Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 06 July 2020

Review of data and knowledge gaps regarding yellow fever vaccine-induced immunity and duration of protection
- J. Erin Staples ORCID: orcid.org/0000-0002-1446-4071 1 ,
- Alan D. T. Barrett 2 ,
- Annelies Wilder-Smith 3 , 4 &
- Joachim Hombach 5
npj Vaccines volume 5 , Article number: 54 ( 2020 ) Cite this article
6916 Accesses
41 Citations
9 Altmetric
Metrics details
- Public health
- Viral infection
Yellow fever (YF) virus is a mosquito-borne flavivirus found in Sub-Saharan Africa and tropical South America. The virus causes YF, a viral hemorrhagic fever, which can be prevented by a live-attenuated vaccine, strain 17D. Despite the vaccine being very successful at decreasing disease risk, YF is considered a re-emerging disease due to the increased numbers of cases in the last 30 years. Until 2014, the vaccine was recommended to be administered with boosters every 10 years, but in 2014 the World Health Organization recommended removal of booster doses for all except special populations. This recommendation has been questioned and there have been reports of waning antibody titers in adults over time and more recently in pediatric populations. Clearly, the potential of waning antibody titers is a very important issue that needs to be carefully evaluated. In this Perspective, we review what is known about the correlate of protection for full-dose YF vaccine, current information on waning antibody titers, and gaps in knowledge. Overall, fundamental questions exist on the durability of protective immunity induced by YF vaccine, but interpretation of studies is complicated by the use of different assays and different cut-offs to measure seroprotective immunity, and differing results among certain endemic versus non-endemic populations. Notwithstanding the above, there are few well-characterized reports of vaccine failures, which one would expect to observe potentially more with the re-emergence of a severe disease. Overall, there is a need to improve YF disease surveillance, increase primary vaccination coverage rates in at-risk populations, and expand our understanding of the mechanism of protection of YF vaccine.
Similar content being viewed by others
A phase I clinical study to assess safety and immunogenicity of yellow fever vaccine
Immune response induced by standard and fractional doses of 17DD yellow fever vaccine

Prior flavivirus immunity skews the yellow fever vaccine response to cross-reactive antibodies with potential to enhance dengue virus infection
Introduction.
Yellow fever (YF) virus, a mosquito-borne flavivirus, is present in tropical areas of Africa and South America. Infection in humans can produce a hemorrhagic fever and is fatal in 30–60% of persons with severe disease 1 , 2 . Recent decades have witnessed an unprecedented emergence of YF virus activity, including in highly urbanized areas where vaccination coverage was low 3 , 4 , 5 . It has been recently estimated that roughly 400 million individuals require vaccination within at-risk zones to potentially prevent epidemic of the disease though many more might be at risk due to the recent expansion of risk zones, particularly in Brazil 3 , 6
YF vaccine was first developed in the 1930s after successful attenuation of the Asibi strain of YF virus to generate the strain 17D 7 . Today, three substrains (17D-204, 17DD, and 17D-213) are used as vaccines and are manufactured by six companies, of which four are prequalified by the World Health Organization (WHO) 8 . The vaccine is given as one dose either by subcutaneous or intramuscular administration, with 80% of vaccine recipients develop neutralizing antibodies 10 days post immunization and close to 100% by one month post immunization in clinical trials 9 . However, it has been noted that children <2 years of age can have lower seroconversion rates following a single dose of YF vaccine 10 . No human efficacy studies have ever been performed with the vaccine, but protection has been robustly demonstrated. Evidence for this conclusion include (1) reduction of laboratory-associated infections in vaccinated workers, (2) observation following initial use of the vaccine in Brazil and other South American countries that YF occurred only in unvaccinated persons, (3) rapid disappearance of cases during YF vaccination campaigns initiated during epidemics, (4) very few vaccine failures detected in any endemic country, and (5) protection of rhesus monkeys against virulent wild-type (WT) YF virus challenge by neutralizing antibodies generated in response to YF vaccination 11 , 12 , 13 .
A booster dose requirement for YF vaccine was first put into place in 1959 under the precursor to International Health Regulations (IHR), International Sanitary Regulations, with booster doses initially being required every 9 years based on available data 14 , 15 . The booster dose interval was changed in 1965 to every 10 years based on limited evidence from two published studies that showed neutralizing antibodies were present in most vaccine recipients, including those who received the vaccine in childhood, for at least 10 years after vaccination 16 , 17 . Starting in late 2011, the WHO Strategic Advisory Group of Experts (SAGE) on Immunization YF working group conducted a systematic review of ~17 unpublished and published studies that identified a very low number of vaccine failures and high seropositivity rates following vaccination over time 18 , 19 . From these additional, albeit observational data, SAGE concluded that a single primary dose of YF vaccine is sufficient to confer sustained immunity and lifelong protection against YF disease, and that a booster dose is not needed, except for special populations (e.g., immunocompromised and immunosuppressed) 20 . In May 2014, the World Health Assembly adopted the recommendation to remove the 10-year booster dose requirement from the IHR, which was enacted in June 2016 21 . In 2014, the United States Advisory Committee on Immunization Practices (ACIP) YF vaccine working group conducted a similar systematic review of YF vaccine immunogenicity 10 . However, since SAGE’s recommendation removed the IHR requirement for boosters, ACIP working group reviewed the available data to determine whether or not booster doses were needed as ACIP had never recommended a booster dose of the vaccine before. Based on the available data, ACIP voted in 2015 that a single primary dose of YF vaccine provides long-lasting protection and is adequate for most travelers 22 . However, as a precautionary measure, it was noted that a booster dose may be given to travelers who received their last dose of YF vaccine at least 10 years previously and who will be in a higher-risk setting based on season, location, activities, and duration of their travel. This would include travelers who plan to spend a prolonged period in endemic areas or those traveling to highly endemic areas, such as rural West Africa during peak transmission season or an area with an ongoing outbreak.
Subsequent to SAGE and ACIP recommendations that a single dose of YF vaccine is sufficient to provide lifelong protection in most individuals, several have questioned this decision 23 , 24 , 25 , 26 . Furthermore, several recent studies have noted waning antibody titers after vaccination and potential vaccine failures 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 . Below we note what is known about vaccine immunity, review the additional data that have been generated using full-dose YF vaccine since the SAGE recommendation in 2013, and discuss next steps to determine if booster doses of YF vaccine are needed.
What constitutes YF vaccine immunity?
One of the key questions to know whether or not YF vaccine booster doses are needed is what constitutes protective vaccine immunity. The closest correlate of protection that exists for YF vaccination was established in one study of non-human primates vaccinated with YF vaccine and then challenged with virulent WT YF virus 11 . From this study, log 10 neutralization index (LNI) of ≥0.7 was established as a potential cut-off for protective immunity with 51 (94%) of 54 surviving monkeys having a LNI ≥ 0.7. In comparison, only one (8%) of 12 monkeys who died when challenged had a LNI above 0.7. Currently, plaque reduction neutralization tests (PRNTs) are used to establish the quantitative titers of YF virus-specific antibodies as it uses less serum and is typically easier to perform. Current studies typically report either 90% PRNT (PRNT 90 ), PRNT 80 , or PRNT 50 titers. Although a PRNT 90 titer is more specific as it reduces the likelihood of positive results due to cross-reactive neutralizing antibodies from other flaviviruses, it measures at the bottom of the S-shaped neutralization curve, which leads to less variability and can lead to false-negative results for lower virus-specific antibody titers 35 . PRNT 50 titer are at the midpoint or more linear portion of an S-shaped curve making them higher, more variable and sensitive, but less specific. Most clinical trials for flavivirus vaccines use a PRNT 50 assay with a titer of 1 in 10 as a correlate of protection 36 , 37 , 38 . However, LNI and PRNT have never been formally compared using standardized reagents to understand how they might relate. Furthermore, it is unclear if neutralizing antibodies as measured using current assays are the only correlate of protection. Our understanding of the role of cell-mediated immunity in both the initial immunologic response, as well as longer-term protection is advancing, but it also comes with the uncertainty of what might represent protective types and levels immunity that could prevent a person developing WT YF disease. However, there is general agreement that the pool of memory cells needs to be able to quickly proliferate when challenged to protect an individual as the incubation period of YF is typically short ranging from 3 to 6 days 24 , 39 , 40 .
The question of what constitutes vaccine immunologic memory is not unique to YF vaccine. Smallpox vaccine also was utilized before efficacy studies could be performed and the same questions about vaccine immunity are present for live-attenuated vaccines against vaccinia virus 41 . Although detection of antibodies is used to denote protective immunity following measles vaccination, it also has been documented that individuals lacking detectable neutralizing antibodies can develop secondary immune response with revaccination or exposure to measles virus suggesting that alternative types of immunity exist 42 .
Currently, whether or not the absence of detectable neutralizing antibodies represent an absence of protective immunity against WT YF disease is a critical knowledge gap for YF immunity. As noted above, it is also unclear what amount of antibody might be needed to protect someone against developing a symptomatic infection or viremia. Two studies have documented roughly one-third of individuals with preexisting YF virus-specific neutralizing antibodies fail to develop an anamnestic neutralizing antibody response (i.e., ≥4-fold or greater increase in neutralization titers) following a booster dose suggesting sterilizing immunity that is correlated with higher pre-vaccination titers 9 , 43 . If it is correct that an absence of detectable neutralizing antibodies following primary immunization or the development of an amnestic response following a booster vaccine dose means an absence of protection for YF in a primary vaccinee, one might have expected more cases of WT YF disease to be reported in children 4–10 years post-vaccination 33 . However, epidemiologic data from the recent outbreaks in Brazil indicate that very few cases of WT disease occurred in children, with a lower incidence of WT disease in children compared to adults 5 , 44 . Although this might be secondary to who is being exposed or differences in clinical attack rate, the recent outbreaks occurring near and in urban areas as well as the notable occurrence of cases in women tend to suggest children were likely exposed to the virus in these recent outbreaks. Finally, the development of an amnestic response might not equate to a lack of protection, particularly if the kinetics of the immunologic response is fast enough to blunt the viremia due to a WT infection.
Vaccine failures
Since 2013, there has been several reports of vaccine failures, one in peer-reviewed literature plus epidemiologic reports issued by public health authorities 45 , 46 , 47 . The published study, which has been cited by others in editorials and reviews to support the need for booster doses, came out in 2014 during the ACIP deliberations and describe individuals having a history of YF vaccination who later develop WT YF disease 24 , 26 , 45 . The ACIP YF vaccine working group contacted the Brazil Ministry of Health (MOH) to verify that, as stated, 459 (55%) of 831 YF cases in Brazil from 1973 to 2008 were vaccine failures, including 27 (3%) primary vaccine failures (e.g., occurring after the first 10 days of vaccination but within the first 10 years of vaccination) and 432 (52%) secondary vaccine failures (e.g., occurring more than 10 years after vaccination potentially due to waning antibody titers) 45 . The Brazil MOH provided data to the working group noting that there were seven vaccine failures in Brazil from 1973 to 2008; five constituting primary vaccine failures, and two secondary vaccine failures occurring at 20 and 27 years post vaccination 10 , 45 , 48 , 49 , 50 . Unfortunately, there has never been a publication to clarify that the data were not accurate and it continues to be cited as evidence to support the need for booster doses 33 .
From data reported to the Pan American Health Organization (PAHO) during 2000–2014 and published on their website, 83 (7%) of 1164 of sylvatic YF cases reported from Bolivia, Brazil, Colombia, and Peru occurred in individuals who reported receiving YF vaccine 46 . More recently during the large outbreaks of YF in Brazil, an epidemiologic bulletin noted at least 11 cases of WT YF in individuals who were previously vaccinated and several more cases have been noted during a recent meeting 47 , 51 . Unfortunately, the information about these additional cases is very limited. It is unknown if these cases represent primary or secondary vaccine failures, whether and what confirmatory laboratory testing was performed, and the underlying medical history of the cases (e.g., immunosuppressed or compromised) that might have impacted their initial immunologic response to the vaccine or longer-term immunologic memory. Critically, given that YF IgM antibodies can persist for years following vaccination 52 , obtaining information about how the diagnosis of WT YF disease was made is important to interpret these results. Furthermore, it is important to note that not all individuals respond to YF vaccination; there is a median seroconversion rate of 99% (range 81–100%) in clinical trials 8 . Critically, for a state like Minas Gerais in Brazil with a population over 20 million, this means that even with 100% vaccination coverage more than 200,000 individuals who were vaccinated would fail to develop an immune response to the vaccine and would be at risk for developing disease if exposed.
Seropositivity in vaccinated individuals
Since the SAGE recommendations in 2013, a number of articles have been published related to the immune response seen following YF vaccine, including cohorts of individuals in endemic and non-endemic locations, of different ages, and at different time points following vaccination. All studies used PRNT or microneutralization test for the detection of neutralizing antibodies against YF virus. However, the percent plaque reduction cut-off used and the definition of seropositivity or protection varied by study such that quantity of neutralizing antibodies measured in different studies are difficult to compare 35 . Furthermore, several of the studies did not use the international standard making comparison of seropositivity or antibody concentrations between studies further challenging 53 . The findings of these studies are summarized below.
Humoral immunity in adults
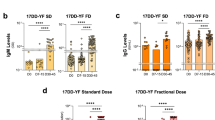
There are data on longer-term humoral immunity for at least eight distinct cohorts of adults in both YF endemic and non-endemic areas of the world who received a full dose of YF vaccine (Table 1 ) 27 , 28 , 31 , 32 , 54 , 55 , 56 , 57 . Notably, there were no apparent differences between studies undertaken in endemic and non-endemic countries. In the first 5 years post-vaccination, seropositivity in the cohorts was >90%. At ≥10 years post-vaccination, the rates of seropositivity were generally lower ranging from 67% to 88% using PRNT 50 –PRNT 90 , except for a small cohort of healthy volunteers in the Netherlands where 97% (34/35) of individuals vaccinated with a full-dose of the vaccine were seropositive at 10 years when measured with PRNT 80 57 . Interestingly, several of the studies saw higher rates of seropositivity 30–35 years post-vaccination compared to rates at 10–20 years post vaccination 54 , 56 . However, the number of individuals in the later vaccination time points are quite limited and they likely received an older vaccination formulation, which have differing quantities of vaccine virus 8 , impacting the generalizability of these results. Several other factors likely impact the overall rates of seropositivity in these studies, such as (1) proof of vaccination 27 , (2) different seropositivity cut-offs 28 , 32 , 35 , (3) different individuals at each time point post-vaccination often with different demographic (e.g., age of vaccination) 27 , 28 , 30 , 56 , (4) potential natural boosting for residents and travelers to endemic areas, and (5) potentially receiving an additional doses of YF vaccine 31 .
Humoral immunity in children
There have been four additional published studies with short-term and long-term immunogenicity for children receiving a full dose of YF vaccine (Table 2 ). The published studies contain cohorts of children who received YF vaccination at 9–23 months of age. Of the two studies published evaluating the seroconversion rate following YF vaccination in children, the rates are highly variable within one of the studies and between the studies 58 , 59 . In a study of 595 children living in Colombia and Peru who received YF vaccine alone or with a tetravalent dengue vaccine on a YF vaccine backbone, the rate of seroconversion was noted to be 99.8–100% when measured by PRNT 50 and titer ≥10 58 . These rates were similar though slightly higher than the rates seen in Mali (95–98%) among children who received a meningococcal A (Men A) vaccine either concurrently or serially with YF vaccine 59 . However, in the same Men A vaccine study, children in Ghana only achieved 68–79% seroconversion rates following YF vaccination. This same trend in lower rates of detectable antibodies between the two populations in the Men A study was seen when the cohorts were followed up at 2–6 years post-vaccination 34 . Seropositivity rates as low as 28% were reported for children in Ghana at 2.3 years post-vaccination, though the rate increased at 6 years post-vaccination to 43%, compared to 50% seropositivity among the children in Mali at 4.5 years post-vaccination 34 . When demographic (age of vaccination, sex), vaccination and exposure history (season of vaccination and pre-vaccination titers), and nutritional status were compared between the children in Mali and Ghana, no significant differences were identified to explain the different rates of seropositivity between these two populations 60 . In the second study evaluating longer-term immunity in different cohorts of children in Brazil up to 10 years post-vaccination, a substantial decline was noted in the seropositivity rates over time 33 . Using a titer ≥10 with PRNT 50 , 54% of children were not seropositive at 7 years post-vaccination. Although the rates of seropositivity increased when using a lower titer cut-off (PRNT ≥ 5), 36% of children at 7 years post-vaccination lacked detectable neutralizing antibodies.
One potential explanation for the varying immune response both initially and potentially longer-term among the pediatric studies could be the age at which the children received their vaccine. Younger age groups might be expected to have a less robust initial immune response, potential immunologic interference from maternal antibodies, or more concomitant infections lead to a decreased immune response 61 , 62 . The cohorts in Mali, Ghana, and some of the children in the Brazil study received YF vaccine at 9 months of age. This is compared to children in Colombia and Peru who received the vaccine at 12 months of age and others in the Brazil cohort who were as old as 23 months when they were vaccinated. However, when the age of vaccination was assessed by the ACIP YF working group relative to the seroconversion rates, the analysis of results from aggregated studies found no difference in seroconversion rates when the children were vaccinated at 9 months of age compared to 12 months 10 , 22 .
With these new pediatric data, there are seemingly more questions than answers to the variability of the results between the pediatric cohorts. The authors of the studies and associated editorials question what contributes to the variability in results hypothesizing that it could be due to differences in immune microenvironment, vaccine substrains used, how the samples were handled, the test used, and potential difference in vaccine handling 33 , 61 , 63 , 64 . Furthermore, in both Ghana and Brazil, the authors questioned whether or not children had received another dose of the vaccine as the proportion seropositive was higher at later time points 33 , 34 .
Additional immunogenicity data
Since 2013, several studies have been published regarding cellular immunity, including CD8+, CD4+, and memory phenotypes, formed in response to YF vaccine 30 , 54 , 55 , 65 . However, the specific impact of alternative types of immunologic memory and their role in protecting persons against disease is not well-characterized or known.
The studies published since SAGE and ACIP made their recommendation that one dose of YF vaccine is sufficient to provide lifelong protection in most individuals provide additional data on YF vaccine immunity. Given the heterogeneity of results, in particular for the pediatric cohorts, further studies would be welcomed.
However, the basic questions that were debated in the discussions of both SAGE and ACIP still remain, how durable is the immunity elicited by YF vaccine and what constitutes protective immunity against YF virus infection and disease? To truly address these questions, additional research and data are needed. Increased transparency and sharing of information on potential vaccine failures are critical to better understand of the >800 million doses the vaccine that have been administered how many might have failed to provide both short-term and long-term protective immunity. With this is the need to continue improving and strengthening YF disease surveillance and laboratory testing 66 , not only to detect possible vaccine failures but also to obtain samples early enough to make a definitive diagnosis of WT disease by molecular testing. In addition, every effort must be made to ascertain the vaccination status of the patient. As noted above, using standards and evaluating the correlation between neutralization titers determined by LNI and PRNT would improve our ability to compare studies and begin to set thresholds as to what antibodies levels are needed to potentially prevent WT disease. Furthermore, additional research is needed to determine the kinetics of the immune response when a vaccinee receives a booster vaccine dose or has a WT infection (e.g., does an amnestic response mean a lack of adequate protection?) and to validate the immune correlate of protection following YF vaccination using more modern knowledge and techniques (e.g., assessing the role of cellular immunity). WHO currently plans to receive input from subject matter experts on how best to proceed with measuring YF vaccine immunity in a consistent manner to allow for comparability between studies.
Overall, we expect the debate of whether or not to give booster doses of YF vaccine to continue in lieu of more data. However, one clear public health action that can and should be taken now is to improve YF vaccination coverage among children living in at risk areas. Based on WHO and UNICEF estimates of vaccine coverage (WUENIC), YF vaccination rates among children living in YF endemic areas ranges from 42% to 97% (median of 85%) in the Americas and 29–94% (median: 68%) in Africa 67 . The current large outbreaks of measles throughout the world, including in YF endemic areas where the vaccines are often given at the same visit, reinforces poor YF vaccination rates that exist among children. If children do not even receive their first dose of YF vaccine, it is hard to focus on whether they might need a booster dose. We encourage researchers, clinicians, and public health officials to continue to evaluate and publish quality data on YF vaccine immunity and vaccine failures to inform public health policy related to YF vaccine use and optimize our ability to prevent YF.
Johansson, M. A., Vasconcelos, P. F. & Staples, J. E. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 108 , 482–487 (2014).
PubMed PubMed Central Google Scholar
Ho, Y. L. et al. Severe yellow fever in Brazil: clinical characteristics and management. J. Travel Med. 26 , https://doi.org/10.1093/jtm/taz040 (2019).
Rezende, I. M. et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 12 , e0006538 (2018).
Pan American Health Organization. Epidemiologic Update: Yellow Fever. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=yellow-fever-2194&alias=43619-16-february-2018-yellow-fever-epidemiological-update-619&Itemid=270&lang=en (2018).
Moussallem, T. M. et al. Yellow fever outbreak in a rural–urban mixed community of Espirito Santo, Brazil: epidemiological aspects. Rev. Panam. salud publ. = Pan Am. J. Public Health 43 , e29 (2019).
Google Scholar
Shearer, F. M. et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect. Dis. 17 , 1209–1217 (2017).
Barrett, A. D. Yellow fever vaccines. Biologicals 25 , 17–25 (1997).
CAS PubMed Google Scholar
Staples, J. E., Monath, T. P., Gershman, M. D. & Barrett, A. D. T. In Plotkin’s Vaccines (eds Plotkin, S. A., Orenstein, W. A., Offit, P. A. & Edwards, K. M.) 1181–1265 (Elsevier Inc., 2018).
Casey, R. M. et al. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak—final report. N. Engl. J. Med. 381 , 444–454 (2019).
Advisory Committee on Immunization Practices (ACIP). Grading of Recommendations, Assessment, Development, And Evaluation (GRADE) for Use of Yellow Fever Vaccine Booster Doses. https://www.cdc.gov/vaccines/acip/recs/grade/yf-vac-boost.html (2015).
Mason, R. A., Tauraso, N. M., Spertzel, R. O. & Ginn, R. K. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 25 , 539–544 (1973).
CAS PubMed PubMed Central Google Scholar
Staples, J. E., Gershman, M. & Fischer, M. Centers for Disease Control & Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 59 (RR07), 1–27 (2010).
Elliott, M. Yellow fever in the recently inoculated. Trans. R. Soc. Trop. Med. Hyg. 38 , 231–234 (1944).
Courtois, G. [Duration of immunity after yellow fever vaccination]. Ann. Soc. Belg. Med. trop. 34 , 9–12 (1954).
CAS Google Scholar
Dick, G. W. & Gee, F. L. Immunity to yellow fever nine years after vaccination with 17D vaccine. Trans. R. Soc. Trop. Med. Hyg. 46 , 449–458 (1952).
Groot, H. & Riberiro, R. B. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull. World Health Organ. 27 , 699–707 (1962).
Rosenzweig, E. C., Babione, R. W. & Wisseman, C. L. Jr. Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am. J. Trop. Med. Hyg. 12 , 230–235 (1963).
Gotuzzo, E., Yactayo, S. & Cordova, E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 89 , 434–444 (2013).
World Health Organization. SAGE Yellow fever work. Background Paper Yellow Fever Vaccines. http://www.who.int/entity/immunization/sage/meetings/2013/april /1_Background_Paper_Yellow_Fever_Vaccines.pdf?ua=1 (2013).
World Health Organization. Vaccines and vaccination against yellow fever. WHO position paper–June 2013. Wkly. Epidemiol. Rec. 88 , 269–283 (2013).
World Health Organization. International and Traveler Health: World—Yellow Fever Vaccination Booster . http://www.who.int/ith/updates/20140605/en/ (2014).
Staples, J. E., Bocchini, J. A. Jr., Rubin, L. & Fischer, M. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine booster doses: recommendations of the Advisory Committee on Immunization Practices, 2015. Morbid. Mortal. Wkly. Rep. 64 , 647–650 (2015).
Grobusch, M. P. et al. Yellow fever revaccination guidelines change—a decision too feverish? Clin. Microbiol. Infect. 19 , 885–886 (2013).
Plotkin, S. A. Ten yearly yellow fever booster vaccinations may still be justified. J. Travel Med. 25 , https://doi.org/10.1093/jtm/tay130 (2018).
Vasconcelos, P. F. Single shot of 17D vaccine may not confer life-long protection against yellow fever. Mem. Inst. Oswaldo Cruz 113 , 135–137 (2018).
PubMed Google Scholar
Amanna, I. J. & Slifka, M. K. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev. Vaccines 15 , 1519–1533 (2016).
Kareko, B. W. et al. Persistence of neutralizing antibody responses among yellow fever virus 17D vaccinees living in a nonendemic setting. J. Infect. Dis. 221 , 2018–2025 (2020).
Collaborative group for studies on yellow fever. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 32 , 4977–4984 (2014).
Collaborative group for studies on yellow fever. Duration of immunity in recipients of two doses of 17DD yellow fever vaccine. Vaccine 37 , 5129–5135 (2019).
Campi-Azevedo, A. C. et al. 17DD yellow fever revaccination and heightened long-term immunity in populations of disease-endemic areas, Brazil. Emerg. Infect. Dis. 25 , 1511–1521 (2019).
Miyaji, K. T. et al. Prevalence and titers of yellow fever virus neutralizing antibodies in previously vaccinated adults. Rev. Inst. Med. Trop. Sao Paulo 59 , e2 (2017).
de Menezes Martins, R. et al. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine 36 , 4112–4117 (2018).
de Noronha, T. G. et al. Duration of post-vaccination humoral immunity against yellow fever in children. Vaccine 37 , 7147–7154 (2019).
Domingo, C. et al. Long-term immunity against yellow fever in children vaccinated during infancy: a longitudinal cohort study. Lancet Infect. Dis. 19 , 1363–1370 (2019).
Barrett, A. D. T. Yellow fever vaccine: the conundrum of 2 doses, one dose, or one-fifth dose to induce and maintain protective immunity. J. Infect. Dis. 221 , 1922–1924 (2020).
World Health Organization. Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. https://apps.who.int/iris/bitstream/handle/10665/69687/who_ivb_07.07_eng.pdf;jsessionid=19BF765D9B3FDAD85EF3F217B0165953?sequence=1 (2007).
Hombach, J., Solomon, T., Kurane, I., Jacobson, J. & Wood, D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 23 , 5205–5211 (2005).
Roehrig, J. T., Hombach, J. & Barrett, A. D. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 21 , 123–132 (2008).
Johansson, M. A., Arana-Vizcarrondo, N., Biggerstaff, B. J. & Staples, J. E. Incubation periods of Yellow fever virus. Am. J. Trop. Med. Hyg. 83 , 183–188 (2010).
Visser, L. G., Veit, O. & Chen, L. H. Waning immunity after single-dose yellow fever vaccination: who needs a second shot? J. Travel Med. 26 , https://doi.org/10.1093/jtm/tay134 (2019).
Kennedy, R. B., Lane, J. M., Henderson, D. A. & Poland, G. A. in Plotkin’s Vaccine (eds Plotkin, S. A., Orenstein, W. A., Offit, P. A. & Edwards, K. M.) 1001–1030 (Elsevier Inc., 2018).
Erdman, D. D., Heath, J. L., Watson, J. C., Markowitz, L. E. & Bellini, W. J. Immunoglobulin M antibody response to measles virus following primary and secondary vaccination and natural virus infection. J. Med. Virol. 41 , 44–48 (1993).
Hepburn, M. J. et al. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine 24 , 2843–2849 (2006).
Brasil Ministerio da Saude. [Yellow Fever Seasonal Monitoring Brasil—2017/2018] https://portalarquivos2.saude.gov.br/images/pdf/2018/outubro/08/Informe-FA.pdf (2018).
Camara, F. P., de Carvalho, L. M. & Gomes, A. L. Demographic profile of sylvatic yellow fever in Brazil from 1973 to 2008. Trans. R. Soc. Trop. Med. Hyg. 107 , 324–327 (2013).
Pan American Health Organization. Yellow Fever: Vaccination Status Among Confirmed Cases in the Americas, 2000–2014. http://ais.paho.org/phip/viz/ed_yellowfever.asp (2019).
Minas Gerais Secretaria de Estado de Saude. [Epidemiologic Information on Yellow Fever]. http://www.saude.mg.gov.br/fornecimento-de-medicamentos/story/10284-informe-epidemiologico-da-febre-amarela-27-02 (2018).
Tuboi, S. H., Costa, Z. G., da Costa Vasconcelos, P. F. & Hatch, D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans. R. Soc. Trop. Med. Hyg. 101 , 169–175 (2007).
Filippis, A. M. et al. Isolation and characterization of wild type yellow fever virus in cases temporally associated with 17DD vaccination during an outbreak of yellow fever in Brazil. Vaccine 22 , 1073–1078 (2004).
Saraiva, M. et al. Historical analysis of the records of sylvan yellow fever in the State of Amazonas, Brazil, from 1996 to 2009. Rev. Soc. Bras. Med. Trop. 46 , 223–226 (2013).
Fereguetti, T. Novel clinical findings associated with YF in rural Brazil in Symposium 69: American Committee on Arthropod-Borne Viruses (ACAV) Symposium II: Everything Old Is New Again - The Re-Emergence of Yellow Fever. 68th Annual Meeting of American Society of Tropical Medicine and Hygiene (National Harbor, MD, USA, 2019).
Gibney, K. B. et al. Detection of anti-yellow fever virus immunoglobulin m antibodies at 3–4 years following yellow fever vaccination. Am. J. Trop. Med. Hyg. 87 , 1112–1115 (2012).
Ferguson, M. Collaborative study to assess the suitability of a candidate International Standard for yellow fever vaccine. Biologicals 32 , 195–205 (2004).
Wieten, R. W. et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS ONE 11 , e0149871 (2016).
Campi-Azevedo, A. C. et al. Short-lived immunity after 17DD yellow fever single dose indicates that booster vaccination may be required to guarantee protective immunity in child. Front. Immunol. 10 , 2192 (2019).
Lindsey, N. P. et al. Persistence of yellow fever virus-specific neutralizing antibodies after vaccination among US travellers. J. Travel Med. 25 , https://doi.org/10.1093/jtm/tay108 (2018).
Roukens, A. H. E., van Halem, K., de Visser, A. W. & Visser, L. G. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann. Intern. Med. 169 , 761–765 (2018).
Lopez, P. et al. Immunogenicity and safety of yellow fever vaccine (stamaril) when administered concomitantly with a tetravalent dengue vaccine candidate in healthy toddlers at 12–13 months of age in Colombia and Peru: a randomized trial. Pediatr. Infect. Dis. J. 35 , 1140–1147 (2016).
Roy Chowdhury, P. et al. Immunogenicity of yellow fever vaccine coadministered with MenAfriVac in healthy infants in Ghana and Mali. Clin. Infect. Dis. 61 , S586–593 (2015).
Idoko, O. T. et al. Antibody responses to yellow fever vaccine in 9 to 11-month-old Malian and Ghanaian children. Expert Rev. Vaccines 18 , 867–875 (2019).
Muyanja, E. et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J. Clin. Investig. 124 , 3147–3158 (2014).
Albrecht, P., Ennis, F. A., Saltzman, E. J. & Krugman, S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91 , 715–718 (1977).
Vasconcelos, P. F. C. & Barrett, A. D. T. Are booster doses of yellow fever vaccine needed?. Lancet Infect. Dis. 19 , 1275–1276 (2019).
Domingo, C. & Niedrig, M. Safety of 17D derived yellow fever vaccines. Expert Opin. Drug Saf. 8 , 211–221 (2009).
Kongsgaard, M. et al. Adaptive immune responses to booster vaccination against yellow fever virus are much reduced compared to those after primary vaccination. Sci. Rep. 7 , 662 (2017).
Berkley, S. Health security’s blind spot. Science 359 , 1075 (2018).
Adrien, N. et al. Differences between coverage of yellow fever vaccine and the first dose of measles-containing vaccine: a desk review of global data sources. Vaccine 37 , 4511–4517 (2019).
Wieten, R. W. et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J. Infect. 72 , 713–722 (2016).
Martins, R. M. et al. 17DD yellow fever vaccine: a double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum. Vaccines Immunother. 9 , 879–888 (2013).
Download references
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and World Health Organization.
Author information
Authors and affiliations.
Arboviral Diseases Branch, U.S. Centers for Disease Control and Prevention, Fort Collins, CO, USA
J. Erin Staples
Department of Pathology and Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston, TX, USA
Alan D. T. Barrett
Institute of Public Health, University of Heidelberg, Heidelberg, Germany
Annelies Wilder-Smith
London School of Hygiene and Tropical Medicine, London, UK
World Health Organization, Geneva, Switzerland
Joachim Hombach
You can also search for this author in PubMed Google Scholar
Contributions
J.E.S., A.D.T.B., and J.H. contributed to the conception of the manuscript; J.E.S., A.D.T.B., A.W.-S., and J.H. contributed to reviewing and interpreting available literature; J.E.S., A.D.T.B., and A.W.-S. contributed to drafting the manuscript; and J.E.S., A.D.T.B., A.W.-S., and J.H. contributed to reviewing and editing the manuscript. All authors approved the submitted version and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Correspondence to J. Erin Staples .
Ethics declarations
Competing interests.
J.E.S., A.W.-S., J.H. declares no competing interests. A.D.T.B. is Editor-in-Chief of npj Vaccines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Staples, J.E., Barrett, A.D.T., Wilder-Smith, A. et al. Review of data and knowledge gaps regarding yellow fever vaccine-induced immunity and duration of protection. npj Vaccines 5 , 54 (2020). https://doi.org/10.1038/s41541-020-0205-6
Download citation
Received : 13 January 2020
Accepted : 29 May 2020
Published : 06 July 2020
DOI : https://doi.org/10.1038/s41541-020-0205-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genomic diversity contributes to the neuroinvasiveness of the yellow fever french neurotropic vaccine.
- Florian Bakoa
- Christophe Préhaud
- Nolwenn Jouvenet
npj Vaccines (2021)
A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate
- Lorena Sanchez-Felipe
- Thomas Vercruysse
- Kai Dallmeier
Nature (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Loading metrics
Open Access
Peer-reviewed
Research Article
Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data
Affiliation MRC Centre for Outbreak Analysis, Department of Infectious Disease Epidemiology, Imperial College London, United Kingdom
Affiliation World Health Organization, Geneva, Switzerland
Affiliation Immunization and Vaccine Development, World Health Organization, Ouagadougou, Burkina Faso
Affiliation Ottawa Public Health, Ottawa, Ontario, Canada
Affiliation Arboviral Disease Branch, Centers for Disease Control and Prevention, Fort Collins, Colorado, United States of America
* E-mail: [email protected]
¶ Membership of the Yellow Fever Expert Committee is provided in the Acknowledgments.
- Tini Garske,
- Maria D. Van Kerkhove,
- Sergio Yactayo,
- Olivier Ronveaux,
- Rosamund F. Lewis,
- J. Erin Staples,
- William Perea,
- Neil M. Ferguson,
- for the Yellow Fever Expert Committee

- Published: May 6, 2014
- https://doi.org/10.1371/journal.pmed.1001638
- Reader Comments
Yellow fever is a vector-borne disease affecting humans and non-human primates in tropical areas of Africa and South America. While eradication is not feasible due to the wildlife reservoir, large scale vaccination activities in Africa during the 1940s to 1960s reduced yellow fever incidence for several decades. However, after a period of low vaccination coverage, yellow fever has resurged in the continent. Since 2006 there has been substantial funding for large preventive mass vaccination campaigns in the most affected countries in Africa to curb the rising burden of disease and control future outbreaks. Contemporary estimates of the yellow fever disease burden are lacking, and the present study aimed to update the previous estimates on the basis of more recent yellow fever occurrence data and improved estimation methods.
Methods and Findings
Generalised linear regression models were fitted to a dataset of the locations of yellow fever outbreaks within the last 25 years to estimate the probability of outbreak reports across the endemic zone. Environmental variables and indicators for the surveillance quality in the affected countries were used as covariates. By comparing probabilities of outbreak reports estimated in the regression with the force of infection estimated for a limited set of locations for which serological surveys were available, the detection probability per case and the force of infection were estimated across the endemic zone.
The yellow fever burden in Africa was estimated for the year 2013 as 130,000 (95% CI 51,000–380,000) cases with fever and jaundice or haemorrhage including 78,000 (95% CI 19,000–180,000) deaths, taking into account the current level of vaccination coverage. The impact of the recent mass vaccination campaigns was assessed by evaluating the difference between the estimates obtained for the current vaccination coverage and for a hypothetical scenario excluding these vaccination campaigns. Vaccination campaigns were estimated to have reduced the number of cases and deaths by 27% (95% CI 22%–31%) across the region, achieving up to an 82% reduction in countries targeted by these campaigns. A limitation of our study is the high level of uncertainty in our estimates arising from the sparseness of data available from both surveillance and serological surveys.
Conclusions
With the estimation method presented here, spatial estimates of transmission intensity can be combined with vaccination coverage levels to evaluate the impact of past or proposed vaccination campaigns, thereby helping to allocate resources efficiently for yellow fever control. This method has been used by the Global Alliance for Vaccines and Immunization (GAVI Alliance) to estimate the potential impact of future vaccination campaigns.
Please see later in the article for the Editors' Summary
Editors' Summary
Yellow fever is a flavivirus infection that is transmitted to people and to non-human primates through the bites of infected mosquitoes. This serious viral disease affects people living in and visiting tropical regions of Africa and Central and South America. In rural areas next to forests, the virus typically causes sporadic cases or even small-scale epidemics (outbreaks) but, if it is introduced into urban areas, it can cause large explosive epidemics that are hard to control. Although many people who contract yellow fever do not develop any symptoms, some have mild flu-like symptoms, and others develop a high fever with jaundice (yellowing of the skin and eyes) or hemorrhaging (bleeding) from the mouth, nose, eyes, or stomach. Half of patients who develop these severe symptoms die. Because of this wide spectrum of symptoms, which overlap with those of other tropical diseases, it is hard to diagnose yellow fever from symptoms alone. However, serological tests that detect antibodies to the virus in the blood can help in diagnosis. There is no specific antiviral treatment for yellow fever but its symptoms can be treated.
Why Was This Study Done?
Eradication of yellow fever is not feasible because of the wildlife reservoir for the virus but there is a safe, affordable, and highly effective vaccine against the disease. Large-scale vaccination efforts during the 1940s, 1950s, and 1960s reduced the yellow fever burden for several decades but, after a period of low vaccination coverage, the number of cases rebounded. In 2005, the Yellow Fever Initiative—a collaboration between the World Health Organization (WHO) and the United Nations Children Fund supported by the Global Alliance for Vaccines and Immunization (GAVI Alliance)—was launched to create a vaccine stockpile for use in epidemics and to implement preventive mass vaccination campaigns in the 12 most affected countries in West Africa. Campaigns have now been implemented in all these countries except Nigeria. However, without an estimate of the current yellow fever burden, it is hard to determine the impact of these campaigns. Here, the researchers use recent yellow fever occurrence data, serological survey data, and improved estimation methods to update estimates of the yellow fever burden and to determine the impact of mass vaccination on this burden.
What Did the Researchers Do and Find?
The researchers developed a generalized linear statistical model and used data on the locations where yellow fever was reported between 1987 and 2011 in Africa, force of infection estimates for a limited set of locations where serological surveys were available (the force of infection is the rate at which susceptible individuals acquire a disease), data on vaccination coverage, and demographic and environmental data for their calculations. They estimate that about 130,000 yellow fever cases with fever and jaundice or hemorrhage occurred in Africa in 2013 and that about 78,000 people died from the disease. By evaluating the difference between this estimate, which takes into account the current vaccination coverage, and a hypothetical scenario that excluded the mass vaccination campaigns, the researchers estimate that these campaigns have reduced the burden of disease by 27% across Africa and by up to 82% in the countries targeted by the campaigns (an overall reduction of 57% in the 12 targeted countries).
What Do These Findings Mean?
These findings provide a contemporary estimate of the burden of yellow fever in Africa. This estimate is broadly similar to the historic estimate of 200,000 cases and 30,000 deaths annually, which was based on serological survey data obtained from children in Nigeria between 1945 and 1971. Notably, both disease burden estimates are several hundred-fold higher than the average number of yellow fever cases reported annually to WHO, which reflects the difficulties associated with the diagnosis of yellow fever. Importantly, these findings also provide an estimate of the impact of recent mass vaccination campaigns. All these findings have a high level of uncertainty, however, because of the lack of data from both surveillance and serological surveys. Other assumptions incorporated in the researchers' model may also affect the accuracy of these findings. Nevertheless, the framework for burden estimation developed here provides essential new information about the yellow fever burden and the impact of vaccination campaigns and should help the partners of the Yellow Fever Initiative estimate the potential impact of future vaccination campaigns and ensure the efficient allocation of resources for yellow fever control.
Additional Information
Please access these websites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1001638 .
- The World Health Organization provides detailed information about yellow fever (in several languages), including photo stories about vaccination campaigns in the Sudan and Mali; it also provides information about the Yellow Fever Initiative (in English and French)
- The GAVI Alliance website includes detailed of its support for yellow fever vaccination
- The US Centers for Disease Control and Prevention provides information about yellow fever for the public, travelers, and health care providers
- The UK National Health Service Choices website also has information about yellow fever
- Wikipedia has a page on yellow fever that includes information about the history of the disease (note that Wikipedia is a free online encyclopedia that anyone can edit; available in several languages)
Citation: Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, et al. (2014) Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data. PLoS Med 11(5): e1001638. https://doi.org/10.1371/journal.pmed.1001638
Academic Editor: Simon I. Hay, University of Oxford, United Kingdom
Received: June 7, 2013; Accepted: March 27, 2014; Published: May 6, 2014
This is an open-access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Funding: The research leading to these results has received funding from the Medical Research Council, the Bill & Melinda Gates Foundation, and the European Union Seventh Framework Programme [FP7/2007–2013] under Grant Agreement n°278433-PREDEMICS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: AUC, area under the curve; BIC, Bayesian Information Criterion; EPI, Enhanced Programme for Immunization; EVI, enhanced vegetation index; GAVI, Global Alliance for Vaccines and Immunization; MCMC, Markov Chain Monte Carlo; MIR, middle infrared reflectance; YFSD, yellow fever surveillance database
Introduction
Yellow fever is a flavivirus infection that is transmitted primarily by mosquitoes of the species Aedes ssp. and Haemagogus spp., with humans and non-human primates being the main vertebrate hosts. It is endemic in tropical areas of Africa and Central and South America. The clinical course of infection in humans shows a wide spectrum of severity including asymptomatic infection, mild illness with flu-like symptoms, and severe disease including fever with jaundice or haemorrhage and death.
Several different transmission cycles have been defined, depending on which host and vector species are involved in transmission: in the sylvatic cycle, tree-dwelling mosquitoes of Aedes spp. (Africa) or Haemagogus spp. (Americas) transmit the virus to non-human primates. In this cycle, spill-over infection of humans occurs when they encroach on this jungle habitat. Conversely, in the urban transmission cycle, humans are the main hosts with transmission occurring via domestic mosquito species. The typical urban vector is Aedes aegyptii , which also serves as the main vector for dengue virus transmission. If yellow fever is introduced into urban areas, large explosive outbreaks can occur, which can be difficult to control. In Africa, there is also an intermediate transmission cycle that occurs in rural areas typically at the edges of forests with humans as well as non-human primates affected, and transmission driven by domestic and semi-domestic mosquito species [1] , [2] .
While eradication of yellow fever is not feasible due to the sylvatic reservoir, a high level of control is achievable owing to the availability of an efficacious and safe vaccine that confers long-lasting immunity from a single dose. Visas for many countries worldwide require proof of previous vaccination against yellow fever, particularly if travelers come from or have visited yellow fever endemic areas, in order to prevent the importation of the disease.
Quantifying the burden of disease caused by yellow fever is made challenging by the wide spectrum of clinical severity, with non-specific symptoms in the majority of infections making diagnosis difficult. In addition, there are considerable limitations in the surveillance and health care systems across much of the affected regions. However, it is clear that yellow fever is substantially underreported [3] , [4] . Previous estimates from the early 1990s placed the burden of disease at 200,000 cases and 30,000 deaths annually [5] , [6] . These estimates relied heavily on data from serological surveys performed in children in Nigeria between 1945 and 1971 [7] . These data still form the basis of more recent efforts to quantify disease burden or the cost-effectiveness of vaccines [8] , [9] . More recent approaches to quantify yellow fever circulation have focused on producing risk maps [10] – [12] , frequently employing regression techniques similar to the approach we adopt [10] , [12] , or relying on expert advice regarding local yellow fever distribution [11] , [12] . However, there are no recent estimates of the yellow fever burden that take into account more recent surveillance and serological data and that account for vaccination coverage.
In 2005, the Yellow Fever Initiative was launched as a collaboration between WHO and the United Nations Children's Fund (UNICEF) with support from the Global Alliance for Vaccines and Immunization (GAVI Alliance). The aim was to secure the precarious yellow fever vaccine supply by creating a vaccine stockpile to be used in outbreak response campaigns as well as to increase the vaccination coverage in the most affected areas by implementation of large preventive mass vaccination campaigns in 12 of the most affected countries in West Africa. Between 2006 and 2012, these campaigns have been implemented in all of these countries apart from Nigeria because of larger than anticipated vaccine needs and limited vaccine supplies. In the same time frame, the Central African Republic, though not covered under the Yellow Fever Initiative, also performed mass vaccination campaigns with support from the GAVI Alliance.
During the October 2011 meeting of the advisory committee on Quantitative Immunization and Vaccine Related Research ([QUIVER], currently named Immunization and Vaccines related Implementation Research [IVIR]), the Advisory Committee recommended that WHO establish a working group to generate updated yellow fever disease burden estimates for Africa. This paper reports the results of this activity, presenting new estimates of the disease burden caused by yellow fever in Africa and the impact of preventive vaccination campaigns carried out under the Yellow Fever Initiative. The estimates are derived from a coherent model framework that integrates all available data including incidence, serology, and vaccination coverage.
We fitted a generalised linear model to the locations where yellow fever was reported in the 25-year period between 1987 and 2011. This model estimated, for each location, the probability of at least one yellow fever report over the observation period. The number of infections required to give rise to these probabilities of occurrence was then estimated by taking into account the probability of detection of yellow fever cases in each country. Estimated numbers of infections were converted to estimates of the force of infection using data on the population size, age distribution, and age-specific vaccination coverage in the observation period. Again using demographic and vaccination coverage data, the burden in terms of the number of infections, severe cases presenting with fever and jaundice or haemorrhage, or deaths can then be obtained from the estimates of the force of infection for each location for any year in the past or future, given assumptions on population growth and size of future vaccination campaigns.
The model was fitted at a spatial resolution of the first sub-national administrative unit (which in many countries is called “province”; this is the terminology adopted throughout this manuscript), so all datasets were resolved or aggregated to this level as appropriate.
Yellow fever occurrence.
A database of the locations of reported outbreaks between 1987 and 2011 was compiled from various sources including the Weekly Epidemiological Record (WER) [13] , the WHO disease outbreak news (DON) [14] , an internal WHO database of outbreaks between 1980 and 2007, and the published literature. Locations were resolved to the province level, and data were recorded for each outbreak on the year of occurrence, size, and control measures implemented. Outbreak reports that could not be located at the province level were excluded.
In 2005, the African Regional Office of WHO established a yellow fever surveillance database (YFSD) of reports of suspected yellow fever cases (based primarily on a case definition of fever with jaundice) across 21 countries in West and central Africa. Data fields recorded for each case included age, gender, location, disease onset date, and the status of laboratory confirmation. The locations of all lab-confirmed cases between 2005 and 2011, resolved to the province level, were combined with the outbreaks dataset to generate an overall dataset of the areas of yellow fever occurrence, recording for each province whether or not there had been at least one yellow fever outbreak or case report in the period from 1987 to 2011.
Due to the very low proportion of suspected cases actually being attributed to yellow fever in the YFSD, the majority of cases reported likely had other causes (for instance viral hepatitis). Hence the national incidence of suspected cases is best interpreted as a measure of the effort put into yellow fever surveillance rather than a measure of yellow fever incidence itself. The incidence of suspected cases was aggregated at the country level and divided by the national population to be used as a covariate in the regression models fitted throughout.
Disease severity.
The proportion of infections presenting as severe cases and the proportion of severe cases resulting in death varies substantially between settings, depending on previous exposure to other flaviviruses, but also factors such as clinical care and importantly detection bias due to surveillance coverage or case definitions used [1] , [15] – [20] . Recent work by Johansson and colleagues [21] has estimated the proportion of infections that are asymptomatic, cause mild symptoms (excluding jaundice and haemorrhage), or severe symptoms (including jaundice, haemorrhage, or death), as well as the proportion of severe cases leading to death. We use these estimates of 13% (95% CI 5%–28%) of infections presenting as severe cases, and 46% (95% CI 31%–60%) of severe cases resulting in death to estimate the number of severe cases and deaths from the number of infections estimated by our model.

Vaccination coverage.
No comprehensive dataset of yellow fever vaccination coverage in the endemic area in Africa was available, so vaccination coverage was estimated using data on (i) large-scale mass vaccination activities in French West Africa during the 1940s to 1960s [22] ; (ii) outbreak response campaigns since 1970, as reported in outbreak reports in the WER or DONs [13] , [14] ; (iii) routine infant yellow fever vaccination occurring as part of the Enhanced Programme for Immunization (EPI) [23] ; and (iv) mass vaccination campaigns in 11 West African countries under the Yellow Fever Initiative and the Central African Republic from 2006 to 2012 [24] , [25] .
Information on yellow fever vaccination was compiled into a dataset of age-specific vaccination coverage at the second sub-national administrative level (district), taking into account the location and extent of each campaign as well as the demographics of the targeted populations. This dataset allowed the achieved coverage to be tracked through time for each birth cohort in each district.
The available information on vaccination activities varied greatly from country to country, sometimes specifying the coverage achieved in a certain area, sometimes the number of doses administered during a vaccination campaign, and sometimes both. If the area targeted by a campaign was well defined geographically we used information on the vaccination coverage achieved by that campaign in preference to the number of doses administered in order to avoid uncertainty in population size affecting our estimates. If no information on the coverage achieved was available or the target population was not sufficiently well defined, we calculated vaccination coverage as the number of doses administered divided by the population size, assuming that individuals from all targeted age groups had an equal chance of receiving the vaccine, and that vaccination was performed irrespective of previous vaccination or disease history.
From the vaccination coverage achieved in individual vaccination campaigns the coverage at the population level over time was obtained by tracking vaccination coverage in each birth cohort. In compiling the vaccination coverage dataset, population movements were ignored, and 100% vaccine efficacy was assumed, with lifelong protection. The last two assumptions are supported by data showing that 99% of individuals seroconvert within 30 days of vaccination [1] , [26] , and neutralising antibodies have been measured 35 years post vaccination [26] – [28] .
In estimating the impact of potential future vaccination campaigns we assumed that no further outbreak response vaccination campaigns would be undertaken and that the country-specific coverage in the infant immunization campaigns would be held constant at the levels estimated for 2011 (see Table S1 ) [23] .
Serological surveys.
Serological surveys have been used historically to assess overall levels of transmission. All literature on yellow fever serologic surveys conducted in Africa and published since 1980 were reviewed and the results collated [21] . For the analysis of transmission intensity, only surveys that had samples tested for yellow fever virus specific neutralising antibodies and were not part of an outbreak investigation were considered [29] – [34] , as surveys conducted in outbreak situations are typically not representative. Even if random population samples are obtained in an outbreak-associated survey, serology would be expected to yield information on the attack rate for that specific outbreak rather than the average force of infection over a longer time period.
Demographic data.
Demographic data on population size and age distribution at a sub-national level were used to interpret the data on vaccination campaigns as well as for estimating the burden. We used UN World population prospects (WPPs) [35] estimates of the population size by country in 5-year age bands for each year between 1950 and 2100. In order to achieve a higher spatial resolution of the population distribution, these estimates were combined with the LandScan 2007 dataset [36] , [37] , which gave estimates for the year 2007 of the total population on a grid of resolution of 1/120 degree latitude and longitude, which is approximately 1 km at the equator. By allocating each grid point to the second sub-national administrative unit (which in many countries is the district), the proportion of each country's population living in any particular district was estimated. In the absence of more detailed datasets, it was assumed that the age distributions were homogeneous within each country, neglecting local differences, for instance between rural and urban areas. We furthermore assumed that population growth was homogeneous within a country, and that the population proportions for each district obtained from the LandScan 2007 dataset were applicable to all other years. Thus we did not capture trends in urbanisation or other shifts in the relative population sizes of different districts over time.
We disaggregated the 5-year age bands of the UN WPP dataset into annual birth cohorts using the method described in Text S1 .
Population based variables for the regression model included the total population for each province, the logarithm of the population size and the proportion of the population living in urban areas (defined as LandScan 2007 dataset pixels with a population density of ≥386 people per sq km [38] ).
Environmental data.
Environmental datasets on rainfall [39] , day- and night-time air temperatures [40] , land cover classifications [41] , [42] , the enhanced vegetation index (EVI), the middle infrared reflectance (MIR) [43] , longitude, latitude, and altitude [44] , [45] were used as potential covariates in the generalised linear model. These data were available as gridded datasets of various spatial resolutions between about 1 km and 10 km, and were aggregated to province level by calculating the mean value for each variable, weighted by the population size attributed to each grid cell in order to obtain values representative of the conditions where human populations are concentrated.
For the land cover classification, the proportion of pixels (weighted by population size) for each category was aggregated for each province to obtain scalar variables. In the endemic zone, some of the 17 defined land cover classes occurred very scarcely or not at all, so we only considered those that accounted to over 5% of the area in at least one province as potential covariates. This resulted in the four categories of evergreen needleleaf forest, deciduous needleleaf forest, mixed forests, and snow and ice being excluded.
For each time-varying variable, the annual mean and the average annual minimum and maximum levels were considered, on the basis of 4-year time series obtained for the period from 2003 to 2006. To evaluate the average annual minimum and maximum, time series were smoothed using Fourier transforms as described by Garske and colleagues [40] . The minimum and maximum of these smoothed curves determined the typical annual minimum and maximum used here. The variable that varied with time were the night- and day-time air temperatures [40] , EVI, MIR [43] , and rainfall [39] .
Prior to fitting, all variables were scaled to unit variance in order to improve model convergence and make the fitted slope parameters comparable.
Model Structure and Fitting
The overall model consisted of several components that were fitted jointly using standard Markov Chain Monte Carlo (MCMC) techniques [46] , [47] .
Generalised linear model for the presence/absence of yellow fever reports.
As the occurrence of yellow fever certainly depends on environmental factors such as climate, land cover, but also the human population size, several environmental variables were considered as potential covariates. However, the number of such potential covariates was large, so the first step in variable selection was to fit univariate models to the dataset including each of the potential covariates in turn. Any variables that were not significantly associated with the data at the 10% confidence limit were excluded from further consideration. Some of the remaining variables were highly correlated, and inclusion of highly correlated variables in regression models can lead to instabilities in the parameter estimates. In order to avoid these problems, covariates were clustered into highly correlated groups, where the absolute pairwise correlation between any two variables within a group was above 0.75. A single variable from each group was then selected as a potential covariate in the regression modeling.
Multivariate models were fitted using the function glm in R version 2.14.2. These models included an intercept, the log surveillance quality indicator at the country level obtained from the YFSD and a factor for each country not included in that database as well as any possible combination of up to 12 additional environmental covariates. The model fit was evaluated using the Bayesian Information Criterion (BIC) [49] , and the 15 best models were further investigated in the full model framework.
From model predictions to transmission intensity.
Serological surveys and detection probability.
Estimating the burden from transmission intensity.
While the number of infections is the most relevant quantity for assessing the degree of transmission of yellow fever, morbidity and mortality estimates are required to assess the impact on populations and health care systems. In order to calculate the number of severe cases and deaths from the infections, we fitted beta distributions to the point estimates and 95% credibility intervals of the proportion of cases among infections and the case fatality ratio estimated by Johansson and colleagues [21] and generated samples from both distributions that we then multiplied by the number of infections estimated during each MCMC iteration. This approach allowed us to include the uncertainty of the severity spectrum in our burden estimates.
Model fitting.
The model fit of the full model was evaluated via BIC, as this takes into account both fit quality (measured by the log likelihood) while penalizing models with a large number of parameters. In addition, we calculated receiver operator characteristic (ROC) curves comparing the regression model predictions with the yellow fever presence/absence data to which the regression models were fitted, and the area under the ROC curve (AUC), which quantifies how well the regression model predictions matched the data [50] . A lower value of the BIC indicates a better model fit, whereas a value of the AUC of 0.5 indicates that model predictions are no better than chance, and a value of 1 corresponds to a perfect fit to the data.
Sensitivity analyses.
While the model inference framework adopted gives parameter estimates and credible intervals around these, there were however other sources of uncertainty that were more difficult to quantify, some of which were assessed in sensitivity analyses.
The impact of the choice of covariates included in the regression model was assessed by comparing the final burden estimates obtained for a number of the best fitting regression models. The model that ranked best in the initial fits of the linear regression model was used as the baseline model and is presented in the main paper, whereas results from the remaining models are shown in Text S2 .
Sensitivity to the magnitude of the standard deviation of the Gaussian prior distribution on the country factors was explored (see Text S3 ).
The vaccination coverage dataset compiled for this study suffers from a number of uncertainties in the input datasets that are difficult to quantify, including uncertain population sizes that impact directly the vaccination coverage achieved with a given number of doses, uncertainties about the completeness and accuracy of the records of past vaccination activities, and the influence of population movements on vaccination coverage. In order to explore the potential impact of these sources of uncertainty on the burden estimates, we generated five alternative vaccination coverage scenarios: assuming only 90% vaccine efficacy, alternative lower or higher population sizes, non-random vaccine allocation, and an alternative scenario of the historic mass vaccination campaigns based on different records [51] . We used these to assess the impact of uncertainty of coverage estimates on the overall estimates of disease burden (see Text S4 for further details).
Last, we also considered two refined model structures that relaxed the assumption that the probability of case detection via routine surveillance was constant through time (see Text S5 ).
Yellow Fever Occurrence
Between 1980 and 2012, 150 yellow fever outbreaks in 26 countries in Africa were reported to WHO ( Figure S1 ). A high number of large outbreaks occurred in the late 1980s and early 1990s, particularly in Nigeria, as well as a large number of relatively smaller outbreaks in West and later central Africa since the turn of the century.
The YFSD contained records of 29,237 suspected cases of yellow fever from 21 countries reported between 2005 and 2011, 302 of which were lab-confirmed, 231 classified as epidemiologically linked to a lab-confirmed case, and 416 as compatible with yellow fever based on symptoms and epidemiology, with the remaining cases considered not due to yellow fever after investigation. The locations of the lab-confirmed, linked, and compatible cases resolved to the province level are shown in Figure S2A , whereas the combined dataset of the presence or absence of yellow fever reports by province is shown in Figure 1A .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
(A) Presence/absence of yellow fever over a 25-year period, by province. White, absence; red, presence of yellow fever reports. (B) Model predictions giving the estimated probability of at least one yellow fever report. (C) Estimates of the annual force of infection at the province level in the 32 countries considered endemic for yellow fever. (D) Estimates of the country-specific detection probability per infection. Countries not considered endemic for yellow fever are shown in navy (A, B, and D) or white (C).
https://doi.org/10.1371/journal.pmed.1001638.g001
The country-specific surveillance quality (defined as the mean annual number of reported suspected cases divided by the national population) is shown in Figure S2B . While there were suspect cases reported from 21 countries, the YFSD included only five suspect cases reported from Angola, none of which were confirmed. It was therefore assumed that this country did not participate effectively in the YFSD, reducing the number of countries included to 20.
Vaccination Coverage
The estimated vaccination coverage over time clearly shows the success of the mass vaccination campaigns in French West Africa between 1940 and 1960, and declining levels of immunity in the following decades caused by low vaccination levels, the birth of new unvaccinated cohorts, and the gradual depletion of the older protected cohorts through mortality. Between 1960 and 2000 there was limited vaccination activity across Africa resulting from disjointed reactive vaccination campaigns. Mass vaccination campaigns implemented since 2006 in the framework of the GAVI investment have achieved much higher coverage levels in West Africa ( Figure S3 ). The impact of infant immunization on coverage at the population level will take time to develop, but if this is pursued in the future and high coverage of new birth cohorts is achieved, it will eventually lead to a high coverage even in countries with currently low population-wide coverage. In countries that currently have high population-level coverage, infant immunization will prevent a repetition of the decline in vaccination coverage observed from the 1960s onwards.
Regression Model Fitting and Variable Selection
All models included log[surveillance quality] and country factors for those countries for which surveillance quality data were not available (due to non-participation in YFSD). In addition a total of 34 potential covariates were evaluated, nine of which were not significantly associated with the data at the threshold of p = 0.1 (see Table S2 ). The remaining 25 variables were clustered into 18 groups (see Figure S4 for the correlations between variables and Figure S5 for maps of the 18 covariates considered in the multivariate regression models), leading to a total of 249,527 models fitted with standard regression software. The 15 best models further investigated in the full model framework included three to five additional covariates ( Table 1 ). These models were investigated further by MCMC, fitting simultaneously the regression parameters and the force of infection from the serological surveys. For identification, these models were indexed with their BIC rank from the initial model fit. Time series and autocorrelation plots of the model parameters for the baseline model (model 1) are shown in Figures S6 and S7, respectively.
https://doi.org/10.1371/journal.pmed.1001638.t001
One would expect the number of cases to be proportional to the population size, leading to a dependence of the model predictions (probability of detecting yellow fever) on the log[population size], and this covariate was indeed included in all of the 15 best fitting models with a regression parameter value around 1, indicating linear dependence of the number of cases on population size. Most models included longitude, mimicking the strong gradient in risk that is observed in yellow fever epidemiology. Latitude, mean EVI, mean MIR, and the land cover category indicating a mosaic of cropland and natural vegetation were included in about half of the models, with typically each model including either mean EVI or mean MIR. The land cover categories of deciduous broadleaf forest, open shrubland, and barren areas were only included in few models, and no further of the 18 potential covariates considered were included in the 15 best fitting models.
The differences in goodness of fit between the models were small compared with the uncertainty inherent in the BIC and AUC estimates ( Figure 2 ), although the BIC indicated a slightly better fit for the models with a smaller index in Table 1 compared with those with a larger index, mirroring the BIC rank in the pure regression models. AUC values were high, averaging just below 0.9, showing a good match between data and regression model predictions. The AUC indicated a better match between regression model predictions and data for models 1, 4, 13, and 15 than the other models, but again, the differences were small.
(A) BIC and (B) AUC values for the 15 models investigated with MCMC, with a prior standard deviation on the country factors of 2. Circles show posterior means, lines the 95% posterior range.
https://doi.org/10.1371/journal.pmed.1001638.g002
Outputs from the Baseline Model
The high values for the AUC seen for the model predictions testified to a good model fit, so it is unsurprising that the spatial distribution of the model predictions matched the dataset of presence or absence of yellow fever reports very well ( Figure 1A and 1B ). The model successfully captures the gradient of transmission intensity from west to east as well as the focus of transmission being in sub-Sahel and tropical latitudes, which is reflected in both the model predictions ( Figure 1B ) and the force of infection estimates ( Figure 1C ).
There was substantial uncertainty in the force of infection estimates, with the highest values of the coefficient of variation being in areas with the lowest force of infection estimates: Rwanda, Burundi, and western parts of Tanzania ( Figure S8 ). Due to the very low force of infection estimates in these areas, this uncertainty has little impact on the burden estimates.
The estimated country-specific detection probability per infection varied over nearly two orders of magnitude between countries. Countries with a higher estimated force of infection also had higher estimates of the case detection probability, with the highest values found in the Central African Republic and Togo and the lowest in Guinea-Bissau, Ethiopia, and Tanzania ( Figure 1D ), Notably the detection probability was estimated to be very low in Nigeria, which has a substantial impact on the burden estimates due to its large population.
The annual number of yellow fever infections, severe clinical cases, and deaths expected from the estimated force of infection were estimated for selected years ( Table 2 ). Between 1995 and 2005, the overall vaccination coverage remained roughly similar across the continent. The moderate increase in estimated burden between these years therefore reflects overall population growth. However, the large preventive mass vaccination campaigns performed between 2006 and 2012 increased the vaccination coverage in the participating countries, substantially outweighing population growth effects and resulting in a 2013 burden estimate of 180,000 (95% CI 51,000–380,000) severe cases presenting with fever and jaundice or haemorrhage including 78,000 (95% CI 19,000–180,000) deaths. We estimate that the recent preventive mass vaccination campaigns between 2006 and 2012 reduced the annual burden evaluated for 2013 by 27% (95% CI 22%–31%), which equates to an overall reduction of 57% (95% CI 54%–59%) in the 12 targeted countries. In these campaigns, the number of targeted provinces and districts and therefore the impact achieved varied by country, with the highest reductions achieved in Benin, Togo, and Cote d'Ivoire, where an estimated 82%, 77%, and 73%, respectively, of the burden was prevented in 2013. The reduction at the national level of participating countries reflects both vaccinated and non-vaccinated regions within each country.
https://doi.org/10.1371/journal.pmed.1001638.t002
Disease burden was estimated to be distributed very unevenly between countries, with by far the largest burden estimated for Nigeria, owing to the moderately high force of infection, low vaccination coverage, and a large population size ( Figure 3 ). The country contributing the next largest number of cases and deaths was the Democratic Republic of the Congo, followed by countries in West Africa with a high force of infection, some of which have recently benefited from the GAVI-funded mass vaccination campaigns ( Table 3 ).
Red bars show the number of deaths estimated assuming implementation of no mass vaccination campaigns between 2006 and 2012, orange bars show the number of deaths estimated for the actual vaccination. Lines show the 95% credibility intervals of the estimated number of deaths. Countries are ordered west to east.
https://doi.org/10.1371/journal.pmed.1001638.g003
https://doi.org/10.1371/journal.pmed.1001638.t003
Mass vaccination campaigns can be extremely effective at reducing the burden in populations with low immunity, with the effect being immediate and long lasting ( Figure 4 ). The impact wanes over the course of decades only as new birth cohorts join the populations ( Figure 4A and 4D ). In this context, routine infant immunization as performed in the EPI in many African countries since the 1980s ( Table S1 ) serves an important purpose by ensuring good vaccination coverage in new birth cohorts and thus preventing any long term decrease in population immunity. As the sole tool to increase population immunity infant immunization is less effective, as it takes decades for such a program to substantially increase the immunity of the whole population. Figure 4B and 4E shows the burden in Ghana and Liberia assuming no infant immunization ever in these two countries. The results illustrate how a high infant immunization coverage is crucial to sustaining low levels of burden (as in Ghana with 91% coverage), whereas low coverage levels (as in Liberia with 39% coverage) will reduce the burden a little but are too low to sustain a low level of burden in the future. A combination of mass vaccination campaigns and infant immunization at good coverage level is therefore likely to reduce the burden quickly and sustain it at low levels.
Thick lines show the point estimate, hashed areas the 95% credibility intervals. Baseline scenario (black) in (A–F) includes past mass vaccination and infant immunization, plus continuing infant immunization at 2011 coverage levels. Alternative scenario (red): (A and D): as baseline, but excluding the mass vaccination campaigns; (B and E): as baseline, but assuming no infant immunization at any time; (C and F): as baseline, but including mass vaccination campaigns targeting children under 5 every 5 years at a coverage of 90% instead of future infant immunization.
https://doi.org/10.1371/journal.pmed.1001638.g004
Some countries achieve high coverage in their routine infant immunization but the coverage in other countries is low. Conversely, the mass vaccination campaigns achieved high coverage levels in most countries targeted. If it is difficult to reach a substantial proportion of infants with routine immunization, one could instead consider repeated mass vaccination campaigns. Figure 4C and 4F shows the effect of repeating mass vaccination campaigns targeting children under 5 every 5 years is similar to what is achieved using routine infant immunization reaching a high proportion of infants. Such age-targeted campaigns would cost less than repeated mass vaccination campaigns targeting all age groups while being similarly effective.
We compared our estimates of mortality due to yellow fever to all-cause crude mortality estimates obtained from the UN WPP [35] for all endemic countries. For the period from 2005 to 2010, the estimates varied between eight and 18 deaths per year per 1,000 population, equating to 9.4 million deaths annually from any cause in the endemic region (calculated using 2010 population estimates). Our estimate of 78,000 deaths from yellow fever for 2013 therefore corresponds to 0.8% of all-cause mortality, but the proportion of the all-cause mortality that would be attributed to yellow fever based on our burden estimates varied substantially between countries ( Figure 5 ), ranging from close to zero in many east African countries to values typically between 1% and 3% in West Africa, with the highest values just under 6% in Mauritania and Guinea-Bissau.
Grey bars indicate the point estimates, black lines the range spanned by the 95% CIs of the burden estimates. Countries are ordered west to east.
https://doi.org/10.1371/journal.pmed.1001638.g005
In this study, we estimated the burden of yellow fever in terms of the number of infections, severe cases, and deaths across Africa by fitting generalised linear regression models to datasets of yellow fever reports between 1987 and 2011. We evaluated the impact of recent large-scale preventive mass vaccination campaigns undertaken between 2006 and 2012 under the Yellow Fever Initiative by estimating the burden expected had these vaccination campaigns not taken place.
We estimate that currently there are between 51,000–380,000 severe cases of yellow fever annually in Africa, resulting in an estimated 19,000–180,000 deaths. These figures are to be compared with previous global estimates of 200,000 cases and 30,000 deaths annually for the early 1990s, around 90% of which occur in Africa [5] , [6] , [52] . It is encouraging that both sets of estimates are broadly similar, particularly since the new estimates take into account all existing data on yellow fever that are currently available. The analysis provided here also gives a better understanding of the spatial and temporal distribution of yellow fever across Africa. The model framework developed takes into account a variety of different data sources, including information on population vaccination coverage over time, which can be used to evaluate the impact of past and potential future vaccination campaigns.
The average annual number of yellow fever cases officially reported to WHO by countries in the endemic zone [53] was 1,165 for the period from 1987 to 2011 considered in this analysis, and 656 for the period between 2005 and 2011 covered by the YFSD (note that this is a different dataset than the YFSD, containing only aggregate numbers). This was in contrast to the estimated annual burden of around 180,000 severe cases (which were defined as presenting with fever and jaundice or haemorrhage), meaning that for each officially reported case there might actually be as many as 50 to 500 severe cases. This is consistent with the 10–1,000-fold under-ascertainment of yellow fever morbidity and mortality recognized in past work [3] , [4] . Such levels of under-ascertainment highlight the difficulties inherent in yellow fever surveillance, which relies on clinical case definitions. Syndromic surveillance is challenging due to the variety of clinical manifestations seen in severe disease that do not include jaundice and therefore might be mistaken for other infections (notably malaria) [26] . In addition, not all jaundice is caused by yellow fever, with other causes including malaria, liver pathogens, and other conditions.
The detection probabilities fitted in our model are of the order of 10 −5 , but these describe the probability that an infection would be reported into either the YFSD or as an outbreak. In the YFSD, there were on average around 135 cases reported annually. Comparing this to our burden estimates of around 1.5 million infections annually in the time period covered by the YFSD, this would lead to an empirical detection probability of the order of 10 −4 across Africa, an order of magnitude larger than the values fitted in our model. However, the detection probabilities fitted in our model represent an average over 25 years, and detection was considerably poorer prior to the introduction of the YFSD.
The proportion of the all-cause mortality that would be attributed to yellow fever based on our burden estimates varied between countries with plausible estimates of less than 3% for most countries, with the exception of Mauritania and Guinea Bissau where nearly 6% of the all-cause mortality would be attributed to yellow fever on the basis of our estimates. The estimates for these two countries may appear unrealistically large, but it should be kept in mind that the uncertainty in the force of infection and consequently in the burden estimate is relatively high in Mauritania ( Figures 3 and S7 ), whereas for Guinea-Bissau, the estimated detection probability is the lowest estimated for any country ( Figure 1D ) due to its low rate of reporting suspected cases to the YFSD. If the overall surveillance quality in this country was not well represented by the participation in the YFSD, the burden estimate here would be over-inflated.
The datasets of yellow fever incidence used to fit the models rely on surveillance recognizing yellow fever cases. Typically the case definition is based on fever with jaundice and/or haemorrhaging symptoms, but of course the sensitivity and specificity of this case definition might vary between settings. In our analysis, we have allowed for the sensitivity to vary between countries by estimating the country-specific surveillance quality. The specificity of the case definition in our datasets should be high across the board, as only laboratory confirmed cases or cases closely linked epidemiologically were included in our analysis. There might however be substantial differences in the severity spectrum of yellow fever between settings, depending on factors such as previous exposure to other flaviviruses, the general immune status of the populations, or the access to health care facilities, although there is no treatment for yellow fever apart from general life support. While we were not able to include any of these effects, we used estimates of the severity with measures of uncertainty by Johansson and colleagues based on the limited available data [21] , capturing the variability seen across different settings. Our model estimates first the number of infections and infers the disease burden in terms of the number of severe cases and deaths from this, so the uncertainty of our burden estimates is inflated by the uncertainty of the severity spectrum. Nevertheless we have chosen to report mainly the number of deaths as cases and deaths are more relevant in terms of disease burden and health care needs than the number of infections, the majority of which are likely to be very mild or asymptomatic.
The credible intervals around the burden estimates presented here also reflect the fact that a range of values for the force of infection estimates yield a similarly good model fit. However, while the credible intervals represent the uncertainty in model parameter estimates, there are further potential sources of uncertainty that are not captured by credible intervals. Firstly, the choice of covariates included in the model could have an effect. However, the 15 models investigated here showed a similarly good fit to the dataset, and the burden estimates from all models were very similar (see Text S2 ).
Second, in order to prevent the country factors (which determine the detection probabilities in countries not participating in the YFSD) from taking infinite values, we assumed a Gaussian prior distribution for these within the Bayesian framework used for model fitting. The standard deviation of this prior distribution was chosen relatively arbitrarily; however, in the sensitivity analyses we have shown that the burden estimates again are fairly independent of the particular value chosen (see Text S3 ).
The dataset of vaccination coverage compiled from various sources reporting on vaccination activities in the last century contains a number of potential sources of uncertainty that are very difficult to quantify. Uncertainty in historical population sizes by age generates uncertainty in vaccination coverage estimates if those estimates are generated from records of the number of vaccine doses used. There are also concerns about the completeness and accuracy of the reports on vaccination activity. Furthermore, the effect of population movements on vaccination coverage could not be taken into account owing to lack of data. Our simplifying assumptions of a 100% vaccine efficacy and lifelong immunity conferred by the vaccine can also be questioned. To evaluate the impact of these uncertainties we undertook sensitivity analyses that carried vaccine effectiveness and the coverage achieved in historical campaigns, but found the effects on burden estimates to be slight (see Text S4 ). While we omitted reactive vaccination campaigns before 1970 in the generation of all vaccination coverage scenarios as these data were not routinely reported prior to this time, this is likely to have little impact on vaccination coverage levels due to the low yellow fever activity and resulting low number and extent of vaccination campaigns in this period.
Uncertainty in demographic data across Africa has a very direct impact on the burden estimates, as such estimates are directly proportional to the population size. This uncertainty is not captured in the confidence intervals given in this paper, as it was not possible to quantify the level of uncertainty.
There are substantial uncertainties regarding the spatial distribution of yellow fever occurrence, which were taken into account in our model by allowing infection risk and detection probabilities to vary between countries. However, the baseline model presented above did not allow for detection probabilities to vary over time, while activities such as the introduction of the YFSD in 2005 were clearly intended to improve surveillance. We therefore investigated two alternative model structures that both allow for a change in the detection probabilities at the time of introduction of the YFSD, both of which estimated an increased probability of case detection in the countries participating in the YFSD following its introduction. The overall burden estimates from these models were very similar to those obtained from the baseline model though there were subtle differences in the spatial distribution of the transmission intensity, with one of the alternative models showing a slightly less pronounced gradient in transmission strength from west to east (see Text S5 ).
Similarly, while we allowed the force of infection for yellow fever to vary in space, we assumed it was constant throughout the 25-year observation period, as well as homogeneous by gender and age. While clearly there will be differences in exposure between age groups and genders, particularly in areas where non-human primates play an important role in transmission, the relatively crude nature of the yellow fever occurrence data did not support a model that would be able to estimate these differences. Our estimates are therefore representative of the overall population but do not reflect the age- and sex-specific exposure likely to be found in many places. The assumption of constant force of infection throughout time means we have not taken into account changes in transmission due to factors such as changed land use or climate change, which might influence the transmission intensity. Clearly yellow fever activity is not constant, but epidemic amplifications and reductions of transmission intensity happen over the timescale of decades. Epidemics are driven, at least in part, by the rapid removal and slow replenishment of susceptible hosts in both humans and wildlife, as illustrated by the widespread epidemics in much of western Africa, and particularly Nigeria, in the 1990s, and a reduction in epidemic activity since then. Furthermore, a serological survey in Central African Republic testing samples collected in 2006 and in 2009 found evidence of an increase in yellow fever exposure over this period [29] , mirroring the increasing number of cases reported from that region in recent years. Therefore our results should be seen as representative of the past 25 years, averaging over the large fluctuations that occur in reality, although the burden estimates for specific years do reflect the population size, age structure, and vaccination coverage pertaining to the time.
Burden estimates were strongly determined by the force of infection estimated from serological surveys [29] – [34] . However, the only surveys available were conducted in central Africa and Nigeria, with these results extrapolated to the remainder of the endemic zone in West and East Africa using the spatial distribution of transmission intensity estimated from the regression model. While all model structures reproduced the gradient in transmission intensity from west to east that is seen in yellow fever epidemiology, this gradient was more pronounced in the baseline model presented in the main paper than in the alternative model that was fitted to an annual dataset of yellow fever reports (see Text S5 ). In the absence of further reliable serological data outside central Africa it is presently not possible to distinguish which model better reflects reality. There are several serological surveys under way or close to completed in east African countries including Sudan, Rwanda, Uganda, Kenya, South Sudan, and Ethiopia. These data, once available, will substantially reduce model uncertainty, allowing us to discriminate between different model assumptions and resulting in more reliable estimates.
Cohort studies collecting data on case incidence and the severity spectrum of disease could also reduce the level of uncertainty. The relatively low incidence of yellow fever implies the need for large cohorts, which would be prohibitively expensive if performed for yellow fever alone. However, including yellow fever diagnostics into ongoing cohort studies (e.g., focused on HIV or malaria) might be a cost-effective way to improve basic understanding of yellow fever epidemiology. A further advantage of studies focusing on multiple diseases would be to understand interactions between infections (most notably cross-immunity between flaviviruses).
Our analysis does not take into account the epidemic character of yellow fever transmission, but rather assumes cases are distributed evenly over time according to a force of infection that is independent of the incidence of cases in the population. Consequently, the impact of vaccination campaigns will be underestimated, as lower transmission in a population due to vaccination also provides indirect protection to unvaccinated individuals (herd immunity). While the impact of herd immunity can be easily quantified in situations where there is only one type of host, this is currently impossible with yellow fever as it is unknown what proportion of cases arise through inter-human transmission via mosquito vectors, and what proportion through the sylvatic cycle. While this question cannot be answered with the methodology employed in the present study, it is an important topic for future work.
Keeping this limitation in mind, we conservatively estimate that the recent mass vaccination campaigns have reduced the yellow fever burden in the 12 participating countries for 2013 by 57% (95% CI 54%–59%) relative to a counterfactual scenario in which these campaigns were not conducted, by vaccinating 78 million people, who make up around 55% of the population of these countries. Across Africa, this amounts to a reduction of the total burden of yellow fever by 27% (95% CI 22%–31%), by vaccinating around 10% of the population in the endemic zone.
Partly as a result of the estimates presented here, in late 2013 the GAVI Alliance Board decided to make available support for additional yellow fever vaccination campaigns, targeting 144 million people across the endemic region in Africa [54] , [55] . Furthermore, the GAVI Alliance is now using our estimates for evaluating the past and future impact of their yellow fever vaccination activities.
The impact of both past and future mass vaccination campaigns will prevent a substantial proportion of yellow fever disease burden for years to come, with a gradual decrease in impact over the next decades as new birth cohorts that have not benefitted from these campaigns enter the population. This effect of slowly declining vaccination coverage following the abandonment of mass vaccination campaigns was seen since the 1960s, and was the cause of the gradual resurgence of yellow fever over the following decades. However, the achievements of the current mass vaccination campaigns could be sustained if a high level of immunization is achieved through a strong EPI program and preventive vaccination of populations that remain at-risk, such as migrants or populations from as yet unvaccinated districts. While the coverage achieved in the routine infant immunization is variable between countries, the coverage achieved in recent mass vaccination campaigns has generally been high. An alternative for countries struggling to reach high EPI coverage levels might therefore be to repeat mass vaccination campaigns targeted at children every few years, although the organizational and financial costs would probably be substantially higher than the existing EPI.
Yellow fever is a disease that is difficult to diagnose and confirm, whose symptoms can be mild and mistaken for other infections, and that occurs in some of the most resource-poor settings globally. Consequently surveillance data reflect patterns of endemicity and emergence of infection in new zones and provide sentinel data on imminent or ongoing outbreaks, but do not reflect the actual disease burden. The most recent estimates of the disease burden stemmed from the early 1990s and therefore an update taking into account the changes in demography, ecology, and vaccination coverage, such as the estimates provided in the present study, was long overdue. The framework for burden estimation developed here is also a useful tool for the evaluation and planning of effective vaccination campaigns. As such, it is being used by the partners of the Yellow Fever Initiative for planning their yellow fever vaccination strategy for the next decade.
Supporting Information
Map of the outbreaks recorded in Africa between 1980 and 2012. Outbreak size indicated by the symbol size, outbreak year coded by the colour.
https://doi.org/10.1371/journal.pmed.1001638.s001
(A) map of the number lab-confirmed, epi-linked, and compatible yellow fever cases reported in the YFSD by province. (B) Annual reporting rate of suspected cases per 100,000 population by country.
https://doi.org/10.1371/journal.pmed.1001638.s002
Estimated vaccination coverage at the first administrative level in the countries endemic for yellow fever on the African continent throughout the decades. Non-endemic countries are shown in grey. The estimate for 2015 is a projection that assumes infant immunization continues at the same levels as in 2011, and no other vaccination campaigns are implemented.
https://doi.org/10.1371/journal.pmed.1001638.s003
Absolute values of the pairwise correlations between the 25 potential covariates significant at the p = 0.1 level from 0 (red) to 1 (white). Clusters are highlighted by a lack of separating lines, and variables not considered for the multivariate models printed in grey.
https://doi.org/10.1371/journal.pmed.1001638.s004
Maps of the 18 variables considered in the multivariate modeling as potential covariates. Colour scale from navy (low) to red (high). A, longitude; B, latitude; C, altitude; D LC, deciduous broadleaf forest; E LC, closed shrubland; F LC, open shrubland; G LC, woody savannas; H LC, urban and built-up; I LC, cropland/natural vegetation mosaic; J LC, barren or sparsely vegetated; K, mean day temperature; L, min day temperature; M, min night temperature; N, max night temperature; O, max EVI; P, min MIR; Q, min rainfall; R, max rainfall.
https://doi.org/10.1371/journal.pmed.1001638.s005
MCMC posterior trace plots of model parameter estimates for the baseline model, thinned by a factor 800.
https://doi.org/10.1371/journal.pmed.1001638.s006
Auto-correlation in posterior estimates of the model parameters for the baseline model. Posterior MCMC samples were thinned by a factor 800.
https://doi.org/10.1371/journal.pmed.1001638.s007
Coefficient of variation of the force of infection estimates. Countries not considered endemic for yellow fever are shown in white.
https://doi.org/10.1371/journal.pmed.1001638.s008
Coverage and year of introduction of the yellow fever vaccine into the routine Enhanced Programme of Immunization by country.
https://doi.org/10.1371/journal.pmed.1001638.s009
Covariates considered in the regression modeling, significance level in univariate models and cluster association.
https://doi.org/10.1371/journal.pmed.1001638.s010
Demographic data analysis.
https://doi.org/10.1371/journal.pmed.1001638.s011
Sensitivity analysis: impact of the covariates included.
https://doi.org/10.1371/journal.pmed.1001638.s012
Sensitivity analysis: impact of the standard deviation of the prior distribution on the country factors.
https://doi.org/10.1371/journal.pmed.1001638.s013
Sensitivity analysis: impact of alternative vaccination coverage scenarios.
https://doi.org/10.1371/journal.pmed.1001638.s014
Sensitivity Analysis: alternative model structures.
https://doi.org/10.1371/journal.pmed.1001638.s015
Acknowledgments
In addition to the author list, the Yellow Fever Expert Committee includes: Donald Burke, Fernando De La Hoz, Bryan Grenfell, Peter M Hansen, and Raymond Hutubessy.
The authors would like to thank Michael Johansson for helpful discussions and sharing his estimates on the yellow fever severity spectrum, Mark Kuniholm for the southern Cameroon dataset, Emily Jentes for helpful discussions, Kara Durski for providing data on several yellow fever vaccination campaigns, and Véronique Millot for support in collected data from countries. The Modis 12Q1 and 13A2 data were obtained through the online Data Pool at the NASA Land Processes Distributed Active Archive Center (LP DAAC), USGS/Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota ( http://lpdaac.usgs.gov/get_data ).
Author Contributions
Conceived and designed the experiments: TG MDVK SY WP NMF. Analyzed the data: TG NMF. Wrote the first draft of the manuscript: TG MDVK NMF. Contributed to the writing of the manuscript: TG MDVK SY OR RL JES WP NMF. ICMJE criteria for authorship read and met: TG MDVK SY OR RL JES WP NMF. Agree with manuscript results and conclusions: TG MDVK SY OR RL JES WP NMF. Provided input and advice on the methods: RL WP OR JES SY.
- View Article
- Google Scholar
- 3. World Health Organization (1986) Prevention and control of yellow fever in Africa. Geneva: World Health Organization.
- 5. World Health Organization. Yellow fever fact sheet no 100. Available: http://www.who.int/mediacentre/factsheets/fs100/en/ . Accessed 19 January 2014.
- 6. World Health Organization Expanded Programme on Immunization (1992) The Resurgence of deadly yellow fever: prevention using EPI. Geneva: WHO.
- 13. World Health Organization. Weekly Epidemiological Record. Available: http://www.who.int/wer/en/ . Accessed 11 June 2012.
- 14. World Health Organization. Disease Outbreak News. Available: http://www.who.int/csr/don/en/ . Accessed 11 June 2012.
- 21. Johansson MA, Staples JE (2013) The whole yellow fever iceberg: estimating the incidence of infection from the number of severe cases. Epidemics 4. Amsterdam: Elsevier.
- 22. Durieux C (1956) Mass yellow fever vaccination in French Africa south of the Sahara. Monograph Series World Health Organization. Geneva: WHO. pp. 115–122.
- 23. World Health Organization (2012) Immunization surveillance, assessment and monitoring. Available: http://www.who.int/immunization_monitoring/data/data_subject/en/index.html . Accessed 11 June 2012.
- 24. World Health Organization, unicef (2009) Yellow Fever Initiative Progress Report 2006 to 2009. Geneva: World Health Organization.
- 25. World Health Organization, unicef (2010) Yellow Fever Initiative Joint WHO and UNICEF 2010 Progress Report. Geneva: World Health Organization.
- 29. Diallo M, Janusz K, Lewis R, Manengu C, Sall AA, et al.. (2010) Rapid assessment of yellow fever viral activity in the Central African Republic. Geneva: World Health Organization.
- 35. United Nations, Department of Economic and Social Affairs, Population Division, Population Estimates and Projections Section. World Population Prospects: The 2010 Revision. Available: http://esa.un.org/wpp/Excel-Data/population.htm . Accessed 11 June 2011.
- 36. Bright EA, Coleman PR, King AL, Rose AN (2008) LandScan 2007. Oak Ridge (Tennessee). Available: http://www.ornl.gov/landscan/ . Accessed 10 December 2013.
- 42. NASA Land Processes Distributed Active Archive Center (LP DAAC) Land Cover Type Yearly L3 Global 1 km SIN Grid (12Q1). USGS/Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota. Available: http://lpdaac.usgs.gov/get_data . Accessed 13 July 2012.
- 43. NASA Land Processes Distributed Active Archive Center (LP DAAC) Vegetation Indices 16-Day L3 Global 1 km (13A2). USGS/Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota. Available: http://lpdaac.usgs.gov/get_data . Accessed 13 July 2012.
- 44. Hijmans RJ, Cameron SE, Parra JL. WorldClim. Available: http://www.worldclim.org/ . Accessed 12 January 2012.
- 46. Brooks S, Gelman A, Jones GL, Meng X-L, editors (2011) Handbook of Markov chain Monte Carlo. Boca Raton (Florida): Chapman & Hall/CRC.
- 47. Gilks WR, Richardson S, Spiegelhalter DJ (1996) Markov chain Monte Carlo in practice. Boca Raton (Florida): Chapman & Hall/CRC.
- 53. World Health Organization (2012) Yellow fever reported cases. Available: http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidenceyfe.htm . Accessed 24 April 2013.
- 54. GAVI Alliance. Vaccine investment strategy. Available: http://www.gavialliance.org/about/strategy/vaccine-investment-strategy/ . Accessed 18 January 2014.
- 55. GAVI Alliance (2013) Yellow fever vaccine investment strategy. Background document #5. GAVI Alliance. Available: http://www.gavialliance.org/library/gavi-documents/strategy/final-vis-analysis-2013—yellow-fever/ . Accessed 18 January 2014.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
An Overview of Yellow Fever Virus Disease
Affiliations.
- 1 Neuro-Infectious Diseases Group, Department of Neurology, Division of Infectious Diseases, University of Colorado School of Medicine, Aurora, CO, USA.
- 2 Department of Epidemiology, Colorado School of Public Health, Aurora, CO, USA.
- PMID: 28974992
- PMCID: PMC5613873
- DOI: 10.1177/1941874417708129
PubMed Disclaimer
Similar articles
- Wild-Type Yellow Fever Virus RNA in Cerebrospinal Fluid of Child. Marinho PES, Alvarenga PPM, Crispim APC, Candiani TMS, Alvarenga AM, Bechler IM, Alves PA, Dornas FP, de Oliveira DB, Bentes AA, Christo PP, Kroon EG. Marinho PES, et al. Emerg Infect Dis. 2019 Aug;25(8):1567-1570. doi: 10.3201/eid2508.181479. Emerg Infect Dis. 2019. PMID: 31310221 Free PMC article.
- Predictors of mortality in patients with yellow fever: an observational cohort study. Kallas EG, D'Elia Zanella LGFAB, Moreira CHV, Buccheri R, Diniz GBF, Castiñeiras ACP, Costa PR, Dias JZC, Marmorato MP, Song ATW, Maestri A, Borges IC, Joelsons D, Cerqueira NB, Santiago E Souza NC, Morales Claro I, Sabino EC, Levi JE, Avelino-Silva VI, Ho YL. Kallas EG, et al. Lancet Infect Dis. 2019 Jul;19(7):750-758. doi: 10.1016/S1473-3099(19)30125-2. Epub 2019 May 16. Lancet Infect Dis. 2019. PMID: 31104909
- Japanese encephalitis virus/yellow fever virus chimera is safe and confers full protection against yellow fever virus in intracerebrally challenged mice. Yang H, Yang H, Li Z, Liu L, Wang W, He T, Fan F, Sun Y, Liu J, Li Y, Zeng X. Yang H, et al. Vaccine. 2018 Apr 25;36(18):2450-2455. doi: 10.1016/j.vaccine.2018.03.038. Epub 2018 Mar 23. Vaccine. 2018. PMID: 29580643
- The 17D-204 and 17DD yellow fever vaccines: an overview of major similarities and subtle differences. Ferreira CC, Campi-Azevedo AC, Peruhype-Magalhāes V, Costa-Pereira C, Albuquerque CP, Muniz LF, Yokoy de Souza T, Oliveira ACV, Martins-Filho OA, da Mota LMH. Ferreira CC, et al. Expert Rev Vaccines. 2018 Jan;17(1):79-90. doi: 10.1080/14760584.2018.1406800. Epub 2017 Nov 27. Expert Rev Vaccines. 2018. PMID: 29172832 Review.
- [Recent knowledge on the pathogenesis of yellow fever and questions for the future]. Marianneau P, Desprès P, Deubel V. Marianneau P, et al. Bull Soc Pathol Exot. 1999 Dec;92(5 Pt 2):432-4. Bull Soc Pathol Exot. 1999. PMID: 11000957 Review. French.
- Assessment of the yellow fever outbreak in Angola from December 2015 through December 2016: A retrospective study. Manuel E, Armando A, Francisco M, Paixão J, Aramburu J, de Oliveira MDS, Freitas H, Pedro AM, Jandondo D, Carderon PB, Lamezon SL, Fortes F, Mariscal J, Cardoso Y, Moreira R, Morais J, Francisco NM. Manuel E, et al. Health Sci Rep. 2024 Feb 15;7(2):e1924. doi: 10.1002/hsr2.1924. eCollection 2024 Feb. Health Sci Rep. 2024. PMID: 38444843 Free PMC article.
- An evaluation of the diagnostic performance characteristics of the Yellow Fever IgM immunochromatographic rapid diagnostic test kit from SD Biosensor in Ghana. Ofosu-Appiah LH, Amelor DK, Ayensu B, Akyereko E, Rabiwu NI, Opare D, Owusu-Okyere G, Laryea DO, Asiedu-Bekoe F, Mingle JAA. Ofosu-Appiah LH, et al. PLoS One. 2022 Jan 7;17(1):e0262312. doi: 10.1371/journal.pone.0262312. eCollection 2022. PLoS One. 2022. PMID: 34995319 Free PMC article.
- The influence of vector-borne disease on human history: socio-ecological mechanisms. Athni TS, Shocket MS, Couper LI, Nova N, Caldwell IR, Caldwell JM, Childress JN, Childs ML, De Leo GA, Kirk DG, MacDonald AJ, Olivarius K, Pickel DG, Roberts SO, Winokur OC, Young HS, Cheng J, Grant EA, Kurzner PM, Kyaw S, Lin BJ, Lopez RC, Massihpour DS, Olsen EC, Roache M, Ruiz A, Schultz EA, Shafat M, Spencer RL, Bharti N, Mordecai EA. Athni TS, et al. Ecol Lett. 2021 Apr;24(4):829-846. doi: 10.1111/ele.13675. Epub 2021 Jan 27. Ecol Lett. 2021. PMID: 33501751 Free PMC article. Review.
- Thromboelastometry identifies coagulopathy associated with liver failure and disseminated intravascular coagulation caused by yellow fever, guiding specific hemostatic therapy: a case report. Crochemore T, Savioli FA, Guerra JCC, Kalmar EMDN. Crochemore T, et al. Rev Bras Ter Intensiva. 2020 Jul-Sep;32(3):474-478. doi: 10.5935/0103-507X.20200078. Rev Bras Ter Intensiva. 2020. PMID: 33053039 Free PMC article.
- Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Miao J, Chard LS, Wang Z, Wang Y. Miao J, et al. Front Immunol. 2019 Oct 1;10:2329. doi: 10.3389/fimmu.2019.02329. eCollection 2019. Front Immunol. 2019. PMID: 31632404 Free PMC article. Review.
- Centers for Disease Control and Prevention. Yellow fever. https://www.cdc.gov/yellowfever/index.html . Accessed April 26, 2017.
- Monath TP, Gershman M, Staples JE, Barrett A. Yellow fever vaccine In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia, PA: Saunders; 2012:870–968.
- World Health Organization. Yellow fever. http://www.who.int/topics/yellow_fever/en/ . Accessed April 26, 2017.
- Staples JE, Gershman MD, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-7):1–27. - PubMed
- Lindsey NP, Rabe IB, Miller ER, Fischer M, Staples JE. Adverse event reports following yellow fever vaccination, 2007-13. J Travel Med. 2016;23(5). - PubMed
Grants and funding
- I01 BX003863/BX/BLRD VA/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
WHO position papers on Yellow fever

Vaccines and vaccination against yellow fever: WHO Position Paper – June 2013
Additional materials, additional who position paper on the use of fractional doses for yellow fever (june 2017).
This policy on the use of fractional doses of yellow fever vaccines can be applied to all WHO prequalified vaccines.
Yellow fever vaccine: WHO position on the use of fractional doses – June 2017 Yellow fever vaccine: WHO position on the use of fractional doses – June 2017
COVID-19 and TDR
- Standing Committee
- Joint Coordinating Board
- Scientific and Technical Advisory Committee
- Scientific Working Groups

- Malaria research
- Tuberculosis research
- Vector-borne diseases
- Neglected tropical diseases research
Implementation research training materials
- Massive open online course (MOOC) on implementation research
- Postgraduate training scheme
- Regional training centres
- Clinical Research Leadership fellowship programme
TDR Global profiles
- Women in science
- TDR impact research grants scheme
- Gender and infectious disease research
- Knowledge management
- SORT IT operational research and training
All feature stories
All Publications
TDR Strategy 2024-29
Global Health Matters podcast »
Grants and other funding opportunities
eTDR portal
Our partnerships
ESSENCE on Health Research
Social Innovation in Health Initiative
The Access and Delivery Partnership
Engaging community leaders to tackle yellow fever in Douala, Cameroon
In response to the detection of yellow fever cases in Douala, Cameroon, a collaborative effort involving the Eliminate Yellow Fever Epidemics (EYE) Secretariat in WHO, WHO representation in Cameroon, and TDR (the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases) aims to strengthen the capacity of Douala communities to reduce the transmission of the yellow fever virus by managing aquatic mosquito habitats such as discarded tires and drinking troughs. Epidemics and outbreaks are one of the four global health challenges TDR focuses on as outlined in the TDR Strategy 2024-2029 .
In urban settings, the yellow fever virus is transmitted by Aedes aegypti mosquitoes. By controlling this mosquito vector, transmission of the virus can be significantly reduced. While vaccination is the primary method of controlling yellow fever outbreaks, vector control can reduce the risk of infection in urban settings and can be implemented early before full protection from vaccination is achieved.

These habitats included discarded tires, storage containers, drinking troughs, sheet metal, and even discarded shoes. This underscores the critical role of community engagement in managing Aedes larval sources to reduce the risk of infection.
As many community leaders were unaware of the link between Aedes mosquitoes and the transmission of yellow fever virus, TDR, the EYE Secretariat, and WHO Cameroon conducted a workshop to train community leaders on managing Aedes habitats.
More than 50 participants, including community leaders, communication professionals, and health area heads from all nine health districts in Douala, took part. The training covered topics such as the role of Aedes mosquitoes in the transmission of yellow fever virus, the role of the community in Aedes habitats, Aedes larval source management, and community engagement strategies. Participants also observed Aedes mosquito larvae and pupae during the session.

Training of trainers session in Douala, Cameroon, April 2024 Credit: TDR / A. Gildas Yahouedo
Dr Raymond Tabue, Head of the Integrated Vector Control Unit at National Malaria Control Programme of Cameroon, emphasized the importance of this training effort in addressing yellow fever risk in Douala. “The training of community leaders in Douala for managing Aedes aquatic habitats is an unprecedented and pioneering effort in controlling yellow fever in Douala,” he said.
Following the training, several priority actions were identified, including training of community members by community leaders, cleaning of gutters and drains to prevent water stagnation, educating the public and allocation of resources for field investigations. WHO Cameroon will closely monitor these actions in collaboration with national authorities. Urban yellow fever outbreaks remain the biggest threat to global health security, and the EYE Secretariat in WHO and TDR will continue to develop effective strategies, with community engagement playing a pivotal role.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Yellow fever.
Leslie V. Simon ; Muhammad F. Hashmi ; Klaus D. Torp .
Affiliations
Last Update: August 7, 2023 .
- Continuing Education Activity
Yellow fever is a mosquito-borne viral illness found in tropical and subtropical areas in South America and Africa. Transmission is primarily via Aedes and Haemagogus mosquitos. It can present with varying clinical features ranging from a self-limited, mild febrile illness to severe hemorrhage and liver disease. This activity reviews the evaluation and management of yellow fever and highlights the role of the interprofessional team in the recognition and management of this condition.
- Review the pathophysiology of yellow fever.
- Identify the typical presentation of a patient with yellow fever.
- Summarize reasons for a delayed diagnosis of yellow fever.
- Explain the interprofessional team strategies for improving care coordination and communication regarding the management of patients with yellow fever.
- Introduction
Yellow fever is a mosquito-borne viral illness found in tropical and subtropical areas in South America and Africa. Transmission is primarily via Aedes and Haemagogus species of mosquito. It can present with varying clinical features ranging from a self-limited, mild febrile illness to severe hemorrhage and liver disease. The “yellow” comes from jaundice that affects some patients with severe disease. The disease is diagnosed by history travel to an endemic area, exposure to infected mosquitoes, vaccination history, symptoms, and laboratory findings. Most cases are self-limited and resemble many other common viral infections. Of those who develop severe disease mortality can approach 50%. Unlike many other mosquito-transmitted viruses, infected humans are not dead-end hosts and may infect mosquitoes during periods of viremia and spread the virus. There is no specific antiviral therapy, but there is an effective vaccine recommended for travelers to endemic areas. Other than vaccination, prevention of mosquito bites is the best way to avoid contracting the virus. [1] [2] [3]
The virus is an RNA virus of the genus Flavivirus , closely related to the viruses that cause West Nile, St. Louis, and Japanese encephalitis. Tree-hole breeding mosquitoes, such as Aedes aegypti and Haemagogous species, transmit yellow fever during the rainy season. The yellow fever virus has three distinct transmission cycles: jungle, intermediate, and urban. The jungle cycle involves transmission between non-human primates (monkeys) and mosquitoes. Humans are infected through infected mosquito bites while visiting or working in the jungle. The intermediate cycle occurs in the African savannah and involves humans who live or work in jungle border areas. Transmission may be between monkeys and humans or humans via mosquito vectors. The urban cycle involves a viremic human who contracted the virus in either the jungle or intermediate cycle who then returns to an urban area. Humans develop significant viremia to infect mosquitoes, which can then transmit the virus to other humans in urban areas. Person to person or primate to human transmission has not been reported without the involvement of a mosquito vector. [4] [5]
- Epidemiology
Vaccination has decreased worldwide epidemics of yellow fever, but the infection has reemerged in many parts of Africa and South America. No one is immune from yellow fever, and it occurs in people of all ages and races. The highest mortality rates are reported in infants and the elderly, who often have depressed immune systems. yellow fever is very rare in the United States. Most cases are diagnosed in unvaccinated travelers to sub-Saharan Africa or South America. While most people develop a self-limited infection, those who develop severe disease. [6]
- Pathophysiology
The incubation period is 3 to 6 days. Once acquired, the virus quickly spreads to multiple organs in the body. The liver is the most important organ affected by yellow fever. It produces profound jaundice due to liver damage. The kidneys also undergo similar pathological alterations and can lead to acute renal failure. When the upper gastrointestinal (GI) tract is involved, the gastric acid mixed with blood produces what is known as black vomit. Central nervous system (CNS) features include cerebral edema and hemorrhage. Encephalopathy is also a common feature of yellow fever. [7] [8]
- History and Physical
The diagnosis requires a thorough travel history and record of immunization. Patients may present with headache, malaise, jaundice, and myalgias with severe back pain commonly reported.
The incubation period is 3-7 days, with most individuals having mild flu like illness. About 15% of cases have severe symptoms including chills, low back pain, headache, and fever.
There is a period of remission which may last 24-48 hours. This may be followed by a return of symptoms and marked intoxication. During this stage, the hepatorenal disease is common and carries a high mortality.
A physical exam may reveal the Faget sign or pulse fever dissociation, facial flushing, and conjunctival injection. During the most toxic phase, patients develop jaundice, dark urine, and vomiting. Bleeding may occur from mucous membranes and in the gastrointestinal tract. Symptoms may mimic those of malaria, leptospirosis, viral hepatitis, other hemorrhagic fevers, dengue, and other flavivirus infections.
Rapid detection methods include the detection of yellow fever antigen using monoclonal enzyme immunoassay in serum specimens and detection of viral genome sequences using polymerase chain reaction (PCR) assay. Yellow fever can be diagnosed using ELISA and serology titers of antibodies. Other investigations depend on what organ is involved. If there is evidence of altered mentation, a lumbar puncture and a CT scan are performed. Blood work may reveal leukopenia with elevated transaminase levels. Neutropenia is common during the first week of the infection.
If the liver is involved, the coagulation profile may be abnormal. Elevation in creatinine, hypoglycemia and metabolic acidosis are strongly associated with a very poor prognosis.
Most yellow fever specific testing can be done at the CDC, but reports will be sent to the state health department. When sending blood samples directly to the CDC, the health department should be informed. [3]
A chest x-ray is done in patients with respiratory distress because of the pulmonary edema. ECG may identify prolonged QT and PR intervals. Arrhythmias are common when the myocardium is affected.
- Treatment / Management
Yellow fever is a reportable infection. Once the virus is contracted, symptoms develop after 3 to 6 days. There is no specific treatment, but severe cases require aggressive supportive care and hydration. Patients should be managed in the intensive care unit (ICU) and closely monitored for disseminated intravascular coagulation (DIC), hemorrhage, kidney, and liver dysfunction. Coagulopathy is managed with fresh frozen plasma, and renal failure may require dialysis. Even though yellow fever is not transmitted from person to person, isolation of the individual should be undertaken until the diagnosis is confirmed. Universal precautions are required when looking after patients with yellow fever although person-person transmission of the virus is unlikely. Infected patients should avoid mosquitoes, as they may transmit the virus to mosquitoes, which can serve as vectors for infection other patients.
Since there is no effective treatment or vaccine, prevention is critical. This is best accomplished by avoiding mosquito bites entirely. Even very short periods outdoors can lead to exposure to mosquito bites, so people should wear proper protective clothing. This protection includes long sleeves, long pants, socks, and closed-toe shoes. Pant legs can be tucked into socks to prevent bites to exposed ankles. Transmission is common during the warmer months, and mosquitoes may bite through very thin clothing, so treating clothing with repellents containing permethrin, DEET, oil of lemon eucalyptus, or other EPA-registered insect repellants will reduce this risk. Permethrin should not be applied directly to the skin, but when applied to clothing, it provides protection even after the clothing is washed. Transmission is most frequent when mosquitoes feed, between dawn and dusk, so outdoor activities during this period should be avoided. However, one of the mosquitos responsible for transmitting the virus, Aedes Aegypty, feeds during the daytime; so there is no safe time during the day for a traveler without repellent and wearing protective clothing. Travelers should sleep in air-conditioned spaces or use mosquito nets or screens to prevent bites during sleep. Standing water is a breeding ground for mosquitoes, so flower pots, buckets, and other containers should be drained. Children’s wading pools should be emptied and stored on their sides, and tire swings should have holes drilled into the bottom to allow trapped water to drain.
There is a safe and highly effective live-attenuated vaccine available to prevent yellow fever. A single dose confers lifelong immunity and is effective within 30 days for 99% of patients. Patients with relative contraindications to live attenuated vaccine who plan to travel to endemic areas should review the recommendations for vaccination prior to travel. [9] [10] [11]
- Differential Diagnosis
The differential diagnosis of yellow fever is broad and makes a careful travel history important. It includes:
- Viral hemorrhagic fevers
- Viral hepatitis
- Lassa fever
- Ebola virus
- Typhoid fever
- Dengue fever
- Disseminated Intravascular Coagulation
- Louse-borne relapsing fever
- West Nile virus encephalitis
- Japanese encephalitis
- Herpes simplex encephalitis
- Eastern and Western equine encephalitis
- Venezuelan Equine encephalitis
- Enterovirus meningitis
- Mycoplasma meningitis
- Cytomegalovirus infection in immunocompromised host
- Tuberculous meningitis
- Nipah virus infection
- Rocky Mountain spotted fever
- Fungal meningitis
- Leptospirosis
- Neurocysticercosis
- Amebic meningoencephalitis
Most cases are subclinical or mildly symptomatic with an excellent prognosis. About 15% of symptomatic patients will develop severe disease. Most will recover, but after a bout of yellow fever, full recovery may take weeks or months. In most cases, there is a reversal of the liver and renal dysfunction. Death occurs in 30% to 50% of patients with severe disease. All travelers to endemic areas should be vaccinated if they are candidates for the live attenuated vaccine.
Death often occurs within 2 weeks during the toxic phase of the infection. Unvaccinated travelers to endemic areas are at high risk for developing symptomatic disease compared to the natives, who have acquired immunity. Rare cases of neurologic and viscerotropic disease have been reported following vaccination.
- Complications
- Multiorgan failure
- Respiratory failure
- Myocarditis
- Encephalitis
- Pearls and Other Issues
Continuous medical education can be obtained through the CDC website regarding yellow fever and yellow fever vaccine.
- Enhancing Healthcare Team Outcomes
Yellow fever is endemic in many parts of the world. The key to this infection is prevention. While the acute infection is managed by an interprofessional group of healthcare professionals, prevention is best done by a coordinated effort of educating patients by the primary care provider, infectious disease nurse, and pharmacist. All travelers to endemic regions should be educated about the vaccine, which confers lifelong immunity. Rarely some individuals may require a booster dose after ten years before traveling to an endemic area. Further, all laboratory workers who regularly handle yellow fever containing blood samples should have their neutralizing antibody titers measured every ten years to determine if they need a booster shot. The pharmacist and nurse should also educate the traveler on wearing long-sleeved garments, sleeping under a net and using DEET containing repellant spray.
It is very unlikely that yellow fever will be eradicated anytime soon. The mosquitoes also transmit the sylvatic form via nonhuman primates. Additionally, deforestation and urbanization have reintroduced the virus into the cities. Plus, there is a limited amount of resources available. [12] [13] (Level V)
Yellow fever can be self-limited or in some cases be life-threatening. Data indicate that about 10-25% of patients will develop severe symptoms that include jaundice, fever, renal and liver failure. The case fatality rates are slightly lower in West Africa compared to South America. However, the ultimate mortality depends on the virulence of the infecting strain and patient susceptibility. Overall, about 3-70% of patients die after contracting yellow fever; the higher mortality is usually in patients with liver and renal damage. Deaths tend to occur within the first 10 days of the toxic phase. Both infants and the elderly are more likely to die than other individuals. Individuals who are unvaccinated usually develop more severe disease than natives. Finally, there are rare cases of post-vaccination neurological deficits and viscerotropic disease leading to death. [14] [15] (Level V)
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Mosquito-Borne Diseases. Mosquitoes are carriers of various diseases, including Zika, dengue fever, West Nile fever, chikungunya, yellow fever, and malaria. National Institute of Allergy and Infectious Diseases, National Institutes of Health
Aedes species mosquito Image courtesy of S Bhimji MD
Faget sign Image courtesy Statpearls
Disclosure: Leslie Simon declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Klaus Torp declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Simon LV, Hashmi MF, Torp KD. Yellow Fever. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Yellow fever: the recurring plague. [Crit Rev Clin Lab Sci. 2004] Review Yellow fever: the recurring plague. Tomori O. Crit Rev Clin Lab Sci. 2004; 41(4):391-427.
- Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). [MMWR Recomm Rep. 2010] Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Staples JE, Gershman M, Fischer M, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010 Jul 30; 59(RR-7):1-27.
- Review Yellow fever: a disease that has yet to be conquered. [Annu Rev Entomol. 2007] Review Yellow fever: a disease that has yet to be conquered. Barrett AD, Higgs S. Annu Rev Entomol. 2007; 52:209-29.
- Review Yellow fever: an update. [Lancet Infect Dis. 2001] Review Yellow fever: an update. Monath TP. Lancet Infect Dis. 2001 Aug; 1(1):11-20.
- Review Prevention of yellow fever in persons traveling to the tropics. [Clin Infect Dis. 2002] Review Prevention of yellow fever in persons traveling to the tropics. Monath TP, Cetron MS. Clin Infect Dis. 2002 May 15; 34(10):1369-78. Epub 2002 Apr 25.
Recent Activity
- Yellow Fever - StatPearls Yellow Fever - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
1. Introduction. Yellow fever (YF) is a mosquito-borne viral illness caused by an arbovirus of the family Flaviviridae, genus Flavivirus, encompassing positive-single-stranded RNA viruses.The virus was isolated for the first time in 1927 in a male patient [].Transmission is primarily by mosquitoes [].After an incubation period of 3-6 days, YF infection can cause the onset of different ...
A BRIEF HISTORY OF YELLOW FEVER RESEARCH. YF originated in Africa, and was imported into Europe and the Americas as a consequence of the slave trade between these continents. 1 In the Western Hemisphere, the first recorded epidemic of disease believed to have been YF occurred in the Yucatan in 1648. 2 Throughout the eighteenth and nineteenth centuries explosive YF outbreaks ravaged tropical ...
Clinically, many people infected with YFV are asymptomatic. 1, 3 Others develop symptoms including sudden fever, chills, headache, low back pain, myalgia, nausea, vomiting, and/or fatigue after an incubation period of approximately 3 to 6 days. 1, 3 Most with YFV disease improve within 3 to 4 days. 3 However, roughly 15% go on to develop a more ...
Author summary Yellow fever, one of the most feared lethal zoonotic disease is re-emerging as a public health threat to tropical and sub-tropical countries of South America and Africa. Despite the existence of an effective yellow fever vaccine that is administered through mass vaccination campaigns and in routine immunization programs. against this disease, the mortality remains very high ...
Yellow fever (YF) virus still represents a major threat in low resource countries in both South America and Africa despite the presence of an effective vaccine. YF outbreaks are not only due to insufficient vaccine coverage for insufficient vaccine supply, but also to the increase in people without history of vaccination living in endemic areas
Yellow fever (YF), an acute viral hemorrhagic disease transmitted by infected mosquitoes, has the potential to spread rapidly and cause serious public health impact. The disease predominantly ...
Yellow fever (YF) virus is a mosquito-borne flavivirus found in Sub-Saharan Africa and tropical South America. The virus causes YF, a viral hemorrhagic fever, which can be prevented by a live ...
Cholera, Plague and Yellow fever - have returned, and new ones have emerged - SARS, Pandemic Influenza, MERS, Ebola and Zika. These epidemics and their impact on global public health have convinced the world's governments of the need for a collective and coordinated defense against emerging public
Yellow fever is a viral hemorrhagic fever that has inflicted stigma, illness, and death among human societies. From the 17 th to the 19 th centuries, yellow fever remained a mysterious illness that predominantly affected tropical regions in Africa, the Caribbean and the Americas. The disease was as feared as cholera or smallpox, and played a ...
Yellow fever, a mosquito-borne flavivirus disease occurs in tropical areas of South America and Africa. It is a disease of major historical importance, but remains a threat to travelers to and residents of endemic areas despite the availability of an effective vaccine for nearly 70 years. An important aspect is the receptivity of many non ...
Yellow fever, a viral infectious disease transmitted by mosquitoes, has re-emerged as a major international public health threat.1 Yellow fever is endemic in 34 countries in Africa and in 13 countries in central and South America.2 Yellow fever epidemiology is affected by factors such as urbanisation, population movements, deforestation, and climate change.3 The risk of yellow fever ...
Yellow fever is the most severe arbovirus ever to circulate in the Americas, and although vaccination campaigns and vector-control efforts have eliminated it from many areas, sylvatic transmission ...
For centuries, yellow fever virus infection generated substantial fear among explorers, tourist travellers, workers, military personnel, and others entering areas of transmission. Currently, there is transmission only in some areas of tropical South America and sub-Saharan Africa. When symptomatic, yellow fever infection causes severe liver dysfunction and coagulopathy with elevated mortality ...
Abstract. Yellow Fever (YF) is an arbovirus endemic in tropical regions of South America and Africa and it is estimated to cause 78,000 deaths a year in Africa alone. Climate change may have substantial effects on the transmission of YF and we present the first analysis of the potential impact on disease burden.
Yellow fever, the original viral haemorrhagic fever, was one of the most feared lethal diseases before the development of an effective vaccine. Today the disease still affects as many as 200 000 persons annually in tropical regions of Africa and South America, and poses a significant hazard to unvaccinated travellers to these areas. Yellow fever is transmitted in a cycle involving monkeys and ...
Background. Yellow fever is a vector-borne disease affecting humans and non-human primates in tropical areas of Africa and South America. While eradication is not feasible due to the wildlife reservoir, large scale vaccination activities in Africa during the 1940s to 1960s reduced yellow fever incidence for several decades.
An Overview of Yellow Fever Virus Disease. An Overview of Yellow Fever Virus Disease Neurohospitalist. 2017 Oct;7(4):157-158. doi: 10.1177/1941874417708129. Epub 2017 May 10. Authors Ian McGuinness 1 , J David Beckham 1 , Kenneth L Tyler 1 , Daniel M Pastula 1 2 Affiliations 1 Neuro-Infectious Diseases Group, Department of ...
In parts of tropical South American, African, and Asian countries, yellow fever (YF) is an endemic disease. It has a high case-fatality rate (20-50 percent) and causes hemorrhagic fever.[1] ... Search calls for papers Journal Suggester Open access publishing ... Technology and Research, Guntur, Andhra Pradesh, India, Ratna Prakash Kondapalli ...
Fact sheet. Yellow feverKey factsYellow fever is an acute viral haemorrhagic disease transmitted. by infected mosquitoes. The "yellow" in the name refers to the jaundice tha. affects some patients.Up to 50% of severely affected persons without treatment wil. die from yellow fever.There are an estimated 200 000 cases of yellow fever, causing ...
The gathered evidence suggests that a single dose of yellow fever vaccination provides lifelong protection in travellers. However, in people living with HIV and children (younger than 2 years), booster doses might still be required because lower proportions of vaccinees were seroprotected 10 or more years post-vaccination. Lower observed seroprotection rates among residents of endemic areas ...
Yellow fever is prevented by a vaccine, which is safe and affordable. A single dose of yellow fever vaccine is sufficient to grant life-long protection. A modelling study based on African data sources estimated the burden of yellow fever during 2013 was 84 000-170 000 severe cases and 29 000-60 000 deaths (1).
The disease yellow fever (YF) is prevented by a live-attenuated vaccine, termed 17D, which has been in use since the 1930s. One dose of the vaccine is thought to give lifelong (35+ years) protective immunity, and neutralizing antibodies are the correlate of protection. Despite being a vaccine-preventable disease, YF remains a major public ...
Research; Funding; Partners; Health emergency appeal; ... WHO position papers on Yellow fever. 5 July 2013. Vaccines and vaccination against yellow fever: WHO Position Paper - June 2013 ... This policy on the use of fractional doses of yellow fever vaccines can be applied to all WHO prequalified vaccines. 1 June 2017
In response to the detection of yellow fever cases in Douala, Cameroon, a collaborative effort involving the Eliminate Yellow Fever Epidemics (EYE) Secretariat in WHO, WHO representation in Cameroon, and TDR (the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases) aims to strengthen the capacity of Douala communities to reduce the transmission of the ...
Yellow fever is a mosquito-borne viral illness found in tropical and subtropical areas in South America and Africa. Transmission is primarily via Aedes and Haemagogus species of mosquito. It can present with varying clinical features ranging from a self-limited, mild febrile illness to severe hemorrhage and liver disease. The "yellow" comes from jaundice that affects some patients with ...