Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Collection 15 August 2018

Top 10 in Biochemistry and Molecular Biology
Explore our most highly accessed biochemistry and molecular biology articles in the first quarter of 2018. Featuring authors from around the World, these papers highlight valuable research within biochemistry and molecular biology from an international community.

Peptide ion channel toxins from the bootlace worm, the longest animal on Earth
- Erik Jacobsson
- Håkan S. Andersson
- Ulf Göransson
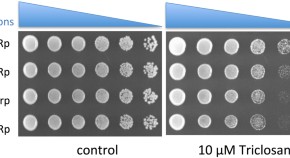
Plasmodium dihydrofolate reductase is a second enzyme target for the antimalarial action of triclosan
- Elizabeth Bilsland
- Liisa van Vliet
- Stephen G. Oliver
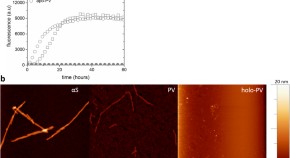
Abundant fish protein inhibits α-synuclein amyloid formation
- Tony Werner
- Ranjeet Kumar
- Pernilla Wittung-Stafshede
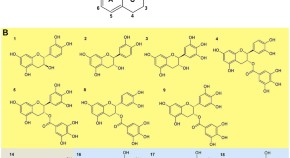
Natural polyphenols as sirtuin 6 modulators
- Minna Rahnasto-Rilla
- Ruin Moaddel
In vivo fluorescence bioimaging of ascorbic acid in mice: Development of an efficient probe consisting of phthalocyanine, TEMPO, and albumin
- Takanori Yokoi
- Takayuki Otani
- Kazuyuki Ishii
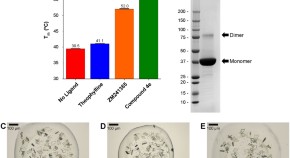
Towards high throughput GPCR crystallography: In Meso soaking of Adenosine A 2A Receptor crystals
- Prakash Rucktooa
- Robert K. Y. Cheng
- Andrew S. Doré
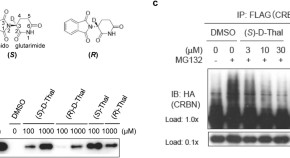
Structural basis of thalidomide enantiomer binding to cereblon
- Tomoyuki Mori
- Toshio Hakoshima
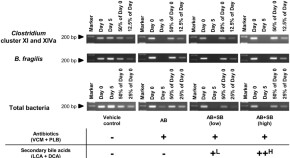
Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels
- Takuya Kuno
- Mio Hirayama-Kurogi
- Sumio Ohtsuki
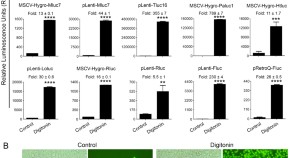
Development and characterization of a novel luciferase based cytotoxicity assay
- Hittu Matta
- Ramakrishnan Gopalakrishnan
- Preet M. Chaudhary
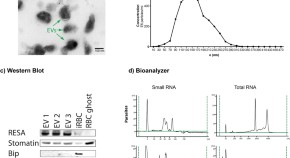
Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles
- Kehinde Adebayo Babatunde
- Smart Mbagwu
- Pierre-Yves Mantel
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Biochemistry and Biotechnology
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- HEAVY METAL (LEAD) TOXICITY AND OXIDATIVE STRESS Follow Following
- Structure Based Drug Design Follow Following
- Medical Biochemistry Follow Following
- Biochemistry Follow Following
- Biotechnology Follow Following
- Molecular Biology Follow Following
- Food Biochemistry Follow Following
- Platelet Rich Plasma Follow Following
- MicroRNA Follow Following
- Molecular docking Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Aims and scope
Biochemistry and Biotechnology Research publishes articles on innovative work from all areas related to Biotechnology and Biochemistry.
Biochemistry and Biotechnology Research is a peer reviewed open access journal, which publishes innovative work from all areas related to Biotechnology and Biochemistry. It covers from molecular biology and the chemistry of biological process to aquatic and earth environmental aspects, as well as computational applications, policy and ethical issues directly related to Biotechnology. Molecular biology, genetic engineering, microbial biotechnology, plant biotechnology, animal biotechnology, marine biotechnology, environmental biotechnology, biological processes, industrial applications, bioinformatics, biochemistry of the living cell, bioenergetics, inorganic biochemistry, innovation in biotechnology and bio-ethics, biotechnology in the developed and developing world, management and economics of biotechnology, political and social issues and others are some of the main subjects considered.
Biochemistry and Biotechnology Research is published quarterly by Net Journals.
Open Access
All articles published by Biochemistry and Biotechnology Research are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Authors of articles published in Biochemistry and Biotechnology Research are the copyright holders of their articles and have granted to any third party, in advance and in perpetuity, the right to use, reproduce or disseminate the article, according to the Net Journals copyright and license agreement.
Article-processing charges
Open access publishing is not without costs. Biochemistry and Biotechnology Research therefore levies an article-processing charge of $400 for each article accepted for publication. We routinely waive charges for authors from low-income countries. A limited number of waivers for article-processing charges are also available at the editors' discretion, and authors wishing to apply for these waivers should contact the editors.
Publication and peer review process
Content overview.
• Regular articles: These should describe new and carefully confirmed findings, and experimental procedures should be given in sufficient detail for others to verify the work. The length of a full paper should be the minimum required to describe and interpret the work clearly.
• Reviews: Submissions of reviews and perspectives covering topics of current interest are welcome and encouraged. Reviews should be concise and no longer than 4-6 printed pages (about 12 to 18 manuscript pages). Reviews manuscripts are also peer-reviewed.
•Short Communications: A Short Communication is suitable for recording the results of complete small investigations or giving details of new models or hypotheses, innovative methods, techniques or apparatus. The style of main sections need not conform to that of full-length papers. Short communications are 2 to 4 printed pages (about 6 to 12 manuscript pages) in length.
Ethical guidelines
Submission of a manuscript to Biochemistry and Biotechnology Research implies that all authors have read and agreed to its content, and that any experimental research that is reported in the manuscript has been performed with the approval of an appropriate ethics committee. Research carried out on humans must be in compliance with the Helsinki Declaration, and any experimental research on animals must follow internationally recognized guidelines. A statement to this effect must appear in the Methods section of the manuscript, including the name of the body which gave approval, with a reference number where appropriate. Informed consent must also be documented. Manuscripts may be rejected if the editorial office considers that the research has not been carried out within an ethical framework, e.g. if the severity of the experimental procedure is not justified by the value of the knowledge gained. For all articles that include information or clinical photographs relating to individual patients, written and signed consent from each patient to publish must also be made available if requested by the editorial staff.
Biochemistry and Biotechnology Research 's publisher, Net Journals, has a legal responsibility to ensure that its journals do not publish material that infringes copyright, or that includes libellous or defamatory content. If, on review, your manuscript is perceived to contain potentially libellous content the journal Editors, with assistance from the publisher if required, will work with authors to ensure an appropriate outcome is reached. The involvement of scientific writers or anyone else who assisted with the preparation of the manuscript content should be acknowledged.
Data and materials release
Submission of a manuscript to Biochemistry and Biotechnology Research implies that readily reproducible materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes. Nucleic acid sequences, protein sequences, and atomic coordinates should be deposited in an appropriate database in time for the accession number to be included in the published article. In computational studies where the sequence information is unacceptable for inclusion in databases because of lack of experimental validation, the sequences must be published as an additional file with the article.Any 'in press' articles cited within the references and necessary for the reviewers' assessment of the manuscript should be made available if requested by the editorial office.
Competing interests
Biochemistry and Biotechnology Research requires authors to declare any competing financial or other interest in relation to their work. All competing interests that are declared will be listed at the end of published articles. Where an author gives no competing interests, the listing will read 'The author(s) declare that they have no competing interests'.
Plagiarism detection
In cases of suspected plagiarism CrossCheck is available to the editors of Biochemistry and Biotechnology Research to detect instances of overlapping and similar text in submitted manuscripts. CrossCheck is a multi-publisher initiative allowing screening of published and submitted content for originality.
Authors of articles published in Biochemistry and Biotechnology Research retain the copyright of their articles and are free to reproduce and disseminate their work.
- About Journal
- Instruction for Authors
- Submit Manuscript
- Publication Ethics
- Current Issue
- Search Menu
- Sign in through your institution
- Advance Articles
- Special issues
- Analytical Chemistry
- Biochemistry and Molecular Biology
- Environmental Science
- Food and Nutrition Science
- Microbiology and Fermentation Technology
- Organic Chemistry
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Why Publish with BBB?
- Call for Papers
- About Bioscience, Biotechnology, and Biochemistry
- About Japan Society for Bioscience, Biotechnology, and Agrochemistry
- Editorial Board
- Advertising and Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Materials and methods, data availability, author contribution, disclosure statement, acknowledgments.
- < Previous
Pharmacokinetics of soy-derived lysophosphatidylcholine compared with that of glycerophosphocholine: a randomized controlled trial
- Article contents
- Figures & tables
- Supplementary Data
Ryohei Tanaka-Kanegae, Hiroyuki Kimura, Koichiro Hamada, Pharmacokinetics of soy-derived lysophosphatidylcholine compared with that of glycerophosphocholine: a randomized controlled trial, Bioscience, Biotechnology, and Biochemistry , Volume 88, Issue 6, June 2024, Pages 648–655, https://doi.org/10.1093/bbb/zbae031
- Permissions Icon Permissions
Lysophosphatidylcholine (LPC) is present in various foods and contains a choline moiety such as in glycerophosphocholine (GPC). However, the potential of LPC as a choline source remains unclear. This study investigated the single-dose pharmacokinetics of 480 mg soy-derived LPC in 12 healthy men compared with that of either soy oil with the same lipid amount (placebo) or GPC with the same choline amount. Both LPC and GPC supplementation increased plasma choline, serum phospholipid, and serum triglyceride concentrations, but neither of them significantly elevated plasma trimethylamine N -oxide concentration. In addition, although the intake of LPC slightly increased plasma LPC16:0, LPC18:2, and total LPC concentrations, their concentrations remained within physiological ranges. No adverse events were attributed to the LPC supplementation. To the best of our knowledge, this study is the first to compare LPC and GPC pharmacokinetics in humans and shows that LPC can be a source of choline.

Pharmacokinetics of lysophosphatidylcholine was evaluated and compared with that of placebo (triglyceride) and glycerophosphocholine in a randomized trial.
Choline (Ch) is an essential nutrient and important for the structural integrity of cell membranes, methyl metabolism, cholinergic neurotransmission, transmembrane signaling, and lipid and cholesterol transport and metabolism. Although the adequate intake values for Ch were defined by the Food and Nutrition Board of the USA (Institute of Medicine 1998 ), the amounts of Ch consumed by most Americans are still below these values (Wallace et al. 2018 ). Because Ch is found predominantly in animal-derived foods, vegetarians and vegans possibly have a greater risk for inadequacy. Therefore, finding and developing new Ch sources, particularly from plants, are practical.
Lysophosphatidylcholine (LPC) is a lysophospholipid widely found in living organisms and food (Weihrauch and Son 1983 ; Tan et al. 2020 ). Although it contains a Ch moiety, LPC has gained less attention as a Ch source and cognitive enhancer candidate than other Ch sources, such as glycerophosphocholine (GPC) and phosphatidylcholine (PC) (Tayebati and Amenta 2013 ; Traini et al. 2020 ). This is because levels of saturated LPC16:0 and LPC18:0 and monounsaturated LPC18:1 above the physiological ranges can cause pro-inflammatory actions and produce reactive oxygen species (ROS) (Tan et al. 2020 ). However, LPC concentrations in the body are tightly regulated by enzymes for LPC production and clearance, including lecithin-cholesterol acyltransferase, lipoprotein-associated phospholipase A2, and LPC acyltransferase (Law et al. 2019 ). We previously showed in vivo that orally supplemented soy-derived LPC, which is commercially available as enzyme-modified lecithin (EML or lysolecithin), restored brain acetylcholine levels without elevating blood LPC16:0, 18:0, and 18:1 levels and exhibited cholinergic activity comparable with that of GPC (Tanaka-Kanegae, Kimura and Hamada 2023 ). Moreover, pharmacokinetic studies of GPC involved humans (Traini, Bramanti and Amenta 2013 ; Böckmann et al. 2023 ), whereas those of LPC were limited to animals (Nilsson 1968 ; Thies et al. 1994 ). Therefore, in the present study, we aimed to investigate the pharmacokinetics of a soy-derived LPC supplement in comparison with that of a GPC supplement in healthy male volunteers. This pharmacokinetic study may serve as a reference for using LPC as a novel Ch source.
The study was approved by the institutional review board of Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan; approval number 1802, Q115), registered in the UMIN Clinical Trials Registry (registration number UMIN000052385), and conducted at the Saga Nutraceuticals Research Institute of Otsuka Pharmaceutical Co., Ltd. in compliance with the Declaration of Helsinki.
Human volunteers
Twelve healthy adult males were recruited for the study. Participants were included in the study when they had no health conditions preventing them from fulfilling the study requirements, as judged by the principal investigator based on the laboratory screening test. The main exclusion criteria were as follows: Those who had a digestive disorder that could affect the absorption of the supplement, those who were regularly taking oral medicines, those who had blood pressure considerably deviating from the normal range, those who had donated blood and/or participated in another clinical trial within a month of the study commencement, and those who were allergic to soy. Accordingly, 12 eligible men who provided informed consent were enrolled.
Test supplements
Three supplements were prepared:
Placebo: 11 soft capsules containing 3.3 g of soy oil,
LPC: 11 soft capsules containing 3.3 g of soy-derived EML (SLP-PasteLyso), 480 mg of LPC, and 66 mg of a Ch moiety,
GPC: 11 hard capsules containing 162 mg of GPC (LIPOID GPC) and 66 mg of a Ch moiety.
Soy oil, SLP-PasteLyso, and LIPOID GPC were purchased from The Nisshin OilliO Group, Ltd. (Tokyo, Japan), Tsuji Oil Mills Co., Ltd. (Mie, Japan), and H. Holstein Co., Ltd. (Tokyo, Japan), respectively. The capsules were manufactured by Sankyo Co., Ltd. (Shizuoka, Japan).
The placebo supplement contained soy oil with the same amount of lipid as the LPC supplement. SLP-PasteLyso is an EML that was used to assess the cholinergic activity of LPC in vivo (Tanaka-Kanegae, Kimura and Hamada 2023 ). Although EML is used as an emulsifier and is generally recognized as safe ( FDA ), its tolerable limits for consumption remain to be established. In the present study, the dose of SLP-PasteLyso was set at 480 mg from a formulation perspective; this dose was deemed safe, considering that EML can be added to beverages at a concentration of 1% ( w/w ) ( FDA 2014 ). Meanwhile, the GPC dose was determined such that the same amount of Ch would be ingested as that of the EML. However, the formulations (soft/hard capsules) were inconsistent due to differences in their physical properties. The purity of LPC in SLP-PasteLyso was 14.6%, whereas that of GPC in LIPOID GPC was more than 98%. Although EMLs with higher LPC purities are commercially available, SLP-PasteLyso, which contained fat (55% acetone-soluble matter), was selected because the absorption of LPC through the lymphatic pathway is improved when ingested with fat (Nilsson 1968 ; Tanaka-Kanegae, Kimura and Hamada 2023 ). Additionally, a negligible amount of PC is present in SLP-PasteLyso. The fatty acid composition of LPC in SLP-PasteLyso was determined using the method described below and is shown in Table 1 .
Fatty acid composition of LPC in SLP-PasteLyso
Levels of LPC16:0 (palmitoyl), 18:0 (stearoyl), 18:1 (oleoyl), 18:2 (linoleoyl), and 18:3 (linolenoyl) were quantified. The ratio of each species to the total LPC, which is the sum of the concentrations of the five LPC species, is presented. Quantification was performed three times using the same lot, and the average value was used. LPC, lysophosphatidylcholine.
Study design
This was a randomized, open-label, controlled study with a crossover consumption of placebo, LPC, and GPC. More than 6 days were left between different supplementations as a washout period. A previous study (Böckmann et al. 2023 ) investigated the metabolism of deuterium-labelled (D9) Ch supplements including GPC and PC at the dose of 2.7 mg kg −1 D9-Ch equivalent. The study demonstrated that blood D9 Ch concentration returned to 0 µmol L −1 within 24 h for all the supplements. Regarding the amount of Ch, the dose used in the present study (66 mg Ch per head) was lower than that in the previous study and can be ingested in a daily diet. Therefore, we considered a washout period of 6 days was adequate. Those who assessed the outcomes were blinded to the supplement assignment. The participants consumed standard meals for a day prior to the study. After overnight fasting, the participants ingested their assigned supplement immediately after consuming a standard meal (two pouches of jelly, Ch was not detected in this meal and the fatty acid composition is presented in Table S1 ). Blood samples were collected before and at 0.5, 1, 2, 4, 6, and 8 h after supplementation. Tubes coated with sodium heparin were used for plasma collection. At 4 h after blood collection, the participants consumed the same standard meal. The participants were allowed to drink mineral water freely but refrained from smoking until the final blood collection. The blood samples were centrifuged (1200× g , 15 min, 4 °C), and the plasma and serum were preserved at −80 °C until measurement. All adverse events during the study days were reported.

Chemical analysis
Ingested LPC and GPC are partially degraded into Ch and converted to PC before absorption (Nilsson and Duan 2019 ). Therefore, changes in plasma Ch and LPC and serum phospholipid concentrations were measured after supplementation. Moreover, serum triglyceride (TG) levels were quantified as increased blood PC may facilitate lipid export from the liver into the blood (Olthof et al. 2005 ). Furthermore, the levels of plasma trimethylamine N -oxide (TMAO), which is associated with ROS production and atherosclerosis development (Zhu et al. 2022 ), were evaluated.
Plasma Ch levels were measured following a previously reported method (Tanaka-Kanegae and Hamada 2021 ) with minor modifications. Briefly, the plasma was mixed with isopropyl homocholine as an internal standard and delipidated using chloroform. The supernatant was subjected to solid-phase extraction using an Oasis WCX (Waters Corporation, Milford, MA, USA). Extraction was completed by elution with 5% formic acid in 2-propanol–acetonitrile (60:40, v/v ). After drying with N 2 , the analytes were reconstituted in phosphate buffer (pH 4.4) and injected into the high-performance liquid chromatography (HPLC) electrochemical detection system.
LPC species in the plasma and SLP-PasteLyso were measured as previously reported (Tanaka-Kanegae, Kimura and Hamada 2023 ). Briefly, plasma LPC was extracted using the Bligh and Dyer method (Bligh and Dyer 1959 ) after adding LPC19:0 (nonadecanoyl) as an internal standard. The extract was dried under reduced pressure and reconstituted in methanol. After filtration, the analytes were injected into the HPLC system coupled to an evaporative light-scattering detector. The detected areas for the different concentrations of the LPC16:0 (palmitoyl) standards were converted to a logarithmic scale to generate a fitting curve, and the levels of LPC16:0, 18:0 (stearoyl), 18:1 (oleoyl), 18:2 (linoleoyl), and 18:3 (linolenoyl) were quantified. Total LPC levels were calculated as the sum of these five LPC species. Serum phospholipid and TG levels were determined enzymatically (Takayama et al. 1977 ; Tamaoku et al. 1982 ) by SRL, Inc. (Tokyo, Japan). Plasma TMAO levels were quantified as previously described (Kanemitsu et al. 2017 ).
Statistical analyses
For kinetic parameters, the delta C max was determined by adjusting the 0 h value to 0, and the incremental area under the curve (iAUC) until 8 h after supplementation was calculated using the trapezoidal method (Le Floch et al. 1990 ). Data are expressed as the mean ± standard deviation. Statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). The effects of the supplement were assessed using a linear mixed-effects model, with the supplement, period, and sequence as fixed factors, and the participants as the random factor. Considering the exploratory nature of the study, correction for multiple testing was not conducted when differences among the supplements were assessed. Differences from the baseline were analyzed using a two-tailed Dunnett's post-hoc test after analysis of variance for randomized block designs. Differences in the frequency of adverse events between placebo and LPC groups were analyzed using Fisher exact probability test (two-sided). Differences were considered statistically significant at p < 0.05.
All 12 test participants completed the study. Although some adverse events, such as headache, were reported during the study, the frequency of such events was not statistically different between placebo and LPC groups ( Table S2 ). In addition, no abnormal changes in laboratory test results were observed after the study. Hence, no adverse events were attributed to LPC supplementation. The plasma Ch concentrations of all the participants are shown in Table S3 . The initial Ch levels of participant ID 12 were considerably above the normal range (7.0-9.3 µmol L −1 , Holm et al. 2003 ) and the levels constantly decreased until 1 h after supplementation, regardless of the content. As a disturbance in Ch metabolism was suspected, that participant was excluded from subsequent analyses. The demographic data of the study participants excluding ID 12 are presented in Table 2 , and the data including ID 12 are presented in Table S4 .
Demographics of the study participants included in analyses
Numbers in brackets indicate the ranges. One participant was excluded from analyses as a disturbance in Ch metabolism was suspected.
Changes in the plasma concentrations, delta C max , and iAUC of Ch are shown in Table 3 (individual data are shown in Table S3 ). A significant supplement effect ( p = 0.036) on the Ch concentrations was observed. Specifically, the Ch concentrations were significantly higher in the LPC and GPC groups than in the placebo group at 2 and 4 h after supplementation ( p = 0.0044 and 0.0027 for LPC, respectively; p = 0.0022 and 0.0005 for GPC, respectively). However, no significant difference in plasma Ch concentrations was found between the LPC and GPC groups at any time point. Additionally, GPC intake significantly increased plasma Ch levels at 4 h compared with the baseline ( p = 0.017). Moreover, significant differences in the delta C max of Ch were observed between the LPC and placebo groups ( p = 0.030) and between the GPC and placebo groups ( p = 0.0085). Furthermore, the iAUC of Ch concentration was significantly higher in the LPC and GPC groups than in the placebo group ( p = 0.0098 and 0.0003 for LPC and GPC, respectively).
Concentrations, delta C max , and iAUC of plasma Ch
Plasma Ch concentration was measured until 8 h after supplementation. Data are presented as the mean ± SD ( n = 11). Different letters indicate statistically significant differences between the supplements ( p < 0.05). * p < 0.05 vs. 0 h. iAUC, incremental area under the curve; Ch, choline; GPC, glycerophosphocholine.
Changes in the plasma concentrations, delta C max , and iAUC of the LPC species are shown in Table 4 (individual data are shown in Table S5 ). The concentration of LPC18:3 was below the determination limit. No significant supplement effect was observed on any of the pharmacokinetic parameters of the LPC species. However, time effects were observed on some of the LPC species. Specifically, significant increases were observed for the LPC16:0 concentration at 6 h after LPC supplementation ( p = 0.017), LPC18:2 concentration at 6 and 8 h after placebo supplementation ( p = 0.0001 and 0.049, respectively) and at 6 h after LPC supplementation ( p = 0.0029), and the total LPC concentration at 6 h after LPC supplementation ( p = 0.0062).
Concentrations, delta C max , and iAUC of plasma LPC species
Concentrations of plasma LPC were measured until 8 h after supplementation. LPC18:3 was below the determination limit. Data are presented as the mean ± SD ( n = 11). * p < 0.05, ** p < 0.01 vs. 0 h.
Changes in the serum concentrations, delta C max , and iAUC of phospholipids are shown in Table 5 (individual data are shown in Table S6 ). No significant supplement effect was observed on any of the pharmacokinetic parameters. However, serum phospholipid levels significantly increased in the LPC and GPC groups ( p = 0.0019 for LPC at 8 h and p < 0.0001 for GPC at 6 and 8 h).
Concentrations, delta C max , and iAUC of serum phospholipids
Serum phospholipid concentration was measured until 8 h after supplementation. Data are presented as the mean ± SD ( n = 11). ** p < 0.01 vs. 0 h.
Changes in the serum concentrations, delta C max , and iAUC of TG are shown in Table 6 (individual data are shown in Table S7 ). A significant supplement effect on the TG concentrations ( p = 0.0085) was observed. Specifically, the TG concentrations were significantly higher in the LPC and GPC groups than in the placebo group at 6 and 8 h after supplementation ( p = 0.023 and < 0.0001 for LPC, respectively; p = 0.041 and 0.0013 for GPC, respectively). However, no significant difference in serum TG concentrations was found between the LPC and GPC groups at any time point. Additionally, significant increases in TG concentration were observed at 4 h in the placebo group ( p = 0.021); at 2, 4, 6, and 8 h in the LPC group ( p = 0.022, 0.0007, 0.0014, and 0.0067, respectively); and at 4, 6, and 8 h in the GPC group ( p = 0.0033, 0.0002, and 0.031, respectively). Moreover, the delta C max of TG was significantly higher in the LPC group than in the placebo and GPC groups ( p = 0.014 vs. placebo and 0.036 vs. GPC). The iAUC of TG concentration was higher in the LPC group than in the other two groups, but the supplement effect assessed by the linear mixed-effects model was not significant ( p = 0.097).
Concentrations, delta C max , and iAUC of serum TG
Serum TG concentration was measured until 8 h after supplementation. Data are presented as the mean ± SD ( n = 11). Different letters indicate statistically significant differences between the supplements ( p < 0.05). * p < 0.05, ** p < 0.01 vs. 0 h. TG, triglyceride.
Changes in the plasma TMAO concentrations are shown in Table 7 (individual data are shown in Table S8 ). No significant supplement or time effect was observed in the plasma TMAO concentrations.
Changes in plasma TMAO concentration
Plasma TMAO concentration was measured until 8 h after supplementation. Data are presented as the mean ± SD ( n = 11). TMAO, trimethylamine N -oxide.
Only males were enrolled in this study as estrogen affects Ch metabolism (Fischer et al. 2010 ). If the supplement doses in the present trial were considered as the amount of Ch (66 mg), they would be lower than those used in previous trials (Kawamura et al. 2012 ; Böckmann et al. 2022 ; Im et al. 2022 ). Therefore, the delta C max and iAUC were calculated to detect the pharmacokinetic changes after supplementation (Judy 2022 ; Le Floch et al. 1990 ; Tobin, Kiens and Galbo 2008 ). Our results demonstrated that the supplementation of LPC increased plasma Ch concentrations similar to that of GPC, and the average peaks were observed at 4 h after both LPC and GPC supplementation. However, previous studies investigating GPC blood kinetics reported varying but faster peak times for blood Ch concentration. Specifically, Kawamura et al. ( 2012 ) reported the peak at 1 h after 1000 mg GPC intake, Böckmann et al. ( 2022 ) after 2 h (1358 mg GPC), and Im et al. ( 2022 ) after 3 h (1200 mg GPC). Of note, this inconsistency is well reflected by significant inter-personal differences in the blood Ch response observed in the present ( Table S3 ) and previous studies (Böckmann et al. 2022 ). Additionally, blood was not collected 3 h after supplementation in our study, when the peak Ch concentration following GPC consumption may be observed. An animal study supports the possibility that the administration of varying doses of Ch precursors result in different peak times for blood Ch concentration (López-Coviella et al. 1995 ). This could be explained by different ratios of chyloportal partition among individuals, by which degraded Ch is absorbed through portal vein, whereas when Ch is converted to PC in the intestine, it is absorbed through lymphatic vessel. Differences in various enzyme activities related to Ch metabolism in the liver probably add to the individual variability in blood Ch response (Li and Vance 2008 ; Nilsson and Duan 2019 ). The results of the present study did not show a clear tendency that the peak blood Ch concentration after LPC supplementation occurs later than that after GPC supplementation. Future studies are warranted to investigate whether the absorption rates of GPC and LPC are similar.
The SLP-PasteLyso used for LPC ingestion consisted mainly of LPC16:0, 18:0, 18:1, 18:2, and 18:3 (Table 1 ). Therefore, the measurement of these five LPC species in the plasma was prioritized, and the sum of the concentrations was calculated as the total LPC concentration. Plasma LPC16:0, 18:2, and total LPC concentrations increased 6 h after LPC supplementation. However, the increases observed were slight, and plasma concentrations did not exceed their respective physiological ranges (Semba 2020 ; Tan et al. 2020 ). Moreover, a similar increase in plasma LPC18:2 concentration was observed after placebo supplementation, and no significant differences in the delta C max or iAUC of any LPC species were found between the LPC and placebo groups. A previous study showed that the intake of corn oil increased plasma LPC18:2 concentrations (Del Bas et al. 2016 ). Given that corn oil is similar to soy oil in being rich in linoleic acid (18:2), the fatty acid that constituted the ingested oil was possibly assimilated and reused as LPC. In contrast, the intake of GPC, which does not contain fatty acids, did not increase the plasma concentration of any LPC species. Low plasma LPC16:0 levels have been associated with insulin resistance and type 2 diabetes development (Takahashi et al. 2015 ; Zhong et al. 2022 ), while low plasma LPC18:2 concentration is a marker of type 2 diabetes, obesity, coronary artery disease, memory impairment, and a decline in gait speed (Mapstone et al. 2014 ; Gonzalez-Freire et al. 2019 ; Bellot et al. 2023 ). Considering the results of the present and previous studies, we do not consider the slight and temporal increases in LPC16:0 and 18:2 concentrations to be detrimental. However, whether supplementation with these LPC species can counteract the above-mentioned negative outcomes remains unclear. To examine this possibility, future studies should focus on the correlation between the dietary intake of LPC and blood LPC concentrations and changes in the activity of enzymes associated with LPC metabolism and the negative outcomes related to low LPC concentrations.
Supplementation with LPC or GPC elevated serum phospholipid concentrations to a similar extent. This finding is consistent with the notion that ingested LPC and GPC are absorbed into enterocytes as LPC or Ch and partially converted to PC via the cytidine diphosphate-Ch pathway and then enter the circulation (Nilsson and Duan 2019 ). Interestingly, these supplements increased the serum TG levels more than the placebo at certain time points. This may be because PC was used to produce very-low-density lipoprotein (VLDL) particles, which eventually enhanced the transportation of TG pooled in hepatic and intestinal cells into the circulation. Previous studies showed that PC is required for VLDL secretion by the small intestine as well as the liver (Tso et al. 1984 ; Yao and Vance 1988 ; Ko et al. 2020 ) and that a 2-week supplementation with PC slightly increased blood TG levels (Olthof et al. 2005 ). Moreover, the delta C max was higher in the LPC group than in the GPC group. This result indicated that the ingested LPC was partially degraded into GPC and fatty acid (Nilsson and Duan 2019 ), and the liberated fatty acid was utilized to synthesize TG, whereas the same phenomenon did not occur for the ingested GPC.
The metabolism of Ch-containing compounds can generate TMAO, and PC produces less TMAO than GPC given the same amount of Ch (Böckmann et al. 2022 ; Böckmann et al. 2023 ). Thus, we hypothesized that the same phenomenon could be observed for LPC as part of the ingested LPC follows the metabolic pathway of PC (Nilsson and Duan 2019 ), and this hypothesis is well supported by a preceding animal study (Duan et al. 2021 ). Although the plasma TMAO concentrations after supplementation were measured, no significant changes were observed. We confirmed that the coefficient values of our analysis system for intra-/inter-day variation in plasma TMAO were less than 7% ( Table S9 ). However, we found no significant increase in TMAO concentrations, possibly because of the considerable differences among individuals, relatively low supplementation doses, and limited sample size. TMAO production in the body is affected by age, intestinal microflora, and other factors; moreover, the previously reported baseline concentrations of TMAO differed across studies (Gatarek and Kaluzna-Czaplinska 2021 ). Therefore, higher doses of LPC and a larger sample size should be applied in future studies to investigate the dose dependency of LPC on blood TMAO concentrations and compare the effects of LPC and GPC supplementation on blood TMAO concentrations.
Regarding the limitation of this study, we did not impose an inclusion criterion on participant's body weight. Either setting such a criterion or adjusting the supplementation dose by body weight may have yielded less inter-personal variability in blood concentrations of the measured compounds. Another limitation was that we did not measure the levels of inflammatory markers. In addition, lysophosphatidic acid that is metabolized by autotaxin from LPC, has been associated with cancer and other inflammatory diseases (Law et al. 2019 ). Therefore, changes in inflammatory status and lysophosphatidic acid concentrations after LPC supplementation should be investigated to ensure the safety of LPC supplementation.
To the best of our knowledge, this study is the first to investigate the pharmacokinetics of LPC and compare it with that of GPC in humans. These results demonstrate that LPC can function as a Ch source, similar to GPC. Given the clinical efficacy of GPC (Traini, Bramanti and Amenta 2013 ; Traini et al. 2020 ), LPC supplementation may also enhance human cognitive function. Its usability and superiority over other Ch sources require further investigation.
The data underlying this article are available in Supplementary Tables.
R.T.-K.: conceptualization, data curation, formal analysis, investigation, methodology, validation, and writing of the original draft. H.K.: LPC measurement and validation. K.H.: project administration, writing—review and editing.
This study was conducted using resources provided by Otsuka Pharmaceutical Co., Ltd. The funder provided support in the form of salaries to the authors but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No potential conflict of interest was reported by the authors.
R.T.-K. thanks his colleagues and Dr. K.H. for their helpful discussions. The authors are grateful to Dr. O.M. and the nurses who cooperated for the study and T.H. for providing the TMAO measuring service.
Bellot P , Moia MN , Reis BZ et al. Are phosphatidylcholine and lysophosphatidylcholine body levels potentially reliable biomarkers in obesity? A review of human studies . Mol Nutr Food Res 2023 ; 67 : e2200568 .
Google Scholar
Bligh EG , Dyer WJ. A rapid method of total lipid extraction and purification . Can J Biochem Physiol 1959 ; 37 : 911 - 7 .
Böckmann KA , Franz AR , Minarski M et al. Differential metabolism of choline supplements in adult volunteers . Eur J Nutr 2022 ; 61 : 219 - 30 .
Böckmann KA , Franz AR , Shunova A et al. Different choline supplement metabolism in adults using deuterium labelling . Eur J Nutr 2023 ; 62 : 1795 - 807 .
Del Bas JM , Caimari A , Rodriguez-Naranjo MI et al. Impairment of lysophospholipid metabolism in obesity: altered plasma profile and desensitization to the modulatory properties of n-3 polyunsaturated fatty acids in a randomized controlled trial . Am J Clin Nutr 2016 ; 104 : 266 - 79 .
Duan XF , Wang CC , Xue CH et al. Trimethylamine N -oxide generation from choline-containing precursors is closely associated with their molecular structure . J Agric Food Chem 2021 ; 69 : 2933 - 5 .
Fischer LM , da Costa KA , Kwock L et al. Dietary choline requirements of women: effects of estrogen and genetic variation . Am J Clin Nutr 2010 ; 92 : 1113 - 9 .
Gatarek P , Kaluzna-Czaplinska J. Trimethylamine N-oxide (TMAO) in human health . EXCLI J 2021 ; 20 : 301 - 19 .
Gonzalez-Freire M , Moaddel R , Sun K et al. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the Baltimore Longitudinal Study of Aging . J Gerontol A Biol Sci Med Sci 2019 ; 74 : 62 - 7 .
Holm PI , Ueland PM , Kvalheim G , Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry . Clin Chem 2003 ; 49 : 286 - 94 .
Im AG , Choi GW , Kang DW et al. Population pharmacokinetic modeling and simulation of choline in healthy Korean subjects after oral administration of choline alfoscerate . J Pharm Investig 2022 ; 52 : 331 - 9 .
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline . Washington (DC) : National Academies Press (US) , 1998 , 390 - 422 .
Judy WV. The single-dose absorption and steady-state bioavailability of different coenzyme Q10 formulations . Integr Med (Encinitas, Calif) 2022 ; 21 : 28 - 34 .
Kanemitsu Y , Asaji K , Matsumoto Y et al. Simultaneous quantitative analysis of uremic toxins by LC-MS/MS with a reversed-phase/cation-exchange/anion-exchange tri-modal mixed-mode column . J Chromatogr B Analyt Technol Biomed Life Sci 2017 ; 1068-9 : 1 - 8 .
Kawamura T , Okubo T , Sato K et al. Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults . Nutrition 2012 ; 28 : 1122 - 6 .
Ko CW , Qu J , Black DD , Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease . Nat Rev Gastroenterol Hepatol 2020 ; 17 : 169 - 83 .
Law SH , Chan ML , Marathe GK et al. An updated review of lysophosphatidylcholine metabolism in human diseases . Int J Mol Sci 2019 ; 20 : 1149 .
Le Floch JP , Escuyer P , Baudin E et al. Blood glucose area under the curve: methodological aspects . Diabetes Care 1990 ; 13 : 172 - 5 .
Li Z , Vance DE. Phosphatidylcholine and choline homeostasis . J Lipid Res 2008 ; 49 : 1187 - 94 .
López-Coviella I , Agut J , Savci V et al. Evidence that 5'-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels . J Neurochem 1995 ; 65 : 889 - 94 .
Mapstone M , Cheema AK , Fiandaca MS et al. Plasma phospholipids identify antecedent memory impairment in older adults . Nat Med 2014 ; 20 : 415 - 8 .
Nilsson Å , Duan RD. Pancreatic and mucosal enzymes in choline phospholipid digestion . Am J Physiol Gastrointest Liver Physiol 2019 ; 316 : G425 - 45 .
Nilsson Å. Intestinal absorption of lecithin and lysolecithin by lymph fistula rats . Biochim Biophys Acta 1968 ; 152 : 379 - 90 .
Olthof MR , van Vliet T , Verhoef P et al. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans . PLoS Med 2005 ; 2 : e135 .
Semba RD. Perspective: the potential role of circulating lysophosphatidylcholine in neuroprotection against Alzheimer disease . Adv Nutr (Bethesda, MD) 2020 ; 11 : 760 - 72 .
Takahashi H , Goto T , Yamazaki Y et al. Metabolomics reveal 1-palmitoyl lysophosphatidylcholine production by peroxisome proliferator-activated receptor α . J Lipid Res 2015 ; 56 : 254 - 65 .
Takayama M , Itoh S , Nagasaki T , Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids . Clin Chim Acta 1977 ; 79 : 93 - 8 .
Tamaoku K , Ueno K , Akiura K , Ohkura Y. New water-soluble hydrogen donors for the enzymatic photometric determination of hydrogen peroxide. II. N-ethyl-N-(2-hydroxy-3-sulfopropyl) aniline derivatives . Chem Pharm Bull 1982 ; 30 : 2492 - 7 .
Tan ST , Ramesh T , Toh XR , Nguyen LN. Emerging roles of lysophospholipids in health and disease . Prog Lipid Res 2020 ; 80 : 101068 .
Tanaka-Kanegae R , Hamada K. A novel in vitro assay model developed to measure both extracellular and intracellular acetylcholine levels for screening cholinergic agents . PLoS One 2021 ; 16 : e0258420 .
Tanaka-Kanegae R , Kimura H , Hamada K. Oral administration of egg- and soy-derived lysophosphatidylcholine mitigated acetylcholine depletion in the brain of scopolamine-treated rats . Nutrients 2023 ; 15 : 3618 .
Tayebati SK , Amenta F. Choline-containing phospholipids: relevance to brain functional pathways . Clin Chem Lab Med 2013 ; 51 : 513 - 21 .
Thies F , Pillon C , Moliere P et al. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain . Am J Physiol 1994 ; 267 : R1273 - 9 .
Tobin LW , Kiens B , Galbo H. The effect of exercise on postprandial lipidemia in type 2 diabetic patients . Eur J Appl Physiol 2008 ; 102 : 361 - 70 .
Traini E , Bramanti V , Amenta F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline), an old choline-containing phospholipid with a still interesting profile as a cognition-enhancing agent . Curr Alzheimer Res 2013 ; 10 : 1070 - 9 .
Traini E , Carotenuto A , Fasanaro AM , Amenta F. Volume analysis of brain cognitive areas in Alzheimer's disease: interim 3-year results from the ASCOMALVA Trial . J Alzheimer's Dis 2020 ; 76 : 317 - 29 .
Tso P , Drake DS , Black DD , Sabesin SM. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine . Am J Physiol 1984 ; 247 : G599 - 610 .
U.S. Food and Drug Administration . Direct Food Substances Affirmed as Generally Recognized as Safe (GRAS), Enzyme-Modified Lecithin, Code of Federal Regulations. Title 21, Volume 3, 21CFR184.1063 . https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1063 (16 December 2023, date last accessed) .
U.S. Food and Drug Administration . GRAS Determination for Canola-derived Lecithin and Lysolecithin in Food . 2014 . https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=533&sort=GRN_No&order=DESC&startrow=1&type=basic&search=canola (16 December 2023, date last accessed) .
Wallace TC , Blusztajn JK , Caudill MA et al. Choline: the underconsumed and underappreciated essential nutrient . Nutr Today 2018 ; 53 : 240 - 53 .
Weihrauch JL , Son Y-S. Phospholipid content of foods . J Am Oil Chem Soc 1983 ; 60 : 1971 - 8 .
Yao ZM , Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes . J Biol Chem 1988 ; 263 : 2998 - 3004 .
Zhong J , Cheung CYY , Su X et al. Specific triacylglycerol, diacylglycerol, and lyso-phosphatidylcholine species for the prediction of type 2 diabetes: a ∼ 16-year prospective study in Chinese . Cardiovasc Diabetol 2022 ; 21 : 234 .
Zhu B , Ren H , Xie F et al. Trimethylamine N-oxide generated by the gut microbiota: potential atherosclerosis treatment strategies . Curr Pharm Des 2022 ; 28 : 2914 - 9 .
Supplementary data
Email alerts, citing articles via.
- Recommend to your librarian
- Journals Career Network
Affiliations
- Online ISSN 1347-6947
- Print ISSN 0916-8451
- Copyright © 2024 Japan Society for Bioscience, Biotechnology, and Agrochemistry
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
In Silico Repurposing of a Novel Inhibitor (drug) of EGFR and VEGFR-2 Kinases of Cancer by Pharmacokinetics, Toxicity, Molecular Docking, and Molecular Dynamics Simulation
- Original Article
- Published: 24 May 2024
Cite this article

- Mona H. Ibraheim 1 ,
- Ibrahim Maher ORCID: orcid.org/0000-0002-9240-3872 1 &
- Ibrahim Khater 2
Vascular endothelial growth factor is an angiogenic that promotes the development and metastasis of tumors (VEGF). The epidermal growth factor receptor, or EGFR, controls the division, growth, and death of cells via several signaling pathways. It has been found that most of the tyrosine kinase EGFR/VEGFR-2 inhibited by drugs that the FDA has approved are so far. The main objective of the present study was to identify an efficacious and selective dual inhibitor of VEGFR-2/EGFR for the treatment of cancer. Out of the 400 ligands tested against the kinases, 12 compounds demonstrated the best docking scores through molecular docking for the two kinases. Of these, only compound SCHEMBL2435814 inhibited the kinases with the highest score values when compared to a reference, vandetanib, as a dual inhibitor of EGFR/VEGFR-2 kinases through interaction with the identified active sites pocket. Following drug-likeness score toxicity and pharmacokinetic testing, the two compounds, SCHEMBL2435814 and vandetanib, were analyzed to determine how the two kinases interacted with each other. The results of calculations of π-cation interactions, hydrogen bonds, and hydrophobic interactions demonstrated a strong interaction between the two kinases and SCHEMBL2435814. Eventually, molecular dynamic modeling was used to assess the stability of complexes. This demonstrated many characteristics, including RMSF, RMSD, SASA, RG, and H-bond analysis, which demonstrated that SCHEMBL2435814 with the two kinases was more stable than vandetanib over a 100ns simulation period. By suppressing EGFR/VEGFR-2, chemical SCHEMBL2435814 may be able to postpone the signaling pathway of proteins that are essential to the advancement of cancer.
Graphical Abstract

This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data Availability
All data is available and attached.
Bray, F., Laversanne, M., Weiderpass, E., & Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer, 127 (16), 3029–3030. https://doi.org/10.1002/cncr.33587
Article PubMed Google Scholar
Al-Warhi, T., Almahli, H., Maklad, R. M., Elsayed, Z. M., El Hassab, M. A., Alotaibi, O. J., ..., El-Ashrey, M. K. (2023). 1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as novel anticancer agents: Synthesis, biological evaluation and molecular modeling insights. Molecules , 28 (7). https://doi.org/10.3390/molecules28073203
Al-Warhi, T., Elbadawi, M. M., Bonardi, A., Nocentini, A., Al-Karmalawy, A. A., Aljaeed, N., ..., Eldehna, W. M. (2022). Design and synthesis of benzothiazole-based SLC-0111 analogues as new inhibitors for the cancer-associated carbonic anhydrase isoforms IX and XII. Journal of Enzyme Inhibition and Medicinal Chemistry , 37 (1), 2635–2643. https://doi.org/10.1080/14756366.2022.2124409
Elbadawi, M. M., Eldehna, W. M., Abd El-Hafeez, A. A., Somaa, W. R., Albohy, A., Al-Rashood, S. T., ..., Abe, M. (2022). 2-Arylquinolines as novel anticancer agents with dual EGFR/FAK kinase inhibitory activity: Synthesis, biological evaluation, and molecular modelling insights. Journal of Enzyme Inhibition and Medicinal Chemistry , 37 (1), 349–372. https://doi.org/10.1080/14756366.2021.2015344
Sabt, A., Eldehna, W. M., Al-Warhi, T., Alotaibi, O. J., Elaasser, M. M., Suliman, H., & Abdel-Aziz, H. A. (2020). Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: Synthesis, biological evaluation and in silico insights. Journal of Enzyme Inhibition and Medicinal Chemistry, 35 (1), 1616–1630. https://doi.org/10.1080/14756366.2020.1806259
Article CAS PubMed PubMed Central Google Scholar
Eliwa, E. M., Frese, M., Halawa, A. H., Soltan, M. M., Ponomareva, L. V., Thorson, J. S., ..., Sewald, N. (2021). Metal-free domino amination-Knoevenagel condensation approach to access new coumarins as potent nanomolar inhibitors of VEGFR-2 and EGFR. Green Chemistry Letters and Reviews , 14 (4), 578–599. https://doi.org/10.1080/17518253.2021.1981462
Amin, K. M., Barsoum, F. F., Awadallah, F. M., & Mohamed, N. E. (2016). Identification of new potent phthalazine derivatives with VEGFR-2 and EGFR kinase inhibitory activity. European Journal of Medicinal Chemistry, 123 , 191–201. https://doi.org/10.1016/j.ejmech.2016.07.049
Article CAS PubMed Google Scholar
Boraei, A. T. A., Ashour, H. K., El Tamany, E. S. H., Abdelmoaty, N., El-Falouji, A. I., & Gomaa, M. S. (2019). Design and synthesis of new phthalazine-based derivatives as potential EGFR inhibitors for the treatment of hepatocellular carcinoma. Bioorganic Chemistry, 85 , 293–307. https://doi.org/10.1016/j.bioorg.2018.12.039
Zhang, H. Q., Gong, F. H., Li, C. G., Zhang, C., Wang, Y. J., Xu, Y. G., & Sun, L. P. (2016). Design and discovery of 4-anilinoquinazoline-acylamino derivatives as EGFR and VEGFR-2 dual TK inhibitors. European Journal of Medicinal Chemistry, 109 , 371–379. https://doi.org/10.1016/j.ejmech.2015.12.032
Dank, M., Zaluski, J., Barone, C., Valvere, V., Yalcin, S., Peschel, C., ..., Bugat, R. (2008). Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Annals of Oncology , 19 (8), 1450–1457. https://doi.org/10.1093/annonc/mdn166
Falchook, G. S., & Kurzrock, R. (2015). VEGF and dual-EGFR inhibition in colorectal cancer. Cell Cycle, 14 (8), 1129–1130. https://doi.org/10.1080/15384101.2015.1022071
Gaber, A. A., Bayoumi, A. H., El-Morsy, A. M., Sherbiny, F. F., Mehany, A. B. M., & Eissa, I. H. (2018). Design, synthesis and anticancer evaluation of 1H-pyrazolo[3,4-d]pyrimidine derivatives as potent EGFR(WT) and EGFR(T790M) inhibitors and apoptosis inducers. Bioorganic Chemistry, 80 , 375–395. https://doi.org/10.1016/j.bioorg.2018.06.017
Sigismund, S., Avanzato, D., & Lanzetti, L. (2018). Emerging functions of the EGFR in cancer. Molecular Oncology, 12 (1), 3–20. https://doi.org/10.1002/1878-0261.12155
Tabernero, J. (2007). The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Molecular Cancer Research, 5 (3), 203–220. https://doi.org/10.1158/1541-7786.mcr-06-0404
Folkman, J., & D’Amore, P. A. (1996). Blood vessel formation: What is its molecular basis? Cell, 87 (7), 1153–1155. https://doi.org/10.1016/s0092-8674(00)81810-3
Olsson, A.-K., Dimberg, A., Kreuger, J., & Claesson-Welsh, L. (2006). VEGF receptor signalling ? in control of vascular function. Nature Reviews Molecular Cell Biology, 7 (5), 359–371. https://doi.org/10.1038/nrm1911
Ezelarab, H. A. A., Ali, T. F. S., Abbas, S. H., Sayed, A. M., Beshr, E. A. M., & Hassan, H. A. (2023). New antiproliferative 3-substituted oxindoles inhibiting EGFR/VEGFR-2 and tubulin polymerization. Molecular Diversity . https://doi.org/10.1007/s11030-023-10603-z
Article PubMed PubMed Central Google Scholar
Shibuya, M. (2011). Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes & Cancer, 2 (12), 1097–1105. https://doi.org/10.1177/1947601911423031
Article CAS Google Scholar
Ghosh, S., Sullivan, C. A. W., Zerkowski, M. P., Molinaro, A. M., Rimm, D. L., Camp, R. L., & Chung, G. G. (2008). High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Human Pathology, 39 (12), 1835–1843. https://doi.org/10.1016/j.humpath.2008.06.004
Modi, S. J., & Kulkarni, V. M. (2019). Vascular endothelial growth factor receptor (VEGFR-2)/KDR inhibitors: Medicinal chemistry perspective. Medicine in Drug Discovery, 2 , 100009. https://doi.org/10.1016/j.medidd.2019.100009
Article Google Scholar
Dhuguru, J., & Skouta, R. (2020). Role of indole scaffolds as pharmacophores in the development of anti-lung cancer agents. Molecules , 25 (7). Retrieved from https://doi.org/10.3390/molecules25071615
Wan, Y., Li, Y., Yan, C., Yan, M., & Tang, Z. (2019). Indole: A privileged scaffold for the design of anti-cancer agents. European Journal of Medicinal Chemistry, 183 , 111691. https://doi.org/10.1016/j.ejmech.2019.111691
Holmes, K., Roberts, O. L., Thomas, A. M., & Cross, M. J. (2007). Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cellular Signalling, 19 (10), 2003–2012.
Alanazi, M. M., Mahdy, H. A., Alsaif, N. A., Obaidullah, A. J., Alkahtani, H. M., Al-Mehizia, A. A., ..., Eissa, I. H. (2021). New bis ([1, 2, 4] triazolo)[4, 3-a: 3′, 4′-c] quinoxaline derivatives as VEGFR-2 inhibitors and apoptosis inducers: Design, synthesis, in silico studies, and anticancer evaluation. Bioorganic Chemistry , 112 , 104949.
Eissa, I. H., Ibrahim, M. K., Metwaly, A. M., Belal, A., Mehany, A. B., Abdelhady, A. A., ..., Mahdy, H. A. (2021). Design, molecular docking, in vitro, and in vivo studies of new quinazolin-4 (3H)-ones as VEGFR-2 inhibitors with potential activity against hepatocellular carcinoma. Bioorganic Chemistry , 107 , 104532.
El-Metwally, S. A., Abou-El-Regal, M. M., Eissa, I. H., Mehany, A. B., Mahdy, H. A., Elkady, H., ..., Elkaeed, E. B. (2021). Discovery of thieno [2, 3-d] pyrimidine-based derivatives as potent VEGFR-2 kinase inhibitors and anti-cancer agents. Bioorganic Chemistry , 112 , 104947.
Hernandez-Davies, J. E., Zape, J. P., Landaw, E. M., Tan, X., Presnell, A., Griffith, D., ..., Sakamoto, K. M. (2011). The multitargeted receptor tyrosine kinase inhibitor linifanib (ABT-869) induces apoptosis through an Akt and glycogen synthase kinase 3β-dependent pathway. Molecular Cancer Therapeutics, 10 (6), 949–959. https://doi.org/10.1158/1535-7163.mct-10-0904
Lang, S. A., Schachtschneider, P., Moser, C., Mori, A., Hackl, C., Gaumann, A., ..., Stoeltzing, O. (2008). Dual targeting of Raf and VEGF receptor 2 reduces growth and metastasis of pancreatic cancer through direct effects on tumor cells, endothelial cells, and pericytes. Molecular Cancer Therapeutics , 7 (11), 3509–3518. https://doi.org/10.1158/1535-7163.mct-08-0373
Xi, L., Zhang, J.-Q., Liu, Z.-C., Zhang, J.-H., Yan, J.-F., Jin, Y., & Lin, J. (2013). Novel 5-anilinoquinazoline-8-nitro derivatives as inhibitors of VEGFR-2 tyrosine kinase: Synthesis, biological evaluation and molecular docking. Organic & Biomolecular Chemistry, 11 (26), 4367–4378.
Ani, F. E., Ibeji, C. U., Obasi, N. L., Kelani, M. T., Ukogu, K., Tolufashe, G. F., ..., Kruger, H. G. (2021). Crystal, spectroscopic and quantum mechanics studies of Schiff bases derived from 4-nitrocinnamaldehyde. Scientific Reports , 11 (1), 8151.
Oyeneyin, O., Ipinloju, N., Nathanael, O., & Akerele, D. (2021). Structural modification of ibuprofen as new NSAIDs via DFT, molecular docking and pharmacokinetics studies. International Journal of Advances in Engineering and Pure Sciences, 33 (4), 614–626.
Oyeneyin, O. E., Adejoro, I. A., Obadawo, B. S., Amoko, J. S., Kayode, I. O., Akintemi, E. O., & Ipinloju, N. (2021). Investigation into the molecular properties of 3-(4-hydroxyphenyl) Prop-2-en-1-one 4-phenyl Schiff base and some of its derivatives-DFT and molecular docking studies. Science Letters, 9 (1), 4–11.
Oyeneyin, O. E., Obadawo, B. S., Orimoloye, S. M., Akintemi, E. O., Ipinloju, N., Asere, A. M., & Owolabi, T. O. (2021). Prediction of inhibition activity of BET bromodomain inhibitors using grid search-based extreme learning machine and molecular docking. Letters in Drug Design & Discovery, 18 (11), 1039–1049.
Kikiowo, B., Ogunleye, A. J., Inyang, O. K., Adelakun, N. S., Omotuyi, O. I., Metibemu, D. S., ..., Ijatuyi, T. T. (2020). Flavones scaffold of Chromolaena odorata as a potential xanthine oxidase inhibitor: Induced Fit Docking and ADME studies. BioImpacts: BI , 10 (4), 227.
Lionta, E., Spyrou, G., Vassilatis, D. K., & Cournia, Z. (2014). Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Current Topics in Medicinal Chemistry, 14 (16), 1923–1938. https://doi.org/10.2174/1568026614666140929124445
Murali, M., Gowtham, H. G., Ansari, M. A., Alomary, M. N., Alghamdi, S., Almehmadi, M., ..., Amruthesh, K. N. (2022). Repositioning therapeutics for SARS-CoV-2: Virtual screening of plant-based anti-HIV compounds as possible inhibitors against COVID-19 viral RdRp. : Current Pharmaceutical Design , 28 (12), 969–980. https://doi.org/10.2174/1381612828666220428120939
Anandan, S., Gowtham, H. G., Shivakumara, C. S., Thampy, A., Singh, S. B., Murali, M., ..., Glossman-Mitnik, D. (2022). Integrated approach for studying bioactive compounds from Cladosporium spp. against estrogen receptor alpha as breast cancer drug target. Scientific Reports , 12 (1), 22446. https://doi.org/10.1038/s41598-022-22038-x
Murali, M., Ahmed, F., Gowtham, H. G., Aribisala, J. O., Abdulsalam, R. A., Shati, A. A., ..., Amruthesh, K. N. (2023). Exploration of CviR-mediated quorum sensing inhibitors from Cladosporium spp. against Chromobacterium violaceum through computational studies. Scientific Reports , 13 (1), 15505. https://doi.org/10.1038/s41598-023-42833-4
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., ..., Wilson, M. (2017). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research , 46 (D1), D1074-D1082. https://doi.org/10.1093/nar/gkx1037
Kundu, D., & Dubey, V. K. (2021). Potential alternatives to current cholinesterase inhibitors: An in silico drug repurposing approach. Drug Development and Industrial Pharmacy, 47 (6), 919–930. https://doi.org/10.1080/03639045.2021.1952216
Schrodinger, L. (2021). he fPyMOLg molecular graphics system, version 2.5.
Larsen, A. K., Ouaret, D., El Ouadrani, K., & Petitprez, A. (2011). Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacology & Therapeutics, 131 (1), 80–90. https://doi.org/10.1016/j.pharmthera.2011.03.012
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31 (2), 455–461. https://doi.org/10.1002/jcc.21334
Daina, A., Michielin, O., & Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7 (1), 42717. https://doi.org/10.1038/srep42717
Salentin, S., Schreiber, S., Haupt, V. J., Adasme, M. F., & Schroeder, M. (2015). PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Research, 43 (W1), W443-447. https://doi.org/10.1093/nar/gkv315
Adasme-Carreño, F., Muñoz-Gutierrez, C., Caballero, J., & Alzate-Morales, J. H. (2014). Performance of the MM/GBSA scoring using a binding site hydrogen bond network-based frame selection: The protein kinase case. Physical Chemistry Chemical Physics, 16 (27), 14047–14058. https://doi.org/10.1039/C4CP01378F
Pronk, S., Páll, S., Schulz, R., Larsson, P., Bjelkmar, P., Apostolov, R., ..., Lindahl, E. (2013). GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics, 29 (7), 845–854. https://doi.org/10.1093/bioinformatics/btt055
Ipinloju, N., Ibrahim, A., da Costa, R. A., Adigun, T. B., Olubode, S. O., Abayomi, K. J., ..., Oyeneyin, O. E. (2023). Quantum evaluation and therapeutic activity of (E)-N-(4-methoxyphenyl)-2-(4-(3-oxo-3-phenylprop-1-en-1-yl) phenoxy) acetamide and its modified derivatives against EGFR and VEGFR-2 in the treatment of triple-negative cancer via in silico approach. Journal of Molecular Modeling , 29 (5), 159.
Usui, T., Ban, H. S., Kawada, J., Hirokawa, T., & Nakamura, H. (2008). Discovery of indenopyrazoles as EGFR and VEGFR-2 tyrosine kinase inhibitors by in silico high-throughput screening. Bioorganic & Medicinal Chemistry Letters , 18 (1), 285–288. https://doi.org/10.1016/j.bmcl.2007.10.084
Gupta, M., Lee, H. J., Barden, C. J., & Weaver, D. F. (2019). The blood-brain barrier (BBB) score. Journal of Medicinal Chemistry, 62 (21), 9824–9836. https://doi.org/10.1021/acs.jmedchem.9b01220
Lipinski, C. A., Lombardo, F., Dominy, B. W., & Feeney, P. J. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews, 46 (1–3), 3–26. https://doi.org/10.1016/s0169-409x(00)00129-0
Bilal, M. S., Ejaz, S. A., Zargar, S., Akhtar, N., Wani, T. A., Riaz, N., ..., Alkahtani, H. M. (2022). Computational investigation of 1, 3, 4 oxadiazole derivatives as lead inhibitors of VEGFR 2 in comparison with EGFR: Density functional theory, molecular docking and molecular dynamics simulation studies. Biomolecules , 12 (11), 1612.
Rahman, M. O., & Ahmed, S. S. (2022). Anti-angiogenic potential of bioactive phytochemicals from Helicteres isora targeting VEGFR-2 to fight cancer through molecular docking and molecular dynamics simulation. Journal of Biomolecular Structure and Dynamics , 1–16. https://doi.org/10.1080/07391102.2022.2122568
BorjianBoroujeni, M., ShahbaziDastjerdeh, M., Shokrgozar, M., Rahimi, H., & Omidinia, E. (2021). Computational driven molecular dynamics simulation of keratinocyte growth factor behavior at different pH conditions. Informatics in Medicine Unlocked, 23 , 100514. https://doi.org/10.1016/j.imu.2021.100514
Rajagopal, S., & Vishveshwara, S. (2005). Short hydrogen bonds in proteins. FEBS Journal, 272 (8), 1819–1832. https://doi.org/10.1111/j.1742-4658.2005.04604.x
Download references
Author information
Authors and affiliations.
Physics Department, Faculty of Science, Zagazig University, P.O.44519, Zagazig, Egypt
Mona H. Ibraheim & Ibrahim Maher
Biophysics Department, Faculty of Science, Cairo University, Giza, Egypt
Ibrahim Khater
You can also search for this author in PubMed Google Scholar
Contributions
M.H.I. designed research; performed research; contributed analytic tools; analyzed data; and reviewed the paper. I.M. helped in designing the research, carried the experiments, analyzed data, and wrote the paper. I.K. helped in designing the research, supervised the experiments, analyzed data, and reviewed the paper.
Corresponding author
Correspondence to Ibrahim Maher .
Ethics declarations
Ethics approval.
Not available.
Informed Consent
Consent to publish, human ethics and consent to participate statement.
Our manuscript was not applied on human beings and thus requires no ethical approval.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ibraheim, M.H., Maher, I. & Khater, I. In Silico Repurposing of a Novel Inhibitor (drug) of EGFR and VEGFR-2 Kinases of Cancer by Pharmacokinetics, Toxicity, Molecular Docking, and Molecular Dynamics Simulation. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04958-8
Download citation
Accepted : 16 April 2024
Published : 24 May 2024
DOI : https://doi.org/10.1007/s12010-024-04958-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dual inhibitors
- Molecular dynamic simulation
- Molecular docking
- Find a journal
- Publish with us
- Track your research
- Animal Biotechnology & Biomedical Sciences Graduate Program

The Animal Biotechnology & Biomedical Sciences (ABBS) program provides rigorous and comprehensive training at the graduate level through the Department of Veterinary & Animal Sciences. The general requirements for the MS and PhD degrees in Animal Biotechnology & Biomedical Sciences are those of the Graduate School .
The ABBS graduate program offers research opportunities that foster creative excellence, technical mastery, intellectual independence, and expertise in the field of molecular and cellular biology. Our faculty members' research encompass areas of immunology, infectious disease, developmental biology, reproductive biology and toxicology. Research topics focus on issues directly relevant to humans and agriculturally important animals. The strong commitment of our faculty members to quality graduate education is evidenced by the continued placement of ABBS graduates in excellent career and post-doctoral positions.
The ABBS graduate program is housed primarily in the Integrated Sciences Building, a newly built, state-of-the-art research facility that houses laboratories equipped for tissue culture, micro-manipulation of cells and embryos, protein purification, genetic engineering, molecular biology, fluorescence microscopy and flow cytometry.

ABBS Graduate Program Director
Kathleen Arcaro, PhD 240 Thatcher Road LSL 1 Room N529 Amherst, MA 01003-9298 413-577-1823 @email
Veterinary and Animal Sciences Department Head
Lisa M. Minter, PhD, 427A Integrated Sciences Building 413-545-6327 @email
Degrees offered
The ABBS graduate program offers the following degrees:
- Master of Science (MS)
- Doctor of Philosophy (PhD)
Program goals
- To prepare scientists for future careers in academia or industry in the areas of animal biotechnology and/or biomedicine.
- To provide research opportunities in areas of immunology, infectious disease, developmental biology, reproductive biology, and toxicology.
- To instill in students an understanding of hypothesis-driven experimental design and the desire to deepen scientific knowledge.
The core of the graduate program is the deep commitment of the active research faculty to provide a quality graduate education. The collaborative environment of our research groups and physically centralized laboratories strengthens the learning environment for graduate students. The involvement of faculty within interdisciplinary programs brings a wide variety of students to our laboratories and seminars and promotes interactions across campus. The faculty attract post-doctoral fellows from around the world and their presence invigorates the research enterprise, thus students do not work in isolation but are part of both the departmental program as well as interdepartmental research communities including the Molecular and Cellular Biology Program , Neuroscience and Behavior Program , and Organismic and Evolutionary Biology Program .
Research in the ABBS graduate program is supported by a variety of sources. Faculty hold grants from the United States Department of Agriculture , the National Institutes of Health , the National Science Foundation , the United States Agency for International Development , as well as from private industry, foundations, and the Massachusetts Agricultural Experiment Station . Our graduates are continually placed in excellent career and post-doctoral positions, nationally and internationally.
Program requirements
See the ABBS Graduate Student Handbook for details on program requirements, courses, rules, and milestones.
The main emphasis of the graduate program is hands-on laboratory research. Students supplement lab work with a variety of courses as well as research seminars and journal clubs. Required courses provide a strong foundation in biochemistry, molecular and cellular biology, immunology and infectious diseases, genetics, and developmental biology. Doctoral students must pass written and oral comprehensive qualifying exams and both Ph.D. and M.S. students must pass a final oral defense of their thesis research. All graduate students form a thesis committee of faculty members who meet regularly with the student to provide guidance and assess progress.
The Fifth Year Master's Degree program is designed for students who are undergraduate students in the department and who begin research in a laboratory during their junior year. During their senior year, they develop their research skills and take six credits of coursework toward their master's degree. To complete the fifth-year master's degree requires 12-15 months after the BS degree and includes a combination of research work for independent study credit plus six – eight additional coursework credits.
Application and admission
Please view the list of research faculty who may be accepting new students into their labs.
Our program accepts new students for the Fall semester; (rotating PhD students are only accepted for the fall semester) we generally accept 1 – 4 students per year. The application deadline is January 15. Generally, master's with a thesis and PhD students are accepted by individual faculty members directly into their laboratories. Each year one or two candidates are admitted as “rotation students” but are still encouraged to contact individual faculty members directly about their research programs and the availability of space and financial support.
Applicants must include a completed online application form and two letters of recommendation. The Graduate Record Examination (GRE) is optional. International students who are not from an English-speaking country (i.e., one whose national language is English) must also take the Test of English as a Foreign Language (TOEFL) and score at least 550 (paper-based test) or 80 (computer-based test). This includes students from non-English speaking countries even though their degrees may have been awarded in English.
Applications can be obtained from the address below. For general information, visit the UMass Graduate School website.
Graduate admissions Phone: 413-545-0722 (8:30 a.m. – 5 p.m. Mon-Fri EST) Email: @email
Check on the status of your application.
The ABBS program has a number of application fee waivers it can grant prospective PhD students interested in studying molecular and cellular biology. These waivers include international applicants, who have faced adversity, such as societal, economic, or academic disadvantages. We also will consider application fee waiver requests from applicants who are first-generation students and students who have been traditionally underrepresented in graduate education in molecular and cellular biology. Applicants should contact Kathleen Arcaro to inquire about an application fee waiver. For further inquiries about the program please contact:
Graduate Program Director Kathleen Arcaro, PhD 240 Thatcher Road LSL 1 Room N529 Amherst, MA 01003-9298 413-577-1823 @email
Financial Aid
Students (U.S. citizens and foreign) are eligible for research or teaching assistantships. Except for non-thesis master's students, graduate students accepted into the program are provided with financial support from individual faculty members (that is, the research supervisor). Research assistants and teaching assistants receive full-time re-numeration in addition to having tuition and medical insurance paid for. Students admitted to the program are responsible for finding their own transportation to and from Amherst, Massachusetts. International students must remain registered as full-time students during their stay to maintain visa status. In addition to the research and teaching assistantships, students may apply for competitive pre-doctoral fellowships at:
- UMass Graduate School
- National Science Foundation Pre-doctoral Fellowship (GRFP)
- National Institutes of Health NRSA Pre-doctoral Training Fellowship (F31, selected NIH components)
- Grant Search for Graduate Students
Interdepartmental graduate programs
Students admitted through other graduate programs on campus may join the research groups in our department if the graduate advisor (PI) is a member of that program. Interdepartmental programs that our students may be interested in include:
- Neuroscience and Behavior Program
- Molecular and Cellular Biology Program
- Organismic and Evolutionary Biology Program
- Typical Course of Study
- Graduate Research Faculty
- Current Graduate Students
- Graduate Handbook
- Graduate Forms
- Awards and Fellowships
431A Integrated Sciences Building 661 North Pleasant Street Amherst, MA 01003-9301
Phone: 413-545-2312 Fax: 413-545-6326 Email: [email protected]

IMAGES
VIDEO
COMMENTS
Applied Biochemistry and Biotechnology is a journal dedicated to publishing innovative papers in biochemistry and biotechnology. Includes reports of technological subjects in the proof-of-concept stage. Provides a forum for case studies, reviews, news items, and more. Authors have the choice to publish using either the traditional publishing ...
Published by Oxford University Press from 2021. Bioscience, Biotechnology, and Biochemistry publishes high-quality papers providing chemical and biological analyses of vital phenomena exhibited by animals, plants, and microorganisms, the chemical structures and functions of their products, and related matters.
Bioscience, Biotechnology, and Biochemistry authors can access the number of online views of their article, the number of citations received, and the paper's Altmetric score. These metrics can be accessed by clicking on the 'View Metrics' link on each article web page. Find out more.
Biochemistry articles from across Nature Portfolio. Biochemistry is the study of the structure and function of biological molecules such as proteins, nucleic acids, carbohydrates and lipids ...
Biotechnology and Applied Biochemistry publishes original full-length articles, short communications, and reviews of biotechnology. The journal is dedicated to rapid publication of high quality, cutting-edge research at the interface between life sciences and their technological exploitation. The Editors will consider papers for publication based on their novelty, their immediate or future ...
Bioscience, Biotechnology, and Biochemistry, Volume 88, Issue 6, June 2024, Pages 585-593, ... Regular Paper. ... It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Biochemistry is a Transformative Journal. Biochemistry has been certified as a transformative journal by cOAlition S, committing to a transition to 100% open access in the future. If your research funder has signed Plan S, your open access charges may be covered by your funder through December 31, 2024. Please visit the ACS Open Science website ...
In the next 25 years, the watchwords must be "change" and "inclusiveness.". Biological technology has the potential to alter many aspects of human life, and it will transform them in ways ...
Explore our most highly accessed biochemistry and molecular biology articles in the first quarter of 2018. Featuring authors from around the World, these papers highlight valuable research from an ...
Starting in the mid-1980s, biotechnology became a very popular word in the title of research publications, appearing in papers concerning business, industry, biomedicine, chemical engineering, agricultural sciences, and even social sciences (Kennedy, 1991). In short, biotechnology signifies a new biological approach to a wide range of industries.
Department of Biotechnology, Central University of Haryana, Mahendragarh, Haryana, India. Dr. Santosh K. Gupta. DBT-National Institue of Plant Genome Research. Aruna Asif Ali Marg, New Delhi, India. Dr Gunvant B. Patil. Assistant Professor, Institute of Genomics for Crop Abiotic Stress Tolerance,
Biotechnology and Applied Biochemistry publishes original full-length articles, short communications, and reviews of biotechnology. The journal is dedicated to rapid publication of high quality, cutting-edge research at the interface between life sciences and their technological exploitation. The Editors will consider papers for publication based on their novelty, their immediate or future ...
Explore a collection of the most read and most cited articles making an impact in Bioscience, Biotechnology, and Biochemistry published within the past two years.This collection will be continuously updated with the journal's leading articles so be sure to revisit periodically to see what is being read and cited.
Biochemical and Biophysical Research Communications (BBRC) is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research.For short communication papers, we offer the fastest submission to acceptance of any journal within life sciences, with "accept" decisions rendered within three weeks of ...
Process Biochemistry is an application-orientated research journal devoted to reporting advances with originality and novelty, in the science and technology of the processes involving bioactive molecules and living organisms.These processes concern the production of useful metabolites or materials, or the removal of toxic compounds using tools and methods of current biology and engineering.
Accordingly, Critical Reviews in Biochemistry and Molecular Biology publishes high-quality reviews that organize, evaluate, and present the current status of high-impact, current issues in the area of biochemistry and molecular biology. Topics are selected on the advice of an advisory board of outstanding scientists, who also suggest authors of ...
Preparative Biochemistry & Biotechnology is an international forum for rapid dissemination of high quality research results dealing with all aspects of preparative techniques in biochemistry, biotechnology and other life science disciplines. Scope includes: Techniques pertinent to clarification, isolation, enrichment, and purification of ...
Biotechnology and Applied Biochemistry; Biotechnology and Bioengineering; Biotechnology Journal; Biotechnology Progress; Cell Biochemistry & Function; Drug Development Research; Drug Testing and Analysis; Electroanalysis; Electrophoresis; Engineering in Life Sciences; Journal of Applied Toxicology ... Search for more papers by this author ...
Overview. Applied Biochemistry and Microbiology is a peer-reviewed journal focusing on practical applications of biochemistry and microbiology research. Covers a wide range of topics, with a particular focus on enzymology, metabolism of microorganisms, phytoimmunity, biosensors, biomedical research, bioactive compounds and many more.
Over 8500 institutions contributed to these biotechnology publications, with the top 5 most productive ones scattered over France, China, the United States of America, Spain, and Brazil. Over 140 countries/regions contributed to the biotechnology research literature, led by the United States of America, China, Germany, Brazil, and India.
Microalgae synthesize a wide range of structural, storage, and bioactive lipids. •. Lipids are produced via plastid-encoded and/or nuclear-encoded synthetic pathways. •. Algal lipids have many uses in fuels, food, feedstocks, and therapeutics. •. Genetic tools have the potential to enhance the synthesis of target lipids.
Biochemistry and Biotechnology Research publishes articles on innovative work from all areas related to Biotechnology and Biochemistry. ... The style of main sections need not conform to that of full-length papers. Short communications are 2 to 4 printed pages (about 6 to 12 manuscript pages) in length.
Biotechnology and Applied Biochemistry publishes original full-length articles, short communications, and reviews of biotechnology. The journal is dedicated to rapid publication of high quality, cutting-edge research at the interface between life sciences and their technological exploitation. The Editors will consider papers for publication based on their novelty, their immediate or future ...
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the ...
Biotechnology and Applied Biochemistry publishes original full-length articles, short communications, and reviews of biotechnology. The journal is dedicated to rapid publication of high quality, cutting-edge research at the interface between life sciences and their technological exploitation. The Editors will consider papers for publication based on their novelty, their immediate or future ...
Call for Papers Purchase Alerts About About Bioscience, Biotechnology, and Biochemistry About Japan Society for Bioscience, Biotechnology, and Agrochemistry Editorial Board Advertising and Corporate Services
Applied Biochemistry and Biotechnology - Vascular endothelial growth factor is an angiogenic that promotes the development and metastasis of tumors (VEGF). ... M.H.I. designed research; performed research; contributed analytic tools; analyzed data; and reviewed the paper. I.M. helped in designing the research, carried the experiments, analyzed ...
The main emphasis of the graduate program is hands-on laboratory research. Students supplement lab work with a variety of courses as well as research seminars and journal clubs. Required courses provide a strong foundation in biochemistry, molecular and cellular biology, immunology and infectious diseases, genetics, and developmental biology.