- Case report
- Open access
- Published: 14 August 2018

Unilateral pulmonary edema: a case report and review of the literature
- Rangani Handagala 1 ,
- Udaya Ralapanawa ORCID: orcid.org/0000-0002-7416-7984 2 &
- Thilak Jayalath 1
Journal of Medical Case Reports volume 12 , Article number: 219 ( 2018 ) Cite this article
15k Accesses
17 Citations
4 Altmetric
Metrics details
Unilateral pulmonary edema is an uncommon condition and is a rare clinical entity that is often misdiagnosed at the initial stages. In a majority of patients it occurs in the upper lobe of the right lung. There are many causes of unilateral pulmonary edema, but the commonest is the presence of a grade 3 mitral regurgitation. Due to its rare presentation, a high index of suspicion is required, and correct management is necessary to reduce the morbidity and mortality.
Case presentation
We present a case of right-sided unilateral pulmonary edema in an 86-year-old Sinhalese Sri Lankan woman who presented with acute onset dyspnea with cardiogenic shock due to acute non-ST elevation myocardial infarction, complicated with grade 3 mitral regurgitation. She had clinical features of heart failure and pulmonary edema, but a chest X-ray showed unilateral infiltrates only on the right side. Distinguishing pneumonia from pulmonary edema according to chest X-ray findings was a challenge initially, and she was therefore initially treated for both conditions. She had remarkable clinical and radiological improvement after 12 hours of intravenously administered furosemide and glyceryl trinitrate therapy. Her brain natriuretic peptide level was elevated and further supported and confirmed the diagnosis retrospectively.
Conclusions
Unilateral pulmonary edema is a completely reversible condition with good patient outcome if it is suspected early and treated early. Even in the absence of readily available echocardiogram skills, a clinical examination is of paramount importance in making a clinical decision in low-resource settings to reduce mortality.
Peer Review reports
Unilateral pulmonary edema (UPE) is uncommon, accounting for 2% of cardiogenic pulmonary edemas, and usually involves the upper lobe of the right lung [ 1 ]. The mechanism of UPE has been attributed to various causes [ 2 ]. Cardiogenic UPE is often misdiagnosed at first [ 1 ]. We present this case with UPE involving the entire right lung. Our patient had a grade 2 mitral regurgitation (MR) which she tolerated fairly well; however, she deteriorated into acute grade 3 MR following a non-ST elevation myocardial infarction (NSTEMI) which explains the importance of hemodynamics driving the cardiac filling pressures on an ischemic heart.
An 86-year-old Sinhalese Sri Lankan woman who had been previously diagnosed as having hypertension, grade 2 MR, and ischemic heart disease with congestive cardiac failure, presented to our preliminary care unit with sudden onset shortness of breath at night while sleeping. She had eaten her dinner and taken her usual medications before sleeping. She had a New York Heart Association (NYHA) heart failure grade of class 2, and could manage her day-to-day activities without support. She could walk 25 meters and could climb 3–4 steps without becoming dyspneic. Apart from her usual symptoms she did not have fever, cough, or chest pain before admission. She is a housewife and mother of five children. She does not smoke tobacco or drink alcohol. At presentation she was on captopril 12.5 mg twice a day, atorvastatin 20 mg at night, soluble aspirin 75 mg at night, bisoprolol 2.5 mg once a day, and furosemide 40 mg in the morning.
On examination, she was found to be dyspneic, drowsy, pale, diaphoretic, and restless. Her body temperature was 37.0 °C. Her blood pressure (BP) was 90/60 mmHg, with a regular, low volume pulse rate of 102 beats per minute. Her heart sounds were unremarkable. Cardiac apex was not palpable. There was a pansystolic murmur at cardiac apex. Her respiratory rate was 26/minute. Her trachea was central and right-sided chest expansion was reduced. Bilateral crepitations and rhonchi were present more significantly on the right side. Her initial oxygen saturation checked by pulse-oximetry was 56% in room air. Her abdomen was not distended and there was mild right hypochondrial tenderness. There was no hepatosplenomegaly. Her cranial nerve examination was normal. Her limbs examination was normal with normal tone, power, and reflexes.
An electrocardiogram showed ST depression in leads V5–V6 and poor R wave progression in leads V1–V4. Her chest X-ray revealed alveolar-interstitial infiltrates and a fluid collection around horizontal fissure in her right lung (Fig. 1 ). Laboratory tests showed a white blood cell count of 12,000/μL with 91.8% neutrophils, hemoglobin of 9.5 g/dL, packed cell volume of 30.3, mean corpuscular volume of 75 fl, mean corpuscular hemoglobin of 23.8 pg, mean corpuscular hemoglobin concentration of 31.4 g/dl, creatinine of 221.5 μmol/l, sodium level of 139 mEq/L, potassium level of 4.4 mEq/L, B-natriuretic peptide (BNP) of 2437.2 pg/ml (normal 450 for NYHA class 2), C-reactive protein (CRP) 7.56 mg/dL (< 10), and troponin I 59.2 ng/mL (< 0.01).
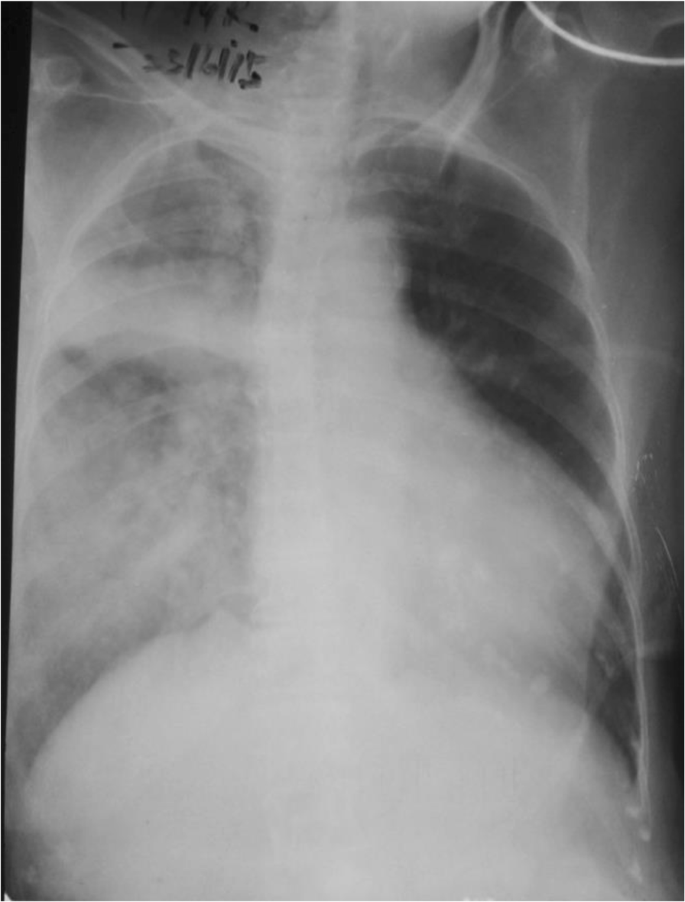
Chest X-ray on admission
Although our patient’s temperature was normal, pneumonia could not be initially excluded in this older patient in the presence of a unilateral pulmonary infiltrate with effusion along the horizontal fissure, in combination with leukocytosis and awaiting CRP level (which took 4 hours to get the report), treatment with intravenously administered broad spectrum antibiotics (ceftriaxone 1 g twice a day and clarithromycin 500 mg twice a day) was initiated to cover severe community acquired pneumonia, and oseltamivir was started since there was an epidemic of influenza H1N1 at the time.
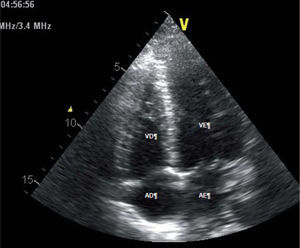
An emergency two-dimensional echocardiogram facility is not available in the preliminary care unit in our set up and our patient was not in a condition to be transferred to a place where a good quality echocardiogram machine was available to assess the severity of MR accurately. Echocardiography was done on third day of admission which disclosed: an ejection fraction of 25–30% with severe left ventricular (LV) dysfunction; and hypokinesia of anterior wall, LV apex, and lower 2/3 of interventricular septum, with an apical aneurysm. A two-dimensional echocardiogram showed grade 3 MR (Fig. 2 ). Although her BNP level was found to be high it took 4 days to get the report due to delays in laboratory processing. Therefore it helped us to support the diagnosis retrospectively.

2D Echocardiogram showing mitral regurgitation
With all these challenges our patient was treated for severe acute on chronic heart failure although radiological evidence was unfavorable. Interestingly, she showed a remarkable improvement with preload reduction with loop diuretics and nitrates. After availability of troponin I levels she was treated for a NSTEMI on top of heart failure with intravenous heparin 500 units/hour infusion (her weight was 40 kg). Her condition was stabilized with adjustment of medical therapy for heart failure including diuretics, nitrates, and opioids. She had persistently low BP for which she needed inotropic support with dopamine and dobutamine which were tailed off subsequently. Repeat chest radiography taken 12 hours later showed complete resolution of the UPE (Fig. 3 ). Subsequently, her CRP was normal and antibiotics were de-escalated after 24 hours, but oseltamivir was continued. She had a fast and remarkable recovery to her preadmission state on day 5 of admission after which she was discharged. She refused any further cardiac intervention. Two weeks after discharge she was reviewed at a medical clinic and found to have NYHA class 2 heart failure. Her BP was120/80 mmHg and pulse rate was 70/minute. Her medications were uptitrated and she was followed up in a medical clinic. After 6 month she had a two-dimensional echocardiogram and revealed ejection fraction of 40% with grade 2–3 MR.
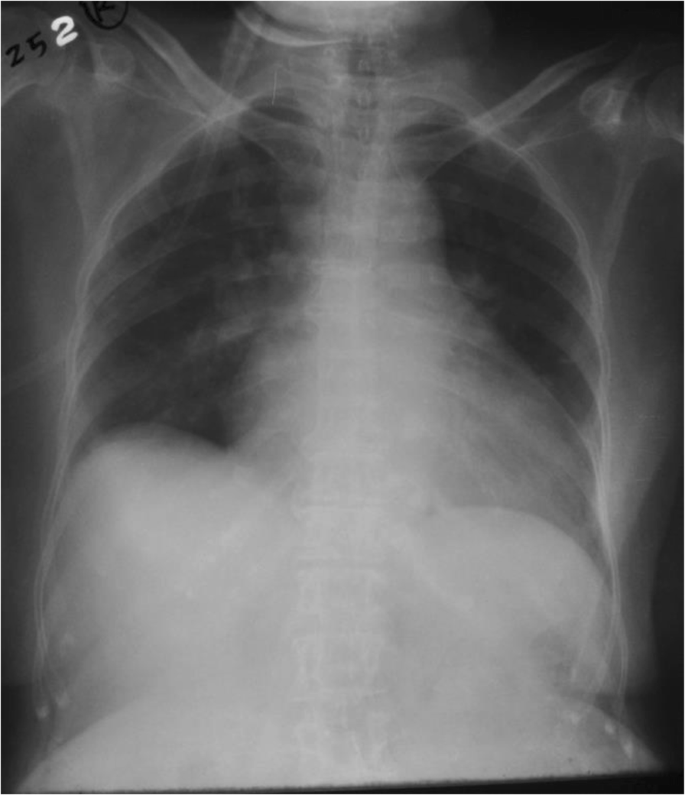
Chest X-ray taken after 12 hours. This X-ray was taken after intravenous furosemide/glyceryl trinitrate infusion
An 86-year-old woman previously diagnosed as having hypertension, grade 2 MR, and ischemic heart disease with congestive cardiac failure, presented with sudden onset shortness of breath. She had clinical features of heart failure and pulmonary edema, but a chest X-ray showed unilateral infiltrates only on the right side. Distinguishing pneumonia from pulmonary edema according to the chest X-ray findings was a challenge initially, and she was treated for both conditions. She had a remarkable clinical and radiological improvement after 12 hours of intravenously administered furosemide and glyceryl trinitrate therapy. Subsequently she was diagnosed as having NSTEMI, complicated with grade 3 MR. Her BNP level was elevated and further supported and confirmed the diagnosis of cardiogenic UPE retrospectively. UPE is a completely reversible condition with good patient outcome if it is suspected early and treated early. Even in the absence of readily available echocardiogram skills, a clinical examination is of paramount importance in making a clinical decision in low-resource settings to reduce mortality as discussed in this case report. Also this case highlights that acute cardiac ischemia can contribute to worsening a preexisting MR and precipitate UPE.
Acute cardiogenic pulmonary edema is a critical condition associated with high mortality, and may be caused by a variety of cardiac diseases, including coronary artery disease. The usual radiographic finding in acute cardiogenic pulmonary edema is bilateral symmetrical opacities in the central zones of the lungs, resulting in the classic “butterfly shadow” [ 3 , 4 ]. UPE is a rare entity that can be mistaken for other causes of unilateral infiltrate on chest radiography, especially pneumonia. UPE has been reported after congestive heart failure, prolonged rest on one side in patients with cardiac decompensation or receiving large amounts of fluids, in cases of rapid expansion of the lungs after pleural effusion, and pneumothorax [ 5 , 6 , 7 ]. It is also seen in a normal lung in patients with unilateral pulmonary disease such as MacLeod syndrome and unilateral pulmonary artery hypoplasia or agenesis, pulmonary artery compression from aortic dissection or LV pseudo aneurysm, and pulmonary venous obstruction from mediastinal fibrosis [ 1 ]. However, it is mainly reported in association with severe MR [ 6 ].
Most cases of UPE associated with left-sided heart failure affect the right lung [ 4 ]. A possible explanation is the poorer lymphatic drainage of the right lung by the small-caliber right bronchomediastinal trunk in comparison with that of the left lung by the large-caliber thoracic duct. Another explanation relates to the left-sided cardiac enlargement that develops in most patients with heart failure and that may physically impede blood flow in the left pulmonary artery, thereby reducing capillary volume. However, severe MR remains the main cause of UPE [ 6 ]. An MR jet affecting predominantly the upper right pulmonary vein, can lead to a larger increase in mean capillary pressure on the right side and, consequently, a greater degree of right acute pulmonary edema. The main mechanism of MR in UPE is mitral leaflet prolapse, but functional MR may also be involved [ 6 , 7 ].
During the early phase of acute myocardial infarction (AMI), transient ischemic MR is common and sometimes causes hemodynamic compromise. However, when several chordate tendineae or papillary muscle rupture occurs, this can lead to abrupt hemodynamic deterioration with cardiogenic shock. It is important to have a high index of suspicion for acute MR in any patient with acute pulmonary edema in the setting of AMI, especially if LV systolic function is well preserved [ 1 ]. Our patient did not have echocardiographic evidence of rupture of papillary muscles, but probably she had ischemia-induced papillary muscle dysfunction due to NSTEMI which would have pushed her to grade 3 MR during this admission causing right UPE. Table 1 , which shows a summary of a recent literature review, highlights that almost all the patients with UPE have some degree of MR.
A unilateral radiography pattern may lead to a false diagnosis of pneumonia and so delay management. Although the induction of an acute phase reaction and an elevated peripheral leukocyte count, especially of neutrophils, in patients with AMI has been reported to be related to the extent of myocardial infarction and with prognosis, the association of unilateral pulmonary infiltrates with leukocytosis and/or acute respiratory distress often leads to antibiotic therapy, despite the absence of fever, especially in older patients [ 1 ]. Furthermore, patients with UPE present with a higher risk of death than patients with bilateral pulmonary edema, and delay in adequate treatment of UPE may be one explanation for this increased mortality [ 1 ]. The absence of fever, a history of sudden onset of dyspnea, and elevated levels of BNP, may help to differentiate UPE from other diagnoses. However, in our case BNP levels helped us to diagnose retrospectively. A murmur on examination can be useful, especially for organic MR. However, the intensity of the murmur is low and correlates poorly with the degree of regurgitation in acute ischemic MR. Echocardiography is useful in determining the severity of MR and its cause. The availability of a good quality echocardiogram on admission would have reduced the morbidity further and improved patient outcome and reduced the hospital stay [ 7 ]. Cardiogenic UPE can easily be mistaken for pneumonia or some other pulmonary pathological condition. The availability of correct resources at an appropriate time would make a dramatic difference in management. Since we cannot guarantee the reliability of supplementary medical backup in our set up, parameters such as physical examination findings and the clinical course should also be taken into account. Where time factor decides the mortality, skills in clinical examination are of paramount importance in making a clinical decision in a low-resource setting to reduce mortality.
In this case, the patient was treated empirically for both pulmonary edema and chest infection and subsequent de-escalation of antibiotics. Cardiogenic shock developed due to NSTEMI on top of a weak heart. Our patient was a diagnosed case of grade 2 MR, but it is doubtful that grade 2 MR would be a cause of pulmonary edema. Acute myocardial ischemia could exacerbate diastolic dysfunction with afterload mismatch and possibly the reduced systolic function, as well as transient ischemic MR due to papillary muscle dysfunction, may cause grade 3 MR, which was seen in this admission [ 1 ]. When regurgitant volume increases suddenly due to transient ischemic MR, the acute rise in left atrial (LA) pressure can be transmitted back to the pulmonary circulation, generating pulmonary edema. UPE can occur not only because the direction of the MR jet affects predominantly the upper right pulmonary vein, but also because of differences between the mechanisms controlling tissue osmotic pressure in the two sides of the lung. For example, in the case of a sudden increase of LA pressure, the right lung parenchyma can develop edema due to the low excretory capability of its lymphatic drainage compared to that of the left lung, precipitating an event of right-sided UPE [ 1 , 8 ]. Recovery of the ischemic papillary muscle and the limited number of episodes of LA hypertension, which are the main determinants of pulmonary edema after AMI, may have contributed to the resolution of the pulmonary edema with treatment over a day. The lesson of this case is that it is important to acknowledge that AMI can present as UPE, even in patients without severe MR or any preexisting pulmonary disease affecting the vasculature or parenchyma of a lung.
The most important take home message from this case is that even with minimal resources available one can save a life in a critical situation if one’s clinical suspicion and diagnosis overrides the supplementary evidence.
UPE is a completely reversible condition with good patient outcome if it is suspected early and treated early.
Abbreviations
Acute myocardial infarction
B-natriuretic peptide
Blood pressure
C-reactive protein
Left atrial
Left ventricular
- Mitral regurgitation
Non-ST elevation myocardial infarction
New York Heart Association
- Unilateral pulmonary edema
Shin JH, Kim SH, Park J, et al. Unilateral pulmonary edema: a rare initial presentation of cardiogenic shock due to acute myocardial infarction. J Korean Med Sci. 2012;27(2):211–4. https://doi.org/10.3346/jkms.2012.27.2.211 .
Article PubMed PubMed Central Google Scholar
Hacking C, Hsu C, et al. Unilateral pulmonary oedema. In: Radiopaedia. 2015. [online] http://radiopaedia.org/articles/unilateral-pulmonary-oedema . [Accessed 14 Sept 2015].
Pandya GJ, Mukhopadhyay A, Ping CA, Lim TK. Unilateral Lobar Pulmonary Edema. JAPI. 2012;60:60–62.
Google Scholar
Nessa CB, Rigler LG. The roentgenological manifestations of pulmonary edema. Radiology. 1941;37:35–45.
Article Google Scholar
Nitzan O, Saliba WR, Goldstein LH, Elias MS. Unilateral pulmonary edema: a rare presentation of congestive heart failure. Am J Med Sci. 2004 Jun;327(6):362–4.
Article PubMed Google Scholar
Legriel S, Tremey B, Mentec H. Unilateral pulmonary edema related to massive mitral insufficiency. Am J Emerg Med. 2006;24:372.
Childress ME, Moy G, Mottram M. Unilateral pulmonary edema resulting from treatment of spontaneous pneumothorax. Am RevRespir Dis. 1971;104:119–21.
CAS Google Scholar
Attias D, Mansencal N, Auvert B, et al. Prevalence, characteristics, and outcomes of patients presenting with cardiogenic unilateral pulmonary edema. Circulation. 2010;122(11):1109–15.
Kashiura M, Tateishi K, et al . Unilateral cardiogenic pulmonary edema associated with acute mitral regurgitation. Acute Med Surg. 2016;4(1):119–122. https://doi.org/10.1002/ams2.234 .
Mehta V, Macduff A. Unilateral Pulmonary Oedema. J Anesth Clin Res 2016;7:11. https://doi.org/10.4172/2155-6148.1000I101 .
Doshi H, El Accaoui R. Unilateral pulmonary edema in acute coronary syndrome: A sinister sign. Eur J Intern Med. 2016;30:e5–e6. https://doi.org/10.1016/j.ejim.2015.11.009 .
Venugopal K, Kushal DP, Shyamala G, Kiran Chand N. Unilateral pulmonary oedema: Rare manifestation of scorpion Sting. Indian Journal of Anaesthesia. 2015;59(7):452–453.
Omran Ja, Jain K et al . Acute Unilateral Pulmonary Edema in Non-Cardiac Settings. J Med Cases. 2014;5(7):417–419.
Warraich HJ, Bhatti UA et al . Unilateral Pulmonary Edema Secondary to Mitral Valve Perforation. Circulation. 2011;124:1994–1995.
Gowrinath K, Attur RP et al . An unusual case of unilateral pulmonary edema in patients with chronic kidney disease. Respiratory Medicine CME. 2009;2:130–133.
Peña C, l Jaquet M et al . Asymmetric Pulmonary Perfusion Causing Unilateral Pulmonary Edema As a Complication of Acute Myocardial Infarction. Rev. Esp Cardiol. 2005;58(7):875–7.
Mokta JK, Mahajan SK et al . Life Threatening Unilateral Pulmonary Oedema at Moderate Altitude. The Indian Journal of Chest Diseases & Allied Sciences. 2004;46:113–116.
Lesieur O, Lorillard R et al . Unilateral pulmonary oedema complicating mitral regurgitation: diagnosis and demonstration by transoesophageal echocardiography. Intensive Care Med. 2000;26:466–470
Download references
Acknowledgements
We all express our gratitude to the patient who kindly gave consent for this case to be presented in this paper.
Author information
Authors and affiliations.
Teaching Hospital, Peradeniya, Sri Lanka
Rangani Handagala & Thilak Jayalath
Department of Medicine, University of Peradeniya, Peradeniya, Sri Lanka
Udaya Ralapanawa
You can also search for this author in PubMed Google Scholar
Contributions
Analysis and interpretation of patient data and literature review were done by RH and UR. UR and TJ guided the other authors in reporting this case and corrected the final manuscript. All authors were involved in the management of the patient and read and approved the final manuscript.
Corresponding author
Correspondence to Udaya Ralapanawa .
Ethics declarations
Authors’ information.
RH (MBBS, Dep. Crit. Care Med) is a registrar in critical care medicine, Teaching Hospital, Peradeniya, Sri Lanka. UR (MBBS, MD, MRCP, FRCP) is a Senior Lecturer and Consultant Physician, Department of Medicine, University of Peradeniya, Sri Lanka. TJ (MBBS, MD, MRCP, FRCP, FRCPE, FACP, FCCP) is a Professor in Medicine and Consultant Physician, Department of Medicine, University of Peradeniya, Sri Lanka.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Handagala, R., Ralapanawa, U. & Jayalath, T. Unilateral pulmonary edema: a case report and review of the literature. J Med Case Reports 12 , 219 (2018). https://doi.org/10.1186/s13256-018-1739-3
Download citation
Received : 17 September 2017
Accepted : 06 June 2018
Published : 14 August 2018
DOI : https://doi.org/10.1186/s13256-018-1739-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain natriuretic peptide
- Heart failure
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Unilateral pulmonary edema: a case report and review of the literature
Affiliations.
- 1 Teaching Hospital, Peradeniya, Sri Lanka.
- 2 Department of Medicine, University of Peradeniya, Peradeniya, Sri Lanka. [email protected].
- PMID: 30103814
- PMCID: PMC6090641
- DOI: 10.1186/s13256-018-1739-3
Background: Unilateral pulmonary edema is an uncommon condition and is a rare clinical entity that is often misdiagnosed at the initial stages. In a majority of patients it occurs in the upper lobe of the right lung. There are many causes of unilateral pulmonary edema, but the commonest is the presence of a grade 3 mitral regurgitation. Due to its rare presentation, a high index of suspicion is required, and correct management is necessary to reduce the morbidity and mortality.
Case presentation: We present a case of right-sided unilateral pulmonary edema in an 86-year-old Sinhalese Sri Lankan woman who presented with acute onset dyspnea with cardiogenic shock due to acute non-ST elevation myocardial infarction, complicated with grade 3 mitral regurgitation. She had clinical features of heart failure and pulmonary edema, but a chest X-ray showed unilateral infiltrates only on the right side. Distinguishing pneumonia from pulmonary edema according to chest X-ray findings was a challenge initially, and she was therefore initially treated for both conditions. She had remarkable clinical and radiological improvement after 12 hours of intravenously administered furosemide and glyceryl trinitrate therapy. Her brain natriuretic peptide level was elevated and further supported and confirmed the diagnosis retrospectively.
Conclusions: Unilateral pulmonary edema is a completely reversible condition with good patient outcome if it is suspected early and treated early. Even in the absence of readily available echocardiogram skills, a clinical examination is of paramount importance in making a clinical decision in low-resource settings to reduce mortality.
Keywords: Brain natriuretic peptide; Heart failure; Mitral regurgitation; Unilateral pulmonary edema.
PubMed Disclaimer
Conflict of interest statement
RH (MBBS, Dep. Crit. Care Med) is a registrar in critical care medicine, Teaching Hospital, Peradeniya, Sri Lanka. UR (MBBS, MD, MRCP, FRCP) is a Senior Lecturer and Consultant Physician, Department of Medicine, University of Peradeniya, Sri Lanka. TJ (MBBS, MD, MRCP, FRCP, FRCPE, FACP, FCCP) is a Professor in Medicine and Consultant Physician, Department of Medicine, University of Peradeniya, Sri Lanka.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-chief of this journal.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chest X-ray on admission
2D Echocardiogram showing mitral regurgitation
Chest X-ray taken after 12…
Chest X-ray taken after 12 hours. This X-ray was taken after intravenous furosemide/glyceryl…
Similar articles
- Unilateral pulmonary edema: a rare initial presentation of cardiogenic shock due to acute myocardial infarction. Shin JH, Kim SH, Park J, Lim YH, Park HC, Choi SI, Shin J, Kim KS, Kim SG, Hong MK, Lee JU. Shin JH, et al. J Korean Med Sci. 2012 Feb;27(2):211-4. doi: 10.3346/jkms.2012.27.2.211. Epub 2012 Jan 27. J Korean Med Sci. 2012. PMID: 22323871 Free PMC article.
- Right upper lobe pulmonary edema caused by acute mitral regurgitation. Diagnosis by transesophageal echocardiography. Roach JM, Stajduhar KC, Torrington KG. Roach JM, et al. Chest. 1993 Apr;103(4):1286-8. doi: 10.1378/chest.103.4.1286. Chest. 1993. PMID: 8131490
- Complicated acute myocardial infarction. Heart failure, shock, mechanical complications. Cercek B, Shah PK. Cercek B, et al. Cardiol Clin. 1991 Nov;9(4):569-93. Cardiol Clin. 1991. PMID: 1811867 Review.
- [Unilateral pulmonary edema in two patients with mitral regurgitation]. Niedeggen A, Janssens U. Niedeggen A, et al. Dtsch Med Wochenschr. 2008 Nov;133(47):2454-7. doi: 10.1055/s-0028-1100938. Epub 2008 Nov 12. Dtsch Med Wochenschr. 2008. PMID: 19006044 German.
- Rare roentgenologic manifestations of pulmonary edema. Myrianthefs P, Markou N, Gregorakos L. Myrianthefs P, et al. Curr Opin Crit Care. 2011 Oct;17(5):449-53. doi: 10.1097/MCC.0b013e328347f501. Curr Opin Crit Care. 2011. PMID: 21670669 Review.
- Redefining Unilateral Pulmonary Edema after Mitral Valve Surgery on Chest X-ray Imaging Using the RALE Scoring System. Mostafa K, Wolf C, Seehafer S, Horr A, Pommert N, Haneya A, Lutter G, Pühler T, Both M, Jansen O, Langguth P. Mostafa K, et al. J Clin Med. 2023 Sep 19;12(18):6043. doi: 10.3390/jcm12186043. J Clin Med. 2023. PMID: 37762983 Free PMC article.
- A Case of Isolated Right Lung Edema and Liver Injury Induced by High Altitude. Takeuchi I, Muramatsu KI, Ota S, Yanagawa Y. Takeuchi I, et al. J Emerg Trauma Shock. 2023 Apr-Jun;16(2):71-72. doi: 10.4103/jets.jets_139_22. Epub 2023 May 23. J Emerg Trauma Shock. 2023. PMID: 37583381 Free PMC article. No abstract available.
- Bilateral Upper Lobe Pulmonary Oedema and Primary Mitral Regurgitation. Hein A, Wai YH. Hein A, et al. Cureus. 2022 Dec 9;14(12):e32347. doi: 10.7759/cureus.32347. eCollection 2022 Dec. Cureus. 2022. PMID: 36628016 Free PMC article.
- Lung Ultrasound Estimates the Overhydration and Benefits Blood Pressure Control in Normal or Mild Symptomatic Hemodialysis Patients. Trirattanapikul A, Kongpetch S, Lukkanalikitkul E, Ahooja A, Seesuk P, Sharma A, Anutrakulchai S. Trirattanapikul A, et al. Int J Nephrol Renovasc Dis. 2022 Dec 19;15:383-395. doi: 10.2147/IJNRD.S374569. eCollection 2022. Int J Nephrol Renovasc Dis. 2022. PMID: 36570492 Free PMC article.
- A rare case of left dominant pulmonary edema in acute mitral regurgitation. Kang CY, Khamooshi P, Pinzon VR, Tottleben JM. Kang CY, et al. Respir Med Case Rep. 2022 Oct 13;40:101746. doi: 10.1016/j.rmcr.2022.101746. eCollection 2022. Respir Med Case Rep. 2022. PMID: 36324338 Free PMC article.
- Shin JH, Kim SH, Park J, et al. Unilateral pulmonary edema: a rare initial presentation of cardiogenic shock due to acute myocardial infarction. J Korean Med Sci. 2012;27(2):211–214. doi: 10.3346/jkms.2012.27.2.211. - DOI - PMC - PubMed
- Hacking C, Hsu C, et al. Unilateral pulmonary oedema. In: Radiopaedia. 2015. [online] http://radiopaedia.org/articles/unilateral-pulmonary-oedema . [Accessed 14 Sept 2015].
- Pandya GJ, Mukhopadhyay A, Ping CA, Lim TK. Unilateral Lobar Pulmonary Edema. JAPI. 2012;60:60–62. - PubMed
- Nessa CB, Rigler LG. The roentgenological manifestations of pulmonary edema. Radiology. 1941;37:35–45. doi: 10.1148/37.1.35. - DOI
- Nitzan O, Saliba WR, Goldstein LH, Elias MS. Unilateral pulmonary edema: a rare presentation of congestive heart failure. Am J Med Sci. 2004;327(6):362–4. doi: 10.1097/00000441-200406000-00013. - DOI - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations
- Genetic Alliance

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
- Previous Issue
- Previous Article
- Next Article
Case Report
Case scenario: acute postoperative negative pressure pulmonary edema.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
- Search Site
David J. Krodel , Edward A. Bittner , Raja Abdulnour , Robert Brown , Matthias Eikermann , Bruno Riou; Case Scenario: Acute Postoperative Negative Pressure Pulmonary Edema. Anesthesiology 2010; 113:200–207 doi: https://doi.org/10.1097/ALN.0b013e3181e32e68
Download citation file:
- Ris (Zotero)
- Reference Manager
FORMATION of noncardiogenic pulmonary edema has been observed after a variety of inciting events, including upper airway obstruction (negative pressure pulmonary edema [NPPE]), 1 acute lung injury, 2 anaphylaxis, 3 fluid maldistribution, 4 and severe central nervous system trauma (neurogenic pulmonary edema). 5 Both the diagnosis of pulmonary edema and an understanding of its underlying pathophysiology have important implications for treatment. Patients with severe postoperative noncardiogenic pulmonary edema who require mechanical ventilation should be ventilated with a low-tidal volume, 6 administration of positive end-expiratory pressure, and low plateau airway pressures. 7,8 Recent studies suggest that noninvasive respiratory support might be a viable approach for the treatment of patients with postoperative respiratory dysfunction, including postoperative NPPE. 9
A 25-yr-old man (weight, 68 kg; height, 183 cm) presented to the surgery center for excision of back and thigh schwannomas on the same day. The patient's medical history was significant only for his history of multiple schwannoma resections and a history of smoking one pack of cigarettes per week for the past 5 yr. He denied previous problems with general anesthesia, and his baseline peripheral oxygen saturation was 99% on ambient air.
The patient was premedicated with 2 mg midazolam, and anesthesia was induced with 250 mg fentanyl, 500 mg thiopental, and 8 mg vecuronium given for facilitation of tracheal intubation. He was atraumatically intubated with a 7-mm ID endotracheal tube using a no. 3 Macintosh laryngoscope (Teleflex Medical, Research Triangle Park, NC) on the first attempt with direct visualization of the vocal cords. The patient was turned prone, bilateral breath sounds were reconfirmed, and schwannoma excisions were performed on the left thigh and the left flank. A total of 0.5 mg hydromorphone was administered for analgesia. The intraoperative course was unremarkable. The patient was hemodynamically stable with minimal blood loss and was easily ventilated and oxygenated. A total of 500 ml lactated Ringer's solution was administered during the 65-min surgical procedure. The patient was returned to the supine position for emergence and extubation. Nondepolarizing motor blockade was not reversed because train-of-four monitoring of the ulnar nerve showed a train-of-four ratio of greater than 90%, demonstrating adequate spontaneous recovery.
Immediately after extubation, the patient developed inspiratory stridor consistent with laryngospasm; the anesthesiologist had difficulty in mask ventilating the patient, and peripheral oxygen saturation decreased to less than 80%. Laryngospasm was treated by 50 mg propofol and manual positive pressure mask ventilation with 100% inspired oxygen. Peripheral oxygen saturation improved gradually, and the patient was transported to the postanesthesia care unit for further supportive treatment.
In the postanesthesia care unit, the patient's oxygen saturation was maintained with 100% oxygen administered via a nonrebreather facemask. The patient coughed pink, frothy sputum during the course of the first postoperative hour. Physical examination revealed crackles bilaterally at the lung bases, and a chest radiograph was performed, showing diffuse, bilateral, hazy, and interstitial opacity throughout both lungs, with normal lung volumes, normal heart size, and no pleural effusions ( fig. 1 ). A diagnosis of NPPE was made, and the patient was admitted to the inpatient postoperative recovery room for overnight observation. With supplemental oxygen, diuretic treatment, and bronchodilator inhalation, his respiratory status continued to improve with peripheral oxygen saturations greater than 94% on ambient air 10 h after surgery. Examination on the morning of the first postoperative day revealed clear lungs bilaterally and peripheral oxygen saturation of 95–97% on ambient air. He was discharged later that morning without signs or symptoms of respiratory compromise on oral analgesics and usual surgical follow-up in 1–2 weeks.

Fig. 1. Chest radiograph taken in the postoperative recovery room, revealing diffuse, bilateral, hazy, and interstitial opacity throughout both lungs, with increased visibility of small lung vessels, normal lung volumes, normal heart size, and no pleural effusions.
Postoperative Recovery Room Diagnostic Evaluation and Treatment
A chest radiograph taken immediately after postanesthesia care unit admission showed diffuse bilateral opacities, a finding that was observed despite conservative intraoperative fluid management ( fig. 1 ). The patient's history, operating room course, and clinical and radiologic findings were most consistent with pulmonary edema with NPPE as the likely cause; however, aspiration pneumonitis (Mendelsohn syndrome) 10 and diffuse alveolar hemorrhage resulting from upper airway obstruction 11 were also included in the differential diagnosis.
When considering the differential diagnosis of acute-onset perioperative pulmonary edema, both cardiac and noncardiac causes should be taken into account ( table 1 ; fig. 2 ). Cardiogenic edema is usually preceded by new-onset left heart dysfunction and may be caused by acute ischemia, infarct, and/or severe arrhythmia, and the diagnosis is confirmed by echocardiography or measurement of the pulmonary artery occlusion pressure. It is likely that a combination of cardiogenic and noncardiogenic mechanisms contributes to the pathogenesis of postoperative pulmonary edema in many cases. For instance, although fluid overload itself can cause pulmonary edema in the presence of normal or even increased cardiac output, 12 intraoperative intravascular fluid overload can exacerbate chronic compensated heart failure.
Table 1. Characteristics of Different Etiologies of Pulmonary Edema in the Perioperative Period

Fig. 2. An algorithm for the clinical differentiation of postoperative pulmonary edema. When considering the differential diagnosis of acute-onset perioperative pulmonary edema, both cardiogenic and noncardiogenic causes should be taken into account. Before making the diagnosis of negative pressure pulmonary edema (NPPE), other causes of pulmonary edema must be considered, particularly those requiring a rapid intervention (fluid maldistribution, anaphylaxis, and cardiogenic pulmonary edema). In the absence of evidence of upper airway obstruction typically leading to NPPE, an adult respiratory distress syndrome or an acute lung injury should be considered. Please note that the algorithm is based on clinical experience and has not yet been validated.
Pulmonary edema caused by anaphylaxis is seen in the setting of exposure to a known or unknown allergen. In the perioperative setting, these often include neuromuscular blocking agents, antibiotics, anesthetics, or latex. 13 The onset is sudden and is typically accompanied by rash, urticaria, and swelling, but bronchospasm and hemodynamic collapse are frequently presenting symptoms. The clinical picture, time course, and severity, and its occurrence after administration of an allergen, help the clinician to relate signs and symptoms of pulmonary edema to an anaphylactic mechanism. The increased histamine and tryptase levels obtained immediately after the reaction are consistent with anaphylaxis. Radioallergosorbent tests and skin tests performed 4–6 weeks after a presumed reaction can help to confirm the clinical diagnosis and identify the inciting allergen. 13
Neurogenic pulmonary edema typically occurs in the setting of a recent severe brain insult, such as subarachnoid hemorrhage, stroke, status epilepticus, trauma, or intracranial mass. Neurogenic pulmonary edema is typically accompanied by unregulated sympathetic discharge leading to pulmonary hypertension, 14 which induces stress failure of pulmonary capillaries and subsequent high permeability pulmonary edema. 15
Acute respiratory distress syndrome and acute lung injury represent a heterogeneous group of severe hypoxic lung diseases. Activation of and damage to the pulmonary endothelium are the hallmark of acute lung injury or acute respiratory distress syndrome, 16 which is caused by a variety of inciting events such as sepsis, systemic inflammatory response syndrome, aspiration, caustic inhalation, blood transfusions, or trauma. Diagnosis is made by exclusion of other causes, as outlined in figure 2 . The severity of hypoxic respiratory failure, chest radiographic findings, and the time course to recovery are key elements that need to be considered for making diagnosis of acute lung injury or acute respiratory distress syndrome. The edema fluid to plasma protein ratio is an additional method to discriminate between cardiogenic pulmonary edema and acute lung injury. Ware et al . 2 compared protein concentration (Biuret method) in the pulmonary edema fluid (taken via a suction catheter inserted into the endotracheal tube) and blood. Using a predefined cutoff of 0.65, the edema fluid to plasma protein ratio had a sensitivity of 81% and a specificity of 81% for the diagnosis of acute lung injury.
Before making the diagnosis of NPPE, other causes of pulmonary edema ( table 2 ; fig. 2 ), particularly those requiring a rapid intervention (fluid maldistribution, anaphylaxis, and cardiogenic pulmonary edema), must be considered. In this patient, intraoperative fluid overload as a mechanism of pulmonary edema was not considered reasonable because the patient had only 500 ml isotonic solution administered intraoperatively, no history of left heart failure, and had been fasting overnight. There was no evidence of cardiogenic or neurogenic pathology and no signs or symptoms of anaphylaxis. Aspiration pneumonitis can be of increased concern in the prone position given the potential for increased abdominal pressure. Our patient was positioned on chest bolsters that allowed the abdomen to hang freely, which might help to decrease intraabdominal pressure. In addition, the radiologic picture of symmetric bilateral pulmonary interstitial infiltrates would be unusual for aspiration pneumonitis, which typically shows a localized infiltrate. In the immediate setting, we could not rule out acute lung injury or acute respiratory distress syndrome, but the severity of respiratory failure and the time course of clinical and radiologic recovery were not ultimately consistent with this etiology. Residual postoperative curarization is associated with reduced pharyngeal muscle tone and possible resulting upper airway obstruction. 17 In our patient, direct measurement of the train-of-four ratio by accelerometry showed a train-of-four ratio greater than 0.9, reflecting adequate recovery from muscle relaxant effects. 18 Coupling these considerations with the clinical picture of laryngospasm, we concluded that the patient's pulmonary edema was likely induced by negative intrathoracic pressure, potentially resulting from strong inspiratory efforts in the setting of laryngospasm.
Table 2. Negative Pressure Pulmonary Edema

In accordance with the reported data, symptoms and clinical signs of pulmonary edema resolved rapidly. 19 Although not performed in this patient, and typically unnecessary to make the diagnosis, hemodynamic measurements, including pulmonary artery occlusion pressure, pulmonary arterial pressure, and central venous pressure, taken after the development of edema, are typically normal. 20
In this patient, conservative treatment with supplemental oxygen administered as 100% oxygen by a nonrebreather mask (flow, 15 l/min), 10 mg furosemide intravenously, and bronchodilators was started. 21 The patient's symptoms of pulmonary edema improved rapidly, such that noninvasive pressure support ventilation was not required. The rapid improvement of the patient's disease represents a typical case of acute postoperative pulmonary edema ( table 2 ).
Epidemiology
Postoperative NPPE typically occurs in response to an upper airway obstruction, where patients can generate high negative intrathoracic pressures, leading to a postrelease pulmonary edema. The current literature regarding its epidemiology is sparse. Young, healthy, athletic patients seem to be at risk for this disorder, 22 and the prevalence of postoperative NPPE is approximately 0.1%. 22,23 In patients developing acute postoperative upper airway obstruction, NPPE has been reported at an incidence of up to 11% ( table 2 ). 24
Typical events leading to acute upper airway obstruction accompanied by perioperative NPPE include laryngospasm and endotracheal tube occlusion by biting. Less typically, NPPE can also occur after foreign body aspiration, oropharyngeal surgery, or postoperative residual curarization, 25 which typically impairs the upper airway dilator muscle strength while preserving inspiratory muscle function. 26 Case reports and retrospective data suggest that the patient characteristics that increase the risk of NPPE seem to include younger patients in American Society of Anesthesiologists physical status categories I and II, who are thought to be most capable of generating highly negative intrathoracic pressures during an obstructing event. Procedural characteristics increasing the risk of NPPE may include oropharyngeal surgery (especially for tumors or other potentially obstructing masses) although the true incidence and hazard ratios have not been reported. 23
Pathogenesis of Noncardiac Pulmonary Edema
Diagnosis of noncardiogenic pulmonary edema requires an understanding of the pulmonary fluid homeostasis. The Starling equation describes the equilibrium of fluid flow through a semipermeable membrane:
where Q = the net transvascular flow of fluid, K = the membrane permeability, P mv = hydrostatic pressure in the microvessels, P pmv = hydrostatic pressure in the perimicrovascular interstitium, πmv = plasma protein osmotic pressure in the peripheral vessels, and πpmv = protein osmotic pressure in the perimicrovascular interstitium.
The osmotic pressure is exerted by solutes in the blood versus those in the interstitium, which cannot cross the semipermeable membrane. Under normal conditions, most of this filtered fluid from the capillaries is returned to the systemic circulation by lymphatics. 27 The alveolar spaces, because of tight junctions in the alveolar epithelium, have very low permeability and do not fill with fluid. Disturbances of pulmonary fluid homeostasis can be induced by four pathways that can lead to increased interstitial fluid: increased hydrostatic pressure in the pulmonary capillary bed (or conversely, decreased pressure in the interstitium), decreased osmotic pressure of plasma, increased permeability of the membrane, and decreased return of fluid to the circulation via lymphatics. 27,28
Pathogenesis of NPPE
During upper airway obstruction and forceful inspiration, pressure in the trachea and lower airways will decrease markedly. The pressure in the pleural space decreases by exactly the same amount, and the pressure in the pulmonary vessels decreases by much less, thus increasing the pressure difference between inside and outside the capillaries and accelerating the formation of interstitial fluid.
Two different mechanisms may explain the development of pulmonary edema during airway obstruction. The most likely mechanism relates to the observation that high negative intrathoracic pressures cause significant fluid shifts from the microvessels to the perimicrovascular interstitium, as seen in patients with congestive heart failure or fluid maldistribution states. The second proposed mechanism involves the disruption of the alveolar epithelium and pulmonary microvascular membranes from severe mechanical stress, leading to increased pulmonary capillary permeability and protein-rich pulmonary edema.
Evidence for a hydrostatic mechanism of NPPE comes from experimental and clinical data. 29,30 In an experimental model of NPPE, Loyd et al . 29 induced a negative inspiratory pressure in sheep (a 9 mmHg decrease in mean central airway pressure). Left atrial pressure decreased by 8 mmHg, and lung lymph flow was increased twice at baseline. Pulmonary arterial pressure was unchanged. The authors concluded that inspiratory loading is associated with an increase in the pulmonary transvascular hydrostatic gradient, possibly by causing a greater decrease in interstitial pressure than in microvascular pressure. Healthy human subjects can generate very high levels of negative inspiratory pressure (>100 mmHg), which in turn increases the return of blood to the right side of the heart, concomitantly increases pulmonary venous pressures, and decreases “downstream” pulmonary interstitial perivascular pressure. The negative intrathoracic pressures generated during the Mueller maneuver (inspiratory effort against a closed glottis) will result in an increased afterload, 31 which in turn will augment the pulmonary capillary hydrostatic pressures. Consequently, a marked increase in hydrostatic pulmonary pressure gradient can be generated, such that fluid filters out of the microcirculation and into the lung interstitium. When a critical quantity of edema fluid collects in the interstitial compartment, alveolar flooding occurs. 32
Clinical Management
Although many patients with NPPE recover with conservative management as in this case, some patients with severe NPPE (or underlying cardiopulmonary disease) require temporary intubation and mechanical ventilation with positive end-expiratory pressure. 33 Diuretics are often administered, but their use is controversial and may even be unnecessary. 19
The patient's wheezing was thought to represent bronchoconstriction, which we treated with inhaled bronchodilators; however, wheezing is caused by air flow through narrowed airways, and this may not necessarily be due to bronchospasm. Turbulence within bronchi, irrespective of the cause, including interstitial edema induced narrowing of bronchial lumina, may account for the development of the clinical symptom wheezing. In vitro and in vivo studies in human and animal models show that β agonists may increase the rate of alveolar fluid clearance via increased active cation transport. 34 Although it is unclear how much nebulized salbutamol arrived at the alveolar epithelium in our patient, it is possible that bronchodilator administration may have accelerated regression of symptoms of pulmonary edema.
An alternative to intubation is noninvasive respiratory support ( i.e ., noninvasive positive pressure ventilation or treatment with continuous positive airway pressure). Recent data suggest that noninvasive respiratory support may be an important tool to prevent or treat acute respiratory failure while avoiding intubation. The aims of noninvasive respiratory support in the context of NPPE include: to partially compensate for the affected respiratory function by reducing the work of breathing; to improve alveolar recruitment with better gas exchange; and to reduce left ventricular afterload, increasing cardiac output and improving hemodynamics. 35 Evidence suggests that noninvasive respiratory support may be an effective strategy to reduce intubation rates, intensive care unit and hospital lengths of stay, and morbidity and mortality in postoperative patients. 9,35 Ultimately, NPPE is a generally benign condition typically resulting in full recovery in 12–48 h when recognized early and necessary supportive treatment is instituted for hypoxemic and/or hypercapnic respiratory failure.
Knowledge Gap
The immediate consequence of the Mueller maneuver is a markedly negative intrathoracic pressure, leading to increased pulmonary transvascular hydrostatic pressure and vulnerability to accumulation of filtered fluid in the interstitium and, ultimately, in the alveoli.
In addition to a hydrostatic mechanism of NPPE, there is evidence that the increased wall stress (circumferential wall tension caused by the transmural pressure) will alter the permeability coefficient ( K ) of the endothelial barrier. A classic paper by John B. West, M.D., Ph.D., D.Sc. (Distinguished Professor of Medicine and Physiology, School of Medicine, University of California, San Diego, San Diego, California), et al . 36 studied the effects of increased capillary transmural pressure in isolated rabbit lungs. The number of breaks in the endothelium increased with perfusion pressures, suggesting that high capillary hydrostatic pressures cause major changes in the ultrastructure of the walls of the capillaries, leading to a high-permeability form of edema. This suggestion was subsequently translated into a human model of increased capillary transmural pressure. This study was performed in six healthy athletes 1 h after an extensive cycling exercise. Analysis of bronchoalveolar lavage in healthy athletes after cycling exercise revealed a higher erythrocyte count and increased protein and albumin content compared with controls, indicating disruption of the endothelial membrane and stress failure. This suggests that acute increases in transmural pressures such as in NPPE may lead to increased permeability of the endothelial barrier. 37
Some information is available on the molecular mechanisms involved in increased endothelial barrier permeability in response to wall stress. When an acute increase in transmural pressure occurs, the radial expansion of the capillary wall translates into linear cellular stretch. Compared with shear stress from laminar flow, the response of endothelial cells to linear stretch is maladaptive. 38,39 Oxidative stress is one mechanism for injury that seems to be up-regulated by increased linear stretch. In fact, increasing levels of cyclic linear stretch result in up-regulation of inducible nitric oxide synthase 40 and xanthine oxidoreductase, as has been shown by Abdulnour et al ., 41 both of which have been repeatedly implicated in cellular injury and increased vascular permeability. Future studies will show whether these mechanisms of increased vascular permeability are clinically relevant in patients presenting with NPPE.
The authors thank Deborah Pederson, M.D. (Instructor in Anesthesia, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts), for reviewing the case scenario and Fumito Ichinose, M.D. (Associate Professor of Anesthesia, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School), for his suggestions regarding the algorithm for making a diagnosis of negative pressure pulmonary edema.
Citing articles via
Most viewed, email alerts, related articles, social media, affiliations.
- ASA Practice Parameters
- Online First
- Author Resource Center
- About the Journal
- Editorial Board
- Rights & Permissions
- Online ISSN 1528-1175
- Print ISSN 0003-3022
- Anesthesiology
- ASA Monitor

- Terms & Conditions Privacy Policy
- Manage Cookie Preferences
- © Copyright 2024 American Society of Anesthesiologists
This Feature Is Available To Subscribers Only
Sign In or Create an Account

The Portuguese Journal of Cardiology, the official journal of the Portuguese Society of Cardiology, was founded in 1982 with the aim of keeping Portuguese cardiologists informed through the publication of scientific articles on areas such as arrhythmology and electrophysiology, cardiovascular surgery, intensive care, coronary artery disease, cardiovascular imaging, hypertension, heart failure and cardiovascular prevention. The Journal is a monthly publication with high standards of quality in terms of scientific content and production. Since 1999 it has been published in English as well as Portuguese, which has widened its readership abroad. It is distributed to all members of the Portuguese Societies of Cardiology, Internal Medicine, Pneumology and Cardiothoracic Surgery, as well as to leading non-Portuguese cardiologists and to virtually all cardiology societies worldwide. It has been referred in Medline since 1987.
Indexed in:
Index Medicus/Medline, Science Citation Index Expanded/Journal of Citation Reports, Scopus
The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years. © Clarivate Analytics, Journal Citation Reports 2022
CiteScore measures average citations received per document published.
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact.
SNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field.
- Palavras-chave
- Introduction
- Case report
- Conflicts of interest
- Bibliography
We report the case of a 21-year-old man who underwent appendectomy under general anesthesia and developed acute pulmonary edema immediately after extubation. We then review the literature, focusing on the pathophysiology and the most important aspects of diagnosis and treatment of post-extubation pulmonary edema.
Apresentamos o caso de um homem de 21 anos, que foi submetido a apendicectomia sob anestesia geral e que imediatamente após a extubação desencadeou quadro de edema pulmonar agudo. A propósito, faremos uma breve revisão da literatura, enfatizando os mecanismos fisiopatológicos subjacentes e os aspectos mais importantes do diagnóstico e tratamento.
Although acute pulmonary edema (APE) as a complication of extubation is uncommon, it has been well characterized. It occurs most frequently in young healthy individuals in the period immediately after extubation, and its severity can worsen the prognosis of low-risk surgery, require invasive treatment and delay hospital discharge 1-3 . The high negative intrathoracic pressure generated by forceful inspiration against the closed glottis is assumed to be the main pathophysiological mechanism behind this form of APE 1,3 . This potentially fatal complication is described in a young, previously healthy adult who underwent appendectomy under general anesthesia for acute appendicitis.
A 21-year-old man, black, a football player, was admitted via the emergency department with a clinical and laboratory diagnosis suggestive of acute appendicitis. There was no relevant family or personal history. Physical examination showed no abnormalities other than in the abdominal region. He underwent laparoscopic appendectomy under balanced general anesthesia, both of which were uneventful. The patient was extubated at the end of the procedure following reversal of neuromuscular blockade. Immediately after extubation, he developed a setting of respiratory distress, with abundant pink frothy sputum and significant arterial desaturation. Physical examination and chest X-ray revealed the presence of APE ( Figure 1 ). After orotracheal reintubation and connection to a ventilator, the patient was transferred to our unit. Following treatment including high-flow oxygen therapy and administration of intravenous boluses of furosemide and isosorbide dinitrate, he showed rapid improvement and was extubated around four hours later ( Figure 2 ). Other laboratory tests, together with electrocardiographic and echocardiographic assessment ( Figures 3 and 4 ) excluded heart disease. There were no further complications.
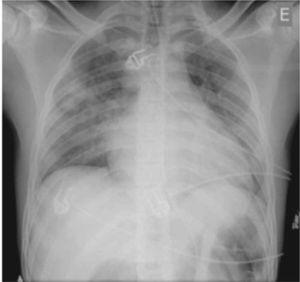
Anteroposterior chest X-ray in dorsal decubitus at 30º immediately after onset of acute pulmonary edema.
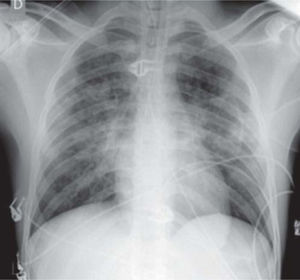
Anteroposterior chest X-ray with the patient seated after resolution of acute pulmonary edema, excluding other pleuropulmonary disease.
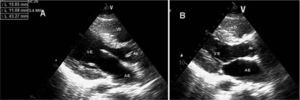
Transthoracic echocardiogram in parasternal long-axis view, in diastole (A) and systole (B), showing no left ventricular dilatation or hypertrophy, preserved systolic function and no structural valve disease. AE: left atrium; Ao: aorta; VD: right ventricle; VE: left ventricle.
Transthoracic echocardiogram in 4-chamber apical view, showing no dilatation of the cardiac chambers. AD: right atrium; AE: left atrium; VD: right ventricle; VE: left ventricle.
APE in the immediate postoperative period can be cardiogenic, following a cardiac event during surgery, or non-cardiogenic, resulting from aspiration or laryngospasm. Post-extubation APE is an example of the latter; it is uncommon, occurring in 0.1% of patients undergoing surgery under general anesthesia 3 . The triggering factor, usually secondary to laryngospasm, is forceful inspiration against the closed glottis, which generates high negative intrathoracic pressures that can reach − 100 cmH 2 O 1-3 .
The high incidence of this form of APE in young men, as shown by published series 2-5 , suggests that considerable muscular strength is required to generate marked negative intrathoracic pressure. As the negative pressure rises, it increases venous return to the right side of the heart, which in turn raises pulmonary venous pressure 1 . A transpulmonary gradient is thus created, with transudation of fluid into pulmonary alveoli and interstices, resulting in the clinical symptoms and signs of APE 1,3 . Mechanical stress also contributes to this situation, due to increased capillary permeability following disruption of alveolar epithelium and pulmonary capillary membranes 1-3 .
APE often resolves itself with support measures and oxygen therapy alone, but in some cases patients need to be reintubated and ventilated, which can be difficult and dangerous due to laryngospasm 2-5 . Even in these circumstances, prognosis is usually excellent, with rapid resolution and complete recovery 2-5 .
The case presented, of a young athlete who developed APE immediately after extubation and in whom the situation was rapidly and completely resolved despite requiring mechanical ventilation, is similar to those reported in the literature 2,4,5 . We have taken this opportunity to briefly review this uncommon but serious complication of upper airway obstruction.
The authors have no conflicts of interest to declare.
- Subscribe to our newsletter
- Send to a friend
- Export reference

- Instructions for authors
- Submit an article
- Ethics in publishing
- Editorial statute

- Current Issue
- Open Access Option
- Language Editing services
- Aims and scope
- Editorial Board
- Advertising
- Most often read
- All metrics
- Open access
- Download PDF
Are you a health professional able to prescribe or dispense drugs?
Você é um profissional de saúde habilitado a prescrever ou dispensar medicamentos
Pulmonary edema
On this page, when to see a doctor, risk factors, complications.
Pulmonary edema is a condition caused by too much fluid in the lungs. This fluid collects in the many air sacs in the lungs, making it difficult to breathe.
In most cases, heart problems cause pulmonary edema. But fluid can collect in the lungs for other reasons. These include pneumonia, contact with certain toxins, medications, trauma to the chest wall, and traveling to or exercising at high elevations.
Pulmonary edema that develops suddenly (acute pulmonary edema) is a medical emergency that needs immediate care. Pulmonary edema can sometimes cause death. Prompt treatment might help. Treatment for pulmonary edema depends on the cause but generally includes additional oxygen and medications.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Pulmonary edema symptoms may appear suddenly or develop over time. Symptoms depend on the type of pulmonary edema.
Sudden (acute) pulmonary edema symptoms
- Difficulty breathing (dyspnea) or extreme shortness of breath that worsens with activity or when lying down
- A feeling of suffocating or drowning that worsens when lying down
- A cough that produces frothy sputum that may have blood in it
- A rapid, irregular heartbeat (palpitations)
- Anxiety, restlessness or a feeling that something bad is about to happen
- Cold, clammy skin
- Wheezing or gasping for breath
Long-term (chronic) pulmonary edema signs and symptoms
- Awakening at night with a cough or breathless feeling that may be relieved by sitting up
- Difficulty breathing with activity or when lying flat
- More shortness of breath than usual when you're physically active
- New or worsening cough
- Rapid weight gain
- Swelling in the legs and feet
High-altitude pulmonary edema (HAPE) signs and symptoms
high-altitude pulmonary edema (HAPE) can occur in adults and children who travel to or exercise at high altitudes. Symptoms are similar to those that occur with acute pulmonary edema and can include:
- Headache, which may be the first symptom
- Shortness of breath with activity, which becomes shortness of breath at rest
- Not being able to exercise as much as you once could
- Dry cough, at first
- Later, a cough that produces frothy sputum that may look pink or have blood in it
- A very fast heartbeat (tachycardia)
Symptoms of high-altitude pulmonary edema (HAPE) tend to get worse at night.
Pulmonary edema that comes on suddenly (acute pulmonary edema) is life-threatening. Call 911 or emergency medical help if you have any of the following acute symptoms:
- Shortness of breath, especially if it comes on suddenly
- Trouble breathing or a feeling of suffocating (dyspnea)
- A bubbly, wheezing or gasping sound when breathing
- Coughing up phlegm that looks pink or has blood in it
- Breathing difficulty with a lot of sweating
- A blue or gray color to the skin
- A big drop in blood pressure that causes lightheadedness, dizziness, weakness or sweating
- A sudden worsening of any of pulmonary edema symptoms
Don't drive yourself to the hospital. Instead, call 911 or emergency medical care and wait for help.

From Mayo Clinic to your inbox
The causes of pulmonary edema vary. Pulmonary edema falls into two categories, depending on where the problem starts.
- If a heart problem causes the pulmonary edema, it's called cardiogenic pulmonary edema. Most often, the fluid buildup in the lungs is due to a heart condition.
- If pulmonary edema is not heart related, it's called noncardiogenic pulmonary edema.
- Sometimes, pulmonary edema can be caused by both a heart problem and a nonheart problem.
Understanding the relationship between the lungs and the heart can help explain why pulmonary edema may occur.
How the lungs work
Lungs contain many small, elastic air sacs called alveoli. With each breath, these air sacs take in oxygen and release carbon dioxide. Typically, this exchange of gases occurs without problems.
But sometimes, the alveoli fill with fluid instead of air. This keeps the bloodstream from taking in oxygen.
How the heart works
The typical heart is made of two upper and two lower chambers. The upper chambers (the right and left atria) receive incoming blood and pump it into the lower chambers (right and left ventricles). The lower chambers pump blood out of the heart.
Typically, blood without oxygen from all over the body enters the right atrium then the right ventricle. From there it's pumped through large blood vessels (pulmonary arteries) to the lungs. There, the blood releases carbon dioxide and picks up oxygen as it flows by the alveoli.
The oxygen-rich blood then returns to the left atrium through the pulmonary veins. It then flows through the mitral valve into the left ventricle. Finally, it leaves the heart through the body's main artery (aorta).
The heart valves keep blood flowing in the right direction. The aortic valve keeps the blood from flowing backward into the heart. From the aorta, the blood travels to the rest of the body.

Chambers and valves of the heart
A typical heart has two upper and two lower chambers. The upper chambers, the right and left atria, receive incoming blood. The lower chambers, the more muscular right and left ventricles, pump blood out of the heart. The heart valves, which keep blood flowing in the right direction, are gates at the chamber openings.
Heart-related (cardiogenic) pulmonary edema
Cardiogenic pulmonary edema is caused by increased pressures in the heart.
It's usually a result of heart failure. When a diseased or overworked left lower heart chamber (left ventricle) can't pump out enough of the blood it gets from the lungs, pressures in the heart go up. The increased pressure pushes fluid through the blood vessel walls into the air sacs.
Medical conditions that can cause heart failure and lead to pulmonary edema include:
Coronary artery disease. Over time, the arteries that supply blood to the heart muscle can become narrow from fatty deposits (plaques). A slow narrowing of the coronary arteries can weaken the left ventricle.
Sometimes, a blood clot forms in one of these narrowed arteries. The clot blocks blood flow and damages part of the heart muscle, resulting in a heart attack. A damaged heart muscle can no longer pump as well as it should.
- Cardiomyopathy. This term means heart muscle damage. With cardiomyopathy, the heart must pump harder, and pressures rise. Then the heart might not be able to work harder when needed, such as during exercise or with an infection or a rise in blood pressure. When the left ventricle can't keep up with the demands that are placed on it, fluid backs up into the lungs.
- Heart valve problems. Narrowing (stenosis) of the aortic or mitral heart valves or a valve that leaks or doesn't close properly affects blood flow into the heart. A valve leak that develops suddenly might cause sudden and severe pulmonary edema.
- High blood pressure (hypertension). Untreated or uncontrolled high blood pressure can enlarge the heart.
- Other heart problems. Inflammation of the heart muscle (myocarditis), heart problems present at birth (congenital heart defects) and irregular heart rhythms (arrhythmias) also may cause pulmonary edema.
- Kidney disease. High blood pressure due to narrowed kidney arteries (renal artery stenosis) or fluid buildup due to kidney disease can cause pulmonary edema.
- Chronic health conditions. Thyroid disease and a buildup of iron (hemochromatosis) or protein (amyloidosis) also may contribute to heart failure and cause pulmonary edema.
Non-heart-related (noncardiogenic) pulmonary edema
Pulmonary edema that is not caused by increased pressures in the heart is called noncardiogenic pulmonary edema.
Causes of noncardiogenic pulmonary edema include:
- Acute respiratory distress syndrome (ARDS). This serious disorder occurs when the lungs suddenly fill with fluid. Many conditions can cause acute respiratory distress syndrome (ARDS), including severe injury (trauma), widespread infection (sepsis), pneumonia and severe bleeding.
- Drug reaction or drug overdose. Many drugs — ranging from aspirin to illegal drugs such as heroin and cocaine — are known to cause pulmonary edema.
- Blood clot in the lungs (pulmonary embolism). A blood clot moving from the blood vessels in the legs to the lungs can cause pulmonary edema.
- Exposure to certain toxins. Inhaling toxins or breathing in some stomach contents when vomiting (aspiration) causes intense irritation of the small airways and air sacs, resulting in fluid buildup.
- High altitudes. Pulmonary edema has been seen in mountain climbers, skiers, hikers and other people who travel to high elevations, usually above 8,000 feet (about 2,400 meters). High-altitude pulmonary edema (HAPE) generally occurs in those who don't take the days or weeks needed to become used to the elevation. But people who live at high altitudes can get HAPE with no elevation change if they have a respiratory illness.
- Near drowning. Inhaling water causes fluid buildup in the lungs.
- Negative pressure pulmonary edema. A blocked upper airway causes negative pressure in the lungs from trying to breathe through the blockage. With treatment, most people with this type of pulmonary edema recover in about 24 hours.
- Nervous system conditions or surgeries. A type of pulmonary edema called neurogenic pulmonary edema can occur after a head injury, seizure or brain surgery.
- Smoke inhalation. Smoke from a fire contains chemicals that damage the membrane between the air sacs and the capillaries. The damage allows fluid to enter the lungs.
- Transfusion-related lung injury. Blood transfusions may cause fluid overload in the left ventricle, leading to pulmonary edema.
- Viral illnesses. Viruses such as the hantavirus and dengue virus can cause pulmonary edema.

- High-altitude pulmonary edema
Air sacs in the lungs, called alveoli, take in oxygen and release carbon dioxide. In high-altitude pulmonary edema (HAPE), it's believed that blood vessels in the lungs squeeze together (constrict), increasing pressure. This causes fluid to leak from the blood vessels to the lung tissues and eventually into the air sacs.
Heart failure and other heart conditions that raise pressure in the heart increase the risk of pulmonary edema. Risk factors for heart failure include:
- Irregular heart rhythms (arrhythmias)
- Alcohol use
- Congenital heart disease
- Coronary artery disease
- Heart valve disease
- High blood pressure
- Sleep apnea
Some nervous system conditions and lung damage due to near drowning, drug use, inhaling smoke, viral illnesses and blood clots also raise the risk.
People who travel to high-altitude locations above 8,000 feet (about 2,400 meters) are more likely to develop high-altitude pulmonary edema (HAPE). It usually affects those who don't take the time — a few days to a week or more — to get used to the elevation.
Children who already have pulmonary hypertension and structural heart defects may be more likely to get HAPE .
Complications of pulmonary edema depend on the cause.
In general, if pulmonary edema continues, the pressure in the pulmonary artery can rise (pulmonary hypertension). Eventually, the heart becomes weak and begins to fail, and pressures in the heart and lungs go up.
Pulmonary edema complications may include:
- Breathing difficulty
- Swelling of the legs, feet and belly area
- Buildup of fluid in the membranes that surround the lungs (pleural effusion)
- Congestion and swelling of the liver
Immediate treatment is necessary for acute pulmonary edema to prevent death.
You may be able to prevent pulmonary edema by managing existing heart or lung conditions and following a healthy lifestyle.
For example, controlling cholesterol and blood pressure can help lower the risk of heart disease. Follow these tips to keep your heart healthy:
- Eat a healthy diet rich in fresh fruits, vegetables, whole grains, fat-free or low-fat dairy, and a variety of proteins.
- Don't smoke.
- Get regular exercise.
- Limit salt and alcohol.
- Manage stress.
- Manage weight.
Preventing high-altitude pulmonary edema (HAPE)
To prevent HAPE , gradually ascend to high elevations. Although recommendations vary, most experts advise increasing elevation no more than 1,000 to 1,200 feet (about 300 to 360 meters) a day once you reach 8,200 feet (about 2,500 meters).
Some climbers take prescription medications such as acetazolamide or nifedipine (Procardia) to help prevent symptoms of HAPE . To prevent HAPE , start taking the medication at least one day before ascent. Ask your health care provider how long you need to take the medication after you've arrived at your high-altitude destination.
May 27, 2022
- Mason RJ, et al. Pulmonary edema. In: Murray and Nadel's Textbook of Respiratory Medicine. 6th ed. Saunders Elsevier; 2016. https://www.clinicalkey.com. Accessed April 2, 2022.
- Pinto DS, et al. Pathophysiology of cardiogenic pulmonary edema. https://www.uptodate.com/contents/search. Accessed April 2, 2022.
- Ferri FF. Pulmonary edema. In: Ferri's Clinical Advisor 2022. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 2, 2022.
- Givertz MM. Noncardiogenic pulmonary edema. https://www.uptodate.com/contents/search. Accessed April 2, 2022.
- Wemple M, et al. Neurogenic pulmonary edema. https://www.uptodate.com/contents/search. Accessed April 2, 2022.
- What is heart failure? National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/heart-failure. Accessed Sept. 11, 2020.
- Pulmonary edema. Merck Manual Professional Version. https://www.merckmanuals.com/professional/cardiovascular-disorders/heart-failure/pulmonary-edema. Accessed April 2, 2022.
- Tintinalli JE, et al., eds. High altitude disorders. In: Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw Hill; 2020. http://accessmedicine.mhmedical.com. Accessed Sept. 11, 2020.
- Din-Lovinescu C, et al. Systematic review of negative pressure pulmonary edema in otolaryngology procedures. The Annals of Otology, Rhinology, and Laryngology. 2020; doi:10.1177/0003489420938817.
- Giesenhagen AM, et al. High altitude pulmonary edema in children: A single referral center evaluation. Journal of Pediatrics. 2019; doi:10.1016/j.jpeds.2019.02.028.
- What is ARDS? National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/ards. Accessed Sept. 11, 2020.
- What is the heart? National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/how-heart-works. Accessed Sept. 11, 2020.
- 2015-2020 Dietary Guidelines for Americans. U.S. Department of Health and Human Services and U.S. Department of Agriculture. https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines. Accessed Sept. 11, 2020.
- Pulse oximetry. American Lung Association. http://www.lung.org/lung-health-and-diseases/lung-procedures-and-tests/pulse-oximetry.html. Accessed Sept. 11, 2020.
- What is coronary heart disease? National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/coronary-heart-disease. Accessed Sept. 11, 2020.
- Loscalzo J, et al., eds. Cardiogenic shock and pulmonary edema. In: Harrison's Principles of Internal Medicine. 21st ed. McGraw-Hill; 2022. https://accessmedicine.mhmedical.com. Accessed April 4, 2022.
- Conde MV, et al. Overview of the management of postoperative pulmonary complications. https://www.uptodate.com/contents/search. Accessed April 2, 2022.
- Levitzky MG. Blood flow to the lung. In: Pulmonary Physiology. 9th ed. McGraw Hill; 2018. https://accessmedicine.mhmedical.com. Accessed Sept. 14, 2020.
- Olson EJ (expert opinion). Mayo Clinic. Sept. 15, 2020.
- Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2017; doi:10.1161/CIR.0.
- Diseases & Conditions
- Pulmonary edema symptoms & causes
More Information
Associated procedures.
- Cardiac catheterization
- Chest X-rays
- Echocardiogram
- Electrocardiogram (ECG or EKG)
CON-XXXXXXXX
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
Fatal diving: could it be an immersion pulmonary edema? Case report
- Case Report
- Open access
- Published: 14 March 2022
- Volume 136 , pages 713–717, ( 2022 )
Cite this article
You have full access to this open access article

- France Evain ORCID: orcid.org/0000-0001-5303-4835 1 ,
- Pierre Louge 2 ,
- Rodrigue Pignel 2 ,
- Tony Fracasso 1 &
- Frédéric Rouyer 3
2733 Accesses
4 Citations
1 Altmetric
Explore all metrics
Immersion pulmonary edema is a rare, underrecognized, and potentially lethal pathology developing during scuba diving and other immersion-related activities (swimming or apnoea). Physiopathology is complex and not fully understood, but its mechanisms involve an alteration of the alveolo-capillary barrier caused by transcapillary pressure elevation during immersion, leading to an accumulation of fluid and blood in the alveolar space. Diagnosis remains a challenge for clinicians and forensic practionner. The symptoms begin during ascent, with cough, frothy sputum, and hemoptysis. Auscultation reveals signs of pulmonary edema. Pulmonary CT scan, which is the radiological exam of choice, shows ground glass opacities and interlobular thickening, eventually demonstrating a patterned distribution, likely in the anterior segments of both lungs. Apart from the support of vital functions, there is no specific treatment and hyperbaric oxygen therapy is not systematically recommended. We present a case of fatal IPE occurring in a recreational diver who unfortunately died shortly after his last dive. Diagnosis was made after complete forensic investigations including post-mortem-computed tomography, complete forensic autopsy, histological examination, and toxicological analysis.
Similar content being viewed by others

Pulmonary Barotrauma of Diving
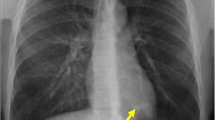
A diver’s dilemma – a case report on bronchopulmonary sequestration

Immersion Pulmonary Edema
Avoid common mistakes on your manuscript.
Introduction
Most diving injuries and deaths are related to decompression injuries. Immersion pulmonary edema (IPE) was first described in 1989 [ 1 ]. This rare and acute pathology develops during scuba diving or other immersion-related activities (swimming and apnoea) [ 2 , 3 ], even in individuals in general good health [ 2 , 4 ], but more often in males over 50 years of age [ 5 , 6 ]. The symptoms begin during ascent, with cough, frothy sputum, and hemoptysis [ 5 ]. Pulmonary CT scan shows ground glass opacities and interlobular thickening, eventually demonstrating a patterned distribution [ 5 , 6 , 7 ], likely in the anterior segments of both lungs. The occurrence of IPE is favored by cold water, hypertension, strenuous exercise, and thigh wetsuits. Its incidence is estimated at 1.1% of all diving accidents [ 3 , 7 , 8 , 9 , 10 ]. However, IPE is largely underdiagnosed, since it can remain asymptomatic, and is often underrecognized by medical and forensic practitioners [ 6 ]. The recurrence rate is estimated at 30%, although former divers may have stopped this activity after the first episode [ 3 , 5 , 8 , 11 ]. Symptoms often develop within 10 min after immersion and worsen during ascent [ 6 , 12 ]. Patients classically experience dyspnoea, frothy sputum, and/or haemoptysis, with possible dizziness, loss of consciousness, and even death [ 3 , 4 , 7 , 8 , 10 ].
Case presentation
One morning in late December, a 56-year-old man in generally good health was diving in Geneva Lake with an experienced diver using air open-circuit and was dressed with a neoprene wet diving suit. He was a recreational diver who started this activity 6 months prior. At this time of year, the water temperature was rather cold (mean temperature during the dive, 7 °C).
After 10 min at 19.5 m under the surface, he started to cough and had difficulty to breath. Both divers started an ascent, without violation of the decompression procedure and keeping the regulator in their mouths. When he arrived at the surface, he was spitting up blood. The total diving time was 13 min. Back on the boat, he lost consciousness and had cardiac arrest.
A rescuer present on the boat immediately performed basic life support (BLS) (no-flow = 0 min). The patient arrived on the beach 5 min later undergoing BLS, and a resuscitation team with an emergency physician immediately started advanced cardiac life support (ACLS).
The first cardiac rhythm identified was non-shockable. Cardiopulmonary resuscitation (CPR) was conducted according to guidelines. The patient presented with an alternating shockable and non-shockable rhythms and was intubated. There was bloody foam in the oropharynx. The patient was transported by helicopter to Geneva University Hospitals, which is the only hospital in the area equipped with a hyperbaric chamber. Advanced CPR was continued during the flight. On admission to the emergency department, low-flow was estimated at 84 min. Pulseless electrical activity (PEA) alternating with ventricular fibrillation episodes requiring two electric shocks was recorded. No cardiac mechanical activity was identified during a focused cardiac ultrasound performed during resuscitation. Arterial blood gas analysis showed severe metabolic acidosis (pH = 6.38 adjusted to temperature, normal 7.35–7.45) with hyperlactatemia (27 mmol/L, normal: 0.5–1.6 mmol/L). After multidisciplinary discussion, the option of implanting an ECMO was not chosen. Death was pronounced 20 min after his admission to the hospital and 104 min of unsuccessful CPR.
A medicolegal autopsy was performed the day after. According to police investigations, the diving equipment did not show any dysfunction. Post-mortem computed tomography (PMCT) revealed diffuse bilateral interstitial thickening, panlobar air space consolidations in the anterior segments of each lobe (Fig. 1 ), and pulmonary emphysema.
Post-mortem computed tomography (PMCT) showing diffuse bilateral interstitial thickening and panlobar air space consolidations in anterior segments of each lobes
Gross autopsy findings included mild obesity (body mass index of 33 kg/m 2 ), small amount of foam and blood in the distal airways, heavy and firm lungs (940 g right and 820 g left), global cardiac hypertrophy (540 g, normalized for BMI), fatty streaks at the pulmonary and coronary arteries, and nonspecific stress/agonal-related findings (subpleural petechiae, pinpoint hemorrhages of gastric mucosa). Neither typical signs of drowning (such as emphysema aquosum or subpleural hemorrhages known as Paltauf spots) nor fresh myocardial damage were found at gross observation and microscopic examination. The histology of the lungs showed intra-alveolar hemorrhage, fluid in the alveolar spaces (Fig. 2 ), and a few hemosiderophages. Post-mortem biochemistry revealed a normal NT-proBNP level at 132 ng/L (normal < 900 ng/L for patients 50 to 75 years old) and elevated level of hs-troponin T (1319 ng/L, normal < 14 ng/L) due to hemolysis of the sample. Toxicological analyses were negative.
Upper left lobe lung parenchyma showing intra-alveolar hemorrhage (star) with fluid in alveolar spaces (arrow) (hematoxylin and eosin, × 200)
In many cases of diving fatalities, IPE initiating cardiac decompensation or loss of consciousness in water is suspected. As a result, it is difficult to interpret eventual drowning-related findings at autopsy. In our case, the diving device remained in the mouth of the victim and the initial loss of consciousness occurred on the support boat.
Lung lesions found at autopsy could also be attributed to CPR, but their location and type are not typical of those usually found [ 13 , 14 ]. Post-mortem examination revealed no typical signs of drowning. Therefore, the lesions observed at autopsy and virtopsy can only be secondary to IPE.
IPE is an underrecognized pathology in swimming and scuba diving activities. Confusion with other scuba diving pathologies is frequent but the treatment of IPE, when isolated, does not require systematic hyperbaric oxygen therapy.
IPE stems from anatomical and functional alterations of the alveolo-capillary barrier secondary to elevation of transcapillary pressure following immersion, with subsequent capillary damage (capillary stress failure) and transudation of fluid and blood into the alveolar space [ 6 ]. Several mechanisms are proposed:
Immersion increases hydrostatic pressure on the body, leading to central redistribution of peripheral circulation (blood pooling) with elevation of pulmonary circulation and cardiac output [ 15 ]. Intensification of ventricular preload and afterload, as well as central blood pooling, leads to drastic elevation of the pulmonary capillary pressure and capillary stress failure [ 5 , 16 ], with leakage of fluid and blood into the alveolar space [ 11 , 17 ]. This phenomenon can eventually be intensified by exposition to cold water (peripheral vasoconstriction) [ 7 ], hypertension, cardiac hypertrophy, emotional stress, thigh diving suit, overhydration [ 18 ], and strenuous exercise [ 5 ].
Hydrostatic pressure with central blood pooling following immersion causes an increase of work in the right ventricle, a mismatch of output volume between both ventricles [ 7 , 14 , 16 ], and an increase of pulmonary capillary pressure. The latter results in capillary stress failure and edema formation. Left ventricular dysfunction and valvular disease [ 4 , 16 ] may foster mismatch of output volume and occurrence of IPE.
Elevation of pulmonary blood pressure and ambient hydrostatic pressure during immersion lessens lung compliance [ 6 ]. Moreover, the air inhaled from the diving cylinder is dense and its flowing resistance is high [ 5 ]. These elements result in an enhancement of respiratory work with diminution of alveolar pressure during inspiration, stretching of alveolar walls [ 5 ], and an increase of transcapillary pressure, leading to capillary damage [ 3 , 15 ]. A maladjusted regulator (inducing further airflow resistance), emotional stress (associated with hyperpnoea and/or jerky breathing), and exercise may enhance respiratory work and foster capillary damage [ 5 ].
Symptoms of IPE are known to worsen during the ascent [ 6 , 12 ]. This can be explained by the natural evolution of the disease, damage of pulmonary capillaries due to filtration of air bubbles during decompression (according to Henry’s law), and expansion of intrathoracic gas (according to Boyle’s law) with redistribution of pulmonary edema. Moreover, the drop of alveolar pressure further increases the already elevated alveolo-capillary pressure gradient, worsening capillary damage [ 6 , 12 ].
The diagnosis relies on clinical history (beginning of symptoms during the dive and worsening during ascent, cough, frothy sputum, and haemoptysis) [ 5 ]. Furthermore, pulmonary CT scan shows ground glass opacities, dilatation of pulmonary veins, interlobular thickening, and/or pleural effusion [ 6 ]. Ground glass opacities and interlobular thickening may demonstrate a patterned distribution [ 5 , 6 , 7 ], likely in the anterior segments of both lungs as in the case described above. This can be explained by the manifestation of pulmonary lesions in a sloping position (areas of greater transmural pressure during the dive) combined with the horizontal position of the diver in the water [ 6 ]. Blood testing (whether ante- or post-mortem) includes cardiac biomarkers (troponins, BNP), which are usually elevated (as opposed to decompression sickness) [ 3 , 10 ]. However, normal levels of these biomarkers do not exclude the diagnosis, since their elevation takes several hours [ 2 , 3 ].
The first measure is to remove the victim from the water to release the effects of hydrostatic pressure, removal of the wetsuit, and warming up to lessen central blood pooling [ 19 ]. Decompression stops should be respected if possible but should not delay the diver’s ascent and exit from the water. If no life-threatening symptom is suspected, the victim should be placed in the sitting position and receive highly concentrated oxygen with a facial mask before being transported to a hospital with an intensive care unit and a hyperbaric chamber. Invasive or non-invasive ventilation (NIV) with continuous positive airway pressure can be useful to ensure correct oxygenation and treat pulmonary edema. Hyperbaric oxygen therapy is indicated only in case of associated decompression sickness. A continuous infusion of nitroglycerin could be indicated in order to reduce right ventricular preload and left ventricular afterload. Diuretics are not systematically indicated due to the frequent dehydration associated with swimming and scuba diving activities. Inhaled beta 2 agonist drugs could be an alternative favoring water alveolar clearance. More data is required to collect proof of efficacy and standardize the treatment of IPE. In most cases, the patient remains in the hospital for 2–3 days and is banned from diving and other swimming activities for 1 month. Further investigations must be carried out to look for an unknown comorbidity, such as hypertension, cardiomyopathy, diabetes, or dyslipidaemia. Evaluation of respiratory function is recommended [ 20 ]. Fostering factors should be avoided (dive in temperate water, well-fitted wetsuit, set regulator, limitation in depth and duration of diving, and avoidance of overhydration and hyperoxia).
IPE is a rare and underestimated pathology in scuba diving and swimming activities, but it can lead to a fatal issue. Post-mortem diagnosis of IPE is challenging. It rests on police investigation (technical expertise in order to exclude malfunction of diving devices, diving profile analysis, audition of witnesses, water temperature), access to medical information (classical cough, frothy sputum and haemoptysis, worsening during ascent), complete medicolegal autopsy (including histological examination, notably of the lungs) to exclude alternative cause of death, signs of drowning and assess comorbid conditions, and toxicological analysis. PMCT is an important diagnosis tool and shows characteristic ground glass opacities and interlobular thickening of the lungs, often demonstrating a distribution in the anterior segments of both lungs.
Wilmshurst PT, Crowther A, Nuri M et al (1989) Cold-induced pulmonary oedema in scuba divers and swimmers and subsequant development of hypertension. The Lancet 8629:62–65. https://doi.org/10.1016/s0140-6736(89)91426-8
Article Google Scholar
Hageman SM, Chakraborty RK, Murphy-Lavoie HM (2021) Immersion pulmonary edema. StatPearls Publishing, Treasure Island
Google Scholar
Louge P, Coulange M, Beneton F et al (2016) Pathophysiological and diagnostic implications of cardiac biomarkers and antidiuretic hormone release in distinguishing immersion pulmonary edema from decompression sickness. Medicine 95(26):e4060. https://doi.org/10.1097/md.0000000000004060
Article CAS PubMed PubMed Central Google Scholar
Peacher DF, Martina SD, Otteni CE et al (2015) Immersion pulmonary edema and comorbidities: case series and updated review. Med Sci Sports Exerc 47:1128–1134. https://doi.org/10.1249/mss.0000000000000524
Article PubMed Google Scholar
Coulange M, Boussuges A, Barthelemy A et al (2015) Oedème pulmonaire en plongée sous-marine: facteurs de risques individuels et contraintes environnementales. Bull Medsuhyp 47:1128–1134
Gempp E (2016) Œdème pulmonaire en plongée sous-marine. Arch Mal Vaiss Prat 76:1–5
Kumar M, Thompson PD (2019) A literature review of immersion pulmonary edema. Phys Sportsmed 47:148–151. https://doi.org/10.1080/00913847.2018.1546104
Edmonds C (2009) Scuba divers’s pulmonary oedema. A review. Diving Hyperb Med 39:232–3
PubMed Google Scholar
Slade JB, Hattori T, Ray CS et al (2001) Pulmonary edema associated with scuba diving: case reports and review. Chest 120:1686–1694. https://doi.org/10.1378/chest.120.5.1686
Marlinge M, Deharo P, Joulia F et al (2018) Troponins in scuba divers with immersion pulmonary edema. Biosci Rep 38:5. https://doi.org/10.1042/bsr20181024
Article CAS Google Scholar
Vinkel J, Bak P, JuelThiis Knudsen P et al (2018) Forensic case reports presenting immersion pulmonary edema as a differential diagnosis in fatal diving accidents. J Forensic Sci 63:299–304. https://doi.org/10.1111/1556-4029.13526
Edmonds C (2016) The evolution of scuba divers pulmonary edema. Undersea Hyperb Med 43:83–91
Ondruschka B, Baier C, Bayer R et al (2018) Chest compression-associated injuries in cardiac arrest patients treated with manual chest compressions versus automated chest compression devices (LUCAS II) - a forensic autopsy-based comparison. Forensic Sci Med Pathol 14:515–525
Hashimoto Y, Moriya F, Furumiya J (2007) Forensic aspects of complications resulting from cardiopulmonary resuscitation. Leg Med 9:94–99. https://doi.org/10.1016/j.legalmed.2006.11.008
Bates ML, Farrell ET, Eldridge MW (2011) The curious question of exercise-induced pulmonary edema. Pulm Med 2011:361931. https://doi.org/10.1155/2011/361931
Article PubMed PubMed Central Google Scholar
Castagna O, Gempp E, Poyet R et al (2017) Cardiovascular mechanisms of extravascular lung water accumulation in divers. Am J of Cardiol 119:929–932. https://doi.org/10.1016/j.amjcard.2016.11.050
Koehle MS, Lepawsky M, McKenzie DC (2005) Pulmonary oedema of immersion. Sports Med 35:183–190. https://doi.org/10.2165/00007256-200535030-00001
Smart DR, Sage M, Davis FM (2014) Two fatal cases of immersion pulmonary oedema - using dive accident investigation to assist the forensic pathologist. Diving Hyperb Med 44:97–100
Gempp E, Louge P, Blatteau J-E (2016) Diving-induced pulmonary edema. Sci Sports 31:362–367
Gempp E, Demaistre S, Louge P (2014) Hypertension is predictive of recurrent immersion pulmonary edema in scuba divers. Int J Cardiol 172:528–529. https://doi.org/10.1016/j.ijcard.2014.01.021
Download references
Open access funding provided by University of Geneva
Author information
Authors and affiliations.
Forensic Pathology, University Center of Legal Medicine, Geneva University Hospitals and University of Geneva, rue Michel-Servet 1, CH-1211, Geneva 4, Switzerland
France Evain & Tony Fracasso
Acute Medicine Department, Hyperbaric Medicine Unit, Geneva University Hospitals, rue Gabrielle-Perret-Gentil 4, 1205, Geneva, Switzerland
Pierre Louge & Rodrigue Pignel
Critical Care Unit of the Emergency Division, Acute Medicine Department, Geneva University Hospitals, rue Gabrielle-Perret-Gentil 4, 1205, Geneva, Switzerland
Frédéric Rouyer
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to France Evain .
Ethics declarations
Ethical statement.
The authors are accountable for all aspect of the work in ensuring that questions related to the accuracy of any part of the work are appropriately investigated and resolved. Formal authorization for anonymous publication of the case was delivered by the local prosecutor (patient informed consent not applicable).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Evain, F., Louge, P., Pignel, R. et al. Fatal diving: could it be an immersion pulmonary edema? Case report. Int J Legal Med 136 , 713–717 (2022). https://doi.org/10.1007/s00414-022-02809-x
Download citation
Received : 04 January 2022
Accepted : 28 February 2022
Published : 14 March 2022
Issue Date : May 2022
DOI : https://doi.org/10.1007/s00414-022-02809-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case report
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- JNMA J Nepal Med Assoc
- v.58(227); 2020 Jul

Negative Pressure Pulmonary Edema: A Case Report
Anisha budhathoki.
1 Department of Anesthesiology, Third affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Negative pressure pulmonary edema is an uncommon complication of the extubation of the endotracheal tube. An increase in intrathoracic pressure and negative pressure of the lung caused by acute laryngeal spasm results from acute upper respiratory obstruction causing life-threatening pulmonary edema by alveolar-capillary damage is called negative pressure pulmonary edema. We here describe 28-years old female case the preoperative diagnosis of pelvic inflammatory disease undergoing exploratory laporoscopy caused negative pressure pulmonary edema while extubation. With the immediate treatment, the patient was discharged without any abnormalities.
INTRODUCTION
Negative pressure pulmonary edema (NPPE) is noncardiogenic pulmonary edema, induce due to an increase in intrathoracic pressure and negative pressure of the lung as a result of acute laryngeal spasm during extubation. NPPE is a life-threatening condition during the extubation after general anesthesia. The prevalence of NPPE occurs in 0.05% to 0.1%. 1 It usually occurs in young healthy patients and during extubation due to which it is also called “post-extubation pulmonary edema.” 2 We here discuss a case of 28 years old female case with a preoperative diagnosis of pelvic inflammatory disease occurring NPPE during extubation.
CASE REPORT
A 28-years old lady presented to the third affiliated hospital of Guangzhou Medical University with complaints of severe abdominal pain in the lower abdomen and pelvic, fever, and pain during intercourse for 3 months. Normal past medical history without any comorbidity. There was no history of any past surgery, trauma, allergies, smoking, drug addiction, and infectious diseases. No specific family history was found.
On physical examination, there was tenderness in lower abdominal parts and pale skin. All the other preoperative assessments including laboratory examinations, imaging examinations, and physical examinations were under normal range.
She was scheduled for exploratory laparoscopy surgery with general anesthesia. The patient was fasted for 12 hours. American society of anesthesiologists (ASA) of degree I with height 155 cm, weight 70 kg was reported. Her Mallampati grade was II degree. Preoperative vital signs were stable with blood pressure of 110/60 mmHg, heart rate 60/m, and SpO 2 100%.
For Anesthesia induction drugs used sufentany l20 μg, propofol 120 mg, rocurium 50 mg, perecoxib 40 mg, remifentanyl 0.1 μg/kg/min continuous infusion, and desflurance 5% continuous infusion. Operation start time 8:45 am. Her vital signs during the operation were stable. EtCO 2 was under normal range. The minimum alveolar concentration (MAC) was 6 to 6.2. Intraabdominal carbondioxide pressure was less than 15 mmHg. Rocurium 10 mg was given with 40 mins interval. Total operation duration was 2 hours. A total of 1500 mL of ringer lactate solution was administered during the surgery, the urine output was approximately 200 mL, and blood loss was less than 20 mL.
Extubation was done at 11:00 am with self spontaneous breathing observation, tidal volume of 400 L, and when she obeyed the command of opening eyes. Right after extubation patient had breathing difficulties, with mask ventilation positive pressure SpO 2 was 88%. But it was ineffective, the further deterioration of SpO 2 was observed with 75% to 60%. Pink frothy sputum was found around the mouth and during suction. Atropine 0.5 mg, neostigmine 1 mg was given. Bilateral pulmonary rales were auscultated at the time. With failure to raise in SpO 2 patient was reintubated at 11:17 am with sevoflurance 1.8% continuous inhalation, fursemide 20 mg, methyprednisolone 80 mg, aminophyline 0.125 mg, dexmedetomide 20 ug, and ephidrine 6 mg was given. The SpO 2 gradually returned to normal and the patient was taken to the Post-operative care unit (PACU) with an endotracheal tube. In PACU physical warmer, cis-atracurium 5 mg was used with continuous observation. The blood gas analysis and chest X-ray was taken immediately. The vital signs and urine output was normal in PACU. After 4 hours of observation, the patient was extubated successfully. The patient was sent to the ward without any discomfort and abnormalities.
A chest X-ray is taken when the patient was sent to PACU and after 4 hours ( Figure 1 ). The chest X-ray at the left side taken when the patient was sent to PACU showed bilateral diffuse, bilateral, hazy, interstitial opacity, pulmonary infiltration throughout both lungs. The chest X-ray at the right side taken after 4 hours patient was sent to PACU showed resolution of the process.

ABG at left side taken when the patient was sent to PACU and right side was taken after 4 hours showed improved SpO 2 ( Table 1 ).
| Parameters | ABG at PACU | ABG after 4 hours |
|---|---|---|
| Base excess (BE) | 1.0 mmol/L | 1.0 mmol/L |
| Calcium (Ca) | 1.10 mmol/L | 1.10 mmol/L |
| Chlorine (Cl) | 101 mEq/L | 102 mEq/L |
| Glucose (Glu) | 7.3 mmol/L | 7 mmol/L |
| Haemoglobin (Hb) | 123 g/dl | 120 g/dl |
| Bicarbonate (Hco ) | 24 mEq/L | 23.3 mEq/L |
| Haematocrit (Hct) | 36% | 37% |
| Potassium (K) | 3.86 mEq/L | 3.96 mEq/L |
| Sodium (Na ) | 140 mEq/L | 143 mEq/L |
| Partial pressure of carbondioxide (PaCO ) | 44 mmHg | 42 mmHg |
| Acidity (pH) | 7.32 | 7.35 |
| Partial pressure of oxygen (PaO ) | 60 mmHg | 100 mmHg |
| Oxygen saturation (SPO ) | 70% | 97.6% |
After the patient was sent to ward ABG report was found under normal ranges, timely vitals, and urine output observation was done. The blood gas analysis (ABG) and chest X-ray was taken regularly with no suspicion and abnormalities. Then the patient was discharged without any abnormalities after 3 days.
Negative pressure pulmonary edema is an uncommon complication of the extubation of the endotracheal tube. NPPE has been reported mainly by anesthetists as a consequence of postoperative laryngospasm. 3 The pathophysiology of NPPE begins with acute laryngeal spasm leading to severe upper airway obstruction. Intrathoracic pressure during acute upper airway obstruction is (> -100 cm H 2 O) whereas normal inspiratory chest pressure is (-2 to -5 cm H 2 O). The absolute value of intrathoracic transpulmonary negative pressure leads to the reduce of alveolar-capillary membrane pressure causing disruption of capillary barrier and alveolar epithelial barrier. All these causes exudation of blood vessels, which makes the red blood cells leak into the alveoli and causes significant lung or bronchial bleeding. These changes lead to red blood cell filtration into the lung and pulmonary edema. 4 The increase in pulmonary blood volume occurs due to the increase in systemic arterial pressure secondary to the release of norepinephrine in response to hypoxia, hypercapnia, and agitation. Frothy pink pulmonary secretions is one of the most important syndrome to diagnose NPPE during extubation. And before making the diagnosis of NPPE, other causes of pulmonary edema particularly those requiring a rapid intervention (fluid maldistribution, anaphylaxis, and cardiogenic pulmonary edema), must be considered. 5 The main diagnosis is done by incidence and symptoms, chest radiograph for pulmonary edema, and measure of pulmonary edema fluid/plasma protein ration. With proper treatment, NPPE can rapidly dissipate. The most important treatments include re-establishment of the airway by relief airway obstruction. Adequate oxygenation should be given, encourage the patient to cough after extubation. Application of positive airway pressure via face mask or LMA or Endotracheal intubation with ventilator support. Although NPPE does not result from fluid overload, it is recommended gentle diuresis using low-dose furosemide. 6 The clinical syndromes can usually improves after 12-24 hours. 7 But in our case, the symptoms were improved within 4 hours without any abnormalities. Patients with NPPE history should be kept in mind and prevention methods can be performed accordingly. It is also believed that the incidence of EPPN is higher than that found in the literature due to the fact that the cases are poorly diagnosed and, in many cases, confused with pulmonary edema due to fluid overload, aspiration pneumonitis and bronchospasm. 8
ACKNOWLEDGEMENTS
We would like to thank Dr. Liu Jie for his valuable support and guidance.
JNMA Case Report Consent Form was signedby the patient and the original article is attached withthe patient's chart.
Conflict of Interest

Pulmonary edema - old left ventricular infarct
Citation, doi, disclosures and case data.
At the time the case was submitted for publication Henry Knipe had the following disclosures:
- Integral Diagnostics, Shareholder (ongoing)
- Micro-X Ltd, Shareholder (ongoing)
These were assessed during peer review and were determined to not be relevant to the changes that were made.
Presentation
Breathlessness.
Patient Data
Pulmonary venous congestion with peribronchial cuffing and mid to lower zone Kerley B lines . Widened cardiac silhouette. Curvilinear calcification over the left ventricular apex. Cardiac pacemaker/AICD. Coronary stent.
Case Discussion
Findings of interstitial pulmonary edema with the likely cause in this case heart failure with the curvilinear calcification at left ventricular apex typical for an old myocardial infarct .
5 public playlists include this case
- chest x-ray 2 by Emil Michalski
- cardiac by Emil Michalski
- Cardiovascular by Saurabh Srivastava
- X RAY by Saeed Moradi
- Ax by Dr. Mo
Related Radiopaedia articles
- Cardiac calcification
- Ischaemic cardiomyopathy
- Myocardial infarction
- Pulmonary interstitial oedema
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers


IMAGES
VIDEO
COMMENTS
Cardiogenic pulmonary edema is typically characterized by dyspnea, hypoxemia, and bilateral opacities. ... There are no findings on imaging studies that would suggest a chronic fibrotic process ...
Unilateral pulmonary edema is an uncommon condition and is a rare clinical entity that is often misdiagnosed at the initial stages. In a majority of patients it occurs in the upper lobe of the right lung. There are many causes of unilateral pulmonary edema, but the commonest is the presence of a grade 3 mitral regurgitation. Due to its rare presentation, a high index of suspicion is required ...
Presentation of Case. Dr. Jonathan E. Eisen: A 47-year-old woman presented to this hospital early during the pandemic of coronavirus disease 2019 (Covid-19), the disease caused by severe acute ...
Discussion. Pulmonary oedema is one of the most common and potentially fatal forms of acute respiratory distress. The clinical presentation is characterised by the development of dyspnoea, associated with rapid accumulation of protein-poor fluid within the lung's interstitial and/or alveolar spaces, almost always the result of acutely elevated cardiac filling pressures. 1 However, the ...
CT Pulmonary Angiogram. ct. Airspace opacity in a central peribronchovascular distribution classic of acute pulmonary edema. There is also smooth thickening of the interlobular septae in the lung bases and apices consistent with interstitial pulmonary edema and correlating with the radiographic finding of Kerley lines.
Conclusion: We report the first case of pulmonary edema following diuretic therapy to stress the need of physicians to follow guidelines of clinical practice. Maintaining an appropriate volume status and treatment of β-receptor blockers is the key to reversing the progress of this adverse effect. In this process, POCUS is a reliable diagnostic ...
Abstract. Background: Unilateral pulmonary edema is an uncommon condition and is a rare clinical entity that is often misdiagnosed at the initial stages. In a majority of patients it occurs in the upper lobe of the right lung. There are many causes of unilateral pulmonary edema, but the commonest is the presence of a grade 3 mitral regurgitation.
Pulmonary edema can be defined as an abnormal accumulation of extravascular fluid in the lung parenchyma. This process leads to diminished gas exchange at the alveolar level, progressing to potentially causing respiratory failure. Its etiology is either due to a cardiogenic process with the inability to remove sufficient blood away from the pulmonary circulation or non-cardiogenic precipitated ...
The edema fluid to plasma protein ratio is an additional method to discriminate between cardiogenic pulmonary edema and acute lung injury. Ware et al .2compared protein concentration (Biuret method) in the pulmonary edema fluid (taken via a suction catheter inserted into the endotracheal tube) and blood. Using a predefined cutoff of 0.65, the ...
Pulmonary edema is the accumulation of excessive fluid in the alveolar walls and alveolar spaces of the lungs. It can be a life-threatening condition in some patients with high mortality and requires immediate assessment and management. This activity reviews the pathophysiology, clinical presentation, evaluation, and management of cardiogenic ...
Case Discussion Classic example of the lines (A to C) Kerley described in pulmonary edema. Kerley D lines are diagnosed on a lateral radiograph. All of these lines are now termed septal lines. Not a bad example of bat wing edema either! Case courtesy of Dr Nivene Saad.
Chest x-ray 10 days earlier. x-ray. Single lead permanent pacemaker (PPM) in situ. Heart is enlarged, and there is some prominence of the pulmonary vasculature, without evidence of interstitial or alveolar edema.
A 70-year-old man with a history of hypertension, hyperlipidemia, type 2 diabetes, heart failure with preserved ejection fraction, and atrial fibrillation presented to an outside hospital for sudden onset of shortness of breath. He denied chest pain, progressive dyspnea, weight gain, or other symptoms. After being stabilized with positive ...
This case was a 5-year-old girl with tumor on saddle area, her hormones level were abnormal preoperatively, such as cortisol, adrenocorticotrophic hormone, free T4 and total T4. During the stage of induction, negative pressure pulmonary edema took place due to mild upper airway obstruction. And the instant chest Computer tomography proved ...
Introduction. Although acute pulmonary edema (APE) as a complication of extubation is uncommon, it has been well characterized. It occurs most frequently in young healthy individuals in the period immediately after extubation, and its severity can worsen the prognosis of low-risk surgery, require invasive treatment and delay hospital discharge 1-3.The high negative intrathoracic pressure ...
This patient presented with acute onset of dyspnea. The frontal chest radiograph is the key to diagnosis of acute pulmonary edema. It shows evidence of both interstitial and alveolar edema. Alveolar edema manifests as ill-defined nodular opacities tending to confluence (see image with arrows). Interstitial edema can be seen as peripheral septal ...
A blood clot moving from the blood vessels in the legs to the lungs can cause pulmonary edema. Exposure to certain toxins. Inhaling toxins or breathing in some stomach contents when vomiting (aspiration) causes intense irritation of the small airways and air sacs, resulting in fluid buildup. High altitudes.
Immersion pulmonary edema (IPE) is used by many as an umbrella term for scuba divers' pulmonary edema (SDPE) and swimming-induced pulmonary edema (SIPE). Both of these conditions share similar presenting symptoms, diagnostics, and treatment. Case studies from the 1980s to the present time highlight differences in pathophysiology and certain ...
Immersion pulmonary edema is a rare, underrecognized, and potentially lethal pathology developing during scuba diving and other immersion-related activities (swimming or apnoea). Physiopathology is complex and not fully understood, but its mechanisms involve an alteration of the alveolo-capillary barrier caused by transcapillary pressure elevation during immersion, leading to an accumulation ...
Negative pressure pulmonary edema is an uncommon complication of the extubation of the endotracheal tube. NPPE has been reported mainly by anesthetists as a consequence of postoperative laryngospasm. 3 The pathophysiology of NPPE begins with acute laryngeal spasm leading to severe upper airway obstruction. Intrathoracic pressure during acute ...
Knipe H, Pulmonary edema - old left ventricular infarct. Case study, Radiopaedia.org (Accessed on 07 Jun 2024) https://doi.org/10.53347/rID-189728