

Hydatidiform mole: Recognition and management
Molar pregnancies may be associated with serious morbidity so prompt diagnosis, appropriate management, and follow-up are essential.
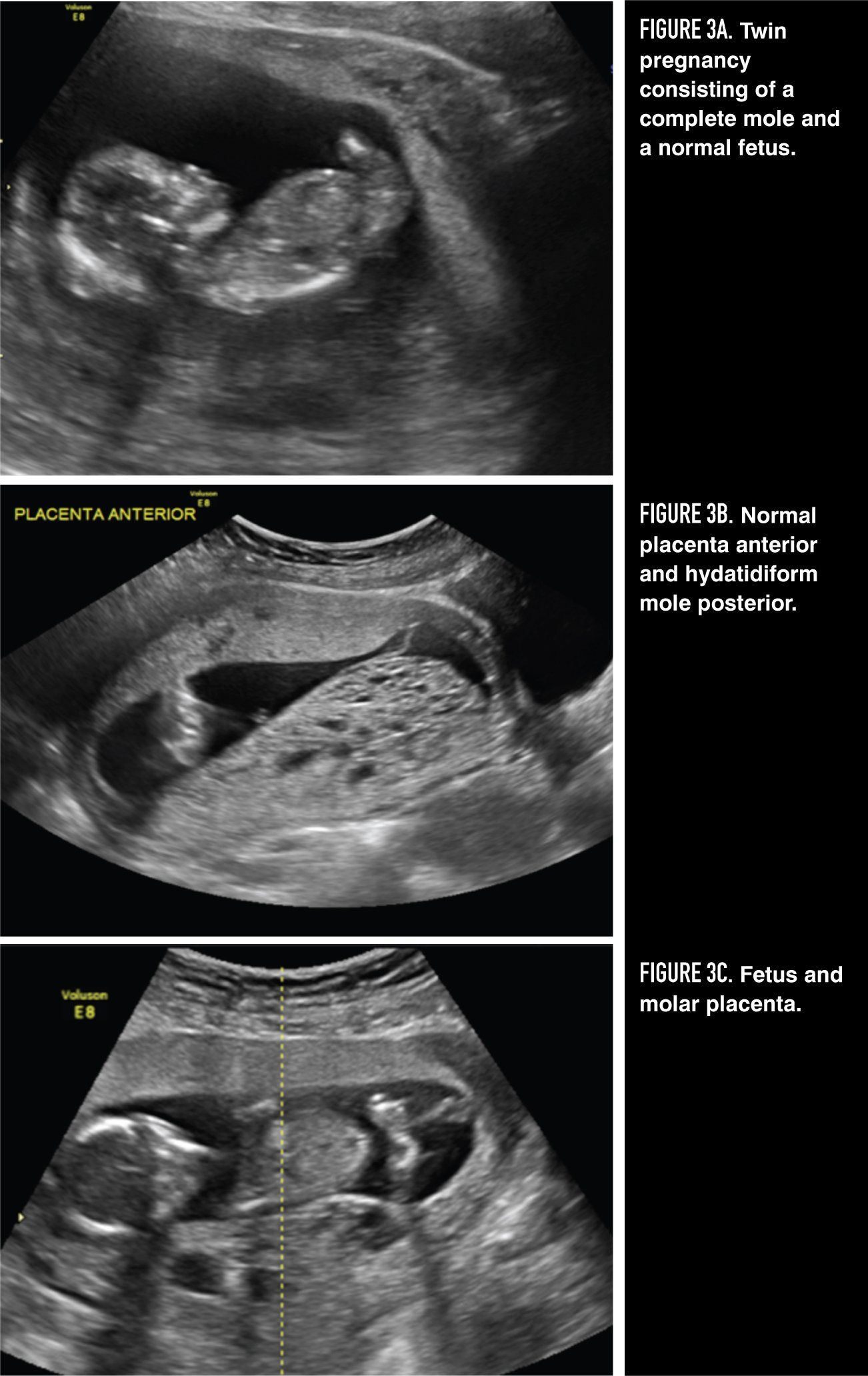
Figures 3A, 3B, 3C
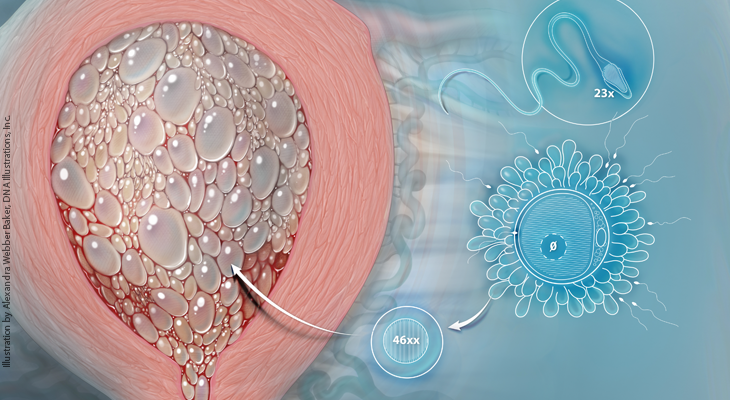
Hydatidiform mole is an abnormal pregnancy characterized by varying degrees of trophoblastic proliferation (both cytotrophoblast and syncytiotrophoblast) and vesicular swelling of placental villi associated with an absent or abnormal fetus/embryo. Two types of hydatidiform mole, complete and partial, have been described based on both morphologic and cytogenetic criteria (Table 1). 1,2
Epidemiology
Epidemiologic studies have reported wide regional variations in the incidence of molar pregnancies. Estimates from studies in North America, Europe, Australia and New Zealand have shown incidence rates ranging from 0.57-1.1 per 1000 pregnancies, whereas studies in Southeast Asia and Japan have suggested an incidence rate as high as 2.0 per 1000 pregnancies. These reported differences may be related to lack of standardization of data collection and reporting rather than true incidence differences. However, socioeconomic status and diet rather than genetic or cultural factors may also contribute to these reported differences in incidence rates. Declining incidence of molar pregnancies in Asia has been attributed to increasing western diet and improved standard of living. The overall incidence of molar pregnancies in the United States and Europe is about 1/1000 pregnancies for both complete and partial moles. 1,2
Several potential etiologic risk factors for development of molar pregnancy have been evaluated (Table 2). 3 For complete hydatidiform moles, two well-established risk factors have emerged: (1) extremes of maternal age; and (2) prior molar pregnancy. Both advanced and very young maternal age have consistently correlated with higher rates of complete mole. Compared to risk in women aged 21 to 35 years, risk of complete mole is 1.9 times higher for women both < 21 years and > 35 years and 7.5 times higher for women > 40 years, including 1 in 3 pregnancies for women > 50 years. These observations suggest that ova of very young or older women are predisposed to abnormal fertilization events that lead to complete hydatidiform moles. Prior complete molar pregnancy increases risk of developing a subsequent complete molar pregnancy.
Risk of a repeat molar pregnancy after one mole is approximately 1%, about 10 to 20 times the risk for the general population, while after two moles, the risk of a third mole is 15% to 20%. History of prior spontaneous abortion also appears to increase risk of a molar pregnancy (both complete and partial) 2- to 3-fold compared to women without a history of prior miscarriage. Dietary deficiency of β-carotene and animal fat has been linked to an increase in complete moles. There appears to be a possible increased risk of molar pregnancy (partial and complete) with a history of oral contraceptive use, while ovulation induction regimens may be associated with an increase in twin pregnancies consisting of a normal fetus(es) and a complete mole.
While several definite etiologic risk factors have been identified for complete moles, the epidemiologic characteristics of partial moles differ and are less well defined. Importantly, the association between maternal age and complete molar pregnancies is not seen in women with partial molar pregnancies. Furthermore, partial molar pregnancies are more common in women with a history of irregular menses, miscarriage, and oral contraceptive use for > 4 years, but are not associated with ethnicity, ovulation induction, or dietary factors.
Complete hydatidiform moles usually arise when an ovum without maternal chromosomes is fertilized by one sperm which then duplicates its DNA, resulting in a 46, XX androgenic karyotype in which all the chromosomes are paternally derived. About 10% of complete moles are 46, XY or 46, XX arising from fertilization of an “empty ovum” by two sperm. Bipaternal diploid complete moles are associated with a maternal autosomal-recessive missense gene mutation, most commonly NLRP7 on chromosome 19q, which results in repetitive molar pregnancies. Partial hydatidiform moles have a triploid karyotype, usually 69, XXY, resulting from dispermic fertilization of an apparently normal ovum (Figure 1). 2
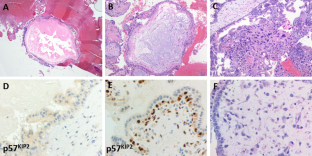
Complete hydatidiform moles undergo early and uniform hydatid enlargement of villi in the absence of an ascertainable fetus or embryo, the trophoblast is consistently hyperplastic with varying degrees of atypia, and villous capillaries are absent. Partial hydatidiform moles demonstrate identifiable fetal or embryonic tissue, chorionic villi of varying size and shape with focal edema, scalloping and prominent stromal inclusions, a functioning villous circulation, as well as focal trophoblastic hyperplasia with only mild atypia. Immunohistochemical staining for p57 (a parentally imprinted, maternally expressed gene) may be useful for differentiating a positive partial mole from a negative complete mole, but cannot be used to distinguish a partial mole from a nonmolar abortus both of which are positive. 4-6
Clinical presentation
Complete hydatidiform moles most commonly present with vaginal bleeding, usually occurring at 6 to 16 weeks of gestation in 90% of cases. The other classical clinical signs and symptoms, such as uterine enlargement greater than expected for gestational dates (28%), hyperemesis (8%), and toxemia, hyperthyroidism, and trophoblastic embolization (< 1%), occur less frequently in more recent years due to earlier diagnosis as a result of widespread use of ultrasonography and accurate tests for human chorionic gonadotrophin (hCG). Bilateral theca lutein cyst enlargement of the ovaries occurs in approximately 15% of cases, hCG levels are often > 100,000 mIU/mL, and fetal heart sounds are absent. 7,8
Partial hydatidiform moles do not have the same presenting features as complete moles. Although the main presenting symptom is also vaginal bleeding, which occurs in about 75% of patients, excessive uterine enlargement, hyperemesis, pregnancy-induced hypertension, hyperthyroidism, and theca lutein cysts develop infrequently. Fewer than 10% have hCG levels > 100,000 mIU/mL. More than 90% of patients with partial moles have symptoms and ultrasound findings consistent with an incomplete or missed abortion, and the diagnosis is usually made only after histologic examination of uterine curettage specimens. 9
Ultrasonography plays a critical role in the diagnosis of both complete and partial molar pregnancy, and it has virtually replaced all other means of preoperative diagnosis. Because the chorionic villi of complete moles exhibit diffuse hydropic swelling, a characteristic vesicular ultrasonographic pattern can be observed consisting of multiple echoes (holes) within the placental mass and usually no fetus (Figure 2). Ultrasonography may also facilitate early diagnosis of a partial mole by demonstrating focal cystic spaces within the placenta and an increase in the transverse diameter of the gestational sac. 12
hCG is a disease-specific tumor marker produced by the trophoblast of hydatidiform moles and gestational trophoblastic neoplasms as well as normal pregnancy. Hydatidiform moles are commonly associated with markedly elevated hCG levels above those of normal pregnancy. Approximately 50% of complete moles have pre-evacuation hCG levels > 100,000 mIU/mL. However, a single hCG level is seldom helpful in differentiating a compete mole from another type of pregnancy. Partial moles, on the other hand, are most often not associated with such elevated hCG levels, as noted previously. 13
Despite earlier diagnosis of complete moles resulting in fewer complications, there has not been a simultaneous reduction in incidence of postmolar gestational trophoblastic neoplasia (GTN).
Once the diagnosis of molar pregnancy is suspected based on history, physical examination, hCG level, and ultrasound findings, the patient should be evaluated for the presence of medical complications (anemia, preeclampsia, hyperthyroidism), which may need to be corrected. Basic laboratory tests should include complete blood count, comprehensive metabolic panel, thyroid function test, urinalysis, and chest x-ray, as well as blood type and screen with cross match if anemic or uterus ≥ 16-week gestational size. An electrocardiogram and coagulation profile may also be indicated. Once the patient is determined to be hemodynamically stable, the most appropriate method of molar evacuation should be decided upon. 1, 2,14
Suction evacuation and curettage is the preferred method of evacuation of a hydatidiform mole, independent of uterine size, for patients who wish to maintain their fertility. After anesthesia is achieved, the cervix is dilated to allow a 12- to 14-mm suction cannula to pass into the lower uterine segment and then rotated as the intrauterine contents are removed, preferably under ultrasound guidance. Suction evacuation should be followed by gentle sharp curettage. Uterotonic drugs should be started after initiation of evacuation of the uterus, although oxytocin receptors may be absent. Because risk of excessive bleeding increases with uterine size, 2 units of blood should be immediately available when the uterus is ≥ 16-week gestational size. Attention to blood and crystalloid replacement decreases pulmonary complications. It is clear that with judicious use of appropriate equipment, access to blood products, careful intraoperative monitoring, and early anticipation of complications, patient outcomes improve. Patients who are Rh-negative should receive Rho(D) immune globulin at the time of evacuation, as Rh D factor is expressed on trophoblastic cells.
Hysterectomy is an alternative to suction curettage in patients who do not wish to preserve fertility or are older and at increased risk for development of postmolar GTN. The adnexa may be left intact even in the presence of theca lutein cysts. In addition to evacuating the molar pregnancy, hysterectomy provides permanent sterilization and eliminates risk of local myometrial invasion as a cause of persistent disease. Because of the potential for metastatic disease even after hysterectomy, risk of postmolar GTN still remains at 3% to 5%, thereby requiring continued hCG follow up.
Medical induction of labor and hysterotomy are not recommended for molar evacuation. These methods increase maternal morbidity, such as blood loss, incomplete evacuation requiring curettage, and the requirement for cesarean delivery in subsequent pregnancies. They also increase trophoblastic dissemination and the development of postmolar GTN requiring chemotherapy.
Prophylactic chemotherapy at the time or immediately after evacuation of a molar pregnancy is associated with a reduction in incidence of postmolar GTN from approximately 15% to 20% down to 3% to 8%. 15 Use of prophylactic chemotherapy should be limited, however, to special situations in which risk of postmolar GTN is much greater than normal (age > 40 years, hCG > 100,000 mIU/mL, excessive uterine enlargement, theca lutein cysts > 6 cm, medical complications) and/or when adequate hCG follow-up is unavailable or unreliable. Essentially all patients who are followed with serial hCG testing after molar evacuation and are found to have persistent GTN can be cured with appropriate chemotherapy.
Twin pregnancy consisting of a complete mole and a coexisting normal fetus is estimated to occur once in every 22,000 to 100,000 pregnancies (Figure 3). It must be distinguished from a partial mole (triploid pregnancy with fetus). The diagnosis can usually be established by ultrasound, but cytogenetics may be used to differentiate between chromosomally normal, potentially viable fetuses and triploid nonviable fetuses. Patients with a normal fetus/complete mole twin pregnancy should be cautioned that they may be at increased risk for hemorrhage, medical complications, and development of persistent GTN. Suction evacuation and curettage in the operating room under ultrasound guidance is recommended for desired pregnancy termination, bleeding, or medical complications. However, up to 40% of these pregnancies will result in normal viable births if allowed to continue. 16,17
After evacuation of a hydatidiform mole, follow-up is essential to detect trophoblastic sequelae (invasive mole and choriocarcinoma), which develop in approximately 15% to 20% of patients with complete mole and 1% to 5% with partial mole. 1,2,14,18 Clinical findings of prompt uterine involution, ovarian cyst regression, and cessation of bleeding are all reassuring signs, however, definitive follow-up requires serial serum hCG measurements every 1 to 2 weeks until three consecutive tests show normal hCG levels, after which hCG levels should be determined at 3-month intervals for 6 months after the spontaneous return to normal. Contraception is recommended during the follow-up period for 6 months after the first normal hCG result. Oral contraceptives are preferred because they have the advantage of suppressing endogenous luteinizing hormone (LH), which may interfere with the measurement of hCG at low levels, and do not increase the risk of postmolar GTN. Indications for treatment of postmolar GTN are: plateauing hCG levels x4 values over 3 weeks, rising hCG levels ≥ 10% x three values over 2 weeks, persistently elevated hCG levels 6 months after evacuation, a histopathologic diagnosis of choriocarcinoma or intermediate trophoblastic tumor, or detection of metastases. 19-21 In all future pregnancies, pathologic examination of the placenta or other products of conception as well as determination of a 6-week postpartum hCG level are recommended.
Disclosures:
The author reports no potential conflicts of interest with regard to this artic
References:
- Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203:531-539.
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717-729
- Strohl AE, Lurain JR. Clinical epidemiology of gestational trophoblastic disease. Curr Obstet Gynecol. Rep 2014;3 40-43
- Szulman AE, Surti U. The syndromes of hydatidiform mole. I: cytogenetic and morphologic correlations. Am J Obstet Gynecol. 1978;131:665-671.
- Szulman AE, Surti U. The syndromes of hydatidiform mole. II: morphologic evolution of the complete and partial mole. Am J Obstet Gynecol. 1978;132:20-27.
- Castrillon DH, Sun D, Weremowicz S, et al. Discrimination of complete hydatidiform mole from its mimics by immunohistochemistry of paternally imprinted gene product p57K1P2. Am J Surg Pathol. 2001;25:1225-1230.
- Soto-Wright V, Bernstein MR, Goldstein DP, et al. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86:775-779.
- Hou JL, Wan XR, Xiang Y, et al. Changes in clinical features in hydatidiform mole: analysis of 113 cases. J Reprod Med. 2008:53:629-633.
- Berkowitz RS, Goldstein DP, Bernstein MR. Natural history of partial molar pregnancy. Obstet Gynecol. 1985;66:677-681.
- Santos-Ramos R, Forney JP, Schwarz BE. Sonographic findings and clinical correlations in molar pregnancy. Obstet Gynecol. 1980;56:186-192.
- Benson CB, Genset DR, Bernstein MR, et al. Sonographic appearance of first trimester complete hydatidiform mole. Obstet Gynecol. 1989;73:414-418.
- Fine C, Bundy AL, Berkowitz RS, et al. Sonographic diagnosis of partial hydatidiform mole. Obstet Gynecol. 1989;73:414-418.
- Berkowitz RS, Ozturk M, Goldstein DP, et al. Human chorionic gonadotropin and free subunits’ serum levels in patients with partial and complete hydatidiform moles. Obstet Gynecol. 1989;74:212-216.
- Berkowitz RS, Goldstein DP. Clinical practice. Molar pregnancy. N Engl J Med. 2009;360:1639-1645.
- Wang Q, Fu J, Hu L, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2017;9:CD 007289.
- Sebire NJ, Foskett M, Paradinas FJ, et al. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359:2165-2166.
- Lin LH, Maesta I, Braga A, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: a retrospective multicenter cohort and literature review. Gynecol Oncol. 2017;145:88-95.
- Lurain JR, Brewer JI, Torok E, Halpern B. Natural history of hydatidiform mole after primary evacuation. Am J Obstet Gynecol. 1983;145:591-595.
- Ngan HYS, Bender H, Benedet JL, et al. Gestational trophoblastic neoplasia, FIGO staging and classification. Int J Gynecol Obstet. 2003;83:175-177.
- Lurain JR. Gestational trophoblastic disease. II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11-18.
- NCCN Guidelines: gestational trophoblastic neoplasia, version 1. 2019, 2018.

Metformin during pregnancy not linked to adverse birth outcomes
An analysis from Harvard shows there is no significantly increased newborn risk when continuing metformin to treat type 2 diabetes in pregnant women.

S1E4: Dr. Kristina Adams-Waldorf: Pandemics, pathogens and perseverance
This episode of Pap Talk by Contemporary OB/GYN features an interview with Dr. Kristina Adams-Waldorf, Professor in the Department of Obstetrics and Gynecology and Adjunct Professor in Global Health at the University of Washington (UW) School of Medicine in Seattle.

Survey: State abortion access impacted OB/GYN residency applications
National survey data presented at ACOG 2024 shows many medical students applying for OB/GYN residencies prioritized states with abortion access.

S1E3: Dr. Emily S. Miller: Placental pathology and MVM evidence
This episode of Pap Talk by Contemporary OB/GYN features an interview with Dr. Emily S. Miller, with Northwestern Medicine in Chicago.

Second trimester COVID-19 linked to increased preeclampsia risk
New ACOG 2024 data suggest SARS-CoV-2 in the early stages of pregnancy can lead to a higher likelihood of preeclampsia, as well as more severe disease.

Identifying gaps in syphilis treatment and prenatal care among pregnant individuals
Preventing congenital syphilis comes down to quick diagnosis and treatment of the infection in pregnancy, and the number of missed opportunities to do so in the United States continues to grow.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Patient Care & Health Information
- Diseases & Conditions
- Molar pregnancy
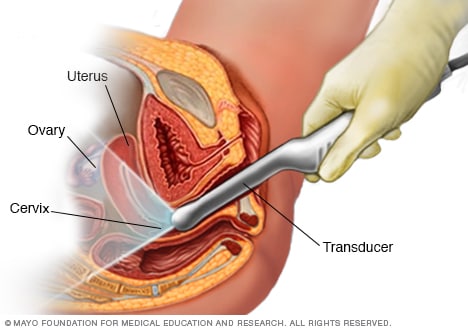
Transvaginal ultrasound
During a transvaginal ultrasound, a healthcare professional or technician uses a wandlike device called a transducer. The transducer is inserted into your vagina while you lie on your back on an exam table. The transducer emits sound waves that generate images of your pelvic organs.
A health care provider who suspects a molar pregnancy is likely to order blood tests and an ultrasound. During early pregnancy, a sonogram might involve a wandlike device placed in the vagina.
As early as eight or nine weeks of pregnancy, an ultrasound of a complete molar pregnancy might show:
- No embryo or fetus
- No amniotic fluid
- A thick cystic placenta nearly filling the uterus
- Ovarian cysts
An ultrasound of a partial molar pregnancy might show:
- A fetus that's smaller than expected
- Low amniotic fluid
- Placenta that appears unusual
After finding a molar pregnancy, a health care provider might check for other medical issues, including:
- Preeclampsia
- Hyperthyroidism
More Information
A molar pregnancy can't be allowed to continue. To prevent complications, the affected placental tissue must be removed. Treatment usually consists of one or more of the following steps:
Dilation and curettage (D&C). This procedure removes the molar tissue from the uterus. You lie on a table on your back with your legs in stirrups. You receive medicine to numb you or put you to sleep.
After opening the cervix, the provider removes uterine tissue with a suction device. A D&C for a molar pregnancy usually is done in a hospital or surgery center.
- Removal of the uterus. This occurs rarely if there's increased risk of gestational trophoblastic neoplasia (GTN) and there's no desire for future pregnancies.
HCG monitoring. After the molar tissue is removed, a provider keeps measuring the HCG level until it goes down. A continuing high level of HCG in the blood might require more treatment.
After treatment for the molar pregnancy is complete, a provider might check HCG levels for six months to make sure no molar tissue is left. For people with GTN , HCG levels are checked for one year after chemotherapy is completed.
Because pregnancy HCG levels also increase during a regular pregnancy, a provider might recommend waiting 6 to 12 months before trying to become pregnant again. The provider can recommend a reliable form of birth control during this time.
- Dilation and curettage (D&C)
Coping and support
Losing a pregnancy can be very hard. Give yourself time to grieve. Talk about your feelings and allow yourself to feel them fully. Turn to your partner, family or friends for support. If you're having trouble handling your emotions, talk to your pregnancy care provider or a counselor.
Preparing for your appointment
You're likely to start by seeing your family care provider or pregnancy care provider. Here's some information to help you get ready for your appointment.
What you can do
Ask a friend or family member to go with you to your appointment, if possible. Having someone there may help you remember the information you get. Make a list of the following:
- Your symptoms, including when they started and how they've changed over time.
- The date of your last menstrual period, if you remember it.
- Key personal information, including other medical conditions you have.
- All medications , vitamins or supplements you take, including doses.
- Questions to ask your provider.
For molar pregnancy, some questions to ask include:
- What is likely causing my symptoms?
- What tests do I need?
- What treatment do you recommend?
- Do I need to follow any restrictions?
- What emergency symptoms should I watch for at home?
- What are my chances of giving birth in the future?
- How long should I wait before trying to become pregnant again?
- Does my condition increase my risk of developing cancer in the future?
- Do you have brochures or printed material that I can have? What websites do you recommend?
Don't hesitate to ask other questions you have.
What to expect from your doctor
Your health care provider might ask you questions, such as:
- Have your symptoms been ongoing or occasional?
- Are you having pain?
- Compared with your heaviest days of menstrual flow, is your bleeding more, less or about the same? Have you passed grapelike cysts from your vagina?
- Have you been lightheaded or dizzy?
- Have you had a past molar pregnancy?
- Do you wish to become pregnant in the future?
- Ferri FF. Molar pregnancy. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed Oct. 3, 2022.
- Berkowitz RS, et al. Hydatidiform mole: Epidemiology, clinical features, and diagnosis. https://www.uptodate.com/contents/search. Accessed Oct. 3, 2022.
- Walls RM, et al., eds. Complications of pregnancy. In: Rosen's Emergency Medicine Concepts and Clinical Practice. 10th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Oct. 3, 2022.
- About gestational trophoblastic disease. American Cancer Society. https://www.cancer.org/cancer/gestational-trophoblastic-disease.html. Accessed Oct. 3, 2022.
- Berkowitz RS, et al. Hydatidiform mole: Treatment and follow-up. https://www.uptodate.com/contents/search. Accessed Oct. 3, 2022.
- Ning F, et al. Understanding and management of gestational trophoblastic disease . F1000 Faculty Review. 2019; doi:10.12688/f1000research.14953.1. Accessed Oct. 3, 2022.
- Frequently asked questions. Dilation and curettage FAQ062. American College of Obstetricians and Gynecologists. https://www.acog.org/womens-health/faqs/dilation-and-curettage. Accessed Oct. 3, 2022.
- Horowitz NS, et al. Epidemiology, diagnosis and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidence-based review and recommendation. Gynecologic Oncology; 2021. doi:10.1016/j.ygyno.2021.10.003.
Associated Procedures
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.
Advertisement
Molar Pregnancy: Epidemiology, Diagnosis, Management, Surveillance
- Family Planning (A Roe and S Sonalkar, Section Editors)
- Published: 19 February 2022
- Volume 11 , pages 133–141, ( 2022 )
Cite this article

- Alice J. Darling ORCID: orcid.org/0000-0002-4708-9247 1 ,
- Benjamin B. Albright 2 ,
- Kyle C. Strickland 3 &
- Brittany A. Davidson 2
545 Accesses
2 Citations
Explore all metrics
Purpose of Review
This review describes recommendations for the diagnosis and management of molar pregnancy, with focus on emerging evidence in recent years, particularly as it pertains to nuances of diagnosis, risk stratification, and surveillance of post-molar malignant trophoblastic disease.
Recent Findings
Topics discussed include advances in histopathologic diagnosis of molar pregnancy to standardize analysis, most recent estimations of post-molar pregnancy malignancy, and updated surveillance guidelines.
Hydatidiform molar pregnancy, resulting from an abnormal fertilization event, is the proliferation of abnormal pregnancy tissue with malignant potential. With increased availability of first trimester ultrasound, early detection of molar pregnancy has increased. While challenging to diagnose radiologically and histologically at early stages, standardization of tissue analysis allows improved detection and increased accuracy of incidence estimate for both complete and partial molar pregnancy. Treatment of molar pregnancy requires evacuation of tissue. Prophylactic chemotherapy or repeat curettage have been explored but not favored. As new molecular markers are sought, our ability to predict malignant transformation following molar pregnancies will allow for more streamlined surveillance. Recent data support a reduction in the length of surveillance following normalization of human chorionic gonadotropin levels after evacuation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Recurrent Molar Pregnancy
Clinicopathological Study of Gestational Trophoblastic Disease (GTD) in a Tertiary Care Hospital
Evaluation of a routine second curettage for hydatidiform mole: a cohort study
Papers of particular interest, published recently, have been highlighted as:, • of importance.
• Albright BB, Shorter JM, Mastroyannis SA, Ko EM, Schreiber CA, Sonalkar S. Gestational trophoblastic neoplasia after human chorionic gonadotropin normalization following molar pregnancy: a systematic review and meta-analysis. Obstet Gynecol. 2020;135(1):12–23. https://doi.org/10.1097/AOG.0000000000003566 . A systematic review and meta-analysis of post-molar gestational trophoblastic neoplasia incidence. This review found a very low (64/18,357, 0.35%, 95% CI 0.27-0.45%) cumulative incidence of GTN development after hCG normalization following a complete molar pregnancy. This rate was even lower for partial moles (5/14,864, 0.03%, 95% CI 0.01-0.08%) .
Article Google Scholar
Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(6):vi39–50. https://doi.org/10.1093/annonc/mdt345 .
• Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet. 2018;143(S2):79–85. https://doi.org/10.1002/ijgo.12615 . FIGO Cancer Report of 2018 reviewing Gestational Trophoblastic Disease. Includes updated FIGO guidelines-most notably removing elevated hCG at ≥6 months after uterine evacuation from GTN diagnostic criteria and specifying hCG followup intervals including reduced surveillance length after partial molar pregnancy .
Soper JT. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet Gynecol. 2021;137(2):355–70. https://doi.org/10.1097/aog.0000000000004240 .
Brown J, Naumann RW, Seckl MJ, Schink J. 15 years of progress in gestational trophoblastic disease: scoring, standardization, and salvage. Gynecol Oncol. 2017;144(1):200–7. https://doi.org/10.1016/J.YGYNO.2016.08.330 .
Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203(6):531–9. https://doi.org/10.1016/J.AJOG.2010.06.073 .
Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. The Lancet. 2010;376(9742):717–29. https://doi.org/10.1016/S0140-6736(10)60280-2 .
Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecol Oncol. 2009;112(3):654–62. https://doi.org/10.1016/J.YGYNO.2008.09.005 .
Article CAS Google Scholar
Maisenbacher MK, Merrion K, Kutteh WH. Single-nucleotide polymorphism microarray detects molar pregnancies in 3% of miscarriages. Fertil Steril. 2019;112(4):700–6. https://doi.org/10.1016/j.fertnstert.2019.06.015 .
Cozette C, Scheffler F, Lombart M, Massardier J, Bolze PA, Hajri T, et al. Pregnancy after oocyte donation in a patient with NLRP7 gene mutations and recurrent molar hydatidiform pregnancies. J Assist Reprod Genet. 2020;37(9):2273–7. https://doi.org/10.1007/s10815-020-01861-z .
Eagles N, Sebire NJ, Short D, Savage PM, Seckl MJ, Fisher RA. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30(9):2055–63. https://doi.org/10.1093/humrep/dev169 .
Gockley AA, Melamed A, Joseph NT, Clapp M, Sun SY, Goldstein DP, et al. The effect of adolescence and advanced maternal age on the incidence of complete and partial molar pregnancy. Gynecol Oncol. 2016;140(3):470–3. https://doi.org/10.1016/J.YGYNO.2016.01.005 .
Savage PM, Sita-Lumsden A, Dickson S, Iyer R, Everard J, Coleman R, et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J Obstet Gynaecol. 2013;33(4):406–11. https://doi.org/10.3109/01443615.2013.771159 .
Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG Int J Obstet Gynaecol. 2002;109(1):99–102. https://doi.org/10.1111/j.1471-0528.2002.t01-1-01037.x .
Sato A, Usui H, Shozu M. ABO blood type compatibility is not a risk factor for gestational trophoblastic neoplasia development from androgenetic complete hydatidiform moles. Am J Reprod Immunol. 2020;83(6):e13237. https://doi.org/10.1111/aji.13237 .
Shamshiri Milani H, Abdollahi M, Torbati S, Asbaghi T, Azargashb E. Risk Factors for hydatidiform mole: is husband’s job a major risk factor?. Asian Pac J Cancer Prev. 2017;18(10):2657–62. https://doi.org/10.22034/apjcp.2017.18.10.2657 .
Berkowitz RS, Bernstein MR, Harlow BL, Rice LW, Lage JM, Goldstein DP, et al. Case-control study of risk factors for partial molar pregnancy. Am J Obstet Gynecol. 1995;173(3 Pt 1):788–94. https://doi.org/10.1016/0002-9378(95)90342-9 .
Melamed A, Gockley AA, Joseph NT, Sun SY, Clapp MA, Goldstein DP, et al. Effect of race/ethnicity on risk of complete and partial molar pregnancy after adjustment for age. Gynecol Oncol. 2016;143(1):73–6. https://doi.org/10.1016/j.ygyno.2016.07.117 .
Sundvall L, Lund H, Niemann I, Jensen UB, Bolund L, Sunde L. Tetraploidy in hydatidiform moles. Hum Reprod. 2013;28(7):2010–20. https://doi.org/10.1093/humrep/det132 .
Sebire NJ, Savage PM, Seckl MJ, Fisher RA. Histopathological features of biparental complete hydatidiform moles in women with NLRP7 mutations. Placenta. 2013;34(1):50–6. https://doi.org/10.1016/j.placenta.2012.11.005 .
Fisher RA, Hodges MD, Newlands ES. Familial recurrent hydatidiform mole: a review. J Reprod Med. 2004;49(8):595–601.
Google Scholar
King JR, Wilson ML, Hetey S, Kiraly P, Matsuo K, Castaneda AV et al. Dysregulation of placental functions and immune pathways in complete hydatidiform moles. Int J Mol Sci. 2019;20(20). https://doi.org/10.3390/ijms20204999 .
Fisher RA, Maher GJ. Genetics of gestational trophoblastic disease. Best Pract Res Clin Obstet Gynaecol. 2021;74:29–41. https://doi.org/10.1016/j.bpobgyn.2021.01.004 .
Soellner L, Begemann M, Degenhardt F, Geipel A, Eggermann T, Mangold E. Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. Eur J Hum Genet. 2017;25(8):924–9. https://doi.org/10.1038/ejhg.2017.94 .
Sun SY, Melamed A, Joseph NT, Gockley AA, Goldstein DP, Bernstein MR, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2016;26(2):367–70. https://doi.org/10.1097/IGC.0000000000000608 .
Sun SY, Melamed A, Goldstein DP, Bernstein MR, Horowitz NS, Moron AF, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia?. Gynecol Oncol. 2015;138(1):46–9. https://doi.org/10.1016/J.YGYNO.2015.05.002 .
Winder AD, Mora AS, Berry E, Lurain JR. The “hook effect” causing a negative pregnancy test in a patient with an advanced molar pregnancy. Gynecol Oncol Rep. 2017;21:34–6. https://doi.org/10.1016/j.gore.2017.06.008 .
Li P, Koch CD, El-Khoury JM. Perimenopausal woman with elevated serum hCG and abdominal pain. Clin Chim Acta. 2021;522:141–3. https://doi.org/10.1016/j.cca.2021.08.018 .
Ross JA, Unipan A, Clarke J, Magee C, Johns J. Ultrasound diagnosis of molar pregnancy. Ultrasound. 2018;26(3):153–9. https://doi.org/10.1177/1742271x17748514 .
Savage JL, Maturen KE, Mowers EL, Pasque KB, Wasnik AP, Dalton VK, et al. Sonographic diagnosis of partial versus complete molar pregnancy: a reappraisal. J Clin Ultrasound. 2017;45(2):72–8. https://doi.org/10.1002/jcu.22410 .
Ronnett BM. Hydatidiform moles: ancillary techniques to refine diagnosis. Arch Pathol Lab Med. 2018;142(12):1485–502. https://doi.org/10.5858/arpa.2018-0226-RA .
Hui P, Buza N, Murphy KM, Ronnett BM. Hydatidiform moles: genetic basis and precision diagnosis. Annu Rev Pathol. 2017;12:449–85. https://doi.org/10.1146/annurev-pathol-052016-100237 .
Madi JM, Braga A, Paganella MP, Litvin IE, Wendland EM. Accuracy of p57(KIP)(2) compared with genotyping to diagnose complete hydatidiform mole: a systematic review and meta-analysis. BJOG. 2018;125(10):1226–33. https://doi.org/10.1111/1471-0528.15289 .
Zheng XZ, Qin XY, Chen SW, Wang P, Zhan Y, Zhong PP, et al. Heterozygous/dispermic complete mole confers a significantly higher risk for post-molar gestational trophoblastic disease. Mod Pathol. 2020;33(10):1979–88. https://doi.org/10.1038/s41379-020-0566-4 .
Lin LH, Maestá I, St Laurent JD, Hasselblatt KT, Horowitz NS, Goldstein DP, et al. Distinct microRNA profiles for complete hydatidiform moles at risk of malignant progression. Am J Obstet Gynecol. 2021;224(4):372.e1-e30. https://doi.org/10.1016/j.ajog.2020.09.048 .
Braga A, Maestá I, Rocha Soares R, Elias KM, Custódio Domingues MA, Barbisan LF, et al. Apoptotic index for prediction of postmolar gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2016;215(3):336.e1-.e12. https://doi.org/10.1016/j.ajog.2016.04.010 .
Padrón L, Rezende Filho J, Amim Junior J, Sun SY, Charry RC, Maestá I, et al. Manual compared with electric vacuum aspiration for treatment of molar pregnancy. Obstet Gynecol. 2018;1-. https://doi.org/10.1097/AOG.0000000000002522 .
Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker RT. Hydatidiform mole: diagnosis, management, and long-term followup of 347 patients. Obstet Gynecol. 1975;45(1):1–8.
CAS Google Scholar
• Zhao P, Lu Y, Huang W, Tong B, Lu W. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):13. https://doi.org/10.1186/s12885-018-5168-x . A systematic review and meta-analysis which demonstrated a risk reduction in post-molar GTN of more than 80% in patients ≥40 years old following hysterectomy compared to those receiving uterine evacuations for molar pregnancy treatment .
Giorgione V, Bergamini A, Cioffi R, Pella F, Rabaiotti E, Petrone M, et al. Role of surgery in the management of hydatidiform mole in elderly patients: a single-center clinical experience. Int J Gynecol Cancer. 2017;27(3):550–3. https://doi.org/10.1097/igc.0000000000000903 .
Eysbouts YK, Massuger L, IntHout J, Lok CAR, Sweep F, Ottevanger PB. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017;145(3):536–42. https://doi.org/10.1016/j.ygyno.2017.03.018 .
Yamamoto E, Nishino K, Niimi K, Watanabe E, Oda Y, Ino K, et al. Evaluation of a routine second curettage for hydatidiform mole: a cohort study. Int J Clin Oncol. 2020;25(6):1178–86. https://doi.org/10.1007/s10147-020-01640-x .
Yamamoto E, Trinh TD, Sekiya Y, Tamakoshi K, Nguyen XP, Nishino K, et al. The management of hydatidiform mole using prophylactic chemotherapy and hysterectomy for high-risk patients decreased the incidence of gestational trophoblastic neoplasia in Vietnam: a retrospective observational study. Nagoya J Med Sci. 2020;82(2):183–91. https://doi.org/10.18999/nagjms.82.2.183 .
Wang Q, Fu J, Hu L, Fang F, Xie L, Chen H, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database of Syst Rev. 2017;9:CD007289-CD. https://doi.org/10.1002/14651858.CD007289.pub3 .
Jiao LZ, Wang YP, Jiang JY, Zhang WQ, Wang XY, Zhu CG, et al. Clinical significance of centralized surveillance of hydatidiform mole. Zhonghua Fu Chan Ke Za Zhi. 2018;53(6):390–5. https://doi.org/10.3760/cma.j.issn.0529-567x.2018.06.006 .
Braga A, Biscaro A, do Amaral Giordani JM, Viggiano M, Elias KM, Berkowitz RS, et al. Does a human chorionic gonadotropin level of over 20,000 IU/L four weeks after uterine evacuation for complete hydatidiform mole constitute an indication for chemotherapy for gestational trophoblastic neoplasia?. Eur J Obstet Gynecol Reprod Biol. 2018;223:50–5. https://doi.org/10.1016/j.ejogrb.2018.02.001 .
Ngu SF, Ngan HYS. Surgery including fertility-sparing treatment of GTD. Best Pract Res Clin Obstet Gynaecol. 2021;74:97–108. https://doi.org/10.1016/j.bpobgyn.2020.10.005 .
Zilberman Sharon N, Maymon R, Melcer Y, Jauniaux E. Obstetric outcomes of twin pregnancies presenting with a complete hydatidiform mole and coexistent normal fetus: a systematic review and meta-analysis. BJOG. 2020;127(12):1450–7. https://doi.org/10.1111/1471-0528.16283 .
Lin LH, Maestá I, Braga A, Sun SY, Fushida K, Francisco RPV, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: A retrospective multicenter cohort and literature review. Gynecol Oncol. 2017;145(1):88–95. https://doi.org/10.1016/j.ygyno.2017.01.021 .
• Albright BB, Myers ER, Moss HA, Ko EM, Sonalkar S, Havrilesky LJ. Surveillance for gestational trophoblastic neoplasia following molar pregnancy: a cost-effectiveness analysis. Am J Obstet Gynecol. 2021. https://doi.org/10.1016/j.ajog.2021.05.031 . Cost-effectiveness analysis of post-molar GTN surveillance finding reduction or elimination of hCG surveillance would be cost effective and clinically reasonable given the rarity of malignant following hCG normalization. Additionally, found a single hCG test 3 months after uterine evacuation was a cost-effective alternative .
Massad LS, Abu-Rustum NR, Lee SS, Renta V. Poor compliance with postmolar surveillance and treatment protocols by indigent women. Obstet Gynecol. 2000;96(6):940–4. https://doi.org/10.1016/s0029-7844(00)01064-4 .
Blok LJ, Frijstein MM, Eysbouts YK, Custers J, Sweep F, Lok C, et al. The psychological impact of gestational trophoblastic disease: a prospective observational multicentre cohort study. BJOG. 2021. https://doi.org/10.1111/1471-0528.16849 .
Jewell EL, Aghajanian C, Montovano M, Lewin SN, Baser RE, Carter J. Association of ß-hCG surveillance with emotional, reproductive, and sexual health in women treated for gestational trophoblastic neoplasia. J Womens Health (Larchmt). 2018;27(3):387–93. https://doi.org/10.1089/jwh.2016.6208 .
Stafford L, McNally OM, Gibson P, Judd F. Long-term psychological morbidity, sexual functioning, and relationship outcomes in women with gestational trophoblastic disease. Int J Gynecol Cancer. 2011;21(7):1256–63. https://doi.org/10.1097/IGC.0b013e3182259c04 .
Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol. 2018;148(2):254–7. https://doi.org/10.1016/j.ygyno.2017.12.008 .
Management of Gestational Trophoblastic Disease: Green-top Guideline No. 38 - June 2020. BJOG. 2021;128(3):e1-e27. https://doi.org/10.1111/1471-0528.16266 .
Horowitz NS, Eskander RN, Adelman MR, Burke W. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: a Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021;163(3):605–13. https://doi.org/10.1016/j.ygyno.2021.10.003 .
Lybol C, Sweep FC, Ottevanger PB, Massuger LF, Thomas CM. Linear regression of postevacuation serum human chorionic gonadotropin concentrations predicts postmolar gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2013;23(6):1150–6. https://doi.org/10.1097/IGC.0b013e31829703ea .
Hardman S. Use of hormonal contraception after hydatidiform mole. BJOG Int J Obstet Gynaecol. 2016;123(8):1336. https://doi.org/10.1111/1471-0528.13691 .
Gaffield ME, Kapp N, Curtis KM. Combined oral contraceptive and intrauterine device use among women with gestational trophoblastic disease. Contraception. 2009;80(4):363–71. https://doi.org/10.1016/j.contraception.2009.03.022 .
Dantas PRS, Maestá I, Filho JR, Junior JA, Elias KM, Howoritz N, et al. Does hormonal contraception during molar pregnancy follow-up influence the risk and clinical aggressiveness of gestational trophoblastic neoplasia after controlling for risk factors? Gynecol Oncol. 2017;147(2):364–70. https://doi.org/10.1016/j.ygyno.2017.09.007 .
Braga A, Maestá I, Short D, Savage P, Harvey R, Seckl M. Hormonal contraceptive use before hCG remission does not increase the risk of gestational trophoblastic neoplasia following complete hydatidiform mole: a historical database review. BJOG Int J Obstet Gynaecol. 2016;123(8):1330–5. https://doi.org/10.1111/1471-0528.13617 .
Morbidity and Mortality Weekly Report: Classifications for Intrauterine Devices. Centers for Disease Control and Prevention. 2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr59e0528a6.htm . Accessed 25 Oct 2021.
Tuncer ZS, Bernstein MR, Goldstein DP, Lu KH, Berkowitz RS. Outcome of pregnancies occurring within 1 year of hydatidiform mole. Obstet Gynecol. 1999;94(4):588–90. https://doi.org/10.1016/S0029-7844(99)00395-6 .
Joneborg U, Coopmans L, van Trommel N, Seckl M, Lok CAR. Fertility and pregnancy outcome in gestational trophoblastic disease. Int J Gynecol Cancer. 2021;31(3):399–411. https://doi.org/10.1136/ijgc-2020-001784 .
Vargas R, Barroilhet LM, Esselen K, Diver E, Bernstein M, Goldstein DP, et al. Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy, and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med. 2014;59(5–6):188–94.
Matsui H, Iitsuka Y, Suzuka K, Seki K, Sekiya S. Subsequent pregnancy outcome in patients with spontaneous resolution of HCG after evacuation of hydatidiform mole: comparison between complete and partial mole. Hum Reprod. 2001;16(6):1274–7. https://doi.org/10.1093/humrep/16.6.1274 .
Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. Gestational Trophoblastic Neoplasia, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(11):1374–91. https://doi.org/10.6004/jnccn.2019.0053 .
Download references
Author information
Authors and affiliations.
Department of Obstetrics & Gynecology, Duke University, Durham, NC, USA
Alice J. Darling
Department of Obstetrics & Gynecology, Division of Gynecologic Oncology, Duke University, Durham, NC, USA
Benjamin B. Albright & Brittany A. Davidson
Department of Pathology, Duke University, Durham, NC, USA
Kyle C. Strickland
You can also search for this author in PubMed Google Scholar
Contributions
AD: Project design, literature review, manuscript draft, critical revision, final approval. BA: Project conception and design, literature review, critical revision, final approval. KS: Collecting and preparing specimens for manuscript figure, critical revision, final approval. BD: Project conception and design, literature review, critical revision, final approval.
Ethics declarations
Ethics approval.
Not applicable, this article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Conflict of interest.
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Family Planning
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Darling, A.J., Albright, B.B., Strickland, K.C. et al. Molar Pregnancy: Epidemiology, Diagnosis, Management, Surveillance. Curr Obstet Gynecol Rep 11 , 133–141 (2022). https://doi.org/10.1007/s13669-022-00327-6
Download citation
Accepted : 09 February 2022
Published : 19 February 2022
Issue Date : June 2022
DOI : https://doi.org/10.1007/s13669-022-00327-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Molar pregnancy
- Hydatidiform mole
- Complete mole
- Partial mole
- Gestational Trophoblastic Disease
- Surveillance
- Find a journal
- Publish with us
- Track your research

Hydatidiform Mole Clinical Presentation
- Author: Lisa E Moore, MD, MS, FACOG, RDMS; Chief Editor: Leslie M Randall, MD, MAS, FACS more...
- Sections Hydatidiform Mole
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Laboratory Studies
- Imaging Studies
- Histologic Findings
- Medical Care
- Surgical Care
- Long-Term Monitoring
- Questions & Answers
- Media Gallery
Complete mole
The typical clinical presentation of complete molar pregnancies has changed with the advent of high-resolution ultrasonography. Most moles are now diagnosed in the first trimester before the onset of the classic signs and symptoms. [ 32 , 33 , 34 ]
Vaginal bleeding
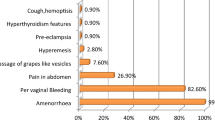
The most common classic symptom of a complete mole is vaginal bleeding. Molar tissue separates from the decidua, causing bleeding. The uterus may become distended by large amounts of blood, and dark fluid may leak into the vagina. This symptom occurs in 50% of cases.
Hyperemesis
Patients may also report severe nausea and vomiting. This is due to extremely high levels of human chorionic gonadotropin (hCG). This is reported to occur in 4% of patients diagnosed at 5-9 weeks of gestation, and in 23% when the diagnosis is made after 10 weeks' gestation.
Hyperthyroidism
Signs and symptoms of hyperthyroidism can be present due to stimulation of the thyroid gland by the high levels of circulating hCG or by a thyroid stimulating substance (ie, thyrotropin) produced by the trophoblasts. [ 35 ] Clinical hyperthyroidism has been reported in 3.7% of women with a hydatidiform mole diagnosed after the 10th week of gestation.
Partial mole
Patients with partial mole do not have the same clinical features as those with complete mole. These patients usually present with signs and symptoms consistent with an incomplete or missed abortion, including vaginal bleeding and absence of fetal heart tones.
In a retrospective study (1994-2013) at a Brazilian trophoblastic disease center, investigators evaluated the clinical presentations and incidence of postmolar gestational trophoblastic neoplasia (GTN) among 355 women with complete mole (n =186) or partial mole (n = 169), with the following findings [ 36 ] :
- Risk of vaginal bleeding, biochemical hyperthyroidism, anemia, uterine size larger than dates, and hyperemesis: Reduced risk in women with partial mole
- Preevacuation serum hCG levels: Lower in women with partial mole
- Median gestational age at evacuation: complete mole, 9 weeks; partial mole, 12 weeks
- Development of GTN: women with complete mole, 17.7%; women with partial mole, 4.1%
Those with complete mole were diagnosed more commonly before evacuation than women with partial mole because they presented more often with signs/symptoms of molar disease. [ 36 ]
Note the following:
Size inconsistent with gestational age: A uterine enlargement greater than expected for gestational age is a classic sign of a complete mole. Unexpected enlargement is caused by excessive trophoblastic growth and retained blood. However, patients also present with size appropriate or smaller than expected for the gestational age.
Preeclampsia : Pelvic ultrasonography has resulted in the early diagnosis of most cases of hydatidiform mole and preeclampsia is seen in less than 2% of cases. [ 33 ]
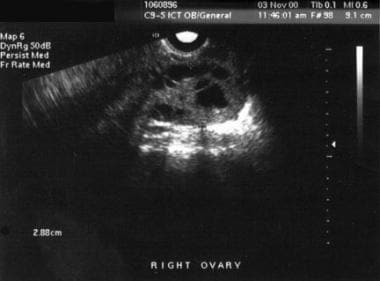
Theca lutein cysts: These are ovarian cysts greater than 6 cm in diameter and accompanying ovarian enlargement. These cysts are not usually palpated on bimanual examination but are identified by ultrasonography. Patients may report pressure or pelvic pain. Because of the increased ovarian size, torsion is a risk. These cysts develop in response to high levels of beta-hCG. They are reported in 11% of cases diagnosed at longer than10-weeks' gestational age. The cysts spontaneously regress after the mole is evacuated, but it may take up to 12 weeks for complete regression.
Uterine enlargement and preeclampsia is reported in only 5% of patients. [ 37 ] Theca lutein cysts, hyperemesis, and hyperthyroidism are extremely rare.
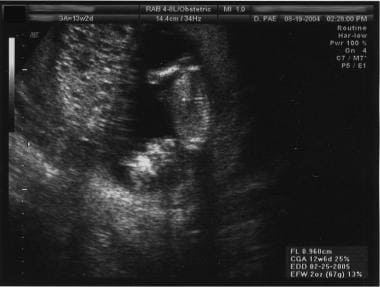
Twinning with a complete mole and a fetus with a normal placenta has been reported (see image below). Cases of healthy infants in these circumstances have been reported. [ 13 , 38 , 39 ]
Women with coexistent molar and normal gestations are at higher risk for developing persistent disease and metastasis. [ 22 ] Termination of pregnancy is a recommended option.
The pregnancy may be continued as long as the maternal status is stable, without hemorrhage, thyrotoxicosis, or severe hypertension. The patient should be informed of the risk of severe maternal morbidity from these complications. [ 40 ]
Prenatal genetic diagnosis by chorionic villus sampling or amniocentesis is recommended to evaluate the karyotype of the fetus.
Soper JT. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet Gynecol . 2021 Feb 1. 137 (2):355-70. [QxMD MEDLINE Link] . [Full Text] .
Schorge JO, Goldstein DP, Bernstein MR, Berkowitz RS. Recent advances in gestational trophoblastic disease. J Reprod Med . 2000 Sep. 45(9):692-700. [QxMD MEDLINE Link] .
Ito Y, Maehara K, Kaneki E, et al. Novel nonsense mutation in the NLRP7 gene associated with recurrent hydatidiform mole. Gynecol Obstet Invest . 2015 Nov 26. [QxMD MEDLINE Link] .
Wolf NG, Lage JM. Genetic analysis of gestational trophoblastic disease: a review. Semin Oncol . 1995 Apr. 22(2):113-20. [QxMD MEDLINE Link] .
Slim R, Mehio A. The genetics of hydatidiform moles: new lights on an ancient disease. Clin Genet . 2007 Jan. 71(1):25-34. [QxMD MEDLINE Link] .
Fisher RA, Hodges MD. Genomic imprinting in gestational trophoblastic disease--a review. Placenta . 2003 Apr. 24 Suppl A:S111-8. [QxMD MEDLINE Link] .
Al-Hussaini TK, Abd el-Aal DM, Van den Veyver IB. Recurrent pregnancy loss due to familial and non-familial habitual molar pregnancy. Int J Gynaecol Obstet . 2003 Nov. 83(2):179-86. [QxMD MEDLINE Link] .
Fallahian M. Familial gestational trophoblastic disease. Placenta . 2003 Aug. 24(7):797-9. [QxMD MEDLINE Link] .
Hodges MD, Rees HC, Seckl MJ, et al. Genetic refinement and physical mapping of a biparental complete hydatidiform mole locus on chromosome 19q13.4. J Med Genet . 2003 Aug. 40(8):e95. [QxMD MEDLINE Link] .
Lawler SD, Fisher RA, Dent J. A prospective genetic study of complete and partial hydatidiform moles. Am J Obstet Gynecol . 1991 May. 164(5 Pt 1):1270-7. [QxMD MEDLINE Link] .
Deveault C, Qian JH, Chebaro W, et al. NLRP7 mutations in women with diploid androgenetic and triploid moles: a proposed mechanism for mole formation. Hum Mol Genet . 2009 Mar 1. 18(5):888-97. [QxMD MEDLINE Link] .
Andreasen L, Christiansen OB, Niemann I, Bolund L, Sunde L. NLRP7 or KHDC3L genes and the etiology of molar pregnancies and recurrent miscarriage. Mol Hum Reprod . 2013 Nov. 19 (11):773-81. [QxMD MEDLINE Link] .
Watson EJ, Hernandez E, Miyazawa K. Partial hydatidiform moles: a review. Obstet Gynecol Surv . 1987 Sep. 42(9):540-4. [QxMD MEDLINE Link] .
Atrash HK, Hogue CJ, Grimes DA. Epidemiology of hydatidiform mole during early gestation. Am J Obstet Gynecol . 1986 Apr. 154(4):906-9. [QxMD MEDLINE Link] .
Grimes DA. Epidemiology of gestational trophoblastic disease. Am J Obstet Gynecol . 1984 Oct 1. 150(3):309-18. [QxMD MEDLINE Link] .
Joyce CM, Fitzgerald B, McCarthy TV, Coulter J, O'Donoghue K. Advances in the diagnosis and early management of gestational trophoblastic disease. BMJ Med . 2022. 1 (1):e000321. [QxMD MEDLINE Link] . [Full Text] .
Jeffers MD, O'Dwyer P, Curran B, Leader M, Gillan JE. Partial hydatidiform mole: a common but underdiagnosed condition. A 3-year retrospective clinicopathological and DNA flow cytometric analysis. Int J Gynecol Pathol . 1993 Oct. 12(4):315-23. [QxMD MEDLINE Link] .
Palmer JR. Advances in the epidemiology of gestational trophoblastic disease. J Reprod Med . 1994 Mar. 39(3):155-62. [QxMD MEDLINE Link] .
Melamed A, Gockley AA, Joseph NT, Sun SY, Clapp MA, Goldstein DP, et al. Effect of race/ethnicity on risk of complete and partial molar pregnancy after adjustment for age. Gynecol Oncol . 2016 Oct. 143 (1):73-76. [QxMD MEDLINE Link] .
Bandy LC, Clarke-Pearson DL, Hammond CB. Malignant potential of gestational trophoblastic disease at the extreme ages of reproductive life. Obstet Gynecol . 1984 Sep. 64(3):395-9. [QxMD MEDLINE Link] .
Bracken MB. Incidence and aetiology of hydatidiform mole: an epidemiological review. Br J Obstet Gynaecol . 1987 Dec. 94(12):1123-35. [QxMD MEDLINE Link] .
Hurteau JA. Gestational trophoblastic disease: management of hydatidiform mole. Clin Obstet Gynecol . 2003 Sep. 46(3):557-69. [QxMD MEDLINE Link] .
Vargas R, Barroilhet LM, Esselen K, et al. Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy, and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med . 2014 May-Jun. 59(5-6):188-94. [QxMD MEDLINE Link] .
Gadducci A, Cosio S, Fanucchi A, et al. Prognosis of Patients with Gestational Trophoblastic Neoplasia and Obstetric Outcomes of Those Conceiving After Chemotherapy. Anticancer Res . 2016 Jul. 36 (7):3477-82. [QxMD MEDLINE Link] .
Joneborg U, Eloranta S, Johansson AL, Marions L, Weibull CE, Lambe M. Hydatidiform mole and subsequent pregnancy outcome: a population-based cohort study. Am J Obstet Gynecol . 2014 Dec. 211(6):681.e1-7. [QxMD MEDLINE Link] .
Lurain JR, Brewer JI, Torok EE, Halpern B. Natural history of hydatidiform mole after primary evacuation. Am J Obstet Gynecol . 1983 Mar 1. 145(5):591-5. [QxMD MEDLINE Link] .
Goto S, Yamada A, Ishizuka T, Tomoda Y. Development of postmolar trophoblastic disease after partial molar pregnancy. Gynecol Oncol . 1993 Feb. 48(2):165-70. [QxMD MEDLINE Link] .
Cheung AN, Khoo US, Lai CY, et al. Metastatic trophoblastic disease after an initial diagnosis of partial hydatidiform mole: genotyping and chromosome in situ hybridization analysis. Cancer . 2004 Apr 1. 100(7):1411-7. [QxMD MEDLINE Link] .
Menczer J, Girtler O, Zajdel L, Glezerman M. Metastatic trophoblastic disease following partial hydatidiform mole: case report and literature review. Gynecol Oncol . 1999 Aug. 74(2):304-7. [QxMD MEDLINE Link] .
Twiggs LB, Morrow CP, Schlaerth JB. Acute pulmonary complications of molar pregnancy. Am J Obstet Gynecol . 1979 Sep 15. 135(2):189-94. [QxMD MEDLINE Link] .
Sebire NJ, Fisher RA, Foskett M, et al. Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy. BJOG . 2003 Jan. 110(1):22-6. [QxMD MEDLINE Link] .
Mangili G, Garavaglia E, Cavoretto P, Gentile C, Scarfone G, Rabaiotti E. Clinical presentation of hydatidiform mole in northern Italy: has it changed in the last 20 years?. Am J Obstet Gynecol . 2008 Mar. 198(3):302.e1-4. [QxMD MEDLINE Link] .
Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol . 1995 Nov. 86(5):775-9. [QxMD MEDLINE Link] .
Sun SY, Melamed A, Goldstein DP, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia?. Gynecol Oncol . 2015 Jul. 138 (1):46-9. [QxMD MEDLINE Link] .
Amir SM, Osathanondh R, Berkowitz RS, Goldstein DP. Human chorionic gonadotropin and thyroid function in patients with hydatidiform mole. Am J Obstet Gynecol . 1984 Nov 15. 150(6):723-8. [QxMD MEDLINE Link] .
Sun SY, Melamed A, Joseph NT, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer . 2015 Nov 19. [QxMD MEDLINE Link] .
Berkowitz RS, Goldstein DP, Bernstein MR. Natural history of partial molar pregnancy. Obstet Gynecol . 1985 Nov. 66(5):677-81. [QxMD MEDLINE Link] .
Fishman DA, Padilla LA, Keh P, Cohen L, Frederiksen M, Lurain JR. Management of twin pregnancies consisting of a complete hydatidiform mole and normal fetus. Obstet Gynecol . 1998 Apr. 91(4):546-50. [QxMD MEDLINE Link] .
Wang G, Cui H, Chen X. A complete hydatidiform mole and coexisting viable fetus in a twin pregnancy: a case report with literature review. J Matern Fetal Neonatal Med . 2023 Dec. 36 (1):2183746. [QxMD MEDLINE Link] .
Steller MA, Genest DR, Bernstein MR, Lage JM, Goldstein DP, Berkowitz RS. Natural history of twin pregnancy with complete hydatidiform mole and coexisting fetus. Obstet Gynecol . 1994 Jan. 83(1):35-42. [QxMD MEDLINE Link] .
Florio P, Severi FM, Cobellis L, et al. Serum activin A and inhibin A. New clinical markers for hydatidiform mole. Cancer . 2002 May 15. 94(10):2618-22. [QxMD MEDLINE Link] .
Fulop V, Mok SC, Berkowitz RS. Molecular biology of gestational trophoblastic neoplasia: a review. J Reprod Med . 2004 Jun. 49(6):415-22. [QxMD MEDLINE Link] .
Fukunaga M. Immunohistochemical characterization of p57(KIP2) expression in early hydatidiform moles. Hum Pathol . 2002 Dec. 33(12):1188-92. [QxMD MEDLINE Link] .
Genest DR, Dorfman DM, Castrillon DH. Ploidy and imprinting in hydatidiform moles. Complementary use of flow cytometry and immunohistochemistry of the imprinted gene product p57KIP2 to assist molar classification. J Reprod Med . 2002 May. 47(5):342-6. [QxMD MEDLINE Link] .
Thaker HM, Berlin A, Tycko B, et al. Immunohistochemistry for the imprinted gene product IPL/PHLDA2 for facilitating the differential diagnosis of complete hydatidiform mole. J Reprod Med . 2004 Aug. 49(8):630-6. [QxMD MEDLINE Link] .
Benachi A, Garritsen HS, Howard CM, Bennett P, Fisk NM. Absence of expression of RhD by human trophoblast cells. Am J Obstet Gynecol . 1998 Feb. 178 (2):294-9. [QxMD MEDLINE Link] .
Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet . 2018 Oct. 143 Suppl 2:79-85. [QxMD MEDLINE Link] .
Eddy GL, Schlaerth JB, Nalick RH, Gaddis O Jr, Nakamura RM, Morrow CP. Postmolar trophoblastic disease in women using hormonal contraception with and without estrogen. Obstet Gynecol . 1983 Dec. 62(6):736-40. [QxMD MEDLINE Link] .
Agarwal R, Teoh S, Short D, et al. Chemotherapy and human chorionic gonadotropin concentrations 6 months after uterine evacuation of molar pregnancy: a retrospective cohort study. Lancet . 2012 Jan 14. 379(9811):130-5. [QxMD MEDLINE Link] .
Sebire NJ, Foskett M, Short D, et al. Shortened duration of human chorionic gonadotrophin surveillance following complete or partial hydatidiform mole: evidence for revised protocol of a UK regional trophoblastic disease unit. BJOG . 2007 Jun. 114(6):760-2. [QxMD MEDLINE Link] .
Batorfi J, Vegh G, Szepesi J, et al. How long should patients be followed after molar pregnancy? Analysis of serum hCG follow-up data. Eur J Obstet Gynecol Reprod Biol . 2004 Jan 15. 112(1):95-7. [QxMD MEDLINE Link] .
Feltmate CM, Batorfi J, Fulop V, et al. Human chorionic gonadotropin follow-up in patients with molar pregnancy: a time for reevaluation. Obstet Gynecol . 2003 Apr. 101(4):732-6. [QxMD MEDLINE Link] .
Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol . 2018 Feb. 148 (2):254-257. [QxMD MEDLINE Link] .
Horowitz NS, Berkowitz RS, Elias KM. Considering changes in the recommended human chorionic gonadotropin monitoring after molar evacuation. Obstet Gynecol . 2020 Jan. 135 (1):9-11. [QxMD MEDLINE Link] .
Garner EI, Lipson E, Bernstein MR, Goldstein DP, Berkowitz RS. Subsequent pregnancy experience in patients with molar pregnancy and gestational trophoblastic tumor. J Reprod Med . 2002 May. 47(5):380-6. [QxMD MEDLINE Link] .
Amezcua CA, Bahador A, Naidu YM, Felix JC. Expression of human telomerase reverse transcriptase, the catalytic subunit of telomerase, is associated with the development of persistent disease in complete hydatidiform moles. Am J Obstet Gynecol . 2001 Jun. 184(7):1441-6. [QxMD MEDLINE Link] .
Wang Q, Fu J, Hu L, Fang F, Xie L, Chen H, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev . 2017 Sep 11. 9:CD007289. [QxMD MEDLINE Link] .
Zhao P, Chen Q, Lu W. Comparison of different therapeutic strategies for complete hydatidiform mole in women at least 40 years old: a retrospective cohort study. BMC Cancer . 2017 Nov 9. 17 (1):733. [QxMD MEDLINE Link] .
- Theca lutein cysts.
- Complete mole.
- Complete mole with an area of clot near cervix consistent with bleeding.
- Twin gestation. Complete mole and normal twin.

Contributor Information and Disclosures
Lisa E Moore, MD, MS, FACOG, RDMS Professor, Department of Obstetrics and Gynecology, Chief, Division of Maternal-Fetal Medicine, Medical Director of Labor and Delivery, Texas Tech University Health Sciences Center, Paul L Foster School of Medicine Lisa E Moore, MD, MS, FACOG, RDMS is a member of the following medical societies: American Congress of Obstetricians and Gynecologists , Central Association of Obstetricians and Gynecologists , International Society of Ultrasound in Obstetrics and Gynecology, Society for Maternal-Fetal Medicine , Society of Diagnostic Medical Sonography Disclosure: Nothing to disclose.
Enrique Hernandez, MD, FACOG, FACS Chairman, Department of Obstetrics and Gynecology, J Robert Willson Professor of Obstetrics, Gynecology and Reproductive Science, Professor of Pathology, Temple University Hospital, Lewis Katz School of Medicine at Temple University Enrique Hernandez, MD, FACOG, FACS is a member of the following medical societies: Alpha Omega Alpha , American Cancer Society , American College of Obstetricians and Gynecologists , American College of Surgeons , American Gynecological and Obstetrical Society , American Medical Association , American Society for Colposcopy and Cervical Pathology , Association of Professors of Gynecology and Obstetrics , Johns Hopkins Medical and Surgical Association , Pennsylvania Medical Society , Philadelphia County Medical Society , Society of Gynecologic Oncology Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape.
A David Barnes, MD, MPH, PhD, FACOG Consulting Staff, Department of Obstetrics and Gynecology, Mammoth Hospital (Mammoth Lakes, CA), Pioneer Valley Hospital (Salt Lake City, UT), Warren General Hospital (Warren, PA), and Mountain West Hospital (Tooele, UT) A David Barnes, MD, MPH, PhD, FACOG is a member of the following medical societies: American College of Forensic Examiners Institute , American College of Obstetricians and Gynecologists , The Society of Federal Health Professionals (AMSUS) , American Medical Association , Utah Medical Association Disclosure: Nothing to disclose.
Leslie M Randall, MD, MAS, FACS Professor and Director, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Diane Harris Wright Professor of Gynecologic Oncology Research, Massey Cancer Center, Virginia Commonwealth University School of Medicine Leslie M Randall, MD, MAS, FACS is a member of the following medical societies: Academy for Innovation in Medical Education, American College of Surgeons , American Medical Association , American Society of Clinical Oncology , International Gynecologic Cancer Society , Society of Gynecologic Oncology Disclosure: Nothing to disclose.
Jordan G Pritzker, MD, MBA, FACOG Adjunct Professor of Obstetrics/Gynecology, Hofstra North Shore-LIJ School of Medicine at Hofstra University; Attending Physician, Department of Obstetrics and Gynecology, Long Island Jewish Medical Center; Medical Director, Aetna, Inc; Private Practice in Gynecology Disclosure: Nothing to disclose.
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Hydatidiform Mole
- Hydatidiform Mole Imaging
- Gestational Trophoblastic Neoplasia
- Human Chorionic Gonadotropin (hCG)
- Gestational Trophoblastic Tumor Staging
- Gynecologic Tumor Markers

- Radiological Case: Hyperreactio Luteinalis With Partial Molar Pregnancy
- Drug Interaction Checker
- Pill Identifier
- Calculators

Molar pregnancies
Highlights & basics.
- Diagnostic Approach
- Risk Factors
History & Exam
- Differential Diagnosis
- Tx Approach
- Emerging Tx
- Complications
PATIENT RESOURCES
- Patient Instructions
Molar pregnancies (MPs; hydatidiform moles) are chromosomally abnormal pregnancies that have the potential to become malignant.
There is a higher possibility of MP in women less than 20 years of age or over 35 years of age, and in those who have experienced MP in a previous pregnancy.
The most common presenting symptom is vaginal bleeding.
Suction evacuation (electrical or manual) is the preferred management option in women who desire preservation of fertility.
Hysterectomy may be considered in women who do not want to preserve fertility.

Quick Reference
Key Factors
Other Factors
Diagnostics Tests
Treatment Options
Epidemiology
Pathophysiology.

Ultrasound showing multiple cystic areas in the uterine cavity giving a "snowstorm appearance" suggestive of molar pregnancy.
Key Articles
Horowitz NS, Eskander RN, Adelman MR, et al. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021 Dec;163(3):605-13. [Abstract] [Full Text]
Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021 Oct;155 Suppl 1(suppl 1):86-93. [Abstract] [Full Text]
Tidy, J, Seckl, M, Hancock, BW, on behalf of the Royal College of Obstetricians and Gynaecologists. Management of gestational trophoblastic disease. BJOG 2021;128: e1-e27. [Full Text]
Lok C, van Trommel N, Massuger L, et al. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020 May;130:228-40. [Abstract]
Referenced Articles
1. Hancock BW. Staging and classification of gestational trophoblastic disease. Clin Obstet Gynecol Best Pract Res. 2003 Dec;17(6):869-83. [Abstract]
2. Eiriksson L, Dean E, Sebastianelli A, et al. Guideline no. 408: management of gestational trophoblastic diseases. J Obstet Gynaecol Can. 2021 Jan;43(1):91-105.e1. [Abstract] [Full Text]
3. Horowitz NS, Eskander RN, Adelman MR, et al. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021 Dec;163(3):605-13. [Abstract] [Full Text]
4. Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021 Oct;155 Suppl 1(suppl 1):86-93. [Abstract] [Full Text]
5. Altieri A, Franceschi S, Ferlay J, et al. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003 Nov;4(11):670-8. [Abstract]
6. Kim SJ, Bae SN, Kim JH, et al. Epidemiology and time trends of gestational trophoblastic disease in Korea. Int J Gynaecol Obstet. 1998 Apr;60 Suppl 1:S33-8. [Abstract]
7. Braga A, Uberti EM, Fajardo Mdo C, et al. Epidemiological report on the treatment of patients with gestational trophoblastic disease in 10 Brazilian referral centers: results after 12 years since International FIGO 2000 Consensus. J Reprod Med. 2014 May-Jun;59(5-6):241-7. [Abstract]
8. Sebire NJ, Fisher RA, Foskett M, et al. Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy. BJOG. 2003 Jan;110(1):22-6. [Abstract]
9. Vargas R, Barroilhet LM, Esselen K, et al. Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy, and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med. 2014 May-Jun;59(5-6):188-94. [Abstract]
10. Altman AD, Bentley B, Murray S, et al. Maternal age-related rates of gestational trophoblastic disease. Obstet Gynecol. 2008 Aug;112(2 Pt 1):244-50. [Abstract]
11. Savage PM, Sita-Lumsden A, Dickson S, et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J Obstet Gynaecol. 2013 May;33(4):406-11. [Abstract] [Full Text]
12. Gockley AA, Melamed A, Joseph NT, et al. The effect of adolescence and advanced maternal age on the incidence of complete and partial molar pregnancy. Gynecol Oncol. 2016 Mar;140(3):470-3. [Abstract]
13. Di Cintio E, Parazzini F, Rosa C, et al. The epidemiology of gestational trophoblastic disease. Gen Diagn Pathol. 1997 Nov;143(2-3):103-8. [Abstract]
14. Braga A, Growdon WB, Bernstein M, et al. Molar pregnancy in adolescents. J Reprod Med. 2012 May-Jun;57(5-6):225-30. [Abstract]
15. Soares RR, Maestá I, Colón J, et al. Complete molar pregnancy in adolescents from North and South America: Clinical presentation and risk of gestational trophoblastic neoplasia. Gynecol Oncol. 2016 Sep;142(3):496-500. [Abstract]
16. Zheng XZ, Qin XY, Chen SW, et al. Heterozygous/dispermic complete mole confers a significantly higher risk for post-molar gestational trophoblastic disease. Mod Pathol. 2020 Oct;33(10):1979-88. [Abstract] [Full Text]
17. Demond H, Anvar Z, Jahromi BN, et al. A KHDC3L mutation resulting in recurrent hydatidiform mole causes genome-wide DNA methylation loss in oocytes and persistent imprinting defects post-fertilisation. Genome Med. 2019 Dec 17;11(1):84. [Abstract] [Full Text]
18. Sanchez-Delgado M, Martin-Trujillo A, Tayama C, et al. Absence of maternal methylation in biparental hydatidiform moles from women with NLRP7 maternal-effect mutations reveals widespread placenta-specific imprinting. PLoS Genet. 2015 Nov;11(11):e1005644. [Abstract] [Full Text]
19. Lin LH, Maestá I, St Laurent JD, et al. Distinct microRNA profiles for complete hydatidiform moles at risk of malignant progression. Am J Obstet Gynecol. 2021 Apr;224(4):372.e1-372.e30. [Abstract]
20. Szulman AE, Surti U. The syndromes of hydatidiform mole. I. Cytogenetic and morphologic correlations. Am J Obstet Gynecol. 1978 Jul 15;131(6):665-71. [Abstract]
21. Szulman AE, Surti U. The syndromes of hydatidiform mole. II. Morphologic evaluation of the complete and partial mole. Am J Obstet Gynecol. 1978 Sep 1;132(1):20-7. [Abstract]
22. Soper JT. Gestational trophoblastic disease. Obstet Gynecol. 2006 Jul;108(1):176-87. [Abstract]
23. Berkowitz RS, Goldstein DP. Chorionic tumors. N Engl J Med. 1996 Dec 5;335(23):1740-8. [Abstract]
24. Braga A, Moraes V, Maestá I, et al. Changing trends in the clinical presentation and management of complete hydatidiform mole among Brazilian women. Int J Gynecol Cancer. 2016 Jun;26(5):984-90. [Abstract]
25. Sun SY, Melamed A, Goldstein DP, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia? Gynecol Oncol. 2015 Jul;138(1):46-9. [Abstract]
26. Ramos MM, Maesta I, de Araújo Costa RA, et al. Clinical characteristics and thyroid function in complete hydatidiform mole complicated by hyperthyroidism. Gynecol Oncol. 2022 Apr;165(1):137-42. [Abstract]
27. Berkowitz RS, Cramer DW, Bernstein MR, et al. Risk factors for complete molar pregnancy from a case-control study. Am J Obstet Gynecol. 1985 Aug 15;152(8):1016-20. [Abstract]
28. Parazzini F, La Vecchia C, Mangili G, et al. Dietary factors and risk of trophoblastic disease. Am J Obstet Gynecol. 1988 Jan;158(1):93-9. [Abstract]
29. Ferraz L, Ramos CAB, Braga A, et al. Association between antioxidant vitamins and oxidative stress among patients with a complete hydatidiform mole. Clinics (Sao Paulo). 2020;75:e1724. [Abstract] [Full Text]
30. Lok C, Frijstein M, van Trommel N. Clinical presentation and diagnosis of gestational trophoblastic disease. Best Pract Res Clin Obstet Gynaecol. 2021 Jul;74:42-52. [Abstract] [Full Text]
31. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 175: ultrasound in pregnancy. Dec 2016 [internet publication]. [Full Text]
32. Tranoulis A, Georgiou D, Sayasneh A, et al. Gestational trophoblastic neoplasia: a meta-analysis evaluating reproductive and obstetrical outcomes after administration of chemotherapy. Int J Gynecol Cancer. 2019 Jul;29(6):1021-31. [Abstract]
33. Beguin Y, Huebers HA, Josephson B, et al. Transferrin receptors in rat plasma. Proc Natl Acad Sci U S A. 1988 Jan;85(2):637-40. [Abstract] [Full Text]
34. Tidy, J, Seckl, M, Hancock, BW, on behalf of the Royal College of Obstetricians and Gynaecologists. Management of gestational trophoblastic disease. BJOG 2021;128: e1-e27. [Full Text]
35. Soto-Wright V, Bernstein M, Goldstein DP, et al. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995 Nov;86(5):775-9. [Abstract]
36. Sun SY, Melamed A, Joseph NT, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2016 Feb;26(2):367-70. [Abstract]
37. Lurain JR. Hydatidiform mole: recognition and management. Contemporary OB/GYN. 2019;64(3). [Full Text]
38. Yamazaki K, Sato K, Shizume K, et al. Potent thyrotropic activity of human chorionic gonadotropin variants in terms of 125I incorporation and de novo synthesized thyroid hormone release in human thyroid follicles. J Clin Endocrinol Metab. 1995 Feb;80(2):473-9. [Abstract]
39. Bhatia N, Mitharwal SM. Hydatidiform mole with uncontrolled hyperthyroidism: an anesthetic challenge. J Anaesthesiol Clin Pharmacol. 2016 Oct-Dec;32(4):537-8. [Abstract] [Full Text]
40. Norman RJ, Green-Thompson RW, Jialal I, et al. Hyperthyroidism in gestational trophoblastic neoplasia. Clin Endocrinol (Oxf). 1981 Oct;15(4):395-401. [Abstract]
41. Coyle C, Short D, Jackson L, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol. 2018 Feb;148(2):254-7. [Abstract]
42. Lok C, van Trommel N, Massuger L, et al. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020 May;130:228-40. [Abstract]
43. American College of Radiology. ACR-ACOG-AIUM-SRU practice parameter for the performance of obstetrical ultrasound. 2018 [internet publication]. [Full Text]
44. Matthews A, Haas DM, O'Mathúna DP, Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Syst Rev. 2015 Sep 08;(9):CD007575. [Full Text]
45. Coukos G, Makrigiannakis A, Chung J, et al. Complete hydatidiform mole. A disease with a changing profile. J Reprod Med. 1999 Aug;44(8):698-704. [Abstract]
46. American College of Radiology. ACR appropriateness criteria®: first trimester vaginal bleeding. 2017 [internet publication]. [Full Text]
47. Soper JT. Gestational trophoblastic disease: current evaluation and management. Obstet Gynecol. 2021 Feb 1;137(2):355-70. [Abstract] [Full Text]
48. Pereira JV, Lim T. Hyperthyroidism in gestational trophoblastic disease - a literature review. Thyroid Res. 2021 Jan 14;14(1):1. [Abstract] [Full Text]
49. American College of Obstetricians and Gynecologists. Practice bulletin no. 181: prevention of Rh D alloimmunization. Aug 2017 [internet publication]. [Full Text]
50. Padrón L, Rezende Filho J, Amim Junior J, et al. Manual compared with electric vacuum aspiration for treatment of molar pregnancy. Obstet Gynecol. 2018 Apr;131(4):652-9. [Abstract]
51. Faculty of Sexual & Reproductive Healthcare. FSRH GL on contraception after pregnancy. Jan 2017 [internet publication]. [Full Text]
52. Braga A, Maestá I, Short D, et al. Hormonal contraceptive use before hCG remission does not increase the risk of gestational trophoblastic neoplasia following complete hydatidiform mole: a historical database review. BJOG. 2016 Jul;123(8):1330-5. [Abstract] [Full Text]
53. Dantas PRS, Maestá I, Filho JR, et al. Does hormonal contraception during molar pregnancy follow-up influence the risk and clinical aggressiveness of gestational trophoblastic neoplasia after controlling for risk factors? Gynecol Oncol. 2017 Nov;147(2):364-70. [Abstract]
54. Zhao P, Lu Y, Huang W, et al. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: a systematic review and meta-analysis. BMC Cancer. 2019 Jan 7;19(1):13. [Abstract] [Full Text]
55. Braga A, Lima L, Parente RCM, et al. Management of symptomatic uterine arteriovenous malformations after gestational trophoblastic disease: the Brazilian experience and possible role for depot medroxyprogesterone acetate and tranexamic acid treatment. J Reprod Med. 2018; 63: 228-39. [Full Text]
56. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 222: gestational hypertension and preeclampsia. Jun 2020 [internet publication]. [Full Text]
57. National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. 25 Jun 2019 [internet publication]. [Full Text]
58. Medicines and Healthcare products Regulatory Agency. Magnesium sulfate: risk of skeletal adverse effects in the neonate following prolonged or repeated use in pregnancy. May 2019 [internet publication]. [Full Text]
59. Food and Drug Administration. Drug Safety Communication: FDA recommends against prolonged use of magnesium sulfate to stop pre-term labor due to bone changes in exposed babies. May 2013 [internet publication]. [Full Text]
60. Lin LH, Maestá I, Braga A, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: a retrospective multicenter cohort and literature review. Gynecol Oncol. 2017 Apr;145(1):88-95. [Abstract]
61. Wang Q, Fu J, Hu L, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2017 Sep 11;(9):CD007289. [Abstract] [Full Text]
62. UK Teratology Information Service. Official response statement: use of ondansetron in the first trimester of pregnancy. Sep 2019 [internet publication]. [Full Text]
63. Huybrechts KF, Hernández-Díaz S, Straub L, et al. Association of maternal first-trimester ondansetron use with cardiac malformations and oral clefts in offspring. JAMA. 2018 Dec 18;320(23):2429-37. [Abstract] [Full Text]
64. Medicines and Healthcare products Regulatory Agency. Ondansetron: small increased risk of oral clefts following use in the first 12 weeks of pregnancy. Jan 2020 [internet publication]. [Full Text]
65. Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018 Jul;13:291-310. [Abstract] [Full Text]
66. Bose S, Gothoskar BP, Coutinho WG, et al. Differential sensitivity of protein synthesis in human cell lines to actinomycin D. Exp Cell Res. 1967 Jun;46(3):599-603. [Abstract]
67. Horowitz NS, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: biology, natural history, and treatment modalities. Gynecol Oncol. 2017 Jan;144(1):208-14. [Abstract]
68. Kaur B, Short D, Fisher RA, et al. Atypical placental site nodule (APSN) and association with malignant gestational trophoblastic disease; a clinicopathologic study of 21 cases. Int J Gynecol Pathol. 2015 Mar;34(2):152-8. [Abstract]
69. Gilman Barber AR, Rhone SA, Fluker MR. Curettage and Asherman's syndrome-lessons to (re-) learn? J Obstet Gynaecol Can. 2014 Nov;36(11):997-1001. [Abstract] [Full Text]
70. Lee SH, Aggarwal BB, Rinderknecht E, et al. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083-6. [Abstract]
Sign in to access our clinical decision support tools
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.26(3); 2018 Aug
Ultrasound diagnosis of molar pregnancy
Introduction.
The primary aims of this study were to establish what proportion of ultrasonically suspected molar pregnancies were proven on histological examination and what proportion of histologically diagnosed molar pregnancies were identified by ultrasound pre-operatively. The secondary aim was to review the features of these scans to help identify criteria that may improve ultrasound diagnosis.
This was a retrospective observational study conducted in the Early Pregnancy Unit at King’s College Hospital London over an 11-year period. Cases of ultrasonically suspected molar pregnancy or other gestational trophoblastic disease were identified and compared with the final histopathological diagnosis. In addition, cases which were diagnosed on histopathology that were not suspected on ultrasound were also examined. In discrepant cases, the images were reviewed unblinded by two senior sonographers. Statistical analysis for likelihood ratio and post-test probabilities was performed.
One hundred eighty-two women had gestational trophoblastic disease suspected on ultrasound examination (1:360, 0.3%); 106/182 (58.2%, 95% CI 51.0 to 65.2%) had histologically confirmed gestational trophoblastic disease. The likelihood ratio for gestational trophoblastic disease after a positive ultrasound was 607.27, with a post-test probability of 0.628.The sensitivity of ultrasound for gestational trophoblastic disease was 70.7% (95% CI 62.9% to 77.4%) with an estimated specificity of 99.88% (95% CI 99.85% to 99.91%); 102/143 (71.3%, 95% CI 63.4 to 78.1%) molar pregnancies were suspected on pre-op ultrasound; 60/68 (88.2%, 95% CI 78.2 to 94.2%) of complete moles were suspected on pre-op ultrasound, compared with 42/75 (56.0%, 95% CI 44.7 to 66.7%) of partial moles. On retrospective review of the pre-op ultrasound images, there were cases that could have been suspected prior to surgery.
Detecting molar pregnancy by ultrasound remains a diagnostic challenge, particularly for partial moles. These data suggest that there has been an increase in both the predictive value and the sensitivity of ultrasound over time, with a high LR and post-test probability; however, the diagnostic criteria remain ill-defined and could be improved.
Gestational trophoblastic disease (GTD) comprises a group of disorders including complete (CM) and partial (PM) molar pregnancies, invasive moles, choriocarcinomas and placental site trophoblastic tumours. Molar pregnancies are the commonest and are categorised as complete or partial, occurring in 1:1000 and 3:1000 pregnancies in the UK, respectively. 1 The incidence of molar pregnancy is rising in the UK and Western Europe, in part due to an increasing number of women having pregnancies at a later age. 2
The typical clinical presentation of molar pregnancy includes vaginal bleeding, hyperemesis gravidarum, early embryonic demise, an enlarged uterus, early pre-eclampsia, hyperthyroidism and abdominal distension 3 . The characteristic ultrasound appearance of hydatidiform mole was first described by Donald in the 1960s as a ‘uterus full of dots’ or a ‘snowstorm’. 4 – 6 This traditional description is of the late features of the disease that are seen in the second trimester. Over the last 20 years in the UK, increasingly sensitive home pregnancy tests and Early Pregnancy Units (EPUs) equipped with transvaginal ultrasound have brought the clinical presentation forward to the first trimester, when the symptoms and ultrasound findings are more subtle.
Concurrently, there has been a move away from routine surgical treatment of miscarriage and increasing use of expectant and medical treatments with no histological examination of pregnancy tissue. Although a pregnancy test can be performed three weeks after a miscarriage to exclude persistent GTD, the lack of diagnosis denies women appropriate follow up in subsequent pregnancies. If a woman is known to have had a molar pregnancy, her follow-up is co-ordinated by our UK regional GTD units and she has an increased risk of a recurrent mole in future pregnancies, particularly after a CM. 7 Ultrasound identification of a possible molar pregnancy allows women to choose surgery over other management options allowing histopathological examination of pregnancy remains.
The primary aims of this study were to establish (a) what proportion of ultrasonically suspected molar pregnancies were proven on histological examination and (b) what proportion of histologically diagnosed molar pregnancies were identified by ultrasound pre-operatively. The secondary aim was to analyse the features of the pre-op scans to help identify criteria that may improve ultrasound diagnosis.
This was a retrospective observational study conducted in the EPU at King’s College Hospital London. Women accessed EPU as self-referred patients, referrals from general practitioners, midwives, fetal medicine unit or the emergency department. The EPU is not part of routine antenatal care, but is for women with clinical problems in the first trimester such as abdominal pain or vaginal bleeding. Clinical and ultrasound data were collected prospectively and stored electronically (ViewPoint, GE Healthcare). All patients had a clinical assessment and transvaginal pelvic ultrasound performed by Gynaecologist sonographers working in the EPU (Voluson E6 and/or E8 Expert, GE Healthcare). If the uterus was enlarged, this was supplemented by a transabdominal approach. The ultrasound criteria for suspecting molar pregnancy were cystic changes, irregularity, or increased echogenicity in the decidua, chorionic tissue or myometrium. 8 , 9 The ultrasound criteria for suspecting malignant GTD were a hypoechoic or heterogenous, predominantly solid tumour within the uterine cavity in the presence of a positive pregnancy test. 10 Patients with histopathologically diagnosed GTDs were identified using electronic patient records and Charing Cross Hospital Trophoblastic Disease Service records.
Inclusion criteria for the primary aims were an ultrasound scan in the first trimester with the diagnosis of a suspected molar pregnancy or other GTD, or histopathological diagnosis of trophoblastic disease confirmed at Charing Cross Hospital over an 11-year period, January 2005 to December 2015.
Unblinded, retrospective review of USS images was performed by two senior sonographers JR and JJ.
Statistical analysis for likelihood ratio and post-test probabilities was performed using University of California’s online calculators for scientific research ( http://www.sample-size.net/post-probability-calculator-test-new/ accessed 28/03/2017). The study protocol was approved by the local Research & Development team.
There were a total of 65,536 pregnancies during the study period of which 182 had suspected GTD on ultrasound examination (1:360, 0.3%); 106/182 (58.2%, 95% CI 51.0 to 65.2%) had histologically confirmed GTD, including a patient with a pregnancy that was unclassifiable histologically, thought to be most likely to be a non-molar pregnancy, but as an atypical mole could not be excluded, she was followed up as per the molar pregnancy protocol; 70/182 (38.5%, 95% CI 31.7 to 45.7%) were non molar miscarriages on histological examination, 2/182 had ongoing pregnancies in which the placental or decidual cysts resolved by the end of the first trimester and they delivered normal babies at term, 2/182 miscarried spontaneously with no tissue available for histology and 2/182 patients had their surgery in the private sector with no histology results available locally.
There were 44 cases of GTD diagnosed histologically with no documented suspicion of the diagnosis on the pre-operative ultrasound. One of these patients presented with abnormal vaginal bleeding at the age of 54 years, was not known to have a positive urinary pregnancy test and the diagnosis of choriocarcinoma was made by outpatient endometrial sampling. Another had a partial molar tubal ectopic pregnancy. Details of the histological subtypes of GTD are shown in Table 1 . Assuming the approximation that there was no additional GTD in patients with negative scans who did not have histological tissue for analysis, the sensitivity of ultrasound was 70.7% (95% CI 62.9% to 77.4%) with a specificity of 99.88% (95% CI 99.85% to 99.91%). The likelihood ratio for GTD after a positive ultrasound was 607.27, with a post-test probability of 0.628. Considering molar pregnancies alone, 60/68 (88.2%, 95% CI 78.2 to 94.2%) of complete moles (CM) were suspected on ultrasound preoperatively, compared with 42/75 (56.0%, 95% CI 44.7 to 66.7%) of partial moles (PM). Overall, 102/143 (71.3%, 95% CI 63.4 to 78.1%) molar pregnancies were suspected on pre-op ultrasound.
Histological subtypes of gestational trophoblastic disease 2005–2015 inclusive
| Complete mole | Partial mole | Invasive mole | Choriocarcinoma | Placental site tumour | Unclassifiable | |
|---|---|---|---|---|---|---|
| Suspected on USS ( = 106) | 60 | 42 | 0 | 2 | 1 | 1 |
| Unsuspected on USS ( = 44) | 8 | 33 | 1 | 1 | 1 | 0 |
| total ( = 150) | 68 | 75 | 1 | 3 | 2 | 1 |
We looked back at examples of the ultrasound images of six of the eight patients with false negative ultrasound scans who had complete moles ( Figure 1 ). Two patients only had scans in the fetal medicine unit and their ultrasound images were not available for review. The cases shown in Figure 1(a) and ( (b) b ) demonstrated cystic changes in the chorionic tissue typical of molar pregnancies. In case 1(a), the Gynaecologist who performed the scan commented that tissue should be sent for histological examination, but was not explicit in stating that this was to check for GTD. Figure 1(c) to ( (f) f ) shows more subtle changes; 1(c) shows small cysts in the chorionic tissue and a relatively high proportion of trophoblast for a small gestational sac. 1(d) and (e) shows abundant chorionic tissue with loss of the normal sac-like architecture. Figure 1(f) showed a small irregular gestational sac only and we were unable to see any features that could indicate a complete mole.

Missed complete moles (false negative ultrasound scans).
There were 33 cases of partial molar pregnancies that were not recorded as having been suspected on pre-operative ultrasound. One of these was the tubal mole. On pre-op ultrasound, this was a 3 cm, predominantly solid ectopic pregnancy. The trophoblast appeared echogenic, but otherwise it was unremarkable ( Figure 2 ). Thirteen cases of PM were referred from the Fetal Medicine Unit and there were six of these with no images available to review. Of the remaining 26 cases, reviewing the images retrospectively and independently, 8/26 had USS features that could have indicated a partial mole ( Figure 3 ). However, the reviewers disagreed in six cases (k = 0.115) indicating a generally poor strength of agreement.

Tubal ectopic partial molar pregnancy.

Missed partial molar pregnancies (false negative ultrasound scans).
This study has shown that just over half of the pregnancies, we suspect to be molar on ultrasound are proven to be so, and that we are able to detect a higher proportion of molar pregnancies by pre-operative ultrasound than previously reported in the literature.
An overview of previous studies showed that 533/1210 (44%) of molar pregnancies were suspected on USS pre operatively, with the US sensitivity for CM moles being much higher than for PM ( Table 2 ). The overall increase in ascertainment in the current study was due to a lower proportion of PM in our population compared with other studies. This may reflect an increasing use of non-surgical treatment of miscarriage over time, but our data were fairly consistent year on year. We treat approximately 20% non-surgically, which may be higher than in some other units – and we do not routinely try to collect tissue from non-surgically managed miscarriages for histopathological examination. This means that unsuspected cases of PM may have been missed as they were treated non-surgically. It may also reflect the fact that we have an older EPU population than in some of the other studies, as CM shows a more pronounced increase with age, 14 but these data were not available for comparison.
Overview of studies reporting USS detection of molar pregnancies.
| Study | CM | CM suspected on US | PM | PM suspected on US |
|---|---|---|---|---|
| Lazarus 1999 | 21 | 57% | – | – |
| Sebire 2001 | 64 | 58% | 91 | 17% |
| Johns 2005 | 11 | 90% | 33 | 49% |
| Fowler 2006 | 253 | 79% | 606 | 29% |
| Kirk 2007 | 20 | 95% | 41 | 20% |
| Savage 2017 | 22 | 86% | 48 | 42% |
| Ross 2017 (current study) | 68 | 88% | 75 | 56% |
| Total | 459 | 78% | 894 | 31% |
Complete mole (CM); Partial mole (PM); Ultrasound (US)
Since modern transvaginal ultrasound has been used routinely for the assessment of early pregnancies, the proportion of molar pregnancies suspected preoperatively has risen. 11 – 13 Older studies from the UK relied upon registration data provided by local hospitals, and with paper-based clinical notes and results, the documentation of preoperative ultrasound was likely to have been less reliable than with contemporary electronic systems, so this may also account for some of the increase.
One of the strengths of our study was that we were able to identify and follow-up pregnancies that were thought could be molar on ultrasound and establish whether the diagnosis was proven on histology so as to assess the value of a positive scan. This is important for sonographers and clinicians so that we can counsel our patients regarding the odds of molar pregnancy before they choose the treatment of their miscarriage. Other than Kirk et al. who found a positive predictive value of 48% for the diagnosis of molar pregnancy, previous studies have only looked at cases where the diagnosis of a molar pregnancy was made histologically to give an estimate of sensitivity. It would be interesting to see whether our data are replicated in other units with a different clinical set ups, staffing and degrees of supervision, to see whether this pattern of diagnosis is consistent across modern practice.
Our study was limited by the retrospective analysis of data. We assumed that all pregnancies that were thought to be molar were explicitly stated as such in the ultrasound reports. It is possible that our Gynaecologist sonographers may have recommended surgical management of miscarriage, but not made it expressly clear in the report that this was because they suspected an underlying molar pregnancy and wanted the remains to be examined histologically. We also had to assume that there was no additional GTD in patients with negative scans who did not have histological tissue for analysis. This was likely to be the case for malignant or invasive GTD, but it is quite possible that there were some cases of molar pregnancy that resolved with expectant or medical management of miscarriage without ever being suspected or detected. Without histopathological examination of all miscarriage tissue, the true false negative rate of ultrasound is impossible to gauge.
Can we improve ultrasound detection of molar pregnancy? We have no diagnostic criteria that have been subject to testing for accuracy or reproducibility. Savage et al. 13 retrospectively examined USS images of proven moles in an to attempt to grade the cystic changes in the placenta and vascularity; they found that PM were more likely to have recognisable embryonic and extraembryonic structures, were more vascular and less likely to consist of cystic placental tissue with no recognisable sac. In their study, hCG did not appear to help to distinguish the two. Johns et al. 11 showed that there may be a role for hCG, but it is more likely to be raised in CM than PM, which is easier to diagnose on ultrasound anyway. Our retrospective, unblinded review of images showed that there were some cases of CM that could have been suspected by more experienced sonographers on USS prior to surgery, due to abundant chorionic tissue with loss of the normal architecture of the gestational sac, but that the main diagnostic difficulty is in distinguishing PM from uncomplicated first trimester miscarriage (i.e. early embryonic demise). Without a prospective study using predefined assessment criteria, the diagnostic criteria will never be rigorously assessed.
Do we need to improve ultrasound detection of molar pregnancy, particularly PM? Will it alter how the miscarriage is managed? There is an ongoing debate in the UK about the financial cost and value of histological examination of the tissue obtained from surgical treatment of miscarriage. 15 What is the value of knowing the diagnosis of PM when it is easy to do a urinary pregnancy test after a miscarriage to check for the rare cases of persistent GTD? In the UK, it is no longer advised that women wait six months before conceiving again after a PM, so delaying a pregnancy is no longer a potential reason to check histology, and the risk of a CM after a PM is 0.1%, as recurrent CM is almost exclusively a problem of CM. 7 It may be that knowledge of an underlying PM needlessly increases women’s anxiety in future pregnancies, when the risk of recurrence is very low. Making the diagnosis could also have the opposite effect, reducing anxiety; however, there is no data available from which to draw a conclusion as to whether there is any psychological benefit of knowing the diagnosis.
Detecting molar pregnancy by ultrasound remains a diagnostic challenge, particularly for PM. These data suggest that there has been an increase in both the predictive value and the sensitivity of ultrasound over time; however, the diagnostic criteria remain ill defined. Awareness of the possibility of molar pregnancy prior to management of miscarriage will guide treatment and allow appropriate follow-up. The recent increase in non-surgical management of miscarriage may result in missed cases but this may well be almost exclusively in PM where the value of a diagnosis is less clear.
Acknowledgements
The authors would like to thank doctors Laura Ferrara, Heena Mehra and Anna Graham who helped in data acquisition for the study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributors
The authors were jointly responsible for the study design, data acquisition & validation, drafting and approval of the manuscript for publication.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search

The changing clinical presentation of complete molar pregnancy
Affiliation.
- 1 New England Trophoblastic Disease Center, Department of Obstetrics and Gynecology, Brigham and Women's Hospital, Boston, Massachusetts, USA.
- PMID: 7566847
- DOI: 10.1016/0029-7844(95)00268-V
Objective: To determine if the clinical presentation of complete hydatidiform mole has changed in recent years compared with historic controls (1965-1975).
Methods: Chart review of all 74 patients referred to the New England Trophoblastic Disease Center for the primary management of complete hydatidiform mole during 1988-1993 was performed and comparison made to historic controls (1965-1975).
Results: Vaginal bleeding remained the most common presenting symptom, occurring in 62 of 74 (84%) current patients, compared with 297 of 306 (97%) controls (P = .001). However, anemia was present in only four of 74 (5%) current patients, compared with 165 of 306 (54%) controls (P = .001). Excessive uterine size, preeclampsia, and hyperemesis occurred in only 21 of 74 (28%), one of 74 (1.3%), and six of 74 (8%) current patients, respectively, compared with 156 of 306 (51%), 83 of 306 (27%), and 80 of 306 (26%), respectively, of historic controls (P = .001). No cases of clinical hyperthyroidism or respiratory distress were found in recent years. Ultrasound diagnosed complete hydatidiform mole before the onset of clinical symptoms in seven of 69 (10%) current patients. Among patients not receiving chemoprophylaxis, persistent gestational trophoblastic tumor developed in 23% of current patients and 18.6% of historic controls.
Conclusion: Fewer current patients with complete hydatidiform mole present with the traditional symptoms of complete hydatidiform mole (excessive uterine size, anemia, preeclampsia, hyperthyroidism, or hyperemesis) when compared with historic controls. However, there has been no statistically significant change in the development of persistent gestational trophoblastic tumor in current patients compared with historic controls.
PubMed Disclaimer
Similar articles
- Changes of clinical features in hydatidiform mole: analysis of 113 cases. Hou JL, Wan XR, Xiang Y, Qi QW, Yang XY. Hou JL, et al. J Reprod Med. 2008 Aug;53(8):629-33. J Reprod Med. 2008. PMID: 18773629
- Clinical presentation of hydatidiform mole in northern Italy: has it changed in the last 20 years? Mangili G, Garavaglia E, Cavoretto P, Gentile C, Scarfone G, Rabaiotti E. Mangili G, et al. Am J Obstet Gynecol. 2008 Mar;198(3):302.e1-4. doi: 10.1016/j.ajog.2007.09.036. Epub 2008 Feb 21. Am J Obstet Gynecol. 2008. PMID: 18177836 Review.
- Persistent trophoblast disease following partial molar pregnancy. Wielsma S, Kerkmeijer L, Bekkers R, Pyman J, Tan J, Quinn M. Wielsma S, et al. Aust N Z J Obstet Gynaecol. 2006 Apr;46(2):119-23. doi: 10.1111/j.1479-828X.2006.00539.x. Aust N Z J Obstet Gynaecol. 2006. PMID: 16638033
- Complete molar pregnancy. Clinical trends at King Fahad Hospital, Riyadh, Kingdom of Saudi Arabia. Felemban AA, Bakri YN, Alkharif HA, Altuwaijri SM, Shalhoub J, Berkowitz RS. Felemban AA, et al. J Reprod Med. 1998 Jan;43(1):11-3. J Reprod Med. 1998. PMID: 9475143
- Natural history of twin pregnancy with complete hydatidiform mole and coexisting fetus. Steller MA, Genest DR, Bernstein MR, Lage JM, Goldstein DP, Berkowitz RS. Steller MA, et al. Obstet Gynecol. 1994 Jan;83(1):35-42. Obstet Gynecol. 1994. PMID: 8272304 Review.
- Changing Trends in the Clinical Presentation and Incidence of Molar Pregnancy in Saudi Arabia: A 30-Year Retrospective Analysis. Altalib A, Al Qahtani N, Alosaimi SS, Al Hashem MS, Almowallad R, Al-Rufiei M, Alhumaid LI. Altalib A, et al. Cureus. 2023 Dec 22;15(12):e50936. doi: 10.7759/cureus.50936. eCollection 2023 Dec. Cureus. 2023. PMID: 38259393 Free PMC article.
- High placental expression of FLT1, LEP, PHYHIP and IL3RA - In persons of African ancestry with severe preeclampsia. Aisagbonhi O, Bui T, Nasamran CA, St Louis H, Pizzo D, Meads M, Mulholland M, Magallanes C, Lamale-Smith L, Laurent LC, Morey R, Jacobs MB, Fisch KM, Horii M. Aisagbonhi O, et al. Placenta. 2023 Dec;144:13-22. doi: 10.1016/j.placenta.2023.10.008. Epub 2023 Oct 20. Placenta. 2023. PMID: 37949031 Free PMC article.
- Partial molar pregnancy with hydrops fetalis causing intrauterine fetal demise: A case report. Panthi A, Bhattarai M, Katwal S, Bhandari S, Baral R, Bhusal M, Khaniya B. Panthi A, et al. Clin Case Rep. 2023 Sep 30;11(10):e8006. doi: 10.1002/ccr3.8006. eCollection 2023 Oct. Clin Case Rep. 2023. PMID: 37786454 Free PMC article.
- A case report of atypical preeclampsia with severity criteria for hydatidiform complete mole. Pérez-Nieto OR, Herrera-Venegas CA, Pozos-Cortés KP, Flores-Ramírez R, Ugalde-Real JS, Argüello-Bolaños J, Puente MEP, Zamarrón-López ÉI, Deloya-Tomas E. Pérez-Nieto OR, et al. Clin Case Rep. 2023 Jun 7;11(6):e7470. doi: 10.1002/ccr3.7470. eCollection 2023 Jun. Clin Case Rep. 2023. PMID: 37305892 Free PMC article.
- Histopathologic and Transcriptomic Profiling Identifies Novel Trophoblast Defects in Patients With Preeclampsia and Maternal Vascular Malperfusion. Horii M, To C, Morey R, Jacobs MB, Li Y, Nelson KK, Meads M, Siegel BA, Pizzo D, Adami R, Zhang-Rutledge K, Lamale-Smith L, Laurent LC, Parast MM. Horii M, et al. Mod Pathol. 2023 Feb;36(2):100035. doi: 10.1016/j.modpat.2022.100035. Epub 2023 Jan 10. Mod Pathol. 2023. PMID: 36853788 Free PMC article.
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Elsevier Science
- Ovid Technologies, Inc.
- Wolters Kluwer
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 26 August 2024
Pregnancy complications and autoimmune diseases in women: systematic review and meta-analysis
- Megha Singh ORCID: orcid.org/0000-0003-3680-7124 1 ,
- Fathima Fazla Ahamed Fayaz ORCID: orcid.org/0000-0003-0788-6492 1 ,
- Jingya Wang ORCID: orcid.org/0000-0003-1498-2693 1 ,
- Steven Wambua ORCID: orcid.org/0000-0003-2300-7670 1 ,
- Anuradha Subramanian ORCID: orcid.org/0000-0001-8875-7363 1 ,
- John A. Reynolds ORCID: orcid.org/0000-0002-8962-4404 2 ,
- Krishnarajah Nirantharakumar ORCID: orcid.org/0000-0002-6816-1279 1 &
- Francesca Crowe ORCID: orcid.org/0000-0003-4026-1726 1
on behalf of MuM-PreDiCT
BMC Medicine volume 22 , Article number: 339 ( 2024 ) Cite this article
Metrics details
Pregnancy complications might lead to the development of autoimmune diseases in women. This review aims to summarise studies evaluating the association between pregnancy complications and the development of autoimmune diseases in women.
Medline, CINAHL, and Cochrane databases were searched up to January 2024. Nineteen pregnancy complications and 15 autoimmune conditions were included. Title, abstract, full-text screening, data extraction, and quality assessment were performed by two reviewers independently. Data were synthesised using narrative and quantitative methods. Results were presented using odds ratios (OR), relative risks (RR), incidence rate ratios (IRR), and 95% confidence intervals (CI).
Thirty studies were included. One study reported composite exposure to pregnancy complications had a risk of any autoimmune disease RR 3.20 (2.90–3.51) compared to women without pregnancy complications. Women with hyperemesis gravidarum had a higher risk of developing coeliac disease ( n = 1) IRR 1.98 (1.27–2.94), Crohn’s disease ( n = 1) IRR 1.61 (1.25–2.04), psoriasis ( n = 1) IRR 1.33 (1.01–1.71), and rheumatoid arthritis ( n = 2) IRR 1.35 (1.09–1.64). Miscarriage associated with subsequent diagnosis of Sjogren syndrome ( n = 2) IRR 1.33 (1.06–2.81) and rheumatoid arthritis ( n = 4) OR 1.11 (1.04–1.20). Gestational hypertension/preeclampsia was linked with the development of systemic sclerosis ( n = 2) IRR 2.60 (1.10–4.60) and T1DM ( n = 2) IRR 2.37 (2.09–2.68). Stillbirth associated with composite autoimmune conditions ( n = 2) RR 5.82 (95% CI 4.87–6.81) and aIRR 1.25 (1.12–1.40). Postpartum psychosis was associated with autoimmune thyroid disease ( n = 1) aIRR2.26 (1.61–2.90).
Conclusions
Women with pregnancy complications subsequently had a higher risk of being diagnosed with autoimmune conditions. Whether this is due to pre-existing undiagnosed health conditions or being causally linked to pregnancy complications is not known.
Peer Review reports
What is already known about this subject?
The prevalence of autoimmune conditions and pregnancy complications has increased globally.
Women with pregnancy complications are at higher risk of cardiometabolic conditions in later life.
What does this study add?
This systematic review consolidates evidence from studies which have studied the association of pregnancy complications and the later development of autoimmune diseases in women.
This review provides new knowledge to help establish the association of pregnancy complications and autoimmune diseases and identifies the need for further research to establish the true association between few conditions like the development of SLE or rheumatoid arthritis followed by miscarriage.
How might this impact on clinical practice?
This study will be useful for health professionals and policymakers to navigate the research findings and identify the need for clinical guidelines beyond postnatal care for women with pregnancy complications.
The prevalence of autoimmune diseases has been increasing globally over the last decade [ 1 ], and in the UK, 1 in 10 people have an autoimmune disease [ 1 , 2 , 3 ]. The majority of autoimmune diseases are more common in women than men [ 4 ] and are a leading cause of death in women between the age of 65 and 75 in the US and UK [ 5 , 6 ]. Although the aetiology of autoimmunity is still not fully understood, the increased prevalence of autoimmune disease has been linked to defective X chromosome inactivation [ 7 , 8 ] and the effects of female hormones [ 9 ].
During pregnancy, there are significant fluctuations in hormone levels and increased physiological stress. Women with pre-existing autoimmune diseases may experience flare-ups or a decrease in their symptoms. For example, rheumatoid arthritis, Grave’s disease, or psoriasis may improve during pregnancy [ 10 , 11 , 12 ], whilst patients with systemic lupus erythematosus (SLE) or multiple sclerosis are at an increased risk of disease exacerbations [ 13 , 14 ]. With an increasing trend in pregnancy complications due to factors such as older age at pregnancy and women entering pregnancy with pre-existing long-term health conditions [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ], it is important to study the role of pregnancy complications in the development of autoimmune diseases. Whilst it is well-established that women with autoimmune diseases have an increased risk of fertility problems and adverse pregnancy outcomes such as miscarriage and foetal growth restriction [ 24 , 25 , 26 , 27 , 28 , 29 , 30 ], less is known about the risk of developing autoimmunity in women who experience pregnancy complications [ 31 ]. Some studies have shown an association between parity and increased risk of Hashimoto thyroiditis, Sjögren’s syndrome, Graves’ disease, and rheumatoid arthritis [ 32 , 33 ]. Moreover, the association between gestational diabetes mellitus (GDM) and the development of type 1 diabetes (T1DM) is well established [ 34 ]. Some pregnancy complications such as hyperemesis gravidarum and gestational hypertension have been associated with the development of rheumatoid arthritis [ 35 , 36 ], whilst other studies have reported that pregnancy loss and gestational hypertension are associated with the development of SLE and systemic sclerosis [ 37 , 38 ]. But other studies conducted on these associations have reported contradictory/inconsistent findings [ 39 , 40 ].
This systematic review aims to determine the association between a wide range of pregnancy complications and the development of autoimmune diseases in women.
This systematic review and meta-analysis have been conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Reporting Guidelines for Meta-analyses of Observational Studies (MOOSE) (Additional file 1 table 2 and Additional file 2 table 1) [ 41 ]. The protocol for this review was registered with Prospero CRD42023412549.
Inclusion and exclusion criteria
Cohort, cross-sectional, or case-control studies reporting on the associations between pregnancy complications and the risk of autoimmune diseases were included. No language restrictions were applied. The population considered were pregnant women without any age restriction. The pregnancy complications (19) and autoimmune diseases (15) selected for inclusion were those that were more common in women, after consultation with experts in the subject (obstetricians, obstetric physicians, rheumatologists, and epidemiologists), and after input from patient and public involvement and engagement (PPIE) group members.
The pregnancy complications included are listed in Table 1 and autoimmune disease in Table 2 .
Search strategy
Medline, CINAHL, and Cochrane Library were searched for studies from 2010 till January 2024.
The search strategies used pre-defined keywords of the exposures (pregnancy complications) and outcomes (autoimmune disease). Terms/keywords for each of the pregnancy complications (early adj3 pregnancy loss*, mp.miscarriage.mp, GDM) and autoimmune diseases (for example arthritis, rheumatoid systemic lupus, or SLE) were used in the search strategy. Google Scholar was searched to identify other grey literature. In addition, the reference list of the included studies, systematic reviews, and scoping reviews were searched manually to minimise the possibility of missing any relevant studies. Letters, commentaries, or editorials were excluded and studies that did not involve humans were also excluded. The searches were repeated periodically to identify newly published studies. The detailed search strategy for Medline is presented in the Additional file 1 table 1 (Table 1 ). This search strategy was adapted for use in other databases (CINAHL and Cochrane library). Pragmatic approach was taken given there was a substantial number of studies that needed screening ( n = 24,340) and the study period was limited from 2010 to 2023 ( n = 13,234). But this was complimented by a secondary search strategy looking at references of included studies, systematic reviews, scoping reviews, and by discussing with topic experts (KN, FC) to minimise the possibility of missing any study before 2010.
Study selection
EndNote reference manager [ 42 ] was used for the title and abstract screening by two researchers independently (MS, JW). Full text of the eligible reviews was screened by two researchers independently (MS, FF). Covidence software [ 43 ] was used for full-text screening and data extraction. A third senior researcher was consulted to resolve any discrepancies in the selection of the studies (FC, KN).
Data extraction
Two reviewers extracted data from the included studies. The data extraction form was adapted from JBI (Joanna Briggs Institute) data extraction form [ 44 ]. A standardised data extraction form was used and was piloted before use. The data were extracted for the following fields: author/s, year of publication, geographical area, aim of the study, population, exposures, comparator, outcomes, covariates, study design, definition of exposure, risk of bias assessment tool and result, number of participants included in the study, summary estimates, authors’ conclusion, and study limitations. The data extraction form is enclosed in Additional file Table 6.
Quality assessment
The quality of included cohort, cross-sectional, and case-control studies was assessed using the Newcastle–Ottawa scale that measures study quality based on selection, comparability of the exposure and comparator groups, and the ascertainment of outcomes and exposures [ 45 ]. The scale has an overall score of 8 points for cohort or case–control studies, and 7 for cross-sectional studies with a maximum of 1 point for each numbered item within the selection and outcome/exposure categories and a maximum of 2 points for the comparability category. We defined studies with a score of ≥ 7 points as low-risk of bias studies (very good), studies with a score of 6 points as moderate-risk of bias studies (good), and those with a score of ≤ 5 points as high-risk of bias studies (satisfactory).
Data synthesis
The effect estimates were reported as adjusted incidence rate ratios (aIRR), adjusted hazards ratios (aHR), adjusted odds ratios (aOR), or adjusted relative risks (aRR) and 95% confidence intervals (CI). We converted these effect estimates using appropriate methods (where possible) to maintain uniformity across studies [ 46 ]. Where more than one study reported the same exposure and outcome, a meta-analysis was conducted using a random effects model to generate a summary estimate. Statistical heterogeneity was estimated using the I 2 statistic. To deal with potentially missing data (sample size, number exposed and unexposed), Additional file 1 of each included study was checked thoroughly, and the authors of the studies were contacted to request the data. If the data was not available and a meta-analysis could not be conducted, then effect estimates were reported as they were published. Where statistical pooling was not possible, the findings were presented in a narrative form including tables and figures to aid data presentation. R (3.3.0) and R Studio (12.1) were used to conduct statistical analysis [ 47 , 48 , 49 ].
Patient and public involvement
Patient and public involvement and engagement (PPIE) representatives (RP and NM) participated in formulating the research question. They have also played key role in collaboration with clinicians and researchers to identify and consider the list of pregnancy complications and autoimmune diseases in the study. They will play a key role in disseminating the results.
Out of the 13,234 records identified from the search and after full-text screening of 85 studies, 30 studies were included [ 31 , 34 , 35 , 36 , 39 , 40 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 ]. Studies were excluded if they did not qualify in the study inclusion criteria based on the study design ( n = 22), population ( n = 5), intervention (2), outcome (23), and comparator(3). The selection process is shown in the PRISMA diagram (Fig. 1 ) [ 41 ]. The list of excluded studies is provided in Additional file 1 table 3.
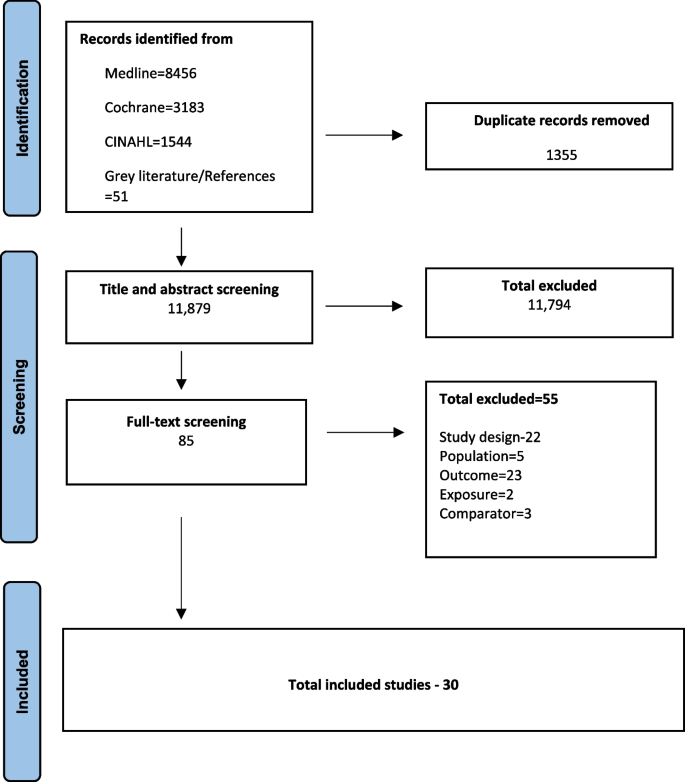
Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram
Characteristics of the included studies are reported in Table 3 . Out of the 30 studies, the majority were prospective cohort studies ( n = 21), 8 were retrospective case–control studies, and 1 was a cross-sectional study. There were 23 studies conducted in Europe, the remainder were in Taiwan (2), China (2), South Korea (1), and the United States (2). In most cohort studies ( n = 14), information about the pregnancy complications and autoimmune diseases was collected through medical records. Medical records were used to establish the autoimmune diseases in case–control studies, and questionnaires were used to determine the pregnancy complications. The follow-up period of the cohort studies varied from 9 months to 26 years with a median of 12 years. Out of the 21 cohort studies, 9 used the data from the same cohort (Danish national registry) [ 31 , 36 , 39 , 53 , 54 , 63 , 64 , 69 ]. In the instance of two studies reporting the same exposure and outcome, the most recent study was used to avoid duplication. We have done this in accordance with the Cochrane handbook for systematic reviews [ 74 ]. For instance, two studies, Mikkelsen et al. [ 63 ] and Nielsen et al. [ 64 ], were both reporting the association of pregnancy complications and the future development of multiple sclerosis in women using Danish Civil Registration System. Mikkelsen et al.’s study was used to report the findings in this review. Details have been added in Additional file tables 1 and 5 [ 75 , 76 ]. A total of 18 different pregnancy complications and 12 autoimmune diseases (including an overall “all autoimmune diseases”) were investigated across the studies. Rheumatoid arthritis (8 studies) and SLE (5 studies) were two of the most included outcomes. The meta-analysis performed in this systematic review is included in Additional file 1 figure 1.1–1.11.
Results of the quality assessment of the studies using the Newcastle–Ottawa scale are shown in Fig. 2 and Additional file 1 Table 4.1–4.3 [ 77 ]. Eighteen out of 30 studies had a low risk of bias with an overall “very good” rating. The principal areas of concern were the comparability of cohorts and the adequacy of follow-up.
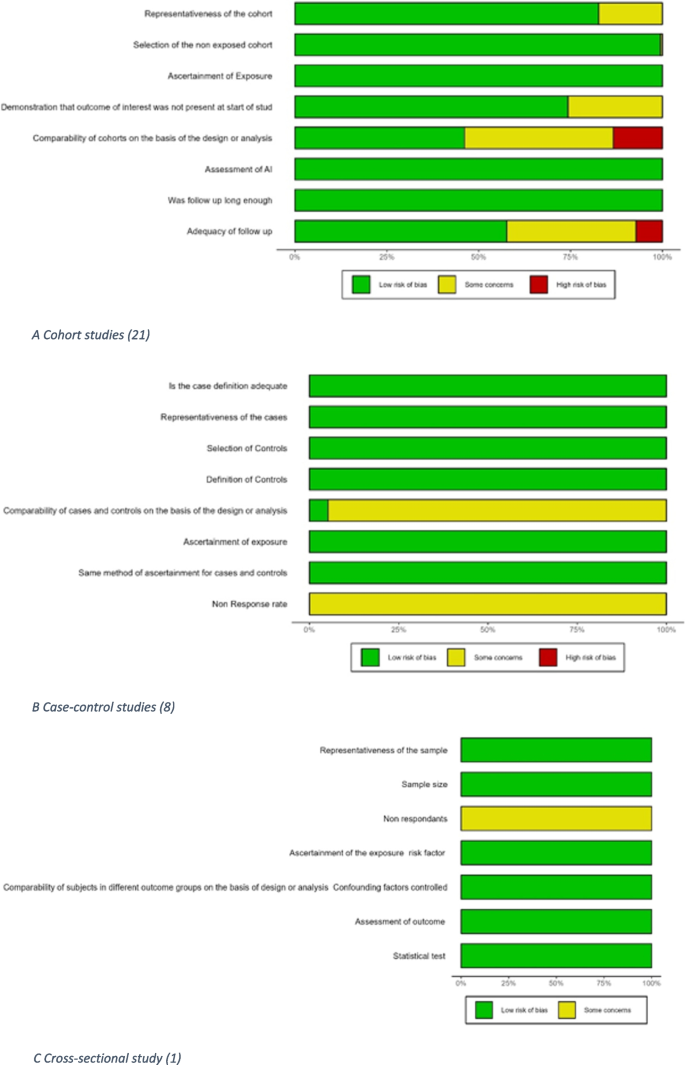
Quality assessment of included studies (Newcastle–Ottawa scale). A Cohort studies (21). B Case–control studies (8). C Cross-sectional study (1)
All autoimmune diseases
There was more than a threefold higher risk of developing autoimmune diseases ( n = 7) in women with pregnancy complications ( n = 6) RR 3.20 (95% CI 2.90–3.51) when compared with women without pregnancy complications [ 58 ]. Out of the pregnancy complications studied individually, two studies reported, women with previous miscarriage RR 3.41 (3.03–3.85) and aIRR 1.10 (1.07–1.14) was reported to have higher risk [ 58 ]. One cohort and one case–control study reported a higher risk of autoimmune diseases in women with gestational hypertension or pre-eclampsia; RR 2.05 (1.70–2.48) and aIRR 1.21 (1.16–1.26) [ 54 , 58 ], respectively. Women with stillbirth were reported to have higher chances to have autoimmune disease in later life reported by two studies RR 5.82 (4.97–6.81) [ 58 ] and aIRR 1.25 (1.12–1.40) [ 54 ]. There was a significantly higher risk of developing autoimmune diseases for women with preterm birth RR 2.35 (1.89–2.92) [ 58 ]. There was little association reported with caesarean section, induced abortion, or postpartum depression with the development of autoimmune diseases [ 31 , 54 , 61 ]. However, a study reported a higher risk of developing autoimmune diseases in women with perinatal depression aHR 1.52 (1.46–1.58), with antenatal depression aHR 1.50 (1.43–1.58), and postpartum depression aHR 1.55 (1.45–1.65) [ 73 ].
Autoimmune thyroid diseases
Hyperemesis gravidarum aIRR 1.49 (1.28–1.72), gestational hypertension or pre-eclampsia aIRR 1.20 (1.10–1.30), and postpartum depression aHR 1.57 (1.05–2.33) [ 54 , 60 ] were all associated with a higher risk of Grave’s disease but there was no significant association between ectopic pregnancy and Grave’s disease aIRR 1.04 (0.93–1.17) [ 54 ]. Gestational hypertension/pre-eclampsia aIRR 1.41 (1.17–1.68) was associated with a higher risk of Hashimoto’s thyroiditis but there was little association of hyperemesis gravidarum or ectopic pregnancy with Hashimoto’s thyroiditis; aIRR 1.38 (0.95–1.92) and aIRR 0.92 (0.68–1.21), respectively [ 54 ]. Two cohort studies reported a greater risk of autoimmune thyroid disease in women with postpartum psychosis aOR 2.78 (1.08–7.17) at 9 months and aIRR 2.26 (1.61–2.90) with 2 years follow-up postpartum when compared with women without postpartum psychosis [ 50 , 51 ].
Coeliac disease
Women who experienced hyperemesis gravidarum had almost a twofold risk of coeliac disease compared to women without; aIRR 1.98 (1.27–2.94) [ 54 ]. None of the other pregnancy complications were significantly associated with coeliac disease; ectopic pregnancy aIRR 1.12 (0.75–1.61), gestational hypertension, pre-eclampsia aIRR 1.19 (0.89–1.56), and intrahepatic cholestasis of pregnancy aHR 1.20 (0.82–1.74) [ 54 , 72 ].
Inflammatory bowel disease (Crohn’s disease and ulcerative colitis)
Out of the five pregnancy complications reported for IBD (hyperemesis gravidarum, missed abortion, gestational hypertension, pre-eclampsia, and Caesarean section), none of these associations were statistically significant. However, studies reporting ulcerative colitis and Crohn’s disease separately found significant associations. Hyperemesis was significantly associated with the development of both ulcerative colitis and Crohn’s disease, aIRR 1.34 (1.09–1.62) and aIRR 1.61 (1.25–2.04), respectively [ 54 ]. Furthermore, a higher risk of Crohn’s disease was also observed in women with intrahepatic cholestasis of pregnancy, HR 1.55 (1.14–2.10) [ 72 ]. No other pregnancy complications were associated with the development of IBD as reported in Fig. 4 .
Ankylosing spondylitis
Out of the three pregnancy complications studied with the development of ankylosing spondylitis in women, there was no significant association for hyperemesis gravidarum IRR 1.63 (0.96–2.25) or ectopic pregnancy IRR 1.02 (0.66–1.50); a significant association was noted with gestational hypertension and pre-eclampsia IRR 1.40 (1.06–1.82) [ 54 ] (Fig. 5 ).
Rheumatoid arthritis
Out of the five studies reporting the association of miscarriage and rheumatoid arthritis, four were meta-analysed to estimate 11% higher odds: pooled OR 1.11 (1.04–1.20) with the other study showing a slightly elevated risk that was not statistically significant aIRR 1.06 (0.97–1.15) [ 35 , 36 , 54 , 57 , 66 , 67 ]. A significant association was also reported with hyperemesis, gestational hypertension, and pre-eclampsia with aIRR 1.35 (1.09–1.64), aIRR 1.18 (1.05–1.31), respectively [ 54 ]. [ 35 , 36 , 54 , 57 , 66 , 67 ]. Whilst three studies were pooled to derive a significant association between rheumatoid arthritis and induced abortion 1.46 OR (1.01–2.12). Women with any pregnancy loss were reported to be at higher risk of developing the disease in one study aIRR 1.12 (1.06–1.12) and others reported no association aIRR 1.01 (0.67–1.44), and this will require further research to establish the true association. The association for induced abortion or any pregnancy loss with rheumatoid arthritis reported mixed findings with significant association reported by few studies and insignificant by others as shown in Fig. 5 [ 31 , 35 , 36 , 54 , 57 , 66 , 67 ]. A higher risk of developing rheumatoid arthritis was observed in women who delivered “extremely low birth weight” babies (< 1000 g) with RR 3.70 (1.00–13.20) or “low birth weight” babies (< 2500 g) with RR 1.40 (1.00–2.10) when compared to women who delivered normal birth weight babies [ 40 ]. An increased risk of rheumatoid arthritis was also reported for women with postpartum depression with aHR 2.62 (1.28–5.39) [ 60 ]. No other pregnancy complications studied in relation with the development of rheumatoid arthritis were statistically significant (Fig. 5 ). There was also no significant association with the development of rheumatoid arthritis as reported in women who delivered very low birth weight babies (< 1500 g) in a study with a small sample size ( n = 20) [ 40 ].
Rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis (composite outcome)
There were no other significant associations with rheumatoid arthritis or the composite outcome with other pregnancy complications studied (gestational hypertension or pre-eclampsia, caesarean section, postpartum haemorrhage, or mothers delivering preterm births or low birth weight babies) (Fig. 5 ) [ 71 ]. Women who delivered small for gestational age babies were more likely to have the composite outcome of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis aOR 1.60 (1.00–2.56) when compared to women with normal for gestational age babies.
Connective tissue diseases (systemic lupus erythematosus, Sjogren syndrome, systemic sclerosis)
Connective tissue disease.
Seven pregnancy complications were reported in association with connective tissue disease (CTD). Women with placental abruption or preterm birth had significantly higher risk of CTD; RR 3.39 (1.96–5.89) and RR 1.78 (1.12–2.82), respectively, [ 58 ] in Fig. 6 . The associations of gestational hypertension, intrauterine growth restriction (IUGR), miscarriage, stillbirth, and composite pregnancy complications with CTD were not statistically significant [ 58 ].
Systemic lupus erythematosus (SLE)
Results for an association between miscarriage and SLE were mixed with one study reporting aIRR of 1.43 (1.08–1.88), and a pooled RR for two smaller studies showing no significant association 0.94 (0.37–2.40) [ 54 , 58 , 66 , 69 ]. However, there was a significant association reported with any pregnancy loss and future development of the disease with OR 1.87 (1.31–2.67) [ 52 , 55 ]. Missed abortions were associated with a higher risk of SLE; IRR 2.13 (1.48–2.98) [ 54 , 69 ]. Women with history of IUGR had almost a fivefold higher risk of SLE; RR 4.80 (1.60–14.50) when compared with women with no IUGR [ 55 ]. Results from three studies showed that a history of stillbirth was associated with a four times higher risk of SLE, pooled RR 4.01 (3.11–5.17) and pooled IRR 3.29 (3.22–4.88) [ 54 , 58 , 66 , 69 ].
Systemic sclerosis
There was an association between gestational hypertension or pre-eclampsia and systemic sclerosis in two studies: OR 2.60 (1.10–4.60). Kamper et al. [ 56 ] reported a significant association with the development of localised scleroderma in women with pre-eclampsia with IRR 1.69 (1.02–2.80) but a nonsignificant association with subset of systemic disease aIRR 1.46 (0.75–2.80) [ 56 , 70 ]. There was a three- to fourfold higher risk of systemic sclerosis for women with IUGR compared with women with normal foetal growth OR 3.90 (1.20–12.30) [ 70 ] and caesarean birth compared to vaginal birth RR 3.09 (1.96–4.63) [ 59 ].
Sjögren’s syndrome
There were seven pregnancy complications examined in relation to Sjögren’s syndrome with results showing a greater risk with hyperemesis gravidarum, aIRR 1.79 (1.06–2.81); miscarriage, aIRR 1.33 (1.08–1.63); induced abortion, aIRR 1.18 (1.01–1.38); and gestational hypertension or pre-eclampsia, aIRR 1.43 (1.09–1.85) [ 54 ]. The associations for ectopic pregnancy, missed abortion, or preterm birth, aIRR 1.18 (0.79–1.68), aIRR 1.12 (0.80–1.51), and RR 0.09 (0.04–18.09), respectively [ 54 , 58 , 66 ], were not statistically significant.
Type 1 diabetes mellitus (T1DM)
Hyperemesis gravidarum aIRR 1.05 (0.74–1.45) and ectopic pregnancy aIRR 1.06 (0.58–1.77) were not significantly associated with T1DM [ 34 , 54 , 65 , 68 ] in Fig. 7 . Results from one cohort study showed that gestational hypertension or pre-eclampsia was associated with a twofold higher risk of T1DM; aIRR 2.37 (2.09–2.68) [ 68 ], whereas in the other study, the association was higher but not statistically significant; OR 1.80 (0.80–3.80) [ 65 ]. Results from two studies showed that the risk of T1DM for women with gestational diabetes was considerably higher; pooled OR 40.89 (24.31–68.78) [ 34 , 65 ]. There was almost a fourfold higher risk of T1DM in women who delivered large for gestational age babies compared to women delivering normal weight for gestational age babies aHR 3.60 (3.23–4.01) [ 68 ]. There was no significant association for women who delivered small for gestational age babies and T1DM; aHR 1.11 (0.94–1.30) [ 62 , 71 ].
Out of the seven pregnancy complications, five complications were associated with a higher risk of psoriasis: hyperemesis gravidarum HR 1.33 (1.01–1.71), ectopic pregnancy aIRR 1.28 (1.07–1.53), induced abortions aIRR 1.33 (1.24–1.42), gestational hypertension or pre-eclampsia aIRR 1.22 (1.06–1.40), and intrahepatic cholestasis of pregnancy aIRR 1.27 (1.07–1.51) [ 54 , 61 , 72 ]. Missed abortion and postpartum depression were not significantly associated with the risk of psoriasis (Fig. 7 ) [ 54 , 61 ].
Associations of pregnancy complications with other miscellaneous autoimmune conditions
No significant association was reported with pregnancy complications studied (hyperemesis, ectopic pregnancy, miscarriage, or gestational hypertension) and the development of multiple sclerosis as mentioned in Fig. 7 [ 64 ]. The association of postpartum depression and alopecia areata (HR 1.97, 0.72–5.37) [ 60 ], and recurrent pregnancy loss and myasthenia gravis (RR 0.85, 0.54–1.31) were not statistically significant [ 63 ].
Timing of developing autoimmune diseases following pregnancy/pregnancy complications
Four studies reported the occurrence of autoimmune diseases following pregnancy over different follow-up times [ 31 , 51 , 54 , 69 ]. Following a pregnancy complication, a woman’s risk of developing Grave’s disease or SLE was higher in the early years after childbirth in comparison to later in life [ 35 , 39 , 54 , 63 , 69 ]. For instance, there was a higher risk of SLE in women with pregnancy loss in the first year postpartum IRR 2.64 (1.18–6.29), whereas there was no significant association noted after two or more years postpartum IRR 1.90 (0.87–4.48) [ 69 ]. Conversely, the risk of developing rheumatoid arthritis (RR 1.05; 0.98–1.13, 5+ years: RR 2.24; 1.58–3.05, and multiple sclerosis) (RR 1.00; 0.91–1.09, 5+ years: RR 2.20; 1.72–2.77) was greater after 5 or more years postpartum. Also, women with hyperemesis gravidarum were at a greater risk of developing rheumatoid arthritis in the first 4 years post birth; this reduced after 5 years, IRR 1.40 (1.09–1.76) and IRR 1.02 (0.59–1.11), respectively [ 54 , 69 ]
This systematic review provides an overview of the associations of 18 pregnancy complications with the risk of developing 15 autoimmune diseases. This review compiles all the available evidence on pregnancy complications linked to the development of autoimmune diseases in women in later life (Figs. 2 , 3 , 4 , 5 , 6 and 7 ) and generates new evidence by quantitative or qualitative analysis of the studies studying the same exposure and outcomes (Additional file 1 figure 1.10). This also further points out the differences in the results observed in two or more studies analysing the association of the same pregnancy complication and autoimmune disease (Additional file 1 fig. 1.11).
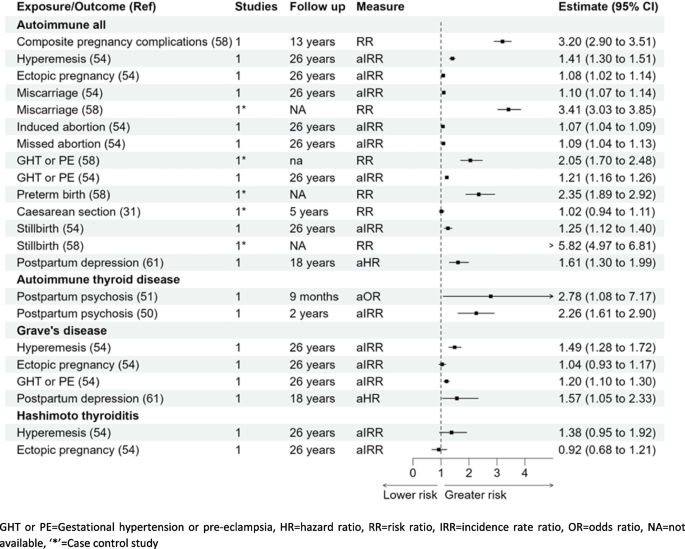
Forest plot association of pregnancy complications and autoimmune diseases (overall) and autoimmune thyroid diseases
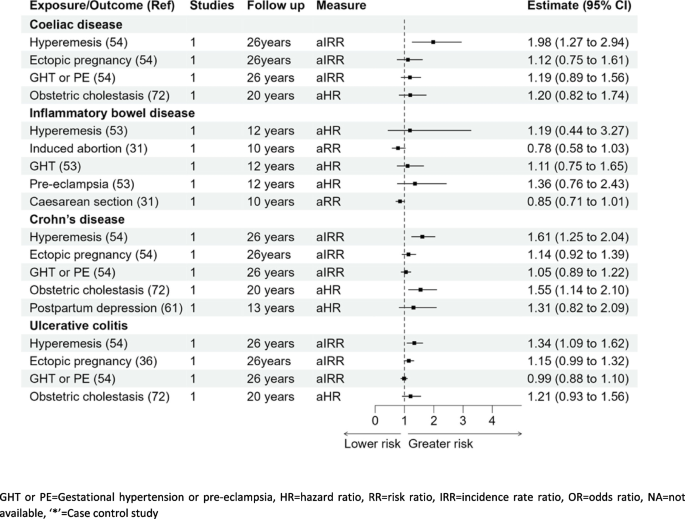
Forest plot-association of pregnancy complications and coeliac disease or inflammatory bowel disease (Crohn’s disease and ulcerative colitis)
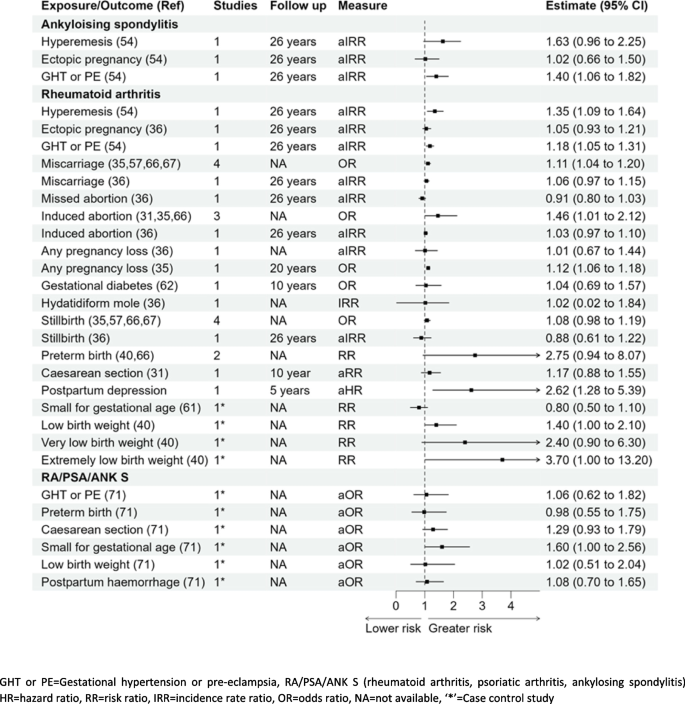
Forest plot- association of pregnancy complications and Ankylosing spondylitis, rheumatoid arthritis, or RA/PSA/ANK S
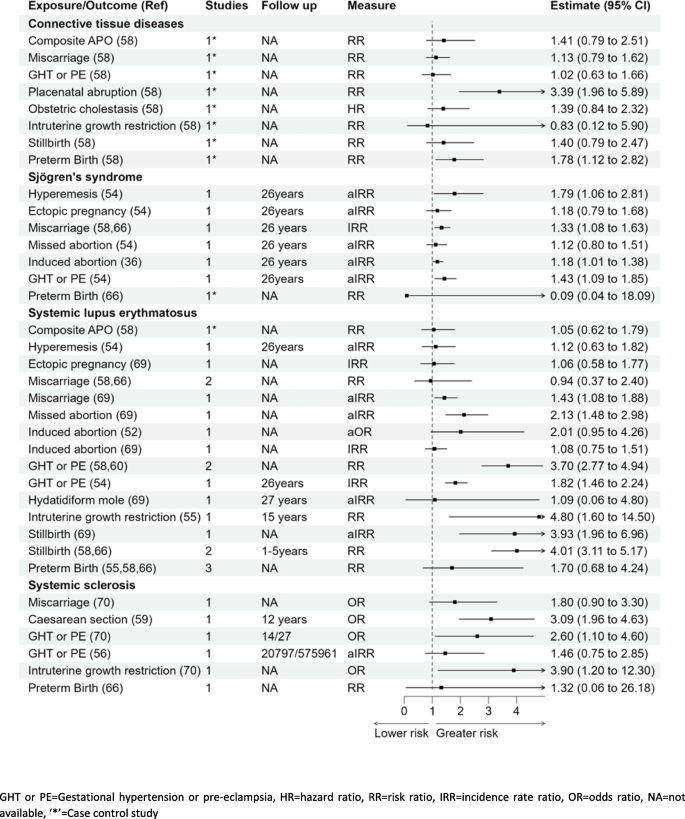
Forest plot showing the association of pregnancy complications and connective tissue diseases
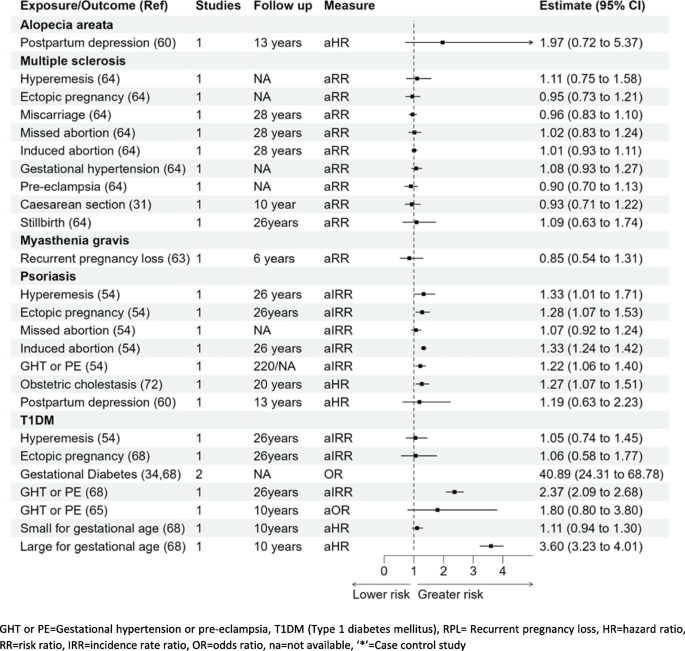
Forest plot showing the association of pregnancy complications and alopecia areata, multiple sclerosis, myasthenia gravis, psoriasis, or T1DM
Studies reported associations for gestational hypertension/preeclampsia followed by preterm birth, hyperemesis gravidarum and ectopic pregnancy, and future autoimmunity. However, there was little or no research on some complications such as molar pregnancy or placental disorders. From the perspective of autoimmune disease outcomes, most studies examined the associations with rheumatoid arthritis followed by autoimmune thyroid diseases, Sjögren’s syndrome, and psoriasis. In contrast, there were very few studies that included vitiligo or myasthenia gravis.
Many of the pregnancy complications increased the risk of overall autoimmune diseases almost threefold, particularly hyperemesis gravidarum, miscarriage, gestational hypertension, stillbirth, and antenatal/postpartum depression. Apart from the known association of gestational diabetes and T1DM, results from this review showed that IUGR or stillbirth were associated with almost three- to fourfold increased risk of systemic sclerosis or systemic lupus erythematosus. There was a higher risk of rheumatoid arthritis with preterm birth and low birth weight babies.
There are findings which require further research, for example, the association of miscarriage and development of SLE [ 58 , 65 , 69 ] and the association of gestational hypertension with T1DM [ 54 , 65 ]. The difference in the findings could possibly be due to the varying study designs or difference in the sample size of the studies. There had been mixed findings amongst the studies included and these may be due to the varying sample size or the study designs.
Earlier studies focused on the association of pregnancy complications with child outcomes such as caesarean birth or pre-eclampsia and the association with long-term health conditions in babies [ 78 , 79 , 80 , 81 , 82 , 83 , 84 ]. However, more recently, studies have reported the association of pregnancy complications and the development of long-term conditions in the mother [ 31 , 85 ]. Reproductive factors and pregnancy complications were found to be associated with later development of metabolic conditions [ 86 , 87 , 88 ]. An association between pregnancy itself, irrespective of pregnancy complications, and the development of autoimmune diseases has been reported [ 31 ]. Some studies identified the association between parity and the development of systemic sclerosis; however, the findings have been conflicting [ 89 , 90 , 91 , 92 ].
It is not clear whether the observed pregnancy complications occur in women with preclinical autoimmune disease or whether these events directly pre-dispose to the development of autoimmune disease [ 93 ]. In terms of the former, women with undifferentiated connective tissue disease (UCTD), who have features compatible with a CTD but do not have a defined CTD [ 94 ], have an increased risk of pregnancy complications including premature delivery, pre-eclampsia, and stillbirth [ 95 ]. As approximately 30% of UCTD may progress to CTD, typically SLE, it is possible that some of the pregnancy complications occurred in women who were in the initial stages of UCTD, i.e. the pregnancy complications were due to a subclinical autoimmune disease.
On the other hand, pregnancy/pregnancy complications bring about fluctuations in female sex hormones accompanied by physiological stress [ 96 ]. The blood levels of both oestrogen and progesterone increase rapidly from the middle of the second trimester, peaking at term. Oestrogen and progesterone have broad effects on the function of both innate and adaptive immune cells (including monocytes/macrophages, neutrophils, dendritic cells, and T and B lymphocytes) [ 97 ]. In pregnancy, placental production of oestriol (E3) increases dramatically. Oestriol has potent anti-inflammatory effects including reducing pro-inflammatory cytokine production, increasing anti-inflammatory cytokines, and reducing CD4+ and CD8+ T cells [ 98 ]. Similarly, progesterone increases regulatory T cells and reduces natural killer cell function systemically and within the placenta [ 98 ]. It is possible, therefore, that hormonal fluctuations and the loss of this anti-inflammatory state postpartum could accelerate the development of autoimmune disease. Furthermore, oestrogens reduce B cell apoptosis which, whilst contributing to maternal humoral immunity, may promote autoreactive B cell survival and drive the immune system toward autoimmunity [ 99 ].
A key driver of future autoimmune disease may be foetal microchimerism [ 100 ]. Foetal cells are present at a low frequency in the maternal circulation postpartum and may persist for decades [ 57 , 101 , 102 , 103 ]. Foetal origin microchimerism is observed at increased rates during pregnancy complications such as miscarriage, pre-eclampsia, foetal growth restriction [ 104 ], or premature labour [ 102 , 105 ]. The mechanisms by which foetal microchimeric cells mediate an increased risk of autoimmunity is not understood although an increased number of these cells is observed in the thyroid gland of women with autoimmune thyroid disease [ 106 ]. To date, a pathogenic role for foetal microchimeric cells has not been demonstrated, and these cells may induce maternal tolerance to foetal antigens and via a bystander effect reduce the severity of some autoimmune diseases such as RA during pregnancy [ 106 ].
Our study has several strengths. The scope of our review was broad and summarises the association of pregnancy complications and the subsequent development of a wide range of autoimmune diseases, and we were able to perform a meta-analysis of studies reporting the same exposure and outcome where possible. We employed rigorous methodology with a pre-specified protocol, and our systematic search was conducted without language restriction and two reviewers screened, extracted data, and appraised the quality of the studies.
There are, however, some limitations. A meta-analysis could not be performed for some of the studies due to missing data such as the sample size or number of exposed/unexposed. Some of the results reported therefore are as reported in one study. Also, nine studies were conducted using the same cohort (Danish birth cohort); this may have a disproportionate effect on our findings. However, efforts were made to avoid duplication in the reporting of results. This study is not able to determine causality and there is a possibility that the women already have undiagnosed preclinical autoimmune diseases, which increased their risk of pregnancy complications in studies, especially those with shorter follow-up time.
Additional research is required that incorporates a comprehensive analysis of pregnancy complications and characterise the phenotype and functionality of persistent foetal origin cells in women with autoimmune diseases compared with healthy women. The exact pathophysiology behind the development of these conditions remains unclear and we do not know why some pregnancy complications have a larger effect than others. To address these questions, prospective longitudinal studies following up on women who experienced pregnancy complications are needed, observing when autoantibodies are first detected [ 107 ]. Furthermore, larger epidemiological studies would be required to define whether autoimmune disease is more prevalent in women who have experienced pregnancy complications and if there is a clear underlying association.
This review has reported that there is an association between pregnancy complications and the subsequent development of autoimmune diseases in women. To further address this question, prospective longitudinal studies following up on women who experienced pregnancy complications are needed, observing when autoantibodies are first detected. Meanwhile, clinicians should be vigilant and detect autoimmune conditions early in women with a history of pregnancy complications.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
95% Confidence intervals
Adjusted hazard ratio
Adjusted incidence risk ratio
Adjusted odds ratio
Adjusted risk ratio
Axial spondyloarthropathy
Clusters of differentiation
Caesarean section
Connective tissue diseases
Gestational diabetes mellitus
Gestational hypertension or pre-eclampsia
Haemolysis, elevated liver enzymes, and low platelet syndrome
Inflammatory bowel disease
Intrauterine growth retardation
Low birth weight
Medical subject headings
Meta-analyses of observational studies
Multiple sclerosis
Not applicable
Newcastle–Ottawa scale
Patient and public involvement and engagement
Preferred reporting items for systematic review and meta-analysis
Rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis
Small for gestational age
Systemic lupus erythematosus
Type 1 diabetes mellitus
Undifferentiated connective tissue disease
United Kingdom
United States
Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. 2023;80:102266.
Article PubMed Google Scholar
Conrad N, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90.
Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2015;3(4):151–5.
Article Google Scholar
Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–69.
Thomas SL, et al. Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health. 2010;100(11):2279–87.
Article PubMed PubMed Central Google Scholar
Mallampalli MP, et al. Role of environment and sex differences in the development of autoimmune diseases: a roundtable meeting report. J Womens Health. 2013;22(7):578–86.
Miquel CH, Faz-Lopez B, Guéry JC. Influence of X chromosome in sex-biased autoimmune diseases. J Autoimmun. 2023;137:102992.
Souyris M, et al. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin Immunopathol. 2019;41(2):153–64.
Merrheim J, et al. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun Rev. 2020;19(3):102468.
Nelson JL, et al. Remission of rheumatoid arthritis during pregnancy and maternal-fetal class II alloantigen disparity. Am J Reprod Immunol. 1992;28(3–4):226–7.
Zakarija M, McKenzie JM. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves’ disease and the relationship to neonatal hyperthyroidism*. J Clin Endocrinol Metab. 1983;57(5):1036–40.
Boyd AS, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35(3):169–72.
Khamashta MA, Ruiz-Irastorza G, Hughes GR. Systemic lupus erythematosus flares during pregnancy. Rheum Dis Clin North Am. 1997;23(1):15–30.
Schubert C, et al. Postpartum relapse risk in multiple sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023;94(9):718–25.
Castles A, et al. Effects of smoking during pregnancy: five meta-analyses. Am J Prev Med. 1999;16(3):208–15.
Cnattingius S, Lambe M. Trends in smoking and overweight during pregnancy: prevalence, risks of pregnancy complications, and adverse pregnancy outcomes. Semin Perinatol. 2002;26(4):286–95. https://doi.org/10.1053/sper.2002.34771 .
DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. Eur J Gen Pract. 1995;1(3):117–117.
Meltzer HM, et al. Effect of dietary factors in pregnancy on risk of pregnancy complications: results from the Norwegian Mother and Child Cohort Study. Am J Clin Nutr. 2011;94(suppl_6):1970S-1974S.
Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118(2 Part 1):305–12.
Ramachenderan J, Bradford J, Mclean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol. 2008;48(3):228–35.
Jaffar F, Laycock K, Huda MSB. Type 1 diabetes in pregnancy: a review of complications and management. Curr Diabetes Rev. 2022;18(7):e051121197761.
Naik VD, et al. Effects of nutrition and gestational alcohol consumption on fetal growth and development. Nutr Rev. 2022;80(6):1568–79.
Upala S, Yong WC, Sanguankeo A. Association between primary Sjögren’s syndrome and pregnancy complications: a systematic review and meta-analysis. Clin Rheumatol. 2016;35(8):1949–55.
Khizroeva J, et al. Infertility in women with systemic autoimmune diseases. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101369.
Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38(2–3):J266–74.
Bobotsis R, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175(3):464–72.
Arvanitakis K, et al. Adverse pregnancy outcomes in women with celiac disease: a systematic review and meta-analysis. Ann Gastroenterol. 2023;36(1):12–24.
PubMed Google Scholar
Smyth A, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–8.
Finkelsztejn A, et al. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118(7):790–7.
Wei S, et al. Systemic lupus erythematosus and risk of preterm birth: a systematic review and meta-analysis of observational studies. Lupus. 2017;26(6):563–71.
Khashan AS, et al. Pregnancy and the risk of autoimmune disease. PLoS One. 2011;6(5):e19658.
Jørgensen KT, et al. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J Autoimmun. 2012;38(2–3):J81–7.
Sobanski V, et al. Special considerations in pregnant systemic sclerosis patients. Expert Rev Clin Immunol. 2016;12(11):1161–73.
Auvinen AM, et al. Type 1 and type 2 diabetes after gestational diabetes: a 23 year cohort study. Diabetologia. 2020;63(10):2123–8.
Hee JY, et al. Pregnancy loss and the risk of rheumatoid arthritis in Chinese women: findings from the China Kadoorie biobank. BMC Public Health. 2022;22(1):1768.
Jorgensen KT, et al. National cohort study of reproductive risk factors for rheumatoid arthritis in Denmark: a role for hyperemesis, gestational hypertension and pre-eclampsia? Ann Rheum Dis. 2010;69(2):358–63.
Gleicher N. Reproductive failure prior to the onset of clinical autoimmune disease. Rheumatology. 1999;38(6):485–7.
Chakravarty E. Pre-disease pregnancy complications and systemic sclerosis: pathogenic or pre-clinical? Arthritis Res Ther. 2012;14(1):102.
Jorgensen KT, et al. Increased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth Cohort. Rheumatology. 2014;53(8):1513–9.
Ma KK, et al. Adverse pregnancy outcomes and risk of subsequent rheumatoid arthritis. Arthritis Rheumatol. 2014;66(3):508–12.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
Team TE. EndNote, Philadelphia,PA. Clarivate. EndNote 20. 64 bit. 2013.
Covidence Systematic Review Software. Veritas Health Innovation, Melbourne Australia. www.covidence.org . Accessed July 2024.
Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global . https://doi.org/10.46658/JBIMES-20-01 .
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta analysis. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Assessed on Nov 2023.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100.
StataCorp. Stata statistical software: release 17. College Station: StataCorp LLC; 2021.
Google Scholar
RStudio Team. RStudio: integrated development for R. Boston: RStudio, Inc.; 2019. http://www.rstudio.com/ .
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. https://www.R-project.org/ .
Bergink V, et al. Prevalence of autoimmune thyroid dysfunction in postpartum psychosis. Br J Psychiatry. 2011;198(4):264–8.
Bergink V, et al. Comorbidity of autoimmune thyroid disorders and psychiatric disorders during the postpartum period: a Danish nationwide register-based cohort study. Psychol Med. 2018;48(8):1291–8.
Hardy C, et al. Pregnancy outcome and family size in systemic lupus erythematosus: a case-control study. Rheumatology (Oxford). 1999;38(6):559–63.
Harpsoe MC, et al. Risk of inflammatory bowel disease according to self-rated health, pregnancy course, and pregnancy complications: a study within the Danish National Birth Cohort. PLoS One. 2013;8(3):e59698.
Jørgensen KT, et al. Hyperemesis, gestational hypertensive disorders, pregnancy losses and risk of autoimmune diseases in a Danish population-based cohort. J Autoimmun. 2012;38(2):J120–8.
Julkunen H, et al. Fetal outcome in lupus pregnancy: a retrospective case-control study of 242 pregnancies in 112 patients. Lupus. 1993;2(2):125–31.
Kamper-Jorgensen M, Gammill HS, Nelson JL. Preeclampsia and scleroderma: a prospective nationwide analysis. Acta Obstet Gynecol Scand. 2018;97(5):587–90.
Kay A, Bach F. Subfertility before and after the development of rheumatoid arthritis in women. Ann Rheum Dis. 1965;24(2):169–73.
Kither H, et al. Adverse pregnancy outcomes and subsequent development of connective tissue disease in the UK: an epidemiological study. BJOG. 2020;127(8):941–9.
Lee KA, et al. Pregnancy-associated risk factors and incidence of systemic sclerosis in primiparous women: a nationwide population-based cohort study. Mod Rheumatol. 2022;32(1):149–54.
Lin CY, et al. Postpartum depression and subsequent autoimmune diseases in Taiwan. Int J Environ Res Public Health. 2018;15(8):20.
Lin LT, et al. Increased risk of systemic lupus erythematosus in pregnancy-induced hypertension: a nationwide population-based retrospective cohort study. Medicine. 2016;95(30):e4407.
Mao Y, et al. Association between history of gestational diabetes mellitus and the risk of arthritis in women. Front Public Health. 2022;10:878845.
Mikkelsen AP, et al. Pregnancy loss and risk of multiple sclerosis and autoimmune neurological disorder: a nationwide cohort study. PLoS One. 2022;17(3):e0266203.
Nielsen NM, et al. Reproductive history and risk of multiple sclerosis. Epidemiology. 2011;22(4):546–52.
Savitz DA, et al. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):41–4.
Siamopoulou-Mavridou A, et al. Outcome of pregnancy in patients with autoimmune rheumatic disease before the disease onset. Ann Rheum Dis. 1988;47(12):982–7.
Spector TD, Silman AJ. Is poor pregnancy outcome a risk factor in rheumatoid arthritis? Ann Rheum Dis. 1990;49(1):12.
Stuart AE, Amer-Wahlin I, Kallen KBM. Neonatal delivery weight and risk of future maternal diabetes. Int J Gynaecol Obstet. 2018;140(1):111–7.
Ulff-Møller CJ, et al. Reproductive factors and risk of systemic lupus erythematosus: nationwide cohort study in Denmark. J Rheumatol. 2009;36(9):1903–9.
van Wyk L, et al. Increased incidence of pregnancy complications in women who later develop scleroderma: a case control study. Arthritis Res Ther. 2011;13(6):R183.
Wallenius M, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis Rheum. 2011;63(6):1534–42.
WikstromShemer EA, et al. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: a population-based cohort study. J Hepatol. 2015;63(2):456–61.
Brann E, et al. Bidirectional association between autoimmune disease and perinatal depression: a nationwide study with sibling comparison. Mol Psychiatry. 2024;09:09.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from www.training.cochrane.org/handbook .
Tramèr MR, et al. Impact of covert duplicate publication on meta-analysis: a case study. BMJ. 1997;315(7109):635–40.
von Elm E, et al. Different patterns of duplicate publication: an analysis of articles used in systematic reviews. JAMA. 2004;291(8):974–80.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Bi S, et al. Long-term effects of preeclampsia on metabolic and biochemical outcomes in offspring: what can be expected from a meta-analysis? Obes Rev. 2022;23(5):e13411.
Black M, et al. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271–9.
Bruce A, Black M, Bhattacharya S. Mode of delivery and risk of inflammatory bowel disease in the offspring: systematic review and meta-analysis of observational studies. Inflamm Bowel Dis. 2014;20(7):1217–26.
Cardwell CR, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53(4):641–51.
Dalla Costa G, et al. Caesarean section and infant formula feeding are associated with an earlier age of onset of multiple sclerosis. Mult Scler Relat Disord. 2019;33:75–7.
Nielsen NM, et al. Maternal diabetes and risk of multiple sclerosis in the offspring: a Danish nationwide register-based cohort study. Mult Scler. 2021;27(11):1686–94.
Nakamura N, et al. Mortality and neurological outcomes in extremely and very preterm infants born to mothers with hypertensive disorders of pregnancy. Sci Rep. 2021;11(1):1729.
Jones WR. Autoimmune disease and pregnancy. Aust N Z J Obstet Gynaecol. 1994;34(3):251–8.
Neiger R. Long-term effects of pregnancy complications on maternal health: a review. J Clin Med. 2017;6(8):76.
Panaitescu AM, et al. Pregnancy complications can foreshadow future disease-long-term outcomes of a complicated pregnancy. Medicina (Kaunas). 2021;57(12):1320.
Okoth K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502.
Cockrill T, Del Junco DJ, Arnett FC, Assassi S, Tan FK, McNearney T, et al. Separate influences of birth order and gravidity/parity on the development of systemic sclerosis. Arthritis Care Res. 2010;62(3):418–24. https://doi.org/10.1002/acr.20096 .
Pisa FE, et al. Reproductive factors and the risk of scleroderma: an Italian case-control study. Arthritis Rheum. 2002;46(2):451–6.
Russo PA, Lester S, Roberts-Thomson PJ. Systemic sclerosis, birth order and parity. Int J Rheum Dis. 2014;17(5):557–61.
Lambe M, et al. Childbearing and the risk of scleroderma: a population-based study in Sweden. Am J Epidemiol. 2004;159(2):162–6.
Beneventi F, et al. Impact of pregnancy on progression of preclinical autoimmune disorders: a prospective cohort study. Rheumatology. 2022;62(9):2971–8.
Antunes M, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. 2018;4(Suppl 1):e000786.
Spinillo A, et al. The effect of newly diagnosed undifferentiated connective tissue disease on pregnancy outcome. Am J Obstet Gynecol. 2008;199(6):632.e1-6.
Assad S, et al. Role of sex hormone levels and psychological stress in the pathogenesis of autoimmune diseases. Cureus. 2017;9:e1315. https://doi.org/10.7759/cureus.1315 .
Serena C, et al. Undifferentiated connective tissue disease in pregnancy: a topic yet to be explored. Front Pharmacol. 2022;13:820760.
Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–71.
Ding J, Zhu BT. Unique effect of the pregnancy hormone estriol on antigen-induced production of specific antibodies in female BALB/c mice. Steroids. 2008;73(3):289–98.
Ubeda F, Wild G. Microchimerism as a source of information on future pregnancies. Proc R Soc Lond B Biol Sci. 2023;290(2005):20231142.
Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33(8):421–7.
Bianchi DW, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705–8.
Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37(5–6):631–44.
Al-Mufti R, et al. Fetal cells in maternal blood of pregnancies with severe fetal growth restriction. Hum Reprod. 2000;15(1):218–21.
Leung TN, et al. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352(9144):1904–5.
Fugazzola L, Cirello V, Beck-Peccoz P. Microchimerism and endocrine disorders. J Clin Endocrinol Metab. 2012;97(5):1452–61.
Luiro K, et al. Autoantibodies predict type 1 diabetes after gestational diabetes - a 23-year cohort study. Front Endocrinol. 2023;14:1286375.
Download references
Acknowledgements
MuM-PreDiCT consortium
This work was funded by the Strategic Priority Fund ‘Tackling multimorbidity at scale’ programme (grant number-MR/W014432/1) delivered by the Medical Research Council and the National Institute for Health and Care Research in partnership with the Economic and Social Research Council and in collaboration with the Engineering and Physical Sciences Research Council.
Author information
Authors and affiliations.
Institute of Applied Health Research, University of Birmingham, Birmingham, UK
Megha Singh, Fathima Fazla Ahamed Fayaz, Jingya Wang, Steven Wambua, Anuradha Subramanian, Krishnarajah Nirantharakumar & Francesca Crowe
Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK
John A. Reynolds
You can also search for this author in PubMed Google Scholar
Contributions
MS was responsible for the analysis and drafting of the manuscript. FF/JW were the second reviewers for the study selection, data extraction check, and quality appraisal. FC and KN were the third reviewers and provided their inputs and guidance at each step of the review. KN, JR, AS, FC, and SW were responsible for revising the manuscript critically and for important intellectual content. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @Nirantharakumar (Krishnarajah Nirantharakumar), @StevenWambua (Steven Wambua), @meghasingh_16 (Megha Singh)
Corresponding author
Correspondence to Krishnarajah Nirantharakumar .
Ethics declarations
Ethics approval and consent to participate.
Since this review analyses the data from the prior systematic reviews, no ethical approval is required. Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
Co-author AS was part of university of Birmingham when this review was initiated but moved to work with Astrazeneca. She currently holds an honorary contract with the University of Birmingham. All the other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3550_moesm1_esm.docx.
Additional file 1: Table 1. Search Strategy MEDLINE. Table 2. The Preferred Reporting Items for Systematic reviews and Meta-Analyseschecklist. Table 3. List of excluded studies. Table 4.1. Quality assessment of the Cohort studies using NOS. Table 4.2. Quality assessment of the case control studies using NOS. Table 4.3. Quality assessment of the cross-sectional studies using NOS. Figure 1.1. Meta-analysis of two studies reporting association of miscarriage and future development of SLE. Figure 1.2. Meta-analysis of two studies reporting association of miscarriage and future development of rheumatoid arthritis. Figure 1.5. Meta-analysis of two studies reporting association of stillbirth and future development of SLE. Figure 1.6. Meta-analysis of two studies reporting association of gestational hypertension or pre-eclampsia and future development of SLE. Figure 1.7. Meta-analysis of two studies reporting association of preterm birth and future development of SLE. Figure 1.8. Meta-analysis of two studies reporting association of preterm birth and future development of rheumatoid arthritis. Figure 1.9. Meta-analysis of two studies reporting association of gestational diabetes and future development of T1DM. Figure 1.10. The new findings from this review. Figure 1.11. The mixed findings of this review. Table 5. Cohort studies with same or overlapping cohorts. Table 6. Data Extraction form.
Additional file 2.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Singh, M., Fayaz, F.F.A., Wang, J. et al. Pregnancy complications and autoimmune diseases in women: systematic review and meta-analysis. BMC Med 22 , 339 (2024). https://doi.org/10.1186/s12916-024-03550-5
Download citation
Received : 17 April 2024
Accepted : 29 July 2024
Published : 26 August 2024
DOI : https://doi.org/10.1186/s12916-024-03550-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pregnancy complications
- Autoimmune disease
BMC Medicine
ISSN: 1741-7015
- General enquiries: [email protected]

IMAGES
COMMENTS
A hydatiform mole (also known as a molar pregnancy) is a gestational trophoblastic disease (GTD), which originates from the placenta and can metastasize. It is unique in that the tumor originates from gestational tissue rather than from maternal tissue. Hydatiform moles (HM) are categorized as complete and partial and are usually considered the ...
The diagnosis of molar pregnancy is usually made during the second trimester, and classical signs and symptoms include large uterine size, toxemia, anemia, hyperemesis, respiratory distress, and hypothyroidism. In recent years, clinical presentation of molar pregnancy has changed largely because of diagnosis of CM at early gestational age.
A molar pregnancy may seem like a regular pregnancy at first. But most molar pregnancies cause symptoms that can include: Dark brown to bright red bleeding from the vagina during the first three months. Severe nausea and vomiting. Sometimes grapelike cysts that pass from the vagina. Pelvic pressure or pain.
Hydatidiform mole (HM) was first described by Hippocrates around 400 BCE as "dropsy of the uterus." Since that time, HM (also referred to as molar pregnancy or mole) has been of clinical and research interest. Molar pregnancy is part of a group of diseases classified as gestational trophoblastic disease (GTD), which originate in the placenta ...
The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86:775-779. Hou JL, Wan XR, Xiang Y, et al. Changes in clinical features in hydatidiform mole: analysis of 113 cases. ... Sonographic findings and clinical correlations in molar pregnancy. Obstet Gynecol. 1980;56:186-192. Benson CB, Genset DR, Bernstein MR, et ...
The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995 Nov. 86(5):775-9. [QxMD MEDLINE Link]. ... et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2015 Nov 19. [QxMD ...
Diagnosis. A health care provider who suspects a molar pregnancy is likely to order blood tests and an ultrasound. During early pregnancy, a sonogram might involve a wandlike device placed in the vagina. As early as eight or nine weeks of pregnancy, an ultrasound of a complete molar pregnancy might show:
This review will highlight important clinical aspects of molar pregnancy specifically, with a focus on new developments in diagnosis and surveillance in the past 5 years. ... Gockley AA, Goldstein DP, Bernstein MR, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center ...
The Clinical Problem Molar pregnancy comprises two distinct entities, partial and complete mole, which ... tain symptoms and signs were common at the time of presentation, including exces - sive ...
Hydatidiform mole (HM) is one of a group of diseases that develop from abnormal proliferation of trophoblasts and are classified as gestational trophoblastic disease (GTD). The two distinct types of HM, complete mole and partial mole, have different karyotypes, gross and microscopic histopathology, clinical presentations, and prognoses [ 1-3 ].
Diagnosis. The diagnosis of a molar pregnancy might be suspected based on a number of clinical features: abnormal vaginal bleeding in early pregnancy is the most common presentation; uterus large for dates (25%); pain from large benign theca-lutein cysts (20%); vaginal passage of grape-like vescicles (10%); exaggerated pregnancy symptoms including hyperemesis (10%), hyperthyroidism (5%), early ...
Molar pregnancy: presentation and diagnosis. Clin Obstet Gynecol 1984;27:181-191. Crossref. ... The changing clinical presentation of complete molar pregnancy. Obstet Gynecol 1995;86:775-779.
The typical clinical presentation of complete molar pregnancies has changed with the advent of high-resolution ultrasonography. Most moles are now diagnosed in the first trimester before the onset of the classic signs and symptoms. [ 32, 33, 34] Vaginal bleeding. The most common classic symptom of a complete mole is vaginal bleeding.
The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995 Nov;86(5):775-9. 36. Sun SY, Melamed A, Joseph NT, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades.
A molar pregnancy. A molar pregnancy occurs when an abnormal egg or sperm join. In a complete molar pregnancy, there is no fetus. There are two sets of genes from the man. In a partial molar pregnancy, a fetus develops but it will be abnormal and cannot survive. At most, the fetus might survive for around three months.
The hydatidiform mole, also known as molar pregnancy, is a rare gynecological disease rising from the trophoblastic tissue with a ranging incidence of 0.6-11.5 per 1000 deliveries worldwide [1,2]. Two forms of molar pregnancy can be distinguished: (1) the complete hydatidiform mole (CHM) occurs when an enucleated egg is fertilized by two spermatozoa or one haploid duplicating spermatozoon ...
Abstract. We can now detect molar pregnancy at a much earlier gestational age than before by using high resolution vaginal ultrasonography. As a result, the current clinical presentation of complete hydatidiform moles has clearly changed compared to that of the classic type of mole. The diagnosis of molar pregnancy is nearly always made by ...
clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole John R. Lurain, MD G ... and prior molar pregnancy. Advanced or very young maternal age has consis-tently correlated with higher rates of completehydatidiformmole.Compared
The typical clinical presentation of molar pregnancy includes vaginal bleeding, hyperemesis gravidarum, early embryonic demise, an enlarged uterus, early pre-eclampsia, hyperthyroidism and abdominal distension 3. ... If a woman is known to have had a molar pregnancy, ...
4 CLINICAL PRESENTATION, INVESTIGATIONS, AND DIAGNOSIS 4.1 Molar pregnancy. Patients usually present with second trimester vaginal bleeding. As diagnosis is often made in the first trimester with ultrasound examination, complications such as hyperemesis gravidarum, pre-eclampsia, and hyperthyroidism are less and less common.
Gemer et al 5 from Israel have also reported the changing current clinical presentation of complete molar pregnancy in 41 patients. In their paper, the mean maternal age was 30.1 years and the mean gestational age at evacuation was 10 weeks with a range of 7-14 weeks. The mean uterine size was compatible with 10 weeks' gestation.
Objective: To determine if the clinical presentation of complete hydatidiform mole has changed in recent years compared with historic controls (1965-1975). Methods: Chart review of all 74 patients referred to the New England Trophoblastic Disease Center for the primary management of complete hydatidiform mole during 1988-1993 was performed and comparison made to historic controls (1965-1975).
Pregnancy complications might lead to the development of autoimmune diseases in women. This review aims to summarise studies evaluating the association between pregnancy complications and the development of autoimmune diseases in women. Medline, CINAHL, and Cochrane databases were searched up to January 2024. Nineteen pregnancy complications and 15 autoimmune conditions were included.