
+254 709 983000/ +254 709 983676

Our History
Our policies, partnerships and collaborators, major research themes, research platforms, scientific departments.
- Policy Briefs
- Publications
- Capacity Strengthening
- Data Access

Established in 1989
Who is KEMRI Wellcome Trust?
The KEMRI Wellcome Trust Research Programme (KWTRP) is based within the KEMRI Centre for Geographic Medical Research – (Coast). Our core activities are funded by the Wellcome Trust. We conduct integrated epidemiological, social, laboratory and clinical research in parallel, with results feeding into local and international health policy. Our research platforms include state-of-the-art laboratories, a demographic surveillance system covering a quarter of a million residents, partnership with Kilifi County Hospital in health care and hospital surveillance, a clinical trials facility, a vibrant engagement programme and a dedicated training facility.
The KWTRP leadership includes the Ag. Executive Director Edwine Barasa, the KEMRI Centre Director Dr. Joseph Mwangangi, Deputy Director George Warimwe and the Chief Operating Officer Catherine Kenyatta.
- Our mission
To undertake cutting-edge and novel research relevant to national, regional and global needs.
To conduct high quality, purposeful, and relevant research in human health, building sustainable research capacity and leadership.
- To conduct research to the highest international scientific and ethical standards on the major causes of morbidity and mortality in the region in order to provide the evidence base to improve health.
- To train an internationally competitive cadre of Kenyan and African research leaders to ensure the long term development of health research in Africa

The Kenyan Medical Research Institute (KEMRI) was established through the Science and Technology (Amendment) Act of 1979, which has since been amended to Science, Technology and Innovation Act, 2013 with the mandate to carry out health science research in Kenya. The Kilifi-based Centre for Geographic Medicine Coast (CGMR-C) is among the 12 KEMRI centers.
The Wellcome Trust was established in 1936 by an endowment left by Henry Wellcome in his will. Henry Wellcome was a rich businessman and philanthropist in the UK. He also travelled in Sudan and Egypt, where he took an interest in malaria control and commissioned a floating laboratory on the Nile. The Wellcome Trust continues to support research in Africa and was one of the first research institutions to partner with the new independent Government of Kenya in 1964, creating the Wellcome Trust Research Laboratories in Nairobi. In the 1980s a few Wellcome Trust funded scientists including Stephen Oppenheimer and Bill Watkins began working in Kilifi District Hospital in collaboration with KEMRI. In 1989 the KEMRI-Wellcome Trust Research Programme (KWTRP) was formed, led by Kevin Marsh and Norbert Peshu. Due to the continued growth of research activities in Kilifi, in 1995 KEMRI granted the Kilifi Station a full Centre status as “KEMRI Centre for Geographic Medicine Research – Coast (KEMRI CGMRC).
Finding ways to beat malaria; Initial work focused on malaria, a devastating disease that was causing the deaths of many children. Working closely with colleagues in the Ministry of Health, researchers conducted studies on insecticide-treated bed nets, antimalarial drugs to prevent malaria in pregnant women and descriptions of severe malaria. Over the last 15 years there has been impressive improvements in malaria control across Africa with Kilifi registering 90 percent drop in malaria cases. Today KWTRP continues to build on its early success in bed net trials with work led from Nairobi that provides information and advice to the national malaria control Programme’s of 14 countries, supported by statistical models to generate maps of malaria risk.
In Kilifi Hospital, KWTRP facilitated the creation of a High Dependency Paediatric Unit where severely sick children had intensive monitoring and treatment. The first studies on clinical definitions of severe malaria in children were based on simple observation by the bedside. Work on severe malaria continues today with increasingly sophisticated laboratory work, DNA sequencing and retinal imaging, and clinical trials that have informed the use of anti-epilepsy drugs and fluids in severely unwell children.
As the Programme developed it was apparent that malaria was just one of a host of health problems confronting children on the coast and that often these problems were interrelated. The focus therefore expanded to include work including pneumonia, meningitis, HIV and malnutrition. Other studies included social science and health systems perspectives, as well as research on community involvement.
In early studies KWTRP engaged shopkeepers with a training programme to ensure they gave anti-malarials at the correct dose. If done well this prevents the need for hospital admissions. These successes have been built on in recent times, with KWTRP studies now showing that SMS messages can help doctors and patients to use anti-malarials correctly. KWTRP has also shown how training and support packages can help doctors and nurses in hospital to improve their patient care. These interventions result in patients getting the correct treatment and saving lives.
The cornerstone of research success is community involvement. This is done through regular interactions with opinion leaders and a network of community representatives, the Schools Engagement Programme, social media and regular open days. The programme builds mutual understanding between researchers and the community, and ensures that research is responsive to community views and interests. This research findings have done much to improve the health of the partnering community in Kilifi, and health around Africa. The work has been enhanced by modern technology which now includes online interaction with secondary schools.
Apart from conducting the highest quality research, one of the objectives is to support the development of scientific leadership in Kenya through deliberate capacity building initiatives. KWTRP has a particular focus on building links with schools in the community, which involves promoting better understanding of science and research. In addition, it has a structured internship programme for introducing Kenyan graduates to research training master’s degree and PhD students. Young researchers are supported through a system of career development to join other research institutions in the region or to develop careers with the programme.
The Kenya Medical Research Institute is a national body mandated by an Act of Parliament to provide overall leadership and guidance for health research in Kenya. The KEMRI-Wellcome Trust Research Programme is embedded within the KEMRI Centre for Geographic Medicine Research-Coast as one of the KEMRI center’s in Kenya.
Wellcome Trust
The Wellcome Trust is an independent charity funding research to improve human and animal health. Established in 1936 and with an endowment of around 13 billion, it is the UK’s largest non-governmental source of funds for biomedical research. As a privately endowed charity, it is independent from governments, from industry and from donors.
The governing document of the Wellcome Trust is its constitution. This represents an updated version of the will of Sir Henry Wellcome, through which the Wellcome Trust was established in 1936. Ultimate responsibility for all activities lies with the Board of Governors.
The Wellcome Trust has since 1989 funded the Core activities of the KEMRI|Wellcome Trust research programme. Which includes administrative support, and support of the key platforms of clinical services, community engagement, Laboratory’s and the Kilifi Health Demographic Surveillance system. This has over time ensured continuation of the research work undertaken by the Programme.
The University of Oxford is internationally renowned for the quality and diversity of its research, with over 3000 academic staff and 3000 postgraduate students working on research. The university’s position as a centre of excellence is enhanced by the ongoing development of interdisciplinary research centers, and collaboration with international academic and industrial partners. The University has a critical mass of supported researchers both local and international, who work within the Programme. This collaboration has also resulted into a well-defined research capacity building platform for researchers in Africa.
The Department of Health in the County provides overall leadership in health service delivery, and facilitates a cordial co-existence with our research centre. Medical staff from the county and periphery health facilities participates in research activities, including clinical research.
The Nairobi programme has close working relations with the Nairobi county health department working closely with the various health institutions under its mandate to facilitate the research work done. Focus is mainly on policy and improving of health care systems. This has over time been extended to include research in key populations in the city.
In Nairobi, the Programme has forged strong links with the Ministries of Health and Education, with several of the Programme researchers acting as technical advisers to the Kenyan Government departments and playing a major part in the development of national strategies.
A series of clinical trials focusing on in-patient hospital care have led to collaborations in Eastern Uganda, notably in Soroti and Mbale District Hospitals. Mbale Clinical Research Unit has been founded on the grounds of Mbale District Hospital and includes a collaboration with Busitema University.
The work of the KEMRI-Wellcome Trust Research Programme is also based on other partnerships. Key researchers hold academic positions at a number of institutions including the universities of Oxford and Warwick, the Liverpool School of Tropical Medicine, London School of Hygiene and Tropical Medicine, Imperial College, and the Institute of Child Health in London.
KWTRP Researchers are also committed to engaging with the local community, to discuss their research and to build mutual understanding and trust between researchers and the community by responding to various concerns. By developing such strong links, the Programme can ensure that its activities are accepted and approved by local communities – a prerequisite for its long-term studies. Moreover, the close juxtaposition of research and application can help ensure that its activities are focused on delivering practical benefits.
And finally, in a national and international context, the key relationships with policy makers and health officials help to ensure that important research findings make a difference to medical care – and thus have an influence far beyond the geographic areas in which the team works.

The Programme has two main bases in Kenya but works in many other parts of the country and is increasingly involved in regional collaborations to support research in neighbouring countries.
The Ugandan unit is based in Mbale town within the Mbale Regional Referral Hospital and was founded in 2008 in response to the need for evidenced based clinical guidelines.
- EAHRC Conference
- EAHRC Journals
- Your health
- Travel health
READ Health
Kenya medical research institute (kemri).
- Health Professionals
- Health Services
- Academic Programmes
- Research Programmes
- Pharmaceutical Products
- Drugs & Medications A-Z

Kenya Medical Research Institute (KEMRI) is a State Corporation established through the Science and Technology (Amendment) Act of 1979, which has since been amended to the Science, Technology and Innovation Act 2013. The 1979 Act established KEMRI as a National body responsible for carrying out health research in Kenya.
To be a leading centre of excellence in research for human health
To improve human health and quality of life through research, capacity building, innovation and service delivery
Research Capacity and Excellence
- Research in human health
- Capacity building
- Service provision
Academic programmes
Research programmes, research ethics review and regulations.
Scientific and Ethics Review Unit (SERU) located at KEMRI Headquarters
Sign up for our alerts
- West Africa
- Microdata Portal
APHRC @20 highlights
The African Population and Health Research Center (APHRC) will culminate its 20th anniversary in 2023. For the last two decades, the Center has been at the forefront of groundbreaking research, tackling Sub-Saharan Africa’s most pressing development issues: health, education, population, aging, urbanization, and well-being. Currently, the Center is implementing over 100 projects in 35 African countries involving global, regional, and national stakeholders. In addition, we engage policy-makers to inform action, influence policy decisions, and collaborate on interventions. APHRC milestones over the last 20 years are numerous and include global recognition and awards.
WHAT'S NEW?
Crossroads of empowerment and vulnerability: perspectives from the dreams (kenya) initiative.
- April 4, 2024
By Isabel Radoli
Imagine this- you are a 14-year-old girl. A middle child in a family of five. Your dad, a casual laborer, […]
Read More…
BLOGS/MEDIA HITS
Recent blog.
Imagine this- you are a 14-year-old girl. A middle child…

We are in the era of free education but majority of children from the urban poor are excluded
On going project, world health organization human reproduction program alliance research and knowledge transfer hub.
The Gates Data System. […]
DATA SCIENCE PROGRAM
POPULATION DYNAMICS AND URBANIZATION IN AFRICA
Maternal and Child Wellbeing
HEALTH AND WELLBEING
HUMAN DEVELOPMENT
Aging and Development
Urbanization and Wellbeing
Research and related capacity strengthening.
Individual Capacity Strengthening
The Individual Capacity Strengthening (ICS) unit seeks to strengthen the technical, intrapersonal, and interpersonal aspects of an individual’s capacity to undertake high-quality research or related functions that contribute to the development and implementation of Africa’s self-sufficiency in R&D.
Capacity Strengthening for Institutions
The Capacity Strengthening for Institutions (CSI) unit seeks to strengthen institutional capacities to collaboratively develop/enhance systems, structures, and processes that promote research and development in Africa.
R&D Ecosystem Strengthening
The R&D Ecosystem Strengthening unit seeks to strengthen support structures, regulatory framework, and linkages for African research institutions and researchers to become critical voices and drivers of the development agenda in Africa.
TRAINING PROGRAMS
Over the last plan period, APHRC designed and piloted a number of training programs to meet growing demand..
POLICY ENGAGEMENT AND COMMUNICATIONS
PEC’s emphasis until now has been on the Communications portfolio, providing support to research programs and..
We are broadening our knowledge management portfolio to include learning, in order to provide the time, space and..
Acknowledging that policy engagement is not a finite or binary proposition, we will promote engagement that is enduring..

HJFMRI Receives Grant from the Bill & Melinda Gates Foundation
HJFMRI was awarded $1.3M by the Bill & Melinda Gates Foundation to support cutting-edge research on COVID-19's impact on antenatal, intrapartum and postnatal care in Kenya.

Fighting Sepsis in Austere Environments
Since 2013 HJF has served as an integrating coordinator for the Austere environments Consortium for Enhanced Sepsis Outcomes (ACESO), which is an established consortium consisting of government, non-profit, university, and industry partners dedicated to improving survival for patients with sepsis.

Preparing for Infectious Disease Outbreak
For 20 years, HJFMRI has been a global leader in international medical research programs related to infectious disease. Our team leverages its existing global infrastructure to better understand, treat and prevent infectious diseases.
Growing HJFMRI Impact
Founded in 2020 in Kisumu, Kenya, the HJFMRI Western Kenya Office has been able to pursue new opportunities and attract new grants from existing partners due to increased client satisfaction and improved service delivery.
Collaborating with CAMRIS
HJF, of which HJFMRI is a subsidiary, acquired CAMRIS International LLC, in October 2020. HJFMRI closely collaborates with CAMRIS International LLC. CAMRIS has supported dozens of government programs over the past 20 years focused on medical research and public health, including many programs overseas and has capacity to quickly start up efforts in new countries within months.

In Search of a Malaria Vaccine Despite the COVID-19 Pandemic
HJFMRI is committed to supporting KEMRI/CDC efforts to create a malaria vaccine. To prevent the COVID-19 pandemic from completely disrupting the study, the HJFMRI team developed innovative and efficient methods to support the clinical trial by ensuring day to day running of the study.

Mobile Mortuary
The HJFMRI team in Nairobi, Kenya successfully coordinated stakeholders and teammates alike in designing, procuring and equipping a mobile mortuary that is a first of the kind in the country and most likely the only one in the region.

First Lab in Kenya Accredited by the College of American Pathologists
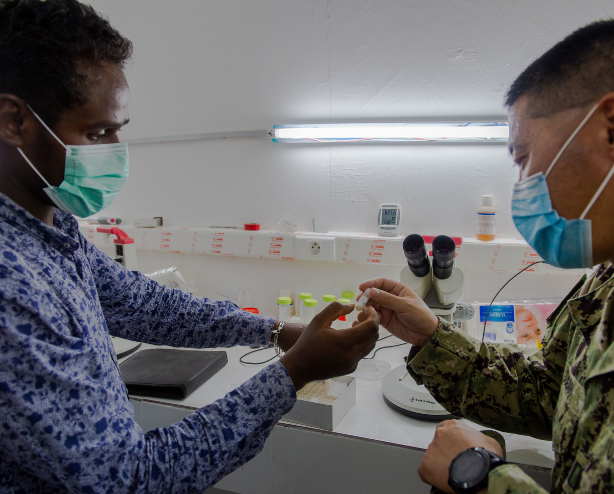
The Kericho Clinical Research Center’s lab, staffed jointly by HJFMRI and the Kenya Medical Research Institute (KEMRI), was the first in Kenya to be accredited by the College of American Pathologists.

Siaya Morgue Renovation
In April 2021 the HJFMRI team provided technical guidance on the renovation of the Siaya County Referral Hospital. The HJFMRI team supervised the entire project to completion, and now that the facility is completed, MITS procedures are being conducted with greater ease and efficiency.

Building Sustainable Clinical Research Infrastructure
HJFMRI LTD GTE helped stand up a new Clinical Research Center in Abuja, Nigeria with the Walter Reed Army Institute of Research (WRAIR) to conduct clinical trials to combat infectious diseases. The CRC in Abuja conducts HIV studies and has pivoted to combat emerging health threats such as Ebola, Lassa fever and COVID-19.

Preventing HIV and Transforming Lives
Since 2003, HJFMRI has successfully supported the U.S. President’s Emergency Plan for AIDS Relief ( PEPFAR ). Working with the U.S. Military HIV Research Program at WRAIR, the DoD and local partners in Africa, HJF has helped develop and implement comprehensive HIV prevention, care and treatment programs in Kenya, Nigeria, Tanzania and Uganda. These programs prevent HIV, save lives and strengthen global health security.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- East Afr Health Res J
- v.3(1); 2019

Research Dissemination Strategies Used by Kenya Medical Research Institute Scientists
James n kariuki.
a Centre for Public Health Research, Kenya Medical Research Institute, Nairobi, Kenya
Joysline Kaburi
b Centre for Biotechnology Research and Development, Kenya Medical Research Institute, Nairobi, Kenya
Rosemary Musuva
c Centre for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya
Doris W Njomo
d Eastern and Southern African Centre for International Parasite Control, Kenya Medical Research Institute, Nairobi, Kenya
Doris Night
e Marketing Department, Kenya Medical Research Institute, Nairobi, Kenya
Carolyne Wandera
James wodera.
f Corporate Affairs Department, Kenya Medical Research Institute, Nairobi, Kenya
Pauline N Mwinzi
Background:.
Dissemination of research findings is acknowledged as an important component of any research process. Implementation of research findings into practice or policy is necessary for improving outcomes in the targeted community. Given the context and dynamic environment in which researchers operate, there is need to find out existing gaps in terms of disseminating research findings to key stakeholders. The objective of this study was to investigate the health research dissemination strategies used by Kenya Medical Research Institute (KEMRI) researchers.
This was a mixed-method study employing concurrent sequence (use of both qualitative and quantitative) methods of data collection. The study was conducted in KEMRI's 10 centres spread in 3 geographical areas: Kisumu, Kilifi, and Nairobi counties. Potential respondents were identified through purposive sampling. Three inter-related data collection methods were employed in this study. These methods included key informant interviews with: (a) MoH officials from county government; (b) KEMRI researchers; and (c) key KEMRI departments, namely Corporate Affairs and the library. Additionally, secondary sources of information, such as scientific reports, KEMRI annual reports, and financial statements, were also reviewed.
Publication of papers in peer-reviewed journals was mentioned as the most common method of dissemination of research findings. Scientists published in 353 peer-reviewed journals (or publishing houses) between the years 2002 and 2015. Over 92.7% of these publications were in international peer-reviewed journals. Conferences and workshops were also mentioned. In the absence of a centralised electronic KEMRI publication database, the research team extracted and collated a publication lists from KEMRI annual reports and financial statements. This was limiting since it did not have an exhaustive list of all publications by KEMRI scientists. Only 3 respondents mentioned having written policy briefs or engaged the media as part of dissemination channels. The media representatives cited the use of social media (Facebook and Twitter) as another channel that KEMRI scientists could exploit. Challenges in dissemination included lack of knowledge on research translation leading to poor synthesis of research outputs as well as selective reporting by the media.
Conclusion:
Publications in peer-reviewed journals was the most preferred channel of communicating scientific outputs. Conferences and writing of policy briefs were the other sources of dissemination. We recommend that KEMRI dissemination channels should go well beyond simply making research available through the traditional vehicles of journal publications and scientific conference presentations but establish institutional mechanism which would facilitate extracting the main messages or key implications derived from research results and communicating them to stakeholders in attractive ways that would encourage them to factor the research implications into their work.
INTRODUCTION
Dissemination is acknowledged as an important component of the research process. The dissemination and implementation of research findings into practice is necessary so as to apply research findings to improve outcomes in the broader community. 1 Innovative models to facilitate more rapid uptake of research findings into practice are urgently needed. 2 Previous studies indicate that a number of research findings which if implemented would have significantly improve health or behavioural outcomes, failed to be translated into meaningful public health interventions across multiple contexts. 3 , 4 Barriers to dissemination and implementation may occur at multiple levels of research and health-care delivery namely at the researcher level, patient level, organisational level, or the market/policy level. 5
Moving the field of scientific dissemination forward will require studies that identify mechanisms and approaches to package and convey the evidence-based information necessary to improve public health and clinical care services in ways relevant to local settings and that balance fidelity and adaptation. 3 Nonetheless, the communication of research findings in a rural sub-Saharan African setting is less straightforward and presents significant challenges with respect to literacy, language, logistics, and confidentiality. In recent years, the Internet and television have revolutionised dissemination as well as introduced new sets of challenges. 6 There is need to find out what the challenges are in disseminating research findings, from researchers, Ministry of Health (MoH) officials and the media, who are key stakeholders in this process.
Interventions developed in the context of efficacy and effectiveness trials are rarely transferable without adaptations to specific settings and additional tools and guidance to support uptake and implementation. Therefore, research is needed to examine the process of transferring interventions into local settings, which may be similar to but also somewhat different from the ones in which the concepts were developed and tested. The most prevalent strategy for dissemination has been to target scientists to increase their dissemination efforts. A combination of education, incentives, and admonishments are required to encourage health scientists who develop and test incentives to also find innovative ways to disseminate results. This approach has however been criticised as being misguided on the basis that asking scientists to be central players in dissemination when they lack the necessary training and usually operate in organisational environments that lack the infrastructure and reward structure to motivate and support systematic dissemination, is unrealistic. 6
As a necessary prerequisite for unpacking how information which can lead to intervention or service changes, we need to understand how and why information on physical and behavioural health, preventive services, disease management, decision making, and other interventions may or may not reach various stakeholders, or why they might not be able to utilise it when it reaches them. We need to understand what underlies the creation, transmission, and reception of information on evidence-based pharmacological, behavioural, genomic, policy and systems interventions. 6 Successful dissemination of health information (including evidence about underutilised interventions) may occur quite differently depending on whether the audience consists of consumers, caregivers, practitioners, policymakers, employers, administrators, or other multiple stakeholder groups. 7 Unless health research findings are communicated effectively, there will be a little chance of those changes happening. 8 The question arises as to how those findings should be disseminated to them in a suitable format when they become relevant. By practice, it is known that researchers at the institute publish their finds in various journals, but to the best of our knowledge, this has not been documented. The objective of this study was, therefore, to investigate and document dissemination strategies used by Kenya Medical Research Institute (KEMRI) scientists and their effectiveness to stakeholders.
This study was conducted in KEMRI's 10 centres located in Nairobi, Coast and Western Kenya. The institute is a state corporation established by an Act of Parliament as the national body responsible for carrying out research for human health in Kenya. The majority of these centres are located in Nairobi County, and they include the Centre for Biotechnology Research and Development (CBRD), Centre for Clinical Research (CCR), Centre for Microbiology Research (CMR), Centre for Public Health Research (CPHR), Centre for Traditional Medicines and Drug Research (CTDMR), Centre for Virus Research (CVR), Centre for Respiratory Diseases Research (CRDR), and Eastern Southern Africa Centre for International Parasitic Control (ESACIPAC). Other centres outside Nairobi include: Centre for Global Health Research (CGHR) in Kisumu County, Centre for Geographic Medicine Research – Coast (CGMR–C) in Kilifi County and Centre for Infectious and Parasitic Diseases Control Research (CIPDCR) in Busia County. MoH programme managers were targeted in the 4 counties where the KEMRI Centre are located. Journalists from media houses in Nairobi were purposefully selected for inclusion into the study.
Study Design
This was a mixed methods study employing a concurrent sequential method of data collection. That is, it involved the collection of qualitative and quantitative data simultaneously. A semistructured questionnaire and interview guide were the 2 tools that were used to collecte quantitative and qualitative data, respectively.
Study Population
The total number of research staff as at the time of conducting the survey were 250 scientists who have diverse qualifications and skills in matters of health. In addition, there are over 300 technologists and technicians who provide research support to the scientific community. All the research scientists were eligible for consideration to participate in the study.
The study established that at the time of undertaking the survey, a number of research officers were either out in the field collecting data or were officially on leave. Thus, all the researchers who were found at their respective workstations were interviewed. No sampling of respondents was necessary. Potential respondents of the in-depth interviews were identified through purposive sampling. Researchers were identified on the basis of i) Principal investigators with more than 1 study concluded, ii) Scientists from the same centre working on different disease profiles to give diversity on thematic areas of interest iii) Scientists who have been in KEMRI for more than 7 years to give depth on issues of dissemination iv) Scientists who provided informed verbal consent. Also included in the interview list, were respondents from KEMRI's Corporate Affairs Department and the Library. Additional interviews were also carried out with health journalists from established media houses, as well as MoH representatives/heads of policy development departments at the county government levels to assess their uptake of health research findings from KEMRI researchers.
Data Collection Methods
The following 3 data collection methods were employed in this study. In-depth interviews with MoH officials from the county government as well as key KEMRI departments (Corporate Affairs and the library). Review of secondary sources of information such as scientific reports and KEMRI annual reports and financial statements. In-depth interviews with KEMRI researchers
In-depth Interviews with MoH Officials
An interview guide containing questions addressing the broad areas of the baseline study was formulated. These themes included 1) policy changes implemented in the last 10 years; 2) what influenced policy change; 3) interaction with KEMRI; (4) views on how interactive with KEMRI could be improved; 5) What research from KEMRI had benefited their work or any interventions they had implemented. Sociodemographic information of respondents was also collected. A total of 3 KIIs were carried out and were conducted in English.
Review of Secondary Sources of Information
In the absence of a centralised electronic KEMRI publication database, the research team extracted and collated a publication list from previous KEMRI annual and financial statements reports from the year 2002 to 2016. These annual and financial reports contained a list of publications by staff as part of the annexure section for each year under review and thus provided an objective and verifiable source document. However, it was found to be limiting since it did not have an exhaustive list of all publications by KEMRI scientists and in some instances had duplication of publications by the same authors. To examine the preferred journal and content of KEMRI publications, a content analysis was performed on papers published. A content analysis provided a means for objective, systematic, and quantitative consideration of published articles. It also allowed for an interpretation of the direction in which KEMRI scientists are taking in terms of priorities of publications. Two reviewers examined the list of publications and coded them into pre-determined themes. A third reviewer was contacted whenever there was a disagreement.
Interviews with KEMRI Researchers, KEMRI Corporate Department, and Library
Key informant interviews (KIIs) were carried out targetting specific departments. The checklist consisted of questions relating to dissemination practices, preferences, and future demand for KEMRI research outputs. This survey targeted to conduct at least 5 KIIs per group, with an option of conducting more until a point of response saturation was attained. The main focus in these guides included methods used for data sharing; challenges in dissemination of research findings; interaction with the media and MoH; how that interaction can be improved; factors that have influenced research use in policy making; factors that have hindered research use in policy making; health issues popularly published; interaction with KEMRI researchers; research packaging by KEMRI scientists.
Data Management and Analysis
Qualitative data were transcribed verbatim. The team of qualitative researchers first familiarised themselves with the transcripts, after which independent coding was done and the codes collectively finalised for each tool. In case of a disagreement on a theme, a third party was called to break the deadlock. The conceptual model for considering diffusion, dissemination and implementation of innovations in health service delivery 9 was used to tease out categorisation of the data collected.
Ethical Approval
Before the commencement of the survey, scientific and ethical approval was sought and received from the national Scientific and Ethical Review Unit (SERU), based at KEMRI. In addition, approval was sought from the directors of each of the 10 centres that constitute KEMRI. During the interview process, informed consent was obtained from the study participants. Additional consent was sought when interviews were to be tape-recorded. Permission to publish this manuscript was also received from the KEMRI Director's Office.
Data Limitation
At the time of conducting this survey, a number of scientists were out of their workstation on official duties. Repeated visits to the stations did not yield much in terms of interviewing more staff members. This was a limitation, especially when compiling the findings. In addition, KEMRI did not have a centralised electronic publication database which would facilitate data mining. The researchers were referred to online journals so as to compile an institutional list of publications. This resulted in duplication of effort. Furthermore, scattered database and profiles were located in different Centre and departments. The Monitoring and Evaluation (M&E) Department of KEMRI had a more organised but not exhaustive list of staff publications. The list of publications from this department formed the basis of secondary desktop review as it was conveniently located.
Quantitative Findings
A total of 37 KEMRI scientists were interviewed during the survey. Their sociodemographic profiles are shown in Table 1 .
Sociodemographic Characteristics (N=37)
Abbreviation: CI, confidence interval
Publications in Peer-Reviewed Journals
A total of 1,639 publications were published by KEMRI researchers between the period 2002 to 2016. During the period under review, KEMRI's scientists published in 353 peer-reviewed journals of which 92.7% were published in international journals. The East African Medical Journal was the only journal from a developing country listed among the top 10 preferred journals, accounting for 7.3% of KEMRI publications. Among the top 10 journals preferred by KEMRI researchers, the PLoS series of journals accounted for 18.7%, Malaria Journal (13.7%), while the American Journal of Tropical Medicine and Hygiene accounted for 12.8%, as shown in Table 2 .
The Top 10 Peer-Reviewed Journals in Terms of KEMRI Publications Between 2002 and 2015
All the respondents (n=37) reported they also attend and present the findings of their research outputs at international conferences. The choice of which conference to attend and funding depends on researchers preferences and the availability of additional funds. The most commonly mentioned conference include American Society of Tropical Medicine and Hygiene (ASTMH) Annual Conferences (48.7%) as well as the annual Pan Africa Mosquito Control Association (PAMCA) conference (27.0%). Table 3 profiles the most commonly attended conferences as reported by the respondents.
Most Commonly Mentioned Conferences and Seminars Attended by KEMRI Staff to Disseminate Research Findings (N=37)
Qualitative Findings
Publication of papers in peer-reviewed journals was the frequently mentioned method of dissemination of KEMRI research findings. Other dissemination channels included presentations at conferences, seminars, workshops and generation of reports to KEMRI and research clients. Only a few participants mentioned having written policy briefs or engaged the media.
Male researcher, Kilifi Centre: “ Scientists don't have training in writing media and policy briefs. That could be 1 reason why we don't use those methods… I think there are many levels of approval before one can use the media. That is discouraging, so mainly we will just publish in journals ”.
Respondents reported that they were not motivated to publish. The numbers of publications had no influence on job promotions or assignment of responsibilities.
Female researcher, CPHR: “… honestly can't say that as a KEMRI scientist I am motivated to publish. We do it because it's part of the job. The promotions are not even based on that. You will see someone with 1 publication getting promoted and another with 10 getting stuck... We get more recognition outside than right here ”.
Respondents involved in the IDIs expressed their frustrations with journals citing long turnaround periods, which sometimes render data obsolete. Other issues of concern included a lack of knowledge about research translation leading to poor synthesis, limited funding to attend conferences, and selective reporting by media.
Male researcher, CMR: “… sometimes I think we people in science talk to ourselves and I think it is important for us to learn to simplify our language and our findings so that you know it is usable to the other people ”. Male researcher, C-GHRC: “ I think there is a lot of bias. Coverage will be given to Zika virus, Ebola or when there is an outbreak of a disease… So you will find others equally detrimental to health are left out ”.
The majority of researchers pointed out that there is a disconnect between the KEMRI departments responsible for research dissemination and the centres, which further aggravates the lack of research being taken up as policy or practice.
Female researcher, CRDR: “ There is a department… which is supposed to link us to the media or people out there. They are the ones to take up the issue. Now that department has not been doing that. I have not heard ”. Male researcher, CBRD: “ Now if someone is working in that department and they don't come around they don't find the interesting finding that is 1 reason why it has not worked… there is a disconnect ”. Male researcher, CTDMR: “ … there is a particular department… like now in marketing. You should take [up] the challenge because during eeh… events that is where you should engage the KEMRI scientist to come and maybe speak or talk about what they are doing. KEMRI is a research institution, so why are you not engaging the scientist in every one of those activities? ”
Policy and Practice Changes Impacted by Research Done at KEMRI
In-depth interviews with KEMRI scientists revealed that most of their research had influenced changes in policy and practice in the country. It was interesting to note that this view was not necessarily acknowledged by the MoH officials. The MoH did not attribute any changes in policy and practice with research done at KEMRI. This could partly be attributed to the frequent reshuffling of officers in the various ministries as well as limited access to published material, as mentioned by the respondents.
Male researcher, CCR: “ I can't boast about it as my work, but together with others it has contributed like change of policy from chloroquine to SP, from SP to ACTs and right now we are working on the issue of correcting ACTs into schistosomiasis. It's still on an early stage, but I believe that there are discussions on very high levels… even the transfusion guidelines in Kenya. The studies that we did in Siaya yeah have contributed into those guidelines because initially, it was like if you have haemoglobin of 5, but our studies showed transfuse the patient and not the lab result. Yeah (Laughs) ” Male researcher, CVR: “ For example, look at the HIV testing among infants that started as a research thing around here initially around 2006 all the way to 2008. Do you know that programme was taken up by the ministry, and now it is a national programme that's how the infants are being tested for HIV all over the country? That's a clear area that showed that research showed that this can work because that is molecular testing ”.
Barriers to KEMRI Research use by Decision Makers
Majority of the MoH officials and media journalists mentioned poor synthesis of research as a major factor contributing to research not being taken up as policy or practice. The scientific language limits the audience to fellow researchers who may not necessarily have a say in policy direction, thus the gap.
Female Journalist, 31 years:” well eeh.. scientist you usually communicate in a very technical language, and journalist eeh communicate in a very simple easy to understand language everybody can understand so aaaah I know the scientist eeh… communicate in that kind of language because of the nature of their work. It will be good for them if they communicate in a language that eeh it is eeh friendly to the journalist and… for to the public ”. Male researcher, EASCIPAC: “… I think it is a problem everywhere. Scientists conduct their research all the time, everywhere in the whole world, but… translating this item into policy findings… there is a disconnect between the researchers and the policy makers. Sometimes even the policy makers do not understand your language so I know in certain institutions they form partnerships with private companies to uptake the data coming from their scientists and convert them into a product that is sellable so that way we are not saying we gave our data to the Ministry of Health… and they did not act on it so KEMRI in itself through this private companies can actually make a product out of it …” Female respondent, 33 years, MoH: “ You know, we don't have access to Internet here… people don't know where to find those journals… Even the reports that people bring here are collecting dust. But if you come to the office, call the officers concerned and share your results, then I think that's the best way to proceed. People can ask questions, and everyone is satisfied and understands what it is about ”.
Other impediments mentioned included the choice of dissemination method, financial implications involved in implementing policy changes, donor-driven research that does not address local needs, priorities of media house and policy makers, delays in ethical clearance from KEMRI and ‘media phobia’ from scientists.
Female Journalist, 31 years: “ I don't know whether the scientists have been sensitised about how to deal with the journalist or they do not know how the journalist profession works… so they are quite hesitant when it comes to providing this research information that the scientist has undertaken. So much valuable information is not out there because scientists are afraid to talk to journalists. We need to work together ”. Female researcher, CCR: “… for donor-driven research, it mostly starts as a collaboration, but later, they want to bully and boss you out, even overtake you as the local researcher and run the show. Now, in the end, your objective becomes a small component of the study. So when you want to sell the idea, no one buys it… Because what does it address anyway? ” Male researcher, CBRD: “ We do not have experience or training in writing policy briefs or media briefs so in the end, who are we targeting? We will publish in peer-reviewed journals, but not everybody has access to that. Not everyone is going online to look. So there is a gap; there are important people not accessing this data. How will it even inform policy then? ”
Suggested Way Forward by Researchers
Researchers mentioned the need to have systems put in place in KEMRI that ensures dissemination of research results. Another key factor mentioned was that researchers need more training on re-packaging of findings to improve chances of research products and outcomes being taken up as policy or practice. Other factors mentioned included functional links between the KEMRI researchers and the corporate department; advocacy for KEMRI research findings to partners and stakeholders; having in place a digital repository in the library; and use of social media.
Male respondent, 45 years, MoH: “ There was a time representatives from KEMRI used to attend our meetings, and it worked well because we were informed of what the scientists are doing. That was a while back. KEMRI now has no visibility here ”. Male researcher, CPHR: “ You see research and policy are somehow detached, especially where institutions don't work like together they are working as separate entities. So for us, I think one of the things is to become proactive in all the areas like doing a lot of lobbying …” Female Librarian: “… we should be able to reflect on what KEMRI does and what KEMRI has been doing for the past, and it would just be nice if someone can access from wherever. We need a digital institutional repository which will work hand in hand with the digital library. I believe, if implemented, it will create a good working information library system that will now uplift our digital level on the electronic part… once we start working with departments, we will be able to get information from centres and the researchers. The repository will bring this together …”
This study provides insights into strategies used by KEMRI researchers and barriers that hinder the dissemination of research findings. The insights are summarised as follows.
Dissemination Channels
This survey established that KEMRI scientists' most preferred avenue of dissemination is through publication in peer-reviewed journals. For researchers, the assessment of productivity and contribution to science is highly pegged by quantifiable means such as publications. Given that the success of a scientific paper partly depends on its outcome, researchers tend to publish their findings in high impact peer-reviewed journals 10 , 11 as well as in open access options 12 that provide the likelihood of it being cited by other authors. By extension, publications that appeared in high-end peer-reviewed journals were associated with knowledge prowess on a particular subject or discipline. Apart from contributing to the knowledge base, publications also inform tenure and future funding directions. 13
Best Practices in Dissemination of Research Findings
Only 3 (8.1%) scientists reported that they had exposure to media engagement (television and radio shows). From the findings, the publication of research findings in local print and electronic media was limited. Use of social media was cited as another channel that is becoming popular with KEMRI scientists. This survey did not establish the impact of the use of social media on the dissemination or advertisement of research findings.
Uptake of Health Research Findings
This study established that there was a disconnect between researchers' work contributing to national policy formulation and inputs into decision making processes. Scientists pointed out circumstances in which their outputs were used to inform policy and practice. However, the potential consumers of KEMRI's research findings, namely the policy makers and journalists reported that they did not share this view. Synthesis of research into policy/practice by government bodies, organisations and other stakeholders is gravely undermined by the different levels of research awareness and experiences within these teams. 14
Barriers to research dissemination and implementation may occur at multiple levels, namely individual researcher level, organisational, and at market/policy level. 15 These barriers are discussed in the subsequent paragraphs.
At Individual Level
The instructions to authors usually guide the scientific language to be used and how the information is packaged. 16 Many researchers have limited exposer to media. Only a handful of scientists have had previous training in writing and handling media. A strategy is required to overcome ‘ media phobia ’ by scientists. Potential users of research outputs face challenges of synthesising research articles arising from various KEMRI publications. This is consistent with studies conducted elsewhere. 15 , 17 This problem is partially aggravated by the high impact journals which have structured guidelines that emphasis on form-over-substance.
Organisational Level
Prior to a change in policy directive, all publications and related outputs had to seek ethical approval from the Office of Director KEMRI. This resulted in publication delays and a backlog of manuscripts, as researchers sort additional authority-to-publish from the institute. By the time of undertaking this survey, there was a policy directive that manuscripts should be cleared for publication by the centre scientific committees. This was aimed at reducing pile-ups of manuscripts and the time lags that it takes to publish them. One of the participants mentioned that a number of studies carried out in KEMRI are funded by external donors, hence by extension, they partially determine the type of research to be conducted as well as where the findings will be published. Empirical studies have augmented that local utilisation of research outputs will occur once research can address local needs. 18 , 19 This can only be realised if the national and county governments prioritise their research needs and source for funding for the same.
Market and Policy Level
The current survey established that priority changes by policy makers and preferences to certain health stories also contributed to the “slow” uptake of KEMRI researchers. Usually, these changes and preferences are not communicated to researchers. This gap probably explained why many KEMRI publications are not used to inform policy and practice.

RECOMMENDATIONS
From this study, the following are the recommendations:-
- Establish knowledge management and knowledge translation mechanisms at the institute to facilitate the collation, synthesis, packaging, and communication of research findings to decision makers and members of the public.
- Encourage extensive use of social, online, and print media. This will offer a convenient way of accessing evidence anywhere at any given anytime. These platforms will also offer the chance of a back-and-forth engagement and not just passive dissemination.
- Continue building on existing dissemination structures and processes which can help the uptake of research outputs. These include the annual KEMRI Annual Scientific Health (KASH) conferences and use of in-house bulletins such as the Bulletin and the Researcher. These will act as aids towards influencing decision making processes, especially when policy makers and implementers require evidence within the shortest period possible.
Dissemination strategies at KEMRI should go well beyond making research available through the traditional vehicles of journal publications and scientific conference presentations. This survey established that there are a number of publications generated for local context were of high quality (methodology). Thus, we postulate that it is not the absence of information, but lack of an institutional mechanism which would facilitate extracting the main messages or key implications derived from research results. The re-packaged or synthesised research publications would possibly be communicated effectively to targeted groups of decision makers and other stakeholders using innovative ways as this would encourage them to factor the research outputs into policy formulation as well as guide practice.
Acknowledgements:
The authors would like to thank all the study participants drawn from ministries of health in the various counties, journalists, and KEMRI scientific staff. In addition, we thank the Director, KEMRI, for the funding support and the permission to publish this paper. This study received funds from the KEMRI / IRG /GoK funds during the financial year 2016/2017.
Peer Reviewed
Competing Interests : None declared.

Global Reach
For more 50 than years, the U.S. military has maintained a substantial program for infectious disease research in Kenya. These collaborations have expanded to eight countries under the U.S. Army Medical Research Directorate–Africa (USAMRD-A), which is headquartered on the campus of the Kenya Medical Research Institute (KEMRI) in Nairobi.
WRAIR helped establish the KEMRI-Kericho Clinical Research Centre (CRC) 1999 in collaboration with KEMRI and HJF, and a new CRC opened in 2012 where more than 50 clinical studies have been completed. The Kericho CRC has conducted randomized, double-blind, placebo-controlled and IND/FDA-regulated studies from Phase I to IV.
- Vaccines against HIV, Ebola and polio; therapeutics against HIV, diarrhea, tuberculosis and opportunistic diseases/ malignancies
- Chosen by several Shigella vaccine manufacturers to conduct three large scale multivalent Shigella vaccine trials
- NIH Networks – Part of National Institutes of Health Division of AIDS/AIDS Clinical Trials Group (ACTG) and IMPAACT, the maternal/child HIV treatment and prevention research network funded by the National Institute of Child Health and Human Development

Medical Research & Programs
- Promote, facilitate and coordinate research and programs
- Build capacity of staff to conduct research
- Spearhead conduct of clinical audits to identify service gaps and areas of improvement
- Provide administrative and fiscal management support for research studies funded internally and externally
- Facilitate dissemination of research findings

- May be self-delivered
- Have health benefits for men
Emergency cases
Please make a call to our help desk.
CCI - Cancer Care International Ground floor, Jadala Place, Ngong Lane Off Ngong Road, Nairobi , Kenya
The Future of Cancer
Where we are going.
To transform the cancer care environment by bringing core clinical services to one central place.
GET WELL SOON
Cci services, oncology consultation, radiotherapy consultation, molecular diagnosis, cancer screening, injections and dressing.

If your primary care physician suspects cancer after diagnosing your symptoms,
it is likely that you’ll be referred to a medical oncologist (cancer specialist) for further diagnosis and treatment. At this point, it’s important to understand that the process of correctly diagnosing the type of cancer, how far it has spread throughout your body and the best possible treatment options will require the services of more than one cancer specialist.
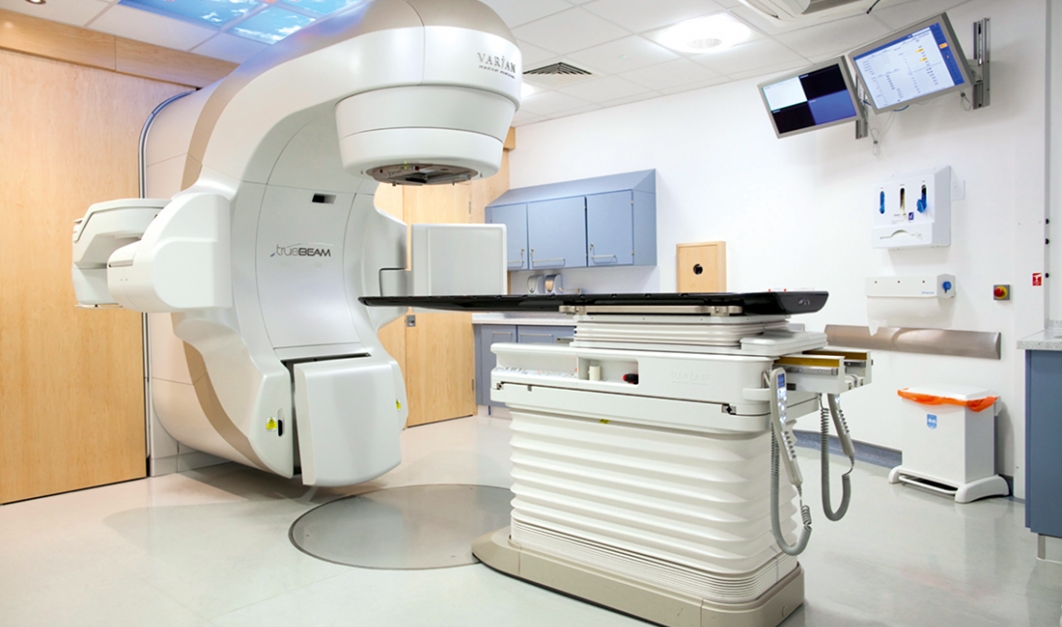
A radiotherapy consultation is the first step in seeking radiation therapy.
This is provided by a radiation oncologist who is part of the patient’s medical care team. Since this is the first visit the patient will make to a particular doctor, no radiotherapy treatment will be performed. Radiotherapy is one of the treatment options available to people who are suffering from cancer. Even in cases where the cancer is incurable, this procedure is still effective in controlling the symptoms that patients feel as a result of their condition. The treatment aims to destroy cancer cells using high-energy radiation that causes them to sink.

There are multiple applications of molecular tests in clinical oncology.
Molecular diagnostics are increasingly used to guide patient management, from diagnosis to treatment, particularly in the fields of cancer.
Deciding to participate in a cancer screening is a personal health decision that should be based on a conversation between you and your doctor.
The risk of developing many types of cancer can be reduced by practicing healthy lifestyle habits, such as eating a healthy diet, getting regular exercise, and not smoking. Also, the sooner a cancer is found and treatment begins, the better the chances are that the treatment will be successful.

Simple Dressing Change
The health care provider chooses the appropriate sterile technique and necessary supplies based on the clinical condition of the patient, the cause of the wound, the type of dressing procedure, the goal of care, and agency policy.

What to expect from counselling
At your appointment, you'll be encouraged to talk about your feelings and emotions with a trained therapist, who'll listen and support you without judging or criticising. The therapist can help you gain a better understanding of your feelings and thought processes, and find your own solutions to problems.
CCI Capabilities and Infrastructure
- Day Care Chemotherapy
- Medical Oncology
- Education and Counseling

The CCI is an exclusive center for day care chemotherapy.
The center has been using the day care concept for administration of single and multi-drug chemotherapy since day one. Daycare chemotherapy has the advantage of avoiding disruption in the day-to-day life of cancer patients and their families, while minimizing treatment costs.
Being amongst the main clinics of one of the leading medical oncologist in the country; Dr. Gladwell Kiarie,
CCI intends to offer top notch medical oncology works towards preventing, diagnosing and treating cancer within the latest guidelines and customized multimodality therapies. The team works closely with the radiation oncology department to offer a combined chemo-radiation approach to a wide variety of cancers.
patients and their family members about the line of treatment planned, the risks, benefits and side effects of their treatment course. We also educate them in advance, on how to cope with the side effects and understand the warning signs which need to be brought to the attention of the doctor.
We have a pharmacy to provide medicines without any delay to increase the comfort of a patient's stay in the center.
The CCI has an excellent team of qualified oncologists, onco-nursing and support staff to manage the center effectively. Every cancer has different angles of approach and every patient needs personalized treatment. We believe in providing a treatment that fits the needs of every patient. Our center is recognized by several corporate and other companies for the treatment of their beneficiaries.
our doctors
Experienced team.

Dr. Gladwell Kiarie

Dr. Gunjan Baijal

Dr. Sridhar P.S.
Mbchb, mmed.int med. (uon), medical oncology (london), frcp (edin), consultant medical oncologist.
Current Chair division of medicine nairobi hospital and member of medical advisory committee Former secretary KESHO Won UICC grant for screening in poor resource settings 2014 Passionate about breast and cervical cancer screening for early diagnosis. Taught in the department of medicine and therapeutics UON as senior lecturer up to 2018.
Dr. Gladwell Wanjiru Gichuru Kiarie completed her Bachelor of Medicine and Surgery Degree (MBChB) from University of Nairobi in the year 1999. She then completed her Post Graduation in Internal Medicine at University of Nairobi in 2005. She attained her Doctors Limited Registration in UK. In February 2007 Dr. Gladwell Wanjiru Gichuru Kiarie got registered as a Consultant Physician with the Kenya Medical Practitioners and Dentists Board and in April 2008 she was registered as a Consultant Medical Oncologist with the Kenya Medical Practitioners and Dentists Board. Dr. Gladwell Wanjiru Gichuru Kiarie has also been in the forefront when it comes to papers presented for publications. She has also presented a number of papers in conferences, seminars and workshops. She is also a part a number of ongoing academic and research activities and papers. Dr. Gladwell Wanjiru Gichuru Kiarie is also a part of number of medical organisations. She is a member of AFYA RESOURCE ASSOCIATES. She is also a member of Kenya Society of Hematology-Oncology, Kenya Medical Association, member of Kenya Association of Women Doctors, American Society of Clinical Oncologists as well as European Society of Medical Oncologists. She is also a technical Advisor at Kenya Cancer Organisation.

- +254-719581035
- intlpatients@apolloinformationcentre.com
- intlpatients@apolloinformationcentre.com

Top Cancer Centres and Hospitals in Nairobi for Cancer Treatment
March 6, 2024 | Like | Leave a comment | 2024-03-06 06 March 2024
If you or a loved one is facing a cancer diagnosis, the choice of where to receive treatment is a critical decision. Nairobi, the capital city of Kenya, is home to several top Cancer Centres and Hospitals in Nairobi that offer top-notch care and cutting-edge treatments. In this article, we’ll delve into the top cancer centers in Nairobi, with a special focus on the Apollo Hospitals Information Centre.
Background and Reputation

Apollo Hospitals Information Centre has established itself as a leader in healthcare globally, and its Nairobi branch is no exception. With a rich legacy of delivering quality medical care, Apollo Hospitals has become synonymous with excellence. The Nairobi center, in particular, is renowned for its state-of-the-art infrastructure and world-class medical expertise.
Services and Specialties Offered
Apollo Hospitals Information Centre provides a comprehensive range of services for cancer patients. From diagnostics to treatment and ongoing support, they prioritize a holistic approach to cancer care. The center specializes in various cancer types, ensuring that patients receive specialized and personalized treatment plans tailored to their unique needs.
Patient-Centric Approach
What sets Apollo Hospitals apart is its unwavering commitment to putting patients first. The compassionate and patient-centric approach ensures that individuals battling cancer not only receive the best medical care but also experience a supportive and empathetic environment. The center’s staff goes the extra mile to make the journey through cancer treatment as comfortable and stress-free as possible.
Top Cancer Centers in Nairobi: A Comprehensive Overview
Advanced Technology in Cancer Treatment
The landscape of cancer treatment is rapidly evolving, thanks to advancements in technology. Nairobi’s top cancer centers are at the forefront of incorporating state-of-the-art diagnostic tools and innovative treatment methods. These technological interventions not only aid in early detection but also enhance the effectiveness of treatment, improving outcomes for patients.
Personalized and Patient-Centric Care
In the realm of cancer treatment, a one-size-fits-all approach falls short. Recognizing this, the top cancer centers in Nairobi prioritize personalized treatment plans. These plans consider the unique characteristics of each patient, ensuring that the chosen treatment aligns with their specific needs and circumstances.
Patient-centric care goes beyond medical interventions; it encompasses emotional and psychological support. The journey through cancer is daunting, and the top cancer centers in Nairobi understand the importance of addressing the holistic well-being of patients.
Community Outreach Programs: Spreading Awareness and Support
Cancer awareness is a crucial aspect of the battle against this formidable disease. The top cancer centers in Nairobi actively engage in community outreach programs, conducting free screening camps, workshops, and educational initiatives. By spreading awareness and providing early detection opportunities, these programs play a pivotal role in the fight against cancer.
The Cost of Cancer Treatment: Navigating Financial Challenges
While receiving quality cancer care is paramount, the financial aspect cannot be overlooked. Cancer treatment can be expensive, and the top cancer centers in Nairobi acknowledge this challenge. Many centers offer financial assistance programs, ensuring that patients have access to the care they need without being burdened by exorbitant costs.
Testimonials and Success Stories: Inspiring Hope
Real-life accounts of cancer survivors serve as beacons of hope for those currently undergoing treatment. The top cancer centers in Nairobi proudly share testimonials and success stories, highlighting the positive impact of their services. These stories not only inspire hope but also provide valuable insights into the quality of care provided by these institutions.
Challenges in Cancer Treatment: A Call for Ongoing Improvement
Despite the remarkable progress in cancer care, challenges persist. Limited access to healthcare in certain regions, late-stage diagnoses, and the need for more affordable treatment options are among the ongoing challenges. The top cancer centers in Nairobi actively address these issues, working towards continuous improvement in cancer care delivery.
Future of Cancer Treatment in Nairobi: Emerging Trends and Innovations
As technology advances and medical research progresses, the future of cancer treatment in Nairobi looks promising. Emerging trends such as precision medicine, immunotherapy, and targeted therapies are paving the way for more effective and personalized cancer treatments. The top cancer centers in Nairobi are at the forefront of embracing these innovations, ensuring that patients have access to the best possible care.
Choosing Excellence in Cancer Care
When it comes to choosing the right cancer treatment center in Nairobi, Kenya, patients are presented with a variety of options. Whether considering the cutting-edge services of Apollo Hospitals Information Centre, the comprehensive care at Nairobi Hospital Cancer Center, or the innovative approaches of Aga Khan University Hospital and MP Shah Hospital, each facility has its unique strengths. However, for serious cancer patients in Kenya, Apollo Hospitals Information Centre in Nairobi stands out as the best choice.
Apollo Hospitals Information Centre: Unparalleled Excellence in Cancer Care

Apollo Hospitals Information Centre in Nairobi, Kenya, has earned a stellar reputation for its unparalleled excellence in cancer care. With a global legacy of delivering world-class healthcare, the Nairobi branch continues to set the standard for comprehensive and cutting-edge cancer treatments.
Advanced Technology and Expertise
One key factor that places Apollo Hospitals at the forefront is its commitment to leveraging advanced technology in cancer treatment. The center boasts state-of-the-art diagnostic tools and treatment methodologies, ensuring that patients receive the most advanced and effective care available.
The Future of Cancer Care: Apollo Proton Cancer Centre
Compassionate and Patient-Centric Approach
Beyond technological advancements, Apollo Hospitals Information Centre is renowned for its compassionate and patient-centric approach. Recognizing the emotional and psychological challenges that accompany a cancer diagnosis, the center’s staff goes the extra mile to provide not only medical expertise but also unwavering support to patients and their families.
Personalized Treatment Plans
For serious cancer patients, the importance of personalized treatment plans cannot be overstated. Apollo Hospitals Information Centre excels in tailoring treatment strategies to each patient’s unique needs, ensuring that the approach aligns with the specific characteristics of the cancer and the individual’s overall health.
Pancreatic Cancer in Kenya – Causes, Symptoms, and Treatment
Holistic Well-Being
Apollo Hospitals Information Centre goes beyond medical interventions, prioritizing the holistic well-being of patients. The center understands that a successful cancer treatment journey involves not only physical health but also emotional and psychological support, making it the ideal choice for those facing serious cancer diagnoses in Kenya.
Testimonials of Cancer patients in Kenya
In conclusion, while Nairobi offers a range of reputable cancer treatment options, Apollo Hospitals Information Centre emerges as the top choice for serious cancer patients in Kenya. The combination of cutting-edge technology, personalized care, and a compassionate approach positions Apollo Hospitals as a beacon of hope for individuals embarking on the challenging journey of cancer treatment.
For those seeking the best in cancer care, Nairobi’s top cancer centers are beacons of hope, offering not just medical expertise but also compassionate and patient-centric support. Remember, your choice of where to receive cancer treatment can make a significant difference in your journey towards recovery.
Related Posts

Leave a Comment Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
START TYPING AND PRESS ENTER TO SEARCH
- Contact
- Locate a Clinic
- International Patients
- Book appointment

Medical oncology
meet our specialists.

- M.D., University of Heidelberg, West Germany (1980)
- Doctoral Research - Max Planck Institute for Medical Research, Department of Biophysics and Biochemistry, Heidelberg, West Germany (1980)
- Residency in Internal Medicine, Henry Ford Hospital, Detroit, Michigan (1983)
- Clinical/Research Fellow, Division of Haematology/Oncology, University of Alabama at Birmingham (1986)
- Professor of Medicine, Division of Hematology/Oncology, Scientist, Comprehensive Cancer Center, University of Alabama at Birmingham / Professor of Pathology, Department of Pathology, University of Alabama at Birmingham
- Director of the Phase I Program within the Clinical Center for Translational Science, serving the institution as a whole
- Clinical Research in Targeted Therapy of Cancer
- American Society of Clinical Oncology (ASCO) - Member
- American Society of Hematology (ASH) - Member
- American Association of Cancer Research (AACR) - Member
- American Medical Association (AMA) - Member

Qualifications
- Diploma in Medical Oncology, University Tunis El Manar 2017
- Residency in medical oncology, University Tunis El Manar 2016
- MD, University Tunis El Manar 2012
Areas of interest
- General Medical Oncology
- Oncology Research and clinical trials
- Gynecological and Genitourinary cancers
Professional associations
- American Society of Clinical Oncology
- European Society of Medical Oncology
- Tunisian Society of Medical Oncology
- Kenya Society of Haematology and Oncology

- MBChB (UoN) (2004)
- MMed (AKU) (2009)
- SCE Oncology (RCP UK) (2013)
- MSc Oncology(Ulm University, Germany) (2017)
- FRCP (Edin) (2018)
- Breast cancer
- Eradication of cervical cancer
- Chronic myeloid leukaemia
- Kenya Society of Haematology and Oncology - Chair
- American Society of Medical Oncology
- Kenya Physician Association
- Kenya Medical Association

Consultant Medical Oncologist at Aga Khan University Hospital, Nairobi
- MD Istanbul University,Turkey (2010)
- MMed, Internal Medicine- University of Nairobi (2017)
- Fellowship in Medical Oncology- University of Nairobi (2020)
- SCE Oncology- RCP UK (2020)
Areas of Interest
- Breast Cancer
- Gastrointestinal Cancers
- Cancer Research, translational Research
Professional Associations
Enter your e-mail address,and we promise not to send spam mails.
DNDi signs memorandum of understanding with Kenya Medical Research Institute

The Kenya Medical Research Institute (KEMRI) and the Drugs for Neglected Diseases initiative (DNDi) have signed a memorandum of understanding (MOU) to collaborate in the execution of research and development (R&D) projects and bridge the existing research gaps on neglected diseases. The MOU, which seeks to increase access to diagnosis and treatment, also aims to support policy change in Kenya.
The ten-year agreement was signed on 12 March 2024 by the Acting Chief Executive Officer and Director-General of KEMRI, Elijah Songok; the Executive Director of DNDi, Dr Luis Pizarro; and DNDi Eastern Africa Director, Prof. Sam Kariuki.
In 2003, KEMRI was among the seven health institutions worldwide that established DNDi. Since then, both organizations worked together in Kenya to find new tools for neglected tropical diseases (NTDs) particularly leishmaniasis and other infectious diseases affecting vulnerable populations.
‘For a long time, we have been talking about enhancing collaboration with our founding partner,’ said Prof. Samuel Kariuki, DNDi Eastern Africa Director. ‘This MOU marks a significant milestone because now, we are even going beyond clinical trials collaboration. We will also collaborate to support the achievement of Universal Health Coverage, including access to quality essential healthcare services and access to safe, effective, quality, and affordable essential medicines for all.’
Under the agreement, the MOU will reinforce and build on existing clinical capacities in endemic areas, and address infrastructure needs where necessary, including providing on-site training in clinical research and facilitating technology transfers in R&D for neglected diseases.
DNDi and KEMRI have also agreed to enhance their cooperation to improve resource mobilization with key donors. They will work together on areas such as pandemic preparedness, climate-sensitive diseases, and data management and biostatistics. Furthermore, they will focus on accelerating the development and adoption of new tools for disease control. This includes making new drugs available for patients and strengthening the regulatory environment to allow drug adoption in a timely manner.
The development of these activities will be conducted through regular sharing of information and interactions.
The Drugs for Neglected Diseases initiative (DNDi) is a not-for-profit medical research organization that discovers, develops, and delivers safe, effective, and affordable treatments for neglected people. DNDi is developing medicines for sleeping sickness, leishmaniasis, Chagas disease, river blindness, mycetoma, dengue, paediatric HIV, advanced HIV disease, cryptococcal meningitis, and hepatitis C. Its research priorities include children’s health, gender equity and gender-responsive R&D, and diseases impacted by climate change. Since its creation in 2003, DNDi has joined with public and private partners across the globe to deliver 13 new treatments, saving millions of lives.
About KEMRI
The Kenya Medical Research Institute (KEMRI) is a state corporation established through the Science and Technology (Amendment) Act of 1979, as the national body responsible for carrying out health research in Kenya. Since its inception, KEMRI has developed a critical mass of scientists and technical personnel, to enable it to mount a competitive research infrastructure to rank as a leading centre of excellence in health research both in Africa as well as globally.
Media contacts
Paul Barasa – DNDi Eastern Africa Mobile:+254 719703195 E-mail: [email protected]
Photo credit: Lameck Ododo-DNDi
Read, watch, share

Ravidasvir journey

2023 R&D portfolio in review: Pandemic preparedness

2023 R&D portfolio in review: Chagas

2023 R&D portfolio in review: Hepatitis C

2023 R&D portfolio in review: HIV

2023 R&D portfolio in review: Mycetoma

2023 R&D portfolio in review: Leishmaniasis

2023 R&D portfolio in review: Filaria – river blindness
Help neglected patients.
To date, we have delivered thirteen new treatments , saving millions of lives.
Our goal is to deliver 25 new treatments in our first 25 years. You can help us get there.

- Neglected tropical diseases
- R&D portfolio
- Policy advocacy
Get in touch
- Our offices
- Integrity Line
- Subscribe to eNews
Work with us
- Join research networks
- Terms of Use
- Acceptable Use Policy
- Privacy Policy
- Cookie Policy
- Our policies
- Except for images, films and trademarks which are subject to DNDi’s Terms of Use, content on this site is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Switzerland License
WORLD CHAGAS DAY 2X MATCH
Double your impact for children and families with neglected diseases like Chagas before April 14 at midnight
This paper is in the following e-collection/theme issue:
Published on 11.4.2024 in Vol 26 (2024)
A Perspective on Crowdsourcing and Human-in-the-Loop Workflows in Precision Health
Authors of this article:

- Peter Washington, PhD
Information and Computer Sciences, University of Hawaii at Manoa, Honolulu, HI, United States
Corresponding Author:
Peter Washington, PhD
Information and Computer Sciences
University of Hawaii at Manoa
1680 East-West Road
Honolulu, HI, 96822
United States
Email: [email protected]
Modern machine learning approaches have led to performant diagnostic models for a variety of health conditions. Several machine learning approaches, such as decision trees and deep neural networks, can, in principle, approximate any function. However, this power can be considered to be both a gift and a curse, as the propensity toward overfitting is magnified when the input data are heterogeneous and high dimensional and the output class is highly nonlinear. This issue can especially plague diagnostic systems that predict behavioral and psychiatric conditions that are diagnosed with subjective criteria. An emerging solution to this issue is crowdsourcing, where crowd workers are paid to annotate complex behavioral features in return for monetary compensation or a gamified experience. These labels can then be used to derive a diagnosis, either directly or by using the labels as inputs to a diagnostic machine learning model. This viewpoint describes existing work in this emerging field and discusses ongoing challenges and opportunities with crowd-powered diagnostic systems, a nascent field of study. With the correct considerations, the addition of crowdsourcing to human-in-the-loop machine learning workflows for the prediction of complex and nuanced health conditions can accelerate screening, diagnostics, and ultimately access to care.
Introduction
Crowdsourcing, a term first coined in 2006 [ 1 ], is the use of distributed human workers to accomplish a central task. Crowdsourcing exploits the “power of the crowd” to achieve goals that are only feasible with a distributed group of humans collaborating, either explicitly or implicitly, toward a common goal. Crowdsourcing has often been applied to public health surveillance [ 2 ], such as for tracking epidemics [ 3 , 4 ], quantifying tobacco use [ 5 ], monitoring water quality [ 6 ], tracking misinformation [ 7 ], and understanding the black-market price of prescription opioids [ 8 ]. In the context of health care, crowdsourcing is most often used for public health, a domain that can clearly benefit from scalable and distributed assessments of health status. Although sampling bias can be an issue in epidemiological uses of crowdsourcing [ 9 ], approaches that account for these issues have performed quite robustly.
A smaller but potentially transformative effort to apply crowdsourcing to precision health rather than population health has recently emerged. In precision health contexts, the goal is to provide a diagnosis using information labeled by crowd workers. There are several variations to this basic setup. Crowdsourcing workflows for diagnostics can diverge with respect to the underlying task, worker motivation strategies, worker training, worker filtering, and privacy requirements.
Here, I describe the existing research in the relatively small and early but growing field of crowdsourcing for precision health. I then discuss ongoing challenges and corresponding opportunities that must be addressed as this field matures.
Existing Examples of Crowdsourcing in and Adjacent to Health Care
There are relatively few examples of crowdsourcing in precision health. The vast successes of machine learning for health [ 10 - 15 ] and the human labor costs required for crowdsourcing make purely automated approaches more appealing when they are possible and feasible. However, the crowdsourcing approaches that have been tested tend to perform well for prediction tasks that are beyond the scope of current automated approaches, especially in psychiatry and the behavioral sciences.
I want to begin by highlighting successes in science, as they can often be applied to health and have started to lead to improvements in diagnostics. Framing crowdsourcing tasks as “citizen science” opportunities can be an effective incentive mechanism [ 16 ]. Oftentimes, these projects are “gamified.” Gamification refers to the incorporation of engaging elements into traditionally burdensome workflows, and in particular game-like affordances, to foster increased participation. A combination of large crowd sizes, worker training procedures, and easy identification tasks have led to previous success in the existing gamified citizen science experiments applied to precision health. For example, in a study involving nearly 100,000 crowd workers who scored images on a citizen science platform, cancer was correctly identified with an area under the receive operating characteristic of around 95% [ 17 ]. In the BioGames app, users who performed with greater than 99% accuracy in a training tutorial were invited to diagnose malaria [ 18 , 19 ]. It was discovered that with a large crowd size, the aggregated diagnostic accuracy of nonexpert crowd workers approached that of experts [ 20 ]. Another citizen science malaria diagnosis application, MalariaSpot, resulted in 99% accuracy in the diagnosis of malaria from blood films [ 21 ]. If the annotation task is relatively simple and nonexperts can be trained with minimal onboarding efforts, then citizen science can be an effective and affordable approach.
“Gamified” crowdsourcing for citizen science has also been successful without explicitly requiring workers to undergo a formal training process. Foldit [ 22 - 25 ] and EteRNA [ 26 - 31 ] are 2 games where players with no biology or chemistry background can explore the design space of protein and RNA folding, respectively. These are both NP-hard (ie, computationally complex) problems, and human players in aggregate have designed solutions that outcompete state-of-the-art computational approaches. These solutions have been used to solve health challenges, such as finding a gene signature for active tuberculosis, which can potentially be used in tuberculosis diagnostics [ 32 ]. Other gamified experiences have been used to build training libraries for complex classification tasks in precision psychiatry. Notably, GuessWhat is a mobile charades game played between children with autism and their parents [ 33 , 34 ]. While the game provides therapeutic benefits to the child with autism [ 35 ], the game simultaneously curates automatic labels of behaviors related to autism through the metadata associated with gameplay [ 36 , 37 ]. These automatically annotated video data have been used to develop state-of-the-art computer vision models for behaviors related to the diagnosis of autism, such as facial expression evocation [ 38 - 41 ], eye gaze [ 42 ], atypical prosody [ 43 ], and atypical body movements [ 44 , 45 ].
An alternative incentive mechanism is paid crowdsourcing. The most popular paid crowdsourcing platform, by far, is Amazon Mechanical Turk (MTurk) [ 46 ]. While paid crowdsourcing specifically for precision health is a relatively nascent field, the general study of paid crowdsourcing (particularly on MTurk) is quite mature. Studies have explored worker quality management [ 47 ], understanding crowd worker demographics [ 48 ], the generation of annotations for use in the training of machine learning models [ 49 - 53 ], the rights of crowd workers [ 54 - 56 ], and understanding crowd worker communities and economics [ 57 - 59 ]. Preliminary studies of paid crowdsourcing have yielded mixed success. Around 81% of images were correctly classified on MTurk in a study involving the grading of diabetic retinopathy from images, with workers failing to correctly indicate the level of severity [ 60 ]. In a separate binary labeling task for glaucomatous optic neuropathy, workers achieved sensitivity in the 80s but reached a specificity below 50% [ 61 ].
In a broader classification task of various medical conditions, workers consistently labeled the “easy” cases while struggling to correctly label and even refusing to label more complicated and nuanced tasks [ 62 ]. Clearly, there is a need for extensive innovations to the traditional paid crowdsourcing workflow to translate this methodology to precision health.
I have extensively investigated the utility of paid crowdsourcing for the diagnosis of autism from unstructured home videos, achieving relatively high diagnostic performance [ 63 - 66 ]. In these experiments, untrained annotators watched short videos depicting children with and without autism and answered questions about the behaviors depicted within the videos. These annotations were provided as input into previously developed machine learning models, achieving binary test performance in the 90s across performance metrics due to the reduction of the complex feature space (unstructured videos) into a low-dimensional representation (vectors of a few categorical ordinal values). This pipeline combining crowdsourcing and machine learning can possibly be extended to other diagnostic domains in psychiatry where the input feature space is complex, heterogeneous, and subjective.
Ongoing Challenges and Corresponding Opportunities
Since crowdsourcing for precision health care is an emerging field of study, numerous challenges must be solved for clinical translation to develop. In the proceeding sections, I highlight several areas that are pressing for the field and for which preliminary work has been published.
Worker Identification and Training
While traditional crowdsourcing can work with minimal to no worker training, complex annotation tasks require the identification of qualified workers. I have found that worker identification can occur through the quantification of their performance on test tasks [ 67 , 68 ] and training promising workers [ 66 ]. Such crowd filtration paradigms will require domain-specific procedures. There is ample room to develop new crowdsourcing systems that inherently support natural worker identification and training procedures for crowdsourcing workflows that require well-designed training processes.
Worker Retention
Once proficient workers are identified, continually engaging and retaining these workers is critical. I have found that workers who are repeatedly encouraged by a human (or human-like chatbot) and treated as members of a broader research team tend to enjoy paid work and even ask for more tasks after the completion of the study [ 69 ]. Thus, it is possible that the guarantee of job security can lead to long-term worker retention. However, worker retention in unpaid settings that rely on intrinsic motivation will require additional innovations. For example, there exists an opportunity to explore the creation of crowd worker communities to provide a means of intrinsic motivation leading to worker retention.
Task Assignment
Certain workers perform exceptionally well on a subset of tasks while underperforming on other assigned tasks [ 70 , 71 ]. There is an opportunity to develop algorithmic innovations involving the effective and optimal assignment of workers to subtasks in a dynamic manner. Reinforcement learning could be a promising approach but has yet to be explored in such scenarios.
Privacy of Human Participants
Data in psychiatry and behavioral sciences are particularly sensitive. Ensuring that sensitive health information is handled appropriately and that workers’ privacy is maintained is essential from an ethical perspective. There are 2 general families of approaches to achieving privacy in crowd-powered health care. First, the data can be modified to obscure sensitive information without removing information required for a diagnosis. I have explored privacy-preserving alterations to video data that obfuscate the identity of participants while maximizing the capacity for workers to annotate behaviors of interest [ 70 , 71 ]. For example, in the case of video analytics on bodily movements, the face can be tracked and blurred, or the body can be converted to a stick figure using a pose-based computer vision library. Sometimes, however, it is impossible to modify the data without severely degrading the diagnostic performance. Therefore, the second family of approaches involves carefully vetting crowd workers, training them, and onboarding them into a secure computing environment. In my previous experiences with this process [ 40 ], I discovered that crowd workers were enthusiastic about the prospect of the “job security” that is implied from the thorough vetting procedure and were, therefore, willing to complete extra privacy and security training (in our case, Research, Ethics, Compliance, and Safety training). There is ample room to expand upon these methods and to develop new paradigms and systems for crowdsourcing involving identifiable and protected health information.
Ensuring Reliability and Reproducibility
An intrinsic challenge when incorporating human workers into precision health workflows is the variability in human responses, both within workers and between workers. I have found that while most crowd workers are inconsistent in their annotation patterns, there are workers who provide consistently sensitive and specific annotations across a wide spectrum of data points [ 67 ]. It is therefore critical to measure both internal consistency and consistency against a gold standard when recruiting crowd workers for precision health care workflows.
Handling Financial Constraints
The crowdsourcing method with the lowest setup barriers is paid crowdsourcing. In such scenarios, financial constraints can limit the scalability of crowdsourcing workflows. One approach is to migrate from a paid system to a gamified system or another means of providing intrinsic motivation to crowd workers. However, achieving critical mass for large-scale pipelines is likely unattainable for such unpaid solutions. Paid crowd workers who consistently perform well could be recruited as full-time or long-term part-time employees for companies and organizations providing crowd-powered services. Integrating such workflows into a Food and Drug Administration (FDA)–approved process can be challenging, but it is worth exploring if it turns out that crowd-powered solutions for digital psychiatry continue to remain superior to pure-artificial intelligence (AI) approaches in the coming years.
Translation Outside of Research Contexts
While pure machine learning approaches for precision health are beginning to translate to clinical settings through formal FDA approval procedures, the prospect of translating human-in-the-loop methods that integrate crowd workers rather than expert clinicians is daunting, especially in light of the challenges mentioned above. However, if such approaches lead to clinical-grade performance for certain conditions that are challenging to diagnose using machine learning alone, then the extra implementation and regulatory effort required to migrate these methods into production-level workflows are likely to be warranted.
While machine learning for health has enabled and will continue to enable more efficient, precise, and scalable diagnostics for a variety of conditions, such models are unlikely to generalize to more difficult scenarios such as psychiatry and the behavioral sciences, which require the ability to identify complex and nuanced social human behavior. Crowd-powered human-in-the-loop workflows have the potential to mitigate some of these current limitations while still offering a high degree of automation. I invite researchers in the fields of digital phenotyping [ 72 - 76 ], mobile sensing [ 77 - 83 ], affective computing [ 84 - 90 ], and related subjects to consider integrating crowdsourcing and human-in-the-loop approaches into their methods when pure-AI leads to suboptimal performance.
Acknowledgments
This project is funded by the NIH Director’s New Innovator Award (DP2) from the National Institutes of Health (award DP2-EB035858).
Conflicts of Interest
None declared.
- Howe J. The rise of crowdsourcing. Wired. Jun 01, 2006. URL: https://sistemas-humano-computacionais.wdfiles.com/local--files/capitulo%3Aredes-sociais/Howe_The_Rise_of_Crowdsourcing.pdf [accessed 2024-03-29]
- Brabham DC, Ribisl KM, Kirchner TR, Bernhardt JM. Crowdsourcing applications for public health. Am J Prev Med. 2014;46(2):179-187. [ CrossRef ] [ Medline ]
- Leung GM, Leung K. Crowdsourcing data to mitigate epidemics. Lancet Digit Health. 2020;2(4):e156-e157. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stockham N, Washington P, Chrisman B, Paskov K, Jung JY, Wall DP. Causal modeling to mitigate selection bias and unmeasured confounding in internet-based epidemiology of COVID-19: model development and validation. JMIR Public Health Surveill. 2022;8(7):e31306. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kraemer JD, Strasser AA, Lindblom EN, Niaura RS, Mays D. Crowdsourced data collection for public health: a comparison with nationally representative, population tobacco use data. Prev Med. 2017;102:93-99. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jakositz S, Pillsbury L, Greenwood S, Fahnestock M, McGreavy B, Bryce J, et al. Protection through participation: crowdsourced tap water quality monitoring for enhanced public health. Water Res. 2020;169:115209. [ CrossRef ] [ Medline ]
- Ghenai A, Mejova Y. Catching Zika fever: application of crowdsourcing and machine learning for tracking health misinformation on Twitter. arXiv. Preprint posted online Jul 12, 2017. [ CrossRef ]
- Dasgupta N, Freifeld C, Brownstein JS, Menone CM, Surratt HL, Poppish L, et al. Crowdsourcing black market prices for prescription opioids. J Med Internet Res. 2013;15(8):e178. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wazny K. "Crowdsourcing" ten years in: a review. J Glob Health. 2017;7(2):020602. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chen PHC, Liu Y, Peng L. How to develop machine learning models for healthcare. Nat Mater. 2019;18(5):410-414. [ CrossRef ] [ Medline ]
- Dua S, Acharya UR, Dua P, editors. Machine Learning in Healthcare Informatics, Volume 56. Berlin, Heidelberg. Springer; 2014.
- Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24-29. [ CrossRef ] [ Medline ]
- Ghassemi M, Naumann T, Schulam P, Beam AL, Chen IY, Ranganath R. A review of challenges and opportunities in machine learning for health. AMIA Jt Summits Transl Sci Proc. 2020;2020:191-200. [ FREE Full text ] [ Medline ]
- Shailaja K, Seetharamulu B, Jabbar MA. Machine learning in healthcare: a review. 2018. Presented at: 2018 Second International Conference on Electronics, Communication and Aerospace Technology (ICECA); March 29-31, 2018;910-914; Coimbatore, India. [ CrossRef ]
- Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2(10):719-731. [ CrossRef ] [ Medline ]
- Das R, Keep B, Washington P, Riedel-Kruse IH. Scientific discovery games for biomedical research. Annu Rev Biomed Data Sci. 2019;2(1):253-279. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dos Reis FJC, Lynn S, Ali HR, Eccles D, Hanby A, Provenzano E, et al. Crowdsourcing the general public for large scale molecular pathology studies in cancer. EBioMedicine. 2015;2(7):681-689. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mavandadi S, Dimitrov S, Feng S, Yu F, Sikora U, Yaglidere O, et al. Distributed medical image analysis and diagnosis through crowd-sourced games: a malaria case study. PLoS One. 2012;7(5):e37245. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ozcan A. Educational games for malaria diagnosis. Sci Transl Med. 2014;6(233):233ed9. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Feng S, Woo M, Chandramouli K, Ozcan A. A game-based platform for crowd-sourcing biomedical image diagnosis and standardized remote training and education of diagnosticians. In: Optics and Biophotonics in Low-Resource Settings, Volume 9314. 2015. Presented at: SPIE BIOS; February 7-12, 2015; San Francisco, CA. [ CrossRef ]
- Luengo-Oroz MA, Arranz A, Frean J. Crowdsourcing malaria parasite quantification: an online game for analyzing images of infected thick blood smears. J Med Internet Res. 2012;14(6):e167. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cooper S, Khatib F, Makedon I, Lu H, Barbero J, Baker D, et al. Analysis of social gameplay macros in the Foldit cookbook. 2011. Presented at: FDG '11: Proceedings of the 6th International Conference on Foundations of Digital Games; June 29, 2011 - July 1, 2011;9-14; Bordeaux, France. [ CrossRef ]
- Curtis V. Motivation to participate in an online citizen science game: a study of Foldit. Sci Commun. 2015;37(6):723-746. [ CrossRef ]
- Eiben CB, Siegel JB, Bale JB, Cooper S, Khatib F, Shen BW, et al. Increased Diels-Alderase activity through backbone remodeling guided by Foldit players. Nat Biotechnol. 2012;30(2):190-192. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kleffner R, Flatten J, Leaver-Fay A, Baker D, Siegel JB, Khatib F, et al. Foldit standalone: a video game-derived protein structure manipulation interface using Rosetta. Bioinformatics. 2017;33(17):2765-2767. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Andreasson JOL, Gotrik MR, Wu MJ, Wayment-Steele HK, Kladwang W, Portela F, et al. Crowdsourced RNA design discovers diverse, reversible, efficient, self-contained molecular switches. Proc Natl Acad Sci U S A. 2022;119(18):e2112979119. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Krüger A, Watkins AM, Wellington-Oguri R, Romano J, Kofman C, DeFoe A, et al. Community science designed ribosomes with beneficial phenotypes. Nat Commun. 2023;14(1):961. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee J, Kladwang W, Lee M, Cantu D, Azizyan M, Kim H, et al. RNA design rules from a massive open laboratory. Proc Natl Acad Sci U S A. 2014;111(6):2122-2127. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shi J, Das R, Pande VS. SentRNA: improving computational RNA design by incorporating a prior of human design strategies. arXiv. Preprint posted online Mar 06, 2019. [ CrossRef ]
- Treuille A, Das R. Scientific rigor through videogames. Trends Biochem Sci. 2014;39(11):507-509. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wayment-Steele HK, Kladwang W, Strom AI, Lee J, Treuille A, Becka A, et al. RNA secondary structure packages evaluated and improved by high-throughput experiments. Nat Methods. 2022;19(10):1234-1242. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med. 2016;4(3):213-224. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kalantarian H, Jedoui K, Washington P, Wall DP. A mobile game for automatic emotion-labeling of images. IEEE Trans Games. 2020;12(2):213-218. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kalantarian H, Washington P, Schwartz J, Daniels J, Haber N, Wall D. A gamified mobile system for crowdsourcing video for autism research. 2018. Presented at: 2018 IEEE International Conference on Healthcare Informatics (ICHI); June 4-7, 2018;350-352; New York, NY. [ CrossRef ]
- Penev Y, Dunlap K, Husic A, Hou C, Washington P, Leblanc E, et al. A mobile game platform for improving social communication in children with autism: a feasibility study. Appl Clin Inform. 2021;12(5):1030-1040. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kalantarian H, Washington P, Schwartz J, Daniels J, Haber N, Wall DP. Guess what? Towards understanding autism from structured video using facial affect. J Healthc Inform Res. 2019;3(1):43-66. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kalantarian H, Jedoui K, Washington P, Tariq Q, Dunlap K, Schwartz J, et al. Labeling images with facial emotion and the potential for pediatric healthcare. Artif Intell Med. 2019;98:77-86. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hou C, Kalantarian H, Washington P, Dunlap K, Wall DP. Leveraging video data from a digital smartphone autism therapy to train an emotion detection classifier. medRxiv. Preprint posted online Aug 01, 2021. [ CrossRef ]
- Kalantarian H, Jedoui K, Dunlap K, Schwartz J, Washington P, Husic A, et al. The performance of emotion classifiers for children with parent-reported autism: quantitative feasibility study. JMIR Ment Health. 2020;7(4):e13174. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Kalantarian H, Kent J, Husic A, Kline A, Leblanc E, et al. Improved digital therapy for developmental pediatrics using domain-specific artificial intelligence: machine learning study. JMIR Pediatr Parent. 2022;5(2):e26760. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Kalantarian H, Kent J, Husic A, Kline A, Leblanc E, et al. Training an emotion detection classifier using frames from a mobile therapeutic game for children with developmental disorders. arXiv. Preprint posted online Dec 16, 2020 . [ CrossRef ]
- Varma M, Washington P, Chrisman B, Kline A, Leblanc E, Paskov K, et al. Identification of social engagement indicators associated with autism spectrum disorder using a game-based mobile app: comparative study of gaze fixation and visual scanning methods. J Med Internet Res. 2022;24(2):e31830. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chi NA, Washington P, Kline A, Husic A, Hou C, He C, et al. Classifying autism from crowdsourced semistructured speech recordings: machine learning model comparison study. JMIR Pediatr Parent. 2022;5(2):e35406. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lakkapragada A, Kline A, Mutlu OC, Paskov K, Chrisman B, Stockham N, et al. The classification of abnormal hand movement to aid in autism detection: machine learning study. JMIR Biomed Eng. 2022;7(1):e33771. [ FREE Full text ] [ CrossRef ]
- Washington P, Kline A, Mutlu OC, Leblanc E, Hou C, Stockham N, et al. Activity recognition with moving cameras and few training examples: applications for detection of autism-related headbanging. 2021. Presented at: CHI '21: CHI Conference on Human Factors in Computing Systems; May 8-13, 2021;1-7; Yokohama, Japan. [ CrossRef ]
- Paolacci G, Chandler J, Ipeirotis PG. Running experiments on Amazon Mechanical Turk. Judgm Decis Mak. 2010;5(5):411-419. [ FREE Full text ] [ CrossRef ]
- Ipeirotis PG, Provost F, Wang J. Quality management on Amazon Mechanical Turk. 2010. Presented at: HCOMP '10: Proceedings of the ACM SIGKDD Workshop on Human Computation; July 25, 2010;64-67; Washington DC. [ CrossRef ]
- Lee YJ, Arida JA, Donovan HS. The application of crowdsourcing approaches to cancer research: a systematic review. Cancer Med. 2017;6(11):2595-2605. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ross J, Zaldivar A, Irani L, Tomlinson B. Who are the turkers? Worker demographics in Amazon Mechanical Turk. ResearchGate. 2009. URL: https://www.researchgate.net/publication/268427703_Who_are_the_Turkers_Worker_Demographics_in_Amazon_Mechanical_Turk [accessed 2024-03-22]
- Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, et al. ImageNet large scale visual recognition challenge. Int J Comput Vis. 2015;115:211-252. [ CrossRef ]
- Sheng VS, Zhang J. Machine learning with crowdsourcing: a brief summary of the past research and future directions. Proc AAAI Conf Artif Intell. 2019;33(01):9837-9843. [ CrossRef ]
- Sorokin A, Forsyth D. Utility data annotation with Amazon Mechanical Turk. 2008. Presented at: 2008 IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops; June 23-28, 2008;1-8; Anchorage, AK. [ CrossRef ]
- Xintong G, Hongzhi W, Song Y, Hong G. Brief survey of crowdsourcing for data mining. Expert Syst Appl. 2014;41(17):7987-7994. [ CrossRef ]
- Irani LC, Silberman MS. Turkopticon: interrupting worker invisibility in Amazon Mechanical Turk. 2013. Presented at: CHI '13: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems; April 27, 2013 - May 2, 2013;611-620; Paris, France. [ CrossRef ]
- Irani L, Silberman MS. From critical design to critical infrastructure: lessons from turkopticon. Interactions. 2014;21(4):32-35. [ CrossRef ]
- Kummerfeld JK. Quantifying and avoiding unfair qualification labour in crowdsourcing. arXiv. Preprint posted online May 26, 2021. [ CrossRef ]
- Hansson K, Ludwig T. Crowd dynamics: conflicts, contradictions, and community in crowdsourcing. Comput Support Coop Work. 2019;28:791-794. [ FREE Full text ] [ CrossRef ]
- Shen XL, Lee MKO, Cheung CMK. Exploring online social behavior in crowdsourcing communities: a relationship management perspective. Comput Human Behav. 2014;40:144-151. [ CrossRef ]
- Wu W, Gong X. Motivation and sustained participation in the online crowdsourcing community: the moderating role of community commitment. Internet Res. 2021;31(1):287-314. [ CrossRef ]
- Brady CJ, Villanti AC, Pearson JL, Kirchner TR, Gupta OP, Shah C. Rapid grading of fundus photographs for diabetic retinopathy using crowdsourcing. J Med Internet Res. Oct 30, 2014;16(10):e233. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mitry D, Peto T, Hayat S, Blows P, Morgan J, Khaw KT, et al. Crowdsourcing as a screening tool to detect clinical features of glaucomatous optic neuropathy from digital photography. PLoS One. 2015;10(2):e0117401. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cheng J, Manoharan M, Zhang Y, Lease M. Is there a doctor in the crowd? Diagnosis needed! (For less than $5). iConference 2015 Proceedings. 2015. URL: https://www.ideals.illinois.edu/items/73844 [accessed 2024-03-22]
- Leblanc E, Washington P, Varma M, Dunlap K, Penev Y, Kline A, et al. Feature replacement methods enable reliable home video analysis for machine learning detection of autism. Sci Rep. 2020;10(1):21245. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tariq Q, Daniels J, Schwartz JN, Washington P, Kalantarian H, Wall DP. Mobile detection of autism through machine learning on home video: a development and prospective validation study. PLoS Med. 2018;15(11):e1002705. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tariq Q, Fleming SL, Schwartz JN, Dunlap K, Corbin C, Washington P, et al. Detecting developmental delay and autism through machine learning models using home videos of Bangladeshi children: development and validation study. J Med Internet Res. 2019;21(4):e13822. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Tariq Q, Leblanc E, Chrisman B, Dunlap K, Kline A, et al. Crowdsourced privacy-preserved feature tagging of short home videos for machine learning ASD detection. Sci Rep. 2021;11(1):7620. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Leblanc E, Dunlap K, Penev Y, Kline A, Paskov K, et al. Precision telemedicine through crowdsourced machine learning: testing variability of crowd workers for video-based autism feature recognition. J Pers Med. 2020;10(3):86. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Leblanc E, Dunlap K, Penev Y, Varma M, Jung JY, et al. Selection of trustworthy crowd workers for telemedical diagnosis of pediatric autism spectrum disorder. Pac Symp Biocomput. 2021;26:14-25. [ FREE Full text ] [ Medline ]
- Washington P, Kalantarian H, Tariq Q, Schwartz J, Dunlap K, Chrisman B, et al. Validity of online screening for autism: crowdsourcing study comparing paid and unpaid diagnostic tasks. J Med Internet Res. 2019;21(5):e13668. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Chrisman B, Leblanc E, Dunlap K, Kline A, Mutlu C, et al. Crowd annotations can approximate clinical autism impressions from short home videos with privacy protections. Intell Based Med. 2022;6:100056. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA. 2017;318(13):1215-1216. [ CrossRef ] [ Medline ]
- Huckvale K, Venkatesh S, Christensen H. Toward clinical digital phenotyping: a timely opportunity to consider purpose, quality, and safety. NPJ Digit Med. 2019;2:88. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Onnela JP. Opportunities and challenges in the collection and analysis of digital phenotyping data. Neuropsychopharmacology. 2021;46(1):45-54. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Onnela JP, Rauch SL. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology. 2016;41(7):1691-1696. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Washington P, Mutlu CO, Kline A, Paskov K, Stockham NT, Chrisman B, et al. Challenges and opportunities for machine learning classification of behavior and mental state from images. arXiv. Preprint posted online Jan 26, 2022. [ CrossRef ]
- Baumeister H, Montag C, editors. Digital Phenotyping and Mobile Sensing: New Developments in Psychoinformatics. Cham, Switzerland. Springer International Publishing; 2019.
- Laport-López F, Serrano E, Bajo J, Campbell AT. A review of mobile sensing systems, applications, and opportunities. Knowl Inf Syst. 2020;62(1):145-174. [ CrossRef ]
- Macias E, Suarez A, Lloret J. Mobile sensing systems. Sensors (Basel). 2013;13(12):17292-17321. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nazir S, Ali Y, Ullah N, García-Magariño I. Internet of things for healthcare using effects of mobile computing: a systematic literature review. Wirel Commun Mob Comput. 2019;2019:1-20. [ FREE Full text ] [ CrossRef ]
- Silva BMC, Rodrigues JJPC, de la Torre Díez I, López-Coronado M, Saleem K. Mobile-health: a review of current state in 2015. J Biomed Inform. 2015;56:265-272. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sim I. Mobile devices and health. N Engl J Med. 2019;381(10):956-968. [ CrossRef ]
- Yürür O, Liu CH, Sheng Z, Leung VCM, Moreno W, Leung KK. Context-awareness for mobile sensing: a survey and future directions. IEEE Commun Surv Tutor. 2014;18(1):68-93. [ CrossRef ]
- Picard RW. Affective Computing. Cambridge, MA. MIT Press; 2000.
- Picard RW. Affective computing: challenges. Int J Hum-Comput Stud. 2003;59(1-2):55-64. [ CrossRef ]
- Poria S, Cambria E, Bajpai R, Hussain A. A review of affective computing: from unimodal analysis to multimodal fusion. Inf Fusion. 2017;37:98-125. [ CrossRef ]
- Scherer KR, Bänziger T, Roesch E, editors. A Blueprint for Affective Computing: a Sourcebook and Manual. Oxford, United Kingdom. Oxford University Press; 2010.
- Tao J, Tan T. Affective computing: a review. In: Affective Computing and Intelligent Interaction. Berlin, Heidelberg. Springer; 2005. Presented at: First International Conference, ACII 2005; October 22-24, 2005;981-995; Beijing, China. [ CrossRef ]
- Wang Y, Song W, Tao W, Liotta A, Yang D, Li X, et al. A systematic review on affective computing: emotion models, databases, and recent advances. Inf Fusion. 2022;83-84:19-52. [ CrossRef ]
- Zhao S, Wang S, Soleymani M, Joshi D, Ji Q. Affective computing for large-scale heterogeneous multimedia data: a survey. ACM Trans Multimed Comput Commun Appl. 2019;15(3s):1-32. [ CrossRef ]
Abbreviations
Edited by A Mavragani; submitted 22.07.23; peer-reviewed by E Vashishtha, MO Khursheed, L Guo; comments to author 02.09.23; revised version received 15.11.23; accepted 30.01.24; published 11.04.24.
©Peter Washington. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 11.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

Op-Ed: Transition and Gender-Affirming Care Is Well-Documented Medical Fact
W e at Centre LGBT+ are concerned about an upcoming visit to our community from an anti-transgender activist who espouses a dangerous narrative about the trans community and the experience of “de-transitioning.”
We would like to present a research-supported look at the experiences of transgender people, their medical care and why some trans people de-transition.
Gender-affirming care — which ranges from social transition steps that include simply calling someone by their correct name and pronouns, to medical treatments including hormone replacement therapies and surgery — is medical fact validated by every major medical association. The American Medical Association, American Academy of Pediatrics, American Psychiatric Association, World Health Organization and many, many more all recognize gender affirming care as medically necessary, life-saving, life-affirming treatment for transgender people.
It is important to explicitly clarify that transness itself is a not a medical issue that requires “fixing.” Instead, gender affirming care aims to treat “dysphoria” by helping trans people align their bodies with who they truly are, as best fits their unique sense of self and transition goals. Instead of dysphoria, some people prefer to focus on gender “euphoria”— a feeling of joy and correctness that comes with their true gender. Every individual’s transition experience, goals, and needs will vary, but transition, understanding, and support are universally recognized as the appropriate care for transgender people.
Research has consistently found extremely high rates of satisfaction with gender affirming medical care, including hormone replacement therapies and surgery.
The 2022 U.S. Transgender Study , compiling insights from over 92,000 respondents, found that the overwhelming majority of individuals receiving hormone replacement therapy — 98% — report their transition has made them more satisfied with their life.
Furthermore, research published in 2022 from the Stanford University School of Medicine explicitly found that starting gender-affirming hormone treatment in adolescence is linked to better mental health for trans people than waiting until later in adulthood.
A 2023 study published in the Journal of the American Medical Association (JAMA) , notes that the regret rate for gender-affirming surgery is estimated at 1% — with not a single respondent to their study having requested or undergone a reversal of their gender-affirming procedure. They also found that trans individuals who received gender-affirming surgery reported lower rates of both depression and anxiety, contributing to greater overall well-being and positive mental health outcomes.
Other examinations of available research have found similar results. A review of 27 scientific studies, published in 2021 in the Journal of the American Society of Plastic and Reconstructive Surgery , confirmed that the regret rate for gender affirming surgery is 1%.
As an illustrative comparison , regret for knee replacement surgery ranges as high as 30% of patients . One might notice that there is no coordinated political action to ban knee surgery, and no touring speakers on the dangers of knee surgery, despite being up to 30 times more likely to negatively impact patients than gender-affirming procedures.
It is worth noting that gender affirming care for youth almost exclusively takes the form of social transitioning and hormone treatments, with surgeries being rarely performed. A 2023 study published in JAMA found that about 92% of gender affirming surgeries are performed on individuals who are 18 or older, with less than 8% performed on individuals younger than 18 years old.
So with gender affirming medical care being associated with extremely high rates of satisfaction, greater life satisfaction and well-being, and reduced levels of anxiety and depression, why do some transgender people de-transition?
Well, most don’t. The U.S. Transgender Survey has found that only 8% of people detransition , and that the majority of those who do de-transition ultimately resume their transition later in their life.
This minority of trans people who de-transition do so — simply put — because society collectively is often not very kind to transgender people. The top four reasons for de-transitioning reported in the U.S. Transgender Survey are 1) pressure from a parent, 2) transitioning was too hard, 3) they faced too much harassment and discrimination, and 4) they had trouble getting a job.
Similarly, a 2021 study found that over 82% of individuals who de-transitioned did so because of external pressure, lack of acceptance, social stigma, and instances of violence and harassment.
To recap: gender affirming care is overwhelmingly associated with extremely high levels of satisfaction and better mental health outcomes. Those who de-transition are a small minority. Those who de-transition and remain de-transitioned are an even smaller minority. Those who de-transition and remain de-transitioned for reasons other than being treated poorly for being trans are an even smaller minority still, representing the smallest possible sliver of transgender experience.
Yet despite representing the smallest possible fraction of trans experience, “de-transitioners” like the speaker visiting our community wield an outsized influence on public discourse around transgender rights and gender-affirming care — often spearheading or supporting attempts to restrict or ban the medical care that is endorsed by every professional medical organization.
Centre LGBT+ encourages all members of the local transgender, nonbinary, and gender nonconforming communities (as well as their allies, friends, and families) to find community and solidarity through the resources we offer. We offer a gender identity support group, a support group for those questioning or developing their gender identity, and a support group for families. We also offer a wide range of social groups for LGBT+ folks of all identities and ages to make friends and find community. We also offer trainings to businesses, congregations, and community groups who want to learn more.
Please visit centrelgbtplus.org or reach out to us at [email protected] to learn more about the resources we offer and how you can help support our local LGBT+ community.
This commentary was authored by the members of the Centre LGBT+ Board .
The post Op-Ed: Transition and Gender-Affirming Care Is Well-Documented Medical Fact appeared first on StateCollege.com .


CENTER FOR GLOBAL HEALTH RESEARCH (CGHR)-KISUMU

The Centre for Global Health Research (CGHR) is located in Kisumu, Western Kenya. The facility was originally inherited from the former East African Medical Research Council.
In 1984, the station was named Malaria and Other Protozoal Diseases Centre (MOPDC) later changing to Vector Biology and Control Research Centre (VBCRC), then Centre for Vector Biology and Control Research (CVBCR) and finally Center for Global Health Research (CGHR). The Centre is today one of the 15 Research Centres of the Kenya Medical Research Institute (KEMRI). The Centre is strategically located in Kisumu City, western Kenya region, in an area that is endemic for major infectious diseases. READMORE
Our mission is to improve human health and quality of life through research, capacity building, innovation and service delivery
Our vision is to be a leading centre of excellence in research for human health.
- To conduct evidence based research aimed at providing solutions for the reduction of infectious, parasitic, non-infectious diseases and other causes of ill-health in Kenya.
- Provide training and capacity building in public health
- Provide leadership in human health research READMORE.
Achievements
Evidence on best Deworming strategies to be employed for Mass Drug Administration (MDA) against Helminth infections:
Preventive chemotherapy is considered the key component in the control of Schistosomiasis and intestinal worms, and among questions that have been addressed on our research platform is how different deworming strategies (whether community-wide or school-based) impact on morbidity in areas with different infection prevalence thresholds. Some of the data from our Schistosomiasis Consortium for Operational Research (SCORE) studies is currently being shared with the Ministry of Health in order to provide evidence on the best combination of deworming strategies based on prevalence thresholds, both in terms of duration and frequency of MDAs.
Evidence of Schistosomiasis in pre-school age children (< 5 years) and need for change in deworming policy: The WHO recommended control strategy of Schistosomiasis is based on preventive chemotherapy interventions targeting the majority of the at-risk population. READMORE
Ongoing Projects
- A demographic surveillance system (DSS) in the previous bed net study area aimed at establishing a high quality surveillance system in a population of 135,000 and provide an infrastructure that will yield reliable demographic data and serve as a platform to evaluate the impact of public health interventions on morbidity and mortality.
- Population genetics of malaria vectors.
- The effects of climate change on human health.
- The epidemiology and immunology of malaria epidemics in the highlands. READMORE
- A community-based trial on the impact of insecticide-treated bed nets on childhood morbidity and mortality in an area of intense and perennial malaria transmission in western Kenya,
- The interaction of malaria and HIV in pregnant women and the immuno-epidemiology of malaria in western Kenya,
- The Asembo Bay Cohort Project.

Dr. STEPHEN MUNGA
Contact info.
Kenya Medical Research Institute
Centre for global health research
P.O Box 20778-00202 Kisumu Tel: +254 020 2726781
+254 020 2722541, +254 020 2713349, 0722205901
0733400003.
Email: gro.irmek obfsctd @rhgc

IMAGES
COMMENTS
The Kenya Medical Research Institute (KEMRI) is a State Corporation established in Kenya in 1979 through the Science and Technology (Repealed) Act, Cap 250 of the Laws of Kenya operated under the Science Technology and Innovation Act, 2013 as the national body responsible for carrying out research in human health in Kenya.
Centre for Biotechnology Research and Development (CBRD) CENTRE FOR DISEASE CONTROL & SURVEILLANCE, MANDERA ... Reach us at Tel: 0722 205 901/020 272 254 1 or [email protected]. ABOUT US. Kenya Medical Research Institute (KEMRI) is a State Corporation established through the Science and Technology (Amendment) Act of 1979, which has since ...
The KEMRI Wellcome Trust Research Programme is a. world-renowned health research unit of excellence. The programme was formed in 1989 when the Kenya Medical Research Institute formed a partnership with the Wellcome Trust and the University of Oxford. The Programme has grown from a small group of 12 to a state of the art facility hosting over ...
The Centre for Biotechnology Research and Development (CBRD) is mandated to undertake basic and biotechnology-related research on human diseases in Kenya, with the overall goal of contributing to the improvement of human health and welfare. ... Kenya Medical Research Institute ... Nairobi, Kenya. Center for Geographic Medicine Research, Coast P ...
The KEMRI Wellcome Trust Research Programme (KWTRP) is based within the KEMRI Centre for Geographic Medical Research - (Coast). Our core activities are funded by the Wellcome Trust. We conduct integrated epidemiological, social, laboratory and clinical research in parallel, with results feeding into local and international health policy. Our ...
The Kenya Medical Research Institute (KEMRI) is a state corporation established through the Science and Technology (Amendment) Act of 1979, (since amended to the Sciences, Technology and Innovation Act 2013), during the tenure of Nicholas Biwott as Minister of State, as the national body responsible for carrying out health research in Kenya.. KEMRI is the medical research arm of the Kenya ...
Kenya Medical Research Institute (KEMRI) | 122,700 followers on LinkedIn. In Search of Better Health | Kenya Medical Research Institute (KEMRI) is a State Corporation established through the ...
Kenya Medical Research Institute, Nairobi, Kenya. 24,807 likes · 383 talking about this · 1,846 were here. Established in 1979, KEMRI is the national... Established in 1979, KEMRI is the national body mandated to carry out Human Health Research...
Kenya Medical Research Institute (KEMRI) is a State Corporation established through the Science and Technology (Amendment) Act of 1979, which has since been amended to the Science, Technology and Innovation Act 2013. The 1979 Act established KEMRI as a National body responsible for carrying out health research in Kenya. Vision. To be a leading ...
APHRC @20 highlights. The African Population and Health Research Center (APHRC) will culminate its 20th anniversary in 2023. For the last two decades, the Center has been at the forefront of groundbreaking research, tackling Sub-Saharan Africa's most pressing development issues: health, education, population, aging, urbanization, and well-being.
Find 584 researchers and browse 4 departments, publications, full-texts, contact details and general information related to Kenya Medical Research Institute | Nairobi, Kenya | KEMRI
HJFMRI LTD GTE helped stand up a new Clinical Research Center in Abuja, Nigeria with the Walter Reed Army Institute of Research (WRAIR) to conduct clinical trials to combat infectious diseases. The CRC in Abuja conducts HIV studies and has pivoted to combat emerging health threats such as Ebola, Lassa fever and COVID-19. Since 2003, HJFMRI has ...
The Centre for clinical research (CCR) was established in 1985 as a clinical trials unit to support development of drugs and vaccines aimed at tackling priority disease conditions in the country. ... During this inception years, CCR was a must visit site for the university of Nairobi Medical students to understand the value of research in drug ...
The objective of this study was to investigate the health research dissemination strategies used by Kenya Medical Research Institute (KEMRI) researchers. ... Other centres outside Nairobi include: Centre for Global Health Research (CGHR) in Kisumu County, Centre for Geographic Medicine Research - Coast (CGMR-C) in Kilifi County and Centre ...
These collaborations have expanded to eight countries under the U.S. Army Medical Research Directorate-Africa (USAMRD-A), which is headquartered on the campus of the Kenya Medical Research Institute (KEMRI) in Nairobi. WRAIR helped establish the KEMRI-Kericho Clinical Research Centre (CRC) 1999 in collaboration with KEMRI and HJF, and a new ...
The Chief Executive Officer, Kenyatta National Hospital, P.O. Box 20723-00202 Nairobi Tel. 020 2726300, 0709854000, 0730643000 Email: [email protected] Quick Links Frequently Asked Questions
Medscore Research Centre(Centre for medical research)) is a registered private firm that operates in collaboration with various researchers and healthcare facilities in Kenya to analyze infectious disease-related protocols, conduct data collection and publish baseline data in peer reviewed journals with a mission to help various stakeholders and promote attainment of the highest possible ...
Cancer Care International Nairobi, Kenya. To create a fulfilling environment where doctors and staff. can bring in the future of cancer care through cutting-edge patient care, clinical research and advanced technology; enabling patients to live longer and stronger lives. view services.
Apollo Hospitals Information Centre in Nairobi, Kenya, has earned a stellar reputation for its unparalleled excellence in cancer care. With a global legacy of delivering world-class healthcare, the Nairobi branch continues to set the standard for comprehensive and cutting-edge cancer treatments. Advanced Technology and Expertise.
The Centre for Microbiology Research (CMR) is one of the oldest research centres of the Kenya Medical Research Institute (KEMRI). CMR has many laboratories spread in four sub-centres located in Nairobi (Mbagathi and Kenyatta National Hospital complex), Kisumu and the coastal town of Kwale. The centre has a total of 37 scientific and 13 support ...
Medical oncology clinic. Telephone: +254 (0) 111 011 888 /+254 (0) 730 011 888 . Email: [email protected] . Me et our specialists. Professor Mansoor Saleh. Chair Department of Haematology-oncology and Consultant Medical Oncologist at Aga Khan University Hospital, Nairobi. Qualifications: M.D., University of Heidelberg, West Germany (1980)
Nairobi, Kenya — 3 Apr 2024. The Kenya Medical Research Institute (KEMRI) and the Drugs for Neglected Diseases initiative (DNDi) have signed a memorandum of understanding to collaborate in the execution of research and development (R&D) projects and bridge the existing research gaps on neglected diseases.
This paper is in the following e-collection/theme issue: Demographics of Users, Social & Digital Divide (651) E-Health Policy and Health Systems Innovation (192) Equity and Digital Divide (155) Use and User Demographics of mHealth (299) Health Care Quality and Health Services Research (209) Digital Health, Telehealth and e-Innovation in Clinical Settings (313)
Centre for Virus Research (CVR) is the oldest of the centers in KEMRI. ... Kenya Medical Research Institute (KEMRI) is a State Corporation established through the Science and Technology (Amendment) Act of 1979, which has since been amended to Science, Technology and Innovation Act 2013. ... Nairobi, Kenya. Center for Geographic Medicine ...
Modern machine learning approaches have led to performant diagnostic models for a variety of health conditions. Several machine learning approaches, such as decision trees and deep neural networks, can, in principle, approximate any function. However, this power can be considered to be both a gift and a curse, as the propensity toward overfitting is magnified when the input data are ...
Other examinations of available research have found similar results. A review of 27 scientific studies, published in 2021 in the Journal of the American Society of Plastic and Reconstructive ...
Kenya Medical Research Institute. Centre for global health research. P.O Box 20778-00202 Kisumu Tel: +254 020 2726781 +254 020 2722541, +254 020 2713349, 0722205901. 0733400003. ... Nairobi, Kenya. Center for Geographic Medicine Research, Coast P.O. Box 230-80108, Kilifi Kenya.