- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
This article has a correction. Please see:
- New advances in type 1 diabetes - June 03, 2024
- Savitha Subramanian , professor of medicine ,
- Farah Khan , clinical associate professor of medicine ,
- Irl B Hirsch , professor of medicine
- University of Washington Diabetes Institute, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA, USA
- Correspondence to: I B Hirsch ihirsch{at}uw.edu
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to modify disease course and preserve β cell function have expanded our broad understanding of this condition. Biomarkers of type 1 diabetes are detectable months to years before development of overt disease, and three stages of diabetes are now recognized. The advent of continuous glucose monitoring and the newer automated insulin delivery systems have changed the landscape of type 1 diabetes management and are associated with improved glycated hemoglobin and decreased hypoglycemia. Adjunctive therapies such as sodium glucose cotransporter-1 inhibitors and glucagon-like peptide 1 receptor agonists may find use in management in the future. Despite these rapid advances in the field, people living in under-resourced parts of the world struggle to obtain necessities such as insulin, syringes, and blood glucose monitoring essential for managing this condition. This review covers recent developments in diagnosis and treatment and future directions in the broad field of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune condition that occurs as a result of destruction of the insulin producing β cells of the pancreatic islets, usually leading to severe endogenous insulin deficiency. 1 Without treatment, diabetic ketoacidosis will develop and eventually death will follow; thus, lifelong insulin therapy is needed for survival. Type 1 diabetes represents 5-10% of all diabetes, and diagnosis classically occurs in children but can also occur in adulthood. The burden of type 1 diabetes is expansive; it can result in long term complications, decreased life expectancy, and reduced quality of life and can add significant financial burden. Despite vast improvements in insulin, insulin delivery, and glucose monitoring technology, a large proportion of people with type 1 diabetes do not achieve glycemic goals. The massive burden of type 1 diabetes for patients and their families needs to be appreciated. The calculation and timing of prandial insulin dosing, often from food with unknown carbohydrate content, appropriate food and insulin dosing when exercising, and cost of therapy are all major challenges. The psychological realities of both acute management and the prospect of chronic complications add to the burden. Education programs and consistent surveillance for “diabetes burnout” are ideally available to everyone with type 1 diabetes.
In this review, we discuss recent developments in the rapidly changing landscape of type 1 diabetes and highlight aspects of current epidemiology and advances in diagnosis, technology, and management. We do not cover the breadth of complications of diabetes or certain unique scenarios including psychosocial aspects of type 1 diabetes management, management aspects specific to older adults, and β cell replacement therapies. Our review is intended for the clinical reader, including general internists, family practitioners, and endocrinologists, but we acknowledge the critical role that people living with type 1 diabetes and their families play in the ongoing efforts to understand this lifelong condition.
Sources and selection criteria
We did individual searches for studies on PubMed by using terms relevant to the specific topics covered in this review pertaining to type 1 diabetes. Search terms used included “type 1 diabetes” and each individual topic—diagnosis, autoantibodies, adjuvant therapies, continuous glucose monitoring, automated insulin delivery, immunotherapies, diabetic ketoacidosis, hypoglycemia, and under-resourced settings. We considered all studies published in the English language between 1 January 2001 and 31 January 2023. We selected publications outside of this timeline on the basis of relevance to each topic. We also supplemented our search strategy by a hand search of the references of key articles. We prioritized studies on each highlighted topic according to the level of evidence (randomized controlled trials (RCTs), systematic reviews and meta-analyses, consensus statements, and high quality observational studies), study size (we prioritized studies with at least 50 participants when available), and time of publication (we prioritized studies published since 2003 except for the landmark Diabetes Control and Complications Trial and a historical paper by Tuomi on diabetes autoantibodies, both from 1993). For topics on which evidence from RCTs was unavailable, we included other study types of the highest level of evidence available. To cover all important clinical aspects of the broad array of topics covered in this review, we included additional publications such as clinical reviews as appropriate on the basis of clinical relevance to both patients and clinicians in our opinion.
Epidemiology
The incidence of type 1 diabetes is rising worldwide, possibly owing to epigenetic and environmental factors. Globally in 2020 an estimated 8.7 million people were living with type 1 diabetes, of whom approximately 1.5 million were under 20 years of age. 2 This number is expected to rise to more than 17 million by 2040 ( https://www.t1dindex.org/#global ). The International Diabetes Federation estimates the global prevalence of type 1 diabetes at 0.1%, and this is likely an underestimation as diagnoses of type 1 diabetes in adults are often not accounted for. The incidence of adult onset type 1 diabetes is higher in Europe, especially in Nordic countries, and lowest in Asian countries. 3 Adult onset type 1 diabetes is also more prevalent in men than in women. An increase in prevalence in people under 20 years of age has been observed in several western cohorts including the US, 4 5 Netherlands, 6 Canada, 7 Hungary, 8 and Germany. 9
Classically, type 1 diabetes presents over the course of days or weeks in children and adolescents with polyuria, polydipsia, and weight loss due to glycosuria. The diagnosis is usually straightforward, with profound hyperglycemia (often >300 mg/dL) usually with ketonuria with or without ketoacidemia. Usually, more than one autoantibody is present at diagnosis ( table 1 ). 10 The number of islet autoantibodies combined with parameters of glucose tolerance now forms the basis of risk prediction for type 1 diabetes, with stage 3 being clinical disease ( fig 1 ). 11 The originally discovered autoantibody, islet cell antibody, is no longer used clinically owing to variability of the assay despite standardisation. 12
Autoantibody characteristics associated with increased risk of type 1 diabetes 10
- View inline

Natural history of type 1 diabetes. Adapted with permission from Insel RA, et al. Diabetes Care 2015;38:1964-74 11
- Download figure
- Open in new tab
- Download powerpoint
Half of all new cases of type 1 diabetes are now recognized as occurring in adults. 13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people. 14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable. The term latent autoimmune diabetes of adults (LADA) was introduced 30 years ago to identify adults who developed immune mediated diabetes. 15 An international consensus defined the diagnostic criteria for LADA as age >30 years, lack of need for insulin use for at least six months, and presence of islet cell autoantibodies. 16 However, debate as to whether the term LADA should even be used as a diagnostic term persists. The American Diabetes Association (ADA) Standards of Care note that for the purpose of classification, all forms of diabetes mediated by autoimmune β cell destruction are included in the classification of type 1 diabetes. 17 Nevertheless, they note that use of the term LADA is acceptable owing to the practical effect of heightening awareness of adults likely to have progressive autoimmune β cell destruction and thereby accelerating insulin initiation by clinicians to prevent diabetic ketoacidosis.
The investigation of adults with suspected type 1 diabetes is not always straightforward ( fig 2 ). 18 Islet cell autoantibodies such as glutamic acid decarboxylase antibody (GADA), tyrosine phosphatase IA2 antibody, and zinc transporter isoform 8 autoantibody act as markers of immune activity and can be detected in the blood with standardized assays ( table 1 ). The presence of one or more antibodies in adults with diabetes could mark the progression to severe insulin deficiency; these individuals should be considered to have type 1 diabetes. 1 Autoantibodies, especially GADA, should be measured only in people with clinically suspected type 1 diabetes, as low concentrations of GADA can be seen in type 2 diabetes and thus false positive measurements are a concern. 19 That 5-10% of cases of type 1 diabetes may occur without diabetes autoantibodies is also now clear, 20 and that the diabetes autoantibodies disappear over time is also well appreciated. 21

Flowchart for investigation of suspected type 1 diabetes in adults, based on data from white European populations. No single clinical feature in isolation confirms type 1 diabetes. The most discriminative feature is younger age at diagnosis (<35 years), with lower body mass index (<25), unintentional weight loss, ketoacidosis, and glucose >360 mg/dL at presentation. Adapted with permission from Holt RIG, et al. Diabetes Care 2021;44:2589-625 1
Genetic risk scoring (GRS) for type 1 diabetes has received attention to differentiate people whose classification is unclear. 22 23 24 Developed in 2019, the T1D-GRS2 uses 67 single nucleotide polymorphisms from known autoimmune loci and can predict type 1 diabetes in children of European and African ancestry. Although GRS is not available for routine clinical use, it may allow prediction of future cases of type 1 diabetes to allow prevention strategies with immune intervention (see below).
A major change in the type 1 diabetes phenotype has occurred over the past few decades, with an increase in obesity; the reasons for this are complex. In the general population, including people with type 1 diabetes, an epidemic of sedentary lifestyles and the “westernized diet” consisting of increased processed foods, refined sugars, and saturated fat is occurring. In people with type 1 diabetes, the overall improvement in glycemic control since the report of the Diabetes Control and Complications Trial (DCCT) in 1993 (when one or two insulin injections a day was standard therapy) has resulted in less glycosuria so that the typical patient with lower body weight is uncommon in high income countries. In the US T1D Exchange, more than two thirds of the adult population were overweight or obese. 25
Similarly, obesity in young people with type 1 diabetes has also increased over the decades. 26 The combination of autoimmune insulin deficiency with obesity and insulin resistance has received several descriptive names over the years, with this phenotype being described as double diabetes and hybrid diabetes, among others, 26 27 but no formal nomenclature in the diabetes classification exists. Many of these patients have family members with type 2 diabetes, and some patients probably do have both types of diabetes. Clinically, minimal research has been done into how this specific population responds to certain antihyperglycemic oral agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists, given the glycemic, weight loss, and cardiovascular benefits seen with these agents. 28 These patients are common in most adult diabetes practices, and weight management in the presence of insulin resistance and insulin deficiency remains unclear.
Advances in monitoring
The introduction of home blood glucose monitoring (BGM) more than 45 years ago was met with much skepticism until the report of the DCCT. 29 Since then, home BGM has improved in accuracy, precision, and ease of use. 30 Today, in many parts of the world, home BGM, a static measurement of blood glucose, has been replaced by continuous glucose monitoring (CGM), a dynamic view of glycemia. CGM is superior to home BGM for glycemic control, as confirmed in a meta-analysis of 21 studies and 2149 participants with type 1 diabetes in which CGM use significantly decreased glycated hemoglobin (HbA 1c ) concentrations compared with BGM (mean difference −0.23%, 95% confidence interval −3.83 to −1.08; P<0.001), with a greater benefit if baseline HbA 1c was >8% (mean difference −0.43%, −6.04 to −3.30; P<0.001). 31 This newer technology has also evolved into a critical component of automated insulin delivery. 32
CGM is the standard for glucose monitoring for most adults with type 1 diabetes. 1 This technology uses interstitial fluid glucose concentrations to estimate blood glucose. Two types of CGM are available. The first type, called “real time CGM”, provides a continuous stream of glucose data to a receiver, mobile application, smartwatch, or pump. The second type, “intermittently scanned CGM,” needs to be scanned by a reader device or smartphone. Both of these technologies have shown improvements in HbA 1c and amount of time spent in the hypoglycemic range compared with home BGM when used in conjunction with multiple daily injections or “open loop” insulin pump therapy. 33 34 Real time CGM has also been shown to reduce hypoglycemic burden in older adults with type 1 diabetes ( table 2 ). 36 Alerts that predict or alarm with both hypoglycemia and hyperglycemia can be customized for the patient’s situation (for example, a person with unawareness of hypoglycemia would have an alert at a higher glucose concentration). Family members can also remotely monitor glycemia and be alerted when appropriate. The accuracy of these devices has improved since their introduction in 2006, so that currently available sensors can be used without a confirmation glucose concentration to make a treatment decision with insulin. However, some situations require home BGM, especially when concerns exist that the CGM does not match symptoms of hypoglycemia.
Summary of trials for each topic covered
Analysis of CGM reports retrospectively can assist therapeutic decision making both for the provider and the patient. Importantly, assessing the retrospective reports and watching the CGM in real time together offer insight to the patient with regard to insulin dosing, food choices, and exercise. Patients should be encouraged to assess their data on a regular basis to better understand their diabetes self-management. Table 3 shows standard metrics and targets for CGM data. 52 Figure 3 shows an ambulatory glucose profile.
Standardized continuous glucose monitoring metrics for adults with diabetes 52

Example of ambulatory glucose profile of 52 year old woman with type 1 diabetes and fear of hypoglycemia. CGM=continuous glucose monitoring; GMI=glucose management indicator
Improvements in technology and evidence for CGM resulting in international recommendations for its widespread use have resulted in greater uptake by people with type 1 diabetes across the globe where available and accessible. Despite this, not everyone wishes to use it; some people find wearing any device too intrusive, and for many the cost is prohibitive. These people need at the very least before meal and bedtime home BGM.
A next generation implantable CGM device (Sensionics), with an improved calibration algorithm that lasts 180 days after insertion by a healthcare professional, is available in both the EU and US. Although fingerstick glucose calibration is needed, the accuracy is comparable to that of other available devices. 53
Advances in treatments
The discovery of insulin in 1921, resulting in a Nobel Prize, was considered one of the greatest scientific achievements of the 20th century. The development of purified animal insulins in the late 1970s, followed by human insulin in the early 1980s, resulted in dramatic reductions in allergic reactions and lipoatrophy. Introduction of the first generation of insulin analogs, insulin lispro in the mid-1990s followed by insulin glargine in the early 2000s, was an important advance for the treatment of type 1 diabetes. 54 We review the next generation of insulin analogs here. Table 4 provides details on available insulins.
Pharmacokinetics of commonly used insulin preparations
Ultra-long acting basal insulins
Insulin degludec was developed with the intention of improving the duration of action and achieving a flatter profile compared with the original long acting insulin analogs, insulin glargine and insulin detemir. Its duration of action of 42 hours at steady state means that the profile is generally flat without significant day-to-day variability, resulting in less hypoglycemia compared with U-100 glargine. 39 55
When U-100 insulin glargine is concentrated threefold, its action is prolonged. 56 U-300 glargine has a different kinetic profile and is delivered in one third of the volume of U-100 glargine, with longer and flatter effects. The smaller volume of U-300 glargine results in slower and more gradual release of insulin monomers owing to reduced surface area in the subcutaneous space. 57 U-300 glargine also results in lesser hypoglycemia compared with U-100 glargine. 58
Ultra-rapid acting prandial insulins
Rapid acting insulin analogs include insulin lispro, aspart, and glulisine. With availability of insulin lispro, the hope was for a prandial insulin that better matched food absorption. However, these newer insulins are too slow to control the glucose spike seen with ingestion of a high carbohydrate load, leading to the development of insulins with even faster onset of action.
The first available ultra-rapid prandial insulin was fast acting insulin aspart. This insulin has an onset of appearance approximately twice as fast (~5 min earlier) as insulin aspart, whereas dose-concentration and dose-response relations are comparable between the two insulins ( table 4 ). 59 In adults with type 1 diabetes, mealtime and post-meal fast acting aspart led to non-inferior glycemic control compared with mealtime aspart, in combination with basal insulin. 60 Mean HbA 1c was 7.3%, 7.3%, and 7.4% in the mealtime faster aspart, mealtime aspart, and post‐meal faster aspart arms, respectively (P<0.001 for non-inferiority).
Insulin lispro-aabc is the second ultra-rapid prandial insulin. In early kinetic studies, insulin lispro-aabc appeared in the serum five minutes faster with 6.4-fold greater exposure in the first 15 minutes compared with insulin lispro. 61 The duration of exposure of the insulin concentrations in this study was 51 minutes faster with lispro-aabc. Overall insulin exposure was similar between the two groups. Clinically, lispro-aabc is non-inferior to insulin lispro, but postprandial hyperglycemia is lower with the faster acting analog. 62 Lispro-aabc given at mealtime resulted in greater improvement in post-prandial glucose (two hour post-prandial glucose −31.1 mg/dL, 95% confidence interval −41.0 to −21.2; P<0.001).
Both ultra-rapid acting insulins can be used in insulin pumps. Lispro-aabc tends to have more insertion site reactions than insulin lispro. 63 A meta-analysis including nine studies and 1156 participants reported increased infusion set changes on rapid acting insulin analogs (odds ratio 1.60, 95% confidence interval 1.26 to 2.03). 64
Pulmonary inhaled insulin
The quickest acting insulin is pulmonary inhaled insulin, with an onset of action of 12 minutes and a duration of 1.5-3 hours. 65 When used with postprandial supplemental dosing, glucose control is improved without an increase in hypoglycemia. 66
Insulin delivery systems
Approved automated insulin delivery systems.
CGM systems and insulin pumps have shown improvement in glycemic control and decreased risk of severe hypoglycemia compared with use of self-monitoring of blood glucose and multiple daily insulin injections in type 1 diabetes. 67 68 69 Using CGM and insulin pump together (referred to as sensor augmented pump therapy) only modestly improves HbA 1c in patients who have high sensor wear time, 70 71 but the management burden of diabetes does not decrease as frequent user input is necessary. Thus emerged the concept of glucose responsive automated insulin delivery (AID), in which data from CGM can inform and allow adjustment of insulin delivery.
In the past decade, exponential improvements in CGM technologies and refined insulin dosing pump algorithms have led to the development of AID systems that allow for minimization of insulin delivery burden. The early AID systems reduced hypoglycemia risk by automatically suspending insulin delivery when glucose concentrations dropped to below a pre-specified threshold but did not account for high glucose concentrations. More complex algorithms adjusting insulin delivery up and down automatically in response to real time sensor glucose concentrations now allow close replication of normal endocrine pancreatic physiology.
AID systems (also called closed loop or artificial pancreas systems) include three components—an insulin pump that continuously delivers rapid acting insulin, a continuous glucose sensor that measures interstitial fluid glucose at frequent intervals, and a control algorithm that continuously adjusts insulin delivery that resides in the insulin pump or a smartphone application or handheld device ( fig 4 ). All AID systems that are available today are referred to as “hybrid” closed loop (HCL) systems, as users are required to manually enter prandial insulin boluses and signal exercise, but insulin delivery is automated at night time and between meals. AID systems, regardless of the type used, have shown benefit in glycemic control and cost effectiveness, improve quality of life by improving sleep quality, and decrease anxiety and diabetes burden in adults and children. 72 73 74 Limitations to today’s HCL systems are primarily related to pharmacokinetics and pharmacodynamics of available analog insulins and accuracy of CGM in extremes of blood glucose values. The iLet bionic pancreas, cleared by the US Food and Drug Administration (FDA) in May 2023, is an AID system that determines all therapeutic insulin doses for an individual on the basis of body weight, eliminating the need for calculation of basal rates, insulin to carbohydrate ratios, blood glucose corrections, and bolus dose. The control algorithms adapt continuously and autonomously to the individual’s insulin needs. 38 Table 5 lists available AID systems.

Schematic of closed loop insulin pump technology. The continuous glucose monitor senses interstitial glucose concentrations and sends the information via Bluetooth to a control algorithm hosted on an insulin pump (or smartphone). The algorithm calculates the amount of insulin required, and the insulin pump delivers rapid acting insulin subcutaneously
Comparison of commercially available hybrid closed loop systems 75
Unapproved systems
Do-it-yourself (DIY) closed loop systems—DIY open artificial pancreas systems—have been developed by people with type 1 diabetes with the goal of self-adjusting insulin by modifying their individually owned devices. 76 These systems are built by the individual using an open source code widely available to anyone with compatible medical devices who is willing and able to build their own system. DIY systems are used by several thousand people across the globe but are not approved by regulatory bodies; they are patient-driven and considered “off-label” use of technology with the patient assuming full responsibility for their use. Clinicians caring for these patients should ensure basic diabetes skills, including pump site maintenance, a knowledge of how the chosen system works, and knowing when to switch to “manual mode” for patients using an artificial pancreas system of any kind. 76 The small body of studies on DIY looping suggests improvement in HbA 1c , increased time in range, decreased hypoglycemia and glucose variability, improvement in night time blood glucose concentrations, and reduced mental burden of diabetes management. 77 78 79 Although actively prescribing or initiating these options is not recommended, these patients should be supported by clinical teams; insulin prescription should not be withheld, and, if initiated by the patient, unregulated DIY options should be openly discussed to ensure open and transparent relationships. 78
In January 2023, the US FDA cleared the Tidepool Loop app, a DIY AID system. This software will connect the CGM, insulin pump, and Loop algorithm, but no RCTs using this method are available.
β cell replacement therapies
For patients with type 1 diabetes who meet specific clinical criteria, β cell replacement therapy using whole pancreas or pancreatic islet transplantation can be considered. Benefits of transplantation include immediate cessation of insulin therapy, attainment of euglycemia, and avoidance of hypoglycemia. Additional benefits include improved quality of life and stabilization of complications. 80 Chronic immunosuppression is needed to prevent graft rejection after transplantation.
Pancreas transplantation
Whole pancreas transplantation, first performed in 1966, involves complex abdominal surgery and lifelong immunosuppressive therapy and is limited by organ donor availability. Today, pancreas transplants are usually performed simultaneously using two organs from the same donor (simultaneous pancreas-kidney transplant (SPKT)), sequentially if the candidate has a living donor for renal transplantation (pancreas after kidney transplant (PAKT)) or on its own (pancreas transplantation alone). Most whole pancreas transplants are performed with kidney transplantation for end stage diabetic kidney disease. Pancreas graft survival at five years after SPKT is 80% and is superior to that with pancreas transplants alone (62%) or PAKT (67%). 81 Studies from large centers where SPKT is performed show that recipients can expect metabolic improvements including amelioration of problematic hypoglycemia for at least five years. 81 The number of pancreas transplantations has steadily decreased in the past two decades.
Islet transplantation
Islet transplantation can be pursued in selected patients with type 1 diabetes marked by unawareness of hypoglycemia and severe hypoglycemic episodes, to help restore the α cell response critical for responding to hypoglycemia. 82 83 Islet transplantation involves donor pancreas procurement with subsequent steps to isolate, purify, culture, and infuse the islets. Multiple donors are needed to provide enough islet cells to overcome islet cell loss during transplantation. Survival of the islet grafts, limited donor supply, and lifelong need for immunosuppressant therapy remain some of the biggest challenges. 84 Islet transplantation remains experimental in the US and is offered in a few specialized centers in North America, some parts of Europe, and Australia. 85
Disease modifying treatments for β cell preservation
Therapies targeting T cells, B cells, and cytokines that find use in a variety of autoimmune diseases have also been applied to type 1 diabetes. The overarching goal of immune therapies in type 1 diabetes is to prevent or delay the loss of functional β cell mass. Studies thus far in early type 1 diabetes have not yet successfully shown reversal of loss of C peptide or maintenance of concentrations after diagnosis, although some have shown preservation or slowing of loss of β cells. This suggests that a critical time window of opportunity exists for starting treatment depending on the stage of type 1 diabetes ( fig 1 ).
Teplizumab is a humanized monoclonal antibody against the CD3 molecule on T cells; it is thought to modify CD8 positive T lymphocytes, key effector cells that mediate β cell death and preserves regulatory T cells. 86 Teplizumab, when administered to patients with new onset of type 1 diabetes, was unable to restore glycemia despite C peptide preservation. 87 However, in its phase II prevention study of early intervention in susceptible individuals (at least two positive autoantibodies and an abnormal oral glucose tolerance test at trial entry), a single course of teplizumab delayed progression to clinical type 1 diabetes by about two years ( table 2 ). 43 On the basis of these results, teplizumab received approval in the US for people at high risk of type 1 diabetes in November 2022. 88 A phase III trial (PROTECT; NCT03875729 ) to evaluate the efficacy and safety of teplizumab versus placebo in children and adolescents with new diagnosis of type 1 diabetes (within six weeks) is ongoing. 89
Thus far, targeting various components of the immune response has been attempted in early type 1 diabetes without any long term beneficial effects on C peptide preservation. Co-stimulation blockade using CTLA4-Ig abatacept, a fusion protein that interferes with co-stimulation needed in the early phases of T cell activation that occurs in type 1 diabetes, is being tested for efficacy in prevention of type 1 diabetes ( NCT01773707 ). 90 Similarly, several cytokine directed anti-inflammatory targets (interleukin 6 receptor, interleukin 1β, tumor necrosis factor ɑ) have not shown any benefit.
Non-immunomodulatory adjunctive therapies
Adjunctive therapies for type 1 diabetes have been long entertained owing to problems surrounding insulin delivery, adequacy of glycemic management, and side effects associated with insulin, especially weight gain and hypoglycemia. At least 50% of adults with type 1 diabetes are overweight or obese, presenting an unmet need for weight management in these people. Increased cardiovascular risk in these people despite good glycemic management presents additional challenges. Thus, use of adjuvant therapies may tackle these problems.
Metformin, by decreasing hepatic glucose production, could potentially decrease fasting glucose concentrations. 91 It has shown benefit in reducing insulin doses and possibly improving metabolic control in obese/overweight people with type 1 diabetes. A meta-analysis of 19 RCTs suggests short term improvement in HbA 1c that is not sustained after three months and is associated with higher incidence of gastrointestinal side effects. 92 No evidence shows that metformin decreases cardiovascular morbidity in type 1 diabetes. Therefore, owing to lack of conclusive benefit, addition of metformin to treatment regimens is not recommended in consensus guidelines.
Glucagon-like peptide receptor agonists
Endogenous GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion and enhances glucose induced insulin secretion, suppresses glucagon secretion, delays gastric emptying, and induces satiety. 93 GLP-1 promotes β cell proliferation and inhibits apoptosis, leading to expansion of β cell mass. GLP-1 secretion in patients with type 1 diabetes is similar to that seen in people without diabetes. Early RCTs of liraglutide in type 1 diabetes resulted in weight loss and modest lowering of HbA 1c ( table 2 ). 49 50 Liraglutide 1.8 mg in people with type 1 diabetes and higher body mass index decreased HbA 1c , weight, and insulin requirements with no increased hypoglycemia risk. 94 However, on the basis of results from a study of weekly exenatide that showed similar results, these effects may not be sustained. 51 A meta-analysis of 24 studies including 3377 participants showed that the average HbA 1c decrease from GLP-1 receptor agonists compared with placebo was highest for liraglutide 1.8 mg daily (−0.28%, 95% confidence interval −0.38% to−0.19%) and exenatide (−0.17%, −0.28% to 0.02%). The estimated weight loss from GLP-1 receptor agonists compared with placebo was −4.89 (−5.33 to−4.45) kg for liraglutide 1.8 mg and −4.06 (−5.33 to−2.79) kg for exenatide. 95 No increase in severe hypoglycemia was seen (odds ratio 0.67, 0.43 to 1.04) but therapy was associated with higher levels of nausea. GLP-1 receptor agonist use may be beneficial for weight loss and reducing insulin doses in a subset of patients with type 1 diabetes. GLP-1 receptor agonists are not a recommended treatment option in type 1 diabetes. Semaglutide is being studied in type 1 diabetes in two clinical trials ( NCT05819138 ; NCT05822609 ).
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter 2 (SGLT-2), a protein expressed in the proximal convoluted tubule of the kidney, reabsorbs filtered glucose; its inhibition prevents glucose reabsorption in the tubule and increases glucose excretion by the kidney. Notably, the action of these agents is independent of insulin, so this class of drugs has potential as adjunctive therapy for type 1 diabetes. Clinical trials have shown significant benefit in cardiovascular and renal outcomes in type 2 diabetes; therefore, significant interest exists for use in type 1 diabetes. Several available SGLT-2 inhibitors have been studied in type 1 diabetes and have shown promising results with evidence of decreased total daily insulin dosage, improvement in HbA 1c , lower rates of hypoglycemia, and decrease in body weight; however, these effects do not seem to be sustained at one year in clinical trials and seem to wane with time. Despite beneficial effects, increased incidence of diabetic ketoacidosis has been observed in all trials, is a major concern, and is persistent despite educational efforts. 96 97 98 Low dose empagliflozin (2.5 mg) has shown lower rates of diabetic ketoacidosis in clinical trials ( table 2 ). 47 Favorable risk profiles have been noted in Japan, the only market where SGLT-2 inhibitors are approved for adjunctive use in type 1 diabetes. 99 In the US, SGLT-2 inhibitors are approved for use in type 2 diabetes only. In Europe, although dapagliflozin was approved for use as adjunct therapy to insulin in adults with type 1 diabetes, the manufacturer voluntarily withdrew the indication for the drug in 2021. 100 Sotagliflozin is a dual SGLT-1 and SGLT-2 inhibitor that decreases renal glucose reabsorption through systemic inhibition of SGLT-2 and decreases glucose absorption in the proximal intestine by SGLT-1 inhibition, blunting and delaying postprandial hyperglycemia. 101 Studies of sotagliflozin in type 1 diabetes have shown sustained HbA 1c reduction, weight loss, lower insulin requirements, lesser hypoglycemia, and more diabetic ketoacidosis relative to placebo. 102 103 104 The drug received authorization in the EU for use in type 1 diabetes, but it is not marketed there. Although SGLT inhibitors are efficacious in type 1 diabetes management, the risk of diabetic ketoacidosis is a major limitation to widespread use of these agents.
Updates in acute complications of type 1 diabetes
Diabetic ketoacidosis.
Diabetic ketoacidosis is a serious and potentially fatal hyperglycemic emergency accompanied by significant rates of mortality and morbidity as well as high financial burden for healthcare systems and societies. In the past decade, increasing rates of diabetic ketoacidosis in adults have been observed in the US and Europe. 105 106 This may be related to changes in the definition of diabetic ketoacidosis, use of medications associated with higher risk, and admission of patients at lower risk. 107 In a US report of hospital admissions with diabetic ketoacidosis, 53% of those admitted were between the ages of 18 and 44, with higher rates in men than in women. 108 Overall, although mortality from diabetic ketoacidosis in developed countries remains low, rates have risen in people aged >60 and in those with coexisting life threatening illnesses. 109 110 Recurrent diabetic ketoacidosis is associated with a substantial mortality rate. 111 Frequency of diabetic ketoacidosis increases with higher HbA 1c concentrations and with lower socioeconomic status. 112 Common precipitating factors include newly diagnosed type 1 diabetes, infection, poor adherence to insulin, and an acute cardiovascular event. 109
Euglycemic diabetic ketoacidosis refers to the clinical picture of an increased anion gap metabolic acidosis, ketonemia, or significant ketonuria in a person with diabetes without significant glucose elevation. This can be seen with concomitant use of SGLT-2 inhibitors (currently not indicated in type 1 diabetes), heavy alcohol use, cocaine use, pancreatitis, sepsis, and chronic liver disease and in pregnancy 113 Treatment is similar to that for hyperglycemic diabetic ketoacidosis but can require earlier use and greater concentrations of a dextrose containing fluid for the insulin infusion in addition to 0.9% normal saline resuscitation fluid. 114
The diagnosis of diabetic ketoacidosis has evolved from a gluco-centric diagnosis to one requiring hyperketonemia. By definition, independent of blood glucose, a β-hydroxybutyrate concentration >3 mmol/L is required for diagnosis. 115 However, the use of this ketone for assessment of the severity of the diabetic ketoacidosis is controversial. 116 Bedside β-hydroxybutyrate testing during treatment is standard of care in many parts of the world (such as the UK) but not others (such as the US). Concerns have been raised about accuracy of bedside β-hydroxybutyrate meters, but this is related to concentrations above the threshold for diabetic ketoacidosis. 116
Goals for management of diabetic ketoacidosis include restoration of circulatory volume, correction of electrolyte imbalances, and treatment of hyperglycemia. Intravenous regular insulin infusion is the standard of care for treatment worldwide owing to rapidity of onset of action and rapid resolution of ketonemia and hyperglycemia. As hypoglycemia and hypokalemia are more common during treatment, insulin doses are now recommended to be reduced from 0.1 u/kg/h to 0.05 u/kg/h when glucose concentrations drop below 250 mg/dL or 14 mM. 115 Subcutaneous rapid acting insulin protocols have emerged as alternative treatments for mild to moderate diabetic ketoacidosis. 117 Such regimens seem to be safe and have the advantages of not requiring admission to intensive care, having lower rates of complications related to intravenous therapy, and requiring fewer resources. 117 118 Ketonemia and acidosis resolve within 24 hours in most people. 115 To prevent rebound hyperglycemia, the transition off an intravenous insulin drip must overlap subcutaneous insulin by at least two to four hours. 115
Hypoglycemia
Hypoglycemia, a common occurrence in people with type 1 diabetes, is a well appreciated effect of insulin treatment and occurs when blood glucose falls below the normal range. Increased susceptibility to hypoglycemia from exogenous insulin use in people with type 1 diabetes results from multiple factors, including imperfect subcutaneous insulin delivery tools, loss of glucagon within a few years of diagnosis, progressive impairment of the sympatho-adrenal response with repeated hypoglycemic episodes, and eventual development of impaired awareness. In 2017 the International Hypoglycemia Study Group developed guidance for definitions of hypoglycemia; on the basis of this, a glucose concentration of 3.0-3.9 mmol/L (54-70 mg/dL) was designated as level 1 hypoglycemia, signifying impending development of level 2 hypoglycemia—a glucose concentration <3 mmol/L (54 mg/dL). 119 120 At approximately 54 mg/dL, neuroglycopenic hypoglycemia symptoms, including vision and behavior changes, seizures, and loss of consciousness, begin to occur as a result of glucose deprivation of neurons in the central nervous system. This can eventually lead to cerebral dysfunction at concentrations <50 mg/dL. 121 Severe hypoglycemia (level 3), denoting severe cognitive and/or physical impairment and needing external assistance for recovery, is a common reason for emergency department visits and is more likely to occur in people with lower socioeconomic status and with the longest duration of diabetes. 112 Prevalence of self-reported severe hypoglycemia is very high according to a global population study that included more than 8000 people with type 1 diabetes. 122 Severe hypoglycemia occurred commonly in younger people with suboptimal glycemia according to a large electronic health record database study in the US. 123 Self- reported severe hypoglycemia is associated with a 3.4-fold increase in mortality. 124 125
Acute consequences of hypoglycemia include impaired cognitive function, temporary focal deficits including stroke-like symptoms, and memory deficits. 126 Cardiovascular effects including tachycardia, arrhythmias, QT prolongation, and bradycardia can occur. 127 Hypoglycemia can impair many activities of daily living, including motor vehicle safety. 128 In a survey of adults with type 1 diabetes who drive a vehicle at least once a week, 72% of respondents reported having hypoglycemia while driving, with around 5% reporting a motor vehicle accident due to hypoglycemia in the previous two years. 129 This contributes to the stress and fear that many patients face while grappling with the difficulties of ongoing hypoglycemia. 130
Glucagon is highly efficacious for the primary treatment of severe hypoglycemia when a patient is unable to ingest carbohydrate safely, but it is unfortunately under-prescribed and underused. 131 132 Availability of nasal, ready to inject, and shelf-stable liquid glucagon formulations have superseded the need for reconstituting older injectable glucagon preparations before administration and are now preferred. 133 134 Real time CGM studies have shown a decreased hypoglycemic exposure in people with impaired awareness without a change in HbA 1c . 34 135 136 137 138 CGM has shown benefit in decreasing hypoglycemia across the lifespan, including in teens, young adults, and older people. 36 139 Although CGM reduces the burden of hypoglycemia including severe hypoglycemia, it does not eliminate it; overall, such severe level 3 hypoglycemia rates in clinical trials are very low and hard to decipher in the real world. HCL insulin delivery systems integrated with CGM have been shown to decrease hypoglycemia. Among available rapid acting insulins, ultra-rapid acting lispro (lispro-aabc) seems to be associated with less frequent hypoglycemia in type 1 diabetes. 140 141
As prevention of hypoglycemia is a crucial aspect of diabetes management, formal training programs to increase awareness and education on avoidance of hypoglycemia, such as the UK’s Dose Adjustment for Normal Eating (DAFNE), have been developed. 142 143 This program has shown fewer severe hypoglycemia (mean 1.7 (standard deviation 8.5) episodes per person per year before training to 0.6 (3.7) episodes one year after training) and restoration of recognition of hypoglycemia in 43% of people reporting unawareness. Clinically relevant anxiety and depression fell from 24.4% to 18.0% and from 20.9% to 15.5%, respectively. A structured education program with cognitive and psychotherapeutic aspects for changing hypoglycemia related behaviors, called the Hypoglycemia Awareness Restoration Program despite optimized self-care (HARPdoc), showed a positive effect on changing unhelpful beliefs around hypoglycemia and improved diabetes related and general distress and anxiety scores. 144
Management in under-resourced settings
According to a recent estimate from the International Diabetes Federation, 1.8 million people with type 1 diabetes live in low and middle income countries (LMICs). 2 In many LMICs, the actual burden of type 1 diabetes remains unknown and material resources needed to manage type 1 diabetes are lacking. 145 146 Health systems in these settings are underequipped to tackle the complex chronic disease that is type 1 diabetes. Few diabetes and endocrinology specialist physicians are available owing to lack of specific postgraduate training programs in many LMICs; general practitioners with little to no clinical experience in managing type 1 diabetes care for these patients. 146 This, along with poor availability and affordability of insulin and lack of access to technology, results in high mortality rates. 147 148 149 In developed nations, low socioeconomic status is associated with higher levels of mortality and morbidity for adults with type 1 diabetes despite access to a universal healthcare system. 150 Although global governments have committed to universal health coverage and therefore widespread availability of insulin, it remains very far from realization in most LMICs. 151
Access to technology is patchy and varies globally. In the UST1DX, CGM use was least in the lowest fifth of socioeconomic status. 152 Even where technology is available, successful engagement does not always occur. 153 In a US cohort, lower CGM use was seen in non-Hispanic Black children owing to lower rates of device initiation and higher rates of discontinuation. 154 In many LMICs, blood glucose testing strips are not readily available and cost more than insulin. 151 In resource limited settings, where even diagnosis, basic treatments including insulin, syringes, and diabetes education are limited, use of CGM adds additional burden to patients. Need for support services and the time/resources needed to download and interpret data are limiting factors from a clinician’s perspective. Current rates of CGM use in many LMICs are unknown.
Inequities in the availability of and access to certain insulin formulations continue to plague diabetes care. 155 In developed countries such as the US, rising costs have led to insulin rationing by around 25% of people with type 1 diabetes. 156 LMICs have similar trends while also remaining burdened by disproportionate mortality and complications from type 1 diabetes. 155 157 With the inclusion of long acting insulin analogs in the World Health Organization’s Model List of Essential Medicines in 2021, hope has arisen that these will be included as standard of care across the world. 158 In the past, the pricing of long acting analogs has limited their use in resource poor settings 159 ; however, their inclusion in WHO’s list was a major step in improving their affordability. 158 With the introduction of lower cost long acting insulin biosimilars, improved access to these worldwide in the future can be anticipated. 160
Making insulin available is not enough on its own to improve the prognosis for patients with diabetes in resource poor settings. 161 Improved healthcare infrastructure, better availability of diabetes supplies, and trained personnel are all critical to improving type 1 diabetes care in LMICs. 161 Despite awareness of limitations and barriers, a clear understanding of how to implement management strategies in these settings is still lacking. The Global Diabetes Compact was launched in 2021 with the goal of increasing access to treatment and improving outcomes for people with diabetes across the globe. 162
Emerging technologies and treatments
Monitoring systems.
The ability to measure urinary or more recently blood ketone concentrations is an integral part of self-management of type 1 diabetes, especially during acute illness, intermittent fasting, and religious fasts to prevent diabetic ketoacidosis. 163 Many people with type 1 diabetes do not adhere to urine or blood ketone testing, which likely results in unnecessary episodes of diabetic ketoacidosis. 164 Noting that blood and urine ketone testing is not widely available in all countries and settings is important. 1 Regular assessment of patients’ access to ketone testing (blood or urine) is critical for all clinicians. Euglycemic diabetic ketoacidosis in type 1 diabetes is a particular problem with concomitant use of SGLT-2 inhibitors; for this reason, these agents are not approved for use in these patients. For sick day management (and possibly for the future use of SGLT-2 inhibitors in people with type 1 diabetes), it is hoped that continuous ketone monitoring (CKM) can mitigate the risks of diabetic ketoacidosis. 165 Like CGM, the initial CKM device measures interstitial fluid β-hydroxybutyrate instead of glucose. CKM use becomes important in conjunction with a hybrid closed loop insulin pump system and added SGLT-2 inhibitor therapy, where insulin interruptions are common and hyperketonemia is frequent. 166
Perhaps the greatest technological challenge to date has been the development of non-invasive glucose monitoring. Numerous attempts have been made using strategies including optics, microwave, and electrochemistry. 167 Lack of success to date has resulted in healthy skepticism from the medical community. 168 However, active interest in the development of non-invasive technology with either interstitial or blood glucose remains.
Insulin and delivery systems
In the immediate future, two weekly basal insulins, insulin icodec and basal insulin Fc, may become available. 169 Studies of insulin icodec in type 1 diabetes are ongoing (ONWARDS 6; NCT04848480 ). How these insulins will be incorporated in management of type 1 diabetes is not yet clear.
Currently available AID systems use only a single hormone, insulin. Dual hormone AID systems incorporating glucagon are in development. 170 171 Barriers to the use of dual hormone systems include the need for a second chamber in the pump, a lack of stable glucagon formulations approved for long term subcutaneous delivery, lack of demonstrated long term safety, and gastrointestinal side effects from glucagon use. 74 Similarly, co-formulations of insulin and amylin (a hormone co-secreted with insulin and deficient in people with type 1 diabetes) are in development. 172
Immunotherapy for type 1 diabetes
As our understanding of the immunology of type 1 diabetes expands, development of the next generation of immunotherapies is under active pursuit. Antigen specific therapies, peptide immunotherapy, immune tolerance using DNA vaccination, and regulatory T cell based adoptive transfer targeting β cell senescence are all future opportunities for drug development. Combining immunotherapies with metabolic therapies such as GLP-1 receptor agonists to help to improve β cell mass is being actively investigated.
The quest for β cell replacement methods is ongoing. Transplantation of stem cell derived islets offers promise for personalized regenerative therapies as a potentially curative method that does away with the need for donor tissue. Since the first in vivo model of glucose responsive β cells derived from human embryonic stem cells, 173 different approaches have been attempted. Mesenchymal stromal cell treatment and autologous hematopoietic stem cells in newly diagnosed type 1 diabetes may preserve β cell function without any safety signals. 174 175 176 Stem cell transplantation for type 1 diabetes remains investigational. Encapsulation, in which β cells are protected using a physical barrier to prevent immune attack and avoid lifelong immunosuppression, and gene therapy techniques using CRISPR technology also remain in early stages of investigation.
Until recently, no specific guidelines for management of type 1 diabetes existed and management guidance was combined with consensus statements developed for type 2 diabetes. Table 6 summarizes available guidance and statements from various societies. A consensus report for management of type 1 diabetes in adults by the ADA and European Association for the Study of Diabetes became available in 2021; it covers several topics of diagnosis and management of type 1 diabetes, including glucose monitoring, insulin therapy, and acute complications. Similarly, the National Institute for Health and Care Excellence also offers guidance on management of various aspects of type 1 diabetes. Consensus statements for use of CGM, insulin pump, and AID systems are also available.
Guidelines in type 1 diabetes
Conclusions
Type 1 diabetes is a complex chronic condition with increasing worldwide prevalence affecting several million people. Several successes in management of type 1 diabetes have occurred over the years from the serendipitous discovery of insulin in 1921 to blood glucose monitoring, insulin pumps, transplantation, and immunomodulation. The past two decades have seen advancements in diagnosis, treatment, and technology including development of analog insulins, CGM, and advanced insulin delivery systems. Although we have gained a broad understanding on many important aspects of type 1 diabetes, gaps still exist. Pivotal research continues targeting immune targets to prevent or delay onset of type 1 diabetes. Although insulin is likely the oldest of existing modern drugs, no low priced generic supply of insulin exists anywhere in the world. Management of type 1 diabetes in under resourced areas continues to be a multifaceted problem with social, cultural, and political barriers.
Glossary of abbreviations
ADA—American Diabetes Association
AID—automated insulin delivery
BGM—blood glucose monitoring
CGM—continuous glucose monitoring
CKM—continuous ketone monitoring
DCCT—Diabetes Control and Complications Trial
DIY—do-it-yourself
FDA—Food and Drug Administration
GADA—glutamic acid decarboxylase antibody
GLP-1—glucagon-like peptide 1
GRS—genetic risk scoring
HbA1c—glycated hemoglobin
HCL—hybrid closed loop
LADA—latent autoimmune diabetes of adults
LMIC—low and middle income country
PAKT—pancreas after kidney transplant
RCT—randomized controlled trial
SGLT-2—sodium-glucose cotransporter 2
SPKT—simultaneous pancreas-kidney transplant
Questions for future research
What future new technologies can be helpful in management of type 1 diabetes?
How can newer insulin delivery methods benefit people with type 1 diabetes?
What is the role of disease modifying treatments in prevention and delay of type 1 diabetes?
Is there a role for sodium-glucose co-transporter inhibitors or glucagon-like peptide 1 receptor angonists in the management of type 1 diabetes?
As the population with type 1 diabetes ages, how should management of these people be tailored?
How can we better serve people with type 1 diabetes who live in under-resourced settings with limited access to medications and technology?
How patients were involved in the creation of this manuscript
A person with lived experience of type 1 diabetes reviewed a draft of the manuscript and offered input on important aspects of their experience that should be included. This person is involved in large scale education and activism around type 1 diabetes. They offered their views on various aspects of type 1 diabetes, especially the use of adjuvant therapies and the burden of living with diabetes. This person also raised the importance of education of general practitioners on the various stages of type 1 diabetes and the management aspects. On the basis of this feedback, we have highlighted the burden of living with diabetes on a daily basis.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: SS and IBH contributed to the planning, drafting, and critical review of this manuscript. FNK contributed to the drafting of portions of the manuscript. All three authors are responsible for the overall content as guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SS has received an honorarium from Abbott Diabetes Care; IBH has received honorariums from Abbott Diabetes Care, Lifescan, embecta, and Hagar and research support from Dexcom and Insulet.
Provenance and peer review: Commissioned; externally peer reviewed.
- DeVries JH ,
- Hess-Fischl A ,
- Gregory GA ,
- Robinson TIG ,
- Linklater SE ,
- International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group
- Harding JL ,
- Wander PL ,
- Dabelea D ,
- Mayer-Davis EJ ,
- SEARCH for Diabetes in Youth Study
- Lawrence JM ,
- SEARCH for Diabetes in Youth Study Group
- Fazeli Farsani S ,
- Souverein PC ,
- van der Vorst MM ,
- Sutherland J ,
- Rokszin G ,
- Stahl-Pehe A ,
- Kamrath C ,
- Atkinson MA ,
- Bingley PJ ,
- Bonifacio E ,
- Leslie RD ,
- Evans-Molina C ,
- Freund-Brown J ,
- Thomas NJ ,
- Zimmet PZ ,
- Rowley MJ ,
- Knowles W ,
- Fourlanos S ,
- Greenbaum CJ ,
- ElSayed NA ,
- American Diabetes Association
- Miller RG ,
- Secrest AM ,
- Sharma RK ,
- Songer TJ ,
- McDonald T ,
- Greenfield JR
- Tridgell DM ,
- Spiekerman C ,
- Greenbaum CJ
- Glessner J ,
- Foster NC ,
- Miller KM ,
- Becker DJ ,
- Khawandanah J
- Pedrosa MR ,
- Franco DR ,
- Gieremek HW ,
- Nathan DM ,
- Diabetes Control and Complications Trial Research Group
- Klonoff DC ,
- Parkes JL ,
- Kovatchev BP ,
- Raghinaru D ,
- iDCL Trial Research Group
- Riddlesworth T ,
- DIAMOND Study Group
- Leelarathna L ,
- Neupane S ,
- FLASH-UK Trial Study Group
- Polonsky W ,
- Hirsch IB ,
- Pratley RE ,
- Kanapka LG ,
- Rickels MR ,
- Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group
- Tauschmann M ,
- APCam11 Consortium
- Russell SJ ,
- Damiano ER ,
- Bionic Pancreas Research Group
- Bailey TS ,
- Group Information ,
- Bergenstal RM ,
- Dellva MA ,
- Mathieu C ,
- Herold KC ,
- Type 1 Diabetes TrialNet Study Group
- Libman IM ,
- DiMeglio LA ,
- T1D Exchange Clinic Network Metformin RCT Study Group
- Petrie JR ,
- Chaturvedi N ,
- REMOVAL Study Group
- Dandona P ,
- Phillip M ,
- DEPICT-1 Investigators
- Rosenstock J ,
- Marquard J ,
- Laffel LM ,
- Hemmingsson JU ,
- ADJUNCT ONE Investigators
- Pieber TR ,
- ADJUNCT TWO Investigators
- Reynolds J ,
- Battelino T ,
- Liljenquist D ,
- Becker RH ,
- Bergmann K ,
- Lehmann A ,
- Cheng AYY ,
- Stender-Petersen K ,
- Hövelmann U ,
- Carlson AL ,
- Komatsu M ,
- Coutant DE ,
- Stamati A ,
- Karagiannis T ,
- Christoforidis A
- Heinemann L ,
- Baughman R ,
- Akturk HK ,
- Snell-Bergeon JK ,
- Prabhu JN ,
- Seyed Ahmadi S ,
- Westman K ,
- Pivodic A ,
- Hermann JM ,
- Freiberg C ,
- DPV Initiative
- Dicembrini I ,
- Cosentino C ,
- Mannucci E ,
- Abelseth J ,
- Karageorgiou V ,
- Papaioannou TG ,
- Weisman A ,
- Cardinez M ,
- Kramer CK ,
- Asarani NAM ,
- Reynolds AN ,
- Elbalshy M ,
- Jennings P ,
- Burnside MJ ,
- Williman JA ,
- Fridell JA ,
- Stratta RJ ,
- Gruessner AC
- Robertson RP
- Shapiro AM ,
- Pepper AR ,
- Shapiro AMJ
- Walker JT ,
- Saunders DC ,
- Brissova M ,
- Gitelman SE ,
- Bluestone JA
- Hagopian W ,
- Ferry RJ Jr . ,
- Protégé Trial Investigators
- von Scholten BJ ,
- Kreiner FF ,
- Gough SCL ,
- von Herrath M
- Type 1 Diabetes TrialNet Abatacept Study Group
- Hardie DG ,
- Dejgaard TF ,
- Christiansen E ,
- ADJUNCT ONE and ADJUNCT TWO Investigators
- Yunasan E ,
- Peters AL ,
- Palanca A ,
- van Nes F ,
- Ampudia Blasco FJ ,
- Dobbins RL ,
- Greenway FL ,
- Zambrowicz BP ,
- Brodovicz K ,
- Soleymanlou N ,
- Wissinger E ,
- Juhaeri J ,
- Mayer-Davis EJ
- Dhatariya KK ,
- Glaser NS ,
- Umpierrez GE
- Ramphul K ,
- Umpierrez G ,
- Korytkowski M
- Weinstock RS ,
- T1D Exchange Clinic Network
- Eshkoli T ,
- Brandstaetter E ,
- Jotkowitz A
- Dhatariya KK
- Joint British Diabetes Societies for Inpatient Care
- Kilpatrick ES ,
- Butler AE ,
- Ostlundh L ,
- Koufakis T ,
- Agiostratidou G ,
- International Hypoglycaemia Study Group
- van de Ven KC ,
- Heerschap A ,
- van der Graaf M ,
- de Galan BE
- Alsifri S ,
- Aronson R ,
- HAT Investigator Group
- Pettus JH ,
- Shepherd L ,
- Van Houten HK ,
- Ziegenfuss JY ,
- Wermers RA ,
- Schernthaner G ,
- Anderson J ,
- Saunders AL ,
- Snell-Bergeon J ,
- Forlenza GP ,
- Bispham J ,
- Gabbay RA ,
- Pontiroli AE ,
- Tagliabue E
- T1D Exchange Intranasal Glucagon Investigators
- Freckmann G ,
- Ehrmann D ,
- El Laboudi A ,
- Spanudakis E ,
- Anantharaja S ,
- Peleckis AJ ,
- Dalton-Bakes C ,
- van Beers CA ,
- Kleijer SJ ,
- CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group ,
- Piras de Oliveira C ,
- Ribeiro A ,
- Chigutsa F ,
- Malecki MT ,
- Hopkins D ,
- Lawrence I ,
- Mansell P ,
- Stanton-Fay SH ,
- Hamilton K ,
- Chadwick PM ,
- DAFNEplus study group
- Goldsmith K ,
- Yudkin JS ,
- Buntinx F ,
- Mapatano MA ,
- De Clerck M ,
- Truyers C ,
- Chambers D ,
- O’Cathain A
- Klatman EL ,
- Auzanneau M ,
- Tanenbaum ML ,
- Iturralde E ,
- Lipman TH ,
- Bhutta ZA ,
- Pfiester E ,
- Thieffry A ,
- Ballhausen H ,
- Gajewska KA ,
- O’Donnell S
- Wareham NJ ,
- Joosse HJ ,
- Raposo JF ,
- de Courten M
- Hemmingsen B ,
- Abouhassan T ,
- Albanese-O’Neill A ,
- Jacobsen L ,
- Haller MJ ,
- Castorino K ,
- Lovblom LE ,
- Cardinez N ,
- Nguyen KT ,
- Andersen G ,
- Meiffren G ,
- Famulla S ,
- Castellanos LE ,
- Balliro CA ,
- Sherwood JS ,
- Tsoukas MA ,
- Bernier-Twardy S ,
- Martinson LA ,
- Carlsson PO ,
- Schwarcz E ,
- Korsgren O ,
- Oliveira MC ,
- Stracieri AB ,
- Buzzetti R ,
- Mauricio D ,
- McGibbon A ,
- Ingersoll K ,
- Tugwell B ,
- Diabetes Canada Clinical Practice Guidelines Expert Committee
- Fleming GA ,
- McCall AL ,
- Gianchandani R ,
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The promise of once-weekly insulins for type 1 diabetes: update on progress
Affiliations.
- 1 Division of Endocrinology, University of Virginia, Charlottesville, VA 22903, USA.
- 2 Division of Endocrinology, University of Virginia, Charlottesville, VA 22903, USA; Center for Diabetes Technology, University of Virginia, Charlottesville, VA 22903, USA. Electronic address: [email protected].
- PMID: 39306455
- DOI: 10.1016/S0140-6736(24)01917-2
PubMed Disclaimer
Conflict of interest statement
KML has received research product support from Dexcom and receives grant support from the US National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases; she has received speaking honorarium from AdventHealth (Orlando, FL, USA). SAB has received research support through her institution from Dexcom, Insulet, Roche, Tandem Diabetes Care, and Tolerion and has participated on a data safety monitoring board for MannKind (non-compensated); she has also received support from the National Institutes of Health and Juvenile Diabetes Research Foundation.
- Search in MeSH
LinkOut - more resources
Full text sources.
- ClinicalKey
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- News Archive
Large study sheds new light on the complex type 1 diabetes genetics landscape and potential drug targets

In new research, scientists analyzed genetic contributors to type 1 diabetes by studying over 61,000 participants (with and without the disease), including individuals from diverse ancestries. This approach led to the identification of 36 new gene regions associated with type 1 diabetes, some of which are also associated with other autoimmune diseases. Additionally, the scientists did “fine mapping” studies to pinpoint the specific disease-causing variants in genetic regions previously associated with type 1 diabetes, toward elucidating the role these variants may play in the disease process. In another set of experiments, they used their genetic association results with data about other autoimmune diseases to identify 12 genes that could potentially be drug targets for type 1 diabetes. Drugs targeting these genes (by affecting the proteins the genes encode) have been studied in clinical trials for other autoimmune diseases, suggesting they could be repurposed for type 1 diabetes. Some of the targets are already being studied in type 1 diabetes clinical trials of various therapies, but others could potentially be explored in future trials to prevent type 1 diabetes.
This large research study with a diverse population has provided important knowledge about the complex type 1 diabetes genetics landscape, revealing new genetic regions associated with the disease, shedding light on how some genetic variants may influence disease, and identifying potential drug targets. Understanding what genes play a role in type 1 diabetes, and what role they play, paves the way to identify new targets to prevent or treat this autoimmune disease.
Robertson CC, Inshaw JRJ, Onengut-Gumuscu S,…Rich SS. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes . Nat Genet 53: 962-971, 2021.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Suicide among female doctors gets a closer look
To assess a smoker’s lung cancer risk, think years — not packs

Eat this. Take that. Get skinny. Trust us.

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Epidemiologist discusses research, shrinking gap between rates of male, female physicians, what can be done
Far more cases get caught when screening guidelines consider duration of habit regardless of intensity, study finds — especially among Black patients

Popularity of newest diet drugs fuel ‘dumpster fire’ of risky knock-offs, questionable supplements, food products, experts warn
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Do phones belong in schools?
Banning cellphones may help protect classroom focus, but school districts need to stay mindful of students’ sense of connection, experts say.

Study provides preliminary evidence in favor of a new type 1 diabetes treatment

Type 1 diabetes is an autoimmune disease that causes the body's immune system to attack and destroy insulin-producing beta cells in the pancreas. Traditional management of type 1 diabetes has primarily involved replacing the missing insulin with injections which, though effective, can be expensive and burdensome. A new study led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment.
The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that plays a key role in cellular metabolism. The latest translational results are a culmination of years of research: In 2010, while corresponding author Raghu Mirmira, MD, PhD , was at Indiana University, he and his lab performed fundamental biochemistry experiments on beta cells in culture. They found that suppressing the metabolic pathway altered by DFMO helped protect the beta cells from environmental factors, hinting at the possibility of preserving and even restoring these vital cells in patients diagnosed with type 1 diabetes.
The researchers confirmed their observations preclinically in zebrafish and then in mice before senior author Linda DiMeglio, MD, MPH, Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a clinical trial to evaluate the safety and tolerability of the drug in type 1 diabetes patients. The results of the trial, which was funded by the Juvenile Diabetes Research Foundation (JDRF) and used DMFO provided by Panbela Therapeutics, indicated that the drug is safe for type 1 diabetes patients and can help keep insulin levels stable by protecting beta cells.
“As a physician-scientist, this is the kind of thing we’ve always strived for – to discover something at a very basic, fundamental level in cells and find a way to bring it into the clinic,” said Mirmira, who is now Professor of Medicine and an endocrinologist at UChicago Medicine. “It definitely underscores the importance of supporting basic science research.”
"It's been truly thrilling to witness the promising results in the pilot trial after this long journey, and we're excited to continue our meaningful collaboration," said DiMeglio.
Importantly, DFMO has already been FDA-approved as a high dose injection since 1990 for treating African Sleeping Sickness and received breakthrough therapy designation for neuroblastoma maintenance therapy after remission in 2020. Pre-existing regulatory approval could potentially facilitate its use in type 1 diabetes, saving effort and expense and getting the treatment to patients sooner.
“For a drug that’s already approved for other indications, the approval timeline can be a matter of years instead of decades once you have solid clinical evidence for safety and efficacy,” said Mirmira. “Using a new formulation of DFMO as a pill allows patients to take it by mouth instead of needing to undergo regular injections, and it has a very favorable side effect profile. It’s exciting to say we have a drug that works differently from every other treatment we have for this disease.”
To follow up on the recently published results, first and co-corresponding author Emily K. Sims, MD, Associate Professor of Pediatrics at IU School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a multi-center clinical trial, also funded by JDRF – with UChicago among the trial sites – to gather even stronger data regarding the efficacy of DFMO as a type 1 diabetes treatment.
"With our promising early findings, we hold hope that DFMO, possibly as part of a combination therapy, could offer potential benefits to preserve insulin secretion in individuals with recent-onset type 1 diabetes and ultimately also be tested in those who are at risk of developing the condition," said Sims.
“A new era is dawning where we’re thinking of novel ways to modify the disease using different types of drugs and targets that we didn’t classically think of in type 1 diabetes treatment,” said Mirmira.
The study, “Inhibition of Polyamine Biosynthesis Preserves β-Cell Function in Type 1 Diabetes,” was published in Cell Medicine Reports in November 2023. Co-authors include Emily K. Sims, Abhishek Kulkarni, Audrey Hull, Stephanie E. Woerner, Susanne Cabrera, Lucy D. Mastrandrea, Batoul Hammoud, Soumyadeep Sarkar, Ernesto S. Nakayasu, Teresa L. Mastracci, Susan M. Perkins, Fangqian Ouyang, Bobbie-Jo Webb-Robertson, Jacob R. Enriquez, Sarah A. Tersey, Carmella Evans-Molina, S. Alice Long, Lori Blanchfield, Eugene W. Gerner, Raghavendra Mirmira, and Linda A. DiMeglio.
- Utility Menu
GA4 tracking code

A new therapy for treating Type 1 diabetes
Promising early results show that longstanding harvard stem cell institute (hsci) research may have paved the way for a breakthrough treatment of type 1 diabetes. utilizing research from the melton lab, vertex pharmaceuticals has developed vx-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (t1d). in conjunction with immunosuppressive therapy, vx-880 produced robust restoration of islet cell function on day 90 in the first patient in its phase 1/2 clinical trial..

The patient was treated with a single infusion of VX-880 at half the target dose in conjunction with immunosuppressive therapy. The patient, who was diagnosed with T1D 40 years ago and has been dependent on exogenous (injected) insulin, achieved successful engraftment and demonstrated rapid and robust improvements in multiple measures. These included increases in fasting and stimulated C-peptide, improvements in glycemic control, including HbA1c, and decreases in exogenous insulin requirement, signifying the restoration of insulin-producing islet cells.
VX-880 is not only a potential breakthrough in the treatment of T1D, it is also one of the very first demonstrations of the practical application of embryonic stem cells, using stem cells that have been differentiated into functional islets to treat a patient, explained Doug Melton, Ph.D., co-director of HSCI, is the Xander University Professor at Harvard and an Investigator of the Howard Hughes Medical Institute. Unlike prior treatments, this innovative therapy gives the patient functional hormone producing cells that control glucose metabolism. This potentially obviates the lifelong need for patients with diabetes to self-inject insulin as the replacement cells “provide the patient with the natural factory to make their own insulin,” explained Melton.
These results from the first patient treated with VX-880 are unprecedented. What makes these results truly remarkable is that they were achieved with only half the target dose,” said Bastiano Sanna, Ph.D., Executive Vice President and Chief of Cell and Genetic Therapies at Vertex. “While still early, these results support the continued progression of our VX-880 clinical studies, as well as future studies using our encapsulated islet cells, which hold the potential to be used without the need for immunosuppression.”
“As a surgeon who has worked in the field of islet cell transplantation for decades, this approach, which obviates the need for an organ donor, could be a game changer,” said James Markmann, M.D., Ph.D., Professor of Surgery and Chief of the Division of Transplant Surgery at Massachusetts General Hospital. “We are excited to progress this unique and potentially transformative medicine through clinical trials and to patients.”
“More than a decade ago our lab had a vision for developing an islet cell replacement therapy to provide a functional cure to people suffering from T1D,” said Melton, a founder and one of the first co-chairs of the Harvard Stem Cell and Regenerative Biology Department. “These promising results bring great hope that stem cell-derived, fully differentiated islet cells could deliver a life-changing therapy for people who suffer from the relentless life-long burden of T1D. I'm so grateful that Harvard and the Harvard Stem Cell Institute have supported this work.”
Latest News
How a small fish could lead to better strategies to repair tendon tears, jason buenrostro lands macarthur ‘genius grant’, new model for in vitro production of human brown fat cells lays groundwork for obesity, diabetes cell therapy, share this story, news by topic.
- Alzheimer's Disease (8)
- Bioengineering (31)
- Blood Diseases (52)
- Cancer (55)
- Cardiovascular Disease (25)
- Diabetes (30)
- Endocrine Disease (1)
- Eye Diseases (10)
- Fibrosis (6)
- Gastrointestinal Disease (8)
- Genetics/Epigenetics (24)
- Graft-Versus-Host Disease (1)
- Hearing Loss (5)
- Immunology (15)
- Kidney Disease (12)
- Liver Disease (6)
- Lung Disease (9)
- Multiple Sclerosis (1)

Apr. 27, 2023
Study unlocks potential breakthrough in type 1 diabetes treatment, rice u. scientists optimize biomaterials screening, identify ‘winning’ formulations.
For the over 8 million people around the globe living with Type 1 diabetes , getting a host immune system to tolerate the presence of implanted insulin-secreting cells could be life-changing.
Rice University bioengineer Omid Veiseh and collaborators identified new biomaterial formulations that could help turn the page on Type 1 diabetes treatment, opening the door to a more sustainable, long-term, self-regulating way to handle the disease.
To do so, they developed a new screening technique that involves tagging each biomaterial formulation in a library of hundreds with a unique “barcode” before implanting them in live subjects.
According to the study in Nature Biomedical Engineering , using one of the alginate formulations to encapsulate human insulin-secreting islet cells provided long-term blood sugar level control in diabetic mice. Catheters coated with two other high-performing materials did not clog up. “This work was motivated by a major unmet need,” said Veiseh, a Rice assistant professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar. “In Type 1 diabetes patients, the body’s immune system attacks the insulin-producing cells of the pancreas. As those cells are killed off, the patient loses the ability to regulate their blood glucose.”

For decades, scientists labored toward what Veiseh called a “‘holy grail’ goal of housing islet cells inside a porous matrix made out of a protective material that would allow the cells to access oxygen and nutrients without getting clobbered by the host’s immune system.”
However, materials with optimal biocompatibility proved very hard to find, due in part to screening constraints. On one hand, immune system response to a given implanted biomaterial can only be assessed in a live host.
“The problem is the immune response needs to be investigated inside the body of these diabetic mice, not in a test tube,” said Boram Kim, a graduate student in the Veiseh lab and co-lead author on the study. “That means that if you want to screen these hundreds of alginate molecules, then you need to have hundreds of animal test subjects. Our idea was to screen for hundreds of biomaterials at the same time, in the same test subject.”

On the other hand, different biomaterial formulations look the same, making it impossible to identify high-performing ones in the absence of some telltale trait. This made testing more than one biomaterial per host unfeasible.
“They are different materials but they look the same,” Veiseh said. “And once they are implanted in the body of a test subject and then taken out again, we cannot distinguish between the materials and we would be unable to identify which material formulation worked best.”
To overcome these constraints, Veiseh and collaborators came up with a way to tag each alginate formulation with a unique ‘barcode’ that allowed them to identify the ones that performed best.

“We paired each modified biomaterial with human umbilical vein endothelial cells (HUVEC) from a different donor,” Kim said.
“The HUVEC cells, because they come from unique donors, act as a barcode that allows us to tell what material was used initially,” Veiseh added. “The winners are the ones that have live cells in them. Once we found them, we sequenced the genome of those cells and figured out which material was paired with it. That’s how we uncovered the greatest hits.”
Trials are underway for stem cell-derived islet cell use in diabetic patients. However, current islet treatments require immunosuppression, making it a taxing way to treat Type 1 diabetes. “Currently, in order to use implanted islet cells in diabetic patients, you have to suppress the entire immune system, just as if you were trying to do an organ transplant,” Veiseh said. “That comes with a lot of complications for the patient.

“They can develop cancer, they can’t fight infections, so, for the vast majority of patients, it’s better to actually do the insulin therapy where they inject themselves. With this biomaterial-encapsulation strategy, no immunosuppression is needed.”
Placing actual HUVEC cells inside the biomaterial capsules increased the likelihood that the host immune system would detect a foreign presence. This makes the experiment more robust than simply testing for immune response to the biomaterials alone.
“We wanted to test a library of these materials, with the selection pressure of having cells inside the beads that makes it harder for the material to not get noticed by the immune system,” Veiseh said. “There’s a lot of interest from all the islet cell manufacturers to be able to get rid of immunosuppression and instead use these alginate hydrogel matrices to protect the implanted cells.” The new high-throughput “barcoding” approach can be deployed to screen for other medical applications using fewer live test subjects.

“That actually feeds into a lot of other projects in my lab where we’re doing biologic production from cells for other disease indications,” Veiseh said. “The same modifications can be applied to all types of materials that go into the body. This is not limited only to cell transplantation. The technology we developed can be paired with a lot of different device concepts.
“For instance, some diabetic patients use automated pump systems to self-administer insulin. The catheters on those pump systems have to be replaced every few days because they get clogged. We were able to show that coating the catheters with these new materials prevented clogging.”
“With this new cell-based barcoding technology, biomaterials research just got an unprecedented boost that will accelerate the translation to clinically applicable products, and make it more affordable,” said Dr. José Oberholzer, a transplant surgeon and bioengineer at the University of Virginia.
“This is a real paradigm shift. With this method, we can now screen hundreds of biomaterials at once and select those that the human body does not reject. We can protect cellular grafts from the assaults of the immune system, without the need for immunosuppressive medications,” Oberholzer added.

Former Rice bioengineering professor and current NuProbe U.S. CEO David Zhang noted that “high-throughput DNA sequencing has revolutionized many biomedical fields.”
“I am pleased to work with Omid to enable the development of improved biomaterials using my team's expertise in DNA sequencing,” added Zhang, who was a co-investigator on the grant. “These improved biomaterials can enable durable implanted cell therapies to function as living drug factories, and can have a positively disruptive impact on patients with a variety of chronic diseases.” The National Institutes of Health (R01 DK120459), JDRF (3-SRA-2021-1023-S-B), the National Science Foundation (CBET1626418), the Rice University Academy Fellowship and Rice’s Shared Equipment Authority supported the research.
Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate | Nature Biomedical Engineering | DOI: 10.1038/s41551-023-01016-2 Authors: Sudip Mukherjee, Boram Kim, Lauren Cheng, Michael David Doerfert, Jiaming Li, Andrea Hernandez, Lily Liang, Maria Ruocco, Peter Rios, Sofia Ghani, Irax Joshi, Douglas Isa, Trisha Ray, Tanguy Terlier, Cody Fell, Ping Song, Roberto Miranda, Jose Oberholzer, David Yu Zhang and Omid Veiseh https://www.nature.com/articles/s41551-023-01016-2
https://news-network.rice.edu/news/files/2023/03/230310_Veiseh-Lab_LG.jpg CAPTION: Omid Veiseh is a Rice assistant professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar. (Photo by Gustavo Raskoksy/Rice University) https://news-network.rice.edu/news/files/2023/03/230310_Boram_Kim_LG.jpg CAPTION: Boram Kim is a Rice graduate student and co-lead author on the study. (Photo by Gustavo Raskosky/Rice University) https://news-network.rice.edu/news/files/2023/03/230310_Veiseh_Kim_LG.jpg CAPTION: Omid Veiseh and Boram Kim. Kim is holding a medical-grade catheter similar to ones used in the study experiments. (Photo by Gustavo Raskosky/Rice University) https://news-network.rice.edu/news/files/2023/03/230310_Veiseh-Lab_capsules1.jpg CAPTION: Different biomaterial formulations look the same, making it impossible to identify high-performing ones in the absence of some telltale trait. To overcome these constraints, Rice researchers tagged each alginate formulation with a unique “barcode” that allowed them to identify the ones that performed best. The multicolored beads illustrate the barcoded biomaterial capsules. (Photo by Gustavo Raskosky/Rice University) https://news-network.rice.edu/news/files/2023/03/230310_Veiseh-Lab_capsules2.jpg CAPTION: The new high-throughput “barcoding” approach can be deployed to screen for other medical applications using fewer live test subjects. (Photo by Gustavo Raskosky/Rice University) https://news-network.rice.edu/news/files/2023/03/José_Oberholzer_LG.jpeg CAPTION: Dr. José Oberholzer is a researcher and transplant surgeon at the University of Virginia. (Photo courtesy of José Oberholzer) https://news-network.rice.edu/news/files/2023/03/Lauren_Cheng_LG.jpg CAPTION: Lauren Cheng is a Rice alum and co-lead author on the study. (Photo courtesy of Lauren Cheng) https://news-network.rice.edu/news/files/2023/03/Sudip_Mukherjee_LG.jpg CAPTION: Sudip Mukherjee, a former Rice postdoctoral associate, is an assistant professor in the School of Biomedical Engineering at the Indian Institute of Technology Varanasi and co-lead author on the study. (Photo courtesy of Sudip Mukherjee)
Fats help tag medical implants as friend or foe: https://news.rice.edu/news/2023/fats-help-tag-medical-implants-friend-or-foe Upgraded tumor model optimizes search for cancer therapies: https://news.rice.edu/news/2023/upgraded-tumor-model-optimizes-search-cancer-therapies HIV ‘drug factory’ implant promises once-a-year therapy: https://news.rice.edu/news/2022/hiv-drug-factory-implant-promises-once-year-therapy Rice lab’s ‘drug factory’ implants cleared for human trials: https://news.rice.edu/news/2022/rice-labs-drug-factory-implants-cleared-human-trials
Department of Bioengineering: https://bioengineering.rice.edu/ Veiseh lab: https://veisehlab.rice.edu/team Cancer Prevention and Research Institute of Texas (CPRIT): https://www.cprit.state.tx.us/
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,552 undergraduates and 3,998 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Current and future therapies for type 1 diabetes
Bernt johan von scholten.
1 Global Chief Medical Office, Novo Nordisk A/S, Søborg, Denmark
Frederik F. Kreiner
Stephen c. l. gough, matthias von herrath.
2 Type 1 Diabetes Center, The La Jolla Institute for Immunology, La Jolla, CA USA
Associated Data
Graphical abstract.
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts based on immune suppression and modulation have been only partly successful in preventing the key pathophysiological feature in type 1 diabetes: the immune-mediated derangement or destruction of beta cells in the pancreatic islets of Langerhans, leading to low or absent insulin secretion and chronic hyperglycaemia. Evidence now warrants a focus on the beta cell itself and how to avoid its dysfunction, which is putatively caused by cytokine-driven inflammation and other stress factors, leading to low insulin-secretory capacity, autoantigen presentation and immune-mediated destruction. Correspondingly, beta cell rescue strategies are being pursued, which include antigen vaccination using, for example, oral insulin or peptides, as well as agents with suggested benefits on beta cell stress, such as verapamil and glucagon-like peptide-1 receptor agonists. Whilst autoimmune-focused prevention approaches are central in type 1 diabetes and will be a requirement in the advent of stem cell-based replacement therapies, managing the primarily cardiometabolic complications of established type 1 diabetes is equally essential. In this review, we outline selected recent and suggested future attempts to address the evolving profile of the person with type 1 diabetes.

Supplementary Information
The online version contains a slide of the figure for download available at 10.1007/s00125-021-05398-3.
Introduction
In addition to prolonging the life expectancy of people living with type 1 diabetes, the discovery of insulin a century ago revolutionised the management of this chronic autoimmune disease. Today, type 1 diabetes is the most common type of diabetes in children, and estimates suggest that around 100,000 children develop the disease every year [ 1 ]. Unfortunately, despite the availability of advanced insulins, affected individuals remain at high risk of serious complications, including cardiovascular mortality [ 2 – 4 ]. New interventions are, therefore, urgently required to improve the prognosis for the increasing number of people who are diagnosed with type 1 diabetes each year.
The profile of the person with type 1 diabetes is evolving and, with that, our understanding of the disease. The overall pathophysiological feature is loss of functional beta cell mass in the pancreatic islets of Langerhans (Fig. (Fig.1) 1 ) [ 5 ]. Hypotheses suggest that the loss of functional beta cell mass occurs in a chain of events analogous to an ‘assisted suicide’ [ 6 , 7 ], where the demise of the beta cell is likely due to a combination of a dysfunctional beta cell that becomes more visible to the immune system, which, in turn, overreacts and destroys the beta cell.

Hallmarks of the evolving profile of the individual with type 1 diabetes, and current and future options for the prevention of this disease and for the management of its associated complications. a According to some recent evidence [ 124 – 130 ]. This figure is available as a downloadable slide
In its early stage (Stage 1), type 1 diabetes is usually asymptomatic; however, the development of autoimmunity is often detectable in early life, with circulating autoantibodies targeting insulin or other proteins, such as GAD65, insulinoma-associated protein 2 (IA2) or zinc transporter 8 (ZNT8) [ 5 ]. When a large portion of the beta cell mass has become dysfunctional or lost, asymptomatic dysglycaemia (Stage 2) and, later, symptoms of hyperglycaemia (Stage 3) ensue due to insufficient or absent insulin secretion.
Type 1 diabetes is a polygenic disorder, in which susceptibility loci or genetic variation contributes to disease risk. The HLA region on chromosome 6 is the main susceptibility locus and, in recent years, many other loci across the genome have been associated with an increasing risk of the disease [ 8 ]. However, from studies in monozygotic twins, for whom the onset of type 1 diabetes can vary considerably [ 9 ], it has become evident that non-genetic factors play a major role in triggering or perpetuating overt type 1 diabetes. A multitude of efforts have failed at robustly identifying such factors, strongly indicating that no single pathogen is responsible. Viral infections have been suggested, including enteroviruses and human herpesvirus-6 [ 10 – 13 ]. Of note, however, studies (mainly in animals) have also suggested that several viral infections may prevent the development of type 1 diabetes [ 14 , 15 ], in line with the ‘hygiene hypothesis’ [ 16 , 17 ].
People living with type 1 diabetes remain dependent on exogenous insulins as the cornerstone therapeutic option [ 18 ]. Since the isolation of insulin in 1921, novel and versatile formulations, analogues and delivery vehicles have been introduced [ 19 , 20 ]. Together with much improved glucose monitoring, these advances have contributed to the increases in the survival and life expectancy of individuals with type 1 diabetes [ 21 ]. Still, only a minority of people with type 1 diabetes achieve recommended glycaemic and time-in-range targets [ 22 ], and hyperglycaemia continues to be a risk factor for short-term metabolic and long-term macro- and microvascular complications [ 2 , 23 – 25 ]. Further, the use of exogenous insulins requires unremitting glycaemic monitoring and dose titration to mitigate the risk of hypoglycaemia. The all-cause mortality risk is around threefold higher for the individual with type 1 diabetes than for the general population [ 2 – 4 , 26 ], and type 1 diabetes has been shown to be linked to cardiovascular outcomes more than any other disease, including type 2 diabetes [ 2 ].
As mentioned earlier, novel interventions are needed for the prevention and management of type 1 diabetes. Whilst progress has been limited, the evolving profile of a person with type 1 diabetes suggests that beyond ensuring accurate titration of exogenous insulin, efficient management of the disease should rely on other additional principles. First, there is an obvious need to act early to prevent or delay the destruction of functional beta cell mass by immunomodulatory intervention or other disease-modifying means. Second, stimulating or reprogramming the remaining beta cell mass to secrete insulin in a balanced way is required to avoid major blood glucose excursions with the lowest possible exogenous insulin dose. Third, reducing the risk of long-term complications, such as cardiovascular and renal outcomes, seems increasingly important (Fig. (Fig.1). 1 ). Below we review selected current and in-development interventions meeting these three criteria (Table (Table1 1 ).
Non-insulin agents for the prevention and management of type 1 diabetes
| Mechanism of action/target | Agent | Reference of selected main studies or ClinicalTrials.gov registration no. |
|---|---|---|
| Systemic approaches | ||
| T effector cells | Teplizumab (anti-CD3) | [ – , ]; NCT03875729 |
| Otelixizumab (anti-CD3) | [ ] | |
| ATG | [ ] | |
| Abatacept (anti-CD80 and anti-CD86) | [ , ] | |
| Alefacept | [ ] | |
| Anti-IL-21 antibody | [ , , , ] | |
| B cells | Rituximab (anti-CD20) | [ , ] |
| T regulatory cell expansion | Low-dose IL-2 | [ – ] |
| Anti-inflammation | Infliximab, adalimumab, etanercept, golimumab (anti-TNF-α) | [ – ] |
| Tocilizumab (anti-IL-6R) | [ , ]; NCT02293837 | |
| GLP-1 RAs | [ – ] | |
| Islet/beta cell-specific approaches | ||
| Islet-antigen tolerisation/immunisation | Oral insulin | [ – ] |
| GAD65 | [ ] | |
| Peptides | [ , ] | |
| Beta cell stress relief and stimulation | GLP-1 RAs | [ , , – , – ] |
| Verapamil | [ , ] | |
| Cardiometabolic improvements | ||
| SGLT inhibition | Dapagliflozin, empagliflozin, sotagliflozin | [ – , , ] |
| GLP-1 agonism | Exenatide, liraglutide, dulaglutide, semaglutide | [ – , – ] |
| Other/unspecific | Amylin (pramlintide) | [ , ] |
| Metformin | [ – ] | |
a Including blood glucose levels, body weight, blood lipids, blood pressure and cardiorenal risk
Immune-focused therapies
The overarching goal of immune-focused therapies in type 1 diabetes is to prevent or delay the loss of functional beta cell mass. The traditional understanding of autoimmunity in type 1 diabetes has focused on systemic immune dysregulation and on autoreactive T cells that have evaded thymic selection and migrated to the periphery, where they destroy islets. This view on the pathogenesis of type 1 diabetes has been referred to as T cell-mediated ‘homicide’ [ 6 ]. Thus, recent efforts have concentrated on cell- or cytokine-directed interventions, which have been successful in other autoimmune diseases. Targeting T cells or proinflammatory cytokines remain valid efforts and many agents are in active development; so far, however, these approaches have been only partly successful. This arguably indicates a need to refocus hypotheses, as discussed later in this review (see ‘ Future perspectives ’ section), where we outline how the beta cell itself contributes to its own demise (the ‘assisted suicide’ hypothesis).
Cell-directed interventions
In line with the traditional immune-centric view on the pathogenesis of type 1 diabetes, many immunomodulatory strategies have focused on antibodies targeting T effector cells. The anti-CD3 antibodies teplizumab and otelixizumab have shown some attenuation of loss of beta cell function [ 27 – 30 ]. A Phase II trial with relatives with a high risk of developing type 1 diabetes indicated a more than 50% risk reduction with teplizumab (HR 0.41 vs placebo) and clinical type 1 diabetes diagnosis was delayed by 1.5–2 years [ 31 ]. Accordingly, teplizumab has recently been granted a breakthrough therapy status by the US Food and Drug Administration. An ongoing Phase III trial (PROTECT; ClinicalTrials.gov registration no. NCT03875729) aims to evaluate the benefits and safety of teplizumab in children and adolescents with recently diagnosed type 1 diabetes.
The presence of autoantibodies against beta cell antigens, such as GAD65 and insulin, has spurred attempts targeting B cell-related molecules. These efforts have been somewhat successful in animal models [ 32 , 33 ], as well as clinically, most prominently with the B cell-depleting anti-CD20 antibody rituximab. Although rituximab led to detectable protraction of beta cell function [ 34 ], the effect was transient [ 35 ], exemplifying the fact that B cell-directed therapy alone does not appear to sustainably prevent or ameliorate beta cell autoimmunity. So far, however, B cell-directed agents have not been tested in the early disease stage, precluding conclusions regarding the usefulness of such interventions in delaying or even preventing progression to later stages.
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [ 36 – 38 ]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the clinical utility of the approach remains to be established. ATG in combination with granulocyte colony stimulating factor (GCSF) was also explored based on the hypothesis of a synergistic benefit of the combination of transient T cell depletion via low-dose ATG with the upregulation of activated T regulatory cells and tolerogenic dendritic cells induced by GCSF. However, the combination did not appear to offer a synergistic effect; in contrast to the use of ATG alone, ATG plus GCSF did not appear to be better than placebo in preserving C-peptide secretion [ 37 ].
Tissue-resident memory T effector cells, which likely play a role in many organ-specific autoimmune diseases, such as type 1 diabetes, are very difficult to eliminate. Alefacept, a T cell-depleting fusion protein that targets CD2 and, therefore, memory T effector cells, was tested in adolescents and young adults with Stage 3 type 1 diabetes in the T1DAL trial [ 39 ]. Although the trial did not complete enrolment as planned, it reported a trend for benefits with regard to beta cell preservation, reduced insulin requirements and low risk of hypoglycaemia that persisted throughout the follow-up of 15 months after treatment.
Importantly, whether considering the targeting of the T or B cell in type 1 diabetes, sufficient long-term benefits via systemic cell pool depletion comes with an inherent risk of introducing equally long-term or even irreversible changes to the immune system. Such changes may predispose the patient to a less favourable prognosis for chronic viral infections. For example, reactivation of Epstein-Barr virus (EBV) has been observed after anti-CD3 therapies [ 40 , 41 ]. Mitigating such risks may be achieved using carefully tailored dosing regimens and monitoring; still, the seriousness of the risks may indicate an unfavourable benefit:risks balance. Therefore, non-depleting immunomodulation has been explored. For example, 24-month blockade of CD80 and CD86 via the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-immunoglobulin fusion molecule abatacept markedly prolonged beta cell function in new-onset type 1 diabetes and was accompanied by increased numbers of naive T cells [ 42 , 43 ].
Cytokine-directed interventions
Anti-inflammatory cytokine-specific compounds, which are successfully used, for example, in rheumatic diseases, have been tested as alternatives to directly targeting the T or B cell in type 1 diabetes, as briefly summarised below. In addition, to stimulate an increase in T regulatory cells, low-dose IL-2 treatment has also been tested and the results have been somewhat promising [ 44 – 48 ], with recent developments mitigating earlier caveats, which included an arguably narrow dose range and lack of full specificity for T regulatory cells.
Blockade or antagonism of the central proinflammatory cytokine TNF-α using infliximab, adalimumab or the receptor fusion protein etanercept have shown some potential in type 1 diabetes, with indications of improved glycaemic control and C-peptide secretion [ 49 , 50 ]. More recently, a C-peptide-sparing effect of TNF-α blockade was reported with golimumab use, after 1 year in children and young adults with type 1 diabetes [ 51 ].
IL-6 is another proinflammatory cytokine that has been targeted with success in multiple other autoimmune diseases [ 52 ]. Although its role in type 1 diabetes is not established, IL-6 has been suggested as a target [ 53 ]. Of note, IL-6 has been shown to protect the beta cell from oxidative stress and is constitutively expressed by pancreatic alpha and beta cells, indicating important physiological roles [ 54 ]. In type 1 diabetes, the EXTEND Phase II trial of tocilizumab, a monoclonal antibody against the IL-6 receptor, was recently completed ( ClinicalTrials.gov registration no. NCT02293837).
IL-21 has been proposed as an attractive target in type 1 diabetes [ 55 , 56 ]. Physiologically, IL-21 is important not only for the function of T helper (Th) cells (Th17 and T follicular helper cells) but also for the generation and migration of CD8 + T cells. CD8 + T cells are now considered the chief T cell type accumulating in and around islets [ 57 , 58 ] with pre-proinsulin emerging as a pivotal autoantigen driving their infiltration in type 1 diabetes [ 59 ]. IL-21 neutralisation has been shown to prevent diabetes in mice [ 60 ], and a C-peptide-sparing benefit of anti-IL-21 alone or in combination with the glucagon-like peptide-1 (GLP-1) receptor agonist (RA) liraglutide has been observed in a clinical proof-of-concept study [ 61 ], as described further below. Reassuringly, non-clinical models, including a viral type 1 diabetes model, showed a minor impact of IL-21 blockade on the immune repertoire [ 55 ].
Antigen vaccination
With the appeal of having no expected effect on acquired immunity, the overall aim of beta cell antigen vaccination is to induce tolerance by balancing the T cell population between auto-aggressive T effector cells and autoantigen-specific T regulatory cells. Induction of T regulatory cells carries the potential benefit of also downregulating the activity of proinflammatory antigen-presenting cells. The topic has been extensively reviewed in the past [ 62 ]. Briefly, inspired by successes with vaccination against, for example, peanut allergy, tolerisation of T effector cells has been attempted using administration of whole antigens, such as oral insulin, or of peptides. Whilst the concepts are promising and under active investigation, their effectiveness in humans is yet to be proven. For example, in at-risk children, oral insulin administration has previously failed to prevent type 1 diabetes [ 63 , 64 ], speculatively due to a suboptimal dose level or unclear effects across risk-specific subgroups [ 65 , 66 ], including those defined by insulin gene polymorphisms. Similar results and considerations have been reported for immunisation with GAD65 [ 67 ] and for peptide-based therapies [ 68 , 69 ]. Further, the lack of full clarity regarding the mechanisms at play with antigen-based therapies outlines a number of shortcomings, including the fact that no biomarker is currently available to assist in establishing the optimal dose regimen.
Non-immunomodulatory adjunctives
We next focus on selected compounds that have gained attention due to their potential benefits as adjuncts to insulin in type 1 diabetes.
Amylin deficiency is a recognised feature of type 1 diabetes [ 70 ]. As a neuroendocrine hormone, amylin inhibits glucagon secretion and contributes to reducing postprandial glucose variability. As an adjunct to meal-time insulin, the injectable amylin analogue pramlintide is approved only in the USA for the treatment of type 1 and type 2 diabetes alike [ 71 ]. In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [ 72 ]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
Metformin is a low-cost agent with glucose-lowering effects that mainly occur via decreased hepatic glucose production. It is not a guideline-recommended option in type 1 diabetes. However, partly because of its ameliorating effect on insulin resistance, metformin has been somewhat promising in managing the disease, especially in children and adolescents, as well as in obese people with type 1 diabetes, with studies indicating reduced insulin requirements and body weight reduction [ 73 – 75 ]. In the large REducing With MetfOrmin Vascular Adverse Lesions (REMOVAL) trial, however, metformin did not reduce the long-term insulin needs or improve glycaemic control in people with long-standing type 1 diabetes and multiple cardiovascular risk factors [ 76 ].
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter (SGLT) inhibitors lower blood glucose levels by restraining the absorption of glucose in the small intestine and promoting the renal excretion of glucose [ 77 ]. Results with dapagliflozin, empagliflozin and sotagliflozin have indicated benefits of SGLT inhibition in managing type 1 diabetes when added to insulin [ 78 – 83 ]. Significant benefits included reduced insulin dose requirements, improved glycaemic control and reduced body weight [ 84 ]. So far, sotagliflozin and dapagliflozin are approved in Europe and Japan (but not the USA) as adjuncts to insulin for the management of overweight or obese people with type 1 diabetes when optimally titrated insulin alone does not provide adequate glycaemic control. Importantly, however, data suggest that the use of SGLT inhibitors in type 1 diabetes is associated with markedly increased risk of diabetic ketoacidosis [ 85 – 87 ]; for sotagliflozin, a 5–17-fold risk increase was noted [ 88 ]. These observations prompted the formation of an international consensus on recommendations for the use of SGLT inhibition in type 1 diabetes [ 89 ] as well as a suggestion that treatment should be overseen by specialists [ 88 ].
GLP-1 is a hormone of the incretin system that is secreted upon food intake. A marked uptake has been seen in the use of GLP-1 RAs in type 2 diabetes due to their pleiotropic glucose-dependent effects that improve glycaemic control and reduce body weight [ 90 ]. In contrast, GLP-1 agonism for the treatment of type 1 diabetes remains unproven, with initial results from smaller investigator-conceived studies being inconclusive. Recently, Phase II findings with the short-acting GLP-1 RA exenatide in adults with type 1 diabetes were negative. In two larger Phase III trials (ADJUNCT ONE and ADJUNCT TWO), the GLP-1 analogue liraglutide used as an adjunct to insulin appeared well-tolerated and improved HbA 1c and reduced body weight [ 91 , 92 ]. Both ADJUNCT trials indicated a minor increase in the risk of hypoglycaemia and hyperglycaemia with ketosis with liraglutide use, whereas the risk of diabetic ketoacidosis was negligible. Subsequently, a plethora of investigations have reached similar conclusions [ 93 – 101 ]. Nonetheless, the use of GLP-1 RAs in type 1 diabetes remains potentially useful, as discussed below.
Verapamil is a common calcium-channel blocker used for decades as an anti-hypertensive agent. In mouse models of type 1 diabetes, verapamil promoted survival of functional beta cells via a mechanism that involves reduced expression of the cellular redox regulator thioredoxin-interacting protein [ 102 ]. In a smaller Phase II trial, verapamil was better than placebo for preserving meal-stimulated C-peptide secretion in adults with type 1 diabetes and no safety concerns were identified [ 103 ]. Despite these findings, however, the place for verapamil as a disease-modifying agent in type 1 diabetes remains to be fully established.
Future perspectives
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the immunopathophysiology of type 1 diabetes, whilst also exploring the promise of combination therapies that integrate tried or new treatment modalities. In addition, lessons learned from type 2 diabetes with regard to the beneficial effects of certain agents on, for example, body weight and cardiorenal risk may also prove relevant in type 1 diabetes. We review selected future prospects addressing these aspects below.
Of further note, the lack of sufficient efficacy of previously tested therapies may also be related to the fact that type 1 diabetes is a heterogenous disease with diverse disease stages (Stages 1 to 3) and modifiers, such as age of onset or clinical diagnosis. Identifying the optimal timing of each type of intervention relative to the disease stages and the age of the patient is, therefore, important. For example, initiating an immunomodulatory intervention at Stage 1 (i.e. prior to clinical diagnosis) is not a straightforward decision and may be associated with clinical inertia. Moreover, an increased focus on disease endotypes (i.e. different biological processes under the type 1 diabetes umbrella) was recently suggested to ensure a precision-medicine approach to type 1 diabetes research and management [ 104 ].
Immune interventions
It is becoming increasingly clear that autoreactivity to islet antigens is also present in healthy individuals [ 59 ] and autoimmunity recurs after autologous nonmyeloablative haematopoietic stem cell transplantation [ 105 , 106 ]. Thus, in line with the ‘assisted suicide’ theory introduced earlier [ 6 , 7 ], it is also increasingly apparent that the development of type 1 diabetes does not only involve dysfunctional islets, but also beta cells that ‘unmask’ themselves to immune recognition and destruction. This notion supports two central realisations; first, it might explain why, in previous studies, immune therapy alone has failed to protect beta cell function over longer periods of time after onset of diabetes. Second, looking forward, novel type 1 diabetes therapies should pursue the holy grail of type 1 diabetes immune therapy: essentially agents that act locally in the islets, within the pancreas, either targeting the immune cells destroying the beta cell or the beta cell itself. Knowledge gained over the years regarding the beta cell has suggested multiple, yet putative reasons for the ‘unmasking’ of these cells. Potential reasons include the facts that beta cells are especially biosynthetically active and systemically exposed [ 107 ] and, therefore, susceptible to stress-induced production of autoantigenic proteins during, for example, infections [ 108 – 110 ]. Moreover, the beta cell might be vulnerable to both cytokine-mediated destruction [ 111 ] and various types of endoplasmic reticulum stress [ 112 ]. Relieving the beta cell of these burdens may provide an opportunity to save the beta cell without resorting to aggressive immune suppression.
With this in mind, we see the following two promising avenues as deserving increased focus going forward: (1) therapies aimed at inducing tolerance to beta cell antigens; and (2) the use of GLP-1 RAs that directly target the beta cells to enhance their function whilst also protecting them from immune-mediated inflammatory stress.
As discussed above, achieving antigenic tolerance has, so far, proven elusive but carries the crucial potential of leaving the overall capacity of the immune system intact whilst suppressing only the diabetogenic cell populations. Future studies need to establish whether inducing tolerance in humans can be achieved by clonal anergy or clonal deletion of effector cells, or whether antigen-specific regulatory cells may be able to suppress autoreactivity locally. Moreover, it needs to be clarified to what extent tissue-resident memory effector cells can be eliminated.
Recent evidence from rodent models indicates a role for GLP-1 RAs in protecting beta cells from apoptosis and in promoting beta cell replication and mass [ 113 – 117 ]. As such, although this remains to be confirmed, it is conceivable that GLP-1 RAs may offer a way to prevent the ‘unmasking’ of the beta cell to immune effector cells, for example, by downregulating expression of MHC class I proteins. Intriguingly, unpublished non-clinical evidence shows that liraglutide also limits immune cell infiltration into pseudo-islets (M. von Herrath, unpublished results). In addition, studies in NOD mice have shown that GLP-1 RAs administered in combination with various immunomodulatory agents, including anti-CD3 compounds [ 118 ], were more efficient in inducing diabetes remission than when given as monotherapy [ 119 ]. Furthermore, the anti-inflammatory effects of GLP-1 RAs are well-documented, with liraglutide being associated with reduced systemic levels of C-reactive protein and of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 [ 120 – 123 ]. Whilst these findings have mainly been observed in animal models or in type 2 diabetes, their relevance to (clinical) type 1 diabetes is conceivable but, so far, largely unexplored.
Management of cardiometabolic complications
A person diagnosed with type 1 diabetes faces a high risk of serious complications and of premature death, primarily for cardiovascular causes. This warrants a therapeutic focus on the broad pathophysiology of the disease.
Further, whilst the exact connections between excess body weight and type 1 diabetes remain debatable [ 124 ], the increased incidence of type 1 diabetes seems to coincide with the rapid rise in the prevalence of obesity [ 125 , 126 ]. Recent evidence suggests that a high BMI may exacerbate the early-stage immune-mediated beta cell destruction in type 1 diabetes, especially in children and adolescents [ 127 ]. Evidence also points to an impact of rapid growth in early childhood [ 128 ], and a positive correlation between the age of type 1 diabetes onset and BMI has been observed [ 129 ]. The ‘accelerator hypothesis’ views high BMI and low insulin sensitivity as triggers for type 1 diabetes onset [ 130 ] and the term ‘double diabetes’ has been suggested to describe an amalgam of type 1 diabetes with parallel and separate pathophysiological processes typically associated with type 2 diabetes, such as obesity and insulin resistance [ 131 ].
Use of SGLT inhibitors or GLP-1 RAs as adjuncts to insulin admittedly holds promise in ameliorating multiple type 1 diabetes complications. For example, evidence suggests that SGLT inhibitors offer cardiorenal protection [ 132 , 133 ], at least in type 2 diabetes, putatively owing to clinically unproven mechanisms of action beyond improved glucose homeostasis [ 134 ]. Moreover, a few GLP-1 RAs (dulaglutide, liraglutide and semaglutide) are now indicated to reduce cardiovascular risk in people with type 2 diabetes and established cardiovascular disease, and a protective effect of GLP-1 RAs on the kidneys is suggested from a range of cardiovascular outcome trials (CVOTs) in type 2 diabetes [ 135 – 138 ]. In addition, both SGLT inhibitors and GLP-1 RAs, especially second-generation GLP-1 RAs (e.g., semaglutide), are associated with a meaningful reducing effect on body weight.
Combination therapies
Combination therapies that work via two mechanistically distinct targets to integrate immune modulation with a beta cell-specific component have been suggested [ 139 – 141 ] and encouraged [ 142 ]. Truly advantageous combination therapies are arguably those in which the components target different pathogenic pathways (for example, systemic vs beta cell-specific pathways), thereby synergising in terms of the beneficial effects. These combination therapies should also be safe and well-tolerated alone and in combination.
Known ongoing efforts are sparse but include the combination of ATG and GCSF (as discussed above) and the combination of targeted immune modulation via an anti-IL-21 antibody in combination with a GLP-1 analogue (liraglutide). In addition to the potential of preserving functional beta cell mass by leveraging the immunomodulatory and anti-inflammatory properties of both the anti-IL-21 antibody and liraglutide, their combination addresses the need to manage the symptoms and complications of established type 1 diabetes, as discussed earlier. As previously mentioned, results from a clinical proof-of-concept trial recently found that anti-IL-21 plus liraglutide was significantly better than placebo in preserving C-peptide secretion over a period of 54 weeks [ 61 ]. The benefits diminished after treatment cessation; however, the treatment appeared safe and well-tolerated.
Stem cell replacement therapy
On the horizon, we approach the promise of stem cell-based therapies [ 143 ], offering a potential cure by replacing or supplementing beta cells that have been lost or have become dysfunctional. Stem cell-derived beta cells, however, also need to be rescued from immune-mediated destruction, suggesting that some degree of immunomodulation will be needed, even in the advent of viable stem cell therapy in type 1 diabetes, unless a fully effective immune-defying capsule is available [ 144 ]. In this context, better prevention or treatment regimens will also be useful for enabling longer-term beta cell graft acceptance.
Closing thoughts
Whilst many intriguing non-insulin therapies have failed to fully meet their potential in the past few decades, hope remains that the knowledge gained has carved out paths towards better options for the prevention and management of type 1 diabetes. Taken together, in our view, stem cell replacement therapies and a refocused development of safe and well-tolerated combination therapies are the most promising emerging preventive or therapeutic avenues. In parallel, reinforced efforts to predict or diagnose type 1 diabetes as soon as possible are equally important in light of the fact that even the best interventions need to be introduced as early as possible to effectively preserve or rescue beta cells in individuals with this condition.
(PPTX 230 kb)
Authors’ relationships and activities
All authors are employees of Novo Nordisk A/S, Denmark.
Abbreviations
| ATG | Anti-thymocyte globulin |
| GCSF | Granulocyte colony stimulating factor |
| GLP-1 | Glucagon-like peptide-1 |
| RA | Receptor agonist |
| SGLT | Sodium-glucose cotransporter |
| Th | T helper |

Contribution statement
All authors contributed substantially to the preparation of the review and approved the version to be published.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Overview & Types
- Symptoms & Diagnosis
- Understanding Blood Sugar
- Diet & Exercise
- Better Living
- Complications
- Related Conditions
- Symptoms & Causes
- Diagnosis & Tests
- Prevention & Treatment
- Living & Managing
- Complications & Related Diseases
- Treating & Managing
- Complications & Related Conditions
- Gestational Diabetes
- Appointment Prep
- View Full Guide
Stem Cell Therapy Implant Shows Promise For Type 1 Diabetes
Dec. 11, 2023 – An experimental device containing millions of stem cells significantly reduced the need for insulin shots among people with type 1 diabetes , according to a new study – a treatment researchers say may someday provide a cure for the chronic, life-altering condition.
Researchers from the University of British Columbia and Vancouver Coastal Health used tiny implants filled with lab-grown pancreatic cells known as VC-02.
The study, published in the journal Nature Biotechnology , involved 10 people who at the start of the study could not produce insulin naturally. After 6 months with the implant, three of them showed significant improvement. Their bodies spent more time within the normal blood sugar range, reducing their need for external insulin.
“The hope is to get these cells strong enough to help stop requiring insulin injections all together,” said David Thompson, MD, principal investigator at the Vancouver trial site and clinical director of the Vancouver General Hospital Diabetes Centre. “I believe this is going to turn into a cure as soon as 2024.”
Type 1 diabetes is a condition in which the immune system destroys insulin-making cells in the pancreas, known as beta cells. Insulin is a hormone that regulates sugar in the blood. The condition – sometimes called juvenile diabetes – is most commonly diagnosed between the ages of 4 and 6 and in early puberty.
In the United States, people who are non-Hispanic and White are most likely to have type 1 diabetes, and it affects men and women at about the same rates. Having a close family member with the illness increases risk. About 1.24 million people in the United States live with type 1 diabetes; that number is expected to reach 5 million by 2050.
With type 1 diabetes, it's as if the body's insulin factory has shut down. People who have the disease need to take insulin from the start.
This differs from type 2 diabetes , in which the body doesn't use insulin properly. It can be managed with lifestyle changes, medications, and sometimes external insulin shots.
Until a century ago – when insulin was discovered – diabetes was a death sentence . A 14-year-old boy who lay dying from diabetes in a Toronto hospital was the first person to receive the new treatment in 1922. Within 24 hours, his high blood glucose levels dropped to near-normal levels.
“Insulin therapy for people with type 1 diabetes is better than it has ever been, but it's still not a cure,” Thompson said. “This is probably the first wave of a new era of medicine using cell therapy.”
The trial tested an experimental cell therapy developed by biotechnology company ViaCyte.
Thompson and his colleagues used devices implanted just beneath the skin, about the size of a small bandage. Unlike a glucose monitor – which is also inserted beneath the skin but only estimates blood glucose levels – the stem cell device delivers a steady supply of insulin to the body.
The trial builds off of a 2021 study that showed this approach could help the human body produce insulin. The latest study increased the number of devices for each person and improved the design to help the lab-grown cells survive.
All the people in the study started out with no insulin production and had surgery at sites in Vancouver, Belgium, and the U.S. to get up to 10 device implants each. After 6 months, three of them showed clear signs of insulin production that stayed steady throughout the yearlong study. One person in the study had showed notable improvement, spending more time in the target blood sugar range and reducing their need for extra daily insulin by 44%.
“Each device is like a miniature insulin-producing factory,” said co-author Timothy Kieffer, PhD, a professor with the departments of surgery and cellular and physiological sciences at the University of British Columbia, and past chief scientific officer of ViaCyte. The cells are “packaged into the device to essentially re-create the blood sugar-regulating functions of a healthy pancreas.”
A cure for type 1 diabetes would also mean preventing several other health complications related to the illness: blindness, kidney problems, limb loss, and even life-threatening blood sugar drops during sleep. Diabetes also significantly heightens the chances of having a heart attack or stroke.
The trial has two big limitations, said Robert Gabbay, MD, chief scientific and medical officer of the American Diabetes Association, who was not involved in the trial. Not only is it small, but the technology failed to normalize blood glucose levels, which is the goal.
But it shows promise, he said. Cell replacement therapies have previously faced a major barrier: The immune system attacks the implanted cells, requiring potentially harmful immunosuppressive drugs.
“This is particularly problematic for people with type I diabetes since the initial cause of type 1 is an autoimmune destruction of beta cells,” Gabbay said. “Placing beta cells sequestered from the immune system has been something that a number of investigative teams have worked on. This early study shows some proof of concept.”

Top doctors in ,
Find more top doctors on, related links.
- Diabetes Health Center Reference
- Diabetes Health Center Slideshows
- Diabetes Health Center Quizzes
- Diabetes Blogs
- Diabetes Health Center Videos
- Diabetes Health Center Medications
- Find an Endocrinologist
- Book: Take Control of Your Diabetes Risk
- General Diabetes
- Type 1 Diabetes
- Type 2 Diabetes
- Prediabetes
- Blood Sugar Control
- Diabetic Neuropathy
- Eye Problems Assessment
- Heart Disease
- Metabolic Syndrome
- Diabetes Overview
- Diabetes Symptoms
- Diabetes Causes
- Diabetes Diagnosis
- Diabetes Treatment
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
New Type 1 Diabetes Treatment Eliminates Need for Insulin
Key takeaways.
- The FDA has approved a new drug called Lantidra to manage low blood sugar in people with type 1 diabetes.
- The drug helps control blood sugar levels through an infusion of donor pancreatic cells that create insulin in the body so that patients no longer need to take external insulin.
- This drug is for people with severe and recurrent hypoglycemia who are unable to achieve target blood sugar levels.
In June, the FDA approved Lantidra—the first donor cell therapy for people with type 1 diabetes who struggle with severe and recurrent low blood sugar. The drug eliminates the need for external insulin, and avoids something as invasive as an islet cell transplant.
Lantidra (donislecel-jujn) is for people who have trouble managing their blood sugar and suffer from hypoglycemia, or for people who have hypoglycemia unawareness—a condition where patients are unable to detect their dropping blood sugar and might not be able to treat it before it drops to potentially dangerous levels. This can be a life-threatening condition that is not easily treatable with medication.
“Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a FDA press release about Lantidra. “Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels.”
Developed by CellTrans, Lantidra is an infusion of islet cells from a deceased donor into the liver portal vein of the diabetic patient. Because the infused cells restore functional pancreatic islet cells in people with type 1 diabetes, external insulin is no longer necessary.
Small—But Successful—Clinical Trials Led to Approval
Lantidra’s safety was studied in two non-randomized trials with 30 participants who live with type 1 diabetes and hypoglycemic unawareness. The patients received at least one Lantidra infusion and a maximum of three infusions, the FDA said in its press release.
After their infusions, 21 patients did not need to take insulin for a year or more. Eleven patients did not need insulin for one to five years and 10 participants did not need insulin for more than five years. Five patients required external insulin after the infusions, and did not achieve any insulin independence.
During the trials there were two deaths—one from multiorgan failure with sepsis about one-and-a-half years after the first infusion, and one from progressive confusion, global atrophy and micro-ischemic disease almost 10 years after the first dose. Both subjects were on immunosuppression at the time.
What to Know About Lantidra
This drug was specifically approved to tackle ongoing and severe low blood sugar in patients with type 1 diabetes who are unable to reach target blood sugar levels despite intensive diabetes management and education.
Type 1 diabetes is a disease where the body's own immune system starts attacking the insulin producing cells of the pancreas, Omid Veiseh, PhD , an associate professor of bioengineering at Rice University, told Verywell. Once those cells are killed off, the patient can no longer regulate their blood glucose, meaning they need to rely on external insulin.
“This new drug gets those insulin producing cells from deceased donors…then they’re purified to be safe in terms of pathogens and whatnot—and then they’re infused into type 1 diabetic recipients,” Veiseh said. “But because the disease is autoimmune mediated, and the cells are from a donor, the patient will receive immunosuppression [medication]. The immune suppression basically protects the cells from the host’s immune response.”
Up until now, patients with recurrent and severe hypoglycemia have had to work with their endocrinologist to manage blood sugar as best as they can through the dosing of their insulin as well as diet and exercise. In severe cases, the use of glucagon , which helps rescue patients from an episode of hypoglycemia, is used, said Fernando Ovalle, MD , director of the Division of Endocrinology, Diabetes and Metabolism at the University of Alabama at Birmingham School of Medicine.
“You don’t want to have hypoglycemia, especially severe or repeated,” Ovalle told Verywell, explaining that a severe low blood sugar can result in incidents like falling and hitting your head, cardiac arrest, or seizures.
For patients for whom ongoing hypoglycemia poses a great threat to their health, including death, an islet cell transplant might be considered. During this procedure, islets are taken from the pancreas of a deceased organ donor, then transferred into a patient with type 1 diabetes. Once the cells are implanted in the liver, they are able to make insulin.
Lantidra introduces healthy islet cells without requiring a transplant procedure.
Veiseh said advancements like Lantidra are giving hope to the medical and type 1 diabetes community. In the future, advancements will likely include replaceable cell types that can be lab-grown—so you don’t have to rely on donors—as well as localized immunosuppression strategies. This means that only the site that cells are infused into will need to be immunosuppressed, not the entire body.
“I think, in the next five or 10 years, we’re going to see a lot more of these islet replacement therapies, on the market,” Veiseh said. “And it’s going to be great for patients, because the cure is to replace those missing cells that have been killed off.”
How Lantidra Is Administered
Lantidra is a prescription medication that is administered by a healthcare professional via infusion into the liver. The recommended minimum dose is 5,000 equivalent islet number (EIN) per kg for the first infusion, and 4,500 EIN/kg for subsequent infusions, the drugmaker said.
The drug is currently only recommended for people who experience recurrent and severe hypoglycemia or have hypoglycemia unawareness. It is not for all people with type 1 diabetes, or for people with type 2 diabetes. It’s best for any patient considering the drug to first consult with their healthcare provider to see if they are the right candidate.
Lantidra is given in a single infusion dose. An additional dose or two may be necessary depending on whether the patient responds to the first infusion and achieves independence from external insulin within one year. In other words, if the first infusion is not successful, another dose might be needed. There is currently no data on the effectiveness or safety for patients who have more than three infusions.
How Does Lantidra Work?
Lantidra is an infusion of islet cells from a deceased donor into the liver portal vein of the diabetic patient. The infused cells restore functional pancreatic islet cells in people with type 1 diabetes, removing the need for external insulin.
Known Side Effects
In clinical trials, there were a series of adverse reactions associated with Lantidra. The drugmaker said these varied with each participant and depended on the number of infusions they received.
Ninety percent of trial participants had at least one serious adverse reaction, and the major causes were attributed to the infusion procedure and immunosuppression drugs. Some of these side effects meant that the patient needed to stop taking the immunosuppressants, which stopped the drug from working.
Some of the most common reactions were nausea, fatigue, anemia, diarrhea, and abdominal pain.
Aside from the side effects of Lantidra itself, there are risks with taking immunosuppressants for a long period of time, including risk of infections, lymphomas and anemia.
“These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient,” the FDA said in its press release.
While the safety of taking Lantidra while pregnant has not been assessed, there are known risks for being on immunosuppressants while expecting. Fetal malformations are associated with some of the suppression drugs, therefore the drugmaker warns patients should have a confirmed negative pregnancy test before starting Lantidra.
How to Get Lantidra
Veiseh said because Lantidra relies on donor cells, CellTrans has a limited supply of the drug.
“You’re sort of at the mercy of donors being available,” he said. “I think the best estimates put this at 2,000 to 4,000 patients per year that you could treat just because of the limitation of organ availability.”
However, Veiseh said Lantidra is a step in the right direction for patients with type 1 diabetes who suffer from severe, ongoing low blood sugar. It is giving them another option instead of an islet cell transplant—a procedure that is only available in certain healthcare settings.
“In other countries, this kind of therapy has been available and reimbursed by insurance for years, but in the U.S., it was sort of a patchwork system of various surgeons that did it but it wasn’t really available to everyone,” said Veiseh. The National Institute of Diabetes and Digestive and Kidney Diseases has said that islet transplantation is considered an experimental procedure, and until it’s approved as a treatment for type 1 diabetes, it can only be performed for research purposes through clinical trials and is generally not covered by insurance.
“I think this is really a big step forward towards making this a potential solution that is uniformly available to all the patients as opposed to just certain ones,” Veiseh said.
University of California San Francisco Department of Surgery. Islet transplant for type 1 diabetes .
Food and Drug Administration. FDA approves first cellular therapy to treat patients with type 1 diabetes .
Food and Drug Administration. Lantidra [drug label].
By Laura Hensley Hensley is an award-winning health and lifestyle journalist based in Canada. Her work has appeared in various outlets, including Best Health Magazine, Refinery29, Global News, and the National Post.
Breakthrough diabetes study could lead to end of regular insulin injections, researchers say
Topic: Health
Researchers say they have made a breakthrough in the treatment of type 1 diabetes which could replace the need for regular insulin injections.
Research published by Baker Heart and Diabetes Institute scientists shows they have manipulated existing pancreatic stem cells to prompt them to produce insulin.
The study from the Melbourne researchers builds on previous work by Monash University scientists, using two existing cancer drugs.
The research is still in its early days and the next step will be pre-clinical animal trials.
But lead researcher and Baker institute scientist, Sam El-Osta, said the potential treatment could be viable for children and adults in the future.
"What we've discovered is the ability to harness the patient's remaining pancreatic cells to influence those cells to behave like insulin-producing beta cells," Professor El-Osta told ABC Radio Melbourne.
"This could potentially modify the course of diabetes and potentially eliminate the need for round-the-clock insulin injections in some people living with type 1 diabetes."
Researchers say the breakthrough could lead to an alternative treatment to regular insulin injections. ( ABC Tropical North: Sophie Meixner )
Broadly, people with diabetes do not naturally produce enough insulin, or their bodies do not use the hormone as they should.
For many people with diabetes, it means multiple insulin injections are required daily to manage the illness.
Research could be 'holy grail'
The two cancer drugs used in the research are already approved by the US Food and Drug Administration.
Researchers said the potential treatment could be "rapid" compared to current treatment options for type 1 diabetes.
"We've been able to repurpose these drugs to determine whether we could influence the trajectory by using these small molecule inhibitors in pancreatic ductal cells," Professor El-Osta said.
"We can quickly influence insulin restoration in a number of days in a dish from tissues derived from type 1 diabetes donors, both children and adults."
Diabetes Australia estimates around 134,000 people in Australia are living with type 1 diabetes, which represents about 10 per cent of all diabetes cases.
The Baker Heart and Diabetes Institute researchers are optimistic their work could potentially help people living with insulin-dependent type 2 diabetes.
The research has been published in a Nature scientific journal, Signal Transduction and Targeted Therapy.
It's estimated more than 130,000 people in Australia are living with type 1 diabetes. ( ABC Local: Damien Larkins )
Chief executive of the Australian Diabetes Society and University of Melbourne associate professor, Sof Andrikopoulos, labelled the research as "remarkable".
"For the 135,000 Australians with type 1 diabetes, this is the holy grail. This is it," he said.
"There's a potential here that this research might lead to the cure of type 1 diabetes, at some point down the road.
"It also has the potential to make a significant improvement in type 2 diabetes."
Dr Andrikopoulos, who was not involved with the study, said the research would reduce the burden of the disease.
"This research has the potential for the body itself, to produce and secrete insulin. So you can see that you're getting rid of needles, you're getting rid of insulin pumps, you're getting rid of finger pricking, you're getting rid of continuous glucose monitors," he said.
While he was hopeful for the future, Dr Andrikopoulos warned steps towards a cure would require consistent funding for diabetes research.
Breakthrough research unveils β-cell dynamics in Type 1 diabetes
- Download PDF Copy
About eight million people live with Type 1 diabetes (T1D) worldwide, a chronic autoimmune condition in which the body attacks and destroys its own insulin-producing β-cells (pronounced "beta") in the pancreas, leading to a lack of insulin and inability to regulate blood sugar. It's not known why the body suddenly perceives its own β-cells as the enemy; some lines of evidence suggest environmental factors such as viral infections may trigger the onset of T1D, others suggest genetics may also play some role.
Groundbreaking research by investigators at Joslin Diabetes Center sheds new light on the specific changes β-cells go through at the onset of T1D. Their findings-;published in Nature Cell Biology -;offer new avenues for targeted interventions for the chronic autoimmune condition.
In the field of Type 1 diabetes, research has largely focused on understanding the immune component, but our study argues that the β-cell is a significant player. Our findings suggest that the β-cell could be initiating key events which then promote the autoimmune mechanism to go awry. It's a paradigm shifting approach." Rohit N. Kulkarni, M.D., Ph.D., Margaret A. Congleton Chair and Co-Head of the Section on Islet & Regenerative Biology at Joslin Diabetes Center
In a series of experiments with β-cells taken from a mouse model of T1D, as well as from humans with established T1D, Kulkarni and colleagues teased out the complex cascade of biochemical steps called a signaling pathway that controls the innate immune response at the onset of T1D. The team identified one pathway that influences the immune characteristics of β-cells, acting like control switches that identify them as friend or foe to the body. These control switches can be imagined as tiny tags. One specific tag the investigators focused on-;called N6-methyladenosine (m6A)-;plays a vital role in the response of β-cells during T1D onset. By adjusting these control switches, the researchers were able to influence the levels of a crucial protein along this pathway, leading to a notable delay in the progression of the disease in a mouse model of T1D.
Dario F. De Jesus MSc, Ph.D., lead author of the study and Research Associate in the Kulkarni Lab, identified the key enzyme METTL3 as crucial for regulating β-cell antiviral defenses. In the late stages of T1D, when METTL3 levels were low, it hinted that higher METTL3 levels shield β-cells from dysfunction. By enhancing METTL3 production in the mouse model, the team successfully delayed progression of disease.
"This discovery suggests that interventions to boost METTL3 levels is a potential strategy to protect β-cells and slow down progression of Type 1 diabetes," emphasized De Jesus, who is also an Instructor in Medicine at Harvard Medical School.
Related Stories
- Reducing ultra-processed foods can lower type 2 diabetes risk
- Semaglutide vs. liraglutide: One-year weight loss efficacy in obesity and type 2 diabetes
- Early pregnancy HbA1c predicts gestational diabetes, reducing the need for complex tests
Taken together, these several lines of evidence paint a clearer picture of the immune events surrounding the still mysterious onset of T1D, including a novel mechanism that could be harnessed for β-cell protection. They also demonstrated that the enzyme METTL3 has the potential to promote β-cell survival and function during disease progression.
"It is notable that this pathway has commercially available compounds that have been used in the context of other diseases," said Kulkarni, who is also a professor of medicine at Harvard Medical School. "While it's a different target, it's an approach which has been shown to work. Among our next steps, we will focus on identifying specific molecules and pathways that can be harnessed to enhance protection of the β-cell."
Co-authors included Natalie K. Brown, Ling Xiao, Jiang Hu, Garrett Fogarty, Sevim Kahraman and Giorgio Basile of Joslin Diabetes Center; Zijie Zhang, Jiangbo Wei and Chuan He of the University of Chicago; Xiaolu Li, Wei-Jun Qian and Matthew J. Gaffrey of Pacific Northwest National Laboratory; Tariq M. Rana of University of California, San Diego; Clayton Mathews and Mark A. Atkinson of the University of Florida College of Medicine; Alvin C. Powers of the Vanderbilt University Medical Center; Audrey V. Parent of University of California, San Francisco; Sirano Dhe-Paganon of Harvard Medical School; and Decio L. Eizirik of Université Libre de Bruxelles.
This work is supported by the National Institutes of Health (grants R01 DK67536, UC4 DK116278, RM1 HG008935, and R01 DK122160). Portions of the mass spectrometry work were performed in the Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, a national scientific user facility sponsored by the Department of Energy under Contract DE AC05-76RL0 1830. R.N.K. acknowledges support from the Margaret A. Congleton Endowed Chair and C.H. is a Howard Hughes Medical Institute Investigator. DFDJ acknowledges support from Mary K Iacocca Junior Postdoctoral Fellowship, American Diabetes Association (grant #7-21-PDF-140, and NIH K99 DK135927).
Joslin Diabetes Center
F. De Jesus, D., et al. (2024). Redox regulation of m6A methyltransferase METTL3 in β-cells controls the innate immune response in type 1 diabetes. Nature Cell Biology . doi.org/10.1038/s41556-024-01368-0 .
Posted in: Medical Science News | Medical Research News | Medical Condition News
Tags: Blood , Blood Sugar , Cell , Cell Biology , Chronic , Diabetes , Education , Enzyme , Genetics , Health Care , Immune Response , Insulin , Laboratory , Mass Spectrometry , Medical School , Medicine , Mouse Model , Pancreas , pH , Protein , Research , Signaling Pathway , Spectrometry , Type 1 Diabetes
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 18, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Bacterial infections could be trigger for type 1 diabetes, new research suggests
by Cardiff University

For the first time, scientists have found that proteins from bacteria can trigger the immune system to attack insulin-producing cells, leading to the development of type 1 diabetes.
The new research showed that killer T-cells—a type of white blood cell that's involved in tackling bacterial infections—can cause type 1 diabetes when activated by bacteria. The researchers showed that proteins from bacterial species known to infect humans could generate killer T-cells that could kill insulin-producing cells.
This research, led by Professor Andrew Sewell at Cardiff University's School of Medicine, expands on their previous studies, which demonstrated that killer T-cells play a major role in initiating type 1 diabetes by killing insulin-producing cells .
Professor Sewell said, "Type 1 diabetes is an autoimmune disease that usually affects children and young adults , where the cells that produce insulin are attacked by the patient's own immune system. This leads to a lack of insulin, meaning that people living with type 1 diabetes need to inject insulin multiple times a day to control their blood sugar levels.
"There is currently no cure for type 1 diabetes and patients require life-long treatment. People living with type 1 diabetes may also develop medical complications later in life, so there is an urgent need to understand the underlying causes of the condition to help us find better treatments."
In laboratory experiments, the researchers introduced bacterial proteins into cell lines from healthy donors and monitored the reaction of killer T-cells from these donors. They found that strong interaction with the bacterial proteins triggered killer T-cells to attack cells that make insulin.
The clinical lead for this study, Dr. Lucy Jones, said, "We observed this in relation to a specific HLA— human leukocyte antigen —a gene that codes for proteins that help the immune system differentiate between our own cells and invading cells.
"The specific HLA associated with the bacterial infection that triggers diabetes is only present in around 3% of the population in the UK. So the bacterial pathogens that can generate anti-insulin T-cells are caused by a rare infection in a small minority of people."
Professor Sewell added, "Killer T-cells are able to target and attack body cells that produce a specific protein . We found that after encountering proteins from some infectious bacteria, killer T-cells could mistakenly also kill cells producing the insulin protein. We found activated T-cells with this same 'cross-reactivity' in the blood of patients with type 1 diabetes suggesting that what we saw in laboratory experiments could have triggered the disease."
The research, published in the Journal of Clinical Investigation , provides the first evidence of how proteins from bacterial germs can trigger the type of killer T-cells seen in patients with type 1 diabetes. The team hopes that knowing more about this process, will allow new ways to diagnose, prevent, or even halt the development of type 1 diabetes.
"We hope that understanding how T-cells trigger diseases like type 1 diabetes will allow us to diagnose and treat disease before the onset of symptoms. Early treatment is known to result in a better prognosis as the healthy pancreatic beta cells that are being attacked can be protected before they are destroyed," said Garry Dolton, the study's first author.
Dr. Lucy Jones said, "Thanks to patients and health care staff being research active, this strong research collaboration between Cwm Taf Morgannwg University health board and Cardiff University has improved our understanding of diabetes."
Explore further
Feedback to editors

Research identifies nearly 200 potential breast carcinogens in food packaging materials
8 minutes ago

New software guards the public from airborne radiation
7 hours ago

Study reveals link between microbiome and aggression in mice
8 hours ago

Biological findings open the door to improved outcomes for young adults with sarcoma

New models quantify how 'nociception' could help improve management of surgical pain
9 hours ago

Wearable sensors, machine learning system could pinpoint Parkinson's
10 hours ago

B-cells hold promise for treating glioblastoma

Small-molecule drug shows potential for hard to treat cancers
11 hours ago

Garage sale study provides yet another reason to sleep on it before making an important decision

Diagnostic guidelines don't catch all rare cancers, study finds
Related stories.

Psoriasis drug shows promise for treating childhood diabetes
Jul 30, 2024

What is type 1.5 diabetes? It's a bit like type 1 and a bit like type 2—but it's often misdiagnosed
Aug 27, 2024

Stem cell therapy could be breakthrough against type 1 diabetes
Jun 25, 2024
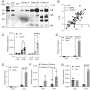
Examining the protein that protects insulin-producing cells
Mar 21, 2024
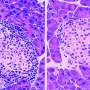
Small protein protects pancreatic cells in model of type 1 diabetes
Aug 16, 2021
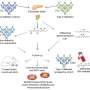
Research provides further evidence that epigenetic changes can cause type 2 diabetes
Dec 13, 2023
Recommended for you

Researchers 'turbocharge' vaccine delivery by jolting 'bystander' immune cells into action in animal models
12 hours ago

Study sheds new light on immune dysfunction in children with severe infections and inflammatory diseases

Fever drives enhanced activity and mitochondrial damage in a subset of T cells, study finds
Sep 20, 2024
New blood test could be an early warning for diabetes in children

Researchers discover immune response to dengue can predict risk of severe reinfections
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Type 1 Diabetes Research At-a-Glance
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management and much more. For specific examples of projects currently funded by the ADA, see below.
Martin John Hessner, PhD
Medical College of Wisconsin
Project: Probiotic normalization of innate immunity in type 1 diabetes
"My passion for immunology and type 1 diabetes stems from a personal interest in autoimmunity. This award enables the translation of our rodent studies where we have delayed and prevented type 1 diabetes by modulating the intestinal microbiota, to newly diagnosed patients."
The problem: The human intestinal tract harbors billions of bacteria – collectively termed the microbiome – which representing a significant environmental contact. Evidence suggests that modern lifestyles may promote growth of an altered, sub-optimal intestinal flora. While development of type 1 diabetes (T1D) has a strong genetic basis, this potentially modifiable environmental factor may explain the increase in T1D incidence observed over the past half-century.
The project: This project extends studies of an elevated inflammatory state that is present in T1D patients as well as their healthy family members. In animal models of T1D, dietary or antibiotic protocols that promote growth of anti-inflammatory bacteria delay/prevent development of T1D. Dr. Hessner’s ADA-funded project will test whether probiotic supplementation reduces inflammation and improves insulin secretion in people newly diagnosed with T1D.
The potential outcome: This project has the potential to identify a safe, broadly applicable, environmental modifier that influences T1D progression, which is critical for development of preventive and therapeutic approaches.
Elizabeth D. Cox, MD, PhD
University of Wisconsin-Madison
Project: Identifying actionable self-management barriers for adults with T1D
“I’m a pediatrician by training, and most of my work has focused on improving the way healthcare is delivered to children with diabetes and other chronic diseases. With this grant from the American Diabetes Association, I will be able to build on what I have learned in studying kids and expand that work to improve the healthcare provided to adults with type 1 diabetes.”
The problem: Many adults with type 1 diabetes (T1D) want self-management help. The researchers have previously designed a self-management help questionnaire for youth with T1D that uses child and parent answers to understand the self-management challenges the family faces. The children and their parents then receive resources designed specifically for their needs. This has been very successful. However, no such program exists for adults with T1D.
The project: Dr. Cox now plans to institute design a self-help management program specifically for adults with T1D. Based on feedback from adults with T1D, she will design a questionnaire by working directly with adults with T1D. Following this, the researchers will then test whether the answers predict important things like how often someone checks their blood sugar, how well their diabetes is controlled, or how well their life is going.
The potential outcome: At the conclusion of this project, the Dr. Cox and her team expect to have a survey that doctors and nurses can use to recommend self-management help that might best meet the patient's needs. Being able to recommend specific types of self-management help is expected to improve the lives of adults with T1D.
Karen Cerosaletti, PhD
University of Washington
Project: Single cell RNAseq analysis of islet antigen reactive memory CD4 T cells during T1D progression and therapy
“My interest is understanding how the immune system malfunctions in type 1 diabetes so that we can more accurately predict who will develop type 1 diabetes, when they will develop it, and how we can prevent or block disease onset or progression. This grant will allow us to determine the role of T cells of the immune system in destroying pancreatic islets, resulting in loss of insulin secretion.”
The problem: Type 1 diabetes (T1D) results from immune destruction of insulin-producing islets in the pancreas. The researchers have identified a specific variant of an immune cell, called a T cell, that is found in patients with T1D but not in control people. However, it is unclear to what degree this specific immune cell type is responsible for the initiation and progression of T1D.
The project: The goal of Dr. Cerosaletti and her team is to investigate the role of this unique immune cell in people at high-risk for developing T1D and in patients recently diagnosed with T1D. She will use powerful new technologies to determine how these cells change over the course of T1D progression and assess if these cells play a role in the destruction of insulin-producing islets.
The potential outcome: The significance of this study to T1D is two-fold: the results will advance the understanding of the immune mechanisms underlying the destruction of the insulin producing islets in T1D. Further, these findings will determine the feasibility of using this unique subset of islet T cells as markers to predict or monitor disease onset and progression during T1D.

Give Today and Change lives!
With your support, the American Diabetes Association® can continue our lifesaving work to make breakthroughs in research and provide people with the resources they need to fight diabetes.
- Article Information
Probability was adjusted for age and sex. Among 1303 patients, 93 had cancer diagnoses, including skin (n = 27), other (n = 21), breast (n = 15), reproductive (n = 8), digestive (n = 6), head and neck (n = 5), bone or blood (n = 4), prostate (n = 4), urinary (n = 2), thoracic (n = 2), and unknown (n = 2).
- Methodological Issues in Study of Daily Insulin Dose and Cancer Risk JAMA Oncology Comment & Response February 1, 2023 Haydee Verduzco-Aguirre, MD; Maria Eugenia Penados-Ovalle, MD; Leonardo Guadalupe Mancillas-Adame, MD
- Methodological Issues in Study of Daily Insulin Dose and Cancer Risk JAMA Oncology Comment & Response February 1, 2023 Rachel G. Miller, PhD; Tina Costacou, PhD
- Methodological Issues in Study of Daily Insulin Dose and Cancer Risk JAMA Oncology Comment & Response February 1, 2023 Yin Zhang, MD, MPH; Edward L. Giovannucci, MD, ScD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Zhong W , Mao Y. Daily Insulin Dose and Cancer Risk Among Patients With Type 1 Diabetes. JAMA Oncol. 2022;8(9):1356–1358. doi:10.1001/jamaoncol.2022.2960
Manage citations:
© 2024
- Permissions
Daily Insulin Dose and Cancer Risk Among Patients With Type 1 Diabetes
- 1 Merck Research Labs, Merck & Co Inc, West Point, Pennsylvania
- 2 Diabetes Institute, Ohio University, Athens
- 3 Diabetes and Endocrinology Clinic, OhioHealth Castrop Center, Athens
- Comment & Response Methodological Issues in Study of Daily Insulin Dose and Cancer Risk Haydee Verduzco-Aguirre, MD; Maria Eugenia Penados-Ovalle, MD; Leonardo Guadalupe Mancillas-Adame, MD JAMA Oncology
- Comment & Response Methodological Issues in Study of Daily Insulin Dose and Cancer Risk Rachel G. Miller, PhD; Tina Costacou, PhD JAMA Oncology
- Comment & Response Methodological Issues in Study of Daily Insulin Dose and Cancer Risk Yin Zhang, MD, MPH; Edward L. Giovannucci, MD, ScD JAMA Oncology
Previous studies among persons with type 1 diabetes (T1D) found a higher incidence of certain cancers in this population compared with the general population. 1 However, no studies have evaluated the risk factors of cancer incidence in T1D. In the present study, using data from the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study, we explored the associations of risk factors with cancer incidence in patients with T1D over a 28-year follow-up period.
Detailed descriptions of the DCCT/EDIC study have been published. 2 Briefly, 1441 patients from 29 centers in North America were enrolled in the DCCT between 1983 and 1989. At the end of the DCCT in 1993, 1375 surviving participants volunteered to continue in the EDIC follow-up study. By year 18 of the EDIC study in 2012, 1304 patients had completed the annual cancer history update, and 1 patient with a cancer diagnosis before enrollment was excluded. This cohort study was approved by The Ohio University Office of Research Compliance, which waived the informed consent requirement because publicly available data were used. We followed the STROBE reporting guideline.
Cancer incidence was calculated with 95% CIs. Associations of risk factors (eMethods in the Supplement ) with cancer incidence were evaluated individually using Cox proportional hazards regression models adjusted for age and sex. Race and ethnicity were collected but not analyzed because most participants were White individuals. Two-sided P < .05 was considered significant, and statistically significant variables were included in multivariable models for further assessment. Because daily insulin dose (time-dependent variable) remained significant in the multivariable model, it was also examined as a fixed variable (mean dose during follow-up). We examined cancer incidence by 3 categories of mean daily insulin dose: low (<0.5 units/kg), medium (≥0.5 and <0.8 units/kg), and high (≥0.8 units/kg).
Of the 1303 patients in the cohort, 93 (7%) had cancer diagnoses after a total of 33 813 person-years follow-up, and the incidence rate was 2.8 (95% CI, 2.2-3.3) per 1000 person-years. At first diagnosis, the mean age was 50 years, and the mean duration of diabetes was 25 years. Among 93 patients, 57 were female individuals (61%), 36 were male individuals (39%), 8 (9%) developed cancer within 10 years, 31 (33%) developed cancer between 11 and 20 years, and 54 (58%) developed cancer between 21 and 28 years.
Age and sex were associated with cancer incidence (hazard ratio [HR] for age, 1.08 [95% CI, 1.05-1.12]; for female sex, 1.74 [95% CI, 1.15-2.64]). Among other variables, exercising habit and high-density lipoprotein cholesterol were inversely associated and daily insulin dose was associated with cancer incidence after adjusting for age and sex. Daily insulin dose remained associated with cancer incidence in the multivariable model 1 (HR, 5.93; 95% CI, 1.21-29.06) and model 2 (HR, 4.13; 95% CI, 1.13-15.17) ( Table ). Cancer incidence was 2.11, 2.87, and 2.91 per 1000 person-years in the low-, medium-, and high-dose groups, respectively ( Figure ).
Hyperinsulinemia is a recognized risk factor for cancer. 3 However, clinical data in type 2 or unspecified diabetes cohorts showed conflicting results; the association was suggested in several studies. 4 , 5 A meta-analysis reported no association between exogenous insulin treatment and cancer risk in 13 of 16 studies, although 4 studies found an association between glargine and breast cancer. 6 Daily insulin doses in those cohorts were low (usually <0.3 units/kg), and many patients discontinued insulin temporarily or permanently during follow-up. 6
This study showed that daily insulin dose was associated with cancer risk in T1D. The HRs were significantly higher in the high-dose vs low-dose group.
A limitation of this study is the relatively small sample size, which precluded analyses with specific cancer types and resulted in a wide CI for daily insulin dose. Moreover, the association found may be subject to residual confounding and was not necessarily causal. Furthermore, larger studies in T1D are needed to validate this association.
Accepted for Publication: May 26, 2022.
Published Online: July 28, 2022. doi:10.1001/jamaoncol.2022.2960
Corresponding Author: Yuanjie Mao, MD, PhD, Diabetes Institute, Ohio University, Parks Hall 149, Athens, OH 45701 ( [email protected] ).
Author Contributions: Drs Zhong and Mao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: All authors.
Administrative, technical, or material support: Mao.
Supervision: Mao.
Conflict of Interest Disclosures: None reported.
Funding/Support: The Diabetes Control and Complications Trial (DCCT) and its follow-up the Epidemiology of Diabetes Interventions and Complications (EDIC) study were supported by grants and contracts from the National Institutes of Health (NIH) and the General Clinical Research Center Program of the National Center for Research Resources. Data from the DCCT/EDIC study were supplied by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repositories.
Disclaimer: This research report was not prepared under the auspices of the DCCT/EDIC study and does not represent analyses or conclusions of the DCCT/EDIC study group, the NIDDK Central Repositories, or the NIH.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

With a $2.7 million grant from the National Institutes of Health, researchers from the Keck School of Medicine of USC will lead a multi-site study to understand and optimize brain development in children with type 1 diabetes. (Photo/Adobe Stock
USC launches large-scale nationwide study of type 1 diabetes and brain development
A new large-scale longitudinal study, led by the Keck School of Medicine of USC, will unite 12 research centers across the United States to explore how type 1 diabetes affects children during a window of time known to be critical for healthy brain development.
Ultimately, the findings could help refine clinical guidelines for managing type 1 diabetes, including what glucose levels are safest in terms of healthy brain development. The study could also aid in the creation of targeted treatments for the condition, including changes to sleep, diet, and physical activity that can help specific patients.
The five-year study is supported by a grant of more than $2.7 million from the National Institutes of Health.
To read the full story, click here .
Share This Story, Choose Your Platform!
- Recent News
- News Archive
- For the Media
- Issue Archive
Recent Post
- NIH-funded exposomics coordinating center to advance precision environmental health September 23, 2024
- Damon Clark, MD, wins volunteerism award for humanitarian efforts September 23, 2024
- USC launches large-scale nationwide study of type 1 diabetes and brain development September 18, 2024
- Keck Hospital of USC named a 2024 top performer by Vizient, Inc. September 18, 2024
- USC COMPASS undergraduate program prepares juniors and seniors for stem cell careers September 17, 2024
- Exposure to air pollution during pregnancy increases postpartum depression risk September 16, 2024
- Generous gift from Jerre and Mary Joy Stead boosts neurosurgery at USC September 12, 2024
- The Keck School of Medicine of USC names 11 endowed faculty members September 11, 2024
- USC Roski Eye Institute celebrates new Glendale location September 10, 2024
- New multidisciplinary program aims to help breakers prevent and manage injuries September 10, 2024
- Study shows cells get involved in unhealthy relationships after acute kidney injury September 9, 2024
- Papadopoulos elected Fellow by Royal Society of Canada for 2024 September 6, 2024

Cure-Focused Diabetes Research
The Diabetes Research Institute houses teams of scientists, engineers, and clinicians with the expertise required to tackle diabetes from many angles. This integration of medicine and technology drives the vision behind the DRI strategy, a comprehensive, multidisciplinary approach to cure diabetes. The strategy builds upon decades of cure-focused research and addresses the major challenges that stand in the way of a biological cure.
A cure is within reach.
A cure would mean restoring natural insulin production and normalizing blood sugar levels without imposing other risks.
DRI clinical trials are already dramatically improving the lives of some people with type 1 diabetes who are now living insulin-free.
The Institute’s scientists are addressing the major research challenges that stand in the way of a biological cure. But continuing this research is only possible with your support.
News & Events
Visit the DRI Foundation Virtual Tour >
Tour the DRI’s facilities and meet our team in the metaverse! Learn how you can help us find a cure…

Inside Our Labs…

Hamptons Garden Gala…

Diabetes Research…
Get the latest news in your inbox.
Hear from our team.
Meet our team in the search for a cure. Hear the stories of the researchers, patients, and supporters who are helping drive our research forward.

Diabetes and Family…

Advancements and…

DRIF 50 Years…
Help our team find a cure.
Dri virtual tour.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 15 December 2022
FDA approves first drug to delay type 1 diabetes
- Thiago Carvalho 0
Lisbon, Portugal.
You can also search for this author in PubMed Google Scholar
Image credit: Denis Pobytov / DigitalVision Vectors / Getty
The US Food and Drug Administration (FDA) approved Provention Bio’s teplizumab on 17 November 2022 as the first preventive treatment for type 1 diabetes. A phase 2 trial of 76 high-risk people showed a doubling of time to the diagnosis of diabetes (the trial’s primary endpoint), from 24.4 months in the control group to 48.8 months in those treated with the monoclonal antibody. The most common adverse effects were transient lymphopenia and a rash that spontaneously resolved.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature Medicine 29 , 280 (2023)
doi: https://doi.org/10.1038/d41591-022-00115-y
The Clinical Pipeline is a column on translational and clinical research, from bench to bedside.
Reprints and permissions
Locum Editor, BMC Infectious Diseases
As an Editor with a background in biomedical sciences, you will handle editorial content for BMC Infectious Diseases.
London or Heidelberg– Hybrid Working Model
Springer Nature Ltd
Survey Interviewer - St. Jude LIFE Research Study
Memphis, Tennessee (US)
St. Jude Children's Research Hospital (St. Jude)
Faculty Position, Department of Hematology
Cell assay senior or lead scientist - blue sky kinases.
Memphis, Tennessee
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Diabetes Australia
Grant awarded for type 2 diabetes research

Dr Rochelle Sleaby, Professor Lena Sanci, Professor Elif Ekinci, Dr Alex Lee, and Dr Kartik Kishore are the recipients of the joint 2024 RACGP Foundation/Diabetes Australia Research Grant .
The grant is for the project “Preparing Primary Care for Precision Diabetes”, which will study how precision medicine (tailored to individual needs) can improve the management and prevention of type 2 diabetes in Australia.
The research will look at how data analytics can be used to get the best health outcomes in type 2 diabetes management in primary care.
Congratulations to Dr Sleaby and her team for focusing on how to accommodate individual needs and responses to therapy.
Research like this supports people living with diabetes to get the best care possible.
Related Articles

Groundbreaking CDE qualification for Aboriginal Health Practitioner

New Australian Diabetes Clinical Trials Network will change lives
Wegovy to be available in Australia
What state or territory do you live in?

IMAGES
VIDEO
COMMENTS
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. ... Latest Research and Reviews ...
Accurate prediction of blood glucose level (BGL) has proven to be an effective way to help in type 1 diabetes management. The choice of input, along with the fundamental choice of model structure ...
To the Editor: Most patients with new-onset type 1 diabetes have substantial intact beta-cell reserve. 1 Thus, we analyzed the efficacy of semaglutide, an agonist of glucagon-like peptide 1 (GLP-1 ...
Hummel, S. et al. Children diagnosed with presymptomatic type 1 diabetes through public health screening have milder diabetes at clinical manifestation. Diabetologia 66 , 1633-1642 (2023).
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
The promise of once-weekly insulins for type 1 diabetes: update on progress Lancet. 2024 Sep 21;404(10458):1081-1083. doi: 10.1016/S0140-6736(24)01917-2. Authors Kaitlin M Love 1 , Sue A Brown 2 Affiliations 1 Division of Endocrinology, University of Virginia ...
Some of the targets are already being studied in type 1 diabetes clinical trials of various therapies, but others could potentially be explored in future trials to prevent type 1 diabetes. This large research study with a diverse population has provided important knowledge about the complex type 1 diabetes genetics landscape, revealing new ...
Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that's when I changed my research plan. And my daughter, who's four years older than my son, became diabetic about 10 years later, when she was 14. When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs ...
A new study led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment. The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that ...
A new therapy for treating Type 1 diabetes. October 20, 2021. Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived ...
For the over 8 million people around the globe living with Type 1 diabetes, getting a host immune system to tolerate the presence of implanted insulin-secreting cells could be life-changing.. Omid Veiseh and Boram Kim. Kim is holding a medical-grade catheter similar to ones used in the study experiments. (Photo by Gustavo Raskosky/Rice University)
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [36-38]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the ...
About 1.24 million people in the United States live with type 1 diabetes; that number is expected to reach 5 million by 2050. With type 1 diabetes, it's as if the body's insulin factory has shut down.
"Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures," said Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, in a FDA press release about Lantidra. "Today's approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 ...
"For the 135,000 Australians with type 1 diabetes, this is the holy grail. This is it," he said. "There's a potential here that this research might lead to the cure of type 1 diabetes, at some ...
Type 1 diabetes mellitus (T1DM) can be predicted, and immune therapy can alter the progression of the disease. ... Research Highlight | 22 March 2021. New combination therapy shows promise for ...
In the field of Type 1 diabetes, research has largely focused on understanding the immune component, but our study argues that the β-cell is a significant player. Our findings suggest that the β ...
The new research showed that killer T-cells—a type of white blood cell that's involved in tackling bacterial infections—can cause type 1 diabetes when activated by bacteria. The researchers ...
The American Diabetes Association funds a productive research portfolio that offers significant progress and hope for improved outcomes for people with type 1 diabetes. Identifying type 1 diabetes before beta cell loss. Dr. Hessner is investigating so-called "biomarkers," which are components in blood or tissue samples that can be measured ...
Identification of a new player in type 1 diabetes risk. Type 1 diabetes is caused by an autoimmune attack of insulin-producing beta-cells. While genetics and the environment are known to play important roles, the underlying factors explaining why the immune system mistakenly recognize beta-cells as foreign is not known.
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
Liam Drew. Encapsulated stem cell-derived islets could shield β cells from the immune system. Credit: Ref. 8. Insulin has been one of the most transformative discoveries in medicine. The ...
1. Zhong W, Mao Y. Daily Insulin Dose and Cancer Risk Among Patients With Type 1 Diabetes. JAMA Oncology. 2022. 2. He M-m, Lo C-H, Wang K, et al. Immune-Mediated Diseases Associated With Cancer Risks. JAMA Oncology. 2022;8(2):209-219. 3. Gearty SV, Dündar F, Zumbo P, et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes.
A new large-scale longitudinal study, led by the Keck School of Medicine of USC, will unite 12 research centers across the United States to explore how type 1 diabetes affects children during a window of time known to be critical for healthy brain development. Ultimately, the findings could help refine clinical guidelines for managing type
The Diabetes Research Institute houses teams of scientists, engineers, and clinicians with the expertise required to tackle diabetes from many angles. This integration of medicine and technology drives the vision behind the DRI strategy, a comprehensive, multidisciplinary approach to cure diabetes. The strategy builds upon decades of cure ...
MONDAY, Sept. 23, 2024 (HealthDay News) -- Diabetes can worsen the state of your gums, but a new study suggests that diabetes medications may undo some of that damage. Researchers in Japan found a ...
Nature Medicine explores the latest translational and clinical research news, with an immunotherapy that delays the onset of type 1 diabetes in high-risk children and adults.
Incidence of type 2 diabetes is worldwide progressively increasing. It is generally assumed that this increase is due to changes in environmental rather than genetic factors. Diets and food supplies are increasingly based on ultra-processed foods (UPFs).1 UPFs are defined as industrially manufactured, ready-to-eat/heat formulations that are largely devoid of whole foods. Although UPFs form are ...
The research will look at how data analytics can be used to get the best health outcomes in type 2 diabetes management in primary care. Congratulations to Dr Sleaby and her team for focusing on how to accommodate individual needs and responses to therapy. Research like this supports people living with diabetes to get the best care possible.