Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 March 2024

Identifying childhood malaria hotspots and risk factors in a Nigerian city using geostatistical modelling approach
- Taye Bayode 1 , 2 &
- Alexander Siegmund 1 , 2
Scientific Reports volume 14 , Article number: 5445 ( 2024 ) Cite this article
507 Accesses
1 Altmetric
Metrics details
- Preventive medicine
- Risk factors
- Socioeconomic scenarios
Malaria ranks high among prevalent and ravaging infectious diseases in sub-Saharan Africa (SSA). The negative impacts, disease burden, and risk are higher among children and pregnant women as part of the most vulnerable groups to malaria in Nigeria. However, the burden of malaria is not even in space and time. This study explores the spatial variability of malaria prevalence among children under five years (U5) in medium-sized rapidly growing city of Akure, Nigeria using model-based geostatistical modeling (MBG) technique to predict U5 malaria burden at a 100 × 100 m grid, while the parameter estimation was done using Monte Carlo maximum likelihood method. The non-spatial logistic regression model shows that U5 malaria prevalence is significantly influenced by the usage of insecticide-treated nets—ITNs, window protection, and water source. Furthermore, the MBG model shows predicted U5 malaria prevalence in Akure is greater than 35% at certain locations while we were able to ascertain places with U5 prevalence > 10% (i.e. hotspots) using exceedance probability modelling which is a vital tool for policy development. The map provides place-based evidence on the spatial variation of U5 malaria in Akure, and direction on where intensified interventions are crucial for the reduction of U5 malaria burden and improvement of urban health in Akure, Nigeria.
Similar content being viewed by others
Spatio-temporal analysis of association between incidence of malaria and environmental predictors of malaria transmission in Nigeria
Oluyemi A. Okunlola & Oyetunde T. Oyeyemi
Geo-epidemiology of malaria incidence in the Vhembe District to guide targeted elimination strategies, South-Africa, 2015–2018: a local resurgence
Sokhna Dieng, Temitope Christina Adebayo-Ojo, … Jean Gaudart
Spatial variation and risk factors of malaria and anaemia among children aged 0 to 59 months: a cross-sectional study of 2010 and 2015 datasets
Jecinta U. Ibeji, Henry Mwambi & Abdul-Karim Iddrisu
Introduction
Infectious disease like malaria has been a public health burden for generations. Though there have been tremendous advances in its management and treatment, but the public health challenge still lingers. According to the recent World Malaria Report 1 progress towards fighting malaria is being stalled as there was an increase in malaria cases for the second consecutive year. However, some improvement of 1% fewer malaria-related deaths were recorded in 2021. In 2018, Sub-Saharan Africa (SSA) accounted for 94% of global malaria deaths. Furthermore, children under the age of five (U5) accounted for 70% of malaria-related mortality in the SSA region 2 , 3 . An increase to 96% of malaria-related death is recorded in WHO African Region in 2021, and the top 16 malaria-affected countries are all situated in SSA. While pregnant women are at heightened malaria exposure risk, about 80% of malaria deaths were from U5 in WHO African Region 1 .
From these worrisome malaria burden statistics, Nigeria takes a large share of the global numbers. In 2021, Nigeria accounted for about 26.6% of malaria cases and 31.3% of malaria-related deaths globally 1 . Descriptively, this amounts to over 50 million and 100,000 of malaria cases and deaths respectively in Nigeria. As averred by 4 , 5 , about 60% of outpatient hospital visits can be attributed to malaria in Nigeria. In Nigeria, U5 children are the most vulnerable group—they experience about an average of 2–4 bouts per year, and account for about 90% of national mortality from malaria 6 . Furthermore, Nigeria accounted for 38.4% of global malaria deaths in children under five 1 . In case of severe type of malaria, comorbidity such as anaemia, respiratory distress and prostration can be experience by the child 6 .
Coupled with the recent slow progress in malaria reduction in SSA, the recent global pandemic—coronavirus disease (COVID-19)—has further contributed to the interruption of malaria control undertakings in malaria endemic regions of the world. Park et al. 7 reported the high levels of surfeit malaria morbidity and mortality in Low and Middle Income Countries (LMICs), which could be attributed to poor community engagement and limited malaria tests. For example, the work of Ilesanmi, Afolabi and Iyiola 8 identifies limited acquisition of malaria tests to healthcare providers as a barrier against visiting health facilities. This could have been because of less funding going towards malaria control because of COVID-19 8 . Thus, the pandemic worsened the healthcare problems such as already weak health systems, ineffective and inefficient health management, and inequitable distribution of human resources between urban and rural areas in Nigeria identified by 9 .
This study sets out to estimate the burden of U5 malaria and variability in the rapidly urbanising medium-sized city of Akure. Overcrowding, environmental degradation, and likely substantial increase in malaria transmission are challenges of rapidly urbanising areas or places in Nigeria such as Akure 10 , 11 . In Nigeria, small or fine scale (e.g. cities) level variations in the burden of malaria and malaria risk factors are not yet sufficiently understood. National or regional-level surveys may not capture intra-urban specific characteristics and risks of malaria burden 10 . Furthermore, national or regional surveys may miss out on adequate sample sizes or tilt to those who use public health facilities and largely exclude socioeconomic data, behaviours, and a well-defined catchment population 12 . Some studies have explored the risk factors of malaria in Nigeria, however, mostly with the use of descriptive and regression statistical techniques to assess a combination of data from blood testing and questionnaires 13 , 14 . A few studies have attempted spatial risk analyses of malaria in Nigeria. For example, using Kriging to develop predictive risk factor maps, 15 assessed the spatial distribution and socio-demographic risk factors of U5 malaria in Nigeria. A close attempt at spatial statistical modelling of malaria incidence and hotspots was made by 16 . These authors used Moran’s diagram, index of local Moran’s I, and spatial regression models to conduct a spatiotemporal analysis of the association between malaria incidence and environmental predictors in Nigeria. In particular 17 , applied Bayesian geostatistical technique to model malaria risk in Nigeria using malaria indicator survey (MIS) data and environmental/climatic data. These studies have all been done at national level which could mask small/local scale spatial variation. Hence, there is sparse use of spatial predictive modelling and the development of probability models with certainty levels to guide the deployment of limited public health resources at sub-national scales in Nigeria which is one of the vital applications of Model-Based Geostatistics (MBG). MBG is a known risk mapping approach which provides robust information on the spatial distribution of infections and facilitates the design and implementation of intervention or control programmes 18 . In addition, MBG modelling method have the capacity to deliver expected precision result for improved decision-making 19 . According to literatures, Model-Based Geostatistics (MBG) is considered as well-established statistical tool for modelling spatial correlation generated by unmeasured risk factors to predict disease prevalence in location of interest or investigation 20 , 21 . MBG is a principled likelihood-based approach with effective applicability in low resource settings and places characterised with incomplete or non-existent disease registries. With MBG, it is possible to provide probability metrics or quantification for pragmatic policy relevant thresholds. Furthermore, MBG allows for quantification of uncertainty and intrinsic variability in small area predictions 22 . Hence, our assessment is sacrosanct and provides city-level information that contributes to understanding specific characteristics of the area (place) and the people (residents) of such places. Till date and to the authors knowledge, no known works have used MBG explicitly to model the fine scale spatial variation of malaria risk and estimation particularly in Nigeria. Our study aims to fill this gap with the aim of identifying U5 malaria prevalence hotspots while considering the social determinants of malaria which are often not available because of incomplete or lack of health registry especially when local scale is concerned. Our study is significant in supporting public health planning by unveiling areas of high malaria prevalence and associated risk factors. This will lead to allocating already scarce resources necessary to reduce malaria's burden in malaria hotspots.
We recognise that spatial dimensions are crucial when managing infectious diseases. Also, as countries are experiencing a reduction in malaria burden, spatial targeting of the disease control efforts towards malaria risk factors and high-risk locations, which our study supports, is pertinent. Identifying hotspots based on the level of certainty and uncertainty, which the MBG affords us, increases our findings’ usefulness for further research, health policy formulation, decision-making, resource planning, allocation, and implementation. Specific gains include the distribution of limited health resources in particular places where they are most required. We expect that our study will create the needed awareness of using MBG in disease modelling for resource-scarce regions to identify disease hotspots and probability levels for increased attention.
Study setting
The study area, Akure is a medium-sized rapidly urbanising city of Ondo State, which is one of the south-western states of Nigeria as shown in Fig. 1 . The fusion of two Local Government Areas (LGA)—Akure North and Akure South—makes up Akure. Since the city became the capital of Ondo State in 1976, several other factors such as being the seat of government, home to the Federal-government owned tertiary institutions such as University of Technology and a College of Agriculture, well-connected transportation routes with proximity to Idanre Hills (a famous tourist centre), have collectively attributed to making Akure the most populated and developed city in Ondo State.
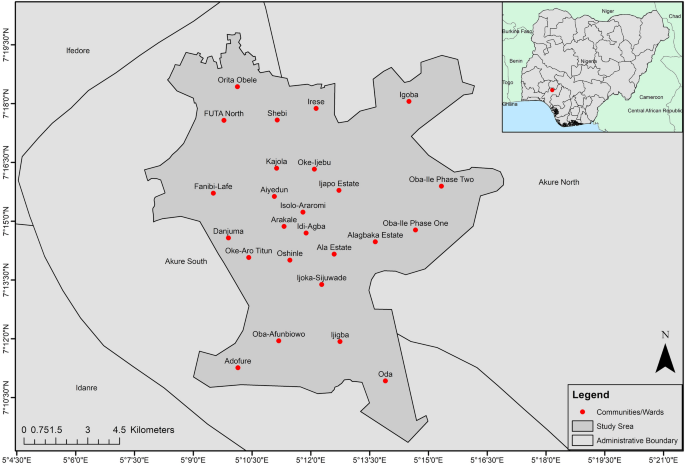
The city of Akure and communities in national context. (Note: The map was drawn by the author with ArcGIS 10.4.1, Esri Inc, http://ww.esri.com . The Nigeria administrative boundaries were gotten from https://datacatalog.worldbank.org/search/dataset/0039368 ; the boundaries for other countries were gotten from https://datacatalog.worldbank.org/search/dataset/0038272/World-Bank-Official-Boundaries ).
According to ( Population and Housing Census 2006 , n.d.), the population of Akure increased from 239,124 in 1991 to 353,211 in 2006 23 . Since the 2006 census is not reliable 24 , we adopted a practical and reliable estimation from the place-based Geographic, Population and Demographic Data project ( https://geopode.world ). Based on derived estimate, the city has over one million residents (1,283,541). From the estimate, U5 comprises of about 12% (162,975) of the estimated population. Akure like Ondo State lies in the tropics which is characterised with humid and derived savanna agroecological zones; dry and wet seasons climate 25 making it a perfect condition for the propagation of vectors (mosquitoes) and transmission of malaria.
Epidemiologic data and explanatory variables
Epidemiologic data (U5 malaria) for this study was obtained with the aid of a Malaria Indicator Questionnaire (MIQ). U5 malaria was determined by a verbal report based on obtained microscopy/clinical test from health centres/laboratories and response to malaria prescribed treatment. We strictly adopted combinations of these two criteria to reduce our bias about the definition of malaria since we do not have the ethical right, qualification, and skills to carry out malaria test on our study participants. To further reduce bias in our studies, cross-checked questions were included. The purpose of some of these questions is to limit the chances of false confirmation of diseases with similar malaria symptoms according to the studies of 26 , 27 . Furthermore, the MIQ was utilised to capture malaria explanatory variables within the frame of social determinants of health (SDH) similar to the study of 28 . The considered SDH variables are within the scope of socio-demographic characteristics (child sex, child age, ethnicity etc.); socioeconomic characteristics (household income, father’s education, mother’s education etc.); preventive behaviour (insecticide-treated bednets—ITN, availability of health infrastructure etc.); built-environmental factors (Window protection, covered roof eaves etc.); and the environmental health factors (drainage condition and covering, toilet facility, proximity to waste disposal point etc.). The considered variables for the analysis were determined after considering the extensive works of 28 , 29 , 30 . MIQ is known to be effective in places of low disease reporting rate and paucity of malaria data 29 , 31 .
Sample size and sampling technique
In most cases, available secondary malaria data from hospital visits lack the important characteristics (socio-economic status (SES) and sociodemographic) and absolute spatial reference (coordinates) thereby making such data unsuitable for our study objectives. These peculiarities are prevalent in SSA particularly in local settings/scale and Akure is no exception. To deal with this challenge, we randomly sampled 1000 buildings like in the study of 32 , with the hope that we would be able to obtain about 600 valid study participants. The estimation of Nigeria’s population, particularly children below the age of five, poses challenges due to infrequent and biased government censuses 24 . Additionally, identifying households with young children in the country beforehand is nearly unfeasible due to lack of antecedent knowledge of houses or households with U5 children. To address these constraints, our study leveraged previous research to determine the sample size, taking budgetary limitations into account. We utilized building sampling as a spatial reference to locate households during our field visits. By importing the extracted building data into ArcGIS Pro, we were able to generate accurate locations of the sampled buildings in relation to the GPS coordinates used during our field survey. The buildings in Akure were extracted following the methodology described on the Picterra platform with a paid subscription ( https://picterra.ch/geospatial-imagery-analysis ).
The study samples are within the scope of other cross-sectional studies and population proportion sampling method of 33 , 34 . According to our knowledge of local demography, most households with U5 children have only one child under five years. In rare cases where there were more than one U5 child i.e. two, we selected the youngest and subsequently selected the eldest in next household with such a similar characteristic as our aim is to model individual-level variability of childhood malaria in Akure. With random sampling, each child, house, or household has equal chances of being selected thereby reducing the risk of selection bias.
Data collection and informed consent
The lead author assisted with five research assistants visited each of the pre-identified houses with the MIQ to gather evidence on active malaria cases after the rainy season. The survey period for this study was between October and December in the year 2019. According to 35 , dry season in Akure is from November to March while the rainy season is from April to October. The sampled houses (families) were visited between 4:30 pm and 6:00 pm to enhance effective targeting of the respondents. Upon visiting a sampled location, the guardian, parents, or adult relative (> 18 years) with the child’s health history was interviewed. Privacy and ethical consent procedures were observed and strictly followed. We obtained informed consent from guardians, parents or adult relative to participate in the study or partake in the interview. Furthermore, we assured, maintained, and adhered to the anonymity of the data and presentation of results obtained from analyses of collected data. The MIQ for the study was created in English language, however, with the option of conducting the interview in Yoruba language (native language in Akure) in case a guardian/parent has a low level of English literacy. This approach ultimately improved the level of inclusion in this study since the lead author and field assistants understands English and Yoruba language. This research was performed in accordance with relevant guidelines and regulations. The methods of data collection and interpretation are in accordance to declaration of Helsinki ethical principles and codes.
Exploratory analysis
Before the development of the geostatistical models for this study, we preliminarily carried out an exploratory analysis of the data. The purpose of this is to provide insights and guides into development of best fit geostatistical model for U5 malaria prevalence 36 . The objectives and focus on this stage of analysis are:
Establish the determinants variables or factors of U5 malaria prevalence. This can be accomplished by utilisation of bivariate analysis such as Chi-square ( \({\mathcal{X}}^{2} )\) to build a table summary of the association between U5 malaria and the covariates. This formed the basis of non-spatial analysis discussed in the later section of this paper.
Explore the association between U5 malaria prevalence and covariates i.e. explanatory or independent variables. In this stage, we fitted a non-spatial generalised linear model (GLM) to observed and assess the relationship (magnitude and direction) of the covariates with U5 malaria prevalence. The selected model has the least Akaike Information Criterion (AIC) from the stepwise forward approach that was conducted. In addition, Variance Inflation Factor (VIF)/generalized Variance Inflation Factor (GVIF) was used as regression diagnostic measure to detect the presence of collinear variables in order to avoid multicollinearity in our model and reduce standard error of model coefficients according to the works of 37 , 38 . Furthermore, we evaluated our designed model accuracy using cross-validation ( k -fold) technique. The purpose of this is to test the effectiveness of our model against data points which were not used during the training of the model (new data sets). During the model training randomly selected subset of the data (training set) is used to inform predictions at location of remainder of the data (test set) 36 . The combination of these methods (GVIF and cross-validation) guide against correlation among model explanatory variables, overfitting of our model, evaluate prediction accuracy, and provide insight on variable importance and selection asides the retention of variables based on their p-values (p < 0.05). In the final step, the odds ratio (OR) which determines risk factors of U5 was computed. Given the exposure or factor, OR greater than 1 means the U5 malaria is likely to occur; OR less than 1 means the event (U5 malaria) is less likely to occur while OR equals 1 means the likelihood of malaria does not change.
Examine spatial dependency of U5 malaria by testing for spatial correlation on the residuals i.e. to examine spatial dependency in step (ii). The focus is to determine if variation in the residuals i.e. variation that is not captured by the retained variables reveal evidence of spatial correlation by using empirical variogram 36 . The choice of spatial model is determined by the detection of spatial correlation in the residual .
Geostatistical modelling
Unlike non-spatial/standard statistical modelling, spatial data and modelling observe the assumption of spatial dependence (autocorrelation) between neighbouring locations due to observed common exposure 39 , 40 , 41 . Spatial autocorrelation in this context refers to the relationship between U5 malaria of a child ( Y ij ) in location j with itself in another neighbouring location within the same geographical space 39 . Spatial autocorrelation expresses the degree of similarity among the observation values within the geographical space of interest 42 . Therefore, to account for spatial dependency, we formulated a geostatistical model which follows the geostatistical model for prevalence surveys by 43 . The model is within the generalised linear mixed model framework or spatial generalised linear mixed models (SGLMMs) which relates disease prevalence data with potential linear predictors, binomial error distribution, logistic-link function and latent Gaussian process by adding random effects at the observed locations 43 , 44 . Model-based geostatistics has its origin from Kriging which is a method of interpolating (predicting values at unmeasured locations) or smoothing spatial data. Particularly, MBG is termed as application of explicit parametric stochastic models and likelihood-based methods of inference to geostatistical problems 45 . The interpolation is based on observation data pairs while correlation is a function of distance between the data pairs 43 , 46 .
Equation ( 1 ) describes the likelihood-based Binomial Geostatistical Model adopted for this study. This is an extension of a binary logistic regression model by the inclusion of random effects and spatially correlated random effects i.e. spatial Gaussian process. Hence, let U5 malaria status Y ij of a child i at location j take the value of 1 if a child has malaria, and 0 otherwise. The dependent variable—Yij follows a Bernoulli probability distribution with P(Y ij = 1) = \({\mathcal{P}}_{ij}\) which is conditional on a stationary Gaussian process \(\left( x \right){ }\) and an additional set of study location specific and unobserved random effects \(Z_{i}\) , the linear predictor of the model assumes the form:
where \({\mathcal{X}}_{i}\) is the vector of a child, with individual-level covariates with associated regression coefficient \(\beta\) ; S = { \(\left( x \right)\) : \(x\) \(\in\) R 2 } is a Gaussian process with mean zero, variance \(\sigma^{2}\) , and correlation function p ( \(x,x^{\prime}\) ) = Corr { \(\left( x \right)\) ,S( \(x^{\prime}\) )}. The Gaussian process ( \(S)\) is stationary and isotropic, while the correlation function is a function of euclidean distance 47 . The aim of study location random effects \(Z_{i}\) is to account for the unexplained nonspatial variation which could be small scale spatial variation or measurement error. This is also known as the nugget effect ( \({\uptau }^{2} )\) . The random effects are independent normal, (i.e. Zi ~ N (0, \({\uptau }^{2}\) )) variates.
In Eq. ( 1 ), we write \(\tau^{2}\) for the variance of \(Z_{i}\) and model S \(\left( x \right)\) as a stationary Gaussian process with variance \(\sigma^{2}\) and matérn correlation function 48 . Matérn model is an efficient method for modelling correlation function as strongly recommended by 45 , 49 , 50 . It contains kappa (k) which determines the smoothness of the process. The matérn correlation function is given by:
where \(\emptyset\) > 0 is a scale parameter which regulates the rate at which the spatial correlation goes to zero or decays as the distance increases 51 , 52 ; k > 0 is the shape parameter which determines the smoothness of \(\left( x \right)\) . Kk (.) is the modified Bessel function of the second kind of order k > 0, and \(u\) is the distance between two sampled locations. Kappa is difficult to estimate reliably since this will involve large data collected at small distances. Hence three discrete set of values (0.5, 1.5, 2.5) corresponding to different level of smoothness have been defined for Kappa 44 . These values correspond to the discontinuity of the different level of smoothness. For this study, we adopted 0.5 for Kappa according to the documentation and works of 36 , 44 . Kappa of 0.5 corresponds to exponential correlation function i.e. the Matérn covariogram becomes the exponential one 44 , 53 . Furthermore, most functions available in PrevMap package in R, the Matérn shape parameter \(\kappa\) is treated as fixed because not all parameters in the Matérn class can be estimated consistently. Matérn class has the capacity to model the behaviours of variogram and it consists of exponential variograms as a special case unlike other popular covariograms such as exponential, powered-exponential, gaussian or spherical covariograms. For more technical details, we refer the reader to the works of 44 , 46 , 53 .
Monte Carlo maximum likelihood and spatial prediction
In this study, Monte Carlo maximum likelihood methods (MCML) was utilised for parameter estimation as documented in the PrevMap package in R 44 . MCML is based on importance sampling techniques approximation of the high-dimensional intractable integral that defines the likelihood function 54 . It enables flexibility in fitting complex models and avoids asymptotic inference and computational challenges encountered in solely likelihood-based fitting 55 . The likelihood function for parameters \(\beta\) and \(\theta^{{\text{T}}}\) = ( \(\sigma^{2}\) , \(\emptyset , {\uptau }^{2}\) ) is obtained by integrating out the random effects included in Eq. ( 1 ), where \(\sigma^{2}\) is the variance, \(\emptyset\) is the range, and \({\uptau }^{2}\) is the nugget effect. We map the risk of U5 malaria over 100 × 100 m grid. Spatial distribution maps of U5 malaria prevalence, likelihood-based geostatistical modelling and spatial prediction were developed in R statistical programming (R version 3.6.3). To improve the model predictions, the covariates are included. The selected covariates for the spatial model were carefully considered according to their significance level as discussed in earlier section i.e. Exploratory analysis. Often, the development of public health policies are based on the exceedance, or non-exceedance of a predefined prevalence or incidence thresholds say t 36 . Therefore, the exceedance probability (EP) of U5 malaria prevalence predictions in each location above the predefined thresholds t can be expressed or defined as:
It is necessary to note that the resulting estimates at each locations have uncertainties that need to be taken to consideration 52 . The exceedance probability can help to overcome this challenge and prevent unjustifiable policy decisions by quantifying how likely \({\mathcal{P}}\left( {\mathcal{X}} \right){ }\) is to be above a threshold t as shown in Eq. (3). For this study, we set prevalence threshold to be 10% (0.1) which can be categorised as hotspots of U5 malaria in Akure. According to 56 , places with annual malaria prevalence of 10–35% have moderate transmission while area of high transmission are above 35%. However, the recently implemented fifth National Malaria Strategic Plan (NMSP) covering the period of 2021–2025 in Nigeria aims to achieve parasite prevalence of less than 10% 57 . We therefore adopted 10% as our exceedance threshold to determine hotspots of malaria prevalence in Akure. If EP is close to 100%, this shows that U5 malaria prevalence to be above the threshold t is very high ; if EP is close to 0%, the prevalence of U5 malaria is highly likely to be below t. EP close to 50% suggests high level of uncertainty which means that prevalence of U5 malaria is equally likely to be above or below t.
Ethical approval
We received ethical approval from the institutional review board at the Institute of Geography, Heidelberg University, Germany. We obtained informed consent from parents, guardians or adult relative who participated in the interview, and we adhered to the anonymity of the data and presented results. Before the interview was carried out, ethical clearance was obtained from the Ondo State Ministry of Health.
Non- spatial analysis
We effectively obtained about 60% valid responses (n = 568). As mentioned earlier, we do not have previous knowledge of houses or households with U5 children. This has contributed to the low responses coupled with budget constraints to sample more houses. Furthermore, we expunged participants who had spent less than two weeks in the location depending on the week of survey to reduce risk of imported malaria. Nevertheless, the obtained valid responses were deemed sufficient after carrying out statistical power analysis with open source G*Power tool, version 3.1.9.6 58 . The point prevalence of U5 malaria in Akure, Nigeria based on verbal confirmation according to the study definition of positive malaria was 22.5%. Malaria prevalence among the female children (23.3%) is higher compared to malaria prevalence among male children (21.9%) according to Table 1 . According to the study, about 40% of the children have ITN. Further to the study findings in Table 1 on the usage of ITN and its impact on malaria prevalence, children who sleep under ITN have lower prevalence of malaria (16.7%) compared to children who do not sleep under ITN (26.4%). This further implies that usage of ITN is malaria risk factor with significant reduced odds of U5 malaria and serves as protection against mosquito bites.
The study findings show that vector-proof houses are determinant factor of malaria. Vector-proof houses protect against malaria. The houses in good condition characterised with good window screening have a lower prevalence of malaria (18.6%), while children living in substandard houses characterised with poor or defected window covering recorded higher prevalence of malaria (39.9%). The condition and source of drinking water also plays significant role in the burden of malaria. According to the study findings, almost half (48%) of the survey households depend on Dug well as water source. Despite this large figure, the burden of U5 malaria is higher (28%) among households with Dug wells compared to affluent households that depends on piped water source (15.9%). As shown in Table S1 (supplementary file) , drainage with covering is a determinant and risk factor of malaria. U5 children living in places with covered drainage records less burden of malaria (13.3%) compared with U5 children dwelling in places with poor drainage facilities (24.3%). According to Table S1 (supplementary file) , Education, Income and type of employment further illustrate effect of social determinants or socioeconomic characteristics on health. U5 children whose fathers are employed in the formal sector have lower burden of malaria (18.2%) compared with U5 children whose fathers are either work in informal sector (26%) or unemployed (28.6%). This phenomenon is similar to the study findings on effect of income level on U5 malaria. According to our study findings, the burden of malaria reduces as income level increases ( Table S1 as supplementary file ). U5 malaria prevalence is lower among mothers who have obtained tertiary education (18.4%) compared to mothers with no education (33.3%).
Supplementary Table S1 online contains additional table summary of mostly non-significant covariates in this study. We have discussed some selected covariates in the manuscript.
Model results
The results reported in Table 2 describes the significant predictors and parameter estimates from the binomial logistic model for this study as documented in Eq. ( 1 ). The sigma sq \((\sigma^{2} )\) is the variance of the Gaussian process, \(\emptyset\) is the scale parameter which represents the extent of the spatial correlation in metres, while tau sq ( \({\uptau }^{2} )\) is the non-spatial variation. After further exploration of the model results particularly because of the binary response at each sampled locations we fitted the model without Z terms i.e. tau sq ( \({\uptau }^{2} )\) . This pragmatic decision further led to the improvement of the model fit. According to the model result, the model accuracy from the k -fold cross-validation was 0.75 (75%) and Cohen’s kappa was 0.01, which could be considered “slight” given the thresholds of 59 relatively indicating good performance. ITN, window protection and piped water source are significant with high variable importance. In addition, these variables are not correlated to each other according to the GVIF values (see Table 2 ). Therefore no added uncertainty in the model estimates and almost non-multicollinearity have been maintained since the VIF values are very close to 1 and lower than threshold of 5 as explicitly discussed in 60 , 61 . The usage of ITN reduces the risk of malaria burden. Concurrently, vector-proof houses with good window protection have a negative relationship with the likelihood of positive malaria outcomes. Water sources (i.e. piped) have a negative association with the probability of malaria, while other sources of water are non-significant.
For this study, point referenced U5 malaria prevalence data were analysed using MBG models to outline and map areas where prevalence of U5 malaria is above or below a set policy threshold. We predicted the prevalence of U5 malaria at a fine scale (100 × 100 m resolution map). The predictive power of the model increases when disease predictors are considered. According to Fig. 3 (left panel), the predicted prevalence of U5 malaria in Akure is slightly above 35%, while it is about 35% when the predictors are not considered as seen in the left panel of Fig. 2 . Furthermore, the probability that U5 malaria prevalence is above 10% is shown in the right panel of Fig. 3 . We used the 10% exceedance threshold to determine hotspots for this study associated with the level of certainty. Therefore, areas with \(\ge\) 80% probability of exceeding the threshold were considered hotspots. The certainty level is captured with the contour lines. The uncertainty in the estimates is quantified using the standard errors as shown in right panel of Fig. 2 . A set of diagnostic plots that provide checks on the convergence of the MCMC is provided in Fig. S1 (supplementary file) .
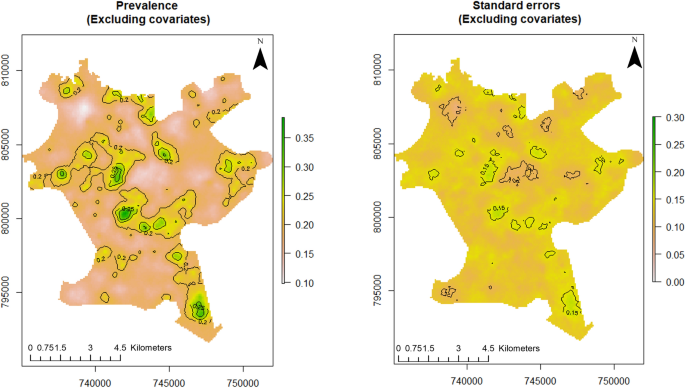
Predictive distribution of U5 malaria in Akure (left panel) and standard errors of the predictions (right panel). The figure was created with R version 3.6.3, https://cran.rstudio.com/ .
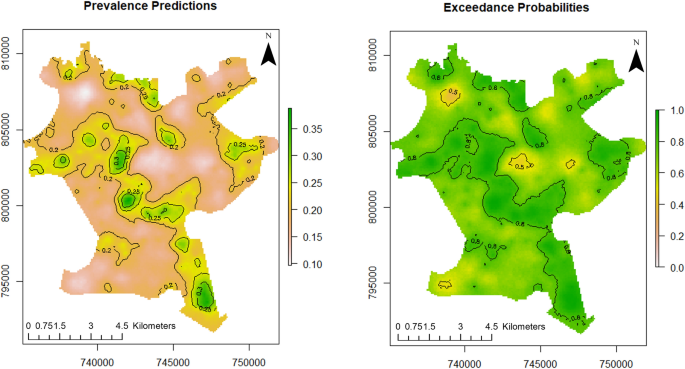
Predictive distribution of U5 malaria in Akure (left panel) and exceedance probabilities (right panel). The figure was created with R version 3.6.3, https://cran.rstudio.com/ .
Spatially targeted policy and healthcare intervention are pertinent to eradicating disease transmission. e.g., malaria. Therefore, spatial modelling of disease remains an important public health tool. Through disease models, hotspots can be determined for prioritising timely intervention in resources-scarce contexts.
The reduction in malaria burden has stalled. The recent figures of global malaria burden according to 1 is the same level as before 2011, with much increase in the last two years. Furthermore, the national-level statistics may not reflect what is obtainable at lower administrative levels. Since countries are experiencing reduction in malaria burden between 2010 and 2019 as reported by 1 , coupled with the scarce availability of health resources, spatial targeting of intervention for maximum utilisation of resources is essential. Geostatistical methods as seen in this study provides the opportunity for precision in hotspot determination.
There is variability in U5 malaria spatial distribution in Akure. The spatial predicted burden of U5 malaria is higher in the poor and low-income communities such as Arakale, Isolo-Araromi, Ayedun, and Oda. The high malaria transmission might have been due to the lack of suitable housing infrastructure. Based on the morphology of Akure as documented by 62 , Arakale and Isolo-Araromi are communities in the city centre characterised by old and substandard buildings, poor drainage facilities and below-minimum space between buildings. Collectively, these features aid high transmission of malaria. Conversely, Oba-Ile Phase Two and Oda which are newly emerging areas (suburbs) and outlying districts of Akure are also characterised with high burden of malaria as shown in the exceedance probability model (right panel of Fig. 3 ). These places have prevalence greater than 10% with 80% certainty. These newly emerging places show element of poor planning control 11 , 24 with fragmented sites which are suitable vector breeding sites 63 . Lower transmission of U5 malaria was observed in affluent neighbourhoods such as Oba-Ile Phase One, Ijapo Estate and Alagbaka Estate. These areas have standard building structures and better facilities such as good rood conditions, drainage, a good water supply and less vegetation 62 and robust urban planning development control.
Local spatial estimations of disease allow us to identify locations of disease clusters where disease prevalence is above the geographical average (hotspots). In this study, U5 malaria hotspots were determined through the exceedance probability model as shown in the right panel of Fig. 3 . 10% cut off was adopted as the threshold to identify malaria hotspots which is in accordance with the NMSP target set by the Nigerian government. According to the exceedance probabilities model, the dark green areas show locations where U5 malaria prevalence is above 10% with certainty level of 80% and above. These places such as Isolo-Araromi, Arakale, Aiyedun, Kajola, Idi-Agba, Fanibi-Lafe, Oba-Ile Phase Two, Oda, Orita Obele and Irese. The identified places require targeted malaria control effort by the health authorities towards malaria elimination in order to meet the NMSP target.
The study analyses elucidate the risk factors of U5 malaria prevalence. Based on our model results, several factors determine the risk of malaria among U5 in Akure. Although not significant, child’s gender is one of these factors. Male children exhibited a slightly lower malaria burden than their female counterparts. A similar study conducted in Cameron shows a non-significant association between child sex and malaria with a lower burden among male children 64 , while the studies of 65 , 66 show significant lower burden of malaria among males compared to females. However, among older children, males are more prone to malaria because of their higher engaging outdoor activities compared to female 13 , 67 . The reason for our findings could be difference in background immunity between male and female children.
The availability and usage of ITNs is another significant and important factor that affects malaria exposure. According to our study, the usage of ITNs reduces the likelihood of childhood malaria by 56% (OR = 0.56; 95% CI = 0.36–0.86) in Akure, Nigeria compared the children who do not sleep under ITN. Our findings agrees with the following studies in Ghana 30 , Nigeria 68 , Uganda 69 , and Kenya 70 . Good ITN protects against mosquito vectors by reducing the vector-to-human contacts. This mechanically prevents or stops mosquito bites.
The impact of urban agriculture on the susceptibility of malaria among children under five was not significant which is in agreement to the studies of in Ibadan Nigeria 13 and 71 in Malawi. According to our study, households that practice urban agriculture are 1.23 times likely to have malaria (OR = 1.23; 95% CI = 0.79–1.88) compared to household who do not practice urban agriculture. Few studies have investigated intra-urban impact of urban agriculture on U5 malaria unlike rural–urban studies. For example 52 , found a positive association between positive malaria outcome and children living in rural areas of Ghana, as well as 40 in Mozambique. Rural areas are usually highly vegetated, serving as a suitable habitat for the breeding of mosquitoes. In addition, we do not find an association between the adopted mode of waste disposal method and U5 malaria prevalence. One of the challenges of urbanisation is increasing waste generation as this has consequences on the health of urban residents. Good waste management practices such as regular trash disposal reduce the risk of malaria as there would be less mosquito breeding, clogging and flooding 72 .
Also, the study findings show a non-significant increasing trend in the burden of malaria with each increasing age categories similar to the outcomes observed in the studies of 13 , 52 . The lower risk of malaria burdens in younger children could be because of the passive immunity acquired from mothers through breastfeeding as observed in the studies of 2 , 52 . Intuitively, this observed phenomenon might also be due to the fact that older children are less likely to sleep under ITN when there are not enough ITN to serve the younger and older children among poor households.
Lower risk of malaria exists among U5 children in vector-proof houses such as window protection (OR = 0.51; 95% CI = 0.34–0.77). Similar findings are reported by 28 , 73 . Houses in good condition i.e. mosquito-proofing houses offer significant advantage of equitably protecting all members of particular households even those that are not sleeping under a bed net 73 . Window screening prevent mosquitoes from entering the houses or places of abode. According to our study, households with a piped source of water have reduced odds of U5 malaria (OR = 0.48; 95% CI = 0.31–0.77). In this study, since wealth index was not considered access to piped water is used as a surrogate for wealth index, which explains the reduced odds for households with a piped source of water and window protection. Poor households are likelier to live in substandard houses with avenues for malaria vectors to find their way into the building. These findings are in agreement with the studies of 17 , 69 , 74 , 75 , 76 where highest wealth status households or better off households are noted to afford malaria preventive measures. Some of these measures include appropriate housing facilities with screens that block or hinder vectors resulting in reduced vector-human contact, insecticide-treated bed nets to reduce malaria transmission, quick diagnosis and acquiring of drugs in case of infection without depending on public facilities. Moreover, malaria in Africa have been described as a disease of rural population and communities which are homes of the poorest of the poor 77 , as further illustrated by income level in Table S1 (supplementary file) . The higher the income level, the lower the odds of U5 malaria.
Study limitations and future research
There are some limitations in this study that should be considered when interpreting the study findings. The epidemiologic variable—presence or absence of malaria—retrospectively determined by verbal report might lead to recall bias. Furthermore, not all research variables that influences the transmission of malaria are considered in this study. Therefore, robust health routine survey data with associated environmental factors and SES void of bias should be considered in future study. Nevertheless, this research primarily considered social factors and cross-checked questions on definition of malaria to limit bias was maintained.
It is pertinent to note that the study’s sample size is relatively small with potential to introduce some biases in the study results such as the low proportion of malaria-positive cases. This might have impacted the low Cohens kappa measure i.e. measurement of agreement of the two categorical variable outcomes (positive and negative malaria outcome). However, the obtained results from statistical power analysis test and cross-validation model accuracy have led to improvement of the study validity. Therefore, an extensive future study with more samples should be strongly considered. Lastly, since this is a cross-sectional study, the impact of seasonality on malaria prevalence should be considered while interpreting the results since the burden of malaria varies seasonally.
Conclusions
This study demonstrated steps toward understanding the spatial structure of U5 malaria through the application of Model-based Geostatistical modelling to a very-fine scale mapping in places of low resource settings such as Akure, Nigeria. The map provides place-based evidence on the spatial variation of U5 malaria in Akure and serves as a guide to locations that require crucial and intensified interventions for the reduction of malaria burden.
The study shows spatially predicted variability of U5 malaria risk in Akure, with high prevalence within the centre of the city, transition zone, and newly developed places/suburbs which are characterised with low urban planning development control. The study further shows low prevalence of U5 malaria burden in the affluent communities such as Alagbaka, Oba-ile etc. According to our findings, the usage of ITNs, window protection, and a piped-water source reduces the risk of U5 malaria. Therefore, interventions addressing these risk factors are germane while also ensuring continuous monitoring of malaria prevalence and intervention assessment should be considered. This is however predicated on the availability of malaria covariates data especially at local level. Hence, barriers on data availability should be addressed. The health challenges of the twenty-first century are complex and requires multiple discipline and approaches to tackle these challenges. Therefore, urban planning control and development in the city core and outlying districts should be intensified.
Geographical or spatial targeting of public health control efforts in U5 malaria hotspots developed in accordance to the exceedance probability model will aid the elimination of malaria in Akure, Nigeria. The evidence-based policy formulation and implementation directed towards places of high malaria risk and transmission can lead to malaria elimination and achieving set target according to the Nigeria’s NMSP. In addition, this can also contribute towards Nigeria’s achievement of Sustainable Development Goals 3 and 11 which are to: (1) Ensure health lives and promote well-being for all at all ages and (2) Making cities and human settlement inclusive, safe resilient and sustainable.
Data availability
Data can be made available from the corresponding author upon reasonable request. However, the R scripts for the exploratory analysis, cross-validation, parameter estimation and spatial prediction are freely available at: https://github.com/Taye20/MBG/tree/main .
Abbreviations
Akaike information criterion
- Exceedance probability
Insecticide-treated nets
Model-based Geostatistics
Monte Carlo maximum likelihood
Malaria Indicator Questionnaire
Malaria Indicator Survey
National Malaria Strategic Plan
Socio-economic status
Social determinants of health
Spatial generalised linear mixed models
Sub- Saharan Africa
Children under five years
Variance Inflation Factor
World Health Organization
World Health Organization. World Malaria Report 2022 . www.who.int/teams/global-malaria-programme (2022).
Dao, F. et al. Burden of malaria in children under five and caregivers’ health-seeking behaviour for malaria-related symptoms in artisanal mining communities in Ghana. Parasit. Vect. 14 , 418 (2021).
Article CAS Google Scholar
Iddrisu, D. & Moyer, C. A. Using the Ghana malaria indicator survey to understand the difference between female and male-headed households and their prevention and testing for malaria among children under 5. Malar. J. 21 , 112 (2022).
Article PubMed PubMed Central Google Scholar
Beargie, S. M. et al. The economic impact of substandard and falsified antimalarial medications in Nigeria. PLOS ONE 14 , e0217910 (2019).
Article CAS PubMed PubMed Central Google Scholar
Onwujekwe, O. et al. The economic burden of malaria on households and the health system in Enugu State Southeast Nigeria. PLoS ONE 8 , e78362 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Edelu, B., Ndu, I., Igbokwe, O. & Iloh, O. Severe falciparum malaria in children in Enugu, South East Nigeria. Niger. J. Clin. Pract. 21 , 1349 (2018).
Article CAS PubMed Google Scholar
Park, J. et al. Barriers against and strategies for malaria control during the COVID-19 pandemic in low- and middle-income countries: A systematic review. Malar. J. 22 , 41 (2023).
Ilesanmi, O., Afolabi, A. & Iyiola, O. Effect of the COVID-19 pandemic on malaria intervention coverage in Nigeria: Analysis of the Premise Malaria COVID-19 Health Services Disruption Survey 2020. Popul. Med. 3 , 1–10 (2021).
Article Google Scholar
Muhammad, F., Abdulkareem, J. H. & Chowdhury, A. A. Major Public Health Problems in Nigeria: A review. South East Asia J. Public Health 7 , 6–11 (2017).
Ozodiegwu, I. D. et al. Field Assessment of the Burden And Determinants Of Malaria Transmission To Inform Tailoring of Interventions (microstratification) in Ibadan and Kano metropolis: Study protocol . https://doi.org/10.1101/2023.01.20.23284766 (2023).
Bayode, T. & Siegmund, A. Tripartite relationship of urban planning, city growth, and health for sustainable development in Akure, Nigeria. Front. Sustain. Cities 5 , 1301397 (2024).
Alegana, V. A., Okiro, E. A. & Snow, R. W. Routine data for malaria morbidity estimation in Africa: Challenges and prospects. BMC Med. 18 , 121 (2020).
Awosolu, O. B., Yahaya, Z. S., Farah Haziqah, M. T., Simon-Oke, I. A. & Fakunle, C. A cross-sectional study of the prevalence, density, and risk factors associated with malaria transmission in urban communities of Ibadan, Southwestern Nigeria. Heliyon 7 , e05975 (2021).
Dawaki, S. et al. Is Nigeria winning the battle against malaria? Prevalence, risk factors and KAP assessment among Hausa communities in Kano State. Malar. J. 15 , 351 (2016).
Ugwu, C. L. J. & Zewotir, T. Spatial distribution and sociodemographic risk factors of malaria in Nigerian children less than 5 years old. Geospat. Health 15 , 2 (2020).
Okunlola, O. A. & Oyeyemi, O. T. Spatio-temporal analysis of association between incidence of malaria and environmental predictors of malaria transmission in Nigeria. Sci. Rep. 9 , 17500 (2019).
Article ADS PubMed PubMed Central Google Scholar
Adigun, A. B., Gajere, E. N., Oresanya, O. & Vounatsou, P. Malaria risk in Nigeria: Bayesian geostatistical modelling of 2010 malaria indicator survey data. Malar. J. 14 , 156 (2015).
Kyomuhangi, I. & Giorgi, E. Geostatistical modeling of variation in disease risk: Continuous or binary data?. Int. J. Infect. Dis. 79 , 110 (2019).
Amoah, B. et al. Model-based geostatistics enables more precise estimates of neglected tropical-disease prevalence in elimination settings: Mapping trachoma prevalence in Ethiopia. Int. J. Epidemiol. 51 , 468–478 (2022).
Article PubMed Google Scholar
Diggle, P. J., Amoah, B., Fronterre, C., Giorgi, E. & Johnson, O. Rethinking neglected tropical disease prevalence survey design and analysis: A geospatial paradigm. Trans. R. Soc. Trop. Med. Hyg. 115 , 208–210 (2021).
Chipeta, M. G. et al. Geostatistical analysis of Malawi’s changing malaria transmission from 2010 to 2017. Wellcome Open Res. 4 , 57 (2019).
Macharia, P. M. et al. Spatio-temporal analysis of Plasmodium falciparum prevalence to understand the past and chart the future of malaria control in Kenya. Malar. J. 17 , 340 (2018).
Population and Hosuing Census 2006 . https://catalog.ihsn.org/index.php/catalog/3340 (2006).
Tofowomo, A. The Planning Implications of Urban Sprawl in Akure . Available at: https://www.isocarp.net/Data/case_studies/1131.pdf (2008).
Omonijo, A. G., Matzarakis, A., Oguntoke, O. & Adeofun, C. O. Influence of weather and climate on malaria occurrence based on human-biometeorological methods in Ondo State, Nigeria. J. Environ. Sci. Eng. 5 , 1215–1228 (2011).
Google Scholar
Ngom, R. & Siegmund, A. Urban malaria in Africa: An environmental and socio-economic modelling approach for Yaoundé, Cameroon. Nat. Hazards 55 , 599–619 (2010).
Ngom, R. & Siegmund, A. The key role of socio-demographic and socio-environmental factors in urban malaria occurrence and control—an illustration using the city of Yaoundé. Soc. Sci. Med. 133 , 269–279 (2015).
Hasyim, H., Dale, P., Groneberg, D. A., Kuch, U. & Müller, R. Social determinants of malaria in an endemic area of Indonesia. Malar. J. 18 , 134 (2019).
Bayode, T. & Siegmund, A. Social determinants of malaria prevalence among children under five years: A cross-sectional analysis of Akure, Nigeria. Sci. Afr. 16 , e01196 (2022).
Nyarko, S. H. & Cobblah, A. Sociodemographic determinants of malaria among under-five children in Ghana. Malar. Res. Treat. 2014 , 1–6 (2014).
Ngatu, N. R. et al. Environmental and sociodemographic factors associated with household malaria burden in the Congo. Malar. J. 18 , 53 (2019).
Koukouli, S., Vlachonikolis, I. & Philalithis, A. Socio-demographic factors and self-reported funtional status: The significance of social support. BMC Health Serv. Res. 2 , 20 (2002).
Gahutu, J.-B. et al. Prevalence and risk factors of malaria among children in southern highland Rwanda. Malar. J. 10 , 134 (2011).
Tsegaye, A. T., Ayele, A. & Birhanu, S. Prevalence and associated factors of malaria in children under the age of five years in Wogera district, northwest Ethiopia: A cross-sectional study. PLOS ONE 16 , e0257944 (2021).
Makinde, O. S., Abiodun, G. J. & Ojo, O. T. Modelling of malaria incidence in Akure, Nigeria: Negative binomial approach. GeoJournal 86 , 1327–1336 (2021).
Giorgi, E. et al. Model building and assessment of the impact of covariates for disease prevalence mapping in low-resource settings: To explain and to predict. J. R. Soc. Interface 18 , 20210104 (2021).
Kianfar, N. & Mesgari, M. S. GIS-based spatio-temporal analysis and modeling of COVID-19 incidence rates in Europe. Spat. Spatio-Temporal Epidemiol. 41 , 100498 (2022).
Kianfar, N., Mesgari, M. S., Mollalo, A. & Kaveh, M. Spatio-temporal modeling of COVID-19 prevalence and mortality using artificial neural network algorithms. Spat. Spatio-Temporal Epidemiol. 40 , 100471 (2022).
Bayode, T. et al. Spatial variability of COVID-19 and its risk factors in Nigeria: A spatial regression method. Appl. Geogr. 138 , 102621 (2022).
Ejigu, B. A. Geostatistical analysis and mapping of malaria risk in children of Mozambique. PLOS ONE 15 , e0241680 (2020).
Ejigu, B. A. & Wencheko, E. Spatial Prevalence and Determinants of Malaria among under-five Children in Ghana. https://doi.org/10.1101/2021.03.12.21253436 (2021).
Lin, C.-H. & Wen, T.-H. How spatial epidemiology helps understand infectious human disease transmission. Trop. Med. Infect. Dis. 7 , 164 (2022).
Diggle, P. J., Tawn, J. A. & Moyeed, R. A. Model-based geostatistics. J. R. Stat. Soc. Ser. C Appl. Stat. 47 , 299–350 (1998).
Article MathSciNet Google Scholar
Giorgi, E. & Diggle, P. J. PrevMap: An R package for prevalence mapping. J. Stat. Softw. 78 , 1456 (2017).
Diggle, P. J., Ribeiro, P. J. & Christensen, O. F. An introduction to model-based geostatistics. In Spatial Statistics and Computational Methods, vol. 173 (ed. Møller, J.) 173 43–86 (Springer, 2003).
Diggle, P. & Ribeiro, P. J. Model-Based Geostatistics (Springer, 2007).
Book Google Scholar
Diggle, P. J. & Giorgi, E. Model-based geostatistics for prevalence mapping in low-resource settings. J. Am. Stat. Assoc. 111 , 1096–1120 (2016).
Article MathSciNet CAS Google Scholar
Matérn, B. Spatial Variation Vol. 36 (Springer, 1986).
Stein, M. L. Interpolation of Spatial Data (Springer, 1999). https://doi.org/10.1007/978-1-4612-1494-6 .
Stein, M. L. & Stein, M. L. Interpolation of Spatial Data: Some Theory for Kriging (Springer, 1999).
Amoah, B., Giorgi, E., Heyes, D. J., van Burren, S. & Diggle, P. J. Geostatistical modelling of the association between malaria and child growth in Africa. Int. J. Health Geogr. 17 , 7 (2018).
Yankson, R., Anto, E. A. & Chipeta, M. G. Geostatistical analysis and mapping of malaria risk in children under 5 using point-referenced prevalence data in Ghana. Malar. J. 18 , 67 (2019).
Zhang, H. Inconsistent estimation and asymptotically equal interpolations in model-based geostatistics. J. Am. Stat. Assoc. 99 , 250–261 (2004).
Christensen, O. F. Monte Carlo maximum likelihood in model-based geostatistics. J. Comput. Graph. Stat. 13 , 702–718 (2004).
Geyer, C. J. & Thompson, E. A. Constrained Monte Carlo maximum likelihood for dependent data. J. R. Stat. Soc. Ser. B Methodol. 54 , 657–683 (1992).
MathSciNet Google Scholar
World Health Organization. A Framework for Malaria Elimination (World Health Organization, 2017).
National Malaria Elimination Programme (NMEP), National Population Commission (NPC) & ICF. In Nigeria Malaria Indicator Survey 2021 Final Report (2022).
Faul, F., Erdfelder, E., Albert-Georg, L. & Axel, B. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39 , 175–191 (2007).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33 , 159 (1977).
Shrestha, N. Detecting multicollinearity in regression analysis. Am. J. Appl. Math. Stat. 8 , 39–42 (2020).
Daoud, J. I. Multicollinearity and regression analysis. J. Phys. Conf. Ser. 949 , 012009 (2017).
Akinbamijo, O. B. & Fasakin, J. O. Spatial disparities in residential housing health—an application of models to Akure, South—West Nigeria. Soc. Sci. 1 , 158–163 (2006).
De Silva, P. M. & Marshall, J. M. Factors contributing to urban malaria transmission in sub-Saharan Africa: A systematic review. J. Trop. Med. 2012 , 1–10 (2012).
Talipouo, A. et al. Malaria prevention in the city of Yaoundé: Knowledge and practices of urban dwellers. Malar. J. 18 , 167 (2019).
Okiring, J. et al. Gender difference in the incidence of malaria diagnosed at public health facilities in Uganda. Malar. J. 21 , 22 (2022).
Workineh, L. et al. Prevalence of Malaria and associated factors among children attending health institutions at South Gondar Zone, Northwest Ethiopia: A cross-sectional study. Glob. Pediatr. Health 8 , 110591 (2021).
Woday, A., Mohammed, A., Gebre, A. & Urmale, K. Prevalence and associated factors of malaria among febrile children in Afar Region, Ethiopia: A health facility based study. Ethiop. J. Health Sci. 29 , 5 (2019).
Yusuf, O. B., Adeoye, B. W., Oladepo, O. O., Peters, D. H. & Bishai, D. Poverty and fever vulnerability in Nigeria: A multilevel analysis. Malar. J. 9 , 235 (2010).
Ssempiira, J. et al. Geostatistical modelling of malaria indicator survey data to assess the effects of interventions on the geographical distribution of malaria prevalence in children less than 5 years in Uganda. PLOS ONE 12 , e0174948 (2017).
Landis-Lewis, Z. et al. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 74 , 376–385 (2006).
Kazembe, L. N. & Mathanga, D. P. Estimating risk factors of urban malaria in Blantyre, Malawi: A spatial regression analysis. Asian Pac. J. Trop. Biomed. 6 , 376–381 (2016).
Bempah, S., Curtis, A., Awandare, G., Ajayakumar, J. & Nyakoe, N. The health-trash nexus in challenging environments: A spatial mixed methods analysis of Accra, Ghana. Appl. Geogr. 143 , 102701 (2022).
Ogoma, S. B. et al. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar. J. 8 , 221 (2009).
Gosoniu, L., Msengwa, A., Lengeler, C. & Vounatsou, P. Spatially explicit burden estimates of malaria in Tanzania: Bayesian geostatistical modeling of the malaria indicator survey data. PLoS ONE 7 , e23966 (2012).
Ibeji, J. U., Mwambi, H. & Iddrisu, A.-K. Spatial variation and risk factors of malaria and anaemia among children aged 0 to 59 months: A cross-sectional study of 2010 and 2015 datasets. Sci. Rep. 12 , 11498 (2022).
Wanzira, H. et al. Factors associated with malaria parasitaemia among children under 5 years in Uganda: A secondary data analysis of the 2014 Malaria Indicator Survey dataset. Malar. J. 16 , 191 (2017).
Spielman, A., Sachs, J. & Malaney, P. THE malaria gap. Am. J. Trop. Med. Hyg. 71 , 141–146 (2004).
Download references
Acknowledgements
The study was based on data collected by the lead author and five trained field assistants Ibrahim Adeniran, Peter Durojaye, Ayadi Pius Akinwande, Oluwatosin Clement Adeola, Akindolire Ayobami Desmond who are post-graduate students from FUTA under the supervision of the lead author. The supports of Emmanuel Eze, Olatunji Johnson, Peter Macharia, and Tobias Matusch during the development of this work are appreciated. This work was supported by Pädagogische Hochschule Heidelberg and open-access publication fee financial support was made available by Heidelberg University, Germany.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Institute of Geography & Heidelberg Centre for Environment (HCE), Heidelberg University, Heidelberg, Germany
Taye Bayode & Alexander Siegmund
Department of Geography-Research Group for Earth Observation (rgeo), UNESCO Chair on World Heritage and Biosphere Reserve Observation and Education, Heidelberg University of Education, Heidelberg, Germany
You can also search for this author in PubMed Google Scholar
Contributions
T.B: conceptualisation; data curation; formal analysis; methodology; validation; visualisation; writing—original draft, writing—review & editing; project administration. A.S: supervision; project administration; writing—review & editing.
Corresponding author
Correspondence to Taye Bayode .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bayode, T., Siegmund, A. Identifying childhood malaria hotspots and risk factors in a Nigerian city using geostatistical modelling approach. Sci Rep 14 , 5445 (2024). https://doi.org/10.1038/s41598-024-55003-x
Download citation
Received : 11 October 2023
Accepted : 19 February 2024
Published : 05 March 2024
DOI : https://doi.org/10.1038/s41598-024-55003-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Urban health
- Spatial variability
- Childhood malaria
- Geostatistics
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Advanced search
- Peer review
AHRO Reviews of Nursing & Midwifery
Human malaria infection in nigeria: critical review of prevention and control techniques.
- Knowledge, Attitude And Uptake Of Covid-19 Vaccine Among Health Care Workers Of University Of Maiduguri Teaching Hospital, Nigeria
- Record : found
- Abstract : found
- Article : found

- Download PDF
- Review article
- Invite someone to review
Abstract
Human malaria infection is among the leading global parasitic diseases which have substantial effects on all facets of human life. A series of measures have been devised to prevent and control malaria infection, including vaccines and prophylaxis. Nigeria, the most populous country in Sub Saharan Africa, is burdened by the effect of malaria infection. This review critical analysis various preventive and control measures employed in malaria infection with a focus on Nigeria.
Author and article information
Affiliations, author notes, author information.
This work has been published open access under Creative Commons Attribution License CC BY 4.0 , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Conditions, terms of use and publishing policy can be found at www.scienceopen.com .
Uzochukwu Benjamin SC, Chiegboka Lausdeus O, Enwereuzo Chibuike, Nwosu Usonwanne, Okorafor David, Onwujekwe Obinna E, Uguru Nkoli P, Sibeudu Florence T, Ezeoke Ogochukwu P. Examining appropriate diagnosis and treatment of malaria: availability and use of rapid diagnostic tests and artemisinin-based combination therapy in public and private health facilities in south east Nigeria. BMC Public Health . Vol. 10(1)2010. Springer Science and Business Media LLC. [ Cross Ref ]
Arora Navneet, Anbalagan Lokhesh C, Pannu Ashok K. Towards Eradication of Malaria: Is the WHO’s RTS,S/AS01 Vaccination Effective Enough? Risk Management and Healthcare Policy . Vol. Volume 14:1033–1039. 2021. Informa UK Limited. [ Cross Ref ]
Islam Md. Saiful, Sujan Md. Safaet Hossain, Tasnim Rafia, Sikder Md. Tajuddin, Potenza Marc N., van Os Jim. Psychological responses during the COVID-19 outbreak among university students in Bangladesh. PLOS ONE . Vol. 15(12)2020. Public Library of Science (PLoS). [ Cross Ref ]
Comment on this article
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: malaria molecular epidemiology current situation in africa.

- 1 Department of Epidemiology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 2 Laboratory for Antimalarial Resistance Monitoring and Malaria Operational Research, National Institute of Communicable Diseases (NCID), Johannesburg, South Africa
- 3 Biosciences Department, Kenya Medical Research Institute (KEMRI) Wellcome Trust Research Programme, Kilifi, Kenya
Editorial on the Research Topic Malaria molecular epidemiology current situation in Africa
Malaria remains a significant public health burden in many of the 85 malaria-endemic countries, the majority of which are in sub-Saharan Africa (sSA) ( 1 ). The value of molecular surveillance in evidence-based decision-making was clearly demonstrated during the COVID-19 pandemic. National Malaria Control Programmes (NMCPs) across Africa are now using of malaria molecular surveillance and epidemiology data to guide intervention selection and targeting to help them eliminate malaria. The four articles in this research topic highlight the importance of molecular epidemiology in informing and improving malaria surveillance strategies.
One of the threats to malaria elimination is the emergence and spread of Plasmodium falciparum parasites with deletions in the histidine-rich protein 2 and 3 ( hrp2/3 ) genes. Malaria parasites with these deletions evade detection by hrp2 -based RDTs, the preferred point-of-care diagnostic across most of sSA, increasing the risk malaria-related morbidity and mortality as well as the chances of onward transmission ( 2 , 3 ). For evidence-based decision on malaria diagnostics, it is essential that NMCPs have accurate and current data on the prevalence and distribution of parasites carrying these deletions.
Duah-Quashie et al. , determined the prevalence of parasites with hrp2/3 gene deletions in symptomatic children from 10 sentinel sites located across three different ecological regions in Ghana between 2015 and 2020. Sequence data generated from archived dried blood spots were analyzed for deletions and polymorphisms in the hrp2 and hrp3 genes. Of the 2,540 samples analyzed, 30.7% carried hrp2 deletions and 17.2% hrp3 deletions, with the prevalence of these gene deletions increasing over time. These findings suggested a possible decrease in sensitivity in the ability of hrp2 -based RDTs to detect malaria in Ghana and calls for increased surveillance.
Similar to Duah-Quashie et al. , Okanda et al. , investigated the prevalence of hrp2/3 gene deletions in malaria parasites collected from symptomatic and uncomplicated malaria patients in Kilifi, Kenya between November 2019 and February 2020. From the 345 samples collected none of the 11 RDT-negative and microscopy positive samples carried both the hrp2 or hrp3 gene deletion. However, an extension of the criteria to increase the sample size using qPCR positive samples identified a low prevalence of both hrp2 and hrp3 gene deleted parasites at 2.1%. The findings from these studies underscore the importance of constant surveillance and the need for novel cost-effective point-of care malaria diagnostic.
The third article in this collection by Matrevi et al. , investigated the almost inevitable problem of the emergence and spread of drug-resistant malaria parasites. Artemisinin-based combination therapies (ACTs) are the recommended treatment for uncomplicated malaria as they are fast acting and highly efficacious. Currently these drugs are the most widely used antimalarials in Africa, so the emergence and spread of resistance to ACTs poses a significant risk to sSA's malaria control/elimination efforts ( 4 ). Monitoring the prevalence of molecular markers associated with antimalarial resistance enables the early detection of and response to emerging resistance. In their study, Matrevi et al. , determined the prevalence of mutations in nine P. falciparum genes associated with resistance to artemisinin derivatives, lumefantrine, chloroquine, quinine, sulphadoxine and pyrimethamine in Ghana. The 1,170 parasite samples assessed were collected over five transmission seasons between 2007 and 2018 from symptomatic children aged 9 years and younger with uncomplicated malaria. The prevalence of parasites carrying mutations in the P. falciparum falcipain 2 gene, potentially associated with artemisinin-partial resistance, increased over the study duration, while no known mutations associated with artemisinin-partial resistance were detected in the P. falciparum coronin gene. Mutation in the P. falciparum cycteine desulfurase gene, possibly associated with lumefantrine resistance, also increased over the study period. These increases in mutation prevalence may be associated with recent reports of decreasing ACT efficacy in Ghana and highlights the need for sustained molecular surveillance to mitigate the risk of drug resistant parasites becoming established in Ghana.
The final paper of this research topic by Arambepola et al. , investigated the impacts of how and when sampling is conducted on the determinants of P. falciparum population structure. Malaria genomic data have been used to understand changes in transmission intensity and parasite relatedness. The more closely related parasites are, the closer they are on a transmission network, potentially suggesting a foci of local transmission. This information can be used by NMCPs to inform control strategies. However, in areas of moderate to high transmission, the complexity of infections makes inferring relatedness challenging.
Arambepola et al. , used two measures of relatedness to investigate population structure in a moderate transmission setting in Kenya. The model developed was then used to assess the power of genomic data to determine population structure under different sampling schemes, levels of missing data and transmission settings. The study revealed that infections sampled closer in time were more likely to genetically similar and less differentiated compared to those sampled further apart. However, there was limited evidence of spatial (village-level) structure. Power to estimate relatedness decreased as the level of missing data increased but was not impacted when only sampling symptomatic individuals. Data from this study suggest that active dense sampling can detect population structure, even when certain data are missing, but not when there are high levels of connectivity between different regions. More research is required to address these short comings.
Considering other epidemiological factors that were not discussed in the articles under this topic is the invasion of Anopheles stephensi into the Horn of Africa which is rapidly spreading in the region into East Africa and as far west into Nigeria and Ghana. This new occurrence calls for effective entomological surveys in African countries as this vector will enhance urban malaria spread and P. vivax transmission. It is worth mentioning the promising gains that can be made by the recent exciting efficacy data from the R21 vaccine bringing hope to reduction of malaria prevalence in Africa.
The work presented in this supplement, highlights the value of malaria molecular epidemiology in guiding evidence-based strategic planning and surveillance strategy implementation by NMCPs to advance elimination efforts. Malaria molecular epidemiology is an essential tool in the elimination toolbox of all NMCPs.
Author contributions
KT: Writing – original draft. ND-Q: Writing – review & editing. JR: Writing – review & editing. LO-O: Conceptualization, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. (2023). World Malaria Report 2023.
2. Greenwood B. Artemisinin-resistant and HRP-negative malaria parasites in Africa. N Engl J Med . (2023) 389:1162–4. doi: 10.1056/NEJMp2309142
PubMed Abstract | Crossref Full Text | Google Scholar
3. Mihreteab S, Platon L, Berhane A, Stokes BH, Warsame M, Campagne P, et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N Engl J Med . (2023) 389:1191–202. doi: 10.1056/NEJMoa2210956
4. Siddiqui FA, Liang X, Cui L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int J Parasitol Drugs Drug Resist . (2021) 16:102–18. doi: 10.1016/j.ijpddr.2021.05.007
Keywords: hrp2/3 , drug resistance, molecular epidemiology, molecular surveillance, molecular markers
Citation: Tandoh KZ, Duah-Quashie NO, Raman J and Ochola-Oyier LI (2024) Editorial: Malaria molecular epidemiology current situation in Africa. Front. Epidemiol. 4:1400612. doi: 10.3389/fepid.2024.1400612
Received: 13 March 2024; Accepted: 19 March 2024; Published: 3 April 2024.
Edited and Reviewed by: Shailendra Saxena , King George’s Medical University, India
© 2024 Tandoh, Duah-Quashie, Raman and Ochola-Oyier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lynette Isabella Ochola-Oyier [email protected]
This article is part of the Research Topic
Malaria molecular epidemiology current situation in Africa
- Research article
- Open access
- Published: 14 April 2016
Malaria Parasitaemia and the use of insecticide-treated nets (INTs) for malaria control amongst under-5 year old children in Calabar, Nigeria
- Anthony Achizie Iwuafor 1 ,
- Chukwudi Charles Egwuatu 2 ,
- Agwu Ulu Nnachi 3 ,
- Ita Okokon Ita 1 ,
- Godwin Ibitham Ogban 1 ,
- Comfort Nneka Akujobi 2 &
- Tenny Obiageli Egwuatu 4
BMC Infectious Diseases volume 16 , Article number: 151 ( 2016 ) Cite this article
4744 Accesses
11 Citations
3 Altmetric
Metrics details
Malaria remains a major cause of febrile illness in Nigeria and interventions to reduce malaria burden in Nigeria focus on the use of insecticide-treated nets. This study determined the prevalence of malaria parasitaemia and the use of insecticide-treated nets (ITNs) for the control of malaria amongst under-five year old children in Calabar, Nigeria.
A total of 270 under-5 year old children were recruited and structured questionnaires were used to obtain information on the background characteristics of the respondents from their caregivers. Capillary blood samples were collected from each of the patients through finger-pricking and tested for malaria parasites by Rapid Diagnostic Test and microscopy.
An overall parasitaemia prevalence of 32.2 % (by Rapid diagnostic test kit [RDT]) and 40.1 % (by microscopy) were obtained in this study. Forty-six (45.5 %) of the febrile patients had malaria parasitaemia (by RDT) or 41 (59.4 %) by microscopy. One hundred and fifty (55.6 %) of the caregivers acknowledged the use of nets on doors and windows for malaria prevention and control. One hundred and thirty-nine (51.5 %) mentioned sleeping under mosquito net while 138 (51.1 %) acknowledged the use of insecticide sprays. Although 191 (71.5 %) of the households possessed at least one mosquito net, only 25.4 % of the under-5 children slept under any net the night before the survey. No statistically significant reduction in malaria parasitaemia was observed with the use of mosquito nets among the under-5 children. Almost all the respondents (97.8 %) identified mosquito bite as the cause of malaria. Fever was identified by the majority of the respondents (92.2 %) as the most common symptom of malaria.
Conclusions
The findings of the study showed high prevalence of parasitaemia and that fever was significantly associated with malaria parasitaemia. Mosquito net utilization among the under-fives was low despite high net ownership rate by households. Therefore, for effective control of malaria, public health education should focus on enlightening the caregivers on signs/symptoms of both uncomplicated and complicated malaria as well as encourage the use of ITNs especially among the under-fives.
Peer Review reports
Febrile illness is the most common and important component of malaria syndrome in sub-Saharan Africa [ 1 ]. Malaria remains one of the most widespread diseases affecting human race in tropical and sub-tropical regions of the world [ 2 ]. According to World malaria report, an estimated 3.3 billion people were at risk of malaria in 2010. Of this total, 1.2 billion were at high risk (>1 case per 1000 population), 47 % of them were living in Africa while 37 % came from South-East Asia [ 3 ]. There were 216 million episodes of malaria in 2010, and approximately 81 % or 174 million cases were in African Region. There were an estimated 655,000 malaria deaths in 2010 of which 91.0 % occurred in the African Region, and 86.0 % of the deaths involved children under the age of five years [ 3 ]. Malaria is caused by five different species of Plasmodium parasites and transmitted by female Anopheles mosquito [ 4 ]. In Nigeria, Plasmodium falciparum is the most dominant malaria parasites (>95.0 %), with P. ovale and P. malariae being responsible for the remainder. Dominant vector species are Anopheles gambiaes. l . and the Anopheles funestus group with some other groups playing a minor role [ 5 ].
Reductions in malaria disease burden, as documented in the recent World Malaria Reports [ 6 , 7 ], have coincided with the massive scale-up of malaria prevention measures, of which vector control was the predominant component, particularly in sub-Saharan Africa. The core malaria vector control interventions are insecticide-treated nets (ITNs) and indoor residual spraying (IRS), both of which deploy insecticides to kill malaria-transmitting mosquitoes [ 8 ].
The Federal Government of Nigeria, therefore, developed the National Malaria Control Strategic Plan 2000–2005, 2006–2010 which due to limited resources was targeted on the vulnerable groups of pregnant women and children under 5 years old. The interventions focused on the use of Long Lasting Insecticidal Nets [LLINs]/Insecticide-Treated Nets [ITNs] and Artemisinin Combination Therapy (ACT). The distribution of LLINs was integrated with Ante Natal Care, immunization as well as stand-alone campaigns [ 9 ]. Also, other organizations which include Faith-based organizations, Non-governmental organizations, and World Bank, with the goal of achieving universal access for the at-risk population of under 5 year old and pregnant women have been involved in free distribution of LLINs/ITNs [ 9 ]. Use of ITNs has been proven to be very effective in reducing malaria and malaria-associated morbidity among preschool children [ 10 ].
The role caregivers, especially mothers, play in attending to their febrile child is very important in reducing morbidity and mortality due to malaria. This is most important where the place is considered high risk for malaria, i.e., if > 5 % of fevers among children is caused by malaria. For example, a child with fever in a high-risk area who does not appear to have any other underlying reason for the fever, e.g., measles on physical evaluation should be considered as having malaria. Such a child should receive anti-malarial drugs. This is the WHO programme guidelines for Integrated Management of Childhood Illness [IMCI], used in resource limited settings to evaluate and treat children [ 11 ].
Despite the evidence-based benefits of sleeping under ITNs, and the efforts made by the Federal Government and Non-governmental Organizations to tame the public health scourge of malaria in Nigeria, some geopolitical zones of the country still record low average number of ITNs ownership/usage per household [ 12 ]. Different reasons have been advanced for poor ownership and usage of ITNs, by caregivers. Hence, this study investigated the prevalence of malaria infection and the use of insecticide-treated nets (ITNs) for malaria control among under-five children in Calabar, Nigeria.
Study design/setting
The study is descriptive and cross-sectional in design. It was carried out from November, 2012 to December, 2013 to determine malaria parasitaemia and the perception and practices of care-givers of under-five children on the use of ITNs amongst the under-five children. It was carried out in the University of Calabar Teaching Hospital, Calabar which is a second generation Teaching hospital in the country, Nigeria. The hospital currently has over 600 beds distributed between the three annexes and renders services in specialized areas in medicine such as paediatric surgery, haemodialysis, neuro-surgery, ophthalmologic surgery and maternal health.
Participants
The study target population consisted of women/men aged 15 – 50 years who had the responsibility of taking care of at least one Under-five year old child. A total of 270 under-5 children who came to the hospital as outpatients with their care-givers were recruited in the study.
Ethical considerations
Approval was obtained from the Research and Ethics Committee of the University of Calabar Teaching Hospital, Calabar, Nigeria. Informed consent was also obtained from the patients’ relatives. Those who declined consent were excluded from the study.
Sample size
Single population proportion formula (N = Z 2 pq/d 2 ) was used to determine the sample size assuming the ITN usage rate among under five children in the South-South zone of Nigeria to be 20.0 % [ 12 ] at 95 % confidence interval, 5 % marginal error, and 10 % non-response rate. This gave a sample size of 270 under five children.
Data collection
Data collection procedures.
A convenient sampling method was employed to select the calculated sample size of under-five caregivers/Under-five year old respondents. As many of the respondents who gave consent on each of their clinic day was enrolled into the study during the study period until the sample size was complete.
Each caregiver who attended clinic with their child (ren) was interviewed by trained interviewers using structured questionnaires (Additional file 1 ) adapted from NPC-NMCP Nigeria Malaria Indicator Survey [ 12 ]. The selection of the interviewers was based on the respondent’s ability to understand English and the local language (Efik/Ibibio language) because where necessary, the interviewer had to interpret the questionnaire in the language of the respondents. The questionnaire was pre-tested to check for comprehensibility of the questions as well as the procedures for conducting the interviews. The questionnaire elicited information on: background characteristics of respondents, knowledge of malaria symptoms, causes of malaria, ways to avoid malaria and knowledge of prompt treatment of children with fever. Other information that was captured by the questionnaire included: household possession and use of mosquito nets, source and cost of mosquito nets, reasons for non-use of nets and febrile illness-associated mortalities one year prior to survey (Additional file 1 ).
Sample collection and processing
Fresh capillary blood samples were collected aseptically from the recruited under-5 children using finger-pricking method as documented by Cheesbrough [ 13 ]. The sample was processed immediately using Paracheck Pf® Rapid diagnostic Test kit (Orchid Biomedical Systems, India). In this, a drop of the whole fresh capillary blood was applied to the sample well ‘A’ and immediately, the specimen was blotted. Six drops of the clearing buffer was then made into well ‘B’ and the setup was allowed to stand undisturbed for 15 min. At the end of 15 min, results were read as follows: if only one pink-coloured band appeared in the control window, test was interpreted as negative. In addition to the control band, if a distinct pink coloured band also appeared in the test window, test was interpreted as positive. Test was considered invalid/inconclusive if no bands appeared on the device. In that case, test was repeated with new device ensuring that the test procedure was followed accurately. One hundred and sixty seven (167) of the rapid diagnostic tests carried out were correlated with microscopy. In this, thick blood smears and thin blood films were made in the field (clinics) and transported to the Paediatrics side-laboratory, where it was stained using 10 % Giemsa for 10 min by standard techniques [ 13 ]. Each slide was examined for the presence or absence of malaria parasites. Each slide was declared positive if at least one parasite was found per 100 high power fields; else, it was reported as negative. In this study, finding of at least one malaria parasite per 100 high power fields is considered positive parasitaemia. Quality of the microscopic slides was ensured by cross checking both the negative and positive slides by other trained Microscopist. Fever was measured with clinical thermometer and was defined as an auxiliary temperature of ≥ 37.5 °C.
Statistical analyses
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software (version 20.0, SPSS Inc., Chicago, IL., USA). Continuous variables were presented as the mean ± standard deviation. Categorical variables were presented as actual numbers and percentages in table forms, or figures. All categorical variables were compared using Pearson’s Chi-square test or Fisher’s exact test. P -values < 0.05 were considered significant for all tests. The outcome variables considered were ownership of ITNs, Use of ITNs a night prior to interview and the effect of ITN usage on malaria parasitaemia amongst under-five year children. Multivariate logistic regression analysis was employed to explore the impact of independent variables such as the child’s age, care-givers age, care-givers tribe and care-givers level of education on the outcome variables. The regression model used predicted the logit, which is the natural log of the odds of having made one or the other decision:
ln (Odds) = ln (Ý/1-Ý) = b 0 + b 1 X 1 + b 2 X 2 + … + b p X p , where Ý is the predicted probability of the event which is coded with ‶0″ (Did not own ITN, Did not sleep under ITN and Negative Parasitaemia) rather than “1” (Own at least one ITN, Slept under an ITN, and Positive Parasitaemia). “1-Ý” is the predicted probability of the other decision and X 1 through X p are distinct independent (predictor) variables and b 0 through b p are the regression coefficients. The “-2 Log Likelihood” statistics and Hosmer-Lemeshow test were used to show how well the model predicts the decisions. Two tailed P -values was reported, odds ratios and 95 % Confidence interval was used to estimate the association between dependent (outcome) variables and independent variables.
Baseline characteristics
A summary of the baseline characteristics of the respondents is given in Table 1 . A total of 270 care-givers (all female) participated in the study. The mean age of the participants was 29.7 ± 5.6 standard deviation. Seventy seven percent of them fell into age-group of 26–35 years. The mean age-group (months) of the under-5 year old children whose care-givers were interviewed was 25.5 ± 17.3 standard deviation. The infants constituted 21.8 % of the children. The Efik tribe (53 %), followed by Igbo (19.5 %), were the most populous tribe in the study. Most of the respondents had tertiary education (58.9 %), only three (1.1 %) did not have any formal education whatsoever. One hundred (40.3 %) of the care-givers were civil servants,87 (35.1 %) were self-employed while 25 (10.1 %) were house-wives.
ITNs ownership and usage
Table 2 shows a summary of ITN-associated questions and answers. The minimum and maximum numbers of ITNs owned by any household were 1 and 5 respectively, with mean, standard deviation of 2.4 and ±1.8. More than two-thirds (191/267; 71.5 %) of the care-givers had at least one ITN per household. Of the number that had at least one ITN, one hundred and seventy eight (93.2 %) of them obtained the nets free of charge, only 13 (6.8 %) procured theirs via purchasing.
Majority of the households (129/191; 67.5 %) that owned at least one net got them from the Primary health centre closest to them, twenty-nine (15.2 %) of them got theirs from Non-Governmental Organizations. Only 2 (1.0 %) got theirs from the Patent medicine store. About half the population of the respondents obtained their nets within 2–12 Months prior to the study, only 14 (7.5 %) got their nets more than 2 years prior to the study. Almost all the nets (93.7 %) were already-treated nets by the time they were procured. Twenty-one (11.0 %) care-givers admitted a secondary treatment of their nets by themselves after procurement.
Sixty-eight (25.4 %) of the children studied were reported to have slept under any net the night before the survey. Amongst thosewho had nets (191), only 68 (35.6 %) had at least one under-5 year old child who slept under the net the night before the study. Those care-givers’ household in which no child slept under a net the night before this study gave different reasons for not sleeping under the net. Some of the reasons included: ‘weather was too hot’ (77.2 %), ‘difficulty at hanging the net’ (7.3 %), ‘there were no mosquitoes’ (7.3 %) (Fig. 1 ).
Reasons for not sleeping under ITN, the night prior to study
Reasons given for not having at least one ITN included: ‘nets not available (65.3 %), ‘don’t like to use nets’ (13.9 %), and ‘there is no mosquito’ (12.5 %).
Caring for a febrile child
Figure 2 shows what the care-givers do when any under-5 year old child under them develops fever. This question applied only to those who admitted that any of the children under their care developed fever within two weeks prior to the study. Fifty-two (48 %) would take the child to any government hospital nearby, 31 (28.4 %) would administer ‘self-treatment’, while 11 (10.1 %) would consult a pharmacist. Other treatment modalities included taking the child to government health centre 7 (6.9 %), Private hospital 5 (4.9 %), and Chemist shop 3 (2.9 %).
The first treatment modalities embarked upon by the Care-givers on instances of febrile illness
Malaria parasitaemia
Two hundred and seventy (270) patients were recruited, RDT tests were carried out on 258 patients and 32.2 % (83/258) had positive parasitaemia. On the hand, one hundred and sixty seven (167) were tested using slide microscopic method, 40.1 % (67/167) had positive slide (parasitaemia).
Two hundred and sixty-four (97.8 %) care-givers correctly identified mosquito bite as the cause of malaria. One care-giver (0.4 %) admitted that it was due to ‘too much intake of oily food’ while 5 (1.8 %) did not know the cause of malaria. Majority of the respondents, 249 (92.2 %) correctly identified ‘hotness of the body or fever’ as the most common sign and symptom of malaria. Other signs and symptoms identified included: ‘generalized body weakness’ (59.3 %), ‘loss of appetite’ (47.4 %), and headache (43.0 %). On malaria prevention, one-hundred and fifty (55.6 %) admitted that ‘using mosquito nets at doors/windows’ can be a means of preventing mosquito bite and of course malaria, one hundred and thirty nine (51.5 %) of them accepted that ‘sleeping under ITN prevents malaria, while 79 (29.3 %) opted for ‘spraying insecticide every night’.
Fever and mortality
One hundred and nine respondents (41.6 %) admitted that at least one under-5 year child under their care had fever 2 weeks prior to the index study; one hundred and fifty respondents (57.3 %) did not have any child with fever for the same period as in the above. For the question to determine incidence of under-five mortality from among the care-givers, a total of 35 under-5 deaths were recorded. Out of the 35 deaths, fourteen (40.0 %) were fever-associated, twelve (34.3 %) were non-fever associated, while for 9 (25.7 %), it could not be ascertained whether the deaths were associated with febrile illness or not.
There was significant association between those with fever and presence of parasitaemia. Those with parasitaemia either by RDT or microscopy testing were more likely to develop fever than those with no parasitaemia, ( p < 0.001). There was no significant difference between tribe, care-givers’ age and ownership of ITNs.
The proportion of febrile patients that had parasitaemia by RDT testing was 55.4 % (46/83), while that by microscopy was 61.2 % (41/67).
The proportion of children under the age of five years that had positive parasitaemia was less among care-givers who had tertiary education (46.3 %), than among those who did not (53.7 %) ( p > 0.05). Malaria parasitaemia increased with the age of the child; it was 15.5 % for age group 1–11 months, 38.0 % for age group 12–35 months and 46.5 % for age group 36–59 months ( p > 0.05).
Table 3 shows the result of logistic regression of household ITNs use on parasitaemia among under-5. The predictor (independent) variables included were under-5 child-ITN usage, caregiver’s age, caregiver’s education and child’s age. The outcome (dependent) variable measured was presence or absence of parasitaemia among under-5 children that slept under any mosquito net and those that did not. Here, under-5 child sleeping under a mosquito net a night before survey, lowering child age, increasing caregivers age, and higher caregivers educational level were associated with lower odds of developing parasitaemia, though none was statistically significant. Table 3 also shows no significant association between treatment modalities for a febrile child and tribe, age or educational levels of the care-givers.
A statistically significant association was observed between ownership of ITNs and care-givers education ( p < 0.05) (Table 4 ). No significant association was found between sleeping under an ITN and tribe, child’s age, educational levels of the care-givers ( p > 0.05) (Table 4 ).
However, the result of the multivariate logistic regression shows that care-givers with no, primary or secondary education were less likely to have bed nets than their counterparts with tertiary education even after other determinants –age and tribe were adjusted for ( p < 0.05; OR = 0.52). A similar model was fitted for possible predictors for ‘sleeping under the net’. Only care-givers age gave a statistically significant result, with younger care-givers not likely going to have under-five children that will sleep under a net ( p > 0.05) (Table 4 ).
In this study, the prevalence of parasitaemia (by RDT) was 32.2 % while that by microscopy was 40.1 %. The proportion of febrile patients that had parasitaemia by RDT was 55.4 % while that by microscopy was 61.2 % ( p < 0.05). This malaria prevalence of 40.1 % was higher than 12 % reported in Tanzania [ 14 ] and 6 % reported in Pakistan [ 15 ] and lower than 53.8 % reported in a relatively similar study in Nigeria [ 12 ].
Mazigo et al . [ 14 ], in their study found out that 52.7 % of the children that had positive parasitaemia were also febrile. In a similar study in Gabon, about 40 % of the children in a hospital who were presented with fever or history of fever also had malaria parasite-positive blood film [ 16 ]. Nigeria Malaria Indicator Survey reported a much lower proportion of febrile children who tested positive for malaria: 11 % using RDT and 12 % using microscopy than obtained in our study [ 12 ]. This result indicates that for the majority of the children, malaria parasitaemia occurred without fever whereas in this index study, more than half (61.2 %) of the children who had malaria parasitaemia also had fever.
Earlier studies had reported higher proportion of febrile patients that were parasitaemic. Ejezie and Ezedinachi [ 17 ], in their study in Calabar, found that 74.9 % of the parasitaemic subjects had high grade temperatures of 38 °C and above. Mabunda et al . [ 18 ] also reported that 72.4 % of the febrile children in their study were parasitaemic. Acquired protective immunity could offer an acceptable reason for presence of malaria parasitaemia without febrile illness and it has been shown to increase with age [ 19 ]. Difference in season of study could be a plausible reason for the variation in malaria prevalence [ 20 ]. Malaria prevalence, in this study, though not statistically significant, was found to increase with the age of the child regardless of the test used. This was in agreement with the findings of Nigerian Malaria Indicator Survey of 2010 [ 12 ].

Malaria control and prevention
During the survey, caregivers were asked if they knew specific measures to prevent malaria attack. Fifty-five point six percent (55.6 %) of the caregivers mentioned using nets on doors and windows, 51.5 % mentioned sleeping under a mosquito net, while other responses were: destroying mosquito breeding places (51.1 %) and spraying insecticides every night (29.3 %). The Nigerian Malaria indicator survey of 2010 [ 12 ] reported sleeping under a mosquito net (77.2 %, South-South region), destroying mosquito breeding places (8 %), and spraying insecticides (20 %). There is no readily available answerto the 25 % decline in awareness concerning sleeping under the mosquito nets as an effective means of preventing malaria. Perhaps, the perceived rise in distribution of ITNs over the region was not accompanied by adequate information, education and communication (IEC).
ITNs Ownership
Net ownership, as determined by possession of at least one mosquito net in a household was found to be 71.5 %. The proportion of households who had at least one mosquito net has risen far above the reported proportion of 42 % obtained in 2010 [ 12 ], 8 % in 2008 (NDHS, 2008) and 2 % in 2003 [ 21 ]. This finding was consistent with the finding of one recent study in Abuja, Nigeria [ 22 ] in which mosquito net ownership pre-and post-intervention study were 58 % and 100 % respectively. Daboer et al . [ 23 ] in their survey in Jos, Nigeria reported that 55.3 % of caregivers of under-5 children owned nets; a rise the authors attributed to the on-going campaigns of ITN distribution. A contrary finding was obtained in a study in Guinea [ 24 ] in which there was a decline in net ownership from 97 % in 2008 to 65 % in 2009. This sharp scale-up in bed net ownership by households in this current study is traceable to more aggressive ITN mass distribution through the support of the Global Fund, DFID, World Bank and Support for the National Malaria Control Programme, and the MDG-assisted funds in Nigeria. The nets were given free of charge most of the time, via Primary Health Centres which are usually at close proximities to households including rural dwellers.
Some variables are known to influence ownership of mosquito nets. Some of these factors have been reported to include level of education, wealth index, family size and residence among others [ 25 ]. In this study, variables like caregiver’s tribe, age and education were tested; only caregiver’s educational level was statistically significantly associated with net ownership. It can also be used as a predictor for net ownership, with care-givers with lower educational attainments more likely to own at least one mosquito net than those with higher educational status. Oresanya et al . [ 26 ] observed that the presence of an educated caregiver in the household raised the odds of owning a net by 42 % in the north, while this was not predictive in the south part of Nigeria after controlling other variables. Whereas the above mentioned study tested education and none education, ours, conducted also in the southern part of Nigeria examined higher and lower educational status. Contrary to our finding, a similar study in Kenyareported that higher education was associated with possession of mosquito nets. Higher education, we expected, should have the ability to better equip caregivers with necessary information about the importance of ownership and utilization of mosquito nets in malaria prevention and control. Obtaining a contrary finding in our study is indeed puzzling.
About two-third of the respondents who do not own at least a net stated that ‘nets were not available’ as a reason for not having one. For most of them, the non-availability of the nets meant they did not know where/how to get one. Other reasons given for not having mosquito net included: ‘does not like to use net’ and ‘there is no mosquito in my residence’. Misconceptions about causes of malaria and prevention modalities are also valid reasons for non-ownership and utilization of nets [ 27 ].
ITNs Utilization
Twenty five-point four percent (25.4 %, 68/267) of children under age 5 among those interviewed slept under a net the night before the survey. Compared to previous NDHS and NMIS surveys in Nigeria, the sustained rise in net utilization was lost. The percentage of children under age five who slept under ITNs has increased steadily and substantially from 6 % in 2003 [ 21 ], to 12 % in 2008 [ 28 ] and to 26.7 % in 2010 by NMIS survey (26.7 % specifically for South-South zone where Calabar belongs, and 30.3 % generally) [ 12 ]. The decline in net utilization was also noticed among households that owned at least one ITN, only 35.6 % slept under an ITN the night before the survey as against 55.1 % in NMIS survey [ 12 ]. The rate of net use has varied over time and in different geopolitical regions in Nigeria. Oresanya et al . [ 26 ] in their study in Abuja reported net utilization of 11.5 %, while 37.2 % was reported in Rivers State by Tobin-West and Alex-Hart [ 29 ].
This current study showed that whereas 71.5 % caregivers with under- 5 children owned at least one ITN, only 25.4 % of the children used a net a night before the survey. The high discrepancy (46 %) between ownership and utilization of ITN in this study could not easily be explained out and as such calls for great concern. It probably shows that there is need for adequate motivation before ownership will translate to utilization. It does appear that in the last 2 years before this index study, large scale net campaigns and distribution was carried out in Calabar and since ITNs were given free of charge, caregivers were poised to have them; however, they lacked the motivation to use them. Tobin-West and Alex-Hart [ 29 ] also reported a similar finding in their study where only one-third of those that owned nets, slept under a net the night before survey. In another study, out of the 55.3 % caregivers with under- 5 children that owned ITNs, only 40 % utilization was recorded a night before study.
The most common reason given among the caregivers; that had at least one ITN for not using it was that the weather was too hot (77.2 %). This same reason has also been reported in other similar studies and has been attributed to the hot tropical climate of the sub-Saharan African region [ 12 , 26 , 30 , 31 ]. Other reasons were: having difficulties hanging it, and that there were no mosquitoes around their residence and these findings were consistent with an earlier study [ 12 ].
Higher educational levels in previous studies [ 22 , 26 ] have been associated with appropriate net usage. In our study, possession of higher education was not statistically associated with net utilization ( p > 0.05). Demographic characteristics like child age, care-givers’ age, education, and ethnicity have been known as possible predictors of net use in other studies [ 26 ]. In this study, only care-givers age was established as predictor ( p < 0.05). Our study showed that older care-givers are more likely to have their children sleep under net than younger care-givers. This finding could have been as a result of past experience these older care-givers have had with caring and parenthood.
Impact of ITNs on the under-five children
Assessment of the impact of ITN coverage and/or utilization on health outcomes is usually difficult. This is usually due to poor routine health information and vital registration systems; making determination of malaria-specific mortality and morbidity almost impossible [ 32 ]. Few studies that have attempted analysing the impact of mosquito net ownership and usage on children have used different approaches [ 33 , 34 ]. In this study, the relationship between household use of ITNs among under-5 children and malaria parasitaemia was analysed using logistic regression analysis. We observed a 32 % reduction in malaria parasitaemia among under-5 net users which was not statistically significant. Lack of statistical significance may have stemmed from the small sample size involved. Previous studies by Lim et al. [ 32 ] reported a pooled relative of 24 % reductionin parasitaemia prevalence in children while Lengeler [ 35 ] reported a 50 % reduction in clinical episodes and malaria parasitaemia.
Care-givers knowledge of malaria and treatment decision to under-5 febrile child
Malaria prevention and control measures aim at preventing mortality and reducing morbidity and also malaria-associated economic losses. Lack of knowledge about malaria and its mode of transmission will hamper appropriate preventive measures. In our study, care-givers were asked questions to ascertain their knowledge of causes, signs and symptoms, and means of prevention of malaria. Almost all the caregivers (97.8 %) identified mosquito bite as the cause of malaria. This finding was consistent with those of previous studies [ 12 , 36 ] but higher than the finding by Oreagba et al . [ 37 ]. This awareness is a good one and could have contributed to the high level of ownership of mosquito nets among care-givers observed in this study. Most of the care-givers also identified fever as the commonest symptom of malaria. This finding was in agreement with the findings of previous studies [ 38 , 39 ]. The recognition of fever by the majority of the caregivers as a symptom of malaria is a welcome development because early treatment depends on prompt recognition of symptoms and signs of malaria in the household [ 40 ]. A worrisome finding was that only 4.1 % of the caregivers acknowledged that disorientation/incoherent speech, which occurs in severe malaria, was a complication of malaria. The implication of this finding is that most caregivers would exclude malaria much the same way they behave when their children have febrile convulsion and might resort to other means of intervention like going to the Traditional healer [ 41 ]. The association between ‘incoherent speech’ and severe childhood malaria should be highlighted and incorporated into health education and health promotion programmes. This will correct anomalies in care-givers’ treatment seeking behaviour. Ability of the caregivers to recognize danger signs of malaria is an important factor for early home management or for seeking treatment at health facility [ 42 ].
Treatment seeking behaviour among caregivers has been shown to be related to the cost, availability and cultural beliefs about the causes and effective cures for malaria-like symptoms [ 43 ]. Among the caregivers who had under-5 children with febrile illness two weeks prior to this survey, 47.7 % sought for treatment first at government hospital nearby. This finding was low compared to reported value of 65.6 % in a previous study in Nigeria [ 23 ] and 71.5 % in a study in Ethiopia [ 36 ]. The finding in this study that 28.4 % of the caregivers would resort to self-treatment at home was fairly high compared to 1.4 % found in Ethiopia [ 36 ] and 3 % in Nigeria. Only few caregivers (10.1 %) resorted first to Chemist/Patent medicine vendors compared to 37 % [ 37 ] and 57.4 % [ 12 ] reported by previous studies. Unlike other previous similar studies in Nigeria [ 44 , 45 ], where traditional/herbal homes were among preferred health facilities care-givers sought after, none of the care-givers in this study accepted ever going to the herbalist for treatment of their febrile children. Perhaps, variation in study areas between the previous studies which were carried out in rural areas and this current study carried out in the metropolitan town of Calabar, Nigeria could explain the difference. The preferred choice of the care-givers to seek treatment first in government hospital for their febrile children may not be unconnected with the high literacy level of the respondents who probably knew they would get better care delivery from such centres. The cost of health care delivery has been one of foremost determinants of treatment seeking behaviour of care-givers [ 46 ]. The free medical services in government hospital in Ethiopia could have contributed to higher proportion of health seekers that used government hospitals there, than it was found in this study. The high number of under-5 care-givers that indulged in self-treatment of their febrile children at home in this study highlights the need for Health extension workers to educate care-givers on home-based management of malaria. Such enlightenment programmes should include recommended anti-malarial drugs and dosages and to be able to detect signs and symptoms of severe malaria that may demand expertise management.
Limitations of the study
Our study has some limitations. First, we tried to replicate a Nigerian Malaria Indicator Survey (NMIS) of 2010 [ 12 ], however we believed that the findings would not very much compare with NMIS; in that, ours was facility-based and the tools used were different. We observed that our sample size was small, and thought this could have been responsible for the study’s lack of power to detect many significant relationships from our data. The tool used in our research (for example questionnaire) encouraged “self-reported data”, not allowing for independent verification. Self-reported data has many sources of potential bias we considered as limitations such as selective memory and exaggeration. Finally, the sampling technique we used (convenience sampling) helped us to have easy access to the study participants in good time, however it could have introduced sampling bias, not allowing for good representation of the entire population.
A parasitaemia prevalence of 40.1 % obtained in this study can still be seen to be high considering recent scale up in malaria prevention campaigns in the area. Fever was significantly associated with malaria parasitaemia. This means a lot of febrile illnesses among the under-five children in this area might still be due to malaria infection. Respondents identified various methods that are used to prevent/control malaria infection, with majority acknowledging putting net at windows and doors, followed by sleeping under mosquito nets and the use of insecticide sprays. Household ownership of nets was very high compared to many recent studies, however, the net ownership did not translate to use as there was much discrepancy between ownership and usage of the net. There was no statistically significant reduction in malaria parasitaemia with the use of mosquito nets over non-use among the under five children studied, an effect that could have arisen due to smallness of sample size. The respondents demonstrated good knowledge of the cause and symptoms of uncomplicated malaria, however, only few knew the signs and symptoms of severe malaria. Majority of the respondents also demonstrated the deadliness of malaria among the under five children via their treatment seeking behaviours. Most of them would prefer to take their children to government hospital first, possibly hoping to obtain best care delivery there. Fairly good number of care-givers would rather prefer to ‘try their luck’ by giving self-medication at home first.
Abbreviations
artemisinin-combination therapy
confidence interval
department for international development
information, education and communication
integrated management of childhood illness
indoor residual spraying
insecticide-treated net
long lasting insecticidal nets
millennium develoment goal
Nigeria demographic and health survey
national malaria control programme
Nigerian malaria indicator survey
national population commission (NPC)
rapid diagnostic test
statistical package for social sciences
Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–98.
Article CAS PubMed Google Scholar
Ketema T, Bacha K, Alemayehu E, Ambelu A. Incidence of Severe Malaria Syndromes and status of immune responses among Khat Chewer malaria patients in Ethiopia. PLoS One. 2015;10(7), e0131212.
Article PubMed PubMed Central Google Scholar
World Health Organization. World malaria report. Geneva: World Health Organization; 2011.
Google Scholar
White NJ. How antimalarial drug resistance affects post-treatment prophylaxis. Malar J. 2008;7:9.
Federal Ministry of Health [FMOH]/National Malaria Control Programme [NMCP]. Strategic Plan 2009–2013: A road Map for Malaria control in Nigeria, Abuja. 2008.
World Health Organization. World malaria report. Geneva: World Health Organization; 2013.
World Health Organization. World malaria report. Geneva: World Health Organization; 2014.
Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:282.
National Malaria Control Programme [NMCP]/Roll Back Malaria [RBM]. NMCP/RBM Business Plan (2009–2010), Nigeria. 2009.
Muller O, Traore C, Kouyate B, Ye Y, Frey C, Coulibaly B, Becher H. Effects of insecticide-treated bednets during early infancy in an African area of intense malaria transmission: a randomized controlled trial. Bull World Health Organ. 2006;84:120–6.
World Health Organization. Handbook: Integrated Management of Childhood Illness [IMCI]. Geneva: WHO; 2005. p. 239–50.
National Population Commission (NPC) [Nigeria]. National Malaria Control Programme (NMCP) [Nigeria], and ICF International. Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: NPC, NMCP, and ICF International; 2012.
Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 1. Second edition. New York, USA: Cambridge University Press; 2006.
Book Google Scholar
Mazigo HD, Meza W, Ambrose EE, Kidenya BR, Kweka EJ. Confirmed malaria cases among children under five with fever and history of fever in rural western Tanzania. BMC Res Notes. 2011;4:359.
Hozhabri S, Luby SP, Rahbar MH, Akhtar S. Clinical diagnosis of Plasmodium falciparum among children with history of fever, Sindh, Pakistan. Int J Infect Dis. 2002;6(3):233–5.
Article PubMed Google Scholar
Dzeing-Ella A, Nze-Obiang PC, Tchoua R, Planche T, Mboza B, Mbounja M, et al. Severe falciparum malaria in Gabonese children: clinical and laboratory features. Malar J. 2005;4:1.
Ejezie GC, Ezedinachi EN. Malaria parasite density and body temperature in children under 10 years of age in Calabar, Nigeria. Trop Geogr Med. 1992;44(1–2):97–101.
CAS PubMed Google Scholar
Mabunda S, Aponte JJ, Tiago A, Alonso P. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malar J. 2009;8:74.
Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. ClinMicrobiol Rev. 2009;22(1):13–36.
CAS Google Scholar
Nkuo-Akenji T, Ntonifor NN, Ndukumu MB, Kimbi HK, Abongwa EL, Nkwescheu A, et al. Environmental factors affecting malaria parasite prevalence in rural Bolifamba, South-West Cameroon. Afr J Health Sci. 2006;13:40–4.
PubMed Google Scholar
National Population Commission (NPC) [Nigeria] and ORC Macro. Nigeria Demographic and Health Survey, NDHS, 2003. Abuja, Nigeria: NPC and ORC Macro; 2004.
Ashikeni MA, Envuladu EA, Zoakah AI. Malaria and the use of the Insecticide Treated Net (ITN) among under-five children in Kuje Area Council of the Federal Capital Territory Abuja, Nigeria. Int J Mosq Res. 2013;3(6):45–53.
Daboer JC, Chingle MP, Ogbonna C. Malaria Parasitaemia and Household use of Insecticide Treated Bed Nets: a cross-sectional survey of under-five in Jos, Nigeria. Niger Med J. 2010;51(1):5–9.
Garcia-Basteiro BL, Schwabe C, Aragon C, Baltazar G, Rehman AM, Matias A. Determinants of bed net use in children under five and household bed net ownership on Bioko Island, Equatorial Guinea. Malar J. 2011;10:179.
Stratton L, O’Neill MS, Kruk MS, Bell ML. The persistent problem of malaria: addressing the fundamental causes of a global killer. Soc Sci Med. 2008;67(5):854–62.
Oresanya OB, Hoshen M, Sofola OT. Utiliszation of insecticide-treated nets by under-five children in Nigeria: assessing progress towards the Abuja targets. Malar J. 2008;7:145.
Arogundade ED, Adebayo SB, Anyanti J, Nwokolo E, Ladipo O, Ankoma A. Relationship between care-givers misconceptions and non-use of ITNs by under-five Nigerian children. Malar J. 2011;10(170):1–10.
National Population Commission (NPC) [Nigeria] and ICF Macro. Nigeria Demographic and Health Survey, NDHS, 2008. Abuja, Nigeria: NPC and ICF Macro; 2009.
Tobin-West CI, Alex-Hart BA. Insecticide-treated bednet ownership and utilisation in Rivers State, Nigeria before a state-wide net distribution campaign. J Vector Dis. 2011;48:133–7.
Ordinioha B. The use of insecticide-treated bednet in a semi-urban community in south Nigeria. Niger J Med. 2007;16:223–6.
Eisele TP, Keating J, Littrell M, Larsen D, Macintyre K. Assessment of insecticide-treated bednet use among children and pregnant women across 15 countries using standardized national surveys. Am J Trop Med Hyg. 2009;80:2009–214.
Lim SS, Fullman N, Stokes A, Ravishankar N, Masiye F, Murray CJL, et al. Net benefits: A multi-country analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PLoS Med. 2011;8, e1001091.
Abdulla S, Schellenberg JA, Nathan R, Mukasa O, Marchant T, et al. Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under- 2 years in Tanzania: community cross sectional study. BMJ. 2001;322:270–3.
Article CAS PubMed PubMed Central Google Scholar
Noor AM, Moloney G, Borle M, Fegan GW, Shewchuk T, et al. The use of mosquito nets and the prevalence of Plasmodium falciparum infection in rural south central Somalia. PLoS One. 2008;3, e2081.
Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. The Cochrane Collaboration, Lengeler C, ed. Cochrane Database of Systematic Reviews. Chichester: John Wiley & Sons, Ltd. Available at: http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD000363/abstract.html ; 2004.
Yewhalaw D, Kassahun W, Woldemichael K, Tushune K, Sudaker M, Kaba D, et al. The influence of the Gilgel-Gibe hydroelectric dam in Ethiopia on care-givers’ knowledge, perceptions and health-seeking behaviour towards childhood malaria. Malar J. 2010;9:47.
Oreagba AL, Onajole AT, Olayemi SO, Mabadeje AFB. Knowledge of malaria amongst care-givers of young children in rural and urban communities in Southwest Nigeria. Trop J Pharm Res. 2004;3(1):299–304.
Sanjana P, Barcus MJ, Bangs MJ, Ompusunggu S, Elyazar I, Marwoto H, et al. Survey of community knowledge, attitudes and practices during a malaria epidemic in central Java, Indonesia. Am J Trop Med Hyg. 2006;75:785–9.
Deressa W, Ali A, Birhane Y. Maternal responses to childhood febrile illnesses in an area of seasonal malaria transmission in rural Ethiopia. Acta Trop. 2007;102:1–9.
Tanner M, Vlassoff C. Treatment-seeking behaviour for malaria: a typology based on endemicity and gender. Soc Sci Med. 1998;46:523–32.
Baume C, Helitzer-Allen D, Kachur P. Patterns of care for children malaria in Zambia. Soc Sci Med. 2000;51:1491–503.
Patel VL, Eisemon TO, Arocha JF. Causal reasoning and the treatment of diarrhoeal diseases by mothers in Kenya. Soc Sci Med. 1988;159:1061.
Lars O, Beth E. Malaria in the United Republic of Tanzania: cultural considerations and health seeking behaviour. Bull World Health Organ. 2000;78(11):1352–7.
Chukwuocha MU, Nwankwo OB, Amadi NA, Esomonu CO, Dozie INS, Ikegwuoha EA, et al. Treatment seeking behaviour of mothers for febrile children in some rural parts of Imo state Nigeria: Implications for Home management of malaria in Endemic areas. Int J Trop Med. 2009;4(3):132–5.
Agu AP, Nwojiji JO. Childhood malaria: mothers’ perception and treatment-seeking behavior in a community in Ebonyi State, South East Nigeria. J Com Med Prim Health Care. 2005;17(1):45–50.
Hill ZC, Kendali P, Arthur B, Kirikwood E, Adjei E. Recognizing childhood illness and their traditional explanations: exploring options for care-seeking interventions in the context of the IMCI strategy in rural Ghana. Trop Med IntHealth. 2003;8:668–76.
Article Google Scholar
Download references
The authors declare that no external funding was received for this study.
Author information
Authors and affiliations.
Department of Medical Microbiology and Parasitology, College of Medical Sciences, University of Calabar, Calabar, Nigeria
Anthony Achizie Iwuafor, Ita Okokon Ita & Godwin Ibitham Ogban
Department of Medical Microbiology and Parasitology, Faculty of Medicine, Nnamdi Azikiwe University, Nnewi Campus, Nigeria
Chukwudi Charles Egwuatu & Comfort Nneka Akujobi
Department of Immunology, Faculty of Medicine, Nnamdi Azikiwe University, Nnewi Campus, Nigeria
Agwu Ulu Nnachi
Department of Medical Microbiology and Parasitology, Faculty of Science, University of Lagos, Akoka, Lagos, Nigeria
Tenny Obiageli Egwuatu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Agwu Ulu Nnachi .
Additional information
Competing interests.
No competing interests of any kind.
Authors’ contributions
AAI conceived of the study, participated in its design and served as the principal investigator. CCE participated in study design, data acquisition and sample collection. AUN participated in the study design and the statistical analysis, and drafted the manuscript for publication. IOI participated in sample collection and processing. GIO participated in data acquisition and sample processing. CANparticipated in the design of the study and the statistical analysis. TOE participated in sample processing and drafting of the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1:.
Questionnaire. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Iwuafor, A.A., Egwuatu, C.C., Nnachi, A.U. et al. Malaria Parasitaemia and the use of insecticide-treated nets (INTs) for malaria control amongst under-5 year old children in Calabar, Nigeria. BMC Infect Dis 16 , 151 (2016). https://doi.org/10.1186/s12879-016-1459-5
Download citation
Received : 13 August 2015
Accepted : 09 March 2016
Published : 14 April 2016
DOI : https://doi.org/10.1186/s12879-016-1459-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Insecticide-treated net (ITNs)
- Parasitaemia
- Plasmodium falciparum
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 03 February 2023
Leveraging innovation technologies to respond to malaria: a systematized literature review of emerging technologies
- Moredreck Chibi 1 ,
- William Wasswa 1 ,
- Chipo Ngongoni 1 ,
- Ebenezer Baba 2 &
- Akpaka Kalu 2
Malaria Journal volume 22 , Article number: 40 ( 2023 ) Cite this article
5210 Accesses
2 Altmetric
Metrics details
In 2019, an estimated 409,000 people died of malaria and most of them were young children in sub-Saharan Africa. In a bid to combat malaria epidemics, several technological innovations that have contributed significantly to malaria response have been developed across the world. This paper presents a systematized review and identifies key technological innovations that have been developed worldwide targeting different areas of the malaria response, which include surveillance, microplanning, prevention, diagnosis and management.
A systematized literature review which involved a structured search of the malaria technological innovations followed by a quantitative and narrative description and synthesis of the innovations was carried out. The malaria technological innovations were electronically retrieved from scientific databases that include PubMed, Google Scholar, Scopus, IEEE and Science Direct. Additional innovations were found across grey sources such as the Google Play Store, Apple App Store and cooperate websites. This was done using keywords pertaining to different malaria response areas combined with the words “innovation or technology” in a search query. The search was conducted between July 2021 and December 2021. Drugs, vaccines, social programmes, and apps in non-English were excluded. The quality of technological innovations included was based on reported impact and an exclusion criterion set by the authors.
Out of over 1000 malaria innovations and programmes, only 650 key malaria technological innovations were considered for further review. There were web-based innovations (34%), mobile-based applications (28%), diagnostic tools and devices (25%), and drone-based technologies (13%.
Discussion and conclusion
This study was undertaken to unveil impactful and contextually relevant malaria innovations that can be adapted in Africa. This was in response to the existing knowledge gap about the comprehensive technological landscape for malaria response. The paper provides information that countries and key malaria control stakeholders can leverage with regards to adopting some of these technologies as part of the malaria response in their respective countries.
The paper has also highlighted key drivers including infrastructural requirements to foster development and scaling up of innovations. In order to stimulate development of innovations in Africa, countries should prioritize investment in infrastructure for information and communication technologies and also drone technologies. These should be accompanied by the right policies and incentive frameworks.
In sub-Saharan Africa, malaria is the leading cause of death for children under 5. It has been reported that malaria infection during pregnancy increases the risk of maternal mortality and neonatal mortality [ 1 ]. According to the World Health Organization (WHO), there were 229 million cases of malaria in 2019 compared to 228 million cases in 2018. The estimated number of malaria deaths stood at 409,000 in 2019, compared with 411,000 deaths in 2018. Children under 5 years of age are the most vulnerable group affected by malaria and in 2019 they accounted for 67% (274,000) of all malaria deaths worldwide. The WHO African Region continues to carry a disproportionately high share of the global malaria burden. In 2019, the region was home to 94% of all malaria cases and deaths with six countries accounting for approximately half of all malaria deaths worldwide: Nigeria (23%), the Democratic Republic of the Congo (11%), United Republic of Tanzania (5%), Burkina Faso (4%), Mozambique (4%) and Niger (4%) [ 2 ].
Knowledge, learning and innovation are key to addressing, minimizing and tackling these disparities. One example of this is the knowledge hub developed by WHO called MAGICapp which aims to give living evidence and resources for tackling malaria interventions. It contains all official WHO recommendations for malaria prevention (vector control and preventive chemotherapies) and case management (diagnosis and treatment). The resources serve as a guide on the strategic use of information to drive impact, surveillance, monitoring and evaluation; operational manuals, handbooks, and frameworks; and a glossary of key terms and definitions. So, this paper aligns with identifying and adding discourse into the importance of reviews especially from a technological perspective.
To understand the advances in malaria services, various scholars have undertaken reviews across vast thematic areas of malaria interventions. In a quest to inform policy, Garner et al. [ 3 ] conducted an analysis of why Cochrane Reviews are important in malaria interventions. They noted that it is important for researchers to collaborate across regions and in understanding new preventive interventions. Their aim was to inform policymakers to understand the importance of reviews in identification of trends that are occurring in malaria interventions. Other aspects that have been looked at through reviews are the costs and cost-effectiveness aligned with malaria control interventions. White et al. [ 4 ] looked at interventions from studies published between 2000 and 2010 looking at the role of infection detection technologies for malaria elimination and eradication and the costs related to them in order to assess how accessible interventions are across regions. More recently, Conteh et al. [ 5 ] also carried on with assessing the unit cost and cost-effectiveness of malaria control during the period of January 1, 2005, and August 31, 2018. The aim was to see how resource allocation can be planned proactively according to costs, though they did highlight that care in methodological and reporting standards is required to enhance data transferability.
In a bid to combat malaria epidemic, several technological innovations have been developed all over the world that have contributed significantly to malaria response. Adeola et al. [ 6 ] reviewed the use of spatial technology for malaria epidemiology in South Africa between 1930 and 2013. The focus was on the use of statistical and mathematical models as well as geographic information science (GIS) and remote sensing (RS) technology for malaria research to create a robust malaria warning system. The mathematical modelling is also aligned with agent-based modelling which Smith et al. [ 7 ] highlighted through their analysis of 90 articles published between 1998 and May 2018 characterizing agent-based models (ABMs) relevant to malaria transmission. The aim was to provide an overview of key approaches utilized in malaria prevention. Such technologies feed into modelling sites and interventions to project various outcomes. From a platform centric perspective, Vasiman et al. [ 8 ] analysed how different mobile phone devices and handheld microscopes work as diagnostic platforms for malaria in low-resource settings. Malaria diagnostics tests and methods have also been reviewed as being key in the successful control and elimination programmes [ 9 ]. Mobile health has been found to play a key role in supporting health workers in the diagnosis and treatment of malaria in sub-Saharan Africa [ 10 ].
To add to this discourse, this paper presents a holistic systematized review of key technological innovations that have been developed worldwide targeting different areas of the malaria response, which include surveillance, microplanning, prevention, diagnosis, and management. A systematized review was utilized in this study as data sources that included unconventional grey sources was utilized and the review gravitated more towards being narrative with tabular accompaniments as compared to the systematic literature reviews that are less narrative [ 11 ]. The study was undertaken with the view to provide African countries and key stakeholders with information relating to technologies that can be adapted in their different contexts as they strengthen malaria response strategies.
Scientific databases literature search
This study adopted a systematic search strategy to identify the publications with innovations related to malaria surveillance, microplanning, prevention, diagnosis, and management from 5 scientific databases (PubMed, Google Scholar, Scopus, IEEE and Science Direct). The keywords used were malaria surveillance, microplanning, prevention, diagnosis and management combined with the words “innovations” or “technologies” in a search query. Innovations deemed not relevant to the scope of this research by the authors include drugs, vaccines, social programmes. Only papers reporting design, implementation or evaluation of malaria technological innovations were considered in this paper. The process was shown in Fig. 1 . The quality of technological innovations included was based on reported impact and judgement by the authors.
PRISMA flow chart for the malaria innovations literature search
Search through technology platforms e.g., google play store and apple app store
This study also adopted a systematic search strategy to identify the mobile apps related to malaria surveillance, microplanning, prevention, diagnosis and management available in the Google Play and Apple App stores. Keywords such as malaria surveillance, microplanning, prevention, diagnosis, and management were used in the search. The search was conducted between July 2021 and December 2021. The applications had to have a description, be in English, have 1000 + installs and reviews to be included in the analysis. The applications that did not meet these criteria were excluded. The core research question was: What mobile-based innovations are available for malaria interventions that can be adopted by the countries in the WHO Africa region for use across the continuum of the malaria response ? The resultant apps considered for this study were 260 as shown in Fig. 2 .
PRISMA flow chart for the mobile apps
Web search using a custom web-content mining algorithm
A custom web-content mining algorithm was also developed to search for malaria innovations and technologies published on different cooperate organizational websites, social media channels like twitter, and media channels like legit news websites like CNN. These technological innovations were collated between July 2021 and December 2021. The innovation name, description, Intellectual Property owner, web link to the innovation and geographical location were collated. Innovations that did not have functional and tested prototypes and were not related to addressing malaria interventions were excluded. The number of innovations surpassed 1000 however after screening, only 240 key technological innovations were selected that best fit the selection criteria.
A total of 650 malaria innovations (260 from Google play and Apple App store, 150 from scientific databases and 240 from web content mining) were considered for detailed review.
The review has identified innovations for malaria in the following technological thematic areas; web-based innovations (34%), mobile-based applications (28%), diagnostic tools and other devices (25%), and drone-based technologies (13%).
Web-based innovations
The web-based technologies include GIS systems [ 12 ]. An example is the Malaria Atlas Project (MAP), developed at the Telethon Kids Institute, Perth, Western Australia. MAP is a web platform that displays time aware raster and survey point data for malaria incidence, endemicity, and mosquito distribution. MAP has been designated as a WHO Collaborating Centre in Geospatial Disease Modelling. The impact of the Atlas Project has been validated in Sokoto Nigeria by Nakakana et al. [ 13 ]. The study concluded that the prevalence of malaria and its transmission intensity in Sokoto are similar to the Malaria Atlas Project predictions for the area and that is essential in modellings various aspects of malaria control planning purposes.
Other innovations like malariaAtlas which is an open-access R-interface on the Malaria Atlas Project, collates malariometric data, providing reproducible means of accessing such data within a freely available and commonly used statistical software environment [ 14 ]. A team from the University of Queensland developed a GIS-based spatial decision support system (SDSS) used to automatically locate and map the distribution of confirmed malaria cases, rapidly classify active transmission foci, and guide targeted responses in elimination zones. This has been implemented and evaluated in the Solomon Islands and Vanuatu in a study by Kelly et al. [ 15 ] and 82.5% of confirmed malaria cases were automatically geo-referenced and mapped at the household level, with 100% of remaining cases geo-referenced at a village level using the system. The GIS-based spatial decision support system has also been implemented in other countries like Vietnam. In Korea, the Malaria Vulnerability Map Mobile System which consists of a system database construction, malaria risk calculation function, visual expression function, and website and mobile application has been developed for use in Incheon [ 16 ]. The Malaria Decision Analysis Support Tool (MDAST) project promotes evidence-based, multi-sectoral malaria control policy-making in Kenya, Tanzania, and Uganda, serving as a pilot for such a programme in other malaria-prone countries [ 17 ].
In Zanzibar, the Malaria Case Notification (MCN) System was developed and the performance evaluation of the tool by Khandekar [ 18 ] showed that while a surveillance system can automate data collection and reporting, its performance will still rely heavily on health worker performance, community acceptance, and infrastructure within a country. A study by Mody et al. [ 19 ] showed that the use of telemedicine and e-health technologies shows promise for the remote diagnosis of malaria and hence several systems been developed. ProMED Mail (PMM) is an open and free to use, global, e-health based surveillance system from the International Society for Infectious Diseases with several use cases for malaria [ 20 , 21 ]. The Epidemic Prognosis Incorporating Disease and Environmental Monitoring for Integrated Assessment (EPIDEMIA) computer system was designed and implemented to integrate disease surveillance with environmental monitoring in support of operational malaria forecasting in the Amhara region of Ethiopia [ 22 ]. Table 1 summarizes some of the technologies.
Mobile applications-based technologies
This study has also revealed that several mobile-based malaria innovations have been developed which include smart mobile apps, Short Message Service (SMS) based apps and Unstructured Supplementary Service Data (USSD) based applications for use across the continuum of the malaria response. In India the Mobile-based Surveillance Quest using IT (MoSQuIT) is being used to automate and streamline malaria surveillance for all stakeholders involved, from health workers in rural India to medical officers and public health decision-makers. Malaria Epidemic Early Detection System (MEEDS) is a groundbreaking mHealth system used in Zanzibar by health facilities to report new malaria cases through mobile phones. Coconut Surveillance is an open-source mobile software application designed by malaria experts specifically for malaria control and elimination and it has become an essential tool for the Zanzibar Malaria Elimination Programme [ 23 ]. The SMS for Life initiative is a ‘public-private’ project that harnesses everyday technology to eliminate stock-outs and improve access to essential medicines in sub-Saharan Africa with a health focus on malaria and other vector borne diseases. This has been implemented and evaluated in Tanzania [ 24 ]. In Mozambique Community Health Workers (CHWs) use inSCALE CommCare tool for decision support, immediate feedback and multimedia audio and images to improve adherence to protocols.
Additional surveillance apps include the likes of the DHS mobile app for Malaria Indicator Surveys and Solution for Community Health-workers (SOCH) mobile app is a comprehensive mobile application tool for disease surveillance, workforce management and supply chain management for malaria elimination [ 25 ]. The National Malaria Case-Based Reporting App (MCBR) is a mobile phone application for malaria case-based reporting to advance malaria surveillance in Myanmar [ 26 ]. Mobile apps have also been used to support distribution of medicines like the Net4Schs App, an android application that is used for data capturing, processing and reporting on School Long-lasting insecticidal nets (LLINs) distribution activities. Apps have also been developed to support malaria screening and diagnosis for example the NLM Malaria Screener is a diagnostic app that assists users in the diagnosis of malaria and in the monitoring of malaria patients. This has been validated in several studies and it is reported that it makes the screening process faster, more consistent, and less dependent on human expertise [ 27 ]. Additional diagnostic apps include the Malaria System MicroApp which is a mobile device-based tool for malaria diagnosis [ 28 ], the Malaria Hero app is a web based mobile app for diagnosis of malaria, and LifeLens is a smartphone app that can detect malaria. Some key technologies are summarized in Table 2 .
Other notable mobile apps that have also been used in malaria management include CommCare’s usage in in Mozambique for integrated community case management in the remote communities. This has been reported to strengthen Community-Based Health [ 29 ]. Another app, FeverTracker, has been used for malaria surveillance and patient information management in India. There has also been a number of educational and knowledge base apps. These are the likes of Malaria Consultant, a mobile application designed to educate individuals on malaria and its prevention; the WHO Malaria toolkit App that brings together the content of the latest world malaria report and of the consolidated WHO Guidelines for malaria. This includes operational manuals for carrying out malaria interventions and other technical documents in one easy to navigate resource. Another interesting area where mobile apps have been used is in malaria prevention and such apps include those that scare away mosquitoes using high frequency sounds, and these include Anti Mosquito Repellent Sound App.
Drone-based technologies
This review has revealed that drone technologies can greatly help in malaria control programmes. The drones can be used in developing genetically-based vector control tools [ 30 ], delivering massive aerial spraying to kill mosquito larvae [ 31 ], identifying mosquito larvae sites using aerial imaging [ 32 ] and in delivering drugs and vaccines [ 33 ]. Anti-malaria drones have been widely used to spray biological insecticides in rice fields and swamps to reduce the emerging mosquito populations. This has been successful in Kenya, Tanzania, India, Rwanda and Zanzibar. In Zanzibar, the Agras MG-1S drones were used to spray 10 L of a biodegradable agent called Aquatain; a chemical that has been used to cover drinking water basins. Drones have also been used to collect data to identify mosquito breeding sites so that the larvae can be controlled, reducing the number of adult mosquitoes able to spread malaria. For example in Malawi and near Lake Victoria the DJI Phantom low-cost drones are being used to survey and find mosquito breeding grounds. A new trial using ‘gene drive’ technology is currently taking place in Burkina Faso where the trial will see the release of genetically modified mosquitoes in an attempt to wipe out the female carriers of the disease [ 34 ].
Diagnostic tools including other devices developed for malaria interventions
Devices that have been developed to respond to malaria include the SolarMal device, a solar-powered mosquito trapper being piloted in Kenya [ 35 ]. The Solar Powered Mosquito Trap (SMOT) is baited with a synthetic odor blend that mimics human odor to lure host-seeking malaria mosquitoes. Other devices such as the ThermaCell Patio Shield Mosquito Repellants developed by ThermaCell are shield lanterns that repel mosquitoes by creating a 15-foot zone of protection. Several devices have also been developed to improve malaria diagnosis and these include the Nanomal DNA analyzer a simple, rapid and affordable point-of-care (POC) handheld diagnostic nanotechnology device to confirm malaria diagnosis and detect drug resistance in malaria parasites in minutes and at the patient’s side, by analysis of mutations in malaria DNA using a range of proven nanotechnologies. Medication Events Monitoring Device (MEMS) have also been greatly used to monitor medication adherence to malaria drugs [ 36 ]. Malaria Rapid Diagnostic Tests (RDTs), sometimes called dipsticks or Malaria Rapid Diagnostic Devices (MRDDS), are simple immunochromatographic tests that identify specific antigens of malaria parasites in whole or peripheral blood. They are categorized into dipstick, cassette or hybrids. Dipstick RDTs are cheap and readily available on market [ 37 ]. An example is the OptiMAL dipstick [ 38 ]. Cassette RDTS are complex and require much time for results to be read but are much safer to use.
This research has culminated into insightful conclusions from the systematized review of the malaria technological Innovations and has been the foundation of the collated database that can be accessed via the WHO AFRO marketplace platform. This is a platform that has been developed to showcase various technologies and innovations that can be applied for different disease areas. This focused on technologies relevant for malaria response. The identified intervention technologies and focus areas provide ways of identifying key leverage points in strengthening the health systems and making tangible impact towards various mandates to fight the scourge of malaria. More importantly highlighting these trends empowers innovators and policy makers on the continent to make informed decisions on applying frugal design to develop affordable, locally manufactured, functional and sustainable innovations fit for the African continent. Furthermore, the marketplace platform provides implementation insights to African nations on the adoption of some of the technological innovations from this study.
The review has highlighted that mobile applications are a vital component of malaria response programmes and are increasingly being used along the different response areas, such as surveillance (malaria data capturing apps like Coconut Surveillance and DHS mobile app), microplanning (drug delivery and distribution management apps like Net4Schs App), prevention (mosquito repelling like Anti Mosquito Repellent Sound App), diagnosis (AI driven slide analysis apps like LifeLens and Malaria Screener), management (telehealth like the Malaria Consultant) and the provision of support for health services [decision support like the solution for Community Health-workers (SOCH) app] as outlined in Fig. 3 . Their impact has been validated in several studies [ 27 , 39 ].
Analysis of the innovations by category, application and target outcome
In 2019, 93% of the global population was covered by a mobile broadband signal. In Sub-Saharan Africa, 3G coverage expanded to 75% compared to 63% in 2017, while 4G doubled to nearly 50% compared to 2017 [ 40 ]. This implies that mobile solutions can substantially mitigate many of the health system limitations prevalent mostly in African countries where malaria is endemic. A substantial number of mobile applications have been developed for surveillance of malaria control programs in Africa such as inSCALE (Mozambique), Coconut Surveillance (Zanzibar), CommCare (Senegal) and DHIS2 (Zimbabwe, and South Africa). This shows that mobile-based apps give a larger footprint and a high level of agility to malaria response. Nevertheless, limited connectivity and erratic energy supplies have been key factors affecting the levels of adoption and some apps have been reported to have a high level of complexity. This has also been reported in other studies [ 41 , 42 ].
Moreover, it has been noted that most of these apps are independent with limited capability for interoperability. Hence there is a need to develop open standards for mobile technologies for malaria control. For example, surveillance applications should be able to have geolocation capabilities and use exiting open-source platforms like OpenStreetMap, OpenDataKit & OpenMapKit; work online and offline mode to enable usage in resource constraints areas, ease of use to enable usage with little or no training and should support different languages including local languages. This calls for more research and implementation of natural language processing frameworks for use in mobile apps in Africa, which can assist with data analytics as well. Furthermore, aligning app development with standards such as the Fast Healthcare Interoperability Resources (FHIR) which facilitate interoperability between legacy health care systems and technology is important.
Superseding technological interoperability, there needs to be platform integration and overall visibility particularly on innovations that target malaria diagnosis, surveillance and management. However, it should be noted that systemically there has been launching of different applications for different malaria interventions which may confuse the public in terms of usage. Therefore, a single application or platform integrating several services such as Coconut Surveillance and owned and managed by a reputable malaria organization or the ministries of health may benefit citizens by allowing them to access services from a single and trusted application. Misinformation and misdiagnosis from publicly available medical apps is a health threat to the public as reported by [ 43 ].
Most of the reviewed web systems depend on data or are used to collect large amounts of malaria data to support decision-making. Hence a need for national malaria control and elimination information systems that can utilize regional and global structures, prioritizing cross-border intelligence sharing information regarding disease transmission hotspots, outbreaks, and human movement. Such systems can also be very useful in responding to pandemics like COVID-19 and other infectious outbreaks. There is also a need to have malaria related data centrally stored and managed by the Ministry of Health or malaria control programmes to guide decision-making at all levels of malaria response among the different stakeholders. Hospitals and clinics have also developed standalone patient information management systems in addition to the national health information management systems like OpenMRS and DHIS2. However, there is no communication between the different patient’s information management systems hence a need for development of open data standard driven systems and APIs to enforce interoperability among health systems in Africa. An effective information system must receive data from other sources, process it and send it back to other systems being used in malaria programme, particularly at the community level.
In malaria control, larval source management is very difficult to archive in rural areas due to perceived difficulties in identifying target areas [ 44 ]. Drones can capture extremely detailed images of the landscape, opening the possibility of replacing the time-consuming hunt for mosquito larvae on the ground with identifying habitat through aerial imagery. The review has shown that this has been used in several countries for example in Malawi and near Lake Victoria using DJI Phantom; low-cost drones that survey wilderness to find mosquito breeding grounds using Geospatial technology. Geospatial technology is rapidly evolving and now can be archived using remotely sensed data [ 45 ]. In Zanzibar, drones have been used to spray rice fields with a thin, non-toxic film as a strategy to eliminate mosquitoes. The review has shown that drones are a possible solution in malaria control programmes as also indicated in other studies [ 45 , 46 ]. The review also showed that rapid diagnostics tools offer fast turnaround services while circumventing obstacles faced when using microscopy in peripheral health care settings, including cost of equipment, reagents, and the need for electricity and skilled personnel [ 47 ].
This study has reviewed key emerging technologies used in malaria control programmes. The review revealed various technological applications that have been developed in response to malaria including surveillance, microplanning, prevention, diagnosis and management. Although breakthrough innovative platforms have been made available, one key challenge remained, which is lack of integration of key end-to-end components and functionalities to facilitate effective and efficient malaria response and to reduce fragmentation.
The review has also revealed several stakeholders in malaria control hence a need for mechanisms that promote the exchange of evidence between scientific, policy, and programme management communities for analysing the potential outcomes of the different malaria control strategies and interventions. In many malaria-endemic areas in Africa, the communication gap between policy makers, health workers, and patients is a significant barrier to efficient malaria control.
Furthermore, artificial intelligence (AI) has been widely used in the reviewed technological innovations, however there is an urgent need to provide reliable datasets, develop local AI expertise among WHO African member states, implement data protection and privacy acts; and put in place health innovation clusters to bring the different stakeholders together to develop and adopt appropriate technologies to solve the intended challenges.
Limitations of this work and future prospects
The main limitation of this work was that some applications were overlapping among the response areas and hence the decision to place an innovation under a given category was based on the judgement of the authors. Another limitation is the fact that this work is not aimed at analysing the total landscape of all malaria innovations. Only those that met the inclusion criteria and deemed relevant by the authors were included hence some innovations might not have been captured but we will be subjected to continuous update on the global database for malaria innovations at https://innov.afro.who.int/emerging-technological-innovations/7-malaria-innovations . Future research can focus on reviewing the technologies that are open source dedicated to malaria, and publishing findings that can be used by medical practitioners, application developers, and governments to collaborate in the process of containing the spread of malaria.
Availability of data and materials
The data used in this report is available to readers.
Ryan SJ, Lippi CA, Zermoglio F. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malar J. 2020;19:170.
Article Google Scholar
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Google Scholar
Garner P, Gelband H, Graves P, Jones K, Maclehose H, Olliaro P, et al. Systematic reviews in malaria: global policies need global reviews. Infect Dis Clin North Am. 2009;23:387–404.
White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J. 2011;10:337.
Conteh L, Shuford K, Agboraw E, Kont M, Kolaczinski J, Patouillard E. Costs and cost-effectiveness of malaria control interventions: a systematic literature review. Value Health. 2021;24:1213–22.
Adeola AM, Botai JO, Olwoch JM, Rautenbach AC, Kalumba AM, Tsela PL, et al. Application of geographical information system and remote sensing in malaria research and control in South Africa: a review. South Afr J Infect Dis. 2015;30:114–21.
Smith NR, Trauer JM, Gambhir M, Richards JS, Maude RJ, Keith JM, et al. Agent-based models of malaria transmission: a systematic review. Malar J. 2018;17:299.
Vasiman A, Stothard JR, Bogoch II. Mobile phone devices and handheld microscopes as diagnostic platforms for malaria and neglected tropical diseases (NTDs) in low-resource settings: a systematic review, historical perspective and future outlook. Adv Parasitol. 2019;103:151–73.
Mbanefo A, Kumar N. Evaluation of malaria diagnostic methods as a key for successful control and elimination programs. Trop Med Infect Dis. 2020;5:E102.
Osei E, Kuupiel D, Vezi PN, Mashamba-Thompson TP. Mapping evidence of mobile health technologies for disease diagnosis and treatment support by health workers in sub-Saharan Africa: a scoping review. BMC Med Inform Decis Mak. 2021;21:11.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26:91–108.
Kurland KS, Gorr WL. GIS tutorial for health. ESRI, Inc.; 2007.
Nakakana UN, Mohammed IA, Onankpa BO, Jega RM, Jiya NM. A validation of the malaria Atlas project maps and development of a new map of malaria transmission in Sokoto, Nigeria: a cross-sectional study using geographic information systems. Malar J. 2020;19:149.
Pfeffer DA, Lucas TCD, May D, Harris J, Rozier J, Twohig KA, et al. Malaria Atlas: an R interface to global malariometric data hosted by the malaria Atlas project. Malar J. 2018;17:352.
Kelly GC, Hale E, Donald W, Batarii W, Bugoro H, Nausien J, et al. A high-resolution geospatial surveillance-response system for malaria elimination in Solomon Islands and Vanuatu. Malar J. 2013;12:108.
Kim JY, Eun SJ, Park DK. Malaria vulnerability map mobile system development using GIS-based decision-making technique. Mob Inf Syst. 2018;12:1–9.
Brown Z, Kramer R, Mutero C, Kim D, Miranda ML, Amenshewa B, et al. Stakeholder development of the malaria decision analysis support tool (MDAST). Malar J. 2012;11(Suppl 1):P15.
Khandekar E. Performance evaluation of zanzibar’s malaria case notification (MCN) surveillance system: the assessment of timeliness and stakeholder interaction. Thesis, MSc Duke Global Health Institute, 2015.
Murray CK, Mody RM, Dooley DP, Hospenthal DR, Horvath LL, Moran KA, et al. The remote diagnosis of malaria using telemedicine or e-mailed images. Mil Med. 2006;171:1167–71.
Madoff LC, Freedman DO. Detection of infectious diseases using unofficial sources infectious diseases. In: Petersen E, Chen LH, Schlagenhauf P, editors. A geographic guide. Hoboken: Wiley Online; 2011.
Woodall JP. Global surveillance of emerging diseases: the ProMED-mail perspective. Cad Saúde Pública. 2001;17:147–54.
Merkord CL, Liu Y, Mihretie A, Gebrehiwot T, Awoke W, Bayabil E, et al. Integrating malaria surveillance with climate data for outbreak detection and forecasting: the EPIDEMIA system. Malar J. 2017;16:89.
Article CAS Google Scholar
Sanches P, Brown B. Data bites man: the production of malaria by technology. Proc ACM Hum-Comput Interact. 2018;2:153.
Barrington J, Wereko-Brobby O, Ward P, Mwafongo W, Kungulwe S. SMS for life: a pilot project to improve anti-malarial drug supply management in rural Tanzania using standard technology. Malar J. 2010;9:298.
Rajvanshi H, Jain Y, Kaintura N, Soni C, Chandramohan R, Srinivasan R, et al. A comprehensive mobile application tool for disease surveillance, workforce management and supply chain management for malaria elimination demonstration project. Malar J. 2021;20:91.
Oo Win Han, Htike Win, Cutts JC, Win KM, Thu KM, Oo MC, et al. A mobile phone application for malaria case-based reporting to advance malaria surveillance in Myanmar: a mixed methods evaluation. Malar J. 2021;20:167.
Yu H, Yang F, Rajaraman S, et al. Malaria screener: a smartphone application for automated malaria screening. BMC Infect Dis. 2020;20:825.
Oliveira AD, Prats C, Espasa M, Serrat FZ, Sales CM, Silgado A, et al. The malaria system microapp: a new, mobile device-based tool for malaria diagnosis. JMIR Res Protoc. 2017;6: e70.
Svoronos T. CommCare: automated quality improvement to strengthen community-based health the need for quality improvement for CHWs. Weston: D-Tree Int Publisher; 2010.
James S, Collins FH, Welkhoff PA, Emerson C, Godfray HC, Gottlieb M, et al. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in sub-Saharan Africa: recommendations of a scientific working group. Am J Trop Med Hyg. 2018;98(Suppl 6):1–49.
Choi L, Majambere S, Wilson AL. Larviciding to prevent malaria transmission. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD012736.pub2 .
Thompson DR, de la Torre JM, Barker CM, Holeman J, Lundeen S, Mulligan S, et al. Airborne imaging spectroscopy to monitor urban mosquito microhabitats. Remote Sens Environ. 2013;137:226–33.
Stanford Graduate School of Business. Zipline : Lifesaving deliveries by drone. Stanford Business. 2019. https://www.gsb.stanford.edu/faculty-research/case-studies/zipline-lifesaving-deliveries-drone .
Pare Toe L, Barry N, Ky AD, Kekele S, Meda W, Bayala K, et al. Small-scale release of non-gene drive mosquitoes in Burkina Faso: from engagement implementation to assessment, a learning journey. Malar J. 2021;20:395.
Hiscox A, Maire N, Kiche I, Silkey M, Homan T, Oria P, et al. The SolarMal project: innovative mosquito trapping technology for malaria control. Malar J. 2012;11(Suppl 1):O45.
Teshome EM, Oriaro VS, Andango PEA, Prentice AM, Verhoef H. Adherence to home fortification with micronutrient powders in Kenyan pre-school children: self-reporting and sachet counts compared to an electronic monitoring device. BMC Public Health. 2018;18:205.
Jelinek T, Grobusch MP, Nothdurft HD. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. J Travel Med. 2000;7:175–9.
Tagbor H, Bruce J, Browne E, Greenwood B, Chandramohan D. Performance of the OptiMAL ® dipstick in the diagnosis of malaria infection in pregnancy. Ther Clin Risk Manag. 2008;4:631–6.
Visser T, Ramachandra S, Pothin E, Jacobs J, Cunningham J, Le Menach A, et al. A comparative evaluation of mobile medical APPS (MMAS) for reading and interpreting malaria rapid diagnostic tests. Malar J. 2021;20:39.
GSMA. Mobile Internet Connectivity 2019 Sub-Saharan Africa Factsheet. 2019. https://www.gsma.com/mobilefordevelopment/wp-content/uploads/2019/07/Mobile-Internet-Connectivity-SSA-Factsheet.pdf .
Murugesan S. Mobile apps in Africa. IT Prof. 2013. https://doi.org/10.1109/MITP.2013.83 .
Shead DC, Chetty S. Smartphone and app usage amongst South African anaesthetic service providers. South Afr J Anaesth Analg. 2021;27:76–82.
Swire-Thompson B, Lazer D. Public health and online misinformation: challenges and recommendations. Annu Rev Public Health. 2019;41:433–51.
Stanton MC, Kalonde P, Zembere K, Spaans RH, Jones CM. The application of drones for mosquito larval habitat identification in rural environments: a practical approach for malaria control? Malar J. 2021;20:244.
Kullmann K. The drone’s eye: applications and implications for landscape architecture. Landscape Res. 2018;43:7.
Liardon JL, Hostettler L, Zulliger L, Kangur K, Shaik NS, Barry DA. Lake imaging and monitoring aerial drone. HardwareX. 2018;3:146–59.
Boyce MR, O’Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health. 2017;17:470.
Download references
Acknowledgements
Author information, authors and affiliations.
World Health Organization Africa Region, Brazzaville, Republic of Congo
Moredreck Chibi, William Wasswa & Chipo Ngongoni
Tropical and Vector Borne Diseases, Universal Health Coverage/Communicable and Non Communicable Disease Cluster, World Health Organization Africa Region, Brazzaville, Republic of Congo
Ebenezer Baba & Akpaka Kalu
You can also search for this author in PubMed Google Scholar
Contributions
MC lead the conceptualization and designing of the study, and writing of the manuscript. WW contributed with data mining, analytics and writing the manuscript. CN contributed with systematized literature review and reviewing the manuscript. EB contributed to conceptualizing the study and reviewing of the draft manuscript. AK contributed to reviewing the draft manuscript and providing expert oversight. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Moredreck Chibi .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
The author reports no conflicts of interest for this work and given their consent for the publication.
Competing interests
The authors declared no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chibi, M., Wasswa, W., Ngongoni, C. et al. Leveraging innovation technologies to respond to malaria: a systematized literature review of emerging technologies. Malar J 22 , 40 (2023). https://doi.org/10.1186/s12936-023-04454-0
Download citation
Received : 23 January 2022
Accepted : 14 January 2023
Published : 03 February 2023
DOI : https://doi.org/10.1186/s12936-023-04454-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Emerging technologies
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.3(1); Jan-Mar 2014
Knowledge, Attitude and Practices on Malaria Among the Rural Communities in Aliero, Northern Nigeria
Rupashree singh.
Department of Biological Sciences, Kebbi State University of Science and Technology, Aliero, Kebbi, Nigeria
Jamila Musa
Sanjay singh.
1 Department of Family Medicine, Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria
Ukatu Victoria Ebere
Families' perceptions, beliefs, and attitudes about malaria causation, symptom identification, treatment of malaria, and prevention are often overlooked in malaria control efforts. This study was conducted to understand these issues, which can be an important step towards developing strategies, aimed at controlling malaria.
Materials and Methods:
A community based descriptive cross-sectional study in four villages: Danwarai, Gehuru, Jiga, and Kashin Zama of Aliero local government area in Kebbi Sate, in northern Nigeria. Two hundred household were randomly selected and interviewed using standardized questionnaire.
Knowledge of the role of mosquitoes in malaria transmission (11.8%) and cause of malaria (9.6%) was observed to be low among the study population. Comprehensive knowledge about malaria prevention measures was high (90%), but not reflecting in their practice (16%). They have good knowledge of mosquito behavior (breeding areas (64.5%), resting places (70%) and biting time (81%)). Seeking hospital care for a febrile child was a good practice (68.5%) observed. Attitudes regarding the best antimalarial therapy was limited (56.7%) to chloroquine.
Conclusions:
Misconceptions about malaria transmission and its cause still exist. Knowledge about preventive measures does not necessarily translate into improvement in practices. There is a need for targeted educational programs to increase the communities' efforts to develop desirable attitude and practices regarding malaria and their participation for malaria control.
Introduction
Malaria is unique among diseases because its roots lie so deep within human communities.[ 1 ] Malaria beliefs and practices are often related to culture, and can influence the effectiveness of control strategies;[ 2 ] thus, local knowledge and practice related to malaria is important for the implementation of culturally appropriate, sustainable, and effective interventions.[ 3 ]
Globally, an estimated half of world populations are at risk of malaria. Malaria is endemic in Africa with an estimated 80% of cases and 90% of deaths of the global burden occurring there, especially amongst children and pregnant women. Together, the Democratic Republic of the Congo and Nigeria account for over 40% of the estimated total of malaria deaths globally.[ 4 ] Malaria is a major public health problem in Nigeria with an estimated 100 million malaria cases and over 300,000 deaths per year. It accounts for 60% of outpatient visits, 30% of hospitalizations among children under 5 years of age, and 11% maternal mortality.[ 5 ]
Twelve years after the first Abuja declaration, Nigeria failed to halve the malaria burden in 2010. In the next 2 years leading up to the Millennium Development Goals' (MDG) deadline, Nigeria is still recording high prevalence (98.4%) of malaria,[ 6 ] hence it is doubtful if Nigeria could halt by 2015 and begin to reverse the incidence of malaria.
The failure to consider community's knowledge, attitude, and practice (KAP) about malaria has contributed to the inability of programs to achieve sustainable control.[ 7 ] People's behavior may increase malaria risk, but to change such behavior is not easy. Indeed, there are many reasons why particular behaviors exist and they often are tied to considerable benefits in areas quite distinct from health. Thus, it is not usually the case that “these people don't know any better”, but rather that their native logic and rationality make sense within the realities and limitations of their local circumstances.[ 1 ]
Families are the primary context within which most health problems and illnesses occur and have a powerful influence on health. Most health belief and behavior are developed and maintained within the family.[ 8 ] Community perceptions, beliefs, and attitudes about malaria causation, symptom identification, treatment of malaria, and prevention influence efforts to address malaria and are often overlooked in control efforts[ 9 ] and it vary from community to community and among individual households.[ 10 ] Considering these issues it can be an important step towards developing strategies aimed at controlling the malaria.[ 11 ] Understanding who already knows about malaria and malaria prevention, who has adopted malaria prevention and mosquito avoidance practices, and who is at risk of malaria infection is a necessary precursor to identifying and targeting vulnerable populations and ensuring successful implementation and sustainability of malaria control efforts.[ 12 ]
There is paucity of data on KAP studies on malaria in northwestern Nigeria. Studies on KAP[ 10 , 13 ] have demonstrated that, direct interaction with community plays an important role in circumventing malaria spread. Healthcare provider like family physician can focus both on traditional physician-patient model and complement it with population based medicine for primary prevention of malaria as domiciliary care and primary prevention are defining characteristics of family medicine. So, in order to create a synergy between primary care physician and community efforts and governmental/nongovernmental organized malaria control interventions in north Nigeria in particular, there is an urgent need to determine the people's knowledge, attitude, and practice of malaria and its control.
Materials and Methods
The study was carried out in the four villages Danwarai, Gehuru, Jiga, and Kashin Zama of Aliero Local Government Area. Aliero is approximately located at latitudes 4°23'S and 12°26'40“N and longitudes 3°6'W and 4°27'35“E. It was created in 1996, with a total land mass of 412.25 km 2 [ 14 ] and has a total population of 67,078.[ 15 ]
Study design and data collection
The study was a community based cross-sectional study. A structured questionnaire was used for interview. The questionnaire was administered to 200 randomly selected households in July and August 2012. Only one adult was interviewed per household. The interviewees were the heads of households. In their absence, a responsible adult above 18 years, chosen by the family was interviewed. The questionnaire was prepared in English language but translated and communicated in local languages when necessary.
Ethical clearance
The study was approved by Kebbi State University of Science and Technology. The objectives of the study were explained to community leaders and local government executives before the permission was granted. Full verbal explanation of the study was given to members of selected households and consent was obtained before inclusion as participants. Respondents were given the right to refuse to take part in the study as well as to withdraw any time during the interview. Privacy and confidentiality were maintained throughout the study.
Data analysis
The data were entered into a Microsoft Excel - Worksheet and analyzed using Epi Info, version 3.5.3. Descriptive statistics were carried out to measure relative frequencies, percentages, averages, and relative frequencies of the variables.
Sociodemographic characteristics of the study population
A total of 200 households' heads were interviewed, 36 from Danwarai, 80 from Gehuru, 39 from Jiga, and 45 from Kashin Zama. There were 68 (34.0%) females and 132 (66.0%) males. Islam was the predominant religion with 198 (99.0%) respondents and Christianity with only two (1.0%) respondents. The sociodemographic and household characteristics of the study population are presented in Table 1 .

Knowledge of malaria, its transmission, cause, and symptom
When asked, “Have you heard of malaria?”; 187 respondents (93.5%) stated yes and 13 (6.5%) stated no. Of the ‘yes’ group, 80 (42.8%) knew malaria because they suffered it. Only 22 (11.8%) respondent correctly stated that mosquitoes which has bitten a malaria patient, was the mode of transmission, while most 139 (74.3%) reported by bites of any mosquito. Plasmodium organism as the main cause of malaria was correctly identified by only 18 (9.6%). Majority of them, 103 (55.1%) reported mosquito bite as cause of malaria. The most commonly mentioned symptom was fever with shivering by 122 respondents (65.2%) [Tables [Tables2 2 and and3 3 ].
Knowledge of malaria and its transmission

Knowledge of malaria causes and symptoms

Knowledge of preventive measures
About 180 (90.0%) of the respondents reported any bed nets as the most common known protective method against malaria, while 128 (64.0%) respondent had knowledge of insecticide treated bed net (ITNs). Second most common known preventive measure was use of mosquito coils by 79 (37.8%) respondent. The knowledge of ways to prevent mosquito breeding, by cleaning of house surroundings was reported by 97 (48.5%) and draining of stagnant water by 58 (29.0%) [ Table 4 ].
Knowledge of malaria preventive measures

Knowledge of mosquito behavior
Stagnant water was reported as mosquito breeding area by most, 129 (64.5%) respondent. Knowledge about mosquito biting time was relatively high, almost all 162 (81.0%) knew that night time is the biting time of mosquito. Majority of respondents 140 (70.0%) identified the indoor dark resting place during day as the resting places of mosquitoes [ Table 5 ].

Attitudes and practices against malaria and its control
When asked what they would do if their child had fever, most caregivers 137 (68.5%) reported that they would go to a hospital. The most important factor was condition of child when deciding to seek formal care for child with fever, reported by 104 (52.0%) followed by perceived cost by 77 (38.5%) respondent. Attitude regarding the best antimalarial therapy was limited to chloroquine, reported by most of them, 106 (56.7%). About 160 (80.0%) of the respondents reported to own any bed nets as the most common protective method against malaria in practice, while 40 (20.0%) reported not using the mosquito net. Those who reported using bed nets only 51 (31.9%) reported using ITNs and 109 (68.1%) reported using untreated nets. Second most common preventive measure in practice was mosquito coil reported by 35 (17.5%). Although 32 (16.0%) reported using no preventive measures, about 110 (68.8%) reported that everyone in their family was sleeping under bed nets [Tables [Tables6 6 and and7]. 7 ]. In practice, 89 (47.6%) respondents reported home treatment with antimalarial.
Attitudes against malaria and its control

Practices against malaria and its control

KAP of malaria and its control were observed to be low among the study population. This result is in agreement with previous findings of other similar studies[ 9 , 12 , 13 , 16 ] and in contrast to other similar studies.[ 17 , 18 , 19 ] Majority of respondents reported to have ever heard about malaria. This was consistent with other studies[ 13 , 16 , 19 ] that shows almost all the respondents have heard about malaria. The main source of information was from individuals' experiences with malaria as reported earlier in a study in Nigeria,[ 20 ] followed by health centers; consistent with another Nigerian study.[ 21 ] There was gaps in knowledge by 8.1% of the respondents stating that they did not know the mode of transmission and more than half (74.3%) of the study participants mentioned bites of any mosquito as a mode of malaria transmission. Only a small proportion of respondents correctly mentioned about malaria transmission and its cause. Thus, the knowledge level of respondents about the mode of malaria transmission was very low when compared to the findings in previous studies reported across Africa.[ 2 , 6 , 11 , 19 , 22 , 23 ] This may be attributable to low level of education in the rural community. Knowledge of mosquito behavior is important to take appropriate malaria preventive actions and it was relatively high among participants of the present study.
This study has demonstrated that respondent had a good knowledge about malaria signs and symptoms. Majority of the respondents mentioned fever (with shivering) as the most common symptom of malaria and is consistent with observations from other similar studies.[ 9 , 16 , 24 , 25 , 26 ] This high level of awareness of the clinical features of malaria might be due to increased access to mass media, health education by health workers, and self experience of malaria. Environmental vector control through elimination of the vector habitat at an early stage is an important primary preventive measure for malaria. In this study, the respondents had good knowledge on environmental preventive measures, consistent with other studies in Nigeria[ 18 , 20 ] and in Ethiopia;[ 19 ] but the knowledge does not necessarily translate into improved practice of preventive measures; an observation reflected in this study. This might be due to poor socioeconomic status and low level of formal education of the rural communities.
Knowledge on the use of bed net as a preventive measure against mosquito bite was high among the respondents (90%) in this study, but only 80% reported use of any bed nets (ITNs or non ITNs). The remaining 10% were aware of their effectiveness in prevention of malaria but could not afford them. Similar high level of knowledge on preventive use of bed net had been observed in other studies in Ethiopia[ 19 , 27 ] and in Malawi.[ 22 ] The awareness of ITNs was high among respondents, but only 31.9% of respondents were actually using it. The added advantage of treating bed nets with residual insecticides should be made known to the communities. Since the cost is reported a major reason for its low utilization in this study and in other study in Nigeria,[ 18 ] government should consider subsidizing mosquito nets to enable all families to invest in them.
Use of hospital for treatment was uniformly advocated, which is similar with a study in Ethiopia.[ 19 ] This might reflect issues of accessibility and quality in the health facilities. But in practice 47.6% of respondent reported giving home treatment, which is consistent with findings reported from other studies in Nigeria[ 26 , 28 , 29 ] and other countries.[ 30 , 31 ] The use of home treatment might be because most of them could not afford hospital and needed prompt treatment. The knowledge of proper administration of antimalarials was also limited as reported in other studies.[ 17 , 31 ] Thus, malaria control policies should recognize the role of home treatment in the management of malaria and provide trainings for the adequate use of antimalarial drugs. An encouraging finding of our study was that only 8% of respondent mentioned traditional healer as a choice of treatment, which was consistent with previous study in Nigeria.[ 32 ] Although local shops were not the popular treatment source in rural areas of Aliero, but sometimes they were the alternative to medical facilities. As a result, a significant portion of the population were receiving initial treatment for perceived malaria from local shops, whose knowledge and capacity for curative treatment is questionable[ 33 ] and should be considered when designing treatment and intervention programs. There is potential to improve malaria care by educating local shopkeepers on the symptoms, appropriate treatment, and dosages of antimalarial drugs. Such educational interventions have been shown to change malaria treatment seeking behavior in other malaria endemic areas.[ 34 , 35 ] In addition, ensuring that shopkeepers understand the proper drug regimen for treating an episode of malaria may help protect against the development of drug resistance by limiting overuse and underdosing.[ 36 ]
Misconceptions about malaria transmission still exist. Increasing the knowledge about malaria transmission and benefits of using available effective preventive and control measures by the individual households and the community could contribute much to the overall reduction of the malaria burden. Educational messages must be culturally sensitive and capitalize on the positive beliefs and behaviors that already exist in local communities. Likewise, programs that mobilizes communities can play a critical role in the adoption of preventive behaviors. An understanding of how these strategies reach the population together with the identification of the main determinants that influence protective behavior are required to monitor evaluate the progress of the malaria control efforts.
Perhaps solution of malaria control lies -in primary care physicians such as family physician or community health workers working in the rural communities. The result of this study will increase their current knowledge for health education and promotion on malaria at the first contact either in the health facilities or in the patient's family house upon home visit.
Acknowledgment
We wish to thank the villagers of Danwarai, Gehuru, Jiga, and Kashi Zama in Aliero Local Government community who participated in the study. I am grateful to their Chairman for his cooperation and logistic support during the study period.
Source of Support: Nil.
Conflict of Interest: None declared.
Severe falciparum malaria in children in Enugu, South East Nigeria
Affiliations.
- 1 Department of Paediatrics, College of Medicine, University of Nigeria, Enugu Campus, Enugu, Nigeria.
- 2 Department of Paediatrics, College of Medicine, Enugu State University of Science and Technology, Enugu, Nigeria.
- PMID: 30297570
- DOI: 10.4103/njcp.njcp_140_18
Introduction: Severe malaria remains one of the leading causes of morbidity and mortality in sub-Saharan Africa and parts of Asia despite several efforts in prevention and management. The prevalence and pattern of presentation may vary from one location to another and from one age group to another.
Objectives: This study was undertaken to review the prevalence and pattern of severe malaria among children presenting in the two tertiary hospitals in Enugu, south-east Nigeria.
Methods: The case records of children presenting with malaria in the two tertiary hospitals in the state were retrieved and the necessary information were obtained using a structured questionnaire.
Results: The children aged from 1 month to 184 months (15 years), with a median age of 36 months and mean age of 49.2 ± 42.7 months. About two-thirds (68/102, 66.7%) of the children were under the age of 5 years, with only 6 of them (8.8%) being 6 months and below. There were significantly more males than females (χ2 = 6.48, P = 0.01); with a M:F ratio of 1.55:1. The peak of presentation was from August and November. Prostration, respiratory distress and severe anaemia were the commonest features of severe malaria, while shock, acute renal failure and abnormal bleeding were the least presenting features Of all the features, only severe anaemia was significantly related to age, (χ2 = 5.027, P = 0.02). Sixty-one (59.8%) of the children had one or more co-morbidities. There were 2 deaths, giving a case fatality rate of 1.96%.
Conclusion: Early presentation will significantly reduce blood transfusions, prolonged admission and death in children with severe malaria.
Keywords: Children; Enugu; Nigeria; falciparum; malaria.
- Anemia / epidemiology*
- Anemia / etiology
- Blood Transfusion
- Child, Preschool
- Comorbidity
- Hospitalization / statistics & numerical data*
- Malaria, Falciparum / diagnosis
- Malaria, Falciparum / epidemiology*
- Nigeria / epidemiology
- Retrospective Studies
- Surveys and Questionnaires

IMAGES
COMMENTS
Introduction. Malaria is most common in tropical and subtropical regions [1].Sub-Saharan Africa is the most affected region. In 2021, the region accounted for almost 95% (234 million) of all malaria cases, and 96% (593,000) of all deaths due to the disease [2], [3].Four countries, namely, Nigeria, the Democratic Republic of Congo, Niger, and the United Republic of Tanzania, accounted for more ...
1.2 Malaria control and elimination in Nigeria. Nigeria accounts for 56% of malaria cases in the West African sub-region. Microscopy detected malaria prevalence in Nigeria dropped from 42% in 2010 to 27.4% in 2015. However, great variations still exist among regions within the country.
Background Quantifying disease costs is critical for policymakers to set priorities, allocate resources, select control and prevention strategies, and evaluate the cost-effectiveness of interventions. Although malaria carries a very large disease burden, the availability of comprehensive and comparable estimates of malaria costs across endemic countries is scarce. Methods A literature review ...
Malaria control in Nigeria is guided by National Malaria Strategic Plans. The goal of the NMSP (2014-2020) is 'to reduce malaria burden to pre-elimination levels and bring malaria-related mortality to zero' using strategies under seven strategic objectives. The theme for the 2017 World Malaria Day activities was 'End Malaria for Good'.
2.1. Study settings. With 95 percent of the general population at risk and 50 percent of the adult population undergoing at least one episode annually, Nigeria carries the heaviest malaria burden in the world.17 Situated in the North Central region and considered the largest state in the country, Niger State is no exception. Malaria accounts for 65 percent of all outpatient visits and ...
In Nigeria, U5 children are the most vulnerable group—they experience about an average of 2-4 bouts per year, and account for about 90% of national mortality from malaria 6. Furthermore ...
These surfaces of PR are then used to predict malaria clinical incidence at 5 × 5 km resolutions across Africa for each year between 2000 and 2015. We used these modelled predictions of changing clinical incidence of malaria to compare spatially matched clinical cases identified in our literature review over the same time period.
Death registration in Nigeria: a systematic literature review of its performance and challenges. Glob Health Action. 2020; ... Malaria was the second-leading cause of YLLs in Nigeria in 1998 and was among the top four causes of YLLs across all countries in the region, except for Cape Verde. By 2019, malaria was the leading cause of YLLs in ...
located. The largest city in Nigeria is Lagos, the second-largest metropole in Africa. Malaria is a major public health concern in Nigeria, with an estimated 68 million cases and 194 000 deaths due to the disease in 2021. Nigeria has the highest burden of malaria globally, accounting for nearly 27% of the global malaria burden.
Human malaria infection is among the leading global parasitic diseases which have substantial effects on all facets of human life. A series of measures have been devised to prevent and control malaria infection, including vaccines and prophylaxis. Nigeria, the most populous country in Sub Saharan Africa, is burdened by the effect of malaria infection.
Background: Malaria is a severe health issue in Nigeria, particularly for pregnant women and children <5 y of age, despite all malaria control programs. From the standpoint of major stakeholders in Nigeria, this study explored both promoting and limiting factors affecting the implementation of malaria policy.
antimalarial medicines. The objectives of this review is to enable health professionals to understand the magnitude of malaria treatment services in Nigeria, to improve knowledge for rational malaria management within different health system contexts with a view to improving access to malaria treatment. The review therefore looks at the following areas: clinical disease and epidemiology; the ...
For further reading, the following articles, referenced in this video, are available at the Journal's website: A Malaria Vaccine for Africa — An Important Step in a Century-Long Quest, in the ...
diseases. 1-3. EFFECTS OF MALARIA IN PREGNANCY. Malaria is responsible for 20 per cent of still births and. 11 per cent of all maternal deaths by way of spontaneous. abortion, maternal anaemia ...
The four articles in this research topic 9 highlight the importance of molecular epidemiology in informing and improving malaria 10 surveillance strategies. 11 12One of the threats to malaria elimination is the emergence and spread of Plasmodium 13 falciparum parasites with deletions in the histidine-rich protein 2 and 3 (hrp2/3) genes.
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
A systematic literature review following PRISMA statement [] was conducted to identify studies measuring the efficacy of ABT or QBT in pregnant women with parasitologically confirmed uncomplicated falciparum malaria, regardless of trimester or clinical symptoms.Seven databases (MEDLINE, Embase, Global Health, Cochrane Library, Scopus, Web of Science and LILACS) and two clinical trial ...
A systematic analysis of recent literature on the prevalence of malaria in pregnancy from many authors was carried out and the facts synthesized to make an easy read. ... , title={Malaria in Pregnancy in Nigeria: A Literature Review}, author={Joseph A. Omang and Antor O. Ndep and Dominic Asuquo Offiong and Fidelis Takim Otu and Kenneth Onyejose ...
Methods: This literature review aims to describe research that has identified characteristics that clients are looking for in the providers they approach for their health care needs, specifically for malaria in Africa. Keywords of 'malaria' and 'treatment seek*' or 'health seek*' and 'Africa' were searched for in the following databases: Web of ...
Background Malaria remains a major cause of febrile illness in Nigeria and interventions to reduce malaria burden in Nigeria focus on the use of insecticide-treated nets. This study determined the prevalence of malaria parasitaemia and the use of insecticide-treated nets (ITNs) for the control of malaria amongst under-five year old children in Calabar, Nigeria. Methods A total of 270 under-5 ...
Abstract. Following the World Health Organization 2021 report of Nigeria being the leading country among the four African countries responsible for half of the malaria mortality all over the world, the President of Nigeria, on August 16, 2022, inaugurated the Nigeria End Malaria Council to reduce the malaria burden in the country, serves as a ...
In 2019, an estimated 409,000 people died of malaria and most of them were young children in sub-Saharan Africa. In a bid to combat malaria epidemics, several technological innovations that have contributed significantly to malaria response have been developed across the world. This paper presents a systematized review and identifies key technological innovations that have been developed ...
Twelve years after the first Abuja declaration, Nigeria failed to halve the malaria burden in 2010. In the next 2 years leading up to the Millennium Development Goals' (MDG) deadline, Nigeria is still recording high prevalence (98.4%) of malaria, hence it is doubtful if Nigeria could halt by 2015 and begin to reverse the incidence of malaria.
Introduction: Severe malaria remains one of the leading causes of morbidity and mortality in sub-Saharan Africa and parts of Asia despite several efforts in prevention and management. The prevalence and pattern of presentation may vary from one location to another and from one age group to another. Objectives: This study was undertaken to review the prevalence and pattern of severe malaria ...