351 BC. There are several reasons experts think his reign was marked by a ...
Today, malaria affects about 250 million people, mostly in developing nations. Tuberculosis kills more than a million people each year. And smallpox and polio have only recently been eradicated or ...
The latest world malaria report states that in 2022, there were an estimated 249 million malaria cases and over 600,000 malaria deaths across the globe. 94% of malaria cases and 95% of malaria ...
Español. Ministers of Health from African countries with the highest burden of malaria committed today to accelerated action to end deaths from the disease. They pledged to sustainably and equitably address the threat of malaria in the African region, which accounts for 95% of malaria deaths globally. The Ministers, gathering in Yaoundé ...
As a result of the interplay between many factors, the control of this disease can be challenging. However, few studies have demonstrated malaria's complexity, control, and modeling although this perspective could lead to effective policy recommendations. This paper aims to be a didactic material providing the reader with an overview of malaria.