- Patient Care & Health Information
- Diseases & Conditions
- Lung cancer
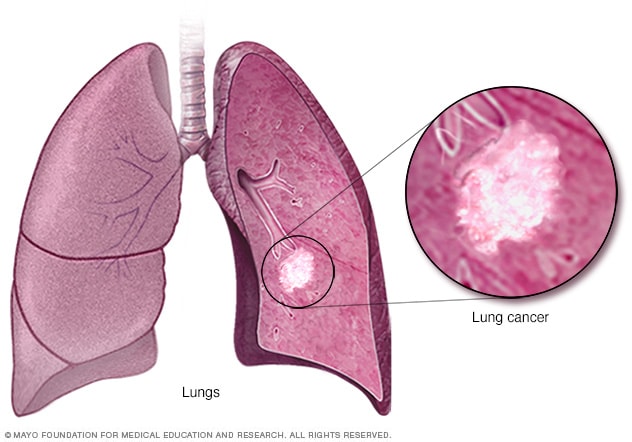
Lung cancer begins in the cells of the lungs.
Lung cancer is a kind of cancer that starts as a growth of cells in the lungs. The lungs are two spongy organs in the chest that control breathing.
Lung cancer is the leading cause of cancer deaths worldwide.
People who smoke have the greatest risk of lung cancer. The risk of lung cancer increases with the length of time and number of cigarettes smoked. Quitting smoking, even after smoking for many years, significantly lowers the chances of developing lung cancer. Lung cancer also can happen in people who have never smoked.

Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Lung cancer typically doesn't cause symptoms early on. Symptoms of lung cancer usually happen when the disease is advanced.
Signs and symptoms of lung cancer that happen in and around the lungs may include:
- A new cough that doesn't go away.
- Chest pain.
- Coughing up blood, even a small amount.
- Hoarseness.
- Shortness of breath.
Signs and symptoms that happen when lung cancer spreads to other parts of the body may include:
- Losing weight without trying.
- Loss of appetite.
- Swelling in the face or neck.
When to see a doctor
Make an appointment with your doctor or other healthcare professional if you have any symptoms that worry you.
If you smoke and haven't been able to quit, make an appointment. Your healthcare professional can recommend strategies for quitting smoking. These may include counseling, medicines and nicotine replacement products.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Lung cancer happens when cells in the lungs develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. In healthy cells, the DNA gives instructions to grow and multiply at a set rate. The instructions tell the cells to die at a set time. In cancer cells, the DNA changes give different instructions. The changes tell the cancer cells to make many more cells quickly. Cancer cells can keep living when healthy cells would die. This causes too many cells.
The cancer cells might form a mass called a tumor. The tumor can grow to invade and destroy healthy body tissue. In time, cancer cells can break away and spread to other parts of the body. When cancer spreads, it's called metastatic cancer.
Smoking causes most lung cancers. It can cause lung cancer in both people who smoke and in people exposed to secondhand smoke. But lung cancer also happens in people who never smoked or been exposed to secondhand smoke. In these people, there may be no clear cause of lung cancer.
How smoking causes lung cancer
Researchers believe smoking causes lung cancer by damaging the cells that line the lungs. Cigarette smoke is full of cancer-causing substances, called carcinogens. When you inhale cigarette smoke, the carcinogens cause changes in the lung tissue almost immediately.
At first your body may be able to repair this damage. But with each repeated exposure, healthy cells that line your lungs become more damaged. Over time, the damage causes cells to change and eventually cancer may develop.
Types of lung cancer
Lung cancer is divided into two major types based on the appearance of the cells under a microscope. Your healthcare professional makes treatment decisions based on which major type of lung cancer you have.
The two general types of lung cancer include:
- Small cell lung cancer. Small cell lung cancer usually only happens in people who have smoked heavily for years. Small cell lung cancer is less common than non-small cell lung cancer.
- Non-small cell lung cancer. Non-small cell lung cancer is a category that includes several types of lung cancers. Non-small cell lung cancers include squamous cell carcinoma, adenocarcinoma and large cell carcinoma.
Risk factors
A number of factors may increase the risk of lung cancer. Some risk factors can be controlled, for instance, by quitting smoking. Other factors can't be controlled, such as your family history.
Risk factors for lung cancer include:
Your risk of lung cancer increases with the number of cigarettes you smoke each day. Your risk also increases with the number of years you have smoked. Quitting at any age can significantly lower your risk of developing lung cancer.
Exposure to secondhand smoke
Even if you don't smoke, your risk of lung cancer increases if you're around people who are smoking. Breathing the smoke in the air from other people who are smoking is called secondhand smoke.
Previous radiation therapy
If you've had radiation therapy to the chest for another type of cancer, you may have an increased risk of developing lung cancer.
Exposure to radon gas
Radon is produced by the natural breakdown of uranium in soil, rock and water. Radon eventually becomes part of the air you breathe. Unsafe levels of radon can build up in any building, including homes.
Exposure to cancer-causing substances
Workplace exposure to cancer-causing substances, called carcinogens, can increase your risk of developing lung cancer. The risk may be higher if you smoke. Carcinogens linked to lung cancer risk include asbestos, arsenic, chromium and nickel.
Family history of lung cancer
People with a parent, sibling or child with lung cancer have an increased risk of the disease.
Complications
Lung cancer can cause complications, such as:
Shortness of breath
People with lung cancer can experience shortness of breath if cancer grows to block the major airways. Lung cancer also can cause fluid to collect around the lungs and heart. The fluid makes it harder for the affected lung to expand fully when you inhale.
Coughing up blood
Lung cancer can cause bleeding in the airway. This can cause you to cough up blood. Sometimes bleeding can become severe. Treatments are available to control bleeding.
Advanced lung cancer that spreads can cause pain. It may spread to the lining of a lung or to another area of the body, such as a bone. Tell your healthcare professional if you experience pain. Many treatments are available to control pain.
Fluid in the chest
Lung cancer can cause fluid to accumulate in the chest, called pleural effusion. The fluid collects in the space that surrounds the affected lung in the chest cavity, called the pleural space.
Pleural effusion can cause shortness of breath. Treatments are available to drain the fluid from your chest. Treatments can reduce the risk that pleural effusion will happen again.
Cancer that spreads to other parts of the body
Lung cancer often spreads to other parts of the body. Lung cancer may spread to the brain and the bones.
Cancer that spreads can cause pain, nausea, headaches or other symptoms depending on what organ is affected. Once lung cancer has spread beyond the lungs, it's generally not curable. Treatments are available to decrease symptoms and to help you live longer.
There's no sure way to prevent lung cancer, but you can reduce your risk if you:
Don't smoke
If you've never smoked, don't start. Talk to your children about not smoking so that they can understand how to avoid this major risk factor for lung cancer. Begin conversations about the dangers of smoking with your children early so that they know how to react to peer pressure.
Stop smoking
Stop smoking now. Quitting reduces your risk of lung cancer, even if you've smoked for years. Talk to your healthcare team about strategies and aids that can help you quit. Options include nicotine replacement products, medicines and support groups.
Avoid secondhand smoke
If you live or work with a person who smokes, urge them to quit. At the very least, ask them to smoke outside. Avoid areas where people smoke, such as bars. Seek out smoke-free options.
Test your home for radon
Have the radon levels in your home checked, especially if you live in an area where radon is known to be a problem. High radon levels can be fixed to make your home safer. Radon test kits are often sold at hardware stores and can be purchased online. For more information on radon testing, contact your local department of public health.
Avoid carcinogens at work
Take precautions to protect yourself from exposure to toxic chemicals at work. Follow your employer's precautions. For instance, if you're given a face mask for protection, always wear it. Ask your healthcare professional what more you can do to protect yourself at work. Your risk of lung damage from workplace carcinogens increases if you smoke.
Eat a diet full of fruits and vegetables
Choose a healthy diet with a variety of fruits and vegetables. Food sources of vitamins and nutrients are best. Avoid taking large doses of vitamins in pill form, as they may be harmful. For instance, researchers hoping to reduce the risk of lung cancer in people who smoked heavily gave them beta carotene supplements. Results showed the supplements increased the risk of cancer in people who smoke.
Exercise most days of the week
If you don't exercise regularly, start out slowly. Try to exercise most days of the week.
Lung cancer care at Mayo Clinic
Living with lung cancer?
Connect with others like you for support and answers to your questions in the Lung Cancer support group on Mayo Clinic Connect, a patient community.
Lung Cancer Discussions

90 Replies Sun, Apr 21, 2024

37 Replies Fri, Apr 19, 2024

104 Replies Thu, Apr 18, 2024
- Non-small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed Dec. 4, 2023.
- Small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462. Accessed Dec. 4, 2023.
- Niederhuber JE, et al., eds. Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 4, 2023.
- Non-small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Lung cancer – non-small cell. Cancer.Net. https://www.cancer.net/cancer-types/lung-cancer/view-all. Accessed Dec. 4, 2023.
- Lung cancer – small cell. Cancer.Net. https://www.cancer.net/cancer-types/33776/view-all. Accessed Dec. 4, 2023.
- Detterbeck FC, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; doi:10.1378/chest.12-2377.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed Dec. 4, 2023.
- Lung cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lung-cancer. Accessed Dec. 4, 2023.
- Cairns LM. Managing breathlessness in patients with lung cancer. Nursing Standard. 2012; doi:10.7748/ns2012.11.27.13.44.c9450.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Jan. 13, 2020.
- Brown AY. Allscripts EPSi. Mayo Clinic. July 30, 2019.
- Searching for cancer centers. American College of Surgeons. https://www.facs.org/search/cancer-programs. Accessed Dec. 4, 2023.
- Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine. 2010; doi:10.1056/NEJMoa1000678.
- Dunning J, et al. Microlobectomy: A novel form of endoscopic lobectomy. Innovations. 2017; doi:10.1097/IMI.0000000000000394.
- Leventakos K, et al. Advances in the treatment of non-small cell lung cancer: Focus on nivolumab, pembrolizumab and atezolizumab. BioDrugs. 2016; doi:10.1007/s40259-016-0187-0.
- Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Medicine. 1999;5:1365.
- Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 2011; doi:10.1056/NEJMoa1102873.
- Infographic: Lung Cancer
- Lung cancer surgery
- Lung nodules: Can they be cancerous?
- Super Survivor Conquers Cancer
Associated Procedures
- Ablation therapy
- Brachytherapy
- Bronchoscopy
- Chemotherapy
- Lung cancer screening
- Positron emission tomography scan
- Proton therapy
- Radiation therapy
- Stop-smoking services
News from Mayo Clinic
- Science Saturday: Study finds senescent immune cells promote lung tumor growth June 17, 2023, 11:00 a.m. CDT
- Era of hope for patients with lung cancer Nov. 16, 2022, 03:00 p.m. CDT
- Mayo Clinic Q&A podcast: Survivorship after surgery for lung cancer Nov. 15, 2022, 01:30 p.m. CDT
- Mayo Clinic Minute: Understanding lung cancer Nov. 02, 2022, 04:00 p.m. CDT
- Lung cancer diagnosis innovation leads to higher survival rates Nov. 02, 2022, 02:30 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been recognized among the top Pulmonology hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 May 2019
Presentation of lung cancer in primary care
- D. P. Weller 1 ,
- M. D. Peake 2 &
- J. K. Field 3
npj Primary Care Respiratory Medicine volume 29 , Article number: 21 ( 2019 ) Cite this article
5196 Accesses
17 Citations
13 Altmetric
Metrics details
- Respiratory signs and symptoms
- Signs and symptoms
Survival from lung cancer has seen only modest improvements in recent decades. Poor outcomes are linked to late presentation, yet early diagnosis can be challenging as lung cancer symptoms are common and non-specific. In this paper, we examine how lung cancer presents in primary care and review roles for primary care in reducing the burden from this disease. Reducing rates of smoking remains, by far, the key strategy, but primary care practitioners (PCPs) should also be pro-active in raising awareness of symptoms, ensuring lung cancer risk data are collected accurately and encouraging reluctant patients to present. PCPs should engage in service re-design and identify more streamlined diagnostic pathways—and more readily incorporate decision support into their consulting, based on validated lung cancer risk models. Finally, PCPs should ensure they are central to recruitment in future lung cancer screening programmes—they are uniquely placed to ensure the right people are targeted for risk-based screening programmes. We are now in an era where treatments can make a real difference in early-stage lung tumours, and genuine progress is being made in this devastating illness—full engagement of primary care is vital in effecting these improvements in outcomes.
Similar content being viewed by others
A systematic review of interventions to recognise, refer and diagnose patients with lung cancer symptoms
Mohamad M. Saab, Megan McCarthy, … Josephine Hegarty

Implications of incidental findings from lung screening for primary care: data from a UK pilot
Emily C. Bartlett, Jonathan Belsey, … Anand Devaraj
Personalised lung cancer risk stratification and lung cancer screening: do general practice electronic medical records have a role?
Bhautesh Dinesh Jani, Michael K. Sullivan, … Frank M. Sullivan
Introduction
Lung cancer poses a significant public health burden around the world; it is the most common cause of cancer mortality in the UK and it accounts for >20% of cancer deaths. 1 There is significant variation in survival rates around the world and this has been largely attributed to the stage at which the cancer is diagnosed. 2 The International Cancer Benchmarking Partnership has demonstrated that survival rates in the UK lag behind those of other countries, and late diagnosis is thought to be a major underlying factor. 3 , 4 Importantly, patients with early-stage disease have a much better prognosis; stage 1 non-small-cell lung cancer can have a 5-year survival rate as high as 75%. 5 Even within the UK, however, there is wide variation in lung cancer survival rates and in the proportion of patients diagnosed with early-stage disease. 6
In the UK, most cancers present symptomatically in primary care (most commonly to a general practitioner, or ‘GP’, the medical lead of a primary care team), and the diagnosis is made after a referral for either investigations or directly to secondary care. 7 Many of the symptoms of lung cancer are very common but non-specific in primary care practice: these include chest pain, cough and breathlessness; 8 hence, lung cancer poses a very significant diagnostic challenge—a primary care practitioner (PCP) working full time is likely to only diagnose 1 or 2 cases per year. Further, lung cancer often emerges on a background of chronic respiratory disease and symptoms of chronic cough—typically in patients who smoke. It can be very difficult to identify changes in these chronic symptoms that might indicate the development of a lung tumour.
Smoking remains the principal aetiological factor and smoking cessation is the key public health initiative to reduce mortality from this disease; 9 indeed, at almost any age smoking cessation can produce health benefits. Hence, public health campaigns to promote smoking cessation, supplemented by strategies in primary care based on nicotine replacement therapies should be encouraged. 10 The role of e-cigarettes is not yet fully understood, 11 although any strategy that reduces exposure to tobacco smoke has a potential for producing significant benefits.
How do patients respond to lung cancer symptoms?
There is a significant body of research around patient response to symptoms that might potentially indicate lung cancer. Because symptoms often present within the context of chronic respiratory symptomatology, changes associated with the development of a tumour may go un-noticed or be dismissed. 8 It is known that patients often delay their help seeking through a range of psychological mechanisms including denial and nihilism—hence, there can often be significant delays before patients present to primary care. 12 , 13
There is evidence for variation in the timeliness of presentation of lung cancer in between countries; people with lung cancer often have symptoms for a considerable period of time before they present to primary care and this is a major source of delay in the diagnostic process with potential adverse impact on survival; 14 , 15 this patient interval does, however, vary between studies. It is important that PCPs understand some of the psychological mechanisms that either promote or inhibit early presentation among their patients.
Public awareness of lung cancer
Over the past few years, there have been campaigns run throughout the UK designed to make the public more aware of symptoms associated with lung cancer—for example the ‘Be clear on Cancer’ campaign run by Public Health England and ‘Diagnose Cancer Early’ in Scotland 16 , 17 (see Fig. 1 ). These campaigns have demonstrated an ability to diagnose additional cancers and effect modest increases in the proportion of patients having tumours diagnosed at stages where they are amenable to resection. 18 , 19

Posters used in the ‘Be Clear on Cancer’ campaign
Of course, lung cancer early detection programmes need to be focussed on the hard-to-reach population and those who will benefit most from involvement; there are often concerns expressed over burdening services with patients with insignificant symptoms 18 and an emerging consensus that all stakeholders should be closely engaged in the campaigns. Nevertheless, available evidence suggests that lung cancer could be diagnosed earlier through these public awareness campaigns, 19 particularly when associated with systems to help primary care physicians risk stratify their patients for lung cancer more effectively—indeed, further work to identify patients who might benefit from targeted interventions should be a priority.
Community-based social marketing interventions have a potential key role; 20 they can increase the likelihood of patients attending PCPs and increase primary care diagnostic activity (such as chest X-ray referrals)—as well as increases in lung cancer diagnostic rates. The level of suspicion at which PCPs consider a referral is a key factor in response to these campaigns—and there are concerns over ‘system overload’ through encouragement to present with symptoms. 13 Ideally, campaigns might preferentially target those at greater risk of lung cancer, such as people with significant smoking histories or occupational exposure.
Primary care response to lung cancer symptoms
In the UK, GPs will on average only diagnose one or two cases of lung cancer per year (if they are in full-time practice). 21 However, during that year, GPs will see hundreds of patients with common symptoms, such as cough, breathlessness and chest pain—hence, there are significant difficulties in identifying, diagnosing and referring these patients in a timely manner.
The 2015 NICE lung cancer guidelines on recognition and referral 22 have underpinned some important strategies to enhance timely lung cancer diagnosis; in many regions of the UK, there are now accelerated diagnostic pathways that assist GPs in identifying and referring patients appropriately. 23 Audit data demonstrate that there are typically several consultations prior to a diagnosis of lung cancer being made. 24 Evidence from significant event analysis in the UK has suggested that there is timely recognition and referral of symptoms in primary care; 25 longer intervals are typically attributed to factors such as X-rays being reported as normal, patient-mediated factors and presentations complicated by co-morbidity. The importance of safety netting has also been emphasised in presentations where a diagnosis of lung cancer is possible. 26
There needs to be continued work to counteract the ‘nihilism’ associated with lung cancer; PCPs are very well aware of patients who may suspect they have lung cancer but fail to present either because they blame themselves (through a history of smoking) or because they believe that if a cancer is diagnosed there is little that can be done about it. 27 This, coupled with the tendency for patients in the UK to be concerned about ‘bothering the doctor’, 28 can have detrimental effects on early diagnosis.
While public campaigns can do much to overcome barriers to presentation, it is vital that PCPs become more pro-active in achieving more timely diagnosis in their practice populations. It is been recommended that they should recognise the psychological mechanisms that might underlie patient delay and tackle nihilistic attitudes through educational and motivational strategies. 29 Indeed, there is cause for cautious optimism with new treatments, and this should be conveyed to patients; for example, the use of stereotactic radiotherapy and volume-sparing surgery means that patients who previously could not be offered curative treatment due to co-morbidities are often now eligible. 30
Audits that systematically identify at-risk patients who may be failing to present are a potential way forward; interventions which identify and target high-risk patients appear feasible in primary care. 31 Crucially, patients should be reassured that PCPs are always happy to see them if they are worried about potential cancer symptoms.
Risk assessment and lung cancer
It is vital in assessing lung cancer risk to look carefully at lifestyle factors and past medical history; only one in seven cases of lung cancer occur in people who have never smoked, and the presence of chronic obstructive pulmonary disease doubles the risk independent of smoking history. 32 A previous history of head and neck, bladder and renal cancers and other factors such as exposure to asbestos or living in high radon exposure areas are all important in lung cancer risk assessment. Family history produces an excess of risk and should be included in risk assessment—as should the symptom of fatigue, a common feature of lung cancer. Cancer decision support tools such as the ‘Caper’ instrument or ‘Q cancer’ have emerged in recent years in the UK, enabling GPs to make assessments of cancer risk based on presenting symptoms; 33 , 34 they have been incorporated into clinical systems in primary care with mixed results.
Beyond these symptom-based models, a number of lung cancer risk models have been developed based on validated epidemiological criteria—for example, the Liverpool Lung Project (LLP) risk model 35 ( www.MyLungRisk.org ), which was subsequently used in the UK Lung Cancer Screening Trial. 36 The LLP v2 risk model has also been used in the Liverpool Healthy Lung project, 37 which has accommodated the risk model within primary care practice and produced risk assessments that are useful in clinical decision making is now running into its third year. The Manchester lung cancer pilot study 38 has used the PLCO 2012 risk prediction model 39 and the recent Yorkshire Lung cancer screening trial 40 is using both the LLP v2 and the PLCO 2012 risk models. Models such as these provide a systematic way of assessing lung cancer risk, taking into account a range of factors, including smoking duration, previous respiratory disease, family history of lung cancer, age, previous history of malignancy and asbestos exposure.
Risk stratification in primary care is clearly a key priority. We need to look at instruments such as the LLP model and identify ways that lung cancer risk stratification can be made easy and convenient in primary care. At present, it is not possible to recommend a specific risk assessment tool for use in primary care; current ongoing research in primary care is externally validating existing tools and will compare their efficacy. 41 Acceptability and feasibility also need to be examined; complex algorithms that place extra burden on practitioners are unlikely to succeed. However, we do need to ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC. We also need a better understanding of ways to maximise benefits of these models—while minimising potential harms such as over-medicalisation, anxiety and false reassurance. 42 Machine learning or neuro-linguistic programming, whereby data from multiple practice-based and external sources might be examined to develop risk estimates, are also likely to play a significant role in the future. 43
Diagnostic pathways
Early diagnosis lung cancer clinics based on multi-disciplinary teams (MDTs) are an ideal option for expediting diagnosis—ideally with an urgent (2-week wait) referral; 44 there is good evidence that these specialist MDT clinics are associated with improved outcomes. Another important consideration is involving the whole primary care team and including other practitioners such as pharmacists who see a lot of patients with, for example, repeat purchases of cough medicine. There has been a push to change referral practices in some parts of the UK—for example, to lower the threshold that PCPs refer for chest X-ray 45 and to encourage practitioners to repeat the investigation after a few months if symptoms persist; critically a normal chest X-ray does not exclude diagnosis of lung cancer. One highly successful programme in Leeds included the option for people to self-refer for chest X-rays in walk-in clinics 19 —a crucial element was the engagement of primary care in the design and implementation of the programme.
Diagnostic pathways have been closely examined and tested over recent years, an example being CRUK’s ACE programme (accelerate, coordinate and evaluate) initiated in June 2014 in England and Wales. 23 Patients often have complex pathways that can lead to delays; important initiatives in the ACE programme and elsewhere include risk-stratified computed tomographic (CT) screening criteria for ‘straight to CT’ referrals following normal chest X-rays and a focus on diagnostic paths for patients with vague symptoms.
Work needs to continue on diagnostic pathways that might expedite lung cancer diagnosis. It is important, for example, that we get more evidence on the impact or potential impact of direct access to investigations such as spiral CT from primary care—at present, there is not sufficient evidence or resource to universally implement this strategy, and there is evidence that delays can occur in primary care (for example, through ordering too many chest X-rays. 46 Nevertheless, GPs in the UK often indicate that direct access to investigations would help streamline diagnosis. 7
Lung cancer screening
A major challenge for primary care is the lack of symptoms in very early stage lung cancer, highlighting the importance of examining the potential of screening. The US National Lung Cancer Screening Trial, which used low-dose CT scanning in high-risk patients, showed a 20% reduction in lung cancer-specific mortality and almost a 7% reduction in all-cause mortality—and the US Preventive Task Force on Lung cancer Screening recommended that lung cancer screening should be implemented in high-risk populations. 47 , 48 Accordingly, Medicare agreed to pay for lung cancer screening within certain criteria—however, the current uptake in the US is only ~2% of high-risk individuals.
The recent report on the NELSON trial at the World Lung Cancer Conference, Toronto 49 has demonstrated an encouragingly low rate of false positives and a mortality benefit of 26% in men and between 39% and 61% in women—depending on the number of years of follow-up (i.e. 8–10 years). These results provide further impetus for the introduction of spiral CT scanning for individuals at high risk of cancer in the UK. Figure 2 illustrates the process for identifying an appropriate screening population, recruiting them and implementing screening—in many ways more complex than existing cancer screening programmes where recruitment is based principally on age and gender.
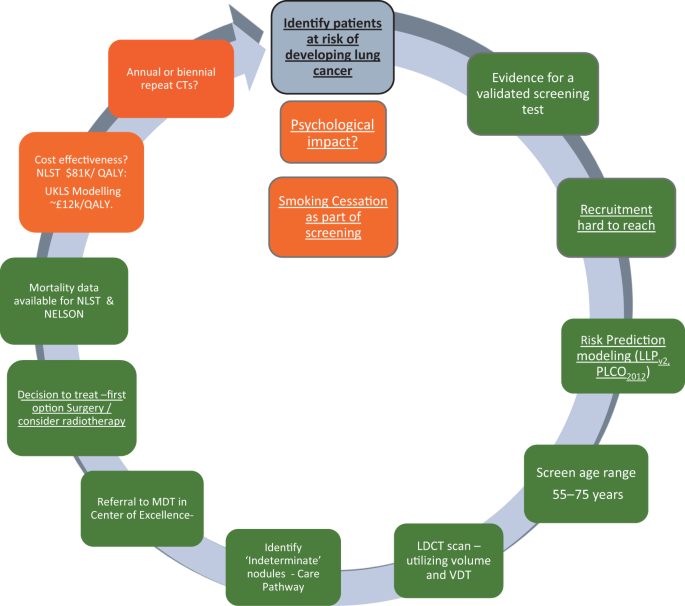
Levels of evidence for the implementation of lung cancer screening in Europe. The colour codes refer to the current status March 2019; traffic lights: green—ready, amber—borderline evidence. Underlined text indicates particular relevance for primary care 53
If we are, indeed, on the cusp of a new screening programme, there are important implications for primary care; the key issue in lung cancer screening is identifying the right patients to invite. This is a task that would involve primary care which currently lacks the systems and the processes to undertake the kind of population- based lung cancer risk assessment required. It is important, therefore, that we plan for an era where high-risk patients are screened for lung cancer (implemented, ideally, in tandem with smoking cessation programmes). We should be refining current strategies to risk stratify patients in primary care in preparation for this new era. 50 , 51 Screening alone, however, is not the total answer and a high level of awareness in both the public and the primary care community will remain vital elements in what needs to be a multi-pronged approach. 52
Conclusions and recommendations
Mortality rates for lung cancer remain stubbornly high; if we are to improve lung cancer outcomes, it is important that early diagnosis and screening efforts achieve their maximum potential. We need to:
identify ways of raising awareness of symptoms potentially associated with lung cancer in ways that encourage people at higher risk to come forward—this will require refinement of the messages delivered in awareness-raising strategies
counter the nihilistic beliefs often associated with lung cancer—early diagnosis CAN lead to improved outcomes
continually strive to improve the primary care response to patients with symptoms of lung cancer, supported by better diagnostic pathways and risk-based decision support
identify ‘fail-safe’ mechanisms by which patients advised to ‘watch and wait’ are not lost to follow-up; it is vital that patients understand these safety netting and follow-up advice
ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC
refine methods to implement lung cancer risk assessment model approaches; this is key to improving diagnosis of early lung cancer—and we should aim for risk estimates that can be readily incorporated into the various kinds of practice software used in primary care practices
continue to improve diagnostic pathways; at present, many different models are being evaluated, including those which give primary care more direct access to investigations such as spiral CT. The key task will be implementation and appropriate support once the best models are determined
fully engage primary care with the likely implementation of spiral CT lung cancer screening in the next few years—this will require the best possible risk-stratification approaches to ensure screening is directed at those who stand to benefit the most from it. It is vital that primary care rises to this challenge
Primary care needs to play a central role in efforts to diagnose lung cancer earlier, if there is to be an improvement in lung cancer outcomes in the years ahead. Research over the past decade gives us a much clearer idea of what needs to be done in refining primary care-based strategies; with adequate commitment and resources primary care will, in conjunction with other health care sectors, help reduce the burden from this disease.
Ferkol, T. & Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 11 , 404–406 (2014).
Article PubMed Google Scholar
Torre, L. A., Lindsey, A., Rebecca, L., Siegel, R. L. & Jemal, A. “Lung cancer statistics.” Lung cancer and personalized medicine. Adv. Exp. Med Biol. 893 , 1–19 (2016).
Coleman, M. P., Forman, D., Bryant, H., Butler, J. & Rachet, B. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377 , 127–138 (2011).
Tørring, M. L., Frydenberg, M., Hansen, R. P., Olesen, F. & Vedsted, P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur. J. Cancer 49 , 2187–2198 (2013).
Walters, S. et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 68 , 551–564 (2013).
Royal College of Physicians. National Lung Cancer Audit Annual report 2017. Available via www.nlcaudit.co.uk
Wagland, R. et al. Facilitating early diagnosis of lung cancer amongst primary care patients: the views of GPs. Eur. J. Cancer Care https://doi.org/10.1111/ecc.12704 (2017).
Article PubMed Central Google Scholar
Walter, F. M. et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br. J. Cancer 112 (suppl 1), S6–S13 (2015).
Article PubMed PubMed Central Google Scholar
Forman, D. et al. Time for a European initiative for research to prevent cancer: a manifesto for Cancer Prevention Europe (CPE). J. Cancer Policy 17, 15–23 (2018).
Article Google Scholar
Smith, S. S. et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch. Intern. Med. 14 (169), 2148–2155 (2009).
Brown, J., Beard, E., Kotz, D., Michie, S. & West, R. Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction 109 , 1531–1540 (2014).
Hansen, R. P., Vedsted, P., Sokolowski, I., Søndergaard, J. & Olesen, F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 11 , 284 (2011).
Niksic, M. et al. Cancer symptom awareness and barriers to symptomatic presentation in England—are we clear on cancer? Br. J. Cancer 28 (113), 533–542 (2015).
Biswas, M., Ades, A. E. & Hamilton, W. Symptom lead times in lung and colorectal cancers: what are the benefits of symptom based approaches to early diagnosis? Br. J. Cancer 112 , 271–277 (2015).
Article CAS PubMed Google Scholar
O’Dowd, E. L. et al. What characteristics of primary care and patients are associated with early death in patients with lung cancer in the UK? Thorax 70 , 161–168 (2015).
Peake, M. D. Be Clear on Cancer: regional and national lung cancer awareness campaigns 2011 to 2014, Final evaluation results. National Cancer Registration and Analysis Service, Public Health England, February 2018. Available via: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/be_clear_on_cancer/
Calanzani, N., Weller, D. & Campbell, C. Development of a Methodological Approach to Evaluate the Detect Cancer Early Programme in Scotland Cancer Research UK Early Diagnosis Conference. https://www.cancerresearchuk.org/sites/default/files/edrc17_poster-42_nataliacalanzani_monteiro_detect_cancer_early_programme_in_scotland.pdf
Ironmonger, L. et al. An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br. J. Cancer 112 , 207 (2015).
Kennedy, M. P. T. et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax 73 , 1128–1136 (2018).
Athey, V. L., Suckling, R. J., Tod, A. M., Walters, S. J. & Rogers, T. K. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax 67 , 412–417 (2012).
Hamilton, W., Peters, T. J., Round, A. & Sharp, D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 60 , 1059–1065 (2005).
Article CAS PubMed PubMed Central Google Scholar
NICE. Suspected Cancer: Recognition and Referral NICE Guideline [NG12] , National Centre for Health and Care Excellence, London (2015).
Fuller, E., Fitzgerald, K. & Hiom, S. Accelerate, Coordinate, Evaluate Programme: a new approach to cancer diagnosis. Br. J. Gen. Pract. 66 , 176–177 (2016).
Lyratzopoulos, G., Neal, R. D., Barbiere, J. M., Rubin, G. P. & Abel, G. A. Variation in number of general practioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 13 , 353–365 (2012).
Mitchell, E. D., Rubin, G. & Macleod, U. Understanding diagnosis of lung cancer in primary care: qualitative synthesis of significant event audit reports. Br. J. Gen. Pract. 63 , e37–e46 (2013).
Evans, J. et al. GPs’ understanding and practice of safety netting for potential cancer presentations: a qualitative study in primary care. Br. J. Gen. Pract. 68 , e505–e511 (2018).
Corner, J., Hopkinson, J., Fitzsimmons, D., Barclay, S. & Muers, M. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax 60 , 314–319 (2005).
Forbes, L. J. et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br. J. Cancer 108 , 292–300 (2013).
Rubin, G. et al. The expanding role of primary care in cancer control. Lancet Oncol. 16 , 1231–1272 (2015).
Jones, G. S. & Baldwin, D. R. Recent advances in the management of lung cancer. Clin. Med. 18 (suppl 2), s41–s46 (2018).
Wagland, R. et al. Promoting help-seeking in response to symptoms amongst primary care patients at high risk of lung cancer: a mixed method study. PLoS ONE 11 , e0165677 (2016).
Ten Haaf, K., De Koning, H. & Field, J. Selecting the risk cut off for the LLP Model. J. Thorac. Oncol. 12 , S2174 (2017).
Hamilton, W. et al. Evaluation of risk assessment tools for suspected cancer in general practice: a cohort study. Br. J. Gen. Pract. 63 , e30–e36 (2013).
Hippisley-Cox, J. & Coupland, C. Identifying patients with suspected lung cancer in primary care: derivation and validation of an algorithm. Br. J. Gen. Pract. 61 , e715–e723 (2011).
Cassidy, A. et al. The LLP risk model: an individual risk prediction model for lung cancer. Br. J. Cancer 98 , 270–276 (2008).
Field, J. K. et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol. Assess. 20 , 1–146 (2016).
Field, J. K. et al. Abstract 4220: Liverpool Healthy Lung Project: a primary care initiative to identify hard to reach individuals with a high risk of developing lung cancer. Cancer Res . https://doi.org/10.1158/1538-7445.AM2017-4220 (2017).
Crosbie, P. A. et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax 0 , 1–5 (2018).
Google Scholar
Tammemagi, C. M. et al. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J. Natl. Cancer Inst. 103 , 1058–1068 (2011).
ISRCTN Registry. The Yorkshire Lung Screening Trial ISRCTN42704678. (2019). https://doi.org/10.1186/ISRCTN42704678
Schmidt-Hansen, M., Berendse, S., Hamilton, W. & Baldwin, D. R. Lung cancer in symptomatic patients presenting in primary care: a systematic review of risk prediction tools. Br. J. Gen. Pract. 67 , e396–e404 (2017).
Usher-Smith, J., Emery, J., Hamilton, W., Griffin, S. J. & Walter, F. M. Risk prediction tools for cancer in primary care. Br. J. Cancer 113 , 1645 (2015).
Goldstein, B. A., Navar, A. M., Pencina, M. J. & Ioannidis, J. Opportunities and challenges in developing risk prediction models with electronic health records data: a systematic review. J. Am. Med. Inf. Assoc. 24 , 198–208 (2017).
Aldik, G., Yarham, E., Forshall, T., Foster, S. & Barnes, S. J. Improving the diagnostic pathway for patients with suspected lung cancer: analysis of the impact of a diagnostic MDT. Lung Cancer 115 , S12–S13 (2018).
Neal, R. D. et al. Immediate chest X-ray for patients at risk of lung cancer presenting in primary care: randomised controlled feasibility trial. Br. J. Cancer 116 , 293 (2017).
Guldbrandt, L. M., Rasmussen, T. R., Rasmussen, F. & Vedsted, P. Implementing direct access to low-dose computed tomography in general practice—method, adaption and outcome. PLoS ONE 9 , e112162 (2014).
Moyer, V. A. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 160 , 330–338 (2014).
PubMed Google Scholar
Cheung, L. C., Katki, H. A., Chaturvedi, A. K., Jemal, A. & Berg, C. D. Preventing lung cancer mortality by computed tomography screening: the effect of risk-based versus US Preventive Services Task Force eligibility criteria, 2005–2015. Ann. Intern. Med. 168 , 229–232 (2018).
IASLC. NELSON Study Shows CT Screening for Nodule Volume Management Reduces Lung Cancer Mortality by 26 Percent in Men. 9th World Congress on Lung Cancer . https://wclc2018.iaslc.org/media/2018%20WCLC%20Press%20Program%20Press%20Release%20De%20Koning%209.25%20FINAL%20.pdf
Field, J. K., Duffy, S. W. & Baldwin, D. R. Patient selection for future lung cancer computed tomography screening programmes: lessons learnt post National Lung Cancer Screening Trial. Transl. Lung Cancer Res. 7 (suppl 2), S114–S116 (2018).
Yousaf-Khan, U. et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 72 , 819–824 (2017).
Peake, M. D., Navani, N. & Baldwin, D. The continuum of screening and early detection, awareness and faster diagnosis of lung cancer. Thorax 73 , 1097–1098 (2018).
Field, J. K., Devaraj, A., Duffy, S. W. & Baldwin, D. R. CT screening for lung cancer: is the evidence strong enough? Lung Cancer 91 , 29–35 (2016).
Download references
Author information
Authors and affiliations.
Usher Institute, University of Edinburgh, Edinburgh, UK
D. P. Weller
Centre for Cancer Outcomes, University College London Hospitals Cancer Collaborative, University of Leicester, NCRAS/PHE, London, UK
M. D. Peake
Roy Castle Lung Cancer Research Programme, Department of Molecular and Clinical Cancer Medicine, The University of Liverpool, Liverpool, UK
J. K. Field
You can also search for this author in PubMed Google Scholar
Contributions
D.P.W. led on literature searching and draft manuscript preparation. J.K.F. and M.D.P. provided input to early drafts and added text in their areas of expertise.
Corresponding author
Correspondence to D. P. Weller .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Weller, D.P., Peake, M.D. & Field, J.K. Presentation of lung cancer in primary care. npj Prim. Care Respir. Med. 29 , 21 (2019). https://doi.org/10.1038/s41533-019-0133-y
Download citation
Received : 22 November 2018
Accepted : 12 April 2019
Published : 22 May 2019
DOI : https://doi.org/10.1038/s41533-019-0133-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Anti-proliferative activity, molecular genetics, docking analysis, and computational calculations of uracil cellulosic aldehyde derivatives.
- Asmaa M. Fahim
- Sawsan Dacrory
- Ghada H. Elsayed
Scientific Reports (2023)
Lung cancer and Covid-19: lessons learnt from the pandemic and where do we go from here?
- Susanne Sarah Maxwell
- David Weller
npj Primary Care Respiratory Medicine (2022)
LncRNA CBR3-AS1 potentiates Wnt/β-catenin signaling to regulate lung adenocarcinoma cells proliferation, migration and invasion
Cancer Cell International (2021)

Is the combination of bilateral pulmonary nodules and mosaic attenuation on chest CT specific for DIPNECH?
- Bilal F. Samhouri
- Chi Wan Koo
Orphanet Journal of Rare Diseases (2021)
Evaluation of a national lung cancer symptom awareness campaign in Wales
- Grace McCutchan
- Stephanie Smits
British Journal of Cancer (2020)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
What Is Lung Cancer?
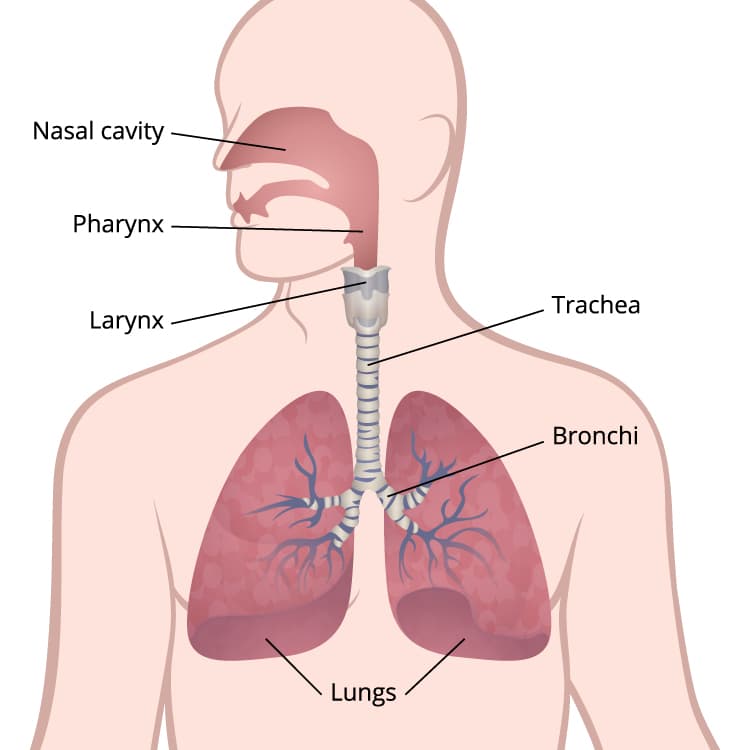
This illustration of the respiratory system shows the lungs, bronchi, trachea, larynx, pharynx, and nasal cavity.
Cancer is a disease in which cells in the body grow out of control. When cancer starts in the lungs, it is called lung cancer.
Lung cancer begins in the lungs and may spread to lymph nodes or other organs in the body, such as the brain. Cancer from other organs also may spread to the lungs. When cancer cells spread from one organ to another, they are called metastases.
Lung cancers usually are grouped into two main types called small cell and non-small cell (non-small cell includes adenocarcinoma and squamous cell carcinoma). These types of lung cancer grow differently and are treated differently. Non-small cell lung cancer is more common than small cell lung cancer. For more information, visit the National Cancer Institute’s Lung Cancer.
Stay Informed
Exit notification / disclaimer policy.
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Airway diseases pp 1–19 Cite as
Clinical Presentation of Lung Cancer
- Pınar Akın Kabalak 5 &
- Ülkü Yılmaz 5
- First Online: 21 September 2023
690 Accesses
Among lung cancer patients, 5–15% are asymptomatic despite experiencing serious morbidity and mortality [1]. Symptoms vary as a result of cell type, localization, diameter, stage, concomitant pulmonary disease, and concomitant paraneoplastic syndromes. The prognosis for patients who are symptomatic at the time of diagnosis has been shown to be worse than that for asymptomatic patients [2]. Therefore, to increase survival, it may be possible to reduce the cancer-related mortality at an early stage as a result of screening individuals in the high-risk group using low-dose tomography [3].
This is a preview of subscription content, log in via an institution .
Chute CG, Greenberg ER, Baron J, et al. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107–11.
Article CAS PubMed Google Scholar
Santos-Martínez MJ, Curull V, Blanco ML, et al. Lung cancer at a university hospital: epidemiological and histological characteristics of a recent and a historical series. Arch Bronconeumol. 2005;41(6):307–12. https://doi.org/10.1016/s1579-2129(06)60230-9 .
Article PubMed Google Scholar
Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013;382(9893):732–41.
Ost DE, Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer. Chest. 2013;143(5 Suppl):e121S–41S.
Article CAS PubMed PubMed Central Google Scholar
Hyde L, Hayde CI. Clinical manifestations of lung cancer. Chest. 1974;65:299. PMID: 4813837.
Beckles MA, Spiro SG, Gen Colice GL, et al. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123(1 Suppl):97S–104S.
Kvale PA, Simoff M, Prakash UBS, et al. Lung cancer palliative care. Chest. 2003;123(1 Suppl):284S–311S.
Athey VL, Walters SJ, Rogers TK. Symptoms at lung cancer diagnosis are associated with major differences in prognosis. Thorax. 2018;73(12):1177. Epub 2018 Apr 17
Chute CG, Greenberg ER, Baron J, et al. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107.
Colice GL. Detecting lung cancer as a cause of hemoptysis in patients with a normal chest radiograph: bronchoscopy vs CT. Chest. 1997;111:877.
Abraham PJ, Capobianco DJ, Cheshire WP. Facial pain as the presenting symptom of lung carcinoma with Normal chest radiograph. Headache. 2003;43(5):499–504.
Farber SM, Mandel W, Spain DM. Diagnosis and treatment of tumors of the chest. New York: Grune & Stratton; 1960.
Google Scholar
Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117:354–7.
Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24:898–904.
Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56(4):905–9.
Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83(2):235–50.
Decker DA, Dines DE, Payne WS, et al. The significance of a cytologically negative pleural effusion in bronchogenic carcinoma. Chest. 1978;74(6):640.
Imazio M, Colopi M, De Ferrari GM. Pericardial diseases in patients with cancer: contemporary prevalence, management and outcomes. Heart. 2020;106(8):569–74.
Friedman T, Quencer KB, Kishore SA, et al. Malignant venous obstruction: superior vena cava syndrome and beyond. Semin Intervent Radiol. 2017;34(4):398–408.
Article PubMed PubMed Central Google Scholar
Gundepalli SG, Tadi P. Cancer, lung pancoast (superior sulcus tumour). Treasure Island: StatPearls Publishing; 2020.
Stankey RM, Roshe J, Sogocio RM. Carcinoma of the lung and dysphagia. Dis Chest. 1969;55(1):13–7. https://doi.org/10.1378/chest.55.1.13 .
Marmor S, Cohen S, Fujioka N, et al. Dysphagia prevalence and associated survival differences in older patients with lung cancer: a SEER-Medicare Population-Based Study. J Geriatr Oncol 2020;S1879-4068(19)30426-6.
Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94.
Wang CY, Zhang XY. (99m)Tc-MDP wholebody bone imaging in evaluation of the characteristics of bone metastasis of primary lung cancer. Zhonghua Zhong Liu Za Zhi. 2010;32(5):382–6.
PubMed Google Scholar
Husaini HA, Price PW, Clemons M, et al. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol. 2009;4:251–9.
Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21(1):1.
Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Arch Neurol. 1988;45(7):741.
Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol. 1992;31(3):268–73.
Nutt SH, Patchell RA. Intracranial haemorrhage associated with primary and secondary tumours. Neurosurg Clin N Am. 1992;3(3):591.
Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–19.
Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–5.
Omuro AM, Lallana EC, Bilsky MH, et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625–7.
Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–55.
Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol. 2002;56:95–101.
Article Google Scholar
Raz DJ, Lanuti M, Gaissert HC, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg. 2011;92(5):1788–92.
Kagohashi K, Satoh H, Ishikawa H, et al. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 2003;20(1):25–8.
https://www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/liver-metastases/?region=on
Khaja M, Mundt D, Dudekula RA, et al. Lung cancer presenting as skin metastasis of the back and hand: a case series and literature review. Case Rep Oncol. 2019;12:480–7.
Joll CA. Metastatic tumours of bone. Br J Surg. 1923;11:38–72.
Afshar A, Farhadnia P, Khalkhali H. Metastases to the hand and wrist: an analysis of 221 cases. J Hand Surg Am. 2014;39(5):923–32.e17.
Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15:51–8.
Mavrogenis AF, Mimidis G, Kokkalis ZT, et al. Acrometastases. Eur J Orthop Surg Traumatol. 2014;24:279–83.
Unsal M, Kutlar G, Sullu Y, et al. Tonsillar metastasis of small cell lung carcinoma. Clin Respir J. 2016;10(6):681–3.
Liyang Z, Yuewu L, Xiaoyi L, et al. Metastases to the thyroid gland. A report of 32 cases in PUMCH. Medicine. 2017;96(36):e7927.
Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016;16:149.
Kızılgöz D, Kabalak PA, Cengiz Tİ, et al. Splenic metastasis in lung cancer. Turk Klin Arch Lung. 2017;18(2):47–51.
Efthymiou C, Spyratos D, Kontakiotis T. Endocrine paraneoplastic syndromes in lung cancer. Hormones. 2018;17:351–8.
Anwar A, Jafri F, Ashraf S, et al. Paraneoplastic syndromes in lung cancer and their management. Ann Transl Med. 2019;7(15):359.
Rossato M, Zabeo E, Burei M, et al. Lung cancer and paraneoplastic neurologic syndromes. Case report and review of the literature. Clin Lung Cancer. 2013;14(3):301–9.
Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–54.
Thomas L, Kwok Y, Edelman MJ. Management of paraneoplastic syndromes in lung cancer. Curr Treat Options in Oncol. 2004;5(1):51–62.
Makiyama Y, Kikuchi T, Otani A, et al. Clinical and immunological characterization of paraneoplastic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(8):5424–31.
Stoyanov GS, Dzhenkov DL, Tzaneva M. Thrombophlebitis Migrans (Trousseau syndrome) in pancreatic adenocarcinoma: an autopsy report. Cureus. 2019;11(8):e5528.
PubMed PubMed Central Google Scholar
Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–82.
Download references
Author information
Authors and affiliations.
Department of Pulmonology, Health Sciences University Atatürk Chest and Chest Surgery Hospital, Ankara, Turkey
Pınar Akın Kabalak & Ülkü Yılmaz
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Otorhinolaryngology, Eskişehir Osmangazi University, Eskisehir, Türkiye
Cemal Cingi
Department of Pulmonology, Celal Bayar University, Manisa, Türkiye
Arzu Yorgancıoğlu
Department of Otorhinolaryngology, Kırıkkale University, Kırıkkale, Türkiye
Nuray Bayar Muluk
Federal University of Bahia School of Medicine and ProAR Foundation, Salvador - Bahia, Brazil
Alvaro A. Cruz
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter.
Kabalak, P.A., Yılmaz, Ü. (2023). Clinical Presentation of Lung Cancer. In: Cingi, C., Yorgancıoğlu, A., Bayar Muluk, N., Cruz, A.A. (eds) Airway diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-22483-6_60-1
Download citation
DOI : https://doi.org/10.1007/978-3-031-22483-6_60-1
Received : 11 November 2022
Accepted : 11 November 2022
Published : 21 September 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-22482-9
Online ISBN : 978-3-031-22483-6
eBook Packages : Medicine Reference Module Medicine
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Lung Cancer: Clinical Presentation and Diagnosis
Affiliation.
- 1 US Naval Hospital Sigonella Italy, PSC 836 Box 2670, FPO, AE 09636.
- PMID: 29313654
In the absence of screening, most patients with lung cancer are not diagnosed until later stages, when the prognosis is poor. The most common symptoms are cough and dyspnea, but the most specific symptom is hemoptysis. Digital clubbing, though rare, is highly predictive of lung cancer. Symptoms can be caused by the local tumor, intrathoracic spread, distant metastases, or paraneoplastic syndromes. Clinicians should suspect lung cancer in symptomatic patients with risk factors. The initial study should be chest x-ray, but if results are negative and suspicion remains, the clinician should obtain a computed tomography scan with contrast. The diagnostic evaluation for suspected lung cancer includes tissue diagnosis, staging, and determination of functional capacity, which are completed simultaneously. Tissue samples should be obtained using the least invasive method possible. Management is based on the individual tumor histology, molecular testing results, staging, and performance status. The management plan is determined by a multidisciplinary team consisting of a pulmonology subspecialist, medical oncology subspecialist, radiation oncology subspecialist, and thoracic surgeon. The family physician should remain involved with the patient to ensure that patient priorities are supported and, if necessary, to arrange for end-of-life care.
Written permission from the American Academy of Family Physicians is required for reproduction of this material in whole or in part in any form or medium.
Publication types
- Adenocarcinoma / complications
- Adenocarcinoma / diagnostic imaging*
- Adenocarcinoma / pathology
- Adenocarcinoma / physiopathology
- Carcinoma, Large Cell / complications
- Carcinoma, Large Cell / diagnostic imaging*
- Carcinoma, Large Cell / pathology
- Carcinoma, Large Cell / physiopathology
- Carcinoma, Squamous Cell / complications
- Carcinoma, Squamous Cell / diagnostic imaging*
- Carcinoma, Squamous Cell / pathology
- Carcinoma, Squamous Cell / physiopathology
- Cough / etiology
- Dyspnea / etiology
- Hemoptysis / etiology
- Lung Neoplasms / complications
- Lung Neoplasms / diagnostic imaging*
- Lung Neoplasms / pathology
- Lung Neoplasms / physiopathology
- Neoplasm Staging
- Paraneoplastic Syndromes / etiology
- Respiratory Function Tests
- Small Cell Lung Carcinoma / complications
- Small Cell Lung Carcinoma / diagnostic imaging*
- Small Cell Lung Carcinoma / pathology
- Small Cell Lung Carcinoma / physiopathology
Lung Cancer Clinical Presentation
There are no specific signs and symptoms for lung cancer, and the clinical presentation of lung cancer may vary from patient to patient. At the time of diagnosis, the vast majority of patients with lung cancer have advanced disease. The frequent absence of symptoms until locally advanced or metastatic disease is present reveals the aggressive nature of lung cancer and also highlights the urgent need to expand efforts to routinely screen patients at high risk. Research indicates that many cases of lung cancer are often detected incidentally via chest imaging. In about 7% to 10% of cases, lung cancer is detected and diagnosed in asymptomatic patients when a chest radiograph is performed to diagnose other conditions. At initial diagnosis, 20% of patients have localized disease, 25% of patients have regional metastasis, and 55% of patients have distant disease spread. In some cases, high-risk patients may be diagnosed while asymptomatic through screening with low-dose computed tomography. About three-fourths of nonscreened patients with lung cancer present with one or more symptoms at the time of diagnosis. The most common symptoms include cough, dyspnea, and hemoptysis. Although the clinical presentation of lung cancer is not specific to the classification or histology of the cancer, certain obstacles may be more likely with different types. One study noted that the most common symptoms at presentation were cough (55%), dyspnea (45%), pain (38%), and weight loss (36%), as well as hemoptysis. The new onset of cough in a smoker or former smoker should raise suspicion for lung cancer. The clinical manifestations of lung cancer may be due to intrathoracic effects of the tumor (e.g., cough, hemoptysis, pleural disease), extrathoracic metastases (most commonly liver, bone, brain), or paraneoplastic phenomena (e.g., hypercalcemia, Cushing syndrome, hypercoagulability disorders, various neurologic syndromes). Squamous cell and small cell cancers usually cause a cough early due to the involvement of the central airways. Hemoptysis is a significant symptom in anyone with a history of smoking. Although bronchitis is the most frequent cause of hemoptysis, 20% to 50% of patients with underlying lung cancer present with hemoptysis. While rare, patients with lung cancer may present with shoulder pain, Horner syndrome, and hand-muscle atrophy, and this group of symptoms is referred to as Pancoast syndrome . Pancoast syndrome is most frequently due to lung cancers arising in the superior sulcus. Metastasis from lung cancer to bone is generally symptomatic, and pain in the back, chest, or extremity and elevated levels of serum alkaline phosphatase are frequently present in patients who have bone metastasis. Moreover, levels of serum calcium may be elevated due to extensive bone disease, and an estimated 20% of patients with non–small cell lung cancer have bone metastases at the time of diagnosis. The American Cancer Society (ACS) indicates that in addition to the signs and symptoms mentioned above, patients with lung cancer may also experience hoarseness, new-onset wheezing, and fatigue. The ACS also encourages individuals to seek medical care early if they are experiencing any symptoms, since early detection and treatment may improve overall clinical outcomes in some patients. The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.

Related Content
Contemporary developments in met-selective kinase inhibitors in advanced non–small-cell lung cancer.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- NPJ Prim Care Respir Med

Presentation of lung cancer in primary care
D. p. weller.
1 Usher Institute, University of Edinburgh, Edinburgh, UK
M. D. Peake
2 Centre for Cancer Outcomes, University College London Hospitals Cancer Collaborative, University of Leicester, NCRAS/PHE, London, UK
J. K. Field
3 Roy Castle Lung Cancer Research Programme, Department of Molecular and Clinical Cancer Medicine, The University of Liverpool, Liverpool, UK
Survival from lung cancer has seen only modest improvements in recent decades. Poor outcomes are linked to late presentation, yet early diagnosis can be challenging as lung cancer symptoms are common and non-specific. In this paper, we examine how lung cancer presents in primary care and review roles for primary care in reducing the burden from this disease. Reducing rates of smoking remains, by far, the key strategy, but primary care practitioners (PCPs) should also be pro-active in raising awareness of symptoms, ensuring lung cancer risk data are collected accurately and encouraging reluctant patients to present. PCPs should engage in service re-design and identify more streamlined diagnostic pathways—and more readily incorporate decision support into their consulting, based on validated lung cancer risk models. Finally, PCPs should ensure they are central to recruitment in future lung cancer screening programmes—they are uniquely placed to ensure the right people are targeted for risk-based screening programmes. We are now in an era where treatments can make a real difference in early-stage lung tumours, and genuine progress is being made in this devastating illness—full engagement of primary care is vital in effecting these improvements in outcomes.
Introduction
Lung cancer poses a significant public health burden around the world; it is the most common cause of cancer mortality in the UK and it accounts for >20% of cancer deaths. 1 There is significant variation in survival rates around the world and this has been largely attributed to the stage at which the cancer is diagnosed. 2 The International Cancer Benchmarking Partnership has demonstrated that survival rates in the UK lag behind those of other countries, and late diagnosis is thought to be a major underlying factor. 3 , 4 Importantly, patients with early-stage disease have a much better prognosis; stage 1 non-small-cell lung cancer can have a 5-year survival rate as high as 75%. 5 Even within the UK, however, there is wide variation in lung cancer survival rates and in the proportion of patients diagnosed with early-stage disease. 6
In the UK, most cancers present symptomatically in primary care (most commonly to a general practitioner, or ‘GP’, the medical lead of a primary care team), and the diagnosis is made after a referral for either investigations or directly to secondary care. 7 Many of the symptoms of lung cancer are very common but non-specific in primary care practice: these include chest pain, cough and breathlessness; 8 hence, lung cancer poses a very significant diagnostic challenge—a primary care practitioner (PCP) working full time is likely to only diagnose 1 or 2 cases per year. Further, lung cancer often emerges on a background of chronic respiratory disease and symptoms of chronic cough—typically in patients who smoke. It can be very difficult to identify changes in these chronic symptoms that might indicate the development of a lung tumour.
Smoking remains the principal aetiological factor and smoking cessation is the key public health initiative to reduce mortality from this disease; 9 indeed, at almost any age smoking cessation can produce health benefits. Hence, public health campaigns to promote smoking cessation, supplemented by strategies in primary care based on nicotine replacement therapies should be encouraged. 10 The role of e-cigarettes is not yet fully understood, 11 although any strategy that reduces exposure to tobacco smoke has a potential for producing significant benefits.
How do patients respond to lung cancer symptoms?
There is a significant body of research around patient response to symptoms that might potentially indicate lung cancer. Because symptoms often present within the context of chronic respiratory symptomatology, changes associated with the development of a tumour may go un-noticed or be dismissed. 8 It is known that patients often delay their help seeking through a range of psychological mechanisms including denial and nihilism—hence, there can often be significant delays before patients present to primary care. 12 , 13
There is evidence for variation in the timeliness of presentation of lung cancer in between countries; people with lung cancer often have symptoms for a considerable period of time before they present to primary care and this is a major source of delay in the diagnostic process with potential adverse impact on survival; 14 , 15 this patient interval does, however, vary between studies. It is important that PCPs understand some of the psychological mechanisms that either promote or inhibit early presentation among their patients.
Public awareness of lung cancer
Over the past few years, there have been campaigns run throughout the UK designed to make the public more aware of symptoms associated with lung cancer—for example the ‘Be clear on Cancer’ campaign run by Public Health England and ‘Diagnose Cancer Early’ in Scotland 16 , 17 (see Fig. Fig.1). 1 ). These campaigns have demonstrated an ability to diagnose additional cancers and effect modest increases in the proportion of patients having tumours diagnosed at stages where they are amenable to resection. 18 , 19

Posters used in the ‘Be Clear on Cancer’ campaign
Of course, lung cancer early detection programmes need to be focussed on the hard-to-reach population and those who will benefit most from involvement; there are often concerns expressed over burdening services with patients with insignificant symptoms 18 and an emerging consensus that all stakeholders should be closely engaged in the campaigns. Nevertheless, available evidence suggests that lung cancer could be diagnosed earlier through these public awareness campaigns, 19 particularly when associated with systems to help primary care physicians risk stratify their patients for lung cancer more effectively—indeed, further work to identify patients who might benefit from targeted interventions should be a priority.
Community-based social marketing interventions have a potential key role; 20 they can increase the likelihood of patients attending PCPs and increase primary care diagnostic activity (such as chest X-ray referrals)—as well as increases in lung cancer diagnostic rates. The level of suspicion at which PCPs consider a referral is a key factor in response to these campaigns—and there are concerns over ‘system overload’ through encouragement to present with symptoms. 13 Ideally, campaigns might preferentially target those at greater risk of lung cancer, such as people with significant smoking histories or occupational exposure.
Primary care response to lung cancer symptoms
In the UK, GPs will on average only diagnose one or two cases of lung cancer per year (if they are in full-time practice). 21 However, during that year, GPs will see hundreds of patients with common symptoms, such as cough, breathlessness and chest pain—hence, there are significant difficulties in identifying, diagnosing and referring these patients in a timely manner.
The 2015 NICE lung cancer guidelines on recognition and referral 22 have underpinned some important strategies to enhance timely lung cancer diagnosis; in many regions of the UK, there are now accelerated diagnostic pathways that assist GPs in identifying and referring patients appropriately. 23 Audit data demonstrate that there are typically several consultations prior to a diagnosis of lung cancer being made. 24 Evidence from significant event analysis in the UK has suggested that there is timely recognition and referral of symptoms in primary care; 25 longer intervals are typically attributed to factors such as X-rays being reported as normal, patient-mediated factors and presentations complicated by co-morbidity. The importance of safety netting has also been emphasised in presentations where a diagnosis of lung cancer is possible. 26
There needs to be continued work to counteract the ‘nihilism’ associated with lung cancer; PCPs are very well aware of patients who may suspect they have lung cancer but fail to present either because they blame themselves (through a history of smoking) or because they believe that if a cancer is diagnosed there is little that can be done about it. 27 This, coupled with the tendency for patients in the UK to be concerned about ‘bothering the doctor’, 28 can have detrimental effects on early diagnosis.
While public campaigns can do much to overcome barriers to presentation, it is vital that PCPs become more pro-active in achieving more timely diagnosis in their practice populations. It is been recommended that they should recognise the psychological mechanisms that might underlie patient delay and tackle nihilistic attitudes through educational and motivational strategies. 29 Indeed, there is cause for cautious optimism with new treatments, and this should be conveyed to patients; for example, the use of stereotactic radiotherapy and volume-sparing surgery means that patients who previously could not be offered curative treatment due to co-morbidities are often now eligible. 30
Audits that systematically identify at-risk patients who may be failing to present are a potential way forward; interventions which identify and target high-risk patients appear feasible in primary care. 31 Crucially, patients should be reassured that PCPs are always happy to see them if they are worried about potential cancer symptoms.
Risk assessment and lung cancer
It is vital in assessing lung cancer risk to look carefully at lifestyle factors and past medical history; only one in seven cases of lung cancer occur in people who have never smoked, and the presence of chronic obstructive pulmonary disease doubles the risk independent of smoking history. 32 A previous history of head and neck, bladder and renal cancers and other factors such as exposure to asbestos or living in high radon exposure areas are all important in lung cancer risk assessment. Family history produces an excess of risk and should be included in risk assessment—as should the symptom of fatigue, a common feature of lung cancer. Cancer decision support tools such as the ‘Caper’ instrument or ‘Q cancer’ have emerged in recent years in the UK, enabling GPs to make assessments of cancer risk based on presenting symptoms; 33 , 34 they have been incorporated into clinical systems in primary care with mixed results.
Beyond these symptom-based models, a number of lung cancer risk models have been developed based on validated epidemiological criteria—for example, the Liverpool Lung Project (LLP) risk model 35 ( www.MyLungRisk.org ), which was subsequently used in the UK Lung Cancer Screening Trial. 36 The LLP v2 risk model has also been used in the Liverpool Healthy Lung project, 37 which has accommodated the risk model within primary care practice and produced risk assessments that are useful in clinical decision making is now running into its third year. The Manchester lung cancer pilot study 38 has used the PLCO 2012 risk prediction model 39 and the recent Yorkshire Lung cancer screening trial 40 is using both the LLP v2 and the PLCO 2012 risk models. Models such as these provide a systematic way of assessing lung cancer risk, taking into account a range of factors, including smoking duration, previous respiratory disease, family history of lung cancer, age, previous history of malignancy and asbestos exposure.
Risk stratification in primary care is clearly a key priority. We need to look at instruments such as the LLP model and identify ways that lung cancer risk stratification can be made easy and convenient in primary care. At present, it is not possible to recommend a specific risk assessment tool for use in primary care; current ongoing research in primary care is externally validating existing tools and will compare their efficacy. 41 Acceptability and feasibility also need to be examined; complex algorithms that place extra burden on practitioners are unlikely to succeed. However, we do need to ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC. We also need a better understanding of ways to maximise benefits of these models—while minimising potential harms such as over-medicalisation, anxiety and false reassurance. 42 Machine learning or neuro-linguistic programming, whereby data from multiple practice-based and external sources might be examined to develop risk estimates, are also likely to play a significant role in the future. 43
Diagnostic pathways
Early diagnosis lung cancer clinics based on multi-disciplinary teams (MDTs) are an ideal option for expediting diagnosis—ideally with an urgent (2-week wait) referral; 44 there is good evidence that these specialist MDT clinics are associated with improved outcomes. Another important consideration is involving the whole primary care team and including other practitioners such as pharmacists who see a lot of patients with, for example, repeat purchases of cough medicine. There has been a push to change referral practices in some parts of the UK—for example, to lower the threshold that PCPs refer for chest X-ray 45 and to encourage practitioners to repeat the investigation after a few months if symptoms persist; critically a normal chest X-ray does not exclude diagnosis of lung cancer. One highly successful programme in Leeds included the option for people to self-refer for chest X-rays in walk-in clinics 19 —a crucial element was the engagement of primary care in the design and implementation of the programme.
Diagnostic pathways have been closely examined and tested over recent years, an example being CRUK’s ACE programme (accelerate, coordinate and evaluate) initiated in June 2014 in England and Wales. 23 Patients often have complex pathways that can lead to delays; important initiatives in the ACE programme and elsewhere include risk-stratified computed tomographic (CT) screening criteria for ‘straight to CT’ referrals following normal chest X-rays and a focus on diagnostic paths for patients with vague symptoms.
Work needs to continue on diagnostic pathways that might expedite lung cancer diagnosis. It is important, for example, that we get more evidence on the impact or potential impact of direct access to investigations such as spiral CT from primary care—at present, there is not sufficient evidence or resource to universally implement this strategy, and there is evidence that delays can occur in primary care (for example, through ordering too many chest X-rays. 46 Nevertheless, GPs in the UK often indicate that direct access to investigations would help streamline diagnosis. 7
Lung cancer screening
A major challenge for primary care is the lack of symptoms in very early stage lung cancer, highlighting the importance of examining the potential of screening. The US National Lung Cancer Screening Trial, which used low-dose CT scanning in high-risk patients, showed a 20% reduction in lung cancer-specific mortality and almost a 7% reduction in all-cause mortality—and the US Preventive Task Force on Lung cancer Screening recommended that lung cancer screening should be implemented in high-risk populations. 47 , 48 Accordingly, Medicare agreed to pay for lung cancer screening within certain criteria—however, the current uptake in the US is only ~2% of high-risk individuals.
The recent report on the NELSON trial at the World Lung Cancer Conference, Toronto 49 has demonstrated an encouragingly low rate of false positives and a mortality benefit of 26% in men and between 39% and 61% in women—depending on the number of years of follow-up (i.e. 8–10 years). These results provide further impetus for the introduction of spiral CT scanning for individuals at high risk of cancer in the UK. Figure Figure2 2 illustrates the process for identifying an appropriate screening population, recruiting them and implementing screening—in many ways more complex than existing cancer screening programmes where recruitment is based principally on age and gender.

Levels of evidence for the implementation of lung cancer screening in Europe. The colour codes refer to the current status March 2019; traffic lights: green—ready, amber—borderline evidence. Underlined text indicates particular relevance for primary care 53
If we are, indeed, on the cusp of a new screening programme, there are important implications for primary care; the key issue in lung cancer screening is identifying the right patients to invite. This is a task that would involve primary care which currently lacks the systems and the processes to undertake the kind of population- based lung cancer risk assessment required. It is important, therefore, that we plan for an era where high-risk patients are screened for lung cancer (implemented, ideally, in tandem with smoking cessation programmes). We should be refining current strategies to risk stratify patients in primary care in preparation for this new era. 50 , 51 Screening alone, however, is not the total answer and a high level of awareness in both the public and the primary care community will remain vital elements in what needs to be a multi-pronged approach. 52
Conclusions and recommendations
Mortality rates for lung cancer remain stubbornly high; if we are to improve lung cancer outcomes, it is important that early diagnosis and screening efforts achieve their maximum potential. We need to:
- identify ways of raising awareness of symptoms potentially associated with lung cancer in ways that encourage people at higher risk to come forward—this will require refinement of the messages delivered in awareness-raising strategies
- counter the nihilistic beliefs often associated with lung cancer—early diagnosis CAN lead to improved outcomes
- continually strive to improve the primary care response to patients with symptoms of lung cancer, supported by better diagnostic pathways and risk-based decision support
- identify ‘fail-safe’ mechanisms by which patients advised to ‘watch and wait’ are not lost to follow-up; it is vital that patients understand these safety netting and follow-up advice
- ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC
- refine methods to implement lung cancer risk assessment model approaches; this is key to improving diagnosis of early lung cancer—and we should aim for risk estimates that can be readily incorporated into the various kinds of practice software used in primary care practices
- continue to improve diagnostic pathways; at present, many different models are being evaluated, including those which give primary care more direct access to investigations such as spiral CT. The key task will be implementation and appropriate support once the best models are determined
- fully engage primary care with the likely implementation of spiral CT lung cancer screening in the next few years—this will require the best possible risk-stratification approaches to ensure screening is directed at those who stand to benefit the most from it. It is vital that primary care rises to this challenge
Primary care needs to play a central role in efforts to diagnose lung cancer earlier, if there is to be an improvement in lung cancer outcomes in the years ahead. Research over the past decade gives us a much clearer idea of what needs to be done in refining primary care-based strategies; with adequate commitment and resources primary care will, in conjunction with other health care sectors, help reduce the burden from this disease.
Author contributions
D.P.W. led on literature searching and draft manuscript preparation. J.K.F. and M.D.P. provided input to early drafts and added text in their areas of expertise.
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
The Most Common Types of Lung Cancer
These vary by age, sex, and smoking status
- Lung Cancer Types
- Men vs. Women
- Age Differences
- Smoking Status
It's easy to think that lung cancer is one diagnosis with one possible outcome. The truth is that there are several types of lung cancer with key differences in what causes them. There are also differences in how each type of lung cancer develops in the body and how it is treated.
Most lung cancer diagnoses fall into a few types and subtypes. However, all people with lung cancer have unique experiences, even if they have the same disease.
This article will help you learn more about these types of lung cancer and how they might vary based on age, sex, smoking status, and other factors.
Main Types of Lung Cancer
Primary lung cancers are those that start in the lungs rather than spreading ( metastasizing ) to the lungs from somewhere else in the body.
There are two main types of primary lung cancers:
- Non-small cell lung cancer (NSCLC) is the most common type of lung cancer overall. It accounts for 80% to 85% of lung cancers in the United States.
- Small cell lung cancers (SCLC) are diagnosed in 10% to 15% of lung cancer cases.
A third type, called a carcinoid tumor , is less common in the lungs. It accounts for just 1% to 2% of lung cancers.
NSCLC and SCLC are also broken down into specific subtypes. The names are based on the kinds of cells that make up the tumors when they're seen under a microscope.
Non-Small Cell Lung Cancer
NSCLC typically grows and spreads more slowly than SCLC. Both are linked with smoking, but NSCLC is also the most common type of lung cancer in younger people and in people who have never smoked.
The risks and causes may depend on what subtype of NSCLC is diagnosed. There are three main subtypes:
- Lung adenocarcinoma accounts for 40% to 50% percent of NSCLC cases.
- Squamous cell carcinoma occurs in some 20% to 30% of all NSCLC cases.
- Large cell lung cancer is responsible in about 3% to 10% of all NSCLC cases.
Small Cell Lung Cancer
SCLC is typically an aggressive, fast-growing cancer.
It is strongly linked with smoking, although other factors including radon exposure may be involved. ( Radon is an odorless, colorless gas that seeps into homes from the soil around it.)
Two subtypes of SCLC are:
- Small cell carcinoma
- Combined small cell carcinoma (cells mixed with another type)
The two main types of lung cancer are non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC, the most common one, has three main subtypes.
Differences Based on Sex
Men continue to have higher incidence rates of lung cancer than women; however, the gap is closing largely due to health policy changes and smoking cessation.
NSCLC is a common lung cancer diagnosis in both men and women, with the adenocarcinoma subtype being the most prevalent. However, researchers describe lung adenocarcinomas as "a different disease in women and men."
Among women , lung adenocarcinomas account for 40% to 60% of their lung cancer cases. Roughly half of these cancers in women are related to smoking, compared to 85% of the cases in men who are smokers. Women also tend to be younger at diagnosis and respond differently to treatments.
Men with NSCLC are more likely to develop squamous cell lung cancer, which is linked with smoking. However, they are less likely to be diagnosed with SCLC than women.
Differences Based on Age
Lung cancer, as with many diseases, is generally more common in older people, with the average age of diagnosis being 70. Only 5% of all cancers are diagnosed in young adults ages 18-39.
Lung adenocarcinoma is the most common type of lung cancer in young adults, accounting for nearly 50% of cases. Carcinoid lung tumors tend to be found most frequently in people aged 45-55, but can be diagnosed at any age, including in children and adolescents. On the other hand, SCLC is relatively rare in young people, seen in less than 5% of lung cancer cases.
Young adults with lung cancer are much more likely to have a genetic factor that contributes to their lung cancer. For this reason, they may have genetic changes that may be treated with newer therapies that target specific mutations.
Smokers vs. Non-Smokers
In many ways, lung cancer in non-smokers is quite different from lung cancer in people who smoke. This applies to even the most common types of the disease.
Both NSCLC and SCLC are linked with a history of smoking , though SCLC's association is much stronger.
Of the three NSCLC subtypes, lung adenocarcinoma is the one most likely to be found in people who never smoked (50% to 60%). Around 10% to 20% of people who never smoked are diagnosed with squamous cell carcinoma. Only 6% to 8% of people who never smoked are diagnosed with SCLC.
Carcinoid tumors do not appear to be associated with smoking. They are found in smokers and non-smokers in numbers that are similar to those seen in the general population.
Current and former smokers develop SCLC and the squamous cell subtype of NSCLC more often than other people. The lung adenocarcinoma subtype of NSCLC is seen more often in women, younger people, and those who have never smoked.
Rates and the Role of Genetics
Lung cancer rates have shifted over time. That's partly because people began to quit or avoid smoking because of the health impacts.
One of the biggest questions, though, is why lung cancer rates have climbed in younger people and those who have never smoked.
Environmental factors like air pollution may be part of the reason, but that doesn't explain many of the cases. Research led by the National Cancer Institute and National Institutes of Health points to genetics.
Their September 2021 study looked at changes in the genes of 232 people with NSCLC diagnoses who never smoked, comparing their normal tissue with tumor samples.
Of the study group, 189 had lung adenocarcinomas while the rest were other types. The study found that gene mutations from natural processes inside the body were associated with the lung cancers.
The researchers also reported three new genetic subtypes of lung cancer in these never-smokers.
While they caution that more research is needed, the findings point to the possibility of future treatment targets.
Targetable Mutations in Lung Cancer
Science continues to delve deeper into the genetic links to lung and other cancers. Still, many healthcare providers and cancer specialists (oncologists) recommend genetic testing for people with cancer.
That's especially true for people with NSCLC lung cancer. Targeted therapies are available or in development for people with specific cancer-related changes (mutations) in genes, including:
Tumors that have treatable mutations are more commonly found in young adults, never-smokers, and women. However, many other people with lung cancer may benefit from targeted therapies as well.
Smoking is a main, but not the only, cause of lung cancer. Genetics may play a key role, especially in younger people, women, and never-smokers. Genetic testing may be recommended to see if you have a mutation for which there is an available treatment.
There are several types and subtypes of lung cancer, most of which are forms of either non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC). There are differences among these types.
One of the most important may be that smoking remains a top reason for some lung cancers, and is associated with both NSCLC and SCLC types, but many other lung cancers are diagnosed in people who have never smoked.
Radon and other environmental causes may contribute to these cancers. In some cases, particularly among younger people, there may be an underlying genetic reason.
A Word From Verywell
NSCLC tends to grow more slowly than small cell lung cancer and have a better prognosis . That said, long-term survival from any form of lung cancer is higher when found in the earlier stages of the disease .
Lung cancer screening is recommended for people ages 50 to 80 with at least a 20 pack-year history of smoking , and who smoked or quit smoking in the past 15 years.
If you've never smoked, you may still want to ask about screening. A 2019 study in the Journal of Thoracic Oncology found that low-dose computerized tomography (CT) imaging helped find early-stage cancers that would have otherwise been missed in both smokers and never-smokers.
American Cancer Society. Key statistics for lung cancer .
American Cancer Society. Key statistics for lung carcinoid tumor .
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: Epidemiology, etiology, and prevention . Clinics in Chest Medicine . 2011;32(4):605-644. doi:10.1016/j.ccm.2011.09.001
Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities . Clinical Cancer Research . 2012;18(9):2443-2451. doi:10.1158/1078-0432.CCR-11-2370
National Organization of Rare Diseases. Small cell lung cancer .
United States Environmental Protection Agency. Health risks of radon .
National Cancer Institute. Small cell lung cancer treatment (PDQ®)–patient version .
American Lung Association. Lung cancer prevalence and incidence .
North CM, Christiani DC. Women and lung cancer: What is new? . Semin Thorac Cardiovasc Surg . 2013;25(2):87-94. doi:10.1053%2Fj.semtcvs.2013.05.002
Rodriguez-Lara V, Avila-Costa MR. An overview of lung cancer in women and the impact of estrogen in lung carcinogenesis and lung cancer treatment . Front Med . 2021;8:600121. doi:10.3389/fmed.2021.600121
Bray F, Tyczynski JE, Parkin DM. Going up or coming down? The changing phases of the lung cancer epidemic from 1967 to 1999 in the 15 European Union countries . Eur J Cancer 2004;40:96-125. doi:10.1016/j.ejca.2003.08.005
Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer—relative risk estimates for the major histological types from a pooled analysis of case-control studies . Int J Cancer . 2012;131(5):1210-1219. doi:10.1002/ijc.27339
Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women . Lung Cancer (Auckl) . 2012;3:79-89. doi:10.2147/LCTT.S37319
American Cancer Society. Types of cancers that develop in young adults .
Liu B, Quan X, Xu C, et al. Lung cancer in young adults aged 35 years or younger: A full-scale analysis and review . J Cancer . 2019;10(15):3553-3559. doi:10.7150%2Fjca.27490
Rich AL, Khakwani A, Free CM, et al. Non-small cell lung cancer in young adults: Presentation and survival in the English National Lung Cancer Audit . QJM . 2015;108(11):891-897. doi:10.1093/qjmed/hcv052
American Cancer Society. Risk factors for lung carcinoid tumors .
Kozielski J, Kaczmarczyk G, Porębska I, Szmygin-Milanowska K, Gołecki M. Lung cancer in patients under the age of 40 years . Contemporary Oncology . 2012;5:413-415. doi:10.5114%2Fwo.2012.31770
Lee MH, Qureshi MM, Suzuki K, Everett P, Tapan U, Mak KS. Small cell lung cancer in young patients: Trends in sociodemographic factors, diagnosis, treatment, and survival . Journal of Thoracic Disease . 2022;14(8).
Centers for Disease Control and Prevention. Lung cancer among people who never smoked .
Zhang T, Joubert P, Ansari-Pour N, et al. Genomic and evolutionary classification of lung cancer in never smokers . Nat Genet . 2021;53(9):1348-1359. doi:10.1038/s41588-021-00920-0
American Cancer Society. Targeted drug therapy for non-small cell lung cancer .
United States Preventive Services Taskforce. Lung cancer: Screening .
Kang HR, Cho JY, Lee SH, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers . Journal of Thoracic Oncology . 2019;14(3):436-444. doi:10.1016/j.jtho.2018.11.002
National Cancer Institute. Non-Small Cell Lung Cancer Treatment (PDQ) – Health Professional Version .
National Cancer Institute. Small Cell Lung Cancer Treatment (PDQ) – Health Professional Version .
By Lynne Eldridge, MD Lynne Eldrige, MD, is a lung cancer physician, patient advocate, and award-winning author of "Avoiding Cancer One Day at a Time."

Lung cancer
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Frank Gaillard had no recorded disclosures.
At the time the article was last revised Calum Worsley had no financial relationships to ineligible companies to disclose.
- Bronchogenic cancer
- Lung cancer: general
- Primary lung cancer
- Bronchogenic malignancy
- Primary lung malignancy
- Bronchogenic carcinoma
- Lung cancer: overview
- Primary lung cancers
- Primary lung carcinoma
Primary lung cancer is a broad term referring to the main histological subtypes of primary lung malignancies that are mainly linked with inhaled carcinogens, with tobacco smoke being a key risk factor.
This article will broadly discuss all the histological subtypes as a group, focussing on their common aspects; for further details please refer to the specific articles on each subtype described below.
On this page:
Terminology, epidemiology, clinical presentation, treatment and prognosis, differential diagnosis.
- Related articles
- Cases and figures
- Imaging differential diagnosis
Bronchogenic carcinoma is a term that is frequently used as a synonym for lung cancer although is not strictly accurate, in that lung cancers can arise from the lung parenchyma and not just the airways 17 .
Lung cancer is a leading type of cancer, equal in prevalence to breast cancer 13 . It is the leading cause of cancer mortality worldwide; accounting for ~20% of all cancer deaths 1 .
Risk factors
The major risk factor is tobacco smoking, which is implicated in 90% of cases and increases the risk of lung cancer (by histological subtype) 10 :
squamous cell lung cancer: 11x (men), 15x (women)
small cell lung cancer: 10x (men), 25x (women)
large cell lung cancer: 7x (men), 8x (women)
lung adenocarcinoma: 4x (men and women)
Other risk factors:
asbestos : 5x increased risk
occupational exposure: uranium, radon, arsenic, chromium
diffuse lung fibrosis: 10x increased risk
chronic obstructive pulmonary disease
Associations
Various paraneoplastic syndromes can arise in the setting of lung cancer:
endocrine/metabolic
SIADH causing hyponatremia: small-cell subtype
ACTH secretion ( Cushing syndrome ): carcinoid and small-cell subtypes
carcinoid syndrome
gynecomastia
adrenal insufficiency (Addison disease): from bilateral metastases 7
hyperparathyroidism : NSCLC can produce parathyroid hormone (extremely rare) 8
hypocalcemia: occurs in the setting of skeletal metastases; especially associated with NSCLC 6
PTH-related peptide (PTHrp) causing hypercalcemia: squamous cell carcinoma
neurological
polyneuropathy
limbic encephalitis : particularly associated with SCLC 9
cerebellar degeneration
Lambert-Eaton myasthenia syndrome
finger clubbing
hypertrophic pulmonary osteoarthropathy (HPOA) : non-small cell 18
nephrotic syndrome
polymyositis 3
dermatomyositis 3
eosinophilia
acanthosis nigricans
thrombophlebitis : adenocarcinoma subtype
Patients with lung cancer may be asymptomatic in up to 50% of cases. Cough and dyspnea are rather non-specific symptoms that are common amongst those with lung cancer.
Central tumors may result in hemoptysis and peripheral lesions with pleuritic chest pain.
Pneumonia, pleural effusion, wheeze, lymphadenopathy are not uncommon. Other symptoms may be secondary to metastases (bone, contralateral lung, brain, adrenal glands, and liver, in frequency order for NSCLC 12 ) or paraneoplastic syndromes .
The term bronchogenic carcinoma is somewhat loosely used to refer to primary malignancies of the lung that are associated with inhaled carcinogens 1 and includes four main histological subtypes. These are broadly divided into non-small cell carcinoma and small cell carcinoma as they differ clinically regarding presentation, treatment, and prognosis:
non-small cell lung cancer (NSCLC) (80%)
adenocarcinoma (35%)
most common cell type overall
most common in women
most common cell type in non-smokers but still most patients are smokers
squamous cell carcinoma (30%)
strongly associated with smoking
most common carcinoma to cavitate
poor prognosis
large-cell carcinoma (15%)
peripherally located
very large, usually >4 cm
small cell lung cancer (SCLC) (20%)
almost always in smokers
metastasizes early
most common primary lung malignancy to cause paraneoplastic syndromes and SVC obstruction
worst prognosis
Other malignant pulmonary neoplasms include lymphoma and sarcoma (rare).
Each subtype has different radiographic appearances, demographics, and prognoses:
squamous-cell carcinoma of the lung
adenocarcinoma of the lung
large cell carcinoma of the lung
small cell carcinoma of the lung
Several antibodies or markers from tissue samples may be useful in the diagnosis and prognostication of disease. These include
programmed death-ligand 1 (PD-L1)-targeted monoclonal antibodies
thyroid transcription factor 1 (TTF-1) : expressed in most lung cancer except squamous cell cancer
ROS1 mutation : 1-2% of NSCLC 15 ; more common in females 14
ALK mutation : 2-5% of NSCLC; more common in males, younger, light/never smokers, and more likely to be adenocarcinoma presenting with advanced disease 16 (see: main article )
according to the IASLC (International Association for the Study of Lung Cancer) 8 th edition lung cancer staging system
previously small cell and non-small cell lung cancers were staged differently, but since 2013 all lung cancers are staged the same way
Treatment and prognosis vary not only with stage but also with cell type. In general, surgery, chemotherapy, and radiotherapy are offered according to the stage , resectability, operability, and functional status. Targeted treatments depend on molecular testing, e.g. ALK mutated lung cancers can be treated with ALK-inhibitors (e.g. crizotinib) 16 .
Non-small cell carcinoma
operable disease (stage I to IIIA): surgery
unresectable disease: neoadjuvant chemotherapy, radiotherapy
advanced disease: palliative combined chemotherapy
prognosis (5-year survival rates) :
local (stage I): 55-67%
locally advanced (stages II-IIIA): 23-40%
advanced (stages IIIB and IV): 1-3%
Small-cell carcinoma
limited disease: chemoradiotherapy
extensive disease: palliative combined chemotherapy
prognosis : poor
limited: 5-year survival rate 15-25%
extensive: 2-year survival 20% (with palliative combined chemotherapy and supportive care)
fibrosing mediastinitis
pulmonary tuberculosis
venous collateral pathways - venous varices
CT guided thoracic biopsy
- lung cancer staging
Quiz questions
- 1. Rosado-de-Christenson M, Templeton P, Moran C. Bronchogenic Carcinoma: Radiologic-Pathologic Correlation. Radiographics. 1994;14(2):429-46; quiz 447. doi:10.1148/radiographics.14.2.8190965 - Pubmed
- 2. Fauci A, Braunwald E, Kasper D et al. Harrison's Principles of Internal Medicine, 17th Edition. (2008) ISBN: 0071466339 - Google Books
- 3. Kubo M, Ihn H, Yamane K et al. Serum KL-6 in Adult Patients with Polymyositis and Dermatomyositis. Rheumatology (Oxford). 2000;39(6):632-6. doi:10.1093/rheumatology/39.6.632 - Pubmed
- 4. Talley N, O'Connor S. Clinical Examination. (2005) ISBN: 9780729537629 - Google Books
- 5. Webb W, Higgins C. Thoracic Imaging. (2010) ISBN: 9781605479767 - Google Books
- 6. Body J, Bone H, de Boer R et al. Hypocalcaemia in Patients with Metastatic Bone Disease Treated with Denosumab. Eur J Cancer. 2015;51(13):1812-21. doi:10.1016/j.ejca.2015.05.016 - Pubmed
- 7. Carvalho F, Louro F, Zakout R. Adrenal Insufficiency in Metastatic Lung Cancer. World J Oncol. 2015;6(3):375-7. doi:10.14740/wjon890w - Pubmed
- 8. Suliburk J & Perrier N. Primary Hyperparathyroidism. Oncologist. 2007;12(6):644-53. doi:10.1634/theoncologist.12-6-644 - Pubmed
- 9. Alamowitch S, Graus F, Uchuya M, Reñé R, Bescansa E, Delattre J. Limbic Encephalitis and Small Cell Lung Cancer. Clinical and Immunological Features. Brain. 1997;120 ( Pt 6)(6):923-8. doi:10.1093/brain/120.6.923 - Pubmed
- 10. Khuder S. Effect of Cigarette Smoking on Major Histological Types of Lung Cancer: A Meta-Analysis. Lung Cancer. 2001;31(2-3):139-48. doi:10.1016/s0169-5002(00)00181-1
- 11. Christianson B, Gupta S, Vyas S, Spartz H, Keshavamurthy J. A Diagnostic Challenge: An Incidental Lung Nodule in a 48-Year-Old Nonsmoker. Lung India. 2018;35(3):251-5. doi:10.4103/lungindia.lungindia_212_17 - Pubmed
- 12. Tamura T, Kurishima K, Nakazawa K et al. Specific Organ Metastases and Survival in Metastatic Non-Small-Cell Lung Cancer. Mol Clin Oncol. 2015;3(1):217-21. doi:10.3892/mco.2014.410 - Pubmed
- 13. World Health Organisation. “Cancer.” Accessed February 8, 2019. https://www.who.int/news-room/fact-sheets/detail/cancer.
- 14. Zinsky R. Metaanalysis of ROS1-Positive Lung Cancer Cases. 111 Lung Cancer. 2016;48(suppl 60). doi:10.1183/13993003.congress-2016.pa2867
- 15. Lin J & Shaw A. Recent Advances in Targeting ROS1 in Lung Cancer. J Thorac Oncol. 2017;12(11):1611-25. doi:10.1016/j.jtho.2017.08.002 - Pubmed
- 16. Le T & Gerber D. ALK Alterations and Inhibition in Lung Cancer. Semin Cancer Biol. 2017;42:81-8. doi:10.1016/j.semcancer.2016.08.007 - Pubmed
- 17. National Cancer Institute [website]. Definition of bronchogenic carcinoma. U.S. Department of Health and Human Services. HTML . Accessed May 5, 2023.
- 18. Yap F, Skalski M, Patel D et al. Hypertrophic Osteoarthropathy: Clinical and Imaging Features. Radiographics. 2017;37(1):157-95. doi:10.1148/rg.2017160052 - Pubmed
Incoming Links
- Intussusception
- Neoplastic intracranial aneurysm
- Radiation-induced lung disease
- Linear atelectasis
- Finger in glove sign (lung)
- Dural metastases
- Pneumatocele
- Calcified pulmonary nodules
- Endobronchial metastases
- Posterior wall of bronchus intermedius
- Primary pulmonary synovial sarcoma
- Adenocarcinoma in situ, minimally invasive adenocarcinoma and invasive adenocarcinoma of lung
- Sclerosing mesenteritis
- Solitary pulmonary nodule
- Lymphangitic carcinomatosis
- Gynaecomastia
- Lung carcinomas of the salivary gland type
- HIV/AIDS (pulmonary and thoracic manifestations)
- Small cell carcinoma of the lung metastasizing to the liver
- Cystic squamous cell lung cancer
- Hepatisation of the lung
- Lung adenocarcinoma
- Pneumonectomy
- Recurrent laryngeal nerve palsy due to hilar lung cancer
- Primary sarcomatoid carcinoma of the lung
- Brain and skull metastases from lung carcinoma
- Lung cancer metastasis to adrenal gland
- Apical lung cancer
- Right upper lobe lung cancer obscured by the first rib
- Non-small cell lung carcinoma
- Esophagopleural fistula and bronchopleural fistulas from lung cancer
- Cerebellar metastasis
- Lung cancer metastatic to brain
- Lepidic-predominant adenocarcinoma of the lung
- Pulmonary blastoma
- Right paratracheal mass
- Question 2615
- Question 2614
- Question 2217
- Question 2214
- Question 2205
- Question 2198
- Question 1775
- Question 1491
- Question 1147
- Question 1129
- Question 1128
- Question 1127
- Question 1097
- Question 1031
- Question 867
- Question 449
Related articles: Chest
- lateral projection
- lateral decubitus
- congenital heart disease
- medical devices in the thorax
- evaluation of nasogastric tube position
- endobronchial intubation
- esophageal intubation
- Groshong catheter
- Hickman catheter
- pulmonary artery ( Swan-Ganz) catheter
- peripherally inserted central catheters
- differential of left paramediastinal catheter positions
- esophageal temperature probe
- tracheostomy tube
- pleural catheters
- cardiac conduction devices
- prosthetic heart valve
- review areas
- acute unilateral airspace opacification (differential)
- acute bilateral airspace opacification (differential)
- acute airspace opacification with lymphadenopathy (differential)
- chronic unilateral airspace opacification (differential)
- chronic bilateral airspace opacification (differential)
- right upper lobe consolidation
- right middle lobe consolidation
- right lower lobe consolidation
- left upper lobe consolidation
- left lower lobe consolidation
- resorptive (obstructive) atelectasis
- passive (relaxation) atelectasis
- compressive atelectasis
- cicatrisation atelectasis
- adhesive atelectasis
- gravity dependant atelectasis
- linear atelectasis
- segmental atelectasis
- subsegmental atelectasis
- round atelectasis
- osteophyte induced adjacent pulmonary atelectasis and fibrosis
- right upper lobe collapse
- right middle lobe collapse
- right lower lobe collapse
- left upper lobe collapse
- left lower lobe collapse
- adult chest x-ray in the exam setting
- pediatric chest x-ray in the exam setting
- neonatal chest x-ray in the exam setting
- aortic nipple
- right atrial enlargement
- left atrial enlargement
- right ventricular enlargement
- left ventricular enlargement
- cardiothoracic ratio
- cavoatrial junction
- moguls of the heart
- vascular pedicle
- apical zone
- tracheal bifurcation angle
- azygos fissure
- superior accessory fissure
- inferior accessory fissure
- left horizontal fissure
- vertical fissure line
- flattening of the diaphragm
- gastric bubble
- nipple marker
- anterior junction line
- posterior junction line
- right paratracheal stripe
- left paratracheal stripe
- posterior tracheal stripe/tracheo-esophageal stripe
- posterior wall of bronchus intermedius
- right paraspinal line
- left paraspinal line
- aortic-pulmonary stripe
- aortopulmonary window
- deviation of the azygo-esophageal recess
- increased retrosternal airspace
- obliteration of the retrosternal airspace
- retrotracheal airspace
- air bronchogram
- big rib sign
- coin lesion
- continuous diaphragm sign
- dense hilum sign
- double contour sign
- egg-on-a-string sign
- extrapleural sign
- finger in glove sign
- flat waist sign
- Fleischner sign
- ginkgo leaf sign
- Golden S sign
- Hampton hump
- haystack sign
- hilum convergence sign
- hilum overlay sign
- Hoffman-Rigler sign
- holly leaf sign
- incomplete border sign
- juxtaphrenic peak sign
- Kirklin sign
- medial stripe sign
- melting ice cube sign
- more black sign
- Naclerio V sign
- pericardial fat tag sign
- cervicothoracic sign
- thoracoabdominal sign
- snowman sign
- spinnaker sign
- steeple sign
- straight left heart border sign
- third mogul sign
- tram-track sign
- walking man sign
- water bottle sign
- Westermark sign
- HRCT terminology
- secondary pulmonary lobule
- ground-glass nodules
- calcified lung nodules
- centrilobular lung nodules
- random pulmonary nodules
- perifissural pulmonary nodules
- acute bronchitis
- chronic bronchitis
- small airways disease
- broncho-arterial ratio
- cystic fibrosis
- allergic bronchopulmonary aspergillosis
- Williams Campbell syndrome
- congenital tracheobronchomegaly (a.k.a. Mounier Kuhn syndrome )
- primary ciliary dyskinesia
- central bronchiectasis
- upper lobe bronchiectasis
- middle lobe bronchiectasis
- lower lobe bronchiectasis
- tracheobronchomalacia
- diffuse tracheal narrowing (differential)
- diffuse airway narrowing (differential)
- tracheal diverticulum
- bronchial diverticulum
- interstitial edema
- alveolar edema
- cardiogenic pulmonary edema
- non-cardiogenic pulmonary edema
- Anti-Jo-1 antibody-positive interstitial lung disease
- amiodarone lung
- bleomycin lung toxicity
- leflunomide-induced acute interstitial pneumonia
- methotrexate lung disease
- acute hypersensitivity pneumonitis
- subacute hypersensitivity pneumonitis
- chronic hypersensitivity pneumonitis
- bird fancier's lung: pigeon fancier's lung
- farmer's lung
- cheese workers' lung
- mushroom worker’s lung
- malt worker’s lung
- maple bark disease
- hot tub lung
- wine maker’s lung
- woodsman’s disease
- thatched roof lung
- tobacco grower’s lung
- potato riddler’s lung
- summer-type pneumonitis
- dry rot lung
- machine operator’s lung
- humidifier lung
- shower curtain disease
- furrier’s lung
- miller’s lung
- lycoperdonosis
- saxophone lung
- acute interstitial pneumonia (AIP)
- cryptogenic organizing pneumonia (COP)
- desquamative interstitial pneumonia (DIP)
- fibrotic non-specific interstitial pneumonia
- cellular non-specific interstitial pneumonia
- idiopathic pleuroparenchymal fibroelastosis
- lymphoid interstitial pneumonia (LIP)
- respiratory bronchiolitis–associated interstitial lung disease (RB-ILD)
- diagnostic HRCT criteria for UIP pattern - ATS/ERS/JRS/ALAT (2011)
- diagnostic HRCT criteria for UIP pattern - Fleischner society guideline (2018)
- asbestos-related diseases
- berylliosis
- progressive massive fibrosis
- domestically acquired particulate lung disease (hut lung)
- giant cell interstitial pneumonia
- mixed dust pneumoconiosis
- silico-asbestosis
- pulmonary siderosis
- pulmonary baritosis
- pulmonary stannosis
- adenocarcinoma in situ (AIS)
- atypical adenomatous hyperplasia
- minimally invasive adenocarcinoma
- lepidic predominant adenocarcinoma (formerly non-mucinous BAC)
- acinar predominant adenocarcinoma
- papillary predominant adenocarcinoma
- micropapillary predominant adenocarcinoma
- solid predominant with mucin production
- invasive mucinous adenocarcinoma (formerly mucinous BAC)
- colloid adenocarcinoma
- fetal adenocarcinoma
- enteric adenocarcinoma
- pseudocavitation
- lung cancer associated with cystic airspaces
- adenosquamous carcinoma
- large cell carcinoma
- pleomorphic carcinoma of the lung
- spindle cell carcinoma of the lung
- giant cell carcinoma of the lung
- carcinosarcoma of lung
- pulmonary blastoma
- squamous cell carcinoma
- mucoepidermoid carcinoma
- adenoid cystic carcinoma
- acinic cell carcinoma
- epithelial-myoepithelial carcinoma
- small-cell lung cancer
- bronchial carcinoid tumors
- peripheral pulmonary carcinoid tumors
- large cell neuroendocrine cell carcinoma of the lung
- squamous dysplasia of lung
- squamous cell carcinoma in situ (CIS) of lung
- atypical adenomatous hyperplasia (AAH)
- adenocarcinoma in situ (AIS)
- minimally invasive adenocarcinoma of the lung
- pulmonary tumourlet
- tumor spread through air spaces (STAS)
- presence of non-lepidic patterns such as acinar, papillary, solid, or micropapillary
- myofibroblastic stroma associated with invasive tumor cells
- pleural invasion
- vascular invasion
- Pancoast tumor
- calcifying fibrous pseudotumor of the lung
- clear cell tumor of the lung
- focal lymphoid hyperplasia of the lung
- pulmonary chondroma
- pulmonary mesenchymal cystic hamartoma
- pulmonary sclerosing pneumocytoma
- cannonball metastases ( mnemonic )
- cavitatory pulmonary metastases
- cystic pulmonary metastases
- Lung-RADS
- IASLC (International Association for the Study of Lung Cancer) 8th edition (current)
- IASLC (International Association for the Study of Lung Cancer) 7th edition (superseeded)
- 1996 AJCC-UICC Regional Lymph Node Classification for Lung Cancer Staging
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 40 years of smoking, she survived lung cancer thanks to new treatments

Yuki Noguchi

Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission. Denise Lee hide caption
Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission.
Denise Lee grew up in Detroit in the mid-1970s and went to an all-girls Catholic high school. She smoked her first cigarette at age 14 at school, where cigarettes were a popular way of trying to lose weight.
Instead, her nicotine addiction lasted four decades until she quit in her mid-50s.
"At some point it got up as high as 2.5 packs a day," Lee, 62, recalls.
Yet she didn't think about lung cancer risk — until she saw a billboard urging former smokers to get screened. Lee, a retired lawyer living in Fremont, Calif., used to drive past it on her way to work.
"The thing that caught my attention was the fact that it was an African American female on the front," she recalls.

Shots - Health News
The american cancer society says more people should get screened for lung cancer.
She eventually got the low-dose CT scan recommended for current and former smokers. When doctors found an early, but dangerous, tumor, Lee cried and panicked. Her mother had cared for her father, who'd died of prostate cancer. "My biggest concern was telling my mom," she says.
But that was six years ago, and Lee is cancer free today. Surgery removed the 2-inch tumor in her lung, then new treatments also boosted her immune system, fighting off any recurrence.
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year – more than breast, prostate and colon cancer combined – which is why many people still think of a diagnosis as synonymous with a death sentence. But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
"If you're gonna have lung cancer, now is a good time," Lee says of the advances that saved her.

Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective. Denise Lee hide caption
Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective.
The key breakthrough, says Robert Winn, a lung cancer specialist at Virginia Commonwealth University, is the ability to better pinpoint the mutations of a patient's particular form of cancer. In the past, treatments were blunt tools that caused lots of collateral damage to healthy parts of the body while treating cancer.
"We've gone from that to molecular characterization of your lung cancer, and it has been a game changer," Winn says. "This is where science and innovation has an impact."
One of those game-changing treatments is called targeted therapy . Scientists identify genetic biomarkers in the mutated cancer cells to target and then deliver drugs that attack those targets, shrinking tumors.

CRISPR gene-editing may boost cancer immunotherapy, new study finds
Another is immunotherapy, usually taken as a pill, which stimulates the body's own defense system to identify foreign cells, then uses the immune system's own power to fight the cancer as if it were a virus.
As scientists identify new cancer genes, they're creating an ever-broader array of these drugs.
Combined, these treatments have helped increase national survival rates by 22% in the past five years – a rapid improvement over a relatively short time, despite the fact that screening rates are very slow to increase. Winn says as these treatments get cheaper and readily available, the benefits are even reaching rural and Black populations with historic challenges accessing health care.
The most remarkable thing about the drugs is their ability to, in some cases, reverse late-stage cancers. Chi-Fu Jeffrey Yang, a thoracic surgeon at Massachusetts General Hospital and faculty at Harvard Medical School, recalls seeing scans where large dark shadows of tumor would disappear: "It was remarkable to see the lung cancer completely melting away."
To Yang, such progress feels personal. He lost his beloved grandfather to the disease when Yang was in college. If he were diagnosed today, he might still be alive.
"Helping to take care of him was a big reason why I wanted to be a doctor," Yang says.
But the work of combating lung cancer is far from over; further progress in lung cancer survival hinges largely on getting more people screened.
Low-dose CT scans are recommended annually for those over 50 who smoked the equivalent of a pack a day for 20 years. But nationally, only 4.5% of those eligible get those scans , compared to rates of more than 75% for mammograms.
Andrea McKee, a radiation oncologist and spokesperson for the American Lung Association, says part of the problem is that lung cancer is associated with the stigma of smoking. Patients often blame themselves for the disease, saying: "'I know I did this to myself. And so I don't I don't think I deserve to get screened.'"
McKee says that's a challenge unique to lung cancer. "And it just boggles my mind when I hear that, because, of course, nobody deserves to die of lung cancer."
Denise Lee acknowledges that fear. "I was afraid of what they would find," she admits. But she urges friends and family to get yearly scans, anyway.
"I'm just so grateful that my diagnosis was early because then I had options," she says. "I could have surgery, I could have chemotherapy, I could be a part of a clinical trial."
And all of that saved her life.
- lung cancer screening
- immunotherapy
- lung cancer

IMAGES
VIDEO
COMMENTS
Smoking is the most common cause of lung cancer. It is estimated that 90% of lung cancer cases are attributable to smoking. ... Most patients already have advanced disease at the time of presentation. Lung cancer symptoms occur due to local effects of the tumor, such as cough due to bronchial compression by the tumor due to distant metastasis ...
Lung cancer is the third most common cancer in the U.S. It's caused by harmful cells in your lungs growing unchecked. Treatments include surgery, chemotherapy, immunotherapy, radiation and targeted drugs. Screening is recommended if you're at high risk. Advances in treatments have caused a significant decline in lung cancer deaths in recent ...
One study noted that the most common symptoms at presentation were cough (55 percent), dyspnea (45 percent), pain (38 percent), and weight loss (36 percent) ( table 1) [ 3 ]. This discussion will present the clinical manifestations of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Screening and risk factors for lung ...
Signs and symptoms of lung cancer that happen in and around the lungs may include: A new cough that doesn't go away. Chest pain. Coughing up blood, even a small amount. Hoarseness. Shortness of breath. Wheezing. Signs and symptoms that happen when lung cancer spreads to other parts of the body may include: Bone pain.
Lung cancer can cause several symptoms that may indicate a problem in the lungs. The most common symptoms include: cough that does not go away. chest pain. shortness of breath. coughing up blood (haemoptysis) fatigue. weight loss with no known cause. lung infections that keep coming back.
Presentation, initial evaluation, and prognosis of malignant pleural mesothelioma. View in. Solitary fibrous tumor. View in. Systemic treatment for unresectable malignant pleural mesothelioma ... Systemic therapy for advanced non-small cell lung cancer with an activating mutation in the epidermal growth factor receptor. View in
Lung cancer remains the second most common cancer diagnosis, and top cancer killer, in the United States. Early diagnosis is key for patient survival, but only 16% of cases are currently caught in the early stages. The primary care provider is uniquely poised to intervene with high-risk patients through careful monitoring and screening of select patients. This article includes discussion of ...
Lung cancer poses a significant public health burden around the world; it is the most common cause of cancer mortality in the UK and it accounts for >20% of cancer deaths. 1 There is significant ...
As a result, the 20th century also experienced significant increases in mortality from lung cancer becoming the leading cancer death for men in the 1950s, and remains the leading cause for men and women today. 1 Lung cancer is the second most common cancer diagnosis in both sexes, causing 25.9% of cancer deaths, with only 18.1% of patients ...
Lung cancer remains the second most common cancer diagnosis, and top cancer killer, in the United States. Early diagnosis is key for patient survival, but only 16% of cases ... PRESENTATION Lung cancer primarily affects those between the ages of 55 and 84, with the median age at diagnosis around 70. Lung cancer is more common in men
Statistics. In 2021, it's estimated there were 235,760 new cases of lung cancer, with an estimated 131,880 deaths from the disease. Once much more common in men, the rate has increased in women over the past few decades and now occurs at a rate of 60.1 cases/100,000 men and 47.9 cases/100,000 women. The median age at diagnosis is 71, with over ...
Patient Characteristics and Clinical Presentation. Lung cancer primarily affects those between the ages of 55 and 84, with the median age at diagnosis around 70. Lung cancer is more common in men than women and disproportionately affects African American males. 1 The overwhelming majority (85% to 90%) of patients diagnosed with lung cancer have ...
Cancer is a disease in which cells in the body grow out of control. When cancer starts in the lungs, it is called lung cancer. Lung cancer begins in the lungs and may spread to lymph nodes or other organs in the body, such as the brain. Cancer from other organs also may spread to the lungs. When cancer cells spread from one organ to another ...
Lung cancer is the most common disease associated with the development of malignant pleural effusion. Although it is evident in all histopathological types, pleural effusion is most commonly seen with adenocarcinoma, which tends to localize in the lung periphery. In the course of the disease, the frequency of developing effusion is 7-23% . In ...
Abstract. In the absence of screening, most patients with lung cancer are not diagnosed until later stages, when the prognosis is poor. The most common symptoms are cough and dyspnea, but the most specific symptom is hemoptysis. Digital clubbing, though rare, is highly predictive of lung cancer. Symptoms can be caused by the local tumor ...
Lung cancer is a heterogenous disease with wide-ranging clinicopathological features. 17. Lung cancer is classified broadly as NSCLC (85% of total diagnoses) or SCLC (15% of total diagnoses). Within NSCLC classifications, adenocarcinomas are the most common subtype of lung cancer, 18. followed by squamous-cell carcinomas ( figure 1 ).
The most common symptoms include cough, dyspnea, and hemoptysis. Although the clinical presentation of lung cancer is not specific to the classification or histology of the cancer, certain obstacles may be more likely with different types. One study noted that the most common symptoms at presentation were cough (55%), dyspnea (45%), pain (38% ...
Introduction. Lung cancer poses a significant public health burden around the world; it is the most common cause of cancer mortality in the UK and it accounts for >20% of cancer deaths. 1 There is significant variation in survival rates around the world and this has been largely attributed to the stage at which the cancer is diagnosed. 2 The International Cancer Benchmarking Partnership has ...
There are two main types of primary lung cancers: Non-small cell lung cancer (NSCLC) is the most common type of lung cancer overall. It accounts for 80% to 85% of lung cancers in the United States. Small cell lung cancers (SCLC) are diagnosed in 10% to 15% of lung cancer cases. A third type, called a carcinoid tumor , is less common in the lungs.
Clinical presentation. Patients with lung cancer may be asymptomatic in up to 50% of cases. Cough and dyspnea are rather non-specific symptoms that are common amongst those with lung cancer. Central tumors may result in hemoptysis and peripheral lesions with pleuritic chest pain. Pneumonia, pleural effusion, wheeze, lymphadenopathy are not ...
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year - more than breast, prostate and colon cancer combined - which is why many people still think of ...