Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Cell culture articles from across Nature Portfolio
Cell culture is a method for growing or maintaining cells in vitro under controlled conditions. Primary cell cultures refer to dispersed cells that are cultured directly from tissues and have limited lifespan, whereas cell lines refer to immortalized cells that can be cultured indefinitely.
Latest Research and Reviews
Imidazolium salt’s toxic effects in larvae and cells of Aedes aegypti and Aedes albopictus (Diptera: Culicidae)
- Wellington Junior da Silva
- Leonardo Francisco Diel
- Onilda Santos da Silva
Influence of relatively short-term culture on adult porcine islets for xenotransplantation
- Naoaki Sakata
- Gumpei Yoshimatsu
- Shohta Kodama
Hepatocytes differentiate into intestinal epithelial cells through a hybrid epithelial/mesenchymal cell state in culture
Hepatocytes dedifferentiate into progenitor-like cells in culture. Here, authors elucidate the dynamics and mechanisms of hepatocyte dedifferentiation and find an unexpected differentiation potential of hepatocytes into intestinal epithelial cells.
- Shizuka Miura
- Kenichi Horisawa
- Atsushi Suzuki
Reprogramming fibroblast into human iBlastoids
A protocol for the generation of induced blastoids, an in vitro integrated model of the human blastocyst derived via somatic reprogramming. This model overcomes restrictions associated with the use of human blastocysts in embryology research.
- Jia Ping Tan
- Xiaodong Liu
- Jose M. Polo
ReLo is a simple and rapid colocalization assay to identify and characterize direct protein–protein interactions
Characterising interactions between proteins that are large and poorly soluble remains challenging. Here, the authors describe ReLo, a rapid and versatile eukaryotic cell culture-based method for detecting and studying direct interactions between structurally complex proteins.
- Harpreet Kaur Salgania
- Mandy Jeske
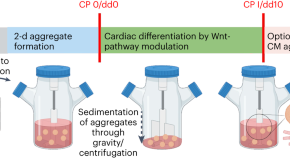
Standardized production of hPSC-derived cardiomyocyte aggregates in stirred spinner flasks
We present a protocol for achieving efficient generation of hPSC-CM aggregates in suspension culture, emphasizing process simplicity, robustness and GMP compliance. The strategy promotes clinical translation and other applications that require large numbers of CMs.
- Nils Kriedemann
- Wiebke Triebert
- Robert Zweigerdt
News and Comment
Setting standards for stem cells.
New traceability and reporting standards aim to improve transparency in stem cell research.
Organoid systems to recapitulate cervical cancer
Human cervical organoids mimic dynamics of cancer and sexually transmitted infections.
- Madhura Mukhopadhyay
Human tonsils in a dish
Human tonsil organoids recapitulate key features of the antigen-specific B cell response.
T cell development in a dish
Mind your media
Cell culture media are typically selected on the basis of common laboratory practices but have major effects on the validity, reproducibility and physiological relevance of the scientific findings. We provide arguments and quantitative examples of why choosing an appropriate cell culture medium matters, particularly in metabolic studies.
- Shoval Lagziel
- Eyal Gottlieb
- Tomer Shlomi
Making limb-like structures from mouse PSCs
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 11 September 2022
Three-dimensional in vitro culture models in oncology research
- Camille Jubelin 1 , 2 , 3 na1 ,
- Javier Muñoz-Garcia 1 , 2 na1 ,
- Laurent Griscom 4 ,
- Denis Cochonneau 2 ,
- Emilie Ollivier 2 ,
- Marie-Françoise Heymann 2 ,
- François M. Vallette 5 ,
- Lisa Oliver 5 , 6 &
- Dominique Heymann ORCID: orcid.org/0000-0001-7777-0669 1 , 2 , 7
Cell & Bioscience volume 12 , Article number: 155 ( 2022 ) Cite this article
14k Accesses
91 Citations
2 Altmetric
Metrics details
Cancer is a multifactorial disease that is responsible for 10 million deaths per year. The intra- and inter-heterogeneity of malignant tumors make it difficult to develop single targeted approaches. Similarly, their diversity requires various models to investigate the mechanisms involved in cancer initiation, progression, drug resistance and recurrence. Of the in vitro cell-based models, monolayer adherent (also known as 2D culture) cell cultures have been used for the longest time. However, it appears that they are often less appropriate than the three-dimensional (3D) cell culture approach for mimicking the biological behavior of tumor cells, in particular the mechanisms leading to therapeutic escape and drug resistance. Multicellular tumor spheroids are widely used to study cancers in 3D, and can be generated by a multiplicity of techniques, such as liquid-based and scaffold-based 3D cultures, microfluidics and bioprinting. Organoids are more complex 3D models than multicellular tumor spheroids because they are generated from stem cells isolated from patients and are considered as powerful tools to reproduce the disease development in vitro. The present review provides an overview of the various 3D culture models that have been set up to study cancer development and drug response. The advantages of 3D models compared to 2D cell cultures, the limitations, and the fields of application of these models and their techniques of production are also discussed.
Introduction
With around 10 million deaths per year, cancer is one of the main causes of mortality in industrial countries after cardiovascular diseases and is consequently a major public health problem [ 1 ]. The decline in overall cancer mortality observed over the last two decades can be attributed more to better prevention and early detection methods than to breakthroughs in treatment even though significant pharmacological and therapeutic progress has been made [ 2 ]. However, these successes vary greatly depending on the type of cancer [ 3 ]. Cancer is a multifactorial disease arising from cells that adopt abnormal behaviors, including sustained proliferative molecular networks (e.g. drug resistance, cell death resistance, angiogenesis) and immune evasion properties leading to the replicative immortality and invasion/migration characteristics associated with metastases [ 4 ].
Malignant tumors are most often considered as heterogeneous tissues including inter- and intra-tumor heterogeneity [ 5 , 6 ]. Inter-tumor heterogeneity refers to the composition and organization diversity observed for a given type of cancer between patients. Intra-tumor heterogeneity corresponds to the intrinsic cellular and molecular heterogeneity within tumor tissue. Intra-tumor heterogeneity does not only refer to the clonal diversity of cancer cells, but also to the heterogeneity of the tumor microenvironment [ 7 ]. Both are frequently amplified by selective pressures, and particularly by therapies that lead to the acquisition of drug resistance profiles in the cancer cells responsible for therapeutic failure [ 8 , 9 ]. Intra-tumor heterogeneity can be considered a dynamic process (e.g. modification of genetic features through selection mechanisms, as well as morphological and phenotypical changes over time and space) that impose continual therapeutic adaptation.
To investigate the mechanisms involved in cancer initiation, progression and drug resistance, diverse models have been proposed. In vivo models (e.g. mice, rats, dogs, monkeys, pig) are based on spontaneous or induced malignant tumors (e.g. inoculation of cancer cells). Spontaneous models consist of de novo generation of cancer, either using genetically engineered animal models (GEMs) that reproduce germline mutations observed in patients [ 10 ], or subjecting animals to radiation, virus or chemical carcinogens [ 11 , 12 ]. The spontaneous models have the advantage of mimicking all the steps in tumor growth in immunocompetent mice, however, the tumors generated are the result of oncogenic events that may be quite different from the natural history of cancer cells. In addition, the tumor microenvironment remains of murine origin, which may limit some therapeutic developments. Allografts or xenografts are faster methods for generating tumors in vivo. However, the frequency of tumor development may be lower, and once again the local microenvironment is murine [ 13 ]. In vivo experiments make it possible to investigate cancer in a highly complex microenvironment similar but not identical to that observed in patients, but their drawbacks include complex analyses, major ethical concerns, and such experiments are time- and resource-consuming requiring trained staff.
In vitro cultures entail growing cells derived from multicellular organisms in plastic or glass culture dishes. In vitro cultures have the advantage of being highly controllable, making possible easily repeatable experiments, being mostly inexpensive, and leading to a vast range of applications. The most widely used in vitro technique is two-dimensional (2D) cell culture, where cells grow as adherent monolayers on culture vessels. However, 2D cultures remain over-simplified models that do not mimic tissue organization in vivo, and in particular the tumor microenvironment (TME). The TME is composed of cellular components, such as immune cells (T cells, B cells, Natural killer cells, macrophages, neutrophils, dendritic cells) and stromal cells (endothelial cells, cancer-associated fibroblasts (CAFs), adipocytes), and of non-cellular components such as the extracellular matrix (ECM) which is a network of macromolecules (mainly proteoglycans, collagen, laminin, elastin, fibronectin, and enzymes), water, cytokines and growth factors [ 14 , 15 , 16 ]. The composition and proportion of each component of the TME depend on the tumor host tissue, the type of cancer and the patient [ 17 , 18 , 19 ]. The TME has been shown to play a major role in tumorigenesis, cancer progression and cancer resistance, and should be taken into account when studying cancer in vitro. The enrichment of 2D cell cultures with other cell types from the TME and/or non-cellular components improves the 2D culture models by partially reproducing the in vivo microenvironment. But 2D cell cultures cannot depict the dimensional organization of a complete tumor mass. Three-dimensional (3D) culture models have the potential to bridge the gap between 2D in vitro models and in vivo models. The present review aims to describe the main 3D culture models currently available for studying cancer development and drug screening in oncology, and to discuss their added value and specific limitations.
In vitro cancer modelling: 2D or not 2D?
Cancer cells cultured in 2D do not have the same morphology, heterogeneous phenotype, extracellular matrix (ECM) or gene and protein expression profile as cells cultured in 3D.
2D cell cultures do not depict the biological realities of tumors
After tissue cultures were established by Harrison in the early twentieth century [ 20 ], mammalian cell cultures developed rapidly with various culture dishes with or without treated surfaces, different cell culture media, and the creation of cell repositories around the world. Because 2D adherent monolayer cell cultures (in temperature, hygrometry and CO 2 controlled environment) produce reproducible results and are easy to implement, inexpensive, and easy to analyze, they have become a widely used tool in biological studies and as well as being the standard for in vitro cancer research. Admittedly, 2D cultures have contributed tremendously to expand knowledge of cancer; however, it has become obvious that these simplistic models cannot depict the biological reality of tumors and their TME.
All the limitations of 2D cultures are inherently linked to the proliferation of the cells as an adherent monolayer on a plastic surface, as opposed to the 3D arrangement observed in vivo, and the morphology is the main differential feature between the culture modes. In 2D, adherent cells spread on the surface of the culture dishes with a flattened morphology. These cells have the ability to translate the mechanical forces of their environment into biochemical signals through mechano-transduction [ 21 ]. It has been shown in noncancerous cell lines that the cells can detect the stiffness of the substrate to which they attach [ 22 ], leading to cytoskeleton remodeling [ 23 ], differential proliferation and cell death [ 24 ]. In the case of chondrocytes, their spreading when cultured as adherent monolayers was associated with the initiation of a dedifferentiated phenotype and cytoskeletal changes in comparison to the in vivo and 3D cell culture phenotype [ 25 , 26 ].
In addition, the physical organization of the monolayer greatly limits cell-to-cell interactions and prevents the formation of a transport gradient. In 2D cultures, cells have equal and unlimited access to the nutrients and their response to molecular cues such as chemotherapy is different from the in vivo situation. Furthermore, the spatial and molecular composition of the ECM produced by cancer cells is strongly affected by the culture method [ 27 ]. The ECM plays a crucial role in tumorigenesis, tumor progression, and migration by modulating signaling events through contact and growth factors binding to cell-surface receptors [ 16 , 28 , 29 , 30 , 31 , 32 , 33 ]. In 3D cultures and in vivo , cells are present as multilayers and as such are not exposed to the same concentration of oxygen, nutrients and signaling molecules depending on their distance from blood vessels. Various physiological processes are based on this transport gradient, i.e. proliferation and angiogenesis. In this context, to stay close to the in vivo tumor organization, pseudo-3D cell cultures (also called 2.5D cell cultures) were developed, such as growing cells as an adherent monolayer on ECM-coated culture vessels [ 34 ], using micropatterned platforms [ 35 ] or culturing cancer cell spheroids on top of a cell-sheet [ 36 ]. However, these pseudo-3D cell cultures do not efficiently replicate tumors and their microenvironment, because all the cells are still attached to their substrate on one side with the other side exposed to the liquid media.
Taking a step further in mimicking the disease with spheroids and organoids
Spheroids are generated either by self-assembling cancer cell lines in suspension or dissociating patient tumor tissue. They are easy to produce and handle, and they are especially powerful for studying micrometastases or avascular tumors [ 37 , 38 ]. It is possible to improve their relevance by integrating cells from the TME (e.g. CAFs, immune cells, vascular cells) into the spheroids [ 39 , 40 ]. For all these reasons, since their first use in 1970 by Inch et al . [ 41 ], spheroids are the most used 3D model in cancer research.
However, spheroids remain simple models that only partially represent the in vivo organization and microenvironment of tumors. Organoids are more complex 3D models. Organoids are self-organized organotypic cultures that arise from tissue-specific adult stem cells (ASC), embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC). ESC- or iPSC-derived organoids make it possible to generate the complex structure of adult organs in which all cells are fully differentiated. They may contain mesenchymal, epithelial, and even endothelial components [ 42 ]. However, these organoids tend to retain fetal properties and contain cells that should not be found in this type of tissue [ 43 ]. On the other hand, ASC-derived organoids are not as complex and can only be generated from adult tissues that retain regenerative properties, but they better reproduce adult tissues [ 44 ]. Although ESC- and iPSC-derived organoids are particularly useful for studying organogenesis and genetic pathologies, ASC-derived organoids have the advantage of mimicking both physiological and pathological adult tissues such as tumors. In 2009, Sato et al . developed a protocol for producing organoids of the intestinal epithelium by growing leucine-rich repeat-containing G protein-coupled receptor 5 positive (LGR5+) intestinal stem cells in medium containing stem cell niche-recapitulating and tissue-specific growth factors. At the same time, another organoid culture method was proposed by Ootani et al . [ 45 ]. Instead of growing isolated stem cells in a submerged manner, they used the Boyden chambers system. Tissue fragments containing both epithelial and stromal cells were embedded in a layer of ECM gels in direct contact with air. This layer sat on a porous membrane that allowed nutrients to diffuse from the bottom compartment containing medium. The advantage of this method over the submerged method is that it allows better oxygenation of the organoids and thus grows large multicellular organoids. Moreover, the stromal cells are enough to support organoid survival and no growth factor supplementation is required. In both culture methods, matrix is required to grow the organoids.
A search for primary articles in PUBMED concerning organoids used in cancer research with the query [(((Cancer) OR (Neoplasms)) AND ((organoid) OR (tumoroid))) NOT (Review)] yields the results of 261 publications on this theme between 2011 and 2015, and 1693 between 2016 and 2020. A similar search using the query [(((Cancer) OR (Neoplasms)) AND ((spheroid) OR (tumorosphere))) NOT (Review)] displays 2,078 publications about spheroids and cancer between 2011 and 2015, and 4,126 between 2016 and 2020. While spheroid research led to almost twice as many publications in the past 5 years compared to the first half of the decade, the publication rate on organoids increased more than six times in the same time interval (Fig. 1 ). This underlines the increasing interest of the scientific community in organoids as oncology research models.
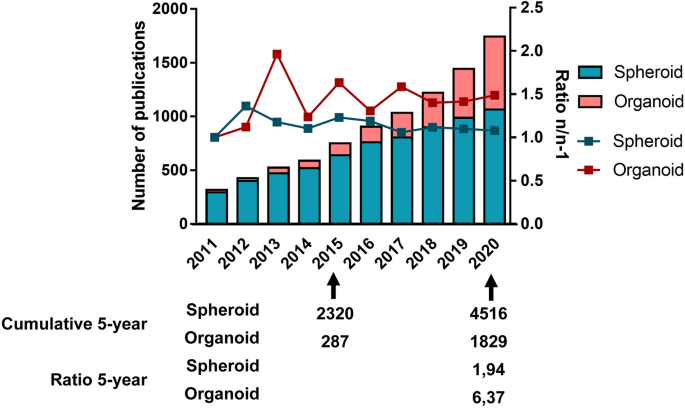
Comparison of the publication rate on spheroids and organoids over the past decade. For spheroids, the query used was: [(((Cancer) OR (Neoplasms)) AND ((spheroid) OR (tumorosphere))) NOT (Review)]. For organoids, the query used was: [(((Cancer) OR (Neoplasms)) AND ((organoid) OR (tumoroid))) NOT (Review)]
Why and how 3D can bridge the gap between in vitro and in vivo studies
A tumor mass is characterized by the coexistence of a heterogenic population of cancer cells in close contact with the ECM and a particular microenvironment. In this context, 3D cell cultures in the form of spheroids or organoids form a biological tool capable of replicating the cellular heterogeneity present in tumors—as opposed to the homogeneity observed in 2D cell cultures. Moreover, as the transport gradient of oxygen, nutrients and cellular waste is generally limited to 150–200 µm, spheroids or organoids with a diameter of more than 500 µm present a stratified structure with a proliferating cell population localized at their periphery and a core which is composed of non-dividing and necrotic cells [ 46 ]. This spherical organization is very similar to what is observed in vivo in avascular tumor masses and is related to drug sensitivity. In addition, 3D cell cultures at least partially reproduce the tumor microenvironment by restoring cell-to-cell and cell-to-ECM interaction. For instance, the cell adhesion molecule e-cadherin, which generates epithelial cell–cell binding and is also involved in cancer initiation and progression, was shown to be present in higher levels in epithelial breast carcinoma MCF-7 cells or colon adenocarcinoma Lovo cells when cultured in 3D, which also resulted in higher chemo-resistance to cisplatin, 5-fluorouracil and Adriamycin. When these cells were treated with an anti-e-cadherin neutralizing antibody, chemosensitivity was restored and the result was similar to that detected in 2D cultures [ 47 ]. Similarly, cell-to-ECM interactions are involved in drug resistance [ 48 ].
3D cultures greatly contributed to better understanding various aspects of cancer biology, such as tumor progression, tumor microenvironment, gene and protein expression, pro-oncogenic signaling pathways, and drug resistance. 3D cultures also seem to be a promising platform for drug developments and screenings, including immunotherapies.
Tumor progression
Tumor progression involves various processes, including carcinogenesis, angiogenesis and metastasis. For each of these mechanisms, the tumor microenvironment is a key player. Roulis et al . demonstrated the role of the microenvironment on the initiation of colorectal cancer (CRC) in mice and patients. Using organoids containing epithelial and mesenchymal cells, these authors showed that colorectal carcinogenesis of mutated stem cells is controlled by neighboring Ptsg2-expressing fibroblasts through paracrine signaling. These fibroblasts secrete prostaglandin E2 (PGE2) that dephosphorylates the oncoprotein Yes-associated protein (YAP) in the stem cells, leading to YAP nuclear translocation, where it can activate the genes involved in cell proliferation and inhibit apoptotic genes. Roulis et al . also observed that the culture of the organoids with PGE2 drives the formation of a spheroid-like structure that is associated with poor differentiation and increased stemness, whereas inhibition of the PGE2 receptor in stem cells allowed organoids to form budding organoids with crypt-villus architecture [ 49 ]. Vasculogenesis is a physiological process observed during organ development and regeneration by which endothelial cells vascularize tissues in an organotypic manner. It is also a process found in cancer. Palikuqi et al . transduced adult endothelial cells with the ETS Variant Transcription Factor 2(ETV2) gene, which is normally only expressed during vasculogenesis in embryos. These authors observed that ETV2 expression allowed endothelial cells to (re)acquire vasculogenic and adaptable properties. When grown in 3D in a matrix composed of a mixture of laminin, entactin and type-IV collagen (LEC matrix), this ETV-expressing endothelial cells were able to form stable vessels. The authors call them R-VEC for ‘reset’ vascular endothelial cells. Using a microfluidic device base in fibrin gel, R-VEC cells were able to grow into a sprouting vascular network that supported gravity-perfused transport of whole blood without losing its integrity. In in vivo experiments in mice, R-VEC cells injected into LEC matrix formed durable non-leaky vessels that were anastomosing to the mouse vasculature. Moreover, R-VEC co-cultured with organoids were able to arborize these organoids. Palikuqi et al . observed differences in the clustering pattern and gene expression of R-VECs when co-cultured with healthy colon-organoids (COs) or with patient-derived colorectal cancer (CRC) organoids. A signature typical of tumor endothelial cells was detected when R-VECs were co-cultured with CRC organoids. With this work, Palikuqi et al . established a model of vasculogenesis that could help study the vascular niches of tumors [ 50 ]. The metastatic process is a multi-step event that involves migration of cancer cells from the primary tumor site through vasculature, invasion into secondary sites and metastatic development. During migration, cancer cells have to make their way into a confining environment. By culturing single breast cancer cells into a hydrogel made of basement membrane and alginate with tunable plasticity, Wisdom et al . showed that cancer cells were able to deform the matrix through the mechanical action of their invadopodia and migrate into the pore they carved. When lowering the plasticity of the matrix, this protease-independent migration was reduced [ 51 ]. To study collective cell migration [ 52 ], which is another form of migration than the traditional single cell migration described in the metastatic process, Huang et al . co-cultured tumorigenic breast cancer cell lines and non-tumorigenic epithelial breast cell lines as spheroids that they embedded into a collagen matrix inside a microfluidic platform. They observed that the architecture of the spheroids varied over time and that this organization was responsible for different invasive properties, underlying the importance of 3D co-culture models for fully understanding the role of cell–cell interaction in the migration process [ 53 ].
Signaling pathways
Differences in cancer cell behavior from 3D compared to 2D cultures come from changes in signaling pathways. Shifts in the catabolism pathways used by cancer cells can be observed when cultured in 2D vs. 3D [ 54 ]. Transformed MCF10A H-Ras mammary epithelial cells and human and murine breast cancer cell lines consumed proline when grown in 3D, whereas they secreted proline in 2D. This proline catabolism helped 3D growth by sustaining ATP production. On the other hand, non-transformed mammary epithelial cells did not use this pathway during acini formation in 3D, implying that proline catabolism is specific to breast cancer cells cultured in 3D. Furthermore, proline catabolism is higher in metastases compared to primary breast cancers in patients and mice, and inhibition of this pathway impairs the formation of lung metastases in mice. Therefore, by growing cancer cells in 3D, Elia et al . discovered a potential drug target against metastasis formation in breast cancer [ 54 ]. The secretome profile of cancer cells is also different in 2D or 3D. Hela cervical cancer cells cultured in 3D secreted extracellular vesicles (EVs) with a miRNA profile much more similar (96%) to cervical cancer patients’ circulating EVs, compared to 2D-derived EVs. The culture condition, however, did not affect the DNA profile of the EVs. Taken together, these results suggest that 3D culture methods may be more relevant for studying the miRNA secretome profile of cancer cells [ 55 ]. Similarly, other signaling pathways involved in cancer are affected by the method of culture and can explain differences in treatment responses observed in traditional 2D cultures compared to in vivo . When cultured in 3D, various colon cancer cell lines displayed a reduced AKT/mTOR/S6K pathway compared to 2D cultures, which is a signaling pathway that is involved in carcinogenesis, cancer cell migration and resistance to treatments [ 56 ]. 3D cultures of ER + /Her2 + breast cancer cell lines promote switching of the AKT to MAPK pathway, leading to reduced sensitivity to treatments [ 57 ].
Gene and protein expression
The gradient of nutrients, oxygen and cellular waste, the hypoxic conditions, the cell-to-cell and cell-to-ECM interactions generated in 3D cultures are all molecular cues that affect cellular physiology and, in turn, alter the gene and protein expression that can be involved in drug resistance. Zschenker et al . observed differences in gene expression profiles between 2 and 3D cell cultures. When cultured in 3D, A549 lung tumor cells and UT-SCC15 squamous carcinoma cells showed better survival rates after radio- and chemotherapy as compared to 2D culture conditions. In addition, the cancer cells grown in 3D also displayed significant altered gene expression with regard to tissue development, cell adhesion and defense responses [ 58 ]. Changes in gene expression profiles that are involved in mechanisms such as autophagy, stemness, cell cycle, apoptosis, cell migration and invasion have also been described [ 59 , 60 , 61 , 62 ]. The microenvironment is also involved in differential gene expression. CRC organoids cultured alone lacked the gene expression involved in cell–cell communication with the microenvironment that were detected in the original tumor tissues. When the CRC organoids were co-cultured with CAFs, the organoids started to re-express in a patient-dependent manner various genes that were present in the original tumor tissue and that have been reported as holding oncogenic functions [ 63 ]. Moreover, spheroid and organoid models mimic the in vivo tumor [ 64 , 65 , 66 , 67 ]. Genetic analyses have shown that organoids established from biopsies of metastatic CRC patients were similar to their initial metastasis. Ninety percent of somatic mutations were shared between tumor biopsy and organoid cultures, and none of the mutations that are specific to the tumor or organoid model are found in driver genes or genes that could serve as a drug target [ 66 ]. The histological and cytological properties, markers and mutations observed in the tumors of rectal cancer patients with or without metastatic disease and treated or not were retained by organoids derived from these different patients in a tissue-of-origin manner. [ 67 ].
Drug discovery and screening, immunotherapies, and personalized medicine
Drug discovery is another interesting perspective for 3D cell cultures. The development of drug candidates is a long and stringent process that goes through in vitro and in vivo tests before reaching clinical trials. During this process, hundreds of promising drugs end up being a failure [ 68 ]. Better screening of the drug candidates early in the development process may raise the success rate and represent a major economic benefit. 3D cell cultures may be a relevant screening model, especially since cells cultured in 2D and 3D do not respond similarly to treatments [ 69 ].
Firstly, the limited diffusion distance within the spheroids does not only concern biological molecules, but also therapeutic agents [ 70 , 71 ]. When comparing the penetration of doxorubicin into hepatocellular carcinoma C3A cells grown in an adherent monolayer versus C3A spheroids, cell nuclei in 3D cultures integrated less doxorubicin than in 2D cultures [ 72 ]. Similar results were described using HeLa cells [ 73 ]. These results correlate with the resistance to chemotherapeutics that can be observed in vivo. Secondly, tumor hypoxia contributes significantly to the failure of conventional anti-cancer treatments. The hypoxia-inducible factor (HIF) is stabilized by hypoxic conditions that contribute to anti-cancer drug resistance, such as increasing drug efflux, inducing anti-apoptosis effects, favoring genetic instability or reducing cell proliferation [ 74 ]. Lastly, as most anti-cancer drugs target fast-dividing cancer cells (e.g. cisplatin, paclitaxel, doxorubicin, 5-fluorouracil), they are thus inefficient on the quiescent cells inside the spheroids. The sensitivity of ER-positive, HER2-amplified and triple-negative breast cancer cell lines to paclitaxel, doxorubicin and 5-fluorouracil was compared between 2 and 3D cultures [ 38 ]. The cell lines BT-549, BT-474 and T-47D that aggregated into dense spheroids showed relative resistance to paclitaxel and doxorubicin compared to 2D cell cultures. This was partially attributed to the hypoxia-induced cell-cycle arrest. Some of the cell lines that developed dense spheroids had a lower Ki-67 labelling index, which correlates with a greater number of cells in the G0 phase of the cell cycle [ 69 ].
Tumor organoids also have the potential to be an in vitro avatar for patient tumors by reproducing their molecular and phenotypic heterogeneity. In this sense, tumor organoids could be used to predict responses in individual patients in a personalized medicine manner. Ganesh et al. were able to generate 65 organoids derived from RC patients and observed a correlation between clinical and tumoroid responses to chemo- and radiotherapies. They further established an in vivo model of RC organoid xenografted mice that reproduced cancer progression. Although the cohort is small, it is very promising for evaluating patients’ responses to therapeutics and adapting their treatments [ 67 ]. This was further validated by Pasch et al . with tumor organoids derived from various cancers (i.e., breast, colorectal, lung, neuroendocrine, ovarian, pancreatic and prostate), biopsy sample types (i.e., core needle biopsies, paracentesis or surgery) and clinical settings (i.e., patient underwent chemotherapy and/or radiotherapy or not) [ 75 ].
Immune cells can be found in the tumor microenvironment and cancer cells often hijack their pathways to create an immunosuppressive environment that will protect them. Of all the types of chemotherapy, immunotherapy is a rapidly progressing field that also benefits from 3D cultures. Using T cells obtained from peripheral blood mononuclear cells (PBMC) and tumor cells from resection taken from the same patients, Dijkstra et al. developed a co-culture platform of patient-derived autologous T cells and mismatch repair-deficient CRC or non-small lung cancer (NSLC) organoids. With this co-culture model, they successfully expanded circulating tumor-reactive T cells that could potentially be used for adoptive T cell transfer [ 76 ]. Tumor spheroids can also help to evaluate anti-tumor drug-induced immune responses. For example, exposing spheroids from CRC cell lines co-cultured with Vδ2 T cells from healthy donors to Zoledronate or Cetuximab triggers Vδ2 T cells to kill CRC cells [ 77 ]. Following their unprecedented efficiency against hematological malignancies, the use of chimeric antigen receptor T (CAR-T) cells has recently been approved by the U.S. Food and Drug Administration (FDA) as an immunotherapy against B-cell acute lymphoblastic leukemia [ 78 ] and diffuse large B cell lymphoma [ 79 ]. CAR-T cells are genetically engineered T cells that target tumor-specific antigens. To evaluate the lethal properties of CAR-T cells that target the EGFRvIII variant commonly found in glioblastomas, Jacob et al . produced glioblastoma organoids (GBOs) that retain the cellular heterogeneity of the patient tumors from which they are derived. When co-cultured together for 72 h, they observed that CAR-T cells were able to infiltrate the organoids and specifically kill the EGFRvIII + cancer cells, while ignoring the EGFRvII I− cells. These results underline the potential of GBOs for measuring antigen-specific CAR-T cell treatment responses depending on the patients [ 80 ].
Most drug candidates that are successful in preclinical in vivo models do not lead to efficient drugs when assessed in humans [ 81 , 82 ]. This underlines the fact that, even if animals are an unavoidable step in setting up clinical trials, species specificity is important. Concerning what has been discussed above, 3D models could help ensure the 3Rs (Replacement, Reduction and Refinement) by eliminating drug candidates that do not work in vitro in 3D cultures and by reducing the number of animals used.
Types of 3D culture and their application in oncology research
When talking about in vitro 3D cultures, it is important to clearly define each model. Firstly, the 3D culture can be homotypic (only one type of cell is present) or heterotypic (two or more types of cell are present) and are called monoculture and co-culture respectively. Secondly, the cells used can be either established cell lines or primary cells. Established cell lines have acquired homogeneous genotypes and phenotypes following multiple passages. They are easier to handle, but less representative of the tissue from which they originate. On the other hand, primary cells are obtained directly from the tissue of a donor and better represent the biological variability between individuals. However, some of these primary cells may have a limited lifespan and be harder to maintain in culture. This could be the case for the cells present in the tumor microenvironment that do not replicate indefinitely, like CAFs, or stem cells which can differentiate in vitro. It should however be noted that huge improvements have been made in the culture and preservation of organoids, even making it possible to establish organoid biobanks of various cancers such as CRC [ 83 ], breast cancer [ 84 ], prostate cancer [ 85 ], lung cancer [ 86 ] or glioblastoma [ 80 ]. Finally, 3D cancer models can be categorized according to the method used to produce the 3D cultures and their final organization: (1) organ-slice cultures, obtained from cultured sections of tumor tissues [ 87 , 88 ]; (2) multi-layered cell cultures, cultures of adherent cells with the property of growing in multilayers after confluence [ 89 , 90 ]; (3) spherical models corresponding to tumor cells growing in spheres. Spherical models have now been used for several decades but a precise nomenclature of the different types of 3D model has not been clearly established and some terms are used interchangeably in the literature [ 91 ]. Here, we will focus on spherical models, which include spheroids and organoids, and which can be generated thanks to liquid-, scaffold-based 3D culture methods or other techniques such as microfluidic platforms or bioprinting.
Liquid-based 3D culture systems
The main characteristic of liquid-based 3D culture systems is that cell adherence to the substrate is restricted by coating the culture vessel, using gravity, or by creating fluid movement to stop the cells from settling at the bottom of the culture vessel. In these conditions, cell–cell interaction is supported, and cells can aggregate. Therefore, liquid-based 3D culture systems are mostly used to generate spheroids, whose inherent morphology reproduces the physical and chemical gradients observed in solid tumors such as pH, oxygen gradient and soluble factors. Because of these particularities, spheroids may be good mimics of avascular tumors and micrometastases. Tumor growth can be divided into two phases: avascular and vascular. The avascular phase corresponds to tumors that receive nutrients and oxygen from the surrounding tissue, limiting their growth. These avascular tumors have cellular heterogeneity similar to that observed in spheroids cultured in vitro, with a necrotic core surrounded by dormant or slow-cycling cells and a ring of fast-dividing cells at the periphery [ 46 ]. Micrometastases are small clusters of cells (between 0.2 and 2 mm in size) that spread from the primary tumor and seed to other tissues [ 92 ]. Micrometastases are mostly avascular and require an angiogenic switch to grow into macrometastases (size > 2 mm) [ 93 ]. In the last two decades, a theory concerning the existence of a subpopulation of cancer cells known as cancer stem-like cells (CSCs) has emerged [ 94 , 95 , 96 ]. Even if it remains a highly debated topic in the scientific community [ 97 ], there is evidence of the existence of cancer cells with self-renewing [ 94 ], differentiation [ 96 ], and tumorigenicity properties [ 95 ], much like healthy stem cells. CSCs seems to contribute to treatment resistance and may thus be important targets for new anti-cancer therapies [ 94 , 95 , 96 ]. As for normal stem cell, CSCs can be identified and enriched by using a sphere-forming assay, which involves liquid-based 3D culture methods [ 98 ].
Liquid-overlay 3D culture models are for the most part simple to use, relatively low-priced, and are easily scalable for high throughput screening (HTS). Moreover, spheroids generated in a liquid environment are exposed to similar conditions (culture media density of around 1 g/cm 3 and viscosity of 0.7–1 mPa s) as blood compartments (viscosity of 3.5–5.5 mPa s and density of 1.0–1.1 g/cm 3 ) [ 99 , 100 , 101 , 102 ]. Otherwise, this liquid surrounding is very different from the environment that surround tumors in vivo, which should be taken into consideration when designing an experiment.
Liquid overlay culture
Liquid overlay culture is the simplest technique for producing spheroids. Cells are seeded on a non-adhesive surface, which promotes cell–cell adhesion, leading to spontaneous spheroid formation (Fig. 2 A). To create this non-adherent condition, the culture vessels can be coated with substrates such as agarose or poly-hydroxymethyl methacrylate [ 103 , 104 , 105 , 106 ]. Non-adherent culture vessels (with low attachment properties) are now commercially available (e.g. Greiner CELLSTAR ® , Corning ® Costar ® Ultra-Low Attachment Surface, Nunclon™ Sphera™). The liquid overlay culture method does not require any specialized equipment and is easily implementable. It can be used to evaluate the stemness of cancer cells through the spheroid formation assay. Zhang et al . observed that 64% of the samples from patients with breast cancer and who underwent therapies were enriched with the ROR1 orphan receptor, which is known to be associated with cancer stemness. When cultured with the liquid overlay technique, ROR1 high samples had a better capacity for forming spheroids than ROR1 low samples. This data was supported by other functional assays such as invasion, engraftment in immune-deficient mice, and survival after treatment, confirming the stemness characteristics of the ROR1 high samples, which may therefore be a target of interest [ 107 ].
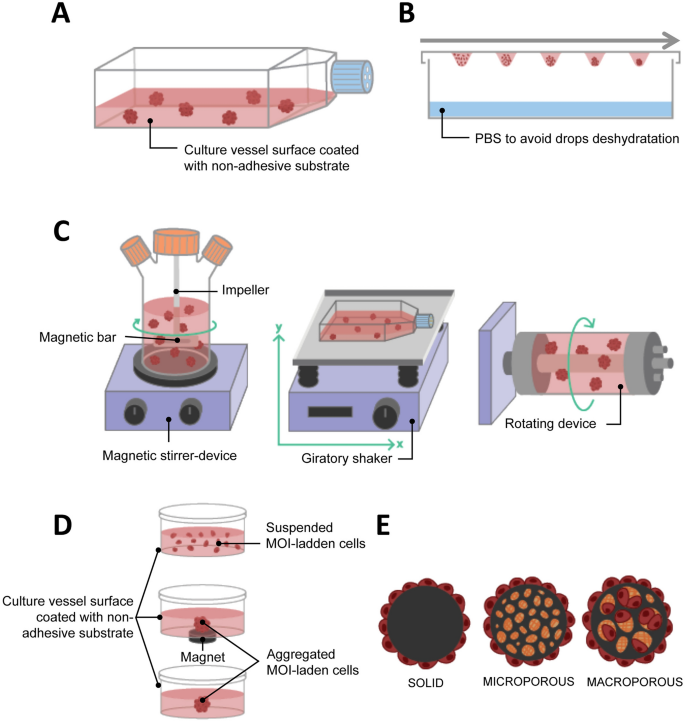
Liquid-based 3D cultures. A Liquid overlay; B Hanging drop; C Agitation-based: spinner flask (left), gyratory shaking (middle), rotary cell culture system/rotating wall vessel (right); D Magnetic levitation; E Microcarrier beads
However, not all cells are capable of spontaneously forming spheroids when cultured under non-adherent conditions (Fig. 3 ) [ 108 ]. Therefore, to promote spheroid formation some adjustments may be required, such as adding extracellular matrix components to the medium [ 109 ], coating the culture dish with bioactive materials such as hyaluronic acid, laminin or poly- d -Lysine [ 110 , 111 , 112 ], or using defined medium supplemented with growth factors and cytokines [ 113 , 114 , 115 ]. The major limitation of the liquid overlay culture method is the variability of the size and/or spherical morphology of the spheroids. In this way, extensively modulating the initial cell number, or using U-bottom or micropatterned plates such as Agrewell plates (Corning™) may be necessary to obtain reproducible spheroids.
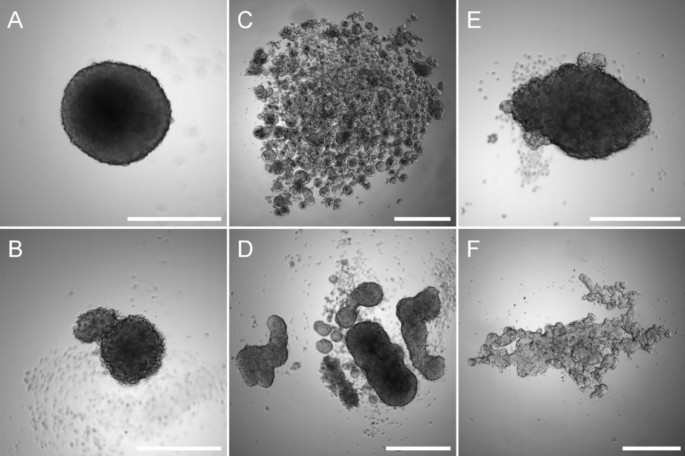
Liquid overlay technique culture. Osteosarcoma MNNG/HOS ( A ) and SAOS-2 cells ( B ), colorectal adenocarcinoma Caco-2 ( C ), colon cancer HT29 ( D ), glioblastoma U251 ( E ) or prostate carcinoma LnCaP ( F ) cells were seeded into a 96-well low-attachment plates and cultured for 7 days. Scale bar corresponds to 500 µm
Hanging drop
The hanging drop culture method relies on the use of gravity for the formation of spheroids and has been used to differentiate embryonic stem cells into embryoid bodies [ 116 ]. A cell suspension volume of less than 30 µL is pipetted onto the surface of a non-coated culture dish, usually the cover, which is then inverted. The drop will not spread on the surface thanks to gravity and surface tension allowing a spheroid to be produced at the bottom of the drop (Fig. 2 B). It is possible to improve spheroid formation by using media additives such as methylcellulose and/or collagen [ 117 ]. The hanging drop culture method has multiple advantages, including its simplicity of implementation since it does not require expensive tools. It also promotes cell–cell contacts with few cells. Used as early as 1910 by R. Harrison, who cultured neuronal cells [ 20 ], it has since been widely used in cancer 3D cultures and is as a consequence a well-documented technique for different cancer cells, including breast [ 108 , 118 ], prostate [ 119 ] and ovarian cancer [ 120 ]. In the case of ovarian cancer, liquid-based 3D culture methods are especially relevant since ovarian cancer cells often metastasize as multicellular aggregate in ascites that accumulate in the abdomen. Using hanging drop to generate epithelial ovarian cancer spheroids, Al Habyan et al . showed that cancer cells can detach spontaneously from the primary tumor as clusters that are less sensitive to apoptosis. They validated these observations in vivo [ 121 ].
At the same time, the technique also evolved and hanging drop platforms have been developed for high-throughput 3D spheroid cultures [ 122 ]. However, this method has several drawbacks. Even though the hanging drop method makes it possible to better control spheroid formation than other methods using larger volumes of cell suspension, it could prove difficult to handle the change of media or treatment of the spheroids with drugs if the spheroids are not transferred to a non-adherent culture dish after assembly.
Agitation-based
Another method for producing spheroids is to culture cells in a liquid environment which is in continual agitation, thus preventing their adherence to the culture recipient and increasing the collision between cells. Under these conditions, cells tend to spontaneously aggregate into spheroids. To create the agitation either the media is in continual movement through stirring or the culture bottle is in continual movement.
Stirring bioreactors include spinner flasks. Spinner flasks are three-neck flasks that can contain hundreds of milliliters of cell suspension. Gas exchanges are possible through the two side arms with filter caps. Fluid movement is produced by an impeller linked to a magnetic bar and the speed of the rotation is controlled by the magnetic-stirrer device (Fig. 2 C left). The main problem with the spinner flask technique is the high shear stress associated with liquid movement, which may impede the culture of certain cell types. Because the spinner flask technique makes it possible to produce large size spheroids with a hypoxic core, various studies on oxygenation have been conducted [ 123 , 124 ]. Durand and Sutherland used spinner flasks to produce spheroids of V79-171 B CHO and compared their response to radiation with single cells [ 125 ]. They observed that spheroids were more resistant to radiation damage and suggested both that cellular response can differ depending on the microenvironment conditions and that cell–cell contact could help in radiation damage repair. Co-culture is also possible with spinner flasks, and a heterotypic spheroid culture model containing FaDu head and neck squamous cell carcinoma cells and peripheral blood mononuclear cells was developed to evaluate the efficacy of immunotherapy with catumaxomab binding to CD3 and EpCAM [ 126 ]. The second method for creating liquid movement is by shaking the culture flasks. The easiest way is to use an orbital shaker that will shake the culture vessel in a 3D gyratory motion (Fig. 2 C, center). The fluid movement effectively produces shear stress, but it can also help mimic in vivo conditions. Along those lines, Masiello et al . used this gyratory motion to reproduce the fluid shear stress that primary ovarian spheroids undergo during the transcoelomic metastasis process [ 127 ].
The rotary cell culture system (RCCS) or rotating wall vessel (RWV) was designed in the 90 s by NASA engineers to study cell cultures in a microgravity environment. This rotary bioreactor consists of a cylindrical vessel that spins slowly around a horizontal axis, thus subjecting the cells it contains to continuous free fall. In this condition, the cells are maintained in suspension and can aggregate into spheroids (Fig. 2 C, right). This technique has the advantage of creating low shear stress. One of the first scientific articles published using the RCCS/RWV method described the growth pattern of several human tumors (e.g. glioma, prostate, urinary, bladder and breast cancer, and metastatic brain tumors) [ 128 ]. More recently, McNeill et al . showed the advantages of such 3D culture systems compared to standard monolayer cultures in malignant bone disease, and cultured viable osteosarcoma patient-derived xenografts for up to 8 days [ 129 ]. By developing a co-culture model involving osteogenically enhanced human mesenchymal stem cells and DDK1-overexpressing MOSJ cells, or Dkk-1 positive patient-derived xenografts, they were able to reproduce the mechanisms of osteo-inhibition observed in malignant bone disease. The RCCS/RWV technique, compared to other liquid-based 3D cultures such as hanging drop and magnetic levitation, makes it possible to produce a higher number of spheroids from various cell lines that are larger than 500 µm. These spheroids can then be distributed into 96-well plates for drug screening [ 130 ]. This highlights the potential for RCCS/RWV to generate spheroids for HTS. However, microgravity models should be used carefully, as such conditions do not translate well physiologically in humans.
Thanks to agitation-based techniques, large numbers of 3D spheroids can be produced extensively as fluid movement participates in the transport of nutrients and oxygen to the spheroids and aids with waste disposal. However, these rotation devices present certain limitations such as the variability in spheroid size and shape within a culture vessel [ 130 ] and the impossibility of following in real time the formation of the spheroids because of the continuous movement. To increase homogeneity in spheroid size, microcarrier beads have been used. This method cannot be directly used for HTS because of the continuous stirring, but it remains useful for mass production of spheroids. This technique requires specific equipment that can be expensive and challenging to properly assemble in the case of the RCCS/RWV method.
Microcarrier beads
Microcarrier beads are usually small spheres ranging from 100 to 300 nm in size that can be of different materials, such as plastic, glass, silica, cellulose or gelatin and coated with protein (collagen, fibronectin) or polysaccharide [glycosaminoglycans (GAGs), dextran]. Mainly used in large-scale cell culture production, they have been developed in oncology research to support anchorage-dependent cells that do not spontaneously aggregate to form spheroids, especially in 3D agitation-based models such as spinner flasks and RCCS/RWV. There are three types of microcarrier: solid, microporous and macroporous (Fig. 2 E). With solid microcarriers, cells grow in a monolayer on their surface, and it is in fact identical to 2D adherent cell culture, except for the spherical, rather than flat, configuration. Cells growing on the surface of microporous carriers are effectively still in an adherent monolayer similar to 2D culture, but they are able to secrete ECM inside the pores of the microcarriers, creating an environment on the inside that is different to that on the outside. Finally, macroporous microcarriers with a pore diameter of more than 10 µm allow cells to invade the macropores and proliferate to form 3D cultures [ 131 ].
Microcarrier beads can be a solution if a cell type does not spontaneously form spheroids in liquid 3D culture systems, and in particular in dynamic 3D systems such as spinner flasks or RCCS/RWV [ 129 , 132 ], but the procedure for harvesting the cells may be difficult, especially for cells cultured with macroporous microcarriers. Moreover, if the microcarrier used is large and made of small pores, it can be a hindrance for the diffusion of nutrients and signaling molecules to some cells. Finally, some materials used to produce microcarriers can limit microscopic observation.
Magnetic levitation
The 3D cell cultured using the magnetic levitation method was developed in 2010 [ 64 ] and relies on biocompatible magnetic iron oxide (MIO) nanoparticles to bio-assemble cells into spheroids. The technology is currently developed by N3D Biosciences and commercialized by Greiner Bio-One Ltd. Cells that are incubated using MIO nanoparticles assimilate them. Brief exposure to a magnetic field promotes cell aggregation and spheroid formation, coupled to cell–cell interaction and ECM synthesis (Fig. 2 D). This is usually done in multi-well plates, and it produces a single spheroid per well. Pan et al . used magnetic levitation to highlight the involvement of miR-509-3p in attenuating spheroid formation in six ovarian cancer cell lines. The authors suggested that this microRNA may be a potential therapeutic drug that could disrupt the metastasis process in epithelial ovarian cancer that relies on the spreading of cancer spheroids into the peritoneal fluid [ 133 ]. Noel et al . developed a downstream metabolic assay from spheroids formed from the co-cultures of pancreatic cancer cells and CAFs [ 39 ]. This technique has the advantage of facilitating rapid and gentle cell aggregation and does not require an artificial substrate, or specialized media or equipment except for the magnets and MIO. In addition, it is not limited to a specific cell type. Co-cultures can be carried out aggregating numerous different cell types into spheroids [ 39 , 40 ]. It is even possible to control to some extent the organization of the cells inside the spheroid at the beginning of the culture, by adding each cell type at different times during magnetic bio-assembly. The first cell type will undergo the magnetic action of the magnet and aggregate. Then, the second cell type is added, and cells aggregate around the previous layer and so on, creating a multi-layered spheroid [ 134 ]. The fact that each spheroid is cultured in a single well and undergoes the same magnetic field as the neighboring spheroids makes possible highly reproducible experiments. Finally, this method is suitable for HTS. However, magnetic levitation has some disadvantages. Firstly, it has been proven that the MIO does not have a direct effect on cell behavior [ 64 , 135 ], however magnetic fields of more than 30 mT affect angiogenesis [ 136 ], tumor spheroid growth [ 137 ], and cell migration [ 138 ]. Moreover, the magnetic nanoparticles color the cells brown because of the iron oxide, which can hinder assays involving colorimetric reagents that generate a brown product upon reaction with an enzyme, such as 3,3′-Diaminobenzidine or o -phenylenediamine.
Scaffold-based 3D culture systems
Scaffold-based 3D culture systems act as a structural support for cell attachment and growth and thus reproduce the ECM to a certain extent. The porosity, solubility, compliance and composition of scaffolds affect the cellular response [ 139 ] and consequently, the choice of biomaterial and synthesis method used to produce the scaffold will depend on the origin and stage of the cancer, the microenvironment cells, and the investigations carried out. A change in ECM composition has been shown in different stages of colorectal cancer, with an increase in the expression of type I collagen, MMP-2 and MMP-9, and a decrease in the expression of type IV collagen and TIMP-3 in the late stages. The ECM of colorectal cancer is associated with a higher proliferation rate for cancer cells compared to the ECM of normal colons [ 140 ]. Similarly, different extracellular matrix signatures were detected between normal colons, primary colon tumors and their metastases in the liver [ 141 ]. To address the multiple types of ECM observed in vivo, a variety of scaffolds have been developed. The scaffolds used for 3D cell cultures are either of natural or synthetic origin (Table 1 ) and the methods used produce a hydrogel, porous or fibrous scaffold (Table 2 ). Hydrogels are polymer networks containing a high percentage of water that can be obtained from natural sources or be synthesized. Hydrogels are synthesized when hydrophilic polymers undergo gelatinization following physical and/or chemical crosslinking. By reproducing the hydrophilic and gel-like structure of the natural ECM, hydrogels recreate an in vivo-like environment to support cell growth in vitro. Porous scaffolds contain pores around 100 µm in diameter. Fibrous scaffolds are composed of polymer fibers (Table 2 ).
Scaffolds of natural origin
Natural scaffolds include protein-based, polysaccharide-based and decellularized ECM scaffolds. Collagen and its derivatives gelatin and gelatin methacryloyl (GelMA) scaffolds belong to the protein-based systems. Collagen is the main fibrous protein in the ECM in connective tissue, representing one-third of the whole-body protein content [ 142 ]. Collagen scaffolds have been used for numerous 3D cultures of cancer cells such as breast [ 143 ] and ovarian cancer [ 144 ], pancreatic ductal adenocarcinoma [ 145 ], head and neck squamous cell carcinoma [ 146 ] and liver cancer [ 147 ].
Matrigel™ is a complex mixture of basement membrane proteins, growth factors and cytokines that are secreted by Engelberth-Holm-Swarm mouse sarcoma cells. Its main proteins are laminin, collagen IV, heparan sulphate and entactin [ 148 ]. Due to its cancer origin, Matrigel™ has been extensively used in oncology research, particularly for studying cell invasion and metastasis, CSCs and cancer resistance [ 149 ]. Recently, Zhang et al . developed a dumbbell model to directly observe the physical interaction between CAFs and cancer cells. Fibroblasts and cancer cells were suspended in Matrigel™, seeded in two separated droplets, and linked to each other by a Matrigel™ causeway. This model was validated using BHK-21 fibroblasts co-cultured with either CaKi-1 kidney carcinoma cells, HeLa cervical cancer cells, A375 human melanoma cells or A549 lung adenocarcinoma cells [ 150 ]. Matrigel™ has been also used to identify CSCs and study their characteristics and properties [ 151 ]. Bodgi et al . used this method to assess the radiosensitivity of bladder cancer in vitro. They observed that cancer cells did not respond in the same way to irradiation when cultured in monolayers compared to 3D cultures in which cells were predominantly more resistant to irradiation. They hypothesized that 2D cultures did not favor CSC generation in contrast to 3D cultures in Matrigel™ that may be responsible for the reduced radiosensitivity [ 152 ]. Moreover, when exposed to doxorubicin, MDA-MB-231 spheroids cultured in Matrigel™ were less sensitive to drugs than cells cultured in PuraMatrix™ (a synthetic peptide hydrogel that is devoid of animal-derived materials), which underlines the role of ECM proteins in chemoresistance [ 48 ]. Finally, Matrigel™ is the most commonly used matrix to support organoid growth [ 153 , 154 , 155 ]. However, using Matrigel™ can result in high and unequal background signals between batches because of contaminants and batch-to-batch variability. Depending on the downstream analyses, different strategies have been developed to minimize these background signals. For example, acetone precipitation of peptide digest from Matrigel™-embedded samples before liquid chromatography with tandem mass spectrometry (LC–MS/MS) makes it possible to increase the number of spectra identified as peptides. This method was applicable to large- and small-sized samples obtained from CRC patient-derived organoids. It thus improved the potency of phosphoproteomic studies as assays for the profiling of individual phosphorylation patterns that may be the mirror of cancer progression and treatment response in patients [ 156 ]. Matrigel™ is also an issue for matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI). MALDI-MSI is a technology that uses a molecule’s mass-to-charge ratio for its identification without any other probes, making it possible to image a thousand molecules (i.e. peptides, lipids, proteins, glycans, and metabolites) in a single experiment. Overlapping signals between Matrigel™ and organoid cells can thus be detected. By adding a centrifugation step at 4 °C, Johnson et al . successfully extracted the organoids from the Matrigel™, and could then transfer them to a gelatin mold that did not generate background noise with MALDI-MSI. With this method, it was even possible to do subject the organoids to HTS. The organoids extracted from the Matrigel™ were transferred to a microarray grid composed of micro-wells. After centrifugation, all organoids were thus aligned on the same z-axis, which makes it possible to have them all in a single section instead of having to section them one-by-one [ 157 ].
A second type of natural polymer is composed of polysaccharide-based scaffolds. GAGs are linear polysaccharides that are capable of attaching covalently to ECM proteins or with other GAGs, and play major roles in stabilizing the ECM. Their structures (chain length and sulfation patterns) differ between cancerous and healthy tissues, and between primary and metastatic tissues [ 158 ]. Hyaluronic acid (HA)-based hydrogels are the most used in oncology research. They have been used to elaborate 3D models [ 159 , 160 , 161 ], to study cell behavior in response to microenvironment modifications [ 162 , 163 , 164 ] or for high throughput screening [ 165 , 166 ]. Alginate and chitosan are also polysaccharides respectively found in the cell walls of brown algae and the exoskeletons of arthropods. Liu et al . created a composite collagen-alginate hydrogel whose stiffness was comparable to the matrix found in human breast tumors and studied the functional impacts of their structural (e.g. gel porosity) and biological modifications (e.g. addition of a chemotactic gradient) on tumor invasion [ 167 ]. By using a collagen-HA composite scaffold, Rao et al . analyzed the behavior of glioblastoma cells. They observed that the nature of the collagen used in the composite scaffold influenced cell morphology, and that the HA concentrations led to the regulation of cancer cell migration, highlighting the major signaling role of the cancer microenvironment [ 139 ]. Maloney et al. also used a collagen-HA composite scaffold as a bio-ink to bioprint organoids in a HTS platform for drug screening. This bio-ink was more efficient in generating spheroids than the commercially available HyStem HA-polyethylene glycol (PEG) hydrogel ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074680/ ), which is a composite of natural and synthetic hydrogels that has been used to expand stem cells [ 168 ].
It remains challenging to reproduce the in vivo microenvironment by crosslinking several polymers into a single scaffold. Tissue decellularization is a good alternative for producing an ECM similar to the original tissue/organ. During this process, all cells are removed from the tissue, and only the ECM and its structure remain. Decellularization is done by physical (e.g. temperature, electroporation, hydrostatic pressure), chemical (e.g. ionic and non-ionic surfactants, acids and bases) and enzymatic (e.g. trypsin, nuclease, dispase) means [ 169 ]. By decellularizing normal and tumor tissues resected from colorectal cancer patients, Pinto et al . showed that the tumor microenvironment induced M2 macrophage polarization, which are known to be anti-inflammatory and pro-angiogenic agents that promote cancer progression [ 170 ]. Hoshiba and Tanaka showed that using decellularized ECM derived from different malignant stages affected the 5-FU sensitivity of HT-29 colorectal cancer cells. When cultured in late-stage cancer decellularized ECM, HT-29 were more resistant to 5-FU treatment compared to low malignant derived decellularized ECM [ 171 ].
Since all these scaffolds come from natural sources, inter-batch variability is an issue and some of the polymers can be hard to extract in large quantities (e.g. collagen). Moreover, their structure is complex, not well defined and could lead to non-reproducible properties from one scaffold to another. Therefore, scientists developed synthetic scaffolds to reduce the cost of production and to limit the variability in the hydrogels produced.
Synthetic scaffolds
In contrast to biopolymers of natural origin, the chemical composition of synthetic scaffolds is fully controlled. It is possible to fine-tune their biochemical and mechanical properties (i.e. stiffness, biodegradability and bioactivity) and these scaffolds are highly reproducible.
PEG is a synthetic polymer that swells to form a hydrogel in a few minutes when exposed to an aqueous solution. Incorporating biochemical and biological functionalities into the PEG polymer backbone makes it possible to directly study their impact on cancer cells [ 172 ]. Sieh et al . used a PEG-based hydrogel, functionalized with the arginine-glycine-aspartic acid (RGD) motif (which provides binding sites for cells through integrin) and matrix metalloproteinase (MMP) cleavage sites (which allows cells to degrade the gel). With this model, the authors showed the effect of 3D cultures on the morphology, gene expression and protein synthesis of LnCaP prostate cancer cells compared to adherent monolayers [ 173 ]. Another recent study showed that by changing the chemical and mechanical properties of a PEG-based hydrogel, it was possible to control the phenotype state of MDA-MB-231 breast cancer cells, directing them toward a highly proliferating, moderately proliferating, or dormancy phenotype [ 174 ]. PEG hydrogel was also hybridized with natural polymers such as collagen [ 175 , 176 , 177 ], chitosan [ 178 , 179 , 180 ] or Matrigel™ [ 181 ] to improve the biocompatibility of the scaffold.
Other commonly used synthetic materials for scaffold production are aliphatic polyesters such as polycaprolactone (PCL), poly(glycolic acid) (PGA), poly(lactic acid) (PLA) or poly(lactic-co-glycolic acid) (PLGA). As with PEG, polyesters have high biocompatibility and tunability [ 182 ]. The major difference between these polyesters is their biodegradation rate. There is an inverted relationship between hydrophobicity and degradability. The more hydrophobic a polymer is, the less degradable it will be. A list of polymers with slow to fast degradability can then be defined: PCL < PLA < PLGA < PGA [ 183 ]. When cultivated into a PCL scaffold, TC-17 Ewing sarcoma (EWS) cells exhibited proliferative rates and anti-cancer drug responses similar to in vivo EWS tumor xenografts, in contrast to cells cultured in 2D [ 184 ]. PCL scaffolds also allowed CAFs to retain their pro-inflammation properties, while these properties were lost in 2D cultures. When xenotransplanted in vivo, these 3D cultured CAFs promoted inflammatory cell infiltration at the tumor site and the invasiveness of cancer cells [ 185 ]. In another study involving PCL scaffolds, organoids were generated by transient culture of CAFs on the scaffold before removing it, followed by culture of primary breast cancer cells [ 186 ]. CAFs deposited ECM on the PCL scaffold, encouraging cancer cell adhesion, survival and proliferation. On the other hand, cancer cells grown on the PCL scaffold without pre-culture with CAFs failed to form organoids even after 10 days in culture. In addition, this hybrid PCL-CAFs ECM scaffold made it possible to study patient-specific responses to treatment by exposing the tumoroids to doxorubicin or mitoxanthrone. This 3D culture model could therefore serve as a personalized medicine platform [ 186 ]. Girard et al . developed a PLGA/PGA/PEG co-polymer scaffold in which they induced the formation of spheroids from various cancer cell lines (melanoma, breast, prostate, ovarian, and lung cancers). These spheroids underwent epithelial-to-mesenchymal transition (EMT), losing the expression of E-Cadherin and acquiring vimentin expression. They also displayed higher resistance to cytotoxic drugs than cells cultured in 2D [ 187 ].
Self-assembling peptides (SAPs) are short molecules that spontaneously assemble into supramolecular nanofiber structures [ 188 ] when exposed to modified pH, temperature or enzymatic treatment [ 189 ]. SAPs have been used as carriers for drug delivery systems [ 190 , 191 ], but they also show great potential for 3D cell cultures in cancer research [ 192 ]. They are highly biocompatible [ 193 ], can be easily tuned, and offer a fibrous network organized similarly to the ECM [ 194 ]. Moreover, the short length of the peptides (< 20 amino acids) renders their synthesis easy, rapid and non-expensive. Of the variety of existing SAPs (Table 2 ), RADA16 is the most commonly used and is commercially available under the name PuraMatrix ® . RADA16 hydrogels make it possible to culture different types of cancer cell such as pancreatic ductal adenocarcinoma [ 195 ], breast carcinoma [ 150 ], hepatocellular carcinoma [ 160 ] and leukemia [ 193 ].
Emerging methods for 3D tumor models
Microfluidic platforms.
Microfluidic platforms are based on the manipulation of fluids, in small volumes and spaces (in the micro range) within a network of channels. This small dimension creates reproducible and predictable laminar flow that facilitates the formation of homogeneous spheroids [ 196 ]. Although microfluidic devices have been used to separate cancer cells, such as circulating tumor cells (CTCs) from blood [ 197 ], their use has been extended to cancer cell cultures either in 2D or in 3D in the past two decades (Fig. 4 ). 3D microfluidic platforms can take various forms, be made of different materials (Table 3 ), and incorporate scaffolds of multiple types to better mimic the in vivo tumor microenvironment. This variety of platforms was developed to study the wide variety of cancer types and their multiple mechanisms. Jeon et al . developed a co-culture 3D microfluidic model made with polydimethylsiloxane (PDMS) to study the metastatic process of breast cancer cells [ 198 ]. They investigated the ability of a bone-seeking clone of the MDA-MB-231 breast cancer metastatic cell line to extravasate into a bone-mimicking microenvironment, into a muscle-mimicking microenvironment or into an acellular collagen matrix. They observed a significantly higher extravasation rate for the breast cancer cells in the bone-mimicking microenvironment compared to the other two microenvironments, highlighting the seed-and-soil theory. With the aim of studying metastatic processes, Toh et al . also proposed a monoculture 3D microfluidic PDMS model [ 199 ]. These authors induced the aggregation of MX-1 breast cancer metastatic cells inside the microfluidic platform before adding a chemoattractant to stimulate cell motility. Cancer cells exhibit amoeboid-like motility (the cells are amorphous and change direction rapidly) and collective motility (cells retain their cell–cell contacts and invade as a group) that have only been observed in 3D in vitro models or in in vivo models. By using this system, Toh et al. observed in real-time the migration and invasion of the cancer cells across a collagen barrier. This approach could be highly useful in anti-metastasis drug assays. Other cancer mechanisms were studied, such as tumor angiogenesis. Recently, Miller et al . used a co-culture 3D microfluidic PDMS model to study endothelial sprouting induced by primary human clear cell renal cell carcinoma (ccRCC) [ 200 ]. Their model reproduced the pro-angiogenic activity of ccRCC cultured in 3D in contrast to 2D transwell assays. Moreover, pharmacological angiogenesis blockade could be then modelled, demonstrating the major value of this type of platform for screening anti-angiogenic drugs.
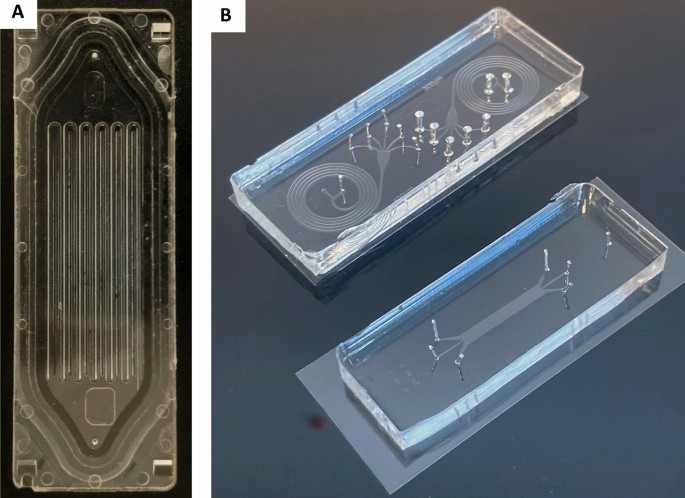
Microfluidic platforms. A Parsortix™ microfluidic platform for isolating circulating tumor cells based on their size and their deformability properties; B Image of PDMS microsystems dedicated to particle separation: spiral microfluidic systems (top); deterministic lateral displacement particle separation system (down). Both are placed on 60 × 22 mm coverslips
Microfluidics platforms have also been used to expand organoids. Pinho et al. observed that growing CRC cells in the microfluidic platform that they developed promoted organoid growth compared to traditional organoid cultures in 12-well plates. These results may be imputed to the continuous perfusion of the organoids with fresh culture media in the microfluidic platform. Moreover, this microfluidic platform was compatible with drug screening, although not in a high throughput way since the microfluidic chip has only four seeding wells [ 201 ]. However, microfluidic devices do not preclude HTS. Indeed, Schuster et al . developed an automated microfluidic platform for dynamic and combinatorial drug screening. Their platform could hold up to 200 samples sub-divided into 20 units of 10 wells each. Each unit could be perfused with independent fluidic conditions (i.e. drug solutions). Moreover, their device was compatible with live-cell time-lapse fluorescence microscopy for the whole duration of the experiment. With this microfluidic platform, they grew pancreatic ductal adenocarcinoma organoids from three different patients that they subjected to constant-dose monotherapy, one-shot combinatorial therapies, or sequential drug administration. They observed that the latter was the most effective therapy. In addition, it is possible to harvest the organoids when an experiment is completed for downstream analysis. Their microfluidic platform could serve as a screening platform for personalized medicine [ 202 ].
PDMS is the main substrate used for the biofabrication of 3D microfluidic platforms. PDMS is biocompatible, can be easily modeled, and is transparent, which facilitates imaging. In addition, PDMS is permeable to gases, facilitating simple gas exchanges. However, PDMS also has multiple disadvantages. It is permeable to water evaporation, which can lead to samples drying out. The porosity of the material is also responsible for high adsorption of cytokines and other signaling molecules, which could produce misleading results [ 203 ]. To overcome this disadvantage, microfluidic platforms based on polystyrene and other materials have been proposed [ 204 ]. 3D microfluidic platforms go a step further in mimicking in vivo tumors than the 3D culture models presented previously, but they require interdisciplinary collaborations (physics, biochemistry, engineering, and biology) and high-cost material/equipment.
- Bioprinting
3D-bioprinting is an innovative approach based on automatic additive manufacturing that offers the potential of assembling tissue-like structures by controlling and positioning cells, tissues and biodegradable biomaterials within a prescribed organization to accomplish one or more biological functions [ 205 , 206 ]. As such, 3D bioprinting offers flexibility in dispensing cells and biomaterials spanning the disparity between artificially engineered tissue and native tissue [ 35 ]. This flexibility facilitates the formation of complex architectures and features that may affect tissue function, which could be the stepping stone to personalized medicine [ 207 ]. For example, the construction of a “mini-brain” which includes the incorporation of different cell types capable of interacting with each other was used to test a range of chemotherapeutic agents [ 208 ]. The hydrogel-based biomaterials used in bioprinting are called bio-inks that must possess adequate viscoelastic properties to guarantee detailed layer by layer deposits, resulting in high fidelity 3D printed constructs [ 209 ]. Depending on the biomaterial present in the bio-ink, the 3D structures formed can be solidified through three different mechanisms: physical (temperature or light) [ 210 ], enzymatic [ 211 ], or chemical crosslinking (pH and ionic compound) [ 212 ]. 3D bioprinters differ through their bioprinting modalities and can thus be classified into 4 categories: (i) droplet-based bioprinting, (ii) laser-based bioprinting, (iii) extrusion-based bioprinting and (iv) stereolithography bioprinting. Each of these bioprinting modalities uses various strategies (Table 4 ).
Inkjet bioprinting is adapted from conventional inkjet printing, which delivers droplets on to a print controlled by thermal, piezoelectric, or microvalve methods [ 206 , 213 ]. The droplets of solution can be positioned in a highly precise mode at high speed, allowing for the construction of complex 3D structures. Inkjet bioprinting offers some distinct advantages, such as high-speed printing of up to 10,000 droplets per second, moderately high resolution suitable for biological constructs (50–300 μm), low cost and control of the concentration of cells and growth factors in the bio-ink. However, inkjet bioprinting is restricted to low viscosity bio-inks (< 10 mPa/s), preventing the assembly of thicker vertical structures [ 214 ] since the more viscous the bio-ink, the greater the force required to eject droplets from the printing nozzle, thereby limiting its applicability [ 215 ]. Furthermore, encapsulated cells in the bio-ink increase the viscosity of the solution, thereby limiting the number of encapsulated cells tolerated in the bio-ink [ 216 ]. Due to this limitation, fabrication of thick complex tissues poses a huge challenge. On the other hand, it is a powerful printing method for generating organoids for drug screening in a high throughput and rapid manner. Jiang et al . developed a homemade droplet printer that involved microfluidics and 3D scaffold-based cultures. By printing Matrigel™ droplets laden with around 1,500 cells from mouse or human lung, kidney and stomach tumors loaded into 96-well plates, they produced organoids within one week with a success rate of 95%. These organoids were representative of inter-organoid homogeneity and inter-patient heterogeneity, as shown by the similar gene mutation signature and response to drugs between the organoids and the tumor they were derived from. While this organoid platform still needs improvement, especially since the number of organoids that can be produced is limited by the tumor samples, the one-week duration required to generate these organoids is far shorter than the time usually required by conventional protocols [ 154 ] and could therefore fit within a personalized medicine context [ 155 ].
Microextrusion bioprinters are extrusion-based, with bio-inks driven through single or multiple nozzles by a pneumatic (air-pressure or mechanical screw/piston-driven) dispensing system [ 217 ]. This approach is a combination of a fluid-dispensing system and an automated robotic cartesian system for extrusion and bioprinting, where bio-inks are spatially disposed under computer-controlled motion, resulting in the precise depositing of cells encapsulated within the bio-ink as micrometric cylindrical filaments making possible the desired 3D custom-shaped structures. This rapid fabrication technique provides better structural integrity compared to inkjet bioprinted constructs due to the continuously deposited filaments. Filaments are expelled mechanically using a pneumatic pump, piston, or screw to drive the fluid flow and build up, layer-upon-layer, a 3D structure using a robotic stage and a printhead capable of x–y–z directional mobility [ 218 ]. Microextrusion bioprinting typically uses soft biomaterials in the form of a hydrogel. Similar to inkjet bioprinting, the materials can be crosslinked ionically, enzymatically, chemically, or with ultraviolet light, as well as thermally [ 219 ]. The resolution of the printed filaments depends on a number of factors, including the size of the nozzle used, the flow rate of the extruded material, and the speed of the printhead while dispensing the biomaterial. The main advantage of microextrusion bioprinting is the versatility of the technique. The use of mechanical force to dispense the materials and an adjustable nozzle or needle inner diameter makes possible a high working range of material viscosities (30 mPa/s to > 600 kPa/s) and the ability to print a high concentration of cells or cellular aggregates similar to the numbers of cells seen in natural tissues [ 219 , 220 ]. Higher resolution would require a smaller nozzle diameter and imposes higher shear stress on the cells, requiring higher pressure to extrude the material and this could have an effect on cell viability. Other challenges with this technique include nozzle clogging and insufficient interlayer bonding, depending on the crosslinking method. Despite these minor challenges, microextrusion bioprinting makes it possible to manufacture constructs of clinically relevant sizes and is often regarded as the most promising bioprinting technique [ 221 , 222 ]. However, the homogeneity of the organoids generated when scaling up is a source of issues, even when using extrusion bioprinting. Substrates with small-sized wells make the process of printing bio-ink in the form of a bead difficult, because it spreads to the wall of the well. Maloney et al . suggested a new method based on extrusion bioprinting in an immersion bath made of gelatin. With the right concentration of gelatin, printing in this immersion bath allowed the bio-ink loaded with cells to remain spherical in shape until crosslinking. They were able to produce viable organoids from different cell lines and from glioblastoma and fibrosarcoma patient samples. Moreover, they proved that their method was compatible with drug screening by subjecting patient-derived organoids to three concentrations of two different drugs [ 223 ].
Laser-assisted bioprinting relies on the use of a donor slide of biomaterial covered with a laser energy absorbing layer which locally evaporates and projects the donor slide material on to the substrate [ 224 ]. This nozzle-free (and clog free) system has a significant advantage given that it is capable of depositing biomaterials containing high cell densities while maintaining high cell viability and resolution [ 220 ]. The resolution is of the order of single cells in a droplet of 20–80 μm in diameter [ 219 ]. However, laser-assisted bioprinting requires a material that is moderately low in viscosity (1–300 mPa/s) and has a fast gelatinization mechanism to achieve high fidelity in the shape of the 3D bioprinted constructs [ 214 ]. Furthermore, preparation of donor slides is time-consuming and challenging for printing multiple materials or cell types. These technical limitations, along with the cost of laser sources, inhibit the generation of clinically relevant 3D constructs and the widespread use of this system.
Stereolithography is an additional nozzle-free technique in which a reservoir of photo cross-linkable material or resin is irradiated either by a laser or patterned UV light [ 225 ]. Exposure to the light source crosslinks the material, allowing for a layer-by-layer construction of thick, complex 3D structures. Stereolithographic printers make possible very rapid fabrication of complex structures with unequalled resolution of 6 μm [ 226 ]. In addition, stereographically printed structures exhibit strong interlayer bonding [ 219 ]. Due to the nature of this technique, the only method for crosslinking is photo-induced, which requires the addition of photo-initiating chemicals to initiate crosslinking [ 206 ]. Unfortunately, the most commonly used photo-initiating chemicals and UV light can also affect cell viability due to their interference with growth signaling pathways [ 227 ]. The material limitations of this technique require a viscosity of < 5 Pa/s and the ability to be photo-crosslinked, thereby considerably reducing the variety of printable materials for use in tissue engineering applications to either modified natural polymers or synthetic polymers. Furthermore, the need for a reservoir of material for printing limits the material to only one cell type, preventing the formation of complex tissues with multiple cell types or regions.
Conclusion and perspectives
The multiplicity of factors responsible for the different forms of cancer has encouraged scientists to use various in vitro study systems. The first and most widely used method is 2D cell culture. With 2D cell cultures, it is possible to control experiment parameters with high precision by reducing these parameters to the bare minimum. If 2D cell culture simplicity is its added value, it is a reductive model that cannot depict the complexity of cancer. 3D culture is a promising cell-based method. Here, we described and compared the advantages and limitations of the techniques that have been developed over the years to decipher the development of cancer. These techniques, which include liquid-based, scaffold-based and emerging 3D culture systems such as microfluidic platforms and bioprinting, incorporate morphological features that cannot be attained by 2D cultures and that influence the behavior of cancer cells and their microenvironment (Fig. 5 ).
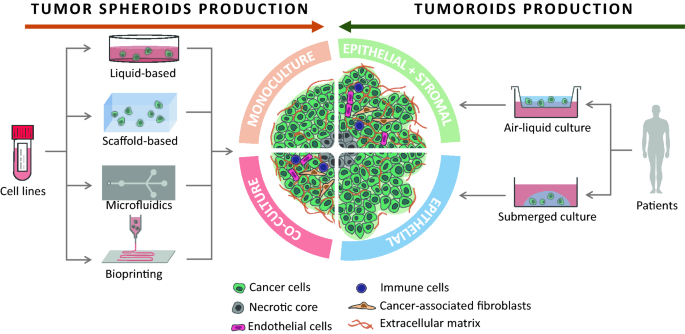
3D culture models for spheroids or tumoroids production. Tumor spheroids are often generated from cell lines, through liquid based-, scaffold based-, microfluidics or bioprinting methods. Depending on the cells added to the model, the tumor spheroid will be mainly composed of cancer cells and other cells and components of the microenvironment can be added. Tumor spheroids often show a round shape. Tumor organoids (or tumoroids) are usually generated from patient tissue samples by using two methods: (i) The submerged culture method that allows the amplification of epithelial cancer stem cells which are then able to produce ECM; (ii) The air–liquid culture method that allows the inclusion of stromal components to the tumoroids. Since tumoroids are self-organizing tissues, they will have a more complex structure than spheroids
As promising as these 3D models are, various challenges come from their use. First, the choice of the 3D culture methods depends on the scientific question raised and consequently each culture method responds to different purposes. Spheroids generated with liquid-based approaches such as the liquid overlay technique are the most straightforward way to do HTS and allow functional studies of compounds on cancer cell such viability or differentiation. However, these approaches may not consider the physical and chemical role of the microenvironment regarding drug resistance. For instance, for osteosarcoma that specifically arises in bone microenvionment, the use of mineralized scaffolds reproduce the “natural” CSCs niche and are much more adapted than soft extracellular matrix [ 228 ]. Similarly, using stationary model with or without scaffold may be inadequate to study processes that involve fluid movements such as the metastatic mechanism and in that case, microfluidic platforms would be more appropriate. Using even more complex 3D models like organoids or 3D bioprinting should allow to better mimic the diseases and to develop personal medicine programs. The use of complex 3D models may be not adapted to HTS. In addition, the increased structural complexity of 3D cultures could also complicate their analysis.
Incorporating in silico models to biological experiments may resolve the data complexity issue. Named by analogy to the words in vitro and in vivo, in silico refers to experiments performed through computer simulation. It is strongly based on results from laboratory experiments, inference, mathematical modelling and can be associated to artificial intelligence. In silico models can be multiscale, ranging from the biomolecular level to individual cell-based and systems models [ 229 ]. Beyond their capacity to provide biological insights and serve as analysis assisting tools, in silico models can also help to refine in vitro and in vivo experiments and increase the quantity and quality of data obtained in conformity with the 3Rs principles [ 230 ].
The choice of the most adapted 3D model to the question raised is dependent to the analytical processes that will be performed. Indeed, the great majority of the current analysis methods were developed for traditional 2D cell cultures, are often not adaptable to 3D culture, and require extensive validation steps [ 231 ]. Microscopic imaging of the 3D specimen will be challenging for the following reasons: (i) the physical properties of light lead to light-scattering in very thick samples, which optical sectioning and clearing techniques can not always resolve; (ii) fluorescent probes targeting specific molecules or organelles (e.g. fluorochrome-linked antibodies, DAPI, etc.) may diffuse non-homogeneously into the spheroids, with saturation of the probes on the outer layer and may be a limitation of successful imaging [ 232 ]; (iii) similarly, the poor diffusion and imaging probes to the central core of larger spheroids may be problematic [ 233 ]. Extensive preliminary setup experiments are required, since the size, the charge, or the affinity of probes to its ligand can impair the results obtained and lead to biased characterisation of spheroids [ 234 ]. 3D culture model have a much more developed ECM that can act as a barrier or a trap for the chemical, compounds and unfortunately may be associated to diffusion issue in lysis or metabolic assays [ 231 ]. However, these issues should not be seen as setbacks. Similar to 2D culture methods, increasing use of these 3D models will lead to the development of new analytic methods.
3D culture has the potential to bridge the gap between in vitro and in vivo models. By increasing the complexity of the 3D models, it is possible to approach what is observed in vivo, and still be experimenting on human cells instead of those of another species. Moreover, by adding computer modelling, it may be possible to include the 3D models in a more systemic environment. Therefore, the future of oncology research, and especially personalized medicine, will rely on the interdisciplinary collaboration of various scientific fields such as biology, medicine, physics, engineering, bioinformatics, and mathematics.
Abbreviations
Three-dimensional
Adult stem cells
Cancer-associated fibroblasts
Chimeric antigen receptor T
Clear cell renal cell carcinoma
Colon organoids
Colorectal cancer
Cancer stem-like cells
Circulating tumor cells
Extracellular matrix
Embryonic stem cells
ETS variant transcription factor 2
Extracellular vesicles
Food and Drug Administration
Glycosaminoglycans
Glioblastoma organoids
Gelatin methacryloyl
Genetically engineered animal models
Hyaluronic acid
Hypoxia-inducible factor
High throughput screening
Induced pluripotent stem cells
Liquid chromatography with tandem mass spectrometry
Laminin, entactin and type-IV collagen matrix
Leucine-rich repeat-containing G protein-coupled receptor 5
Matrix-assisted laser desorption/ionization mass spectrometry imaging
Magnetic iron oxide
Non-small lung cancer
Peripheral blood mononuclear cells
Polydimethylsiloxane
Polyethylene glycol
Polyglycolic acid
Prostaglandin E2
Polylactic acid
Polycaprolactone
Polylactic-co-glycolic acid
‘Reset’ vascular endothelial cells
Rotary cell culture system
Arginine-glycine-aspartic acid motif
Rotating wall vessel
Self-assembling peptides
Tumor microenvironment
Yes-associated protein
Heron M, Anderson RN (2016) Changes in the leading cause of death: recent patterns in heart disease and cancer mortality. NCHS Data Brief. 1–8.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551 .
Article Google Scholar
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. https://doi.org/10.3389/fphar.2018.01300 .
Article CAS PubMed PubMed Central Google Scholar
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013 .
Article CAS PubMed Google Scholar
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–17. https://doi.org/10.1016/j.bbcan.2009.11.002 .
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37. https://doi.org/10.1038/nature12624 .
Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. https://doi.org/10.1038/nature12626 .
Brown HK, Tellez-Gabriel M, Cartron P-F, et al. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: myth or reality? Drug Discov Today. 2019;24:763–72. https://doi.org/10.1016/j.drudis.2018.11.017 .
Article PubMed Google Scholar
Vallette FM, Olivier C, Lézot F, et al. Dormant, quiescent, tolerant and persister cells: four synonyms for the same target in cancer. Biochem Pharmacol. 2019;162:169–76. https://doi.org/10.1016/j.bcp.2018.11.004 .
Kersten K, De Visser KE, Van Miltenburg MH, Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9:137–53. https://doi.org/10.15252/emmm.201606857 .
Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. https://doi.org/10.1038/nprot.2009.120 .
Kemp CJ. Animal models of chemical carcinogenesis: driving breakthroughs in cancer research for 100 years. Cold Spring Harb Protoc. 2015;2015:865–74. https://doi.org/10.1101/pdb.top069906 .
Article PubMed PubMed Central Google Scholar
Son W-C, Gopinath C. Early occurrence of spontaneous tumors in CD-1 mice and Sprague-Dawley rats. Toxicol Pathol. 2004;32:371–4. https://doi.org/10.1080/01926230490440871 .
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921–5. https://doi.org/10.1016/j.cub.2020.06.081 .
Arneth B (2019) Tumor microenvironment. Medicina (Kaunas) 56. https://doi.org/10.3390/medicina56010015 .
Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. https://doi.org/10.1242/jcs.023820 .
Cacho-Díaz B, García-Botello DR, Wegman-Ostrosky T, et al. Tumor microenvironment differences between primary tumor and brain metastases. J Transl Med. 2020;18:1. https://doi.org/10.1186/s12967-019-02189-8 .
Kim S, Kim A, Shin J-Y, Seo J-S. The tumor immune microenvironmental analysis of 2,033 transcriptomes across 7 cancer types. Sci Rep. 2020;10:9536. https://doi.org/10.1038/s41598-020-66449-0 .
Kim G, Pastoriza JM, Condeelis JS, et al. The contribution of race to breast tumor microenvironment composition and disease progression. Front Oncol. 2020;10:1022. https://doi.org/10.3389/fonc.2020.01022 .
Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1910;142:5–73. https://doi.org/10.1002/jez.1401420103 .
Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. https://doi.org/10.1096/fj.05-5424rev .
Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. https://doi.org/10.1126/science.1116995 .
Solon J, Levental I, Sengupta K, et al. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–61. https://doi.org/10.1529/biophysj.106.101386 .
Nemir S, West JL. Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng. 2010;38:2–20. https://doi.org/10.1007/s10439-009-9811-1 .
Parreno J, Nabavi Niaki M, Andrejevic K, et al. Interplay between cytoskeletal polymerization and the chondrogenic phenotype in chondrocytes passaged in monolayer culture. J Anat. 2017;230:234–48. https://doi.org/10.1111/joa.12554 .
Zhou Y, Chen H, Li H, Wu Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J Cell Mol Med. 2017;21:1073–84. https://doi.org/10.1111/jcmm.12946 .
Jakubikova J, Cholujova D, Hideshima T, et al. A novel 3D mesenchymal stem cell model of the multiple myeloma bone marrow niche: biologic and clinical applications. Oncotarget. 2016;7:77326–41. https://doi.org/10.18632/oncotarget.12643 .
Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20:139–45. https://doi.org/10.1016/j.semcancer.2010.04.004 .
Nguyen-Ngoc K-V, Cheung KJ, Brenot A, et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci USA. 2012;109:E2595-2604. https://doi.org/10.1073/pnas.1212834109 .
Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. https://doi.org/10.1083/jcb.201102147 .
Kauppila S, Stenbäck F, Risteli J, et al. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–8. https://doi.org/10.1002/(SICI)1096-9896(1998110)186:3%3c262::AID-PATH191%3e3.0.CO;2-3 .
Senthebane DA, Jonker T, Rowe A, et al (2018) The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int J Mol Sci. 19. https://doi.org/10.3390/ijms19102861 .
Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51. https://doi.org/10.1530/JOE-10-0377 .
Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11:293. https://doi.org/10.1186/1471-2407-11-293 .
Zhang Y, Liao K, Li C, et al (2017) Progress in integrative biomaterial systems to approach three-dimensional cell mechanotransduction. Bioengineering (Basel) 4. https://doi.org/10.3390/bioengineering4030072 .
Lee J, Shin D, Roh J-L. Development of an in vitro cell-sheet cancer model for chemotherapeutic screening. Theranostics. 2018;8:3964–73. https://doi.org/10.7150/thno.26439 .
Al-Ramadan A, Mortensen AC, Carlsson J, Nestor MV. Analysis of radiation effects in two irradiated tumor spheroid models. Oncol Lett. 2018;15:3008–16. https://doi.org/10.3892/ol.2017.7716 .
Stöhr D, Schmid JO, Beigl TB, et al. Stress-induced TRAILR2 expression overcomes TRAIL resistance in cancer cell spheroids. Cell Death Differ. 2020;27:3037–52. https://doi.org/10.1038/s41418-020-0559-3 .
Noel P, Muñoz R, Rogers GW, et al. Preparation and metabolic assay of 3-dimensional spheroid co-cultures of pancreatic cancer cells and fibroblasts. J Vis Exp. 2017. https://doi.org/10.3791/56081 .
Jaganathan H, Gage J, Leonard F, et al. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci Rep. 2014;4:6468. https://doi.org/10.1038/srep06468 .
Inch WR, McCredie JA, Sutherland RM. Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth. 1970;34:271–82.
CAS PubMed Google Scholar
Azar J, Bahmad HF, Daher D, et al. The use of stem cell-derived organoids in disease modeling: an update. Int J Mol Sci. 2021;22:7667. https://doi.org/10.3390/ijms22147667 .
Wu H, Uchimura K, Donnelly EL, et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869-881.e8. https://doi.org/10.1016/j.stem.2018.10.010 .
Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. https://doi.org/10.1038/nature07935 .
Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–6. https://doi.org/10.1038/nm.1951 .
Curcio E, Salerno S, Barbieri G, et al. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials. 2007;28:5487–97. https://doi.org/10.1016/j.biomaterials.2007.08.033 .
Nakamura T, Kato Y, Fuji H, et al. E-cadherin-dependent intercellular adhesion enhances chemoresistance. Int J Mol Med. 2003;12:693–700.
Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18:41. https://doi.org/10.1186/s12885-017-3953-6 .
Roulis M, Kaklamanos A, Schernthanner M, et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524–9. https://doi.org/10.1038/s41586-020-2166-3 .
Palikuqi B, Nguyen D-HT, Li G, et al (2020) Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature. 1–7. https://doi.org/10.1038/s41586-020-2712-z .
Wisdom KM, Adebowale K, Chang J, et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat Commun. 2018;9:4144. https://doi.org/10.1038/s41467-018-06641-z .
Yang Y, Zheng H, Zhan Y, Fan S. An emerging tumor invasion mechanism about the collective cell migration. Am J Transl Res. 2019;11:5301–12.
CAS PubMed PubMed Central Google Scholar
Huang YL, Shiau C, Wu C, et al. The architecture of co-culture spheroids regulates tumor invasion within a 3D extracellular matrix. Biophys Rev Lett. 2020;15:131–41. https://doi.org/10.1142/s1793048020500034 .
Elia I, Broekaert D, Christen S, et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat Commun. 2017;8:15267. https://doi.org/10.1038/ncomms15267 .
Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:13012. https://doi.org/10.1038/s41598-019-49671-3 .
Riedl A, Schlederer M, Pudelko K, et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–18. https://doi.org/10.1242/jcs.188102 .
Gangadhara S, Smith C, Barrett-Lee P, Hiscox S. 3D culture of Her2+ breast cancer cells promotes AKT to MAPK switching and a loss of therapeutic response. BMC Cancer. 2016;16:345. https://doi.org/10.1186/s12885-016-2377-z .
Zschenker O, Streichert T, Hehlgans S, Cordes N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS ONE. 2012;7: e34279. https://doi.org/10.1371/journal.pone.0034279 .
Bingel C, Koeneke E, Ridinger J, et al. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis. 2017;8: e3013. https://doi.org/10.1038/cddis.2017.398 .
Ahmed EM, Bandopadhyay G, Coyle B, Grabowska A. A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell Oncol (Dordr). 2018;41:319–28. https://doi.org/10.1007/s13402-018-0374-8 .
Article CAS Google Scholar
Melissaridou S, Wiechec E, Magan M, et al. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019;19:16. https://doi.org/10.1186/s12935-019-0733-1 .
Jia W, Jiang X, Liu W, et al. Effects of three-dimensional collagen scaffolds on the expression profiles and biological functions of glioma cells. Int J Oncol. 2018;52:1787–800. https://doi.org/10.3892/ijo.2018.4330 .
Naruse M, Ochiai M, Sekine S, et al. Re-expression of REG family and DUOXs genes in CRC organoids by co-culturing with CAFs. Sci Rep. 2021;11:2077. https://doi.org/10.1038/s41598-021-81475-2 .
Souza GR, Molina JR, Raphael RM, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–6. https://doi.org/10.1038/nnano.2010.23 .
Tan PHS, Aung KZ, Toh SL, et al. Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology. Biomaterials. 2011;32:6131–7. https://doi.org/10.1016/j.biomaterials.2011.04.084 .
Weeber F, van de Wetering M, Hoogstraat M, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci USA. 2015;112:13308–11. https://doi.org/10.1073/pnas.1516689112 .
Ganesh K, Wu C, O’Rourke KP, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25:1607–14. https://doi.org/10.1038/s41591-019-0584-2 .
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273–86. https://doi.org/10.1093/biostatistics/kxx069 .
Imamura Y, Mukohara T, Shimono Y, et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep. 2015;33:1837–43. https://doi.org/10.3892/or.2015.3767 .
Ward JP, King JR. Mathematical modelling of drug transport in tumour multicell spheroids and monolayer cultures. Math Biosci. 2003;181:177–207. https://doi.org/10.1016/s0025-5564(02)00148-7 .
Gong X, Lin C, Cheng J, et al. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE. 2015;10: e0130348. https://doi.org/10.1371/journal.pone.0130348 .
Ong S-M, Zhao Z, Arooz T, et al. Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials. 2010;31:1180–90. https://doi.org/10.1016/j.biomaterials.2009.10.049 .
Ma H, Jiang Q, Han S, et al. Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Mol Imaging. 2012;11:487–98.
Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. https://doi.org/10.1016/j.drup.2011.03.001 .
Pasch CA, Favreau PF, Yueh AE, et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin Cancer Res. 2019;25:5376–87. https://doi.org/10.1158/1078-0432.CCR-18-3590 .
Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174:1586-1598.e12. https://doi.org/10.1016/j.cell.2018.07.009 .
Varesano S, Zocchi MR, Poggi A. Zoledronate triggers Vδ2 T cells to destroy and kill spheroids of colon carcinoma: quantitative image analysis of three-dimensional cultures. Front Immunol. 2018;9:998. https://doi.org/10.3389/fimmu.2018.00998 .
Research C for DE and (2019) FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA.
Research C for DE and (2019) FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. FDA.
Jacob F, Salinas RD, Zhang DY, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180:188-204.e22. https://doi.org/10.1016/j.cell.2019.11.036 .
Perel P, Roberts I, Sena E, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334:197. https://doi.org/10.1136/bmj.39048.407928.BE .
Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–8.
PubMed PubMed Central Google Scholar
van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–45. https://doi.org/10.1016/j.cell.2015.03.053 .
Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373-386.e10. https://doi.org/10.1016/j.cell.2017.11.010 .
Beshiri ML, Tice CM, Tran C, et al. A PDX/Organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clin Cancer Res. 2018;24:4332–45. https://doi.org/10.1158/1078-0432.CCR-18-0409 .
Li YF, Gao Y, Liang BW, et al. Patient-derived organoids of non-small cells lung cancer and their application for drug screening. Neoplasma. 2020;67:430–7. https://doi.org/10.4149/neo_2020_190417N346 .
Vaira V, Fedele G, Pyne S, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci USA. 2010;107:8352–6. https://doi.org/10.1073/pnas.0907676107 .
Naipal KAT, Verkaik NS, Sánchez H, et al. Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer. 2016;16:78. https://doi.org/10.1186/s12885-016-2119-2 .
Miura S, Suzuki H, Bae YH. A multilayered cell culture model for transport study in solid tumors: evaluation of tissue penetration of polyethyleneimine based cationic micelles. Nano Today. 2014;9:695–704. https://doi.org/10.1016/j.nantod.2014.10.003 .
Movia D, Bazou D, Volkov Y, Prina-Mello A. Multilayered cultures of NSCLC cells grown at the air-liquid interface allow the efficacy testing of inhaled anti-cancer drugs. Sci Rep. 2018;8:12920. https://doi.org/10.1038/s41598-018-31332-6 .
Weiswald L-B, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. https://doi.org/10.1016/j.neo.2014.12.004 .
Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–5. https://doi.org/10.1245/s10434-018-6486-6 .
Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. https://doi.org/10.1126/science.1150224 .
Hu J, Mirshahidi S, Simental A, et al. Cancer stem cell self-renewal as a therapeutic target in human oral cancer. Oncogene. 2019;38:5440–56. https://doi.org/10.1038/s41388-019-0800-z .
Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. https://doi.org/10.1186/1471-2407-14-173 .
Arima Y, Nobusue H, Saya H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020;111:2689–95. https://doi.org/10.1111/cas.14504 .
Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203–5. https://doi.org/10.1016/j.stem.2009.02.003 .
Ma X-L, Sun Y-F, Wang B-L, et al. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19:760. https://doi.org/10.1186/s12885-019-5963-z .
Poon C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. 2020.
Fröhlich E, Bonstingl G, Höfler A, et al. Comparison of two in vitro systems to assess cellular effects of nanoparticles-containing aerosols. Toxicol In Vitro. 2013;27–360:409–17. https://doi.org/10.1016/j.tiv.2012.08.008 .
Article CAS PubMed Central Google Scholar
Nader E, Skinner S, Romana M, et al. Blood rheology: key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front Physiol. 2019;10:1329. https://doi.org/10.3389/fphys.2019.01329 .
Kenner T. The measurement of blood density and its meaning. Basic Res Cardiol. 1989;84:111–24. https://doi.org/10.1007/BF01907921 .
Carlsson J, Yuhas JM. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1–23. https://doi.org/10.1007/978-3-642-82340-4_1 .
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–24. https://doi.org/10.1038/nprot.2008.226 .
Xiang X, Phung Y, Feng M, et al. The development and characterization of a human mesothelioma in vitro 3D model to investigate immunotoxin therapy. PLoS ONE. 2011;6: e14640. https://doi.org/10.1371/journal.pone.0014640 .
Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11:922–32. https://doi.org/10.1177/1087057106292763 .
Zhang S, Zhang H, Ghia EM, et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci USA. 2019;116:1370–7. https://doi.org/10.1073/pnas.1816262116 .
Froehlich K, Haeger J-D, Heger J, et al. Generation of multicellular breast cancer tumor spheroids: comparison of different protocols. J Mammary Gland Biol Neoplasia. 2016;21:89–98. https://doi.org/10.1007/s10911-016-9359-2 .
Dubois C, Dufour R, Daumar P, et al. Development and cytotoxic response of two proliferative MDA-MB-231 and non-proliferative SUM1315 three-dimensional cell culture models of triple-negative basal-like breast cancer cell lines. Oncotarget. 2017;8:95316–31. https://doi.org/10.18632/oncotarget.20517 .
Carvalho MP, Costa EC, Correia IJ. Assembly of breast cancer heterotypic spheroids on hyaluronic acid coated surfaces. Biotechnol Prog. 2017;33:1346–57. https://doi.org/10.1002/btpr.2497 .
Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. https://doi.org/10.1038/nmeth1015 .
Kano J, Ishiyama T, Nakamura N, et al. Establishment of hepatic stem-like cell lines from normal adult porcine liver in a poly- d -lysine-coated dish with NAIR-1 medium. In Vitro Cell Dev Biol Anim. 2003;39:440–8. https://doi.org/10.1290/1543-706X(2003)039%3c0440:EOHSCL%3e2.0.CO;2 .
Frøen RC, Johnsen EO, Petrovski G, et al. Pigment epithelial cells isolated from human peripheral iridectomies have limited properties of retinal stem cells. Acta Ophthalmol. 2011;89:e635-644. https://doi.org/10.1111/j.1755-3768.2011.02198.x .
Fleurence J, Cochonneau D, Fougeray S, et al. Targeting and killing glioblastoma with monoclonal antibody to O -acetyl GD2 ganglioside. Oncotarget. 2016;7:41172–85. https://doi.org/10.18632/oncotarget.9226 .
Maliszewska-Olejniczak K, Brodaczewska KK, Bielecka ZF, Czarnecka AM. Three-dimensional cell culture model utilization in renal carcinoma cancer stem cell research. Methods Mol Biol. 2018;1817:47–66. https://doi.org/10.1007/978-1-4939-8600-2_6 .
Wang X, Yang P. In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J Vis Exp. 2008. https://doi.org/10.3791/825 .
Leung BM, Lesher-Perez SC, Matsuoka T, et al. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci. 2015;3:336–44. https://doi.org/10.1039/c4bm00319e .
Raghavan S, Mehta P, Horst EN, et al. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7:16948–61. https://doi.org/10.18632/oncotarget.7659 .
Eder T, Eder IE. 3D hanging drop culture to establish prostate cancer organoids. Methods Mol Biol. 2017;1612:167–75. https://doi.org/10.1007/978-1-4939-7021-6_12 .
Létourneau IJ, Quinn MCJ, Wang L-L, et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer. 2012;12:379. https://doi.org/10.1186/1471-2407-12-379 .
Al Habyan S, Kalos C, Szymborski J, McCaffrey L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene. 2018;37:5127–35. https://doi.org/10.1038/s41388-018-0317-x .
Tung Y-C, Hsiao AY, Allen SG, et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–8. https://doi.org/10.1039/c0an00609b .
Sutherland RM, Sordat B, Bamat J, et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46:5320–9.
Hystad ME, Rofstad EK. Oxygen consumption rate and mitochondrial density in human melanoma monolayer cultures and multicellular spheroids. Int J Cancer. 1994;57:532–7. https://doi.org/10.1002/ijc.2910570416 .
Durand RE, Sutherland RM. Effects of intercellular contact on repair of radiation damage. Exp Cell Res. 1972;71:75–80. https://doi.org/10.1016/0014-4827(72)90265-0 .
Hirschhaeuser F, Leidig T, Rodday B, et al. Test system for trifunctional antibodies in 3D MCTS culture. J Biomol Screen. 2009;14:980–90. https://doi.org/10.1177/1087057109341766 .
Masiello T, Dhall A, Hemachandra LPM, et al. A dynamic culture method to produce ovarian cancer spheroids under physiologically-relevant shear stress. Cells. 2018; 7. https://doi.org/10.3390/cells7120277 .
Ingram M, Techy GB, Saroufeem R, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell DevBiol-Animal. 1997;33:459–66. https://doi.org/10.1007/s11626-997-0064-8 .
McNeill EP, Reese RW, Tondon A, et al. Three-dimensional in vitro modeling of malignant bone disease recapitulates experimentally accessible mechanisms of osteoinhibition. Cell Death Dis. 2018; 9. https://doi.org/10.1038/s41419-018-1203-8 .
Zanoni M, Piccinini F, Arienti C, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6:19103. https://doi.org/10.1038/srep19103 .
Chen AK-L, Chen X, Choo ABH, et al. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res. 2011;7:97–111. https://doi.org/10.1016/j.scr.2011.04.007 .
Santini MT, Rainaldi G, Indovina PL. Multicellular tumour spheroids in radiation biology. Int J Radiat Biol. 1999;75:787–99. https://doi.org/10.1080/095530099139845 .
Pan Y, Robertson G, Pedersen L, et al. miR-509-3p is clinically significant and strongly attenuates cellular migration and multi-cellular spheroids in ovarian cancer. Oncotarget. 2016;7:25930–48. https://doi.org/10.18632/oncotarget.8412 .
Urbanczyk M, Zbinden A, Layland SL, et al. Controlled heterotypic pseudo-islet assembly of human β-cells and human umbilical vein endothelial cells using magnetic levitation. Tissue Eng Part A. 2020;26:387–99. https://doi.org/10.1089/ten.TEA.2019.0158 .
Guo WM, Loh XJ, Tan EY, et al. Development of a magnetic 3D spheroid platform with potential application for high-throughput drug screening. Mol Pharm. 2014;11:2182–9. https://doi.org/10.1021/mp5000604 .
Wang Z, Yang P, Xu H, et al. Inhibitory effects of a gradient static magnetic field on normal angiogenesis. Bioelectromagnetics. 2009;30:446–53. https://doi.org/10.1002/bem.20501 .
Zablotskii V, Polyakova T, Lunov O, Dejneka A. How a high-gradient magnetic field could affect cell life. Sci Rep. 2016;6:37407. https://doi.org/10.1038/srep37407 .
Hashimoto Y, Kawasumi M, Saito M. Effect of static magnetic field on cell migration. Electr Eng Japan. 2007;160:46–52. https://doi.org/10.1002/eej.20203 .
Rao SS, Dejesus J, Short AR, et al. Glioblastoma behaviors in three-dimensional collagen-hyaluronan composite hydrogels. ACS Appl Mater Interfaces. 2013;5:9276–84. https://doi.org/10.1021/am402097j .
Li Z-L, Wang Z-J, Wei G-H, et al. Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells. World J Gastrointest Oncol. 2020;12:267–75. https://doi.org/10.4251/wjgo.v12.i3.267 .
Naba A, Clauser KR, Whittaker CA, et al. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer. 2014;14:518. https://doi.org/10.1186/1471-2407-14-518 .
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58. https://doi.org/10.1146/annurev.biochem.77.032207.120833 .
Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–12. https://doi.org/10.1016/j.biomaterials.2011.07.001 .
Zheng L, Hu X, Huang Y, et al. In vivo bioengineered ovarian tumors based on collagen, matrigel, alginate and agarose hydrogels: a comparative study. Biomed Mater. 2015;10: 015016. https://doi.org/10.1088/1748-6041/10/1/015016 .
Dangi-Garimella S, Sahai V, Ebine K, et al. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PLoS ONE. 2013;8: e64566. https://doi.org/10.1371/journal.pone.0064566 .
Ayuso JM, Vitek R, Swick AD, et al. Effects of culture method on response to EGFR therapy in head and neck squamous cell carcinoma cells. Sci Rep. 2019;9:12480. https://doi.org/10.1038/s41598-019-48764-3 .
Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun. 2013;433:327–32. https://doi.org/10.1016/j.bbrc.2013.03.008 .
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. https://doi.org/10.1016/j.semcancer.2005.05.004 .
Benton G, Arnaoutova I, George J, et al. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79–80:3–18. https://doi.org/10.1016/j.addr.2014.06.005 .
Zhang Y, Jiang B, Lee MH. A novel 3D model for visualization and tracking of fibroblast-guided directional cancer cell migration. Biology (Basel) 2020; 9. https://doi.org/10.3390/biology9100328 .
Bahmad HF, Cheaito K, Chalhoub RM, et al. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 2018;8:347. https://doi.org/10.3389/fonc.2018.00347 .
Bodgi L, Bahmad HF, Araji T, et al. Assessing radiosensitivity of bladder cancer in vitro: a 2D vs. 3D approach. Front Oncol. 2019;9:153. https://doi.org/10.3389/fonc.2019.00153 .
Boj SF, Hwang C-I, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–38. https://doi.org/10.1016/j.cell.2014.12.021 .
Seppälä TT, Zimmerman JW, Sereni E, et al. Patient-derived organoid pharmacotyping is a clinically tractable strategy for precision medicine in pancreatic cancer. Ann Surg. 2020;272:427–35. https://doi.org/10.1097/SLA.0000000000004200 .
Jiang S, Zhao H, Zhang W, et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Rep Med. 2020;1: 100161. https://doi.org/10.1016/j.xcrm.2020.100161 .
Abe Y, Tada A, Isoyama J, et al. Improved phosphoproteomic analysis for phosphosignaling and active-kinome profiling in Matrigel-embedded spheroids and patient-derived organoids. Sci Rep. 2018;8:11401. https://doi.org/10.1038/s41598-018-29837-1 .
Johnson J, Sharick JT, Skala MC, Li L. Sample preparation strategies for high-throughput mass spectrometry imaging of primary tumor organoids. J Mass Spectrom. 2020;55: e4452. https://doi.org/10.1002/jms.4452 .
Weyers A, Yang B, Yoon DS, et al. A structural analysis of glycosaminoglycans from lethal and nonlethal breast cancer tissues: toward a novel class of theragnostics for personalized medicine in oncology? OMICS. 2012;16:79–89. https://doi.org/10.1089/omi.2011.0102 .
Campbell JJ, Davidenko N, Caffarel MM, et al. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS ONE. 2011;6: e25661. https://doi.org/10.1371/journal.pone.0025661 .
Xu X, Gurski LA, Zhang C, et al. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33:9049–60. https://doi.org/10.1016/j.biomaterials.2012.08.061 .
Xiao W, Zhang R, Sohrabi A, et al. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res. 2018;78:1358–70. https://doi.org/10.1158/0008-5472.CAN-17-2429 .
Gurski LA, Xu X, Labrada LN, et al. Hyaluronan (HA) interacting proteins RHAMM and hyaluronidase impact prostate cancer cell behavior and invadopodia formation in 3D HA-based hydrogels. PLoS ONE. 2012;7: e50075. https://doi.org/10.1371/journal.pone.0050075 .
Shen Y-I, Abaci HE, Krupsi Y, et al. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater Sci. 2014;2:655–65. https://doi.org/10.1039/C3BM60274E .
Liu H-Y, Korc M, Lin C-C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell–matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials. 2018;160:24–36. https://doi.org/10.1016/j.biomaterials.2018.01.012 .
Gurski LA, Jha AK, Zhang C, et al. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30:6076–85. https://doi.org/10.1016/j.biomaterials.2009.07.054 .
Tang Y, Huang B, Dong Y, et al. Three-dimensional prostate tumor model based on a hyaluronic acid-alginate hydrogel for evaluation of anti-cancer drug efficacy. J Biomater Sci Polym Ed. 2017;28:1603–16. https://doi.org/10.1080/09205063.2017.1338502 .
Liu C, Lewin Mejia D, Chiang B, et al. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018;75:213–25. https://doi.org/10.1016/j.actbio.2018.06.003 .
Chen D, Qu Y, Hua X, et al. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye (Lond). 2017;31:962–71. https://doi.org/10.1038/eye.2017.8 .
Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017;2017:9831534. https://doi.org/10.1155/2017/9831534 .
Pinto ML, Rios E, Silva AC, et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials. 2017;124:211–24. https://doi.org/10.1016/j.biomaterials.2017.02.004 .
Hoshiba T, Tanaka M. Decellularized matrices as in vitro models of extracellular matrix in tumor tissues at different malignant levels: mechanism of 5-fluorouracil resistance in colorectal tumor cells. Biochim Biophys Acta. 2016;1863:2749–57. https://doi.org/10.1016/j.bbamcr.2016.08.009 .
Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. https://doi.org/10.1016/j.biomaterials.2010.02.044 .
Sieh S, Taubenberger AV, Rizzi SC, et al. Phenotypic characterization of prostate cancer LNCaP cells cultured within a bioengineered microenvironment. PLoS ONE. 2012;7: e40217. https://doi.org/10.1371/journal.pone.0040217 .
Pradhan S, Slater JH. Fabrication, characterization, and implementation of engineered hydrogels for controlling breast cancer cell phenotype and dormancy. MethodsX. 2019;6:2744–66. https://doi.org/10.1016/j.mex.2019.11.011 .
Liang Y, Jeong J, DeVolder RJ, et al. A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials. 2011;32:9308–15. https://doi.org/10.1016/j.biomaterials.2011.08.045 .
Kaphle P, Li Y, Yao L. The mechanical and pharmacological regulation of glioblastoma cell migration in 3D matrices. J Cell Physiol. 2019;234:3948–60. https://doi.org/10.1002/jcp.27209 .
Reynolds DS, Bougher KM, Letendre JH, et al. Mechanical confinement via a PEG/collagen interpenetrating network inhibits behavior characteristic of malignant cells in the triple negative breast cancer cell line MDA.MB.231. Acta Biomater. 2018;77:85–95. https://doi.org/10.1016/j.actbio.2018.07.032 .
Tsao C-T, Kievit FM, Wang K, et al. Chitosan-based thermoreversible hydrogel as an in vitro tumor microenvironment for testing breast cancer therapies. Mol Pharm. 2014;11:2134–42. https://doi.org/10.1021/mp5002119 .
Chang F-C, Tsao C-T, Lin A, et al. PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells. Polymers (Basel) 2016; 8. https://doi.org/10.3390/polym8040112 .
Chang F-C, Levengood SL, Cho N, et al. Crosslinked chitosan-PEG hydrogel for culture of human glioblastoma cell spheroids and drug screening. Adv Ther (Weinh). 2018; 1. https://doi.org/10.1002/adtp.201800058 .
Beck JN, Singh A, Rothenberg AR, et al. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials. 2013;34:9486–95. https://doi.org/10.1016/j.biomaterials.2013.08.077 .
You Z, Cao H, Gao J, et al. A functionalizable polyester with free hydroxyl groups and tunable physiochemical and biological properties. Biomaterials. 2010;31:3129–38. https://doi.org/10.1016/j.biomaterials.2010.01.023 .
Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–14. https://doi.org/10.1016/0142-9612(96)85755-3 .
Fong ELS, Lamhamedi-Cherradi S-E, Burdett E, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci USA. 2013;110:6500–5. https://doi.org/10.1073/pnas.1221403110 .
Balachander GM, Talukdar PM, Debnath M, et al. Inflammatory role of cancer-associated fibroblasts in invasive breast tumors revealed using a fibrous polymer scaffold. ACS Appl Mater Interfaces. 2018;10:33814–26. https://doi.org/10.1021/acsami.8b07609 .
Nayak B, Balachander GM, Manjunath S, et al. Tissue mimetic 3D scaffold for breast tumor-derived organoid culture toward personalized chemotherapy. Colloids Surf B Biointerfaces. 2019;180:334–43. https://doi.org/10.1016/j.colsurfb.2019.04.056 .
Girard YK, Wang C, Ravi S, et al. A 3D fibrous scaffold inducing tumoroids: a platform for anticancer drug development. PLoS ONE. 2013;8: e75345. https://doi.org/10.1371/journal.pone.0075345 .
Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. https://doi.org/10.1038/nbt874 .
Lee S, Trinh THT, Yoo M, et al. Self-assembling peptides and their application in the treatment of diseases. Int J Mol Sci 2019; 20. https://doi.org/10.3390/ijms20235850 .
Ashworth JC, Thompson JL, James JR, et al. Peptide gels of fully-defined composition and mechanics for probing cell–cell and cell–matrix interactions in vitro. Matrix Biol. 2020;85–86:15–33. https://doi.org/10.1016/j.matbio.2019.06.009 .
Liu J, Huang W, Pang Y, et al. Molecular self-assembly of a homopolymer: an alternative to fabricate drug-delivery platforms for cancer therapy. Angew Chem Int Ed Engl. 2011;50:9162–6. https://doi.org/10.1002/anie.201102280 .
Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels—versatile matrices for 3D cell culture in cancer medicine. Front Oncol. 2015;5:92. https://doi.org/10.3389/fonc.2015.00092 .
Tang C, Shao X, Sun B, et al. The effect of self-assembling peptide RADA16-I on the growth of human leukemia cells in vitro and in nude mice. Int J Mol Sci. 2009;10:2136–45. https://doi.org/10.3390/ijms10052136 .
Mi K, Wang G, Liu Z, et al. Influence of a self-assembling peptide, RADA16, compared with collagen I and Matrigel on the malignant phenotype of human breast-cancer cells in 3D cultures and in vivo. Macromol Biosci. 2009;9:437–43. https://doi.org/10.1002/mabi.200800262 .
Betriu N, Semino CE. Development of a 3D co-culture system as a cancer model using a self-assembling peptide scaffold. Gels. 2018; 4. https://doi.org/10.3390/gels4030065 .
Wu LY, Di Carlo D, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008;10:197–202. https://doi.org/10.1007/s10544-007-9125-8 .
Tellez-Gabriel M, Cochonneau D, Cadé M, et al. Circulating tumor cell-derived pre-clinical models for personalized medicine. Cancers (Basel). 2018; 11. https://doi.org/10.3390/cancers11010019.
Jeon JS, Bersini S, Gilardi M, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci USA. 2015;112:214–9. https://doi.org/10.1073/pnas.1417115112 .
Toh Y-C, Raja A, Yu H, van Noort D. A 3D microfluidic model to recapitulate cancer cell migration and invasion. Bioengineering (Basel). 2018;5. https://doi.org/10.3390/bioengineering5020029 .
Miller CP, Tsuchida C, Zheng Y, et al. A 3D human renal cell carcinoma-on-a-chip for the study of tumor angiogenesis. Neoplasia. 2018;20:610–20. https://doi.org/10.1016/j.neo.2018.02.011 .
Pinho D, Santos D, Vila A, Carvalho S. Establishment of colorectal cancer organoids in microfluidic-based system. Micromachines (Basel). 2021;12:497. https://doi.org/10.3390/mi12050497 .
Schuster B, Junkin M, Kashaf SS, et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat Commun. 2020;11:5271. https://doi.org/10.1038/s41467-020-19058-4 .
Berthier E, Young EWK, Beebe D. Engineers are from PDMS-land, biologists are from polystyrenia. Lab Chip. 2012;12:1224–37. https://doi.org/10.1039/c2lc20982a .
Ko J, Ahn J, Kim S, et al. Tumor spheroid-on-a-chip: a standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip. 2019;19:2822–33. https://doi.org/10.1039/c9lc00140a .
Kačarević ŽP, Rider PM, Alkildani S, et al. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel). 2018; 11. https://doi.org/10.3390/ma11112199 .
Rider P, Kačarević ŽP, Alkildani S, et al. Bioprinting of tissue engineering scaffolds. J Tissue Eng. 2018;9:2041731418802090. https://doi.org/10.1177/2041731418802090 .
Gómez-Oliva R, Domínguez-García S, Carrascal L, et al. Evolution of experimental models in the study of glioblastoma: toward finding efficient treatments. Front Oncol 2021; 10. https://doi.org/10.3389/fonc.2020.614295 .
Heinrich MA, Bansal R, Lammers T, et al. 3D-bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv Mater. 2019;31: e1806590. https://doi.org/10.1002/adma.201806590 .
Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. https://doi.org/10.1186/s40824-018-0122-1 .
Kim SH, Yeon YK, Lee JM, et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun. 2018;9:1620. https://doi.org/10.1038/s41467-018-03759-y .
Petta D, Armiento AR, Grijpma D, et al. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication. 2018;10: 044104. https://doi.org/10.1088/1758-5090/aadf58 .
Rutz AL, Hyland KE, Jakus AE, et al. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater. 2015;27:1607–14. https://doi.org/10.1002/adma.201405076 .
Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. https://doi.org/10.1016/j.biomaterials.2016.06.012 .
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. https://doi.org/10.1038/nbt.2958 .
Hölzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8: 032002. https://doi.org/10.1088/1758-5090/8/3/032002 .
Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3:144–56. https://doi.org/10.1016/j.bioactmat.2017.11.008 .
Kirchmajer DM, Gorkin Iii R, In Het Panhuis M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J Mater Chem B. 2015;3:4105–17. https://doi.org/10.1039/c5tb00393h .
Ning L, Chen X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol J. 2017; 12. https://doi.org/10.1002/biot.201600671 .
Pedde RD, Mirani B, Navaei A, et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater. 2017; 29:. https://doi.org/10.1002/adma.201606061 .
Malda J, Visser J, Melchels FP, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–28. https://doi.org/10.1002/adma.201302042 .
Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338:921–6. https://doi.org/10.1126/science.1226340 .
Ferris CJ, Gilmore KG, Wallace GG, In het Panhuis M. Biofabrication: an overview of the approaches used for printing of living cells. Appl Microbiol Biotechnol. 2013;97:4243–58. https://doi.org/10.1007/s00253-013-4853-6 .
Maloney E, Clark C, Sivakumar H, et al. Immersion bioprinting of tumor organoids in multi-well plates for increasing chemotherapy screening throughput. Micromachines (Basel). 2020;11:208. https://doi.org/10.3390/mi11020208 .
Article PubMed Central Google Scholar
Catros S, Fricain J-C, Guillotin B, et al. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 2011;3: 025001. https://doi.org/10.1088/1758-5082/3/2/025001 .
Miri AK, Nieto D, Iglesias L, et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater. 2018;30: e1800242. https://doi.org/10.1002/adma.201800242 .
Soman P, Chung PH, Zhang AP, Chen S. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol Bioeng. 2013;110:3038–47. https://doi.org/10.1002/bit.24957 .
Xu L, Sheybani N, Yeudall WA, Yang H. The effect of photoinitiators on intracellular AKT signaling pathway in tissue engineering application. Biomater Sci. 2015;3:250–5. https://doi.org/10.1039/C4BM00245H .
Bassi G, Panseri S, Dozio SM, et al. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci Rep. 2020;10:22294. https://doi.org/10.1038/s41598-020-79448-y .
Edelman LB, Eddy JA, Price ND. In silico models of cancer. Wiley Interdiscip Rev Syst Biol Med. 2010;2:438–59. https://doi.org/10.1002/wsbm.75 .
Jean-Quartier C, Jeanquartier F, Jurisica I, Holzinger A. In silico cancer research towards 3R. BMC Cancer. 2018;18:408. https://doi.org/10.1186/s12885-018-4302-0 .
Riss T, Trask OJ Jr. Factors to consider when interrogating 3D culture models with plate readers or automated microscopes. In Vitro Cell Dev Biol -Animal. 2021;57:238–56. https://doi.org/10.1007/s11626-020-00537-3 .
Thurber GM, Wittrup KD. Quantitative spatiotemporal analysis of antibody fragment diffusion and endocytic consumption in tumor spheroids. Cancer Res. 2008;68:3334–41. https://doi.org/10.1158/0008-5472.CAN-07-3018 .
Tchoryk A, Taresco V, Argent RH, et al. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjug Chem. 2019;30:1371–84. https://doi.org/10.1021/acs.bioconjchem.9b00136 .
Smyrek I, Stelzer EHK. Quantitative three-dimensional evaluation of immunofluorescence staining for large whole mount spheroids with light sheet microscopy. Biomed Opt Express. 2017;8:484–99. https://doi.org/10.1364/BOE.8.000484 .
Download references
Acknowledgements
Not applicable.
The present work was supported by internal grant of the Institut de Cancérologie de l’Ouest, Saint-Herblain, France (Recipient: Prof. D. Heymann).
Author information
Camille Jubelin and Javier Muñoz-Garcia contributed equally to the work
Authors and Affiliations
Nantes Université, CNRS, US2B, UMR 6286, 44000, Nantes, France
Camille Jubelin, Javier Muñoz-Garcia & Dominique Heymann
Institut de Cancérologie de l’Ouest, Tumor Heterogeneity and Precision Medicine Lab.. Université de Nantes, 44805, Saint-Herblain, France
Camille Jubelin, Javier Muñoz-Garcia, Denis Cochonneau, Emilie Ollivier, Marie-Françoise Heymann & Dominique Heymann
Atlantic Bone Screen, Saint-Herblain, France
Camille Jubelin
Univ Rennes, CNRS, INSERM, Biosit UAR 3480 US_S 018, BIM3D core facility, Rennes, France
Laurent Griscom
Nantes Université, INSERM, CRCINA, UMR1232, Nantes, France
François M. Vallette & Lisa Oliver
CHU de Nantes, Nantes, France
Lisa Oliver
Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK
Dominique Heymann
You can also search for this author in PubMed Google Scholar
Contributions
CJ and JMG wrote the first draft of the manuscript that have been corrected and approved by all authors. CJ and JMG contributed equally to the works. DH coordinated the works. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Dominique Heymann .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
With the submission of this manuscript we would like to undertake that all authors of this paper have read and approved the final version submitted.
Competing interests
Atlantic Bone Screen paid the salary of Camille Jubelin . The authors declare that they have no known conflicting financial interests or personal relationships that could have influenced the work reported in this manuscript.
Availability of data and materials
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jubelin, C., Muñoz-Garcia, J., Griscom, L. et al. Three-dimensional in vitro culture models in oncology research. Cell Biosci 12 , 155 (2022). https://doi.org/10.1186/s13578-022-00887-3
Download citation
Received : 19 January 2022
Accepted : 18 August 2022
Published : 11 September 2022
DOI : https://doi.org/10.1186/s13578-022-00887-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 3D cell culture
- Multicellular tumor spheroid
- Liquid-based 3D culture
- Scaffold-based 3D culture
- Microfluidics
Cell & Bioscience
ISSN: 2045-3701
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
In vitro plant tissue culture: means for production of biological active compounds
Affiliations.
- 1 Tecnologico de Monterrey, Campus Monterrey, Escuela de Ingeniería y Ciencias, Ave. Eugenio Garza Sada 2501, 64849, Monterrey, NL, México.
- 2 Tecnologico de Monterrey, Campus Monterrey, Escuela de Ingeniería y Ciencias, Ave. Eugenio Garza Sada 2501, 64849, Monterrey, NL, México. [email protected].
- PMID: 29736623
- PMCID: PMC7088179
- DOI: 10.1007/s00425-018-2910-1
Plant tissue culture as an important tool for the continuous production of active compounds including secondary metabolites and engineered molecules. Novel methods (gene editing, abiotic stress) can improve the technique. Humans have a long history of reliance on plants for a supply of food, shelter and, most importantly, medicine. Current-day pharmaceuticals are typically based on plant-derived metabolites, with new products being discovered constantly. Nevertheless, the consistent and uniform supply of plant pharmaceuticals has often been compromised. One alternative for the production of important plant active compounds is in vitro plant tissue culture, as it assures independence from geographical conditions by eliminating the need to rely on wild plants. Plant transformation also allows the further use of plants for the production of engineered compounds, such as vaccines and multiple pharmaceuticals. This review summarizes the important bioactive compounds currently produced by plant tissue culture and the fundamental methods and plants employed for their production.
Keywords: Bioactive; In vitro plant culture; Plant pharmaceuticals; Plant tissue culture; Secondary metabolites; Transformation.
PubMed Disclaimer
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Diagram of current methods employed…
Diagram of current methods employed for the large-scale production of bioactive compounds using…
Similar articles
- Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Niazian M, Sabbatini P. Niazian M, et al. Planta. 2021 Oct 30;254(6):111. doi: 10.1007/s00425-021-03771-5. Planta. 2021. PMID: 34718882 Review.
- An update on biotechnological intervention mediated by plant tissue culture to boost secondary metabolite production in medicinal and aromatic plants. Prashant SP, Bhawana M. Prashant SP, et al. Physiol Plant. 2024 Jul-Aug;176(4):e14400. doi: 10.1111/ppl.14400. Physiol Plant. 2024. PMID: 38945697 Review.
- Current approaches toward production of secondary plant metabolites. Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M. Hussain MS, et al. J Pharm Bioallied Sci. 2012 Jan;4(1):10-20. doi: 10.4103/0975-7406.92725. J Pharm Bioallied Sci. 2012. PMID: 22368394 Free PMC article.
- Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Hasnain A, Naqvi SAH, Ayesha SI, Khalid F, Ellahi M, Iqbal S, Hassan MZ, Abbas A, Adamski R, Markowska D, Baazeem A, Mustafa G, Moustafa M, Hasan ME, Abdelhamid MMA. Hasnain A, et al. Front Plant Sci. 2022 Oct 13;13:1009395. doi: 10.3389/fpls.2022.1009395. eCollection 2022. Front Plant Sci. 2022. PMID: 36311115 Free PMC article. Review.
- Specialized Plant Metabolism Characteristics and Impact on Target Molecule Biotechnological Production. Matsuura HN, Malik S, de Costa F, Yousefzadi M, Mirjalili MH, Arroo R, Bhambra AS, Strnad M, Bonfill M, Fett-Neto AG. Matsuura HN, et al. Mol Biotechnol. 2018 Feb;60(2):169-183. doi: 10.1007/s12033-017-0056-1. Mol Biotechnol. 2018. PMID: 29290031 Review.
- Effect of In Vitro Micropropagation on the Chemical, Antioxidant, and Biological Characteristics of Senecio nutans Sch. Bip., an Endemic Plant of the Atacama Desert Andean Region. Parra C, Muñoz-Torres P, Escobar H, Simirgiotis MJ, Contreras-Contreras G, Ruiz-Fernández Á, Maulen C, Martínez-Cifuentes M, Mariotti-Celis MS. Parra C, et al. Plants (Basel). 2024 Mar 7;13(6):755. doi: 10.3390/plants13060755. Plants (Basel). 2024. PMID: 38592747 Free PMC article.
- Ceratophyllum demersum the submerged macrophyte from the mining subsidence reservoir Nadrybie Poland as a source of anticancer agents. Masłyk M, Lenard T, Olech M, Martyna A, Poniewozik M, Boguszewska-Czubara A, Kochanowicz E, Czubak P, Kubiński K. Masłyk M, et al. Sci Rep. 2024 Mar 20;14(1):6661. doi: 10.1038/s41598-024-57375-6. Sci Rep. 2024. PMID: 38509188 Free PMC article.
- Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus scutellarioides in a Cell Model. Kowalczyk T, Sikora J, Merecz-Sadowska A, Kukula-Koch W, Synowiec E, Majda A, Juda D, Śliwiński T, Sitarek P. Kowalczyk T, et al. Int J Mol Sci. 2024 Jan 15;25(2):1043. doi: 10.3390/ijms25021043. Int J Mol Sci. 2024. PMID: 38256118 Free PMC article.
- Nutritional characteristics and antiradical activity of turmeric ( Curcuma longa L.), beetroot ( Beta vulgaris L.), and carrot ( Daucus carota L.) grown in Bangladesh. Moulick SP, Jahan F, Islam MB, Bashera MA, Hasan MS, Islam MJ, Ahmed S, Karmakar D, Ahmed F, Saha T, Dey SS, Boby F, Saha M, Saha BK, Bhuiyan MNH. Moulick SP, et al. Heliyon. 2023 Oct 31;9(11):e21495. doi: 10.1016/j.heliyon.2023.e21495. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027870 Free PMC article.
- Total Content of Saponins, Phenols and Flavonoids and Antioxidant and Antimicrobial Activity of In Vitro Culture of Allochrusa gypsophiloides (Regel) Schischk Compared to Wild Plants. Mursaliyeva VK, Sarsenbek BT, Dzhakibaeva GT, Mukhanov TM, Mammadov R. Mursaliyeva VK, et al. Plants (Basel). 2023 Oct 10;12(20):3521. doi: 10.3390/plants12203521. Plants (Basel). 2023. PMID: 37895985 Free PMC article.
- Abdallah NA, Prakash CS, McHughen AG. Genome editing for crop improvement: challenges and opportunities. GM Crops Food. 2015;6:183–205. doi: 10.1080/21645698.2015.1129937. - DOI - PMC - PubMed
- Afolayan AJ, Adebola PO. In vitro propagation: a biotechnological tool capable of solving the problem of medicinal plants decimation in South Africa. Afr J Biotechnol. 2004;3:683–687.
- Ahloowalia BS, Savangikar VA (2003) Low cost options for energy and labour. Low cost options for tissue culture technology in developing countries. In: Proceedings of a technical meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and held in Vienna, 26–30 August 2002 (pp. 41–46). Viennam Austria: International Atomic Energy Agency. ISBN 92–0–115903–X
- Ahloowalia BS, Prakash J, Savangikar VA, Savangikar C (2003) Plant tissue culture. Low cost options for tissue culture technology in developing countries. In: Proceedings of a technical meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and held in Vienna, 26–30 August 2002 (pp. 3–10). Vienna, Austria: International Atomic Energy Agency. ISBN 92–0–115903–X
- Ali RM, Abbas HM. Response of salt stressed barley seedlings to phenylurea. Plant Soil Environ. 2003;49:158–162. doi: 10.17221/4107-PSE. - DOI
Publication types
- Search in MeSH
Related information
Grants and funding.
- 00000025/CONACYT
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
In vitro technology in plant conservation: relevance to biocultural diversity.

1. Introduction
2. the loss of plant diversity: threats, drivers, and magnitude, biocultural diversity and plant conservation, 3. plant conservation and social dimension of technoscience, 3.1. studies on the conservation of plants: limitations and opportunities, 3.2. indigenous plants, 3.3. social dimension, technoscience and plant relationships: concerns, 4. the nexus of in vitro technology and plant conservation, 4.1. micropropagation and cryopreservation methods, 4.2. advantages of in vitro technology.
- In vitro methods facilitate tapping into the abilities of plant tissue to reproduce vegetatively from limited starting material, thus reducing the need to harvest whole plants or numerous plants from the wild, preventing the depletion of vulnerable populations in their natural habitats. Plant multiplication by in vitro technology is achieved by proliferation of the apical or axillary meristems, which consist of rapidly growing cells, are generally genetically consistent, relatively virus-free, and bear greater capacity for multiplication compared to non-meristematic tissues. Alternately, plants can be propagated by regeneration, in which individual plant cells express their inherent capacity, referred to as “totipotency”, to divide and differentiate to form complete plants. Both modes of plant propagation have specific advantages. While multiplication using pre-existing meristems is known to produce genetically identical clones that can be used to enrich a specific population, the plants produced by regeneration may exhibit genetic variations that can be exploited to create genetically diverse plant populations. Thus, in vitro technology allows regeneration of fully functional specimens from small amounts of tissue, saving time and money and reducing the need to harvest numerous specimens. The mass production of rare or useful plants via micropropagation diverts from overexploitation of wild specimens for commercial use [ 131 , 169 ].
- In vitro grown specimens of threatened or rare plants can also help populate ex situ collections in botanical gardens and other research institutions that can be later re-introduced in natural habitats [ 92 , 170 , 171 ]. This allows for the short and long-term storage of germplasm, which protects it from current threats, and also facilitates the selection of genotypes for future use [ 172 ].
- These techniques allow for an unlimited amount of explant production to supply material for scientific experiments, allowing one to replicate tests ad-lib under rigorous standards and to engage in large trial-and-error conservation interventions [ 129 , 173 , 174 ].
4.3. Limitations of In Vitro Technologies
4.4. challenges and opportunities at the interface of plant conservation, in vitro technology, and indigenous plants, 5. final considerations about in vitro technology in biocultural conservation, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Barnhill-Dilling, S.K.; Delborne, J.A. Whose Intentions? What Consequences? Interrogating “Intended Consequences” for Conservation with Environmental Biotechnology. Conserv. Sci. Pract. 2021 , 3 , e406. [ Google Scholar ] [ CrossRef ]
- Pretty, J.; Adams, B.; Berkes, F.; Ferreira, S.; Dudley, N.; Hunn, E.; Maffi, L.; Milton, K.; Rapport, D.; Robbins, P.; et al. The Intersections of Biological Diversity and Cultural Diversity: Towards Integration. Conserv. Soc. 2009 , 7 , 100–112. [ Google Scholar ]
- Dunn, L.; Burney, L. Ethnobotany, the Science of Survival: A Declaration from Kaua’i. Econ. Bot. 2007 , 61 , 1–2. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Isbell, F.; Calcagno, V.; Hector, A.; Connolly, J.; Harpole, W.S.; Reich, P.B.; Scherer-Lorenzen, M.; Schmid, B.; Tilman, D.; van Ruijen, J.; et al. High Plant Diversity Is Needed to Maintain Ecosystem Services. Nature 2011 , 477 , 199–202. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Heywood, V.H. Plant Conservation in the Anthropocene–Challenges and Future Prospects. Plant Divers. 2017 , 39 , 314–330. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Amos, R. Reassessing the Role of Plants in Society. Int. J. Law Context 2017 , 13 , 295–315. [ Google Scholar ] [ CrossRef ]
- Cunsolo, A.; Ellis, N.R. Ecological Grief as a Mental Health Response to Climate Change-Related Loss. Nat. Clim. Chang. 2018 , 8 , 275–281. [ Google Scholar ] [ CrossRef ]
- Benn, J. What Is Biodiversity? United Nations Environment Programme, 2. ; World Conservation Monitoring Centre: New York, NY, USA, 2010; Available online: https://www.unesco.pl/fileadmin/user_upload/pdf/BIODIVERSITY_FACTSHEET.pdf (accessed on 14 September 2021).
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A. Biodiversity Loss and Its Impact on Humanity. Nature 2012 , 486 , 59–67. [ Google Scholar ] [ CrossRef ]
- Mace, G.M. The Ecology of Natural Capital Accounting. Oxf. Rev. Econ. Policy 2019 , 35 , 54–67. [ Google Scholar ] [ CrossRef ]
- Pimm, S.L.; Alibhai, S.; Bergl, R.; Dehgan, A.; Giri, C.; Jewell, Z.; Joppa, L.; Kays, R.; Loarie, S. Emerging Technologies to Conserve Biodiversity. Trends Ecol. Evol. 2015 , 30 , 685–696. [ Google Scholar ] [ CrossRef ]
- Harfoot, M.B.; Tittensor, D.P.; Knight, S.; Arnell, A.P.; Blyth, S.; Brooks, S.; Burgess, N.D. Present and Future Biodiversity Risks from Fossil Fuel Exploitation. Conserv. Lett. 2018 , 11 , e12448. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bradshaw, C.J.; Ehrlich, P.R.; Beattie, A.; Ceballos, G.; Crist, E.; Diamond, J.; Dirzo, R.; Ehrlich, A.; Harte, M.E.; Pyke, G.; et al. Underestimating the Challenges of Avoiding a Ghastly Future. Front. Conserv. Sci. 2021 , 1 , 9. [ Google Scholar ] [ CrossRef ]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S. Benefits of Increasing Plant Diversity in Sustainable Agroecosystems. J. Ecol. 2017 , 105 , 871–879. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Zhang, Y.; Lü, X.; Isbell, F.; Stevens, C.; Han, X.; He, N.; Zhang, G.; Yu, Q.; Huang, J.; Han, X. Rapid Plant Species Loss at High Rates and at Low Frequency of N Addition in Temperate Steppe. Glob. Chang. Biol. 2014 , 20 , 3520–3529. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.; Palmer, T. Accelerated Modern Human–Induced Species Losses: Entering the Sixth Mass Extinction. Sci. Adv. 2015 , 1 , e1400253. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- De Vos, J.; Joppa, L.; Gittleman, J.L.; Stephens, P.R.; Pimm, S.L. Estimating the Normal Background Rate of Species Extinction. Conserv. Biol. 2015 , 29 , 452–462. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the Brink as Indicators of Biological Annihilation and the Sixth Mass Extinction. Proc. Natl. Acad. Sci. USA 2020 , 117 , 13596–13602. [ Google Scholar ] [ CrossRef ]
- Hawkins, B. Plants for Life: Medicinal Plant Conservation and Botanic Gardens ; BGCI: Richmond, UK, 2007. [ Google Scholar ]
- Pandit, R.; Pörtner, H.O.; Scholes, R.J.; Agard, J.; Archer, E.; Arneth, A.; Bai, X.; Barnes, D.; Burrows, M.; Chan, L.; et al. Scientific Outcome of the IPBES-IPCC Co-Sponsored Workshop on Biodiversity and Climate Change ; IPBES Secretariat—UWA School of Agriculture: Bonn, Germany, 2021; p. 256. [ Google Scholar ]
- Schmidt, B.; Gemeinholzer, B.; Treolar, A. Open Data in Global Environmental Research: The Belmont Forum’s Open Data Survey. PLoS ONE 2016 , 11 , e0146695. [ Google Scholar ] [ CrossRef ]
- Hanspach, J.; Haider, L.J.; Oteros-Rozas, E.; Olafsson, A.S.; Gulsrud, N.M.; Raymond, C.M.; Torralba, M.; Martín-López, B.; Bieling, C.; Garcia-Martin, M. Biocultural Approaches to Sustainability: A Systematic Review of the Scientific Literature. People Nat. 2020 , 2 , 643–659. [ Google Scholar ] [ CrossRef ]
- Elands, B.; Vierikko, K.; Andersson, E.; Fischer, L.; Goncalves, P.; Haase, D.; Kowarik, I.; Luz, A.; Niemelä, J.; Santos-Reis, M. Biocultural Diversity: A Novel Concept to Assess Human-Nature Interrelations, Nature Conservation and Stewardship in Cities. Urban For. Urban Green. 2019 , 40 , 29–34. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hemstrom, K.; Palmer, H. On participatory research, knowledge integration and societal transformation. In Anatomy of a 21st-Century Sustainability Project: The Untold Stories ; Dymitrow, M., Ingelhag, K., Eds.; Mistra Urban Futures; Chalmers University of Technology: Gothenburg, Sweden, 2019; pp. 29–37. [ Google Scholar ]
- Mayhew, P.J. Global Climate and Extinction: Evidence from the fossil records. In Climate Change, Ecology and Systematics ; Hodkinson, T.R., Jones, M.B., Waldren, S., Parnell, J.A.N., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 99–121. [ Google Scholar ]
- Mayhew, P.J.; Jenkins, G.B.; Benton, T.G. A Long-Term Association between Global Temprature and Biodiversity. Origination and Extinction in the Fossil Record. Proc. R. Soc. B Biol. Sci. 2008 , 275 , 47–53. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Vellend, M. Habitat Loss Inhibits Recovery of Plant Diversity as Forests Regrow. Ecology 2003 , 84 , 1158–1164. [ Google Scholar ] [ CrossRef ]
- Watson, J.E.; Evans, T.D.; Venter, O.; Maxwell, S.L. Manage Forests as Protection against Warming. Nature 2019 , 567 , 311–312. [ Google Scholar ] [ CrossRef ]
- Turney, C.; Ausseil, A.-G.; Broadhurst, L. Urgent Need for an Integrated Policy Framework for Biodiversity Loss and Climate Change. Nat. Ecol. Evol. 2020 , 4 , 996. [ Google Scholar ] [ CrossRef ]
- Thomas, K.; Hardy, R.D.; Lazrus, H.; Mendez, M.; Orlove, B.; Rivera-Collazo, I.; Roberts, J.T.; Rockman, M.; Warner, B.P.; Winthrop, R. Explaining Differential Vulnerability to Climate Change: A Social Science Review. Wiley Interdiscip. Rev. Clim. Chang. 2019 , 10 , e565. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate Change Threats to Plant Diversity in Europe. Proc. Natl. Acad. Sci. USA 2005 , 102 , 8245–8250. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Petelka, J.; Plagg, B.; Säumel, I.; Zerbe, S. Traditional Medicinal Plants in South Tyrol (Northern Italy, Southern Alps): Biodiversity and Use. J. Ethnobiol. Ethnomed. 2020 , 16 , 1–15. [ Google Scholar ] [ CrossRef ]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G. Ten Golden Rules for Reforestation to Optimize Carbon Sequestration, Biodiversity Recovery and Livelihood Benefits. Glob. Chang. Biol. 2021 , 27 , 1328–1348. [ Google Scholar ] [ CrossRef ]
- Allan, R.P.; Hawkins, E.; Bellouin, N.; Collins, B. IPC. Summary for policymakers. In Climate Change 2021: The Physical Science Basis ; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM_final.pdf (accessed on 7 November 2021).
- Oldekop, J.A.; Sims, K.R.E.; Karna, B.K.; Whittingham, M.J.; Agrawal, A. Reductions in Deforestation and Poverty from Decentralized Forest Management in Nepal. Nat. Sustain. 2019 , 2 , 421–428. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Strassburg, B.B.N.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Braga Junqueira, A.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Global Priority Areas for Ecosystem Restoration. Nature 2020 , 586 , 724–729. [ Google Scholar ] [ CrossRef ]
- Verschuuren, B. Conclusions: How the cultural, spiritual and philosophical underpinnings of sacred natural sites can make conservation in Asia more effective and sustainable. In Asian Sacred Natural Sites ; Routledge: London, UK, 2016; pp. 319–331. ISBN 1-315-67627-3. [ Google Scholar ]
- Zecca, G.; Casazza, G.; Piscopo, S.; Minuto, L.; Grassi, F. Are the Responses of Plant Species to Quaternary Climatic Changes Idiosyncratic? A Demographic Perspective from the Western Alps. Plant Ecol. Divers. 2017 , 10 , 273–281. [ Google Scholar ] [ CrossRef ]
- Christmas, M.J.; Breed, M.F.; Lowe, A.J. Constraints to and Conservation Implications for Climate Change Adaptation in Plants. Conserv. Genet. 2016 , 17 , 305–320. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sayre, M.; Stenner, T.; Argumedo, A. You Can’t Grow Potatoes in the Sky: Building Resilience in the Face of Climate Change in the Potato Park of Cuzco, Peru. Cult. Agric. Food Environ. 2017 , 39 , 100–108. [ Google Scholar ] [ CrossRef ]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017 , 355 , eaai9214. [ Google Scholar ] [ CrossRef ]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.E.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction Debt of High-Mountain Plants under Twenty-First-Century Climate Change. Nat. Clim. Chang. 2012 , 2 , 619–622. [ Google Scholar ] [ CrossRef ]
- Salick, J.; Fang, Z.; Byg, A. Eastern Himalayan Alpine Plant Ecology, Tibetan Ethnobotany, and Climate Change. Glob. Environ. Chang. 2009 , 19 , 147–155. [ Google Scholar ] [ CrossRef ]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated Increase in Plant Species Richness on Mountain Summits Is Linked to Warming. Nature 2018 , 556 , 231–234. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pertoldi, C.; Bach, L.A. Evolutionary Aspects of Climate-Induced Changes and the Need for Multidisciplinarity. J. Therm. Biol. 2007 , 32 , 118–124. [ Google Scholar ] [ CrossRef ]
- Corradi, E.; Abbet, C.; Gafner, F.; Hamburger, M.; Potterat, O. Screening of Alpine Plant Extracts as Protective Agents against UV-Induced Skin Damage. Planta Med. 2013 , 79 , PC3. [ Google Scholar ] [ CrossRef ]
- Descola, P. Beyond Nature and Culture ; University of Chicago Press: Chicago, IL, USA, 2013. [ Google Scholar ]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Barnard, P.; Moomaw, W.R. World Scientists’ Warning of a Climate Emergency. BioScience 2020 , 70 , 8–12. [ Google Scholar ] [ CrossRef ]
- Maffi, L. Linguistic, Cultural, and Biological Diversity. Annu. Rev. Anthropol. 2005 , 34 , 599–617. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Corlett, R.T. The Anthropocene Concept in Ecology and Conservation. Trends Ecol. Evol. 2015 , 30 , 36–41. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Simlai, T.; Sandbrook, C. Digital surveillance technologies in conservation and their social implications. In Conservation Technology ; Wich, S., Piel, A., Eds.; Oxford University Press: Oxford, UK, 2021; pp. 239–249. [ Google Scholar ]
- Gavin, M.C.; McCarter, J.; Mead, A.; Berkes, F.; Stepp, J.R.; Peterson, D.; Tang, R. Defining Biocultural Approaches to Conservation. Trends Ecol. Evol. 2015 , 30 , 140–145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Meine, C. Conservation Biology: Past and Present—Conservation Biology for All ; Meine, C., Ed.; Oxford University Press: Oxford, UK, 2010; pp. 1–5. [ Google Scholar ]
- Lorimer, J. Wildlife in the Anthropocene: Conservation after Nature ; University of Minnesota Press: Milwaukee, WI, USA, 2015; ISBN 1-4529-4429-6. [ Google Scholar ]
- Bain, R. Technology and State Government. Am. Sociol. Rev. 1937 , 2 , 860–874. [ Google Scholar ] [ CrossRef ]
- Stiegler, B. Technics and Time: The Fault of Epimetheus ; Stanford University Press: Redwood City, CA, USA, 1998. [ Google Scholar ]
- Arthur, W.B. The Nature of Technology: What It Is and How It Evolves ; Simon and Schuster: New York, NY, USA, 2009; ISBN 1-4391-6578-5. [ Google Scholar ]
- Jørgensen, D. Not by Human Hands: Five Technological Tenets for Environmental History in the Anthropocene. Environ. Hist. 2014 , 20 , 479–489. [ Google Scholar ] [ CrossRef ]
- Verbeek, P.-P. Toward a theory of technological mediation. In Technoscience and Postphenomenology ; Friis, J., Crease, R.P., Eds.; The Manhattan Papers; Lexington Books: London, UK, 2015; pp. 189–203. [ Google Scholar ]
- Latour, B. The more manipulations, the better. In Representation in Scientific Practice Revisited ; Coopmans, C., Vertesi, J., Lynch, M., Woolgar, S., Eds.; Massachusetts Institute of Technology: Cambridge, MA, USA, 2014; pp. 10–19. [ Google Scholar ]
- Sismondo, S. An Introduction to Science and Technology Studies ; Wiley-Blackwell Chichester: Chichester, UK, 2010. [ Google Scholar ]
- Holmes, G.; Cavanagh, C.J. A Review of the Social Impacts of Neoliberal Conservation: Formations, Inequalities, Contestations. Geoforum 2016 , 75 , 199–209. [ Google Scholar ] [ CrossRef ]
- Berger-Tal, O.; Lahoz-Monfort, J.J. Conservation Technology: The next Generation. Conserv. Lett. 2018 , 11 , e12458. [ Google Scholar ] [ CrossRef ]
- Arneth, A.; Shin, Y.-J.; Leadley, P.W.; Rondinini, C.; Bukvareva, E.; Kolb, M.; Midgley, G.F.; Oberdorff, T.; Palomo, I.; Saito, O. Post-2020 Biodiversity Targets Need to Embrace Climate Change. Proc. Natl. Acad. Sci. USA 2020 , 49 , 30882–30891. [ Google Scholar ] [ CrossRef ]
- O’Connor, L.M.J.; Fugère, V.; Gonzalez, A. Evolutionary Rescue Is Mediated by the History of Selection and Dispersal in Diversifying Metacommunities. Front. Ecol. Evol. 2020 , 8 , 437. [ Google Scholar ] [ CrossRef ]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Plantform on Biodiversity and Ecosystem Services ; IPBES Secretariat: Bonn, Germany, 2019; p. 56. [ Google Scholar ]
- Brey, P. Is Information Ethics Culture-Relative? Int. J. Technol. Hum. Interact. 2007 , 3 , 13. [ Google Scholar ] [ CrossRef ]
- Maffi, L. Biocultural diversity and sustainability. In The Sage Handbook of Environment and Society ; Sage Publishing: London, UK, 2007; pp. 267–277. [ Google Scholar ]
- Maffi, L.; Woodley, E. Biocultural Diversity Conservation: A Global Sourcebook ; Routledge: New York, NY, USA, 2012; ISBN 1-136-54426-7. [ Google Scholar ]
- Upreti, B.; Tewari, L.; Tewari, A. Role of Plants Used in Religious and Cultral System by Local Inhabitnats of Sacred Forests of District Pothoragarth Kumaun Himalaya. Biolife 2017 , 5 , 7–11. [ Google Scholar ]
- Ticktin, T.; Mondragón, D.; Lopez-Toledo, L.; Dutra-Elliott, D.; Aguirre-León, E.; Hernández-Apolinar, M. Synthesis of Wild Orchid Trade and Demography Provides New Insight on Conservation Strategies. Conserv. Lett. 2020 , 13 , e12697. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Loh, J.; Harmon, D. Biocultural Diversity: Threatened Species, Endangered Languages ; WWF Netherlands: Zeist, The Netherlands, 2014; Volume 1, pp. 30–43. [ Google Scholar ]
- Cámara-Leret, R.; Bascompte, J. Language Extinction Triggers the Loss of Unique Medicinal Knowledge. Proc. Natl. Acad. Sci. USA 2021 , 118 , e2103683118. [ Google Scholar ] [ CrossRef ]
- Mackey, B.; Claudie, D. Points of Contact: Integrating Traditional and Scientific Knowledge for Biocultural Conservation. Environ. Ethics 2015 , 37 , 341–357. [ Google Scholar ] [ CrossRef ]
- Sandbrook, C. The Social Implications of Using Drones for Biodiversity Conservation. Ambio 2015 , 44 , 636–647. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Tripathi, M. Critical Review of Some Important Medicinal and Aromatic Plants of Western Himalaya. Res. J. Pharm. Sci. 2020 , 9 , 7–16. [ Google Scholar ]
- Manuel-Navarrete, D. Double Coupling: Modeling Subjectivity and Asymmetric Organization in Social-Ecological Systems. Ecol. Soc. 2015 , 20 , 1–10. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bonneau, A. Indigenous Knowledge Systems Often Overlooked in Academia. 2021. Available online: https://thediscourse.ca/okanagan/indigenous-knowledge-systems-overlooked-in-academia (accessed on 5 November 2021).
- Lewis, D.; Williams, L.; Jones, R.A. A Radical Revision of the Public Health Response to Environmental Crisis in a Warming World: Contributions of Indigenous Knowledges and Indigenous Feminist Perspectives. Can. J. Public Health 2020 , 111 , 897–900. [ Google Scholar ] [ CrossRef ]
- Institutional Labour Organization (ILO) C169—Indigenous and Tribal Peoples Convention; Article 1. 2021. Available online: https://www.ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:12100:0::NO::P12100_ILO_CODE:C169 (accessed on 6 June 2021).
- Ouane, A.; Glanz, C. Optimising Learning, Education and Publishing in Africa: The Language Factor—A Review and Analysis of Theory and Practice in Mother-Tongue and Bilingual Education in Sub-Saharan Africa ; UNESCO Institute for Lifelong Learning Press: Hamburg, Germany, 2011. [ Google Scholar ]
- McGregor, D. Indigenous Knowledge Systems in Environmental Governance in Canada. KULA 2021 , 5 , 1–10. [ Google Scholar ] [ CrossRef ]
- Turner, N.J.; Luczaj, L.; Migliorini, P.; Pieroni, A.; Dreon, A.L.; Sacchetti, L.E.; Paoletti, M.G. Edible and Tended Wild Plants, Traditional Ecological Knowledge and Agroecology. Crit. Rev. Plant Sci. 2011 , 30 , 198–225. [ Google Scholar ] [ CrossRef ]
- Love, T.R. Indigenous Knowledges, Priorities and Processes in Qualitative Organization and Management Research. Qual. Res. Organ. Manag. Int. J. 2020 , 15 , 6–20. [ Google Scholar ] [ CrossRef ]
- Batumike, R.; Imani, G.; Bisimwa, B.; Urom, C.; Mambo, H.; Kalume, J.; Kavuba, F.; Cuni-Sanchez, A. From Tree Species to Forest Services: Ethnic Differences in Lomami, Democratic Republic of the Congo. Econ. Bot. 2021 , 1–12. [ Google Scholar ] [ CrossRef ]
- Whyte, K.P. Our ancestors’ dystopia now: Indigenous conservation and the anthropocene. In The Routledge Companion to the Environmental Humanities ; Heise, U., Christensen, J., Niemann, M., Eds.; Routledge: New York, NY, USA, 2017; pp. 208–215. [ Google Scholar ]
- Reid, A.J.; Eckert, L.; Lane, J.-F.; Young, N.; Hinch, S.; Darimont, C.; Cooke, S.; Ban, N.C.; Marshall, A. “Two-Eyed Seeing”: An Indigenous Framework to Transform Fisheries Research and Management. Fish Fish. 2021 , 22 , 243–261. [ Google Scholar ] [ CrossRef ]
- Broadhead, L.-A.; Howard, S. Confronting the Contradictions between Western and Indigenous Science: A Critical Perspective on Two-Eyed Seeing. AlterNative Int. J. Indig. Peoples 2021 , 17 , 111–119. [ Google Scholar ] [ CrossRef ]
- Ross, A.; Sherman, K.P.; Snodgrass, J.G.; Delcore, H.D.; Sherman, R. Indigenous Peoples and the Collaborative Stewardship of Nature: Knowledge Binds and Institutional Conflicts ; Routledge: Walnut Creek, CA, USA, 2016; ISBN 1-315-42661-7. [ Google Scholar ]
- Kimmerer, R. Braiding Sweetgrass: Indigenous Wisdom, Scientific Knowledge and the Teachings of Plants , 2nd ed.; Milkweed Editions: Minneapolis, MN, USA, 2020; ISBN 978-1-57131-335-5. [ Google Scholar ]
- Ludwig, D.; Macnaghten, P. Traditional Ecological Knowledge in Innovation Governance: A Framework for Responsible and Just Innovation. J. Responsible Innov. 2020 , 7 , 26–44. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.-C. Cryobiotechnologies: Tools for Expanding Long-Term Ex Situ Conservation to All Plant Species. Biol. Conserv. 2020 , 250 , 108736. [ Google Scholar ] [ CrossRef ]
- Barnes, J.C.; Delborne, J.A. The Politics of Genetic Technoscience for Conservation: The Case of Blight-Resistant American Chestnut. Environ. Plan. E Nat. Space 2021 , 25148486211024910. [ Google Scholar ] [ CrossRef ]
- Rossi, J. Genes Are Not Information: Rendering Plant Genetic Resources Untradeable through Genetic Restoration Practices. Geoforum 2014 , 55 , 66–75. [ Google Scholar ] [ CrossRef ]
- Jacobs, D.F.; Dalgleish, H.J.; Nelson, C.D. A Conceptual Framework for Restoration of Threatened Plants: The Effective Model of American Chestnut (Castanea Dentata) Reintroduction. New Phytol. 2013 , 197 , 378–393. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Barnhill-Dilling, S.K.; Rivers, L.; Delborne, J.A. Rooted in Recognition: Indigenous Environmental Justice and the Genetically Engineered American Chestnut Tree. Soc. Nat. Resour. 2020 , 33 , 83–100. [ Google Scholar ] [ CrossRef ]
- Barnes, J.C. Engineering Conservation: The Biogeography, Biopolitics, and Biotechnology of American Chestnut Restoration. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2018. [ Google Scholar ]
- Foster, A.D.; Rosenzweig, M.R. Microeconomics of Technology Adoption. Annu. Rev. Econ. 2010 , 2 , 395–424. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Delborne, J.A.; Binder, A.R.; Rivers, L.; Barnes, J.C.; Barnhill-Dilling, S.; George, D.; Sudweeks, J. Biotechnology, the American Chestnut Tree, and Public Engagement (Workshop Report) ; Genetic Engineering and Society Center, North Carolina State University: Raleigh, NC, USA, 2018. [ Google Scholar ]
- Juiling, S.; Leon, S.; Jumian, J.; Tsen, S.; Lee, Y.L.; Khoo, E.; Sugau, J.B.; Nilus, R.; Pereira, J.; Damit, A.; et al. Conservation Assessment and Spatial Distribution of Endemic Orchids in Sabah, Borneo. Nat. Conserv. Res. 2020 , 5 , 136–144. [ Google Scholar ] [ CrossRef ]
- Sharrock, S.; Hoft, R.; Dias, B.F.D.S. An Overview of Recent Progress in the Implementation of the Global Strategy for Plant Conservation—A Global Perspective. Rodriguésia 2018 , 69 , 1489–1511. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wandersee, J.H.; Schussler, E.E. Preventing Plant Blindness. Am. Biol. Teach. 1999 , 61 , 82–86. [ Google Scholar ] [ CrossRef ]
- Shannon, L. Plant Speak. PAN 2012 , 1 , 65–71. [ Google Scholar ] [ CrossRef ]
- Pouteau, S. Plants as Open Beings: From Aesthetics to plant–human ethics. In Plant Ethics ; Kallhof, A., Di Paola, M., Schorgenhumer, M., Eds.; Routledge: London, UK, 2018; pp. 82–97. ISBN 1-315-11439-9. [ Google Scholar ]
- Balding, M.; Williams, K. Plant Blindness and the Implications for Plant Conservation. Conserv. Biol. 2016 , 30 , 1192–1199. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Margulies, J.; Bullough, L.-A.; Hinsley, A.; Ingram, D.; Cowell, C.; Goettsch, B.; Klitgard, B.; Lavorgna, A.; Sinovas, P.; Phelps, J. Illegal Wildlife Trade and the Persistence of “Plant Blindness”. Plants People Planet 2019 , 1 , 173–182. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Walters, R. Eco-crime and green activism. In Forging a Scio-Legal Approach to Environmental Harms ; Bergin, T., Orlando, E., Eds.; Routledge: Oxon, UK, 2017; pp. 220–236. [ Google Scholar ]
- Cousins, S.R.; Williams, V.L.; Witkowski, E.T.F. Uncovering the Cycad Taxa (Encephalartos Species) Traded for Traditional Medicine in Johannesburg and Durban, South Africa. S. Afr. J. Bot. 2012 , 78 , 129–138. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kenny, J.G. Conservation and Murder: The Plight of Indigenous Land Defenders in Mexico, Costa Rica, New Zealand and the Philippines. Ph.D. Thesis, Fordham University, New York, NY, USA, 2021. [ Google Scholar ]
- Medin, D.L.; García, S.G. Conceptualizing Agency: Folkpsychological and Folkcommunicative Perspectives on Plants. Cognition 2017 , 162 , 103–123. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ojalehto, B.L.; Medin, D.L.; Horton, W.S.; Garcia, S.G.; Kays, E.G. Seeing Cooperation or Competition: Ecological Interactions in Cultural Perspectives. Top. Cogn. Sci. 2015 , 7 , 624–645. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, Invasibility and the Role of Environmental Stress in the Spread of Non-Native Plants. Perspect. Plant Ecol. Evol. Syst. 2000 , 3 , 52–66. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pleasant, J.M. Food Yields and Nutrient Analyses of the Three Sisters: A Haudenosaunee Cropping System. Ethnobiol. Lett. 2016 , 7 , 87–98. [ Google Scholar ]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen Fixation in a Landrace of Maize Is Supported by a Mucilage-Associated Diazotrophic Microbiota. PLoS Biol. 2018 , 16 , e2006352. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Balick, M.J.; Cox, P.A. Plants, People, and Culture: The Science of Ethnobotany , 2nd ed.; Garland Science CRC Press: Boca Raton, FL, USA, 2020; ISBN 1-00-009848-6. [ Google Scholar ]
- Sandbrook, C.; Luque-Lora, R.; Adams, W.M. Human Bycatch: Conservation Surveillance and the Social Implications of Camera Traps. Conserv. Soc. 2018 , 16 , 493–504. [ Google Scholar ] [ CrossRef ]
- Bonga, J. Can Explant Choice Help Resolve Recalcitrance Problems in in Vitro Propagation, a Problem Still Acute Especially for Adult Conifers? Trees 2017 , 31 , 781–789. [ Google Scholar ] [ CrossRef ]
- Carlín, A.P.; Tafoya, F.; Solís, A.G.A.; Pérez-Molphe-Balch, E. Effects of Different Culture Media and Conditions on Biomass Production of Hairy Root Cultures in Six Mexican Cactus Species. In Vitro Cell. Dev. Biol.-Plant 2015 , 51 , 332–339. [ Google Scholar ] [ CrossRef ]
- CITES Turbinicarpus. Available online: https://cites.org/eng/taxonomy/term/9622 (accessed on 17 November 2021).
- Muneta, J.D. Peyote Crisis Confronting Modern Indigenous Peoples: The Declining Peyote Population and the Demand for Conservation. Am. Indian Law J. 2020 , 9 , 135. [ Google Scholar ]
- Fitz Maurice, W.A.; Sanchez, E.; Fitz Maurice, B.; Guadalupe Martinez, J. Turbinicarpus Alonsoi; The IUCN Red List of Threatened Species; International Union for the Conservation of Nature and Natural Resources. Available online: https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species (accessed on 9 November 2021).
- Stephens, L.; Fuller, D.; Boivin, N.; Rick, T.; Gauthier, N.; Kay, A.; Marwick, B.; Armstrong, C.G.; Barton, C.M.; Denham, T. Archaeological Assessment Reveals Earth’s Early Transformation through Land Use. Science 2019 , 365 , 897–902. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Climer Vigueira, C.; Denham, T.; Dobney, K.; et al. Current Perspectives and the Future of Domestication Studies. Proc. Natl. Acad. Sci. USA 2014 , 111 , 6139. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Corlett, R.T. A Bigger Toolbox: Biotechnology in Biodiversity Conservation. Trends Biotechnol. 2017 , 35 , 55–65. [ Google Scholar ] [ CrossRef ]
- Lee, K.; Seo, P.J. Dynamic Epigenetic Changes during Plant Regeneration. Trends Plant Sci. 2018 , 23 , 235–247. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011 , 47 , 5–16. [ Google Scholar ] [ CrossRef ]
- Shahzad, A.; Parveen, S.; Sharma, S.; Shaheen, A.; Saeed, T.; Yadav, V.; Akhtar, R.; Ahmad, Z.; Upadhyay, A. Plant tissue culture: Applications in plant improvement and conservation. In Plant Biotechnology: Principles and Applications ; Abdin, M., Kiran, U., Kamaluddin, A.A., Eds.; Springer: Singapore, 2017; pp. 37–72. [ Google Scholar ]
- Saxena, A.; Shukla, M.R.; Saxena, P.K. Synthetic seeds: Relevance to endangered germplasm conservation in vitro. In Synthetic Seeds ; Faisal, M., Alatar, A.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 21–60. [ Google Scholar ]
- Thorpe, T.A. History of Plant Tissue Culture. Mol. Biotechnol. 2007 , 37 , 169–180. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ashmore, S.E.; Hamilton, K.N.; Offord, C.A. Conservation Technologies for Safeguarding and Restoring Threatened Flora: Case Studies from Eastern Australia. In Vitro Cell. Dev. Biol.-Plant 2011 , 47 , 99–109. [ Google Scholar ] [ CrossRef ]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020 , 9 , 345. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In Vitro Propagation of Medicinal and Aromatic Plants: The Case of Selected Greek Species with Conservation Priority. In Vitro Cell. Dev. Biol. 2019 , 55 , 635–646. [ Google Scholar ] [ CrossRef ]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An Efficient Micropropagation Protocol for an Endangered Ornamental Tree Species (Magnolia Sirindhorniae Noot. & Chalermglin) and Assessment of Genetic Uniformity through DNA Markers. Sci. Rep. 2019 , 9 , 9634. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Komakech, R.; Kim, J.; Kim, W.J.; Omujal, F.; Yang, S.; Moon, B.C.; Okello, D.; Rahmat, E.; Nambatya Kyetune, G.; Matsabisa, M.G. A Micropropagation Protocol for the Endangered Medicinal Tree Prunus Africana (Hook f.) Kalkman: Genetic Fidelity and Physiological Parameter Assessment. Front. Plant Sci. 2020 , 11 , 1871. [ Google Scholar ] [ CrossRef ]
- Singh, R.K.; Anandhan, S.; García-Pérez, L.M.; Ruiz-May, E.; Nava Pérez, E.; Quiroz-Figueroa, F.R. An Efficient Protocol for in Vitro Propagation of the Wild Legume Cicer microphyllum Benth., a Crop Wild Relative of Chickpea ( Cicer arietinum L.). In Vitro Cell. Dev. Biol.-Plant 2019 , 55 , 9–14. [ Google Scholar ] [ CrossRef ]
- Benson, E. Plant Conservation Biotechnology ; CRC Press: Boca Raton, FL, USA, 1999; ISBN 1-4822-7303-9. [ Google Scholar ]
- Bi, W.; Saxena, A.; Ayyanath, M.-M.; Harpur, C.; Shukla, M.R.; Saxena, P.K. Conservation, Propagation, and Redistribution (CPR) of Hill’s Thistle: Paradigm for Plant Species at Risk. Plant Cell Tissue Organ Cult. 2021 , 145 , 75–88. [ Google Scholar ] [ CrossRef ]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020 , 9 , 1634. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reed, B.M. Plant Cryopreservation: A Continuing Requirement for Food and Ecosystem Security. In Vitro Cell. Dev. Biol.-Plant 2017 , 53 , 285–288. [ Google Scholar ] [ CrossRef ]
- Popova, E.; Shukla, M.R.; Kim, H.H.; Saxena, P.K. Plant Cryopreservation for biotechnology and breeding. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools ; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Berlin, Germany, 2015; pp. 63–93. [ Google Scholar ]
- Salama, A.; Popova, E.; Jones, M.P.; Shukla, M.R.; Fisk, N.S.; Saxena, P.K. Cryopreservation of the Critically Endangered Golden Paintbrush ( Castilleja levisecta Greenm.): From Nature to Cryobank to Nature. In Vitro Cell. Dev. Biol.-Plant 2018 , 54 , 69–78. [ Google Scholar ] [ CrossRef ]
- Rathwell, R.; Popova, E.; Shukla, M.R.; Saxena, P.K. Development of Cryopreservation Methods for Cherry Birch ( Betula lenta L.), an Endangered Tree Species in Canada. Can. J. For. Res. 2016 , 46 , 1284–1292. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Whiteley, S.E.; Bunn, E.; Menon, A.; Mancera, R.L.; Turner, S.R. Ex Situ Conservation of the Endangered Species Androcalva Perlaria (Malvaceae) by Micropropagation and Cryopreservation. Plant Cell Tissue Organ Cult. 2016 , 125 , 341–352. [ Google Scholar ] [ CrossRef ]
- Popova, E.V.; Shukla, M.R.; McIntosh, T.; Saxena, P.K. In Vitro and Cryobiotechnology Approaches to Safeguard Lupinus rivularis Douglas Ex Lindl., an Endangered Plant in Canada. Agronomy 2021 , 11 , 37. [ Google Scholar ] [ CrossRef ]
- Barnhill-Dilling, S.K.; Delborne, J.A. The Genetically Engineered American Chestnut Tree as Opportunity for Reciprocal Restoration in Haudenosaunee Communities. Biol. Conserv. 2019 , 232 , 1–7. [ Google Scholar ] [ CrossRef ]
- Sharma, N. In Vitro Conservation of Gentiana Kurroo Royle: An Indigenous Threatened Medicinal Plant. Indian J. Plant Genet. Resour. 2001 , 14 , 99–100. [ Google Scholar ]
- McComb, J.; Bennett, I.; Tonkin, C. In Vitro Propagation of Eucalyptus Species. In Tissue Culture of Australian Plants ; Taki, A., Williams, R., Eds.; University of New England: Armidale, NSW, Australia, 1996; pp. 112–156. [ Google Scholar ]
- Reshi, N.A.; Sudarshana, M.; Girish, H. In Vitro Micropropagation of Rhinacanthus nasutus (L) Kurz. Int. J. Biodivers. Conserv. 2018 , 10 , 357–364. [ Google Scholar ] [ CrossRef ]
- Daniëls, C.W. A Study of the Propagation and Cultivation of Gethyllis multifolia and G. villosa . Master’s Thesis, Cape Peninsula University of Technology, Cape Town, Western Cape, South Africa, June 2007. [ Google Scholar ]
- Witbooi, H. In Vitro Propagation of Agathosma Betulina an Indigenous Plant of Economic Importance. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, Western Cape, South Africa, June 2013. [ Google Scholar ]
- Mridula, M.R.; Nair, A.S. Rapid Micro-Propagation of Wrightia Tinctoria (ROXB.) R Br: A Medicinal Tree. Int. J. Bot. Stud. 2018 , 3 , 126–131. [ Google Scholar ]
- Supinrach, S.; Supinrach, I. Study of cuttings and cutting environment of dutchman’s pipe ( Aristolochia ringens Vahl.). In Proceedings of the 52nd Kasetsart University Annual Conference, Kasetsart University, Bankok, Thailand, 7 February 2014; pp. 355–362. [ Google Scholar ]
- Chinnadurai, V.; Viswanathan, P.; Kalimuthu, K.; Vanitha, A.; Ranjitha, V.; Pugazhendhi, A. Comparative Studies of Phytochemical Analysis and Pharmacological Activities of Wild and Micropropagated Plant Ethanol Extracts of Manihot Esculenta. Biocatal. Agric. Biotechnol. 2019 , 19 , 101166. [ Google Scholar ] [ CrossRef ]
- Barron, R. In Vitro Regeneration, Rooting, and Cloning of Artemisia Tridentata. Master’s Thesis, Boise State University, Boise, ID, USA, 6 July 2020. [ Google Scholar ]
- He, T.; Xu, J.; Yang, L.; Wang, H. An Efficient Method for Plant Regeneration from Calli of Swertia Mussotii, an Endangered Medicinal Herb. Am. J. Plant Sci. 2012 , 3 , 904–908. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bose, B.; Kumaria, S.; Choudhury, H.; Tandon, P. Assessment of Genetic Homogeneity and Analysis of Phytomedicinal Potential in Micropropagated Plants of Nardostachys Jatamansi, a Critically Endangered, Medicinal Plant of Alpine Himalayas. Plant Cell Tissue Organ Cult. 2016 , 124 , 331–349. [ Google Scholar ] [ CrossRef ]
- Monteuuis, O.; Doulbeau, S.; Verdeil, J.-L. DNA Methylation in Different Origin Clonal Offspring from a Mature Sequoiadendron Giganteum Genotype. Trees 2008 , 22 , 779. [ Google Scholar ] [ CrossRef ]
- Murch, S.J.; Ragone, D.; Shi, W.L.; Alan, A.R.; Saxena, P.K. In vitro conservation and sustained production of breadfruit (artocarpus altilis, moraceae): Modern technologies for a traditional tropical crop. In Protocols for Micropropagation of Woody Trees and Fruits ; Jain, S., Hagman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 95, pp. 279–288. ISBN 978-1-4020-6352-7. [ Google Scholar ]
- Kim, J.; Soh, S.Y.; Bae, H.; Nam, S.-Y. Antioxidant and Phenolic Contents in Potatoes ( Solanum tuberosum L.) and Micropropagated Potatoes. Appl. Biol. Chem. 2019 , 62 , 1–9. [ Google Scholar ] [ CrossRef ]
- Vollmer, R.; Espirilla, J.; Villagaray, R.; Cardenas, J.; Castro, M.; Sanchez, J.C.; Anglin, N.L. Cryopreservation of Potato Shoot Tips for Long-Term Storage. In Solanum Tuberosum, Methods and Protocols ; Dobnik, D., Gruden, K., Ramsak, Z., Coll, A., Eds.; Humana Press: Totowa, NJ, USA, 2021; pp. 21–54. [ Google Scholar ]
- Uchendu, E.E.; Shukla, M.; Saxena, P.K.; Keller, J.E.R. Cryopreservation of Potato Microtubers: The Critical Roles of Sucrose and Desiccation. Plant Cell Tissue Organ Cult. 2016 , 124 , 649–656. [ Google Scholar ] [ CrossRef ]
- Parra-Rondinel, F.; Casas, A.; Begazo, D.; Paco, A.; Márquez, E.; Cruz, A.; Segovia, J.; Torres-García, I.; Zarazúa, M.; Lizárraga, L. Natural and Cultural Processes Influencing Gene Flow Among Wild (Atoq Papa), Weedy (Araq Papa and k’ipa Papa), and Crop Potatoes in the Andean Region of Southern Peru. Front. Ecol. Evol. 2021 , 9 , 327. [ Google Scholar ] [ CrossRef ]
- Fretz, A.; Jahne, A.; Lorz, H. Cryopreservation of Embryogenic Suspension Cultures of Barley ( Hordeum vulgare L.). Botanica Acta 1992 , 105 , 140–145. [ Google Scholar ] [ CrossRef ]
- da Silva, P.; Contim, L.; de Freitas, D.; Aride, P.; dos Santos, A. In Vitro Establishment of Kapok Tree ( Ceiba pentandra L. Gaertn) Apical Shoots. Sci. Agrar. 2010 , 11 , 437–443. [ Google Scholar ]
- Usman, I.S.; Abdulmalik, M. Cryopreservation of Embryonic Axes of Maize ( Zea mays L.) by Vitrification Protocol. Afr. J. Biotechnol. 2010 , 9 , 8955–8957. [ Google Scholar ]
- Perez, J.; Araya-Valverde, E.; Garro, G.; Abdelnour-Esquivel, A. Analysis of Stress Indicators during Cryopreservation of Seeds of Landrace Maize (Zea Mays). CryoLetters 2017 , 38 , 445–454. [ Google Scholar ]
- Rossetto, M.; Yap, J.-Y.S.; Lemmon, J.; Bain, D.; Bragg, J.; Hogbin, P.; Gallagher, R.; Rutherford, S.; Summerell, B.; Wilson, T.C. A Conservation Genomics Workflow to Guide Practical Management Actions. Glob. Ecol. Conserv. 2021 , 26 , e01492. [ Google Scholar ] [ CrossRef ]
- Sheikholeslami, B.; Shukla, M.R.; Turi, C.E.; Harpur, C.; Saxena, P.K. Saving Threatened Plant Species: Reintroduction of Hill’s Thistle (Cirsium Hillii (Canby) Fernald) to Its Natural Habitat. PLoS ONE 2020 , 15 , e0231741. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and Conservation of Plant Biodiversity. Resources 2013 , 2 , 73–95. [ Google Scholar ] [ CrossRef ]
- Wang, M.-R.; Chen, L.; Teixeira da Silva, J.A.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology of Apple ( Malus spp.): Development, Progress and Future Prospects. Plant Cell Rep. 2018 , 37 , 689–709. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reiter, N.; Whitfield, J.; Pollard, G.; Bedggood, W.; Argall, M.; Dixon, K.; Davis, B.; Swarts, N. Orchid Re-Introductions: An Evaluation of Success and Ecological Considerations Using Key Comparative Studies from Australia. Plant Ecol. 2016 , 217 , 81–95. [ Google Scholar ] [ CrossRef ]
- Bunn, E.; Turner, S.; Panaia, M.; Dixon, K.W. The Contribution of in Vitro Technology and Cryogenic Storage to Conservation of Indigenous Plants. Aust. J. Bot. 2007 , 55 , 345–355. [ Google Scholar ] [ CrossRef ]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018 , 248 , 1–18. [ Google Scholar ] [ CrossRef ]
- Shaw, R.; Greggor, A.; Plotnik, J. The Challenges of Replicating Research on Endangered Species. Anim. Behav. Cogn. 2021 , 8 , 240–246. [ Google Scholar ] [ CrossRef ]
- Sandbrook, C.; Fisher, J.A.; Holmes, G.; Luque-Lora, R.; Keane, A. The Global Conservation Movement Is Diverse but Not Divided. Nat. Sustain. 2019 , 2 , 316–323. [ Google Scholar ] [ CrossRef ]
- Ryder, O.A. Cloning Advances and Challenges for Conservation. Trends Biotechnol. 2002 , 20 , 231–232. [ Google Scholar ] [ CrossRef ]
- Snaddon, J.; Petrokofsky, G.; Jepson, P.; Willis, K.J. Biodiversity Technologies: Tools as Change Agents. Biol. Lett. 2013 , 9 , 20121029. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Zimov, S. Pleistocene Park: Return of the Mammoth’s Ecosystem. Science 2005 , 308 , 796–798. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Kordyum, E.; Hasenstein, K.H. Plant Biology for Space Exploration—Building on the Past, Preparing for the Future. Life Sci. Space Res. 2021 , 29 , 1–7. [ Google Scholar ] [ CrossRef ]
- Preil, W. In vitro propagation and breeding of ornamental plants: Advantages and disadvantages of variability. In Genetic Manipulation in Plant Breeding ; Preil, W., Ed.; De Gruyter Press: Ahrensburg, Germany, 2019; pp. 377–404. ISBN 3-11-087194-7. [ Google Scholar ]
- United Nations. Convention on Biological Diversity. 1992. Available online: https://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 7 October 2021).
- Antonelli, A.; Smith, R.J.; Simmonds, M.S.J. Unlocking the Properties of Plants and Fungi for Sustainable Development. Nat. Plants 2019 , 5 , 1100–1102. [ Google Scholar ] [ CrossRef ]
- Sas-Rolfes, M.; Challender, D.W.S.; Hinsley, A.; Veríssimo, D.; Milner-Gulland, E.J. Illegal Wildlife Trade: Scale, Processes, and Governance. Annu. Rev. Environ. Resour. 2019 , 44 , 201–228. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- De, L.; Pathak, P. Conservation, Management and Utilization of Orchid Genetic Resources. Orchid Soc. India 2018 , 32 , 81–91. [ Google Scholar ]
- Hinsley, A.; Nuno, A.; Ridout, M.; St John, F.; Roberts, D. Estimating the Extent of CITES Noncompliance among Traders and End-consumers; Lessons from the Global Orchid Trade. Conserv. Lett. 2017 , 10 , 602–609. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hinsley, A.; De Boer, H.J.; Fay, M.F.; Gale, S.W.; Gardiner, L.M.; Gunasekara, R.S.; Kumar, P.; Masters, S.; Metusala, D.; Roberts, D.L. A Review of the Trade in Orchids and Its Implications for Conservation. Bot. J. Linn. Soc. 2018 , 186 , 435–455. [ Google Scholar ] [ CrossRef ]
- Popova, E.; Kim, H.H.; Saxena, P.K.; Engelmann, F.; Pritchard, H.W. Frozen Beauty: The Cryobiotechnology of Orchid Diversity. Biotechnol. Adv. 2016 , 34 , 380–403. [ Google Scholar ] [ CrossRef ]
- Brand, R.; Fischer, J. Overcoming the Technophilia/Technophobia Split in Environmental Discourse. Environ. Politics 2013 , 22 , 235–254. [ Google Scholar ] [ CrossRef ]
- Solis-Castañeda, G.J.; Zamilpa, A.; Cabañas-García, E.; Bahena, S.M.; Pérez-Molphe-Balch, E.; Gómez-Aguirre, Y.A. Identification and Quantitative Determination of Feruloyl-Glucoside from Hairy Root Cultures of Turbinicarpus Lophophoroides (Werderm.) Buxb. & Backeb. (Cactaceae). In Vitro Cell. Dev. Biol.-Plant 2020 , 56 , 8–17. [ Google Scholar ] [ CrossRef ]
- Gallia, M.C.; Echeverri Del Sarto, J.; Bongiovanni, G.A. Sustainable and Efficient Protocols for in Vitro Germination and Antioxidants Production from Seeds of the Endangered Species Araucaria Araucana. J. Genet. Eng. Biotechnol. 2021 , 19 , 181. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gehlot, H.S.; Tak, N.; Dagla, H.R.; Davis, T.D. Indigenous and Modern Scientific Strategies for Characterization, Conservation and Sustainable Utilization of Bio-Resources of the Indian Thar Desert. J. Arid. Land Stud. 2014 , 24 , 5–8. [ Google Scholar ]
- Goswami, D.; Dagla, H.R. In vitro culture of haloxylon recurvum and haloxylon salicornicum: Valuable source of food additives and pharmaceutical and nutritional components from extreme arid zone. In Exploring Plant Cells for the Production of Compuounds of Interest ; Malik, S., Ed.; Springer: Cham, Switzerland, 2021; pp. 335–339. [ Google Scholar ]
- Moon, K.; Blackman, D. A Guide to Understanding Social Science Research for Natural Scientists. Conserv. Biol. 2014 , 28 , 1167–1177. [ Google Scholar ] [ CrossRef ]
- Kelly, R.; Mackay, M.; Nash, K.L.; Cvitanovic, C.; Allison, E.H.; Armitage, D.; Bonn, A.; Cooke, S.J.; Frusher, S.; Fulton, E.A. Ten Tips for Developing Interdisciplinary Socio-Ecological Researchers. Socio-Ecol. Pract. Res. 2019 , 1 , 149–161. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Romeis, J.; Meissle, M.; Brunner, S.; Tschamper, D.; Winzeler, M. Plant Biotechnology: Research behind Fences. Trends Biotechnol. 2013 , 31 , 222–224. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moyo, M.; Bairu, M.; Amoo, S.; Van Staden, J. Plant Biotechnology in South Africa: Micropropagation Research Endeavours, Prospects and Challenges. S. Afr. J. Bot. 2011 , 77 , 996–1011. [ Google Scholar ] [ CrossRef ] [ Green Version ]
Click here to enlarge figure
| Scientific Name | Traditional Uses | In Vitro Method Used | Geographical Location | Reference |
|---|---|---|---|---|
| Castanea americana | food, wood | transgenic * modification * | Northeastern USA | [ ] |
| Turbinicarpus sp. | medicinal, ceremonial | tissue culture *^ | Mexico | [ ] |
| Gentiana kurroo | medicinal | shoot culture * | India | [ ] |
| Eucalyptus spp. | medicinal | tissue culture * | Australia, Tasmania | [ ] |
| Rhinacanthus nasutus | medicinal, dye | tissue culture * | Southwest Bengal | [ ] |
| Gethyllis multifolia | medicinal | hydro culture * | Worcester, South Africa | [ ] |
| Agathosma betulina | medicinal, food | micropropagation * | Western Cape, South Africa | [ ] |
| Wrightia tinctoria | medicinal | stem cuttings * | India | [ ] |
| Aristolochia ringens | medicinal | root, stem cuttings * | Nigeria | [ ] |
| Manihot esculenta | medicinal, food | micropropagation * | Global distribution | [ ] |
| Artemisia tridentata | medicinal, ceremonial | micropropagation * | Western North America | [ ] |
| Swertia mussotii | medicinal, ceremonial | micropropagation * | Qinghai-Tibet Plateau, China | [ ] |
| Nardostachys jatamansi | medicinal | micropropagation * | Himalayan region | [ ] |
| Sequoiadendron giganteum | ornamental | meristem culture | Sierra Nevada, USA | [ ] |
| Artocarpus altilis | medicinal, food | meristem culture *^ | Pacific Islands | [ ] |
| Solanum tuberosum | food, ceremonial | nodal explant tissue culture *^ shoot tip, micro tuber cryopreservation | Global distribution | [ , , , ] |
| Hordeum vulgare | food | embryo cryopreservation * | SW Asia, Himalayas | [ ] |
| Ceiba pentandra | medicinal, wood | apical shoot culture | Tropical forests, global distribution | [ ] |
| Zea mays | food, ceremonial | embryo, seed cryopreservation | Global distribution | [ , ] |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants 2022 , 11 , 503. https://doi.org/10.3390/plants11040503
Kulak V, Longboat S, Brunet ND, Shukla M, Saxena P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants . 2022; 11(4):503. https://doi.org/10.3390/plants11040503
Kulak, Verena, Sheri Longboat, Nicolas D. Brunet, Mukund Shukla, and Praveen Saxena. 2022. "In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity" Plants 11, no. 4: 503. https://doi.org/10.3390/plants11040503
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Development of Eucommia ulmoides Oliver tissue culture for in vitro production of the main medicinal active components
- Plant Tissue Culture
- Published: 08 August 2024
Cite this article

- Siqiu Xiao 1 , 2 , 3 na1 ,
- Xuchen Tian 1 , 2 , 3 na1 ,
- Ying Zhang 1 , 2 , 3 ,
- Jiayi Wu 1 , 2 , 3 ,
- Chengyu Qin 1 , 2 , 3 ,
- Hongling Wei 1 , 2 , 3 ,
- Shengnan Xie 1 , 2 , 3 ,
- Jing Yang 1 , 2 , 3 ,
- Dewen Li 1 , 2 , 3 &
- Ying Liu ORCID: orcid.org/0000-0002-5322-0010 1 , 2 , 3
Explore all metrics
Eucommia ulmoides Oliver ( E. ulmoides ) is rich in a variety of medicinally active compounds which have a very high economic value. Various types of explants (stem, stem with axillary bud, terminal bud, and leaves) were cultured on Murashige and Skoog (MS) medium with different types and concentrations of growth regulators. The present study found that the cultured stem explants induced callus. Terminal bud and the stem with axillary bud–cultured explants induced clustered regenerated plantlets. The terminal bud was designated as clustered regenerated plantlets I after culture, while the stem with axillary bud was labeled as clustered regenerated plantlets II after culture. The highest induction rate of clustered regenerated plantlets was 66.67% after culture on medium supplemented with 0.5 mg ּL −1 6-benzylaminopurine (6-BA) and 0.01 mg ּL −1 α-naphthaleneacetic acid (NAA). The highest induction rate of callus tissue was 80.66% following culture on medium containing 0.09 mg ּL −1 NAA and 0.5 mg ּL −1 6-BA. The medicinal active ingredients of clustered regenerated plantlets and callus tissue were compared with those in natural seedlings and demonstrated that there was little difference in the types of compounds between clustered regenerated plantlets and natural seedlings. The contents of phenylpropanoids, phenols, flavonoids, lignin, and iridoids in different induced tissues were determined and showed that the best material for obtaining lignin compounds was clustered regenerated plantlets induced by terminal bud, and the best material for obtaining phenylpropanoids, phenols, and iridoids was clustered regenerated plantlets induced by stems with axillary bud. The theoretical basis and technical support provided in this study are essential for the establishment of an asexual propagation system for E. ulmoides and the commercial regeneration of plants. Furthermore, in vitro culture can efficiently produce a significant amount of medicinally active components, thereby decreasing reliance on wild resources. Research on the variations of medicinal components in E. ulmoides not only offers a theoretical foundation but also supplies experimental materials for the development of novel drugs.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbasi BH, Siddiquah A, Tungmunnithum D, Bose S, Younas M, Garros L, Drouet S, Giglioli-Guivarc’h N, Hano C (2019) Isodon rugosus (Wall ex Benth) Codd in vitro cultures: establishment, phytochemical characterization and in vitro antioxidant and anti-aging activities. Int J Mol Sci 20:452
Article PubMed PubMed Central Google Scholar
Cao RZ (2018) Effects of different growth regulators and trace elements on the growth and secondary metabolites content of Eucommia ulmoides Oliv. Northwest A&F University, MA thesis
Dang SN, Gao RM, Zhang YQ, Feng YM (2022) In vitro regeneration and its histological characteristics of Dioscorea nipponica Makino. Sci Rep 12:18436–18436
Dilkalal A, Annapurna AS, Umesh TG (2021) In vitro regeneration, antioxidant potential, and genetic fidelity analysis of Asystasia gangetica (L.)T. Anderson. In Vitro Cell Dev Biol - Plant 57:447–459
Dominguez MM, Padilla CS, Mandadi KK (2022) A versatile Agrobacterium -based plant transformation system for genetic engineering of diverse citrus cultivars. Front Plant Sci 13:878335
Dong NQ, Lin HX (2021) Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J Integrat Plant Biol 63:180–209
Article CAS Google Scholar
Gu Y, Chen Y, Yue X, Xiong P, Pan D, Song P, Luo B (2022) LF-NMR/MRI determination of different 6-benzylaminopurine concentrations and their effects on soybean moisture. Front Plant Sci 13:885804
Hu Y, Javed HH, Asghar MA, Peng X, Brestic M, Skalický M, Ghafoor AZ, Cheema HN, Zhang FF, Wu YC (2022) Enhancement of lodging resistance and lignin content by application of organic carbon and silicon fertilization in Brassica napus L. Front Plant Sci 13:807048
Huang TK, Plesha MA, Falk BW, Dandekar AM, McDonald KA (2010) Bioreactor strategies for improving production yield and functionality of a recombinant human protein in transgenic tobacco cell cultures. Biotechnol Bioeng 102:508–520
Article Google Scholar
Jaime A, Teixeira S, Zeng S, Godoy-Hernández G, Rivera-Madrid R, Dobránszki J (2019) Bixa orellana L. (achiote) tissue culture: a review. In Vitro Cell Dev Biol - Plant 55:231–241
Jamwal K, Bhattacharya S, Puri S (2018) Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J Appl Res Med Aromat Plants 9:26–38
Google Scholar
Kang HH, Naing AH, Kim CK (2020) Protoplast isolation and shoot regeneration from protoplast-derived callus of petunia hybrida Cv. Mirage Rose Biol (basel) 9:228
CAS Google Scholar
Karakas FP, Turker AU (2013) An efficient in vitro regeneration system for Bellis perennis L. and comparison of phenolic contents of field-grown and in vitro -grown leaves by LC-MS/MS. Ind Crops Prod 48:162–170
Karakas FP, Turker AU (2016) Improvement of shoot proliferation and comparison olsecondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. In Vitro Cell Dev Biol - Plant 52:608–618
Karakas PF (2020) Efficient plant regeneration and callus induction from nodal and hypocotyl explants of goji berry ( Lycium barbarum L.) and comparison of phenolic profiles in calli formed under different combinations of plant growth regulators. Plant Physiol Biochem 146:384–391
Article CAS PubMed Google Scholar
Kim S, Jeong YJ, Park SH, Park SC, Lee SB, Lee J, Kim SW, Ha BK, Kim HS, Kim H (2020) The synergistic effect of co-treatment of methyl jasmonate and cyclodextrins on pterocarpan production in Sophora Flavescens cell cultures. Int J Mol Sci 21:3944
Article CAS PubMed PubMed Central Google Scholar
Kim WS, Ha JH, Jeong SH, Lee JI, Lee BW, Jeong Y, Kim CY, Park JY, Ryu BY, Kwon HJ, Lee AC (2022) Immunological effects of Aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells 11:2805
Kulus D, Tymoszuk A (2020) Induction of callogenesis, organogenesis, and embryogenesis in non-meristematic explants of bleeding heart and evaluation of chemical diversity of key metabolites from callus. Int J Mol Sci 21:5826
Lei YN, Cui JJ, Zhang XB (2019) Effects of exogenous hormones on chlorogenic acid content in Eucommia ulmoides callus. Shaanxi Agricultural Sci 65:24–28
Li J, Sun M, Li H, Ling Z, Wang D, Zhang J, Shi L (2022) Full-length transcriptome-referenced analysis reveals crucial roles of hormone and wounding during induction of aerial bulbils in lily. BMC Plant Biol 22:415
Li L, Liu M, Shi K, Yu ZJ, Zhou Y, Fan RS, Shi QQ (2019) Dynamic changes in metabolite accumulation and the transcriptome during leaf growth and development in Eucommia ulmoides . Int J Mol Sci 20:4030
Liu W, Zhang J, Jiao C, Yin X, Fei Z, Wu Q, Chen K (2019) Transcriptome analysis provides insights into the regulation of metabolic processes during postharvest cold storage of loquat ( Eriobotrya japonica ) fruit. Hortic Res 6:49
Mao J, Ma D, Niu C, Ma X, Li K, Tahir MM, Chen S, Liu X, Zhang D (2022) Transcriptome analysis reveals the regulatory mechanism by which MdWOX11 suppresses adventitious shoot formation in apple. Hortic Res 9:80
Mitrofanova I, Ivanova N, Kuzmina T, Mitrofanova O, Zubkova N (2021) In vitro regeneration of clematis plants in the nikita botanical garden via somatic embryogenesis and organogenesis. Front Plant Sci 12:541171
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Palacio L, Baeza MC, Cantero JJ, Cusido R, Goleniowski ME (2008) In vitro propagation of Jarilla ( Larrea divaricate Cav.) and secondary metabolite production. Biol Pharm Bull 31:2321–2325
Park ES, Moon WS, Song MJ, Kim MN, Chung KH, Yoon JS (2001) Antimicrobial activity of phenol and benzoic acid derivatives. Int Biodeter Biodegr 47:209–214
Ramabulana AT, Steenkamp P, Madala N, Dubery IA (2020) Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 10:178
Sadat-Hosseini M, Arab MM, Soltani M, Eftekhari M, Soleimani A, Vahdati K (2022) Predictive modeling of Persian walnut ( Juglans regia L.) in vitro proliferation media using machine learning approaches: a comparative study of ANN KNN and GEP Models. Plant Methods 18:48
Salih AM, Al-Qurainy F, Khan S, Tarroum M, Nadeem M, Shaikhaldein HO, Alabdallah NM, Alansi S, Alshameri A (2021) Mass propagation of Juniperus procera Hoechst. Ex Endl. From seedling and screening of bioactive compounds in shoot and callus extract. BMC Plant Biol 21:192
Shi Y, Zhang S, Peng D, Wang C, Zhao D, Ma K, Wu J, Huang L (2019) Transcriptome analysis of Clinopodium chinense (Benth.) O. Kuntze and identification of ginvolved in triterpenoid saponin biosynthesis. Int J Mol Sci 20:2643
Singh M, Agrawal S, Afzal O, Altamimi ASA, Redhwan A, Alshammari N, Patel M, Adnan M, Elasbali AM, Khan S (2022) Optimization of elicitation conditions to enhance the production of potent metabolite withanolide from Withania somnifera (L.). Metabolites 12:854
Szopa A, Ekiert H (2014) Production of biologically active phenolic acids in Aronia melanocarpa (Michx.) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. Plant Growth Regul 72:51–58
Wang CY, Tang L, Li L, Zhou Q, Li YJ, Li J, Wang Y (2020) Geographic authentication of Eucommia ulmoides leaves using multivariate analysis and preliminary study on the compositional response to environment. J Front Plant Sci 11:79
Wetzstein HY, Porter JA, Janick J, Ferreira JFS (2018) Selection and clonal propagation of high artemisinin genotypes of Artemisia annua . Front Plant Sci 9:358
Wu JY (2023) Study on the Optimization of culture in vitro condition and the effect on the content of major medicinal active ingredients in Eucommia ulmoides . Northeast Forestry University, MA thesis
Wu MF, Liu PY, Wang SY, Zhong C, Zhao XH (2021) Ultrasonic microwave-assisted micelle combined with fungal pretreatment of Eucommia ulmoides leaves significantly improved the extraction efficiency of total flavonoids and gutta-percha. Foods 10:2399
Wuyts N, De Waele D, Swennen R (2006) Extraction and partial characterization of polyphenol oxidase from banana ( Musa acuminata Grande naine) roots. Plant Phv Bio 44:308–314
Yang L, Zhang CL, Su ZJ, Zhao L, Wu JX, Sun XY, Zhang XJ, Hu XQ (2022) Inactivation of Salmonella typhimurium SLL344 by chlorogenic acid and the impairment of cellular integrity. Front Microbiol 13:887950
Yazdanpanah P, Jonoubi P, Zeinalabedini M, Rajaei H, Ghaffari MR, Vazifeshenas MR, Abdirad S (2021) Seasonal metabolic investigation in pomegranate ( Punica granatum L.) highlights the role of amino acids in genotype and organ-specific adaptive responses to freezing stress. Front Plant Sci 12:699139
Zeng MN, Ren YJ, Zhang BB, Wang SC, Liu M, Jia JF, Guo PL, Zhang QQ, Zheng XK, Feng WS (2021) In vitro non-small cell lung cancer inhibitory effect by new diphenylethane isolated from stems and leaves of Dioscorea oppositifolia L. via ERβ-STAT3 pathway. Front Pharmacol 12:622681
Zhang H, Guo MQ, Wu QN, Zhao MQ, Li RP, Deng XM, Xi RC (2022) Efficient regeneration of mature Castanopsis hystrix from in vitro stem explants. Front Plant Sci 13:914652
Zhang WP, Wen Z, Zhang L, Fu DM, Zhang ZD, Zhang L, Zu YG (2020) Contents of main active ingredients and correlation with different ages and parts of Eucommia ulmoides oliv. Non-Wood Forest Res 38:46–57
Zhang Z, Lu ZF, Wu HM, Zhang Z, Lv ZF, Zhou ZQ (2016) Phenolic compositions and antioxidant capacity of the fruit pulp of Popular pomelo cultivars in Chongqing. Food Sci 37:83–88
Zhu MQ, Xu WZ, Wen JL, Zhu YH, Li Y, Su YQ, Zhang Q, Sun RC (2017) Dynamic changes of photosynthetic properties and chemical compositions of Eucommia ulmoides Oliver under two planting models. Ind Crops Prod 96:46–56
Download references
This research was funded by the Heilong jiang province natural science fund project (No. LH2021C014), Key Research and Development Program of Heilongjiang Province (JD22A008), and Horizontal project (No. 2021).
Author information
Siqiu Xiao and Xuchen Tian contributed equally to this work.
Authors and Affiliations
College of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin, 150040, China
Siqiu Xiao, Xuchen Tian, Ying Zhang, Jiayi Wu, Chengyu Qin, Hongling Wei, Shengnan Xie, Jing Yang, Dewen Li & Ying Liu
Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University, Harbin, 150040, China
Engineering Research Center of Forest Bio-Preparation, Ministry of Education, Northeast Forestry University, Harbin, 150040, China
You can also search for this author in PubMed Google Scholar

Contributions
D-W. L. and Y. L. conceived the project and critically edited the manuscript; S-Q. X., X-C. T., and J-Y. W. conducted the experiments and wrote the manuscript; Y. Z., J-Y. W., H–L. W., and J. Y. assisted with the experiments; C-Y. Q. and S–N. X. analyzed the data; D-W. L. and Y. L. provided guidance on experimental methods. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Dewen Li or Ying Liu .
Ethics declarations
Consent to participate.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
11627_2024_10441_MOESM1_ESM.docx
Supplementary file1 Additional information. See appendix for detection and analysis of differential compounds. (DOCX 39 KB)
Rights and permissions
Reprints and permissions
About this article
Xiao, S., Tian, X., Zhang, Y. et al. Development of Eucommia ulmoides Oliver tissue culture for in vitro production of the main medicinal active components. In Vitro Cell.Dev.Biol.-Plant (2024). https://doi.org/10.1007/s11627-024-10441-0
Download citation
Received : 27 December 2023
Accepted : 15 June 2024
Published : 08 August 2024
DOI : https://doi.org/10.1007/s11627-024-10441-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Eucommia ulmoides Oliver ( E. ulmoides )
- In vitro culture
- Plant growth regulator concentrations
- Secondary metabolites
- Clustered regenerated plantlets
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 06 August 2024
Research progress of brain organoids in the field of diabetes
- Ying Su 1 , 2 na1 ,
- Aimei Liu 1 na1 ,
- Hongguang Chen 1 ,
- Qingjie Chen 1 ,
- Bo Zhao 1 , 2 ,
- Runze Gao 1 , 2 ,
- Kangwei Zhang 2 ,
- Tie Peng 3 ,
- Zhenwang Zhang 1 ,
- Changhan Ouyang 1 , 2 &
- Dan Zhu 1
Molecular Brain volume 17 , Article number: 53 ( 2024 ) Cite this article
191 Accesses
1 Altmetric
Metrics details
Human embryonic stem cells and human induced pluripotent stem cells may be used to create 3D tissues called brain organoids. They duplicate the physiological and pathological characteristics of human brain tissue more faithfully in terms of both structure and function, and they more precisely resemble the morphology and cellular structure of the human embryonic brain. This makes them valuable models for both drug screening and in vitro studies on the development of the human brain and associated disorders. The technical breakthroughs enabled by brain organoids have a significant impact on the research of different brain regions, brain development and sickness, the connections between the brain and other tissues and organs, and brain evolution. This article discusses the development of brain organoids, their use in diabetes research, and their progress.
Introduction
Diabetes mellitus is a metabolic disorder that affects over 400 million people globally. It is characterized by hyperglycemia and may have a number of serious side effects, including early death [ 1 ].Beta-cell dysfunction is a consequence of type 1 diabetes (T1D), an autoimmune disorder. Type 2 diabetes (T2D), the most common form of adult diabetes, is characterized by peripheral insulin resistance and significantly incorrect insulin production [ 2 ]. Furthermore, rare monogenic diabetes mellitus is becoming more prevalent. These disorders, which include neonatal diabetes (ND) and mature-onset diabetes of the young (MODY), are caused by mutations in a single gene that is necessary for the development or function of pancreatic beta cells [ 2 , 3 ]. Regretfully, there is still a great deal to learn about effective treatment options and the genesis of diabetes.
The term “organoids” refers to the technique of using stem cells cultured in vitro in a specific three-dimensional environment to create tissues with an architecture and functions similar to the original organ. The ability of stem cells to form complex tissue architectures and self-organize is necessary for the development of organoids. These self-organizing structures may include portions that reflect different brain areas; they are commonly referred to as “brain organoids” because of how frequently human brain regions are seen throughout the body. Cerebral organoids may have structural characteristics that are distinct to a particular region of the brain.
The novel technology known as organoids makes new models for the study of developmental biology and disease conceivable [ 4 ]. Human neurons are now widely used in in vitro systems that enable a wide range of phenotypic and mechanistic studies due to the recent and rapid advancements in stem cell technology, such as the ability to differentiate pluripotent stem cells (PSCs) and reprogramme somatic cells into induced pluripotent stem cells (iPSCs) [ 5 , 6 ]. Modeling neuropsychiatric and neurological diseases using organoids derived from induced pluripotent stem cells (iPSCs) might be useful for drug discovery (Fig. 1 ). These efforts have recently led to the development of three-dimensional (3D) brain organoids, which are being used as experimental models to study the pathophysiology of disease and normal organogenesis [ 4 , 7 ]. These organoids imitate the developing nervous system.
Brain organoid generation and therapeutic potential. Brain organoids can be generated from patient induced pluripotent stem cells (iPSCs) derived from adult fibroblasts and can be used to simulate human neurological disorders. Drug screening may be one of the potential applications for predicting drug efficacy before treatment
Brain organoids have significant promise for a variety of applications, including as drug screening, gene editing, modeling nervous system illnesses, exploring the evolutionary rules governing brain development, and modifying the brain’s evolutionary processes. There are several options for treating diabetes thanks to the recent and ongoing advancements in brain organoid technology. The composition, development, and use of brain organoids in diabetes are discussed in this article.
Construction and development of brain organoids
Research progress of brain organoid construction methods.
Though not all 3D systems for neuronal culture could be regarded as brain organoids, methods to stimulate neuronal differentiation from RGCs into 3D neuronal structures have been pursued since the early 1990s. Brain organoids may replicate the brain’s developmental process and reflect the physiological, pathological, and pharmacological characteristics of the brain. They also have many anatomical and cellular similarities with the real brain [ 8 , 9 ].
The creation of brain organoid technology is based on early research into the two-dimensional induction of neuroectodermal cells and the three-dimensional differentiation of embryoid bodies (EBs). Researchers also started looking at EB differentiation approaches because of the relatively basic cell types in two-dimensional (2D) culture systems, the stark disparities between cell interactions and actual tissue, and the challenge of directly examining human brain tissue. Advances in stem cell technology have made it possible for researchers to employ human induced pluripotent stem cells (hiPSCs) to construct brain-like tissues and organs from a 3D viewpoint. Researchers have also started to investigate neural cell differentiation procedures of PSCs [ 10 , 11 ].
The creation of innovative procedures for brain organoid formation was made possible by the initial groundbreaking studies of differentiation employing 2D monolayer cultures [ 12 , 13 , 14 , 15 , 16 ] and the groundbreaking work on 3D cultures by the Sasai and Kleber group [ 17 ]. Generally speaking, there are two primary methods for creating brain organoids: VSC self-assembly and external sensor inputs. Using neural guiding molecules and extracellular arrays, the research groups of Sasai and Knoblich conducted experiments on 3D brain organoid culture systems [ 11 , 13 ]. Key features of the fetal brain are mimicked by human brain organoids; nevertheless, the inability to create 3D brain structures that accurately represent late fetal development is due to the drawbacks of existing organoid techniques, including intrinsic hypoxia and cell death [ 18 ]. The development of a technique by Gordon et al. to create three-dimensional organoids of the human cerebral cortex with characteristics of the neonatal period is astounding [ 19 ] (Fig. 2 ).
Advances in methodology of brain organoid generation. ( A ) A simple method using a minimum of medium and extracellular matrix to create self-organized brain organoids. ( B ) Synthetic materials promote organoid maturation. ( C ) Organoids with mixed systems, such as neuromuscular organoids, make it possible to study the interaction between organs. ( D ) Microfluidics develop vasculature in organoids
Brain organoids have progressed from non-directional whole brain organoids to several brain organoids with distinct regional features, including cortex [ 20 , 21 ], midbrain [ 22 , 23 ], hippocampus [ 24 ], cerebellum [ 25 ], and spinal cord [ 26 ]. Specifically, realized brain organoid vascularity and regional brain organoids.
Currently, brain organoid cultures are classified into two categories based on whether or not targeted differentiation is carried out. First, stem cell differentiation produces organoid structures that, when grown in cultures, produce multi-brain organoids without the need for external morphogenetic agents. The second approach involves timing the addition of exogenous morphogenetic and neurotrophic substances to cultivate organoids in certain brain areas in accordance with the regulatory systems of the human brain development process (Fig. 3 ).
Unguided and guided approaches for making brain organoids. Unguided methods take use of hPSCs’ inherent signaling and self-organization abilities to allow them to naturally differentiate into tissues that resemble the growing brain. The resultant brain organoids often have diverse tissues that mimic various parts of the brain. Directed techniques use growth factors and tiny chemicals to create spheres that symbolize a particular tissue type. Organoid techniques specific to brain regions include the early usage of modular variables to influence the destiny of stem cells. Later phases of differentiation subsequently eliminate these components. Moreover, alignment techniques may be used to create two or more spheres or organoids that symbolize the identities of various brain areas. These can then be combined to create “class assemblies” that simulate the interactions of various brain regions
The non-oriented brain organoids
Serum-free culture was primarily used to create neuroectoderm during the early stages of brain organoid differentiation [ 27 ], while matrigel and bioreactors were used to accomplish long-term neuronal differentiation culture [ 11 ], among other methods. The SFEBq culture method, for example, successfully differentiates embryoid bodies of mouse embryonic stem cells (mESCs) into telencephalic tissue by simultaneously adding inducers for neuronal differentiation (e.g., Wnt antagonists and Nodal antagonists). Sasai’s team [ 28 ] produced a serum-free liquid culture of embryoid body-like aggregates with rapid reaggregation in 2005. By blocking the Notch pathway, the team used SFEBq to create human and mouse embryonic stem cells (ESCs) that resemble the retina in terms of composition and cell structure, so mimicking the retina’s developing process to some degree. He established the foundation for the creation of brain organoids [ 18 , 29 ].
Using the SFEBq technique, Lancaster et al. used hiPSCs for the first time in 2013 to promote differentiation into entire brain organoids [ 11 ] (Fig. 2 ). They stimulated and directed the differentiation of hiPSC embryoid structures with endoderm, mesoderm, and ectoderm. After that, they went through neuroectoderm and neuroepithelium to produce structures that resembled the early embryo’s cerebral cortex, which may have represented the early embryo’s development of the human brain.
Cells linked to the hippocampus, retina, forebrain, midbrain, hindbrain, and other areas with apico-basal polarity are seen in whole-brain organoids [ 11 ]. Additional research has shown that the integration of tissue engineering and three-dimensional culture may enhance the tissue structure of brain organoids and boost the repeatability of differentiation [ 30 ]. Researchers used microfilaments of poly (lactide-co-glycolic acid) (PLGA) fibers as scaffolding to generate microfilament-engineered brain organoids (MEOs) in 2017 [ 31 ]. The brain organoid scaffolds made from microfilms are part of a system that advances cortical development and encourages the production of neuronal ectoderms [ 31 ]. He used air-liquid interface culture of whole brain organoids (ALI-COR) in 2019; this method improves axonal development and neuron survival in whole brain organoids [ 32 ].
To put it briefly, the unguided brain organoids created in this work mimic the cellular makeup and anatomical structure of the brain in vivo, can model the brain’s developmental process, and can represent the physiological and pathological characteristics of the brain. These capabilities open up new avenues for research on brain function, disease simulation, drug discovery, and other related topics.
Region-specific brain organoids
Organoids made out of the whole brain exhibit unique heterogeneity [ 33 ]. Brain organoids were developed to mimic specific functional properties of individual brain regions in order to better understand the functions of various brain regions and their interregulation, as well as to look into patterns of neuronal development and the onset and progression of diseases in particular brain regions. Organoids of the brain were created to mimic the properties of several brain areas, including the cerebellum, midbrain, and forebrain (Table 1 ).
The optic nerve, hippocampus, thalamus, hypothalamus, and other brain areas are included in the forebrain. In 2011, Sasai et al. caused PSCs to spontaneously generate upper hemisphere vesicles [ 18 ], inhibiting the WNT pathway and concurrently activating the WNT pathway to establish a proximal-distal axis. The distal portion folds inward to create the optic cup structure, while the proximal section includes retinal features including inner nuclear layer (INL) and retinal ganglion cells (RGCs). The research produced organoids called optic cups that contained several kinds of retinal cells. After that, the scientists constructed a 3D culture system that resembled cortical growth [ 21 , 22 ], setting the stage for the production of organoids of the brain that are particular to a certain area. 2015 Sasai and colleagues optimized cortical organoids and produced hippocampal organoids by inhibiting SMAD pathways and activating WNT and BMP pathways [ 25 ]. In 2016, Qian et al. created a hypothalamus organoid by inhibiting the SMAD route and activating the WNT and SHH signaling pathways [ 34 ]. In order to create neuroectodermal organization and increase BMP7, Xiang et al. blocked the SMAD pathway in 2019 [ 35 ]. This resulted in the development of thalamic organoids.
In the pathophysiology and therapy of Parkinson’s disease (PD), the midbrain—which regulates information transmission, motor control, and sensory processing between the forebrain and spinal cord—has drawn a lot of interest. Jo et al. produced midbrain organoids in 2016 by adding SHH/FGF8 to induce ceiling structure [ 24 ], activating the WNT pathway, and inhibiting the SMADs pathway. By identifying dopamine production, they opened up a new avenue for research on Parkinson’s and other illnesses. Furthermore, in 2015, MONZEL et al. stimulated neuroepithelial stem cells to produce organoids in the midbrain using SHH inhibitors and GSK3 inhibitors [ 23 ].
The brain’s motor control center, the cerebellum, has the capacity to develop into distinct neuronal groupings. To form cerebellar organoids, Muguruma et al. created a boundary structure between the midbrain and cerebellum by blocking the SMADs pathway, adding FGF2 and insulin to promote caudation of cerebellar organoids, and then adding FGF19 and SDF1 to induce cells to promote neuroepithelial formation in the cerebellar lamina [ 26 ]. Because of the diverse cell types and fragile structure of the cerebellum area, the cerebellar organoid culture system still needs a long-term culture system.
In addition to neurons, microgila also play a pivotal role in the brain’s functionality. Currently, the lack of microglia with the ability to reshape neuronal networks and phagocytose apoptotic cells and debris is a major shortcoming of the midbrain organoid system. Moreover, modeling of diabetes-related neurological complications is not possible in the absence of microglia. By co-culturating hiPSCs-derived mesodermal progenitor cells (Brachyury + ) with neurospheres, Worsdorfer et al. renewably generated vascularized neuroorganoids that included vasculoid structures (CD31 + ) and microglia-like cells [ 36 ]. This study provided a model for studying angiogenesis and neurodevelopment, but did not investigate the function of microglia in the organoids. In the another study, Fagerlund et al. reported that hiPSCs-derived eythro-myeloid progenitors (CD41 + ) migrated into human brain organoids [ 37 ]. Differentiated into microglia-like cells, and interacted with synaptic material. Whole-cell patch-clamp and multi-electrode array recording showed that microglia within organoids promoted the maturation of neural networks. A recent study that co-cultured human midbrain organoids with hiPSCs-derived macrophage progenitor cells also reported that microglia integration let to increased nerve maturity and function [ 38 ]. Whole-cell patch-clamp and multi-electrode array recordings showed that lower action potential generation thresholds and shorter peak-to-peak intervals were observed in midbrain organoids with microglia integration, suggesting that microglia integration improve neural maturation.
The methodologies for constructing region-specific brain organoids are also examined. The development of these organoids continues to advance, integrating developmental inducers, biomaterials, and bioreactor systems. It is anticipated that more precise realization of region-specific brain organoids can be achieved. The aim is to replicate the maturation processes of various brain regions and establish relevant disease models. These may be used to research the control of neurodevelopment and the beginning of illness in certain brain areas. To more accurately mimic the physiological structure of the brain, however, further work has to be done on the precision of the generated brain areas and the repeatability of the cells.
Brain organoid fusion
Although brain organoids may be utilized to model many interacting brain areas, their sizes and spatial configurations are very varied and unpredictable. Researchers have attempted to mimic the structure and environment of the genuine human brain by integrating several brain organoid areas in an effort to create a more realistic brain organoid design that can replicate the development of different brain regions and model disorders. 2017, Team Pasca performed neural induction of PSC by SFEBq to induce the formation of dorsal and ventral telencephalic organoids by regulating WNT and SHH signalling [ 39 ], spontaneously fused ventral and dorsal telencephalic organoids and observed irregular migration of interneurons in the cortical tissue. Interneurons in fused organoids from Timothy syndrome patients migrated abnormally. Bagley et al. fused the ventral telencephalon and whole brain organoids to form brain organoids fused to the dorsoventral axis [ 40 ]. Based on this, Xiang et al. studied the migration of CXC chemokine receptor 4 (CXCR4) dependent interneurons from ventral to dorsal migration [ 41 ]. Medial ganglionic neurite (MGE) organoids were constructed, and then fused with cortical organoids to examine CXC chemokine receptor (CXCR4) dependent interneurons.
To better imitate the mutual projection of the thalamus and cortex, XIANG et al. produced thalamocortical fusion organoids by physically combining thalamic and cortical organoids [ 35 ]. Studies of neurological conditions including schizophrenia and autism spectrum disorders may be conducted using the biaxial projection between the thalamus and cortex, which replicates synaptic connections in the body. Subsequently, MIURA et al. employing fusion of striatal and cortical organoids, showed that cortical neurons project axons to striatal organoids and make synaptic connections with neutral invertebrate neurons, showing enhanced electrical features and calcium activity [ 42 ]. By combining the two kinds of organoids, functional integration was accomplished in these four investigations. In an in vitro three-dimensional culture media, they replicated the tangential movement of human endoneurons [ 39 , 40 , 41 , 42 ].
Brain organoid fusion methods provide a potent platform for investigating the relationships between various brain regions/tissues, including the impact of tissue growth centers on brain organoid development and the investigation of cell-cell interactions in vitro. Nevertheless, in order to create functioning circuits and provide helpful instruments for researching brain function, current fusion organoid manufacturing techniques need to be further refined to represent particular brain space projections and physiological reactions.
Vascularization of brain organoids
Organoids in the brain still differ significantly from the genuine human brain. Lack of a circulatory system is one of the main obstacles. Gas penetration, nutrition delivery, neuron differentiation, and other processes are all impacted by vascular function [ 43 , 44 ]. Necrotic regions will form in the organoid center as a result of inadequate oxygen and nutrient penetration, which will interfere with the proper growth of brain organoids and the neuronal migration route [ 45 ]. Thus, the development of a vascular network is a critical requirement for the optimization of brain organoids. As of right now, there are primarily two methods for vascularizing brain organoids: creating blood vessels in the organoids by in vivo transplantation and creating blood vessels in vitro.
In 2018, researchers transplanted brain organoids into the cerebral cortex of NOD-SCID immunodeficient mice, and the blood vessels of mice infiltrated into the implanted brain organoids within 14 days after transplantation; Compared with non-vascularized brain organoids in vitro, the in vivo development environment improves cell maturation and survival in brain organoids [ 46 ]. For the purpose of achieving the functional link between human axons and neurons in the mouse brain, brain organoids that have been transplanted may produce a lot of new neurons and live for over 200 days [ 46 ]. To create human-mouse vascular tissue linkages in the grafts, human vascularized organoids (vOrganoids) co-cultured with human umbilical vein endothelial cells were inserted into the mouse S1 cortex. Compared to non-vascularized brain organoids, vascularized brain organoid transplantation increases blood vessel development and cell survival [ 47 ]. As a result, a series of transplantation experiments have demonstrated the importance of vascularization in the maturation of brain organoids.
In terms of in vitro vascularization, in 2019, Cakir et al. co-differentiated human embryonic stem cells expressing ETV2 (ETS variant 2) with wild-type embryonic stem cells to achieve directional induction of vascular endothelial cell differentiation in cortical organoids, based on which vascularization cortical organoids were constructed [ 48 ]. vascularized human cortical organoids (vhCOs) form perfusable blood vessels; Compared with control cortical organoids, the cell survival rate in vascularized cortical organoids was significantly improved [ 48 ]. In addition, by co-culturing with venous endothelial cells, researchers were able to establish vascularized brain organoids in vitro, in which venous endothelial cells can form well-developed reticular or tubular vascular systems, as confirmed by single-cell RNA sequencing. This vascularized brain organoid system has similar molecular properties and cell types to the human fetal telencephalon [ 47 ]. The integration of brain organoids and vascular system under in vitro culture will help to improve the phenomenon of central necrosis during the long-term cultivation of brain organoids.
Application of brain organoid research technology in the field of diabetes
As a novel in vitro cultivation technology, brain organoids may not only mimic early brain development in vitro, but also help to understanding brain development and developmental paths. In addition, brain organoids provide novel tools for neurological illness modeling, in vitro drug screening, gene therapy, and the simulation of human brain development (Fig. 4 ).
Applications of brain organoid research. As part of a regenerative medicine therapy, pluripotent stem cells (PSCs) from brain organoids may be implanted to brain injury areas to heal damaged tissue or utilized to investigate brain illnesses. To simulate vascular and infectious disorders and investigate their interactions with organoid cells, non-CNS derived entities including microglia, blood arteries, and viruses may be incorporated into brain organoids. The genesis of disorders affecting the nervous system may be studied using brain organoids obtained from patients or genetically engineered using CRISPR-Cas9 to carry disease-associated genetic abnormalities
The uses of brain organoids for illness [ 49 ], drug development [ 50 ], evolution [ 51 ], and brain development [ 40 ] may be further expanded by combining gene editing methods with brain organoids to generate various sorts of mutations. Brain organoids may also be used in conjunction with single-cell sequencing technology, which is crucial for understanding how the brain develops and figuring out how diseases are caused. Recent years have seen significant developments in the field of diabetes, offering fresh perspectives on the management and prevention of diabetes-related disorders, thanks to the quick growth of brain organoid research technologies.
An organoid brain model associated with KCNJ11 p.V59M was used to examine the pathogenic mechanism of neonatal diabetes
The brain organoid approach is a good model for studying neuronal differentiation and the consequences of genetic variety on brain development and disease gene expression [ 52 , 53 , 54 ]. Neonatal diabetes (NDM) has been studied through the direct effects of P. Vir59met (V59M) in the KCNJ11 gene on precursor neurons and neuronal cells. Gokhan et al. generated brain organoids from martyrs or hiPSCs with the KCNJ11 V59M mutant allele to isolate confounding effects associated with NDM [ 55 ].
The pancreas and the brain express the KCNJ11 gene, which genes for Kir6.2, a crucial subunit of the ATP-sensitive potassium channel (KATP). NDM may result from the acquisition of a functional mutation in heterozygosity in the KCNJ11 gene. A dominant heterozygous mutation in the KCNJ111 gene causes NDM, a monogenic illness that affects around 30% of the population. These mutations often cause the KATP channel to be permanently activated, which keeps the cell persistently hyperpolarized. Due to the altered functionality of these KATP channels, the beta cells within the islets are incapable of secreting insulin, Consequently, this results in elevated blood sugar levels [ 56 ]. Neural stem cells in V59M organisms often do not develop and move, according to data. As a result, there are abnormalities in the development and function of brain circuits. This lowers neurogenesis. Tolbutamide (Tol), a KATP channel blocker, may be used as a medication to treat mutant organoids, which can partly correct the molecular flaws brought on by the cell membrane’s hyperpolarization. In brain tissue taken from HIPSC patients, this work offers the first concrete proof that the mutant KCNJ11 channel results in neurological impairment.
New drug therapies for people with inherited diseases can typically be found thanks to advancements in personalised medicine platforms that use stem cell-derived tissue [ 57 , 58 ]. Additionally, pathology linked to mutations in the KCNJ11 gene for neonatal diabetes can be detected thanks to hiPSC-derived brain organoid platforms. Using a brain organoid platform, confounding effects associated with neonatal diabetes were differentiated from the direct impact of V59M mutations on neurocytes and neurons. It may be inferred from this that the development of brain organoids offers valuable insights into the fundamental principles of cellular and neurophysiological events linked to intricate metabolic disorders.
Treatment of diabetic retinopathy with cerebral organoids containing optic vesicles
In recent years, various 3D brain organoids including hiPSC-derived neuroretinal discs have presented new chances to research retinal illnesses [ 39 , 59 , 60 ].
By altering the growth conditions to transform iPSCs into neural tissue, Elke et al. were able to successfully create bilaterally symmetric visual slices in brain organoids in 2021 [ 61 ]. The researchers began the induction culture with a decreased cell density. After that, during the neuroectodermal growth phase, retinol acetate (RA) was given to the media at various concentrations. Staining structures, most likely the original “eyes,” emerged in the tissue cultivated with retinol acetate after about 30 days in culture (Fig. 5 ). Immunofluorescence labeling indicated considerable expression of the eye-associated marker genes RAX, Pax6 and FOXG1 in the stained areas of these organoids.
Schematic showing steps of OVB-organoid generation from iPSCs. The results of the research demonstrated the use of IPSC-derived human brain organoids in the generation of bilateral forebrain-connected OV, cellular diversity, and complexity reduction. The brain organoids start to gather OV at day 30, and during the next 60 days, they manifest as distinct structures
Subsequent examination revealed that FOXG1 expression was gradient in the SOX2-positive invagination zone, suggesting that the visual and forebrain regions in these organoids were distinct, in line with the eye’s maturation process throughout human embryonic development. After a growth period of 50–60 days, the rudimentary “eyes” transformed into one or two mature, visible optic nerve cell structures (Fig. 5 ), and such organoids are designated optic vesicle cell-brain organoids (OVB organoids). This work is the first to functionally connect brain organoids with retinal structure. It helps to explore the interplay between “brain and eye” during embryonic development and offers a strong tool for the pathophysiology and treatment of diabetic retinal disorders.
Based on this, scientists have cultivated exosomes from tissues resembling OVB, which are involved in both retinal development and retinal disorders including diabetic retinopathy [ 62 ].
The primary symptom of diabetic microangiopathy, which causes distinct alterations in the retinal lesions, is diabetic retinopathy. Furthermore, retinal ischemia-reperfusion damage (IRI) is a significant factor in the development of DR [ 62 ]. Directly after retinal ischemia are transient hypoxia and nutritional depletion. Reperfusion produces excessive reactive oxygen species (ROS), which leads to oxidative stress and an exaggerated inflammatory response [ 63 ]. The significance of mesenchymal stem cells (MSCs) in vitro exosome secretion in the eye has gained more interest since studies have shown that intravitreal injection of LV-derived exosomes may alleviate diabetic retinopathy (DR) [ 64 ]. Moissiev et al. established the impact of exosomes on retinal ischemia by intravitreally injecting hypoxia-grown exosomes containing angiogenic active components and control saline into mice models of oxygen-induced retinopathy (OIR) [ 65 ]. Exosome therapy decreased and avoided retinal thinning in comparison to the control group.
Moreover, Liu et al. discovered that exosomes may transport endogenous miR-579 and circulating RNA cPWWP2A, and that these effects on retinal vascular function in diabetes patients are mediated by differences in the expression levels of these two molecules [ 66 ]. 148 Adipose tissue mesenchymal stem cell exosomes carrying miR-192 or miR-222 have been shown by Safwat et al. to be able to suppress angiogenesis and inflammatory responses in the DR [ 67 ].
The potential to cure diabetic retinopathy has increased with the discovery of OVB organoids. OVB organoids are a significant tool for disease modeling, high-throughput drug screening as an alternative to animal models. An affordable substitute for animal models in illness research and medication screening are in vitro models. Exosomes produced from brain organoids seem to offer tremendous promise for medication delivery and the treatment of ocular illnesses, according to recent research on the therapeutic effects of exosomes in ocular diseases. Furthermore, a great deal of obstacles still need to be addressed, such as the absence of microglia and blood arteries, which are essential for preserving the long-term survival of organoids and accurately replicating the retina. In addition, novel biotechnologies including 3D bioprinting, oxygen delivery systems, and retina-on-a-chip are being created to meet these difficulties.
Building novel and emerging organoid culture systems to simulate organoid co-culture microenviroment in diabetes disease modeling
The properties and uses of brain organoid systems can currently be further increased when paired with additional engineering methods. Organ-on-a-chip technology offers a critical platform for the facile manipulation of the microenvironment and nutrient supply, and it constitutes a pivotal method for the concurrent culture of diverse cell and tissue types within organoid systems [ 68 ]. Furthermore, organ chips mitigate the challenge of co-culturing distinct organs of the same type in a singular medium to a considerable extent. The ability to replicate the interplay of several organs in an in vitro setting is extremely important, particularly for complicated metabolic illnesses that impact multiple tissues, like diabetes mellitus. A microfluidic in vitro model is employed for simulating neural tube development. For instance, an organ-on-a-chip configuration may utilize soluble factor-infused microchannels as entry and convergence zones, facilitating the establishment of a consistent morphogenetic gradient via diffusive processes within a central culture chamber. This system was able to replicate the Sonic Hedgehog (SHH) signaling gradients and the bone morphogenetic protein (BMP) gradients along the dorsoventral axis of the neural tube, thereby facilitating the induction of neural tube development models [ 69 ].
In 3D cultured organoids, material diffusion and transport are not enough to meet the growing metabolic demand, so it is difficult to ensure long-term growth and maturity. The establishment of functional vascular system is a necessary condition for the continuous healthy cultivation of brain organoids. Capabilities of perfusable blood vessels can be emulated through the utilization of organ-on-a-chip technology [ 70 , 71 , 72 ]. In addition, microchips or micro-bioreactors have also been constructed for brain organoids, such as in microfluidic systems that reduce necrotic areas in midbrain organoids by ensuring the supply of media through continuous laminar flow [ 73 ], which utilizes forced convection and media mixing to enhance nutrient supply. Therefore, organ chips is also an important solution to solve the difficulties faced in the process of organoid culture [ 68 ].
Summary and prospect
Recent developments in the field of organoid technology have yielded substantial improvements in our comprehension of the principles behind human brain development and the etiology of neurological disorders. By combining various technologies, scientists have made significant progress in understanding the evolutionary laws of brain development and the mechanisms that regulate brain development. These models, which include non-targeted, regional, and combined brain organoids, hold promise for modeling nervous system diseases, drug testing, gene editing, and other areas.
Nevertheless, the present technology for producing brain organoids has not been created yet and has significant limitations because to the constraints of culture and induction approaches. The size of organoids, the maturation of neurons, and the subsequent generation of more complete cell types are all limited by the conditions of in vitro culture. Additionally, because brain organoids lack complete neuronal circuits and functional zoning, it is difficult to predict internal structures such as oligodendrocytes and astrocytes. Finally, the application of brain organoids is limited because they lack essential cell types like immune cells, which cannot form complex neuronal circuits. Thirdly, brain organoids’ metabolic properties vary greatly from those of the real brain. Fourthly, the growing conditions of brain organoids and the chemical combinations introduced vary due to the heterogeneity of iPSC cells in various labs, resulting in wildly disparate brain organoid models. Fifth, the cell composition, morphological features, and differentiation efficiency of the various batches of brain organoids vary as well [ 8 ].
The use of brain organoids in diabetes therapy is still in its infancy. In 2021, Gokhan et al. separated the direct effects of the V59M mutation on neuroprecursors and neurons from the disruptive effects linked to neonatal diabetes using the brain organoid platform [ 55 ]. Electrophysiological investigations have revealed that mutant brain organoids may create functioning neural networks whose excitability is reduced under baseline circumstances, even if mutant KCNJ11 channel activity hinders the formation of neuronal precursor cells. Furthermore, raising extracellular potassium levels erased the difference between the amount of mutant brain organoids and spontaneous active control. According to the findings, in human samples, mutant KCNJ11 channel activity does not control network excitability or circuit development. The method by which brain organoids may more effectively regulate the cellular and neurophysiological processes that accompany complicated metabolic disorders is supported by the findings of this research. This is a novel approach to the treatment of diabetic neuropathy using brain organoids in research.
Furthermore, new therapeutic approaches for diabetic retinopathy have been made possible by the discovery of OVB organoids as well as complicated brain and retinal multiorganoids. CPWWWP2A cyclin RNA was found in exosomes produced from OVB organoids by Liu et al. [ 66 ]. This finding may indirectly affect retinal vascular function in diabetes patients. Exosomes rich in miR-192 or miR-222 produced from mesenchymal stem cells were discovered by Safwat et al. [ 67 ]. These miRNAs may suppress angiogenesis and the inflammatory response in DR.
In addition, it is challenging to guarantee long-term viability and maturity in 3D cultivated organoids because material diffusion and transport are insufficient to fulfill the increasing metabolic requirement. For the proper culture of brain organoids to continue, a functioning circulatory system must be established. Organoid chips can be used to replicate blood arteries with the capacity to perfuse [ 70 , 71 , 72 ]. Microfluidic systems that minimize necrotic areas in midbrain organoids by guaranteeing the supply of media through continuous laminar flow [ 73 ] a technique that makes use of forced convection and media mixing to enhance nutrient supply have also been developed for brain organoids, as have microchips or micro-bioreactors. Organ chips are therefore a significant way to address the challenges associated with organoid cultivation [ 68 ]. Conversely, the microbiome exerts influences on neurodevelopment and the functionality of the central nervous system, which employing co-culture techniques with microbial entities or their by-products within tissue cultures may elucidate these intricate interactions, and the amalgamation of microbiota and immune elements within an organ-on-a-chip platform could also enhance its fidelity as a model [ 74 ]. Moreover, the deployment of neural tissue proliferation molecules aligns with the utilization of dorsal forebrain organoids (FGF2) and epidermal growth factor (EGF), as well as ventral forebrain organoids [ 75 ]. Upon identification, growth factors were incorporated into the media for both dorsal and ventral forebrain organoids to facilitate neural differentiation and maturation. For purpose of generating oligodendrocytes containing forebrain organoids, additional insulin-like growth factor (IGF), platelet-derived growth factor AA (PDGF-AA), hepatocyte growth factor (HGF) was used during differentiation and maturation. These promising culture conditions and using growth factors will improve the long-term viability and maturation of organoids [ 76 , 77 ].
The convergence of bioengineering with the organoid domain has facilitated the advancement of automation and miniaturization in detection processes, alongside the capacity for real-time acquisition of biological data during culturing. Bioengineered hydrogels have been developed and evaluated for their efficacy in supporting the three-dimensional cultivation of organoids, albeit they typically exhibit less promotional impact on growth compared to hydrogels derived from the extracellular matrix. A hydrogel constitutes a three-dimensional matrix of insoluble water-containing polymers. The dimensions of the micropores are capable of accurately replicating the size of organoids [ 78 ]. Furthermore, the system can be engineered to capture cells or their contents post-experimentation. This facilitates the examination of interactions between various organoids, with the intent to mirror physiological interactions observed in organs.
As to scale up organoid production, automation and miniaturization techniques are born at the right time, expect for the microfluidic systems, recently, an emerging frontier is combining organoids with artificial intelligence (AI) systems, known as “organoid intelligence” [ 79 , 80 ]. The goal is to use stem cell-derived organoids not only as models, but also as active components integrated into biological hybrid systems that demonstrate cognition and learning. Osteogenesis imperfecta provides an unprecedented opportunity to elucidate neurophysiological mechanisms and advance the pharmacology and toxicology related to the brain. By summarizing the composition of human brain cells [ 81 ] and adding structure, OI systems allow direct experimental access to investigate the processes underlying neural signals and network activity. OI also has the potential to inspire new directions in neuromorphic engineering and proceed unprecedented biological computing capabilities [ 74 ]. Since the study elucidates how brain organoids exhibit learning and information processing based on dynamic neuronal signals, these findings could guide the development of hardware and scale up organoid production.
The creation and use of brain organoids will remain a crucial topic for the life sciences in the future due to the quick development of new technologies and the increased interest in brain organoids in recent years. These developments have created new opportunities for a better understanding of human brain development, function, evolution, and disease. Although brain organoids can simulate the cellular, molecular and functional characteristics of brain development, due to the challenges associated with maintaining long-term healthy culture, brain organoids created in vitro are mostly limited in their ability to describe embryonic brain properties during their brief culture period. The significant variability in organoid generation and differentiation is a concern. Despite there are a large number of standardizing protocols to improve reproducibility across different laboratories about brain organoids, such as promising culture systems, growth factors and small molecule [ 75 ], its research in the field of diabetes remains to be further explored. More research is still needed to build brain organoids with more sophisticated and developed neural networks. Among them, it is anticipated that the creation of functionally vascularized brain organoids will enable the long-term growth of brain organoids. In summary, brain organoids represent a novel technology that has garnered significant attention and quick growth in recent years, presenting both potential and obstacles for research. It is anticipated that as this technology advances, it will offer a crucial resource for comprehending the human brain and examining a wide range of biological and medical issues.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition [J]. Diabetes Res Clin Pract. 2019;157:107843.
Article PubMed Google Scholar
Ashcroft F M Rorsmanp. Diabetes mellitus and the β cell: the last ten years [J]. Cell. 2012;148(6):1160–71.
Article PubMed PubMed Central Google Scholar
Bishay R H, Greenfield JR. A review of maturity onset diabetes of the young (MODY) and challenges in the management of glucokinase-MODY [J]. Med J Aust. 2016;205(10):480–5.
Shih HP, Wang A. Pancreas organogenesis: from lineage determination to morphogenesis [J]. Annu Rev Cell Dev Biol. 2013;29:81–105.
Article CAS PubMed Google Scholar
Cabrera O, Berman D M, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function [J]. Proc Natl Acad Sci U S A. 2006;103(7):2334–9.
Aamodt K I, Powers AC. Signals in the pancreatic islet microenvironment influence β-cell proliferation [J]. Diabetes Obes Metab. 2017;19(Suppl 1):124–36.
Schutgens F. Human organoids: tools for understanding Biology and Treating diseases [J]. Annu Rev Pathol. 2020;15:211–34.
Chiaradia I, Lancaster MA. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo [J]. Nat Neurosci. 2020;23(12):1496–508.
Sharf T, Van der molen T, Glasauer S M K, et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids [J]. Nat Commun. 2022;13(1):4403.
Article CAS PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors [J]. Cell. 2006;126(4):663–76.
Lancaster M A, Renner M, Martin C A, et al. Cerebral organoids model human brain development and microcephaly [J]. Nature. 2013;501(7467):373–9.
Ying Q L, Stavridis M. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture [J]. Nat Biotechnol. 2003;21(2):183–6.
Zhang SC, Duncan I D Wernigm, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells [J]. Nat Biotechnol. 2001;19(12):1129–33.
Shi Y, Kirwan P, Smith J et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses [J]. Nat Neurosci, 2012, 15(3): 477 – 86, s1.
Gaspard N, Bouschet T, Hourez R, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells [J]. Nature. 2008;455(7211):351–7.
Chambers S M, Fasano C A, Papapetrou E P, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling [J]. Nat Biotechnol. 2009;27(3):275–80.
Sato T, Vries R G, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche [J]. Nature. 2009;459(7244):262–5.
Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture [J]. Nature. 2011;472(7341):51–6.
Qian X, SU Y, Adam C D et al. Sliced human cortical organoids for modeling distinct cortical layer formation [J]. Cell Stem Cell, 2020, 26(5): 766 – 81.e9.
Yoon S J Gordona, Tran S S, et al. Long-term maturation of human cortical organoids matches key early postnatal transitions [J]. Nat Neurosci. 2021;24(3):331–42.
Mariani J, Simonini M V Palejevd, et al. Modeling human cortical development in vitro using induced pluripotent stem cells [J]. Proc Natl Acad Sci U S A. 2012;109(31):12770–5.
Kadoshima T, Sakaguchi H, Nakano T, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex [J]. Proc Natl Acad Sci U S A. 2013;110(50):20284–9.
Monzel A S, Smits L M, Hemmer K, et al. Derivation of human midbrain-specific organoids from Neuroepithelial Stem cells [J]. Stem Cell Rep. 2017;8(5):1144–54.
Article Google Scholar
Jo J, Xiao Y, Sun A X, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons [J]. Cell Stem Cell. 2016;19(2):248–57.
Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue [J]. Nat Commun. 2015;6:8896.
Muguruma K, Nishiyama A, Kawakami H, et al. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells [J]. Cell Rep. 2015;10(4):537–50.
Ogura T, Sakaguchi H, Miyamoto S et al. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells [J]. Development, 2018, 145(16).
Eiraku M, Watanabe K, Matsuo-takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals [J]. Cell Stem Cell. 2008;3(5):519–32.
Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells [J]. Nat Neurosci. 2005;8(3):288–96.
Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs [J]. Cell Stem Cell. 2012;10(6):771–85.
Lancaster M A, Corsini N S, Wolfinger S, et al. Guided self-organization and cortical plate formation in human brain organoids [J]. Nat Biotechnol. 2017;35(7):659–66.
Giandomenico S L, Mierau S B, Gibbons G M, et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output [J]. Nat Neurosci. 2019;22(4):669–79.
Quadrato G, Nguyen T, Macosko E Z, et al. Cell diversity and network dynamics in photosensitive human brain organoids [J]. Nature. 2017;545(7652):48–53.
Qian X, Nguyen H N, Song MM, et al. Brain-region-specific Organoids using mini-bioreactors for modeling ZIKV exposure [J]. Cell. 2016;165(5):1238–54.
Xiang Y, Tanaka Y, Cakir B et al. hESC-Derived thalamic Organoids Form reciprocal projections when fused with cortical organoids [J]. Cell Stem Cell, 2019, 24(3): 487 – 97.e7.
Wörsdörfer P, Dalda N. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells [J]. Sci Rep. 2019;9(1):15663.
Fagerlund I, Dougalis A, Shakirzyanova A et al. Microglia-like cells promote neuronal functions in cerebral organoids [J]. Cells, 2021, 11(1).
Sabate-Soler S, Nickels S L, Saraiva C, et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality [J]. Glia. 2022;70(7):1267–88.
Birey F, Andersen J, Makinson C D, et al. Assembly of functionally integrated human forebrain spheroids [J]. Nature. 2017;545(7652):54–9.
Bagley JA, Reumann D. Fused cerebral organoids model interactions between brain regions [J]. Nat Methods. 2017;14(7):743–51.
Xiang Y, Tanaka Y, Patterson B et al. Fusion of regionally specified hPSC-Derived Organoids models Human Brain Development and Interneuron Migration [J]. Cell Stem Cell, 2017, 21(3): 383 – 98.e7.
Miura Y, Li M Y, Birey F, et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells [J]. Nat Biotechnol. 2020;38(12):1421–30.
Yin X, Mead B E, Safaee H, et al. Eng Stem Cell Organoids [J] Cell Stem Cell. 2016;18(1):25–38.
Article CAS Google Scholar
Shen Q, Goderie S K Jinl, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells [J]. Science. 2004;304(5675):1338–40.
Giandomenico S L, Lancaster M A. Probing human brain evolution and development in organoids [J]. Curr Opin Cell Biol. 2017;44:36–43.
Mansour A A, Gonçalves JT, Bloyd C W, et al. An in vivo model of functional and vascularized human brain organoids [J]. Nat Biotechnol. 2018;36(5):432–41.
Shi Y, Sun L, Wang M, et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo [J]. PLoS Biol. 2020;18(5):e3000705.
Cakir B, Xiang Y, Tanaka Y, et al. Engineering of human brain organoids with a functional vascular-like system [J]. Nat Methods. 2019;16(11):1169–75.
Bian S, Repic M, Guo Z, et al. Genetically engineered cerebral organoids model brain tumor formation [J]. Nat Methods. 2018;15(8):631–9.
Zhou T, Cederquist G Y Tanl et al. High-content screening in hPSC-Neural progenitors identifies drug candidates that inhibit Zika Virus infection in fetal-like organoids and adult brain [J]. Cell Stem Cell, 2017, 21(2): 274 – 83.e5.
Mora-bermúdez F, Badsha F, Kanton S et al. Differences and similarities between human and chimpanzee neural progenitors during cerebral cortex development [J]. Elife, 2016, 5.
Velasco S, Paulsen B. 3D brain organoids: studying Brain Development and Disease outside the embryo [J]. Annu Rev Neurosci. 2020;43:375–89.
Lancaster M A, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies [J]. Science. 2014;345(6194):1247125.
Di Lullo E, Kriegstein A R. The use of brain organoids to investigate neural development and disease [J]. Nat Rev Neurosci. 2017;18(10):573–84.
Dalgin G, Tryba A K, Cohen A P, et al. Developmental defects and impaired network excitability in a cerebral organoid model of KCNJ11 p.V59M-related neonatal diabetes [J]. Sci Rep. 2021;11(1):21590.
Gloyn A L, Pearson E R, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes [J]. N Engl J Med. 2004;350(18):1838–49.
Clevers H. Modeling Development and Disease with organoids [J]. Cell. 2016;165(7):1586–97.
Sahu S, Sharan SK. Translating embryogenesis to Generate Organoids: Novel approaches to Personalized Medicine [J]. iScience. 2020;23(9):101485.
Gabriel E, Wason A, Ramani A, et al. CPAP promotes timely cilium disassembly to maintain neural progenitor pool [J]. Embo j. 2016;35(8):803–19.
Fernando M, Lee S, Wark J R, et al. Differentiation of brain and retinal organoids from confluent cultures of pluripotent stem cells connected by nerve-like axonal projections of optic origin [J]. Stem Cell Rep. 2022;17(6):1476–92.
Gabriel E, Albanna W. Human brain organoids assemble functionally integrated bilateral optic vesicles [J]. Cell Stem Cell. 2021;28(10):1740–e578.
Walker R. Diabetic retinopathy: protecting the vision of people with diabetes [J]. Br J Community Nurs. 2004;9(12):545–7.
Mu H, Wang Y, Wei H, et al. Collagen peptide modified carboxymethyl cellulose as both antioxidant drug and carrier for drug delivery against retinal ischaemia/reperfusion injury [J]. J Cell Mol Med. 2018;22(10):5008–19.
Zhang W, Wang Y. Exosomes Derived from mesenchymal stem cells modulate miR-126 to ameliorate Hyperglycemia-Induced retinal inflammation Via Targeting HMGB1 [J]. Invest Ophthalmol Vis Sci. 2019;60(1):294–303.
Anderson J D Moisseieve. Protective Effect of Intravitreal Administration of exosomes derived from mesenchymal stem cells on retinal ischemia [J]. Curr Eye Res. 2017;42(10):1358–67.
Liu C, Ge H M, Liu B H, et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction [J]. Proc Natl Acad Sci U S A. 2019;116(15):7455–64.
Safwat A, Sabry D. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits [J]. J Circ Biomark. 2018;7:1849454418807827.
Park SE, Georgescu A. Organoids-on-a-chip [J]. Science. 2019;364(6444):960–5.
Demers C J, Soundararajan P, Chennampally P, et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device [J]. Development. 2016;143(11):1884–92.
Kim S, Lee H, Chung M, et al. Engineering of functional, perfusable 3D microvascular networks on a chip [J]. Lab Chip. 2013;13(8):1489–500.
Hasan A, Paul A, Memic A, et al. A multilayered microfluidic blood vessel-like structure [J]. Biomed Microdevices. 2015;17(5):88.
Kolesky D B, Homan K A, Skylar-scott M A, et al. Three-dimensional bioprinting of thick vascularized tissues [J]. Proc Natl Acad Sci U S A. 2016;113(12):3179–84.
Berger E, Magliaro C, Paczia N, et al. Millifluidic culture improves human midbrain organoid vitality and differentiation [J]. Lab Chip. 2018;18(20):3172–83.
Smirnova L, Hartung T. The Promise and potential of Brain organoids [J]. Adv Healthc Mater, 2024: e2302745.
Mulder L A, Depla J A, Sridhar A, et al. A beginner’s guide on the use of brain organoids for neuroscientists: a systematic review [J]. Stem Cell Res Ther. 2023;14(1):87.
Nevin Z S Madhavanm, Shick H E, et al. Induction of myelinating oligodendrocytes in human cortical spheroids [J]. Nat Methods. 2018;15(9):700–6.
Marton RM, Miura Y, Sloan S A, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures [J]. Nat Neurosci. 2019;22(3):484–91.
Glieberman A L, Pope B D, ZIMMERMAN JF, et al. Synchronized stimulation and continuous insulin sensing in a microfluidic human islet on a chip designed for scalable manufacturing [J]. Lab Chip. 2019;19(18):2993–3010.
Smirnova L, Morales Pantoja I E, Hartung T. Organoid intelligence (OI) - the ultimate functionality of a brain microphysiological system [J]. Altex. 2023;40(2):191–203.
Morales Pantoja I E Smirnoval, Muotri A R, et al. First Organoid Intelligence (OI) workshop to form an OI community [J]. Front Artif Intell. 2023;6:1116870.
Morales Pantoja I E Dingl, Leite P E C, A Novel Approach to Increase Glial Cell Populations in Brain Microphysiological Systems [J]. Advanced, Biology et al. n/a(n/a): 2300198.
Qian X, Jacob F, Song MM, et al. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor [J]. Nat Protoc. 2018;13(3):565–80.
Ozone C, Suga H, Eiraku M, et al. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells [J]. Nat Commun. 2016;7:10351.
Download references
Acknowledgements
Not applicable.
This study was supported by the Natural Science Foundation of Hubei Province (Grant Number 2024AFB1025 to Dan Zhu), and Hubei University of Science and Technology “Medical Research Special Fund” (Grant Number 2022YKY18 to Dan Zhu), and Foundation of Hubei University of Science and Technology “Doctoral Initiation Fund” (Grant Number BK202414 to Dan Zhu), and Foundation of Hubei University of Science and Technology “Horizontal Research Projects” (Grant Number 2023HX121 to Dan Zhu).
Author information
Ying Su, Aimei Liu, Hongguang Chen and Qingjie Chen contributed equally to this work.
Authors and Affiliations
Hubei Key Laboratory of Diabetes and Angiopathy, Xianning Medical College, Hubei University of Science and Technology, No.88, Xianning Avenue, Xianan District, Xianning, 437000, Hubei Province, P. R. China
Ying Su, Aimei Liu, Hongguang Chen, Qingjie Chen, Bo Zhao, Runze Gao, Zhenwang Zhang, Changhan Ouyang & Dan Zhu
School of Phamacy, Hubei University of Science and Technology, Xianning, 437000, Hubei Province, P. R. China
Ying Su, Bo Zhao, Runze Gao, Kangwei Zhang & Changhan Ouyang
Hubei University of Science and Technology, Xianning, 437100, P. R. China
You can also search for this author in PubMed Google Scholar
Contributions
Ying Su, Aimei Liu and Hongguang Chen wrote, edited and revised the manuscript, and confirmed the authenticity of the raw data. Qingjie Chen and Bo Zhao drawed the schematic illustrations. Runze Gao and Tie Peng participated in the collation of article tables. Zhenwang Zhang, Changhan Ouyang and Dan Zhu provided direction and guidance throughout the preparation of this manuscript. All authors read and approved final version of manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Zhenwang Zhang , Changhan Ouyang or Dan Zhu .
Ethics declarations
Ethics approval and consent to participate, patient consent for publication, competing interests.
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Su, Y., Liu, A., Chen, H. et al. Research progress of brain organoids in the field of diabetes. Mol Brain 17 , 53 (2024). https://doi.org/10.1186/s13041-024-01123-4
Download citation
Received : 23 May 2024
Accepted : 25 July 2024
Published : 06 August 2024
DOI : https://doi.org/10.1186/s13041-024-01123-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain organoids
- Pluripotent stem cells
- Gene editing
- Retinopathy
Molecular Brain
ISSN: 1756-6606
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18.

Autoimmunity: From Bench to Bedside [Internet].
Chapter 45 cell culture and cell analysis.
María-Teresa Arango , Paula Quintero-Ronderos , John Castiblanco , and Gladis Montoya-Ortíz .
- Introduction
In vitro cell culture is a method used for studying the behavior of animal cells in a controlled environment, free of systemic variations. Currently, different types of cell cultures have been adapted and developed. Animal cell cultures have been applied for studying basic cell biology, interactions of drugs and other chemicals with cells, production of vaccines and proteins, etc. This chapter covers a brief summary of the main features, types, requirements and applications of cell culture methodology. Also, we describe applications and principles for cell separation techniques, ranging from basic to more advanced applications.
- Development of cell culture
Cell culture was developed in the early twentieth century as a method to study the behavior of animal cells in an environment free of the systemic changes that can be found in an animal during the normal homeostasis and stress of an experiment. The first model chosen for cell culture were amphibian cells, presumably for being exothermic animals and given that their cells would not require successive incubations. Later, medical science breakthroughs led to an interest in endothermic animals, where the normal and pathological development is similar to that of humans. The advent of mouse strains genetically pure brought mammals to the research laboratories. While the embryos provided a wide range of cell types in primary culture, mouse models had the advantage of continuous cell lines and a substantial tumor cell repertoire.
The progression of cell culture as a modern and sophisticated technique is based mostly on the needs of two major medical branches: virology and oncology. Cell culture has also been welcomed in many medicine and manufacturing routine applications. Cytogenetically analysis of amniocentesis derived-cells has the ability to disclose genetic disorders in the fetus. Likewise, viral infections can be evaluated quantitatively and qualitatively in host-cultured cells. Toxic effects of potential pharmaceutical compounds and contaminants can be evaluated also by using cell cultures.
Cell culture applications
The main uses of cell culture systems include:
Experimental model systems in basic and medical sciences. Cell culture offers certain advantages over the environmental and biological variability of other models. In addition, the use of genetically defined and characterized cell lines can simplify the analysis of experimental data. On the other hand, results obtained with specific cellular systems may not be representative of a wide range of other types of cells.
Study of physiological requirements for certain cell types. These include studies on positive effects of growth factors, growth-promoting substances, negative effects of cytotoxic compounds or xenobiotics and events related to programmed cell death (apoptosis), as well as cell proliferation, cell activation, cell signaling or any other cellular process.
Studies of cell development and differentiation. these include aspects of cell cycle and gene expression.
Pathological studies. these are enclosed in the characterization of cells using karyotyping to determine their genetic status.
Genetic Manipulation. cell culture techniques have played an essential role in the development of molecular biology, through the development of methods such as transfection.
Biotechnology. based on the manufacturing and industrial production of therapeutic proteins, vaccines and monoclonal antibodies.
Animal cell culture systems
Cellular systems can be established from whole organisms (e.g., chicken embryo), discrete organs (e.g., mouse liver), or blood cells (e.g., lymphocytes). While, in theory, it is possible to grow nucleated cells from any source, in practice, the highest probability of success is achieved with active young cells. The main considerations for the development of cell cultures are:
Biosafety. It is important to be aware of the potential infection risks when culturing animal cells. Although animal cells have a reduced risk of disease transmission compared to human cells, cultures should always be handled as a potential source of pathogenic microorganisms.
Using primary culture or continuous cell lines. freshly isolated cells more easily reflect the biochemical dynamics of the cells in vivo , although having a limited life span. Continuous cell lines are easy to use and offer the advantage of a priori knowledge of their specific growth requirements.
Culture media requirements. These include the provision of inorganic ions (such as a balanced salt solution), a carbon source, organic nutrients and other supplements that include antimicrobial agents to counteract the risk of contamination. For growth support, usually a basal medium supplemented with serum (e.g., fetal bovine serum) or a homologous serum supplement with a set mixture composition of proteins, polypeptides, hormones, lipids, and trace elements. Levels of CO 2 and O 2 should be considered; many cultures are buffered with bicarbonate and should be kept in a CO 2 rich atmosphere.
Advantages and disadvantages of cell culture
Clearly, the study of cellular activity in vitro has several advantages and disadvantages. The main advantage is the consistency and reproducibility of results that can be obtained from using a batch of clonal cells. Cell cultures have a highly control of the physicochemical environment (i.e., pH, temperature, osmotic pressure, oxygen, and carbon dioxide tension) which can be controlled very accurately, and the control of physiological conditions, which can be constantly examined.
The disadvantages of cell culture are: highly skilled personnel, techniques must be performed using strict asepsis techniques because animal cells grow slower than many of the common contaminants (e.g., bacteria, viruses and fungi). Additionally, animal cells may not survive when isolated and therefore are not capable of an independent sustainable existence without providing a complex environment. One of the main limitations of cell culture is the expense and effort that has to be applied to obtain a relatively low amount of cells.
In addition, tissue composition is variable and heterogeneous. Replicas from the same sample have various constituents. To replicate an experimental result, cell lines must be manipulated many times in serial passages. For instance, every culture is going to be different from the original and less uniform in its constitution. In order to resolve this issue, the replicas are randomly mixed in each passage and the selective pressure of growing conditions tends to produce an optimal prevalent phenotype.
Main types of cell culture
Cell culture is a collection of techniques and resources in which cells that were part of an organism are growth in an artificial controlled environment. Usually, tissue must be previously treated to disrupt it by mechanical or enzymatic processes depending on the origin of the tissue and the purpose of the cell culture. However, some cells can be culture without this treatment such as in the case of liquid samples.
During tissue culture, tissue characteristics and architecture must be retained, at least partially. For instance, growth is slow and restricted to the use of embryonic tissue or 3D cell culture procedures. The 3D cell culture mimics the microenvironment, architectural design and functionality of a normal tissue under control conditions. In some cases, cell migration is observed through the solid phase when a piece of tissue is placed on a solid liquid-interface. Thus generating primary explants that grow outside the original tissue
The Primary Culture is the first culture that grows successfully after the cell isolation from a tissue. As mentioned above, cell should be subculture in a series of passages in order to keep the best condition for cell growth. As result, these cell lines go into senescence after the thirtieth division cycle. For this reason, cells are storage in a cell bank system to maintain them for long periods of time. In some cases, the cells can be immortalized, e.g., B cell lymphocyte can be immortalized with the Epstein- Barr virus to confer them the ability to proliferate indefinitely. These transformed cells have the advantage of unlimited availability, but they have the disadvantage of losing its initial characteristics. Figure 1 shows the main ways to start cell cultures (for further information please see recommended readings).
Main type of sources for cell cultures. Adapted from (1).
- Preliminary preparation and sampling
Cell samples are obtained and prepared from organs, tissues and biological fluids depending on the target cell type, type of genetic material and type of analysis to be performed ( Table 1 ). In all cases one must follow processes to ensure the quality of the sample. Generally, the preparation is performed after obtaining the sample, however, if this is not possible, conditions should be provided to ensure that the sample does not undergo degradation.
Some types of cell cultures and their applications.
The success of each technique depends on the proper acquisition, processing and preservation of the sample.
Considerations for blood samples
Blood-drawing is a critical step for the experimental design. There are many factors that influence the conditions of blood components such as nutritional status, hormone levels and the circadian rhythms. If an experiment or project depends on the analysis and comparison of multiple parameters in blood samples collected from different individuals, it must be ensured that blood drawing should be carried out under the same conditions. For example, nutritional state and circulating hormones and cytokines may have important effect on the performance and behavior of the cells. Thus, in fasting state, the leptin levels in the blood are diminished and as a result, there are differences in T cell processes such as differentiation and increasing IFNγ secretion and decrease IL-4 production. In addition, differences in leptin levels have been shown to interfere with the T-regulatory cells proliferation.
Another important factor for evaluating immune functions in blood samples is the time when the sample is collected as circadian rhythms play an important role. One example is the response to the toxoid of Clostridium tetani . Typically, a higher inflammatory response is seen around 3 a.m (represented high IFNγ/IL-10 ratio) and the lowest point during the day is late morning and evening (10 a.m and 8 p.m). The highest values correspond to the peak of plasma melatonin, while the lowest values are related with higher levels of cortisol. These two hormones have opposing immune effects; cortisol naturally inhibits the pro-inflammatory response while melatonin stimulates it. As a consequence, the levels of these hormones and the way that cells respond to stimulation depend on the level of hormones and cytokines that are also influenced by the circadian rhythm.
Regarding the anticoagulant used for getting blood samples, it is very important to have in mind the effect that it may have on the cells. As for the more common anticoagulant the ethylenediamine tetraacetic acid (EDTA), one of its properties is to chelate metals, especially calcium which is extremely important for the cell activation through the calcium channels. For this reason it is recommended using other anticoagulants such as acid citrate-dextrose (ACD), sodium heparin, lithium heparin or sodium citrate. Also, the time that cells are exposed to the anticoagulant must be very short, to ensure proper cell recovery, viability and function. Temperature for blood storage is also critical. Therefore, it is recommended to storage the blood at room temperature to avoid abrupt changes that may have effects on the cells.
Cell separation methods
To obtain tissue samples suitable for laboratory analysis, several procedures like separation, fractionation and characterization are usually performed and they are applied based on the properties of each cell type in the sample. The choice of each protocol depends on the desired degree of separation, preservation of viability, and technical analyses that would be studied.
Cell separation techniques have the advantage to allow high yield and recovery in a shorter time. Some examples are separation by density sedimentation and/or flow cytometry. In general, high performance techniques used for cell separations rely on differences like: 1. Cell size, 2. Cell density (specific gravity), 3. Cell load, 4. Cell surface chemistry, 5. cellular complexity and 6. fluorescence emission of two or more cellular constituents or adsorbed antibody.
Cell separation by sedimentation and centrifugation Methods
Centrifugation depends not only on the centrifugal force but also on other factors which modify sedimentation and are dependent on the cell characteristics.
Differential centrifugation
This process is normally the most simple in practice, given that it only can separate cells showing large differences in size (at least 10 times) ( Figure 2a ). In the case of blood with anticoagulant, at 200 g (g = gravities) erythrocytes sediment in the lower zone, leukocytes appear at the interface and at the supernatant there will be a platelet rich plasma phase. It is possible to obtain plasma with less platelets using higher speed (3.000g) ( Figure 2b ).
Differential centrifugation. Separation is done according to size and the sedimentation coefficient, which is dependent on the mass. A. Peripheral blood separation. B. Differential centrifugation by size.
Density gradient centrifugation
Barrier methods (centrifugation through a continuous centrifugation media). To achieve more effective separations each sample is centrifuged on a bed of density intermediate between the two cell types that are to be separated.
Zonal centrifugation or sedimentation rate. This method is used to separate cell types whose sedimentation coefficient differs. To be able to perform this separation it is necessary to form a density gradient, which favors the concentration of each cell type in a narrow band or zone. The density gradient is generated before adding the sample, using several different solutions with suitable concentration of a compound (Ficoll, sucrose, albumin, fetal bovine serum).
After the gradient is formed, a small volume of sample is deposited upon it and centrifuged for a short time. Typically, for this process a gradient less than the maximum density of cells is used and therefore it may not reach the sedimentation equilibrium. Always shorter times are used, if cells are centrifuged for longer times they will leak and end up in the background, this is how the result is dependent on the time of centrifugation. This technique can separate all blood cell types, viable sperm, viable and non-viable cells from disaggregated tissues and suspension cell samples ( Figure 3a ).
A. Zonal centrifugation, separation depending on the coefficient of sedimentation. Centrifugation stops before the sedimentation equilibrium is reached. B. Centrifugation on a gradient of constant centrifugation media, separation media have constant density (more...)
Peripheral mononuclear blood cells separation. The peripheral blood mononuclear cells (PBMCs) include lymphocytes, monocytes and macrophages. They share different characteristics as the presence of a circular nucleus and their density. As a consequence of these characteristics they can be isolated by different techniques (see above-Cell separation methods) and also by the use of special type of tubes as the BDVacutainer® CPT™ which is a vacuum-driven drawing tube containing anti-coagulant and a cell separation medium. The procedure to isolate the cells may also affect them, as for the use of different components to the separation and the serial washes to clean them.
Using Ficoll-Hypaque, which has a density of 1.077 g/ mL, density identical to that of lymphocytes and monocytes, it is possible to recover PBMCs. The Ficoll-Hypaque is a combination of a polymer of high molecular weight sucrose (Ficoll) and an organic compound (sodium diatrizoate: 3–5 bis acetylamino-2, 4, 6 tri-iodobenzoic acid). Granulocytes and erythrocytes have a higher density and when peripheral blood is centrifuged in a Ficoll-Hypaque gradient, it passes through a package formed on the bottom of the tube. Platelets have a lesser density and remain in the plasma and the mononuclear fraction located at the interface ( Figure 3b ).
There are other solutions for the separation of mononuclear cells using density gradients, the most effective one is the use of Percoll. To separate mononuclear, the commercial solutions most commonly used is Lymphoprep, Hystopaque and Lymphopure. Several media solutions are available with suitable densities for the separation of other specific cell types: Nycoprep -1.077 for mononuclear cells, Nycoprep -1.068 for monocytes, Polymorphoprep for polymorphonuclear cells and Nycoprep -1.063 for platelets.
Isopycnic centrifugation or sedimentation equilibrium. This is a method in which cells are separated solely according to its density using another centrifugation variant, called isopycnic (der. Greek. Similar density). It is also performed on a density gradient, but in this case the centrifugation time is sufficiently long to reach the sedimentation equilibrium. To achieve the sedimentation equilibrium continuous gradients are used to cover the entire range of cell densities: at the bottom of the tube has to have the greater density than the denser cells. Thus, independent of the time of centrifugation, the cells will never sediment at the bottom but instead will reach a stable intermediate position in the gradient.
Centrifugal elutriation. This process involves the separation of cells according to their sedimentation rate. The original process consists on performing successive cycles of sedimentation and decanting provided by the incorporation into a system of liquid flow suspension. Finally, when it is combined with the effect of the centrifugal force it results in centrifugal elutriation. Here the cells are exposed to two opposing forces; firstly by centrifugal force and the drag force by the continuous flow of the medium counter force ( Figure 4a & b ).
Elutriation. A. Elutriation separation chamber. B. Elutriation process. 1. Introduction of the cell suspension. 2. The centrifugal force pushes the cells to the bottom of the chamber, continuously introducing a liquid medium, which opposes the centrifugal (more...)
The above method yields a versatile, rapid and effective form of separation for cell subpopulations according to their size from a mixture. Moreover this process, admits high cell concentrations and even allows for a greater recovery rate of viable cells compared to the original cell.
- Blood cell storage: cryopreservation and thawing process
Sometimes cells are not necessarily cultured immediately after the blood drawing, thus it is necessary to keep and storage them for future experiments. Freezing is the best way to storage cells. However, freezing affects the proliferation and cytokine secretion as well as the protein production and mRNA expression. Other consequences of the freeze-thaw process are mechanical injuries produced by crystal formation, alteration of physical properties and shape of cellular structures. That is why protocols for freezing cells include cryoprotectant substances in the freezing media that prevent crystal formation and avoid osmotic injury. Some examples are the dymethylsulphoxide (DMSO), glycerol, ethylene glycol or hydroxyethyl.
Hence, it is necessary to take into account many considerations when freeze-thawing cells. Firstly, it is extremely important to process and store the cells within a period between 8 to 24 hours after samples are collected and they should be conserved in especial freezing media. Secondly, it could be helpful to include in the freezing media caspase inhibitors to avoid the apoptosis of the cells as a consequence of the stress. And finally, the rate by which the temperature will decrease should be slow enough to avoid the rupture of the cells. This is done using special containers that have alcohols (i.e Isopropanol) which surround the sample tubes. Thus, the rate of temperature decreasing is gradually, and close to 1°C/min to -70°C. After this process frozen samples should be transferred to liquid nitrogen promptly within the next 24 – 72 hours.
As for the thawing process it is recommended to thaw the cells rapidly by transferring the cryovials directly from liquid nitrogen to 37°C. Immediately after the samples are thawed, they should be diluted and washed to eliminate the cryopreservant that could be toxic for the cells. The rapid change of temperature and media diminish the osmotic variation and protects the integrity of the cells. However, despite all these considerations there may be some problems, for example, cell clumping is frequent following the thawing process as a consequence of the release of DNA of dead cells. In that case, DNase can be used to avoid the aggregation of cells. Finally, it is recommended giving the cells a resting period before the experiment so they can eliminate the components that they were producing before the freezing process. This resting period allows them also to get used to the new conditions and favors to normalize the conditions for future comparisons. General recommendations about the treatment of blood samples for cell culture are summarized in table 2 .
General guidelines for sample blood collection and cell processing.
- Characterization and separation of cells by cellular markers
Flow cytometry
Today, flow cytometry is an important method in biomedical research and clinical laboratories, especially for its ability to analyze automatically different cell suspensions. Flow cytometry is based on the transportation of a cell suspension (e.g., blood cells, bone marrow aspiration and dissociated tissues) driven by the flow of an isotonic solution to the measuring point or flow chamber. Flow cytometry uses include analysis (biomarker detection) and separation (sorting) of cells previously labeled with fluorochromes ( Figure 5 ). Some flow cytometers only perform the first, while others carry out both.
Flow cytometry. Principles of operation of the flow cytometer (see text). Adapted from (1).
Characterization of a cell population. Briefly, the cell suspension arrives in laminar flow conditions, forming a very fine line containing individual cells in succession. These cells pass one by one through a laser whose wavelength allows excitation of previously incorporated fluorescent markers. The light emerging from each cell is analyzed for scattering and fluorescence intensity.
Cell characterization is accomplished by measuring multiple physical characteristics of the cells, as they flow to a beam of light. The properties measured include the relative size, relative granularity or internal complexity, and relative fluorescence intensity given by the use of fluorocromes. In order to make these measurements, the cytometer has three main systems: fluidics, optics, and electronics. The first one transports the cells to the interrogation point where the laser bean pass through. The second one consists of the different lasers that illuminate the cells in the interrogation point, and directs the light to the filters and detectors. And finally the third one is the electronic system that helps to convert the changes in light signals from the detectors to values that can be interpreted by the computer.
This technique has multiple functional applications. study different cell surface markers and intracellular signaling by using monoclonal antibodies, assessment of DNA and RNA content of the cell and the determination of its shape and size.
Characterization of a cell population by flow cytometry
The first characteristics than can be determined by the simplest cytometer are the shape and the internal complexity of the cells. These characteristics are measured by the changes in the light scattering. It occurs when a cell deflects incident laser light. Hence there are many cellular factors that affect light scattering such as cell’s membrane, nucleus, and any granular material inside the cell. Also, the cell shape and surface topography can contribute to the total light scatter.
There are two ways to measure the scattered light. First, the forward-scattered light (FSC) is proportional to cell-surface area or size. As a consequence, FSC can be interpreted as the shadow projected by the cell, finally the detection of FSC is done parallel to the lasers. On the other hand, the side-scattered light (SSC) is proportional to cell granularity or internal complexity. For instance, SSC is the measurement of mostly refracted and reflected light that occurs at any interface within the cell, it means that SSC indicated how much the light is diverted from the original source as a consequence of the content of the cells. As results, SSC is collected at approximately 90 degrees to the laser beam ( figure 6 ).
A. Schematic representation of side and forward scatter light (SSC and FSC). B. Results of FSC vs SSC analysis of leucocytes from whole blood.
In addition, superficial and internal cell markers can be detected by flow cytometry and they allow a better characterization of the cell population within a sample. In order to do these analyses it is necessary to use antibodies that bind to the markers of a specific cell population, but there is not enough binding of the antibodies to identify the cell. As a result, the antibodies should be labeled with different flurochromes. As an example, a cytometer with three lasers can detect 8 colors. It means that it can be used to find 8 different cell markers plus SSC and FSC.
Fluorochromes
Typically, a fluorescent compound absorbs light energy over a range of wavelengths that is characteristic for that compound. The absorbed light causes excitement of electrons in the compound raising them to a higher energy level. Finally, when the source energy finishes the excited electrons quickly decay to their basal state, emitting the excess energy as photon light. This process is called fluorescence.The range over which a fluorescent compound is excited is known absorption spectrum. On the other hand, the range of emitted wavelengths for a particular compound is known as emission spectrum.
Fluorochromes are fluorescent components that are excited by different wavelength, and they emit light in specific wavelengths in the visual spectra that can be detected by an instrument ( Figure 7 ). Hence, this property is used for labeling antibodies; commonly, a fluorescent dye is conjugated to a monoclonal antibody, then it can be used to identify a particular cell type based on the characteristic cell markers ( Figure 8 ).
Fluorochrome characteristics. A. Specific wavelength values of excitation and emission. (Colors represented the fluorescence emission visible color for each fluorochrome). B. Relative brightness scale of common fluorochromes. It is recommended to use (more...)
Antibodies labeled with fluorochromes against specific cell markers allow the characterization of a cell population within a sample.
The correct detection and characterization of cell populations depends on the right choice of fluorochromes and the correct calibration of the Flow cytometry. In the first case, it is important to know which cells are going to be analyzed and how is the expression pattern of the marker that is going to be used for their characterization. For example, a marker which has a low expression in the cell surface should be labeled with a strong fluorochrome in order to intensify the signal. On the contrary, a marker highly expressed can be labeled with a fluorochrome with mild intensity ( Figure 7 ).
Secondly, the cytometer should be calibrated in order to provide good quality results. One of these calibration processes is called compensation and it should be done every time that an experiment is run. Compensation is the correction for the spectral overlap during multicolor flow cytometry experiments. The goal of color compensation is to correctly quantify each dye with which a particular cell is labeled. This is done by subtracting the portion of the signal overlapping between fluorochromes ( Figure 9 . For further information please go to the recommended lectures).
Compensation. In an experiment with more than two fluorochromes there can be spillover. Spillover takes place when the presence of the other fluorescent reagent can contribute significantly to optical background in proportion to the brightness of a specific (more...)
All this together, allows the characterization of a mixed population of cells by using different fluorochromes. This method leads to distinguishing and characterizing the subpopulations within the sample in combination with FCS and SSC. The combination of different markers, FCS or SSC enables the definition and sub-analysis of populations known as “gating’. ( Figure 10 )
Gating approach to analyze peripheral blood mononuclear cells (PBMC). Left panel shows the results for the stained control and right panel shows the results for the unstained/negative control, which includes all the events registered in panel A. Panel (more...)
Cell separation by flow cytometry or fluorescence activated cell sorting (FACS)
This process allows the separation of cell samples fractions according to their morphologic and fluorescent characteristics. A regular flow cytometry analysis must be done before to define which populations will be sorted. Then, cells are separated in different fractions when the flow passing through the detection point is transformed into small droplets. These droplets contain a single cell (as a result of an ultrasonic vibration). Finally, a voltage pulse which provides an electrical charge is applied according with the cell characteristics. This pulse allows the cells separation when the droplet passes through an electrical field. Then, cells are deflected according to their charge, thus falling into different sample collection tubes ( Figure 5 ).
- Affinity separation by magnetic particles
This process is of great application. Currently in use are commercially available microspheres (beads) in several sizes (0.5 - 10 microns) for different applications and formed by a super-paramagnetic material (iron oxide) coated with a thin layer of plastic polymer which allows the absorption and/or covalently binding of different molecules. Often an antibody is bound to magnetic or immunomagnetic microspheres antibodies ( Figure 11 ).
Affinity separation using magnetic beads. A. Direct labeling: microsphere covalently linked to the Fc domain specific antibody for the target cells. B. Indirect labeling: uses two types of reagents, an unmodified primary antibody covalently linked to (more...)
- Count and cell viability
The accurate determination of the amount and viability of the cells is very important for correct standardization of reagents and conditions for cell culture experiments. Counting is performed using a hemacytometer counting chamber consisting of a central chamber (double counting chamber) which is divided into two parts by a transverse slit of 1mm. Each chamber consists of a silver film etched in a grid of 3×3 mm. Each rack is divided into nine side frames, each of 1×1 mm. Boxes in the corners are divided into 16 squares and center box in 25. The hemacytometer is accompanied by a thin cover glass slide with a weight that determines the exact depth when placed on the chamber ( Figure 12 ). Alternatively, cells can be counted by using flow cytometry according to the number of events registered in the analysis or the use of special tubes which allows the determination of absolute cell counts (Further information can be found in recommended readings or in the Becton Dickinson website http://www.bdbiosciences.com/research/ ).
Hemocytometer. The coverslip is 0.1 mm above the grid, and the lines etched on the grid are at preset dimensions. The four outer squares, marked 1–4, each cover a volume of 10–4 mL. The inner square, marked as 5, also covers a volume of (more...)
Cell viability refers to the ability of a cell to perform its biochemical and physiological processes, particularly in regards to its metabolism and ability to divide. However, in practice the term is relative as it is used with different criteria; viability is commonly spoken of when referring to cell integrity or metabolic activity or their proliferative capacity. In fact, with the use of a vital dye exclusion is possible to determine cell viability base on the integrity of the membrane from living cells. This membrane excludes certain dyes such as trypan blue, eosin, 7-AAD or propidium iodide, whilst dead cells allow their passage into the cytoplasm ( Figure 13 ). For this method, the cell suspension is mixed with a known volume of dye and examined visually to determine the dead and live cells. In the case of trypan blue, cytoplasm is seen in refractive (clear) when the cell is alive while the cytoplasm of dead cells is seen blue. Propidium iodide stains dead cells red when observed in the fluorescence microscope. Alternative, flow cytometry can be used instead of the microscope with the colorants 7-AAD or propidium iodide ( Figure 14 ).
Cell viability - Exclusion assays These tests determine the number of viable cells present in a cell suspension. Live cells possess intact cell membranes that exclude certain dyes like trypan blue. If the cell membrane is damaged (dead cells) trypan blue (more...)
Cell viability - Exclusion assays in flow cytometry. The 7-AminoActinomycin D (7-AAD) has the ability of inserting itself between the tops of successive CG bases of the DNA double strand. This occurs when the interior of the cell and the nuclear chromatin (more...)
- Recommended readings
- Cite this Page Arango MT, Quintero-Ronderos P, Castiblanco J, et al. Cell culture and cell analysis. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 45.
- PDF version of this title (39M)
In this Page
Related information.
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Cell culture and cell analysis - Autoimmunity Cell culture and cell analysis - Autoimmunity
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
REVIEW article
Are we ready to integrate 3d culture systems in acute myeloid leukemia and bone marrow microenvironment research.

- 1 Department of Biomedical Engineering, University of Rochester, Rochester, NY, United States
- 2 Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, NY, United States
- 3 Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, NY, United States
- 4 Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY, United States
Acute myeloid leukemia (AML) is the most aggressive adult leukemia and results in a dismal 5-year survival rate of less than 30%. While research has primarily focused on identifying intrinsic mutations driving leukemogenesis, the role of the bone marrow microenvironment (BMME) in disease progression remains poorly understood. For this purpose, conventional 2D cultures inadequately replicate the complex BMME interactions crucial for the maintenance of normal hematopoiesis and leukemia pathogenesis. In recent years, 3D cultures or microphysiological systems (MPS), have emerged as promising tools for in vitro modeling of the human BMME. These approaches provide a promise for a more physiologically relevant platform for investigating the mechanistic underpinnings of AML interactions with BMME components, as well as exploring chemoresistance mechanisms and facilitating drug discovery efforts. This review discusses the considerations in biomaterials, biophysical, and biochemical factors to develop the BMME in vitro for AML studies, the state-of-the-art 3D models of the BMME, and the challenges and prospects of adopting MPS for AML research.
1 Introduction
Approximately 300 billion mature blood cells are produced daily originating from a small pool of 11,000 – 50,000 hematopoietic stem cells (HSCs) in the bone marrow ( 1 ). Hematopoiesis is the process of producing, differentiating, and mobilizing hematopoietic cells into circulation and secondary lymphoid organs, ensuring a sufficient supply of blood cells for normal physiology and in response to injury or infection. The activity of HSCs is regulated by a complex network of cell-intrinsic factors such as transcriptional, epigenetic, and metabolic regulators as well as local and extrinsic long-range humoral cues ( 2 ). The hematopoietic system must efficiently produce and mobilize sufficient blood cells during injury and inflammation without depleting the limited stem cell population ( 2 ). In malignancies such as acute myeloid leukemia (AML), this tightly regulated process can be severely disrupted. AML is an aggressive blood cancer in the blood and bone marrow that is associated with failure of the hematopoietic system. It is the most common type of acute leukemia in adults, characterized by the expansion of immature and abnormal blast cells derived from the myeloid lineage (AML blast cells) ( 3 ). Initiation of AML is typically thought to involve hematopoietic stem cells and progenitor cells (HSPCs) acquiring mutations and transforming into leukemic stem cells (LSCs), also known as leukemia-initiating cells (LICs). The combinations of cytogenetic and genetic abnormalities seen in AML patients underlie the heterogeneous nature of the disease with various mutations in signaling pathways, DNA methylation, transcription factors, tumor suppressors, etc. ( 4 ). AML blast cells accumulate excessively in the bone marrow and peripheral blood, impairing the production of healthy blood cells such as red blood cells, white blood cells, and platelets ( 4 ). The loss of normal hematopoiesis is associated with bone marrow failure, anemia, neutropenia, and thrombocytopenia ( 4 ). Although AML is relatively uncommon, the steady rise in AML incidences and deaths globally over the last decade is alarming ( 3 , 5 , 6 ).
Incidence is particularly higher in the elderly population with the median age of diagnosis at 68 and overall 5-year survival rates of less than 10% ( 7 ). The poor prognosis in this patient population is attributed to poor long-term response to chemotherapy, high relapse rates after treatment, and lack of effective therapy for relapsed patients ( 3 ). The standard of care for most AML patients is the ‘3 + 7’ daunorubicin (DNR) and cytrabine (Ara-C) chemotherapy which many patients do not qualify for and nearly 70% of patients in the most affected group succumb to the disease within 1 year of chemotherapy ( 3 , 8 – 10 ). This poorly tolerable chemotherapy strategy, which was first discovered in the 1970s, remained largely unchanged ( 4 , 9 ). Henceforth, identifying novel treatment approaches is crucial for improving care for vulnerable patients who cannot tolerate conventional chemotherapies and stem cell transplantation. Molecular profiling of AML has led to the further stratification of AML subtypes and the development of targeted therapies based on specific genomic alterations. Currently, there are several FDA-approved therapies available including inhibitors of FLT3 (gilteritinib), IDH1 (ivosidenib), and IDH2 (enasidenib). Although therapy options are becoming more available, progress is slower for patients with higher-risk forms of AML such as ones with TP53 mutations, and older adults ( 11 ). Overall, although the majority of AML patients undergo intensive induction chemotherapy achieve complete remission, relapse remains high ( 12 ). Due to the heterogeneity of AML and variable responses to therapies, finding a one-size-fits-all cure is difficult. Therefore, it is necessary to explore alternative targets that are not exclusive to the blast cells and LICs.
One of the main challenges in improving therapies for AML is the limited understanding of the role of the bone marrow microenvironment (BMME) in the progression and treatment of AML. In various leukemias, research has demonstrated that the interplay between leukemic cells and the BMME influences the progression and relapse of disease, while also modulating drug response and contributing to chemotherapy resistance ( 13 , 14 ). In vivo and in vitro studies showed that AML blast cells modulate endothelial cell activation, establish crosstalk with fibroblasts, and inhibit osteoblast activity ( 14 – 19 ) ( Figure 1 ). The alteration in the BMME leads to failure of the bone marrow and also provides a leukemia-favorable environment that can support the self-renewal and quiescence of LSCs and the proliferation of AML blast cells ( 14 – 19 ). LSCs that reside in the BMME are often resistant to chemotherapy, and relapse is common due to persistent leukemic cells sequestered in the BMME (associated with minimal residual disease where a certain number of cells are still present after treatment) ( 4 , 15 ). While chemotherapeutic agents are effective at targeting rapidly cycling AML blasts, they are ineffective against the quiescent LSCs ( 23 ). One of the mechanistic explanations for chemoresistance is through cell adhesion mediated by the BMME ( 24 – 27 ). In vivo studies also showed that alterations within the BMME can initiate disease in hematological malignancies such as myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and AML ( 2 , 21 , 28 , 29 ). It is no surprise that AML research focus has expanded toward understanding where the BMME fits in the pathological puzzle. What the field knows so far is that the BMME contributes to AML pathophysiology in several ways including: 1) abnormalities in the cellular components can drive neoplasia; 2) remodeling or dysregulation can support the survival and progression of AML cells; and 3) it can act as a sanctuary for AML blast cells and LICs to evade chemotherapy ( 30 ).
Figure 1 (A) The BMME supports hematopoeiesis through cellular and non-cellular interactions. The BMME is composed of non-hematopoietic cells such as bone marrow stromal/stem cells (BMSCs), endothelial cells, and osteoblasts that regulate proliferation, self-renewal, and lineage determination of HSPCs ( 15 , 20 ). The complex regulatory network includes interactions with secreted proteins (including CXCL12, IL-6, MCP-1, SCF, and RANKL) and cell-bound proteins (such as VCAM1, E-selectin, and N-cadherin) ( 15 , 21 , 22 ). Disruption of the network can disrupt maintenance of hematopoiesis and can lead to initiation of malignancies. Dotted arrows represent differentiation. (B) AML cell signaling dysregulates BMME components and lead to loss of support for normal hematopoiesis. In vivo and in vitro studies showed that AML cells hijack the BMME to create a permissive environment for AML cell proliferation and maintenance of leukemic stem cells (LSC). (1) During AML, BMSCs are dysregulated and expressed higher Ang-1, VCAM1, and CXCL12. In addition, BMSCs can confer chemotherapy resistance to AML cells via VLA4/VCAM1, E-selectin, and N-cadherin. (2) AML cells can dysregulate osteoblastic cells through CCL3 and TPO ( 21 ). Osteopontin secreted from dysregulated osteoblast contribute to protection of AML cells from apoptosis. (3) AML cells release inflammatory cytokines such as TNF-α and CXCL12 that induce increased expression of E-selection on endothelial cells surfaces which AML cells can bind to. These interactions contribute to the cell-adhesion mediated drug resistance (CAM-DR) that sustains LSC in the BMME. The overall functional disruption of the BMME leads to loss of support for HSPCs and dysregulation of normal hematopoiesis. Red arrows indicate signaling from the cell source. Created using BioRender.com .
The expanding understanding of interactions between the BMME and leukemia cells has led to the emergence of innovative therapies that could potentially supplement existing treatments. These newer forms of targeted therapies include targeting supportive cells in the BMME rather than leukemia cells directly such as the ongoing clinical trial of abaloparatide and bevacizumab in myeloid dysplastic syndrome (MDS) ( 31 ). Abalopratide is a parathyroid hormone analog that can increase BMSC and osteoblastic support for HSC while bevacizumab blocks VEGF which decreases angiogenesis in the BMME. So far, Phase 1 of the trial showed promising evidence of BMME remodeling as seen with a significant increase in bone marrow stem/stromal cells (BMSCs) in patients ( 31 ). Another approach to targeting the BMME involves using uproleselan (GMI-1271), an E-selectin antagonist that disrupts the adhesion of leukemic cells to endothelial cells in the BMME, thereby mitigating adhesion-induced chemotherapy resistance ( 30 , 32 ). These trials demonstrate a shift in the development of therapies for leukemia, now considering the BMME as a major targetable factor. As research evolves rapidly, the drug discovery tools must keep pace, and 3D culture systems can offer a significant advantage.
2 The need for a 3D culture for the BMME
Most in vitro AML studies rely on conventional 2D cultures of AML cells with HSPCs on a supportive layer of BMSCs ( 30 , 33 , 34 ). Although these models have shown protection of leukemic cells from chemotherapy due to stromal cell interactions, they do not fully reflect the complex interactions within the BMME. Particularly, 2D AML cell cultures lack cues from the extracellular matrix (ECM), growth factors, and signaling molecules stored in the ECM, as well as cellular interactions present in the BMME ( 24 , 35 – 42 ). A comprehensive study published recently by Liebers et al. demonstrated the feasibility of ex vivo drug response profiling in guiding the treatment of AML patients undergoing daunorubicin and cytarabine therapy ( 43 ). AML cells sampled from either lymph node, peripheral blood, or bone marrow, were tested with chemotherapy agents in 2D culture, and the association between ex vivo response versus in vivo responses was analyzed. The study showed that ex vivo chemotherapy resistance predicted poor in vivo response, with the strongest correlation observed in high-risk AML patients based on the ELN-22 (European LeukemiaNet 2022) ( 44 ) stratifications. The authors noted study limitations, including the inability to account for microenvironmental factors that can affect drug response in vivo . Incorporation of microenvironmental factors is imperative to improve prediction accuracy ( 43 ).
In the last decade, researchers have taken an integrated approach to developing 3D cultures or microphysiological system (MPS) models (also Organ-on-chips) of various organs and tissues to facilitate mechanistic studies and drug screening for diseases. Applications of MPS include modeling complex tissue systems such as the blood-brain barrier (BBB), lung-on-a-chip, and cardiovascular tissue models ( 45 – 48 ). The strategy is advantageous as it can recapitulate the complex tissue microstructures while reducing reliance on animal models ( 49 – 55 ). By incorporating human cells from patients or cell lines, 3D models provide a valuable tool for studying disease progression, offering insights that are difficult to obtain from human patients ( 56 ). In the United States, the recent announcement of the FDA Modernization Act 2.0 emphasizes the need to utilize alternative testing methods such as MPS in replacement of animal models in the preclinical testing phase for new drug development ( 48 , 57 ). The goal of this decision could improve clinical outcomes and decrease the number of animals used in drug testing, potentially revolutionizing drug development and personalized medicine. The global interest in MPS is rapidly expanding, as evidenced by the initiation of collaborative networks such as the European Organ-On-Chip Society (EUROoCS), International MPS Society (IMPSS), National Institute of Health (NIH) Tissue Chip Consortiums, IQ Microphysiological Systems Affiliate (IQ-MPS). These networks unite funding agencies, pharmaceutical companies, MPS manufacturers, and academic institutions globally to promote the integration of MPS into biomedical research ( 58 ). Meetings and conferences hosted by these organizations facilitate open discussions, allowing the sharing of new technologies and promising advances in the MPS field, while also addressing critical challenges such as standardization, affordability, and adoption.
Recognizing the potential of MPS, several groups have developed 3D in vitro models of the BMME using hydrogel technologies and microfluidics platforms. These models can maintain the long-term function of HSPCs by incorporating hydrogels and niche components known to regulate normal hematopoiesis ( 59 – 69 ). The approach is advantageous in providing a platform that can accommodate various requirements of modeling the physiological BMME which is challenging in 2D culture settings. These requirements include incorporating 1) various cellular components in BMME that are known to support HSPCs; 2) extracellular matrix that provides structural, mechanical, and signaling support for HSPCs; and 3) extrinsic molecular cues such as growth factors and cytokines provided by culture media. A recent review by Busch et al. details the cellular components in BMME that are important for HSPC regulation such as BMSCs, endothelial cells, and osteolineage cells, as well as the biophysical and biochemical cues such as ECM components and mechanical properties found in the native BMME ( 70 ). These 3D culture systems can improve in vitro or ex vivo studies by providing higher relevance to human BMME and increasing research throughput. Besides performing physiological functions such as sustaining HSPCs, these platforms have also demonstrated the development of leukemic cell drug resistance conferred by the BMME ( 60 , 62 ). The models can serve the purpose of elucidating the mechanism of AML interactions with the BMME, identifying new therapeutic targets to treat AML, drug characterization, and toxicity profiling, and high-throughput screening (HTS) studies to test the efficacy of new therapies ( 34 ).
In this review, we will highlight the frontline 3D models developed within the last decade that have expanded our understanding of the BMME role in AML. These advances encompass the use of synthetic and natural biomaterials, bioreactors, and microfluidics approaches for applications in mechanistic studies and chemotherapeutic investigations. The focus of the view is on the considerations for the development of 3D models of the AML BMME. This review is performed by literature search using PubMED, PubMED Central, SCOPUS, Web of Science, and Google Scholar for keywords including ‘Acute Myeloid Leukemia’, ‘3D culture’, ‘ in vitro’ , and ‘bone marrow microenvironment’. We reviewed articles published from 2008 – 2024.
3 The BMME and AML cell interactions
HSPCs reside in specialized ‘niches’ in the BMME including the endosteal surface of trabecular bone (endosteal niche), central marrow region (central medullary niche). And regions close to vascular structures including sinusoidal endothelium (perivascular niche) and arterioles (arteriolar niche) ( 71 ). The niches are defined by cellular compositions, biochemical and biophysical properties ( 30 , 71 , 72 ). The cellular components include both hematopoietic cells such as megakaryocytes, macrophages, regulatory T-cells, and non-hematopoietic cells such as MSCs, endothelial cells, osteolineage cells, fibroblasts, neuronal cells, and adipocytes. The cells that make up the niches are interconnected via vascular and innervated networks within the bone marrow ( 2 ) ( Figure 1A ). Together, these interactions play a crucial role in regulating the proliferation, self-renewal, and lineage determination of HSPCs ( 20 , 21 , 73 ). Studies that selectively deplete specific BMME populations demonstrate a loss of support for HSCs and thus reducing quiescent HSCs ( 13 ). The BMME actively coordinates the balance between the maintenance of quiescence and the activation of HSPCs during hemostasis or in response to an injury or inflammation event ( 13 ).
The two commonly discussed niches are the endosteal and perivascular niches. The first is comprised of osteoblasts and osteocytes that are thought to support HSC quiescence and self-renewal ( 30 , 70 ) ( Figure 1A ). Meanwhile, the latter is composed of endothelial cells that line up the arterial and sinusoidal vessels and are thought to promote more proliferative and committed HSPCs ( 30 , 70 ). Both niches are enriched with supportive BMSCs. However, the function of the specific niches is still subject to research and debate as these regions are so close to each other ( 30 ). The endosteal matrix niche is characterized by a stiff extracellular matrix, high ionic calcium levels, and hypoxic ( 70 ). The hypoxic environment can promote lower ATP levels in HSCs which supports a quiescent state however the topic of oxygen concentrations within the BMME is still in debate as the precise levels of oxygen in the bone marrow are still unclear ( 71 , 74 ). In vitro , evidence has shown that hypoxic conditions are potentially better for HSCs maintenance ( 71 ). In response to oxygen levels in the BMME, the transcription factor hypoxia-inducible factor α (Hif1-α) controls the expression of CXCL12 and CXCR4 that regulates HSC ( 74 ). HSCs in the sinusoidal endothelium are thought to be involved in blood cell production as they are more differentiated and ready to enter the bloodstream ( 2 ). The perivascular niche is enriched with perivascular BMSCs and specific subtypes such as CXCL12-abundant reticular cells (CAR cells) and macrophages. The cells release factors such as E-selectin, CXCL12, stem cell factor (SCF), leptin, and nestin.
Cells in the BMME are embedded within a rich ECM containing fibrous proteins such as collagen and elastin, and glycoproteins such as laminin, proteoglycans, vitronectin, and fibronectin that sequester growth factors, cytokines, and metalloproteinases that directly affect HSPC fate ( 70 , 74 , 75 ). These proteins are mostly deposited by cells in the stroma such as BMSCs, endothelial cells, reticular cells, adipocytes, and smooth muscle cells ( 74 ). The distribution of proteins may vary between the endosteal and perivascular niches with collagens (type I-XI) making up to 90% of the ECM in the BMME ( 75 ). ECM in the endosteal niche is composed predominantly of collagen type I and fibronectin while laminin is more present in vascular regions ( 70 ). Mechanical properties of the ECM were predominantly measured using murine or bovine samples, so it remains unclear if human bone marrow exhibits similar values. Reported Young’s modulus of the central marrow to the endosteal surface ranges from 0.1 kPA to 50 kPA ( 70 , 76 , 77 ). The stiffness of the perivascular regions may be similar to the basement membrane measured in endothelial basement membrane at 2–3 kPA ( 78 ). The BMME components that support HSPCs including the cell types and ECM components are reviewed in various publications ( 70 , 79 – 82 ). Deregulation of the BMME can occur during chronic and acute inflammation, as well as aging and malignancies ( 13 ). Malignant cells alter the BMME to create a leukemic niche that supports blast and LISc at the expense of hematopoiesis ( 83 ). The comprehensive findings on AML and BMME cell interactions can be found in several published review articles ( 2 , 21 , 30 ). Here we highlight the AML and BMME cell interactions that are to be considered in modeling the BMME in vitro .
3.1 Bone marrow stromal cells
BMSCs are a heterogeneous population within the stroma and make up ~20% of the cellular volume in the bone marrow ( 84 ). According to single-cell RNAseq of human bone marrow aspirates, BMSCs are predicted to be the main source of cellular signaling in the BMME ( 84 ). BMSCs span multiple cell subtypes based on expressions of markers such as Leptin receptors ( Lepr ), Adiponectin ( Adipoq ), NG2 ( Cspg4 ), and CxCL12 , and can differentiate into osteoblasts, adipocytes, and chondrocytes ( 85 ). BMSC regulates the hematopoietic compartment through several key niche factors such as CxCL12, Kitl (SCF), IL-7, angiopoietin-1 (Ang-1) ( 84 , 85 ) ( Figure 1A ). Moreover, these cells can confer chemoresistance to AML cells as reported in vitro and in vivo through VLA-4/VCAM-1 signaling which activates the nuclear factor κB (NF-κB) ( 21 ) ( Figure 1B ). This mechanism is termed cell adhesion-mediated drug resistance (CAM-DR) and is also associated N-cadherin and E-selectin ( 70 ). Although the 2D culture of BMSCs with AML cells may involve these pathways, it lacks other adhesion-mediated interactions with niche cells and from the 3D ECM itself. These limitations contribute to the loss of ‘stem-ness’ of LSCs in conventional stroma cultures which can impact the translation of in vitro findings to in vivo and human studies. Additionally, BMSCs give rise to osteolineage cells that include osteoprogenitors, preosteoblasts, and mature osteoblasts ( 85 – 88 ). Osteolineage cells regulate HSPC homing, maturation, and function, and the loss of osteoblastic function is associated with the development of myelodysplasia and leukemia ( 2 , 21 ).
3.2 Osteolineage cells
Osteoblastic cells mineralize the collagen fiber network to synthesize new bone matrix and regulate electrolyte homeostasis between extracellular fluid and bones ( 30 ). Osteoblasts that line up the endosteum play a crucial role in bone remodeling and differentiation of osteoclast precursor cells into mature osteoclasts ( 30 ). When terminally differentiated, osteoblasts become osteocytes and account for up to 95% of the total cells in the bone ( 30 ). Osteoblasts in the endosteal niche promote HSC quiescence through the Notch pathway, Ang-1, and thrombopoietin (TPO) ( 70 ). CXCL12, osteopontin and SCF secreted by osteoblasts are also associated with the maintenance of HSPCs ( 79 ). HSPCs retention in the bone marrow by osteoblasts is associated with CXCL12 signaling ( 79 ). When G-CSF is reduced, the number of osteoblasts and CXCL12 expression decreases which results in the egress of HSPCs into the blood circulation ( 79 ). During AML, leukemic cells can disrupt BMSC differentiation into osteolineage cells ( Figure 1B ). Single-cell RNA sequencing (scRNA-seq) analysis showed that emerging AML cells impaired osteogenesis in BMSCs and reduced regulatory molecules that support hematopoiesis ( 85 ). Additionally, osteoblasts can also regulate the protection of leukemic cells against chemotherapy. Particularly, these cells can secrete osteopontin which binds to the αVβ3 receptor on AML cells and protects the cells from apoptosis ( 36 , 57 ). Our group also showed that AML can functionally inhibit osteoblastic cell activity through CCL3 signaling as seen in the leukemic mice model, blast crisis chronic myelogenous leukemia (bcCML) ( 14 ) ( Figure 1B ).
3.3 Fibroblasts
BMSCs can also give rise to fibroblasts that support the hematopoietic microenvironment. A subset of fibroblasts in murine BM is shown to express Cxcl12 and Ang-1 which indicate a potential niche regulatory function similar to cancer associated-fibroblasts (CAFs) ( 85 ). In solid tumor microenvironment and B cell acute lymphoblastic leukemia (B-ALL), CAFs can support chemoresistance which is associated with poor prognosis ( 21 ). In addition, malignant CAFs may remodel the BMME and transform it into a more aggressive niche ( 89 ). In a retrospective study by Zhai et al. of 63 primary AML patient bone marrow biopsies, immunohistochemistry showed the abundance of reticular fibers in the bone marrow of AML patients which is associated with a higher frequency of relapse and mortality ( 90 ). In the bone marrow samples, CAFs widely expressed high levels of fibroblast-specific protein 1 (FSP-1), alpha smooth muscle actin (α-SMA), and fibroblast activation protein (FAP) ( 90 ). When the CAFs were cultured with leukemia cell lines, THP-1 or K562, leukemia cells were seen to be less sensitive to chemotherapy. Previously, In vitro experiments by Ryningen et al. demonstrated that coculturing human AML blast cells with fibroblasts resulted in increased blast cell proliferation, and reduced apoptosis mediated by dysregulated cytokine levels such as IL-8 ( 18 ). Although the mechanism underlying the role of CAFs in AML is still being explored, pieces of evidence in AML and other cancers showed that the presence of CAFs in the BMME is associated with bone marrow fibrosis, leukemia progression, and sensitivity to chemotherapies ( 89 ).
3.4 Endothelial cells
Bone marrow is highly vascularized allowing hematopoietic cells to leave or enter the circulation ( 2 ). Imaging studies show that HSPCs were found co-localizing with vascular structures within the bone marrow ( 71 ). In addition, perivascular endothelial cells were shown to be important for HSC activation during bone marrow stess ( 2 , 13 , 91 ). The types of endothelial cells present in the BMME include arteriolar endothelial cells (AECs) and sinusoid endothelial cells (SECs) that vary between marker expressions ( 2 ). Evidence has shown that E-selectin coupled with chemotherapy doubled the survival time of leukemic mice compared to receiving chemotherapy alone and thus acts as a potential BMME-targeted therapy to improve AML survival ( 21 ). Furthermore, evidence shows remodeling of the vasculature during AML indicated by increased permeability, increased nitric oxide (NO), reduced perfusion ( 2 ). In mouse and human AML, an increase in angiogenesis is seen however this result is contradicted by intravital imaging studies that showed a selective reduction of blood vessels near the endosteum ( 2 ). The reduction is seen due to the accumulation of pro-inflammatory and antiangiogenic cytokines secreted by AML cells in the BMME such as TNF-α and CXCL12 ( 21 ) ( Figure 1B ). When the vessel defects were rescued, the loss of healthy HSC number was prevented and blast cells were more sensitive to chemotherapy ( 2 , 92 ). Similarly, when endothelial cell-derived NO is inhibited, vascular leakiness is rescued in AML xenograft experiments ( 93 ).
3.5 ECM components and matrix remodeling
The ECM anchors HSC to the endosteal and vascular niches and allows for the appropriate hematopoietic signaling to occur in the BMME. Hijacking of this ECM feature leads AML cells to develop resistance to chemotherapy and sequestering of minimal residual disease in the BMME ( 94 ). For example, CD44 expression on LSC and blast cells which bind to hyaluronic acid, osteopontin, fibronectin, selectins, laminins, collagens, and matrix metalloproteinases (MMPs), is associated with increased chemotherapy resistance and cancer aggressiveness ( 95 ). CD44 is also implicated in the migration, proliferation, differentiation, and survival of HSPCs. In addition, it also regulates the homing of HSPCs in the bone marrow and the homing of LSCs to intra and extra-medullary niches ( 96 ). On the other hand, MMP9 is typically downregulated in AML patients, and its high expression is potentially linked to a better prognosis ( 94 ). Furthermore, the biomechanical properties of the ECM may also be altered during AML. An in vitro study comparing ECM deposited by BMSCs from different MDS subtypes and healthy controls found that MDS-derived ECM was thicker and more compliant compared to, measured by atomic force microscopy ( 97 ). The ECM content differed from the control samples, exhibiting higher levels of glycosaminoglycans (GAGs), sulfated GAGs, and hyaluronic acid ( 97 ).
4 Biomaterial, biophysical, and biochemical considerations to recapitulate the BMME in vitro
4.1 biomaterials considerations.
Tissue engineering strategies often involve the use of hydrogels and complex 3D culture devices to provide a matrix structure and dynamic microenvironment, similar to native tissue. Considerations for designing the BMME in vitro must include selecting the appropriate biomaterials, emulating the biophysical and biochemical cues, and incorporating niche cells ( Figure 2 ). The selection of biomaterials is contingent upon achieving biocompatibility to sustain cell growth, differentiation, and function without inducing immune responses or toxicity reactions ( 85 ). Particularly in modeling the BMME, where multiple cell types are involved, considerations must be made for cell-matrix interactions that can influence cellular functions, such as proangiogenic or osteogenic responses in BMSCs, immune cell responses, and HSPC differentiation ( 57 , 98 ). Mechanical properties of biomaterials like stiffness, elasticity, and viscoelasticity must be considered as they can influence cellular behavior and response ( 75 ). Additionally, the porosity and permeability of hydrogels must be tuned specifically to the tissue of interest to ensure appropriate nutrient and oxygen diffusion, waste removal, and cell-cell interactions. A recent study by Li et al. showed that larger pore size (80 um) and higher Young’s modulus of 70 kPA were favorable for the proliferation of HSPCs and maintenance of stemness phenotypes ( 99 ). The biomaterials chosen should maintain structural integrity and mechanical properties over extended culture periods to support long-term studies. Various synthetic and naturally derived biomaterials have been used in modeling the bone marrow stroma ( Table 1 ). Decisions made on the use of the desired materials revolve around cost-effectiveness, ease of synthesis, and biocompatibility. The biomaterials include synthetically derived polymers such as polyacrylamide (pAAm), polyurethane (PU), poly-L-lactic acid (PLLA), polyglycolic acid, polystyrene (PS), poly(ethylene glycol) (PEG), and hydroxyapatite. Incorporating cell-adhesive peptides, growth factors, or signaling molecules can also promote cell attachment, differentiation, and tissue organization, thus increasing biocompatibility. On the other hand, various naturally occurring materials have been used to mitigate the biocompatibility of synthetic matrices by using alginate, decellularized Wharton’s jelly matrix, Matrigel, and fibrinogen.
Figure 2 Considerations of designing the BMME in vitro . The BMME is a highly complex network of cells and matrix components that regulate HSPCs self-renewal, proliferation, differentiation, and mobilization. To accurately recapitulate the BMME, it is essential to consider factors such as biomaterials, biophysical properties, biochemical signals, and cellular composition. These elements must be carefully integrated to create a model that closely resembles the native human BMME.
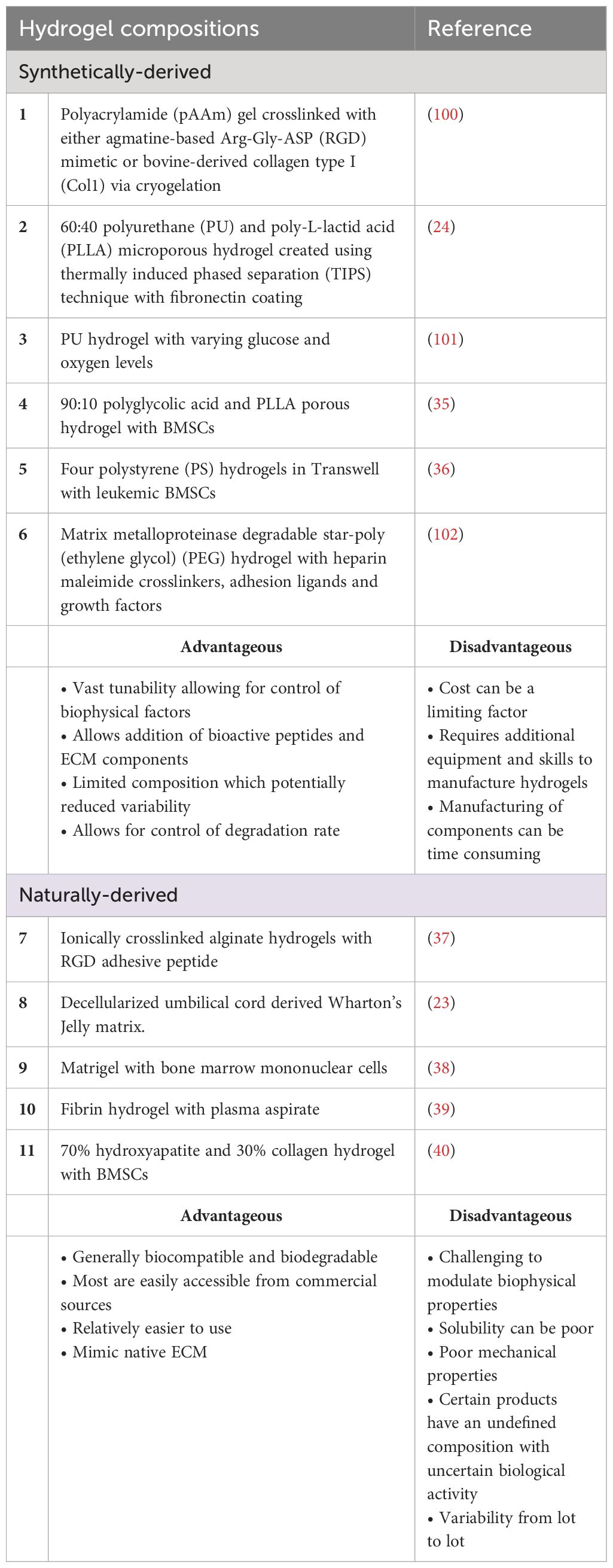
Table 1 Summary of materials used as hydrogels for static 3D AML in vitro culture.
Earlier efforts in culturing AML cells include using synthetically derived-biocompatible hydrogels ( 24 , 37 , 100 ). The benefits of using these hydrogels include the vast tunability of the mechanical properties that can be tailored based on the tissue or disease of interest. For example, bioactive peptides can be incorporated into the hydrogel backbone such as peptides containing the Arg-Gly-Asp (RGD) integrin-binding motif. Dainiak et al., 2008 fabricated a 3D hydrogel composed of pAAm crosslinked with either agmatine-based RGD mimetic or bovine-derived collagen type I (Col1) via cryogelation ( 100 ). The resulting hydrogels are macroporous, having a pore diameter of 10–100 μm and very elastic. pAAm-based hydrogel is used as it is drainage-protected, highly tunable, and provides mechanical strength that can resemble tissue-like elasticity. KG-1a cell line (ATCC CCL-246.1), a promyeloblast macrophage cell line isolated from an AML patient, was cultured either in hydrogel containing RGD, hydrogel containing Col 1, or in a 2D culture plate. The authors found that KG-1a cells form aggregates in Col1 and RGD-incorporated pAAM hydrogel while the cells stay as single cells in control unmodified hydrogel. Chemoresistance was tested by treating the culture systems with Ara-C. The authors found that the KG-1a cell aggregates in 3D conditions were more resistant to Ara-C treatment compared to single cells in the unmodified hydrogel. This higher resistance, however, may be due to the protective effect of aggregates. Critically, the high porosity of the hydrogels caused the release of non-adherent KG-1a cells from the unmodified hydrogel into the culture with every media change or addition of treatments. Therefore, the comparison of Ara-C effects on KG-1a cells between the control unmodified hydrogels with RGD and Col1 is not conclusive.
The loss of cells due to the large pores of the hydrogel can be alleviated by fabricating hydrogels with smaller pore sizes. Nair et al. (2015) found that a 60:40 combination of PU and PLLA created a hydrogel with dual nano and microporous property via thermally induced phased separation (TIPS) technique ( 24 ). This formulation showed the highest fibronectin adsorption compared to other combinations and thus was used to culture KG-1a cells. VLA-4 integrin expressed on AML cells can bind to stromal fibronectin and the downstream effects confer CAM-DR to chemotherapy ( 103 , 104 ). The authors found that KG-1a cells cultured in fibronectin-coated PU/PLLA have increased cell adhesion and that the original CD34 + /CD33 - phenotype was maintained after 7-day culture as compared to a reduction of the phenotype in fibronectin-coated tissue culture plate control ( 24 ). CAM-DR was observed by the lack of KG-1a cell death in the hydrogel after treatment of Ara-C as compared to profound cell loss in 2D conditions. When treated with DNR, KG-1a cells in the hydrogel remained drug-resistant for a longer time as compared to controls ( 24 ). These findings lead the authors to investigate the mechanism that contributes to chemoresistance in KG-1a cells cultured in the 3D culture. It was shown that the upregulation of the anti-apoptotic Bcl2 and p27 kip1 , which can result in cell growth arrest, was associated with chemoresistance. Subsequently, the inhibition of Bcl2 via the inhibitor ABT737 increased cell death of KG-1a cells in the presence of Ara-C and DNR ( 24 ).
Natural hydrogels can offer superior biocompatibility with cells, which explains why several research groups prefer to explore the utilization of these hydrogels for culturing HSPC and AML cells in vitro . In a paper published by Shin et al. (2016), the researchers used alginate hydrogel ionically crosslinked with RGD peptide to culture leukemic cells with distinct genetic mutations; MOLM-14 (MLL-AF9), U-937 (without MLL-AF9), and K-562 (CML cells with BCR-ABL) in a 96-well plate format. The authors investigated the proliferation of leukemic cell lines in 3D hydrogels with a range of physiological tissue stiffness relevant to the hematopoietic system stiffness (Young’s modulus, E = 0.075 kPa ~ 3 kPa) ( 37 ). These cells express α 5 β 1 which binds to the RGD sequence in fibronectin ( 37 ). The authors found that leukemia cells formed amorphous aggregates in viscous matrix while forming single large spheroids in solid matrices. Shin et al. (2016) revealed that the MOLM-14 shows increased proliferation responses across matrix stiffness whereas K562 cells show a stiffness-dependent decrease in cell number ( 37 ). The authors found that RGD and a softer matrix confer resistance of AML cell lines to multiple drugs. The study advanced the field by showing that cell-extrinsic factors can have different impacts depending on the AML cell used and that genetic mutations and physical environments can have varying relationships ( 37 ).
Li et al. (2018) explored the use of human-derived decellularized Wharton’s Jelly matrix processed from umbilical cords ( 23 ). The rationale for using this matrix is that it contains similar components to bone marrow ECM, such as collagen, fibronectin, hyaluronic acid, and sulfated proteoglycans. The study used three different cell lines: HL60; Kasumi-1; and MV4-11 and tested the responses of the AML cell lines to Ara-C treatment. The results showed that AML cells in the Wharton’s Jelly matrix had reduced proliferation compared to cells in 2D suspension culture, but there was no difference in apoptosis. Additionally, the cells in the 3D matrix had characteristics of LSCs and a significant population of cells that were resistant to chemotherapy. The study also found that the AML cells in the Wharton’s Jelly matrix had an increased ability to self-renew and were more resistant to the chemotherapy drug Ara-C ( 23 ). The study further corroborated the association between elevated N-cadherin expression in 3D cells and heightened chemotherapeutic resistance of AML cells.
In the study by Xu et al. (2019), the authors explored the response signatures of Ara-C in bone marrow mononuclear cells (BMMCs) from both AML patients and healthy patients encapsulated in Matrigel, a commercial hydrogel rich in laminin and collagen IV ( 38 ). The researchers first optimized the growth conditions to recapitulate the BMME using BMMCs from healthy patients. They found that BMMSCs were capable of multilineage differentiation, including both hematopoietic lineages and stromal cells. Next, the researchers characterized the ex vivo growth and sensitivity to Ara-C of cryopreserved BMMCs from 49 AML patients. RNA-sequencing analysis was performed to determine differential gene expression patterns between 3D and 2D culture plates. The data showed unique gene expression signatures and novel genetic mutations associated with sensitivity to Ara-C treatment. This publication demonstrates the ability to integrate genomic approaches with 3D cultures of human cells and shows the feasibility of using these platforms to study human cell interactions with chemotherapies.
An alternative natural hydrogel that is commonly used for cell culture is fibrinogen hydrogels. Fibrinogen is derived from the polymerization of fibrin in the presence of thrombin which takes place during blood clotting response. Alhallak et al. (2021) proposed a fibrinogen hydrogel that is incorporated with plasma aspirate from a human donor. The combination of plasma culture with fibrinogen gel is rationalized to promote the proliferation and drug resistance of AML cells ( 39 ). Plasma contains a plethora of cytokines, chemokines, and growth factors that mimic the in vivo environment, whereas synthetic hydrogels lack these essential components. In this research, the fibrinogen and plasma culture hydrogels were used to culture both leukemic cell lines (KO52 and NOMO-1) and primary AML cells. The results showed that the AML cell line had a much higher expansion rate at days 3 and 7 in hydrogels compared to 2D cultures. Additionally, primary AML cells cultured in 2D showed no expansion on day 3, but when cultured in 3D hydrogel, the cells expanded 4.5-fold. The researchers also investigated the drug resistance of AML cells to Alisertib (Ali), Ara-C, and Venetoclax. In 2D culture, the drugs induced significant AML cell killing, exceeding 50%. However, in 3D culture, the drugs induced mild to no killing of cells. These findings suggest the chemoresistance of AML cells due to the protective fibrinogen and plasma culture hydrogel similar to native BMME.
4.2 Introduction of niche cells into hydrogels
4.2.1 bmsc stromal cells.
The incorporation of niche cells into hydrogels is an important next step to improve the modeling the BMME. Aljitawi et al. (2014) took the approach of incorporating stromal support into a porous hydrogel by co-culturing the leukemic cells with human BMSCs. In the study, a 3D porous hydrogel was created using a combination of 90:10 polyglycolic acid and PLLA. The hydrogel was highly porous, allowing for the free diffusion of molecules up to 1000 Dalton ( 35 ). The study aimed to investigate the cytotoxic and apoptotic effects of chemotherapy agents on leukemic cell lines HL-60, Kasumi-1, and MV4-11 in co-culture with BMSCs. The results showed that the proliferative response to chemotherapy in 3D conditions was cell-line specific, with Kasumi-1 cells having a higher proliferative response across all DNR treatment doses compared to HL-60 cells. Additionally, HL-60 cells were found to be more resistant to drug-induced apoptosis in 3D conditions compared to 2D stromal culture and suspension culture. The study also found that protection from chemotherapy in 3D conditions may be due to soluble factors released by the cells and that higher doses of chemotherapy can abolish this protective effect. The study also investigated whether the protection was due to the CAM-DR mediated by N-cadherin and found that HL-60 in 3D had higher N-cadherin expression compared to 2D, whereas Kasumi-1 cells did not express significant differences in N-cadherin in 2D and 3D. Similar to Shin et al. (2016), these findings suggest that the mechanism of chemoresistance can vary between cell lines and that consideration of which cell type to use for studies is highly crucial ( 35 , 37 ).
Shen et al. (2016) co-cultured osteoblasts differentiated from BMSCs of leukemia patients with MV4–11 cell line on hydrogel sheets made out of PS. This model attempted to emulate the interactions between the leukemic BMME and leukemia cells. The model is composed of four-layer PS porous sheets arranged within a Transwell insert, featuring pores ranging in size from 150 μm to 200 μm in diameter. The model is in contrast with the previously discussed hydrogel systems that contained dense and randomized 3D microstructures that can absorb and retain liquid. The culture received osteogenic differentiation media which included dexamethasone, β-glycerophosphate, and retinoic acid to induce differentiation of leukemic BMSCs into mature osteoblasts. The authors investigated the effect of blocking the interaction of osteopontin and the receptors, αvβ3 and CD44, via a cyclic peptide containing the RGD sequence that binds to osteopontin. The authors found that BMSCs cultured in 3D secrete more alkaline phosphatase (ALP) and osteopontin compared to 2D culture of BMSCs in culture plate ( 36 ). In the presence of the osteoblasts in 2D and 3D, MV4–11 cells are more resistant to treatment with Ara-C indicated by reduced apoptosis. When the cyclic RGD peptide was introduced into the culture, the adhesion and migration between MV4–11 cells and the matrix were disrupted which caused the leukemia cell to leave the 3D environment into the media and become more sensitive to Ara-C treatment ( 36 ). Another publication from the same group also showed that treatment with AMD3100 and G-CSF with Ara-C reduced the apoptosis of leukemia cells in 3D compared to 2D culture with osteoblast ( 105 ). These findings validated the protective role of the microenvironment, in particular osteoblastic cells, to leukemia cells in vitro .
Borella et al. (2021) created a hydrogel composed of 70% hydroxyapatite and 30% collagen to culture AML cells collected from pediatric patients with leukemic BMSCs isolated from the same patient or healthy donors. Leukemic BMSCs were isolated at the time of diagnosis and cultured for 7 days in the 3D hydrogel before adding AML cells. The models allow for the culture of primary AML cells for at least 21 days. The authors explored three different cell-stroma conditions which are leukemic BMSCs only, leukemic BMSCs with BMSCs-derived osteoblasts, and leukemic BMSCs with HUVECs and their effects on AML cell proliferation. It was found that AML cells formed a physical connection with BMSCs indicated by the presence of membrane nanotubes and gap junctions positive for connexin-43 (CX43) staining. The authors found that this interaction modulates transcriptome reprogramming which includes aberrant cell proliferation and differentiation and compromises their immunomodulatory capacity ( 40 ). Using this 3D model, the researchers conducted a high-throughput screening using 480 candidates and found Lercanidipine as a potential drug to target leukemic BMSCs. This molecule was able to reduce the proliferation rate of leukemic BMSCs without toxicity to healthy CD34+ HSPCs cells ( 40 ). The authors investigated the potential of dual targeting the AML cells and BMSCs using Ara-C and Lercanidipine to reduce leukemia proliferation and found a synergistic effect in reducing the proliferation of leukemic cells in 3D. This dual approach also showed success in AML mice when treated with both Ara-C and Lercanidipine ( 40 ).
4.2.2 Endothelial cells
Vascular cell incorporation has also been studied as an additional component to recapitulate the AML BMME in vitro. Bray et al. (2017) aimed to create a vascular niche for leukemia cells using a star-peg heparin gel with adhesion ligands and pro-angiogenic factors ( 102 ). The gel was made of MMP-sensitive PEG and heparin that was functionalized with adhesion ligands and pro-angiogenic factors. The resultant gel had a storage modulus of 200–300 Pa, which is optimal for endothelial network formation. As the hydrogel is MMP-sensitive, cells can cleave and remodel their environment during the culturing period. The vascular niche was created by culturing human mesenchymal stem cells (hMSCs) and human umbilical vein endothelial cells (HUVECs) together with leukemia cell lines KG1a, MOLM13, MV4–11, and OCI-AML3, or patient samples. The researchers explored the effects of chemotherapy on the cells grown in this matrix and compared it to a 2D suspension culture. Leukemia cells grown in the 3D matrix were more resistant to the Ara-C and DNR compared to 2D culture but with variable effects depending on the cell types and drugs. Particularly, MOLM13 cells were more resistant to DNR doses in 3D than in 2D, and MV4–11 cells and OCI-AML3 cells had increased resistance to both DNR and Ara-C in 3D compared to 2D ( 102 ). The authors investigated the effect of inhibiting the CXCR4/CXCR12 axis that plays a protective role against chemotherapy via the stromal microenvironment ( 106 – 108 ). AMD3100, CXCR4 inhibitor, showed mobilization of AML cells from the vascular network but it does not increase the efficiency of DNR in 3D cultures. In both cell lines and patient cells, this inhibition showed variable effects on cell-adhesion. The authors also observed that AML cell lines formed a heterogeneous mixture of spheroids and loose cell clumps in contact with the vascular network in the hydrogel ( 102 ). Meanwhile, primary AML cells proliferated slower compared to AML cell lines and formed clumps rather than spheroids in the hydrogel. Particularly, the cells exhibited a preference for single cell adherence and growth to the HUVEC-hMSC networks which resemble AML cells phenotype in vivo ( 102 ). Flow cytometry showed similar marker expression of cells in 2D and 3D, with a varying change that depended on the type of cells used. The combination of DNR and Ara-C completely obliterated cells in 3D cultures of primary AML cells and cell lines at day 14 after treatment ( 102 ).
A recent research article by Alhattab et al. (2023) uses robotic 3D printing to establish a high throughput BMME drug screening platform ( Figure 2 ) ( 109 ). The authors explored a class of ultrashort tetramer peptides (IIZK, Ac-Ile-Ile-Cha-Lys-NH2) capable of self-assembling into stable hydrogels, which formed a highly porous network of nanofibers with mechanical properties akin to the stromal matrix. Leveraging automated robotic bioprinting, the aim was to fabricate a 3D BMME-like structure comprising primary leukemia cells or cell lines, BMSCs, and endothelial cells. The investigation encompassed assessments of cell biocompatibility, functionalities, and drug responses, alongside RNAseq and gene expression analyses. Notably, BMSCs grown in 3D exhibited elevated expression of osteogenic and adipogenic differentiation markers, including osteopontin (spp1), BMP-2, and FOXO-1 ( 109 ). The hydrogel supported the growth of primary AML cells, endothelial cells, and BMSCs, with high viability observed in leukemia cell lines KG1a, HL-60, and MV 4–11. AML cells cultured in 3D displayed quiescence and chemoresistance, along with increased colony formation compared to 2D cultures. Furthermore, BMSCs in 3D demonstrated enhanced protection of leukemia cells against chemotherapy agents. RNAseq analysis revealed potential pathways contributing to AML drug resistance and disease relapse through the modulated expression of HGF, FGF1, CCL2, and IL6.
4.3 Biochemical cues in 3D BMME
Velliou et al. (2015) explored the effect of environmental factors on the proliferation and metabolic evolution of AML cells in 3D and 2D. In this model, a similar hydrogel was used as described before by Nair et al. ( 24 ). In this model, K-562 cell line (ATCC CCL-243), lymphoblast cells isolated from the bone marrow of a 53-year-old patient with chronic myelogenous leukemia (CML), were cultured in a PU-based 3D hydrogel coated with Col1 ( 101 ). The authors investigated the effects of different levels of oxidative stress and glucose on the phenotype of K-562 in 3D culture. The PU-based hydrogel showed great maintenance of K-562 cells in vitro as compared to other hydrogels tested ( 110 ). In this study, varying glucose concentrations were tested which were: (1) optimal tissue culture media concentration (4.3 g/L); (2) maximum physiological glucose concentration (1.3 g/L); and (3) minimum physiological glucose concentration (0.6 g/L). The glucose concentrations were tested against hypoxic (5% O 2 ) and normoxic (20% O 2 ) conditions. Understanding the impact of these factors on 3D culture systems is essential for future drug testing, as the microenvironment may synergistically interact with environmental factors, influencing drug response ( 101 ). All in all, the authors found varying effects of oxidative stress and glucose levels between 2D and 3D cultures. Specifically, under hypoxia-induced oxidative stress, glucose levels had a more profound impact on the proliferation of K-562 cells in 3D compared to 2D. In 3D under hypoxia, the highest level of glucose induces higher cell proliferation meanwhile the lowest level of glucose suppresses the expansion of K-562 cells ( 101 ). In addition, cells cultured in 2D showed higher metabolic activity indicated by increased metabolite levels in media such as lactate and glutamate. The findings suggest that the mode of culture can have a significant impact on the responses towards oxidative stress and glucose levels.
4.4 Incorporation of biophysical cues
4.4.1 mimicking interstitial flow and osteoblastic niche.
While hydrogels can be valuable in providing the initial 3D environment for cells, they often lack precise control of biophysical stimulation ( 111 ). Therefore, microfluidic and perfusable hydrogels platforms have been popular in tissue-on-chip approaches as it provide an opportunity to incorporate flow to mimic the endothelial blood flow or interstitial fluid flow ( Figure 3 ). These platforms can provide spatial refinements that can recreate tissue inter-compartmentalization. The combination of both hydrogels and microfluidics approaches represents a highly advantageous strategy, offering a dynamic 3D environment to recapitulate the complex BMME interactions. More recently, several groups including ours have published multi-niche BMME microfluidics systems containing the endosteal and/or vascular niche as a physiological microenvironment to culture HSPCs in vitro ( 62 , 113 – 115 ).
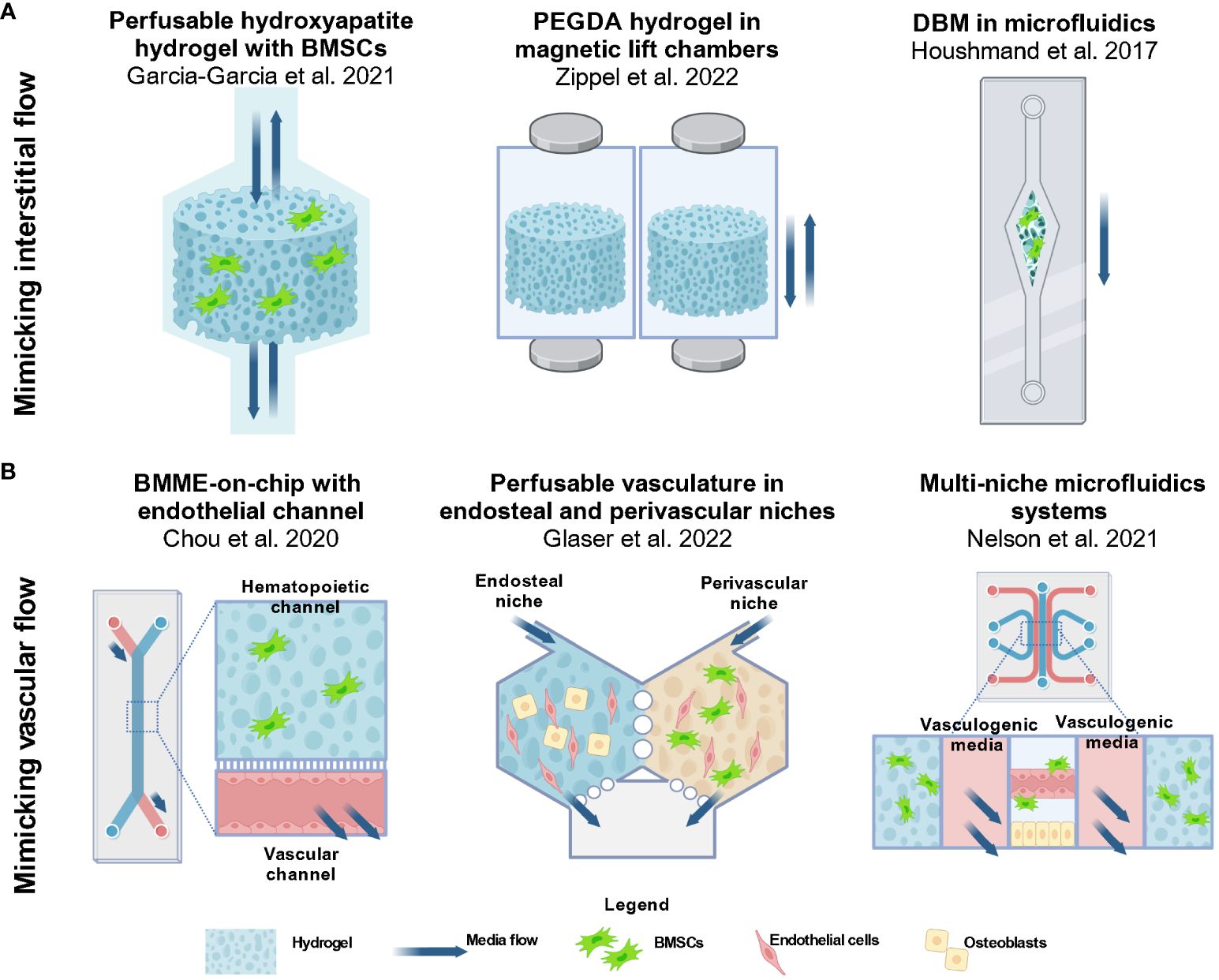
Figure 3 Approaches in 3D modeling of the BMME for AML studies involve utilizing perfusable hydrogels and microfluidics devices. (A) Several groups aimed to create dynamic models with biophysical factors that are present in the BMME. To incorporate interstitial flow in the matrix, groups have incorporated perfusability in the design of hydrogel scaffolds ( 42 , 112 ). The approach includes a bioreactor system with media flowing through a porous hydroxyapatite hydrogel by Garcia-Garcia et al., 2021 and magneticized PEGDA hydrogel enabling non-contact perfusion in a culture chamber as demonstrated by Zippel et al., 2022. An alternative way to introduce flow is via microfluidics as demonstrated by Houshmand et al., 2017 where a demineralized bone matrix scaffold is placed in a single channel microfluidics systems ( 41 ). Images were reproduced using BioRender.com . (B) The functional vasculature is an important component of the BMME that supports the niches and cells present. Among the approaches include a BMME-on-chip microfluidic device by Chou et al., 2020 that consist of a a hematopoietic channel with a fibrinogen matrix and an endothelial channel with media flow ( 60 ). Glaser et al. and Nelson et al. designed microfluidics devices with multiple niches to recapitulate the perivascular niche and endosteal niche ( 62 , 113 ). Currently these models are not being used for AML studies, however they provide innovative approaches to recapitulating the dynamic BMME in vitro. BMSC, bone marrow stromal/stem cells; MSC, mesenchymal stromal/stem cells; HUVEC, human umbilical vein endothelial cells; OB, osteoblastic cells; EC, endothelial cells. Images were reproduced from ( 60 , 62 , 113 ) using BioRender.com .
Several groups have incorporated fluid flow by incorporating perfusion-based approaches designed based on the principle of interstitial flow through the trabecular-like bone matrix and bone marrow ECM ( Figure 3A ). Garcia-Garcia et al. (2021) used a bioreactor-based 3D system with a perfusable porous hydrogel containing an osteoblastic niche by using a hydroxyapatite hydrogel seeded with BMSCs and HSPCs ( 42 ). Hydroxyapatite was chosen because it shares similar mineral components to the trabecular bone. Similar to Shin et al. (2016), this model allowed the researchers to investigate the fate of HSPCs and AML cells that remained in the niche, and those that exited the hydrogel ( 37 ). Previously it was shown that HSPCs that remained in the hydrogel were quiescent while more committed populations were found in the supernatant ( 64 ). This differential effect was also seen in the AML model which incorporates CD34 + BM cells from patients with AML and myeloproliferative neoplasms (MPN) at least 3 weeks in culture. The authors found that leukemic committed progenitor cells were enriched in the hydrogel while mature leukemic cells (CD34 - CD38 - ) and mature leukemic blasts (CD33 + ) were found in the supernatant. Gene expression analysis showed that cells in the niche region highly expressed CXCL12, VCAM1, and IL6 which regulate leukemic cells. Meanwhile, cells in the supernatant expressed macrophage colony-stimulating factor (M-CSF) that attracts and regulates mature myeloid cell differentiation. The results showed that the distribution and differentiation of AML cells were dependent on niche-specific signals similar to the native BMME. The authors also explored the cell interaction with an angiogenic niche by culturing human adipose-derived stromal vascular fraction (SVF) cells within a collagen-based hydrogel. These cells contain both stromal progenitors and endothelial lineage cells. In the hydrogel, the cells form 3D networks with positive CD31 + and NG2 + staining indicating the presence of endothelial cells and perivascular cells. To investigate the behavior of malignant cells in the distinct niches, UCSD-AML1 cell line was cultured in either the hydrogel with the osteoblastic niche or the SVF. A significant observation found was that UCSD-AML1 cells cultured in the SVF-containing hydrogel had lower expression of ANG1 , higher levels of ANG2 and proinflammatory cytokines within 1 week of culture as compared to cells cultured in the osteoblastic niche hydrogel. The data suggest that the different niches can distinctly regulate leukemic cell expansion and differentiation ( 42 ).
A publication from Zippel et al. (2022) detailed a similar approach of culturing HSPC, AML cells, and BMSCs in perfusable porous hydrogels ( 112 ). The hydrogel is composed of a PEG diacrylate (PEGDA) backbone crosslinked with a cell adhesive peptide sequence RGD (RGD-acrylate) and, methacrylated magnetic nanoparticles ( Figure 3A ). The magnetic hydrogels are suspended in the wells of deep 12-well plates housed in a magnetic lift system which provides a controlled movement of the hydrogel. The models overcome the diffusion-limited transport of growth factors and media components in 3D culture without the need for stirring, shaking, or pumping ( 116 ). In addition, the parallelized system allows for simultaneous testing for up to 12 conditions or treatments at a time. AML cell line KG-1a, a promyeloblast macrophage line, was cultured in either a coculture system with BMSCs isolated from human BM or a triculture system with umbilical blood-derived HSPCs and BMSCs in the magnetic hydrogel. Treatments of the hydrogels with cyclophosphamide (CPA) and 5-fluorouracil (5-FU) showed a higher cell viability of KG-1a cells in 3D coculture as compared to static 2D culture indicating a higher chemotherapeutic resistance in 3D ( 112 ). To enable continuous monitoring of the cell conditions, the authors proposed non-invasive metabolic profiling of the supernatant during chemotherapeutic treatments. Particularly, lactate, glucose, and adenosine concentrations were measured at different days after treatments. These metabolites are chosen because active cells consume glucose and produce lactate and adenosine ( 112 ). The authors showed that the metabolic profile can potentially vary depending on the chemotherapeutic chosen. In the 3D coculture of KG-1a cells with BMSCs, CPA caused a decreased metabolic activity while imatinib (IMA) caused the opposite reaction.
Houshmand et al., 2017 described a hydrogel system composed of demineralized bone matrix (DBM) coated with collagen in a single-channel microfluidics assembly to mimic interstitial fluid flow in the bone marrow stroma ( 41 ) ( Figure 3A ). TF-1 cells were cultured with BMSCs in the 3D hydrogel in a microfluidics setup and 2D condition. Scanning electron microscopy showed that TF-1 cells are in proximity with BMSCs, indicating that the cells invaded the microenvironment and formed a comfortable niche in the 3D culture. The findings also show that in the 3D condition, TF-1 cells exhibited retained CD34 + /CD38 - phenotype, increased proliferation, and elevated chemoresistance to azacitidine and Ara-C in 3D culture as compared to 2D microfluidic culture. qPCR showed that BCL2 is elevated after chemotherapy treatment which recapitulated one of the mechanisms of drug resistance shown before ( 95 ).
4.4.2 Mimicking vasculature flow and osteoblastic niche
Incorporation of the vascular component with media flow can mimic the blood flow in microvasculature in the BMME. We believe that a dynamic vascular component is a requirement for future BMME MPS developments. Recreating distinct arteriole and sinusoidal niches requires either intricate cell isolation procedures from these niches or robust differentiation protocols that can generate both components. However, neither approach has been fully established yet. Currently, state of the art approaches includes creating an endothelium mimetic with flow in a microfluidic channels or perfusable self-assembled endothelium tubes in hydrogels ( Figure 3B ).
In 2020, the Chou et al. published an article demonstrating the design of a primary human bone marrow chip in PDMS microfluidic device with two-channels separated by a porous membrane ( 60 ) ( Figure 3B ). The top channel is the hematopoietic channel with a fibrin hydrogel containing BMSC, CD34 + HSPCs while the bottom vascular channel consist of an endothelium lining each surface of the channel. The authors showed sustained myeloerythroid proliferation of HSPCs and predicted clinically relevant hematopoietic toxicity to 5-FU ( 60 ). Our approach relied on the use of a commercial version of the described platform by systems from Emulate Inc, Chip-S1 ® . By using a commercially available device, we can focus on studying the BMME cell interactions with HSPCs without spending the resources on fabrication. In this setup, we incorporated an endosteal niche by culturing osteoblastic cells differentiated from BMSCs, BMSCs, and HSPCs in fibrin hydrogel in the top channel, and endothelial cell-lined bottom channel with fluid flow. We showed that HSPCs from mice were maintained for at least 2 weeks in culture and were able to engraft in mice and differentiate into mature myeloid and lymphoid cells ( 114 ).
The approach described in Glaser et al. (2022), consists of a two hexagonal chambers connected by two-way ports that separate an osteoblastic niche and a perivascular niche ( 113 ) ( Figure 3B ). The vascular niche is created using human cord blood-derived endothelial cells and primary human BMSC in a fibrin hydrogel. When interstitial flow is introduced, the endothelial cells self-assemble into perfusable microvascular network via vasculogenesis. The osteoblastic niche consists of osteoblast cell line hFOB 1.19 with endothelial cells in fibrin. After 7 days of culture, SCF, ICAM-1, VCAM-1 and E-selectin were observed on the vessels of both chambers. The authors indicated that the presence of VCAM-1 may demonstrate recapitulation of arterioles in the bone marrow ( 113 ). The approach resulted in both chambers with perfusable vasculature networks with different permeability that support CD34+ HSPCs. Nelson et al. (2021), took a similar approach of developing a perivascular niche and a vascularized endosteal niche in a custom-made 5-channel PDMS microfluidics systems using soft-lithography ( 62 ) ( Figure 3B ). The resulting device consists of various cells including BMSCs, pericytes, osteoblasts, CAR cells, hematopoietic cells, and adipose cells. Among the analyses performed was an investigation into the protection of HSPCs from ionizing radiation. The authors demonstrated that the endosteal niche reduced apoptosis in HSPCs ( 62 ). Although these systems are not yet optimized for AML studies, the findings demonstrate that culturing endosteal and vascular niches present in the BMME is feasible, highlighting the need for further research to develop a physiological BMME in vitro .
5 Considerations and future directions
5.1 the need for validation of in vivo studies in the mps model.
Validation of clinical and physiological relevance of in vitro findings from MPS models are still a major obstacle. Currently, there is still a lack of conclusive evidence that 3D cell-based systems can accurately recapitulate disease pathophysiology ( 117 ). Sufficient quantitative and reproducible data must be provided to replace current 2D models already used in academic and industry laboratories ( 117 ). Indeed, the majority of 3D culture models discussed in this review have shown that when AML cells, whether derived from cell lines or primary AML cells, are cultured within hydrogel systems, they exhibit chemoresistance. While the findings from these studies validate a crucial phenotype within the leukemic BMME, there remain additional phenotypes that require validation to ensure the achievement of a comprehensive physiological recapitulation of the BMME. The majority of the articles reviewed in the previous section have been mainly focused on validating CAM-DR that is seen in vivo , one that conventional 2D cultures struggle to replicate. Particularly, the findings supported the idea that BMSCs, osteoblastic cells, endothelial cells, and matrix interactions can provide a protective niche for leukemic cells to evade chemotherapies. In addition, the goal of the 3D culture systems was focused on maintaining AML cell numbers and stemness in the presence of an artificial matrix or BMME niche components. AML drug research that uses established cell lines often lacks proper healthy cell controls such as healthy HSPCs ( 118 ). For drug discovery purposes, the drugs must show efficacy to AML cells and at the same time the absence of toxicity to the BMME cells and HSPCs ( 118 ).
More importantly, we believe that there needs to be an emphasis on the validation of key phenotypes of AML interactions with BMME components that were shown to promote disease progression. For example, we and others have shown that AML cells can modulate the BMME by functionally inhibiting osteoblastic cell activity and BMSC differentiation into mature osteoblasts ( 14 , 92 , 119 – 122 ). Leukemic cells can disrupt osteoblastic cell activity through secreted factors such as TPO and CCL3 ( 14 , 21 ). As osteoblastic cells and BMSCs modulate HSPC quiescence, the modulation of their function negatively impacts the hematopoietic compartment in the bone marrow. In addition, in AML, the endosteal vascular niche is remodeled from anti-angiogenic and inflammatory cytokines secreted by leukemic cells such as TNF and CXCL12 ( 21 ). The downstream effects of these interactions include damage to BMSCs, endothelial cells, and osteoblasts which lead to impaired ability of the endosteal niche to support HSC maintenance ( 21 ). Findings such as these, which were shown in vivo experiments, must be validated in 3D culture systems to ensure that the system can mimic not only the AML maintenance but also the leukemic cell interactions with the BMME components in vitro . Validation of works performed in vitro and in vivo must be done to ensure a faithful recapitulation of the AML BMME. Findings from such experiments can provide evidence to drive the adoption of 3D culture systems in AML research.
5.2 Accounting cytogenetic heterogeneity in AML
The underlying genomic and molecular complexity of AML makes it challenging to find therapies that will benefit the majority of AML patients. As evident from previous studies, responses to hydrogel materials and chemotherapies in 3D culture can vary depending on the AML cell source ( 35 , 39 , 102 , 109 ). These works suggest the need for exploring multiple cell types, either primary cells or cell lines to capture the heterogenous responses associated with cytogenetic abnormalities in AML. Multiple immortalized cell lines have been established throughout the years to study AML cell mechanisms, chemoresistance, and drug discovery which have provided valuable findings to the AML field. Skopek et al. (2023) provided a comprehensive review of the AML cell types commonly used in research ( 118 ). A summary of the cell lines used in the 3D culture systems is listed in Table 2 . MPS developers must carefully select AML cell lines that take into account the genetic mutations. For example, the NPM1 mutations are the most common genetic alteration in adult AML, but the occurrence of the mutation is only in about 30% of total cases ( 118 , 125 ). For instance, researchers exploring phenotypes linked to NPM1 mutations may find it beneficial to select cell lines like OCI-AML3 to narrow down the focus of their 3D culture investigations.
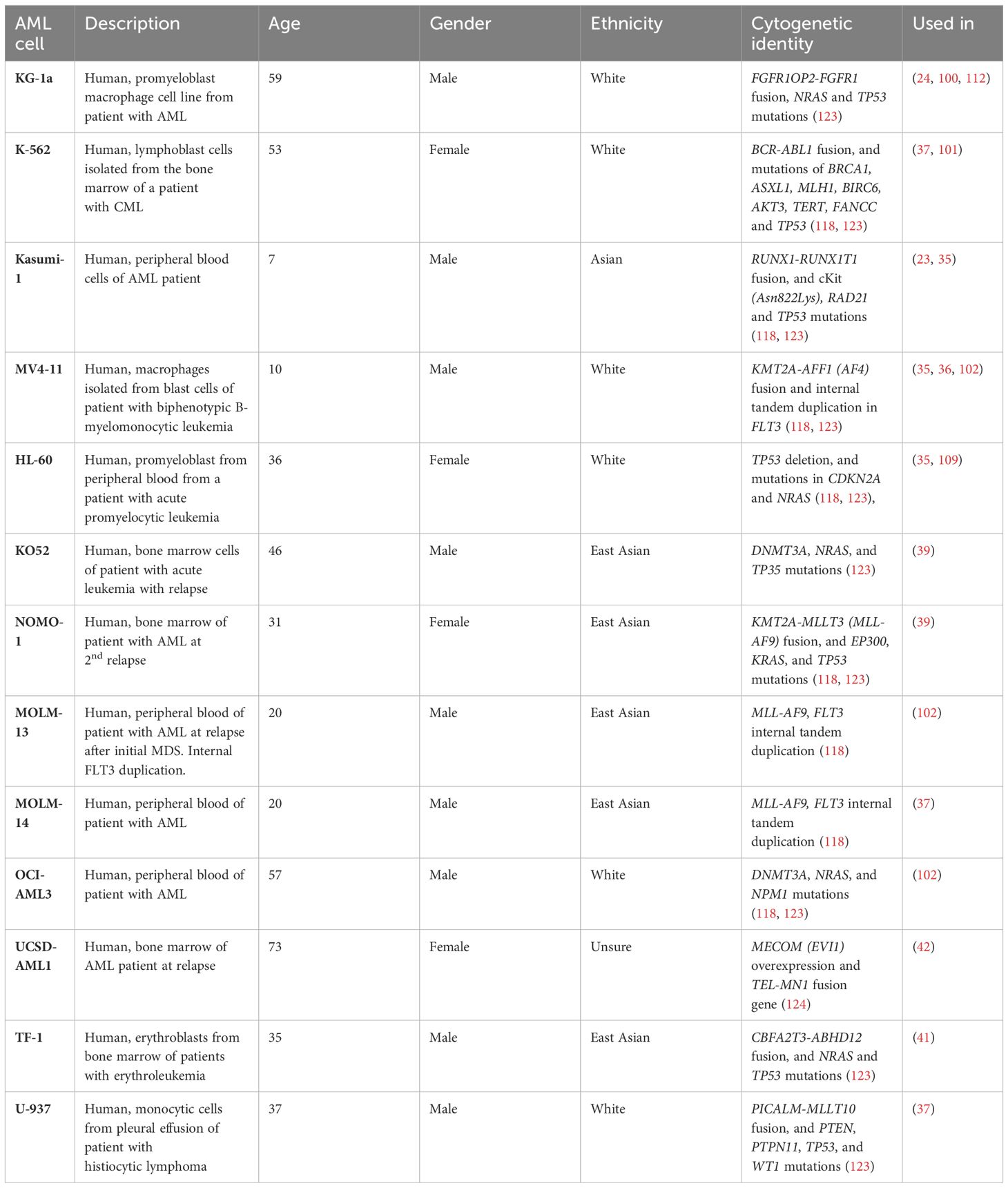
Table 2 Summary of human cell lines used in AML 3D culture development.
5.3 Considerations for sex, race and ethnicities considerations
The American Cancer Society estimated that there will be 62,770 new cases and 23,670 deaths of leukemia in 2024 based on the NCI SEER cancer statistics ( 126 ). Specifically in AML, the estimated new cases and deaths for males are higher as compared to females. In most invasive cancers, men are at higher risk of being diagnosed with cancer (41.6%) compared to women (39.6%). This is believed to be caused by greater exposure to carcinogenic environmental and lifestyle factors ( 126 , 127 ). However, male predominance is still largely unexplained by risk factors and therefore the role of sex-related biological factors such as endogenous hormone exposure and immune function must be further investigated ( 126 – 128 ). Most AML cell lines were derived from male patients of white or Asian descent potentially due to the predominance of AML incidence in males ( Table 2 ). In B-cell chronic lymphocytic leukemia (CLL), in which the predominance is also in males, varying DNA methylation patterns between men and women may contribute to the sex-related difference in CLL risk ( 129 ). Although results in intrinsic sex differences are still limited, 3D culture systems could potentially serve as a tool to study cellular sex differences in the BMME and malignancy.
Although the role of race and ethnicity is not fully understood in cell culture, the potential implications should be considered especially in the context of preclinical drug testing. The lack of racial and ethnic diversity can potentially contribute to cancer health disparities. A review in lung cancer recently published found over 800 lung cancer cell lines came mostly from white and Asian patients although lung cancer disproportionately impacts Black individuals in the US ( 130 ). In addition, the authors highlighted that no cell lines were identified from other groups such as Hispanic/latin(x), American Indian/American Native, or Native Hawaiian or other Pacific Islander ( 130 ). The review paper highlighted the need to establish additional cell lines to ensure the representation of all population groups in critical pre-clinical research. In the context of AML, the disparities in clinical trial enrollment, cancer cell biobanks, and in-hospital death rates in AML, highlight the need to acknowledge the lack of representation in research and care settings, and preclinical studies ( 131 ). In terms of cytogenetic landscape, multiple findings have associated ancestry-associated differences that contribute to molecular features in AML ( 131 ). For example, studies have reported that Black AML patients have lower frequencies of NPM1 and WT1 mutations and higher frequencies of IDH1/2- mutation ( 131 , 132 ). As the understanding of the influence of race and ethnicity emerges, the field should anticipate the integration of these factors into basic and preclinical research.
5.4 Readiness of 3D culture platforms in AML biomedical research
MPS and 3D culture platforms are advantageous in drug discovery as they can recapitulate the complex tissue microstructures while reducing the exhaustive requirements of animal models ( 49 – 54 ). The field is still at its early stages with new models developed with modified approaches such as composite hydrogels with varying stiffness and bioprinted HTS systems ( 109 , 133 ). However, the adoption of MPS and 3D culture approaches in AML research remains limited. MPS is still an emerging field facing challenges with validation, standardization, and acceptance ( 134 ). Standardization will be necessary to ensure the adoptability of 3D culture systems into preclinical studies. One of the primary hurdles in embracing 3D culture techniques, particularly concerning hydrogel construction in AML research, stems from the need to adopt the material fabrication techniques and equipment associated. As evident in this review, researchers must also select from a wide range of biomaterials as there is no standard hydrogel. This is a significant challenge for basic science or clinical labs lacking backgrounds in tissue engineering and biomaterials. Most AML 3D culture systems detailed in literature are custom-built, leading to significant challenges in seamlessly transferring these systems to other research laboratories. In this regard, using a commercially available device may be beneficial; however, cost must be considered, as these devices are newer to the market and can be expensive. Consideration must also be given to the cost of animal models, including the development of new syngeneic or genetic models to study specific BMME and AML cell interactions. Additionally, there is a cost associated with untranslatable findings from 2D culture models to clinical trials.
So far, this review has covered various 3D models encompassing static hydrogels, perfusable hydrogels, and microfluidics-based bone marrow on-a-chip. These models have provided findings that showed recapitulation of chemotherapy resistance of AML cells from matrix and niche cell interactions, and establishment of functional niches that support HSPC maintenance. In addition, the models have also shown distinct responses dependent on AML cell sources, whether primary or cell lines, that may reflect patient-specific responses to chemotherapies and thus provide more relevance to human studies compared to 2D or animal studies. To facilitate the adoption of 3D culture systems into preclinical studies, developers perhaps should consider aligning their approach to a specific context of use (CoU). Specific CoUs for MPS platforms currently include toxicology pharmacokinetics (ADME), pharmacodynamics, efficacy, and drug safety. By defining the CoU or particular goals of the devices, developers can define the strengths and limitations of their platform to convince stakeholders and researchers to adopt their systems. Examples of CoU or goals that are ready to be investigated in the 3D culture of AML BMME include investigating: 1) gender disparities using different cell sources; 2) aging BMME and AML cell progression; 3) initiation of disease due to perturbation of BMME in human cells; 4) development of therapy-related AML (t-AML); and 5) polypharmacy effects in BMME.
Author contributions
AS: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. BF: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from the Wilmot Cancer Institute and the American Cancer Society Discovery grant (RSG #135889) awarded to BF.
Acknowledgments
We would like to acknowledge Dr. Danielle Benoit, at the University of Oregon for her mentorship and support for AS’s thesis research. We also thank the reviewers for providing feedback and comments that helped refined the writing of this review article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hoggatt J, Pelus LM. The Factory of Blood Production: Hematopoietic Stem Cells. In: Pelus LM, Hoggatt J, editors. Hematopoietic Stem Cells: Methods and Protocols . Springer US, New York, NY (2023). p. 3–7.
Google Scholar
2. Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol . (2019) 20:303–20. doi: 10.1038/s41580-019-0103-9
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Wiese M, Daver N. Unmet clinical needs and economic burden of disease in the treatment landscape of acute myeloid leukemia. Am J Manag Care . (2018) 24:S347–s55.
PubMed Abstract | Google Scholar
4. Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, et al. Acute myeloid leukaemia. Nat Rev Dis Primers . (2016) 2:16010. doi: 10.1038/nrdp.2016.10
5. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev . (2019) 36:70–87. doi: 10.1016/j.blre.2019.04.005
6. Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol . (2020) 13:72. doi: 10.1186/s13045-020-00908-z
7. Surveillance, Epidemiology, and End Results (SEER). SEER*Explorer Database: Acute Myeloid Leukemia (AML) SEER Relative Survival by Time Since Diagnosis, 2000-2018 (2000-2018). Available online at: www.seer.cancer.gox https://seer.cancer.gov/statistics-network/explorer/application.html (Accessed March 12 2023).
8. Acute Myeloid Leukemia (AML) SEER 5-year Relative Survival Rates, 2012-2018 (2012-2018). Available online at: https://seer.cancer.gov/statistics-network/explorer/application.html (Accessed March 12 2023).
9. Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J . (2021) 11:41–. doi: 10.1038/s41408-021-00425-3
10. Roussel X, Daguindau E, Berceanu A, Desbrosses Y, Warda W, Neto da Rocha M, et al. Acute myeloid leukemia: from biology to clinical practices through development and pre-clinical therapeutics. Front Oncol . (2020) 10:599933–. doi: 10.3389/fonc.2020.599933
11. Bhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol . (2023) 16:29. doi: 10.1186/s13045-023-01424-6
12. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med . (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
13. Fröbel J, Landspersky T, Percin G, Schreck C, Rahmig S, Ori A, et al. The hematopoietic bone marrow niche ecosystem. Front Cell Dev Biol . (2021) 9:705410. doi: 10.3389/fcell.2021.705410
14. Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood . (2012) 119:540–50. doi: 10.1182/blood-2011-04-348151
15. Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA. The bone marrow microenvironment – Home of the leukemic blasts. Blood Rev . (2017) 31:277–86. doi: 10.1016/j.blre.2017.03.004
16. Staversky RJ, Byun DK, Georger MA, Zaffuto BJ, Goodman A, Becker MW, et al. The chemokine CCL3 regulates myeloid differentiation and hematopoietic stem cell numbers. Sci Rep . (2018) 8:14691. doi: 10.1038/s41598-018-32978-y
17. Krevvata M, Silva BC, Manavalan JS, Galan-Diez M, Kode A, Matthews BG, et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood J Am Soc Hematol . (2014) 124:2834–46. doi: 10.1182/blood-2013-07-517219
CrossRef Full Text | Google Scholar
18. Ryningen A, Wergeland L, Glenjen N, Gjertsen BT, Bruserud Ø. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leuk Res . (2005) 29:185–96. doi: 10.1016/j.leukres.2004.06.008
19. Pezeshkian B, Donnelly C, Tamburo K, Geddes T, Madlambayan GJ. Leukemia mediated endothelial cell activation modulates leukemia cell susceptibility to chemotherapy through a positive feedback loop mechanism. PloS One . (2013) 8:e60823. doi: 10.1371/journal.pone.0060823
20. Ngo S, Papazoglou D, Huerga Encabo H, Bonnet D. Exploring the intricate cross-talk between clonal expansion and the bone marrow niche. Front Hematol . (2024) 3. doi: 10.3389/frhem.2024.1334807
21. Soto CA, Lo Celso C, Purton LE, Frisch BJ. From the niche to Malignant hematopoiesis and back: reciprocal interactions between leukemia and the bone marrow microenvironment. JBMR Plus . (2021) 5:e10516. doi: 10.1002/jbm4.10516
22. Arai F, Hosokawa K, Toyama H, Matsumoto Y, Suda T. Role of N-cadherin in the regulation of hematopoietic stem cells in the bone marrow niche. Ann N Y Acad Sci . (2012) 1266:72–7. doi: 10.1111/j.1749-6632.2012.06576.x
23. Li D, Lin TL, Lipe B, Hopkins RA, Shinogle H, Aljitawi OS. A novel extracellular matrix-based leukemia model supports leukemia cells with stem cell-like characteristics. Leuk Res . (2018) 72:105–12. doi: 10.1016/j.leukres.2018.08.012
24. Nair MS, Mony U, Menon D, Koyakutty M, Sidharthan N, Pavithran K, et al. Development and molecular characterization of polymeric micro-nanofibrous scaffold of a defined 3-D niche for in vitro chemosensitivity analysis against acute myeloid leukemia cells. Int J Nanomed . (2015) 10:3603–22. doi: 10.2147/ijn.s80397
25. Huang Y, Wang Y, Tang J, Qin S, Shen X, He S, et al. CAM-DR: mechanisms, roles and clinical application in tumors. Front Cell Dev Biol . (2021) 9. doi: 10.3389/fcell.2021.698047
26. Kim HN, Ruan Y, Ogana H, Kim Y-M. Cadherins, selectins, and integrins in CAM-DR in leukemia. Front Oncol . (2020) 10. doi: 10.3389/fonc.2020.592733
27. Zhuang L, Darley R, Ottmann OG, Zabkiewicz J, Alvares C. Bone marrow stromal cells mediate adhesion based drug resistance in acute myeloid leukaemia through reciprocal feedback of the β-catenin/CD44 signalling axis. Blood . (2018) 132:2776. doi: 10.1182/blood-2018-99-113811
28. Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature . (2010) 464:852–7. doi: 10.1038/nature08851
29. Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature . (2014) 506:240–4. doi: 10.1038/nature12883
30. Dozzo A, Galvin A, Shin J-W, Scalia S, O’Driscoll CM, Ryan KB. Modelling acute myeloid leukemia (AML): What’s new? A transition from the classical to the modern. Drug Deliv Transl Res . (2022) 13(8):2110–41. doi: 10.1007/s13346-022-01189-4
31. Moore JW, Mendler JH, Loh KP, Azadniv M, O'Dwyer KM, Huselton EJ, et al. Therapeutic modulation of the marrow microenvironment in MDS: A phase I trial of abaloparatide and bevacizumab. Blood . (2022) 140:4050–2. doi: 10.1182/blood-2022-159515
32. DeAngelo DJ, Jonas BA, Liesveld JL, Bixby DL, Advani AS, Marlton P, et al. Phase 1/2 study of uproleselan added to chemotherapy in patients with relapsed or refractory acute myeloid leukemia. Blood . (2022) 139:1135–46. doi: 10.1182/blood.2021010721
33. Chramiec A, Vunjak-Novakovic G. Tissue engineered models of healthy and Malignant human bone marrow. Adv Drug Delivery Rev . (2019) 140:78–92. doi: 10.1016/j.addr.2019.04.003
34. Cartledge Wolf DM, Langhans SA. Moving myeloid leukemia drug discovery into the third dimension. Front Pediatr . (2019) 7. doi: 10.3389/fped.2019.00314
35. Aljitawi OS, Li D, Xiao Y, Zhang D, Ramachandran K, Stehno-Bittel L, et al. A novel three-dimensional stromal-based model for in vitro chemotherapy sensitivity testing of leukemia cells. Leuk Lymph . (2014) 55:378–91. doi: 10.3109/10428194.2013.793323
36. Shen ZH, Zeng DF, Wang XY, Ma YY, Zhang X, Kong PY. Targeting of the leukemia microenvironment by c(RGDfV) overcomes the resistance to chemotherapy in acute myeloid leukemia in biomimetic polystyrene scaffolds. Oncol Lett . (2016) 12:3278–84. doi: 10.3892/ol.2016.5042
37. Shin JW, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc Natl Acad Sci U S A . (2016) 113:12126–31. doi: 10.1073/pnas.1611338113
38. Xu H, Muise ES, Javaid S, Chen L, Cristescu R, Mansueto MS, et al. Identification of predictive genetic signatures of Cytarabine responsiveness using a 3D acute myeloid leukaemia model. J Cell Mol Med . (2019) 23:7063–77. doi: 10.1111/jcmm.14608
39. Alhallak K, de la Puente P, Jeske A, Sun J, Muz B, Rettig MP, et al. 3D tissue engineered plasma cultures support leukemic proliferation and induces drug resistance. Leuk Lymph . (2021) 62:2457–65. doi: 10.1080/10428194.2021.1919657
40. Borella G, Da Ros A, Borile G, Porcù E, Tregnago C, Benetton M, et al. Targeting the plasticity of mesenchymal stromal cells to reroute the course of acute myeloid leukemia. Blood . (2021) 138:557–70. doi: 10.1182/blood.2020009845
41. Houshmand M, Soleimani M, Atashi A, Saglio G, Abdollahi M, Nikougoftar Zarif M. Mimicking the acute myeloid leukemia niche for molecular study and drug screening. Tissue Eng Part C Methods . (2017) 23:72–85. doi: 10.1089/ten.tec.2016.0404
42. García-García A, Klein T, Born G, Hilpert M, Scherberich A, Lengerke C, et al. Culturing patient-derived Malignant hematopoietic stem cells in engineered and fully humanized 3D niches. Proc Natl Acad Sci U.S.A . (2021) 118. doi: 10.1073/pnas.2114227118
43. Liebers N, Bruch P-M, Terzer T, Hernandez-Hernandez M, Paramasivam N, Fitzgerald D, et al. Ex vivo drug response profiling for response and outcome prediction in hematologic Malignancies: the prospective non-interventional SMARTrial. Nat Cancer . (2023) 4:1648–59. doi: 10.1038/s43018-023-00645-5
44. Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood . (2022) 140:1345–77. doi: 10.1182/blood.2022016867
45. Truskey GA. Human microphysiological systems and organoids as in vitro models for toxicological studies. Front Public Health . (2018) 6. doi: 10.3389/fpubh.2018.00185
46. Baptista LS, Porrini C, Kronemberger GS, Kelly DJ, Perrault CM. 3D organ-on-a-chip: The convergence of microphysiological systems and organoids. Front Cell Dev Biol . (2022) 10. doi: 10.3389/fcell.2022.1043117
47. Campisi M, Lim SH, Chiono V, Kamm RD. 3D self-organized human blood-brain barrier in a microfluidic chip. Methods Mol Biol . (2021) 2258:205–19. doi: 10.1007/978-1-0716-1174-6_14
48. Criscione J, Rezaei Z, Hernandez Cantu CM, Murphy S, Shin SR, Kim D-H. Heart-on-a-chip platforms and biosensor integration for disease modeling and phenotypic drug screening. Biosensors Bioelectron . (2023) 220:114840. doi: 10.1016/j.bios.2022.114840
49. Kelm JM, Lal-Nag M, Sittampalam GS, Ferrer M. Translational in vitro research: integrating 3D drug discovery and development processes into the drug development pipeline. Drug Discovery Today . (2019) 24:26–30. doi: 10.1016/j.drudis.2018.07.007
50. Low LA, Tagle DA. Tissue chips - innovative tools for drug development and disease modeling. Lab Chip . (2017) 17:3026–36. doi: 10.1039/C7LC00462A
51. Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol . (2011) 21:745–54. doi: 10.1016/j.tcb.2011.09.005
52. Low LA, Tagle DA. Microphysiological systems (Tissue chips) and their utility for rare disease research. Adv Exp Med Biol . (2017) 1031:405–15. doi: 10.1007/978-3-319-67144-4_23
53. Low LA, Sutherland M, Lumelsky N, Selimovic S, Lundberg MS, Tagle DA. Organs-on-a-chip. Adv Exp Med Biol . (2020) 1230:27–42. doi: 10.1007/978-3-030-36588-2_3
54. Osaki T, Sivathanu V, Kamm RD. Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering. Curr Opin Biotechnol . (2018) 52:116–23. doi: 10.1016/j.copbio.2018.03.011
55. U.S. Food, Drug Administration. Advancing Alternative Methods at FDA . Available online at: https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda (Accessed January 5, 2020).
56. Chatterjee C, Schertl P, Frommer M, Ludwig-Husemann A, Mohra A, Dilger N, et al. Rebuilding the hematopoietic stem cell niche: Recent developments and future prospects. Acta Biomater . (2021) 132:129–48. doi: 10.1016/j.actbio.2021.03.061
57. Zushin PH, Mukherjee S, Wu JC. FDA Modernization Act 2.0: transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J Clin Invest . (2023) 133. doi: 10.1172/jci175824
58. da Silva RGL, Blasimme A. Organ chip research in Europe: players, initiatives, and policies. Front Bioeng Biotechnol . (2023) 11:1237561. doi: 10.3389/fbioe.2023.1237561
59. Sieber S, Wirth L, Cavak N, Koenigsmark M, Marx U, Lauster R, et al. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J Tissue Eng Regener Med . (2018) 12:479–89. doi: 10.1002/term.2507
60. Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng . (2020) 4:394–406. doi: 10.1038/s41551-019-0495-z
61. Di Maggio N, Piccinini E, Jaworski M, Trumpp A, Wendt DJ, Martin I. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. Biomaterials . (2011) 32:321–9. doi: 10.1016/j.biomaterials.2010.09.041
62. Nelson MR, Ghoshal D, Mejías JC, Rubio DF, Keith E, Roy K. A multi-niche microvascularized human bone marrow (hBM) on-a-chip elucidates key roles of the endosteal niche in hBM physiology. Biomaterials . (2021) 270:120683. doi: 10.1016/j.biomaterials.2021.120683
63. Cook MM, Futrega K, Osiecki M, Kabiri M, Kul B, Rice A, et al. Micromarrows—Three-dimensional coculture of hematopoietic stem cells and mesenchymal stromal cells. Tissue Eng Part C: Methods . (2011) 18:319–28. doi: 10.1089/ten.tec.2011.0159
64. Bourgine PE, Klein T, Paczulla AM, Shimizu T, Kunz L, Kokkaliaris KD, et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc Natl Acad Sci . (2018) 115:E5688–e95. doi: 10.1073/pnas.1805440115
65. Congrains A, Bianco J, Rosa RG, Mancuso RI, Saad STO. 3D scaffolds to model the hematopoietic stem cell niche: applications and perspectives. Mater (Basel) . (2021) 14:569. doi: 10.3390/ma14030569
66. Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro . Nat Methods . (2014) 11:663–9. doi: 10.1038/nmeth.2938
67. Rödling L, Schwedhelm I, Kraus S, Bieback K, Hansmann J, Lee-Thedieck C. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci Rep . (2017) 7:4625. doi: 10.1038/s41598-017-04808-0
68. Abarrategi A, Mian SA, Passaro D, Rouault-Pierre K, Grey W, Bonnet D. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. J Exp Med . (2018) 215:729–43. doi: 10.1084/jem.20172139
69. Khan AO, Rodriguez-Romera A, Reyat JS, Olijnik AA, Colombo M, Wang G, et al. Human bone marrow organoids for disease modeling, discovery, and validation of therapeutic targets in hematologic Malignancies. Cancer Discovery . (2023) 13:364–85. doi: 10.1158/2159-8290.CD-22-0199
70. Busch C, Nyamondo K, Wheadon H. Complexities of modeling the bone marrow microenvironment to facilitate hematopoietic research. Exp Hematol . (2024). 135:104233. doi: 10.1016/j.exphem.2024.104233
71. Choi JS, Mahadik BP, Harley BAC. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol J . (2015) 10:1529–45. doi: 10.1002/biot.201400758
72. Hoggatt J, Pelus LM. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem Cell Res Ther . (2011) 2:13. doi: 10.1186/scrt54
73. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells . (1978) 4:7–25.
74. Krause DS, Scadden DT, Preffer FI. The hematopoietic stem cell niche—home for friend and foe? Cytom Part B: Clin Cytom . (2013) 84B:7–20. doi: 10.1002/cyto.b.21066
75. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta . (2014) 1840:2506–19. doi: 10.1016/j.bbagen.2014.01.010
76. Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell . (2014) 14:81–93. doi: 10.1016/j.stem.2013.10.009
77. Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science . (2013) 341:1240104. doi: 10.1126/science.1240104
78. Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int . (2015) 2015:167025. doi: 10.1155/2015/167025
79. Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh Migr . (2014) 8:563–77. doi: 10.4161/19336918.2014.968501
80. He N, Zhang L, Cui J, Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res . (2014) 2014:128436. doi: 10.1155/2014/128436
81. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature . (2014) 505:327–34. doi: 10.1038/nature12984
82. Greim H, Kaden DA, Larson RA, Palermo CM, Rice JM, Ross D, et al. The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann N Y Acad Sci . (2014) 1310:7–31. doi: 10.1111/nyas.12362
83. Wobus M, Bornhäuser M. Editorial: Deciphering the bone marrow microenvironment in hematologic Malignancies. Front Oncol . (2023) 13. doi: 10.3389/fonc.2023.1231467
84. Chen L, Pronk E, van Dijk C, Bian Y, Feyen J, van Tienhoven T, et al. A single-cell taxonomy predicts inflammatory niche remodeling to drive tissue failure and outcome in human AML. Blood Cancer Discovery . (2023) 4:394–417. doi: 10.1158/2643-3230.BCD-23-0043
85. Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell . (2019) 177:1915–32.e16. doi: 10.1016/j.cell.2019.04.040
86. Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell . (2012) 10:259–72. doi: 10.1016/j.stem.2012.02.003
87. Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell . (2015) 160:269–84. doi: 10.1016/j.cell.2014.11.042
88. Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell . (2014) 15:154–68. doi: 10.1016/j.stem.2014.06.008
89. Gu L, Liao P, Liu H. Cancer-associated fibroblasts in acute leukemia. Front Oncol . (2022) 12. doi: 10.3389/fonc.2022.1022979
90. Zhai Y, Zhang J, Wang H, Lu W, Liu S, Yu Y, et al. Growth differentiation factor 15 contributes to cancer-associated fibroblasts-mediated chemo-protection of AML cells. J Exp Clin Cancer Res . (2016) 35:147. doi: 10.1186/s13046-016-0405-0
91. Scadden DT. Nice neighborhood: Emerging concepts of the stem cell niche. Cell . (2014) 157:41–50. doi: 10.1016/j.cell.2014.02.013
92. Duarte D, Hawkins ED, Akinduro O, Ang H, De Filippo K, Kong IY, et al. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell . (2018) 22:64–77.e6. doi: 10.1016/j.stem.2017.11.006
93. Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell . (2017) 32:324–41.e6. doi: 10.1016/j.ccell.2017.08.001
94. Izzi V, Lakkala J, Devarajan R, Savolainen ER, Koistinen P, Heljasvaara R, et al. Expression of a specific extracellular matrix signature is a favorable prognostic factor in acute myeloid leukemia. Leuk Res Rep . (2018) 9:9–13. doi: 10.1016/j.lrr.2017.12.001
95. Zöller M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol . (2015) 6. doi: 10.3389/fimmu.2015.00235
96. Izzi V, Lakkala J, Devarajan R, Ruotsalainen H, Savolainen ER, Koistinen P, et al. An extracellular matrix signature in leukemia precursor cells and acute myeloid leukemia. Haematologica . (2017) 102:e245–e8. doi: 10.3324/haematol.2017.167304
97. Bains AK, Behrens Wu L, Rivière J, Rother S, Magno V, Friedrichs J, et al. Bone marrow mesenchymal stromal cell-derived extracellular matrix displays altered glycosaminoglycan structure and impaired functionality in Myelodysplastic Syndromes. Front Oncol . (2022) 12. doi: 10.3389/fonc.2022.961473
98. Salazar-Terreros MJ, Vernot JP. In vitro and in vivo modeling of normal and leukemic bone marrow niches: cellular senescence contribution to leukemia induction and progression. Int J Mol Sci . (2022) 23:7350. doi: 10.3390/ijms23137350
99. Li W, Liang H, Ao Y, Tang B, Li J, Li N, et al. Biophysical cues of bone marrow-inspired scaffolds regulate hematopoiesis of hematopoietic stem and progenitor cells. Biomaterials . (2023) 298:122111. doi: 10.1016/j.biomaterials.2023.122111
100. Dainiak MB, Savina IN, Musolino I, Kumar A, Mattiasson B, Galaev IY. Biomimetic macroporous hydrogel scaffolds in a high-throughput screening format for cell-based assays. Biotechnol Prog . (2008) 24:1373–83. doi: 10.1002/btpr.30
101. Velliou EG, Dos Santos SB, Papathanasiou MM, Fuentes-Gari M, Misener R, Panoskaltsis N, et al. Towards unravelling the kinetics of an acute myeloid leukaemia model system under oxidative and starvation stress: a comparison between two- and three-dimensional cultures. Bioprocess Biosyst Eng . (2015) 38:1589–600. doi: 10.1007/s00449-015-1401-z
102. Bray LJ, Binner M, Körner Y, von Bonin M, Bornhäuser M, Werner C. A three-dimensional ex vivo tri-culture model mimics cell-cell interactions between acute myeloid leukemia and the vascular niche. Haematologica . (2017) 102:1215–26. doi: 10.3324/haematol.2016.157883
103. Matsunaga T, Fukai F, Miura S, Nakane Y, Owaki T, Kodama H, et al. Combination therapy of an anticancer drug with the FNIII14 peptide of fibronectin effectively overcomes cell adhesion-mediated drug resistance of acute myelogenous leukemia. Leukemia . (2008) 22:353–60. doi: 10.1038/sj.leu.2405017
104. Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med . (2003) 9:1158–65. doi: 10.1038/nm909
105. Shen ZH, Zeng DF, Kong PY, Ma YY, Zhang X. AMD3100 and G-CSF disrupt the cross-talk between leukemia cells and the endosteal niche and enhance their sensitivity to chemotherapeutic drugs in biomimetic polystyrene scaffolds. Blood Cells Mol Dis . (2016) 59:16–24. doi: 10.1016/j.bcmd.2016.03.009
106. Tavor S, Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Semin Cancer Biol . (2010) 20:178–85. doi: 10.1016/j.semcancer.2010.07.001
107. Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood . (2000) 96:2655–63. doi: 10.1182/blood.V96.8.2655
108. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood . (2006) 107:1761–7. doi: 10.1182/blood-2005-08-3182
109. Alhattab DM, Isaioglou I, Alshehri S, Khan ZN, Susapto HH, Li Y, et al. Fabrication of a three-dimensional bone marrow niche-like acute myeloid Leukemia disease model by an automated and controlled process using a robotic multicellular bioprinting system. Biomater Res . (2023) 27:111. doi: 10.1186/s40824-023-00457-9
110. Blanco TM, Mantalaris A, Bismarck A, Panoskaltsis N. The development of a three-dimensional scaffold for ex vivo biomimicry of human acute myeloid leukaemia. Biomaterials . (2010) 31:2243–51. doi: 10.1016/j.biomaterials.2009.11.094
111. Maharjan S, Ma C, Singh B, Kang H, Orive G, Yao J, et al. Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications. Adv Drug Delivery Rev . (2024) 115237. doi: 10.1016/j.addr.2024.115237
112. Zippel S, Dilger N, Chatterjee C, Raic A, Brenner-Weiß G, SChadzek P, et al. A parallelized, perfused 3D triculture model of leukemia for in vitro drug testing of chemotherapeutics. Biofabrication . (2022) 14:035011. doi: 10.1088/1758-5090/ac6a7e
113. Glaser DE, Curtis MB, Sariano PA, Rollins ZA, Shergill BS, Anand A, et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials . (2022) 280:121245. doi: 10.1016/j.biomaterials.2021.121245
114. Sharipol A, Lesch ML, Soto CA, Frisch BJ. Bone marrow microenvironment-on-chip for culture of functional hematopoietic stem cells. Front Bioeng Biotechnol . (2022) 10. doi: 10.3389/fbioe.2022.855777
115. Cairns J, Leonard E, Khan K, Parks C, Maglennon G, Zhang B, et al. Optimal experimental design for efficient toxicity testing in microphysiological systems: A bone marrow application. Front Pharmacol . (2023) 14:1142581. doi: 10.3389/fphar.2023.1142581
116. Rödling L, Volz EM, Raic A, Brändle K, Franzreb M, Lee-Thedieck C. Magnetic macroporous hydrogels as a novel approach for perfused stem cell culture in 3D scaffolds via contactless motion control. Adv Healthc Mater . (2018) 7:1701403. doi: 10.1002/adhm.201701403
117. Carragher N, Piccinini F, Tesei A, Trask OJ Jr, Bickle M, Horvath P. Concerns, challenges and promises of high-content analysis of 3D cellular models. Nat Rev Drug Discov . (2018) 17:606–. doi: 10.1038/nrd.2018.99
118. Skopek R, Palusińska M, Kaczor-Keller K, Pingwara R, Papierniak-Wyglądała A, Schenk T, et al. Choosing the right cell line for acute myeloid leukemia (AML) research. Int J Mol Sci . (2023) 24:5377. doi: 10.3390/ijms24065377
119. Ackun-Farmmer MA, Soto CA, Lesch ML, Byun D, Yang L, Calvi LM, et al. Reduction of leukemic burden via bone-targeted nanoparticle delivery of an inhibitor of C-chemokine (C-C motif) ligand 3 (CCL3) signaling. FASEB J . (2021) 35:e21402. doi: 10.1096/fj.202000938rr
120. Tomasoni C, Arsuffi C, Donsante S, Corsi A, Riminucci M, Biondi A, et al. AML alters bone marrow stromal cell osteogenic commitment via Notch signaling. Front Immunol . (2023) 14:1320497. doi: 10.3389/fimmu.2023.1320497
121. Schroeder T, Geyh S, Germing U, Haas R. Mesenchymal stromal cells in myeloid Malignancies. Blood Res . (2016) 51:225–32. doi: 10.5045/br.2016.51.4.225
122. Shahrokh B, Allahbakhshian FM, Ahmad G, Fatemeh F, Hossein MM. AML-derived extracellular vesicles negatively regulate stem cell pool size: A step toward bone marrow failure. Curr Res Transl Med . (2023) 71:103375. doi: 10.1016/j.retram.2022.103375
123. Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech . (2018) 29(2):25. doi: 10.7171/jbt.18-2902-002
124. Oval J, Smedsrud M, Taetle R. Expression and regulation of the evi-1 gene in the human factor-dependent leukemia cell line, UCSD/AML1. Leukemia . (1992) 6(5):446–51.
125. Falini B, Brunetti L, Sportoletti P, Martelli MP. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood . (2020) 136:1707–21. doi: 10.1182/blood.2019004226
126. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: A Cancer J Clin . (2024) 74:12–49. doi: 10.3322/caac.21820
127. Jackson SS, Marks MA, Katki HA, Cook MB, Hyun N, Freedman ND, et al. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer . (2022) 128:3531–40. doi: 10.1002/cncr.34390
128. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol . (2016) 16:626–38. doi: 10.1038/nri.2016.90
129. Lin S, Liu Y, Goldin LR, Lyu C, Kong X, Zhang Y, et al. Sex-related DNA methylation differences in B cell chronic lymphocytic leukemia. Biol Sex Differ . (2019) 10:2. doi: 10.1186/s13293-018-0213-7
130. Leon C, Manley E, Neely AM, Castillo J, Ramos Correa M, Velarde DA, et al. Lack of racial and ethnic diversity in lung cancer cell lines contributes to lung cancer health disparities. Front Oncol . (2023) 13. doi: 10.3389/fonc.2023.1187585
131. Eisfeld A-K. Disparities in acute myeloid leukemia treatments and outcomes. Curr Opin Hematol . (2024) 31:58–63. doi: 10.1097/MOH.0000000000000797
132. Bhatnagar B, Kohlschmidt J, Mrózek K, Zhao Q, Fisher JL, Nicolet D, et al. Poor survival and differential impact of genetic features of black patients with acute myeloid leukemia. Cancer Discovery . (2021) 11:626–37. doi: 10.1158/2159-8290.CD-20-1579
133. Hong J, Zhu Z, Cui L, Wang Z, Hao Y, Tian X, et al. Bone marrow-inspired hydrogel/graphene composite scaffolds to support in vitro expansion of hematopoietic stem cells. J Mater Chem B . (2024) 12:2354–63. doi: 10.1039/D3TB02448B
134. Holloway PM. Novel, emerging chip models of the blood-brain barrier and future directions. In: Stone N, editor. The Blood-Brain Barrier: Methods and Protocols . NY Springer US, New York (2022). p. 193–224.
Keywords: acute myeloid leukemia, in vitro , bone marrow microenvironment, microphysiological systems, 3D culture, AML, MPS, BMME
Citation: Sharipol A and Frisch BJ (2024) Are we ready to integrate 3D culture systems in acute myeloid leukemia and bone marrow microenvironment research? Front. Hematol. 3:1407698. doi: 10.3389/frhem.2024.1407698
Received: 27 March 2024; Accepted: 02 July 2024; Published: 06 August 2024.
Reviewed by:
Copyright © 2024 Sharipol and Frisch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin J. Frisch, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
The objective of this review is, therefore, to summarize the main molecules currently being produced using plant cell/tissue culture, their applications in areas such as medicine and food technology, and the plant material cultured for their production. The review also covers new trends in in vitro cell/tissue culture and plant transformation.
In the 1800s, scientists discovered a useful alternative to in vivo research, the in vitro cell culture system. Cell culture has been widely used in academia and industry for diverse issues such as cell biology research to vaccine production and cancer drug discoveries. This article will provide a brief introduction and summary of cell culture history with some key milestones and discuss the ...
Cell culture is a very versatile tool in the investigation of basic scientific and translation research questions. The advantage of using cell lines in scientific research is their homogeneity and associated reproducibility in data generated. This chapter introduces the principles behind the setup of a cell culture lab and the guidelines that ...
In vitro culture is a method applied for the growth and development of plant cells, tissues, and organs that uses a nutritive culture medium under controlled sterilized conditions.
Three-dimensional (3D) culture systems are becoming increasingly popular due to their ability to mimic tissue-like structures more effectively than the monolayer cultures. In cancer and stem cell research, the natural cell characteristics and architectures ...
Microfluidic systems designed for cell culture are emerging platforms for in vitro drug screening and cell therapies. This review addresses 2D and 3D cell culture methods, their main information, and...
In vitro culture systems have been reported as a substitute for the large scale production of metabolites of pharmaceutical interest [ 28, 130 ]. The overproduction of naphtodianthrones from cell suspension cultures of H. perforatum have been customized using phytohormones and different elicitors [ 47, 127, 131 ].
Precise knowledge of all aspects controlling plant tissue culture and in vitro plant regeneration is crucial for plant biotechnologists and their correlated industry, as there is increasing demand for this scientific knowledge, resulting in more productive and resilient plants in the field. However, the development and application of cell and tissue culture techniques are usually based on ...
Research has shifted toward improving in vitro models by increasing their complexity in order to understand how mature intricate tissues form ( Holloway et al., 2019 ). An increase in complexity can be accomplished by culturing different cell types together in one culture, called co-culturing.
Cell culture is a method for growing or maintaining cells in vitro under controlled conditions. Primary cell cultures refer to dispersed cells that are cultured directly from tissues and have ...
This review presents an overview of the culture media and practices used in plant tissue culture and developmental biology. The compositions of the most co
Three-dimensional (3D) culture models have the potential to bridge the gap between 2D in vitro models and in vivo models. The present review aims to describe the main 3D culture models currently available for studying cancer development and drug screening in oncology, and to discuss their added value and specific limitations.
The review also focuses on major constraints associated with the use of plant tissue culture technique that need to be addressed for sustainable industrial applications. Various useful products can be obtained as food products, pharmaceutical products and cosmetics via in vitro plant regeneration ( Niazian et al., 2017; Chandran et al., 2020 ).
Such in vitro culture methods exploit all the available genetic variability and reduce the period of the breeding program to develop tolerant and resistant genotypes [ 6 ]. Primarily, plant tissue culture is used in vegetatively propagated crops and self-pollinated crops, especially with narrower genetic bases.
In vitro plant cell and tissue culture techniques are the basis of many micropropagation and breeding programs for scientific research. Plant tissue culture (PTC) involves organogenesis and embryogenesis, and the outcome depends on the different conditions to which the tissue is exposed. PTC is a stressful environment—high relative humidity, low ventilation rate, high concentrations of plant ...
Plant tissue culture as an important tool for the continuous production of active compounds including secondary metabolites and engineered molecules. Novel methods (gene editing, abiotic stress) can improve the technique. Humans have a long history of reliance on plants for a supply of food, shelter and, most importantly, medicine.
Tissue culture is in vitro maintenance and propagation of isolated cells tissues or organs in an appropriate artificial environment. Many animal cells can be induced to grow outside of their organ or tissue of origin under defined conditions when supplemented with a medium containing nutrients and growth factors.
However, the large-scale proliferation and improvement of the gladiolus have been accomplished with the aid of plant tissue culture and other biotechnological techniques. The current review includes a thorough examination of the growth and development parameters required for successful in vitro gladiolus development as well as cormel formation.
In vitro plant tissue culture (PTC) techniques have greatly contributed to industry and agriculture in the last century by exploiting the economic potential of several commercial crop plants.
The use of cell-based systems has increasingly replaced in vivo research and the application of in vitro models enjoys an ever-growing popularity. To avoid repeating past mistakes, high standards of reproducibility and reliability must be established and maintained in the field of in vitro biomedical research.
Herpes simplex virus-1 (HSV-1) establishes a latent infection in peripheral neurons and periodically reactivates in response to a stimulus to permit transmission. In vitro models using primary neurons are invaluable to studying latent infection because they use bona fide neurons that have undergone differentiation and maturation in vivo. However, culture conditions in vitro should remain as ...
In vitro culture is a key foundation to study the function of rumen ciliates. It is impossible to culture any ruminal ciliate species axenically in vitro, and rumen ciliates are isolated from rumen fluid and cultured as monocultures or mixed cultures in vitro to better study them [4, 5, 6].
Respectful and just collaboration can facilitate a shared research space among plant biologists, social scientists, and Indigenous Peoples to better understand the role of in vitro technology in the successful conservation of biocultural environments.
Furthermore, in vitro culture can efficiently produce a significant amount of medicinally active components, thereby decreasing reliance on wild resources. Research on the variations of medicinal components in E. ulmoides not only offers a theoretical foundation but also supplies experimental materials for the development of novel drugs.
In vitro culture technologies can potentially grow any plant anywhere and offer the added value of overproduction of plant cultures, enhanced production of secondary metabolites, and the generation of novel medicinal compounds.
The technical breakthroughs enabled by brain organoids have a significant impact on the research of different brain regions, brain development and sickness, the connections between the brain and other tissues and organs, and brain evolution. ... The integration of brain organoids and vascular system under in vitro culture will help to improve ...
Embryo culture is crucial to achieve successful outcomes in assisted reproductive technology (ART) for cattle. This study explored the innovative use of dry incubators integrated with time-lapse monitoring systems for bovine embryo culture, building on their advantages in human medicine, such as reduced contamination risk, stable temperature control, and lower gas consumption. Our research ...
In vitro cell culture is a method used for studying the behavior of animal cells in a controlled environment, free of systemic variations. Currently, different types of cell cultures have been adapted and developed. Animal cell cultures have been applied for studying basic cell biology, interactions of drugs and other chemicals with cells, production of vaccines and proteins, etc. This chapter ...
Natural hydrogels can offer superior biocompatibility with cells, which explains why several research groups prefer to explore the utilization of these hydrogels for culturing HSPC and AML cells in vitro. In a paper published by Shin et al. (2016), the researchers used alginate hydrogel ionically crosslinked with RGD peptide to culture leukemic ...