

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
Climate Change and California’s Drought
Take an aerial tour of one of California’s drought-stricken landscapes in this clip from Years of Living Dangerously .
Geography, Social Studies, Civics
In this video, Don Cheadle takes an aerial tour of one of California’s drought -stricken landscapes —a disappearing reservoir called Folsom Lake. Flying with him is Felicia Marcus, the top water official for the American state, who explains that four years of drought and no snowpack in the Sierra Nevada mountains have severely depleted the reservoir, one of the state’s main water supplies.
“This is absolutely what our future looks like under climate change,” Marcus states.
Find more of this story in the episode titled “Uprooted,” part of the National Geographic Channel’s Years of Living Dangerously series.
In 2016, California took the lead among U.S. states in addressing climate change by extending legislation to curb greenhouse gas emissions. Targeting both power plants and vehicles, the state committed to the goal of curbing carbon emissions to 40% below 1990 levels by 2030. Laws passed in 2006 had already set targets to reduce emissions to 1990 levels by 2020.
Together, two American politicians, Democratic California Governor Jerry Brown, and Republican former Governor Arnold Schwarzenegger, took a message about fighting climate change to the United Nations’ Climate Change Conference in Paris, France, in 2015. Brown also helped design an effort urging leaders of states, cities, and provinces around the world to commit to standards beyond what national leaders would adopt.
California, and most western U.S. states, rely heavily on snowpack each winter to resupply surface water streams and lakes. Lack of winter storms and warmer temperatures results in low snowmelt levels and depleted water supplies.
Articles & Profiles
Instructional links, media credits.
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Last Updated
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

National Integrated Drought Information System
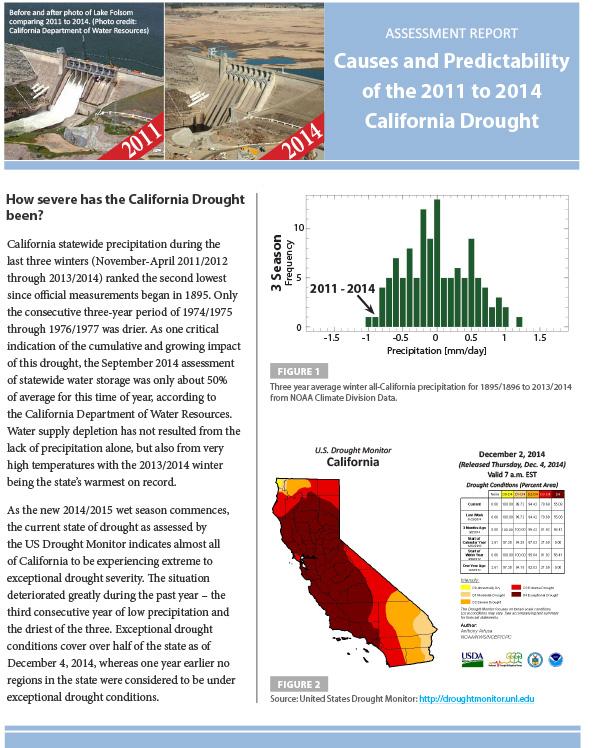
Summary: Causes and Predictability of the 2011 to 2014 California Drought Assessment Report
Two-page summary of the Drought Task Force I Assessment Report: Causes and Predictability of the 2011 to 2014 California Drought .
The report and summary were produced as part of the NOAA Drought Task Force I organized by the NOAA Modeling, Analysis, Predictions, and Projections Program (MAPP) in partnership with the National Integrated Drought Information System (NIDIS).
California Water Library
Economic Analysis of the 2014 Drought for California Agriculture
Richard Howitt, Josué Medellín–Azuara, Duncan MacEwan, Jay R. Lund, Daniel Sumner | July 15th, 2014
California is enduring its third driest year on record as agricultural, urban and environmental demands for water are at an all-time high. This report presents an assessment of the economic impacts of the 2014 drought on crop production, livestock and dairies using a suite of models.
This analysis extends the preliminary estimates of the Central Valley drought impacts released May 19, 2014 (Howitt et al. 2014) to include:
•Broadened coverage of the Statewide Agricultural Production Model (SWAP) to include majoragricultural areas on California’s central and south coasts and inland farms of the Imperial,Coachella and Palo Verde valleys,
•Updated SWAP agricultural production and economic impact estimates for the Central Valleyusing the most current data available,
•Estimated Central Valley impacts if the drought persists through 2015 and 2016, includingeconomic effects and the impact of increasing groundwater depletion and pumping depths usingthe California Department of Water Resources’ (DWR) C2VSIM model,
•Estimated fallowing of cropland due to drought using the SWAP model,
•Estimated losses to dairies and livestock using a supply elasticity approach based on pasturelosses and feed crop prices, and
•Comments on the preliminary draft from various state agencies.
The study finds that the 2014 drought will result in a 6.6 million acre-foot reduction in surface water available to agriculture. This loss of surface water will be partially replaced by increasing groundwater pumping by 5 million acre-feet.
The resulting net water shortage of 1.6 million acre-feet will cause losses of $810 million in crop revenue and $203 million in dairy and other livestock value, plus additional groundwater pumping costs of $454 million. These direct costs to agriculture total $1.5 billion. The total statewide economic cost of the 2014 drought is $2.2 billion, with a total loss of 17,100 seasonal and part-time jobs. Table ES-1 summarizes the key findings of the study.
agriculture , Central Valley , drought , economic analysis
Search the California Water Library
- Best Management Practice
- Biological Opinion
- Book/Textbook
- Bulletin 118
- California Water Plan
- Conference proceedings
- Congressional Testimony
- Environmental Documents
- Groundwater Sustainability Plan (GSP) review findings
- Guidance Document
- Handbook/Guide
- Issue Paper
- Journal Article
- Legal document
- Legislation
- NGO Position Paper
- Policy - state
- Policy Brief
- Research Article
- Research Brief
- Science Panel Report
- Technical Report
- Water Right Decision
- White Paper
- Adaptive Management
- Agriculture
- Basic Information
- California Water Fix/BDCP/Delta Conveyance
- Climate Change
- Drinking Water
- Drought & Hydrology
- Desalination
- Ecosystem & Species Mgt
- Ecosystem Restoration
- Invasive Species
- Wetland Mitigation
- Environmental Justice
- Flood Management
- Geochemistry
- Geohydrology
- Groundwater
- Human Right to Water
- Infrastructure
- Integrated Regional Water Management
- Landscape Reform
- People & Water
- Planning & Management
- Recycled Water
- Sacramento-San Joaquin Delta
- Science Management
- Transfers & Markets
- Tribal Water Issues
- Upper Watershed Management
- Water & Energy
- Water Project Operations
- Water Rights, Water Law
- Water Supply
- Water Quality
- Federal Water Quality
- Non Point Pollution Sources
- Point Pollution Sources
- State Water Quality
- Water Quality Monitoring
- Water Use & Efficiency
- Uncategorized
- adaptive management
- adjudicated basins
- agricultural drainage
- agriculture
- agriculture water use and efficiency
- allocations
- anadromous fish
- atmospheric rivers
- basic information
- basin characterization
- benthic macroinvertebrates
- best management practices (BMPs)
- bioassessment
- biological opinion (BiOp)
- brine management
- Central Valley
- Central Valley Project (CVP)
- climate change
- cloud seeding
- coastal aquifers
- Colorado River
- conjunctive use
- debris flow
- Delta conveyance
- Delta Stewardship Council (DSC)
- desalination
- direct potable reuse
- disadvantaged communities (DACs)
- drinking water
- economic analysis
- ecosystem management
- ecosystem restoration
- endangered species
- environmental justice
- flood management
- floodplain restoration
- forest management
- fugitive dust
- Glen Canyon Dam
- Gravity Recovery and Climate Experiment (GRACE)
- Great Basin Aquifer
- groundwater
- Groundwater Ambient Monitoring and Assessment (GAMA) Program
- groundwater contamination
- groundwater dependent ecosystems
- Groundwater Exchange
- groundwater pumping impacts
- groundwater recharge
- Groundwater Sustainability Plan (GSP)
- groundwater-surface water interaction
- habitat restoration
- human right to water
- hydraulic fracturing
- infrastructure
- interbasin flow
- invasive species
- managed aquifer recharge (MAR) - also see Groundwater Recharge
- microplastics
- Monterey amendments
- native fish
- ocean acidification
- oil and gas
- outreach and engagement
- planning and management
- privatization
- public trust doctrine
- Quantification Settlement Agreement
- reasonable use doctrine
- recycled water
- Regional Water Quality Control Plan
- risk assessment
- Sacramento–San Joaquin Delta
- science management
- sea level rise
- seawater intrusion
- State Water Project (SWP)
- Subscription/Donation
- Surface Water Ambient Monitoring Program (SWAMP)
- Sustainable Groundwater Management Act (SGMA)
- Trading - See Water Markets
- transboundary aquifers
- Transfers - See Water Markets
- tribal water issues
- uncategorized
- upper watershed management
- urban water conservation
- vernal ponds
- water and energy
- water budget
- water management - see planning and management
- water markets
- water pricing
- water project operations
- water quality
- water rights
- water supply
- water supply forecasting
- water transfers
- water use efficiency
Hydrological Region
- Central Coast
- Lower Basin States and Mexico - Colorado River
- North Coast
- North Lahontan
- Sacramento River
- San Francisco Bay
- San Joaquin River
- South Coast
- South Lahontan
- Tulare Lake
- Upper Basin States - Colorado River
- Acton Valley 4-005
- Adobe Lake Valley 6-010
- Ames Valley 7-016
- Amos Valley 7-034
- Antelope Valley 6-044
- Arroyo Santa Rosa Valley 4-007
- Arroyo Seco Valley 7-037
- Avawatz Valley 6-026
- Batiquitos Lagoon Valley 9-022
- Bear Valley 8-009
- Bessemer Valley 7-015
- Bicycle Valley 6-025
- Big Meadows Valley 8-007
- Big Valley (Lake) 5-015
- Borrego Valley - Ocotillo Wells 7-024.02
- Bristol Valley 7-008
- Broadwell Valley 6-032
- Buck Ridge Fault Valley 7-054
- Butte Valley 1-003
- Cadiz Valley 7-007
- Cady Fault Area 6-090
- Cahuilla Valley 9-006
- Calzona Valley 7-041
- Campo Valley 9-028
- Canebrake Valley 7-046
- Carpinteria 3-018
- Carrizo Plain 3-019
- Carson Valley 6-006
- Castac Lake Valley 5-029
- Caves Canyon Valley 6-038
- Chemehuevi Valley 7-043
- Chocolate Valley 7-032
- Cholame Valley 3-005
- Chuckwalla Valley 7-005
- Coachella Valley - Desert Hot Springs 7-021.04
- Coachella Valley - Indio 7-021.01
- Coachella Valley - Mission Creek 7-021.02
- Coachella Valley - San Gorgonio Pass 7-021.04
- Coastal Plain of Los Angeles - Central 4-011.04
- Coastal Plain of Los Angeles - Hollywood 4-011.02
- Coastal Plain of Los Angeles - Santa Monica 4-011.01
- Coastal Plain of Los Angeles - West Coast 4-011.03
- Coastal Plain of Orange County 8-001
- Coastal Plain of San Diego 9-033
- Collins Valley 7-055
- Conejo 4-010
- Copper Mountain Valley 7-011
- Corralitos - Pajaro Valley 3-002.01
- Cottonwood Valley 9-027
- Coyote Lake Valley 6-037
- Coyote Wells Valley 7-029
- Cronise Valley 6-035
- Cuddeback Valley 6-050
- Cuyama Valley 3-013
- Dale Valley 7-009
- Darwin Valley 6-057
- Davies Valley 7-061
- Deadman Valley - Deadman Lake 7-013.01
- Deadman Valley - Surprise Spring 7-013.02
- Death Valley 6-018
- Deep Springs Valley 6-015
- Denning Spring Valley 6-078
- Downtown 2-040
- East Salton Sea 7-033
- Eel River Valley 1-010
- El Cajon Valley 9-016
- El Mirage Valley 6-043
- Elsinore - Bedfored-Coldwater 8-004.02
- Elsinore - Elsinore Valley 8-004.01
- Escondido Valley 9-009
- Fenner Valley 7-002
- Fish Lake Valley 6-014
- Fremont Valley 6-046
- Gilroy-Hollister Valley - Llagas Area 3-003.01
- Goldstone Valley 6-048
- Goleta 3-016
- Harper Valley 6-047
- Helendale Fault Valley 7-048
- Hemet Lake Valley 8-006
- Hidden Valley 4-016
- Imperial Valley 7-030
- Indian Wells Valley - 6-054
- Iron Ridge Area 7-050
- Ivanpah Valley 6-030
- Jacumba Valley 7-047
- Johnson Valley - Soggy Lake 7-018.01
- Johnson Valley - Upper Johnson Valley 7-018.02
- Joshua Tree 7-062
- Kane Wash Area 6-089
- Kelso Valley 6-031
- Lanfair Valley 7-001
- Langford Valley - Irwin 6036.02
- Langford Valley - Langford Well Lake 6-036.01
- Langford Valley 6-036.02
- Las Posas Valley 4-008
- Lavic Valley 7-014
- Leach Valley 6-027
- Lockwood Valley 4-017
- Long Valley 6-011
- Los Osos Valley - Los Osos 3-008.01
- Los Osos Valley - Warden Creek 3-008.02
- Lost Horse Valley 7-051
- Lower Kingston Valley 6-021
- Lower Mojave River Valley 6-040
- Lucerne Valley 7-019
- Malibu Valley 4-022
- Means Valley 7-017
- Mesquite Valley 6-029
- Middle Amargosa Valley - 6-020
- Middle Mojave River Valley 6-041
- Mission Valley 9-014
- Modoc) 5-004
- Morongo Valley 7-020
- Morro Valley 3-041
- Napa-Sonoma Valley - Napa-Sonoma Lowlands 2-002.03
- Needles Valley 7-044
- Ocotillo-Clark Valley 7-025
- Ogilby Valley 7-035
- Ojai Valley 4-002
- Orocopia Valley - 7-031
- Owens Valley - Fish Slough 6-012.02
- Owens Valley 6-012.01
- Owl Lake Valley 6-088
- Pahrump Valley 6-028
- Palo Verde Mesa 7-039
- Palo Verde Valley 7-038
- Pamo Valley 9-024
- Panamint Valley 6-058
- Peach Tree Valley 3-032
- Petaluma Valley 2-001
- Pilot Knob Valley 6-051
- Pinto Valley 7-006
- Pipes Canyon Fault Valley 7-049
- Pittsburgh Plain 2-004
- Piute Valley 7-045
- Pleasant Valley 4-006
- Potrero Valley 9-029
- Poway Valley 9-013
- Raymond 4-023
- Red Pass Valley 6-024
- Redding Area - Anderson 5-006.03
- Redding Area - Bowman 5-006.01
- Redding Area - Enterprise 5-006.04
- Redding Area - Millville 5-006.05
- Redding Area - South Battle Creek 5-006.06
- Rice Valley 7-004
- Riggs Valley 6-023
- Russell Valley 4-020
- Sacramento Valley - Antelope 5-021.54
- Sacramento Valley - Bend 5-021.53
- Sacramento Valley - Butte 5-021.70
- Sacramento Valley - Colusa 5-021.52
- Sacramento Valley - Corning 5-021.51
- Sacramento Valley - Los Molinos 5-021.56
- Sacramento Valley - North American (5-021.64)
- Sacramento Valley - North Yuba 5-021.60
- Sacramento Valley - Red Bluff 5-021.50
- Sacramento Valley - Solano 5-021.66
- Sacramento Valley - South American 5-021.65
- Sacramento Valley - South Yuba 5-021.61
- Sacramento Valley - Sutter 5-021.62
- Sacramento Valley - Vina 5-021.57
- Sacramento Valley - Wyandotte Creek 5-021.69
- Sacramento Valley - Yolo 5021.67
- Salinas Valley - 180/400 Foot Aquifer 3-004.01
- Salinas Valley - Atascadero Area 3-004.11
- Salinas Valley - East Side Aquifer 3-004.02
- Salinas Valley - Forebay Aquifer 3-004.04
- Salinas Valley - Langley Area 3-004.09
- Salinas Valley - Monterey 3-004.10
- Salinas Valley - Paso Robles Area 3-004.06
- Salinas Valley - Upper Valley Aquifer 3-004.05
- Saline Valley 6-017
- Salt Wells Valley 6-053
- San Antonio Creek Valley 3-014
- San Dieguito Creek 9-012
- San Elijo Valley 9-023
- San Felipe Valley 7-027
- San Fernando Valley 4-012
- San Gabriel Valley 4-013
- San Jacinto 8-005
- San Joaquin Valley - Chowchilla 5-022.05
- San Joaquin Valley - Cosumnes (5-022.16)
- San Joaquin Valley - Delta-Mendota 5-022.07
- San Joaquin Valley - East Contra Costa 5-022.19
- San Joaquin Valley - Eastern San Joaquin 5-022.01
- San Joaquin Valley - Kaweah 5-022.11
- San Joaquin Valley - Kern 5-022.14
- San Joaquin Valley - Kings 5-022.08
- San Joaquin Valley - Madera 5-022.06
- San Joaquin Valley - Merced 5-022.04
- San Joaquin Valley - Modesto 5-022.02
- San Joaquin Valley - Pleasant Valley 5-022.10
- San Joaquin Valley - Tracy 5-022.15
- San Joaquin Valley - Tulare Lake 5-022.12
- San Joaquin Valley - Tule 5-022.13
- San Joaquin Valley - Turlock 5-022.03
- San Joaquin Valley - Westside 5-022.09
- San Joaquin Valley - White Wolf 5-022.18
- San Juan Valley 9-001
- San Luis Obispo Valley 3-009
- San Luis Rey Valley - Lower San Luis Rey Valley 9-007.02
- San Luis Rey Valley - Upper San Luis Rey Valley 9-007.01
- San Marcos Area 9-032
- San Mateo Valley 9-002
- San Onofre Valley 9-003
- San Pasqual Valley 9-010
- Santa Barbara 3-017
- Santa Clara River Valley - Fillmore 4-004.05
- Santa Clara River Valley - Mound 4-004.03
- Santa Clara River Valley - Oxnard 4-004.02
- Santa Clara River Valley - Piru 4-004.06
- Santa Clara River Valley - Santa Clara River Valley East 4-004.07
- Santa Clara River Valley - Santa Paula 4-004.04
- Santa Clara Valley - East Bay Plain 2-009.04
- Santa Clara Valley - Niles Cone 2-009.01
- Santa Clara Valley - San Mateo Plain 2-009.03
- Santa Clara Valley - Santa Clara 2-009.02
- Santa Cruz Mid-County 3-001
- Santa Margarita 3-027
- Santa Maria River Valley - Arroyo Grande 3-012.02
- Santa Maria River Valley - Santa Maria 3-012.01
- Santa Maria River Valley 3-012.02
- Santa Maria Valley 9-011
- Santa Rosa Valley - Santa Rosa Plain 1-055.01
- Santa Ynez River Valley 3-015
- Scott River Valley 1-005
- Searles Valley 6-052
- Seven Oaks Valley 8-008
- Shasta Valley 1-004
- Sierra Valley 5-012.01
- Silver Lake Valley 6-034
- Simi Valley 4-009
- Soda Lake Valley 6-033
- South San Diego River Valley 9-015
- Superior Valley 6-049
- Temecula Valley 9-005
- Terwilliger Valley 7-026
- Thousand Oaks Area 4-019
- Tule Lake 1-002-01
- Twentynine Palms Valley 7-010
- Ukiah Valley 1-052
- Upper Kingston Valley 6-022
- Upper Mojave River Valley 6-042
- Upper Ojai Valley 4-001
- Upper Santa Ana Valley - Chino 8-002.01
- Upper Santa Ana Valley - Cucamonga 8-002.02
- Upper Santa Ana Valley - Rialto-Colton 8-002.04
- Upper Santa Ana Valley - Riverside-Arlington 8-002.03
- Upper Santa Ana Valley - San Bernardino 8-002.06
- Upper Santa Ana Valley - San Timoteo 8-002.08
- Upper Santa Ana Valley - Temescal 8-002.09
- Upper Santa Ana Valley - Yucaipa 8-002.07
- Vallecito-Carrizo Valley 7-028
- Ventura River Valley - Lower Ventura River 4-003.02
- Ventura River Valley - Upper Ventura River 4-003.01
- Vidal Valley 7-042
- Ward Valley 7-003
- Warren Valley 7-012
- West Salton Sea
- Wingate Valley 6-019
- Yuma Valley 7-036
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 September 2024
Continental-scale associations of Arabidopsis thaliana phyllosphere members with host genotype and drought
- Talia L. Karasov ORCID: orcid.org/0000-0002-3592-6597 1 , 2 ,
- Manuela Neumann ORCID: orcid.org/0000-0003-2778-3028 2 nAff7 ,
- Laura Leventhal 3 , 4 ,
- Efthymia Symeonidi 1 ,
- Gautam Shirsekar 2 nAff8 ,
- Aubrey Hawks 1 ,
- Grey Monroe ORCID: orcid.org/0000-0002-4025-5572 2 nAff9 ,
- Pathodopsis Team ,
- Moisés Exposito-Alonso 3 , 4 nAff10 nAff11 ,
- Joy Bergelson 5 ,
- Detlef Weigel ORCID: orcid.org/0000-0002-2114-7963 2 , 6 &
- Rebecca Schwab 2
Nature Microbiology ( 2024 ) Cite this article
Metrics details
- Biological techniques
Plants are colonized by distinct pathogenic and commensal microbiomes across different regions of the globe, but the factors driving their geographic variation are largely unknown. Here, using 16S ribosomal DNA and shotgun sequencing, we characterized the associations of the Arabidopsis thaliana leaf microbiome with host genetics and climate variables from 267 populations in the species’ native range across Europe. Comparing the distribution of the 575 major bacterial amplicon variants (phylotypes), we discovered that microbiome composition in A. thaliana segregates along a latitudinal gradient. The latitudinal clines in microbiome composition are predicted by metrics of drought, but also by the spatial genetics of the host. To validate the relative effects of drought and host genotype we conducted a common garden field study, finding 10% of the core bacteria to be affected directly by drought and 20% to be affected by host genetic associations with drought. These data provide a valuable resource for the plant microbiome field, with the identified associations suggesting that drought can directly and indirectly shape genetic variation in A. thaliana via the leaf microbiome.
The widely different environments in which the cosmopolitan species Arabidopsis thaliana is found today 1 have left strong signatures of selection throughout its genome 2 . While geographic differences in abiotic factors are well appreciated, similar differences in the resident microbiota are also likely to influence local plant fitness 3 . A recent survey of A. thaliana root microbiomes 4 found regional differentiation, often reflecting the composition of the soil microbiota. Host location was similarly significantly correlated with both root- and leaf-associated microbial composition of another crucifer, Boechera stricta 5 .
We already know that host genetics can influence microbiome composition 5 , 6 , 7 , 8 , and geographic differences in host genetics may in turn structure the resident microbiome, but the two might also be independently affected by physical distance, including abiotic factors that vary geographically 4 , 5 . For example, pH is a significant predictor of bacteria in the A. thaliana rhizosphere 4 , consistent with pH as a major driver of soil bacterial communities 9 . Similarly, precipitation can be a significant predictor of plant microbiome composition 10 .
Because previous studies have typically been limited in the number of populations 4 or the geographic range surveyed 3 , it has been difficult to disentangle the effects of host genetics, geography and abiotic factors on the plant-associated microbiome. In this Resource, we use a continental-scale assessment of bacteria that colonize A. thaliana leaves to identify environmental and host genetic factors that are strongly associated with distinct microbiome types. We then determine the environmental variables that best predict microbiome composition. Finally, we follow up with a controlled field experiment to test the relative contributions of host genetics and of water availability to these predictable patterns and a direct demonstration that a common bacterial taxon can provide drought protection. Our results indicate that differential plant survival in low-water environments might in part be due to different bacteria colonizing drought-adapted and drought-susceptible plants.
From February to May 2018, we visited 267 European A. thaliana populations around the end of their vegetative growth and close to the onset of flowering 11 (Fig. 1a,b ). At each site we collected whole rosettes from two individuals, along with a neighbouring crucifer (family Brassicaceae, primarily Capsella bursa-pastoris ), if present, and two soil samples. We evaluated A. thaliana life history traits (Fig. 1c and Extended Data Fig. 1 ) and extracted information on climate variables for the collection sites 12 . We assessed the microbial composition of the leaf and soil samples by sequencing the V3–V4 region of the 16S ribosomal RNA locus and identifying amplicon sequence variants (ASV) using DADA 13 . Each ASV was considered a distinct bacterial lineage or phylotype. Host genetics and absolute microbe abundance were assessed by shotgun sequencing plant tissue, which generates reads of host and microbial genomes 14 .

a , b , A. thaliana plants were collected from distinct ecosystems. a , Examples of aspects of collection locations. b , Latitude/longitude of all locations. MOG is an acronym for Moguériec, France, and Vdc for Villaviciosa de Córdoba, Spain. c , Based on images of individual plants taken at each site, we assessed plant health and development. The x axis represents qualitative values ( Methods ), except for the rosette diameter, which is classified in intervals of (1) 0–1 cm, (2) 1–2 cm and so on. The disease index corresponds to different macroscopic disease symptoms as indicated ( Hpa , Hyaloperonospora arabidopsidis ). The central horizontal line in each box indicates the median, the bounds indicate the upper and lower quartiles and the number above the boxes indicates the individuals in each group.
Phyllosphere composition is distinct from the soil and is host species specific
There is considerable debate as to the origin of the microbes that colonize plants, although soil often has a measurable influence 4 , 15 , 16 . A study across 17 European A. thaliana populations 4 found differentiation between root and non-root-associated microbes, but no significant differences between A. thaliana and neighbouring grasses 4 . Intra-species comparisons in a common garden experiment had suggested that host genetics can explain about 10% of the variance among A. thaliana leaf bacteria 17 . At the basis of these comparisons is the question of how much the host influences microbiome assembly, either because of active recruitment of specific microbes, or because of the differential ability of microbes to colonize their hosts.
To explicitly test for enrichment of specific taxa in the phyllosphere, we compared soil and plant leaves across all 267 sites via multi-dimensional scaling (MDS; Hellinger transformation). As expected, there was broad-scale separation between the phyllosphere and the soil (Fig. 2a,b ). Modelling 18 the effect of compartment on the microbial core phylotypes in the phyllosphere revealed differential abundance of 91% (524/575) of phylotypes between the A. thaliana phyllosphere and soil (False Discovery Rate (FDR) <0.01). Focusing on differences among host species 18 , we found 36% (205/575) of phylotypes to distinguish A. thaliana from neighbouring crucifers (Extended Data Fig. 2 ). This indicates that inter-host species differences in genetics or phenology have a strong influence on microbiome composition. On a phylotype-by-phylotype basis, abundance in A. thaliana was poorly predicted by a phylotype’s abundance in soil or in the surrounding companion plants (Extended Data Fig. 2 ).

a , b , Ordination on a Hellinger transformation of the samples. Arabidopsis thaliana leaf microbiomes are significantly differentiated from that of surrounding soil ( a ) and less so, but still significantly, from surrounding crucifers (Brassicaceae) ( b ). c , d , k -means clustering ( k = 2) ( c ) identified two microbiome types that turned out to have a north–south latitudinal cline ( d ). e , Distribution of higher taxonomic levels across the southern and northern clusters. f , Comparison of extent of seasonal variation in south-west Germany (winter and spring) with the European geographic variation (clusters 1 and 2). g , Absence of correlation in fold changes (FCs) in phylotype abundance between the northern and southern clusters ( y axis) and between the winter and spring samples from south-western Germany ( x axis). Colour indicates association with the two north–south clusters 1 and 2.
Phyllosphere microbial composition varies with latitude
We tested the geographic differentiation of microbiomes using dimensionality reduction for the entire community and assessment of the spatial distribution for each bacterial phylotype. The former reveals global trends in composition, while the latter provides information on individual microbes contributing to such trends. Loadings on both the first and second principal coordinate axes (Fig. 2c ) correlated with latitude (Pearson’s r = 0.75, P = 2.2 × 10 −16 , and r = −0.24, P = 1.35 × 10 −7 , respectively), suggesting geographic structure in the phyllosphere microbiome. Because silhouette scoring 19 indicated that A. thaliana phyllosphere microbiomes were best characterized as two distinct types, we used k -means clustering of the Hellinger-transformed counts table to classify our samples (Fig. 2c and Extended Data Fig. 3 ). We found that the two microbiome types were strongly differentiated by geography, with one dominating in Northern and the other in Southern Europe (Fig. 2d,e ). Among individual phylotypes, the relative abundance of one third (33%) was significantly associated with latitude (linear regression, FDR <0. 01), but only a small minority, 2%, was correlated with longitude, confirming that Northern and Southern European A. thaliana reproducibly harbour different microbiota. One percent of the plant-associated phylotypes were also significantly correlated in the soil with latitude, suggesting that the latitudinal contrast is formed via colonization.
The phyllosphere changes with plant development and the seasons 20 . To test whether the observed latitudinal phyllosphere contrast could be explained by seasonal and developmental differences, we compared our samples with a multi-year dataset from a single location in Germany 21 . Projecting seasonal phylotype composition into the MDS biplots of our pan-European samples did not reveal any preferential association of collection season with microbiome type (Fig. 2f ). Comparing changes in the abundance of single phylotypes between seasons and between the two major microbiome types (Fig. 2g ) similarly did not point to the latitudinal contrast reflecting environmental variation being caused by local seasonal differences (Wald test of multinomial frequency estimates, P > 0. 01).
The association between latitude and phylotype abundance was phylotype specific, differing within and between bacterial families (Fig. 3a and Extended Data Fig. 3 ). Pseudomonas and Sphingomonas are abundant across A. thaliana populations 21 , 22 , 23 and both genera can affect A. thaliana health 21 , 24 , 25 . Linear regression of each core phylotype onto latitude revealed that four of the five most abundant sphingomonads have latitudinal clines (Fig. 3a,b , FDR <0. 01), while the most abundant pseudomonad phylotypes did not show long-distance variation (Fig. 3b–e ). Rhizobiaceae were also latitudinally differentiated. A consequence of phylotype-specific association with latitude was that the two major microbiome types were significantly differentiated at the phylotype level, but not at higher taxonomic levels (Fig. 2e and Extended Data Fig. 3 ). Thus, even though A. thaliana is colonized by different individual phylotypes in Northern and Southern Europe, the bacterial classes remain broadly the same (Fig. 2e ).

a , Linear relationships between relative abundance (RA) of the most common phylotypes. The y axis represents −log 10 -transformed FDR-corrected P values obtained when regressing the abundance of a phylotype on latitude (linear regression). Phylotypes are grouped by families, which are indicated on the bottom. b , c , There is a strong latitudinal cline for the RA of the most abundant sphingomonads ( b ) but not for the most abundant pseudomonads ( c ; note the difference in RA scale). d , e , Interpolation of the abundance of the top sphingomonad phylotype ( d ) and of ATUE5 ( e ), the top pseudomonad phylotype and a known opportunistic pathogen, revealed a continuous spatial gradient for the top sphingomonad ( d ), but a patchy distribution with regional hotspots for the top pseudomonad ( e ). f , The relationship between microbiome type and polymorphism in plant immune genes was assessed with the F st population differentiation index. The most extreme F st values were found in the immune regulator ACD6 . Data in b and c are presented as the estimated regression value ± s.e.m. Chr, chromosome.
Common phylotypes differ in their geographic distributions
A single Pseudomonas phylotype, ATUE5 (previously OTU5), is a common opportunistic pathogen in local populations in south-west Germany, where it is an important driver of total microbial load 21 . Because ATUE5 was also the most abundant pseudomonad in our study, we wanted to learn how its distribution was geographically structured (Fig. 3c ). ATUE5 was the seventh most common phyllosphere phylotype overall, with a relative abundance of up to 64% (mean of 1.8%). ATUE5 was found in 56% of samples, but without significant latitudinal differentiation (Pearson’s r = 0.01, P = 0.92).
Despite ATUE5 being a common phyllosphere member, its distribution was disjoint, and ordinary Kriging interpolation across the sampled range confirmed a very patchy presence (Fig. 3c ). In contrast, the most frequent Sphingomonas phylotype (and most frequent phylotype overall) showed a significant latitudinal cline (Fig. 3b ). High ATUE5 abundance was largely limited to single populations or populations very close to each other, with a spatial autocorrelation restricted to distances of under 50 km (Extended Data Fig. 6 ). In summary, the Pseudomonas pathogen ATUE5 is widely yet very unevenly distributed.
Drought metrics predict microbiome composition
Common garden experiments have indicated that environmental factors strongly shape bacterial microbiome composition 17 . Our continental-scale data enabled us to test which abiotic factors are most correlated with geographic structure of the phyllosphere microbiome.
We tested for associations between climate variables and microbiome composition, including developmental and health traits as potential confounders 26 . Altogether, we considered 39 covariates that could influence microbiome composition (Extended Data Fig. 7 and Extended Data Table 1 ). We first removed covariates that were highly correlated with others and then performed random forest classification using the two microbiome types as response variables (Fig. 4 and Extended Data Fig. 8 ). The covariate with greatest explanatory power was the Palmer Drought Severity Index (PDSI) mean from the six pre-collection months, a metric of recent dryness 27 . PDSI was similarly the best predictor for the loading of a sample on MDS1. In general, environmental covariates were better predictors than were plant traits. In contrast, environmental covariates (including PDS1) had poor predictive power for plant-associated phylotypes in the soil microbiome, explaining less than 1% of the variance in the loading on the first principal coordinate axis.

a , Random forest modelling was used to determine environmental variables associated with microbiome type. The abbreviations are explained in Methods . b , PDSI of the location was the best predictor of microbiome type, explaining more than 50% of the variance. The upper and lower hinges of the boxes represent the first and third quartiles and the central line the median, with n = 269 plants in cluster 1 and n = 192 plants in cluster 2. c , The mean PDSI throughout Europe for January to April 2018.
Because PDSI is correlated with latitude, we tested whether information about both variables improves prediction outcomes. Inclusion of PDSI significantly improved predictive capacity ( P = 4.2 × 10 −7 for logistic regression with microbiome type and P = 2.7 × 10 −7 for linear regression on MDS1), indicating that the association between microbiome type and PDSI extends beyond latitudinal correlation. PDSI was also predictive for microbiome composition within geographic regions and their corresponding sampling tours ( P = 2.3 × 10 −7 for logistic regression with cluster identity and P = 0. 047 for linear regression on MDS1).
From mixed-effects modelling, we estimated the marginal R 2 for PDSI to be 50%. Together with previous work supporting the importance of water availability in determining host-associated microbiomes 9 , we conclude that water availability affects which microbes can access the host plant and/or proliferate on the host. Drought might do so directly by affecting plant physiology, indirectly by shaping host genetics or by a combination of the two. Additionally, drought affects the abundances of microbes in the abiotic environment, and hence which microbes are present for colonization.
Host genetics is associated with microbiome composition
Arabidopsis thaliana exhibits strong population structure across Europe, with a pattern of isolation by distance 28 and greater latitudinal than longitudinal differentiation 1 . Climate-driven selective pressures, particularly water availability and drought 29 , along with different groups of insect predators 30 have contributed to the geographic structure of A. thaliana genetic diversity.
To determine whether this extends to the phyllosphere microbiome, we extracted heritability estimates for phyllosphere phylotypes from eight common garden experiments in which 200 A. thaliana accessions had been grown in four Swedish locations across 2 years 8 . Two thirds (368/575; 64%) of our core phylotypes had been observed in this study 8 . We were able to obtain heritability estimates for 251 of these phylotypes, almost all of which (247; 98.4%) had significant positive heritability in at least one of the eight experiments. Genetic differences are therefore very likely to contribute to the observed geographic differentiation of the A. thaliana phyllosphere microbiome across Europe. However, heritability does not necessarily imply direct host control of each phylotype, as it can also be exerted indirectly via microbial hub taxa 8 .
To determine how microbiome composition in our study might be influenced by host genetics, which was representative of previous surveys 1 (Extended Data Fig. 4 ), we fitted a mixed-effects model that included relatedness as a random effect and the loading on the first axis of the decomposition of the microbiome composition as the phenotypic response variable. Plant genotype alone explains 68% of the variance in the loading along MDS1 and 52% of the variance in the MDS2 loading (pseudo h 2 0.68, standard error of the mean (s.e.m.) 0.10 for MDS1 and pseudo h 2 0.52, s.e.m. 0.12 for MDS2). MDS1 explains 8% and MDS2 5% of the variance in microbiome composition, consistent with host genetics probably playing only a subordinate role in structuring the microbiome 8 , 17 , 31 . In a mixed-effects model, PDSI was associated with MDS1, whereas several genetic principal components were associated with MDS2 (Extended Data Tables 2 – 4 ).
Because immune genes are prime targets for interactions with microbes 32 , 33 , we tested whether specific immune gene alleles are associated with the two microbiome types. Among a generous, though not exhaustive, list of 1,103 genes with connection to pathogen response and defense 34 , the top single-nucleotide polymorphism (SNP) was in ACD6 (empirical P = 0.0001) (Fig. 3f and Extended Data Fig. 5 ). ACD6 alleles can differentially impact pathogen resistance through constitutive effects on immunity 35 . The full ACD6 haplotypes associated with each microbiome type have not yet been reconstructed, as the short reads used for genotypic comparisons did not allow for resolution of full-length alleles. Nonetheless, our results demonstrate a striking association between microbiome type and polymorphisms in a central regulator of immune activation. Whether resident microbiota select for ACD6 allele type, or instead ACD6 allele type influences microbiome type, remains to be determined.
Are genetic alleles responsible for microbiome variation across geography? For defense genes such as R genes, this is probably not the case as variation tends to be maintained within local populations of A. thaliana 36 , 37 . We do not know whether this extends to genes that control the non-pathogenic microbiota. A previous study found ~150 SNPs to be significantly associated with heritable microbiome composition in A. thaliana 31 . When we tested the geographic differentiation of these SNPs across Europe (Extended Data Fig. 5 ), we found that they had significantly higher global F st values than the genome-wide background, consistent with different A. thaliana populations selecting for different microbiota.
Host adaptation to drought influences microbial abundance
To disentangle the impact of drought from that of plant genetics, we conducted a common garden field experiment in California. Using a setup similar to our previous work in Europe 29 , we grew A. thaliana accessions (Extended Data Table 5 ) under a high- and low-watering regimen. Focusing on accessions that had previously been identified as drought adapted or susceptible based on genetic loci associated with adaptation to drought 29 , we assessed differences in phyllosphere composition after drought stress. Of the 575 core phylotypes in the European field collections, 154 were present in California and 20 were sufficiently common to enable us to determine the relative influences of genetics and drought treatment on their relative abundances (Extended Data Tables 2 – 4 ). Of these 20 phylotypes, 3 were significantly influenced by host genetic classification of drought-adapted versus susceptible accessions, and 3/20 showed a significant interaction between drought treatment and host genotype (Extended Data Table 6 ). Two out of 20 showed a significant response to the abiotic drought treatment alone. The phylotypes that were significantly associated with plant genotype in the California field experiment accounted for an appreciable fraction of the total microbiome in the European wild collections—an average of 13.2% of the total microbial community in a plant and as high as 71.9% total relative abundance in a plant (Extended Data Fig. 9 ). The most abundant phylotype across the European collection (Extended Data Fig. 9 ) was significantly associated with plant genotypic classification. In total, these results indicate that genetic adaptation to drought has an impact on some of the most abundant bacteria that colonize a plant.
Common phylotypes alter drought effects on A. thaliana
Finally, we tested whether water availability can influence the abundance of a common phylotype, the opportunistic pathogen ATUE5. In growth chambers, we exposed 5-week-old plants of the Col-0 reference accession to a week-long drought, followed by syringe inoculation with the ATUE5 p25.c2 strain 21 . Three days after infection, we compared bacterial growth and green tissue in drought-stressed and well-watered plants. Drought significantly reduced the ability of ATUE5 to proliferate in planta (Extended Data Fig. 10 ; two-sided Wilcoxon rank-sum test, P = 0.003), a result consistent with Pseudomonas pathogens relying on water availability to spread and multiply 38 . Drought also significantly reduced the green, photosynthetically active leaf area (Extended Data Fig. 10 ), with ATUE5 infection blunting this negative effect of drought.
These results indicate that infection by an opportunistic pathogen may be conditionally beneficial, conferring drought tolerance under specific conditions. ATUE5 was previously shown to influence A. thaliana growth in a genotype-specific manner 39 , indicating that the interaction between drought and ATUE5 infection is likely to differ between plant populations. This is reminiscent of viral infection reducing drought-based mortality 40 and in agreement with plant growth promoting effects of microbes under drought 41 , as discussed in a recent review 42 of the diverse mechanisms of microbe-mediated drought tolerance. Moreover, there is precedence for cryptic A. thaliana pathogens providing environment-specific fitness benefits 43 .
Our results reveal several robust trends. Firstly, colonization of A. thaliana leaves imposes a strong bottleneck on the microbes that arrive from the surrounding soil and other plants, with most microbes differing in abundance between the soil and A. thaliana leaves and more than a quarter differing between A. thaliana and companion plants from the same family. Host genetics clearly matters for determining which microbes manage to establish in and on the plant. Our results indicate that these trends, observed before over small regions 4 , 7 , 8 , are reproducible and ubiquitous on a continental scale. Secondly, geography and associated abiotic factors significantly influence the microbes on A. thaliana : a plant in Spain will very probably be colonized by a different suite of microbes than a plant in Sweden. Our field experiment begins to disentangle the direct contribution of geography-dependent climate differences on the microbiome from those that are mediated by adaptive differences in host genetics. We note, however, that both genetic population structure and environmental variables exhibit autocorrelation, hence the variance explained by plant genotype is invariably confounded by correlated environmental factors, with the exact extent being difficult to discern. We identify genetic variation in an immunity gene, ACD6 , to be associated with microbiome type and with PDSI. Specific alleles of ACD6 confer drought tolerance 44 , adding further complexity to our understanding of the relationship between drought, microbes and plant genetics. Lastly, our analyses suggest that microbial colonization of plants is strongly dictated by water availability and the attendant microbiota. This again raises the question of how different microbial communities influence plant phenotype. Drought not only plays a major selective role in A. thaliana populations 29 , but it is also known to affect the ability of plants to withstand pathogen attack. An important question will be whether different background microbiomes in plants that are more likely to experience drought in the wild will help or hamper defense against pathogens 45 .
Sample collection
Arabidopsis thaliana and other crucifers were sampled during local springtime in 2018. Most crucifer companion samples were Capsella bursa-pastoris , and the rest were Cardamine hirsuta . A full list of sampling locations and dates is provided in Extended Data Table 1 . Rosettes were separated from the roots using alcohol wipe-sterilized scissors and forceps, then washed with water and ground with a sharp disposable spatula (Roth) in RNAlater (Sigma, now Thermo Fisher). For each A. thaliana plant for which soil was accessible, one to three tablespoons of soil were collected from the location where the plant had been removed and placed in a clean airtight bag. Samples were then maintained in electrical coolers (Severin Kühlbox KB2922) until the end of the sampling trip (which were 1–12 days long). In the lab, samples were stored at 4 °C. Within 0–3 days, RNAlater was removed from plant samples. Samples were centrifuged for 1 min at 1,000 g , the supernatant was removed and samples were washed with 1 ml autoclaved water. For storage at −80 °C, plant tissue was transferred with ethanol sterilized forceps to screw cap freezer tubes containing 1.0 mm Garnet Sharp Particles (BioSpec Products, Cat. No. 11079110GAR). A ~200 mg aliquot from each soil sample was transferred to a screw cap freezer tube using an ethanol sterilized spatula, with great effort to exclude plant and insect pieces. Before aliquoting, soil bags were kept at −80 °C and defrosted at 4 °C overnight, unless aliquoting was done immediately upon arrival in the lab at the end of the sampling trip.
Nagoya Protocol Compliance
Respective national authorities of all sampled countries that are party to the Nagoya Protocol were contacted. Where needed, advised measures were taken and resulted in sampling and export permits: KC3M-160/11. 04. 2018 (Bulgaria), ABSCH-IRCC-FR-253846-1 (France) and ABSCH-IRCC-ES-259169-1 (Spain).
Plant phenotyping
Scores presented in Fig. 1 and Extended Data Fig. 1 are
Developmental state: vegetative (1), just bolting (2), flowering (3), mature (4) and drying (5)
Herbivory index: no (1), weak (2), strong (3) and very strong (4) herbivory
For rosette diameter, a 1 cm rosette diameter classification corresponds to any rosette diameter ≤1 cm.
DNA extraction
DNA was extracted from plant samples according to the protocol from ref. 21 . Soil DNA was extracted using Qiagen Mag Attract PowerSoil DNA EP Kit (384) (cat. 27100-4-EP). On dry ice, soil samples were transferred from tubes to PowerBead DNA plates using sterile individual funnels. Plates were stored up to 2 weeks at −80 °C until processing. The Qiagen protocol was adapted to a 96-well-pipette (Integra Viaflo96). PowerBead solution and SL Solution were pre-warmed at 55–60 °C to avoid precipitation. RNase A was added to the PowerBead solution just before use. From step 17 of the protocol, instead of starting epMotion protocol, the following steps were performed: to each well of the 2 ml deep-well plate containing maximum 850 µl of supernatant, 750 µl of Bead Solution was added and mixed with Eppendorf MixMate at 650 rpm for 10–20 min. Plates were placed on a magnet for 5 min, the supernatant solution discarded and the beads washed three times with 500 µl wash solution. Beads were eluted with 100 µl elution buffer. The eluate was transferred to PCR plates and stored at −20 °C until library preparation.
Drought treatment with infection
Plants of the A. thaliana Col-0 reference accession were grown for 35 days at 23 °C under short day conditions (8 h light:16 h dark) with normal watering (approximately 1 l water per tray once soil moisture dropped below a reading of 3; XLUX Soil Moisture Meter). At 35 days, plants were randomized into new trays and watering treatments started. Soil moisture was measured every day. Control plants were watered normally once the soil moisture readings were between 2 and 3. Drought-stressed trays were dried down to an average soil moisture reading of 1, kept ≤1 for a full day, then maintained between a reading of 1 and 2 with minimal watering. The plants were exposed to these contrasting water conditions for seven days before infection. On day 7, control trays were watered normally (until soil moisture averaged a reading of 5–6 per tray) and drought trays were watered at 0.4× normal water per tray (reaching an average soil moisture reading of 2–3). After having been watered, two leaves per plant were syringe-infiltrated with either MgSO 4 (control) or ATUE5 p25.c2 at an OD 600 of 0.0002. Each treatment had approximately 96 plants, divided over four trays. Plants were photographed every other day, starting at 35 days after planting. Plant growth and health were estimated by measuring green pixel area per plant using plantCV 46 (Supplementary Data Table 1 ). At 3 days after infection, hole punches were taken from two leaves per plant, ground and resuspended in dilutions 10 mM MgSO 4 . Colonies were counted after 2–3 days of growth on selective lysogeny broth agar plates with 100 µg ml −1 nitrofurantoin to select for Pseudomonas (Supplementary Data Table 2 ). No statistical methods were used to pre-determine sample sizes but sample sizes are similar to or greater than those reported in previous publications 47 .
Field experiment
A total of 110 A. thaliana accessions were planted in a common garden experiment with water manipulation in a common garden field site at the Carnegie Institution for Science (37.42857020996903° N, 122.17944689424299° W) in Stanford in the spring of 2023 (Extended Data Table 5 ). We selected two groups of accessions based on their predicted contrasts in ability to survive drought in two consecutive field experiments at two locations. Based on survival data under low watering in Spain 29 , polygenic scores were trained on 515 accessions following state-of-the-art methods 48 using PLINK v2.00a2.3 49 . Conducting polygenic scores with different sets of SNPs (varying P value of their association with survival from 10 −3 to 10 −9 ), we verified a broad overlap of accessions in the top 30 and bottom 30 of the rank distribution. We utilized a threshold of 0.001 to select such 30 top and 30 bottom accessions. In a second round of experiments in California, a pilot study for the current work, polygenic scores were trained on total fitness (survival and fruit production) under drought conditions in 245 accessions. Polygenic score analyses used the software GEMMA and the Bayesian Sparse Linear Mixed Model 50 . This approach utilized genome-wide SNP information and their estimated parameters (probability of causal effect and the effect size) to make polygenic score predictions. We again selected 30 accessions with the highest and lowest polygenic scores. Finally, from the two polygenic score prediction rounds we identified 57 accessions with a high score in drought survival and 59 with a low score to conduct field experiments and microbiome analyses (3 and 1 accessions, respectively, did not have enough seeds for our experiment size). As there was some overlap in selected accessions from the first to the second year, only a total of 110 unique accessions were sown.
We planted seeds from selected accessions in 464 individual, randomized pots on 16 November 2022 in a common garden field site at the Carnegie Institution for Science. Five to ten seeds were planted in each pot within a 60-pot tray with Nutrient Ag Solutions PROMIX PGX Biofungicide Plug & Germination mix. The trays were gently watered for 2 weeks until germinants were established. We thinned each pot to have a single plant, before imposing a high and low precipitation treatment. For the well-watered treatment, the plants received an additional 144 min of rainfall every 2 days from December 2022 to May 2023 (about 600 additional mm for the entire growing season) on top of the natural rainfall at this location. The drought treatment consisted of only natural rainfall, which in California typically leads to water stress and visible mortality of A. thaliana plants.
Microbiome study
On 5 April 2023, we collected two true leaves from every plant that had not begun to senesce or decay (386 plants in total). All tools were sterilized between plant sampling. Tubes with tissue were immediately submerged in liquid nitrogen and transferred to a −80 °C freezer.
16S rDNA ASV identification
Oligonucleotide primers targeting the consensus V3–V4 ribosomal DNA (rDNA) region from 341 bp (5′-CCTACGGGAGGCAGCAG-3′) to 806 bp (5′-GGACTACNVGGGTWTCTAAT-3′) were used to amplify 16S rDNA sequences with the protocol described in ref. 21 . Briefly, amplification was achieved with a two-step PCR protocol in which 100 µM peptide nucleic acid was used in the initial PCR to block amplification of chloroplasts. Amplicons were sequenced on the MiSeq (Illumina) platform using the MiSeq Reagent Kit v3 (600 cycle). Samples with lower coverage were preferentially sequenced to greater depth in subsequent runs in a total of four runs of the Miseq. Output from all runs was pooled for downstream analysis. Primer sequences were removed before analysis with a combination of usearch (version 11, ref. 51 ) and custom bash scripting. The 16S rDNA sequences were quality trimmed using DADA2 13 (version 1.10.1). The forward read was truncated at position 260 and the reverse read at position 210 due to decreased quality of the second read. Reads were truncated when the quality score dropped to less than or equal to 2 (trunQ=2). Chimeras were removed with the removeBimeraDenovo function (method=‘consensus’) and ASVs called de novo using DADA2. The resulting reads were then aligned using AlignSeqs from the DECIPHER package 52 (version 2.8.1). A phylogenetic tree of the de novo called ASVs was constructed using fasttreeMP 53 (version 2.1.11). Taxonomic assignment of reads was performed with comparisons of 16S rDNA sequences to the Silva database 54 (nr v132 training set).
Only samples with at least 1,000 reads after filtering for mitochondria and chloroplast reads were included. We began with 939 samples (including soil samples and neighbouring non- A. thaliana plants), in which we found 195,545 ASVs. A total of 918 samples had a sufficient number of reads (>1,000 reads) and after removing ASVs that were not found in any single sample with more than 50 reads, we were left with 10,566 ASVs. We identified a core set of 575 ASVs by filtering for those ASVs that were present in at least 5% of A. thaliana samples. The ASVs classified as belonging to the taxonomic class Cyanobacteria were removed from the dataset to eliminate possible misassignment of plant chloroplast DNA that can vary between plant genotypes and skew subsequent analyses.
For the Californian field experiment, we sequenced the 16S rDNA amplicons as above and processed ASVs with the same pipeline used for the European wild samples. In the Californian ASV table, we identified ASVs present in 10% or more of the samples, and merged these ASV identifiers with those of the European collections to call the intersection of observed ASVs.
Climate variables
The majority of climate variables were obtained from Terraclimate 12 using the data for 2018 ( http://www.climatologylab.org/terraclimate.html ), a dataset with approximately 4 km spatial resolution. For random forest modelling and climate associations, we calculated the average value of each climate metric over the 6 months preceding the date of collection. The following variables were included in the random forest modelling from the Terraclimate dataset: tmax, maximum temperature; tmin, minimum temperature; vp, vapour pressure: ppt, precipitation accumulation; srad, downward surface shortwave radiation; ws, wind speed; pet, reference evapotranspiration (ASCE Penman–Montieth); q , runoff; aet, actual evapotranspiration; def, climate water deficit soil and soil moisture; swe, snow water equivalent; PDSI; and vpd, vapour pressure deficit.
We further analysed associations with Koeppen–Geiger climatic zones 55 , 56 , which were inferred in R using the package kgc and the regional classifications from ref. 57 . Initial assessments of the density of microbes throughout Europe were calculated via ordinary Kriging using the R package automap 58 (version 1.0-14). Four models were tested during variogram fitting, namely ‘Sph’, ‘Exp’, ‘Gau’ and ‘Ste’. Interpolation was performed either on the abundance data untransformed or on log 10 -transformed values with 0. 0001 added to allow for zero counts to be included. Global information on the major vegetation types was obtained using the Globcover 2009 map (released December 2010) from the European Space Agency ( http://due.esrin.esa.int/page_globcover.php ). Measures of soil properties were obtained using the International Soil Reference and Information Centre (ISRIC, global gridded soil information) Soil Grids ( https://soilgrids.org/#!/?layer=geonode:taxnwrb_250m ).
At the time of collection we took several measurements of the soil and air temperature and humidity (Soil temp, Air temp, Soil hum and Air hum), the surrounding plant community and the location type: distance between the focal and the closest neighbouring A. thaliana plant (Ath.Ath), distance between the focal and the closest other plant (Ath.other), immediate plant density (Ground cover), visible H. arabidopsidis infection on focal plant (Hpa plant) or at site (Hpa site), visible Albugo spp. infection on focal plant (Albugo tour), fraction of herbal plants in the surrounding (Strata herbs), and estimated sun exposure (Sun), slope (Slope) and ground humidity (Humidity ground). Measurements are listed and detailed in Extended Data Table 1 .
Feature selection and random forest modelling
Features of interest were first identified by feature selection in the R package caret 59 (version 6.0-86) using repeated cross-validation (three repeats). Prediction variables were preprocessed by centring, scaling and nearest-neighbour imputation for samples that lacked data for a variable. A training set was generated with 75% of the data. Random forest regression was performed to minimize the root mean squared error with repeated cross-validation. Variable importance was assessed via generalized cross-validation in the package caret 59 .
ASV differential abundance analysis
Differential abundance of ASVs in the soil versus A. thaliana , and A. thaliana versus other Brassicaceae was assessed using the edgeR 18 package in R (version 3.28.1). We estimated a common negative binomial dispersion parameter, and abundance-dispersion trends by Cox–Reid approximated profile likelihoods 60 . We then fit a quasi-likelihood negative binomial generalized log-linear model to the count data. We tested for differential abundance by a likelihood ratio test.

Phylotype classification and regression
Phylotypic clusters were identified by k -means clustering of Hellinger-transformed ASV count matrices. The optimal number of clusters was determined through both partitioning around medioids 61 using the pamk function in the R package fpc 62 (version 2.2.9) and through silhouette analysis 19 in the cluster (version 2.1.2) package in R 63 .
To determine the relative effect sizes of drought, latitude and plant identity on MDS loadings, phenotypes were modelled using restricted expectation maximum likelihood with the lmekin package in R with kinship as a random effect 64 . The kinship matrix was constructed using several methods including the R package gaston 64 as well as the centred kinship matrix in gemma (version 0.98.3) 65 . The different methods yielded unstable estimates of kinship, probably due to the low coverage of the plant genomes. To account for the low coverage, we employed a method designed for kinship estimation in low coverage data, SEEKIN 66 using the homogeneous parameter. Mixed-effects modelling with a kinship matrix was computed both with lmekin 67 and with GEMMA. The data distribution was assumed to be normal but this was not formally tested. The proportion of phenotypic variance explained by the environmental covariates was estimated with the function ‘r.squaredLR’ from the package MuMIn (version 1.43.1) and the pseudo-heritability was estimated using the kinship matrix and lmekin as well as in GEMMA (-gk = 1, maf = 0.1). In the paper we report the lower estimate for pseudo-heritability as estimated in GEMMA with the centred kinship matrix also estimated in GEMMA.
To test for the relative effects of genotype, latitude and PDSI in a single model, we estimated the first five principal components of the plant genotype relatedness matrix 68 and included the eigenvectors as covariates in our models for microbiome type and the loading on MDS1 and MDS2 (Fig. 2 ). The data distribution was assumed to be normal but this was not formally tested. Regressions used the lm and glm functions (logit link) in the R stats package. The relative importance of PDSI and Tour ID were tested with the models in glm glmer(cluster identity) ~ PDSI + 1|Tour_ID, family = ‘binomial’) or with lmer(MDS1 ~ PDSI + 1|Tour_ID).
Plant polymorphism calling and filtering
Raw reads were mapped to the TAIR10 reference genome of A. thaliana with bwa-mem (bwa 0. 7. 15) 69 . SNP calling was performed using GATK (version 3.5) HaplotypeCaller using recommended best practices 70 with some modifications. Filtering for individuals with greater than 25% missing data (across all the SNPs) and bi-allelic SNPs with greater than 25% missing data (across all the individuals) resulted in a final set of 527 individuals with 409,850 bi-allelic SNPs for further analysis.
Population structure analysis of A. thaliana
Wright’s fixation index ( F st ) was calculated using the method of Cockeram and Weir 71 . The 1001 Genomes 1 dataset (without individuals from North America) was merged with the dataset from this study to perform principal component analysis. Genotypes from this study were projected into the principal component space of the 1001 Genomes genotypes using the SmartPCA tool of EIGENSOFT (version 6) 72 .
Heritability comparisons
For comparison of ASV distributions and heritability estimates, we identified related OTUs from four microbiome common garden experiments in Sweden 8 . OTU sequences from ref. 8 were downloaded from https://forgemia.inra.fr/bbrachi/microbiota_paper , as were heritability estimates for the OTUs. Correspondence between Swedish OTUs (called from sequenced V5–V7 region of 16S rDNA) and the ASVs in our study (identified from sequenced V3–V4 regions of the 16S rDNA locus) was established using the Qiime2 73 fragment insertion method using sepp-refs-gg-13-8 as the reference database. Correspondence between the OTUs and ASVs was established with divergence of less than 1% on the Green Genes tree.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The V3–V4 16S rDNA sequence data and metagenomic sequencing data of plants were deposited in the European Nucleotide Archive (ENA) under the Primary Accession ENA: PRJEB44379 . Metadata and processed read data sets including phyloseq objects are available at Zenodo via https://doi.org/10.5281/zenodo.11187761 (ref. 74 ).
Code availability
Scripts for data processing, analyses and figure generation can be accessed at GitHub via https://github.com/tkarasov/pathodopsis .
1001 Genomes Consortium. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana . Cell 166 , 481–491 (2016).
Article Google Scholar
Hancock, A. M. et al. Adaptation to climate across the Arabidopsis thaliana genome. Science 334 , 83–86 (2011).
Article CAS PubMed Google Scholar
Bartoli, C. et al. In situ relationships between microbiota and potential pathobiota in Arabidopsis thaliana . ISME J. 12 , 2024–2038 (2018).
Article PubMed PubMed Central Google Scholar
Thiergart, T. et al. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 4 , 122–131 (2020).
Article PubMed Google Scholar
Wagner, M. R. et al. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 7 , 12151 (2016).
Article CAS PubMed PubMed Central Google Scholar
Bodenhausen, N., Horton, M. W. & Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana . PLoS One 8 , e56329 (2013).
Mittelstrass, J., Sperone, F. G. & Horton, M. W. Using transects to disentangle the environmental drivers of plant-microbiome assembly. Plant Cell Environ. 44 , 3515–3525 (2021).
Brachi, B. et al. Plant genetic effects on microbial hubs impact host fitness in repeated field trials. Proc. Natl Acad. Sci. USA 119 , e2201285119 (2022).
Delgado-Baquerizo, M. et al. A global atlas of the dominant bacteria found in soil. Science 359 , 320–325 (2018).
Roux, F., Frachon, L. & Bartoli, C. The genetic architecture of adaptation to leaf and root bacterial microbiota in Arabidopsis thaliana . Mol. Biol. Evol. 40 , msad093 (2023).
Wagner, M. R. et al. Natural soil microbes alter flowering phenology and the intensity of selection on flowering time in a wild Arabidopsis relative. Ecol. Lett. 17 , 717–726 (2014).
Abatzoglou, J. T., Dobrowski, S. Z., Parks, S. A. & Hegewisch, K. C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 5 , 170191 (2018).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13 , 581–583 (2016).
Karasov, T. L., Neumann, M. & Duque-Jaramillo, A. The relationship between microbial biomass and disease in the Arabidopsis thaliana phyllosphere. Preprint at bioRxiv https://doi.org/10.1101/828814 (2019).
Lundberg, D. S. et al. Defining the core Arabidopsis thaliana root microbiome. Nature 488 , 86–90 (2012).
Bonito, G. et al. Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol. Ecol. 23 , 3356–3370 (2014).
Horton, M. W. et al. Genome-wide association study of Arabidopsis thaliana leaf microbial community. Nat. Commun. 5 , 5320 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 , 139–140 (2010).
Rousseeuw, P. J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20 , 53–65 (1987).
Beilsmith, K., Perisin, M. & Bergelson, J. Natural bacterial assemblages in Arabidopsis thaliana tissues become more distinguishable and diverse during host development. mBio 12 , e02723–20 (2021).
Karasov, T. L. et al. Arabidopsis thaliana and pseudomonas pathogens exhibit stable associations over evolutionary timescales. Cell Host Microbe 24 , 168–179.e4 (2018).
Regalado, J. et al. Combining whole-genome shotgun sequencing and rRNA gene amplicon analyses to improve detection of microbe–microbe interaction networks in plant leaves. ISME J. 14 , 2116–2130 (2020).
Lundberg, D. S. et al. Contrasting patterns of microbial dominance in the Arabidopsis thaliana phyllosphere. Proc. Natl Acad. Sci. USA 119 , e2211881119 (2021).
Innerebner, G., Knief, C. & Vorholt, J. A. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 77 , 3202–3210 (2011).
Shalev, O. et al. Commensal Pseudomonas strains facilitate protective response against pathogens in the host plant. Nat. Ecol. Evol. 6 , 383–396 (2022).
McMullan, M. et al. Evidence for suppression of immunity as a driver for genomic introgressions and host range expansion in races of Albugo candida , a generalist parasite. eLife 4 , e04550 (2015).
Palmer, W. C. Meteorological Drought (US Department of Commerce Weather Bureau, 1965).
Platt, A. et al. The scale of population structure in Arabidopsis thaliana . PLoS Genet. 6 , e1000843 (2010).
Exposito-Alonso, M. et al. Natural selection on the Arabidopsis thaliana genome in present and future climates. Nature 573 , 126–129 (2019).
Züst, T. et al. Natural enemies drive geographic variation in plant defenses. Science 338 , 116–119 (2012).
Bergelson, J., Mittelstrass, J. & Horton, M. W. Characterizing both bacteria and fungi improves understanding of the Arabidopsis root microbiome. Sci. Rep. 9 , 24 (2019).
Teixeira, P. J. P., Colaianni, N. R., Fitzpatrick, C. R. & Dangl, J. L. Beyond pathogens: microbiota interactions with the plant immune system. Curr. Opin. Microbiol. 49 , 7–17 (2019).
Ma, K.-W. et al. Coordination of microbe–host homeostasis by crosstalk with plant innate immunity. Nat. Plants 7 , 814–825 (2021).
Glander, S. et al. Assortment of flowering time and immunity alleles in natural Arabidopsis thaliana populations suggests immunity and vegetative lifespan strategies coevolve. Genome Biol. Evol. 10 , 2278–2291 (2018).
Todesco, M. et al. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana . Nature 465 , 632–636 (2010).
Bakker, E. G., Toomajian, C., Kreitman, M. & Bergelson, J. A genome-wide survey of R gene polymorphisms in Arabidopsis . Plant Cell 18 , 1803–1818 (2006).
Karasov, T. L. et al. The long-term maintenance of a resistance polymorphism through diffuse interactions. Nature 512 , 436–440 (2014).
Aung, K., Jiang, Y. & He, S. Y. The role of water in plant–microbe interactions. Plant J. 93 , 771–780 (2018).
Duque-Jaramillo, A. et al. The genetic and physiological basis of Arabidopsis thaliana tolerance to Pseudomonas viridiflava . New Phytol. 240 , 1961–1975 (2023).
González, R. et al. Plant virus evolution under strong drought conditions results in a transition from parasitism to mutualism. Proc. Natl Acad. Sci. USA 118 , e2020990118 (2021).
Ma, Y., Dias, M. C. & Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11 , 591911 (2020).
Shaffique, S. et al. Research progress in the field of microbial mitigation of drought stress in plants. Front. Plant Sci. 13 , 870626 (2022).
Hiruma, K. et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165 , 464–474 (2016).
Okuma, E., Nozawa, R., Murata, Y. & Miura, K. Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis . Plant Signal. Behav. 9 , e28085 (2014).
Colaianni, N. R. et al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe 29 , 635–649.e9 (2021).
Berry, J. C., Fahlgren, N., Pokorny, A. A., Bart, R. S. & Veley, K. M. An automated, high-throughput method for standardizing image color profiles to improve image-based plant phenotyping. PeerJ 6 , e5727 (2018).
Goel, A. K. et al. The Pseudomonas syringae type III effector HopAM1 enhances virulence on water-stressed plants. Mol. Plant. Microbe Interact. 21 , 361–370 (2008).
Choi, S. W., Mak, T. S.-H. & O’Reilly, P. F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 15 , 2759–2772 (2020).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4 , 7 (2015).
Zhou, X., Carbonetto, P. & Stephens, M. Polygenic modeling with bayesian sparse linear mixed models. PLoS Genet. 9 , e1003264 (2013).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 , 2460–2461 (2010).
Wright, E. S. Using DECIPHER v2. 0 to analyze big biological sequence data in R. R J . 8 , 352–359 (2016).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 5 , e9490 (2010).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 , D590–D596 (2013).
Koppen, W. Das geographische System der Klimate. Handbuch der Klimatologie I , 1–44 (1936).
Köppen, W. Versuch einer Klassifikation der Klimate, vorzugsweise nach ihren Beziehungen zur Pflanzenwelt. Geogr. Z. 6 , 593–611 (1900).
Google Scholar
Rubel, F. & Kottek, M. Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol. Z. 19 , 135–141 (2010).
Hiemstra, P. automap: automatic interpolation package. R package version 1.0-14 . https://cran.r-project.org/web/packages/automap/automap.pdf (2013).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28 , 1–26 (2008).
Cox, D. R. & Reid, N. Parameter orthogonality and approximate conditional inference. J. R. Stat. Soc. Ser. B 49 , 1–39 (1987).
Kaufman, L. & Rousseeuw, P. J. in Finding Groups in Data: An Introduction to Cluster Analysis 344 , 68–125 (Wiley, 1990).
Hennig, C. fpc: flexible procedures for clustering. R package version 2.2-12. CRAN https://CRAN.R-project.org/package=fpc (2024).
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M. & Hornik, K. cluster: cluster analysis basics and extensions. R package version 2.1.5. (CRAN, 2023).
Perdry, H. & Dandine-Roulland, C. gaston: genetic data handling (QC, GRM, LD, PCA) and linear mixed models version 1. CRAN https://cran.r-project.org/web/packages/gaston/gaston.pdf (2023).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44 , 821–824 (2012).
Dou, J. et al. Estimation of kinship coefficient in structured and admixed populations using sparse sequencing data. PLoS Genet. 13 , e1007021 (2017).
Therneau, T. M. & Therneau, M. T. M. coxme: mixed effects cox models. CRAN https://cran.r-project.org/web/packages/coxme/index.html (2015).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88 , 76–82 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25 , 1754–1760 (2009).
Van der Auwera, G. A. et al. From FastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43 , 11.10.1–11.10.33 (2013).
PubMed Google Scholar
Weir, B. S. & Cockerham, C. C. Estimating F -statistics for the analysis of population structure. Evolution 38 , 1358–1370 (1984).
CAS PubMed Google Scholar
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2 , e190 (2006).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37 , 852–857 (2019).
Continental-scale associations of Arabidopsis thaliana phyllosphere members with host genotype and drought. Zenodo https://doi.org/10.5281/zenodo.11187761 (2024).
Schliep, K. P. phangorn: phylogenetic analysis in R. Bioinformatics 27 , 592–593 (2011).
Download references
Acknowledgements
We thank J. Keck, T. Hagmaier, A. Rütten, T. Vaupel, K. Poersch, N. Vasilenko, H. Vo-Gia, J. Elis, C. Tahtsidou, T. Schlegel and F. Vogt for aliquoting soil. We thank F. Roux, H. Burbano, A. Duque and M. Collenberg for comments on the paper. We thank M. Horton for providing global SNP F st values. We thank H. Burbano, M. Horton and B. Brachi for discussion. This work was funded by an HFSPO Long-term Fellowship LT000348/2016-L EMBO LRTF 1483-2015 and NIH grant R35 GM150722-01 (T.L.K.), ERC-SyG PATHOCOM (J.B. and D.W.) and the Max Planck Society (D.W.).
Open access funding provided by Max Planck Society.
Author information
Manuela Neumann
Present address: Robert Bosch GmbH, Renningen, Germany
Gautam Shirsekar
Present address: Department of Entomology and Plant Pathology, Institute of Agriculture, University of Tennessee, Knoxville, TN, USA
Grey Monroe
Present address: Department of Plant Sciences, University of California Davis, Davis, CA, USA
Moisés Exposito-Alonso
Present address: Department of Integrative Biology, University of California Berkeley, Berkeley, CA, USA
Present address: Howard Hughes Medical Institute, University of California Berkeley, Berkeley, CA, USA
Authors and Affiliations
School of Biological Sciences, University of Utah, Salt Lake City, UT, USA
Talia L. Karasov, Efthymia Symeonidi & Aubrey Hawks
Department of Molecular Biology, Max Planck Institute for Biology Tübingen, Tübingen, Germany
Talia L. Karasov, Manuela Neumann, Gautam Shirsekar, Grey Monroe, A. Cristina Barragán, Ilja Bezrukov, Claudia Friedemann, Alba González Hernando, Anette Habring, Julia Hildebrandt, Sonja Kersten, Patricia Lang, Sergio M. Latorre, Miriam Lucke, Derek S. Lundberg, Ulrich Lutz, Fiona Paul, Fernando A. Rabanal, Julian Regalado, Thanvi Srikant, Bridgit Waithaka, Anjar T. Wibowo, Wei Yuan, Detlef Weigel & Rebecca Schwab
Department of Biology, Stanford University, Stanford, CA, USA
Laura Leventhal & Moisés Exposito-Alonso
Department of Plant Biology, Carnegie Institution for Plant Science, Stanford, CA, USA
Center for Genomics and Systems Biology, Department of Biology, New York University, New York, NY, USA
Joy Bergelson
Institute for Bioinformatics and Medical Informatics, University of Tübingen, Tübingen, Germany
Detlef Weigel
You can also search for this author in PubMed Google Scholar
Pathodopsis Team
- A. Cristina Barragán
- , Ilja Bezrukov
- , Claudia Friedemann
- , Alba González Hernando
- , Anette Habring
- , Julia Hildebrandt
- , Sonja Kersten
- , Patricia Lang
- , Sergio M. Latorre
- , Miriam Lucke
- , Derek S. Lundberg
- , Ulrich Lutz
- , Fiona Paul
- , Fernando A. Rabanal
- , Julian Regalado
- , Thanvi Srikant
- , Bridgit Waithaka
- , Anjar T. Wibowo
- & Wei Yuan
Contributions
T.L.K., R.S., G.S., J.B., M.E.-A. and D.W. devised the study. T.L.K., R.S., G.S., M.N., L.L., E.S. and the PATHODOPSIS collection team collected and prepared the samples. T.L.K., G.S. and M.N. processed the samples. T.L.K., R.S. and G.S. analysed the data. G.M. provided climate data. A.H. performed infection experiments. T.L.K., D.W. and R.S. wrote the paper.
Corresponding authors
Correspondence to Talia L. Karasov or Detlef Weigel .
Ethics declarations
Competing interests.
D.W. holds equity in Computomics, which advises plant breeders. D.W. consults for KWS SE, a plant breeder and seed producer. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Microbiology thanks Maggie Wagner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 distribution of sampled a. thaliana plants with various developmental and health states..
Arbitrary scales (see Methods) except for rosette size (cm).
Extended Data Fig. 2 Differential abundance of phylotypes in soil, A. thaliana phyllospheres, and phyllospheres of other Brassicaceae.
a , 91% of phylotypes were differentially abundant between A. thaliana and soil. b , 36% of phylotypes were differentially abundant between A. thaliana and other Brassicaceae. Bold points indicate significance with an FDR ≤ 0.01. c , Within-site correlation of phylotype abundance. Correlation coefficients (scale on top left) were calculated for the co-occurrence of a phylotype within a site between the two A. thaliana plants collected at the site, A. thaliana x A. thaliana (third ring from the outside), other Brassicaceae x A. thaliana (second ring from the outside), and soil x A. thaliana (outermost ring). The central tree in the Circos plot represents the maximum likelihood tree of phylotypes, plotted without inferred branch lengths 75 .
Extended Data Fig. 3 Contrasts in phylotype abundances between Southern and Northern microbiome clusters.
a , Silhouette scores for membership assignment to either of the two main microbiome types, cluster 1 (Southern) and cluster 2 (Northern). For each cluster, number of individuals and average distance between a sample and members of the other cluster is indicated. b , Differential abundance of phylotypes between Southern and Northern microbiome clusters. y-axis shows the log 2 (Fold Change) for the relative abundance difference of a phylotype between clusters. Bold points indicate significance with an FDR ≤ 0.01.
Extended Data Fig. 4 Projection of A. thaliana genotypes from this study into genotypic PC space from the 1001 Genomes Project.
Individuals from this study (‘Pathodopsis’) align well with the broader 1001 Genomes ( https://1001genomes.org ) collection of primarily Eurasian accessions.
Extended Data Fig. 5 F st around ACD6 and globally.
a , Cockerham and Weir’s fixation index F st was estimated for SNPs in a list of known immune-associated genes. The genome-wide most extreme F st values are concentrated in a region on chromosome 4 that includes the immune regulator ACD6 (At4g14400). Reference genome positions (in nt) on chromosome 4 given at the bottom. b , Bergelson and colleagues 32 identified A. thaliana SNPs associated with (and likely to influence) microbiome composition. We compared the geographic differentiation of these SNPs (F st ) to the genome-wide distribution. Microbiome-associated SNPs exhibit significantly higher F st values than the remainder of the genomic SNPs (Wilcoxon rank-sum test p < 2.2x10 −16 ). The central horizontal line in each box indicates the median, the bounds indicate the upper and lower quartiles and the whiskers indicate 1.5*inner quartile range.
Extended Data Fig. 6 Distance-Semivariance plot for ATUE5.
Relationship between the geographic distance between two sampled A. thaliana plants, and the correlation of the abundance of ATUE5 between these two plants.
Extended Data Fig. 7 Correlogram of relationship between environmental and developmental covariates used in random forest modeling.
Covariates are detailed in Methods.
Extended Data Fig. 8 Biplots of the correlation of environmental and physiological variables on the MDS axes in Fig. 2 .
a , Environmental variables derived from Terraclimate . b , Environmental variables measured at time of collection. Correlations were assessed with the envfit() function in vegan, and vector length corresponds to strength of correlation. Long-term climate variables ( a ) are better predictors of microbiome composition than are more temporary weather and physiological variables measured at the time of collection ( b ).
Extended Data Fig. 9 Relative abundance of phylotypes is significantly associated with plant genotype classification.
Four out of 20 phylotypes that were shared between the Eurasian collections and California field experiment were significantly associated with plant genotype. a , Histograms for the relative abundance of each of the four significant phylotypes across all plants collected in Eurasia. b , Histogram of the total relative abundance per plant of the sum of all four phylotypes (mean = 13.2% RA).
Extended Data Fig. 10 Impact of a common phylotype on plant growth as a function of drought status.
Arabidopsis thaliana plants were exposed to combinations of drought and infection with ATUE5 strain p25.c2. a , The change in plant leaf area from day 0 to day 10 as calculated based on daily images and extracting green pixels from images. Infection with ATUE5 reduced the negative effect of drought on plant growth (ANOVA, p = 0.0063 in ANOVA). b , measured colony forming units (cfu) on day 3 post infection. 5/41 (12%) drought-treated plants had established infection on day 3, whereas 17/23 (42%) of control plants had established infection at this same timepoint (Wilcoxon rank-sum p = 0.0027).
Supplementary information
Reporting summary, peer review file, supplementary data.
Supplementary Data Tables 1 and 2 in .xlsx format with tabs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Karasov, T.L., Neumann, M., Leventhal, L. et al. Continental-scale associations of Arabidopsis thaliana phyllosphere members with host genotype and drought. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01773-z
Download citation
Received : 27 April 2022
Accepted : 02 July 2024
Published : 06 September 2024
DOI : https://doi.org/10.1038/s41564-024-01773-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.

IMAGES
COMMENTS
California relies on Sierra Nevada precipitation for much of its water sup- ply. In the mountains, water year 2014 (fall of 2013 through summer of 2014) was almost as dry as the record-setting year 1977. Moreover, the state has had below-average precipitation every year since 2007, except for 2011. Having the record dry year of 2014 a$ er years ...
This report was prepared in response to the dry conditions of 2012-14 (Figure 1.1) and particularly in response to the very dry hydrology of water year 2014. Water year 2014 ranked as the third driest on record in terms of statewide precipitation, with the three-year period of water years 2012-14 ranking as the driest consecutive three-year ...
The study finds that the 2014 drought will result in a 6.6 million acre-foot reduction in surface water available to agriculture. This loss of surface water will be partially replaced by increasing groundwater ... Table ES-1. 2014 Drought and California Agriculture Summary . Drought impact Loss quantity . Water supply . Surface water reduction ...
Or, sign up to receive drought alerts when the U.S. Drought Monitor or U.S. Drought Outlook updates for your city/zip code. A multi-agency partnership that coordinates drought monitoring, forecasting, planning, and information at national, state, and local scales. CNAP newsletter summarizes 2014 drought in California.
California experienced the most severe drought in its recorded history during the years 2012-2016, characterized by record high temperatures and precipitation deficits (Swain et al., 2014; Ullrich ...
Despite local problems, the effect of major drought on California's statewide economy was quite small (Howitt et al. 2014, 2015a, b). Urban areas, supporting most of the people and economic activity, have developed diversified portfolios of water supply and conservation activities that were quite successful during the California drought;
For the past three years (2012-2014), California has experienced the most severe drought conditions in its last century. But how unusual is this event? Here we use two paleoclimate reconstructions of drought and precipitation for Central and Southern California to place this current event in the context of the last millennium.
U.S. Drought Monitor California December 2, 2014 (Released Thursday, Dec. 4, 2014) Valid 7 a.m. EST Drought Conditions (Percent Area) 2011 2014 ASSESSMENT REPORT Causes and Predictability of the 2011 to 2014 California Drought Before and after photo of Lake Folsom comparing 2011 to 2014. (Photo credit: California Department of Water Resources ...
The 1 year 2014 California drought was a multicentury-scale event; The cumulative 2012-2014 California drought was a multimillennial-scale event; ... In this case, the calibration period is the complete period of overlap between the two data sets: 1895 to 2006. Here two procedures are compared: (1) "mean/std adjustment" of the NADA tree ...
Climate change and California drought in the 21st century. ... J Christian-Smith, M Levy, PH Gleick, Maladaptation to drought: A case report from California. Sustain Sci 9, 1-11 (2014). ... D Griffin, KJ Anchukaitis, How unusual is the 2012-2014 California drought? Geophys Res Lett 41, 9017-9023 (2014). Crossref. Google Scholar. 10.
This case study demonstrates that California's current strategies for dealing with long or severe droughts are less successful than previ-ously thought when short- and long-term impacts are evalu-ated together. This finding is particularly relevant given. Handled by Soontak Lee, Yeungnam University, Korea.
In 2014, more than 80% of California was in the grip of an "extreme drought". Against this backdrop, Oroville's capacity fell to 30% - a historic low level. As the water level receded to hundreds ...
Abstract The state of California has experienced the worst drought in its historical record during 2012-2015. ... The ongoing situation in California holds the potential to become an important case study both for scientists interested in understanding the causes of underlying temperature and precipitation anomalies and also for decision ...
There is growing interest in understanding the effects of hydrometeorological variability, and especially drought, on the economic and environmental performance of bulk power systems and electricity markets (van Vliet et al 2012; van Vliet et al 2016b, Voisin et al 2016).In the United States (U.S.), California is particularly vulnerable to drought due to its reliance on in-state and imported ...
In 2016, California took the lead among U.S. states in addressing climate change by extending legislation to curb greenhouse gas emissions. Targeting both power plants and vehicles, the state committed to the goal of curbing carbon emissions to 40% below 1990 levels by 2030. Laws passed in 2006 had already set targets to reduce emissions to ...
Case Study Summary Sheet for the California Drought 2014 (MEDC) Where did it happen? California is a state located on the western coast of the USA. A severe drought in California has depleted snow packs, rivers, and lakes, and groundwater use has soared to make up the shortfall. ... $5 billion loses in 2014 alone due to losses incurred in the ...
Two-page summary of the Drought Task Force I Assessment Report: Causes and Predictability of the 2011 to 2014 California Drought. The report and summary were produced as part of the NOAA Drought Task Force I organized by the NOAA Modeling, Analysis, Predictions, and Projections Program (MAPP) in partnership with the National Integrated Drought Information System (NIDIS).
Now, California has launched a landmark effort to save its groundwater. In 2014, deep in drought, the state passed a law to protect its aquifers; since then, local water managers have developed sustainability plans for those deemed the most imperiled. ... California is a case study in the challenges of protecting those resources. Farm interests ...
Abstract California has experienced severe drought in 2012-2014 (which appears to be continuing into 2015), with especially low winter precipitation and mountain snowpack in winter 2013-2014. ... this is not the case over the entire record of 1920-2014, ... These results strongly suggest that while the warming climate might have slightly ...
These direct costs to agriculture total $1.5 billion. The total statewide economic cost of the 2014 drought is $2.2 billion, with a total loss of 17,100 seasonal and part-time jobs. Table ES-1 summarizes the key findings of the study. Get Document Email.
See how severe drought impacted people in California and learn how communities adapted to the water shortage. Use this resource to provide relevance and context for learning about drought and opportunities for students to evaluate adaptation strategies.
In August 2015 44% of rivers had what% of their normal water flow? 10%. Some short-term impacts. Shortage of fruit and veg meant that prices rose by 6%, 17,000 agriculture jobs were lost, most HEP dams stopped producing electricity and a 36% increase in wildfires which killed wildlife and damaged farming land.
In a second round of experiments in California, a pilot study for the current work, polygenic scores were trained on total fitness (survival and fruit production) under drought conditions in 245 ...
Using the 2021 Heat Dome as a case study, a post-event analysis using online news media articles (n = 2909) from 5 subscription news databases and a grey literature search was conducted to identify the socio-economic impacts of the extreme heat event in Canada. ... Yu Y. Compound heat wave, drought, and dust events in California. J Clim. 2022 ...