Can Science Extend the Human Lifespan?
Geiger, the man who learned how to count radioactivity, openmind books, scientific anniversaries, nanomedicine to fight cancer, featured author, latest book, mary lyon: the geneticist who discovered that women are (cellular) mosaics.
Sixty years ago, British geneticist Mary Frances Lyon (1925-2014) proposed a hypothesis stating that a specific inactivation process occurs in women’s sex chromosomes — and those of female mammals in general — transforming them into cellular mosaics. This hypothesis, which has been known ever since as lyonization, has been proven fully valid in our times, having led to interesting advances and discoveries.


LYONIZATION
At the end of the 1950s, it was discovered that the pair of sex chromosomes in males is comprised of a normally sized chromosome, the X chromosome inherited from the mother, and a very small chromosome, the Y, inherited from the father. In women, however, this pair consists of two X chromosomes, one from the father and one from the mother. It was then deduced that women have double the X chromosome genes than men, which is no small matter given that the X chromosome contains more than a thousand genes, whereas the Y has only about 75. Thus arose the enigma: are genes from both X chromosomes expressed within the female’s cells or is there some kind of compensation for women’s double dose of genes (compared to males).
Faced with this dilemma, Lyon first proposed that one of the somatic cell’s two X chromosomes in women — and in female mammals, in general — is inactivated in the phase when genes are expressed, in interphase1. Specifically what happens is that the inactivated X chromosome is completely condensed — a process that is called heterochromatinization — whereas the other X chromosome and the rest of the chromosomes are decondensed and expressed in RNA and proteins. The inactive X chromosome would be the origin of the sex chromatin or Barr body, a more darkly stained body observed in the nucleus of a female cell and which is absent in male cells. Even though female cells have a double dose of X-linked genes, the process of inactivation caused by heterochromatinization results in only one of the set — the one that has not been converted into heterochromatin — being active, thus compensating for the double dose in females, as compared to males who only have one X.

Secondly, Lyon proposed that inactivation occurs in the first phases of female development — in fact just two short hours from fertilization — and at random: in some cells the X chromosome inherited from the father is inactivated and in others it is the X chromosome from the mother. Thirdly, she sustained that once a specific X chromosome has been inactivated in a specific embryonic cell, all of the cells that originate from it will inherit said inactivation, with the result that all of the cells in the area of the adult body that this particular cell gave rise to will be inactive. This explains why organisms with coloration patches in the fur are usually female and not male. This is the case with tabby cats with black and orange patches on a white background. These cats would be heterozygous for the gene that determines the colors orange and black, which is located in the X chromosomes. The patches of color reflect the zones that have developed from cells that have inactivated one or the other of the X chromosomes. This is why in some zones one of the color alleles — the orange — is expressed and in other zones the other allele — the black — is expressed.
Therefore, and in conclusion, what has been known as lyonization causes mammalian females to be mosaics, where the genes of one or the other of the two X chromosomes carried in the cells are expressed, a phenomenon that does not happen in males because their cells contain only genes of a single X chromosome, in addition to the Y
REVOLUTIONARY GENETIC CONCEPTS RELATED TO LYONIZATION
Although Mary Lyon’s hypothesis about the inner workings of women’s (and mammalian females’) X chromosomes was at first met with some reservations, in later years it was generally accepted. Particularly because much of the research that her hypothesis prompted (some of it even conducted by her) received convincing genetic and cytologic results which supported the theory. Once confirmed, it represented the starting point for several discoveries, some of interest for theoretical biology and others with implications for clinical genetics.
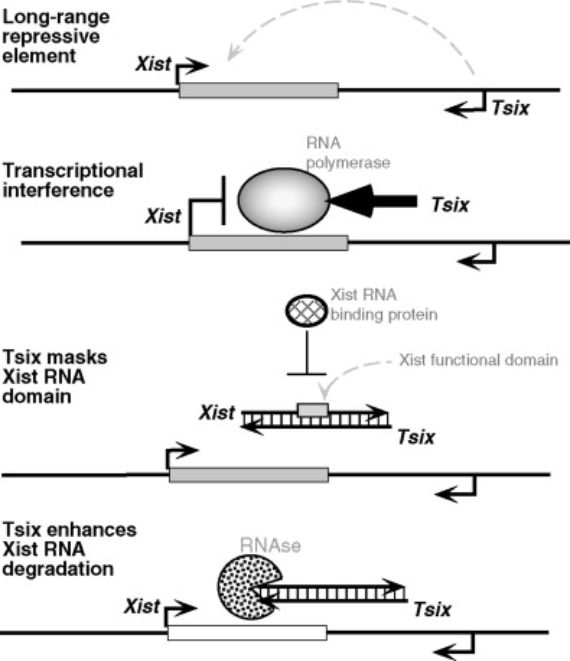
Thirty years after Lyon’s initial work on the process, it was discovered2 that the chromosome X-inactivation in females is controlled by the very X chromosome to be inactivated. And as opposed to what normally happens, this is not because a protein comes from some other chromosome or from the same X chromosome. It happens because in the X chromosome where a gene is going to be inactivated, the Xist (for “X inactive specific transcript”) gene synthesizes a long RNA — it has more than 15,000 ribonucleotides — that in binding itself along the chromosome subjects the chromosome to the heterochromatinization process, inactivating it, and thus preventing the transcription process that occurs first with the messenger RNA and later with proteins. This was one of the first times that it was found that a long non-coding RNA (a non-protein coding transcript) could have a gene activation regulatory function, something that was later seen to occur in various biological situations.
Another exceptional genetic mechanism has been found to be related to female active X chromosomes. Specifically what happens in this chromosome is that another RNA (different from the previously mentioned one) is synthesized from the Xist gene. But this happens from the opposite strand of the gene’s double helix — and in the opposite direction — from that which is transcribed in the inactive chromosome. This RNA is called Tsix, Xist backwards. It is even longer than the Xist; it has 40,000 bases, and with its synthesis in the active X chromosome, it prevents the synthesis of the Xist RNA with the inactivating function3. It is also one of the first cases in which it was observed that two different RNAs were transcribed from a single gene: one in one direction of the gene’s double helix and another in the other direction, and where, in addition, one of the RNAs interferes with the other: if the Tsix impedes the Xist synthesis, it would be an anti-Xist.
CLINICAL IMPLICATIONS OF LYONIZATION
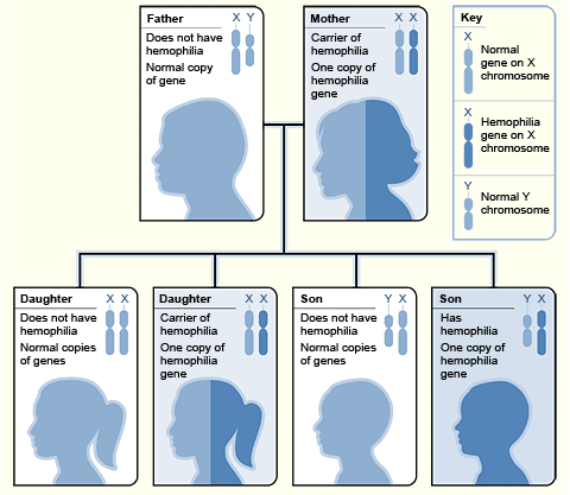
The phenomenon of lyonization that occurs during the development of women and mammalian females in general could also have clinical ramifications. Women are generally less likely than men to suffer diseases caused by gene mutations of the X chromosome. This is due to the fact that women have two X chromosomes, and if one carries a mutated gene, it can be substitute with the normal gene that comes from the other X chromosome. This does not occur in males because they only have one X chromosome. This is what happens with diseases like hemophilia, which involves hemorrhaging caused by a lack of a coagulation factors. In this case, men can be hemophiliacs due to a genetic mutation of their single X chromosome from which these factors are synthesized. In contrast, although women can be carriers of the mutated gene in one of their X chromosomes, with the normal gene in the other X, enough coagulation proteins are produced to prevent hemorrhaging, event though it is only expressed in half of her cells and that only fifty percent of the normal coagulation factors are produced. This level is adequate to protect them from hemorrhaging, especially because the coagulation factors are released from the cells and circulate through the bloodstream where they can act upon the whole body.
But there are other situations in which the presence of an X chromosome with a functioning gene opposite a mutated gene in the other X chromosome of women does not protect them from the disease. This is what occurs, for example, in Rett syndrome, which is mainly prevalent in women with symptoms that include a serious cognitive disability — a severe form of autism — that becomes increasingly worse throughout the patient’s life4. This syndrome occurs as a result of a mutation in a gene in the X chromosome from which a protein is synthesized. The protein binds to the DNA in various locations and regulates the expression of many other genes. This occurs most of all in the brain. Whereas women with the mutation in both X chromosome genes or men with the mutation in their single X chromosome would not be viable, heterozygous (with one normal and one mutated gene) women are viable, but suffer from this syndrome. In this case what happens is that only half the brain cells of the patients express the normal gene — the cells that have inactivated the mutated X. But the other half, having inactivated the X with the normal gene, does not produce the protein. Half of the nerve cell production of the protein is not adequate for normal brain function.
PROSPECTS AND CONCLUSION
As we have said, the lyonization hypothesis has continued to prove valid through the present day. Though it continues to be a field of active research in many respects. First of all, much about the inactivation mechanism itself is being investigated: research on all the protein factors that interact with the RNA that react first, research on the effects of these factors on the DNA structure and the genes that are inactivated, etc. Secondly, there is ongoing research on the possible deviations in the process: there are cases in which inactivation does not occur randomly, but rather in some women activation occurs with a preference for either the paternal or maternal X chromosome; and research on the existence of genes that escape inactivation in the X chromosome that is heterochromatinized. All this research is also of clinical interest, for example, the latter two phenomena can play a role in certain illnesses and kinds of cancer. Likewise, v ery recently there has been investigation into to what degree men’s greater sensitivity to the coronavirus (SARS-CoV-19) could be related to their greater production of the cell membrane protein, ACE2 (Angio-tensin Converting Enzyme 2), which the virus uses to enter MRC-5 cells. The gene that controls this enzyme is located in the X chromosome, and if what happens is that men produce greater amounts of ACE2 than women and are therefore more susceptible to infections, the question is: How is this difference produced when Lyon’s hypothesis predicts that there should be equal production between the two sexes. Could this difference be due to the behavior of male hormones on the gene?
The pioneer behind this entire field of research, Mary F. Lyon, saw her hypothesis accepted and her work recognized as she was invited to join such important scientific societies such as the Royal Society (although she met with opposition) or the National Academy of Sciences in the U.S. She even lived to see her her name on a laboratory in the institute where she developed her work: the Mary Lyon Centre in Harwell, and in 2014 the establishment of the Mary Lyon Medal from the U.K. Genetics Society. However, she was not awarded the Nobel Prize, which many scientists claim she deserved for her revolutionary, comprehensive, and productive hypothesis.
Bibliography
- Lyon, MF. 1961.Gene Action in the X-chromosome of the mouse. Nature, 190:372-373. The first in a set of works in which the hypothesis is developed.
- Brown, C.J. et al.1991. A gene from the region of the human X inactive centre is expressed exclusively from the Inactive X chromosome. Nature, 349: 38-44.
- Lee JT et al.1999. Tsix, a gene antisense to Xist at the X inactivation center. Nat Genet, 21(4):400-4.
- Bienvenu, T. et al. 2000. MECP2 mutation account for most cases of typical forms of Rett syndrome. Hum. Mol. Genet. 9:1377-1384
- Sama, I.E et al. 14 May-2020. Circulating plasma concentration of Angiotensin Converting Enzyme 2 in men and women. Eur.Heart Journal. 41(19): 1810-1817.
Manuel Ruiz Rejón
Related publications.
- A Hidden Genetic Code?
- Jumping Genes: Disease and Health
- The Diseases That Hide in Our Genes
More about Science
Environment, leading figures, mathematics, scientific insights, more publications about manuel ruiz rejón, comments on this publication.
Morbi facilisis elit non mi lacinia lacinia. Nunc eleifend aliquet ipsum, nec blandit augue tincidunt nec. Donec scelerisque feugiat lectus nec congue. Quisque tristique tortor vitae turpis euismod, vitae aliquam dolor pretium. Donec luctus posuere ex sit amet scelerisque. Etiam sed neque magna. Mauris non scelerisque lectus. Ut rutrum ex porta, tristique mi vitae, volutpat urna.
Sed in semper tellus, eu efficitur ante. Quisque felis orci, fermentum quis arcu nec, elementum malesuada magna. Nulla vitae finibus ipsum. Aenean vel sapien a magna faucibus tristique ac et ligula. Sed auctor orci metus, vitae egestas libero lacinia quis. Nulla lacus sapien, efficitur mollis nisi tempor, gravida tincidunt sapien. In massa dui, varius vitae iaculis a, dignissim non felis. Ut sagittis pulvinar nisi, at tincidunt metus venenatis a. Ut aliquam scelerisque interdum. Mauris iaculis purus in nulla consequat, sed fermentum sapien condimentum. Aliquam rutrum erat lectus, nec placerat nisl mollis id. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Nam nisl nisi, efficitur et sem in, molestie vulputate libero. Quisque quis mattis lorem. Nunc quis convallis diam, id tincidunt risus. Donec nisl odio, convallis vel porttitor sit amet, lobortis a ante. Cras dapibus porta nulla, at laoreet quam euismod vitae. Fusce sollicitudin massa magna, eu dignissim magna cursus id. Quisque vel nisl tempus, lobortis nisl a, ornare lacus. Donec ac interdum massa. Curabitur id diam luctus, mollis augue vel, interdum risus. Nam vitae tortor erat. Proin quis tincidunt lorem.
The Astronomer who Looked Inside Trees
Do you want to stay up to date with our new publications.
Receive the OpenMind newsletter with all the latest contents published on our website
OpenMind Books
- The Search for Alternatives to Fossil Fuels
- View all books
About OpenMind
Connect with us.
- Keep up to date with our newsletter
- Subscriber Services
- For Authors
- Publications
- Archaeology
- Art & Architecture
- Bilingual dictionaries
- Classical studies
- Encyclopedias
- English Dictionaries and Thesauri
- Language reference
- Linguistics
- Media studies
- Medicine and health
- Names studies
- Performing arts
- Science and technology
- Social sciences
- Society and culture
- Overview Pages
- Subject Reference
- English Dictionaries
- Bilingual Dictionaries
Recently viewed (0)
- Save Search
- Share This Facebook LinkedIn Twitter
Related Content
Related overviews.
sex chromatin
More Like This
Show all results sharing this subject:
Lyon hypothesis
Quick reference.
The hypothesis that gene dosage imbalance between males and females, because of the presence of two X chromosomes in females (XX) as opposed to only one in males (XY), is compensated for by random inactivation of one of the X chromosomes in the somatic cells of females. The inactivated X chromosome becomes the Barr body ( see sex chromatin). M. F. Lyon (1925), British geneticist
From: Lyon hypothesis in Concise Medical Dictionary »
Subjects: Medicine and health
Related content in Oxford Reference
Reference entries.
View all related items in Oxford Reference »
Search for: 'Lyon hypothesis' in Oxford Reference »
- Oxford University Press
PRINTED FROM OXFORD REFERENCE (www.oxfordreference.com). (c) Copyright Oxford University Press, 2023. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use (for details see Privacy Policy and Legal Notice ).
date: 24 March 2024
- Cookie Policy
- Privacy Policy
- Legal Notice
- Accessibility
- [66.249.64.20|195.158.225.244]
- 195.158.225.244
Character limit 500 /500
C2005/F2401 '07 Lecture 21
© Copyright 2007 Deborah Mowshowitz and Lawrence Chasin Department of Biological Sciences Columbia University New York, NY. Last Update 11/25/2007 12:09 PM
Handouts: You need 21A (Crosses -- not on web) & 21B (for dominance & pedigrees).
Note: There are many examples of genetic conditions described here. The basic principles, but not all the details, will be covered in class. Be sure you understand all the examples -- ask your TA or email Dr. M if you have any questions.
I. Inactive X's and Barr bodies -- Why extra or missing X's are usually tolerated and extra or missing autosomes are not. (This = topic VI of last lecture.)
A. Lyon Hypothesis = inactive X Hypothesis
The idea that extra X's are genetically inert is called the Lyon hypothesis (or the inactive X hypothesis). According to the Lyon hypothesis, every female is a mosaic, since some of her cells use her maternal X to make proteins and some use her paternal X.
B. Barr bodies
You can actually see the inactive X during interphase because it forms a Barr body. There are 2 X chromosomes in every female cell, but (according to the inactive X hypothesis) only 1 of them works (is transcribed) most of the time. In general, if there are extra X chromosomes, all the extras are inactive, whether the cell is male or female. The inactive X's remain tightly coiled during interphase and are called Barr bodies. (So you can tell the sex of the cell without doing a karyotype.) Note that inactive X chromosomes are replicated, but not transcribed.
C. How is mosaic detected? Intro. to Genetic Terminology
Consider coat color in cats. This is how Lyon actually figured out the inactive X existed. In cats, a gene controlling coat color is on the X. The position of the gene is known as the l ocus of the gene. This gene has two alleles (alternate forms); one → black coat color and the other → orange. One of the alleles is present at the coat color locus on every X. The Y chromosome does not carry an allele of the coat color gene. Males have only one X, which carries either the black or the orange allele, so normal male cats are all black or all orange. (They may have regular stripes, superimposed on the black or orange, but the background color is either all black or all orange -- they don't have areas of orange and areas of black). Females have two X's, so they carry two alleles of the coat color gene -- one on each X. A female can be homozygous black (have 2 black alleles), be homozygous orange (have 2 orange alleles), or be heterozygous (have one allele of each color), as shown in figure. Females can be orange, black or patchy (with areas of each color). Only heterozygous females are patchy. All this makes sense if only one copy of the X works in each patch so only one copy of the coat color gene works per cell (and per patch). Rare patchy males are XXY (Klinefelter's Kats).
Note "Patchy" is called tortoise shell, not tabby; calico = patchy plus white. (Tabby = regular pattern of stripes that occurs in both males and females.) D. When do Barr bodies form? How do you get the mosaic?
Fertilized egg (zygote) → ball of cells → each cell inactivates one X at random → each cell divides by mitosis → descendants with same X on/off. Once an X is inactivated, it generally remains inactivated through succeeding mitoses, so all mitotic descendants of a single cell have the same X on and the same X off → all cells in one area (or with same lineage) have same X on/off.
Germ line cells (which will go through meiosis) turn both X's back on before gametes are made, before meiosis occurs. So either one of the two X chromosomes can be used or inactivated in the next generation. To review Genetic Terminology so far, try 8R-1. (Also see Becker fig. 20-2 [18-2].)
II. Patterns of Inheritance -- An example and the general principles -- See Top half of Handout 21A
A. What are the Big Issues to consider?
1. How are genes/genotypes inherited , and
2. How does a particular genotype (state of the genetic information) determine phenotype (appearance, function, etc)?
We'll start by looking more closely at the example of orange/black coat color in cats and then go on to other examples and the general case.
B. How do you figure out the pattern of Inheritance? -- an example for a gene on the X For a different classic example of inheritance of a sex-linked trait, see Sadava 10.23. (For this, and many of the other figures in this lecture, the fig. or table # is the same in the 7th & 8th editions of Purves/Sadava.)
Note: The term "trait" is used in several different ways. It usually means whatever property you are following. Depending on the circumstances, it can mean the overall property you are considering such as coat color, OR it can mean the form (phenotype) of that property that you are following, such as orange coat color. So people speak of "the fur color trait" or "the orange color trait" depending on the context. ("Trait" is also sometimes used to refer to the carrier or heterozygous condition, as in "she has the sickle cell trait" meaning she has no symptoms but carries one allele for sickle cell.)
Consider a gene on the X such as the one that determines orange vs black coat color. Suppose you mate a tortoiseshell female cat X orange male (see handout 21A). How do you figure out what will happen? Follow the steps below. (Each step is drawn on handout for each parent.)
1. Draw parental chromosomes with proper alleles .
a. Number of gene copies. For genes on the X: Male has only one copy (allele) of the gene, female has two copies (alleles). For a genes on an autosome (discussed below), both male and female have two alleles of each gene.
b. Terminology (for an individual with two alleles). If both alleles are the same, individual is said to be homozygous or a homozygote. In this case, a homozygous cat can be either homozygous black or homozygous orange. If the two alleles are different, the individual is said to be heterozygous or to be a heterozygote. A tortoiseshell cat must be heterozygous.
2. Go through DNA replication to double DNA, chromatids/chromosome and # alleles/cell. Note sister chromatids are identical (if no crossing over**) but homologs need not be.
a. Sister chromatids must be identical since they are the 2 products of a single, semi-conservative, DNA replication. (See ** below.)
b. Homologs need not be identical -- one came from the mother and one the father. Homologs DO need to have the same genes (loci) lined up in the same order -- they just don't have to have the same alleles of these genes. In this case, for the heterozygous female cat, one homolog has the orange allele of the coat color gene (at the coat color locus), and the other homolog has the black allele of the coat color gene.
3. Go through meiosis : Homologs separate at first division and sister chromatids separate at second division. This produces 4 gametes -- two different kinds, but in equal proportions (again assuming no crossing over**). **Note: Crossing over does not make any significant difference here because you are following only one gene at a time. When you start considering two or more genes at a time, then you have to take crossing over into account, and we'll explain how to do that later. We're ignoring it now, because the gametes come out the same (for the one gene under consideration) whether there is crossing over or not. See Becker fig 20-14 [18-14] or Sadava 10.19. Crossing over occurs in both figures, but you still get two gametes with one allele of the gene (Y in Becker or B in Sadava) and two gametes with the other allele (y or b).
4. Do fusion of gametes from both parents to get zygotes (cats). You can use a Punnett square (or simple probability) to keep track of all combinations and proportions. This gives you the genotypes of the offspring (zygotes).
5. Look at genotypes and infer phenotypes of offspring = what develops from zygotes. Consider all possible combos and what proportions they occur in. Note that in this case there is no dominance, so phenotype follows directly from genotype. If cat has only black (or only orange) alleles, you get a black (or orange) cat. If cat has both alleles, you get a tortoiseshell cat. Cat is black in areas where X with B allele is on active X; cat is orange in areas where X with O allele is on active X. (See below for mechanism of determination of black vs. orange.)
6. Terminology (for genes on the X): In this course, and in many other contexts, the terms 'sex-linked' and 'X-linked' are used interchangeably, so sex linked = 'on the X chromosome.' If a gene is on the Y chromosome, a different term is used. Some biologists use the term 'sex-linked' to refer to genes on either the X or the Y. However, since there are very few genes on the Y, genes referred to as 'sex-linked' are almost always on the X (no matter how the term 'sex-linked' is used).
C. How does genotype determine phenotype?
How does a gene specify orange or black? In all cases, to figure out how genotype and phenotype correspond, you need to consider the enzymes and pathways involved. Current understanding of this case is as follows:
1. Black Part -- how is black pigment made?
Gene A is probably on an autosome.
2. Orange part -- how is orange pigment made?
A gene on the X (call it gene C) codes for an enzyme that converts the black stuff into orange stuff. (The difference in color is probably caused by a different arrangement of pigment granules.) This gene has two alleles, called "O" and "B" above.
3. What determines orange vs black?
What differs between orange and black cats is the activity of the second enzyme. If the second enzyme is active, the black pigment is converted to orange. If the second enzyme is inactive, the black pigment remains black.
- The O allele → working peptide → catalyzes conversion of black stuff into orange.
- The B allele → no working peptide → no conversion of black stuff into orange so black color shows up (black not masked).
4. General Case .
- We will see many cases like this where one allele → working peptide and other allele does not.
- This is not always the case -- sometimes one allele → working peptide and other allele gives an altered, but working, peptide, as in HbA vs HbS or bloodtypes A and B.
- What happens to phenotype (in a heterozygote) usually depends on job of peptide, and whether gene is on X or autosome. See handout 21B and below for examples.
For a sample problem on sex linked inheritance, try problem 9-9 A & C. (Part B depends on a discussion of dominance, which will be considered later.)
III . How does inheritance work for autosomal genes? See bottom half of Handout 21A.
A. Blue (bl) vs Brown (br) eye color -- an example (For a different classic example of inheritance of an autosomal trait see Sadava figs. 10.3 & 10.4 (for bkg info see previous figs. & table 10.1) or Becker fig. 20-11 & 20-12 [18-11 & 18-12].)
1. Crosses to consider : Suppose you start with homozygous bl X homozygous br (= parental generation) to get heterozygous offspring (first filial generation or F 1 ); then you cross two heterozygous F 1 's to get the next (F 2 ) generation.
2. Genotypes of gametes and zygotes -- genotypes are determined as shown on handout, and the steps are the same as in the case above (steps 1-4). However the types of gametes, genotypes of zygotes, and their proportions, are different, because coat color gene is on X chromosome and eye color gene is on an autosome. Important difference: for autosomal genes, the gametes produced by a heterozygous male or female are identical.
3. Phenotypes (step 5) -- phenotypes depend on the roles of the products of the bl and br alleles. There is no inactivation of autosomes, so you need to figure out what phenotype of heterozygote will be. What will it look like? The usual answer is, "it will be brown, because brown is dominant." But why is br dominant to bl? What is the underlying mechanism?
br allele → enzyme; catalyzes conversion of colorless stuff → brown bl allele → no active enzyme; no brown stuff made. Eye appears blue (due to light scattering) in absence of brown pigment.
br allele → enzyme → brown pigment. Presence of bl allele has no effect on action of br allele or its products. Therefore eyes of heterozygote are brown.
c. This situation (dominance of allele coding for active enzyme) is common -- Usual Features
Enzyme is produced constitutively; number of copies of gene determine amount of mRNA and protein made. One allele → active enzyme → job done; other allele → no (active) enzyme; without active enzyme job doesn't get done. In heterozygote, one allele → active enzyme; other allele → no enzyme. Total amount of enzyme is 1/2 the amount in a normal (homozygote), but it is enough to get the job done. Therefore, effects of allele that produces enzyme override effects of allele that → no enzyme. Note it is the products of the alleles (the enzymes, proteins, etc.) that determine what will happen in a heterozygote, not the alleles themselves. In this case, the normal allele is dominant to the allele that makes no product. This is the usual case, but there are exceptions. Other possibilities are discussed below.
4. Dominance -- terminology and symbols.
- Whenever effects of one allele (really the products of the allele) override effects of another allele, the first allele is said to be dominant and the second allele is said to be recessive.
- In this case br is dominant and bl is recessive. In that case, the symbols B and b (upper and lower case of same letter) are often used for the pair of alleles. Use of two forms of the same letter emphasizes we are dealing with two forms (alleles) of the same gene, not different genes.
- Whether you use B & b or bl & br, it is usually wise to use one set of symbols for the phenotypes (blue and brown) and a DIFFERENT set of symbols for the alleles (bl and br or B and b.) Using the same terms (blue & brown) for alleles/genotypes and traits/phenotypes can cause lots of unnecessary confusion.
5. Summary of results for parents → F1 → F2
For a review of the terminology and a sample cross with an autosomal gene, try problem 9-1. For more practice, try 9-3 & 9-4.
Note that Becker and Sadava both have genetics problems at the end of the respective chapters. (Answers to problems in Sadava are in the back of the book. Answers to problems in Becker are in the Solutions Manual, which should be on reserve in the Biology Library, 601 Fairchild.)
B. General Case -- see bottom of 21A for genotypes; see below and bottom of 21B for phenotypes)
Assume A = normal allele. A* = mutant or altered allele.
2. Crosses -- Genotypes
a. P X P →F1 Parents = AA X A*A* What are gametes from each parent? Mix gametes to get zygotes; Get all heterozygotes in first generation of offspring (F 1 ) b. When cross two F1's → F2 Parents = AA* X AA* What are gametes from each parent? Mix gametes to get zygotes . Not all zygotes are the same. You get: 1:2:1 genotype = AA:2AA*:A*A* in second generation of offspring (F 2 )
Assume A → normal enzyme or peptide A* → altered peptide or none.
Phenotype of AA* depends on how altered the product of A* is, whether it interferes with action of normal enzyme, etc. AA* can look like AA, A*A* or in between. A* can be dominant to A, or A and A* can be co-dominant, or either allele can be only partially dominant over the other. It all depends on what the job of gene A is and how different the product of allele A* is from the product of allele A. See next section for details.
IV. Details of Phenotypes -- Dominance Relationships
A. A* is recessive -- Bl vs Br or PKU
1. Mechanism. In these cases, lack of an enzyme usually causes a block in metabolism. The resulting symptoms (for a disease) can be due to the build up of intermediates and/or to the lack of end product. The individual is normal as long as there is at least one A (normal) allele that → some working enzyme. So AA and AA* or A_ genotypes have normal phenotypes (they look and act the same). But A*A* individuals have no enzyme; they have a block in metabolism and have the disease.
2. Examples:
- Phenylketonuria (PKU) -- can't break down the amino acid phenylalanine → tyrosine. See Sadava fig. 17.1.
- Galactosemia -- can't break down and get energy from galactose
- Tay-Sachs disease (TS) -- can't break down a complex lipid in nerve cells
- Cystic fibrosis (CF) -- can't transport ions properly in and out of cells. See Sadava fig.17.3 (b).
3. Treatment by alteration of diet. In some cases, manipulating the diet gets around the block and prevents symptoms. Examples:
a. PKU -- Block is in conversion of the amino acid phenylalanine (phe) to tyrosine. You need to provide just enough phe so person can make proteins and grow, but you need to keep phe levels low so none is left over, since excess can not be broken down and disposed of. Excess phe (or compounds derived from it) interfere with brain development and cause severe mental retardation. The altered diet is extremely successful -- average IQ of untreated person (on normal diet) is 53; average IQ of treated person is 93. All newborns in the US are screened for this condition. See Sadava fig. 17.11 (17.10).
b. Galactosemia -- block is in conversion of galactose to glucose. Need to eat a diet without galactose (that means without milk, since the sugar in milk is lactose, a disaccharide of glu and gal). Provide glucose instead as an energy source.
4. Protein or Gene replacement
a. Why can't you just add the missing protein? The protein usually gets broken down before it reaches its target cells. This is what usually happens; only a few proteins (mostly those that function in blood such as insulin and clotting factors) can be supplied from outside.
b. Why Gene therapy. It should be easier to target intact genes to the right cells than it is to target intact proteins. Also, once the genes reach the correct cells, they should continue to make proteins for a long time, perhaps for the life of the person. Everyone assumes this will work eventually, but so far, gene therapy has not been very helpful, and there have been some recent serious problems. See Sadava 17.21 (17.20) for outline of the procedure.
5. In some cases you can't do anything to avoid the block in metabolism (except prevention -- see below). Protein (or gene) replacement isn't feasible, and changing diet doesn't help. Examples:
a. TS (Tay Sachs Disease) . Lipid is made irrespective of diet -- it is continually made and broken down ("turned over") in nerve cells of normal people; in people with TS the lipid is made but can't be removed so it just piles up until it destroys the nerve cells and causes death, usually by age 5.
b. CF (cystic fibrosis) -- Defect is in protein needed for ion transport in and out of cells. Protein is not made or is not transported to the right place in the cell. You can't get around actual defect but can relieve symptoms (accumulation of mucus in lungs, etc.) and extend length and quality of life; gene therapy is in the works. Eventually added genes should produce protein that is targeted correctly to the right part of the cell, and allow normal ion transport.
For a problem involving a typical recessive autosomal disease see 9-8. For a problem on genetic testing see 9-13.
B. A* is dominant -- for example, HD (Huntington's Disease)
1. Mechanisms are various. Either
a. Not enough (normal) protein. 1/2 usual amount of protein isn't enough; protein can be catalytic and not shovel fast enough or structural and not hold up the roof. Example: HC.
b. Presence of abnormal protein. Abnormal protein can cause problems directly; functioning of abnormal protein can interfere with normal. Example: HD.
2. Examples of dominant diseases :
a. Hypercholesteremia (HC) . HC is due to a defect in the carrier (receptor) for uptake of cholesterol from the blood. Heterozygotes have half the usual carrier level, and half the rate of uptake. Blood cholesterol gets too high, causing premature heart attacks. See Sadava 17.3A (17.3a). Homozygotes (who are quite rare) have no receptor and have even higher levels of blood cholesterol; they have heart attacks at extremely early ages. This disease (or the mutant allele that causes the disease) is considered dominant, although it is really partially dominant -- homozygotes have less function and more severe symptoms than heterozygotes.
b. Huntington's Disease (HD) . A defective protein gums up the brain causing degeneration of nerve function. Symptoms do not occur until late in life, usually well after reproductive age. In this case, the altered protein encoded by the mutant allele appears to interfere with the functioning protein made by the normal allele. This disease is completely dominant -- homozygotes and heterozygotes have similar symptoms.
3. Treatment:
a. HC. Diet and cholesterol-lowering drugs (statins) are the standard treatment. The drugs inhibit enzymes of cholesterol synthesis. If gene therapy is tried, it will probably not involve adding a normal copy of the defective gene, as one normal copy is already present (in heterozygotes). Instead, the genetic engineering methods will attempt to change regulation ( increase gene expression = up-regulate) the normal allele which is already there.
b. HD. No treatment currently exists. Prevention is the only option. Therapeutic abortion is problematic here, as affected individuals can be asymptomatic for 40-50 years before they develop the disease. If gene therapy is tried here, it will involve attempts to silence (down-regulate) the abnormal allele, possibly by anti-sense technology (adding RNA complementary to mRNA or the equivalent RNAi technique). The issue here is to decrease gene expression. Pre-symptomatic diagnosis is possible -- you can determine from DNA tests if a person (who is still healthy) carries the defective allele and will develop HD eventually. For a discussion of the issues involved here, both biological and otherwise, see Mapping Fate by Alice Wexler. (She is from a family with the condition.)
For a problem on inheritance of a dominant condition, see 9-12. For a review of the relationship between dominance and enzyme function, see 9-18.
C. Incomplete or partial dominance. Example -- red vs white vs pink flower color.
In cases above, AA* looked like one parent. What if the heterozygote looks or acts in between? For example, in some plants, crosses between red-flowered plants and white-flowered homozygous plants → F 1 offspring with pink flowers. This is usually called incomplete or partial dominance. In this case, the flower color gene codes for an enzyme that catalyzes pigment production. An AA* plant makes 1/2 of the enzyme found in an AA; this catalyzes production of 1/2 the amount of red pigment found in an AA. The red pigment is spread more thinly giving a pink appearance. See Sadava fig. 10.12 (10.13). Incomplete dominance occurs because the amount of enzyme is limiting in pigment production. (Incomplete and partial dominance are synonymous; they are similar to, but not exactly the same as, co-dominance, the next case.)
Look at problem 9-7. One of the cases here (you figure out whether it's A or B) is an example of incomplete dominance.
D. Co-dominance.
1. Idea: In all cases so far, one allele → inactive or defective product. What if each allele produces a working product, but the products are different? Then each allele/product will "do its thing" unaffected by the other.
2. Example #1 -- MN blood type : This is controlled by one gene with two co-dominant alleles. Each allele codes for a slightly different version of the same cell surface protein. (The protein is not an enzyme; its normal function is unknown.)
3. Example #2 -- ABO blood type. This is controlled by one gene with 3 alleles; 2 of the alleles are co-dominant. See Sadava 10.13 (10.14).
a. Role of gene product: The gene codes for an enzyme that adds sugars to the surfaces of red blood cells (RBC's). b. Role of Different Alleles The 3 alleles of the gene code for variant forms of the same enzyme. c. Dominance Relationships : Two of the alleles (I A and I B ) are co-dominant to each other and both are dominant over the third allele (i). d. Mechanism (1) The two co-dominant alleles -- code for variant (working) forms of the same enzyme. The substrate binding sites of the two forms are slightly different. Therefore the two different forms of the enzyme bind slightly different sugars, and add different sugars to the surfaces of red blood cells (RBC's). (a). I B -- One working form of the enzyme, coded for by the I B allele, adds the sugar galactose to the surface of RBC's. RBC with galactose → B blood type. (In picture below, G on cell surface = galactose.) (b). I A -- The other working type of enzyme, coded for by the I A allele, adds the modified sugar acetyl galactosamine. RBC with galactosamine → A blood type. (In picture below, A on cell surface = acetyl galactosamine.) (2) The i allele -- codes for an inactive (non working) enzyme; cells with ii genotype → no enzyme → no sugars added. If no sugars → O blood type. e. Heterozygotes: How genotype & phenotype match up. (1). Type AB: I A I B individuals have a mixture of both enzymes and a mixture of sugars on their RBC → AB blood type. (2). Type A & B: Both homozygous I A and heterozygous I A i have only one type of enzyme and acetyl galactosamine on their RBC's and are type A; similarly both I B I B and I B i have galactose and are type B. f. Multiple alleles: This gene has 3 different alleles. Any gene can have many alleles because many different variants of the same DNA sequence are possible. Often the alleles divide into two classes, those → functional enzyme and those → nonfunctional enzyme. In this case, two of the alleles → two slightly different but functional enzymes. For another example of multiple alleles, see Sadava 10.11 (10.12). For role of ABO in transfusions, see Sadava 10.13 (10.14).
See problem 9-2 for a simple case of co-dominant inheritance. See 9-5 for a review of the genetics of the ABO blood type. V. Dominance & Genetic Conditions -- some additional features to consider
A. Dominance/recessiveness for sex linked conditions. How can mutations on the X chromosome be recessive?
You would think mutations on the X would show up in a heterozygous female because of the inactive X, just as black color shows up in a heterozygous O/B cat. Since only one X chromosome works per cell, a heterozygous female should be a mosaic. Some cells should use the X with the mutant allele and have a mutant phenotype; some cells should use the normal X and have a normal phenotype. This is what occurs, so there is no dominance on the cell level in a female, But what about the whole organism? If half the cells have a normal phenotype, the organism as a whole may have a normal phenotype. Therefore, even with the inactive X, you can have dominance on an organismic level for genes on the X.
Example: hemophilia. Hemophilia is caused by a lack of a clotting factor encoded by a gene on the X chromosome. Gene has alleles H and h. (H = good allele; h = inactive allele) In a heterozygous Hh female, only one X chromosome works per cell, so woman is mosaic -- the H allele is used in some cells and the h allele in the others. Therefore 1/2 her cells will make good clotting factor and half will make none. However the clotting factor is secreted into the blood, so her blood will contain clotting factor. Even though some cells make clotting protein and some don't, her blood overall will contain enough clotting protein so there'll be no problem. (Remember the usual case is that 1/2 of the usual amount of enzyme is enough; the clotting factor is an enzyme.)
Another example: colorblindness . A female heterozygous for color blindness, also on the X, has normal vision since aprox. every other cell has good light receptors. In this case, the mosaic is so finely grained it isn't noticeable. See Sadava 10.24.
To review issues of dominance of X-linked traits, look at 9-9B and 9-14.
B. Disease vs variation: Some of the examples above involve "normal variation" -- blue vs brown, AB vs O etc. Neither blue nor brown is considered "abnormal" or "mutant." Some examples involve genetic diseases, where one phenotype is clearly "abnormal" and the other is not. In some cases, it is not clear cut what is "normal" and what is not.
C. Inherited Diseases -- how to define a disease as dominant or recessive. Terminology for genetic diseases
1. Dominant conditions: If one A* causes disease = effects of A* show up even in presence of A = A*_ is sick (whatever allele is in the blank), then you call A* and/or the disease it causes dominant. It doesn't matter if if two A** alleles are worse than one, and A*A* is sicker than A* A -- as long as both homozygotes and heterozygotes have symptoms, the disease is considered dominant, although strictly speaking it should be considered partially dominant.
2. Recessive conditions: If it takes two A* alleles to cause disease, so only homozygotes (A*A*) are affected, allele and/or disease it causes are called recessive.
3. How is this terminology different than usual? In many cases, A* is considered dominant if AA* and A*A* have the same phenotype. A is considered dominant if AA* and AA have the same phenotype. If AA* has an "in between" phenotype, then neither allele is considered to be dominant or A* is said to be partially dominant. For genetic diseases, A* is usually considered dominant if AA* has symptoms, even if they are not as severe as for A*A* (as for hypercholesterolemia.)
VI. Summary of Options for Treatment, Diagnosis & Prevention of Genetic Diseases
A. Alteration of Diet -- in some cases altering the diet can avoid the consequences of genetic blocks. Details above for PKU, galactosemia.
B. Drugs -- in some cases drugs can alter enzyme production or activity and circumvent (or alleviate) consequences of genetic blocks. (Details above for Hypercholesterolemia ). Drugs are often more effective when combined with an alteration of diet.
C. Protein or Gene replacement -- Protein replacement works in a few cases, and gene therapy is under development; see above.
D. Prevention -- what can you do if there is no treatment?
a. Why carrier testing? (For recessive conditions.)
A*A* or aa individuals come primarily from marriages of two heterozygotes (affected individuals don't usually marry and reproduce). If one parent is AA and one is Aa, child can not inherit the disease since child cannot inherit two a's. In other words, a couple (with normal pheno) is at risk of having an affected child only if both parents are Aa. If potential parents are tested, and are both Aa, they can avoid having an aa child by various procedures -- adoption, artificial insemination, egg donation, etc. Or they can do prenatal testing as follows.
b. How do you do Carrier Testing for recessive conditions?
How can you detect carriers if their phenotype is normal? Carriers or heterozygotes usually produce about 1/2 as much enzyme as homozygous normal. This occurs because transcription of structural genes is often not regulated; all copies of the gene are expressed (transcribed) constitutively. AA individuals have two copies of the gene, so they make two units of mRNA and a corresponding amount of protein (100%). AA* (Aa) heterozygotes have only one copy of the (working) gene so they make one unit of (working) mRNA and 1/2 as much protein (50%). As long as some enzyme is present, there is no block in metabolism and the "job gets done." That is why Aa and AA have the same gross phenotype. But their enzyme levels are different, and this allow testing and detection of carriers. (DNA tests can also detect many carrier states.)
c. Amniocentesis, prenatal testing & therapeutic abortion
Cells of fetus can be harvested by amniocentesis (sampling of fluid surrounding the fetus). Fetal cells can be tested for DNA and/or enzyme levels and for chromosomal abnormalities. If mutation causing a disease is known, DNA can be tested directly. For recessive conditions, enzyme levels in fetal cells can be tested; in an aa fetus, enzyme level will be zero. (DNA must be tested if cells in amniotic fluid do not express the enzyme/gene.) If parents wish to do so, an affected fetus can be aborted. Whether a therapeutic abortion is appropriate or not is an ethical question to which there is no simple answer; the question is more difficult when the outcome of the disease is uncertain, or symptoms normally develop only at an advanced age (as for Huntington's).
VII. Pedigrees and Ratios -- How do you follow the pattern of inheritance?
A. Ratios: How do you tell if a condition is dominant or recessive?
For experimental plants and animals, you start with parents of known genotype, say a homozygous normal X homozygous affected, and cross them to get descendants (first an F 1 and then an F 2 generation). Each case should produce characteristic ratios of phenotypes (normal: in between: mutant) in the F 2 . (See handout 21B, bottom.)
1. If gene is autosomal , in F 2 expect 3:0:1 (known as 3:1), or 1:0:3 (known as 1:3) or 1:2:1 ratios in phenotypes. Note that dominance relationships depend on structure and function of gene product, not gene itself.
Problem 9-7 is an example of the use of ratios.
2. If a gene is sex-linked (on the X), the ratios of normal to affected will be different than those given above, and (at least in some cases) ratios will be different depending on which parent (normal or affected) is the female and which is the male. See Sadava 10.23 & intro to chapter 10.
For inheritance of X-linked traits (using ratios), see Problem 9-10.
B. Pedigrees -- How do you tell if a new human condition is dominant or recessive?
The obvious thing is to do the crosses as above and look at the ratios of phenotypes. But you can't cross people! So you look at pedigrees = crosses already done for you.
1. Example of a pedigree = top example on handout 21B. See also Sadava 10.10 (10.10 && 10.11) & 10.24.
Note: a ffected means "has whatever condition we are talking about." It is not the same as in fected, which means "has been invaded by an infectious organism," such as a bacterium or a virus. 2. General procedure for analyzing pedigrees :
a. Use trial and error! You make a guess -- say, let's assume condition is autosomal and dominant. Then you try to assign each individual in the pedigree a genotype consistent with this assumption. If it works, that means condition could be autosomal dominant. If it doesn't work, you've ruled out autosomal dominant. You try to narrow down the possibilities as much as possible.
b. Don't Use Proportions : In most pedigrees the proportions of affected : normal in each marriage are not significant because the sample sizes are too small. If a Aa marries an aa and has a few kids, you don't expect to get an exact 1:1 ratio of Aa: aa in the offspring. Proportions are only useful in very large pedigrees or families with many descendants. What matters in most pedigrees is whether the pedigree is possible -- could these parents have these kids and vice versa? You test for what is possible or not, not whether proportions from each individual cross fit the expected.
3. How you analyze top case on handout 21B:
a. How you rule out dominance -- because affected person (filled symbol) has normal parents. (Normal kids are consistent with a dominant parent; normal parents are not consistent with a dominant kid.)
b. How you show this condition could be recessive -- assign a genotype to each individual. Start with the affected person (II-1), who must be aa, then parents (must be Aa), then kids (also Aa). Spouse of affected person is A_ -- could be Aa or AA; if condition is rare, you assume spouse is AA.
c. Sex Linkage -- is it possible here? Check it out and see! You should be able to see why condition in this pedigree can not be recessive and sex linked. (Could affected mom have normal son?)
4. Things to note about second case shown on handout 21B.
a. This pedigree is consistent with dominant inheritance (but see below). Note that parents of II-1 have the condition being traced but her children do not. This is perfectly consistent with dominant inheritance -- If II-1 is Dd, one of her parents must have had D, but her kids can be dd.
b. This pedigree is consistent with recessive inheritance if I-1 is a heterozygote (= carrier). It is unlikely that I-1 is a carrier of the same condition affecting his spouse if the condition is rare.
c. Could this condition be sex-linked? Try it for yourself. You should be able to see why condition could be dominant & sex-linked but not recessive & sex-linked.
For typical problems involving pedigrees, see 9-11 & 9-15.
Next time: What if you follow the inheritance of more than one gene at a time?
© 2007 Deborah Mowshowitz and Lawrence Chasin Department of Biological Sciences Columbia University New York, NY .
Mary Lyon and the hypothesis of random X chromosome inactivation
Affiliation.
- 1 Institute of Medical Genetics, School of Medicine, Cardiff University, Cardiff, UK. [email protected]
- PMID: 21643983
- DOI: 10.1007/s00439-011-1013-x
The 50th anniversary of Mary Lyon's 1961 Nature paper, proposing random inactivation in early embryonic life of one of the two X chromosomes in the cells of mammalian females, provides an opportunity to remember and celebrate the work of those involved. While the hypothesis was initially put forward by Lyon based on findings in the mouse, it was founded on earlier studies, notably the work of Susumu Ohno; it was also suggested independently by Beutler and colleagues using experimental evidence from a human X-linked disorder, glucose-6-phosphate dehydrogenase deficiency, and has proved to be of as great importance for human and medical genetics as it has for general mammalian genetics. Alongside the hypothesis itself, previous cytological studies of mouse and human chromosomes, and the observations on X-linked mutants in both species deserve recognition for their essential role in underpinning the hypothesis of random X-inactivation, while subsequent research on the X-inactivation centre and the molecular mechanisms underlying the inactivation process represent some of the most outstanding contributions to human and wider mammalian genetics over the past 50 years.
Publication types
- Historical Article
- Biological Evolution*
- Developmental Biology / history*
- Genetics / history*
- History, 20th Century
- Research / history*
- X Chromosome Inactivation / genetics
- X Chromosome Inactivation / physiology*
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Journal Club
- Published: 13 September 2023
- Epigenetics
Mary Lyon and the birth of X-inactivation research
- Marnie E. Blewitt ORCID: orcid.org/0000-0002-2984-1474 1 , 2
Nature Reviews Genetics volume 25 , page 6 ( 2024 ) Cite this article
1297 Accesses
1 Citations
16 Altmetric
Metrics details
- Chromosomes
- Development
- Gene expression
As geneticists, we take for granted that female mammals have one of their two X chromosomes inactivated to achieve dosage compensation of X-linked gene expression with XY males, and that this inactive X chromosome influences the presentation of X-linked traits. However, it was a seminal single-author hypothesis paper by Mary Lyon, published in 1961, that first proposed X-inactivation and how it would affect phenotypes in female mammals. Lyon’s insight was visionary, especially at a time when sex-specific differences in the underlying biology, clinical manifestation and treatment of disease were largely invisible — and their implications for females unknown — because mostly only male subjects were studied.
Lyon’s paper built on the discovery of a densely staining nuclear body in the cells of female but not male mammals that Ohno and Hauschka proposed to be an X chromosome. On the basis of simple microscopy images and mouse phenotypes, Lyon made several critical predictions. She first hypothesized that the densely staining (probably) X chromosome was transcriptionally inactive, that is, she proposed the existence of X-inactivation. For decades, X-inactivation was called Lyonization in recognition of ‘The Lyon hypothesis’. Her reasoning was that XO female mice were viable and fertile, meaning only a single X chromosome was necessary for development and the densely staining one in XX cells could be inactive.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout

Original article
Lyon, M. F. Gene action in the X-chromosome of the mouse ( Mus musculus L.). Nature 190 , 372–373 (1961)
Article CAS PubMed Google Scholar
Download references
Author information
Authors and affiliations.
Epigenetics and Development Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia
Marnie E. Blewitt
Department of Medical Biology, University of Melbourne, Parkville, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Marnie E. Blewitt .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Blewitt, M.E. Mary Lyon and the birth of X-inactivation research. Nat Rev Genet 25 , 6 (2024). https://doi.org/10.1038/s41576-023-00655-0
Download citation
Published : 13 September 2023
Issue Date : January 2024
DOI : https://doi.org/10.1038/s41576-023-00655-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.2(5367); 1963 Nov 16
The Lyon Hypothesis
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (405K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References .
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LYON MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22; 190 :372–373. [ PubMed ] [ Google Scholar ]
- GORMAN JG, DI RE J, TREACY AM, CAHAN A. The application of -Xga antiserum to the question of red cell mosaicism in female heterozygotes. J Lab Clin Med. 1963 Apr; 61 :642–649. [ PubMed ] [ Google Scholar ]

Encyclopedia of Genetics, Genomics, Proteomics and Informatics pp 1130–1131 Cite as
Lyonization
- Reference work entry
- First Online: 01 January 2016
151 Accesses
Variegation in mammalian females as predicted by the Lyon hypothesis. In an XX mammalian female one of the X chromosomes remains in a condensed state (see Fig. L57 ). It replicates its DNA asynchronously and its genes are not transcribed after the blastocyst stage, 3.5–4.5 dpc in the trophectoderm and 5.5–6.5 dpc in the embryo cell initials of the female mouse. In the germline, the inactive X is reactivated at the time of beginning of meiosis (12.5–13.5 dpc; the average time of gestation in the mouse is 19 days). In the male, X-chromosomal inactivation is limited to the duration of meiosis, presumably to restrict deleterious recombination with the Y chromosome. After fertilization, and before implantation, inactivation recurs in the female. The inactive X chromosome moves to the perinuclear position during S phase where it is silenced (Zhang L-F et al 2007 Cell 129:693). The other X chromosome displays a more open structure and its gene content is expressed. The majority of the genes...
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Rights and permissions
Reprints and permissions
Copyright information
© 2008 Springer Science+Business Media
About this entry
Cite this entry.
(2008). Lyonization. In: Encyclopedia of Genetics, Genomics, Proteomics and Informatics. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6754-9_9661
Download citation
DOI : https://doi.org/10.1007/978-1-4020-6754-9_9661
Published : 16 July 2016
Publisher Name : Springer, Dordrecht
Print ISBN : 978-1-4020-6753-2
Online ISBN : 978-1-4020-6754-9
eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- To save this word, you'll need to log in. Log In
Lyon hypothesis
Medical Definition of Lyon hypothesis
Dictionary entries near lyon hypothesis, cite this entry.
“Lyon hypothesis.” Merriam-Webster.com Medical Dictionary , Merriam-Webster, https://www.merriam-webster.com/medical/Lyon%20hypothesis. Accessed 24 Mar. 2024.
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
8 grammar terms you used to know, but forgot, homophones, homographs, and homonyms, commonly misspelled words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, popular in wordplay, the words of the week - mar. 22, 12 words for signs of spring, 9 superb owl words, 'gaslighting,' 'woke,' 'democracy,' and other top lookups, 10 words for lesser-known games and sports, games & quizzes.


COMMENTS
The inactive X hypothesis or the Lyon's hypothesis or the Dosage Compensation is widely known from 1961 which states that only one of the two X chromosomes in the homogametic sex is functional while the other condenses and is inactivated. The X inactivated in some cells would be that from the father, in other cells it would be that from the ...
Time 7 to read. Sixty years ago, British geneticist Mary Frances Lyon (1925-2014) proposed a hypothesis stating that a specific inactivation process occurs in women's sex chromosomes — and those of female mammals in general — transforming them into cellular mosaics. This hypothesis, which has been known ever since as lyonization, has been ...
The hypothesis that gene dosage imbalance between males and females, because of the presence of two X chromosomes in females (XX) as opposed to only one in males (XY), is compensated for by random inactivation of one of the X chromosomes in the somatic cells of females. The inactivated X chromosome becomes the Barr body (see sex chromatin). M. F.
A. Lyon Hypothesis = inactive X Hypothesis The idea that extra X's are genetically inert is called the Lyon hypothesis (or the inactive X hypothesis). According to the Lyon hypothesis, every female is a mosaic, since some of her cells use her maternal X to make proteins and some use her paternal X. ... Note: The term "trait" is used in several ...
Lyon hypothesis (now Lyon Law) to explain X chromosome dosage: •One X is inactivated in females •X-inactivation occurs in early development •~2 weeks after fertilization, ~100's cell stage/blastocyst •Must be re-activated in the germline •X-inactivation is random •X inactivation is clonal (females are essentially mosaics for X ...
Lyon Hypothesis. Postulates that in mammalian cells with more than one X chromosome, usually all but one are heteropycnotic (highly condensed and thus dark-stained at all stages) and form n-1 Barr bodies. The heteropycnosis may not affect the entire length of the chromosome equally. Mary Lyon, a British geneticist, suggested that the ...
1. Introduction. Lyon hypothesized in 1961 that one X chromosome in female mice became inactivated [].In 1962, she followed this prescient paper with an extension of the hypothesis to humans, including the suggestion that genes with Y homologues would escape from 'X-chromosome inactivation' (XCI) [].In the intervening 55 years, we have learned much about the process of inactivation, and ...
The Journal of Pediatrics. The Lyon hypothesis. A curious imbalance appears to occur in nature—the possession of two X chromosomes by one sex (usually the female) compared with only one X in the other sex. Thus, all genes on the X in the female are present in a double dose when compared with the male. Yet most such genes produce the same ...
Mosaicism. In 1961 Mary Lyon, an English scientist, hypothesized that one of the two X chromosomes in females becomes genetically silent early in a female embryo's development.To understand how she arrived at this idea, which has come to be known as "the Lyon Hypothesis," we need to understand what was known about the sex chromosomes.
gained from Lyon's obituary of Charles Ford (Lyon 2001). Background to X-inactivation The mammalian X chromosome had been the subject of considerable research interest for more than a decade before Mary Lyon's proposal of random X chromosome inactivation, and her hypothesis drew extensively from this earlier work.
Abstract. THE Lyon hypothesis 1,2 suggests that in the XX mouse and human female one X chromosome is genetically inactive after a certain stage in embryogenesis; that this 'inactive' X ...
The 50th anniversary of Mary Lyon's 1961 Nature paper, proposing random inactivation in early embryonic life of one of the two X chromosomes in the cells of mammalian females, provides an opportunity to remember and celebrate the work of those involved. While the hypothesis was initially put forward by Lyon based on findings in the mouse, it was founded on earlier studies, notably the work ...
Lyon died aged 89, on Christmas Day 2014, after drinking a glass of sherry, eating her Christmas lunch and settling down in her favourite chair for a nap. She was born in Norwich, UK, in 1925, the ...
The 50th anniversary of Mary Lyon's 1961 Nature paper, proposing random inactivation in early embryonic life of one of the two X chromosomes in the cells of mammalian females, provides an opportunity to remember and celebrate the work of those involved. While the hypothesis was initially put forward by Lyon based on findings in the mouse, it ...
X-inactivation (also called Lyonization, after English geneticist Mary Lyon) is a process by which one of the copies of the X chromosome is inactivated in therian female mammals. The inactive X chromosome is silenced by being packaged into a transcriptionally inactive structure called heterochromatin.
A curious imbalance appears to occur in nature—the possession of two X chromosomes by one sex (usually the female) compared with only one X in the other sex. Thus, all genes on the X in the female are present in a double dose when compared with the male. Yet most such genes produce the same effect in both sexes. An intriguing theory to account for this equalization of expression has recently ...
Search for: Biology Glossary search by EverythingBio.com. The hypothesis, named after Mary Lyon who stated it, suggesting that doseage compensation in mammals is by inactivation of all but one X chromosome in cells with more than one X chromosome. The Barr body, visible in some female mammalian cells, is an inactivated X chromosome.
The Lyon hypothesis refers directly to a Barr body. It was proposed by English geneticist Mary Frances Lyon (1925-) in 1961 that a Barr body is actually an inactivated X chromosome. According to this hypothesis, female mammals sequester one X chromosome in each of their cells during the early stages of development.
However, it was a seminal single-author hypothesis paper by Mary Lyon, published in 1961, that first proposed X-inactivation and how it would affect phenotypes in female mammals. Lyon's insight ...
LYON MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372-373. [ PubMed] [ Google Scholar] GORMAN JG, DI RE J, TREACY AM, CAHAN A. The application of -Xga antiserum to the question of red cell mosaicism in female heterozygotes. J Lab Clin Med. 1963 Apr;61:642-649.
Variegation in mammalian females as predicted by the Lyon hypothesis. In an XX mammalian female one of the X chromosomes remains in a condensed state (see Fig. L57).It replicates its DNA asynchronously and its genes are not transcribed after the blastocyst stage, 3.5-4.5 dpc in the trophectoderm and 5.5-6.5 dpc in the embryo cell initials of the female mouse.
The meaning of LYON HYPOTHESIS is a hypothesis explaining why the phenotypic effect of the X chromosome is the same in the mammalian female which has two X chromosomes as it is in the male which has only one X chromosome: one of each two somatic X chromosomes in mammalian females is selected at random and inactivated early in embryonic development.