Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article

Trisha Prentice, MD, PhD (Neonatologist), Comments
Jessica wallace, rn (nicu nurse), and paul mann, md (neonatologist), comments, kate robson (mother of preterm infant and parent representative), comments, annie janvier, md, phd (neonatologist, clinical ethicist), comments, outcome of the case (annie janvier), john d. lantos, md, comments, does it matter if this baby is 22 or 23 weeks.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Annie Janvier , Trisha Prentice , Jessica Wallace , Kate Robson , Paul Mann , John D. Lantos; Does It Matter if This Baby Is 22 or 23 Weeks?. Pediatrics September 2019; 144 (3): e20190113. 10.1542/peds.2019-0113
Download citation file:
- Ris (Zotero)
- Reference Manager
A 530-g girl born at 22 weeks and 6 days’ gestation (determined by an ultrasound at 11 weeks) was admitted to the NICU. Her mother had received prenatal steroids. At 12 hours of age, she was stable on low ventilator settings. Her blood pressure was fine. Her urine output was good. After counseling, her parents voiced understanding of the risks and wanted all available life-supporting measures. Many nurses were distressed that doctors were trying to save a “22-weeker.” In the past, 4 infants born at 22 weeks’ gestation had been admitted to that NICU, and all had died. The attending physician on call had to deal with many sick infants and the nurses’ moral distress.
Recent studies reveal that, with active treatment, infants born at 22 weeks’ gestation can achieve survival rates of 25% to 50%. 1 Nevertheless, many hospitals do not offer life-sustaining interventions for such infants. For NICU clinicians, then, hospital policies and/or customary practices may conflict with clinical judgment and evidence-based outcome studies. Such conflicts can create moral distress. In this Ethics Rounds, we present a case that reveals these dilemmas and analyze possible solutions.
Domenica was 36 hours old when Dr Jane took over her care. Dr Jane was on nights, covering the delivery room and 72-bed NICU with only a junior resident to help her. Domenica was born at 22 weeks and 6 days’ gestation (determined by an ultrasound at 11 weeks.) Her birth weight was 530 g. She was in her “honeymoon”: stable on low ventilator settings at 30% oxygen. Her blood pressure was fine. Her urine output was good. The mother had received prenatal steroids.
When Dr Jane was charged with Domenica’s care, she could feel the tension in the voice of the attending day team. The nurses were distressed that doctors were trying to save a “22-weeker.” They thought that she could not survive and that treatment would just cause pain and prolong the inevitable dying process. In that unit, many infants born at 23 weeks’ gestation received interventions and often survived, but the 4 infants born at 22 weeks’ gestation that had been admitted to the NICU had died. Dr Jane spoke to Domenica’s parents. They were realistic and knew she would probably die but they still hoped to beat the odds.
Dr Jane admired the NICU nurses. She knew how devoted they were and how they had to do all the tough work: pricking, poking, prodding, and suctioning while supporting and comforting the parents.
As midnight approached, Dr Jane realized that a disproportionate amount of her call had been spent managing the nurses’ distress and validating their concerns. Her interventions seemed to work. She gradually felt less hostility from the nurses. Then, at midnight, the nurses changed shifts and it seemed a revolution was starting in the nurses’ staff room. She realized that she would need to start counseling the new nurses; this was taking time away from her caring for the other 71 infants in the NICU.
On the spur of the moment, she changed the identification tag on Domenica’s incubator, threw away the 1 that said “22 weeks,” and replaced it with 1 that said “23 weeks.” When a nurse asked, she said that the gestational age had been uncertain, and that Domenica was in fact probably born at 23 weeks’ gestation. The nurse taking care of Domenica let out a big sigh and said, “Well, there is some hope. We are working for something.” Moral distress disappeared for the rest of the night. Dr Jane told the resident, “Don’t do this at home, not the best thing to do. I know I will pay for this, but I don’t have any ideas for tonight, do you?”
Did Dr Jane do the right thing?
The presence of moral distress is sometimes palpable when you enter a NICU; its influence can be far reaching and at times underappreciated. Traditionally, moral distress has been described as an organizational or systems problem that constrains a clinician into providing care that they judge is not in a patient’s interests. 2 , 3 Professionals suffer when they believe that they are just causing pain with no hope of benefit. To respond to such distress, we must consider the appropriateness or accuracy of the judgment that there is no hope of benefit.
Much moral distress occurs within the context of medical uncertainty. A difference of 24 hours between the gestational ages of 22 weeks and 6 days and 23 weeks does not carry with it the significant change in prognosis worthy of the discrepant moral or emotional response that the altered bassinette tag brings. Although Domenica may indeed be in a “honeymoon phase” and difficult days may be ahead, there is no evidence-based reasoning to justify the belief that a trial of therapy is any more unethical for her than for an infant born at 23 weeks’ gestation. The arbitrary lines that institutions draw between impermissible and permissible (or between futility and hope) on the basis of estimates of gestational age may be well intentioned. They seek to limit expensive and burdensome treatments of limited benefit. Yet, we know that gestational age alone is inadequate for accurate prognostication. 4 We do not yet know if Domenica will have a good outcome. We do know that not offering intensive care will surely lead to her death.
Despite these facts, the moral distress felt by the nurses is real. They remain certain in their conviction that intensive care is not in Domenica’s interests because of their own fixed beliefs and values. The objective evidence is 1 thing. Their own experiences are another. They feel that they are being compelled to do the wrong thing and thus that their moral integrity is being compromised. This moral distress needs to be addressed and managed.
The physician has appropriately endeavored to hear and validate concerns and communicate clear goals of care. This has managed but not resolved the distress. The effects do not carry over from nursing shift to the next. Furthermore, the process is time consuming and likely emotionally draining for the physician.
This case highlights the hidden costs of moral distress. This case will have far-reaching ramifications. One could imagine that the care provided to Domenica may differ from that provided to a more mature infant who is “worth fighting for.” Should Domenica die, it would solidify the convictions of the nurses that treatment of “22-weekers” is futile. Her death would heighten the distress response should another infant with a similar gestation be admitted to the unit. 5 , 6 The moral residue of Domenica’s case may have a powerful effect on responses to future cases.
Meanwhile, the physician is struggling with the need to care for and support the morally distressed nurses while managing the needs of Domenica and many other patients in the unit. As the clinical leader whose tasks include managing the emotions of the NICU team, the physician is expected to deal with the moral distress. She is painfully aware that the nurses are hoping that Dominica’s life-sustaining interventions will be withdrawn. The physician, however, believes that this infant might survive. The parents want Dominica to receive life-sustaining interventions. Thus, Dr Jane takes a moral shortcut and deceives the nurses about Dominica’s gestational age.
By deceiving the nurses, the physician has killed her own moral integrity to protect the integrity of the distressed nurses, ensure ongoing intensive care, and allow her to do her job. This desperate strategy has also modeled deceptive conduct to an impressionable physician in training. The physician does not appear proud of her actions, but she struggles to find a more appropriate strategy to manage the interests of all involved. Given the situation, her actions were ethically appropriate. But they should be taken as a signal that there are deeper institutional problems in this NICU that need to be addressed.
Was there a better approach? It is unlikely that any single isolated intervention will be sufficient because there are both organizational and individual factors at play. Institutions need to work hard to create ethical environments in which clinicians are trained to critically appraise ethical issues before they are raised by emergent clinical situations and where their concerns are heard and validated. Individuals need support to grapple with the subjectivity and uncertainty surrounding clinical decisions and be open to having their moral judgments challenged.
Although the experience of moral distress is real, it does not necessarily follow that the clinical plan or situation that causes that distress is itself unethical or requires change. 7 When moral distress cannot be resolved because of moral subjectivity, individuals need to be mindful of the ways in which their own distress may affect others. These hidden costs of moral distress require further acknowledgment within the dialogue of moral distress.
Deception in health care is rarely justified. The physician felt that, in this case, deception was justified, but it could have a big cost. If the nurses found out the attending physician had been dishonest about the gestational age, trust among the entire health care team and unit morale could be jeopardized.
As health care professionals, we have a duty to interact honestly with our patients, their families, and interprofessionally. This obligation was successfully met in the first part of the shift, with the physician candidly engaging the nursing staff, discussing the goals of care for the patient, and trying to address the nurses’ clinical concerns. The importance of such deliberations to address moral distress and ethical dilemmas in the NICU cannot be overstated. 8 Both nurses and physicians report that lack of communication among team members is a common contributor to moral distress. 9 , 10 Deception could end up exacerbating moral distress rather than relieving it.
The basis for the nurses’ emotional distress in this scenario is likely multifactorial. Nurses frequently have a limited voice in decisions regarding resuscitative efforts and ongoing clinical care for extremely premature infants. They are expected, however, to unequally shoulder the burden of hands-on care for periviable infants and to meet the emotional needs of their parents even when they feel therapeutic interventions are not in the best interests of the patient. 1 , 11 , 12 Routine neonatal nursing care (eg, obtaining cuff blood pressures and changing a diaper) can cause life-threating skin breakdown in the friable, gelatinous skin of periviable infants. Standard nursing interventions such as intravnous insertions, laboratory draws, suctioning, securing endotracheal tubes, line placement, and the care of drains may cause significant pain and discomfort for infants. When infants are extremely fragile, even opening an isolette door can cause distress. 13
There are not many evidence-based guidelines to support best nursing practice for periviable infants, leaving many nurses to feel that they are experimenting on patients by trial and error. Nurses who participate in such care often feel like they are abandoning their oath to “do no harm” and that painful interventions are not in the infant’s best interest.
When the infants have a good chance of a good outcome, nurses feel that the pain is worth it. 1 A nurse in this case is quoted as saying, “Well, there is some hope. We are working for something.” But was there actually more hope for an infant born at 23 weeks’ gestation than for an infant born at 22 weeks and 6 days’ gestation? Neonatal nurses frequently overestimate poor outcomes for premature infants. Such outcome misconception is highest in nurses who infrequently care for periviable infants. 14 Nurses frequently hear about patients who have poor long-term outcomes. They have limited opportunities to see NICU survivors who are thriving. 15 It is always hard to know whether to base clinical judgments on peer-reviewed multicentre outcome studies that show improving outcomes for periviable infants 16 or, instead, to base judgments on local experiences in their own NICU.
Educating the nursing staff on the unique clinical features of Domenica’s case is therefore of the utmost importance. Some factors suggest a higher than average likelihood of a good outcome. Domenica is a girl of average birth weight who received prenatal steroids and was born at a center that provided active treatment at the time of delivery. These variables are all associated with an improved likelihood of survival. 17 Furthermore, given the margin of error on ultrasound dates, 18 Domenica could in fact truly have been born at 23 weeks’ gestation.
Given all these factors, the doctor’s deception in this case regarding Dominica’s presumed gestational age, although expedient for a shift, becomes a missed opportunity for a teachable moment. Even if Domenica does well, the nursing staff will remain reluctant to provide treatment to other neonates born at 22 weeks’ gestation. There is a clear disconnect between the physician’s beliefs about possible clinical outcomes for neonates born at 22 weeks’ gestation and the nurses who think that they “don’t survive.” 19
There are almost no meaningful distinctions to be made regarding treatment of an infant born at 22 weeks and 6 days’ gestation compared with an infant who has achieved 23 weeks’ gestation. The attending physician needs to continue to engage each shift of nurses with as much energy as she can spare; this is the only way to move the clinical care team away from gestational age–based thinking and toward a more holistic care approach that can accommodate the certainty of clinical uncertainty for all periviable infants.
This case fills me with feelings of profound sympathy for all involved: the frightened parents, the exhausted doctor, the nurses struggling with their complex feelings, and, most of all, for the infant. A NICU can feel like a war zone at times with so many competing priorities and life-or-death situations. The drama that is the everyday normal of the NICU can cloud our sense of what is ethical in the moment.
If the noise around the central issue is eliminated; that is to say, if one takes away the fatigue of the medical staff, the lateness of the hour, the doubts or questions 1 caregiver had about intentions or abilities of another, the vulnerability of the parents, and the confusion around discussions of gestational age and viability, a clearer question is left: is it ever ethical for a doctor to lie to nurses about a patient? In this case, Dr Jane’s lying is understandable but ultimately not justifiable. Honesty underpins the trust that is necessary for a team to work together in the NICU. Although it is sometimes difficult to figure out what the truth is, if we know something to be true, it must be acknowledged.
The intention of this lie was to protect the patient but the telling of the lie opened up numerous other channels of potential harm. Once the lie was uncovered, what would that do to the trust relationship between different members of the medical team? What would it mean to the next family with an infant born at 22 weeks’ gestation? Or the next family of an infant whom the doctor claims was born at 23 weeks’ gestation? We cannot confine the impact of our actions to 1 moment or 1 relationship. The impact can continue to spiral in directions we could not possibly anticipate.
It is especially hard to deal with institutional ethical issues at the bedside. Ideally, ethical disputes and discussions should happen up the line (in general theoretical discussions) or well down the line (during debriefs of critical incidents). They should never happen in the room with the patient and family, where the focus should only be on the human being who needs care. The question of whether we should resuscitate “22-weekers” does not belong in the patient’s room. If we create ample opportunities to discuss such questions in more appropriate environments, we will reduce the risk of them occurring in the moment when they are most likely to do harm. Creating these opportunities for exploring ethical issues far away from the bedside helps caregivers manage moral distress and be more present for their actual patient in the moment when their skills are needed.
I am the parent of an infant who spent time in a NICU. I am filled with appreciation for the actions of the doctor. Although I cannot find a way to ethically excuse Dr Jane, I see her as a doctor desperately trying to do the best for 1 small patient at a particularly significant and vulnerable moment. So, although it may seem like I am throwing this doctor under the bus, my emotional response is just the opposite. This is exactly the type of doctor I would wish for in this type of situation, one so dedicated that she or he is willing to entertain personal risk to help Domenica and her parents. My hope is that this 1 action did not lead to harm for this doctor, this infant, or this family and that it in fact advanced or improved care for infants at the edge of viability by helping caregivers gain a new understanding of both the perils and possibilities facing infants born so early.
We know gestational age is imprecise: approximately one-half of the “23-weekers” we take care of are in fact “22-weekers.” We have also known for a long time that gestational age is only 1 of several factors that predicts survival. 4 Despite our knowledge, many guidelines regarding intervention in the periviable period divide infants on the basis of completed 7-day periods of gestation. Such guidelines are neither rational nor ethically defensible. 20 – 22 Infants who are premature, like all other patients, should be assessed as individuals. The aim should be to establish individualized goals of care for each patient and with each family while recognizing uncertainty rather than acting on gestational age labels. 23
Dr Jane violated the nurses’ trust. Honesty is essential for trusting, collegial relationships. But it is easy to say that as an outsider looking in. It is easy for me to write it while sitting in my pink writing chair with my perfect cappuccino. But what were Dr Jane’s alternatives? None of them was much better.
Dr Jane was aware of the empirical evidence about gestational age. She listened to and discussed these issues with the nurses working in the evening. But taking more hours for “debriefing and educating” the night shift would have caused a threat to patient safety. The difficult shift described in the case was not the time or the place to question interventions for fragile infants at risk of death and disability.
These questions need to be discussed in other forums. I wonder if in a large unit such as the one described, the “Time-out: not now/later” approach would have worked. This approach is easier in small open-bay units where all nurses can be reached at the same time and doctors know each nurse individually. In single-room large units, interactions are less efficient and personal. The organization of the provider shifts are also important: interactions between providers are often easier in units with 12-hour shifts and rotating schedules.
This “Time-out: not now/later” approach also requires that the moral distress be dealt with constructively. Taking care of ill patients is difficult. The nurse, resident, and physician taking care of Domenica need to know about goals of care. The other providers need to support them, not add fuel to the fire. Disorganized and excessive “group therapy” can be harmful. Too often, nurses will spend their well-deserved break speaking about “Domenica cases” instead of re-energizing themselves for the rest of their hard shifts. Doctors do the same. A frequent (and worse) situation is when doctors and nurses agree. This can result in unsolved frustration and resentment for those directly dealing with such difficult cases. But whose job is it (or should it be) to manage the nurses’ moral distress during evening and night shifts?
Perhaps Dr Jane felt the most distress that night? Many physicians would have just told the nurses to do their jobs, to face the music, and to stop complaining. Dr Jane took a more creative approach; one that was, admittedly, ethically questionable. By adding several hours to the gestational age, Dr Jane improved Domenica’s care but also created new problems that will have to be dealt with later.
I was biased in commenting on this case because I am Dr Jane.
After the end of the night shift, I disclosed to Domenica’s night nurse that I had changed the gestational age. I told her I was sorry, that I was exhausted, that I did not know what to do to help. I explained that Domenica was born at 22 weeks and 6 days’ gestation, ±5 days, so she may well have been born at 23 weeks’ gestation. Domenica’s nurse smiled. She told me “her 22-weeker” was still stable and this made her hope for the best.
I have reflected a lot about this case. Had Domenica’s parents not been present at bedside, I would not have changed the gestational age. I would have been able to discuss the case with the nurse privately. Because the parents were present, the nurse had limited opportunities to discuss her distress with other providers. She heard the resident and me speak with the parents. She understood what was going on and knew the parents were realistic.
We reflected about the fact that we felt like a team: Domenica was more than “a 22-weeker.” She was “our patient.”
Although I was Dr Jane in this case, I am not the only Dr Jane. Most neonatologists have dealt with similar cases. I have realized through the years that who Dr Jane is matters. It matters that she is a woman. Her age matters. Doctors’ views and styles are shaped by their own experiences with previous patients and perhaps with their own children.
I work with 300 nurses in a large unit. I did my residency where I now work. Some of the nurses I work with saw me grow personally and professionally over the years. They helped me become who I am. Over the years, I shared their moral distress. When I was a resident 22 years ago, like many of them, I was morally distressed about “24-weekers.” Neonatologists listened to us, told us about the outcomes, and gave us articles, but our distress did not decrease. Education, science, and rationality are not enough in these cases. We needed to personally see infants survive and do well. We did not need graphs, percentages, or to debrief or be listened to. Over the years, I have seen tiny infants who survive and do well. I have also seen infants survive and do badly. I also delivered preterm at 24 weeks’ gestation. Doing neonatal follow-up as a fellow gave me a lot of humility. Being in contact with families and on family social media groups helps me remain curious and humble. I see how often my prognostications turned out wrong.
Adding 1 day to Domenica’s gestational age made many providers have a different attitude about her care. It was an effective, if unprofessional, intervention. I have never repeated this; it could have had serious negative impacts in other circumstances. Since Domenica’s case, we now have dedicated support sessions for nurses that are organized by nurses. Sometimes, we do this on an emergency basis. There is still so much more to do.
Domenica had a difficult NICU course, but she survived. She is now 6 years old. Her parents are thrilled about how well she is doing. They are now family stakeholders who help us improve care, teaching, and research. With other parents, they wrote an article called “our child is not just a gestational age” 24 that is closely related to the case.
This case presents a straightforward conflict between bad policy and bad behavior. It is never good for professional morale for doctors to deceive nurses. It is never good for patients when clinicians make decisions that do not reflect the best scientific evidence and clinical judgment. In this case, a doctor was trying to do what she thought was best for her patient and her parents. She was inhibited by many nurses’ perception that such treatment was futile. These perceptions reflect a widely held but erroneous belief that treatment of babies born at 22 weeks is futile. As this case and many studies show, it is not. Decisions for babies born at 22 weeks should be made the way all good clinical decisions are made, by taking into account all the relevant clinical information and the parents’ preferences then making an individualized clinical judgment.
Dr Janvier conceptualized and drafted the introduction, case, and affiliated comment; Drs Prentice, Mann, and Lantos and Ms Wallace and Ms Robson conceptualized the ethical dilemma and drafted the affiliated comment; and all authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
FUNDING: No external funding.
Competing Interests
Reply: does it matter if this baby is 22 or 23 weeks.
Dear Sir or Madam:
I read with great interest the article by Janvier et al. It elegantly discusses providers’ moral dilemma caring for infants born at 22 weeks. I was surprised a case where intervention was refused was not included for a contrasting viewpoint. As a pediatric subspecialty surgeon and mother of a deceased 22-weeker, I feel compelled to offer that perspective.
After 4 years and numerous medical interventions, at nearly 40 years old I was pregnant with my only child. When I began having cervical change while out of town, my husband and I were nervous but remained hopeful. After a cerclage and several days convalescence, we were ready to return home. At hours shy of 22 weeks, our son had a chance. Unfortunately, en route home my condition changed. My husband and I drove directly to our local hospital where I was likely in labor and possibly leaking amniotic fluid. A friend and I inquired about steroids and magnesium to improve my son’s outcome and tocolytics to slow his delivery, but were repeatedly told these interventions “don’t work until 23 weeks.” When my friends, family, and I continued to ask, refusal was portrayed as hospital policy. We were given numerous reasons: I “could get an infection,” steroids and magnesium “aren’t effective at 22 weeks,” my son “probably wouldn’t survive delivery;” and the real kicker because I should have known better, “they don’t make breathing tubes small enough” for my 500g child.
Nearly 24 hours later, my water broke. My son’s arm popped out, he got stuck, I struggled to deliver him, and he died in the process. In my subsequent weeks of maternity/bereavement leave I invested time exploring literature on periviable birth. Going into the hospital, I was aware of thriving 22-weekers and wondered who these super-human babies were if proactive treatment was not physiologically beneficial.
The authors discuss, “deception in healthcare,” in a case where gestational age was misrepresented to facilitate care. Where is discussion of “deception” as it pertains to patients? The information given to me was not rooted in current evidence and I have learned from other mothers this is a common experience. In a study from Germany, only 2 of 47 infants died during delivery demonstrating it is possible to safely deliver a 22-weeker (1). Moreover, their survival rate was 61% with complication rates no different than 23-weekers. In Sweden, another study demonstrated a 53% survival rate with similar morbidity (2). Within North America, the University of Iowa reports a 59% survival rate with 22-weekers (3) and recently demonstrated an amazingly low rate of morbidity with proactive treatment (4).
Working in a center that treats some of the sickest children from around the world, I appreciate moral distress. I have felt it more than once. But as a patient I offer a perspective that hopefully shows the value of persevering in difficult situations. This experience shattered my faith in medicine and it is difficult to cope with aspects of my own pediatric career; prenatal counseling, consulting on children in the NICU, caring for healthy infants. The downstream ramifications impact the colleagues who cover for me and the patients I should have been caring for. I worry I will not last in pediatric medicine.
The next time you or your colleague encounters a 22-weeker feared futile, I’d ask you consider the parents’ perspective. What has shaken my trust most is not that my son died, because I recognize it was a grim situation, but that nobody would even try to help him. In my own professional life, I have had failures but have also been part of amazing “saves” and have learned to imagine the possibilities in our ever-advancing medical environment. I will always wonder, “what if someone had tried when it was my child?”
References:
1. Mehler K, Oberthuer A, Keller T, et al. Survival Among Infants Born at 22 or 23 Weeks’ Gestation Following Active Prenatal and Postnatal Care. JAMA Pediatr. 2016;170(7):671–677.
2. Backes, C.H., Söderström, F., Ågren, J. et al. Outcomes following a comprehensive versus a selective approach for infants born at 22 weeks of gestation. J Perinatol 39, 39–47 (2019)
3. University of Iowa Childrens Hospital. Accessed December 15, 2019. https://uichildrens.org/health-library/15-questions-you-should-ask-about...
4. Watkins PL, Dagle JM, Bell EF, and Colaizy TT. Outcomes at 18 to 22 Months of Corrected Age for Infants Born at 22 to 25 Weeks of Gestation in a Center Practicing Active Management. J Pediatr. 2019 Oct 9 [Epub ahead of print].
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Getting Pregnant
- Registry Builder
- Baby Products
- Birth Clubs
- See all in Community
- Ovulation Calculator
- How To Get Pregnant
- How To Get Pregnant Fast
- Ovulation Discharge
- Implantation Bleeding
- Ovulation Symptoms
- Pregnancy Symptoms
- Am I Pregnant?
- Pregnancy Tests
- See all in Getting Pregnant
- Due Date Calculator
- Pregnancy Week by Week
- Pregnant Sex
- Weight Gain Tracker
- Signs of Labor
- Morning Sickness
- COVID Vaccine and Pregnancy
- Fetal Weight Chart
- Fetal Development
- Pregnancy Discharge
- Find Out Baby Gender
- Chinese Gender Predictor
- See all in Pregnancy
- Baby Name Generator
- Top Baby Names 2023
- Top Baby Names 2024
- How to Pick a Baby Name
- Most Popular Baby Names
- Baby Names by Letter
- Gender Neutral Names
- Unique Boy Names
- Unique Girl Names
- Top baby names by year
- See all in Baby Names
- Baby Development
- Baby Feeding Guide
- Newborn Sleep
- When Babies Roll Over
- First-Year Baby Costs Calculator
- Postpartum Health
- Baby Poop Chart
- See all in Baby
- Average Weight & Height
- Autism Signs
- Child Growth Chart
- Night Terrors
- Moving from Crib to Bed
- Toddler Feeding Guide
- Potty Training
- Bathing and Grooming
- See all in Toddler
- Height Predictor
- Potty Training: Boys
- Potty training: Girls
- How Much Sleep? (Ages 3+)
- Ready for Preschool?
- Thumb-Sucking
- Gross Motor Skills
- Napping (Ages 2 to 3)
- See all in Child
- Photos: Rashes & Skin Conditions
- Symptom Checker
- Vaccine Scheduler
- Reducing a Fever
- Acetaminophen Dosage Chart
- Constipation in Babies
- Ear Infection Symptoms
- Head Lice 101
- See all in Health
- Second Pregnancy
- Daycare Costs
- Family Finance
- Stay-At-Home Parents
- Breastfeeding Positions
- See all in Family
- Baby Sleep Training
- Preparing For Baby
- My Custom Checklist
- My Registries
- Take the Quiz
- Best Baby Products
- Best Breast Pump
- Best Convertible Car Seat
- Best Infant Car Seat
- Best Baby Bottle
- Best Baby Monitor
- Best Stroller
- Best Diapers
- Best Baby Carrier
- Best Diaper Bag
- Best Highchair
- See all in Baby Products
- Why Pregnant Belly Feels Tight
- Early Signs of Twins
- Teas During Pregnancy
- Baby Head Circumference Chart
- How Many Months Pregnant Am I
- What is a Rainbow Baby
- Braxton Hicks Contractions
- HCG Levels By Week
- When to Take a Pregnancy Test
- Am I Pregnant
- Why is Poop Green
- Can Pregnant Women Eat Shrimp
- Insemination
- UTI During Pregnancy
- Vitamin D Drops
- Best Baby Forumla
- Postpartum Depression
- Low Progesterone During Pregnancy
- Baby Shower
- Baby Shower Games
Breech, posterior, transverse lie: What position is my baby in?

Fetal presentation, or how your baby is situated in your womb at birth, is determined by the body part that's positioned to come out first, and it can affect the way you deliver. At the time of delivery, 97 percent of babies are head-down (cephalic presentation). But there are several other possibilities, including feet or bottom first (breech) as well as sideways (transverse lie) and diagonal (oblique lie).
Fetal presentation and position
During the last trimester of your pregnancy, your provider will check your baby's presentation by feeling your belly to locate the head, bottom, and back. If it's unclear, your provider may do an ultrasound or an internal exam to feel what part of the baby is in your pelvis.
Fetal position refers to whether the baby is facing your spine (anterior position) or facing your belly (posterior position). Fetal position can change often: Your baby may be face up at the beginning of labor and face down at delivery.
Here are the many possibilities for fetal presentation and position in the womb.
Medical illustrations by Jonathan Dimes
Head down, facing down (anterior position)
A baby who is head down and facing your spine is in the anterior position. This is the most common fetal presentation and the easiest position for a vaginal delivery.
This position is also known as "occiput anterior" because the back of your baby's skull (occipital bone) is in the front (anterior) of your pelvis.
Head down, facing up (posterior position)
In the posterior position , your baby is head down and facing your belly. You may also hear it called "sunny-side up" because babies who stay in this position are born facing up. But many babies who are facing up during labor rotate to the easier face down (anterior) position before birth.
Posterior position is formally known as "occiput posterior" because the back of your baby's skull (occipital bone) is in the back (posterior) of your pelvis.
Frank breech
In the frank breech presentation, both the baby's legs are extended so that the feet are up near the face. This is the most common type of breech presentation. Breech babies are difficult to deliver vaginally, so most arrive by c-section .
Some providers will attempt to turn your baby manually to the head down position by applying pressure to your belly. This is called an external cephalic version , and it has a 58 percent success rate for turning breech babies. For more information, see our article on breech birth .
Complete breech
A complete breech is when your baby is bottom down with hips and knees bent in a tuck or cross-legged position. If your baby is in a complete breech, you may feel kicking in your lower abdomen.
Incomplete breech
In an incomplete breech, one of the baby's knees is bent so that the foot is tucked next to the bottom with the other leg extended, positioning that foot closer to the face.
Single footling breech
In the single footling breech presentation, one of the baby's feet is pointed toward your cervix.
Double footling breech
In the double footling breech presentation, both of the baby's feet are pointed toward your cervix.
Transverse lie
In a transverse lie, the baby is lying horizontally in your uterus and may be facing up toward your head or down toward your feet. Babies settle this way less than 1 percent of the time, but it happens more commonly if you're carrying multiples or deliver before your due date.
If your baby stays in a transverse lie until the end of your pregnancy, it can be dangerous for delivery. Your provider will likely schedule a c-section or attempt an external cephalic version , which is highly successful for turning babies in this position.
Oblique lie
In rare cases, your baby may lie diagonally in your uterus, with his rump facing the side of your body at an angle.
Like the transverse lie, this position is more common earlier in pregnancy, and it's likely your provider will intervene if your baby is still in the oblique lie at the end of your third trimester.
Was this article helpful?
What to know if your baby is breech

What's a sunny-side up baby?

What happens to your baby right after birth

Perineal massage

BabyCenter's editorial team is committed to providing the most helpful and trustworthy pregnancy and parenting information in the world. When creating and updating content, we rely on credible sources: respected health organizations, professional groups of doctors and other experts, and published studies in peer-reviewed journals. We believe you should always know the source of the information you're seeing. Learn more about our editorial and medical review policies .
Ahmad A et al. 2014. Association of fetal position at onset of labor and mode of delivery: A prospective cohort study. Ultrasound in obstetrics & gynecology 43(2):176-182. https://www.ncbi.nlm.nih.gov/pubmed/23929533 Opens a new window [Accessed September 2021]
Gray CJ and Shanahan MM. 2019. Breech presentation. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448063/ Opens a new window [Accessed September 2021]
Hankins GD. 1990. Transverse lie. American Journal of Perinatology 7(1):66-70. https://www.ncbi.nlm.nih.gov/pubmed/2131781 Opens a new window [Accessed September 2021]
Medline Plus. 2020. Your baby in the birth canal. U.S. National Library of Medicine. https://medlineplus.gov/ency/article/002060.htm Opens a new window [Accessed September 2021]

Where to go next


- Delivery Room Management
- Ethics, Decision making, and Quality of Life
- Hematology; blood and platelet transfusions
- Hypoxic Ischemic Encephalopathy
- Oxygen Therapy
- Pulmonary hypertension, ECMO and inhaled nitric oxide
- Retinopathy
- Sepsis and Necrotising Enterocolitis
- Argentinian Birds
- Australian Birds Part 1
- Australian Birds Part 2
- Australian Wildlife, non-avian
- Birds of Quebec
- California Birds
- English Birds
- Florida Birds
- New Zealand Birds
- Wildlife of the Canadian Rockies
- Wildlife of the West coast, Vancouver Island
- Birds of Hawai’i
- AAP, Scottsdale AZ 2014
- Atlanta 2013
- Brigham and Women’s Hospital September 2016
- Buenos Aires 2017
- Helsinki 2013
- Melbourne 2012
- Neonatologie aan de Maas 2014
- New Brunswick 2013
- PAS 2016- Prognosis Symposium
- Pediatric Academic Societies meeting, Boston 2012
- Saudi Arabia 2012
- Nitric Oxide in the Preterm
- Présentations françaises
- Publications
Active intensive care at 22 weeks gestation
Even the New England Journal are getting in on the act ( Lee CD, et al. Neonatal Resuscitation in 22-Week Pregnancies. N Engl J Med. 2022;386(4):391-3 ), I guess that someone talked to the editors about the practice variation in resuscitation of profoundly immature babies, and in response they have published this short vignette with 2 somewhat opposing views, Leif Nelin who promotes the idea that we should recommend universal active intervention, and Elizabeth Foglia who is in favour of recommending selective resuscitation.
I find it very interesting that there is not a 3rd author promoting an approach which still happens in many centres, i.e. recommending universal comfort care.
It is also interesting that there is no real disagreement on the facts, that without active intensive care mortality is 100%; that with intensive care some babies survive, and the majority of the survivors have good lives. The actual proportion of survivors is, of course, very variable, and it requires a commitment of both obstetrics and neonatology to work together to achieve the best results.
Dr Foglia says 2 things that require some reflection, she notes that “almost all extremely preterm infants require resuscitative interventions after birth to survive” which is sort of true, but depends on what you mean by “resuscitative interventions”, in most centres all such babies have endotracheal intubation shortly after birth, but further “resuscitative interventions” are uncommon. The second thing is “The current limit of viability is 22 weeks’ gestation.” That is stated as a verity, but it ignores 3 things, 1. we never know exactly what the GA is, except after IVF, so if you actively intervene for all 22 week GA babies, you will have intervened for some at 21 weeks. 2. If survival at 23 weeks can be as high as 60%, surely at 21 weeks and 6 days it would not suddenly drop to zero! 3. There are reported survivors who were thought to be <22 weeks.
Current guidelines do not often recommend antenatal steroids at 22 and 23 weeks, which is partly because of a lack of such infants in randomized controlled trials, but we are unlikely to have substantial numbers of mother in trials at those gestations for a while, if ever, so observational data are all we are likely to have. Rossi RM, et al. Association of Antenatal Corticosteroid Exposure and Infant Survival at 22 and 23 Weeks. Am J Perinatol. 2021(EFirst) . This article, as one example, calculated the probability of survival at 22 and 23 weeks of GA, according to whether steroids were given prior to delivery. The data source they used had no information of timing of steroids, it was just a checkbox, yes or no. It probably includes, therefore, many babies with brief steroid exposure. Survival is only presented for babies who received active neonatal intensive care. The overall survival at 22 weeks, to one year of age, is shown below, divided by birth weight categories.
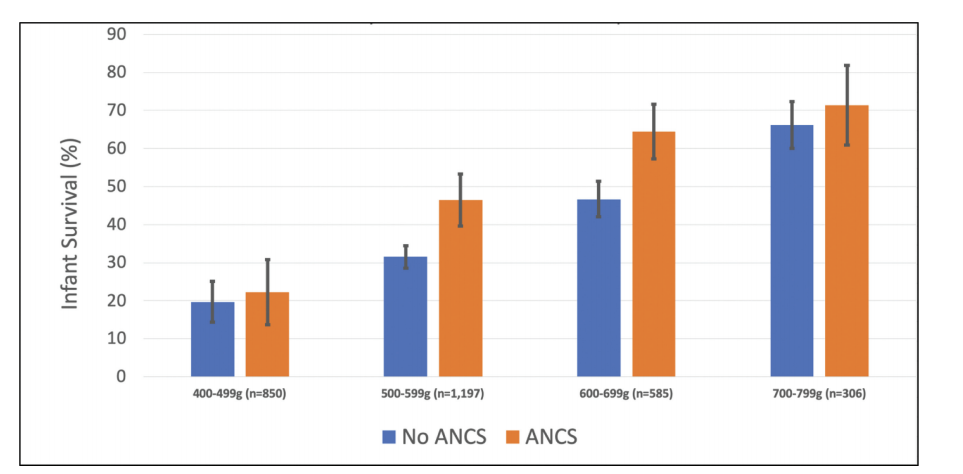
They don’t have the same sort of birth weight breakdown for the 23 week babies, but overall 1 year survival was 58% after antenatal steroids, and 48% without steroids. Relative risk 1.5 (95% compatibility intervals, 1.3-1.6). 62% of the 22 week deaths of babies who had antenatal steroids were before 7 days of age, as were 53% of the 23 weeks infants.
Currently all the data about such deliveries is consistent, ANS administration is associated with a major improvement in survival, the NNT is actually smaller than at any later GA. All the studies, unfortunately, suffer also from the same biases, which are sort of self-evident.
What is also consistent, is that centres with the best results , have a co-ordinated approach with obstetrics, and routinely give steroids as soon as the mothers are admitted.
As for my response to the NEJM article? I would perhaps phrase it a little differently, I think that active neonatal intensive care should be offered as an option to all mothers presenting with an increased risk of delivering at 22 to 24 weeks gestation, and that option should be presented as a reasonable choice which will be supported by the whole team, who will then do whatever they can to have the best possible outcome. When additional risk factors are present, such as growth restriction, imminent delivery without benefit of significant ANS exposure, then the discussion of the options must recognize those facts. When increased risk is very great, such as estimated weight <400g or florid chorioamnionitis, then it is vitally important to be realistic. It is also important to recognize that the decision to give steroids, as soon as possible, does not mandate active neonatal care, but will give the best chance for the baby if the later decision is indeed to proceed with intensive care. And that a decision for such care does not mandate a cesarean delivery, which should be considered a separate (obviously related) decision, which takes into account additional factors, including maternal age, risk factors etc.
Share this:
About Keith Barrington
2 responses to active intensive care at 22 weeks gestation.
Hi Keith, You might be interested in our new publication reporting rates of active care and infant survival rates in Victoria in 2009-2017 in a whole-of -state population of babies born at 22-24 weeks’ gestation. We found lower rates of ANC exposure in babies not offered active care (as you would expect) and higher rates in those for whom active care had been provided. That was seen in babies born at 22, 23 and 24 weeks’ gestation. Boland, RA., et al. (2021). Temporal changes in rates of active management and infant survival following live birth at 22–24 weeks’ gestation in Victoria. ANZJOG, doi: https://doi.org/10.1111/ajo.13309 . But we also found that many clinicians providing care and parent counselling did not have accurate perceptions of outcome of the babies born at 22-25 weeks- underestimating survival and overestimating rates of major disability. And, that clinicians were less accurate in their estimations of outcome in 2020 than they were a decade ago in 2010. Boland, RA., et al. (2021). Disparities between perceived and true outcomes of infants born at 23–25 weeks’ gestation. ANZJOG Online First), 1-8. doi: https://doi.org/10.1111/ajo.13443 So the questions we pose are: What are we telling the parents about their infant’s potential for a good outcome? And how do misconceptions about outcome affect parent counselling and decision-making in babies born at 22 and 23 weeks? More research needed to explore this! Dr Rose Boland Melbourne, Victoria
Thank you Keith. I think we have to remind ourselves that it is not our baby or the hospital’s baby. We must inform the parent’s of what might happen and then their desires and opinions should be followed. If they want the baby to have “a fair go” as the Aussies would say that should be respected. Even if the baby only survives a few hours, or days, they can feel they have tried and done their best. As obstetricians and neonatologists we should be prepared to give the baby the best treatment we know and not be half hearted about it. After all, it is the parents who will have the responsibility and hard work of caring for the child. The era of paternalistic doctors making all the decisions should now be behind us.
Leave a comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Search for:
Recent Posts
- 22 to 23 weeks gestation, what is so special?
- Oxygen is toxic in older kids too!
- Donor human milk, not toxic after all!
- Is there any indication to close the PDA?
- Time to open the DOOR
breathe, baby, breathe

Follow Neonatal Research via Email
Enter your email address to follow this blog and receive notifications of new posts by email.
Email Address:
Follow Neonatal Research
- Follow @NeonatalResearc
- antenatal steroids
- antibiotics
- anticonvulsants
- Assisted ventilation
- Breast-feeding
- breast milk
- Congenital Heart Disease
- Convulsions
- Delayed Cord Clamping
- diaphragmatic hernia
- End-of-life decisions
- endotracheal intubation
- enteral feeding
- erythropoietin
- Gastro-oesophageal reflux
- Global Neonatal Health
- Head Ultrasound
- Health Care Organization
- Heart Surgery
- Hemodynamics
- High-Flow cannula
- Hypoglycemia
- Hypotension
- Hypothermia
- hypoxic ischemic encephalopathy
- infection control
- intracranial hemorrhage
- Lactoferrin
- long term outcomes
- lung compliance
- Necrotising Enterocolitis
- Nitric Oxide
- oxygen therapy
- oxygen toxicity
- Preventing Prematurity
- pulmonary physiology
- Randomized Controlled Trials
- Research Design
- respiratory support
- Resuscitation
- Retinopathy of Prematurity
- surfactant treatment
- Systematic Reviews
- transfusion
Respire, bébé, respire!

Canadian Premature Babies Foundation

Sainte Justine Hospital

Canadian Neonatal Network

Préma-Québec

- Advocating for impaired children (23)
- Clinical Practice Guidelines (7)
- Neonatal Research (1,010)
- Not neonatology (28)
- The CPS antenatal counselling statement (14)
Transport Néonatal

- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- January 2022
- October 2021
- September 2021
- August 2021
- January 2021
- December 2020
- October 2020
- September 2020
- August 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- February 2019
- December 2018
- November 2018
- October 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- Entries feed
- Comments feed
- WordPress.com
Posts with most views, last 48 hours
- 22 to 23 weeks gestation, what is so special?
- They really are CRAP! C-ReActive Protein: "Hazardous Waste".
- Oxygen is toxic in older kids too!
- Not futile any more; survival and long term outcomes at 22 weeks.
- Breast milk fortifiers, a new systematic review
- Early routine surfactant, method and outcomes
- Is there any indication to close the PDA?
- To bolus or not to bolus? Not really a question...
- What is hypoglycemia? part 2
- Does tactile stimulation in the delivery room actually do anything?
- 1,507,863 hits

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
Association between mode of delivery and infant survival at 22 and 23 weeks of gestation
Affiliations.
- 1 Department of Obstetrics and Gynecology, University of Cincinnati College of Medicine, Cincinnati, OH (Drs Czarny, Forde, Rossi, and DeFranco);. Electronic address: [email protected].
- 2 Department of Obstetrics and Gynecology, University of Cincinnati College of Medicine, Cincinnati, OH (Drs Czarny, Forde, Rossi, and DeFranco).
- 3 Department of Obstetrics and Gynecology, University of Cincinnati College of Medicine, Cincinnati, OH (Drs Czarny, Forde, Rossi, and DeFranco);; Perinatal Institute, Cincinnati Children's Hospital Medical Center, Cincinnati, OH (Drs DeFranco and Hall).
- 4 Perinatal Institute, Cincinnati Children's Hospital Medical Center, Cincinnati, OH (Drs DeFranco and Hall); Translational Data Science and Informatics, Geisinger, Danville, PA, USA (Dr Hall).
- PMID: 33652159
- DOI: 10.1016/j.ajogmf.2021.100340
Background: Cesarean delivery is currently not recommended before 23 weeks' gestation unless for maternal indications, even in the setting of malpresentation. These recommendations are based on a lack of evidence of improved neonatal outcomes and survival following cesarean delivery and the maternal risks associated with cesarean delivery at this early gestational age. However, as neonatal resuscitative measures and obstetrical interventions improve, studies evaluating the potential neonatal benefit of periviable cesarean delivery have reported inconsistent findings.
Objective: This study aimed to compare the survival rates at 1 year of life among resuscitated infants delivered by cesarean delivery with those delivered vaginally at 22 and 23 weeks of gestation.
Study design: We conducted a population-based cohort study of all resuscitated livebirths delivered between 22 0/7 and 23 6/7 weeks of gestational age in the United States between 2007 and 2013. The primary outcome was the rate of infant survival at 1 year of life for different routes of delivery (cesarean vs vaginal delivery) at both 22 and 23 weeks of gestation. The secondary outcome variables included infant survival rates for neonates who survived beyond 24 hours of life, neonatal survival, and the length of survival. A secondary analysis also included a comparison of the infant survival rates between the different routes of delivery cohorts stratified by fetal presentation, steroid exposure, and ventilation. Information about composite adverse maternal outcomes were limited to infants who were delivered between 2011 and 2013 (when these items were first reported) and were defined as a requirement for blood transfusion, an unplanned operating room procedure following delivery, unplanned hysterectomy, and intensive care unit admission; the composite adverse maternal outcomes were also compared between the different delivery route cohorts for deliveries occurring between 22 and 23 weeks of gestation. Multivariable logistic regression analysis was used to determine the association between cesarean delivery and infant survival and other neonatal and maternal outcomes.
Results: Resuscitated infants delivered by cesarean delivery had higher rates of survival at 22 weeks (44.9 vs 23.0%; P<.001) and at 23 weeks (53.3 vs 43.4%; P<.001) of gestation regardless of fetal presentation. Multivariable logistic regression analysis demonstrated that infants who were delivered by cesarean delivery at 22 weeks (adjusted relative risk, 2.3; 95% confidence interval, 1.9-2.8) and 23 weeks (adjusted relative risk, 1.4; 95% confidence interval, 1.2-1.5) of gestation were more likely to survive than those delivered vaginally. When the cohort was limited to neonates who survived beyond the first 24 hours of life, vertex neonates born by cesarean delivery were not more likely to survive at 22 weeks (adjusted relative risk, 1.2; 95% confidence interval, 0.9-1.7) or 23 weeks (adjusted relative risk, 1.1; 95% confidence interval, 0.9-1.3) of gestation. An increased risk for composite adverse maternal outcomes (adjusted relative risk, 1.7; 95% confidence interval, 1.1-2.7) was associated with cesarean delivery at 22 to 23 weeks of gestation.
Conclusion: Cesarean delivery is associated with increased survival at 1 year of life among resuscitated, periviable infants born between 22 0/7 and 23 6/7 weeks of gestation, especially in the setting of nonvertex presentation. However, cesarean delivery is associated with increased maternal morbidity.
Keywords: antenatal corticosteroids; infant survival; neonatal survival; periviable.
Copyright © 2021 Elsevier Inc. All rights reserved.
- Cesarean Section*
- Cohort Studies
- Delivery, Obstetric*
- Gestational Age
- Infant, Newborn
- Retrospective Studies
- United States

Variation in fetal presentation
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created The Radswiki had no recorded disclosures.
At the time the article was last revised Yuranga Weerakkody had no financial relationships to ineligible companies to disclose.
- Delivery presentations
- Variation in delivary presentation
- Abnormal fetal presentations
There can be many variations in the fetal presentation which is determined by which part of the fetus is projecting towards the internal cervical os . This includes:
cephalic presentation : fetal head presenting towards the internal cervical os, considered normal and occurs in the vast majority of births (~97%); this can have many variations which include
left occipito-anterior (LOA)
left occipito-posterior (LOP)
left occipito-transverse (LOT)
right occipito-anterior (ROA)
right occipito-posterior (ROP)
right occipito-transverse (ROT)
straight occipito-anterior
straight occipito-posterior
breech presentation : fetal rump presenting towards the internal cervical os, this has three main types
frank breech presentation (50-70% of all breech presentation): hips flexed, knees extended (pike position)
complete breech presentation (5-10%): hips flexed, knees flexed (cannonball position)
footling presentation or incomplete (10-30%): one or both hips extended, foot presenting
other, e.g one leg flexed and one leg extended
shoulder presentation
cord presentation : umbilical cord presenting towards the internal cervical os
- 1. Fox AJ, Chapman MG. Longitudinal ultrasound assessment of fetal presentation: a review of 1010 consecutive cases. Aust N Z J Obstet Gynaecol. 2006;46 (4): 341-4. doi:10.1111/j.1479-828X.2006.00603.x - Pubmed citation
- 2. Merz E, Bahlmann F. Ultrasound in obstetrics and gynecology. Thieme Medical Publishers. (2005) ISBN:1588901475. Read it at Google Books - Find it at Amazon
Incoming Links
- Obstetric curriculum
- Cord presentation
- Polyhydramnios
- Footling presentation
- Normal obstetrics scan (third trimester singleton)
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Fetal Presentation, Position, and Lie (Including Breech Presentation)
, MD, Children's Hospital of Philadelphia
Variations in Fetal Position and Presentation
- 3D Models (0)
- Calculators (0)
- Lab Test (0)

Presentation refers to the part of the fetus’s body that leads the way out through the birth canal (called the presenting part). Usually, the head leads the way, but sometimes the buttocks (breech presentation), shoulder, or face leads the way.
Position refers to whether the fetus is facing backward (occiput anterior) or forward (occiput posterior). The occiput is a bone at the back of the baby's head. Therefore, facing backward is called occiput anterior (facing the mother’s back and facing down when the mother lies on her back). Facing forward is called occiput posterior (facing toward the mother's pubic bone and facing up when the mother lies on her back).
Lie refers to the angle of the fetus in relation to the mother and the uterus. Up-and-down (with the baby's spine parallel to mother's spine, called longitudinal) is normal, but sometimes the lie is sideways (transverse) or at an angle (oblique).
For these aspects of fetal positioning, the combination that is the most common, safest, and easiest for the mother to deliver is the following:
Head first (called vertex or cephalic presentation)
Facing backward (occiput anterior position)
Spine parallel to mother's spine (longitudinal lie)
Neck bent forward with chin tucked
Arms folded across the chest
If the fetus is in a different position, lie, or presentation, labor may be more difficult, and a normal vaginal delivery may not be possible.
Variations in fetal presentation, position, or lie may occur when
The fetus is too large for the mother's pelvis (fetopelvic disproportion).
The fetus has a birth defect Overview of Birth Defects Birth defects, also called congenital anomalies, are physical abnormalities that occur before a baby is born. They are usually obvious within the first year of life. The cause of many birth... read more .
There is more than one fetus (multiple gestation).

Position and Presentation of the Fetus
Some variations in position and presentation that make delivery difficult occur frequently.
Occiput posterior position
In occiput posterior position (sometimes called sunny-side up), the fetus is head first (vertex presentation) but is facing forward (toward the mother's pubic bone—that is, facing up when the mother lies on her back). This is a very common position that is not abnormal, but it makes delivery more difficult than when the fetus is in the occiput anterior position (facing toward the mother's spine—that is facing down when the mother lies on her back).
Breech presentation
In breech presentation, the baby's buttocks or sometimes the feet are positioned to deliver first (before the head).
When delivered vaginally, babies that present buttocks first are more at risk of injury or even death than those that present head first.
The reason for the risks to babies in breech presentation is that the baby's hips and buttocks are not as wide as the head. Therefore, when the hips and buttocks pass through the cervix first, the passageway may not be wide enough for the head to pass through. In addition, when the head follows the buttocks, the neck may be bent slightly backwards. The neck being bent backward increases the width required for delivery as compared to when the head is angled forward with the chin tucked, which is the position that is easiest for delivery. Thus, the baby’s body may be delivered and then the head may get caught and not be able to pass through the birth canal. When the baby’s head is caught, this puts pressure on the umbilical cord in the birth canal, so that very little oxygen can reach the baby. Brain damage due to lack of oxygen is more common among breech babies than among those presenting head first.
Breech presentation is more likely to occur in the following circumstances:
Labor starts too soon (preterm labor).
Sometimes the doctor can turn the fetus to be head first before labor begins by doing a procedure that involves pressing on the pregnant woman’s abdomen and trying to turn the baby around. Trying to turn the baby is called an external cephalic version and is usually done at 37 or 38 weeks of pregnancy. Sometimes women are given a medication (such as terbutaline ) during the procedure to prevent contractions.
Other presentations
In face presentation, the baby's neck arches back so that the face presents first rather than the top of the head.
In brow presentation, the neck is moderately arched so that the brow presents first.
Usually, fetuses do not stay in a face or brow presentation. These presentations often change to a vertex (top of the head) presentation before or during labor. If they do not, a cesarean delivery is usually recommended.
In transverse lie, the fetus lies horizontally across the birth canal and presents shoulder first. A cesarean delivery is done, unless the fetus is the second in a set of twins. In such a case, the fetus may be turned to be delivered through the vagina.

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada)—dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge .
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Edition
- IN THIS TOPIC
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Fetal Presentation, Position, and Lie (Including Breech Presentation)
, MD, Children's Hospital of Philadelphia
- 3D Models (0)
- Calculators (0)

Abnormal fetal lie or presentation may occur due to fetal size, fetal anomalies, uterine structural abnormalities, multiple gestation, or other factors. Diagnosis is by examination or ultrasonography. Management is with physical maneuvers to reposition the fetus, operative vaginal delivery Operative Vaginal Delivery Operative vaginal delivery involves application of forceps or a vacuum extractor to the fetal head to assist during the second stage of labor and facilitate delivery. Indications for forceps... read more , or cesarean delivery Cesarean Delivery Cesarean delivery is surgical delivery by incision into the uterus. The rate of cesarean delivery was 32% in the United States in 2021 (see March of Dimes: Delivery Method). The rate has fluctuated... read more .
Terms that describe the fetus in relation to the uterus, cervix, and maternal pelvis are
Fetal presentation: Fetal part that overlies the maternal pelvic inlet; vertex (cephalic), face, brow, breech, shoulder, funic (umbilical cord), or compound (more than one part, eg, shoulder and hand)
Fetal position: Relation of the presenting part to an anatomic axis; for transverse presentation, occiput anterior, occiput posterior, occiput transverse
Fetal lie: Relation of the fetus to the long axis of the uterus; longitudinal, oblique, or transverse
Normal fetal lie is longitudinal, normal presentation is vertex, and occiput anterior is the most common position.
Abnormal fetal lie, presentation, or position may occur with
Fetopelvic disproportion (fetus too large for the pelvic inlet)
Fetal congenital anomalies
Uterine structural abnormalities (eg, fibroids, synechiae)
Multiple gestation
Several common types of abnormal lie or presentation are discussed here.

Transverse lie
Fetal position is transverse, with the fetal long axis oblique or perpendicular rather than parallel to the maternal long axis. Transverse lie is often accompanied by shoulder presentation, which requires cesarean delivery.
Breech presentation
There are several types of breech presentation.
Frank breech: The fetal hips are flexed, and the knees extended (pike position).
Complete breech: The fetus seems to be sitting with hips and knees flexed.
Single or double footling presentation: One or both legs are completely extended and present before the buttocks.
Types of breech presentations
Breech presentation makes delivery difficult ,primarily because the presenting part is a poor dilating wedge. Having a poor dilating wedge can lead to incomplete cervical dilation, because the presenting part is narrower than the head that follows. The head, which is the part with the largest diameter, can then be trapped during delivery.
Additionally, the trapped fetal head can compress the umbilical cord if the fetal umbilicus is visible at the introitus, particularly in primiparas whose pelvic tissues have not been dilated by previous deliveries. Umbilical cord compression may cause fetal hypoxemia.

Predisposing factors for breech presentation include
Preterm labor Preterm Labor Labor (regular uterine contractions resulting in cervical change) that begins before 37 weeks gestation is considered preterm. Risk factors include prelabor rupture of membranes, uterine abnormalities... read more
Multiple gestation Multifetal Pregnancy Multifetal pregnancy is presence of > 1 fetus in the uterus. Multifetal (multiple) pregnancy occurs in up to 1 of 30 deliveries. Risk factors for multiple pregnancy include Ovarian stimulation... read more
Uterine abnormalities
Fetal anomalies
If delivery is vaginal, breech presentation may increase risk of
Umbilical cord prolapse
Perinatal death
It is best to detect abnormal fetal lie or presentation before delivery. During routine prenatal care, clinicians assess fetal lie and presentation with physical examination in the late third trimester. Ultrasonography can also be done. If breech presentation is detected, external cephalic version can sometimes move the fetus to vertex presentation before labor, usually at 37 or 38 weeks. This technique involves gently pressing on the maternal abdomen to reposition the fetus. A dose of a short-acting tocolytic ( terbutaline 0.25 mg subcutaneously) may help. The success rate is about 50 to 75%. For persistent abnormal lie or presentation, cesarean delivery is usually done at 39 weeks or when the woman presents in labor.

Face or brow presentation
In face presentation, the head is hyperextended, and position is designated by the position of the chin (mentum). When the chin is posterior, the head is less likely to rotate and less likely to deliver vaginally, necessitating cesarean delivery.
Brow presentation usually converts spontaneously to vertex or face presentation.
Occiput posterior position
The most common abnormal position is occiput posterior.
The fetal neck is usually somewhat deflexed; thus, a larger diameter of the head must pass through the pelvis.
Progress may arrest in the second phase of labor. Operative vaginal delivery Operative Vaginal Delivery Operative vaginal delivery involves application of forceps or a vacuum extractor to the fetal head to assist during the second stage of labor and facilitate delivery. Indications for forceps... read more or cesarean delivery Cesarean Delivery Cesarean delivery is surgical delivery by incision into the uterus. The rate of cesarean delivery was 32% in the United States in 2021 (see March of Dimes: Delivery Method). The rate has fluctuated... read more is often required.
Position and Presentation of the Fetus
If a fetus is in the occiput posterior position, operative vaginal delivery or cesarean delivery is often required.
In breech presentation, the presenting part is a poor dilating wedge, which can cause the head to be trapped during delivery, often compressing the umbilical cord.
For breech presentation, usually do cesarean delivery at 39 weeks or during labor, but external cephalic version is sometimes successful before labor, usually at 37 or 38 weeks.
Drugs Mentioned In This Article

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the Merck Manuals and our commitment to Global Medical Knowledge.
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Manual
- IN THIS TOPIC
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Glob J Health Sci
- v.8(2); 2016 Feb
A Triplet Pregnancy with Spontaneous Delivery of a Fetus at Gestational Age of 20 Weeks and Pregnancy Continuation of Two Other Fetuses until Week 33
Maryam ghorbani.
1 Qom University of Medical Sciences, Qom, IR Iran
Somayeh Moghadam
2 Alborz University of Medical Sciences, Alborz, IR Iran
Introduction:
The prevalence of pregnancies with triplet or more has been increased due to using assisted reproductive treatments. Meanwhile, multiple pregnancies have higher risks and long-term maternal-fetal complications compared to twin and singleton pregnancies. Delayed interval delivery (DID) is a new approach in the management of multiple pregnancies following delivery or abortion. The purpose of this paper is to evaluate the benefits of DID and presents a case that used this method.
This paper covers a report on a case of triplet pregnancy resulting from assisted reproductive techniques with spontaneous delivery of a fetus at gestational age of 20 weeks and the use of conservative DID for two other fetuses until the 33 rd week.
In our case, the delivery of two other fetuses occurred spontaneously at gestational age of 33 weeks after the delivery of the first fetus at week 20.
Conclusions:
Using DID is a useful and reliable method, but requires careful monitoring, especially in patients with a history of infertility.
1. Introduction
The prevalence of pregnancies with triplet or more has been increased due to using assisted reproductive treatments and is directly correlated to the number of embryos transferred into the uterus after IVF. In the United States, the incidence of pregnancies with triplet or more reached 184 per 100,000 from 37 between 1980 and 2002. Although a slight decrease was observed in the incidence in 1998 ( Papageorghiou, Avgidou, Bakoulas, Sebire, & Nicolaides 2006 ), the rate of pregnancies with triplet or more began to fall in 1990, but the rate of twin pregnancies generally increased to 25 percent during this period ( Hasson, Shapira, Many, Jaffa, & Har-Toov, 2011 ).
Multiple pregnancies have higher risks and long-term maternal-fetal complications compared to twin and singleton pregnancies. The two major complications in triplet pregnancies are fetal loss before 24 weeks and early preterm delivery before 32 weeks ( Papageorghiou et al., 2006) . As all multiple pregnancies are on the rise, the incidence of prematurity and its complications are significantly correlated with the number of embryos ( Biard, Bernard, Thomas, & Hobinont 2000 ). Other complications include increased risk of preeclampsia, hypertension, and premature rupture of membranes compared to singleton pregnancies. In these pregnancies, the risk of prematurity and low birth weight is 10 times, the risk of fetal death is 5 times, neonatal death is 7 times and cerebral palsy is 4 times higher compared to singleton pregnancies. Also twin pregnancy has long-term consequences such as increased risk of neurological complications in future such as epilepsy and learning disabilities. Maternal morbidity is specifically increased due to hypertension, preeclampsia, thromboembolism, urinary tract infection, placental abruption, placenta previa and maternal mortality. Delayed interval delivery (DID) is a new strategy in the management of multiple pregnancies following delivery or abortion. This approach was first introduced by Carson in 1880. This technique is sometimes suggested in elective multiple pregnancies in which uterine contractions cease after the abortion of the first fetus. This method can be an appropriate technique to manage the remaining embryos. It seeks to avoid termination of pregnancy and aims to keep fetuses until they are viable ( Ismail Temur, 2013 ). There is no exact evidence due to the use of antibiotics, tocolysis, or cerclage in prolonging multiple pregnancies. When there are no signs of infection and membranes are intact, prophylactic antibiotics are controversial. In all cases with interval management delivery antibiotic prophylaxis continued. But in some reports they use only in the presence of bacterial colonization of the amniotic fluid. Tocolysis has been used after delivery of the first twin, with different combinations, including β-2agonists, calcium channel blockers, anti-inflammatory drugs, MgSO 4 , and progesterone. Using cerclage in interval management delivery is not consensus. The invasive nature of cerclage and the increased risk of chorioamnionitis due to the closure of a potentially infected amniotic sac is certainly a great concern. Once such a procedure had been done, strict bed rest in the hospital was suggested. Infection, abruption, fetal demise, and fetal anomaly must be recognized and treated appropriately. But after delivery of the first fetus, for delaying the delivery of subsequent fetuses, therapy should be started. Although there is no conclusive evidence supporting the antibiotics, cerclage, and tocolysis should be used for improveing the outcome of the remaining fetuses ( Kao, Hsu, & Ding, 2006 ). A number of case reports show that DID of the second embryo or other embryos is a practical approach in some cases and this approach can lead to a reduction in perinatal mortality ( Zhang, Martin, & Trumble, 2004 ). Therefore, due to controversies in applying this approach, further studies are needed to evaluate the effectiveness of this method in case reports and this paper is presented with the aim of presenting a report on the successful application of this method.
2. Case Report
A 22-year-old gravid woman with a triplet pregnancy of 20±3 weeks was admitted to Hazrat Zahra Hospital, Qom on March 2014. Vaginal examination performed on admission showed an open cervix and the amniotic sac inside the vagina. Patient’s history revealed that she had been married for about 3 years and got pregnant with IUI treatment due to infertility for a year. She was cerclaged in early pregnancy and had gestational diabetes. Upon admission, ampicillin was administered intravenously, and ringer serum plus hyoscine were administered to relieve pain. Due to patient’s increased pains and the amniotic sac inside the vagina, cerclage was removed and she was injected with 50 mg pethidine intramuscularly. About 6 hours later, vaginal examination was performed, when limbs of the first fetus could be touched inside the cervix. The first fetus began to expel at 9:25 a.m., and her hand could be observed out of the vagina. The first baby girl was born with Apgar score of 0.0 at 14:50. But, the placenta was not expelled and umbilical cord was visible out of the vagina. On the same day, as the placenta was not expelled, 2 grams ampicillin was injected in umbilical arteries, but the placenta was not expelled. The patient and her husband did not consent to the use of oxytocin and about 2 hours later, the patient was transferred to the midwifery ward to rest. About an hour later, the expelled umbilical cord was spontaneously separated, but the placenta was not expelled. On March 21, 2014 the following report was presented by ultrasound of the fetuses: the right fetus has variable presentation, normal amniotic fluid, and a posterior placenta at gestational age of 20 weeks and 6 days and the left fetus has variable presentation, normal amniotic fluid, and a posterior placenta at gestational age of 20 weeks and 3 days. A diamniotic dichorionic pregnancy is confirmed. Cervical canal length was about 41 mm, and its os was open. A heterogeneous area with dimensions of 48 × 28 is observed in lower part of the uterus near internal os that might be the aborted placenta. Pregnancy ultrasound was again performed on March 27, 2014 and it was reported that both cephalic fetuses were male with normal amniotic fluid and both placentas had right lateral posterior position at 21-22 weeks gestation. The expelled placenta was not observed yet. The patient was hospitalized and monitored for about 2 weeks in the midwifery ward and then discharged against medical advice with good general condition and without vaginal bleeding. During the hospitalization, she was checked every other day for CBC, diff, ESR and CRP tests which were all within the normal range. During pregnancy, the patient received 40 mg clexane subcutaneously. About 90 days later she was readmitted on June, 2014 at gestational age of 33± 4weeks due to the beginning of pain and uterine contractions, and vaginal examination showed that the cervix was dilated 9 cm. So emergency cesarean section was performed to deliver fetuses who had good Apgar scores and were transferred to the NICU.

3. Discussion and Conclusion
In a study conducted by Temur in 2013, a case of DID was presented as follows:
The case was a 31-year-old pregnant woman with 8 years past her first pregnancy and primary infertility. Her husband had oligoasthenospermia. The patient was pregnant with twins after ICSI. Her pregnancy was not high risk until 16 weeks, but the first fetus was aborted spontaneously at 17 weeks and 6 days without abortion symptoms (pain, bleeding, and abdominal cramping). The first fetus was born dead and premature rupture of membranes was observed just before the abortion. The aborted fetus had no major abnormalities. After abortion, the health of the second fetus was examined by ultrasound. It had a normal amount of amniotic fluid and a normal gestational sac. The cervix length was reduced to less than 25 mm; internal os was open with dimensions of 10 × 15mm, but external os was closed. According to the results, after reviewing all interventions (cerclage, tocolysis, bed rest, prolonged hospitalization and antibiotic therapy) and discussing risky complications (maternal and neonatal sepsis, severe bleeding, the need for hysterectomy and risk of death), the patient was advised about all treatment options and a consent form was obtained from her. Under general anesthesia in the operating room, the umbilical cord was cut as closely as possible to internal os and the placenta was left in the uterus and McDonald cerclage was performed. The patient was locally and generally monitored for infection, bleeding and coagulopathy disorders. On the day of surgery, the patient was checked for infection and the results were as follows: body temperature of 37.5 °C, leukocytes 12,000 per cubic millimeter, and CRP ++ (measured qualitatively). Interventions were started as follows: broad-spectrum antibiotic therapy (ceftriaxone sodium 4 gr per day intravenously), tocolysis with ritodrine HCL (150 mg per hour) and clindamycin vaginal cream twice daily for 14 days. CRP was reported ++++ two days later, but it was negative after 5 days. The patient was hospitalized during pregnancy up to 132 days i.e. at 36 weeks and 6 days. All interventions were stopped after 14 days, but the patient was monitored for maternal and fetal infection symptoms, any symptoms of premature delivery and fetal health. CRP level was measured weekly, white blood cells and complete blood count were measured at baseline and then monthly and all were insignificant. A dose of 12 mg of betamethasone was administered to promote lung maturation at 28 and 32 weeks. No anticoagulant therapy was undertaken. The baby was born by cesarean section at 36 weeks and 5 days due to breech position. The first minute Apgar score was 8 and the fifth minute Apgar score was 10 and the baby did not need hospitalization in the NICU. The baby and mother were discharged from the hospital in good health 5 days after delivery. The neonatal development and postpartum period did not have a problem. There were no maternal complications. Mother and baby were discharged in good health 5 days after delivery. The placentas had no histopathological problem. In addition, the patient was pregnant with her second baby without any treatment ( Ismail Temur, 2013 ).
The present study has some differences and similarities with our study, in both studies the placenta was remained in uterus and umbilical cord was cut. But in Temur study the patient was hospitalized all the time between birth of first fetus and second fetus. But in our study, our patient discharged after two weeks with personal consent and readmitted about 90 days later again with uterine contraction.
A quadruplet pregnancy occurred in a 32-year-old female which had primary infertility because of endometriosis. And pregnancy was result of oocyte retrieval and tubal embryo transfer. In second trimester, one fetus reduced by using intracardiac KCl injection. But spontaneous rupture of membranes occurred at 29 weeks of gestation. Tocolytic and prophylactic antibiotic treatment were began for patient. The birth of first fetus occurred four days later and a male fetus was delivered with weight of 1,235 g and Apgar scores of 7 and 9 at 1 and 5 minutes, respectively. There was no evidence of placenta abruption, vaginal bleeding, or contraction of uterine. The treatment with tocolytics, steroids, and antibiotics were continued. Complete blood count and C-reactive protein had normal range. Preterm labor occurred at 31 weeks of gestation. Emergency cesarean section was performed because of breech presentation. The birth weight of two male fetuses was 1,440 and 1,285 g and Apgar scores were 7 and 9 at 1 and 5 minutes, respectively. They were taken to the neonatal intensive care unit for observation. After follow-up visits for 2 years, the first infant had retinopathy while the other 2 infants were healthy without any deficits ( Kao et al., 2006) .
The present study has some differences with our study. In this study spontaneous rupture of membranes occurred at 29 weeks of gestation but in our study this problem was found in gestational age of 20 weeks. In study done by Kao et al, tocolysis began after birth of first fetus but in our study this way (Except use of Hyoscine) was not selected because physicians tendency in our research was to terminate pregnancy. In our study, placenta of first fetus was remained in cervix and delayed delivery was done spontaneously about 13 weeks later that was an approximately longer time than other current studies. But researchers in current study did not mention any thing about placenta of first fetus and delayed delivery of other fetuses happened about 2 weeks after first delivery.
Petousis et al in 2012 done a study. The goal was examining the efficacy of delaying the delivery of the second fetus with tocolysis and emergency cervical cerclage after delivery of first fetus. They entered 5 cases with dichorionic twin pregnancies after miscarriage of the first fetus (<24 weeks). And they analyzed the obstetric and neonatal outcomes. They concluded emergency cervical cerclage has beneficial effect on obstetric and neonatal outcomes for second fetus. In this study emergency cerclage was applied after birth of first fetus but in our study cervical cerclage was opened due to termination of pregnancy ( Petousis et al., 2012) .
Several studies have examined the consequences of applying DID as follows:
Rosbergen et al. (2005) investigated short-term and long-term results of twin pregnancies after DID. They reported that DID of the remaining fetus had a positive effect on short-term results i.e. these infants were similar to infants with the same gestational age ( Rosbergen et al., 2005) .
In the study of Wouter et al. (2009) , the first fetus was born at 18 weeks and 6 days and the second fetus was born at 37 weeks and 6 days. Using prophylactic tocolysis with antibiotics and administration of corticosteroids after delivery of the first fetus led to the prolongation of pregnancy more than three months and a term infant was born in year ( Wouters, Gianottn, Bayram, & Doornbos, 2009 ).
Zhang et al. and Oyelese et al. independently conducted two population-based studies using similar data for multiple birth records between 1995 and 1998. Zhang et al. reviewed 200 twin pregnancies in which the first fetus was born between 17 and 29 weeks and the second fetus was born two or more days later. They compared the data obtained with the data of 374 twin pregnancies in which the second fetus was born on the same day or the next day in terms of perinatal and fetal survival outcomes. They concluded that DID of the second fetus for 2 days or more before 30 weeks will lead to improved infant survival ( Zhang, Martin, & Trumble 2004 ). Oyelese et al. conducted a retrospective cohort study in 2005 based on the data of 4257 twin pregnancies between 1995 and 1998, in which the first fetus was delivered vaginally at 22 to 28 weeks and investigated the effect of DID on pregnancy outcomes between the two groups. In the first group, the first fetus was delivered between 22 and 23 weeks and the second one with delay of less than 3 weeks and in the second group, the second fetus was born immediately after the first one. They concluded that DID of the second baby leads to improved infant survival and reduced infant mortality ( Oyelese, Ananth, Smulian, & Vintzileos, 2005 ).
4. Conclusion
According to our study and literature review, we conclude that DID is a reasonable strategy needed especially in cases of infertility, especially if patients are monitored carefully and accurately.
Almost in all reported cases, treatment strategies for the first fetus included cutting the umbilical cord in the internal os, using broad-spectrum oral and intravenous antibiotics, leaving the placenta of the first fetus inside the uterus, and bed rest for a specific time or throughout pregnancy. However, in most cases, the use of cerclage is still controversial and it is used less than other methods.
- Biard J. M, Bernard P, Thomas K, Hobinont C. Conservative management of triplet pregnancy after delivery of one foetus. 2000; 3 :71–75. [ PubMed ] [ Google Scholar ]
- Hasson J, Shapira A, Many A, Jaffa A, Har-Toov J. Reduction of twin pregnancy to singleton: Does it improve pregnancy outcome? Early Online. 2011:1–5. [ PubMed ] [ Google Scholar ]
- Kao S. P, Hsu S, Ding D. C. Delayed interval delivery in a triplet pregnancy. Chin Med Assoc. 2006; 69 (2):92–94. http://dx.doi.org/10.1016/S1726-4901(09)70121-2 . [ PubMed ] [ Google Scholar ]
- Oyelese Y, Ananth C. V, Smulian J. C, Vintzileos A. M. Delayed interval delivery in twin pregnancies in the United States: Impact on perinatal mortality and morbidity. Am J Obstet Gynecol. 2005; 192 :439–444. http://dx.doi.org/10.1016/j.ajog.2004.07.055 . [ PubMed ] [ Google Scholar ]
- Papageorghiou A. T, Avgidou K, Bakoulas V, Sebire N. J, Nicolaides K. H. Risks of miscarriage and early preterm birth in trichorionic triplet pregnancies with embryo reduction versus expectant management. New Data and Systematic Review. 2006; 21 (7):1912–1917. [ PubMed ] [ Google Scholar ]
- Petousis S, Goutzioulis A, Margioula-Siarkou C, Katsamagkas T, Kalogiannidis I, Agorastos T. Emergency cervical cerclage after miscarriage of the first fetus in dichorionic twin pregnancies: Obstetric and neonatal outcomes of delayed delivery interval. Arch Gynecol Obstet. 2012; 286 :613–617. http://dx.doi.org/10.1007/s00404-012-2362-y . [ PubMed ] [ Google Scholar ]
- Rosbergen M, Vogt H. P, Baerts W, Eyck J. V, Arabin B, Marjolein J. M, Lingen V. Long-term and short term outcome after delayed-interval delivery in multi-fetal pregnancies. Eur J Obstet Gynecology Reprod Biol. 2005; 122 :66–72. http://dx.doi.org/10.1016/j.ejogrb.2004.11.036 . [ PubMed ] [ Google Scholar ]
- Temur I. A Twin Pregnancy Provided with ICSI, an abortion of one first fetus at 18th week and live birth of the second fetus at the end of the 36 week. A case report and literature review. 2013; 26 (13):1355–1358. [ PubMed ] [ Google Scholar ]
- Wouters K. A, Gianottn J, Bayram N, Doornbos J. P. R. Term life birth after late abortion of first twin. Acta Obstet Gynecol. 2009; 88 :1148–1152. http://dx.doi.org/10.1080/00016340903171066 . [ PubMed ] [ Google Scholar ]
- Zhang J, Martin B, Trumble J. Delayed interval delivery and infant survival: A population-base study. Am J Obstet Gynecol. 2004; 191 :470–476. http://dx.doi.org/10.1016/j.ajog.2004.03.002 . [ PubMed ] [ Google Scholar ]
- Trying to Conceive
- Signs & Symptoms
- Pregnancy Tests
- Fertility Testing
- Fertility Treatment
- Weeks & Trimesters
- Staying Healthy
- Preparing for Baby
- Complications & Concerns
- Pregnancy Loss
- Breastfeeding
- School-Aged Kids
- Raising Kids
- Personal Stories
- Everyday Wellness
- Safety & First Aid
- Immunizations
- Food & Nutrition
- Active Play
- Pregnancy Products
- Nursery & Sleep Products
- Nursing & Feeding Products
- Clothing & Accessories
- Toys & Gifts
- Ovulation Calculator
- Pregnancy Due Date Calculator
- How to Talk About Postpartum Depression
- Editorial Process
- Meet Our Review Board
Week 22 of Your Pregnancy
Verywell / Bailey Mariner
- Baby Development
Self-Care Tips
Advice for partners.
- Doctor Visits
Special Considerations
At 22 weeks, you are more than halfway through your pregnancy. You may now be able to hear your baby's heartbeat with a stethoscope. You may also feel your first, mild, practice contractions.
22 Weeks Pregnant Is How Many Months? 5 months and 2 weeks
Which Trimester? Second trimester
How Many Weeks to Go? 18 weeks
Your Baby's Development at 22 Weeks
At 22 weeks, a baby is typically over 7 1/2 inches (19.2 centimeters) from the top of the head to the bottom of the buttocks (known as the crown-rump length ). The baby's height is approximately 10 3/4 inches (27.4 centimeters) from the top of the head to the heel (crown-heel length). This week, the baby weighs around 16 3/4 ounces or just over a pound (476 grams).
Eye Developments
The baby’s tear ducts are developing.
By 22 weeks, the baby's heartbeat can usually be heard through a stethoscope . You can tell the difference between the pregnant parent's and baby's heartbeat by the number of beats in a minute. An adult's heart rate is between 60 and 100 beats per minute. The baby's heart beats faster and will be between 110–160 beats per minute.
Making Strong Bones
The baby is growing rapidly and taking in more calcium for healthy development, especially of the bones and teeth. The skeleton continues to harden.
Baby's hands can now move independently. Baby can touch one hand with the other, cross the hands, and can even grasp the umbilical cord.
Explore a few of your baby's week 22 milestones in this interactive experience.
Stay Calm Mom: Episode 10
Watch all episodes of our Stay Calm Mom video series and follow along as our host Tiffany Small talks to a diverse group of parents and top doctors to get real answers to the biggest pregnancy questions.
How to Deal With Unwanted Pregnancy Advice
Your common symptoms this week.
During the second trimester, you may be experiencing leg cramps, forgetfulness , food cravings, nasal congestion, or skin changes . A new symptom that you may begin to feel this week is the tightening of the uterus.
Uterine Contractions
Your uterus is contracting, but you might not notice it. Braxton Hicks contractions begin as early as 20 weeks , and they become more common as pregnancy progresses.
Braxton Hicks contractions are a tightening of the muscles in the uterus. When the muscles get tight, your belly may feel hard. These contractions are normal. They are typically mild and irregular, but they can feel strong.
Braxton Hicks contractions are often considered "practice contractions." They aren't dangerous. However, it's important to know the difference between these practice contractions and the real thing :
- Braxton Hicks contractions are irregular while labor contractions are more predictable in their pattern. Real labor pains get stronger and more frequent over time.
- Braxton Hicks contractions are not accompanied by other symptoms, while labor contractions may be joined by back pain, vaginal bleeding, or a gush of fluid.
- Braxton Hicks contractions tend to go away with movement or exercise, but true labor continues.
You have a better chance of staying healthy during pregnancy if you get a little exercise , stay within the recommended guidelines for weight gain , take your prenatal vitamins, attend all of your prenatal appointments , and follow your doctor or midwife's advice.
This week you can take a closer look at your diet to be sure it's balanced and includes enough calcium. You may also want to think about how you feel about others discussing or touching your body.
Setting Boundaries
As your pregnant belly grows, so does the attention you receive. Loved ones, friends, coworkers, and, yes, strangers often remark on—and touch—your body freely.
You may not mind it at all and feel flattered or excited by these gestures. But, it could make you feel uncomfortable or intruded upon.
What Experts Say
“That can be especially true if you have a history of body image issues or abuse. In these moments, it’s completely appropriate to set a boundary and to say something like, 'Thank you so much for your good wishes, but I'm not comfortable with others touching me.' This way, you can both acknowledge people’s good intentions, but also communicate an appropriate personal request and boundary.”
—Shara Marrero Brofman, PsyD
Either reaction is OK, of course. It is also perfectly acceptable to tell people that you’re uncomfortable with their commentary or actions.
Getting Your Calcium
Your baby needs calcium to build strong bones. The amount of calcium that the baby needs increases in the second half of pregnancy. If you don't get enough calcium from your diet, your baby will take it out of your bones.
Experts recommend that pregnant people over the age of 18 get 1,000mg of calcium each day. You should take your prenatal vitamin and try to get enough calcium in your daily diet by choosing calcium-rich foods like:
- Canned salmon or sardines
- Dark leafy green vegetables like kale, broccoli, collard greens, and bok choy
- Fortified foods such as orange juice, cereals, bread, and tofu
- Low-fat dairy products, including milk, cheese, yogurt, and ice cream (or almonds and almond milk as a non-dairy alternative)
Your Week 22 Checklist
- Continue to take your prenatal vitamins .
- Choose snacks and foods that can help you reach your daily calcium requirements .
- Learn the difference between practice and real contractions .
- Speak up about your body boundaries .
- Look into newborn care classes .
While childbirth classes are always at the top of the mind (for good reason), you may also want to look into newborn care classes, parenting classes, and infant CPR training. These classes cover the basics like diapering , bathing, and feeding . But perhaps more importantly, they can help you and your partner feel empowered.
“Many new parents and parents-to-be seem to feel that they should know what to do with a new baby. Or, they may even receive messages from others about how they should just follow their instincts about what their baby needs. But these messages can leave parents feeling helpless.”
It can be overwhelming to have a newborn, especially if it's your first baby. It can be difficult for you and your partner to think clearly when you have this new responsibility—all while you're sleep-deprived. But, by going into your new situation with some information and a little newborn knowledge, it may reduce your stress and boost your confidence.
You can find out about classes by asking your healthcare provider for recommendations or speaking to someone at your hospital or birthing center.
At Your Doctor’s Office
If you are scheduled for prenatal testing, this week is typically the last week to have a few tests, including:
- The maternal serum screening or quad screen blood test is typically performed between 15 weeks and 22 weeks.
- The Level 2 ultrasound is usually conducted between 18 weeks and 22 weeks.
- If needed, the fetal echocardiogram generally takes place between 18 weeks and 22 weeks.
Upcoming Doctor’s Visits
- Your next regular prenatal visit will likely be around week 24 .
- A screening test for gestational diabetes or high blood sugar during pregnancy is typically scheduled between week 24 and week 28 .
High blood pressure is a health condition that some people have before they become pregnant, but sometimes it develops during pregnancy.
High Blood Pressure
Blood pressure is the pressure or force of the blood against the walls of the blood vessels as the heart pumps. It is written as two numbers: The top number is the systolic pressure, and the bottom number is the diastolic pressure. The unit of measurement for pressure is millimeters of mercury (mmHg).
Healthy blood pressure is classified as systolic levels of less than 120 mmHg and diastolic levels less than 80 mmHg (written as >120/80 mmHg or spoken as "less than 120 over 80"). When the blood pressure is higher than the healthy guideline two separate times at least four hours apart, it is called hypertension.
Doctors classify hypertension in several stages:
- Elevated : Systolic levels ranging from 120 to 129 mmHg AND diastolic levels less than 80 mmHg
- Stage 1 Hypertension: Systolic levels ranging from 130 to 139 mmHg AND/OR diastolic levels at 80 to 89 mmHg
- Stage 2 Hypertension: Systolic levels 140 mmHg or higher AND/OR diastolic levels 90 mmHg or higher
- Stage 3 Hypertensive Crisis: Systolic levels higher than 180 mmHg AND/OR diastolic levels above 120 mmHg
High Blood Pressure in Pregnancy
High blood pressure affects about 1 in every 12 to 17 pregnancies. During pregnancy, there are two main types of high blood pressure.
- If you had high blood pressure before you become pregnant or before 20 weeks of pregnancy, it is called chronic hypertension .
- If you develop high blood pressure after the 20th week of pregnancy, it's called gestational or pregnancy-related hypertension .
Complications
Untreated hypertension can cause problems for pregnant people and their babies:
- Cesarean delivery
- Growth problems in the baby
- Heart or kidney problems in biological parent
- Placental abruption (the placenta detaching from the uterus)
- Preeclampsia
- Premature birth
Treatment of high blood pressure during pregnancy depends on the type of high blood pressure you have, your blood pressure levels, and your symptoms. Treatment may include:
- Monitoring your blood pressure more often either at the office or at home
- Regular blood tests and urine tests
- Referral to a cardiologist or a perinatologist
- Monitoring the baby's growth through ultrasound
Concerning Symptoms
High blood pressure is often a silent condition that doesn't necessarily have any symptoms. However, there are symptoms that could mean your blood pressure is getting worse or developing into preeclampsia, such as:
- Severe headache or a headache that will not go away
- Problems with your vision like seeing spots
- Swelling in your hands or face
- Quickly gaining weight
- Stomach pain
- Nausea and vomiting
- Breathing issues
A Word From Verywell
Your belly is growing higher, and by week 22 the top of your uterus (known as the fundus) is now above your belly button. Your uterus may even occasionally feel hard for a few seconds as you being to experience practice contractions.
Inside the uterus, your baby is continuing to grow and gain weight. If you hold a 1-pound bag of coffee or a pound of butter, you can feel just how much your baby weighs this week. Next week is the last week of your fifth month.
Oyer CE, Sung CJ, Friedman R, et al. Reference values for valve circumferences and ventricular wall thicknesses of fetal and neonatal hearts . Pediatr Dev Pathol . 2004;7(5):499-505. doi:10.1007/s10024-004-1117-6
Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight . PLoS Med . 2017;14(3):e1002284. doi:10.1371/journal.pmed.1002220
American College of Obstetrics and Gynecologists. FAQ156. How Your Fetus Grows During Pregnancy .
U.S. National Library of Medicine. National Institutes of Health. U.S. Department of Health and Human Services. MedlinePlus. Fetal Development .
Pulse & Heart Rate. Cleveland Clinic.
Hofmeyr F, Groenewald CA, Nel DG, Myers MM, Fifer WP, Signore C, Hankins GD, Odendaal HJ; PASS Network. Fetal heart rate patterns at 20 to 24 weeks gestation as recorded by fetal electrocardiography . J Matern Fetal Neonatal Med . 2014;27(7):714-8. doi:10.3109/14767058.2013.836485
Kumar A, Kaur S. Calcium: A nutrient in pregnancy. J Obstet Gynaecol India . 2017 Oct;67(5):313-318. doi:10.1007/s13224-017-1007-2
Fagard J, Esseily R, Jacquey L, O'Regan K, Somogyi E. Fetal origin of sensorimotor behavior . Front Neurorobot . 2018;12:23. doi:10.3389/fnbot.2018.00023
University of Michigan. Michigan Medicine. Contractions During Pregnancy: What to Expect .
National Institutes of Health. NIH Osteoporosis and Related Bone Diseases National Resource Center. NIH Pub. No. 18-7881. Pregnancy, Breastfeeding and Bone Health .
Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation . Med Clin North Am . 2016;100(6):1199-1215. doi:10.1016/j.mcna.2016.06.004
U.S. Department of Health and Human Services. National Institutes of Health Office of Dietary Supplements. Calcium: Fact Sheet for Health Professionals .
American College of Obstetricians and Gynecologists. Screening for fetal aneuploidy. Practice bulletin no. 163 . Obstet Gynecol . 2016;127(5):e123-37. doi:10.1097/aog.0000000000001406
American College of Obstetricians and Gynecologists. Ultrasound in pregnancy. Practice Bulletin No. 175 . Obstet Gynecol. 2016;128: e241–56. doi:10.1097/aog.0000000000001815
Donofrio MT, Moon-grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association . Circulation . 2014;129(21):2183-242. doi:10.1161/01.cir.0000437597.44550.5d
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 190: gestational diabetes mellitus . Obstet Gynecol . 2018;131(2):e49-64. doi:10.1097/AOG.0000000000002501
Centers for Disease Control and Prevention. High Blood Pressure. Facts About Hypertension .
American College of Obstetrician and Gynecologists. FAQ034. Preeclampsia and High Blood Pressure During Pregnancy .
Centers for Disease Control and Prevention. High Blood Pressure. High Blood Pressure During Pregnancy .
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy . Obstet Gynecol . 2019;133(1):e26-e50. doi:10.1097/AOG.0000000000003020
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia . Obstet Gynecol . 2019;133(1):e1-e25. doi:10.1097/AOG.0000000000003018
Seely EW, Ecker J. Chronic hypertension in pregnancy . Circulation . 2014;129(11):1254-61. doi:10.1161/CIRCULATIONAHA.113.003904
By Holly Pevzner Holly Pevzner is an award-winning writer who specializes in health, nutrition, parenting, and family travel.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 May 2018
Birth weight variants are associated with variable fetal intrauterine growth from 20 weeks of gestation
- L. Engelbrechtsen ORCID: orcid.org/0000-0002-0666-0830 1 , 2 ,
- D. Gybel-Brask 3 ,
- Y. Mahendran 1 , 2 ,
- M. Crusell 1 ,
- T. H. Hansen ORCID: orcid.org/0000-0001-5948-8993 1 ,
- T. M. Schnurr ORCID: orcid.org/0000-0002-6573-4959 1 , 2 ,
- E. Hogdall 4 ,
- L. Skibsted 3 ,
- T. Hansen ORCID: orcid.org/0000-0001-8748-3831 1 &
- H. Vestergaard ORCID: orcid.org/0000-0003-3090-269X 1 , 5
Scientific Reports volume 8 , Article number: 8376 ( 2018 ) Cite this article
4714 Accesses
4 Citations
1 Altmetric
Metrics details
- Genetics research
- Paediatric research
Fetal intrauterine growth is influenced by complex interactions between the maternal genes, environment and fetal genes. The aim of this study was to assess the effect of GWAS-identified genetic variants associated with birth weight on intrauterine fetal growth in 665 children. Fetal growth was estimated by two-dimensional ultrasound scans at 20, 25 and 32 weeks of gestation and growth trajectories were modeled using mixed linear regression. A genetic risk score (GRS) of birth weight-raising variants was associated with intrauterine growth showing an attenuating effect on the unconditional daily reduction in proportional weight gain of 8.92 × 10 −6 percentage points/allele/day (p = 2.0 × 10 −4 ), corresponding to a mean difference of 410 g at 40 weeks of gestation between a child with lowest and highest GRS. Eight variants were independently associated with intrauterine growth throughout the pregnancy, while four variants were associated with fetal growth in the periods 20–25 or 25–32 weeks of gestation, indicating that some variants may act in specific time windows during pregnancy. Four of the intrauterine growth variants were associated with type 2 diabetes, hypertension or BMI in the UK Biobank, which may provide basis for further understanding of the link between intrauterine growth and later risk of metabolic disease.
Similar content being viewed by others

The influence of insulin-related genetic variants on fetal growth, fetal blood flow, and placental weight in a prospective pregnancy cohort
Pauline K. Reim, Line Engelbrechtsen, … Torben Hansen
Distinction between the effects of parental and fetal genomes on fetal growth
Thorhildur Juliusdottir, Valgerdur Steinthorsdottir, … Kari Stefansson
Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors
Nicole M. Warrington, Robin N. Beaumont, … Rachel M. Freathy
Introduction
Fetal intrauterine growth is influenced by complex interactions between the maternal genes and environment, and fetal genes 1 . Maternal factors, such as obesity and diabetes, can result in abnormal fetal growth patterns leading to large-for-gestational age (LGA) fetuses, whereas acquired complications during pregnancy such as pre-eclampsia can lead to small-for-gestational age (SGA) fetuses 1 , 2 , 3 . Abnormal fetal growth can increase the risk of perinatal adverse effects, but also the risk of morbidity and mortality in adult life 4 , 5 , 6 .
An association between fetal growth and the occurrence of metabolic syndrome later in life was first noted by Barker et al . in 1993 7 . This lead to a number of observational studies, which demonstrated that adverse environmental factors during fetal development increase the lifelong risk of metabolic and cardiovascular complications 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 . Subsequently, the maternal factors associated with birth weight have been carefully studied in numerous epidemiological studies 2 , 8 , 10 , 12 .
Knowledge about the fetal genetic contribution to intrauterine growth is limited. A recent genome-wide association study (GWAS) identified 60 loci associated with birth weight and concluded that 15% of the variance in birth weight can be explained by fetal genetic variation 13 . Similarly, a number of genetic association studies have identified single gene variants associated with abnormal fetal growth. Many of these variants are additionally known to be associated with type 2 diabetes (T2D), maturity-onset diabetes of the young (MODY) subtypes, or rare congenital diseases leading to metabolic alterations 13 , 14 , 15 , 16 . Most studies have used birth weight as a surrogate of intrauterine growth and have not assessed fetal genetic effects during specific gestational growth periods. Therefore, little is known on how fetal genetic variants may affect intrauterine growth, and if genetic variants have specific gestational periods, in which they are more important for fetal growth. Thus, we aimed to evaluate the prenatal effect of the recently GWAS identified birth weight variants by testing the impact of variants combined in a genetic risk score (GRS) on overall fetal growth during 2 nd and 3 rd trimester and independently during specific gestational weeks of fetal development. Subsequently, we hypothesized that the variants with the largest effect on fetal intrauterine growth, in this study, may represent essential growth pathways that could provide insight into the well-established link between intrauterine growth and adult disease. We therefore explored if the variants associated with fetal intrauterine weight were also associated with development of hypertension, T2D and BMI in adulthood using data from the UK Biobank.
Prenatal data from 665 newborn children were included in the analyses. The children were born of mothers with mean ± SD age of 30.23 ± 4.70 years and a pre-pregnancy BMI of 24.78 ± 4.93 kg/m 2 (Table 1 ). The children had a mean birth weight of 3580 ± 474 g and were born at a mean gestational age of 279 ± 10 days (Table 1 ).
Fetal weight estimated by ultrasound at 20, 25, and 32 weeks of gestation displayed a curvilinear trajectory similar to previous reports of intrauterine growth 17 , 18 , 19 (Fig. 1B ), with a 4.7 (95%CI: 4.6, 4.7) % basal daily weight increment (linear time effect) and a 0.014 (95%CI: 0.013, 0.014) percentage point per day reduction in the daily proportional weight gain (quadratic time effect). The growth trajectory including weight measured at birth showed a similar trend with a 4.0 (3.9, 4.1) % basal daily weight increment and a 0.010 (95%CI: 0.010, 0.011) percentage point per day reduction in the daily proportional weight gain (Table 2 ).
Fetal growth curves. Growth curves of fetal weight estimated by ultrasound with ( A ) and without ( B ) birth weight, as well as abdominal circumference ( C ), femur length ( D ), biparietal diameter ( E ) and occipito-frontal diameter ( F ) measured by ultrasound. Gestational age is estimated at the nuchal translucency scan (11–13 weeks of gestation) and used as reference throughout pregnancy. Lines represents the mean trend with 95% confidence (dark grey) and prediction intervals (light grey) from unconditional growth models fitted using mixed linear regression.
Abdominal circumference, biparietal diameter, occipitofrontal diameter and femur length measured by ultrasound also displayed curvilinear growth with comparable trajectories and daily proportional increments ranging from 1.9 to 2.5% per day and a reduction in the daily proportional increment ranging from 0.006 to 0.009 percentage points per day (Table 2 , Fig. 1 ).
Genetic risk score of birth weight associates with intrauterine weight and fetal girth
We tested the effect of an unweighted birth weight-raising GRS on intrauterine growth using mixed linear regression models. We found a significant effect of the GRS, which attenuated the reduction in the daily proportional weight gain by 7.78 × 10 −6 (95%CI: 1.64 × 10 −6 , 1.33 × 10 −5 ) percentage points per day per birth weight-raising allele (Table 3 ). The association was stronger when including weight measured at birth showing a per allele effect of 8.92 × 10 −6 (95%CI: 4.32 × 10 −6 , 1.4 × 10 −5 ) percentage points per day, corresponding to a mean difference at term of 410 g (95%CI: 115, 798) g between a child with the lowest and highest GRS (Fig. 2 ).
Fetal growth according to high and low GRS. Growth curves of fetal weight estimated by ultrasound and birth weight. Gestational age was estimated at the nuchal translucency scan (11–13 weeks of gestation) and used as reference throughout pregnancy. Lines represent the mean trend with 95% confidence intervals from conditional growth models fitted using mixed linear regression.
Fetal weight estimated by ultrasound is a compound measure based on femoral length, abdominal circumference and head circumference, the latter itself based on biparietal diameter and occipitofrontal diameter. When testing the impact of the GRS on each of these individual measurements we found a 3.22 × 10 −6 (95%CI: 6.02 × 10 −6 , 5.82 × 10 −5 ) percentage point lower reduction in daily proportional increase in abdominal circumference per birth weight-raising allele (Table 3 ).
We tested the impact of the GRS on specific fetal growth periods, week 20–25, week 25–32 and week 32-birth by multiple linear regression models adjusted for gestational age, weight and gestational age at previous ultrasound scan, maternal pre-pregnancy BMI and fetal gender. We found an association between the GRS and fetal growth in the period week 20–25 (β = 0.94 ± 0.60 g per day per birth weight-raising allele, p = 0.011), and in the period week 25–32 (β = 1.75 ± 1.45 g per day per birth weight-raising allele, p = 0.023), an additionally a strong association in the period week 32 to birth (β = 8.10 ± 2.99 g per day per birth weight-raising allele, p = 0.007).
We tested if the GRS was associated with abnormal fetal weight measured as either LGA or SGA. We found no association between the GRS and LGA (OR 1.07 (95%CI: 0.98, 1.17)) and similarly, no association between the GRS and SGA was found (OR 0.96 (95%CI: 0.90, 1.02)).
Associations and interactions between maternal traits and GRS on intrauterine growth and birth weight
We hypothesized that maternal environmental traits such as smoking, BMI and glucose levels might influence the effect of the GRS, and tested if there were any interactions between the additive per allele effect of the GRS on change in daily proportional weight gain per day (GA 2 × GRS) and the given traits. There was no interaction between maternal pre-pregnancy BMI and intrauterine growth (p = 0.47) or birth weight (p = 0.45), and similarly no interactions when testing across tertiles of the GRS (p = 0.89).
However, we did observe marginal effects of maternal pre-pregnancy BMI on several fetal growth parameters (Table 3 ). Maternal BMI weakened the reduction in the daily proportional weight gain by 8.2 × 10 −6 (95%CI: 3.74 × 10 −6 , 1.25 × 10 −5 ) percentage points per day per birth weight-raising allele (Table 3 ). Same effects were seen for fetal AC, OFD and femur length growth, but no effect on BPD.
We assessed the effect of co-conditioning of maternal BMI on fetal growth (including birth weight) and observed a small effect of BMI on the attenuation of the daily proportional weight gain by 5.01 × 10 −5 (95%CI: −1.1 × 10 −5 , 1.1 × 10 −4 ) percentage points per day per birth weight-raising allele compared to 8.92 × 10 −6 (95%CI: 4.32 × 10 −6 , 1.40 × 10 −5 ) without co-conditioning of BMI.
We then tested if maternal glucose levels were interacting with the GRS. We had measures of 2 hour glucose levels from oral glucose tolerance tests (OGTTs) in 155 women and included the interaction term (GA 2 × GRS × glucose) in the mixed linear regression model of intrauterine growth. We found no interaction of maternal glucose levels on intrauterine weight (p = 0.76) or birth weight (p = 0.90).
Sixty-two of the mothers were smoking during pregnancy. We tested the interaction (GA 2 × GRS × smoking) and found no effect of maternal smoking on intrauterine weight (p = 0.82) or birth weight (p = 0.10).
Genetic variants associated with intrauterine weight
We tested the independent effect of all variants included in the GRS on intrauterine weight (Suplementary Table 1 ). Eight variants were associated with overall intrauterine growth. We then tested the impact of variants in growth periods from 20–25 weeks, from 25–32 weeks and from 32 weeks to birth. Two variants (rs61830764 and rs61862780) showed association with fetal growth in the period week 20–25. Similarly, two variants (rs6989280 and rs28510415) were associated with fetal growth in week 25–32, while four variants were associated with fetal growth from week 32 to birth (Suplementary Table 1 ).
Genetic variants associated with adult onset disease
The eight variants, which were associated with intrauterine fetal growth, were tested for association with T2D, hypertension and BMI in the UK Biobank (Table 4 ). This was done to test, if the variants with the largest genetic effect on intrauterine growth trajectories would have an effect on development of adult metabolic disease. The variant, rs700059, which was positively associated with overall intrauterine growth, was inversely correlated with T2D in a subsample of 19,630 individuals in the UK Biobank (β = −0.002 ± 0.001, p = 0.0007). Same direction of effect was reported in the DIAGRAM GWAS of 152,000 individuals (p = 0.032), but not reaching the GWAS significance level 20 . In contrast, rs10830963, showed a strong positive association with development of T2D (β = 0.003 ± 0.001, p = 6.37 × 10 −11 ), with the same direction of effect in the DIAGRAM GWAS (p = 1.7 × 10 −7 ). We tested for association with BMI and found an inverse association with rs700059 (β = −0.030 ± 0.014, p = 0.026), and a positive associations with rs10830963 (β = 0.028 ± 0.010, p = 0.008) and rs72851023 (β = 0.051 ± 0.018, p = 0.004). The GIANT GWAS of 339,224 individuals has previously reported same direction of effect for association between variants and BMI (p = 0.0005, p = 0.069 and p = 0.012, respectively), but none of them reaching the GWAS significance threshold (p < 5 × 10 −8 ) 21 .
In this study, we evaluated the combined effect of GWAS-identified birth weight-raising variants on intrauterine fetal growth from 20 gestational weeks to birth. We demonstrate that the birth weight-raising GRS was associated with overall intrauterine fetal growth, suggesting that the fetal genetic contribution to birth weight is mediated throughout pregnancy. We found a difference in birth weight of 410 g in children with low and high GRS, indicating that the combined effect of genetic variants has a marked effect on intrauterine growth, which is measurable at birth. We found eight independent variants with positive effects on intrauterine fetal growth, and our findings suggest that some variants are associated with overall intrauterine weight, while others seem to act in specific gestational time windows. We used an explorative approach to test if the variants, associated with intrauterine growth in this study, were associated with adult metabolic disease in the UK Biobank. We demonstrate that, in particular, two of the variants, rs700059 and rs10830963, are associated with T2D, hypertension or BMI in the UK Biobank. These findings emphasize the importance of the fetal genetic contribution to intrauterine growth, which may provide the basis for further understanding of the link between intrauterine growth and risk of later metabolic disease.
Birth weight has previously been used for assessment of fetal intrauterine growth, since it is a simple measure, obtained on most individuals at birth, and easy to compare across cohorts. However, birth weight does not give comprehensive information on growth trajectories during pregnancy. In this study, we used ultrasonic measures of fetal weight, which enabled us to draw a detailed picture of the impact of genetic variants on fetal growth at specific time points during pregnancy. We found a significant effect of the GRS on overall intrauterine growth from 20 weeks of gestation to birth (p = 2.0 × 10 −4 ) corresponding to an attenuation of the reduction in daily proportional weight gain by 8.92 × 10 −6 percentage points per day per birth weight-raising allele. We hypothesized that maternal environmental factors might influence the effect of the GRS and found a marginal independent effect of maternal pre-pregnancy BMI. Previous studies, have assessed the impact of maternal BMI on fetal growth, and it is well-established that maternal obesity increases the risk of a large-for-gestational age fetus 19 , 22 . Similarly, greater fetal biometries can be detected in obese women as early as 21 weeks of gestation and estimated fetal weight is progressively greater from 30 weeks of gestation in compared to normal weight women 19 . However, despite these previous associations between maternal obesity and fetal growth, our study implies that maternal BMI only has a marginal effect on genetic growth trajectories of the fetus. The effect of maternal BMI on genetic growth trajectories could be mediated through a stronger genetic predisposition to obesity, which in the mother leads to a higher BMI, and cause a similar phenotype in the fetus with increased intrauterine growth and higher birth weight 23 .
When assessing the impact of fetal genetics on intrauterine growth, it is important to acknowledge that 50% of genes are shared between mother and fetus. In general, the maternal genotypes contribute relatively little to birth weight 13 , 24 , 25 , unless the maternal genotype directly influences the intrauterine environment 14 , 15 . This has been demonstrated in carriers of rare heterozygous glucokinase (GCK) mutations, which causes altered pancreatic glucose-sensing. A fetal GCK mutation causes a ~530 g decrease in birth weight, whereas a maternal GCK mutation causes a ~600 g increase in birth weight; but if the GCK mutation is present in both mother and fetus, no effect on birth weight is observed 15 . A similar effect has been observed for TCF7L2 , a mutation in which causes an increase in birth weight when carried by the fetus and/or mother 14 ; an effect likely mediated by elevated fasting plasma glucose levels. Our results do not indicate that the combined genetic effect of birth weight variants on intrauterine growth is mediated or modified by stimulated plasma glucose. However, it is possible that maternal obesity-related traits could lead to higher birth weight regardless of fetal genotype as we see in the independent effect of BMI on intrauterine growth 26 , 27 .
We identified eight variants positively associated with intrauterine growth during pregnancy. All eight variants were exerting their effect on fetal growth throughout pregnancy and not in specific gestational weeks. In contrast, two variants (rs61830764 and rs61862780) showed association with fetal growth in the period week 20–25, suggesting that these may be more important for fetal growth in the second trimester of pregnancy. Similarly, two variants (rs6989280 and rs28510415) were associated with fetal growth in week 25–32, indicating that their effect is most important in late second trimester and early third trimester.
The variant, rs7964361 had the largest effect size, attenuating the unconditional reduction in daily proportional weight gain and is located in the insulin-like growth factor 1 ( IGF1 ) gene, which encodes the insulin-like growth factor 1 hormone (IGF1). In mice, maternal IGF1 has been shown to stimulate fetal growth by increasing the transfer of nutrients across the placenta in vivo 28 , 29 , while in vitro studies have suggested that fetal IGF1 stimulates fetal growth 30 . Concurrently, ablation or differential methylation of IGF1 has been shown to cause abnormal fetal growth in mice 29 , 31 . In line with these findings, our results suggest that rs7964361 may play a role in fetal intrauterine growth.
A number of observational studies have highlighted the importance of the intrauterine environment in relation to disease risk later in life, such as development of T2D. The association between birth weight and T2D is mediated through both non-genetic and genetic factors, among which the genetic effects during fetal life may play a substantial role 13 , 32 . A number of common genetic variants have been associated with both birth weight and T2D 13 ; however, only a small proportion of the heritability of both traits can be explained by these variants 13 , 32 . We hypothesized that the variants with the largest effect on fetal intrauterine growth, in this study, may represent essential growth pathways that could provide insight into the well-established link between intrauterine growth and adult disease 7 , 8 . We found an association between intrauterine growth and rs10830963; a variant which is strongly associated with T2D in the UK Biobank (p = 6.37 × 10 −11 ). rs10830963 is an intronic variant located in the Melatonin receptor 1B gene ( MTNR1B ), which encodes one of the two receptors of melatonin, a hormone involved in circadian rhythms. Previous studies have reported a strong effect of rs10830963 on fasting glucose levels, insulin secretion and disposition index 33 , 34 , 35 , as well as risk of T2D identified through large GWAS 36 , 37 . Similarly, GWAS significant association of rs10830963 and offspring birth weight in maternal carriers have recently been reported 38 . The association between maternal rs10830963 and birth weight is likely mediated through elevated maternal fasting glucose levels, causing increased placental transfer of glucose, which leads to higher fetal insulin secretion and thereby increased birth weight. Our study suggests that the effect of the fetal variant also leads to increased fetal intrauterine growth. However, whether the effect is caused by maternal transmission of the variant, is mediated through altered maternal glucose levels 38 or if the variant affects fetal glucose regulation per se is unknown. Further studies are needed to investigate this.
There are some limitations to this study. First of all, we used an unweighted GRS comprised of GWAS-identified birth weight variants 13 , that assumes an additive and equal effect of all variants at each time point during pregnancy, which may not be completely accurate. We chose this method, because we expect the effect sizes to vary substantially from second to third trimester and the reported effect estimates are based on birth weight alone 13 . However, using this method does not take into account any interactions between variants or genes that may be present. Additionally, we have used a cohort of 665 children, which for genetic studies may be of relatively small size and of limited power to detect associations. However, we used birth weight variants identified by GWAS, so it is thus plausible that these variants should be associated during prenatal growth without a strict statistical threshold. Another limitation to this study is that we used fetal weight calculated by the Hadlock formula as an estimation of fetal growth, and there may be some inaccuracies in the estimations. In particular, we expect to have some uncertainties in weight estimations in the third trimester, where it is difficult to achieve precise measures of the fetal head and abdominal circumferences. However, we used the same sonographers throughout the study period, which may limit the intra-observer and inter-observer variations. We did not have data on maternal genotypes in this study, which would have made it possible to assess how maternal genotypes would affect fetal growth and adjust our analyses accordingly. In line with this, detailed information and adjustment for maternal environmental factors such as diet and gestational weight gain would have strengthened the analyses.
In conclusion, we demonstrate that a GRS of birth weight-raising variants is associated with intrauterine fetal growth suggesting that the fetal genetic contribution to birth weight is mediated throughout pregnancy. We show that some variants influence fetal growth in specific gestational weeks and may be more important for fetal growth in these time windows. Additionally, our study supports that variants associated with fetal intrauterine growth may also have an impact on development of metabolic disease later in life.
Pregnant women (>18 years of age) attending a nuchal translucency scan at 12 weeks of gestation at Roskilde University Hospital were invited for participation in this study, as previously described 39 , 40 .
In addition to the nuchal translucency scan at 12 weeks of gestation, the women were invited for a malformation ultrasound scan at 20 weeks of gestation, and for fetal growths scans at 25 and 32 weeks of gestation. A total of 753 pregnancies were included in the study. Exclusion criteria were missing genotypes, maternal gestational diabetes, pre-eclampsia, and twins gestations, incomplete follow-up, inclusion of pregnancy twice, or presence of an acardiac twin, leaving 665 pregnancies in the study.
Ultrasound scans
Ultrasound scans were performed on ultrasound scanners from GE Healthcare (Buckinghamshire, UK) by trained midwifes or medical doctors. Estimation of gestational age was based on ultrasound measurements of the fetal crown–rump length (CRL) at the nuchal translucency scan. Assessment of fetal weight were based on two-dimensional ultrasound and included measurements of abdominal circumference (AC) in mm, bi-parietal diameter (BPD) in mm, occipital-frontal diameter (OFD) in mm and length of the femur bone (FL) in mm. BPD was measured using an ultrasound probe in an axial plane, traversing the thalami, and cavum septum pellucidum with the transducer perpendicular to the central axis of the head. The distance from the outer edge of the near calvarial parietal wall to the inner edge of the far parital calvarial wall was termed the BPD, while distance from the near occipital wall to the far frontal calvarial wall was measured as the OFD. FL was measured as the longest length of the femoral bone in a horizontal plane. AC was measured as the transverse abdominal circumference at the level of the fetal ventricle and portal vein.
Fetal weight in grams was calculated based on the Hadlock formula 18 :
The definition of small-for-gestational-age (SGA) was a newborn child with a birth weight below −15% (<10 th percentile) for gestational age at birth. The definition of large-for-gestational-age (LGA) was a child with a birth weight >22% (>90 th percentile) for given gestational age at birth 41 , 42 .
Pregnancy outcome
The outcome of the pregnancy was retrieved from patients’ medical records including data on birth weight, mode of delivery and gender of the baby. Parity was defined as birth of a baby after 22 weeks of gestation and registered as 0–5. Women who had not previously given birth (parity = 0), women who had given birth once (parity = 1), twice (parity = 2), three times (parity = 3) or four times (parity = 4) prior to the examined pregnancy.
Anthropometric measurements
All women reported their age, pre-pregnancy weight and height at the first visit. BMI was calculated as BMI = weight (kg)/height 2 (m). Additionally, the mothers reported if they were smoking or not during pregnancy. If they had any medical diseases before or during pregnancy it was reported. Similarly if the women were treated with medication during pregnancy it was recorded.
Biochemical measures
Women with a pre-pregnancy body mass index (BMI) ≥ 27 kg/m 2 , family history of diabetes, glucosuria or previous birth of baby weighing ≥ 4500 g were offered an oral glucose tolerance test (OGTT) according to clinical practice in Denmark. The OGTT was performed at 14–20 weeks if the women had two risk factors or previous GDM or at 28–30 weeks of gestation if the woman had only one risk factor. The OGTT was based on intake of 75 g glucose after a minimum 10-hour overnight fast, with plasma glucose concentration measured two hours after ingestion.
Acid-base testing of the cord blood was performed following delivery in both vaginal and cesarean deliveries. After the sample for acid-base testing was obtained, a sample of cord blood was taken for genotyping. Due to this procedure, it was not possible to obtain blood for genotyping in all children due to either clotting of the cord vessels, not enough blood for sample, or unawareness from the midwife/doctor. In total 753 cord blood samples were retrieved and kept in a −80 °C freezer until DNA extraction.
Genotyping and QC
We used DNA from cord blood samples from 753 children for genotyping using the Illumina Infinium HumanCoreExome Beadchip platform (Illumina, San Diego, CA). Genotypes were called using the Genotyping module (version 1.9.4) of GenomeStudio software (version 2011.1, Illumina). We excluded, duplicates, ethnic outliers, and samples with extreme inbreeding coefficients, mislabeled or missing sex, or call rate <95%, leaving 701 children who passed all quality control criteria.
Genetic risk score
The genetic variants selected for the GRS were based on 60 GWAS-identified birth weight-raising loci 13 . Variants or proxies ( r 2 ≥ 0.90) for the 60 loci associated with BW were retrieved from the Human ExomeBeadChip. Two loci were not present on the array; rs139975827 was not captured by any proxy and rs11096402 was located on the X-chromosome, which was not imputed. Hence, we included 58 loci in an unweighted GRS, which was constructed by summing the dosages for each birth weight-raising allele. The GRS ranged from 47.5 to 78.8 alleles and was normally distributed. The cut-offs for tertiles were based on the distribution of GRS. The cut-offs for tertiles were GRS < 53.1 (1 st tertile), GRS 53.1–56.6 (2 nd tertile), GRS > 56.6 (3 rd tertile).
Statistical analyses
Data analysis was performed in R version 3.4.1 ( www.r-project.org ). Two-tailed testing was used and statistical significance was defined as a two-sided p-value below 0.05.
Overall intrauterine growth
To model the curvilinear increase in fetal weight estimates from week 20 to birth, we fitted an unconditional growth model with a linear and quadratic time trend, using mixed linear regression with a random intercept and random growth trajectory of each fetus:
To assess the impact of the GRS on overall intrauterine growth, an interaction with the quadratic time term was added, thereby conditioning the change in weight over time on the GRS:
A fixed effect interactions with the linear time component was not specified, as this represents the initial growth rate at time = 0; too early for the GRS to have any measurable biological effect. In order to adjust for effects of fetal sex and maternal pre-pregnancy BMI, interactions between these covariates and the quadratic time term were added to the conditional model:
Fetal weight in gram was log-transformed prior to analysis to address the issue of increasing heteroscedasticity of weight measurements during pregnancy. Models were fitted by restricted maximum likelihood using a Gaussian spatial structure to model the correlation between temporally non-equidistant weight estimates. Model assumptions were assessed visually by inspection of normal probability plots and residual plots.
To assess the impact of the individual genetic variants on overall intrauterine growth, similar conditional growth models with and without adjustment for fetal sex and maternal pre-pregnancy BMI were fitted. Models of GRS and individual variant effects were fitted with and without birth weight measurements to assess whether birth weight was the sole driver of identified associations.
Interactions between the GRS and maternal environment traits such as BMI, smoking and glucose levels were tested by including the respective interaction terms in the model (i.e. GA 2 × GRS × BMI).
Specific growth periods
To assess the impact of the GRS during specific gestational growth periods, we used multiple linear regression models. For instance, to assess the impact on growth from the malformation scan at week 20 to the first study-specific scan at 25 weeks of gestation, we specified a model describing weight at 25 weeks as a function of the GRS, including weight at 20 weeks to adjust for growth prior to the period in question and actual gestational age at both measurements to adjust for variation in the actual age at which the ultrasound scans were performed (Weight 25weeks ~ GRS + Weight 20weeks + GA 20weeks + GA 25weeks ).
Growth from 25 to 32 weeks and from 32 weeks to birth was modeled in the same way. In order to adjust for effects of fetal sex and maternal pre-pregnancy BMI, main effects of these covariates were added to the model (Weight 25weeks ~ GRS + Weight 20weeks + GA 20weeks + GA 25weeks + Sex + BMI).
To assess the impact of individual variants during specific gestational growth periods, similar models with and without adjustment for fetal sex and maternal pre-pregnancy BMI were fitted. No correction for multiple testing was performed, since these loci were previously identified for association with BW, and it is therefore plausible to assume that they are associated with fetal growth.
Birth weight
The impact of maternal pre-pregnancy BMI on birth weight was assessed by simple linear regression, whereas multiple linear regression adjusted for fetal sex, maternal pre-pregnancy BMI and gestational age at birth was applied to test for associations between the GRS and birth weight.
Adult disease in UK Biobank
We used birth weight-raising variants in the GRS to test for association with intrauterine growth. The variants have previously been tested for association with T2D, hypertension and BMI in a birth weight GWAS 13 . In line with this, we wanted to test if the variants associated with intrauterine growth (and not only birth weight) were involved in development of metabolic disease using data from the UK Biobank of 490,000 individuals. We hypothesized that this would provide insight into the genetic aspects of fetal growth trajectories, and which direction of effect they have on later metabolic disease. The association between intrauterine growth variants and T2D or hypertension (ICD-10) was tested using logistic regression, while association with BMI measured at baseline was tested using simple linear regression. We additionally tested all direction of effects of associations with BMI in the GIANT GWAS summary statistics and for T2D in the DIAGRAM GWAS summary statistics 20 , 21 .
The study was approved by the Ethics Committee for Region Zealand (SJ-55 and SJ-347) and the Danish Data Protection Agency and was registered in ClinicalTrials.gov (Identifier: NCT00836524). The study was conducted in accordance with the principles of the Helsinki Declaration. All study participants provided written informed consent.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available from the Novo Nordisk Foundation Center for Basic Metabolic Research, Section of Metabolic Genetics whose authors may be contacted.
Bloomfield, F. H., Spiroski, A.-M. & Harding, J. E. Fetal growth factors and fetal nutrition. Semin . Fetal . Neonatal Med ., https://doi.org/10.1016/j.siny.2013.03.003 (2013).
Moore, T. R. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am. J. Obstet. Gynecol. 202 , 643–649 (2010).
Article PubMed Google Scholar
Hung, T.-H., Hsieh, T.-T. & Chen, S.-F. Risk of abnormal fetal growth in women with early- and late-onset preeclampsia. Pregnancy Hypertens ., https://doi.org/10.1016/j.preghy.2017.09.003 (2017).
Hattersley, A. T. & Tooke, J. E. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet Lond. Engl. 353 , 1789–1792 (1999).
Article CAS Google Scholar
Gluckman, P. D., Hanson, M. A., Beedle, A. S. & Raubenheimer, D. Fetal and neonatal pathways to obesity. Front. Horm. Res. 36 , 61–72 (2008).
Hales, C. N. et al . Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303 , 1019–1022 (1991).
Article PubMed PubMed Central CAS Google Scholar
Barker, D. J. et al . Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36 , 62–67 (1993).
Article PubMed CAS Google Scholar
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359 , 61–73 (2008).
Pulizzi, N. et al . Interaction between prenatal growth and high-risk genotypes in the development of type 2 diabetes. Diabetologia 52 , 825–829 (2009).
Wright, C. S. et al . Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am. J. Hypertens. 22 , 215–220 (2009).
Article MathSciNet PubMed Google Scholar
Desai, M., Beall, M. & Ross, M. G. Developmental origins of obesity: programmed adipogenesis. Curr. Diab. Rep. 13 , 27–33 (2013).
Article PubMed PubMed Central Google Scholar
Clausen, T. D. et al . Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 94 , 2464–2470 (2009).
Horikoshi, M. et al . Genome-wide associations for birth weight and correlations with adult disease. Nature 538 , 248–252 (2016).
Freathy, R. M. et al . Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am. J. Hum. Genet. 80 , 1150–1161 (2007).
Hattersley, A. T. et al . Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat. Genet. 19 , 268–270 (1998).
Gloyn, A. L. et al . Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes. N. Engl. J. Med. 350 , 1838–1849 (2004).
Olsen, I. E., Groveman, S. A., Lawson, M. L., Clark, R. H. & Zemel, B. S. New Intrauterine Growth Curves Based on United States Data. Pediatrics 125 , e214–e224 (2010).
Hadlock, F. P., Harrist, R. B. & Martinez-Poyer, J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 181 , 129–133 (1991).
Zhang, C. et al . Association of Maternal Obesity With Longitudinal Ultrasonographic Measures of Fetal Growth: Findings From the NICHD Fetal Growth Studies-Singletons. JAMA Pediatr. 172 , 24–31 (2018).
Scott, R. A. et al . An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 66 , 2888–2902 (2017).
Locke, A. E. et al . Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015).
Ay, L. et al . Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. - PubMed - NCBI. Available at: https://www.ncbi.nlm.nih.gov/pubmed/?term=ay+bjog+2009 . (Accessed: 25th January 2018).
Tyrrell, J. et al . Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 315 , 1129–1140 (2016).
Horikoshi, M. et al . New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 45 , 76–82 (2013).
Rice, F. & Thapar, A. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Hum. Dev. 86 , 425–432 (2010).
Kulkarni, S. R. et al . Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 36 , 2706–2713 (2013).
Misra, V. K., Trudeau, S. & Perni, U. Maternal serum lipids during pregnancy and infant birth weight: the influence of prepregnancy BMI. Obes . Silver Spring . Md 19 , 1476–1481 (2011).
CAS Google Scholar
Takeda, Y. & Iwashita, M. Role of growth factors on fetal growth and maturation. Ann. Acad. Med. Singapore 22 , 134–141 (1993).
PubMed CAS Google Scholar
Sferruzzi-Perri, A. N., Owens, J. A., Pringle, K. G., Robinson, J. S. & Roberts, C. T. Maternal insulin-like growth factors-I and -II act via different pathways to promote fetal growth. Endocrinology 147 , 3344–3355 (2006).
Hill, D. J., Crace, C. J., Strain, A. J. & Milner, R. D. Regulation of amino acid uptake and deoxyribonucleic acid synthesis in isolated human fetal fibroblasts and myoblasts: effect of human placental lactogen, somatomedin-C, multiplication-stimulating activity, and insulin. J. Clin. Endocrinol. Metab. 62 , 753–760 (1986).
Nawathe, A. R. et al . Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin . Epigenetics 8 (2016).
Beaumont, R. N., Horikoshi, M., McCarthy, M. I. & Freathy, R. M. How Can Genetic Studies Help Us to Understand Links Between Birth Weight and Type 2 Diabetes? Curr . Diab . Rep . 17 (2017).
Bouatia-Naji, N. et al . A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 41 , 89–94 (2009).
Prokopenko, I. et al . Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 41 , 77–81 (2009).
Sparsø, T. et al . G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes 58 , 1450–1456 (2009).
Dupuis, J. et al . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42 , 105–116 (2010).
Voight, B. F. et al . Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42 , 579–589 (2010).
Beaumont, R. N. et al . Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum . Mol . Genet . https://doi.org/10.1093/hmg/ddx429 .
Gybel-Brask, D., Johansen, J. S., Christiansen, I. J., Skibsted, L. & Høgdall, E. V. S. Serum YKL-40 and gestational diabetes - an observational cohort study. APMIS Acta Pathol . Microbiol . Immunol . Scand ., https://doi.org/10.1111/apm.12573 (2016).
Gybel-Brask, D., Høgdall, E., Johansen, J., Christensen, I. J. & Skibsted, L. Serum YKL-40 and uterine artery Doppler–a prospective cohort study, with focus on preeclampsia and small-for-gestational-age. Acta Obstet. Gynecol. Scand. 93 , 817–824 (2014).
Clausson, B., Gardosi, J., Francis, A. & Cnattingius, S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG Int. J. Obstet. Gynaecol. 108 , 830–834 (2001).
Chang, T. C., Robson, S. C., Boys, R. J. & Spencer, J. A. Prediction of the small for gestational age infant: which ultrasonic measurement is best? Obstet. Gynecol. 80 , 1030–1038 (1992).
Download references
Acknowledgements
This work was supported by a research grant from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Author information
Authors and affiliations.
Novo Nordisk Foundation Center for Basic Metabolic Research, Section of Metabolic Genetics, University of Copenhagen, Copenhagen, Denmark
L. Engelbrechtsen, Y. Mahendran, M. Crusell, T. H. Hansen, T. M. Schnurr, T. Hansen & H. Vestergaard
Danish Diabetes Academy, Odense, Denmark
L. Engelbrechtsen, Y. Mahendran & T. M. Schnurr
Department of Gynecology and Obstetrics, Section of Fetal Medicine, Roskilde University Hospital, Roskilde, Denmark
D. Gybel-Brask & L. Skibsted
Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Herlev, Denmark
Steno Diabetes Center Copenhagen, Gentofte, Denmark
H. Vestergaard
You can also search for this author in PubMed Google Scholar
Contributions
T.H. and H.V. planned the study. Statistical tests were performed by L.E., Y.M. and T.H.H. L.E., T.H.H., Y.M., M.C., T.S., T.H., E.H., D.B. and H.V. analyzed the results. L.E. wrote the first draft. All authors participated in editing the manuscript.
Corresponding author
Correspondence to H. Vestergaard .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Engelbrechtsen, L., Gybel-Brask, D., Mahendran, Y. et al. Birth weight variants are associated with variable fetal intrauterine growth from 20 weeks of gestation. Sci Rep 8 , 8376 (2018). https://doi.org/10.1038/s41598-018-26752-3
Download citation
Received : 14 February 2018
Accepted : 17 May 2018
Published : 30 May 2018
DOI : https://doi.org/10.1038/s41598-018-26752-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Pauline K. Reim
- Line Engelbrechtsen
- Torben Hansen
Scientific Reports (2023)
Transforming obstetric ultrasound into data science using eye tracking, voice recording, transducer motion and ultrasound video
- Lior Drukker
- Harshita Sharma
- Aris T. Papageorghiou
Scientific Reports (2021)
Genetic variants in the glucocorticoid pathway genes and birth weight
- Michael O. Schneider
- Theresa Hübner
- Eva Schwenke
Archives of Gynecology and Obstetrics (2021)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
22 Weeks Pregnant

Your baby is the size of a
At 22 weeks pregnant you may be feeling well and truly pregnant as your baby bump expands and you prepare for your little one’s arrival. With so much going on in your life right now, it’s possible to feel overwhelmed, which is why we've created a helpful guide to what happens at 22 weeks pregnant, covering symptoms you may experience, updates on your baby’s development, and questions for your healthcare provider. So relax and read on!
Highlights at 22 Weeks Pregnant
Following are a few highlights from 22 weeks pregnant:
Your little one now has tiny eyebrows!
Your baby is becoming more responsive to noises around 22 weeks, and you may observe them reacting to a sound during an ultrasound.
At 22 weeks pregnant, your baby's sense of touch has developed.
Soon you could experience mild contraction-like feelings in your abdomen—these may be Braxton Hicks contractions, which are totally normal and not actually contractions!
At 22 weeks pregnant, you may be experiencing many changes to your body as your baby bump grows and you gain some weight. If you’re concerned about weight gain, keep an eye on your pregnancy diet , and remember, you’re carrying around a tiny human right now! Your healthcare provider will check your weight and offer you advice if needed. You can also check out our weight gain calculator below to help you stay on track at 22 weeks pregnant.
Pregnancy Weight Gain Calculator
Fill out your details:.
This is a mandatory field.
I don't know my week
22 Weeks Pregnant: Your Baby's Development
Your little one is making amazing developments during this period. Check out some of the developments happening inside your belly at 22 weeks pregnant:
When you are 22 weeks pregnant, your baby’s eyelids are still fused shut, but the eyes themselves are starting to move.
Tear ducts are also forming and your baby now has eyebrows—little tufts of fine white hair. Your little one may be furrowing those tiny brows!
Your baby is becoming more and more responsive to external stimuli. If you were to have an ultrasound at 22 weeks and a loud noise occurred during the scan, you might see your baby react. For example, your baby might pull their arms and legs closer together in response to the sound.
Your baby’s brain is rapidly developing, and nerve endings are forming. Around this time your baby will have developed a sense of touch, which means they might be experimenting with this new sense by stroking a body part that they happen to reach or sucking their thumb.
Your baby is now starting to put on layers of what’s called brown fat, which helps keep them warm.
How Many Months Is 22 Weeks Pregnant?
How far along is 22 weeks? At 22 weeks, you are approaching the end of your fifth month of pregnancy. If you’re wondering what trimester that is, you’re in the second trimester.
How Big Is a Baby at 22 Weeks Pregnant?
Wondering about the size of your baby when you are 22 weeks pregnant? Your little one could weigh about 1 pound and be almost 7 1/2 inches long, crown to rump.
Your Baby: What Does 22 Weeks Pregnant Look Like?
Check out this illustration to help you get a sense of what your little one might look like this week:
Your Body at 22 Weeks Pregnant
As your bump becomes more prominent around 22 weeks, you might find that more and more people are able to tell that you’re pregnant, making this new stage in your life seem all the more real for you.
As your belly grows, you may struggle with your body image. Some days you may love your pregnant body; other days you may feel uncomfortable with these physical changes and worry that you’ll never look the same again.
Having these kinds of feelings is normal, and it might help to speak to your loved ones or your healthcare provider about what you’re going through. Eating healthily and getting regular exercise may also help you feel better.
22 Weeks Pregnant: Your Symptoms
At 22 weeks pregnant, here are some of the symptoms you may be experiencing:
Heartburn . That burning sensation in your throat and chest during pregnancy could be heartburn. Despite the name, heartburn has nothing to do with your heart! Heartburn happens when stomach acids leak into the esophagus. It’s a common complaint during pregnancy, as pregnancy hormones relax the valve that usually keeps the acids out. Eating small meals throughout the day, staying upright after eating a meal, and avoiding spicy and fried foods might help. Check with your healthcare provider if you’re struggling with heartburn.
Hot flashes. Hormonal changes and a faster metabolism are probably responsible for you feeling hotter and sweatier than normal. The best you can do is to try and stay cool, so wear loose clothes, drink lots of water, and put on the fan or air conditioner.
Racing heart. Did you know that your heart is pumping up to 30 to 50 percent more blood now that you’re pregnant? This is actually good news—more oxygen and nutrients are being delivered to your baby via the placenta. For this reason, having a racing heart can be normal during pregnancy. If you also feel short of breath or you feel your heart racing and it stays that way, call your provider ASAP.
Pelvic pain. Pregnancy hormones loosen your joints, helping them become more flexible. But this flexibility may be causing you some pain. To help, try not to lift heavy objects and avoid standing for too long. Learn more about hip and pelvic pain .
Abdominal pain or cramping. As well as the common lower back pain you may be feeling, you might also experience mild uterine cramps, abdominal pain, or what feels like mild contractions at 22 weeks pregnant, or at another time during this trimester. This could be normal—for example, you could be experiencing Braxton Hicks “practice” contractions , which help your body get ready for labor, or you could be feeling your abdominal muscles and ligaments stretch as your belly grows. However, you'll want to contact your healthcare provider if you have abdominal pain or cramping that doesn't go away or gets worse, or is accompanied by diarrhea; or if you're feeling increased pressure in the pelvic area; or you're experiencing bleeding. Of course, feel free to reach out to your provider if you have questions or concerns about any symptoms at 22 weeks pregnant.
How Big Is a Pregnant Belly at 22 Weeks?
Every pregnancy is unique, so it’s possible for your bump to be very obvious at 22 weeks pregnant or perhaps it’s still easily disguised. Either way, your belly is continuing to grow as your baby and uterus expand. If you recently had a prenatal checkup (perhaps including an ultrasound exam) at 20 to 22 weeks, your healthcare provider may have measured your fundal height, which is the distance from your pubic bone to the top of your uterus.
What Does 22 Weeks Pregnant Look Like?
For a better idea of what size your belly might be around 22 weeks pregnant, in your fifth month of pregnancy, check out the image below.
22 Weeks Pregnant: Things to Consider
From picking baby names to maintaining intimacy with your partner, being 22 weeks pregnant brings with it many things to consider. Read on for some helpful tips and suggestions.
Try some stress-relief methods. It’s normal to worry during pregnancy. However, it’s better for both you and your baby if you try to keep your stress levels under control. This is easier said than done, but strategies such as cutting back on the amount of hours you work, delegating tasks to others, exercising regularly, and speaking to someone you trust about your fears and anxieties could really help.
Sex during pregnancy is generally safe if you’re having a healthy and normal pregnancy, and when both you and your partner feel up for it. You won’t hurt your baby—the amniotic sac and the muscles of your uterus keep your baby protected. You may find that your sex drive ebbs and flows during pregnancy. Some moms-to-be report an increased desire for sex during this trimester, as their energy levels have now returned after the first stage of pregnancy. Seeing some spots of blood or having mild cramping after sex can be normal, but if you have heavy bleeding or persistent cramping, contact your healthcare provider. If you have pregnancy complications—for example, if you’re at an increased risk of preterm labor—your provider may recommend abstaining from sex during your pregnancy.
Keep looking for those baby names! If you’re struggling to find inspiration, check out our list of the top baby boy names and our list of the top baby girl names . If you have a few favorite names but you’re struggling to pick, consider throwing a baby naming party —perhaps your loved ones can help you make your mind up.
If you have other children, consider how you will let them know about the new arrival and think about how you would like to involve them in your pregnancy.
Start planning your baby’s nursery and think about what changes need to be made to the existing room to make it comfortable for your baby. If your baby will be sharing the space with your toddler, read our article on creating a room for two .
22 Weeks Pregnant: Consult Your Healthcare Provider
Throughout your pregnancy, your healthcare provider is there to support you and answer any questions you have. Here are some common questions at 22 weeks:
How am I doing with regard to weight gain at 22 weeks pregnant? If I’m not where I should be, what changes should I make to get back on track? Keep in mind that you can also play around with our Pregnancy Weight Gain Calculator .
Do I need a flu shot? What should I do if I come down with the flu?
What should I do if I come in contact with someone who has chicken pox ?
What hospitals or birth centers do you recommend? When do I need to pre-register to deliver?
22 Weeks Pregnant: Your Checklist
For a little help along the way, check out our to-dos:
□ If you now know your little one’s gender and would like to do a big reveal for family and friends, start organizing your gender reveal party. To help you out, we've put together some gender reveal game ideas and a more general list of ideas for revealing your baby’s gender .
□ a few months to go until your due date, now is a good time to start thinking about and researching your options for labor and delivery. Consult your healthcare provider and find out what’s available to you. You might like to enquire about pain relief and other comfort measures that might be options for you. If a birth plan is something you might like to have, take a look at our downloadable birth plan guide.
□ Start to think about who you would like with you during labor and delivery. It could be your partner, a relative, or a friend. Some moms-to-be hire a doula —someone who has received special training in labor support and childbirth. If you would like to hire a doula, then start asking around for referrals—you could ask your healthcare provider or the person who runs your birthing class. Schedule appointments with potential doulas to get to know them a little better, and don’t forget to ask them about their fees, as the cost may not be covered by any insurance you have.
□ If you’re thinking of breastfeeding, ask your healthcare provider or other moms in your area for tips on finding a good lactation consultant or lactation classes that you could sign up for.
How We Wrote This Article The information in this article is based on the expert advice found in trusted medical and government sources, such as the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. You can find a full list of sources used for this article below. The content on this page should not replace professional medical advice. Always consult medical professionals for full diagnosis and treatment.
- ACOG. “How Your Fetus Grows During Pregnancy.”
- American College of Obstetricians and Gynecologists. Your Pregnancy and Childbirth: Month to Month, 6th ed. (Washington, DC: American College of Obstetricians and Gynecologists, 2015).
- Cleveland Clinic. “Fetal development: Stages of Growth.”
- Kids Health. “Pregnant Mental Health.”
- Kids Health. “Week 22.”
- Mayo Clinic. “Fetal Development: The Second Trimester.”
- Mayo Clinic. “Fetal Positions.”
- Mayo Clinic. “Fundal Height.”
- Mayo Clinic. Guide to a Healthy Pregnancy, 2nd ed. (Rochester, MN: Mayo Clinic Press, 2018).
Review this article:
Read more about pregnancy.
- Pregnancy Announcement
- Pregnancy Calendar
- Pregnancy Symptoms
- Baby Shower & Registry
- Prenatal Health and Wellness
- Preparing For Your New Baby
- Giving Birth
- Due Date Calculator

IMAGES
VIDEO
COMMENTS
Indeed, for each of those 3 examples, survival may be lower, and long term complications just as uncertain, as the baby born at 22 weeks gestation. Surely all high risk infants, whatever their gestational age, have outcomes that are uncertain and variable, require individualized decision-making with parental involvement, and deserve long term ...
Abstract We investigated changes in the frequencies of four primary types of singleton fetal lie/presentation for each gestational week from 18 to 39 weeks in ... ultrasound examinations were used. From 22 to 36 weeks of gestation, the prevalence of cephalic presentation increased from 47% (45-50%) to 94% (91-96%), before and after which ...
Resuscitated infants delivered by cesarean delivery had higher rates of survival at 22 weeks (44.9 vs 23.0%; P<.001) and at 23 weeks (53.3 vs 43.4%; P<.001) of gestation regardless of fetal presentation.Multivariable logistic regression analysis demonstrated that infants who were delivered by cesarean delivery at 22 weeks (adjusted relative risk, 2.3; 95% confidence interval, 1.9-2.8) and 23 ...
In a study from Germany, only 2 of 47 infants died during delivery demonstrating it is possible to safely deliver a 22-weeker (1). Moreover, their survival rate was 61% with complication rates no different than 23-weekers. In Sweden, another study demonstrated a 53% survival rate with similar morbidity (2).
babies born at 22 weeks' gestation in England and Wales. DEsign with a threefold increase in 22 weeks' gestation Population based cohort study. sEtting England and Wales, comprising routine data for births and hospital records. PartiCiPants Babies alive at the onset of care in labour at 22 weeks+0 days to 22 weeks+6 days
Active treatment was provided to 6657 infants. The rate at 22 weeks was 19.4% and increased with each advancing week, and was significantly higher for infants born between days 4 and 6 at 22 or 23 weeks of gestation compared with those born between days 0 and 3 (26.2% and 78.3%, respectively, vs 14.1% and 65.9%, respectively; P < .001).
The clinical care of infants born at 22 weeks' gestation must be well-designed and standardized if optimal results are to be expected. Although several approaches to care in this vulnerable population are possible, protocols should be neither random nor inconsistent. We describe the approach taken at the University of Iowa Stead Family Children's Hospital neonatal intensive care unit with ...
Fetal presentation, or how your baby is situated in your womb at birth, is determined by the body part that's positioned to come out first, and it can affect the way you deliver. At the time of delivery, 97 percent of babies are head-down (cephalic presentation).
Survival is only presented for babies who received active neonatal intensive care. The overall survival at 22 weeks, to one year of age, is shown below, divided by birth weight categories. They don't have the same sort of birth weight breakdown for the 23 week babies, but overall 1 year survival was 58% after antenatal steroids, and 48% ...
Decision-making on resuscitation in the delivery room in neonates born between 22 and 24 weeks of gestation is complex and varies according to institutions [1, 2].Despite advances in perinatal and ...
United States. Cesarean delivery is associated with increased survival at 1 year of life among resuscitated, periviable infants born between 22 0/7 and 23 6/7 weeks of gestation, especially in the setting of nonvertex presentation. However, cesarean delivery is associated with increased maternal morbidity.
These studies show a wide variation in survival rates following live birth at periviable gestational ages, ranging from 0-37% at 22 weeks', 1-64% at 23 weeks', 31-78% at 24 weeks' and 59-86% at 25 weeks' of gestation. The variation in survival rates following periviable birth among cohorts in developed nations ( Figure 2) is ...
breech presentation: fetal rump presenting towards the internal cervical os, this has three main types. frank breech presentation (50-70% of all breech presentation): hips flexed, knees extended (pike position) complete breech presentation (5-10%): hips flexed, knees flexed (cannonball position) footling presentation or incomplete (10-30%): one ...
When a fetus faces up, the neck is often straightened rather than bent,which requires more room for the head to pass through the birth canal. Delivery assisted by a vacuum device or forceps Operative Vaginal Delivery Operative vaginal delivery is delivery using a vacuum extractor or forceps. A vacuum extractor consists of a small cup made of a rubberlike material that is connected to a vacuum.
During routine prenatal care, clinicians assess fetal lie and presentation with physical examination in the late third trimester. Ultrasonography can also be done. If breech presentation is detected, external cephalic version can sometimes move the fetus to vertex presentation before labor, usually at 37 or 38 weeks.
On March 21, 2014 the following report was presented by ultrasound of the fetuses: the right fetus has variable presentation, normal amniotic fluid, and a posterior placenta at gestational age of 20 weeks and 6 days and the left fetus has variable presentation, normal amniotic fluid, and a posterior placenta at gestational age of 20 weeks and 3 ...
Heartbeat. By 22 weeks, the baby's heartbeat can usually be heard through a stethoscope. You can tell the difference between the pregnant parent's and baby's heartbeat by the number of beats in a minute. An adult's heart rate is between 60 and 100 beats per minute. The baby's heart beats faster and will be between 110-160 beats per minute.
A congenital anomaly scan (CAS), sometimes called congenital anatomy scan or 20-week scan, is done in the second trimester, between 18 to 22 weeks. The International Society of Ultrasound in Obstetrics and Gynecology ( ISUOG) recommends that CAS ultrasound be performed as part of the routine prenatal care for pregnant all women.
Birth month group discussions. Your baby's grip, vision and hearing are all getting stronger now. By week 22 of pregnancy, your little one has also achieved a big milestone: breaking the 1-pound mark! Meanwhile, you may be noticing more changes, including a protruding navel and possibly even slightly bigger feet — the result of all those ...
22 weeks pregnant: Symptoms, tips, and baby development. Product. Tracking cycle. Getting pregnant. Pregnancy. Help Center. Find out what's happening in your body at 22 weeks pregnant, the size of your growing baby, and what symptoms you might have at this stage.
We then tested the impact of variants in growth periods from 20-25 weeks, from 25-32 weeks and from 32 weeks to birth. Two variants (rs61830764 and rs61862780) showed association with fetal ...
When you are 22 weeks pregnant, your baby's eyelids are still fused shut, but the eyes themselves are starting to move. Tear ducts are also forming and your baby now has eyebrows—little tufts of fine white hair. Your little one may be furrowing those tiny brows! Your baby is becoming more and more responsive to external stimuli.