- What Is Back Pain?
- Tests & Diagnosis
- Living With
- View Full Guide

An Overview of Spondylolisthesis

What Is Spondylolisthesis?
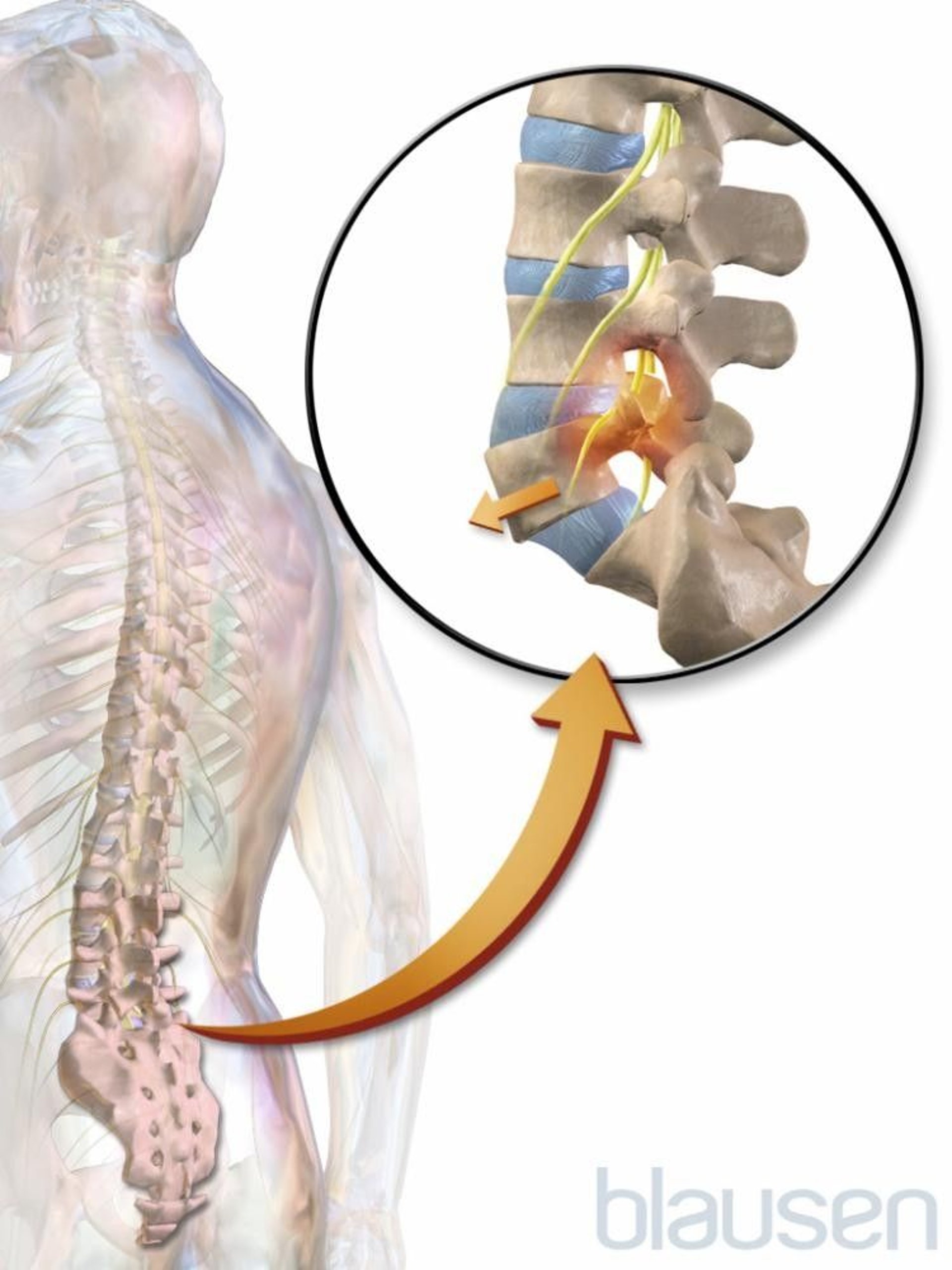
Spondylolisthesis (pronounced spahn-duh-low-liss-thee-sus) is a condition in which one of the bones in your spine (the vertebrae) slips out of place and moves on top of the vertebra next to it.
It usually happens at the base of your spine (lumbar spondylolisthesis). When the slipped vertebra puts pressure on a nerve, it can cause pain in your lower back or legs.
Spondylolisthesis Symptoms
Sometimes, people with this condition don't notice anything is wrong. But you can have symptoms that include:
- Lower back pain
- Muscle tightness and stiffness
- Pain in your buttocks
- Pain that spreads down your legs (due to pressure on nerve roots)
- Pain that gets worse when you move around
- Tight hamstrings (muscles in the back of your thighs)
- Trouble standing or walking
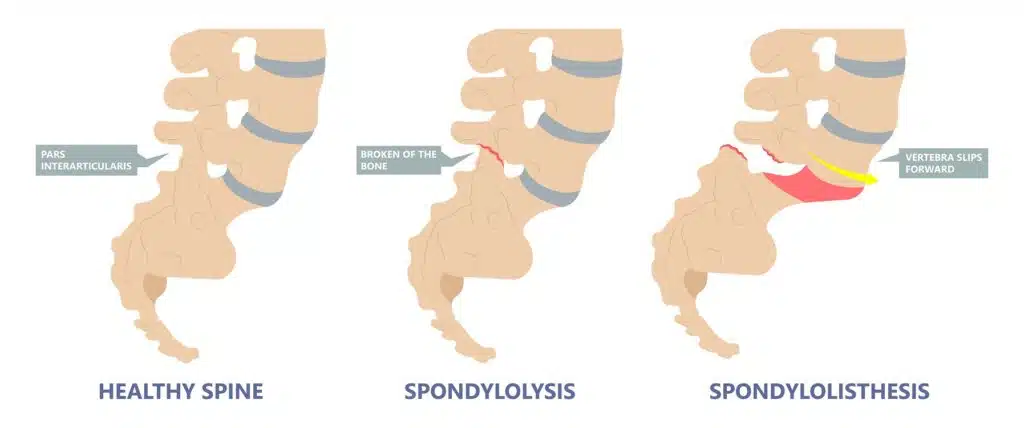
Spondylolisthesis vs. Spondylolysis
Spondylolysis (pronounced spahn-duh-loll-iss-us) and spondylolisthesis are different conditions of the spine, though they're sometimes related. Both conditions cause pain in your lower back .
Spondylolysis is a weakness or small fracture (crack) in one of your vertebrae. This usually affects your lower back, but it can also happen in the middle of your back or your neck. It's most often found in kids and teens, especially those involved in sports that repeatedly overstretch the lower spine, like football or gymnastics.
It's not uncommon for people with spondylolysis to also have spondylolisthesis. That's because the weakness or fracture in your vertebra may cause it to move out of place.
Types of Spondylolisthesis
Doctors divide this condition into six main types, determined by cause.
Degenerative spondylolisthesis: This is the most common type. As people age, the disks that cushion vertebrae can become worn, dry out, and get thinner. This makes it easier for the vertebra to slip out of place.
Isthmic spondylolisthesis: This type is caused by spondylosis. A crack in the vertebra can lead it to slip backward, forward, or over a bone below. It may affect kids and teens who do gymnastics, do weightlifting, or play football because they repeatedly overextend their lower backs. But it also sometimes happens when you're born with vertebrae whose bone is thinner than usual.
Congenital spondylolisthesis: Also known as dysplastic spondylolisthesis, this happens when your vertebrae are aligned incorrectly due to a birth defect.
Traumatic spondylolisthesis: In this type, an injury (trauma) to the spine causes the vertebra to move out of place.
Pathological spondylolisthesis: This type is caused by another spine condition, such as osteoporosis or a spinal tumor.
Postsurgical spondylolisthesis: Also called iatrogenic spondylolisthesis, this happens when a vertebra slips out of place after spinal surgery.
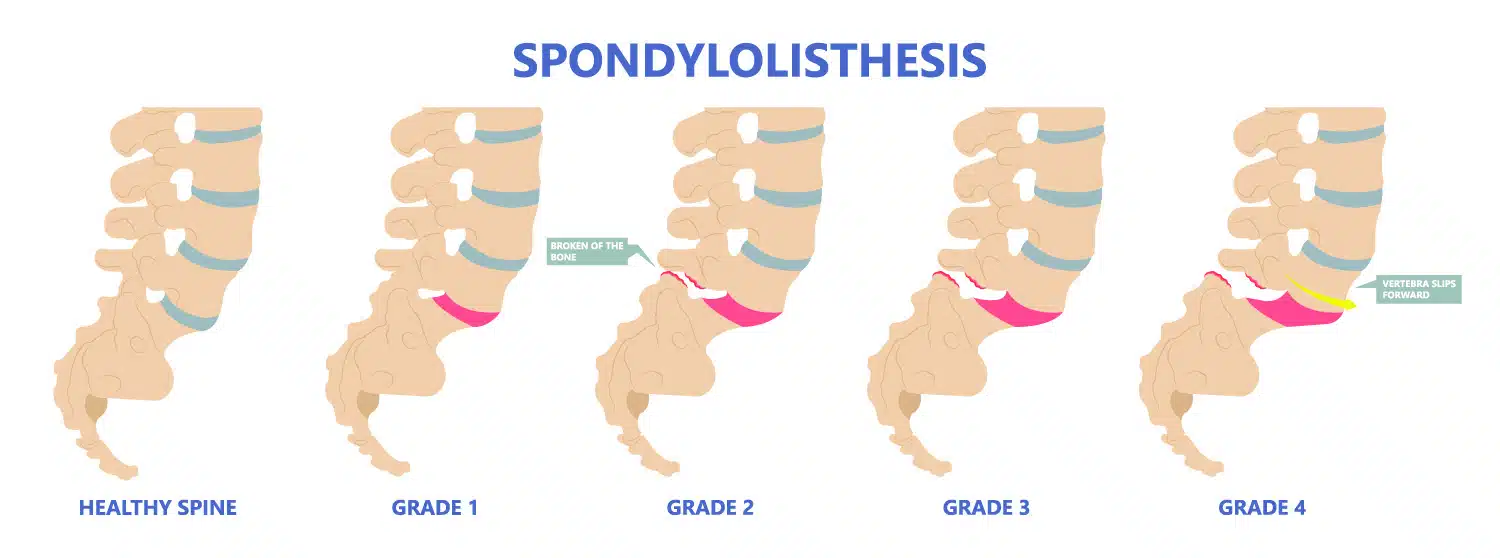
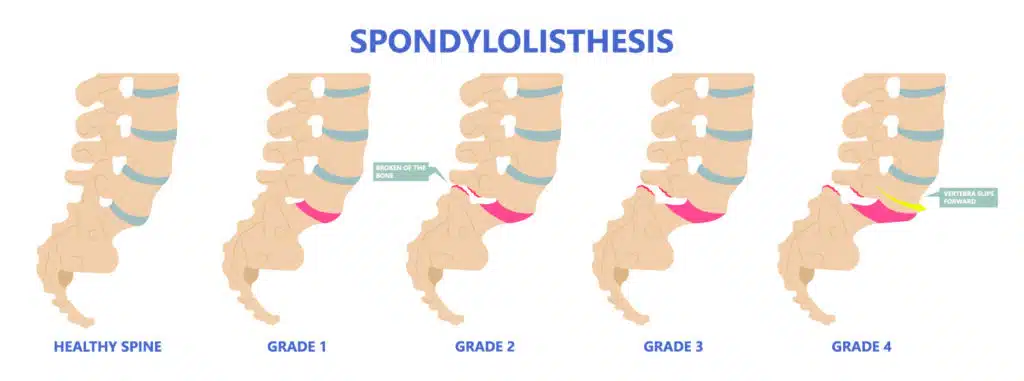
Grades of Spondylolisthesis
Your doctor may give your spondylolisthesis a grade based on how serious it is. The most common grading system is called Meyerding's classification and includes:
- Grade I : The most common grade, this is defined as 1%-25% slippage of the vertebra
- Grade II : Up to 50% slippage of the vertebra
- Grade III : Up to 75% slippage
- Grade IV : 76%-100% slippage
- Grade V : More than 100% slippage, also known as spondyloptosis
Grades I and II are considered low grade. Grades III and up are considered high grade.
Spondylolisthesis Causes and Risk Factors
Causes of spondylolisthesis include:
- Wear and tear with age
- Birth defects
- Spondylolysis
- Injury to the spine
- Another condition such as a spinal tumor or osteoporosis
- Spinal surgery
You're more likely to get this condition if you:
- Take part in sports that put stress on your spine
- Were born with thinner areas of vertebrae that are prone to breaking and slipping
- Are 50 or older
- Have a degenerative spinal condition
Spondylolisthesis Diagnosis
If your doctor thinks you might have this condition, they'll ask about your symptoms and run imaging tests to see if a vertebra is out of place. These tests may include:
These tests can also help your doctor determine a grade for your spondylolisthesis.
Spondylolisthesis Treatments
The treatment you'll need depends on what grade of spondylolisthesis you have, as well as your age, symptoms, and your medical history. Low grade can usually be treated with physical therapy or medications. With high grade, you may need surgery, especially if you're in a lot of pain.
Nonsurgical treatment options include:
- Rest : You may need to take some time off from sports and other vigorous activities.
- Medications : Your doctor may recommend over-the-counter anti-inflammatory medicines to relieve your pain, such as ibuprofen or naproxen.
- Injections : Steroid shots in the area where you have pain can bring relief.
- Physical therapy : Daily exercises that stretch and strengthen your supportive abdominal and lower back muscles can lower your pain.
- Braces : For children with fractures in the vertebrae (spondylolysis), a back brace can restrict movement so the fractures can heal.
Spondylolisthesis Surgery
If you have high-grade spondylolisthesis or if you still have serious pain and disability after nonsurgical treatments, you may need surgery. This usually means spinal decompression, often along with spinal fusion.
Spinal surgery is always done under general anesthesia , which means you're asleep during the operation.
Spinal decompression: Decompression lessens the pressure on the nerves in your spine to relieve pain. There are several techniques your surgeon can use to give your nerves more room. They may remove bone from your spine, take out part or all of a disk, or make the opening in your spinal canal larger. Your surgeon might need to use all these methods during your surgery.
Spinal fusion: In spinal fusion, the doctor joins, or fuses, the affected vertebrae together to prevent them from slipping again. After this surgery, you may have a bit less flexibility in your spine.
Pars repair: This surgery repairs fractures in the vertebrae using small wires or screws. Sometimes, a bone graft is used to reinforce the fracture so it can heal better.
After spinal surgery, you'll likely need to stay in the hospital for at least a day. Most people can go home within a week. You may be able to stand or even walk the day after the operation. You may go home with pain medication to ensure that your recovery is as easy as possible.
You'll need to limit physical activity for 8-10 weeks after your surgery so your spine can heal. But you should still move around and even walk every day. This can make your recovery go faster and help keep complications at bay.
Around 10-12 weeks after your surgery, you'll start physical therapy to stretch and strengthen your muscles and help you move more easily. Ideally, you should have physical therapy for a year.
For the first year after your surgery, you'll need to see your surgeon about every 3 months. You'll likely have X-rays taken at these follow-ups to make sure your spine is healing well.
Spondylolisthesis Complications
Serious spondylolisthesis sometimes leads to another condition called cauda equina syndrome . This is a serious condition in which nerve roots in part of your lower back called the cauda equina get compressed. It can cause you to lose feeling in your legs. It also can affect your bladder.
This is a medical emergency. If left untreated, cauda equina syndrome can lead to a loss of bladder control and paralysis.
See your doctor if you:
- Have trouble controlling your bladder or bowels
- Notice numbness or a strange sensation between your legs or on your buttocks, inner thighs, backs of your legs, feet, or heels
- Have pain or weakness in a leg or both legs that may cause stumbling
The symptoms may come on slowly and vary in how serious they are.
Spondylolisthesis Outlook
For most people, rest and nonsurgical treatments bring long-term relief within several weeks. But sometimes, spondylolisthesis comes back again after treatment. This happens more often when it was a higher grade.
If you've had surgery, you'll most likely do well afterward. Most people get back to normal activities within a few months. But your spine may not be as flexible as it was before.
Spondylolisthesis is when one of your vertebrae moves out of place. This sometimes leads to back pain and other symptoms. It can be usually treated with rest, medication, and/or physical therapy. But serious cases may require surgery.
Spondylolisthesis FAQs
What is the main cause of spondylolisthesis?
In adults, it most often happens when cartilage and bones in the spine become worn from conditions such as arthritis. It's more common in people age 50 and older. In kids and teens, the most common causes are either a spinal birth defect or injury to the spine.
Is spondylolisthesis a serious condition?
For most people, it's not serious. Many people have few symptoms or no symptoms at all. It's only a problem when it causes pain or limits your ability to move. If that happens, you'll need to see a doctor for treatment.
Top doctors in ,
Find more top doctors on, related links.
- Back Pain News
- Back Pain Reference
- Back Pain Slideshows
- Back Pain Quizzes
- Back Pain Videos
- Back Pain Medications
- Find a Neurologist
- Find a Pain Medicine Specialist
- WebMDRx Savings Card
- Ankylosing Spondylitis
- Drug Interaction Checker
- Osteoporosis
- Pain Management
- Pill Identifier
- Second Opinions
- SI Joint Pain
- More Related Topics
Spondylolisthesis: Definition, Causes, Symptoms, and Treatment
by Dave Harrison, MD • Last updated November 26, 2022
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Reddit (Opens in new window)
What is Spondylolisthesis?
The spine is comprised of 33 bones, called vertebra , stacked on top of each other interspaced by discs . Spondylolisthesis is a condition where one vertebra slips forward or backwards relative to the vertebra below. More specifically, retrolisthesis is when the vertebra slips posteriorly or backwards, and anterolisthesis is when the vertebra slips anteriorly or forward.
Spondylosis vs Spondylolisthesis
Spondylosis and Spondylolisthesis are different conditions. They can be related but are not the same. Spondylosis refers to a fracture of a small bone, called the pars interarticularis, which connects the facet joint of the vertebra to the one below. This may lead to instability and ultimately slippage of the vertebra. Spondylolisthesis, on the other hand, refers to slippage of the vertebra in relation to the one below.
Types and Causes of Spondylolisthesis
There are several types of spondylolisthesis, often classified by their underlying cause:
Degenerative Spondylolisthesis
Degenerative spondylolisthesis is the most common cause, and is due to general wear and tear on the spine. Overtime, the bones and ligaments which hold the spine together may become weak and unstable.
Isthmic Spondylolisthesis
Isthmic spondylolisthesis is the result of another condition, called “ spondylosis “. Spondylosis refers to a fracture of a small bone, called the pars interarticularis, which connects the facet joint of the vertebra to the one below. If this interconnecting bone is broken, it can lead to slippage of the vertebra. This can sometimes occur during childhood or adolsence but go unnoticed until adulthood when degenerative changes cause worsening slippage.
Congenital Spondylolisthesis
Congenital spondylolisthesis occurs when the bones do not form correctly during fetal development
Traumatic Spondylolisthesis
Traumatic spondylolisthesis is the result of an injury such as a motor vehicle crash
Pathologic Spondyloslisthesis
Pathologic spondylolisthesis is when other disorders weaken the points of attachment in the spine. This includes osteoporosis, tumors, or infection that affect the bones and ligaments causing them to slip.
Iatrogenic Spondylolisthesis
Iatrogenic spondylolisthesis is the result of a prior surgery. Some operations of the spine, such as a laminectomy, may lead to instability. This can cause the vertebra to slip post operatively.
Spondylolisthesis Grades
Spondylolisthesis is classified based on the degree of slippage relative to the vertebra below
- Grade 1 : 1 – 25 % forward slip. This degree of slippage is usually asymptomatic.
- Grade 2: 26 – 50 % forward slip. May cause mild symptoms such as stiffness and pain in your lower back after physical activity, but it’s not severe enough to affect your everyday activities.
- Grade 3 : 51 – 75 % forward slip. May cause moderate symptoms such as pain after physical activity or sitting for long periods.
- Grade 4: 76 – 99% forward slip. May cause moderate to severe symptoms.
- Grade 5: Is when the vertebra has slipped completely of the spinal column. This is a severe condition known as “spondyloptysis”.
Symptoms of Spondylolisthesis
Spondylolisthesis can cause compression of spinal nerves and in severe cases, the spinal cord. The symptoms will depend on which vertebra is affected.
Cervical Spondylolisthesis (neck)
- Arm numbness or tingling
- Arm weakness
Lumbar Spondylolisthesis (low back)
- Buttock pain
- Leg numbness or tingling
- Leg weakness
Diagnosing Spondylolisthesis
Your doctor may order imaging tests to confirm the diagnosis and determine the severity of your spondylolisthesis. The most common imaging tests used include:
- X-rays : X-rays can show the alignment of the vertebrae and any signs of slippage.
- CT scan: A CT scan can provide detailed images of the bones and soft tissues in your back, allowing your doctor to see any damage or abnormalities.
- MRI: An MRI can show the spinal cord and nerves, as well as any herniated discs or other soft tissue abnormalities.
Treatments for Spondylolisthesis
Medications.
For those experiencing pain, oral medications are first line treatments. This includes non-steroidal anti-inflammatory medications (NSAIDs) such as ibuprofen, acetaminophen, or in severe cases opioids or muscle relaxants (with extreme caution). Topical medications such as lidocaine patches are also sometimes used.
Physical Therapy
Physical therapy can help improve mobility and strengthen muscles around your spine to stabilize your neck and lower back. You may also receive stretching exercises to improve flexibility and balance exercises to improve coordination.
Surgery is reserved for severe cases of spondylolisthesis in which there is a high degree of instability and symptoms of nerve compression.
In these cases a spinal fusion may be necessary. This surgery joins two or more vertebra together using rods and screws, in order to improve stability.
Reference s
- Alfieri A, Gazzeri R, Prell J, Röllinghoff M. The current management of lumbar spondylolisthesis. J Neurosurg Sci. 2013 Jun;57(2):103-13. PMID: 23676859.
- Stillerman CB, Schneider JH, Gruen JP. Evaluation and management of spondylolysis and spondylolisthesis. Clin Neurosurg. 1993;40:384-415. PMID: 8111991.
About the Author
Dave Harrison, MD
Dr. Harrison is a board certified Emergency Physician with a part time appointment at San Francisco General Medical Center and is an Assistant Clinical Professor-Volunteer at the UCSF School of Medicine. Dr. Harrison attended medical school at Tufts University and completed his Emergency Medicine residency at the University of Southern California. Dr. Harrison manages the editorial process for SpineInfo.com.
- Degenerative Spondylolisthesis Symptoms
By: Marco Funiciello, DO, Physiatrist
Peer-Reviewed
Degenerative spondylolisthesis typically causes low back pain along with a cluster of symptoms and signs in one or both legs.
Degenerative Spondylolisthesis: Common Symptoms and Signs
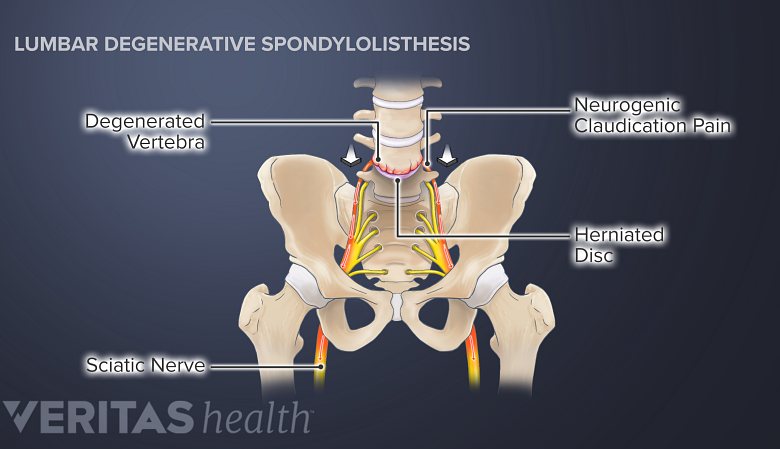
Degenerative spondylolisthesis symptoms include neurogenic claudication, sciatica, and radiculopathy.
In degenerative spondylolisthesis, the degenerated facet joints and other parts of the vertebral bone tend to increase in size. The enlarged, abnormal bone then encroaches upon the central canal and/or nerve hole (foramen) causing spinal stenosis or foraminal stenosis.
In This Article:
- Degenerative Spondylolisthesis
- Degenerative Spondylolisthesis Treatment
- Surgery for Degenerative Spondylolisthesis
Degenerative Spondylolisthesis Video
These changes typically result in some combination of the following symptoms and signs.
Persistent low back pain
Low back pain caused by degenerative spondylolisthesis is usually persistent and described as a consistent dull ache, 1 Cushnie D, Johnstone R, Urquhart JC, Gurr KR, Bailey SI, Bailey CS. Quality of Life and Slip Progression in Degenerative Spondylolisthesis Treated Nonoperatively. Spine (Phila Pa 1976). 2018;43(10):E574-E579. doi:10.1097/BRS.0000000000002429 but it may also feel like a sharp, stabbing sensation for some individuals.
The pain is typically localized in the low back region and may worsen with physical activity, standing, or walking.
Neurogenic claudication
Intermittent neurogenic claudication affects around 75% of people with degenerative spondylolisthesis. It is characterized by episodes of low back pain that radiate to both legs, along with accompanying sensations of tingling, a sensation of weakness, and hamstring spasm. 2 Li N, Scofield J, Mangham P, Cooper J, Sherman W, Kaye A. Spondylolisthesis. Orthop Rev (Pavia). 2022 Jul 27;14(4):36917. doi: 10.52965/001c.36917. PMID: 35910544; PMCID: PMC9329062. , 3 García-Ramos CL, Valenzuela-González J, Baeza-Álvarez VB, Rosales-Olivarez LM, Alpizar-Aguirre A, Reyes-Sánchez A. Degenerative spondylolisthesis I: general principles. Espondilolistesis degenerativa lumbar I: principios generales. Acta Ortop Mex. 2020;34(5):324-328.. , 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
It is possible to have any combination of symptoms and they typically occur during walking variable distances or prolonged standing. 2 Li N, Scofield J, Mangham P, Cooper J, Sherman W, Kaye A. Spondylolisthesis. Orthop Rev (Pavia). 2022 Jul 27;14(4):36917. doi: 10.52965/001c.36917. PMID: 35910544; PMCID: PMC9329062.
Sciatica: Radiating leg pain
Back pain may radiate into the buttock, thighs, and into the leg and foot. 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
Radiating leg pain is commonly known as sciatica . This pain occurs due to the irritation, compression, or inflammation of spinal nerve roots in the lower back. 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
Radiculopathy: Abnormal sensations, weakness, and loss of muscle reflexes
When the spinal nerve roots are compressed or sufficiently inflamed and neurologic deficits are present, the condition is called radiculopathy . Radiculopathy may cause leg weakness and affect muscle reflexes. Additionally, numbness may be felt in the thigh, leg, and/or foot. 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
It may be challenging to perform activities that require strength, such as walking, climbing stairs, or lifting objects.
Little Known Symptoms of Degenerative Spondylolisthesis
As degenerative spondylolisthesis progresses, the symptoms may lessen due to compensatory mechanisms of the spine that increase spinal stability and prevent further progression.
However, in some individuals, the progression may continue and cause the following symptoms and signs.
Sleep disturbances
Back pain and leg pain may cause disturbed sleep or trouble falling asleep. 5 Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J. 2008;17(3):327-335. doi:10.1007/s00586-007-0543-3
For this reason, some individuals may choose to sleep in the fetal position (sleeping on the side with knees bent close to the chest) to relieve leg symptoms. 5 Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J. 2008;17(3):327-335. doi:10.1007/s00586-007-0543-3
Restless leg syndrome
Leg pain and claudication may sometimes cause restless legs syndrome. In this condition, aching or burning pain in the calves causes an irresistible urge to move the legs continuously, causing disturbed sleep. 5 Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J. 2008;17(3):327-335. doi:10.1007/s00586-007-0543-3
Difficulty walking and imbalance
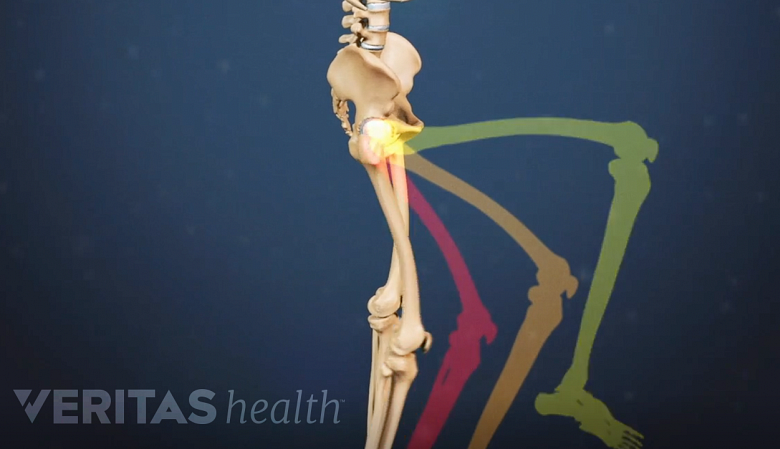
Degenerative spondylolisthesis may cause difficulty walking and maintaining balance.
As degenerative spondylolisthesis progresses, difficulties with walking and maintaining balance may be experienced. These signs arise from nerve compression caused by the slipped vertebra and associated degenerative changes, Altered posture, muscle weakness and reduced coordination may result. 6 Studnicka K, Ampat G. Lumbosacral Spondylolisthesis. [Updated 2022 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560679/
There are many nerves in our legs that are responsible for relaying information to the brain about position and balance. If these nerves are irritated or compressed in the spine then the brain may not get the necessary information needed for good balance and posture control.
These changes can impact mobility and function, making it harder to engage in normal daily activities.
Limited range of motion
Degenerative spondylolisthesis can affect lumbar range of motion due to the degenerative bone changes that prevent full segmental motion. Muscle spasm and stiffness may result.
Individuals may find it challenging to twist or engage in activities that involve spinal movement. This restricted range of motion can contribute to discomfort and stiffness in the affected area.
Menopause-Related Spondylolisthesis Symptoms
The onset of menopause may accelerate normal degenerative changes of the lumbar vertebrae, discs, facet joints, and ligaments. 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
Typically, the symptoms associated with this progression include low back pain, stiffness, and/or pain radiating down the leg (sciatica). 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
Read more about Sciatica Symptoms
Diagnosis of Degenerative Spondylolisthesis
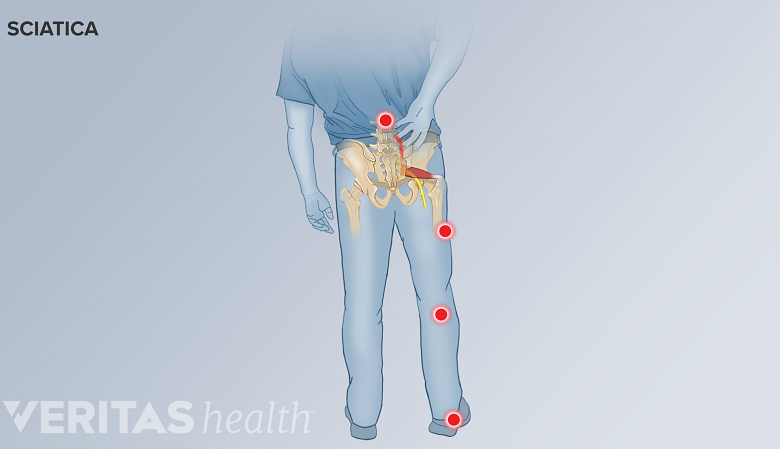
Radiating sciatica pain may occur in degenerative spondylolisthesis.
A physician trained in musculoskeletal conditions can help diagnose degenerative spondylolisthesis.
A comprehensive assessment of the patient’s history, past medical history, thorough physical examination, and review of any prior tests and imaging studies are performed.
During the review of patient history and the physical examination, physicians typically check for 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526 :
- Pain pattern. Physicians ask about localized or radiating pain and the pattern of pain distribution to check if sciatica is present.
- Postural effects. In degenerative spondylolisthesis, pain is exacerbated while bending backward and relieved when bending forward.
- History of symptoms. Neurogenic claudication and hamstring spasm while walking or standing for variable periods of time may indicate spinal stenosis caused by degenerative spondylolisthesis.
If these symptoms and signs are noticed, the physician may order imaging tests to further investigate the condition.
Imaging Tests for Degenerative Spondylolisthesis
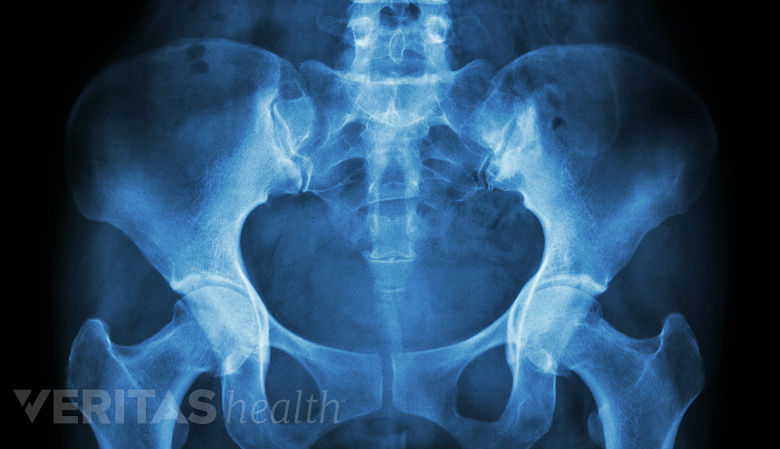
X-rays are helpful in diagnosing and assessing the extent of degenerative spondylolisthesis.
Imaging tests may help confirm the diagnosis of degenerative spondylolisthesis and provide evidence of the extent of progression of the condition.
- Standing lateral radiographs are considered the most reliable and standard test for diagnosing degenerative spondylolisthesis. 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526
- Flexion-extension radiographs are used to determine if there is any motion of one vertebra upon the other (translation) and/or instability during spinal movements. 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526
- Magnetic resonance imaging (MRI) scans may be used to check for spinal stenosis, nerve root compression, spinal cord involvement, and disc degeneration. 3 García-Ramos CL, Valenzuela-González J, Baeza-Álvarez VB, Rosales-Olivarez LM, Alpizar-Aguirre A, Reyes-Sánchez A. Degenerative spondylolisthesis I: general principles. Espondilolistesis degenerativa lumbar I: principios generales. Acta Ortop Mex. 2020;34(5):324-328.. , 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526 Some researchers consider MRI scans as the most reliable test to diagnose spinal stenosis in degenerative lumbar spondylolisthesis. 8 Matz PG, Meagher RJ, Lamer T, et al. North American Spine Society. Clinical Guidelines for Multidisciplinary Spine Care. Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. 2nd ed.; 2016.
- CT scans are used if bone involvement such as spondylolysis or isthmic spondylolisthesis is suspected, as these scans provide detailed evaluation of bone integrity.
If an MRI is not possible, computed tomography (CT) scans with myelography may be used as an alternative test. 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526 , 8 Matz PG, Meagher RJ, Lamer T, et al. North American Spine Society. Clinical Guidelines for Multidisciplinary Spine Care. Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. 2nd ed.; 2016.
MRI scans or CT scans may also be used if severe neurogenic claudication is present, bowel and/or bladder incontinence is reported, and/or tumors are suspected.
- 1 Cushnie D, Johnstone R, Urquhart JC, Gurr KR, Bailey SI, Bailey CS. Quality of Life and Slip Progression in Degenerative Spondylolisthesis Treated Nonoperatively. Spine (Phila Pa 1976). 2018;43(10):E574-E579. doi:10.1097/BRS.0000000000002429
- 2 Li N, Scofield J, Mangham P, Cooper J, Sherman W, Kaye A. Spondylolisthesis. Orthop Rev (Pavia). 2022 Jul 27;14(4):36917. doi: 10.52965/001c.36917. PMID: 35910544; PMCID: PMC9329062.
- 3 García-Ramos CL, Valenzuela-González J, Baeza-Álvarez VB, Rosales-Olivarez LM, Alpizar-Aguirre A, Reyes-Sánchez A. Degenerative spondylolisthesis I: general principles. Espondilolistesis degenerativa lumbar I: principios generales. Acta Ortop Mex. 2020;34(5):324-328..
- 4 Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2016;11:39-52. Published 2016 Dec 1. doi:10.1016/j.jot.2016.11.001
- 5 Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J. 2008;17(3):327-335. doi:10.1007/s00586-007-0543-3
- 6 Studnicka K, Ampat G. Lumbosacral Spondylolisthesis. [Updated 2022 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560679/
- 7 Akkawi I, Zmerly H. Degenerative Spondylolisthesis: A Narrative Review. Acta Biomed. 2022;92(6):e2021313. Published 2022 Jan 19. doi:10.23750/abm.v92i6.10526
- 8 Matz PG, Meagher RJ, Lamer T, et al. North American Spine Society. Clinical Guidelines for Multidisciplinary Spine Care. Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. 2nd ed.; 2016.
Dr. Marco Funiciello is a physiatrist with Princeton Spine and Joint Center. He has a decade of clinical experience caring for spine and muscle conditions with non-surgical treatments.
- Degenerative Spondylolisthesis Symptoms "> Share on Facebook
- Degenerative Spondylolisthesis Symptoms "> Share on Pinterest
- Degenerative Spondylolisthesis Symptoms "> Share on X
- Subscribe to our newsletter
- Print this article
- Degenerative Spondylolisthesis Symptoms &body=https://www.spine-health.com/conditions/spondylolisthesis/degenerative-spondylolisthesis-symptoms&subject= Degenerative Spondylolisthesis Symptoms "> Email this article
Editor’s Top Picks
Spondylolysis and spondylolisthesis, leg pain and numbness: what might these symptoms mean, sciatica symptoms, lumbar radiculopathy, isthmic spondylolisthesis symptoms.
Popular Videos

Sciatica Causes and Symptoms Video
Cervical Disc Replacement Surgery Video

Lower Back Strain Video

3 Gentle Stretches to Prevent Neck Pain Video
Undergoing a Spinal Fusion?
Learn how bone growth stimulation therapy can help your healing process
Sponsored by Orthofix
Health Information (Sponsored)
- Learn How Bone Growth Therapy Can Help You
- Don't Push Through the Pain: Contact the Experts at Cedars-Sinai
- Suffering from Lumbar Spinal Stenosis? Obtain Long Term Pain Relief
- Take the Chronic Pain Quiz
Spondylolisthesis
- Diagnosis |
- Treatment |
Spondylolisthesis is partial displacement of a bone in the lower back.
Injuries or a degenerative condition can cause this disorder.
Pain is felt in the low back and may travel down one or both legs.
The diagnosis is based on the results of imaging tests.
Treatment includes measures to relieve pain.
The spine (spinal column) consists of back bones (vertebrae) stacked one on top of another. In lumbar spondylolisthesis, a vertebrae in the lower back slips forward. This disorder usually occurs during adolescence or young adulthood (often in athletes). It is usually caused by a birth defect or an injury that causes fractures (breaks) in a part of the vertebra. If both sides of the vertebra are involved, the vertebra can then slip forward over the one below it. Spondylolisthesis can also occur in older adults, mainly as the result of degeneration of the discs between the vertebrae or osteoarthritis . People who develop spondylolisthesis as adults are at risk of developing lumbar spinal stenosis .
Symptoms of Spondylolisthesis
Mild to moderate spondylolisthesis may cause little or no pain, particularly in young people.
When pain occurs in adolescents, it is felt on only one side of the spine and may travel down a leg. The pain may accompany a fracture.
When pain occurs in adults, it is felt over a specific part of the spine and travels down both legs. In these cases, the pain results from a degenerative condition.
Pain is worsened by standing or leaning back. It can be accompanied by numbness, weakness, or both in the legs.
Diagnosis of Spondylolisthesis
Imaging tests
Doctors base the diagnosis of spondylolisthesis on imaging tests, usually x-rays taken of the lower spine.
Other imaging tests, such as magnetic resonance imaging (MRI) or sometimes computed tomography (CT), may be done.
Treatment of Spondylolisthesis
Measures to relieve pain and stabilize the spine
One to two days of bed rest may provide pain relief for people with spondylolisthesis. Longer bed rest weakens the core muscles and increases stiffness, thus worsening back pain and prolonging recovery. Sleeping in a comfortable position on a medium mattress is recommended. People who sleep on their back can place a pillow under their knees. People who sleep on their side should use a pillow to support their head in a neutral position (not tilted down toward the bed or up toward the ceiling). They should place another pillow between their knees with their hips and knees bent slightly if that relieves their back pain. People can continue to sleep on their stomach if they are comfortable doing so.
Applying cold (such as ice packs) or heat
Physical therapy and exercises to strengthen and stretch the muscles in the abdomen, buttocks, and back (the core muscles) may help. (See also Low Back Pain: Prevention .)

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences

Spondylolisthesis
Spondylolisthesis is where one of the bones in your spine, called a vertebra, slips forward. It can be painful, but there are treatments that can help.
It may happen anywhere along the spine, but is most common in the lower back.
Check if you have spondylolisthesis
The main symptoms of spondylolisthesis include:
- pain in your lower back, often worse when standing or walking and relieved when sitting or bending forward
- pain spreading to your bottom or thighs
- tight hamstrings (the muscles in the back of your thighs)
- pain, numbness or tingling spreading from your lower back down 1 leg ( sciatica )
Spondylolisthesis does not always cause symptoms.
Spondylolisthesis is not the same as a slipped disc . This is when the tissue between the bones in your spine pushes out.
Non-urgent advice: See a GP if:
- you have lower back pain that does not go away after 3 to 4 weeks
- you have pain in your thighs or bottom that does not go away after 3 to 4 weeks
- you're finding it difficult to walk or stand up straight
- you're worried about the pain or you're struggling to cope
- you have pain, numbness and tingling down 1 leg for more than 3 or 4 weeks
What happens at your GP appointment
If you have symptoms of spondylolisthesis, the GP may examine your back.
They may also ask you to lie down and raise 1 leg straight up in the air. This is painful if you have tight hamstrings or sciatica caused by spondylolisthesis.
The GP may arrange an X-ray to see if a bone in your spine has slipped forward.
You may have other scans, such as an MRI scan , if you have pain, numbness or weakness in your legs.
Treatments for spondylolisthesis
Treatments for spondylolisthesis depend on the symptoms you have and how severe they are.
Common treatments include:
- avoiding activities that make symptoms worse, such as bending, lifting, athletics and gymnastics
- taking anti-inflammatory painkillers such as ibuprofen or stronger painkillers on prescription
- steroid injections in your back to relieve pain, numbness and tingling in your leg
- physiotherapy to strengthen and stretch the muscles in your lower back, tummy and legs
The GP may refer you to a physiotherapist, or you can refer yourself in some areas.
Waiting times for physiotherapy on the NHS can be long. You can also get it privately.
Surgery for spondylolisthesis
The GP may refer you to a specialist for back surgery if other treatments do not work.
Types of surgery include:
- spinal fusion – the slipped bone (vertebra) is joined to the bone below with metal rods, screws and a bone graft
- lumbar decompression – a procedure to relieve pressure on the compressed spinal nerves
The operation is done under general anaesthetic , which means you will not be awake.
Recovery from surgery can take several weeks, but if often improves many of the symptoms of spondylolisthesis.
Talk to your surgeon about the risks and benefits of spinal surgery.
Causes of spondylolisthesis
Spondylolisthesis can:
- happen as you get older – the bones of the spine can weaken with age
- run in families
- be caused by a tiny crack in a bone (stress fracture) – this is more common in athletes and gymnasts
Page last reviewed: 01 June 2022 Next review due: 01 June 2025

- Our Reviews
- Our Hospitals and Treatment Centers
- Neurologist
- Atypical Face Pain
- Carpal Tunnel Syndrome
- Complex Regional Pain Syndrome
- Degenerative Disc Disease
- Failed Back Surgery
- Fibromyalgia
- Headaches and Migraines
- Minimally Invasive Spine Surgery
- Muscle Spasms
- Pancreatitis
- Pelvic Pain
- Peripheral Neuropathy
- Peripheral Vascular Disease
- Phantom Limb Pain
- Post-Operative Pain
- Anterior Cervical Discectomy And Fusion
- Caudal Epidural With Lysis Of Adhesions
- Electroencephalography
- Electromyography and Nerve Conduction Velocity Studies
- Epidural Steroid Injections
- Facet Injections
- IFuse Implant System
- Intrathecal Pump
- Kyphoplasty
- Laminectomy And Fusion
- Medial Branch Blocks and Neurotomies
- Microdiscectomy
- Pain Management
- Peripheral Field Stimulators
- Selective Nerve Root Blocks
- Small And Large Joint Injections
- Patient Portal
- Accepted Insurance
- Data Breach Notification
- Notice of Privacy Practices
Spondylolisthesis: Understanding Causes, Symptoms & Treatment
Are you experiencing lower back pain that won't go away? Have you or a loved one recently been diagnosed with spondylolisthesis? If so, you're not alone. Spondylolisthesis is a common condition that affects the spine, and understanding its causes, symptoms, and treatment is crucial for managing and improving your quality of life.
This blog post will explore everything you need about spondylolisthesis, including its various forms, underlying causes, and effective treatment options. So, whether you're dealing with this condition or simply looking to educate yourself on this joint spine issue, keep reading to understand better spondylolisthesis and how to address it effectively.

What is Spondylolisthesis?
Spondylolisthesis is a common condition that affects the spine and can cause discomfort and pain for those with it. It occurs when one vertebra (bone in the spine) slips forward over another vertebra, causing the spinal column to become misaligned. This condition can affect people of all ages, but it is most commonly seen in adults over 50 .
What is the root cause of Spondylolisthesis?
The most common cause of spondylolisthesis is a fracture or defect in the pars interarticularis , a small bony section of the vertebra. This fracture can be caused by repetitive stress due to sports or activities that pressure the spine, such as weightlifting, gymnastics, or football. It can also happen due to congenital conditions or degenerative diseases like arthritis. Sometimes, spondylolisthesis can be caused by sudden trauma, such as a car accident or a fall.
What are the signs and symptoms of Spondylolisthesis?

The symptoms of spondylolisthesis vary depending on the severity of the condition. In mild cases, there may be no noticeable symptoms, but as the condition progresses, symptoms may include:
- Lower back pain
- Muscle spasms in the back
- Stiffness in the back
- Numbness or tingling in the legs
- Difficulty standing or walking
- Decreased range of motion in the back
- Weakness in the legs
How do you stop spondylolisthesis from progressing?
How exactly do you stop spondylolisthesis from worsening? There are practical strategies for managing and halting the progression of spondylolisthesis. Get ready to take control of your spinal health and stop spondylolisthesis in its tracks.
- Exercise regularly – Regular exercise helps to strengthen the muscles in your back and abdomen, providing better support for your spine. However, if you have spondylolisthesis, some exercises may be harmful. Consult a physical therapist to create a safe, individualized exercise plan for your condition.
- Avoid high-impact activities – Jumping and landing on the feet, such as running or basketball, can put additional stress on the spine. Instead, opt for low-impact exercises like swimming or cycling.
- Practice good posture – Poor posture can contribute to spondylolisthesis. Make a conscious effort to maintain good posture throughout the day, whether sitting, standing, or bending over. Consider using a lumbar support cushion if you spend much time sitting.
- Lose weigh t – Being overweight stresses the spine, which can worsen spondylolisthesis. Maintaining a healthy weight can help ease symptoms and stop the condition from progressing.
- Avoid lifting heavy objects – Putting strain on the lower back can worsen spondylolisthesis. If you need to lift heavy objects, use proper lifting techniques, such as bending your knees and keeping your back straight.
- Consider chiropractic care – Chiropractic manipulation and adjustments can help improve joint function and decrease pain in spondylolisthesis patients.
- Seek medical treatment – If you have persistent symptoms of spondylolisthesis, it's crucial to seek medical treatment. Your doctor may recommend physical therapy, pain medication, or in severe cases, surgery.
What are the 5 stages of spondylolisthesis?
Understanding the stages of spondylolisthesis is essential to identify its severity and manage it effectively. These are the five stages of spondylolisthesis and the accompanying symptoms.
Stage 1: Grade 1 Spondylolisthesis
The first stage of spondylolisthesis is also known as mild spondylolisthesis and is characterized by the slippage of less than 25% of one vertebra over another. In this stage, the symptoms may be minimal, and most people may not experience any. However, some common symptoms of grade 1 spondylolisthesis include mild back pain, stiffness, and muscle tightness in the lower back.
Stage 2: Grade 2 Spondylolisthesis
Grade 2 spondylolisthesis is characterized by the slippage of 26% to 50% of one vertebra over another. At this stage, the symptoms can become more noticeable, including increased back pain, numbness or tingling in the legs or feet, and difficulty standing or walking for extended periods. This stage may also lead to changes in posture and decreased flexibility in the lower back.
Stage 3: Grade 3 Spondylolisthesis
In this stage, the slippage increases to 51% to 75% of one vertebra over another. At this point, the spinal deformity may become apparent. Patients may experience severe back pain that radiates to the hips and legs, making it difficult to perform daily activities. Nerve compression is also standard in this stage, leading to symptoms like weakness, numbness, and tingling in the legs.
Stage 4: Grade 4 Spondylolisthesis
Grade 4 spondylolisthesis is characterized by the slippage of more than 75% of one vertebra over another. This stage can be severely debilitating, causing extreme back pain, nerve compression, and even difficulty in controlling bladder and bowel movements. Patients may also experience weakness and numbness in the legs, making it challenging to walk or stand for extended periods.
Stage 5: Grade 5 Spondylolisthesis
The final stage of spondylolisthesis, grade 5, is also known as spondyloptosis. In this stage, the slippage is more than 100% of one vertebra over another, meaning the vertebra has completely slipped off the one below it. At this point, the spinal deformity is severe and can lead to life-altering symptoms, including severe back pain, nerve damage, and loss of motor control in the legs.
Treatment options for Spondylolisthesis
Various treatment options for spondylolisthesis can help manage and relieve its symptoms. Let’s explore these treatment options and how they can help those with spondylolisthesis.
- Physical therapy:
Physical therapy is often the first line of treatment for spondylolisthesis. A physical therapist will work with the patient to strengthen the muscles in the back and abdomen, which can help stabilize the spine and prevent further slippage. They will also teach the patient proper posture and body mechanics to reduce pressure on the affected area. Physical therapy can also include exercises to increase flexibility and range of motion, which can help alleviate pain and stiffness.
- Medications:
Over-the-counter pain relievers such as ibuprofen and acetaminophen can help manage the pain caused by spondylolisthesis. Sometimes, a doctor may prescribe more vital pain medication or muscle relaxants if the pain is severe. However, these medications should only be used under the supervision of a doctor and are not a long-term solution for managing the condition.
- Bracing:
In some cases, a back brace may be recommended to provide support and stability to the affected area. This can help alleviate pain and prevent further slippage. It is crucial to work with a physical therapist to ensure the proper fit and usage of the brace.
- Steroid injections:
If other treatment options do not provide enough relief, a doctor may recommend steroid injections. These injections can help reduce inflammation and pain in the affected area. They are generally used as a short-term solution and may need to be repeated periodically.
- Surgery:
In severe cases of spondylolisthesis, surgery may be required. The most common surgery for this condition is spinal fusion, where the affected vertebrae are fused together to prevent slippage. This surgery can help alleviate pain and prevent further damage to the spine and nerves.
Get lasting relief from Spondylolisthesis!
Ready to take control of your Spondylolisthesis and find lasting relief? Look no further than Neuro Spine & Pain Center - your top choice for comprehensive treatment and expert care for Miami pain management .
Our team of renowned spine specialists in Miami understands the complexity of Spondylolisthesis and is dedicated to creating personalized treatment plans to address its underlying causes. From advanced imaging techniques to cutting-edge therapies, we have the tools to help you overcome this condition and live your life to the fullest.
Don't let Spondylolisthesis hold you back any longer, schedule a consultation with our experts today and let us guide you towards a pain-free and active lifestyle.

The material on this site is for informational purposes only and DOES NOT CONSTITUTE THE PROVIDING OF MEDICAL ADVICE, and is not intended to be a substitute for independent professional medical judgment, advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions or concerns you may have regarding your health.
Patient center
FAQ Notice of Privacy Practices Data Breach Notification Accepted Insurance
Usefull Links
Home Referring Providers About Us Contact Us
Copyright © 2023 Neuro Spine and Pain Center

- See All Locations
- Primary Care
- Urgent Care Facilities
- Emergency Rooms
- Surgery Centers
- Medical Offices
- Imaging Facilities
- Browse All Specialties
- Diabetes & Endocrinology
- Digestive & Liver Diseases
- Ear, Nose & Throat
- General Surgery
- Neurology & Neurosurgery
- Obstetrics & Gynecology
- Orthopaedics
- Pain Medicine
- Pediatrics at Guerin Children’s
- Urgent Care
- Medical Records Request
- Insurance & Billing
- Pay Your Bill
- Advanced Healthcare Directive
- Initiate a Request
- Help Paying Your Bill
Spondylolisthesis
Spondylolisthesis is a displacement of a vertebra in which the bone slides out of its proper position onto the bone below it. Most often, this displacement occurs following a break or fracture.
Surgery may be necessary to correct the condition if too much movement occurs and the bones begin to press on nerves.
Other complications may include:
- Chronic back pain
- Sensation changes
- Weakness of the legs
- Temporary or permanent damage of spinal nerve roots
- Loss of bladder control
When a vertebra slips out of proper alignment, discs can be damaged. To surgically correct this condition, a spinal surgeon removes the damaged disc. The slipped vertebra is then brought back into line, relieving pressure on the spinal nerve.
Types of spondylolisthesis include:
- Dysplastic spondylolisthesis , caused by a defect in part of the vertebra
- Isthmic spondylolisthesis , may be caused by repetitive trauma and is more common in athletes exposed to hyperextension motions
- Degenerative spondylolisthesis , occurs with cartilage degeneration because of arthritic changes in the joints
- Traumatic spondylolisthesis , caused by a fracture of the pedicle, lamina or facet joints as a result of direct trauma or injury to the vertebrae
- Pathologic spondylolisthesis , caused by a bone defect or abnormality, such as a tumor
Symptoms may vary from mild to severe. In some cases, there may be no symptoms at all.
Spondylolisthesis can lead to increased lordosis (also called swayback), and in later stages may result in kyphosis, or round back, as the upper spine falls off the lower.
Symptoms may include:
- Lower back pain
- Muscle tightness (tight hamstring muscle)
- Pain, numbness or tingling in the thighs and buttocks
- Tenderness in the area of the vertebra that is out of place
- Weakness in the legs
- Stiffness, causing changes in posture and gait
- A semi-kyphotic posture (leaning forward)
- A waddling gate in advanced cases
- Lower-back pain along the sciatic nerve
- Changes in bladder function
Spondylolisthesis may also produce a slipping sensation when moving into an upright position and pain when sitting and trying to stand.
Spondylolisthesis may appear in children as the result of a birth defect or sudden injury, typically occurring between the fifth bone in the lower back (lumbar vertebra) and the first bone in the sacrum (pelvis).
In adults, spondylolisthesis is the result of abnormal wear on the cartilage and bones from conditions such as arthritis , trauma from an accident or injury, or the result of a fracture, tumor or bone abnormality.
Sports that place a great deal of stress on bones may cause additional deterioration, fractures and bone disease, which may cause the bones of the spine to become weak and shift out of place.
A simple X-ray of the back will show any cracks, fractures or vertebrae slippage that are the signs of spondylolisthesis.
A CT scan or an MRI may be used to further diagnose the extent of the damage and possible treatments.
Treatment for spondylolisthesis will depend on the severity of the vertebra shift. Stretching and exercise may improve some cases as back muscles strengthen.
Non-invasive treatments include:
- Heat/Ice application
- Pain medicine (Tylenol and/or NSAIDS)
- Physical therapy
- Epidural injections
Surgery may be needed to fuse the shifted vertebrae if the patient has:
- Severe pain that does not get better with treatment
- A severe shift of a spine bone
- Weakness of muscles in a leg or both legs
Surgical process realigns the vertebrae, fixing them in place with a small rod that is attached with a pedicle screw, adding stability to the spine with or without the addition to an interbody (bone graft or cage) placed between the vertebra from the side or front.
Choose a doctor and schedule an appointment.
Get the care you need from world-class medical providers working with advanced technology.
Cedars-Sinai has a range of comprehensive treatment options.
(1-800-233-2771)
Available 7 days a week, 6 am - 9 pm PT
Expert Care for Life™ Starts Here
Looking for a physician.

Advanced non-surgical solutions to relieve pain and discomfort.
Applying modern technology to provide patients with safer, more precise, and minimally invasive procedures.

Preserving full range of motion for patients through Disc Replacement Surgery.
A highly customized option that combines Disc Replacement and traditional Spinal Fusion Surgery.
Utilizing the body’s natural healing process to rebuild damaged tissue and heal injuries.

Utilizing your stem cells to naturally promote healing.
Harnessing your blood’s healing properties to overcome pain.
Using your microfragmented adipose tissue to resolve pain and discomfort.
Promoting natural healing for ligaments and joints.
Naturally restoring hair loss & rejuvenating skin with PRP.

Our care for you doesn’t end after treatment, discover how we support you through the recovery process.
One-on-one manual therapy performed by licensed therapists.
Minimizes stress on joints while strengthening and improving flexibility and balance.

Comprehensive hands-on treatment from your licensed physical therapist
An effective technique to relieve muscle knots, ease tension, reduce pain, and increase mobility.

Take your game to the next level with sport-specific strength and agility training.
Our in-house neurology helps to ensure quick diagnosis and treatment
Non-surgical solutions for athletic pain and injuries – including most orthopedic conditions.
- Physician Assistants
- Physical Therapists
- Patient Victories
- Conditions We Treat
- In The Media
- Research Outcomes
- Patient Portal
- Medical Records Request Form
- Patient Forms
- Financial Services
Spondylolisthesis
UNDERSTANDING THE SYMPTOMS, CAUSES AND TREATMENTS
Understanding Spondylolisthesis
Spondylolisthesis is a medical diagnosis to describe the forward slippage of one vertebral body in relation to the vertebra below. The spine is made of several motion segments stacked on top of one another to allow for smooth movement in all directions. Each of these segments has three major points of contact including two facet joints and an intervertebral disc. If the facet joint and intervertebral discs degenerate or experience trauma this could lead to abnormal motion and misalignment. Another common cause of spondylolisthesis often diagnosed and treated by the specialists at VSI is a stress fracture in the vertebra.
Symptoms of spondylolisthesis depend on the severity of slippage. Symptoms can include pain, discomfort, stiffness, or muscle spasms in the low back. Symptoms of radiculopathy may appear including numbness, tingling, pain, or weakness in the legs. If the slippage is severe and causes detrimental pressure on the spinal nerves, you may develop symptoms of cauda equina syndrome. These include numbness in the groin area or down the legs, loss of bowel or bladder control, urinary urgency, or difficulty with balance or walking. Cauda equina is a spinal emergency and if you are experiencing these symptoms seek immediate evaluation.
When to Seek Treatment
If you’re noticing symptoms associated with Spondylolisthesis and suspect a spinal issue, it’s crucial to consider consulting a board-certified spinal specialist. Reach out promptly to a certified spine surgeon for an accurate diagnosis and timely treatment. Early intervention can significantly improve your overall well-being and provide a broader range of treatment options, which may decrease as symptoms persist. The key to a successful and speedy recovery lies in addressing the root of the pain with your spine specialist as soon as symptoms arise.
While many people experience day-to-day back or neck pain, dismissing it as soreness, this may not be the case for everyone. If your pain persists for more than 10 days, it should be taken more seriously. Evaluate such prolonged pain with a spine surgeon to identify the root issue and determine the appropriate treatment. Additionally, be attentive to other signs related to back or neck pain that should not be ignored, including pain accompanied by fever, pain associated with loss of bladder control, and weakness/tingling/numbness in your arms or legs.
It’s important to note that these are general guidelines based on our expertise in spine care over the past three decades, recognizing that each patient’s symptoms may be unique.

Common Causes
The spine is made up of bones, discs, soft tissues, and nerves. There are 7 cervical, 12 thoracic, and 5 lumbar vertebral bodies to make up the spinal column. Each of these vertebral bodies is stacked one on top of another. Between the vertebral bodies are intervertebral discs which act as the shock absorbers of the spine which naturally degenerate as we age. There are two different types of spondylolisthesis: degenerative and isthmic.
A pars fracture also known as spondylolysis is a fracture of the pars interarticularis. Isthmic spondylolisthesis is the medical term for slippage due to this type of fracture. In non-medical terms, this means the fracture caused instability, and over time the vertebral body slipped forward. This type of fracture may be the result of direct trauma or from a genetic weakness in this area of the bone and commonly occurs in adolescence.
Degenerative spondylolisthesis is caused by arthritic changes to the facet joints or degeneration of the intervertebral disc. With degenerative disc disease, intervertebral discs progressively break down. The discs lose hydration, there’s a decrease in disc height and function. They are no longer able to provide good structural stability. Recall facet joints are part of the three-joint complex stabilizing the spine. As the facet joints degenerate small ligaments supporting the joint wear down and loosen. This laxity allows the joint to separate more often contributing to a slippage or spondylolisthesis.
Diagnosing Spondylolisthesis
To properly diagnose a spondylolisthesis, weight-bearing x-rays are required. Typical imaging studies include AP, lateral, flexion, and extension lumbar views. These vital imaging studies allow your spinal specialists not only to diagnose a spondylolisthesis but also to grade the severity of this slippage. There are 5 different grades of spondylolisthesis and the higher the grade the increased risk of neurologic symptoms.
Treatment Options
There are many different treatment options for spondylolisthesis depending on the severity of the patient’s symptoms and the degree of slippage. Often, non-operative treatments are started initially. These include spine-specialized physical therapy, core strengthening, manual massage, dry needling, low-impact exercise, and maintaining an overall healthy lifestyle.
If patients fail non-operative treatment or are developing worsening neurologic symptoms, surgery is often discussed. There are many different approaches for surgical intervention which your spinal specialist at VSI will discuss with you in detail. The goal of surgical intervention is to stabilize the spine, alleviate pressure from the nerves, and correct any structural deformity. Every surgical plan is personalized to our patients depending on his or her symptoms or spinal needs.
Frequently Asked Questions about Spondylolisthesis
Does a spondylolisthesis always cause pain.
No, often mild degrees of slippage are asymptomatic meaning they cause no pain. Often, patients do not realize they have a spondylolisthesis until x-ray imaging confirms this diagnosis. If the spondylolisthesis worsens over time patients are more likely to experience symptoms.
Does a Spondylolisthesis get worse over time?
Life is a degenerative process. It is normal for joints to become arthritic or discs to degenerate as we use our back. If you are diagnosed with spondylolisthesis, especially at a young age, this does tend to worsen over time. Therefore following up with your spinal specialist at VSI is important to continue monitoring your physical exam for any changes in strength or reflexes and x-ray imaging to assess for any progression of the spondylolisthesis.
What activities should I avoid doing if I have a Spondylolisthesis?
High impact activities that put the spine at risk for trauma should be avoided in someone with a Spondylolisthesis. Building strong abdominal core muscles and maintaining an active healthy lifestyle are extremely important to aid in the stability of your spine. Activities to avoid include extreme impact sports, heaving weight lifting, diving, gymnastics, etc. unless cleared by a medical practitioner.
Can a Spondylolisthesis be reversed without surgery?
No, unfortunately spondylolisthesis is a structural instability of the spine. Non-operative treatments such as physical therapy and building core muscle strength aid in the stability of your spine and can decrease symptoms but they cannot change the underlying structural issue.
Get The Solutions You’ve Been Looking For
Reviewed by: Dr. Christopher Good, Dr. Colin Haines, Dr. Ehsan Jazini, Dr. Thomas Schuler, Dr. William Kemp

What Is Spondylolisthesis?
What to do about your slipped vertebra.
When you describe someone who’s tough, sturdy, and strong, people often say that they have a “backbone.” Or if you’re discussing the unshakable core of something, you also might use the word “backbone.” But as it turns out, those metaphors for the spine might not be as realistic as you may think.
Like any part of the body, your spine is prone to its fair share of unsteadiness. Specifically, a condition called spondylolisthesis in which a vertebra moves and slips out of place, causing intense lower back pain among other symptoms. The once stable spinal column is anything but as a result, and it can lead to complications.
The Cleveland Clinic reports that around 4% to 6% of adults live with this condition.

Spondylolisthesis occurs when one vertebra slips forward over the vertebra below it. The term is pronounced spon-duh-low-liss-thee-sis and is derived from the Greek language: spondylo means vertebra and listhesis means to slip .
There are several types or causes of spondylolisthesis ; a few are listed below.
Congenital spondylolisthesis means the disorder is present at birth.
Isthmic spondylolisthesis occurs when a defect called a pars fracture occurs in a bony supporting vertebral structure at the back of the spine called the pars interarticularis.
Degenerative spondylolisthesis is more common and is often associated with degenerative disc disease , wherein the discs (e.g., due to the effects of growing older) lose hydration and resiliency and provide less protection.
Any vertebra may slip out of place, but spondylolisthesis tends to be the most common in the lower back. With the vertebrae out of place and without proper positioning, the entire spinal column is out of whack, which can lead to problems if it’s not addressed.
Spondylolisthesis Symptoms
Each form of this condition shares similar symptoms. But often, spondylolisthesis doesn’t present any symptoms at all—it’s asymptomatic, and it may take years to finally develop any symptoms.
These symptoms can include:
Lower back pain
Pain that extends to the buttocks and thighs
Pain that worsens with activity
Stiff muscles, which can include tight hamstrings or muscle spasms in the hamstrings
Difficulty with standing or walking
Tired feeling, tingling, numbness, or weakness in legs
Curvature of the spine, also known as kyphosis
The symptoms show up differently in each person. While one person may complain of low back pain , another may primarily feel pain in their legs.
There are some complications that may arise from spondylolisthesis. The pain in one’s low back and legs can become chronic, along with feelings of numbness, weakness, or tingling. Additionally, infection may occur, or the spinal nerves can be permanently damaged. A less common result of spondylolisthesis can be loss of bladder or bowel control.
How Spondylolisthesis May Develop
The spine is connected to everything else in the body in one way or another. It really carries the brunt of every move we make, from walking to jumping to twisting to micromovements you barely notice.
Medically speaking, this means that the lumbar spine , or the lower region of your spinal column, is constantly exposed to directional pressures while it carries, absorbs, and distributes most of your body's weight at rest and during activity.
In other words, while your lumbar spine is carrying and absorbing body weight, it also moves in different directions (e.g., rotate, bend forward). Sometimes, this combination causes excessive stress to the vertebra and/or its supporting structures (which may already be weak) and may lead to a vertebral body slipping forward over the vertebra beneath.
For a visual, picture riding on a horse. If the horse gets wet or is sweating, the saddle may slip forward more easily. The saddle is on an unstable surface, which causes it to continually slip forward. This would make for a very unsteady riding experience, and the same happens with the spine when spondylolisthesis occurs.
Some people can be more at risk for developing spondylolisthesis than others. For instance, if a family member has spondylolisthesis, your risk for developing the disorder may be greater.
Some activities make you more susceptible to spondylolisthesis. Gymnasts, linemen in football, and weight lifters all put significant pressure and weight on their low backs. Spondylolisthesis can develop as a result of repeated excessive strains and stress.
Degenerative spondylolisthesis is more common in those older than 50 and tends to be diagnosed more often in women than men. Bone disease and fractures —also more common as you age—can additionally lead to spondylolisthesis. Health-wise, a tumor can also result in spondylolisthesis. On the flip side, children can experience spondylolisthesis through a birth defect, athletics (particularly in kids who have overextended their spines), or traumatic injury.
Causes of Spondylolisthesis
There are multiple causes of spondylolisthesis. However, there is a growing consensus that it’s typically the result of instability of the spine in general, and specifically the lumbar spine, the most common type of spondylolisthesis. This spinal instability often leads to pain that radiates to the affected limb.
When the spine is unstable, daily repetitive motions and acute stresses associated with sports or work fatigue the soft tissue structures ( muscles , tendons , and ligaments ) that keep the spine aligned and fully functional. As a result of this instability, the vertebrae become unstable and begin to slip. Then, spondylolisthesis can develop.
Another cause of spondylolisthesis is the development of spinal osteoarthritis . Osteoarthritis causes vertebral bones to degenerate, leading to facet joints that slowly lose their normal structural support. This results in slippage. As a result, spinal discs begin to degenerate further, contributing to the development of spondylolisthesis.
If your doctor suspects spondylolisthesis, they will begin with a discussion about your medical history and when you first noticed the pain. Next, the doctor will perform a physical exam, which usually involves moving the legs to find out where the back pain is originating.
Your doctor will also need to use a differential diagnosis approach, or ruling things out, as they work to reach a conclusion. Spondylolisthesis can also present as general low back pain, spondylolysis (or a pars fracture , a stress fracture in the spine), or a pinched nerve .
If spondylolisthesis is suspected, the doctor will move onto what’s generally considered the best way to diagnose spondylolisthesis—an X-ray.
The X-ray below shows you a good example of a lumbar spondylolisthesis. Look at the area to which the arrow is pointing: You can see that the vertebra above the arrow isn't in line with the vertebra below it. It's slipped forward; it's spondylolisthesis.

Sometimes a CT scan or MRI scan will be used to diagnose spondylolisthesis if a doctor needs to view smaller details of the spine or see the soft tissues, which can include nerves and discs.
There’s also a grading system that doctors use to denote the severity of a spondylolisthesis using five descriptive categories. Although there are several factors your doctor considers when evaluating your spondylolisthesis, the grading scale (below) is based on how far forward a vertebral body has slid forward over the vertebra beneath. Often, the doctor uses a lateral (side view) X-ray to examine and grade a spondylolisthesis. Grade I is a smaller slip than Grade IV or V.
Grade I: Less than 25% slip
Grade II: 25% to 49% slip
Grade III: 50% to 74% slip
Grade IV: 75% to 99% slip
Grade V: The vertebra has fallen forward off the vertebra below it. This is the most severe type of spondylolisthesis and is termed spondyloptosis.
Complications of Spondylolisthesis
As the soft tissues become more unstable and the vertebrae slip and degenerate, nerves are entrapped and irritated, causing pain and discomfort. When this pain becomes chronic, it may prevent people from participating in enjoyable activities and hobbies. It can also interfere with daily domestic tasks, such as cooking, cleaning, and taking care of children and pets. As tasks mount up and there are fewer positive outlets for stress (such as exercise), all of this can result in a lowered quality of life.
If left untreated, spondylolisthesis may lead to lumbar spinal stenosis , a condition in which the spinal canal narrows and compresses the surrounding nerves and blood vessels. Spinal stenosis causes weakness and/or intense chronic pain in the back that often travels down the legs, making it difficult to walk. A diagnosis of spinal stenosis requires the use of imaging technology, such as computed tomography scans or magnetic resonance imaging, to precisely identify the affected areas of the spine as well as the scope of degeneration.
Because of the stresses placed on the vertebrae and the degenerative nature of arthritis, people with spondylolisthesis are at greater risk for lumbar spinal fractures. The risk is even high among older adults and those with osteoporosis . When the vertebrae fractures due to stress, spinal instability is increased, and the compromised facets may irritate the corresponding spinal nerves and cause pain.
If pinched nerves, spinal stenosis, and degenerative arthritis cannot be managed with conservative measures, such as rest, medication, or physical therapy , surgery may be necessary to restore proper function and eliminate debilitating pain. In particular, children with high-grade spondylolisthesis who continually experience radial pain often need surgery.
However, because of the inherent risks associated with spinal surgery, all conservative measures should be exhausted first.
Spondylolisthesis Treatment
If you’re searching for the right spondylolisthesis treatment , there are several options.
Nonsurgical Treatment
Your doctor may want to start with a more conservative approach before moving on to surgery. These options can include:
Rest, or taking a break from activities or sports that have either caused or will continue to exacerbate the condition.
Over-the-counter pain medications , such as acetaminophen or NSAIDs (e.g., ibuprofen). If the pain can’t be lessened by these medications, your doctor may write you a prescription for an oral steroid or administer steroid shots . The goal is to reduce pain and inflammation at the source.
Wearing a back brace , specifically a corset or one that’s geared toward low back pain. However, be cautious as overuse of a back brace is felt to weaken the muscles of the spine.
Physical therapy, which can be one of the most effective ways to treat spondylolisthesis. Techniques like deep tissue massage, TENS (transcutaneous electrical nerve stimulation ), and exercises that focus on core stability and range of motion can help to strengthen the spinal muscles and teach you to prevent even more injury.
Core strengthening
Weight loss
Applying heat and/or ice (ask your doctor for specific recommendations on how long and how often to apply each)
For a medical treatment that lands between conservative approaches and surgery, you could turn to something called regenerative injection therapy, in which a doctor will extract cells from one part of the body and inject them at the site of the spondylolisthesis. These include platelet-rich plasma (PRP) therapy and stem cell therapy and are less invasive than surgery. But be forewarned that while these two treatments are heavily marketed, they remain unproven in scientific research.
Spondylolisthesis Surgery
Let’s say that you’ve tried everything and you’re still experiencing painful symptoms. Or perhaps the severity of your case falls within the higher grades. In this case, your doctor may recommend surgery.
If your spondylolisthesis is stable (does not change with position), you may be a candidate for a simple decompression. This involves removing the bone, cartilage, and/or disc material that is compressing the nerve. This is done through procedures such as:
Foraminotomy
In cases where there is instability, the bones are actively sliding when bending and extending the back. These cases typically require spinal fusion . The goal of fusion is to realign the spine and fixate it in the proper alignment with hardware. The hardware acts to stabilize the segment while bony fusion, or bone growth, across the joint occurs.
The hardware anchors the bones together with screws and rods or plates. A fusion often incorporates a cage which is a spacer used in place of the disc. This provides even greater stability.
The commonly accepted term for this surgery is lumbar interbody fusion (LIF) with an extra word (or letter) at the beginning indicating where your surgeon will make the incision to access your spine:
Anterior (ALIF) —from stomach side
Transforaminal (TLIF) or Posterior (PLIF) —the back side
Oblique (OLIF)—off-center back
Lateral (LLIF)—the side of the spine (under the ribs), which is also known by its brand name, XLIF (Extreme Lateral Interbody Fusion)
While no patient is excited about the prospect of a fusion, the potential for improvement in pain far outweighs the risks of the procedure and the mild to moderate loss of movement attained.
Spondylolisthesis Outlook
The outlook for the majority of those with mild or moderate (low-grade) forms of spondylolisthesis is optimistic. Many people with low-grade spondylolisthesis respond well to a combination of rest, physical therapy, medication, and stretching and strengthening of the lower back.
Lifestyle changes (like getting more exercise, quitting smoking, and improving cardiovascular health) are also important components of managing the pain and complications associated with spondylolisthesis. These measures also help improve recovery should surgery be chosen in the future. Because spinal stability is a core consideration in the management and treatment of spondylolisthesis, any nonoperative measures should aim at strengthening the core muscles that support the spine.
Although spinal surgery carries inherent risks and involves significant recovery time for those with high-grade spondylolisthesis, it often allows younger people with severe spinal issues to improve their quality of life, especially in light of evolving surgical options. Older patients, and in particular those with complications such as osteoporosis, have more risks associated with spinal surgery. Because of this, outlook is improved when spondylolisthesis is diagnosed and treated early in the course of the disease, before these complications arise.
Cho, Youp Il., Park, Young S., Lee, Soon H. “MRI findings of lumbar spine instability in degenerative spondylolisthesis.” Journal of Orthopaedic Surgery. 2017;25(2). doi:10.1177/2309499017718907
Goel A. “Is the symptom of cervical or lumbar radiculopathy an evidence of spinal instability?” J Craniovertebr Junction Spine. 2018 Apr-Jun;9(2):81-82. doi: 10.4103/jcvjs.JCVJS_52_18.
MacDonald, J., Stuart, E., Rodenberg, R. “Musculoskeletal Low Back Pain in School-aged Children: A Review.” JAMA Pediatrics. 2017;171(3):280–287. doi:10.1001/jamapediatrics.2016.3334
Yi Xiang, J., Wang, Zoltán Káplár, Min Deng, Jason, et al. “Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence.” Journal of Orthopaedic Translation , Volume 11, 2017, Pages 39-52, ISSN 2214-031X, https://doi.org/10.1016/j.jot.2016.11.001.
Sengupta, D.K., Herkowitz, H.N. “Degenerative spondylolisthesis: review of current trends and controversies.” Spine (Phila Pa 1976). 2005 Mar 15. doi: 10.1097/01.brs.0000155579.88537.8e
Deer, T., Sayed, D., Michels, J., et al. “A Review of Lumbar Spinal Stenosis with Intermittent Neurogenic Claudication: Disease and Diagnosis.” Pain Med. 2019 Dec 1;20(Suppl 2):S32-S44. doi: 10.1093/pm/pnz161. PMID: 31808530; PMCID: PMC7101166.
Wang, P., Wang F., Gao Y.L., et al. “Lumbar spondylolisthesis is a risk factor for osteoporotic vertebral fractures: a case-control study.” J Int Med Res. 2018 Sep;46(9):3605-3612. doi: 10.1177/0300060518776067. Epub 2018 May 29. PMID: 29808735; PMCID: PMC6136001.
Metzger, R., Chaney, S. “Spondylolysis and spondylolisthesis: What the primary care provider should know.” Wiley Online Library. https://journals.lww.com/jaanp/Abstract/2014/01000/Spondylolysis_and_spondylolisthesis__What_the.3.aspx Published October 1, 2013. Accessed September 27, 2022.
Cavalier, R., Herman, M.J., Cheung, E.V., et al. “Spondylolysis and spondylolisthesis in children and adolescents: I. Diagnosis, natural history, and nonsurgical management.” J Am Acad Orthop Surg. 2006 Jul;14. doi:10.5435/00124635-200607000-00004.
Williams, Keith D. Campbell's Operative Orthopaedics, 14th Edition. Chapter 40, Spondylolisthesis, 1802-1831.e1, 2021. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323672177000407?scrollTo=%23hl0000985
Lionel, N., Metz, B.S., Vedat, D. “Neurosurgery Clinics of North America, Volume 18, Issue 2: Low-Grade Spondylolisthesis,” Pages 237-248, https://www.clinicalkey.com/#!/content/journal/1-s2.0-S1042368007000216
Studnicka, K., Ampat, G. “Lumbar Stabilization.” [Updated 2021 Nov 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562179/?report=classic
Goh, G.S., Tay, Y.W.A., Yue, W.M., et al. “What Are the Patient-reported Outcomes, Complications, and Radiographic Results of Lumbar Fusion for Degenerative Spondylolisthesis in Patients Younger Than 50 Years?” Clin Orthop Relat Res. 2020 Aug;478(8):1880-1888. doi: 10.1097/CORR.0000000000001252.
Bice, M., Anderson, Paul A. “Benzel's Spine Surgery, Fifth Edition. Chapter 8: Bone Physiology and Osteoporosis.” https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323636681000082?scrollTo=%23hl0000350
- Share via facebook
- Share via pinterest
- Share via twitter
- Share via mail
More Like This
How to prevent pars fractures.

What Makes Spondylolisthesis Worse

Retrolisthesis Differs From Spondylolisthesis

Spondylolisthesis and Pars Fractures

More Stories You Might Like...

Drugs and Medications for Spondylolisthesis

Degenerative Spondylolisthesis of the Lumbar Spine

Spinal Bracing: A Treatment Option for Spondylolisthesis

Utility Menu
- Request an Appointment
Popular Searches
- Adult Primary Care
Orthopedic Surgery
Spondylolisthesis.
Select your language:
In spondylolisthesis, one of the bones in your spine — called a vertebra — slips forward and out of place. This may occur anywhere along the spine, but is most common in the lower back (lumbar spine). In some people, this causes no symptoms at all. Others may have back and leg pain that ranges from mild to severe.
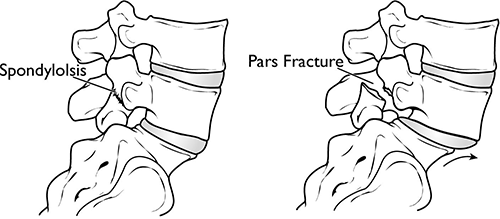
(Left) In spondylolysis, a fracture often occurs at the pars interarticularis. (Right) Because of the pars fracture, only the front part of the bone slips forward.

What are the different types of spondylolisthesis?
Many types of spondylolisthesis can affect adults. The two most common types are degenerative and spondylolytic. There are other less common types of spondylolisthesis, such as slippage caused by a recent, severe fracture or a tumor.
What is degenerative spondylolisthesis?
As we age, general wear and tear causes changes in the spine. Intervertebral discs begin to dry out and weaken. They lose height, become stiff, and begin to bulge. This disc degeneration is the start to both arthritis and degenerative spondylolisthesis (DS).
As arthritis develops, it weakens the joints and ligaments that hold your vertebrae in the proper position. The ligament along the back of your spine (ligamentum flavum) may begin to buckle. One of the vertebrae on either side of a worn, flattened disc can loosen and move forward over the vertebra below it. This can narrow the spinal canal and put pressure on the spinal cord. This narrowing of the spinal canal is called spinal stenosis and is a common problem in patients with DS.
Women are more likely than men to have DS, and it is more common in patients who are older than 50. A higher incidence has been noted in the African-American population.
What is spondylolytic spondylolisthesis?
One of the bones in your lower back can break and this can cause a vertebra to slip forward. The break most often occurs in the area of your lumbar spine called the pars interarticularis.
In most cases of spondylolytic spondylolisthesis, the pars fracture occurs during adolescence and goes unnoticed until adulthood. The normal disc degeneration that occurs in adulthood can then stress the pars fracture and cause the vertebra to slip forward. This type of spondylolisthesis is most often seen in middle-aged men.
Because a pars fracture causes the front (vertebra) and back (lamina) parts of the spinal bone to disconnect, only the front part slips forward. This means that narrowing of the spinal canal is less likely than in other kinds of spondylolisthesis, such as DS in which the entire spinal bone slips forward.
What are the symptoms of degenerative spondylolisthesis?
Patients with DS often visit the doctor's office once the slippage has begun to put pressure on the spinal nerves. Although the doctor may find arthritis in the spine, the symptoms of DS are typically the same as symptoms of spinal stenosis. For example, DS patients often develop leg and/or lower back pain. The most common symptoms in the legs include a feeling of vague weakness associated with prolonged standing or walking.
Leg symptoms may be accompanied by numbness, tingling, and/or pain that is often affected by posture. Forward bending or sitting often relieves the symptoms because it opens up space in the spinal canal. Standing or walking often increases symptoms.
What are the symptoms of spondylolytic spondylolisthesis?
Most patients with spondylolytic spondylolisthesis do not have pain and are often surprised to find they have the slippage when they see it in x-rays. They typically visit a doctor with low back pain related to activities. The back pain is sometimes accompanied by leg pain.
How is a spondylolisthesis diagnosed?
Doctors diagnose both DS and spondylolytic spondylolisthesis using the same examination tools.
After discussing your symptoms and medical history, your doctor will examine your back. This will include looking at your back and pushing on different areas to see if it hurts. Your doctor may have you bend forward, backward, and side- to-side to look for limitations or pain.
Other tests which may help your doctor confirm your diagnosis include:
X-rays. These tests visualize bones and will show whether a lumbar vertebra has slipped forward. X-rays will show aging changes, like loss of disc height or bone spurs. X-rays taken while you lean forward and backward are called flexion-extension images. They can show instability or too much movement in your spine.
Magnetic resonance imaging (MRI). This study can create better images of soft tissues, such as muscles, discs, nerves, and the spinal cord. It can show more detail of the slippage and whether any of the nerves are pinched.
Computed tomography (CT). These scans are more detailed than x-rays and can create cross-section images of your spine.
How is spondylolisthesis treated without surgery?
Although nonsurgical treatments will not repair the slippage, many patients report that these methods do help relieve symptoms.
Physical therapy and exercise . Specific exercises can strengthen and stretch your lower back and abdominal muscles.
Medication . Pain killers and non-steroidal anti-inflammatory medicines may relieve pain.
Steroid injections . Cortisone is a powerful anti-inflammatory. Cortisone injections around the nerves or in the "epidural space" can decrease swelling, as well as pain. It is not recommended to receive these, however, more than three times per year. These injections are more likely to decrease pain and numbness, but will not relieve weakness of the legs.
When should someone with degenerative spondylolisthesis be treated with surgery?
Patients should consider surgery for degenerative spondylolisthesis if they have tried the nonsurgical treatments for 3 to 6 months with no improvement.
Before committing to surgery, your provider will take a close look at the extent of the arthritis in your spine and whether your spine has excessive movement.
DS patients who are candidates for surgery are usually not able to walk or stand, and have a poor quality of life due to the pain and weakness.
When should someone with spondylolytic spondylolisthesis be treated with surgery?
Patients should consider surgery for spondylolytic spondylolisthesis if they have tried the nonsurgical treatments for at least 6 to 12 months with no improvement.
If the slippage is getting worse or the patient has progressive neurologic symptoms, such as weakness, numbness, or falling, and/or symptoms of cauda equina syndrome, surgery may help.
How is spondylolisthesis treated with surgery?
Surgery for both DS and spondylolytic spondylolisthesis includes removing the pressure from the nerves and spinal fusion.
Removing the pressure involves opening up the spinal canal. This procedure is called a laminectomy. Spinal fusion is essentially a "welding" process. The basic idea is to fuse together the painful vertebrae so that they heal into a single, solid bone.
Departments and Programs Who Treat This Condition
Spine surgery.

Popular Services
- Patient & Visitor Guide
Committed to improving health and wellness in our Ohio communities.
Health equity, healthy community, classes and events, the world is changing. medicine is changing. we're leading the way., featured initiatives, helpful resources.
- Refer a Patient
Spondylolisthesis
We design a unique treatment plan for your condition of spondylolisthesis and take into account your life goals., what is spondylolisthesis.
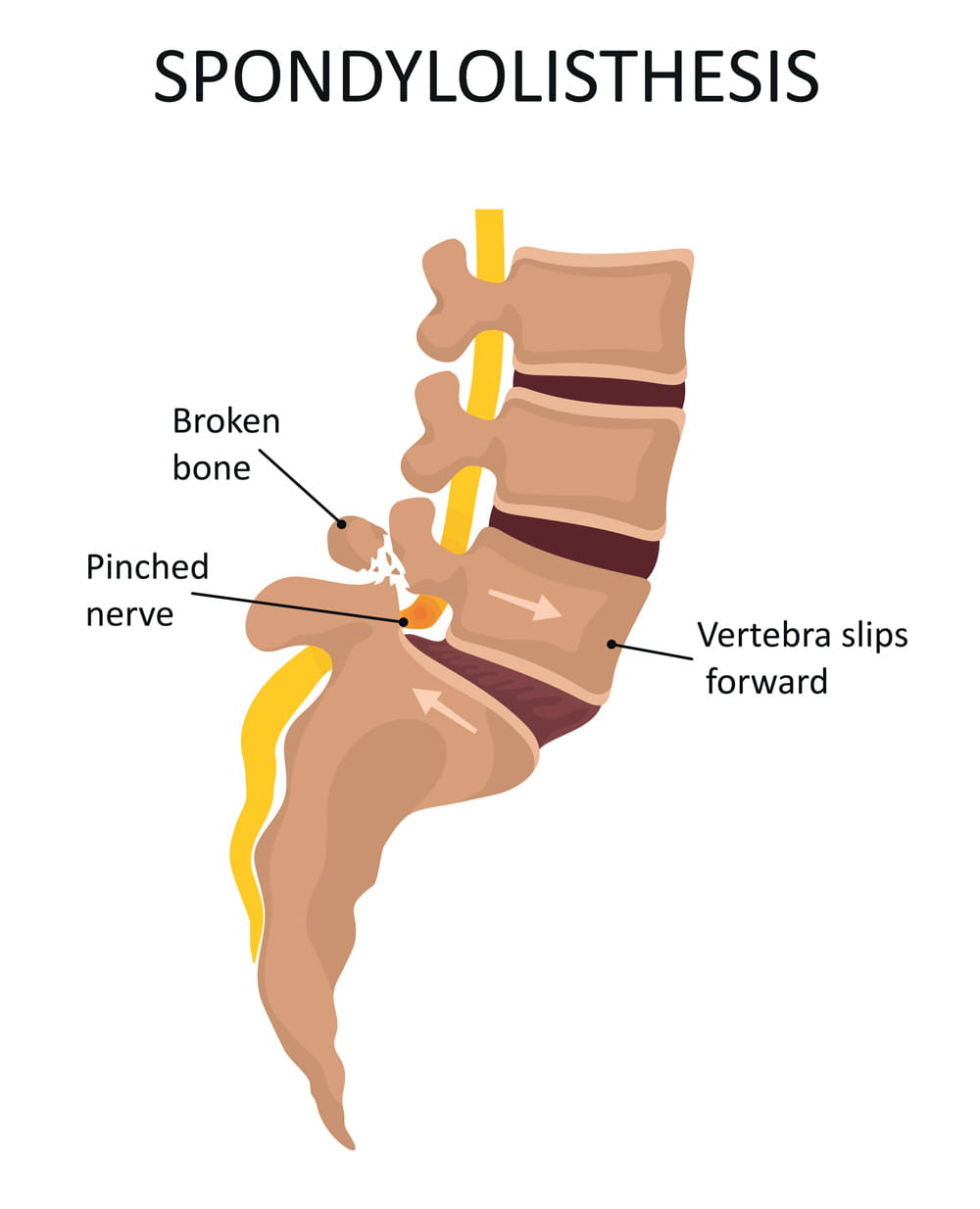
Low back pain, leg pain and weakness in the legs can happen if the bone that’s out of position significantly narrows the spinal column and begins to press on nerves.
Causes of spondylolisthesis
- Birth defect of the vertebral joint – This usually occurs in the lower spine where the lumbar spine and sacrum come together
- Stress “micro-fracture” in the bone due to overstretching and overuse – This can occur with sports activities such as gymnastics, weight lifting, ice skating and football
- Aging or overuse-related wear on the spinal joints
Rest and anti-inflammatory medication resolve most cases.
If it’s more severe, you may need physical therapy or surgery.
Spondylolisthesis grades
Doctors commonly describe spondylolisthesis as either high-grade or low-grade, depending on how severe your condition is. Grades are from 1 to 4.
- Low-grade (grade 1 and grade 2) usually occurs in adolescents and is considered less severe. Low-grade doesn’t typically require surgery.
- High-grade (grade 3 and grade 4) may require surgery if you’re experiencing severe pain.
The grade of your condition is based on how far away from proper alignment your spine has become.
Spondylolisthesis symptoms
In many cases, people who have spondylolisthesis don’t have any symptoms. You may not be aware you have the condition until an X-ray is taken for an unrelated reason. If you do have symptoms, the most common are:
- Lower back pain that feels like a muscle strain
- Muscle spasms or tightness in your hamstring
- Lower back pain that worsens with activity and improves with rest
- Difficulty walking or standing
- Pain when bending over
- Stiffness in your back
- Pain extending down from your lower back to your thighs
If you have high-grade spondylolisthesis, you may experience tingling, numbness or weakness in one or both legs.
Diagnosing spondylolisthesis
Following a thorough medical history, physical and neurological exams, our spine surgeons may recommend any of the following tests to confirm whether a bone in your spine is out of alignment. All tests are available at Ohio State Spine Care :
- Computed tomography (CT) scan
- Magnetic resonance imaging (MRI) scan
- Electromyography (EMG) to test your muscles and nerves
Spondylolisthesis treatment
Ohio State’s Spine Care team has the benefit of extra expertise from treating many people with spondylolisthesis. Because of this, the Spine Care team, composed of orthopedic and neurological specialists, is uniquely qualified to determine whether you’re likely to benefit from nonsurgical treatment. We also recommend lifestyle changes to prevent future problems with your spine.
We offer treatments ranging from physical therapy to the most complex spine surgeries. Physicians, therapists and other care providers work together to provide you with options that increase mobility and reduce pain.
Most people who come to Ohio State Spine Care don’t require surgery.
Lifestyle changes
- Exercise, such as Pilates or yoga, to strengthen muscles in your back
- Quitting smoking
- Guidance on weight loss to reduce pressure on your spine
Nonsurgical treatments
- Physical therapy – We’ll work with you one-on-one to customize a treatment plan for your needs and goals
- Spine orthobiologics use substances in your body to activate the healing process naturally
- Wearing a back brace to limit spine movement
- Medication for pain management
Most people return slowly to full function, including athletic activity.
Spondylolisthesis surgery
You may need surgery if a spinal bone that has slipped is likely to cause damage to nerves and the surrounding spinal structure, or if it’s causing severe pain or muscle weakness in one or both legs.
Our surgeons can perform minimally invasive surgery to correct the symptoms of spondylolisthesis. The surgeon makes tiny incisions in the back and works through a tube to minimize skin and muscle damage, reduce blood loss and reduce postsurgical pain.
At Ohio State, we can use both minimally invasive surgery and conventional surgical techniques for these procedures:
- Decompression surgery (laminectomy) to remove part of the vertebra and relieve pressure on your spinal cord or nerves
- Spinal fusion surgery to fuse a severely slipped bone with the vertebra below it and restore stability to the spinal column
Most people who have decompression or fusion surgery can return to full function, including athletic activities.
Ohio State conducts innovative research in the laboratory, as well as through clinical trials.
Those who have a pinched nerve may be eligible to participate in one of the following areas of research at The Ohio State University Wexner Medical Center.
Biomechanical testing: We’re doing biomechanical testing to assess the spine before and after surgery. A specialized vest helps us assess your spinal movement and measure the effectiveness of surgery. It ultimately may provide valuable information about which treatment methods will best increase mobility and function of the spine.
Back pain consortium: We’re members of the International Consortium for Health Outcomes Measurement (ICHOM). Membership in this elite organization allows us to engage with other top U.S. medical centers in global research studies on back pain. As we measure our results against established international standards, we share best practices and elevate our standard of care.
Enroll in a clinical trial
- Learn more about spine care at Ohio State Download our guide
Patient Education Animation Library
How would you like to schedule.
Don’t have MyChart? Create an account
Subscribe. Get just the right amount of health and wellness in your inbox.
- GP practice services
- Health advice
- Health research
- Medical professionals
Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skin, nail and hair health
Travel and vaccinations
Treatment and medication
Women's health
Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z
Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
- Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Spondylolisthesis and spondylolysis
Peer reviewed by Dr Laurence Knott Last updated by Dr Colin Tidy, MRCGP Last updated 20 Nov 2021
Meets Patient’s editorial guidelines
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Cervical spondylosis article more useful, or one of our other health articles .
In this article :
What is spondylolisthesis, spondylolisthesis vs spondylolysis.
- Who gets spondylolisthesis and spondylolysis? (Epidemiology)
Spondylolisthesis causes (aetiology)
- Types of spondylolisthesis
- Presentation
Differential diagnosis
Investigations.
- Spondylolisthesis treatment and management
Complications of surgical repair
Spondylolisthesis prognosis.
Continue reading below
Spondylolisthesis is the movement of one vertebra relative to the others in either the anterior or posterior direction due to instability. Degenerative spondylolisthesis is a common pathology, often causing lumbar canal stenosis 1 .
Anatomy of the vertebrae
The vertebrae can be divided into three portions:
Centrum - involved in weight bearing. This is the body of the vertebra and is formed of cancellous bone.
Dorsal arch - surrounds and protects the spinal cord. It carries the upper and lower facet joints of each vertebra which articulate with the facet joints of the vertebra above and below, respectively. The part of the vertebral arch between them is the thinnest part and is called the pars interarticularis, or the isthmus.
Posterior aspect - protrudes and can be palpated on the lower back.
Lumbar vertebra 1 inferior surface
Lumbar vertebra 1 anterior surface
Images by Anatomography, via Wikimedia Commons . Click here to see a lumbar vertebra 1 close-up superior surface animation.
Spondylolysis and spondylolisthesis are separate conditions, although spondylolysis often precedes spondylolisthesis.
Spondylolysis is a bony defect (commonly due to a stress fracture but it may be a congenital defect) in the pars interarticularis of the vertebral arch, separating the dorsum of the vertebra from the centrum. It may occur unilaterally or bilaterally. It most commonly affects the fifth lumbar vertebra and may cause back pain.
Spondylolisthesis refers to the anterior slippage of one vertebra over another (or the fifth vertebra over the sacrum). There are five forms:
Isthmic : the most common form, usually acquired in adolescence as a consequence of spondylolysis but often unnoticed until adulthood.
Degenerative : developing in older adults as a result of facet joint osteoarthritis and bone remodelling.
Traumatic (rare): resulting from fractures of the neural arch.
Pathologic : from metastases or metabolic bone disease.
Dysplastic : (rare): congenital, resulting from malformation of the pars.
Spondylosis is a general term for degenerative osteoarthritic changes in the spine. It involves dehydration of the intervertebral discs with consequent narrowing of the intervertebral spaces. There may be changes in the facet joints with osteophyte formation and this may put pressure on the nerve roots, causing motor and sensory disturbance.
Who gets spondylolisthesis and spondylolysis? (Epidemiology) 2
Spondylolysis is a common diagnosis with a high prevalence in children and adolescents complaining of low back pain.
There is an increased risk of spondylolysis in young athletes like gymnasts, presumably due to impact-related stress fractures . However most cases are low-grade. At-risk activities include gymnastics, diving, tennis, cricket, weightlifting, football and rugby.
Isthmic spondylolisthesis affects around 5% of the population but is more common in young athletes. 60-80% of people with spondylolysis have associated spondylolisthesis 3 4 .
The majority of cases of spondylolysis and spondylolisthesis affect L5 and most of the remainder affect L4.
Degenerative spondylolisthesis is more common in older people, particularly women.
Traumatic, metastatic and dysplastic spondylolistheses are relatively rare.
Many cases of spondylolisthesis are asymptomatic.
Spondylolisthesis commonly occurs due to a fracture or defect in the pars interarticularis, the narrowest part of the posterior vertebral arch between the upper and lower facet joints. When this is breached, the upper facet joint may no longer be able to hold the vertebra in place against the downward force of body weight and forward/downward slippage occurs.
Risk factors that increase the risk of spondylolysis developing into spondylolisthesis include 5 :
Female gender.
Presence of spina bifida or spina bifida occulta .
Vertebral wedging.
Hyperlordosis.
Positive family history.
Certain high-impact sports, as evidenced by increased rates in athletes and gymnasts 3 .
Types of spondylolisthesis 2
Stable or unstable.
Asymptomatic or symptomatic.
Graded according to degree of slippage; the Meyerding classification is based on the ratio of the overhanging part of the superior vertical body to the anterio-posterior length of the inferior vertebral body:
Grade I: 0-25%.
Grade II: 26-50%.
Grade III: 51-75%.
Grade IV: 76-100%.
Grade V (spondyloptosis): >100%.
Graded according to type; the Wiltse classification (1976):
Type I: dysplastic (congenital).
Type II: isthmic: secondary to a lesion involving the pars interarticularis:
Subtype A: secondary to stress fracture.
Subtype B: result of multiple healed stress fractures resulting in an elongated pars.
Subtype C: acute pars fracture (rare).
Type III: degenerative.
Type IV: post-traumatic: fracture in a region other than the pars.
Type V: pathological: diffuse or local disease.
Type VI: iatrogenic.
Presentation 4
Spondylolysis symptoms.
Most cases of spondylolysis are asymptomatic and identified incidentally.
It may present with low back pain provoked by lumbar extension, paraspinal spasm and tight hamstrings.
It frequently does not show on X-ray. It is important to consider it in the differential diagnosis of back pain, as its identification can prevent progression and avoid the potential need for aggressive intervention.
Spondylolisthesis symptoms
Presentation varies slightly by type although common spondylolisthesis symptoms include exercise-related back pain, radiating to the lower thighs, which tends to be eased by rest, particularly in positions of spinal flexion.
Isthmic spondylolisthesis
Most patients are asymptomatic, even with progressing slippage.
Symptoms often begin around the adolescent growth spurt.
Back pain - worse with activity (particularly back extension) - this may come on acutely or insidiously.
Pain may flare with sudden or trivial activities and is relieved by resting.
Pain is worse with higher grades of disease.
Pain may radiate to buttocks or thighs
There are usually no neurological features with lower grades of slippage but radicular pain becomes common with larger slips. Pain below the knee due to nerve root compression or disc herniation would suggest more severe slippage. High degrees of spondylolisthesis may present with neurogenic claudication or even cauda equina impingement.
Tightened hamstrings are very common
There may be enhanced lordosis and a waddling gait with shortened step length.
There may be gluteal muscular wasting.
Degenerative spondylolisthesis
Pain is aching in nature and insidious in onset.
Pain is in the low back and posterior thighs.
Neurogenic claudication may be present with lower-extremity symptoms worsening with exercise.
Symptoms are often chronic and progressive, sometimes with periods of remission.
If lumbar stenosis is also present, reflexes may be diminished.
Dysplastic spondylolisthesis
Presentation and physical findings are similar to isthmic spondylolisthesis but with a greater likelihood of neurological compromise.
Traumatic spondylolisthesis
Patients will have experienced acute trauma and are likely to have significant pain.
Severe slips may cause cauda equina compression with bladder and bowel dysfunction, radicular symptoms or neurogenic claudication.
Physical findings are as for the other types.
Pathological spondylolisthesis
Symptoms may be insidious in onset and associated with radicular pain.
Other causes of back pain need to be ruled out - eg:
Osteoarthritis .
Ankylosing spondylitis .
Mechanical lower back pain .
Spinal cord lesion.
Multiple myeloma .
Vertebral compression fracture .
Lumbar disc prolapse.
Discitis/other spinal disc problems .
Blood tests - looking for infection, myeloma, hypercalcaemia/hypocalcaemia.
Lateral spinal X-rays - will show spondylolisthesis. These are best performed in the position of maximal pain.
Oblique spinal X-rays - may (but will often not) detect spondylolysis.
Radionuclide scintigraphy and CT may help in cases of spondylolysis in distinguishing progressing lesions of the pars from stable lesions.
MRI is often performed perioperatively to look at relationships between the bony and neurological structures in the compromised area.
Spondylolisthesis treatment and management 1 2 4
The goal of treatment is to relieve pain, stabilise the spinal segment and stop or reverse the slippage. Patients need to be evaluated for the presence of instability, as if there is an unstable segment early surgery will be needed.
Depending on the severity of the spondylolysis and symptoms associated it may be treated either conservatively or surgically, both of which have shown significant success.
Conservative treatments such as bracing and decreased activity have been shown to be most effective with patients who have early diagnosis and treatment. Low-intensity pulsed ultrasound in addition to conservative treatment appears to achieve a higher rate of bony union. Surgery may be required if conservative treatment, for at least six months, failed to give sustained pain relief for the activities of daily living.
For degenerative spondylolisthesis, surgery is indicated mainly for perceived functional impairment. Improvement in neurological symptoms is one of the main treatment objectives. For this, it is useful to perform radicular decompression. The most frequent technique is direct posterior decompression.
Conservative treatment
Complete bed rest for 2-3 days can be helpful in relieving pain, particularly in spondylolysis, although longer periods are likely to be counterproductive. Patients should try to sleep on their side as much as possible, with a pillow between the knees.
Activity modification to prevent further injury. This may mean avoidance of activities if there is >25% slippage.
Analgesia - eg, paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), codeine phosphate.
Steroid and local anaesthetic injections are sometimes used around compressed nerve roots or even into the fracture area of the pars for diagnostic purposes.
Bracing: a brace or corset may be recommended for a pars interarticularis fracture which is likely to heal. Bracing with exercise may be beneficial for patients with mild or even more severe degrees of slippage.
Physiotherapy: this includes massage, ultrasound, bracing, mobilisation, biomechanical correction, hydrotherapy, exercises for flexibility, strength and core stability and a gradual return to activity programme.
More than 80% of children treated non-surgically will have full resolution of symptoms.
A meta-analysis of observation studies suggested that around 80% of all patients treated non-operatively would have a successful clinical outcome after one year. Lesions diagnosed at the acute stage and unilateral lesions were the best subgroups 6 .
Surgical treatment
If there is evidence of progression or if conservative measures are ineffective then surgical therapy may be offered. This depends also on degree and aetiology.
Surgical intervention involves a prolonged rehabilitation period so it is generally not considered until conservative treatments have failed. An exception would be in the case of significant instability or neurological compromise and in high-grade slips.
Surgical therapy involves fusing the affected vertebra with a neighbouring normally aligned vertebra (both anteriorly and posteriorly). The intervertebral disc is usually also removed, as it is inevitably damaged. The slipped vertebra may be realigned.
Whilst most surgeons agree that decompression of the nerves is of benefit to patients, the benefit of realigning slipped vertebrae is uncertain. For example, when the spondylolisthesis is very gradual in onset, or in cases of congenital spondylolisthesis, compensatory changes in the spine and musculature occur so that realignment may increase the possibility of further injury.
There is good evidence that surgical treatment of symptomatic spondylolisthesis is significantly superior to non-surgical management in the presence of 7 :
Significant neurological deficit.
Failed response to conservative therapy.
Instability with neurological symptoms.
Degree of subluxation of III or more.
Unremitting pain affecting quality of life.
A large systematic review concluded that reduction of displacement carried benefits over fusion alone, although a large retrospective review showed high complication rates, particularly for older patients with more severe disease 8 9 10 11 .
Fusion techniques can be associated with neurological complications in older patients with degenerative spondylolisthesis, but in adolescent patients outcomes are good 9 .
Surgery is commonly complicated by pseudoarthrosis (non-union) which may result in chronic pain years down the line.
In the case of spondylolysis, if surgery is offered it would involve pinning the defect. However, most cases are managed conservatively.
Implant failure.
Pseudoarthrosis (failure of bone healing leading to a 'false joint').
Poor alignment of the fusion.
Neurological damage: foot drop, spinal cord compression . Chronic nerve injury/inflammation: neuropathic pain can persist in the face of apparent surgical success, possibly due to permanent changes in the nerves or a deregulation of pain control mechanisms.
Spondylolisthesis is generally a benign condition; however, it runs a chronic course and is therefore a cause of much morbidity and disability. In degenerative spondylolisthesis this will relate in part to the progress and prognosis of the underlying changes.
Dr Mary Lowth is an author or the original author of this leaflet.
Further reading and references
- Guigui P, Ferrero E ; Surgical treatment of degenerative spondylolisthesis. Orthop Traumatol Surg Res. 2017 Feb;103(1S):S11-S20. doi: 10.1016/j.otsr.2016.06.022. Epub 2016 Dec 30.
- Gagnet P, Kern K, Andrews K, et al ; Spondylolysis and spondylolisthesis: A review of the literature. J Orthop. 2018 Mar 17;15(2):404-407. doi: 10.1016/j.jor.2018.03.008. eCollection 2018 Jun.
- Toueg CW, Mac-Thiong JM, Grimard G, et al ; Prevalence of spondylolisthesis in a population of gymnasts. Stud Health Technol Inform. 2010;158:132-7.
- Syrmou E, Tsitsopoulos PP, Marinopoulos D, et al ; Spondylolysis: a review and reappraisal. Hippokratia. 2010 Jan;14(1):17-21.
- Sadiq S, Meir A, Hughes SP ; Surgical management of spondylolisthesis overview of literature. Neurol India. 2005 Dec;53(4):506-11.
- Klein G, Mehlman CT, McCarty M ; Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009 Mar;29(2):146-56. doi: 10.1097/BPO.0b013e3181977fc5.
- Alfieri A, Gazzeri R, Prell J, et al ; The current management of lumbar spondylolisthesis. J Neurosurg Sci. 2013 Jun;57(2):103-13.
- Weinstein JN, Lurie JD, Tosteson TD, et al ; Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009 Jun;91(6):1295-304. doi: 10.2106/JBJS.H.00913.
- Sansur CA, Reames DL, Smith JS, et al ; Morbidity and mortality in the surgical treatment of 10,242 adults with spondylolisthesis. J Neurosurg Spine. 2010 Nov;13(5):589-93. doi: 10.3171/2010.5.SPINE09529.
- Kasliwal MK, Smith JS, Kanter A, et al ; Management of high-grade spondylolisthesis. Neurosurg Clin N Am. 2013 Apr;24(2):275-91. doi: 10.1016/j.nec.2012.12.002. Epub 2013 Feb 21.
- Longo UG, Loppini M, Romeo G, et al ; Evidence-based surgical management of spondylolisthesis: reduction or arthrodesis in situ. J Bone Joint Surg Am. 2014 Jan 1;96(1):53-8. doi: 10.2106/JBJS.L.01012.
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 19 Nov 2026
20 nov 2021 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Spondylolisthesis.
Steven Tenny ; Andrew Hanna ; Christopher C. Gillis .
Affiliations
Last Update: May 22, 2023 .
- Continuing Education Activity
Spondylolisthesis is a condition that occurs when one vertebral body slips with respect to the adjacent vertebral body causing radicular or mechanical symptoms or pain. It is graded based on the degree of slippage of one vertebral body on the adjacent vertebral body. Any pathological process that can weaken the supports keeping vertebral bodies aligned can allow spondylolisthesis to occur. This activity illustrates the evaluation and management of spondylolisthesis and reviews the role of the interprofessional team in improving care for patients with this condition.
- Describe the pathophysiology of spondylolisthesis.
- Review the workup of a patient with spondylolisthesis.
- Summarize the treatment options for spondylolisthesis.
- Describee the importance of collaboration and communication among the interprofessional team in encouraging weight loss in patients to reduce symptoms and increase the quality of life in those with spondylolisthesis.
- Introduction
Spondylolisthesis is the slippage of one vertebral body with respect to the adjacent vertebral body causing mechanical or radicular symptoms or pain. It can be due to congenital, acquired, or idiopathic causes. Spondylolisthesis is graded based on the degree of slippage of one vertebral body on the adjacent vertebral body. [1]
Spondylolisthesis commonly classifies as one of five major etiologies: degenerative, isthmic, traumatic, dysplastic, or pathologic. Degenerative spondylolisthesis occurs from degenerative changes in the spine without any defect in the pars interarticularis. It is usually related to the combined facet joint and disc degeneration leading to instability and forward movement of one vertebral body relative to the adjacent vertebral body. Isthmic spondylolisthesis results from defects in the pars interarticularis. The cause of isthmic spondylolisthesis is undetermined, but a possible etiology includes microtrauma in adolescence related to sports such as wrestling, football, and gymnastics, where repeated lumbar extension occurs. Traumatic spondylolisthesis occurs after fractures of the pars interarticularis or the facet joint structure and is most common after trauma. Dysplastic spondylolisthesis is congenital and secondary to variation in the orientation of the facet joints to an abnormal alignment. In dysplastic spondylolisthesis, the facet joints are more sagittally oriented than the typical coronal orientation. Pathologic spondylolisthesis can be from systemic causes such as bone or connective tissue disorders or a focal process, including infection, neoplasm, or iatrogenic origin. Additional risk factors for spondylolisthesis include a first-degree relative with spondylolisthesis, scoliosis, or occult spina bifida at the S1 level. [1]
- Epidemiology
Spondylolisthesis most commonly occurs in the lower lumbar spine but can also occur in the cervical spine and rarely, except for trauma, in the thoracic spine. Degenerative spondylolisthesis predominately occurs in adults and is more common in females than males with increased risk in the obese. Isthmic spondylolisthesis is more common in the adolescent and young adult population but may go unrecognized until symptoms develop in adulthood. There is a higher prevalence of isthmic spondylolisthesis in males. Dysplastic spondylolisthesis is more common in the pediatric population, with females more commonly affected than males. Current estimates for prevalence are 6 to 7% for isthmic spondylolisthesis by the age of 18 years, and up to 18% of adult patients undergoing MRI of the lumbar spine. Grade I spondylolisthesis accounts for 75% of all cases. Spondylolisthesis most commonly occurs at the L5-S1 level with an anterior translation of the L5 vertebral body on the S1 vertebral body. The L4-5 level is the second most common location for spondylolisthesis.
- Pathophysiology
Any process that can weaken the supports keeping vertebral bodies aligned can allow spondylolisthesis to occur. As one vertebra moves relative to the adjacent vertebrae, local pain can occur from mechanical motion or radicular or myelopathic pain can occur due to compression of the exiting nerve roots or spinal cord, respectively. Pediatric patients are more likely to increase spondylolisthesis grade when going through puberty. Older patients with lower grades I or II spondylolistheses are less likely to progress to higher grades over time.
- History and Physical
Patients typically have intermittent and localized low back pain for lumbar spondylolisthesis and localized neck pain for cervical spondylolisthesis. The pain is exacerbated by flexing and extending at the affected segment, as this can cause mechanic pain from motion. Pain may be exacerbated by direct palpation of the affected segment. Pain can also be radicular in nature as the exiting nerve roots become compressed due to the narrowing of nerve foramina as one vertebra slips on the adjacent vertebrae, the traversing nerve root (root to the level below) can also be impinged through associated lateral recess narrowing, disc protrusion, or central stenosis. Pain can sometimes improve in certain positions such as lying supine. This improvement is due to the instability of the spondylolisthesis that reduces with supine posture, thus relieving the pressure on the bony elements as well as opening the spinal canal or neural foramen. Other symptoms associated with lumbar spondylolisthesis include buttock pain, numbness, or weakness in the leg(s), difficulty walking, and rarely loss of bowel or bladder control.
Anteroposterior and lateral plain films, as well as lateral flexion-extension plain films, are the standard for the initial diagnosis of spondylolisthesis. One is looking for the abnormal alignment of one vertebral body to the next as well as possible motion with flexion and extension, which would indicate instability. In isthmic spondylolisthesis, there may be a pars defect, which is termed the "Scotty dog collar." The "Scotty dog collar" shows a hyperdensity where the collar would be on the cartoon dog, which represents the fracture of the pars interarticularis. Computed tomography (CT) of the spine provides the highest sensitivity and specificity for diagnosing spondylolisthesis. Spondylolisthesis can be better appreciated on sagittal reconstructions as compared to axial CT imaging. MRI of the spine can show associated soft tissue and disc abnormalities, but it is relatively more challenging to appreciate bony detail and a potential pars defect on MRI. [2] [3]
- Treatment / Management
For grade I and II spondylolisthesis, treatment typically begins with conservative therapy, including nonsteroidal anti-inflammatory drugs (NSAIDs), heat, light exercise, traction, bracing, and/or bed rest. Approximately 10% to 15% of younger patients with low-grade spondylolisthesis will fail conservative treatment and need surgical treatment. No definitive standards exist for surgical treatment. Surgical treatment includes a varying combination of decompression, fusion with or without instrumentation, or interbody fusion. Patients with instability are more likely to require operative intervention. Some surgeons recommend a reduction of the spondylolisthesis if able as this not only decreases foraminal narrowing but also can improve spinopelvic sagittal alignment and decrease the risk for further degenerative spinal changes in the future. The reduction can be more difficult and riskier in higher grades and impacted spondylolisthesis. [4] [5] [6] [7] [8] [2] [9] [10]
- Differential Diagnosis
- Degenerative Lumbar Disc Disease
- Lumbar Disc Problems
- Lumbosacral Disc Injuries
- Lumbosacral Discogenic Pain Syndrome
- Lumbosacral Facet Syndrome
- Lumbosacral Radiculopathy
- Lumbosacral Spine Acute Bony Injuries
- Lumbosacral Spondylosis
- Myofascial Pain in Athletes
- Pearls and Other Issues
Meyerding’s classification of spondylolisthesis is the most commonly used grading method. Its basis is on the percentage of anterior translation relative to the adjacent level. Grade I spondylolisthesis is 1 to 25% slippage, grade II is up to 50% slippage, grade III is up to 75% slippage, and grade IV is 76-100% slippage. If there is more than 100% slippage, it is known as spondyloptosis or grade V spondylolisthesis.
Subclasses of isthmic spondylolisthesis are subtype A (stress fractures of the pars), subtype B (elongation of the pars without overt fracture), subtype C (acute fracture of the pars).
Subclasses of pathologic spondylolisthesis are subtype A (systemic causes) and subtype B (focal processes).
- Enhancing Healthcare Team Outcomes
An interprofessional team consisting of a specialty-trained orthopedic nurse, a physical therapist, and an orthopedic surgeon or neurosurgeon will provide the best outcome and long-term care of patients with degenerative spondylolisthesis. Chiropractors may also have involvement, as they may be the first to encounter the condition on X-rays. The treating clinician will decide on the management plan, and then have the other team members engaged - surgical cases with include the nursing staff in pre-, intra-, and post-operative care, and coordinating with PT for rehabilitation. In non-operative cases, the PT will keep the rest of the team informed of progress or lack thereof. The team should encourage weight loss as weight reduction may reduce symptoms and increase the quality of life. Interprofessional collaboration, as above, will drive patient outcomes to their best results. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Lumbar Spine Sagittal CT of L5-S1, Grade II Spondylolisthesis Contributed by Christopher Gillis, MD, and Steven Tenny, MD
Disclosure: Steven Tenny declares no relevant financial relationships with ineligible companies.
Disclosure: Andrew Hanna declares no relevant financial relationships with ineligible companies.
Disclosure: Christopher Gillis declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Tenny S, Hanna A, Gillis CC. Spondylolisthesis. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PubMed Links to PubMed
Similar articles in PubMed
- Isthmic Spondylolisthesis. [StatPearls. 2024] Isthmic Spondylolisthesis. Burton MR, Dowling TJ, Mesfin FB. StatPearls. 2024 Jan
- High-grade slippage of the lumbar spine in a rat model of spondylolisthesis: effects of cyclooxygenase-2 inhibitor on its deformity. [Spine (Phila Pa 1976). 2006] High-grade slippage of the lumbar spine in a rat model of spondylolisthesis: effects of cyclooxygenase-2 inhibitor on its deformity. Komatsubara S, Sairyo K, Katoh S, Sakamaki T, Higashino K, Yasui N. Spine (Phila Pa 1976). 2006 Jul 15; 31(16):E528-34.
- [Three vertebral reduction and fixation for revision of lumbar spondylolisthesis]. [Zhongguo Gu Shang. 2014] [Three vertebral reduction and fixation for revision of lumbar spondylolisthesis]. Li CS. Zhongguo Gu Shang. 2014 Sep; 27(9):717-21.
- Review A review of the pathomechanism of forward slippage in pediatric spondylolysis: the Tokushima theory of growth plate slippage. [J Med Invest. 2015] Review A review of the pathomechanism of forward slippage in pediatric spondylolysis: the Tokushima theory of growth plate slippage. Sairyo K, Nagamachi A, Matsuura T, Higashino K, Sakai T, Suzue N, Hamada D, Takata Y, Goto T, Nishisho T, et al. J Med Invest. 2015; 62(1-2):11-8.
- Review Spondylolisthesis. [Orthop Rev (Pavia). 2022] Review Spondylolisthesis. Li N, Scofield J, Mangham P, Cooper J, Sherman W, Kaye A. Orthop Rev (Pavia). 2022; 14(4):36917. Epub 2022 Jul 27.
Recent Activity
- Spondylolisthesis - StatPearls Spondylolisthesis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

- 086558 85566
- Request Appointment --> Request Appointment
Request Appointment
Enter your details and we will be in touch with you shortly; Or call 08655885566 between 8 am and 8 pm.
QI Spine Blog
- Everyday Tips
- Success Stories

Spondylolisthesis L5-S1: Symptoms, Causes & Treatment
Qi spine clinic, 4 years ago, what do you mean by spondylolisthesis l5-s1, difference between spondylolysis & spondylolisthesis, overview of l5-s1, spondylolisthesis – types & grades, signs & symptoms of spondylolisthesis l5-s1, other common symptoms are: –.
- Lower back pain is the most common one.
- Weakness in one or both thighs/legs
- Pain might radiate to buttocks or thighs
- Decreased ability to control bowel/bladder movements (in type IV and above)
- The muscles in the back of thigh i.e. hamstrings feel tight
- Difficulty in standing, walking.
Causes of Spondylolisthesis L5-S1
- Birth defect
- Followed by trauma
Other common problems at L5-S1 are as follows: –
- Spondylolysis
- Disc herniation
- Facet joint arthropathy
- Nerve compression
Diagnosing L5-S1 Spondylolisthesis
Treatment for spondylolisthesis l5-s1, the non-surgical treatment methods include:-, pain management.
- Rest – Take relative rest. Taking a break from all the strenuous activities can help in relieving the pain but it won’t reverse the condition.
- Medication – You can ease your pain with the help of OTC medicines.
- Injections – You might be suggested to get steroid medications/injections directly into the affected area.
- Physical Therapy – Specific exercises can help you in strengthening your abdomen and back. Frequency specific microcurrent and certain exercises can relieve pain.
To stabilize the spine for a long term effect
- Spine rehab & accurate treatment by targeting specific muscle – This can be achieved by exercise plan focusing on the weaker muscles around abdomen and back. Regular exercise can relieve pain.
- Brace – A brace can help in stabilizing your spine. It limits movement so that fractures can heal.
Spondylolisthesis L5-S1 Exercises
Below are the exercises that may help in decreasing the pain..
- Pelvic tilt – It strengthens your lower abdominal muscles and also stretches your lower back.
- Lower trunk rotation – It increases the mobility and flexibility of your spine.
- Partial curl
- Pelvic bridging
Frequently Asked Questions about L5-S1 Spondylolisthesis
What do you mean by l5-s1, what is the best exercise for l5-s1 spondylolisthesis, what does spondylolisthesis mean, what are the common symptoms of l5-s1 spondylolisthesis.
- Pain in the lower back and/or associated leg pain
- Pain, numbness, weakness or tingling in legs or feet
- Pain that gets worse with activity
- Change in posture and gait caused by hamstring tightness
- Intermittent shooting pain that passes from buttocks down to legs
What are the different grades of spondylolisthesis?
What is the objective of l5-s1 spondylolisthesis treatment, what are the necessary diagnostic tests to be done for l5-s1 spondylolisthesis, what are the main causes of l5-s1 spondylolisthesis, what are the other problems that may occur at l5-s1.
Visit our nearest clinic for your first consultation
Recommended Articles

Have You Been Recommended Back Surgery?
Dr. garima anandani, 5 years ago.
Disc Degenerative L4-L5: Causes And Treatment
Qi spine clinic, 3 years ago.
Managing pain and mobility with sacroiliac joint d...
Qi spine clinic, 9 months ago.
We will notify you when our specialist answer your question.
Leave us your details
We will notify you when your question is answered
Our doctors usually respond within 24 hours
Enter your details and we will be in touch with you shortly Or call 086558 85566 between 8 am and 8 pm
We will be in touch with you shortly. Our clinic working hours are 8AM to 8PM.
Modal Header
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Chronic low back pain: history, symptoms, pain mechanisms, and treatment.

1. Introduction
2. evaluation of pain, 3. red flags, 4. yellow flags, psychosocial, 5. lifestyle, 5.1. function and disability, 5.3. smoking, 6. co-morbid conditions, 6.1. cardiovascular, 6.2. rheumatologic, 6.2.1. rheumatoid arthritis (ra), 6.2.2. axial spondyloarthritis (axspa), 6.3. diabetes, 6.4. malignancy, 6.5. gynecologic, 6.6. nephrology and urologic, 7. vertebral column pain, 7.1. anterior element pain, 7.2. posterior element pain, 7.2.1. facet joint pain, 7.2.2. other posterior spinal structures, 8. extra-vertebral column pain, 8.1. sacroiliac joint, 8.2. soft tissue structures, 8.2.1. maigne’s syndrome, 8.2.2. iliolumbar ligament pain, 8.2.3. spinal segmental instability and muscular dysfunction, 9. conclusions, author contributions, conflicts of interest.
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017 , 389 , 736–747. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018 , 392 , 1789–1858, Erratum in: Lancet 2019 , 393 , e44. https://doi.org/10.1016/S0140-6736(19)31047-5 . [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018 , 391 , 2368–2383. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Agence Nationale d’Accréditation et d’Evaluation en Santé. Diagnostic, Prise en Charge et Suivi des Malades Atteints de Lombalgie Chronique ; ANAES: Paris, France, 2000. [ Google Scholar ]
- Manchikanti, L.; Boswell, M.V.; Singh, V.; Benyamin, R.M.; Fellows, B.; Abdi, S.; Buenaventura, R.M.; Conn, A.; Datta, S.; Derby, R.; et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009 , 12 , 699–802. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dillane, J.B.; Fry, J.; Kalton, G. Acute back syndrome: A study from general practice. Br. Med. J. 1966 , 2 , 82–84. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021 , 398 , 78–92. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barrey, C.Y.; Le Huec, J.C.; French Society for Spine Surgery. Chronic low back pain: Relevance of a new classification based on the injury pattern. Orthop. Traumatol. Surg. Res. 2019 , 105 , 339–346. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Deyo, R.A.; Diehl, A.K. Cancer as a cause of back pain: Frequency, clinical presentation, and diagnostic strategies. J. Gen. Intern. Med. 1988 , 3 , 230–238. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Verhagen, A.P.; Downie, A.; Popal, N.; Maher, C.; Koes, B.W. Red flags presented in current low back pain guidelines: A review. Eur. Spine J. 2016 , 25 , 2788–2802. [ Google Scholar ] [ CrossRef ]
- Carragee, E.J.; Hannibal, M. Diagnostic evaluation of low back pain. Orthop. Clin. N. Am. 2004 , 35 , 7–16. [ Google Scholar ] [ CrossRef ]
- Kendall, N.A.; Linton, S.J.; Main, C.J. Guide to Assessing Psychosocial Yellow Flags in Acute Low Back Pain: Risk Factors for Long-Term Disability and Work Loss ; Accident Rehabilitation and Compensation Insurance Corporation of New Zealand and the National Health Committee: Wellington, New Zealand, 1997. [ Google Scholar ]
- Pinheiro, M.B.; Ferreira, M.L.; Refshauge, K.; Maher, C.G.; Ordoñana, J.R.; Andrade, T.B.; Tsathas, A.; Ferreira, P.H. Symptoms of depression as a prognostic factor for low back pain: A systematic review. Spine J. Off. J. N. Am. Spine Soc. 2016 , 16 , 105–116. [ Google Scholar ] [ CrossRef ]
- Kreiner, D.S.; Matz, P.; Bono, C.M.; Cho, C.H.; Easa, J.E.; Ghiselli, G.; Ghogawala, Z.; Reitman, C.A.; Resnick, D.K.; Watters, W.C.; et al. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J. 2020 , 20 , 998–1024, Erratum in Spine J. 2021 , 21 , 726–727. https://doi.org/10.1016/j.spinee.2021.02.006 . [ Google Scholar ] [ CrossRef ]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006 , 15 (Suppl. S2), S192–S300. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020 , 161 , 1976–1982. [ Google Scholar ] [ CrossRef ]
- Linton, S.J.; Shaw, W.S. Impact of Psychological Factors in the Experience of Pain. Phys. Ther. 2011 , 91 , 700–711. [ Google Scholar ] [ CrossRef ]
- Bener, A.; El-Rufaie, O.F.; Kamran, S.; Georgievski, A.B.; Farooq, A.; Rysavy, M. Disability, depression and somatization in low back pain population. Int. J. Rheum. Dis. 2006 , 9 , 257–263. [ Google Scholar ] [ CrossRef ]
- Magni, G.; Caldieron, C.; Rigatti-Luchini, S.; Merskey, H. Chronic musculoskeletal pain and depressive symptoms in the general population. An analysis of the 1st National Health and Nutrition Examination Survey data. Pain 1990 , 43 , 299–307. [ Google Scholar ] [ CrossRef ]
- He, C.; Chen, H.; Guo, L.; Xu, L.; Liu, Q.; Zhang, J.; Hu, X. Prevalence and factors associated with comorbid depressive symptoms among people with low back pain in China: A cross-sectional study. Front. Psychiatry 2022 , 13 , 922733. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, S.M.; Kim, H.J.; Jang, S.; Kim, H.; Chang, B.S.; Lee, C.K.; Yeom, J.S. Depression is Closely Associated With Chronic Low Back Pain in Patients Over 50 Years of Age: A Cross-sectional Study Using the Sixth Korea National Health and Nutrition Examination Survey (KNHANES VI-2). Spine 2018 , 43 , 1281–1288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fernandez, M.; Colodro-Conde, L.; Hartvigsen, J.; Ferreira, M.L.; Refshauge, K.M.; Pinheiro, M.B.; Ordoñana, J.R.; Ferreira, P.H. Chronic low back pain and the risk of depression or anxiety symptoms: Insights from a longitudinal twin study. Spine J. 2017 , 17 , 905–912. [ Google Scholar ] [ CrossRef ]
- Hurwitz, E.L.; Morgenstern, H.; Yu, F. Cross-sectional and longitudinal associations of low-back pain and related disability with psychological distress among patients enrolled in the UCLA Low-Back Pain Study. J. Clin. Epidemiol. 2003 , 56 , 463–471. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, H.; Hurwitz, E.L.; Li, J.; de Luca, K.; Tavares, P.; Green, B.; Haldeman, S. Bidirectional Comorbid Associations between Back Pain and Major Depression in US Adults. Int. J. Environ. Res. Public Health 2023 , 20 , 4217. [ Google Scholar ] [ CrossRef ]
- Corrêa, L.A.; Mathieson, S.; Meziat-Filho, N.A.M.; Reis, F.J.; Ferreira, A.S.; Nogueira, L.A.C. Which psychosocial factors are related to severe pain and functional limitation in patients with low back pain? Psychosocial factors related to severe low back pain. Braz. J. Phys. Ther. 2022 , 26 , 100413. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Akhtar, E.; Ballew, A.T.; Orr, W.N.; Mayorga, A.; Khan, T.W. The Prevalence of Post-Traumatic Stress Disorder Symptoms in Chronic Pain Patients in a Tertiary Care Setting: A Cross-Sectional Study. Psychosomatics 2019 , 60 , 255–262. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Valat, J.P.; Goupille, P.; Védere, V. Low back pain: Risk factors for chronicity. Rev. Rhum. Engl. Ed. 1997 , 64 , 189–194. [ Google Scholar ] [ PubMed ]
- Ochsmann, E.B.; Rueger, H.; Letzel, S.; Drexler, H.; Muenster, E. Over-indebtedness and its association with the prevalence of back pain. BMC Public Health 2009 , 9 , 451. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Department of Health Human Services FDA Center for Drug Evaluation Research; U.S. Department of Health Human Services FDA Center for Biologics Evaluation Research; U.S. Department of Health Human Services FDA Center for Devices Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006 , 4 , 79. [ Google Scholar ] [ CrossRef ]
- Thigpen, C.; Shanley, E. Clinical assessment of upper extremity injury outcomes. J. Sport Rehabil. 2011 , 20 , 61–73. [ Google Scholar ] [ CrossRef ]
- Verbunt, J.A.; Westerterp, K.R.; van der Heijden, G.J.; Seelen, H.A.; Vlaeyen, J.W.; Knottnerus, J.A. Physical activity in daily life in patients with chronic low back pain. Arch. Phys. Med. Rehabil. 2001 , 82 , 726–730. [ Google Scholar ] [ CrossRef ]
- van Weering, M.G.; Vollenbroek-Hutten, M.M.; Tönis, T.M.; Hermens, H.J. Daily physical activities in chronic lower back pain patients assessed with accelerometry. Eur. J. Pain 2009 , 13 , 649–654. [ Google Scholar ] [ CrossRef ]
- Citko, A.; Górski, S.; Marcinowicz, L.; Górska, A. Sedentary Lifestyle and Nonspecific Low Back Pain in Medical Personnel in North-East Poland. BioMed Res. Int. 2018 , 2018 , 1965807. [ Google Scholar ] [ CrossRef ]
- Björck-van Dijken, C.; Fjellman-Wiklund, A.; Hildingsson, C. Low back pain, lifestyle factors and physical activity: A population based-study. J. Rehabil. Med. 2008 , 40 , 864–869. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pinto, R.Z.; Ferreira, P.H.; Kongsted, A.; Ferreira, M.L.; Maher, C.G.; Kent, P. Self-reported moderate-to-vigorous leisure time physical activity predicts less pain and disability over 12 months in chronic and persistent low back pain. Eur. J. Pain 2014 , 18 , 1190–1198. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mikkelsson, L.O.; Nupponen, H.; Kaprio, J.; Kautiainen, H.; Mikkelsson, M.; Kujala, U.M. Adolescent flexibility, endurance strength, and physical activity as predictors of adult tension neck, low back pain, and knee injury: A 25 year follow up study. Br. J. Sports Med. 2006 , 40 , 107–113. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lin, C.C.; McAuley, J.H.; Macedo, L.; Barnett, D.C.; Smeets, R.J.; Verbunt, J.A. Relationship between physical activity and disability in low back pain: A systematic review and meta-analysis. Pain 2011 , 152 , 607–613. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Griffin, D.W.; Harmon, D.C.; Kennedy, N.M. Do patients with chronic low back pain have an altered level and/or pattern of physical activity compared to healthy individuals? A systematic review of the literature. Physiotherapy 2012 , 98 , 13–23. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ryan, C.G.; Grant, P.M.; Dall, P.M.; Gray, H.; Newton, M.; Granat, M.H. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: An observational study. Aust. J. Physiother. 2009 , 55 , 53–58. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Spenkelink, C.D.; Hutten, M.M.; Hermens, H.J.; Greitemann, B.O. Assessment of activities of daily living with an ambulatory monitoring system: A comparative study in patients with chronic low back pain and nonsymptomatic controls. Clin. Rehabil. 2002 , 16 , 16–26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bahouq, H.; Allali, F.; Rkain, H.; Hmamouchi, I.; Hajjaj-Hassouni, N. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol. Int. 2013 , 33 , 1277–1281. [ Google Scholar ] [ CrossRef ]
- Alsaadi, S.M.; McAuley, J.H.; Hush, J.M.; Maher, C.G. Prevalence of sleep disturbance in patients with low back pain. Eur. Spine J. 2011 , 20 , 737–743, Erratum in Eur. Spine J. 2012 , 21 , 554–560. https://doi.org/10.1007/s00586-011-1954-8 . [ Google Scholar ] [ CrossRef ]
- Kelly, G.A.; Blake, C.; Power, C.K.; O’keeffe, D.; Fullen, B.M. The association between chronic low back pain and sleep: A systematic review. Clin. J. Pain 2011 , 27 , 169–181. [ Google Scholar ] [ CrossRef ]
- Alsaadi, S.M.M.; McAuley, J.H.; Hush, J.M.; Lo, S.; Bartlett, D.J.; Grunstein, R.R.; Maher, C.G. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin. J. Pain 2014 , 30 , 755–765. [ Google Scholar ] [ CrossRef ]
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Nordstoga, A.L. Influence of sleep problems and co-occurring musculoskeletal pain on long-term prognosis of chronic low back pain: The HUNT Study. J. Epidemiol. Community Health 2020 , 74 , 283–289. [ Google Scholar ] [ CrossRef ]
- Sribastav, S.S.; Long, J.; He, P.; He, W.; Ye, F.; Li, Z.; Wang, J.; Liu, H.; Wang, H.; Zheng, Z. Risk Factors Associated with Pain Severity in Patients with Non-specific Low Back Pain in Southern China. Asian Spine J. 2018 , 12 , 533–543. [ Google Scholar ] [ CrossRef ]
- Ditre, J.W.; Heckman, B.W.; LaRowe, L.R.; Powers, J.M. Pain Status as a Predictor of Smoking Cessation Initiation, Lapse, and Relapse. Nicotine Tob. Res. 2021 , 23 , 186–194. [ Google Scholar ] [ CrossRef ]
- Jacob, L.; Freyn, M.; Kalder, M.; Dinas, K.; Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: A retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 2018 , 9 , 17420–17429. [ Google Scholar ] [ CrossRef ]
- McDaniel, J.C.; Browning, K.K. Smoking, chronic wound healing, and implications for evidence-based practice. J. Wound Ostomy Cont. Nurs. 2014 , 41 , 415–423. [ Google Scholar ] [ CrossRef ]
- Akmal, M.; Kesani, A.; Anand, B.; Singh, A.; Wiseman, M.; Goodship, A. Effect of nicotine on spinal disc cells: A cellular mechanism for disc degeneration. Spine 2004 , 29 , 568–575. [ Google Scholar ] [ CrossRef ]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart. 2024 , 19 , 11. [ Google Scholar ] [ CrossRef ]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020 , 76 , 2982–3021, Erratum in J. Am. Coll. Cardiol. 2021 , 77 , 1958–1959. [ Google Scholar ] [ CrossRef ]
- Gaziano, T.; Reddy, K.S.; Paccaud, F.; Horton, S.; Chaturvedi, V. Cardiovascular Disease. In Disease Control Priorities in Developing Countries , 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006; Chapter 33. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11767/ (accessed on 13 June 2024).
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021 , 21 , 401. [ Google Scholar ] [ CrossRef ]
- Oliveira, C.B.; Maher, C.G.; Franco, M.R.; Kamper, S.J.; Williams, C.; Silva, F.G.; Pinto, R.Z. Co-occurrence of chronic musculoskeletal pain and cardiovascular diseases: A systematic review with meta-analysis. Pain Med. 2020 , 21 , 1106–1121. [ Google Scholar ] [ CrossRef ]
- Zhu, K.; Devine, A.; Dick, I.M.; Prince, R.L. Association of back pain frequency with mortality, coronary heart events, mobility, and quality of life in elderly women. Spine 2007 , 32 , 2012–2018. [ Google Scholar ] [ CrossRef ]
- Shcherbina, A.; Longacre, M. The Association Between Atherosclerosis and Low Back Pain: A Systematic Review. PM&R 2017 , 9 , 1144–1156. [ Google Scholar ]
- Goodlin, S.J.; Wingate, S.; Albert, N.M.; Pressler, S.J.; Houser, J.; Kwon, J.; Chiong, J.; Storey, C.P.; Quill, T.; Teerlink, J.R. Investigating Pain in Heart Failure Patients: The Pain Assessment, Incidence, and Nature in Heart Failure (PAIN-HF) Study. J. Card. Fail. 2012 , 18 , 776–783. [ Google Scholar ] [ CrossRef ]
- Chen, J.; Zhang, Y.; Simonsick, E.; Starkweather, A.; Chen, M.-H.; McCauley, P.; Chyun, D.; Cong, X. Back pain and heart failure in community-dwelling older adults: Findings from the Health ABC study. Geriatr. Nurs. 2021 , 42 , 643–649. [ Google Scholar ] [ CrossRef ]
- Samartzis, D.; Bow, C.; Karppinen, J.; Luk, K.D.K.; Cheung, B.M.Y.; Cheung, K.M.C. Hypertension is independently associated with lumbar disc degeneration: A large-scale population-based study. Glob. Spine J. Conf. World Forum Spine Res. 2014 , 4 , s-0034-1376579. [ Google Scholar ] [ CrossRef ]
- Leino-Arjas, P.; Kaila-Kangas, L.; Solovieva, S.; Riihimäki, H.; Kirjonen, J.; Reunanen, A. Serum lipids and low back pain: An association? A follow-up study of a working population sample. Spine 2006 , 31 , 1032–1037. [ Google Scholar ] [ CrossRef ]
- Leino-Arjas, P.; Solovieva, S.; Kirjonen, J.; Reunanen, A.; Riihimaki, H. Cardiovascular risk factors and low-back pain in a long-term follow-up of industrial employees. Scand. J. Work. Environ. Health 2006 , 32 , 12–19. [ Google Scholar ] [ CrossRef ]
- Zhang, T.T.; Liu, Z.; Liu, Y.L.; Zhao, J.J.; Liu, D.W.; Tian, Q.B. Obesity as a Risk Factor for Low Back Pain: A Meta-Analysis. Clin. Spine Surg. 2018 , 31 , 22–27. [ Google Scholar ] [ CrossRef ]
- Peng, T.; Perez, A.; Pettee Gabriel, K. The Association among Over weight, Obesity, and Low Back Pain in U.S. Adults: A Cross-Sectional Study of the 2015 National Health Interview Survey. J. Manip. Physiol. Ther. 2018 , 41 , 294–303. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pozzobon, D.; Ferreira, P.H.; Dario, A.B.; Almeida, L.; Vesentini, G.; Harmer, A.R.; Ferreira, M.L. Is there an association between diabetes and neck and back pain? A systematic review with meta-analyses. PLoS ONE 2019 , 14 , e0212030. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, C.H.; Min, K.B.; Min, J.Y.; Kim, D.H.; Seo, K.M.; Kim, D.K. Strong association of type 2 diabetes with degenerative lumbar spine disorders. Sci. Rep. 2021 , 11 , 16472. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vogt, M.T.; Nevitt, M.C.; Cauley, J.A. Back problems and atherosclerosis. The Study of Osteoporotic Fractures. Spine 1997 , 22 , 2741–2747. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Smith, D.; Wilkie, R.; Croft, P.; McBeth, J. Pain and mortality in older adults: The influence of pain phenotype. Arthritis Care Res. 2017 , 70 , 236–243. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Celano, C.M.; Villegas, A.C.; Albanese, A.M.; Gaggin, H.K.; Huffman, J.C. Depression and Anxiety in Heart Failure: A Review. Harv. Rev. Psychiatry 2018 , 26 , 175–184. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rutledge, T.; Reis, V.A.; Linke, S.E.; Greenberg, B.H.; Mills, P.J. Depression in Heart Failure. A Meta-Analytic Review of Prevalence, Intervention Effects, and Associations with Clinical Outcomes. J. Am. Coll. Cardiol. 2006 , 48 , 1527–1537. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Conley, S.; Feder, S.; Redeker, N.S. The relationship between pain, fatigue, depression and functional performance in stable heart failure. Hear. Lung 2015 , 44 , 107–112. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Whooley, M.A.; de Jonge, P.; Vittinghoff, E.; Otte, C.; Moos, R.; Carney, R.M.; Ali, S.; Dowray, S.; Na, B.; Feldman, M.D.; et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008 , 300 , 2379–2388. [ Google Scholar ] [ CrossRef ]
- Pantilat, S.Z.; Riordan, D.L.O.; Rathfon, M.A. Etiology of Pain and Its Association with Quality of Life among Patients with Heart Failure. J. Palliat. Med. 2016 , 19 , 1254–1259. [ Google Scholar ] [ CrossRef ]
- Kauppila, L.I.; Mikkonen, R.; Mankinen, P.; Pelto-Vasenius, K.; Maenpaa, I. MR aortography and serum cholesterol levels in patients with long-term nonspecific lower back pain. Spine 2004 , 29 , 2147–2152. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Turgut, A.T.; Sonmez, I.; Cakit, B.D.; Kosar, P.; Kosar, U. Pineal gland calcification, lumbar intervertebral disc degeneration and abdominal aorta calcifying atherosclerosis correlate in low back pain subjects: A cross-sectional observational CT study. Pathophysiology 2008 , 15 , 31–39. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kearney, P.M.; Baigent, C.; Godwin, J.; Halls, H.; Emberson, J.R.; Patrono, C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006 , 332 , 1302–1308. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cannon, C.P.; Curtis, S.P.; FitzGerald, G.A. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: A randomised comparison. Lancet 2006 , 368 , 1771–1781. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS Study: A randomized controlled trial. JAMA 2000 , 284 , 1247–1255. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chan, T.Y. Adverse interactions between warfarin and nonsteroidal antiinflammatory drugs: Mechanisms, clinical significance, and avoidance. Ann. Pharmacother. 1995 , 29 , 1274–1283. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gary, R.A.; Dunbar, S.B.; Higgins, M.K.; Musselman, D.L.; Smith, A.L. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J. Psychosom. Res. 2010 , 69 , 119–131. [ Google Scholar ] [ CrossRef ]
- Helliwell, P.S.; Zebouni, L.N.; Porter, G.; Wright, V. A clinical and radiological study of back pain in rheumatoid arthritis. Br. J. Rheumatol. 1993 , 32 , 216–221. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Baykara, R.A.; Bozgeyik, Z.; Akgul, O.; Ozgocmen, S. Low back pain in patients with rheumatoid arthritis: Clinical characteristics and impact of low back pain on functional ability and health related quality of life. J. Back Musculoskelet. Rehabil. 2013 , 26 , 367–374. [ Google Scholar ] [ CrossRef ]
- Kothe, R.; Kohlmann, T.; Klink, T.; Rüther, W.; Klinger, R. Impact of low back pain on functional limitations, depressed mood and quality of life in patients with rheumatoid arthritis. Pain 2007 , 127 , 103–108. [ Google Scholar ] [ CrossRef ]
- Miura, K.; Morita, O.; Hirano, T.; Watanabe, K.; Fujisawa, J.; Kondo, N.; Netsu, T.; Hanyu, T.; Shobugawa, Y.; Endo, N. Prevalence of and factors associated with dysfunctional low back pain in patients with rheumatoid arthritis. Eur. Spine J. 2019 , 28 , 976–982. [ Google Scholar ] [ CrossRef ]
- Van Der Heijde, D.; Landewe, R.; Van Vollenhoven, R.; Fatenejad, S.; Klareskog, L. Level of radiographic damage and radiographic progression are determinants of physical function: A longitudinal analysis of the TEMPO trial. Ann. Rheum. Dis. 2008 , 67 , 1267–1270. [ Google Scholar ] [ CrossRef ]
- Wang, R.; Ward, M.M. Epidemiology of axial spondyloarthritis: An update. Curr. Opin. Rheumatol. 2018 , 30 , 137–143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Winkler, A.E.; Miller, M. Update on Axial Spondyloarthritis. Mo. Med. 2022 , 119 , 79–83. [ Google Scholar ]
- Chen, S.; Liao, M.; Li, J.; Peng, H.; Xiong, M. The correlation between microvessel pathological changes of the endplate and degeneration of the intervertebral disc in diabetic rats. Exp. Ther. Med. 2013 , 5 , 711–717. [ Google Scholar ] [ CrossRef ]
- Poddubnyy, D.; Rudwaleit, M. Early spondyloarthritis. Rheum. Dis. Clin. N. Am. 2012 , 38 , 387–403. [ Google Scholar ]
- Dubreuil, M.; Deodhar, A.A. Axial spondyloarthritis classification criteria: The debate continues. Curr. Opin. Rheumatol. 2017 , 29 , 317–322. [ Google Scholar ] [ CrossRef ]
- McVeigh, C.M.; Cairns, A.P. Diagnosis and management of ankylosing spondylitis. BMJ 2006 , 333 , 581–585. [ Google Scholar ] [ CrossRef ]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020 , 10 , 107–111. [ Google Scholar ] [ CrossRef ]
- Kakadiya, G.; Gandbhir, V.; Soni, Y.; Gohil, K.; Shakya, A. Diabetes Mellitus-A Risk Factor for the Development of Lumbar Disc Degeneration: A Retrospective Study of an Indian Population. Glob. Spine J. 2022 , 12 , 215–220. [ Google Scholar ] [ CrossRef ]
- Arkkila, P.E.; Gautier, J.F. Musculoskeletal disor-ders in diabetes mellitus. Best Pract. Res. Clin. Rheumatol. 2003 , 17 , 945–970. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kong, J.G.; Park, J.B.; Lee, D.; Park, E.Y. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 2015 , 9 , 155–161. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van den Brande, R.; Cornips, E.M.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022 , 35 , 100446. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Perrin, R.G.; Laxton, A.W. Metastatic spine disease: Epidemiology, pathophysiology, and evaluation of patients. Neurosurg. Clin. N. Am. 2004 , 15 , 365–373. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression ; National Collaborating Centre for Cancer (UK): Cardiff, UK, 2008. [ PubMed ]
- Ziu, E.; Viswanathan, V.K.; Mesfin, F.B. Spinal Metastasis. [Updated 14 August 2023]. In StatPearls [Internet] ; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441950/ (accessed on 8 June 2024).
- Shakil, H.; Malhotra, A.K.; Badhiwala, J.H.; Karthikeyan, V.; Essa, A.; He, Y.; Fehlings, M.G.; Sahgal, A.; Dea, N.; Kiss, A.; et al. Contemporary trends in the incidence and timing of spinal metastases: A population-based study. Neurooncol. Adv. 2024 , 6 , vdae051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Janjan, N.A. Radiation for bone metastases: Conventional techniques and the role of systemic radiopharmaceuticals. Cancer 1997 , 80 (Suppl. S8), 1628–1645. [ Google Scholar ] [ CrossRef ]
- Sutcliffe, P.; Connock, M.; Shyangdan, D.; Court, R.; Kandala, N.B.; Clarke, A. A systematic review of evidence on malignant spinal metastases: Natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol. Assess. 2013 , 17 , 1–274. [ Google Scholar ] [ CrossRef ]
- Chin, H.; Kim, J. Bone Metastasis: Concise Overview. Fed Pract. 2015 , 32 , 24–30. [ Google Scholar ]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017 , 6 , 34–41. [ Google Scholar ] [ CrossRef ]
- Brott, N.R.; Le, J.K. Mittelschmerz. In StatPearls [Internet] ; StatPearls Publishing: Treasure Island, FL, USA, 2024. [ Google Scholar ] [ PubMed ]
- Patti, L.; Leslie, S.W. Acute Renal Colic. [Updated 2024 Jun 6]. In StatPearls [Internet] ; StatPearls Publishing: Treasure Island, FL, USA, 2024. [ Google Scholar ]
- Pirola, G.M.; Verdacchi, T.; Rosadi, S.; Annino, F.; De Angelis, M. Chronic prostatitis: Current treatment options. Res. Rep. Urol. 2019 , 11 , 165–174. [ Google Scholar ] [ CrossRef ]
- Roy, P.J.; Weltman, M.; Dember, L.M.; Liebschutz, J.; Jhamb, M.; HOPE Consortium. Pain management in patients with chronic kidney disease and end-stage kidney disease. Curr. Opin. Nephrol. Hypertens. 2020 , 29 , 671–680. [ Google Scholar ] [ CrossRef ]
- Hedayati, S.S.; Yalamanchili, V.; Finkelstein, F.O. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012 , 81 , 247–255. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P.; Lancet Low Back Pain Series Working Group. Low back pain: A call for action. Lancet 2018 , 391 , 2384–2388. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- DePalma, M.J.; Ketchum, J.M.; Saullo, T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011 , 12 , 224–233. [ Google Scholar ] [ PubMed ]
- Ohtori, S.; Miyagi, M.; Inoue, G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg. Relat. Res. 2018 , 2 , 11–17. [ Google Scholar ]
- Fields, A.J.; Liebenberg, E.C.; Lotz, J.C. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014 , 14 , 513–521. [ Google Scholar ]
- Lotz, J.C.; Fields, A.J.; Liebenberg, E.C. The role of the vertebral end plate in low back pain. Glob. Spine J. 2013 , 3 , 153–163. [ Google Scholar ]
- Fischgrund, J.S.; Rhyne, A.; Macadaeg, K.; Moore, G.; Kamrava, E.; Yeung, C.; Truumees, E.; Schaufele, M.; Yuan, P.; DePalma, M.; et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur. Spine J. 2020 , 29 , 1925–1934. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988 , 166 , 193–199. [ Google Scholar ] [ CrossRef ]
- Arana, E.; Kovacs, F.M.; Royuela, A.; Estremera, A.; Asenjo, B.; Sarasíbar, H.; Amengual, G.; Galarraga, I.; Alonso, A.; Casillas, C.; et al. Modic changes and associated features in Southern European chronic low back pain patients. Spine J. 2011 , 11 , 402–411. [ Google Scholar ] [ CrossRef ]
- Sheng-Yun, L.; Letu, S.; Jian, C.; Mamuti, M.; Jun-Hui, L.; Zhi, S.; Chong-Yan, W.; Shunwu, F.; Zhao, F. Comparison of modic changes in the lumbar and cervical spine, in 3167 patients with and without spinal pain. PLoS ONE 2014 , 9 , e114993. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Herlin, C.; Kjaer, P.; Espeland, A.; Skouen, J.S.; Leboeuf-Yde, C.; Karppinen, J.; Niinimäki, J.; Sørensen, J.S.; Storheim, K.; Jensen, T.S. Modic changes-their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE 2018 , 13 , e0200677. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Czaplewski, L.G.; Rimmer, O.; McHale, D.; Laslett, M. Modic changes as seen on MRI are associated with nonspecific chronic lower back pain and disability. J. Orthop. Surg. Res. 2023 , 18 , 351. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Chou, D.; Samartzis, D.; Bellabarba, C.; Patel, A.; Luk, K.D.K.; Kisser, J.M.S.; Skelly, A.C. Degenerative Magnetic Resonance Imaging Changes in Patients with Chronic Low Back Pain. Spine 2011 , 36 , S43–S53. [ Google Scholar ] [ CrossRef ]
- Mohd Isa, I.L.; Teoh, S.L.; Mohd Nor, N.H.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022 , 24 , 208. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Nachemson, A. Measurement of intradiscal pressure. Acta Orthop. 1959 , 28 , 269–289. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Althoff, I.; Brinckmann, P.; Frobin, W.; Sandover, J.; Burton, K. An improved method of stature measurement for quantitative determination of spinal loading. Application to sitting postures and whole body vibration. Spine 1992 , 17 , 682–693. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, J.Q.; Kwong, W.H.; Chan, Y.L.; Kawabata, M. Comparison of in vivo Intradiscal Pressure between Sitting and Standing in Human Lumbar Spine: A Systematic Review and Meta-Analysis. Life 2022 , 12 , 457. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Rohlmann, A.; Zander, T.; Graichen, F.; Bergmann, G. Lifting up and laying down a weight causes high spinal loads. J. Biomech. 2013 , 46 , 511–514. [ Google Scholar ]
- Dolan, K.J.; Green, A. Lumbar spine reposition sense: The effect of a ‘slouched’ posture. Man. Ther. 2006 , 11 , 202. [ Google Scholar ] [ CrossRef ]
- Méndez-Gutiérrez, A.; Marín Navas, F.; Acevedo-González, J.C. Frequency of use of discography findings for the diagnosis of low back pain of discogenic origin. Systematic review of the literature. Rev. Esp. Cir. Ortop. Traumatol. 2024 , 68 , 209–222. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cuellar, J.M.; Stauff, M.P.; Herzog, R.J.; Carrino, J.A.; Baker, G.A.; Carragee, E.J. Does provocative discography cause clinically important injury to the lumbar intervertebral disc? A 10-year matched cohort study. Spine J. 2016 , 16 , 273–280. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peng, B.; Pang, X.; Wu, Y.; Zhao, C.; Song, X. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain 2010 , 149 , 124–129. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kallewaard, J.W.; Wintraecken, V.M.; Geurts, J.W.; Willems, P.C.; van Santbrink, H.; Terwiel, C.T.M.; van Kleef, M.; van Kuijk, S.M.J. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: The IMBI study. Pain 2019 , 160 , 945–953. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Freeman, B.J.; Mehdian, R. Intradiscal electrothermal therapy, percutaneous discectomy, and nucleoplasty: What is the current evidence? Curr. Pain Headache Rep. 2008 , 12 , 14–21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bibby, S.R.; Urban, J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004 , 13 , 695–701. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Jiang, Y.; Wang, J.; Sun, D.; Liu, Z.; Qi, L.; Du, M.; Wang, J.; Li, Y.; Zhu, C.; Huang, Y.; et al. A hydrogel reservoir as a self-contained nucleus pulposus cell delivery vehicle for immunoregulation and repair of degenerated intervertebral disc. Acta Biomater. 2023 , 170 , 303–317. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schneider, B.J.; Hunt, C.; Conger, A.; Qu, W.; Maus, T.P.; Vorobeychik, Y.; Cheng, J.; Duszynski, B.; McCormick, Z.L. The effectiveness of intradiscal biologic treatments for discogenic low back pain: A systematic review. Spine J. 2022 , 22 , 226–237. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Glover, J.R. Arthrography of the joints of the lumbar vertebral arches. Orthop. Clin. N. Am. 1977 , 8 , 37–42. [ Google Scholar ] [ CrossRef ]
- Bogduk, N. Clinical Anatomy of the Lumbar Spine and Sacrum , 3rd ed.; Churchill Livingstone: Edinburgh, UK, 1997; pp. 127–144. [ Google Scholar ]
- Goldthwaite, J.E. The lumbosacral articulation: An explanation of many cases of lumbago, sciatica, and paraplegia. Boston. Med. Surg. J. 1911 , 164 , 365–372. [ Google Scholar ] [ CrossRef ]
- Hirsch, C.; Ingelmark, B.E.; Miller, M. The anatomical basis for low back pain: Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta. Orthop. Scand. 1963 , 33 , 1–17. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cyron, B.M.; Hutton, W.C. The tensile strength of the capsular ligaments of the apophyseal joints. J. Anat. 1981 , 132 , 145–150. [ Google Scholar ]
- Yang, K.H.; King, A.I. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine 1984 , 9 , 557–565. [ Google Scholar ] [ CrossRef ]
- Dory, M.A. Arthrography of the lumbar facet joints. Radiology 1981 , 140 , 23–27. [ Google Scholar ] [ CrossRef ]
- Revel, M.; Poiraudeau, S.; Auleley, G.R.; Payan, C.; Denke, A.; Nguyen, M.; Chevrot, A.; Fermanian, J. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: Proposed criteria to identify patients with painful facet joints. Spine 1998 , 23 , 1972–1976. [ Google Scholar ] [ CrossRef ]
- Chen, X.; Tang, H.; Lin, J.; Zeng, R. Temporal trends in the disease burden of osteoarthritis from 1990 to 2019, and projections until 2030. PLoS ONE 2023 , 18 , e0288561. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- O’Leary, S.A.; Paschos, N.K.; Link, J.M.; Klineberg, E.O.; Hu, J.C.; Athanasiou, K.A. Facet Joints of the Spine: Structure-Function Relationships, Problems and Treatments, and the Potential for Regeneration. Annu. Rev. Biomed. Eng. 2018 , 20 , 145–170. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Falco, F.J.E.; Manchikanti, L.; Datta, S.; Sehgal, N.; Geffert, S.; Onyewu, O.; Singh, V.; Bryce, D.A.; Benyamin, R.M.; Simopoulos, T.T.; et al. An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician 2012 , 15 , E869–E907. [ Google Scholar ] [ CrossRef ]
- Manchikanti, L.; Singh, V.; Pampati, V.; Damron, K.S.; Barnhill, R.C.; Beyer, C.; A Cash, K. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 2001 , 4 , 308–316. [ Google Scholar ] [ CrossRef ]
- MacVicar, J.; MacVicar, A.M.; Bogduk, N. The Prevalence of “Pure” Lumbar Zygapophysial Joint Pain in Patients with Chronic Low Back Pain. Pain Med. 2021 , 22 , 41–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Matar, H.E.; Navalkissoor, S.; Berovic, M.; Shetty, R.; Garlick, N.; Casey, A.T.; Quigley, A.M. Is hybrid imaging (SPECT/CT) a useful adjunct in the management of suspected facet joints arthropathy? Int. Orthop. 2013 , 37 , 865–870. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Dolan, A.L.; Ryan, P.J.; Arden, N.K.; Stratton, R.; Wedley, J.R.; Hamann, W.; Fogelman, I.; Gibson, T. The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Br. J. Rheum. 1996 , 35 , 1269–1273. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carrera, G.F.; Williams, A.L. Current concepts in evaluation of the lumbar facet joints. Crit. Rev. Diagn. Imaging 1984 , 21 , 85–104. [ Google Scholar ] [ PubMed ]
- Raymond, J.; Dumas, J.M.; Lisbona, R. Nuclear imaging as a screening test for patients referred for intraarticular facet block. J. Can. Assoc. Radiol. 1984 , 35 , 291–292. [ Google Scholar ] [ PubMed ]
- Revel, M.E.; Listrat, V.M.; Chevalier, X.J.; Dougados, M.; Nguyen, M.P.; Vallee, C.; Wybier, M.; Gires, F.; Amor, B. Facet joint block for low back pain: Identifying predictors of a good response. Arch. Phys. Med. Rehabil. 1992 , 73 , 824–828. [ Google Scholar ] [ PubMed ]
- Lanuzzi, A.; Little, J.S.; Chiu, J.B.; Baitner, A.; Kawchuk, G.; Khalsa, P.S. Human lumbar facet joint capsule strains, I. During physiological motions. Spine J. 2004 , 4 , 141–152. [ Google Scholar ]
- Little, J.S.; Ianuzzi, A.; Chiu, J.B.; Baitner, A.; Khalsa, P.S. Human lumbar facet joint capsule strains: I.I. Alteration of strains subsequent to anterior interbody fixation. Spine J. 2004 , 4 , 153–162. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stuber, K.; Lerede, C.; Kristmanson, K.; Sajko, S.; Bruno, P. The diagnostic accuracy of the Kemp’s test: A systematic review. J. Can. Chiropr. Assoc. 2014 , 58 , 258–267. [ Google Scholar ] [ PubMed ] [ PubMed Central ]
- Cohen, S.P.; Bhaskar, A.; Bhatia, A.; Buvanendran, A.; Deer, T.; Garg, S.; Hooten, W.M.; Hurley, R.W.; Kennedy, D.J.; McLean, B.C.; et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg. Anesth. Pain Med. 2020 , 45 , 424–467. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Bogduk, N. On the rational use of diagnostic blocks for spinal pain. Neurosurg. Quart. 2009 , 19 , 88–100. [ Google Scholar ] [ CrossRef ]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain 1994 , 58 , 195–200. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manchikanti, L.; Pampati, V.; Fellows, B.; Bakhit, C.E. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr. Rev. Pain. 2000 , 4 , 337–344. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bogduk, N. On diagnostic blocks for lumbar zygapophysial joint pain. F1000 Med. Rep. 2010 , 2 , 57. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Bogduk, N.; Holmes, S. Controlled zygapophysial joint blocks: The travesty of cost-effectiveness. Pain Med. 2000 , 1 , 25–34. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Leggett, L.E.; Soril, L.J.; Lorenzetti, D.L.; Noseworthy, T.; Steadman, R.; Tiwana, S.; Clement, F. Radiofrequency ablation for chronic low back pain: A systematic review of randomized controlled trials. Pain Res. Manag. J. Can. Pain Soc. 2014 , 19 , e146–e153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Leclaire, R.; Fortin, L.; Lambert, R.; Bergeron, Y.M.; Rossignol, M. Radiofrequency facet joint denervation in the treatment of low back pain: A placebo-controlled clinical trial to assess efficacy. Spine 2001 , 26 , 1411–1416 discussion 1417. [ Google Scholar ] [ CrossRef ]
- Lee, C.-H.; Chung, C.K.; Kim, C.H. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: A meta-analysis of randomized controlled trials. Spine J. Off. J. N. Am. Spine Soc. 2017 , 17 , 1770–1780. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maas, E.T.; Ostelo, R.W.; Niemisto, L.; Jousimaa, J.; Hurri, H.; Malmivaara, A.; van Tulder, M.W. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst. Rev. 2015 , 2015 , CD008572. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Juch, J.N.S.; Maas, E.T.; Ostelo, R.W.J.G.; Groeneweg, J.G.; Kallewaard, J.W.; Koes, B.W.; Verhagen, A.P.; van Dongen, J.M.; Huygen, F.J.P.M.; van Tulder, M.W. Effect of Radiofrequency Denervation on Pain Intensity Among Patients with Chronic Low Back Pain: The Mint Randomized Clinical Trials. JAMA 2017 , 318 , 68–81, Erratum in: JAMA 2017 , 318 , 1188. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Du, T.; Lu, G.; Li, J.; Ni, B.; Shu, W.; Sun, T.; Yang, D.; Zhu, H. Pain-Free Survival after Endoscopic Rhizotomy Versus Radiofrequency for Lumbar Facet Joint Pain: A Real-World Comparison Study. Pain Physician 2022 , 25 , E87–E94. [ Google Scholar ] [ PubMed ]
- Lilius, G.; Laasonen, E.M.; Myllynen, P.; Harilainen, A.; Grönlund, G. Lumbar Facet Joint Syndrome. A Randomised Clinical Trial. Bone Jt. J. 1989 , 71 , 681–684. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carette, S.; Marcoux, S.; Truchon, R.; Grondin, C.; Gagnon, J.; Allard, Y.; Latulippe, M. A Controlled Trial of Corticosteroid Injections into Facet Joints for Chronic Low Back Pain. N. Engl. J. Med. 1991 , 325 , 1002–1007. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barnsley, L.; Lord, S.M.; Wallis, J.; Bogduk, N. Lack of Effect of Intraarticular Corticosteroids for Chronic Pain in the Cervical Zygapophyseal Joints. N. Engl. J. Med. 1994 , 330 , 1047–1050. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kennedy, D.J.; Fraiser, R.; Zheng, P.; Huynh, L.; Levin, J.; Smuck, M.; Schneider, B.J. Intra-articular Steroids vs. Saline for Lumbar Z-Joint Pain: A Prospective, Randomized, Double-Blind Placebo-Controlled Trial. Pain Med. 2019 , 20 , 246–251. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Greater Manchester Combined Authority. Greater Manchester EUR Policy Statement on: Facet Joint Injections for Neck and Back Pain. GM Ref: GM070. 2019. Version 2.2. Available online: http://northwestcsu.nhs.uk/BrickwallResource/GetResource/d5f134e0-aab1-4be3-9475-3f973f126423 (accessed on 20 April 2024).
- Boswell, M.V.; Manchikanti, L.; Kaye, A.D.; Bakshi, S.; Gharibo, C.G.; Gupta, S.; Jha, S.S.; Nampiaparampil, D.E.; Simopoulos, T.T.; Hirsch, J.A. A Best-Evidence Systematic Appraisal of the Diagnostic Accuracy and Utility of Facet (Zygapophysial) Joint Injections in Chronic Spinal Pain. Pain Physician 2015 , 18 , E497–E533. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schneider, B.J.; Doan, L.; Maes, M.K.; Martinez, K.R.; Cota, A.G.; Bogduk, N. Systematic Review of the Effectiveness of Lumbar Medial Branch Thermal Radiofrequency Neurotomy, Stratified for Diagnostic Methods and Procedural Technique. Pain Med. 2020 , 21 , 1122–1141. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gillespie, K.A.; Dickey, J.P. Biomechanical role of lumbar spine ligaments in flexion and extension: Determination using a parallel linkage robot and a porcine model. Spine 2004 , 29 , 1208–1216. Available online: https://insights.ovid.com/pubmed?pmid=15167660 (accessed on 20 April 2024). [ CrossRef ] [ PubMed ]
- Keller, M.S. Gymnastics injuries and imaging in children. Pediatr. Radiol. 2009 , 39 , 1299–1306. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Baastrup, C.I. On the Spinous Processes of the Lumbar Vertebrae and the Soft Tissues between Them, and on Pathological Changes in That Region. Acta Radiol. 1933 , 14 , 52–55. [ Google Scholar ] [ CrossRef ]
- Hatgis, J.; Granville, M.; Jacobson, R.E. Baastrup’s Disease, Interspinal Bursitis, and Dorsal Epidural Cysts: Radiologic Evaluation and Impact on Treatment Options. Cureus 2017 , 9 , e1449. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Beks, J. Kissing spines: Fact or fancy? Acta Neurochir. 1989 , 100 , 134–135. [ Google Scholar ] [ CrossRef ]
- Lamer, T.J.; Tiede, J.M.; Fenton, D.S. Fluoroscopically-guided injections to treat “kissing spine” disease. Pain Physician 2008 , 11 , 549–554. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mitra, R.; Ghazi, U.; Kirpalani, D.; Cheng, I. Interspinous ligament steroid injections for the management of Baastrup’s disease: A case report. Arch. Phys. Med. Rehabil. 2007 , 88 , 1353–1356. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Filippiadis, D.K.; Mazioti, A.; Argentos, S.; Anselmetti, G.; Papakonstantinou, O.; Kelekis, N.; Kelekis, A. Baastrup’s disease (kissing spines syndrome): A pictorial review. Insights Imaging 2015 , 6 , 123–128. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shokri, P.; Zahmatyar, M.; Falah Tafti, M.; Fathy, M.; Rezaei Tolzali, M.; Ghaffari Jolfayi, A.; Nejadghaderi, S.A.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Non-spinal low back pain: Global epidemiology, trends, and risk factors. Health Sci. Rep. 2023 , 6 , e1533. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Tsoi, C.; Griffith, J.F.; Lee, R.K.L.; Wong, P.C.H.; Tam, L.S. Imaging of sacroiliitis: Current status, limitations and pitfalls. Quant. Imaging Med. Surg. 2019 , 9 , 318–335. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cher, D.; Polly, D.; Berven, S. Sacroiliac joint pain: Burden of disease. Med. Devices 2014 , 7 , 73–81. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schuit, D.; McPoil, T.G.; Mulesa, P. Incidence of sacroiliac joint malalignment in leg length discrepancies. J. Am. Podiatr. Med Assoc. 1989 , 79 , 380–383. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Herzog, W.; Conway, P.J. Gait analysis of sacroiliac joint patients. J. Manip. Physiol Ther. 1994 , 17 , 124–127. [ Google Scholar ]
- Marymont, J.V.; Lynch, M.A.; Henning, C.E. Exercise-related stress reaction of the sacroiliac joint: An unusual cause of low back pain in athletes. Am. J. Sports Med. 1986 , 14 , 320–323. [ Google Scholar ] [ CrossRef ]
- Schoenberger, M.; Hellmich, K. Sacroiliac dislocation and scoliosis. Hippokrates 1964 , 35 , 476–479. [ Google Scholar ]
- Albert, H.; Godskesen, M.; Westergaard, J. Prognosis in four syndromes of pregnancy-related pelvic pain. Acta Obstet. Gynecol. Scand. 2001 , 80 , 505–510. [ Google Scholar ] [ PubMed ]
- Berg, G.; Hammar, M.; Mollernielsen, J.; Linden, U.; Thorblad, J. Low back pain during pregnancy. Obs. Gynecol. 1988 , 71 , 71–75. [ Google Scholar ] [ CrossRef ]
- Katz, V.; Schofferman, J.; Reynolds, J. The sacroiliac joint: A potential cause of pain after lumbar fusion to the sacrum. J. Spinal Disord. Tech. 2003 , 16 , 96–99. [ Google Scholar ] [ CrossRef ]
- Cibulka, M.T.; Sinacore, D.R.; Cromer, G.S.; Delitto, A. Unilateral hip rotation range of motion asymmetry in patients with sacroiliac joint regional pain. Spine 1998 , 23 , 1009–1015. [ Google Scholar ] [ CrossRef ]
- Krishnamoorthy, V.P.; Beck, E.C.; Kunze, K.N.; Cancienne, J.M.; Krivicich, L.M.; Suppauksorn, S.; Ayeni, O.R.; Nho, S.J. Radiographic prevalence of sacroiliac joint abnormalities and clinical outcomes in patients with femoroacetabular impingement syndrome. Arthroscopy 2019 , 35 , 2598–2605.e1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fortin, J.D.; Tolchin, R.B. Sacroiliac arthrograms and post-arthrography computerized tomography. Pain Physician 2003 , 6 , 287–290. [ Google Scholar ] [ CrossRef ]
- Slipman, C.W.; Jackson, H.B.; Lipetz, J.S.; Chan, K.T.; Lenrow, D.; Vresilovic, E.J. Sacroiliac joint pain referral zones. Arch. Phys. Med. Rehabil. 2000 , 81 , 334–338. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fortin, J.D.; Dwyer, A.P.; West, S.; Pier, J. Sacroiliac joint: Pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine 1994 , 19 , 1475–1482. [ Google Scholar ] [ CrossRef ]
- Fortin, J.D.; Falco, F.J. The Fortin finger test: An indicator of sacroiliac pain. Am. J. Orthop. 1997 , 26 , 477–480. [ Google Scholar ]
- Telli, H.; Telli, S.; Topal, M. The validity and reliability of provocation test in the diagnosis of sacroiliac joint dysfunction. Pain Physician 2018 , 21 , E367. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schneider, B.J.; Ehsanian, R.; Rosati, R.; Huynh, L.; Levin, J.; Kennedy, D.J. Validity of Physical Exam Maneuvers in the Diagnosis of Sacroiliac Joint Pathology. Pain Med. 2020 , 21 , 255–260. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saueressig, T.; Owen, P.J.; Diemer, F.; Zebisch, J.; Belavy, D.L. Diagnostic Accuracy of Clusters of Pain Provocation Tests for Detecting Sacroiliac Joint Pain: Systematic Review with Meta-analysis. J. Orthop. Sports Phys. Ther. 2021 , 51 , 422–431. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stelzer, W.; Stelzer, D.; Stelzer, E.; Sammer, A.; Aichner, E.; Braune, M.; Schneider, B.J.; Duller, C.; Feigl, G. Success Rate of Intra-articular Sacroiliac Joint Injection: Fluoroscopy vs. Ultrasound Guidance-A Cadaveric Study. Pain Med. 2019 , 20 , 1890–1897. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Simopoulos, T.T.; Manchikanti, L.; Gupta, S.; Aydin, S.M.; Kim, C.H.; Solanki, D.R.; Nampiaparampil, D.E.; Singh, V.; Staats, P.S.; Hirsch, J.A. Systematic review of the diagnostic accuracy and therapeutic effectiveness of sacroiliac joint interventions. Pain Physician 2015 , 18 , E713–E756. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manchikanti, L.; Falco, F.J.; Benyamin, R.M.; Kaye, A.D.; Boswell, M.V.; Hirsch, J.A. A modified approach to grading of evidence. Pain Physician 2014 , 17 , E319–E325. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kristoff, T.J.; Sinopoli, J.T.; Farley, T.; Rabah, N.; Thompson, N.R.; Goyal, K. The therapeutic effectiveness of fluoroscopically guided intra-articular sacroiliac joint injections in patients with sacroiliac joint dysfunction, an observational study. Interv. Pain Med. 2023 , 2 , 100269. Available online: https://www.sciencedirect.com/science/article/pii/S2772594423001012 (accessed on 10 May 2024). [ CrossRef ]
- Schmidt, G.L.; Bhandutia, A.K.; Altman, D.T. Management of Sacroiliac Joint Pain. J. Am. Acad. Orthop. Surg. 2018 , 26 , 610–616. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Goodwin, B.; Averell, N.; Al-Shehab, U.; Ernazarov, A.; Price, L.; Choudhary, A.; Jermyn, R. Efficacy of platelet-rich plasma for sacroiliac joint dysfunction: A qualitative systematic review with pooled analysis. Regen. Med. 2023 , 18 , 505–514. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mehkri, Y.; Tishad, A.; Nichols, S.; Scott, K.W.; Arias, J.; Lucke-Wold, B.; Rahmathulla, G. Outcomes After Minimally Invasive Sacroiliac Joint Fusion: A Scoping Review. World Neurosurg. 2022 , 168 , 120–132. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chang, E.; Rains, C.; Ali, R.; Wines, R.C.; Kahwati, L.C. Minimally invasive sacroiliac joint fusion for chronic sacroiliac joint pain: A systematic review. Spine J. 2022 , 22 , 1240–1253. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Randhawa, S.; Garvin, G.; Roth, M.; Wozniak, A.; Miller, T. Maigne syndrome—A potentially treatable yet underdiagnosed cause of low back pain: A review. J. Back Musculoskelet. Rehabil. 2022 , 35 , 153–159. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maigne, J.Y.; Lazareth, J.P.; Surville, H.G.; Maigne, R. The lateral cutaneous branches of the dorsal rami of the thoraco-lumbar junction. Surg. Radiol. Anat. 1989 , 11 , 289–293. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kuniya, H.; Aota, Y.; Kawai, T.; Kaneko, K.-I.; Konno, T.; Saito, T. Prospective study of superior cluneal nerve disorder as a potential cause of low back pain and leg symptoms. J. Orthop. Surg. Res. 2014 , 9 , 139. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine 1994 , 19 , 801–806. [ Google Scholar ] [ CrossRef ]
- Georgetti, L.J.; Sims, A.C.; Amabile, A.H. An Anatomical Exploration of the Structures Associated with Low Back Pain Caused by Maigne’s Syndrome. FASEB J. 2018 , 32 , 644.11. [ Google Scholar ] [ CrossRef ]
- Maigne, R.; Nieves, W.L. Diagnosis and Treatment of Pain of Vertebral Origin , 1st ed.; Koonja: Seoul, Republic of Korea, 2008. [ Google Scholar ]
- Frisch, H. Programmierte Untersuchung des Bewegungsapparates ; Springer: Berlin/Heidelberg, Germany, 1983. [ Google Scholar ]
- Lee, H.; Chae, H.; Ryu, M.; Yang, C.; Kim, S. Acupuncture for patients with Maigne’s syndrome: A case series. Medicine 2023 , 102 , e33999. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Pun, W.K.; Luk, K.; Yeong, J. Influence of the erect posture on the development of the lumbosacral region. Surg. Radiol. Anat. 1987 , 9 , 69–73. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dąbrowski, K.; Ciszek, B. Anatomy and morphology of iliolumbar ligament. Surg. Radiol. Anat. 2023 , 45 , 169–173. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Maigne, J.Y.; Maigne, R. Trigger point of the posterior iliac crest: Painful iliolumbar ligament or cutaneous dorsal ramus pain? An anatomical study. Arch. Phys. Med. Rehabil. 1991 , 72 , 734–737. [ Google Scholar ] [ CrossRef ]
- Sims, J.A.; Moorman, S.J. The role of the iliolumbar ligament in low back pain. Med Hypotheses 1996 , 46 , 511–515. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Janssen, A.; Tomasella, M. Lombalgie localisée au niveau de la crête iliaque postérieure [Posterior iliac crest low back pain]. Rev. Med. Liege. 2023 , 78 , 503–509. [ Google Scholar ] [ PubMed ]
- Nayak, B.K.; Singh, D.K.; Kumar, N.; Jaiswal, B. Recovering from nonspecific low back pain despair: Ultrasound-guided intervention in iliolumbar syndrome. Indian J. Radiol. Imaging 2020 , 30 , 448–452. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019 , 49 , 464–476. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bogduk, N. Functional anatomy of the spine. Handb Clin. Neurol. 2016 , 136 , 675–688. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Matheve, T.; Hodges, P.; Danneels, L. The Role of Back Muscle Dysfunctions in Chronic Low Back Pain: State-of-the-Art and Clinical Implications. J. Clin. Med. 2023 , 12 , 5510. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Russo, M.; Deckers, K.; Eldabe, S.; Kiesel, K.; Gilligan, C.; Vieceli, J.; Crosby, P. Muscle Control and Non-specific Chronic Low Back Pain. Neuromodulation 2018 , 21 , 1–9. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ PubMed Central ]
- Macedo, L.G.; Maher, C.G.; Hancock, M.J.; Kamper, S.J.; McAuley, J.H.; Stanton, T.R.; Stafford, R.; Hodges, P.W. Predicting response to motor control exercises and graded activity for patients with low back pain: Preplanned secondary analysis of a randomized controlled trial. Phys. Ther. 2014 , 94 , 1543–1554. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Steele, J.; Bruce-Low, S.; Smith, D. A review of the specificity of exercises designed for conditioning the lumbar extensors. Br. J. Sports Med. 2015 , 49 , 291–297. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gilligan, C.; Volschenk, W.; Russo, M.; Green, M.; Gilmore, C.; Mehta, V.; Deckers, K.; De Smedt, K.; Latif, U.; Sayed, D.; et al. Five-Year Longitudinal Follow-up of Restorative Neurostimulation Shows Durability of Effectiveness in Patients with Refractory Chronic Low Back Pain Associated with Multifidus Muscle Dysfunction. Neuromodulation 2024 . [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Farley, T.; Stokke, J.; Goyal, K.; DeMicco, R. Chronic Low Back Pain: History, Symptoms, Pain Mechanisms, and Treatment. Life 2024 , 14 , 812. https://doi.org/10.3390/life14070812
Farley T, Stokke J, Goyal K, DeMicco R. Chronic Low Back Pain: History, Symptoms, Pain Mechanisms, and Treatment. Life . 2024; 14(7):812. https://doi.org/10.3390/life14070812
Farley, Tyler, Jesse Stokke, Kush Goyal, and Russell DeMicco. 2024. "Chronic Low Back Pain: History, Symptoms, Pain Mechanisms, and Treatment" Life 14, no. 7: 812. https://doi.org/10.3390/life14070812
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
The page could not be loaded. The CMS.gov Web site currently does not fully support browsers with "JavaScript" disabled. Please enable "JavaScript" and revisit this page or proceed with browsing CMS.gov with "JavaScript" disabled. Instructions for enabling "JavaScript" can be found here. Please note that if you choose to continue without enabling "JavaScript" certain functionalities on this website may not be available.

An official website of the United States government
Here's how you know
The .gov means it's official.
Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure.
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Tracking Sheet
Cervical fusion, document note, note history, contractor information.
| Contractor Name | Contract Type | Contract Number | Jurisdiction | States |
|---|---|---|---|---|
| A and B MAC | 09101 - MAC A | J - N | Florida | |
| A and B MAC | 09102 - MAC B | J - N | Florida | |
| A and B MAC | 09201 - MAC A | J - N | Puerto Rico Virgin Islands | |
| A and B MAC | 09202 - MAC B | J - N | Puerto Rico | |
| A and B MAC | 09302 - MAC B | J - N | Virgin Islands |
LCD Information
Document information.
CPT codes, descriptions and other data only are copyright 2023 American Medical Association. All Rights Reserved. Applicable FARS/HHSARS apply.
Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein.
Current Dental Terminology © 2023 American Dental Association. All rights reserved.
Copyright © 2023 , the American Hospital Association, Chicago, Illinois. Reproduced with permission. No portion of the American Hospital Association (AHA) copyrighted materials contained within this publication may be copied without the express written consent of the AHA. AHA copyrighted materials including the UB‐04 codes and descriptions may not be removed, copied, or utilized within any software, product, service, solution or derivative work without the written consent of the AHA. If an entity wishes to utilize any AHA materials, please contact the AHA at 312‐893‐6816.
Making copies or utilizing the content of the UB‐04 Manual, including the codes and/or descriptions, for internal purposes, resale and/or to be used in any product or publication; creating any modified or derivative work of the UB‐04 Manual and/or codes and descriptions; and/or making any commercial use of UB‐04 Manual or any portion thereof, including the codes and/or descriptions, is only authorized with an express license from the American Hospital Association. The American Hospital Association (the "AHA") has not reviewed, and is not responsible for, the completeness or accuracy of any information contained in this material, nor was the AHA or any of its affiliates, involved in the preparation of this material, or the analysis of information provided in the material. The views and/or positions presented in the material do not necessarily represent the views of the AHA. CMS and its products and services are not endorsed by the AHA or any of its affiliates.
This policy is developed as a multi-MAC collaboration to provide an evidence-based policy for cervical fusion.
Changes between proposed and final include formatting revisions, clarification of covered indications, limitations, and exceptions to conservative therapy. There were also minor grammatical revisions made to the Summary of Evidence and Analysis of Evidence.
CMS National Coverage Policy
This LCD supplements but does not replace, modify or supersede existing Medicare applicable National Coverage Determinations (NCDs) or payment policy rules and regulations for cervical fusion. Federal statute and subsequent Medicare regulations regarding provision and payment for medical services are lengthy. They are not repeated in this LCD. Neither Medicare payment policy rules nor this LCD replace, modify or supersede applicable state statutes regarding medical practice or other health practice professions acts, definitions and/or scopes of practice. All providers who report services for Medicare payment must fully understand and follow all existing laws, regulations and rules for Medicare payment for cervical fusion and must properly submit only valid claims for them. Please review and understand them and apply the medical necessity provisions in the policy within the context of the manual rules. Relevant CMS manual instructions and policies may be found in the following Internet-Only Manuals (IOMs) published on the CMS Web site:
IOM Citations:
- Chapter 13, Section 13.5.4 Reasonable and Necessary Provision in an LCD
Social Security Act (Title XVIII) Standard References:
- Title XVIII of the Social Security Act, Section 1862(a)(1)(A) states that no Medicare payment may be made for items or services which are not reasonable and necessary for the diagnosis or treatment of illness or injury.
- Title XVIII of the Social Security Act, Section 1862(a)(7). This section excludes routine physical examinations.
Code of Federal Regulations (CFR) References:
- Title 42, Ch. IV, § 410.74 Physician assistants’ services, §410.75 Nurse practitioners’ services and § 410.76 Clinical nurse specialists’ services.
Coverage Guidance
Compliance with the provisions in this LCD may be monitored and addressed through post payment data analysis and subsequent medical review audits.
History/Background and General Information
Fusion of the cervical spine alone or in conjunction with other cervical spine procedures is performed for the management and treatment of various spinal conditions such as cervical radiculopathy, cervical myelopathy, and unstable spine. Cervical radiculopathy is pain that radiates from the neck through the arm. Cervical myelopathy describes compression of the spinal cord at the cervical level that results in neurologic deficits. Unstable spine has been defined as destruction of either the anterior or posterior elements of the spine making them nonfunctional, more than 3.5 mm of displacement of one vertebra in relation to another and/or greater than 11 degrees of rotational difference between adjacent vertebrae. 1 These three conditions may have multiple etiologies including herniated disc, spinal stenosis, spinal degeneration, synovial cyst, and symptomatic pseudoarthrosis from non-union of prior fusion. Physical assessment and imaging findings must correspond with the deficits attributable to the affected region of the cervical spine.
Covered Indications
A. Cervical fusion surgery is considered reasonable and necessary for the decompression of symptomatic cervical nerve root impingement when all of the following requirements are met:
- Persistent or recurrent moderate or severe arm pain (4 or more on the visual analog scale [VAS] or equivalent) present for a minimum of 12 weeks within the current episode of neck pain with documented failure to respond to multimodal conservative management (as tolerated) in the absence of exceptional circumstances (below) AND
- Nerve compression negatively impacts activities of daily living (ADLs) AND
- All other potential sources of pain/neurological deficit have been excluded AND
- Imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) evidence of central, lateral recess or foraminal stenosis at the level corresponding with clinical myotome signs or symptoms and including at least 1 of the following:
- Cervical degenerative disc disease as indicated by the presence of 1 or more of the following findings: herniated nucleus pulposus, narrowing of the intervertebral disc, disc osteophytes, facet hypertrophy, or synovial cysts.
- Tumors (primary or metastatic)
- Post infection radiographic findings
- Spinal instability as defined by subluxation or translation more than 3.5 mm on static lateral views or dynamic radiographs OR sagittal plane angulation of more than 11 degrees between adjacent segments. 1
Limitations
The following is considered not reasonable and necessary for the decompression of symptomatic cervical nerve root impingement:
- Isolated chronic axial cervical pain
Exceptions to conservative therapy requirement for decompression of symptomatic cervical nerve root impingement:
- Cervical myelopathy class III or above OR
- Progression of neurological deficits during the trial of conservative treatment.
- Presenting with progressive motor weakness OR
- Significant motor weakness interfering with ADLs OR
- Severe radicular pain defined as pain limiting ability to perform ADLs and ≥7/10 on VAS or equivalent scale 2 AND associated with confirmatory imaging (CT, MRI) and clinical-radiological correlation.
- Loss of bowel or bladder control
B. Cervical fusion surgery is considered reasonable and necessary for the decompression of symptomatic cervical canal stenosis when the following requirements are met:
- Persistent or recurrent moderate or severe arm pain (4 or more on the VAS or equivalent) present for a minimum of 12 weeks within the current episode of arm pain with documented failure to respond to multimodal conservative management (as tolerated) in the absence of exceptional circumstances (below) OR
- Nerve compression negatively impacts ADLs OR
- Spastic gait, loss of manual dexterity, problems with sphincter control AND
- Imaging (MRI or CT) evidence of central stenosis at the level corresponding with clinical signs or symptoms and including at least 1 of the following:
- Cervical degenerative disc disease as indicated by the presence of 1 or more of the following findings: herniated nucleus pulposus, narrowing of the intervertebral disc, disc osteophytes, facet hypertrophy or synovial cysts.
- Congenital short pedicles
- Ossification of the posterior longitudinal ligament.
- Spinal instability as defined by subluxation or translation more than 3.5 mm on static lateral views or dynamic radiographs OR sagittal plane angulation of more than 11 degrees between adjacent segments.
- Cord compression with or without increased cord signal.
The following are considered not reasonable and necessary for decompression of symptomatic cervical canal stenosis:
- Isolated chronic axial cervical pain.
- Asymptomatic myelopathy (regardless of severity on imaging findings).
Exceptions to conservative therapy requirement for decompression of symptomatic cervical canal stenosis:
C. Cervical fusion surgery is considered reasonable and necessary for the decompression or stabilization of the cervical spine for the following indications:
- Fractures or dislocations are likely to result in spinal instability without neurological defects OR
- Fractures or dislocations associated with neurological defects are present at the affected level OR
- Instability is present.
- Presence of malignant or benign tumors which have caused instability or neurologic deficit where treatment of the tumor will likely require stabilization of the spine 4 OR
- Expected treatment of the tumor, whether by chemotherapy or radiation therapy or surgery will likely cause spinal instability or neurologic deficits 4 OR
- Infection involving the spine in the form of discitis, osteomyelitis, or epidural abscess when 3 :
- Imaging or other studies (MRI, biopsy, bone aspirate) demonstrating infection AND
- Imaging evidence of vertebral body destruction OR documentation that spinal debridement will cause vertebral instability 5,6 OR
- Cervical kyphosis associated with cord compression or Atlantoaxial (C1-C2) subluxation or Basilar invagination of the odontoid process into the foramen magnum; or Subaxial (C2-T1) instability kyphosis, head drop syndrome, post-laminectomy deformity are present OR
- Symptomatic pseudarthrosis (non-union of prior fusion) with radiological (e.g., CT or MRI) demonstration of non-union of prior fusion (lack of bridging bone or abnormal motion at fused segment) after 12 months since fusion surgery or with radiographic evidence of hardware failure (fracture or displacement) is present OR
- Spinal instability is present after laminectomy OR
- Rheumatoid arthritis with associated instability is present OR
- Cervical degenerative spondylolisthesis with spinal instability (Anterolisthesis/Posterolisthesis) is present AND
- Substantial functional limitation is present such as severe neck pain, difficulty ambulating and decrease ability to perform ADLs or ability to maintain forward gaze OR
- Progression of deformity is present
Cervical Fusion for the decompression or stabilization of the cervical spine is not reasonable and necessary when all the above criteria are not fulfilled.
Provider Qualifications
The Medicare Program Integrity Manual states services will be considered reasonable and necessary only if performed by appropriately trained providers.
Patient safety and quality of care mandate that healthcare professionals who perform cervical fusion are appropriately trained and credentialed by a formal residency/fellowship program. Credentialing or privileges are required for procedures performed in inpatient and outpatient settings.
All aspects of care must be within the provider’s medical licensure and scope of practice.
Notice: Services performed for any given diagnosis must meet all the indications and limitations stated in this LCD, the general requirements for medical necessity as stated in CMS payment policy manuals, all existing CMS national coverage determinations, and all Medicare payment rules.
Definitions
Acute Pain – An unpleasant sensory and emotional experience associated with actual or potential tissue damage which is present for up to 6 weeks. 7
Baseline Pain- An initial measurement of the pain which is taken at a specified time point and used for comparison over time to look for changes in the pain levels.
Cervical Radiculopathy- Pain in a radicular pattern in 1 or both upper extremities related to compression and irritation of 1 or more cervical nerve roots. 8
Chronic Pain – The temporal definition of pain persisting at least 12 weeks after the onset of the acute pain.
Conservative Therapy – Consists of an appropriate combination of medication in therapeutic dosages (e.g., non-steroidal anti-inflammatory [NSAIDs], serotonin and norepinephrine reuptake inhibitors [SNRIs], analgesics, etc.) administered for a sufficient amount of time to determine efficacy, in combination with either physical therapy, spinal manipulation therapy, cognitive behavioral therapy (CBT), home exercise program, acupuncture, or other interventions based on the individual’s specific presentation, physical findings, and imaging results.
Consistent Improvement – The progressive, incremental, and clinically meaningful improvement of physical signs or symptoms.
Disability – Activity limitations or participation restrictions in an individual with a health condition, disorder or disease. 9
Functional Impairment - A physical, functional, or physiological impairment causing deviation from the normal function of a tissue or organ. This results in a significantly limited, impaired, or delayed capacity to move, coordinate actions, or perform physical activities and is exhibited by difficulties in 1 or more of the following areas: physical and motor tasks; independent movement; performing basic life functions. 9
GRADE – A system developed by the GRADE Working Group to address the shortcomings of present grading systems in healthcare. The GRADE system uses a common, sensible, and transparent approach to grading the quality of evidence. The results of applying the GRADE system to clinical trial data are displayed in a table known as a GRADE profile.
Hangman’s Fracture - A bilateral fracture traversing the pars interarticularis of cervical vertebrae 2 (C2) with an associated traumatic subluxation of C2 on cervical vertebrae 3 (C3). 10
Multidisciplinary Biopsychosocial Rehabilitation (MBR ) – Targets physical as well as psychological and social aspects of pain and involves a team of healthcare providers with different professional backgrounds and training.
Myelopathy- An inclusive term describing a compression of the spinal cord resulting in neurological deficits e.g., spasticity (sustained muscle contractions), hyperreflexia, pathologic reflexes, paresthesia affecting the extremities, muscle weakness, digit/hand clumsiness, or gait disturbance. 11,12
Nonspecific low back pain – Back pain that cannot be attributed to a specific disease or spinal pathology .
Session- A session is a time period, which includes all procedures (e.g., Sacroiliac joint injections and radiofrequency ablations) that are performed during the same day.
Sustained and Constant Pain Relief - The pain relief must continue for the defined time period and without interruption or regression to the primary (index) pain.
Symptomatic pseudarthrosis - Non-union of prior fusion with radiological (e.g., CT or MRI) evidence (lack of bridging bone or abnormal motion at fused segment) and 12 months or more since fusion surgery or with radiographic evidence of hardware failure (fracture or displacement). 13
Unstable Spine- Cervical spinal instability has been defined as destruction of either the anterior or posterior elements of the spine making them nonfunctional, more than 3.5 mm of displacement of one vertebra in relation to another or greater than 11 degrees of rotational difference between adjacent vertebrae. 1,14
Conservative Treatment
Treatment of cervical radiculopathy can range from conservative management to surgery. Conservative care is considered initial management for cervical radiculopathy from degenerative disorders as most cases will be self-limited and resolve spontaneously over a variable length of time without specific intervention. 8 Conservative management for cervical radiculopathy may include oral analgesics or short course of oral glucocorticoids, avoidance of provocative activities, short term immobilization with cervical collar or pillow, active physical therapy, manual therapy, and cervical traction. 15,16 In a published analysis of clinical practice guidelines for neck pain and radiculopathy the following interventions were listed as recommended components of conservative therapy 16 :
- Pharmacotherapies: analgesics, Paracetamol (Acetaminophen), NSAIDs, SNRIS, opioids (including tramadol), topical medications including NSAIDS.
- Non-Pharmacologic Interventions: exercise programs/physical therapy, thermotherapy, manual therapy (combined with other treatment), acupuncture, epidural steroid injection.
Studies have shown that surgery and conservative interventions achieve similar long-term results, and that conservative treatment is often the preferred option because it avoids the risks of surgical treatment. 8,17 A systematic review found sufficient low-quality evidence to support the role of conservative measures as a first-line approach for the management of cervical radiculopathy. 18 Observational data reports 40-80% of patients with cervical radiculopathy will improve with conservative measures. 15 Exercise alone or in combination with other treatments was shown to be beneficial for cervical radiculopathy in a systematic review and meta-analysis. 19 Physical therapy accompanied by home exercise for 6 weeks has been shown in a randomized trial to substantially reduce neck and arm pain for patients with cervical radiculopathy. 20 A RCT compared cervical collar or physical therapy and found improvement over a wait and see approach suggesting that an active treatment approach results in better outcomes. 21 A RCT demonstrated that patients who proceed to surgery for management of cervical radiculopathy had more rapid resolution of symptoms compared to those who remained in physical therapy but outcomes at 2 years were similar and conclude structured physiotherapy should be considered first line management. 22 Further investigation with the same author reported anterior cervical decompression and fusion (ACDF) plus PT was superior to PT alone. 23 Subject matter experts agreed that conservative therapy is an acceptable first line approach and that there are modifiable risk factors such as diabetes management, smoking cessation, and weight loss that can impact disease course and surgical outcomes. 24
NASS Guidelines state: “As the majority of patients with cervical radiculopathy will improve with nonoperative treatment, particularly within 3 months from onset, most practitioners would agree that a 6- to 12-week period of nonoperative care seems reasonable. Specific provisions of what should be included during the course of nonoperative treatment were not outlined as there is no universally agreed upon group of modalities. The types of treatment that one would consider, however, should include physical therapy, anti-inflammatory pain medications, and epidural steroid injections.” 3
A multi-disciplinary, international panel established consensus-based guidance on effective nonsurgical treatment modalities for individuals in different stages (i.e., acute, subacute, and, chronic) of cervical radiculopathy. 25 These guidelines include patient education with positive reinforcing and non-nocebo content, spinal manipulative therapy, exercise, sustaining pain-relieving positions, cognitive behavioral therapy, general aerobic exercise, strength training, postural education, and workplace and vocational/ergonomic assessment.
Berman, et al 26 compared the American College of Radiology appropriateness criteria for MRI to the specific criteria in 56 commercial policies and clinical management organizations in the USA, for neck pain both with and without radicular symptoms. While the focus was on coverage criteria for MRIs, the study also summarized organizational positions on conservative care.
- Different definitions of conservative care are used e.g., “a multimodality approach consisting of a combination of active and inactive components” and “a combination of strategies to reduce inflammation, alleviate pain, and improve function.” 26
- All organizations require the failure of at least 6 weeks of conservative management. Some organizations require conservative management within the preceding 3 or 6 months, while others do not specify how recently conservative treatment needs to take place.
- Conservative interventions are ‘provider-directed treatment’ that includes education, activity modification, NSAIDs, narcotic and non-narcotic analgesic medications, oral or injectable corticosteroids, a provider-directed home exercise/stretching program, cross-training, avoidance of aggravating activities, physical/occupational therapy, spinal manipulation, interventional pain procedure, and other pain management techniques. Only a single organization requires 2 separate forms of conservative treatment, although the guidelines do not specify what these are.
Imaging Modalities
North American Spine Society (NASS) 8 Evidence-Based Clinical Guidelines on the Diagnosis and Treatment for Cervical Radiculopathy from Degenerative Disorders state MRI is the recommended study for correlative compressive lesions in cervical spine patients who have failed conservative therapy and are being considered for intervention based on Grade B recommendation. The consensus of the group was that CT can be considered in those who have a contraindication to MRI. Computed tomography myelography was recommended for patients with discordant findings on MRI or contraindication to MRI based on Grade B Recommendation. There was insufficient evidence to make a recommendation for or against the use of electromyography (EMG) for cervical radiculopathy evaluation.
Cervical Radiculopathy
Cervical radiculopathy is a common condition that usually occurs when a nerve root is inflamed by a herniated disc or bone spur. As a result, the inflamed nerve root may result in neck pain, numbness or muscle weakness, and sensory symptoms. This condition impacts approximately 85 out of 100,000 people. 27
Clinical Outcomes
Seven evidence syntheses with publication dates ranging from 2016-2022 that assessed clinical outcomes were identified. 28-34 Two reviews included only RCTs. 28,30 Four studies included both non-randomized studies of interventions (NRSI) and a small number (range 1-6) of RCTs. 29,31-33 The number of primary studies in the syntheses ranged from 7-23, with generally small sample sizes. Half the studies diagnosed participants with single-level, unilateral cervical radiculopathy. Comparators included different fusion techniques, foraminotomy, arthroplasty, and conservative interventions. Anterior cervical discectomy and fusion (ACDF) was an intervention in each study. Timing to follow-up ranged from 24 to 47 months.
Broekema, et al 28 conducted a systematic review with meta-analysis that included 1,567 participants described in 21 RCTs. Thirteen of the studies had data from less than 100 participants. The clinical effects of ACDF were compared with posterior cervical foraminotomy (PCF), anterior cervical discectomy without intervertebral spacer (ACD), ACD with poly-methylmethacrylate as an intervertebral spacer (PMMA), autologous bone graft (ABG), ACDF with autologous bone graft plus plating (ABGP), cervical disc arthroplasty (CDA), and physical therapy. Anterior cervical discectomy and fusion had a significantly better success rate compared to ACD. Among the remaining comparisons, there were no significant differences in success rates. Six subgroup comparisons found no significant differences in work status across interventions. Aside from a significant effect favoring ACDF over CDA, there were no differences in disability outcomes at mean follow-up. Improvement in arm pain favored ACDF with a cage over ABGP, ACD over ACDF with a cage, and ACDF over CDA. The clinical significance of these differences was not described. Limitations in this evidence synthesis included an overall high risk of bias (only 3 of 21 included studies had a low risk of bias), indirectness (the mean age across studies was 43.8 years), and imprecision due to the small sample sizes across sizes, with 62% of studies having less than 100 participants.
Fang, et al 29 included 3 RCTs and 12 NRSI with a total of 52,705 (~51K from a single cohort study) participants diagnosed with single-level unilateral cervical radiculopathy in a systematic review with meta-analysis. Outcomes comparing ACDF with PCF were measured at a mean of 47 months. No statistically significant differences were identified for measures of disability (function), neck pain, arm pain, or satisfaction. Limitations impacting the certainty of the evidence included inherent drawbacks due to most of the primary study designs (12 of 15 studies were retrospective cohort designs) and the 3 RCTs either did not contribute to or contributed minimally to the pooled analyses, indirectness (the mean ages across studies ranged from 39 to 53.8 years), and significant heterogeneity was observed in subgroup analyses of ACDF vs. PCF using open and minimally invasive techniques.
In a network meta-analysis, Gao, et al 30 performed direct and indirect comparisons of ABG, ACD, ACF, PCF, ACDFP (ACDF plus plating), CDA, PMMA, and physical therapy. Twenty-three RCTs (N=1,844) were assessed at a mean of 26 months. There were no statistical differences among the different interventions in measures of success rate, arm/neck pain, or disability. There were limitations in this network meta-analysis including an overall high risk of bias (only 13% [3 of 23] of the studies had a low risk of bias), indirectness (the mean/median age among the included studies ranged from 41 to 50.5 years and only 2 of 23 studies were conducted within the United States), imprecision (sample sizes were small [27-209]), and inconsistency (clinical and statistical heterogeneity).
Goedmakers, et al 31 systematically reviewed 6 RCTs and 2 NRSI (N=777) in comparing ACDF with CDA. Timing to follow-up in the studies ranged from 1-7 years. A single study reported a statistically significant difference between interventions for disability; however, the difference did not achieve clinical relevance. Another 5 studies found no significant difference between CDA and ACDF. None of the studies reported a significant difference in neck pain after 2 years post-surgery. Limitations of this systematic review included all articles showed intermediate to high risk of bias, indirectness (mean age was ~45 years), imprecision (sample sizes were small [41-209]), and inconsistency (high heterogeneity precluded pooling the results of individual studies).
A systematic review with meta-analysis of 658 patients (1 RCT; 11 NRSI) with single-level cervical radiculopathy compared traditional ACDF vs. stand-alone ACDF vs. CDA. 32 The reviewers found no statistically significant differences in disability measures at a minimum of 1 year for the different interventions. Limitations included the drawbacks inherent in observational studies (11 of 12 included studies were cohort designs), uncertainty about the internal validity of the studies (risk of bias of the included studies was not assessed), imprecision (the mean sample size was 54.8), and inconsistency (high heterogeneity was present in the pooled analysis for disability outcomes).
Liu, et al 33 produced a systematic review of 1,336 patients with cervical radiculopathy (3 RCTs, 7 NRSI) who underwent either ACDF or PCF. At a mean of 3.7 years following the procedure, there were no between-group differences in patient-reported measures of pain and disability. Based on a single study, ACDF showed a significantly higher success rate (93.6%) than the PCF group (85.1%). Limitations of this review included the drawbacks inherent in observational studies (7 of the 10 studies were retrospective cohort designs), all the RCTs were judged to have a high risk of bias, and indirectness (the mean age of the included participants was ~46 years [39-53.3]).
Zou, et al 34 conducted a systematic review with a meta-analysis of 1,175 patients with unilateral cervical radiculopathy. One RCT and 6 NRSI compared clinical outcomes for groups receiving ACDF or MI-PCF (minimally invasive posterior cervical fusion). At a mean post-surgical follow-up of 47 months, no statistically significant differences were observed in patient-reported disability, neck pain, or arm pain. Limitations in this evidence synthesis included those inherent in observational study designs, indirectness (the mean age of the participants was 48.9 years), and inconsistency (high heterogeneity was reported for arm pain outcomes).
In aggregate, the clinical effects associated with the different surgical and conservative interventions did not result in statistically significant or clinically relevant differences. The only exception was ACDF, which showed a better success rate than ACD. Overall, the certainty of the evidence was determined to be very low. Limitations included those inherent in observational study designs, high risk of bias in most RCTs, indirectness, imprecision, and heterogeneous outcomes.
Surgical Outcomes
Three evidence syntheses reported on perioperative measures. Operative time was assessed in all studies. 29,30,34 Length of stay (LOS) was included in 2 reviews. 29,34 A single study analyzed blood loss. 29
Fang, et al 29 compared ACDF with open PCF or MI-PCF for patients with single-level, unilateral cervical radiculopathy in a systematic review with meta-analysis. The reviewers reported that compared to ACDF, open PCF had a shorter operation time, while there was no difference between ACDF and MI-PCF. Based on data from 2 studies, there was no significant difference in blood loss among the surgical techniques. The PCF group was associated with a significantly shorter length of hospitalization than the ACDF group. Limitations impacting the certainty of the evidence included inherent drawbacks due to most of the primary study designs (12 of 15 studies were retrospective cohort designs) and the 3 RCTs either did not contribute to or contributed minimally to the pooled analyses, indirectness (the mean ages across studies ranged from 39 to 53.8 years), and significant heterogeneity was observed in subgroup analyses of ACDF vs. PCF using open and minimally invasive techniques.
Gao, et al 30 assessed operative time for different surgical procedures in a network meta-analysis. Direct and indirect comparisons showed ABGP, ACD, ACDF, and ACDFP had shorter surgery times compared with ABG. Anterior cervical discectomy and fusion had a shorter surgery time compared with PMMA. There were limitations in this network meta-analysis including an overall high risk of bias (only 13% [3 of 23] of the studies had a low risk of bias), indirectness (the mean/median age among the included studies ranged from 41 to 50.5 years and only 2 of 23 studies were conducted within the United States), imprecision (sample sizes were small [27-209]), and inconsistency (clinical and statistical heterogeneity).
Zou, et al 34 performed a systematic review with meta-analysis comparing ACDF with MI-PCF. The analysis did not find any significant difference in operative time between the 2 cohorts. Data from 3 retrospective studies observed a statistically significant difference in LOS favoring MI-PCF. Limitations in this evidence synthesis included those inherent in observational study designs, and indirectness (the mean age of the participants was 48.9 years).
In summary, the observed perioperative results varied by the comparators and the type of outcome. Compared to open PCF, ACDF had significantly longer operative time and LOS. Minimally invasive posterior cervical fusion also had a significantly less LOS than ACDF. When ACDF was compared to other surgical techniques, no significant differences were found in operative time and blood loss. The overall certainty of the evidence was rated as very low due to the high risk of bias in the included RCTs, limitations inherent in observational studies, and uncertainty about the applicability of the results to the US Medicare population (indirectness).
Radiographic Outcomes
Two evidence syntheses described post-surgical radiographic results associated with individuals diagnosed with cervical radiculopathy. 32,33
Katsuura, et al 32 performed a systematic review with meta-analysis that compared traditional ACDF vs. stand-alone ACDF vs. CDA. At a minimum of 1-year post-surgical follow-up, no significant differences in the C2-C7 (Cobb) angle were observed. Limitations of this evidence synthesis included the drawbacks inherent in observational studies (11 of 12 included studies were cohort designs), uncertainty about the internal validity of the studies (risk of bias of the included studies was not assessed), and imprecision (the mean sample size was 54.8).
Liu, et al 33 systematically reviewed data from 3 RCTs and 7 NRSI, encompassing 1,335 patients; however, only a single RCT provided data regarding radiographic outcomes. The postoperative ROM of the caudal adjacent segment was 11.33° ± 5.07° in the ACDF group and 8.73° ± 5.92° in the PCF group. The significance of this difference was not described. Limitations of this review included the drawbacks inherent in observational studies (7 of the 10 studies were retrospective cohort designs), all the RCTs were judged to have a high risk of bias, and indirectness (the mean age of the included participants was ~46 years [39-53.3]).
Overall, there was limited evidence concerning radiographic outcomes following cervical fusion in patients diagnosed with cervical radiculopathy. No significant between-group differences were reported for Cobb angle or ROM. The overall certainty of the evidence was judged as very low due to a high/unknown risk of bias, study design limitations, imprecise data, and indirectness.
Undesirable Effects
Six evidence syntheses described undesired effects associated with cervical fusion surgery. 28,30,31,33-35 Assessment timeframes ranged from 24 to 57 months.
Broekema, et al 28 performed a systematic review with meta-analysis of 1,567 participants diagnosed with cervical radiculopathy who underwent anterior or posterior surgical interventions and were compared with other surgical techniques. There was a higher complication rate in the group with ABG from the iliac crest. This effect disappeared when donor-site complications were left out. No other significant effect was observed in the comparison between ACDF with ABG and ACDF with a cage. Subgroup comparisons found no significant differences in reoperation rate across the different surgeries. Limitations in this evidence synthesis included an overall high risk of bias (only 3 of 21 included studies had a low risk of bias), indirectness (the mean age across studies was 43.8 years), and imprecision due to the small sample sizes with 62% of studies having less than 100 participants.
Gao, et al 30 conducted a network meta-analysis of 23 RCTs that included direct and indirect comparisons of ACDF, ABG, ACD, ACF, PCF, ACDFP, CDA, PMMA, and physical therapy. There were no statistical differences among the various interventions, including physical therapy for complication and reoperation rates. There were limitations in this network meta-analysis including an overall high risk of bias (only 13% [3 of 23] of the studies had a low risk of bias), indirectness (the mean/median age among the included studies ranged from 41 to 50.5 years and only 2 of 23 studies were conducted within the United States), imprecision (sample sizes were small [27-209]), and inconsistency (clinical and statistical heterogeneity).
Goedmakers, et al 31 systematically reviewed 777 participants with cervical radiculopathy from 6 RCTs and 2 NRSI in comparing CDA to ACDF. The authors concluded the reporting on the level of reoperation is heterogeneous and incomplete; however, the results suggested reoperations were most frequent at the adjacent level for the ACDF group and at the index level for the CDA group. Four articles described adjacent segment disease (ASD), of which only 1 article described a significantly higher incidence of ASD in ACDF patients. No other statistically significant differences in complication rates were described between groups. Limitations of this systematic review included all articles showed intermediate to high risk of bias, indirectness (mean age was ~45 years), imprecision (sample sizes were small [41-209]), and inconsistency (high heterogeneity precluded pooling the results of individual studies).
Gutman, et al 35 performed a systematic review with meta-analysis that focused on the comparative undesired effects of participants with single-level cervical radiculopathy who prospectively underwent ACDF, CDA, or MI-PCF. The rate of revision surgery following ACDF (14.46) was greater than double that of MI-PCF (7.00) and CDR (6.49). Compared to ACDF (21.29), the rate of adverse events was significantly less for MI-PCF (3.00), but greater for CDA (27.27). Limitations in this review included the risk of bias of the primary studies was not assessed, indirectness (the mean age for each of the included studies was less than 50 years [43-49.3]), imprecision (the number of studies was few [4], with small sample sizes), and uncertainty concerning inconsistency (heterogeneity was not assessed).
Liu, et al 33 assessed the overall complication rate in a systematic review that compared patients who had received either ACDF or PCF. The mean complication rate was 7% in the ACDF group and 4% in the PCF group. The difference was not statistically significant. Limitations of this review included the drawbacks inherent in observational studies (7 of the 10 studies were retrospective cohort designs), all the RCTs were judged to have a high risk of bias, and indirectness (the mean age of the included participants was ~46 years [39-53.3]).
Zou, et al 34 employed a systematic review with meta-analysis of 1,175 patients who underwent either ACDF or PCF. In the ACDF group, complication rates ranged between 1.8% and 10.5%. In the MIS-PCF group, the rate was between 0% and 14.3%. The between-group difference was not significant. The reoperation rate was between 0-7.5% in the ACDF group and 0 -14.3% in the MIS-PCF group. The difference was not significant. Limitations in this evidence synthesis included those inherent in observational study designs, and indirectness (the mean age of the participants was 48.9 years).
In aggregate, the complication rates associated with different surgical procedures and physical therapy are generally similar; however, the rate of adverse events with ACDF appears to be greater than MI-PCF, and the rate of ASD is lower with CDA vs. ACDF. The overall results of the different evidence syntheses were heterogeneous concerning reoperation rates. No significant differences were found among the surgical interventions studied apart from a small systematic review (Gutman, et al.; 2018) that concluded the revision surgery rate of ACDF was double that of CDA and MI-PCF. The credibility of these findings was very low due to a lack of quality appraisal (risk of bias) for the included RCTs, the small numbers of studies (4) and participants (506), uncertain applicability to the US Medicare population, and omitting an assessment of heterogeneity. Overall, the certainty of the evidence was judged to be very low due to multiple limitations i.e., study designs, high/unclear risk of bias, indirectness, imprecise results, and unexplained heterogeneity.
Societal Input
The North American Spine Society (NASS) has published appropriateness criteria for cervical fusion surgery as a treatment for cervical radiculopathy. 3
Cervical radiculopathy: from degenerative disorders (either from disc herniation or bony stenosis), as an adjunct to disc excision, that meets ALL the following criteria:
- Pattern of radiculopathy explained by imaging.
- 6 to 12 weeks of an appropriate course of nonoperative treatment.
- Severity of symptoms prevents the patient from working.
- Functionally limiting motor weakness.
Cervical fusion may NOT be indicated in cases that do not fulfill the above criteria including cervical radiculopathy from isolated foraminal stenosis treated with a partial medial facetectomy/foraminotomy.
Degenerative Cervical Myelopathy
Six evidence syntheses with publication dates ranging from 2014-2022 that assessed clinical outcomes were identified. 36-41 Three of the syntheses included only non-randomized studies of an intervention (NRSI). 37,39,40 Three studies included both NRSI and a small number (1-4) of RCTs. 36,38,41 The number of primary studies in the syntheses ranged from 5-18, with generally small sample sizes. Participants were mostly diagnosed with multilevel cervical spondylotic myelopathy (CSM). Comparators included different fusion techniques, arthroplasty, and hybrid surgery. Anterior cervical discectomy and fusion was an intervention in each study. Timing to follow-up was variable (1-60+ months).
Fei, et al 36 conducted a systematic review with meta-analysis that compared 2 fusion techniques, ACDF and ACCF (anterior cervical corpectomy and fusion). Eighteen primary studies (1 RCT, 17 NRSI) were included in the review. Outcomes were assessed at a minimum of 6 months post-operation. Neurological status (success) and pain intensity outcomes were similar between surgical groups. There were several limitations in this study including those associated with observational studies (17 of 18 studies were cohort or case-control designs), indirectness (17 of 18 studies included participants located in China or South Korea, and all the studies had a mean/median between 32-59 years), imprecision (all studies had small sample sizes with confidence intervals that crossed the indicator for no effect), and inconsistency of effects across studies (significant heterogeneity for all secondary outcomes).
In a systematic review with meta-analysis, Han, et al 37 reported on 14 NRSI that compared ACDF and ACCF. At a postoperative minimum of 24 months, all clinical measures (success rate, neurological status, pain, and function [disability]) were statistically similar ( p >0.05). Limitations of this review involved the inclusion of only low-quality studies (all included studies were observational cohort or case-control designs), indirectness (mean ages ranged from 44-58.74 yrs.), imprecision (all studies had small sample sizes and pain outcomes had small numbers of pooled data), and high statistical heterogeneity (inconsistency) was present for functional outcomes.
Li, et al 38 performed a network meta-analysis that included 4 RCTs and 12 NRSI. The analysis included direct and indirect comparisons of ACDF, ACCF, cervical disc arthroplasty [replacement] (CDA), and hybrid surgery. At a mean of 33.3 months following surgery, CDA demonstrated a statistically significant difference in disability outcomes compared to the other surgical procedures. The clinical significance of these results was not described. Limitations in this network meta-analysis were associated with low-quality studies (12 of 16 studies were case-control designs), indirectness (indirect comparisons were a core component of the methodology and 14 studies took place in Asia [China, South Korea, Singapore]), and imprecision (all studies had small sample sizes).
Montano, et al 39 systematically reviewed and meta-analyzed 5 NRSI (487 participants) with multilevel CSM. No difference in neurological status was found between ACDF and laminoplasty at a mean of 35.4 months post-surgery (MD = -0.053; 95% CI, -0.511 to 0.404; p = 0.819). Limitations involved the low-quality of all included studies (observational cohort or case-control designs), indirectness (all studies had mean ages between 54 and 56.8 years.), imprecision (too few events for all outcomes and wide confidence intervals for all pooled outcomes measured).
A systematic review without meta-analysis of 11 primary studies (2 RCTs, 9 NRSI) included a total of 570 participants with single or multilevel CSM. 41 Clinical outcomes were assessed between 12 and 60 months postoperatively. No statistically significant differences in ACDF and ACCF were found for neurological status, pain, or function (disability). Review limitations included the overall low-quality primary study designs (9 of the 11 included studies were observational cohort or case-control), indirectness (none of the studies were performed in the United States and all studies had a mean age between 46.8 and 57.7 years), imprecision (all the studies had small sample sizes), and inconsistency (I 2 test revealed high heterogeneity [>75%] across studies for all outcomes).
Wang, et al 40 included 14 NRSI with wide variation in sample sizes (40-2188) that comparatively assessed ACDF and ACCF. There was no between-group difference in patient-reported function; however, improvement in neurological status at 1-60+ months follow-up favored ACDF (OR = 0.49, 95% CI: 0.06 to 0.91; p = 0.02). Limitations involved the selection of low-quality studies (all studies were retrospective observational cohorts or case-control designs) and indirectness (11 of the studies were conducted in Asia [China, South Korea]).
Primary Studies
One RCT that was not included in any evidence synthesis was identified. El-Ghandour, et al 42 compared ACDF with posterior laminectomy with or without fusion in the management of degenerative cervical myelopathy (DCM). Sixty-eight patients (mean age 53 years) were enrolled in a 1:1 randomized trial. Clinical outcomes were assessed after 1 year. There were significantly better outcomes in patient-reported disability (function) and pain scores in the ACDF group compared to posterior surgical approaches. The Nurick myelopathy grading scale showed a nonsignificant improvement with using the posterior approach. The authors noted several methodologic limitations including a high risk of bias in the randomization process, small sample size, and short-term follow-up.
In aggregate, clinical outcomes were found to be largely equivalent regardless of surgical technique. The overall certainty of the evidence was judged to be very low due to limitations inherent in the included study designs, indirectness (uncertainty about the applicability to the US Medicare population), imprecision (variability within studies in the direction and clinical relevance of effects), and inconsistencies across studies in the magnitude of effect.
A total of 7 evidence syntheses evaluated surgical (perioperative) outcome measures. 36-40,43,44 All syntheses included NRSI, while RCTs were represented in 2 reviews. 4,5 Sample sizes were small, patients were diagnosed with multilevel CSM, comparisons were between surgical interventions, and follow-up assessments were variable.
Fei, et al 38 reported on data from 1,246 patients included in 18 primary studies (1 RCT, 17 NRSI). Compared to ACCF, patients who underwent ACDF had superior perioperative outcomes (length of stay [LOS], operative time, and blood loss). Limitations included the preponderance of low-quality studies (17 of 18 studies were of observational designs), indirectness (17 of 18 studies included participants located in China or South Korea, and all the studies had a mean/median between 32-59 years), imprecision (all studies had small sample sizes with confidence intervals that crossed the indicator for no effect), and inconsistency (significant heterogeneity for all secondary outcomes).
Han, et al 37 included 15 NRSI (1,372 participants) in comparing ACDF and ACCF. No significant difference in operative time was found (MD = -9.34, 95% CI = -42.99 to 24.31; p = 0.59). Length of stay (MD = -5.60, 95% CI = -7.09 to - 4.11; p <0.00001) and blood loss (MD = -151.35, 95% CI = -253.22 to -49.48; p = 0.004) were both less (better) in the ACDF group vs. the ACCF group. Limitations involved the low-quality of all included studies (observational cohort or case-control designs), indirectness (mean ages ranged from 44-58.74 years), imprecision (all studies had small sample sizes), and inconsistency (high statistical heterogeneity was present for blood loss and operative time).
A network meta-analysis that included 1,639 patients with multilevel CSM found that ACDF resulted in a shorter operative time compared (directly and indirectly) to other surgical techniques (ACCF, CDA, and hybrid surgery). 38 Limitations included low-quality studies (12 of 16 studies were case-control designs), indirectness (indirect comparisons were a core component of the methodology, 14 studies took place in Asia [China, South Korea, Singapore], and only a single study included a US population), and imprecision (all studies had small sample sizes).
Montano, et al 39 compared the results of ACDF and laminoplasty in 5 NRSI (487 patients). There was a statistically significant difference favoring ACDF regarding blood loss (MD = -131.381; 95% CI, -259.522 to -3.240; p = 0.044). No between-group difference was seen in operative time (MD = -10.231; 95% CI, -58.612 to 38.149; p = 0.679). Limitations related to the low-quality of included studies (all were observational cohort or case-control designs), indirectness (all studies had mean ages between 54 and 56.8 years), imprecision (too few events for all outcomes and wide confidence intervals for all pooled outcomes measured), and inconsistency (high heterogeneity involving operative time and blood loss).
A total of 5,249 patients from 14 NRSI provided data on surgical outcomes for a systematic review with meta-analysis. 40 Compared to ACCF, blood loss was significantly less in patients that underwent ACDF (OR = -528.63, 95% CI: -586.86, -470.39; p < 0.00001). Operative time was comparable between the 2 surgical groups. Limitations were due to the low-quality of the included studies (all studies were retrospective observational cohorts or case-control designs) and indirectness (11 of the studies were conducted in Asia [China, South Korea]).
Xu, et al 43 pooled data from 6 NRSI (379 patients) in comparing ACDF with posterior laminoplasty (LAMP). No significant differences between the 2 surgical groups were found for operative time (MD = 32.81, 95% CI = -26.76 to 92.38; p = 0.28) and blood loss MD = -24.16, 95% CI = -174.47 to 126.15; p = 0.75). Limitations included the low-quality study designs (All studies were retrospective observational cohorts or case-control), indirectness (all studies had mean ages between 44.7 and 67 years), imprecision (data were derived from small studies with wide confidence intervals that crossed the indicator for no effect), and inconsistency (very high unexplained heterogeneity).
A systematic review with meta-analysis of 669 participants (4 NRSI) assessed surgical outcomes between ACDF and hybrid surgery. 44 Blood loss significantly favored ACDF over hybrid surgery (SMD = -30.29, 95% CI = -45.06, -15.52; p <.00001), while operative time was similar. The limitations of this review involved the low-quality study designs (all studies were retrospective observational cohorts or case-control designs), and indirectness (none of the studies were performed in the United States, and the mean age among studies ranged from 46.1 to 54.36 years).
One RCT that was not included in any evidence synthesis was identified. El-Ghandour, et al 42 compared ACDF with posterior laminectomy with or without fusion in the management of degenerative cervical myelopathy (DCM). Sixty-eight patients (mean age 53 years) were enrolled in the 1:1 randomized trial. The mean operative duration was significantly longer in the ACDF group (p < 0.001). The mean hospital stay was significantly longer in the posterior group (p < 0.001). The authors noted several methodologic limitations including a high risk of bias in the randomization process, small sample size, and short-term follow-up.
Overall, the observed perioperative results varied by the comparators and the type of outcome. Compared to open PCF, ACDF had significantly longer operative time and LOS. Minimally invasive posterior cervical fusion also had a significantly less LOS than ACDF. When ACDF was compared to other surgical techniques, no significant differences were found in operative time and blood loss. The overall certainty of the evidence was rated as very low due to the high risk of bias in the included RCTs, limitations inherent in observational studies, and uncertainty about the applicability of the results to the US Medicare population (indirectness).
Five evidence syntheses included assessments of radiographic outcomes (fusion rate, range of motion [ROM], alignment, and lordotic. 36,37,39,40
Fusion rate was recorded in a systematic review with meta-analysis of 18 primary studies (1 RCT, 17 NRSI). 36 After a minimum of 6 months post-surgery, the authors found the fusion rate was 100% for patients that underwent ACDF and ACCF. Review limitations involved the low-quality of selected studies (17 of 18 studies were of observational designs), indirectness (17 of 18 studies included participants located in China or South Korea, and all the studies had a mean/median between 32-59 years), imprecision (all studies had small sample sizes with confidence intervals that crossed the indicator for no effect), and inconsistency (there was significant heterogeneity for all secondary outcomes except fusion rate).
In another systematic review with meta-analysis, Han, et al (2014) also reported no difference in fusion rates between ACDF and ACCF (OR = 1.17, 95% CI = 0.34 to 4.11; p = 0.80). 37 Cobb and segmental angles were measured at a minimum of 24 months follow-up. Anterior cervical discectomy and fusion showed statistically significant outcomes compared to ACCF. The limitations in this review included the low-quality of the selected studies (all studies were observational cohort or case-control designs), indirectness (mean ages ranged from 44-58.74 years), imprecision (all studies had small sample sizes), and inconsistency (high statistical heterogeneity was present for fusion rate and Cobb angle).
Montano, et al 39 conducted a systematic review with meta-analysis that compared radiographic outcomes reported in 5 NRSI between ACDF and laminoplasty. The Cobb angle (cervical lordosis) results were significantly better for the ACDF group (MD = 5.63; 95% CI, 1.663-8.469; p = 0.004), while ROM favored laminoplasty (MD = -5.399; 95% CI, -10.584 to -0.214; p = 0.041). Limitations included the low-quality of analyzed studies (all were observational cohort or case-control designs), indirectness (all studies had mean ages between 54 and 56.8 years) imprecision (too few events for all outcomes and wide confidence intervals for all pooled outcomes measured), and inconsistency (high heterogeneity involving cervical lordosis and ROM.
A systematic review with meta-analysis of 14 NRSI (5,249 patients diagnosed with multilevel CSM) reported statistically significant results favoring ACDF versus ACCF in sagittal and segmental angles, as well as fusion rate. 40 Limitations were due to the low-quality of the included studies (all studies were retrospective observational cohorts or case-control designs) and indirectness (11 of the studies were conducted in Asia [China, South Korea]).
Zhao, et al 44 evaluated the radiographic effects of ACDF compared to hybrid surgery. The meta-analysis of 669 patients (4 NRSI) found no significant differences in fusion rate and C2-C7 (Cobb) angle at 24 to 38 months post-surgery. Review limitations included the low-quality of the selected studies (all were retrospective observational cohorts or case-control designs), indirectness (none of the studies were performed in the United States and the mean age among studies ranged from 46.1 to 54.36 years), imprecision (wide confidence angles that represented variable outcomes were found in the pooled data fusion rate), and inconsistency (heterogeneity of effects in the pooled analyses for C2-C7 angle and fusion rate).
In aggregate, radiographic outcomes – obtained at variable time points – showed equitable or favorable results in fusion rate, angular measurements, and alignment for ACDF compared to ACCF, laminoplasty, and hybrid surgery. Laminoplasty was superior to ACDF in preserving ROM. The certainty of the evidence was determined to be very low due to the types of included study designs (mostly retrospective NRSI), uncertainty about the generalizability to the US Medicare population, imprecise results, and heterogeneity of findings.
Six evidence syntheses provided findings regarding a range of undesirable effects associated with cervical fusion surgeries. 36-40,44 Four reviews focused on the total or overall complication rate. 36-39 In addition to the total complication rate, 2 syntheses detailed the results of a range of undesirable effects. 40,44
Fei, et al 36 reported that the total complication rate was significantly lower for individuals with ACDF than ACCF (RR= 0.51, 95% CI: 0.33, 0.80; p = 0.003). This result was based on a meta-analysis of 1,246 patients, with a minimum of 6 months follow-up. Limitation of this evidence synthesis were the low-quality of the selected studies (17 of 18 studies were of observational designs), indirectness (17 of 18 studies included participants located in China or South Korea, and all the studies had a mean/median between 32-59 years), imprecision (all studies had small sample sizes with confidence intervals that crossed the indicator for no effect), and significant heterogeneity was present.
Another systematic review with meta-analysis of 1,372 patients diagnosed with multilevel CSM also determined the incidence of overall complications was significantly greater in the ACCF group compared to those who had ACDF surgery (OR = 0.50, 95% CI = 0.35 to 0.73; p = 0.0003). 37 In this review the minimum follow-up period was 24 months. Limitations involved the low-quality of all included studies (observational cohort or case-control designs), indirectness (mean ages ranged from 44-58.74 years), and imprecision (all studies had small sample sizes)
A network meta-analysis calculated both direct and indirect comparisons of overall complications for patients who had undergone ACDF, ACCF, CDA, or hybrid surgery. 38 At a post-operative mean of 33.3 months, CDA resulted in a lower incidence of complications than different types of cervical fusion procedures. The limitations of this network meta-analysis included the low-quality of most study designs (12 of 16 studies were case-control designs), indirectness (indirect comparisons were a core component of the methodology, and only a single study included a US population), and imprecision (all studies had small sample sizes).
A systematic review with meta-analysis included 5 NRSI (N=487) that compared outcomes (mean 35.4 months) between ACDF and laminoplasty. 39 There was no statistically significant difference regarding the complication rate (OR = 1.604; 95% CI, 0.972-2.648; p = 0.065). Limitations included the low-quality of the selected studies (all included studies were observational cohort or case-control designs), indirectness (all studies had mean ages between 54 and 56.8 years), and imprecision (wide confidence intervals for all pooled data).
Wang, et al 40 included data from 5,249 participants (14 NRSI) in a systematic review with meta-analysis. The number of total complications was significantly less for individuals that received ACDF compared to ACCF (OR=0.56, 95% CI: 0.48-0.65; p < 0.00001). This study provided additional details about specific undesired effects. No significant difference between ACDF and ACCF was found for dysphagia, hoarseness, cerebrospinal fluid (CSF) leakage, infection rate, epidural hematoma, axial pain, hardware breakage, and pseudoarthrosis. Anterior cervical discectomy and fusion demonstrated statistically superior outcomes regarding the event rate of C5 palsy, revision surgeries, graft subsidence, and graft dislodgement. The limitations of this review were related to the low-quality of all the selected studies (retrospective observational cohorts or case-control designs), and indirectness (11 of the studies were conducted in China or South Korea).
Zhao, et al 44 compared ACDF to hybrid surgery, pooling data from 4 NRSI that comprised 669 patients. Post-surgical outcomes were captured between 24 and 38 months. There was a significant difference in total complications favoring ACDF (OR = 0.66, 95% CI = 0.44-0.98; P = .04). At the discrete outcome level, there was no significant difference between ACDF and hybrid surgery seen for graft subsidence, C5 palsy, infection, CSF leakage, dysphagia, or epidural hematoma. Limitations related to the low-quality of all the selected studies (retrospective observational cohorts or case-control designs), and indirectness (none of the studies were performed in the US, and mean age among studies ranged from 46.1 and 54.36 years), and imprecision (wide confidence intervals, representative of variable outcomes, were found in the pooled data subgroup analyses for graft subsidence, infection rate, and CSF leakage).
One RCT that was not included in any evidence synthesis was identified. El-Ghandour, et al 42 compared ACDF with posterior laminectomy with or without fusion in the management of degenerative cervical myelopathy (DCM). Sixty-eight patients (mean age 53 years) were enrolled in the 1:1 randomized trial. No significant difference in postoperative complications was found, except postoperative dysphagia was significantly higher in the anterior group (p < 0.05). The authors noted several methodologic limitations including a high risk of bias in the randomization process, small sample size, and short-term follow-up.
A retrospective cohort compared the short-term outcomes for laminoplasty, laminectomy with fusion, and ACDF. 45 Data from 546 patients resulted in the authors concluding that laminectomy with fusion carries the highest risk for morbidity, mortality, and unplanned readmissions in the short-term postoperative period. Laminoplasty and ACDF cases carry similar short-term complications and risks. Study limitations include the retrospective design, short-term (30-day) outcomes, and clinical heterogeneity.
Overall, apart from CDA, the complication rate favored ACDF over other types of fusion and non-fusion surgeries. The certainty of the evidence was judged to be very low due to inherent study design limitations, indirectness, imprecision involving subgroup analyses, and significant heterogeneity.
Cervical Myeloradiculopathy (Mixed)
Cervical myelopathy occurs when there is compression of the spinal cord in the neck area. Patients present with issues with fine motor skills, pain or stiffness in the neck and loss of balance. Causes of cervical myelopathy may include rheumatoid arthritis, cervical spine trauma, spinal infection, spinal tumors or cancer. 46
Myelopathy and cervical spine instability severity is classified based on the criteria put forth by Ranawat. 47 , 48
- Class I – No neurologic deficit
- Class II – Subjective weakness with hyperreflexia and dysesthesia
- Class IIIA – Objective weakness with long-tract signs; remains ambulatory
- Class IIIB – Objective weakness with long-tract signs; nonambulatory and quadriparetic
Four evidence syntheses with publication dates ranging from 2013-2022 that assessed clinical outcomes were identified. 49-52 All the syntheses included only randomized controlled trials. The number of primary studies in the syntheses ranged from 8 to 30, with generally small sample sizes. The eligibility criteria included participants that were diagnosed with single or multilevel cervical radiculopathy, myelopathy, or both (myeloradiculopathy). All reviews compared the effects of ACDF to CDA. Timing to follow-up was variable (29 months to ~8 years).
Núñez, et al 49 published a systematic review with meta-analysis that comprised 9 RCTs with sample sizes ranging from 44 to 541. Clinical outcomes were assessed at a mean of 7.8 years post-surgery. The overall success rate in the CDA group was significantly higher than in the ACDF group (OR = 1.98, 95% CI: 1.57–2.49, p < 0.001) with moderate heterogeneity (I 2 = 36%, p = 0.16). There were no significant differences between the 2 groups regarding neck pain (SMD = − 0.17, 95% CI: − 0.37–0.04, p = 0.11) with high heterogeneity (I 2 = 74%, p = 0.002). Patient-reported arm pain results significantly favored the CDA group (SMD = − 0.16, 95% CI: − 0.29 to − 0.04, p = 0.01) with moderate heterogeneity (I 2 = 31%, p = 0.20). The physical SF-36 (quality of life) component significantly favored the CDA group (SMD = 0.13 95% CI: 0.03–0.23, p = 0.01) with very low heterogeneity (I 2 = 1%, p = 0.40). No significant differences were found between the 2 groups in the mental SF-36 component (SMD = 0.19, 95% CI: − 0.03–0.41, p = 0.10), with substantial heterogeneity (I 2 = 69%, p = 0.02). Limitations of this review included a high or unclear risk of bias involving all RCTs, indirectness (the mean age across studies was ~44.5 years), some imprecision involving overall treatment success, significant heterogeneity was present in all pooled clinical results.
Peng, et al 50 produced a systematic review with meta-analysis of 9,329 participants with single or multilevel cervical radiculopathy or myelopathy. At multiple follow-up time points, overall success favored CDA (total, OR 1.91, 95% CI 1.73 to 2.11, p=0.000). Neurological success results also favored CDA in all follow-up periods (total, OR 1.74, 95% CI 1.49 to 2.04, p = 0.000). Disability results in all follow-up periods favored CDA (total, OR 1.70, 95% CI 1.49 to 1.94, p = 0.000). Limitations of this analysis included all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age among the studies ranged between 40-50 years), and imprecision (all pooled analyses for each follow-up period had confidence intervals that crossed the null value).
Xing, et al 51 included 8 RCTs with sample sizes ranging from 19 to 541. Participants (N=1,617) had single-level cervical radiculopathy or myelopathy. Outcomes were measured between 24 and 48 months. For overall success, CDA was significantly superior to ACDF (OR = 1.84, 95% CI = 1.43 to 2.36; p < 0.00001), with no heterogeneity (I 2 = 0%). The neurological success rate results showed CDA was significantly superior to ACDF (OR = 1.75, 95% CI = 1.20 to 2.55; p = 0.004), with no heterogeneity (I 2 = 0%). There was no significant difference between the 2 treatment groups concerning disability measures. For arm pain, there was a significant difference between the 2 groups favoring CDA (MD = -4.86, 95% CI = -6.42 to -3.30; p < 0.00001) with low heterogeneity (I 2 = 8%). There was a significant difference between the 2 groups favoring CDA (MD = -7.90, 95% CI = -10.36 to -5.44; p < 0.00001) with no heterogeneity (I 2 = 0%) regarding neck pain outcomes. Limitations of this evidence synthesis included all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age of included studies was 43 years), imprecision (6 of 8 [75%] of studies had sample sizes smaller than 400 and all pooled analyses exhibited confidence intervals that crossed the null value), and inconsistency (moderate heterogeneity in the pooled results for disability).
Zhang, et al 52 included 1,238 participants from 13 RCTs in a systematic review with meta-analysis. Outcomes were assessed at a mean of 83 months. The overall success rate significantly favored the CDA group compared to the ACDF group (OR = 1.68, 95% CI: 1.29–2.19, p < 0.001) with low heterogeneity (I 2 = 0%, p = 0.62). The CDA group had significantly higher neurological success than the ACDF group (OR = 1.54, 95% confidence interval [CI]: 1.14– 2.08, p = 0.004) with moderate heterogeneity (I 2 = 34.0%, p = 0.18). For disability results, the CDA group was significantly improved versus the ACDF group (MD = − 0.20, 95% CI: − 0.36 to − 0.05, p = 0.009), with moderate heterogeneity (I 2 = 37.8%, p = 0.20); however, the effect size did not reach clinical relevance. Measures of quality-of-life were better in the CDA group; however, neither statistical nor clinical significance was achieved. Both neck and arm pain were significantly better in the CDA group than in the ACDF group (MD = − 0.20, 95% CI: − 0.35 to − 0.05, p = 0.01 and MD = − 0.23, 95% CI: − 0.38 to − 0.07, p = 0.004, respectively), with low heterogeneity (I 2 = 31.6%, p = 0.23 and I 2 = 24.3%, p = 0.27, respectively). Limitations of this evidence synthesis included studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age across studies was ~44.5 years), imprecision (confidence intervals crossed the null effect), and inconsistency (moderate to substantial heterogeneity was found in the pooled analyses for neurological success and disability outcomes).
In aggregate, CDA demonstrated statistically significant improvement across clinical outcomes apart from quality-of-life measures, which were similar to ACDF. The clinical significance of the results was not described when assessing most pooled results for specific outcomes. Overall, the certainty of evidence was judged to be very low. All the included RCTs were rated as having unclear (some concerns) or a high risk of bias. The mean age among the studies ranged between 40-50 years and clinical relevance was not usually assessed (indirectness). More than 50% of studies had small sample sizes and wide confidence intervals suggesting variable effects (imprecision).
A single evidence synthesis provided data regarding surgical results.
Peng, et al 50 conducted a systematic review with meta-analysis of 30 RCTs. Participants (N=9,329) with single or multilevel cervical radiculopathy or myelopathy randomly underwent ACDF or CDA. The pooled data showed ACDF had a significantly longer operative duration (OR, − 0.28; 95% CI, − 0.37 to − 0.2; p = 0.000). There were no significant differences between CDA and ACDF for blood loss and LOS (OR, 0.63; 95% CI, − 2.83 to 4.1; p = 0.72; OR, − 0.01; 95% CI, − 0.08 to 0.05; p = 0.68, respectively). The limitations of this analysis included all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age among the studies ranged between 40-50 years), and imprecision (all pooled analyses for each follow-up period had confidence intervals that crossed the null value).
Based on limited data, CDA demonstrated more than a 25% reduction in operative time compared to ACDF. There were no other perioperative outcomes that revealed significant differences between CDA and ACDF. The certainty of the evidence was rated as very low. All included primary studies had unclear (some concerns) or a high risk of bias. There was uncertainty about the applicability of the results to the US Medicare population (indirectness). All pooled analyses for each follow-up period had confidence intervals that crossed the null value (imprecision).
Three evidence syntheses reported on radiographic results in comparing ACDF with CDA. 49,51,52
Núñez, et al 49 systematically reviewed and meta-analyzed data from 9 RCTs (N=2,664) who received either ACDF or CDA. The fusion rate was 94.06% in the ACDF group, with no data for CDA. The heterotopic ossification rate for CDA = 10.3%, with no data for ACDF. The ROM rate in the CDA group was significantly higher (SMD = 1.86, 95% CI: 1.63–2.08, p < 0.001) with no heterogeneity (I 2 = 0%, p = 0.52). Less superior adjacent segment disease (ASD) was reported in the CDA group (OR = 0.33, 95% CI: 0.17–0.65, p = 0.001) with substantial heterogeneity (I 2 = 81%, p < 0.001). Less inferior ASD was reported in the CDA group (OR = 0.31, 95% CI: 0.15–0.66, p = 0.002), with substantial heterogeneity (I 2 = 75.1%, p = 0.05). Limitations of this review included a high or unclear risk of bias involving all RCTs, indirectness (the mean age across studies was ~44.5 years), and inconsistency (significant heterogeneity was present in all pooled results except ROM).
Xing, et al 51 measured radiological success as part of a systematic review with meta-analysis. There was no significant difference between the ACDF and CDA groups (OR = 0.87, 95% CI = 0.36 to 2.09; p = 0.76). Limitations of this evidence synthesis included all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age of included studies was 43 years), and imprecision 6 of 8 {75%} of studies had sample sizes smaller than 400 and all pooled analyses exhibited confidence intervals that crossed the null value).
Zhang, et al 52 pooled data from 13 RCTs (N=1,238) in assessing ROM between patients who had ACDF or CDA. The CDA group had significantly larger ROM than the ACDF group (MD = 1.76, 95% CI: 1.57– 1.94, p < 0.001) with low heterogeneity (I 2 = 0%, p = 0.38). Limitations of this evidence synthesis included all studies having unclear (some concerns) or high risk of bias, indirectness (the mean age across studies was ~44.5 years), imprecision (confidence intervals crossed the null effect), and inconsistency (moderate heterogeneity regarding ASD outcomes).
Overall, apart from overall success, CDA demonstrated statistically greater radiological results when compared to ACDF. The certainty of the evidence was determined to be very low. Limitations included high or uncertain risk of bias across all included trials, indirectness, imprecision, and heterogeneity (ASD outcomes).
Four evidence syntheses reported the pooled results of undesirable effects associated with cervical spine surgeries. All studies were conducted as systematic reviews with meta-analysis of RCTs. 49-52
Núñez, et al 49 described outcomes for 2,664 participants obtained from 9 RCTs. For overall complication rate, no significant differences were found between CDA (33.1%) and ACDF (38.9%) (OR = 0.84, 95% CI: 0.56–1.27, p = 0.42), with moderate heterogeneity ( I 2 = 37%, p = 0.13). Reoperations occurred in 4.4% of CDA patients, a significantly lower rate compared with 15.6% of the ACDF group (OR = 0.26, 95% CI: 0.19–0.37, p < 0.001), with no heterogeneity ( I 2 = 0.0%, p = 0.97). The limitations of this review included a high or unclear risk of bias involving all RCTs, indirectness (the mean age across studies was ~44.5 years), and inconsistency (significant heterogeneity was present in the pooled results).
Peng, et al 50 included 30 RCTs (N=9,329) in the review comparing ACDF with CDA. In assessing implant events, no significant difference was found between the CDA and ACDF groups in short-term, mid-term, and long-term follow-up and total analysis (total, OR 1.14, 95% CI 0.92 to 1.42, p = 0.23). The incidence of ASD was higher in the ACDF group in long-term follow-up and total analysis (total, OR 0.71, 95% CI 0.55 to 0.94, p = 0.01). The incidence of dysphagia and dysphonia was higher in ACDF in short-term follow-up. There were no significant differences in total, mid- and long-term follow-up between CDA and ACDF (total, OR 0.9, 95% CI 0.73 to 1.09, p = 0.28). The reoperation rate was higher in ACDF in all follow-ups (total, OR 0.37, 95% CI 0.29 to 0.46, p = 0.000). This analysis had limitations including all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age among the studies ranged between 40-50 years), and imprecision (all pooled analyses for each follow-up period had confidence intervals that crossed the null value).
Xing, et al 51 compared the overall complication rates of ACDF and CDA for a mixed (cervical radiculopathy or myelopathy) population. The meta-analysis found there was no significant difference between the 2 surgical methods (RR = 0.77, 95% CI = 0.48 to 1.23; p = 0.27) with moderate heterogeneity ( I 2 = 39%). Limitations of this evidence synthesis included all primary studies were rated as having unclear (some concerns) or high risk of bias, indirectness (the mean age of included studies was 43 years), and imprecision (6 of 8 {75%} of studies had sample sizes smaller than 400 and all pooled analyses exhibited confidence intervals that crossed the null value).
Zhang, et al 52 provided a comprehensive meta-analysis of undesired effects for 1,238 participants who underwent ACDF or CDA. The difference in complication rates was not significant between the 2 groups (OR = 1.01, 95% CI: 0.77–1.32, p = 0.96), with low heterogeneity (I 2 = 8.4%, p = 0.37). For reoperations at the index level, the rate was significantly lower in the CDA group than in the ACDF group (OR = 0.41, 95% CI: 0.25–0.69, p = 0.001), with substantial heterogeneity (I 2 = 61.0%, p = 0.004). For reoperation at an adjacent level, the rate was significantly lower in the CDA group than in the ACDF group (OR = 0.34, 95% CI: 0.26–0.46, p < 0.001), with low heterogeneity (I 2 = 23.4%, p = 0.22). Limitations of this evidence synthesis included all studies having unclear (some concerns) or high risk of bias, indirectness (the mean age across studies was ~44.5 years), imprecision (confidence intervals crossed the null effect), and inconsistency (substantial heterogeneity regarding the reoperation rate).
In aggregate, ACDF and CDA demonstrate similar overall complication rates; however, CDA resulted in fewer reoperations and less ASD. Anterior cervical discectomy and fusion and CDA result in equitable long-term dysphagia and dysphonia outcomes. The overall certainty of the evidence was determined to be very low due to the high or unclear risk of bias in the included RCTs, indirectness, imprecise results, and inconsistency (heterogeneity) affecting multiple outcomes.
Societal Guidance
North American Spine Society (NASS) 3 appropriateness criteria for cervical fusion surgery as a treatment for cervical myelopathy states “While there are some non-fusion procedures available to decompress the spinal canal such as laminoplasty and anterior cervical discectomy without fusion, the workhorses of surgical treatments for cervical spondylotic myelopathy (CSM) are ACDF, anterior corpectomy and fusion, and posterior laminectomy and fusion. It is well-accepted that non-operative care is acceptable in patients with mild myelopathy and that surgery should be considered in most. Anterior cervical decompression and fusion has become the most common surgical approach and technique for treating patients with CSM. With the availability of modern posterior cervical screw fixation, posterior cervical laminectomy and fusion has become an additional workhorse for treatment of CSM, with equivalent results as anterior surgery.”
Cervical myelopathy: (either from disc herniation, bony stenosis, or ossification of posterior longitudinal ligament [OPLL]) may be considered as an adjunct to decompression when anterior cervical discectomy, corpectomy or posterior laminectomy is planned for decompression of the spinal cord.
A multidisciplinary expert panel defined appropriate use criteria (AUC) of cervical fusion for the treatment of degenerative conditions of the cervical spine [Reitman]. Appropriate use criteria were developed using the RAND/UCLA appropriateness methodology. All scenarios included a surgical plan as either cervical fusion (nonspecific) or specified as anterior cervical fusion (ACF), posterior cervical fusion (PCF), or combined (APCF). Clinical scenarios involving myelopathy was most strongly associated with an “Appropriate” rating. A total of 92% of scenarios with myelopathy, and well controlled medical or mild psychosocial comorbidities, or smokers, received a final rating of “Appropriate.” All (100%) scenarios with myelopathy and poorly controlled medical or mild psychosocial comorbidities, or smokers, received a final rating of “Appropriate” for cervical fusion surgery.
AOSpine North America and the Cervical Spine Research Society 53 published clinical practice guidelines that outline recommendations about how to best manage (1) patients with mild, moderate, and severe myelopathy and (2) nonmyelopathic patients with evidence of cord compression with or without clinical symptoms of radiculopathy.
Recommendations based on 5 systematic reviews of the literature concerning the value of surgery and nonsurgical approaches for CSM were as follows: (1) “We recommend surgical intervention for patients with moderate and severe DCM.” (2) “We suggest offering surgical intervention or a supervised trial of structured rehabilitation for patients with mild DCM. If initial nonoperative management is pursued, we recommend operative intervention if there is neurological deterioration and suggest operative intervention if the patient fails to improve.” (3) “We suggest not offering prophylactic surgery for nonmyelopathic patients with evidence of cervical cord compression without signs or symptoms of radiculopathy. We suggest that these patients be counseled as to potential risks of progression, educated about relevant signs and symptoms of myelopathy, and be followed clinically.” (4) “Non-myelopathic patients with cord compression and clinical evidence of radiculopathy with or without electrophysiological confirmation are at a higher risk of developing myelopathy and should be counseled about this risk. We suggest offering either surgical intervention or nonoperative treatment consisting of close serial follow-up or a supervised trial of structured rehabilitation. In the event of myelopathic development, the patient should be managed according to the recommendations above.”
The World Federation of Neurosurgical Societies (WFNS) The Spine Committee of the World Federation of Neurosurgical Societies (WFNS) 54 formulated an evidence-based consensus meeting on the management of CSM to develop recommendations for global applicability. With some adaptations, the WFNS Spine Committee endorsed the guidelines of AOSpine North America and the Cervical Spine Research Society. 53 Additional recommendations include: using modified Japanese Orthopedic Association scale (mJOA) or its regional modifications to classify CSM as severe, moderate or mild and suggest offering surgical intervention or rehabilitation for patients with mild CSM (mJOA) score 15–17. They state there is a consistent lack of evidence regarding the value of nonoperative treatment of cervical myelopathy in the literature hence nonoperative treatment may not be the final decision in most cases. They also share that predicting factors that indicate a possible deterioration during nonoperative, important predictors of myelopathy development include the presence of symptomatic radiculopathy, prolonged motor evoked potentials and somatosensory evoked potentials and electromyography signs of anterior horn cell lesions (low evidence), patients are likely to achieve a better result after surgery if they have a shorter duration of symptoms (low evidence) and a call for additional RCTs.
World Federation of Neurosurgical Societies (WFNS) Spine Committee recommendations include:
- In patients with CSM, the indications for surgery include persistent or recurrent radiculopathy nonresponsive to conservative treatment (3 years); progressive neurological deficit; static neurological deficit with severe radicular pain when associated with confirmatory imaging (CT, MRI) and clinical-radiological correlation.
- The indications of anterior surgery for patients with CSM include straightened spine or kyphotic spine with a compression level below.
They clarify in the elderly age groups with bony ankylosis due to osteophytes at C5–6–7, CSM may manifest at higher levels where motion segments are preserved, especially the C3–4 level and also at lower levels such as the C7–T1 level. These guidelines provide directives for future research with suggested outcome measures, variabilities to consider as well as patient and surgery selection guidance.
The Italian Neurosurgical Society (SINch) 55 analyzed and proposed their own recommendations for the management of CSM in accordance with the recommendations published by the spine committee of the WFNS. The majority of WFNS recommendations were adopted with a few modifications.
The Japanese Orthopaedic Association (JOA) 56 published 2023 clinical practice guidelines on the management of cervical spondylotic myelopathy(CSM). The recommendations relevant to this evidence analysis are reported here:
- For mild cases of CSM, conservative treatment is primarily selected; however, at present there is insufficient evidence regarding treatment outcomes. Surgery is considered suitable for progressive myelopathy in which conservative treatment is unsuccessful.
- It is possible that conservative treatment can delay the progression of mild-to-moderate CSM, and therefore, performing conservative treatment is weakly recommended. For severe and progressive CSM, surgery is likely the first choice of treatment. However, there are few reports regarding the choice of treatment for mild and moderate CSM, and thus, it is difficult to draw a conclusion.
- There is no clear recommendation regarding which surgeries to perform
Additional Systematic Review/Meta-Analysis
Youssef et al 57 conducted a systematic review and meta-analysis to evaluate the patient-reported and clinical outcomes of adult patients who underwent subaxial posterior cervical fusion with decompression for spondylosis, spinal stenosis and degenerative disc disease resulting in radiculopathy or myelopathy. PubMed and Embase were searched for applicable literature resulting in a total of 33 and 31 articles which included in the systematic review and the meta-analysis, respectively. Changes in values of preoperative to postoperative patient-reported outcomes (visual analog scales for arm pain and neck pain, Neck Disability Index (NDI), JOA score, modified JOA score, and Nurick pain scale) were evaluated. Patient reported outcomes, successful fusion, revision surgeries, and complications/adverse events were considered. A subgroup analysis was performed in 2 instances; 1 for studies that included surgical indications for only myelopathy or radiculopathy (or combination) and another for studies that included surgical indications for only myelopathy or ossifications of the posterior longitudinal ligament (or combination). Cumulative changes in PROMs improved for all surgical indications including the 2 subgroup analyses. All surgical indications resulted in pooled outcomes rates of 98.25% for successful fusion, 1.09% for revision, and 9.02% for complications/adverse events. Axial pain, C5 palsy, transient neurological worsening, and wound infection were the most common complications. Limitations include high risk of bias, reporting bias, confounding, high heterogeneity, included 20/31 retrospective studies, and combining of different PROMs. Authors report many conflicts of interest but none that seem to impact this study; however, the study was funded by Providence Medical Technology, Inc.
Unstable Spine
Systematic Review/Meta-Analysis
Mahmoud and colleagues 58 conducted a systematic review comprised of 627 patients and 36 articles to review the surgical indications, complications, and functional outcomes of different approaches for hangman fractures. Selected literature contained at least 1 of the primary outcomes: functional outcomes, complication rates, operation time, and blood loss. Minimally invasive surgery, C2 direct pedicle screw, C2- C3 fusion, and ACDF techniques were reviewed. Visual analog scores fell in all 4 groups when comparing pre- and postoperative scores, with the lowest VAS scores observed in the minimally invasive surgery group. In unstable fracture patterns, the literature showed that ACDF was preferred. Complications remained low in all groups with the greatest blood loss occurring in the posterior approach (255.9 mL in open posterior approach, 75.8 mL in MIS, and 64.3 mL in ACDF). Authors concluded if indicated, posterior approach may be preferred due to lower blood loss and disc access. When considering posterior approach, MIS may improve outcomes and have fewer complications. Limitations of the present study are inclusion of studies with retrospective study design, variations in reporting methods making data points challenging to categorize and lack comparative outcome studies.
Lee and associates 59 performed a meta-analysis comparing biomechanical and clinical outcomes between anterior-only and combined anterior and posterior fusions to determine which method of cervical fusion yielded better results for unstable cervical injuries. Ultimately, the analysis included 12 studies (8 biomechanical and 4 clinical studies) which included published dates between 2000-2019. Authors concluded cervical stability can be successfully restored following subaxial cervical injuries by anterior-only and combined anterior and posterior methods. No significant differences in clinical outcomes were observed while some advantages were associated with combined fusion in terms of biomechanical stability. Selective use of methods should be determined based on type of injury. Authors also note need for control of technical factors of surgery that may have impacted results of cervical fusion. Limitations include a small sample size, in vitro studies, low number of studies included for analysis, and risk of bias.
Prospective Studies
Yang et et al 60 performed a prospective study to propose a novel classification and scoring system called the posterior ligament-bone injury classification and severity score (PLICS) that offers a quantitative score to guide the need for posterior stabilization in addition to anterior reconstruction for subaxial cervical fracture dislocations (SCFDs). The study included 456 SCFD patients. High risk patients were defined as having PLICS ≥ 7 together with extremely unstable lateral mass fracture (EULMF). All others were defined as low-risk patients. Anterior only reconstruction was performed in the low-risk patients and additional posterior lateral mass fixation and fusion was performed after anterior reconstruction in the high-risk patients. The VAS, the NDI, and the American Spinal Injury Association (ASIA) impairment scale were utilized for outcomes. At 12 month follow up, 321 patients in the low-risk group and 49 patients in the high-risk group were still participating. Visual analog scores from preoperative to 12 months significantly improved (from 6.1 + 0.3 to 1.1 + 0.2 in the low-risk group, P < 0.001; from 6.4 + 0.2 to 1.4 + 0.2 in the high-risk group, P < 0.001). In the low-risk group at 12 month follow up the average NDI score was statistically low 8.8 + 2.5 vs 13.8 + 3.4, P = 0.034). With regards to the ASIA scale, 80.5% of patients experienced > 1 grade improvement. Authors concluded for SCFD patients, EULMF and a PLICS score of 7 or greater may serve as a threshold for posterior stabilization in addition to anterior reconstruction. Limitations include the study design, patient selection methods, and exclusion of ankylosing spondylitis (AS) patients.
Madan et al 61 conducted a prospective cohort study to assess the functional, neurological, and radiological outcomes of the patients with traumatic cervical spine instability. A total of 99 patients with subaxial cervical spine injuries were admitted and operated on at a single center between February 2014 and February 2016 and included prospectively. In all patients, bony fusion, neurological recovery, NDI and complications were assessed during the mean follow up period of 27 months (range 12–42 months). Corpectomy procedures that involved 1 level were 77.8% (n=77), 2 levels were 19.2% (n=19) and 3 levels were 3% (n=3). Neck Disability Index resulted in a mean of 7.57 ± 5.42. Grade 1 fusion was reported in 64.6% of cases. The most frequent adverse event was dysphagia 79.8% (n=79). Authors concluded an appropriate treatment option for subaxial cervical spine injuries is anterior cervical corpectomy and stabilization with cage filled with bone and cervical reflex locking plate. Authors further state this procedure is associated with favorable fusion rates and is likely a procedure of choice for posttraumatic multiple disc prolapse. Limitations of this study include study design, single center inclusion, risk of bias and sample size.
A prospective cohort study sponsored by the International Spine Study Group (ISSG) followed 77 patients surgically treated for adult cervical deformities for 1-year. 62 Diagnoses included cervical sagittal imbalance (56%), cervical kyphosis (55%), proximal junctional kyphosis (7%), and coronal deformity (9%). Posterior fusion was performed in 85% (mean levels = 10), and anterior fusion was performed in 53% (mean levels = 5). At 1 year after surgery the cohort reported statistically significant improvement in neck pain, ability to perform usual activities, and reduced pain and discomfort measured by standardized scales. Limitations include lack of control group, short term follow-up, performance at high volume centers with experienced surgeons that can reduce generalizability, and lack of standardized selection for surgery.
Retrospective Studies
Zheng and associates 63 conducted a single center, retrospective, observational study comprised of 79 patients to investigate the role of ACDF in alleviating symptoms in patients with cervical vertigo associated with cervical instability. A significant relief of vertigo and dizziness was reported following anterior cervical surgery during the 2-year follow up period.
Yang and associates 64 performed a retrospective chart review to investigate the efficacy and safety of halo vest application before and during surgery in 25 patients with AS and severe thoracic kyphosis who underwent surgical treatment of cervical fracture-dislocation. Posterior or combined anterior-posterior surgery was performed on all patients. Authors conclude that use of the halo vest before and during the surgery is safe and effective while assisting with positioning, awake nasoendotracheal intubation, nursing, and the procedure by providing satisfactory reduction and immobilization.
Wang et al 65 performed a retrospective chart review comprised of 36 patients to investigate the safety and efficacy of the halo-vest in the treatment of cervical fracture in patients with ankylosing spondylitis (AS) and kyphosis. Authors concluded The AS patient should undergo early surgical stabilization utilizing a halo-vest to aid in spinal deformity correction and to avoid neurological decline.
Skeppholm and associates 66 conducted a cohort study of 28 patients with artificial disc replacement (ADR) or anterior cervical decompression which were recruited from a larger RCT cohort to evaluate in vivo motion and stability of implanted artificial discs. Authors concluded most of the ADRs resulted in favorable mobility and proper attachment years after implantation with instability detected in only 8% of patients.
Rustagi and colleagues 67 conducted a retrospective chart review of 29 patients to present a series of posterior-only operated occiput-cervical fixation (OCF) cases following metastasis to the upper cervical spine (UCS) and craniocervical junction (CCJ). Authors concluded posterior OCF without tumor resection and anterior reconstruction can effectively manage symptomatic metastasis while offering pain relief and improved quality of life.
Luksanapruksa and associates 68 conducted a retrospective cohort study of 33 patients to evaluate surgical outcomes and complications of cervical spine fractures in ankylosing spondylitis (CAS) patients who were treated using either the posterior (P) or combined approach after experiencing neck pain after a fall. Authors conclude, although not statistically significant, posterior surgery was associated with lower blood loss, rate of complications, and shorter length of stay as compared to the combined approach. Limitations include study design, small sample size, and moderate lost to follow up rate.
Li et al 69 conducted a retrospective study comprised of 38 consecutive patients to describe the authors’ method of anterior discectomy/corpectomy and fusion combined with internal fixation for the treatment of unstable hangman’s fractures and to evaluate the clinical and radiological outcomes. Authors conclude study results indicate to address unstable hangman’s fractures, an anterior discectomy/corpectomy and fusion combined with internal fixation is a favorable approach.
Lee and associates 70 conducted a retrospective review of radiologic images of patients (n=34) who underwent C1-2 fusion. Patients were divided into 1 of 2 groups (the C-arm group or O-arm group). Authors concluded in cases of unstable C1-2 pathologies posterior fixation can reduce the operative time by utilizing intraoperative cone-beam CT scans for spinal navigation.
Lang and colleagues 71 conducted a cohort study comprised of 33 patients to assess the radiological and mid-term patient-reported outcome of traumatic subaxial cervical fractures treated with different plate systems. Authors concluded regarding a fragile fracture, that dynamic plate provided adequate stability with no significant loss of reduction when compared to the rigid plates. Patient-reported outcomes were satisfactory in both groups.
Kong and associates 72 retrospectively analyzed clinical data of 46 patients who underwent C2-3 ACDF combined with internal fixation for unstable hangman’s fractures to investigate the changes in the sagittal parameters of the cervical spine and the clinical efficacy of C2-3 ACDF combined with internal fixation. Authors conclude that anterior reconstruction of the anterior and middle columns with plate internal fixation can successfully achieve stability, correct displacement and angulation of C2, and restore sagittal balance while maintaining a high rate of fusion with few complications.
Kim and colleagues 73 conducted a retrospective analysis of 65 patients who were treated for a C1 fracture at single site between 1999 and 2016. Authors concluded type 3 fractures are most likely to be unstable while type 2 fracture and MVC are associated with higher rates of fusion failure. Most fractures were managed conservatively.
Bakhsheshian 74 performed a systematic case review comprised of 8 case series and 64 patients to evaluate studies that utilized C2 lag screw placement in patients with traumatic spondylolisthesis of the axis (TSA). Authors concluded while freehand placement of C2 pedicle lag screws may be a reasonable option in some cases, C2 lag screw fixation resulted in positive fusion in most TSA patients. However, this study does not demonstrate superiority over conservative treatment or other surgical methodologies and evidence is limited to level IV studies.
Jin et al 75 conducted a retrospective clinical study to assess clinical outcomes and sagittal balance after unstable hangman fracture in 45 patients. Authors concluded that both anterior and posterior approaches are effective in treating unstable hangman fractures. They further state recovery of cervical sagittal balance was achieved by utilizing the posterior approach as compared to the anterior approach.
Garrido and colleagues 76 conducted a retrospective data review of 71 cases to assess the clinical outcomes of both rigid and nonrigid occipitocervical (OC) fusion constructs of a multicenter cervical spine study group. Authors conclude a statistically significant decrease in complication rates are associated with rigid occipitocervical construct as compared to nonrigidly fixed.
Dagtekin et al 77 conducted a single center retrospective study comprised of 88 patients to evaluate OCJ injuries and to discuss the treatment modalities of these traumas and examine cadaveric studies for a better understanding. Authors concluded while it is essential to consider fracture pathophysiology and fracture type, they believe the most appropriate modality for atlantoaxial stabilization is C1-C2 segmental stabilization.
Clark et al 78 conducted a retrospective chart review comprised of 43 consecutive patients to evaluate 30-day and 1-year mortality rates and review complications associated with posterior C1–2 fusion in an octogenarian cohort. Authors conclude in the octogenarian population a greater mortality rate is associated with initial fracture displacement. Lower mortality rates resulted with posterior C1-2 fusions with unstable type II odontoid fractures as compared to nonoperative management mortality rates.
Candura et al 79 performed a retrospective case series on 64 consecutive patients with Type II odontoid fractures who presented to the Vertebral Surgery Unit. Authors conclude odontoid Type II fractures can be successfully treated with conservative treatment modalities in elderly patients.
Aldrian et al 80 conducted a retrospective chart review on 46 patients to evaluate outcomes following surgical or nonoperative treatment of Hadley type IIA odontoid fractures. Authors conclude following nonoperative management, Hadley type IIA odontoid fractures are associated with increased risk for secondary loss of reduction and bony nonunion.
Surgical Approach
Multiple studies have compared surgical approaches and have not demonstrated superiority of 1 approach over another. A RCT compared ventral to dorsal surgical approach for CSM and found among 163 subjects the approaches shared similar outcomes at 1 year. 81 Other studies report similar results with some advantages and disadvantages found for each approach. A meta-analysis reports on 4,348 subjects and concludes that laminectomy and fusion techniques offer comparable clinical outcomes. 82 A 2023 systematic review and meta-analysis also found no significant difference in functional outcomes from ACDF vs. posterior discectomy (PD) with moderate evidence per GRADE analysis. 83 The subject matter experts summarize that an individualized approach should be tailored to the individual patient’s pathology to determine the optimal surgical route.
A 2023 systematic review on cervical degenerative disease treatment by the Agency for Healthcare Research and Quality 84 included 57 RCTs, 56 nonrandomized studies and 1 systematic review that enrolled patients with radiculopathy and/or myelopathy at 1 or more levels and analyzed various surgical approaches used. They report there were few comparative studies of non-operative treatments, and most studies were rated moderate risk of bias with most evidence rated lower insufficient strength to draw conclusions on comparative benefits and harms. They included ACDF, anterior versus posterior approach, standalone cage versus plate and cage in ACDF, and laminoplasty versus laminotomy with fusion. They conclude there were few differences in benefits between surgical approaches and techniques for the treatment of cervical degenerative disease however there was some differences in the frequency of adverse events for some comparison.
The Washington State Dept. of Labor & Industries’ Industrial Insurance Medical Advisory Committee (IIMAC) published a “Guideline for Diagnosis and Treatment of Cervical Radiculopathy and Myelopathy” Labor & Industries 85 and states: “The ideal surgical approach for radiculopathy related to herniated disc remains a matter of debate. Various studies have compared the different surgery types and found no significant difference among them. Cervical surgeries can be divided into 2 major approaches: anterior (with or without fusion) and posterior. Except for hybrid surgeries, the choice of surgical procedure is left to the discretion of the surgeon. Hybrid surgeries combine artificial disc replacements and anterior cervical discectomy with fusion at select vertebral bodies (adjacent or non-adjacent) in a single procedure. They state there is insufficient evidence in medical literature to permit conclusions on its safety and efficacy.” 85
North American Spine Society (NASS) Evidence-Based Clinical Guidelines on the Diagnosis and Treatment for Cervical Radiculopathy from Degenerative Disorders agrees that there is a lack of evidence that 1 surgical approach is superior. They reviewed multiple surgical approaches and found outcomes comparable with a Grade B Recommendation. 8
Axial Neck Pain
Axial neck pain is a prevalent condition that causes significant morbidity and productivity loss. 86 Nonspecific (primary) axial neck pain is distinguished from neck pain associated with specific causes (e.g., radiculopathy, myelopathy, stenosis, fracture). The pain distribution is localized to the neck and immediate surrounding structures and does not involve dysfunction of the arms, hands, fingers, or other body regions. It is characterized primarily by dull, achy pain in the nuchal region (posterior) neck. 87 The pain can sometimes travel to the base of the skull, shoulder, or scapulae. Other symptoms may include neck stiffness, headaches, and localized areas of muscle pain or paresthesia. Most axial neck pain is diagnosed based on ruling out specific causes of neck pain. There is a wide range of pharmacologic and non-pharmacologic treatment options for axial neck pain. The appropriateness of surgery for axial neck pain is uncertain. 88 The purpose of this analysis is to summarize the evidence on the impact of fusion surgery procedures for the management of primary axial neck pain.
Literature Analysis
In a systematic review with meta-analysis, van Middelkoop, et al 89 identified 10 RCTs that compared additional fusion upon anterior decompression surgical techniques. The reviewers found there was no additional benefit of fusion techniques applied within an anterior discectomy procedure in patients with neck pain without radiculopathy on pain, recovery, and return to work. For recovery, the pooled risk difference in the short-term follow-up was -0.06 (95% CI -0.22 to 0.10) and -0.07 (95% CI -0.14 to 0.00) in the long-term follow-up. The pooled risk differences for pain and return to work all demonstrated no differences.
Riew, et al 90 conducted a systematic review that examined the clinical outcome in patients undergoing ACDF for axial neck pain without radicular or myelopathic symptoms. No comparative studies were identified; however, 3 case series were evaluated. All studies showed a mean improvement of pain of at least 50% approximately 4 years following surgery. Functional outcomes improved between 32% and 52% from baseline. Most patients reported satisfaction with surgery, 56% in 1 study and 79% in another. Complications varied among studies ranging from 1% to 10% and included pseudoarthrosis (9%), nonunion and revision (3%), and screw removal (1%). The authors concluded, “There is low evidence suggesting that patients with axial neck pain without radicular or myelopathic symptoms may receive some improvement in pain and function following ACDF. However, whether this benefit is greater than nontreatment or other treatments cannot be determined with the present literature.” The authors reported several limitations in their review. These included low-quality primary study designs (noncomparative case series), indirectness (the mean age of patients ranged from 42-56 years) imprecise data (small sample sizes, and the proportion of patients who achieved a clinically meaningful improvement in pain and function >30% was not reported), and inconsistency regarding the selection of fusion levels and integrity of discs adjacent to the operated levels, and the role of provocative testing).
As part of a Neck Pain Task Force, Carragee, et al 91 conducted a best-evidence synthesis of literature from 1980 through 2006 on surgical interventions for neck pain alone in the absence of serious pathologic disease. The reviewers found that cervical fusion for neck pain without radiculopathy was not supported by current evidence. The literature search identified 4 frequently cited studies, which were deemed to be scientifically inadmissible, pertaining to cervical fusion for non-radicular neck pain with only common degenerative changes.
- Palit, et al 92 retrospectively reported on the outcomes of 38 patients out of a possible 175 subjects (22%) who underwent ACDF for neck pain and degenerative disc disease (DDD). No concurrent, historical, or retrospective controls were identified. Fusion levels were determined by a painful and concordant response to disc injections. An unknown number of patients were excluded by the authors or declined surgery. Some potential patients (again, the number is unknown) were excluded because of psychological risk factors. The number of subjects lost to follow-up or who refused follow-up was not reported. There was no apparent standard assessment interval, and the intervals between the surgery and the reported assessment varied widely, from 2 to 7 years. Other interventions that the subjects might have received during this period— which could have affected outcomes—are not reported. Of the reported cases, the mean numerical pain rating after surgery remained greater than 4 (of 10), and the Oswestry Disability Index score showed moderate-to-serious impairment in most of the select group of patients followed. No neck-specific functional outcomes were assessed. Only 18 patients out of an unknown number (between 38 and 175) who were operated on by the authors for neck pain and degenerative disc disease said that the surgery had ‘met their expectations’.
- Garvey, et al 93 reported on 87 of 112 (78%) retrospectively identified patients who underwent ACDF for a diagnosis of ‘mechanical cervical spine pain’ (defined by the authors as patients who had more neck pain than arm pain). Patients were evaluated 5 to 10 years after surgery. The number of patients evaluated or considered for surgery is unknown. The selection process and screening were not detailed. Other treatments received were not reported.
The group was heterogeneous for diagnosis: an unknown proportion of patients had some radiculopathy, radiographic instability, or cervical deformity. The validity of the outcomes reported is uncertain. For example, it is not clear if pain and functional impairment measurements were recorded both before and after surgery; if validated functional assessments for neck pain were used; and whether subjects considered the occurrence of surgery to have been advantageous to their litigation claim (78%). Only 58 of these 112 patients (52%) reported feeling ‘somewhat better’ or ‘much better’ than they did before their surgery; only 25 patients (23%) reported feeling at least ‘somewhat satisfied’ with their neck condition on follow-up. The authors cited historical controls treated by nonoperative care of neck pain alone, 21% of whom reported complete pain relief and 49% who reported partial relief. These cited ‘control’ outcomes were similar to the authors’ reported surgical outcomes.
- Two additional studies are frequently cited to support surgical treatment of neck pain. 94,95 Both are retrospective case series with poorly reported recruitment procedures and follow few acceptable study design methods for outcome evaluation. For example, in these studies surgical outcomes are based solely on surgeons’ perceptions of patient improvement as opposed to validated outcome instruments. Simmons, et al report that the operating surgeon determined that 30 of 31 (97%) patients undergoing ACDF for neck pain were “all found to have immediate lessening” of symptoms after surgery; they further state that in every case “all pain was gone in a week after surgery.” 95 This observation is unlike any recorded by validated outcomes measures or collected by independent examiners.
Carragee, et al 96 go on to state, “It is well documented that neck pain without serious underlying disease shows wide and spontaneous variations in severity and any accompanying impairment. Thus, none of these frequently cited, uncontrolled studies can confidently estimate how much, if any, of the reported improvement was due to a surgical intervention, how much was due to natural history, and how much might be explained by various nonspecific and unidentified factors. Although these studies are frequently cited as demonstrating clear efficacy of cervical fusion for primary neck pain, none of these were found to be scientifically admissible by the Neck Pain Task Force. Instead, after critical review of the methods and data we found no clinical evidence, even in the best-known studies purporting definitive efficacy, to support the use of either cervical fusion or cervical disc arthroplasty in patients with neck pain without radiculopathy or serious underlying pathology.”
In addition to the methodological shortcomings of these studies described by Carragee, et al 96 , there were further limitations. There was indirectness regarding the Medicare population (mean ages ranged from 42 to 45 years) and the target condition, nonspecific axial neck pain, (the study authored by Simmons, et al included mostly [64%] of participants with specific causes of neck pain i.e., fracture, radiculopathy, myelopathy). All the studies had small sample sizes (n= 34-84) producing imprecise results.
In addition to these earlier primary studies, a single more recent RCT compared anterior fusion surgery with multidisciplinary rehabilitation for the treatment of individuals who were refractory to conservative treatment for chronic whiplash-associated disorders (WAD). 97 This study included 49 patients (mean ages were 38 and 40 years for the surgery and rehabilitation groups, respectively) with predominate midline neck pain, who had no neurological abnormalities and no specific changes seen on X-ray and MRI. The primary endpoint was the patient’s perceived change in neck pain assessed on the follow-up, using the disease-specific Balanced Inventory for Spinal Disorders (BIS) questionnaire. Follow-up evaluations were performed at variable time points ranging from 17 to 50 months. Analyses were performed by intention-to-treat and per-protocol. At follow-up, 67% of the patients in the surgery group and 23% (95% CI 15 to 64) in the rehabilitation group assessed improvements in the ITT analysis. Corresponding proportions in the per-protocol analysis were 83% and 12%, respectively. This study was judged to have a high risk of bias due to the lack of blinding or incomplete blinding, and the outcome measurement (self-reported change in pain perception) was likely to be influenced by the lack of blinding. Additionally, there was very serious indirectness (all participants had neck pain caused by a motor vehicle accident and the mean ages were well below the Medicare population), and imprecision (small sample size and wide confidence intervals suggesting variable effects).
Taking into consideration all the relevant studies, the certainty of evidence was judged to be very low due to study design and execution limitations, indirectness, and imprecision.
Appropriate Use Criteria/Coverage Recommendations
The North American Spine Society (NASS) assessed the appropriateness of cervical fusion for axial neck pain without stenosis and concluded that the surgical procedure was never considered appropriate. 98 The authors additionally commented, “There was a trend to be rarely appropriate for 2 or greater level fusions, whereas there was more uncertainty for 1 level fusions. Although uncertain, anterior procedures were overall favored over posterior or anterior and posterior procedures.” 98
In the 2023 NASS Coverage Recommendations 3 , the committee states “Concerning the scenarios in which cervical fusion is not indicated, recent evidence-based medicine reviews have concluded that there is little to no evidence that fusion is an effective treatment for axial neck pain without neurological symptoms.” 3
Evidence-based, clinical practice decision-support guidance explicitly does not recommend surgical intervention for the management of non-radicular neck pain in adults due to a lack of high-quality data demonstrating benefit and the possible risk of harm. 98
Cervical fusion alone or in combination with other spine surgical procedures is often performed for the management of multiple spinal conditions. There are 3 overall categories: cervical radiculopathy, cervical myelopathy, and unstable spine. There are multiple etiologies which can be revealed on imaging including herniated disc, spinal stenosis, spinal degeneration, synovial cyst and symptomatic pseudoarthrosis from non-union of prior fusion. In all cases the history, physical exam findings and imaging must correlate with the neurological deficits attributable to the affected region of the cervical spinal cord and other causes must be excluded.
The natural disease course of cervical radiculopathy is improvement with time and conservative measures so surgical management is reserved for refractory cases or when motor weakness creates significant functional limitations necessitating escalation of treatment. While the evidence supporting conservative therapy is low quality there are multiple RCTs and systematic review/meta-analysis as well as societal input and subject matter expert agree on a trial of conservative measures prior to surgical interventions. There is a paucity of data on how long the conservative period should be with reports ranging from 6 weeks to 3 or more months. As the data on disease course finds most improvement within the first 3 months a 12-week trial of conservative management is established, however the policy does acknowledge the need for some patients with progression during this time to shift to surgical management.
Cervical myelopathy (degenerative and mixed) is typically treated surgically due to the higher rate of neurological symptoms and risk of progression. In mild cases resolution with conservative therapy is possible and a trial of conservative treatment is indicated with education on progressive symptoms and intervention if worsening. Guidelines consist of a 6–12-week trial and 12 weeks was selected to allow time for the natural history of resolution or progression to determine if surgical intervention is necessary. In cases where there are progressive symptoms with neurological deficits surgical intervention prior to the 12-week time period can be considered.
In cases of unstable spine surgical intervention is necessary and there are few alternative options to consider. Malignant or benign spinal tumors leading to intractable pain or instability or anticipated instability due to treatment may necessitate stabilization with fusion. In some cases, spinal infection with destruction of bone and cervical kyphosis with cord compression may require surgical intervention to stabilize the spine.
There is not sufficient evidence to support surgical intervention for axial neck pain. Systematic review evaluating outcome for ACDF for axial neck pain with and without radiculopathy or myelopathy reported low quality evidence and the benefit of the surgery over time could not be established as compared to nonsurgical treatment options. The remaining evidence reviewed concludes very low quality and therefore this is not considered reasonable and necessary. This is consistent with societal guidelines.
Investigation into the optimal surgical procedure has explored outcomes with various approaches. While there are clear benefits and disadvantages of the various approaches the overall outcome is largely equivalent. As there is no evidence to support 1 surgical approach over another the decision for the optimal surgical approach is determined by the surgeon and patients with consideration of the pathology and evaluation of risk and benefits of the various approaches. This is consistent with societal guidance and expert opinion.
Proposed Process Information
| Changes | Fields Changed |
|---|---|
| N/A |
| Meeting Date | Meeting States | Meeting Information |
|---|
| Meeting Date | Meeting States | Meeting Information |
|---|
| Requestor Name | Requestor Letter |
|---|---|
Coding Information
Bill type codes.
| Code | Description |
|---|
Revenue Codes
CPT/HCPCS Codes
Icd-10-cm codes that support medical necessity, icd-10-cm codes that do not support medical necessity, additional icd-10 information, general information.
Please refer to the related Local Coverage Article: Billing and Coding: Cervical Fusion (A59674) for documentation requirements, utilization parameters and all coding information as applicable.
This bibliography presents those sources that were obtained during the development of this policy. The Contractor is not responsible for the continuing viability of Website addresses listed below.
- White III AA, Johnson RM, Panjabi MM, Southwick WO. Biomechanical analysis of clinical stability in the cervical spine. Clinical Orthopaedics and Related Research®. 1975;109:85-96.
- Boonstra AM, Preuper HRS, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain®. 2014;155(12):2545-2550.
- (NASS) NASS. Cervical fusion: defining appropriate coverage positions. NASS Coverage Recommendations 2023 (May). North American Spine Society (NASS). www.spine.org. Accessed11/21/23.
- Surgeons AAoN. Spinal Tumors. https://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Spinal-Tumors. Accessed Nov. 2, 2023.
- Nagashima H, Tanishima S, Tanida A. Diagnosis and management of spinal infections. J Orthop Sci. 2018;23(1):8-13.
- Elmajee M, Munasinghe C, Aljawadi A, Elawady K, Shuweihde F, Pillai A. Posterior stabilisation without formal debridement for the treatment of non-tuberculous pyogenic spinal infection in frail and debilitated population–A systematic review and meta-analysis. Journal of Clinical Orthopaedics and Trauma. 2021;15:9-15.
- Raja SN CD, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. . The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976-1982.
- Bono CM, Ghiselli G, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. The Spine Journal. 2011;11(1):64-72.
- Rondinelli RD, Genovese E, Katz RT, et al. AMA guides to the evaluation of permanent impairment, Sixth Edition. 2008.
- Menger DLSGWSMRP. Hangman's Fractures. Published 2023. Updated August 13, 2023. Accessed Jan 2023.
- Donnally III CJ, Hanna A, Odom CK. Cervical myelopathy. StatPearls Publishing. StatPearls [Internet] Web site. https://www.ncbi.nlm.nih.gov/books/NBK482312/ Published 2023. Accessed 10-27, 23.
- Marquis BO, Capone PM. Myelopathy. Handbook of clinical neurology. 2016;136:1015-1026.
- Wirth FP, Dowd GC, Sanders HF, Wirth C. Cervical discectomy: a prospective analysis of three operative techniques. Surgical neurology. 2000;53(4):340-348.
- Panjabi MM, White III AA. Basic biomechanics of the spine. Neurosurgery. 1980;7(1):76-93.
- Kothari MJ, Chuang Kathy Treatment and prognosis of cervical radiculopathy. UpToDate. https://www.uptodate.com/. Published 2023. Updated 2/28/23. Accessed11/1/2023.
- Corp N, Mansell G, Stynes S, et al. Evidence-based treatment recommendations for neck and low back pain across Europe: a systematic review of guidelines. European Journal of Pain. 2021;25(2):275-295.
- Nikolaidis I, Fouyas IP, Sandercock PAG, Statham PF. Surgery for cervical radiculopathy or myelopathy. Cochrane Database of Systematic Reviews. 2010(1).
- Plener J, Csiernik B, To D, et al. Conservative Management of Cervical Radiculopathy: A Systematic Review. Clin J Pain. 2023;39(3):138-146.
- Liang L, Feng M, Cui X, et al. The effect of exercise on cervical radiculopathy: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(45):e17733.
- Walker MJ, Boyles RE, Young BA, et al. The Effectiveness of Manual Physical Therapy and Exercise for Mechanical Neck Pain: A Randomized Clinical Trial. Spine. 2008;33(22):2371-2378.
- Kuijper B, Tans JT, Beelen A, Nollet F, de Visser M. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomised trial. Bmj. 2009;339:b3883.
- Engquist M, Löfgren H, Öberg B, et al. Factors Affecting the Outcome of Surgical Versus Nonsurgical Treatment of Cervical Radiculopathy. Spine. 2015;40(20):1553-1563.
- Engquist M, Löfgren H, Öberg B, et al. A 5-to 8-year randomized study on the treatment of cervical radiculopathy: anterior cervical decompression and fusion plus physiotherapy versus physiotherapy alone. Journal of Neurosurgery: Spine. 2017;26(1):19-27.
- Harrop JS, Mohamed B, Bisson EF, et al. Congress of neurological surgeons systematic review and evidence-based guidelines for perioperative spine: preoperative surgical risk assessment. Neurosurgery. 2021;89(Supplement_1):S9-S18.
- Thoomes E, Thoomes-de Graaf M, Cleland JA, Gallina A, Falla D. Timing of evidence-based nonsurgical interventions as part of multimodal treatment guidelines for the management of cervical radiculopathy: a Delphi study. Physical Therapy. 2022;102(5):pzab312.
- Berman D, Holtzman A, Sharfman Z, Tindel N. Comparison of Clinical Guidelines for Authorization of MRI in the Evaluation of Neck Pain and Cervical Radiculopathy in the United States. Journal of the American Academy of Orthopaedic Surgeons. 2023;31(2):64-70.
- Cervical Radiculopathy (Pinched Nerve). Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/22639-cervical-radiculopathy-pinched-nerve Published 2022. Updated 03/29/2022. Accessed October 18, 2023.
- Broekema AE, Groen RJ, de Souza NFS, et al. Surgical interventions for cervical radiculopathy without myelopathy: a systematic review and meta-analysis. JBJS. 2020;102(24):2182-2196.
- Fang W, Huang L, Feng F, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy for the treatment of single-level unilateral cervical radiculopathy: a meta-analysis. J Orthop Surg Res. 2020;15(1):202.
- Gao Q-Y, Wei F-L, Zhu K-L, et al. Clinical Efficacy and Safety of Surgical Treatments in Patients With Pure Cervical Radiculopathy. Frontiers in Public Health. 2022;10:892042.
- Goedmakers CMW, Janssen T, Yang X, Arts MP, Bartels R, Vleggeert-Lankamp CLA. Cervical radiculopathy: is a prosthesis preferred over fusion surgery? A systematic review. Eur Spine J. 2020;29(11):2640-2654.
- Katsuura Y, York PJ, Goto R, et al. Sagittal Reconstruction and Clinical Outcome Using Traditional ACDF, Versus Stand-alone ACDF Versus TDR: A Systematic Review and Quantitative Analysis. Spine (Phila Pa 1976). 2019;44(19):E1151-E1158.
- Liu WJ, Hu L, Chou PH, Wang JW, Kan WS. Comparison of Anterior Cervical Discectomy and Fusion versus Posterior Cervical Foraminotomy in the Treatment of Cervical Radiculopathy: A Systematic Review. Orthop Surg. 2016;8(4):425-431.
- Zou T, Wang PC, Chen H, Feng XM, Sun HH. Minimally invasive posterior cervical foraminotomy versus anterior cervical discectomy and fusion for cervical radiculopathy: a meta-analysis. Neurosurg Rev. 2022;45(6):3609-3618.
- Gutman G, Rosenzweig DH, Golan JD. Surgical Treatment of Cervical Radiculopathy: Meta-analysis of Randomized Controlled Trials. Spine (Phila Pa 1976). 2018;43(6):E365-E372.
- Fei Q, Li J, Su N, et al. Comparison between anterior cervical discectomy with fusion and anterior cervical corpectomy with fusion for the treatment of cervical spondylotic myelopathy: a meta-analysis. Ther Clin Risk Manag. 2015;11:1707-1718.
- Han YC, Liu ZQ, Wang SJ, Li LJ, Tan J. Is anterior cervical discectomy and fusion superior to corpectomy and fusion for treatment of multilevel cervical spondylotic myelopathy? A systemic review and meta-analysis. PLoS One. 2014;9(1):e87191.
- Li Z, Long C, Li B, Wei J. Efficacy and safety of surgical interventions for treating multilevel cervical spondylotic myelopathy via anterior approach: A network meta-analysis. Pain Physician. 2019;22(4):E275.
- Montano N, Ricciardi L, Olivi A. Comparison of Anterior Cervical Decompression and Fusion versus Laminoplasty in the Treatment of Multilevel Cervical Spondylotic Myelopathy: A Meta-Analysis of Clinical and Radiological Outcomes. World Neurosurg. 2019;130:530-536 e532.
- Wang T, Guo J, Long Y, Hou Z. Comparison of Two Anterior Reconstructive Techniques in the Treatment of 3-Level and 4 Level Cervical Spondylotic Myelopathy: A Meta-analysis of Last Decade. Geriatr Orthop Surg Rehabil. 2022;13:21514593221124415.
- Schuermans VNE, Smeets A, van de Kar LGC, et al. A Systematic Review on Neurological Outcomes for Cervical Degenerative Myelopathy After Anterior Decompression Surgery: Motion Preservation vs Fusion. Int J Spine Surg. 2022;16(6):969-976.
- El-Ghandour NMF, Soliman MAR, Ezzat AAM, Mohsen A, Zein-Elabedin M. The safety and efficacy of anterior versus posterior decompression surgery in degenerative cervical myelopathy: a prospective randomized trial. J Neurosurg Spine. 2020:1-9.
- Xu L, Sun H, Li Z, Liu X, Xu G. Anterior cervical discectomy and fusion versus posterior laminoplasty for multilevel cervical myelopathy: A meta-analysis. Int J Surg. 2017;48:247-253.
- Zhao CM, Chen Q, Zhang Y, Huang AB, Ding WY, Zhang W. Anterior cervical discectomy and fusion versus hybrid surgery in multilevel cervical spondylotic myelopathy: A meta-analysis. Medicine (Baltimore). 2018;97(34):e11973.
- Lee NJ, Kim JS, Park P, Riew KD. A comparison of various surgical treatments for degenerative cervical myelopathy: a propensity score matched analysis. Global spine journal. 2022;12(6):1109-1118.
- Medicine JH. Cervical Myelopathy. Johns Hopkins Medicine. Cervical Myelopathy. Accessed 11/14, 2023.
- Ranawat CS, O'Leary P, Pellicci P, Tsairis P, Marchisello P, Dorr L. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61(7):1003-1010.
- Gillick JL, Wainwright J, Das K. Rheumatoid arthritis and the cervical spine: a review on the role of surgery. International journal of rheumatology. 2015;2015.
- Nunez JH, Escudero B, Omiste I, et al. Outcomes of cervical arthroplasty versus anterior cervical arthrodesis: a systematic review and meta-analysis of randomized clinical trials with a minimum follow-up of 7-year. Eur J Orthop Surg Traumatol. 2022.
- Peng Z, Hong Y, Meng Y, Liu H. A meta-analysis comparing the short- and mid- to long-term outcomes of artificial cervical disc replacement(ACDR) with anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease. Int Orthop. 2022;46(7):1609-1625.
- Xing D, Ma XL, Ma JX, Wang J, Ma T, Chen Y. A meta-analysis of cervical arthroplasty compared to anterior cervical discectomy and fusion for single-level cervical disc disease. J Clin Neurosci. 2013;20(7):970-978.
- Zhang Y, Lv N, He F, et al. Comparison of cervical disc arthroplasty and anterior cervical discectomy and fusion for the treatment of cervical disc degenerative diseases on the basis of more than 60 months of follow-up: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):143.
- Fehlings MG, Martin AR, Tetreault LA, et al. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Role of Baseline Magnetic Resonance Imaging in Clinical Decision Making and Outcome Prediction. Global Spine J. 2017;7(3 Suppl):221S-230S.
- Zileli M, Maheshwari S, Kale SS, Garg K, Menon SK, Parthiban J. Outcome Measures and Variables Affecting Prognosis of Cervical Spondylotic Myelopathy: WFNS Spine Committee Recommendations. Neurospine. 2019;16(3):435-447.
- Costa F, Anania CD, Agrillo U, et al. Cervical Spondylotic Myelopathy: From the World Federation of Neurosurgical Societies (WFNS) to the Italian Neurosurgical Society (SINch) Recommendations. Neurospine. 2023;20(2):415-429.
- Watanabe M, Chikuda H, Fujiwara Y, et al. Japanese Orthopaedic Association (JOA) Clinical practice guidelines on the Management of Cervical Spondylotic Myelopathy,2020 - Secondary publication. J Orthop Sci. 2023;28(1):1-45.
- Youssef JA, Heiner AD, Montgomery JR, et al. Outcomes of posterior cervical fusion and decompression: a systematic review and meta-analysis. Spine J. 2019;19(10):1714-1729.
- Mahmoud A, Shanmuganathan K, Montgomery A. Surgical Management of Hangman's Fracture: A Systematic Review. Int J Spine Surg. 2023.
- Lee DY, Park YJ, Song MG, Kim KT, Kim DH. Comparison of anterior-only versus combined anterior and posterior fusion for unstable subaxial cervical injuries: a meta-analysis of biomechanical and clinical studies. Eur Spine J. 2021;30(6):1460-1473.
- Yang JS, Liu P, Liu TJ, et al. When is the circumferential stabilization necessary for subaxial cervical fracture dislocations? The posterior ligament-bone injury classification and severity score: a novel treatment algorithm. Eur Spine J. 2021;30(2):524-533.
- Madan A, Thakur M, Sud S, Jain V, Singh Thakur RP, Negi V. Subaxial Cervical Spine Injuries: Outcomes after Anterior Corpectomy and Instrumentation. Asian J Neurosurg. 2019;14(3):843-847.
- Ailon T, Smith JS, Shaffrey CI, et al. Outcomes of Operative Treatment for Adult Cervical Deformity: A Prospective Multicenter Assessment With 1-Year Follow-up. Neurosurgery . 2018;83(5):1031-1039. doi:10.1093/neuros/nyx574.
- Yang H, Wang H, Zhang B, Sun Y, Wang L, Lu X. Cervical spine fracture-dislocation in patients with ankylosing spondylitis and severe thoracic kyphosis: Application of halo vest before and during surgical management. Clin Neurol Neurosurg. 2021;207:106744.
- Wang L, Wang H, Wang C, Zhang B, Yang H, Lu X. Comparative study of halo-vest reduction and skull traction reduction in the treatment of cervical fracture dislocation in patients with ankylosing spondylitis. Front Surg. 2023;10:1129809.
- Skeppholm M, Svedmark P, Noz ME, Maguire GQ, Jr., Olivecrona H, Olerud C. Evaluation of mobility and stability in the Discover artificial disc: an in vivo motion study using high-accuracy 3D CT data. J Neurosurg Spine. 2015;23(3):383-389.
- Rustagi T, Mashaly H, Mendel E. Posterior occiput-cervical fixation for metastasis to upper cervical spine. J Craniovertebr Junction Spine. 2019;10(2):119-126.
- Luksanapruksa P, Millhouse PW, Carlson V, Ariyawatkul T, Heller J, Kepler CK. Comparison of Surgical Outcomes of the Posterior and Combined Approaches for Repair of Cervical Fractures in Ankylosing Spondylitis. Asian Spine J. 2019;13(3):432-440.
- Li Z, Li F, Hou S, et al. Anterior discectomy/corpectomy and fusion with internal fixation for the treatment of unstable hangman's fractures: a retrospective study of 38 cases. J Neurosurg Spine. 2015;22(4):387-393.
- Lee JS, Son DW, Lee SH, Ki SS, Lee SW, Song GS. Comparative Analysis of Surgical Outcomes of C1-2 Fusion Spine Surgery between Intraoperative Computed Tomography Image Based Navigation-Guided Operation and Fluoroscopy-Guided Operation. J Korean Neurosurg Soc. 2020;63(2):237-247.
- Lang S, Neumann C, Fiedler L, Alt V, Loibl M, Kerschbaum M. Does Dynamic Anterior Plate Fixation Provide Adequate Stability for Traumatic Subaxial Cervical Spine Fractures at Mid-Term Follow-Up? J Clin Med. 2021;10(6).
- Kong W, Yang X, Li Z, Hu B, Song Y. Analysis of the Cervical Sagittal Alignment in Patients with Unstable Hangman Fracture Under C2 approximately 3 Anterior Discectomy and Fusion. World Neurosurg. 2020;137:e1-e8.
- Kim HS, Cloney MB, Koski TR, Smith ZA, Dahdaleh NS. Management of Isolated Atlas Fractures: A Retrospective Study of 65 Patients. World Neurosurg. 2018;111:e316-e322.
- Bakhsheshian J, Sizdahkhani S, Ohiorhenuan I, Buchanan IA, Strickland B, Pham MH. Transpedicular lag screw placement in traumatic cervical spondylolisthesis: Case report and systematic review of the literature. J Clin Neurosci. 2019;63:256-262.
- Jin C, Xie N, Ren Y, et al. How Does Cervical Sagittal Balance Change After Hangman Fracture Treated with Anterior or Posterior Approach Surgery? World Neurosurg. 2020;138:e767-e777.
- Garrido BJ, Myo GK, Sasso RC. Rigid versus nonrigid occipitocervical fusion: a clinical comparison of short-term outcomes. J Spinal Disord Tech. 2011;24(1):20-23.
- Dagtekin A, Avci E, Hamzaoglu V, et al. Management of occipitocervical junction and upper cervical trauma. J Craniovertebr Junction Spine. 2018;9(3):148-155.
- Clark S, Nash A, Shasti M, et al. Mortality Rates After Posterior C1-2 Fusion for Displaced Type II Odontoid Fractures in Octogenarians. Spine (Phila Pa 1976). 2018;43(18):E1077-E1081.
- Candura D, Perna A, Velluto C, et al. Conservative management of Anderson Type II odontoid fractures in octogenarians: is radiological union what we are searching for? Eur Rev Med Pharmacol Sci. 2022;26(1 Suppl):33-42.
- Aldrian S, Erhart J, Schuster R, et al. Surgical vs nonoperative treatment of Hadley type IIA odontoid fractures. Neurosurgery. 2012;70(3):676-682; discussion 682-673.
- Ghogawala Z, Terrin N, Dunbar MR, et al. Effect of ventral vs dorsal spinal surgery on patient-reported physical functioning in patients with cervical spondylotic myelopathy: a randomized clinical trial. Jama. 2021;325(10):942-951.
- de Dios Perez E. Degenerative cervical myelopathy: Surgical treatment, imaging evaluation, and outcome. 2023.
- Sattari SA, Ghanavatian M, Feghali J, et al. Anterior cervical discectomy and fusion versus posterior decompression in patients with degenerative cervical myelopathy: a systematic review and meta-analysis. J Neurosurg Spine. 2023:1-13.
- Selph SS SA, Jungbauer RM, Brodt E, Blazina I, Philipp TC, Mauer KM, Dettori J, Atchison C, Riopelle D, Stabler-Morris S, Fu R, Yu Y, Chou R. . Cervical Degenerative Disease Treatment: A Systematic Review. Comparative Effectiveness Review No. 266. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 75Q80120D00006.). AHRQ Publication No. 24-EHC001.Rockville, MD: Agency for Healthcare Research and Quality;. https://effectivehealthcare.ahrq.gov/products/cervical-degenerative-disease/research. Accessed11/28/23.
- Industries. WSDoL. Labor and Industries’ Industrial Insurance Medical Advisory Committee (IIMAC). Guideline for diagnosis and treatment of cervical radiculopathy and myelopathy. . https://www.lni.wa.gov/patient-care/treating-patients/treatment-guidelines-and-resources/_docs/2014CervicalGuideline-FINAL.pdf [lni.wa.gov]. Updated Effective December 2014, Hyperlinks and minor corrections June 2023. . Accessed11/28/23.
- Cohen SP. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin Proc. 2015;90(2):284-299.
- Ohnari H, Sasai K, Akagi S, Iida H, Takanori S, Kato I. Investigation of axial symptoms after cervical laminoplasty, using questionnaire survey. Spine J. 2006;6(3):221-227.
- (NASS) NASS. Appropriate Use Criteria: Cervical Fusion (2013) North American Spine Society (NASS). North American Spine Society: Appropriate Use Criteria for Cervical Fusion Web site. https://www.spine.org/Portals/0/assets/downloads/ResearchClinicalCare/CervicalFusionAUC.pdf. Published 2013. Accessed 11/14/, 2023.
- van Middelkoop M, Rubinstein SM, Ostelo R, et al. No additional value of fusion techniques on anterior discectomy for neck pain: a systematic review. Pain. 2012;153(11):2167-2173.
- Riew KD, Ecker E, Dettori JR. Anterior cervical discectomy and fusion for the management of axial neck pain in the absence of radiculopathy or myelopathy. Evid Based Spine Care J. 2010;1(3):45-50.
- Carragee EJ, Hurwitz EL, Cheng I, et al. Treatment of neck pain: injections and surgical interventions: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S153-169.
- Palit M, Schofferman J, Goldthwaite N, et al. Anterior discectomy and fusion for the management of neck pain. Spine (Phila Pa 1976). 1999;24(21):2224-2228.
- Garvey TA, Transfeldt EE, Malcolm JR, Kos P. Outcome of anterior cervical discectomy and fusion as perceived by patients treated for dominant axial-mechanical cervical spine pain. Spine (Phila Pa 1976). 2002;27(17):1887-1895; discussion 1895.
- Whitecloud TS, 3rd, Seago RA. Cervical discogenic syndrome. Results of operative intervention in patients with positive discography. Spine (Phila Pa 1976). 1987;12(4):313-316.
- Simmons EH, Bhalla SK. Anterior cervical discectomy and fusion. A clinical and biomechanical study with eight-year follow-up. J Bone Joint Surg Br. 1969;51(2):225-237.
- Carragee EJ, Hurwitz EL, Cheng I, et al. Treatment of neck pain: injections and surgical interventions: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. J Manipulative Physiol Ther. 2009;32(2 Suppl):S176-193.
- Nystrom B, Svensson E, Larsson S, Schillberg B, Mork A, Taube A. A small group Whiplash-Associated-Disorders (WAD) patients with central neck pain and movement induced stabbing pain, the painful segment determined by mechanical provocation: Fusion surgery was superior to multimodal rehabilitation in a randomized trial. Scand J Pain. 2016;12:33-42.
- Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013;13(7):734-743.
- Isaac Z, Atlas SJ, K L. Management of non-radicular neck pain in adults. UpToDate. www.uptodate.com. Published 2023. Updated Oct. 4, 2023. Accessed Oct. 30, 2023.
Revision History Information
| Revision History Date | Revision History Number | Revision History Explanation | Reasons for Change |
|---|
Associated Documents
| Updated On | Effective Dates | Status | |
|---|---|---|---|
| 06/21/2024 | 08/11/2024 - N/A | Future Effective | You are here |
Read the LCD Disclaimer
Email this document to yourself or someone else
An asterisk ( * ) indicates a required field. This email will be sent from you to the recipient email address(es) you enter. Please do not use this feature to contact CMS. To submit a comment or question to CMS, please use the Feedback/Ask a Question link available at the bottom of every MCD page.
Contractor Detail
Lcd disclaimer, license agreements.
Please review and accept the agreements in order to view Medicare Coverage documents, which may include licensed information and codes.
LICENSE FOR USE OF PHYSICIANS' CURRENT PROCEDURAL TERMINOLOGY, FOURTH EDITION ("CPT")
End User Point and Click Amendment: CPT codes, descriptions and other data only are copyright 2023 American Medical Association. All Rights Reserved (or such other date of publication of CPT). CPT is a trademark of the American Medical Association (AMA).
You, your employees and agents are authorized to use CPT only as agreed upon with the AMA internally within your organization within the United States for the sole use by yourself, employees and agents. Use is limited to use in Medicare, Medicaid or other programs administered by the Centers for Medicare and Medicaid Services (CMS). You agree to take all necessary steps to insure that your employees and agents abide by the terms of this agreement.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT for resale and/or license, transferring copies of CPT to any party not bound by this agreement, creating any modified or derivative work of CPT, or making any commercial use of CPT. License to use CPT for any use not authorized herein must be obtained through the AMA, CPT Intellectual Property Services, AMA Plaza 330 N. Wabash Ave., Suite 39300, Chicago, IL 60611-5885. Applications are available at the AMA Web site, http://www.ama-assn.org/go/cpt .
Applicable FARS\DFARS Restrictions Apply to Government Use.
AMA Disclaimer of Warranties and Liabilities.
CPT is provided "as is" without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. No fee schedules, basic unit, relative values or related listings are included in CPT. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this file/product is with CMS and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
CMS Disclaimer
The scope of this license is determined by the AMA, the copyright holder. Any questions pertaining to the license or use of the CPT should be addressed to the AMA. End Users do not act for or on behalf of the CMS. CMS DISCLAIMS RESPONSIBILITY FOR ANY LIABILITY ATTRIBUTABLE TO END USER USE OF THE CPT. CMS WILL NOT BE LIABLE FOR ANY CLAIMS ATTRIBUTABLE TO ANY ERRORS, OMISSIONS, OR OTHER INACCURACIES IN THE INFORMATION OR MATERIAL CONTAINED ON THIS PAGE. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information or material. Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled "I Accept".
LICENSE FOR USE OF CURRENT DENTAL TERMINOLOGY (CDT TM )
End User License Agreement: These materials contain Current Dental Terminology (CDT TM ), copyright© 2023 American Dental Association (ADA). All rights reserved. CDT is a trademark of the ADA.
The license granted herein is expressly conditioned upon your acceptance of all terms and conditions contained in this agreement. By clicking below on the button labeled "I accept", you hereby acknowledge that you have read, understood and agreed to all terms and conditions set forth in this agreement.
If you do not agree with all terms and conditions set forth herein, click below on the button labeled "I do not accept" and exit from this computer screen.
If you are acting on behalf of an organization, you represent that you are authorized to act on behalf of such organization and that your acceptance of the terms of this agreement creates a legally enforceable obligation of the organization. As used herein, "you" and "your" refer to you and any organization on behalf of which you are acting.
- Subject to the terms and conditions contained in this Agreement, you, your employees and agents are authorized to use CDT only as contained in the following authorized materials and solely for internal use by yourself, employees and agents within your organization within the United States and its territories. Use of CDT is limited to use in programs administered by Centers for Medicare & Medicaid Services (CMS). You agree to take all necessary steps to ensure that your employees and agents abide by the terms of this agreement. You acknowledge that the ADA holds all copyright, trademark and other rights in CDT. You shall not remove, alter, or obscure any ADA copyright notices or other proprietary rights notices included in the materials.
- Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CDT for resale and/or license, transferring copies of CDT to any party not bound by this agreement, creating any modified or derivative work of CDT, or making any commercial use of CDT. License to use CDT for any use not authorized herein must be obtained through the American Dental Association, 211 East Chicago Avenue, Chicago, IL 60611. Applications are available at the American Dental Association web site, http://www.ADA.org .
- Applicable Federal Acquisition Regulation Clauses (FARS)/Department of Defense Federal Acquisition Regulation supplement (DFARS) Restrictions Apply to Government Use. U.S. Government Rights Provisions.
- Organizations who contract with CMS acknowledge that they may have a commercial CDT license with the ADA, and that use of CDT codes as permitted herein for the administration of CMS programs does not extend to any other programs or services the organization may administer and royalties dues for the use of the CDT codes are governed by their commercial license.
- ADA DISCLAIMER OF WARRANTIES AND LIABILITIES. CDT is provided "as is" without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. No fee schedules, basic unit, relative values or related listings are included in CDT. The ADA does not directly or indirectly practice medicine or dispense dental services. The sole responsibility for software, including any CDT and other content contained therein, is with CMS; and no endorsement by the ADA is intended or implied. The ADA expressly disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice to you if you violate the terms of this Agreement. The ADA is a third party beneficiary to this Agreement.
- CMS DISCLAIMER. The scope of this license is determined by the ADA, the copyright holder. Any questions pertaining to the license or use of the CDT should be addressed to the ADA. End Users do not act for or on behalf of the CMS. CMS disclaims responsibility for any liability attributable to end user use of the CDT. CMS will not be liable for any claims attributable to any errors, omissions, or other inaccuracies in the information or material covered by this license. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information or material. The license granted herein is expressly conditioned upon your acceptance of all terms and conditions contained in this agreement. If the foregoing terms and conditions are acceptable to you, please indicate your agreement by clicking below on the button labeled "I Accept". If you do not agree to the terms and conditions, you may not access or use software. Instead you must click below on the button labeled "I Do Not Accept" and exit from this computer screen.
License For Use of National Uniform Billing Committee (NUBC) UB-04
These materials contain NUBC Official UB-04 Specifications (UB-04 Data), which is copyrighted by the American Hospital Association (AHA).
THE LICENSE GRANTED HEREIN IS EXPRESSLY CONDITIONED UPON YOUR ACCEPTANCE OF ALL TERMS AND CONDITIONS CONTAINED IN THIS AGREEMENT. BY CLICKING BELOW ON THE BUTTON LABELED "I ACCEPT", YOU HEREBY ACKNOWLEDGE THAT YOU HAVE READ, UNDERSTOOD AND AGREED TO ALL TERMS AND CONDITIONS SET FORTH IN THIS AGREEMENT.
IF YOU DO NOT AGREE WITH ALL TERMS AND CONDITIONS SET FORTH HEREIN, CLICK BELOW ON THE BUTTON LABELED "I DO NOT ACCEPT" AND EXIT FROM THIS COMPUTER SCREEN. IF YOU ARE ACTING ON BEHALF OF AN ORGANIZATION, YOU REPRESENT THAT YOU ARE AUTHORIZED TO ACT ON BEHALF OF SUCH ORGANIZATION AND THAT YOUR ACCEPTANCE OF THE TERMS OF THIS AGREEMENT CREATES A LEGALLY ENFORCEABLE OBLIGATION OF THE ORGANIZATION. AS USED HEREIN, "YOU" AND "YOUR" REFER TO YOU AND ANY ORGANIZATION ON BEHALF OF WHICH YOU ARE ACTING.
- Subject to the terms and conditions contained in this Agreement, you, your employees, and agents are authorized to use UB-04 Data only as contained in the following authorized materials and solely for internal use by yourself, employees and agents within your organization within the United States and its territories. Use of UB-04 Data is limited to use in programs administered by Centers for Medicare & Medicaid Services (CMS). You agree to take all necessary steps to ensure that your employees and agents abide by the terms of this Agreement. You acknowledge that the AHA holds all copyright, trademark, and other rights in UB-04 Data. You shall not remove, alter, or obscure any AHA copyright notices or other proprietary rights notices included in the materials.
- Any use not authorized herein is prohibited, including, by way of illustration and not by way of limitation, making copies of UB-04 Data for resale and/or license, transferring copies of UB-04 Data to any party not bound by this agreement, creating any modified or derivative work of UB-04 Data, or making any commercial use of UB-04 Data. License to use UB-04 Data for any use not authorized herein must be obtained through the American Hospital Association, 155 N. Wacker Drive, Suite 400, Chicago, Illinois, 60606. Applications are available at the NUBC website, http://www.nubc.org/ .
- The UB-04 Data included in this product is commercial technical data and/or computer databases and/or commercial computer software and/or commercial computer software documentation, as applicable, which was developed exclusively at private expense by the American Hospital Association, 155 N. Wacker Drive, Suite 400, Chicago, Illinois 60606. U.S. Government rights to use, modify, reproduce, release, perform, display, or disclose these technical data and/or computer data bases and/or computer software and/or computer software documentation are subject to the limited rights restrictions of DFARS 252.227-7015(b)(2) (November 1995) and/or subject to the restrictions of DFARS 227.7202-1(a) (June 1995) and DFARS 227.7202-3(a) (June 1995), as applicable for U.S. Department of Defense procurements and the limited rights restrictions of FAR 52.227-14 (December 2007) and FAR 52.227-19 (December 2007), as applicable, and any applicable agency FAR Supplements, for non-Department of Defense Federal procurements.
- AHA DISCLAIMER OF WARRANTIES AND LIABILITIES. UB-04 Data is provided "as is" without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. The sole responsibility for the software, including any UB-04 Data and other content contained therein, is with the Medicare/Medicaid Contractor or the CMS; and no endorsement by the AHA is intended or implied. The AHA expressly disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice to you if you violate the terms of this Agreement. The AHA is a third-party beneficiary to this Agreement.
- CMS DISCLAIMER. The scope of this license is determined by the AHA, the copyright holder. Any questions pertaining to the license or use of the UB-04 Data should be addressed to the AHA. End users do not act for or on behalf of the CMS. CMS DISCLAIMS RESPONSIBILITY FOR ANY LIABILITY ATTRIBUTABLE TO END USER USE OF THE UB-04 DATA. CMS WILL NOT BE LIABLE FOR ANY CLAIMS ATTRIBUTABLE TO ANY ERRORS, OMISSIONS, OR OTHER INACCURACIES IN THE INFORMATION OR MATERIAL COVERED BY THIS LICENSE. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information or material.
Page Help for LCD - Cervical Fusion (L39799)
Introduction.
This page displays your requested Local Coverage Determination (LCD). The document is broken into multiple sections. You can use the Contents side panel to help navigate the various sections. A Local Coverage Determination (LCD) is a decision made by a Medicare Administrative Contractor (MAC) on whether a particular service or item is reasonable and necessary, and therefore covered by Medicare within the specific jurisdiction that the MAC oversees. Please note that codes (CPT/HCPCS and ICD-10) have moved from LCDs to Billing & Coding Articles.
More information
MACs are Medicare contractors that develop LCDs and process Medicare claims. MACs develop an LCD when there is no national coverage determination (NCD) or when there is a need to further define an NCD for the specific jurisdiction. LCDs outline how the contractor will review claims to ensure that the services provided meet Medicare coverage requirements. Before an LCD becomes final, the MAC publishes Proposed LCDs, which include a public comment period. LCD document IDs begin with the letter "L" (e.g., L12345). Proposed LCD document IDs begin with the letters "DL" (e.g., DL12345). The guidelines for LCD development are provided in Chapter 13 of the Medicare Program Integrity Manual. The Social Security Act, Sections 1869(f)(2)(B) and 1862(l)(5)(D) define LCDs and provide information on the process
The LCD Tracking Sheet is a pop-up modal that is displayed on top of any Proposed LCD that began to appear on the MCD on or after 1/1/2022. The Tracking Sheet provides key details about the Proposed LCD, including a summary of the issue, who requested the new/updated policy, links to key documents, important process-related dates, who to contact with questions about the policy, and the history of previous policy considerations. The information displayed in the Tracking Sheet is pulled from the accompanying Proposed LCD and its correlating Final LCD and will be updated as new data becomes available. The Tracking Sheet modal can be closed and re-opened when viewing a Proposed LCD.
Printing a Document to PDF

Frequently Asked Questions (FAQs)
Are you a provider and have a question about billing or coding.
Please contact your Medicare Administrative Contractor (MAC). MACs can be found in the MAC Contacts Report .
Do you have questions related to the content of a specific Local Coverage Determination (LCD) or an Article?
Are you a beneficiary and have questions about your coverage, are you looking for codes (e.g., cpt/hcpcs, icd-10), local coverage.
For the most part, codes are no longer included in the LCD (policy). You will find them in the Billing & Coding Articles. Try using the MCD Search to find what you're looking for. Enter the code you're looking for in the "Enter keyword, code, or document ID" box. The list of results will include documents which contain the code you entered.
Please Note: For Durable Medical Equipment (DME) MACs only, CPT/HCPCS codes remain located in LCDs. All other Codes (ICD-10, Bill Type, and Revenue) have moved to Articles for DME MACs, as they have for the other Local Coverage MAC types.
National Coverage
NCDs do not contain claims processing information like diagnosis or procedure codes nor do they give instructions to the provider on how to bill Medicare for the service or item. For this supplementary claims processing information we rely on other CMS publications, namely Change Requests (CR) Transmittals and inclusions in the Medicare Fee-For-Service Claims Processing Manual (CPM).
In order for CMS to change billing and claims processing systems to accommodate the coverage conditions within the NCD, we instruct contractors and system maintainers to modify the claims processing systems at the national or local level through CR Transmittals. CRs are not policy, rather CRs are used to relay instructions regarding the edits of the various claims processing systems in very descriptive, technical language usually employing the codes or code combinations likely to be encountered with claims subject to the policy in question. As clinical or administrative codes change or system or policy requirements dictate, CR instructions are updated to ensure the systems are applying the most appropriate claims processing instructions applicable to the policy.
How do I find out if a specific CPT code is covered in my state?
Enter the CPT/HCPCS code in the MCD Search and select your state from the drop down. (You may have to accept the AMA License Agreement.) Look for a Billing and Coding Article in the results and open it. (Or, for DME MACs only, look for an LCD.) Review the article, in particular the Coding Information section.
If you need more information on coverage, contact the Medicare Administrative Contractor (MAC) who published the document. The contractor information can be found at the top of the document in the Contractor Information section (expand the section to see the details).
If you don’t find the Article you are looking for, contact your MAC .
Did you receive a Medicare coverage denial?
Was your Medicare claim denied? Here are some hints to help you find more information:
1) Check out the Beneficiary card on the MCD Search page.
2) Try using the MCD Search and enter your information in the "Enter keyword, code, or document ID" box. Your information could include a keyword or topic you're interested in; a Local Coverage Determination (LCD) policy or Article ID; or a CPT/HCPCS procedure/billing code or an ICD-10-CM diagnosis code. Try entering any of this type of information provided in your denial letter.
3) Contact your MAC .
4) Visit Medicare.gov or call 1-800-Medicare.
It is Thursday and the weekly MCD data isn’t refreshed?
Are you having technical issues with the medicare coverage database (mcd), mcd session expiration warning.
Your MCD session is currently set to expire in 5 minutes due to inactivity. If your session expires, you will lose all items in your basket and any active searches. If you would like to extend your session, you may select the Continue Button.
Reset MCD Search Data
If you are experiencing any technical issues related to the search, selecting the 'OK' button to reset the search data should resolve your issues.

IMAGES
VIDEO
COMMENTS
Spondylolisthesis (pronounced spahn-duh-low-liss-thee-sus) is a condition in which one of the bones in your spine (the vertebrae) slips out of place and moves on top of the vertebra next to it. It ...
In spondylolisthesis, one of the bones in your spine — called a vertebra — slips forward and out of place. This may occur anywhere along the spine, but is most common in the lower back (lumbar spine). In some people, this causes no symptoms at all. Others may have back and leg pain that ranges from mild to severe.
Spondylolysis (spon-dee-low-lye-sis) and spondylolisthesis (spon-dee-low-lis-thee-sis) are common causes of low back pain in children and adolescents. Spondylolysis is a weakness or stress fracture in one of the vertebrae, the small bones that make up the spinal column. This condition or weakness can occur in up to 5% of children as young as ...
Symptoms of Spondylolisthesis. Spondylolisthesis can cause compression of spinal nerves and in severe cases, the spinal cord. ... Surgery is reserved for severe cases of spondylolisthesis in which there is a high degree of instability and symptoms of nerve compression. In these cases a spinal fusion may be necessary. This surgery joins two or ...
Degenerative spondylolisthesis symptoms include neurogenic claudication, sciatica, and radiculopathy. In degenerative spondylolisthesis, the degenerated facet joints and other parts of the vertebral bone tend to increase in size. The enlarged, abnormal bone then encroaches upon the central canal and/or nerve hole (foramen) causing spinal ...
Degenerative spondylolisthesis, as noted above, is caused by spinal osteoarthritis, also known as spondylosis, in which facet joints and discs of the spine deteriorate over time. This is the most common form on spondylolisthesis. Isthmic spondylolisthesis is caused by a pars interarticularis defect, also known as a pars fracture or spondylolysis.
Spondylolisthesis is slippage of a lumbar vertebra in relation to the vertebra below it. Anterior slippage (anterolisthesis) is more common than posterior slippage (retrolisthesis). Spondylolisthesis has multiple causes. It can occur anywhere in the spine and is most common in the lumbar and cervical regions.
In lumbar spondylolisthesis, a vertebrae in the lower back slips forward. This disorder usually occurs during adolescence or young adulthood (often in athletes). It is usually caused by a birth defect or an injury that causes fractures (breaks) in a part of the vertebra. If both sides of the vertebra are involved, the vertebra can then slip ...
The main symptoms of spondylolisthesis include: pain in your lower back, often worse when standing or walking and relieved when sitting or bending forward. pain spreading to your bottom or thighs. tight hamstrings (the muscles in the back of your thighs) pain, numbness or tingling spreading from your lower back down 1 leg ( sciatica)
Stage 2: Grade 2 Spondylolisthesis. Grade 2 spondylolisthesis is characterized by the slippage of 26% to 50% of one vertebra over another. At this stage, the symptoms can become more noticeable, including increased back pain, numbness or tingling in the legs or feet, and difficulty standing or walking for extended periods.
Symptoms. Symptoms may vary from mild to severe. In some cases, there may be no symptoms at all. Spondylolisthesis can lead to increased lordosis (also called swayback), and in later stages may result in kyphosis, or round back, as the upper spine falls off the lower. Symptoms may include: Lower back pain; Muscle tightness (tight hamstring muscle)
Many people with spondylolisthesis will have mild symptoms and very little visible deformity. Often, the first physical sign of spondylolisthesis is a tightening of the hamstring muscles in the legs. ... In patients with an instability of the spine, a spinal fusion may be recommended. The primary objective is to relieve pressure on the nerves ...
Symptoms. Symptoms of spondylolisthesis depend on the severity of slippage. Symptoms can include pain, discomfort, stiffness, or muscle spasms in the low back. Symptoms of radiculopathy may appear including numbness, tingling, pain, or weakness in the legs. ... In non-medical terms, this means the fracture caused instability, and over time the ...
Specifically, a condition called spondylolisthesis in which a vertebra moves and slips out of place, causing intense lower back pain among other symptoms. The once stable spinal column is anything ...
Spondylolisthesis occurs when one of the vertebrae in the spine slips out of position. Symptoms can include difficulty walking, lower back pain, leg weakness, and more. Treatment can include ...
The basic idea is to fuse together the painful vertebrae so that they heal into a single, solid bone. In spondylolisthesis, one of the bones in your spine — called a vertebra — slips forward and out of place. This may occur anywhere along the spine, but is most common in the lower back (lumbar spine). In some people, this causes no symptoms ...
Spondylolisthesis. Spondylolisthesis occurs when one vertebra in the spinal column becomes fractured and the spine slips out of place, usually in the lumbar area. Back pain, numbness in the extremities, or sensory loss can be caused by nerve root compression as a result of the slippage. Related conditions include spondylosis which is arthritis ...
Spondylolisthesis is a condition in which a bone in the spine (a vertebra) slips forward or backward in relation to the bone below it. It occurs most frequently in the lower back, but any vertebra in the spine can be affected. Low back pain, leg pain and weakness in the legs can happen if the bone that's out of position significantly narrows ...
Degenerative spondylolisthesis. Pain is aching in nature and insidious in onset. Pain is in the low back and posterior thighs. Neurogenic claudication may be present with lower-extremity symptoms worsening with exercise. Symptoms are often chronic and progressive, sometimes with periods of remission.
Spondylolisthesis is the slippage of one vertebral body with respect to the adjacent vertebral body causing mechanical or radicular symptoms or pain. It can be due to congenital, acquired, or idiopathic causes. Spondylolisthesis is graded based on the degree of slippage of one vertebral body on the adjacent vertebral body.
Signs & Symptoms of Spondylolisthesis L5-S1. L5-S1 spondylolisthesis can cause back pain as well as numbness, weakness in both the legs. All symptoms may not be prominent. It may happen that one may not feel any pain or numbness until years even after the slippage of the vertebra.
Lumbar spinal stenosis occurs in the lower back and is the most common form of spinal stenosis.Pain or numbness resulting from lumbar stenosis can affect the lower back as well as the legs and ...
Chronic low back pain (cLBP) is the most frequently reported cause of years lived with disability. Identifying the anatomical structures or dysfunction contributing to patients' symptoms is critical to guiding treatment. The etiology of back pain and differential diagnosis is often broad, ranging from non-degenerative cLBP (trauma, tumor, inflammation, infection, etc.) to degenerative (also ...
Zheng and associates 63 conducted a single center, retrospective, observational study comprised of 79 patients to investigate the role of ACDF in alleviating symptoms in patients with cervical vertigo associated with cervical instability. A significant relief of vertigo and dizziness was reported following anterior cervical surgery during the 2 ...