Clinical Research Organization Market

Analysis and Review: Clinical Research Organization Market by Service (Drug Discovery Services, Pre-Clinical Services, Clinical Services, Post-Approval Services), by Production (In-House, Outsourced), by Indication (Oncology, CNS, Cardiovascular Diseases, Metabolic Disorders, Immunology, Respiratory, Musculoskeletal Disorders, Hematological Disorders), by End User (Pharmaceutical & Biotechnology Companies, Medical Device Companies, Governments & Private Firms, Academic Institutions, Others) and By Region- Forecast for 2023 – 2033
Market Insights on Clinical Research Organization Market covering sales outlook, demand forecast and up-to-date key trends
- Report Preview
- Request Methodology
Clinical Research Organization Market Snapshot (2023 to 2033)
According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033.
Market Outlook:
| Data Points | Market Insights |
|---|---|
| Market Value 2022 | US$ 58.02 Billion |
| Market Value 2023 | US$ 62.43 Billion |
| Market Value 2033 | US$ 139.56 Billion |
| CAGR 2023 to 2033 | 8.4% |
| Share of Top 5 Countries | 63.4% |
| Key Players | The key players in the market are Charles River Laboratories, Laboratory Corporation of America Holdings, IQVIA Inc, Parexel International Corporation, ICON plc., Medpace, Syneos Health, CTI Clinical Trial and Consulting Services, Neuroservices Alliance, QPS Neuropharmacology, MD Biosciences, EphyX Neuroscience. |
The Clinical Research Organization (CRO) market refers to the industry segment companies and organizations providing clinical research services to pharmaceutical, biotechnology, and medical device companies. It encompasses the commercial activities and financial transactions associated with outsourcing clinical trials and research studies.
The market is driven by the increasing demand for efficient and cost-effective drug development processes. Pharmaceutical and biotech companies often outsource clinical research activities to CROs to leverage their specialized expertise, infrastructure, and resources. This allows the companies to focus on their core competencies, such as drug discovery and marketing, while relying on CROs for the execution of clinical trials.
Don't pay for what you don't need
Customize your report by selecting specific countries or regions and save 30%!
Sales Analysis of Clinical Research Organization Market from 2018 to 2022 Vs Market Outlook for 2023 to 2033
Sales of the market grew at a CAGR of 6.4% between 2018 to 2022.
With a historical forecast of stable growth, the clinical research organization (CRO) industry has seen significant growth in recent years. The rise in demand for outsourced clinical trials from pharmaceutical, biotechnology, and medical device businesses is what is causing this surge.
The clinical research organization (CRO) market is anticipated to maintain its growth trajectory in the upcoming years, according to the projection. The expansion of the CRO market is anticipated to be fueled by an increase in clinical trials, a rise in the need for personalized medication, and the prevalence of chronic diseases. Various clinical trial processes, including patient recruiting and retention, data analysis, and medication discovery, are using these technologies.
Considering this, FMI expects the global clinical research organization market to grow at a CAGR of 8.4% through the forecasted years.
What are the Key Opportunities in the Clinical Research Organization Market?
CROs can provide specialized services for carrying out clinical studies in this field as personalized medicine and targeted medicines gain popularity. This covers the identification of biomarkers, patient screening, and customized trial planning. CROs now have more potential due to the use of real-world data and virtual trials.
Companies with expertise in these fields can provide pharma/biotech firms with specialized services and assistance for real-world research as well as for virtual studies, which can shorten the trial duration and expense. It can also include telemedicine and digital health technology in clinical trials to raise study compliance, increase patient participation, and offer ongoing monitoring. CROs can be assisted in adopting these technologies and incorporating them into clinical trial designs by businesses offering specialised services.
They are now also able to provide specialized services in data analytics and machine learning due to the increased availability of real-time data in clinical trials. To do this, one can employ predictive analytics to foresee dangers, spot trends, and improve study design, which thereby helps in the growth of the global market.

Principal Consultant
Talk to Analyst
Find your sweet spots for generating winning opportunities in this market.
Which Factors Could Possibly Restrain the Growth of the Clinical Research Organization Market?
Ensuring compliance with regulatory regulations is one of the biggest issues for CRO businesses. Navigating through each nation's unique legislation can be time-consuming and expensive. To make sure that all applicable regulations are followed, CROs must have a strong regulatory staff. Companies in the pharma and biotech industries are increasingly looking for CROs that can offer services at a low cost without sacrificing quality or timeliness. To remain competitive, CROs may need to modify their pricing strategies and business models in response to margin concerns.
Large-scale multicenter trials might be difficult to finance since there is sometimes a lack of funding for clinical trials. To maximize the cost-effectiveness of clinical studies, sponsors, researchers, and CROs must collaborate due to the high cost of drug development and growing cost constraints. The capabilities of CROs may also be constrained by a lack of highly skilled workers, including researchers, data administrators, and statisticians. Lack of qualified personnel may cause inefficiencies, delays in hiring and retaining staff, and a general decline in the quality of project outputs.
Country-wise Insights
What makes the usa the dominating country in the clinical research organization market.
The USA clinical research organization market is expected to register 32.0% in the global market in 2022.
There are several life science businesses in the United States, and these businesses are embracing innovations in clinical research. CROs that offer these services are in demand as technological innovations like telemedicine and virtual trials are used more frequently. Drug development efforts by biotech and pharmaceutical firms are increasing in the USA These businesses are turning more and more to CROs for assistance, including access to facilities and programs for current patients, enrollment and recruiting information, personalized protocol design, higher trial completion rates, and lower costs.
Why is China considered a Lucrative Market for Clinical Research Organization Services?
China accounted for around 9.3% market share in 2022 globally.
The clinical research organization (CRO) market in China is expanding due to several factors. The rapidly ageing and expanding Chinese population is a significant contributing element, which has raised demand for cutting-edge medical cures and treatments. Furthermore, recent reforms and laws adopted by the Chinese government have made it simpler to conduct clinical trials and research there.
The government has put in place many steps to hasten the regulatory approval procedure for novel pharmaceuticals and therapies, luring multinational companies to locate their research and development operations in the nation. Additionally, the development of healthcare and rising income levels in China have raised the demand for advanced healthcare services, such as clinical research and triage.
What Makes India an Emerging Market for Clinical Research Organization?
India holds a 7.4% value share in the global market in 2022.
The clinical research organization (CRO) market is expanding in India for a variety of reasons. Large and diversified patient populations are a significant contributing element, which makes India a desirable location for carrying out clinical studies. Due to the country's accessible healthcare services and cheaper cost of living, clinical trials in India are also more economical than in many other nations.
The supportive regulatory environment created by the Indian government, which promotes clinical research activities by streamlining the approval procedure for clinical trials and cutting the time needed to secure regulatory approvals, is another important aspect. More foreign money has also been invested in India's clinical research sector as a result of the rise in medical tourism there.
Get the data you need at a Fraction of the cost
Personalize your report by choosing insights you need and save 40%!
Category-wise Insights
Which service is largely adopted for clinical research organizations.
The post-approval services segment held the major chunk of about 45.7% of the global market by the end of 2022.
A key factor in the expansion of the clinical research organization (CRO) market is post-approval services. Post-approval services are the actions taken after regulatory bodies or authorities have given their clearance for the use of a product or therapy. These services are essential for maintaining the product's safety and effectiveness as well as for carrying out additional studies that could increase the product or therapy's effectiveness.
In order to diversify their service portfolio and give their clients value-added services, several CROs offer post-approval services. Pharmacovigilance, medical monitoring, safety monitoring, data management, statistical analysis, quality assurance, and regulatory compliance are a few examples of the services that may be provided.
Which production type is largely preferred for the clinical research organization market?
The In-house production segment contribute 58.3% share of the global market in 2022.
The term in-house describes a method of conducting all phases of clinical research within a single organization, often comprising study design, data management, monitoring, and statistical analysis. Clinical research organizations (CRO) market development is still mostly fueled by internal clinical research conducted by pharmaceutical and biotech corporations. The in-house methodology has several benefits, including more control over studies, quicker decision-making, and reduced reliance on outside suppliers.
A pharmaceutical or biotech company's ability to quickly and efficiently react to any noteworthy events or changes in the study protocol can be facilitated by in-house clinical research, which provides better control over study data and technological competence.
Which Indication Dominates the Global Market in 2022?
The oncology by indication segment accounted for a revenue share of 23.4% in the global market at the end of 2022.
One of the markets for clinical research organizations (CROs) that is expanding the quickest is oncology, which has had a big impact on the development of the sector. Cancer research is an appealing subject for CROs to concentrate their attention on because it is a complex and fast-developing field of medicine that calls for a high degree of knowledge and resources.
Many variables, such as the rising cancer burden globally, developments in molecular biology and genomics, and the creation of more advanced and novel immuno-oncology medicines, all contribute to the expansion of oncology research and the CRO business. The need for more advanced oncology therapies that can address patients' complicated requirements is growing as cancer rates are expected to keep rising.
Which End User Segment Propels the Market Growth?
The pharmaceutical and biotechnology companies hold a share of 45.6% of the global market in 2022.
The market for clinical research organizations (CROs) is expanding mostly due to the efforts of the pharmaceutical and biotechnology industries. These businesses invest a lot of money in the discovery of novel treatments and medications, and they frequently contract out some or all of the clinical research work to CROs to increase productivity and access to funding.
A great deal of financing for clinical research studies-particularly those involving new medicinal compounds-comes from pharmaceutical and biotech corporations. These businesses frequently look to collaborate with CROs who can offer specialized knowledge in research design, data administration, and general project management. Pharmaceutical and biotech companies can shorten the time it takes to develop new drugs by outsourcing clinical trial efforts to CROs and concentrating internal resources on core research and development tasks.
Competitive Landscape
The market's key vendors are concentrating on diversifying their product offerings to strengthen their market share in clinical research organizations and to broaden their presence in developing nations. Pricing strategies, market strategies, technology improvements, regulatory compliance, and acquisition and distribution agreements with other companies to extend their business are the main tactics used by manufacturers to acquire a competitive edge in the market.
For instance:
- In January 2020, Charles River announced a scientific collaboration with Takeda Pharmaceutical Company Limited in order to focus on drug discovery in the four core therapeutic areas that Takeda works on. These four therapeutic areas include oncology, gastroenterology, neuroscience, and rare disease. This collaboration focuses on delivering preclinical candidates.
- In July 2022, Labcorp launched a new test called the neurofilament light chain (NfL) blood test, which will help in the identification and confirmation of neurodegenerative diseases like multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and others.
Similarly, recent developments have been tracked by the team at Future Market Insights related to companies in the clinical research organization market space, which are available in the full report
Scope of the Clinical Research Organization Report
| Attribute | Details |
|---|---|
| Forecast Period | 2023 to 2033 |
| Historical Data Available for | 2018 to 2022 |
| Market Analysis | USD Million for Value |
| Key Regions Covered | North America; Latin America; Europe; South Asia; East Asia; Oceania; Middle East; and Africa (MEA) |
| Key Countries Covered | USA, Canada, Brazil, Mexico, Argentina, Germany, UK, France, Italy, Spain, Russia, BENELUX, Nordics, China, Japan, South Korea, India, Thailand, Indonesia, Malaysia, Philippines, Vietnam, Australia, New Zealand, Türkiye, South Africa, North Africa, and GCC Countries |
| Key Segments Covered | Service, Production, Indication, End User, and Region |
| Key Companies Profiled | Charles River Laboratories; Laboratory Corporation of America Holdings; IQVIA Inc; Parexel International Corporation; ICON plc.; Medpace, Syneos Health; CTI Clinical Trial and Consulting Services; Neuroservices Alliance; QPS Neuropharmacology; MD Biosciences; EphyX Neuroscience |
| Report Coverage | Market Forecast, Competition Intelligence, DROT Analysis, Market Dynamics and Challenges, Strategic Growth Initiatives |
| Customization & Pricing | Available upon Request |
Key Segments Covered in Clinical Research Organization Industry Research
By service:.
- Drug discovery Services
- Pre-clinical Services
- Clinical Services
- Post Approval Services
By Production:
By indication:.
- Cardiovascular Diseases
- Metabolic Disorders
- Respiratory
- Musculoskeletal Disorders
- Hematological Disorders
By End User:
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Governments & Private Firms
- Academic Institutions
- North America
- Latin America
- Middle East and Africa (MEA)
Frequently Asked Questions
How big is the clinical research organization market.
The clinical research organization market is slated to attain US$ 62.43 billion in 2023.
What Might be the Market’s Size by 2033?
The market is expected to attain a value of US$ 139.56 billion by 2033.
Which Opportunities are Emerging in the Market?
Increasing popularity of targeted medicines and personalized medicines are creating opportunities for key players.
Which Factors Pull Back the Market Growth?
Strict regulatory regulations and lack of funding for clinical trials are pulling back the market’s growth.
Who are the Key Players in the CRO Industry?
IQVIA Inc, Parexel International Corporation, and ICON plc. are the key players in the CRO industry.
Table of Content
Explore Healthcare Insights
- Get Free Brochure -
Your personal details are safe with us. Privacy Policy*
- Get a Free Sample -
- Request Methodology -
- Customize Now -
I need Country Specific Scope ( -30% )
- Talk To Analyst -
I am searching for Specific Info.
- Download Report Brochure -

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
- Healthcare IT
- Contract Research Organization (CRO) Services Market
"Designing Growth Strategies is in our DNA"
Contract Research Organization (CRO) Services Market Size, Share & Industry Analysis, By Type (Early Phase Development Services [Chemistry, Manufacturing and Controls (CMC), Preclinical Service, and Discovery], Clinical [Phase 1, Phase 2, Phase 3, and Phase 4], Laboratory Service, and Others), By Application (Oncology, Neurology, Cardiology, Infectious Disease, Metabolic, Disorder, Renal/Nephrology, and Others), By End-user (Pharmaceutical & Biotechnological Companies, Medical Device Companies, Academic & Research Institutes, and Others), and Regional Forecast, 2024-2032
Last Updated: August 12, 2024 | Format: PDF | Report ID: FBI100864
- Segmentation
- Methodology
- Infographics
- Request Sample PDF
KEY MARKET INSIGHTS
The global contract research organization (CRO) services market size was valued at USD 79.54 billion in 2023. The market is projected to grow from USD 86.33 billion in 2024 to USD 175.46 billion by 2032, exhibiting a CAGR of 9.3% during the forecast period. Moreover, the U.S. contract research organization services market is projected to grow significantly, reaching an estimated value of USD 77.80 billion by 2032, driven by the increased outsourcing of clinical trials.
Contract research organization (CRO) services are involved in providing preclinical services, data collection services, clinical services, and others to biotechnology, pharmaceutical, and medical device companies. Contract research organization (CRO) service providers help life science companies reduce the costs involved in the development and launch of novel therapeutics and medical devices .
Moreover, the emergence of new small and mid-sized pharmaceutical and biotechnological companies that have limited resources for conducting R&D by themselves has also been fueling the demand for contract research organization (CRO) services to outsource their R&D. In order to fuel this increasing demand for contract research organization (CRO) services, key market players have increased their focus on the adoption of advanced technologies to enhance their offerings.
For instance, in April 2021, Pharmaceutical Product Development, LLC. (PPD), now a part of Thermo Fisher Scientific Inc., collaborated with Science 37, a clinical trial service provider, to use Science 37’s decentralized clinical trial Software as a Service (SaaS) -based technology platform to design, build, test, implement, and execute digital trials.
Due to the COVID-19 lockdown restrictions in 2020, the contract research organization (CRO) services market experienced slow growth as many clinical trials were delayed. However, in 2021 and 2022, the market experienced significant growth due to the initiation of delayed clinical trials and increased demand for vaccines for COVID-19.
Contract Research Organization (CRO) Services Market Trends
Rise in the Outsourcing of Clinical Trials due to High R&D Cost Drive Market Growth
Due to the increasing demand for effective diagnosis and therapeutics, pharmaceutical, biotechnology, and medical device companies have increased their focus on R&D spending. This factor has been fueling the number of clinical trials being conducted globally.
- For instance, as per the data published by the European Federation of Pharmaceutical Industries and Associations (EFPIA), R&D expenditure by pharmaceutical companies in 2022 experienced a growth of 7.2% to reach USD 46,792.8 million.
However, clinical trial studies can be very costly depending upon the type of indication, size of the study, and its duration. R&D expenditures can be high if the success rate of the study is low, as it may take more resources and take a longer period. Rising drug development costs are one of the major reasons for the increasing spending on R&D.
Due to this, many biotechnology and pharmaceutical industry have increased their focus on outsourcing their R&D studies to contract research organization (CRO) services providers. For instance, in March 2023, ICON plc LEO Pharma partnered together to conduct clinical trials to develop new effective medicines for dermatology indications.
The emergence of contract research organization (CRO) services providers has been fueling the R&D studies sponsored by small and mid-sized companies at a significant rate.
Request a Free sample to learn more about this report.
Contract Research Organization (CRO) Services Market Growth Factors
Increasing Demand for Cost-effective Treatment has been Fueling the Market Growth
The burden of chronic diseases, such as cardiovascular diseases, diabetes, cancer, neurological disorders, and others, has been growing significantly globally. For instance, per the data published by the American College of Cardiology Foundation in December 2023, cardiovascular diseases caused 19.8 million deaths globally in 2022, increasing from 12.4 million in 1990, similarly, as per the data published by Deutsches Ärzteblatt in 2023, as of December 2021, around 1.8 million people had dementia in Germany.
The growing burden of these diseases has been fueling the annual spending on diagnosis and treatment of these diseases. For instance, as per the data published by the National Cancer Institute (NCI) in 2023, in the U.S., spending on cancer was estimated to be USD 1,920.20 in 2015. This value experienced a growth of 10.0% and reached USD 208.90 billion in 2020. This cost includes cancer medical services and prescription drug costs.
The high cost associated with the diagnosis and treatment of these diseases is sometimes difficult for the population to cover, especially in low- and middle-income countries, leading to limited treatment options in such regions. Due to this, the pharmaceutical and biotechnological companies have increased their focus on the development and commercialization of new advanced and cost-effective diagnosis and treatment options. This factor has been fueling the outsourcing of R&D by life science companies for the development of cost-effective treatment options, thereby fueling the global contract research organization (CRO) services market growth.
Increasing Number of Clinical Trials to Boost the Adoption of Increased Outsourcing to Contract Research Organization (CRO) Services Provider
Clinical trials are essential for medication development processes all over the world. It helps discover novel treatments for diseases and new techniques for detecting, diagnosing, and preventing disease development. Clinical trials offer the scientific foundation for guiding and treating patients and evaluating novel medications and equipment. Clinical trial results help direct the right direction even though researchers may not get the expected results. Clinical trials in the U.S. are comparatively less than those conducted in other nations worldwide. Many clinical trials are conducted outside the U.S. and the European Union since it is often easier and less expensive.
Clinical trial success rates are highly dependent on the study's stage and the treatments or items being developed. Based on the article from the Biotechnology Innovation Organization, only about 9% of medications make it through phase I to approval. Recently, the number of clinical trials that have been filed has risen significantly. Clinical trials have become increasingly sophisticated, yet they are still necessary to study and develop novel medications and technologies. This increase in the number of clinical trials is anticipated to boost the development of new drugs and is expected to drive the market's growth during the forecast period.
RESTRAINING FACTORS
Lack of Skilled Professionals to Restrain Growth of the Market
The surge in globalization is boosting the rate of innovation and technology. New opportunities are constantly being added in terms of occupation. Furthermore, increasing industrialization and new services have increased the demand for new skills. This trend has also increased competency in job opportunities.
Contract research organization (CRO) services providers face issues attracting and maintaining highly competent experts as they compete for qualified and experienced scientists with biotechnology, pharmaceutical, medical devices businesses, and academic and research institutions. Companies must offer better compensation and incentives to compete better, affecting the finances and outcomes of players, mainly small-scale analytical testing providers.
This scarcity of qualified specialists is a barrier to adopting novel procedures and technologies, restricting market growth. For instance, as per the data published by Labiotech in September 2023, there were more than 600,00 job vacancies in biopharma, indicating a labor shortage of 7.0%.
Moroever, skill development in the life science industry has not been delivered globally, resulting in gaps between the average working age group and basic literacy.
The limited adoption of knowledge and experience, along with the scarcity of the healthcare workforce, has been limiting the market growth.
Contract Research Organization (CRO) Services Market Segmentation Analysis
By type analysis.
Early Phase Development Services Segment Dominated due to Rising Focus of the Lifesciences Companies on the Development of Advanced Products
By type, the market is divided into early phase development services, clinical, laboratory services, and others.
The early phase development services are further segmented into chemistry, manufacturing and controls (CMC), preclinical service, and discovery. Similarly, the clinical segment is further classified into phase 1, phase 2, phase 3, and phase 4.
The early phase development services dominated the market in 2023. The dominance of the segment is attributed to the increasing focus of the market players on boosting the early phase development services for chronic diseases. For instance, in December 2022, Phastar entered into Lean Life Science’s Oncology Development Programme (ODP2) with the aim of identifying and fueling the R&D for academics and early-stage companies.
Moreover, the clinical segment accounted for the second-most major global contract research organization (CRO) services market share in 2023. The segment’s growth in the market is attributed to the emergence of small and mid-sized pharmaceutical and biotechnology companies, which are focusing on the development of novel therapeutics.
Moreover, the laboratory service is expected to grow at a substantial CAGR during the forecast period due to increasing demand for laboratory services by healthcare companies with limited resources.
To know how our report can help streamline your business, Speak to Analyst
By Application Analysis
Oncology Segment to Dominate Led by Continuous Development of Cancer Treatment Solutions
Based on application, the market can be segmented into oncology, neurology, cardiology, infectious disease, metabolic disorders, renal/nephrology, and others.
The oncology segment dominated the market in 2023. The segment’s dominance in the contract research organization (CRO) services market is attributed to the growing focus of life science companies on the development of novel therapeutics.
- For instance, in May 2023, PharmaMar launched its phase 1 clinical trial for its new molecule, PM54, for treating patients suffering from solid tumors. The first-in-human trial was conducted in hospitals in the U.S., Spain, and other countries.
Furthermore, the others segment accounted for the second most dominant portion of the market share in 2023. The growth of the segment is attributed to the growing burden of diseases such as digestive diseases, nutritional deficiencies, respiratory disorders, and others. Moreover, the growing funding by pharmaceutical and biotechnological companies for the effective therapeutic development of these diseases has been fueling the market growth.
- In October 2023, Forbion, a life sciences company, was involved in funding Aiolos Bio, Inc. for conducting phase 2 clinical studies to study the safety and efficacy of drug candidate AIO-001, indicated for treating asthma.
Moreover, the neurology segment is expected to grow at a significant CAGR during the forecast period due to the increasing prevalence of neurological disorders, such as Alzheimer’s, dementia, etc.
By End-User Analysis
Pharmaceutical & Biotechnological Companies to Hold Dominant Share Backed by Increasing R&D
Based on end-user, the market is segmented into pharmaceutical & biotechnological companies, medical device companies, academic & research institutes, and others.
The pharmaceutical & biotechnology companies dominated the market in 2023. Pharmaceutical and biotechnology companies are generally outsourcing the therapeutic and other product development functions to independent service providers to optimize the use of a more flexible cost structure and also avoid maintaining redundant development capabilities globally. This is driving the segmental growth.
Moreover, the medical devices segment is expected to grow at the fastest CAGR during the forecast period due to the rise in the number of registered clinical trials for medical devices. For instance, as per ClinicalTrials.gov, as of January 2022, approximately 40,901 clinical trials were registered for medical devices.
REGIONAL INSIGHTS
In terms of geography, the global market is studied across North America, Europe, Asia Pacific, and the Rest of the World.
North America Contract Research Organization (CRO) Services Market Size, 2023 (USD Billion)
To get more information on the regional analysis of this market, Request a Free sample
The North American contract research organization (CRO) services market was valued at USD 40.01 billion in 2023 and is expected to hold a leading share of the global market in the forecast period. Factors responsible for the region’s dominance include the important pharmaceutical companies located in the region, overall drug development activity, and good healthcare infrastructure. Pharmaceutical organizations have increased their focus on outsourcing clinical trials to treat different disease conditions. Also, these organizations are investing more in R&D activities.
Europe was the second-largest region in the global market in 2023 and is estimated to hold this position during the forecast period. This is due to the surge in the incidence of diseases. Based on the article from Strategic Policy Group, the number of older people aged 65 years or more in the region would increase significantly, rising from around 90.5 million in 2019 to 129.8 million by 2050. Moreover, healthcare expenditure is surging, and pharma companies depend more on contract research organizations to offer more efficiency and productivity.
The Asia Pacific market is projected to grow at the fastest CAGR. The high growth is mainly attributed to the surge in R&D activity and the growing shift toward outsourcing. Furthermore, low-cost resources in the Asia Pacific region are one of the major factors driving the number of clinical trials being conducted in the region, thereby fueling the market growth.
- For instance, as per the World Health Organization (WHO), the number of clinical trials registered in Southeast Asia in 2022 reached 11,030, experiencing a growth of 48.5% from 2019.
The market in the Rest of the World is expected to hold a limited share of the market during the forecast period. However, increasing healthcare expenditure in the countries in the rest of the world is expected to fuel market growth.
Key Industry Players in Contract Research Organization (CRO) Services Market
Labcorp Adopts Merger & Acquisition Strategies to Bolster Portfolio and Strengthen Market Foothold
Labcorp Drug Development accounted for the major shares of the market 2023. The increased growth of the market was majorly due to the company’s strong focus on mergers and acquisition to upskill their services. For instance, in July 2021, Labcorp announced the expansion of its oncology portfolio by acquiring Omniseq. This acquisition upskilled the company’s contract research organization services portfolio.
Other key players, such as IQVIA, Pharmaceutical Product Development, LLC, and ICON pIc, held significant shares in the market. This was due to their strong focus on R&D to expand its product offerings and strengthen its global presence. Furthermore, companies are also focusing on incorporating new technologies, such as artificial intelligence , to increase the efficacy of their service offerings.
LIST OF TOP CRO SERVICES COMPANIES:
- Medpace (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- ICON plc (Ireland)
- IQVIA Inc (U.S.)
- Syneos Health (U.S.)
- Novotech (Australia)
- KCR S.A. (U.S.)
- PSI (Switzerland)
- Ergomed Group (U.K.)
- Thermo Fisher Scientific Inc. (U.S.)
- WuXi Biologics (China)
- Tigermed (China)
- Worldwide Clinical Trials (U.S.)
- Charles River Laboratories (U.S.)
- Microbiologics (U.S.)
- Parexel International (MA) Corporation (U.S.)
KEY INDUSTRY DEVELOPMENTS:
- February 2024 – Ergomed Group announced the expansion of its U.S. presence with a new office at Kendall Square in Cambridge, Massachusetts.
- February 2024 – Charles River Laboratories entered into a partnership with Wheeler Bio to expedite the progression from therapeutic discovery to manufacturing for its clients.
- January 2024 – WuXi Biologics collaborated with BioNTech SE to discover investigational monoclonal antibodies for developing next-generation therapeutic product candidates.
- December 2021 – Thermo Fisher Scientific Inc. completed the acquisition of Pharmaceutical Product Development, LLC. This acquisition enhanced the company's key services and offerings.
- December 2021 – Laboratory Corporation of America Holdings completed the acquisition of Toxikon Corporation. This acquisition bolstered the company’s strong non-clinical development portfolio.
REPORT COVERAGE
An Infographic Representation of Contract Research Organization (CRO) Services Market

To get information on various segments, share your queries with us
The global market report provides a detailed market analysis. It focuses on key aspects such as an overview of the market, penetration of outsourcing in R&D and key countries, pricing analysis. Additionally, it includes key industry developments such as mergers, partnerships, & acquisitions, the impact of COVID-19 on the market, and brand analysis. Besides these, the report offers insights into the market trends and highlights key industry developments. In addition to the aforementioned factors, the report encompasses several factors that have contributed to the growth of the market over recent years. The report also covers a regional analysis of different segments.
To gain extensive insights into the market, Request for Customization
Report Scope & Segmentation
|
|
| 2019-2032 |
| 2023 |
| 2024 |
| 2024-2032 |
| 2019-2022 |
| CAGR of 9.3% from 2024-2032 |
| Value (USD Billion) |
|
|
| |
| |
|

Bhushan Pawar ( Assistant Manager -Healthcare )
Frequently Asked Questions
Fortune Business Insights says that the global market size was USD 79.54 billion in 2023 and is projected to reach USD 175.46 billion by 2032.
In 2023, North America stood at USD 40.01 billion.
Growing at a CAGR of 9.3%, the market will exhibit steady growth in the forecast period.
The clinical segment is expected to be the leading segment in this market during the forecast period.
The increasing number of clinical trials and surge in the cases of chronic disease are some of the major factors driving the markets growth.
Labcorp and IQVIA are some of the major players in the global market.
North America dominated the market in 2023.
The surge in the outsourcing for R&D by the pharmaceutical organizations more efficiency and productivity are factors that drive the adoption of the service.
- STUDY PERIOD: 2019-2032
- BASE YEAR: 2023
- HISTORICAL DATA: 2019-2022
- NO OF PAGES: 168
Personalize this Research
- Granular Research on Specified Regions or Segments
- Companies Profiled based on User Requirement
- Broader Insights Pertaining to a Specific Segment or Region
- Breaking Down Competitive Landscape as per Your Requirement
- Other Specific Requirement on Customization

Related Reports
- Contract Development and Manufacturing Organization (CDMO) Outsourcing Market
- ASEAN Contract Development and Manufacturing Organization (CDMO) Market
- Contract Manufacturing Organization (CMO) Market
- Europe CRO Services Market
- Clinical Trials Market
“We are quite happy with the methodology you outlined. We really appreciate the time your team has spent on this project, and the efforts of your team to answer our questions.”
“Thanks a million. The report looks great!”
“Thanks for the excellent report and the insights regarding the lactose market.”
“I liked the report; would it be possible to send me the PPT version as I want to use a few slides in an internal presentation that I am preparing.”
“This report is really well done and we really appreciate it! Again, I may have questions as we dig in deeper. Thanks again for some really good work.”
“Kudos to your team. Thank you very much for your support and agility to answer our questions.”
“We appreciate you and your team taking out time to share the report and data file with us, and we are grateful for the flexibility provided to modify the document as per request. This does help us in our business decision making. We would be pleased to work with you again, and hope to continue our business relationship long into the future.”
“I want to first congratulate you on the great work done on the Medical Platforms project. Thank you so much for all your efforts.”
“Thank you very much. I really appreciate the work your team has done. I feel very comfortable recommending your services to some of the other startups that I’m working with, and will likely establish a good long partnership with you.”
“We received the below report on the U.S. market from you. We were very satisfied with the report.”
“I just finished my first pass-through of the report. Great work! Thank you!”
“Thanks again for the great work on our last partnership. We are ramping up a new project to understand the imaging and imaging service and distribution market in the U.S.”
“We feel positive about the results. Based on the presented results, we will do strategic review of this new information and might commission a detailed study on some of the modules included in the report after end of the year. Overall we are very satisfied and please pass on the praise to the team. Thank you for the co-operation!”
“Thank you very much for the very good report. I have another requirement on cutting tools, paper crafts and decorative items.”
“We are happy with the professionalism of your in-house research team as well as the quality of your research reports. Looking forward to work together on similar projects”
“We appreciate the teamwork and efficiency for such an exhaustive and comprehensive report. The data offered to us was exactly what we were looking for. Thank you!”
“I recommend Fortune Business Insights for their honesty and flexibility. Not only that they were very responsive and dealt with all my questions very quickly but they also responded honestly and flexibly to the detailed requests from us in preparing the research report. We value them as a research company worthy of building long-term relationships.”
“Well done Fortune Business Insights! The report covered all the points and was very detailed. Looking forward to work together in the future”
“It has been a delightful experience working with you guys. Thank you Fortune Business Insights for your efforts and prompt response”
“I had a great experience working with Fortune Business Insights. The report was very accurate and as per my requirements. Very satisfied with the overall report as it has helped me to build strategies for my business”
“This is regarding the recent report I bought from Fortune Business insights. Remarkable job and great efforts by your research team. I would also like to thank the back end team for offering a continuous support and stitching together a report that is so comprehensive and exhaustive”
“Please pass on our sincere thanks to the whole team at Fortune Business Insights. This is a very good piece of work and will be very helpful to us going forward. We know where we will be getting business intelligence from in the future.”
“Thank you for sending the market report and data. It looks quite comprehensive and the data is exactly what I was looking for. I appreciate the timeliness and responsiveness of you and your team.”
+1 424 253 0390 (US)
+44 2071 939123 (UK)
+91 744 740 1245 (APAC)
[email protected]
- Request Sample

Bhushan is a seasoned professional with nearly a decade of experience in consulting and market research, specializing in biotechnology, life sciences, and pharmaceuticals. His in-depth knowledge and insights have been honed through years of dedicated work, focusing on various segments, including dermatology, oncology, ophthalmology, and vaccines, among others.
With a solid foundation built during his tenure at Ajanta Pharma and Sentiss Pharma, where he spent over 4 years in sales and marketing roles, Bhushan has developed a nuanced understanding of the industry dynamics and market trends. His hands-on experience in sales and marketing has equipped him with a unique perspective that complements his strategic approach to market research and consulting. His proficiency in patent analysis and pipeline assessment adds a crucial dimension to his insights.
Throughout his career, Bhushan has led and contributed to projects for prominent companies such as Fresenius Kabi, Johnson & Johnson, Novo Nordisk, and Sanofi, delivering actionable insights that have driven growth and innovation within the pharmaceutical horizon. His track record of success and his ability to navigate complex market landscapes make him a sought-after industry expert in the field.

The global contract research organization (CRO) services market size is projected to grow from $86.33 billion in 2024 to $175.46 billion by 2032
Read More at:-
- Healthcare & Pharmaceuticals
Clinical Research Organization Market Growth & Trends [2031]
Segments - Global Clinical Research Organization (CRO) Market By Service (Clinical Research Services, Early Phase Development Services, Laboratory Services, Consulting Services, and Others), By Molecule Type (Small molecules and Biologics), By End-user (Pharmaceutical & Biotechnology Company, Research & Academic Institute, and Others), and Regions (North America, Asia Pacific, Europe, Latin America, Middle East, and Africa) - Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2023-2031
Report Description
Clinical research organization market outlook.
The global Clinical Research Organization (CRO) market was estimated at USD 80.6 Billion in 2022 and is anticipated to reach USD 220.3 Billion by 2031 , expanding at a CAGR of 12.2% during the forecast period. A clinical research organization (CRO) provides support to the pharmaceuticals, biotechnology, and medical devices industries in the form of research services outsourced on a contract basis. Clinical research organizations (CROs) play a crucial role in the development of pharmaceuticals, medical devices, and therapies. A sponsor (a business, organization, or institution) who wishes to run a clinical trial hires CROs to plan, coordinate, execute, and manage the lifecycle of the clinical trial, safely and efficiently.
CROs have the knowledge, capabilities, processes, and procedures that are needed to develop and run a successful clinical trial while ensuring trial quality and compliance with national and international standards. A CRO serves as the main contact between the sponsor and other stakeholders throughout the trial and communicates with ethics and compliance committees, regulatory personnel, vendors, physicians, and research coordinators.
Macro-economic Factors
Increasing healthcare expenditure.
Healthcare expenditure refers to the amount of money spent on healthcare goods and services, such as medical treatments, hospitalizations, medical equipment, and other healthcare-related expenses. It includes both public and private spending on healthcare, such as expenditure by governments, insurance companies, and individuals. Global spending on health more than doubled in real terms over the past two decades, reaching around US$ 8.5 trillion in 2019, or 9.8% of global GDP. Increasing healthcare spending raises the amount of funds for clinical research and development. This results in a large number of clinical trials and high demand for the services of CROs. High-income countries accounted for nearly 80% of global spending on health, with the US accounting for more than 40% alone.
Regulatory Environment
The regulatory environment has a significant impact on the clinical rese arch organization (CRO) market. Regulatory requirements for conducting clinical trials vary by country and region and considerably affect the demand for the services of CROs, the cost of conducting clinical trials, and the profitability of CROs. Government policies, such as regulations on quality standards, impact the market significantly. For instance, the Drugs and Cosmetics Act (1940) regulates the import, manufacture, and distribution of drugs and ensures that drugs and cosmetics sold are safe, effective, and conform to essential quality standards.
Clinical Research Organization Market Dynamics
Market drivers.
- Rise in the Number of Drug Development Activities
Rising Drug development activities are one of the positive factors for the clinical research organizations (CROs) market. The demand for CRO services increases drastically, as pharmaceuticals and biotechnology companies continue to invest in research and development to bring new drugs to market.
Drug development is a complex and expensive process that involves various steps, that includes drug discovery, preclinical studies, clinical trials, regulatory approval, and others. CROs offer significant cost savings to a large number of pharmaceutical companies by providing economies of scale. Drug development activities are increasing worldwide, which is a primary driving force of clinical research organizations. The demand for clinical trials to test the safety and efficacy of new drugs and medical devices is increasing, as the pharmaceutical industry continues to expand. For instance, in 2022 , Labcorp Drug Development helped to develop 100% of oncology drugs approved by the FDA (The United States Food and Drug Administration).
- Technology Advancement and Outsourcing of Clinical Trials
Outsourcing of clinical trials has become a common practice in the pharmaceutical and biotechnology industries. Clinical trials are complex and require significant resources, including specialized equipment, facilities, and personnel.
Many pharmaceuticals and biotech companies are outsourcing their clinical trial activities to clinical research organizations (CROs) to reduce costs, streamline operations, and improve efficiency. The demand for CROs increases, as more pharmaceutical companies outsource their clinical trials. This is boosting the market for these organizations.
Market Restraint
Regulatory Compliance
Regulatory compliance is an essential aspect of conducting clinical trials, and it is considered to be a significant restraint for the market. CROs are required to comply with strict regulations and guidelines set by regulatory authorities such as the FDA, EMA (The European Medicines Agency), and other national regulatory agencies.
Protecting the rights, safety, and welfare of people who participate in clinical trials is a critical aspect of the regulations set by the FDA. The organization oversees clinical trials to ensure they are designed, conducted, analyzed, and reported according to federal law and good clinical practice (GCP) regulations. Compliance with these regulations is challenging and time-consuming, which makes it difficult for CROs to compete in the market.
Scope of Clinical Research Organization Market Report
The report on the global Clinical Research Organization (CRO) market includes an assessment of the market, trends, segments, and regional markets. Overview and dynamics have also been included in the report.
|
|
|
|
| – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast |
|
| 2022 |
|
| 2016-2021 |
|
| 2023–2031 |
|
| (Clinical Research Services, Early Phase Development Services, Laboratory Services, Consulting Services, and Others); (Small molecules and Biologics); (Pharmaceutical & Biotechnology Company, Research & Academic Institute, and Others) |
|
| Asia Pacific, North America, Latin America, Europe, Middle East, and Africa |
|
| Company Share, Market Analysis and Size, Competitive Landscape, Growth Factors, and Trends, and Revenue Forecast |
|
| IQVIA Inc, LabCorp, Thermo Fisher Scientific Inc. ICON plc, Syneos Health |
Clinical Research Organization Market Segmental Outlook
On the basis of Service , the global clinical research organization (CRO) market is segmented into clinical research services, early-phase development services, laboratory services, consulting services, and others. The clinical research services segment is expected to expand at a CAGR of 12.4% from 2023 to 2031 . The clinical research services are further categorized into Phase I, Phase II, Phase III, and Phase IV. The growth of the segment is attributed to the increasing prevalence of chronic diseases and the increasing demand for effective medications & diagnostics products across the globe. Furthermore, various contract research organizations offer a wide range of clinical trial research services and support different areas of medical device & drug development. In 2020 , Parexel and Synairgen plc formed a strategic collaboration for conducting a Phase III study of an Interferon-beta (IFN-beta) treatment for COVID - 19 patients. Such strategic initiatives by CROs are expected to minimize the hindrance and boost the segment.
In terms of molecule type , the global clinical research organization (CRO) market is segmented into small molecules and biologics. The small molecules segment is expected to hold 38.5% share of the market during the forecast period, owing to the increasing number of chronic diseases. According to the British Heart Foundation's statistics in August 2022 , around 7.6 billion people in the UK suffered from some form of heart or cardiovascular disease in 2021 . Such an increase in the number of chronic diseases is expected to increase the demand for treatments, and ultimately the demand for small molecules.
Regional Outlook
On the basis of region , the global clinical research organization (CRO) market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America accounted for 40.0% share of the market in 2022 . This is attributed to the presence of major pharmaceutical companies in the region and their widespread drug development activity and excellent healthcare infrastructure. Additionally, several key pharmaceutical companies in the region are outsourcing R&D and clinical trials, due to the changes in competition and reimbursement from generic drugs. This is boosting the Clinical Research Organization (CRO) market in North America.
Key Benefits for Industry Participants & Stakeholders
- In-depth Analysis of the Global Clinical Research Organization (CRO) Market
- Historical, Current, and Projected Market Size in Terms of Value
- Potential & Niche Segments and Regions Exhibiting Promising Growth Covered
- Industry Drivers, Restraints, and Opportunities Covered in the Study
- Recent Industry Trends and Developments
- Competitive Landscape & Strategies of Key Players
- Neutral Perspective on Global Clinical Research Organization (CRO) Market Performance
- Clinical Research Services
- Early Phase Development Services
- Laboratory Services
- Consulting Services
By Molecule Type
- Small molecules
By End-user
- Pharmaceutical & Biotechnology Company
- Research & Academic Institute
- North America
- Asia Pacific
- Latin America
- Middle East & Africa
Key Market Players Profiled in the Report
- Asymchem Laboratories (Tianjin) Co., Ltd
- Charles River Laboratories.
- Dalton Pharma Services
- Eurofins Scientific
- Frontage Holdings Corporation
- Hangzhou Tigermed Consulting Co., Ltd.
- Jubilant Pharmova Limited
- Medpace, Inc.
- Parexel International Corporation
- Pharmaron Beijing Co., Ltd.
- Piramal Enterprises Ltd.
- REPROCELL Inc.
- Sun Pharmaceutical Industries Ltd.
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec
Competitive Landscape
- Manufacturers operating in the global Clinical Research Organization (CRO) market include Asymchem Laboratories (Tianjin) Co., Ltd, Charles River Laboratories., Dalton Pharma Services, Domainex, Eurofins Scientific, Evotec, Frontage Holdings Corporation, Genscript, Hangzhou Tigermed Consulting Co., Ltd., ICON plc, Inotiv, IQVIA Inc, Jubilant Pharmova Limited, LabCorp, Medpace, Inc., Parexel International Corporation, Pharmaron Beijing Co., Ltd., Piramal Enterprises Ltd, REPROCELL Inc., SGS, Sun Pharmaceutical Industries Ltd., Syneos Health, Thermo Fisher Scientific Inc., WuXi AppTec.
- Market players are pursuing key strategies such as acquisitions, collaborations, and geographic expansion to increase their market share.
Frequently Asked Questions
1. what is the major end-user of clinical research organization (cro) that driving the market growth.
The research & academic institute segment held a market share of 33.3% in 2022 and is anticipated to expand at a CAGR of 12.2% during the forecast period. It is typically responsible for scientific research for the advancement of new drugs.
2. Who are the major players operating in the market?
Major manufacturers include IQVIA Inc, LabCorp, Thermo Fisher Scientific Inc., ICON plc, and Syneos Health are among the key players that hold a major chunk of the market.
3. Can I ask for different company profiles?
Additional company profiles are provided on request
4. What are the criteria used for selecting a company profile?
Factors such as competitive strength and market positioning are key areas considered while selecting key companies to be profiled.
5. What are the factors driving the Clinical Research Organization (CRO) market?
The rise in the number of drug development activities, technology advancement and outsourcing of clinical trials are the key driving factors for the market.
6. How big will be the Clinical Research Organization (CRO) market in 2030?
According to this Growth Market Reports report, the Clinical Research Organization (CRO) market is expected to register a CAGR of 12.3 % during the forecast period, 2015-2030, with an anticipated valuation of USD 220.3 Billion by the end of 2030.
7. Which macroeconomic factors affect the market?
Economic Condition, Increasing Healthcare Expenditure, Advancement in Technology, and Regulatory Environment are key macroeconomic factors shaping the market during the forecast period.
8. What is the impact of COVID-19 on the overall market during 2019 -2020?
The pandemic has positive impact on the global Clinical Research Organization (CRO) market. The COVID-19 outbreak spread worldwide, increase in number of clinical research activities has boost the demand of Clinical Research Organization (CRO).
9. What additional data analysis is available in a report?
In addition to market size (in USD Billion), the company market share (in % for the base year 2022), the impact of key regulations, current & future trends, and reimbursement scenario overview: private and public healthcare institutions by region have been provided.
10. What is the base year calculated in the global Clinical Research Organization (CRO) market report and what is the analysis period?
The base year considered for the global Clinical Research Organization (CRO) market report is 2021. The complete analysis period is 2016 to 2031, wherein, 2016 to 2021 are the historic years, and the forecast is provided from 2023 to 2031.
Table Of Content
Methodology, related reports.
Some other reports from this category!
Drugs for Herpes Labialis (Oral Herpes) Market Trends [...
Dengue treatment market size, share & growth report | 2..., mmr vaccine market trends, growth, industry & revenue [..., acromegaly treatment market size, share & growth report..., teenager myopia control market size, share & industry |..., cannabidiol (cbd) market share, opportunities, industry..., prosthetic joint infections treatment market forecast [..., cannabis market size, share, growth & industry trends [..., heart valve replacement market size, share & industry |..., ibuprofen market size, share, industry & opportunities ....
Our Clients

- Request Sample

- Pharmaceuticals
Clinical Research Organization Market
Global Clinical Research Organization Market Size, Trends & Analysis - Forecasts to 2028 By Type (Drug Discovery, Pre-clinical, and Clinical), By Service (Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/ Assurance, Bio-statistics, Investigator Payments, Laboratory, Patient and site Recruitment, Technology, and Others), By Application (Oncology, Cardiovascular, Autoimmune/Inflammation, Central nervous system (CNS), Dermatology, Infectious diseases, Diabetes, Pain, and Others), By End User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, and Others), and By Region (North America, Asia Pacific, Central & South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
- Report Summary
- Table of Content
- Research Methodology
- Customize this research

Global Clinical Research Organization Market Size
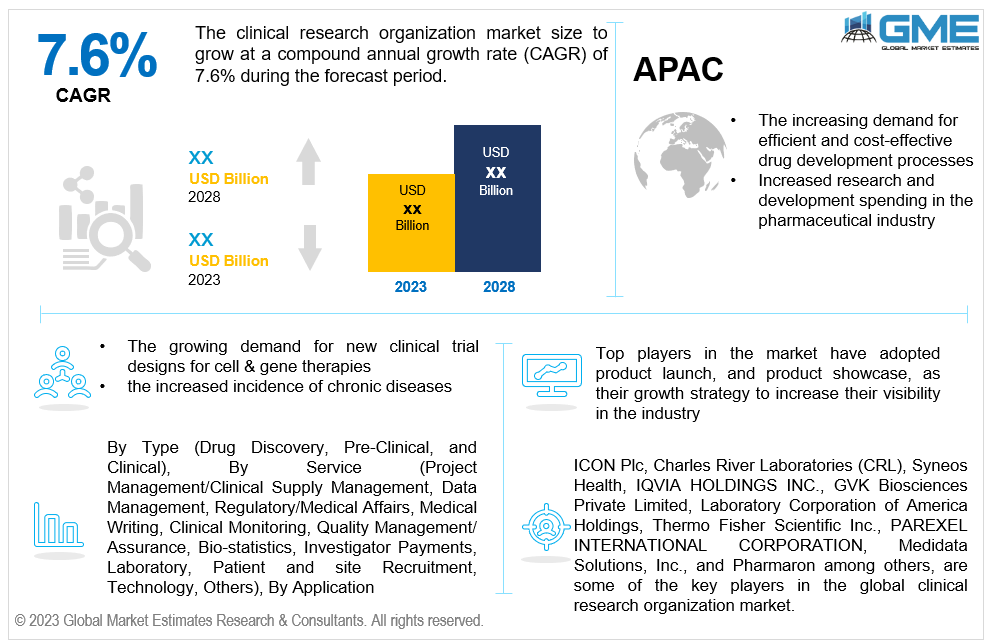
The global clinical research organization market is projected to grow at a CAGR of 7.6% from 2023 to 2028.
Clinical research organizations (CROs) offer outsourced services to the biotechnology and pharmaceutical sectors. They carry out clinical studies for novel medications and treatments. Drug firms themselves or academic institutions doing the trials can hire CROs. CROs are created to save expenses for businesses creating novel medications and pharmaceuticals for specialized markets. They want to make the medication market entrance and development simpler because it is no longer necessary for huge pharmaceutical corporations to handle everything "in-house”. These organizations offer services that can speed up the whole process of bringing a novel technology or medication to market, from concept creation through FDA marketing approval, without the requirement for the drug's sponsor to keep any people on staff to deliver these services. The pharmaceutical and drug development companies devote a lot of resources and funding to CROs supporting more globally coordinated and collaborative research activities as a result of the growing priority placed on research and development. This is the rationale behind the rising importance of strategic alliances in the value chain for novel medication development.
The increasing demand for efficient and cost-effective drug development processes, increased research and development spending in the pharmaceutical industry, and the growing demand for new clinical trial designs for cell & gene therapies are expected to support the growth of the market during the forecast period. Additionally, the market is expanding as a result of the increased incidence of chronic diseases, the high cost of in-house drug research, the focus on rare diseases, and the numerous orphan medications in development.
CROs play a crucial role in clinical trials as they have plenty of opportunities in the fast-expanding therapeutic disciplines of oncology, neurology, cardiology, and vaccines. To increase patient involvement, study compliance, and continuous monitoring, CROs can include telemedicine and digital health technologies in clinical trials, which presents an opportunity for the major players in the global market. However, CROs continue to face a various challenges, such as commoditization, intense competition, and cutting-edge patient engagement models. Due to market segmentation and larger businesses capturing a larger share of the market, smaller CROs are under pressure.
Well-established companies these areas can provide pharmaceutical and biotechnology companies with specialized services and support for both real-world research and virtual studies, which can reduce the length and cost of the trial. They may now also provide specialized services in data analytics and machine learning due to the increasing accessibility of real-time data in clinical trials. To do this, one may use predictive analytics to anticipate risks, identify trends, and enhance research design, which contributes to the expansion of the global market.
Digital therapies are software-based treatments intended to treat or manage medical diseases. CROs have become more involved in the development and assessment of these interventions. To improve patient results, these therapies are frequently utilized in conjunction with conventional pharmacological techniques. CROs were using big data and sophisticated analytics approaches to glean valuable insights from vast amounts of clinical and patient data. This support better decision-making, trend spotting, and trial protocol optimization.
Global Clinical Research Organization Market: By Type
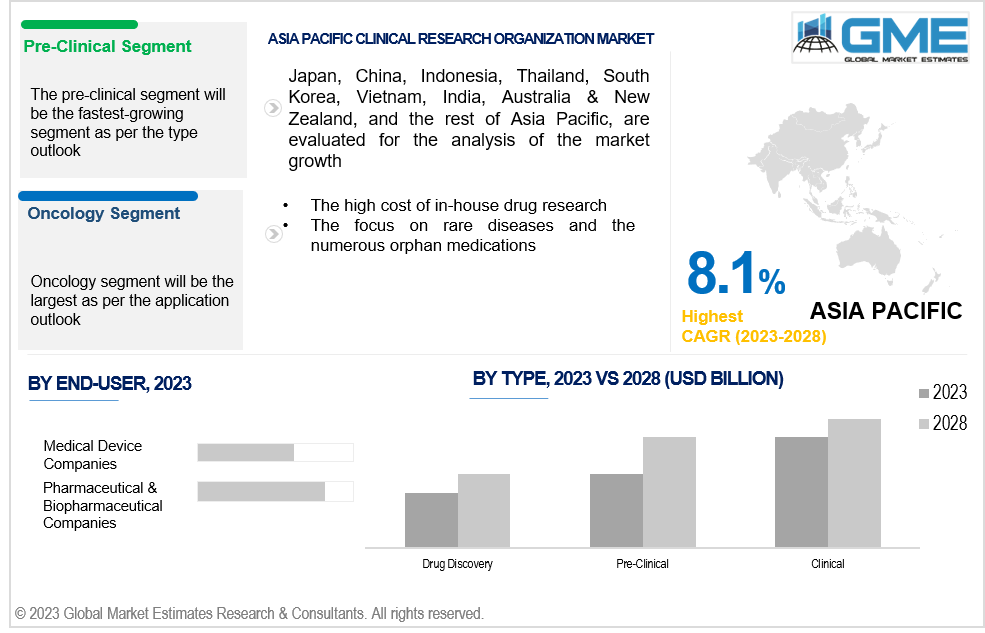
The pre-clinical is expected to be the fastest-growing segment in the market during the forecast period. Leading CROs have amassed a large amount of pre-clinical experience, and CROs are utilizing this expertise to provide highly effective and precise pre-clinical services. Additionally, CROs let small and midsize businesses participate in the difficult drug development process without having to make a substantial investment in capital equipment. Pre-clinical trials are more successful, according to an article by Anju Life Sciences Software that was published in March 2021, due to higher data quality, better safety judgments, lower trial operating expenses, and quicker study execution.
The clinical segment holds the largest share of the market during the forecast period. The segment is expanding due to the rising incidence of chronic diseases and the rising demand for rapid and precise disease diagnostics.
Global Clinical Research Organization Market: By Service
Data management is anticipated to be the fastest-growing segment in the market during the forecast period. To collect data from clinical trial locations, data management entails using electronic data capture (EDC) systems or using alternative data gathering techniques. This can entail making case report forms (CRFs) and guaranteeing that the information gathered conforms with legal requirements.
The project management/clinical supply management segment holds the largest share of the market. Clinical trial design, coordination, and execution are the main goals of these services, which also make sure that clinical supplies are available and managed appropriately. In the CRO industry, project management services entail supervising every component of a clinical study to guarantee its proper execution within specified schedules and financial restrictions. Clinical supply management services prioritize the prompt and effective provision of research medications, medical equipment, and other materials needed for the clinical trial.
Global Clinical Research Organization Market: By Application
The central nervous system (CNS) is anticipated to be the fastest-growing segment in the market during the forecast period. CROs are essential to the management of clinical trials for CNS illnesses including Parkinson's, Alzheimer's, and other similar conditions. They offer knowledge in protocol formulation, location choice, patient recruiting, and trial administration as a whole.
The oncology segment holds the largest share of the market. Preclinical research, clinical trials, and other stages of the drug development process are all supported by a variety of services offered by oncology CROs. Since there are many different medications being developed in the cancer industry, oncology CROs need to have a thorough understanding of the disease process and cutting-edge therapies. Additionally, they must be able to plan and carry out clinical studies that adhere to the exacting requirements of the FDA and other authorities.
Global Clinical Research Organization Market: By End User
The medical device companies is anticipated to be the fastest-growing segment in the market during the forecast period. Clinical trials for new or enhanced medical devices are carried out by CROs on behalf of medical device manufacturers. CROs offer competence in patient recruiting, data management, regulatory compliance, research design, and statistical analysis. They aid manufacturers of medical devices in navigating the challenging world of clinical research and support the efficient and successful conduct of clinical studies.
The pharmaceutical & biopharmaceutical companies segment holds the largest share of the market. Due to the complicated nature of the drug development process, pharmaceutical companies are increasingly depending on CROs for drug research and development services. In order to cut costs and speed up time to market, biotechnology companies are also outsourcing their R&D operations to CROs. Thus, it is anticipated that increasing investment in R&D spending would fuel this segment's growth throughout the forecast period.
Global Clinical Research Organization Market: By Region
Regionally, North America is projected to hold the leading position in the global region in the market. This is due to the region conducting a rising number of clinical trials as well as the existence of several CROs in nations like the United States and Canada. CRO companies are in great demand throughout the region as the desire to outsource R&D operations grows.
Asia Pacific is predicted to have rapid growth during the forecast period. This can be attributed to elements including rising healthcare costs, expanding government funding for clinical research, and an increase in the number of people with chronic illnesses. Clinical trials are being conducted in China at a higher rate thanks to the government's and China's Food and Drug Administration's regulatory flexibility.
Europe market is anticipated to see stable market growth throughout the forecast period due to the favorable reimbursement policies for clinical trials and the rising number of biopharmaceutical businesses.
Global Clinical Research Organization Market Share and Competitor Analysis
Some of the key players in the global clinical research organization market are ICON Plc, Charles River Laboratories (CRL), Syneos Health , IQVIA HOLDINGS INC., GVK Biosciences Private Limited, Laboratory Corporation of America Holdings, Thermo Fisher Scientific Inc., PAREXEL INTERNATIONAL CORPORATION, Medidata Solutions, Inc., and Pharmaron among others..
Please note: This is not an exhaustive list of companies profiled in the report.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
2.4 Data Metrics on Feed Stocks
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY TYPE
4.1 Introduction
4.2 Clinical Research Organization Market: Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2022 & 2028
4.4 Drug Discovery
4.4.1 Drug Discovery Market Estimates and Forecast, 2020-2028 (USD Million)
4.5 Pre-Clinical
4.5.1 Pre-Clinical Market Estimates and Forecast, 2020-2028 (USD Million)
4.6 Clinical
4.6.1 Clinical Market Estimates and Forecast, 2020-2028 (USD Million)
5 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY SERVICE
5.1 Introduction
5.2 Clinical Research Organization Market: Service Scope Key Takeaways
5.3 Revenue Growth Analysis, 2022 & 2028
5.4 Project Management/Clinical Supply Management
5.4.1 Project Management/Clinical Supply Management Market Estimates and Forecast, 2020-2028 (USD Million)
5.5 Data Management
5.5.1 Data Management Market Estimates and Forecast, 2020-2028 (USD Million)
5.6 Regulatory/Medical Affairs
5.6.1 Regulatory/Medical Affairs Market Estimates and Forecast, 2020-2028 (USD Million)
5.7 Medical Writing
5.7.1 Medical Writing Market Estimates and Forecast, 2020-2028 (USD Million)
5.8 Clinical Monitoring
5.8.1 Clinical Monitoring Market Estimates and Forecast, 2020-2028 (USD Million)
5.9 Quality Management/ Assurance
5.9.1 Quality Management/ Assurance Market Estimates and Forecast, 2020-2028 (USD Million)
5.10 Bio-statistics
5.10.1 Bio-statistics Market Estimates and Forecast, 2020-2028 (USD Million)
5.11 Investigator Payments
5.11.1 Investigator Payments Market Estimates and Forecast, 2020-2028 (USD Million)
5.12 Laboratory
5.12.1 Laboratory Market Estimates and Forecast, 2020-2028 (USD Million)
5.13 Patient and site Recruitment
5.13.1 Patient and site Recruitment Market Estimates and Forecast, 2020-2028 (USD Million)
5.14 Technology
5.14.1 Technology Market Estimates and Forecast, 2020-2028 (USD Million)
5.15 Others
5.15.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
6 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY APPLICATION
6.1 Introduction
6.2 Clinical Research Organization Market: Application Scope Key Takeaways
6.3 Revenue Growth Analysis, 2022 & 2028
6.4 Oncology
6.4.1 Oncology Market Estimates and Forecast, 2020-2028 (USD Million)
6.5 Cardiovascular
6.5.1 Cardiovascular Market Estimates and Forecast, 2020-2028 (USD Million)
6.6 Autoimmune/Inflammation
6.6.1 Autoimmune/Inflammation Market Estimates and Forecast, 2020-2028 (USD Million)
6.7 Central nervous system (CNS)
6.7.1 Central nervous system (CNS) Market Estimates and Forecast, 2020-2028 (USD Million)
6.8 Dermatology
6.8.1 Dermatology Market Estimates and Forecast, 2020-2028 (USD Million)
6.9 Infectious diseases
6.9.1 Infectious diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.10 Diabetes
6.10.1 Diabetes Market Estimates and Forecast, 2020-2028 (USD Million)
6.11.1 Pain Market Estimates and Forecast, 2020-2028 (USD Million)
6.12 Others
6.12.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
7 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY END USER
7.1 Introduction
7.2 Clinical Research Organization Market: End User Scope Key Takeaways
7.3 Revenue Growth Analysis, 2022 & 2028
7.4 Pharmaceutical & Biopharmaceutical Companies
7.4.1 Pharmaceutical & Biopharmaceutical Companies Market Estimates and Forecast, 2020-2028 (USD Million)
7.5 Medical Device Companies
7.5.1 Medical Device Companies Market Estimates and Forecast, 2020-2028 (USD Million)
7.6 Others
7.6.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
8 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY REGION
8.1 Introduction
8.2 North America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.1 By Type
8.2.2 By Service
8.2.3 By Application
8.2.4 By End User
8.2.5 By Country
8.2.5.1 U.S. Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.1.1 By Type
8.2.5.1.2 By Service
8.2.5.1.3 By Application
8.2.5.1.4 By End User
8.2.5.2 Canada Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.2.1 By Type
8.2.5.2.2 By Service
8.2.5.2.3 By Application
8.2.5.2.4 By End User
8.2.5.3 Mexico Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.3.1 By Type
8.2.5.3.2 By Service
8.2.5.3.3 By Application
8.2.5.3.4 By End User
8.3 Europe Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.1 By Type
8.3.2 By Service
8.3.3 By Application
8.3.4 By End User
8.3.5 By Country
8.3.5.1 Germany Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.1.1 By Type
8.3.5.1.2 By Service
8.3.5.1.3 By Application
8.3.5.1.4 By End User
8.3.5.2 U.K. Presered Flowers Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.2.1 By Type
8.3.5.2.2 By Service
8.3.5.2.3 By Application
8.3.5.2.4 By End User
8.3.5.3 France Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.3.1 By Type
8.3.5.3.2 By Service
8.3.5.3.3 By Application
8.3.5.3.4 By End User
8.3.5.4 Italy Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.4.1 By Type
8.3.5.4.2 By Service
8.3.5.4.3 By Application
8.3.5.4.4 By End User
8.3.5.5 Spain Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.5.1 By Type
8.3.5.5.2 By Service
8.3.5.5.3 By Application
8.3.5.5.4 By End User
8.3.5.6 Netherlands Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.6.1 By Type
8.3.5.6.2 By Service
8.3.5.6.3 By Application
8.3.5.6.4 By End User
8.3.5.7 Rest of Europe Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.7.1 By Type
8.3.5.7.2 By Service
8.3.5.7.3 By Application
8.3.5.7.4 By End User
8.4 Asia Pacific Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.1 By Type
8.4.2 By Service
8.4.3 By Application
8.4.4 By End User
8.4.5 By Country
8.4.5.1 China Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.1.1 By Type
8.4.5.1.2 By Service
8.4.5.1.3 By Application
8.4.5.1.4 By End User
8.4.5.2 Japan Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.2.1 By Type
8.4.5.2.2 By Service
8.4.5.2.3 By Application
8.4.5.2.4 By End User
8.4.5.3 India Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.3.1 By Type
8.4.5.3.2 By Service
8.4.5.3.3 By Application
8.4.5.3.4 By End User
8.4.5.4 South Korea Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.4.1 By Type
8.4.5.4.2 By Service
8.4.5.4.3 By Application
8.4.5.4.4 By End User
8.4.5.5 Singapore Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.5.1 By Type
8.4.5.5.2 By Service
8.4.5.5.3 By Application
8.4.5.5.4 By End User
8.4.5.6 Malaysia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.6.1 By Type
8.4.5.6.2 By Service
8.4.5.6.3 By Application
8.4.5.6.4 By End User
8.4.5.7 Thailand Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.7.1 By Type
8.4.5.7.2 By Service
8.4.5.7.3 By Application
8.4.5.7.4 By End User
8.4.5.8 Indonesia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.8.1 By Type
8.4.5.8.2 By Service
8.4.5.8.3 By Application
8.4.5.8.4 By End User
8.4.5.9 Vietnam Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.9.1 By Type
8.4.5.9.2 By Service
8.4.5.9.3 By Application
8.4.5.9.4 By End User
8.4.5.10 Taiwan Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.10.1 By Type
8.4.5.10.2 By Service
8.4.5.10.3 By Application
8.4.5.10.4 By End User
8.4.5.11 Rest of Asia Pacific Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.11.1 By Type
8.4.5.11.2 By Service
8.4.5.11.3 By Application
8.4.5.11.4 By End User
8.5 Middle East and Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.1 By Type
8.5.2 By Service
8.5.3 By Application
8.5.4 By End User
8.5.5 By Country
8.5.5.1 Saudi Arabia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.1.1 By Type
8.5.5.1.2 By Service
8.5.5.1.3 By Application
8.5.5.1.4 By End User
8.5.5.2 U.A.E. Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.2.1 By Type
8.5.5.2.2 By Service
8.5.5.2.3 By Application
8.5.5.2.4 By End User
8.5.5.3 Israel Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.4.3.1 By Type
8.5.4.3.2 By Service
8.5.4.3.3 By Application
8.5.5.3.4 By End User
8.5.5.4 South Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.4.1 By Type
8.5.5.4.2 By Service
8.5.5.4.3 By Application
8.5.5.4.4 By End User
8.5.5.5 Rest of Middle East and Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.5.1 By Type
8.5.5.5.2 By Service
8.5.5.5.2 By Application
8.5.5.5.4 By End User
8.6 Central & South America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.1 By Type
8.6.2 By Service
8.6.3 By Application
8.6.4 By End User
8.6.5 By Country
8.6.5.1 Brazil Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.1.1 By Type
8.6.5.1.2 By Service
8.6.5.1.3 By Application
8.6.5.1.4 By End User
8.6.5.2 Argentina Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.2.1 By Type
8.6.5.2.2 By Service
8.6.5.2.3 By Application
8.6.5.2.4 By End User
8.6.5.3 Chile Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.3.1 By Type
8.6.5.3.2 By Service
8.6.5.3.3 By Application
8.6.5.5.4 By End User
8.6.5.4 Rest of Central & South America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.4.1 By Type
8.6.5.4.2 By Service
8.6.5.4.3 By Application
8.6.5.4.4 By End User
9 COMPETITIVE LANDCAPE
9.1 Company Market Share Analysis
9.2 Four Quadrant Positioning Matrix
9.2.1 Market Leaders
9.2.2 Market Visionaries
9.2.3 Market Challengers
9.2.4 Niche Market Players
9.3 Vendor Landscape
9.3.1 North America
9.3.2 Europe
9.3.3 Asia Pacific
9.3.4 Rest of the World
9.4 Company Profiles
9.4.1 ICON Plc
9.4.1.1 Business Description & Financial Analysis
9.4.1.2 SWOT Analysis
9.4.1.3 Products & Services Offered
9.4.1.4 Strategic Alliances between Business Partners
9.4.2 Charles River Laboratories (CRL)
9.4.2.1 Business Description & Financial Analysis
9.4.2.2 SWOT Analysis
9.4.2.3 Products & Services Offered
9.4.2.4 Strategic Alliances between Business Partners
9.4.3 Syneos Health
9.4.3.1 Business Description & Financial Analysis
9.4.3.2 SWOT Analysis
9.4.3.3 Products & Services Offered
9.4.3.4 Strategic Alliances between Business Partners
9.4.4 IQVIA HOLDINGS INC.
9.4.4.1 Business Description & Financial Analysis
9.4.4.2 SWOT Analysis
9.4.4.3 Products & Services Offered
9.4.4.4 Strategic Alliances between Business Partners
9.4.5 Laboratory Corporation of America Holdings
9.4.5.1 Business Description & Financial Analysis
9.4.5.2 SWOT Analysis
9.4.5.3 Products & Services Offered
9.4.5.4 Strategic Alliances between Business Partners
9.4.6 THERMO FISHER SCIENTIFIC INC.
9.4.6.1 Business Description & Financial Analysis
9.4.6.2 SWOT Analysis
9.4.6.3 Products & Services Offered
9.4.6.4 Strategic Alliances between Business Partners
9.4.7 PAREXEL INTERNATIONAL CORPORATION
9.4.7.1 Business Description & Financial Analysis
9.4.7.2 SWOT Analysis
9.4.7.3 Products & Services Offered
9.4.8.4 Strategic Alliances between Business Partners
9.4.8 Medidata Solutions, Inc.
9.4.8.1 Business Description & Financial Analysis
9.4.8.2 SWOT Analysis
9.4.8.3 Products & Services Offered
9.4.9 Pharmaron
9.4.9.1 Business Description & Financial Analysis
9.4.9.2 SWOT Analysis
9.4.9.3 Products & Services Offered
9.4.9.4 Strategic Alliances between Business Partners
9.4.10 GVK Biosciences Private Limited
9.4.10.1 Business Description & Financial Analysis
9.4.10.2 SWOT Analysis
9.4.10.3 Products & Services Offered
9.4.10.4 Strategic Alliances between Business Partners
9.4.11 Other Companies
9.4.11.1 Business Description & Financial Analysis
9.4.11.2 SWOT Analysis
9.4.11.3 Products & Services Offered
9.4.11.4 Strategic Alliances between Business Partners
10 RESEARCH METHODOLOGY
10.1 Market Introduction
10.1.1 Market Definition
10.1.2 Market Scope & Segmentation
10.2 Information Procurement
10.2.1 Secondary Research
10.2.1.1 Purchased Databases
10.2.1.2 GMEs Internal Data Repository
10.2.1.3 Secondary Resources & Third Party Perspectives
10.2.1.4 Company Information Sources
10.2.2 Primary Research
10.2.2.1 Various Service of Respondents for Primary Interviews
10.2.2.2 Number of Interviews Conducted throughout the Research Process
10.2.2.3 Primary Stakeholders
10.2.2.4 Discussion Guide for Primary Participants
10.2.3 Expert Panels
10.2.3.1 Expert Panels Across 30+ Industry
10.2.4 Paid Local Experts
10.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
10.3 Market Estimation
10.3.1 Top-Down Approach
10.3.1.1 Macro-Economic Indicators Considered
10.3.1.2 Micro-Economic Indicators Considered
10.3.2 Bottom Up Approach
10.3.2.1 Company Share Analysis Approach
10.3.2.2 Estimation of Potential Product Sales
10.4 Data Triangulation
10.4.1 Data Collection
10.4.2 Time Series, Cross Sectional & Panel Data Analysis
10.4.3 Cluster Analysis
10.5 Analysis and Output
10.5.1 Inhouse AI Based Real Time Analytics Tool
10.5.2 Output From Desk & Primary Research
10.6 Research Assumptions & Limitations
10.7.1 Research Assumptions
10.7.2 Research Limitations
LIST OF TABLES
1 Global Clinical Research Organization Market, By Type, 2020-2028 (USD Mllion)
2 Drug Discovery Market, By Region, 2020-2028 (USD Mllion)
3 Pre-Clinical Market, By Region, 2020-2028 (USD Mllion)
4 Clinical Market, By Region, 2020-2028 (USD Mllion)
5 Global Clinical Research Organization Market, By Service, 2020-2028 (USD Mllion)
6 Project Management/Clinical Supply Management Market, By Region, 2020-2028 (USD Mllion)
7 Data Management Market, By Region, 2020-2028 (USD Mllion)
8 Regulatory/Medical Affairs Market, By Region, 2020-2028 (USD Mllion)
9 Medical Writing Market, By Region, 2020-2028 (USD Mllion)
10 Clinical Monitoring Market, By Region, 2020-2028 (USD Mllion)
11 Quality Management/ Assurance Market, By Region, 2020-2028 (USD Mllion)
12 Bio-statistics Market, By Region, 2020-2028 (USD Mllion)
13 Investigator Payments Market, By Region, 2020-2028 (USD Mllion)
14 Laboratory Market, By Region, 2020-2028 (USD Mllion)
15 Patient and site Recruitment Market, By Region, 2020-2028 (USD Mllion)
16 Technology Market, By Region, 2020-2028 (USD Mllion)
17 Others Market, By Region, 2020-2028 (USD Mllion)
18 Global Clinical Research Organization Market, By Application, 2020-2028 (USD Mllion)
19 Oncology Market, By Region, 2020-2028 (USD Mllion)
20 Cardiovascular Market, By Region, 2020-2028 (USD Mllion)
21 Autoimmune/Inflammation Market, By Region, 2020-2028 (USD Mllion)
22 Central nervous system (CNS) Market, By Region, 2020-2028 (USD Mllion)
23 Dermatology Market, By Region, 2020-2028 (USD Mllion)
24 Infectious diseases Market, By Region, 2020-2028 (USD Mllion)
25 Diabetes Market, By Region, 2020-2028 (USD Mllion)
26 Pain Market, By Region, 2020-2028 (USD Mllion)
27 Others Market, By Region, 2020-2028 (USD Mllion)
28 Global Clinical Research Organization Market, By END USER, 2020-2028 (USD Mllion)
29 Pharmaceutical & Biopharmaceutical Companies Market, By Region, 2020-2028 (USD Mllion)
30 Medical Device Companies Market, By Region, 2020-2028 (USD Mllion)
31 Others Market, By Region, 2020-2028 (USD Mllion)
32 Regional Analysis, 2020-2028 (USD Mllion)
33 North America Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
34 North America Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
35 North America Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
36 North America Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
37 North America Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
38 U.S Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
39 U.S Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
40 U.S Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
41 U.S Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
42 Canada Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
43 Canada Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
44 Canada Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
45 CANADA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
46 Mexico Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
47 Mexico Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
48 Mexico Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
49 mexico Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
50 Europe Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
51 Europe Clinical Research Organization Market, By service, 2020-2028 (USD Million)
52 Europe Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
53 europe Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
54 europe Clinical Research Organization Market, By country, 2020-2028 (USD Million)
55 Germany Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
56 Germany Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
57 Germany Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
58 germany Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
59 UK Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
60 UK Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
61 UK Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
62 U.kClinical Research Organization Market, By End User, 2020-2028 (USD Million)
63 France Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
64 France Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
65 France Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
66 france Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
67 Italy Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
68 Italy Clinical Research Organization Market, By T Service Type, 2020-2028 (USD Million)
69 Italy Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
70 italy Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
71 Spain Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
72 Spain Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
73 Spain Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
74 spain Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
75 Rest Of Europe Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
76 Rest Of Europe Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
77 Rest of Europe Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
78 REST OF EUROPE Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
79 Asia Pacific Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
80 Asia Pacific Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
81 Asia Pacific Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
82 asia Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
83 Asia Pacific Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
84 China Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
85 China Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
86 China Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
87 china Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
88 India Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
89 India Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
90 India Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
91 india Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
92 Japan Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
93 Japan Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
94 Japan Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
95 japan Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
96 South Korea Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
97 South Korea Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
98 South Korea Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
99 south korea Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
100 Middle East and Africa Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
101 Middle East and Africa Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
102 Middle East and Africa Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
103 MIDDLE EAST and AFRICA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
104 Middle East and Africa Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
105 Saudi Arabia Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
106 Saudi Arabia Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
107 Saudi Arabia Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
108 saudi arabia Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
109 UAE Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
110 UAE Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
111 UAE Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
112 uae Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
113 Central & South America Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
114 Central & South America Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
115 Central & South America Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
116 CENTRAL & SOUTH AMERICA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
117 Central & South America Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
118 Brazil Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
119 Brazil Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
120 Brazil Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
121 brazil Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
122 ICON Plc: Products & Services Offering
123 Charles River Laboratories (CRL): Products & Services Offering
124 Syneos Health: Products & Services Offering
125 IQVIA HOLDINGS INC.: Products & Services Offering
126 Laboratory Corporation of America Holdings: Products & Services Offering
127 THERMO FISHER SCIENTIFIC INC.: Products & Services Offering
128 PAREXEL INTERNATIONAL CORPORATION : Products & Services Offering
129 Medidata Solutions, Inc.: Products & Services Offering
130 Pharmaron, Inc: Products & Services Offering
131 GVK Biosciences Private Limited: Products & Services Offering
132 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Clinical Research Organization Market Overview
2 Global Clinical Research Organization Market Value From 2020-2028 (USD Mllion)
3 Global Clinical Research Organization Market Share, By Type (2022)
4 Global Clinical Research Organization Market Share, By Service (2022)
5 Global Clinical Research Organization Market Share, By Application (2022)
6 Global Clinical Research Organization Market Share, By End User (2022)
7 Global Clinical Research Organization Market, By Region (Asia Pacific Market)
8 Technological Trends In Global Clinical Research Organization Market
9 Four Quadrant Competitor Positioning Matrix
10 Impact Of Macro & Micro Indicators On The Market
11 Impact Of Key Drivers On The Global Clinical Research Organization Market
12 Impact Of Challenges On The Global Clinical Research Organization Market
13 Porter’s Five Forces Analysis
14 Global Clinical Research Organization Market: By Type Scope Key Takeaways
15 Global Clinical Research Organization Market, By Type Segment: Revenue Growth Analysis
16 Drug Discovery Market, By Region, 2020-2028 (USD Mllion)
17 Pre-Clinical Market, By Region, 2020-2028 (USD Mllion)
18 Clinical Market, By Region, 2020-2028 (USD Mllion)
19 Global Clinical Research Organization Market: By Service Scope Key Takeaways
20 Global Clinical Research Organization Market, By Service Segment: Revenue Growth Analysis
21 Project Management/Clinical Supply Management Market, By Region, 2020-2028 (USD Mllion)
22 Data Management Market, By Region, 2020-2028 (USD Mllion)
23 Regulatory/Medical Affairs Market, By Region, 2020-2028 (USD Mllion)
24 Medical Writing Market, By Region, 2020-2028 (USD Mllion)
25 Clinical Monitoring Market, By Region, 2020-2028 (USD Mllion)
26 Quality Management/ Assurance Market, By Region, 2020-2028 (USD Mllion)
27 Bio-statistics Market, By Region, 2020-2028 (USD Mllion)
28 Investigator Payments Market, By Region, 2020-2028 (USD Mllion)
29 Laboratory Market, By Region, 2020-2028 (USD Mllion)
30 Patient and site Recruitment Market, By Region, 2020-2028 (USD Mllion)
31 Technology Market, By Region, 2020-2028 (USD Mllion)
32 Others Market, By Region, 2020-2028 (USD Mllion)
33 Global Clinical Research Organization Market: By Application Scope Key Takeaways
34 Global Clinical Research Organization Market, By Application Segment: Revenue Growth Analysis
35 Oncology Market, By Region, 2020-2028 (USD Mllion)
36 Cardiovascular Market, By Region, 2020-2028 (USD Mllion)
37 Autoimmune/Inflammation Market, By Region, 2020-2028 (USD Mllion)
38 Central nervous system (CNS) Market, By Region, 2020-2028 (USD Mllion)
39 Dermatology Market, By Region, 2020-2028 (USD Mllion)
40 Infectious diseases Market, By Region, 2020-2028 (USD Mllion)
41 Diabetes Market, By Region, 2020-2028 (USD Mllion)
42 Pain Market, By Region, 2020-2028 (USD Mllion)
43 Others Market, By Region, 2020-2028 (USD Mllion)
44 Global Clinical Research Organization Market: By End User Scope Key Takeaways
45 Global Clinical Research Organization Market, By End User Segment: Revenue Growth Analysis
46 Pharmaceutical & Biopharmaceutical Companies Market, By Region, 2020-2028 (USD Mllion)
47 Medical Device Companies Market, By Region, 2020-2028 (USD Mllion)
48 Others Market, By Region, 2020-2028 (USD Mllion)
49 Regional Segment: Revenue Growth Analysis
50 Global Clinical Research Organization Market: Regional Analysis
51 North America Clinical Research Organization Market Overview
52 North America Clinical Research Organization Market, By Type
53 North America Clinical Research Organization Market, By Service
54 North America Clinical Research Organization Market, By Application
55 North America Clinical Research Organization Market, By End User
56 North America Clinical Research Organization Market, By Country
57 U.S. Clinical Research Organization Market, By Type
58 U.S. Clinical Research Organization Market, By Service
59 U.S. Clinical Research Organization Market, By Application
60 U.S. Clinical Research Organization Market, By End User
61 Canada Clinical Research Organization Market, By Type
62 Canada Clinical Research Organization Market, By Service
63 Canada Clinical Research Organization Market, By Application
64 Canada Clinical Research Organization Market, By End User
65 Mexico Clinical Research Organization Market, By Type
66 Mexico Clinical Research Organization Market, By Service
67 Mexico Clinical Research Organization Market, By Application
68 Mexico Clinical Research Organization Market, By End User
69 Four Quadrant Positioning Matrix
70 Company Market Share Analysis
71 ICON Plc: Company Snapshot
72 ICON Plc: SWOT Analysis
73 ICON Plc: Geographic Presence
74 Charles River Laboratories (CRL): Company Snapshot
75 Charles River Laboratories (CRL): SWOT Analysis
76 Charles River Laboratories (CRL): Geographic Presence
77 Syneos Health: Company Snapshot
78 Syneos Health: SWOT Analysis
79 Syneos Health: Geographic Presence
80 IQVIA HOLDINGS INC.: Company Snapshot
81 IQVIA HOLDINGS INC.: Swot Analysis
82 IQVIA HOLDINGS INC.: Geographic Presence
83 Laboratory Corporation of America Holdings: Company Snapshot
84 Laboratory Corporation of America Holdings: SWOT Analysis
85 Laboratory Corporation of America Holdings: Geographic Presence
86 Thermo Fisher Scientific Inc.: Company Snapshot
87 Thermo Fisher Scientific Inc.: SWOT Analysis
88 Thermo Fisher Scientific Inc.: Geographic Presence
89 Parexel International Corporation : Company Snapshot
90 Parexel International Corporation : Swot Analysis
91 Parexel International Corporation : Geographic Presence
92 Medidata Solutions, Inc.: Company Snapshot
93 Medidata Solutions, Inc.: SWOT Analysis
94 Medidata Solutions, Inc.: Geographic Presence
95 Pharmaron, Inc.: Company Snapshot
96 Pharmaron, Inc.: SWOT Analysis
97 Pharmaron, Inc.: Geographic Presence
98 GVK Biosciences Private Limited: Company Snapshot
99 GVK Biosciences Private Limited: SWOT Analysis
100 GVK Biosciences Private Limited: Geographic Presence
101 Other Companies: Company Snapshot
102 Other Companies: SWOT Analysis
103 Other Companies: Geographic Presence
The Global Clinical Research Organization Market has been studied from the year 2019 till 2028. However, the CAGR provided in the report is from the year 2023 to 2028. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Clinical Research Organization Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

1. Discounted price for multiple reports across domains
2. Complimentary 10 hours free analyst time for market review
3. Free business intelligence platform with subscription
4. Free upgrade to enterprise license (allows to share across all company locations)
5. Choose reports from a database of more than 10,000 reports
6. Free trial, before you make a purchase decision. No purchase commitment .
Frequently Asked Questions

- Base Year for Estimate 2023
- Report Id 4289
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
Or view our licence options:
We never share your personal data. Privacy Policy
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS

- Known globally as a renowned B2B information integrator and analyser providing actionable insights with disruptive research that correlates markets, technologies, new use cases and companies
- 5500+ companies worldwide approach us every year for their revenue growth initiatives
- 80% of Fortune 2000 Companies rely on our research to identify new revenue sources
- Patent-pending in-house 'Artificial Intelligence powered Real-Time Competitive Analytics Tool‘
- 95% Renewal Rate
- Health, Pharma & Medtech ›
Pharmaceutical Products & Market
Industry-specific and extensively researched technical data (partially from exclusive partnerships). A paid subscription is required for full access.
- Leading global contract research organizations based on revenue 2023
In 2023, the leading contract research organizations worldwide included Laboratory Corporation of America Holdings, Thermo Fisher Scientific, and IQVIA. The first ranked company, Thermo Fisher Scientific, which acquired Pharmaceutical Product Development (PPD) in 2021, generated over 23 billion U.S. dollars in revenue through its clinical research business. Contract research organizations Contract research organizations (CROs) are companies that are contracted by larger pharmaceutical companies to manage select aspects of clinical trials. Today, CROs are essential for pharmaceutical research and development. Pharmaceutical companies are spending more money than ever on research and development, and there is no indication that spending will decrease in the future. Evidence suggests that CROs will become even more valuable. The clinical CRO market is expected to increase to over 100 billion U.S. dollars by the beginning of the next decade. Pharmaceutical research and development Globally, Roche and Johnson & Johnson will be the top pharmaceutical companies based on their projected research and development spending . There are regional differences in research and development among pharmaceutical companies. Of all pharmaceutical companies globally, those based in the U.S. develop the highest number of new chemical or biological entities.
Leading global contract research organizations (CROs) based on 2023 revenue (in million U.S. dollars)
| Characteristic | Revenue in million U.S. dollars |
|---|---|
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
To access all Premium Statistics, you need a paid Statista Account
- Immediate access to all statistics
- Incl. source references
- Download as PDF, XLS, PNG and PPT
Additional Information
Show sources information Show publisher information Use Ask Statista Research Service
* Only Laboratory Products & Biopharma Services segment. No separate information about pure clinical research-related revenues.
** Estimated.
*** Went private during 2023, thus no data available.
Other statistics on the topic Outsourcing in the pharmaceutical industry
- Pharmaceutical market: worldwide revenue 2001-2023
- Total global pharmaceutical R&D spending 2014-2028
- Top 50 pharmaceutical companies - Rx sales and R&D spending 2023
- Global pharmaceutical market in-house vs outsourcing services share 2014-2023

To download this statistic in XLS format you need a Statista Account
To download this statistic in PNG format you need a Statista Account
To download this statistic in PDF format you need a Statista Account
To download this statistic in PPT format you need a Statista Account
As a Premium user you get access to the detailed source references and background information about this statistic.
As a Premium user you get access to background information and details about the release of this statistic.
As soon as this statistic is updated, you will immediately be notified via e-mail.
… to incorporate the statistic into your presentation at any time.
You need at least a Starter Account to use this feature.
- Immediate access to statistics, forecasts & reports
- Usage and publication rights
- Download in various formats
* For commercial use only
Basic Account
- Free Statistics
Starter Account
- Premium Statistics
The statistic on this page is a Premium Statistic and is included in this account.
Professional Account
- Free + Premium Statistics
- Market Insights
1 All prices do not include sales tax. The account requires an annual contract and will renew after one year to the regular list price.
Statistics on " Outsourcing in the pharmaceutical industry "
- Select activities outsourced by biopharmaceutical companies 2022
- Enterprise value of top contract pharma research organizations 2022
- CRO market size worldwide forecast 2032
- Top contract research organization transaction deals until 2023
- Value of select contract pharma manufacturing companies 2022
- CDMO market size worldwide forecast 2023-2033
- CDMO market size worldwide forecast by type 2020-2030
- Share of CDMOs worldwide by modality and region 2022
- Share of CDMOs worldwide by value chain service and region 2022
- Top CDMO companies by number of M&A deals 2017-2021
- Number of CDMO merger and acquisition deals worldwide 2012-2021
- Value of CDMO merger and acquisition deals worldwide 2012-2021
- Number of CDMO merger and acquisition deals worldwide by country 2017-2021
- Value of CDMO merger and acquisition deals worldwide by country 2017-2021
- Preferred outsourcing in clinical development among biopharma companies by size 2023
- Outsourcing in clinical development among global biopharma companies by size 2023
- Likelihood of outsourcing drug development activities by biopharma companies 2023
- Change in biopharma outsourcing model usage 2021 vs. 2023
Other statistics that may interest you Outsourcing in the pharmaceutical industry
- Premium Statistic Pharmaceutical market: worldwide revenue 2001-2023
- Premium Statistic Total global pharmaceutical R&D spending 2014-2028
- Premium Statistic Top 50 pharmaceutical companies - Rx sales and R&D spending 2023
- Basic Statistic Global pharmaceutical market in-house vs outsourcing services share 2014-2023
- Premium Statistic Select activities outsourced by biopharmaceutical companies 2022
- Premium Statistic Enterprise value of top contract pharma research organizations 2022
- Premium Statistic Leading global contract research organizations based on revenue 2023
- Basic Statistic CRO market size worldwide forecast 2032
- Premium Statistic Top contract research organization transaction deals until 2023
- Basic Statistic Value of select contract pharma manufacturing companies 2022
- Premium Statistic CDMO market size worldwide forecast 2023-2033
- Premium Statistic CDMO market size worldwide forecast by type 2020-2030
- Basic Statistic Share of CDMOs worldwide by modality and region 2022
- Basic Statistic Share of CDMOs worldwide by value chain service and region 2022
- Premium Statistic Top CDMO companies by number of M&A deals 2017-2021
- Basic Statistic Number of CDMO merger and acquisition deals worldwide 2012-2021
- Basic Statistic Value of CDMO merger and acquisition deals worldwide 2012-2021
- Premium Statistic Number of CDMO merger and acquisition deals worldwide by country 2017-2021
- Premium Statistic Value of CDMO merger and acquisition deals worldwide by country 2017-2021
- Basic Statistic Preferred outsourcing in clinical development among biopharma companies by size 2023
- Premium Statistic Outsourcing in clinical development among global biopharma companies by size 2023
- Premium Statistic Likelihood of outsourcing drug development activities by biopharma companies 2023
- Basic Statistic Change in biopharma outsourcing model usage 2021 vs. 2023
Further related statistics
- Basic Statistic Revenue forecast of CRO market 2016-2021, by location
- Premium Statistic Clinical CRO market share worldwide by company 2016
- Basic Statistic CRO market size worldwide 2016, by function
- Basic Statistic CROs market share worldwide 2016
- Premium Statistic Estimated total CRO market worldwide in 2018, by function
- Premium Statistic Preclinical CRO market share worldwide by company 2016
- Premium Statistic CRO market share worldwide 2016, by function
- Premium Statistic Total addressable CRO market worldwide in 2018, by function
- Basic Statistic Veterinary CRO market revenues worldwide 2024-2029
- Premium Statistic Market revenue of CRO in APAC 2015-2020
- Premium Statistic Clinical trial density in APAC 2016, by country
- Premium Statistic Pharmaceutical CRO market value in China 2015-2024, by research stage
- Premium Statistic R&D expenditure of the major CROs in China 2017-2019
- Premium Statistic Total addressable CRO market worldwide 2013-2020
- Premium Statistic Revenue breakdown of the major CROs in China 2019, by segment
- Premium Statistic China's share of global clinical CRO market 2015-2024
- Basic Statistic Percent of registered clinical studies worldwide by type 2024
- Basic Statistic Percent of clinical studies with posted results worldwide by type 2024
- Premium Statistic Unsuccessful attempts and new drugs for cancer types 1998-2014
Further Content: You might find this interesting as well
- Revenue forecast of CRO market 2016-2021, by location
- Clinical CRO market share worldwide by company 2016
- CRO market size worldwide 2016, by function
- CROs market share worldwide 2016
- Estimated total CRO market worldwide in 2018, by function
- Preclinical CRO market share worldwide by company 2016
- CRO market share worldwide 2016, by function
- Total addressable CRO market worldwide in 2018, by function
- Veterinary CRO market revenues worldwide 2024-2029
- Market revenue of CRO in APAC 2015-2020
- Clinical trial density in APAC 2016, by country
- Pharmaceutical CRO market value in China 2015-2024, by research stage
- R&D expenditure of the major CROs in China 2017-2019
- Total addressable CRO market worldwide 2013-2020
- Revenue breakdown of the major CROs in China 2019, by segment
- China's share of global clinical CRO market 2015-2024
- Percent of registered clinical studies worldwide by type 2024
- Percent of clinical studies with posted results worldwide by type 2024
- Unsuccessful attempts and new drugs for cancer types 1998-2014
- Clinical Research Organization Market
Clinical Research Organization Market Size, Share, Opportunities, And Trends By Service Type (Early Phase Development Services, Clinical Research Services, Laboratory Services, Regulatory Consulting Services), By Therapeutic Area (Oncology, Clinical Pharmacology, Cardiology, Infectious Disease, Neurology, Gastroenterology & Hepatology, Ophthalmology, Others), By End-user (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, Academic Institutes), And By Geography - Forecasts From 2024 To 2029
- Published : Mar 2024
- Report Code : KSI061616785
- Pages : 145
- Description
- Table Of Contents
- Companies Profiled
Offered with this report
Market Data and Estimates in Excel
2 Months of Post Sale Analyst Support
Requirement Specific Customization
The clinical research organization market is anticipated to grow significantly over the forecast period.
The market for clinical research organizations (CROs) has grown significantly due to factors such as the demand for specialized knowledge, growing R&D expenses, and the complexity of clinical trials. Pharmaceutical, biotechnology, and medical device firms can benefit from a variety of services provided by CROs, including data management, regulatory compliance, clinical trial management, and pharmacovigilance. The number of clinical trials that have been outsourced to CROs has increased recently because of their capacity to offer affordable solutions, access to patient populations throughout the world, and proficiency in handling regulatory requirements in various geographic areas. The CRO industry has expanded because of technological breakthroughs like artificial intelligence and electronic data capture, which have improved data analysis capabilities and streamlined trial procedures. The CRO industry is very cutthroat, with both big international companies and specialty suppliers competing for market share. CROs frequently use mergers and acquisitions as a means of growing their service offerings and geographic reach. In the upcoming years, it is anticipated that the CRO market will continue to rise because of the rising demand for novel therapeutics and strict regulatory requirements.
Market Drivers
- The increasing demand for outsourcing is expected to drive the clinical research organization market.
The demand for Clinical Research Organizations to handle clinical trial outsourcing is significantly increasing in the biotechnology and pharmaceutical industries. The need to reduce expenses, shorten the time it takes to create new drugs and get access to specialized knowledge is what is driving this trend. Businesses can reduce operational risk, maximize resource allocation, and concentrate on their core strengths by utilizing the services of CROs.
Furthermore, outsourcing makes it possible to connect with a worldwide network of researchers and patients, which makes it easier to carry out trials in a variety of geographical locations. All things considered, the growing demand for outsourcing highlights how essential it is to boost productivity and creativity within the drug development ecosystem.
Chronic diseases increase will also drive the market. According to NCBI, 2023, between 2020 and 2050, there will be a 61.11% increase in the number of Americans 50 years of age and older, from 137.25 million to 221.13 million. The number of people 50 years of age and older who have at least one chronic illness is predicted to rise from 71.522 million in 2020 to 142.66 million in 2050, a 99.5% increase. Simultaneously, it is anticipated that the number of people with multimorbidity will rise from 7.8304 million in 2020 to 14.968 million in 2050, a 91.16% increase. According to race, by 2050, non-Hispanic whites (64.6%), non-Hispanic blacks (61.47%), and Hispanics and other races (64.5%) are expected to have one or more chronic illnesses.
- Globalization of clinical trials is expected to boost the market.
Clinical trial globalization is a reflection of a major change in the pharmaceutical and biotechnology sectors toward conducting research across a variety of geographical locations. Regulatory incentives, economic benefits in emerging markets, and access to larger and more diversified patient populations are some of the elements driving this trend. Companies can ensure wider applicability of data, improve variety in research populations, and speed up patient enrollment by expanding trial locations abroad.
Globalization does, however, come with drawbacks, like complicated regulations, disparities in culture, and logistical difficulties. Notwithstanding these obstacles, clinical trials' globalization is nonetheless vital to the advancement of medical research and the enhancement of patients' access to cutting-edge therapies on a global scale.
- Advancing technology is a significant driver for the clinical research organization market.
Technological developments are transforming clinical research by improving patient involvement, data quality, and efficiency. More accurate patient selection, predictive modeling, and real-time trial progress monitoring are made possible by innovations like artificial intelligence, machine learning, and big data analytics. Wearables and remote monitoring tools are examples of digital health technology that enable decentralized trial conduct, enhancing patient convenience and cutting expenses.
Moreover, computerized data capture technologies expedite the process of developing new drugs by streamlining data collecting and processing. All things considered, these technological developments enable researchers to make data-driven choices, maximize trial results, and eventually accelerate the release of safe and efficient treatments onto the market.
IQVIA received recognition for its AI software in June 2023. In order to enable real-world data research, the program, which incorporates natural language processing technology, assists in the analysis of complicated and unstructured patient data, offering distinctive insights into patient care and disease states. What makes the AI software unique is its capacity to offer insights that can be put into practice. It opens up important perspectives on population health, such as the social determinants of health (SDoH), which determine most health outcomes. One of the biggest healthcare systems in Illinois, Northshore-Edward-Elmhurst Health, is using this technology to help doctors identify and screen 56% more at-risk patients based on SDoH, enabling patients in need to receive focused intervention.
Asia Pacific region is expected to grow significantly.
The clinical research organization (CRO) market in Asia-Pacific is expanding significantly as a result of several important factors. The growing incidence of chronic illnesses, growing investments in healthcare infrastructure, and the development of the biotechnology and pharmaceutical industries are the main drivers of this rise. In addition, the area has cheaper operating expenses than Western markets and a sizable and diversified patient base. In nations like China, India, and South Korea, the conduct of clinical trials is further facilitated by regulatory reforms and advantageous government policies.
Consequently, the CRO market has experienced significant expansion, with Asia-Pacific emerging as a major hub for outsourcing clinical research activities. For instance, In November 2021, Based on the encouraging pre-clinical outcomes of LG00034053, a novel medication candidate for the treatment of osteoarthritis, LG Chem declared on the 4th that the company has been granted approval by the Korean Ministry of Food and Drug Safety for the Phase 1b/2 clinical trials.LG Chem aims to expedite the creation of novel medications by creating clinical trials that connect Phases 1 and 2. With the help of this approval, LG Chem will carry out studies at Samsung Medical Center to determine the best dose for patients with mild to moderate knee osteoarthritis (K&L2-3) by assessing factors like safety and tolerability, pharmacokinetics (the process of drug absorption, distribution, metabolism, and excretion), and effectiveness.
Market Restraints
- Increasing competition and consolidation could constraint the market
Demand growth is causing the Clinical Research Organization market to become more competitive and consolidated. As more businesses join the market, the rivalry for talent and contracts gets fiercer, which puts pressure on prices. Consolidation of the sector is fueled by larger CROs acquiring smaller ones to increase capabilities and reach globally. Even while there are advantages like economies of scale, smaller CROs could find it difficult to compete. Players in the CRO sector face a great deal of difficulty in striking a balance between competitiveness and consolidation.
Market Developments
- January 2024- Labcorp, a global leader of innovative and comprehensive laboratory services, and Hawthorne Effect, Inc., a complete clinical trials solution integrating technology, announced a strategic collaboration to advance centralized clinical trial capabilities for sponsors seeking to increase patient diversity and inclusion, decrease site burden, and accelerate enrollment and clinical study timelines.
- October 2023- Through creative and integrated technology-enabled pharmacovigilance (PV) safety services and solutions, IQVIA, a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, announced a strategic collaboration with argenx to advance treatment for patients with rare autoimmune diseases.
Segmentation
- Early phase development services
- Clinical research services
- Laboratory services
- Regulatory consulting services
- Clinical pharmacology
- Infectious disease
- Gastroenterology & Hepatology
- Ophthalmology
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Academic Institutes
- United Kingdom
- Saudi Arabia
- South Korea
1. INTRODUCTION
1.1. Market Overview
1.2. Market Definition
1.3. Scope of the Study
1.4. Market Segmentation
1.5. Currency
1.6. Assumptions
1.7. Base, and Forecast Years Timeline
1.8. Key Benefits to the Stakeholder
2. RESEARCH METHODOLOGY
2.1. Research Design
2.2. Research Processes
3. EXECUTIVE SUMMARY
3.1. Key Findings
3.2. Analyst View
4. MARKET DYNAMICS
4.1. Market Drivers
4.2. Market Restraints
4.3. Porter’s Five Forces Analysis
4.3.1. Bargaining Power of Suppliers
4.3.2. Bargaining Power of Buyers
4.3.3. Threat of New Entrants
4.3.4. Threat of Substitutes
4.3.5. Competitive Rivalry in the Industry
4.4. Industry Value Chain Analysis
4.5. Analyst View
5. CLINICAL RESEARCH ORGANIZATION MARKET BY SERVICE TYPE
5.1. Introduction
5.2. Early phase development services
5.2.1. Market Trends and Opportunities
5.2.2. Growth Prospects
5.2.3. Discovery services
5.2.4. Chemistry, manufacturing & control (CMC)
5.2.5. Preclinical services
5.2.5.1. Pharmacokinetics/Pharmacodynamics (PK/PD)
5.2.5.2. Toxicology services
5.2.5.3. Others
5.3. Clinical research services
5.3.1. Market Trends and Opportunities
5.3.2. Growth Prospects
5.3.3. Phase I
5.3.4. Phase II
5.3.5. Phase III
5.3.6. Phase IV
5.4. Laboratory services
5.4.1. Market Trends and Opportunities
5.4.2. Growth Prospects
5.4.3. Bioanalytical testing services
5.4.4. Analytical testing services
5.4.5. Physical Characterization
5.4.6. Raw Material Testing
5.4.7. Batch Release Testing
5.4.8. Stability Testing
5.4.9. Others
5.5. Regulatory consulting services
5.5.1. Market Trends and Opportunities
5.5.2. Growth Prospects
6. CLINICAL RESEARCH ORGANIZATION MARKET BY THERAPEUTIC AREA
6.1. Introduction
6.2. Oncology
6.2.1. Market Trends and Opportunities
6.2.2. Growth Prospects
6.3. Clinical pharmacology
6.3.1. Market Trends and Opportunities
6.3.2. Growth Prospects
6.4. Cardiology
6.4.1. Market Trends and Opportunities
6.4.2. Growth Prospects
6.5. Infectious disease
6.5.1. Market Trends and Opportunities
6.5.2. Growth Prospects
6.6. Neurology
6.6.1. Market Trends and Opportunities
6.6.2. Growth Prospects
6.7. Gastroenterology & Hepatology
6.7.1. Market Trends and Opportunities
6.7.2. Growth Prospects
6.8. Ophthalmology
6.8.1. Market Trends and Opportunities
6.8.2. Growth Prospects
6.9. Others
6.9.1. Market Trends and Opportunities
6.9.2. Growth Prospects
7. CLINICAL RESEARCH ORGANIZATION MARKET BY END-USER
7.1. Introduction
7.2. Pharmaceutical & Biopharmaceutical Companies
7.2.1. Market Trends and Opportunities
7.2.2. Growth Prospects
7.3. Medical Device Companies
7.3.1. Market Trends and Opportunities
7.3.2. Growth Prospects
7.4. Academic Institutes
7.4.1. Market Trends and Opportunities
7.4.2. Growth Prospects
8. CLINICAL RESEARCH ORGANIZATION MARKET BY GEOGRAPHY
8.1. Introduction
8.2. North America
8.2.1. By Service Type
8.2.2. By Therapeutic Area
8.2.3. By End User
8.2.4. By Country
8.2.4.1. United States
8.2.4.1.1. Market Trends and Opportunities
8.2.4.1.2. Growth Prospects
8.2.4.2. Canada
8.2.4.2.1. Market Trends and Opportunities
8.2.4.2.2. Growth Prospects
8.2.4.3. Mexico
8.2.4.3.1. Market Trends and Opportunities
8.2.4.3.2. Growth Prospects
8.3. South America
8.3.1. By Service Type
8.3.2. By Therapeutic Area
8.3.3. By End User
8.3.4. By Country
8.3.4.1. Brazil
8.3.4.1.1. Market Trends and Opportunities
8.3.4.1.2. Growth Prospects
8.3.4.2. Argentina
8.3.4.2.1. Market Trends and Opportunities
8.3.4.2.2. Growth Prospects
8.3.4.3. Others
8.3.4.3.1. Market Trends and Opportunities
8.3.4.3.2. Growth Prospects
8.4. Europe
8.4.1. By Service Type
8.4.2. By Therapeutic Area
8.4.3. By End User
8.4.4. By Country
8.4.4.1. United Kingdom
8.4.4.1.1. Market Trends and Opportunities
8.4.4.1.2. Growth Prospects
8.4.4.2. Germany
8.4.4.2.1. Market Trends and Opportunities
8.4.4.2.2. Growth Prospects
8.4.4.3. France
8.4.4.3.1. Market Trends and Opportunities
8.4.4.3.2. Growth Prospects
8.4.4.4. Italy
8.4.4.4.1. Market Trends and Opportunities
8.4.4.4.2. Growth Prospects
8.4.4.5. Spain
8.4.4.5.1. Market Trends and Opportunities
8.4.4.5.2. Growth Prospects
8.4.4.6. Others
8.4.4.6.1. Market Trends and Opportunities
8.4.4.6.2. Growth Prospects
8.5. Middle East and Africa
8.5.1. By Service Type
8.5.2. By Therapeutic Area
8.5.3. By End User
8.5.4. By Country
8.5.4.1. Saudi Arabia
8.5.4.1.1. Market Trends and Opportunities
8.5.4.1.2. Growth Prospects
8.5.4.2. UAE
8.5.4.2.1. Market Trends and Opportunities
8.5.4.2.2. Growth Prospects
8.5.4.3. Others
8.5.4.3.1. Market Trends and Opportunities
8.5.4.3.2. Growth Prospects
8.6. Asia Pacific
8.6.1. By Service Type
8.6.2. By Therapeutic Area
8.6.3. By End User
8.6.4. By Country
8.6.4.1. Japan
8.6.4.1.1. Market Trends and Opportunities
8.6.4.1.2. Growth Prospects
8.6.4.2. China
8.6.4.2.1. Market Trends and Opportunities
8.6.4.2.2. Growth Prospects
8.6.4.3. India
8.6.4.3.1. Market Trends and Opportunities
8.6.4.3.2. Growth Prospects
8.6.4.4. South Korea
8.6.4.4.1. Market Trends and Opportunities
8.6.4.4.2. Growth Prospects
8.6.4.5. Taiwan
8.6.4.5.1. Market Trends and Opportunities
8.6.4.5.2. Growth Prospects
8.6.4.6. Thailand
8.6.4.6.1. Market Trends and Opportunities
8.6.4.6.2. Growth Prospects
8.6.4.7. Indonesia
8.6.4.7.1. Market Trends and Opportunities
8.6.4.7.2. Growth Prospects
8.6.4.8. Others
8.6.4.8.1. Market Trends and Opportunities
8.6.4.8.2. Growth Prospects
9. COMPETITIVE ENVIRONMENT AND ANALYSIS
9.1. Major Players and Strategy Analysis
9.2. Market Share Analysis
9.3. Mergers, Acquisitions, Agreements, and Collaborations
9.4. Competitive Dashboard
10. COMPANY PROFILES
10.1. Charles River Laboratories, Inc.
10.2. Laboratory Corporation of America Holdings
10.3. IQVIA
10.4. Pharmaceutical Product Development
10.5. PAREXEL International
10.6. Syneos Health
10.7. ICON plc.
10.8. Medpace
10.9. CTI Clinical Trial and Consulting Services
10.10. QPS Neuropharmacology
Charles River Laboratories, Inc.
Laboratory Corporation of America Holdings
Pharmaceutical Product Development
PAREXEL International
Syneos Health
CTI Clinical Trial and Consulting Services
QPS Neuropharmacology
Related Reports
| Report Name | Published Month | Get Sample PDF |
|---|---|---|
| Mar 2022 | ||
| Dec 2023 | ||
| Dec 2023 | ||
| Oct 2023 | ||
| Mar 2024 |
License Type
Explore custom options available with this study.
- Customize this report
- Buy sections of the study
- Buy country specific report
Subscribe Us
Let us know the industries you are interested in, request free sample.
Clinical Research Organizations
- Veterinary Equipment
- Animal Drugs
- Animal Vaccines
- Purified Proteins
- Bioservices
- Biotechnology Research Equipment
- Protein Engineering
- Electro Therapy
- Nuclear Medicine
- Cardiovascular
- Dental Care
- Diabetes Care
- Ophthalmology
- Pulmonology
- Drug Filled Devices With Needle
- Clinical IT
- Healthcare Analytics
- Aesthetic Devices
- Clinical Diagnostic Instrument
- Immune System Diagnostics
- Infectious Disease Testing
- Handheld Monitoring Devices
- Single time Monitoring Devices
- Molecular Diagnostic Devices
- Medical Optical Imaging
- Nuclear Medicine Imaging
- Fluoroscopy
- Mammography
- Surgical Imaging
- Joint Reconstruction
- Drug Delivery Products
- Personal Protective Equipment
- Dental Medical Instruments
- Hospital and Clinical Use
- Ablation Devices
- General Surgical Devices
- Minimally Invasive Surgery Devices
- Bariatric Surgery Devices
- Surgical Access Devices
- Wound Care Devices
- Contraceptive Devices
- Anesthesia Equipment
- Insulin Pumps
- Hyperglycemics
- Behavioural Disorder Drugs
- Diabetes Care Drugs
- Oncology Drugs
- Developmental Anomalities
- External Condition Drugs
- Immune System Drugs
- Infectious Disease Drugs
- OB/GYN Drugs
Filter Reports
By Countries
9 Clinical Research Organizations Reports
Study Period: 2019 - 2029
Regions Covered: North America, Europe, Asia-Pacific, Middle East and Africa, South America
Major Players: Bio-Rad Laboratories Inc., PerkinElmer Inc., Thermo Fisher Scientific Inc., Merck KGaA, Agilent Technologies Inc.

Major Players: Bio-Rad Laboratories Inc., Illumina Inc., Beckman Coulter Inc. (Danaher Corporation), Becton, Dickinson, and Company, Fluidigm Corporation

Regions Covered: North America, Europe, Asia Pacific, Middle East and Africa, South America
Major Players: Anika Therapeutics, Inc., Arthro-Kinetics, JRL Orthopaedic Ltd, Geistlich Group (Geistlich Pharma AG), B. Braun SE

Major Players: Crown Bioscience Inc., Charles River Laboratories, Inc., ICON plc, Taconic Biosciences, Inc., Covance Inc.

Major Players: Agilent Technologies, Thermo Fischer Scientific, Danaher Corporation, Bio Rad Laboratories Inc., General Electric Company (GE Healthcare)

Study Period: 2021 - 2029
Major Players: Perkinelmer Inc., Danaher Corporation, Thermo Fisher Scientific Inc., Agilent Technologies, BD (Becton, Dickinson and Company)

Major Players: Epithelix Sarl, Mattek Corporation, AlveoliX AG, ATCC Global, Tissuse GmbH

Major Players: Illumina, Inc., Thermo Fisher Scientific, F. Hoffmann-La Roche Ltd., Agilent Technologies, Myriad Genetics

Major Players: KEOFITT A/S, Saint Gobain, GEA Group, Merck KGaA, Sartorius AG (Sartorius Stedim Biotech)

- Asia Clinical Research Organizations Research
- Europe Clinical Research Organizations Research
- Middle East and Africa Clinical Research Organizations Research
- North America Clinical Research Organizations Research
- South America Clinical Research Organizations Research
- Argentina Clinical Research Organizations Research
- Australia Clinical Research Organizations Research
- Brazil Clinical Research Organizations Research
- Canada Clinical Research Organizations Research
- China Clinical Research Organizations Research
- France Clinical Research Organizations Research
- Germany Clinical Research Organizations Research
- India Clinical Research Organizations Research
- Italy Clinical Research Organizations Research
- Japan Clinical Research Organizations Research
- Mexico Clinical Research Organizations Research
- South Africa Clinical Research Organizations Research
- South Korea Clinical Research Organizations Research
- Spain Clinical Research Organizations Research
- UK Clinical Research Organizations Research
- US Clinical Research Organizations Research
Related Clinical Research Organizations Reports
- Biotechnology
Please be sure to check your spam folder too.

Get a free sample of this report
Please enter your name
Business Email
Please enter a valid email
Please enter your phone number

Sorry! Payment Failed. Please check with your bank for further details.
Want to use this image? X
Please copy & paste this embed code onto your site:
Images must be attributed to Mordor Intelligence. Learn more
About The Embed Code X
Mordor Intelligence's images may only be used with attribution back to Mordor Intelligence. Using the Mordor Intelligence's embed code renders the image with an attribution line that satisfies this requirement.
In addition, by using the embed code, you reduce the load on your web server, because the image will be hosted on the same worldwide content delivery network Mordor Intelligence uses instead of your web server.
- / Market Insights
- U.S. Contract Research Organization (CROs) Market Analysis

U.S. CONTRACT RESEARCH ORGANIZATION (CROS) MARKET ANALYSIS
U.s. contract research organization (cros) market, by service typ (drug discovery, preclinical studies, early phase i - iia, phase iia - iii, phase iiib – iv, medical coding and writing, monitoring, clinical data management, and others), by therapeutic application (oncology, cardiovascular diseases, central nervous system diseases, infectious diseases, immunological disorders, respiratory disorders, and others), by size of cros (small size, medium size, and large size).
- Published In : Apr 2024
- Code : CMI3179
- Pages : 301
- Formats : Excel and PDF
- Industry : Pharmaceutical
- U.S. Contract Research Organization (CROs) Market Size and Trends
The U.S. contract research organization (CROs) market is estimated to be valued at USD 19.44 Bn in 2024 and is expected to reach USD 43.61 Bn by 2031 , exhibiting a compound annual growth rate (CAGR) of 12.2% from 2024 to 2031.

To learn more about this report, request sample copy
The U.S. contract research organization (CROs) market has been witnessing positive growth over the past few years. Rising R&D expenditure of pharmaceutical and biopharmaceutical companies and the emergence of new drug pipelines with complex molecules are expected to drive the market growth during the forecast period. In addition, the growing demand for specialized testing services and strategic partnerships between pharmaceutical companies and CROs to maximize outsourcing and reduce development costs are further expected to support the market expansion. However, pricing pressures, intense competition, and lack of harmonization of regulations globally may challenge market players over the next few years. Overall, increased R&D spending and outsourcing activities indicate a promising outlook for the U.S. contract research organization (CROs) market during the forecast years.
Market Driver – Increasing Number of Clinical Trials
Pharmaceutical and biotechnology companies are investing heavily in research and development (R&D) to bring new drugs and therapies to the market. As a result, there is a greater need for conducting clinical trials to test the safety and efficacy of these products. This increased research and development (R&D) expenditure boosts the demand for contract clinical research organization services. Increasing number of clinical trials globally is a key driver propelling the growth and expansion of the global contract clinical research organization market. For instance, according to the data published by Honeycomb Worldwide Inc., a company that operates in the pharmaceuticals industry, on May 17, 2023, there were 452,604 registered clinical trials globally on ClinicalTrials.gov. Out of the total registered studies, 64,838 are actively recruiting participants. This represents a significant increase from the over 365,000 registered trials reported in early 2021. It is clear that the clinical research landscape continues to expand at a substantial rate.
- Market Concentration and Competitive Landscape

Pharmaceutical and biotechnology industries have been continuously increasing their expenditure on research and development activities, in order to introduce new drugs in the market. Companies invest huge amounts in R&D to develop advanced healthcare solutions through clinical research. However, conducting clinical trials requires specialized infrastructure and expertise that most companies may not have in-house. This boosts the global contract clinical research organization market growth. For instance, Merck & Co., Inc., a multinational pharmaceutical company, announced that its R&D expenses were US$ 9.6 billion in the fourth quarter of 2023 as compared to US$ 3.8 billion in the fourth quarter of 2022. R&D expenses were US$ 30.5 billion for 2023 as compared to US$ 13.5 billion for 2022.

Data privacy and security concerns represent a significant restraining factor for the contract clinical research organization market due to several key reasons: Complexity of Data Handling: Clinical trials generate large volumes of sensitive and personally identifiable information (PII) from participants. This includes medical history, genetic data, and other confidential details. Managing and securing such complex datasets require specialized expertise and resources. International Data Transfers: Many clinical trials are conducted across multiple countries, necessitating the transfer of data across borders. Compliance with diverse data protection laws and regulations in different jurisdictions adds complexity and potential legal risks for CROs. Ethical Considerations: Protecting the privacy and confidentiality of participants is an ethical imperative in clinical research. Any compromise in data security not only violates ethical principles but also undermines the integrity of the research process.
Market Opportunities – Increasing Adoption of Inorganic Growth Strategies by Key Players
Key market players are focused on adopting inorganic growth strategies, such as partnership agreements, in order to expand their presence globally. For instance, in June 2023, ITOCHU Corporation, a company involved in domestic trading, import/export, and overseas trading of various products, announced that its subsidiary, A2 Healthcare Corporation, had signed the first partnership agreement with NRG Oncology-Japan a non-profit organization dedicated to supporting clinical trials. NRG promotes multicenter joint clinical research within Japan as an affiliate of the National Cancer Institute. Based on the agreement, A2 Healthcare will continue to engage in the clinical trial support business for drugs that have not yet been approved in Japan to address drug lag and drug loss, which are recent social issues.

The service type segment includes drug discovery, preclinical studies, early phase I - IIa, phase IIa - III, phase IIIb – IV, medical coding and writing, monitoring, clinical data management, and others. The drug discovery subsegment is expected to have 28.5% of the market share in 2024 owing to growing outsourcing of drug discovery activities and increasing research and development investments by pharmaceutical companies. CROs offer end-to-end drug discovery services right from target identification and validation to lead optimization. They employ advanced technologies such as genomic screening, bioinformatics and artificial intelligence which help clients accelerate the discovery process. Outsourcing drug discovery to specialized CROs allows pharmaceutical companies to focus on their core competencies while obtaining expert services in a cost-effective manner. It also provides access to latest tools and infrastructure not available in-house. Furthermore, intense patent cliff pressures on large pharma have driven them to increase discovery investments to replenish pipelines, benefiting CRO drug discovery revenues. The expertise of CROs in novel areas like oncology and rare diseases further enhances their appeal. Overall, the need to boost productivity and flexibility at reduced costs continues to support strong demand growth for outsourced drug discovery services.
Insights, By Therapeutic Application: Increasing Prevalence of Cancer
The therapeutic application segment includes oncology, cardiovascular diseases, central nervous system diseases, infectious diseases, immunological disorders, respiratory disorders, and others. The oncology subsegment is expected to have 29.3% of the market share in 2024 driven by a soaring cancer drug pipeline. With the prevalence of cancer on the rise, pharmaceutical firms are devoting huge R&D investments towards developing innovative oncology therapies. Additionally, immunotherapy and personalized medicine are opening new opportunities in this field requiring specialized expertise. CROs have emerged as strategic partners to biopharmaceutical industry in oncology with their dedicated centers housing state-of-the-art technologies and a pool of trained oncology researchers. Their multi-disciplinary approach aids faster design and execution of clinical trials. Further, their global networks help find the best suited patients and sites for studies. These advantages coupled with rising complexity of oncology studies have made CROs an invaluable resource, driving the largest revenues in the oncology segment.
Insights, Size of CROs: Extensive Service Portfolios
The size of CROs segment includes small size (less than 100 employees), medium size (100-500 employees), and large size (more than 500 employees). The large CROs (more than 500 employees) subsegment is expected to have 41.2% of the market share in 2024. Large CROs have extensive service portfolios covering the entire drug development lifecycle from pre-clinical to post-marketing. Their economies of scale allow investments in advanced lab infrastructure, large teams of specialists, and a global delivery footprint. This enables centralized project management of even the most complex multi-country studies. Large CROs also have robust quality systems and regulatory compliance processes certified by global health authorities. Furthermore, their financial might supports constant innovation through strategic acquisitions and partnerships. Pharmaceutical clients therefore prefer large trusted CROs to handle their most high-profile programs globally. In addition, large CROs have dominated the market through organic as well as inorganic growth over the past decades to cement their leadership positions.
- Market Report Scope
U.S. Contract Research Organization (CROs) Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2023 | Market Size in 2024: | US$ 19.44 Bn |
| Historical Data for: | 2019 To 2023 | Forecast Period: | 2024 To 2031 |
| Forecast Period 2024 to 2031 CAGR: | 12.2% | 2031 Value Projection: | US$ 43.61 Bn |
| Geographies covered: | |||
| Segments covered: | Drug Discovery , Preclinical studies , Early Phase I - IIa , Phase IIa - III , Phase IIIb – IV , Medical coding and writing , Monitoring , Clinical Data Management , and Others Oncology , Cardiovascular Diseases , Central Nervous System Diseases , Infectious Diseases , Immunological Disorders , Respiratory Disorders , and Others Small Size (Less than 100 employees) , Medium Size (100-500 employees) , and Large Size (More than 500 employees) | ||
| Companies covered: | IQVIA INC., ICON plc, Labcorp, Syneos Health, PROMETRIKA, LLC. , Premier Research, Caidya, Charles River Laboratories, Inc., Parexel International Corporation, Medpace, Inc., WuXi AppTec, Courante Oncology, EPS Corporation, Worldwide Clinical Trials, and Tigermed | ||
| Growth Drivers: | |||
| Restraints & Challenges: | |||
- Key Developments
- In March 2024, Veeda Clinical Research Limited, a full-service contract research organization (CRO) with a proven record of drug development success, announced that it had acquired Heads, a privately-held European CRO which specializes in conducting clinical trials in oncology. Through this acquisition, Veeda Clinical Research Limited entered the league of global CROs with integrated capabilities to extend contract research services from discovery to clinical development extending to post commercial launch.
- In November 2023, Ichor Life Sciences, a full-service contract research organization (CRO) and longevity biotechnology company, announced the launch of Ichor Clinical Trial Services. Ichor Clinical offers a spectrum of customized clinical trial solutions, ranging from individual service options to full CRO assistance.
- In September 2023, Parexel International Corporation , a contract research organization (CRO), and Partex, a data-to-drugs pharma platform, announced a partnership aiming to leverage artificial intelligence (AI)-powered solutions to accelerate drug discovery and the development for biopharmaceutical customers worldwide and de-risk the assets in their portfolios. Under the partnership, Partex’s clinical trial execution by the Partex group of companies will be managed by Parexel as the preferred CRO provider. The organizations will also collaborate on improving clinical trial execution for customers through the Partex-validated artificial intelligence (AI) platform, adding to and expanding Parexel’s existing AI tools and capabilities.
- In July, 2023, NAMSA, a world-leading MedTech Contract Research Organization (CRO) offering global end-to-end development services, announced its acquisition of CRI – The Clinical Research Institute, a Germany-based full service CRO. Combining the expertise of both organizations will position them as the leading MedTech CRO specializing in cardiovascular research services worldwide.
- In December 2021, IQVIA INC ., a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, launched new research nursing and phlebotomy services to offer access to a global network of high-quality, credentialed professionals for patients participating in clinical trials. The IQVIA research nursing and phlebotomy teams possess the capability to conduct a diverse range of assessments, collect data and specimens, and provide assistance in the direct delivery and administration of investigational products to patients.
*Definition: The U.S. Contract Research Organization (CROs) Market involves companies that provide outsourced clinical trial and drug development services to pharmaceutical, biotechnology, and medical device companies. CROs take over portions of the drug development process, including study protocol design, patient recruitment, clinical evaluations, and regulatory submissions. By outsourcing various stages of clinical testing to well-established CROs, biopharma companies can control costs and focus internal resources on core drug development.
- Market Segmentation
- Drug Discovery
- Preclinical studies
- Early Phase I - IIa
- Phase IIa - III
- Phase IIIb – IV
- Medical coding and writing
- Clinical Data Management
- Cardiovascular Diseases
- Central Nervous System Diseases
- Infectious Diseases
- Immunological Disorders
- Respiratory Disorders
- Small Size (Less than 100 employees)
- Medium Size (100-500 employees)
- Large Size (More than 500 employees)
- Syneos Health
- PROMETRIKA, LLC.
- Premier Research
- Charles River Laboratories, Inc.
- Parexel International Corporation
- Medpace, Inc.
- WuXi AppTec
- Courante Oncology
- EPS Corporation
- Worldwide Clinical Trials
Please Share :
About Author
Ghanshyam Shrivastava
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions. Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
U.S. Contract Research Organization (CROs) Market Report Snapshots
- U.S. Contract Research Organization (CROs) Companies
- U.S. Contract Research Organization (CROs) Market Challenges And Opportunities
- U.S. Contract Research Organization (CROs) Market News
Frequently Asked Questions
What will be the CAGR of the U.S. Contract Research Organization (CROs) market from 2024 to 2031?
What are the major factors driving the u.s. contract research organization (cros) market growth, what are the key factors hampering the growth of the u.s. contract research organization (cros) market, which is the leading service type in the u.s. contract research organization (cros) market, which are the major players operating in the u.s. contract research organization (cros) market.
Jump to Content
- Market Size and Trends
- Key Takeways from Lead Analyst
- Insights, By Service Type
- Insights, By Therapeutic Application
- Insights, Size of CROs
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports
Want to Buy a Report but have a Limited Budget?
We help clients to procure the report or sections of the report at their budgeted price. Kindly click on the below to avail
Press Release
U.S. Contract Research Organization (CROs) Market is estimated to be valued at USD 19.44 Bn in 2024
Reliability and Reputation

EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.

U.S. Contract Research Organization (CROs) Market
- Report Code: 3179,
- Published on: Apr 2024,
- Format: PDF
This Report Addresses
- Market size from 2024 to 2031
- Expected market growth until 2031
- Supplier and Demand Analysis
- Detailed analysis on market Drivers, Restraints, and Future Opportunities, and Trends of the industry
- Cross sectional analysis for all the Segments, across regions and 30 countries.
- Competitive landscape comprising of Company Market Share Analysis and Snapshot of Key Players.
- In-depth analysis on financials and sustainability strategies adopted by market players.
- Analyst recommendations
To request a Sample Copy , please complete the form below

Top 12 Clinical Research Organisations (CROs) in 2023

- Author Company: PharmiWeb.Jobs
- Author Name: Lucy Walters
- Author Email: [email protected]
- Author Website: https://www.pharmiweb.jobs/
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world , highlighting the companies driving this considerable growth and accelerating research and development across the globe.
Recognised as the world’s largest and most comprehensive CRO powered by Healthcare Intelligence, ICON provides outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. ICON has a global network of offices in 46 countries, working on specialities including Medical Devices, Therapeutics, Government and Public Health Solutions, Clinical Research Services, Commercialisation and Outcomes, and more.
With approximately 70,000 employees, IQVIA is a leading provider of advanced analytics, technology solutions and clinical research services to the life sciences industry. Operating in more than 100 countries, IQVIA’s Clinical Research solutions include Protocol Design, Phase I Trials, Phase IIb / III Trials, Site Identification, Patient Recruitment and Global Laboratories.
With more than 20,000 employees globally, Parexel is one of the world’s largest CROs and provides contract research, consulting, and medical communications services to the worldwide pharmaceutical, biotechnology, and medical device industries. Parexel provides the full range of Phase I to IV clinical development services, with global study locations in Baltimore, US, London, UK, Los Angeles, US, and Berlin, Germany.
Syneos Health
Syneos Health is the only fully integrated biopharmaceutical solutions organisation built to accelerate customer success, with 29,000+ employees working across more than 110 countries. The company’s Clinical Development services include Decentralized Solutions, Bioanalytical Solutions, Early Phase, Phase II–III, Phase IIIb – IV, Real World and Late Phase, and Medical Device and Diagnostics. In 2022, Syneos Health was the winner of the Life Sciences Award for Most Innovative CRO.
Labcorp Drug Development
With more than 70,000 employees, Labcorp is a leading global life sciences company with unparalleled diagnostics and drug development capabilities. The company is driven to help medical, biotech, and pharmaceutical companies transform ideas into innovations, and supports clinical trial activities in approximately 100 countries. Labcorp plans to spin-off its clinical development business, to be named Fortrea, in mid-2023.
Part of Thermo Fisher Scientific, PPD is a leading global CRO with 130,000+ employees, working to make the world healthier, cleaner, and safer. The company offers a broad range of clinical development and analytical services, helping to accelerate medicines from early development through regulatory approval and market access. PPD works with a broad range of therapeutic areas, including Biosimilar Development, Cell & Gene Therapy, Immunology and Rheumatology, Infectious Diseases, Women’s Health, and more.
In 2022, Medpace celebrated 30 years of accelerating the development of medical therapeutics, with approximately 5,000 employees working across 41 countries. The company provides medical and regulatory leadership and guidance throughout the clinical development life cycle, along with efficient execution of studies around the world, from Early Phase to Late Phase. Medpace also earned the 2022 CRO Leadership Awards for Capability, Compatibility, Expertise, Quality, and Reliability.
Charles River Laboratories
With 20,000+ employees worldwide, Charles River Laboratories provides essential products and services that help pharmaceutical and biotechnology companies, government agencies and leading academic institutions across the globe to accelerate their research and drug development efforts. The company currently operates more than 110 facilities across 20+ countries, working on therapeutic areas including COVID-19, Cardiovascular Disease, Immunology, Infectious Diseases, and Rare Diseases.
WuXi AppTec
WuXi AppTec is a global company providing a broad portfolio of R&D and manufacturing services to help pharmaceutical and healthcare companies across the globe advance discoveries and deliver ground-breaking treatments. The company currently has more than 45,000 employees globally, including 42,000 scientists. WuXi Clinical, a wholly owned subsidiary of WuXi AppTec, is a global CRO providing a comprehensive range of clinical development services for the development of pharmaceuticals, biologics, and medical devices.
CTI is one of the 20 largest CROs in the world, with associates in 60+ countries across six continents, including 12 locations in Europe. The company is the highest-ranked CRO in the world after being named a winner in all 5 core categories of the 2022 CRO Leadership Award. CTI provides its partners with a full range of clinical services, across therapeutic areas including Rare / Orphan Diseases, Regenerative Medicine / Gene Therapy, Immunology, Transplantation, and Hematology / Oncology.
Worldwide Clinical Trials
With approximately 3,000 employees and operating in more than 60 countries, Worldwide Clinical Trials is a global, midsize CRO providing top-performing bioanalytical and Phase I-IV clinical development services to biotechnology and pharmaceutical companies. The company’s major therapeutic focus areas include Cardiovascular, Metabolic, Neuroscience, Oncology, and Rare Diseases. In 2022, they were recognised for excellence and honoured in 5 categories in the CRO Leadership Awards.
PSI CRO is a privately-owned, full-service CRO operating globally, with 27,000+ employees in more than 60 countries. Committed to being the best CRO in the world, the company specialises in the planning and execution of global pivotal registration clinical trials, supporting trials across multiple countries and continents. PSI was named a 2022 CRO Leadership Award Champion in the categories of Compatibility, Quality, and Reliability.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
Home > Healthcare > Pharmaceuticals > Pharma Manufacturing > Clinical Trials Market
Clinical Trials Market - By Phase (I, II, III, IV), Study Design (Interventional, Observational, Expanded Access), Service Type (Outsourcing, In-house), Therapeutic Area (Oncology, Dermatology, Neurology, Cardiology) – Global Forecast (2024 – 2032)
- Report ID: GMI5029
- Published Date: Jun 2024
- Report Format: PDF
- Clinical Trials Market Size
Clinical Trials Market size was valued at USD 55.8 billion in 2023 and is estimated to grow at 5.4% CAGR from 2024 to 2032. The market has seen considerable growth due to the increasing prevalence of chronic diseases and the subsequent demand for innovative therapies.

The growing incidence of chronic conditions such as cancer, diabetes, and cardiovascular diseases has underscored the need for cutting-edge and effective treatments, thereby fueling the demand for novel therapies and interventions that aim to expand clinical studies and improve patient outcomes. According to the data by ClinicalTrials.gov reports, as of May 17, 2023, a total of 452,604 registered clinical trials, with 64,838 actively recruiting participants globally. This marked a significant rise from the roughly 365,000 trials recorded in early 2021.
| Report Attribute | Details |
|---|---|
| Base Year: | 2023 |
| Clinical Trials Market Size in 2023: | USD 55.8 Billion |
| Forecast Period: | 2024 - 2032 |
| Forecast Period 2024 - 2032 CAGR: | 5.4% |
| 2032 Value Projection: | USD 89.8 Billion |
| Historical Data for: | 2021 – 2023 |
| No. of Pages: | 140 |
| Tables, Charts & Figures: | 278 |
| Segments covered: | Phase, Study Design, Service Type, Therapeutic Area, and Region |
| Growth Drivers: | |
| Pitfalls & Challenges: |
The evident growth featured the continuous expansion of clinical studies at a significant pace, drawing significant investments from pharmaceutical companies and healthcare organizations, thereby expanding market growth. Additionally, advancements in biotechnology and personalized medicine are accelerating the development of targeted therapies, further driving the growth of the clinical trials market.
Clinical trials is a prospective biomedical or behavioral research study involving human participants to evaluate the safety and efficacy of experimental medical treatments or behavioral interventions. It is designed to answer specific questions about new treatments, including novel vaccines, drugs, dietary choices, dietary supplements, and medical devices. Clinical trials generate data on dosage, safety, and efficacy and are conducted after receiving approval from health authorities and ethics committees.
- Clinical Trials Market Trends
High trial complexity, coupled with robust pipelines and stringent regulations for conducting trials are factors influencing the spending on clinical development services outsourcing. The complexity involves a greater number of trial procedures, access to specialized patient populations, regulatory challenges in emerging markets, and mandated documentation such as REACH legislation and other regulatory requirements that have diverted the companies towards outsourcing. This has resulted in significant outsourcing of clinical trials, thereby propelling the market growth.
- Research indicates that, on average, approximately 64% of clinical development services are outsourced, contrasting with only 38% to 40% conducted internally. More than 60% of sponsors have expanded outsourcing by forming strategic partnerships and utilizing functional outsourcing. This increasing preference for outsourcing is a significant driver of market growth, facilitating enhanced efficiency, access to specialized expertise, and streamlined operations in clinical trials.
- Additionally, leveraging contract research organization services enables manufacturers and sponsors to focus on production capacity and improve internal processes. The wide range of available services, from drug discovery to post-marketing surveillance, simplified operations for mid-sized and small-scale pharmaceutical and biotechnology firms, allowing them to outsource research and development activities and reduce infrastructure investments.
- For instance, in November 2023, Syneos Health expanded its clinical trial capabilities in China through a collaboration with GoBroad Healthcare Group. This collaborative agreement aimed to expand the opportunities in the China market.
- Clinical Trials Market Analysis

Based on the phase, the market is categorized as phase I, phase II, phase III, and phase IV. The phase II segment is set to lead the market, accounting for the largest revenue of USD 23.4 billion in 2023, anticipating its dominance throughout the forecast period with a CAGR of 5.2%.
- The high segmental revenue is due to the growing number of industry-sponsored and non-industry-sponsored clinical trials in phase II, the complexity associated with phase II clinical trials, and the globalization of clinical trials.
- Phase II trials are the second most expensive stage after phase III studies, involving two parts that include exploring a range of doses and efficacy studies and finalizing the dose. The FDA estimated that around 33% of investigational drugs are usually under phase II trial, and numerous therapeutics and vaccines are currently in phase II for oncology treatment.
- For instance, according to the World Health Organization’s International Clinical Trials Registry Platform (ICTRP), as of February 2023, out of 271,906 clinical trials in a known phase of development, 35% were in Phase II. By May 2023, Phase II trials accounted for 74,432 clinical studies, constituting 36% of the total registrations. This significant representation of Phase II trials underscored their pivotal role in evaluating the efficacy and safety of new treatments before advancing to larger-scale Phase III trials and eventual regulatory approval processes.

Based on study design, the clinical trials market is classified into an interventional study, observational study, and expanded access study. The interventional study segment holds the largest market share of 76.6% in 2023 and is expected to maintain dominance throughout the analysis period.
- The interventional study segment dominated the market due to its widespread use and effectiveness in evaluating new medical interventions.
- Interventional studies account for the largest percentage of all clinical investigations, with the majority focused on testing new drugs, biologics, clinical procedures, behavioral interventions, and medical devices.
- Also, they are preferred for researchers as it allows to actively control and manipulate the variables being studied, minimizing confounding effects and efficiently detecting even small to moderate clinically important impacts. Therefore, growing number of industry-sponsored and non-industry-sponsored interventional trials is a key driver of the segment's dominance in the market.
Based on the service type, the clinical trials market is segmented into outsourcing service and in-house service. The outsourcing service segment is projected to reach USD 58.3 billion by 2032.
- The outsourcing segment dominated the market due to the increasing complexity of clinical trials and the need for specialized expertise. Pharmaceutical and biotechnology companies outsource clinical trials to CROs to streamline operations, reduce costs, and access specialized services. Also, CROs provide comprehensive services, including clinical trial management, data management, regulatory affairs, and patient recruitment.
- Thus, the growing demand for innovative treatments, advancements in technology, and the need for efficient resource utilization drive the outsourcing trend further expanding the market growth.
Based on the therapeutic area, the clinical trials market is segmented into autoimmune disease, oncology , cardiology, infectious disease, dermatology, ophthalmology, neurology, hematology, and other therapeutic areas. The oncology segment dominated the market with the highest share in 2023 exhibiting a growth rate of 4.5% during the analysis period.
- The prevalence of cancer continues to rise globally, necessitating continuous innovation in treatments and therapies.
- Oncology trials often involve a diverse range of studies, including those focused on chemotherapy, immunotherapy, targeted therapy, and combination therapies, addressing various types and stages of cancer. For instance, according to the WHO ICTRP, the number of registered oncology clinical trials has steadily increased, from approximately 19,211 in 2013 to 26,396 in 2022. The uptrend was observed due to the significant breakthroughs in immunotherapies, precision medicine , gene therapy , and combination therapy, thereby solidifying the oncology prominence.
- Further, pharmaceutical and biotech companies are heavily investing in oncology research to capitalize on the significant market potential and address the pressing need for effective treatments. Similarly, advancements in molecular biology and genetics have enabled the development of personalized oncology treatments, further driving the demand for clinical trials in the oncology segment.

The U.S. dominated the North American clinical trials market accounting for USD 27.3 billion in 2023 and is anticipated to show considerable growth over the analysis period.
- The U.S. holds a leading position in the North American market driven by a well-established healthcare system and infrastructure, with significant government and private investments in R&D. For instance, according to the National Institutes of Health (NIH), the U.S. federal government's investment in biomedical research reached USD 42.1 billion in 2022, providing a strong foundation for clinical trials.
- In addition, the high prevalence of chronic diseases, such as cancer, cardiovascular diseases, and diabetes, drives the demand for new and innovative treatments in the U.S. market. For instance, according to the American Cancer Society, cancer is the second leading cause of death in the U.S., with an estimated 1.9 million new cases and 609,360 deaths in 2022. Thus, the high prevalence of chronic diseases led to a surge in demand for personalized medicines, which in turn accelerated the research activities and expanded clinical trials studies in the country.
- Additionally, the well-established regulatory framework for clinical trials, providing guidelines streamlining approval processes, and the presence of leading pharmaceutical and CRO companies further bolsters the U.S. market's growth.
Germany exhibited a high growth potential in the European clinical trials market.
- Germany boasts a strong research base with large and diverse pharmaceutical industry players and academic institutions actively involved in clinical trials. According to a report published by Germany Trade & Invest, Germany has a robust research infrastructure, with 45 university hospitals and 118 clinical institutes involved in clinical trials, providing a strong foundation for conducting clinical trials.
- In addition, Germany's prominence in the European market is further bolstered by regulatory support for clinical research. Initiatives such as the Federal Ministry of Education and Research (BMBF) and the German Research Association (DFG) provide funding for clinical trials, solidifying Germany's position as a hub for research studies in the region.
The Asia Pacific clinical trials market is poised for rapid growth with a CAGR of 6% during the forecast period.
- The rising prevalence of chronic disease emerged the need for novel treatment options to explore opportunities number in the Asia Pacific region market. For instance, over the past five years, the number of clinical trials in the APAC region has consistently risen, from 11,571 trials in 2019 to 14,346 trials in 2023. This rise in trials is owing to supportive environment, patient population and per patient recruitment cost.
- This has further aimed to enhance the APAC's clinical research capabilities, with countries such as Australia, that offer incentives such as the Research and Development Tax Incentive (R&DTI), while Taiwan provides additional incentives to attract global clinical trials, including extended market exclusivity and higher reimbursement rates for drugs tested in the country.
- Thus, growing government support and research investment fosters the market in the region.
- Clinical Trials Market Share
The global clinical trials industry is marked by a diverse range of stakeholders, including pharmaceutical and biotechnology companies, CROs, academic institutions, and healthcare providers. Key players in the market have established themselves through comprehensive service offerings that include clinical trial management , clinical trial data management systems, regulatory affairs, and patient recruitment. In addition, the companies are leveraging cutting-edge technologies such as artificial intelligence (AI) and machine learning to streamline trial efficiency and enhance data analysis capabilities to further solidify their market presence.
Clinical Trials Market Companies
Few of the prominent players operating in the clinical trials industry include:
- Charles River Laboratories (MPI Research)
- Clinipace Inc.
- Eli Lilly and Company
- Laboratory Corporation of America Holdings (Covance Inc)
- Medpace, Inc.
- Merck & Co
- Parexel International Corporation
- Pfizer Inc.
- Syneos Health
- The Emmes Company, LLC
- Thermo Fisher Scientific (PPD Inc)
- Veeda Clinical Research
- Worldwide Clinical Trials
- WuXi AppTech
Clinical Trials Industry News
- In April 2024, Hanmi Pharmaceutical has partnered with MSD (Merck & Co.) to collaborate on clinical trials and supply agreements for BH3120, an immuno-oncology drug. The Phase I trial will evaluate BH3120's safety and efficacy in combination with Keytruda (pembrolizumab), MSD's anti-PD-1 therapy. This collaboration aimed to accelerate the clinical development of BH3120 for potential future treatments in oncology.
- In April 2024, Parexel forged a strategic partnership with Palantir Technologies Inc. to utilize AI in accelerating the delivery of safe and effective clinical trials for global biopharmaceutical customers. Through this collaboration, Parexel will harness Palantir’s Foundry and Artificial Intelligence Platform (AIP) to bolster its clinical data platform, aiming to enhance trial efficiency while upholding stringent safety and regulatory standards.
The clinical trials market research report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue in USD Million from 2021 – 2032 for the following segments: Click here to Buy Section of this Report
Market, By Phase
Market, By Study Design
- Interventional study
- Observational study
- Expanded access study
Market, By Service Type
- Outsourcing service
- In-house service
Market, By Therapeutic Area
- Autoimmune disease
- Infectious disease
- Dermatology
- Ophthalmology
- Other therapeutic areas
The above information is provided for the following regions and countries:
- Netherlands
- Switzerland
- Rest of Europe
- South Korea
- Philippines
- Rest of Asia Pacific
- Rest of Latin America
- Saudi Arabia
- South Africa
- Rest of Middle East and Africa
Click here to buy sections of this report
Frequently Asked Questions (FAQ) :
How big is the clinical trials market, why is the need for phase ii clinical trials growing, why is the use of outsourcing clinical trials rising, what is the value of the u.s. clinical trials industry, clinical trials market scope, premium report details.
- Base Year: 2023
- Companies covered: 11
- Tables & Figures: 278
- Countries covered: 30

Your personal details will remain secure and confidential.
Is your requirement urgent? Please give us your business email for a speedy delivery!
Enter your business email *
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
Top 15 Contract Research Organizations (CROs) in 2024
Post-pandemic, as drug development businesses recover and grow, CROs around the world continue to undergo profound transformation. As in previous years, 2023 will be all about mergers and acquisitions, with big players getting even bigger and smaller companies surviving thanks to niche advantages such as technological know-how or brilliant scientists among their employees.
The top 15 CROs globally would be in the following order based on 2023 revenue :
- LabCorp (Covance) , with 15.05 billion USD (2023) and 75000 employees ( Labcorp , 2024). In February 2015, Labcorp completes its $6 billion purchase of Covance, Inc., creating the world’s leading health care diagnostics company. The combination of Covance’s drug development leadership and Labcorp’s medical testing expertise builds the market leader in central laboratory and bioanalysis services. Labcorp’s clinical trials companies, Labcorp Clinical Trials and Tandem Laboratories, align under the Covance brand.
- IQVIA , with 14.85 billion USD (2023) and 86000 employees (IQVIA, 2022). IQVIA, formerly Quintiles and IMS Health, Inc., is an American Fortune 500 and S&P 500 multinational company serving the combined industries of health information technology and clinical research. IQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. With approximately 86,000 employees, IQVIA conducts operations in more than 100 countries.
- ICON (PRA) , with 7.74 billion USD (2022) and 41160 employees (ICON, 2023). From a small team of 5 people in 1990, ICON now employs over 41,100 people. ICON has been recognised as one of the world’s leading Contract Research Organisations through a number of high-profile industry awards .
- Thermo Fisher Scientific (PPD) , with 7.02 billion USD (2023) and 35000 employees (Businesswire, 2023). Recognized as a global industry leader in accelerating promising medicines from early development through regulatory approval and market access, we serve pharma, biotech, medical device and government organizations with custom-tailored solutions, including full-service partnerships and functional service partnerships. As a strategic partner in clinical development and analytical services, we apply cutting edge technologies, therapeutic expertise and a firm commitment to quality to help customers deliver life-changing therapies.
- Lonza - 6.5 billion USD, 17896 employees. Though being a contract development and manufacturing organization (CDMO), rather than a CRO, takes the 10th position with the 5.9 billion USD in sales and over 17500 employees worldwide (2023), offering “proprietary line of in silico and in vitro services for manufacturability, immunogenicity, potency assessment, humanization and protein engineering” ( https://pharma.lonza.com , 2022). Founded in 1897 in the Swiss Alps, today, Lonza operates across five continents. With approximately 17,500 full-time employees, it comprises high-performing teams and individual talent that make a meaningful difference to our own business, as well as to the communities in which we operate. The business benefits from global supply chains, but we have worked to maintain the agility to address marketplace needs on a local level. CDMOs become reliable partners for Pharma companies as their “services reduce R&D costs while improving productivity and are essential to providing safe and effective treatment to patients” ( https://pharma.lonza.com , 2022.
- Wuxi AppTec with 5.8 billion USD revenue in 2021 and 44360 employees (Companiesmarketcap, 2022). As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the pharmaceutical and healthcare industry around the world to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, and cell and gene therapies CTDMO (Contract Testing, Development and Manufacturing Organization), helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received AA ESG rating from MSCI in 2023 and its open-access platform is enabling more than 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that "every drug can be made and every disease can be treated."
Syneos Health® is a leading fully integrated biopharmaceutical solutions organization built to accelerate customer success. We translate unique clinical, medical affairs and commercial insights into outcomes to address modern market realities.
We bring together a talented team of professionals, who work across more than 110 countries, with a deep understanding of patient and physician behaviors and market dynamics. Together we share insights, use the latest technologies and apply advanced business practices to speed our customers’ delivery of important therapies to patients.
Cell and Gene Therapy CDMO Solutions has supported the development of 11 FDA approved cell and gene therapies and have conducted more than 900 studies in this field over the past year. Unsurpassed end-to-end offering results in enhanced access to scientific and regulatory expertise via multidisciplinary bench of experts to help you problem solve.
Parexel (EQT Private Equity and Goldman Sachs Asset Management) with 3.8 billion USD in 2022 and 21000 employees (Parexel, 2023). Parexel is among the world’s largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders, emerging innovators, and sites to design and deliver clinical trials with patients in mind, increasing access and participation to make clinical research a care option for anyone, anywhere.
- Medpace Holdings, Inc. with 1.135 billion USD revenue in 2021 and 5400 employees (Medpace, 2023). Integrating core clinical trial services delivers efficient and streamlined execution and higher quality results. Through its wholly-owned subsidiaries, Medpace offers clinical pharmacology, as well as supporting laboratory services including central labs, bioanalytical lab, ECG core lab, and imaging core labs.
Worldwide Clinical Trials – 0.6 billion USD , 3000 employees, 60 countries.
Worldwide Clinical Trials started out more than 30 years ago as a small science team with a big goal: To always provide authentic, personalized attention to our partners. And as the industry has grown, CROs have merged, and trials have grown increasingly complex, we haven’t forgotten what truly matters: People.
With more than 3,000 employees in more than 60 countries around the world, we’re still staying true to those roots. Our team is dedicated to staying accessible, flexible, and solution-focused to ensure our partners not only have the best possible outcomes – but they also know their Worldwide team is only a call away.
Allucent (CATO SMS and Pharm-Olam) – 0.36 billion USD, 1200+ employees .
Allucent originated with CATO SMS, itself created by the merger of Cato Research and SMS-oncology in 2019. Cato Research, founded in 1988, was known for its ability to design and execute successful development strategies and guide creative new products through the regulatory process.
Previous acquisitions by CATO SMS included Array Biostatistics, a full-service biostatistical and statistical programming CRO, and Nuventra Pharma Sciences, one of the industry’s leading providers of clinical pharmacology science and services. With these acquisitions, CATO SMS expanded its services to offer biostatistical consulting, analysis, programming, and cutting-edge modeling and simulation techniques to inform clinical trial designs and predict trial outcomes.
In early 2022, CATO SMS merged with Pharm-Olam, a global clinical research organization delivering clinical trial services to organizations around the world.
KCR – 0.15 billion USD, 700 employees
KCR is a clinical development solutions provider creating value for biotechnology and pharmaceutical organizations.
Founded in 1997, our expert teams support clients with full-service clinical development capabilities across three main areas: Trial Execution, Consulting, and Placement. KCR operates across North America, Europe, and Australia, with main office locations in Boston, US, Berlin, Germany, and Warsaw, Poland.
Our geographical coverage across 25+ countries, cutting-edge technical capabilities and tailored offerings allow for the optimized delivery of solutions to develop life-changing therapies. KCR offers access to an estimated population of 1.1 billion people.
Advanced Clinical – 0.1 billion USD , 1100+ employees
Today, Advanced Clinical has grown organically, with coverage across North America, Eastern Europe, Western Europe and Asia-Pacific, providing contract research organization (CRO), FSP and strategic resourcing solutions. A research services and strategic resourcing organization committed to providing a better clinical experience by delivering comprehensive Contract Research Organization (CRO), Functional Service Provider (FSP) and Strategic Resourcing services that optimize effectiveness for both patients and sponsors throughout the clinical research journey.
Using our decades of experience, we strive to improve the lives of everyone touched by clinical research and help our clients achieve better outcomes through candid conversations, foresight, resilience and innovative solutions.
CTI Clinical Trial and Consulting Services – 0.08 billion USD, 750 employees . CTI has grown consistently and significantly, becoming a global organization with associates in more than 60 countries across the world. We have worked on more than 10,000 projects, worked on every continent except Antarctica, and have contributed to more than 150 new drug and device approvals through global regulatory agencies such as the FDA, EMA, and others. Currently, we work with approximately 250 pharmaceutical and emerging biotechnology and medical device companies.
Among recent reasons for mergers and the whole new approach to conducting clinical research, apart from pursuit of new clients, talents, expertise, products, and markets, there have been notorious coronavirus crisis and military conflicts worldwide. We faced the advent of globalization, decentralization, remote monitoring, and personalized approach to drugs R&D. But we are now looking into the era of technology in clinical research. Processes that allow remote audits and monitoring, previously considered too risky, are now risk mitigators (Bahls, Christine, 2021), at least from that point of view. Clinical trial participants enrollment, and even participation itself, constantly moves into tech dimension.
Only few among the leading CROs escaped M&As for now, but this seems to be the matter of time. Savlovschi – Wicks, Theodora (2022) estimated that “in 2021, the global CRO market is expected to reach an impressive US$88 billion by 2028”. According to IQVIA , only in biopharma sector the total transaction value grew by 70% to reach 152 billion USD by the end of 2023, featuring prominent $43Bn Pfizer-Seagen deal, and other considerable M&As for BMS-Karuna ($14Bn), Merck-Prometheus ($10.2Bn), AbbVie-Immunogen ($10.1Bn), AbbVie-Cerevel ($8.7Bn), Biogen-Reata ($7.3Bn) and Roche-Telavant ($7.1Bn).
Among the CRO businesses the following M&As took place lately:
- Charles River Labs acquires MPI Research (2018), Citoxlab (2019), HemaCare (2020) and Cognate BioServices (2021)
- IQVIA (formerly Quintiles and IMS Health) acquired Clintec and 40% of Q2 Solutions (2021)
- ICON purchased PRA Health Services (2021)
- Thermo Fisher Scientific bought PPD (2021)
- Labcorp purchased Covance and Chiltern
- Inotiv acquired Envigo (2021)
- INC Research and inVentiv Health merged to later become Syneos Health
- Parexel become the property of EQT Private Equity and Goldman Sachs Asset Management
- CATO SMS and Pharm-Olam merged in 2022
- Triley Bidco acquired Clinigen (2022)
The article by Nataliia Vietchinkina, MS in Clinical Research Administration
University of Liverpool.
References:
- Christine Bahls (2021) ‘The Post-Pandemic CRO Landscape’, Applied Clinical Trials, Applied Clinical Trials-09-01-2021, Volume 30, Issue 9 [online] Available from: https://www.appliedclinicaltrialsonline.com/view/the-post-pandemic-cro-landscape
- Savlovschi – Wicks, Theodora (2022) ‘Top 10 CROs to watch in 2022’ [online]. Available from: https://www.proclinical.com/blogs/2022-3/top-10-cros-to-watch-in-2022
- Labcorp (2022) Labcorp Announces 2021 Fourth Quarter and Full-Year Results, 10 February 2022 [online]. Available from” https://ir.labcorp.com/news-releases/news-release-details/labcorp-announces-2021-fourth-quarter-and-full-year-results#:~:text=Revenue%20was%20%2416.12%20billion%20%2C%20an,by%20divestitures%20of%20(0.1%25)
- IQVIA (2022) IQVIA Reports Fourth-Quarter and Full-Year 2021 Results; Raises Full-Year 2022 Profit Guidance, 15 February 2022 [online]. Available from: https://ir.iqvia.com/press-releases/press-release-details/2022/IQVIA-Reports-Fourth-Quarter-and-Full-Year-2021-Results-Raises-Full-Year-2022-Profit-Guidance/default.aspx#:~:text=Revenue%20of%20%2413%2C874%20million%20for,12.4%20percent%20at%20constant%20currency
- ICON (2022) ICON Reports Fourth Quarter and Full Year 2021 Results, 2022 [online]. Available from: https://www.iconplc.com/news-events/press-releases/icon-reports-fourth-quarter-and-full-year-2021-results/
- Businesswire (2021) ‘PPD Reports Fourth Quarter and Full Year 2020 Results’, 23 February 2021 [online]. Available from: https://www.businesswire.com/news/home/20210223006094/en/PPD-Reports-Fourth-Quarter-and-Full-Year-2020-Results
- Macrotrends (2022) ‘Syneos Health Revenue 2012-2021 | SYNH’ [online]. Available from: https://www.macrotrends.net/stocks/charts/SYNH/syneos-health/revenue
- https://companiesmarketcap.com/ (2022) ‘Revenue for Charles River Laboratories (CRL)’ [online]. Available from: https://companiesmarketcap.com/charles-river-laboratories/revenue/
- Vinluan, Frank (2021) ‘CRO Parexel changes private equity hands again, this time for $8.5B’ [online]. Available from: https://medcitynews.com/2021/07/cro-parexel-changes-private-equity-hands-again-this-time-for-8-5b/
- Medpace (2021) ‘Medpace Holdings, Inc. Reports Third Quarter 2021 Results’ [online]. Available from: https://investor.medpace.com/news-releases/news-release-details/medpace-holdings-inc-reports-third-quarter-2021-results#:~:text=The%20Company%20forecasts%202021%20revenue,%24176.0%20million%20to%20%24180.0%20million%20 .
- Companiesmarketcap (2022) ‘Revenue for WuXi AppTec (2359.HK) Revenue in 2021 (TTM)’ [online]. Available from: https://companiesmarketcap.com/wuxi-apptec/revenue/
- https://pharma.lonza.com (2022) ‘Design, assess and optimize for clinical success’ [online]. Available from: https://pharma.lonza.com/offerings/early-development-services .
Clinical Research News
Upcoming clinical trials.
- University Health Network, Toronto Sunnybrook Health Sciences Centre; Hamilton Health Sciences Corporation Not yet recruiting Gemcitabine/Cisplatin/Nab-Paclitaxel and Rilvegostomig in Resectable iCCA (NEOLANGIO) Cholangiocarcinoma Resectable Canada
- University of Michigan National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Not yet recruiting Diabetic Foot Ulcer (DFU) Rapid Pathogen Identification Diabetes Mellitus | Diabetic Foot Ulcer | Wound United States
- Ricardo A Gutierrez Ramirez, MD, MSc, FACOG Recruiting Adverse Neonatal Outcomes With a Shortened Clinical Regimen of Dexamethasone. (UNODEXA) Preterm Birth | Distress Respiratory Syndrome Honduras
- Cairo University Recruiting Evaluation of Dentin Graft and I-PRF With and Without Vitamin C for Post-extraction Socket Preservation Socket Preservation Egypt
- Brigham and Women's Hospital Columbia University; National Institute on Aging (NIA); Boston Medical Center Not yet recruiting Refinement and Testing of Recruitment Methodology for Behavioral Medication Adherence Interventions Using Behavioral Science-based Approaches Diabetes | Medication Adherence | Pharmacist-Patient Relations
- Kaiser Permanente University of California, San Diego; National Institute on Minority Health... and other collaborators Not yet recruiting Filipino Family Health Initiative 1.0 (FFHI) Depression | Anxiety | Parenting
- Mayo Clinic National Cancer Institute (NCI) Not yet recruiting Genetic Testing for the Prevention of Cancer in Indigenous American Communities (JUNIPER Trial) Malignant Solid Neoplasm | Hematopoietic and Lymphatic System Neoplasm United States
- Abhishek Parolia Recruiting Bacterial Reduction After Supplementary Disinfection Procedures in Infected Root Canals Dental Pulp Necroses United States
- M.D. Anderson Cancer Center Recruiting A Phase II Trial Utilizing Metronidazole to Optimize the Microbiome of Rectal Adenocarcinoma Undergoing Neoadjuvant Therapy Rectal Adenocarcinoma United States
- Overseas Pharmaceuticals, Ltd. Shanghai ShouYan Clinical Development Co.,Ltd. Not yet recruiting Comparative Pharmacokinetic Study of Pirfenidone Modified-Release Tablets in Healthy Subjects Under Fasting and Fed Conditions Idiopathic Pulmonary Fibrosis China
- University of Colorado, Denver National Institute on Drug Abuse (NIDA) Recruiting A Randomized, Placebo Controlled Study of Cannabidiol in Young Adult Cannabis Users Cannabis Use Disorder United States
- Koç University Not yet recruiting Education Program on Evidence-based Practices on Nurses' Competencies and Clinical Decision-Making Levels (RCT) Evidence Based Practice Turkey
Clinical Research Jobs
Sponsors and Collaborators
- Assiut University
- GlaxoSmithKline
- National Cancer Institute (NCI)
- Cairo University
- AstraZeneca
- Assistance Publique - Hôpitaux de Paris
- Mayo Clinic
- M.D. Anderson Cancer Center
- Novartis Pharmaceuticals
- National Institute of Allergy and Infectious Diseases (NIAID)
- Massachusetts General Hospital
- National Taiwan University Hospital
- Boehringer Ingelheim
- Hoffmann-La Roche
- Merck Sharp & Dohme LLC
- Stanford University
- University of California, San Francisco
- Eli Lilly and Company
- Duke University
- View Full List
Medical Conditions
- Breast Cancer
- Hypertension
- Prostate Cancer
- HIV Infections
- Coronary Artery Disease
- Heart Failure
- Diabetes Mellitus, Type 2
- Colorectal Cancer
- Lung Cancer
- Schizophrenia
- Cardiovascular Diseases
- Atrial Fibrillation
Drug Interventions
- Cyclophosphamide
- Carboplatin
- Dexamethasone
- Gemcitabine
- Capecitabine
- Bevacizumab
- Pembrolizumab
- Methotrexate
- Oxaliplatin
- Dexmedetomidine
- Fludarabine
Please enter your Full Name
Please enter your Email
Please enter a valid Phone Number
Please enter your Company Name
Please enter your Partnership
Please enter your Job Title
Please select one option.
Please verify captcha
Clinical Research Organizations
Outsourced Market for Patient trials.
Beroe LiVE.Ai™
AI-powered self-service platform for all your sourcing decision needs across 1,200+ categories like Clinical Research Organizations.
Market Data, Sourcing & Supplier Intelligence, and Price & Cost Benchmarking.
The World’s first Digital Market Analyst
Abi, the AI-powered digital assistant brings together data, insights, and intelligence for faster answers to sourcing questions
Abi is now supercharged with GPT4 AI engine. Enjoy the ease of ChatGPT, now on Abi
Clinical Research Organizations Suppliers
Find the right-fit clinical research organizations supplier for your specific business needs and filter by location, industry, category, revenue, certifications, and more on Beroe LiVE.Ai™.

Up to 3 months
The Supplier Evaluation Risk (SER) Rating is Dun & Bradstreet’s proprietary scoring system used to assess the probability that a business will seek relief from creditors or cease operations within the next 12 months. SER ratings range from 1 to 9, with 9 indicating the highest risk of failure. We’ve prepared an infographic to help business owners better understand what influences their SER Rating.

Related Categories
AI & Digital Ops In Patient Access
Artificial Intelligence in Patient Engagement
Cell Therapies
Clinical Trial Supply (CTS)
Connected Devices and Wearables
Drug Development Biometrics Services
Drug Development Core Lab
Early Phase Clinical Trials
Lab Animals
Lab Equipment and Supplies
Lab Equipment and Supplies -Trends, Pricing and Contract Analysis
Patient Recruitment

Use the Clinical Research Organizations market, supplier and price information for category strategy creation and Quaterly Business Reviews (QRBs)
Clinical Research Organizations market report transcript
Global market outlook on clinical research organizations.
The global CRO market was estimated at $31.6 billion in 2018, and it is expected to grow at a CAGR of 12 percent to reach $45.2 billion by 2022
This is due to the increased outsourcing (>50 percent) seen from the pharmaceutical and biotechnology companies, with 85 percent comprised by the clinical segment
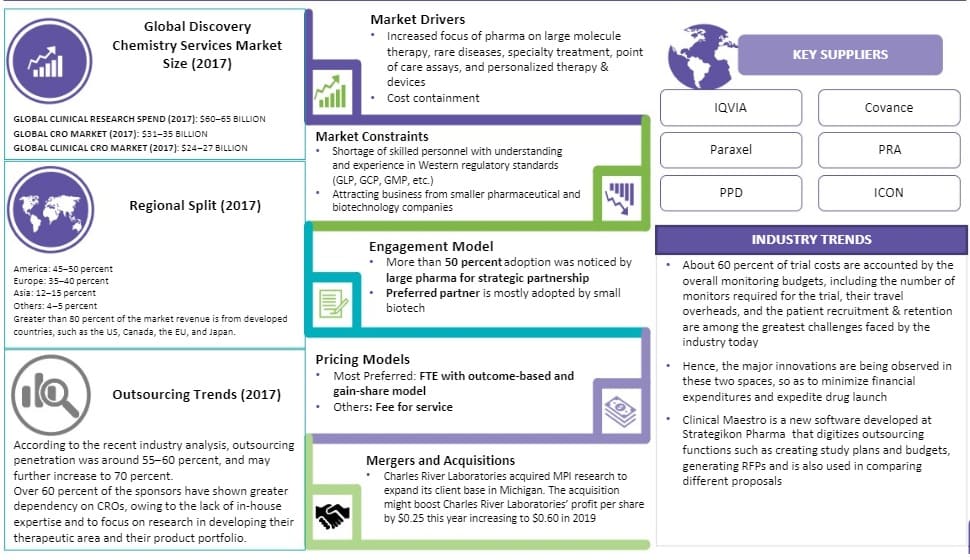
- Drug discovery is the largest segment by service type and accounts for approximately 33%,of the total CRO market
- Although spend on specialty focus, such as rare diseases, personalized medicines, and specialty oncology, has increased, the larger portfolio by spend size would continue to drive the programmatic CRO market
- The clinical segment of the CRO market is valued at $30.6 billion in 2017 and is expected to grow at a CAGR of 7 percent. Almost 85 percent of the global CRO market revenue comes from the clinical CRO market. This is directly related to about 50 percent of Phase II–IV activities already being outsourced by pharma companies
- On the other hand, the preclinical segment was valued at $2.8-3 billion in 2017 and is expected to grow at a CAGR 10 percent, as more in-house preclinical activities such as discovery and early phase studies are beginning to be outsourced by pharma companies at a range of 38-40 percent
- The CRO market is split regionally as America: 45–50 percent, Europe: 35–40 percent, Asia: 12–15 percent, Others: 4–5 percent. Greater than 80 percent of the market revenue is from developed countries, such as the US, Canada, the EU, and Japan.
- The top CROs are IQVIA, Paraxel, PPD, Covance, and PRAICON.
- Constraints in the contract research organization market include shortage of skilled personnel with understanding and experience in western regulatory standards (GLP, GCP, GMP, etc.) and challenges in attracting business from smaller pharmaceutical and biotechnology companies.
Regional Analysis
North America and Europe hold greater than 80 percent of the CRO market, as pharma spend ~70 percent and 18 percent of their clinical trial R&D spending in these regions, respectively
- Though the cost of conducting clinical trials in emerging countries will be 60 percent less than that of developed countries, greater than 80 percent of the market revenue is from developed countries - US, Canada, the EU, Japan
- APAC contributes to 10 –15 percent of the overall CRO revenue, which makes this region the third largest market share holder with increased trials being seen in China, Russia and South Korea
- Increased outsourcing of clinical services such as Clinical Data Management, Biostatistics, Pharmacovigilance, etc., by large pharma to emerging market of India is being seen. The reason being low cost labor availability, which will further drive up the revenue of Asia-Pacific region in the future
CRO Market Drivers
- Pharma companies have increased outsourcing their in-house R&D activities to CROs
- Large molecule development outsourcing has increased from ~35 percent to 45 percent, due to more supply and availability of reliable single use technology
Cost Containment
- Pharmaceutical companies one of the major factors is to reduce costs, by lowering their internal capacities in manufacturing and R&D. This leads to expenses being diverted toward outsourcing, making CROs play an integral part of pharma to increase their productivity, access new capabilities, shift fixed to variable costs, and improve their global reach
Real World –Data and Analytics
- As regulators and payers place increasing importance on RWD, pharma is seen to partner with CRO, to support with predictive modeling, analytics, etc. This is driving CROs to either acquire or partner with specialists in this area
- E.g., PPD is working to expand its expertise for RWD by joining forces with a division of insurance giant Anthem
Emerging Markets
- The emerging markets of Brazil, China, Russia are witnessing increased trials, creating demand for regional CROs, with strong regulatory knowledge. For example, OCT, Intrials, etc.
- Examples: Offshoring of HEOR, pharmacovigilance services to India offer cost saving of ~30–45 percent. The acquisition of Quantum Solutions India by Parexel is an example of this growing trend. South Korea is emerging as the next early phase destinations, with a 8.22 percent CAGR between 2012 and 2016
New Areas of Research and Regulations
- The increased focus of pharma on large molecule therapy, rare diseases, specialty treatment, point of care assays, and personalized therapy & devices will require clinical trial testing and submission of data to the relevant regulatory authorities. For example, >80 percent of CRAs of INC Research are principally focused on one therapeutic area
- Also, the depth of regulatory scrutiny has increased, such as early RWD collection and reporting every adverse events, driving increased clinical trials
Clinical Research Organizations Market Overview
Currently, EDC and CTMS are the most commonly used data sources for clinical data management market, however, with the growing adoption of RBM, majority of the companies will move to more sophisticated technologies for clinical research organization.
60 percent of trial costs are accounted by the overall monitoring budgets, including the number of monitors required for the trial, their travel overheads, and the patient recruitment & retention. Hence, the major innovations are being observed in these two spaces, so as to minimize financial expenditures and expedite drug launch.
The fragmented contract research organizations market witnessed continuous mergers and acquisitions from 2012 to 2017. This has, in turn, resulted in continued consolidation of the contract research organization market, with the top 10 suppliers, holding >55 percent of the market share.
Why You Should Buy This Report
- The Beroe analysis report gives the CRO market drivers, technology trends and innovations and outsourcing penetration analysis.
- The report lists out the market share of the top contract research organizations, key mergers and acquisitions, recent trends and innovations and best industry practices.
- It lists the major suppliers in the clinical data management market.
Interesting Reads:
Discover the world of market intelligence and how it can elevate your business strategies.
Learn more about how market intelligence can enable informed decision-making, help identify growth opportunities, manage risks, and shape your business's strategic direction.
Get Ahead with AI-Enabled Market Insights Schedule a Demo Now
Schedule a Demo Now
Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk
Unlock hidden opportunities in the Construction industry

Published: May 10, 2023 Report Code: GDCON398905-MP-L5
- Share on Twitter
- Share on LinkedIn
- Share on Facebook
- Share on Threads
- Share via Email
- Report Overview
Methodology
Equip yourself with the essential tools needed to make informed and profitable decisions with our Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk report.
Note: This is an on-demand report that will be delivered upon request. The report will be delivered within 2 to 3 business days of the purchase, excluding weekends and holidays. Certain sections of the report may be removed or altered based on data availability and relevance.
Why choose our report?
- Unleash the full story: Dive deep into the Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk project and gain access to vital information such as its value, progress, and key dates. By building a complete picture, you stay one step ahead of the competition.
- Meet the influencers: Get a view of the key stakeholders involved in the Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk project. From developers and contractors to architects, this report introduces you to the key players with whom you can forge valuable connections and unlock exciting collaboration opportunities.
- Uncover new business opportunities: Are you in the business of supplying products or services related to the construction industry? This report is your guide to identifying lucrative opportunities within the Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk project, showcasing your offerings, and boosting your chances of securing valuable contracts.
- Turbocharge your business development: Time is precious, and we understand that. With this report in your hands, you can save valuable hours that would otherwise be spent tirelessly researching and prospecting. The comprehensive insights provided will expedite your new business development efforts, giving you a head start in seizing exciting opportunities.
What is included in the report?
Project name, value, location, stage, sector, announcement quarter, start quarter, end quarter, scope, background, description, key stakeholders.
Data in the Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk report has been gathered from tracking over 60,000 news, company and government sources, as well as primary research with direct contact with key project stakeholders.
Following wealth of information on Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk is covered in the scope of this report:
• Key Stakeholders
Reasons to Buy
• Save time developing new business opportunities
Key Players
Frequently asked questions.
- Currency Conversion is for Indicative purpose only. All orders are processed in US Dollars only.
- USD - US Dollar
- AUD — Australian Dollar
- BRL — Brazilian Real
- CNY — Yuan Renminbi
- GBP — Pound Sterling
- INR — Indian Rupee
- JPY — Japanese Yen
- ZAR — South African Rand
- USD — US Dollar
- RUB — Russian Ruble
Can be used by individual purchaser only
Can be shared by unlimited users within one corporate location e.g. a regional office
Can be shared globally by unlimited users within the purchasing corporation e.g. all employees of a single company
Undecided about purchasing this report?
Get in touch to find out about multi-purchase discounts.
[email protected] Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Sample Report
Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk was curated by the best experts in the industry and we are confident about its unique quality. However, we want you to make the most beneficial decision for your business, so we offer free sample report to help you:
- Assess the relevance of the report
- Evaluate the quality of the report
- Justify the cost
Download your copy of the sample report and make an informed decision about whether the full report will provide you with the insights and information you need.
Below is a sample report to help you understand the structure of the report you are buying

“The GlobalData platform is our go-to tool for intelligence services. GlobalData provides an easy way to access comprehensive intelligence data around multiple sectors, which essentially makes it a one-for-all intelligence platform, for tendering and approaching customers.
GlobalData is very customer orientated, with a high degree of personalised services, which benefits everyday use. The highly detailed project intelligence and forecast reports can be utilised across multiple departments and workflow scopes, from operational to strategic level, and often support strategic decisions. GlobalData Analytics and visualisation solutions has contributed positively when preparing management presentations and strategic papers.”
“COVID-19 has caused significant interference to our business and the COVID-19 intelligence from GlobalData has helped us reach better decisions around strategy. These two highlights have helped enormously to understand the projections into the future concerning our business units, we also utilise the project database to source new projects for Liebherr-Werk to use as an additional source to pitch for new business.”
Your daily news has saved me a lot of time and keeps me up-to-date with what is happening in the market, I like that you almost always have a link to the source origin. We also use your market data in our Strategic Business Process to support our business decisions. By having everything in one place on the Intelligence Center it has saved me a lot of time versus looking on different sources, the alert function also helps with this.
Having used several other market research companies, I find that GlobalData manages to provide that ‘difficult-to-get’ market data that others can’t, as well as very diverse and complete consumer surveys.
Our experience with GlobalData has been very good, from the platform itself to the people. I find that the analysts and the account team have a high level of customer focus and responsiveness and therefore I can always rely on. The platform is more holistic than other providers. It is convenient and almost like a one stop shop. The pricing suite is highly competitive and value for our organisation.
I like reports that inform new segments such as the analysis on generation Z, millennials, the impact of COVID 19 to our banking customers and their new channel habits. Secondly the specialist insight on affluent sector significantly increases our understanding about this group of customers. The combination of those give us depth and breadth of the evolving market.
I’m in the business of answering and helping people make decisions so with the intelligence center I can do that, effectively and efficiently. I can share quickly key insights that answer and satisfy our country stakeholders by giving them many quality studies and primary research about competitive landscape beyond the outlook of our bank. It helps me be seen as an advisory partner and that makes a big difference. A big benefit of our subscription is that no one holds the whole data and because it allows so many people, so many different parts of our organisation have access, it enables all teams to have the same level of knowledge and decision support.
“I know that I can always rely on Globaldata’s work when I’m searching for the right consumer and market insights. I use Globaldata insights to understand the changing market & consumer landscape and help create better taste & wellbeing solutions for our customers in food, beverage and healthcare industries.
Globaldata has the right data and the reports are of very high quality compared to your competitors. Globaldata not only has overall market sizes & consumer insights on food & beverages but also provides insights at the ingredient & flavour level. That is key for B2B companies like Givaudan. This way we understand our customers’ business and also gain insight to our unique industry”
GlobalData provides a great range of information and reports on various sectors that is highly relevant, timely, easy to access and utilise. The reports and data dashboards help engagement with clients; they provide valuable industry and market insights that can enrich client conversations and can help in the shaping of value propositions. Moreover, using GlobalData products has helped increase my knowledge of the finance sector, the players within it, and the general threats and opportunities.
I find the consumer surveys that are carried out to be extremely beneficial and not something I have seen anywhere else. They provided an insightful view of why and which consumers take (or don’t) particular financial products. This can help shape conversations with clients to ensure they make the right strategic decisions for their business.
One of the challenges I have found is that data in the payments space is often piecemeal. With GD all of the data I need is in one place, but it also comes with additional market reports that provide useful extra context and information. Having the ability to set-up alerts on relevant movements in the industry, be it competitors or customers, and have them emailed directly to me, ensures I get early sight of industry activity and don’t have to search for news.
Related reports

Every Company Report we produce is powered by the GlobalData Intelligence Center.
Subscribing to our intelligence platform means you can monitor developments at Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk in real time.
- Access a live Omsk Oblast Gov – Omsk Waste Treatment Plant – Omsk dashboard for 12 months, with up-to-the-minute insights.
- Fuel your decision making with real-time deal coverage and media activity.
- Turn insights on financials, deals, products and pipelines into powerful agents of commercial advantage.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Biden Economy
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds Rates
- Top Mutual Funds
- Options: Highest Open Interest
- Options: Highest Implied Volatility
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Stock Comparison
- Advanced Chart
- Currency Converter
- Credit Cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Credit Card Offers
- Best Free Checking
- Student Loans
- Personal Loans
- Car insurance
- Mortgage Refinancing
- Mortgage Calculator
- Morning Brief
- Market Domination
- Market Domination Overtime
- Asking for a Trend
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Financial Freestyle
- Capitol Gains
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Omsk, oblast of -- moody's announces completion of a periodic review of ratings of omsk, oblast of.
Announcement of Periodic Review: Moody's announces completion of a periodic review of ratings of Omsk, Oblast of
Global Credit Research - 21 Jul 2020
London, 21 July 2020 -- Moody's Investors Service ("Moody's") has completed a periodic review of the ratings of Omsk, Oblast of and other ratings that are associated with the same analytical unit. The review was conducted through a portfolio review in which Moody's reassessed the appropriateness of the ratings in the context of the relevant principal methodology(ies), recent developments, and a comparison of the financial and operating profile to similarly rated peers. The review did not involve a rating committee. Since 1 January 2019, Moody's practice has been to issue a press release following each periodic review to announce its completion.
This publication does not announce a credit rating action and is not an indication of whether or not a credit rating action is likely in the near future. Credit ratings and outlook/review status cannot be changed in a portfolio review and hence are not impacted by this announcement. For any credit ratings referenced in this publication, please see the ratings tab on the issuer/entity page on www.moodys.com for the most updated credit rating action information and rating history.
Key rating considerations are summarized below.
The credit profile of the Oblast of Omsk (Ba3) incorporates low likelihood of extraordinary support from the federal government. In addition, it reflects the region's historically moderate or weak operating performance, some concentration of tax revenues and substantial refinancing risks given the uneven repayment schedule with bulky repayments. The region suffers from elevated population migration given the subdued regional economic wealth compared to national average. Its economy demonstrates weak growth which translates into modest dynamics of tax revenues. Moderate operating performance and a consistent need for market access for debt refinancing mean the region remains relatively vulnerable to an ongoing supply of credit and possible adverse economic scenarios. More positively, the credit profile incorporates some resilience of revenues to economic cycles due to relative stability of the local economy and authorities' clear policy to reduce leverage which declined to moderate levels during the last three years.
This document summarizes Moody's view as of the publication date and will not be updated until the next periodic review announcement, which will incorporate material changes in credit circumstances (if any) during the intervening period.
The principal methodology used for this review was Regional and Local Governments published in January 2018. Please see the Rating Methodologies page on www.moodys.com for a copy of this methodology.
This announcement applies only to EU rated and EU endorsed ratings. Non EU rated and non EU endorsed ratings may be referenced above to the extent necessary, if they are part of the same analytical unit.
This publication does not announce a credit rating action. For any credit ratings referenced in this publication, please see the ratings tab on the issuer/entity page on www.moodys.com for the most updated credit rating action information and rating history.
© 2020 Moody's Corporation, Moody's Investors Service, Inc., Moody's Analytics, Inc. and/or their licensors and affiliates (collectively, "MOODY'S"). All rights reserved.
CREDIT RATINGS ISSUED BY MOODY'S INVESTORS SERVICE, INC. AND/OR ITS CREDIT RATINGS AFFILIATES ARE MOODY'S CURRENT OPINIONS OF THE RELATIVE FUTURE CREDIT RISK OF ENTITIES, CREDIT COMMITMENTS, OR DEBT OR DEBT-LIKE SECURITIES, AND MATERIALS, PRODUCTS, SERVICES AND INFORMATION PUBLISHED BY MOODY'S (COLLECTIVELY, "PUBLICATIONS") MAY INCLUDE SUCH CURRENT OPINIONS. MOODY'S INVESTORS SERVICE DEFINES CREDIT RISK AS THE RISK THAT AN ENTITY MAY NOT MEET ITS CONTRACTUAL FINANCIAL OBLIGATIONS AS THEY COME DUE AND ANY ESTIMATED FINANCIAL LOSS IN THE EVENT OF DEFAULT OR IMPAIRMENT. SEE MOODY'S RATING SYMBOLS AND DEFINITIONS PUBLICATION FOR INFORMATION ON THE TYPES OF CONTRACTUAL FINANCIAL OBLIGATIONS ADDRESSED BY MOODY'S INVESTORS SERVICE CREDIT RATINGS. CREDIT RATINGS DO NOT ADDRESS ANY OTHER RISK, INCLUDING BUT NOT LIMITED TO: LIQUIDITY RISK, MARKET VALUE RISK, OR PRICE VOLATILITY. CREDIT RATINGS, NON-CREDIT ASSESSMENTS ("ASSESSMENTS"), AND OTHER OPINIONS INCLUDED IN MOODY'S PUBLICATIONS ARE NOT STATEMENTS OF CURRENT OR HISTORICAL FACT. MOODY'S PUBLICATIONS MAY ALSO INCLUDE QUANTITATIVE MODEL-BASED ESTIMATES OF CREDIT RISK AND RELATED OPINIONS OR COMMENTARY PUBLISHED BY MOODY'S ANALYTICS, INC. AND/OR ITS AFFILIATES. MOODY'S CREDIT RATINGS, ASSESSMENTS, OTHER OPINIONS AND PUBLICATIONS DO NOT CONSTITUTE OR PROVIDE INVESTMENT OR FINANCIAL ADVICE, AND MOODY'S CREDIT RATINGS, ASSESSMENTS, OTHER OPINIONS AND PUBLICATIONS ARE NOT AND DO NOT PROVIDE RECOMMENDATIONS TO PURCHASE, SELL, OR HOLD PARTICULAR SECURITIES. MOODY'S CREDIT RATINGS, ASSESSMENTS, OTHER OPINIONS AND PUBLICATIONS DO NOT COMMENT ON THE SUITABILITY OF AN INVESTMENT FOR ANY PARTICULAR INVESTOR. MOODY'S ISSUES ITS CREDIT RATINGS, ASSESSMENTS AND OTHER OPINIONS AND PUBLISHES ITS PUBLICATIONS WITH THE EXPECTATION AND UNDERSTANDING THAT EACH INVESTOR WILL, WITH DUE CARE, MAKE ITS OWN STUDY AND EVALUATION OF EACH SECURITY THAT IS UNDER CONSIDERATION FOR PURCHASE, HOLDING, OR SALE.
MOODY'S CREDIT RATINGS,ASSESSMENTS, OTHER OPINIONS, AND PUBLICATIONS ARE NOT INTENDED FOR USE BY RETAIL INVESTORS AND IT WOULD BE RECKLESS AND INAPPROPRIATE FOR RETAIL INVESTORS TO USE MOODY'S CREDIT RATINGS, ASSESSMENTS, OTHER OPINIONS OR PUBLICATIONS WHEN MAKING AN INVESTMENT DECISION. IF IN DOUBT YOU SHOULD CONTACT YOUR FINANCIAL OR OTHER PROFESSIONAL ADVISER.
ALL INFORMATION CONTAINED HEREIN IS PROTECTED BY LAW, INCLUDING BUT NOT LIMITED TO, COPYRIGHT LAW, AND NONE OF SUCH INFORMATION MAY BE COPIED OR OTHERWISE REPRODUCED, REPACKAGED, FURTHER TRANSMITTED, TRANSFERRED, DISSEMINATED, REDISTRIBUTED OR RESOLD, OR STORED FOR SUBSEQUENT USE FOR ANY SUCH PURPOSE, IN WHOLE OR IN PART, IN ANY FORM OR MANNER OR BY ANY MEANS WHATSOEVER, BY ANY PERSON WITHOUT MOODY'S PRIOR WRITTEN CONSENT.
MOODY'S CREDIT RATINGS,ASSESSMENTS, OTHER OPINIONS AND PUBLICATIONS ARE NOT INTENDED FOR USE BY ANY PERSON AS A BENCHMARK AS THAT TERM IS DEFINED FOR REGULATORY PURPOSES AND MUST NOT BE USED IN ANY WAY THAT COULD RESULT IN THEM BEING CONSIDERED A BENCHMARK.
All information contained herein is obtained by MOODY'S from sources believed by it to be accurate and reliable. Because of the possibility of human or mechanical error as well as other factors, however, all information contained herein is provided "AS IS" without warranty of any kind. MOODY'S adopts all necessary measures so that the information it uses in assigning a credit rating is of sufficient quality and from sources MOODY'S considers to be reliable including, when appropriate, independent third-party sources. However, MOODY'S is not an auditor and cannot in every instance independently verify or validate information received in the rating process or in preparing its Publications.
To the extent permitted by law, MOODY'S and its directors, officers, employees, agents, representatives, licensors and suppliers disclaim liability to any person or entity for any indirect, special, consequential, or incidental losses or damages whatsoever arising from or in connection with the information contained herein or the use of or inability to use any such information, even if MOODY'S or any of its directors, officers, employees, agents, representatives, licensors or suppliers is advised in advance of the possibility of such losses or damages, including but not limited to: (a) any loss of present or prospective profits or (b) any loss or damage arising where the relevant financial instrument is not the subject of a particular credit rating assigned by MOODY'S.
To the extent permitted by law, MOODY'S and its directors, officers, employees, agents, representatives, licensors and suppliers disclaim liability for any direct or compensatory losses or damages caused to any person or entity, including but not limited to by any negligence (but excluding fraud, willful misconduct or any other type of liability that, for the avoidance of doubt, by law cannot be excluded) on the part of, or any contingency within or beyond the control of, MOODY'S or any of its directors, officers, employees, agents, representatives, licensors or suppliers, arising from or in connection with the information contained herein or the use of or inability to use any such information.
NO WARRANTY, EXPRESS OR IMPLIED, AS TO THE ACCURACY, TIMELINESS, COMPLETENESS, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE OF ANY CREDIT RATING, ASSESSMENT, OTHER OPINION OR INFORMATION IS GIVEN OR MADE BY MOODY'S IN ANY FORM OR MANNER WHATSOEVER.
Moody's Investors Service, Inc., a wholly-owned credit rating agency subsidiary of Moody's Corporation ("MCO"), hereby discloses that most issuers of debt securities (including corporate and municipal bonds, debentures, notes and commercial paper) and preferred stock rated by Moody's Investors Service, Inc. have, prior to assignment of any credit rating, agreed to pay to Moody's Investors Service, Inc. for credit ratings opinions and services rendered by it fees ranging from $1,000 to approximately $2,700,000. MCO and Moody's investors Service also maintain policies and procedures to address the independence of Moody's Investors Service credit ratings and credit rating processes. Information regarding certain affiliations that may exist between directors of MCO and rated entities, and between entities who hold credit ratings from Moody's Investors Service and have also publicly reported to the SEC an ownership interest in MCO of more than 5%, is posted annually at www.moodys.com under the heading "Investor Relations — Corporate Governance — Director and Shareholder Affiliation Policy."
Additional terms for Australia only: Any publication into Australia of this document is pursuant to the Australian Financial Services License of MOODY'S affiliate, Moody's Investors Service Pty Limited ABN 61 003 399 657AFSL 336969 and/or Moody's Analytics Australia Pty Ltd ABN 94 105 136 972 AFSL 383569 (as applicable). This document is intended to be provided only to "wholesale clients" within the meaning of section 761G of the Corporations Act 2001. By continuing to access this document from within Australia, you represent to MOODY'S that you are, or are accessing the document as a representative of, a "wholesale client" and that neither you nor the entity you represent will directly or indirectly disseminate this document or its contents to "retail clients" within the meaning of section 761G of the Corporations Act 2001. MOODY'S credit rating is an opinion as to the creditworthiness of a debt obligation of the issuer, not on the equity securities of the issuer or any form of security that is available to retail investors.
Additional terms for Japan only: Moody's Japan K.K. ("MJKK") is a wholly-owned credit rating agency subsidiary of Moody's Group Japan G.K., which is wholly-owned by Moody's Overseas Holdings Inc., a wholly-owned subsidiary of MCO. Moody's SF Japan K.K. ("MSFJ") is a wholly-owned credit rating agency subsidiary of MJKK. MSFJ is not a Nationally Recognized Statistical Rating Organization ("NRSRO"). Therefore, credit ratings assigned by MSFJ are Non-NRSRO Credit Ratings. Non-NRSRO Credit Ratings are assigned by an entity that is not a NRSRO and, consequently, the rated obligation will not qualify for certain types of treatment under U.S. laws. MJKK and MSFJ are credit rating agencies registered with the Japan Financial Services Agency and their registration numbers are FSA Commissioner (Ratings) No. 2 and 3 respectively.
MJKK or MSFJ (as applicable) hereby disclose that most issuers of debt securities (including corporate and municipal bonds, debentures, notes and commercial paper) and preferred stock rated by MJKK or MSFJ (as applicable) have, prior to assignment of any credit rating, agreed to pay to MJKK or MSFJ (as applicable) for credit ratings opinions and services rendered by it fees ranging from JPY125,000 to approximately JPY250,000,000.
MJKK and MSFJ also maintain policies and procedures to address Japanese regulatory requirements.
- Partner with us
- Apply Online

Omsk State Medical University
The university.
- Recognitions
- Eligibility
- Fees Structure
Omsk State Medical University was established in 1920 in the Omsk city of the Russian Federation and is one of the oldest medical universities in Russia. It is also one of the largest as well top-ranked medical universities in Russia, constantly securing its position in the list of 100 best universities in Russia.
For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment. In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University.
Omsk State Medical University is duly approved by the Medical Council of India (MCI) and offers a 6-Year MBBS Program for local as well as international students. Medical aspirants from India who have qualified NEET can apply for direct admission to the MBBS Program of Omsk State Medical University.

Ministry of Science and Higher Education, Russia

World Directory of Medical Schools (WDOMS)

Medical Council of India (MCI)

Educational Commission for Foreign Medical Graduates (ECFMG)

Medical Council of Canada (MCC)

Foundation for Advancement of International Medical Education and Research (FAIMER)
To get admission to the MBBS Program of Omsk State Medical University, the student must qualify NEET-UG (National Eligibility cum Entrance Test-Undergraduate).
Besides NEET-UG, there is no requirement to go through any additional entrance examination.
|
|
|
|
|
|
|
| Fees for Tuition, Hostel, and Medical Insurance | Yearly Payments | Fees for Year I | US$ 5,000 |
| Fees for Year II | US$ 4,200 | |||
| Fees for Year III | US$ 4,200 | |||
| Fees for Year IV | US$ 4,200 | |||
| Fees for Year V | US$ 4,200 | |||
| Fees for Year VI | US$ 4,200 | |||
|
| Mess Charges | Yearly Payments | Mess charges | US$ 1200 per year |
RUS EDUCATION SUPPORT

INDIAN FOOD
MODERN CLASSROOMS
Medical Laboratories
Clinical Training
Recreational Facilities
Ensured Safety
FMGE (Foreign Medical Graduates Examination) Preparation

USMLE (United States Medical Licensing Examination) Preparation

- Omsk State Medical University was established in 1920 in the Omsk city of Russia.
- Omsk State Medical University constantly ranks in the list of 100 best universities in Russia.
- Since its inception, more than 35,000 doctors have been trained at Omsk State Medical University.
- For the clinical training of the students, Omsk State Medical University has affiliations with 33 hospitals and clinics.
- Library of Omsk State Medical University has a collection of more than 6,00,000 books.
University Address
Mbbs program, admission & support, medical licensing examination support, student life.

In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University, which is indeed a remarkable achievement. Now, every year, more than 8,500 students graduate from the University. The University has a team of about 500 faculty members. University’s campus is divided into six buildings.
For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment.
Library of Omsk State Medical University is one of the oldest and leading university libraries in the Russian Federation. It houses a collection of more than 6,00,000 books and also provides access to eBooks, online medical journals, and other internet resources. To help the students easily find and borrow books, an electronic system is installed in the library. Students are given access to the library database in Russian as well as the English language.
The University is actively involved in various research projects related to medicine and healthcare. Students at Omsk State Medical University get an opportunity to participate in those projects, be a part of the future of medicine, and develop significant skills during the program.
Quality of Omsk State Medical University’s education system is also certified by the International Organization for Standardization (ISO). For a personalized and modern learning experience, the University has well-equipped classrooms and follows an apt-student teacher ratio. On the campus, the students also have access to various laboratories, including Physics lab, Chemistry lab, and Physiology lab.
The University preserves its 100 years of glorious history and achievements with the help of a dedicated museum on the Campus, which is open for all students and University visitors.
Omsk State Medical University is also associated with many medical universities and other institutions in Russia as well as other countries to increase joint initiatives, share resources, and facilitate student exchange programs.
Omsk State Medical University is well-recognized in Russia as well as internationally. It is listed in the World Directory of Medical Schools (WDOMS) and certified by the Educational Commission for Foreign Medical Graduates (ECFMG), United States of America. Also, Omsk State Medical University is duly approved by the Medical Council of India (MCI) and the Medical Council of Canada (MCC). Omsk State Medical University offers a 6-Year MBBS Program for local as well as international students. Medical aspirants from India who have qualified NEET can apply for direct admission to the MBBS Program of Omsk State Medical University.

Omsk State Medical University Ulitsa Lenina, 12, Omsk, Omsk Oblast, Russia, 644099

Omsk State Medical University offers a 6-Year MBBS Program in Russian Medium. For international students, classes for initial years may be conducted in English language.
The Program for MBBS in Russia is focused on building a strong academic base with a pragmatic approach to education and medical research. To provide hands-on clinical experience, the students studying MBBS in Russia are involved in clinical training from the second year of MBBS. While education in classrooms and laboratories helps the students develop academic skills and sound theoretical understanding, clinical training in University-affiliated hospitals help them apply their knowledge into practice.

To get admission to the MBBS Program of Omsk State Medical University, you can apply online at Rus Education website.
Rus Education is exclusively authorized by the Russian Centre for Science and Culture (Cultural Department of The Embassy of the Russian Federation in India) to promote Russian Education among Indian Citizens. Rus Education is also an authorized associate of Omsk State Medical University. We facilitate one-window admission to the MBBS Program of Omsk State Medical University with no requirement of any donation or capitation and without any entrance examination.

| FMGE (Foreign Medical Graduates Examination) Preparation |
|
| USMLE (United States Medical Licensing Examination) Preparation |
|

For the living arrangement of students, Omsk State Medical University maintains 6 dormitories. Hostel facilities are well-furnished and equipped with facilities required for daily student life, including shared kitchens, sports facilities and study space. Two or three students share one hostel room.
The University maintains its own sports and recreational facilities, and interested students can play the sports of their choice to relax and stay fit.
To arrange recreational activities and community programs for the students, the University has a dedicated Department of Extracurricular and Social Activities. To involve students in social work, University runs different volunteering and charity activities. Depending on their interests, students can also join creative activities like dance, singing, and humor organized by student clubs.
For promoting healthy lifestyle and fitness among students, the University has 22 sports clubs and also operates two gyms and five fitness centers on the campus. These facilities open up a wide array of options for the students to play their favorite sports and stay energetic.
On off-days, students can also go out to explore Omsk city, which is one of the largest cities in Russia, offering plenty of avenues for amusement. Omsk has numerous museums, historical architectures, classic theaters, and educational institutions that students can explore. While food is available through the mess facility of the University, occasionally, students can go out for dining. To get around in Omsk, students don’t face any problems as the city has well-regulated means for public transport, including buses, taxis, metro, and railways. Being a city where people of many nationalities and religions live together, Omsk offers a high culture and friendly environment for strangers and locals alike.
So, Omsk State Medical University and the Omsk city offers the very best of education as well as life, making student life not only fruitful but exciting, fulfilling, and full of experiences, and memories.
TOP MEDICAL UNIVERSITIES IN RUSSIA
.jpg)
Perm State Medical University
.jpg)
Tver State Medical University
.jpg)
Orenburg State Medical University

Mari State University
.jpg)
Siberian State Medical University
A php error was encountered.
Severity: Notice
Message: Undefined variable: countries
Filename: includes/footer.php
Line Number: 26
File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 26 Function: _error_handler
File: /home/mbbsinrussia/public_html/application/controllers/University.php Line: 46 Function: view
File: /home/mbbsinrussia/public_html/index.php Line: 315 Function: require_once
Severity: Warning
Message: Invalid argument supplied for foreach()
Message: Undefined variable: state
Line Number: 44
File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 44 Function: _error_handler
©2024-25 Rus Education.

IMAGES
COMMENTS
Clinical Research Organization Market Snapshot (2023 to 2033) According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033. Market Outlook: The key players in the market are Charles River ...
The global contract research organization (CRO) services market size was valued at USD 79.54 billion in 2023. The market is projected to grow from USD 86.33 billion in 2024 to USD 175.46 billion by 2032, exhibiting a CAGR of 9.3% during the forecast period. Moreover, the U.S. contract research organization services market is projected to grow ...
Contract Research Organization Market size accounted for USD 56.7 billion in 2022 and is estimated to grow at 6.9% CAGR between 2023 and 2032. Rising adoption of advanced technologies for efficient R&D outcomes and the increasing outsourcing trends in the clinical trials industry has largely influenced the global market growth.
Global Clinical Research Organization (CRO) Market Overview 18 Market Segmentation by Select Geographies 19 to 20 ... Clinical Research Organization is an organization contracted by a pharma, bio-pharma or a medical device company to take the lead in managing that companys trials and medical
The global Clinical Research Organization (CRO) market was estimated at USD 80.6 Billion in 2022 and is anticipated to reach USD 220.3 Billion by 2031, expanding at a CAGR of 12.2% during the forecast period. A clinical research organization (CRO) provides support to the pharmaceuticals, biotechnology, and medical devices industries in the form ...
Contract Research Organization Market Analysis. The Global Contract Research Organization Market is expected to register a CAGR of 7% during the forecast period. The COVID-19 pandemic had a huge effect on the contract research organization (CRO) market because clinical trials were still going on to find effective treatments and vaccines for the ...
The global clinical research organization market is projected to grow at a CAGR of 7.6% from 2023 to 2028. Clinical research organizations (CROs) offer outsourced services to the biotechnology and pharmaceutical sectors. They carry out clinical studies for novel medications and treatments. Drug firms themselves or academic institutions doing ...
Healthcare CRO Market Size & Trends. The global healthcare contract research organization (CRO) market size was valued at USD 50.55 billion in 2023 and is anticipated to grow at a CAGR of 7.0% from 2024 to 2030. Increasing investment in R&D programs, preference for outsourcing activities due to time & cost constraints, and patent expiration of blockbuster drugs are key factors anticipated to ...
In 2023, the leading contract research organizations worldwide included Laboratory Corporation of America Holdings, Thermo Fisher Scientific, and IQVIA. The first ranked company, Thermo Fisher ...
The clinical research organization market is anticipated to grow significantly over the forecast period. The market for clinical research organizations (CROs) has grown significantly due to factors such as the demand for specialized knowledge, growing R&D expenses, and the complexity of clinical trials. ...
Clinical Research Organizations. 9 comprehensive market analysis studies and industry reports on the Clinical Research Organizations sector, offering an industry overview with historical data since 2019 and forecasts up to 2029. This includes a detailed market research of 87 research companies, enriched with industry statistics, industry ...
The global clinical trials market size was valued at USD 80.7 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.49% from 2024 to 2030. The market growth spiked in 2020 owing to the COVID-19 pandemic. This growth pattern was witnessed by both virtual clinical trials and traditional ones.
The pharmaceutical & biotechnology companies dominated the global clinical research organization market withholding the total market share of about 45.6% by the end of 2022, owing to the ...
This boosts the global contract clinical research organization market growth. For instance, Merck & Co., Inc., a multinational pharmaceutical company, announced that its R&D expenses were US$ 9.6 billion in the fourth quarter of 2023 as compared to US$ 3.8 billion in the fourth quarter of 2022. R&D expenses were US$ 30.5 billion for 2023 as ...
The global clinical trials market was estimated to be worth $38.7 billion in 2021 and is expected to reach £52 billion by 2026. In this article, we look at the top 12 CROs in the world, highlighting the companies driving this considerable growth and accelerating research and development across the globe.. ICON. Recognised as the world's largest and most comprehensive CRO powered by ...
2023/2024 CLINICAL RESEARCH ORGANIZATION INSIGHTS REPORT 9 Year 1 Year 2 Year 3 Year 4 Year 5 Skills Pay Given that market rates have not kept up with inflation, once a CRA gains experience, they often look for a more lucrative opportunity. If they stay with the same company, assuming standard merit increases, once a CRA has three to five years of
The clinical trials market size was valued at USD 55.8 billion in 2023 and is estimated to grow at 5.4% CAGR from 2024 to 2032 driven by increasing prevalence of chronic diseases across the globe. ... leveraging contract research organization services enables manufacturers and sponsors to focus on production capacity and improve internal ...
In early 2022, CATO SMS merged with Pharm-Olam, a global clinical research organization delivering clinical trial services to organizations around the world. KCR - 0.15 billion USD, 700 employees . KCR is a clinical development solutions provider creating value for biotechnology and pharmaceutical organizations.
Clinical Research Organizations market report transcript. The global CRO market was estimated at $31.6 billion in 2018, and it is expected to grow at a CAGR of 12 percent to reach $45.2 billion by 2022. This is due to the increased outsourcing (>50 percent) seen from the pharmaceutical and biotechnology companies, with 85 percent comprised by ...
Tsentral'nyy Rynok is a market in Omsk Oblast, Western Siberia. Mapcarta, the open map.
Business Intelligence & Marketing Manager, SAL Heavy Lift "COVID-19 has caused significant interference to our business and the COVID-19 intelligence from GlobalData has helped us reach better decisions around strategy. ... Having used several other market research companies, I find that GlobalData manages to provide that 'difficult-to-get ...
Moody's Investors Service ("Moody's") has completed a periodic review of the ratings of Omsk, Oblast of and other ratings that are associated with the same analytical unit. The review was ...
For the clinical training of the students, the University has affiliations with 33 hospitals and clinics in the city. Students are provided education with state-of-the-art infrastructure, including research labs and modern medical equipment. In a century-long time, more than 35,000 doctors have been trained at Omsk State Medical University.