An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
2024 Alzheimer's disease facts and figures
- PMID: 38689398
- PMCID: PMC11095490
- DOI: 10.1002/alz.13809
This article describes the public health impact of Alzheimer's disease (AD), including prevalence and incidence, mortality and morbidity, use and costs of care and the ramifications of AD for family caregivers, the dementia workforce and society. The Special Report discusses the larger health care system for older adults with cognitive issues, focusing on the role of caregivers and non-physician health care professionals. An estimated 6.9 million Americans age 65 and older are living with Alzheimer's dementia today. This number could grow to 13.8 million by 2060, barring the development of medical breakthroughs to prevent or cure AD. Official AD death certificates recorded 119,399 deaths from AD in 2021. In 2020 and 2021, when COVID-19 entered the ranks of the top ten causes of death, Alzheimer's was the seventh-leading cause of death in the United States. Official counts for more recent years are still being compiled. Alzheimer's remains the fifth-leading cause of death among Americans age 65 and older. Between 2000 and 2021, deaths from stroke, heart disease and HIV decreased, whereas reported deaths from AD increased more than 140%. More than 11 million family members and other unpaid caregivers provided an estimated 18.4 billion hours of care to people with Alzheimer's or other dementias in 2023. These figures reflect a decline in the number of caregivers compared with a decade earlier, as well as an increase in the amount of care provided by each remaining caregiver. Unpaid dementia caregiving was valued at $346.6 billion in 2023. Its costs, however, extend to unpaid caregivers' increased risk for emotional distress and negative mental and physical health outcomes. Members of the paid health care and broader community-based workforce are involved in diagnosing, treating and caring for people with dementia. However, the United States faces growing shortages across different segments of the dementia care workforce due to a combination of factors, including the absolute increase in the number of people living with dementia. Therefore, targeted programs and care delivery models will be needed to attract, better train and effectively deploy health care and community-based workers to provide dementia care. Average per-person Medicare payments for services to beneficiaries age 65 and older with AD or other dementias are almost three times as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 22 times as great. Total payments in 2024 for health care, long-term care and hospice services for people age 65 and older with dementia are estimated to be $360 billion. The Special Report investigates how caregivers of older adults with cognitive issues interact with the health care system and examines the role non-physician health care professionals play in facilitating clinical care and access to community-based services and supports. It includes surveys of caregivers and health care workers, focusing on their experiences, challenges, awareness and perceptions of dementia care navigation.
Keywords: Alzheimer's dementia; Alzheimer's disease; Biomarkers; COVID‐19; Care navigation; Care navigator; Caregivers; Dementia; Dementia care navigation; Dementia workforce; Diagnostic criteria; Family caregiver; Health care costs; Health care expenditures; Health care professional; Health care utilization; Home and community‐based services; Incidence; Long‐term care costs; Long‐term care utilization; MCI due to Alzheimer's disease; Medicaid spending; Medicare spending; Mild cognitive impairment; Morbidity; Mortality; Navigator; Prevalence; Primary care physician; Risk factors.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
PubMed Disclaimer
Alzheimer's disease (AD) continuum. Although…
Alzheimer's disease (AD) continuum. Although these arrows are of equal size, the components…
Number and ages of people…
Number and ages of people 65 or older with Alzheimer's dementia, 2024. Percentages…
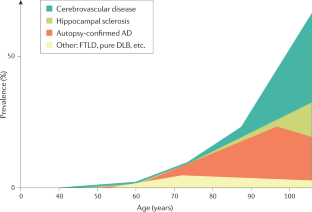
Prevalence of Alzheimer's disease in…
Prevalence of Alzheimer's disease in the 50 U.S. states, including the 10 counties…
Estimated lifetime risk for Alzheimer's…
Estimated lifetime risk for Alzheimer's dementia, by sex, at ages 45 and 65.…
Projected number of people age…
Projected number of people age 65 and older (total and by age) in…
Deaths due to Alzheimer's and…
Deaths due to Alzheimer's and other dementias in the United States in 2020…
Percentage changes in selected causes…
Percentage changes in selected causes of death (all ages) between 2000 and 2021.…
U.S. annual Alzheimer's death rate…
U.S. annual Alzheimer's death rate (per 100,000 people) by year. Created from data…
Proportion of caregivers of people…
Proportion of caregivers of people with Alzheimer's or other dementias versus caregivers of…
Percentage of caregivers who report…
Percentage of caregivers who report high to very high stress due to caregiving.…
Work‐related changes among caregivers of…
Work‐related changes among caregivers of people with Alzheimer's or other dementias who had…
Person‐centered care delivery. Created from…
Person‐centered care delivery. Created from data from the Alzheimer's Association.
Distribution of aggregate costs of…
Distribution of aggregate costs of care by payment source for Americans age 65…
Percentage changes in emergency department…
Percentage changes in emergency department visits per 1,000 fee‐for‐service Medicare beneficiaries with selected…
Place of death due to…
Place of death due to Alzheimer's disease, 2002 to 2021. Created from data…
Hospital stays per 1,000 Medicare…
Hospital stays per 1,000 Medicare beneficiaries age 65 and older with specified coexisting…
Today's dementia care.
Difficulties and stressors that caregivers…
Difficulties and stressors that caregivers experience. *Percentages of bars may not total the…
Stressors when getting health care…
Stressors when getting health care for care recipients.
Navigator's professional role.
Preferred channels for communicating with…
Preferred channels for communicating with a care navigator.
Access to care navigators with…
Access to care navigators with cultural competency by race/ethnicity of care recipient.
Health care professionals’ views on…
Health care professionals’ views on cultural competence.
Anticipated outcomes for care recipients…
Anticipated outcomes for care recipients of a navigator program.
Anticipated outcomes for caregivers of…
Anticipated outcomes for caregivers of a navigator program.
Care navigator services that would…
Care navigator services that would be valuable to dementia caregivers.
Community‐based services that would be…
Community‐based services that would be valuable to dementia caregivers.
Time spent on navigator‐type services…
Time spent on navigator‐type services and percentage of patients with cognitive issues receiving…
Focus of dementia care support…
Focus of dementia care support provided.
Services provided that support dementia…
Services provided that support dementia care for patients and their families.
Most valuable services in supporting…
Most valuable services in supporting dementia care for patients and their families.
Health care workers’ views on…
Health care workers’ views on the effectiveness of dementia care navigation.
Barriers to dementia care navigation.
Professionals who help dementia patients…
Professionals who help dementia patients and caregivers navigate health care.
The ideal future state of…
The ideal future state of dementia care.
Similar articles
- 2023 Alzheimer's disease facts and figures. [No authors listed] [No authors listed] Alzheimers Dement. 2023 Apr;19(4):1598-1695. doi: 10.1002/alz.13016. Epub 2023 Mar 14. Alzheimers Dement. 2023. PMID: 36918389
- 2022 Alzheimer's disease facts and figures. [No authors listed] [No authors listed] Alzheimers Dement. 2022 Apr;18(4):700-789. doi: 10.1002/alz.12638. Epub 2022 Mar 14. Alzheimers Dement. 2022. PMID: 35289055
- 2021 Alzheimer's disease facts and figures. [No authors listed] [No authors listed] Alzheimers Dement. 2021 Mar;17(3):327-406. doi: 10.1002/alz.12328. Epub 2021 Mar 23. Alzheimers Dement. 2021. PMID: 33756057
- Dementia -- Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review [Internet]. Swedish Council on Health Technology Assessment. Swedish Council on Health Technology Assessment. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. PMID: 28876770 Free Books & Documents. Review.
- Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Lin JS, O'Connor E, Rossom RC, Perdue LA, Burda BU, Thompson M, Eckstrom E. Lin JS, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. PMID: 24354019 Free Books & Documents. Review.
- A Review of the Current Status of Disease-Modifying Therapies and Prevention of Alzheimer's Disease. Parums DV. Parums DV. Med Sci Monit. 2024 May 13;30:e945091. doi: 10.12659/MSM.945091. Med Sci Monit. 2024. PMID: 38736218 Free PMC article. Review.
- Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid ß deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. - PubMed
- Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: A case‐control study. Lancet Neurol. 2012;11(2):1048‐1056. - PMC - PubMed
- Jack CR, Lowe VJ, Weigand SD, et al. Serial PiB and MRI in normal, mild cognitive impairment and Alzheimer's disease: Implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355‐1365. - PMC - PubMed
- Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795‐804. - PMC - PubMed
- Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: A longitudinal study. Lancet Neurol. 2018;17(3):241‐250. - PMC - PubMed
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Seven recent papers amplify advances in Alzheimer’s research
Alzheimer's Disease Biomarkers Dementias Neuroscience

AMP AD uses an open-science research model that makes all data and methods rapidly available to the research community at large through the data sharing infrastructure, the AD Knowledge Portal. Since the Portal’s launch in 2015, more than 3,000 researchers world wide from the academic, biotech, and pharmaceutical industry sectors have used the data resources for research on Alzheimer’s and related dementias.
Alzheimer’s is a complex disease, and as it slowly develops, many normal biological processes in the brain and the body go awry, from inflammation, to blood vessels damage and neuronal death. Seven recent AMP AD reports showcase research advances related to the discovery of new drug candidate targets, identification of molecular subtypes of the disease, and new potential biomarkers that can serve as the basis for a precision medicine approach to therapy development.
Identifying ATP6VA1 gene as a candidate target for treatment: Researchers at the Icahn School of Medicine at Mount Sinai in New York generated several types of molecular data from 364 brain donors at different stages of Alzheimer’s. Using network modeling, a way to show data and its relationships, the team identified large sets of genes associated with the disease. Among the thousands of molecular changes associated with Alzheimer’s, the expression of a set of neuronal genes (neuronal network) was the most disrupted. Their analyses identified ATP6VA1 as a master regulator gene of this neuronal network and demonstrated that increasing its expression genetically, or by using a pharmacologic agent, led to improving neuronal function in cultured cells and in flies. These findings were published in Neuron and pave the way for new drug discovery efforts targeting ATP6VA1 .
Finding and validating VGF gene as key regulator of Alzheimer’s: Another AMP AD study led by researchers at Icahn School of Medicine identified the VGF gene and protein as having a key role in protecting the brain against Alzheimer’s. This discovery was made possible by combining computational analyses that integrate large human Alzheimer’s molecular datasets, clinical features of Alzheimer’s, DNA variation, and data on gene- and protein expression with experimental studies in mouse models. The findings provide a new target for researchers seeking to develop drugs to treat or prevent Alzheimer’s. The report of the discovery of this gene as a key driver and its validation in mouse studies was published in Nature Communications .
Identifying different types of microglia associated with Alzheimer’s: An AMP AD research team at Columbia University conducted a study that measured the expression of genes in individual microglial cells purified from human brain samples obtained at autopsy and during neurosurgical procedures. This single cell profiling technology identified several molecular subtypes of microglia based on the pattern of gene expression. Follow-up validation studies in post mortem brain tissue showed that this microglia subtype was less abundant in Alzheimer's brains compared to control brains. These results, published in Nature Communications , will help design larger, more specific studies of the role of microglia subtypes in Alzheimer’s.
Using data to unfold and predict disease process: An AMP AD team led by researchers at Sage Bionetworks in Seattle used innovative computational approaches to make predictions about the sequence of molecular changes that lead to Alzheimer’s. The team used RNA sequencing data collected from a large collection of post-mortem tissue from Alzheimer’s and control brains. This modeling method, called the manifold learning method, predicted early-stage disease processes, such as RNA-splicing, mitochondrial function, and protein transport. Additionally, the method predicted several distinct molecular subtypes of late-onset Alzheimer’s. These predictions speak to the complex nature of the disease and the need to verify these observations in longitudinal studies where molecular signatures can be linked to different clinical features of the disease. These findings were published in Nature Communications .
Network modeling identifies molecular subtypes of Alzheimer’s: Using a large collection of human brain samples from different studies, a team led by researchers at Icahn School of Medicine also analyzed RNA sequencing data and identified three major molecular subtypes of Alzheimer’s. The subtypes, which are independent of age and disease stage, and are replicated across multiple brain regions, show how different combinations of biological pathways lead to brain degeneration. With further research and validation in larger groups, these molecular subtypes may help reveal how Alzheimer’s progresses and potential ways to slow or stop it. Their findings were published in Science Advances .
Identifying new biomarkers in spinal fluid: AMP AD researchers at Emory University identified groups of proteins (protein panels) associated with Alzheimer’s that could be identified in both brain and spinal fluid. These overlapping protein panels detected in the spinal fluid reflected changes in multiple biological process in the brain. The researchers found this by measuring 3,500 proteins in spinal fluid, and 12,000 proteins in a collection of postmortem brain samples, from patients with Alzheimer’s and cognitively normal study participants. The study also showed that these changes in the protein expression pattern were specific for Alzheimer’s. This work lays the foundation for the discovery of new fluid biomarkers for Alzheimer’s. These findings were published in Science Advances .
Investigating how being female may increase risk of Alzheimer’s: Duke University researchers and members of the Alzheimer’s Disease Metabolomic Consortium (ADMC) participating in the AMP AD program, analyzed the changes in the levels of 180 metabolites in the blood from more than 1,500 people who took part in the NIA-supported Alzheimer’s Disease Neuroimaging Initiative . The researchers reported that there are differences in a subset of blood metabolites associated with Alzheimer's based on sex and ApoE4 status. ApoE4 is the strongest Alzheimer's risk factor gene. Women with Alzheimer’s who carry the ApoE4 gene have a distinct metabolic pattern in blood. These metabolic changes suggest that females have a greater impairment of brain energy production than males. Dissecting metabolic differences in Alzheimer’s can identify specific pathways within specific patient subgroups and guide the way to personalized medicine.
The data and methods from the above studies are available and can be accessed by researchers across the world through the AD Knowledge Portal . The portal is the data repository for the AMP AD Target Discovery Program, and other NIA-supported team-science programs operating under open-science principles. Now in its sixth year, AMP AD is demonstrating the power of open science to enable the scientific community to investigate difficult scientific questions and jumpstart new drug discovery projects.
The AMP AD research teams are funded by NIA grants U01AG046152, U01AG046170, U01AG046139, U01AG046161, R01AG046171, R01AG046174, U19AG010483, U01AG042791, U01AG061357, U01AG061359, U01AG061835, and U24AG061340.
The studies outlined here were also supported by the following NIA grants (in order of appearance):
- ATP6VA1: NIA grants U01AG046170, RF1AG054014, RF1AG057440, R01AG057907, U01AG052411, R01AG062355, U01AG058635, and R01AG068030
- VGF: NIA grants U01AG046170, R01AG046170, RF1AG054014, RF1AG057440, R01AG057907, R01AG055501, U01AG046161, P50AG025688, 5R01AG053960, and 5R01AG062355
- Microglia: NIA grants U01AG046152, R01AG036836, R01AG048015, and RF1AG057473
- Disease process: NIA grants U54AG054345, RF1AG057443, P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, U01AG46152, P50AG016574, R01AG032990, U01AG046139, R01AG018023, U01AG006576, U01AG006786, R01 AG025711, R01AG017216, and R01AG003949
- Subtypes: U01AG046170, RF1AG054014, RF1AG057440, R01AG057907, U01AG052411, R01AG062355, U01AG058635, R01AG068030, P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, U01AG46152, U01AG52411, K01AG062683, and U01AG058635
- Spinal fluid biomarkers: NIA grants R01AG053960, R01AG057911, R01AG061800, RF1AG057471, RF1AG057470, R01AG061800, R01AG057911, R01AG057339, U01AG046161, and U01AG061357
- Female risk: NIA grants U01AG024904, P30AG10161, R01AG15819, R01AG17917, U01AG46152, U01AG61356, R01AG059093, R01AG046171, RF1AG051550, and U01AG024904, RF1AG058942, R01AG057452, R03AG054936, and RF1AG061872
These AMP AD activities relate to NIH’s AD+ADRD Research Implementation Milestone 2.A , “Create new research programs that use data-driven, systems-based approaches to integrate the study of fundamental biology of aging with neurobiology of aging and research on neurodegeneration, AD and AD-related dementias to better understand the mechanism(s) of vulnerability and resilience in AD across all levels of biologic complexity (from cellular to population level) and to gain a deeper understanding of the complex biology and integrative physiology of healthy and pathologic brain aging;” Milestone 9.B , "Accelerate the development of the next generation CNS imaging ligands and biofluid molecular signatures targeting a variety of disease processes (neuroinflammation, bioenergetic/metabolic compromise, oxidative stress, synaptic pathology) that can be used as research tools or developed into diagnostic, prognostic, theragnostic or target engagement biomarkers;" and Milestone 9.F , “Initiate studies to develop minimally invasive biomarkers for detection of cerebral amyloidosis, AD and AD-related dementias pathophysiology.”
References:
Wang M, et al. Transformative network modeling of multi-omics data reveals detailed circuits, key regulators, and potential therapeutics for Alzheimer's disease . Neuron . 2021;109(2):257-272.e14. doi:10.1016/j.neuron.2020.11.002.
Beckmann ND, et al. Multiscale causal networks identify VGF as a key regulator of Alzheimer's disease . Nature Communications. 2020;11(1): 3942. doi:10.1038/s41467-020-17405-z.
Olah M, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease . Nature Communications . 2020;11(1):6129. doi:10.1038/s41467-020-19737-2.
Mukherjee S, et al. Molecular estimation of neurodegeneration pseudotime in older brains . Nature Communications . 2020;11(1):5781. doi:10.1038/s41467-020-19622-y.
Neff RA, et al. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets . Science Advances . 2021;7(2):eabb5398. doi: 10.1126/sciadv.abb5398.
Higginbotham L, et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer's disease . Science Advances . 2020;6(43):eaaz9360. doi:10.1126/sciadv.aaz9360.
Arnold M, et al. Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome . Nature Communications . 2020;11(1): 1148. doi:10.1038/s41467-020-14959-w.
nia.nih.gov
An official website of the National Institutes of Health
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 May 2021
Alzheimer disease
- David S. Knopman ORCID: orcid.org/0000-0002-6544-066X 1 ,
- Helene Amieva 2 ,
- Ronald C. Petersen 1 ,
- Gäel Chételat 3 ,
- David M. Holtzman 4 ,
- Bradley T. Hyman ORCID: orcid.org/0000-0002-7959-9401 5 ,
- Ralph A. Nixon 6 , 7 &
- David T. Jones ORCID: orcid.org/0000-0002-4807-9833 1
Nature Reviews Disease Primers volume 7 , Article number: 33 ( 2021 ) Cite this article
45k Accesses
815 Citations
217 Altmetric
Metrics details
- Alzheimer's disease
- Diagnostic markers
- Translational research
Alzheimer disease (AD) is biologically defined by the presence of β-amyloid-containing plaques and tau-containing neurofibrillary tangles. AD is a genetic and sporadic neurodegenerative disease that causes an amnestic cognitive impairment in its prototypical presentation and non-amnestic cognitive impairment in its less common variants. AD is a common cause of cognitive impairment acquired in midlife and late-life but its clinical impact is modified by other neurodegenerative and cerebrovascular conditions. This Primer conceives of AD biology as the brain disorder that results from a complex interplay of loss of synaptic homeostasis and dysfunction in the highly interrelated endosomal/lysosomal clearance pathways in which the precursors, aggregated species and post-translationally modified products of Aβ and tau play important roles. Therapeutic endeavours are still struggling to find targets within this framework that substantially change the clinical course in persons with AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Is Alzheimer disease a disease?

The Amyloid-β Pathway in Alzheimer’s Disease

Emerging diagnostics and therapeutics for Alzheimer disease
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10 , 844–852 (2014).
PubMed PubMed Central Google Scholar
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256 , 183–194 (2004).
CAS PubMed Google Scholar
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 7 , 263–269 (2011).
Petersen, R. C. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology 91 , 395–402 (2018).
Nelson, P. T. et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121 , 571–587 (2011).
Boyle, P. A. et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann. Neurol. 83 , 74–83 (2018). A clinical-neuropathological analysis of >1,000 persons demonstrating how multiple aetiologies relate to late-life cognition.
CAS PubMed PubMed Central Google Scholar
Schneider, J. A., Arvanitakis, Z., Leurgans, S. E. & Bennett, D. A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 66 , 200–208 (2009).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134 , 171–186 (2017).
Karanth, S. et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 77 , 1299–1307 (2020).
Brodaty, H. et al. The world of dementia beyond 2020. J. Am. Geriatr. Soc. 59 , 923–927 (2011).
PubMed Google Scholar
Wu, Y. T. et al. The changing prevalence and incidence of dementia over time — current evidence. Nat. Rev. Neurol. 13 , 327–339 (2017).
Satizabal, C. L. et al. Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med. 374 , 523–532 (2016).
Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11 , 1006–1012 (2012).
Tom, S. E. et al. Trends in incident dementia and early life socieoeconomic status by birth cohort in the adult changes in thought study. JAMA Open 3 , e2011094 (2020).
Google Scholar
Kovari, E., Herrmann, F. R., Bouras, C. & Gold, G. Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology 82 , 326–331 (2014).
Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F. & Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia 32 , 523–532 (2017).
Hy, L. X. & Keller, D. M. Prevalence of AD among whites: a summary by levels of severity. Neurology 55 , 198–204 (2000).
Gillis, C., Mirzaei, F., Potashman, M., Ikram, M. A. & Maserejian, N. The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement. 11 , 248–256 (2019).
Petersen, R. C. et al. Mild cognitive impairment due to Alzheimer’s disease: criteria in the community. Ann. Neurol. 74 , 199–208 (2013).
Degenhardt, E. K. et al. Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics 57 , 208–216 (2016).
Robinson, J. L. et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141 , 2181–2193 (2018).
Prince, M. et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 8 , 23 (2016).
Röhr, S. et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res. Ther. 12 , 167 (2020).
Petersen, R. C. et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90 , 126–135 (2018).
Petersen, R. C. et al. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology 75 , 889–897 (2010).
Mielke, M. M., Vemuri, P. & Rocca, W. A. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6 , 37–48 (2014).
Thambisetty, M., An, Y. & Tanaka, T. Alzheimer’s disease risk genes and the age-at-onset phenotype. Neurobiol. Aging 34 , 2696.e1–5 (2013).
CAS Google Scholar
Haass, C., Kaether, C., Thinakaran, G. & Sisodia, S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2 , a006270 (2012).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488 , 96–99 (2012).
van der Lee, S. J. et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17 , 434–444 (2018).
Bellenguez, C. et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol. Aging 59 , 220.e1–220.e9 (2017).
Leonenko, G. et al. Polygenic risk and hazard scores for Alzheimer’s disease prediction. Ann. Clin. Transl. Neurol. 6 , 456–465 (2019).
Karch, C. M., Cruchaga, C. & Goate, A. M. Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83 , 11–26 (2014).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 , 414–430 (2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 396 , 413–446 (2020).
Singh-Manoux, A. et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74 , 712–718 (2017).
Gottesman, R. F. et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 74 , 1246–1254 (2017).
Samieri, C. et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 320 , 657–664 (2018).
Gottesman, R. F. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317 , 1443–1450 (2017).
Vemuri, P. et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138 , 761–771 (2015).
Golde, T. E., DeKosky, S. T. & Galasko, D. Alzheimer’s disease: the right drug, the right time. Science 362 , 1250–1251 (2018).
Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 18 , 794–799 (2015).
Zlokovic, B. V. et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 16 , 1714–1733 (2020).
Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R. & Van Hoesen, G. W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1 , 103–116 (1991). Graphic demonstration of the different regional distributions of Aβ and tau at autopsy.
Montine, T. J. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 123 , 1–11 (2012).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8 , 101–112 (2007).
Holtzman, D. M., Morris, J. C. & Goate, A. M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 3 , 77sr71 (2011).
Thinakaran, G. & Koo, E. H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283 , 29615–29619 (2008).
Haass, C. & Willem, M. Secreted APP modulates synaptic activity: a novel target for therapeutic intervention? Neuron 101 , 557–559 (2019).
Cirrito, J. R. et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48 , 913–922 (2005).
Kang, J. E. et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 326 , 1005–1007 (2009).
Boespflug, E. L. & Iliff, J. J. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-β, and sleep. Biol. Psychiatry 83 , 328–336 (2018).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid β and tau at synapses in Alzheimer’s disease. Neuron 82 , 756–771 (2014).
Benarroch, E. E. Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: emerging mechanisms. Neurology 91 , 125–132 (2018).
Rice, H. C. et al. Secreted amyloid-β precursor protein functions as a GABA(B)R1a ligand to modulate synaptic transmission. Science 363 , eaao4827 (2019).
Müller, U. C., Deller, T. & Korte, M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18 , 281–298 (2017).
Small, S. A. & Petsko, G. A. Endosomal recycling reconciles the Alzheimer’s disease paradox. Sci. Transl. Med. 12 , eabb1717 (2020).
Gallardo, G. & Holtzman, D. M. Amyloid-β and tau at the crossroads of Alzheimer’s disease. Adv. Exp. Med. Biol. 1184 , 187–203 (2019).
Pooler, A. M., Noble, W. & Hanger, D. P. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 76 (Pt A), 1–8 (2014).
Eftekharzadeh, B. et al. Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s disease. Neuron 99 , 925–940.e7 (2018).
Kent, S. A., Spires-Jones, T. L. & Durrant, C. S. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 140 , 417–447 (2020).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211 , 387–393 (2014).
Wu, J. W. et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19 , 1085–1092 (2016).
de Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73 , 685–697 (2012).
Dujardin, S. et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26 , 1256–1263 (2020). A clinical-neuropathological-molecular demonstration of how post-translational modifications of tau might play a role in the rate of clinical progression.
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549 , 523–527 (2017).
Gratuze, M. et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Invest. 130 , 4954–4968 (2020).
Shi, Y. et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 216 , 2546–2561 (2019).
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70 , 960–969 (2011).
Braak, H. & Braak, E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82 , 239–259 (1991).
Delacourte, A. et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52 , 1158–1165 (1999).
Price, J. L. & Morris, J. C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 45 , 358–368 (1999).
Delacourte, A. et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp. Gerontol. 37 , 1291–1296 (2002).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128 , 755–766 (2014).
Jack, C. R. et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain 142 , 3230–3242 (2019).
Hanseeuw, B. J. et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76 , 915–924 (2019).
Ossenkoppele, R. et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139 , 1551–1567 (2016).
Graff-Radford, J. et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20 , 222–234 (2021).
Kim, J., Basak, J. M. & Holtzman, D. M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63 , 287–303 (2009).
Huynh, T. V., Davis, A. A., Ulrich, J. D., Holtzman, D. M. & Apolipoprotein, E. and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 58 , 824–836 (2017).
Reiman, E. M. et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106 , 6820–6825 (2009).
Jack, C. R. Jr. et al. Age, sex, and APOE e4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 72 , 511–519 (2015).
van der Kant, R., Goldstein, L. S. B. & Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 21 , 21–35 (2020).
Small, S. A. & Duff, K. Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60 , 534–542 (2008).
Busche, M. A. & Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23 , 1183–1193 (2020).
Kurt, M. A., Davies, D. C., Kidd, M., Duff, K. & Howlett, D. R. Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol. Dis. 14 , 89–97 (2003).
Le, R. et al. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J. Neuropathol. Exp. Neurol. 60 , 753–758 (2001).
Leyns, C. E. G. et al. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci. 22 , 1217–1222 (2019).
Small, S. A., Simoes-Spassov, S., Mayeux, R. & Petsko, G. A. Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 40 , 592–602 (2017).
Nixon, R. A. & Yang, D. S. Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis. 43 , 38–45 (2011).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17 , 777–792 (2016).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30 , 572–580 (1991).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27 , 457–464 (1990). A real-time demonstration of a close correspondence between synapse loss and degree of cognitive impairment in brain biopsies from patients with AD.
Spires, T. L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25 , 7278–7287 (2005).
Arbel-Ornath, M. et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 12 , 27 (2017).
Zhou, L. et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8 , 15295 (2017).
Masliah, E., Hansen, L., Albright, T., Mallory, M. & Terry, R. D. Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 81 , 428–433 (1991).
Henstridge, C. M. et al. Post-mortem brain analyses of the Lothian Birth Cohort 1936: extending lifetime cognitive and brain phenotyping to the level of the synapse. Acta Neuropathol. Commun. 3 , 53 (2015).
Lleo, A. et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s Disease cerebrospinal fluid. Mol. Cell Proteom. 18 , 546–560 (2019).
DeVos, S. L. et al. Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front. Neurosci. 12 , 267 (2018).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53 , 337–351 (2007).
Keskin, A. D. et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl Acad. Sci. USA 114 , 8631–8636 (2017).
Busche, M. A. et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321 , 1686–1689 (2008).
Kuchibhotla, K. V. et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59 , 214–225 (2008).
Menkes-Caspi, N. et al. Pathological tau disrupts ongoing network activity. Neuron 85 , 959–966 (2015).
Busche, M. A. et al. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 22 , 57–64 (2019).
Anderson, K. J., Scheff, S. W. & DeKosky, S. T. Reactive synaptogenesis in hippocampal area CA1 of aged and young adult rats. J. Comp. Neurol. 252 , 374–384 (1986).
Geddes, J. W. & Cotman, C. W. Plasticity in hippocampal excitatory amino acid receptors in Alzheimer’s disease. Neurosci. Res. 3 , 672–678 (1986).
Hyman, B. T., Kromer, L. J. & Van Hoesen, G. W. Reinnervation of the hippocampal perforant pathway zone in Alzheimer’s disease. Ann. Neurol. 21 , 259–267 (1987).
Liu, L. et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7 , e31302 (2012).
Rauch, J. N. et al. LRP1 is a master regulator of tau uptake and spread. Nature 580 , 381–385 (2020).
Kounnas, M. Z. et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 82 , 331–340 (1995).
Rebeck, G. W., Reiter, J. S., Strickland, D. K. & Hyman, B. T. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11 , 575–580 (1993).
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron-glia signaling in synapse elimination. Annu. Rev. Neurosci. 42 , 107–127 (2019).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19 , 983–997 (2013).
Menzies, F. M. et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93 , 1015–1034 (2017).
Nixon, R. A. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 31 , 2729–2743 (2017).
Shehata, M. et al. Autophagy enhances memory erasure through synaptic destabilization. J. Neurosci. 38 , 3809–3822 (2018).
Glatigny, M. et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29 , 435–448.e8 (2019).
Cataldo, A. M. et al. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157 , 277–286 (2000).
Van Acker, Z. P., Bretou, M. & Annaert, W. Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: impact of genetic risk factors. Mol. Neurodegener. 14 , 20 (2019).
Suire, C. N. et al. Cathepsin D regulates cerebral Aβ42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimers Res. Ther. 12 , 80 (2020).
Kwart, D. et al. A large panel of isogenic APP and PSEN1 mutant human iPSC neurons reveals shared endosomal abnormalities mediated by APP β-CTFs, not Aβ. Neuron 104 , 256–270.e5 (2019). Evidence from induced pluripotent stem cells on the critical role of endosomal dysregulation in the pathogenesis of Aβ and tau-induced disease.
Pensalfini, A. et al. Endosomal dysfunction induced by directly over-activating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer’s disease. Cell Rep. 33 , 108420 (2020).
Lauritzen, I. et al. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 132 , 257–276 (2016).
Lee, J. H. et al. Presenilin 1 maintains lysosomal Ca 2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12 , 1430–1444 (2015).
Lee, J. H. et al. β2-adrenergic agonists rescue lysosome acidification and function in PSEN1 deficiency by reversing defective ER-to-lysosome delivery of ClC-7. J. Mol. Biol. 432 , 2633–2650 (2020).
Morel, N. & Poea-Guyon, S. The membrane domain of vacuolar H + ATPase: a crucial player in neurotransmitter exocytotic release. Cell. Mol. Life Sci. 72 , 2561–2573 (2015).
Higashida, H., Yokoyama, S., Tsuji, C. & Muramatsu, S. I. Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J. Physiol. Sci. 67 , 11–17 (2017).
El Far, O. & Seagar, M. A role for V-ATPase subunits in synaptic vesicle fusion? J. Neurochem. 117 , 603–612 (2011).
Peng, K. Y. et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain 142 , 163–175 (2019).
Liu, R. Q. et al. Membrane localization of β-amyloid 1-42 in lysosomes: a possible mechanism for lysosome labilization. J. Biol. Chem. 285 , 19986–19996 (2010).
Morishita, H. & Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 35 , 453–475 (2019).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176 , 11–42 (2019).
Bordi, M. et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12 , 2467–2483 (2016).
Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A. & Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 1802 , 2–10 (2010).
Nixon, R. A. The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta Proteins Proteom. 1868 , 140443 (2020).
Whyte, L. S., Lau, A. A., Hemsley, K. M., Hopwood, J. J. & Sargeant, T. J. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? J. Neurochem. 140 , 703–717 (2017).
Pensalfini, A. et al. Intracellular amyloid and the neuronal origin of Alzheimer neuritic plaques. Neurobiol. Dis. 71 , 53–61 (2014).
Adalbert, R. et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132 , 402–416 (2009).
Nixon, R. A. & Yang, D. S. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb. Perspect. Biol. 4 , a008839 (2012).
Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regenerat. Res. 15 , 25–29 (2020).
Lowry, J. R. & Klegeris, A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull. 139 , 144–156 (2018).
Sarlus, H. & Heneka, M. T. Microglia in Alzheimer’s disease. J. Clin. Invest. 127 , 3240–3249 (2017).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13 , 722–737 (2013).
Cho, M. H. et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10 , 1761–1775 (2014).
Jay, T. R. et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J. Neurosci. 37 , 637–647 (2017).
Lewcock, J. W. et al. Emerging microglia biology defines novel therapeutic approaches for Alzheimer’s disease. Neuron 108 , 801–821 (2020).
Kim, H. J. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 22 , 1576–1584 (2017).
Saido, T. & Leissring, M. A. Proteolytic degradation of amyloid β-protein. Cold Spring Harb. Perspect. Med. 2 , a006379 (2012).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17 , 1016–1024 (2018).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124 , 1–38 (2008). Demonstration of the relationship between the default mode network and amyloid accumulation.
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101 , 4637–4642 (2004).
Buckner, R. L. et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29 , 1860–1873 (2009).
Agosta, F. et al. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol. Aging 33 , 1564–1578 (2012).
Whitwell, J. L. et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol. Aging 36 , 1245–1252 (2015).
Townley, R. A. et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2 , fcaa068 (2020).
Franzmeier, N. et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat. Commun. 11 , 347 (2020).
Sintini, I. et al. Tau and amyloid relationships with resting-state functional connectivity in atypical Alzheimer’s disease. Cereb. Cortex https://doi.org/10.1093/cercor/bhaa319 (2020).
Article PubMed Central Google Scholar
Jones, D. T. et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex 97 , 143–159 (2017).
Sepulcre, J. et al. Hierarchical organization of tau and amyloid deposits in the cerebral cortex. JAMA Neurol. 74 , 813–820 (2017).
Jack, C. R. Jr. et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81 , 1732–1740 (2013).
Protas, H. D. et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 70 , 320–325 (2013).
Franzmeier, N. et al. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci. Adv. 6 , eabd1327 (2020). Analysis showing how regional tau expansion follows connectivity patterns.
D’Onofrio, G. et al. Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr. Alzheimer Res. 9 , 759–771 (2012).
Lehmann, M. et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136 , 844–858 (2013).
Crutch, S. J. et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 13 , 870–884 (2017).
Gorno-Tempini, M. L. et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 71 , 1227–1234 (2008).
Bergeron, D. et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann. Neurol. 84 , 729–740 (2018).
Ossenkoppele, R. et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138 , 2732–2749 (2015).
Murray, M. E. et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10 , 785–796 (2011).
Clarfield, A. M. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch. Intern. Med. 163 , 2219–2229 (2003).
Beach, T. G., Monsell, S. E., Phillips, L. E. & Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at national institute on aging Alzheimer disease centers, 2005-2010. J. Neuropathol. Exp. Neurol. 71 , 266–273 (2012).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89 , 88–100 (2017).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134 , 2456–2477 (2011).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76 , 1006–1014 (2011).
Botha, H. et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain 141 , 1201–1217 (2018).
Petersen, R. C. & Yaffe, K. Issues and questions surrounding screening for cognitive impairment in older patients. JAMA 323 , 722–724 (2020).
Patnode, C. D. et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services TaskFforce. JAMA 323 , 764–785 (2020).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 , 535–562 (2018).
Chetelat, G. et al. Amyloid-PET and 18 F-FDG-PET in the diagnostic investigation of Alzheimer”s disease and other dementias. Lancet Neurol. 19 , 951–962 (2020).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55 , 306–319 (2004).
Jack, C. R. J. et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer Association Workgroups. Alzheimer’s Dement. 7 , 257–262 (2011).
Knopman, D. S. et al. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol. Aging 46 , 32–42 (2016).
Schröder, J. & Pantel, J. Neuroimaging of hippocampal atrophy in early recognition of Alzheimer’s disease–a critical appraisal after two decades of research. Psychiatry Res. Neuroimaging 247 , 71–78 (2016).
Petersen, C. et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 138 , 597–612 (2019).
Ridha, B. H. et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 5 , 828–834 (2006).
Greenberg, S. M. et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol. 16 , 30–42 (2020).
Graff-Radford, J. et al. Cerebral microbleeds: prevalence and relationship to amyloid burden. Neurology 92 , e253–e262 (2019).
Li, X. et al. The significant effects of cerebral microbleeds on cognitive dysfunction: an updated meta-analysis. PLoS ONE 12 , e0185145 (2017).
Laforce, R. Jr. et al. Parallel ICA of FDG-PET and PiB-PET in three conditions with underlying Alzheimer’s pathology. Neuroimage Clin. 4 , 508–516 (2014).
Caroli, A. et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology 84 , 508–515 (2015).
Iaccarino, L., Sala, A. & Perani, D. Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET. Ann. Clin. Transl. Neurol. 6 , 1113–1120 (2019).
Villemagne, V. L., Doré, V., Burnham, S. C., Masters, C. L. & Rowe, C. C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 14 , 225–236 (2018).
Clark, C. M. et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11 , 669–678 (2012).
Murray, M. E. et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138 , 1370–1381 (2015).
Klunk, W. E. et al. The centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11 , 1–15.e1–4 (2015).
Landau, S. M. et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol. 74 , 826–836 (2013).
Leuzy, A. et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain 139 , 2540–2553 (2016).
Grothe, M. J. et al. In vivo staging of regional amyloid deposition. Neurology 89 , 2031–2038 (2017).
Jagust, W. J. & Landau, S. M. Temporal dynamics of β-amyloid accumulation in aging and Alzheimer’s disease. Neurology 96 , e1347–e1357 (2021).
Jack, C. R. Jr. et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using THE National Institute on Aging-Alzheimer’s association research framework. JAMA Neurol. 76 , 1174–1183 (2019).
PubMed Central Google Scholar
Johnson, K. A. et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer’s association. J. Nucl. Med. 54 , 476–490 (2013).
Rabinovici, G. D. et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321 , 1286–1294 (2019). A very large pragmatic trial of the value of Aβ imaging in clinical dementia practice.
de Wilde, A. et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 75 , 1062–1070 (2018).
Jeong, H. J. et al. [ 18 F]THK5351 PET imaging in patients with mild cognitive impairment. J. Clin. Neurol. 16 , 202–214 (2020).
Pascoal, T. A. et al. 18 F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143 , 2818–2830 (2020).
Leuzy, A. et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 77 , 955–965 (2020).
Ossenkoppele, R. et al. Discriminative accuracy of [ 18 F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 320 , 1151–1162 (2018). Demonstration of the remarkable specificity of an elevated tau PET signal outside of the medial temporal lobe for persons with elevated Aβ.
Aschenbrenner, A. J., Gordon, B. A., Benzinger, T. L. S., Morris, J. C. & Hassenstab, J. J. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91 , e859–e866 (2018).
Brier, M. R. et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med. 8 , 338ra366 (2016).
Harrison, T. M. et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann. Neurol. 85 , 229–240 (2019).
Lu, M. et al. Aggregated tau measured by visual interpretation of flortaucipir positron emission tomography and the associated risk of clinical progression of mild cognitive impairment and Alzheimer disease: results from 2 phase III clinical trials. JAMA Neurol. 78 , 445–453 (2021).
Lowe, V. J. et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141 , 271–287 (2018).
Pontecorvo, M. J. et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140 , 748–763 (2017).
Shaw, L. M. et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 14 , 1505–1521 (2018).
Molinuevo, J. L. et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 136 , 821–853 (2018).
Hansson, O. et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 1 , 30029–30023 (2018).
Hansson, O. et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 23 , 316–320 (2007).
Wiltfang, J. et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J. Neurochem. 101 , 1053–1059 (2007).
Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302 , 385–393 (2009).
Skillbäck, T. et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138 , 2716–2731 (2015).
Buerger, K. et al. No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 130 , e82 (2007).
Seppälä, T. T. et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78 , 1568–1575 (2012).
Barthélemy, N. R. et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 26 , 398–407 (2020).
Janelidze, S. et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 11 , 1683 (2020).
Kern, S. et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 76 , 187–193 (2019).
Zetterberg, H. & Bendlin, B. B. Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26 , 296–308 (2020).
Tarawneh, R. et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 73 , 561–571 (2016).
Schindler, S. E. et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93 , e1647–e1659 (2019).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19 , 422–433 (2020).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324 , 772–781 (2020).
de Wolf, F. et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 143 , 1220–1232 (2020).
Ashton, N. J. et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 16 , 265–284 (2020).
Wattmo, C. & Wallin A, K. Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res. Ther. 9 , 70 (2017).
Wilson, R. S. et al. Cognitive decline in incident Alzheimer’s disease in a community population. Neurology 74 , 951–955 (2010).
van Harten, A. C. et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology 91 , e300–e312 (2018).
Kryscio, R. J. et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 83 , 1359–1365 (2014).
Stewart, R. et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br. J. Psychiatry 198 , 199–205 (2011).
Amariglio, R. E. et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50 , 2880–2886 (2012).
Petersen, R. C. et al. Randomized controlled trials in mild cognitive impairment: sources of variability. Neurology 88 , 1751–1758 (2017).
Roberts, R. O. et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 75 , 970–979 (2018).
Vos, S. J. et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138 , 1327–1338 (2015).
Jack, C. R. Jr. et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 321 , 2316–2325 (2019).
Knopman, D. S. et al. Entorhinal cortex tau, amyloid-β, cortical thickness and memory performance in non-demented subjects. Brain 142 , 1148–1160 (2019).
Vos, S. J. et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12 , 957–965 (2013).
Serrano-Pozo, A., Qian, J., Monsell, S. E., Betensky, R. A. & Hyman, B. T. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol. 77 , 917–929 (2015).
Craft, S. et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology 51 , 149–153 (1998).
Butler, M. et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 52–62 (2018).
Fink, H. A. et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 39–51 (2018).
Brasure, M. et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168 , 30–38 (2018).
Kane, R. L. et al. in Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia (Agency for Healthcare Research and Quality, 2017).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385 , 2255–2263 (2015). A first demonstration of non-pharmacological means of delaying cognitive decline in elderly persons at risk for dementia.
Debette, S. et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77 , 461–468 (2011).
Williamson, J. D. et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321 , 553–561 (2019).
Reuben, D. B. et al. D-CARE: the dementia care study: design of a pragmatic trial of the effectiveness and cost effectiveness of health system-based versus community-based dementia care versus usual dementia care. J. Am. Geriatr. Soc. 68 , 2492–2499 (2020).
Fisk, J. D., Beattie, B. L., Donnelly, M., Byszewski, A. & Molnar, F. J. Disclosure of the diagnosis of dementia. Alzheimers Dement. 3 , 404–410 (2007).
Alpinar-Sencan, Z. & Schicktanz, S. Addressing ethical challenges of disclosure in dementia prediction: limitations of current guidelines and suggestions to proceed. BMC Med. Ethics 21 , 33 (2020).
Amieva, H. et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int. Psychogeriatr. 28 , 707–717 (2016).
Tricco, A. C. et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 318 , 1687–1699 (2017).
Mohs, R. C. et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 57 , 481–488 (2001).
Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15 , 73–88 (2019).
Rogers, M. B. https://www.alzforum.org/news/research-news/aducanumab-still-needs-prove-itself-researchers-say (2020).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med . https://doi.org/10.1056/NEJMoa2100708 (2021).
Shugart, J. https://www.alzforum.org/news/conference-coverage/banish-av-ban2401-antibody-makes-its-move-phase-3-program (2020).
VandeVrede, L., Boxer, A. L. & Polydoro, M. Targeting tau: clinical trials and novel therapeutic approaches. Neurosci. Lett. 731 , 134919 (2020).
Rios-Romenets, S. et al. Baseline demographic, clinical, and cognitive characteristics of the Alzheimer’s prevention initiative (API) autosomal-dominant Alzheimer’s disease Colombia trial. Alzheimers Dement 16 , 1023–1030 (2020).
Insel, P. S., Donohue, M. C., Sperling, R., Hansson, O. & Mattsson-Carlgren, N. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann. Clin. Transl. Neurol. 7 , 776–785 (2020).
Ballard, C. et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 17 , 213–222 (2018).
Jennings, L. A. et al. Patient and caregiver goals for dementia care. Qual. Life Res. 26 , 685–693 (2017).
Bannon, S. et al. In it together: a qualitative meta-synthesis of common and unique psychosocial stressors and adaptive coping strategies of persons with young-onset dementia and their caregivers. Gerontologist https://doi.org/10.1093/geront/gnaa169 (2020).
Moon, H. & Adams, K. B. The effectiveness of dyadic interventions for people with dementia and their caregivers. Dementia 12 , 821–839 (2013).
van Charante, E. P. et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388 , 797–805 (2016).
Andrieu, S. et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16 , 377–389 (2017).
Sink, K. M. et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trial. JAMA 314 , 781–790 (2015).
De Jager, P. L., Yang, H. S. & Bennett, D. A. Deconstructing and targeting the genomic architecture of human neurodegeneration. Nat. Neurosci. 21 , 1310–1317 (2018).
Rehiman, S. H. et al. Proteomics as a reliable approach for discovery of blood-based Alzheimer’s disease biomarkers: a systematic review and meta-analysis. Ageing Res. Rev. 60 , 101066 (2020).
Mahajan, U. V. et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLoS Med. 17 , e1003012 (2020).
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21 , 1370–1379 (2018).
van der Kant, R. et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 24 , 363–375.e9 (2019).
American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders 5th ed. (American Psychiatric Association, 2013).
Albert, M. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging– Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7 , 270–279 (2011).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13 , 614–629 (2014).
Thal, D. R., Rub, U., Orantes, M. & Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58 , 1791–1800 (2002).
Download references
Acknowledgements
The authors acknowledge research support from NIH (D.S.K. and R.C.P, P30 AG062677 and U01 AG006786; B.T.H., P30AG062421; R.A.N. P01 AG017617 and R01 AG062376).
Author information
Authors and affiliations.
Department of Neurology, Mayo Clinic, Rochester, MN, USA
David S. Knopman, Ronald C. Petersen & David T. Jones
Inserm U1219 Bordeaux Population Health Center, University of Bordeaux, Bordeaux, France
Helene Amieva
Normandie Univ, UNICAEN, INSERM, U1237, PhIND “Physiopathology and Imaging of Neurological Disorders”, Institut Blood and Brain @ Caen-Normandie, Cyceron, Caen, France
Gäel Chételat
Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA
David M. Holtzman
Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
Bradley T. Hyman
Departments of Psychiatry and Cell Biology, New York University Langone Medical Center, New York University, New York, NY, USA
Ralph A. Nixon
NYU Neuroscience Institute, New York University Langone Medical Center, New York University, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (D.S.K.); Epidemiology (H.A.); Mechanisms/pathophysiology (D.T.J., R.A.N., B.T.H. and D.M.H.); Diagnosis, screening and prevention (G.C., R.C.P. and D.S.K.); Management (R.C.P. and D.S.K.); Quality of life (D.S.K.); Outlook (D.S.K.); Overview of Primer (D.S.K.).
Corresponding author
Correspondence to David S. Knopman .
Ethics declarations
Competing interests.
D.S.K. served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety Monitoring Board for a tau therapeutic for Biogen but receives no personal compensation. He is a site investigator in a Biogen aducanumab trial. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche and Alzeca Biosciences but receives no personal compensation. He receives research support from the NIH. G.C. serves on the Scientific Advisory Board of the Fondation Vaincre Alzheimer but receives no personal compensation. She receives personal fees from Fondation d’entreprise MMA des Entrepreneurs du Futur and from Fondation Alzheimer as she serves in the Operational Committee. She receives research support from European Union Horizon 2020 research and innovation programme (grant agreement number 667696), Inserm, Fondation d’entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées and Fondation Vaincre Alzheimer. R.C.P. is a consultant for Biogen, Inc., Roche, Inc., Merck, Inc., Genentech Inc. and Eisai, Inc., has given educational lectures for GE Healthcare, receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), UpToDate, and receives research support from the NIH. B.T.H. has a family member who works at Novartis and owns stock in Novartis; he serves on the SAB of Dewpoint and owns stock. He serves on a scientific advisory board or is a consultant for Biogen, Novartis, Cell Signalling, the US Dept of Justice, Takeda, Vigil, W20 group and Seer. His laboratory is supported by sponsored research agreements with Abbvie, F Prim, and research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, Brightfocus and the JPB Foundation. H.A. serves on the Scientific Advisory Board of the Observatoire des Mémoires but receives no personal compensation. She receives research support from Spoelberch Foundation, Association France Alzheimer et maladies apparentées, the Regional Health Agency of Aquitaine and National Research Agency. D.M.H. reports being a Co-founder for C2N Diagnostics LLC and participating in scientific advisory boards/consulting for Genentech, C2N Diagnostics, Denali, Merck and Idorsia. He is an inventor on patents licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies (this anti-tau antibody programme is licensed to Abbvie) and to Eli Lilly on the therapeutic use of an anti-amyloid-β antibody. His laboratory receives research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, the JPB Foundation, Good Ventures, Centene, BrightFocus and C2N Diagnostics. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks K. Iqbal, D. Selkoe, H. Soininen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Knopman, D.S., Amieva, H., Petersen, R.C. et al. Alzheimer disease. Nat Rev Dis Primers 7 , 33 (2021). https://doi.org/10.1038/s41572-021-00269-y
Download citation
Accepted : 09 April 2021
Published : 13 May 2021
DOI : https://doi.org/10.1038/s41572-021-00269-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Journal Articles by Date
Professional and scientific articles from the Alzheimer’s Disease Program authors whose names are in bold face . Bibliographies are listed by year.
Suchsland, M.Z., Gaster, B., Raetz, J., Belza, B., McGuire, L. , Olivari, B. , Tracy, K., & Fitzpatrick, A.L. Developing a cognitive assessment toolkit for primary care: qualitative assessment of providers’ needs and perceptions of usability in clinical practice. BMC Health Serv Res 23, 1006 (2023). https://doi.org/10.1186/s12913-023-09991-7
Eustaquio PC, Salmon-Trejo LA, McGuire LC , Ellington SR. Epidemiologic and Clinical Features of Mpox in Adults Aged >50 Years — United States, May 2022–May 2023. MMWR Morb Mortal Wkly Rep 2023; 72:893–896. DOI: http://dx.doi.org/10.15585/mmwr.mm7233a3 .
Jackson EMJ , O’Brien K, McGuire LC , Baumgart M, Gore J , Brandt K, Levey AI, Lamont H. Promoting Healthy Aging: Public Health as a Leader for Reducing Dementia Risk, Public Policy & Aging Report , 2023;, prad011, https://doi.org/10.1093/ppar/prad011
Gore J, Denno B, Omura JD, Baumgart M, McGuire LC, O’Brien K. Promoting Healthy Aging to Reduce the Risk of Dementia: A Public Health Imperative. Generations Journal, Vol. 47, No. 1 (Spring 2023). DOI: https://generations.asaging.org/promoting-healthy-aging-reduce-risk-dementia
Miyawaki, C. E., Bouldin, E. D., Taylor, C. A., McGuire, L. C. , & Markides, K. S. (2023). Characteristics of Asian American Family Caregivers of Older Adults Compared to Caregivers of Other Racial/Ethnic Groups: Behavioral Risk Factor Surveillance System 2015–2020. Journal of Applied Gerontology, 42(5), 1101–1107. https://doi.org/10.1177/07334648221146257
Wooten KG, McGuire LC, Olivari BS, Jackson EM, Croft JB . Racial and Ethnic Differences in Subjective Cognitive Decline — United States, 2015–2020. MMWR Morb Mortal Wkly Rep 2023; 72: 250-255. DOI: https://www.cdc.gov/mmwr/volumes/72/wr/mm7210a1.htm
Jackson EM , Omura JD , Boring MA, Odom EL, Foster AL, Olivari BS , McGuire LC , Croft JB . Prevalence and Characteristics of Arthritis Among Caregivers — 17 States, 2017 and 2019. MMWR Morb Mortal Wkly Rep 2022;71:1389–1395. DOI: http://dx.doi.org/10.15585/mmwr.mm7144a1 .
Omura JD, McGuire LC, Patel R , Baumgart M, Lamb, R, Jeffers EM, Olivari BS, Croft JB, Thomas CW, Hacker K . Modifiable Risk Factors for Alzheimer Disease and Related Dementias Among Adults Aged ≥45 Years — United States, 2019. MMWR Morb Mortal Wkly Rep 2022;71:680–685. DOI: http://dx.doi.org/10.15585/mmwr.mm7120a2external icon .
Downing KF, Oster ME, Olivari BS , Farr SL. Early-onset dementia among privately-insured adults with and without congenital heart defects in the United States, 2015-2017. Int J Cardiol. 2022 Jul 1;358:34-38. doi: 10.1016/j.ijcard.2022.04.019. Epub 2022 Apr 11. PMID: 35417738. https://pubmed.ncbi.nlm.nih.gov/35417738/
Samson ME , Yeung LF, Rose CE, Qi YP, Taylor CA , Crider KS, Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011–2014, The American Journal of Clinical Nutrition , Volume 116, Issue 1, July 2022, Pages 74–85, https://doi.org/10.1093/ajcn/nqac065
Olivari BS, Jeffers EM, Tang KW, McGuire LC . Improving Brain Health for Populations Disproportionately Affected by Alzheimer’s Disease and Related Dementias. Clin Gerontol. 2022;1-5. doi: 10.1080/07317115.2022.2043977
Jeffers EM , Bouldin ED, McGuire LC , Knapp KA, Patel R, Guglielmo D, Taylor CA, Croft JB . Prevalence and Characteristics of Subjective Cognitive Decline Among Unpaid Caregivers Aged ≥45 Years – 22 States, 2015-2019. MMWR Morb Mortal Wkly Rep. 2021 Nov 19;70(46):1591-1596. doi: 10.15585/mmwr.mm7046a1.PMID: 34793418
Miyawaki CE, Bouldin ED, Taylor CA, McGuire LC. Baby Boomers Who Provide Informal Care for People Living with Dementia in the Community. Int J Environ Res Public Health. 2021 Sep 15;18(18):9694. doi: 10.3390/ijerph18189694.PMID: 34574619
Yang Q, Tong X, Coleman King S, Olivari BS , Merritt RK. Stroke Hospitalizations Before and During COVID-19 Pandemic Among Medicare Beneficiaries in the United States. Stroke. 2021 Jul 29:STROKEAHA121034562. doi: 10.1161/STROKEAHA.121.034562. Online ahead of print. PMID: 34320816
Havers FP, Whitaker M, Self JL, … Taylor CA ; COVID-NET Surveillance Team. Hospitalization of Adolescents Aged 12-17 Years with Laboratory-Confirmed COVID-19 – COVID-NET, 14 States, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021 Jun 11;70(23):851-857. doi: 10.15585/mmwr.mm7023e1. PMID: 34111061
Flatt JD, Cicero EC, Lambrou NH, Wharton W, Anderson JG, Bouldin ED, McGuire LC, Taylor CA . Subjective cognitive decline higher among sexual and gender minorities in the United States, 2015—2018. https://doi.org/10.1002/trc2.12197
Barrett J, Olivari BS , Price A, Taylor CA . Cognitive Decline and Dementia Risk Reduction: Promoting Healthy Lifestyles and Blood Pressure Control. 2021. Am J Prev Med 2021;1−4. https://doi.org/10.1016/j.amepre.2021.03.005
Olivari BS, Baumgart M , Taylor CA, McGuire, LC . Population measures of subjective cognitive decline: A means of advancing public health policy to address cognitive health. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2021; 7:e12142. https://doi.org/10.1002/trc2.12142
Bouldin ED, Taylor CA, Knapp KA, Miyawaki CE, Mercado NR, Wooten KG, McGuire LC. Unmet needs for assistance related to subjective cognitive decline among community-dwelling middle-aged and older adults in the US: prevalence and impact on health-related quality of life. Int Psychogeriatr. 2021 Jul;33(7):689-702. doi: 10.1017/S1041610220001635. Epub 2020 Sep 4.PMID: 32883384
Matthews KA, Gaglioti AH, Holt JB, McGuire LC , Greenlund KJ. County-Level Concentration of Selected Chronic Conditions Among Medicare Fee-for-Service Beneficiaries and Its Association with Medicare Spending in the United States, 2017. Popul Health Manag. 2021 Apr;24(2):214-221. doi: 10.1089/pop.2019.0231. Epub 2020 Apr 1.PMID: 32233970
Quinn K, Miyawaki CE, Croff R, Vogel MT, Belza B, Souza AM, Liu M, Edwards VJ , Friedman DB. Terms and Measures of Cognitive Health Associated With Dementia and Alzheimer’s Disease: A Scoping Review. Res Aging. 2020 Jun-Jul;42(5-6):174-185. doi: 10.1177/0164027520911284.
Omura JD, Brown DR, McGuire LC, Taylor CA , Fulton JE, Carlson SA. Cross-sectional association between physical activity level and subjective cognitive decline among US adults aged ≥45 years, 2015. Prev Med. 2020 Oct 6;141:106279. doi: 10.1016/j.ypmed.2020.106279 .
Bouldin ED , Taylor CA, Knapp KA, Miyawaki CE, Mercado NR, Wooten KG , McGuire LC . Unmet needs for assistance related to subjective cognitive decline among community-dwelling middle-aged and older adults in the US: prevalence and impact on health-related quality of life. Int Psychogeriatr . 2020;1-14. doi:10.1017/S1041610220001635
Miyawaki CD, Bouldin ED , Taylor CA, McGuire LC. Baby Boomers as Caregivers: Results From the Behavioral Risk Factor Surveillance System in 44 States, the District of Columbia, and Puerto Rico, 2015–2017. Prev Chronic Dis 2020;17:200010.
Edwards VJ, Bouldin ED , Taylor CA , Olivari BS , McGuire LC . Characteristics and Health Status of Informal Unpaid Caregivers—44 States, District of Columbia, and Puerto Rico, 2015–2017. MMWR Morb Mortal Wkly Rep 2020;69:183-188. DOI: http://dx.doi.org/10.15585/mmwr.mm6907a2
Taylor, C.A ., Bouldin, E.D. , Greenlund, K.J., McGuire, L.C . Comorbid Chronic Conditions Among Older Adults with Subjective Cognitive Decline, United States, 2015–2017. Innovation in Aging, Volume 4(1).
Olivari BS , French ME, McGuire LC. The Public Health Road Map to Respond to the Growing Dementia Crisis. Innovation in Aging, Volume 4(1) .
Brody DJ, Kramarow EA, Taylor CA , McGuire LC (2019). Cognitive Performance in Adults Aged 60 and Over: National Health and Nutrition Examination Survey, 2011–2014. National Health Statistics Reports ; no. 126. Hyattsville, MD: National Center for Health Statistics. https://www.cdc.gov/nchs/data/nhsr/nhsr126-508.pdf [PDF 659 KB]
Saydah S, Gerzoff RB, Taylor CA , Ehrlich JR, Saaddine J (2019). Vision Impairment and Subjective Cognitive Decline–Related Functional Limitations — United States, 2015–2017. MMWR Morb Mortal Wkly Rep , 68(20): 453–457. https://www.cdc.gov/mmwr/volumes/68/wr/mm6820a2.htm?s_cid=mm6820a2_w
Peterson RL, Carvajal SC, McGuire LC , Fain MJ, Bell ML (2019). State Inequality, Socioeconomic Position, and Subjective Cognitive Decline in the United States. SSM Popul Health , 7: 100357. https://www.ncbi.nlm.nih.gov/pubmed/30886886
Matthews KA, Wei X, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019). Racial and Ethnic Estimates of Alzheimer’s Disease and Related Dementias in the United States (2015 – 2060) in Adults Aged ≥65 Years. Alzheimer’s & Dementia , 15(1): 17–24. https://www.ncbi.nlm.nih.gov/pubmed/30243772
Bouldin, E. D. , Shaull, L., Andresen, E. M., Edwards, V. J. , & McGuire, L. C. (2018). Financial and Health Barriers and Caregiving-Related Difficulties Among Rural and Urban Caregivers. The Journal of Rural Health , 34(3), 263-274. http://dx.doi.org/10.1111/jrh.12273
Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C . (2018). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia . https://doi.org/10.1016/j.jalz.2018.06.3063 external icon
Kelley, M., Ulin, B., & McGuire, L. C. (2018). Reducing the Risk of Alzheimer’s Disease and Maintaining Brain Health in an Aging Society. Public Health Reports , 133(3), 225-229. http://dx.doi.org/10.1177/0033354918763599
Lee-Kwan, S. H., Park, S., Maynard, L. M., Blanck, H. M., McGuire, L. C. , & Collins, J. L. (2018). Parental Characteristics and Reasons Associated With Purchasing Kids’ Meals for Their Children. American Journal of Health Promotion , 32(2), 264-270. http://dx.doi.org/10.1177/0890117116683797
Olivari, B. S. , Baumgart, M., Lock, S. L., Whiting, C. G., Taylor, C. A. , Iskander, J., . . . McGuire, L. C. (2018). CDC Grand Rounds: Promoting Well-Being and Independence in Older Adults. MMWR. Morbidity and Mortality Weekly Report , 67(37), 1036-1039. http://dx.doi.org/10.15585/mmwr.mm6737a4
Rabarison, K. M., Bouldin, E. D. , Bish, C. L., McGuire, L. C. , Taylor, C. A. , & Greenlund, K. J. (2018). The Economic Value of Informal Caregiving for Persons With Dementia: Results From 38 States, the District of Columbia, and Puerto Rico, 2015 and 2016 BRFSS. American Journal of Public Health , 108(10), 1370-1377. http://dx.doi.org/10.2105/ajph.2018.304573
Taylor, C. A. , Bouldin, E. D. , & McGuire, L. C. (2018). Subjective Cognitive Decline Among Adults Aged ≥45 Years — United States, 2015–2016. MMWR. Morbidity and Mortality Weekly Report , 67(27), 753-757. http://dx.doi.org/10.15585/mmwr.mm6727a1
Bouldin, E. , Trivedi, R., Reiber, G., Rosland, A., Silverman, J., Krieger, J., & Nelson, K. (2017). Associations between having an informal caregiver, social support, and self-care among low-income adults with poorly controlled diabetes. Chronic Illness , 13(4), 239-250. http://dx.doi.org/10.1177/1742395317690032
Bouldin, E. , Wong, E., Liu, C., Littman, A., Taylor, L., Rice, K., & Reiber, G. (2017). Chronic wound care utilization among Veterans using VHA and Medicare. Wound Medicine , 17, 1-6. http://dx.doi.org/10.1016/j.wndm.2017.01.003
Bouldin ED , Shaull L, Andresen EM, Edwards VJ , McGuire LC . Financial and Health Barriers and Caregiving-Related Difficulties Among Rural and Urban Caregivers. J Rural Health . 2017 Sep 23. http://dx.doi.org/10.1111/jrh.12273 .
Cancelliere C, Coronado VG, Taylor CA , Xu L (2017). Epidemiology of Isolated vs. Non-Isolated Mild Traumatic Brain Injury Treated in Emergency Departments in the United States, 2006–2012: Sociodemographic Characteristics. J Head Trauma Rehabil , 32(4):E37–E46 [ http://dx.doi.org/10.1097/HTR.0000000000000260 ].
Edwards VJ , Anderson LA , Thompson WW, Deokar AJ . Mental health differences between men and women caregivers, BRFSS 2009. J Women Aging . 2017;29(5):385-391. http://dx.doi.org/10.1080/08952841.2016.1223916 .
Eisenberg, Y., Bouldin, E . D., Gell, N., & Rosenberg, D. (2017). Planning Walking Environments for People with Disabilities and Older Adults. In Walking: Connecting Sustainable Transport with Health (pp. 187-209). Emerald Publishing Limited.
Haarbauer-Krupa J, Taylor CA , Yue JK, Winkler EA, Pirracchio R, Cooper SR, Burke JF, Stein MB, Manley GT, The TRACK-TBI Investigators (2017). Screening for Post-Traumatic Stress Disorder in a Civilian Emergency Department Population with Traumatic Brain Injury. J Neurotrauma , 34(1): 50–58 [ http://dx.doi.org/10.1089/neu.2015.4158 ].
Littman AJ, Bouldin ED , Haselkorn JK. This is your new normal: A qualitative study of barriers and facilitators to physical activity in Veterans with lower extremity loss. Disabil Health J . 2017 Oct;10(4):600-606. http://dx.doi.org/10.1016/j.dhjo.2017.03.004 .
Lock, S. L., Baumgart, M., Whiting, C. G., McGuire, L.C., Iskander, J. K., Thorpe, P., & Laird, S. (2017). Healthy aging: promoting well-being in older adults. CDC Grand Rounds.
Miyawaki CE, Bouldin ED , Kumar GS, McGuire LC . Associations between Physical Activity and Cognitive Functioning among Middle-Aged and Older Adults. J Nutr Health Aging . 2017;21(6):637-647. http://dx.doi.org/10.1007/s12603-016-0835-6 .
Snowden MB, Steinman LE, Bryant LL, Cherrier MM, Greenlund KJ, Leith KH, Levy C, Logsdon RG, Copeland C, Vogel M, Anderson LA , Atkins DC, Bell JF, Fitzpatrick AL. Dementia and co-occurring chronic conditions: a systematic literature review to identify what is known and where are the gaps in the evidence? Int J Geriatr Psychiatry . 2017 Apr;32(4):357-371. http://dx.doi.org/10.1002/gps.4652 .
Tang W, Kannaley K, Friedman DB, Edwards VJ , Wilcox S, Levkoff SE, Hunter RH, Irmiter C, Belza B. Concern about developing Alzheimer’s disease or dementia and intention to be screened: An analysis of national survey data. Arch Gerontol Geriatr . 2017 Jul;71:43-49. http://dx.doi.org/10.1016/j.archger.2017.02.013 .
Taylor CA , Greenlund SF, McGuire LC , Lu H, Croft JB. Deaths from Alzheimer’s Disease – United States, 1999-2014. MMWR Morb Mortal Wkly Rep . 2017 May 26;66(20):521-526. http://dx.doi.org/10.15585/mmwr.mm6620a1 .
Taylor CA , Bell JM, Breiding MB, Xu L (2017). Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ , 66(No. SS-9): 1–16 [ http://dx.doi.org/10.15585/mmwr.ss6609a1 ].
Wiltz, J. L., Blanck, H. M., Lee, B., Kocot, S. L., Seeff, L., McGuire, L. C ., & Collins, J. (2017). Electronic Information Standards to Support Obesity Prevention and Bridge Services Across Systems, 2010–2015. Preventing Chronic Disease , 14, E103. http://doi.org/10.5888/pcd14.160299
Alzheimer’s Disease and Healthy Aging Program authors are in bold face .
Matthews, K. A., Holt, J., Gaglioti, A. H., Lochner, K. A., Shoff, C., McGuire, L. C. , & Greenlund, K. J. (2016). Peer Reviewed: County-Level Variation in Per Capita Spending for Multiple Chronic Conditions Among Fee-for-Service Medicare Beneficiaries, United States, 2014. Preventing Chronic Disease , 13 . DOI: 10.5888/pcd13.160240.
Eke, P. I., Wei, L., Borgnakke, W. S., Thornton-Evans, G., Zhang, X., Lu, H., McGuire, L. C. and Genco, R. J. (2016). Periodontitis prevalence in adults≥ 65 years of age, in the USA. Periodontology 2000 , 72 (1), 76-95. DOI: 10.1111/prd.12145.
VanFrank B.K., Park S., Foltz J.L., McGuire L.C. , Harris D.M. (2016). Physician Characteristics Associated With Sugar-Sweetened Beverage Counseling Practices. American Journal of Health Promotion , 0890117116680472. DOI: 10.1177/0890117116680472.
Park S., Akinbami L.J., McGuire L.C. , Blanck H.M. Association of sugar-sweetened beverage intake frequency and asthma among US adults, 2013. Preventive medicine , 91 , 58-61. DOI: 10.1016/j.ypmed.2016.08.004.
Park S., Thompson F.E., McGuire L.C. , Pan L., Galuska D.A., Blanck H.M. (2016). Sociodemographic and Behavioral Factors Associated with Added Sugars Intake among US Adults. Journal of the Academy of Nutrition and Dietetics, 116 (10), 1589-98. DOI: 10.1016/j.jand.2016.04.012.
Freedman D.S., Lawman H.G., Pan L., Skinner A.C., Allison D.B., McGuire L.C. , Blanck H.M. (2016). The prevalence and validity of high, biologically implausible values of weight, height, and BMI among 8.8 million children. Obesity , 24 (5), 1132-1139. DOI: 10.1002/oby.21446.
Lee-Kwan S.H., Pan L., Maynard L.M., McGuire L.C. , Park S. (2016). Factors Associated with Self-Reported Menu-Labeling Usage among US Adults. Journal of the Academy of Nutrition and Dietetics, 116 (7), 1127-35. DOI: 10.1016/j.jand.2015.12.015.
VanFrank, B. K., Park, S., Foltz, J., McGuire, L. C., & Harris, D. M. (2016). Physician Counseling on Sugar-Sweetened Beverages: Areas for Improvement. Pediatrics , 137 (Supplement 3), 121A-121A. DOI: 10.1542/peds.137.Supplement_3.121A.
Lee-Kwan, S. H., Park, S., Maynard, L. M., Blanck, H. M., McGuire, L. C. , & Collins, J. L. (2016). Parental Characteristics and Reasons Associated With Purchasing Kids’ Meals for Their Children. American Journal of Health Promotion , 0890117116683797. DOI: 10.1177/0890117116683797.
Jewett, A., Beck, L. F., Taylor, C ., & Baldwin, G. (2016). Bicycle helmet use among persons 5years and older in the United States, 2012. Journal of safety research , 59 , 1-7. DOI: 10.1016/j.jsr.2016.09.001.
Haarbauer-Krupa, J., Taylor, C. A ., Yue, J. K., Winkler, E. A., Pirracchio, R., Cooper, S. R., … & Manley, G. T. (2016). Screening for post-traumatic stress disorder in a civilian emergency department population with traumatic brain injury. Journal of neurotrauma , (ja). DOI: 10.1089/neu.2015.4158.
Edwards, V. J. , Anderson, L. A. , Thompson, W. W., & Deokar, A. J. (2016). Mental health differences between men and women caregivers, BRFSS 2009. Journal of Women & Aging , 1-7. DOI: 10.1080/08952841.2016.1223916.
Nicklett, E. J., Anderson, L. A., & Yen, I. H. (2016). Gardening Activities and Physical Health Among Older Adults A Review of the Evidence. Journal of Applied Gerontology , 35 (6), 678-690. DOI: 10.1177/0733464814563608.
Vandenberg, A. E., Hunter, R. H., Anderson, L. A., Bryant, L. L., Hooker, S. P., & Satariano, W. A. (2016). Walking and Walkability: Is Wayfinding a Missing Link? Implications for Public Health Practice. Journal of physical activity & health , 13 (2). DOI: 10.1123/jpah.2014-0577.
Anderson, L. A., & Slonim, A. (2016). Perspectives on the strategic uses of concept mapping to address public health challenges. Evaluation and Program Planning, 60, 194-201. DOI: 10.1016/j.evalprogplan.2016.08.011
Hunter, R. H., Anderson, L. A., & Belza, B. L. (Eds.). (2016). Community Wayfinding: Pathways to Understanding . Springer. ISBN 978-3-319-31072-5.
Greenlund, K. J., Liu, Y., Deokar, A. J., Wheaton, A. G., & Croft, J. B. (2016). Association of Chronic Obstructive Pulmonary Disease With Increased Confusion or Memory Loss and Functional Limitations Among Adults in 21 States, 2011 Behavioral Risk Factor Surveillance System. Preventing chronic disease , 13 . DOI: 10.5888/pcd13.150428.
Faul, M., Stevens, J. A., Sasser, S. M., Alee, L., Deokar, A. J., Kuhls, D. A., & Burke, P. A. (2016). Older adult falls seen by emergency medical service providers: a prevention opportunity. American journal of preventive medicine , 50 (6), 719-726. DOI: 10.1016/j.amepre.2015.12.011.
Bouldin, E. D., Thompson, M. L., Boyko, E. J., Morgenroth, D. C., & Littman, A. J. (2016). Weight change trajectories after incident lower-limb amputation. Archives of physical medicine and rehabilitation , 97 (1), 1-7. DOI: 10.1016/j.apmr.2015.09.017
Bouldin, E. D., Littman, A. J., Wong, E., Liu, C. F., Taylor, L., Rice, K., & Reiber, G. E. (2016). Medicare‐VHA dual use is associated with poorer chronic wound healing. Wound Repair and Regeneration , 24 (5), 913-922. DOI: 10.1111/wrr.12454
Cannell, M. B., Bouldin, E. D., Teigen, K., Akhtar, W. Z., & Andresen, E. M. (2016). The cross-sectional association between severity of non-cognitive disability and self-reported worsening memory. Disability and health journal , 9 (2), 289-297. DOI: 10.1016/j.dhjo.2015.09.001
CDC Healthy Aging Program authors are in bold face .
Anderson, L. (In Press). Population Aging. In Oxford Bibliographies in Public Health. Ed. David McQueen. New York: Oxford University Press.
Anderson, L.A. , Prohaska, T.R., & Satariano, W.A. (In Press). Prevention. Whitbourne, S.K. (ed.), The Encyclopedia of Adulthood and Aging. Hoboken, NJ: John Wiley & Sons, Inc.
Anderson, L.A., Deokar, A., Edwards, V.J. , Bouldin. E.D., & Greenlund, K.J. (2015). Demographic and Health Status Differences Among People Aged 45 or Older With and Without Functional Difficulties Related to Increased Confusion or Memory Loss, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140429. DOI: http://dx.doi.org/10.5888/pcd12.140429 .
Brady, T.J., Anderson, L.A. , & Kobau, R. (2015). Chronic Disease Self-Management Support: Public Health Perspectives. Frontiers in Public Health , http://dx.doi.org/10.3389/fpubh.2014.00234 .
Deokar, A.J. , Bouldin, E.D., Edwards, V.J., & Anderson, L.A. (2015). Increased Confusion and Memory Loss in Households, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140430. DOI: http://dx.doi.org/10.5888/pcd12.140430 .
Edwards, V.J., Anderson, L.A., & Deokar, A.J. (2015). Proxy Reports About Household Members With Increased Confusion or Memory Loss, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140427. DOI: http://dx.doi.org/10.5888/pcd12.140427 .
Friedman, D.B., Becofsky, K., Anderson, L.A. , et al. (2015). Public perceptions about risk and protective factors for cognitive health and impairment: A review of the literature. International Psychogeriatrics . Jan 16:1-13. doi:10.1017/S1041610214002877. (attached PDF for personal reading)
Phelan, E., Debman, K., Anderson, L.A. , & Owen, S. (2015). A Systematic Review of Intervention Studies to Prevent Hospitalizations of Community-dwelling Older Adults with Dementia. Medical Care . 53(2), 207–213. doi: 10.1097/MLR.0000000000000294
Petrescu-Prahova, M., Belza, B., Leith, K., Allen, P., Coe, N., & Anderson, L. (In Press). Using Social Network Analysis to Assess Mentorship and Collaboration in a Public Health Network. Preventing Chronic Disease .
Shenson, D., Moore, R.T., Benson, W., & Anderson, L.A. (2015). Polling Places, Pharmacies, and Public Health: Vote & Vax 2012. American Journal of Public Health . doi:10.2105/AJPH.2015.302628
Snowden, M., Steinman, L., Carlson, W.L., Mochan, K.N., Abraido-Lanza, A.F., Bryant , L. … & Anderson, L.A. (2015). Effect of Physical Activity, Social Support and Skills Training on Late-Life Emotional Health: A Systematic Literature Review and Implications for Public Health Research” Frontiers in Public Health .
Anderson, L.A. , & Egge, R. (2014). Expanding efforts to address Alzheimer’s disease: The Healthy Brain Initiative. Alzheimer’s & Dementia . 10:S453–S456. [doi:10.1016/j.jalz.2014.05.1748]
Anderson, L.A. , & Prohaska, T.R. (2014). Fostering Engagement and Independence: Opportunities and Challenges for an Aging Society, Health Education & Behavior , 41:5S-9S. [doi:10.1177/1090198114547818]
Anderson, L.A. , Slonim, A., Yen, I.H., Jones, D.L., Allen, P., Hunter, R.H., Goins, R.T., Leith, K.H., Rosenberg, D., Satariano, W.A., & McPhillips-Tangum, C. (2014). Developing a Framework and Priorities to Promote Mobility Among Older Adults. Health Education & Behavior . 41:10S-18S. [doi:10.1177/1090198114537492]
Barbour, K.E., Stevens, J.A., Helmick, C.G., Lou, Y-H, Murphy, L.B., Hootman, .J.M., Theis K., Anderson, L.A. , Baker, N.A., & Sugerman, D.E. (2014). Falls and Fall Injuries Among Adults with Arthritis — United States, 2012. MMWR . 63 (17):379–383.
Barile, J.P., Edwards, V.J. , Dhingra, S.S., & Thompson, W.W. (2014). Associations Among County-Level Social Determinants of Health, Child Maltreatment, and Emotional Support on Health-Related Quality of Life in Adulthood. Psychology of Violence . doi:10.1037/a0038202
Liu, Y., Croft, J.B., Anderson, L.A. , Wheaton, A.G., Presley-Cantrell, L.R., & Ford, E.S. (2014). The Association of Chronic Obstructive Pulmonary Disease, Disability, Engagement in Social Activities, and Mortality Among Older Adults: United States, 1994-2006. International Journal of Chronic Obstructive Pulmonary Disease . 9:75 – 83. [doi:dx.doi.org/10.2147/COPD.S53676]
Nicklett, E., Anderson, L.A. , & Yen, I. (2014). Gardening Activities and Physical Health among Older Adults: A Review of the Evidence. Journal of Applied Gerontology .DOI: 10.1177/0733464814563608
Ory, M.G., Anderson, L.A. , Friedman, D.B., Pulczinskia, J.C., Eugene, N., & Satariano, W.A., (2014). Setting the Stage: Cancer Prevention among Adults Aged 45 to 64. American Journal of Preventive Medicine . 46(3), Supplement 1: S1–S6.
Rao, J., Anderson, L.A. , Lin, F.C., & Laux, J.P. (2014). Completion of Advance Directives Among U.S. Consumers, American Journal of Preventive Medicine . 46(1):65–70.
Yen, I.H., Flood, J.F., Thompson, H., Anderson, L.A. , & Wong, G. (2014). How Design of Places Promotes or Inhibits Mobility of Older Adults: Realist Synthesis of 20 Years of Research. Journal of Aging and Health . Published online 30 April 2014 [doi:10.1177/0898264314527610]
Krist, A.H., Shenson, D., Woolf, S.H., Bradley, C., Liaw, W., Rothemich, S., Slonim, A., Benson, W. & Anderson, L.A. (2013). Clinical and Community Delivery Systems for Preventive Care: An Integration Framework. American Journal of Preventive Medicine . 45(4):508–516.
Liu Y, Croft JB, Chapman DP, Perry GS, Greenlund KJ, Zhao G, Edwards VJ . (2013). Relationship between adverse childhood experiences and unemployment among adults from five US states. Social Psychiatry and Psychiatric Epidemiology , 48, 357-369. doi:10.1007/s00127-012-0554-1.
Slonim, A., Benson, W., Anderson, L.A. , Jones, E. (2013). Strategic Priorities to Increase Use of Clinical Preventive Services Among Older US Adults. Preventing Chronic Disease . 10:120231. [doi:http://dx.doi.org/10.5888/pcd10.120231]
Spencer, L.M., Schooley, M.W., Anderson, L.A. , Kochtitzky, C.S., DeGroff, A..S, Devlin, H.M., & Mercer, S.L. (2013) Seeking Best Practices: A Conceptual Framework for Planning and Improving Evidence-Based Practices. Preventing Chronic Disease . 10:130186. [doi: http://dx.doi.org/10.5888/pcd10.130186]
Wilcox, S., Altpeter, M., Anderson, L.A. , Belza, B., Bryant, L., Jones, D.L., Leith, K.H., Phelan, E.A., & Satariano, W.A. (2013). The Healthy Aging Research Network: Resources for Building Capacity for Public Health and Aging Practice. American Journal of Health Promotion. 28(1):2-6. [doi: 10.4278/ajhp.121116-CIT-564]
Anderson, L.A. , Goodman, R., Holtzman R, Posner, S, & Northridge, M. (2012). Aging in the United States: Opportunities and Challenges for Public Health. American Journal of Public Health , 102(3):393–5.
Furner, S.E., & Anderson, L.A. (2012). Assessing the Health and Quality of Life of Older Populations: Concepts, Resources, and Systems. In Prohaska T, Anderson LA, Binstock R. editors. Public Health for an Aging Society. Baltimore, Maryland: Johns Hopkins University Press.
Holtzman, D., & Anderson, L.A. (2012). Aging and health in America: a tale from two boomers. American Journal of Public Health, 102(3):392.
Prohaska, T., Anderson, L.A. , Binstock. R., editors. (2012). Public Health for an Aging Society. Baltimore, Maryland: Johns Hopkins University Press.
Shenson, D., Adams, M., Bolen, J., Wooten K., Clough, J. Giles, W., & Anderson, L.A. (2012). Developing an Integrated Strategy to Reduce Ethnic and Racial Disparities in the Delivery of Clinical Preventive Services for Older Americans. American Journal of Public Health , On-line, Jun 14, 2012.
Yen, I.H., & Anderson, L.A. (2012). Built Environment and Mobility of Older Adults: Key Policy and Practice Efforts, Journal of the American Geriatrics Society. Article first published online: 9 MAY 2012. DOI: 10.1111/j.1532-5415.2012.03949
Anderson, L.A. Population Aging. Oxford Bibliographies Online, 2011. (Chapter for the Public Health section of Oxford Bibliographics Online. This chapter includes over 100 citations on aging related issues.
Anderson, L.A. , Day, K.L. , & Vandenberg A.E. (2011). Using a concept map as a tool for strategic planning: The Healthy Brain Initiative. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 8(5):A117. http://www.cdc.gov/pcd/issues/2011/sep/10_0255.htm .
Day, K.L. , Friedman, D.B., Laditka, J.N., Anderson, L.A. , Hunter, R., Laditka, S.B., Wu, B., McGuire, L.C., & Coy, M.C. (2011). Prevention of Cognitive Impairment: Physician Perceptions and Practices. Journal of Applied Gerontology. DOI: 10.1177/0733464811401354
Kusano, C.T., Bouldin, E.D., Anderson, L.A. , McGuire, L.C. , Salvail, F.R., Wynkoop Simmons, K., Andresen, E.M. (2011). Adult informal caregivers reporting financial burden in Hawaii, Kansas, and Washington: Results from the 2007 Behavioral Risk Factor Surveillance System. Disability and Health Journal. 4(4):229–237.
Prohaska, T., Anderson, L.A. , Hooker, S.P., Hughes, S.L., & Belza, B. (2011). Editorial: Mobility and Aging: Transference to Transportation. Journal of Aging Research . Article ID 392751, doi:10.4061/2011/392751.
Shenson, D., Adams, M., Bolen, J., & Anderson, L.A. (2011). Routine checkups don’t ensure that seniors get preventive services. The Journal of Family Practice . 60(1): E1–10.
Snowden, M., Steinman, L., Mochan, K., Grodstein, F., Thurman, D., Brown, D., … & Anderson, L. A. (2011). Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. Journal of the American Geriatrics Society . 59:704–716.
Vandenberg, A.E., Price, A.E., Friedman, D.B., Marchman, G, & Anderson, L.A. , (2011). How Do Top Cable News Websites Portray Cognition as an Aging Issue? The Gerontologist . 52 (3): 367–382.
McGuire, L.C., Bouldin, E.L., Andresen, E.M., & Anderson, L.A. (2010). Examining modifiable health behaviors, body weight, and use of preventive health services among caregivers and non-caregivers aged 65 years and older using in Hawaii, Kansas, and Washington using BRFSS, 2007. Journal of Nutrition, Health and Aging . 14(5):373-9.
Rao, J.K. , Anderson, L.A. , Sukumar, B., Beauchesne, D.A., Stein, T., & Frankel, R.M. Engaging communication experts in a Delphi process to identify patient behaviors that could impact communication in medical encounters.BMC Health Services Research 2010, 10:97 (19 April 2010).
Snowden, M., Dhingra, S.S., Keyes, C.L.M., & Anderson, L.A. (2010). Changes in Mental Well-Being in the Transition to Late Life: Findings From MIDUS I and II. American Journal of Public Health . 100(12):2385-2388.
Anderson LA , Logsdon RG, Hochhalter AK, Sharkey JR. Introduction. The Gerontologist .2009; 49(S1):S1-S2.
Anderson LA , Day KL , Beard RL, Reed PS, Wu B. The public’s perceptions about cognitive health and Alzheimer’s disease among the U.S. population: A national review. The Gerontologist . 2009; 49(S1):S3-S11.
Day KL , McGuire LC , Anderson LA .The Centers for Disease Control and Prevention’s Healthy Brain Initiative: emerging implications of cognitive impairment. Generations . 2009; 33(1):11-17.
DeFries EL, McGuire LC , Andresen EM, Brumback BA, Anderson LA. Caregivers of older adults with cognitive impairment. Prev Chronic Dis . 2009 Apr;6(2):A46.
McGuire LC , Strine T, Vachirasudlekha S, Anderson LA ,Berry JT, Mokdad AH. Modifiable characteristics of a healthy lifestyle in US older adults with or without serious psychological distress, 2007 Behavioral Risk Factor Surveillance System. Int J Public Health . 2009;54:S84-S93.
McGuire LC, Strine T, Allen RS, Anderson LA , Mokdad AH. The Patient Health Questionnaire 8: current depressive symptoms among U.S. older adults, 2006 Behavioral Risk Factor Surveillance System. y . 2009 Apr;17(4):324-34.
Rao JK, Abraham L, Anderson LA . Novel approach, using end-of-life issues, for identifying items for public health surveillance. Preventing Chronic Disease . 2009;6(2):A57.
White-Cooper S, Dawkins NU, Kamin SL, Anderson LA. Community-Institutional Partnerships: Understanding Trust Among Partners. Health Educ Behav 2009 36: 334-347.
Ford ES, Mokdad AH, Li C, McGuire LC , Strine TW, Okoro CA, et al. Gender differences in coronary heart disease and health-related quality of life: Findings from 10 states from the 2004 Behavioral Risk Factor Surveillance System. Journal of Women’s Health 2008;17(5):757-768.
Frank E, Carrera JS, Rao JK , Anderson LA . Satisfaction with career choice among U.S. medical students. Archives of Internal Medicine 2008;168(15):1712-1716.
Garrett MD, Baldridge D, Benson WF , McGuire LC . Missing cohorts of caregivers among American Indian and Alaska Native communities. The IHS Primary Care Provider 2008;33:105-111.
Mark H, Irwin K, Sternberg M, Anderson LA , Magid DJ, Stiffman M. Providers’ perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sexually Transmitted Diseases 2008;35(2):184-189.
McGuire LC , Strine TW, Vachirasudlekha S , Mokdad AH, Anderson, LA . The prevalence of depression in older U.S. women, 2006 Behaviorial Risk Factor Surveillance System. Journal of Women’s Health 2008;17:501-507.
Rao JK . Applying the findings of public health research to communities: Balancing ideal conditions with real-world circumstances . Preventing Chronic Disease 2008;5(2):[4 pages].
Shenson D, Benson W , Harris AC . Expanding the delivery of clinical preventive services through community collaboration: The SPARC model . Preventing Chronic Disease 2008;5(1):[8 pages].
Thurman DJ, Stevens JA, Rao JK . Practice parameter: Assessing patients in a neurology practice for risk of falls (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008;70(6):473-479.
Wright DS, Anderson LA , Brownson RC, Gwaltney MK, Scherer J, Cross AW, et al. Engaging partners to initiate evaluation efforts: Tactics used and lessons learned from the Prevention Research Centers Program . Preventing Chronic Disease 2008;5(1):[7 pages].
Anderson LA , McConnell SR. The healthy brain and our aging population: translating science to public health practice. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S1-S2.
Anderson LA , McConnell SR. Cognitive health: an emerging public health issue. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S70-S73.
Cunningham-Sabo L, Carpenter WR, Peterson JC, Anderson LA , Helfrich CD, Davis SM. Utilization of prevention research: searching for evidence. American Journal of Preventive Medicine 2007;33(1S):S9–S20.
Davis SM, Anderson LA (Guest Editors). The dissemination and utilization of prevention research: increasing our knowledge and understanding. American Journal of Preventive Medicine 2007;33(1S).
Ford ES, Ajani UA, McGuire LC , Liu S. Dietary magnesium and the metabolic syndrome among U.S. adults. Obesity 2007;15(5):1139-1146.
Li C, Ford ES, McGuire LC , Mokdad AH. Association of metabolic syndrome and insulin resistance with congestive heart failure: findings from National Health and Nutrition Examination Survey III”. Journal of Epidemiology and Community Health 2007;61(1):67-73.
Li C, Ford E, McGuire LC , Mokdad A. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity 2007;15:217-223.
McGuire LC , Ajani UA, Ford ES. Cognitive functioning in late life: the impact of moderate alcohol consumption. Annals of Epidemiology 2007;17:93-99.
McGuire LC , Anderson LA , Talley RC, Crews JE. Supportive care needs of Americans: a major issue for women as both recipients and providers of such care. Journal of Women’s Health 2007:16(6):784-789.
McGuire LC , Ford ES, Okoro CA. U.S. older adults with disabilities in the face of natural disasters: implications for evacuation. Disasters 2007;31:49-56.
McGuire LC , Strine TW, Okoro CA, Ahluwalia IB, Ford ES. Healthy lifestyle behaviors in U.S. older adults with and without disabilities, Behavioral Risk Factor Surveillance System 2003 . Preventing Chronic Disease 2007:4(1):[11 pages].
McGuire LC , Strine TW, Okoro CA, Ahluwalia, IB, Ford ES. Modifiable characteristics of a healthy lifestyle in U.S. older adults with or without frequent mental distress, Behavioral Risk Factor Surveillance System. American Journal of Geriatric Psychiatry 2007;15:754-761.
McGuire LC , Rao JK, Anderson LA , Ford ES. Completion of a durable power of attorney for health care: what does cognition have to do with it? The Gerontologist :47(4),457-467.
Okoro CA, Strine TW, McGuire LC , Balluz LS, Mokdad AH. Employment status and frequent mental distress among adults with disabilities, Occupational Medicine 2007;57(3):217-220. [Epub ahead of print].
Okoro CA, Denny CH, McGuire LC , Balluz L.S, Goins RT, Mokdad AH. Disability among older American Indians and Alaska Natives: Disparities in prevalence, health-risk behaviors, obesity, and chronic conditions. Ethnicity and Disease 2007;17(4):686-692.
Ory MG, Mier N, Sharkey JR, Anderson LA . Translating science into public health practice: lessons from physical activity interventions. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S52-S57.
Rao JK . Complementary and alternative medicine for arthritis. North Carolina Medical Journal 2007;68(6):453-456.
Rao JK . Looking back and looking forward [editorial] . Preventing Chronic Disease 2007;5(1):[3 pages].
Rao JK , Anderson LA , Frankel RM, Inui TS. Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Medical Care 2007;45:340-349.
Theis KA, Rao JK , Anderson LA , Thompson PM. End-of-life content in Comprehensive Cancer Control Plans: A systematic review. American Journal of Hospice Palliative Care 2007;24(5):390-398.
Anderson LA , Gwaltney MK, Sundra DL, Brownson RC, Kane M, Cross AW, Mack R, Schwartz R, Sims T, White CR. Using concept mapping to develop a logic model for the Prevention Research Centers Program . Preventing Chronic Disease . [serial online] 2006 Jan [date cited].
Chapman DP, Williams SM, Strine TW, Anda RF, Moore MJ . Dementia and its implications for public health . Preventing Chronic Disease . [serial online] 2006 Apr [date cited].
Ford ES, Mokdad AH, Link MW, Garvin WS, McGuire LC , Jiles RB, Balluz LS. Chronic disease in health emergencies: in the eye of the hurricane . Preventing Chronic Disease . [serial online] 2006 Apr [date cited].
Healthy Aging Research Network Writing Group. The prevention research centers healthy aging research network . Preventing Chronic Disease . [serial online] 2006 Jan [date cited].
Kobau R, DiIorio CA, Anderson LA , Price PH. Further validation and reliability testing of the Attitudes and Beliefs about Living with Epilepsy (ABLE) components of the CDC Epilepsy Program Instrument on Stigma . Epilepsy & Behavior . 2006; 8(3): 552–9.
McGuire LC , Ahluwalia IB, Strine TW. Chronic disease-related behaviors in U.S. older women: behavioral risk factor surveillance system, 2003 . Journal of Women’s Health . 2006; 15(1): 3–7.
McGuire LC , Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life . American Journal of Geriatric Psychiatry . 2006; 14(1): 36–42.
McGuire LC , Ford ES, Ajani UA. The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging [PDF–295 KB] . BMC Geriatrics . 2006; 6:8.
Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK . Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate . Medical Decision Making . 2006; 26(2): 194–206.
Simoes EJ, Kobau R, Kapp J, Waterman B, Mokdad A, Anderson LA . Associations of physical activity and body mass index with activities of daily living in older adults. Journal of Community Health . 2006; 31(6): 453–467.
Lang JE, Benson WF, Anderson LA . Aging and public health: partnerships that can impact cardiovascular health programs . American Journal of Preventive Medicine . 2005; 29(5S1): 158–163.
Lang JE, Moore MJ, Harris AC, Anderson LA . Healthy aging: priorities and programs of the Centers for Disease Control and Prevention. Generations . 2005; 29(2): 24–29.
Maylahn C, Alongi S, Alongi J, Moore MJ, Anderson LA . Data needs and uses for older adult health surveillance: perspectives from state health agencies . Preventing Chronic Disease [serial online] 2005 Jul [date cited].
Mokdad AH, Mensah GA, Posner SF, Reed E, Simoes EJ, Engelgau MM, and the Chronic Diseases and Vulnerable Populations in Natural Disasters Working Group. When chronic conditions become acute: prevention and control of chronic diseases and adverse health outcomes during natural disasters . Preventing Chronic Disease [serial online] 2005 Nov [date cited].
Rao JK, Alongi J, Anderson LA , Jenkins L, Stokes GA, Kane M. Development of public health priorities for end-of-life initiatives . American Journal of Preventive Medicine . 2005; 29(5): 453–460.
Shenson, D, Bolen, J, Adams, M, Seeff, L, Blackman, D. Are older adults up-to-date with cancer screening and vaccinations? Preventing Chronic Disease . [serial online] 2005 Jul [date cited].
Wheeler FC, Anderson LA , Boddie-Willis C, Price PH, Kane M. The role of state public health agencies in addressing less prevalent chronic conditions . Preventing Chronic Disease . [serial online] 2005 Jul [date cited].
Wilson RS, Scherr PA, Bienias JL, Mendes de Leon CF, Everson-Rose SA, Bennett DA, Evans DA. Socioeconomic characteristics of the community in childhood and cognition in old age . Experimental Aging Research . 2005; 31(4): 393–407.
Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease . Neuroepidemiology . 2005; 25(1): 8–14.
Anderson LA , Cornell CE. Prevention research in women’s health: studies from the field-introduction . Health Education & Behavior . 2004; 31(4 Suppl): 12S–17S.
Mack KA, Anderson LA , Galuska D, Zablotsky D, Holtzman D, Ahluwalia I. Health and sociodemographic factors associated with body weight and weight objectives for women: 2000 Behavioral Risk factor Surveillance System. Journal of Women’s Health . 2004; 13(9): 1019–1032.
Phelan EA, Anderson LA , LaCroix AZ, Larson EB. Older adults’ views of “successful aging”–how do they compare with researchers’ definitions . Journal of the American Geriatrics Society . 2004; 52(2): 211–216.
Rao JK , Hootman JM. Prevention research and rheumatic disease . Current Opinion in Rheumatology . 2004; 16(2): 119-124.
Rao JK , Weinberger M, Anderson LA , Kroenke K. Predicting reports of unmet expectations among rheumatology patients . Arthritis and Rheumatism . 2004; 51(3): 215–221.
Rein DB, Anderson LA , Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population . American Journal of Managed Care . 2004; 10(12): 917–924.
Caplan LS, Mandelson MT, Anderson LA . Validity of self-reported mammography: examining recall and covariates among older women in a health maintenance organization . American Journal of Epidemiology . 2003; 157(3): 267–272.
Currey SS, Rao JK , Winfield JB, Callahan LF. Performance of a generic health-related quality of life measure in a clinic population with rheumatic disease . Arthritis and Rheumatism . 2003; 49(5): 658–664.
Damush TM, Weinberger M, Perkins SM, Rao JK , Tierney WM, Qi R, Clark DO. Randomized trial of a self-management program for primary care patients with acute low back pain: short-term effects . Arthritis and Rheumatism . 2003; 49(2): 179–186.
Damush TM, Weinberger M, Perkins SM, Rao JK , Tierney WM, Qi R, Clark DO. The long term effects of a self-management program for inner-city primary care patients with acute low back pain . Archives of Internal Medicine . 2003; 163(21): 2632–2638.
Freburger JK, Callahan LF, Currey SS, Anderson LA . Use of the Trust in Physician Scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist . Arthritis & Rheumatism . 2003; 49(1): 51–58.
Rao JK , Kroenke K, Mihaliak KA, Grambow SC, Weinberger M. Rheumatology patients’ use of complementary therapies: results from a one-year longitudinal study . Arthritis and Rheumatism . 2003; 49(5): 619-625.
Swindle RW, Rao JK , Helmy A, Plue L, Zhou XH, Eckert GJ, Weinberger M. Integrating clinical nurse specialists into the treatment of primary care patients with depression . International Journal of Psychiatry in Medicine . 2003; 33(1): 17–37.
Tao G, Branson B, Anderson LA , Irwin KL. Do physicians provide counseling with HIV and STD testing at physician offices or hospital outpatient departments . AIDS. 2003; 17(8): 1243–1247.
Zhang P, Tao G, Anderson LA . Differences in access to health care services among adults in rural America by rural classification categories and age . Australian Journal of Rural Health . 2003; 11(2): 64–72.
Ziemer DC, Berkowitz KJ, Panayioto RM, El-Kebbi IM, Musey VC, Anderson LA, Wanko NS, Fowke ML, Brazier CW, Dunbar VG, Slocum W, Bacha GM, Gallina DL, Cook CB, Phillips LS. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes . Diabetes Care . 2003; 26(6): 1719–24.
Anderson LA , Eyler AA, Galuska D, Brown DL, Brownson RC. Relationship of satisfaction with body size and trying to lose weight in a national survey of overweight and obese women aged 40 and older, United States . Preventive Medicine . 2002; 35(4): 390–396.
Damush TM, Weinberger M, Clark DO, Tierney WM, Rao JK , Perkins SM, Verel K. Acute low back pain self-management intervention for urban primary care patients: rationale, design, and predictors of participation . Arthritis and Rheumatism . 2002; 47(4): 372–379.
Newton KM, Buist DSM, Keenan N, Anderson LA , LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey . Obstetrics & Gynecology . 2002; 100(1): 18–25.
Rao JK , Anderson LA , Smith SM. End of life is a public health priority . American Journal of Preventive Medicine . 2002; 23(3): 215–220.
Rao JK , Koppaka VR. Şanti . Annals of Internal Medicine . 2002; 137(10): 852–854.
Rao JK , Kroenke K, Mihaliak KA, Eckert GJ, Weinberger M. Can guidelines impact the ordering of magnetic resonance imaging studies by primary care providers for low back pain . American Journal of Managed Care . 2002; 8(1): 27–35.
Kennet J, McGuire LC , Willis SL, Schaie KW. Memorability functions in verbal memory: a longitudinal approach . Experimental Aging Research . 2000; 26(2): 121–137.
McGuire LC, Morian A, Codding R, Smyer MA. Older adults’ memory for medical information: influence of elderspeak and note taking. International Journal of Rehabilitation and Health . 2000; 5(2): 117–127.
Rao JK , Weinberger M, Kroenke K. Visit-specific expectations and patient-centered outcomes: a literature review . Archives of Family Medicine . 2000; 9(10): 1148–1155.
To receive email updates about Alzheimer's Disease and Healthy Aging, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Current Alzheimer disease research highlights: evidence for novel risk factors
Editor(s): Ji, Yuan-Yuan
1 Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USA
2 Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
3 Institute of Neurology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, China
4 Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, Sichuan, China
5 Centre for Public Health, Queen's University Belfast, Belfast, UK
6 Global Brain Health Institute, University of California, San Francisco, San Francisco, CA, USA
7 Department of Neurology, University of California, San Francisco, CA, USA.
Correspondence to: Dr. Wei-Dong Le, Institute of Neurology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, ChinaE-Mail: [email protected] ; Dr. Yue Leng, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USAE-Mail: [email protected]
How to cite this article: Brenowitz WD, Xiang Y, McEvoy CT, Yang C, Yaffe K, Le WD, Leng Y. Current Alzheimer disease research highlights: evidence for novel risk factors. Chin Med J 2021;134:2150–2159. doi: 10.1097/CM9.0000000000001706
Received 2 February, 2021
Willa D. Brenowitz and Yang Xiang contributed equally to the work.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0
Alzheimer disease (AD) is the most common type of dementia characterized by the progressive cognitive and social decline. Clinical drug targets have heavily focused on the amyloid hypothesis, with amyloid beta (Aβ), and tau proteins as key pathophysiologic markers of AD. However, no effective treatment has been developed so far, which prompts researchers to focus on other aspects of AD beyond Aβ, and tau proteins. Additionally, there is a mounting epidemiologic evidence that various environmental factors influence the development of dementia and that dementia etiology is likely heterogenous. In the past decades, new risk factors or potential etiologies have been widely studied. Here, we review several novel epidemiologic and clinical research developments that focus on sleep, hypoxia, diet, gut microbiota, and hearing impairment and their links to AD published in recent years. At the frontiers of AD research, these findings and updates could be worthy of further attention.
Introduction
As one of the most common neurodegenerative diseases and causes of dementia, Alzheimer disease (AD) is a critical topic for biomedical research. The main clinical manifestation of AD is the progressive decline of cognitive function and activities of daily living, and the pivotal pathological features of AD are amyloid beta (Aβ) deposition, neurofibrillary tangles, neuroinflammation, synaptic degeneration, and neuronal loss. [1] While research on AD has been ongoing for >100 years, our understanding of AD is also constantly enriched by the new research directions. However, there are still no effective treatments to delay, halt, or even reverse the process of AD.
In the past few decades, researchers have gradually shifted their attention from treatments to the early diagnosis and prevention of AD. [2] There is an interest in identifying the novel risk factors for AD as well as the novel biomarkers to help detect AD before the symptom onset. Several potential risk factors for AD have been studied extensively, including cardiovascular disease (CVD), diabetes, obesity, low education, social isolation, and depression. [3] However, recent epidemiologic and clinical studies are expanding our understanding of potential AD markers and risk factors to other health behaviors and conditions, such as sleep, diet, and hearing loss. For example, sleep disturbance has a complex association with AD and may be either a preclinical biomarker or potential modifiable risk factor for AD. In particular, hypoxia, often caused by severe obstructive sleep apnea syndrome (OSAS), significantly promotes the development of AD, inspiring the attempts to treat AD using the hyperbaric oxygen treatment (HBOT). [4] Dietary patterns are associated with cognition in older adults, and the Mediterranean-style diet (MD) is associated with reduced risk of AD. Gut microbiota may be an intriguing potential mediator between diet and AD. [5] Also, hearing impairment which is often ignored in clinical practice has strong associations with the risk of AD.
Here, we review the latest developments and especially the epidemiological evidence on sleep, hypoxia, diet, gut microbiota, and hearing impairment in the research field of AD published in recent years. These topics are receiving increasing research interest and may point to novel areas for intervention in the treatment and prevention of AD. As the frontiers of AD research, these findings and updates could be worthy of further attention.
Recent Progress in the Research of AD
In this section, we will review and summarize the recent progress in the research of AD focusing on five novel potential risk factors or early disease indicators such as sleep, hypoxia, diet, gut microbiota, and hearing impairment. As these factors are relatively novel, this review focuses on the epidemiologic evidence with some discussion of potential mechanisms as well as areas for future research.
Sleep and AD
One of the most exciting recent highlights in Alzheimer research is the bi-directional relationship between sleep disturbances and the risk of AD. Patients with AD frequently experience sleep disturbances, including insomnia, abnormal sleep duration, poor nighttime sleep quality, excessive daytime sleepiness, and disrupted circadian rhythms. [6] These sleep problems subsequently reduce patient quality of life and increase the risk for premature institutionalization.
Importantly, growing evidence from the epidemiological studies has suggested that 50% to 80% increased risk of dementia is associated with sleep disturbances, including insomnia, sleep-disordered breathing (SDB), disrupted circadian rhythms, and sleep-related movement disorders. [7–12] Excessive daytime napping has also been associated with an increased risk of cognitive impairment in older men, although the underlying mechanisms are less clear. [13] Several studies have found a U-shaped association between sleep duration and risk of dementia, [14,15] indicating the effects of both short and long sleep duration on cognitive aging. Furthermore, one recent study discovered 23 macro- and micro-physiological architecture metrics of sleep, including rapid eye movement sleep duration, features of the electroencephalography power spectra derived from multivariate analysis, and spindle and slow oscillation morphology and coupling, which were all strongly linked with cognitive performance in older adults. [16] Sleep disturbances are increasingly recognized as a preclinical marker or potential modifiable risk factor for AD.
Over the past decade, emerging evidence from animal and human studies has begun to uncover the nature of the association between sleep disturbances and AD. Since the earlier animal studies that identified a close link between sleep-wake cycle and the pathogenesis of AD, [17,18] a growing body of research has suggested sleep deprivation as both a result and trigger of Aβ, the hallmark pathological feature of AD. [19] Prospective analysis also showed that baseline measures of non-rapid eye movement (NREM) sleep slow-wave activity and sleep quality are sensitive in predicting longitudinal trajectory of Aβ deposition in healthy older adults, indicating the role of sleep as a useful biomarker for forecasting Aβ pathological progression before the clinical cognitive impairment. [20] Furthermore, sleep–wake cycle was found to regulate brain interstitial fluid (ISF) tau, and chronic sleep deprivation might increase ISF and cerebrospinal fluid (CSF) tau as well as tau pathology spreading. [21] It was also suggested that SDB might lead to increased tau levels over time in those with normal cognition or mild cognitive impairment (MCI). [22] Interestingly, one recent study found a coherent pattern of electrophysiological, hemodynamic, and CSF oscillations during human NREM sleep, suggesting a link in the neurophysiology of sleep and waste clearance in the brain. [23]
In addition to the use of neuroimaging and biomarkers, recent studies are also starting to use novel statistical approaches, such as Mendelian randomization (MR), to overcome the limitations of observational epidemiological studies and to reveal the causal relationship between sleep and AD. For instance, using the MR approach, it was found that higher genetic risk for AD might predict shorter sleep duration, suggesting that short sleep duration could be part of the AD disease process and thus serve as an early marker for AD. [24] Meanwhile, another MR study showed no causal effect of self-reported or accelerometer-measured sleep traits on AD risk. Given the growing evidence that indicates a bi-directional relationship between sleep disturbances and AD, emerging studies are underway to examine the use of sleep interventions in the prevention and treatment of AD. One recent review summarized the effects of several sleep interventions that have been studied in patients with MCI or mild dementia, including Cognitive Behavioral Therapy-Insomnia (CBT-I), a structured limbs exercise program, aromatherapy, phase locked loop acoustic stimulation, transcranial stimulation, suvorexant, melatonin, donepezil, galantamine, rivastigmine, tetrahydroaminoacridine, and Continuous Positive Airway Pressure (CPAP) and concluded that CBT-I, melatonin, suvorexant, and CPAP for OSA hold the most promises. [25] Since medications might further impair cognition, non-pharmacological interventions are of particular interest for older adults who are at high risk for dementia. Cordone et al [26] highlighted several promising techniques to enhance NREM sleep oscillations that have solid scientific basis for preventing or slowing down AD pathology but remain to be tested in clinical settings.
Overall, while it remains inconclusive whether sleep disturbances are early signs or risk factors for AD, recent research highlights the importance of sleep among older adults. Changes in sleep architecture and electroencephalogram might be considered as a valuable marker of AD before the onset of cognitive symptoms and help with the early detection of the disease. [27] Future research is needed to test whether sleep disturbances could be the risk factors for AD and to explore the use of sleep interventions in patients at high risk for AD. [25]
Hypoxia and AD
Hypoxia can be caused by CVD, hematological diseases, chronic kidney diseases (CKD), respiratory dysfunction, and environmental conditions, which could influence the central nervous system and induce neurodegeneration. [28–33] Acute hypoxia can be induced by stroke, while OSAS, capillary dysfunction, and CKD may lead to chronic hypoxia. Cognitive impairment may also occur in normal adults after hypoxia. [34,35]
Hypoxia is associated with AD. [28–33] Both acute and chronic hypoxia intervention in experimental animals can result in the aggravation of cognitive dysfunction and the AD-type pathological alterations including Aβ deposition, hyperphosphorylation of tau protein, synaptic degeneration, and neuronal loss. [36] Increasing evidence suggests that hypoxia facilitates the pathogenesis of AD through multiple pathways including increasing the production and accelerating the accumulation of Aβ, [36] decreasing the degradation of Aβ, [37] reducing the clearance of Aβ, [38] elevating the hyperphosphorylation of tau, [39] inhibiting the autophagic function, [40] aggravating neuroinflammation [41] and oxidation stress, [42] ruining the mitochondria function, [43] and causing the stress of endoplasmic reticulum. [44] As mentioned above, it is rational to propose that hypoxia is one of the essential factors contributing to the pathogenesis of AD.
Given that hypoxia contributes to the pathogenesis of AD, the development of prevention and treatment targeting hypoxia is promising. HBOT is a safe, effective, and routinely used medical procedure. Growing evidence suggests that HBOT can induce the neuroplasticity and improve the cognitive function in patients suffering from neurocognitive impairment due to stroke and brain injuries. [45,46] Moreover, HBOT cannot only improve cognitive functions and ameliorate the brain glucose metabolism in AD and amnestic MCI (aMCI) patients [4,47] but also induce significant senolytic effects including significantly increasing telomere length and clearance of senescent cells in the aging individuals. [48]
Besides, HBOT is capable of improving the cognitive behavioral performance, reducing Aβ burden and tau hyperphosphorylation, alleviating neuroinflammation by decreasing astrogliosis and microgliosis, reducing proinflammatory cytokines, and elevating phagocytic markers in mouse mole of AD. [49] More recently, HBOT was shown to be able to reduce Aβ accumulation and hippocampal neuritic atrophy, increase hippocampal neurogenesis, and profoundly improve the cognitive deficits through the upregulation of neurotrophic factors. [50] Moreover, HBOT has been proved to inhibit Aβ25–35-induced toxicity, oxidative stress, and neuronal apoptosis. [51,52] In addition, both the cognitive impairment and hippocampal damage can be attenuated by HBOT via NF-κB signaling pathway [53] or p38 mitogen-activated protein kinase (MAPK) in the animal model of AD. [54] However, there is still no research report on whether HBOT has the capacity of preventing or delaying the occurrence of AD in high-risk groups at an early stage. Besides, further experiments are still warranted because of the possibility of oxygen toxicity, even though HBOT itself seems to be beneficial to cognition.
Diet and AD
As poor diet contributes to several AD risk factors including obesity, hypertension, and diabetes, modifying the dietary behavior could be an effective public health strategy to protect against age-related neurodegeneration and AD in late life.
A growing body of evidence has linked several foods (eg, green vegetables, berries, fish, and olive oil), nutrients (eg, B-vitamins, vitamin E, and omega-3 fatty acids), and plant bioactives (eg, flavanoids) to reduce dementia risk. Consuming these nutrients and foods in combination as a dietary pattern is likely to exert greater synergistic effects on the physiological processes underlying neurodegeneration. The MD rich in antioxidants and flavonoids and characterized by high intake of fruits, vegetables, whole grains, olive oil, nuts, and legumes; moderate intake of fish, poultry, and alcohol, and low intake of red meat have proven cardiometabolic benefits [55] and remain the most frequently studied dietary pattern for neuroprotection during ageing. Evidence from prospective studies indicate beneficial associations among MD adherence, slower rate of cognitive decline, and reduced risk of cognitive impairment, in Western [56,57] and Asian [58] populations. However, findings have not been consistent likely because of the differences in populations studied, measures of MD and cognition, length of follow-up, and adjustment for important confounders such as cardiovascular morbidity and baseline cognitive function. To date, only a small number of studies have examined the relationship between diet and incident AD. [59] Among older cognitively healthy U.S. adults, those in the highest tertile of MD adherence had 40% to 54% reduced AD risk compared to those in the lowest MD tertile, but results have not been replicated in French or Swedish populations [59] making it difficult to draw firm conclusions.
The neuroprotective mechanisms of a healthy diet are not fully elucidated, but multiple antioxidants, anti-inflammatory, and vascular pathways are likely to be important. The MD improves vascular function and insulin resistance [55,60] that contribute to the cognitive decline and AD. Experimental and preclinical studies have shown that dietary antioxidants and flavonoids have a direct effect on the brain by inhibiting oxidative stress, cytokine production and pro-inflammatory cell signaling pathways, and suppressing neuroinflammatory processes implicated in AD. Emerging data suggest that diets rich in fruit, vegetables, whole grains, and fiber promote biodiversity of the gut microbiome and decreased pro-inflammatory gut-derived bacteria and toxins are shown to contribute to early neuroinflammatory changes, and AD pathology. [5,60] Evidence from observational studies report a link between greater MD adherence and favorable brain structures and functions that protect against neurodegeneration as well as less Aβ accumulation in AD-vulnerable regions of the brain. [61] Furthermore, dietary restriction [62] achieved by calorie restriction (30%–40%) or intermittent fasting may provide neuroprotection by attenuating neuroinflammation and insulin resistance and promoting synaptic plasticity and neurogenesis. [63] Beneficial effects of DR on AD pathology have been demonstrated in some [64,65] but not all [66] transgenic animal models, and it is not yet clear on how the findings translate to humans. [63]
The effect of diet on AD risk is not yet known; however, randomized controlled trial data evaluating the effect of diet on cognitive performance demonstrated less decline in global cognition, memory, and executive function in response to a MD >4 to 6 years [60] with no convincing benefit for shorter-term MD interventions (up to 12 months) [59] in cognitively healthy older adults, suggesting that several years of dietary exposure may be needed to detect changes in intermediate cognitive tests of AD risk in general populations.
Overall, accumulating data suggest a role for diet in AD prevention but larger adequately powered intervention and prospective studies in diverse populations with clinically relevant endpoints incorporating incident AD, MCI as well as sensitive neurocognitive tests and brain biomarkers associated with preclinical AD risk are required to understand the effect of diet on AD from the earliest to later stages of disease.
Gut microbiota and AD
Given the complex bidirectional communication system that exists between the gut and brain, there is a growing interest in the gut microbiome as a novel and potentially modifiable risk factor for cognitive impairment and AD. Gut dysbiosis has been implicated in the pathogenesis and progression of AD.
Compared with the normal controls, the Bacteroidetes , Actinomyces , Ruminococcus , and Selenomonas in AD patients are significantly different. [67] The cognitively normal elderly do not have an AD-type pattern of gut microbiota compared with patients at the early stage of AD, [68] and specific gut microbiota, especially enriched Enterobacteriaceae , are associated with AD patients compared with aMCI and cognitively healthy controls, [69] indicating the potential of gut microbiota in the differential diagnosis of AD. Besides, the alteration of gut microbiota tends to occur several years before the onset of dementia, even at the early stage of MCI. [68] A cross-sectional study showed that an increase in Bacteroidetes in non-dementia patients is independently associated with the presence of MCI. [70] The abundance increase of Enterobacteriaceae , Akkermansia , Slackia , Christensenellaceae , and Erysipelotriaceae in MCI patients suggests that this special gut microbiota composition may indicate the presence of MCI. [71]
To investigate the causal relationship between gut microbiota and AD, a clinical study found that gamma-aminobutyric acid (GABA) and serotonin may play an important role in the gut microbiota–host interaction in AD patients. [72] A pilot study revealed the characteristics of the MCI-specific gut fungi (mycobiome) signatures and elucidated that the diet-regulated mycobiome are associated with AD markers and fungal–bacterial co-regulation networks in MCI patients. [73] A clinical study showed that Proteobacteria is positively correlated with Aβ42:Aβ40 ratio, but the fecal propionic acid and butyric acid are negatively correlated with Aβ42 level in MCI patients with the MD. [71] Intriguingly, the gut microbiota composition is strongly correlated with apolipoprotein E (ApoE) genotype. The relative abundance of different bacterial groups is significantly different under the influence of ApoE genotype, [74] which is also related to the specific gut microbiota composition of human and ApoE-targeted replacement mice, especially the Prevotellaceae and Ruminococcaceae and several butyrate-producing genera. [75] Moreover, the relative abundance of Prevotella and Ruminococcus in female ApoE4-familial Alzheimers disease (FAD) mice is higher than that of female ApoE3-FAD mice, whereas the relative abundance of Sutterella in male ApoE4-FAD mice is significantly higher than that of female ApoE3-FAD mice, implying a synergistic effect of ApoE and sex on gut microbiota of AD. [76]
The metabolites of gut microbiota are particularly critical in the mechanism of the gut–brain axis. Trimethylamine-n-oxide (TMAO), a kind of gut microbiota metabolites, can be found in human CSF. [77] It was suggested that the gut microbiota metabolites, such as lipopolysaccharide (LPS) and short chain fatty acids, could mediate the systemic inflammation and intracerebral amyloidosis through endothelial dysfunction. [78] A multicenter clinical study found that the serum concentration of primary bile acid (BA) is significantly decreased, whereas the concentrations of secondary BA, deoxycholic acid, and its conjugated form of glycine and taurine are increased in AD patients compared with cognitively normal elderly. [79] Moreover, it was found that the certain blood BA-related indicators are associated with CSF-Aβ, CSF-p-tau 181, CSF-t-tau, glucose metabolism, and brain atrophy in patients with MCI and AD, respectively. [80]
Additionally, there are also many studies exploring the effect and mechanism of different types of intervention on AD using gut microbiota as a potential mediator. High dose of Jatrorrhizine, [81] an essential component of coptidis rhizome, a Chinese traditional herb, is capable of improving the learning and memory ability, reducing Aβ deposition, and altering the abundance of certain gut microbiota composition such as Firmicutes and Bacteroidetes in APP/PS1 mice. [82] Besides, 27-hydroxycholesterol can aggravate AD-type pathological alterations, gut microbiota dysbiosis, and intestinal barrier dysfunction. [83] The fructooligosaccharides derived from Morinda officinalis improves the learning and memory abilities of rats by regulating the interaction between intestinal ecosystem and brain. [84] Xanthoceraside can alleviate the symptoms of AD by affecting the composition and endogenous metabolites of gut microbiota in rats. [85] GV-971, an oligosaccharide sodium, has the capacity of inhibiting gut microbiota dysbiosis and related phenylalanine/isoleucine accumulation, alleviating neuroinflammation, and reversing cognitive dysfunction. [86]
Environmental factors may also influence the pathogenesis of AD through gut microbiota. Long-term exposure to noise alters gut microbiota composition and accelerates age-related neurochemical and inflammatory regulation disorders, resulting in the aggravated AD-type pathological changes in the brain of senescence-accelerated prone mice. [87] Treatment of mid infrared light of peak wavelength 7.7 to 10 mm can attenuate the decline of learning and memory, reduce Aβ deposition, and alter the gut microbiota composition. [88]
Due to the importance of gut microbiota in the pathogenesis of AD, many studies assessed its potential therapeutic value. Clinical studies have found that supplement of multiple probiotics alters the gut microbiota composition and serum tryptophan metabolism in AD patients, [89] and promotes the mental plasticity and stress relief in healthy elderly. [90] A multicenter study published recently has also shown that the MD can alter the composition of the gut microbiota and improve cognitive function in older adults. [5]
In animal study, it revealed that the transplantation of feces from normal wild type mice to AD transgenic mice significantly reduces Aβ burden and tau pathology, attenuates the glial activation, learning and memory impairment, and abnormal expression of genes related to intestinal macrophage activity, and restores the circulating inflammatory monocytes and synaptic plasticity in AD mice. [91,92] The gut microbiota alteration can promote Aβ deposition by activating the MAPK signaling pathway and C/enhancer binding protein β (EBP)/asparagine endopeptidase (AEP) signaling pathway in the brain of AD transgenic mice. [93,94] In addition, the probiotics have the capacity of improving the maze navigation, restoring the long-term potential, and balancing the antioxidant/oxidative biomarkers in mice. [95] In detail, Lactobacillus plantarum inhibits the synthesis of TMAO and reduces the clusterin level. [96] Clostridium butyricum and its metabolites inhibit microglia-mediated neuroinflammation. [97] Bifidobacterium longum regulates NF-κB activation via inhibiting LPS production. [98] Of note, however, clinical studies have found that probiotics supplement does not significantly improve the cognitive and biochemical indicators in patients with severe AD. [99]
Unlike the intestinal probiotics, the effects of antibiotics on AD are more complicated. Antibiotics, such as streptozotocin, can promote the growth of proinflammatory gut microbiota sub-types in animals, leading to learning and memory impairment, as has been used to establish sporadic AD models. [100] Rifampicin and minocycline could decrease the levels of Aβ, glial activation, and inflammatory cytokines in the brain of AD mice. [101,102] Rapamycin not only reduces the level of Aβ and the activation of microglia but also decreases the phosphorylation of tau protein. [103] Nevertheless, despite some encouraging results in the animal studies, the clinical efficacy of antibiotics in patients with AD remains controversial so far.
Hearing impairment and AD
There is an emerging interest in the role of age-related hearing impairment on development of AD and other dementias. Hearing impairments can be caused by changes in the inner ear (eg, peripheral hearing) and/or dysfunction in auditory processing (eg, central hearing). Hearing impairments are common among older adults: affecting up to 40% of adults aged 65 and up to 90% of adults aged >90. [104] Hearing difficulty is commonly reported by patients with AD. [105] Observational studies have found a consistent association between hearing loss and risk of dementia and cognitive decline. [106,107] This work raises the question whether hearing loss may cause AD and dementia; however, alternative mechanisms could also explain this association. [108]
Hearing impairments may directly affect dementia risk through brain atrophy by impairing cognitive processing abilities or by increasing cognitive load. [108] In animal studies, noise-induced (peripheral) hearing loss is associated with increased neurodegeneration in the hippocampus, decreased neurogenesis, and poor memory function. [109] Hearing impairments may also affect “psychosocial wellbeing” including social engagement, mental health, and physical activity, [109] which could lead to increased dementia risk. [110] In epidemiologic studies, peripheral hearing loss measured by pure tone audiometry may offer some of the stronger evidence that hearing loss may cause dementia, since pure tones are less affected by AD-neurodegeneration than central hearing. [110] Peripheral hearing impairment has also been associated with decreased whole brain volumes, reduced temporal lobe or auditory cortex volumes, [111,112] or reduced hippocampal volume. [113] But there are conflicting results. [114]
Hearing loss can often be corrected or mitigated, which could in turn also reduce dementia burden if hearing loss causes dementia. The Lancet commission on dementia prevention in 2020 suggested that treating hearing loss may reduce dementia burden by up to 8%. [110] However, this estimate is based on observational studies which may be biased. Evidence from several small clinical trials has been mixed; some studies are suggestive that treatment of hearing impairments may improve cognition in non-demented patients, [115] but this was not found in AD patients. [116] Although studies often include important confounders in statistical models, unmeasured or residual confounder may remain. Both hearing and cognitive impairments are strongly associated with age, tend to have a gradual onset, and may have shared etiologies, including neurodegeneration, vascular and metabolic diseases, and aging processes. [117] Some studies even question a biologic between hearing and cognition, as many cognitive tests rely on hearing and poor hearing may lead to more errors in hearing-based cognitive tests. [118]
Relatively few studies have examined associations between hearing and AD specifically; one study on dementia sub-types have found associations between hearing impairment and clinical AD but not vascular dementia. [119] One neuroimaging study found an association with pure tone and word recognition hearing loss and in vivo Aβ deposition, [120] while an autopsy study found an association between clinician rated hearing loss and tau neurofibrillary degeneration stage but not Aβ plaque frequency. [121] Higher genetic risk for AD also is associated with increased difficulty hearing in noise in older adults, suggesting a shared biologic pathway and that central hearing loss such as difficulty hearing in noise may be a preclinical marker for AD. [122] Neurodegeneration in AD affects anatomical structures including the auditory pathways: neuritic plaques and tangles have been found in auditory association cortex as well as subcortical auditory pathways, which includes the medial temporal lobe. Several studies find that central auditory processing dysfunction is strongly associated with AD and precedes dementia diagnosis. [123]
Older adults with hearing impairments are a higher risk for dementia and may be an important subgroup for referral for dementia evaluation. Treating hearing impairment may also help to prevent dementia; however, further research is needed to clarify the relationships between hearing impairments, AD, and dementia. Regardless, treatment for hearing impairments should be prioritized to improve quality of life of older adults with hearing loss.
Perspectives
There is no denying that the clinical treatment of AD is currently facing significant bottlenecks. The failure of many clinical trials suggests the importance of early diagnosis and prevention. Therefore, in recent years, researchers have been interested in thinking about AD from a broad perspective and in evaluating novel potential risk factors beyond those traditionally associated with AD such as CVD, diabetes, and education. These findings of the effects of oxygen metabolism, inflammation, and gut microbiota provide novel evidence that systemic effects may impact brain aging. Furthermore, our review highlights the potential importance of underappreciated health factors to healthy aging such as sleep, diet, and hearing. This new research adds further evidence to support a shift from amyloid focused drug targets to multi-domain interventions that may help prevent AD and slow cognitive decline.
However, there is still a lot of work to be done in these areas. In Table 1 , we present main evidence for each topic in our review as well as list key next steps for research. In particular, studies are needed to clarify the causal directions of the association between these potential novel risk factors and AD and to understand the underlying mechanisms and how they are related to AD neuropathogenesis. The clinical application of the study of oxygen metabolism and AD, such as the attempt to treat AD with HBOT; development of new intestinal probiotics; the prevention and treatment effect of specific diet components on AD. As the future direction of AD research, these works will require more multidisciplinary collaboration and the use of innovative research methods.
| Items | Summary of main evidence | Priorities for future research |
| Sleep | There exists a bi-directional relationship between sleep disturbances and dementia, but it remains unclear whether sleep disturbances are early signs or risk factors for AD. | Future research to uncover potential mechanisms and to explore the use of sleep interventions for the prevention and treatment of AD among high-risk older adults. |
| Hypoxia | Chronic hypoxia is one of the important environmental factors contributing to the pathogenesis of AD. | Further research is needed to determine whether prospective prevention and treatment of hypoxia may be helpful to delay or ameliorate the progression of AD by any mechanism. |
| Diet | Certain nutrients (eg, antioxidants) and dietary patterns (eg, Mediterranean diet) might have neuroprotective effects, but results have been inconsistent. | Larger adequately powered intervention and prospective studies in diverse populations with clinically relevant endpoints as well as sensitive neurocognitive tests and brain biomarkers associated with preclinical AD risk are required to understand the effect of diet on AD from the earliest to later stages of disease. |
| Gut microbiome | Numerous evidences have been obtained on the relationship between gut microbiota and AD from clinical studies, animal experiments, and mechanism exploration. | Whether some specific bacteria or combinations of bacteria in the gut microbiota have a role in the prevention and treatment of AD remains to be further clarified. |
| Hearing loss | Peripheral and central hearing loss are associated with lower regional brain volumes and dementia risk | Studies to determine mechanisms and direction of associations. |
- Cited Here |
- View Full Text | PubMed | CrossRef
- PubMed | CrossRef
- View Full Text | PubMed
Alzheimer disease; Sleep; Hypoxia; Diet; Gut microbiota
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Relationship between depression and lifestyle factors in chinese adults using..., association between common cardiovascular drugs and depression, limited value of recovery phase-limited st segment depression of treadmill exercise test</strong>', 'yang hongbo; huang, zheyong; lou, yi; shen, yunli; qian, juying; ge, junbo', 'chinese medical journal', 'february 20, 2014', '127', '4' , 'p 742-746');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> limited value of recovery phase-limited st segment depression of treadmill....
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Aducanumab Discontinued as Alzheimer's Treatment
- Donanemab Approved for Treatment of Early Alzheimer's Disease
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Medicare Treatment Coverage
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- Criteria for Diagnosis and Staging
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

There's No Better Time to Support the Fight Against Alzheimer’s
Alzheimer's association workgroup publishes biology-based criteria for diagnosis and staging of alzheimer's disease.
- Core 1 biomarkers become abnormal early in the disease course. This category of biomarkers (either PET or biofluid) directly measures either amyloid plaques or phosphorylated tau. An individual with an abnormal Core 1 biomarker will nearly always have both plaques and tangles at autopsy in sufficient levels to meet standard criteria for a diagnosis of Alzheimer’s. Therefore, an abnormal Core 1 biomarker result is sufficient to establish a diagnosis of Alzheimer’s and contribute to clinical decision-making throughout the disease continuum.
- Later-changing Core 2 biomarkers reflect deposits of aggregated tau in the brain, and can provide prognostic information. When abnormal, they increase confidence that Alzheimer’s is contributing to symptoms.
- Treatments that target core disease pathology (i.e., anti-amyloid immunotherapies for Alzheimer’s) have received regulatory approval. This highlights the importance of alignment among clinicians, industry, and academia.
- The most significant advance in Alzheimer’s diagnostics in recent years has been the development of blood-based markers with some assays exhibiting accurate diagnostic performance. This makes the biological diagnosis of Alzheimer’s more generally accessible and is projected to revolutionize clinical care and research.
About the Alzheimer's Association
The Alzheimer’s Association is a worldwide voluntary health organization dedicated to Alzheimer’s care, support and research. Our mission is to lead the way to end Alzheimer's and all other dementia — by accelerating global research, driving risk reduction and early detection, and maximizing quality care and support. Our vision is a world without Alzheimer's and all other dementia®. Visit alz.org or call 800.272.3900.
Keep Up With Alzheimer’s News and Events
The first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Comprehensive review on alzheimer’s disease: causes and treatment.

1. Introduction
2. alzheimer’s disease diagnostic criteria, 3. alzheimer’s disease’s neuropathology, 3.1. senile plaques (sp), 3.2. neurofibrillary tangles (nfts), 3.3. synaptic loss, 4. the stages of alzheimer’s disease, 5. causes and risk factors of alzheimer’s disease, 5.1. alzheimer’s disease hypotheses, 5.1.1. cholinergic hypothesis, 5.1.2. amyloid hypothesis, 5.2. alzheimer’s disease risk factors, 5.2.1. aging, 5.2.2. genetics.
- Amyloid Precursor Protein (APP)
- Presenilin-1 (PSEN-1) and Presenilin-2 (PSEN-2)
- Apolipoprotein E (ApoE)
- ATP Binding Cassette Transporter A1 (ABCA1)
- Clusterin Gene (CLU) and Bridging Integrator 1 ( BIN1 )
- Evolutionarily Conserved Signaling Intermediate in Toll pathway (ECSIT)
- Estrogen Receptor Gene (ESR)
- Other Genes
5.2.3. Environmental Factors
- Air Pollution
5.2.4. Medical Factors
- Cardiovascular Disease (CVDs)
- Obesity and Diabetes
6. Treatment
6.1. symptomatic treatment of ad, 6.1.1. cholinesterase inhibitors.
- Rivastigmine
- Galantamine (GAL)
6.1.2. N -methyl d -aspartate (NMDA) Antagonists
6.2. promising future therapies, 6.2.1. disease-modifying therapeutics (dmt), 6.2.2. chaperones.
- Heat Shock Proteins (Hsps)
- Vacuolar sorting protein 35 (VPS35)
6.2.3. Natural Extract
7. conclusions, author contributions, conflicts of interest.
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Sub-Cell. Biochem. 2012 , 65 , 329–352. [ Google Scholar ] [ CrossRef ]
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2011 , 32 , 275–279. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Blass, J.P. Alzheimer’s disease. Dis. A Mon. Dm 1985 , 31 , 1–69. [ Google Scholar ] [ CrossRef ]
- Terry, R.D.; Davies, P. Dementia of the Alzheimer type. Annu. Rev. Neurosci. 1980 , 3 , 77–95. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rathmann, K.L.; Conner, C.S. Alzheimer’s disease: Clinical features, pathogenesis, and treatment. Drug Intell. Clin. Pharm. 1984 , 18 , 684–691. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020 , 12. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020 , 396 , 413–446. [ Google Scholar ] [ CrossRef ]
- Schachter, A.S.; Davis, K.L. Alzheimer’s disease. Dialogues Clin. Neurosci. 2000 , 2 , 91–100. [ Google Scholar ] [ CrossRef ]
- Jatoi, S.; Hafeez, A.; Riaz, S.U.; Ali, A.; Ghauri, M.I.; Zehra, M. Low Vitamin B12 levels: An underestimated cause of minimal cognitive impairment and dementia. Cureus 2020 , 12 , e6976. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cho, H.S.; Huang, L.K.; Lee, Y.T.; Chan, L.; Hong, C.T. Suboptimal baseline serum Vitamin B12 is associated with cognitive decline in people with Alzheimer’s disease undergoing cholinesterase inhibitor treatment. Front. Neurol. 2018 , 9 , 325. [ Google Scholar ] [ CrossRef ]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984 , 34 , 939–944. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Neugroschl, J.; Wang, S. Alzheimer’s disease: Diagnosis and treatment across the spectrum of disease severity. Mt. Sinai J. Med. N. Y. 2011 , 78 , 596–612. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011 , 7 , 263–269. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012 , 2 , a006239. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Yaari, R.; Fleisher, A.S.; Tariot, P.N. Updates to diagnostic guidelines for Alzheimer’s disease. Prim. Care Companion Cns Disord. 2011 , 13 , 11f01262. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011 , 1 , a006189. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014 , 82 , 756–771. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Singh, S.K.; Srivastav, S.; Yadav, A.K.; Srikrishna, S.; Perry, G. Overview of Alzheimer’s disease and some therapeutic approaches targeting abeta by using several synthetic and herbal compounds. Oxidative Med. Cell. Longev. 2016 , 2016 , 7361613. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cras, P.; Kawai, M.; Lowery, D.; Gonzalez-DeWhitt, P.; Greenberg, B.; Perry, G. Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc. Natl. Acad. Sci. USA 1991 , 88 , 7552–7556. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Perl, D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. N. Y. 2010 , 77 , 32–42. [ Google Scholar ] [ CrossRef ]
- Armstrong, R.A. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol. 2009 , 47 , 289–299. [ Google Scholar ] [ PubMed ]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017 , 38 , 1205–1235. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tabaton, M.; Piccini, A. Role of water-soluble amyloid-beta in the pathogenesis of Alzheimer’s disease. Int. J. Exp. Pathol. 2005 , 86 , 139–145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brion, J.P. Neurofibrillary tangles and Alzheimer’s disease. Eur. Neurol. 1998 , 40 , 130–140. [ Google Scholar ] [ CrossRef ]
- Metaxas, A.; Kempf, S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016 , 11 , 1579–1581. [ Google Scholar ] [ CrossRef ]
- Overk, C.R.; Masliah, E. Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochem Pharm. 2014 , 88 , 508–516. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lleo, A.; Nunez-Llaves, R.; Alcolea, D.; Chiva, C.; Balateu-Panos, D.; Colom-Cadena, M.; Gomez-Giro, G.; Munoz, L.; Querol-Vilaseca, M.; Pegueroles, J.; et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol. Cell. Proteom. Mcp 2019 , 18 , 546–560. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer Disease. JAMA Neurol. 2016 , 73 , 561–571. [ Google Scholar ] [ CrossRef ]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016 , 12 , 292–323. [ Google Scholar ] [ CrossRef ]
- Kumar, A.; Sidhu, J.; Goyal, A. Alzheimer Disease. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 8 December 2020).
- Wattmo, C.; Minthon, L.; Wallin, A.K. Mild versus moderate stages of Alzheimer’s disease: Three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy. Alzheimer’s Res. Ther. 2016 , 8 , 7. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Apostolova, L.G. Alzheimer disease. Continuum 2016 , 22 , 419–434. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019 , 57 , 87–105. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013 , 36 , 375–399. [ Google Scholar ] [ CrossRef ]
- Babic, T. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999 , 67 , 558. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016 , 14 , 101–115. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Monczor, M. Diagnosis and treatment of Alzheimer’s disease. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2005 , 5 , 5–13. [ Google Scholar ] [ CrossRef ]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain A J. Neurol. 2018 , 141 , 1917–1933. [ Google Scholar ] [ CrossRef ]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the amyloid hypothesis in Alzheimer’s disease. J. Alzheimer’s Dis. Jad 2019 , 68 , 493–510. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018 , 12 , 25. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ricciarelli, R.; Fedele, E. The amyloid cascade hypothesis in Alzheimer’s disease: It’s time to change our mind. Curr. Neuropharmacol. 2017 , 15 , 926–935. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Guerreiro, R.; Bras, J. The age factor in Alzheimer’s disease. Genome Med. 2015 , 7 , 106. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016 , 160 , 134–147. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019 , 15 , 565–581. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010 , 23 , 213–227. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. Off. J. Am. Coll. Med Genet. 2016 , 18 , 421–430. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Khanahmadi, M.; Farhud, D.D.; Malmir, M. Genetic of Alzheimer’s disease: A narrative review article. Iran. J. Public Health 2015 , 44 , 892–901. [ Google Scholar ]
- Li, N.M.; Liu, K.F.; Qiu, Y.J.; Zhang, H.H.; Nakanishi, H.; Qing, H. Mutations of beta-amyloid precursor protein alter the consequence of Alzheimer’s disease pathogenesis. Neural Regen. Res. 2019 , 14 , 658–665. [ Google Scholar ] [ CrossRef ]
- Tcw, J.; Goate, A.M. Genetics of beta-Amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 2017 , 7 , a024539. [ Google Scholar ] [ CrossRef ]
- Bi, C.; Bi, S.; Li, B. Processing of mutant beta-amyloid precursor protein and the clinicopathological features of familial Alzheimer’s disease. Aging Dis. 2019 , 10 , 383–403. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Dai, M.H.; Zheng, H.; Zeng, L.D.; Zhang, Y. The genes associated with early-onset Alzheimer’s disease. Oncotarget 2018 , 9 , 15132–15143. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Zhao, J.; Liu, X.; Xia, W.; Zhang, Y.; Wang, C. Targeting amyloidogenic processing of APP in Alzheimer’s disease. Front. Mol. Neurosci. 2020 , 13 , 137. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cai, Y.; An, S.S.; Kim, S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging 2015 , 10 , 1163–1172. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Lanoiselee, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017 , 14 , e1002270. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- De Strooper, B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. Embo Rep. 2007 , 8 , 141–146. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017 , 114 , 629–631. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Walker, E.S.; Martinez, M.; Brunkan, A.L.; Goate, A. Presenilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Abeta 42/40 ratios. J. Neurochem. 2005 , 92 , 294–301. [ Google Scholar ] [ CrossRef ]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009 , 63 , 287–303. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013 , 9 , 106–118. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Giau, V.V.; Bagyinszky, E.; An, S.S.; Kim, S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015 , 11 , 1723–1737. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Koldamova, R.; Fitz, N.F.; Lefterov, I. ATP-binding cassette transporter A1: From metabolism to neurodegeneration. Neurobiol. Dis. 2014 , 72 Pt A , 13–21. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Nordestgaard, L.T.; Tybjaerg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015 , 11 , 1430–1438. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s disease: Mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 2019 , 13 , 164. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Holler, C.J.; Davis, P.R.; Beckett, T.L.; Platt, T.L.; Webb, R.L.; Head, E.; Murphy, M.P. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer’s disease brain and correlates with neurofibrillary tangle pathology. J. Alzheimer’s Dis. Jad 2014 , 42 , 1221–1227. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Andrew, R.J.; De Rossi, P.; Nguyen, P.; Kowalski, H.R.; Recupero, A.J.; Guerbette, T.; Krause, S.V.; Rice, R.C.; Laury-Kleintop, L.; Wagner, S.L.; et al. Reduction of the expression of the late-onset Alzheimer’s disease (AD) risk-factor BIN1 does not affect amyloid pathology in an AD mouse model. J. Biol. Chem. 2019 , 294 , 4477–4487. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Soler-Lopez, M.; Badiola, N.; Zanzoni, A.; Aloy, P. Towards Alzheimer’s root cause: ECSIT as an integrating hub between oxidative stress, inflammation and mitochondrial dysfunction. Hypothetical role of the adapter protein ECSIT in familial and sporadic Alzheimer’s disease pathogenesis. Bioessays News Rev. Mol. Cell. Dev. Biol. 2012 , 34 , 532–541. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Mi Wi, S.; Park, J.; Shim, J.H.; Chun, E.; Lee, K.Y. Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-kappaBs in Toll-like receptor 4 signaling. Mol. Biol. Cell 2015 , 26 , 151–160. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soler-Lopez, M.; Zanzoni, A.; Lluis, R.; Stelzl, U.; Aloy, P. Interactome mapping suggests new mechanistic details underlying Alzheimer’s disease. Genome Res. 2011 , 21 , 364–376. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Zhao, L.; Woody, S.K.; Chhibber, A. Estrogen receptor beta in Alzheimer’s disease: From mechanisms to therapeutics. Ageing Res. Rev. 2015 , 24 , 178–190. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sundermann, E.E.; Maki, P.M.; Bishop, J.R. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause 2010 , 17 , 874–886. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yaffe, K.; Lindquist, K.; Sen, S.; Cauley, J.; Ferrell, R.; Penninx, B.; Harris, T.; Li, R.; Cummings, S.R. Estrogen receptor genotype and risk of cognitive impairment in elders: Findings from the Health ABC study. Neurobiol. Aging 2009 , 30 , 607–614. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Goumidi, L.; Dahlman-Wright, K.; Tapia-Paez, I.; Matsson, H.; Pasquier, F.; Amouyel, P.; Kere, J.; Lambert, J.C.; Meirhaeghe, A. Study of estrogen receptor-alpha and receptor-beta gene polymorphisms on Alzheimer’s disease. J. Alzheimer’s Dis. Jad 2011 , 26 , 431–439. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Khorram Khorshid, H.R.; Gozalpour, E.; Saliminejad, K.; Karimloo, M.; Ohadi, M.; Kamali, K. Vitamin D Receptor (VDR) polymorphisms and late-onset Alzheimer’s disease: An association study. Iran. J. Public Health 2013 , 42 , 1253–1258. [ Google Scholar ] [ PubMed ]
- Liu, X.; Jiao, B.; Shen, L. The epigenetics of Alzheimer’s Disease: Factors and therapeutic implications. Front. Genet. 2018 , 9 , 579. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Wainaina, M.N.; Chen, Z.; Zhong, C. Environmental factors in the development and progression of late-onset Alzheimer’s disease. Neurosci. Bull. 2014 , 30 , 253–270. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Grant, W.B.; Campbell, A.; Itzhaki, R.F.; Savory, J. The significance of environmental factors in the etiology of Alzheimer’s disease. J. Alzheimer’s Dis. Jad 2002 , 4 , 179–189. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012 , 2012 , 472751. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Croze, M.L.; Zimmer, L. Ozone atmospheric pollution and Alzheimer’s disease: From epidemiological facts to molecular mechanisms. J. Alzheimer’s Dis. Jad 2018 , 62 , 503–522. [ Google Scholar ] [ CrossRef ]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013 , 2013 , 524820. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-ing: Focusing on Alzheimer’s disease. Oxidative Med. Cell. Longev. 2017 , 2017 , 7039816. [ Google Scholar ] [ CrossRef ]
- Koyama, A.; Hashimoto, M.; Tanaka, H.; Fujise, N.; Matsushita, M.; Miyagawa, Y.; Hatada, Y.; Fukuhara, R.; Hasegawa, N.; Todani, S.; et al. Malnutrition in Alzheimer’s disease, dementia with lewy bodies, and frontotemporal lobar degeneration: Comparison using serum albumin, total protein, and hemoglobin level. PLoS ONE 2016 , 11 , e0157053. [ Google Scholar ] [ CrossRef ]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s disease. J. Alzheimer’s Dis. Jad 2006 , 10 , 145–163. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s disease. Adv. Neurobiol. 2017 , 18 , 183–197. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019 , 431 , 1843–1868. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sochocka, M.; Zwolinska, K.; Leszek, J. The infectious etiology of Alzheimer’s disease. Curr. Neuropharmacol. 2017 , 15 , 996–1009. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Fulop, T.; Itzhaki, R.F.; Balin, B.J.; Miklossy, J.; Barron, A.E. Role of microbes in the development of Alzheimer’s disease: State of the art—An international symposium presented at the 2017 IAGG congress in San Francisco. Front. Genet. 2018 , 9 , 362. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Muzambi, R.; Bhaskaran, K.; Brayne, C.; Smeeth, L.; Warren-Gash, C. Common bacterial infections and risk of incident cognitive decline or dementia: A systematic review protocol. BMJ Open 2019 , 9 , e030874. [ Google Scholar ] [ CrossRef ]
- Stampfer, M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006 , 260 , 211–223. [ Google Scholar ] [ CrossRef ]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. 2017 , 7 , 69–87. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- De Bruijn, R.F.; Ikram, M.A. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014 , 12 , 130. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Alford, S.; Patel, D.; Perakakis, N.; Mantzoros, C.S. Obesity as a risk factor for Alzheimer’s disease: Weighing the evidence. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018 , 19 , 269–280. [ Google Scholar ] [ CrossRef ]
- Pegueroles, J.; Jimenez, A.; Vilaplana, E.; Montal, V.; Carmona-Iragui, M.; Pane, A.; Alcolea, D.; Videla, L.; Casajoana, A.; Clarimon, J.; et al. Obesity and Alzheimer’s disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget 2018 , 9 , 34691–34698. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Anjum, I.; Fayyaz, M.; Wajid, A.; Sohail, W.; Ali, A. Does obesity increase the risk of dementia: A literature review. Cureus 2018 , 10 , e2660. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s disease: Mechanisms and nutritional aspects. Clin. Nutr. Res. 2018 , 7 , 229–240. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Singh, R.; Sadiq, N.M. Cholinesterase Inhibitors. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK544336/ (accessed on 8 December 2020).
- Eldufani, J.; Blaise, G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: A review of recent clinical applications. Alzheimers Dement 2019 , 5 , 175–183. [ Google Scholar ] [ CrossRef ]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019 , 20 , 1479–1487. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Wang, R.; Reddy, P.H. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimer’s Dis. Jad 2017 , 57 , 1041–1048. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kuns, B.; Rosani, A.; Varghese, D. Memantine. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK500025/ (accessed on 8 December 2020).
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016 , 16 , 247–253. [ Google Scholar ] [ CrossRef ]
- Crous-Bou, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017 , 9 , 71. [ Google Scholar ] [ CrossRef ]
- Crismon, M.L. Tacrine: First drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994 , 28 , 744–751. [ Google Scholar ] [ CrossRef ]
- Qizilbash, N.; Birks, J.; Lopez Arrieta, J.; Lewington, S.; Szeto, S. Tacrine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2000 , CD000202. [ Google Scholar ] [ CrossRef ]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007 , 3 , 303–333. [ Google Scholar ] [ PubMed ]
- Kumar, A.; Sharma, S. Donepezil. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK513257/ (accessed on 8 December 2020).
- Dooley, M.; Lamb, H.M. Donepezil: A review of its use in Alzheimer’s disease. Drugs Aging 2000 , 16 , 199–226. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Annicchiarico, R.; Federici, A.; Pettenati, C.; Caltagirone, C. Rivastigmine in Alzheimer’s disease: Cognitive function and quality of life. Ther. Clin. Risk Manag. 2007 , 3 , 1113–1123. [ Google Scholar ] [ PubMed ]
- Muller, T. Rivastigmine in the treatment of patients with Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2007 , 3 , 211–218. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018 , 9 , 171–178. [ Google Scholar ] [ CrossRef ]
- Birks, J.; Grimley Evans, J.; Iakovidou, V.; Tsolaki, M.; Holt, F.E. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2009 , CD001191. [ Google Scholar ] [ CrossRef ]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000 , 60 , 1095–1122. [ Google Scholar ] [ CrossRef ]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010 , 6 , 345–354. [ Google Scholar ] [ CrossRef ]
- Kim, J.K.; Park, S.U. Pharmacological aspects of galantamine for the treatment of Alzheimer’s disease. Excli J. 2017 , 16 , 35–39. [ Google Scholar ] [ CrossRef ]
- Wahba, S.M.; Darwish, A.S.; Kamal, S.M. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovariectomized albino-rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016 , 65 , 151–163. [ Google Scholar ] [ CrossRef ]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019 , 13 , 43. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Huang, Y.J.; Lin, C.H.; Lane, H.Y.; Tsai, G.E. NMDA Neurotransmission dysfunction in behavioral and psychological symptoms of Alzheimer’s disease. Curr. Neuropharmacol. 2012 , 10 , 272–285. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Companys-Alemany, J.; Turcu, A.L.; Bellver-Sanchis, A.; Loza, M.I.; Brea, J.M.; Canudas, A.M.; Leiva, R.; Vazquez, S.; Pallas, M.; Grinan-Ferre, C. A novel NMDA receptor antagonist protects against cognitive decline presented by senescent mice. Pharmaceutics 2020 , 12 , 284. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sanchez-Lopez, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesus, G.; Beas-Zarate, C.; et al. Memantine for the treatment of dementia: A Review on its current and future applications. J. Alzheimer’s Dis. Jad 2018 , 62 , 1223–1240. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cummings, J.; Fox, N. Defining disease modifying therapy for Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2017 , 4 , 109–115. [ Google Scholar ] [ CrossRef ]
- Huang, L.K.; Chao, S.P.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020 , 27 , 18. [ Google Scholar ] [ CrossRef ]
- Neumann, U.; Ufer, M.; Jacobson, L.H.; Rouzade-Dominguez, M.L.; Huledal, G.; Kolly, C.; Luond, R.M.; Machauer, R.; Veenstra, S.J.; Hurth, K.; et al. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. Embo Mol. Med. 2018 , 10 , e9316. [ Google Scholar ] [ CrossRef ]
- Vandenberghe, R.; Riviere, M.E.; Caputo, A.; Sovago, J.; Maguire, R.P.; Farlow, M.; Marotta, G.; Sanchez-Valle, R.; Scheltens, P.; Ryan, J.M.; et al. Active Abeta immunotherapy CAD106 in Alzheimer’s disease: A phase 2b study. Alzheimers Dement 2017 , 3 , 10–22. [ Google Scholar ] [ CrossRef ]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement 2020 , 6 , e12050. [ Google Scholar ] [ CrossRef ]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2020 , 12 , 95. [ Google Scholar ] [ CrossRef ]
- Galimberti, D.; Scarpini, E. Disease-modifying treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2011 , 4 , 203–216. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ghezzi, L.; Scarpini, E.; Galimberti, D. Disease-modifying drugs in Alzheimer’s disease. Drug Des. Dev. Ther. 2013 , 7 , 1471–1478. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Medina, M. An Overview on the clinical development of tau-based therapeutics. Int. J. Mol. Sci. 2018 , 19 , 1160. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Davidowitz, E.J.; Krishnamurthy, P.K.; Lopez, P.; Jimenez, H.; Adrien, L.; Davies, P.; Moe, J.G. In vivo validation of a small molecule inhibitor of tau self-association in htau mice. J. Alzheimer’s Dis. Jad 2020 , 73 , 147–161. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cortez, L.; Sim, V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014 , 8 , 197–202. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Campanella, C.; Pace, A.; Caruso Bavisotto, C.; Marzullo, P.; Marino Gammazza, A.; Buscemi, S.; Palumbo Piccionello, A. Heat shock proteins in Alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018 , 19 , 2603. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wilhelmus, M.M.; de Waal, R.M.; Verbeek, M.M. Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer’s disease. Mol. Neurobiol. 2007 , 35 , 203–216. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Martin-Pena, A.; Rincon-Limas, D.E.; Fernandez-Funez, P. Engineered Hsp70 chaperones prevent Abeta42-induced memory impairments in a Drosophila model of Alzheimer’s disease. Sci. Rep. 2018 , 8 , 9915. [ Google Scholar ] [ CrossRef ]
- Calderwood, S.K.; Murshid, A. Molecular chaperone accumulation in cancer and decrease in Alzheimer’s disease: The potential roles of HSF1. Front. Neurosci. 2017 , 11 , 192. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Repalli, J.; Meruelo, D. Screening strategies to identify HSP70 modulators to treat Alzheimer’s disease. Drug Des. Dev. Ther. 2015 , 9 , 321–331. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Li, X.; Shao, H.; Taylor, I.R.; Gestwicki, J.E. Targeting allosteric control mechanisms in heat shock protein 70 (Hsp70). Curr. Top. Med. Chem. 2016 , 16 , 2729–2740. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Abisambra, J.; Jinwal, U.K.; Miyata, Y.; Rogers, J.; Blair, L.; Li, X.; Seguin, S.P.; Wang, L.; Jin, Y.; Bacon, J.; et al. Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biol. Psychiatry 2013 , 74 , 367–374. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Bohush, A.; Bieganowski, P.; Filipek, A. Hsp90 and its co-chaperones in neurodegenerative diseases. Int. J. Mol. Sci. 2019 , 20 , 4976. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ou, J.R.; Tan, M.S.; Xie, A.M.; Yu, J.T.; Tan, L. Heat shock protein 90 in Alzheimer’s disease. Biomed Res. Int. 2014 , 2014 , 796869. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, B.; Liu, Y.; Huang, L.; Chen, J.; Li, J.J.; Wang, R.; Kim, E.; Chen, Y.; Justicia, C.; Sakata, K.; et al. A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer’s mouse model via an HSF1-mediated mechanism. Mol. Psychiatry 2017 , 22 , 990–1001. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Li, J.G.; Chiu, J.; Ramanjulu, M.; Blass, B.E.; Pratico, D. A pharmacological chaperone improves memory by reducing Abeta and tau neuropathology in a mouse model with plaques and tangles. Mol. Neurodegener. 2020 , 15 , 1. [ Google Scholar ] [ CrossRef ]
- Vagnozzi, A.N.; Li, J.G.; Chiu, J.; Razmpour, R.; Warfield, R.; Ramirez, S.H.; Pratico, D. VPS35 regulates tau phosphorylation and neuropathology in tauopathy. Mol. Psychiatry 2019 . [ Google Scholar ] [ CrossRef ]
- Li, J.G.; Chiu, J.; Pratico, D. Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35. Mol. Psychiatry 2020 , 25 , 2630–2640. [ Google Scholar ] [ CrossRef ]
- Mecozzi, V.J.; Berman, D.E.; Simoes, S.; Vetanovetz, C.; Awal, M.R.; Patel, V.M.; Schneider, R.T.; Petsko, G.A.; Ringe, D.; Small, S.A. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014 , 10 , 443–449. [ Google Scholar ] [ CrossRef ]
- Knight, E.M.; Williams, H.N.; Stevens, A.C.; Kim, S.H.; Kottwitz, J.C.; Morant, A.D.; Steele, J.W.; Klein, W.L.; Yanagisawa, K.; Boyd, R.E.; et al. Evidence that small molecule enhancement of beta-hexosaminidase activity corrects the behavioral phenotype in Dutch APP(E693Q) mice through reduction of ganglioside-bound Abeta. Mol. Psychiatry 2015 , 20 , 109–117. [ Google Scholar ] [ CrossRef ]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019 , 20 , 2313. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ma, Y.; Yang, M.W.; Li, X.W.; Yue, J.W.; Chen, J.Z.; Yang, M.W.; Huang, X.; Zhu, L.L.; Hong, F.F.; Yang, S.L. Therapeutic effects of natural drugs on Alzheimer’s disease. Front. Pharmacol. 2019 , 10 , 1355. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Disease Modifying Agents | Mechanism of Action |
|---|---|
| Phase 3 Clinical Trials | |
| Monoclonal antibody—targets β-amyloid and removes it. | |
| Monoclonal antibody—binds and removes β-amyloid. | |
| Amyloid vaccine—stimulates production of antibodies against β-amyloid. | |
| Monoclonal antibody—reduces protofibrillar β-amyloid. | |
| Tau protein aggregation inhibitor. | |
| Low-dose levetiracetam—improves synaptic function and reduces amyloid-induced neuronal hyperactivity | |
| Mast cell stabilizer and anti-inflammatory—promotes microglial clearance of amyloid | |
| RAGE (Receptor for Advanced Glycation End-products) antagonist—reduces inflammation and amyloid transport into the brain | |
| Glutamate modulator—reduces synaptic levels of glutamate and improves synaptic functioning | |
| Tyrosine kinase inhibitor—modulates inflammatory mast cell and reduces amyloid protein and tau phosphorylation | |
| Monoclonal antibody—targets soluble oligomers and removes β-amyloid | |
| Monoclonal antibody—prevents tau propagation | |
| Active immunotherapy—targets β-amyloid and removes it | |
| Monoclonal antibody—removes amyloid protofibrils and reduces amyloid plaques | |
| Monoclonal antibody—removes tau and reduces tau propagation | |
| Monoclonal antibody—removes amyloid by recognizing aggregated pyroglutamate form of Aβ | |
| Monoclonal antibody—neutralizes soluble tau aggregates | |
| Monoclonal antibody—removes extracellular tau | |
| Alpha-secretase modulator—reduces amyloid | |
| Monoclonal antibody—immunomodulatory that targets CD38 and regulates microglial activity | |
| Tyrosine kinase inhibitor (dasatinib) + flavonoid (quercetin)—reduces senescent cells and tau aggregation | |
| Epigenetic, Tau Antisense oligonucleotide—reduces tau production | |
| Neurotransmitter receptors ion channel modulator—improves neuropsychiatric symptoms | |
| Tyrosine kinase inhibitor—promotes clearance of amyloid and tau proteins | |
| Selective inhibitor of APP—reduces amyloid, tau, and α-synuclein production | |
| Filamin A protein inhibitor—reduces tau hyperphosphorylation, synaptic dysfunction, and stabilizes soluble amyloid and the α7 nicotinic acetylcholine receptor interaction | |
| Glutaminyl cyclase (QC) enzyme inhibitor—reduces amyloid plaques and pyroglutamates Aβ production | |
| Glutamate receptor antagonist—reduces glutamate-mediated excitotoxicity | |
| Activates ABCC1 (ATP binding cassette subfamily C member 1 transport protein)—removes amyloid | |
| Monoclonal antibody—removes tau and reduces tau propagation | |
| Monoclonal antibody—removes tau | |
| Aggregation inhibitor—reduces tau aggregation | |
| Monoclonal antibody—removes amyloid | |
| Stabilizes tubulin-binding, microtubule, and reduces cellular damage mediated by tau | |
| Natural Compounds | Mechanism of Action |
|---|---|
| Aβ formation inhibitors | |
| Reduction of Aβ accumulation | |
| Promotion of Aβ degradation | |
| ), Houttuyniacordata Thunb. (Saururaceae) water extracts, Huperzine A, and ethyl acetate extract from Diospyros kaki L.f | Inhibition of Aβ Neurotoxicity and reduce over-activation of microglial cells, neuroinflammation, oxidative stress, and disruption of calcium homeostasis, which lead to neuron loss |
| L., geniposide from the fruit of G. jasminoides J. Ellis, ginsenoside Rd from Panax ginseng C. A. Mey, crocin from Crocus sativus L., and quinones) | Inhibition of hyperphosphorylated tau protein and its aggregation |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020 , 25 , 5789. https://doi.org/10.3390/molecules25245789
Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules . 2020; 25(24):5789. https://doi.org/10.3390/molecules25245789
Breijyeh, Zeinab, and Rafik Karaman. 2020. "Comprehensive Review on Alzheimer’s Disease: Causes and Treatment" Molecules 25, no. 24: 5789. https://doi.org/10.3390/molecules25245789
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dela J Public Health
- v.7(4); 2021 Sep

Diagnosis and Treatment of Alzheimer’s Disease:
Introduction.
Alzheimer’s Disease (AD) exerts a significant worldwide impact. An estimated 44 million individuals currently live with this Major Neurocognitive Disorder. There are about 6.2 million Americans with AD dementia today and AD kills more people than breast cancer and prostate cancer combined. The National Institute on Aging estimates that the prevalence of AD doubles every five years beyond the age of 65 and as the population ages, a greater proportion of the population is affected. AD will cost the United States over $355 billion in 2021, rising to over $1.5 trillion by 2050 1 imposing a significant economic burden. Early diagnosis and treatment of neurocognitive disorders are critical in determining treatment approaches and shaping policy to prepare for the deluge of cases to come. This review describes current diagnostic approaches and treatment advances for AD.
Current Diagnostic Strategies
The evaluation of a person with suspected memory impairment includes a comprehensive set of assessments aimed at characterizing the etiology of cognitive decline and identifying treatable pathologies. These assessments include a detailed medical history, physical and mental status examinations, basic labs, and neuroimaging studies. Additional tools may also include neuropsychological testing and advanced brain imaging techniques. Once reversible causes have been ruled out, clues for specific causes of major neurocognitive disorder are sought. A history of multiple strokes, for example, may point towards a diagnosis of vascular dementia. A history of head trauma may suggest traumatic encephalopathy. A history of prolonged alcohol use disorder may support the diagnosis of an alcohol-related dementia. In adults over 60, the most frequent cause of progressive cognitive decline is AD. 2

Emerging Diagnostics
Finding earlier and more definitive ways to diagnose AD has been the subject of significant amounts of research, and testing advances have been seen in the last decade with expanded use of positron emission tomography (PET) and magnetic resonance imaging (MRI), as well as in the identification of biomarkers in cerebrospinal fluid (CSF) and more recently serum. While limited, some of these diagnostic advances are available to the public, though typically at a high price.
A high-level overview of emerging diagnostic strategies can be found below.
Volumetric Data
In simple terms, volume changes in specific brain regions can predict the likelihood of progression from mild cognitive impairment (MCI) to AD. These volume assessments can be done by radiologists or with the help of FDA-approved MRI volumetric data software packages such as Neuroquant and Neuoreader. Hippocampal volume changes in particular are regarded as an important AD biomarker. 3 Because of limited sensitivity of this measure in diagnosing AD, however, MRI studies are regarded as a contributor to the diagnostic process but not sufficient in themselves for determining a diagnosis. 4
Diffusion Tensor Imaging
Diffusion Tensor Imaging (DTI) is an advanced neuroimaging technique that uses the diffusion properties of water molecules to generate magnetic resonance images that correspond to changes in macroscopic axonal organization. This technique can be used to evaluate the structure of vertical cellular micro-circuits, termed “minicolumns.” Previous studies have demonstrated that minicolumns are known to be altered in a somewhat predictable and progressive manner during aging, MCI, and AD. 5 Additionally, pathologic changes of cortex columnar architecture are associated with increased plaque load and cognitive decline. 6 With the aid of proprietary software, DTI can be measured and used as a marker of neurodegeneration.
Pathologic species of two proteins, amyloid-β (Aβ) and hyperphosphorylated tau accumulate in the brains of persons with AD. PET scans are able to assess for both proteins and serve as a reliable biomarker. Amyloid accumulation precedes clinically significant cognitive changes and tau accumulation progresses in step with cognitive decline, suggesting the value of PET scans for diagnosis and measurement of disease progression. 7
CSF and Blood Tests
Cerebrospinal fluid (CSF), accessible through lumbar puncture, surrounds the brain. Changes in the levels of Aβ and tau proteins in the CSF develop decades before the onset of clinically significant AD. 8 Among the tests of CSF developed during recent decades, the most prominent are CSF Aβ42:Aβ40 ratio and the CSF tau phosphorylated at threonine 181 (P-tau181). CSF P-tau217, measurable in the peripheral circulation, is hoped to provide a biomarker with very high sensitivity and specificity. 9
Because blood is more easily accessed than CSF, C2N Diagnostics in St. Louis, Missouri has developed and released a blood test called PrecivityAD which is available in most of the U.S. and to the European Union. The test uses mass spectrometry to detect specific species of beta-amyloid in serum which are lower in AD. The test is not presently covered by insurance, representing a significant cost that may be defrayed for eligible individuals through a financial assistance program. The test is not a stand-alone diagnostic tool; rather the results are a probability score and are intended to be interpreted in concert with other testing means. 10 Additionally, research on plasma Aβ42:Aβ40 ratio and P-tau181 suggests potential value. 11 , 12
Implications of New Diagnostic Strategies: Risks vs. Benefits
Although some people express the wish not to know, almost 90% of people surveyed in a large US study expressed a wish to know their diagnosis. Sixty-five percent of respondents said that even if they were asymptomatic they would be likely or somewhat likely to accept a medical test to assess for AD. 13 Early detection offers the benefits of earlier access to medications, inclusion in clinical trials, the opportunity for lifestyle modification, and knowledge useful to families in preparing for the future while the affected individual remains able to participate actively in decision-making.
Early detection and disclosure, however, also carry risks. The mental health effects of receiving a diagnosis are known to be significant not only for patients but also for families. Multiple studies have documented a small increase in death by suicide in those with dementia, most prominently during the first three months after a diagnosis was made. 14 This suggests the need for increased mental health support and monitoring particularly in the early months after a diagnosis is given, as well as the importance of educating family about suicide risk. As treatment options for early stage dementia increase, the benefits and risks of early diagnostic assessment will become a matter of great significance.
Current Therapeutic Strategies and Options
Several mechanisms have been proposed to account for the pathology of AD, and current treatments are counteract these mechanisms. The most widely accepted disease models are the amyloid cascade hypothesis, the tau hypothesis, the cholinergic hypothesis, and the excitotoxicity hypothesis.
Our current AD medications were developed to address the cholinergic deficit that occurs early in AD. The selective loss of cholinergic neurons, an early pathologic finding in AD, results in a profound reduction in the neurotransmitter acetylcholine, which affects learning and memory neuronal circuitry. Facilitating cholinergic transmission was therefore an early approach to AD treatment which resulted in several palliative medications still in use. 15 – 17
Cholinesterase Inhibitors
The primary action of the cholinesterase inhibitors is the reversible inhibition of cholinesterase, the enzyme which breaks down acetylcholine in brain synapses, thereby prolonging the effect of the diminished level of brain acetylcholine. 18 , 19 Three cholinesterase inhibitors are currently used: donepezil, rivastigmine, and galantamine. Meta-analyses have shown that these agents delay decline in cognitive function, slow the decline in global clinical rating, and may delay the decline of activities of daily living (ADL) and emergence of adverse behaviors, as much as 6 to 12 months on average. 20 , 21 Significant side effects include gastrointestinal symptoms, dizziness, vertigo, fatigue, insomnia, hallucinations, bradycardia, syncope, and muscle cramps. 15
Donepezil is a reversible non-competitive acetylcholinesterase inhibitor shown to affect cognitive function, activities of daily living, and global clinical status. Benefits for the 10 mg dose appear marginally larger than for the 5 mg dose. A larger 23-mg dose form is available, with disputed clinical advantages. 20
Rivastigmine is a pseudo-irreversible inhibitor of acetylcholinesterase and butyrylcholinesterase and acts by binding to two active sites of acetylcholinesterase. It is called pseudo-irreversible because it dissociates slower than acetylcholinesterase. 20 Adverse effects of the oral preparation are significant, but the transdermal form is more tolerable for many patients, although it can cause dermatologic reactions. 22 , 23
Galantamine is a reversible competitive acetylcholinesterase inhibitor and modulator of nicotinic acetylcholine receptors. 15 Theoretically, this agent will have greater effect in areas of the brain with low levels of acetylcholine. 24 Its effects are similar to those of the other cholinesterase inhibitors. 24
NMDA Receptor Antagonist
Overstimulation of glutamatergic activity in the brain results in an excitotoxic overload of calcium flux into neurons through N-methyl-D-aspartate (NMDA) receptor ion channels. 25 , 26 Excitotoxicity leads to a gradual loss of synaptic function and eventual neurodegeneration, correlating with the progressive decline in cognition and the pathological anatomy seen in AD. 27 The NMDA receptor plays a critical role in glutamate synaptic transmission and in synaptic plasticity, thought to underlie learning and memory. 28 Memantine, a low-affinity NMDA receptor antagonist, modulates NMDA receptors to reduce glutamate-induced excitotoxicity and is thought to palliate cognitive decline associated with AD in this way. 27 , 28 Memantine is FDA-indicated for moderate to severe AD. 29 It has been shown to improve activities of daily living scores, global function assessment scores, and stage of dementia assessment scores. 30 , 31 It has also been suggested that it can be efficacious in reducing delusions, agitation/aggression, disinhibition, and diurnal rhythm disturbances. 32 Although benefit is clear, its magnitude is modest. 31 It is available as immediate and extended-release formulations, and as part of a combination pill with donepezil. 15 The combination of a cholinesterase inhibitor with memantine appears to have synergistic benefits so it is a standard practice in the treatment of moderate to severe AD. 33
Emerging Treatments
The search for disease-modifying AD therapies has led to the development of medications which target the pathologic forms of amyloid beta (Aβ) protein and tau protein associated with this disease. 34 The amyloid cascade hypothesis proposes that toxic forms of Aβ protein leads to neuronal death and synaptic dysfunction. Aβ pathology is an early finding in the disease. 34 , 35 The tau pathology has been shown to correlate more specifically with the progression of cognitive impairment. 36 , 37
Targeting Amyloid Pathology
The neurodegenerative effects of AD are attributed in part to the effects of beta amyloid and hyperphosphorylated tau, though newer theories raise additional possibilities. Amyloid plaques and tau-containing neurofibrillary tangles remain necessary for a pathological diagnosis of AD. 38 Several familial forms of AD have been linked to genetic mutations which alter the production of amyloid. CSF biomarker studies have also shown that Aβ42 peptides decline one to two decades prior to onset of symptoms in AD. 39 Although insoluble aggregates and soluble dimers of amyloid have been demonstrated to cause synaptic toxicity, the soluble aggregates are considered to correlate better with symptoms of AD and disease severity. 40
Therapeutic agents have been developed to reduce different forms of pathologic Aβ, interrupt Aβ aggregation, or increase Aβ clearance from the CNS. Many tested agents, however, have failed to demonstrate efficacy and some have even caused worsening of cognitive or physical symptoms, raising questions about the amyloid hypothesis. 41 Newer research techniques seek to improve drug evaluation by assuring that adequate measures are used and appropriate subjects enrolled in clinical trials. 42
Passive Immunotherapeutics
Following the early failure of a vaccine intended to develop a beneficial immune response in persons with AD, researchers developed passive immunotherapeutic agents: monoclonal antibody solutions created in biological systems for infusion into human subjects. 43 The objective is to reduce peripheral and central effects of Aβ42. 44 Several passive immunotherapeutic agents have failed clinical trials, but others remain in testing.
Aducanumab (BIIB037) is a human anti-Aβ monoclonal antibody that selectively targets aggregated forms of Aβ, including soluble oligomers and insoluble fibrils. Given as an infusion, aducanumab enters the central nervous system and decreases Aβ in prodromal or mild AD with Aβ PET-confirmed pathology, in a time and dose-dependent manner. Significant plaque reduction has been demonstrated. The main safety finding has been dose-related amyloid-related imaging abnormalities – edema/effusion (ARIA-E) which are more common among Apo-E4 carriers. In subjects who received the highest dose of 10 mg/kg, researchers reported a significant decline in the progression of cognitive impairment (on the CDR-Sum of Boxes). 45 In June of 2021, aducanumab was approved by the FDA for treatment of AD with the stipulation that a phase IV trial carefully assess its efficacy and safety. Subsequently, amidst some controversy about the accelerated approval process which occurred despite limited evidence of treatment benefit, the FDA revised the medication’s indication to target its use toward AD-related mild cognitive impairment or mild dementia.
Lecanemab (BAN2401) is a humanized IgG1 version of a mouse monoclonal antibody which selectively binds to large soluble Aβ protofibrils. Lecanemab has been shown to slow cognitive decline, increase CSF levels of Aβ (which drop in AD), and reduce total tau levels 46 but further validation of current findings is needed to address concerns about the methodology of the initial studies. Three clinical trials are currently in progress, looking at efficacy and safety among early AD subjects ( {"type":"clinical-trial","attrs":{"text":"NCT03887455","term_id":"NCT03887455"}} NCT03887455 ); efficacy and safety among early preclinical and preclinical AD subjects with early and intermediate amyloid ( {"type":"clinical-trial","attrs":{"text":"NCT04468659","term_id":"NCT04468659"}} NCT04468659 ); and safety, efficacy, and tolerability of different dose levels among early AD patients ( {"type":"clinical-trial","attrs":{"text":"NCT01767311","term_id":"NCT01767311"}} NCT01767311 ).
Donanemab (LY3002813) is an immunoglobulin directed towards a molecular target present only in brain amyloid plaques. In a phase II trial among early AD subjects, there was some improvement in composite cognition scores and ability to do activities of daily living (ADLs), but secondary outcomes did not show a significant difference. ARIA-E were observed but were noted to be asymptomatic. 47 A dose escalation study of single and multiple doses explored safety and tolerability and showed 40-50% amyloid reduction and 90% of subjects developed drug antibodies at three months after a single dose. 48
Gantenerumab, an additional monoclonal antibody in testing, has a 20-fold higher affinity for Aβ oligomers than monomers. 49 The earlier phase II trial was terminated for futility but there were dose-dependent effects observed indicating that higher doses may be necessary for efficacy. 50 An analysis of a PET sub-study suggests that at higher doses, there is a robust reduction of amyloid at two years. 51 There are currently ongoing trials evaluating pharmacodynamics of subcutaneous administration ( {"type":"clinical-trial","attrs":{"text":"NCT04592341","term_id":"NCT04592341"}} NCT04592341 ); safety and tolerability of long-term administration ( {"type":"clinical-trial","attrs":{"text":"NCT04339413","term_id":"NCT04339413"}} NCT04339413 ); safety and efficacy among early AD subjects ( {"type":"clinical-trial","attrs":{"text":"NCT03443973","term_id":"NCT03443973"}} NCT03443973 , {"type":"clinical-trial","attrs":{"text":"NCT03444870","term_id":"NCT03444870"}} NCT03444870 ); and safety, tolerability, biomarker, and cognitive efficacy among genetic early onset AD ( {"type":"clinical-trial","attrs":{"text":"NCT01760005","term_id":"NCT01760005"}} NCT01760005 ).
Crenezumab is a monoclonal antibody which binds to monomers and aggregated forms of Aβ with a 10-fold higher affinity for oligomers. 52 Earlier clinical trials did not meet clinical endpoints but there was note of a reduction in clinical decline in the higher dose group, as with gantenerumab. 53 Ongoing trials currently are evaluating crenezumab and its effect on tau burden among presenelin mutation carriers and noncarriers ( {"type":"clinical-trial","attrs":{"text":"NCT03977584","term_id":"NCT03977584"}} NCT03977584 ) and efficacy among preclinical AD ( {"type":"clinical-trial","attrs":{"text":"NCT01998841","term_id":"NCT01998841"}} NCT01998841 ).
BACE Inhibitors
β-site amyloid precursor protein cleaving enzyme (BACE) is an enzyme which performs the initial step in Aβ formation. 41 Several agents have been developed to block BACE activity in order to reduce Aβ accumulation.
Clinical failures of BACE inhibitors among persons with mild to moderate AD and prodromal AD have occurred with lanabecestat (AZD3293, LY3314814), atabecestat (JNJ-54861911), and verbecestat (MK8931). Elenbecestat (CNP520) was the last remaining BACE inhibitor evaluated to potentially slow down the onset and progression of clinical symptoms associated with AD ( {"type":"clinical-trial","attrs":{"text":"NCT02565511","term_id":"NCT02565511"}} NCT02565511 ). The trial was discontinued for safety concerns. 54
BACE inhibitors are successful in inhibiting Aβ formation but they have not been shown to produce cognitive, clinical, or functional benefit in large randomized controlled trials (RCT). Indeed, several BACE inhibitors were found to be poorly tolerated and some of them failed also in patients with prodromal AD. To some investigators, the failure of BACE inhibitors casts doubt on the value of blocking the formation of toxic Aβ in persons with AD. 55
Anti-Aggregation Agents
Another approach to interfering with the amyloid cascade is to block the aggregation of Aβ into oligomers and fibrils into amyloid plaques which may trigger the synaptic dysfunction and neuronal loss in AD. The soluble oligomers are considered the pathogenic form of Aβ associated with neurodegeneration. 56
Scyllo -Inositol (ELND005) has been shown to neutralize toxic effects of Aβ oligomers, including amelioration of oligomer-induced synaptic loss. 57 It is also thought to directly affect both Aβ clearance and myo-inositol regulation to improve cognitive function. 58 Its efficacy outcomes in mild to moderate AD, however, have not been found to be significant. 59 No ongoing trials are addressing its effect on earlier AD stages.
ALZ-801 is an improved prodrug of tramiprosate thought to inhibit the formation of amyloid oligomers without plaque interaction. 60 , 61 It selectively blocks the formation of Aβ oligomers with some clinical efficacy among high risk APOE carriers at a high dose and a dose dependent preservation of hippocampal volume. This is an oral agent which has been shown to have adequate CNS penetration. 60 , 62 Ongoing trials are looking into the effect of ALZ-801 on biomarkers ( {"type":"clinical-trial","attrs":{"text":"NCT04693520","term_id":"NCT04693520"}} NCT04693520 ) and efficacy and safety ( {"type":"clinical-trial","attrs":{"text":"NCT04770220","term_id":"NCT04770220"}} NCT04770220 ), both in APOE carriers.
Tau Directed Therapies
The lack of clear efficacy of amyloid-based therapeutics has led investigators to explore other upstream pathologic processes involving other targets. 46 Tau, a microtubule binding protein which forms neurofibrillary tangles (NFTs), is another histopathologic hallmark which characterizes AD. The accumulation of tau has been found to correlate more closely with severity of dementia than does amyloid load. There is evidence that Aβ accumulation can exacerbate tau pathology and vice versa. 63 Tau protein has also been found to at least partially mediate some of the toxic effects of Aβ leading to synapse loss, dendritic simplification, and eventual cell death in AD. 34 Initial approaches for tau-based therapies have focused on inhibition of kinases or tau aggregation or stabilization of microtubules. Most of these studies have been discontinued due to toxicity or lack of efficacy. 64 Current trials are focused on tau immunotherapies. Post-translational modifications and consequent loss of microtubule binding and tau misfolding lead to elevated levels of tau in the cytosol, making these processes viable targets. 34 , 64 Other significant targets include cytoskeletal disruption and impairments in protein degradation mechanisms. 64
Inhibition of Tau Aggregation
Methylene blue (MB) is a long established medication with broad utility in many conditions due to its role in promoting mitochondrial activity as well as mitigating neuroinflammation. 65 In animal models, MB has been shown to reduce Aβ levels and improved learning and memory thought to be mediated by an increase in Aβ clearance. 66 It has been found to reverse tau aggregation 67 and to promote clearance of tau filaments by inducing autophagy. 64 Although some efficacy was noted in improving cognition and reducing tau pathology in animal studies, it has not demonstrated significant benefits in human trials. This has been attributed to MB reducing the number of tau fibrils but increasing the number of granular tau oligomers, which are thought to be essential for neuronal death. 68
Curcumin is a natural plant product derived from turmeric root, with antioxidant and anti-inflammatory properties. It directly binds to β-pleated sheets of proteins and prevents aggregation. 69 Like MB, it has also been shown in animal studies to reduce tau and Aβ pathology and ameliorate cognitive deficits. 64 Previous trials have not shown any significant cognitive effects. 70 A recent trial involving a small population showed only modest results ( {"type":"clinical-trial","attrs":{"text":"NCT01383161","term_id":"NCT01383161"}} NCT01383161 ). The clinical development of curcumin as a therapeutic agent has been hindered by concern about bioavailability, poor water solubility at neutral or acidic pH, instability at basic pH, and rapid intestinal and first pass glucuronidation. 69
Post Translational Modifications
Protein phosphatase 2A (PP2A) is a protein that regulates signaling pathways. Sodium selenate is an antitumor agent which has been found to be a potent PP2A activator and reduces phosphoprylation of tau in animal studies in TBI 71 and AD. 72 In a phase IIa trial, 73 it was found to be safe and well-tolerated but there were no subsequent efficacy trials. A recent trial utilized sodium selenate as an oral supplement to potentially slow down neurodegeneration, based on the hypothesis that insufficient selenium supply to antioxidant enzymes may contribute to AD pathophysiology. 74
CDK5 inhibitors (flavopiridol and roscovitin) were developed primarily in oncology to prevent cell death. They compete with ATP for binding with CDK5, resulting in reduced activation of this kinase. 75 They have not been tested for neurodegenerative diseases although it has been thought that cell cycle progression and/or mitosis may be valid targets for AD. 76
Glycogen synthase kinase (GSK) 3β is highly expressed in the brain and has been implicated in tau phosphorylation. It is considered an important drug target due to its high specificity as a substrate. 77 Tideglusib is an irreversible GSK3β that does not compete with ATP. A phase II trial on mild AD subjects, however, showed tideglusib to have no clinical benefit. 78 An additional phase II trial showed no benefit to subjects with progressive supranuclear palsy (PSP). Lithium is another inhibitor of GSK3. Studies among patients with MCI and AD have been limited, but a reduction in phospho-tau levels were noted and one study showed stabilization of cognitive symptoms. In a meta-analysis of 5 RCTs on GSK 3 inhibitors, however (two trials on Tideglusib and three trials on lithium), GSK3 inhibitors were deemed ineffective in treating MCI and AD as the studies were found to be too small. 79
Microtubule Stabilization
Compounds that stabilize microtubules may have therapeutic potential as the disruption of microtubule-based transport mechanisms contributes to synaptic degeneration. 80
Epithilone D (BMS-241027) is a small molecule able to penetrate the blood brain barrier. It was found to increase microtubule numbers and reduce the number of axons in animal studies. 34 There was also note of improved cognition and reduced tau pathology in mouse models but the phase I clinical trial was discontinued in 2013 (NT 01492374).
TPI 287 (Abeotaxane) is another microtubule stabilizing compound which was initially found to reduce hyperphosphorylated tau in the brain and to improve performance in animal models. It was subsequently trialed in patients with AD, progressive supranuclear palsy, and corticobasal syndrome. Severe hypersensitivity reactions, however, were observed in AD patients and clinical worsening and biomarker changes were seen in PSP and corticobasal syndrome. 81
Davenutide (NAPVSIPQ) is an 8-amino acid peptide derived from activity-dependent neuroprotective protein (ADNP). ADNP deficiency is thought to lead to tauopathies. 34 In animal models, davenutide was found to play a positive role in attenuating Aβ1-42-induced impairments in spatial memory and synaptic plasticity. 82 It is thought to stabilize microtubules and reduce hyperphosphorylated tau levels. 83 In a phase I trial, it was found to be well tolerated given intranasally among patients with MCI. Although there was note of potential efficacy in two tests of memory and attention, the study failed to detect a statistically significant difference on composite cognitive memory scores. 84
Tau Immunization Approaches
Active and passive immunization against phospho-tau peptides have the potential to modulate tau pathology. Antibodies pass through the blood brain barrier and enter the brain. 34
One candidate active vaccine is AADvac1 which targets nonphsophorylated tau. In its phase I trial, patients were given 3 doses of the vaccine. Almost all developed an IgG immune response. The most common adverse effect was injection site reactions. There were no cases of meningoencephalitis or vasogenic edema after administration. 85 A follow-up study was done on the same population and given three more doses plus two boosters with the primary objective being the determination of long-term safety. The most common adverse event was again local injection site reaction. Again, no cases of meningoencephalitis or vasogenic edema were observed. New micro-hemorrhages were observed in one Apo E4 homozygote. IgG titers did regress over time indicating the need for more frequent boosters. A tendency towards slower atrophy on MRI was observed and there seems to be a slower decline on cognitive assessment in those with higher titers. 86
ACI-35 is another active vaccine that targets phosphorylated tau. 87 In animal studies, there was note of reduction in soluble and insoluble tau. The vaccine also did not induce marked CNS inflammation despite the multiple epitopes. 75 , 88 A phase Ib-IIa trial is currently ongoing to determine safety, tolerability, and immunogenicity. It is expected to complete by 2023 ( {"type":"clinical-trial","attrs":{"text":"NCT04445831","term_id":"NCT04445831"}} NCT04445831 ).
Passive Immunization
Passive immunization potentially provides a possible solution to concerns about immunologic side effects with active immunization. There is greater specificity for the target epitope and the effects of immunization are likely to be transient. 75 Anti-tau antibodies have been shown to enter neurons and bind to a cytosolic receptor which eventually leads to proteosomal degradation of the complex and inhibition of intracellular tau aggregation. 89 – 91
RG7345 (RO6926496) is an antibody that recognizes tau phosphorylated at Ser422. Tau phosphorylated at this site is considered pathological. 92 It has been shown to enter neurons and reduce tau pathology, but this trial was discontinued by Roche likely due to some pharmacokinetic issues ( {"type":"clinical-trial","attrs":{"text":"NCT02281786","term_id":"NCT02281786"}} NCT02281786 ). No apparent safety or efficacy concerns. 75
Gosuranemab (BIIB092) is an IgG4 monoclonal that recognizes a site in the N-terminal region. It was found safe and well-tolerated with no adverse effects in the low and moderate dosage arms. Unbound N-terminal tau in the CSF was reduced but AD biomarkers were not reduced. 93 , 94 There is currently a phase II trial for those with MCI and mild AD assessing safety and tolerability plus immunogenicity and efficacy of multiple doses in slowing cognitive and functional impairment (NTC 03352557). Expected completion of the study is in 2024.
Tilavonemab (ABBV-8E12, C2N-8E12) is an IgG4 antibody intended to work extracellularly. In vitro, this blocks uptake and inhibits seeded tau aggregation. 63 No adverse reactions were reported among PSP patients in phase I. Phase II trials included both PSP patients and AD patients. The trials for PSP were discontinued. The trials for AD patients, however, are still ongoing with the extension study expected to conclude July 2021 ( {"type":"clinical-trial","attrs":{"text":"NCT02880956","term_id":"NCT02880956"}} NCT02880956 , {"type":"clinical-trial","attrs":{"text":"NCT03712787","term_id":"NCT03712787"}} NCT03712787 ).
Zagotenemab (LY3303560) is a humanized version of the IgG1 antibody MC-1 with its primary epitope located in the N-terminal region. 63 The initial phase I trial evaluated safety, tolerability, and pharmacokinetics in healthy individuals ( {"type":"clinical-trial","attrs":{"text":"NCT02754830","term_id":"NCT02754830"}} NCT02754830 ) and among those with mild to moderate AD ( {"type":"clinical-trial","attrs":{"text":"NCT 03019536","term_id":"NCT03019536"}} NCT 03019536 ). A phase II trial is underway evaluating efficacy among early symptomatic AD patients ( {"type":"clinical-trial","attrs":{"text":"NCT03518073","term_id":"NCT03518073"}} NCT03518073 ). Study completion is estimated to be in October 2021.
Semorinemab (RO7105705, MTAU9937A) is an antibody designed to bind and intercept tau in the extracellular brain, blocking cell-to-cell spread. 63 Initial phase I results showed no dose-limiting toxicities and no serious adverse effects. The antibody was also detected in CSF. 95 , 96 The phase II trial on prodromal to mild AD was completed January 2021 and primary endpoints were safety measures and CDR-Sum of Boxes (NCT3289143). The phase II trial on moderate AD is still ongoing at this time ( {"type":"clinical-trial","attrs":{"text":"NCT03828747","term_id":"NCT03828747"}} NCT03828747 ).
BIIB076 (NI-105, 6C5 hulgG1/I) is a human IgG1 recombinant monoclonal antibody. Intravenous and subcutaneous forms were assessed up to 26 times highest predicted dose. Drug levels were measured in the serum and tau levels were measured in the CSF. No adverse effects noted. 92 The phase I trial on ascending doses given to healthy volunteers and AD patients monitored adverse events as well as pharmacokinetics ( {"type":"clinical-trial","attrs":{"text":"NCT03056729","term_id":"NCT03056729"}} NCT03056729 ). This study was completed in March 2020 but no results have been posted or published.
JNJ-63733657 is an IgG1 antibody with affinity for the paired helical filament. 63 It recognizes an epitope in the mid region of tau. The phase I trial of ascending doses in healthy participants found this antibody to be generally safe and well-tolerated ( {"type":"clinical-trial","attrs":{"text":"NCT03689153","term_id":"NCT03689153"}} NCT03689153 ). A second phase I ascending dose study was completed in December 2019 ( {"type":"clinical-trial","attrs":{"text":"NCT03375697","term_id":"NCT03375697"}} NCT03375697 ) with no results posted yet. A phase II trial on efficacy and safety in early AD is currently ongoing and expected to complete by March 2025 ( {"type":"clinical-trial","attrs":{"text":"NCT04619420","term_id":"NCT04619420"}} NCT04619420 ).
Bepranemab (UCB0107) is likely an IgG4 (Alzforum.org 2019b). It also binds to the mid-region of tau, like JNJ-63733657. 92 Two phase I clinical trials were completed in 2018 and 2019 ( {"type":"clinical-trial","attrs":{"text":"NCT03464227","term_id":"NCT03464227"}} NCT03464227 , {"type":"clinical-trial","attrs":{"text":"NCT 03605082","term_id":"NCT03605082"}} NCT 03605082 ). There are two other phase I trials involving safety and tolerability in PSP patients. The phase II study involving AD patients is not yet recruiting at this time ( {"type":"clinical-trial","attrs":{"text":"NCT04867616","term_id":"NCT04867616"}} NCT04867616 ).
Treatment of Noncognitive Symptoms of Dementia
Noncognitive symptoms of dementia (NCSD) are symptoms that contribute significantly to functional decline, caregiver burden, and eventually, the decision for institutionalization. 97 Early treatment, therefore, is essential. These symptoms present intermittently or persistently. The most prevalent and stable is apathy. Other symptoms include depression, anxiety, irritability, and psychosis. The four key symptoms of wandering, aggression/agitation, delusions, and irritability have been found to be associated with more severe illness. 98 Nonpharmacologic approaches are designated as the first line approach for treatment. Pharmacological interventions have been prescribed as well and are often seen as more expedient, though with variable benefit. Their use is complicated by adverse effects, dose-dependent increased mortality, and limited supporting evidence. 99 – 101 The concern for risks associated particularly with antipsychotic use has resulted in boxed warnings by the FDA for atypical and conventional antipsychotics. 99 Furthermore, none of these pharmacologic agents have been indicated for this use by the FDA so their use is off-label. The American Psychiatric Association’s (APA) guideline on antipsychotic use to treat agitation or psychosis in dementia recommends that antipsychotics be used when symptoms of agitation or psychosis are severe, dangerous, or cause significant distress to the patient, titrating doses only to the minimum effective dose, and to taper and withdraw once an adequate response is achieved. 102 Ultimately, among the antipsychotics, there is no single agent that is able to provide both efficacy and safety emphasizing the need to individualize treatment based on a careful balance of benefits and adverse effects. 103
Serotonergic antidepressants offer a more promising pharmacologic approach to the treatment of NCSD. Citalopram, a selective serotonin reuptake inhibitor (SSRI), has been shown to have some efficacy for agitation in dementia. 104 In a placebo controlled, double blind RCT, citalopram was found to meaningfully reduce agitation and caregiver distress. Some associated cognitive decline and cardiac side effects (QTc prolongation), however, hamper its long-term use. 105 , 106 Results suggest that citalopram needs to be given at least nine weeks to allow enough time for a full response. 107 The evidence for its use for this indication, nevertheless, remains compelling, and potentially could be a class effect for all SSRIs. 108 , 109
Some additional novel and/or repositioned agents are being studied as treatments for agitation in dementia. 99 Four will be discussed here.
Dextromethorphan is a sigma-1 receptor agonist which has some mood-modulating properties. 110 It is also a low affinity NMDA antagonist, a serotonin and norepinephrine reuptake inhibitor, a histamine H1 receptor agonist, and a neuronal nicotinic alpha-3 beta-4 receptor antagonist. 99 , 111 Quinidine and deuteration appear to prolong dextromethorphan's plasma half-life, reduce first pass metabolism, and facilitate brain penetration. 112 Deuterated (d6)-dextromethorphan/quinidine (AVP-786) is currently being evaluated as a treatment for agitation in people with AD. 113 Two phase III trials evaluating efficacy, safety, and tolerability have been completed, {"type":"clinical-trial","attrs":{"text":"NCT02442765","term_id":"NCT02442765"}} NCT02442765 (February 2020) and {"type":"clinical-trial","attrs":{"text":"NCT02442778","term_id":"NCT02442778"}} NCT02442778 (August 2020). The two trials reportedly showed mixed findings and failed to confirm the anti-agitation effect, probably due to differences in study design. 113 , 114 Four ongoing trials are currently recruiting subjects ( {"type":"clinical-trial","attrs":{"text":"NCT04464564","term_id":"NCT04464564"}} NCT04464564 , {"type":"clinical-trial","attrs":{"text":"NCT04408755","term_id":"NCT04408755"}} NCT04408755 , {"type":"clinical-trial","attrs":{"text":"NCT03393520","term_id":"NCT03393520"}} NCT03393520 , {"type":"clinical-trial","attrs":{"text":"NCT02446132","term_id":"NCT02446132"}} NCT02446132 ).
Cannabinoids like tetrahydrocannabinol (THC) are agonists at cannabinoid receptors 1 and 2. Cannabinoid receptor activity has behavioral effects and modulates of neuroinflammation and oxidative stress, so these receptors are a potential drug target. 115 Nabilone, a synthetic oral THC which is a partial agonist at CB1/2 receptors, is thought to potentially have some efficacy for agitation in moderate to severe AD. 116 In a recent RCT evaluating nabilone efficacy and safety, it was found to be effective for agitation, although with some dose-related sedation. Observational studies have also shown promising results, particularly in cases with refractory symptoms. 117 Two of three recent meta-analyses were unable to show conclusive results for cannabinoid efficacy in the treatment of agitation or aggression. 118 , 119 The most recent meta-analysis, however, was able to show significant improvement in different NPS instruments and efficacy was associated with baseline dementia severity and dose. 120
Brexpiprazole is a partial receptor agonist (D3, D2, 5-HT1A) and receptor antagonist (5HT2A, alpha1B/2C) which has been shown in phase II trials to be effective for agitation in patients with AD with improved safety profile compared with other second generation antipsychotics. 121 , 122 Of note, brexpiprazole on a slow titration schedule, had higher efficacy and tolerability. There are several ongoing phase III trials ( {"type":"clinical-trial","attrs":{"text":"NCT03594123","term_id":"NCT03594123"}} NCT03594123 , {"type":"clinical-trial","attrs":{"text":"NCT03724942","term_id":"NCT03724942"}} NCT03724942 , {"type":"clinical-trial","attrs":{"text":"NCT03548584","term_id":"NCT03548584"}} NCT03548584 , {"type":"clinical-trial","attrs":{"text":"NCT03620981","term_id":"NCT03620981"}} NCT03620981 ) to evaluate long term use, safety, efficacy.
Prazosin is a centrally acting alpha 1 receptor antagonist indicated for hypertension and symptoms of benign prostatic hypertrophy. It readily crosses the blood brain barrier and in a small double-blind trial, it has shown efficacy for agitation among patients with moderate AD using a flexible dosing titration up to a maximum of 6 mg TDD. It was also well tolerated. 123 A completed but unpublished second trial looked into the efficacy of a fixed dose of 4 mg twice daily given for a longer period of study ( {"type":"clinical-trial","attrs":{"text":"NCT01126099","term_id":"NCT01126099"}} NCT01126099 ). A phase III trial is ongoing to evaluate efficacy and dose titration ( {"type":"clinical-trial","attrs":{"text":"NCT03710642","term_id":"NCT03710642"}} NCT03710642 ).
Mirtazapine ( {"type":"clinical-trial","attrs":{"text":"NCT03031184","term_id":"NCT03031184"}} NCT03031184 , completed 06/2020) and lithium ( {"type":"clinical-trial","attrs":{"text":"NCT02129348","term_id":"NCT02129348"}} NCT02129348 , completed 01/2020) have also been evaluated for agitation in AD but results have yet to be published. Escitalopram is being reevaluated for agitation as well ( {"type":"clinical-trial","attrs":{"text":"NCT03108846","term_id":"NCT03108846"}} NCT03108846 , ongoing recruitment). 99
Public Health Implications
We may be standing at the brink of a new era of AD diagnosis and treatment, a development which will have significant public health implications. If early detection becomes a reality, the detected individuals will burden an already stressed system by new care needs. Resources may need to be directed at increasing public awareness of the health implications of dementia. Dissemination of information about the effectiveness of treatments will be needed as well as work to remove the stigma associated with mental health disorders and the stigma of being treated for them. Furthermore, overall access to mental health services needs to be improved. 124 , 125
The current advances should inspire hope, however, that many cases of dementia can be delayed or prevented as a result of earlier detection, lifestyle modifications, and new treatment approaches. We are on the verge of a paradigm shift in the way we approach AD.
- Open access
- Published: 03 July 2024
Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies
- Agathe Vrillon 1 , 2 , 3 ,
- Olivier Bousiges 4 , 5 ,
- Karl Götze 2 ,
- Catherine Demuynck 6 ,
- Candice Muller 6 ,
- Alix Ravier 6 ,
- Benoît Schorr 6 ,
- Nathalie Philippi 5 , 6 , 7 ,
- Claire Hourregue 1 ,
- Emmanuel Cognat 1 , 2 ,
- Julien Dumurgier 1 ,
- Matthieu Lilamand 1 ,
- Benjamin Cretin 5 , 6 , 7 ,
- Frédéric Blanc 5 , 6 , 7 &
- Claire Paquet 1 , 2
Alzheimer's Research & Therapy volume 16 , Article number: 146 ( 2024 ) Cite this article
56 Accesses
3 Altmetric
Metrics details
Increasing evidence supports the use of plasma biomarkers of amyloid, tau, neurodegeneration, and neuroinflammation for diagnosis of dementia. However, their performance for positive and differential diagnosis of dementia with Lewy bodies (DLB) in clinical settings is still uncertain.
We conducted a retrospective biomarker study in two tertiary memory centers, Paris Lariboisière and CM2RR Strasbourg, France, enrolling patients with DLB ( n = 104), Alzheimer’s disease (AD, n = 76), and neurological controls (NC, n = 27). Measured biomarkers included plasma Aβ40/Aβ42 ratio, p-tau181, NfL, and GFAP using SIMOA and plasma YKL-40 and sTREM2 using ELISA. DLB patients with available CSF analysis ( n = 90) were stratified according to their CSF Aβ profile.
DLB patients displayed modified plasma Aβ ratio, p-tau181, and GFAP levels compared with NC and modified plasma Aβ ratio, p-tau181, GFAP, NfL, and sTREM2 levels compared with AD patients. Plasma p-tau181 best differentiated DLB from AD patients (ROC analysis, area under the curve [AUC] = 0.80) and NC (AUC = 0.78), and combining biomarkers did not improve diagnosis performance. Plasma p-tau181 was the best standalone biomarker to differentiate amyloid-positive from amyloid-negative DLB cases (AUC = 0.75) and was associated with cognitive status in the DLB group. Combining plasma Aβ ratio, p-tau181 and NfL increased performance to identify amyloid copathology (AUC = 0.79). Principal component analysis identified different segregation patterns of biomarkers in the DLB and AD groups.
Conclusions
Amyloid, tau, neurodegeneration and neuroinflammation plasma biomarkers are modified in DLB, albeit with moderate diagnosis performance. Plasma p-tau181 can contribute to identify Aβ copathology in DLB.
Introduction
Dementia with Lewy bodies (DLB) is evaluated to be the third cause of dementia after Alzheimer’s disease (AD) and vascular dementia. DLB is characterized by a poorer prognosis, higher healthcare costs and caregiver burden, and a greater impact on quality of life compared with AD [ 1 , 2 ]. Yet, therapeutic research in DLB has long remained a poorly invested field, and there are no disease-specific treatments currently approved by the European Medicines Agency. However, this field has known some recent expansion, with more than 30 ongoing trials in 2022, including evaluations of disease modifier molecules [ 3 ]. In this context, achieving early and reliable diagnosis should be a priority.
Patients with DLB present with a wide range of cognitive, psychiatric, motor, and dysautonomic symptoms that can vary in-between patients and over time within individuals. The currently used diagnosis criteria, defined by McKeith et al., include imaging and electrophysiological supportive biomarkers in association with core clinical features [ 4 ]. However, no specific markers of pathology are included.
DLB is characterized by the presence of Lewy bodies formed primarily of alpha-synuclein. Amyloid-beta (Aβ) plaques, and to a lesser extent hyperphosphorylated tau (p-tau) tangles, may also be present [ 5 , 6 ]. Cerebral and peripheral inflammation has been demonstrated to be contributing mechanisms, especially at the early stages of the disease [ 7 ]. Specific blood-based assays would benefit diagnostic in clinical practice and facilitate clinical trials, as non-invasive and scalable. Real-time quaking-induced conversion (RT-QuIC) assays are showing high sensitivity and specificity to detect alpha-synuclein in cerebrospinal fluid (CSF) in DLB and Parkinson’s disease (PD [ 8 , 9 , 10 ]. However, they still require further validation, especially in plasma [ 11 ]. Interestingly, number of amyloid and tau plasma biomarkers, as well as axonal and neuroinflammatory markers have been studied in DLB and appear modified. Plasma p-tau has demonstrated good accuracy to identify an AD copathology commonly present in DLB [ 12 , 13 , 14 ]. Indeed, more than half of patients with DLB demonstrate coexisting amyloid lesions, that impact clinical presentation and disease progression [ 5 , 6 ]. Several plasma p-tau isoforms, including plasma p-tau181, p-tau231, and p-tau217, were reported to be higher in DLB patients with CSF or PET evidence of amyloid copathology [ 12 , 14 , 15 ]. Plasma NfL and glial fibrillary acidic protein (GFAP) levels were found to be higher in Lewy body dementia (DLB and Parkinson’s disease dementia) compared to controls but lower compared to AD [ 16 , 17 ]. Glial markers, including soluble triggering receptor expressed on myeloid cells 2 (sTREM2) and human chitinase-3 (YKL-40) have shown inconsistent modifications in DLB, both in CSF and in plasma, but nevertheless suggesting different glial activation patterns than in AD [ 18 , 19 ].
While being nonspecific and considering the presence of neuroinflammation and the possible co-existence of AD pathology, our question is could those existing plasma biomarkers be useful in DLB diagnosis? The aim of this study was to investigate 6 plasma biomarkers, as standalone markers or in combinations to evaluate their usefulness in DLB diagnosis, in comparison to AD patients and control individuals.
In a cross-sectional retrospective bicentric study, we analyzed samples from the Cognitive Neurology Center, Lariboisière Hospital, Université Paris Cité, Paris, France, and the Memory Clinic (CM2R) of Strasbourg, Alsace, France from 2012 to 2021. The cohort consecutively included 27 neurological control subjects (Paris: n = 19, Strasbourg: n = 8), 104 patients with DLB (Paris: n = 56, Strasbourg: n = 48), and 76 patients with AD (Paris: n = 71, Strasbourg: n = 5).
All included subjects underwent comprehensive neurological examination, neuropsychological evaluation, and brain imaging. All controls and AD subjects underwent lumbar puncture as well as 87% (90/104) of DLB patients. Consensus diagnoses were validated after a multidisciplinary review of cases, by neurologists, neuropsychologists, neuroradiologists, and biologists. All DLB patients fulfilled the most recent revised diagnosis criteria for probable DLB, established by McKeith et al. [ 4 ]. Diagnosis of AD, including MCI and dementia patients, was made according to Albert’s et al. criteria and Dubois et al. criteria [ 20 , 21 ]. All patients with AD displayed a CSF amyloid-positive profile. Neurological controls were individuals seen at the clinic but for whom, no evidence of neurocognitive disease was found; they presented with normative or sub-normative cognitive testing, normal morphological brain imaging, and normal CSF profile. Those subjects included some participants in observational research studies and individuals with subjective cognitive complaints in the context of minor depressive symptoms, sleep disorders, chronic pain or chronic fatigue, or systemic disorders. The cognitive status was assessed with the mini-mental state evaluation (MMSE).
Plasma biomarkers measurements
Plasma samples were obtained through venipuncture, in the morning in fasting conditions, on the same day as CSF uptake. After a 20-minute centrifugation at 1,900 x g within 4 h, plasma was aliquoted in polypropylene tubes and stored at − 80 °C until analysis.
All analyses were performed in Inserm U1144, Université Paris Cité, Paris, France.
Plasma Aβ40, Aβ42, NfL, and GFAP were measured with the Simoa Neurology 4-plex E kit from Quanterix®. Plasma p-tau181 was measured with the Simoa p-tau181 Advantage V2 assays also from Quanterix®. Samples were analyzed blinded in singlicate. All samples were above the threshold of quantification. All intra and inter coefficients of variations were below 10% (Aβ40: intraplate CV = 2.7%, interplate CV = 4.2%; Aβ42: intraplate CV = 1.2%, interplate CV;=3.4%; GFAP, intra plate CV = 9.9%, interplate CV = 6.0%; NfL, intraplate CV = 6.1%, interplate CV = 3.0%; p-tau181: intraplate CV = 9.5%, inter plate = 5.3% ).
Plasma sTREM2 and YKL-40 levels were measured using commercial ELISA kits from R&D System (respectively, #DY1828-05 and #DY2599), both validated for plasma measurements. Samples were run blinded in duplicates. Intra- and inter-coefficients of variations were respectively 7.1% and 10.7% for sTREM2 and 5.1% and 6.6% for plasma YKL-40.
Apolipoprotein E (APOE) genotype was determined through analysis of 2 single nucleotide variations (formerly single nucleotide polymorphisms, rs429358, and rs7412) using established standard polymerase chain reaction as described in Dumurgier et al. [ 22 ].
CSF AD biomarkers
CSF AD biomarkers analysis was available for all control subjects, AD patients, and 87% of DLB patients CSF was collected by standard lumbar puncture procedure. CSF samples were collected in polypropylene tubes for CSF AD biomarker measurements. In Paris cohort, CSF Aβ42, Aβ40, t-tau, and p-tau181 were measured by different assays across time, including Innotest Fujirebio® ( n = 30) and Elecsys Roche® for Aβ42, p-tau181 and t-tau and Innotest Fujirebio® for Aβ40 ( n = 101). In Strasbourg cohort, CSF Aβ42, Aβ40, t-Tau, and p-tau181 were measured with Innotest Fujirebio® for the largest part ( n = 58) and with Lumipulse Fujirebio® for n = 3 subjects. Assays and cut-offs are detailed in Supp. Table 1 .
Patients with available CSF AD biomarkers were classified according to the AT(N) classification [ 23 ].
Statistical analysis
Statistical analyses were conducted using SPSS® version 29.0 (IBM statistics) and Graphpad® Prism version 9.0 (GraphPad, San Diego, CA, USA). Continuous nonparametric data are presented as median (interquartile range) and parametric data as mean (standard deviation).
Age, MMSE, and levels of education were compared between diagnostic groups using Kruskall Wallis test and sex and APOE ɛ4 carriership using Chi-2 test. Plasma biomarker levels did not display a normal distribution and were log-transformed before analysis. Association of the biomarkers with age, sex, and APOE ɛ4 carriership was studied unadjusted (with Spearman’s correlation for age and chi-2 test for sex and APOE status) then adjusting for age, sex, and APOE status using linear regression.
One-way analysis of variance adjusted on age and sex with Tukey’s LSD post hoc analysis, adjusting for multiple comparisons, was used to assess biomarker level differences between the diagnostic groups. Effect sizes were estimated with Cohen’s d .
Receiver operating characteristic (ROC) analysis with area under the curve (AUC) calculation was obtained by performing logistic regression, including age and sex as covariates, to study the diagnosis performance of the plasma biomarkers. Combination of biomarkers were studied using logistic binary regression, in a stepwise backward elimination strategy based on Wald to identify the best combination for differentiation between diagnosis groups. Areas under the curve (AUCs) were compared with the Akaike information criterion (AIC).
The AD group was analyzed as two groups, AD-MCI and AD dementia, in secondary analyses.
To explore the association of our plasma biomarkers with amyloid copathology, we stratified DLB patients according to the AT scheme, dichotomizing on the A status defined by CSF Aβ42/Aβ40 ratio (A + versus A-), then by AT status (A + T + versus A-T-). The differences in plasma biomarker levels were studied using one-way analysis of variance adjusting on age and sex. Effect sizes were estimated using eta squared η 2 .
MMSE scores were transformed into the square root of the number of errors (√[30-MMSE]) to ensure normalcy of distribution [ 24 ]. The association of biomarkers with MMSE was studied using Spearman’s correlation and with linear regression adjusting on age, sex, and level of education.
Principal component analysis (PCA) was performed in the DLB and AD groups to explore the pattern of association between the different biomarkers. Outlier values, defined by a value > mean ± 3SD, were excluded for each biomarker before analysis. Kaiser–Meyer–Olkin Measure of Sampling Adequacy test and Bartlett’s Test of Sphericity were used to evaluate the suitability of the dataset. The number of components was determined by the number of eigenvalues greater than one. Variables with a loading factor > 0.4 or < − 0.4 were regarded as representative of the component.
The overall cohort’s demographic characteristics and plasma biomarker levels are presented in Table 1 , and by center in Supp. Table 2 . DLB and AD patients were significantly older than control individuals ( P < 0.001). We observed a higher percentage of males in the DLB group than in the AD and control groups ( P = 0.037). The AD group displayed more frequent APOE ɛ4 carriership than the DLB and NC groups ( P < 0.001). In the AD group, 97% ( n = 74) displayed a CSF A + T + profile and 3% ( n = 2) an A + T- profile. As a sensitivity analysis, the main analyses have been reproduced after the exclusion of the A + T- subjects and yielded similar results (Suppl. Figure 1 ). Additionally, the characteristics of AD-MCI and AD-dementia groups are presented in Supp. Table 3 .
Associations with age, sex, and APOE status are detailed in Supp. Table 4 . In the whole cohort, all plasma biomarkers were associated with age (β = 0.236–0.538, P ≤ 0.002) except for Aβ ratio (β=-0.042, P = 0.593) after adjustment on sex and APOE ɛ4 carriership. Plasma GFAP and YKL-40 levels were higher in females after adjustment on age and APOE status (GFAP: β = 0.258, P < 0.001; YKL-40, β = 0.198, P = 0.008). Plasma Aβ ratio, p-tau181, and sTREM2 levels were associated with APOE ɛ4 carriership in adjusted analysis (β = 0.165-0.228, P ≤ 0.028). Focusing on the DLB group, after adjustment for covariates, we found positive associations between age and plasma p-tau181, NfL, GFAP, and YKL-40 levels (β = 0.247–0.521, P ≤0.030) and between female sex and plasma GFAP levels (β = 0.259, P = 0.008). No association was found between any plasma marker and ApoE4 carriership, after adjustment for age and sex in the DLB group.
Correlations between biomarkers are displayed in Supp. Figure 2 . Focusing on DLB patients, plasma GFAP, p-tau181, and NfL showed significant associations ( r = 0.341–0.560, P < 0.0001 overall). Plasma YKL-40 and sTREM2 were significantly associated ( r = 0.284, P = 0.003), as well as with plasma NfL ( r = 0.406, P < 0.000 both). Plasma GFAP was the only marker significantly associated with the plasma Aβ ratio ( r =-0.325, P < 0.0001), though there was a tendency to association between the Aβ ratio and p-tau181 ( r =-0.185, P = 0.067).
Biomarkers levels across diagnosis groups
Plasma biomarker levels are displayed in Fig. 1 . Patients with DLB displayed lower levels of plasma Aβ ratio ( P = 0.037, d = 0.576) and higher p-tau181 ( P = 0.017, d = 0.644) and a tendency to higher GFAP levels ( P = 0.057, d = 0.057), compared to NC, after adjustment for age and sex. Additionally, patients with DLB displayed significantly lower levels of plasma p-tau181 ( P < 0.001, d = 1.11), NfL ( P = 0.037, d = 0.390), and GFAP ( P < 0.001, d = 0.685) compared with AD patients. DLB patients had higher levels of plasma sTREM2 compared with AD patients ( P = 0.022, d = 0.413). No difference was observed in plasma levels for YKL-40 between diagnostic groups, with or without adjustment. Plasma Aβ ratio levels were lower and p-tau181, GFAP, and NfL levels all higher in AD patients compared with controls, but not plasma sTREM2 and YKL-40.

Plasma biomarkers levels across diagnosis groups. Plasma biomarkers levels across diagnosis groups including a , Aβ ratio; b , p-tau181; c , NfL; d , GFAP; e , sTREM2; and f , YKL-40. P-values were obtained through one-way ANCOVA followed by post hoc Tukey’s test, adjusting for multiple comparisons. Significant differences ( P < 0.05) are reported. The effect size was determined using Cohen’s d . Boxplots display the median, IQR, and value for all participants
Looking at AD stages, plasma p-tau181 levels remained significantly higher in both AD-MCI and AD dementia groups compared with the DLB group (Supp. Figure 3 ). Plasma NfL and GFAP levels were higher and sTREM2 levels lower in the AD dementia group compared with the DLB groups, but did not differ between AD-MCI and DLB.
DLB diagnostic performance
To differentiate DLB from controls, our plasma biomarkers yielded moderate AUCs from 0.74 to 0.78, without significant differences between biomarkers (Fig. 2 a). Plasma p-tau181 yielded the highest AUC of 0.78 (95% CI 0.68–0.87). Combining biomarkers did not outperform p-tau-181 sole (Fig. 2 b).

Plasma biomarkers performance to identify DLB. ROC analysis: a , to compare single biomarkers performance to discriminate between DLB patients and NC; b , to compare biomarkers combination to discriminate between DLB and NC; c , to compare single biomarkers performance to discriminate between DLB and AD patients; d , to compare biomarkers combination to discriminate between DLB and AD patients. ROC analysis results are presented as AUC (95% CI). Combinations of biomarkers were selected through binary logistic regression with backward stepwise elimination, including age and sex as constant variables. a model including p-tau181 outperformed all other models (∂AIC > 4), b no significant difference in the model’s fit with the All markers model (∂AIC < 4).
To differentiate DLB from AD, plasma p-tau181 yielded the highest AUC (0.80) as a standalone biomarker and outperformed the other biomarkers (∂AIC > 4, Fig. 2 c). The optimal combination of markers was the association of plasma p-tau181 and YKL-40, that performed as well as the combination of all biomarkers (all biomarkers model, AUC = 0.84 versus plasma p-tau181 + plasma YKL-40, AUC = 0.83, ∂AIC < 4, Fig. 2 d).
To differentiate AD from controls, plasma p-tau181 had the best performance as a standalone biomarker (AUC = 0.92) and association with other biomarkers did not improve diagnosis performance (Supp. Table 5 ).
The diagnosis performance of the plasma biomarkers used individually was overall similar when analyzing separately AD-MCI and AD dementia cases (Supp. Figure 3 ). The combination of plasma p-tau181 and YKL-40 had the best performance to differentiate DLB patients from AD-MCI (AUC = 0.86, ∂AIC > 4 versus all biomarkers model [AUC = 0.88] and p-tau181 alone [AUC = 0.80]), with the best trade-off between the goodness of fit and parsimony. To distinguish DLB from AD dementia, the association of plasma Aβ ratio, p-tau181, and NfL (AUC = 0.85) was not inferior to the all biomarkers model (AUC = 0.87, ∂AIC < 4).
Identification of amyloid copathology in DLB
CSF analysis was available for 87% (90/104) of DLB patients (Table 1 ). According to the AT(N) classification, 24% of patients presented an AD CSF profile on the AD continuum, 12% being A + T- and 12% A + T+. A + DLB patients displayed higher concentrations of plasma p-tau 181 compared with A- DLB ( P = 0.011, η2 = 0.71) after adjustment on age and sex (Fig. 3 , a-f). A + T + patients displayed higher levels of plasma p-tau181 and NfL levels compared with A-T- DLB (respectively, P = 0.003, η2 = 0.131 and P = 0.036, η2 = 0.062, Fig. 3 , g-l).

Plasma biomarkers levels in relation to amyloid pathology in DLB patients. Plasma biomarkers levels across amyloid-negative (A-) DLB and amyloid-positive (A+) DLB patients including a , Aβ ratio; b , p-tau181; c , NfL; d , GFAP; e , sTREM2; f , YKL-40; and across A-T- DLB and A + T + DLB patients including: g , Aβ ratio; h , p-tau181; i , NfL; j , GFAP; k , sTREM2; l , YKL-40. For biomarker levels comparison, P-values were obtained through one-way ANCOVA adjusting for multiple comparisons. Significant differences ( P < 0.05) are reported in bold. The effect size was determined using η2. Boxplots display the median, IQR, and value for all participants
Plasma biomarkers identified A + DLB patients with overall moderate AUCs ranging from AUC = 0.64 to AUC = 0.75, as standalone biomarkers. Plasma p-tau181 displayed a higher AUC of 0.75, outperforming all other biomarkers (∂AIC > 4). The best combination of markers was the association of plasma p-tau181, GFAP, and NfL, yielding an AUC of 0.79, which was equivalent to the performance of the combination of all 6 plasma markers (AUC = 0.82, ∂AIC < 4 ). Plasma p-tau181 was outperformed by the combinations of all 6 biomarkers (AUC = 0.82 versus AUC = 0.75, ∂AIC = 5.5).
Diagnosis performance of our plasma biomarkers was overall better in discriminating A + T + from A-T- DLB patients (AUC = 0.71–0.85, Fig. 4 , c). Plasma p-tau181 displayed the highest AUC, of 0.85, outperforming all other biomarkers. Combining biomarkers (AUC = 0.87–0.91, Fig. 4 , d) did not statistically outperform plasma p-tau181 sole (AUC = 0.85, ∂AIC <4).

Plasma biomarkers performance for identification of amyloid copathology in DLB patients. ROC analysis: a , to compare single biomarkers performance to discriminate between A- and A + DLB patients; b , to compare biomarkers combination to discriminate between A- and A + DLB patients; c , to compare single biomarkers performance to discriminate between A-T- and A + T + DLB patients; d , to compare biomarkers combination to discriminate between A-T- and A + T + DLB patients. ROC analysis results are presented as AUC (95% CI). Combinations of biomarkers were selected through binary logistic regression with backward stepwise elimination, including age and sex as constant variables. a the model including p-tau181 outperformed all other models (∂AIC > 4). b the model associating plasma p-tau181, GFAP, and NfL was equivalent to the All markers models (∂AIC < 4). c the model including p-tau181 outperformed the All markers model (∂AIC > 4), with the best trade-off between parsimony and performance
Association with cognitive measurement
The associations of the plasma biomarkers with MMSE in diagnosis groups are presented in Supp. Table 6 . In the DLB patients, we found higher plasma levels of p-tau181 levels were correlated with lower MMSE in unadjusted analysis (Spearman’s r = 0.231, P = 0.024). After adjustment on age, sex, and level of education, there remained no significant association (β=-0.176, P = 0.072). In the whole cohort, higher plasma p-tau181 and plasma GFAP levels were significantly associated with lower MMSE, after adjustment on age, sex, and level of education (respectively: β=-0.378 and β=-0.373, P < 0.001). In the AD group, higher plasma GFAP levels were correlated with lower MMSE in unadjusted analysis ( r = -0.253, P = 0.032).
Principal component analysis
Lastly, we performed PCA to investigate the relationship between the different biomarkers in AD and DLB groups (Fig. 5 ). In DLB, we identified 2 principal components that explained 58% of the total variance in the dataset (Fig. 5 , a). Component 1 accounted for 19% of the variance and was associated with plasma Aβ ratio, p-tau181, and GFAP. Component 2 captured 39% of the variance and was associated with neuroinflammatory markers sTREM2 and YKL-40 and axonal damage markers NfL. In the AD group, PCA analysis yielded two principal components as well (Fig. 5 , b). First, a component 1 associated plasma Aβ ratio and neuroinflammatory markers sTREM2 and YKL-40, explaining 20% of the variance. A component 2 clustered plasma p-tau181, GFAP, and axonal damage markers NfL, capturing 36% of the variance.

Principal component analysis of biomarker data in DLB and AD patients. a , Principal component analysis in DLB patients ( n = 103). Component 1 associating plasma Aβ ratio, p-tau181, and GFAP explained 19% of the variance of the biomarkers data. Component 2 associating neuroinflammation sTREM2 and YKL-40 and axonal damage NfL makers explained 40% of variance. b , Principal component analysis in AD patients ( n = 76). Component 1 associating plasma Aβ ratio, p-tau181, and GFAP explained 20% of the variance of the biomarkers data. Component 2 associating neuroinflammation sTREM2 and YKL-40 and axonal damage NfL markers explained 36% of variance
In the present study, we report plasma biomarker modifications, including amyloid and tau, neurodegeneration, and neuroinflammation across a cohort of patients with probable DLB, compared with AD and controls. DLB patients displayed intermediate levels of plasma Aβ ratio, p-tau181and GFAP, falling in between control subjects and AD patients. Plasma p-tau181 was further altered in DLB patients with AD copathology. Subtle changes in plasma sTREM2 levels could be observed.
Plasma Aβ ratio, p-tau181, and GFAP levels were higher in DLB compared with NC but lower than those observed in the AD group. Those findings are in keeping with the previously published literature [ 25 ]. Plasma Aβ ratio was significantly lower in DLB patients compared with NC, even if the size of the effect was moderate compared with those of the decrease observed in the AD groups. Plasma Aβ42/40 has been reported to correlate with 18 F-florbetapir SUVR in DLB [ 17 ].
Regarding plasma p-tau, there is now significant evidence of its increase in DLB, already at the MCI stage [ 12 , 14 , 17 ]. The effect size difference was greater when comparing DLB and AD than between DLB and controls. Regarding diagnostic performance, p-tau181 had the highest performance in differentiating DLB from NC, and combining biomarkers did not improve diagnosis performance. To differentiate DLB from AD, p-tau181 also displayed the best performance, albeit moderate, in line with what has been reported in the literature [ 17 , 25 , 26 ]. Plasma GFAP displayed the largest effect size difference when comparing DLB to controls. It could reflect both copathology as GFAP has been demonstrated to be associated with Aβ mediated astrocytic reactivity, and general neurodegeneration [ 27 ]. In our study, it was not associated with CSF amyloid status, which could indicate Aβ-independent astrocytic activation or neurodegeneration. Indeed, there is emerging evidence that supports the existence of an astrocytic activation in DLB independently of amyloid pathology. Significant tracer uptake in 11 C-PK11195 microglial PET has been observed in DLB with no association with amyloid pathology [ 28 ]. Autopsy studies on DLB brains have demonstrated increased GFAP + astrocyte reactivity, in association with Lewy body pathology [ 29 , 30 ]. If it is established that AD copathology has an important impact on the inflammatory signals detected in DLB, there is emerging evidence for specific astroglial processes related to Lewy body pathology, that could be picked up by plasma biomarkers.
No difference in plasma NfL levels was observed between NC and DLB, whereas there was a significant difference between DLB and AD groups. Previous findings regarding plasma NfL in DLB have been ambiguous, which may partly be because of small sample studies, discrepancies in design, and variability of cohorts, combining sometimes DLB with Parkinson’s disease dementia [ 31 ]. In several studies, plasma NfL was shown to reflect disease progression in later DLB stages as a non-specific marker of worse cognitive and clinical outcomes, as well as a reflection of amyloid copathology [ 32 , 33 ].
In our cohort, no difference in levels of plasmaYKL-40 could be observed. Previous studies had reported no difference in CSF YKL-40 levels between DLB and controls [ 18 ]. In plasma, increased levels of YKL-40 have been described in a cohort of Lewy body dementia patients including DLB patients and Parkinson’s disease dementia patients [ 19 ]. Specific studies focusing on DLB cases will be needed to clearly state if CSF or plasma YKL-40 are consistently altered in DLB. In our work, the combination of plasma p-tau181 and YKL-40 levels increased performance to differentiate AD from DLB, which would still indicate an underlying glial process picked up by YKL-40. Plasma sTREM2 was higher in DLB compared with AD. High levels of CSF sTREM2 in DLB have already been reported [ 18 ]. Similar findings have been observed in PD brain, suggesting a reaction to alpha-synuclein deposition [ 34 ]. Plasma sTREM2 levels did not differ in A- and A + DLB subjects, suggesting that the observed increase in the DLB group is not related to AD pathology. However, it is still unclear if YKL-40 or sTREM2 plasma levels are the reflection of a central process, or of an associated peripherical immune dysregulation. In the brain, the expression and secretion of YKL-40 are attributed to astrocyte activation [ 35 ]. Brain-derived YKL-40 is hypothesized to then be released in the blood and contribute to plasma levels. Regarding sTREM2, while CSF levels are considered to reflect microglial inflammation, there is evidence that blood sTREM2 might reflect the activation of a wider range of myeloid cells [ 36 ]. In addition, there is growing evidence of altered peripherical immune response in DLB. High peripheral levels of cytokines and modified lymphocyte profile have been reported, at both MCI and dementia stages [ 37 , 38 ]. While both the central and peripherical inflammation processes are likely key features of DLB, CSF and plasma neuroinflammation markers might likely provide different information.
Complementary biomarkers reflecting the other pathological mechanisms of DLB, such as, first and foremost, αsynuclein aggregation but also synaptic alterations, and mitochondrial dysfunction, would most likely contribute to diagnosis.
Amyloid deposition is common in dementia with Lewy bodies (DLB), ranging from 40 to 70% in neuropathological studies [ 39 , 40 ]. Approximately half of patients with DLB demonstrate coexisting amyloid lesions, that impact clinical presentation and disease progression [ 41 ]. Plasma p-tau markers, including plasma p-tau181 and p-tau231, were shown to pick up amyloid pathology and correlate with cognitive decline, accordingly to CSF and PET markers [ 12 , 14 ]. In our study, only plasma p-tau181 was significantly higher comparing the A + DLB patients compared with the A-. Comparing A + T + to A-T-, both plasma p-tau181(with a higher effect size) and NfL were increased. This suggests that plasma biomarkers display more significant abnormalities in DLB patients with AD copathology when abnormalities in CSF Aβ and p-tau are both established (A + T + stage). We did not observe a difference in CSF A + and A- DLB groups for plasma GFAP or Aβ ratio, conversely as what has already been reported [ 17 ]. We cannot exclude that our small samples of CSF amyloid-positive patients could have prevented us from measuring existing effects. However, combining p-tau181 to Aβ ratio and NfL significantly increased performance to identify A + patients, compared with the use of p-tau181 sole.
Additionally, we only found an association of MMSE with plasma-tau181 in DLB patients in unadjusted analysis, keeping in line with the reported poorer cognitive status of patients with amyloid copathology [ 12 ]. Thus, we add evidence to existing studies that p-tau181 is a valuable marker of AD co-pathology and expand on the potential of combining biomarkers.
Interestingly, our PCA analysis demonstrated different segregations of our biomarkers in AD and DLB groups, pointing towards differential underlying physiopathology. In DLB, axonal NfL and glial markers sTREM2 and YKL-40 clustered in a 1st component, suggesting neuroinflammation and axonal loss as driving most of the variance in the data set. Plasma, Aβ ratio, p-tau181, and GFAP clustered in an “amyloid component”, that can be hypothesized as reflecting amyloid copathology. Indeed, plasma GFAP has been reported to be an early and independent marker of astrocytosis reactive to Aβ pathology, associating closely with amyloid markers [ 27 , 42 ]. In the AD group, plasma p-tau was associated with plasma GFAP and NfL in a first component explaining a higher part of the variance, in what could be identified as a tau and neurodegeneration component. In a 2nd component, plasma Aβ clustered with plasma glial markers. Studies on the longitudinal course of microglial activation along the AD continuum have reported an early peak at the MCI stage which could explain this segregation [ 43 ].
Our study included well-characterized DLB and AD patients and control subjects. It benefited from the use of biomarkers and reference diagnosis criteria. A strength is that our sample originated from clinical settings and thus brings ‘real-life’ evidence on the use of those novel biomarkers, compared to strictly selected research cohorts. Amyloid ratio, p-tau, NfL, and GFAP were measured with the established and highly accurate Simoa method.
This work does not go without limitations. CSF data about amyloid copathology was lacking for a small part of the cohort. We did not have available measurements of other p-tau isoforms than p-tau-181, notably of p-tau217 or p-tau231, which may be more sensitive and specific in early AD. There is still little evidence currently on possible differences in p-tau isoforms in diagnosis accuracy for DLB [ 12 , 14 , 15 ]. sTREM2 and YKL-40 levels were measured using Elisa, whereas all other biomarkers were measured using Simoa, which might have induced some variability. We had no available measurement of alpha-synuclein pathology, DLB patients being included on clinical diagnosis. However, the clinical criteria used have demonstrated high specificity [ 44 ]. Plasma biomarkers should also be investigated in comparison to other atypical parkinsonian syndromes and FTD syndromes, that constitute potential differential diagnoses for DLB. Finally, exploring the association of plasma biomarkers with the clinical features of the disease could further inform their use, and give some insight on the clinical heterogeneity observed within the DLB spectrum. Additionally, combining plasma biomarkers and other clinical and supportive biomarkers could provide a more accurate diagnosis and prognosis.
In conclusion, we found a specific pattern of impairment in plasma biomarkers of amyloid, tau axonal damage, and neuroinflammation in DLB patients. Plasma p-tau181 levels were elevated in DLB cases with AD comorbid pathology, which could have potential for selecting patients for Aβ targeting therapeutics. The diagnosis performance of our biomarkers for diagnosis remained moderate, underlying the need for further development of specific markers for synucleinopathies and DLB-specific biomarkers.
Data availability
Anonymized data will be shared upon reasonable request after approval by the local research ethics committee.
Abbreviations
- Alzheimer’s disease
Akaike information criteria
area under the receiver operating characteristic curve
confidence interval
dementia with Lewy bodies
neurological controls
receiver operating characteristic curve
van de Beek M, van Steenoven I, Ramakers IHGB, Aalten P, Koek HL, Olde Rikkert MGM, et al. Trajectories and determinants of quality of life in dementia with Lewy Bodies and Alzheimer’s Disease. J Alzheimers Dis. 2019;70(2):389–97.
Article PubMed PubMed Central Google Scholar
Mueller C, Soysal P, Rongve A, Isik AT, Thompson T, Maggi S, et al. Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: a meta-analysis of longitudinal studies. Ageing Res Rev. 2019;50:72–80.
Article PubMed Google Scholar
Abdelnour C, Gonzalez MC, Gibson LL, Poston KL, Ballard CG, Cummings JL, et al. Dementia with Lewy Bodies Drug Therapies in clinical trials: systematic review up to 2022. Neurol Ther. 2023;12(3):727–49.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89(1):88–100.
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;01(7):2181–93.
Article Google Scholar
Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, et al. Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129(5):729–48.
Article CAS PubMed Google Scholar
Amin J, Erskine D, Donaghy PC, Surendranathan A, Swann P, Kunicki AP, et al. Inflammation in dementia with Lewy bodies. Neurobiol Dis. 2022;168:105698.
Bongianni M, Ladogana A, Capaldi S, Klotz S, Baiardi S, Cagnin A, et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol. 2019;6(10):2120–6.
Article CAS PubMed PubMed Central Google Scholar
Hall S, Orrù CD, Serrano GE, Galasko D, Hughson AG, Groveman BR, et al. Performance of αSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol Commun. 2022;10(1):90.
Bentivenga GM, Mammana A, Baiardi S, Rossi M, Ticca A, Magliocchetti F, et al. Performance of a seed amplification assay for misfolded alpha-synuclein in cerebrospinal fluid and brain tissue in relation to Lewy body disease stage and pathology burden. Acta Neuropathol. 2024;147(1):18.
Okuzumi A, Hatano T, Matsumoto G, Nojiri S, Ueno S, ichi, Imamichi-Tatano Y, et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med. 2023;29(6):1448–55.
Gonzalez MC, Ashton NJ, Gomes BF, Tovar-Rios DA, Blanc F, Karikari TK, et al. Association of Plasma p-tau181 and p-tau231 concentrations with Cognitive decline in patients with probable dementia with Lewy Bodies. JAMA Neurol. 2022;79(1):32–7.
Nedelska Z, Schwarz CG, Lesnick TG, Boeve BF, Przybelski SA, Lowe VJ, et al. Association of Longitudinal β-Amyloid Accumulation determined by Positron Emission Tomography with Clinical and Cognitive decline in adults with probable Lewy Body Dementia. JAMA Netw Open. 2019;2(12):e1916439.
Hall S, Janelidze S, Londos E, Leuzy A, Stomrud E, Dage JL, et al. Plasma phospho-tau identifies Alzheimer’s Co-pathology in patients with Lewy Body Disease. Mov Disord. 2021;36(3):767–71.
Quadalti C, Palmqvist S, Hall S, Rossi M, Mammana A, Janelidze S, et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat Med. 2023;29(8):1964–70.
Cousins KAQ, Irwin DJ, Chen-Plotkin A, Shaw LM, Arezoumandan S, Lee EB, et al. Plasma GFAP associates with secondary Alzheimer’s pathology in Lewy body disease. Ann Clin Transl Neurol. 2023;10(5):802–13.
Donaghy PC, Firbank M, Petrides G, Lloyd J, Barnett N, Olsen K, et al. The relationship between plasma biomarkers and amyloid PET in dementia with Lewy bodies. Parkinsonism Relat Disord. 2022;101:111–6.
Morenas-Rodríguez E, Alcolea D, Suárez-Calvet M, Muñoz-Llahuna L, Vilaplana E, Sala I, et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci Rep. 2019;24(1):7803.
Villar-Piqué A, Schmitz M, Hermann P, Goebel S, Bunck T, Varges D, et al. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J Neuroinflammation. 2019;16(1):145.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
Dumurgier J, Laplanche JL, Mouton-Liger F, Lapalus P, Indart S, Prévot M, et al. The screening of Alzheimer’s patients with CSF biomarkers, modulates the distribution of APOE genotype: impact on clinical trials. J Neurol. 2014;261(6):1187–95.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Philipps V, Amieva H, Andrieu S, Dufouil C, Berr C, Dartigues JF, et al. Normalized Mini-mental State examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43(1):15–25.
Hamilton CA, O’Brien J, Heslegrave A, Laban R, Donaghy P, Durcan R et al. Plasma biomarkers of neurodegeneration in mild cognitive impairment with Lewy bodies. Psychol Med. 2023;1–9.
Chouliaras L, Thomas A, Malpetti M, Donaghy P, Kane J, Mak E, et al. Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer’s disease, frontotemporal dementia and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2022;93(6):651–8.
Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease Continuum. JAMA Neurol. 2021;78(12):1471–83.
Surendranathan A, Su L, Mak E, Passamonti L, Hong YT, Arnold R, et al. Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain. 2018;141(12):3415–27.
Erskine D, Ding J, Thomas AJ, Kaganovich A, Khundakar AA, Hanson PS, et al. Molecular changes in the absence of severe pathology in the pulvinar in dementia with Lewy bodies. Mov Disord. 2018;33(6):982–91.
Low CYB, Lee JH, Lim FTW, Lee C, Ballard C, Francis PT, et al. Isoform-specific upregulation of FynT kinase expression is associated with tauopathy and glial activation in Alzheimer’s disease and Lewy body dementias. Brain Pathol. 2021;31(2):253–66.
Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400.
Barba L, Abu-Rumeileh S, Halbgebauer S, Bellomo G, Paolini Paoletti F, Gaetani L, et al. CSF synaptic biomarkers in AT(N)-Based subgroups of Lewy Body Disease. Neurology. 2023;101(1):e50–62.
Götze K, Vrillon A, Bouaziz-Amar E, Mouton-Liger F, Hugon J, Martinet M, et al. Plasma neurofilament light chain in memory clinic practice: evidence from a real-life study. Neurobiol Dis. 2023;176:105937.
Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, et al. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun. 2018;499(4):797–802.
Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22(4):530–46.
Jay TR, Von Saucken VE, Landreth GE. TREM2 in neurodegenerative diseases. Mol Neurodegeneration. 2017;12(1):56.
Amin J, Boche D, Clough Z, Teeling J, Williams A, Gao Y, et al. Peripheral immunophenotype in dementia with Lewy bodies and Alzheimer’s disease: an observational clinical study. J Neurol Neurosurg Psychiatry. 2020;91(11):1219–26.
King E, O’Brien JT, Donaghy P, Morris C, Barnett N, Olsen K, et al. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J Neurol Neurosurg Psychiatry. 2018;89(4):339–45.
Coughlin D, Xie SX, Liang M, Williams A, Peterson C, Weintraub D, et al. Cognitive and pathological influences of Tau Pathology in Lewy Body Disorders. Ann Neurol. 2019;85(2):259–71.
Irwin J, Hurtig DI. H. The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J Alzheimers Dis Parkinsonism [Internet]. 2018 [cited 2023 Oct 31];08(04). https://www.omicsonline.org/open-access/the-contribution-of-tau-amyloidbeta-and-alphasynuclein-pathology-todementia-in-lewy-body-disorders-2161-0460-1000444-103649.html .
Biundo R, Weis L, Fiorenzato E, Pistonesi F, Cagnin A, Bertoldo A, et al. The contribution of beta-amyloid to dementia in Lewy body diseases: a 1-year follow-up study. Brain Commun. 2021;3(3):fcab180.
Pereira JB, Janelidze S, Smith R, Mattsson-Carlgren N, Palmqvist S, Teunissen CE, et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain. 2021;144(11):3505–16.
Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain. 2017;140(3):792–803.
PubMed PubMed Central Google Scholar
Skogseth R, Hortobágyi T, Soennesyn H, Chwiszczuk L, Ffytche D, Rongve A, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies versus Neuropathology. J Alzheimers Dis. 2017;59(4):1139–52.
Download references
Acknowledgements
Not applicable.
This work was funded through a grant from l’Association des Aidants et Malades à Corps de Lewy (A2MCL).
Author information
Authors and affiliations.
AP-HP Nord, Cognitive Neurology Center Hôpital Lariboisière-Fernand Widal, Université Paris Cité, 200 rue du Faubourg Saint-Denis, Paris, 75010, France
Agathe Vrillon, Claire Hourregue, Emmanuel Cognat, Julien Dumurgier, Matthieu Lilamand & Claire Paquet
Université Paris Cité, INSERM, UMRS 1144, Paris, France
Agathe Vrillon, Karl Götze, Emmanuel Cognat & Claire Paquet
University of California San Francisco, San Francisco, USA
Agathe Vrillon
Laboratory of Biochemistry and Molecular Biology, University Hospital of Strasbourg, Strasbourg, France
Olivier Bousiges
University of Strasbourg and CNRS, ICube laboratory UMR 7357 and FMTS (Fédération de Médecine Translationnelle de Strasbourg), team IMIS Strasbourg, Strasbourg, France
Olivier Bousiges, Nathalie Philippi, Benjamin Cretin & Frédéric Blanc
CM2R (Memory Resource and Research Centre), Service of Gerontology Mobile-Neuro-Psy-Research, Geriatrics Department, University Hospital of Strasbourg, Strasbourg, France
Catherine Demuynck, Candice Muller, Alix Ravier, Benoît Schorr, Nathalie Philippi, Benjamin Cretin & Frédéric Blanc
Neuropsychology unit, Service of Neurology Strasbourg, University Hospital of Strasbourg, Strasbourg, France
Nathalie Philippi, Benjamin Cretin & Frédéric Blanc
You can also search for this author in PubMed Google Scholar
Contributions
AV, CP, FB, and OB created the concept and design. Data acquisition was performed by AV, CH, EC, KG, JD, OB, BC, CD, CM, AR, BC, BS, NP, and ML. AV performed data analysis. AV, CP, FB, and OB contributed to sample selection. All authors contributed to the interpretation of data. AV drafted the manuscript and all authors revised it. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Agathe Vrillon .
Ethics declarations
Ethics approval and consent to participate.
All patients or their legal relatives gave written informed consent to their participation in the study. For Paris cohort, the collection and analysis of samples were approved by the local ethic committee of Bichat University, Paris, France (CEERB GHU Nord n°10–037). For Strasbourg cohort, this study was part of the larger cohort study AlphaLewyMA ( https://clinicaltrials.gov/ct2/show/NCT01876459 , registered June 11, 2013), approved by the ethics committee of East France (IV). All procedures were in accordance with the Declaration of Helsinki.
Consent for publication
Competing interests.
F.B. was the national coordinator for France for the Eisai Delphia (E2027), Axovant Headway-DLB and Roche Graduate therapeutic trials; he had received honoraria from Roche, Eisai and Biogen for oral presentations, and from Eisai for a board. C.P. is a member of the International Advisory Boards of Lilly; is a consultant for Fujiribio, Alzhois, Neuroimmune, Ads Neuroscience, Roche, AgenT and Gilead; and is involved as an investigator in several clinical trials for Roche, Esai, Lilly, Biogen, Astrazeneca, Lundbeck, and Neuroimmune. All other authors report no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vrillon, A., Bousiges, O., Götze, K. et al. Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies. Alz Res Therapy 16 , 146 (2024). https://doi.org/10.1186/s13195-024-01502-y
Download citation
Received : 12 March 2024
Accepted : 12 June 2024
Published : 03 July 2024
DOI : https://doi.org/10.1186/s13195-024-01502-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dementia with Lewy bodies
- Plasma biomarkers
- Amyloid pathology
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
Watch CBS News
FDA approves new Alzheimer's treatment, donanemab from Eli Lilly
By Alexander Tin
Edited By Paula Cohen
Updated on: July 3, 2024 / 12:22 PM EDT / CBS News
The Food and Drug Administration approved a new Alzheimer's treatment called donanemab on Tuesday, clearing the way for the third addition to a new class of drugs aimed at slowing the brain's decline in patients facing the early stages of the disease.
Branded as Kisunla by drugmaker Eli Lilly, donanemab's approval follows years of setbacks and delays in getting the experimental Alzheimer's treatment to market, despite promising clinical trial results .
Eli Lilly says the drug will be available within weeks following the approval.
"Kisunla demonstrated very meaningful results for people with early symptomatic Alzheimer's disease, who urgently need effective treatment options. We know these medicines have the greatest potential benefit when people are treated earlier in their disease, and we are working hard in partnership with others to improve detection and diagnosis," Anne White, president of Eli Lilly's neuroscience arm, said in a news release .
CBS News chief medical correspondent Dr. Jon LaPook described it as "an incremental advance" that may slow the decline of cognitive function for people in the early stages of the disease.
"We're not reversing it — it's not a cure. But if you can slow the decline, I think that's important," LaPook said on "CBS Mornings."
The FDA previously rebuffed Eli Lilly's request for accelerated approval last year, citing concerns about its long-term safety data. After Eli Lilly submitted more data to the FDA, the company said it expected the agency would decide on approval by the end of March.
That decision was delayed after the FDA scheduled an advisory committee to wrestle with questions over the drug's safety issues and how effectiveness was measured in its trials. The panel ultimately voted unanimously last month in favor of the drug's benefits outweighing its risks, for patients in the early stages of Alzheimer's disease.
How does donanemab work?
Donanemab is part of a class of Alzheimer's treatments called anti-amyloid monoclonal antibodies, which work to combat the buildup of a protein in the brain called amyloid plaque that has been linked to Alzheimer's disease.
The antibody in donanemab targets amyloid plaques that have built up in patients by binding to and removing them from the brain.
Patients in Eli Lilly's trials were given intravenous donanemab infusions for around half an hour, every four weeks. Depending on brain scans measuring amyloid levels in the brain, patients were able to stop taking the drug after as early as six months.
In its trials , the company says almost half of patients were able to meaningfully clear out amyloid after around a year after taking the drug. Patients saw no "rebound of amyloid plaque" in the year after treatment wrapped up.
The only other Alzheimer's treatment that works in a similar way on the market is lecanemab, branded as Leqembi by drugmakers Eisai and Biogen. An earlier drug called aducanumab ( marketed as Aduhelm ) from Biogen was discontinued in January.
Beyond effectiveness, Eli Lilly has also touted a handful of other reasons that patients might choose their drug instead of lecanemab.
Donanemab infusions are shorter and less frequent. Trial participants were also able to stop using the drug after amyloid plaque was removed, "which can result in lower treatment costs and fewer infusions," a company spokesperson said.
How much will the treatment cost?
Eli Lilly says it will launch with a list price that adds up to $32,000 for 12 months of treatment, though the actual cost will depend on how long patients take the drug. Some patients in the clinical trials were able to stop the treatment after six months, based on results from brain scans, while others took it for 18 months.
Last year, Eisai defended its list price of $26,500 per year when it launched sales of Leqembi.
But most patients also do not pay the full list price for prescription drugs. For patients with Medicare Part B, the Centers for Medicare and Medicaid Services said donanemab will be covered in the same way it covers lecanemab (Leqembi), with patients paying a 20% coinsurance after they meet their deductible. These patients will need to get the drug from doctors enrolled in a study gathering data tracking its effectiveness.
"CMS is committed to helping people get timely access to treatments and improving care for people with Alzheimer's disease and their families," a CMS spokesperson said.
Eli Lilly noted in a statement: "The potential to complete treatment after a limited-duration course of therapy, along with 30-minute infusions once per month, could result in lower patient out-of-pocket treatment costs and fewer infusions compared to other amyloid-targeting therapies."
How effective was the treatment for Alzheimer's symptoms?
Eli Lilly measured donanemab's effectiveness primarily through rating scales designed to measure the cognitive and functional decline caused by dementia symptoms in patients with early stages of Alzhiemer's.
Compared with patients who received only a placebo, Eli Lilly said those who got the drug saw their decline slow. The gap widened over time, slowing by 22% overall at 76 weeks after first starting the donanemab infusions.
"Importantly, the magnitude of impact on these clinical endpoints meets, and in several respects exceeds prior approvals for demonstration of clinical benefit and effectiveness," the company said of the results in a briefing document given to the FDA panel.
The company says this translated to effectively prolonging how long it took until patients stepped down into the next stage of Alzheimer's disease.
What are the side effects of donanemab?
The labels for all of the anti-amyloid treatments greenlighted by the FDA to date for Alzheimer's already carry a boxed warning about "amyloid-related imaging abnormalities" that can show up on MRI scans.
While these abnormalities generally result in no symptoms, they have been linked to rare but serious issues in some patients like brain function issues and seizures.
These abnormalities were seen in around a quarter of participants in Eli Lilly's trials of donanemab. At least five deaths were reported in donanemab recipients in patients with these kinds of abnormalities, mostly from hemorrhages in the brain.
Eli Lilly says their trials of donanemab tested the drug in harder to treat patients than other treatments studied around the same time. That means the trial included older trial participants as well as those with a gene called APOE ε4 that can increase the risk of Alzheimer's as well as these abnormalities.
Close to 1 in 10 trial participants who took donanemab also experienced a reaction to the infusion, compared to 0.5% of placebo participants. The most common symptoms included chills, skin reddening, nausea, shortness of breath, headache and chest pain.
Approximately 3% of donanemab-treated participants developed hypersensitivity to the infusion, including 0.3% who had a severe allergic reaction.
- Alzheimer's Disease
Alexander Tin is a digital reporter for CBS News based in the Washington, D.C. bureau. He covers the Biden administration's public health agencies, including the federal response to infectious disease outbreaks like COVID-19.
More from CBS News

FDA bans ingredient found in some citrus-flavored sodas

Fake therapist fooled hundreds online until she died, state records say

Insurance wouldn't pay for his vasectomy — coverage could be elusive

Watch Live: Biden campaigns in Wisconsin to kick off critical weekend
.jpeg?sfvrsn=816b1756_1)
New Breakthrough in Alzheimer’s Research: UTMB Researchers Develop Nasal Spray Treatment for Alzheimer’s Disease
July 3, 2024 • 2:00 p.m.
Researchers at the University of Texas Medical Branch recently discovered a significant advancement in the fight against neurodegenerative diseases such as Alzheimer’s disease and dementia. The study, published today in Science Translational Medicine, introduces an innovative nasal spray treatment that has shown promising results in clearing harmful tau protein build-up and improving cognitive functions in aged mice models with neurodegenerative diseases.
“This nasal spray approach opens new avenues for non-invasive delivery of tau therapeutic antibodies directly to the brain, and it holds promise for many neurodegenerative diseases.” said Dr. Rakez Kayed, lead author and professor at the Department of Neurology at UTMB.
Tau is a microtubule-associated protein found in human brains that helps stabilize microtubules, part of the framework that gives the cell its shape and helps it stay organized, in neurons. In healthy brains, tau proteins help keep things in order. But in neurodegenerative diseases, they can become abnormally twisted and form tangles that disrupt neuronal function and lead to cognitive decline. Current tau immunotherapies have struggled with efficacy due to their limited ability to penetrate intracellular compartments where these tau buildups reside.
Kayed and his team developed a specific type of antibody, TTCM2, which selectively recognizes and targets toxic tau buildup. The antibody was packaged in particles to enhance its delivery to the brain via the nasal route. This method bypasses the blood-brain barrier, a significant hurdle in neurodegenerative disease treatment, ensuring rapid and effective delivery of the therapy.
“Our research highlights the potential of nasal tau immunotherapy to effectively target intracellular tau aggregates– a primary driver of neurodegeneration and cognitive decline in diseases like Alzheimer’s and other tauopathies,” added Kayed. “This method not only improves the delivery of therapeutic antibodies but also enhances their efficacy in clearing tau aggregates and improving cognitive functions”.
An essential aspect of this approach is that it involves TRIM21, an intracellular receptor for antibodies and E3 ligase, known for mediating the clearance of antibody bound pathogens like viruses. In the study, TRIM21 facilitated the clearance of antibody bound intracellular tau aggregates, thereby enhancing the therapeutic effect and cognitive improvements in the mice model.
“This advancement could significantly impact the treatment strategies for Alzheimer’s and related tauopathies, offering new hope for millions of patients suffering from these debilitating conditions,” said Sagar Gaikwad, first author of the study and postdoctoral fellow at UTMB.
This study highlights the potential impact on future treatments for neurodegenerative diseases. Researchers at UTMB plan to advance this research by conducting further preclinical trials and exploring the potential of TTCM2-ms in human clinical trials. The goal is to translate these promising results into a viable treatment option for patients suffering from Alzheimer’s disease and other tau-related disorders.
The study was funded by grants from the NIH, Alzheimer’s Association and UTMB Claude D. Pepper OAIC Pilot grant. Authors and investigators also include Nicha Puangmalai, Minal Sonawane and Mauro Montalbano from UTMB.
- Alumni News
- Awards & Accolades
- Faculty/Staff News
- Health Care
- In The News
- News Releases
- UTMB Research
- UTMB Support Areas
- UTMB Twitter
- UTMB Facebook
- UTMB Instagram

COMMENTS
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Alzheimer's disease articles from across Nature Portfolio. Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by ...
Memory declines slowly in normal aging (1). Alzheimer's disease is marked by more rapid cognitive decline, often starting earlier in life (2). Current therapies enhance cognition without changing the rate of decline in AD (3). The anticipated effect of novel therapies is reduction in the rate of decline (4). Go to:
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ ...
View Full Text ; View PDF ; Diagnostic performance of plasma pTau 217, pTau 181, Aβ 1-42 and Aβ 1-40 in the LUMIPULSE automated platform for the detection of Alzheimer disease. Recently developed blood markers for Alzheimer's disease (AD) detection have high accuracy but usually require ultra-sensitive analytic tools not commonly available in clinical laboratories, and their performa...
Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer's Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD.
Journal of Neurology (2024) Alzheimer disease (AD) is the most common contributor to dementia in the world, but strategies that slow or prevent its clinical progression have largely remained ...
In studies of potential disease-modifying drugs for Alzheimer's disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says ...
The inexorable progression of Alzheimer's disease exerts a huge toll on patients, families, and society, costing approximately $1 trillion annually, an amount that is likely to increase with the ...
Dementia is a clinical syndrome characterized by impairment in several cognitive domains that prevents an individual from living a fully functional and autonomous life ().The most common cause of dementia is Alzheimer's disease (AD), accounting for nearly 60 to 80% of all cases ().AD is the sixth leading cause of death, with an estimated prevalence of nearly 30 million people worldwide.
This article describes the public health impact of Alzheimer's disease (AD), including prevalence and incidence, mortality and morbidity, use and costs of care and the ramifications of AD for family caregivers, the dementia workforce and society. The Special Report discusses the larger health care s …
More than 50% of people diagnosed with Alzheimer's dementia who were studied at Alzheimer's Disease Research Centers had mixed dementia. 22 In community-based studies, the percentage is considerably higher. 24 Mixed dementia is most common in people age 85 or older. 27, 28: Symptoms vary depending on the combination of brain changes present.
Alzheimer's disease is by far the most common type of dementia, representing about 60-80% of all diagnosed cases of cognitive disorders [ 10 ]. In 2020, around 6.2 million Americans aged 65 or older had Alzheimer's disease, and this number is predicted to increase to 12.7 million Americans by 2050. Figure 5.
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
Aims and scope. Alzheimer's Research & Therapy is the major forum for translational research into Alzheimer's disease. An international peer-reviewed journal, it publishes open access basic research with a translational focus, as well as clinical trials, research into drug discovery and development, and epidemiologic studies.
The National Institute on Aging and the Alzheimer's Association convened three separate work groups in 2011 and single work groups in 2012 and 2018 to create recommendations for the diagnosis and characterization of Alzheimer's disease (AD). The present document updates the 2018 research framework in response to several recent developments.
Seven recent AMP AD reports showcase research advances related to the discovery of new drug candidate targets, identification of molecular subtypes of the disease, and new potential biomarkers that can serve as the basis for a precision medicine approach to therapy development. Identifying ATP6VA1 gene as a candidate target for treatment ...
What thrilled Sperling, who won the award for her work on clinical trials of Alzheimer's treatments, was a sense of hope, which has been conspicuously missing from research into the disease for ...
Indeed, in one study of 184 individuals who met research neuropathological criteria for AD 9, ... C. et al. Contribution to Alzheimer's disease risk of rare variants in TREM2, SORL1, and ABCA7 ...
The healthy brain and our aging population: translating science to public health practice. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 2007;3(S1):S1-S2. Anderson LA, McConnell SR. Cognitive health: an emerging public health issue. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 2007;3(S1):S70-S73.
As one of the most common neurodegenerative diseases and causes of dementia, Alzheimer disease (AD) is a critical topic for biomedical research. The main clinical manifestation of AD is the progressive decline of cognitive function and activities of daily living, and the pivotal pathological features of AD are amyloid beta (Aβ) deposition ...
CHICAGO, June 27, 2024 — A workgroup convened by the Alzheimer's Association has published revised criteria for the diagnosis and staging of Alzheimer's disease that are based on the biology of the disease and reflect recent advancements in Alzheimer's research, diagnostics and treatment. The 2024 update includes an updated biomarker classification system that includes blood-based ...
Potential new target for early treatment of Alzheimer's disease. ScienceDaily . Retrieved July 3, 2024 from www.sciencedaily.com / releases / 2024 / 07 / 240702135544.htm
Alzheimer's disease (AD) is a disorder that causes degeneration of the cells in the brain and it is the main cause of dementia, which is characterized by a decline in thinking and independence in personal daily activities. AD is considered a multifactorial disease: two main hypotheses were proposed as a cause for AD, cholinergic and amyloid hypotheses. Additionally, several risk factors such ...
Alzheimer's Disease (AD) exerts a significant worldwide impact. An estimated 44 million individuals currently live with this Major Neurocognitive Disorder. ... An interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease. Alzheimer's Research & Therapy, 10 (1 ...
Dementia with Lewy bodies (DLB) is evaluated to be the third cause of dementia after Alzheimer's disease (AD) and vascular dementia. DLB is characterized by a poorer prognosis, higher healthcare costs and caregiver burden, and a greater impact on quality of life compared with AD [1, 2].Yet, therapeutic research in DLB has long remained a poorly invested field, and there are no disease ...
Further research on larger cohorts is warranted to definitely ascertain the application of NLR as a possible marker for aMCI and AD. 1. Introduction. Alzheimer's disease (AD) is the most common cause of dementia and one of the leading causes of morbidity and mortality in the older population . Other frequent types of dementia are, ...
FDA approves new treatment to slow progression of early Alzheimer's 02:45. The Food and Drug Administration approved a new Alzheimer's treatment called donanemab on Tuesday, clearing the way for ...
In an 18-month study of people who were in the early stages of Alzheimer's disease, the antibody lecanemab - which the US Food and Drug Administration approved in 2023 - slowed the rate of ...
Researchers at UTMB plan to advance this research by conducting further preclinical trials and exploring the potential of TTCM2-ms in human clinical trials. The goal is to translate these promising results into a viable treatment option for patients suffering from Alzheimer's disease and other tau-related disorders.