- Subject List
- Take a Tour
- For Authors
- Subscriber Services
- Publications
- African American Studies
- African Studies
- American Literature
- Anthropology
- Architecture Planning and Preservation
- Art History
- Atlantic History
- Biblical Studies
- British and Irish Literature
- Childhood Studies
- Chinese Studies
- Cinema and Media Studies
- Communication
- Criminology
- Environmental Science

Evolutionary Biology
- International Law
- International Relations
- Islamic Studies
- Jewish Studies
- Latin American Studies
- Latino Studies
- Linguistics
- Literary and Critical Theory
- Medieval Studies
- Military History
- Political Science
- Public Health
- Renaissance and Reformation
- Social Work
- Urban Studies
- Victorian Literature
- Browse All Subjects
How to Subscribe
- Free Trials
In This Article Expand or collapse the "in this article" section Character Displacement
Introduction, general overviews.
- Terminology
- Ecological Character Displacement
- Reproductive Character Displacement
- Agonistic Character Displacement
- Testing for Character Displacement
- Community-Wide Character Displacement
- Character Displacement and Speciation
- Character Displacement, Phenotypic Plasticity, and Learning
Related Articles Expand or collapse the "related articles" section about
About related articles close popup.
Lorem Ipsum Sit Dolor Amet
Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Aliquam ligula odio, euismod ut aliquam et, vestibulum nec risus. Nulla viverra, arcu et iaculis consequat, justo diam ornare tellus, semper ultrices tellus nunc eu tellus.
- Adaptive Radiation
- Disruptive Selection
- Hybrid Zones
- Male-Male Competition
- Mate Choice
- Natural Selection
- Niche Evolution
- Sexual Selection
- Sexual Selection and Speciation
- Sympatric Speciation
Other Subject Areas
Forthcoming articles expand or collapse the "forthcoming articles" section.
- Bacterial Species Concepts
- Recombination and Evolution
- Selective Sweeps
- Find more forthcoming articles...
- Export Citations
- Share This Facebook LinkedIn Twitter
Character Displacement by Greg Grether LAST REVIEWED: 26 April 2018 LAST MODIFIED: 26 April 2018 DOI: 10.1093/obo/9780199941728-0104
Character displacement is the phenomenon of species at the same trophic level evolving through natural selection in response to each other because of some costly interaction. It can result in a geographic pattern in which closely related species differ more from each other phenotypically (e.g., in morphology, coloration, or behavior) in areas where their ranges overlap than where their ranges do not overlap. The term “character displacement” was originally used to refer to this geographic pattern, and some biologists continue to use it that way. However, since the 1970s, most researchers have used the term to refer to specific evolutionary processes. Two forms of character displacement, ecological character displacement (ECD) and reproductive character displacement (RCD), are widely recognized. A third form, agonistic character displacement (ACD), was proposed more recently and is gaining recognition. ECD is caused by indirect (exploitative) competition between species for common resources (e.g., food, nesting sites) and usually results in reduced resource overlap (i.e., niche divergence), although, in theory, exploitative competition can also cause niche convergence. RCD is caused by reproductive interference between species (e.g., courtship, mating, hybridization) and invariably reduces the frequency or cost of the interaction. RCD is synonymous with “reinforcement” when it increases the level of reproductive isolation between hybridizing species. RCD can result in patterns of divergence in easily observable traits, such as courtship signals and activity schedules, but it can also involve more subtle changes, such as evolved shifts in sensitivity to heterospecific seminal products. ACD occurs in two modes, divergent and convergent. Convergent ACD is the expected mode when interspecific resource defense is adaptive, while divergent ACD is the expected mode when interspecific resource defense is not adaptive. Convergent ACD is caused by competition between species for mates or other resources and can result in convergence in traits involved in competitor recognition (e.g., agonistic signals, neural templates) and enhancements in interspecific fighting ability (e.g., tactics, weaponry). Divergent ACD is caused by aggressive interference between species and reduces the frequency or cost of the interaction, through divergence in traits involved in competitor recognition or that affect the rate of interspecific encounters. Character displacement processes are widely considered to have a major role in structuring ecological communities, in the generation of phenotypic diversity, and in the evolution of barriers to reproduction between populations, culminating in speciation.
Pfennig and Pfennig 2012 is the most comprehensive and current book on character displacement and its myriad consequences. Schluter 2000 dispels the once common view that little or no evidence exists for ecological character displacement (ECD), while also identifying gaps in the evidence. Dhondt 2012 provides a succinct review of some well-supported cases of ECD. Grant and Grant 2014 chronicles a thoroughly documented case of ECD in action on a Galapagos island. Coyne and Orr 2004 reviews the tumultuous history of reinforcement theory and the empirical evidence that spurred theoreticians to figure out how to model it correctly. Nosil 2012 examines the roles of both reinforcement and ECD in speciation. Grether, et al. 2013 reviews agonistic character displacement (ACD) theory and the evidence available for this form of character displacement. See also Ecological Character Displacement , Reproductive Character Displacement , and Agonistic Character Displacement .
Coyne, J. A., and H. A. Orr. 2004. Speciation . Sunderland, MA: Sinauer.
Summarizes the “extraordinarily tortuous history” of the theory of reinforcement, the state of empirical research, and alternative explanations for enhanced prezygotic isolation in sympatry (chapter 10).
Dhondt, A. A. 2012. Interspecific competition in birds . Oxford: Oxford Univ. Press.
Summarizes key evidence for ECD in selected species, including, but not restricted to, birds (chapter 10).
Grant, P. R., and B. R. Grant. 2014. 40 years of evolution: Darwin’s finches on Daphne Major Island . Princeton, NJ: Princeton Univ. Press.
DOI: 10.1515/9781400851300
Most studies of character displacement are based on comparing populations to make inferences about evolution past. This book synthesizes the results of a different approach: studying evolution as it unfolds. One product of this long-term effort is a fully documented case of ECD in the beak of the finch Geospiza fortis after the Island of Daphne Major was colonized by a larger finch, G. magnirostris (chapter 7).
Grether, G. F., C. N. Anderson, J. P. Drury, et al. 2013. The evolutionary consequences of interspecific aggression. Annals of the New York Academy of Sciences 1289.1: 48–68.
DOI: 10.1111/nyas.12082
Compares ACD theory with alternative theoretical frameworks, discusses how to distinguish between character displacement processes empirically, and reviews the state of theory and empirical evidence for ACD, concluding that the evidence is substantial but further research is needed.
Nosil, P. 2012. Ecological speciation . Oxford: Oxford Univ. Press.
DOI: 10.1093/acprof:osobl/9780199587100.001.0001
Discusses various aspects of the hypothesis that reproductive isolation evolves in response to species interactions and emphasizes the difficulty of distinguishing reinforcement from ECD (chapters 3, 4, and 6).
Pfennig, D. W., and K. S. Pfennig. 2012. Evolution’s wedge: Competition and the origins of diversity . Berkeley: Univ. of California Press.
DOI: 10.1525/california/9780520274181.001.0001
The only book devoted entirely to character displacement and one of few advanced treatments to cover both reproductive character displacement and ECD. Character displacement is presented as a unifying principle that can be applied to many fundamental questions in biology.
Schluter, D. 2000. The ecology of adaptive radiation . Oxford: Oxford Univ. Press.
Places research on ECD into a historical context, reviews the underlying theory, and critically reviews the evidence from observational, predictive and experimental studies (chapter 6). Concludes that the evidence supports a role for ECD in evolutionary diversification, but further research is needed.
back to top
Users without a subscription are not able to see the full content on this page. Please subscribe or login .
Oxford Bibliographies Online is available by subscription and perpetual access to institutions. For more information or to contact an Oxford Sales Representative click here .
- About Evolutionary Biology »
- Meet the Editorial Board »
- Amniotes, Diversification of
- Ancient DNA
- Behavioral Ecology
- Canalization and Robustness
- Cancer, Evolutionary Processes in
- Character Displacement
- Coevolution
- Cognition, Evolution of
- Constraints, Evolutionary
- Contemporary Evolution
- Convergent Evolution
- Cooperation and Conflict: Microbes to Humans
- Cooperative Breeding in Insects and Vertebrates
- Creationism
- Cryptic Female Choice
- Darwin, Charles
- Disease Virulence, Evolution of
- Diversification, Diversity-Dependent
- Ecological Speciation
- Endosymbiosis
- Epigenetics and Behavior
- Epistasis and Evolution
- Eusocial Insects as a Model for Understanding Altruism, Co...
- Eusociality
- Evidence of Evolution, The
- Evolution and Development: Genes and Mutations Underlying ...
- Evolution and Development of Individual Behavioral Variati...
- Evolution, Cultural
- Evolution of Animal Mating Systems
- Evolution of Antibiotic Resistance
- Evolution of New Genes
- Evolution of Plant Mating Systems
- Evolution of Specialization
- Evolutionary Biology of Aging
- Evolutionary Biomechanics
- Evolutionary Computation
- Evolutionary Developmental Biology
- Evolutionary Ecology of Communities
- Experimental Evolution
- Field Studies of Natural Selection
- Founder Effect Speciation
- Frequency-Dependent Selection
- Fungi, Evolution of
- Gene Duplication
- Gene Expression, Evolution of
- Genetics, Ecological
- Genome Evolution
- Geographic Variation
- Group Selection
- Heterochrony
- Heterozygosity
- History of Evolutionary Thought, 1860–1925
- History of Evolutionary Thought before Darwin
- History of Evolutionary Thought Since 1930
- Human Behavioral Ecology
- Human Evolution
- Hybrid Speciation
- Hybridization and Diversification
- Identifying the Genomic Basis Underlying Phenotypic Variat...
- Inbreeding and Inbreeding Depression
- Inclusive Fitness
- Innovation, Evolutionary
- Islands as Evolutionary Laboratories
- Kin Selection
- Land Plants, Evolution of
- Landscape Genetics
- Landscapes, Adaptive
- Language, Evolution of
- Latitudinal Diversity Gradient, The
- Macroevolution
- Macroevolution, Clade-Level Interactions and
- Macroevolutionary Rates
- Mass Extinction
- Maternal Effects
- Mating Tactics and Strategies
- Medicine, Evolutionary
- Meiotic Drive
- Modern Synthesis, The
- Molecular Clocks
- Molecular Phylogenetics
- Mutation Rate and Spectrum
- Mutualism, Evolution of
- Natural Selection in Human Populations
- Natural Selection in the Genome, Detecting
- Neutral Theory
- New Zealand, Evolutionary Biogeography of
- Niche Construction
- Non-Human Animals, Cultural Evolution in
- Origin and Early Evolution of Animals
- Origin of Amniotes and the Amniotic Egg
- Origin of Eukaryotes
- Origin of Life, The
- Paradox of Sex
- Parental Care, Evolution of
- Parthenogenesis
- Personality Differences, Evolution of
- Pest Management, Evolution and
- Phenotypic Plasticity
- Phylogenetic Comparative Methods and Tests of Macroevoluti...
- Phylogenetic Trees, Interpretation of
- Phylogeography
- Polyploid Speciation
- Population Genetics
- Population Structure
- Post-Copulatory Sexual Selection
- Psychology, Evolutionary
- Punctuated Equilibria
- Quantitative Genetic Variation and Heritability
- Reaction Norms, Evolution of
- Reinforcement
- Reproductive Proteins, Evolution of
- Selection, Directional
- Selection, Disruptive
- Selection Gradients
- Selection, Natural
- Selection, Sexual
- Selfish Genes
- Sequential Speciation and Cascading Divergence
- Sexual Conflict
- Sexual Size Dimorphism
- Speciation Continuum
- Speciation Genetics and Genomics
- Speciation, Geography of
- Speciation, Sympatric
- Species Concepts
- Species Delimitation
- Sperm Competition
- Systems Biology
- Taxonomy and Classification
- Tetrapod Evolution
- The Philosophy of Evolutionary Biology
- Theory, Coalescent
- Trends, Evolutionary
- Wallace, Alfred Russel
- Privacy Policy
- Cookie Policy
- Legal Notice
- Accessibility
Powered by:
- [66.249.64.20|185.148.24.167]
- 185.148.24.167
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

CHARACTER DISPLACEMENT: ECOLOGICAL AND REPRODUCTIVE RESPONSES TO A COMMON EVOLUTIONARY PROBLEM
Character displacement – trait evolution stemming from selection to lessen resource competition or reproductive interactions between species – has long been viewed as an important mechanism for enabling closely related species to coexist. Yet, the causes and consequences of character displacement have not been fully explored. Moreover, character displacement in traits associated with resource use (ecological character displacement) has been studied largely independently of that in traits associated with reproduction (reproductive character displacement). Here, we underscore the commonalities of these two forms of character displacement and discuss how they interact. We focus on the causes of character displacement and explore how character displacement can have downstream effects ranging from speciation to extinction. In short, understanding how organisms respond to competitive and reproductive interactions with heterospecifics offers key insights into the evolutionary consequences of species coexistence and diversification.
Introduction
How can closely related species coexist in the same habitat? Why are even closely related species often phenotypically different from one another? What role do interactions between species play in the process of diversification? In this article, we describe how the answers to such questions can emerge from knowledge of how organisms respond to a common evolutionary problem. Namely, organisms often face reduced fitness stemming from interactions with other species that reduce access to resources or successful reproduction. Here, we show how selection minimizes competitive or reproductive interactions between species by favoring the evolution of divergent resource-use or reproductive phenotypes. This process, termed “character displacement” ( Brown and Wilson, 1956 ), is potentially a leading cause of adaptive diversification (reviewed in Schluter, 2000 ). In particular, character displacement: favors the evolution of novel resource-use or reproductive traits; drives divergence between sympatric and allopatric conspecific populations; and both initiates and finalizes the process of speciation.
Despite the significance of character displacement, previous research has focused largely on whether or not it occurs (reviewed in Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Dayan and Simberloff, 2005 ). The need exists, however, to move beyond establishing the existence of character displacement in order to discover its full implications. Moreover, research has tended to focus separately on ecological character displacement (character displacement in traits associated with resource use) and reproductive character displacement (character displacement in traits associated with reproduction) [for notable exceptions, see research on stickleback fish (reviewed in Rundle and Schluter, 2004 ) and Darwin's finches (reviewed in Grant and Grant, 2008 )]. Consequently, there has been relatively little cross-fertilization of ideas between researchers who study these two forms of character displacement.
Rather than comprehensively review the evidence for character displacement, as has been done elsewhere ( Howard, 1993 ; Schluter, 2000 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Dayan and Simberloff, 2005 ; Groning and Hochkirch, 2008 ), we highlight future directions for research in character displacement. Our specific goals are threefold. First, we seek to unite ecological and reproductive character displacement under the same conceptual framework. Second, we underscore the value of exploring more fully the ecological and evolutionary causes and consequences of character displacement. In particular, we describe why some species may be especially prone to undergo character displacement and discuss some of character displacement's downstream effects. Third, we evaluate how reproductive and ecological character displacement interact and thereby affect the likelihood that either process will unfold.
Unifying Ecological and Reproductive Character Displacement
We begin by presenting a unified framework for making the fields of ecological and reproductive character displacement parallel in focus. To do so, we first discuss what constitutes character displacement and review, albeit briefly, the problems of definitions that have plagued both ecological and reproductive character displacement. We suggest that applying the conceptual framework developed for ecological character displacement to reproductive character displacement will alleviate confusion and place both fields on equal footing.
What is Character Displacement?
Brown and Wilson (1956) coined the term “character displacement,” but the catalyst for the idea can be traced to Gause (1934) . Gause (1934) showed experimentally that two species cannot stably coexist if they overlap completely in resource requirements [ Darwin (1859) had actually made a similar argument but did not provide empirical support]. In such situations, one species ultimately edges out the other. This hypothesis, termed the competitive exclusion principle ( Hardin, 1960 ), forms a cornerstone of ecology. The competitive exclusion principle has an important corollary: that species can stably coexist if they differ in resource use ( Hardin, 1960 ; Pianka, 2000 ). Therefore, initially identical, interacting species will experience strong selection to evolve differences in resource use ( Lack, 1947 ; Grant, 1972 ; Arthur, 1982 ; Schluter, 1994 ; Pfennig et al., 2007 ). Similarly, such species will experience strong selection to evolve differences in reproductive traits (reviewed in Butlin and Ritchie, 1994 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ); otherwise, one species may drive the other locally extinct through “reproductive” exclusion (also referred to as “sexual exclusion”; Hochkirch et al., 2007 ; Groning and Hochkirch, 2008 ).
Character displacement is likely a general phenomenon in that most species will, at some point in their evolutionary history, confront heterospecifics with which they competitively or reproductively interact. In such situations, individuals most dissimilar from the average resource-use or reproductive traits of another species are expected to procure more resources or successful reproduction than other members of their population ( Slatkin, 1980 ; Taper and Case, 1985 , 1992 ; Abrams, 1986 ; Butlin and Ritchie, 1994 ; Doebeli, 1996 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ). Consequently, these most divergent individuals should experience highest fitness. If heritable variation exists in these traits, each species will evolve to be less like the other (although, as we note briefly later on, asymmetric character displacement can arise if the species differ in whether and how much they diverge; for fuller discussion see Schluter, 2000 ; Cooley, 2007 ). Such selection, acting to lessen competitive or reproductive interactions between species, can promote evolutionary divergence in traits associated with resource use or reproduction; i.e., character displacement ( Figure 1 ; for a review of the theory, see Schluter, 2000 ; Coyne and Orr, 2004 ). In the absence of character displacement, competitive or reproductive exclusion may ensue ( Gause, 1934 ; Liou and Price, 1994 ; Groning and Hochkirch, 2008 ).

(a) Initially, two species encounter each other and overlap in phenotypes associated with resource use or reproduction (indicated here by the two overlapping bell-shaped curves). Character displacement arises when individuals most dissimilar from the average resource-use or reproductive phenotypes of another species are more successful at acquiring resources or reproduction than other members of their population. Consequently, (b) the most divergent individuals should experience highest fitness and the two species should tend to evolve to be less like the other. Character displacement is indicated when the difference between species in mean trait value is greater after selection ( d A ) than before selection ( d B ).
In this article, “character displacement” refers to the evolutionary accentuation of phenotypic differences between species stemming from selection to lessen resource competition or reproductive interactions between them (later, we broaden this definition to include selection acting within species). Character displacement can assume two distinct forms that differ in the agent and target of selection ( Brown and Wilson, 1956 ). “Ecological character displacement” refers to trait evolution stemming from selection to lessen resource competition between species and therefore acts on traits associated with resource use (e.g., morphological structures such as beaks and jaws; Slatkin, 1980 ; Schluter, 2001 ). By contrast, “reproductive character displacement” refers to trait evolution stemming from selection to lessen sexual interactions between species and therefore acts on traits associated with reproduction (e.g., sexual signals or female mate preferences; Brown and Wilson, 1956 ; Crozier, 1974 ).
An important prediction of character displacement is that species should differ in traits associated with resource use or reproduction if they occur together ( Brown and Wilson, 1956 ; Grant, 1972 ; Schluter, 2000 ). Moreover, selection to lessen resource competition or reproductive interactions should act only where species actually co-occur ( Brown and Wilson, 1956 ; Lack, 1947 ). Consequently, character displacement should also produce a distinctive pattern in which species are more dissimilar where they occur together than where each occurs alone ( Figure 2a, b ; Brown and Wilson, 1956 ; Lack, 1947 ). Thus, within each species, populations in sympatry with the heterospecific should differ from those in allopatry ( Figure 2b ; Lack, 1947 ). Character displacement therefore consists of two hallmark features: (1) the process of phenotypic evolution stemming from selection to lessen resource competition or reproductive interactions between species ( Figure 1 ); and ( 2 ) the resulting pattern of geographical variation in which sympatric species show exaggerated divergence, and in which conspecific populations in sympatry with aheterospecific differ from those in allopatry ( Figure 2b ).

(a) For two species that occur in both sympatry with each other and in allopatry, character displacement should produce a distinctive pattern of divergence (b) in which the two species are more dissimilar to each other in sympatry (where there is selection for divergence) than in allopatry (where there is no such selection). Moreover, within such species, populations in sympatry with the heterospecific are expected to diverge from conspecific populations in allopatry. (c) Because the likelihood of encountering heterospecifics may increase along a spatial gradient (e.g., as one moves from the edge to the center of a species' geographical range), character displacement may produce a pattern in which, within each species, the magnitude of divergence increases along a gradient with increasing likelihood of encountering heterospecifics.
Conflation of Process and Pattern
Character displacement has often been conflated with the pattern that is predicted to arise from it (reviewed in Grant, 1972 ; Endler, 1986 ; Schluter, 2000 ; Goldberg and Lande, 2006 ). Defining character displacement as a pattern, however, is problematic, because patterns of divergence between species and populations can be generated via processes other than selection to avoid interactions with heterospecifics ( Grant, 1972 ; Strong et al., 1979 ; Simberloff and Boecklen, 1981 ; Arthur, 1982 ; Endler, 1986 ; Diamond et al., 1989 ). In the case of ecological character displacement, the conflation of process and pattern provoked a lengthy and spirited debate over what constituted “true” character displacement (for a review of this debate, see Schluter, 2001 ). In response, researchers generally agreed to define ecological character displacement as the process described above (i.e., the definition that we presented in the previous section is widely accepted; e.g., see Schluter, 2001 , 2002 ). By defining character displacement in terms of process rather than pattern, researchers could thereby focus strictly on the ecological and evolutionary implications of resource competition between species ( Grant, 1972 ; Schluter, 2000 ).
Unifying the Conceptual Framework of Ecological and Reproductive Character Displacement
Although the field of ecological character displacement is largely reconciled as to what constitutes character displacement, the field of reproductive character displacement has achieved no such resolution. Consequently, conflation of pattern and process is widespread when dealing with reproductive character displacement (for additional discussion see also Butlin and Ritchie, 1994 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ). Yet, as the literature on ecological character displacement clearly illustrates, patterns of trait divergence can be generated via processes other than selection to avoid interactions with heterospecifics (e.g., founder effects; Marko, 2005 ; reviewed in Grant, 1972 ; Schluter, 2000 ). Defining reproductive character displacement as a process, rather than as a pattern, has the same benefit as defining ecological character displacement as a process: researchers can focus specifically on the ecological and evolutionary implications of interactions between species driving reproductive trait divergence. Moreover, taking a parallel process-oriented approach to both ecological and reproductive character displacement allows for a more complete understanding of how they interact. If the two fields continue to define character displacement differently, then the ability to adequately delineate, let alone address, questions regarding how and why ecological and reproductive character displacement interact will be hampered.
Defining reproductive character displacement as a process is not new. Indeed, Butlin and Ritchie (1994) define reproductive character displacement as “the process of divergence in mating signal systems between reproductively isolated species” (p. 62, italics are ours; Butlin and Ritchie, 1994 ). The definition of reproductive character displacement that we use here is a more general form than that of Butlin and Ritchie (1994) . Yet, this more general definition clarifies the relationship of reproductive character displacement to reinforcement – the evolution of traits that minimize hybridization between species ( Dobzhansky, 1940 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ). By the general definition we use here, reinforcement constitutes a special case of reproductive character displacement. This approach is also not new. Indeed, Blair (1974) – who is credited with coining the term “reinforcement” ( Coyne and Orr, 2004 ) – refers to reinforcement as “a rather restricted form of character displacement” (p. 1119, Blair, 1974 ).
Defining reproductive character displacement broadly as the selective process by which reproductive traits diverge in order to minimize costly reproductive interactions with heterospecifics, and including reinforcement as a form of character displacement, emphasizes the general importance of selection as the driving force behind reproductive trait divergence. The definition we use here thereby minimizes confusion about what does, and does not, constitute reproductive character displacement versus reinforcement, and overcomes the issues associated with confounding pattern and process when describing reproductive character displacement (e.g., those who study reinforcement typically consider reproductive character displacement to be a signature pattern resulting from reinforcement; see Howard, 1993 ; Servedio and Noor, 2003 and references therein).
Butlin and Ritchie (1994) argued that reproductive character displacement and reinforcement should be considered separate processes based, in part, on the nature of interactions between species (see also Butlin, 1987 ). Whereas reinforcement was defined as arising from interactions where species could actually exchange genes during mating, reproductive character displacement was deemed to arise from all other mating interactions ( Butlin, 1987 ; Butlin and Ritchie, 1994 ). Yet, in the same way that competition for resources can take different forms [i.e., exploitative (indirect) competition and interference (direct) competition] but still generate ecological character displacement ( Schluter, 2000 ), different types of sexual interactions between species can promote reproductive character displacement. In particular, reproductive interactions between species can take two general forms: direct interactions, in which the two species actually risk hybridizing with one another, and indirect interactions, in which the two species utilize similar aspects of their habitat (e.g., signaling space, pollinators) to seek and attract mates.
Direct interactions can produce wasted mating effort (e.g., in terms of lost gametes or investment in searching for a mate) if no viable offspring are produced (for discussion of hybrid fitness see Barton and Hewitt, 1989 ; Arnold, 1997 ; Coyne and Orr, 2004 ). Even if hybrid offspring are viable, hybridization may still result in low fitness if hybrids have lower survivorship or reduced fertility and fecundity (reviewed in Barton and Hewitt, 1989 ; Arnold, 1997 ; Coyne and Orr, 2004 ). Consequently, selection should generally minimize the risks of hybridization by favoring divergence between species in reproductive traits; i.e., reproductive character displacement. In other words, direct interactions that contribute to gene flow between species can lead to reinforcement, which we consider to be a special case of reproductive character displacement (see also Blair, 1974 ).
By contrast, indirect interactions can generate interference between species that make mate localization difficult and costly in terms of increasing signaling effort or increasing search times and their associated costs ( Butlin and Ritchie, 1994 ; Gerhardt and Huber, 2002 ). For example, species that use acoustic signals can mask, jam, or attenuate aspects of one another's signal properties, making it difficult to discern either signal (reviewed in Gerhardt and Huber, 2002 ). Similarly, plants that compete for pollinators may cope with pollen limitation and pollen interference ( Levin, 1985 ; Caruso, 2000 ; Smith and Rausher, 2008 ). As with the direct reproductive interactions described above, selection should generally minimize indirect reproductive interference by favoring divergence between species in reproductive traits; i.e., reproductive character displacement.
This dichotomy of direct and indirect interactions should not be taken as mutually exclusive –species can interact in both ways, and how they interact may change spatially and temporally. The key point is that, for direct and indirect reproductive interactions, both the agent and target of selection are the same. Consequently, both types of interactions can promote divergence in reproductive traits as a means of minimizing costly reproductive interactions between species. In other words, both can promote reproductive character displacement (according to the definition we use here).
Given this framework for defining ecological and reproductive character displacement in a similar way, we turn to evaluating the causes of character displacement. In particular, we seek to examine what factors facilitate character displacement and thereby make it more likely to occur than the alternative outcomes of competitive or reproductive exclusion.
Causes of Character Displacement
The consensus that has emerged from decades of work is that character displacement is taxonomically widespread ( Schluter, 2000 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Dayan and Simberloff, 2005 ). Yet, why character displacement appears to be more likely to transpire in some circumstances and taxa than in others remains relatively unexplored (reviewed in Schluter 2000 ; Rice and Pfennig 2007 ). Understanding when and why character displacement is more likely to proceed is important, because differences in the occurrence of character displacement could explain ecological and evolutionary patterns of diversity. For example, communities or taxa that are more prone to undergo character displacement will likely be more diverse that those communities or taxa where character displacement does not occur, for at least two reasons. First, species that undergo character displacement are less likely to go extinct through competitive or reproductive exclusion (see above). Second, as we describe later, character displacement may promote speciation. Hence, as part of a more general theory for why some communities or taxa are more diverse than others ( Schluter, 2000 ), it is important to determine what factors facilitate character displacement.
Factors that Facilitate Character Displacement
Four, nonexclusive factors appear to facilitate character displacement and therefore make it more likely to unfold. Two are evolutionary factors: strong selection disfavoring interactions with heterospecifics, and ecological opportunity. The remaining two are proximate factors: initial trait differences between species, and abundant standing variation. Although these factors facilitate adaptive evolution in general, and are therefore not unique to character displacement, studies are needed to examine how they affect character displacement. Below, we describe each factor and its effect on character displacement in turn.
First, character displacement is more likely to occur when selection against interactions with heterospecifics is strong. For example, reproductive character displacement is increasingly likely to occur as the costs of hybridization increase ( Liou and Price, 1994 ). Moreover, differences between species in the strength of selection to avoid interactions with the other species may explain asymmetric character displacement, where one species diverges less than another species ( Cooley, 2007 ). When one species suffers higher costs in the interaction, it may experience greater divergence than the other species (although asymmetric character displacement can occur for other reasons not described here; see Schluter, 2000 ; Cooley, 2007 ). Character displacement should also be more likely to occur when the encounter rate between species is high, and, hence, when selection disfavoring interactions with heterospecifics is strong (see Figure 2c ; for examples, see Pfennig and Murphy, 2002 ; Tynkkynen et al., 2004 ; Pfennig and Pfennig, 2005 ).
Second, character displacement is facilitated by “ecological opportunity,” the availability of different resource types underutilized by other species ( Simpson, 1953 ; Schluter, 2000 ; although the concept of ecological opportunity has traditionally been applied to resources, a similar principle applies to having available signal space in the case of reproductive character displacement). Character displacement often generates new resource-use or reproductive traits in sympatry that differ from the pre-displacement traits in allopatry ( Howard, 1993 ; Schluter, 2000 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Dayan and Simberloff, 2005 ; Groning and Hochkirch, 2008 ). Therefore, for character displacement to occur, exploitable resources or signal space that are not already utilized by another species must be available (i.e., there must be resources or signal space onto which a species can actually be displaced; Pfennig et al., 2006 ; Groning and Hochkirch, 2008 ). In the absence of exploitable resources or signal space, competitive or reproductive exclusion may result ( Pfennig et al., 2006 ; Hochkirch et al., 2007 ; Groning and Hochkirch, 2008 ).
Third, character displacement occurs most readily if interacting species already differ in phenotypic traits under selection when they come into contact with one another ( Slatkin, 1980 ; Liou and Price, 1994 ). Although character displacement can occur without such initial differences, character displacement is facilitated if other factors “jump-start” the divergence, prior to interactions with heterospecifics ( Slatkin, 1980 ). Such factors may act in allopatry before the two species come into contact with one another, and they may include drift or spatially divergent natural or sexual selection ( Schluter, 2000 ). Such differences may then be amplified in sympatry by selection acting to lessen interspecific interactions ( Schluter, 2000 ; Rice and Pfennig, 2007 ). In the absence of initial differences between species, one species will be more likely to drive the other locally extinct; e.g., through competitive or reproductive exclusion (see above). Thus, species that differ initially from heterospecifics should be more prone to undergo character displacement ( Slatkin, 1980 ; Liou and Price, 1994 ; Schluter, 2000 ; Rice and Pfennig, 2007 ).
Finally, character displacement may be more likely to occur when interacting species are phenotypically variable ( Milligan, 1985 ). Phenotypic variation is important, because it increases the chances that character displacement can evolve through the selective filtering of divergent phenotypes in sympatry that were already present in allopatry (for reviews see Rice and Pfennig, 2007 ; Barrett and Schluter, 2008 ). Indeed, because this process should unfold relatively rapidly, abundant standing variation should facilitate character displacement as opposed to competitive or reproductive exclusion ( Rice and Pfennig, 2007 ). Thus, species with abundant standing variation should therefore be especially likely to undergo character displacement ( Rice and Pfennig, 2007 ).
Given that abundant standing variation might facilitate character displacement, what evolutionary and developmental mechanisms generate such variation? Answering this question could explain why some populations are predisposed to undergo character displacement. In the next section, we discuss two such mechanisms: intraspecific competition and phenotypic plasticity.
Intraspecific Character Displacement
As noted above, species with abundant standing variation should be especially prone to undergo character displacement. Therefore, identifying the mechanisms that generate and maintain variation within natural populations is crucial for understanding the factors that facilitate character displacement. One such mechanism is disruptive selection, which arises when extreme phenotypes have a fitness advantage over more intermediate phenotypes ( Mather, 1953 ). By favoring extreme phenotypes, disruptive selection maintains, and may even increase, variation in natural populations ( Rueffler et al., 2006 ). Indeed, such selection could ultimately result in the evolution of resource or mating polymorphism – alternative phenotypes within the same population that differ in resource use or mate acquisition tactics ( Andersson, 1994 ; Smith and Skúkason, 1996 ).
Although numerous agents can generate disruptive selection, intraspecific competition for resources or mates has long been viewed as a leading cause ( Rosenzweig, 1978 ; Wilson and Turelli, 1986 ; Day and Young, 2004 ; Rueffler et al., 2006 ). Such disruptive selection on traits associated with resource or reproduction thereby favors divergence in these characters within populations (for examples, see Smith, 1993 ; Medel et al., 2003 ; Bolnick, 2004 ; Pfennig et al., 2007 ; Bolnick and Lau, 2008 ; Calsbeek and Smith, 2008 ; Hendry et al., 2009 ; Martin and Pfennig, in press ). The resulting trait evolution, arising from interactions within species, is analogous to that stemming from interactions between species ( Dayan and Simberloff, 2005 ) and can be considered as “intraspecific character displacement” (sensu West-Eberhard, 2003 ).
Once a population has undergone intra specific character displacement, it may, in turn, be more prone to undergo inter specific character displacement, for at least two reasons. First, intraspecific character displacement may favor the evolution of alternative resource-use or mate-acquisition phenotypes (Martin and Pfennig, in press). The evolution of such alternative phenotypes, prior to interactions between species, may fuel rapid character displacement via differential success of the alternative phenotypic variants ( Figure 3 ; Rice and Pfennig, 2007 ; Barrett and Schluter, 2008 ). Second, even when disruptive selection does not favor distinct morphs, it does tend to maintain, and even increase, both phenotypic and genetic variation in natural populations ( Mather, 1953 ; Rueffler et al., 2006 ). As noted in the previous section, such abundant standing variation increases the chances that interspecific character displacement will occur ( Rice and Pfennig, 2007 ).

Initially (a, d), a focal species (species 1, whose trait distribution is indicated by the bell-shaped curve) occurs alone in allopatry, either as a monomorphic species (a) or as a polymorphic species (d) consisting of alternative resource use or reproductive morphs (morphs 1, 2), one of which is initially rarer than the other. Later (b, e), a superior competitor, species 2 (whose trait distribution is indicated by the heavy bell-shaped curve), becomes sympatric with species 1 (either because species 2 invades the habitat of species 1 or vice versa). Finally (c, f), because of selection imposed by species 2, species 1 undergoes an evolutionary shift in resource use and associated phenotypic features (ecological character displacement) or in reproductive traits (reproductive character displacement; in both cases, the trait distributions of species 1 before selection are shown by the dashed bell-shaped curves). When there is little standing variation prior to encountering the heterospecific (as in panel c), character displacement unfolds only if for novel phenotypes that are more dissimilar to the competitor arise and spread in sympatry following the invasion of species 2. Because such novel phenotypes, if they do not already exist in the population, can only be generated through mutation, recombination, or introgression – all of which are relatively slow processes – competitive or reproductive exclusion, as opposed to character displacement, are more likely. By contrast, when there is abundant standing variation (as in panel f), character displacement unfolds when the phenotypic variant that is more dissimilar to the competitor (here, morph 1) is selectively favored and thereby increases in frequency at the expense of the alternative morph. Because such a process can unfold rapidly (e.g., potentially, within a single generation), character displacement, as opposed to exclusion, is more likely to transpire. Although we have illustrated this process as involving discrete morphs (which may have arisen through intraspecific character displacement), it could also occur in populations expressing a wide-range of continuously distributed phenotypes. Modified from Rice and Pfennig (2007) .
Phenotypic Plasticity
In the previous section, we focused on a selective agent – intraspecific competition – that favors variation within populations. Such variation might, in turn, predispose populations to subsequently undergo interspecific character displacement. However, it is important to also consider the proximate mechanisms that generate such variation. Elucidating these proximate mechanisms is vital, because different proximate mechanisms can influence the speed at which new phenotypic variants arise. Therefore, different proximate mechanisms may ultimately influence the speed of character displacement, and, hence, whether character displacement even occurs in the first place. In particular, any proximate mechanism that facilitates divergence in resource-use or reproductive phenotypes may render character displacement, as opposed to competitive or reproductive exclusion, more likely to transpire.
An important proximate mechanism for rapidly generating new phenotypic variants is phenotypic plasticity. Phenotypic plasticity is the ability of an individual organism to react to an environmental stimulus with a change in phenotype (reviewed in West-Eberhard, 2003 ). Phenotypic plasticity enables organisms to respond rapidly to the presence of heterospecifics by altering their phenotype adaptively (see reviews by, and references in, Robinson and Wilson, 1994 ; Agrawal, 2001 ; Pfennig and Murphy, 2002 ; Fordyce, 2006 ; Pfennig et al., 2006 ). For example, when faced with resource competition or reproductive interactions from a heterospecific, individuals of many species facultatively express alternative resource-acquisition or reproductive phenotypes that lessen competition or reproductive interactions (e.g., Werner and Hall, 1976 ; Pfennig and Murphy, 2002 ; Pfennig, 2007 ). Such rapid shifts in resource-acquisition or reproductive traits have not traditionally been considered character displacement, because phenotypic plasticity is often regarded as a nongenetic response that is incapable of mediating adaptive evolution ( Grant, 1972 ; Arthur, 1982 ; Schluter, 2000 ). Yet, the magnitude and direction of a plastic response is often genetically variable, and, consequently, subject to natural selection and evolutionary change (reviewed in Schlichting and Pigliucci, 1998 ; West-Eberhard, 2003 ; DeWitt and Scheiner, 2004 ).
Moreover, intergenerational plasticity – specifically, maternal effects – might actually promote a form of “canalization,” in which trait differences between species and populations persist, even when individuals are reared under common conditions (Pfennig and Martin, in press ). Maternal effects occur when the phenotype of a female influences the phenotype of her offspring, independent of the direct effects of her genes on her offspring's phenotype ( Mousseau and Fox, 1998 ). Because these effects can be acted upon by selection ( McAdam and Boutin, 2004 ) and then cause information to be conveyed reliably between generations ( Rossiter, 1996 ; Agrawal et al., 1999 ; Plaistow et al., 2006 ; Allen et al., 2008 ), they may play an important role in mediating adaptive evolution ( Jablonka and Lamb, 1995 ; Maynard Smith, 1998 ).
Maternal effects can facilitate either form of character displacement, but they may be especially important in mediating ecological character displacement. Ecological character displacement causes interacting species to utilize different resources (reviewed in Schluter, 2000 ; Day and Young, 2004 ). When resource quality is asymmetric, one species will gain the more profitable resource, whereas the other will be forced onto a less profitable resource (e.g., Pfennig and Pfennig, 2005 ; Grant and Grant, 2006 ). Consequently, females of the latter species may mature at a smaller body size or in poorer condition (e.g., Gorbushin, 1996 ; Pfennig and Pfennig, 2005 ; Grant and Grant, 2006 ). Because of their smaller size and poorer condition, these females may subsequently produce offspring that are also smaller and in poorer condition, purely because of a maternal effect (e.g., Pfennig and Martin, in press ). As a result of this maternal effect, the offspring may ultimately express a resource-use phenotype less like that expressed by the other species (resource use is often correlated with body size). Moreover, because maternal effects can be transmitted reliably between generations (see above), these differences in trait expression between populations in sympatry with a heterospecific competitor and those in allopatry may persist even when individuals are experimentally reared under common conditions. Such a pattern would give the misleading appearance that genetic differences underlie these trait differences. Thus, trait differences between populations undergoing character displacement may be underlain entirely by a maternal effect (for a possible example, see Pfennig and Martin, in press ).
The discussion above suggests that phenotypic plasticity can mediate rapid phenotypic divergence between species. Phenotypic plasticity might also promote the evolution of genetic differences that stabilize such phenotypic differences ( West-Eberhard, 2003 ). If individuals in a population begin to facultatively express a novel phenotype that lessens costly interactions with a heterospecific, and if there is underlying genetic variation in the degree to which individuals respond to heterospecifics, then selection should favor those alleles or gene combinations that best stabilize, refine, and extend the new trait's expression (a process know as “genetic accommodation”, West-Eberhard, 2003 ). Thus, under persistent selection to minimize competition or reproductive interactions with heterospecifics, divergent traits that are initially plastic may eventually become genetically canalized (i.e., “fixed”) in the population (e.g., Pfennig and Murphy, 2000 , 2002 ). Furthermore, phenotypic shifts mediated by phenotypic plasticity may shield populations from extinction (e.g., via competitive or reproductive exclusion) as genetic evolution proceeds.
Phenotypic plasticity therefore plays a potentially important role in facilitating character displacement ( Wilson, 1992 ; Pfennig and Murphy, 2002 ). Plastic traits themselves may be the targets of selection that initially diverge rapidly between species (i.e., they may undergo character displacement) ( Pfennig and Murphy, 2000 , 2002 ). Additionally, plasticity can promote canalized character displacement (sensu Pfennig and Murphy, 2002 ) or buffer populations from extinction while the evolution of such canalization proceeds (sensu West-Eberhard, 2003 ).
In the above section, we explored the causes of character displacement by highlighting some diverse factors that might promote character displacement. By fostering character displacement as opposed to extinction through competitive or reproductive exclusion, these factors could ultimately explain why some communities or taxonomic groups are more diverse. In other words, any factors that contribute to character displacement may have far reaching implications beyond simply mediating trait divergence between species. Below, we explore such evolutionary and ecological implications in more detail.
Consequences of Character Displacement
As described above, character displacement results in divergent traits between the interacting species as well as divergent traits within each species between allopatric and sympatric populations ( Figure 2b ). This hallmark pattern is not the only significant outcome of character displacement, however. For example, as the above discussion indicates, whether character displacement occurs depends on a number of evolutionary and proximate factors. Consequently, some groups may be more likely to undergo character displacement (and therefore be more diverse) than others. Yet, character displacement's role in generating such macroevolutionary patterns of differential taxonomic diversity is largely unknown (but see Schluter, 2000 ). Similarly, the ecological and evolutionary implications that stem from character displacement remain relatively unexplored.
Here, we discuss how character displacement can influence four key evolutionary processes: correlated evolution, sexual selection, speciation, and extinction. By influencing how these processes unfold, character displacement has potentially far reaching impacts beyond mere trait divergence between species.
Correlated Evolution
During character displacement, sympatric and allopatric populations diverge in traits involved in resource use or reproduction ( Figure 2b ). However, populations may often also diverge in traits that are not directly involved in resource acquisition or reproduction owing to correlated evolution with those traits actually targeted by selection (sensu Conner and Hartl, 2004 ). Such divergence in correlated traits can accentuate differences between interacting species and, within each species, between populations in sympatry and allopatry. As we describe below, these differences could, in turn, enhance reproductive isolation among these groups.
When correlated evolution in response to heterospecifics arises from pleiotropy, fitness trade-offs can arise between the benefits of avoiding deleterious interactions with heterospecifics and the costs accrued in other fitness components ( Pfennig and Pfennig, 2005 ). For example, both reproductive and ecological character displacement have caused spadefoot toads to evolve smaller body size in the presence of a heterospecific competitor ( Pfennig and Pfennig, 2005 ). This shift in size appears to have arisen as a by-product, rather than as a direct target, of character displacement ( Pfennig and Pfennig, 2005 ). Yet, the shift to reduced body size in sympatry is associated with reduced offspring survival, female fecundity, and sexual selection on males ( Pfennig and Pfennig, 2005 ). Thus, character displacement may sometimes represent the “best of a bad situation” in that it lessens competition but at a cost: individuals in sympatry with the displaced phenotype may have higher fitness than those without the displaced trait because they experience reduced competition, but they may have reduced fitness relative to individuals in allopatry ( Pfennig and Pfennig, 2005 ).
Fitness trade-offs associated with the benefits of avoiding deleterious interactions with heterospecifics on the one hand, and the costs accrued in other fitness components on the other hand, may have at least three important consequences. First, depending on the nature of the trade-off and the strength of selection to avoid heterospecific interactions, trade-offs may constrain the evolution of adaptive traits that reduce heterospecific interactions (sensu Conner and Hartl, 2004 ). In other words, pleiotropic interactions may limit evolutionary divergence in response to heterospecifics. Variation within and between species in fitness trade-offs may explain why character displacement varies among populations or why it is sometimes expressed asymmetrically between the interacting species ( Schluter, 2000 ; Cooley, 2007 ). Second, such fitness trade-offs may explain why traits that evolve in sympatry often do not spread back into allopatry, even in the face of high gene flow (for discussion, see Servedio and Noor, 2003 ; Higgie and Blows, 2007 ). Finally, because fitness trade-offs may cause individuals in sympatry to have reduced fitness relative to those in allopatry (as in the spadefoot toad example described in the previous paragraph), sympatric populations may be at higher extinction risk relative to allopatric populations ( Pfennig and Pfennig, 2005 ; see also Webb, 2003 ; Groning and Hochkirch, 2008 ). We will return to this point below.
Sexual Selection
Sexual selection explains much of the diversity in sexual signals and mating behaviors in sexually reproducing organisms ( Andersson, 1994 ), and character displacement can have a profound influence on sexual selection. When character displacement alters the expression of mate choice or traits used in sexual signaling or male competition, it necessarily impacts the expression of sexual selection among populations that differ in interactions with heterospecifics ( Boughman, 2001 , 2007 ; Pfennig and Ryan, 2007 ). Indeed, character displacement may impact sexual selection in at least two ways. First, character displacement can preclude mate choice formale traits that are indicative of mate quality and thereby alter the underlying fitness consequences of mate choice and sexual signaling ( Higgie and Blows, 2008 ; Pfennig, 1998 , 2000 ). Second, character displacement can alter the targets of sexual selection in populations that differ in their interactions with heterospecifics without necessarily affecting the fitness accrued through mating decisions or mate attraction. We discuss each of these impacts separately below.
First, character displacement potentially alters sexual selection by precluding the expression of mate choice for fitness-enhancing conspecific mates ( Higgie and Blows, 2008 ; Pfennig, 1998 , 2000 ). Generally, females should choose mates that provide them with fitness benefits, such as enhanced numbers of offspring or better-quality offspring (reviewed in Andersson, 1994 ). If reproductive character displacement favors the evolution of preferences that ensure mating with the correct species, the resulting preferences that evolve via character displacement may not be those that also enable females to select high-quality conspecific mates ( Pfennig, 1998 , 2000 ; Higgie and Blows, 2007 , 2008 ). For example, sexual selection theory generally predicts that females prefer males with more elaborate or costly signals that are indicative of a male's ability to confer benefits to a female ( Andersson, 1994 ; Bradbury and Vehrencamp, 1998 ). If, however, heterospecifics possess elaborate traits, character displacement may promote the evolution of preferences for less exaggerated signals ( Ryan and Rand, 1993 ; Pfennig, 1998 ; e.g., Pfennig, 2000 ; Rosenthal et al., 2002 ; Higgie and Blows, 2008 ). By adopting such preferences, females may avoid costly heterospecific interactions, but they may concomitantly forego information about a prospective conspecific mate's ability to convey additional fitness benefits (for examples, see Pfennig, 2000 , 2008 ; Higgie and Blows, 2007 , 2008 ).
Such trade-offs will not always arise via character displacement ( Pfennig, 1998 ). For example, if males with the most elaborate characters are also the most dissimilar from heterospecifics, sexual selection and character displacement reinforce each other ( Pfennig, 2000 ). Yet, when trade-offs do arise, their effects may be far-reaching. As with pleiotropic effects described above, trade-offs in mate choice can explain why divergent mating traits that evolve in sympatry do not spread back into allopatry via gene flow ( Pfennig and Pfennig, 2005 ; Higgie and Blows, 2007 ). Indeed, when trade-offs in mate choice arise, sympatric and allopatric populations can experience nearly opposing patterns of mate-choice mediated sexual selection. Consequently, not only will mate preferences diverge between sympatry and allopatry, but sexual signals (and any correlated traits) will also diverge ( Hoskin et al., 2005 ; Pfennig and Pfennig, 2005 ; Pfennig and Ryan, 2006 ; Higgie and Blows, 2007 , 2008 ). As we discuss below, such divergence in mating behavior can lead to reproductive isolation and, ultimately, speciation of allopatric and sympatric populations ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ). Furthermore, over time, selection may favor the resolution of trade-offs by promoting the evolution of preferences for multiple traits that enable females to avoid heterospecific interactions while simultaneously assessing conspecific quality ( Pfennig, 1998 ). Thus, character displacement can contribute not only to divergence in a given aspect of a signal, but it can also indirectly promote the evolution of multiple or complex signals for discriminating mates (reviewed in Pfennig, 1998 ; Gerhardt and Huber, 2002 ; Hebets and Papaj, 2005 ).
A second major way that character displacement may impact sexual selection is by altering the targets of sexual selection in sympatry versus allopatry (e.g., Gerhardt, 1994 ; Pfennig, 2000 ; Higgie and Blows, 2007 ). As we describe below, character displacement can lead to morphological changes in resource-use traits that concomitantly alter the production of sexual signals (e.g., Podos, 2001 ; Huber and Podos, 2006 ). These novel sexual signals might, in turn, become targets for further elaboration by direct sexual selection (e.g., because of their attractiveness to the opposite sex or effectiveness in competition among conspecifics for mates; Andersson 1994 ). Moreover, because ecological and reproductive character displacement often promote habitat shifts, occupancy of these novel habitats will tend to promote new patterns of sexual selection (sensu Endler and Basolo, 1998 ; Boughman, 2002 ). The nature of mate preferences and sexually selected traits often co-vary with habitat, because the transmission and the perception of sexual signals are typically habitat-dependent (reviewed in Wiley, 1994 ; Bradbury and Vehrencamp, 1998 ; Endler and Basolo, 1998 ; Boughman, 2002 ). Thus, any shifts in habitat use that are mediated by character displacement will likely be accompanied by shifts in patterns of sexual selection (e.g., Boughman, 2007 ).
As a result of the above effects of character displacement on sexual selection, sympatric and allopatric populations will potentially diverge in mating behaviors that were not necessarily the direct targets of selection to reduce heterospecific interactions. Because mate choice plays a critical role in reproductive isolation (reviewed in Coyne and Orr, 2004 ), divergent patterns of sexual selection in sympatry versus allopatry could ultimately contribute to speciation ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ). Thus, character displacement may initiate speciation between populations that differ in their interactions with heterospecifics ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ), which is the topic we turn to next.
Character displacement potentially plays a critical role in speciation in two ways. First, character displacement can finalize speciation between already divergent groups (reviewed in Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Grant and Grant, 2008 ). Second, character displacement can initiate divergence and reproductive isolation between populations that differ in their interactions with heterospecifics ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ; Pfennig and Rice, 2007 ). We discuss each of these avenues to speciation in turn.
Character displacement has long been regarded as important in completing the process of speciation (reviewed in Coyne and Orr, 2004 ; Grant and Grant, 2008 ). Ecological character displacement, for example, should cause differentiated, but potentially interbreeding populations (i.e., incipient species) to diverge in resource acquisition traits (reviewed in Grant and Grant, 2008 ). Specialization on alternate resources may reduce contact between the two incipient species and thereby allow for the accumulation of genetic differences between them that, in turn, contributes to enhanced isolation (reviewed in Coyne and Orr, 2004 ; Grant and Grant, 2008 ; Price, 2008 ). Moreover, if the two species interbreed and produce hybrids of low fitness, reproductive character displacement will cause divergence in reproductive traits and thereby preclude hybridization ( Dobzhansky, 1940 ; reviewed in Howard, 1993 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ). This process of reinforcement will therefore finalize speciation by promoting the evolution of complete reproductive isolation ( Dobzhansky, 1940 ; reviewed in Howard, 1993 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ).
That character displacement can also initiate speciation has received relatively little attention (but see Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ; Pfennig and Rice, 2007 ). Character displacement may instigate speciation by driving the evolution of divergent traits between populations that differ in their interactions with heterospecifics ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ; Pfennig and Rice, 2007 ). In particular, because individuals in sympatry will experience a different selective environment than conspecifics in allopatry, conspecific populations in these two types of environments are expected to diverge in resource-use or reproductive traits ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ; Pfennig and Rice, 2007 ). Such divergence may indirectly promote speciation via two non-mutually exclusive routes.
First, character displacement may promote the evolution of post-mating barriers to gene flow between sympatric and allopatric populations ( Pfennig and Rice, 2007 ). In particular, as an indirect consequence of character displacement between species, offspring produced by matings between conspecific individuals from different selective environments (i.e., sympatric male/female × allopatric male/female) may express an intermediate phenotype that is less well adapted to either selective environment than that expressed by offspring produced by matings between individuals from the same selective environment (i.e., sympatric male/female × sympatric male/female or allopatric male/female × allopatric male/female) (sensu Rice, 1987 ; Hatfield and Schluter, 1999 ; Rundle, 2002 ). For example, individuals produced by matings across sympatry and allopatry may express intermediate resource acquisition phenotypes that make them competitively inferior in either sympatry or allopatry (e.g., Pfennig and Rice, 2007 ). Similarly, individuals produced from matings across sympatry and allopatry may engage in mating behaviors that are inappropriate for either selective environment (sensu Hatfield and Schluter, 1996 ; Vamosi and Schluter, 1999 ; Svedin et al., 2008 ; van der Sluijs et al., 2008 ). Such maladaptation essentially serves as post-mating barriers to gene flow between populations in different selective environments.
Second, character displacement may promote the evolution of pre-mating barriers between sympatric and allopatric populations. During reproductive character displacement, female preferences or male traits may become so divergent that females in sympatry fail to recognize allopatric males as acceptable mates (or vice versa). Consequently, populations in sympatry and allopatry will become reproductively isolated from each other ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ). Likewise, ecological character displacement can contribute to pre-mating barriers between conspecific populations in sympatry versus allopatry if shifts in habitat or resource use preclude mating between them (reviewed in Rundle and Schluter, 2004 ).
Differentiation between conspecific populations in sympatry versus allopatry is especially likely to occur if character displacement generates the kinds fitness trade-offs described above. By precluding the spread of traits from sympatry into allopatry (and vice versa; see correlated evolution above), such trade-offs essentially generate a selective barrier between sympatry and allopatry that fosters local adaptation ( Pfennig and Pfennig, 2005 ). Moreover, because of reduced gene flow between sympatry and allopatry, populations in these divergent selective environments may accumulate further differences that exaggerate both pre- and post-mating isolation between them. Thus, speciation between sympatric and allopatric populations may arise as an indirect consequence of selection for divergence between species during interspecific character displacement ( Hoskin et al., 2005 ; Pfennig and Ryan, 2006 ; Pfennig and Rice, 2007 ).
Although we have focused above on interactions between pairs of species, character displacement may also drive numerous, rapid speciation events. If, for example, a given species interacts with different heterospecifics across different populations, local evolution of mating behaviors in response to these interactions may isolate these conspecific populations and generate speciation among them (i.e., “speciation cascades”; Pfennig and Ryan, 2006 ). Thus, multiple speciation events, and possibly even adaptive radiations ( Schluter, 2000 ), may arise as a by-product of interactions between species.
Coexistence Versus Extinction
Generally, character displacement is expected to promote species coexistence by reducing fitness-decrementing interactions that would otherwise lead to competitive or reproductive exclusion (see above and also Losos, 2000 ). Yet, even when character displacement promotes coexistence, populations in sympatry may have reduced survival and reproductive rates as a result of character displacement ( Pfennig and Pfennig, 2005 ). Consequently, sympatric populations may experience higher extinction risk than conspecific populations in allopatry (for review and discussion of how adaptive evolution can lead to extinction risk, see Kokko and Brooks, 2002 ; Webb, 2003 ). Character displacement can contribute to enhanced extinction risk when it involves trade-offs between the benefits of avoiding heterospecific interactions and the costs of expressing the displaced phenotype ( Pfennig and Pfennig, 2005 ). The costs that accrue to individuals in sympatry may reduce population fitness and thereby render sympatric populations more likely to go extinct ( Pfennig and Pfennig, 2005 ). For example, as we described above (see phenotypic plasticity ), ecological character displacement may result in one species being displaced onto a novel resource that is of lower quality or more ephemeral than the pre-displacement resource. Lower quality resources may support smaller populations that are more susceptible to stochastic extinction events, thereby rendering sympatric populations at higher extinction risk relative to allopatric populations. Likewise, displacement onto a more ephemeral resource may make sympatric populations more susceptible to stochastic extinction events than allopatric populations.
Reproductive character displacement also could engender costs if the displaced phenotypes (such as male signals or female preferences) are more costly to express (for discussion of mate choice for costly signals, see Andersson, 1994 ). More costly signals could reduce reproductive rates and limit population size ( Kokko and Brooks, 2002 ). Additionally, extinction risk may depend on how males trade-off sexual and viability selection ( Kokko and Brooks, 2002 ). Novel sexual signaling in sympatry may be more susceptible to trade-offs that enhance the risk of extinction relative to populations in allopatry. Moreover, as described above (see sexual selection ), avoidance of heterospecifics may preclude females from selecting high quality mates and reduce sympatric female fitness relative to allopatric female fitness ( Pfennig, 2000 ; Higgie and Blows, 2008 ; Pfennig, 2008 ). Such trade-offs can reduce female fecundity, rates of reproduction, and even offspring growth or survival ( Pfennig, 2000 , 2008 ). Indeed, if character displacement suppresses condition-dependent sexual selection in sympatry, sympatric populations may be less able to adapt to changing environments (sensu Lorch et al., 2003 ). Thus, relative to conspecifics in allopatry, those in sympatry may be smaller, slower growing, and less able to respond to changes in the environment ( Pfennig and Pfennig, 2005 ). As a result, populations that have undergone character displacement may be more likely to experience extinction.
In sum, character displacement generally promotes species coexistence ( Losos, 2000 ). Depending on the way that character displacement unfolds, however, it may, counter intuitively, also enhance the risk of extinction in populations that are sympatric with heterospecifics relative to those that are not ( Pfennig and Pfennig, 2005 ; see also Kokko and Brooks, 2002 ; Webb, 2003 ). Thus, the distributions of many species may be patchier in areas where they are sympatric with a heterospecific than where they are allopatric, and this patchy distribution may be associated with stochastic factors, rather than because of the deterministic processes of competitive or reproductive exclusion. Moreover, persistence of sympatric populations may be more variable in both space and time. Indeed, coexistence between species may be more dynamic than originally thought, with sympatric populations experiencing chance extinction, followed by recolonization and coexistence. In other words, the outcome of heterospecific interactions may not be merely one or the other of two alternatives: coexistence or exclusion. Instead, character displacement may promote coexistence while increasing the likelihood of chance extinction.
Relationship Between Ecological and Reproductive Character Displacement
Throughout this article, we have referred to character displacement – rather than to ecological or reproductive character displacement – when the concepts being discussed apply to either process. Although the two processes are similar in many ways, relatively few studies have examined how they interact [for notable exceptions, see research on stickleback fish (reviewed in Rundle and Schluter, 2004 ) and Darwin's finches (reviewed in Grant and Grant, 2008 )]. Yet, because species that compete for resources likely interact during mating and vice versa ( Schluter, 2000 ; Rundle and Schluter, 2004 ; Grant and Grant, 2008 ; Price, 2008 ), reproductive and ecological character displacement may often become intertwined. Below, we discuss how ecological character displacement may affect reproductive character displacement and vice versa.
Ecological Character Displacement in Phenotypic Traits as a Promoter of Reproductive Character Displacement
Ecological character displacement can promote reproductive character displacement when shifts in resource-use traits also alter the production of signals used for reproduction ( Huber and Podos, 2006 ; Grant and Grant, 2008 ). If these shifts in signal production reduce deleterious reproductive interactions between species, then ecological selection essentially jump-starts reproductive character displacement. For example, shifts in resource use that lead to changes in bird beak and larynx morphology can cause concomitant shifts in a sexual signal – bird song – that is directly involved in species recognition (reviewed in Podos and Nowicki, 2004 ; Grant and Grant, 2008 ; Price, 2008 ). Indeed, in the medium ground finch, Geospiza fortis , populations that consist of a large-beaked morph and a small-beaked morph – which feed on large and small seeds, respectively – produce distinct song types ( Huber and Podos, 2006 ). Females apparently use these different song types during mate choice and mate assortatively with males of their own beak type ( Huber et al., 2007 ). Thus, ecological selection can also alter sexual signals in a way that affects mate choice, and potentially, reproductive isolation ( Podos, 2001 ; Podos and Nowicki, 2004 ; Podos et al., 2004 ; Huber et al., 2007 ; Grant and Grant, 2008 ).
Although the above example focuses on acoustic signals, shifts in resource use could foster similar changes in other sensory modalities used in sexual signaling. In particular, shifts in resource use could affect visual or olfactory sexual signals depending on how dietary components (e.g., carotenoids) are incorporated into sexual displays. In many fish species, for example, male coloration is diet-dependent (see discussion and references in Andersson, 1994 ; Olson and Owens, 1998 ), and coloration can also play an important role in species recognition (e.g., Seehausen and van Alphen, 1998 ; Boughman, 2001 ). If ecological character displacement causes divergence in resource use, male signaling can be affected if the dietary components used to generate a given signal are no longer available (or are too costly to acquire) with the new diet ( Boughman, 2007 ). Consequently, resource shifts may be accompanied by shifts in sexual signals, which can then be maintained and further elaborated via reproductive character displacement if they minimize deleterious reproductive interactions between species.
Although recent work has focused on how resource shifts can alter male signals, morphological and physiological changes that accompany ecological character displacement can also directly affect female perception, and, consequently, female mate choice (sensu Endler and Basolo, 1998 ; Ryan, 1998 ; Boughman, 2002 ). Changes in jaw morphology to capture prey, divergence in olfactory or visual sensitivity to localize prey, and even shifts in overall body size for specializing on different resources that result from ecological character displacement could simultaneously alter female perception and discrimination of male signals ( Endler and Basolo, 1998 ; Ryan, 1998 ; Boughman, 2002 ). For example, changes in ear morphology caused by changes in jaw structure or body size could affect female perception of and preferences for male calls ( Ryan, 1990 , 1998 ; Boughman, 2002 ). Thus, ecological character displacement could directly alter female mate preferences, and thereby initiate or perpetuate reproductive character displacement, if such preferences also minimize reproductive interactions.
Ecological Character Displacement in Habitat Use as a Promoter of Reproductive Character Displacement
The above discussion illustrates how resource shifts can directly alter male sexual signals or female mate preference, and thereby promote reproductive character displacement. Yet, ecological character displacement may also mediate indirect divergence in reproductive characters. In particular, because habitat critically affects the attenuation and perception of signals (reviewed in Wiley, 1994 ; Bradbury and Vehrencamp, 1998 ; Boughman, 2002 ; Gerhardt and Huber, 2002 ), shifts in habitat use associated with ecological character displacement may promote selection for the evolution of novel sexual signals that are more suited to the new foraging habitat. The sympatric anoles, Anolis cooki and A. cristatellus , for example, display divergent UV light sensitivity that appears to enable them to co-occur in partitioned light microhabitat ( Leal and Fleishman, 2002 ). Such divergent microhabitat use may facilitate co-occurrence by enabling them to partition resources ( Leal and Fleishman, 2002 ). UV reflectance of male dewlaps has also diverged so that male dewlaps contrast most against the light microhabitat in which each species resides, thereby facilitating species recognition ( Leal and Fleishman, 2002 ). Presumably, divergent habitat use simultaneously selects for signals that optimize communication in the novel habitat while also minimizing reproductive interactions between species ( Leal and Fleishman, 2002 ). In this way, ecological character displacement may indirectly foster the evolution of divergent reproductive characters that minimize reproductive interactions.
Changes in habitat or resource use via ecological character displacement may also generate changes in female mate preferences that promote reproductive character displacement. Such changes could occur in two ways. First, novel habitats may exert direct selection on females to evolve preferences for male traits that are most efficiently detected in those new habitats ( Endler and Basolo, 1998 ; Boughman, 2002 ). Second, novel habitats or resources may exert natural selection on female sensory systems to better identify prey ( Ryan, 1998 ; Boughman, 2002 ). These shifts in sensory sensitivity could indirectly alter patterns of female mate choice ( Ryan, 1990 , 1998 ; Endler and Basolo, 1998 ; Boughman, 2002 , 2007 ). If such preferences reduce sexual interactions between species, they may be further enhanced by reproductive character displacement.
In sticklebacks, for example, a benthic ecomorph forages and mates in the littoral zone where red coloration is more difficult to detect. A limnetic ecomorph, by contrast, occurs in open water where red coloration is more discernable ( Boughman, 2001 ). Benthic females are less sensitive to variation in red than are limnetic females, and, unlike limnetic females, benthic females do not tend to prefer redder males ( Boughman, 2001 , 2007 ; reviewed in Boughman, 2002 ). Male red coloration, in turn, is “tuned” to female perception of red color: males are redder in populations where females are actually sensitive to, and thus prefer, redder males ( Boughman, 2001 , 2007 ). Perhaps more critically, the extent to which a given limnetic/benthic species pair is reproductively isolated is negatively correlated with female red sensitivity and preference in a given population ( Boughman, 2001 ). Thus, shifts in mate preference tied to different habitats dictates the degree to which reproductive divergence has occurred ( Boughman, 2001 ). Generally, shifts in resource or habitat use via ecological character displacement may play a critical role in initiating and promoting reproductive character displacement by fostering changes in mate preferences and sexual signals that minimize reproductive interactions between species.
Reproductive Character Displacement as a Promoter of Ecological Character Displacement
Although most empirical work has focused on how shifts in resource or habitat use may dictate shifts in reproductive characters, the reverse scenario could unfold (e.g., Boughman, 2001 ; Podos, 2001 ; Huber and Podos, 2006 ; Boughman, 2007 ). The evolution of reproductive characters stemming from selection to minimize reproductive interference could also cause divergence in traits associated with resource acquisition ( Konuma and Chiba, 2007 ). If, for example, species segregate in space or time to avoid reproductive interactions, they may be concomitantly exposed to novel, underutilized resources, thereby possibly leading to a shift in traits associated with resource use.
Moreover, mate preferences to avoid interactions with heterospecifics may promote the evolution of traits involved in the production of those signals (e.g., body size, beak morphology), which could, in turn, cause a shift in resource use and associated traits ( Konuma and Chiba, 2007 ). In the anole example above, for instance, the evolutionary chain of events is unclear. Although habitat partitioning in different light environments may have fostered the evolution of sexual signals that result in reproductive trait divergence, the converse also could have occurred. That is, reproductive interactions may have generated divergence in perception and signaling that in turn fostered habitat and resource partitioning ( Leal and Fleishman, 2002 ).
Teasing Apart Reproductive and Ecological Character Displacement: Caveats
As discussed above, reproductive and ecological character displacement can promote each other. Yet, correlated evolution in either sexual signals or resource acquisition traits in response to direct selection on the alternative type of trait does not constitute character displacement ( Coyne and Orr, 2004 ). For example, the correlated evolution of sexual signals in response to selection to minimize resource competition would not represent reproductive character displacement per se. Only if those reproductive traits also become the targets of selection to minimize reproductive interactions would divergence in these traits constitute reproductive character displacement. Caution must therefore be exercised when studying character displacement in systems that display divergence in both reproductive and resource use traits ( Rundle and Schluter, 1998 ; Coyne and Orr, 2004 ).
Nevertheless, two species that are similar enough to compete for resources will also likely utilize similar habitat for mate acquisition and vice versa ( Schluter, 2000 ; Rundle and Schluter, 2004 ; Grant and Grant, 2008 ; Price, 2008 ). Thus, initial changes to resource-use traits or sexual signals brought about by one form of character displacement will fuel the alternate form of character displacement, if these differences are subsequently maintained or enhanced by selection to minimize interspecific interactions. As ecological and reproductive character displacement become intertwined, the degree to which one leads the other may become obscure ( Schluter, 2000 ). Studies specifically aimed at teasing apart the relative contribution of each process are needed to determine if both types of character displacement are occurring in a given system (e.g., Rundle and Schluter, 1998 ).
Finally, although we have focused on how reproductive and ecological character displacement might reinforce each other, each process can potentially preclude the other from occurring. The inhibition of one process by the other may be especially likely to occur when either process generates habitat partitioning (either spatially or temporally). When habitat partitioning arises via one process, selection to minimize interactions via the alternate process is effectively shut down. Essentially, the operation of one process negates the selective pressure for the other process to occur.
Conclusions
The consensus that has emerged from previous work is that character displacement is taxonomically widespread and that it can act to lessen both competitive and reproductive interactions between species ( Howard, 1993 ; Schluter, 2000 ; Servedio and Noor, 2003 ; Coyne and Orr, 2004 ; Dayan and Simberloff, 2005 ; Groning and Hochkirch, 2008 ). Having established that character displacement occurs, researchers can now move on to teasing apart what factors that facilitate character displacement. Because character displacement can drive speciation and adaptive radiations ( Schluter, 2000 ; Grant and Grant, 2008 ), understanding what species are especially prone to undergoing character displacement may help explain why some taxonomic groups are more diverse than others. Understanding when character displacement proceeds – and when it does not – may therefore reveal how microevolutionary processes generate macroevolutionary patterns of diversity.
Similarly, by understanding how and when character displacement is likely to occur, we may gain insights into patterns of species coexistence, community diversity, and potentially large-scale patterns of species distribution. Character displacement necessarily mediates species coexistence ( Losos, 2000 ), and it has the further potential to alter population dynamics, extinction risk, and concomitantly, species ranges (see discussion above as well as Brown, 1995 ; Thompson, 2005 ; Groning and Hochkirch, 2008 ). Studying character displacement can therefore potentially reveal how the fitness consequences of interactions between species ultimately translate into macroecological patterns of species richness, distributions, and diversity. Thus, the process by which individuals optimize fitness in response to heterospecifics – character displacement – provides a unifying framework for understanding the origins, abundance, and distribution of biodiversity.
Acknowledgements
For comments and discussion we thank T. Price, A. Rice, E. Wojtowicz, R. Martin, S. Dhole, C. Ledón-Rettig, A. Chunco, L. Exline, and M. Servedio. We also thank J. Wiens and two anonymous reviewers, whose comments greatly improved the manuscript, and M. Noor for moral support and a chocolate shake. The National Science Foundation and the National Institutes of Health support our research on character displacement.
Literature Cited
- Abrams PA. Character displacement and niche shift analyzed using consumer-resource models of competition. Theoretical Population Biology. 1986; 29 :107–160. [ PubMed ] [ Google Scholar ]
- Agrawal AA. Phenotypic plasticity in the interactions and evolution of species. Science. 2001; 294 :321–326. [ PubMed ] [ Google Scholar ]
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999; 401 :60–63. [ Google Scholar ]
- Allen RM, Buckley YM, Marshall DJ. Offspring size plasticity in response to intraspecific competition: An adaptive maternal effect across life-history stages. American Naturalist. 2008; 171 :225–237. [ PubMed ] [ Google Scholar ]
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [ Google Scholar ]
- Arnold ML. Natural hybridization and evolution. Oxford University Press; Oxford, UK: 1997. [ Google Scholar ]
- Arthur W. The evolutionary consequences of interspecific competition. Advances in Ecological Research. 1982; 12 :127–187. [ Google Scholar ]
- Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends in Ecology and Evolution. 2008; 23 :38–44. [ PubMed ] [ Google Scholar ]
- Barton NH, Hewitt GM. Adaptation, speciation and hybrid zones. Nature. 1989; 341 :497–503. [ PubMed ] [ Google Scholar ]
- Blair WF. Character displacement in frogs. American Zoologist. 1974; 14 :1119–1125. [ Google Scholar ]
- Bolnick DI. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004; 58 :608–618. [ PubMed ] [ Google Scholar ]
- Bolnick DI, Lau OL. Predictable patterns of disruptive selection in stickleback in postglacial lakes. American Naturalist. 2008; 172 :1–11. [ PubMed ] [ Google Scholar ]
- Boughman JW. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature. 2001; 411 :944–948. [ PubMed ] [ Google Scholar ]
- Boughman JW. How sensory drive can promote speciation. Trends in Ecology and Evolution. 2002; 17 :571–577. [ Google Scholar ]
- Boughman JW. Condition-dependent expression of red colour differs between stickleback species. Journal of Evolutionary Biology. 2007; 20 :1577–1590. [ PubMed ] [ Google Scholar ]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sinauer Associates, Inc; Sunderland, MA: 1998. [ Google Scholar ]
- Brown JH. Macroecology. University of Chicago Press; Chicago, IL: 1995. [ Google Scholar ]
- Brown WL, Wilson EO. Character displacement. Systematic Zoology. 1956; 5 :49–64. [ Google Scholar ]
- Butlin R. Speciation by reinforcement. Trends in Ecology and Evolution. 1987; 2 :8–13. [ PubMed ] [ Google Scholar ]
- Butlin RK, Ritchie MG. Behaviour and speciation. In: Slater PJB, Halliday TR, editors. Behaviour and speciation. Cambridge University Press; Cambridge, UK: 1994. [ Google Scholar ]
- Calsbeek R, Smith TB. Experimentally replicated disruptive selection on performance traits in a Caribbean lizard. Evolution. 2008; 62 :478–484. [ PubMed ] [ Google Scholar ]
- Caruso CM. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution. 2000; 54 :1546–1557. [ PubMed ] [ Google Scholar ]
- Conner JK, Hartl DL. A primer of ecological genetics. Sinauer Associates, Inc.; Sunderland, MA: 2004. [ Google Scholar ]
- Cooley JR. Decoding asymmetries in reproductive character displacement. Proceedings of the Academy of Natural Sciences of Philadelphia. 2007; 156 :89–96. [ Google Scholar ]
- Coyne JA, Orr HA. Speciation. Sinauer Associates, Inc.; Sunderland, MA: 2004. [ Google Scholar ]
- Crozier RH. Niche shape and genetical aspects of character displacement. American Zoologist. 1974; 14 :1151–1157. [ Google Scholar ]
- Darwin C. On the origin of species by means of natural selection. 1st ed. John Murray; London, UK: 1859. [ Google Scholar ]
- Day T, Young KA. Competitive and facilitative evolutionary diversification. BioScience. 2004; 54 :101–109. [ Google Scholar ]
- Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecology Letters. 2005; 8 :875–894. [ Google Scholar ]
- DeWitt TJ, Scheiner SM. Phenotypic plasticity: functional and conceptual approaches. Oxford University Press; New York, NY: 2004. [ Google Scholar ]
- Diamond J, Pimm SL, Gilpin ME, LeCroy M. Rapid evolution of character displacement in Myzomelid honeyeaters. American Naturalist. 1989; 134 :675–708. [ Google Scholar ]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. American Naturalist. 1940; 74 :312–321. [ Google Scholar ]
- Doebeli M. An explicit genetic model for ecological character displacement. Ecology. 1996; 77 :510–520. [ Google Scholar ]
- Endler JA. Natural selection in the wild. Princeton University Press; Princeton, NJ: 1986. [ Google Scholar ]
- Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends in Ecology and Evolution. 1998; 13 :415–420. [ PubMed ] [ Google Scholar ]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. Journal of Experimental Biology. 2006; 209 :2377–2383. [ PubMed ] [ Google Scholar ]
- Gause GF. The struggle for existence. Williams and Wilkins; Baltimore, MD: 1934. [ Google Scholar ]
- Gerhardt HC. Reproductive character displacement of female mate choice in the gray treefrog, Hyla chrysoscelis. Animal Behaviour. 1994; 47 :959–969. [ Google Scholar ]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press; Chicago, IL: 2002. [ Google Scholar ]
- Goldberg EE, Lande R. Ecological and reproductive character displacement on an environmental gradient. Evolution. 2006; 60 :1344–1357. [ PubMed ] [ Google Scholar ]
- Gorbushin AM. The enigma of mud snail shell growth: asymmetrical competition or character displacement? Oikos. 1996; 77 :85–92. [ Google Scholar ]
- Grant PR. Convergent and divergent character displacement. Biological Journal of the Linnean Society. 1972; 4 :39–68. [ Google Scholar ]
- Grant PR, Grant BR. Evolution of character displacement in Darwin's finches. Science. 2006; 313 :224–226. [ PubMed ] [ Google Scholar ]
- Grant PR, Grant BR. How and why species multiply: the radiation of Darwin's finches. Princeton University Press; Princeton, NJ: 2008. [ Google Scholar ]
- Groning J, Hochkirch A. Reproductive interference between animal species. Quarterly Review of Biology. 2008; 83 :257–282. [ PubMed ] [ Google Scholar ]
- Hardin G. The competitive exclusion principle. Science. 1960; 131 :1292–1297. [ PubMed ] [ Google Scholar ]
- Hatfield T, Schluter D. A test for sexual selection on hybrids of two sympatric sticklebacks. Evolution. 1996; 50 :2429–2434. [ PubMed ] [ Google Scholar ]
- Hatfield T, Schluter D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution. 1999; 53 :866–873. [ PubMed ] [ Google Scholar ]
- Hebets EA, Papaj DR. Complex signal function: developing a framework of testable hypotheses. Behavioral Ecology And Sociobiology. 2005; 57 :197–214. [ Google Scholar ]
- Hendry AP, Huber SK, De Leon LF, Herrel A, Podos J. Disruptive selection in a bimodal population of Darwin's finches. Proceedings of the Royal Society B-Biological Sciences. 2009; 276 :753–759. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Higgie M, Blows MW. Are traits that experience reinforcement also under sexual selection? American Naturalist. 2007; 170 :409–420. [ PubMed ] [ Google Scholar ]
- Higgie M, Blows MW. The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution. 2008; 62 :1192–1203. [ PubMed ] [ Google Scholar ]
- Hochkirch A, Groning J, Bucker A. Sympatry with the devil: reproductive interference could hamper species coexistence. Journal of Animal Ecology. 2007; 76 :633–642. [ PubMed ] [ Google Scholar ]
- Hoskin CJ, Higgie M, McDonald KR, Moritz C. Reinforcement drives rapid allopatric speciation. Nature. 2005; 437 :1353–1356. [ PubMed ] [ Google Scholar ]
- Howard DJ. Reinforcement: origin, dynamics, and fate of an evolutionary hypothesis. In: Harrison RG, editor. Hybrid zones and the evolutionary process. Oxford University Press; New York, NY: 1993. pp. 46–69. [ Google Scholar ]
- Huber SK, De Leon LF, Hendry AP, Bermingham E, Podos J. Reproductive isolation of sympatric morphs in a population of Darwin's finches. Proceedings of the Royal Society B-Biological Sciences. 2007; 274 :1709–1714. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huber SK, Podos J. Beak morphology and song features covary in a population of Darwin's finches ( Geospiza fortis ) Biological Journal of the Linnean Society. 2006; 88 :489–498. [ Google Scholar ]
- Jablonka E, Lamb MJ. Epigenetic inheritance and evolution: the Lamarkian dimension. Oxford University Press; New York, NY: 1995. [ Google Scholar ]
- Kokko H, Brooks R. Sexy to die for? Sexual selection and the risk of extinction. Conference on Extinction Thresholds; Helsinki, Finland. 2002. pp. 207–219. [ Google Scholar ]
- Konuma J, Chiba S. Ecological character displacement caused by reproductive interference. Journal of Theoretical Biology. 2007; 247 :354–364. [ PubMed ] [ Google Scholar ]
- Lack D. Darwin's finches. Cambridge University Press; Cambridge, UK: 1947. [ Google Scholar ]
- Leal M, Fleishman LJ. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002; 269 :351–359. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Levin DA. Reproductive character dispalcement in Phlox. Evolution. 1985; 39 :1275–1281. [ PubMed ] [ Google Scholar ]
- Liou LW, Price TD. Speciation by reinforcement of premating isolation. Evolution. 1994; 48 :1451–1459. [ PubMed ] [ Google Scholar ]
- Lorch PD, Proulx S, Rowe L, Day T. Condition-dependent sexual selection can accelerate adaptation. Evolutionary Ecology Research. 2003; 5 :867–881. [ Google Scholar ]
- Losos JB. Ecological character displacement and the study of adaptation. Proceedings of the National Academy of Sciences, U.S.A. 2000; 97 :5693–5695. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marko PB. An intraspecific comparative analysis of character divergence between sympatric species. Evolution. 2005; 59 :554–564. [ PubMed ] [ Google Scholar ]
- Martin RA, Pfennig DW. Disruptive selection in natural populations: the roles of ecological selection and resource competition. American Naturalist. in press. [ PubMed ] [ Google Scholar ]
- Mather K. The genetical structure of populations. Symposium of the Society for Experimental Biology. 1953; 2 :196–216. [ Google Scholar ]
- Maynard Smith J. Evolutionary genetics. 2nd ed Oxford University Press; New York, NY: 1998. [ Google Scholar ]
- McAdam AG, Boutin S. Maternal effects and the response to selection in red squirrels. Proceedings of the Royal Society B. 2004; 271 :75–79. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Medel R, Botto-Mahan C, Kalin-Arroyo M. Pollinator-mediated selection on the nectar guide phenotype in the Andean monkey flower, Mimulus luteus. Ecology. 2003; 84 :1721–1732. [ Google Scholar ]
- Milligan BG. Evolutionary divergence and character displacement in two phenotypically-variable competing species. Evolution. 1985; 39 :1207–1222. [ PubMed ] [ Google Scholar ]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford University Press; New York, NY: 1998. [ Google Scholar ]
- Olson VA, Owens IPF. Costly sexual signals: are carotenoids rare, risky or required? Trends in Ecology and Evolution. 1998; 13 :510–514. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Martin RA. A maternal effect mediates rapid population divergence and character displacement in spadefoot toads. Evolution. in press. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Murphy PJ. Character displacement in polyphenic tadpoles. Evolution. 2000; 54 :1738–1749. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Murphy PJ. How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution. 2002; 56 :1217–1228. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Rice AM. An experimental test of character displacement's role in promoting postmating isolation between conspecific populations in contrasting competitive environments. Evolution. 2007; 61 :2433–2443. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Rice AM, Martin RA. Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology. 2006; 87 :769–779. [ PubMed ] [ Google Scholar ]
- Pfennig DW, Rice AM, Martin RA. Field and experimental evidence for competition's role in phenotypic divergence. Evolution. 2007; 61 :257–271. [ PubMed ] [ Google Scholar ]
- Pfennig KS. The evolution of mate choice and the potential for conflict between species and mate-quality recognition. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998; 265 :1743–1748. [ Google Scholar ]
- Pfennig KS. Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behavioral Ecology. 2000; 11 :220–227. [ Google Scholar ]
- Pfennig KS. Facultative mate choice drives adaptive hybridization. Science. 2007; 318 :965–967. [ PubMed ] [ Google Scholar ]
- Pfennig KS. Population differences in condition-dependent sexual selection may promote divergence in non-sexual traits. Evolutionary Ecology Research. 2008; 10 :763–773. [ Google Scholar ]
- Pfennig KS, Pfennig DW. Character displacement as the “best of a bad situation”: fitness trade-offs resulting from selection to minimize resource and mate competition. Evolution. 2005; 59 :2200–2208. [ PubMed ] [ Google Scholar ]
- Pfennig KS, Ryan MJ. Reproductive character displacement generates reproductive isolation among conspecific populations: an artificial neural network study. Proceedings of the Royal Society B-Biological Sciences. 2006; 273 :1361–1368. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pfennig KS, Ryan MJ. Character displacement and the evolution of mate choice: an artificial neural network approach. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007; 362 :411–419. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pianka ER. Evolutionary ecology. 6th ed Benjamin/Cummings; San Francisco, CA: 2000. [ Google Scholar ]
- Plaistow SJ, Lapsley CT, Benton TG. Context-dependent intergenerational effects: The interaction between past and present environments and its effect on population dynamics. American Naturalist. 2006; 167 :206–215. [ PubMed ] [ Google Scholar ]
- Podos J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature. 2001; 409 :185–188. [ PubMed ] [ Google Scholar ]
- Podos J, Nowicki S. Beaks, adaptation, and vocal evolution in Darwin's finches. Bioscience. 2004; 54 :501–510. [ Google Scholar ]
- Podos J, Southall JA, Rossi-Santos MR. Vocal mechanics in Darwin's finches: correlation of beak gape and song frequency. Journal of Experimental Biology. 2004; 207 :607–619. [ PubMed ] [ Google Scholar ]
- Price T. Speciation in birds. Roberts and Company Publishers; Greenwood Village, CO: 2008. [ Google Scholar ]
- Rice AM, Pfennig DW. Character displacement: in situ evolution of novel phenotypes or sorting of pre-existing variation? Journal of Evolutionary Biology. 2007; 20 :448–459. [ PubMed ] [ Google Scholar ]
- Rice WR. Speciation via habitat specialization: the evolution of reproductive isolation as a correlated character. Evolutionary Ecology. 1987; 1 :301–315. [ Google Scholar ]
- Robinson BW, Wilson DS. Character release and displacement in fish: a neglected literature. American Naturalist. 1994; 144 :596–627. [ Google Scholar ]
- Rosenthal GG, Wagner WE, Ryan MJ. Secondary reduction of preference for the sword ornament in the pygmy swordtail Xiphophorus nigrensis (Pisces: Poeciliidae) Animal Behaviour. 2002; 63 :37–45. [ Google Scholar ]
- Rosenzweig ML. Competitive speciation. Biological Journal of the Linnean Society. 1978; 10 :274–289. [ Google Scholar ]
- Rossiter MC. Incidence and consequences of inherited environmental effects. Annual Review of Ecology and Systematics. 1996; 27 :451–476. [ Google Scholar ]
- Rueffler C, Van Dooren TJM, Leimar O, Abrams PA. Disruptive selection and then what? Trends in Ecology and Evolution. 2006; 21 :238–245. [ PubMed ] [ Google Scholar ]
- Rundle HD. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution. 2002; 56 :322–329. [ PubMed ] [ Google Scholar ]
- Rundle HD, Schluter D. Reinforcement of stickleback mate preferences: sympatry breeds contempt. Evolution. 1998; 52 :200–208. [ PubMed ] [ Google Scholar ]
- Rundle HD, Schluter D. Natural selection and ecological speciation in sticklebacks. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D, editors. Adaptive speciation. Cambirdge University Press; Cambridge, UK: 2004. pp. 192–209. [ Google Scholar ]
- Ryan MJ. Sensory systems, sexual selection, and sensory exploitation. Oxford Surveys in Evolutionary Biology. 1990; 7 :157–195. [ Google Scholar ]
- Ryan MJ. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998; 281 :1999–2003. [ PubMed ] [ Google Scholar ]
- Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993; 47 :647–657. [ PubMed ] [ Google Scholar ]
- Schlichting CD, Pigliucci M. Phenotypic evolution: a reaction norm perspective. Sinauer; Sunderland, MA: 1998. [ Google Scholar ]
- Schluter D. Experimental evidence that competition promotes divergence in adaptive radiation. Science. 1994; 266 :798–801. [ PubMed ] [ Google Scholar ]
- Schluter D. The ecology of adaptive radiation. Oxford University Press; Oxford, UK: 2000. [ Google Scholar ]
- Schluter D. In: Ecological character displacement. Evolutionary ecology: concepts and case studies. Fox CW, Roff DA, Fairbairn DJ, editors. Oxford University Press; New York, NY: 2001. pp. 265–276. [ Google Scholar ]
- Schluter D. Character displacement. In: Pagel M, editor. Encyclopedia of evolution. Vol. 1. Oxford University Press; Oxford, UK: 2002. pp. 149–150. [ Google Scholar ]
- Seehausen O, van Alphen JJM. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids ( Haplochromis nyererei complex) Behavioral Ecology and Sociobiology. 1998; 42 :1–8. [ Google Scholar ]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annual Review of Ecology Evolution and Systematics. 2003; 34 :339–364. [ Google Scholar ]
- Simberloff DS, Boecklen W. Santa Rosalia reconsidered: size ratios and competition. Evolution. 1981; 35 :1206–1228. [ PubMed ] [ Google Scholar ]
- Simpson GG. The major features of evolution. Columbia University Press; New York, NY: 1953. [ Google Scholar ]
- Slatkin M. Ecological character displacement. Ecology. 1980; 61 :163–177. [ Google Scholar ]
- Smith RA, Rausher MD. Experimental evidence that selection favors character displacement in the ivyleaf morning glory. American Naturalist. 2008; 171 :1–9. [ PubMed ] [ Google Scholar ]
- Smith TB. Disruptive selection and the genetic basis of bill size polymorphism in the African finch Pyrenestes. Nature. 1993; 363 :618–620. [ Google Scholar ]
- Smith TB, Skúkason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics. 1996; 27 :111–133. [ Google Scholar ]
- Strong DR, Jr., Szyska LA, Simberloff DS. Tests for community-wide character displacement against null hypotheses. Evolution. 1979; 33 :897–913. [ PubMed ] [ Google Scholar ]
- Svedin N, Wiley C, Veen T, Gustafsson L, Qvarnstrom A. Natural and sexual selection against hybrid flycatchers. Proceedings of the Royal Society B-Biological Sciences. 2008; 275 :735–744. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Taper ML, Case TJ. Quantitative genetic models for the coevolution of character displacement. Ecology. 1985; 66 :355–371. [ Google Scholar ]
- Taper ML, Case TJ. Coevolution among competitors. Oxford Surveys in Evolutionary Biology. 1992; 8 :63–109. [ Google Scholar ]
- Thompson JN. The geographic mosaic of coevolution. University of Chicago Press; Chicago, IL: 2005. [ Google Scholar ]
- Tynkkynen K, Rantala MJ, Suhonen J. Interspecific aggression and character displacement in the damselfly Calopteryx splendens. Journal of Evolutionary Biology. 2004; 17 :759–767. [ PubMed ] [ Google Scholar ]
- Vamosi SM, Schluter D. Sexual selection against hybrids between sympatric stickleback species: evidence from a field experiment. Evolution. 1999; 53 :874–879. [ PubMed ] [ Google Scholar ]
- van der Sluijs I, Van Dooren TJM, Hofker KD, van Alphen JJM, Stelkens RB, Seehausen O. Female mating preference functions predict sexual selection against hybrids between sibling species of cichlid fish. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008; 363 :2871–2877. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Webb C. A complete classification of Darwinian extinction in ecological interactions. American Naturalist. 2003; 161 :181–205. [ PubMed ] [ Google Scholar ]
- Werner EE, Hall DS. Niche shifts in sunfishes: experimental evidence and significance. Science. 1976; 191 :404–406. [ PubMed ] [ Google Scholar ]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; Oxford, UK: 2003. [ Google Scholar ]
- Wiley RH. Errors, exaggeration, and deception in animal communication. In: Real L, editor. Behavioral mechanisms in ecology. University of Chicago Press; Chicago, IL: 1994. pp. 157–189. [ Google Scholar ]
- Wilson DS, Turelli M. Stable underdominance and the evolutionary invasion of empty niches. American Naturalist. 1986; 127 :835–850. [ Google Scholar ]
- Wilson EO. The diversity of life. Harvard University Press; Cambridge, MA: 1992. [ Google Scholar ]
Development and evolution of character displacement
Affiliation.
- 1 Department of Biology, University of North Carolina, Chapel Hill, North Carolina 27599, USA. [email protected]
- PMID: 22257002
- PMCID: PMC3352989
- DOI: 10.1111/j.1749-6632.2011.06381.x
Character displacement occurs when competition for either resources or successful reproduction imposes divergent selection on interacting species, causing divergence in traits associated with resource use or reproduction. Here, we describe how character displacement can be mediated either by genetically canalized changes (i.e., changes that reflect allelic or genotype frequency changes) or by phenotypic plasticity. We also discuss how these two mechanisms influence the tempo of character displacement. Specifically, we suggest that, under some conditions, character displacement mediated by phenotypic plasticity might occur more rapidly than that mediated by genetically canalized changes. Finally, we describe how these two mechanisms may act together and determine character displacement's mode, such that it proceeds through an initial phase in which trait divergence is environmentally induced to a later phase in which divergence becomes genetically canalized. This plasticity-first hypothesis predicts that character displacement should be generally mediated by ancestral plasticity and that it will arise similarly in multiple, independently evolving populations. We conclude by highlighting future directions for research that would test these predictions.
© 2012 New York Academy of Sciences.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, U.S. Gov't, Non-P.H.S.
- Biodiversity
- Biological Evolution*
- Evolution, Molecular
- Genetic Speciation
- Models, Biological*
- Models, Genetic
- Population Dynamics
Grants and funding
- DP2 OD004436/OD/NIH HHS/United States
- DP2 OD004436-01/OD/NIH HHS/United States
- Search Menu
- Advance Articles
- Virtual Issues
- Special Issues
- Editor's Choice
- Why Publish
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- About Biological Journal of the Linnean Society
- Editorial Board
- Advertising & Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, supporting information, acknowledgements.
- < Previous
Character displacement is a pattern: so, what causes it?
*Corresponding author. E-mail: [email protected]
- Article contents
- Figures & tables
- Supplementary Data
Yoel E. Stuart, S. Andrew Inkpen, Robin Hopkins, Daniel I. Bolnick, Character displacement is a pattern: so, what causes it?, Biological Journal of the Linnean Society , Volume 121, Issue 3, July 2017, Pages 711–715, https://doi.org/10.1093/biolinnean/blx013
- Permissions Icon Permissions
Character displacement was originally defined simply as a pattern – divergence between two species in sympatry but not allopatry – and it was recognized that multiple processes might generate this pattern. However, over time, character displacement has come to be nearly synonymous with the process of adaptive divergence between species caused by selection stemming from resource-competitive interactions (and if not, then from reproductive interactions). This tight link between character displacement and resource competition has generated, and continues to generate, imprecision and confusion in the literature. Here, to address this problem, we suggest unlinking character displacement – the pattern – from any specific process (e.g. natural selection arising from species interactions). That is, character displacement should be documented as a pattern, agnostically with respect to process. Purposeful, direct investigation of what process generated character displacement then naturally follows. This has the benefit of acknowledging that there can be many different avenues to divergence in sympatry.
The ecology and evolution literature is replete with hundreds of cases where two species – usually closely related, ecologically similar species – show divergence from one another when they co-occur in sympatry but are indistinguishable where they exist separately in allopatry. In 1956, Brown and Wilson coined a name for this pattern – character displacement [unless noted otherwise, the use of character displacement in this manuscript refers to the pattern of divergence in sympatry relative to allopatry] – and proposed two processes by which selection for such divergence could arise, reinforcement and resource competition ( Brown & Wilson, 1956 ). In the six decades since, resource competition has become tightly linked with the term ‘character displacement’ such that character displacement and resource competition-driven divergence are nearly synonymous (P.R. Grant, personal communication; Stuart & Losos, 2013 ).
Problematically, the interchangeability between character displacement (that is, the pattern) and resource competition has meant that resource competition is commonly inferred when only divergence in sympatry has been shown ( Connell, 1980 ; Simberloff & Boecklen, 1981 ; Stuart & Losos, 2013 ). Such straightforward inference has inhibited the investigation of the causes of character displacement patterns, thereby slowing progress toward understanding the many ways in which divergence evolves. Indeed, Brown and Wilson ( Brown & Wilson, 1956 ) and many others (e.g. ( Simberloff & Boeklen, 1981 ); reviewed in ( Grant, 1972 ) ( Schluter, 2000 ), ( Pfennig & Pfennig 2012 )) have pointed out that numerous other ecological and evolutionary processes besides resource competition can result in character displacement [ Table 1 ; reviewed in ( Schluter, 2000 )]. So, while documenting the pattern is often straightforward, discerning the processes causing character displacement is more difficult ( Stuart & Losos, 2013 ).
Processes that could generate a pattern of character displacement, and how the processes differ in their target of selection (if selection is involved) and outcomes, all under the pattern-based definition of character displacement we advocate here
*We propose this as a more descriptive name for ecological character displacement, given the ambiguity of the “ecological” qualifier. **That is “reproductive”. ***For an intriguing wrinkle on intraguild predation, see interspecific killing ( Hoogland & Brown, 2016 ). ****Of course, divergence in the expression of plasticity can be genetically based and evolve.
Here, we trace a brief history of character displacement to understand how it changed meanings to refer to the process of divergence due to natural selection from species interactions. We then emphasize a pattern-based definition in line with the original definition that unlinks character displacement from a specific selective force. Our focus on pattern is in contrast to arguments to define character displacement as a process ( Grant, 1972 ) ( Pfennig & Pfennig, 2009 , 2012 ), but, as explained below, we believe that disentangling the pattern from the process results in a simpler nomenclature, and more importantly, actually promotes a more thorough investigation of the ecological and evolutionary forces that cause divergence. Our goal is to encourage researchers to recognize that documenting the pattern of character displacement (across a landscape or through time) is just the first step in a broader task of distinguishing between a variety of processes that might generate such patterns.
In 1953, Brown and Wilson published a paper about geographical variation and the subspecies concept ( Wilson & Brown, 1953 ). This collaboration launched them into a number of “megathought discussions” ( Brown, 1986 ), and they became particularly interested in one of Wilson’s findings in his revision of the ant genus Lasius , namely that “certain pairs of species, distinct morphologically and ecologically where they occurred together … were in other regions replaced by populations that appeared to be intermediate to … the same two species” ( Wilson, 1955 ; Brown, 1986 ). Brown and Wilson began collecting evidence for this pattern, which Brown had started calling “character displacement”, leading to a 1955 paper in which the idea and phrase were first coined ( Wilson & Brown, 1955 ). By 1956, the pair was ready to publish a general description of
… a seldom-recognized and poorly known speciation phenomenon that we consider to be of potential major significance in animal systematics. This condition, which we have come to call ‘character displacement,’ may be roughly described as follows. Two closely related species have overlapping ranges. In the parts of the ranges where one species occurs alone, the populations of that species are similar to the other species and may even be very difficult to distinguish from it. In the area of overlap, where the two species occur together, the populations are more divergent and easily distinguished, i.e., they ‘displace’ one another in one or more characters. The characters involved can be morphological, ecological, behavioral, or physiological; they are assumed to be genetically based. ( Brown & Wilson, 1956 )
In this description, Brown and Wilson defined character displacement as a pattern: divergence in sympatry. That is, for a phenotypic trait X in each of two species, in sympatry, they are divergent ( | X ¯ 1 − X ¯ 2 | > 0 ), while in allopatry they are not ( | X ¯ 1 − X ¯ 2 | ≅ 0 ), where X ¯ 1 and X ¯ 2 are the population means for trait X for species 1 and 2, respectively. [One alternative mathematical formulation is | X ¯ 1 A − X ¯ 2 A | < | X ¯ 1 S − X ¯ 2 S | , where A and S represent allopatry and sympatry, respectively. This is slightly different from Brown and Wilson’s verbal description of the pattern, allowing two species to be different from each other in allopatry and asking whether the two species are even more different in sympatry. This ‘exaggerated divergence’ formulation is both more general and biologically realistic.] To document this pattern, one need not presuppose that the trend is driven by any one process, for example natural selection, sexual selection, drift, ecological sorting, biased migration, and so on.
S everal processes initially proposed
Brown and Wilson also considered process, describing a pair of “interaction[s] in the history of the [species] pair…”, with character displacement arising “most often as a product of the genetic and ecological interaction of two (or more) newly evolved cognate species during their period of first contact” ( Brown & Wilson, 1956 ). The first interaction they considered was “ reinforcement of the reproductive barriers [italics by ( Brown & Wilson, 1956 )]. It may happen that the species continue to interbreed to some extent, and either the resulting inseminations are ineffectual, or the hybrids produced are inviable or sterile, resulting in what geneticists have termed ‘gamete wastage.’ Consequently, any further ethological or genetic divergence reducing this wastage will be strongly favored by selection.” The second process Brown and Wilson considered was “ ecological character displacement [italics by ( Brown & Wilson, 1956 )]. It seems clear from an a priori basis that any further ecological divergence lessening competition between the overlapping populations will be favored by natural selection if it has a genetic basis.” Thus, character displacement was originally proposed as a pattern arising from two species-interaction driven processes: (1) natural selection to reduce unprofitable interspecific matings (reinforcement; we consider reinforcement and reproductive character displacement – RCD – to be the same process. Some authors consider reinforcement to be nested within a broader concept of RCD ( Pfennig & Pfennig, 2009 , 2012 )), and (2) natural selection to reduce interspecific competition (ecological character displacement; as resource competition became the dominant paradigm in ecology through the 1960s-1980s ( Schoener, 1982 ), the rather vague adjective, “ecological”, became synonymous with resource competition in the character displacement literature).
O ne process gets defined
Grant formally linked the pattern of divergence in sympatry to divergent selection arising from species interactions by redefining character displacement as the “process by which a morphological character state of a species changes under natural selection arising from the presence, in the same environment, of one or more species similar to it ecologically and/or reproductively” ( Grant, 1972 ). By defining character displacement thus, a pattern of divergence in sympatry can only be called character displacement ( sensu Grant) if it stems from divergent natural selection imposed by an interacting species. That is, Grant argued that species interactions had to be involved ( Grant, 1972 ). [Though carefully defined by Grant, such that divergent natural selection could come from any kind of species interaction, today, to many workers, one cannot call a pattern of divergence character displacement unless one has proved competition (or reinforcement), even if it is clear that some yet-to-be-determined species interaction is present.]
Was it necessary to restrict the source of divergence to only selection from species interactions? Brown and Wilson clearly were focusing on species interactions: character displacement “aris[es] most often as a product of the … interaction of … species…”, with “two important ways in which the sympatric populations can interact…” ( Brown & Wilson, 1956 ). However, “most often” and “ways in which” indicate that other events besides species interactions could also generate a pattern of divergence in sympatry that still constitutes character displacement ( Brown, 1986 ).
A problem with a process-based definition restricted to species interactions is how to discuss patterns of divergence in sympatry that do not arise from species interactions. One could come up with new terms for each such pattern-process, but that risks obscuring the clear relationships among the processes – that is, they are all trying to explain divergence in sympatry. Brown and Wilson’s pattern-based approach, in contrast, allows any process that generates divergence in sympatry to be considered and compared and synthesized under a single umbrella: (the pattern of) character displacement.
“Qualified” character displacement denotes process
Thus, in our opinion, we should return to the original pattern-based definition of character displacement, unlinking it from the processes that generate it. Agnostic to process, one should first test whether | X 1 − X 2 | > 0 is true in sympatry but not in allopatry. Then, process should be investigated to determine what kind of character displacement has occurred ( Table 1 ). [Grant argued that linking pattern to process was advantageous, in part, because different patterns of divergence could be considered representative of the same selective process. Yet, by reporting mechanism through a qualifier as described below, our approach also works for the other named patterns of character displacement, like trait over-dispersion and species-for-species matching ( Schluter, 2000 ) ( Grant, 1972 ), or cases where divergence increases through time in areas of newly established sympatry. ( Grant, 1972 ).]
How does one denote the various processes driving character displacement if the pattern has been discovered? The nomenclature under our approach is straightforward. Indeed, researchers have already begun to solve this question by putting qualifying adjectives in front of the term “character displacement”. For example, divergence resulting from selection to reduce resource competition has been called “ecological” character displacement ( Schluter, 2000 ); to reduce unprofitable matings (i.e. reinforcement) has been called “reproductive” character displacement ( Pfennig & Pfennig, 2009 ) and to reduce aggressive encounters has been called “agonistic” character displacement ( Grether et al. , 2009 ). In each of these cases, the qualifier denotes the type of process underlying divergence in sympatry. Thus, under a return to Brown and Wilson’s original pattern-based definition, all we must do is adapt the “qualifier” approach by extending the list of qualifiers to describe new processes ( Table 1 ).
How are we to determine the source of divergence itself? Schluter and McPhail codified a set of criteria for testing whether character displacement results from resource competition, and is therefore “ecological” character displacement [Appendix 1; ( Schluter & McPhail, 1992 )]. Similar criteria have been put forth for reinforcement ( Waage, 1979 ; Hopkins, 2013 ) and “agonistic” character displacement ( Grether et al. , 2009 ). This criterion approach can be modified for all the processes in Table 1 (see Table A1), thereby providing a rigorous way to discern among potential processes. We note here that these criteria do not explicitly require measurement of divergent selection to confirm process (for those processes that depend on selection). However, in concert with criteria 6 (what is the source of selection) and 4 (how traits are related to selection) (Appendix Table A1), testing whether there is a cost of coexisting in sympatry and whether trait divergence lessens that cost would bolster a hypothesized selective process. In other words, is mean fitness for at least one of the species positively related to trait divergence: d w ¯ d ( | X ¯ 1 − X ¯ 2 | ) > 0 , where the numerator represents the change in average fitness.[See Slatkin (1980) for detailed theoretical treatment of ecological character displacement. Reviewed in Schluter (2000) .] Of course, character displacement may be a result of selection in the distant past. If the populations have diverged sufficiently, ongoing selection for displacement might not be measurable, except by experimental tests artificially generating less-displaced populations or natural experiments that do the same ( Hopkins, 2013 ; Stuart & Losos, 2013 ).
We know that our inclusive, pattern-based definition runs against common usage ( Grant, 1972 ); nevertheless, we feel that the benefit of describing character displacement as a pattern, and then pursuing process, outweighs any drawbacks that come from changing common usage ( Grant, 1972 ; Pfennig & Pfennig, 2012 ). First, character displacement can be documented and named clearly without requiring process, so as to make findings relevant (and searchable) for syntheses and meta-analyses and to spur future research. Second, there are testable criteria to distinguish among processes once the pattern is documented ( Tables 1 and A1), and the kind of process can be easily noted with a qualifier. But, third, if not all the criteria are met, character displacement is not void as it would be under the existing process-based definition where selection must be shown. Rather the pattern can still be published with careful discussion of those processes that are consistent with any criteria the researchers have tested in their study. Fourth, this framework requires trait divergence, rather than just trait evolution, so other phenomena like character convergence ( Grant, 1972 ) and red-queen escalation are excluded and can be examined separately.
Ecologists and evolutionary biologists are motivated to (1) describe the natural world to find patterns that govern biological diversity and (2) understand the proximate and ultimate processes that generate these patterns. By unlinking character displacement from resource competition, and species interactions generally, we hope to facilitate both these goals.
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Appendix 1. Criteria for ‘ecological’ character displacement, modified from (Schluter & McPhail, 1992).
Table A1. Criteria applied to all processes.
We thank Ambika Kamath, Jonathan Losos and James Stroud for thoughtful comments on this manuscript. We thank J.A. Allen, P.R. Grant and an anonymous reviewer for their insightful comments.
Brown JWL . 1986 . This week’s citation Classic® . Current Contents 20 : 18 .
Google Scholar
Brown WL Wilson EO . 1956 . Character displacement . Systematic Zoology 5 : 49 – 64 .
Case , T.J . 1979 . Character displacement and coevolution in some Cnemidophorus lizards. Fortschritte der Zoologie 25 : 235 – 282 .
Connell JH . 1980 . Diversity and the coevolution of competitors, or the Ghost of Competition Past . Oikos 35 : 131 – 138 .
Grant P . 1972 . Convergent and divergent character displacement . Biological Journal of the Linnean Society 4 : 39 – 68 .
Grether GF Losin N Anderson CN Okamoto K . 2009 . The role of interspecific interference competition in character displacement and the evolution of competitor recognition . Biological Reviews 84 : 617 – 635 .
Holt RD . 1977 . Predation, apparent competition, and the structure of prey communities . Theoretical Population Biology 12 : 197 – 129 .
Hoogland JL Brown CR . 2016 . Prairie dogs increase fitness by killing interspecific competitors . Proceedings of the Royal Society B 283: 20160144
Hopkins R . 2013 . Reinforcement in plants . New Phytologist 197 : 1095 – 1103 .
Losos JB . 1990 . A phylogenetic analysis of character displacement in Caribbean Anolis lizards . Evolution 44 : 558 – 569 .
Meiri S Simberloff D Dayan T . 2011 . Community-wide character displacement in the presence of clines: a test of Holarctic weasel guilds . Journal of Animal Ecology 80 : 824 – 834 .
Pfennig DW Martin RA . 2009 . A maternal effect mediates rapid population divergence and character displacement in spadefoot toads . Evolution 63 : 898 – 909 .
Google Preview
Pfennig DW Pfennig KS . 2012 . Evolution’s Wedge . Berkeley : University of California Press .
Pfennig KS Pfennig DW . 2009 . Character displacement: ecological and reproductive responses to a common evolutionary problem . Quarterly Review of Biology 84 : 253 – 276 .
Polis GA Myers CA Holt RD . 1989 . The ecology and evolution of intraguild predation: potential competitors that eat each other . Annual Review of Ecology and Systematics 20 : 297 – 330 .
Schluter D . 2000 . The ecology of adaptive radiation . Oxford, UK : Oxford University Press .
Schluter D McPhail JD . 1992 . Ecological character displacement and speciation in sticklebacks . American Naturalist 140 : 85 – 108 .
Schluter D Price TD Grant PR . 1985 . Ecological character displacement in Darwin’s Finches . Science (New York, N.Y.) 227 : 1056 – 1059 .
Schoener TW . 1982 . The controversy over interspecific competition: despite spirited criticism, competition continues to occupy a major domain in ecological thought . American Scientist 70 : 586 – 595 .
Simberloff D Boecklen W . 1981 . Santa Rosalia reconsidered: size ratios and competition . Evolution 35 : 1206 – 1228 .
Slatkin M . 1980 . Ecological character displacement . Ecology 61 : 163 – 177 .
Stuart YE Losos JB . 2013 . Ecological character displacement: glass half full or half empty? Trends in Ecology & Evolution 28 : 402 – 408 .
Waage JK . 1979 . Reproductive character displacement in Calopteryx (Odonata: calopterygidae) . Evolution 33 : 104 – 116 .
Wilson EO . 1955 . A monographic revision of the ant genus Lasius . Bulletin of the Museum of Comparative Zoology 113 : 1 – 205 .
Wilson EO Brown WL . 1953 . The subspecies concept and its taxonomic application . Systematic Zoology 2 : 97 – 111 .
Wilson EO Brown WL . 1955 . Revisionary notes on the Sanguinea and Neogagates groups of the ant genus Formica . Psyche 62 : 108 – 129 .
Author notes
Supplementary data, email alerts, citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1095-8312
- Print ISSN 0024-4066
- Copyright © 2024 The Linnean Society of London
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 May 2022
Dynamic character displacement among a pair of bacterial phyllosphere commensals in situ
- Lucas Hemmerle 1 ,
- Benjamin A. Maier 1 ,
- Miriam Bortfeld-Miller 1 ,
- Birgitta Ryback 1 ,
- Christoph G. Gäbelein 1 ,
- Martin Ackermann 2 , 3 &
- Julia A. Vorholt ORCID: orcid.org/0000-0002-6011-4910 1
Nature Communications volume 13 , Article number: 2836 ( 2022 ) Cite this article
4674 Accesses
12 Citations
22 Altmetric
Metrics details
- Microbial communities
- Microbial ecology
Differences between species promote stable coexistence in a resource-limited environment. These differences can result from interspecies competition leading to character shifts, a process referred to as character displacement. While character displacement is often interpreted as a consequence of genetically fixed trait differences between species, it can also be mediated by phenotypic plasticity in response to the presence of another species. Here, we test whether phenotypic plasticity leads to a shift in proteome allocation during co-occurrence of two bacterial species from the abundant, leaf-colonizing families Sphingomonadaceae and Rhizobiaceae in their natural habitat. Upon mono-colonizing of the phyllosphere, both species exhibit specific and shared protein functions indicating a niche overlap. During co-colonization, quantitative differences in the protein repertoire of both bacterial populations occur as a result of bacterial coexistence in planta . Specifically, the Sphingomonas strain produces enzymes for the metabolization of xylan, while the Rhizobium strain reprograms its metabolism to beta-oxidation of fatty acids fueled via the glyoxylate cycle and adapts its biotin acquisition. We demonstrate the conditional relevance of cross-species facilitation by mutagenesis leading to loss of fitness in competition in planta . Our results show that dynamic character displacement and niche facilitation mediated by phenotypic plasticity can contribute to species coexistence.
Similar content being viewed by others

Rapid evolution of bacterial mutualism in the plant rhizosphere

Mapping phyllosphere microbiota interactions in planta to establish genotype–phenotype relationships
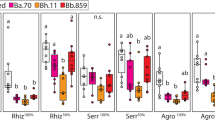
Intracellular Burkholderia Symbionts induce extracellular secondary infections; driving diverse host outcomes that vary by genotype and environment
Introduction.
The mechanisms that generate and maintain genetic and phenotypic variation in natural populations are central to ecology and evolution. Character displacement, the evolutionary divergence of competing species, plays a fundamental role in the assembly of diverse communities 1 , 2 . Sympatric species compete for the same set of limited resources, and thus natural selection might favor diversification of their use. As a result, phenotypes may emerge in which alternative resources are exploited, thereby reducing competition for nutrients 3 , 4 , 5 , 6 . Once separated in niche space, a species may modify the environment and directly or indirectly enhance its own growth and survival 7 , 8 . As a result, competitive interactions are altered among species through mechanisms such as increased access to limiting resources, which in turn has impacts on biodiversity and prevalence 9 .
The nature of the interactions that generate patterns of diversity remains difficult to examine 10 . To provide a mechanistic basis, evolution experiments and evolutionary analysis of bacterial populations have been used to study the processes that lead to diversifications, and these model studies have illustrated how competitive and facilitative interactions between distinct genotypes can evolve and contribute to their coexistence 8 , 11 , 12 , 13 , 14 , 15 . These and other studies have added to our understanding of microbial community assembly by providing exemplary analysis of the evolutionary processes that drive diversity.
A complementary and non-exclusive perspective on the interaction of microbial species that may contribute to coexistence is their potential to dynamically shift their phenotype in response to the presence of other species. This process is referred to as phenotypic plasticity and has been underappreciated in mediating character displacement 16 , 17 , 18 . According to this idea, when two extant species encounter each other and compete for limited resources, they diversify through changes in gene expression and thus become less similar (Fig. 1 ). The latter can be seen as the result of an ecological interaction, but may be the consequence of an evolutionary process in the past. Such phenotypic diversification could be exhibited by competing species to reduce their niche overlap. In fact, it has been hypothesized that phenotypic plasticity may precede genetic differences among species 18 .
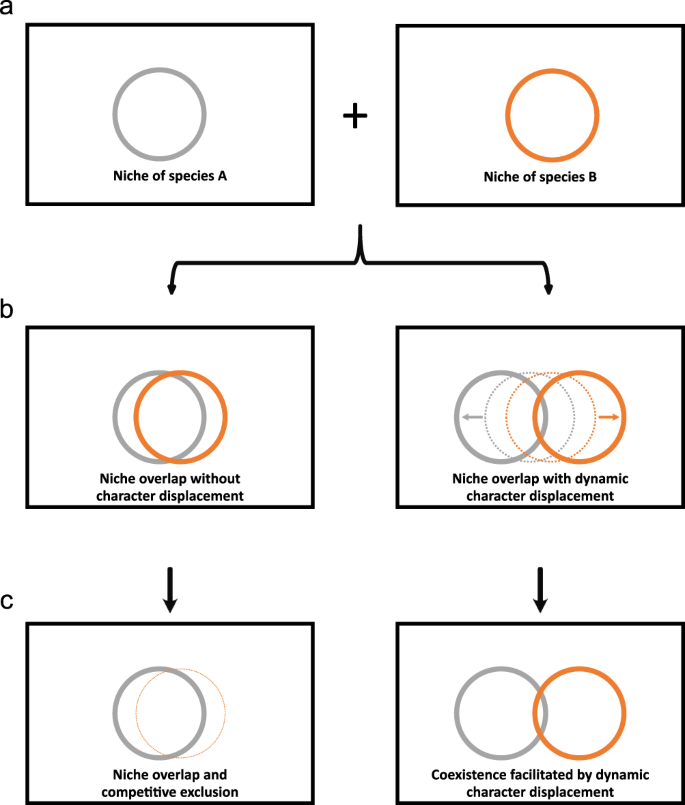
a General niche of two species, A (gray) and B (orange), in case they do not encounter each other in a given habitat. b During co-occurrence, the niches of the two species overlap substantially, leading to competition for shared resources (left) but also to potential for a dynamic character displacement (right, indicated by arrows). c As a consequence, one species may displace the other species from the niche resulting in competitive exclusion (left). Alternatively, a dynamic character displacement may occur, reducing the original niche overlap and leading to coexistence (right). The size of the circle indicates productivity in the ecological niche. In this study, niche occupancy was measured using a proteomics approach.
While measuring differences among species is more straightforward, discerning the processes causing character displacement is inherently difficult 2 , 10 and requires empirical quantifiable differences with appropriate controls. Here, we set out to test the phenotypic plasticity of two commensal species and to examine whether they become phenotypically more dissimilar when co-occurring compared to being present alone. To provide context for their interaction, we chose two bacterial strains isolated from the phyllosphere of the model plant Arabidopsis and tested their interaction in planta . The phyllosphere, the above-ground parts of plants dominated by leaves, is an important ecosystem due to its contribution to carbon dioxide fixation in terrestrial systems and indigenous bacteria may impact plant growth and health 19 , 20 , 21 . In addition, the phyllosphere is also a highly suitable habitat to study microbial interactions because it represents a discrete habitat or, more precisely, a sum of discrete habitats 22 , 23 , 24 and allows quantifying population sizes in a well-defined area. The phyllosphere, a heterogeneously structured and oligotrophic environment, has been shown to maintain high and reproducible microbial biodiversity 25 , 26 . As a result, due to limited resources, it provides the ground for species interactions and opportunities to discover rules for community assembly in this habitat 27 , 28 .
Culture-independent studies on the composition of the phyllosphere microbiota of different plant species revealed that reoccurring phylogenetic structures can be observed 29 , 30 , 31 , 32 , 33 . Bacteria belonging to the phylum Proteobacteria are most commonly found, followed by Actinobacteria, Bacteroidetes, and Firmicutes across distinct plant species 26 , 29 , 34 . These bacteria are mostly found on the surface of leaves, where they can form aggregates 20 , 35 , 36 , 37 , 38 , 39 . These bacterial aggregates can be constituted by a single bacterial species or by several species and are of great interest for the elucidation of bacteria-bacteria interactions 40 , 41 .
Several studies have addressed the physiological adaptation strategies involved in leaf colonization by individual bacterial species of commensals 42 , 43 , 44 , 45 and plant pathogens 46 , 47 , 48 . Complementary studies have provided the first insights into the physiological capacity of the coexisting phyllosphere microbiota in planta using metaproteomics 25 , 49 . While providing a snapshot of the state of microbial communities in situ, the dynamic processes underlying niche occupancy as a consequence of species competition are currently not well understood. Because interspecies interactions in situ are complicated by the presence of a multitude of species, simplified systems are needed, that allow to dissect dynamic behavior of populations.
In the present study, we use an established gnotobiotic Arabidopsis thaliana model system 50 , 51 to test whether two species undergo shifts in the phenotype upon co-colonization. We select two strains representing the most common colonizing proteobacterial families Sphingomonadaceae and Rhizobiaceae and apply mass spectrometry-based proteomics to quantify proteome-level changes in each strain upon co-colonization of plants compared to mono-colonization. In addition, we identify the specific adaption of each strain to the in planta condition compared to growth on artificial media. As expected, we confirm that both species are generalist bacteria that have the potential to utilize a variety of substrates such as glucose or sucrose as an available, yet limiting carbon source on leaves 52 . In addition to facilitation, we observe a dynamic character displacement that might contribute to the coexistence of the populations. We confirm relevant traits by mutagenesis and testing in planta .
Assessment of the interaction of two phyllosphere strains based on population sizes and proteome allocation in planta
To study the interaction between phyllosphere microbiota strains, we selected two representative strains from the Alphaproteobacteria, the most abundant bacterial class found on A. thaliana leaves 26 . Among Alphaproteobacteria the bacterial families of Sphingomonadaceae and Rhizobiaceaea are the most common and include strains with plant beneficial functions 20 , 21 , 29 , 50 , 53 . Previous experiments have shown that the genera of Sphingomonas and Rhizobium have the potential to affect community assembly and structure 27 . Furthermore, both genera have overlapping metabolic capacities and occur in the spatially defined, oligotrophic environment of the A. thaliana phyllosphere 26 , 29 . Specifically, we chose Sphingomonas Leaf257 and Rhizobium Leaf68 and confirmed their consistent colonization capacities in mono-colonization using a gnotobiotic plant growth system (see Methods). Both strains were isolated from leaf washes and colonize leaves as epiphytes 29 , 54 . We also ensured that both strains colonized the phyllosphere upon co-colonization. In addition, we confirmed their co-occurrence during colonization at the microscale (Supplementary Fig. 1 ).
We then conducted four independent large-scale colonization experiments in which we inoculated the plants with either Rhizobium Leaf68 and Sphingomonas Leaf257 or a mixture of both. In addition, we determined the bacterial population sizes upon mono- and co-colonization. Both strains colonized the phyllosphere, with Sphingomonas Leaf257 reaching 1.6 × 10 9 CFUs per gram fresh weight (CFUs g −1 FW) (Fig. 2a ) and Rhizobium Leaf68 of 2.6 × 10 8 CFUs g −1 FW (Fig. 2b ). During co-colonization of the phyllosphere, both strains significantly changed their colonization capacity (Kruskal–Wallis, P -value <0.01). Sphingomonas Leaf257 was reduced to 7.5 × 10 8 (0.46-fold) (Fig. 2a ) and Rhizobium Leaf68 increased to 1.2 × 10 9 CFUs g −1 FW (4.6-fold) (Fig. 2b ). The population sizes indicated an interaction between the two strains during co-colonization in the phyllosphere, as both populations changed in number relative to mono-colonization. Because commensal bacteria can elicit plant responses 55 and may indirectly affect colonization of other bacteria, we tested the interaction of both strains using a selection of plant immunity mutants (Methods). We found that the bacterial interaction was robust in different plant backgrounds (Supplementary Fig. 2 ).
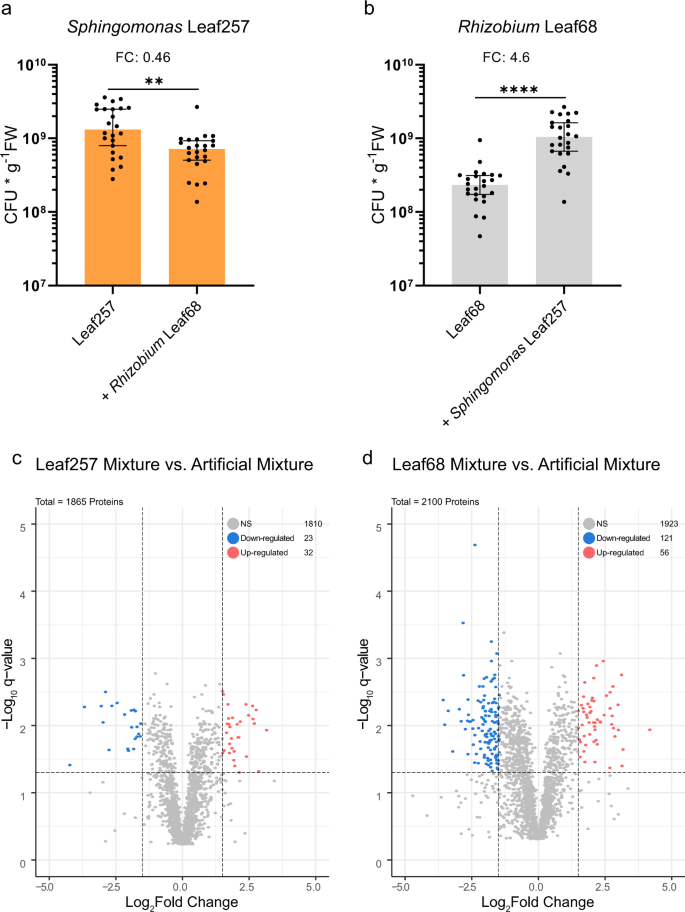
a , b Cell numbers (CFUs per gram fresh weight) of Sphingomonas Leaf257 (orange), or Rhizobium Leaf68 (gray) alone and upon co-colonization harvested after 11 dpi. Average fold-change (FC) between the two conditions is indicated above the bar. Graphs depict the combined data from four independent biological replicates with each dot representing the CFUs obtained from a single plant with six plants per biological replicate ( n = 24). In the graphs, the median and 95% confidence interval are shown. Statistical analysis was performed using the Kruskal–Wallis test ( P -values: * p -value < 0.05, ** p -value < 0.01, *** p -value < 0.001, and **** p -value < 0.0001). Proteome changes are depicted as volcano plots for ( c ) Leaf257 during co-colonization of the plant with Leaf68 and ( d ) Leaf68 during co-colonization of the plant with Leaf257.) Proteomics data were obtained from four independent biological replicates ( n = 4) (see methods). The adjusted P -value ( q -value) cutoff was set to 0.05 (horizontal line) and Log 2 Fold change cutoffs are set to >1.5 or < −1.5 (vertical lines). In the graphs, only proteins detected with at least two unique peptides are shown. The total number of proteins, not significantly regulated (NS), and Down- / Up-regulated are indicated in each graph. For ( c , d ) statistical analysis was performed using one-way ANOVA and resulting P -values were corrected for multiple comparisons using Benjamini–Hochberg. Source data for ( a , b ) are provided as a Source Data file.
We determined the proteomes of the bacterial populations in planta using a washing protocol (Methods) (i.e., Rhizobium Leaf68, Sphingomonas Leaf257 and both upon co-colonization) from the corresponding experiments enumerated above from four biological replicates. For protein determination, we used liquid chromatography-mass spectrometry (LC-MS/MS) operated in data-dependent acquisition mode (Methods, Supplementary Fig. 3a ). Notably, to improve protein quantification and to compensate effects of sample complexity of bacteria during mono-colonization compared to co-colonization, we also measured an artificial mixture from bacteria grown in mono-colonization in planta after cell lysis and protein digestion. In addition to the biological samples of bacteria grown in planta , we harvested the two strains grown each on solidified artificial medium on agar plates incubated under the same light and temperature regime in plant growth chambers in four independent replicates. Our MS-based approach allowed the detection of up to 2500 proteins per strain with normalized protein abundances spanning more than six orders of magnitude (Supplementary Fig. 3b–d ) and the quantification of about 2000 proteins detected with two or more unique peptides for each strain. We identified proteins that were induced or reduced when the bacteria encountered each other in planta , confirming that both bacteria interacted with each other (Fig. 2c, d ), as already suggested by the population sizes (Fig. 2a, b ).
Individualized and common adaptation strategies to colonize the phyllosphere
Before investigating the interaction between both bacteria, we first analyzed the physiological adaptation to the in planta conditions of each strain compared to growth on artificial media using proteomics. For Sphingomonas Leaf257, we identified 184 proteins that were induced in planta (Supplementary Fig. 4a , Supplementary Data 1 ). Among these, a number of proteins indicate an adaptation to environmental stress in terms of desiccation, oxidative stress and light in line with earlier reports on leaf strains 25 , 44 . Exemplarily, these included catalase counteracting oxidative stress as well as deoxyribodipyrimidine photolyase-related protein (COG3046) and UmuC as SOS induced DNA polymerase enabling DNA damage repair. We also identified a glycosyltransferase (ASF14_08960) as a highly induced protein (Log 2 fold-change >35) and its paralog (ASF14_03660, >4-fold) that was also induced upon plant colonization in Sphingomonas melonis Fr1, another phyllosphere commensal 44 . Glycosyltransferases might be involved in lipopolysaccharide and biofilm synthesis 56 , 57 , 58 and their strong induction might highlight their relevance in phyllosphere colonization. In addition, fourteen distinct TonB-dependent receptors were induced upon phyllosphere colonization that might be involved in uptake of iron, carbohydrates or amino acids 25 , 44 .
For Rhizobium Leaf68, we found 356 proteins induced in planta (Supplementary Fig. 4b , Supplementary Data 2 ). The most strongly induced protein (about 1000-fold) in planta was an alcohol dehydrogenase (ASF03_19755), closely followed by several hypothetical proteins. We also identified two proteins that are involved in the detoxification of reactive oxygen species, a peroxidases (ASF03_20400) and a superoxide dismutase (ASF03_03695) with fold changes higher than 10 indicating that the bacterium has to cope with ROS-stress during phyllosphere colonization 25 , 42 . In addition, we found a predicted biotin sulfoxide reductase (ASF03_18405, 5.5-fold) and a biotin synthase (ASF03_03440, 5.2-fold) to be induced, which may suggest the need of Rhizobium Leaf68 to scavenge a biotin derivative. Two proteins (ASF03_19470 and ASF03_19480) involved in the synthesis of succinoglycan, an exopolysaccharide, were also strongly induced with fold changes of more than an order of magnitude (Supplementary Data 2 ). Succinoglycans are exopolysaccharides (EPS) known for their thermal stability, high water retention and viscosifying properties in many soil bacteria, including Rhizobia 59 , 60 , 61 . EPS production is a major factor in the formation of bacterial aggregates and might therefore benefit Rhizobium Leaf68 to colonize and form aggregates in the phyllosphere 26 , 36 . Another phyllosphere-specific response of Rhizobium Leaf68 was the induction of two sulfonate monooxygenases (ASF03_18445, 20.6-fold; ASF03_18375, 5.4-fold) and several ABC transporter binding proteins (CysA, 7.5-fold; CysP, 13.5-fold; SsuA, 32-fold; SsuB, 27-fold), proteins involved in sulfur acquisition during sulfur limiting conditions 62 . Furthermore, we found the two subunits of sulfate adenylyltransferase (ASF03_03950, >20-fold; ASF03_03955, 5.4-fold), an enzyme involved in sulfate assimilation, significantly induced, supporting the hypothesis that sulfur is limiting in the phyllosphere congruent with earlier studies 44 , 63 , 64 .
The induced proteins upon plant colonization in mono-colonization indicated that unique but also shared adaptation might occur. To compare the induced proteome systematically, we analyzed the data for orthologous groups (OGs) of proteins and determined which OGs were significantly regulated and shared between both strains (q-value < 0.05 and Log 2 fold-change >1.5 or <−1.5). From a total of 62 shared OGs, 24 were induced and 38 reduced when comparing in planta regulated proteins compared to in vitro conditions (Supplementary Fig. 4c , Supplementary Data 3 ). Next, we grouped all OGs by their functional category to see which functions are shared between both bacteria during phyllosphere colonization (Supplementary Fig. 4d , Supplementary Data 3 ). Some of the most induced shared protein orthologues are two glycosyltransferases (COG0438, COG0463), proteins involved in the molybdenum cofactor and thiamine biosynthesis, catalases, and one of the key enzymes involved in the glyoxylate cycle, isocitrate lyase (COG2224). The other key enzyme in the glyoxylate cycle, malate synthase (COG2225), was also induced in both strains but only 1.3-fold in Rhizobium Leaf68. The induction of the glyoxylate cycle upon plant colonization was previously observed in another phyllosphere commensal, S. melonis Fr1 44 . Additionally, a pyruvate dehydrogenase (COG0028) was induced in both strains with similar fold changes as well as the histidine kinase sensor protein EnvZ. The EnvZ histidine kinase is involved in the response to changes in osmolarity in E. coli 65 , a stress that bacteria also face during phyllosphere colonization 66 . Furthermore, we observed a glutathione transferase, an alcohol dehydrogenase, acetolactate and glutamine synthetase, and several outer membrane proteins. Interestingly, D-3-phosphoglycerate dehydrogenase was induced in both strains, which is the committing and rate limiting step in the phosphorylated pathway of L-serine biosynthesis, consistent with findings in S. melonis Fr1 44 . Furthermore, both strains downregulated three OGs involved in translation, ribosomal structure and biogenesis, and transcription respectively, which could be due to lower growth rates in planta compared to artificial media. Notably, we also found flagellin (COG1344) reduced in line with earlier studies 44 , 67 . Whether or not this strategy serves to minimize detection by the plant immune system 68 , 69 remains to be shown. Common OGs downregulated by both strains were a glycerol-3-phosphate dehydrogenase (COG0578), an alpha-L-arabinofuranosidase (COG3534) and several hypothetical proteins of which one is potentially a glycosyl transferase (COG0463).
In summary, the majority of shared regulated OGs were associated with intracellular stress responses as well as membrane-, cell wall- and envelop-biogenesis, indicative of the importance of cell-barrier remodeling in bacterial adaptation to the rather hydrophobic and water depleted environment of the phyllosphere in accordance with previous studies 26 , 36 . Importantly also, shared induced OG related to nutrient uptake and metabolism suggested common adaptation to the oligotrophic phyllosphere environment and potential niche overlap.
Proteome changes during co-colonization of the phyllosphere
Next, we analyzed the dynamic niche occupancy upon coexistence based on the proteomes of the two strains. To do so, we compared the proteomes generated upon co-colonization with the ones determined under mono-colonization conditions (upon artificial mixture, see above). Sphingomonas Leaf257 significantly changed 55 proteins upon co-colonization (q-value < 0.05 and Log 2 fold-change >1.5 or <−1.5) of which 32 were induced and 23 were reduced (Fig. 2c , Supplementary Data 4 ). Rhizobium Leaf68 significantly altered 177 proteins ( q -value < 0.05 and Log 2 fold-change >1.5 or <−1.5) of which 56 were induced and 121 reduced (Fig. 2d , Supplementary Data 5 ). These changes indicate a reciprocal interaction between the two strains in the phyllosphere.
For Sphingomonas Leaf257, one of the most induced proteins upon co-colonization with Rhizobium Leaf68 was dipeptidyl peptidase-4 that cleaves X-proline dipeptides from the N-terminus of polypeptides (ASF14_01870, 6.4-fold) (Supplementary Data 4 ) 70 . Proline-rich proteins are a common building block in the glycoproteins found in plant cell walls, where they are used as anchor points for glycan chains 71 . In agreement with this, several polysaccharide-degrading enzymes were also strongly induced, namely a xylosidase/arabinosidase (ASF14_10230, 5.2-fold), a xylan 1,4-beta-xylosidase (ASF14_16825, 5.2-fold), a glucan 1,4-beta-glucosidase (ASF14_10240, 4.2-fold), and another xylanase (ASF14_16480, 3.1-fold) (Fig. 3a , Supplementary Data 4 ). In addition, the induction (2.4-fold) of an ABC transporter (ASF14_10270) genomically encoded close to a gene for a predicted xylosidase suggested an increased metabolization of sugar monomers obtained from xylan degradation, highlighting a possible degradation of plant cell walls. We also found 6-phosphogluconolactonase (ASF14_10560) induced (2.3-fold), which is required, for example, to metabolize glucuronic acid, which forms side chains of xylan, thus supporting the notion of a sugar metabolism from xylan. In addition, Sphingomonas Leaf257 induced the iron transporter FeoB (2.9-fold) as well as a predicted ferrichrome-iron receptor (2.5-fold) indicating increased iron limitation during co-colonization of the plant under competitive conditions. Interestingly, less strongly but consistently induced were several enzymes involved in the Shikimate pathway (Supplementary Data 4 ). Isochorismate pyruvate-lyase (ASF14_15595) catalyzes the reaction from isochorismate to pyruvate and salicylic acid, the latter being observed in other bacteria under iron-limiting conditions 72 , 73 . The most downregulated proteins by Sphingomonas Leaf257 under co-colonization conditions compared to mono-association were a Zn-dependent dipeptidase (ASF14_12990, 18-fold), a putative phosphatase (ASF14_09200, 12-fold) and several hypothetical proteins (>7-fold). In addition, a predicted low-affinity phosphate transporter (ASF14_05655, 3.4-fold) and a phosphate ABC transporter substrate-binding protein (ASF14_05925, 2.4-fold) were downregulated upon co-colonization that were induced in planta upon mono-colonization (Supplementary Data 1 and 4 ). Thus, our results suggest that co-colonization with Rhizobium Lea68 results in increased iron- and phosphate shortage for Sphingomonas Leaf257 and drives the latter to exploit additional carbon sources by metabolizing components of the plant cell wall, most prominently xylan.
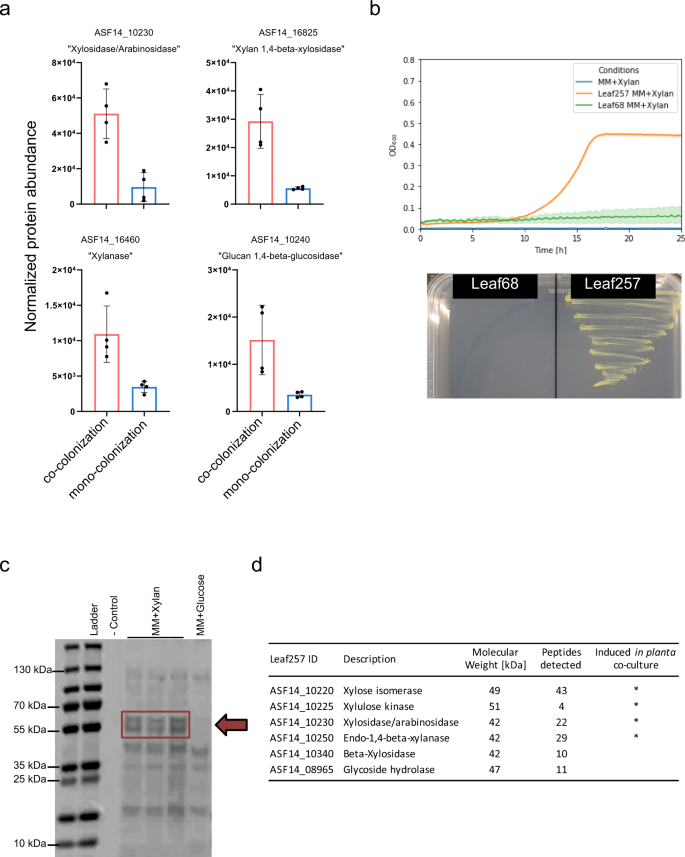
a Normalized abundance of proteins related to xylan degradation obtained from the proteomics workflow (see methods, Supplementary Data 4) upon co-colonization (red) and mono-colonization (blue) for four enzymes potentially involved in xylan degradation. Error bars represent the standard deviation. Data are from 4 independent replicates, n = 4). b Growth curve depicting optical density (OD 600 ) of Sphingomonas Leaf257 (orange), Rhizobium Leaf68 (green) and negative control (blue) in minimal medium with 10 mM xylan in liquid (top) and growth on solid minimal medium with 10 mM xylan for Leaf257, Leaf68 (no growth) (bottom). Error bars (top) represent the standard deviation. Data are from 5 independent replicates, n = 5. c SDS-gel of enriched supernatant (see methods) of Sphingomonas Leaf257 grown in liquid minimal medium with ddH 2 O (control), xylan or glucose. For MM + xylan three biological independent replicates were prepared. One replicate was prepared for the control MM + glucose. The xylan specific double-band at ca. 55 kDa (red box) was extracted and analyzed using LC-MS. d Overview of protein identified in the LC-MS approach. Source data for ( a , b , c ) are provided as a Source data file.
For Rhizobium Leaf68, one of the most induced proteins upon co-colonization with Sphingomonas Leaf257 was an alpha-galactosidase (ASF03_16130, 9-fold). Another alpha-galactosidase (ASF03_16155) was induced 4-fold, and we observed a small but significant induction for an alpha-galactoside-binding protein (ASF03_16135), suggesting adaptation towards the digestion and uptake of alpha-galactosyl moieties that might be derived from polysaccharides of the plant cell wall (Supplementary Data 5 ) 74 , 75 . Several hypothetical proteins were also among the most induced proteins. Interestingly, isocitrate lyase (ASF03_04985; 4-fold) and a glycolate dehydrogenase (ASF03_04840; 6-fold) were induced during co-colonization, suggesting an increased flux towards the glyoxylate cycle as an anabolic pathway. Consistent with this finding, we observed an induction of several enzymes involved in the beta-oxidation of fatty acids (ASF03_04360, 7-fold; ASF03_12360, 2.1-fold; ASF03_12365, 3.4-fold; ASF03_20960, 4.5-fold). Several TonB-dependent receptors, RND efflux systems and other transporters were also induced in response to co-colonization, some of which were predicted ABC transporter for glycerol-3-phosphate (ASF03_16050, 6.4-fold; ASF03_21060, 3.9-fold; ASF03_21075, 3.4-fold; ASF03_18705, 3.3-fold; ASF03_18690, 3.2-fold). In line with this observation, we measured a strong reduction (5-fold) of the glycerol-3-phopshate regulon repressor GlpR (ASF03_11310) indicating an adaptation towards the acquisition of glycerol-3-phosphate through de-repression of the underlying regulon. Glycerol-3-phosphate uptake is notable in the context of fatty acid degradation as this may suggest an adaptation of Rhizobium Leaf68 towards growth on lipids. Furthermore, two proteins involved in phosphonate utilization were significantly induced (ASF03_14895, 8-fold; ASF03_14885, 5.8-fold), suggestive of phosphate starvation during co-colonization. Further highlighting an association with phosphate starvation, the PhoB transcriptional regulator (ASF03_21155) associated with phosphate and other nutrient stress networks 76 was induced (Fig. 4a ). Taken together, this indicated that in response to co-colonization with Sphingomonas Leaf257, Rhizobium Leaf68 adapted to phosphate scarcity by engaging global regulatory mechanisms. One of the most reduced proteins during co-colonization was alcohol dehydrogenase (ASF03_19755; Log 2 fold-change < −6.8), which was striking as the enzyme was highly induced in the mono-colonization compared to artificial media (Fig. 4a , Supplementary Data 2 and 5 ). Another observation was that biotin synthase (ASF03_03440), the last enzyme in the biotin biosynthesis, was significantly reduced in Rhizobium Leaf68 upon co-colonization with Sphingomonas Leaf257.
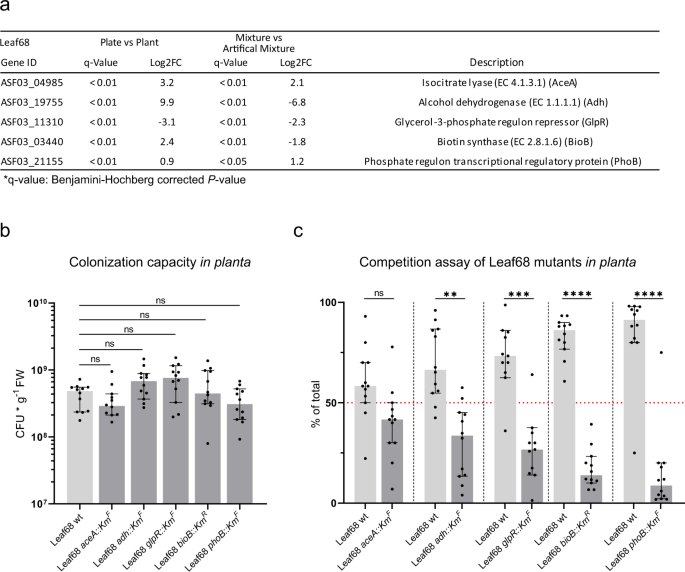
a Overview table of the five genes of Rhizobium Leaf68 selected for mutagenesis and fold-changes of the encoded proteins over the different proteomics conditions (Supplementary Data 2 and 5 ). P- values were calculated using a one-way ANOVA and adjusted for multiple comparisons using Benjamini-Hochberg. b Colonization capacity of each (CFUs per gram fresh weight), Leaf68 wild type (light gray), mutants (dark gray) alone in planta . Statistical analysis was performed using Kruskal–Wallis test. c Competition assay depicting the percentage (%) of total population wild type versus each mutant respectively. A 1:1 mixture is indicated at 50% with a red dotted line. For statistical analysis a two-sided paired t-test was performed ( P -values: * p -value < 0.05, ** p -value < 0.01, *** p -value < 0.001, and **** p -value < 0.0001). In graphs b and c, the median and 95% confidence interval are shown. For both graphs each data point represents the values obtained from a single plant with a total of 12 plants for these experiments. Source data for ( b , c ) are provided as a Source Data file.
In summary, our results suggest that both strains react to each other, encounter enhanced competition for phosphorus and iron and shift towards alternative metabolic pathways.
Sphingomonas Leaf257, a plant-derived xylan degrader and sugar generalist
Our proteomics data suggested that Sphingomonas Leaf257 explores new niches when experiencing species competition. In particular, the data for co-colonization compared to mono-colonization indicated an increased utilization of xylan from the cell wall (Fig. 3a and Supplementary Data 4 ), a polysaccharide predominantly made up of xylose. To verify the ability of Sphingomonas Leaf257 to metabolize plant-derived xylan, we performed growth assays in a minimal medium containing xylan as the sole carbon and energy source. Indeed, we observed growth both in liquid culture as well as on plates, confirming the ability of Sphingomonas Leaf257 to degrade xylan (Fig. 3b ). Growth on xylan requires the secretion of xylan-degrading enzymes. To test for the presence of such proteins, we sampled the spent medium from liquid cultures with xylan, concentrated it and analyzed it using SDS-PAGE. We observed prominent bands at around 55 kDa upon growth on xylan, but not when glucose was the carbon source (Fig. 3c ). LC-MS analysis resulted in the identification of several proteins, six of which were enzymes involved in the degradation of larger polysaccharides such as xylan and its subsequent metabolization (Fig. 3d ). One of these enzymes was already identified as significantly induced during co-colonization (ASF14_10230) (Fig. 3a ) and three others were detected in planta (ASF14_10220, ASF14_10225, ASF14_10250) (Fig. 3d ). The three xylan-degrading enzymes, ASF14_10230, 10250, and 10340, contained a signal peptide, indicating that they are indeed secreted to degrade the xylan polysaccharide. In contrast, both the xylose isomerase and xylulose kinase as well as the glycoside hydrolase did not contain a predicted signal peptide. The presence of these enzymes in culture supernatants might thus be due to partial cell lysis and accumulation in the medium. Nonetheless, their abundance in a minimal medium with xylan is further suggestive of the metabolization of xylose derived from xylan through the pentose phosphate pathway.
The degradation of xylan results in the release of mostly xylose but also additional carbohydrates, including C6 sugars that form side chains on the β-1,4-xylose backbone 77 , as mentioned above. To test if Sphingomonas Leaf257 prefers certain sugars, we determined the growth on different substrates and their combinations in liquid culture. In addition, we took samples of the supernatant for HPLC analysis. We found that Sphingomonas Leaf257 is able to grow on xylose, glucose, and galactose and co-consumes these sugars (Supplementary Fig. 5 ). We did not observe evidence for the accumulation of incompletely metabolized intermediates such as pyruvate, acetate, or other byproducts during growth on the different sugar substrates. Our data therefore indicate that Sphingomonas Leaf257 is a sugar generalist able to consume the carbohydrates derived from xylan degradation.
Rhizobium Leaf68 requires PhoB for phyllosphere fitness and is facilitated by Sphingomonas Leaf257 to replete its biotin pool
Next, we enquired fitness traits of Rhizobium Leaf68 and the molecular basis for its enhanced growth upon co-colonization by Sphingomonas Leaf257. Rhizobium Leaf68 (unlike Sphingomonas Leaf257) was amenable to genetic manipulation and we selected five genes for mutagenesis (Fig. 4a ). We chose the gene encoding the PhoB transcriptional regulator (ASF03_21155) and the gene encoding the GlpR repressor (ASF03_11310) to test whether the underlying regulons are important for phyllosphere colonization in general or play a role in the interaction with Sphingomonas Leaf257. We also mutated adh encoding for a predicted alcohol dehydrogenase (ASF03_19755). To investigate the relevance of the glyoxylate cycle in planta we generated a mutant in aceA encoding isocitrate lyase (ASF03_04985). In addition, we tested BioB (ASF03_03440), an enzyme involved in biotin biosynthesis that was induced in planta in mono-colonization but reduced in presence of Sphingomonas Leaf257. With the exception of the phoB mutant the generated Rhizobium Leaf68 mutants grew similarly compared to the wild type during growth on minimal medium with glucose (Supplementary Fig. 6 ). As expected, the isocitrate lyase mutant was unable to grow on acetate (Supplementary Fig. 6g ) confirming the essentiality of aceA for the growth on the substrate 78 , 79 . Next, we tested whether each mutant retained the ability to colonize the plant and achieved comparable population sizes compared to Rhizobium Leaf68 wild-type, which was the case (Fig. 4b ) (Kurskal–Walllis, P -value = ns). Although cell numbers after plant colonization were comparable, strains might still be at a growth disadvantage when competing with the parental strain. To test this, we conducted in planta assays in which we competed the mutants against the wild-type strain using an initial 1:1 ratio in the inoculum. While the isocitrate lyase (ASF03_04985) mutant showed no apparent fitness defect, the alcohol dehydrogenase and GlpR mutants showed a small competitive disadvantage when challenged against the wild type (Fig. 4c ). The bioB and phoB mutants showed a strong decrease in competitiveness against the wild type (Fig. 4c ). Thus, our data underscore that BioB and PhoB, which are known to be involved in environmental adaptation of Salmonella 80 , 81 , are required for fitness during phyllosphere colonization of Rhizobium Leaf68.
Biotin synthase (BioB) catalyzes the last step in biotin biosynthesis. To determine whether the strain has the potential to produce biotin, we searched the genome of Rhizobium Leaf68 using BLAST for additional genes encoding enzymes involved in the biosynthesis of the coenzyme. We found that the bioD gene encoding ATP-dependent dethiobiotin synthase was absent, suggesting that the strain is an auxotroph for biotin - or the precursor dethiobiotin. We confirmed that Rhizobium Leaf68 was unable to grow on minimal medium containing glucose without vitamin addition (Supplementary Fig. 6a–c ). We observed that Leaf68 wild-type was able to grow with biotin and dethiobiotin alike, and that the bioB mutant required biotin for growth (Supplementary Fig. 6d, e ). We then examined whether the bioB mutant can be rescued in competition against the wild type in planta upon external biotin supplementation compared to mock treatment. Indeed, the supplementation with biotin rescued the bioB mutant during competition with the wild type strain (Fig. 5a ), further confirming the relevance of this trait during plant colonization.
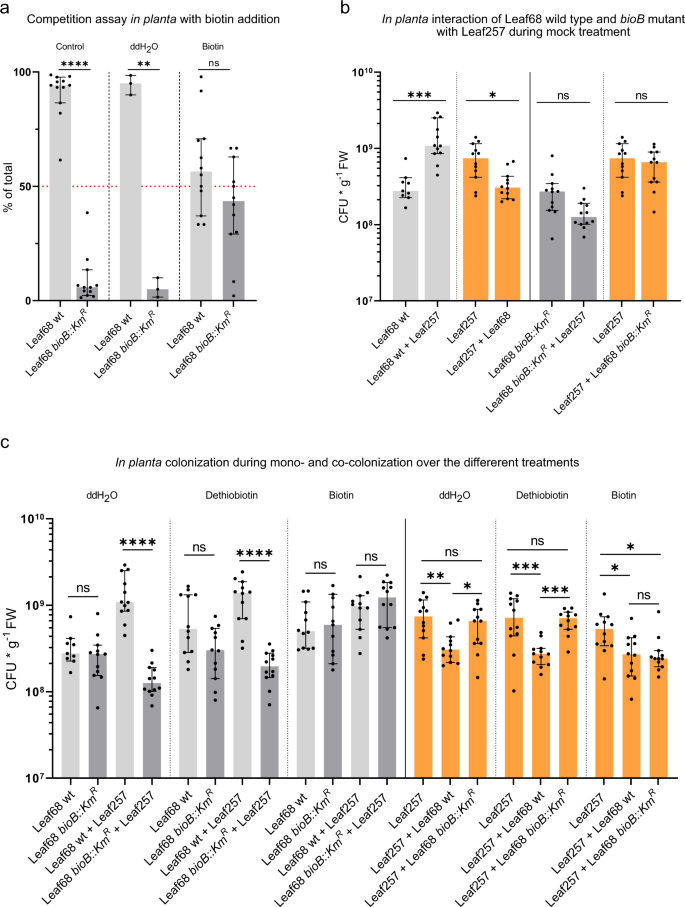
a Competition assay depicting the percentage (%) of total population of Rhizobium Leaf68 wild type (light gray) versus Leaf68 bioB::Km R (dark gray) without treatment (control), mock treatment (ddH 2 O) and biotin treatment post inoculation (p.i) (see methods). For statistical analysis a two-sided paired t-test was performed. A 1:1 mixture is indicated at 50% with the red dotted line. b Depiction of the bacterial colonization (CFUs per gram fresh weight) for Leaf68 wild type (light gray), Leaf68 bioB::Km R (dark gray), Sphingomonas Leaf257 (orange) during mono- and co-colonization and mock (ddH 2 O) treatment of the plant. c Bacterial colonization (CFUs per gram fresh weight) of Leaf68 (light gray), Leaf68 bioB::Km R (dark gray) and Leaf257 (orange) during mono- and co-colonization treatment with either ddH 2 O, dethiobiotin or biotin in planta (see “Methods”). Each data point represents the CFUs of a single plant with a total of 12 plants for this experiment, except for the mock (ddH 2 O) in A due to a contamination. In all graphs, the median and 95% confidence interval are shown. For ( b , c ), statistical analysis was performed using the Kruskal–Wallis test ( P -values: * p -value < 0.05, ** p -value < 0.01, *** p -value < 0.001, and **** p -value < 0.0001). Source data are provided as a Source data file.
The findings described above suggested that dethiobiotin is available in planta and may induce bioB expression to catalyze the last step in biotin biosynthesis. BioB is an instable enzyme that is partially consumed during the reaction 82 , 83 . It was also shown that upon addition of dethiobiotin a significant fraction of the protein is degraded 84 . Therefore, the reduction in BioB in Rhizobium Leaf68 upon co-colonization with the Sphingomonas Leaf257 (Supplementary Data 5 ; Fig. 4a ) may reflect a higher degradation of BioB due to an increase in the dethiobiotin pool available to Rhizobium Leaf68. We thus set out to investigate whether the bioB mutant was impaired in its interaction with Sphingomonas Leaf257. Specifically, we sought to test whether the Rhizobium Leaf68 bioB mutant mitigates the negative interaction towards Sphingomonas Leaf257 and the failure of the latter to benefit the Rhizobium strain. Remarkably, we observed a significant loss of the interaction with the Rhizobium Leaf68 bioB mutant (Fig. 5b ), indicating that an increased dethiobiotin pool provided by Sphingomonas Leaf257 is responsible for the significant increase in the Rhizobium Leaf68 population during co-colonization (Fig. 2b ). In addition, and to complete the analysis of the Rhizobium Leaf68 with respect to its interaction with Sphingomonas Leaf257, we also tested the Rhizobium Leaf68 glpR , phoB , aceA and adh mutants. For the glpR mutant, we observed a significant reduction in population size during co-colonization with Sphingomonas Leaf257 (Supplementary Fig. 7a ). This suggests that GlpR is relevant for the fitness of Rhizobium Leaf68 in competition with the wild type or Sphingomonas Leaf257 in planta (Fig. 4c , Supplementary Fig. 7a ). In contrast, the Rhizobium Leaf68 phoB mutant showed no effect on the interaction between Rhizobium Leaf68 and Sphingomonas Leaf257, suggesting that PhoB is relevant for fitness in planta compared to the wild type but not for the biotic interaction between Leaf68 and Leaf257 (Fig. 4c , Supplementary Fig. 7b ). Neither of the other two Rhizobium mutants, aceA and adh , showed an effect when tested in competition (Supplementary Fig. 7c, d ).
After establishing the dethiobiotin dependence in the interaction of Rhizobium Leaf68 with Sphingomonas Leaf257, we tested whether Sphingomonas Leaf257 was able to complement Rhizobium Leaf68 in vitro in minimal medium (Supplementary Fig. 8c, d ). Indeed, the prototroph Sphingomonas Leaf257 was able to sustain growth of Rhizobium Leaf68 wild type in vitro in the absence of biotin supplementation (Supplementary Fig. 8c, d ). Finally, we tested whether exogenous application of biotin to the phyllosphere was sufficient to recover the interaction of the Rhizobium Leaf68 bioB mutant and Sphingomonas Leaf257. Notably, exogenous application of biotin restored the phenotype of the Rhizobium mutant interacting with Sphingomonas Leaf257 to wild-type levels (Fig. 5c ). In the control treatment (mock) the interaction between Rhizobium Leaf68 wild-type and Sphingomonas Leaf257 remained unchanged (Fig. 5b ). The addition of biotin and its precursor dethiobiotin significantly increased the abundance of Rhizobium Leaf68 wild-type during mono-colonization of the plant (Supplementary Fig. 8a ), while Sphingomonas Leaf257 was unaffected by the treatments (Supplementary Fig. 8b ). As expected, the Leaf68 bioB mutant increased its abundance significantly upon the addition of biotin but remained unchanged during dethiobiotin addition (Supplementary Fig. 8a ).
Taken together, our results indicate that in the presence of Sphingomonas Leaf257, the available dethiobiotin pool on the leaf surface is increased such that it enables Rhizobium Leaf68 to reach a higher colonization capacity. They highlight that the two-sided interaction of Rhizobium Leaf68 and Sphingomonas Leaf257 is triggered by vitamin supplementation in planta and manifests in dynamic niche displacement of the latter toward exploiting an additional substrate, namely xylan as revealed by protein allocation (Fig. 3 ).
Gnotobiotic model systems provide the opportunity to address fundamental ecological questions and dissect the mechanism of bacterial interactions in native-like conditions in a controlled environment 27 , 28 . Omics approaches such as transcriptomics, proteomics and metabolomics offer the possibility to record specific phenotypic states at given time points 25 , 44 , 47 , 49 , 85 , 86 . Here, we characterized the in situ phenotype and interaction of two genome-sequenced bacterial isolates of prevalent bacterial taxa from the At -LSPHERE 29 by mass spectrometry-based proteomics and revealed their ecology and adaptive strategies underlying their coexistence in the phyllosphere.
We first investigated both bacteria colonizing the plant in mono-colonization to establish their general ecological niche occupancy. The proteomics data of the bacteria, Rhizobium Leaf68 and Sphingomonas Leaf257, in planta compared to artificial media as reference indicated adaptive traits that were in line with previous observations in the phyllosphere 25 , 42 , 44 , 47 , 49 , 67 . We observed the expression of species-specific but also shared adaptive strategies towards the phyllosphere environment (Supplementary Fig. 4 ). Overlapping ecological niches makes the pair interesting to study their coexistence with regard to niche separation to evade competition, here referred to as dynamic character displacement. Indeed, both bacteria reacted to each other upon co-colonization, observable in changes in absolute cell abundance and differential expression in the proteome (Fig. 2 ). Niche separation can be observed in Sphingomonas Leaf257, which allocates its proteome towards the degradation of plant-derived xylan and simultaneous utilization of the derived monosaccharides. The niche versatility of Sphingomonas Leaf257 is further underscored by the induction of numerous TonB-dependent receptors and efflux systems also observed in other Sphingomonas under competitive environmental conditions 25 . The tendency of Sphingomonadaceae to utilize a wide plant-associated substrate range might be an evolutionary result of their consistent phyllosphere colonization across different plant species 20 , 26 , 87 , 88 . Especially, the ability to degrade plant-derived xylan revealed in this study (Fig. 3 , Supplementary Fig. 5 ) might be a common resilience trait, enabling Sphingomonas to explore new niches during co-colonization with other phyllosphere microbiota members. While the accessibility of xylan to leaf epiphytes is not fully clear, the leaf contains areas where the cuticle thickness can vary greatly 89 , and accessibility might be higher at veins, leaf margins and the basis of trichomes.
During co-colonization, Rhizobium Leaf68 induces the glyoxylate cycle and allocates proteome resources towards the beta-oxidation of fatty acids. Notably, fatty acids are important structural components and precursors of the plant cuticle making it a potentially resource for nutrients. Overall, the proteomes of both species suggest that both bacteria undergo a niche separation to evade competition. Since changes were inducible and occurred specifically upon coexistence of the two species we refer to dynamic character displacement rather to extend the concept of ecological character displacement that refers to the genetic difference of strains 2 .
We found that facilitation by vitamin sharing contributes to the increase of the Rhizobium Leaf68 cell numbers in the presence of Sphingomonas Leaf257 (Fig. 2b ). We identified the biotin precursor dethiobiotin to be crucial for this population gain: In contrast to wild-type cells, the Rhizobium Leaf68 bioB mutant did not profit from the presence of Sphingomonas Leaf257 nor from dethiobiotin addition but showed an increase in cell numbers after addition of biotin to the phyllosphere (Fig. 5c , Supplementary Fig. 8a ). To understand the nutritional and molecular basis facilitating microbe-microbe interactions, further studies are needed to elucidate the metabolic profile of the phyllosphere regarding free vitamin pool sizes on leaves. However, the finding that the addition of biotin or its precursor dethiobiotin to plants increases the population of Rhizobium Leaf68 indicates that the vitamin is growth limiting, but that some biotin or precursor is available. The microbiota, or as tested here, Sphingomonas Leaf257 helps to supply the biotin precursor dethiobiotin rather than biotin as revealed by the importance of biotin synthase BioB in the interaction and fitness gain of Rhizobium Leaf68. Passive leakage might occur for dethiobiotin as observed for biotin 90 . It is currently unclear why dethiobiotin rather than biotin becomes available. An explanation might be the chemical stability of dethiobiotin compared to biotin. Under mild conditions and in oxygenic environments, such as the phyllosphere, biotin is readily oxidized to biotin sulfoxide, while dethiobiotin is not, due to the absence of sulfur. In line with this conclusion is the observation that Rhizobium Leaf68 induced a biotin sulfoxide reductase during mono-colonization but did not change its expression during co-colonization with Leaf257 (Supplementary Data 2 and 5 ). This suggests that Rhizobium Leaf68 scavenges biotin sulfoxide that could become available in the phyllosphere but relies on the dethiobiotin obtained from Sphingomonas Leaf257.
Metabolic dependencies are thought to strengthen the interaction among bacteria by sharing metabolites, leading to adaptive gene loss 91 , 92 , 93 , 94 , 95 , 96 . Although vitamin sharing by Sphingomonas Leaf257 was shown to be sufficient to explain the growth facilitation of Rhizobium Leaf68, also the release of extracellular enzymes for xylan degradation by Sphingomonas Leaf257 could be used as a shared public good helping other species through active niche construction, making Sphingomonas Leaf257 a potential key stone species. In a previous study Rhizobium Leaf68 was not impacted by the presence or absence of one specific strain in a 62 member community 27 . However, many strains might share vitamins, making the strain the most commonly detected one in a higher complexity microbiota. While Sphingomonas Leaf257 was not tested in this particular complex community, another Sphingomonas strain, Leaf231, was identified as a keystone strain.
In summary, we used MS-based proteomics to characterize metabolic shifts and identified the phenotypic plasticity of interacting species, here referred to as dynamic character displacement. While our data demonstrate that both bacteria respond to each other and become more dissimilar in their respective proteome constitution under mono- versus co-colonization, investigations on the microscale level will remain to be conducted. Specifically, the heterogeneous nature of leaves 22 , 41 , 97 , 98 , 99 will give rise to subpopulations with each potentially expressing different adaptations to their local microenvironment. While our bulk approach captured changes upon co-colonization, further investigation at the single-cell level will be of interest. Here, microscopy-based approaches such as FISH to visualize bacterial populations in situ 40 , 41 , 100 - and fluorescent reporters to monitor expression with spatial resolution 22 , 41 , 97 or par-seqFISH 101 could be applied. Our ability to combine in situ phenotyping and relate it to the genetic potential of phyllosphere colonizing bacteria leads us to speculate that the observed dynamic character displacement, as described here, is an important mechanism during the formation of stable microbial communities, and supports the notion that both genetic diversity and phenotypic diversity promotes coexistence, which remains underexplored 10 , 17 , 18 . This study contributes to our understanding about how bacteria coexist in complex, oligotrophic environments. Such understanding is a prerequisite for the design of stable, biologically relevant synthetic communities. Due to redundant functions of microbiota members, a bottom-up approach of studying pairs of interactions under controlled conditions represents a way to uncover bacteria-bacteria interactions and together with higher complexity synthetic communities and environmental studies will contribute to a better understand the dynamics and structure of biological diverse communities, their assembly and ultimately their astonishing persistence.
Strains and growth conditions
The two bacterial strains Sphingomonas Leaf257 and Rhizobium Leaf68 were selected from the At -LSPHERE collection 29 based on their colonization capacity and previous data collected 27 , 55 . Both strains were grown on R-2A-agar (Sigma-Aldrich, Buchs, Switzerland) supplemented with 0.5% v/v methanol (R2A + M) for 3 days at 22 °C prior usage.
Mutant strain construction in Rhizobium Leaf68
The list of primers used is provided in Supplementary Data 6 . For the generation of deletion mutants of Leaf68 the flanking regions of ~650 bp were amplified using the upstream (HR1) and downstream (HR2) primers for each mutant respectively (Supplementary Data 6 ) followed by digestion with MunI/KpnI (upstream) and KpnI/Nsil (downstream) and inserted into the pREDSIX vector 102 . The construct was cleaved with KpnI between the two flanking regions of the respective gene and a kanamycin-resistances cassette (KmR) was inserted that has been cut from pRGD-KmR 102 with KpnI. The orientation of the cassette was confirmed by PCR with primers binding the flanking region (OR/OF, this study) and kanamycin cassette (Kan-2/-4 described previously by Ledermann et al., 2016 102 ). Both possible insertion directions were selected, and electroporated into Rhizobium Leaf68. For this, 100 μl competent cells were pulsed with 0.5 μg plasmid DNA at 2.2 kV in a 1-mm-gap cuvette (MicroPulser BIO-RAD). After 5 h of recovery in half LB medium at 28 °C cells were plated onto R2A agar plates containing 50 μg/mL Kanamycin. The insertion deletion mutants of all clones were confirmed by PCR using primers inside (IF/IR) and outside (OF/OR) the flanking region, respectively.
Growth assay in micro-titer plates
Overnight pre-cultures were grown in 20 mL minimal medium with 20 mM glucose (MM + G) in in 100 mL baffled shake flasks at 28 °C. For Rhizobium Leaf68 50 µM of biotin, pantothenic acid and niacin were added to complement the auxotrophies. For the validation of the auxotrophies (Supplementary Fig. 5a-e ) another pre-culture in MM + G without vitamins was inoculated. After 2-3 doublings cells were collected. To remove the remaining substrate, cells were spun down at 3220 × g at room-temperature for 10 min. The pellets were washed with two volumes equivalents of 10 mM MgCl 2 and re-suspended in 10 mM MgCl 2 . Next, the OD 600 was adjusted to 0.5 to inoculate the main cultures in 96-well pates (ThermoFischer Scientific Nunclon 96 Flat Bottom Transparent Polystyrol). The plates contained a minimal medium (180 µL) containing the respective carbon source and the three vitamins (biotin, pantothenic acid, niacin) together, in different binary combinations, or each alone. The 96-well plates were inoculated with 20 µL of culture to reach a final OD 600 of 0.05. The OD 600 was measured every 10 min using a Tecan Infinity M200 Pro spectrophotometer (Tecan) with a bandwidth of 9 nm and 25 flashes. The plates were shaken orbital with 1 mm amplitude for 15 s between measurements while incubating at 28 °C. Growth curves were analyzed using the Python-based tools pandas version 1.0.1 ( https://pandas.pydata.org/ ) and matplotlib version 3.1.3 ( https://matplotlib.org/stable/index.html ).
Gnotobiotic growth conditions of Arabidopsis thaliana plants
Arabidopsis thaliana Columbia (Col-0), bak1-5/bkk1-1 103 , jar1-1 104 , rbohd 105 , and npr1-1 106 seeds were surface sterilized according to Schlesier et al. 107 and stratified at 4 °C in the dark for 4 days. Sterile seeds were sown in 24 well cell-culture plates (TPP Techno Plastic Products AG, Switzerland) on full strength Murashige and Skoog (MS) medium (pH5.8, including vitamins) (Sigma-Aldrich, Buchs, Switzerland) supplemented with plant agar (Duchefa, Haarlem, Netherlands) and 3% w/v sucrose. Plants were grown for 1 week under long-day growth conditions (16-h-photoperiod) before switching to short-day conditions (9-h photoperiod) for the rest of the experiment. The temperature in the chamber (CU-41L4, Percival Perry, USA) was set to 24 °C during light and 22 °C during dark cycles at a constant relative humidity of 65% rh.
Plant inoculation
Three days prior to inoculation, the bacterial strains or mutants were pre-grown on R2A + M agar plates. For inoculation after 10 days of plant growth a sterile 1 µL plastic loop was used to collect cell material from each strain and cells were re-suspended in 1 mL of 10 mM MgCl 2 . The tubes containing the bacteria were vortexed and optical density (OD 600 ) was adjusted to a final concentration of 0.02 per strain. Notably, the mixture of both had a final OD 600 of 0.04 with both strains having a concentration of 0.02. The gnotobiotic 10-day-old plants were inoculated with 10 µL of bacterial solution by pipetting. At this growth stage, the plant has four leaves. Each leaf was treated with a drop containing 2 µL, an additional drop of 2 µL was distributed in the middle of the plant. Axenic control plants were mock-inoculated with 10 µL of 10 mM MgCl 2 . Plants were further grown for 11 days (total plant age at harvest time point: 21 days).
Chemical complementation with biotin in planta
Prior the plant treatment a biotin stock of 1 mM in ddH 2 O was prepared, sterile filtrated and further diluted to 10 µM in ddH 2 O. Plants were treated at two time points with the first 30 min after inoculation and the second 3 days later. For treatment either 10 µL 10 µM biotin (treatment) or 10 µL ddH 2 O (mock) or 10 mM MgCl 2 (mock) were carefully pipetted to the phyllosphere without touching the leaves.
Bacterial strains Rhizobium Leaf68 and Sphingomonas Leaf257 expressing a fluorescent protein (mCherry) (Lab stock) were grown on R2A + M and prepared for plant inoculation as described above. Prior to inoculation, strain Leaf68 was fluorescently labeled using Alexa Fluor™ 488 NHS Ester (ThermoFischer Scientific) following the manufacturer’s instructions. The strains were adjusted to a final OD 600 of 1 (Supplementary Fig. 1a ) or 0.1 (Supplementary Fig. 1b-d ) and mixed prior to inoculation. Inoculated plants were grown for 24, 48, and 72 hours in the plant growth chamber at the short-day settings described above. Single leaves were detached and directly mounted on a standard cover slip. Images were acquired using a standard fluorescence microscopy setup (Zeiss Axio Observer Z1, Hamamatsu Orca-ER) using a ×40 objective. Fiji software 108 (ImageJ, v.2.0.0) was used for linear intensity adjustments and to insert scale bars.
Enumeration of bacterial growth in the phyllosphere
To determine bacterial colonization after 11 days post-inoculation colony-forming units (CFUs) were measured using a leaf-wash protocol according to Vogel et al. 51 . For the leaf washing, the above-ground parts were separated from the roots using a sterile scalpel. The resulting phyllosphere of single plants was transferred into phosphate buffer (pH 7, 100 mM) containing surfactant (Silwet L-77, 0.2% v/v) (Leu + Gygax AG, Birmensdorf, Switzerland). Bacteria were washed off as described previously 51 . Briefly, tubes were shaken for 2 × 7.5 min at 25 Hz in a Qiagen Tissue Lyser II (Qiagen) and sonicated for 5 min in an ultrasonic cleaning bath (Branson Ultrasonics). The leaf wash was vortexed and plated on R2A + M -agar using a tenfold dilution series (10 0 -10 −8 ). After 2-3 days of incubation at 22 °C CFUs per g plant fresh weight were determined. On plates, both strains could be distinguished by eye according to the color of the colonies, with Sphingomonas Leaf257 forming yellow and Rhizobium Leaf68 forming white colonies. Bacterial abundance data were analyzed using Prism 9.2 (GraphPad).
Competition Leaf68 wild type and mutants
To determine the percentage of total cells we inoculated and enumerated bacterial growth as described above and plated the leaf was on R2A + M to recover the total CFUs and R2A + M containing 50 μg/mL Kanamycin to recover the mutant CFUs. For each comparison, we inferred the Leaf68 wild type CFUs by subtracting the total CFUs from the mutant CFUs and plotted the percentage of total for each wild type and mutant.

Harvest of phyllosphere bacteria for MS-based proteomics
To recover bacteria from the phyllosphere of A. thaliana , the phyllosphere of 10 plants was separated from the rhizosphere using flame sterilized scalpels and forceps and transferred into 50 mL Falcon tubes containing 25 mL ice-cold TE-P buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5). The epiphytic bacteria were washed off leaves using a previously established protocol that minimizes plant contamination 44 . Briefly, it consists of alternating cycles of vortexing and sonication as well as a soft filtration step through a nylon mesh to remove large plant particles, followed by the concentration of the bacterial fraction. For each biological replicate the enriched cell pellet corresponding to 20 plants was pooled, snap frozen and stored at −80 °C until further processing.
Proteomics of bacteria recovered from the phyllosphere
In total, four biological independent replicates were generated for MS-based proteomics analysis. As a reference to the in planta conditions, both Rhizobium Leaf68 and Sphingomonas Leaf257 were each grown on R2A + M-agar (see above) in the plant growth chamber at the settings described above. Bacterial cell pellets were dissolved and lysed using a lysis buffer containing 100 mM ammonium bicarbonate, 8 M urea and 1× cOmplete EDTA-free protease inhibitor cocktail (Sigma-Aldrich, Buchs, Switzerland) and indirect sonication (3 × 1 min, 100% amplitude, 0.8 cycle time) in a VialTweeter (HIFU, Hielscher, Teltow, Germany) 45 , 109 . Insoluble parts were removed by centrifugation at 13,000 g for 15 min at 4 °C. Protein concentration of supernatant was determined using the Pierce BCA assay kit (Thermo Fischer Scientific, Reinach, Switzerland) according to the manufacturer’s instructions. Protein disulfide bonds were reduced and cysteine residues were alkylated as described previously 45 using 5 mM tris(2-carboxylethyl)phosphine (TCEP, Sigma-Aldrich, Buchs, Switzerland) and 10 mM iodoacetamide respectively (IAA, Sigma-Aldrich, Buchs, Switzerland). Prior to protein digestion, samples were diluted 1 to 5 with freshly prepared 50 mM ammonium bicarbonate buffer to reduce the urea concentration below 2 M. Sequencing grade modified trypsin (Promega AG, Dübendorf, Switzerland) was added at an trypsin to protein ratio of 1:50 and protein digestion was carried out overnight at 37 °C with shaking at 300 rpm. After incubation, trypsin was inactivated using heat incubation in a tabletop shaker at 95 °C for 5 min followed by acidification through addition of formic acid to an approximate final concentration of 1%. Insoluble parts were removed by centrifugation at 20,000 g for 10 min and the supernatant was desalted using Sep-Pak Vac C18 reversed phase columns (Waters Corporation, Baden-Dättswil, Switzerland) as previously described 109 and dried under vacuum. The samples were re-solubilized in 3% acetonitrile (ACN) and 0.1% formic acid (FA) to a final concentration of 0.1-1.0 mg mL −1 .
MS analysis
Mass spectrometry analyses were performed on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fischer Scientific) equipped with a digital PicoView source (New Objective, Littleton, USA) and coupled to an M-Class ultraperformance liquid chromatography (UPLC) system using an ACN/water solvent system containing two channels with 0.1% FA (v/v) in water for channel A and 0.1% FA (v/v), 99.9% ACN (v/v) for channel B. For chromatographic separation 2 µL peptide sample (at a concentration of 0.5 µg µL −1 ) was loaded on a nanoEase M/Z Symmetry C18 trap column (100 A, 5 µm; 180 µm × 20 mm, Waters) followed by a nanoEase M/Z HSS T3 column (100 A, 1.8 µm; 75 µm × 250 mm, Waters). Peptide samples were separated at a flow rate of 300 nL min −1 using the following gradient: 2% to 5% B in 2 min, 25% B in 93 min, 35% B in 10 min, and 95% B in 10 min. The MS was operated in data-dependent acquisition and top-speed mode with a maximum cycle time set to 3 s. Full-scan MS spectra were acquired in the Orbitrap analyzer with a mass range of 300-1500 m/z and a resolution of 120k with an automated gain control (AGC) target value of 5 × 10 5 . Precursor ions were isolated with a window of 1.6 m/z and fragmented using higher energy collisional dissociation (HCD) with a normalized collision energy of 30%. For fragmentation only precursor ions with charge states from +2 to +7 and a signal intensity of at least 5 × 10 3 . Fragment ion spectra (MS/MS) were acquired in the Ion trap operated in rapid scan mode with an AGC value of 8000 using a maximum injection time of 300 ms. The dynamic exclusion was set to 25 s. Sample measurements were acquired using internal lock mass calibration on m/z 371.10124 and 445.12003.
Database search and quantitative analysis
Label-free precursor (MS1) intensity-based quantification was performed using Progenesis QI for proteomics (Nonlinear Dynamics, v.4.0) as previously described 45 , 110 . Briefly, for all samples the automatic alignment was reviewed and manually adjusted before normalization. From each Progenesis peptide ion (default sensitivity in peak picking) a maximum of the top five tandem mass spectra were exported as mascot generic file (*.mgf) using the charge deconvolution and deisotoping option and a maximum number of 200 peaks per MS/MS. The mascot generic files were searched using the Mascot Server (Matrix Science, v.2.6.2) against a decoyed and reversed protein sequence database containing the 4341 annotated proteins of Rhizobium Leaf68 (NCBI Taxon ID: 1736231 ) and the 4024 annotated proteins of Sphingomonas Leaf257 (NCBI Taxon ID: 1736309 ) concatenated with the Arabidopsis Information Resource (TAIR) database (release TAIR10) and 260 known mass spectrometry contaminants. Parameters for precursor ion tolerance and fragment ion tolerance were set to ± 10 ppm and ± 0.5 Da, respectively. The search parameters were as followed: trypsin digestion (two missed cleavages allowed), as fixed modification of the carbamidomethylation of cysteine and as variable modification of the oxidation of methionine, carbamylation of the N-terminus and lysine. The mascot search was imported into Scaffold (Proteome Software, v.4.8.9) using 5% peptide and 10% protein false discovery rate (FDR) as thresholds. The scaffold spectrum reports were imported into Progenesis QI. Normalization was performed on all precursor ions of the corresponding strain. Notably, in the mixture and artificial mixture conditions normalization was performed on all precursor ions from Leaf68 or Leaf257 respectively, i.e. to quantify the Leaf68 proteome in the mixture we only considered precursor ions specific for proteins from Leaf68 for normalization and vice versa. For protein quantification, the three most abundant peptide ions (Hi-3 approach) were used for protein quantification. Only proteins with two or more unique peptides detected were considered for quantification. For statistical testing, one-way ANOVA was applied and the resulting P -values were corrected using the Benjamini-Hochberg procedure directly in Progenesis QI resulting in q-values. If not indicated otherwise the general cutoffs for significantly regulated proteins were q-value < 0.05 and Log 2 fold-change (FC) >1.5 or < -1.5.
Comparison of orthologues protein groups in Leaf257 and Leaf68
The annotated genomes for both strains were obtained from the PATRIC database 111 . Subsequently, the genomes were annotated with orthologous groups (OGs) using eggNog v.4.5 112 . In order to identify shared OGs, the proteomics data of both bacteria colonizing the plant alone during mono-association was compared by identifying shared regulated OGs. For the OG comparison only proteins/OGs detected with at least 3 unique peptides and a q-value < 0.05 and Log 2 fold-change >1.5 or < −1.5 in both bacteria were considered.
Experimental investigation of sugar preferences in Sphingomonas Leaf257 using HPLC
The sugar utilization characterization of Leaf257 was performed in a volume of 20 mL in 100 mL baffled shake flasks at 22 °C and 160 rpm. in a Minitron Incubator (Infors HT). The minimal media contained either of the three sugars (10 mM), glucose, xylose and galactose (Sigma-Aldrich, Buchs, Switzerland) alone as sole carbon source or binary combinations of each. To estimate bacterial growth OD 600 of all conditions was measured over eight-time points and simultaneously 1 mL of the culture was used for further analysis of the supernatant. Supernatant analysis for each sample was performed by high-performance liquid chromatography using an Ultimate 3000 UHPLC device (Thermo Fischer Scientific) equipped with a Rezex ROA Organic Acid H + column (7.8 × 300 mm; Phenomenex) as analytical column and a RI-detector (RefractorMax521). The mobile phase was 2.5 mM H 2 SO 4 at a flow rate of 0.6 mL min −1 and the conditions were isocratic. The sample injection volume was 10 µL and the refractive index (RI) was monitored for metabolite detection.
Purification and identification of xylan-degrading enzymes in Sphingnomonas Leaf257
To test for the secretion of xylan-degrading enzymes, Leaf257 was grown in minimal medium containing 10 mM xylan (Roth AG, Arlesheim, Germany). Supernatant samples were taken from the liquid cultures, centrifuged and sterile filtrated to remove bacterial remains. The supernatant was concentrated in a centrifugal filter with a molecular weight cutoff of 10 kDa (Amicon Ultra, Merck Millipore) and analyzed using SDS-PAGE analysis revealing a protein band specific for growth on minimal medium with xylan (Fig. 3c ), which was identified by in-gel digestion and LC-MS (Fig. 3c, d ).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data obtained from the MS-based proteomics approach has been deposited to the ProteomeXchange Consortium ( http://proteomecentral.proteomexchange.org ) via the PRIDE partner repository 113 with the dataset identifier PXD026619 and 10.6019/PXD026619 [ https://www.ebi.ac.uk/pride/archive/projects/PXD026619 ]. A list of all identified protein is available from the PRIDE dataset. The data generated in this work are provided in the Supplementary Information and Source Data files.
Brown, W. L. Jr. & Wilson, E. O. Character displacement. Syst. Biol. 5 , 49–64 (1956).
Google Scholar
Stuart, Y. E. & Losos, J. B. Ecological character displacement: glass half full or half empty? Trends Ecol. Evol. 28 , 402–408 (2013).
Article PubMed Google Scholar
Schluter, D. & McPhail, J. D. Ecological character displacement and speciation in sticklebacks. Am. Nat. 140 , 85–108 (1992).
Article CAS PubMed Google Scholar
Tilman, D., May, R. M., Lehman, C. L. & Nowak, M. A. Habitat destruction and the extinction debt. Nature 371 , 65–66 (1994).
Article ADS Google Scholar
Ghoul, M. & Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 24 , 833–845 (2016).
Pfennig, D. W., Rice, A. M. & Martin, R. A. Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology 87 , 769–779 (2006).
Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18 , 119–125 (2003).
Article Google Scholar
Day, T. & Young, K. A. Competitive and facilitative evolutionary diversification. Bioscience 54 , 101–109 (2004).
Stachowicz, J. J. Mutualism, facilitation, and the structure of ecological communities. Bioscience 51 , 235–246 (2001).
Stuart, Y. E., Inkpen, S. A., Hopkins, R. & Bolnick, D. I. Character displacement is a pattern: so, what causes it? Biol . J. Linn. Soc. 121 , 711–715 (2017).
Brockhurst, M. A., Hochberg, M. E., Bell, T. & Buckling, A. Character displacement promotes cooperation in bacterial biofilms. Curr. Biol. 16 , 2030–2034 (2006).
Ellis, C. N., Traverse, C. C., Mayo-Smith, L., Buskirk, S. W. & Cooper, V. S. Character displacement and the evolution of niche complementarity in a model biofilm community. Evolution 69 , 283–293 (2015).
Article PubMed PubMed Central Google Scholar
Rainey, P. B., Buckling, A., Kassen, R. & Travisano, M. The emergence and maintenance of diversity: insights from experimental bacterial populations. Trends Ecol. Evol. 15 , 243–247 (2000).
Turner, P. E., Souza, V. & Lenski, R. E. Tests of ecological mechanisms promoting the stable coexistence of two bacterial genotypes. Ecology 77 , 2119–2129 (1996).
Xavier, J. B. & Foster, K. R. Cooperation and conflict in microbial biofilms. Proc. Natl. Acad. Sci. USA 104 , 876–881 (2007).
Article ADS CAS PubMed PubMed Central Google Scholar
Westeberhard, M. J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Evol. Syst. 20 , 249–278 (1989).
Turcotte, M. M. & Levine, J. M. Phenotypic plasticity and species coexistence. Trends Ecol. Evol. 31 , 803–813 (2016).
Pfennig, D. W. & Pfennig, K. S. Development and evolution of character displacement. Ann NY Acad Sci . 1256 , 89–107 (2012).
Article ADS PubMed MATH Google Scholar
Finkel, O. M., Castrillo, G., Herrera Paredes, S., Salas Gonzalez, I. & Dangl, J. L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 38 , 155–163 (2017).
Muller, D. B., Vogel, C., Bai, Y. & Vorholt, J. A. The plant microbiota: systems-level insights and perspectives. Annu. Rev. Genet. 50 , 211–234 (2016).
Schlaeppi, K. & Bulgarelli, D. The plant microbiome at work. Mol. Plant Microbe Interact. 28 , 212–217 (2015).
Leveau, J. H. & Lindow, S. E. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98 , 3446–3453 (2001).
Lindow, S. E. & Leveau, J. H. Phyllosphere microbiology. Curr. Opin. Biotechnol. 13 , 238–243 (2002).
Meyer, K. M. & Leveau, J. H. Microbiology of the phyllosphere: a playground for testing ecological concepts. Oecologia 168 , 621–629 (2012).
Article ADS PubMed Google Scholar
Delmotte, N. et al. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 106 , 16428–16433 (2009).
Vorholt, J. A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10 , 828–840 (2012).
Carlstrom, C. I. et al. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 3 , 1445–1454 (2019).
Vorholt, J. A., Vogel, C., Carlstrom, C. I. & Müller, D. B. Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe. 22 , 142–155 (2017).
Bai, Y. et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528 , 364–369 (2015).
Article ADS CAS PubMed Google Scholar
Bodenhausen, N., Horton, M. W. & Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana . PLoS ONE 8 , e56329 (2013).
Horton, M. W. et al. Genome-wide association study of Arabidopsis thaliana leaf microbial community. Nat. Commun. 5 , 5320 (2014).
Roman-Reyna, V. et al. Characterization of the leaf microbiome from whole-genome sequencing data of the 3000 rice genomes project. Rice (NY) 13 , 72 (2020).
Zarraonaindia, I. et al. The soil microbiome influences grapevine-associated microbiota. mBio . 6 , e02527–14 (2015).
Article PubMed PubMed Central CAS Google Scholar
Laforest-Lapointe, I. & Whitaker, B. K. Decrypting the phyllosphere microbiota: progress and challenges. Am. J. Bot. 106 , 171–173 (2019).
PubMed Google Scholar
Baldotto, L. E. B. & Olivares, F. L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 54 , 918–931 (2008).
Lindow, S. E. & Brandl, M. T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69 , 1875–1883 (2003).
Monier, J. M. & Lindow, S. E. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA 100 , 15977–15982 (2003).
Monier, J. M. & Lindow, S. E. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70 , 346–355 (2004).
Morris, C. E., Monier, J. M. & Jacques, M. A. A technique To quantify the population size and composition of the biofilm component in communities of bacteria in the phyllosphere. Appl. Environ. Microbiol. 64 , 4789–4795 (1998).
Remus-Emsermann, M. N. P. et al. Spatial distribution analyses of natural phyllosphere-colonizing bacteria on Arabidopsis thaliana revealed by fluorescence in situ hybridization. Environ. Microbiol. 16 , 2329–2340 (2014).
Remus-Emsermann, M. N. P. & Schlechter, R. O. Phyllosphere microbiology: at the interface between microbial individuals and the plant host. New Phytol. 218 , 1327–1333 (2018).
Gourion, B., Rossignol, M. & Vorholt, J. A. A proteomic study of Methylobacterium extorquens reveals a response regulator essential for epiphytic growth. Proc. Natl. Acad. Sci. USA 103 , 13186–13191 (2006).
Jacobs, J. L., Carroll, T. L. & Sundin, G. W. The role of pigmentation, ultraviolet radiation tolerance, and leaf colonization strategies in the epiphytic survival of phyllosphere bacteria. Microb. Ecol. 49 , 104–113 (2005).
Müller, D. B., Schubert, O. T., Rost, H., Aebersold, R. & Vorholt, J. A. Systems-level proteomics of two ubiquitous leaf commensals reveals complementary adaptive traits for phyllosphere colonization. Mol. Cell. Proteom. 15 , 3256–3269 (2016).
Article CAS Google Scholar
Ochsner, A. M. et al. Use of rare-earth elements in the phyllosphere colonizer Methylobacterium extorquens PA1. Mol. Microbiol. 111 , 1152–1166 (2019).
Article CAS PubMed PubMed Central Google Scholar
Helmann, T. C., Deutschbauer, A. M. & Lindow, S. E. Genome-wide identification of Pseudomonas syringae genes required for fitness during colonization of the leaf surface and apoplast. Proc. Natl. Acad. Sci. USA 116 , 18900–18910 (2019).
Nobori, T. et al. Transcriptome landscape of a bacterial pathogen under plant immunity. Proc. Natl. Acad. Sci. USA 115 , E3055–E3064 (2018).
Pulawska, J. et al. Transcriptome analysis of Xanthomonas fragariae in strawberry leaves. Sci. Rep . 10 , 20582 (2020).
Knief, C. et al. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 6 , 1378–1390 (2012).
Innerebner, G., Knief, C. & Vorholt, J. A. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 77 , 3202–3210 (2011).
Vogel, C., Innerebner, G., Zingg, J., Guder, J. & Vorholt, J. A. Forward genetic in planta screen for identification of plant-protective traits of Sphingomonas sp Strain Fr1 against Pseudomonas syringae DC3000. Appl. Environ. Microbiol. 78 , 5529–5535 (2012).
Ryffel, F. et al. Metabolic footprint of epiphytic bacteria on Arabidopsis thaliana leaves. ISME J. 10 , 632–643 (2016).
Vogel, C. M., Potthoff, D. B., Schafer, M., Barandun, N. & Vorholt, J. A. Protective role of the Arabidopsis leaf microbiota against a bacterial pathogen. Nat. Microbiol. 6 , 1537–1548 (2021).
Pfeilmeier, S. et al. The plant NADPH oxidase RBOHD is required for microbiota homeostasis in leaves. Nat. Microbiol. 6 , 852–864 (2021).
Maier, B. A. et al. A general non-self response as part of plant immunity. Nat. Plants 7 , 696–705 (2021).
Breton, C., Snajdrova, L., Jeanneau, C., Koca, J. & Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 16 , 29r–37r (2006).
Tao, F., Swarup, S. & Zhang, L. H. Quorum sensing modulation of a putative glycosyltransferase gene cluster essential for Xanthomonas campestris biofilm formation. Environ. Microbiol. 12 , 3159–3170 (2010).
Zhou, M. X., Zhu, F., Dong, S. L., Pritchard, D. G. & Wu, H. A novel glucosyltransferase is required for glycosylation of a serine-rich adhesin and biofilm formation by Streptococcus parasanguinis . J. Biol. Chem. 285 , 12140–12148 (2010).
Becker, A. et al. Regulation of succinoglycan and galactoglucan biosynthesis in Sinorhizobium meliloti . J. Mol. Microbiol. Biotechnol. 4 , 187–190 (2002).
CAS PubMed Google Scholar
Halder, U., Banerjee, A. & Bandopadhyay, R. Structural and functional properties, biosynthesis, and patenting trends of bacterial succinoglycan: a review. Indian J. Microbiol. 57 , 278–284 (2017).
Niehaus, K. & Becker, A. The role of microbial surface polysaccharides in the Rhizobium-legume interaction. Sub-Cell. Biochem. 29 , 73–116 (1998).
Ellis, H. R. Mechanism for sulfur acquisition by the alkanesulfonate monooxygenase system. Bioorg. Chem. 39 , 178–184 (2011).
Marco, M. L., Legac, J. & Lindow, S. E. Pseudomonas syringae genes induced during colonization of leaf surfaces. Environ. Microbiol. 7 , 1379–1391 (2005).
Yu, X. L. et al. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc. Natl. Acad. Sci. USA 110 , E425–E434 (2013).
Cai, S. J. & Inouye, M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli . J. Biol. Chem. 277 , 24155–24161 (2002).
Freeman, B. C. et al. Physiological and transcriptional responses to osmotic stress of two Pseudomonas syringae strains that differ in epiphytic fitness and osmotolerance. J. Bacteriol. 195 , 4742–4752 (2013).
Scheublin, T. R. et al. Transcriptional profiling of gram-positive Arthrobacter in the phyllosphere: induction of pollutant degradation genes by natural plant phenolic compounds. Environ. Microbiol. 16 , 2212–2225 (2014).
Felix, G., Duran, J. D., Volko, S. & Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18 , 265–276 (1999).
Macho, A. P. & Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 54 , 263–272 (2014).
Hopsu-Havu, V. K. & Glenner, G. G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie 7 , 197–201 (1966).
Kavi Kishor, P. B., Hima Kumari, P., Sunita, M. S. & Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 6 , 544 (2015).
Chipperfield, J. R. & Ratledge, C. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals 13 , 165–168 (2000).
Visca, P., Ciervo, A., Sanfilippo, V. & Orsi, N. Iron-regulated salicylate synthesis by Pseudomonas Spp. J. Gen. Microbiol. 139 , 1995–2001 (1993).
Seifert, G. J., Barber, C., Wells, B., Dolan, L. & Roberts, K. Galactose biosynthesis in Arabidopsis : genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Curr. Biol. 12 , 1840–1845 (2002).
Zablackis, E., Huang, J., Muller, B., Darvill, A. G. & Albersheim, P. Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107 , 1129–1138 (1995).
Santos-Beneit, F. The Pho regulon: a huge regulatory network in bacteria. Front. Microbiol. 6 , 402 (2015).
Mortimer, J. C. et al. An unusual xylan in Arabidopsis primary cell walls is synthesised by GUX3, IRX9L, IRX10L and IRX14. Plant J. 83 , 413–426 (2015).
Honer Zu Bentrup, K., Miczak, A., Swenson, D. L. & Russell, D. G. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis . J. Bacteriol. 181 , 7161–7167 (1999).
Reinscheid, D. J., Eikmanns, B. J. & Sahm, H. Characterization of the isocitrate lyase gene from Corynebacterium glutamicum and biochemical analysis of the enzyme. J. Bacteriol. 176 , 3474–3483 (1994).
Groisman, E. A., Chiao, E., Lipps, C. J. & Heffron, F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. USA 86 , 7077–7081 (1989).
Lamarche, M. G., Wanner, B. L., Crepin, S. & Harel, J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32 , 461–473 (2008).
Jameson, G. N., Cosper, M. M., Hernandez, H. L., Johnson, M. K. & Huynh, B. H. Role of the [2Fe-2S] cluster in recombinant Escherichia coli biotin synthase. Biochemistry 43 , 2022–2031 (2004).
Sirithanakorn, C. & Cronan, J. E. Biotin, a universal and essential cofactor: synthesis, ligation and regulation. FEMS Microbiol. Rev. 45 , fuab003 (2021).
Choi-Rhee, E. & Cronan, J. E. Biotin synthase is catalytic in vivo, but catalysis engenders destruction of the protein. Chem. Biol. 12 , 461–468 (2005).
Wilmes, P. et al. Community proteogenomics highlights microbial strain-variant protein expression within activated sludge performing enhanced biological phosphorus removal. ISME J. 2 , 853–864 (2008).
Beier, S., Rivers, A. R., Moran, M. A. & Obernosterer, I. Phenotypic plasticity in heterotrophic marine microbial communities in continuous cultures. ISME J. 9 , 1141–1151 (2015).
Kim, H. et al. High population of Sphingomonas species on plant surface. J. Appl. Microbiol. 85 , 731–736 (1998).
Singh, P., Santoni, S., Weber, A., This, P. & Peros, J. P. Understanding the phyllosphere microbiome assemblage in grape species ( Vitaceae ) with amplicon sequence data structures. Sci. Rep . 9 , 14294 (2019).
Kosma, D. K. et al. The impact of water deficiency on leaf cuticle lipids of Arabidopsis . Plant Physiol. 151 , 1918–1929 (2009).
Piffeteau, A. & Gaudry, M. Biotin uptake: influx, efflux and countertransport in Escherichia coli K12. Biochim. Biophys. Acta 816 , 77–82 (1985).
D’Souza, G. et al. Less is more: selective advantages can explain the prevalent loss of biosynthetic genes in bacteria. Evolution 68 , 2559–2570 (2014).
Article PubMed CAS Google Scholar
Hassani, M. A., Duran, P. & Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 6 , 58 (2018).
Mas, A., Jamshidi, S., Lagadeuc, Y., Eveillard, D. & Vandenkoornhuyse, P. Beyond the black queen hypothesis. ISME J. 10 , 2085–2091 (2016).
Morris, B. E., Henneberger, R., Huber, H. & Moissl-Eichinger, C. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37 , 384–406 (2013).
Pacheco, A. R., Moel, M. & Segre, D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun. 10 , 103 (2019).
Article ADS PubMed PubMed Central CAS Google Scholar
Pande, S. et al. Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J. 8 , 953–962 (2014).
Joyner, D. C. & Lindow, S. E. Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. Microbiol. 146 , 2435–2445 (2000).
Remus-Emsermann, M. N., de Oliveira, S., Schreiber, L. & Leveau, J. H. Quantification of lateral heterogeneity in carbohydrate permeability of isolated plant leaf cuticles. Front. Microbiol. 2 , 197 (2011).
PubMed PubMed Central Google Scholar
Remus-Emsermann, M. N. P., Tecon, R., Kowalchuk, G. A. & Leveau, J. H. J. Variation in local carrying capacity and the individual fate of bacterial colonizers in the phyllosphere. ISME J. 6 , 756–765 (2012).
Peredo, E. L. & Simmons, S. L. Leaf-FISH: microscale imaging of bacterial taxa on phyllosphere. Front. Microbiol . 8 , 2669 (2018).
Dar, D., Dar, N., Cai, L. & Newman, D. K. Spatial transcriptomics of planktonic and sessile bacterial populations at single-cell resolution. Science 373 , eabi4882 (2021).
Ledermann, R., Strebel, S., Kampik, C. & Fischer, H. M. Versatile vectors for efficient mutagenesis of Bradyrhizobium diazoefficiens and other alphaproteobacteria. Appl. Environ. Microbiol. 82 , 2791–2799 (2016).
Roux, M. et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23 , 2440–2455 (2011).
Staswick, P. E., Tiryaki, I. & Rowe, M. L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14 , 1405–1415 (2002).
Torres, M. A., Dangl, J. L. & Jones, J. D. Arabidopsis gp91 phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99 , 517–522 (2002).
Cao, H., Glazebrook, J., Clarke, J. D., Volko, S. & Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 , 57–63 (1997).
Schlesier, B., Breton, F. & Mock, H. P. A hydroponic culture system for growing Arabidopsis thaliana plantlets under sterile conditions. Plant Mol. Biol. Rep. 21 , 449–456 (2003).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 , 676–682 (2012).
Hemmerle, L., Ochsner, A. M., Vonderach, T., Hattendorf, B. & Vorholt, J. A. Mass spectrometry-based approaches to study lanthanides and lanthanide-dependent proteins in the phyllosphere. Methods Enzymol. 650 , 215–236 (2021).
Uhrig, R. G. et al. Diurnal dynamics of the Arabidopsis rosette proteome and phosphoproteome. Plant Cell Environ. 44 , 821–841 (2021).
Davis, J. J. et al. The PATRIC bioinformatics resource center: expanding data and analysis capabilities. Nucleic Acids Res. 48 , D606–D612 (2020).
Huerta-Cepas, J. et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 44 , D286–D293 (2016).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47 , D442–D450 (2019).
Download references
Acknowledgements
We thank Paola Picotti, Sebastian Pfeilmeier and Christopher Field for helpful discussions. We thank Bernd Roschitzki and Jonas Grossmann from the Functional Genomics Center Zurich (FGCZ) for support with the LC-MS/MS setup. We thank Tim Keys and Corina Mathew for support with the HPLC setup and Christine Vogel and Raphael Ledermann for input into generating the mutants of Rhizobium Leaf68. This work was supported by an ERC Advanced grant (PhyMo, no. 668991), a grant from the Swiss National Science Foundation (310030B_201265) and as part of NCCR Microbiomes, a National Centre of Competence in Research, funded by the Swiss National Science Foundation (no. 51NF40_180575).
Author information
Authors and affiliations.
Institute of Microbiology, ETH Zurich, Zurich, Switzerland
Lucas Hemmerle, Benjamin A. Maier, Miriam Bortfeld-Miller, Birgitta Ryback, Christoph G. Gäbelein & Julia A. Vorholt
Department of Environmental Systems Science, ETH Zurich, Zurich, Switzerland
Martin Ackermann
Department of Environmental Microbiology, Eawag, Dubendorf, Switzerland
You can also search for this author in PubMed Google Scholar
Contributions
L.H., M.A. and J.A.V. designed the study; L.H. led the experimental work; B.A.M. and M.B.-M. contributed to performing the plant experiments; M.B.-M. helped with the mutant generation in Rhizobium Leaf68; B.R. contributed to the characterization of the auxotrophies in Rhizobium Leaf68; C.G.G. performed the microscopy analysis; L.H. performed the bioinformatics analysis; L.H. and J.A.V. wrote the manuscript with input from all authors.
Corresponding author
Correspondence to Julia A. Vorholt .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Sara Mitri and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary information, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, supplementary data 5, supplementary data 6, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hemmerle, L., Maier, B.A., Bortfeld-Miller, M. et al. Dynamic character displacement among a pair of bacterial phyllosphere commensals in situ. Nat Commun 13 , 2836 (2022). https://doi.org/10.1038/s41467-022-30469-3
Download citation
Received : 28 June 2021
Accepted : 03 May 2022
Published : 20 May 2022
DOI : https://doi.org/10.1038/s41467-022-30469-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Assessing microbiome population dynamics using wild-type isogenic standardized hybrid (wish)-tags.
- Benjamin B. J. Daniel
- Yves Steiger
- Julia A. Vorholt
Nature Microbiology (2024)
Leaf microbiome dysbiosis triggered by T2SS-dependent enzyme secretion from opportunistic Xanthomonas pathogens
- Sebastian Pfeilmeier
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- Research article
- Open access
- Published: 30 January 2008
Experimental demonstration of ecological character displacement
- Jabus G Tyerman 1 ,
- Melanie Bertrand 1 ,
- Christine C Spencer 2 &
- Michael Doebeli 1 , 3
BMC Evolutionary Biology volume 8 , Article number: 34 ( 2008 ) Cite this article
6240 Accesses
34 Citations
Metrics details
The evolutionary consequences of competition are of great interest to researchers studying sympatric speciation, adaptive radiation, species coexistence and ecological assembly. Competition's role in driving evolutionary change in phenotypic distributions, and thus causing ecological character displacement, has been inferred from biogeographical data and measurements of divergent selection on a focal species in the presence of competitors. However, direct experimental demonstrations of character displacement due to competition are rare.
We demonstrate a causal role for competition in ecological character displacement. Using populations of the bacterium Escherichia coli that have adaptively diversified into ecotypes exploiting different carbon resources, we show that when interspecific competition is relaxed, phenotypic distributions converge. When we reinstate competition, phenotypic distributions diverge.
This accordion-like dynamic provides direct experimental evidence that competition for resources can cause evolutionary shifts in resource-related characters.
When populations of different species occur in sympatry (together), they often have trait values that are more extreme than the values occurring in allopatric (isolated) populations [ 1 ]. For traits associated with resource acquisition or metabolism, this phenomenon is called ecological character displacement, to distinguish it from reproductive character displacement, which describes shifts in traits associated with reproduction. Ecological character displacement is observed in Galapagos finches [ 2 – 4 ], plethodontid salamanders [ 5 ], sticklebacks [ 2 ], Anolis lizards [ 6 ], and spadefoot toads [ 7 , 8 ], and is generally believed to be caused by resource competition. Theory [ 9 – 12 ] predicts that character displacement will result from competition selecting and maintaining extreme phenotypes to minimize phenotypic overlap and thus minimize interspecific competition.
Experiments also support the hypothesis that competition can select for divergence in resource-related traits. Schluter [ 13 ] measured selection in sticklebacks and demonstrated that growth rates and survival were depressed in the presence of competitors, and that selection was frequency dependent [ 14 ]; and Bolnick [ 15 ] showed that competition could generate disruptive selection regimes in natural populations of sticklebacks. However, selection is not evolution, and few studies have shown that interspecific competition for resources leads to evolutionary shifts in phenotypic distributions of resource-related traits [ 16 ]. Taper [ 16 ] demonstrated character shifts using bean weevils, however he failed to detect trade-offs associated with the observed shifts, thus the divergence may have evolved for reasons other than interspecific resource competition. Microbes have been employed to great advantage in studying the generation and sorting of adaptive variation [ 17 – 21 ]. Using microbes to test evolutionary hypotheses is possible because microbes evolve quickly in response to environmental conditions set and controlled by the researcher. Additionally, replicate populations can be studied in order to determine the repeatability of evolutionary response, and microbes can be stored indefinitely at -80° so that assays between ancestors and descendents can be conducted [reviewed in [ 22 ]]. MacLean et al. [ 18 ] used biolog plates to characterize diversification of Pseudomonas bacteria in response to resource competition. This study also demonstrated how diverse genotypes were maintained by frequency dependent interactions likely resulting from competition for resources. Similarly, Barrett et al. [ 20 ] showed that diversification of Pseudomonas generated imperfect generalists in response to competition for substitutable resources. These studies nicely illustrate how metabolic diversification occurs in the face of resource competition. While they show divergence in phenotype space, the phenotypes measured are not functionally linked to competitive performance in the environment experienced during evolution. Therefore, the importance of the phenotype for competition remains unclear. For example, it is not clear whether in experimental populations seeded with only two phenotypes, competition would lead to divergence, i.e. to an increase in the phenotypic distance between these two strains. Similarly, it is unclear what the effects of removing competition from other strains would be on a single focal phenotype.
Using diversified Escherichia coli B populations, we show in this paper that competition for resources can lead to phenotypic divergence of competing strains, i.e., to ecological character displacement, and that absence of competition can lead to phenotypic convergence. We evolved E. coli for 1000 generations in liquid batch cultures with glucose and acetate as sole carbon resources. Ten replicate populations diversified into cultures consisting of two ecotypes that specialized on glucose or acetate [See Additional file 1 ]. When E. coli grows in batch culture, glucose is consumed first, followed by acetate [ 23 ]. This generates a two-phase (i.e., diauxic) growth profile within a single 24 h batch cycle (Figure 1a ). Diauxic growth profiles reveal how bacteria consume one resource (e.g., glucose) and switch to a second resource (e.g., acetate) only when the first is exhausted. Resource exploitation can thus be described as a metabolic reaction norm [ 24 ], and different metabolic reaction norms correspond to different 24 h growth profiles.
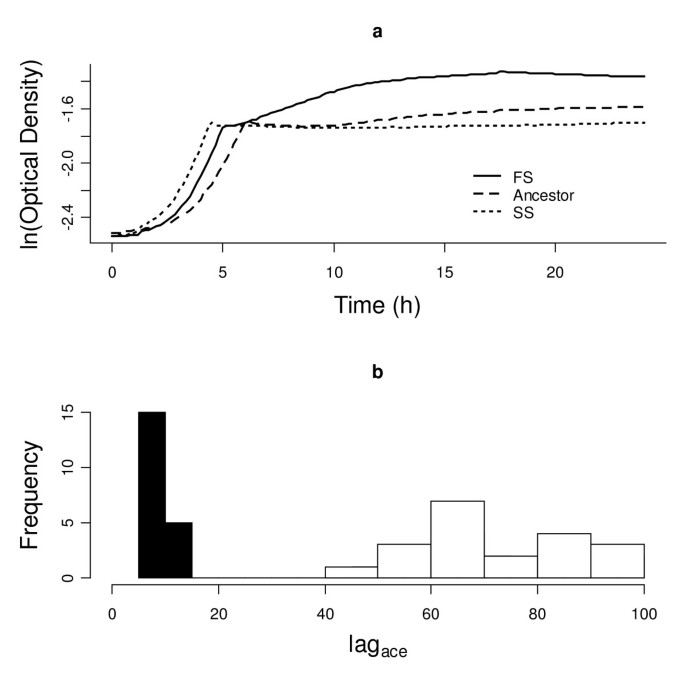
24 h growth curves reveal resource usage differences between ecotypes . (a) Examples of 24 h growth curves for the ancestor and derived ecotypes (Slow-switchers and Fast-switchers) from strain dst1018 after 1000 generations of evolution. (b) Histogram of lag ace reveals two phenotypic clusters (Fast-switchers = black and Slow-switchers = white).
Our evolved cultures had diversified into two ecotypes, identifiable by different 24 h growth profiles (Figure 1a ). These growth profiles were assayed in the absence of competitors of the opposite ecotype and hence are not a plastic response to the presence of a competitor. Instead, they reflect genetically distinct metabolic reaction norms, because offspring clones generate similar 24 h growth profiles as parental clones from which they descend [ 24 ]. We named the two distinct ecotypes Slow-Switchers (SS) and Fast-Switchers (FS) after differences in their relative switching lags ( lag ace ) between diauxic growth phases (Figure 1b ). We extracted lag ace and nine additional quantifiable traits from diauxic growth curve profiles. These phenotypic traits carry the signatures of different strategies for metabolizing resources and have been shaped and maintained by competition for resources [[ 24 ], See Additional file 2 ].
Results & Discussion
We envisage ecotypes occupying different regions of multidimensional phenotype space, characterized by particular values of resource-related traits, z. We can measure the distance between ecotypes, Δz, under transitions from sympatry to allopatry (or vice versa), and ask whether that distance changes due to character displacement as theory predicts [ 9 , 11 , 12 , 25 ]. Under competitive release, i.e., moving from sympatry to allopatry, phenotypic distributions should evolve towards intermediate values and thus appear closer in phenotype space, so that the distance measured in sympatry, Δz SYM is larger than the distance in allopatry, Δz ALLO (i.e., Δz SYM - Δz ALLO > 0). We tested this prediction by evolving FS and SS ecotypes (from three populations) in isolation (i.e., under competitive release) for ~200 generations. Growth curve parameters were extracted at T1 (generation 0), corresponding to sympatry, and at T30 (generation 200), corresponding to allopatry. We measured evolutionary response as the difference in trait value (T1-T30) for each ecotype from each population. We reduced the number of traits by conducting a principle components analysis (PCA, see Additional file 3 ) and characterized SS and FS ecotypes in composite phenotype space (Figure 2a ). We calculated the distances Δz ALLO and Δz SYM , and tested whether Δz SYM - Δz ALLO > 0. Under competitive release, we found strong support for phenotypic convergence (Figure 2b ) between ecotypes from all three populations (randomization test, dst1018: P = 1.0 × 10 -6 , dst1019: P = 1.0 × 10 -6 and dst1020: P = 1.0 × 10 -6 ). Convergence occurred primarily along the first principal component axis, with parallel shifts occurring on the remaining axes. Patterns of evolutionary response differed among populations. For example, convergence in two populations (dst1019 and dst1020) consisted of both ecotypes moving towards one another in phenotype space, but in population dst1018, convergence was due to a shift of both ecotypes in the same direction, but with SS changing to a larger extent (Figure 2a ). We suspect that initial differences in position in phenotype space (dst1018 vs. dst1019 and dst1020) accounted for differences in evolutionary response of these ecotypes when released from competition.
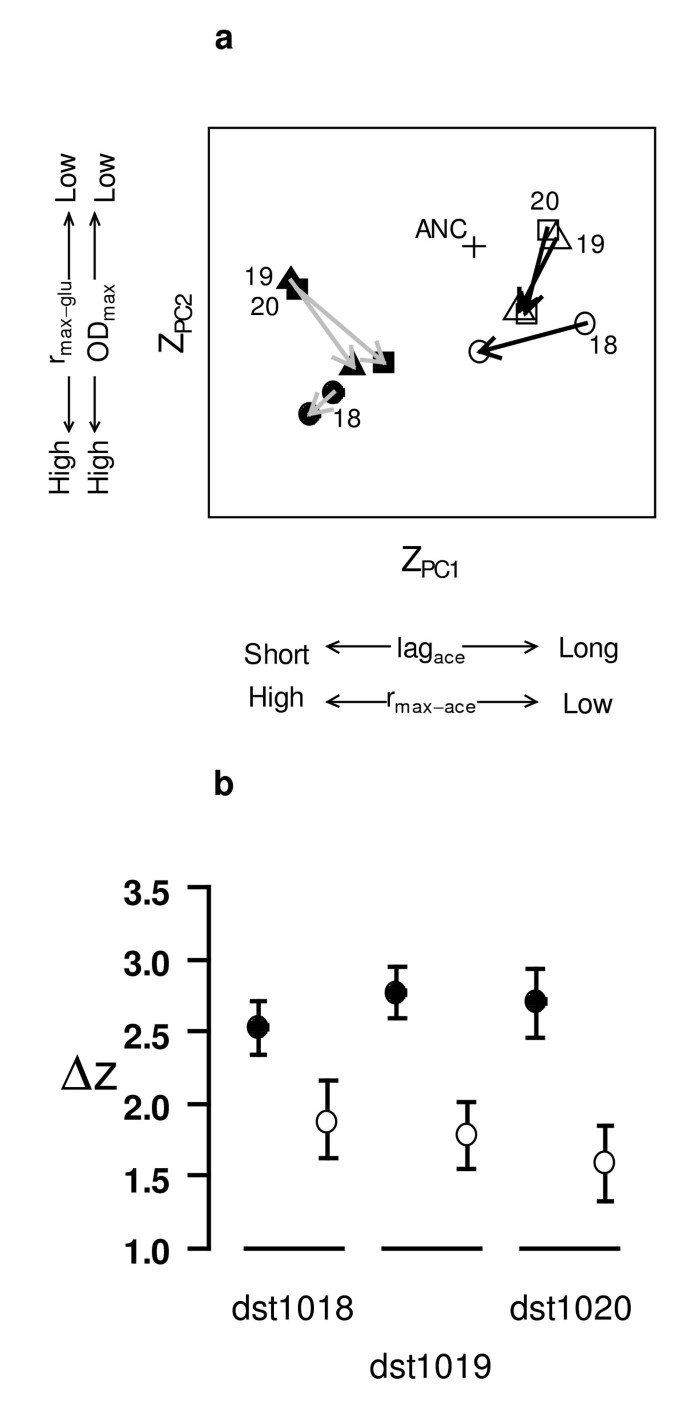
Character displacement under competitive release . (a) Symbols reflect mean ecotype evolutionary response from replicates (n = 20) evolved from each of three source populations (dst1018 = circles; dst1019 = triangles; dst1020 = squares), and arrows show evolutionary trajectories from sympatry to allopatry. Black symbols are FS ecotypes, white symbols are SS ecotypes. The ancestor (+) to the original evolution experiment is illustrated for comparison. Phenotypes are projected into two dimensions using the loadings from PC1 and PC2. (b) Mean distance in trait space, Δz, between ecotypes in sympatry (black) and allopatry (white) during competitive release, for replicates (n = 20) from three populations. Error bars are 95% confidence intervals.
Next, we investigated whether adding competition would induce phenotypic divergence. For this we selected intermediate, convergent genotypes (SS' and FS'), which we isolated from T30 cultures (Figure 3 , see Methods for further description). We competed SS' vs. FS' for 200 generations, after which the frequency of FS'-derived genotypes was <0.1% in 4 of the 10 competition replicates, suggesting that SS'-derived ecotypes were often able to competitively exclude FS'-derived ecotypes. Thus, we isolated genotypes derived from SS' (SS SYM ) or FS' (FS SYM ) from an earlier time point (generation 100, when FS was still present in an appreciable frequency in all cultures), and calculated the mean growth-curve parameters for SS SYM and FS SYM . We projected these parameters using the same composite trait space characterized during competitive release (Figure 4a ). Thus, we explicitly tested whether competition could induce evolutionary divergence by directly reversing the changes that occurred during competitive release. Indeed we found that competition induced divergence (t = 2.73, df = 9, p < 0.02, Figure 4b ).
After 200 generations (T30) of isolated evolution, "convergent" cultures (SS ALLO and FS ALLO ) were assayed for intermediate genotypes . (a) SS' ecotype derived in an ara- culture (dst1018), with FS (dotted) and SS (dashed) ecotypes shown for comparison and (b) FS' ecotype derived from an ara+ culture (dst1019) with FS (dotted) and SS (dashed) ecotypes shown for comparison.
Character displacement after competition was induced between intermediate ecotypes (SS vs. FS) . (a) Phenotypes are projected and scaled as in Figure 2, and gray symbols and arrows illustrate the evolutionary trajectories that occurred during "competitive release" (first phase of study) in the relevant populations for comparison (see Figure 2a). Mean ecotype trajectories for SS' (circles) and FS' (triangles) from allopatry to sympatry. The black arrow shows the mean evolutionary trajectory of SS'-derived genotypes during competition, while the FS'-derived genotypes did not change substantially. (b) Distance in trait space, Δz, between pairs of SS' and FS' competitors in sympatry (white) and allopatry (black).
Interestingly, divergence did not exactly retrace the evolutionary trajectory of convergence (Figure 4a vs. Figure 2a ). Both convergence under competitive release, and subsequent divergence due to competition, occurred along the first composite trait axis. However, under competitive release, both ecotypes contributed to convergence, whereas only the SS' phenotype contributed to divergence. Moreover, the magnitude of the evolutionary response during the divergent phase was smaller than during the convergent phase (Figure 4b vs. Figure 2b ). This difference in magnitude may be because we assayed character displacement after 200 generations in the first phase and only 100 generations in the second phase, allowing less time for evolution. However, the difference in magnitude of evolutionary response may also have ecological reasons. Schluter [ 2 ] argued that the speed of divergence during character displacement is greatest when phenotypic distance (i.e., degree of similarity) between competing species is intermediate. In particular, while very similar species experience intense competition, the speed of divergence is not expected to be high, because an increase in phenotypic distance may not substantially decrease competitive intensity. Instead, divergence becomes faster only after it has progressed considerably (See Figure 6.1 in ref [ 2 ]). Since the phenotypes we competed were rather similar (Figure 3 ), this effect may have delayed the response in our divergence treatments.
Finally, the evolutionary response under competition may be different because divergence may have occurred in phenotypic dimensions not captured by the composite trait space defined by the PCA analysis of the competitive release experiment. We conducted an independent PCA analysis on the data from only the divergence phase of our experiment, which yielded a different composite trait space. In this new trait space divergence is also significant, but the response is of similar magnitude to the evolutionary response initially identified (data not shown).
Ecologists [ 2 , 9 , 11 , 12 ] continue to emphasize a causal role for competition in ecological character displacement. However, other factors, such as predation [ 21 , 26 , 27 ] can also affect adaptive processes of diversification. Grant & Grant [ 3 ] have therefore recently called for a definitive demonstration of competition's causal role in ecological character displacement. Here, we answer this call using experimental tests in bacterial populations. Our evidence for character convergence after competitive release is particularly compelling, and our work supports the trust that ecological theory [ 2 , 11 , 28 , 29 ] has placed on competition for resources as an important driver of character divergence.
Our study demonstrates that interspecific competition for resources can cause resource-related phenotypes to shift as expected in response to competition. The initial adaptive diversification generating SS and FS ecotypes, followed by our manipulations of interspecific competition (by removing and subsequently adding competitors) reveals competition's role in driving accordion-like shifts on distributions of resource-related phenotypes: divergence followed by convergence followed by divergence. Coexistence in the face of interspecific competition for shared resources may demand such an evolutionary response, with the exclusion of the inferior ecotype as an alternative outcome [ 30 ].
Description of evolved strains
Ten replicate populations of E. coli B were alternately initiated from two isogenic lines [ 31 ], which differed with respect to a neutral marker. The isogenic lines differed in their ability to utilize arabinose (ara+/-), which we exploited to discriminate between lineages in mixed cultures (see "Fitness assays" and "Competition induced" sections below). We followed the protocols of Lenski et al. [ 31 ] and others [ 24 , 32 – 36 ] with minor variations. We used large, loosely-covered test tubes, filled with 10 mL of Davis Minimal Salts media (DM) supplemented with 250 μ g/mL glucose and 575 μ g/mL acetate as the sole carbon sources. These resources were selected because diversification in their presence has been shown previously [ 24 , 33 , 36 ]. Cultures were incubated at 37° and vigorously shaken (250 rpm) for 24 h. Each day (i.e., after 24 ± 1 h of growth), 100 μ L of culture was transferred to 10 mL of fresh media (~1/100 dilution) and thus the seasonal cycle was reset. Each batch cycle yielded on average log 2 100 = 6.7 generations.
To test whether adaptation had occurred, we competed three populations (dst1018, dst1019, dst1020) against the ancestor of opposite marker type, and calculated relative fitness as done previously [ 31 ] (see below). These three populations were selected from the initial ten populations because there was a high correlation between colony morphology variation (large vs. small) and ecotype (SS vs. FS), which we exploited for purposes of identification in mixed culture assays. Fitness increased by ~14% [See Additional file 1 ] in all three populations. This suggests that adaptive evolution occurred over the course of 1000 generations.
By generation 1000, two discernable E. coli ecotypes, Fast-switching (FS) and Slow-switching (SS), were identified in all ten replicate populations [See Additional file 1 ], and there was extensive variation in frequency of the two ecotypes. We view the parallel emergence of diversity in each population as an indication that the divergence was adaptive [ 10 ].
To show that there is a functional (i.e., adaptive) explanation for the divergence in our E. coli populations, we assessed whether trade-offs in resource usage were detectable between SS and FS strains. From previous work [ 24 , 33 , 36 ] and this study, it appears that SS was functionally similar to the ancestor, while FS had diverged to exploit acetate earlier in the 24 h growth cycle (indicated by reduced lag ace , Figure 1b ). Presumably this enhanced performance on acetate is associated with reduced performance on glucose. Such a trade-off has previously been found in diversified strains that have evolved under similar conditions [ 24 , 32 ]. To test for trade-offs in resource use, we competed SS and FS in environments that were skewed to having either more glucose or more acetate (see Fitness Assays below). A tradeoff would imply that in a glucose-enhanced/acetate-reduced environment, SS -having a metabolic profile geared towards efficient glucose use – would have higher fitness, while in an acetate-enhanced/glucose-reduced environment, FS – having a metabolic profile geared towards enhanced acetate use – would have higher fitness. Indeed, we found support for the hypothesis that tradeoffs in resource use underlie the maintenance of diversity in metabolic profiles (t = 4.305, p < 0.0005 [See Additional file 2 ]). This tradeoff in resource use strongly supports the hypothesis that resource competition was the selective cause for the divergence into SS and FS ecotypes.
Asexual nature of our lines
E. coli exchange DNA via conjugation, passing plasmids between donor and recipient cells. However, E. coli B has no plasmids and can thus be considered asexual [ 31 ]. We ensured that the ancestral lines (rel606 and rel607) and evolved lines used in this study had no plasmids with a standard mini preparation of genomic DNA isolated from cells grown from each culture (Sigma GenElute Plasmid Miniprep Kit). No plasmids were detected in the ancestors or evolved cells.
Fitness assays
Fitness of each evolved line was determined relative to the ancestor using competition experiments as described in [ 31 ]. Briefly, evolved cultures (mixed sample of SS and FS) from the endpoint of our evolution experiment (generation 1000) and cultures from the ancestors (both marker types) were inoculated from frozen stock into evolutionary media, and grown for 24 h. Evolved culture and ancestor (of opposite marker type) were mixed in equal proportions (by volume) and inoculated into fresh medium (~1/100 dilution) in ten replicates; plated on Tetrazolium agar with arabinose to determine densities at inoculation (T0), and then grown and transferred for two days before being plated to yield T2 densities. Relative fitness was calculated as ln(EV T2 /EV T0 )/ln(ANC T2 /ANC T0 ) (modified from [ 31 ]), where EV is the density of evolved culture and ANC is the density of the ancestor (at times T0 and T2). To determine fitness of SS and FS in skewed resource environments (i.e., 90% [glucose]-10% [acetate] or 10% [glucose]-90% [acetate]), we isolated 10 SS and 10 FS genotypes from strain dst1018, inoculated them individually into fresh medium (50% [glucose] - 50% [acetate]) for 24 hours, and then arbitrarily selected pairs of SS and FS to mix in equal proportions (by volume). We inoculated ten pairs into both extreme environments. We plated T0 and T2 on tetrazolium agar plates (without arabinose) and used colony morphology (large or small colonies, see [ 24 ]) to aid us in determining the densities of both SS and FS ecotypes at each time point. We calculated relative fitness as above, substituting SS and FS for EV and ANC.
Growth parameter extraction
Growth curves were obtained by inoculating ~1.5 μ L of conditioned culture into 150 μ L of fresh evolutionary medium (see above) in individual wells of a 96-well microplate. Microplate cultures were grown in a Biotek 808UI Optical Density reader, under similar conditions to the original evolutionary environment (37°, well shaken). Measurements consisted of optical densities (OD, 600 nm) obtained every 10 min over the course of 24 h. Data files were converted to a usable format using Microsoft Excel, and growth curve parameters were extracted with a program written in object oriented C++.
Table 1 summarizes the parameters extracted from growth curves. These were modified from [ 24 ]. The StartTime was extracted but not used directly in the analysis; it was used indirectly in the calculation of other variables (see Table 1 ). StartTime was the time where the OD (600 nm) of the growing culture first reached 0.08. Slopes were extracted using a moving window algorithm (i.e., linear regression through nine successive time points), and were used for the calculation of r max glu , switching OD , and r max ace . OD max and OD final were the maximum and final optical densities during the 24 h growth period. Means for each ecotype in Table 1 were calculated from twenty SS and twenty FS clones isolated from population dst1018.
Character Displacement experiments
1) competitive release.
We selected three of ten diversified populations for this experiment (dst1018, dst1019, dst1020). From the 1000-generation mark (maintained at -80°) we conditioned these populations in fresh evolutionary media for 24 h, and plated on tetrazolium agar to isolate genotypes. We selected 20 SS and FS genotypes (initially by colony morphology and confirmed by growth profile) from each population, and used these genotypes to initiate allopatric cultures (i.e., no interspecific competition). 1.5 μL of each culture was inoculated into a single well containing 150 μ L evolutionary media of a 96-well microtitre plate (~1/100 dilution). Growth conditions and protocols mirrored the evolutionary conditions, with the exception of differences in volume between test tubes (10 mL in the original evolution experiment) and microplate wells (150 μ L in this experiment). Separate microtitre plates were used for SS and FS cultures to prevent the possibility of cross-contamination between ecotypes. Although growth curves were measured on ecotypes grown in isolation (i.e., no interspecific competition), we assumed that initial growth parameter values (T1) had no mutations, and thus reflected the evolutionary signal of each ecotype under sympatry. This assumption is conservative, because we are actually measuring the parameters in isolation for the sympatric values to compare to later measures in allopatry. All cultures were propagated in isolation (allopatry) for ~200 generations by transferring 1/100 of the culture to fresh media in a new microplate every 24 hours for 30 days. After 30 days of evolution, the values obtained from growth curves were assumed to reflect the mean evolutionary response for each replicate to the treatment of allopatry. A detailed analysis of individual genotypes (beyond this study) is ongoing. Growth parameters from all derived cultures were log transformed.
2) Competition induced
From T30 cultures generated in the first phase of this study (see above) we noted that all cultures were genetically heterogeneous (as determined by variation in growth curve profiles from isolated clones). Generally, there were 2–3 genotypes in FS ALLO (i.e., cultures derived from FS) and 2–5 genotypes in SS ALLO (i.e., cultures derived from SS). In many SS ALLO cultures, we noted one particular recurring genotype that had a decreased lag ace , here labeled SS' (Figure 3a ). Similarly, in FS ALLO cultures, we noted one particular genotype with reduced maximum yields ( OD MAX ) in each phase of diauxic growth (relative to the ancestral FS genotype), here labeled FS' (Figure 3b ). We considered these novel genotypes as intermediate between SS and FS ecotypes, and relatively convergent towards the opposite ecotype, when compared with the ecotype from which they were descended (SS' derived from SS and FS' derived from FS). A single SS-derived genotype (SS') was selected from one of the twenty dst1018 replicates, and a single FS-derived genotype (FS') was isolated from one of the dst1019 replicates. We used single genotypes for each novel ecotype because we wanted to focus on the role of competition (as opposed to extant genetic makeup of initially variable populations) in ecological character displacement. Additionally, a fully replicated design with all possible complimentary pairs of isolated novel genotypes in competition would be impractical.
We initiated ten mixed cultures of SS' vs. FS' (1:1, by volume) and inoculated these treatments into microplate wells (as above). We also inoculated pure SS' or FS' culture to determine z ALLO for each ecotype. We propagated the mixed cultures for 30 days (200 generations) to determine if competition would cause the SS' and FS' to diverge in resource-related phenotype space. Because the frequency of FS'-derived clones <0.1% by T30, we assayed our populations at T15 (See main text). We plated all replicate populations onto Tetrazolium agar (with arabinose) and identified descendent clones by their ara +/- status. Fourteen clones for each of SS'-derived and FS'-derived subpopulations from each replicate mixture were isolated and conditioned for 24 h before being assayed for growth curve parameters. We then calculated the mean parameter value from descendents from each ecotype from each competition replicate for statistical analysis (see below).
Statistical analysis
In both phases of the experiment, we tested the hypothesis that competition caused character displacement such that Δz SYM - Δz ALLO > 0.
1. Competitive release
We calculated the evolutionary response to competitive release (i.e., sympatry to allopatry) for each of ten traits by taking the difference in log-transformed trait values between T1 and T30. We pooled the evolutionary responses for all 120 replicates (3 source populations × 2 ecotypes × 20 replicates/population/ecotype). We conducted a PCA using the correlation matrix of the pooled response data [ 37 ]. We used only the first four principle components as they had eigenvalues > 1 [ 37 ], and accounted for > 81% of the variation in response to allopatry. [See Additional file 3 and Table 2 for a summary of the PCA]. We used the loadings from these four components and the difference data to generate independent (orthogonal) composite trait values. Thus, our ecotypes are described as points in four-dimensional phenotype space. From T1, we calculated:
Δz SYM = z FS-SYM - z SS-SYM
and from T30, we calculated:Δz ALLO = z FS-ALLO - z SS-ALLO
where z is a vector in four dimensional trait space reflecting mean population values for FS or SS ecotypes in sympatry or allopatry. We analyzed the three source populations separately. We determined Δz SYM and Δz ALLO (and 95% C.I.) by randomly sampling 20 distances 1000 times from the fully permutated distance data set. We used a randomization test procedure to determine the probability of obtaining a test statistic (Δz SYM - Δz ALLO ) that was ≥ observed data [ 38 ]. P values in the main text indicate the proportion of 100,000 analogous datasets created having (Δz SYM - Δz ALLO > observed data), after randomly reclassifying all distances into Δz SYM or Δz ALLO datasets.
2. Competition induced
Our competition replicates comprised pairs (n = 10) of SS' and FS' derived genotypes. Thus, we used a paired t-test to determine whether Ha: Δz SYM - Δz ALLO > 0. This allowed us to quantify evolutionary response (i.e., divergence) in each replicate (i.e., Δz SYM ) separately, so that divergence across replicates could arise even if ecotypes made different contributions to divergence in different replicates.
Abbreviations
slow-switching ecotype
fast-switching ecotype
acetate lag
distance in phenotypic (trait) space.
Brown WL, Wilson EO: Character displacement. Syst Zool. 1956, 5: 49-64. 10.2307/2411924.
Article Google Scholar
Schluter D: The ecology of adaptive radiation. 2000, Oxford: Oxford University Press
Google Scholar
Grant P, Grant B: Evolution of character displacement in Darwin's Finches. Science. 2006, 313: 224-226. 10.1126/science.1128374.
Article CAS PubMed Google Scholar
Schluter D, Price T, Grant P: Ecological character displacement in Darwin's Finches. Science. 1985, 227: 1056-59. 10.1126/science.227.4690.1056.
Adams D, Rohlf R: Ecological character displacement in Plethodon: biomechanical differences found from geometric morphometric study. PNAS. 2000, 97: 4106-11. 10.1073/pnas.97.8.4106.
Article PubMed Central CAS PubMed Google Scholar
Losos J: Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Ann Review of Ecol and Systematics. 1994, 25: 467-93. 10.1146/annurev.es.25.110194.002343.
Pfennig D, Murphy P: A test of alternative hypotheses for character divergence between coexisting species. Ecology. 2003, 84: 1288-97. 10.1890/0012-9658(2003)084[1288:ATOAHF]2.0.CO;2.
Pfennig D, Rice M, Martin R: Field and experimental evidence for competition's role in phenotypic divergence. Evolution. 2007, 61: 257-71. 10.1111/j.1558-5646.2007.00034.x.
Article PubMed Google Scholar
Doebeli M: An explicit genetic model for ecological character displacement. Ecology. 1996, 77: 510-520. 10.2307/2265626.
Schluter D: Ecological character displacement in adaptive radiation. Am Nat. 2000, 156: S4-S16. 10.1086/303412.
Slatkin M: Ecological character displacement. Ecology. 1980, 61: 163-77. 10.2307/1937166.
Taper M, Chase T: Quantitative genetic models for the coevolution of character displacement. Ecology. 1985, 66: 355-71. 10.2307/1940385.
Schluter D: Experimental evidence that competition promotes divergence in adaptive radiation. Science. 1994, 266: 798-801. 10.1126/science.266.5186.798.
Schluter D: Frequency dependent natural selection during character displacement in sticklebacks. Evolution. 2003, 57: 1142-1150.
Bolnick D: Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004, 58: 608-18.
Taper M: Experimental character displacement in the adzuki bean weevil, Callosobruchus chinensis. Bruchids and Legumes: economics, ecology and coevolution. Edited by: Fujii K, Gatehouse A, Johnson C, Mitchel R, Yoshida T. 1990, Kluwer, 289-301.
Chapter Google Scholar
Rainey P, Travisano M: Adaptive radiation in a heterogeneous environment. Nature. 1998, 394: 69-72. 10.1038/27900.
MacLean RC, Dickson A, Bell G: Resource competition and adaptive radiation in a microbial microcosm. Ecol Letters. 2005, 8: 38-46. 10.1111/j.1461-0248.2004.00689.x.
Hall AR, Colegrave N: How does resource supply affect evolutionary diversification?. Proc R Soc Lond B. 2007, 274: 73-8. 10.1098/rspb.2006.3703.
Article CAS Google Scholar
Barrett RDH, MacLean RC, Bell G: Experimental Evolution of Pseudomonas fluorescens in Simple and Complex Environments. Am Nat. 2005, 166: 470-480. 10.1086/444440.
Meyer J, Kassen R: The effects of competition and predation on diversification in a model adaptive radiation. Nature. 2007, 446: 432-5. 10.1038/nature05599.
Elena S, Lenski R: Evolutionary experiments with microorganisms: the dynamics and genetics of adaptation. Nature reviews: Genetics. 2003, 4: 457-69. 10.1038/nrg1088.
Mahadevan R, Edwards J, Doyle IFJ: Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophysical J. 2002, 83: 1331-40.
Friesen ML, Saxer G, Travisano M, Doebeli M: Experimental evidence for sympatric ecological diversification due to frequency dependent competition in Escherichia coli. Evolution. 2004, 58: 245-60.
Abrams PA: Character displacement and niche shift analyzed using consumer-resource models of competition. Theor Pop Biol. 1986, 29: 107-60. 10.1016/0040-5809(86)90007-9.
Rundle H, Vamosi S, Schluter D: Experimental test of predation's effect on divergent selection during character displacement in sticklebacks. PNAS. 2003, 100: 14943-8. 10.1073/pnas.2036360100.
Nosil P, Crespi B: Experimental evidence that predation promotes divergence in adaptive radiation. PNAS. 2006, 103: 9090-5. 10.1073/pnas.0601575103.
Dieckmann U, Doebeli M: On the origin of species by sympatric speciation. Nature. 1999, 400: 354-7. 10.1038/22521.
Doebeli M, Dieckmann U: Speciation along environmental gradients. Nature. 2003, 421: 259-64. 10.1038/nature01274.
Hardin G: The competitive exclusion principle. Science. 1960, 131: 1292-7. 10.1126/science.131.3409.1292.
Lenski R, Rose M, Simpson S, Tadler S: Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat. 1991, 138: 1315-41. 10.1086/285289.
Spencer CC, Saxer G, Travisano M, Doebeli M: Seasonal resource oscillations maintain diversity in bacterial microcosms. Evolutionary Ecology Research. 2007, 9: 775-87.
Spencer CC, Bertrand M, Travisano M, Doebeli M: Adaptive diversification in genes regulating resource use in Escherchicia coli . PLoS Genetics. PLoS Genetics. 2007, 3: 83-88. 10.1371/journal.pgen.0030015.
Spencer C, Tyerman J, Bertrand M, Doebeli M: Adaptation increases the likelihood of diversification in an experimental bacterial lineage. PNAS.
Le Gac M, Brazas M, Bertrand M, Tyerman J, Spencer C, Hancock R, Doebeli M: Metabolic changes associated with adaptive diversification in Escherichia coli . Genetics.
Tyerman JG, Havard N, Saxer G, Travisano M, Doebeli M: Unparallel diversification in bacterial microcosms. Proc R Soc Lond B. 2005, 272: 1393-8. 10.1098/rspb.2005.3068.
Dillon W, Goldstein M: Multivariate analysis: methods and applications. 1984, New York, NY: John Wiley & Sons
Manly BF: Randomization, bootstrap and Monte Carlo methods in biology. 1997, London: Chapman & Hall, 2
Download references
Acknowledgements
Financial support provided by NSERC to JGT and MD, and by the James S McDonnell Foundation to MD. Lab assistance was provided by M. Ciou, M. Chan, G. Khaira, R. McBride, and R. Suprin. RE Lenski kindly provided our lab with E. coli B strains (rel606 and rel607) that served as the ancestors in our evolution experiment. M. Friesen helped develop protocols used in extracting parameters from growth curves. Discussions with D. Ally, A. Blachford, R. Blok, J. Fletcher, L. Harmon, M. Le Gac and D. Schluter aided us in preparing the study and manuscript.
Author information
Authors and affiliations.
Dept. Zoology & Centre for Biodiversity, University of British Columbia, 6270 University Blvd., Vancouver, BC, V6T 1Z4, Canada
Jabus G Tyerman, Melanie Bertrand & Michael Doebeli
Dept. Ecology and Evolutionary Biology, University of Toronto, 25 Harbord St., Toronto, Ontario, M5S 3G5, Canada
Christine C Spencer
Dept. Mathematics, University of British Columbia, 6270 University Blvd., Vancouver, BC, V6T 1Z4, Canada
Michael Doebeli
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jabus G Tyerman .
Additional information
Authors' contributions.
JT conceived the experiments, conducted the experiments, analyzed the data, and wrote the manuscript. MB and CCS conducted the experiments. MD conceived the experiments and wrote the manuscript.
Electronic supplementary material
12862_2007_603_moesm1_esm.pdf.
Additional file 1: Figure S1. (a) Relative fitness of the ancestor (Generation 0) and three populations (Generation 1000) versus the ancestor of opposite marker type (ara+/-). The dashed horizontal line is equivalent fitness, error bars indicate 95% confidence intervals, and letters above error bars denote significantly different groups. (b) The proportion of SS (95% CI) in ten replicate populations evolved in glucose-acetate environment (populations in rank order). The dashed horizontal line represents the grand mean for all populations. (PDF 22 KB)
12862_2007_603_MOESM2_ESM.pdf
Additional file 2: Figure S2. Competition experiments in skewed resource environments reveal that mean SS fitness is greater than mean FS fitness when [glucose] is enhanced (from 50% to 90%) and [acetate] reduced (from 50% to 10%) (left) and that mean SS fitness is lower than mean FS fitness when [glucose] is reduced and [acetate] enhanced (right). The horizontal line indicates equal fitness, and the error bars indicate 95% CI. (PDF 17 KB)
12862_2007_603_MOESM3_ESM.pdf
Additional file 3: Figure S3. Principle component analysis (PC1 vs. PC2) on differences between sympatric and allopatric trait values for Slow-switchers (white) and Fast-switchers (black) from replicates initiated from three populations (dst1018 = circles, dst1019 = triangles, dst1020 = squares). (PDF 20 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Tyerman, J.G., Bertrand, M., Spencer, C.C. et al. Experimental demonstration of ecological character displacement. BMC Evol Biol 8 , 34 (2008). https://doi.org/10.1186/1471-2148-8-34
Download citation
Received : 25 September 2007
Accepted : 30 January 2008
Published : 30 January 2008
DOI : https://doi.org/10.1186/1471-2148-8-34
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Interspecific Competition
- Evolutionary Response
- Growth Profile
- Phenotypic Distribution
- Character Displacement
BMC Ecology and Evolution
ISSN: 2730-7182
- General enquiries: [email protected]
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Ecological and community-wide character displacement: the next generation

2005, Ecology Letters
Related Papers
Evolution Since Darwin: The First 150 Years
D. Luke Mahler
Journal of Heredity
Austin Patton
Adaptive radiation plays a fundamental role in our understanding of the evolutionary process. However, the concept has provoked strong and differing opinions concerning its definition and nature among researchers studying a wide diversity of systems. Here, we take a broad view of what constitutes an adaptive radiation, and seek to find commonalities among disparate examples, ranging
Laurence Despres
Biological Journal of the Linnean Society
Patrik Nosil
Ecological Entomology
Laurence Després
S. Andrew Inkpen
Character displacement was originally defined simply as a pattern—divergence between two species in sympatry but not allopatry—and it was recognized that multiple processes might generate this pattern. However, over time, character displacement has come to be nearly synonymous with the process of adaptive divergence between species caused by selection stemming from resource-competitive interactions (and if not that, reproductive interactions). This tight link between character displacement and resource competition has generated, and continues to generate, imprecision and confusion in the literature. Here, to address this problem, we suggest unlinking character displacement the pattern from any specific process (e.g., natural selection arising from species interactions). That is, character displacement should be documented as a pattern, agnostically with respect to process. Purposeful, direct investigation of what process generated character displacement then naturally follows. This has the benefit of acknowledging that there can be many different avenues to divergence in sympatry.
Janette Boughman , Idelle Cooper
Kimmo Kahilainen
RELATED PAPERS
Ecology Letters
Elena Litchman
Journal of Evolutionary Biology
Travis Hagey
Journal of Animal Ecology
Jennie Mallela
Anna Runemark , Kostas Sagonas , Erik Svensson
Annals of the New York Academy of Sciences
Chris Anderson
YASEL U. ALFONSO
NUR ALEEA MOHD YUSSOF
Ecology and Evolution
Donat Petricioli
Gábor Herczeg
Trends in Ecology & Evolution
Rosemary GILLESPIE
Stanley A Sawyer , M. Butler
Biological Journal of The Linnean Society
Patricio Jorge Macchi
Suzanne Gray
Janette Boughman
Malacologia
Debahuti Mukherjee
Evolutionary Biology-new York
The American Naturalist
Louie Yang , James Fordyce
Scott Carroll
Thomas DeWitt
Proceedings. Biological sciences
Joana Meier
Andrew Hendry
Craig Layman
Ingeborg P. Helland
Integrative and Comparative Biology
Journal of evolutionary biology
Erik Svensson
Journal of Zoology
Christopher McClure
Jonathon C. Marshall
Proceedings. Biological sciences / The Royal Society
Andy Purvis
David Reznick
Proceedings of the Royal Society B: Biological Sciences
Anthony Herrel
Luca Santini
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Faculty of Arts and Sciences
- FAS Scholarly Articles
- Communities & Collections
- By Issue Date
- FAS Department
- Quick submit
- Waiver Generator
- DASH Stories
- Accessibility
- COVID-related Research
- Terms of Use
- Privacy Policy
- By Collections
- By Departments
Show simple item record
Character Displacement Is a Pattern: So, What Causes It?
Files in this item.
This item appears in the following Collection(s)
- FAS Scholarly Articles [18292]

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

15.4: Ecological and Evolutionary Consequences of Competition
- Last updated
- Save as PDF
- Page ID 83717
15.4.1 Competitive Exclusion
Figure \(\PageIndex{1}\): 1) A smaller (yellow) species of bird forages across the whole tree. 2) A larger (red) species competes for resources. 3) Red dominates in the middle for the more abundant resources. Yellow shifts to a new niche, avoiding competition.
The competitive exclusion principle postulates that two species which compete for the same limited resource cannot coexist at constant population values. When one species has even the slightest advantage over another, the one with the advantage will dominate in the long term. This leads either to the extinction of the weaker competitor or to an evolutionary or behavioral shift toward a different ecological niche. The principle has been paraphrased in the maxim "complete competitors cannot coexist" (Hardin 1960).
Georgy Gause formulated the law of competitive exclusion based on laboratory competition experiments using two species of Paramecium , P. aurelia and P. caudatum . The conditions were to add fresh water every day and input a constant flow of food. Although P. caudatum initially dominated, P. aurelia recovered and subsequently drove P. caudatum extinct via exploitative resource competition. However, Gause was able to let the P. caudatum survive by differing the environmental parameters (food, water). Thus, Gause's law is valid only if the ecological factors are constant.
Figure \(\PageIndex{2}\): Paramecium aurelia and Paramecium caudatum grow well individually, but when they compete for the same resources, P. aurelia outcompetes P. caudatum .
Competitive exclusion is predicted by mathematical and theoretical models such as the Lotka–Volterra models of competition. However, competitive exclusion is rarely observed in natural ecosystems and many biological communities appear to violate Gause's law. The best-known example is the so-called "paradox of the plankton" (Hutchinson 1960). All plankton species live on a very limited number of resources, primarily solar energy and minerals dissolved in the water. According to the competitive exclusion principle, only a small number of plankton species should be able to coexist on these resources. Nevertheless, large numbers of plankton species coexist within small regions of open sea.
15.4.2 Niche Differentiation
Niche differentiation (also known as niche separation and niche partitioning ) refers to the process by which competing species use the environment differently in a way that helps them to coexist. When two species differentiate their niches, they tend to compete less strongly, and are thus more likely to coexist. Species can differentiate their niches in many ways, such as by consuming different foods, or using different areas of the environment.
As an example of niche partitioning, several anole lizards in the Caribbean islands share common diets—mainly insects. They avoid competition by occupying different physical locations. Although these lizards might occupy different locations, some species can be found inhabiting the same range, with up to 15 in certain areas. For example, some live on the ground while others are arboreal. Species who live in different areas compete less for food and other resources, which minimizes competition between species. However, species who live in similar areas typically compete with each other (Pacala 1985).
Figure \(\PageIndex{3}\): Niche differentiation by size: greater duckweed, lesser duckweed and rootless dwarf duckweed.
Competing species can partition their niche in different ways. This list is not exhaustive, but illustrates several classic examples.
Resource Partitioning is the phenomenon where two or more species divide out resources like food, space, resting sites etc. to coexist. For example, some lizard species appear to coexist because they consume insects of differing sizes (Caldwell & Vitt 1999). Alternatively, species can coexist on the same resources if each species is limited by different resources, or differently able to capture resources. Different types of phytoplankton can coexist when different species are differently limited by nitrogen, phosphorus, silicon, and light (Grover 1997). In the Galapagos Islands, finches with small beaks are more able to consume small seeds, and finches with large beaks are more able to consume large seeds. If a species' density declines, then the food it most depends on will become more abundant (since there are so few individuals to consume it). As a result, the remaining individuals will experience less competition for food. Although "resource" generally refers to food, species can partition other non-consumable objects, such as parts of the habitat. For example, warblers are thought to coexist because they nest in different parts of trees (MacArthur 1958). Species can also partition habitat in a way that gives them access to different types of resources. As previously stated, anole lizards appear to coexist because each uses different parts of the forests as perch locations (Grover 1997). This likely gives them access to different species of insects.
Predator Partitioning occurs when species are attacked differently by different predators (or natural enemies more generally). For example, trees could differentiate their niche if they are consumed by different species of specialist herbivores, such as herbivorous insects. If a species density declines, so too will the density of its natural enemies, giving it an advantage. Thus, if each species is constrained by different natural enemies, they will be able to coexist (Grover 1994). Early work focused on specialist predators; however, more recent studies have shown that predators do not need to be pure specialists, they simply need to affect each prey species differently (Chesson & Kuang 2008, Sedio et al. 2013).
Conditional Differentiation (sometimes called temporal niche partitioning ) occurs when species differ in their competitive abilities based on varying environmental conditions. For example, in the Sonoran Desert, some annual plants are more successful during wet years, while others are more successful during dry years (Angert et al. 2013). As a result, each species will have an advantage in some years, but not others. When environmental conditions are most favorable, individuals will tend to compete most strongly with members of the same species. For example, in a dry year, dry-adapted plants will tend to be most limited by other dry-adapted plants.
Competition-Predation Trade-Off Species can differentiate their niche via a competition-predation trade-off if one species is a better competitor when predators are absent, and the other is better when predators are present. Defenses against predators, such as toxic compounds or hard shells, are often metabolically costly. As a result, species that produce such defenses are often poor competitors when predators are absent. Species can coexist through a competition-predation trade-off if predators are more abundant when the less defended species is common, and less abundant if the well-defended species is common (Holt et al. 1994). This effect has been criticized as being weak, because theoretical models suggest that only two species within a community can coexist because of this mechanism (Chase et al. 2002).
15.4.3 Coexistence
Figure \(\PageIndex{4}\): Coexistence theory attempts to explain the paradox of the plankton - how can ecologically similar species coexist without competitively excluding each other?
Coexistence theory is a framework to understand how competitor traits can maintain species diversity and stave-off competitive exclusion even among similar species living in ecologically similar environments.
Coexistence theory explains the stable coexistence of species as an interaction between two opposing forces: fitness differences between species, which should drive the best-adapted species to exclude others within a particular ecological niche, and stabilizing mechanisms, which maintains diversity via niche differentiation. For many species to be stabilized in a community, population growth must be negative density-dependent, i.e. all participating species have a tendency to increase in density as their populations decline. In such communities, any species that becomes rare will experience positive growth, pushing its population to recover and making local extinction unlikely. As the population of one species declines, individuals of that species tend to compete predominantly with individuals of other species. Thus, the tendency of a population to recover as it declines in density reflects reduced intraspecific competition (within-species) relative to interspecific competition (between-species), the signature of niche differentiation.
Figure \(\PageIndex{5}\): Groundhog and a raccoon eating together.
Two qualitatively different processes can help species to coexist: a reduction in average fitness differences between species or an increase in niche differentiation between species. These two factors have been termed equalizing and stabilizing mechanisms, respectively (Chesson 2000). For species to coexist, any fitness differences that are not reduced by equalizing mechanisms must be overcome by stabilizing mechanisms.
Equalizing mechanisms reduce fitness differences between species. As its name implies, these processes act in a way that merge the competitive abilities of multiple species closer together. Equalizing mechanisms affect interspecific competition (the competition between individuals of different species). For example, when multiple species compete for the same resource, competitive ability is determined by the minimum level of resources a species needs to maintain itself (known as an R*, or equilibrium resource density) (Tilman 1980). Thus, the species with the lowest R* is the best competitor and excludes all other species in the absence of any niche differentiation. Any factor that reduces the differences in R* level between species (like increased harvest of the dominant competitor) is classified as an equalizing mechanism. Environmental variation (which is the focus of the Intermediate Disturbance Hypothesis) can be considered an equalizing mechanism. Since the fitness of a given species is intrinsically tied to a specific environment, when that environment is disturbed (e.g. through storms, fires, volcanic eruptions, etc.) some species may lose components of their competitive advantage which were useful in the previous version of the environment.
Stabilizing mechanisms promote coexistence by concentrating intraspecific competition relative to interspecific competition. In other words, these mechanisms "encourage" an individual to compete more with other individuals of its own species, rather than with individuals of other species. Stabilizing mechanisms increase the low-density growth rate of all species. Resource partitioning (a type of niche differentiation) is a stabilizing mechanism because interspecific competition is reduced when different species primarily compete for different resources. Similarly, if species are differently affected by environmental variation (e.g., soil type, rainfall timing, etc.), this can create a stabilizing mechanism called the storage effect. The theory proposes one way for multiple species to coexist: in a changing environment, no species can be the best under all conditions (Chesson & Warner 1981). Instead, each species must have a unique response to varying environmental conditions, and a way of buffering against the effects of bad years. The storage effect gets its name because each population "stores" the gains in good years or microhabitats (patches) to help it survive population losses in bad years or patches.
15.4.4 Character Displacement
Figure \(\PageIndex{6}\): Several species of Galapagos finches display character displacement. Each closely related species differs in beak size and beak depth, allowing them to coexist in the same region since each species eats a different type of seed: the seed best fit for its unique beak. The finches with the deeper, stronger beaks consume large, tough seeds, while the finches with smaller beaks consume the smaller, softer seeds.
Character displacement is the phenomenon where differences among similar species whose distributions overlap geographically are accentuated in regions where the species co-occur, but are minimized or lost where the species' distributions do not overlap. This pattern results from evolutionary change driven by biological competition among species for a limited resource (e.g. food). The rationale for character displacement stems from the competitive exclusion principle, which contends that to coexist in a stable environment two competing species must differ in their respective ecological niche; without differentiation, one species will eliminate or exclude the other through competition.
For example, Darwin's finches can be found alone or together on the Galapagos Islands. Both species' populations actually have more individuals with intermediate-sized beaks when they live on islands without the other species present. However, when both species are present on the same island, competition is intense between individuals that have intermediate-sized beaks of both species because they all require intermediate sized seeds. Consequently, individuals with small and large beaks have greater survival and reproduction on these islands than individuals with intermediate-sized beaks. Different finch species can coexist if they have traits—for instance, beak size—that allow them to specialize on particular resources. When Geospiza fortis and Geospiza fuliginosa are present on the same island, G. fuliginosa tends to evolve a small beak and G. fortis a large beak. The observation that competing species' traits are more different when they live in the same area than when competing species live in different areas is called character displacement. For the two finch species, beak size was displaced: beaks became smaller in one species and larger in the other species.
Angert, A.L., Huxman, T.E., Chesson, P., & Venable, D.L. (2009). Functional tradeoffs determine species coexistence via the storage effect. Proceedings of the National Academy of Sciences, 106 (28), pp. 11641–11645. doi:10.1073/pnas.0904512106
Caldwell, J.P., & Vitt, L.J. (1999). Dietary asymmetry in leaf litter frogs and lizards in a transitional northern Amazonian rain forest. Oikos, 84 (3), pp. 383–397. doi:10.2307/3546419
Chase, J.M., Abrams, P.A., Grover, J.P., Diehl, S., Chesson, P., Holt, R.D., Richards, S.A., Nisbet, R.M., & Case, T.J. (2002). The interaction between predation and competition: A review and synthesis. Ecology Letters, 5 (2), pp. 302–315. doi:10.1046/j.1461-0248.2002.00315.x
Chesson, P., & Warner, R. (1981). Environmental variability promotes coexistence in lottery competitive systems. The American Naturalist, 117 (6), pp. 923–943. doi:10.1086/283778
Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics, 31 , pp. 343–366. doi:10.1146/annurev.ecolsys.31.1.343
Chesson, P., & Kuang, J.J. (2008). The interaction between predation and competition. Nature, 456 (7219), pp. 235–238. doi:10.1038/nature07248
Grover, J.P. (1997). Resource competition (1st ed.). London: Chapman & Hall . ISBN 978-0412749308
Hardin, G. (1960). The competitive exclusion principle. Science, 131 (3409), pp. 1292–1297. doi:10.1126/science.131.3409.1292
Holt, R.D., Grover, J., & Tilman, D. (1994). Simple rules for interspecific dominance in systems with exploitative and apparent competition. The American Naturalist, 144 (5), pp. 741–771. doi:10.1086/285705
Hutchinson, G.E. (1961). The paradox of the plankton. The American Naturalist, 95 (882), pp. 137–145. doi:10.1086/282171
MacArthur, R.H. (1958). Population ecology of some warblers of northeastern coniferous forests. Ecology, 39 (4), pp. 599-619.
Pacala, S.W., & Roughgarden, J. (1985). Population experiments with the Anolis lizards of St. Maarten and St. Eustatius. Ecology, 66 (1), pp. 129–141. doi:10.2307/1941313
Sedio, B.E., Ostling, A.M. (2013). How specialised must natural enemies be to facilitate coexistence among plants? Ecology Letters, 16 (8), pp. 995–1003. doi:10.1111/ele.12130
Tilman, D. (1980). Resources: A graphical-mechanistic approach to competition and predation. The American Naturalist, 116 (3), pp. 362–393. doi:10.1086/283633
Contributors and Attributions
This chapter was written by N. Gownaris and T. Zallek, with text taken from the following CC-BY resources:
Competitive exclusion principle by Wikipedia, the free encyclopedia
Ecological niche by Wikipedia, the free encyclopedia
Coexistence theory by Wikipedia, the free encyclopedia
Character displacement by Wikipedia, the free encyclopedia

IMAGES
VIDEO
COMMENTS
Character displacement is the phenomenon where differences among similar species whose distributions overlap geographically are accentuated in regions where the species co-occur, but are minimized or lost where the species' distributions do not overlap. This pattern results from evolutionary change driven by biological competition among species ...
Character displacement processes are widely considered to have a major role in structuring ecological communities, in the generation of phenotypic diversity, and in the evolution of barriers to reproduction between populations, culminating in speciation. ... Discusses various aspects of the hypothesis that reproductive isolation evolves in ...
In sum, several case studies provide evidence that is consistent with the plasticity-first hypothesis for character displacement. Nevertheless, much more work is needed to assess how general this mechanism is for explaining character displacement. As we discuss in more detail below, additional theoretical and empirical work is needed to fully ...
Character displacement - trait evolution stemming from selection to lessen resource competition or reproductive interactions between species - has long been viewed as an important mechanism for enabling closely related species to coexist. ... This hypothesis, termed the competitive exclusion principle (Hardin, 1960), forms a cornerstone of ...
A foundational concept in evolutionary ecology is that resource competition and other costly interactions drive trait divergence between closely related species—a process known as "character displacement.". Consistent with this hypothesis is the observation that trait disparity is generally greater between co-occurring (sympatric) versus ...
As character displacement increases the chances of species coexistence, it can play a key role in structuring ecological communities. It has also been implicated in the formation of new species and in large-scale evolutionary phenomena, such as adaptive radiation. Thus, character displacement may be central in the origin, maintenance and ...
Ecological character displacement, mostly seen as increased differences of size in sympatry between closely-related or similar species, is a focal hypothesis assuming that species too similar to one another could not coexist without diverging, owing to interspecific competition.
displacement. Generally, character displacement is well supported empirically, and it remains a vital explanation for how new species arise and diversify. Keywords: competition, Darwin's divergence of character, hybridi-zation, phenotypic plasticity, sexual selection, speciation. Divergence of character … is of high importance on my the-
Character displacement occurs when competition for either resources or successful reproduction imposes divergent selection on interacting species, causing divergence in traits associated with resource use or reproduction. ... This plasticity-first hypothesis predicts that character displacement should be generally mediated by ancestral ...
Character displacement was originally defined simply as a pattern - divergence between two species in sympatry but not allopatry - and it was recognized that multiple processes might generate this pattern. However, over time, character displacement has come to be nearly synonymous with the process of adaptive divergence between species ...
for character displacement research, and our specific goals are threefold. First, we seek to unite ecological and reproductive character displacement under the same conceptual framework. Second, we under-score the value of exploring more fully the ecological and evolutionary causes and consequences of character displacement.
Testing the hypothesis of Ecological Character Displacement between the Sexes Past workers have suggested a number of approaches for testing the hypothesis of ECD between the sexes, ranging from simple morphometric analysis of ecological sex differences (Shine 1989 ), to phenotypic selection analyses (Price 1984 , Hedrick and Temeles 1989 ), to ...
Character displacement is an evolutionary hypothesis, yet the number of examples of adaptive phenotypic plasticity continues to increase 51, 52. Thus, the potential for plastic responses to produce a pattern of ECD must be ruled out. ... Criterion 3: the character displacement pattern results from an evolutionary shift rather than from species ...
In this study, the concept of dynamic character displacement among interacting bacterial species from leaf-colonizing families was empirically tested using a proteomics approach. A phenotypic ...
ABSTRACT Character displacement is the process by which traits evolve in response to selection to lessen resource competition or reproductive interactions between species. Although character displacement has long been viewed as an important mechanism for enabling closely related species to coexist, the causes and consequences of character displacement have not been fully explored. Moreover ...
The evolutionary consequences of competition are of great interest to researchers studying sympatric speciation, adaptive radiation, species coexistence and ecological assembly. Competition's role in driving evolutionary change in phenotypic distributions, and thus causing ecological character displacement, has been inferred from biogeographical data and measurements of divergent selection on ...
Studies of ecological character displacement and community-wide character displacement span a wide range of taxa including plants; the majority support the hypothesis. The studies, however, come in taxonomic clusters of wellstudied test-cases, notably carnivorous mammals, Galapagos finches, Anolis lizards on islands, threespine sticklebacks ...
Character displacement was originally defined simply as a pattern - divergence between two species in sympatry but not allopatry - and it was recognized that multiple processes might generate this pattern. However, over time, character displacement has come to be nearly synonymous with the process of adaptive divergence between species ...
An alternative hypothesis, first suggested by West-Eberhard , is that agonistic signals functioning in social competition may also diverge to reduce the costs of misdirected interspecific aggression—a process now termed agonistic character displacement (ACD) [12-14].
Character displacement, first defined in the animal literature, is frequently used to explain these patterns. Character displacement is the process whereby competing species respond to selection to increase their mean difference in a trait associated with resource use or reproduction (Brown and Wilson 1956; Mayr 1970; Pfennig and Pfennig 2009 ...
15.4.4 Character Displacement. Figure \(\PageIndex{6}\): Several species of Galapagos finches display character displacement.Each closely related species differs in beak size and beak depth, allowing them to coexist in the same region since each species eats a different type of seed: the seed best fit for its unique beak.
Character displacement (1, 2) is an evolu-tionary divergence in resource-exploiting traits such as jaws and beaks that is caused by interspecific competition (3-5). It has the potential to explain nonrandom patterns of co-occurrence and morphological differences between coexisting species (6-10).
Character displacement is an evolutionary divergence that occurs when two similar species inhabit the same environment. In this instance, natural selection favors those organisms that develop ...
The load-displacement curve of six concrete composite slabs with steel lattice girders is displayed in Figure 15 . Basic information of the load-displacement curve of six concrete composite slabs with steel lattice girders is shown in Table 5. These slabs are similar in size to CDBP4812, enabling a direct and accurate comparison.