An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Transplantation Without Overimmunosuppression (TWO) study protocol: a phase 2b randomised controlled single-centre trial of regulatory T cell therapy to facilitate immunosuppression reduction in living donor kidney transplant recipients
Affiliations.
- 1 Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK [email protected].
- 2 Oxford Transplant Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
- 3 Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
- 4 Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
- 5 NIHR Biomedical Research Centre GMP unit, Guy's Hospital, London, UK.
- 6 Peter Gorer Department of Immunobiology, King's College London Faculty of Life Sciences and Medicine, London, UK.
- PMID: 35428650
- PMCID: PMC9014059
- DOI: 10.1136/bmjopen-2022-061864
Introduction: Regulatory T cell (Treg) therapy has been demonstrated to facilitate long-term allograft survival in preclinical models of transplantation and may permit reduction of immunosuppression and its associated complications in the clinical setting. Phase 1 clinical trials have shown Treg therapy to be safe and feasible in clinical practice. Here we describe a protocol for the TWO study, a phase 2b randomised control trial of Treg therapy in living donor kidney transplant recipients that will confirm safety and explore efficacy of this novel treatment strategy.
Methods and analysis: 60 patients will be randomised on a 1:1 basis to Treg therapy (TR001) or standard clinical care (control). Patients in the TR001 arm will receive an infusion of autologous polyclonal ex vivo expanded Tregs 5 days after transplantation instead of standard monoclonal antibody induction. Maintenance immunosuppression will be reduced over the course of the post-transplant period to low-dose tacrolimus monotherapy. Control participants will receive a standard basiliximab-based immunosuppression regimen with long-term tacrolimus and mycophenolate mofetil immunosuppression. The primary endpoint is biopsy proven acute rejection over 18 months; secondary endpoints include immunosuppression burden, chronic graft dysfunction and drug-related complications.
Ethics and dissemination: Ethical approval has been provided by the National Health Service Health Research Authority South Central-Oxford A Research Ethics Committee (reference 18/SC/0054). The study also received authorisation from the UK Medicines and Healthcare products Regulatory Agency and is being run in accordance with the principles of Good Clinical Practice, in collaboration with the registered trials unit Oxford Clinical Trials Research Unit. Results from the TWO study will be published in peer-reviewed scientific/medical journals and presented at scientific/clinical symposia and congresses.
Trial registration number: ISRCTN: 11038572; Pre-results.
Keywords: Clinical trials; IMMUNOLOGY; Nephrology; TRANSPLANT MEDICINE; Transplant surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
PubMed Disclaimer
Conflict of interest statement
Competing interests: PH is an advisor to Sangamo Therapeutics. Envarsus long-acting sustained release tacrolimus is provided by Chiesi on a cost-neutral basis. MJB has received an educational grant and speakers fees from Astellas UK.
Diagrammatic representation of the immunosuppressive…
Diagrammatic representation of the immunosuppressive regimen used in The TWO study.
Overview of maintenance immunosuppression dosing…
Overview of maintenance immunosuppression dosing with minimisation in the TR001 (cell therapy) arm.
Key time points alongside clinical…
Key time points alongside clinical and immune monitoring plans. EQ-5D-5L, EuroQol 5 Dimensions…
Similar articles
- Individualised immunosuppression with intravenously administered donor-derived modified immune cells compared with standard of care in living donor kidney transplantation (TOL-2 Study): protocol for a multicentre, open-label, phase II, randomised controlled trial. Morath C, Schmitt A, Schmitt M, Wang L, Kleist C, Opelz G, Süsal C, Tran TH, Scherer S, Schwenger V, Kemmner S, Fischereder M, Stangl M, Hauser IA, Sommerer C, Nusshag C, Kälble F, Speer C, Benning L, Bischofs C, Sauer S, Schubert ML, Kunz A, Hückelhoven-Krauss A, Neuber B, Mehrabi A, Schwab C, Waldherr R, Sander A, Büsch C, Czock D, Böhmig GA, Reiser J, Roers A, Müller-Tidow C, Terness P, Zeier M, Daniel V, Schaier M. Morath C, et al. BMJ Open. 2022 Nov 11;12(11):e066128. doi: 10.1136/bmjopen-2022-066128. BMJ Open. 2022. PMID: 36368749 Free PMC article.
- Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Harden PN, Game DS, Sawitzki B, Van der Net JB, Hester J, Bushell A, Issa F, Brook MO, Alzhrani A, Schlickeiser S, Scotta C, Petchey W, Streitz M, Blancho G, Tang Q, Markmann J, Lechler RI, Roberts ISD, Friend PJ, Hilton R, Geissler EK, Wood KJ, Lombardi G. Harden PN, et al. Am J Transplant. 2021 Apr;21(4):1603-1611. doi: 10.1111/ajt.16395. Epub 2021 Feb 2. Am J Transplant. 2021. PMID: 33171020 Free PMC article. Clinical Trial.
- Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ, Roberts ISD, Streitz M, Josien R, Böger CA, Scottà C, Markmann JF, Hester JL, Juerchott K, Braudeau C, James B, Contreras-Ruiz L, van der Net JB, Bergler T, Caldara R, Petchey W, Edinger M, Dupas N, Kapinsky M, Mutzbauer I, Otto NM, Öllinger R, Hernandez-Fuentes MP, Issa F, Ahrens N, Meyenberg C, Karitzky S, Kunzendorf U, Knechtle SJ, Grinyó J, Morris PJ, Brent L, Bushell A, Turka LA, Bluestone JA, Lechler RI, Schlitt HJ, Cuturi MC, Schlickeiser S, Friend PJ, Miloud T, Scheffold A, Secchi A, Crisalli K, Kang SM, Hilton R, Banas B, Blancho G, Volk HD, Lombardi G, Wood KJ, Geissler EK. Sawitzki B, et al. Lancet. 2020 May 23;395(10237):1627-1639. doi: 10.1016/S0140-6736(20)30167-7. Lancet. 2020. PMID: 32446407 Free PMC article.
- Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option? Höcker B, Tönshoff B. Höcker B, et al. Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
- Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, Taylor RS. Woodroffe R, et al. Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149 Review.
- The Role of Regulatory T Cells and Their Therapeutic Potential in Hypertensive Disease of Pregnancy: A Literature Review. Headen K, Jakaite V, Mesaric VA, Scotta C, Lombardi G, Nicolaides KH, Shangaris P. Headen K, et al. Int J Mol Sci. 2024 Apr 30;25(9):4884. doi: 10.3390/ijms25094884. Int J Mol Sci. 2024. PMID: 38732104 Free PMC article. Review.
- Promises and Pitfalls of Next-Generation Treg Adoptive Immunotherapy. Christofi P, Pantazi C, Psatha N, Sakellari I, Yannaki E, Papadopoulou A. Christofi P, et al. Cancers (Basel). 2023 Dec 17;15(24):5877. doi: 10.3390/cancers15245877. Cancers (Basel). 2023. PMID: 38136421 Free PMC article. Review.
- Contribution of αβ T cells to macrophage polarization and MSC recruitment and proliferation on titanium implants. Avery D, Morandini L, Gabriec M, Sheakley L, Peralta M, Donahue HJ, Martin RK, Olivares-Navarrete R. Avery D, et al. Acta Biomater. 2023 Oct 1;169:605-624. doi: 10.1016/j.actbio.2023.07.052. Epub 2023 Aug 1. Acta Biomater. 2023. PMID: 37532133
- Opportunities for Treg cell therapy for the treatment of human disease. Bluestone JA, McKenzie BS, Beilke J, Ramsdell F. Bluestone JA, et al. Front Immunol. 2023 Apr 19;14:1166135. doi: 10.3389/fimmu.2023.1166135. eCollection 2023. Front Immunol. 2023. PMID: 37153574 Free PMC article. Review.
- Can regulatory T cells improve outcomes of sensitised patients after HLA-Ab incompatible renal transplantation: study protocol for the Phase IIa GAMECHANgER-1 trial. Dudreuilh C, Jarvis P, Beadle N, Pilecka I, Shaw O, Gardner L, Scottà C, Mamode N, Game DS, Sanchez-Fueyo A, Lombardi G, Learoyd A, Douiri A, Dorling A. Dudreuilh C, et al. BMC Nephrol. 2023 Apr 28;24(1):117. doi: 10.1186/s12882-023-03157-7. BMC Nephrol. 2023. PMID: 37118685 Free PMC article.
- Poggio ED, Augustine JJ, Arrigain S, et al. . Long‐term kidney transplant graft survival-Making progress when most needed. Am J Transplant 2021;21:2824–32. 10.1111/ajt.16463 - DOI - PubMed
- Rama I, Grinyó JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol 2010;6:511–9. 10.1038/nrneph.2010.102 - DOI - PubMed
- Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol 2019;32:389–99. 10.1007/s40620-018-0549-4 - DOI - PMC - PubMed
- Weiner DE, Carpenter MA, Levey AS, et al. . Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant 2012;12:2437–45. 10.1111/j.1600-6143.2012.04101.x - DOI - PMC - PubMed
- Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol 2012;7:2058–70. 10.2215/CJN.04410512 - DOI - PubMed
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
Grants and funding
- MR/N027930/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- White Rose Research Online
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 27 September 2020
Cost-effectiveness of adrenaline for out-of-hospital cardiac arrest
- Felix Achana 1 , 2 ,
- Stavros Petrou 1 , 2 na1 ,
- Jason Madan 1 ,
- Kamran Khan 1 ,
- Chen Ji 1 ,
- Anower Hossain 3 ,
- Ranjit Lall 1 ,
- Anne-Marie Slowther 1 ,
- Charles D. Deakin 4 ,
- Tom Quinn 5 ,
- Jerry P. Nolan 1 , 6 ,
- Helen Pocock 1 , 7 ,
- Nigel Rees 8 ,
- Michael Smyth 1 , 9 ,
- Simon Gates 10 ,
- Dale Gardiner 11 &
- Gavin D. Perkins ORCID: orcid.org/0000-0003-3027-7548 1
for the PARAMEDIC2 Collaborators
Critical Care volume 24 , Article number: 579 ( 2020 ) Cite this article
4441 Accesses
12 Altmetric
Metrics details
The ‘Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration In Cardiac Arrest’ (PARAMEDIC2) trial showed that adrenaline improves overall survival, but not neurological outcomes. We sought to determine the within-trial and lifetime health and social care costs and benefits associated with adrenaline, including secondary benefits from organ donation.
We estimated the costs, benefits (quality-adjusted life years (QALYs)) and incremental cost-effectiveness ratios (ICERs) associated with adrenaline during the 6-month trial follow-up. Model-based analyses explored how results altered when the time horizon was extended beyond 6 months and the scope extended to include recipients of donated organs.
The within-trial (6 months) and lifetime horizon economic evaluations focussed on the trial population produced ICERs of £1,693,003 (€1,946,953) and £81,070 (€93,231) per QALY gained in 2017 prices, respectively, reflecting significantly higher mean costs and only marginally higher mean QALYs in the adrenaline group. The probability that adrenaline is cost-effective was less than 1% across a range of cost-effectiveness thresholds. Combined direct economic effects over the lifetimes of survivors and indirect economic effects in organ recipients produced an ICER of £16,086 (€18,499) per QALY gained for adrenaline with the probability that adrenaline is cost-effective increasing to 90% at a £30,000 (€34,500) per QALY cost-effectiveness threshold.
Conclusions
Adrenaline was not cost-effective when only directly related costs and consequences are considered. However, incorporating the indirect economic effects associated with transplanted organs substantially alters cost-effectiveness, suggesting decision-makers should consider the complexity of direct and indirect economic impacts of adrenaline.
Trial registration
ISRCTN73485024 . Registered on 13 March 2014.
Introduction
Although survival rates following adult out-of-hospital cardiac arrest have increased in recent years, they generally remain below 10% [ 1 , 2 ]. Survivors are at increased risk of cognitive and functional impairment and often take significant time to attain an acceptable quality of life or to return to work [ 3 ].
In some countries, when a patient dies after cardiac arrest, families can be asked to agree to the patient’s organs being donated for the benefit of others. A meta-analysis of 26 studies reported that, on average, 5.8% (95% CI 2.1–10.9%) of patients admitted to intensive care donate their organs for transplantation after brain stem death [ 4 ]. Observational studies suggest that cardiac arrest before organ donation does not adversely affect long-term graft function [ 5 ] and that such transplant programmes are cost-effective [ 6 , 7 ].
The PARAMEDIC2 (Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration In Cardiac Arrest) trial showed that adrenaline improves overall survival, but not long term neurological outcome [ 8 , 9 ]. In this paper, we explore the costs and benefits of adrenaline within the PARAMEDIC2 trial across within-trial and lifetime horizons and model the contribution of organ donation after a cardiac arrest on cost-effectiveness.
Trial background
PARAMEDIC2 (ISRCTN73485024) was a pragmatic, individually randomised, double-blind, controlled trial of 8014 adult patients with out-of-hospital cardiac arrest in the UK [ 9 ]. Patients, treated by five National Health Service (NHS) ambulance services between December 2014 and October 2017, were randomised to either parenteral adrenaline or saline placebo, along with standard care. The primary clinical outcome was the rate of survival at 30 days. Survival, neurological outcomes as indicated by the modified Rankin Scale (mRS) (ranging from 0 [no symptoms] to 6 [death]) [ 10 ] and economic outcomes were followed up from randomisation to 6 months. The South Central–Oxford C Research Ethics Committee (REC: 14/SC/0157) and the Medicines and Healthcare Products Regulatory Authority (EudraCT: 2014-000792-11) approved the study protocol. The trial was funded by the UK National Institute for Health Research Health Technology Assessment Programme (12/127/126). Further details of the trial are reported elsewhere [ 9 ].
Overview of economic evaluation
A cost-utility analysis was conducted, with results expressed in terms of incremental cost per quality-adjusted life year (QALY) gained through the use of adrenaline. The baseline economic evaluation was conducted from the perspective of the UK NHS and personal social services (PSS) [ 11 ], with a time horizon of 6 months post-randomisation. A decision-analytic model was used to extrapolate outcomes beyond trial follow-up and assess the cost-effectiveness of adrenaline over a lifetime horizon. All costs were expressed in British pounds sterling and valued at 2017 prices (with commensurate values in Euros estimated using purchasing power parities). Costs and QALYs accrued beyond the first year of follow-up were discounted to present values at 3.5% per annum in accordance with recommendations from the National Institute for Health and Care Excellence (NICE) [ 11 ]. The economic evaluation adhered to an approved pre-specified analytical plan which is available in the supplementary material (Additional file 1 ).
Measurement and valuation of resource use
Resource inputs associated with the pre-hospital emergency response, until the point of hospital admission or death, were extracted from the trial case report forms completed by research paramedics. These data included the number of emergency response staff/ambulance crew and vehicles in attendance, duration of emergency response, and cumulative doses of adrenaline administered. Unit costs for these resource inputs were drawn from national tariffs (Additional file 2 ).
For patients surviving to hospital admission, the economic costs associated with hospital care were extracted through data linkage with two national population-wide databases: Hospital Episode Statistics (HES) for England and the Patient Episode Database for Wales (PEDW). This provided patient-level profiles of resource use associated with hospital episodes for study patients over the trial time horizon, including emergency department attendances, critical care and other inpatient admissions, covering procedures undertaken including percutaneous coronary intervention, by-pass surgery, implantable cardioverter defibrillators (ICDs), cardiac pacemakers and lengths of stay, day case admissions and outpatient attendances. HealthCare Resource Group (HRG) codes were derived using the NHS HRG4 2016–17 Reference Cost Grouper software version [ 12 ]. The Department of Health and Social Care’s Reference Costs 2016–17 schedule was used to assign costs to derived HRG codes [ 12 ]. For patients without linkage to routine hospital records, hospital resource inputs were extracted from data contained within the trial case report forms and valued using national tariffs.
Surviving patients or their proxies were asked to complete questionnaires at 3 and 6 months post-randomisation, reporting hospital admissions and use of hospital outpatient services following initial discharge, by type and volume, and use of community health and social services, medications and aids and equipment. Further questions captured wider societal attributable resource use, with data collected on time off work, over-the-counter medications, aids and adaptations purchased privately and any additional costs borne by study patients or informal carers. Unit costs for these resource inputs were drawn from national tariffs (Additional file 2 ).
Calculation of health utilities and QALYs
Health-related quality of life was assessed using the EuroQol EQ-5D-5L [ 13 ], completed at 3 and 6 months post-cardiac arrest. Responses to the EQ-5D-5L descriptive system were converted into health utility values, based on the UK EQ-5D-3L tariff using the van Hout interim cross-walk algorithm [ 14 ], in line with current recommendations [ 15 ]. mRS scores collected at hospital discharge were also converted into health utility values, based on the UK EQ-5D-3L tariff, using a published mapping algorithm [ 16 ]. A QALY profile was generated for each trial participant using the area-under-the-curve method, assuming a baseline health utility value of zero [ 17 ] and linear interpolation between utility measurements at hospital discharge and at 3 and 6 months assessment points, accounting for survival [ 18 ]. We used 3- or 6-month mRS-derived health utilities in place of EQ-5D-5L-derived values if the latter were missing.
Analytical methods
Analyses of clinical outcomes, resource use and costs.
Survival outcomes were analysed through the use of fixed-effect regression models with adjustment for age, sex, interval between the emergency call and the ambulance arrival at the scene, interval between the ambulance arrival and the administration of the study drug, cause of cardiac arrest, initial cardiac rhythm, whether the cardiac arrest was witnessed and whether CPR was performed by a bystander [ 9 ]. Between treatment-group differences in mean resource use and mean costs were estimated using two-sample Student’s t tests. Estimates of 95% confidence intervals (CIs) surrounding between-group differences in mean costs were obtained using nonparametric bootstrapping with replacement, based on 1000 replications [ 19 ].
Handling missing data
Multiple imputations using chained equations with predictive mean matching [ 20 ] was used to predict values for missing observations, assuming data were missing at random. Missing costs and health utility values were imputed at the level of resource category, health-related quality of life measure and assessment point, stratified by survival status at hospital discharge and treatment allocation in accordance with current recommendations [ 21 ]. Twenty imputed datasets were generated and used to inform the base-case and subsequent sensitivity and subgroup analyses. Parameter estimates were pooled across the 20 imputed datasets using Rubin’s rule to account for between and within-imputation components of variance terms associated with parameter estimates.
Within-trial economic evaluation
Bivariate linear regressions that take into account the correlation between each patient’s costs and effects were used to model total costs and total QALYs over the 6-month follow-up period. By specifying the treatment group as an indicator within each equation, the incremental costs and QALYs attributable to adrenaline were estimated, while controlling for baseline covariates (age, sex, time to first dose administration, cause of cardiac arrest, whether the cardiac arrest was witnessed, whether a bystander performed CPR and initial cardiac rhythm). Cost-effectiveness was expressed in terms of an incremental cost-effectiveness ratio (ICER), defined as the incremental adjusted cost of adrenaline divided by the incremental adjusted QALYs of adrenaline. The ICER was then compared with a range of cost-effectiveness threshold values for an additional QALY. ICERs falling below or in the region of £20,000 (€23,000) to £30,000 (€34,500) per QALY gained would, in general, be considered as representing a cost-effective use of UK NHS resources [ 11 , 22 ]. Confidence intervals surrounding mean ICER values were not calculated as they are problematic to interpret if the ICER denominator is estimated close to zero and the interval extends to cover negative values [ 23 ]. This is because a negative ICER might equally imply lower costs and more effective treatment or greater costs and less effective treatment compared with placebo. There is no way of differentiating between these two qualitatively different scenarios from a confidence interval alone. Thus, we followed standard practice in economic evaluations to present mean ICER values without accompanying confidence intervals or standard errors. Instead, uncertainty surrounding mean cost-effectiveness estimates were characterised through a Monte Carlo method involving simulating 1000 replicates of the ICER from a joint distribution of incremental costs and incremental QALYs [ 24 ]. By calculating net monetary benefits for each of these simulated ICER values at alternative cost-effectiveness thresholds varying from £0 to £100,000 (€115,000) per QALY gained, the probability of cost-effectiveness of adrenaline (defined as the proportion of positive net monetary benefits at a given threshold level) was calculated and plotted as a cost-effectiveness acceptability curve [ 25 ].
Sensitivity analyses
A series of sensitivity analyses, summarised in Additional file 3 , assessed the impact of varying features of the within-trial economic evaluation on cost-effectiveness. These included extending the perspective of analysis to cover broader societal resource use and costs and extending the time horizon to 12 months after the cardiac arrest event. Further economic modelling (described in the sections that follow) explored how results altered when the time horizon was extended to cover the lifetimes of cardiac arrest survivors and the scope extended to include recipients of donated organs.
Sub-group analyses
A series of pre-specified subgroup analyses, summarised in Additional file 4 , explored whether the cost-effectiveness estimates based on the within-trial data altered by age, gender, time to first dose administration, cause of cardiac arrest, whether the cardiac arrest was witnessed, whether bystander performed CPR or initial cardiac rhythm.
Extrapolations of cost-effectiveness
Controlled organ donation after neurological or cardiac death is available to families for patients who sustain un-survivable severe brain injury. Decision-analytic modelling was used to estimate the incremental cost-effectiveness of adrenaline beyond the parameters of the PARAMEDIC2 trial to include the costs and benefits of organ donation. A Markov state transition model was developed to extrapolate lifetime costs and benefits for out-of-hospital cardiac arrest survivors (Fig. 1 , plot a). We separately adapted a previous economic model of adult lung transplantation [ 26 ] to simulate the impact on the cost-effectiveness of adrenaline resulting from changes in organ donation from deceased PARAMEDIC2 donors who had experienced out-of-hospital cardiac arrest. The model (Fig. 1 , plot b) simulated pre and post-transplantation survival for individuals on the active national transplant waiting list. Epidemiological data for the model were informed by a separate linkage of the PARAMEDIC2 patients to the UK National Transplant Registry. Further details of the model structures, data inputs, analytical methods and description of methods for integrating the direct and indirect effects of adrenaline generated by the two models are provided in Additional file 5 .
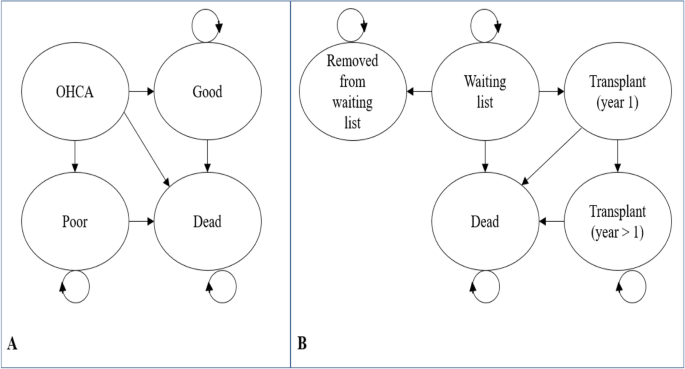
Model diagrams. Plot a : Model to extrapolate cost-effectiveness beyond PARAMEDIC2 trial follow-up. OHCA represents health state for out-of-hospital cardiac arrest patients. Good represents survival with good neurological function. Poor represents survival with poor neurological function. Plot b : Organ donor model adapted from Fisher et al. [ 26 ]
A total of 8014 patients were randomised to either parenteral adrenaline (4015 patients) or saline placebo (3999 patients). In the adrenaline group, 130 patients (3.2%) were alive at 30 days, compared with 94 patients (2.4%) in the placebo group (unadjusted odds of survival, 1.39; 95% confidence interval [CI], 1.06 to 1.82; P = 0.02) [ 9 ]. Severe neurologic impairment at hospital discharge (mRS score of 4 or 5) was more common amongst survivors in the adrenaline group than in the placebo group (39 of 126 patients [31.0%] vs. 16 of 90 patients [17.8%]) [ 9 ].
Figure 2 summarises the flow and completeness of data sources used to inform the economic parameters of the trial. Overall, between 1 and 2% of costs (at the component level) and approximately 1% of QALY data were missing (and subsequently imputed) for the primary analysis.
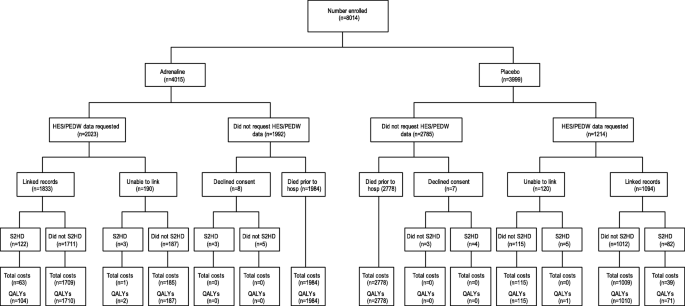
Flow chart of data sources used to inform resource use and costs. S2HD indicates survivors to hospital discharge, HES/PEDW indicates Hospital Episode Statistics/Patient Episode Database for Wales
Resource use reported by the trial participants and or their proxies by treatment arm is summarised in Additional file 6 . More patients were admitted to intensive care in the adrenaline arm (2016 vs 1209, P < 0.001), creating a larger group for assessment for donation after neurological or cardiac death. Linkage of the PARAMEDIC2 patients to the UK National Transplant Registry revealed that there were 99 recipients of organs donated by the adrenaline group compared with 67 recipients of organs donated by the placebo group (from 40 donors in the adrenaline group vs 24 donors in the placebo group). Breakdown by organ type is provided in Table 1 .
Amongst all participants, mean total NHS and PSS costs over the 6-month trial time horizon was £3789 (€4357) in the adrenaline group vs. £2698 (€3103) in the placebo group [unadjusted mean cost difference £1091 (€1255) (bootstrap 95% CI £807 (€928) to £1398 (€1608)); P < 0.001] (Table 2 ). There was also a significant difference in mean total societal costs: £3829 (€4403) in the adrenaline group vs. £2687 (€3090) in the placebo group [unadjusted mean cost difference £1143 (€1314) (bootstrap 95% CI £861 (€990) to £1451 (€1669)); P < 0.001]. Amongst those who survived to hospital discharge, there were no significant differences in mean total NHS and PSS costs. However, amongst survivors to hospital discharge, mean utility values were significantly lower at hospital discharge in the adrenaline group [0.48 vs 0.60, unadjusted mean difference − 0.118 (95% CI − 0.196 to − 0.032); P = 0.002].
The within-trial economic evaluation results are summarised in Table 3 . The base case analysis, over a 6-month time horizon, produced an ICER of £1,693,003 (€1,946,953) per QALY gained, reflecting significantly higher mean costs and only marginally higher mean QALYs in the adrenaline group. Extrapolating the within-trial analysis produced ICERs of £644,308 (€740,954) per QALY gained over 1 year and £81,070 (€93,231) per QALY gained over the lifetimes of survivors. The probability that adrenaline is cost-effective remained below 1% across a range of cost-effectiveness thresholds (plots a and b of Fig. 3 ), while mean net monetary benefits were negative at all thresholds. The cost-effectiveness estimates remained robust to a range of sensitivity and subgroup analyses (Additional file 7 ).
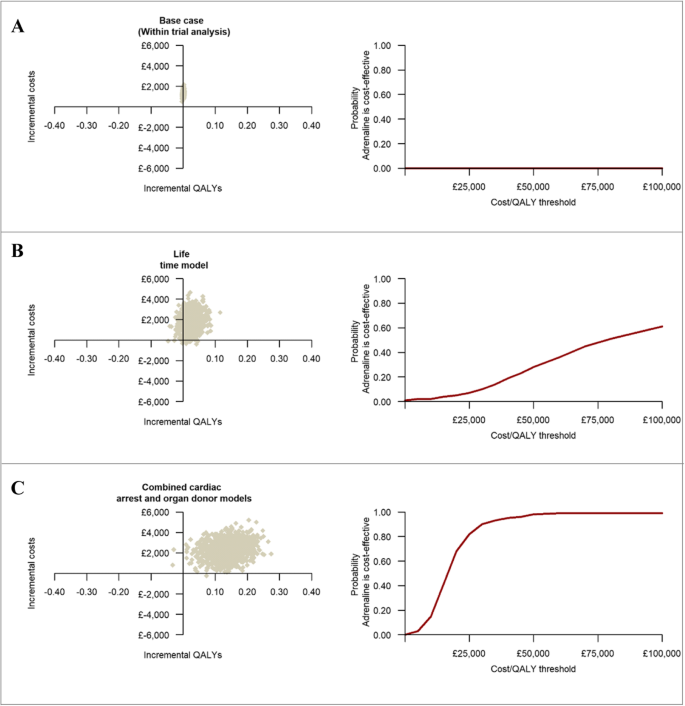
The three graphs on the left-hand side represent the cost-effectiveness plane for the within-trial, lifetime and the combined cardiac arrest and organ donor analyses. The three graphs on the right-hand side represent cost-effectiveness acceptability curves and give the probability that adrenaline is cost-effective compared with placebo at a specified cost-effectiveness threshold. Each cost-effectiveness plane is divided into four quadrants (North-West, North-East, South-West and South-East) by the intersection of the horizontal and vertical axis. South-East quadrant implies adrenaline is less costly and more effective than placebo, North-West quadrant implies adrenaline is less effective and more costly, the North-East quadrant implies adrenaline is more effective but also more costly and the South-West quadrant implies adrenaline is less effective but also less costly
The decision-analytic modelling that estimated combined direct economic effects over the lifetimes of survivors and indirect economic effects in organ recipients revealed that routine use of adrenaline at a UK-wide level in this patient group would result in incremental costs of £49,759,481 (€57,223,403) and 3093 additional QALYs per annum (Table 4 ). The mean ICER at a patient-level was estimated at £16,086 (€18,499) per QALY gained for adrenaline. The probability that adrenaline is cost-effective compared with placebo was 39% at a £15,000 (€17,250) per QALY cost-effectiveness threshold, increasing to 90% at a £30,000 (€34,500) threshold (Table 4 and plot c of Fig. 3 ). Sensitivity analyses revealed that these cost-effectiveness estimates remained robust to a range of modelling assumptions and data inputs with the exception of the estimated impact of the number of organs donated for transplantation on the cost-effectiveness of adrenaline (Additional file 5 ).
This paper presents the first economic evaluation of adrenaline in adults with out-of-hospital cardiac arrest. The study revealed that when the assessment is restricted to the first 6 months that follow out-of-hospital cardiac arrest, adrenaline is associated with significantly higher mean costs, largely driven by the additional costs associated with hospital admissions, and only marginally higher mean QALYs. The base case analysis generated a mean ICER of £1,693,003 (€1,946,953) per QALY gained at 6 months post-randomisation, well in excess of accepted cost-effectiveness thresholds [ 11 ]. Consequently, the probability that adrenaline is cost-effective approximated to zero in the base-case analysis. Moreover, this conclusion remained robust after extensive sensitivity analyses that accounted for different sources of uncertainty. Notably, however, separate decision-analytic modelling that extrapolated cost-effectiveness over the lifetimes of survivors and incorporated additional costs and health consequences for organ recipients significantly altered the cost-effectiveness calculus. The probability that adrenaline is cost-effective increased to 90% at a £30,000 (€34,500) per QALY cost-effectiveness threshold, arguably within the bounds of acceptance by health technology agencies tasked with reimbursement decisions [ 11 ]. The cost-effectiveness conclusions were particularly sensitive to the inclusion of recipients of donated organs into economic modelling. The economic value of the additional survival and QALY benefits to organ recipients associated with adrenaline clearly outweigh the additional costs that result from organ transplantation. Similar findings have been reported for other interventions. In a study evaluating the costs and benefits of traumatic cardiopulmonary arrest resuscitation [ 27 ], the authors concluded that the financial burden associated with the procedure could be offset by expanding the benefits to include organ donation.
Previous economic evaluations of adrenaline were conducted in clinical contexts outside the management of out-of-hospital cardiac arrest [ 28 , 29 , 30 ], and therefore, a comparative assessment of cost-effectiveness evidence is not possible. Similarly, other pharmacological interventions for cardiac arrest, such as anti-arrythmics, have yet to be evaluated from an economic perspective. Our data should, therefore, be of relevance to clinical decision-makers and service planners tasked with the care and management of a condition that causes significant death and disability. Moreover, our economic evaluation is the first, to our knowledge, that has modelled the indirect economic benefits of organ donation arising from an intervention that increases the likelihood of admission to intensive care following an acute out-of-hospital clinical event. It may, therefore, alter methodological thinking on the incorporation of benefits other than directly to the patient into economic evaluations of interventions that affect the likelihood for organ donation. Such a methodological shift will require ethical consideration of how to balance benefits to others with benefits or harms to patients receiving the intervention.
Readers should consider caveats when interpreting the study results. First, our findings may not be applicable outside of the UK as the models of prehospital care, and healthcare costs may differ in other countries. Second, our adapted model of adult lung transplantation excluded the effects of adrenaline on pancreas and heart transplants. A paucity of epidemiological and economic evidence meant that we were unable to parameterise extensions to our decision-analytic model that considered effects beyond lung, liver and kidney transplants. Nevertheless, the number of pancreases and hearts transplanted to recipients of organs donated by the PARAMEDIC2 patients was small (< 10) and therefore unlikely to alter the balance of overall conclusions. Third, as with any health economic evaluation, there are likely to be potentially relevant effects that are not captured by the tools available for measurement and valuation. Specifically, we did not value other potential consequences of adrenaline, for example, the potential benefits to family members arising from the increased likelihood of being able to say goodbye and be present at the time of death [ 31 ]. Finally, in the modelling that extended our assessments of cost-effectiveness of adrenaline over the lifetimes of cardiac arrest survivors and incorporated the indirect economic effects of organ transplantation, a number of assumptions were required to simplify the parameterisation and address data limitations. Details of these assumptions are described in additional file 5 . These included assuming that functional health state does not change over extended periods of survival and that resource use captured in the 3–6 month post-randomisation period can be extrapolated to cover the 6–12 month post-randomisation period. The organ donor modelling required a set of steady or equilibrium-state assumptions (number of organs available each year equals the number of transplantations with no wastage; the probability of death or removal on the waiting list are independent of organ supply) that captured the impact of intervention on the probability of transplantation. Nevertheless, our approach was consistent with current methodological guidance for decision-analytic modelling [ 32 ]. Furthermore, extensive sensitivity analyses were conducted that found that the conclusions from the base-case analyses were robust to doubling and halving input parameter values and alternative specifications of modelling assumptions.
In conclusion, adrenaline use in adults with out-of-hospital cardiac arrest does not represent a cost-effective use of resources when only directly related costs and consequences are considered. However, incorporating the indirect economic effects associated with transplanted organs substantially alters the cost-effectiveness calculus, suggesting decision-makers should consider the complexity of direct and indirect economic impacts of adrenaline.
Availability of data and materials
Not applicable
Abbreviations
Donation after brain death
Donation after circulatory death
Ex vivo lung perfusion
Ex vivo machine perfusion
Hospital Episode Statistics
HealthCare Resource Group
Implantable cardioverter defibrillator
Incremental cost-effectiveness ratio
National Health Service
NHS and Personal Social Services
National Institute for Care Excellence
Patient Episode Database for Wales
Quality-adjusted life year
United Kingdom
United Kingdom Blood and Transplant
Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81(11):1479–87.
Article Google Scholar
Chan PS, McNally B, Tang F, Kellermann A, Group CS. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130(21):1876–82.
Perez CA, Samudra N, Aiyagari V. Cognitive and functional consequence of cardiac arrest. Curr Neurol Neurosci Rep. 2016;16(8):70.
Sandroni C, D'Arrigo S, Callaway CW, Cariou A, Dragancea I, Taccone FS, et al. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: a systematic review and meta-analysis. Intensive Care Med. 2016;42(11):1661–71.
West S, Soar J, Callaway CW. The viability of transplanting organs from donors who underwent cardiopulmonary resuscitation: a systematic review. Resuscitation. 2016;108:27–33.
Snyder RA, Moore DR, Moore DE. More donors or more delayed graft function? A cost-effectiveness analysis of DCD kidney transplantation. Clin Transpl. 2013;27(2):289–96.
Dageforde LA, Feurer ID, Pinson CW, Moore DE. Is liver transplantation using organs donated after cardiac death cost-effective or does it decrease waitlist death by increasing recipient death? HPB (Oxford). 2013;15(3):182–9.
Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther AM, et al. Pre-hospital assessment of the role of adrenaline: measuring the effectiveness of drug administration in cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation. 2016;108:75–81.
Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–21.
Article CAS Google Scholar
Haywood K, Whitehead L, Nadkarni VM, Achana F, Beesems S, Bottiger BW, et al. COSCA (Core outcome set for cardiac arrest) in adults: an advisory statement from the international liaison committee on resuscitation. Resuscitation. 2018;127:147–63.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. London, Department of Health. Available online from https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 . Accessed 09 Sept 2020.
NHS Digital. HRG4+ 2016/17 Reference Costs Grouper 2017 London. 2017. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper .
Google Scholar
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
National Institute for Health and Care Excellence (NICE). Position statement on use of the EQ-5D-5L valuation set for England (updated November 2018). London: NICE; 2018. Report No.: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l .
Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Mak. 2010;30(3):341–54.
Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. Pharmacoeconomics. 2017;35(5):501–15.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–36.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67.
Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70.
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–503 v-vi.
Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766.
Glick HADJ, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2014.
Book Google Scholar
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–87.
Fisher A, Andreasson A, Chrysos A, Lally J, Mamasoula C, Exley C, et al. An observational study of donor ex vivo lung perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 2016;20(85):1–276.
Love KM, Brown JB, Harbrecht BG, Muldoon SB, Miller KR, Benns MV, et al. Organ donation as an outcome of traumatic cardiopulmonary arrest: a cost evaluation. J Trauma Acute Care Surg. 2016;80(5):792–8.
Sumner A, Coyle D, Mitton C, Johnson DW, Patel H, Klassen TP, et al. Cost-effectiveness of epinephrine and dexamethasone in children with bronchiolitis. Pediatrics. 2010;126(4):623–31.
Shaker MS. An economic evaluation of prophylactic self-injectable epinephrine to prevent fatalities in children with mild venom anaphylaxis. Ann Allergy Asthma Immunol. 2007;99(5):424–8.
Fenwick E, Wilson J, Sculpher M, Claxton K. Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive Care Med. 2002;28(5):599–608.
Kentish-Barnes N, Chaize M, Seegers V, Legriel S, Cariou A, Jaber S, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341–52.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Mak. 2012;32(5):667–77.
Download references
Acknowledgements
The PARAMEDIC 2 trial team and the University of Warwick acknowledge the support of the National Institute for Health Research Clinical Research Network (NIHR CRN) and the Applied Research Centre (ARC) West Midlands. This project was funded by the National Institute for Health Research’s Health Technology Assessment Programme (project number 12/127/126).
We acknowledge NHS Blood and Transplant for access to data held on the UK Transplant Registry held by the Organ Donation and Transplantation Directorate and all those who have contributed data to the Registry.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Technology Assessment Programme, NIHR, NHS or the Department of Health.
PARAMEDIC 2 collaborators: Stavros Petrou, Jason Madan, Kamran Khan, Chen Ji, Anower Hossain, Ranjit Lall, Anne-Marie Slowther, Charles D. Deakin, Tom Quinn, Jerry P. Nolan, Helen Pocock, Nigel Rees, Michael Smyth, Simon Gates, Dale Gardiner, Gavin D. Perkins, Matthew Cooke, Sarah Lamb, Andrew Carson (RIP), Ian Jacobs (RIP), Ed England, John Black, Nicola Brock, Claire Godfrey, Sarah Taylor, Michelle Thomson, Isabel Rodriguez-Bachiller, Claire King, Marie Stevens, Johanna Lazarus, Helen Werts, Joshua Golding, Rachel Fothergill, Fionna Moore, Alex Boda, Richard Whitfield, Laura Galligan, Rob Lovett, Jennifer Bradley, Lyndsay O’Shea, Mark Docherty, Imogen Gunsen, Gill Price, Andy Rosser, Garry Parcell, Mindy Jhamat, Josh Miller, Jenny Sears Brown, Alice Pretty, Madison Larden, Emma Harris, Jenny Lumley-Holmes, Rhiannon Boldy, Prudence Horwood, Kyee Han, Karl Charlton, Sonia Byers, Gary Shaw, Matt Limmer, Craig Wynne, Michelle Jackson, Emma Bell, Oliver Gupta, Rima Gupta, Charlotte Scomparin, Susie Hennings, Jessica Horton, James Buck, Sarah Rumble, Hayley Johnson, Eva Kritzer, Chockalingham Muthiah, Adrian Willis, Claire Daffern, Louise Clarkson, Felix Achana, Nicola Cashin, Emma Skilton, Malvenia Richmond, Martin Underwood, Natalie Strickland, Sarah Duggan, Scott Regan, Marie Stevens, Jill Wood; Trial Steering Committee (Jon Nicholl, Neil Bayliss, Helen Snooks, Jonathan Benger, Robert Andrews, David Pitcher, William Lee, Matt Wise); Data Monitoring Committee (Marion Campbell, Jasmeet Soar, Kathy Rowan, Sue Mason). We would also like to thank collaborators at all receiving hospitals, all staff involved at participating ambulance services and our patient and public partners.
This project was funded by the National Institute for Health Research’s Health Technology Assessment Programme (project number 12/127/126).
Author information
Stavros Petrou and Gavin D Perkins are joint senior authors.
Authors and Affiliations
Warwick Clinical Trials Unit, Warwick Medical School, The University of Warwick, Coventry, UK
Felix Achana, Stavros Petrou, Jason Madan, Kamran Khan, Chen Ji, Ranjit Lall, Anne-Marie Slowther, Jerry P. Nolan, Helen Pocock, Michael Smyth, Gavin D. Perkins, Stavros Petrou, Jason Madan, Kamran Khan, Chen Ji, Ranjit Lall, Anne-Marie Slowther, Jerry P. Nolan, Helen Pocock, Michael Smyth & Gavin D. Perkins
Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
Felix Achana, Stavros Petrou & Stavros Petrou
Institute of Statistical Research and Training (ISRT), University of Dhaka, Dhaka, Bangladesh
Anower Hossain & Anower Hossain
Southampton Respiratory Biomedical Research Unit, National Institute for Health Research, Southampton, UK
Charles D. Deakin & Charles D. Deakin
Kingston University and St. George’s, University of London, London, UK
Tom Quinn & Tom Quinn
Critical Care Unit, Royal United Hospital, Bath, UK
Jerry P. Nolan & Jerry P. Nolan
South Central Ambulance Service NHS Foundation Trust, Otterbourne, UK
Helen Pocock & Helen Pocock
Welsh Ambulance Services NHS Trust, Swansea, UK
Nigel Rees & Nigel Rees
West Midlands Ambulance NHS Foundation Trust, Dudley, UK
Michael Smyth & Michael Smyth
Cancer Clinical Trials Unit, University of Birmingham, Birmingham, UK
Simon Gates & Simon Gates
National Clinical Lead for Organ Donation, NHS Blood and Transplant, Bristol, UK
Dale Gardiner & Dale Gardiner
You can also search for this author in PubMed Google Scholar
- Stavros Petrou
- , Jason Madan
- , Kamran Khan
- , Anower Hossain
- , Ranjit Lall
- , Anne-Marie Slowther
- , Charles D. Deakin
- , Tom Quinn
- , Jerry P. Nolan
- , Helen Pocock
- , Nigel Rees
- , Michael Smyth
- , Simon Gates
- , Dale Gardiner
- , Gavin D. Perkins
- , Matthew Cooke
- , Sarah Lamb
- , Andrew Carson
- , Ian Jacobs
- , Ed England
- , John Black
- , Nicola Brock
- , Claire Godfrey
- , Sarah Taylor
- , Michelle Thomson
- , Isabel Rodriguez-Bachiller
- , Claire King
- , Marie Stevens
- , Johanna Lazarus
- , Helen Werts
- , Joshua Golding
- , Rachel Fothergill
- , Fionna Moore
- , Alex Boda
- , Richard Whitfield
- , Laura Galligan
- , Rob Lovett
- , Jennifer Bradley
- , Lyndsay O’Shea
- , Mark Docherty
- , Imogen Gunsen
- , Gill Price
- , Andy Rosser
- , Garry Parcell
- , Mindy Jhamat
- , Josh Miller
- , Jenny Sears Brown
- , Alice Pretty
- , Madison Larden
- , Emma Harris
- , Jenny Lumley-Holmes
- , Rhiannon Boldy
- , Prudence Horwood
- , Karl Charlton
- , Sonia Byers
- , Gary Shaw
- , Matt Limmer
- , Craig Wynne
- , Michelle Jackson
- , Emma Bell
- , Oliver Gupta
- , Rima Gupta
- , Charlotte Scomparin
- , Susie Hennings
- , Jessica Horton
- , James Buck
- , Sarah Rumble
- , Hayley Johnson
- , Eva Kritzer
- , Chockalingham Muthiah
- , Adrian Willis
- , Claire Daffern
- , Louise Clarkson
- , Felix Achana
- , Nicola Cashin
- , Emma Skilton
- , Malvenia Richmond
- , Martin Underwood
- , Natalie Strickland
- , Sarah Duggan
- , Scott Regan
- , Jill Wood
- , Jon Nicholl
- , Neil Bayliss
- , Helen Snooks
- , Jonathan Benger
- , Robert Andrews
- , David Pitcher
- , William Lee
- , Matt Wise
- , Marion Campbell
- , Jasmeet Soar
- , Kathy Rowan
- & Sue Mason
Contributions
FA, SP, KK, JM and DG contributed to the design of the within-trial cost-effectiveness analysis and the economic modelling. CJ, AH, RL and SG contributed to statistical design and analysed the data. AS, CD, TQ, JN, HP, NR, MS and GP contributed to the design of the study and data collection and provided clinical oversight. All authors contributed to drafting and proof-reading of the manuscript for high-quality intellectual work and that the findings and conclusions are consistent with the data. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Gavin D. Perkins .
Ethics declarations
Ethics approval and consent to participate.
The South Central–Oxford C Research Ethics Committee (REC: 14/SC/0157) and the Medicines and Healthcare Products Regulatory Authority (EudraCT: 2014-000792-11) approved the study protocol.
Consent for publication
Competing interests.
MAS reports funding from the National Institute for Health Research. MAS has volunteer roles with the International Liaison Committee on Resuscitation, European and Resuscitation Council UK.
TQ reports grant funding paid to his institution by the National Institute for Health Research and British Heart Foundation. TQ has volunteer roles with the European Society of Cardiology.
GDP reports grant funding paid to his institution from the National Institute for Health Research, Resuscitation Council UK and British Heart Foundation related to this work. GDP has volunteer roles with the International Liaison Committee on Resuscitation, European and Resuscitation Council UK and Intensive Care Foundation. GDP is supported as a NIHR Senior Investigator and serves as an Editor for Resuscitation.
SP is supported by a Senior Investigator award from the UK National Institute of Health Research.
NR reports grants from National Institute for Health Research and Health and Care Research Wales during the conduct of the study.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PARAMEDIC2 HEALTH ECONOMICS ANALYSIS PLAN. Details of the PARAMEDIC2 health economics analysis plan.
Additional file 2.
Unit costs for NHS, non-NHS and personal and social service resource inputs (£ sterling, 2017 prices). Unit costs data used to inform the within trial economic evaluation.
Additional file 3.
Pre-specified and post-hoc sensitivity analyses exploring varying features of the within-trial economic evaluation on the cost-effectiveness results. List of sensitivity analyses.
Additional file 4.
Subgroup analyses conducted that explored potential heterogeneity in the within-trial incremental cost-effectiveness of adrenaline.
Additional file 5.
Economic modelling to extrapolate cost-effectiveness beyond trial follow-up and incorporate the indirect effects of organ donation. List of subgroup analyses.
Additional file 6.
Resources use for NHS and personal and social services by trial arm in the 6 month post-randomisation period; all patients. Resource use results table, within-trial economic evaluation.
Additional file 7.
Cost-effectiveness (£, 2017 prices) of adrenaline based on within-trial economic evaluation; sensitivity analyses and subgroup analyses results. Additional cost-effectiveness results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Achana, F., Petrou, S., Madan, J. et al. Cost-effectiveness of adrenaline for out-of-hospital cardiac arrest. Crit Care 24 , 579 (2020). https://doi.org/10.1186/s13054-020-03271-0
Download citation
Received : 07 May 2020
Accepted : 02 September 2020
Published : 27 September 2020
DOI : https://doi.org/10.1186/s13054-020-03271-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cost-effectiveness of adrenaline
- Cardiac arrest
- Organ donation
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]


Methods and participant characteristics in the Cancer Risk in Vegetarians Consortium: A cross-sectional analysis across 11 prospective studies
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected] [email protected]
- ORCID record for Kabayam M. Venkat Narayan
- ORCID record for Tammy Y. N. Tong
- ORCID record for Hsin-Chou Yang
- ORCID record for Aurora Perez-Cornago
- Info/History
- Supplementary material
- Preview PDF
Background The associations of vegetarian diets with risks for site-specific cancers have not been estimated reliably due to the low number of vegetarians in previous studies. Therefore, the Cancer Risk in Vegetarians Consortium was established.
Objective To describe and compare the baseline characteristics between non-vegetarian and vegetarian diet groups and between the collaborating studies.
Methods We harmonised individual-level data from 11 prospective cohort studies in the UK, US, India, China, and Taiwan. Comparisons of food intakes, sociodemographic and lifestyle factors were made between diet groups and between cohorts using descriptive statistics.
Results 2.3 million participants were included; 66% women and 34% men, with mean ages at recruitment of 57 (SD: 7.8) and 57 (8.6) years, respectively. There were 2.1 million meat eaters, 60,903 poultry eaters, 44,780 pescatarians, 81,165 vegetarians, and 14,167 vegans. Food intake differences between the diet groups varied across the cohorts; for example, fruit and vegetable intakes were generally higher in vegetarians than in meat eaters in all the cohorts except in China. BMI was generally lower in vegetarians, particularly vegans, except for the cohorts in India and China. In general, but with some exceptions, vegetarians were also more likely to be highly educated and physically active and less likely to smoke. In the available resurveys, stability of diet groups was high in all the cohorts except in China.
Conclusions Food intakes and lifestyle factors of both non-vegetarians and vegetarians varied markedly across the individual cohorts, which may be due to differences in both culture and socioeconomic status, as well as differences in questionnaire design. Therefore, care is needed in the interpretation of the impacts of vegetarian diets on cancer risk.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This work has been funded by the World Cancer Research Fund UK (grant 2019/1953). The funder was not involved in any of the following: study design, collection, analysis, interpretation of data, and writing of the report.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Each study in this consortium received ethical clearance from their respective ethical committees which covered future research. The Loma Linda University Institutional Review Board gave ethical approval for the Adventist Health Study 2. Ethical approval for the CARRS study was provided by Institutional Review Boards of PHFI, AIIMS, MDRF Chennai, India, AKU and Emory University. In addition, the study has received regulatory approval from the Health Ministry Screening Committee of India. The EPIC Oxford study was given ethical approval by Royal College of General Practitioners Clinical Research Ethics Committee, the Central Oxford Research Ethics Committee and local research ethics committees. The South Central Oxford C Research Ethics Committee gave ethical approval for the Oxford Vegetarian Study. The Institutional Review Board for ethics in the Buddhist Dalin Tzu Chi Hospital waived ethical approval for the Tzu Chi Health Study. The National Research Ethics Service Committee for Yorkshire and the Humber, Leeds East provided ethical approval for the UKWCS. The Ethical Review Committee of the Chinese Centre for Disease Control and Prevention, Oxford Tropical Research Ethics Committee, University of Oxford gave ethical approval for the China Kadoorie Biobank. The NHS Health Research Authority (approval provided by Anglia and Oxford Multi centre Research Ethics Committee) gave ethical approval for the Million Women Study. The Special Studies Institutional Review Board of the U.S. National Cancer Institute provided ethical clearance for the NIH AARP study. The North West Multi Centre Research Ethics Committee provided ethical approval for the UK Biobank study.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
List of abbreviations: BMI, Body Mass Index; CARRS, Centre for cArdiometabolic Risk Reduction in South Asia; EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, Food Frequency Questionnaire; MET, metabolic equivalent of task; NIH-AARP, National Institutes of Health-AARP Diet and Health Study.
Data described in the manuscript will not be made available because studies pooled by the Cancer Risk in Vegetarians Consortium are not owned by the writing group and so are not available from this consortium. Individual studies may be contacted to request access to their data.
Conflict of interest: None declared.
Data Availability
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Epidemiology
- Addiction Medicine (330)
- Allergy and Immunology (651)
- Anesthesia (174)
- Cardiovascular Medicine (2508)
- Dentistry and Oral Medicine (307)
- Dermatology (210)
- Emergency Medicine (386)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (884)
- Epidemiology (11987)
- Forensic Medicine (10)
- Gastroenterology (721)
- Genetic and Genomic Medicine (3910)
- Geriatric Medicine (366)
- Health Economics (653)
- Health Informatics (2523)
- Health Policy (973)
- Health Systems and Quality Improvement (936)
- Hematology (350)
- HIV/AIDS (813)
- Infectious Diseases (except HIV/AIDS) (13487)
- Intensive Care and Critical Care Medicine (778)
- Medical Education (387)
- Medical Ethics (106)
- Nephrology (417)
- Neurology (3664)
- Nursing (205)
- Nutrition (546)
- Obstetrics and Gynecology (709)
- Occupational and Environmental Health (681)
- Oncology (1905)
- Ophthalmology (555)
- Orthopedics (230)
- Otolaryngology (299)
- Pain Medicine (243)
- Palliative Medicine (71)
- Pathology (463)
- Pediatrics (1075)
- Pharmacology and Therapeutics (446)
- Primary Care Research (435)
- Psychiatry and Clinical Psychology (3293)
- Public and Global Health (6343)
- Radiology and Imaging (1341)
- Rehabilitation Medicine and Physical Therapy (780)
- Respiratory Medicine (847)
- Rheumatology (389)
- Sexual and Reproductive Health (384)
- Sports Medicine (334)
- Surgery (426)
- Toxicology (51)
- Transplantation (180)
- Urology (156)
Informed consent
Information and guidance for researchers, what is informed consent.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research.
Consent should be obtained before the participant enters the research (prospectively), and there must be no undue influence on participants to consent. The minimum requirements for consent to be informed are that the participant understands what the research is and what they are consenting to.
There are two distinct stages to a standard consent process for competent adults:
- Stage 1 (giving information) : the person reflects on the information given; they are under no pressure to respond to the researcher immediately.
- Stage 2 (obtaining consent): the researcher reiterates the terms of the research, often as separate bullet points or clauses; the person agrees to each term (giving explicit consent) before agreeing to take part in the project as a whole. Consent has been obtained.
Researchers should ensure that they comply with the General Data Protection Regulation (GDPR) during and after the consent process, especially if they will be collecting 'special category' (ie sensitive) data or personal data in the course of their research (also refer to the advice on consent in research involving children ). See also the guidance on data protection and research and the data protection checklist for use when preparing an application for ethical review.
Where your research includes filming or photography, you should refer to specific guidance in the Photography and GDPR toolkit .
Written or oral consent – which process suits your project?
Which process to use depends on the research project (its context, design and participants), though an oral process is usually only appropriate where a written process is not feasible. Any consent process must be understandable to the participants concerned. Please see the sections below to find out about different processes which may be used depending on the context, as well as informed consent templates for each process.
Written informed consent process
A written process is used where:
- Reading and signing forms is not problematic.
- The research is complex or has multiple stages.
- First access to the research participants is by providing written information.
Though opinions differ about the legal force of signed consent forms, they provide extra proof that the terms of consent have been understood. This can be especially important when seeking consent for copyright over data, or for future uses of data. Also, future funders or regulators may want written proof of the terms of original consent.
For literate participants who are not put off by written information, a written process is often a straightforward way of communicating the 'research contract'.
Between the provision of information and obtaining consent, the participant should be given a reasonable amount of time to consider whether to consent and to ask questions, though the time given depends on the project design, the context of the research and the participants.
The written consent templates below can be adapted to suit your study.
Oral informed consent process
An oral consent process is where researcher and participant have a conversation to give information and obtain consent. There is no paper form to sign. It is normally used:
- where literacy is a problem
- where there are cultural or political concerns with signing contract-like documents
- where either the researcher and/or the participant could be put at risk by existence of a paper record
- where time for consent is limited, eg a chance interaction between researcher and participant (although you should not use an oral process merely to correct poor planning of research)
- for research conducted via remote video conferencing software
It may also be more appropriate when interviewing elite participants as part of the research.
For all other research, how you arrange the oral process depends on how you will encounter your participants (for example email, phone, an on-the-street-meeting by chance). Between the information-giving and consent stage the participant should be given a reasonable amount of time to consider whether to consent, though this depends on the project design, the type of participants and the context of the research.
When obtaining oral consent, please ensure you are recording the consent process either using a recording device (for example audio recorder if you are conducting an interview that needs to be recorded) or, if participants do not agree to audio recording or if using or keeping audio records is unsafe, by using a researcher record of oral consent template or completing a written consent form on their behalf.
The oral consent templates below can be adapted to suit your study, but careful consideration is required to ensure that these are appropriate for the research and the participants.
Informed consent templates
Written informed consent process (including online surveys).
| Description | File download |
|---|---|
| Any relevant advertising or recruiting material (poster, email text, social media advert) | |
| Template information sheet | |
| Template written consent form | |
| Template information sheet for online research For online tasks only, where there is no face-to-face contact with human participants | |
| Template information sheet for research involving children |
|
| Template assent form for research involving children |
|
| Participant information sheet for CUREC 3 studies | |
| Consent form for CUREC 3 studies | |
| Template email consent form – for low-risk research in the social sciences and humanities that does not involve face-to-face contact with participants |
| Description | File download |
|---|---|
| Any relevant advertising or recruiting material (poster, email text, social media advert) | |
| Oral script | |
| Written information sheet | |
| Assent template for studies recruiting children | |
| Alongside your script, the use of an oral consent record form is recommended. This is a form only you fill in, and helps you keep track of any specific conditions attached to an oral consent |
Template agreements
| Description | File download | |
|---|---|---|
| This legal contribution template should be signed by research participants and Oxford researchers if the latter wish to upload participants’ photographs, videos, films, podcasts, audio recordings, etc to University of Oxford archives. This should be completed in addition to the above informed consent templates | ||
| This legal confidentiality agreement template can be adapted for research assistants/ interpreters/ translators/ transcribers/ local fieldworkers who are non-University employees | ||
| This legal confidentiality agreement template can be adapted for research assistants/ interpreters/ translators/ transcribers/ local fieldworkers who are University employees |
Cases where 'implied consent' may be acceptable (for example online surveys)
Researchers should always aim to inform people fully and obtain appropriate consent. However, in some cases the research may be straightforward enough that a separate, deliberate process for obtaining consent is not needed. In these cases participants, by their actions, imply consent. This is seen most often in research:
BPG 06 Internet-mediated research
Template information sheet for online research
- where no participant personal details are obtained
- where the topic of research is very low risk and no special category data will be collected
- where participation is confined to one small task, eg completing a survey or simple pencil or computer task
Please note: consent cannot be inferred from inaction (eg failure to move away from a camera).
When consent works against the aims of research
If your research employs deception, you will not be able to inform participants fully about your project’s true aims. In this case please check if you can fully apply the CUREC approved procedure on research involving the deception of participants . If the deception raises ethical concerns such that the application is not covered by this procedure please complete a CUREC 2 application form.
Some research settings evolve very rapidly (for example in conflict studies). Similarly, some research participants may only be revealed in time-poor or emergency settings (eg heart attack patients). This infringes on the standard information-giving stage of research. The vulnerability of participants in those settings may justify an expedited or fully waived consent process. Again it is important to describe the research setting clearly. You may need to complete a CUREC 2 application to the relevant committee in these cases: please check with your DREC or your IDREC .
Last updated Thursday 2 December 2021
Related links
- Where and how to apply for ethics review
- Committee information
- Research ethics FAQs and glossary
- Research integrity and ethics policy
- Ethics committee contacts
This site uses session cookies and persistent cookies to improve the content and structure of the site.
By clicking “ Accept All Cookies ”, you agree to the storing of cookies on this device to enhance site navigation and content, analyse site usage, and assist in our marketing efforts.
By clicking ' See cookie policy ' you can review and change your cookie preferences and enable the ones you agree to.
By dismissing this banner , you are rejecting all cookies and therefore we will not store any cookies on this device.
OSCC Research Database
Research type
Research Database
Contact name
John Bithell
Contact email
Research summary
South Central - Oxford B Research Ethics Committee
REC reference
Date of rec opinion.
20 Mar 2018
REC opinion
Favourable Opinion
Research database title
Establishment organisation.
University of Oxford
Establishment organisation address
University Offices
Wellington Square
- Privacy notice
- Terms & conditions
- Accessibility statement
- Feedback or concerns

- Visit Our Blog about Russia to know more about Russian sights, history
- Check out our Russian cities and regions guides
- Follow us on Twitter and Facebook to better understand Russia
- Info about getting Russian visa , the main airports , how to rent an apartment
- Our Expert answers your questions about Russia, some tips about sending flowers

Russian regions
- Belgorod oblast
- Bryansk oblast
- Ivanovo oblast
- Kaluga oblast
- Kostroma oblast
- Kursk oblast
- Lipetsk oblast
- Moskovskaya oblast
- Sergiev Posad
- Orlovskaya oblast
- Ryazan oblast
- Smolensk oblast
- Tambov oblast
- Tula oblast
- Tver oblast
- Vladimir oblast
- Voronezh oblast
- Yaroslavl oblast
- Map of Russia
- All cities and regions
- Blog about Russia
- News from Russia
- How to get a visa
- Flights to Russia
- Russian hotels
- Renting apartments
- Russian currency
- FIFA World Cup 2018
- Submit an article
- Flowers to Russia
- Ask our Expert
Moscow Oblast, Russia
The capital city of Moskovskaya oblast: Moscow .
Moscow Oblast - Overview
Moscow Oblast is a federal subject of Russia located in the Central Federal District. Moscow, the capital city of the country, is the administrative center of Moscow Oblast. At the same time, Moscow is not part of this region, it is a separate federal subject of Russia, a city of federal importance.
The population of Moscow Oblast is about 7,769,000 (2022), the area - 44,379 sq. km.
Moskovskaya oblast flag
Moskovskaya oblast coat of arms.

Moskovskaya oblast map, Russia
Moskovskaya oblast latest news and posts from our blog:.
23 June, 2022 / Natural Spring Gremyachiy Klyuch in Moscow Oblast .
23 March, 2022 / Main Cathedral of the Russian Armed Forces .
31 January, 2022 / Vasilyevsky (Shcherbatovsky) Castle in Moscow Oblast .
1 August, 2021 / Savvino-Storozhevsky Monastery near Moscow .
4 August, 2020 / Sights of Moscow Oblast - the heart of Russia .
More posts..
History of Moscow Oblast
The territory of the Moscow region was inhabited more than 20 thousand years ago. In the first millennium AD, this land was inhabited mostly by the Finno-Ugric peoples (Meryane and Meshchera). In the 9th-10th centuries, the Slavs began active development of the region. The population was engaged in hunting, fisheries, agriculture, and cattle breeding.
In the middle of the 12th century, the territory of the present Moscow region became part of the Vladimir-Suzdal principality, the first towns were founded (Volokolamsk in 1135, Moscow in 1147, Zvenigorod in 1152, Dmitrov in 1154). In the first half of the 13th century, the Vladimir-Suzdal principality was conquered by the Mongols.
In the 14th-16th centuries, Moscow principality became the center of unification of Russian lands. The history of the Moscow region is inextricably linked to military events of the Time of Troubles - the siege of the Trinity-Sergius Monastery by the troops of False Dmitry II, the first and second militias.
More historical facts…
In 1708, by decree of Peter the Great, Moskovskaya gubernia (province) was established. It included most of the territory of present Moscow oblast. In 1712, St. Petersburg became the capital of the Russian Empire and the significance of the Moscow region as the country’s economic center began to decrease.
In 1812, the Battle of Borodino took place near Moscow. It was the biggest battle of the Russian-French War of 1812. In the second half of the 19th century, especially after the peasant reform of 1861, the Moscow province experienced economic growth. In 1851, the first railway connected Moscow and St. Petersburg; in 1862 - Nizhny Novgorod.
The population of the Moscow region increased significantly (in 1847 - 1.13 million people, in 1905 - 2.65 million). On the eve of the First World War, Moscow was a city with a population of more than one million people.
In November, 1917, the Soviet power was established in the region. In 1918, the country’s capital was moved from St. Petersburg to Moscow that contributed to economic recovery of the province. In the 1920s-1930s, a lot of churches located near Moscow were closed, a large number of cultural monuments were destroyed. On January 14, 1929, Moscow Oblast was formed.
In 1941-1942, one of the most important battles of the Second World War took place on the territory of the region - the Battle for Moscow. In the postwar years, the growth of economic potential of the region continued; several science cities were founded (Dubna, Troitsk, Pushchino, Chernogolovka).
In the 1990s, the economy of Moscow Oblast experienced a deep crisis. Since the 1990s, due to the motorization of the population and commuting, road traffic situation in the Moscow region significantly deteriorated. Traffic jams have become commonplace.
Pictures of Moscow Oblast

Moscow Oblast scenery
Author: Mikhail Grizly

At the airport in the Moscow region
Author: Evgeny Davydov

Nature of Moscow Oblast
Author: Alexander Khmelkov
Moscow Oblast - Features
Moscow Oblast is located in the central part of the East European Plain, in the basin of the rivers of Volga, Oka, Klyazma, Moskva. The region stretches from north to south for 310 km, from west to east - 340 km. It was named after the city of Moscow, which however is not part of the region. Part of the administrative authorities of the region is located in Krasnogorsk.
On the territory of the Moscow region, there are 77 cities and towns, 19 of them have a population of more than 100 thousand people. The largest cities are Balashikha (518,300), Podolsk (309,600), Mytishchi (262,700), Khimky (256,300), Korolyov (225,300), Lubertsy (209,600), Krasnogorsk (174,900), Elektrostal (149,000), Odintsovo (138,900), Kolomna (136,800), Domodedovo (136,100).
The climate is temperate continental. Summers are warm, winters are moderately cold. The average temperature in January is minus 10 degrees Celsius, in July - plus 19 degrees Celsius.
One of the most important features of the local economy is its proximity to Moscow. Some of the cities (Odintsovo, Krasnogorsk, Mytishchi) have become in fact the “sleeping districts” of Moscow. The region is in second place in terms of industrial production among the regions of Russia (after Moscow).
The leading industries are food processing, engineering, chemical, metallurgy, construction. Moscow oblast has one of the largest in Russia scientific and technological complexes. Handicrafts are well developed (Gzhel ceramics, Zhostov trays, Fedoskino lacquered miniatures, toy-making).
Moscow railway hub is the largest in Russia (11 radial directions, 2,700 km of railways, the density of railways is the highest in Russia). There are two large international airports - Sheremetyevo and Domodedovo. Vnukovo airport is used for the flights within the country.
Attractions of Moscow Oblast
Moscow Oblast has more than 6,400 objects of cultural heritage:
- famous estate complexes,
- ancient towns with architectural monuments (Vereya, Volokolamsk, Dmitrov, Zaraysk, Zvenigorod, Istra, Kolomna, Sergiev Posad, Serpukhov),
- churches and monasteries-museums (the Trinity Lavra of St. Sergius, Joseph-Volokolamsk monastery, Pokrovsky Khotkov monastery, Savvino Storozhevsky monastery, Nikolo Ugresha monastery).
The most famous estate complexes:
- Arkhangelskoye - a large museum with a rich collection of Western European and Russian art of the 17th-19th centuries,
- Abramtsevo - a literary and artistic center,
- Melikhovo - an estate owned by A.P. Chekhov at the end of the 19th century,
- Zakharovo and Bolshiye Vyazyomy included in the History and Literature Museum-Reserve of Alexander Pushkin,
- House-Museum of the composer P.I. Tchaikovsky in Klin,
- Muranovo that belonged to the poet F.I. Tyutchev,
- Shakhmatovo - the estate of the poet Alexander Blok.
The architectural ensemble of the Trinity Sergius Lavra is a UNESCO World Heritage Site. The largest museum of the Moscow region is located in Serpukhov - Serpukhov Historical and Art Museum.
The places of traditional arts and crafts are the basis of the souvenir industry of Russia:
- Fedoskino - lacquer miniature painting,
- Bogorodskoe - traditional manufacture of wooden toys,
- Gzhel - unique tradition of creating ceramics,
- Zhostovo - painted metal crafts,
- Pavlovsky Posad - fabrics with traditional printed pattern.
Some of these settlements have museums dedicated to traditional crafts (for example, a toy museum in Bogorodskoe), as well as centers of learning arts and crafts.
Moskovskaya oblast of Russia photos
Landscapes of moscow oblast.

Nature of the Moscow region

Country road in the Moscow region

Moscow Oblast landscape
Author: Mikhail Kurtsev
Moscow Oblast views

Author: Asedach Alexander

Country life in Moscow Oblast
Author: Andrey Zakharov

Church in Moscow Oblast
Author: Groshev Dmitrii
Churches of Moscow Oblast

Church in the Moscow region

Cathedral in Moscow Oblast
The questions of our visitors
- Currently 2.85/5
Rating: 2.9 /5 (197 votes cast)
Sponsored Links:
Current time by city
For example, New York
Current time by country
For example, Japan
Time difference
For example, London
For example, Dubai
Coordinates
For example, Hong Kong
For example, Delhi
For example, Sydney
Geographic coordinates of Elektrostal, Moscow Oblast, Russia
Coordinates of elektrostal in decimal degrees, coordinates of elektrostal in degrees and decimal minutes, utm coordinates of elektrostal, geographic coordinate systems.
WGS 84 coordinate reference system is the latest revision of the World Geodetic System, which is used in mapping and navigation, including GPS satellite navigation system (the Global Positioning System).
Geographic coordinates (latitude and longitude) define a position on the Earth’s surface. Coordinates are angular units. The canonical form of latitude and longitude representation uses degrees (°), minutes (′), and seconds (″). GPS systems widely use coordinates in degrees and decimal minutes, or in decimal degrees.
Latitude varies from −90° to 90°. The latitude of the Equator is 0°; the latitude of the South Pole is −90°; the latitude of the North Pole is 90°. Positive latitude values correspond to the geographic locations north of the Equator (abbrev. N). Negative latitude values correspond to the geographic locations south of the Equator (abbrev. S).
Longitude is counted from the prime meridian ( IERS Reference Meridian for WGS 84) and varies from −180° to 180°. Positive longitude values correspond to the geographic locations east of the prime meridian (abbrev. E). Negative longitude values correspond to the geographic locations west of the prime meridian (abbrev. W).
UTM or Universal Transverse Mercator coordinate system divides the Earth’s surface into 60 longitudinal zones. The coordinates of a location within each zone are defined as a planar coordinate pair related to the intersection of the equator and the zone’s central meridian, and measured in meters.
Elevation above sea level is a measure of a geographic location’s height. We are using the global digital elevation model GTOPO30 .
Elektrostal , Moscow Oblast, Russia
MOSCOW - RUSSIA
Ewf b.v east west forwarding.
Edelveis, Right Entrance, 2nd Floor Davidkovskaja, 121352 Moscow, Russia
- Phone: +7 495 938-99-66
- Mobile: +7 495-997-0977
- Fax: +7 495 938-99-67
- email: [email protected]
- web: www.eastwestforwarding.com
Company Profile
- LIST WITH US
To: EWF B.V EAST WEST FORWARDING
Enter the security code:
+7 495 938-99-67
+7 495-997-0977
+7 495 938-99-66
Directory of Freight Forwarders, Cargo Agents, Shipping Companies, Air, Ocean, Land, Logistics and Transportation Brokers

IMAGES
VIDEO
COMMENTS
South Central - Oxford A. Previous name(s) Oxfordshire Research Ethics Committee A Chair Dr Hugh Davies. Approvals Specialist Chris King. Approvals Administrator George Hudson. Phone numbers 0207 104 8118, 0207 104 8100. Email address [email protected]. Region/Nation South Central. Usual meeting venue Meeting held by video-conference via ...
East of England - Cambridge South. East of Scotland Research Ethics Service REC 1. East of Scotland Research Ethics Service REC 2. Health and Social Care Research Ethics Committee A (HSC REC A) Health and Social Care Research Ethics Committee B (HSC REC B) London - Bloomsbury. London - Camden and Kings Cross. London - Central.
As set out in its research ethics policy, the University requires that all such research be subject to appropriate ethics review. The Central University Research Ethics Committee (CUREC) has overall responsibility for the development of this policy and for the University's ethics review process. CUREC's subcommittees are responsible for ...
The interdivisional research ethics committees (IDRECs) for Medical Sciences and Social Sciences and Humanities each have a minimum of three external members. The Oxford Tropical Research Ethics Committee (OxTREC) has four external members. These committees provide ethical review of research involving human participants, human tissue and/or ...
Ethics and dissemination: Ethical approval has been provided by the National Health Service Health Research Authority South Central-Oxford A Research Ethics Committee (reference 18/SC/0054). The study also received authorisation from the UK Medicines and Healthcare products Regulatory Agency and is being run in accordance with the principles of ...
The South Central-Oxford C Research Ethics Committee (REC: 14/SC/0157) and the Medicines and Healthcare Products Regulatory Authority (EudraCT: 2014-000792-11) approved the study protocol. The trial was funded by the UK National Institute for Health Research Health Technology Assessment Programme (12/127/126).
A track record of leading effective teams to successfully deliver clinical research projects. · Experience: South Central - Oxford B Research Ethics Committee · Education: University of Oxford · Location: Greater Oxford Area · 221 connections on LinkedIn. View Dr Maria Breen's profile on LinkedIn, a professional community of 1 billion ...
The EPIC Oxford study was given ethical approval by Royal College of General Practitioners Clinical Research Ethics Committee, the Central Oxford Research Ethics Committee and local research ethics committees. The South Central Oxford C Research Ethics Committee gave ethical approval for the Oxford Vegetarian Study.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research. Consent should be obtained before the participant enters the research ...
amendment to be implemented at NHS sites in England until the outcome of the HRA assessment has been confirmed. 11 April 2023. Professor Christopher Butler University of Oxford Radcliffe Observatory Quarter, Woodstock Road Oxford. OX2 6GG. Dear Professor Butler. Bristol REC Centre Temple Quay House. 2 The Square Temple Quay Bristol. BS1 6PN.
South Central - Berkshire Research Ethics Committee Health Research Authority 2 Redman Place ... Woodstock Road Oxford OX2 6GG Dear Professor Butler, Study title: Platform Adaptive trial of NOvel antiviRals for eArly treatMent of covid-19 In the Community ... South Central - Berkshire Research Ethics Committee Attendance at Sub-Committee of the ...
Research Ethics Service South Central - Oxford A Research Ethics Committee Annual Report 01 April 2015 - 31 March 2016 South Central - Oxford A Research Ethics Committee…
amendment to be implemented at NHS sites in England until the outcome of the HRA assessment has been confirmed. 26 March 2024. Professor Christopher Butler University of Oxford Radcliffe Observatory Quarter Woodstock Road Oxford. OX2 6GG. Dear Professor Butler, Health Research Authority. 2 Redman Place Stratford.
South Central - Oxford C Research Ethics Committee Health Research Authority (Bristol) Ground Floor, Temple Quay House, 2 The Square BS1 6PN 26 August 2022 ... South Central - Oxford C Research Ethics Committee Attendance at Sub-Committee of the REC meeting on 19 August 2022
South Central - Oxford B Research Ethics Committee Whitefriars Level 3, Block B Lewin's Mead Bristol BS1 2NT ... Research protocol or project proposal [STAR protocol] 1.0 27 January 2016 ... Copy to: Mrs Heather House, Oxford University Hospitals NHS foundation trust.
Annual Report 01 April 2016 - 31 March 2017 South Central - Oxford B Research Ethics Committee Annual Report Page 2 Part 1 - Committee Membership and Training Name. ... South Central - Oxford B Research Ethics Committee Annual ; of 29 /29. Match case Limit results 1 per page.
Phone numbers. 0207 1048144, 0207 104 8089, 0207 104 8271. Email address. [email protected]. Region/Nation. South Central. Usual meeting venue. Meeting held by video-conference via Zoom. Usual meeting time.
South Central - Oxford B. Previous name(s) Oxfordshire Research Ethics Committee B Chair Dr Maria Breen. Approvals Specialist Chelsea Phillips. Approvals Administrator Najmin Akhter / Vicky Canfield-Duffy. Phone numbers 0207 104 8134 ...
Gorodskoy Okrug Elektrostal' is in Moscow Oblast. Gorodskoy Okrug Elektrostal' is situated nearby to Shibanovo and Vysokovo. Mapcarta, the open map.
South Central - Oxford B Research Ethics Committee. REC reference. 18/SC/0092. Date of REC Opinion. 20 Mar 2018. REC opinion. Favourable Opinion. Research database title. OSCC Research Database. Establishment organisation. University of Oxford. Establishment organisation address. University Offices. Wellington Square. Oxford. OX1 2JD
Moscow Oblast - Features. Moscow Oblast is located in the central part of the East European Plain, in the basin of the rivers of Volga, Oka, Klyazma, Moskva. The region stretches from north to south for 310 km, from west to east - 340 km. It was named after the city of Moscow, which however is not part of the region.
Graduate Certificate in Ethics in Public Service 354 Family Nurse Practitioner (Post Master's Certificate) 355 ... mentoring, and contributing to society through their cutting-edge research and scholarly activities. Our staff members care about supporting our students and their success. Our students care about making a difference in the world ...
The latitude of the Equator is 0°; the latitude of the South Pole is −90°; the latitude of the North Pole is 90°. Positive latitude values correspond to the geographic locations north of the Equator (abbrev. ... within each zone are defined as a planar coordinate pair related to the intersection of the equator and the zone's central ...
EWF B.V EAST WEST FORWARDING. Edelveis, Right Entrance, 2nd Floor Davidkovskaja, 121352 Moscow, Russia. Phone: +7 495 938-99-66; Mobile: +7 495-997-0977