- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries

The Pathway to Becoming a Clinical Research Associate
Introduction to a Clinical Research Associate Career
The role of a clinical research associate, essential skills for a successful cra, certification and education requirements, clinical research coordinators (crc) and clinical trial assistants (ctas), entry-level cra positions, career progression in research, clinical trial project manager (pm) or clinical trial manager (ctm), clinical trial it specialist, conclusion and future trends in cra careers.
The clinical research enterprise supports the discovery of novel therapies, interventions, and diagnostics in many therapeutic areas and applications. Driven by technological enhancements and gains in precision medicine, clinical research professionals are in high demand. Speculative reports indicate the clinical trial market will reach over 70 billion dollars by 2028. [1] With concerns over the anticipated shortages of clinical research associates (CRAs), there are many training options available to choose from.
CRAs can expect to make between 60K and 120K+ per year, depending on a combination of experience, job requirements, location, and education. Individuals may seek employment opportunities from pharmaceutical companies, contract research organizations (CROs), and the federal government. Since they are part of the enterprise of improving patient outcomes, most find their careers in clinical research highly rewarding.
The primary work of a clinical research associate (CRA) consists of monitoring the activities of a clinical research site. Their scope of responsibility also includes facilitating communications between sponsors and research sites, ultimately helping a clinical study reach a successful completion.
A CRA is tasked with overseeing the implementation of a clinical trial protocol in a manner that prioritizes patient safety and data integrity. Specific activities include monitoring:
- Subject recruitment
- Subject screening and enrolment
- Adherence to Good Clinical Practice
- Generation of complete and accurate documentation
- Recording of study data into case report forms and electronic data capture systems
- Study site management
CRAs serve as the nexus among key study stakeholders, such as pharmaceutical companies, principal investigators, clinical research coordinators (CRCs), and clinical data managers. They are core facilitators in the production of the scientific data essential to the development of life-saving diagnostics and therapeutics. [2]
Becoming a successful CRA typically begins with an interest in clinical research. It is common for individuals who enjoy fast-paced work environments, critical thinking, and helping others to choose a research career.
Successful CRAs demonstrate above-average communication, problem-solving, and organization skills. Industry veterans also tend to display a natural tendency towards empathic-based leadership qualities and the ability to work well under pressure.
The CRA is often in charge of multiple study sites. Subsequently, a clinical study professional should be comfortable with travel and demonstrate a high level of computing proficiency to manage substantial amounts of reporting and documentation.
Complementary skillsets that can contribute to the success of a CRA include:
- A comprehensive understanding of activities related to early drug discovery through commercialization
- Advanced medical or scientific knowledge because study data features complex medical terminology, statistical elements, and medical procedures
- Advanced writing abilities to handle the high density of communications
- Well-developed project management skills
Most CRA positions require a bachelor’s degree in life sciences or related fields and a healthy amount of clinical study experience. Several universities offer graduate certificates or master’s degrees in clinical research or clinical trial management. Obtaining a CRA university credential consists of meeting specific eligibility benchmarks determined by educational background, hours of CRA-related experience, letters of recommendation, and industry-specific training records.
Two industry associations offer professional credentials through certification exams. In order to sit for the exam, an aspiring CRA needs to have completed extensive hours working in the field.
The roadmap to obtaining a CRA certification from the dominant professional society mandates the following requirements and benchmarks [3]:
- A minimum of a bachelor’s degree in a life science-related discipline and 3,000 hours of work in human subjects research
- Or an undergraduate or graduate degree (excluding graduate certificates) in the field of clinical research plus 1,500 hours of work in human subjects research
- And an advanced knowledge of applicable regulations, Good Clinical Practice guidelines, and HIPAA requirements
It should be mentioned that professional association credentialing is not necessary to work as a CRA, although some find certification helps obtain employment in certain circumstances.
Career Pathways and Advancement
As with many chosen career paths, the journey to becoming a CRA is not necessarily linear. Many successful CRAs find that training as a CRC allows for greater industry experience and a more fluid transition into a prominent CRA role. A CRC, and sometimes a research nurse, helps conduct a clinical trial and typically works at one site. The transition to a CRA requires a shift in perspective from one who conducts the trial to one who monitors those who conduct trials at several sites.
Alternatively, CRAs may begin their careers as clinical trial assistants (CTAs). CTAs assist with eligibility assessments, personnel management, and participant needs. They also oversee some of the clinical site’s daily functions, which may include correcting safety hazards, maintaining HIPAA and compliance records, and ordering supplies.
Entry-level CRA positions provide a fantastic opportunity to gain more field experience. To help hone their understanding of what is expected, it is common for entry-level CRAs to work alongside other senior members or clinical trial leads.
Hallmarked by extensive administrative and scheduling duties, research data generation and recording, subject recruitment and retention, and determination of study eligibility, entry-level CRAs should learn all the functions that coincide with a clinical trial. Other job functions entail understanding the U.S. Food and Drug Administration’s (FDA) regulatory requirements for clinical research and product market approval.
There are many lucrative management-level opportunities for senior-level CRAs. Most positions require a minimum of a bachelor’s degree. However, some higher-paying jobs may require candidates to have a master’s or doctoral degree, along with a strong record of accomplishment and qualifying experience. CRAs who enjoy mentoring others may also find that exploring training-type positions may provide new opportunities.
Related Career Opportunities in Research
A person in one of these roles is in charge of planning and overseeing the functional details of clinical trials. Working directly for a pharmaceutical company sponsor or CRO, a PM or CTM organizes the personnel, resources, and conduct of research, from initial trial design through study closeout and the final analysis of trial data. The PM or CTM is also responsible for study site management, data capture, analytics, monitoring, validation, and reporting results.
Pivotal to the applications needed to fuel the trend toward decentralized clinical trials, the clinical trial IT specialist is responsible for maintaining the systems used to conduct participant activities and gather study data. This includes optimizing user interfaces for digital functionality and multi-device capabilities. The IT specialist is often responsible for overseeing the integration of electronic data capture systems and maintaining systems used for clinical data analysis. The clinical IT specialist also represents the first line of defense against patient privacy violations, system threats, and HIPAA violations. [4]
Fueled by the need for improved outcomes, we can expect more innovations to emerge from the research sector in the years ahead. There is also a growing momentum to bring more inclusive trial experiences to marginalized populations. Catapulting off an already robust climate, the demand for clinical research professionals will increase significantly. The high demand could dictate the way that the clinical trial industry approaches staffing decisions by hiring based on specific proficiencies.
Current trends hint at a bolder convergence between real-world data and artificial intelligence that may result in improved predictive disease modeling, clinical decisions, and drug optimization. [5] Algorithmic-based capabilities also carry the potential to inform recruitment strategies by removing some of the barriers that impede participation, thereby improving patient diversity.[6] These trends will shape the future of CRA work, thereby demanding that CRAs stay abreast of changes in how clinical trials are designed and implemented.
With so many revolutionary changes to the medical and clinical trial landscape, there has never been a more exciting time to join the research community. Learn more about CRA opportunities today.
1. Markets and Markets Clinical Statistics. Clinical Trials Market Statistics . Accessed June 17, 2024
2. Kandi, V., & Vadakedath, S. (2023). Clinical Trials and Clinical Research: A Comprehensive Review . Cureus, 15(2).
3. Association of Clinical Research Professionals. “ ACRP-CP Certification .” Accessed June 17, 2024.
4. He Y, Aliyu A, Evans M, Luo C. Health Care Cybersecurity Challenges and Solutions Under the Climate of COVID-19: Scoping Review . J Med Internet Res. 2021 Apr 20;23(4): e21747.
5. Harrer, Pratik Shah, Bhavna Antony, and Jianying Hu. Artificial Intelligence for Clinical Trial Design . Trends in Pharmacological Sciences 40(8):577-591. Accessed June 17, 2024
6. Kurt, A., Semler, L., Meyers, M. et al. Research Professionals’ Perspectives, Barriers, and Recommendations Regarding Minority Participation in Clinical Trials . J. Racial and Ethnic Health Disparities 4, 1166–1174 (2017)

- On Research Podcast – Practical Uses of AI in Research
- NIH Reminder: OLAW Annual Reports Due December 1, 2024
- What CITI Program is Reading – September 25, 2024
- CITI Program Funding Finds – September 24, 2024
- Director, Office of Animal Research Compliance
- Research Integrity Coordinator
- Assistant Director, Pre-Award Services
- Clinical Research Nurse
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| BUY_NOW | This cookie is set to transfer purchase details to our learning management system. | |
| CART_COUNT | This cookie is set to enable shopping cart details on the site and to pass the data to our learning management system. | |
| cookielawinfo-checkbox-advertisement | 1 year | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Advertisement". |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| JSESSIONID | session | Used by sites written in JSP. General purpose platform session cookies that are used to maintain users' state across page requests. |
| PHPSESSID | session | This cookie is native to PHP applications. The cookie is used to store and identify a users' unique session ID for the purpose of managing user session on the website. The cookie is a session cookies and is deleted when all the browser windows are closed. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| XSRF-TOKEN | session | The cookie is set by Wix website building platform on Wix website. The cookie is used for security purposes. |
| Cookie | Duration | Description |
|---|---|---|
| bcookie | 2 years | This cookie is set by linkedIn. The purpose of the cookie is to enable LinkedIn functionalities on the page. |
| lang | session | This cookie is used to store the language preferences of a user to serve up content in that stored language the next time user visit the website. |
| lidc | 1 day | This cookie is set by LinkedIn and used for routing. |
| pll_language | 1 year | This cookie is set by Polylang plugin for WordPress powered websites. The cookie stores the language code of the last browsed page. |
| Cookie | Duration | Description |
|---|---|---|
| _gat | 1 minute | This cookies is installed by Google Universal Analytics to throttle the request rate to limit the colllection of data on high traffic sites. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | This cookie is installed by Google Analytics. The cookie is used to calculate visitor, session, campaign data and keep track of site usage for the site's analytics report. The cookies store information anonymously and assign a randomly generated number to identify unique visitors. |
| _gat_UA-33803854-1 | 1 minute | This is a pattern type cookie set by Google Analytics, where the pattern element on the name contains the unique identity number of the account or website it relates to. It appears to be a variation of the _gat cookie which is used to limit the amount of data recorded by Google on high traffic volume websites. |
| _gat_UA-33803854-7 | 1 minute | This is a pattern type cookie set by Google Analytics, where the pattern element on the name contains the unique identity number of the account or website it relates to. It appears to be a variation of the _gat cookie which is used to limit the amount of data recorded by Google on high traffic volume websites. |
| _gcl_au | 3 months | This cookie is used by Google Analytics to understand user interaction with the website. |
| _gid | 1 day | This cookie is installed by Google Analytics. The cookie is used to store information of how visitors use a website and helps in creating an analytics report of how the website is doing. The data collected including the number visitors, the source where they have come from, and the pages visted in an anonymous form. |
| _hjAbsoluteSessionInProgress | 30 minutes | No description available. |
| _hjFirstSeen | 30 minutes | This is set by Hotjar to identify a new user’s first session. It stores a true/false value, indicating whether this was the first time Hotjar saw this user. It is used by Recording filters to identify new user sessions. |
| _hjid | 1 year | This cookie is set by Hotjar. This cookie is set when the customer first lands on a page with the Hotjar script. It is used to persist the random user ID, unique to that site on the browser. This ensures that behavior in subsequent visits to the same site will be attributed to the same user ID. |
| _hjIncludedInPageviewSample | 2 minutes | No description available. |
| _hjIncludedInSessionSample | 2 minutes | No description available. |
| _hjTLDTest | session | No description available. |
| _uetsid | 1 day | This cookies are used to collect analytical information about how visitors use the website. This information is used to compile report and improve site. |
| BrowserId | 1 year | This cookie is used for registering a unique ID that identifies the type of browser. It helps in identifying the visitor device on their revisit. |
| CFID | session | This cookie is set by Adobe ColdFusion applications. This cookie is used to identify the client. It is a sequential client identifier, used in conjunction with the cookie "CFTOKEN". |
| CFTOKEN | session | This cookie is set by Adobe ColdFusion applications. This cookie is used to identify the client. It provides a random-number client security token. |
| CONSENT | 16 years 5 months 4 days 4 hours | These cookies are set via embedded youtube-videos. They register anonymous statistical data on for example how many times the video is displayed and what settings are used for playback.No sensitive data is collected unless you log in to your google account, in that case your choices are linked with your account, for example if you click “like” on a video. |
| vuid | 2 years | This domain of this cookie is owned by Vimeo. This cookie is used by vimeo to collect tracking information. It sets a unique ID to embed videos to the website. |
| Cookie | Duration | Description |
|---|---|---|
| bscookie | 2 years | This cookie is a browser ID cookie set by Linked share Buttons and ad tags. |
| IDE | 1 year 24 days | Used by Google DoubleClick and stores information about how the user uses the website and any other advertisement before visiting the website. This is used to present users with ads that are relevant to them according to the user profile. |
| MUID | 1 year 24 days | Used by Microsoft as a unique identifier. The cookie is set by embedded Microsoft scripts. The purpose of this cookie is to synchronize the ID across many different Microsoft domains to enable user tracking. |
| test_cookie | 15 minutes | This cookie is set by doubleclick.net. The purpose of the cookie is to determine if the user's browser supports cookies. |
| VISITOR_INFO1_LIVE | 5 months 27 days | This cookie is set by Youtube. Used to track the information of the embedded YouTube videos on a website. |
| YSC | session | This cookies is set by Youtube and is used to track the views of embedded videos. |
| yt-remote-connected-devices | never | These cookies are set via embedded youtube-videos. |
| yt-remote-device-id | never | These cookies are set via embedded youtube-videos. |
| Cookie | Duration | Description |
|---|---|---|
| _app_session | 1 month | No description available. |
| _gfpc | session | No description available. |
| _uetvid | 1 year 24 days | No description available. |
| _zm_chtaid | 2 hours | No description available. |
| _zm_csp_script_nonce | session | No description available. |
| _zm_cta | 1 day | No description |
| _zm_ctaid | 2 hours | No description available. |
| _zm_currency | 1 day | No description available. |
| _zm_mtk_guid | 2 years | No description available. |
| _zm_page_auth | session | No description available. |
| _zm_sa_si_none | session | No description |
| _zm_ssid | session | No description available. |
| AnalyticsSyncHistory | 1 month | No description |
| BNI_persistence | 4 hours | No description available. |
| BrowserId_sec | 1 year | No description available. |
| CookieConsentPolicy | 1 year | No description |
| cred | No description available. | |
| f | never | No description available. |
| L-veVQq | 1 day | No description |
| li_gc | 2 years | No description |
| owner_token | 1 day | No description available. |
| PP-veVQq | 1 hour | No description |
| renderCtx | session | This cookie is used for tracking community context state. |
| RL-veVQq | 1 day | No description |
| twine_session | 1 month | No description available. |
| UserMatchHistory | 1 month | Linkedin - Used to track visitors on multiple websites, in order to present relevant advertisement based on the visitor's preferences. |
| web_zak | past | No description |
| wULrMv6t | No description | |
| zm_aid | past | No description |
| zm_haid | past | No description |
- Skip to main menu
- Skip to user menu
How To Become A Clinical Research Associate - A New Scientist Careers Guide
- Finding a job
- Career guides
What does a clinical research associate do?
Clinical research associates (CRAs) are responsible for running clinical research, which consists of trials designed to test new or current drugs/immunisations and analyse their effectiveness, risks, benefits and safety of use.
CRAs play an important role in the healthcare industry and public health development by helping to design and test new medications, vaccinations and other therapeutic agents.
CRAs can be involved at any stage of a drug development trial, including planning, coordinating and supervising. It is their responsibility to ensure a drug has been appropriately examined and all its risks have been evaluated before it is released to be used publicly.
CRAs most commonly work for pharmaceutical companies or contract research organisations. They might also have to spend some time working in a hospital setting to collect data about the drugs they are analysing. They might also work for universities or public/global health organisations.
As a CRA, you may have a range of responsibilities depending on your employer, project and level of experience. Typically, CRAs will need to complete tasks such as:
- Designing and writing trial protocols and standard operating procedures
- Presenting protocols and procedures to steering committees
- Designing data collection forms
- Requesting ethics approvals and working with ethics committees
- Liaising with staff conducting the trials, such as doctors or consultants
- Training local staff based on trial-specific standards
- Monitoring operations during clinical trial data collection
- Collecting completed data collection forms
- Performing data management and analysis, and discussing the results
- Closing trials and finalising reports with the help of a statistician
CRAs will work in a team of other research professionals, including contract organisation or sponsor staff, principal investigators and clinical research coordinators.
How to become a clinical research associate
To become a CRA, you need to obtain a degree in medical sciences, life sciences or nursing. This can be in subjects such as biomedical science , anatomy, physiology, immunology , pharmacology or broader degree subjects like chemistry and biology .
Alternatively, you can access a career as a CRA by acquiring a higher national diploma (HND). This is a qualification equivalent to the second year of a bachelor’s degree. HNDs can be beneficial to those who want to enter more practical fields, clinical research included.
Occasionally, you can enter a CRA role from an administrative background. For instance, if you begin working as a clinical trials administrator/assistant and decide you would prefer the role of a CRA, you can complete additional qualifications to do this. However, this will take some time and can be difficult.
Most employees view undergraduate qualifications as sufficient, but in some cases a postgraduate degree may be beneficial. Master’s degrees and PhDs can gain you an advantage when applying for competitive positions, and help you gain more experience in research .
Work experience is key to securing a clinical research job. You can get this at any point in your training, and some universities may help with this. The types of work experience most useful for a CRA role include:
- Academic research
- Pharmaceutical research (e.g. via a pharmaceutical industry placement during your degree)
- Laboratory work
- Nursing or care work
- Work in a pharmacy or medical sales
- Other, similar activities
How long does it take to become a clinical research associate?
Becoming a CRA will usually take around three to four years, depending on the access pathway you choose.
If you opt to complete an undergraduate degree, this can take three to four years. You can then apply to job positions as a CRA straight away. However, if you don’t have sufficient work experience, you may need to start at a lower-level position such as a more administrative job. From here, you can gain more experience and reapply for a higher-level position.
If you choose to obtain a HND qualification, this will take two years. Provided you have sufficient experience, you can then apply for a graduate post as a CRA, but again you may need to gain some extra experience/qualifications in some cases.
If you opt to do a postgraduate degree first, this may take an additional one to three years depending on whether it is a master’s degree or a PhD. Many CRA job positions also allow for completion of postgraduate qualifications alongside the job.
A day in the life of a clinical research associate
Most CRAs work about 40 hours a week, during weekdays. There may be an out-of-hours commitment, for instance if working in a hospital setting monitoring a new drug, but this is dependent on your employer and role.
CRAs can work on multiple trials at a time, in multiple different sites. This will depend on the complexity of each trial and what stage each of the trials is in. Therefore, the role may entail some travelling at times, while other times most of your work will be concentrated on one site.
As a CRA, you will carry out a wide variety of tasks. Some days may be spent writing reports. On other days, you will work on site with healthcare staff , or you might go into your office and attend meetings.
No matter your experience level or how senior your role is, as a CRA, you will need to work in a team with other research and healthcare professionals. You will be communicating with research nurses, doctors, health consultants, investigators and managerial and administrative staff from the company requesting the trial.
The role requires good communication skills, as well as good time management and organisation skills, because carrying out a few different studies at once may mean some tasks clash with one another and you need to prioritise the most important ones.
Clinical research associate: Career options
As with most clinical roles, CRAs undergo lots of continuing professional development (CPD) within their role. There are many training courses available to CRAs to build on their existing skills and develop new competencies.
Most training courses are organised by external bodies, and many are paid for by the employer. One of the organisations that runs training courses is the Institute of Clinical Research (ICR). It provides training in areas such as effective project management for clinical trials and advanced clinical trial monitoring, among several others.
The ICR also offers certificates and a diploma that you can complete to evidence your skills in clinical research. Becoming an ICR member and obtaining courses and qualifications from it can also be beneficial to career development, as you will meet other prominent professionals in your field through interacting with this organisation.
You can also opt to complete a postgraduate qualification, such as a PhD or master’s degree in several different areas, including clinical research and clinical pharmacology .
As mentioned, you may need to climb up the professional ladder to become a CRA, and many people start off as clinical trial administrators or junior CRAs. Within these roles, you might complete tasks such as handling documentation and correspondence or helping to set up trial sites.
From here, you can move on to becoming a senior CRA as you gain experience. At this point, you will have more advanced responsibilities, such as project management of whole trials and designing case report forms.
If you develop sufficient experience and gain contacts in the field, there is a possibility of self-employment if you want to become a freelance CRA.
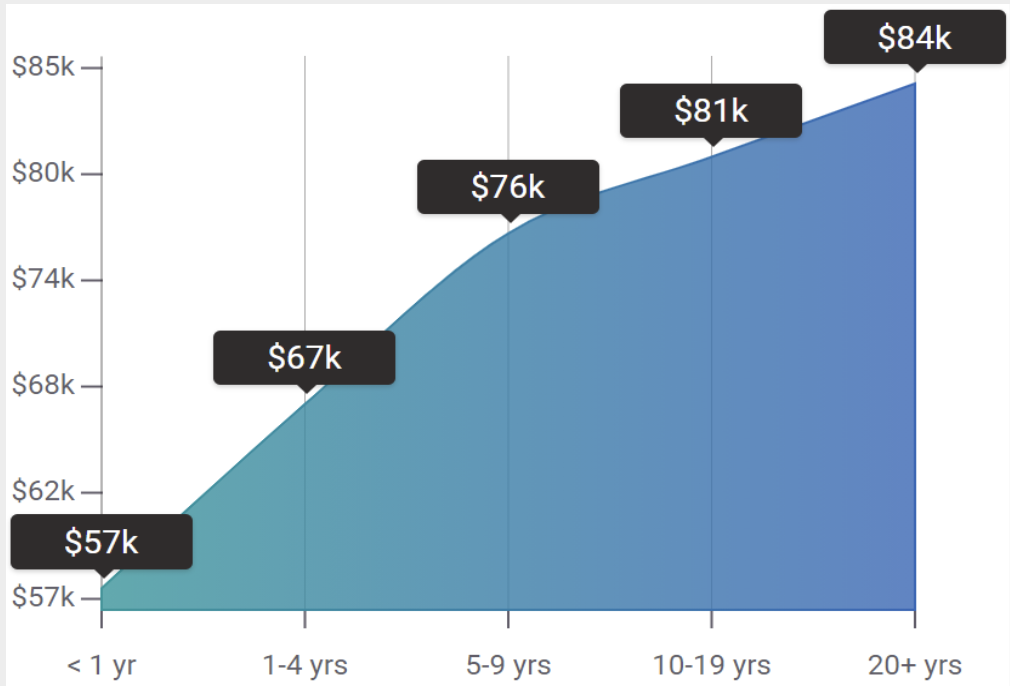
Salary: How much does a clinical research associate earn in the UK and US?
In the UK, starting salaries for CRAs range between £26,000 and £34,000 per year. As a more senior CRA, you might earn between £35,000 and £50,000, and in the most senior positions involving managerial tasks, you might earn upwards of £55,000.
Salaries will vary between regions and employers, as well as depending on your level of experience and responsibilities. Some companies offer additional benefits.
In the US, the average salary for a CRA is $70,000 per year. The range is between $60,200 and $80,900. This can vary depending on the region you work in, your education and experience levels and any additional qualifications you have.
Salaries will also be different as a freelance CRA, and this will depend on the number of clients you have and any business-related expenses you need to cover.
- Prospects. Clinical research associate. Available from: https://www.prospects.ac.uk/job-profiles/clinical-research-associate (accessed Apr 2024)
- CK Group. Clinical research associate (CRA) job profile. Available from: https://ckgroup.co.uk/candidate/job-profiles/clinical-research-associate-cra-job-profile/ (accessed Apr 2024)
- Nikolova, T. The CRA Wizard. How to become a CRA for dummies in 7 steps (or less). Available from: https://www.thecrawizard.com/how-to-become-a-cra-for-dummies (accessed Apr 2024)
- Glassdoor. Clinical research associate career. Available from: https://www.glassdoor.co.uk/Career/how-to-become-clinical-research-associate_KO14,41.htm (accessed Apr 2024)
- Coursera. How to become a clinical research associate. Published Nov 2023. Available from: https://www.coursera.org/articles/clinical-research-associate
- Walters, L. Pharmiweb.jobs. 8 ways to advance your career as a clinical research associate (CRA). Published Sept 2023. Available from: https://www.pharmiweb.jobs/article/8-ways-to-advance-your-career-as-a-clinical-research-associate-cra- (accessed Apr 2024)
- Salary.com. Clinical research assistant salary in the United States. Available from: https://www.salary.com/research/salary/alternate/clinical-research-assistant-salary (available from Apr 2024)
Share this article
Related articles

What jobs can you get with a computer science degree? - A New Scientist Careers Guide

What does a Clinical Physiologist in Neurophysiology do?

What jobs can you get with a physics degree? - A New Scientist Careers Guide
Latest articles.
Your session is about to expire
Clinical research roles: how to become a clinical research associate, clinical research associate job description.
Clinical research associates (CRAs) are key actors in facilitating and ensuring successful clinical trial operations. A CRA in clinical research plays an essential role in the trial’s logistics, acting as a liaison between trial sites and the sponsor, and interacting with patients.
What is a clinical research associate?
A clinical research associate (CRA) is responsible for ensuring that clinical trials follow regulations, protocols, and Good Clinical Practice (GCP) guidelines.[ 1 ] The job description of a clinical research associate is centered on monitoring clinical trials to ensure they are conducted safely and effectively, and facilitating communication and coherence between sites and the sponsor. Their duties could include:
- Site selection: CRAs may be involved to varying degrees in selecting appropriate clinical trial sites as well as performing pre-trial site screening visits to verify their capacity to conduct the trial
- Protocol development: CRAs may help the sponsor/investigators develop the clinical trial protocol
- Study start up: CRAs assist with study start up activities, including obtaining regulatory approvals, collecting documentation, training site staff, and conducting site initiation visits
- Site visits: A core responsibility of the CRA is to visit the trial sites, both during the trial (to ensure protocol and regulatory compliance), and after the trial (to perform site close-out)
- Site monitoring: CRAs verify that sites are following the data management plan and protocols to help ensure data is accurate and complete
- Safety monitoring: CRAs may monitor patient safety throughout the trial, including adverse event reporting and serious adverse event management
- Regulatory compliance: CRAs ensure that clinical trials are conducted according to legal and ethical regulations and GCP guidelines
Other potential names for this position include clinical research monitor, clinical trials coordinator, clinical research scientist, or clinical research manager.
Qualifications: How to become a clinical research associate
To become a clinical research associate, you typically need a bachelor's degree in a relevant field such as biology, life sciences, or nursing. A degree in a field that is related to clinical research is generally preferred but is not required for all positions. Some employers may require additional qualifications, such as a master's degree or specialized certification.
In addition to formal education, experience working in the medical research industry is highly desirable. Many CRAs start their career in clinical research working under the supervision of experienced clinical research professionals.
Some of the skills needed to perform the job functions of a CRA include:
- Strong attention to detail and organizational skills
- Excellent communication and interpersonal skills
- Ability to work both independently and as part of teams
- Knowledge of the pharmaceutical and healthcare industries, health and life sciences, and clinical trial processes, regulations, and guidelines
- Analytical and problem-solving skills
- Proficiency in data management and analysis software
If you do not have a relevant degree or prior experience, there are still ways to break into the field of clinical research as a CRA. Here are a few tips:
- Look for entry-level clinical research jobs: Many companies offer entry-level clinical research associate jobs for individuals who do not have prior experience in clinical research. These positions may involve assisting CRAs with managing study activities or administrative tasks.
- Volunteer: Volunteering for clinical research organizations (CROs), research hospitals conducting trials, or other clinical research companies can provide valuable experience and demonstrate your interest in the field. Further, this experience may count as relevant work experience and could qualify you to take the ACRP CRA certification exam (see next section)
Clinical research associate certification
Several courses and clinical research certifications can help you land a CRA job. Two of the most in-demand clinical research associate certifications include SOCRA’s Certified Clinical Research Professional (CCRP) certification and the Association of Clinical Research Professionals (ACRP)’s Certified Clinical Research Associate (CCRA) certification.[ 2 ],[ 3 ] A CRA certification can help demonstrate your knowledge and skills to potential employers.
Similarly, the Certified Clinical Research Professionals Society (CCRPS) offers an Advanced Clinical Research Associate Certification (ACRAC) and course which covers clinical research protocols, clinical trials regulations set by the FDA, Good Clinical Practice (GCP), ICH guidelines, and more.[ 4 ] On a more general level, Coursera offers a specialized certification in Medical Terminology led by Rice University to help you get started on various career paths in the medical field.[ 5 ]
Where to look for clinical research associate jobs
Clinical research associates typically work for pharmaceutical companies, contract research organizations (CROs), academic institutions, or government agencies involved in conducting clinical research. They work closely with a team of clinical research professionals, including principal investigators (PIs), clinical research coordinators (CRC), data managers, and sponsors.
You may be able to find job openings directly with such companies by surveying their websites for open positions. However, you can also look for CRA jobs on job boards such as Indeed[ 6 ] or LinkedIn[ 7 ].
What is the average clinical research associate salary?
The average clinical research associate salary in the United States is around $81,826 (as of when this article was written), with a lower range of $49,052 and a higher range (senior clinical research associate salary) of around $136,499.[8] Salary will depend on multiple factors: level of experience, education, other certifications/qualifications, skillset, and the specific employer.
Clinical Research Associate job outlook
The job outlook for clinical research associates is quite positive, with the high demand for professionals in this field expected to continue. According to the Bureau of Labor Statistics, the employment of medical and health services managers, which includes clinical research associates, is projected to grow 28% between 2021 and 2031.[ 9 ]
In addition, the pharmaceutical and biotech industries are constantly developing new drugs and therapies, which must go through clinical trials before they can be approved. In the USA alone, there are currently over 400,000 active clinical trials.[ 10 ] Thus, there is high demand for professionals with the skills and knowledge to oversee these trials, ensure they are conducted safely and ethically, and optimize their success, particularly in the context of recent advances in technological adoption and new trial models. Moreover, CRA salaries have increased by an average of 15% over the past five years.[ 11 ]
Becoming a clinical research associate is a challenging yet rewarding career path requiring unique skills and qualifications. If you are passionate about medical research and want to make a difference in patients' lives, and you like working with diverse teams on large projects, this may be a promising career path for you. With the proper education, a CRA training program, and some relevant experience, you can become a vital part of the clinical research industry and contribute to the development of new treatments and therapies.
Other Trials to Consider
Intensity Modulated Radiation Therapy (IMRT)
Onabotulinumtoxina, biological g207, facing your fears: asd/id, weight maintenance + intermittent fasting, burst wave lithotripsy, popular categories.
DCIS Clinical Trials 2024
Clinical Trials in Houston, TX
Clinical Trials in Seattle, WA
Clinical Trials in Sacramento, CA
Clinical Trials in Greensboro, NC
Clinical Trials in Texas
Clinical Trials in Illinois
Paid Clinical Trials in San Diego, CA
Clinical Trials in Montreal, QC
Achromatopsia Clinical Trials 2023
Popular guides.

Clinical Research Associate: A Full Guide on Becoming A CRA

Clinical Research Associate
A complete guide on how to become a clinical research associate.
Over 1.9 million students receive a bachelors of science every year. While a few go on to PhD, Masters, and Medical programs; many are ready to start clinical research certification online to start a career in the frontiers of medical research and patient care.
As a new student applying to the science job market, you may only find internships or recognize that even entry-level science jobs requires 1-2 years of experience. More so, you may realize many of these jobs require intense labor in the lab or just did not meet your expectations for your science degree.
This is why a career as a CRA should be considered with clinical research coordinator training. We train over 500 students each month in clinical research coordinator training and clinical research associate training (depending on prior background).
For those who have always wanted a career in medicine or have a gap year before medical school; Clinical Research Training is the next step to getting a head start in your career.
Because the position is unlike actually working in the lab and more of a management role; you get 1-on-1 connections with physicians and medical staff that can lead to a better application for medical school and other medical careers later on.
Best of all; many of these positions accept remote staff (and some allow you to travel 45-75% with full expenses including travel, accommodation, meals, and other per-dime expenses covered).
Clinical Research Training can help you save money while also increasing your salary. CRA’s with our level of training can expect to make between $6,500-$12,000 a month with an estimated promotion rate of 33% a year: an amount that is uncommon in other science-degree careers.
CCRPS is one of the only major US-based ACCRE, ACCME, ANCC, ACPE, and Transcelerate Biopharma accredited CRA certification courses that accepts students with no prior background for certification. T
his is because our course is thorough and created by Senior CRAs who have been in the field for long enough to understand what you need to know to begin working and applying. The course can be completed in as little as 7 days with dedicated full-day study time.
Clinical Research Associate Certification Qualifications
Foreign Doctors Welcome : A Clinical Research Associate or Coordinator plays a vital role in directing and supervising clinical trials conducted by physicians, nurses, and other science professionals. This career path is particularly attractive to many foreign doctors with completed medical degrees (MBBS) who can utilize their expertise in the US healthcare system by pursuing a CRA career instead of taking the USMLE or repeating residency training. For those interested in coordinating aspects, consider the Clinical Research Coordinator course .
Distinct Skillset : Unlike the traditional medical field you may be familiar with after years of schooling, Clinical Research Associate training provides a distinct and valuable skillset. For comprehensive understanding of Good Clinical Practice, see the ICH-GCP course .
Most Extensive Online Course : Our program goes beyond basic introductions, offering a comprehensive curriculum with over 110 modules – the most extensive Clinical Research Associate course available online. This in-depth training ensures you're well-prepared to secure a coveted CRA position.
Superior Coursework : Securing a CRA role is a strategic career move compared to the limitations of many traditional medical positions. While generic courses abound, we've observed that graduates often struggle due to a lack of substantive content. Our Clinical Research Associate course addresses this gap by providing Senior Clinical Research Associate-level training through 110 intensive modules grounded in the latest scientific principles. For those looking to assist in clinical trials, the Clinical Trials Assistant Training may also be of interest.
Diverse Career Opportunities : This high-demand science-based medical field offers diverse opportunities:
Work in the Private Sector : Pursue a CRA career with renowned pharmaceutical companies like Pfizer. Enhance your skills with the Advanced Clinical Research Project Manager Certification .
Academic Opportunities : Work in the academic sphere at medical schools. Those aiming for higher responsibilities may consider the Advanced Principal Investigator Physician Certification .
Unmatched Flexibility and Knowledge : In addition to our exceptional course content, we boast the largest number of clinical research courses available online, providing you with unmatched flexibility and knowledge. For those interested in safety monitoring of drugs, the Pharmacovigilance Certification and Medical Monitor Certification can enhance your capabilities in these critical areas.
Why Take A CRA Certification Course
The role of the clinical research associate is to ensure that medical devices, new treatments and new drugs are approved for patients' use.
This field is taken as a certificate program course in many schools. For example, you may find associate degree programs. These programs can be completed in two years and can be offered through both the online and the hybrid formats. Hybrid formats combine both online and on-campus courses together.
If you opt for a fully online program, you can still get an immersive education. Different platforms like emails and discussion boards are used to ensure and promote interaction between the students as well as the lecturers.
Online learning platforms are used to upload the syllabus, course materials, lectures and assignments. Some online programs include field work as part of their requirements, in order for students to gain first hand experience working with clinical trials and patients. Depending on the school, they may have a list of approved clinical research institutes and other facilities. Otherwise, you will have to find a facility for yourself and get the school's approval.
These certificate programs are generally designed for professionals that are already in the medical fields (like medical assistants or nurses) and are interested in moving to the field of clinical research.
They may therefore ask for a copy of your CV or resumé or they may ask for a letter from your employers to verify that you have the needed medical experience. Some programs may require just an undergraduate degree in a medical science or life science related field.
Clinical research associates are trained to assist clinical researchers and investigators in the coordination, administration and management of clinical trials.
During this training, different courses will be taught revolving around subjects like safety procedures, subject recruitment, regulatory requirements, drug development, accountability, trial management, medical terminology etc.
The importance of the role of the clinical research associate means that companies that conduct clinical trials are usually very selective. The need to comply with strict regulations often inform their decision when making a choice of their clinical research associate. It is therefore very difficult to get a job as a clinical research associate without previous experience in clinical trials.
Many companies require around at least two years experience in clinical monitoring as a clinical project assistant or clinical trial administrator before considering applicants for this important role.
In applying for the post of a clinical research associate , ensure that you read the job description and indicate or highlights the relevant experience on your curriculum vitae. Your cover letter should be specific to the company you're applying to.
Do not use a one-for-all cover letter. Personalize your cover letter to each company and highlight the skills that fit the specific requirements of the role. Not all companies advertise their vacancies, so you can try to find out about other unadvertised vacancies, you might increase your chances.
Further certification can enhance your resume such as the ACCRE accredited CRA program which contains 110 learning modules for Clinical Research Associate Training and Placement
The Best CRA Certification Course For Entry-Levels
There is a huge shortage of well-trained CRAs, but many companies are reluctant to hire untrained entry-level clinical monitors because of patient and trial safety. Because of this, even the beginner entry-level jobs require certification or training.
Our program is considered one of the top clinical research graduate programs online. Most courses provide very light training that may look good because of the company names, but alone is not sufficient to pass the interview rounds a company conducts.
Because our modules are prepared help even Senior Clinical Research Associates, we find more of our students with no background quickly passing their interview rounds.
CCRPS Course covers double to triple the amount of course content than other courses. While many courses are simply 5-20 simple interactive modules, our course covers 140 dense modules in thorough detail.
After each session, students can ask their questions privately with the course instructor, all of whom have 15+ years of CRA experience.
Currently, 82% of our students are hired within the first month of taking the course. Students with limited background or those looking to gain extra experience are offered a remote internship of up to 6 months during the time they are interviewing.
This advantage allows many students with limited experience to get hired with a higher paying job than previously offered.
While a majority of our students are physicians, a majority of the CRA workforce are Science Grads and Nurses. nonetheless, we train all students at a Senior CRA level regardless of background because clinical research monitoring is vastly different from any lab or science course you may have taken.
Clinical research associates are given the protocol of a study including all medical protocol that must be followed but because they do not diagnose or treat. Medical knowledge is supplemental but not sufficient in this career path.
This is the main reason why our Clinical Research Training includes all possible scenarios you may face at the protocol and guideline level in your future company.
How To Get Experience For Clinical Research Associate Jobs
CCRPS, like other educational institutes, is only associated with educating and certifying clinical research professionals so we do not provide job placement. We want to make sure you apply with your best foot forward. Below are links we readily refer to graduates who are looking for job support. Having a great CV and cover letter are essential to applying for jobs. Recruiters are paid by the company which hires you and thus are free for searching employees. Be realistic but also be driven. Make sure you get continue reaching out until you get a true rejection from any job you apply to as they may never have seen your application if you received no response.
Clinical Research Job Advising: Kunal at ClinicalTrialPodcast
Free Resume Review: TopCV TopCV provides a free review and feedback for your current resume.
Resume Distribution: ResumeRabbit Resume rabbit distributes your resume to 60 job posting sites.
Clinical Research Recruiters: I-Recruit I-Recruit distributes your resume to clinical research recruiters.
Clinical Research Job Bulletin: Indeed Indeed usually provides the most uptodate job bulletin for clinical research jobs
Always use a cover letter specific for the company and job when applying if you are not using a recruiter.
The ICH-GCP in Clinical Research
Regardless of the type of clinical research or function of an IP being tested, it is important that clinical research should meet two critical criteria:
The clinical research process should respect the rights, freedom and dignity of tested patients (human participants).
Data from the clinical research process should be accurately collected, safely stored, rigorously scrutinized and correctly interpreted.
One way to ensure that these requirements are met is to follow a set of internationally recognized and accepted standards for clinical research.
Most countries across the world today follow ICH-GCP, that is, International Committee for Harmonization of Good Clinical Practice guidelines in conducting clinical research on human participants7.
The ICH-GCP outlines procedures and precautions that are essential in order to protect the safety and wellbeing of human research participants during clinical research, and to ensure the integrity of data from clinical research studies.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.z
Qualifications and Qualities of a CRA
According to the International Accrediting Organization for Clinical Research (IAOCR), candidates for CRA positions usually hold either a biological science degree, or one in medicine or nursing10.
The New Scientist recommends that aspiring CRAs should possess a good working knowledge of one or more of the following subjects – anatomy, biology, biochemistry, chemistry, immunology, microbiology, pharmacology, physiology or toxicology11.
In addition to a background in medical or life sciences, a CRA is required to have a good grasp of data management, including Electronic Data Capture (EDC), data analytics and reporting12.
Sketching the CRA work profile, the authors Diane St. Germain and Marjorie Good state that CRAs are the ones who scrutinize clinical study data most closely from start to finish—as a result, they are often the first to notice critical patterns and interesting trends, and to report these to the research team as well as to the CRO13.
Equally if not more importantly, a CRA must possess a high level of emotional and interpersonal savvy. This is a crucial area, since a CRA’s success hin ges upon his/her ability to elicit the best from team members, in terms of both performance and probity.
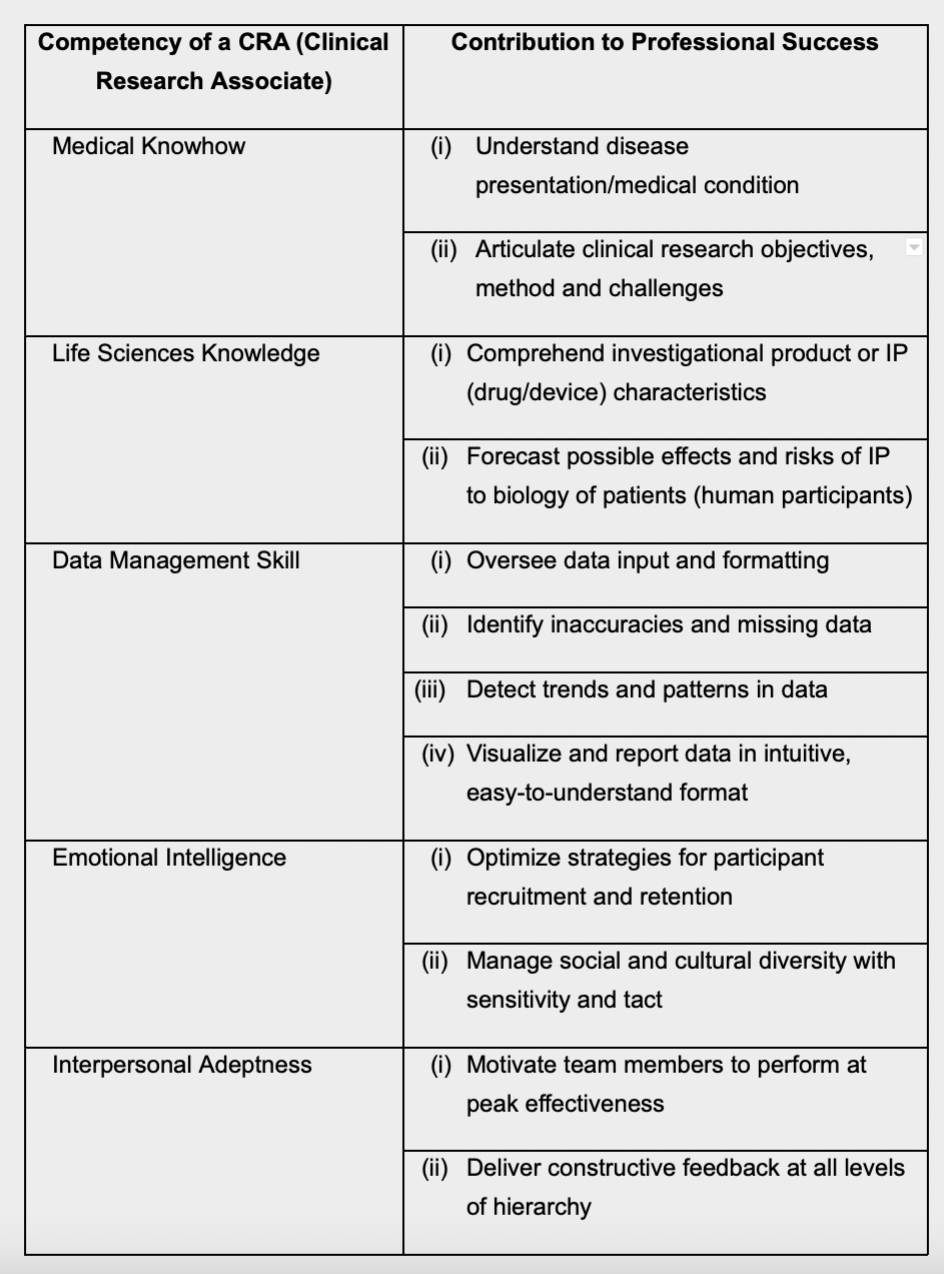
Core Competency Framework for CRAs
To illustrate, the ACRP’s ‘Core Competency
Framework for Clinical Study Monitoring’
requires that a CRA should be able to identify
and correct compliance violations at a study
site. The CRA must not only bring such
violations to the attention of site staff, s/he
must induce them to take corrective action,
as well as reporting the matter and even
escalating it, where necessary14.
The table below summarizes the ideal
competencies of a CRA, and provides
insights on how each ability contributes to
the CRA’s performance.
CRA Career Path
In the past, CRA positions were often filled by individuals with medical or nursing backgrounds, with little thought given to their lack of research training15. As awareness grew about the importance of research experience for a CRA, employers began preferring those with years of experience in clinical research settings, such as Clinical Trials Assistants (CTAs) and Clinical Research Coordinators (CRCs)16.
However, in recent years, the focus has shifted once again from a tenure-based mindset to a skills-based evaluation17. In part, this change has been brought about by the growth in professional courses and training programs in the field.
For instance, many leading US Universities today offer master’s programs in clinical research18. In addition, there are some widely recognized certification programs for clinical research associates, such as those offered by the ACRP19 and the Society of Clinical Research Associates (SOCRA) 20.
Note: You must already be working as a CRA to qualify for the ACRP and SOCRA certification programs.
A Toe in the Door: CRA Certification for a Non-CRA
By this point, you might be wondering, “I have no research experience… I’ve never worked as a Clinical Trials Assistant (CTA) or a s a Clinical Research Coordinator (CRC). Nor do I have a degree in Clinical Research. Can I still become a CRA?”
The simple answer is, yes, you can.
You might be a life sciences graduate looking for a lucrative career in the pharmaceutical or biotechnology sectors. Or, you’re excited by a career in research, but unsure whether the drudgery of a Ph.D. is your thing.
Maybe you’re just looking for a job that represents a great option for someone with your combo of science background plus detail-orientedness.
Whichever of these descriptions best applies to you, a career as a Clinical Research Associate could be exactly right for you.
With the right training, you can be recruited directly to a Clinical Research Associate position, even without a background in clinical research.
So, what kind of training will help me break through the ‘experience’ barrier and land a job as a CRA?
As you’ve already gathered from the table, the skill-set required to be a successful CRA is pretty extensive.
Aside from an in-depth knowledge of scientific and medical concepts and principles, a CRA must have a sound grasp of medical research regulatory requirements, a penchant for being thorough and systematic, as well as a knack for coordinating and managing people with diverse skills, roles and backgrounds.
To our knowledge, CCRPC’s ‘Advanced Clinical Research Associate Certification’ (ACRAC) is one of a kind: The ACRAC is the only multi-accredited* certification program in the US that offers the kind of exhaustive as well as intensive training that equips candidates from a non-clinical background with the abilities and competencies that make a good CRA.
Best of all? The ACRAC is open to fresh graduates holding a B.S. degree in any of the life sciences, with no requirement for prior exposure or experience in clinical research.
*The ACRAC program offered by CCRPC is accredited to ACCRE (Accreditation Council for Clinical Research & Education), ACCME (Accreditation Council for Continuing Medical Education), ACPE (Accreditation Council for Pharmacy Education), ANCC (A merican Nurses Credentialing Center), as well as Transcelerate Biopharma.

Training to be a CRA through CCRPS ACRAC
The ACRAC program includes over 100 course modules that cover all the important knowledge domains and skill-sets required by a CRA.
Designed for a total study time of approximately 250 hours, this training program can be completed at your own pace, or, for those able to dedicate the whole day to study, in as little as two to three weeks.
Starting with a broad overview of clinical research jargon and terminology, the course walks students through the principles of Good Clinical Practice, familiarizing you with the relevant sections of the ICH-GCP and the FDA’s E6(R2).
The program places particular emphasis on ethical practices in research with vulnerable populations.
Students going through the ACRAC are trained in all major aspects of designing a Clinical Trial Protocol in keeping with the Code of Federal Regulations (CFR).
They additionally learn the steps involved in the IRB/IEC approvals process and how to prepare required documents.
Finally, students become aware of the importance of pharmacovigilance and the regulatory process for new drug testing.
A major chunk of the ACRAC certification centers around equipping the CRA for day-to-day responsibilities, such as different types of site visits – preliminary (Site Qualification), preparatory (Site Initiation) and progress monitoring visits (Routine Monitoring).
Crucially, the ACRAC covers essential documentation such as the Case Report Form and Trial Master File, as well as electronic data capture (EDC) and remote monitoring systems.
A vital component of the training program involves empowering students to tackle challenging situations.
For a CRA, these include identifying protocol deviations and violations, and recognizing as well as reporting research fraud and ethical misconduct.
In addition to its comprehensive coverage, the ACRAC certification offers the great advantage of including 17.5 CME credits – that is, course credits that count towards ‘Continuing Medical Education’.
These credits can be used by individuals desiring to further their education and/or careers in healthcare-related fields, including medicine, nursing, pharmacy and research.

Clinical Research Associate Training
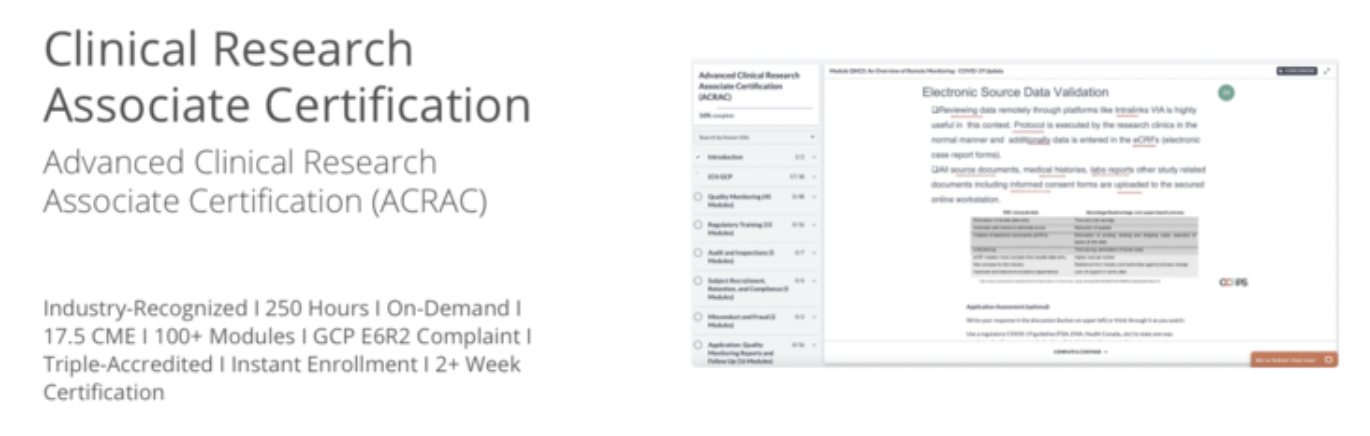
Get ahead in clinical research with advanced accredited online CRA certification for $450. Demo our on-demand course below.
Clinical Research Associate Certification
Advanced clinical research associate certification (acrac).
Chapter 1: Introduction
This chapter orients you to the concept of Continuing Medical Education (CME) and outlines how the CCRPS CRA program contents meets AMA requirements for CME. Given that, across the US, physician practitioners are required to complete between 20 and 50 hours of CME credits yearly, the ACCME-accredited CCRPS CRA course can be used not only to build knowledge and skills in the field of clinical trial management, but also to further a successful medical career. Additionally, the introductory chapter introduces you to the clinical terminology and abbreviations commonly encountered in clinical research, for example, Investigational Product (IP), Good Clinical Practice (GCP), Institutional Review Board (IRB) and so on.
Chapter 2: Roles and Relationships in Clinical Trials
The unit presents the foundational background to beginning and building a career as a clinical research associate (CRA). As you know, a CRA plays a critical role in setting up as well as monitoring the clinical trials process for an investigational product or IP – a medical drug or device under development. In this unit, you will learn how a CRA interacts with other stakeholders, including the Clinical Research Organization (CRO) or Sponsor of the clinical trials, the Principal Investigator (PI) as well as other research site staff, the trials monitoring team including the Clinical Research Coordinator (CRC),other CRAs and the Data Safety Monitoring Board (DSMB), as well as the research ethics committee (Institutional Review Board or IRB).
Chapter 3: Sponsor and Investigator Roles
In this unit, you will gain insight into the ICH-GCP guidelines, particularly addendum E6, sections 2 through 5, which outline procedures and precautions essential for protecting the safety and wellbeing of human research participants during clinical research. These include guidelines for obtaining informed consent from human subjects, maintenance of trial records, reporting of compliance, safety and research progress, as well as procedures for suspension or termination of the trials process. The chapter familiarizes you with the critical importance of monitoring for Adverse Events (AEs), including types of AEs and regulations for documentation and reporting.
Chapter 4: Clinical Trial Design
In this chapter, you will acquire insight into the different phases of the clinical trials process, from the pre-clinical phase through Phases 0 to 4 of clinical testing. The unit will familiarize you with important concepts of clinical trials, such as the structure and goals of each phase of clinical trials, approaches to dosing, toxicology of pharmaceutical products, in vitro and in vivo testing, dose escalation and so on. Finally, the chapter reviews the FDA’s drug approval process.
Chapter 5: ICH-GCP – Overview
The chapter dives deep into GCP, including a review of the history of medical research leading up to the ICH-GCP. The unit covers all four QSEM categories of the guidelines for ensuring Quality, Safety and Efficacy of the IP, as well as Multidisciplinary guidelines (mainly pertaining to documentation and electronic data safety standards). In addition, the chapter includes an overview of MedDRA software that provides a standardized system of terminology and notation for documenting clinical research, as well as principles of budgeting for clinical trials.
Chapter 6: Ethical Research in Vulnerable Populations
The unit provides a detailed walk-through of the regulations and compliance requirements for conducting clinical trials with human subjects who meet the definition of a ‘vulnerable population’, including pregnant women and fetuses, children, mentally incapacitated individuals (those with cognitive functioning impaired by neurolopsychological conditions or chronic substance abuse), as well as prisoners. You will acquire familiarity with the challenges of research in such populations, including the requirement for parental consent, fair but not excessive incentive, justifiable deception or incomplete disclosure, coercive practices and so forth.
Chapter 7: Adverse Events
Through this module, you will gain a bird’s eye view of the protocol for documenting, reporting and responding to AEs or adverse events during the clinical trials process. The unit covers concepts such as expectedness, severity and seriousness of AEs, Adverse Drug Reactions (ADRs) as a sub-category of AEs, Investigational New Drug or IND reports, causality analysis for AEs and so on. In addition, the chapter reviews the responsibilities of both research sponsors as well as IRBs in sharing AE information with subjects.
Chapter 8: Clinical Trial Protocol
The chapter provides an in-depth tutorial on the structure and elements of a CTP or clinical trial protocol, as well as guidelines on writing a CTP. Important concepts reviewed include study Risk Benefit Analysis (RBA), study sample statistics (sample size, statistical power, plan for data analysis), risk management and study administration. Additionally, the module covers concepts central to study sample selection, addressing inclusion and exclusion criteria, especially safety and ethics considerations in sampling.
Chapter 9: Protocol Deviations and Violations
Through this unit, you will gain familiarity with the many potential causes of protocol deviations and violations, learning to distinguish between minor (deviations) and major departures or violations of protocol. Content provides understanding of the most commonly occurring violations, including both minor (off-schedule subject assessments, subjects’ use of prohibited drugs, and so on) as well as major violations (failure to obtain informed consent, failure to report AEs and so forth). Further, the chapter reviews principles for reporting protocol deviations, IRB approval for planned deviations and related concepts.
Chapter 10: IRB and DSMB
This chapter briefly reviews the history of IRBs and examines the principles guiding IRB decision-making. In addition, the unit discusses recent developments in compliance, including sIRB (single IRB) and SmartIRB for institutions that are part of the CTSA (Clinical and Translational Science Awards). The bulk of this module dives into the categories of IRB review, including full board and expedited review, examining criteria for review exemption such as educational or purely behavioral research, as well as studies collecting identifiable data, surveys and interviews.
Chapter 11: Review Questions
The module provides a self-assessment tool by including questions that review the content covered in previous chapters. The set of 71 questions examines all aspects of ICH-GCP previously discussed.
Chapter 12: Site Monitoring Visits
In this module, an overview is provided of the different types of site monitoring visits, including site selection or qualification visit, study initiation visit, routine or progress monitoring visit, as well as study termination or close-out visit. Important concepts discussed include pre-qualification preparations and site feasibility assessment as well as study monitoring criteria (data omission, incorrect entries, inaccurate calculations, documentation of corrections and so on). For each type of site monitoring visit, the chapter reviews relevant documentation.
Chapter 13: Site Qualification Visit (SQV)
The chapter gives an in-depth understanding of the stages and steps involved in selecting a study site. Elements reviewed within the module include the process of investigator selection and criteria for site evaluation (the four P’s: Patient, Protocol, Performance, Profit). Importantly, the module reviews the most common errors in feasibility assessment, including overestimation of sample availability at site, selection of site staff with low motivation, poor-performing sites owing to high competition for personnel and resources (for example, owing to multiple studies running on a single site), and so on.
Chapter 14: Site Initiation Visit (SIV)
The module dives into the details of an SIV or site initiation visit. You will review the procedure for pre-SIV preparation, including filing for IRB and other necessary approvals, permits and licenses. Additionally, the chapter examines elements of the SIV agenda, mainly orientation and training of site staff, creation of important study-related documents such as the Trial Master File (TMF) and post-SIV filing of compliance documents such as FDA form 1572 and Financial Disclosure Form (FDF) for relevant site personnel.
Chapter 15: Routine Monitoring Visit (RMV)
In this unit, the elements of a routine or periodic monitoring visit are discussed in detail. You will become familiar with the agenda of an RMV, which prioritizes receiving updates on AEs from site staff (incidence, documentation, seriousness and so on), as well as oversight of the overall progress of trials. The chapter covers different approaches to site monitoring, contrasting traditional (full-scale) monitoring with risk-based monitoring (RBM), as well as comparing on-site monitoring with remote monitoring. A crucial concept addressed by the unit is Source Data Verification (SDV), which is central to obtaining meaningful, high-quality data from clinical trials.
Chapter 16: Site Close-Out Visit (SCOV)
The module gives you a comprehensive overview of the protocol and procedures involved in terminating or closing out a trial site. Aspects covered in the chapter include pre-SCOV preparations such as IRB notification and schedule coordination among site staff (PI, other investigators, medical staff) and monitoring team (CRC, CRAs and so on), agenda for an SCOV – drug inventory management, database verification and lockdown, subject intimation and completion of all subject-related documents, staff-related documentation as well as other administrative tasks including close-out report compilation.
Chapter 17: Tools for Monitoring Visits
This unit outlines a host of tips and tools that can help a CRA in successfully tackling the complex process of monitoring clinical trials. The chapter lists numerous physical accessories you can use for effective monitoring, including scheduling and calculation aids, ready reckoners for drug information and medical terminology, as well as document templates to speed up the process of obtaining trial updates while also serving as checklists for the site visit agenda. Additionally, the unit highlights helpful strategies that a CRA can use to ensure that site visits go smoothly, from travel advice to team-building suggestions.
Chapter 18: Audit and Inspections
The module deals with one of the most crucial and often most feared aspects of a CRA’s career – audits and inspections by the CRO (sponsor), FDA or other regulatory authority. Starting from the basic distinction between an audit and an inspection, the chapter covers in detail the protocols for both audits and inspections. Crucially, the chapter will enable you to grasp the difference between a routine audit/ inspection and a ‘for-cause’ audit/ inspection. Further, it lays out the sequence of an FDA inspection in full (including a detailed walk-through of the FDA BIMO or Biomedical Research Monitoring Program inspection), and provides important guidelines on the do’s and dont’s for CRAs during an audit/ inspection, such as the critical ‘3 to 5 minute rule’. You will acquire familiarity with important audit and inspection-related documents such as FDA Form 482 (Notice of Inspection) and Form 483 (Notice of Observation) as well as the Establishment Inspection Report (EIR) prepared by the auditor/ inspector. Finally, you will gain insight into the classes of observations provided in an EIR, including NAI (no action indicated), VAI (voluntary action indicated) and OAI (official action indicated)—the last is commonly termed an ‘FDA warning letter’.
Chapter 19: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of clinical trial quality monitoring, including monitoring visits, tools as well as audits and inspections.
Chapter 20: SDV and Informed Consent
In this chapter, the ICH-GCP section 4.8 guidelines on obtaining informed consent from subjects are discussed in detail, highlighting the need for using non-technical language, transparent delineation of risks, consent without undue influence, obtaining consent (and assent) from minors and their Legally Acceptable Representatives (LARs), as well as consent from non-English speakers and sedated subjects. The chapter additionally covers important aspects of Source Data Verification (SDV) with respect to electronic as well as paper-based medical records, and highlights the central goal of SDV, which is to conform to ICH-GCP requirements that subject trial data (as recorded in Case Report Forms or CRFs) must correspond to source data (previous medical records).
Chapter 21: Case Report Form
The module provides an in-depth tutorial on the structure and elements of a Case Report Form or CRF, including the different forms for PI verification, subject enrollment, eligibility and randomization, medical history, physical examination and laboratory data, compliance, adverse events and so on. In addition, the chapter outlines important data notation rules, such as the use of accepted acronyms (‘ND’ for missing data and ‘UNK’ for unknown information, MM-DD-YY format, time-stamp data and so forth), as well as guidelines for the design of CRFs (such as consistency of notation, avoidance of data fields that can be computed and of duplicate data fields and so on).
Chapter 22: Quality Control and Safety
Within this unit, you will learn the central concepts of Quality Control (QC) in the context of clinical trials, including definitions of QC and its relationship with the complementary process of Quality Assurance (QA), the use of Key Performance Indicators (KPIs) in QC, need for a Corrective and Preventive Action (CAPA) plan and so on. Additionally, the module examines the QA process, focusing on the central role of RBM or risk-based monitoring in present-day QA as well as providing guidelines on Quality Metrics (QMs) for evaluating the trials process. The chapter also reviews ICH-GCP guidelines on subject safety, underlining risk-benefit assessment, stoppage rules (for instance, in case of SAEs) and reporting responsibilities. Finally, it introduces the FDA’s Human Research Protection Program (HRPP) as a platform that provides training and support for personnel involved in clinical trials.
Chapter 23: Technology in Trials
In this chapter, an in-depth tutorial is provided of the systems used in modern clinical trials for Electronic Data Capture (EDC) and database management. Systems such as Interactive Response Technologies (IRTs) including IVRS and IWRS (Interactive Voice and Web Response Systems, respectively) as well as RTSM systems for Randomization and Trial Supply Management are examined. The unit reviews the benefits of standardized data management and data sharing, approaches to database management and the concept of an Independent Data Monitoring Committee (IDMC). Critical elements of data integrity, such as proper anonymisation and coding, completeness of data, data safety precautions and logging of site visits and other progress reports are highlighted. The unit further examines the essential features of a good Clinical Data Management (CDM) system that complies with FDA CFR Title 21 and HIPAA regulations, such as setting access privileges, tracking changes and updates, data security and locking, flagging and reconciliation of AEs and so forth. Finally, the chapter looks at CTMSs (Clinical Trial Management Systems) in depth, covering the aspects that allow management of day-to-day trials in multi-site studies.
Chapter 24: Modernized Monitoring (Remote, Risk-based, Centralized)
This chapter offers a detailed walk-through of modern, remote monitoring of clinical trials, which evolved into a full-fledged system in response to the COVID-19 pandemic. Important concepts discussed include the critical site initiation process, Electronic Source Data Verification (ESDV) and FDA regulatory guidance for remote monitoring of clinical trials. In this module, you will learn how FDA’s ALCOA (Attributable, Legible, Contemporaneous, Original and Accurate) criteria for data quality have been adapted to remote monitoring. Further, the unit discusses how HIPAA compliance in remote monitoring is achieved by using limited data sets (wherein sensitive individual information is concealed through anonymous subject codes) regulated by data use agreements. Finally, the unit examines how risk-based monitoring approaches have allowed centralized monitoring to evolve into a cost-effective and safe method for clinical trial monitoring.
Chapter 25: Pharmacovigilance and Regulatory Affairs
Through this unit, you will gain insight into the process and rationale behind pharmacovigilance (PV) and its central role in the clinical trials process. The chapter reviews the statistics on AEs, distinguishes between Type A and Type B AEs, and profiles seriousness of ADRs or Adverse Drug Reactions as well as the iGuard Drug Risk Rating System. Importantly, the unit covers ADR causality assessment in detail, including both severity and probability assessment. An important element of PV addressed in this module is the Individual Case Safety Report (ICSR), its structure, content and role in trial monitoring. Other concepts discussed include types of PV inspections (routine vs. ‘for cause’), PSURs or Periodic Safety Update Reports and study criteria for instituting DSMBs (Data Safety Management Boards). Finally, the module also reviews the domain of Regulatory Affairs (RA) as a function of PV, outlining roles and responsibilities of RA personnel as well as the importance of RA in streamlining the process of drug development by ensuring compliance throughout manufacturing, clinical trials, marketing and advertising.
Chapter 26: Investigational Product
In this chapter, an in-depth review is provided of the protocol for receiving, storing and dispensing the IP or investigational product. At every stage, guidelines lay down strategies for ensuring verifiability, accountability and safety of both study subjects and staff. Thus, IP handling precautions include the need for logging date of manufacture, temperature throughout transit, as well as batch number and individual unit numbers (such as bottle or tube identifiers) carefully and accurately, as well as recording shipping details and filing shipping receipts. Additionally, the unit addresses the need for IP dispensing precautions, such as limiting dispensation to authorized personnel only, as well as maintaining individual subject IP logs.
Chapter 27: Local and Central Labs
The module profiles the evolution of lab testing in clinical trials, from error-prone localized laboratory testing to centralized testing that allows homogeneity of testing procedures and measurements, thus minimizing errors and improving outcomes. The chapter reviews standards for clinical trial laboratories as per the GLCP (Good Clinical Laboratory Practice) and CLIA norms (Clinical Laboratory Improvement Amendments), as well as providing guidelines for lab audits, including fire safety, protective gear, staff training and so forth.
Chapter 28: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of trial documentation (SDV, CRF, ICSR), quality control, pharmacovigilance, as well as IP and lab guidelines.
Chapter 29: Regulatory Documents in Clinical Trials
The chapter reviews essential documentation to be created and maintained throughout the course of the clinical trials, including the Trial Master File (TMF), FDA forms 1571, 1572, 3674, 3454/3455 and CFR Title 21 Form 312, besides ethics approval documents such as the IRB-approved protocol, informed consent form, subject education and study advertising materials. You will acquire in-depth familiarity with each of these forms, and learn the importance of maintaining and updating records, for example by incorporating IRB revisions and amendments, periodic renewals of permissions and licenses and copies of submitted reports. In addition, the unit summarizes the need for filing documents outlining study- and site-specific procedures, including SOP (Standard Operating Procedure), MOP (Manual of Procedures), Investigator Brochure (IB), Delegation of Authority Log (DOAL), site staff CVs, SAE notifications, logs of subject screening and enrollment, IP storage (temperature, humidity, etc.) and all relevant study parameters.
Chapter 30: CFR Title 21 Part 11 – Electronic Signatures
This unit gives you an overview of Title 21 of the FDA Code of Federal Regulations (CFR), including Chapter 1 sections on informed consent (Section 50), IRB approval (Section 56) and so on, Series on food (100), pharmaceuticals (200 and 300) and so on, as well as FDA Drug Schedules. The major part of the module focuses on Part 11 which deals with Electronic Records and Electronic Signatures (ERES), laying down the criteria for determining safety and reliability (trustworthiness) of electronic data and signatures.
Chapter 31: New Drug Application
Through this module, you will gain knowledge of the FDA process for evaluating a drug under development, and the role of a CRA in streamlining this process. An important distinction covered here is the difference between an IND (Investigational New Drug) and an NDA (New Drug Application). The chapter discusses in-depth the criteria used in evaluating an IND, including toxicology and pharmacokinetics data, as well as requirements for different drug classes (oncology vs. non-oncology). Additionally, the unit covers FDA requirements for AE reporting, including assessment of seriousness, expectedness and format for expedited reporting of life-threatening SARs, as well as safety reporting requirements for investigators.
Chapter 32: Trial Master File
The unit provides a detailed breakdown of the organization of a TMF or Trial Master File, listing the various binders that should be included within the TMF, as well as their contents. Thus, the TMF should contain binders pertaining to the study protocol and IRB, investigator qualifications, FDA forms and correspondence, FDFs or Financial Disclosure Forms, communications with the CRO, and other relevant trial aspects. A helpful templatic guide to creating a TMF is also provided in this chapter, as well as a self-assessment quiz of 10 items on important sections of a TMF.
Chapter 33: Disclosures and Payments for PI, Site, Patients
In this chapter, FDA guidelines regulating financial disclosure are discussed in-depth, covering the definition of ‘conflict of interest’ and the stipulations of Title 21 Section 54 on disclosure requirements. The unit helpfully contrasts FDA requirements with Canadian and UK/EU policies. You will study real life case examples of conflict of interest, as well as lawsuits pertaining to financial disclosure disputes to help gain a better understanding of the potential problems arising from failure to disclose financial interests in clinical trials. Another important dimension covered in the module is the regulation of payments to PIs and other investigators as well as patient payments, which must comply with CMS (Center for Medicare and Medicaid Services) policy on ‘fair market value’ as well as the Federal ‘Anti-Kickback Statute’. The unit contains guidelines on clinical trial budgeting and subject payments. Finally, the chapter reviews IRB guidelines on advertising to recruit human participants for clinical trials, including stipulations against misleading and coercive language, as well as excessive incentives.
Chapter 34: Patient Recruitment, Retention and Compliance
The unit provides an overview of the process of patient (subject) recruitment in clinical trials, from population research to identify motives for participation, to media support for building up public awareness and interest, to community and physician outreach for referrals and enrollment. Additionally, the chapter identifies common barriers to meeting recruitment goals and outlines strategies for maximizing recruitment, such as relaxing overly stringent criteria, offering reasonable incentives such as travel reimbursement and highlighting benefits of participation. Similarly, the unit covers common causes of patient drop-out as well as strategies for minimizing drop-outs, such as improving patient experience (increased attention and listening to patients, flexible scheduling of visits to suit patients’ convenience and so on). Finally, the unit discusses novel strategies to increase patient retention and improve compliance in clinical trials; these techniques harness technology to yield better outcomes, for example, simplifying form completion through digitized forms with auto-fill features, gamifying elements of compliance reporting, and so forth.
Chapter 35: Misconduct and Fraud
This module discusses the various motives for committing scientific fraud and the fallout of fraudulent practices in clinical trials. A scale for classifying errors in clinical trial data is presented, with ‘honest, isolated mistake’ at one end of the spectrum and ‘deliberate data falsification with malicious intent’ at the other. Types of clinical data that may be falsified, methods used in falsification (fabrication, substitution, omission), as well as scenarios in clinical trials where falsification may be occurring are presented. Through this chapter, you will gain familiarity with the signs to watch out for during the actual clinical trials process.
Chapter 36: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, including questions on all aspects of regulatory documents, site documents (TMF and contents), trial budgeting and payments, patient recruitment and scientific fraud.
Chapter 37: Site Visit Templates
This module contains a set of templates that you can use for documenting the details of site monitoring as a CRA, either in their current form, or in a form adapted to the needs of your own study. The templates included in this unit include:
Site Qualification Visit (SQV) – checklist for preparations, questionnaire for assessing the site prior to the actual visit, assessment form and follow-up letter
Site Initiation Visit (SIV) – agenda for visit, confirmation letter to request PI attendance during SIV, report following SIV
Routine Monitoring Visit (RMV) – confirmation letter to request PI attendance, report following RMV, follow-up letter
Site Close-Out Visit (SCOV) – confirmation letter to request PI attendance, agenda for SCOV, report following SCOV, follow-up letter
CRA transition letter – document notifying site PI of appointment of new monitor (yourself as CRA)
Chapter 38: Interviewing and Career
In this unit, you will find suggestions and recommendations for making a positive impact in interviews for CRA positions, as well as tips and strategies for making rapid progress in a clinical research career.
Chapter 39: Final Examination
This module comprises a comprehensive 51-item, self-paced quiz to assess your competency in the skills and knowledge required for a Clinical Research Associate position.
https://www.beroeinc.com/category-intelligence/clinical-research-organizations-market/
https://www.linkedin.com/jobs/search?keywords=Clinical%20Research%20Associate&location=United%20States&geoId=103644278&trk=public_jobs_jobs-search-bar_search-submit&position=1&pageNum=0
https://www.centerwatch.com/articles/24791-demand-for-experienced-clinical-trial-professionals-outpacing-supply-acrp-says
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317309/
https://www.niaid.nih.gov/research/dmid-investigational-product
https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
Dixon JR. 1999. The international conference on harmonization good clinical practice guideline. Quality Assurance. 6(2): 65-74. DOI: 10.1080/105294199277860
https://www.fda.gov/files/drugs/published/E6%28R2%29-Good-Clinical-Practice--Integrated-Addendum-to-ICH-E6%28R1%29.pdf
https://www.who.int/groups/research-ethics-review-committee/recommended-format-for-a-research-protocol/
https://iaocr.com/finding-first-clinical-research-job/
https://jobs.newscientist.com/en-au/article/a-career-in-clinical-research/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326906/
St. Germain DC, Good MJ. 2017. Data management in clinical trials. In: Gallin JI, Ognibene FP, Lee Johnson L, editors. Principles and practice of clinical research. San Diego: Academic Press. p. 531-545. ISBN 978-0-12-849905-4
https://acrpnet.org/wp-content/uploads/dlm_uploads/2017/04/clinical-study-monitoring-competencies.pdf
https://www.clinicalleader.com/doc/starting-a-career-in-clinical-research-things-we-wish-we-knew-0001
https://www.proclinical.com/blogs/2021-9/how-to-get-a-job-as-a-clinical-research-associate-cra
https://acrpnet.org/2018/06/11/5-clinical-research-trends-emerge-at-acrp-2018/
https://www.collegechoice.net/sciences/clinical-research/best-masters-degrees/
https://acrpnet.org/certifications/cra-certification/
https://www.socra.org/certification/program-overview/
Pharmacovigilance: A Complete Guide to Pharmacovigilance and Drug Safety Training
The ultimate guide to clinical research monitoring.

A Day in the Life of a Clinical Research Associate
For the 2023-2024 academic year, we have 112 schools in our MHAOnline.com database and those that advertise with us are labeled “sponsor”. When you click on a sponsoring school or program, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Every pill, cream, tonic, and capsule a pharmacist passes over the counter to an individual has taken a long journey from concept to the point of sale. In fact, many medications that do not require a prescription, such as those found in the allergy, cold, and flu aisle, also go through the same arduous process.
Before a drug can be prescribed and sold, it must be proven safe and effective. Pharmaceutical companies, universities, and health organizations put drugs through clinical trials or clinical study processes to obtain this proof. In the clinical trials world, the company that provides financial support to a research group selected to put the drug through clinical trials is known as a contract research organization or CRO. Primarily, CRO organizations offer financial aid for pharmaceutical development and biotechnology for agricultural and medical device industries.
In the case of pharmaceuticals, drugs that have not yet been approved for sale are administered to vetted participants in a controlled manner during a clinical trial. Clinical trials involve a great deal of documentation, analysis, observation, and organization. A team of professionals is engaged in administering a clinical trial, including a clinical research associate (CRA).
The CRA acts as a liaison between the study’s sponsor CRO (e.g., pharmaceutical company) and the clinics where the study occurs. Because the clinical trial results must be kept entirely transparent and not influenced by the sponsor’s interests, this is a critical role. Therefore, a successful CRA will be detail-oriented, highly educated, and communicate clearly with sponsors and clinical representatives.
| Featured Clinical Research Administration Programs | ||
|---|---|---|
| Arizona State University | Clinical Research Management (MS) | |
| Arizona State University | Clinical Research Management - Regulatory Science (MS) | |
| Arizona State University | Regulatory Science (MS) | |
| Arizona State University | Regulatory Science (MS) - Food Safety | |
| Johns Hopkins University - Advanced Academic Programs | MS Regulatory Science | |
| For the 2023-2024 academic year, we have 112 schools in our MHAOnline.com database and those that advertise with us are labeled “sponsor”. When you click on a sponsoring school or program, or fill out a form to request information from a sponsoring school, we may earn a commission. View our for more details. | ||
THANK YOU FOR YOUR INTEREST IN Southern New Hampshire University Online MS - Construction Management
Work environment of clinical research associates.
A clinical research associate works both at clinical sites and sponsor locations. During a trial, the CRA conducts regular site visits—virtually and physically—to ensure good progress and record-keeping on the clinical site.
CRAs are often responsible for multiple trials at one time, meaning significant amounts of travel between these sites. Sometimes, a CRA may be assigned to a specific geographical region, limiting travel.
Clinical Team of a Clinical Research Associate
Typically, a clinical research associate needs to have direct contact with the participants involved in the study. However, CRAs must work in a collaborative environment, coming into frequent contact with the clinical team at the study site and the supervisors from the study sponsor. Thus, clinical research associates work in the middle of the chain of command, which begins at the top with:
- Contract research organization (CRO) or sponsor
- Principal investigator (PI)
- Clinical research associate (CRA)
- Clinical research coordinators (CRC)
In short, a CRA is focused more on data, accuracy, and quality control while a CRC collects data and interacts with patients. Dan Sfera , in his YouTube channel titled The Clinical Trials Guru, summarizes the CRA profession with this description: “CROs select various PIs across the country, and PIs make sure that sites follow good clinical practices and protocols. They ensure this by hiring CRAs who look at all the recorded data and do not interact with patients when they are completing a site visit.”
Typical Daily Responsibilities of Clinical Research Associates
The daily responsibilities of a CRA are mainly dependent on the stage of the trial they are supervising. As such, below is a breakdown of the typical duties of a CRA during the beginning, middle, and end of a clinical study.
Before a Study Begins
Every clinical study must take place in an appropriately equipped clinical location. The CRA plays a critical role in selecting a site for a clinical study and may even be asked to suggest sites based on their previous experiences. CRAs may also evaluate the applications of sites that self-select as eligible for a particular study. A pool of potential study sites is narrowed down by having sites complete and submit a feasibility survey.
When the pool of sites has been narrowed down, the clinical research associate conducts site selection visits with the chosen locations. During these visits, the CRA spends up to half a day confirming the validity of the feasibility study, meeting with the team (mainly the assigned coordinator), and observing the capabilities and equipment at the facility.
Upon completion of the site visit, the CRA compiles a report for the study sponsor and presents their findings and recommendations for proceeding with the study.
During a Study
Once a site has been selected, the CRA is now responsible for ensuring that the site knows the sponsor’s required protocol and is appropriately set up to conduct the study. In addition, the clinical research associate conducts site visits at regular intervals throughout the study to ensure that protocols are followed and data is effectively collected.
Depending on the study, CRAs may conduct in-person site visits as well as virtual visits. In recent years, the use of remote visit technology has allowed CRAs to review paperwork online, for instance, and reserve in-person visits for necessary personal interactions. During site visits, the CRA ensures that the study is always proceeding with good clinical practices. More details on the specific methods are available below.
Ultimately, since the CRA is a liaison, developing and maintaining positive relationships is an essential part of the job. During the trial, the CRA must communicate effectively and help the clinical staff appropriately to ensure the study progresses smoothly.
When a Study Ends
The CRA typically conducts a closeout visit after a study or when it becomes necessary to end a study—e.g. when enrollment is too low.
During the closeout visit, the CRA verifies that all paperwork is in order and that all obligations have been met on both sides. For example, the verification process may mean ensuring the trial drugs are returned or destroyed, completing and adequately filing all documentation, and compiling all information necessary to complete a final report for the study sponsor.
Required Skills & Knowledge of Clinical Research Associates
Based on the responsibilities outlined above, it should be no surprise that CRAs must have a knack for detail. Proper documentation, filing, and storage are all critical parts of the job description. It is up to the CRA to ensure that the sponsor and clinic understand their obligations at every point of the study. All policies and procedures are being followed to ensure successful data collection.
In addition to being detail-oriented, a good CRA also pays heed to the ethics of the position. For example, clinical trials and studies can have extensive consequences for the organizations that sponsor them, as well as for the trial participants, and eventually, for consumers at large. Therefore, CRAs act as vital monitors for ethical issues and should be able to stand up to any perceived transgressions.
Above all, it is essential that a CRA understands and can implement good clinical practices (GCP) successfully. The GCP guidelines are an internationally developed set of standards from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) that details ethically and effectively conducting drug trials with human subjects.
The ICH topics are divided into four categories: quality, efficacy, safety, and multidisciplinary. CRAs can find the guidelines in more detail on the ICH website. The YouTube Channel, GCP Mindset , outlines the different roles and responsibilities of CRAs. In short, upholding the GCP standards is the most critical part of the CRA job, meaning that any CRA must be familiar with GCP and recognize and enforce these practices in a clinical setting.
Certification for Clinical Research Associates
While certification is not a legal requirement to work as a clinical research associate, it can provide an important differentiator when looking for employment or career advancement in many professions.
Two main bodies offer certification for CRAs: the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SOCRA).
Association of Clinical Research Professionals (ACRP) – Certified Clinical Research Associate (CCRA) Certification
To earn the Certified Clinical Research Associate (CCRA) credential from ACRP, applicants have two available pathways:
- Pathway 1 – Clinical research professionals with 3,000 hours of verifiable work experience are eligible to sit for the CCRA Exam.
- Pathway 2 – Clinical research professionals with 1,500 hours of verifiable work experience and a clinical research degree are eligible to sit for the CCRA Exam.
Applicants also have to submit proof of their current job description and resume and pass the CCRA exam. In addition, CCRAs must complete at least 24 hours of continuing education credits and be recertified every two years to maintain certification. Beyond the CCRA certification, ACRP also offers certifications for project managers, research coordinators, and principal investigators, and an umbrella certification for clinical professionals.
Society of Clinical Research Associates (SOCRA) – Certified Clinical Research Professional (CCRP) Certification
To earn the Certified Clinical Research Professional (CCRP) certification from SOCRA, applicants must either:
- Have at least two years of full-time experience as clinical research professionals or 3,500 hours of part-time experience in the past five years
- Have a degree in clinical research plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
- Have a certificate in clinical research (undergraduate or graduate), a degree in science, health science, pharmacy, or a related field (associate’s or bachelor’s), plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
Applicants must also pass the CCRP exam and be recertified every three years. Recertification requires 45 total hours of continuing education credits.
The critical difference between the SOCRA and ACRP certifications is that ACRP expressly certifies CRAs, while the SOCRA certification can apply to other clinical research professionals.
Matt Zbrog is a writer and researcher from Southern California. Since 2018, he’s written extensively about emerging issues in healthcare administration and public health, with a particular focus on progressive policies that empower communities and reduce health disparities. His work centers around detailed interviews with researchers, professors, and practitioners, as well as with subject matter experts from professional associations such as the American Health Care Association / National Center for Assisted Living (AHCA/NCAL) and the American College of Health Care Executives (ACHCA).
Related Programs
- 1 Online Master’s in Clinical Research Administration Programs
- 2 Online Doctor of Health Sciences (DHS)
- 3 Online Master’s Degrees in Food Safety – Regulatory Affairs & Quality Assurance
- 4 Online Master’s in Bioinformatics Programs
- 5 Online Master’s in Biotechnology Programs
Related FAQs
- 1 Clinical Significance vs. Statistical Significance
- 2 How Do I Become a Clinical Trials Research Nurse?
- 3 How Do You Become a Clinical Research Coordinator (CRC)?
- 4 What Can You Do with a Degree in Clinical Research Administration?
- 5 What is Clinical Data Management (CDM)?
Related Posts
A day in the life of a clinical data analyst.
Every day, health organizations like hospitals, clinics, and physician offices collect data about their patients. This information is used to make data-driven decisions in order to provide the highest level of care to their patients as well as reduce expenses and errors. From the outcome of a particular treatment to a large-scale clinical trial, health data is a critical part of the modern healthcare process. Clinical data analysts help to make sense of the extensive data that is at their fingertips, creating stories that turn numbers into actionable intelligence to improve healthcare outcomes.
Healthcare Debates: Does Costly US Healthcare Fund R&D and Medical Research?
This article explores research and development in the context of greater healthcare expenditures, including looking into the main drivers of healthcare spending, influences on pricing, and where R&D fits into the whole ecosystem.
Pharmaceutical Quality Director – A Day in the Life
Consumers and corporations need to know that the drugs dispensed by pharmaceutical companies are manufactured safely and efficiently. Pharmaceutical quality directors ensure that quality control protocols are followed within the industry’s manufacturing, testing, and inspection procedures.
Closing the Gap: Women Leading Healthcare
Women drive healthcare. They are the industry's biggest consumers and workers. They serve as caregivers in their homes and make most decisions for their family's health. Why is it, then, that of the 40 Fortune 500 healthcare companies, not a single one is helmed by a woman?
Clinical Application Analyst – A Day in the Life
Electronic health records (EHR) and other clinical software systems are the new industry standard in healthcare. However, plenty of clinics, hospitals, and private practices have yet to make the full migration. That’s where clinical application analysts come in.
- MTS Health Sciences How to Become a Clinical Research Associate
- Laboratory Technology
- Natural & Clinical Science
- Medical IT & Administrative
- Patient-Facing Technology
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Infection Preventionist
- Medical Laboratory Scientist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Anesthesia Technician & Technologist
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Rehabilitation Technician
- Respiratory Therapist
- Surgical Technologist
Certification Guides
Career guides, interviews & features, how to become a clinical research associate (cra), search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Scientists, researchers, and doctors make discoveries about drugs, surgical procedures, behavioral therapies, or medical devices through their work in laboratories and healthcare settings. This is only the beginning of the journey for pharmaceuticals, therapies, and devices, as bringing the findings from the lab to the street requires a vigorous scientific process known as a clinical trial. Clinical research associates (CRAs) are the professionals responsible for ensuring that clinical trials move forward following established guidelines and regulations for ethics, safety, and reporting.
Clinical research associates, also known as “monitors,” work on behalf of sponsors funding clinical trials for the new or existing drug, device, surgery, or behavioral intervention. Working directly for the sponsor or through a contract research organization, the main task of a CRA is to monitor the progress of an ongoing clinical trial.
Through in-person site visits or remote monitoring systems, a CRA serves as the central point of contact between a sponsor and testing sites; ensures that the trial is being administered per approved protocols; verifies that the clinical trial is being conducted ethically at all sites; and confirms the validity and accuracy of all data being collected and reported at test sites.
In addition to reading, interpreting, and understanding medical technology, clinical research associates must have excellent interpersonal and communication skills. The ability to understand best clinical practices, design protocols, and data standards requires CRAs to have outstanding attention to detail, analytical skills, and the capacity to deliver constructive feedback to participating research sites on their performance.
Although not a requirement, many CRAs travel between multiple research sites for study oversight, which may require a valid driver’s license, the physical capacity to travel, and/or willingness to fly or drive regularly.
This detailed guide explores the education and credentials required to become a clinical research associate (CRA).
Arizona State University
Johns hopkins university (aap), university of west florida, steps to become a clinical research associate (cra).
The pathways to becoming a clinical research associate are numerous and available to anyone with a high school diploma or higher. While formal education is not technically required to enter the field, having a bachelor’s degree or higher can make potential candidates much more competitive.
Certification in the field is also not required, but obtaining certification from the Society of Clinical Research Associates (SOCRA) or the Association of Clinical Research Professionals (ACRP) can result in more opportunities and even more competitive salaries.
Finally, all aspiring CRAs are advised to check out the International Conference on Harmonisation’s (ICH) guidelines for Good Clinical Practice (GCP) to get a feel for the professional expectations and responsibilities.
Here is how to become a CRA depending on one’s level of education. Please note that in the United States, there are two major certification bodies for CRAs: the Society of Clinical Research Associates (SOCRA) and the Association of Clinical Research Professionals (ACRP). Each pathway includes the eligibility requirements to pursue credentialing through either of these entities.
PATH 1: Earn a High School Diploma and Gain Experience
Perhaps the most strenuous route to this career is becoming a certified CRA with a high school diploma and between 3,000 and 3,500 hours of qualifying work experience (depending on the certification entity).
These candidates often start out in support positions assisting a more experienced or certified CRA with mundane tasks. An entry-level worker can earn increased responsibilities through a demonstrated capacity to learn the regulations, protocols, and ethical considerations. To qualify for the following CRA certification exams, high school graduates must:
SOCRA Category 1
- Complete two full-time years of CRA work within five years, or 3,500 hours of part-time work
ACRP CCRA (Certified Clinical Research Associate)
- Complete 3,000 hours performing essential duties
- Submit a resume documenting and demonstrating job performance
Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
PATH 2: Earn an Associate Degree and Gain Experience
Depending on the program, an associate’s degree of applied science (AAS) in clinical research can be a standalone degree or a stepping-stone to a bachelor’s or master’s. Licensed vocational or practical nurse (LVN or LPN) programs are designed specifically for practical, job-ready skills and may qualify aspiring CRAs for the ACRP certification.
Similar to the path taken by those with a high school diploma, having an associate degree, LPN, or LVN can open the door to some entry-level jobs in the industry. At this level, some prospective CRAs assist more experienced CRAs or some engage independently in entry-level tasks related to study monitoring. Those working as CRAs with an associate’s degree, LPN, or LVN can qualify for certification after working a certain number of hours in the field.
To qualify for the following CRA certification exams, associate degree graduates must:
SOCRA Category 2
- Hold a “clinical research” degree
- Complete one full-time year as a CRA or 1,750 hours part-time
ACRP Option 2 (Also for LVN, LPN)
- Hold a “clinical research degree” or complete 1,500 hours performing essential duties
PATH 3: Earn a Bachelor’s Degree and Gain Experience
Most entry-level clinical research associate positions require candidates to have a bachelor’s of science (BS) in a health-related field from an accredited four-year university. In some cases, programs are designed to add practical hours needed to qualify for certification tests.
Those interested in becoming a CRA can study nursing, health sciences, biological sciences, clinical research, clinical research administration, clinical research management, medical technology, or life sciences, among many other subjects. Because many entry-level positions are looking for those with previous work in the field, those earning a BS should seek internships, part-time work, and/or fellowships involving participation in research, if possible.
To qualify for the following CRA certification exams, bachelor’s degree graduates must:
SOCRA Category 3
- Hold a “clinical research” undergraduate degree
ACRP Options 1 & 2
- Complete 3,000 hours performing essential job duties or 1,500 hours of equivalent work experience requirements through ACRP certifications or approved clinical research degree programs accredited by the Council for Higher Education
PATH 4: Earn a Master’s Degree for Opportunities in Management
A master’s program in clinical research is generally designed for those already working as CRAs to expand their skills or to advance into management or supervisory roles within the field. However, for those with non-health science bachelor’s degrees who want to become CRAs, seeking a master’s of science in clinical research or a master’s of science in clinical research management could be a pathway to breaking into the field.
Because many of these programs are offered online, earning a degree is possible for even those students who need full flexibility of schedule to complete the degree. Although requirements for admission into master’s programs vary, those looking to gain admission into a master’s of science for clinical research commonly need the following:
- A bachelor’s degree
- Official transcripts demonstrating specific coursework in science
- A statement of purpose
- Letters of Recommendation or Reference
- A resume or CV
- An application fee
- TOEFL or IELTS scores (international students only)
Clinical Research Associate (CRA) Degree Programs
There is a range of formalized training programs that prepare professionals for this key role in ensuring the safe, and ethical development of medical technologies. Below you will find examples of programs at a range of educational levels available to those interested in a career as a CRA.
Durham Tech – AAS Program
Durham tech, located in Durham, North Carolina, offers a 71-credit hybrid on-campus and online clinical trials research associate (CTRA) associate of applied science (AAS) program. Durham’s CTRA AAS prepares graduates to work on any side of clinical research in an assistant’s role.
While most programs require the student to attend on-campus courses, there are several courses that are offered completely online. The program takes 20 to 21 months and includes coursework in research site management; clinical research management; research protocol design; an introduction to ethics; anatomy and physiology; an introduction to clinical data; pathophysiology; and clinical research terminology.
Graduates of the program may be eligible to sit for national certification examinations and will be prepared for opportunities at medical centers, pharmaceutical industries, hospitals, research facilities, clinics, physicians’ offices, and device companies.
- Location: Durham, NC
- Accreditation: Southern Association of Colleges and Schools Commission on Colleges (SACSCOC); Commission on Accreditation of Allied Health Education Programs (CAAHEP)
- Expected Time to Completion: 20 to 21 months
- Estimated Tuition: $5,396
Washington University in St.Louis University College – BS, MS, Certificates
Washington University in St. Louis, Missouri, has various degree options for CRAs at all stages of their career to work as monitors. Students can enhance their current skills and knowledge in clinical research management, as well as gain a deep mastery regarding how to best move clinical research forward in an ethical, compliant, and safe way.
Those with at least six units of transferable coursework qualify to apply to the 120-credit-hour bachelor of science in clinical research management to start their careers. Anyone with any educational background can pursue University College’s 21-credit undergraduate certificate in clinical research management to enhance career skills or make a resume more competitive.
Students who already have a BA or BS also have options at Washington University. Experienced professionals in the clinical research field who wish to seek formalized training can earn a 21-credit advanced certificate in clinical research management or a 30-credit master of science (MS) in clinical research management. Those with a non-healthcare bachelor’s degree who wish to become high-level CRAs can up their skills and knowledge by choosing the combined bachelor’s and master’s degree options.
Although the coursework in each program varies to suit the level of education, themes across all the programs include the fundamentals of clinical research management; research ethics and regulatory affairs; compliance, legal and regulatory issues; and data and information management in health sciences.
- Location: St. Louis, MO
- Accreditation: Higher Learning Commission (HLC)
- Expected Time to Completion: BS (up to 48 months); certificate (12 months); MS (24 months)
- Estimated Tuition: Undergraduate courses ($695 to 895 per credit); Graduate courses ($665 to 995 per credit)
Barnett International – Online Seminar
Designed for CRAs with two years of experience or less, this online clinical research associate onboarding program by Barnett International prepares entry-level employees to monitor clinical trials at high levels appropriate to industry standards.
Over ten weeks of synchronous online coursework lasting three hours per week, participants will learn topics including informed consent, investigational product accountability, safety definitions and reporting requirements, and regulatory compliance and quality assurance: audits and inspections. Participants receive 30 hours (3.0 CEUs) of continuing education credits.
- Location: Needham, MA
- Accreditation: Accreditation Council for Pharmacy Education
- Expected Time to Completion: Ten weeks
- Estimated Tuition: By Early Bird Deadline ($1,795); After Early Bird Deadline ($1,995); June 10 is the early bird deadline
Continuing Education for Clinical Research Associates (CRAs)
Both CRA certification bodies require continuing education to maintain active certification status.
SOCRA requires recertification every three years. It calls for 45 hours of CE to be completed over the course of the first three years beyond passing the initial test. Twenty-two CE units must be related to clinical research; the remainder can be in the professional or therapeutic area in which one works or specializes. In addition, those looking to maintain or renew certification must complete a “recertification continuing competence learning module.”
The ACRP expects certified CRAs to engage in continuing education (CE) and continuing involvement (CI) to maintain certifications. Continuing education should include coursework in research and healthcare, and continuing involvement requires candidates to engage in activities such as authorship, participating in investigator meetings, or working as a peer reviewer, among other opportunities. Notably, ACRP utilizes an ongoing point system for professionals to maintain their certifications.
CRA Career and Salary
Clinical trials and the objectivity they bring to advances in treatment are extremely important. In an increasingly globalized society, diseases spread across borders, and in an age of increased antibiotic resistance, new ways to fight bacteria will be needed. Furthermore, with an aging U.S. population comes increased rates of chronic conditions and the subsequent reliance on pharmaceuticals to improve people’s quality of life.
It’s not surprising that the Bureau of Labor Statistics (2022) predicted a 7 percent increase in openings for medical and clinical laboratory technicians between 2021 and 2031, much more than the average growth anticipated across all U.S. occupations during that same decade (5 percent). As far as the salaries are concerned, here are the salary percentiles for clinical laboratory technologists and technicians in the US ( BLS May 2022):
| United States | |
|---|---|
| Number of professionals employed | 333,600 |
| Annual mean wage | $59,130 |
| 10th percentile | $35,220 |
| 25th percentile | $40,440 |
| 50th percentile (median) | $57,380 |
| 75th percentile | $74,920 |
| 90th percentile | $84,670 |
Lastly, while the BLS doesn’t track salaries for CRAs, PayScale.com (June 2023)—a site that relies on self-reported data—found that the median annual salary for a CRA was $72,393. Among the 1,391 CRAs reporting their annual salaries, Payscale found these percentiles:
- 10th percentile: $48,000
- 50th percentile (median): $72,393
- 90th percentile: $101,000
Specialized skills in CRA that increased salaries included medical devices (37 percent pay increase over average), team leadership (35 percent), and writing procedures & documentation (20 percent).
Years of experience, predictably, also have an impact on salary. Entry-level CRAs earn 15 percent below the average, while experienced CRAs (ten to 19 years) earn 16 percent above the average and late-career CRAs (20+ years) earn 25 percent above the average.
It is important to note that these figures also vary based on the data source. For illustration, Indeed.com (June 2023) found an average annual salary of $80,957 among United States clinical research associates.

Becca Brewer is building a better future on a thriving earth by healing herself into wholeness, divesting from separation, and walking the path of the loving heart. Previously to her journey as an adventurer for a just, meaningful, and regenerative world, Becca was a formally trained sexuality educator with a master of education.
Related Articles
- Upskilling in the Allied Health Professions
- Single-use Plastics in Medicine Raise Concerns About Sustainability
- Clinical Research Certification - CCRA, CCRC, CPI
- Online Master’s in Clinical and Translational Research Programs
- Online Bachelor’s in Health Science Programs (BSHS Degree)
- Online Master's Programs in Clinical Management and Leadership
- Clinical Lab Science vs. Clinical Research
- BSHS (Health Sciences) vs BSHA (Health Administration) Programs
- Clinical vs Translational Research
- Guide to Health Science Careers
Related Programs
Clinical Research Associate Career Guide
- Career guide intro
- How to become
- Career path
Similar job titles
- Trends and outlook
- Career tips
- Where the jobs are
What is a clinical research associate?
A clinical research associate, or CRA, is like a superhero for modern medicine. They make sure new medical treatments are safe and effective by managing clinical trials. These are tests where scientists try out new drugs or treatments to see if they work and if they’re safe.
CRAs are super important because they make sure everything in the trial runs smoothly. Without them, we wouldn’t be able to trust new medical treatments because there wouldn’t be anyone to double-check all the important details.
Duties and responsibilities
- Setting up trials: CRAs start by getting all the plans approved. They also help create the materials needed for the trial.
- Checking on trials : They visit trial sites regularly to make sure everything goes as planned and follows the rules.
- Handling data : They verify that all the info collected during the trial is correct and complete. This is crucial because it affects the trial’s results.
- Keeping everyone safe : They constantly check to make sure the trial is safe for everyone involved.
Work environment
CRAs often have a mix of office and travel work. When they’re not visiting trial sites, they might work from an office in a hospital or a special research facility.
The places CRAs visit can vary a lot. One day they might be in a lab, another day in a clinic, or traveling to different cities or even countries. Their work environment can change often, which keeps the job exciting and dynamic for those who like variety.
Typical work hours
Most of the time, CRAs work normal office hours—9 AM to 5 PM, Monday through Friday. But their schedule can change depending on the needs of the trial. They might need to start early, stay late, or work on weekends, especially when visiting trial sites or when a trial is really busy. Being flexible is key for these associates because their days can be pretty unpredictable.
How to become a clinical research associate
Want to help develop new medicines and treatments? Here’s how you can become a CRA:
Step 1: Get a bachelor’s degree
Start with a bachelor’s degree in a field like nursing, biology, or any science-related area. These degrees give you the basic knowledge you need about how medical research works.
Step 2: Gain experience
Next, try to get some real-world experience. This could be through internships or jobs in hospitals or labs where you get to work on actual research projects. The more you understand about clinical trials from real experiences, the better.
Step 3: Go for a master’s degree (optional)
If you really want to boost your chances, consider getting a master’s degree. This is especially helpful if you want to learn more about specific areas like drug development or how healthcare policies affect research.
Step 4: Consider a clinical research certificate program
Taking a short course in clinical research can be a great next step. These programs teach you about the nuts and bolts of running clinical trials and making sure they follow legal and ethical standards.
Step 5: Get certified
Getting a certification from a group like the Association of Clinical Research Professionals can make you stand out in the job market. It shows you’ve got the skills and knowledge to do the job well.
Step 6: Start applying for jobs
With your education, experience, and certification in hand, start applying for CRA jobs. Use job sites, networking sites, and university career resources to find job openings.
Step 7: Keep learning
The world of clinical research is always changing. Keep up with new developments by taking online courses or attending workshops. This will make sure you stay good at your job and know the latest in research and treatment developments.
Here are some online classes we recommend:
- The Beginners Course for Clinical Research : A great start to understand the basics of clinical research.
- The Good Clinical Practice : Learn how to review clinical trials critically.
- Clinical Trials Operations Specializations : Dive into the details of how to manage and analyze data from trials.
- Introduction to Systematic Review and Meta-Analysis : Learn how to perform detailed reviews and analyze the results.
How much do clinical research associates make?
The salary of a CRA is influenced by several factors, including their education, years of experience in the field, type of employment (full-time, part-time, contractual), location, and the size and nature of the employer (pharmaceutical companies, hospitals, independent research labs).
Those working in pharmaceutical and manufacturing tend to earn the highest incomes. Geographical location significantly affects earnings, with salaries typically higher in states with a larger concentration of biotech firms and clinical research organizations.
Highest paying industries
- Pharmaceutical and Medicine Manufacturing: $118,380
- Offices of Physicians: $111,580
- Federal Government: $110,460
- Research and Development: $106,320
- Hospitals: $102,220
Highest paying states
- District of Columbia: $106,422
- Alaska: $106,600
- Massachusetts: $103,670
- New Jersey: $102,380
- Connecticut: $101,280
The average national salary for a Clinical Research Associate is:
Browse CRA salary data by market
Types of clinical research associates
CRAs come in different types, depending on the part of a clinical trial they work on or the kind of patients they help. Here’s a breakdown:
- Early-stage trials CRA : These CRAs work on the first steps of testing new treatments, known as Phase I or Phase II trials. They make sure everything goes according to plan at the places where the trials are done and check that all the information collected is correct.
- Late-stage trials CRA : These associates handle the big leagues of clinical trials, called Phase III or Phase IV. They deal with lots of data and manage trials that include many people to see if a treatment really works in the real world.
- Contract CRA : Contract CRAs are like freelancers in the research world. They might work on their own or with a company that specializes in research, and they’re usually hired just for specific parts of a trial, like setting it up, keeping an eye on things, or wrapping it up.
- Oncology CRA : Oncology CRAs focus on finding new ways to treat cancer. This job is super important because it deals with complex research and treatments that need careful handling due to the seriousness of the disease.
- Pediatric CRA : These CRAs specialize in research for kids. They need to know a lot about children’s health and how to handle the special rules that come with working on medical trials that involve young participants.
Top skills for clinical research associates
Here’s what you need to shine in this role:
- Understanding health science : You’ve got to know your stuff when it comes to health and science. Knowing health science helps you make sure the trials are useful and the results make sense.
- Attention to detail : In this job, the little things really matter. You need to be super careful and make sure every single detail is correct during a trial.
- Great communication skills : You’ll talk to a lot of different people, like doctors, scientists, and sometimes even patients. Good people skills help you work well with others and solve problems when they come up.
- Organizational know-how : CRAs have a lot to keep track of—like schedules, data, and lots of tasks all at once. Being organized and good at managing your time helps you handle everything without getting stressed or making mistakes.
- Strong ethics : Working with medical trials is a big deal because you’re dealing with people’s health. It’s super important to always do the right thing, like keeping patient info private and making sure everyone knows what’s happening in a trial.
Looking for a new job?
Browse our national database of CRA job openings and apply today
Clinical research associate career path
If you’re thinking about a career as a CRA, you’ll have lots of options for moving up and trying new things as you gain experience. Here’s a look at some paths you might take:
Climb the ladder in clinical research
As you get better at your job, you could move up to become a senior CRA or even a clinical project manager. In these roles, you’d oversee whole research projects and manage teams, making sure everything runs smoothly from start to finish.
Dive into academia
If you love learning and teaching, you might go back to school and get a doctorate. This could lead to jobs like clinical research scientist, where you’d come up with and guide new studies, or even become a professor who teaches the next generation of researchers.
Switch to regulatory affairs
Another cool option is to move into regulatory affairs. This job is all about making sure that new medical treatments meet all the rules and laws before they go out to the public. You could work as a regulatory affairs associate or climb up to manager or director roles.
Explore business roles
If you’re interested in the business side of things, you might like roles such as clinical trial auditor or business development manager. These jobs involve checking the details of clinical trials, finding ways to grow the business, and keeping everything running smoothly.
- Clinical Data Manager
- Research Scientist
- Biostatistician
- Data Analyst
- Data Scientist
- Regulatory Specialist
- Medical Science Liaison
- Program Manager
- Program Coordinator
- Medical Biller
Clinical research associate position trends and outlook
Here’s a look at what’s shaping the future for CRAs:
- New medical advances : As medicine gets more personalized and new treatments are developed, CRAs are super important in making sure everything goes right. They need to stay sharp and keep up with the latest methods and tech in clinical trials.
- Specialized knowledge is key : Areas like cancer research, infectious diseases, and brain health are growing fast; associates with expertise in these areas are especially needed. Plus, with more clinical trials happening around the world, knowing how to work with different cultures and global rules is a must.
- Tech-savvy required : The digital world is taking over clinical trials too. They need to know how to use digital tools that manage trial data and documents; being good with technology isn’t just nice to have but essential.
Employment projections
Things are looking good for CRAs. Jobs in this field are expected to grow by 16% through 2031, which is much faster than average. This growth is driven by the ongoing need for new meds and health solutions. So, if you’re thinking about this career, it’s a pretty exciting time to get involved.
Clinical research associate career tips
Stay in the know.
The medical field is always changing. Keep up with the latest news, technology, and rules in clinical research. This means regularly checking out new studies, attending industry events, and maybe even following some health science blogs.
Understand the big picture
Get to know everything about the research projects you work on—not just your part. Understanding the goals and the big picture can help you manage your tasks better and make smarter decisions.
Pay attention to details
In clinical trials, the little things can make a big difference. Being organized and careful helps ensure that the data you collect and report is accurate and reliable.
Network, network, network
Connecting with other people in your field can open up new opportunities and help you learn. Consider joining groups like:
- Association of Clinical Research Professionals (ACRP)
- Society for Clinical Research Sites (SCRS)
- American Society of Clinical Oncology (ASCO)
Never stop learning
Clinical research is a field where you never stop learning. Look for courses, workshops, and seminars to improve your skills. Organizations like SoCRA and ACRP offer lots of resources and certifications that can boost your career.
Invest in your soft skills
Besides all the scientific stuff, good communication, problem-solving, and teamwork are crucial. You’ll often work in teams and deal with different kinds of challenges, so being able to work well with others and manage your time is key.
Where the CRA jobs are
Top employers.
- Quintiles IMS
- Pharmaceutical Product Development
- North Carolina
- Pennsylvania
- Massachusetts
Top job sites
- ClinicalTrials.gov
- ResearchGate
What is the role of a clinical research associate in a clinical trial?
In the context of a clinical trial, they play an essential role in ensuring the safety and efficacy of new drugs or medical devices. Their responsibilities include designing and implementing research protocols, monitoring the progress of clinical trials, ensuring compliance with regulatory standards, and liaising with ethical committees, among others.
What qualifications are typically required for a clinical research associate role?
Most positions require at least a bachelor’s degree in a life science field such as biology or biochemistry. In addition, specific training in clinical research and experience, including internships or previous job roles related to clinical trials, could be advantageous. Some employers prefer candidates who have a master’s degree or are certified as clinical research professionals.
What skills are essential for clinical research associates?
An effective CRA should possess strong attention to detail, excellent organizational skills, and an analytical mindset. They should also have a solid understanding of the scientific process and ethical guidelines for clinical trials. Communication and interpersonal skills are also essential since this role often involves interacting with other professionals.
How is a typical day for a clinical research associate?
A typical day depends on the clinical trial stage they oversee. Their workday might involve designing trial protocols, coordinating with ethics committees, monitoring trial progress, assessing and managing risks, ensuring data quality, or preparing status reports. The role often includes a mix of office-based work and travel to the locations where clinical trials occur.
What types of challenges do clinical research associates face?
They can face a variety of challenges. Given the industry’s highly regulated nature, compliance with complex regulations and guidelines can be demanding. They may encounter difficulties in recruiting and retaining trial participants, dealing with unforeseen issues during a trial, managing ethical questions, and addressing problems with data quality or completeness. Also, traveling to the trial site, often part of their job, can present logistical hurdles.
Can a clinical research associate work remotely?
Some aspects of their work can be performed remotely; for example, managing data, preparing reports, and communicating with study team members. However, due to the nature of the role, it often requires travel to the clinical trial site for monitoring visits, inspections, and audits. The potential for remote work depends on the trial’s nature and the employer’s or client’s policies.
Do clinical research associates focus on a specific therapeutic area?
While some specialize in specific therapeutic areas, others may work across a variety of them. Specialization can be advantageous as it enhances their knowledge and understanding of particular diseases, treatment methods, and associated regulations. Conversely, working across multiple therapeutic areas can provide a wide range of experience and flexibility.
What further career opportunities can a clinical research associate pursue?
Becoming a CRA can provide a pathway to various career advancements. With experience, they may move into senior or leadership positions within clinical research, such as a lead CRA, clinical trial manager, or director of clinical operations. There are also opportunities for transitioning to other roles within the pharmaceutical or biotech industry, research institutions, or regulatory bodies.
How can a clinical research associate stay updated on industry trends and regulations?
Staying current is crucial in a rapidly evolving field like clinical research. Attending industry conferences and seminars, completing continuous professional development courses, and subscribing to relevant industry publications can be beneficial. Membership in professional organizations can provide access to a wealth of resources and networking opportunities. It’s also crucial to stay updated with regulatory changes as they occur.
What impact has technology had on the role of a clinical research associate?
Technology has significantly changed the role and responsibilities of a CRA. Advances in data management systems have transformed how data is collected, analyzed, and stored. Remote monitoring technologies have also reduced the need for on-site visits. The use of digital tools and artificial intelligence is increasingly supporting those in risk management and decision-making. As technology continues to evolve, professionals in this role should stay updated with emerging tech trends that might shape the future of clinical research.
Reviewed and verified by Pete Newsome
Ready for a change?
Browse millions of job openings today and find your career zen!
Spotlight on the Clinical Research Associate Career Pathway and Resources
Blog June 24, 2024

Anthony Chew, Clinical Trial Operations, Guardant Health
Standing on the outside of a profession and looking in is no easy thing. Without access to “insider’s knowledge” about how to pursue a career path in which you are interested, how do you even know in which direction to take the first step? For example, many people who are just beginning to explore different roles in clinical research find the duties of a clinical research associate (CRA) very attractive (on paper, at least). However, they may not realize immediately that very few applicants for a CRA position with a sponsor or contract research organization (CRO) will be hired straight into a monitoring role without first having worked as a clinical research coordinator (CRC) for a site or as a clinical trial assistant (CTA) for a sponsor.
In a forthcoming peer-reviewed article for ACRP’s Clinical Researcher journal, Anthony Chew, a clinical trial operations professional and recent graduate of the MS program in Medical Product Development Management at San Jose State University, describes CRAs as serving as “the primary liaison between the sponsor and the site by monitoring and verifying data to ensure accuracy and adherence to protocols. They collaborate with investigators, conduct site visits [including for site feasibility/selection and validation/qualification purposes followed by study initiation and ongoing monitoring], and maintain strict documentation to guarantee the integrity of a trial.”
As part of his graduate studies, Chew surveyed current and former CRAs to learn about their professional backgrounds, the types of employers that initially hired them, the training processes they underwent as new CRAs, and any advice they had for aspiring CRAs. Among the respondents, experience in the clinical research field ranged from two years to more than 25 years, with an average experience of nine years in the field. Nearly half of the respondents held their first CRA job with a CRO, whereas most of the rest began at a sponsor organization and just a handful began at an academic institution/hospital.
Notably, more than two-thirds of respondents were observed to have held positions as CRCs or CTAs prior to becoming CRAs. At the most basic level, CRCs support, facilitate, and coordinate daily research activities on the site end; CTAs provide administrative and project tracking support for clinical trials on the sponsor end.
“Among the respondents who were former CRCs, almost one-third cited their experience as CRCs to have played a major role in helping become an effective CRA,” Chew writes. “Respondents who had experience as a CTA also indicated its benefits.”
According to one of the respondents to Chew’s survey, “I do think one benefit of being a CRC is that if you’re interested in going into the medical field, that can be a really good bridge, because you’re working with patients and physicians in a clinical setting. But…there isn’t quite as much room for [direct] upward mobility” for CRCs at a site. On the other hand, CTAs might be promoted into a CRA position without having to leave their current employer.
Chew notes that characteristics related to “communication skills” and “attention to detail” were listed by most respondents as important for being an effective CRA. He also writes that “the purpose of this survey was not to provide a definitive judgment of where or how CRAs are hired, but rather, to point the aspiring CRA in the ‘general direction’ and offer insight as to the advantages and disadvantages that each type of experience provides. While there is no ‘wrong’ pathway to become a CRA, the responses would suggest that the ideal pathway is to start out as a CRC, then join a large CRO as a full-service alignment CRA [working on multiple trials for different sponsors at once], and then to secure a CRA position with a sponsor. This pathway optimizes the ability for the potential candidate to receive as much experience as possible. …Obviously, this is easier said than done.”
Respondents who did not have CRC or CTA experience before becoming CRAs mentioned networking, continuing education, and having a background in such duties as working in a laboratory, handling clinical samples, and demonstrating an ability to adhere to the protocols for trials as useful, Chew wrote.
“Clinical research is still a developing field, and while the pathway to becoming a CRA can be frustrating, the experiences [noted by current and former CRAs in this survey demonstrate] that persistence can pay off,” Chew concludes.
The full article by Chew will appear in the August 2024 issue of Clinical Researcher .
Resources
Association of Clinical Research Professionals—Ready, Set, Clinical Research!
ACRP Certified Clinical Research Associate (CCRA®) Certification
ACRP CRA Core Competency Foundations(TM) Training Program
Career Navigation in Contract Research Organizations: A Vignette from Clinical Researcher (August 2023)
Available in the Course Catalog as ACRP 2024 Study & Site Management session replays (login required): A Crash Course in the Basics of Clinical Trial Monitoring Fixing Feasibility: Collaborative Approaches for Redefining and Improving Site Selection Site Qualification Visits: From “The First Date” to Going Steady Site Validation/Qualification Visit: Picking the Right Sites
Available in the Course Catalog in the ACRP 2024 Workforce Development session replays (login required): A CRA Training Program for Overcoming Workforce Development Roadblocks
Available in the Course Catalog in the ACRP 2024 Leadership & Professionalism session replays (login required):
A Holistic CRA Evaluation Process for Improving Monitoring Visits
Edited by Gary Cramer
Sorry, we couldn't find any jobs that match your criteria.

The Growing Role of Latinos in Clinical Research: Opportunities, Challenges, and the Path Forward

Mission Accomplished: Coming Back to Earth from the Heights of the ACRP Ride4DEI

Considering the Benefits of Music: Not All Alzheimer’s Treatments are Pills or Shots
Jobs in the acrp career center.
- Experienced CRC
- Clinical Research Coordinator – Interventional Endoscopy
- Clinical Research Coordinator (CRC) I
- Senior Clinical Research Coordinator

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Department of Pathology in Stanford University’s School of Medicine is seeking a Clinical Research Coordinator Associate to apply medical knowledge and experience, under the direction of the principal investigator to oversee and direct clinical course of research participants in clinical trials.
Stanford University is one of the world’s most renowned universities. Sitting in the heart of the San Francisco Bay Area among the valley’s most progressive companies.
The Department of Pathology at Stanford School of Medicine, one of its founding departments, stands as a leader among its peers nationwide. Our preeminent faculty spans from emerging leaders to highly accomplished physicians and scientists, including a Nobel laureate and members of the National Academy. Our mission is to improve the diagnosis, treatment, and basic understanding of the human disease. This is done through discovery (research), education, and clinical care.
You will be working with an unparalleled leading-edge community of faculty and staff that are fundamentally changing the world of health care. You will have the opportunity to influence and drive change with your innovative ideas, the ability to make a difference and participate in human advancements. Our culture is fast-paced, energetic, and growing all of the time.
We offer a variety of benefits beyond traditional medical, dental, retirement, and savings options:
- Events and program for children, sports camps, tuition options
- World-class intellectual stimulation through learning and development classes, workshops, and onsite conferences from leading-edge speakers and faculty
- Work/life and family-friendly policies and reimbursement
- Participation in Stanford’s social responsibility and sustainable programs for a better world
- A vibrant university culture that values the uniqueness of each individual
We are seeking candidates who are progressive thinkers, see challenges as simply problems to solve, and have the spirit and energy to change the world.
About the Department of Pathology:
Comprised of extraordinary faculty and staff, our mission is to improve the ability to diagnose, treat and understand the origin and manifestation of human disease, and to care for those who have or are at risk to develop disease. We accomplish this through our clinical services (in all fields of anatomic and clinical pathology, including molecular and genomic pathology, histocompatibility testing and transfusion medicine) and be research (which includes basic, translational and clinical research into the origins and manifestations of disease, including efforts to improve disease prediction and prevention as part of the goal of achieving precision medicine and health), and also by educating future leaders in pathology and related fields. Everything we do is to achieve the goals of providing the highest quality of clinical services to the patients for whom we passionately care, to advance our ability to understand, diagnose, monitor and ultimately to cure disease or to prevent or delay its occurrence, and to provide outstanding education and career development opportunities to those who share these goals.
For more information about the department visit: http://pathology.stanford.edu/
About the Sean N. Parker Center:
The Sean N. Parker Center for Allergy and Asthma Research at Stanford University is the first of its kind, dedicated to advancing treatments and discovering underlying immune mechanisms to develop lasting cures for allergies and asthma in both children and adults. Our interdisciplinary team of leading scientists, physician-scientists, and specialists from fields such as immunology, gastroenterology, otolaryngology, chemistry, bioengineering, pathology, pulmonology, and genetics collaborates on cutting-edge research aimed at understanding immune dysfunctions that lead to allergic reactions.
As a global leader in allergy research, our Center works collaboratively with researchers worldwide, sharing data through an interlinked network of satellite centers to conduct innovative clinical trials. Our research efforts extend beyond allergies and asthma to encompass a range of immune-related disorders, including eczema, food allergies, eosinophilic conditions, drug allergies, and gastrointestinal diseases.
Through laboratory and computational research, clinical trials, and community outreach, we are committed to developing rational, evidence-based therapies that provide the safest and most effective treatments for patients. By combining innovative science with compassionate care, we strive to transform lives at both local and global levels.
About the Position:
The Sean N. Parker Center for Allergy and Asthma Research at Stanford University is seeking a Clinical Research Coordinator Associate (CRCA) to manage life-changing clinical trials under the direction of project researchers, investigators, or managers.
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
*- The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Desired Qualifications:
- Phlebotomy license.
- Prior experience in clinical research setting.
- Bachelors degree in related field.
Education & Experience: (Required):
- Two-year college degree and two years related work experience or a Bachelor's degree in a related field or an equivalent combination of related education and relevant experience.
Knowledge, Skills and Abilities (Required):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
Certifications & Licenses:
- Working toward certification(s) to perform basic patient measurements and tests, such as phlebotomy and EKG.
Physical Requirements*:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
*- Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
Working Conditions:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
- Due to the nature of the work, this position will work fully on-site.
The expected pay range for this position is $31.84 to $37.79 per hour.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location, and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website ( https://cardinalatwork.stanford.edu/benefits-rewards ) provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You
Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow. We offer career development programs, tuition reimbursement, or course auditing. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture. We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you. Climb our rock wall or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun. Stroll through historic sculptures, trails, and museums.
- Enviable resources. Enjoy free commuter programs, ridesharing incentives, discounts and more
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned. Consistent with its obligations under the law, the university will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Fixed-Term
- Requisition ID: 104721
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Academic
Post Date: Aug 26, 2024
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
RECRUITMENT FRAUD ALERT
It has been brought to our attention that there has been fraudulent activity by scammers attempting to represent themselves as Parexel employees or recruiters. These individuals are attempting to reach potential job seekers through online chat interviews and sending false offer letters, representing Parexel without our consent. If you’re concerned that you’ve been contacted by an unauthorized Parexel recruiter or employee, please notify [email protected] . You may also report suspicious fraudulent activity to your local law enforcement agency. Thank you.

- Global Careers
- China Careers
- Japan Careers
My research opens up new medical possibilities. And I do it
Search Jobs
Radius: Enter distance 5 15 25 35 50
Senior Clinical Research Associate/Clinical Research Associate II
As a Clinical Research Associate (CRA) at Parexel, you act as an integral part to get treatments to patients sooner. Our CRAs' priority is the safety and well-being of the patients. As you travel to investigator sites and perform your monitoring duties, we encourage you to be inquisitive, take accountability, build relationships, and act with integrity.
Join a team with a wide variety of experiences and knowledge, and work on global projects within a broad scope of therapeutic areas. We’re looking for people who want to grow personally and professionally and support their colleagues globally and cross-functionally.
Success Profile
Do you have these soft skills and interpersonal traits to succeed at Parexel?
- Communicator
- Detail-oriented
- Problem-solver
- Self-starter
About This Role
The Clinical Research Associate is the clinical sites’ direct point of contact and accountable for managing site quality and delivery from site identification through close-out. The aim of this role is to build the relationship with the site, to be the sole position accountable for the site performance, including driving the site successfully to initiation, the development of a robust patient recruitment strategy, setting up a system, assessing compliance with regulations and the site’s capability to comply with the study needs and recruitment expectations, and problem solving to address and resolve site issues. This may include various tasks and roles within the CRA framework, contingent upon project phase and country demands, and complexity of the study. The Clinical Research Associate will oversee the conduct of the trial at designated sites, ensuring the rights and well-being of human subjects are protected, evaluating the quality and integrity of the reported data, evaluating the site efficacy of staff training and requiring retraining where necessary, developing strategy regarding patient recruitment, evaluating and building the relationship with the clinical site, using problem-solving to promote positive working relationships with the site and staff, and ensuring the conduct of the trial is in compliance with the currently approved protocol/amendment(s), with GCP and with the applicable regulatory requirement(s). Where available an “initiation Clinical Research Associate“ (iCRA) specializing in Pre SIV activities will be assigned & accountable for managing and driving the strategy for the Pre SIV / startup tasks of the study. The iCRA) also support protocol amendments if applicable.
Key Accountabilities:
Start-up (from site identification through pre-initiation):
Act as Parexel’s direct point of contact with assigned sites, accountable for quality and delivery during the start-up phase.
Build relationships with investigators and site staff.
Conduct, drive and manage country specific feasibility and/or site pre-qualification and qualification activities, which may include:
-Preparation, negotiation, and facilitation of execution of Confidentiality Agreements (CDA), Clinical Site Agreements (CSAs) and any amendments. -Conduct remote Qualification Visits (QVs).
Generate visit/contact reports, using judgment to identify site issues and problem solving to direct resolution.
Develop strategy to configure, distribute, and collect, and review and approve, high quality country specific and/or site specific documents or essential regulatory documents (SRP) and any updated or amended regulatory documentation.
Customize, review, and negotiate as needed, country/site specific Informed Consent Forms (ICF), translations (within parameters of country/regulatory/client requirements), and customize and negotiate any amendments.
Prepare and submit IRB/IEC and MoH/RA (if applicable) application(s), resolving conflicts, determining appropriate follow up until receipt of final approval.
Submit all pertinent documentation to the trial master file as per project plans/sponsor/company policy.
Forecast, develop, manage, and revise plans and strategies for:
-IRB/IEC and MoH / RA submission/approval, -Site activation, -Patient recruitment & retention.
Update and maintain appropriate Clinical Trial Management systems (CTMS) in a timely manner.
Promptly identify, use judgment and knowledge to address and resolve or escalate, any site question and/or issue, including but not limited to: potential issues or risks with site activation timelines, issues with patient recruitment strategy, deficiencies in training, data quality or integrity, study non-compliance, etc.
Facilitate and support allocated sites with access to relevant study systems and ensure they are compliant with all project specific training requirements prior to study start.
Actively participate in Investigator and other external or internal meetings, audits & regulatory inspections
Work in a self-driven capacity, with limited need for oversight.
Proactively keep manager informed about work progress and any issues.
Maintenance (from initiation through close out):
Act as Parexel’s direct contact with assigned sites, assess and ensure overall integrity of study implementation and adherence to study protocol at clinical sites, and perform problem-solving to address and resolve site issues.
Facilitate and support site with access to relevant study systems and ensure sites are compliant with project specific training requirements.
Evaluate if on-site staff assignment is still accurate and determine and implement corrective actions & follow-up, if necessary / relevant.
Address and resolve issues at sites, including the need for additional training, potential deficiencies in documentation, and communication.
Address/evaluate/resolve issues pending from the previous visit, if any.
Follow-up on and respond to appropriate site related questions.
Apply working knowledge and judgment to identify and evaluate potential data quality and data integrity issues. Determine and implement appropriate follow-up action.
Actively participate in Investigator and other external or internal meetings and audits & regulatory inspections as required.
Collect, review, and approve (if applicable) updated/amended site documentation, including regulatory documents as applicable.
Evaluate site recruitment plan in collaboration with the site staff on an ongoing basis and provide strategy for improvements.
Perform on-site visits; this includes Qualification and Initiation visits; apply judgment and knowledge to independently resolve site issues, questions and concerns.
Conduct remote visits/contacts as requested/needed.
Generate visit/contact report.
Evaluate overall compliance and performance of sites and site staff: provide recommendations regarding site-specific actions and use judgment and experience to assess the ability and motivation of site staff.
Assess & manage test article/study supply including supply, accountability and destruction/return status.
Review & follow-up site payment status.
Follow-up on CRF data entry, query status, and SAEs.
Conduct on-site study-specific training (if applicable).
Perform site facilities assessments
Recognize impact of study non-compliance/issues/delays/changes on study timelines and communicate study issues that require immediate action, with proposed strategy for resolution
Overall Accountabilities from Site Identification to Close out:
Ensure timely and accurate completion of project goals and update of applicable trial management systems.
Work with team members to meet project goals, provide strategy for efficient project planning and goal completion, and encourage the support of team members where required.
Update all appropriate Clinical Trial Management Systems (CTMS) on an ongoing basis, including performing regular reviews of site level data in clinical systems (e.g. CTMS, EDC, IVRS, and SIS) and ensure timely and high quality data entry compliance from sites, manage and submit all relevant documents to the Trial Master File (TMF), ensuring first time quality, and distribute study documents to site including configuration of Investigator Site Files if applicable and on-going maintenance for completeness and quality.
Ensure that assigned sites are audit and inspection ready
Monitor and maintain compliance with ICH-GCP and applicable international and local regulations.
Delegate administrative and other tasks to Administrative Support Team as needed and where appropriate. Guide Administrative Support Team members, review work, and provide feedback to manager regarding performance.
Show commitment and perform consistent high quality work.
Maintain a positive, results-orientated work environment, building partnerships and collaborative relationships, communicating with team(s) in an open, balanced, objective manner, modeling the high performance culture values.
Maintain a working knowledge of and ensure compliance with applicable ICH-GCP Guidelines, international and local regulations, Parexel SOPs, other Parexel / Sponsor training requirements and study specific procedures, plans and training.
Ensure basic understanding of project scope, milestones, budgets, and strive for high quality, timely, and efficient delivery.
Provide input and feedback for Performance Development Conversation(s).
Develop expertise to become a subject matter expert.
Complete additional tasks in a timely manner (e.g. timesheets, expenses, metrics, etc.)
Strong problem solving skills
Able to take initiative and work independently, and to proactively seek guidance when necessary.
Excellent presentation skills.
Client focused approach to work.
Ability to interact professionally within a client organization.
Flexible attitude with respect to work assignments and new learning.
Ability to prioritize multiple tasks, and achieve project timelines; utilizing strong analytical skills to make decision autonomously due to the unpredictable nature of the issues that arise.
Strong ability to make appropriate decisions in ambiguous situations.
Willingness to work in a matrix environment and to value the importance of teamwork.
Strong computer skills including but not limited to the knowledge of a Clinical Trial Management System (CTMS), Electronic Document Management System (EDMS), and MS-Office products such as Excel and Word.
Excellent interpersonal, verbal, and written communication skills.
Sense of urgency in completing assigned tasks and ability to assist others to meet study/country deliverables.
Excellent time management in order to meet study needs, team objectives, and department goals.
Proven ability to work across cultures.
Shows commitment to and performs consistently high quality work.
Ability to successfully work in a (‘virtual’) team environment.
Consulting Skills.
Able to accommodate travel time requirements, according to tasks allocation/phase of the study assigned.
Act as a mentor and role model for other team members.
Effectively enlist the support of team members in meeting goals.
Attention to detail.
Holds a driver’s license where required.
Knowledge and Experience:
Substantial Site Management experience or equivalent experience in clinical research, with understanding of clinical trials methodology and terminology
Educated to degree level (biological science, pharmacy, or other health-related discipline preferred) or equivalent nursing qualification or other equivalent experience
EEO Disclaimer
Parexel is an equal opportunity employer. Qualified applicants will receive consideration for employment without regard to legally protected status, which in the United States includes race, color, religion, sex, sexual orientation, gender identity, national origin, disability, or protected veteran status.
Share this job

Explore this location
Check It Out
Potential Career Path
Parexel offers various career paths and internal development programs for CRAs to advance to the next level. This could include enhancing your technical position, moving into management, or shifting to other areas of the business.
- Project Management Subject Matter Expert People Management
Employee Insights

Nick on a day in the life of a CRA
Nick Burger | Clinical Research Associate II
Watch Video

Liliana on CRA tasks and our recognition program
Liliana Belmares Flores | Senior Clinical Research Associate

Marlayna on why Parexel is a great place for CRAs
Marlayna Fitts | APEX CRA Field Coach
TEAM IMPRESSIONS

Why Clinical Research Associates work at Parexel
Patient-focused in everything we do
We push the boundaries of what is possible to create clinical trials that are inclusive, innovative, and patient-focused
Supportive and inclusive environment
We foster collaboration, teamwork, respect, and inclusivity, to work together to achieve common goals.
Career growth and development
We develop your skills through training, mentorship, and career advancement programs.
Flexible work arrangements
We focus on outputs and results, not where and when you work.
Diverse therapeutic areas and project exposure
We continuously learn from a broad exposure from early to late phase clinical trials and our cros-functional global teams.
Advanced Technology
We recognize the importance of first-time quality to bring treatments to patients faster.
Learn About Our Culture

Neuroscience Careers
Neuroscience diseases affect the very core of one’s being and experience. We work diligently to lighten the burden for patients and families alike. Explore opportunities to join our team.

Careers in Inflammation & Immunology
Each day, we’re working toward building a deeper connection and understanding with those who count on us – the patients.

Cell and Gene Careers
Cell and gene therapies (CAGT) have rapidly emerged as among the fastest-growing spaces in all of biopharma R&D, and Parexel was an early entrant into this space.


Our work culture
Learn about our culture, perks, learning opportunities, and our corporate responsibility approach.
Careers in Oncology
Our dedicated people, innovative approaches, and culture of caring all work together to ease the journey for patients and investigators.

Careers in Rare Diseases
In rare disease, every single patient is precious. Discover where your skills can create a life-changing achievement for every person involved.

Emerging Talent Programs
Begin your career journey at Parexel with our emerging talent programs or our internships, placements and apprenticeships. Advance science by keeping the patients at the heart.

About our hiring process
It’s all about finding the right fit, for you and for us. Our recruiters work with you and our hiring managers to bring together a team and culture where everyone can grow and be successful.

Hear about Xoli's role as Sr. Director of Patient Inclusion
Discover Xoli's passion for fostering diversity within the clinical trial industry. Learn about her new role, the importance of patient engagement, and Parexel's commitment to creating an inclusive workplace.

Aida Sabo, Sr. VP DEI, on Diversity, Equity & Inclusion at Parexel
Discover how Aida drives DEI change at Parexel. Learn why it's crucial, goes beyond diversity dimensions, and embraces the advantage of valuing differences.

Follow us on Social Media
Parexel is present on several social media channels where we post our latest updates. Follow, interact, and rate us!

Medical Writing Opportunities
Join Parexel's global team of 700+ medical writers. Deliver impactful, high-quality content and advance your career in medical writing.

Flexible Work Arrangement
In-office, home-based or a mix? What’s your preference? We value the work-life balance of our employees, and as such Parexel is offering maximum flexibility to our employees wherever possible.

Clinical Operations Opportunities
Are you passionate about making a difference in the fight against cancer and beyond? At Parexel's Global Clinical Operations, we are dedicated to putting patients at the heart of every clinical trial. With a global footprint and 5000 Clinical Operations team members worldwide, we are impacting clinical research.

A day in a life of a Project Leader
Christine and Ewa share what a Project Leader does, why to become a Project Leader, and what mindset is needed. Explore how a Project Leader resembles a cheerleader or a coxswain of a rowing boat!

Meet Lola: Senior Clinical Research Associate
Lola shares about her position as a Senior Clinical Research Associate (Sr. CRA), what skills are needed for her role, and what she enjoys outside of work!

Roles within our Functional Service Provider devision
Within our outsourcing model, you are deployed as an experienced colleague for our customers, benefiting from exposure to both the clinical research organization (CRO) and sponsor experience.

Roles within Data Operations
Impact patients with a role in Biostatistics, Statistical Programming and Data Management.

Meet Amrita: Manager, Statistical Programmer
Amrita tells us about how Parexel has helped her grow within her career with working flexibility and opportunities to learn.

Meet Janice: Principal Statistical Programmer FSP
Janice You shares why she choose Parexel FSP. Find out which skills are needed in her role, what she enjoys the most, as well as what challenges her.

Meet Neha: Senior Biostatistician
Neha describes her role as a Senior Biostatistician and why she chose Parexel.

Meet Rahul: Senior Health Economics Associate
Rahul describes his role as a Senior Health Economics Associate in the Health Economics and Outcomes Research team

Meet Joanna: Senior Medical Writer
Joanna provides insights about her role as a Senior Medical Writer. She describes what excites her about the role and what it is like working with highly educated, motivated, and professional colleagues.

Meet Mary: Principal Medical Writer
Mary provides insights about her role as a Principal Medical Writer. She shares what it is like working with her fellow colleagues, how Parexel has supported her career development, and what excites her most about the work she does.

Meet Chanakarn: Clinical Research Associate II
Charnakarn talks about what it is like to be a CRA, and the support she has been given to achieve her career goals.

Rebuilding Careers: How Ashwini rediscovered her confidence at Parexel
Meet Ashwini Somayaji, Senior Manager for Medical Writing Services. Ashwini's career took an unexpected turn with a five-year break. However, her determination and passion led her to Parexel, where she reignited her professional aspirations. With the support and guidance of her colleagues, Ashwini's journey became one of rebuilding confidence and career growth.

Returning with Passion: What Marlayna experienced coming back to Parexel
After a brief departure, Marlayna joined as a Senior Clinical Research Associate and transitioned later into being a field coach and mentor for new CRAs. When returning Marlayna discovered a company more committed than ever to employee well-being and belonging.

Meet Sheryl: Principal Regulatory Affairs Consultant, Regulatory Strategy
Sheryl, Principal Regulatory Affairs Consultant, talks about why she joined Parexel and how she keeps the patient first.

Meet Kanika: Manager in Project Manager
Kanika describes her role within finance operations and why she chose to join Parexel

Meet Cheri & Tarryn: Project Specialist II
Cheri and Tarryn are sisters and Project Specialists at Parexel South Africa. Learn about their lives, roles and the culture of the Project Planning & Support (PPS) department.

Meet Jamie: Diversity & Inclusion
Jamie offers her perspective on Parexel’s inclusivity to LGBTQ+ colleagues, how she feels about being "out" at work, and more.

Meet Kirill: Executive Director, FSP Biometrics
Kirill describes what excites him about his role and how he tries to challenge and encourage his staff.

Meet Santino: Clinical Operations Leader
Santino's career at Parexel, from a Project Specialist to a Clinical Operations Leader, showcases a diverse journey through Clinical Operations, fueled by a passion for the pharmaceutical industry and a commitment to impacting patient lives. His experience highlights Parexel's dedication to professional growth and the embodiment of the "We Care" promise in every facet of their work.

Meet Siddhika: Clinical Data Analyst III
Meet Siddhika, Clinical Data Analyst III as she explains why she decided to apply to Parexel after a six year career break

Meet Jitender: Director, Health Economics
Jitender describes his role as a Director, Health Economics, supporting our clients with strategic recommendations and delivering the value story of new treatments.

Meet Jagan: Director, India Assistant Compliance Officer
Jagan describes his role as a Director within the compliance team based in India

Catalyst Award Winner 2022
Catalyst is advancing workplaces that work for Women - Parexel was recognized for "Leveraging Gender Partnership to Advance Women in Leadership."

Meet Jennifer: Associate Director, Scientific Services, MedCom
Jennifer discusses her role as an Associate Director, Scientific Services in Medical Communications, and the challenges she enjoys.

Meet Simona: Principal Consultant, Regulatory & Access
Simona shares how Parexel has supported her career development, the day-to-day activities of being a Principal Consultant at R&A, and much more!

UK Career Webinar — Accelerate your career in Clinical Project Leadership
View this career webinar to hear from our Clinical Project Leadership team in the UK about their opportunities for growth and the team's culture at Parexel.

Meet Joy: Senior Director, Statistical Programming
Joy joined Parexel in 2006 and has since then built an incredible career and lasting relationships with her colleagues. She is passionate about programming and finds fulfillment in supporting clinical trials. Outside of work, she enjoys hiking and spending time with loved ones.

Meet Celine: Director for Integrated Solutions Strategy
Celine talks about her silver award in The PharmaTimes Clinical Researcher of the Year (Americas) competition, which she received while working as a Senior Project Leader at Parexel.

Meet Tina: Manager, Project Finance Excellence
Meet Tina Huang, Project Finance Excellence Manager, as she discusses hAssociate Director, Scientific Services, MedComer role and what it's like to work at Parexel Taipei

Meet Amelia: Senior Manager, Medical Writing Services
Meet Amelia Young, Senior Manager, Medical Writing Services, as she discusses her role at Parexel

Meet Andrea: Manager, FSP
Andrea discusses why she returned to Parexel, what excites her about being an FSP Manager, and the best career advice she ever received!

Meet Catherine: Associate Project Director
People are Catherine's passion. She enjoys showing a project team how their work fits into the bigger picture, sharing knowledge and celebrating accomplishments. Explore her career advice, newly established behaviors, and more!

Meet Jahanara: Vice President, FSP Biometrics, India
Jahanara is proud of growing within Parexel and our Women in Leadership program.

Meet Nayoung: VP & APAC Head of Enterprise Account Leadership
Nayoung participated in our Women in Leadership program and an MBA program and believes the advice from Parexel colleagues to BE BOLD encouraged her to take the risk to move forward.

Meet Chalermporn: Senior Clinical Research Associate
Chalermporn talks about what it is like to be a CRA at Parexel, and the support she has been given to pursue her career.

Meet Dorothy: Senior Document Quality Reviewer Medical Writing Services
Dorothy shares her story about returning to work after a career break, and how she arrived at Parexel having previously worked for another CRO.

Watch Replay: Why Biotech Matters More Than Ever
Insights on working with Biotech clients to rapidly take new science from the bench through registration.

Career Blog - Should I consider a mentor

Meet Laurias: Manager, Clinical Operations
Laurias shares how Parexel has supported his career development from CRA to Clin Ops Manager and flexibility within the workplace.

Meet Jens: Senior Director, Medical Writing Services
Meet Jens Zurrahn, Senior Director for Medical Writing Services, as he discusses his role at Parexel.

Meet Vivek: Senior Manager, Statistical Programming FSP
Vivek describes his role as a Senior Manager within the Statistical Programming Functional Service Provider (FSP) team.

Watch Replay: Online Seminar Italy Clinical Research Associates Putting the Patients First
View this career seminar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA and working in Clinical Operations at Parexel.

Watch Replay: Online Seminar UK Clinical Research Associates Putting the Patient First
View this webinar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA working in Clinical Operations at Parexel

Watch Replay: Online Seminar EMEA Your Skill Set Could Save Lives Working as a Stats Programmer
View this webinar to hear from our Data Operations colleagues and about the role of Statistical Programming in the Clinical Research Industry

Meet Wipawee: Clinical Research Associate I (CRA I)
Wipawee shares about her role as a Clinical Research Associate (CRA) at the Parexel Thailand office, including the training and CRA job responsibilities. Learn about what she considers to be the most attractive part of working as a CRA in Parexel.

Location: Quakertown Depot
Come work with a supportive team of 30 colleagues in Quakertown to provide packaging, labeling, and global distribution of clinical trial materials.

Meet Ben: Senior Regulatory Affairs Consultant
Ben provides and insight into his role as a regulatory affairs consultant. He also talks openly about being part of the LGBTQ+ community and how Parexel's flexible work arrangements help him as a single father.

Meet iCRA Twins Marina Palumbo and Anna Korelis
Double the passion and double the commitment to working With Heart™. Read on to learn more about these colleagues whose family and work lives are uniquely intertwined at Parexel!

Meet Rebecca: Senior Data Management Lead
Rebecca talks about her day-to-day activities as a Senior Data Management Lead and why Parexel's core value of Empowerment and Accountability stand out to her.

Meet Sanjay: India Country Head & Head CTS&L
Sanjay discusses his dual role, Parexel’s strong collaborative and cohesive working environment, and how our patient-centric culture makes him feel connected.

Location: Germany
At Parexel Germany are 750+ employees, we have an Office, Early Phase Clinical Unit and Logistics Depot, and 40+ nationalities.

Meet Yogeeta: Senior Document Quality Reviewer
Yogeeta talks about returning to work after a 3-year career break, and the support she received from her manager at Parexel India.

Watch Replay: Online Seminar Spain Clinical Research Associates Putting the Patients First
View this career seminar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA working in Clinical Operations at Parexel.

Meet Virginia: Project Quality & Risk Management
Virginia shares what it is like working at Parexel Argentina and how it has given her the opportunity to meet with a wide range of creative-minded people and this is what keeps her on her toes.

Meet Nadia: Senior Data Management Lead
Nadia shares details about her role as a Senior Data Management Lead and what she finds rewarding in her job.

Meet Tom: Medical IT
Tom talks about the knowledge and experience he has gained at Parexel; along with what is different upon him rejoining.

Meet Barbara, a Senior Clinical Operations Leader who generates excitement
Barbara is living her passion for Physics by tutoring kids in her neighborhood and sparking their joy in the subject. Her interest in sparking excitement is coming in handy for her role as a Senior Clinical Operations Leader as well. Do you like to excite your team?

Meet Julia: CVP, Head of Medical Writing and GMBA Ireland
Julia provides leadership insights, inspiration and advice from her 20+ years at Parexel.

Recognition Program
An interview about the value of the Recognition Program and the high engagement of Parexel's employees.

Meet Bob: Biostatistician II FSP
Bob shares about his roles as a Biostatistician II within Parexel FSP, his direct involvement with the client's team and goals, and what is needed to be successful in his role.
Meet Urvashi: Medical Writer I
Urvashi tells us why she chose Parexel and what she enjoys being a Medical Writer I

Meet Reyad: Associate Clinical Operations Leader
Reyad, Associate Clinical Operations Leader, shares how Parexel supports flexibility within the workplace and what he finds to make a great leader.

Meet Margaret: Clinical Research Nurse
Find out why Margaret enjoys working as a Nurse in Clinical Research and working at Parexel, also how Parexel supports her in a way she hasn't experienced anywhere else.

Meet Penny: Senior Clinical Research Associate
Penny, Senior Clinical Research Associate, shares the reason why she kept coming back to Parexel, the responsibilities of her role and how she keeps the patient at the heart of everything she does.

Women at Parexel
Parexel employee base is 70 % female, and we are proud to say 60 % of managers+ and 46 % of VP-level+ are female. Yet we are committed to improving these numbers with several leadership programs for female and male colleagues!

Meet Adriane, a Clinical Operations Leader sharing insights and tips
Discover Adriane's role as a Clinical Operations Leader. She shares insights into her daily responsibilities, the skills crucial for success, and the rewarding challenges she faces. Find out how Parexel supported her career development and get inspired by her advice for professional growth.

Roles within Medical Communications
Make a difference with a role in Medical Communications

Meet Seeba: Regional Director for Project Planning and Support
Seeba describes her role within Project Planning and Support.

Meet Blessy: Data Management Lead I
Blessy describes her role, what excites her about it, and how she came to work in Clinical Data.

Blog: Should I consider engaging with a mentor
Lets review what mentoring really means, and how it can benefit you

Meet Robbin: Associate Manager, Statistical Programming
Robbin joined Parexel for her Placement year in 2014 and has since progressed into a Associate Manager, Statistical Programming. Dedication and communication are her driving skills.

Meet Ekaterina: Senior Statistical Programmer
Ekaterina loves challenges and new tasks! She feels her contribution to clinical trials really helps people and that inspires her.

Great Place to Work - India
In February 2023, Parexel India has been certified as a Great Place to Work®, for the second time in three years — on average, scores for Parexel India increased in all categories by 10 to 15 points.

Meet Swarnalatha: Senior Principal Statistical Programmer FSP
Swarnalatha has been working for over 14 years in the pharma industry as a Statistical Programmer, and has been impressed with the work flexibility and empowerment of female colleagues at Parexel.

Meet Mati: Medical Writer II
Mati provides an insight into his role as a Medical Writer II in the Taipei office in Taiwan. He also openly talks about being part of the LGBTQ+ community at Parexel and how welcoming the Taipei office is!

Meet Angeli: Senior Project Specialist
Angeli shares what it is like to work as a Project Specialist and with her colleagues within the Project Planning & Support department.

Meet Agnieszka: Senior Clinical Operations Leader
Agnieszka shares about her role as a Senior Clinical Operations Leader, the skills needed, the challenges and teamwork. She is looking back on a 17-year career path at Parexel.

Meet Lillie: Clinical Research Associate I
Lillie shares what her role as a CRA I looks like and how she got started at Parexel.

Coming back to Parexel: Looking forward to strong collaboration
After a brief departure, Ira Mills (Senior Scientific Specialist) found himself being drawn back to Parexel. He missed the strong working and personal collaboration with his colleagues and the broad institutional support. Parexel not only cares deeply about patients but also about its employees.

Location: Argentina
Work where you will find flexible working options, a supportive atmosphere, constant learning, and more.

Location: India
Join one of our 5 locations in India. Parexel India employs ~5770 employees, which represents 25% of our global population. We offer a supportive and fun work culture, flexibility, career growth, and learning opportunities.

Re-excel: Return to work
Do you want to return to work after an extended period of time away from the workplace? Parexel has many opportunities for those interesting in re-establishing a meaningful career with heart. Now is the time to re-excel at Parexel!

Roles within Scientific Data Organization
Be at the core of what we do at Parexel with a role in our Scientific Data Organization

Meet Emmanuel: Senior Clinical Research Associate
Emmanuel shares about his day to day duties and how he emphasizes the patients' wellbeing, by running smooth trials. Due to his great work, he was recently awarded for Extraordinary Monitoring Efforts.

Meet Andrea: Initiation Clinical Research Associate II
Andrea talks about what it is like to be an iCRA and the opportunities she has been given to progress her career.

Meet Jayashree: Senior Clinical Data Analyst
Jayashree details what it's like to be a CDA and what skills you need to be successful in the role. Being able to contribute to a good cause through clinical trials is a genuine reason as to why she enjoys her job.

Roles within AI Labs
Discover how Parexel AI Labs is leveraging technology and AI to improve clinical trials, advance patient safety, and transform our everyday work.

Meet Donata, a Senior Project Leader focusing on quality and growth
Donata's advancement from an entry-level position to Senior Project Leader at Parexel showcases her dedication to quality and determination to exceed client expectations. Her journey reflects the supportive and growth-oriented environment at Parexel, where passion and hard work pave the way for making a meaningful impact.

Meet Madalina: Clinical Operations Leader
As a Clinical Operations Leader, Madalina invites people to join the wonderful and life-changing experience of working With Heart and passion for the future of medicine.

Meet Carolina, a Senior Project Leader with a stellar growth story
Carolina's remarkable growth story, achieving 5 promotions within 10 years highlights her supportive team and the importance of personal growth. She progressed from Project Specialist to Senior Project Leader and moved from Argentina to the US.

Meet Kathryn: Clinical Research Associate II
Kathryn, Clinical Research Associate II, tells us why she chose Parexel and how she keeps the patient at the heart of everything she does.

Learning and development
We believe that investing in your professional and personal development is an investment in Parexel, and we want to help you realize your full potential and career. Ensuring we have a fully trained and capable workforce is a key part of delivering quality work and patient safety.

Benefits & Support
At Parexel, we prioritize putting people first, allowing you to achieve your best work. Explore the Flexible Work Program, our "Bravo" Recognition Program, and how we build our well-being in our supportive culture.
Diversity, Equity & Inclusion at Parexel
Learn how Parexel embraces diversity and strives for equity and inclusion in its workforce, clinical trials, and supplier partnerships.

Join our APEX Program and become a CRA
Join Parexel's Accelerated Program of Education, Exposure, and Experience (APEX) and embark on a 6-month journey to become a remarkable Clinical Research Associate (CRA). With hands-on experience and expert guidance, we'll help you excel in the field.

Meet Mwango: SVP & Global Head of Regulatory Strategy
Mwango shares how she came to Parexel after spending 16 years at the FDA to experience the process and considerations from the drug developer’s perspective. Read about her role as a VP-Technical and what valuable advice she offers to those looking to work With Heart™.

Meet Mark, a Corporate Real Estate & Services Manager, proud to be part of Parexel's global mission
Mark shares about his background in the U.S. Navy and how he has been able to transition his skillset to leading a team as a Corporate Real Estate & Services Manager at Parexel.

What is a Clinical Research Organization?
A Clinical Research Organization (CRO) is a company contracted by a pharmaceutical, biological or medical device manufacturer to manage clinical research studies and other services to support product development. Learn about the four phases and the key functions of clinical trials.

Meet Catia, a Director of Clinical Operations talking about leadership learnings
Catia shares her 15-year career journey from Clinical Site Manager to Director, highlighting leadership insights, people management lessons, and the benefit of support.

Parexel's Newsroom
Read our corporate news, press releases, as well as our ESG report.

Medical Writing Careers Webinar Replay
Our expert medical writers share their extensive industry experience. Whether you're a seasoned medical writer or just starting, this webinar caters to all skill levels, offering valuable insight into the life of a Medical Writer and what it is like to work at Parexel.

Why leaders work at Parexel
Learn more about Parexel's exceptional team dedicated to efficient, high-quality clinical development services. With a culture of care, collaboration, and ownership, we put patients first.

Meet Cheng Cai: Director, Clinical Pharmacology Modeling and Simulation (CPMS)
Cheng is passionate about advancing clinical research and improving patient outcomes. He enjoys collaborating with his talented team and building mathematical models in his role. Outside of work, he leads an active lifestyle and values quality time with his family through various activities.

Parexel Military Talent Community
We know ‘serving’ is a core value of many of our military community. At Parexel, you can continue on your mission to serve, by joining an organization dedicated to improving the lives of patients worldwide. The skills and values you have developed in your military career or as a military spouse are transferrable to meaningful careers here at Parexel.

Video on Working With Heart™ - Christina's Clinical Operations Manager Perspective
View how Christina reflects on her work experience, her impact on patients and her management style of respect and growth. Christina is leading a team of Clinical Operations Leaders who manage groundbreaking trials in the biotech space.

Parexel's Careers Blog
Learn more about our teams, our company culture and benefits, hear from our employees, find hiring resources and more.

Blog: Do you take your career seriously
Taking the time to review your career path is an important, but sometimes overwhelming, task that many of us gloss over.

Meet Nadia: Principal Biostatistician
Nadia Seniavina talks about her role as a Principal Biostatistician and what excites her most working in Parexel

Meet Steve, Sr. Director Cloud & Infrastructure Solutions
Discover how his passion for resilient technology solutions is making a difference in global clinical trials and transforming patients' lives worldwide. Steve started as an intern and now leads the team that supports the technology tools he helped build during his early years at Parexel.

A day in a life of a Clinical Operations Leader (COL)
Viviana and Jani share what a Clinical Operations Leader does, what it takes to be in their role, and why to work in Clinical Operations. Joining Parexel as a COL means taking on significant responsibilities, being open-minded and making a meaningful impact in clinical research.

Meet Chrishni, a Senior Project Leader with an exciting global journey
Discover Chrishni's inspiring journey at Parexel, where her love for France led her to relocate from Australia and thrive in her career based in Lyon. She enjoys collaborating with her team and finding a harmonious work-life balance to indulge in her passions.

Meet Doreen, a Project Leader focusing on patient-centric research
Explore Doreen's journey as a Project Leader at Parexel, where she embraces her role in improving patient materials and ensuring patient-friendly and inclusive studies. Additionally, learn about Doreen's onboarding process, her Line Manager's support for work-life balance, and how she enjoys adventures with her children.

Meet Marije, a Clinical Operations Manager in Parexel, is most proud of becoming a people leader
Marije, a former nurse and now Clinical Operations Manager in Parexel, is dedicated to enhancing patients' lives through her involvement in clinical research. Marije's exceptional leadership skills and impressive career growth further highlight her as a motivating individual.

Meet Adrian, a Site Care Partner navigating the complex landscape of clinical trials and prioritizing patient care
Adrian works as an outsourced Site Care Partner (SCP) for a client within the clinical research and pharmaceutical industry. Learn more about Adrian's experience and insights on how he contributes to the success of clinical trials and prioritizing patient care.

Meet Rachel Smith: Global Head of Rare Disease, CoE
Rachel, Global Head of Rare Disease, CoE, advocates for rare disease patients and drives innovation in clinical trials at Parexel. Her own diagnosis gives her a unique perspective on patient experiences and outcomes. Discover Rachel's inspiring projects and her dedication to advancing rare disease research.

Meet Jessica, an Associate Project Director guiding trials through the complex regulatory landscape
Jessica works as an Associate Project Director within our Regulatory and Access Global Project Leadership team. Learn more about Jessica's experience and how she contributes to the success of clinical trials.

Meet Anthony, a Sr. Principal Medical Writer contributing to lifesaving treatments
Explore his career reflections, motivations, and proud moments in contributing to medical advancements through his work.

Why Clinical Operations Leaders work at Parexel
Listen to Elizabeth Edwards, SVP Clinical Operations at Parexel, as she delves into why she works With Heart™, the impact of Clinical Operations Leaders, how Parexel supports their success, and what makes Parexel's culture unique.

A Day in the Life: Discover Sinan's Impactful Journey as a VP Technical of Regulatory Strategy at Parexel
Sinan, VP Technical of Regulatory Strategy, is dedicated to putting patients first by helping to navigate the complex landscapes of clinical trials. He has great satisfaction in guiding clients to innovative products and trial designs,

Meet Julie, a Senior Clinical Assistant, proud of her contribution
Julie welcomes volunteers at the Early Phase Clinic reception in Berlin, manages payments, and caters to the volunteers in the kitchen.

Meet Steve, a VP Technical of Regulatory Strategy responsible for steering clients through the complexities of regulatory requirements
Steve works as a VP Technical within Regulatory Strategy, where he is working to guide clients through the regulatory journey from initial clinical trials to achieving market success. Read more about what a day in the life of a VP Technical at Parexel looks like and how Steve is able to utilize his FDA background and apply his skills to his current position.

Meet Inhye, a Senior Clinical Research Nurse contributing to society
Inhye finds pride in her role in contributing to society. She emphasizes putting patients first and ensuring volunteer safety and comfort during clinical trials. Learn more insights into her journey with Parexel, her reasons for choosing the organization, and the supportive work environment.

Working as a Nurse in Clinical Research at Parexel
Antje and Katharina share insights into working as a Nurse in Clinical Research, where innovative medications are developed through early-phase and first-in-human studies.

Project Leadership Opportunities
Learn how Project Leaders partner with pharmaceutical and biotech clients, manage with determination and With Heart, and our unwavering commitment to patient-centricity.

Meet Rebecca, a Project Leader who is passionate about empathy
Discover Rebecca's insights on effective leadership, collaboration, and patient-centric approaches.

Meet Theodora, a Site Contract Leader
Theodora Chung, Site Contract Leader, offers us an insight into her role, which is to oversee the whole clinical trial budget and contract planning, drafting and negotiations till its execution. She is passionate about working in the team.

Location: Mexico
Learn about Parexel Mexico, where you can be part of a rapidly growing team dedicated to improving patient health. Enjoy flexible work options, a supportive and collaborative culture, and continuous professional growth. Discover how you can make a global impact with us.

New Medicines, Novel Insights Newsletter
Parexel's insight-generation engine, our people, share perspectives on patient-guided clinical research.

New Medicines, Novel Insights: Advancing Precision Oncology
This report examines some obstacles drug developers encounter and describes strategies to help bring precision cancer medicines more quickly and certainly to market—to benefit an ever-increasing proportion of patients.

Meet David, a champion for disability inclusion
His leadership fosters workplace and clinical research inclusivity. Explore David's inspiring journey from combat medic to Parexel VP and Disability Steering Committee co-lead.

Data and Technology Careers at Parexel
Drive innovation in clinical trials, enhance efficiency, and impact patient lives. Grow your career and explore diverse roles in Cyber Security, Digital Architecture, AI, Business Engagement, Technology Quality, and Enterprise Infrastructure.

Parexel's Environmental, Social and Governance Reports
Explore the company’s ESG priorities, strategies, and activities. We are committed to regularly and transparently communicating our ESG efforts.

Meet Matt, a Clinical Operations Leader delivering insights with determination
From CRA to Clinical Operations Leader: Embracing challenges, generating insights, prioritizing candor. Explore the skills to excel in Clinical Research.

Meet Bindu: a Principal Statistical Programmer
Discover Bindu D's inspiring journey as a Principal Statistical Programmer at Parexel. Learn how her passion for patient care drives innovation in clinical research.

Clinical Research Associate 1
- Science and Medical Research
- Opening on: Sep 26 2024
- Cancer Center-Administration
- Research Foundation
- Clinical Research Associate I, E99
Job Summary:
The Clinical Research Associate I will coordinate Oncology research trials for the Cancer Center and associated satellite sites, primarily involving investigational medications and treatments, including early or late phase FDA-regulated trials. Ensure compliance with federal guidelines at every step. Interact and correspond with clinicians, sponsors, cooperative groups, Upstate's Institutional Review Board and regulatory agencies. Enroll, register and screen patients for Oncology clinical research trials; collect and submit clinical trial patient data. Perform various administrative duties, which may include proper collection and shipment of lab samples; regulatory upkeep and maintenance of clinical research study supplies.
Minimum Qualifications:
Bachelor's degree and 2 years related experience or equivalent combination of education and experience. Excellent oral and written communication skills and attention to detail. Ability to work both independently and within a team setting.
Preferred Qualifications:
SoCRA or ACRP certification or Oncology research experience
Monday-Friday days
Message to Applicants:
Recruitment Office: Human Resources
We are an Equal Opportunity Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, age, protected veteran status or disability or other protected classes under State and Federal law.
Share this job
Thank you for sharing!
Recently Posted Jobs
Nursing assistant 2 (hct), administrative specialist, medical assistant, before you go would you like to receive updates and information from suny upstate medical university on available jobs and career opportunitites.

Cookies are small pieces of information that are stored by the user's browser on the hard drive of a personal computer. The use of cookies is a standard practice among Internet Web sites. Most of Upstate's Web sites do not use cookies, however occasional "session cookies" may be used to enhance or customize a visit to Upstate's Web sites. Session cookies can be created automatically on the device used to access Upstate's Web sites, and do not contain personal information and do not compromise privacy or security. We may use the cookie feature to store a randomly generated identifying tag on the device used to access Upstate's Web sites. A session cookie is erased when a browser is closed.

Working together, we can reimagine medicine to improve and extend people’s lives.
Clinical Sciences Associate Director
About the role.
Key Responsibilities:
- Study Leader and/or Clinical Scientist for predominantly high complexity, global studies. May function as a Core Project Team member for assigned projects to drive the Research-Development-Commercial (RDC) continuum. May co-lead project clinical sub-team and reports study/project progress and issues with their resolution plan to project teams and stakeholders. Directs early stages of study design and operational plans.
- Lead a global cross functional Clinical Trial Team (CTT) to ensure all trial deliverables are met; sets stretch goals, promotes realistic planning and timelines, and presents actionable alternatives to accelerate timelines. Proactively lead risk mitigation discussions, risk management and implementation at the trial level. Responsible and accountable for forecasting and managing overall study budget(s) in collaboration with key partners.
- Lead development of strategic and scientific input into study concept, feasibility, and ability to execute; develops and implements study-level operational execution plan in partnership with key cross functional partners, if applicable. Independently lead the clinical protocol development process in collaboration with the Medical Lead and other line functions; responsible author for clinical protocols, amendments, etc.; contribute to the medical/scientific input given for the development of study-related documents and processes which resides in other line functions; contribute to the development of clinical sections of study-level regulatory documents. May provide clinical leadership and strategic input for all clinical deliverables across assigned indication/program or studies within Biomedical Research. May act as Focus/Disease/Platform Area Lead.
- Lead the ongoing medical/scientific review of clinical trial data across assigned studies in collaboration with the medical expert and key line functions, and partners on data analysis and data interpretation, including safety trend analysis, signal detection, development of first interpretable results, reporting clinical study results in Clinical Study Report (CSR), and internal/external publications.
- Prepare and lead dose escalation meetings with investigators. Coordinate the real time availability of quality clinical trial data, to provide consolidated information for dose escalation meetings and Phase II data reviews with relevant stakeholders.
- Responsible for implementation of best practices and standards for trial management, including sharing lessons learned. Represent group on initiatives; may serve as Subject Matter Expert.
Novartis Compensation and Benefit Summary: The pay range for this position at commencement of employment is expected to be between $174,400 - $261,600/year. While salary ranges are effective from 1/1/24 through 12/31/24, fluctuations in the job market may necessitate adjustments to pay ranges during this period. Further, final pay determinations will depend on various factors, including, but not limited to geographical location, experience level, knowledge, skills, and abilities. The total compensation package for this position may also include other elements, including a sign-on bonus, restricted stock units, and discretionary awards in addition to a full range of medical, financial, and/or other benefits (including 401(k) eligibility and various paid time off benefits, such as vacation, sick time, and parental leave), dependent on the position offered. Details of participation in these benefit plans will be provided if an employee receives an offer of employment. If hired, employee will be in an “at-will position” and the Company reserves the right to modify base salary (as well as any other discretionary payment or compensation program) at any time, including for reasons related to individual performance, Company or individual department/team performance, and market factors.
Novartis EVP Manifesto.mp4
Essential Requirements:
- This position will be located at either the Cambridge, MA or the East Hanover, NJ site and will not have the ability to be located remotely. This position will require 0-5% travel as defined by the business (domestic and/ or international).
- Bachelors in life science/healthcare required; Advanced degree or equivalent education/degree in life sciences/ healthcare preferred (PhD/MD/ PharmD/ Masters).
- Approximately 8+ years’ experience in clinical trials/development with 3+ years leading cross-functional teams. Ability to lead multiple complex clinical trials concurrently.
- Strong understanding of oncology/hematology and demonstrates high learning agility. Proficient in clinical trial methodology with an emphasis in early clinical development. Strong operational project and program management experience with an emphasis in early clinical development, including excellent planning, prioritization, problem solving and organizational skills. Demonstrated capability to interpret, discuss and represent trial level data.
- Demonstrated knowledge and ability to confidently drive complex collaborations through unpredictable circumstances and higher paced changes. Demonstrate strong tolerance for ambiguity, willingness to adapt, and willingness to speak-up and challenge. Proven track record of successfully interacting with and influencing with a wide range of people, building strong positive relationships.
- Embraces a culture of diversity, inclusion, quality, innovation, and integrity.
- Maintain expert knowledge of ICH-GCP, external regulations and procedures, and supplements by training and practice of Novartis SOPs and internal policies.
Preferred Requirements:
- Radioligand therapy (RLT) experience preferred.
Benefits and rewards: Read our handbook to learn about all the ways we’ll help you thrive personally and professionally: https://www.novartis.com/careers/benefits-rewards
Commitment to Diversity and Inclusion:
Novartis is committed to building an outstanding, inclusive work environment and diverse teams' representative of the patients and communities we serve.
Why Novartis: Helping people with disease and their families takes more than innovative science. It takes a community of smart, passionate people like you. Collaborating, supporting and inspiring each other. Combining to achieve breakthroughs that change patients’ lives. Ready to create a brighter future together? https://www.novartis.com/about/strategy/people-and-culture
Join our Novartis Network: Not the right Novartis role for you? Sign up to our talent community to stay connected and learn about suitable career opportunities as soon as they come up: https://talentnetwork.novartis.com/network
Benefits and Rewards: Read our handbook to learn about all the ways we’ll help you thrive personally and professionally: https://www.novartis.com/careers/benefits-rewards
EEO Statement:
The Novartis Group of Companies are Equal Opportunity Employers who are focused on building and advancing a culture of inclusion that values and celebrates individual differences, uniqueness, backgrounds and perspectives. We do not discriminate in recruitment, hiring, training, promotion or other employment practices for reasons of race, color, religion, sex, national origin, age, sexual orientation, gender identity or expression, marital or veteran status, disability, or any other legally protected status. We are committed to fostering a diverse and inclusive workplace that reflects the world around us and connects us to the patients, customers and communities we serve.
Accessibility & Reasonable Accommodations
The Novartis Group of Companies are committed to working with and providing reasonable accommodation to individuals with disabilities. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the application process, or to perform the essential functions of a position, please send an e-mail to [email protected] or call +1(877)395-2339 and let us know the nature of your request and your contact information. Please include the job requisition number in your message.


COMMENTS
Clinic Manager (Clinical Research) New. Delricht Research 2.2. Overland Park, KS 66223. $70,000 - $110,000 a year. Full-time. Monday to Friday + 1. Easily apply. DelRicht is a clinical research company dedicated to providing an excellent customer experience to all patients that participate in our clinical trials.
A Clinical Research Associate (CRA) is a professional who oversees clinical trials and ensures that all procedures and regulations are followed. CRAs work directly with sponsors, clinical trial sites, and regulatory agencies to ensure the safety and accuracy of clinical studies.
Here are some steps you can take to pursue a career as a clinical research associate: 1. Pursue a bachelor's degree in a health science-related field. Most clinical research associate positions require candidates to have a bachelor's degree in a health science-related field. For those interested in a position as a clinical research associate ...
A minimum of a bachelor's degree in a life science-related discipline and 3,000 hours of work in human subjects research. Or an undergraduate or graduate degree (excluding graduate certificates) in the field of clinical research plus 1,500 hours of work in human subjects research.
The typical day of a clinical research associate includes planning and managing clinical research projects for pharmaceutical companies. They may recruit participants, coordinate schedules, input data, and oversee trials. In their career, clinical researchers may also be in charge of ensuring that researchers follow all local and federal ...
Here are the top 6 factors to support a successful career as a CRA at IQVIA. 1. Communication. As a CRA, you are the key liaison between management, the study site, and the sponsor. With so many moving parts in clinical research, strong interpersonal skills and good command of English are important. It is also critical to have fluency in the ...
Current Employee in Rapid City, SD, South Dakota. Retirement plan is excellent plan. Search Clinical research associate jobs. Get the right Clinical research associate job with company ratings & salaries. 4,557 open jobs for Clinical research associate.
A day in the life of a clinical research associate. Most CRAs work about 40 hours a week, during weekdays. There may be an out-of-hours commitment, for instance if working in a hospital setting ...
Clinical Research Associates typically work for research facilities, clinical agencies and pharmaceutical companies to coordinate clinical trials. They work closely with other clinical research professionals to test new drugs, procedures and biotechnology that could benefit modern medical practices and patient ailments. Their job is to analyze ...
A clinical research associate (CRA) plays a vital role in the development of new medical treatments. They ensure that clinical trials are conducted ethically and according to protocols, monitoring the progress from start to finish. CRAs are essential for ensuring data integrity and regulatory compliance. The demand for CRAs is increasing due to ...
This role monitors the progress of ongoing clinical trials. Find out how to become a clinical research associate with these steps: Contents. 1. Get a feel for this role's responsibilities. 2. Examine your education. 3. Earn an advanced degree.
Some of the skills needed to perform the job functions of a CRA include: Strong attention to detail and organizational skills. Excellent communication and interpersonal skills. Ability to work both independently and as part of teams. Knowledge of the pharmaceutical and healthcare industries, health and life sciences, and clinical trial ...
The Ultimate Guide to Clinical Research Monitoring. Clinical research associate job requirements Enter the field as a Clinical Research Associate (CRA) with CCRPS's accredited training. Remote roles, $6,500-$12,000 monthly, and 33% annual promotion. 7-day CRA certification for a swift career start.
Clinical trials involve a great deal of documentation, analysis, observation, and organization. A team of professionals is engaged in administering a clinical trial, including a clinical research associate (CRA). The CRA acts as a liaison between the study's sponsor CRO (e.g., pharmaceutical company) and the clinics where the study occurs.
Clinical research associates, also known as "monitors," work on behalf of sponsors funding clinical trials for the new or existing drug, device, surgery, or behavioral intervention. Working directly for the sponsor or through a contract research organization, the main task of a CRA is to monitor the progress of an ongoing clinical trial.
A clinical research associate (CRA), also called a clinical monitor or trial monitor, is a health-care professional who performs many activities related to medical research, particularly clinical trials.Clinical research associates work in various settings, such as pharmaceutical companies, medical research institutes and government agencies.
Anthony Chew, MS. In clinical trial operations, clinical research associates (CRAs) serve as the primary liaison between study sponsors and sites by monitoring and verifying data to ensure accuracy and adherence to protocols. They collaborate with investigators, conduct site visits, and maintain strict documentation to guarantee the integrity ...
The Clinical Research Associate (CRA) will work in close collaboration with cross-functional clinical, regulatory, safety and manufacturing team members, academic partners, and external vendors. This position reports through the Clinical Operations function which is accountable for overall clinical execution and provides scientific input during ...
What is a clinical research associate? A clinical research associate, or CRA, is like a superhero for modern medicine. They make sure new medical treatments are safe and effective by managing clinical trials. These are tests where scientists try out new drugs or treatments to see if they work and if they're safe.
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. Here, you'll find the autonomy and flexibility you need to take your CRA career to the ...
In a forthcoming peer-reviewed article for ACRP's Clinical Researcher journal, Anthony Chew, a clinical trial operations professional and recent graduate of the MS program in Medical Product Development Management at San Jose State University, describes CRAs as serving as "the primary liaison between the sponsor and the site by monitoring and verifying data to ensure accuracy and adherence ...
The Department of Pathology in Stanford University's School of Medicine is seeking a Clinical Research Coordinator Associate to apply medical knowledge and experience, under the direction of the principal investigator to oversee and direct clinical course of research participants in clinical trials. ... May require extended or unusual work ...
Learn more about applying for Senior Clinical Research Associate/Clinical Research Associate II at Parexel ... Why Clinical Research Associates work at Parexel. Patient-focused in everything we do. We push the boundaries of what is possible to create clinical trials that are inclusive, innovative, and patient-focused ...
Job Summary: The Clinical Research Associate I will coordinate Oncology research trials for the Cancer Center and associated satellite sites, primarily involving investigational medications and treatments, including early or late phase FDA-regulated trials. Ensure compliance with federal guidelines at every step. Interact and correspond with clinicians, sponsors, cooperative groups, Upstate's ...
Key Responsibilities: Study Leader and/or Clinical Scientist for predominantly high complexity, global studies. May function as a Core Project Team member for assigned projects to drive the Research-Development-Commercial (RDC) continuum. May co-lead project clinical sub-team and reports study/project progress and issues with their resolution plan to project teams and stakeholders. Directs ...
Clinical Research Associates (CRAs) play a vital role in ensuring our clinical research programs are executed with the quality and excellence our sponsors expect and the care and passion patients deserve. These roles are the backbone of every strong CRO, helping drive breakthrough scientific research and playing a lead role on every clinical trial.