
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


5.4: Ribosomes
- Last updated
- Save as PDF
- Page ID 68562
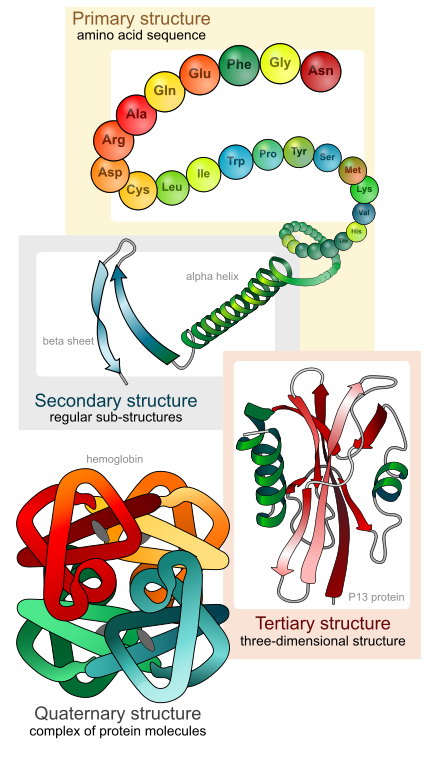
Ribosomes are the cellular structures responsible for protein synthesis. The word “synthesis” means “to combine things to produce something else.” In this context, protein synthesis means combining different amino acids together to form a protein. Ribosomes join amino acids together in a chain to form a protein ( Figure \(\PageIndex{1}\) ). This amino acid chain then folds into a complex 3-dimensional structure. The shape of a protein is what gives the protein its specific function.
Helpful Hint
Proteins are not typically used as a source of energy for the body. Protein from your diet is broken down into individual amino acids which are reassembled by your ribosomes into proteins that your cells need. Ribosomes do not produce energy.
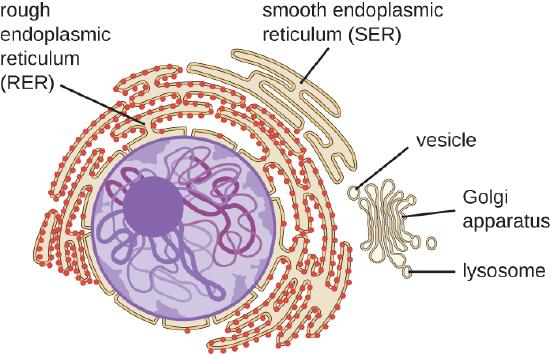
When viewed through an electron microscope, free ribosomes appear as either clusters or single tiny dots floating freely in the cytoplasm. Ribosomes may be attached to either the cytoplasmic side of the plasma membrane or the cytoplasmic side of the rough endoplasmic reticulum (Figure \(\PageIndex{2}\)).
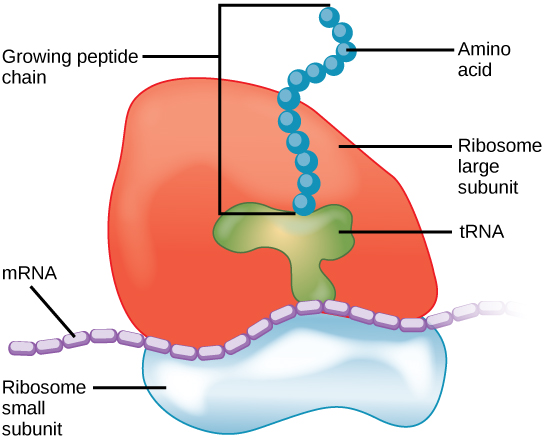
Because protein synthesis is essential for all cells, ribosomes are found in practically every cell, although they are smaller in prokaryotic cells. They are particularly abundant in immature red blood cells for the synthesis of hemoglobin, which functions in the transport of oxygen throughout the body. Electron microscopy has shown us that ribosomes, which are large complexes of protein and RNA, consist of two subunits, aptly called large and small ( Figure \(\PageIndex{3}\) ). Ribosomes receive their “orders” for protein synthesis from the nucleus where the DNA is transcribed into messenger RNA (mRNA). The mRNA travels to the ribosomes, which translate the code provided by the sequence of the nitrogenous bases in the mRNA into a specific order of amino acids in a protein. Amino acids are the building blocks of proteins.
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax .
Text adapted from: OpenStax , Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/[email protected]
15.5 Ribosomes and Protein Synthesis
Learning objectives.
By the end of this section, you will be able to do the following:
- Describe the different steps in protein synthesis
- Discuss the role of ribosomes in protein synthesis
The synthesis of proteins consumes more of a cell’s energy than any other metabolic process. In turn, proteins account for more mass than any other component of living organisms (with the exception of water), and proteins perform virtually every function of a cell. The process of translation, or protein synthesis, involves the decoding of an mRNA message into a polypeptide product. Amino acids are covalently strung together by interlinking peptide bonds in lengths ranging from approximately 50 to more than 1000 amino acid residues. Each individual amino acid has an amino group (NH 2 ) and a carboxyl (COOH) group. Polypeptides are formed when the amino group of one amino acid forms an amide (i.e., peptide) bond with the carboxyl group of another amino acid ( Figure 15.15 ). This reaction is catalyzed by ribosomes and generates one water molecule.
The Protein Synthesis Machinery
In addition to the mRNA template, many molecules and macromolecules contribute to the process of translation. The composition of each component may vary across species; for example, ribosomes may consist of different numbers of rRNAs and polypeptides depending on the organism. However, the general structures and functions of the protein synthesis machinery are comparable from bacteria to human cells. Translation requires the input of an mRNA template, ribosomes, tRNAs, and various enzymatic factors. (Note: A ribosome can be thought of as an enzyme whose amino acid binding sites are specified by mRNA.)
Link to Learning
Click through the steps of this PBS interactive to see protein synthesis in action.
Even before an mRNA is translated, a cell must invest energy to build each of its ribosomes. In E. coli , there are between 10,000 and 70,000 ribosomes present in each cell at any given time. A ribosome is a complex macromolecule composed of structural and catalytic rRNAs, and many distinct polypeptides. In eukaryotes, the nucleolus is completely specialized for the synthesis and assembly of rRNAs.
Ribosomes exist in the cytoplasm of prokaryotes and in the cytoplasm and rough endoplasmic reticulum of eukaryotes. Mitochondria and chloroplasts also have their own ribosomes in the matrix and stroma, which look more similar to prokaryotic ribosomes (and have similar drug sensitivities) than the ribosomes just outside their outer membranes in the cytoplasm. Ribosomes dissociate into large and small subunits when they are not synthesizing proteins and reassociate during the initiation of translation. In E. coli, the small subunit is described as 30S, and the large subunit is 50S, for a total of 70S (recall that Svedberg units are not additive). Mammalian ribosomes have a small 40S subunit and a large 60S subunit, for a total of 80S. The small subunit is responsible for binding the mRNA template, whereas the large subunit sequentially binds tRNAs. Each mRNA molecule is simultaneously translated by many ribosomes, all synthesizing protein in the same direction: reading the mRNA from 5' to 3' and synthesizing the polypeptide from the N terminus to the C terminus. The complete mRNA/poly-ribosome structure is called a polysome .
The tRNAs are structural RNA molecules that were transcribed from genes by RNA polymerase III. Depending on the species, 40 to 60 types of tRNAs exist in the cytoplasm. Transfer RNAs serve as adaptor molecules. Each tRNA carries a specific amino acid and recognizes one or more of the mRNA codons that define the order of amino acids in a protein. Aminoacyl-tRNAs bind to the ribosome and add the corresponding amino acid to the polypeptide chain. Therefore, tRNAs are the molecules that actually “translate” the language of RNA into the language of proteins.
Of the 64 possible mRNA codons—or triplet combinations of A, U, G, and C—three specify the termination of protein synthesis and 61 specify the addition of amino acids to the polypeptide chain. Of these 61, one codon (AUG) also encodes the initiation of translation. Each tRNA anticodon can base pair with one or more of the mRNA codons for its amino acid. For instance, if the sequence CUA occurred on an mRNA template in the proper reading frame, it would bind a leucine tRNA expressing the complementary sequence, GAU. The ability of some tRNAs to match more than one codon is what gives the genetic code its blocky structure.
As the adaptor molecules of translation, it is surprising that tRNAs can fit so much specificity into such a small package. Consider that tRNAs need to interact with three factors: 1) they must be recognized by the correct aminoacyl synthetase (see below); 2) they must be recognized by ribosomes; and 3) they must bind to the correct sequence in mRNA.
Aminoacyl tRNA Synthetases
The process of pre-tRNA synthesis by RNA polymerase III only creates the RNA portion of the adaptor molecule. The corresponding amino acid must be added later, once the tRNA is processed and exported to the cytoplasm. Through the process of tRNA “charging,” each tRNA molecule is linked to its correct amino acid by one of a group of enzymes called aminoacyl tRNA synthetases . At least one type of aminoacyl tRNA synthetase exists for each of the 20 amino acids; the exact number of aminoacyl tRNA synthetases varies by species. These enzymes first bind and hydrolyze ATP to catalyze a high-energy bond between an amino acid and adenosine monophosphate (AMP); a pyrophosphate molecule is expelled in this reaction. The activated amino acid is then transferred to the tRNA, and AMP is released. The term "charging" is appropriate, since the high-energy bond that attaches an amino acid to its tRNA is later used to drive the formation of the peptide bond. Each tRNA is named for its amino acid.
The Mechanism of Protein Synthesis
As with mRNA synthesis, protein synthesis can be divided into three phases: initiation, elongation, and termination . The process of translation is similar in prokaryotes and eukaryotes. Here we’ll explore how translation occurs in E. coli , a representative prokaryote, and specify any differences between prokaryotic and eukaryotic translation.
Initiation of Translation
Protein synthesis begins with the formation of an initiation complex . In E. coli , this complex involves the small 30S ribosome, the mRNA template, three initiation factors (IFs; IF-1, IF-2, and IF-3), and a special initiator tRNA , called tRNA fMet .
In E. coli mRNA, a sequence upstream of the first AUG codon, called the Shine-Dalgarno sequence (AGGAGG), interacts with the rRNA molecules that compose the ribosome. This interaction anchors the 30S ribosomal subunit at the correct location on the mRNA template. Guanosine triphosphate (GTP), which is a purine nucleotide triphosphate, acts as an energy source during translation—both at the start of elongation and during the ribosome’s translocation. Binding of the mRNA to the 30S ribosome also requires IF-3.
The initiator tRNA then interacts with the start codon AUG (or rarely, GUG). This tRNA carries the amino acid methionine, which is formylated after its attachment to the tRNA. The formylation creates a "faux" peptide bond between the formyl carboxyl group and the amino group of the methionine. Binding of the fMet-tRNA fMet is mediated by the initiation factor IF-2. The fMet begins every polypeptide chain synthesized by E. coli , but it is usually removed after translation is complete. When an in-frame AUG is encountered during translation elongation, a non-formylated methionine is inserted by a regular Met-tRNA Met . After the formation of the initiation complex, the 30S ribosomal subunit is joined by the 50S subunit to form the translation complex. In eukaryotes, a similar initiation complex forms, comprising mRNA, the 40S small ribosomal subunit, eukaryotic IFs, and nucleoside triphosphates (GTP and ATP). The methionine on the charged initiator tRNA, called Met-tRNA i , is not formylated. However, Met-tRNA i is distinct from other Met-tRNAs in that it can bind IFs.
Instead of depositing at the Shine-Dalgarno sequence, the eukaryotic initiation complex recognizes the 7-methylguanosine cap at the 5' end of the mRNA. A cap-binding protein (CBP) and several other IFs assist the movement of the ribosome to the 5' cap. Once at the cap, the initiation complex tracks along the mRNA in the 5' to 3' direction, searching for the AUG start codon. Many eukaryotic mRNAs are translated from the first AUG, but this is not always the case. According to Kozak’s rules , the nucleotides around the AUG indicate whether it is the correct start codon. Kozak’s rules state that the following consensus sequence must appear around the AUG of vertebrate genes: 5'-gccRccAUGG-3'. The R (for purine) indicates a site that can be either A or G, but cannot be C or U. Essentially, the closer the sequence is to this consensus, the higher the efficiency of translation.
Once the appropriate AUG is identified, the other proteins and CBP dissociate, and the 60S subunit binds to the complex of Met-tRNA i , mRNA, and the 40S subunit. This step completes the initiation of translation in eukaryotes.
Translation, Elongation, and Termination
In prokaryotes and eukaryotes, the basics of elongation are the same, so we will review elongation from the perspective of E. coli . When the translation complex is formed, the tRNA binding region of the ribosome consists of three compartments. The A (aminoacyl) site binds incoming charged aminoacyl tRNAs. The P (peptidyl) site binds charged tRNAs carrying amino acids that have formed peptide bonds with the growing polypeptide chain but have not yet dissociated from their corresponding tRNA. The E (exit) site releases dissociated tRNAs so that they can be recharged with free amino acids. The initiating methionyl-tRNA, however, occupies the P site at the beginning of the elongation phase of translation in both prokaryotes and eukaryotes.
During translation elongation, the mRNA template provides tRNA binding specificity. As the ribosome moves along the mRNA, each mRNA codon comes into register, and specific binding with the corresponding charged tRNA anticodon is ensured. If mRNA were not present in the elongation complex, the ribosome would bind tRNAs nonspecifically and randomly.
Elongation proceeds with charged tRNAs sequentially entering and leaving the ribosome as each new amino acid is added to the polypeptide chain. Movement of a tRNA from A to P to E site is induced by conformational changes that advance the ribosome by three bases in the 3' direction. The energy for each step along the ribosome is donated by elongation factors that hydrolyze GTP. GTP energy is required both for the binding of a new aminoacyl-tRNA to the A site and for its translocation to the P site after formation of the peptide bond. Peptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. The formation of each peptide bond is catalyzed by peptidyl transferase , an RNA-based enzyme that is integrated into the 50S ribosomal subunit. The energy for each peptide bond formation is derived from the high-energy bond linking each amino acid to its tRNA. After peptide bond formation, the A-site tRNA that now holds the growing peptide chain moves to the P site, and the P-site tRNA that is now empty moves to the E site and is expelled from the ribosome ( Figure 15.18 ). Amazingly, the E. coli translation apparatus takes only 0.05 seconds to add each amino acid, meaning that a 200-amino-acid protein can be translated in just 10 seconds.
Visual Connection
Many antibiotics inhibit bacterial protein synthesis. For example, tetracycline blocks the A site on the bacterial ribosome, and chloramphenicol blocks peptidyl transfer. What specific effect would you expect each of these antibiotics to have on protein synthesis?
Tetracycline would directly affect:
- tRNA binding to the ribosome
- ribosome assembly
- growth of the protein chain
Chloramphenicol would directly affect:
Termination of translation occurs when a nonsense codon (UAA, UAG, or UGA) is encountered. Upon aligning with the A site, these nonsense codons are recognized by protein release factors that resemble tRNAs. The releasing factors in both prokaryotes and eukaryotes instruct peptidyl transferase to add a water molecule to the carboxyl end of the P-site amino acid. This reaction forces the P-site amino acid to detach from its tRNA, and the newly made protein is released. The small and large ribosomal subunits dissociate from the mRNA and from each other; they are recruited almost immediately into another translation initiation complex. After many ribosomes have completed translation, the mRNA is degraded so the nucleotides can be reused in another transcription reaction.
Protein Folding, Modification, and Targeting
During and after translation, individual amino acids may be chemically modified, signal sequences appended, and the new protein “folded” into a distinct three-dimensional structure as a result of intramolecular interactions. A signal sequence is a short sequence at the amino end of a protein that directs it to a specific cellular compartment. These sequences can be thought of as the protein’s “train ticket” to its ultimate destination, and are recognized by signal-recognition proteins that act as conductors. For instance, a specific signal sequence terminus will direct a protein to the mitochondria or chloroplasts (in plants). Once the protein reaches its cellular destination, the signal sequence is usually clipped off.
Many proteins fold spontaneously, but some proteins require helper molecules, called chaperones , to prevent them from aggregating during the complicated process of folding. Even if a protein is properly specified by its corresponding mRNA, it could take on a completely dysfunctional shape if abnormal temperature or pH conditions prevent it from folding correctly.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/biology-2e/pages/1-introduction
- Authors: Mary Ann Clark, Matthew Douglas, Jung Choi
- Publisher/website: OpenStax
- Book title: Biology 2e
- Publication date: Mar 28, 2018
- Location: Houston, Texas
- Book URL: https://openstax.org/books/biology-2e/pages/1-introduction
- Section URL: https://openstax.org/books/biology-2e/pages/15-5-ribosomes-and-protein-synthesis
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 25 September 2017
Ribosomal proteins: insight into molecular roles and functions in hepatocellular carcinoma
- Y Wang 2 &
Oncogene volume 37 , pages 277–285 ( 2018 ) Cite this article
3192 Accesses
25 Citations
1 Altmetric
Metrics details
- Prognostic markers
Ribosomes, which are important sites for the synthesis of proteins related to expression and transmission of genetic information in humans, have a complex structure and diverse functions. They consist of a variety of ribosomal proteins (RPs), ribosomal RNAs (rRNAs) and small nucleolar RNAs. Owing to the involvement of ribosomes in many important biological processes of cells, their major components, rRNAs and RPs, have an important role in human diseases, including the initiation and evolvement of malignancies. However, the main mechanisms underlying the involvement of ribosomes in cancer remain unclear. This review describes the crucial role of ribosomes in various common malignant tumors; in particular, it examines the effects of RPs, including S6, the receptor for activated C-kinase and RPS15A, on the development and progression of hepatocellular carcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
251,40 € per year
only 5,03 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy
Jian Kang, Natalie Brajanovski, … Elaine Sanij
RRP15 deficiency induces ribosome stress to inhibit colorectal cancer proliferation and metastasis via LZTS2-mediated β-catenin suppression
Zhixiong Dong, Jinhai Li, … Chunming Ding

Cancer-associated mutations in the ribosomal protein L5 gene dysregulate the HDM2/p53-mediated ribosome biogenesis checkpoint
Ines Oršolić, Slađana Bursać, … Siniša Volarević
Ruggero D, Pandolfi PP . Does the ribosome translate cancer? Nat Rev Cancer 2003; 3 : 179–192.
Article CAS PubMed Google Scholar
Shenoy N, Kessel R, Bhagat TD, Bhattacharyya S, Yu Y, McMahon C et al . Alterations in the ribosomal machinery in cancer and hematologic disorders. J Hematol Oncol 2012; 5 : 32.
CAS PubMed PubMed Central Google Scholar
Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J et al . Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev 2015; 35 : 225–285.
PubMed Google Scholar
Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M . The structure of the eukaryotic ribosome at 3.0 A resolution. Science 2011; 334 : 1524–1529.
CAS PubMed Google Scholar
Khatter H, Myasnikov AG, Natchiar SK, Klaholz BP . Structure of the human 80 S ribosome. Nature 2015; 520 : 640–645.
Brar GA, Weissman JS . Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol 2015; 16 : 651–664.
Nakhoul H, Ke J, Zhou X, Liao W, Zeng SX, Lu H . Ribosomopathies: mechanisms of disease. Clin Med Insights Blood Disorders 2014; 7 : 7–16.
Danilova N, Gazda HT . Ribosomopathies: how a common root can cause a tree of pathologies. Dis Models Mech 2015; 8 : 1013–1026.
CAS Google Scholar
Teng T, Thomas G, Mercer CA . Growth control and ribosomopathies. Curr Opin Genet Dev 2013; 23 : 63–71.
Stumpf CR, Ruggero D . The cancerous translation apparatus. Curr Opin Genet Dev 2011; 21 : 474–483.
Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ et al . A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 2017; 23 : 461–471.
Wang C, Cigliano A, Jiang L, Li X, Fan B, Pilo MG et al . 4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice. Hepatology (Baltimore, MD) 2015; 61 : 200–213.
Google Scholar
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S et al . Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011; 140 : 1071–1083.
Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J et al . Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology (Baltimore, MD) 2013; 57 : 140–151.
Ruan Y, Sun L, Hao Y, Wang L, Xu J, Zhang W et al . Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J Clin Invest 2012; 122 : 2554–2566.
Lian Z, Liu J, Li L, Li X, Tufan NL, Wu MC et al . Human S15a expression is upregulated by hepatitis B virus X protein. Mol Carcinog 2004; 40 : 34–46.
Xu M, Wang Y, Chen L, Pan B, Chen F, Fang Y et al . Down-regulation of ribosomal protein S15A mRNA with a short hairpin RNA inhibits human hepatic cancer cell growth in vitro . Gene 2014; 536 : 84–89.
Zeng M, Zheng M, Lu D, Wang J, Jiang W, Sha O . Anti-tumor activities and apoptotic mechanism of ribosome-inactivating proteins. Chin J Cancer 2015; 34 : 325–334.
Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M . One core, two shells: bacterial and eukaryotic ribosomes. Nat Struct Mol Biol 2012; 19 : 560–567.
Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI . The multifunctional nucleolus. Nat Rev Mol Cell Biol 2007; 8 : 574–585.
Gruschke S, Ott M . The polypeptide tunnel exit of the mitochondrial ribosome is tailored to meet the specific requirements of the organelle. BioEssays 2010; 32 : 1050–1057.
Desai N, Brown A, Amunts A, Ramakrishnan V . The structure of the yeast mitochondrial ribosome. Science 2017; 355 : 528–531.
Ramesh M, Woolford JL Jr . Eukaryote-specific rRNA expansion segments function in ribosome biogenesis. RNA 2016; 22 : 1153–1162.
Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M . The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA 2008; 14 : 1918–1929.
Orelle C, Carlson ED, Szal T, Florin T, Jewett MC, Mankin AS . Protein synthesis by ribosomes with tethered subunits. Nature 2015; 524 : 119–124.
Zeng F, Chen Y, Remis J, Shekhar M, Phillips JC, Tajkhorshid E et al . Structural basis of co-translational quality control by ArfA and RF2 bound to ribosome. Nature 2017; 541 : 554–557.
Hinnebusch AG, Lorsch JR . The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harbor Perspect Biol 2012; 4 : pii: a011544.
PubMed PubMed Central Google Scholar
Orsolic I, Jurada D, Pullen N, Oren M, Eliopoulos AG, Volarevic S . The relationship between the nucleolus and cancer: current evidence and emerging paradigms. Semin Cancer Biol 2016; 37-38 : 36–50.
Ruggero D . Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Sci Signal 2012; 5 : pe38.
Volarevic S, Stewart MJ, Ledermann B, Zilberman F, Terracciano L, Montini E et al . Proliferation, but not growth, blocked by conditional deletion of 40 S ribosomal protein S6. Science 2000; 288 : 2045–2047.
Kim JH, You KR, Kim IH, Cho BH, Kim CY, Kim DG . Over-expression of the ribosomal protein L36a gene is associated with cellular proliferation in hepatocellular carcinoma. Hepatology (Baltimore, MD) 2004; 39 : 129–138.
Donati G, Montanaro L, Derenzini M . Ribosome biogenesis and control of cell proliferation: p53 is not alone. Cancer Res 2012; 72 : 1602–1607.
Wang H, Zhao LN, Li KZ, Ling R, Li XJ, Wang L . Overexpression of ribosomal protein L15 is associated with cell proliferation in gastric cancer. BMC Cancer 2006; 6 : 91.
Zhan Y, Melian NY, Pantoja M, Haines N, Ruohola-Baker H, Bourque CW et al . Dystroglycan and mitochondrial ribosomal protein L34 regulate differentiation in the Drosophila eye. PLoS One 2010; 5 : e10488.
Da Costa L, Narla G, Willig TN, Peters LL, Parra M, Fixler J et al . Ribosomal protein S19 expression during erythroid differentiation. Blood 2003; 101 : 318–324.
He H, Sun Y . Ribosomal protein S27L is a direct p53 target that regulates apoptosis. Oncogene 2007; 26 : 2707–2716.
Jang CY, Lee JY, Kim J . RpS3, a DNA repair endonuclease and ribosomal protein, is involved in apoptosis. FEBS Lett 2004; 560 : 81–85.
Hegde V, Wang M, Deutsch WA . Human ribosomal protein S3 interacts with DNA base excision repair proteins hAPE/Ref-1 and hOGG1. Biochemistry 2004; 43 : 14211–14217.
Kim J, Chubatsu LS, Admon A, Stahl J, Fellous R, Linn S . Implication of mammalian ribosomal protein S3 in the processing of DNA damage. J Biol Chem 1995; 270 : 13620–13629.
Yang ZY, Jiang H, Qu Y, Wei M, Yan M, Zhu ZG et al . Metallopanstimulin-1 regulates invasion and migration of gastric cancer cells partially through integrin beta4. Carcinogenesis 2013; 34 : 2851–2860.
Narla A, Ebert BL . Ribosomopathies: human disorders of ribosome dysfunction. Blood 2010; 115 : 3196–3205.
Fumagalli S, Thomas G . The role of p53 in ribosomopathies. Semin Hematol 2011; 48 : 97–105.
Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I et al . The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 1999; 21 : 169–175.
Sutcliffe JE, Brown TR, Allison SJ, Scott PH, White RJ . Retinoblastoma protein disrupts interactions required for RNA polymerase III transcription. Mol Cell Biol 2000; 20 : 9192–9202.
Donati G, Bertoni S, Brighenti E, Vici M, Trere D, Volarevic S et al . The balance between rRNA and ribosomal protein synthesis up- and downregulates the tumour suppressor p53 in mammalian cells. Oncogene 2011; 30 : 3274–3288.
Zhou X, Liao WJ, Liao JM, Liao P, Lu H . Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol 2015; 7 : 92–104.
van Riggelen J, Yetil A, Felsher DW . MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 2010; 10 : 301–309.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A . Global cancer statistics, 2012. CA Cancer J Clin 2015; 65 : 87–108.
Whittaker S, Marais R, Zhu AX . The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010; 29 : 4989–5005.
Shukla SK, Kumar V . Hepatitis B virus X protein and c-Myc cooperate in the upregulation of ribosome biogenesis and in cellular transformation. FEBS J 2012; 279 : 3859–3871.
Fatima G, Mathan G, Kumar V . The HBx protein of hepatitis B virus regulates the expression, intracellular distribution and functions of ribosomal protein S27a. J Gen Virol 2012; 93 : 706–715.
Raychaudhuri S, Fontanes V, Barat B, Dasgupta A . Activation of ribosomal RNA transcription by hepatitis C virus involves upstream binding factor phosphorylation via induction of cyclin D1. Cancer Res 2009; 69 : 2057–2064.
Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA . Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol 2005; 7 : 295–302.
Sollner-Webb B, Tower J . Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem 1986; 55 : 801–830.
Yu F, Shen X, Fan L, Yu Z . Analysis of histone modifications at human ribosomal DNA in liver cancer cell. Sci Rep 2015; 5 : 18100.
Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V . A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci 2007; 64 : 29–49.
Wang HD, Trivedi A, Johnson DL . Regulation of RNA polymerase I-dependent promoters by the hepatitis B virus X protein via activated Ras and TATA-binding protein. Mol Cell Biol 1998; 18 : 7086–7094.
Ahuja R, Kapoor NR, Kumar V . The HBx oncoprotein of hepatitis B virus engages nucleophosmin to promote rDNA transcription and cellular proliferation. Biochim Biophys Acta 2015; 1853 : 1783–1795.
Gentilella A, Kozma SC, Thomas G . A liaison between mTOR signaling, ribosome biogenesis and cancer. Biochim Biophys Acta 2015; 1849 : 812–820.
Zinzalla V, Stracka D, Oppliger W, Hall MN . Activation of mTORC2 by association with the ribosome. Cell 2011; 144 : 757–768.
Iadevaia V, Liu R, Proud CG . mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 2014; 36 : 113–120.
Li W, Tan D, Zhang Z, Liang JJ, Brown RE . Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep 2008; 20 : 713–719.
Magnuson B, Ekim B, Fingar DC . Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J 2012; 441 : 1–21.
Plas DR, Thomas G . Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol 2009; 21 : 230–236.
Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M . mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res 2004; 10 : 8421–8425.
Baba HA, Wohlschlaeger J, Cicinnati VR, Hilgard P, Lang H, Sotiropoulos GC et al . Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int 2009; 29 : 399–405.
Ching YP, Wong CM, Chan SF, Leung TH, Ng DC, Jin DY et al . Deleted in liver cancer (DLC) 2 encodes a RhoGAP protein with growth suppressor function and is underexpressed in hepatocellular carcinoma. J Biol Chem 2003; 278 : 10824–10830.
Leung TH, Yam JW, Chan LK, Ching YP, Ng IO . Deleted in liver cancer 2 suppresses cell growth via the regulation of the Raf-1-ERK1/2-p70S6K signalling pathway. Liver Int 2010; 30 : 1315–1323.
Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR et al . Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol 1999; 17 : 676–682.
Sengupta J, Nilsson J, Gursky R, Spahn CM, Nissen P, Frank J . Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol 2004; 11 : 957–962.
McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ . The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol 2002; 62 : 1261–1273.
Wu J, Meng J, Du Y, Huang Y, Jin Y, Zhang J et al . RACK1 promotes the proliferation, migration and invasion capacity of mouse hepatocellular carcinoma cell line in vitro probably by PI3K/Rac1 signaling pathway. Biomed Pharmacother 2013; 67 : 313–319.
Wang WD, Wen Z, Ji W, Ma Y . RACK1 expression contributes to JNK activity, but JNK activity does not enhance RACK1 expression in hepatocellular carcinoma SMMC-7721 cells. Oncol Lett 2015; 9 : 2767–2770.
Zhou S, Cao H, Zhao Y, Li X, Zhang J, Hou C et al . RACK1 promotes hepatocellular carcinoma cell survival via CBR1 by suppressing TNF-alpha-induced ROS generation. Oncol Lett 2016; 12 : 5303–5308.
Zhou T, Lv X, Guo X, Ruan B, Liu D, Ding R et al . RACK1 modulates apoptosis induced by sorafenib in HCC cells by interfering with the IRE1/XBP1 axis. Oncol Rep 2015; 33 : 3006–3014.
Akiyama N, Matsuo Y, Sai H, Noda M, Kizaka-Kondoh S . Identification of a series of transforming growth factor beta-responsive genes by retrovirus-mediated gene trap screening. Mol Cell Biol 2000; 20 : 3266–3273.
Lavoie C, Tam R, Clark M, Lee H, Sonenberg N, Lasko P . Suppression of a temperature-sensitive cdc33 mutation of yeast by a multicopy plasmid expressing a Drosophila ribosomal protein. J Biol Chem 1994; 269 : 14625–14630.
Jimenez L, Becerra A, Landa A . Cloning, expression and partial characterization of a gene encoding the S15a ribosomal protein of Taenia solium. Parasitol Res 2004; 92 : 414–420.
Chen J, Wei Y, Feng Q, Ren L, He G, Chang W et al . Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int J Ocol 2016; 48 : 1628–1638.
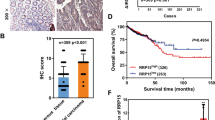
Song MJ, Jung CK, Park CH, Hur W, Choi JE, Bae SH et al . RPL36 as a prognostic marker in hepatocellular carcinoma. Pathol Int 2011; 61 : 638–644.
Kowalczyk P, Woszczynski M, Ostrowski J . Increased expression of ribosomal protein S2 in liver tumors, posthepactomized livers, and proliferating hepatocytes in vitro . Acta Biochim Pol 2002; 49 : 615–624.
Pogue-Geile K, Geiser JR, Shu M, Miller C, Wool IG, Meisler AI et al . Ribosomal protein genes are overexpressed in colorectal cancer: isolation of a cDNA clone encoding the human S3 ribosomal protein. Mol Cell Biol 1991; 11 : 3842–3849.
Kasai H, Nadano D, Hidaka E, Higuchi K, Kawakubo M, Sato TA et al . Differential expression of ribosomal proteins in human normal and neoplastic colorectum. J Histochem Cytochem 2003; 51 : 567–574.
Kobayashi T, Sasaki Y, Oshima Y, Yamamoto H, Mita H, Suzuki H et al . Activation of the ribosomal protein L13 gene in human gastrointestinal cancer. Int J Mol Med 2006; 18 : 161–170.
Huang CJ, Chien CC, Yang SH, Chang CC, Sun HL, Cheng YC et al . Faecal ribosomal protein L19 is a genetic prognostic factor for survival in colorectal cancer. J Cell Mol Med 2008; 12 : 1936–1943.
Huang CJ, Yang SH, Lee CL, Cheng YC, Tai SY, Chien CC . Ribosomal protein S27-like in colorectal cancer: a candidate for predicting prognoses. PLoS One 2013; 8 : e67043.
Guo X, Shi Y, Gou Y, Li J, Han S, Zhang Y et al . Human ribosomal protein S13 promotes gastric cancer growth through down-regulating p27(Kip1). J Cell Mol Med 2011; 15 : 296–306.
Wu Q, Gou Y, Wang Q, Jin H, Cui L, Zhang Y et al . Downregulation of RPL6 by siRNA inhibits proliferation and cell cycle progression of human gastric cancer cell lines. PLoS ONE 2011; 6 : e26401.
Du J, Shi Y, Pan Y, Jin X, Liu C, Liu N et al . Regulation of multidrug resistance by ribosomal protein l6 in gastric cancer cells. Cancer Biol Ther 2005; 4 : 242–247.
Zhang Y, Shi Y, Li X, Du W, Luo G, Gou Y et al . Inhibition of the p53-MDM2 interaction by adenovirus delivery of ribosomal protein L23 stabilizes p53 and induces cell cycle arrest and apoptosis in gastric cancer. J Gene Med 2010; 12 : 147–156.
Shi Y, Zhai H, Wang X, Han Z, Liu C, Lan M et al . Ribosomal proteins S13 and L23 promote multidrug resistance in gastric cancer cells by suppressing drug-induced apoptosis. Exp Cell Res 2004; 296 : 337–346.
Li C, Ge M, Yin Y, Luo M, Chen D . Silencing expression of ribosomal protein L26 and L29 by RNA interfering inhibits proliferation of human pancreatic cancer PANC-1 cells. Mol Cell Biochem 2012; 370 : 127–139.
Muro S, Miyake Y, Kato H, Tsutsumi K, Yamamoto K . Serum anti-60 S ribosomal protein L29 antibody as a novel prognostic marker for unresectable pancreatic cancer. Digestion 2015; 91 : 164–173.
Milosevic N, Kühnemuth B, Mühlberg L, Ripka S, Griesmann H, Lölkes C et al . Synthetic lethality screen identifies RPS6KA2 as modifier of epidermal growth factor receptor activity in pancreatic cancer. Neoplasia 2013; 15 : 1354–1362.
Wei F, Ding L, Wei Z, Zhang Y, Li Y, Qinghua L et al . Ribosomal protein L34 promotes the proliferation, invasion and metastasis of pancreatic cancer cells. Oncotarget 2016; 7 : 85259–85272.
Ray S, Johnston R, Campbell DC, Nugent S, McDade SS, Waugh D et al . Androgens and estrogens stimulate ribosome biogenesis in prostate and breast cancer cells in receptor dependent manner. Gene 2013; 526 : 46–53.
Thakur A, Sun Y, Bollig A, Wu J, Biliran H, Banerjee S et al . Anti-invasive and antimetastatic activities of ribosomal protein S6 kinase 4 in breast cancer cells. Clin Cancer Res 2008; 14 : 4427–4436.
Wang S, Huang J, He J, Wang A, Xu S, Huang S-F et al . RPL41, a small ribosomal peptide deregulated in tumors, is essential for mitosis and centrosome integrity. Neoplasia 2010; 12 : 284–IN288.
Bee A, Ke Y, Forootan S, Lin K, Beesley C, Forrest SE et al . Ribosomal protein l19 is a prognostic marker for human prostate cancer. Clin Cancer Res 2006; 12 : 2061–2065.
Bee A, Brewer D, Beesley C, Dodson A, Forootan S, Dickinson T et al . siRNA knockdown of ribosomal protein gene RPL19 abrogates the aggressive phenotype of human prostate cancer. PLoS One 2011; 6 : e22672.
Wang M, Hu Y, Stearns ME . RPS2: a novel therapeutic target in prostate cancer. J Exp Clin Cancer Res 2009; 28 : 6.
Shen F, Yan C, Liu M, Feng Y, Chen Y . RACK1 promotes prostate cancer cell proliferation, invasion and metastasis. Mol Med Rep 2013; 8 : 999–1004.
McDonald JM, Pelloski CE, Ledoux A, Sun M, Raso G, Komaki R et al . Elevated phospho-S6 expression is associated with metastasis in adenocarcinoma of the lung. Clin Cancer Res 2008; 14 : 7832–7837.
Yang M, Sun H, Wang H, Zhang S, Yu X, Zhang L . Down-regulation of ribosomal protein L22 in non-small cell lung cancer. Med Oncol 2013; 30 : 646.
Rao S, Lee SY, Gutierrez A, Perrigoue J, Thapa RJ, Tu Z et al . Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood 2012; 120 : 3764–3773.
Ni JQ, Liu LP, Hess D, Rietdorf J, Sun FL . Drosophila ribosomal proteins are associated with linker histone H1 and suppress gene transcription. Genes Dev 2006; 20 : 1959–1973.
Lin KY, Tai C, Hsu JC, Li CF, Fang CL, Lai HC et al . Overexpression of nuclear protein kinase CK2 alpha catalytic subunit (CK2alpha) as a poor prognosticator in human colorectal cancer. PLoS One 2011; 6 : e17193.
Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O'Brien SE et al . CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res 2010; 70 : 10288–10298.
Yang M, Sun H, He J, Wang H, Yu X, Ma L et al . Interaction of ribosomal protein L22 with casein kinase 2alpha: a novel mechanism for understanding the biology of non-small cell lung cancer. Oncol Rep 2014; 32 : 139–144.
Chan MW, Wei SH, Wen P, Wang Z, Matei DE, Liu JC et al . Hypermethylation of 18 S and 28 S ribosomal DNAs predicts progression-free survival in patients with ovarian cancer. Clin Cancer Res 2005; 11 : 7376–7383.
Tsofack SP, Meunier L, Sanchez L, Madore J, Provencher D, Mes-Masson AM et al . Low expression of the X-linked ribosomal protein S4 in human serous epithelial ovarian cancer is associated with a poor prognosis. BMC Cancer 2013; 13 : 303.
Kim SH, Jang YH, Chau GC, Pyo S, Um SH . Prognostic significance and function of phosphorylated ribosomal protein S6 in esophageal squamous cell carcinoma. Mod Pathol 2013; 26 : 327–335.
Hagner PR, Mazan-Mamczarz K, Dai B, Balzer EM, Corl S, Martin SS et al . Ribosomal protein S6 is highly expressed in non-Hodgkin lymphoma and associates with mRNA containing a 5' terminal oligopyrimidine tract. Oncogene 2011; 30 : 1531–1541.
Download references
Acknowledgements
This work was supported by the grants from the Natural Science Foundation of Zhejiang Province (LY17H160047), the National Natural Science Foundation of China(81201953, 81772628, 81703310) and the Research Found for the Doctoral Program of High Education of China from the Ministry of Education (20113321120003).
Author information
Authors and affiliations.
Department of Hepatobiliary Surgery, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
X Xie, P Guo, H Yu & G Chen
Research Center of Evidence-Based Medicine and Clinical Epidemiology, School of Public Health and Management, Wenzhou Medical University, Wenzhou, China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to G Chen .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Xie, X., Guo, P., Yu, H. et al. Ribosomal proteins: insight into molecular roles and functions in hepatocellular carcinoma. Oncogene 37 , 277–285 (2018). https://doi.org/10.1038/onc.2017.343
Download citation
Received : 18 April 2017
Revised : 21 June 2017
Accepted : 14 August 2017
Published : 25 September 2017
Issue Date : 18 January 2018
DOI : https://doi.org/10.1038/onc.2017.343
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Ribosomal protein l34 is a potential prognostic biomarker and therapeutic target in hilar cholangiocarcinoma.
- Jianxin Qian
Cell & Bioscience (2020)
DNA methylation signature of smoking in lung cancer is enriched for exposure signatures in newborn and adult blood
- K. M. Bakulski
- J. A. Colacino
Scientific Reports (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Conclusion: General Principles of Ribosome Structure and Function
Cite this chapter.

- Alexander S. Spirin 3
Part of the book series: Cellular Organelles ((CORG))
198 Accesses
In this concluding chapter an attempt to formulate several general principles of the structure and function of the ribosome is undertaken. Our understanding of the ribosome is far from complete, and the formulations reflect only the current level of knowledge in this area. Concerning the ribosome structure, the principles formulated can be considered as a summary of factual information and its generalization, whereas the principles of the function are rather hypothetical and represent just plausible models. Nevertheless, this seems to be the first attempt to give a generalized conceptual vision of the ribosome and to coordinate the structure and the function. From both a scientific and educational point of view, such tentative formulations may be a useful appendix to the main course of experimental ribosomology.
- Ribosomal Protein
- Large Subunit
- Small Subunit
- Ribosomal Subunit
- Large Ribosomal Subunit
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Author information
Authors and affiliations.
Russian Academy of Sciences, Moscow, Russia
Alexander S. Spirin
You can also search for this author in PubMed Google Scholar

Rights and permissions
Reprints and permissions
Copyright information
© 1999 Kluwer Academic/Plenum Publishers, New York
About this chapter
Spirin, A.S. (1999). Conclusion: General Principles of Ribosome Structure and Function. In: Ribosomes. Cellular Organelles. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-7817-8_19
Download citation
DOI : https://doi.org/10.1007/978-1-4615-7817-8_19
Publisher Name : Springer, Boston, MA
Print ISBN : 978-0-306-46146-0
Online ISBN : 978-1-4615-7817-8
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Ribosome Structure, Function, and Early Evolution
Kristopher opron.
1 Bioinformatics Core, University of Michigan, Ann Arbor, MI 48109-0674, USA; moc.liamg@norpok
Zachary F. Burton
2 Department of Biochemistry and Molecular Biology, 603 Wilson Rd., Michigan State University, MI 48824-1319, USA
Ribosomes are among the largest and most dynamic molecular motors. The structure and dynamics of translation initiation and elongation are reviewed. Three ribosome motions have been identified for initiation and translocation. A swivel motion between the head/beak and the body of the 30S subunit was observed. A tilting dynamic of the head/beak versus the body of the 30S subunit was detected using simulations. A reversible ratcheting motion was seen between the 30S and the 50S subunits that slide relative to one another. The 30S–50S intersubunit contacts regulate translocation. IF2, EF-Tu, and EF-G are homologous G-protein GTPases that cycle on and off the same site on the ribosome. The ribosome, aminoacyl-tRNA synthetase (aaRS) enzymes, transfer ribonucleic acid (tRNA), and messenger ribonucleic acid (mRNA) form the core of information processing in cells and are coevolved. Surprisingly, class I and class II aaRS enzymes, with distinct and incompatible folds, are homologs. Divergence of class I and class II aaRS enzymes and coevolution of the genetic code are described by analysis of ancient archaeal species.
1. Introduction
We offer a general and conceptual review of translation in prokaryotic systems. We concentrate on prokaryotes in order to correlate ribosome structure and function with the earliest evolution of translation systems. Ribosomes are considered in bacterial systems, using the Thermus thermophilus ribosome as an example, and numbering throughout is for T. thermophilus . Discussion of transfer ribonucleic acid (tRNA), genetic code, and aminoacyl-tRNA synthetase (aaRS) evolution relies on ancient archaeal species because these functions are the most primitive in archaea. Although some factors have been substituted in evolution, translation mechanisms are similar in archaeal, bacterial, and eukaryotic systems. In our discussion, we stress the homologous binding and functions of IF2, EF-Tu, and EF-G G-protein GTPases in translation initiation and elongation. As part of an ancient pre-ribosome, the 30S subunit and the decoding center appear to be older than an associated 50S subunit, and the 50S subunit may have been recruited from a captured, formerly mobile peptidyl transferase center (PTC). The messenger ribonucleic acid (mRNA) and tRNAs lie across the neck of the 30S subunit between the body and the head/beak. The head/beak reversibly swivels and/or tilts relative to the body to function as a translocation ratchet. The 30S and 50S subunits reversibly rotate relative to one another to form an additional ratchet for elongation and initiation. Interactions between the 30S and 50S subunits and events at the A-site latch regulate translocation. A channel forms between the 30S and the 50S subunits, allowing tRNAs to align, advance, and pass through without major obstructions. We describe translation initiation and elongation using images taken from recent structures. Characteristics of tRNAs that make a relatively stiff and efficient translation adapter are discussed. Translation systems appear to have evolved around tRNA. The referencing in this review is not exhaustive, and we apologize to anyone whose original work we may have overlooked. Excellent and more detailed reviews are available for initiation [ 1 ], elongation [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 ], and translocation [ 10 , 11 ], and we refer the reader to these for a more comprehensive referencing of original literature.
2. Initiation of Translation
2.1. homologous gtpases in initiation and elongation of translation.
Through the initiation and elongation phases of translation, homologous G-protein GTPases IF2, EF-Tu and EF-G play a central role [ 1 , 10 , 12 , 13 , 14 ]. A homology search shows that these three G-proteins are among the four closest homologs in T. thermophilus , along with elongation factor 4, an apparent EF-G mimic [ 15 ]. These GTPases interact homologously with the ribosome, particularly with the GTPase-associated complex (GAC) and the sarcin-ricin loop (SRL) of the 50S subunit. An overlay of relevant structures shows the G-proteins and their homologous residues binding to the same site on the ribosome (see below).
2.2. Mechanism of Initiation
Initially, the 30S and 50S ribosomal subunits are dissociated. The initiation complex can form on the 30S subunit before recruitment of the 50S subunit [ 1 ], possibly indicating that the 30S ribosomal subunit and its decoding center may, in some sense, be older evolutionarily than the 50S subunit. To bring the 30S and 50S subunits together involves: (1) assembly of initiation factors IF1, IF2, and IF3; (2) the binding of the initiator fMet-tRNA fMet (acylated with N -formyl-methionine); and/or (3) the binding of the mRNA on the 30S subunit [ 16 , 17 ]. There may be three primary translocation ratchets for the ribosome. Within the 30S subunit, the head/beak swivels relative to the body. Also, the 30S head/beak can tilt relative to the 30S body. The 50S subunit rotates reversibly relative to the 30S subunit during initiation and elongation. Dynamic motions of the ribosome are important for positioning fMet-tRNA fMet for initiation and also for the stepwise translocation of the mRNA and tRNAs during elongation.
Multiple pathways can lead to assembly of the initiation complex ( Figure 1 and Figure 2 ). A Shine-Dalgarno sequence on mRNA can bind to the ribosome independent of initiation factors and fMet-tRNA fMet [ 1 , 18 ]. The images in Figure 1 indicate the formation of the SD-ASD (Shine-Dalgarno-anti-Shine-Dalgarno) interaction [ 18 ]. An SD on mRNA (e.g., AGGA) pairs with the ASD near the 3′ end of the 16S rRNA (1537-UCCU) [ 19 ], helping to position the mRNA AUG initiation codon at the ribosome P-site. The SD-ASD interaction persists through the assembly of the 50S subunit and early elongation. mRNAs that lack a Shine-Dalgarno sequence can be translated, but these mRNAs probably require previous assembly of IF1, IF2, IF3, and fMet-tRNA fMet . The fMet-tRNA fMet is held in the P-site of the 30S subunit by the initiation factors ( Figure 2 ) [ 16 , 17 ]. IF1 appears to block the A-site, preventing assembly of an initiation complex before the 50S subunit recruitment. IF1 helps to bind, orient, and position IF2 and IF3. The NTD ( N -terminal domain) and CTD (C-terminal domain) of IF3 span the ribosome P-site to position and hold the fMet-tRNA fMet in place. The NTD of IF-3 blocks the E-site to prevent E-site tRNA binding. The CTD of IF3 is highly dynamic in its interactions with the 30S subunit and appears to have a role in positioning the P-site fMet-tRNA fMet . Assembly of intermediate complexes stimulates the swiveling of the 30S subunit head/beak [ 17 ]. Assembly with the 50S subunit induces relative rotation of the 50S and 30S subunits, helping to accommodate the P-site fMet-tRNA fMet for initiation [ 20 ]. Presumably, assembly with the 50S subunit and the release of initiation factors set up conditions to recruit the A-site aa-tRNA, which will enter bound to the IF2 homolog EF-Tu, after the IF2 release, and rotate into the fully accommodated A-site to initiate peptide bond synthesis.

The Shine-Dalgarno-anti-Shine-Dalgarno (SD-ASD) contact in translation initiation on the 30S ribosomal subunit; ( A ) messenger ribonucleic acid (mRNA, red) with an SD sequence binds the ASD (green) near the 3′-end of the 16S rRNA (PDB 1JGQ). The 16S rRNA is beige (body) and black (head/beak). mRNA lies across the neck. tRNA fMet (blue) binds in the P-site. Ribosomal proteins are white; ( B ) detail of the SD-ASD [red-green (carbons)] interaction (PDB 4V4Z).

Formation of a pre-initiation complex on the 30S ribosomal subunit (PDB 5LMV). IF1 (magenta), IF2 (blue), and IF3 (cyan) are present. The fMet-tRNA fMet (yellow) is bound in the P-site. Other colors are as in Figure 1 . The fMet of the P-site fMet-tRNA fMet bound to IF2 is in the space-filling representation.
3. Elongation of Translation
3.1. molecular motor.
Considered as a molecular motor, the ribosome appears to be driven by a complex thermal ratchet translocation mechanism with an overall step length of ~14 angstroms [1 codon (3 nt) on an extended mRNA] [ 21 ]. Because the mRNA is read in the 5′→3′ direction, the mRNA threads through the ribosome in the 3′→5′ direction during stepwise translocation. The ribosome A- (aminoacyl), P- (peptidyl), and E- (exit) tRNA sites are located at the interface (neck) between the 30S subunit body and the head/beak. The mRNA threads through the same crevice (across the neck) in the 16S rRNA as if threaded halfway around a spool. Thus, the mRNA is a neckless (half a choke chain) and the A-, P-, and E-site tRNAs are baubles attached to the chain [ 22 ]. There is an ~18° swiveling motion of the 16S rRNA head and beak versus the body (rotation and/or displacement of the neck) that drives ribosome bound tRNAs into hybrid states and then slides back versus the mRNA that stays in an advanced position. Acting as pawls, tRNA 3′-CCA and codon-anticodon [mRNA-ASL (anticodon stem-loop)] interactions appear to maintain the directionality and phase of the ratchet. The exiting peptide chain may also function as a pawl for translocation [ 23 ]. Another aspect of the ratchet is a reversible ~7° rotation of the 30S versus the 50S ribosomal subunit, which appears to assist mRNA and tRNA displacement [ 22 , 23 ]. Observed from above the 50S subunit looking toward the 30S subunit beneath, forward translocation is a counterclockwise rotation of the 30S subunit relative to the 50S subunit. The ribosome is a weak molecular machine that generates only ~13±2 pN (pico Newton) of force [ 21 ].
Translation elongation is described in a schematic ( Figure 3 ). As noted above, IF2, EF-Tu, and EF-G are G-protein GTPase homologs that occupy the same site on the ribosome, thus only one of these initiation or elongation factors can be present in a particular intermediate. Therefore, during each peptide bond addition cycle, EF-Tu and EF-G must cycle on and off the ribosome. In Figure 4 , ribosome structures were overlaid for ribosomal protein S2, and EF-Tu·GTP and EF-G·GDP from each ribosome structure precisely align for homologous residues and the GTP/GDP-binding site. As noted above, IF2, EF-Tu, and EF-G comprise a set of the closest homologs in T. thermophilus . In Figure 3 , intermediates A and B describe the aa-tRNA·EF-Tu·GTP ternary complex entry to the ribosome. Intermediates C and D describe the EF-Tu proofreading of the codon-anticodon interaction and elbow accommodation (see below). Intermediates C, D, and E describe the tRNA A-site CCA accommodation, which is entry of the A-site aa-tRNA into proximity to the P-site peptidyl-tRNA for peptidyl transfer. Intermediates E and F describe peptidyl transfer in the presence of EF-G·GTP. Intermediates G and H describe translocation in the presence of EF-G leading to EF-G release. Hybrid tRNA states are indicated as the (codon-anticodon position)/(the 3′-CCA position), i.e., pe/E with the mRNA-ASL in a pe hybrid state (p→e) and the 3′-CCA in the full E-state ( Table 1 ). In Figure 3 , some intermediates are highlighted with images from cryo-electron microscopy and X-ray crystallography. The rate of each amino acid addition is ~7 s −1 , and accommodation appears to be the rate-limiting step [ 24 , 25 , 26 ]. Defining contacts for the tRNA anticodon loops and 3′-CCA ends are recorded in Table 1 and in the text.

Stages of the translation elongation cycle; ( A , B ) binding of the aa-tRNA·EF-Tu·GTP ternary complex to the A/T site; ( C ) conformational closing of the 30S subunit and forming the codon-anticodon A-site latch; ( D ) elbow accommodation of aa-tRNA; ( E ) full CCA accommodation of aa-tRNA to the A/A-site, release of EF-Tu·GDP, entry of EF-G·GTP; ( F ) peptidyl transfer; ( G ) EF-G·GTP→GDP and onset of translocation, opening of the codon-anticodon latch, formation of hybrid tRNA states pe/E and ap/A or ap/ap; ( H ) full forward and reverse translocation. Some intermediate x-ray or cryo-electron microscopy structures are shown.

EF-Tu and EF-G are homologs that occupy the same site on the ribosome; ( A ) overlay of ribosome-EF-Tu·GTP (green; PDB 5UYM) and ribosome-EF-G·GDP (red; PDB 4V5M). Overlays were done for ribosomal protein S2. Ribosomes are omitted from the images for simplicity; ( B ) EF-G·GDP structure (red); ( C ) EF-Tu·GTP structure (green). At the right, an alignment of Thermus thermophilus EF-G, EF-Tu, and IF2 is shown. Conserved residues (i.e., EF-Tu, Val21, Ile61, and His86) involved in stimulating GTP hydrolysis are indicated. In the alignment, e-values are versus Escherichia coli EF-G.
Phases of the Translation Elongation Cycle.
Cplx: complex; elbow or EA: elbow accommodation; CCA: CCA accommodation. Steps that are most important for translational accuracy are indicated with exclamation points.
3.2. tRNA as a Relatively Stiff Adapter
The tRNA folds into an L-shape in solution. The bend of the L is the elbow, at which the D loop and T loop interact to form a relatively stiff joint. One end of the L is the anticodon (Ac) loop and the other is the 3′-CCA end to which the amino acid is attached. The anticodon loop is a compact 7-mer loop with a U-turn between the 2nd and 3rd loop bases [ 27 ]. The 7-mer U-turn loop is essential to present a 3 nt anticodon. A 6- or 8-mer loop, for instance, would not support a U-turn or a 3 nt genetic code. The relative stiffness of tRNA at the elbow and anticodon makes tRNA an adequate adapter for translation.
Distortions of tRNAs, however, occur during the aa-tRNA accommodation [ 28 ] and translocation [ 29 ]. tRNA navigates a channel through the ribosome that is approximately the dimensions of a tRNA, but minor obstructions are encountered. Interactions of aa-tRNA with EF-Tu induce tRNA bending [ 28 ]. Interaction with the 50S helix89 (h89) during elbow accommodation causes aa-tRNA deformation [ 30 ]. During translocation, the tRNA contacts the A-site finger [ 31 ] and other micro-pawls [ 32 ]. The 3′-CCA-aa end of tRNA is single-stranded and flexible. During CCA accommodation, the 3′-CCA-aa end must navigate to the appropriate position in the PTC [ 30 , 33 ].
During codon-anticodon latching and A/A-site tRNA accommodation and translocation, tRNA mostly maintains its characteristic L-shape and rotates to assume new positions. tRNA binds the ribosome as an aa-tRNA·EF-Tu·GTP ternary complex with its aminoacylated end (3′-CCA-aa) bound to EF-Tu [the A/T state (aminoacyl/Ternary Complex)] [ 34 ] and rotates into the A/A-site (fully accommodated) after releasing from EF-Tu·GDP [ 30 , 33 , 35 , 36 ]. The 3′-CCA-aa end of the A/A-site tRNA is flexible and must penetrate the PTC for peptide synthesis within a dehydrated environment [ 36 , 37 ]. With minor exceptions, therefore, tRNA L-shapes rotate into position rather than undergoing large conformational distortions. Recognition of tRNA rotations between hybrid states is sensed by the ribosome to stimulate sequential events associated with the amino acid addition cycle, some examples being: (1) in transit, tRNAs encounter micro-pawls; (2) the A-site latch opens and closes; (3) the 30S-50S subunit contacts change during rotation; and (4) EF-Tu and EF-G GTPases enter and exit regulating the timing of GTPase activities.
3.3. tRNA Entry
EF-Tu·GTP binds aa-tRNA to form the aa-tRNA·EF-Tu·GTP ternary complex, and this is the form in which aa-tRNA enters the ribosome [ 34 ]. EF-Tu·GTP binding sequesters the 3′-CCA-aa end of the incoming aa-tRNA, thus the aa-tRNA cannot enter the fully accommodated A/A-site directly without first binding accurately within the ternary complex to mRNA and then releasing EF-Tu·GDP. EF-Tu and EF-G are activated for GTPase activity by the ribosome, acting as a GTPase-activating factor (GAF). In particular, contact of aa-tRNA·EF-Tu·GTP with the 23S sarcin-ricin loop [SRL; h95: A2660, G2661, A2662, G2663] and the GTPase-associated complex (GAC; ribosomal protein L11, h42, h43, h44, and A1067) of the 50S ribosomal subunit stimulates the EF-Tu GTPase activity, leading to aa-tRNA and EF-Tu·GDP dissociation and elbow accommodation. From simulations, elbow accommodation appears to be a reversible step that dissociates aa-tRNA from EF-Tu·GDP, but EF-Tu·GDP does not appear to dissociate from the ribosome until full CCA-aa accommodation. The aa-tRNA elbow (the bend of the L) interacts with the 50S GAC, which acts as an allosteric effector to stimulate EF-Tu·GTP→GDP [ 5 , 38 , 39 , 40 , 41 , 42 ]. With initial ternary complex contact to the ribosome, no EF-Tu·GTP contacts are made to the 50S subunit (complex 1, Table 1 ). Closing of the A-site anticodon stem loop (ASL) latch and hybrid A/T-site aa-tRNA rotation brings aa-tRNA·EF-Tu·GTP into contact with the 50S GAC and the SRL, and these contacts stimulate GTP hydrolysis. Dissociation of aa-tRNA from EF-Tu·GDP during elbow accommodation allows subsequent full CCA-aa accommodation and seating of the aa-tRNA for peptidyl transfer. EF-G·GTP stimulates peptide bond formation, indicating replacement of EF-Tu·GDP with EF-G·GTP before peptide bond synthesis. EF-G·GTP→GDP stimulates translocation [ 10 , 22 ].
3.4. Forming the Accurate Codon-Anticodon Latch and Closing the 30S Subunit Conformation
The accuracy of translation requires the tight closing of the A-site codon-anticodon latch to confirm base pairing ( Figure 5 ). An overlay of open and closed latch structures is shown in Figure 5 A. The overlay image is shown to emphasize that mRNA threads along the 16S rRNA neck (h28) between the head/beak and body. An open conformation of the latch is shown in Figure 5 B [ 43 ], and a closed 30S conformation is shown in Figure 5 C [ 44 ]. The latch is comprised of the A-site codon-anticodon helix and the 16S rRNA nucleotides G530 (G530 loop), A1492 and A1493 (h44), and the 23S rRNA nucleotide A1913 (h69). The ribosomal protein S12 interacts with the closed latch. The latch closure involves G530~A1492 H-bonding and G530 H-bonding to tRNA wobble and central anticodon position ribose rings [ 43 ]. Sealing the latch closes the 30S subunit by bringing the 16S rRNA G530 loop next to the 16S rRNA h44 (A1492, A1493). The closing of the A-site latch is communicated to the 50S subunit through interactions of the 30S h44 (A1492, A1493) and the 50S h69 (A1913), h71, and h62.

Closing of the codon-anticodon latch closes the 30S ribosomal subunit; ( A ) overlay of open and closed latch structures. The head and beak are black (16S: 930–1380). The body is white. tRNA sites locate to the cleft between the head/beak and the body; ( B ) the latch is open (complex 1); ( C ) the latch is closed (complex 3). This transition occurs in three stages ( Table 1 ).
By contrast, a near-cognate tRNA generally fails to maintain a closed conformation of the latch and the 30S subunit, releasing and replacing the aa-tRNA·EF-Tu·GTP/GDP ternary complex before inaccurate aa-tRNA CCA accommodation and aa misincorporation can occur. A near-cognate tRNA would be one with a G~U wobble pair in the 1st or 2nd codon position, which correspond to the 2nd or 3rd anticodon position (reading 5′→3′). The codon-anticodon latch, therefore, is tight enough to accurately select Watson-Crick pairs from wobble pairs in the 1st and 2nd codon positions (the 2nd and 3rd anticodon positions). From x-ray crystallography studies, it appears that near cognate G~U pairs within a tightened latch are forced into Watson-Crick geometry, which requires keto → enol tautomerization of either G or U and is not energetically favorable nor kinetically stable, which leads to near-cognate aa-tRNA release [ 44 ]. By contrast, wobble contacts are generally allowed in the wobble position, which is the 3rd codon position (the 1st anticodon position). Because wobble base pairs are tolerated at the wobble position of the codon-anticodon, the maximum complexity of the genetic code in tRNA is 2 × 4 × 4 = 32 anticodons versus 4 × 4 × 4 = 64 codons in mRNA (see below) [ 45 , 46 ]. Generally, therefore, tRNA anticodon geometry and readout limited the potential size of the genetic code, because for the most part—with an exception of tRNA Ile (UAU), which is very rarely used in prokaryotes versus tRNA Met (CAU)—only pyrimidine-purine discrimination is possible at the wobble position.
The mechanism of EF-Tu GTPase activation by the 50S ribosomal subunit is shown in Figure 6 [ 43 ]. The 23S rRNA SRL has a central role because contact with the SRL opens a hydrophobic gate on EF-Tu that is formed by interaction of Ile61 (Switch 1) and Val21 (P loop; G1 box). Opening the hydrophobic gate allows His86 (Switch 2) to approach the GTP γ-phosphate and activate a water molecule to engage in hydrolysis. Because GTP hydrolysis requires the 50S SRL interaction with aa-tRNA·EF-Tu·GTP, which is dependent on the prior 30S subunit closing and sealing the codon-anticodon latch, EF-Tu regulates the accuracy of codon-anticodon binding and latching and is an initial check on translational accuracy. The A-site codon-anticodon latch remains closed through: (1) EF-Tu GTP hydrolysis and dissociation; (2) accommodation of the aa-tRNA into the A/A-site; (3) EF-G·GTP binding; and (4) formation of the peptide bond. The A-site latch, therefore, can be considered to be a powerful pawl in regulating A-site to P-site translocation, because the latch must open to allow this progression. The regulatory and catalytic residues in EF-Tu are conserved in its homologs and replacements EF-G and IF2 ( Figure 4 ), as are contacts on the ribosome to the SRL and GAC.

50S GTPase-associated complex (GAC) and SRL (ball and stick representation) binding to aa-tRNA·EF-Tu·GTP activates His86 to stimulate GTPase activity. Numbering of EF-Tu residues is as in Figure 4 .
3.5. Accommodation
Accommodation requires a large rotation of the A-site aa-tRNA from the A/T-site to the A/A-site (~100 angstrom transition of the incoming amino acid) ( Figure 7 ). Figure 7 A shows an overlay of A/T and A/A structures. Figure 7 B shows the fully accommodated A/A structure. Figure 7 C shows the A/T structure before the release of EF-Tu from aa-tRNA, elbow accommodation, and CCA accommodation.

Accommodation of aa-tRNA from the A/T-site to the A/A-site. This is a multi-step process that includes elbow accommodation and CCA accommodation (see Figure 3 ; Table 1 ); ( A ) overlay of PDB 5IBB (A/A state) and 5UYM (A/T state); ( B ) the fully accommodated A/A state poised for peptidyl bond formation; ( C ) the A/T state before elbow accommodation and CCA accommodation. EF-Tu·GTP is colored magenta.
Accommodation is described in two major steps: (1) elbow accommodation; and (2) CCA accommodation [ 28 , 30 , 33 ]. It appears that EF-Tu·GDP remains associated with the ribosome through aa-tRNA elbow accommodation, which is a major step in proofreading the accuracy of the anticodon-codon attachment. Elbow accommodation indicates that the A-site tRNA elbow (the bend of the L) is positioned proximal (within ~30 angstroms) to the P-site tRNA elbow. To obtain this position, the aa-tRNA elbow must interact and slide against the h89 of the 50S ribosomal subunit, an interaction that is stimulated by the presence of EF-Tu·GDP, although aa-tRNA must dissociate from EF-Tu·GDP to access the elbow accommodated position. The second step is CCA accommodation, which involves the complete seating of the 3′-CCA-aa within the peptidyl transferase center (PTC). For full A/A-site aa-tRNA accommodation, EF-Tu·GDP dissociates from the ribosome, allowing the entry of EF-G·GTP, which stimulates peptidyl transfer.
Once EF-Tu dissociates, the free 3′-CCA-aa end of the A-site tRNA is single-stranded and flexible and must penetrate the PTC [ 30 , 35 , 36 , 37 ]. It appears that this transition may occur via multiple routes. In the A/A-site, the C75 of the aa-tRNA forms a restraining Watson-Crick base pair with 23S G2553 (h92, A-loop, PTC). The C75:G2553 contact can be considered a defining aspect of the A-site tRNA in the PTC. The P-site tRNA is similarly restrained at its 3′-CCA-peptide end by G:C Watson-Crick base pairs (G2252:C74 and G2251:C75). Accommodation is viewed as the primary proofreading step in translation because cognate aa-tRNA survives the long transition but near-cognate and non-cognate aa-tRNAs, which are less stable at the ASL, dissociate [ 2 , 30 , 44 ]. Because EF-Tu appears to associate with the ribosome through both the A-site aa-tRNA latching step and the elbow accommodation steps that are most important for proofreading the ASL-mRNA contact, EF-Tu is a major fidelity factor for translation.
3.6. Peptide Bond Formation
The codon-anticodon A-site latch remains closed through peptidyl bond formation, but opens to enable translocation. CCA accommodation results in a kink in the mRNA between the P-site and A-site tRNAs in order to position the tRNA 3′-ends for peptidyl transfer ( Figure 8 ). After bond formation, the latch relaxes to allow translocation of the A-site tRNA to the P-site. The P-site 3′-CCA-peptidyl-tRNA is restrained by PTC bases (P-loop) G2251 that pairs peptidyl-tRNA C75, and G2252 that pairs C74 (Watson-Crick pairs). A2451, C2452, and U2585 also make defining P-site peptidyl-tRNA contacts. These P-site 3′-CCA-peptide contacts appear to break only after peptidyl transfer, EF-G GTPase activity, and the onset of translocation ( Figure 8 ). Onset of translocation transfers the 3′-CCA end of the deacylated P-site tRNA into the E-site (U2431, A2432) and its anticodon loop into a pe hybrid state [ 23 , 47 ].

EF-G·GDP (green) in a compact conformation in a pre-translocation or catalytic state. The compact conformation may be more indicative of an EF-G·GTP catalytic structure. Note the induced kink or bend in the mRNA at the latch that helps position P-site and A-site tRNAs in sufficient proximity for peptidyl transfer.
Bringing and finally locking the 3′-CCA-aa end of the A/A-site tRNA close to the P-site tRNA peptide in a dehydrated environment with appropriate molecular positioning and crowding is sufficient to support peptide bond synthesis. The peptide chain is transferred from the P-site tRNA to the A-site tRNA, where the peptide chain mostly remains stationed. In the presence of EF-G·GTP/GDP, reversible excursions between A-site and P-site contacts are observed for peptidyl-tRNA, described as A/A, ap/A, and ap/ap hybrid states [ 47 , 48 ]. In the ap/A←→ap/ap hybrid state, G2553 of the A loop (h92) either forms a Watson-Crick base pair to peptidyl-tRNA C75, which is typical of an A-site contact, or G2251:C75 and G2252:C74 P-loop (h80) Watson-Crick pairs are observed, which are typical of a P-site contact. This dynamic conversion appears to continue until late in translocation [ 49 ]. After full translocation, the peptidyl-tRNA resides in the P-site. It may be that a longer peptide chain, which is missing in most structures, could act as a stronger pawl, driving forward translocation of peptidyl-tRNA from the A-site to the P-site.
The PTC is considered to be a molecular crowding and dehydration chamber generally lacking any particular ribozyme activity [ 50 ]. Although the PTC has been described as a ribozyme, it is not a good one [ 49 ]. It has been reasonably suggested that a generalist bond formation function of the PTC helps to support peptidyl transfer using 20 encoded amino acid substrates. Peptide bond formation is stimulated by EF-G·GTP, thus EF-G·GTP enters the complex and contacts the closed latch before peptide bond formation occurs but after the homolog EF-Tu·GDP release from the shared binding site. For cognate aa-tRNA incorporation, accommodation appears to be the overall rate-limiting and proofreading step for translation [ 51 , 52 ].
The ribosome PTC reaction is described as a two pathway mechanism [ 53 , 54 ]. One pathway is the proton shuttle mechanism in which a zwitterionic transition state is generated after A-site aa-tRNA attack. The proton shuttle mechanism appears to be the major route for protein synthesis, at least with the substrates utilized in these studies (i.e., A-site Phe-tRNA Phe and P-site fMet-tRNA fMet ). A high kinetic solvent isotope effect (hydrogen/deuterium exchange) for protein synthesis indicates proton transfers from water, supporting the proton shuttle pathway. Potentially, some amino acid substrates may prefer the alternate reaction pathway, which is general base catalyzed. The pH dependence of the protein synthesis mechanism so far observed, however, is not generally consistent with heavy use of the base-catalyzed path. In contrast to peptide synthesis, translation termination mechanisms utilize the base-catalyzed path, leading to P-site peptidyl-tRNA hydrolysis and peptide chain release mediated by a protein translation release factor [ 54 , 55 , 56 ]. Because translation termination appears to use the base-catalyzed path, and because the base-catalyzed path does not appear to be energetically inaccessible, the base-catalyzed pathway may also be used for some peptide synthesis reactions.
3.7. Translocation
The ribosome has been discussed as a relatively weak molecular motor with a complex thermal translocation ratchet ( Figure 9 and Figure 10 ) [ 21 ]. The ratchet has three primary modes: (1) the 30S head/beak swivel; (2) the 30S head tilt; and (3) the reversible rotation of the 30S and the 50S subunits relative to one another. Other micro-motions are also identified, such as: (1) the opening and closing of the L1 stalk; (2) the movements of the L11 stalk to regulate GTPases; and (3) the 30S subunit h44 dynamics. In Figure 9 , translocation motions are indicated for a single intermediate. In Figure 10 , two intermediate structures (designated pre and post) are overlaid [ 22 ]. The ratchet is biased forward by a P-site tRNA rotating its 3′-CCA end into the E site, EF-G GTPase activity and, after translocation, reverse ratcheting and swiveling and EF-G·GDP release. A-site, P-site, and E-site tRNAs are located at the interface of the 16S rRNA head and body ( Figure 9 ). The mRNA also threads through the cleft separating the head/beak from the body of the 16S rRNA (across the neck; h28). Swiveling of the 16S head/beak by ~18° appears to advance the mRNA and move tRNAs into hybrid states. For instance, swiveling of the head/beak dissociates the E-site tRNA and moves the P-site tRNA into a pe/E hybrid conformation that makes defining E-site contacts at its 3′-CCA end (23S U2431, A2432) but makes a pe hybrid transition at its ASL. Forward swiveling of the head occludes the empty A-site so the reverse swiveling head/beak motion becomes part of the tRNA binding, latching, and accommodation processes for formation of the next peptide bond.

EF-G GTPase activity and translocation. The tRNA pe/E and ap/A (or ap/ap) hybrid states are observed. The 16S rRNA nucleotides 930–1380 are black (head/beak domain). mRNA (red) occupies the channel between the 16S rRNA body and head/beak (the neck). The codon-anticodon latch is not fully closed and the ap/A tRNA anticodon stem loop (ASL) has disengaged from the latch and has begun to translocate toward the P-site. For the ap/A-site tRNA, the 3′-CCA, where a peptide chain would be attached, makes a typical CCA A-site contact (C75:G2553). The pe/E tRNA 3′-CCA makes a typical E-site contact (U2431, A2432). EF-G·GDP is in an extended conformation supporting forward translocation and acting as a pawl to prevent reverse translocation.

Two thermal rotary motions of the 30S subunit support forward translocation. The 30S subunit reversibly rotates ~7° versus the 50S subunit. The mRNA [red (post translocated) and green (pre-translocated)] threads between the 16S rRNA head/beak domain and the body (the neck), ~18° swiveling of the head/beak domain versus the body drives the mRNA forward and the mRNA:tRNA codon:anticodon attachments into hybrid states. For the pre-translocated state, mRNA and pe/E tRNA are green. 16S rRNA (pre) is white. For the post-translocated state, EF-G·GDP (extended conformation), mRNA, and pe/E site tRNA are yellow, red, and blue. The 16S rRNA (post) is light blue (body) or black (16S head/beak; 930–1380). In the rightmost image, the 16S rRNA body is not shown to simplify the image.
The 30S and the 50S subunits of the ribosome are somewhat loosely attached. Subunit association is stabilized by mRNA binding and Mg 2+ . The somewhat loose binding allows restrained sliding of the subunits relative to one another. Ratcheting of the 30S subunit relative to the 50S subunit by ~7° helps to drive forward translocation [ 22 ]. Before reverse ratcheting and swiveling, the entry of tRNAs that are not in an EF-Tu·GTP complex is inhibited. Movement of the P-site tRNA to the E-site and the entry of aa-tRNA·EF-Tu·GTP support a natural forward directionality and flow to translation. From structures, EF-G·GTP appears to hold the A-site mRNA-ASL codon-anticodon latch for peptidyl transfer. Therefore, EF-G·GTP→GDP might release the A-site latch to support A/A→ap/A←→ap/ap translocation [ 47 , 48 ]. EF-G·GDP likely dissociates from the ribosome after the reverse 30S subunit head/body ratcheting versus the 50S subunit and the reverse 16S rRNA head/beak swiveling relative to the 16S body. Tilting of the 30S head/beak versus the 30S body also occurs in translocation [ 57 ]. From simulations, the order of events in translocation appears to be: (1) ratcheting of the 50S subunit relative to the 30S subunit; (2) swiveling of the 30S head/beak relative to the 30S body; and (3) the 30S head tilt.
3.8. EF-G·GTP/GDP in Translocation
EF-G·GTP/GDP undergoes multiple conformational distortions during binding, peptidyl transfer, GTPase, and translocation [ 3 , 10 , 29 , 58 , 59 ]. PDB 4WPO is characterized as a pre-translocation state of the ribosome, but the conformation is very close to that expected of a catalytic state ( Figure 8 ) [ 60 ]. Most interestingly, EF-G·GDP is covalently locked in a compact conformation, which is the expected form for EF-G·GTP before GTP hydrolysis and at the time of peptidyl transfer from the P-site to the A-site. In PDB 4WPO, the latch is closed, and the mRNA is kinked between the A-site and P-site, as expected for a catalytic intermediate. Subsequent translocation complexes have; (1) a more elongated conformation of EF-G·GDP; (2) an opening latch; and (3) the A-site tRNA ASL-mRNA contact has moved forward into an ap/A or an ap/ap hybrid position ( Figure 9 and Figure 10 ) [ 47 , 48 ]. Translocating ribosomes have been captured in a number of intermediate states that likely represent EF-G·GTP [ 29 , 48 ] and EF-G·GDP [ 59 ] complexes.
3.9. 30S-50S Intersubunit Bridges in Translocation
Because the 30S and the 50S subunits rotate relative to one another in translocation, intersubunit contacts regulate dynamics [ 61 , 62 ]. In the 30S subunit, h44 (A-site latch; A1492, A1493) is a long helix that makes multiple contacts to the 50S subunit, including to h69 (B2a; bridge 2a), h71 (B3), and h62 (B6). B2a 50S h69 is part of the A-site latch, including latch residue A1913. B3 is very close to the center of the ratchet for intersubunit rotation. B6 is very important to maintaining the 30S-50S association. The 30S h44 connects the A-site ASL latch (16S G530, A1492, A1493, 23S A1913) with the 50S subunit (h69). Mutations that disable bridges to 16S h44 are lethal. The 30S S13 protein interacts with the 50S h38 (A-site finger) (B1a). This contact affects the dynamics of the 30S head (i.e., swivel and/or tilt). The 30S S15 protein contacts the 50S h34 (B4). The 30S h23 contacts the 50S h68 (B7a), and the 30S h14 contacts the 50S L14/L19 (B8). Mutations that break B1a, B4, B7a, and B8 accelerate forward and reverse translocation, indicating that these are pawls that help to maintain the mRNA coding and the 30S-50S binding registers.
3.10. Ratchet Pawls
We posit that A-site, P-site, and E-site codon-anticodon (mRNA-ASL), 3′-CCA tRNA attachments, and the exiting peptide chain form the primary pawls that help maintain the ribosome translocation phase. The E-site does not bind acylated tRNA, so tRNA cannot slip from the P-site to the E-site without the transfer of the peptide chain to the A-site. As might have been expected, mutation of the E-site near the 3′-CCA contact causes frameshifting errors [ 63 ]. This is anticipated if the E-site evolved (in part) to limit backwards mRNA slippage and to maintain the translocation phase. The exiting peptide chain may also act as a pawl to help enforce the A-site to P-site and the P-site to E-site tRNA transitions. Because only translocation intermediates with hybrid pe/E and E/E tRNAs have been observed, available data do not appear to address the precise mechanism of EF-G·GDP release or to describe the full 14 angstrom translocation step. Often, translocation by molecular machines that is driven by thermal ratchets involves a partial power stroke (i.e., EF-G·GTP→GDP) followed by forward sliding and the establishment of pawls to inhibit backtracking. Maintaining 2-3 tRNAs on mRNA during translation helps to hold the translocation phase. Therefore, the E-site tRNA helps to maintain the register. The peptide chain and the A-site latch appear to help hold the mRNA. Traversing or relaxing pawls helps move the mRNA stepwise through the ribosome. Based on this analysis, at least 5-7 pawls must be considered in addition to any micro-pawls that may influence transitions. Primary pawls in translation correspond to tRNA codon-anticodon (mRNA-ASL) attachments (2 or 3), tRNA 3′-CCA attachments (2 or 3), and the exiting peptide chain (1). The EF-G·GTP→GDP power stroke and conformational extensions of EF-G have been considered to establish pawls that maintain forward translocation.
The A-site finger (23S 879-898; h38) that contacts the 30S S13 protein might be considered a micro-pawl to maintain the register for translocation. Transitioning from the A/A-site to the ap/ap-site, tRNAs contact the A-site finger [ 31 ]. Type I tRNAs make weaker contact than type II tRNAs (with expanded V loops). Deletion of the A-site finger affects translocation rates and may cause frameshift errors. Another micro-pawl might be the PE loop (16S G1338-U1341). Interaction of the pe/E tRNA ASL with the PE loop and ribosomal protein S13 appears to induce the 30S head tilting [ 57 ]. The 16S rRNA C1397 and A1503, which can intercalate with mRNA bases, have also been considered to be micro-pawls to resist reverse translocation [ 29 ].
3.11. Kink-Turns and Micro-Motions
In concert with the larger ratcheting, tilting, and swiveling motions of the ribosome are smaller movements. The ribosome is described as a flexible, semi-stable, and dynamic molecular motor [ 23 ]. The 50S and the 30S subunits reversibly slide relative to one another in a reversible ratcheting motion, and the 16S rRNA head/beak swivels relative to the 16S body around the neck [ 22 ]. Kink-turns are small loops that cause instabilities in helices, allowing for movements of an arm that may contribute to or resist translocation. Several of these kink-turns have been identified that may be important in regulating GTPase activity and translocation. The GAC that stimulates GTPase activity for IF2, EF-Tu, and EF-G is mounted on a kink-turn (Kt-42) located in 23S rRNA h42. The GAC is comprised of h42, h43, h44, and ribosomal protein L11. A-site to P-site translocation of the decoding center appears to involve an interaction between the 16S rRNA h44 (body) and h28 (neck). This action is associated with: (1) EF-G·GTP→GDP; (2) the opening of the 30S subunit; (3) the opening of the G530~A1492 latch; and (4) the movement of the A-site tRNA codon-anticodon from an A/A-state to a reversible ap/A←→ap/ap hybrid state. This has been observed as an ~8 angstrom movement of the decoding center [ 23 , 48 ]. A kink-turn (Kt-38) in the 23S rRNA h38 is found near a 50S to the 30S subunit contact (affecting the A-site finger interaction with protein S13).
4. Evolution of Translation
4.1. trna evolution.
Translation systems evolved around tRNA. Internal tRNA homologies and tRNA evolution are described in Figure 11 for type I and type II tRNAs (type II tRNAs have expanded V loops) [ 27 , 64 ]. The 17 nt Ac (anticodon) loop and T loop stem-loop-stems are homologous (initially close to 5′-CCGGGUU/CAAAACCCGG; sequence ambiguity is only in the 7 nt loop, not in the 5 nt stems; / indicates the position of the U-turn). The position of the U-turn is the same (between loop positions 2 and 3) in the Ac and T loops. The D loop is derived from a 17 nt UAGCC repeat (initially 5′-UAGCCUAGCCUAGCCUA). The last 5 nt of the D loop region (the yellow segment just 5′ of the Ac stem-loop-stem in Figure 11 ) is homologous to 5′-acceptor stems (As; 5′-As positions 3-7; initially 5′-GGCGG). The 5′-As evolved from a GCG repeat (initially 5′-GCGGCGG). The type I tRNA V loop (5 nt) is homologous to a 3′-As (3′-As nt 69-73 using our revised numbering for tRNAs; initially 5′-CCGCC). The 3′-As evolved from a CGC repeat (initially 5′-CCGCCGC). The type II tRNA V loop (initially 14 nt) is homologous to a 3′-As (7 nt) ligated to a 5′-As (7 nt) (initially 5′-CCGCCGCGCGGCGG).

tRNA evolution. Homologous regions have the same color. Ac and T loop stem-loop-stems (17 nt) are red (initially ~CCGGGUUCAAAACCCGG). 5′-As (initially GCGGCGG) and homologous regions are yellow. 3′-As (initially CCGCCGC) and homologous regions are green. The D loop microhelix UAGCC repeat region (originally 17 nt) is magenta. Green spheres are Mg 2+ ions.
Type I tRNA evolved by the ligation of three 31 nt minihelices followed by two symmetrical 9 nt deletions within ligated 3′- and 5′-acceptor stems [ 27 , 64 , 65 ]. Type II tRNA evolved by the ligation of three 31 nt minihelices followed by one 9 nt deletion within the 5′ ligated 3′- and 5′-acceptor stems [ 64 ]. Type II tRNA is a proposed intermediate in processing to type I tRNA, and, thus, the same model explains both tRNA subtype variants. A minihelix is a 17 nt microhelix flanked by 5′ and 3′ 7 nt acceptor stems [ 27 , 64 ]. Other models for tRNA evolution have been advanced, but no model based on the ligation of two minihelices [ 66 , 67 , 68 , 69 ] can be correct for the many reasons that have previously been explained [ 27 , 64 ]. For instance, in a two minihelix model, the Ac and T loops cannot be homologs, and, therefore, only a three minihelix model can describe tRNA evolution. The three minihelix model is strongly supported using statistical tests [ 27 , 64 ].
4.2. tRNA as Core Evolutionary Intellectual Property
As explained above, tRNA evolved from repeats (GCG, CGC, and UAGCC repeats; 5′-As, 3′-As, and D loop) and inverted repeats (~CCGGGUUCAAAACCCGG; Ac loop and T loop stem-loop-stems) [ 27 , 64 ]. In Figure 12 , a model for abiogenic evolution of tRNA from repeating polymers and inverted repeats is shown. Only a handful of ribozymes are necessary to generate tRNA from minihelix, microhelix, and repeating polymer precursors. Existing tRNAs radiated from these ordered sequences [ 27 , 64 ]. According to the model, tRNA comprises the central biological intellectual property necessary to coevolve rRNA, mRNA, the genetic code, aminoacyl-tRNA synthetase (aaRS) enzymes, and biological coding. Hydration-dehydration cycles are sufficient to generate many biopolymers [ 70 , 71 ], supporting the idea of the PTC as a dehydration chamber [ 50 ].

A model for the evolution of abiogenesis, the RNA-protein world, and cellular life. RNA and ribozyme functions that have been generated in vitro are indicated in red. The central advance in evolution of life on earth and biological coding is tRNA. This figure was modified from [ 64 ].
4.3. Aminoacyl-tRNA Synthetase Evolution
Aminoacyl-tRNA synthetases (aaRS; i.e., GlyRS) charge tRNAs with amino acids [ 72 ]. These enzymes are of two folding classes, designated class I and class II, with structural subclasses A-E (i.e., GlyRS-IIA or IleRS-IA). Class I aaRS enzymes have an active site of parallel β-sheets, described as a “Rossmannoid” fold. Evolutionarily, however, there is no detectable homology comparing class I aaRS enzymes and classical Rossmann fold (β–α) 8 proteins. By contrast to class I aaRS, class II aaRS enzymes have an active site of antiparallel β-sheets. Rodin and Ohno posited that, despite their incompatible folds, class I and class II aaRS enzymes were somehow related, possibly by antisense transcription-translation [ 73 , 74 , 75 , 76 , 77 , 78 ]. Class I and class II aaRS enzymes, however, have been shown to be likely homologs [ 45 ]. Our laboratory has obtained local alignments of IleRS-IA (i.e., Methanobacterium bryantii ) and GlyRS-IIA (i.e., Methanobacterium congolense ) enzymes with e-values as low as 5 × 10 −11 , strongly indicating homology.
In Figure 13 and Figure 14 , archaeal aaRS enzymes are compared. In Figure 13 , the comparison is mostly for Pyrococcus furiosis , an ancient species that is very similar to LUCA (the last universal common cellular ancestor). Because of the proximity of P. furiosis to LUCA, this comparison gives an accessible and qualitative view of early aaRS evolution and radiation. Figure 13 indicates: (1) aaRS editing; (2) aaRS structural subclasses; (3) closest apparent relatives (by e-value); and (4) genetic code columns (see also Figure 14 ). We make the following points from Figure 13 : (1) related aaRS enzymes tend to align in genetic code columns; (2) class I and class II aaRS enzymes appear to be homologs; and (3) aaRS editing is mostly confined to columns 1 and 2 of the genetic code and to hydrophobic (column 1) and neutral (column 2) amino acids, for which accurate aaRS discrimination in the active site may be problematic. Evolution of the genetic code appears to be via tRNA charging errors, and the code goes to universality and closure because of translational fidelity mechanisms that inhibit continued code innovation by suppressing tRNA charging errors [ 46 ].

Aminoacyl-tRNA synthetases (aaRS) evolution (mostly) in Pyrococcus furiosis (Pfu) , an ancient archaea. Columns of the genetic code are indicated by shading: column 1 (red); column 2 (yellow); column 3 (blue); and column 4 (green). Class I and class II aaRS enzymes are indicated with their structural subclasses (A–E). aaRS enzymes with editing active sites are in green type. Boxes are placed around class I aaRS enzymes. Sma: Staphylothermus marinus; Eco: Escherichia coli . AlaX is an editing function missing a synthetic AlaRS active site. This figure is modified from [ 46 ].

In archaea, the genetic code is effectively half as complex in tRNA compared to mRNA. The genetic code is shown as a codon-anticodon (Ac) table. The structural classes of aaRS enzymes are indicated (i.e., GlyRS-IIA is indicated as GLY-IIA). Grey shading indicates aaRS editing. Red bases are not utilized in the tRNA anticodon wobble position. Boxes indicate co-evolution of amino acids and aaRS enzymes in columns. TyrRS-IC and TrpRS-IC (boxed) are related across rows. Significantly, only pyrimidine/purine discrimination is achieved in the wobble position in archaea because of anticodon wobble ambiguity. Ancient archaea tend to have ~44 tRNAs, but tRNA wobble U and C anticodons are effectively synonymous.
The smaller the e-value, the more closely related are the enzymes. From column 1 of the genetic code ( Figure 13 and Figure 14 ), ValRS-IA, IleRS-IA, MetRS-IA, and LeuRS-IA are closely related class IA enzymes that attach hydrophobic amino acids (Val, Ile, Met, Leu) and that edit improper amino acid attachments. Editing by aaRS enzymes involves moving a mischarged aa-tRNA into a proofreading active site and hydrolysis of the inappropriate amino acid attachment. Very clearly, in column 1, hydrophobic amino acids, aaRS-IA enzymes, aaRS editing, and the genetic code structure are co-evolved. From column 2, ThrRS-IIA, ProRS-IIA, and SerRS-IIA are related class IIA enzymes. ThrRS-IIA and SerRS-IIA edit amino acid attachments. Thr and Ser are closely related neutral amino acids with the capacity for one hydrogen bond to the amino acid side chain, limiting the specificity of ThrRS and SerRS discrimination of substrates. ThrRS-IIA appears to be closely related to GlyRS-IIA, which may indicate that a GlyRS once occupied column 2 of the code (see below). In column 3, HisRS-IIA, AspRS-IIB, and AsnRS-IIB appear related, indicating additional code structure. The closely related TyrRS-IC and TrpRS-IC that charge tRNAs with aromatic amino acids locate to the top row of the genetic code, possibly indicating late evolution of the code along rows rather than within largely occupied columns. Phe, Tyr, and Trp are thought to be some of the last amino acids added to the code. We conclude that amino acids, aaRS enzymes, tRNAs, and the genetic code structure are coevolved, as expected. These strong patterns and structures in the genetic code are most apparent when represented as a codon-anticodon table in ancient archaea, such as P. furiosis , which is similar to LUCA.
4.4. rRNA Evolution
rRNA is posited to have evolved after and around tRNA [ 79 , 80 ]. The ribosome may have initially evolved as a scaffold for mRNA (pre-16S rRNA) utilizing a mobile PTC, which was a dehydration and molecular crowding chamber to enable peptide bond formation [ 50 , 65 ]. With the evolution of the 23S rRNA, the PTC became immobile and the translocation ratchet (~7° rotation) could be evolved between the two ribosome subunits. The tRNA passage channel, which is formed in the 50S ribosomal subunit, probably evolved around tRNA to mold the channel shape and size to allow transit of tRNAs. In evolution, major blocks to tRNA passage would have been strongly negatively selected. Minor tRNA-interacting features of the ribosome (i.e., the L1 stalk, the A-site finger, and the P-site finger) may have evolved as pawls to help maintain the mRNA reading frame. The 16S rRNA swivel between the head/beak and the body, therefore, may have been the most primitive translocation mechanism for a standalone pre-16S rRNA scaffold before the recruitment, evolution, and attachment of the 50S subunit. The long 30S h44 connects to the 50S subunits h69, h71, and h62, linking the A-site ASL latch to the 50S subunit and the translocation mechanism.
4.5. Evolution of the Genetic Code
A representation of the genetic code is shown in Figure 14 . The code is displayed as a codon-anticodon table with emphasis on the reduced effective size of the genetic code in tRNA relative to mRNA. Representation of the anticodon is essential because the genetic code evolved around tRNAs [ 45 , 46 ]. For instance, adenine is disallowed in the tRNA anticodon wobble position [ 45 , 46 ]. In archaea and bacteria, moreover, tRNA Ile (UAU) is rarely used. Furthermore, single base discrimination of A, G, C, and U at the anticodon wobble position is difficult. Effectively, only purine versus pyrimidine discrimination is achieved at the wobble position for archaeal tRNAs. At the base of code evolution, therefore, the genetic code capacity in tRNA shrinks to 32 distinct anticodons at most [ 45 , 46 ]. The near universal standard genetic code encodes 20 amino acids + stops. The complexity of the standard code is limited due to the maintenance of 4-codon sectors, which are protected from subdivision by amino acid identity and aaRS editing—two issues of aaRS charging accuracy and translational fidelity. Hydrophobic and neutral amino acids with limited capacity for hydrogen bonding tend to locate to 4-codon sectors (columns 1 and 2) rather than splitting into 2-codon sectors (column 3). Stop codons are recognized by proteins, not tRNAs, so Trp should not be considered to reside in a 1-codon sector. In archaea, tRNA Ile (UAU) is rarely used, so tRNA Ile (UAU) and tRNA Met (CAU) should not be considered 1-codon sectors at the base of code evolution. We prefer the classic structure and representation of the genetic code to circular code diagrams because code structure is most apparent using a codon-anticodon table ( Figure 14 ).
The most primitive ribosomes and tRNAs are posited to have synthesized polyglycine [ 45 , 46 , 81 , 82 ] using any RNA as an mRNA template. Polyglycine is currently a cross-linking component of bacterial cell walls [ 45 , 46 ] and may have had a similar role in stabilizing protocells before LUCA. From such beginnings, the genetic code, which appears to be the crowning achievement of abiogenesis ( Figure 12 ), could be evolved by straightforward Darwinian selection. We posit that Gly was the first encoded amino acid that, at an early stage of evolution, Gly occupied the entire genetic code table [ 45 , 46 ]. Also, SerRS-IIA is split in the code between column 2 and column 4, indicating that, as the code evolved, Ser occupied blocks from which it was subsequently excluded, as we propose for Gly. If the entire genetic code initially encodes glycine, this has two important consequences. First, initially all mRNA sequences encode polyglycine. Second, according to this model, the genetic code, tRNA, and mRNA coevolve via Darwinian selection to a complete and accurate code.
The oldest rRNA sequences appear to be more tRNA-like than more derived species, indicating that tRNA existed before the evolution of 16S and 23S rRNA. The 16S rRNA appears to be more similar in sequence to tRNA than 23S rRNA and 5S rRNA. From a 1-letter code in which every mRNA encodes polyglycine, the code can progress from a 1-letter→4-letter→8-letter→16-letter→21-letter (20 aa + stops) code, as previously described [ 45 , 46 ]. Evolution of the A-site codon-anticodon latch in the 16S rRNA may correspond to the 8-letter→16-letter code transition [ 45 , 46 ]. The 2nd nt in the codon-anticodon position is most important for translational accuracy. However, the latch was necessary to discriminate the 1st codon position (the 3rd anticodon position). This discrimination was required to generate a code complexity of 4 × 4=16-letters. Because the latch also supports accuracy of pairing at the wobble position, evolution of the latch supported the further evolution from a 16-letter to a 21-letter code, bringing the standard genetic code to closure and universality. For other views, see [ 83 , 84 ].
5. Conclusions
The ribosome is a large and dynamic thermal ratchet molecular motor at the heart of information processing in cells. The mRNA and bound tRNAs traverse the 16S rRNA neck. Translocation is supported by a reversible swiveling motion of the 16S rRNA head/beak versus the body, by an apparent tilting of the head/beak relative to the body, and by a reversible ratcheting motion between the 50S and the 30S ribosomal subunits. Directionality of translation appears to be due to GTPase elongation factors EF-Tu and EF-G that bind in turn to the same site on the ribosome. EF-Tu is a primary fidelity factor for translation. EF-G mostly functions in peptidyl transfer and translocation. Attachments of tRNAs to mRNA and to the 50S subunit appear to act as pawls to enforce the direction and the mRNA register of translation. The 30S-50S subunit contacts regulate translocation and appear to communicate events at the A-site ASL latch to translocation ratchets. The coevolution of ribosomes, the genetic code, mRNA, and aaRS enzymes appears to have been around the tRNAs that evolved from ordered repeat sequences and stem-loop-stems that include a U-turn within a 7 nt loop (Ac and T loop).
Acknowledgments
We thank Paul Whitford (Northeastern University) and Sean Connell (Ikerbasque: Basque Foundation for Science) for help identifying relevant ribosome structures and for detailed email discussions. The field mourns the death of Thomas Steitz (Nobel Laureate; Yale University).
Abbreviations
Author contributions.
Conceptualization, Z.F.B.; formal analysis, K.O.; project administration, Z.F.B.; software, K.O.; visualization, Z.F.B.; writing—original draft, Z.F.B.; writing—review & editing, Z.F.B.
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.

COMMENTS
Structure of Ribosomes • A ribosome is made from complexes of RNAs and proteins and is, therefore, a ribonucleoprotein. • Each ribosome is divided into two subunits: 1. A smaller subunit which binds to a larger subunit and the mRNA pattern, and 2. A larger subunit which binds to the tRNA, the amino acids, and the smaller subunit. • Prokaryotes have 70S ribosomes respectively subunits
STRUCTURE AND FUNCTION OF THE RIBOSOME. THE ROYAL SWEDISH ACADEMY OF SCIENCES has as its aim to promote the sciences and strengthen their influence in society. BOX 50005 (LILLA FRESCATIVÄGEN 4 A), SE-104 05 STOCKHOLM, SWEDEN TEL +46 8 673 95 00, FAX +46 8 15 56 70, [email protected] HTTP://KVA.SE.
Ribosomes are the cellular structures responsible for protein synthesis. The word "synthesis" means "to combine things to produce something else.". In this context, protein synthesis means combining different amino acids together to form a protein. Ribosomes join amino acids together in a chain to form a protein ( Figure 5.4.1 5.4. 1 ).
Ribosomes. Even before an mRNA is translated, a cell must invest energy to build each of its ribosomes. In E. coli, there are between 10,000 and 70,000 ribosomes present in each cell at any given time.A ribosome is a complex macromolecule composed of structural and catalytic rRNAs, and many distinct polypeptides. In eukaryotes, the nucleolus is completely specialized for the synthesis and ...
The ribosome word is derived - 'ribo' from ribonucleic acid and 'somes' from the Greek word 'soma' which means 'body'. Ribosomes are tiny spheroidal dense particles (of 150 to 200 A 0 diameters) that are primarily found in most prokaryotic and eukaryotic.. They are sites of protein synthesis.; They are structures containing approximately equal amounts of RNA and proteins and ...
Structural and functional aspects of the eukaryotic ribosome. Interweaving of rRNA and r-proteins on the (A) LSU near ES7 L and ES39 L (Klinge et al. 2011), and (B) SSU near ES3 and ES6 (Rabl et ...
The full assignment of ther-proteins in the yeast and fungal 80S ribosomes, however, only became possible with the im-proved resolution (3.0-3.9 A˚) resulting from the crystal structures of the SSU and LSU from Tetrahymena thermophila (Klinge et al. 2011; Rabl et al. 2011) and the Saccharomyces cerevi-siae 80S ribosome (Figs. 1D,E and 2D,E ...
The ribosome is a macromolecular machine, composed of RNAs and proteins, that. synthesizes proteins by stringing together amino acids according to genetic instructions. Introduction. The ribosome ...
This review describes the crucial role of ribosomes in various common malignant tumors; in particular, it examines the effects of RPs, including S6, the receptor for activated C-kinase and RPS15A ...
A ribosome is an organelle made out of protein and RNA. Its role in protein synthesis is extremely important. And it is the structure of the ribosome that allows it to function as it does. Ribosomes. Ribosomes are small organelles and are the site of protein synthesis (translation). Ribosomes can be found alone or in groups within the cytoplasm.
RIBOSOME - STRUCTURE. Ribosomes are non-membrane bound cell organelles made of RNA and proteins. Ribosomes are complex molecular machine that make proteins from amino acids in the process called protein synthesis or translation. Every cell needs ribosomes to manufacture proteins. Ribosomes are found in both prokaryotic and eukaryotic cells.
Chapter 5 Proteins & Amino Acids 3 mechanical stresses and afford protection to the organisms that produce them. On a cellular level, structural proteins contribute to the physical
The process of eukaryotic ribosome assembly stretches across the nucleolus, the nucleoplasm and the cytoplasm, and therefore relies on efficient nucleocytoplasmic transport. In yeast, the import machinery delivers ~140,000 ribosomal proteins every minute to the nucleus for ribosome assembly. At the same time, the export machinery facilitates translocation of ~2000 pre-ribosomal particles every ...
The assignment of specific ribosomal functions to individual ribosomal proteins is difficult due to the enormous cooperativity of the ribosome; however, important roles for distinct ribosomal ...
The full assignment of the r-proteins in the yeast and fungal 80S ribosomes, however, only became possible with the improved resolution (3.0-3.9 Å) resulting from the crystal structures of the SSU and LSU from Tetrahymena thermophila (Klinge et al. 2011; Rabl et al. 2011) and the Saccharomyces cerevisiae 80S ribosome (Figs. (Figs.1D,E 1 D,E ...
Abstract. In this concluding chapter an attempt to formulate several general principles of the structure and function of the ribosome is undertaken. Our understanding of the ribosome is far from complete, and the formulations reflect only the current level of knowledge in this area. Concerning the ribosome structure, the principles formulated ...
3. Ribosomes - 80 S 3. Ribosomes - 70 S 4. Distinct compartments in the cell 4. No compartments. i.e. the cytoplasm and the nucleus 5. Depending upon the species number of 5. There is only one chromosome per cell. chromosomes per nucleus varies from two to many. 6. Each chromosome is linear with its two 6. The chromosome is circular and remains
Ribosomes are among the largest and most dynamic molecular motors. The structure and dynamics of translation initiation and elongation are reviewed. Three ribosome motions have been identified for initiation and translocation. A swivel motion between the head/beak and the body of the 30S subunit was observed. A tilting dynamic of the head/beak ...
The 80S eukaryote ribosome consists of a 40S and a 60S subunit. In vertebrates the 40S subunit contains 18S rRNA, while the 60S subunit contains 28-29S, 5.8S and 5S rRNA. In plants and invertebrates the 40S subunit contains 16-18S RNA, while the 60S subunit contains 25S and 58 and 5.8S rRNA. here are T
detection of genetic disorders. forensic science. everyday products, e.g., laundry detergents (enzymes that digest 'dirt') emerging new medicines. Manipulation of DNA. restriction nucleases - proteins that cleave DNA at particular locations, enabling fragmentation into smaller parts in a predictable way.
3.3 Bacterial Plasma Membranes. Describe the fluid mosaic model of membrane structure and identify the types of lipids typically found in bacterial membranes. Distinguish macroelements (macronutrients) from micronutrients (trace elements) and provide examples of each. Provide examples of growth factors needed by some microorganisms.
The assignment of individual ribosomal proteins to specific ribosomal functions is difficult due to the enormous cooperativity of the ribosome. However, the role of a few proteins have been ...
1 Assembly of the Bacterial Ribosome with Circularly Permuted rRNA Xiyu Dong1,2, Kai Sheng1,2, Luca F.R. Gebert1, Sriram Aiyer3,4, Ian J. MacRae1, Dmitry Lyumkis3,4, James R. Williamson1,2 1 Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA. 2 Department of Chemistry, and The Skaggs Institute for Chemical Biology, The Scripps
The ribosome, one of the largest molecular machines in living cells, is in charge of protein synthesis. The ribosome translates genetic code into amino acid sequences that fold into protein structures. Ribosomes are the birth place of proteins in living cells. The bacterial ribosome plays also a key role in medicine as a drug target for ...
518 3 510-690-2727 [email protected] KOLAKOWSKI, VICTORIA Civil Direct Calendar 519 3 510-690-2728 [email protected] RILES, ELIZABETH Civil Harassment/Small Claims
Created Date: 4/2/2024 9:19:11 AM