- Case Report
- Open access
- Published: 15 October 2008

Chronic tophaceous gout presenting as acute arthritis during an acute illness: a case report
- Abhijeet Dhoble 1 ,
- Vijay Balakrishnan 1 &
- Robert Smith 1
Cases Journal volume 1 , Article number: 238 ( 2008 ) Cite this article
16k Accesses
5 Citations
Metrics details
Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. It can either manifest as acute arthritis or chronic tophaceous gout.
Case presentation
We present a 39-year-old male patient who developed acute arthritis during his hospital course. Later on, after a careful physical examination, patient was found to have chronic tophaceous gout. The acute episode was successfully treated with colchicines and indomethacin.
Gout usually flares up during an acute illness, and should be considered while evaluating acute mono articular arthritis. Rarely, it can also present with tophi as an initial manifestation.
Gout is a metabolic disease, which is characterized by acute or chronic arthritis, and deposition of monosodium urate crystals in joint, bones, soft tissues, and kidneys [ 1 – 4 ]. In 18 th century, Garrod proposed a causative relationship between elevated uric acid and urate crystal formation, which is underlying pathology for gout [ 4 ]. Gout can either manifest as acute arthritis or chronic arthropathy, which is also called tophaceous gout [ 1 , 2 , 5 ].
A 39-year-old African American male patient was admitted with one-day history of acute left lower quadrant pain, and was diagnosed with acute uncomplicated diverticulitis, confirmed by computed tomography (CT) of the abdomen. His medical and surgical history was unremarkable, and he denied any medication use. He denied smoking or illicit drug use, but admitted occasional alcohol use on every other weekend. He did not follow any particular diet. He had an average built with BMI of 29.6. He was started on intravenous antibiotics and pain medication, which led to significant clinical improvement within two days.
On the third day of hospitalization, he developed acute, severe pain and swelling of the left elbow. Within next few hours, pain worsened and he was unable to move the elbow joint, which was tender, erythematous, and swollen on examination (figure 1 ). Never investigated in the past, we also noted a firm 4 × 6 cm mass on each elbow, and another one surrounding the proximal inter-phalangeal joint of right middle finger (figure 2 ). There was no overlying edema or cellulitis. There were no other swellings or tophi noted especially on toes or ears. When asked particularly, he denied similar episodes in the past. He also denied any episode of swelling of great toe in the past.
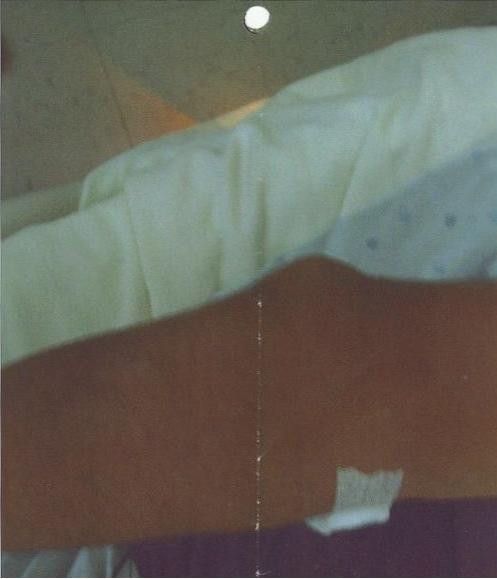
Tophus at the back of right elbow.
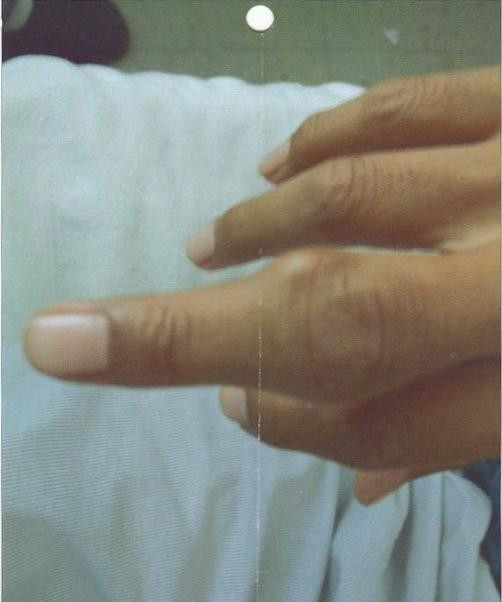
Tophi/tophus around the proximal inter-phalangeal joint of right middle finger.
Plain radiography of left elbow showed joint effusion, and soft tissue swelling. Radiography of other joints including hands and feet was not performed. Laboratory values on the third day are given in table 1 . Liver function test was also performed, and the results were unremarkable. Diagnostic arthocentesis was performed on both the elbows, and revealed negatively birefringent needle-shaped crystals using polarized microscopy in both samples. Detailed analysis of synovial fluid is given in table 2 . The swelling on the right elbow was aspirated to determine the etiology because patient had that swelling for a long time.
The patient responded partially to colchicine, but later had great relief with indomethacin. Colchicine was used at the dose of 0.6 mg every two hourly. He received total of six doses, but it was stopped because he developed severe nausea and vomiting. He admitted that his pain was reduced to 4/10 in intensity from 9/10 before treatment, but swelling was persistent. We initiated indomethacin at 50 mg every eight hourly, and his pain and swelling was relieved to great extent in 48 hours.
Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. All patients have hyperuricemia at some point of their disease. But, either normal or low serum uric acid levels can occur at the time of acute attack; and asymptomatic hyperuricemic individuals may never experience a clinical event resulting from urate crystal deposition [ 1 – 4 ]. Low to normal uric acid concentration can be due to excessive excretion of uric acid, crystal formation, or systemic inflammatory state [ 6 , 7 ]; however, exact mechanism is still not completely understood. A diagnosis of gout is most accurate when supported by visualization of uric acid crystals in a sample of joint or bursal fluid, or demonstrated histologically in excised tissue. Synovial fluid analysis of our patient was consistent with inflammatory arthritis. Mild leucocytosis in this patient was due to systemic inflammatory response.
Visible or palpable tophi, as this patient exhibited, are usually noted only among those patients who are hyperuricemic and have had repeated attacks of acute gout, often over many years. However, presentation of tophaceous deposits in the absence of gouty arthritis is also reported [ 5 , 8 ]. Pain and inflammation are manifested when uric acid crystals activate the humoral and cellular inflammatory processes [ 9 ].
During an acute illness, if systemic inflammatory state prevails, such as in an acute infection, cytokines and chemokines triggers inflammation and cause arthritis in the presence of urate crystals [ 10 , 11 ]. Phagocytosis of these crystals by macrophages in the synovial lining cells precedes influx of neutrophils in the joint [ 9 – 11 ]. This process releases various mediators of inflammation locally [ 12 , 13 ].
Hyperuricemia is often present in patients with tophaceous gout, and they can benefit from uric acid lowering therapy early during the course [ 14 , 15 ]. In our patient, serum uric acid and 24-hour urine uric acid level was within normal limits when measured in the hospital before his discharge from the hospital. It was decided to follow him up in the clinic in two weeks, and measure these values again during 'interval gout' before deciding to start him on any particular medication to prevent further attacks of acute arthritis.
Our patient presented with tophi as an initial presentation of gout, which is very rare, but has been reported [ 5 , 8 ]. Investigational studies due to acute elbow joint pain deciphered the underlying mystery of chronic swelling. Systemic inflammatory response secondary to diverticulitis exposed the joints to the effects of urate.
First-line treatments for an acute flare are either oral colchicine and/or non-steroidal anti-inflammatory agents. Systemic or intra-articular corticosteroids can also be used, and are equally effective, but with more side effects [ 16 , 17 ]. Interleukin-1 inhibitors are still under investigation, and are not approved for an acute attack of gout [ 18 ].
Gout usually flares up during an acute illness, and should always be considered while evaluating acute mono articular arthritis in hospitalized patients. Gout can present with tophi as an initial manifestation of the disease process.
Written informed consent was obtained from the patient for publication of this case report and accompanying images in Journal of Medical Case Reports. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Campion EW, Glynn RJ, DeLabry LO: Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987, 82: 421-10.1016/0002-9343(87)90441-4.
Article CAS PubMed Google Scholar
Hall AP, Barry PE, Dawber TR, McNamara PM: Epidemiology of gout and hyperuricemia: A long term population study. Am J Med. 1967, 42: 27-10.1016/0002-9343(67)90004-6.
Logan JA, Morrison E, McGill PE: Serum uric acid in acute gout. Ann Rheum Dis. 1997, 56: 696-7.
Article PubMed Central CAS PubMed Google Scholar
Garrod AB: The Nature and Treatment of Gout and Rheumatic Gout. 1863, 2
Google Scholar
Wernick R, Winkler C, Campbell S: Tophi as the initial manifestation of gout. Report of six cases and review of literature. Arch intern med. 1992, 152: 873-10.1001/archinte.152.4.873.
Urano W, Yamanaka H, Tsutani H, Nakajima H, Matsuda Y, Taniguchi A, Hara M, Kamatani N: The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002, 29 (9): 1950-3.
CAS PubMed Google Scholar
Simkin PA: The pathogenesis of podagra. Ann Intern Med. 1977, 86: 230.
Hollingworth P, Scott JT, Burry HC: Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983, 26: 98-101. 10.1002/art.1780260117.
Beutler A, Schumacher HR: Gout and 'pseudogout': when are arthritic symptoms caused by crystal disposition?. Postgrad Med. 1994, 95: 103-6.
Schumacher HR, Phelps P, Agudelo CA: Urate crystal induced inflammation in dog joints: sequence of synovial changes. J Rheumatol. 1974, 1: 102.
Gordon TP, Kowanko IC, James M, Roberts-Thomson PJ: Monosodium urate crystal-induced prostaglandin synthesis in the rat subcutaneous air pouch. Clin Exp Rheumatol. 1985, 3: 291.
Malawista SE, Duff GW, Atkins E, Cheung HS, McCarty DJ: Crystal-induced endogenous pyrogen production. A further look at gouty inflammation. Arthritis Rheum. 1985, 28: 1039-10.1002/art.1780280911.
Falasca GF, Ramachandrula A, Kelley KA, O'onnor CR, Reginato AJ: Superoxide anion production and phagocytosis of crystals by cultured endothelial cells. Arthritis Rheum. 1993, 36: 105-10.1002/art.1780360118.
Sutaria S, Katbamna R, Underwood M: Effectiveness of interventions for the treatment of acute and prevention of recurrent gout – a systematic review. Rheumatology (Oxford). 2006, 45: 1422-10.1093/rheumatology/kel071.
Article CAS Google Scholar
Wallace SL, Singer JZ: Therapy in gout. Rheum Dis Clin North Am. 1988, 14: 441.
Janssens HJ, Janssen M, Lisdonk van de EH, van Riel PL, van Weel C: Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 371 (9627): 1854-60. 10.1016/S0140-6736(08)60799-0. 2008 May 31
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Lioté F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentão J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gòrska I, EULAR Standing Committee for International Clinical Studies Including Therapeutics: EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006, 65 (10): 1312-24. 10.1136/ard.2006.055269.
So A, De Smedt T, Revaz S, Tschopp J: A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007, 9: R28-10.1186/ar2143.
Article PubMed Central PubMed Google Scholar
Download references
Acknowledgements
We thank patient for giving us consent for the publication of the case report.
Author information
Authors and affiliations.
Department of Internal Medicine, Michigan State University, East Lansing, Michigan, USA
Abhijeet Dhoble, Vijay Balakrishnan & Robert Smith
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Abhijeet Dhoble .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed equally in collecting patient data, chart review, and editing medical images. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Dhoble, A., Balakrishnan, V. & Smith, R. Chronic tophaceous gout presenting as acute arthritis during an acute illness: a case report. Cases Journal 1 , 238 (2008). https://doi.org/10.1186/1757-1626-1-238
Download citation
Received : 09 October 2008
Accepted : 15 October 2008
Published : 15 October 2008
DOI : https://doi.org/10.1186/1757-1626-1-238
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diverticulitis
- Serum Uric Acid
- Uric Acid Level
Cases Journal
ISSN: 1757-1626
- Case Report
- Open access
- Published: 06 January 2014
Gout in a 15-year-old boy with juvenile idiopathic arthritis: a case study
- Hallie Morris 2 ,
- Kristen Grant 1 ,
- Geetika Khanna 3 &
- Andrew J White 4
Pediatric Rheumatology volume 12 , Article number: 1 ( 2014 ) Cite this article
18k Accesses
15 Citations
10 Altmetric
Metrics details
Joint pain is a common complaint in pediatrics and is most often attributed to overuse or injury. In the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. Rarely, a child has two distinct causes for joint pain. In this case, an obese 15-year-old male was diagnosed with gout, a disease common in adults but virtually ignored in the field of pediatrics. The presence of juvenile idiopathic arthritis (JIA) complicated and delayed the consideration of this second diagnosis. Indeed, the absence of gout from this patient’s differential diagnosis resulted in a greater than two-year delay in receiving treatment. The patients’ BMI was 47.4, and he was also mis-diagnosed with osteochondritis dissecans and underwent medical treatment for JIA, assorted imaging studies, and multiple surgical procedures before the key history of increased pain with red meat ingestion, noticed by the patient, and a subsequent elevated uric acid confirmed his ultimate diagnosis. With the increased prevalence of obesity in the adolescent population, the diagnosis of gout should be an important consideration in the differential diagnosis for an arthritic joint in an overweight patient, regardless of age.
In the pediatric population, there are numerous causes of joint pain, stiffness, and swelling. Many can be attributed to minor activity or overuse-related injury, especially in the overweight and obese populations [ 1 ], but in the face of persistent, severe, or recurrent symptoms, other diagnoses must be considered. These typically fall into two categories in children and adolescents: bony or structural causes [ 2 ], or rheumatologic conditions [ 3 ]. Careful history and physical examination, along with use of imaging and laboratory studies, can often distinguish between the two [ 4 , 5 ]; however, when a complete work-up is performed and no clear answer emerges, the differential must be expanded [ 6 ]. In the rare case in which a firm primary diagnosis has been made, it is more difficult still to consider additional, secondary, causes of joint pain.
The following case describes an adolescent young man with severe ankle pain, as well as multiple other joint complaints, who was correctly diagnosed and treated for polyarticular juvenile idiopathic arthritis. While the majority of his joints improved, his ankle continued to be extremely tender and swollen. After two years of aggressive medical treatment, surgical procedures, and multiple imaging studies, it was careful probing into his history and classic physical examination findings that ultimately led to the additional diagnosis of gout.
Case presentation
A 15-year-old obese Hispanic young man presented to the orthopedic surgery service with right-sided ankle pain. His pain began after a sports injury approximately one year prior to presentation but did not respond to immobilization, physical therapy, or prolonged rest. The pain was located at the medial side of the ankle, was worse with activity, and was accompanied by intermittent swelling. He took ibuprofen at night occasionally that provided modest relief.
His past medical history was unremarkable. Family history was negative for autoimmune disease including JIA. His only medication was occasional ibuprofen, and his immunizations were up to date.
On examination, the patient was heavyset, weighing 142.8 kg and standing 173.6 cm tall, with a BMI of 47.4. His vital signs were normal and he was in no distress. Musculoskeletal exam revealed bilateral limited ankle dorsiflexion, worse on the right. He had tenderness to palpation over the medial aspect of his ankle just anterior to the medial malleolus. All other joints were normal.
His initial work-up consisted of radiographs of his right ankle, showing evidence of a healing osteochondritis dissecans (OCD) lesion. He was instructed to continue physical therapy and follow up in three months with the possibility of arthroscopy of the affected joint if there was no improvement.
At follow-up, his pain was unchanged. Radiographs showed the previously seen presumed OCD lesion, now with a sclerotic border. He was scheduled for an MRI of the ankle, which was read as more consistent with a chondroblastoma versus an inflammatory process.
Several months later, he began complaining of left shoulder pain and decreased range of motion of gradual onset. He denied any trauma causing this new complaint. He was again seen by the orthopedic service and found to have significantly decreased range of motion and AC joint tenderness. Radiographs of the shoulder did not show any abnormalities. He was diagnosed with adhesive capsulitis and instructed to perform stretching exercises and take acetaminophen for pain. An MRI of the shoulder was performed given the unusual age of presentation for adhesive capsulitis, which revealed evidence of inflammation and synovitis consistent with juvenile idiopathic arthritis. He was referred to the rheumatology service.
The patient described daily pain and some morning stiffness for several weeks at a time, which would then subside for several weeks before returning. The pain and stiffness involved the right elbow, left knee, right wrist and several fingers in addition to the left shoulder and right ankle previously described. Examination by the pediatric rheumatologist noted improvement of his range of motion of the left shoulder with his home exercises and was back within normal limits. He did have moderate pain with extension of the shoulder. His left wrist was also tender with limited range of motion. His range of motion was limited bilaterally in the ankles and multiple PIP joints were painful and swollen. Considering the symmetric joint distribution, involvement of PIPs, and an elevated rheumatoid factor of 24.7 IV/mL (nl 0.0-13.9), the patient’s presentation was felt to be most consistent with early onset rheumatoid arthritis, or RF + polyarticular JIA. Subsequently, an anti-CCP antibody test was positive. He was prescribed naproxen and methotrexate. Laboratory studies from this initial visit were also notable for an elevated CRP 6.7 mg/dL, WBC 12.6 K/uL, Hgb of 12.3 g/dL, and ESR of 61 mm/hr. ANA, dsDNA, and HLA-B27 were negative.
The patient presented for follow-up approximately 2.5 months after beginning the naproxen and methotrexate regimen. At that time, the pain in the majority of his joints had improved substantially, however, the right ankle had become more tender and swollen. The pain was worse in the morning, to the point that he began using crutches. He was started on twice weekly etanercept injections, but when he experienced no relief, was subsequently switched to adalimumab and then to rituximab.
Given the lack of response of the patient’s ankle to this therapeutic regimen (Figure 1 ) over the course of the next year, despite improvement in his other joints, arthroscopic exploration of the right ankle with synovectomy and potential OCD curettage was performed. During surgery, the patient was found to have “significant debris” including a “crystalline white substance” within the joint space, which was attributed by the surgeons to “the previous steroid injection.” The fluid was not sent for culture, cell count or crystal studies, despite the fact that the patient had not ever received a steroid injection in that joint. The debris was removed, but not sent for pathology.
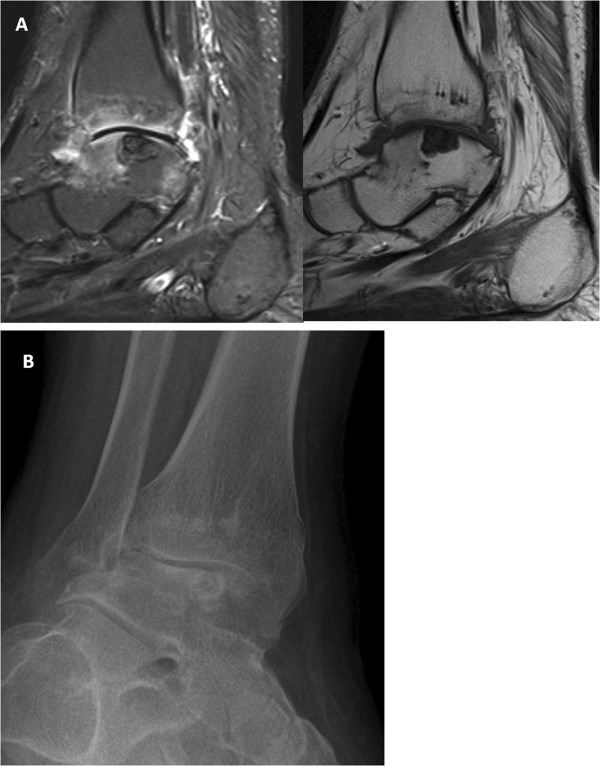
Imaging of gouty joint in an adolescent. (A) Synovitis on MRI with and without IV contrast of the right ankle with osteitis in the distal tibia and talar dome. Talar dome lesion was thought to represent an osteochondritis dissecans/osteonecrosis. (B) Radiographs performed 15 months later show marked joint space loss with persistent talar dome lesion which likely represents an intraosseous tophus.
After recovery from surgery, the patient returned to the rheumatology service for further management of his JIA. He continued to have pain and stiffness of his right ankle as well as several other joints that was difficult to manage. He was eventually started on a low dose of prednisone, previously avoided given his weight, which did offer moderate symptomatic relief. Ultimately the medication regimen of prednisone, etanercept, methotrexate, naproxen, and sulfasalazine had the greatest impact on his JIA symptoms, although his right ankle continued to be the most painful joint.
2.5 years after he first presented, the patient developed multiple non-tender scattered subcutaneous nodules over the extensor surface of his bilateral forearms, his left elbow, and his right knee (Figure 2 ). Their etiology was unclear, but attributed to rheumatoid nodules. Four months later, however, the patient spontaneously noted that his ankle pain seemed to worsen following the ingestion of red meat. With this new data in hand, a uric acid level was sent and was extremely elevated at 13.3 mg/dL. The nodules were deemed to be consistent with tophi, virtually pathognomonic for gout. The patient was started on colchicine and then allopurinol and improved. Over the course of time he did begin to complain of pain in his big toe, a more classic presentation of gout. Uric acid levels however, remained high, running between 11.7 and 13.5 mg/dL over the following year.

X-ray imaging of gouty tophus in an adolescent. Lateral view of the forearm shows subcutaneous nodules along the dorsal aspect of the proximal forearm.
At the time of the patient’s transition to adult care, he continued to exhibit active symptomatology, with joint pain in the knees, elbows, and ankles, limited range of motion in the aforementioned joints as well as his wrists, MCP’s and PIP’s, and difficulty ambulating. He continues to be treated for both JIA and gout.
Joint pain is a common complaint in pediatrics and in the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. In this case, however, diagnosis was complicated because the child had two distinct etiologies causing joint pain. Moreover, the patient’s second diagnosis was gout, a very uncommon condition in a pediatric patient, even in the setting of morbid obesity.
Poly-articular juvenile idiopathic arthritis was considered as the unifying diagnosis when the patient presented with involvement of multiple joints. The typical presenting features of JIA are morning stiffness, pain and swelling of the joints, limited range of motion, and joint contractures [ 7 ]. Radiographs may show some soft tissue swelling and osteopenia early, with subchondral sclerosis and erosions evident after long-standing disease, but MRI has been shown to be more sensitive for bone marrow edema and tenosynovitis, as well as bone erosions, cartilage lesions, and synovial hypertrophy [ 8 ]. JIA is most often seen in children of European descent, but affects children throughout the world. The disease is thought to be idiopathic with its cause poorly understood, although there is mounting evidence that autoimmunity may be involved [ 7 ]. There is also thought to be potential genetic susceptibility in affected individuals [ 9 ]. While the majority of the patient’s overall complaints did seem to fit the diagnosis of JIA both in his clinical presentation and laboratory and imaging results, as well as his response to treatment, his right ankle continued to be refractory to treatment.
The acquisition of additional history led to the consideration of gout as a second diagnosis. Historically, gout has affected predominantly older, overweight men but in more recent years the male to female ratio has fallen to 2:1 [ 10 ]. A resurgence of gout across the population has been noted in recent years, and juvenile gout has also begun to be reported, with many of the cases being due solely to known risk factors such as being overweight [ 11 , 12 ]. On imaging, plain films may show little evidence of gout in early stages, but later in the course can show joint effusions, bony erosions, or tophi within the joint. Gout can appear similar to other arthritides on MRI, with mild bone marrow edema, tenosynovitis, and bony erosions, making diagnosis difficult but important to consider, especially in the setting of an overweight or obese patient. However, if tophi are present, MRI is able to detect this as a potentially differentiating characteristic. Other imaging studies may be of more use to differentiate gout from other diagnoses, as ultrasound may be able to detect crystals within the joint space, and can even differentiate between gout and pseudogout [ 13 ]. The gold standard for diagnosing gout remains the acquisition of urate crystals from synovial fluid. In the absence of this data, however, the presence of 2 of the 3 Rome clinical criteria (uric acid >7.0 mg/dL, history of painful joint with abrupt onset and remission within 2 weeks, and presence of tophus), as were existent in our patient, was found to have a positive predictive value of 76.9%, and a specificity of 88.5% [ 14 ].
While it seems likely the patient did have gout, gout alone does not seem most likely given the clinical picture. Gout simulating JIA is a possibility; a patient with untreated gout can develop bony erosions and deformities, leading to the disappearance of the intercritical periods which are usually pathognomonic of gout. Typically, however, one would expect gout to present as an episodic arthritis. Even in untreated individuals, complete resolution of the earliest attacks nearly always occurs within several weeks, which this patient never experienced. Moreover, the symmetric joint distribution with involvement of the PIPs along with a positive rheumatoid factor and CCP antibody point toward the concurrent JIA diagnosis in this case.
There were several missed opportunities to diagnose this patient earlier in his course. First, gout was not considered as a part of the original differential because of its propensity to affect older individuals. As the epidemic of childhood obesity grows, adult conditions usually a result of long term lifestyle consequences are being seen more frequently in the pediatric population, most notably type II diabetes but also musculoskeletal complaints and, as in this case, gout.
Secondly, if gout had been on the differential, the radiologic features may have been recognized as being consistent with this condition. Third, a uric acid level was not sent until after the additional diet history was obtained. And finally, proper examination of joint fluid, from either aspiration or surgical debridement, would have revealed the presence of negatively birefringent crystals, providing a timely diagnosis.
In conclusion, gout was diagnosed in this teenage patient with longstanding juvenile idiopathic arthritis. The diagnosis of gout should therefore be an important element of the differential for a refractory painful joint in an overweight patient regardless of age, and regardless of pre-existing diagnoses. Failing to consider this diagnosis may result in delay of optimal treatment and cause long-term effects of bone erosion and joint destruction. Sending joint fluid for crystalline analysis, checking uric acid levels, and performing imaging studies, specifically non-invasive, cost effective modalities such as ultrasound, are all reasonable parts of a complete work-up in any child with arthritis.
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Deere KC: Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012, 153 (9): 1932-1938. 10.1016/j.pain.2012.06.006.
Article PubMed Google Scholar
Amendola A, Panarella L: Osteochondral lesions: medial vs. lateral, persistent pain, cartilage restoration options and indications. Foot Ankle Clin. 2009, 14 (2): 215-227. 10.1016/j.fcl.2009.03.004.
Jennings F, Lambert E, Fredericson M: Rheumatologic diseases presenting as sports-related injuries. Sports Med. 2008, 38 (11): 917-930. 10.2165/00007256-200838110-00003.
Punaro M: Rheumatologic conditions in children who may present to the orthopaedic surgeon. J Am Acad Orthop Surg. 2011, 19 (3): 163-169.
PubMed Google Scholar
Wukich DK, Tuason DA: Diagnosis and treatment of chronic ankle pain. Instr Course Lect. 2011, 60: 335-350.
Choudhary S, McNally E: Review of common and unusual causes of lateral ankle pain. Skeletal Radiol. 2011, 40 (11): 1399-1413. 10.1007/s00256-010-1040-z.
Gowdie PJ, Tse SM: Juvenile idiopathic arthritis. Pediatr Clin North Am. 2012, 59 (2): 301-327. 10.1016/j.pcl.2012.03.014.
Breton S, Jousse-Joulin S, Finel E, Marhadour T, Colin D, de Parscau L, Devauchelle-Pensec V: Imaging approaches for evaluating peripheral joint abnormalities in juvenile idiopathic arthritis. Semin Arthritis Rheum. 2012, 41 (5): 698-711. 10.1016/j.semarthrit.2011.08.004.
Prahalad S, Conneely KN, Jiang Y, Sudman M, Wallace CA, Brown MR: Susceptibility to childhood onset rheumatoid arthritis: investigation of a weighted genetic risk score that integrates cumulative effects of variants at five genetic loci. Arthritis Rheum. 2013, 65 (6): 1663-1667. 10.1002/art.37913.
Article PubMed Central CAS PubMed Google Scholar
Zampogna G, Andracco R, Parodi M, Cutolo M, Cimmino MA: Has the clinical spectrum of gout changed over the last decades?. Clin Exp Rheumatol. 2012, 30 (3): 414-416.
CAS PubMed Google Scholar
Chen SY, Shen ML: Juvenile gout in Taiwan associated with family history and overweight. J Rheumatol. 2007, 34 (11): 2308-2311.
Kedar E, Simkin PA: A perspective on diet and gout. Adv Chronic Kidnet Dis. 2012, 19 (6): 392-397. 10.1053/j.ackd.2012.07.011.
Article Google Scholar
Dalbeth N, Doyle AJ: Imaging of gout: an overview. Best Pract Res Clin Rheumatol. 2012, 26 (6): 823-838. 10.1016/j.berh.2012.09.003.
Malik A, Schumacher HR, Dinnella JE, Clayburne GM: Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid analysis. J Clin Rheumatol. 2009, 15 (1): 22-24. 10.1097/RHU.0b013e3181945b79.
Download references
Author information
Authors and affiliations.
Boston Combined Residency Program, PGY1, Boston, Mass, USA
Kristen Grant
Washington University School of Medicine, WUSM IV, St Louis, Missouri, USA
Hallie Morris
Division of Radiology, Washington University School of Medicine, St Louis, Missouri, USA
Geetika Khanna
Division of Pediatric Rheumatology, Washington University School of Medicine, St Louis, Missouri, USA
Andrew J White
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrew J White .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
KG participated in background research and drafting of the manuscript. HM was involved in drafting and revision of the manuscript. GK contributed figures and their impressions. AW conceived of the study, participated in its coordination, and was involved in drafting and revision of the manuscript. All authors read and approved of the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( https://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Morris, H., Grant, K., Khanna, G. et al. Gout in a 15-year-old boy with juvenile idiopathic arthritis: a case study. Pediatr Rheumatol 12 , 1 (2014). https://doi.org/10.1186/1546-0096-12-1
Download citation
Received : 14 October 2013
Accepted : 24 December 2013
Published : 06 January 2014
DOI : https://doi.org/10.1186/1546-0096-12-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Juvenile idiopathic arthritis
- Osteochondritis dissecans
- Treatment failure
Pediatric Rheumatology
ISSN: 1546-0096
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Gout Case Study

In this gout case study, a 66-year-old female has a past medical history of diabetes, neuropathy, depression, migraines, insomnia, gout, and hypertension. She has numerous complaints. Her gout has not been in good control as she is requiring significant indomethacin use for flare management. She says that capsaicin doesn’t help her knee pain. In addition, her blood sugars have been elevated. Her current medication list includes:
- Aspirin 81 mg daily
- Capsaicin cream prn
- Diphenhydramine 25 mg at night
- Metformin 500 mg BID
- hydrochlorothiazide 50 mg daily
- Glipizide 5 mg daily
- Allopurinol 200 mg daily
- Indomethacin 50 mg TID PRN
- Duloxetine 30 mg daily
- Topiramate 50 mg BID
Before I bring up anything else, I always try to address the patient’s issues first; so let’s do that.
First, let’s address the gout flares as we would like to minimize NSAID use. I would be inclined to obtain a uric acid level to see where that is at. From there, we will likely have to consider an increase in allopurinol (I’d also like renal function labs). There are two other considerations that I would think about here as well. Hydrochlorothiazide could increase uric acid and directly oppose uric acid lowering therapy. If it is being used simply for hypertension it would be a good opportunity to select another agent. What agent would make sense? An ACE/ARB would be beneficial in a patient with diabetes but more specifically, losartan may be purported to have some activity that may reduce uric acid levels. Lots of potential wins with a transition off hydrochlorothiazide to an antihypertensive like losartan.
Capsaicin needs to be scheduled to have analgesic relief. Patient education and inquiring about how she was using this medication would be my first step in this situation. If she had tried a scheduled course of at least a few weeks, a topical NSAID would be a consideration. You’d think the knee pain would be improved if she was using indomethacin frequently, but obviously, we’d like to avoid this long-term.
Let’s talk about blood sugars. It is unclear why metformin is at a lower dose. Titration of that medication would be most appropriate. An investigation into whether an SGLT-2 or GLP-1 would be an option would be another consideration with the potential of getting rid of the sulfonylurea. Also, remember that thiazide diuretics may have modest effects on hyperglycemia so maybe changing the hydrochlorothiazide would help for one more reason.
Lastly, I’m never a big fan of diphenhydramine. It is often a cause of the prescribing cascade which I talk about extensively in my latest book Perils of Polypharmacy . I’d seek to inquire about why this medication is being used. This in combination with topiramate could potentially lead to some cognitive issues so I would definitely like to review this with the patient and ensure that these drugs are both safe and effective for what they are being used for.
What else would you look at here in this gout case study?
Did you enjoy this blog post? Subscribers are emailed new blog posts TWICE per week! In addition, you’ll get access to the free giveaways below. Over 6,000 healthcare professionals have subscribed for our FREE Giveaways. Why haven’t you?!
- 30 medication mistakes PDF
- 18+ Page Drug Interaction PDF
- 10 Commandments of Polypharmacy Webinar based on my experiences in clinical practice
Study Materials and Resources For Healthcare Professionals and Students – Amazon Books
I’d also like to take a look at her lifestyle- specifically diet and exercise- for a person who has also had a fair share of gout attacks I have been able to reduce the frequency on diet modifications….
I would like to offer the following comments.
One consideration is whether she has gout. Without a definitive diagnosis (seeing crystals in synovial fluid) this could be something else. Having monoarticular knee pain would make me think more about an incorrect diagnosis of gout. When I had someone like this it was unusual for them to say that anyone had “stuck a needle” into any joint. What can occur is finding crystals in the synovial fluid of the knee even if the presentation is “classic” podagra. Doing arthrocentesis on joints in the toe is harder than the knee. If the history was very clear she had podagra, I would be more likely to believe that gout was a correct diagnosis.
Having drug-induced hyperuricemia is not a disease and does not need to be treated. Since she is prescribed HCTZ, that needs to be sorted. As noted, her glucose might be slightly lower without taking HCTZ.
As noted, It is critical to know her renal function and serum uric acid. I would want to know the serum uric acid after the resolution of an acute painful episode. The serum uric acid can be low, normal, or elevated during acute gouty arthritis. I would also want to know the serum uric acid after stopping HCTZ.
If this is gout, the target for the serum uric acid is <6.5 mg/dl. There is no “dose” of allopurinol in the absence of knowing the serum uric acid. I would also assess the persistence and adherence of refilling allopurinol (and meds in general). Someone who thinks they are doing great and stops taking allopurinol might develop a flare. The dose of allopurinol should not be “increased”. It must be increased to reach the target serum concentration. Rarely, doses up to 900 mg/d are needed.
Losartan is not purported to lower uric acid, it lowers uric acid (unique among ARBs). It is unknown if there are fewer flares of gout if losartan is used. Realistically, hypouricemic therapy will be used rather than just losartan.
I disagree about using NSAIDs for a week. If someone has acute gouty arthritis, I would have no issue treating the patient with high doses of any NSAID (e.g., naproxen 500 mg BID x 5-7 days) except aspirin. Unless the dose of aspirin is in the range of 3.5 g/d, it blocks urate excretion. Every NSAIDs studied reduces pain in acute gout (naproxen, ibuprofen, sulindac, etc.). If someone had another reason to avoid an NSAID (e.g., heart failure and using ACEI and loop diuretics), I would use prednisone 35 mg/d for 7 days (no tapering needed). I would not be concerned about the glucose effect of prednisone for one week. If the pain is more chronic and then flares, I would not use colchicine since it is less likely to be effective if the acute event is longer than 24 hours.
The patient’s weight is not listed. Since T2DM is most often seen in obese patients, I would try to change the diet before adding more drugs. The diet effect on gout is insignificant. The use of SGLT-2 and GLP-1 needs to be considered with the cost of these drugs, especially for Medicare patients who will reach the donut hole. These drugs compared to placebo had less than a 2% absolute benefit over the 2-5 years of the clinical trials. The NNT for these drugs is about 60 to 130 to prevent ONE composite event. The cost of the drug alone to prevent ONE composite event ranges from $1 million to $3 million. The effect of marketing and the ADA has made these drugs sound remarkable. I would change metformin to extended-release for convenience and give 1500 mg/d. The only information I was ever able to find is the labeling to show a small difference between 1500 mg and 2000 mg of metformin. Glipizide can also be increased to 10 mg/d. Knowing the control of diabetes is important before any changes are made.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Notify me of new posts by email.
Submit Comment
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Written By Eric Christianson
December 29, 2021, study materials for pharmacists.

Free 18 Page PDF On The Most Notable Drug Interactions!
Looking for something, recent posts.
- Med List Review – Renal Concerns
- What Is Bad About Paroxetine – 3 Strikes and You’re Out
- Allergy Medications As Deprescribing Targets
- Topiramate-Induced Cognitive Impairment
- Top 3 Drugs That Change Urine Color
Explore Categories
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Recognition of gout in rheumatoid arthritis
A case report.
Editor(s): NA.,
Department of Rheumatology and Clinical Immunology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China.
∗Correspondence: Ping Fu, Department of Rheumatology and Clinical Immunology, The Second Affiliated Hospital of Kunming Medical University, Dian Mian Avenue, PO Box 650101, Kunming, of China (e-mail: [email protected] ).
Abbreviations: CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GCs = glucocorticoids, LEF = leflunomide, MP = methylprednisolone, MTX = methotrexate, NSAIDs = nonsteroidal antiinflammatory drugs, RA = rheumatoid arthritis, RF = rheumatoid factor.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
An informed consent was obtained from the individual included in this study.
The authors have no funding and no conflicts of interest to disclose.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial License 4.0 (CCBY-NC), where it is permissible to download, share, remix, transform, and buildup the work provided it is properly cited. The work cannot be used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc/4.0
Rationale:
Rheumatoid arthritis (RA) and gout are common rheumatic diseases. However, their coexistance has been rarely reported. Here in, we describe a case of a middle aged Chinese woman having RA complicated with atypical gout on both the knee joints.
Patient concerns:
A 44-year-old Chinese woman complained of swelling and tenderness of multiple joints since 10 months. She had a positive rheumatoid factor and high titers of anti-CCP antibody. She was diagnosed with RA, and commenced on methotrexate, leflunomide, and methylprednisolone. Her symptoms of pain and swelling over interphalangeal and wrists joints subsided except the knee joints. She was started with treat to target treatment (TTT) for RA and rest of her medications was adjusted accordingly. Surprisingly, her symptoms did not improve ever after the addition of a biologic agent, tumor necrosis factor (TNF)-α receptor antagonist.
Diagnosis:
Presence of urate crystals in the synovium was viewed under polarization microscope which was extracted from one of the knee joint. Hence, we established the diagnosis of RA complicated with gout.
Interventions:
We commenced her on TNF-α receptor antagonist, colchicines, and febuxostat.
Outcomes:
Her symptoms of pain and swelling improved significantly on both the knees and no longer recurred.

Lessons:
Coexistence of RA and gout has been rarely reported as it is not frequently seen in clinical practice. Hence, when patients with RA with oligoarthritis repeatedly do not respond to TTT, a standard antirheumatism treatment, the possibility of RA complicating with gout should be rule out.
1 Introduction
Rheumatoid arthritis (RA) is one of the most common symmetric, peripheral polyarthritis, chronic inflammatory rheumatic diseases of an unknown etiology. The general prevalence of RA is about 0.5% to 1% in the adult population with a male and female prevalence ratio of 1:3. [1,2] Meanwhile, gout is a deposition of monosodium urate crystals which is present in about 1% to 4% of the general population; where males develop gout in 2:6 ratio compared to females. [3,4] Although both RA and gout are commonly seen in clinical scenario, their coexistence has been rarely reported. There is a popular belief that RA and gout do not, or rarely, coexist with each other. However, it has been reported quite frequently in elderly males. [5] This case report describes a 44-year-old Chinese woman with RA complicated with gout in both the knee joints.
2 Case report
A 44-year-old Chinese woman in 2002 was admitted to our hospital's ward for swelling and tenderness of multiple joints since 10 months. On local examination there was fusiform swelling in proximal and distal interphalangeal joints of right hand, bilateral wrists, and knee joints. In addition, she complained of morning stiffness lasting for about 30 minutes. Her X-ray revealed of joint space narrowing of bilateral wrist and interphalangeal joints ( Fig. 1 ).

Her laboratory tests performed at our hospital revealed of normal serum uric acid level, positive rheumatoid factor (RF), and an elevated RF-immunoglobulin A (IgA) 288.69 IU/mL (normal range, <20 IU/mL). In addition, she had a positive anti-CCP antibody and an elevated CCP-immunoglobulin G (CCP-IgG) 229.78 IU/mL (normal range, <25 IU/mL). There was a strong positivity for antinuclear antibody with the titer of 1:1000, anti-Sjögren's syndrome related antigen (SSA) (+++), Ro 52 (++), RNP/Sm (+++), and ribosomal P protein (RNP) (+++). Meanwhile her acute phase reactants such as erythrocyte sedimentation rate (ESR) 47 mm/h (normal range, <15 mm/h) and C-reactive protein (CRP) 20.67 mg/L (normal range, <10 mg/L) were remarkably elevated.
Our patient fulfilled the 2010 ACR/EULAR Rheumatoid Arthritis Classification Criteria for the diagnosis of RA. [6] She was put on methotrexate (MTX), leflunomide (LEF), and methylprednisolone (MP) 12 mg/d. In a few days of time her symptoms of swelling and tenderness in both the knees subsided. Unfortunately, MTX and LEF had to be stopped due to impaired hepatic functions. We commenced her instead on tumor necrosis factor (TNF)-α receptor antagonist which showed good therapeutic response but later on due to high cost price of TNF-α receptor antagonist, our patient could not afford regularly. She took it with iguratimod only when the symptoms relapsed.
In 2015, she suffered from interstitial lung disease. Hence, low dose of oral MP 4 to 8 mg/d and LEF 10 mg was commenced. Meanwhile, as she was planning to conceive we replaced LEF with iguratimod and TNF-α receptor antagonist 50 mg once a week. During a follow-up visit after about 6 months she complained of mild swelling and tenderness in both the knees, more frequently on right knee during warm temperatures. These symptoms aggravated more after taking MP 4 mg, then 8 mg for 2 to 3 days. An ultrasonography of her right knee showed effusion, synovial thickening, “double track sign” of articular cartilages and bilateral popliteal cysts ( Fig. 2 ). Under polarization microscope, there was presence of birefringence needle-like crystals extracted from knee joint fluid ( Fig. 3 ). Laboratory examination showed raised RF 47.50 IU/mL (normal range, <20 IU/mL), CRP 26.90 mg/L (normal range, <10 mg/L), and ESR 31 mm/h (normal range, <15 mm/h), but a raised uric acid 363 μmol/L (normal range female, 178.4–297.4 μmol/L). Hence, diagnosis of RA complicated with gouty arthritis of knee joints was established. She was commenced on colchicine, febuxostat 40 mg/d, and TNF-α receptor antagonist. In the next follow-up (about 3 years) visit, her symptoms had completely subsided and no longer recurred.

3 Discussion
The RA and gout rarely coexists in the same patient. [7] It is considered urate crystals can block the activation of T and B cells and also possess antioxidant with antiphagocytosis properties which may contribute reducing the incidence of RA with gout. In addition, interleukin-6 in patients with RA may be able to decrease gout attack. [8] Moreover, MTX and LEF are commonly taken by patients with RA that reduce uric acid levels also. [9,10] A recent study has demonstrated coexistence of RA and gout are not uncommon. [11–13] However, a study by Petsch et al claimed incidence of gout with patients with RA do exist but is lower than the general population. [11] Meanwhile, Merdler-Rabinowicz et al. consider incidence of gout in patients with RA is not lower than the general population. [14] Hence, both the studies differ from each other.
Uric acid deposition has been found in most patients with RA with the use of dual energy computer topography (DECT) in gout patient in recent years. [13] RA complicated with gout have been rarely reported previously which may be attributed to following reasons: polyarthritis in patient with RA can dominate the symptoms of atypical gout; long-term use of medicine such as glucocorticoids (GCs), nonsteroidal antiinflammatory drugs (NSAIDs) can prevent gout attack; and diagnosis of atypical gout mainly relies on joint puncture to find out urate crystals in the synovium, but it is often difficult to implement in small joints. Patients with RA with gout mostly are elderly men who have high RF titer and significant level of uric acid levels in serum than those suffering for only RA. [14,15] The patient presented in this case report is a middle aged woman with normal serum uric acid levels and lacks typical gout history. Hence, there was no proper evidence to diagnose her for having gout in the beginning.
After 6 months from discharge during a follow-up, she complained of swelling and tenderness in both the knee joints which aggravated more in warm temperatures. The symptoms relapsed even after the commencement of biologic agent. This manifestation in joints was not consistent with the features of RA. So we checked serum uric acid levels in blood which was slightly raised. Meanwhile, ultrasonography of her knees showed “double track sign,” a typical characteristic image seen in gout. Moreover, for confirmatory diagnosis of gout we extracted the synovial fluid from one of the knee joint and birefringent needle-like crystals were observed under polarization microscope. Meanwhile, the patient's serum uric acid level was slightly raised. It may be due to: Female patients have estrogen and progesterone which can promote the elimination of uric acid such that it can maintain the normal uric acid levels; as our patient was on MTX and LEF for the treatment of RA, it is possible that MTX can inhibit synthesis of purine and increase the level of adenosine, [9] and LEF can increase the elimination of uric acid by reducing the levels of uric acid through regulation of urate transporter in renal epithelial cells [10] ; and RF is likely to prevent the deposition of uric acid crystals. Hence, for these reasons, the level of uric acid presents to be slightly higher, or even normal. In addition, there is usually at least one acute episode of attack that happens in majority of patients with RA with gout. [15] However, as our patient initially denied any history of acute attack with the relapse of symptoms of swelling and tenderness over knees, we misdiagnosed for not reaching the target, clinical remission, or low disease activity. [16] So the patient did not completely stop the treatment with GCs and other drugs. Perhaps, the reason for no attack of gout must have been associated with long term use of GCs, NSAIDs, and biologic agents.
Another interesting aspect seen in our case report is our patient complained of swelling and tenderness in both the knees especially in July, at night or in warm weather. A research has revealed increasing onset of acute gouty arthritis from March to July and highest in July. From July started to decrease till September which was recorded lowest. Moreover, autumn had a significant association with the onset of acute gouty arthritis. This physiochemical changes are associated with increased mean temperature between neighboring days may lead to the formation of monosodium urate crystals. [17] In addition, the onset of gout in the early morning and evening is more common than daytime, possibly due to cortisol level falling to the lowest during midnight and at 4:00 am . [18] Furthermore, high temperature and high humid environment or a high temperature and low humid environment may cause physiologic changes or behavior associated with acute attack of gout and latter more relevant. [19] Therefore, if joint pain is associated with season, weather, and night, we do need to rule out possibility of gout.
4 Conclusion
Coexistance of RA and gout are rarely reported as it is not frequently seen in clinical scenario. We hold an opinion when patients with RA with oligoarthritis repeatedly attacked cannot meet the treat to target, a standard antirheumatism treatment, the possibility of RA complicated gout should be considered or rule out else wise. Hence, it is very important to examine the joint by DECT, and ultrsonography to detect uric acid deposition in tissue and joints and furthermore, it is wise to aspirate synovial fluid and analyze it under polarization microscope to detect urate crystals.
Acknowledgment
The authors thank the patient for her consent to publish her case and the related pictures.
Author contributions
Conceptualization: Xuan Wang.
Supervision: Ping Fu.
Visualization: Ping Fu.
Writing – original draft: Guowang Zhao.
Writing – review & editing: Guowang Zhao.
Guwang Zhao orcid: 0000-0002-2455-9007.
- Cited Here |
- PubMed | CrossRef |
- Google Scholar
- View Full Text | PubMed | CrossRef |
gout; rheumatoid arthritis; treatment
- + Favorites
- View in Gallery
- News and Features
- Conferences
- Clinical Tools
- Special Collections
Aiming for Lower Serum Urate Levels May Help Reduce Gout Flares
Gout flares are less frequent when serum urate (SU) levels are below 6 mg/dL after the first year of urate-lowering therapy (ULT), according to study results published in Annals of the Rheumatic Diseases .
The 2020 Gout Treatment Guidelines from the American College of Rheumatology recommend the use of flare prophylaxis for at least 3 to 6 months after starting ULT. It is also suggested to continue or resume prophylaxis on a case-by-case basis among patients with recurrent flares. However, it has not been determined whether a longer duration of gout flare prophylaxis for all patients initiating ULT would be beneficial.
To address this, researchers conducted a secondary analysis using data from the Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout (CARES) trial to investigate gout flare rates based on repeated measurements of SU levels. Participants in the CARES trial were randomly assigned to receive febuxostat or allopurinol, which was titrated in order to reach the treatment target (SU levels <6 mg/dL). Flare prophylaxis consisted of colchicine 0.6 mg daily or naproxen 250 mg twice/day for 6 months.
In the current analysis, the participants were followed-up every 3 or 6 months, beginning at study enrollment and continuing until dropout, death, or trial completion. The primary outcome was self-reported gout flares.
The analysis included 6183 participants aged a median of 65 years, of which 84% were men. The median follow-up period was 3.2 years and SU levels were measured at months 0, 3, 6, and every 6 months thereafter.
Gout flares were most common when SU levels were 10 mg/dL or higher; flares were least common when SU levels were 3.9 mg/dL or lower. The highest flare rates were seen during the first 3 months after initiating ULT, with a second peak noted between months 6 and 12, when prophylactic treatment was stopped. During the first year of ULT, there were no significant differences in flare rates between different SU level groups, but the rates were consistently highest when SU levels were 10 mg/dL or higher.
After the first year of ULT, a dose-response relationship was observed between SU levels and flare rates. Flare rates were significantly lower after month 12 when SU levels were 3.9 mg/dL or lower vs levels ranging from 4.0 to 5.9 mg/dL. Flare rates were nearly 50% greater during months 12 to 36 and more than 4-times higher during months 36 to 72 when SU levels were 10 mg/dL or higher vs levels ranging from 4.0 to 5.9 mg/dL.
The SU levels of 607 participants decreased by 2.5 to 3.5 mg/dL over 3 months and 116 participants experienced the same decrease over 6 months. Among both groups, the median number of gout flares was 0. The mean number of gout flares was 0.24 over 3 months among those who experienced the SU decrease over a 3-month span. Among the participants who experienced the SU decrease over a 6-month span, the mean number of flares was 0.38 over 6 months.
Study limitations include a decreased sample size during the first year of follow-up, a high number of dropouts in later years, and reliance on self-reporting of gout flares that did not require physician evaluation.
The researchers concluded, “The spike in flares in all SU categories after stopping prophylaxis suggests a prophylaxis for longer than 6 months may be warranted.”
Disclosure: Some study authors report affiliations with biotech, pharmaceutical, and/or device companies. Please see the original reference for a full list of authors’ disclosures .
This article originally appeared on Rheumatology Advisor
References:
Picked For You
Latest News
Want to read more?
Please login or register first to view this content.
Login Register
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Global, regional, and national burden of gout, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021
Collaborators.
- GBD 2021 Gout Collaborators : Marita Cross , Kanyin Liane Ong , Garland T Culbreth , Jaimie D Steinmetz , Ewerton Cousin , Hailey Lenox , Jacek A Kopec , Lydia M Haile , Peter M Brooks , Deborah R Kopansky-Giles , Karsten E Dreinhoefer , Neil Betteridge , Mohammadreza Abbasian , Mitra Abbasifard , Aidin Abedi , Melka Biratu Aboye , Aleksandr Y Aravkin , Al Artaman , Maciej Banach , Isabela M Bensenor , Akshaya Srikanth Bhagavathula , Ajay Nagesh Bhat , Saeid Bitaraf , Rachelle Buchbinder , Katrin Burkart , Dinh-Toi Chu , Sheng-Chia Chung , Omid Dadras , Xiaochen Dai , Saswati Das , Sameer Dhingra , Thanh Chi Do , Hisham Atan Edinur , Ali Fatehizadeh , Getahun Fetensa , Marisa Freitas , Balasankar Ganesan , Ali Gholami , Tiffany K Gill , Mahaveer Golechha , Pouya Goleij , Nima Hafezi-Nejad , Samer Hamidi , Simon I Hay , Samuel Hundessa , Hiroyasu Iso , Shubha Jayaram , Vidya Kadashetti , Ibraheem M Karaye , Ejaz Ahmad Khan , Moien Ab Khan , Moawiah Mohammad Khatatbeh , Ali Kiadaliri , Min Seo Kim , Ali-Asghar Kolahi , Kewal Krishan , Narinder Kumar , Thao Thi Thu Le , Stephen S Lim , Stany W Lobo , Azeem Majeed , Ahmad Azam Malik , Mohamed Kamal Mesregah , Tomislav Mestrovic , Erkin M Mirrakhimov , Manish Mishra , Arup Kumar Misra , Madeline E Moberg , Nouh Saad Mohamed , Syam Mohan , Ali H Mokdad , Kaveh Momenzadeh , Mohammad Ali Moni , Yousef Moradi , Vincent Mougin , Satinath Mukhopadhyay , Christopher J L Murray , Sreenivas Narasimha Swamy , Van Thanh Nguyen , Robina Khan Niazi , Mayowa O Owolabi , Jagadish Rao Padubidri , Jay Patel , Shrikant Pawar , Paolo Pedersini , Quinn Rafferty , Mosiur Rahman , Mohammad-Mahdi Rashidi , Salman Rawaf , Aly M A Saad , Amirhossein Sahebkar , Fatemeh Saheb Sharif-Askari , Mohamed Metwalii Khalifa Saleh , Austin E Schumacher , Allen Seylani , Paramdeep Singh , Amanda E Smith , Ranjan Solanki , Yonatan Solomon , Ker-Kan Tan , Nathan Y Tat , Nigusie Selomon Selomon Tibebu , Yuyi You , Peng Zheng , Osama A Zitoun , Theo Vos , Lyn M March , Anthony D Woolf
- PMID: 38996590
- PMCID: PMC11263476
- DOI: 10.1016/S2665-9913(24)00117-6
Background: Gout is an inflammatory arthritis manifesting as acute episodes of severe joint pain and swelling, which can progress to chronic tophaceous or chronic erosive gout, or both. Here, we present the most up-to-date global, regional, and national estimates for prevalence and years lived with disability (YLDs) due to gout by sex, age, and location from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021, as well as forecasted prevalence to 2050.
Methods: Gout prevalence and YLDs from 1990 to 2020 were estimated by drawing on population-based data from 35 countries and claims data from the USA and Taiwan (province of China). Nested Bayesian meta-regression models were used to estimate prevalence and YLDs due to gout by age, sex, and location. Prevalence was forecast to 2050 with a mixed-effects model.
Findings: In 2020, 55·8 million (95% uncertainty interval 44·4-69·8) people globally had gout, with an age-standardised prevalence of 659·3 (525·4-822·3) per 100 000, an increase of 22·5% (20·9-24·2) since 1990. Globally, the prevalence of gout in 2020 was 3·26 (3·11-3·39) times higher in males than in females and increased with age. The total number of prevalent cases of gout is estimated to reach 95·8 million (81·1-116) in 2050, with population growth being the largest contributor to this increase and only a very small contribution from the forecasted change in gout prevalence. Age-standardised gout prevalence in 2050 is forecast to be 667 (531-830) per 100 000 population. The global age-standardised YLD rate of gout was 20·5 (14·4-28·2) per 100 000 population in 2020. High BMI accounted for 34·3% (27·7-40·6) of YLDs due to gout and kidney dysfunction accounted for 11·8% (9·3-14·2).
Interpretation: Our forecasting model estimates that the number of individuals with gout will increase by more than 70% from 2020 to 2050, primarily due to population growth and ageing. With the association between gout disability and high BMI, dietary and lifestyle modifications focusing on bodyweight reduction are needed at the population level to reduce the burden of gout along with access to interventions to prevent and control flares. Despite the rigour of the standardised GBD methodology and modelling, in many countries, particularly low-income and middle-income countries, estimates are based on modelled rather than primary data and are also lacking severity and disability estimates. We strongly encourage the collection of these data to be included in future GBD iterations.
Funding: Bill & Melinda Gates Foundation and the Global Alliance for Musculoskeletal Health.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of interests R Buchbinder reports grants or contracts from Australian National Health and Medical Research Council, Australian Government, Arthritis Australia, HCF Foundation, Cabrini Foundation; royalties from Wolters Klewer Health for authorship of the chapterPlantar fasciitis in UpToDate; all outside the submitted work. S Das reports voluntary member and leadership of the Association of Diagnostic and Laboratory Medicine and Women in Global Health, outside the submitted work. D R Kopansky-Giles reports support for the present manuscript from Global Alliance for Musculoskeletal Health; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid, with Global Alliance for Musculoskeletal Health with the International Coordinating Council and World Spine Care, Canada, as a member of the Canadian Board of Directors; all outside the submitted work. K Krishan reports non-financial support from the UGC Centre of Advanced Study, CAS II (awarded to the Department of Anthropology, Panjab University, Chandigarh, India), outside the submitted work. L M March reports grants or contracts from National Health and Medical Research Council (NHMRC) Australian Government for Centre of Research Excellence for Better Outcomes in Inflammatory Arthritis, Medical Research Future Fund (MRFF) Australian Government for a Biologic Tapering trial in Adults with rheumatoid arthritis and psoriatic arthritis, and MRFF Australian Government for a biologic tapering trial in children with juvenile idiopathic arthritis; royalties from Wolters Klewer Health for authorship of the chapter Epidemiology and risk factors for osteoarthritis in UpToDate; royalties from Elsevier for being co-editor of The Musculoskeletal System, 3rd Edn, 2022; participation on a Data Safety Monitoring Board (DSMB) or Advisory Board with NHMRC Australian Government as a pro-bono member of DSMB for an investigator initiated text message study for low back pain; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid, with Australian Rheumatology Association as a pro-bono Chair Research Advisory Committee and Global Alliance for MSK Health – Global Alliance for Musculoskeletal Health as a pro-bono Executive Committee member; all outside the submitted work. All other authors declare no competing interests.
Global prevalence of gout by…
Global prevalence of gout by age and sex in 2020 Shaded areas represent…
Age-standardised prevalence of gout by…
Age-standardised prevalence of gout by country for male and female sexes combined and…
Global cases of gout forecast…
Global cases of gout forecast to year 2050 and decomposition of changes in…
Similar articles
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Lancet. 2017 Sep 16;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2. Lancet. 2017. PMID: 28919117 Free PMC article.
- Global, regional, and national burden of neck pain, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. GBD 2021 Neck Pain Collaborators. GBD 2021 Neck Pain Collaborators. Lancet Rheumatol. 2024 Mar;6(3):e142-e155. doi: 10.1016/S2665-9913(23)00321-1. Lancet Rheumatol. 2024. PMID: 38383088 Free PMC article.
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Lancet. 2018 Nov 10;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7. Epub 2018 Nov 8. Lancet. 2018. PMID: 30496104 Free PMC article.
- Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Global Burden of Disease Study 2013 Collaborators. Global Burden of Disease Study 2013 Collaborators. Lancet. 2015 Aug 22;386(9995):743-800. doi: 10.1016/S0140-6736(15)60692-4. Epub 2015 Jun 7. Lancet. 2015. PMID: 26063472 Free PMC article. Review.
- Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T. Degenhardt L, et al. Lancet. 2013 Nov 9;382(9904):1564-74. doi: 10.1016/S0140-6736(13)61530-5. Epub 2013 Aug 29. Lancet. 2013. PMID: 23993281 Review.
- Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50:S11–S16. - PubMed
- Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397:1843–1855. - PubMed
- Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–662. - PubMed
- Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8(suppl 1):S2. - PMC - PubMed
- Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679. 87.e1. - PubMed
Related information
Linkout - more resources, full text sources.
- Elsevier Science

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Introduction
- Conclusions
- Article Information
eTable 1. Participant Characteristics by Sex and Race (Complete Case Analysis)
eTable 2. Sex-Specific Association Between Black Race and Gout and Hyperuricemia, 2007-2008 to 2015-2016 NHANES (Complete Case Analysis)
eTable 3. Association of Risk Factors With Gout and Hyperuricemia Among Women
eTable 4. Association of Risk Factors With Gout and Hyperuricemia Among Men
eFigure. Age-Adjusted Prevalence of Gout Among Black and White US Adults Between NHANES III (1988-1994) and NHANES 2006-2017
- Serum Urate and Recurrent Gout JAMA Original Investigation February 6, 2024 This retrospective study of patients with gout examines whether serum urate levels are associated with subsequent risk of gout flares and hospitalization for gout. Natalie McCormick, PhD; Chio Yokose, MD, MSc; Gregory J. Challener, MD; Amit D. Joshi, MBBS, PhD; Sruthi Tanikella, BS; Hyon K. Choi, MD, DrPH
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
McCormick N , Lu N , Yokose C, et al. Racial and Sex Disparities in Gout Prevalence Among US Adults. JAMA Netw Open. 2022;5(8):e2226804. doi:10.1001/jamanetworkopen.2022.26804
Manage citations:
© 2024
- Permissions
Racial and Sex Disparities in Gout Prevalence Among US Adults
- 1 Clinical Epidemiology Program, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Boston
- 2 Mongan Institute, Department of Medicine, Massachusetts General Hospital, Boston
- 3 Department of Medicine, Harvard Medical School, Boston, Massachusetts
- 4 Arthritis Research Canada, Vancouver, British Columbia
- 5 Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston
- 6 Slone Epidemiology Center at Boston University, Boston, Massachusetts
- 7 Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities, Boston, Massachusetts
- 8 Department of Medicine, University of Auckland, Auckland, New Zealand
- 9 Division of Clinical Immunology and Rheumatology, University of Alabama at Birmingham
- 10 Department of Biochemistry, University of Otago, Dunedin, New Zealand
- Original Investigation Serum Urate and Recurrent Gout Natalie McCormick, PhD; Chio Yokose, MD, MSc; Gregory J. Challener, MD; Amit D. Joshi, MBBS, PhD; Sruthi Tanikella, BS; Hyon K. Choi, MD, DrPH JAMA
Question Are there racial differences in gout prevalence among adults in the US general population, and what might explain these disparities?
Findings In this cross-sectional study of 18 693 participants, gout was 1.8 times more prevalent among Black women than White women and 1.3 times more prevalent among Black men than White men. These associations attenuated most after adjusting for poverty, diet, body mass index, and chronic kidney disease (CKD) among women and for diet and CKD among men; they were nullified in both sexes after adjusting for all risk factors, with similar findings for hyperuricemia.
Meaning These findings suggest that racial disparities in gout may be explained by diet, social determinants of health, and CKD, which could help identify interventions to reduce these disparities.
Importance Emerging data suggest gout and hyperuricemia may now be more frequent among Black adults in the US than White adults, especially Black women. However, national-level, sex-specific general population data on racial differences in gout prevalence and potential socioclinical risk factors are lacking.
Objective To identify sex-specific factors driving disparities between Black and White adults in contemporary gout prevalence in the US general population.
Design, Setting, and Participants This cross-sectional analysis used nationally representative, decadal survey data from successive cycles of the National Health and Nutrition Examination Survey from 2007 to 2016. Data were analyzed from November 1, 2019, through May 31, 2021. Participants included US adults self-reporting Black or White race.
Exposures Self-reported race, excess body mass index, chronic kidney disease (CKD; defined as estimated glomerular filtration rate <60 mL/min/1.73 m 2 , according to latest equations without race coefficient), poverty, poor-quality diet, low educational level, alcohol consumption, and diuretic use.
Main Outcomes and Measures Race- and sex-specific prevalence of physician- or clinician-diagnosed gout and hyperuricemia and their differences before and after adjusting for potential socioclinical risk factors.
Results A total of 18 693 participants were included in the analysis, consisting of 3304 Black women (mean [SD] age, 44.8 [0.4] years), 6195 White women (mean [SD] age, 49.8 [0.3] years), 3085 Black men (mean [SD] age, 43.6 [0.5] years]), and 6109 White men (mean [SD] age, 48.2 [0.3] years). Age-standardized prevalence of gout was 3.5% (95% CI, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5%-2.5%) in White women (age-adjusted odds ratio [OR], 1.81 [95% CI, 1.29-2.53]); prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26 [95% CI, 1.02-1.55]). These associations attenuated after adjusting for poverty, diet, body mass index, and CKD among women and for diet and CKD among men but became null after adjusting for all risk factors (ORs, 1.05 [95% CI, 0.67-1.65] among women and 1.05 [95% CI, 0.80-1.35] among men). Hyperuricemia end point findings were similar.
Conclusions and Relevance In this nationally representative race- and sex-specific cross-sectional study of US adults, gout was more prevalent in adults self-reporting Black race during a recent 10-year period compared with their White counterparts. These racial differences may be explained by sex-specific differences in diet and social determinants of health and clinical factors. Culturally informed efforts focusing on these factors could reduce current gout-related disparities.
Gout has historically been considered a disease of White men who overindulge in gamey meats and other rich foods. 1 However, emerging data suggest gout and hyperuricemia (its causal precursor) impart an even larger burden on other demographic groups, including Black men, Black women, and White women. 2 Indeed, the global frequency and disability burden of gout among women have been rising disproportionately relative to gout among men, 3 with gout among women characterized by a higher frequency of obesity 4 , 5 and related cardiometabolic sequalae. 4 - 7 At the same time, previous studies have suggested that compared with White US residents, Black US residents are at increased risk for gout 2 , 8 , 9 and lower health-related quality of life. 10 However, national-level general population data on sex-specific racial differences in the burden of gout are lacking.
Importantly, potential racial differences may be largely attributable to differences in nongenetic social determinants of health 11 rather than ancestry-specific differences in genes regulating urate handling. 12 For example, excess adiposity, a major risk factor for gout 13 , 14 and hyperuricemia, 15 - 17 is more prevalent among Black women in the US general population than White women. 18 - 21 Moreover, after accounting for body mass index (BMI), alcohol consumption, and other factors, Black men had a lower risk of incident gout and incident hyperuricemia in the all-male Multiple Risk Factor Intervention Trial. 11 However, general population data are lacking for both women and men; sex-specific analysis is critical given that women and men tend to differ in their gout risk factor profiles (eg, higher frequencies of diuretic use 5 , 7 , 22 and obesity 4 , 5 and lower frequency of alcohol consumption 4 , 5 have been observed in women vs men).
Our objective was to investigate current racial differences in gout prevalence among women and men in the US general population and identify sex-specific factors that may explain these differences. We hypothesized these differences would be present at the national level and would be associated, in a sex-specific manner, with racial differences in social and lifestyle factors, diuretic use, and chronic kidney disease (CKD).
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional, nationwide survey in the US that assesses the health and nutritional status of adults and children using interviews, physical examinations, and laboratory data. 23 NHANES uses a complex, multistage probability design to provide a nationally representative sample of the noninstitutionalized US civilian population. The present study included participants 18 years or older with data on gout status and serum urate levels using data collected from the 2007-2008 through 2015-2016 annual NHANES cycles; gout diagnoses were not ascertained in the earlier surveys (1999-2000 through 2005-2006). However, data on gout diagnoses were ascertained in the NHANES III (1988-1994) and were used to compare the current sex- and race-specific estimates with those from more than 2 decades earlier. All procedures in each NHANES were approved by the National Center for Health Statistics Ethics Review Board, and written informed consent was obtained from participants at the time of enrollment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline for cross-sectional studies.
During the in-person interviews, participants reported on their age, sex, race and ethnicity, educational attainment, household size and income, recent dietary intake, medical history (including gout), and use of prescription medications within the past 30 days (including diuretics). Race and ethnicity were categorized as Hispanic (Mexican and non-Mexican Hispanic), non-Hispanic Asian (from 2011 onward), non-Hispanic Black or African American (hereafter referred to as Black), non-Hispanic White (hereafter referred to as White), and other race and ethnicity; the present analysis was limited to those self-identifying as Black or White race. Household income and size were used to determine the poverty to income ratio, a derived variable used in prior NHANES studies 24 - 26 and calculated as household income, adjusted for household size, divided by the year-specific poverty guideline 27 issued by the US Department of Health and Human Services. These guidelines are used to determine eligibility for many federal benefit programs, including the Supplemental Nutrition Assistance Program. 24 - 26 , 28 Alcoholic beverage consumption (number of drinks per week) was computed from responses to questions on the frequency of alcoholic beverage consumption (eg, number of days per week, month, or year) and total number of drinks per day of consumption. Two 24-hour dietary recalls were undertaken to ascertain the types and amounts of all foods and beverages consumed by participants. After mapping to the US Department of Agriculture Food Patterns Equivalents Database, 29 these data were used to compute a diet quality score based on the dietary components emphasized or minimized in the Dietary Approaches to Stop Hypertension (DASH) diet. 30 - 32
At the mobile examination units, participants had their height and weight measured by trained staff using standardized instruments, with BMI calculated by dividing the weight in kilograms by the height in meters squared. Blood was drawn for measurement of serum levels of urate, creatinine, and other biomarkers. Serum creatinine levels were used to determine glomerular filtration rate according to the latest equations, which do not include a coefficient for Black race. 33
During the NHANES home interviews, all participants were asked, “Has a doctor or other health professional ever told you that you had gout?” Serum urate levels were measured in blood samples obtained at the time of enrollment using a colorimetric method where urate is oxidized to allantoin and hydrogen peroxide by uricase using a multichannel analyzer (Hitachi Model 737; Boehringer Mannheim Diagnostics). The quality-control procedures have been described elsewhere. 34 Values are reported in milligrams per deciliter and can be converted to micromoles per liter by multiplying by 59.48. Hyperuricemia was defined according to the top 10th percentile of serum urate levels for women (6.8 mg/dL) and men (7.8 mg/dL), considering the biological urate saturation point as well as statistical efficiency.
Potential risk factors, which were selected on the basis of prior knowledge, 13 , 14 , 31 , 32 , 35 - 39 included low educational level (high school or less [dichotomous]), poverty (household income <130% of the federal poverty guideline [dichotomous]), 25 BMI (continuous), 13 , 14 , 40 , 41 number of alcoholic beverages consumed per week (continuous), poor diet quality (lower level of adherence to the DASH pattern [continuous]), 31 , 32 diuretic use in the past 30 days (dichotomous), 38 - 40 and CKD (estimated glomerular filtration rate <60 mL/min/1.73 m 2 [dichotomous]). 36 To enhance comparability, factors were scored such that their presence (or higher values) corresponded to a greater risk of gout. For example, the continuous DASH score was inverted such that higher scores indicated lower levels of adherence.
Data were analyzed from November 1, 2019, through May 31, 2021. Incorporating sample weights and accounting for the clusters and strata of the complex study design of the NHANES, we calculated the prevalence of gout and hyperuricemia within the US adult Black and White populations according to sex. We next determined the association between each risk factor and the odds of gout among men and women, adjusting for age and race, along with racial differences in the levels and frequencies of each risk factor (specifically, the age-adjusted difference between Black and White women and Black and White men). We then performed stepwise logistic regression by adding potential risk factors one by one to show how the association between self-reported Black race and gout changed with the addition of each variable, among those with complete data available, for women and men separately. We started with educational level, followed by poverty (2 social factors), then consecutively added alcohol consumption, diet score, and BMI (3 lifestyle factors potentially associated with educational level and poverty), and finished with diuretic medication use and CKD status (2 clinical factors potentially associated with the other purported risk factors). Characteristics of these women and men were similar to those of the sample at large (eTable 1 in the Supplement ), as was age-adjusted prevalence of gout and hyperuricemia (eTable 2 in the Supplement ). We calculated 95% CIs for all effect estimates. We used SAS, version 9.4 (SAS Institute, Inc) for all statistical analyses. Two-sided P < .05 indicated statistical significance.
Overall, we included 18 693 participants comprising 3085 Black men (mean [SD] age, 43.6 [0.5] years), 3304 Black women (mean [SD] age, 44.8 [0.4] years), 6109 White men (mean [SD] age, 48.2 [0.3] years), and 6195 White women (mean [SD] age, 49.8 [0.3] years). Table 1 shows the characteristics stratified by sex and race. For both sexes, Black participants tended to be younger than White participants and had a higher frequency of poverty and lower educational attainment. With the exception of alcohol consumption, which was greater among White adults, all risk factors for gout and hyperuricemia were more prevalent in Black adults than White adults.
The age-adjusted prevalence of gout during the 2007-2016 study period was 7.0% (95% CI, 6.2%-7.9%) in Black men, 3.5% (95% CI, 2.7%-4.3%) in Black women, 5.4% (95% CI, 4.7%-6.2%) in White men, and 2.0% (95% CI, 1.5%-2.5%) in White women ( Table 2 ). Black race was associated with greater odds of gout during this period among women (odds ratio [OR], 1.81 [95% CI, 1.29-2.53]), among men (OR, 1.26 [95% CI, 1.02-1.55]) ( Table 2 ), and overall (OR, 1.46 [95% CI, 1.22-1.74]). In contrast, there was no racial difference in the prevalence of gout approximately 2 decades earlier in the 1988-1994 NHANES III (eFigure in the Supplement ), with age-adjusted ORs of 0.98 (95% CI, 0.65-1.47) among women, 0.91 (95% CI, 0.68-1.21) among men, and 0.93 (95% CI, 0.73-1.17) overall.
All risk factors except educational level were associated with gout among women in age- and race-adjusted analysis (eTable 3 in the Supplement ). Notably, poverty was associated with 2.4 times greater odds of gout (OR, 2.36 [95% CI, 1.79-3.10]), whereas current use of diuretic medications was associated with 2.2 times greater odds of gout (OR, 2.17 [95% CI, 1.59-2.95]). Furthermore, all deleterious factors were more frequent (or levels were elevated) in Black women compared with White women, except for alcohol consumption, which was lower among Black women (eTable 3 in the Supplement ). For example, age-adjusted mean BMI was higher in Black women (difference, 3.2 [95% CI, 2.9-3.6]) than White women, and a greater proportion of Black women than White women had incomes below the poverty guideline (difference, 8.4% [95% CI, 6.0%-10.7%]). In our stepwise regression analysis ( Table 3 ), the greatest attenuations in the association between self-reported race and gout occurred after adjustment for poverty, diet, BMI, and CKD; adjustment for CKD, the final variable, rendered a null OR of 1.05 (95% CI, 0.67-1.65).
Most purported risk factors were associated with gout among men, including alcohol consumption, although unlike gout among women, the association with poverty was not significant (OR, 1.14 [95% CI, 0.93-1.40]) (eTable 4 in the Supplement ). As with women, alcohol consumption was lower among Black men than White men, but the racial differences in BMI and poverty status between Black and White men were smaller than those between Black and White women. As such, adjustment for poverty and BMI did not eliminate the association between race and gout in the stepwise regression analysis ( Table 4 ), whereas adjustment for CKD rendered a null OR of 1.05 (95% CI, 0.80-1.35).
The stepwise regression analysis for racial differences in hyperuricemia prevalence followed closely consistent patterns of gout among men and women ( Tables 3 and 4 ), with the greatest attenuations in the association for hyperuricemia among women occurring after adjustment for poverty (OR, 1.71 [95% CI, 1.36-2.15]), BMI (OR, 1.22 [95% CI, 0.96-1.57]), diet (OR, 1.52 [95% CI, 1.19-1.93]), and CKD (OR, 1.01 [95% CI, 0.76-1.35]). Among men, the greatest attenuations were observed after adjustment for diuretic use (OR, 1.24 [95% CI, 0.96-1.61]) and CKD (OR, 1.08 [95% CI, 0.81-1.42]).
In this nationally representative, race- and sex-specific study of US adults, gout was more prevalent in adults who self-reported Black race during a recent 10-year period compared with their White counterparts. This disparity is in contrast with data from 2 decades earlier (the NHANES III), when no differences in gout prevalence were observed between Black and White adults, and parallels a disproportionate worsening of CKD 42 and overweight and obesity 21 among Black adults over time. Indeed, our findings suggest that the current racial disparities (2007-2016) may be explained by differences in the frequencies or levels of key social or lifestyle and clinical factors. Importantly, there were distinct sex-specific patterns, with higher BMI levels and poverty rates among Black vs White women than among Black vs White men. At the same time, alcohol consumption, an established gout risk factor, was greater in White adults than in Black adults. These findings show that gout is no longer exclusive to affluent White men (ie, the “disease of kings”) 43 —if it ever was—while highlighting social determinants of health that could serve as targets for reducing these disparities in the general population.
Excess adiposity is a foremost risk factor for gout 13 , 41 and hyperuricemia, 15 and in the present study, where Black women had higher BMI levels than White women, adjustment for BMI attenuated much of the racial difference in gout and hyperuricemia among women. Poverty and poor-quality diet, factors that may themselves affect BMI, were already present in the model. Higher BMI levels among Black women than White women were also reported in previous NHANES 18 , 20 and other US studies. 2 , 44 - 46 In contrast to the findings among women, our study and previous studies 2 , 18 , 44 - 46 found that BMI levels were similar (or even lower) among Black men compared with White men and that adjustment for BMI did not eliminate the association between race and gout in men. Adiposity increases serum urate levels and gout risk by reducing urate excretion and increasing urate production. 47 - 52 Ancillary analysis of a randomized dietary intervention trial showed healthy weight loss diets could reduce serum urate levels, especially among those with baseline hyperuricemia, 53 whereas bariatric surgery has been associated with reductions in serum urate levels and incidence of gout and hyperuricemia. 54 , 55 Furthermore, a recent sex-specific cohort study 13 found that excess adiposity was associated with risk of incident gout among women and men, corroborating the larger effect size of BMI that we observed among women. Together with the findings of the present study, individual- and societal-level strategies to help Black women achieve and maintain a healthy body weight may help reduce disparities in the incidence of gout among Black and White women.
Another key risk factor, CKD, also attenuated the racial differences for gout and hyperuricemia, particularly in men, even with prior adjustment for documented CKD risk factors, including adiposity, poverty, 56 and DASH adherence. 57 Although the higher prevalence of CKD in Black adults (compared with White adults) has been attributed in part to the APOL1 risk alleles present in African American populations, 58 , 59 evidence suggests that racial differences in sociodemographic, lifestyle, and clinical factors play a greater role, 60 with poorer access to health care among Black individuals also contributing. 61
Although gout has historically been viewed as a disease of wealthy White men who could afford to overindulge in alcohol and purine-rich foods, the association between socioeconomic status and gout and hyperuricemia has garnered little empirical study and remains unclear. 62 Our contemporary findings show that poverty (ie, low household income level) is associated with more than 2 times greater odds of gout among women (OR, 2.36 [95% CI, 1.79-3.10]) and may explain a portion of the racial differences among women with gout, whereas there was no association between poverty and gout among men (OR, 1.14 [95% CI, 0.93-1.40]). Although this may reflect the excess burden of gout-related cardiometabolic and kidney sequalae among lower-income individuals, 24 , 63 given the associations among socioeconomic status, food insecurity, diet quality, and obesity, 25 , 64 especially among women, 65 poverty could have downstream associations with diet and BMI.
The serum urate level–lowering effects of DASH-style diets (vs typical US diets) have been demonstrated in randomized clinical trials, 66 , 67 whereas prospective cohort studies have reported inverse associations between DASH adherence (adjusted for total energy intake) and the clinical end point of incident gout. 31 , 32 However, greater DASH adherence is also associated with higher food costs 68 , 69 ; US adults living in poverty have had lower DASH adherence scores than those with higher incomes, 57 , 68 as have Black adults compared with White adults, 57 , 68 with evidence of higher relative costs to achieve DASH adherence among Black adults than White adults. 68 As such, interventions aimed at promoting healthy eating patterns such as DASH or others described in the Dietary Guidelines for Americans, 31 and reducing barriers 70 to adherence, including for those receiving Supplemental Nutrition Assistance Program benefits (who tend to have poorer-quality diets even compared with eligible individuals not receiving benefits), 71 could reduce racial disparities in the prevalence of gout and hyperuricemia, particularly among women.
Diuretic medication use also contributed to racial differences in both gout and hyperuricemia, with significantly more Black women and men currently using diuretics than their White counterparts. This is likely related to higher frequency of hypertension among Black individuals, particularly among Black women, 72 although choice of antihypertensive agent may vary as well. Antihypertensive agents that lower urate levels (eg, calcium channel blockers or losartan) could be preferred to minimize gout risk, 38 , 73 whereas potassium-sparing diuretics 39 could also be considered for individuals at risk of gout when clinically appropriate. Finally, the inverse racial difference in levels of alcohol consumption is consistent with studies reporting higher levels of alcohol consumption among White women and men than their Black counterparts. 74
The present study had several strengths. Given that NHANES data are collected from community-based samples and weighted to be representative of the US population, our findings are generalizable to Black and White individuals in the US at large. Although the self-reported physician- and clinician-sourced diagnoses of gout were not clinically confirmed, this definition performed well in a validation study, 75 and residual misclassification would likely bias our estimates toward the null. Furthermore, our findings for gout were consistent with those for hyperuricemia, an objective laboratory end point.
This study also has limitations. Our study was cross-sectional, although the plausible temporal associations among race, risk factors, and outcomes were apparent, and any residual plausible reverse causations would likely have made our effect estimates conservative (eg, known gout leading to diet change). The associations between risk factors and outcomes were also consistent with those reported for incident outcomes in prospective cohort studies. 13 , 14 , 31 , 32 , 35 - 39 Nevertheless, as with any observational study, our analyses may be subject to residual or unmeasured confounding. Finally, we acknowledge that the racial differences reported herein may be influenced by racism 76 and other resultant factors not explicitly included in our models, such as stress, lower levels of physical activity, and poorer access to health care. Importantly, however, the investigated factors, which together nullified the racial differences in gout among men and women, are likely correlated with these and other key contributors. For example, the built environment in some poorer neighborhoods may pose barriers to physical activity, which has implications for BMI, whereas clinician bias and intergenerational mistrust can result in Black individuals receiving lower-quality health care, which has implications for the development of CKD. 61
The findings of this nationally representative, cross-sectional study of gout prevalence in US adults suggest that gout may be more common in Black men and women compared with their White counterparts. Moreover, these differences may be explained by racial differences in key socioclinical factors, including excess BMI, poverty, and poor diet as well as CKD in women and by CKD, poor diet, and diuretic use in men. Culturally informed interventions designed to address adiposity and kidney disease and improve diet quality while recognizing the role of poverty in gout among women could help reduce these disparities.
Accepted for Publication: June 27, 2022.
Published: August 15, 2022. doi:10.1001/jamanetworkopen.2022.26804
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 McCormick N et al. JAMA Network Open .
Corresponding Author: Natalie McCormick, PhD, Clinical Epidemiology Program, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, 100 Cambridge Street, Suite 1600, Boston, MA 02114 ( [email protected] ).
Author Contributions: Dr Choi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhang, Choi.
Acquisition, analysis, or interpretation of data: McCormick, Lu, Yokose, Joshi, Sheehy, Rosenberg, Warner, Dalbeth, Merriman, Saag, Choi.
Drafting of the manuscript: McCormick, Choi.
Critical revision of the manuscript for important intellectual content: Lu, Yokose, Joshi, Sheehy, Rosenberg, Warner, Dalbeth, Merriman, Saag, Zhang, Choi.
Statistical analysis: Lu, Joshi, Saag, Zhang, Choi.
Obtained funding: Choi.
Administrative, technical, or material support: McCormick, Warner, Choi.
Supervision: Choi.
Conflict of Interest Disclosures: Dr McCormick reported receiving grants from Canadian Institutes of Health Research and the National Institutes of Health during the conduct of the study. Dr Yokose reported receiving grants from the Rheumatology Research Foundation during the conduct of the study. Dr Joshi reported being a recent employee of Regeneron outside the submitted work. Dr Warner reported receiving personal fees from AstraZeneca and grants from Pfizer Inc outside the submitted work. Dr Dalbeth reported receiving personal fees from AstraZeneca, Dyve Biosciences, Horizon Therapeutics PLC, Selecta Biosciences, Arthrosi Therapeutics, JW Pharmaceutical Corporation, PK MED, PTC Therapeutics, Protalix BioTherapeutics, AbbVie, and Janssen Pharmaceuticals and grants from Amgen Inc outside the submitted work. Dr Saag reported receiving personal fees from AbbVie, Amgen Inc, Arthrosi Therapeutics, Atom Bioscience, Bayer AG, CSL Behring, Daiichi Sankyo Company, Limited, Gilead Sciences, Inc, Horizon Therapeutics PLC, Inflazome, LG Pharma, Mallinkrodt Pharmaceuticals, Radius Health, Inc, Roche/Genetech, Swedish Orphan Biovitrum AB, and Takeda Pharmaceutical Company, Limited, and grants from Amgen Inc, Horizon Therapeutics PLC, Radius Health, Inc, Swedish Orphan Biovitrum AB, and Shanton Pharma outside the submitted work. Dr Choi reported receiving research support from Horizon Therapeutics PLC and consulting fees from LG Pharma, Horizon Therapeutics PLC, Allena Pharmaceuticals, Inc, and Protalix Biotherapeutics outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by grants P50-AR-060772 and R01-AR-065944 from the National Institutes of Health; by a Fellowship Award from the Canadian Institutes of Health Research (Dr McCormick); Career Development Award K99-AR080243 from the National Institutes of Health (Dr McCormick); and a Scientist Development Award from the Rheumatology Research Foundation (Dr Yokose).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentation: This paper was presented as a poster at the 2022 Annual European Congress of Rheumatology; June 3, 2022; Copenhagen, Denmark.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Medindia » News » Research News » Gout Cases Expected to Surge Over 70% by 2050
Gout Cases Expected to Surge Over 70% by 2050
Rise in Global Gout Cases
What is gout.
- Global, regional, and national burden of gout, 1990â2020, and projections to 2050 - (https://www.healthdata.org/research-analysis/library/global-regional-and-national-burden-gout-1990-2020-and-projections-2050

| --> --> |

- Consult Online Doctor
- Take Telemedicine course
- Health Centers
- Health Tools
- Health info by Speciality
- Know Your Body
- Health and Wellness
- Web Stories
- Newsletters
- Health Tips
- Nutrition Facts
- Lifestyle & Wellness
- Beauty Tips
- Diet & Nutrition
- Home Remedies
- Obesity & Weight Loss
- Complementary Medicine
- Special Reports
- Press Releases
- Health Guide
- Health articles
- Health Quiz
- Health Facts
- Travel Health
- Medicine and Movies
- Symptom Articles
- Diabetes Tools
- Pediatric Calculators
- Men's Health
- Women's Health
- Height Weight Tools
- Cardiac Tools
- Pharma Tools
- Wellness Interactive Tools
- Drug Information
- Drugs by Condition
- Drug Price List - Brand Names
- Drug Interaction with Food
- Drug Price List - Generic Names
- Drugs - Side Effects
- Drug Videos
- Drug Database
- Allopathy Doctors
- Alternative Medicine Doctors
- Physiotherapist
- Speech Therapist
- Sexologists
- Hospital Directory
- Diagnostic Lab Directory
- Pharmacy or Chemist
- Medical Equipment Suppliers
- Pharma Directory
- Emergency Services
- PG Education
- Family Medicine
- Other Resources
- Medical Aphorism
- Health Acts in India
- Health Quotations
- Medical Conference
- Amazing Body Facts
- Health poll

- Editorial Team
- Exclusive Interviews
- In the News
- Partners & Affiliates
- Advertise With Us
- हिन्दी
- français
- Español
- 中文
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Effects of wind farm construction on soil nutrients and vegetation: a case study of linxiang wind farm in hunan province.

Share and Cite
Li, L.; Ma, W.; Duan, X.; Wang, S.; Wang, Q.; Gu, H.; Wang, J. Effects of Wind Farm Construction on Soil Nutrients and Vegetation: A Case Study of Linxiang Wind Farm in Hunan Province. Sustainability 2024 , 16 , 6350. https://doi.org/10.3390/su16156350
Li L, Ma W, Duan X, Wang S, Wang Q, Gu H, Wang J. Effects of Wind Farm Construction on Soil Nutrients and Vegetation: A Case Study of Linxiang Wind Farm in Hunan Province. Sustainability . 2024; 16(15):6350. https://doi.org/10.3390/su16156350
Li, Lin, Wenjing Ma, Xiangyi Duan, Shuo Wang, Qiong Wang, Huangling Gu, and Jingsong Wang. 2024. "Effects of Wind Farm Construction on Soil Nutrients and Vegetation: A Case Study of Linxiang Wind Farm in Hunan Province" Sustainability 16, no. 15: 6350. https://doi.org/10.3390/su16156350
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Surg Neurol Int

Acute presentation of spinal gouty arthritis: A case report and review of literature
Background:.
Gout is an inflammatory arthritis that results from faulty purine metabolism, affecting approximately 4% of adults in the US, and predominately affects people in the fourth decade of life. Further, spinal gout is rarely the first presentation of gout, especially in younger individuals.
Case Description:
A 26-year-old male came to the emergency room with acute lower extremity numbness and weakness. The MR demonstrated an enhancing epidural lesion at T6–T8 in the mid-thoracic spine. He subsequently underwent a decompressive laminectomy and fusion at levels T6–T9, resulting in full recovery 1 year later. The pathology demonstrated needle-like monosodium urate crystals consistent with the diagnosis of spinal gouty arthritis.
Conclusion:
Gout rarely initially presents in a young adult in the spine. Here, we reviewed the case of spinal gout in a 26-year-old male who successfully underwent spinal surgery.

INTRODUCTION
Gout, that afflicts approximately 4% of adults in the United States, is an inflammatory arthritis that results from faulty purine metabolism.[ 6 ] It becomes acutely symptomatic when the final product of purine metabolism, uric acid, reaches high saturation levels in the blood. This is attributed either to decreased clearance or overproduction. The uric acid then crystallizes into monosodium urate which is then deposited into tissues; it particularly involves the synovial joints of the feet, hands, and, rarely, the spine. These crystals produce an inflammatory response that results in swollen, tender, and hot joints; the deposits are known as tophi. Here, we report a case of tophaceous gout leading to neurological deficits in a 26-year-old male along with a review of the relevant literature.
CASE REPORT
Clinical findings.
A 26-year-old male presented with 1–2 months of progressive right lower extremity weakness culminating in a 1-day history of the inability to ambulate. On examination, he exhibited 4-/5 hip flexion, knee extension, and knee flexion bilaterally in the lower extremities, 2/5 right-sided plantar flexor weakness, left lower extremity anteromedial hip numbness, and patellar hyperreflexia.
Radiographic presentation
The thoracic MR with and without contrast demonstrated a right posterior-lateral T2 enhancing epidural lesion extending from T6 to T8 contributing to severe canal/cord stenosis and mild cord T6–T9 cord edema [ Figure 1a - -f]. f ]. The noncontrast thoracic computed tomography scan demonstrated multilevel periarticular lytic/erosive lesions involving the T6–T7 to T8–T9 facet joints, accompanied by lobulated high-density juxta-articular material extending into the adjacent canal [ Figure 2a - -e e ].

Magnetic resonance imaging (MRI) images with the right lateral epidural abnormality in the mid-thoracic spine with spinal cord compression. (a) Sagittal T2 sequences, (b) sagittal short-tau inversion recovery sequences, (c) sagittal T1 sequences without contrast, (d) sagittal T1 sequences with contrast, (e) axial T1 MRI with contrast demonstrating extension of a tophus into the spinal canal and compressing the spinal cord to the left, (f) axial T1 magnetic resonance imaging with contrast at a level with no inflammatory or degenerative changes and no spinal cord compression.

Computed tomography (CT) images showing lytic process of bilateral thoracic facet joins with compression of the spinal cord. (a) Sagittal CT scans of the mid-thoracic spine demonstrating erosive changes of the right facet joints, (b) sagittal STIR sequences, (c) sagittal T1 sequences without contrast, (d) sagittal T1 sequences with contrast, (e) axial T1 magnetic resonance imaging with contrast demonstrating extension of a tophus into the spinal canal and compressing the spinal cord to the left.
The next day, he underwent a T6–T9 decompressive laminectomy for the resection of the epidural mass accompanied by pedicle screw instrumentation [ Figure 3a and andb]. b ]. At each level, there was a “white, cheese- like” material causing substantial dural compression; the material was grossly consistent with tophaceous deposits within the facet joints and the pathological confirmation of needle-like monosodium urate crystals.

(a) Postoperative anteroposterior and lateral thoracic X-rays demonstrating a short T6–T9 fusion construct. (b) Postoperative lateral X-rays of the thoracic spine demonstrating short fusion of the thoracic spine.
Postoperative course
On postoperative day 1, the patient was 4+/5 in the lower extremities bilaterally, and the numbness of the anteromedial segment of his left lower extremity was markedly improved. He was discharged 6 days later, and at 1 postoperative year, his neurological examination was normal.
The final pathology demonstrated whitish aggregates of strand-like material surrounded by inflammatory cells, including many foreign body giant cells. Under polarized light, these aggregates revealed strongly negative birefringent needle-shaped crystals consistent with gout.
Further, the postoperative rheumatologic workup documented elevated serum and urine uric acid levels. In addition, an X-ray of the right hand showed a synovial inflammatory process involving the ring finger. The patient was started on colchicine 0.6 mg b.i.d. and allopurinol 200 mg q.d.
Here, we described a rare case of spinal tophaceous gout in a 26-year-old male who newly presented with an acute paraparesis. The diagnosis of gout was not originally entertained, given the patient’s negative history and young age.
In a review of 131 cases, Toprover et al . found that gout can affect the lumbar (38%), cervical (24.8%), and thoracic spine (17.8%).[ 3 ] The most frequent clinical manifestation is typically back pain; however, more severe neurologic symptoms have been described, including the onset of acute weakness.[ 1 ]
The current epidemiologic studies show that the prevalence of gout in patients between the ages of 18 and 44 is <0.3%.[ 4 ] As of 2019, only 159 cases of spinal gout have been reported; gout is typically diagnosed in the fourth decade of life, the average age in men 30 years old.[ 2 ] Around 50% of patients present with neurological sequela requiring surgery if there is a neurological compromise. Here, our patient required surgical decompression/fusion to both establish the diagnosis and treat the acute paraparesis. Only 13 reported cases demonstrate patients who were diagnosed with spinal gout, with only one of those patients having no prior history.[ 5 ]
Here, we presented the rare scenario of a 26-year-old male acutely developing paraparesis secondary to T6–T9 tophaceous gout.
How to cite this article: Akhter AS, Mohyeldin A, Grossbach AJ. Acute presentation of spinal gouty arthritis: A case report and review of literature. Surg Neurol Int 2019;10:232.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.

IMAGES
VIDEO
COMMENTS
Gout is an inflammatory arthritis caused by chronic hyperuricemia. This arthritis is due to deposition of monosodium urate crystals in and around joints and connective tissue.[] Previously were described four different clinical phases of gout progression:[] - Asymptomatic patients with serological findings of hyperuricemia - Acute gouty arthritis - Intercritical gout - Chronic tophaceous gout ...
In fact, 5% of those who develop gout, have uric acid levels above 9 mg/dL . In a study done in 2009, 339 patients who presented with acute gout, 14% had serum uric acid levels ≤6 mg/dL, and 18% had levels between 6 mg/dL and 8 mg/dL . The later presentation usually involves nephrolithiasis and tophi, as well as recurrent episodes of acute ...
Background Gout is a metabolic disease that can manifest as acute or chronic arthritis, and deposition of urate crystals in connective tissue and kidneys. It can either manifest as acute arthritis or chronic tophaceous gout. Case presentation We present a 39-year-old male patient who developed acute arthritis during his hospital course. Later on, after a careful physical examination, patient ...
Gout case •If acute or chronic gout is suspected, a serum uric acid test can be performed. A low serum uric acid level makes a gout diagnosis much less probable, but hyperuricemia and joint pain does not confirm gout. During an acute gout flare, a patient may paradoxically have normal or even low SUA levels.
Recent diuretic use and the risk of recurrent gout attacks: the online case-crossover gout study. J Rheumatol 2006;33:1341-1345[Erratum, J Rheumatol 2006;33:1714.] PubMed. ISI. Google Scholar. 20.
Because these results suggested gout, the patient was ad-vised to start colchicine 0.6 mg twice daily and allopurinol 100 mg daily, titrated every 2 weeks to a target dose of 300 mg daily. He was advised to reduce his daily dosage of aspirin to 81 mg, to discontinue indomethacin, and to take acetaminophen as needed, up to 4 g/day. In addition, the
Gout and incidence of 12 cardiovascular diseases: a case-control study including 152 663 individuals with gout and 709 981 matched controls, The Lancet Rheumatology, 6, 3, (e156-e167), (2024 ...
Alcohol is a well-known risk factor for gout. Studies showed that alcohol consumption is related to the amount consumed. Additionally, the risk for gout and hyperuriceamia depends on the type of different alcoholic drinks. ... Case reports of (PET/CT) in gout showed articular and periarticular FDG (18 F-fluoro-2-deoxy-D-glucose) uptake. Soft ...
A multivariable nested case-control study was performed among 62 574 patients with gout, and a self-controlled case series, adjusted for season and age, was performed among 1421 patients with gout flare and cardiovascular event. Exposures Gout flares were ascertained using hospitalization, primary care outpatient, and prescription records.
Abstract. Gout is a painful arthritic condition that affects many people worldwide. The disease has been associated with hyperuricaemia and life style risk factors such as obesity, alcohol intake, meat and seafood consumption. We present a case of a 67-year-old male with a history of gout, who attended the clinic with a painful 1st ...
The disease has been associated with hyperuricaemia and life style risk factors such as obesity, alcohol intake, meat and seafood consumption. We present a case of a 67-year-old male with a history of gout, who attended the clinic with a painful 1st metatarsophalangeal joint, which had progressively worsened in pain, mobility and deformity in ...
Joint pain is a common complaint in pediatrics and is most often attributed to overuse or injury. In the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. Rarely, a child has two distinct causes for joint pain. In this case, an obese 15-year-old male was diagnosed with gout, a disease ...
Detailed methods of the study design were published previously. 17 Briefly, 950 participants fulfilling American College and Rheumatology gout classification criteria 18 with a serum urate concentration of 6.8 mg/dl or greater were enrolled from 21 sites between 2017 and 2019. This noninferiority, double-blind, double-dummy, 72-week trial (CSP594 Comparative Effectiveness in Gout: Allopurinol ...
In this matched case-control study, we used linked primary and secondary electronic health records from the UK Clinical Practice Research Datalink to assemble a cohort of individuals with a first-time diagnosis of gout between Jan 1, 2000 and Dec 31, 2017, who were aged 80 years or younger at diagnosis, and free of cardiovascular diseases up to 12 months after diagnosis.
GOUT A. GOALS 1. Understand pathogenesis of gouty arthritis 2. Learn pharmacologic treatment for gout B. CASE • 55-year-old man with history of episodic pain and swelling in the 1st MTP joints • Started allopurinol one week earlier • Physical examination showed rock-hard lump on right pina and hot, tender
Conclusion. Our study evaluated general practitioners' knowledge and management of gout at community health service clinics in the Tongzhou district of Beijing. The results indicated a poor understanding of the diagnosis and treatment of gout. This lack of understanding likely resulted in inadequate clinical decisions.
In this gout case study, a 66-year-old female has a past medical history of diabetes, neuropathy, depression, migraines, insomnia, gout, and hypertension. She has numerous complaints. Her gout has not been in good control as she is requiring significant indomethacin use for flare management. She says that capsaicin doesn't help her knee pain.
The Global Burden of Disease Study 2017 published that there are 41 million living with gout worldwide [3]. The prevalence of gout in 1990 was 20.2 million, and this number more than doubled in 2017 to 41.2 million. Even with the advances in the treatment of gout, a failure to comply with a treatment regimen has led to these increasing numbers [5].
Rheumatoid arthritis (RA) is one of the most common symmetric, peripheral polyarthritis, chronic inflammatory rheumatic diseases of an unknown etiology. The general prevalence of RA is about 0.5% to 1% in the adult population with a male and female prevalence ratio of 1:3. [1,2] Meanwhile, gout is a deposition of monosodium urate crystals which ...
Case Study: Gout BC 362, Medical Biochemistry, Julia Jardina, Kassie Brink, and Ashley Shegog Case Backg ro u n d : Robert , a 65 year ol d man was admi t t ed t o t he hospi t al wi t h severe pai n and edema i n hi s ri ght f oot maki ng i t hard f or hi m t o wal k. A f t er gi vi ng hi m an exam, you
Gout flares are less frequent when serum urate (SU) levels are below 6 mg/dL after the first year of urate-lowering therapy (ULT), according to study results published in Annals of the Rheumatic ...
Background: Gout is an inflammatory arthritis manifesting as acute episodes of severe joint pain and swelling, which can progress to chronic tophaceous or chronic erosive gout, or both. Here, we present the most up-to-date global, regional, and national estimates for prevalence and years lived with disability (YLDs) due to gout by sex, age, and location from the Global Burden of Diseases ...
Key Points. Question Are there racial differences in gout prevalence among adults in the US general population, and what might explain these disparities?. Findings In this cross-sectional study of 18 693 participants, gout was 1.8 times more prevalent among Black women than White women and 1.3 times more prevalent among Black men than White men.
Gout, once known as the "disease of kings and king of diseases," is among the most prevalent etiologies of chronic inflammatory arthritis in the United States, characterized by monosodium urate (MSU) monohydrate crystals deposition within tissues.[1][2] Hippocrates first described gout in ancient Greece; hence, it is the most understood and manageable disease among all rheumatic diseases.[3][4]
Rilonacept reduced gout flares over a 16-week period by 70% compared to placebo in subjects with a history of gout. 29 Likewise, a study of 432 patients initiating ULT with allopurinol found that canakinumab provided superior prophylaxis compared with colchicine. 30 Over 16 weeks, >60% reduction in the mean number of flares per patient for ...
A separate study by Harvard University researchers, published in the journal Scientific Reports, attributed the surge in gout cases to the rise in comorbid conditions known to be precursors to ...
Why does gout tend to occur in the feet? * 1 pointgravity and cooler temperatures allow chemicals to crystallize easilycirculation tends to be more sluggish in the feet than elsewherethe joint between the first metatarsal and the great toe is subject to a lot of weight-bearing stressthe first metatarsals are most likely to develop osteophytes Case Study: Your client is a healthy 3 5-year ...
INTRODUCTION. The incidence of gout has doubled over the past two decades and continues to increase.1 Studies have established risk factors for gout including genetic factors, excess alcohol consumption, purine-rich diet, the metabolic syndrome, use of diuretics and chronic renal failure. Increasing trends of these underlying factors in the general population, especially diet and the metabolic ...
Amidst escalating global energy demands, the advancement and utilization of renewable energy sources have emerged as critical strategies for addressing environmental concerns and alleviating energy crises. Among them, wind power, as a renewable and clean energy source, has been widely applied and developed in China. However, the construction of wind farms may have some impact on vegetation ...
The current epidemiologic studies show that the prevalence of gout in patients between the ages of 18 and 44 is <0.3%. As of 2019, only 159 cases of spinal gout have been reported; gout is typically diagnosed in the fourth decade of life, the average age in men 30 years old. Around 50% of patients present with neurological sequela requiring ...