- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Open Access
- Reasons to publish with us
- About Health Education Research
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction.
- < Previous
Young people and healthy eating: a systematic review of research on barriers and facilitators
- Article contents
- Figures & tables
- Supplementary Data
J Shepherd, A Harden, R Rees, G Brunton, J Garcia, S Oliver, A Oakley, Young people and healthy eating: a systematic review of research on barriers and facilitators, Health Education Research , Volume 21, Issue 2, 2006, Pages 239–257, https://doi.org/10.1093/her/cyh060
- Permissions Icon Permissions
A systematic review was conducted to examine the barriers to, and facilitators of, healthy eating among young people (11–16 years). The review focused on the wider determinants of health, examining community- and society-level interventions. Seven outcome evaluations and eight studies of young people's views were included. The effectiveness of the interventions was mixed, with improvements in knowledge and increases in healthy eating but differences according to gender. Barriers to healthy eating included poor school meal provision and ease of access to, relative cheapness of and personal taste preferences for fast food. Facilitators included support from family, wider availability of healthy foods, desire to look after one's appearance and will-power. Friends and teachers were generally not a common source of information. Some of the barriers and facilitators identified by young people had been addressed by soundly evaluated effective interventions, but significant gaps were identified where no evaluated interventions appear to have been published (e.g. better labelling of food products), or where there were no methodologically sound evaluations. Rigorous evaluation is required particularly to assess the effectiveness of increasing the availability of affordable healthy food in the public and private spaces occupied by young people.
Healthy eating contributes to an overall sense of well-being, and is a cornerstone in the prevention of a number of conditions, including heart disease, diabetes, high blood pressure, stroke, cancer, dental caries and asthma. For children and young people, healthy eating is particularly important for healthy growth and cognitive development. Eating behaviours adopted during this period are likely to be maintained into adulthood, underscoring the importance of encouraging healthy eating as early as possible [ 1 ]. Guidelines recommend consumption of at least five portions of fruit and vegetables a day, reduced intakes of saturated fat and salt and increased consumption of complex carbohydrates [ 2, 3 ]. Yet average consumption of fruit and vegetables in the UK is only about three portions a day [ 4 ]. A survey of young people aged 11–16 years found that nearly one in five did not eat breakfast before going to school [ 5 ]. Recent figures also show alarming numbers of obese and overweight children and young people [ 6 ]. Discussion about how to tackle the ‘epidemic’ of obesity is currently high on the health policy agenda [ 7 ], and effective health promotion remains a key strategy [ 8–10 ].
Evidence for the effectiveness of interventions is therefore needed to support policy and practice. The aim of this paper is to report a systematic review of the literature on young people and healthy eating. The objectives were
(i) to undertake a ‘systematic mapping’ of research on the barriers to, and facilitators of, healthy eating among young people, especially those from socially excluded groups (e.g. low-income, ethnic minority—in accordance with government health policy);
(ii) to prioritize a subset of studies to systematically review ‘in-depth’;
(iii) to ‘synthesize’ what is known from these studies about the barriers to, and facilitators of, healthy eating with young people, and how these can be addressed and
(iv) to identify gaps in existing research evidence.
General approach
This study followed standard procedures for a systematic review [ 11, 12 ]. It also sought to develop a novel approach in three key areas.
First, it adopted a conceptual framework of ‘barriers’ to and ‘facilitators’ of health. Research findings about the barriers to, and facilitators of, healthy eating among young people can help in the development of potentially effective intervention strategies. Interventions can aim to modify or remove barriers and use or build upon existing facilitators. This framework has been successfully applied in other related systematic reviews in the area of healthy eating in children [ 13 ], physical activity with children [ 14 ] and young people [ 15 ] and mental health with young people [16; S. Oliver, A. Harden, R. Rees, J. Shepherd, G. Brunton and A. Oakley, manuscript in preparation].
Second, the review was carried out in two stages: a systematic search for, and mapping of, literature on healthy eating with young people, followed by an in-depth systematic review of the quality and findings of a subset of these studies. The rationale for a two-stage review to ensure the review was as relevant as possible to users. By mapping a broad area of evidence, the key characteristics of the extant literature can be identified and discussed with review users, with the aim of prioritizing the most relevant research areas for systematic in-depth analysis [ 17, 18 ].
Third, the review utilized a ‘mixed methods’ triangulatory approach. Data from effectiveness studies (‘outcome evaluations’, primarily quantitative data) were combined with data from studies which described young people's views of factors influencing their healthy eating in negative or positive ways (‘views’ studies, primarily qualitative). We also sought data on young people's perceptions of interventions when these had been collected alongside outcomes data in outcome evaluations. However, the main source of young people's views was surveys or interview-based studies that were conducted independently of intervention evaluation (‘non-intervention’ research). The purpose was to enable us to ascertain not just whether interventions are effective, but whether they address issues important to young people, using their views as a marker of appropriateness. Few systematic reviews have attempted to synthesize evidence from both intervention and non-intervention research: most have been restricted to outcome evaluations. This study therefore represents one of the few attempts that have been made to date to integrate different study designs into systematic reviews of effectiveness [ 19–22 ].
Literature searching
A highly sensitive search strategy was developed to locate potentially relevant studies. A wide range of terms for healthy eating (e.g. nutrition, food preferences, feeding behaviour, diets and health food) were combined with health promotion terms or general or specific terms for determinants of health or ill-health (e.g. health promotion, behaviour modification, at-risk-populations, sociocultural factors and poverty) and with terms for young people (e.g. adolescent, teenager, young adult and youth). A number of electronic bibliographic databases were searched, including Medline, EMBASE, The Cochrane Library, PsycINFO, ERIC, Social Science Citation Index, CINAHL, BiblioMap and HealthPromis. The searches covered the full range of publication years available in each database up to 2001 (when the review was completed).
Full reports of potentially relevant studies identified from the literature search were obtained and classified (e.g. in terms of specific topic area, context, characteristics of young people, research design and methodological attributes).
Inclusion screening
Inclusion criteria were developed and applied to each study. The first round of screening was to identify studies to populate the map. To be included, a study had to (i) focus on healthy eating; (ii) include young people aged 11–16 years; (iii) be about the promotion of healthy eating, and/or the barriers to, or facilitators of, healthy eating; (iv) be a relevant study type: (a) an outcome evaluation or (b) a non-intervention study (e.g. cohort or case control studies, or interview studies) conducted in the UK only (to maximize relevance to UK policy and practice) and (v) be published in the English language.
The results of the map, which are reported in greater detail elsewhere [ 23 ], were used to prioritize a subset of policy relevant studies for the in-depth systematic review.
A second round of inclusion screening was performed. As before, all studies had to have healthy eating as their main focus and include young people aged 11–16 years. In addition, outcome evaluations had toFor a non-intervention study to be included it had to
(i) use a comparison or control group; report pre- and post-intervention data and, if a non-randomized trial, equivalent on sociodemographic characteristics and pre-intervention outcome variables (demonstrating their ‘potential soundness’ in advance of further quality assessment);
(ii) report an intervention that aims to make a change at the community or society level and
(iii) measure behavioural and/or physical health status outcomes.
(i) examine young people's attitudes, opinions, beliefs, feelings, understanding or experiences about healthy eating (rather than solely examine health status, behaviour or factual knowledge);
(ii) access views about one or more of the following: young people's definitions of and/or ideas about healthy eating, factors influencing their own or other young people's healthy eating and whether and how young people think healthy eating can be promoted and
(iii) privilege young people's views—presenting views directly as data that are valuable and interesting in themselves, rather than only as a route to generating variables to be tested in a predictive or causal model.
Non-intervention studies published before 1990 were excluded in order to maximize the relevance of the review findings to current policy issues.
Data extraction and quality assessment
All studies meeting inclusion criteria underwent data extraction and quality assessment, using a standardized framework [ 24 ]. Data for each study were entered independently by two researchers into a specialized computer database [ 25 ] (the full and final data extraction and quality assessment judgement for each study in the in-depth systematic review can be viewed on the Internet by visiting http://eppi.ioe.ac.uk ).
Outcome evaluations were considered methodologically ‘sound’ if they reported:Only studies meeting these criteria were used to draw conclusions about effectiveness. The results of the studies which did not meet these quality criteria were judged unclear.
(i) a control or comparison group equivalent to the intervention group on sociodemographic characteristics and pre-intervention outcome variables.
(ii) pre-intervention data for all individuals or groups recruited into the evaluation;
(iii) post-intervention data for all individuals or groups recruited into the evaluation and
(iv) on all outcomes, as described in the aims of the intervention.
Non-intervention studies were assessed according to a total of seven criteria (common to sets of criteria proposed by four research groups for qualitative research [ 26–29 ]):
(i) an explicit account of theoretical framework and/or the inclusion of a literature review which outlined a rationale for the intervention;
(ii) clearly stated aims and objectives;
(iii) a clear description of context which includes detail on factors important for interpreting the results;
(iv) a clear description of the sample;
(v) a clear description of methodology, including systematic data collection methods;
(vi) analysis of the data by more than one researcher and
(vii) the inclusion of sufficient original data to mediate between data and interpretation.
Data synthesis
Three types of analyses were performed: (i) narrative synthesis of outcome evaluations, (ii) narrative synthesis of non-intervention studies and (iii) synthesis of intervention and non-intervention studies together.
For the last of these a matrix was constructed which laid out the barriers and facilitators identified by young people alongside descriptions of the interventions included in the in-depth systematic review of outcome evaluations. The matrix was stratified by four analytical themes to characterize the levels at which the barriers and facilitators appeared to be operating: the school, family and friends, the self and practical and material resources. This methodology is described further elsewhere [ 20, 22, 30 ].
From the matrix it is possible to see:
(i) where barriers have been modified and/or facilitators built upon by soundly evaluated interventions, and ‘promising’ interventions which need further, more rigorous, evaluation (matches) and
(ii) where barriers have not been modified and facilitators not built upon by any evaluated intervention, necessitating the development and rigorous evaluation of new interventions (gaps).
Figure 1 outlines the number of studies included at various stages of the review. Of the total of 7048 reports identified, 135 reports (describing 116 studies) met the first round of screening and were included in the descriptive map. The results of the map are reported in detail in a separate publication—see Shepherd et al. [ 23 ] (the report can be downloaded free of charge via http://eppi.ioe.ac.uk ). A subset of 22 outcome evaluations and 8 studies of young people's views met the criteria for the in-depth systematic review.

The review process.
Outcome evaluations
Of the 22 outcome evaluations, most were conducted in the United States ( n = 16) [ 31–45 ], two in Finland [ 46, 47 ], and one each in the UK [ 48 ], Norway [ 49 ], Denmark [ 50 ] and Australia [ 51 ]. In addition to the main focus on promoting healthy eating, they also addressed other related issues including cardiovascular disease in general, tobacco use, accidents, obesity, alcohol and illicit drug use. Most were based in primary or secondary school settings and were delivered by teachers. Interventions varied considerably in content. While many involved some form of information provision, over half ( n = 13) involved attempts to make structural changes to young people's physical environments; half ( n = 11) trained parents in or about nutrition, seven developed health-screening resources, five provided feedback to young people on biological measures and their behavioural risk status and three aimed to provide social support systems for young people or others in the community. Social learning theory was the most common theoretical framework used to develop these interventions. Only a minority of studies included young people who could be considered socially excluded ( n = 6), primarily young people from ethnic minorities (e.g. African Americans and Hispanics).
Following detailed data extraction and critical appraisal, only seven of the 22 outcome evaluations were judged to be methodologically sound. For the remainder of this section we only report the results of these seven. Four of the seven were from the United States, with one each from the UK, Norway and Finland. The studies varied in the comprehensiveness of their reporting of the characteristics of the young people (e.g. sociodemographic/economic status). Most were White, living in middle class urban areas. All attended secondary schools. Table I details the interventions in these sound studies. Generally, they were multicomponent interventions in which classroom activities were complemented with school-wide initiatives and activities in the home. All but one of the seven sound evaluations included and an integral evaluation of the intervention processes. Some studies report results according to demographic characteristics such as age and gender.
Soundly evaluated outcome evaluations: study characteristics (n = 7)
| Author/Country/Design | Population | Setting | Objectives | Providers | Programme content |
| Klepp and Wilhelmsen [ ], Norway, CT (+PE) | Seventh grade (13 years old) students | Secondary schools | Teachers and peer educators | ||
| Moon [ ], UK, CT (+PE) | Year 8 and Year 11 pupils (aged 11–16 years) | Secondary schools | |||
| Nicklas [ ], USA, RCT (+PE) | Ninth grade (age range 14–15 years) at start; 3-year longitudinal cohort intervention | High schools | Objective of the ‘Gimme 5’ programme Objective of the parent programme ‘5 a Day For Better Health’: | Teachers, health educators and school catering personnel | |
| Perry [ ], USA, RCT (+PE) | Ninth grade (14- to 15-year-old pupils) | Suburban high school | Teachers administered the programme in general, with 30 class-elected peer leaders leading the class-based sessions | ||
| Vartiainen [ ], Finland, RCT (+PE) | 12- to 16-year-old students | Secondary schools in the Karelia and Kuopio regions of Finland | Health educators, school nurses, peer educators, school teachers | ||
| Walter I and II [ ], USA, RCT (+PE) | Fourth grade (mean age 9 years at start); 5-year longitudinal cohort intervention | Elementary and junior high schools | Teachers delivered the classroom component. Health and education professionals conducted risk factor examination screening |
| Author/Country/Design | Population | Setting | Objectives | Providers | Programme content |
| Klepp and Wilhelmsen [ ], Norway, CT (+PE) | Seventh grade (13 years old) students | Secondary schools | Teachers and peer educators | ||
| Moon [ ], UK, CT (+PE) | Year 8 and Year 11 pupils (aged 11–16 years) | Secondary schools | |||
| Nicklas [ ], USA, RCT (+PE) | Ninth grade (age range 14–15 years) at start; 3-year longitudinal cohort intervention | High schools | Objective of the ‘Gimme 5’ programme Objective of the parent programme ‘5 a Day For Better Health’: | Teachers, health educators and school catering personnel | |
| Perry [ ], USA, RCT (+PE) | Ninth grade (14- to 15-year-old pupils) | Suburban high school | Teachers administered the programme in general, with 30 class-elected peer leaders leading the class-based sessions | ||
| Vartiainen [ ], Finland, RCT (+PE) | 12- to 16-year-old students | Secondary schools in the Karelia and Kuopio regions of Finland | Health educators, school nurses, peer educators, school teachers | ||
| Walter I and II [ ], USA, RCT (+PE) | Fourth grade (mean age 9 years at start); 5-year longitudinal cohort intervention | Elementary and junior high schools | Teachers delivered the classroom component. Health and education professionals conducted risk factor examination screening |
RCT = Randomized Controlled Trial; CT = controlled trial (no randomization); PE = process evaluation.
Separate evaluations of the same intervention in two populations in New York (the Bronx and Westchester County).
The UK-based intervention was an award scheme (the ‘Wessex Healthy Schools Award’) that sought to make health-promoting changes in school ethos, organizational functioning and curriculum [ 48 ]. Changes made in schools included the introduction of health education curricula, as well as the setting of targets in key health promotion areas (including healthy eating). Knowledge levels, which were high at baseline, changed little over the course of the intervention. Intervention schools performed better in terms of healthy food choices (on audit scores). The impact on measures of healthy eating such as choosing healthy snacks varied according to age and sex. The intervention only appeared possibly to be effective for young women in Year 11 (aged 15–16 years) on these measures (statistical significance not reported).
The ‘Know Your Body’ intervention, a cardiovascular risk reduction programme, was evaluated in two separate studies in two demographically different areas of New York (the Bronx and Westchester County) [ 45 ]. Lasting for 5 years it comprised teacher-led classroom education, parental involvement activities and risk factor examination in elementary and junior high schools. In the Bronx evaluation, statistically significant increases in knowledge were reported, but favourable changes in cholesterol levels and dietary fat were not significant. In the Westchester County evaluation, we judged the effects to be unclear due to shortcomings in methods reported.
A second US-based study, the 3-year ‘Gimme 5’ programme [ 40 ], focused on increasing consumption of fruits and vegetables through a school-wide media campaign, complemented by classroom activities, parental involvement and changes to nutritional content of school meals. The intervention was effective at increasing knowledge (particularly among young women). Effects were measured in terms of changes in knowledge scores between baseline and two follow-up periods. Differences between the intervention and comparison group were significant at both follow-ups. There was a significant increase in consumption of fruit and vegetables in the intervention group, although this was not sustained.
In the third US study, the ‘Slice of Life’ intervention, peer leaders taught 10 sessions covering the benefits of fitness, healthy diets and issues concerning weight control [ 41 ]. School functioning was also addressed by student recommendations to school administrators. For young women, there were statistically significant differences between intervention and comparison groups on healthy eating scores, salt consumption scores, making healthy food choices, knowledge of healthy food, reading food labels for salt and fat content and awareness of healthy eating. However, among young men differences were only significant for salt and knowledge scores. The process evaluation suggested that having peers deliver training was acceptable to students and the peer-trainers themselves.
A Norwegian study evaluated a similar intervention to the ‘Slice of Life’ programme, employing peer educators to lead classroom activities and small group discussions on nutrition [ 49 ]. Students also analysed the availability of healthy food in their social and home environment and used a computer program to analyse the nutritional status of foods. There were significant intervention effects for reported healthy eating behaviour (but not maintained by young men) and for knowledge (not young women).
The second ‘North Karelia Youth Study’ in Finland featured classroom educational activities, a community media campaign, health-screening activities, changes to school meals and a health education initiative in the parents' workplace [ 47 ]. It was judged to be effective for healthy eating behaviour, reducing systolic blood pressure and modifying fat content of school meals, but less so for reducing cholesterol levels and diastolic blood pressure.
The evidence from the well-designed evaluations of the effectiveness of healthy eating initiatives is therefore mixed. Interventions tend to be more effective among young women than young men.
Young people's views
Table II describes the key characteristics of the eight studies of young people's views. The most consistently reported characteristics of the young people were age, gender and social class. Socioeconomic status was mixed, and in the two studies reporting ethnicity, the young people participating were predominantly White. Most studies collected data in mainstream schools and may therefore not be applicable to young people who infrequently or never attend school.
Characteristics of young people's views studies (n = 8)
| Study | Aims and objectives | Sample characteristics |
| Dennison and Shepherd [ ] | ||
| Harris [ ] | ||
| McDougall [ ] | ||
| Miles and Eid [ ] | ||
| Roberts [ ] | ||
| Ross [ ] | ||
| Watt and Sheiham [ ] | ||
| Watt and Sheiham [ ] |
| Study | Aims and objectives | Sample characteristics |
| Dennison and Shepherd [ ] | ||
| Harris [ ] | ||
| McDougall [ ] | ||
| Miles and Eid [ ] | ||
| Roberts [ ] | ||
| Ross [ ] | ||
| Watt and Sheiham [ ] | ||
| Watt and Sheiham [ ] |
All eight studies asked young people about their perceptions of, or attitudes towards, healthy eating, while none explicitly asked them what prevents them from eating healthily. Only two studies asked them what they think helps them to eat healthy foods, and only one asked for their ideas about what could or should be done to promote nutrition.
Young people tended to talk about food in terms of what they liked and disliked, rather than what was healthy/unhealthy. Healthy foods were predominantly associated with parents/adults and the home, while ‘fast food’ was associated with pleasure, friendship and social environments. Links were also made between food and appearance, with fast food perceived as having negative consequences on weight and facial appearance (and therefore a rationale for eating healthier foods). Attitudes towards healthy eating were generally positive, and the importance of a healthy diet was acknowledged. However, personal preferences for fast foods on grounds of taste tended to dominate food choice. Young people particularly valued the ability to choose what they eat.
Despite not being explicitly asked about barriers, young people discussed factors inhibiting their ability to eat healthily. These included poor availability of healthy meals at school, healthy foods sometimes being expensive and wide availability of, and personal preferences for, fast foods. Things that young people thought should be done to facilitate healthy eating included reducing the price of healthy snacks and better availability of healthy foods at school, at take-aways and in vending machines. Will-power and encouragement from the family were commonly mentioned support mechanisms for healthy eating, while teachers and peers were the least commonly cited sources of information on nutrition. Ideas for promoting healthy eating included the provision of information on nutritional content of school meals (mentioned by young women particularly) and better food labelling in general.
Table III shows the synthesis matrix which juxtaposes barriers and facilitators alongside results of outcome evaluations. There were some matches but also significant gaps between, on the one hand, what young people say are barriers to healthy eating, what helps them and what could or should be done and, on the other, soundly evaluated interventions that address these issues.
Synthesis matrix
| Young people's views on barriers and facilitators | Interventions which address barriers or build on facilitators identified by young people | ||
| Barriers | Facilitators | Soundly evaluated interventions ( = 7) | Other evaluated interventions ( = 15) |
| ) | ) | ) ) ) ) | ) ) |
| ) | |||
| ) | ) | ||
| ) | ) as well as with adulthood ( ) ) | ) ). | ) ) |
| ) | ) ) | ) (see also ) ) ) ) | |
| ) ) | ) | ) ) ) as above | ) as above |
| ) | ) | ) | |
| ) | ) | ) ) | ) ) |
| ) ) | ) as above None identified—research gap | ||
| ) ) | ) ) | ) ) ) | ) ) |
| ) | |||
| ) | ) | ||
| ) | |||
| Young people's views on barriers and facilitators | Interventions which address barriers or build on facilitators identified by young people | ||
| Barriers | Facilitators | Soundly evaluated interventions ( = 7) | Other evaluated interventions ( = 15) |
| ) | ) | ) ) ) ) | ) ) |
| ) | |||
| ) | ) | ||
| ) | ) as well as with adulthood ( ) ) | ) ). | ) ) |
| ) | ) ) | ) (see also ) ) ) ) | |
| ) ) | ) | ) ) ) as above | ) as above |
| ) | ) | ) | |
| ) | ) | ) ) | ) ) |
| ) ) | ) as above None identified—research gap | ||
| ) ) | ) ) | ) ) ) | ) ) |
| ) | |||
| ) | ) | ||
| ) | |||
Key to young people's views studies: Y1 , Dennison and Shepherd [ 56 ]; Y2 , Harris [ 57 ]; Y3 , McDougall [ 58 ]; Y4 , Miles and Eid [ 59 ]; Y5 , Roberts et al. [ 60 ]; Y6 , Ross [ 61 ]; Y7 , Watt and Sheiham [ 62 ]; Y8 , Watt and Sheiham [ 63 ]. Key to intervention studies: OE1 , Baranowski et al. [ 31 ]; OE2 , Bush et al. [ 32 ]; OE3 , Coates et al. [ 33 ]; OE4 , Ellison et al. [ 34 ]; OE5 , Flores [ 36 ]; OE6 , Fitzgibbon et al. [ 35 ]; OE7 , Hopper et al. [ 64 ]; OE8 , Holund [ 50 ]; OE9 , Kelder et al. [ 38 ]; OE10 , Klepp and Wilhelmsen [ 49 ]; OE11 , Moon et al. [ 48 ]; OE12 , Nader et al. [ 39 ]; OE13 , Nicklas et al. [ 40 ]; OE14 , Perry et al. [ 41 ]; OE15 , Petchers et al. [ 42 ]; OE16 , Schinke et al. [ 43 ]; OE17 , Wagner et al. [ 44 ]; OE18 , Vandongen et al. [ 51 ]; OE19 , Vartiainen et al. [ 46 ]; OE20 , Vartiainen et al. [ 47 ]; OE21 , Walter I [ 45 ]; OE22 , Walter II [ 45 ]. OE10, OE11, OE13, OE14, OE20, OE21 and OE22 denote a sound outcome evaluation. OE21 and OE22 are separate evaluations of the same intervention. Due to methodological limitations, we have judged the effects of OE22 to be unclear. Y1 and Y2 do not appear in the synthesis matrix as they did not explicitly report barriers or facilitators, and it was not possible for us to infer potential barriers or facilitators. However, these two studies did report what young people understood by healthy eating, their perceptions, and their views and opinions on the importance of eating a healthy diet. OE2, OE12, OE16 and OE17 do not appear in the synthesis matrix as they did not address any of the barriers or facilitators.
In terms of the school environment, most of the barriers identified by young people appear to have been addressed. At least two sound outcome evaluations demonstrated the effectiveness of increasing the availability of healthy foods in the school canteen [ 40, 47 ]. Furthermore, despite the low status of teachers and peers as sources of nutritional information, several soundly evaluated studies showed that they can be employed effectively to deliver nutrition interventions.
Young people associated parents and the home environment with healthy eating, and half of the sound outcome evaluations involved parents in the education of young people about nutrition. However, problems were sometimes experienced in securing parental attendance at intervention activities (e.g. seminar evenings). Why friends were not a common source of information about good nutrition is not clear. However, if peer pressure to eat unhealthy foods is a likely explanation, then it has been addressed by the peer-led interventions in three sound outcome evaluations (generally effectively) [ 41, 47, 49 ] and two outcome evaluations which did not meet the quality criteria (effectiveness unclear) [ 33, 50 ].
The fact that young people choose fast foods on grounds of taste has generally not been addressed by interventions, apart from one soundly evaluated effective intervention which included taste testings of fruit and vegetables [ 40 ]. Young people's concern over their appearance (which could be interpreted as both a barrier and a facilitator) has only been addressed in one of the sound outcome evaluations (which revealed an effective intervention) [ 41 ]. Will-power to eat healthy foods has only been examined in one outcome evaluation in the in-depth systematic review (judged to be sound and effective) (Walter I—Bronx evaluation) [ 45 ]. The need for information on nutrition was addressed by the majority of interventions in the in-depth systematic review. However, no studies were found which evaluated attempts to increase the nutritional content of school meals.
Barriers and facilitators relating to young people's practical and material resources were generally not addressed by interventions, soundly evaluated or otherwise. No studies were found which examined the effectiveness of interventions to lower the price of healthy foods. However, one soundly evaluated intervention was partially effective in increasing the availability of healthy snacks in community youth groups (Walter I—Bronx evaluation) [ 45 ]. At best, interventions have attempted to raise young people's awareness of environmental constraints on eating healthily, or encouraged them to lobby for increased availability of nutritious foods (in the case of the latter without reporting whether any changes have been effected as a result).
This review has systematically identified some of the barriers to, and facilitators of, healthy eating with young people, and illustrated to what extent they have been addressed by soundly evaluated effective interventions.
The evidence for effectiveness is mixed. Increases in knowledge of nutrition (measured in all but one study) were not consistent across studies, and changes in clinical risk factors (measured in two studies) varied, with one study detecting reductions in cholesterol and another detecting no change. Increases in reported healthy eating behaviour were observed, but mostly among young women revealing a distinct gender pattern in the findings. This was the case in four of the seven outcome evaluations (in which analysis was stratified by gender). The authors of one of the studies suggest that emphasis of the intervention on healthy weight management was more likely to appeal to young women. It was proposed that interventions directed at young men should stress the benefits of nutrition on strength, physical endurance and physical activity, particularly to appeal to those who exercise and play sports. Furthermore, age was a significant factor in determining effectiveness in one study [ 48 ]. Impact was greatest on young people in the 15- to 16-year age range (particularly for young women) in comparison with those aged 12–13 years, suggesting that dietary influences may vary with age. Tailoring the intervention to take account of age and gender is therefore crucial to ensure that interventions are as relevant and meaningful as possible.
Other systematic reviews of interventions to promote healthy eating (which included some of the studies with young people fitting the age range of this review) also show mixed results [ 52–55 ]. The findings of these reviews, while not being directly comparable in terms of conceptual framework, methods and age group, seem to offer some support for the findings of this review. The main message is that while there is some evidence to suggest effectiveness, the evidence base is limited. We have identified no comparable systematic reviews in this area.
Unlike other reviews, however, this study adopted a wider perspective through inclusion of studies of young people's views as well as effectiveness studies. A number of barriers to healthy eating were identified, including poor availability of healthy foods at school and in young people's social spaces, teachers and friends not always being a source of information/support for healthy eating, personal preferences for fast foods and healthy foods generally being expensive. Facilitating factors included information about nutritional content of foods/better labelling, parents and family members being supportive; healthy eating to improve or maintain one's personal appearance, will-power and better availability/lower pricing of healthy snacks.
Juxtaposing barriers and facilitators alongside effectiveness studies allowed us to examine the extent to which the needs of young people had been adequately addressed by evaluated interventions. To some extent they had. Most of the barriers and facilitators that related to the school and relationships with family and friends appear to have been taken into account by soundly evaluated interventions, although, as mentioned, their effectiveness varied. Many of the gaps tended to be in relation to young people as individuals (although our prioritization of interventions at the level of the community and society may have resulted in the exclusion of some of these interventions) and the wider determinants of health (‘practical and material resources’). Despite a wide search, we found few evaluations of strategies to improve nutritional labelling on foods particularly in schools or to increase the availability of affordable healthy foods particularly in settings where young people socialize. A number of initiatives are currently in place which may fill these gaps, but their effectiveness does not appear to have been reported yet. It is therefore crucial for any such schemes to be thoroughly evaluated and disseminated, at which point an updated systematic review would be timely.
This review is also constrained by the fact that its conclusions can only be supported by a relatively small proportion of the extant literature. Only seven of the 22 outcome evaluations identified were considered to be methodologically sound. As illustrated in Table III , a number of the remaining 15 interventions appear to modify barriers/build on facilitators but their results can only be judged unclear until more rigorous evaluation of these ‘promising’ interventions has been reported.
Finally, it is important to acknowledge that the majority of the outcome evaluations were conducted in the United States, and by virtue of the inclusion criteria, all the young people's views studies were UK based. The literature therefore might not be generalizable to other countries, where sociocultural values and socioeconomic circumstances may be quite different. Further evidence synthesis is needed on barriers to, and facilitators of, healthy eating and nutrition worldwide, particularly in developing countries.
The aim of this study was to survey what is known about the barriers to, and facilitators of, healthy eating among young people with a view to drawing out the implications for policy and practice. The review has mapped and quality screened the extant research in this area, and brought together the findings from evaluations of interventions aiming to promote healthy eating and studies which have elicited young people's views.
There has been much research activity in this area, yet it is disappointing that so few evaluation studies were methodologically strong enough to enable us to draw conclusions about effectiveness. There is some evidence to suggest that multicomponent school-based interventions can be effective, although effects tended to vary according to age and gender. Tailoring intervention messages accordingly is a promising approach which should therefore be evaluated. A key theme was the value young people place on choice and autonomy in relation to food. Increasing the provision and range of healthy, affordable snacks and meals in schools and social spaces will enable them to exercise their choice of healthier, tasty options.
We have identified that several barriers to, and facilitators of, healthy eating in young people have received little attention in evaluation research. Further work is needed to develop and evaluate interventions which modify or remove these barriers, and build on these facilitators. Further qualitative studies are also needed so that we can continue to listen to the views of young people. This is crucial if we are to develop and test meaningful, appropriate and effective health promotion strategies.
We would like to thank Chris Bonell and Dina Kiwan for undertaking data extraction. We would also like to acknowledge the invaluable help of Amanda Nicholas, James Thomas, Elaine Hogan, Sue Bowdler and Salma Master for support and helpful advice. The Department of Health, England, funds a specific programme of health promotion work at the EPPI-Centre. The views expressed in the report are those of the authors and not necessarily those of the Department of Health.
Google Scholar
Google Preview
- healthy diet
| Month: | Total Views: |
|---|---|
| November 2016 | 97 |
| December 2016 | 30 |
| January 2017 | 142 |
| February 2017 | 474 |
| March 2017 | 551 |
| April 2017 | 531 |
| May 2017 | 288 |
| June 2017 | 223 |
| July 2017 | 194 |
| August 2017 | 160 |
| September 2017 | 274 |
| October 2017 | 457 |
| November 2017 | 534 |
| December 2017 | 1,913 |
| January 2018 | 2,369 |
| February 2018 | 2,421 |
| March 2018 | 3,801 |
| April 2018 | 3,998 |
| May 2018 | 2,929 |
| June 2018 | 2,177 |
| July 2018 | 2,422 |
| August 2018 | 2,469 |
| September 2018 | 2,635 |
| October 2018 | 3,102 |
| November 2018 | 4,124 |
| December 2018 | 2,786 |
| January 2019 | 2,687 |
| February 2019 | 3,644 |
| March 2019 | 4,985 |
| April 2019 | 4,055 |
| May 2019 | 3,480 |
| June 2019 | 2,876 |
| July 2019 | 3,013 |
| August 2019 | 2,524 |
| September 2019 | 2,360 |
| October 2019 | 2,100 |
| November 2019 | 2,117 |
| December 2019 | 1,595 |
| January 2020 | 1,884 |
| February 2020 | 2,068 |
| March 2020 | 1,833 |
| April 2020 | 1,953 |
| May 2020 | 970 |
| June 2020 | 1,058 |
| July 2020 | 1,152 |
| August 2020 | 931 |
| September 2020 | 1,518 |
| October 2020 | 1,548 |
| November 2020 | 1,761 |
| December 2020 | 1,207 |
| January 2021 | 1,211 |
| February 2021 | 1,440 |
| March 2021 | 1,910 |
| April 2021 | 1,531 |
| May 2021 | 1,253 |
| June 2021 | 670 |
| July 2021 | 580 |
| August 2021 | 548 |
| September 2021 | 763 |
| October 2021 | 1,058 |
| November 2021 | 1,009 |
| December 2021 | 816 |
| January 2022 | 697 |
| February 2022 | 824 |
| March 2022 | 1,047 |
| April 2022 | 1,053 |
| May 2022 | 935 |
| June 2022 | 504 |
| July 2022 | 391 |
| August 2022 | 436 |
| September 2022 | 694 |
| October 2022 | 909 |
| November 2022 | 790 |
| December 2022 | 489 |
| January 2023 | 764 |
| February 2023 | 601 |
| March 2023 | 938 |
| April 2023 | 755 |
| May 2023 | 686 |
| June 2023 | 594 |
| July 2023 | 452 |
| August 2023 | 419 |
| September 2023 | 582 |
| October 2023 | 840 |
| November 2023 | 593 |
| December 2023 | 522 |
| January 2024 | 811 |
| February 2024 | 717 |
| March 2024 | 866 |
| April 2024 | 1,003 |
| May 2024 | 1,080 |
| June 2024 | 541 |
| July 2024 | 292 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1465-3648
- Print ISSN 0268-1153
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 15 July 2024
The interplay between diet and the gut microbiome: implications for health and disease
- Fiona C. Ross ORCID: orcid.org/0009-0002-9902-8072 1 , 2 , 3 ,
- Dhrati Patangia ORCID: orcid.org/0009-0002-8769-051X 2 , 4 ,
- Ghjuvan Grimaud ORCID: orcid.org/0000-0003-2752-4755 2 , 4 ,
- Aonghus Lavelle 1 , 2 ,
- Eugene M. Dempsey 2 , 3 , 5 ,
- R. Paul Ross ORCID: orcid.org/0000-0003-4876-8839 2 &
- Catherine Stanton ORCID: orcid.org/0000-0002-6724-7011 2 , 3
Nature Reviews Microbiology ( 2024 ) Cite this article
3019 Accesses
243 Altmetric
Metrics details
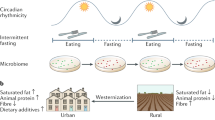
Diet has a pivotal role in shaping the composition, function and diversity of the gut microbiome, with various diets having a profound impact on the stability, functionality and diversity of the microbial community within our gut. Understanding the profound impact of varied diets on the microbiome is crucial, as it will enable us not only to make well-informed dietary decisions for better metabolic and intestinal health, but also to prevent and slow the onset of specific diet-related diseases that stem from suboptimal diets. In this Review, we explore how geographical location affects the gut microbiome and how different diets shape its composition and function. We examine the mechanisms by which whole dietary regimes, such as the Mediterranean diet, high-fibre diet, plant-based diet, high-protein diet, ketogenic diet and Western diet, influence the gut microbiome. Furthermore, we underscore the need for exhaustive studies to better understand the causal relationship between diet, host and microorganisms for the development of precision nutrition and microbiome-based therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Diet–microbiota interactions and personalized nutrition
The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk

The Spanish gut microbiome reveals links between microorganisms and Mediterranean diet
Walker, A. W. & Hoyles, L. Human microbiome myths and misconceptions. Nat. Microbiol. 8 , 1392–1396 (2023).
Article CAS PubMed Google Scholar
Lundgren, S. N. et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 6 , 109 (2018).
Article PubMed PubMed Central Google Scholar
Singh, R. K. et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15 , 73 (2017).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 , 559–563 (2014).
Ghosh, T. S. et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69 , 1218–1228 (2020). This paper demonstrates that a 1-year Mediterranean dietary intervention in elderly individuals can positively alter the gut microbiota, leading to improved markers of lower frailty, cognitive function and reduced inflammation.
Bourdeau-Julien, I. et al. The diet rapidly and differentially affects the gut microbiota and host lipid mediators in a healthy population. Microbiome 11 , 26 (2023).
Article CAS PubMed PubMed Central Google Scholar
Heinken, A. et al. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. 41 , 1320–1331 (2023).
Zampieri, G., Vijayakumar, S., Yaneske, E. & Angione, C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput. Biol. 15 , e1007084 (2019).
Valls-Pedret, C. et al. Mediterranean diet and age-related cognitive decline. JAMA Intern. Med. 175 , 1094 (2015).
Article PubMed Google Scholar
Sánchez-Villegas, A. et al. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med. 11 , 208 (2013).
Martinez-Gonzalez, M. A. & Martin-Calvo, N. Mediterranean diet and life expectancy; beyond olive oil, fruits, and vegetables. Curr. Opin. Clin. Nutr. Metab. Care 19 , 401–407 (2016).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26 , 964–973 (2020).
Johnson, A. J. et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 25 , 789–802.e5 (2019).
Kopp, W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 12 , 2221–2236 (2019).
Shanahan, F., Ghosh, T. S. & O’Toole, P. W. The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160 , 483–494 (2021).
Abdelsalam, N. A., Hegazy, S. M. & Aziz, R. K. The curious case of Prevotella copri . Gut Microbes 15 , 2249152 (2023).
Pasolli, E. et al. Accessible, curated metagenomic data through experimentHub. Nat. Methods 14 , 1023–1024 (2017).
De Filippis, F. et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe 25 , 444–453.e3 (2019).
Hippe, B. et al. Faecalibacterium prausnitzii phylotypes in type two diabetic, obese, and lean control subjects. Benef. Microbes 7 , 511–517 (2016).
Armet, A. M. et al. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe 30 , 764–785 (2022).
KEYS, A. et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 124 , 903–915 (1986).
Muralidharan, J. et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-plus study. Am. J. Clin. Nutr. 114 , 1148–1158 (2021).
Meslier, V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69 , 1258–1268 (2020).
Gómez-Pérez, A. M. et al. Gut microbiota in nonalcoholic fatty liver disease: a PREDIMED-Plus trial sub analysis. Gut Microbes 15 , 2223339 (2023).
Wang, D. D. et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 27 , 333–343 (2021).
Rinott, E. et al. The effects of the green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 14 , 29 (2022).
Waddell, I. S. & Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: from epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 63 , 8752–8767 (2022).
Oliver, A. et al. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. mSystems 6 , e00115–e00121 (2021).
Coker, J. K., Moyne, O., Rodionov, D. A. & Zengler, K. Carbohydrates great and small, from dietary fiber to sialic acids: how glycans influence the gut microbiome and affect human health. Gut Microbes https://doi.org/10.1080/19490976.2020.1869502 (2021).
Benítez-Páez, A. et al. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. mSystems 4 , e00209–e00219 (2019).
Costabile, A. et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br. J. Nutr. 99 , 110–120 (2008).
Wang, Y. et al. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 7 , 129 (2016).
PubMed PubMed Central Google Scholar
Deehan, E. C. et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27 , 389–404.e6 (2020).
Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell 175 , 962–972.e10 (2018).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54 , 2325–2340 (2013).
Article Google Scholar
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 341 , 569–573 (2013).
Siddiqui, M. T. & Cresci, G. A. The immunomodulatory functions of butyrate. J. Inflamm. Res. 14 , 6025–6041 (2021).
van der Hee, B. & Wells, J. M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29 , 700–712 (2021).
Roager, H. M. et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut 68 , 83–93 (2019).
Procházková, N. et al. Advancing human gut microbiota research by considering gut transit time. Gut 72 , 180–191 (2023).
Muegge, B. D. et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332 , 970–974 (2011).
Trefflich, I. et al. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food Sci. Nutr. 60 , 2990–3004 (2020).
Losno, E. A., Sieferle, K., Perez-Cueto, F. J. A. & Ritz, C. Vegan diet and the gut microbiota composition in healthy adults. Nutrients 13 , 2402 (2021).
Miao, Z. et al. Gut microbiota signatures of long-term and short-term plant-based dietary pattern and cardiometabolic health: a prospective cohort study. BMC Med. 20 , 204 (2022).
Cheng, H. et al. Interactions between gut microbiota and polyphenols: a mechanistic and metabolomic review. Phytomedicine 119 , 154979 (2023).
Corrêa, T. A. F., Rogero, M. M., Hassimotto, N. M. A. & Lajolo, F. M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front Nutr. 6 , 188 (2019).
Ross, F. C. et al. Potential of dietary polyphenols for protection from age-related decline and neurodegeneration: a role for gut microbiota? Nutr. Neurosci . https://doi.org/10.1080/1028415X.2023.2298098 (2024).
Selinger, E. et al. Evidence of a vegan diet for health benefits and risks — an umbrella review of meta-analyses of observational and clinical studies. Crit. Rev. Food Sci. Nutr. 63 , 9926–9936 (2022).
Espín, J. C., González-Sarrías, A. & Tomás-Barberán, F. A. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 139 , 82–93 (2017).
Sesso, H. D. et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 115 , 1490–1500 (2022).
Stapleton, P. D., Shah, S., Ehlert, K., Hara, Y. & Taylor, P. W. The β-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus . Microbiology 153 , 2093–2103 (2007).
Chan, C.-L., Gan, R.-Y., Shah, N. P. & Corke, H. Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Control 92 , 437–443 (2018).
Article CAS Google Scholar
Santos, C. A., Lima, E. M. F., de Melo Franco, B. D. G. & Pinto, U. M. Exploring phenolic compounds as quorum sensing inhibitors in foodborne bacteria. Front. Microbiol. 12 , 735931 (2021).
Plamada, D. & Vodnar, D. C. Polyphenols — gut microbiota interrelationship: a transition to a new generation of prebiotics. Nutrients 14 , 137 (2021).
Cortés‐Martín, A., Selma, M. V., Tomás‐Barberán, F. A., González‐Sarrías, A. & Espín, J. C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 64 , 1900952 (2020).
Prochazkova, M. et al. Vegan diet is associated with favorable effects on the metabolic performance of intestinal microbiota: a cross-sectional multi-omics study. Front. Nutr. 8 , 783302 (2022).
Dingeo, G. et al. Phytochemicals as modifiers of gut microbial communities. Food Funct. 11 , 8444–8471 (2020).
Davila, A.-M. et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol. Res. 68 , 95–107 (2013).
Ma, N., Tian, Y., Wu, Y. & Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 18 , 795–808 (2017).
Neis, E., Dejong, C. & Rensen, S. The role of microbial amino acid metabolism in host metabolism. Nutrients 7 , 2930–2946 (2015).
Barreto, F. C. et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4 , 1551–1558 (2009).
Yue, T. et al. Hydrogen sulfide creates a favorable immune microenvironment for colon cancer. Cancer Res. 83 , 595–612 (2023).
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181 , 1263–1275.e16 (2020). This paper shows that a ketogenic diet changes the gut microbiota by depleting bifidobacteria and inhibiting their growth through ketone bodies, leading to a reduction in pro-inflammatory T H 17 cells and emphasizing the importance of chemical communication in mediating host responses to dietary interventions.
Zhu, H. et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 7 , 11 (2022).
Bisanz, J. E., Upadhyay, V., Turnbaugh, J. A., Ly, K. & Turnbaugh, P. J. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26 , 265–272.e4 (2019).
Lindefeldt, M. et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5 , 5 (2019).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173 , 1728–1741.e13 (2018).
Kong, C. et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 6 , 154 (2021).
Ma, D. et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 8 , 6670 (2018).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2 , 50–61 (2020).
Frioux, C. et al. Enterosignatures define common bacterial guilds in the human gut microbiome. Cell Host Microbe 31 , 1111–1125.e6 (2023).
Schnorr, S. L. et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 5 , 3654 (2014).
Martínez, I. et al. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep. 11 , 527–538 (2015). This paper compares the gut microbiota of Papua New Guineans and US residents, indicating that variations in microbial diversity and abundance may result from modern lifestyle factors in industrialized societies, limiting bacterial diversity with implications for human health.
Sun, S. et al. Does geographical variation confound the relationship between host factors and the human gut microbiota: a population-based study in China. BMJ Open 10 , e038163 (2020).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. 107 , 14691–14696 (2010).
De Filippo, C. et al. Diet, environments, and gut microbiota. a preliminary investigation in children living in rural and urban Burkina Faso and Italy. Front. Microbiol. 8 , 1979 (2017).
Zhou, X., Qiao, K., Wu, H. & Zhang, Y. The impact of food additives on the abundance and composition of gut microbiota. Molecules 28 , 631 (2023).
del Pozo, S. et al. Potential effects of sucralose and saccharin on gut microbiota: a review. Nutrients 14 , 1682 (2022).
Wolfson, S. J. et al. Bacterial hydrogen sulfide drives cryptic redox chemistry in gut microbial communities. Nat. Metab. 4 , 1260–1270 (2022).
Naimi, S., Viennois, E., Gewirtz, A. T. & Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 9 , 66 (2021).
Filippou, C. D. et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 11 , 1150–1160 (2020).
Dwiyanto, J. et al. Ethnicity influences the gut microbiota of individuals sharing a geographical location: a cross-sectional study from a middle-income country. Sci. Rep. 11 , 2618 (2021).
Rampelli, S. et al. Metagenome sequencing of the Hadza hunter-gatherer gut microbiota. Curr. Biol. 25 , 1682–1693 (2015).
Gomez, A. et al. Gut microbiome of coexisting BaAka pygmies and Bantu reflects gradients of traditional subsistence patterns. Cell Rep. 14 , 2142–2153 (2016).
Mancabelli, L. et al. Meta‐analysis of the human gut microbiome from urbanized and pre‐agricultural populations. Env. Microbiol. 19 , 1379–1390 (2017).
Ecklu-Mensah, G. et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: the METS-Microbiome study. Nat. Commun. 14 , 5160 (2023).
Jha, A. R. et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 16 , e2005396 (2018).
Carter, M. M. et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell 186 , 3111–3124.e13 (2023).
Huang, Y. et al. Gut microbiota insights into human adaption to high‐plateau diet. iMeta https://doi.org/10.1002/imt2.6 (2022).
Grześkowiak, Ł. et al. Distinct gut microbiota in Southeastern African and Northern European infants. J. Pediatr. Gastroenterol. Nutr. 54 , 812–816 (2012).
Hansen, M. E. B. et al. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol. 20 , 16 (2019).
Tett, A. et al. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 26 , 666–679.e7 (2019). This paper reveals that the gut microorganism P. copri includes a P. copri complex with four distinct clades, predominantly found in non-Western populations, wherein individuals commonly exhibit co-presence of all clades.
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535 , 376–381 (2016).
Dhakan, D. B. et al. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. Gigascience 8 , giz004 (2019).
Dehingia, M. et al. Gut bacterial diversity of the tribes of India and comparison with the worldwide data. Sci. Rep. 5 , 18563 (2015).
Keohane, D. M. et al. Microbiome and health implications for ethnic minorities after enforced lifestyle changes. Nat. Med. 26 , 1089–1095 (2020). This paper shows that Irish Travellers retain similar human gut microbiomes to that of non-industrialized populations, indicating that microbiota composition is associated with non-dietary factors and may be linked to the risk of microbiome-related metabolic diseases.
Vatanen, T. et al. Mobile genetic elements from the maternal microbiome shape infant gut microbial assembly and metabolism. Cell 185 , 4921–4936.e15 (2022).
Ennis, D., Shmorak, S., Jantscher-Krenn, E. & Yassour, M. Longitudinal quantification of Bifidobacterium longum subsp. infantis reveals late colonization in the infant gut independent of maternal milk HMO composition. Nat. Commun. 15 , 894 (2024).
Walsh, C., Lane, J. A., van Sinderen, D. & Hickey, R. M. Human milk oligosaccharides: shaping the infant gut microbiota and supporting health. J. Funct. Foods 72 , 104074 (2020).
Ma, J. et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci. Rep. 10 , 15792 (2020).
Ho, N. T. et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 9 , 4169 (2018).
van den Elsen, L. W. J., Garssen, J., Burcelin, R. & Verhasselt, V. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front. Pediatr. 7 , 47 (2019).
Forbes, J. D. et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases — does a common dysbiosis exist? Microbiome 6 , 221 (2018).
Le Huërou-Luron, I., Blat, S. & Boudry, G. Breast- v . formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 23 , 23–36 (2010).
Taylor, R., Keane, D., Borrego, P. & Arcaro, K. Effect of maternal diet on maternal milk and breastfed infant gut microbiomes: a scoping review. Nutrients 15 , 1420 (2023).
Sindi, A. S. et al. Effect of a reduced fat and sugar maternal dietary intervention during lactation on the infant gut microbiome. Front. Microbiol. 13 , 900702 (2022).
Savage, J. H. et al. Diet during pregnancy and infancy and the infant intestinal microbiome. J. Pediatr. 203 , 47–54.e4 (2018).
Sikder, Md. A. A. et al. Maternal diet modulates the infant microbiome and intestinal Flt3L necessary for dendritic cell development and immunity to respiratory infection. Immunity 56 , 1098–1114.e10 (2023).
Grant, E. T., Boudaud, M., Muller, A., Macpherson, A. J. & Desai, M. S. Maternal diet and gut microbiome composition modulate early‐life immune development. EMBO Mol. Med. 15 , e17241 (2023).
Wang, C. et al. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 14 , 2057779 (2022).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444 , 1027–1031 (2006).
Turnbaugh, P. J., Bäckhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3 , 213–223 (2008).
Bäckhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104 , 979–984 (2007).
Moretti, C. H. et al. Germ‐free mice are not protected against diet‐induced obesity and metabolic dysfunction. Acta Physiol. 231 , e13581 (2021).
Rabot, S. et al. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci. Rep. 6 , 32484 (2016).
Fleissner, C. K. et al. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104 , 919–929 (2010).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102 , 11070–11075 (2005).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473 , 174–180 (2011).
Duncan, S. H. et al. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 32 , 1720–1724 (2008).
Sze, M. A. & Schloss, P. D. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio 7 , e01018–e01116 (2016).
Dalby, M. J. Questioning the foundations of the gut microbiota and obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378 , 20220221 (2023).
Gurung, M. et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51 , 102590 (2020).
Ryan, P. M. et al. Metformin and dipeptidyl peptidase-4 inhibitor differentially modulate the intestinal microbiota and plasma metabolome of metabolically dysfunctional mice. Can. J. Diabetes 44 , 146–155.e2 (2020).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 , 55–71 (2021). This review explores the connection between the gut microbiota, its microbial compounds and their roles in healthy metabolism and in metabolic diseases.
Pascale, A. et al. Microbiota and metabolic diseases. Endocrine 61 , 357–371 (2018).
Chey, W. D., Kurlander, J. & Eswaran, S. Irritable bowel syndrome. JAMA 313 , 949 (2015).
Vervier, K. et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut 71 , 1821–1830 (2022).
Bootz-Maoz, H. et al. Diet-induced modifications to human microbiome reshape colonic homeostasis in irritable bowel syndrome. Cell Rep. 41 , 111657 (2022).
Duan, R., Zhu, S., Wang, B. & Duan, L. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin. Transl. Gastroenterol. 10 , e00012 (2019).
Serrano-Moreno, C. et al. Diets for inflammatory bowel disease: what do we know so far? Eur. J. Clin. Nutr. 76 , 1222–1233 (2022).
Dong, C. et al. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort study. J.Crohns Colitis 16 , 1187–1196 (2022).
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A. & Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr. 11 , 77–91 (2020).
Schmitt, M. & Greten, F. R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 21 , 653–667 (2021).
O’Keefe, S. J. D. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6 , 6342 (2015).
Zhou, Z., Chen, J., Yao, H. & Hu, H. Fusobacterium and colorectal cancer. Front. Oncol . https://doi.org/10.3389/fonc.2018.00371 (2018).
Tjalsma, H., Boleij, A., Marchesi, J. R. & Dutilh, B. E. A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat. Rev. Microbiol. 10 , 575–582 (2012).
Estruch, R. et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378 , e34 (2018).
Delgado-Lista, J. et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 399 , 1876–1885 (2022).
Sobiecki, J. G. et al. A nutritional biomarker score of the Mediterranean diet and incident type 2 diabetes: integrated analysis of data from the MedLey randomised controlled trial and the EPIC-InterAct case-cohort study. PLoS Med. 20 , e1004221 (2023).
Haskey, N. et al. A Mediterranean diet pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: a randomised controlled trial. J. Crohns Colitis 17 , 1569–1578 (2023).
Staudacher, H. M. et al. Clinical trial: a Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 59 , 492–503 (2024).
Barnes, L. L. et al. Trial of the mind diet for prevention of cognitive decline in older persons. N. Engl. J. Med. 389 , 602–611 (2023).
Suskind, D. L. et al. The specific carbohydrate diet and diet modification as induction therapy for pediatric Crohn’s disease: a randomized diet controlled trial. Nutrients 12 , 3749 (2020).
Lewis, J. D. et al. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology 161 , 837–852.e9 (2021).
Wilson, B. et al. Faecal and urine metabolites, but not gut microbiota, may predict response to low FODMAP diet in irritable bowel syndrome. Aliment. Pharmacol. Ther. 58 , 404–416 (2023).
Cox, S. R. et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 158 , 176–188.e7 (2020).
Poslt Königová, M., Sebalo Vňuková, M., Řehořková, P., Anders, M. & Ptáček, R. The effectiveness of gluten-free dietary interventions: a systematic review. Front. Psychol. 14 , 1107022 (2023).
Bonder, M. J. et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 8 , 45 (2016).
Francavilla, A. et al. Gluten-free diet affects fecal small non-coding RNA profiles and microbiome composition in celiac disease supporting a host-gut microbiota crosstalk. Gut Microbes 15 , 2172955 (2023).
Hahn, D., Hodson, E. M. & Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 10 , CD001892 (2020).
PubMed Google Scholar
Hsu, C.-K. et al. Effects of low protein diet on modulating gut microbiota in patients with chronic kidney disease: a systematic review and meta-analysis of international studies. Int. J. Med. Sci. 18 , 3839–3850 (2021).
Garcia-Mazcorro, J. F., Mills, D. A., Murphy, K. & Noratto, G. Effect of barley supplementation on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation in obese db/db mice. Eur. J. Nutr. 57 , 2513–2528 (2018).
Connolly, M. L., Tuohy, K. M. & Lovegrove, J. A. Wholegrain oat-based cereals have prebiotic potential and low glycaemic index. Br. J. Nutr. 108 , 2198–2206 (2012).
Chen, L. et al. Short- and long-read metagenomics expand individualized structural variations in gut microbiomes. Nat. Commun. 13 , 3175 (2022).
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617 , 581–591 (2023).
Finishing the euchromatic sequence of the human genome. Nature 431 , 931–945 (2004).
Abdill, R. J., Adamowicz, E. M. & Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 20 , e3001536 (2022).
Browne, H. P. et al. Boosting microbiome science worldwide could save millions of children’s lives. Nature 625 , 237–240 (2024).
Schei, K. et al. Early gut mycobiota and mother-offspring transfer. Microbiome 5 , 107 (2017).
Zhang, F., Aschenbrenner, D., Yoo, J. Y. & Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 3 , e969–e983 (2022).
Liang, G. & Bushman, F. D. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 19 , 514–527 (2021).
Schulfer, A. et al. Fecal viral community responses to high-fat diet in mice. mSphere 5 , e00833–e00919 (2020).
Pargin, E. et al. The human gut virome: composition, colonization, interactions, and impacts on human health. Front. Microbiol. 14 , 963173 (2023).
Pärnänen, K. et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 9 , 3891 (2018).
Pärnänen, K. M. et al. Early-life formula feeding is associated with infant gut microbiota alterations and an increased antibiotic resistance load. Am. J. Clin. Nutr. 115 , 407–421 (2022).
Stege, P. B. et al. Impact of long-term dietary habits on the human gut resistome in the Dutch population. Sci. Rep. 12 , 1892 (2022).
Oliver, A. et al. Association of diet and antimicrobial resistance in healthy U.S. adults. mBio 13 , e0010122 (2022).
Jacka, F. N. et al. Correction to: a randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 16 , 236 (2018).
Parletta, N. et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr. Neurosci. 22 , 474–487 (2019).
Horn, J., Mayer, D. E., Chen, S. & Mayer, E. A. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl. Psychiatry 12 , 164 (2022). This review compiles evidence from preclinical and clinical studies investigating the impact of dietary interventions on various psychiatric and neurological disorders, to highlight the role of diet-induced microbial alterations and potential benefits for brain health.
Govindpani, K. et al. Vascular dysfunction in Alzheimer’s disease: a prelude to the pathological process or a consequence of it? J. Clin. Med. 8 , 651 (2019).
Kinney, J. W. et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 4 , 575–590 (2018).
Google Scholar
Berding, K. et al. Feed your microbes to deal with stress: a psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 28 , 601–610 (2023).
Adams, J. et al. Comprehensive nutritional and dietary intervention for autism spectrum disorder — a randomized, controlled 12-month trial. Nutrients 10 , 369 (2018).
Ghalichi, F., Ghaemmaghami, J., Malek, A. & Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J. Pediatr. 12 , 436–442 (2016).
Ross, F. C. et al. Existing and future strategies to manipulate the gut microbiota with diet as a potential adjuvant treatment for psychiatric disorders. Biol. Psychiatry 95 , 348–360 (2023).
Download references
Acknowledgements
The authors acknowledge funding from Science Foundation Ireland/APC Microbiome Ireland and the European Union’s Horizon 2020 research and innovation program under grant agreement no. 964590.
Author information
Authors and affiliations.
Department of Anatomy and Neuroscience, University College Cork, Cork, Ireland
Fiona C. Ross & Aonghus Lavelle
APC Microbiome Ireland, University College Cork, Cork, Ireland
Fiona C. Ross, Dhrati Patangia, Ghjuvan Grimaud, Aonghus Lavelle, Eugene M. Dempsey, R. Paul Ross & Catherine Stanton
Department of Paediatrics and Child Health, University College Cork, Cork, Ireland
Fiona C. Ross, Eugene M. Dempsey & Catherine Stanton
Teagasc Moorepark Food Research Centre, Cork, Ireland
Dhrati Patangia & Ghjuvan Grimaud
INFANT Centre, University College Cork, Cork, Ireland
Eugene M. Dempsey
You can also search for this author in PubMed Google Scholar
Contributions
F.R.C., G.G. and D.P. wrote the article and researched data for the manuscript. All authors contributed substantially to the discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Catherine Stanton .
Ethics declarations
Competing interests.
C.S. discloses grant support from Nestle, DuPont Nutrition & Biosciences/International Flavors & Fragrances Inc. (IFF) and Danone Nutricia. E.M.D. and R.P.R. disclose grant funding from IFF. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Ross, F.C., Patangia, D., Grimaud, G. et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-024-01068-4
Download citation
Accepted : 07 June 2024
Published : 15 July 2024
DOI : https://doi.org/10.1038/s41579-024-01068-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Urban poverty and nutrition challenges associated with accessibility to a healthy diet: a global systematic literature review
Affiliations.
- 1 Research Institute for Equitable Development EQUIDE, Universidad Iberoamericana, Prolongación Paseo de Reforma 880, Lomas de Santa Fé, 01219, Mexico City, Mexico. [email protected].
- 2 Research Institute for Equitable Development EQUIDE, Universidad Iberoamericana, Prolongación Paseo de Reforma 880, Lomas de Santa Fé, 01219, Mexico City, Mexico.
- 3 Yale School of Public Health, 60 College Street, New Haven, CT, 06510, USA.
- PMID: 33472636
- PMCID: PMC7816472
- DOI: 10.1186/s12939-020-01330-0
Background: There is an increasing global trend towards urbanization. In general, there are less food access issues in urban than rural areas, but this "urban advantage" does not benefit the poorest who face disproportionate barriers to accessing healthy food and have an increased risk of malnutrition.
Objectives: This systematic literature review aimed to assess urban poverty as a determinant of access to a healthy diet, and to examine the contribution of urban poverty to the nutritional status of individuals.
Methods: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methodology, our review included quantitative and qualitative studies published in English or in Spanish between 2000 and 2019. The articles were eligible if they focused on nutrition access (i.e. access to a healthy diet) or nutrition outcomes (i.e., anemia, overweight and obesity, micronutrient deficiency, micronutrient malnutrition) among urban poor populations. Articles were excluded if they did not meet pre-established criteria. The quality of the quantitative studies was assessed by applying Khan et al.'s methodology. Similarly, we assessed the quality of qualitative articles through an adapted version of the National Institute for Health and Care Excellence (NICE) methodology checklist. Finally, we systematically analyzed all papers that met the inclusion criteria based on a qualitative content and thematic analysis.
Results: Of the 68 papers included in the systematic review, 55 used quantitative and 13 used qualitative methods. Through the analysis of the literature we found four key themes: (i) elements that affect access to healthy eating in individuals in urban poverty, (ii) food insecurity and urban poverty, (iii) risk factors for the nutritional status of urban poor and (iv) coping strategies to limited access to food. Based on the systematization of the literature on these themes, we then proposed a conceptual framework of urban poverty and nutrition.
Conclusions: This systematic review identified distinct barriers posed by urban poverty in accessing healthy diets and its association with poorer nutrition outcomes, hence, questioning the "urban advantage". A conceptual framework emerging from the existing literature is proposed to guide future studies and policies.
Systematic review registration: PROSPERO Registration number: CRD42018089788 .
Keywords: Food security; Nutrition; Public health; Social protection; Urban poverty.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no competing interests.
Preferred Reporting Items for Systematic…
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Diagram
Access measures and nutrition outcomes…
Access measures and nutrition outcomes used as dependent variables in quantitative studies. Note:…
Conceptual Framework of nutrition and…
Conceptual Framework of nutrition and urban poverty
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia. Osborne SR, Alston LV, Bolton KA, Whelan J, Reeve E, Wong Shee A, Browne J, Walker T, Versace VL, Allender S, Nichols M, Backholer K, Goodwin N, Lewis S, Dalton H, Prael G, Curtin M, Brooks R, Verdon S, Crockett J, Hodgins G, Walsh S, Lyle DM, Thompson SC, Browne LJ, Knight S, Pit SW, Jones M, Gillam MH, Leach MJ, Gonzalez-Chica DA, Muyambi K, Eshetie T, Tran K, May E, Lieschke G, Parker V, Smith A, Hayes C, Dunlop AJ, Rajappa H, White R, Oakley P, Holliday S. Osborne SR, et al. Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
- Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low- and middle-income countries (LMIC). Goudet SM, Bogin BA, Madise NJ, Griffiths PL. Goudet SM, et al. Cochrane Database Syst Rev. 2019 Jun 17;6(6):CD011695. doi: 10.1002/14651858.CD011695.pub2. Cochrane Database Syst Rev. 2019. PMID: 31204795 Free PMC article.
- Dietary Patterns and Risk of Cardiovascular Disease: A Systematic Review [Internet]. 2020 Dietary Guidelines Advisory Committee, Dietary Patterns Subcommittee. 2020 Dietary Guidelines Advisory Committee, Dietary Patterns Subcommittee. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul 15. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul 15. PMID: 35294140 Free Books & Documents. Review.
- Dietary Patterns and Growth, Size, Body Composition, and/or Risk of Overweight or Obesity: A Systematic Review [Internet]. Boushey C, Ard J, Bazzano L, Heymsfield S, Mayer-Davis E, Sabaté J, Snetselaar L, Van Horn L, Schneeman B, English LK, Bates M, Callahan E, Butera G, Terry N, Obbagy J. Boushey C, et al. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. PMID: 35129906 Free Books & Documents. Review.
- Food insecurity and academic performance in Spanish adolescents: Results from the EHDLA study. de Camargo EM, Chen S, Jiménez-López E, Victoria-Montesinos D, Smith L, López-Gil JF. de Camargo EM, et al. Heliyon. 2024 Apr 16;10(8):e29489. doi: 10.1016/j.heliyon.2024.e29489. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681539 Free PMC article.
- The Urban Environment and Cardiometabolic Health. Rajagopalan S, Vergara-Martel A, Zhong J, Khraishah H, Kosiborod M, Neeland IJ, Dazard JE, Chen Z, Munzel T, Brook RD, Nieuwenhuijsen M, Hovmand P, Al-Kindi S. Rajagopalan S, et al. Circulation. 2024 Apr 16;149(16):1298-1314. doi: 10.1161/CIRCULATIONAHA.123.067461. Epub 2024 Apr 15. Circulation. 2024. PMID: 38620080 Review.
- Cross-Sectional Analysis of Infant Diet, Outcomes, Consumer Behavior and Parental Perspectives to Optimize Infant Feeding in Response to the 2022 U.S. Infant Formula Shortage. Damian-Medina K, Cernioglo K, Waheed M, DiMaggio DM, Porto AF, Smilowitz JT. Damian-Medina K, et al. Nutrients. 2024 Mar 5;16(5):748. doi: 10.3390/nu16050748. Nutrients. 2024. PMID: 38474876 Free PMC article.
- Evaluation of the Effectiveness of a Bilingual Nutrition Education Program in Partnership with a Mobile Health Unit. French ML, Christensen JT, Estabrooks PA, Hernandez AM, Metos JM, Marcus RL, Thorpe A, Dvorak TE, Jordan KC. French ML, et al. Nutrients. 2024 Feb 23;16(5):618. doi: 10.3390/nu16050618. Nutrients. 2024. PMID: 38474746 Free PMC article.
- Differences in Intakes of Select Nutrients by Urbanization Level in the United States Population 2 Years and Older, NHANES 2013-2018. Wambogo EA, Ansai N, Herrick KA, Reedy J, Hales CM, Ogden CL. Wambogo EA, et al. J Nutr. 2024 Feb;154(2):617-625. doi: 10.1016/j.tjnut.2023.12.030. Epub 2023 Dec 22. J Nutr. 2024. PMID: 38142922
- UN Department of Economic and Social Affairs . 2018 revision of world urbanization prospects: United Nations. 2018.
- UN Development Programme. Sustainable urbanization strategy: UNDP’S support to sustainable, inclusive and resilient cities in the developing world. New York City; 2016.
- Davenport S, Carneiro PT. Governance for development: World Bank. 2015.
- UN . Transforming our world: The 2030 Agenda for Sustainable Development A/RES/70/1. 2015.
- Pérez-Escamilla R. Food security and the 2015-2030 sustainable development goals: from human to planetary health: perspectives and opinions. Curr Dev Nutr. 2017;1(7):e000513. doi: 10.3945/cdn.117.000513. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- UL1 TR001863/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- A-Z Publications
Annual Review of Public Health
Volume 35, 2014, review article, can we say what diet is best for health.
- D.L. Katz 1,2 , and S. Meller 2
- View Affiliations Hide Affiliations Affiliations: 1 Prevention Research Center, Yale University School of Public Health, Griffin Hospital, Derby, Connecticut 06418; email: [email protected] 2 Yale University School of Medicine, New Haven, Connecticut 06510
- Vol. 35:83-103 (Volume publication date March 2014) https://doi.org/10.1146/annurev-publhealth-032013-182351
- © Annual Reviews
Diet is established among the most important influences on health in modern societies. Injudicious diet figures among the leading causes of premature death and chronic disease. Optimal eating is associated with increased life expectancy, dramatic reduction in lifetime risk of all chronic disease, and amelioration of gene expression. In this context, claims abound for the competitive merits of various diets relative to one another. Whereas such claims, particularly when attached to commercial interests, emphasize distinctions, the fundamentals of virtually all eating patterns associated with meaningful evidence of health benefit overlap substantially. There have been no rigorous, long-term studies comparing contenders for best diet laurels using methodology that precludes bias and confounding, and for many reasons such studies are unlikely. In the absence of such direct comparisons, claims for the established superiority of any one specific diet over others are exaggerated. The weight of evidence strongly supports a theme of healthful eating while allowing for variations on that theme. A diet of minimally processed foods close to nature, predominantly plants, is decisively associated with health promotion and disease prevention and is consistent with the salient components of seemingly distinct dietary approaches. Efforts to improve public health through diet are forestalled not for want of knowledge about the optimal feeding of Homo sapiens but for distractions associated with exaggerated claims, and our failure to convert what we reliably know into what we routinely do. Knowledge in this case is not, as of yet, power; would that it were so.
Article metrics loading...
Full text loading...
Literature Cited
- Abdulla M , Andersson I , Asp NG , Berthelsen K , Birkhed D . 1. et al. 1981 . Nutrient intake and health status of vegans. Chemical analyses of diets using the duplicate portion sampling technique. Am. J. Clin. Nutr. 34 : 2464– 77 [Google Scholar]
- Accurso A , Bernstein RK , Dahlqvist A , Draznin B , Feinman RD . 2. et al. 2008 . Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr. Metab. 5 : 9 [Google Scholar]
- Ackermann RT , Finch EA , Brizendine E , Zhou H , Marrero DG . 3. 2008 . Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am. J. Prev. Med. 35 : 357– 63 [Google Scholar]
- Aldana SG , Greenlaw RL , Diehl HA , Salberg A , Merrill RM . 4. et al. 2006 . The behavioral and clinical effects of therapeutic lifestyle change on middle-aged adults. Prev. Chronic Dis. 3 : A05 [Google Scholar]
- Alrabadi NI . 5. 2012 . The effect of lifestyle food on chronic diseases: a comparison between vegetarians and non-vegetarians in Jordan. Glob. J. Health Sci. 5 : 65– 69 [Google Scholar]
- Appel LJ , Sacks FM , Carey VJ , Obarzanek E , Swain JF . 6. et al. 2005 . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 294 : 2455– 64 [Google Scholar]
- Astrup A . 7. 2005 . The role of dietary fat in obesity. Semin. Vasc. Med. 5 : 40– 47 [Google Scholar]
- Atkins R . 8. 2013 . Dr. Atkins' New Diet Revolution. Lanham, MD: Rowman and Littlefield [Google Scholar]
- Bardone-Cone AM , Fitzsimmons-Craft EE , Harney MB , Maldonado CR , Lawson MA . 9. et al. 2012 . The inter-relationships between vegetarianism and eating disorders among females. J. Acad. Nutr. Diet. 112 : 1247– 52 [Google Scholar]
- Barnard ND , Cohen J , Jenkins DJ , Turner-McGrievy G , Gloede L . 10. et al. 2006 . A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 29 : 1777– 83 [Google Scholar]
- Barnard ND , Cohen J , Jenkins DJ , Turner-McGrievy G , Gloede L . 11. et al. 2009 . A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 89 : 1588S– 96S [Google Scholar]
- Barnard ND , Gloede L , Cohen J , Jenkins DJ , Turner-McGrievy G . 12. et al. 2009 . A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J. Am. Diet. Assoc. 109 : 263– 72 [Google Scholar]
- Bastian B , Loughnan S , Haslam N , Radke HR . 13. 2012 . Don't mind meat? The denial of mind to animals used for human consumption. Personal. Soc. Psychol. Bull. 38 : 247– 56 [Google Scholar]
- Belza A , Ritz C , Sorensen MQ , Holst JJ , Rehfeld JF , Astrup A . 14. 2013 . Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr. 97 : 980– 89 [Google Scholar]
- Best D , Grainger P . 15. 2007 . Low-fat or low-carbohydrate diet for cardiovascular health. Can. J. Cardiovasc. Nurs. 17 : 19– 26 [Google Scholar]
- Bjerregaard P , Young TK , Hegele RA . 16. 2003 . Low incidence of cardiovascular disease among the Inuit—What is the evidence?. Atherosclerosis 166 : 351– 57 [Google Scholar]
- Blumenthal JA , Babyak MA , Hinderliter A , Watkins LL , Craighead L . 17. et al. 2010 . Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch. Intern. Med. 170 : 126– 35 [Google Scholar]
- Boden G , Sargrad K , Homko C , Mozzoli M , Stein TP . 18. 2005 . Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 142 : 403– 11 [Google Scholar]
- Boling CL , Westman EC , Yancy WS Jr . 19. 2009 . Carbohydrate-restricted diets for obesity and related diseases: an update. Curr. Atheroscler. Rep. 11 : 462– 69 [Google Scholar]
- Brehm BJ , Seeley RJ , Daniels SR , D'Alessio DA . 20. 2003 . A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 88 : 1617– 23 [Google Scholar]
- Brinsden H , Lobstein T . 21. 2013 . Comparison of nutrient profiling schemes for restricting the marketing of food and drink to children. Pediatr. Obes. 8 : 325– 37 [Google Scholar]
- Cairns G , Angus K , Hastings G , Caraher M . 22. 2013 . Systematic reviews of the evidence on the nature, extent and effects of food marketing to children: a retrospective summary. Appetite 62 : 209– 15 [Google Scholar]
- Carey VJ , Bishop L , Charleston J , Conlin P , Erlinger T . 23. et al. 2005 . Rationale and design of the Optimal Macro-Nutrient Intake Heart Trial to Prevent Heart Disease (OMNI-Heart). Clin. Trials 2 : 529– 37 [Google Scholar]
- 24. Cent. Disease Control. (CDC) 2013 . National Health and Nutrition Examination Survey ( NHANES ) Updated Nov. 25. Atlanta, Ga. http://www.cdc.gov/nchs/nhanes.htm [Google Scholar]
- Chandalia M , Garg A , Lutjohann D , von Bergmann K , Grundy SM , Brinkley LJ . 25. 2000 . Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 342 : 1392– 98 [Google Scholar]
- Chandon P , Wansink B . 26. 2012 . Does food marketing need to make us fat? A review and solutions. Nutr. Rev. 70 : 571– 93 [Google Scholar]
- Chiuve SE , Sampson L , Willett WC . 27. 2011 . The association between a nutritional quality index and risk of chronic disease. Am. J. Prev. Med. 40 : 505– 13 [Google Scholar]
- Cordain L , Eaton SB , Miller JB , Mann N , Hill K . 28. 2002 . The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur. J. Clin. Nutr. 56 : Suppl. 1 S42– 52 [Google Scholar]
- Cordain L , Miller JB , Eaton SB , Mann N . 29. 2000 . Macronutrient estimations in hunter-gatherer diets. Am. J. Clin. Nutr. 72 : 6 1589– 92 [Google Scholar]
- Craig WJ . 30. 2009 . Health effects of vegan diets. Am. J. Clin. Nutr. 89 : 1627S– 33S [Google Scholar]
- Daley CA , Abbott A , Doyle PS , Nader GA , Larson S . 31. 2010 . A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 9 : 10 [Google Scholar]
- Dansinger ML , Gleason JA , Griffith JL , Selker HP , Schaefer EJ . 32. 2005 . Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293 : 43– 53 [Google Scholar]
- Davis B , Melina V . 33. 2000 . Becoming Vegan Summertown, TN: Book [Google Scholar]
- Davis W . 34. 2011 . Wheat Belly Emmaus, PA: Rodale [Google Scholar]
- de Lorgeril M , Salen P . 35. 2005 . Dietary prevention of coronary heart disease: the Lyon Diet Heart Study and after. World Rev. Nutr. Diet. 95 : 103– 14 [Google Scholar]
- de Lorgeril M , Salen P . 36. 2006 . The Mediterranean diet in secondary prevention of coronary heart disease. Clin. Investig. Med. 29 : 154– 58 [Google Scholar]
- de Lorgeril M , Salen P , Martin JL , Monjaud I , Delaye J , Mamelle N . 37. 1999 . Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99 : 779– 85 [Google Scholar]
- Delichatsios HK , Welty FK . 38. 2005 . Influence of the DASH diet and other low-fat, high-carbohydrate diets on blood pressure. Curr. Atheroscler. Rep. 7 : 446– 54 [Google Scholar]
- Denke M . 39. 2001 . Metabolic effects of high-protein, low-carbohydrate diets. Am. J. Cardiol. 88 : 59– 61 [Google Scholar]
- Dewell A , Weidner G , Sumner MD , Chi CS , Ornish D . 40. 2008 . A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J. Am. Diet. Assoc. 108 : 347– 56 [Google Scholar]
- Due A , Larsen TM , Mu H , Hermansen K , Stender S , Astrup A . 41. 2008 . Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am. J. Clin. Nutr. 88 : 1232– 41 [Google Scholar]
- Eaton S , Eaton SB III , Konner MJ . 42. 1997 . Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. Eur. J. Clin. Nutr. 51 : 207– 16 [Google Scholar]
- Estruch R , Ros E , Salas-Salvado J , Covas MI , Corella D . 43. et al. 2013 . Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368 : 1279– 90 [Google Scholar]
- Feinman RD . 44. 2011 . Fad diets in the treatment of diabetes. Curr. Diabet. Rep. 11 : 128– 35 [Google Scholar]
- 45. Food Nutr. Board, Inst. Med 2005 . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids ( Macronutrients ). Washington, DC: Natl. Acad. [Google Scholar]
- Ford ES , Bergmann MM , Kröger J , Schienkiewitz A , Weikert C , Boeing H . 46. 2009 . Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch. Intern. Med. 169 : 1355– 62 [Google Scholar]
- Forsythe CE , Phinney SD , Fernandez ML , Quann EE , Wood RJ . 47. et al. 2008 . Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 43 : 65– 77 [Google Scholar]
- Foster GD , Wyatt HR , Hill JO , Makris AP , Rosenbaum DL . 48. et al. 2010 . Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann. Intern. Med. 153 : 147– 57 [Google Scholar]
- Frassetto LA , Schloetter M , Mietus-Synder M , Morris RC Jr , Sebastian A . 49. 2009 . Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur. J. Clin. Nutr. 63 : 947– 55 [Google Scholar]
- Galimanis A , Mono ML , Arnold M , Nedeltchev K , Mattle HP . 50. 2009 . Lifestyle and stroke risk: a review. Curr. Opin. Neurol. 22 : 60– 68 [Google Scholar]
- Gallop R . 51. 2010 . The G.I. Diet. New York: Workman [Google Scholar]
- Gardner CD , Kiazand A , Alhassan S , Kim S , Stafford RS . 52. et al. 2007 . Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 297 : 969– 77 [Google Scholar]
- Giacosa A , Barale R , Bavaresco L , Gatenby P , Gerbi V . 53. et al. 2013 . Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 22 : 90– 95 [Google Scholar]
- Giugliano D , Ceriello A , Esposito K . 54. 2006 . The effects of diet on inflammation: emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 48 : 677– 85 [Google Scholar]
- Gopinath B , Rochtchina E , Flood VM , Mitchell P . 55. 2010 . Healthy living and risk of major chronic diseases in an older population. Arch. Intern. Med. 170 : 208– 9 [Google Scholar]
- Gregg EW , Chen H , Wagenknecht LE , Clark JM , Delahanty LM . 56. et al. 2012 . Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308 : 2489– 96 [Google Scholar]
- Harris JL , Pomeranz JL , Lobstein T , Brownell KD . 57. 2009 . A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Annu. Rev. Public Health 30 : 211– 25 [Google Scholar]
- Hietaniemi M , Jokela M , Rantala M , Ukkola O , Vuoristo JT . 58. et al. 2009 . The effect of a short-term hypocaloric diet on liver gene expression and metabolic risk factors in obese women. Nutr. Metab. Cardiovasc. Dis. 19 : 177– 83 [Google Scholar]
- Horton J . 59. 2010 . The Global Burden of Disease Study 2010. Lancet 380 : 9859 http://www.elsevierdigital.com/The-Lancet/GBD/ [Google Scholar]
- Hu FB . 60. 2003 . Plant-based foods and prevention of cardiovascular disease: an overview. Am. J. Clin. Nutr. 78 : 544S– 51S [Google Scholar]
- Hu FB . 61. 2009 . Diet and lifestyle influences on risk of coronary heart disease. Curr. Atheroscler. Rep. 11 : 257– 63 [Google Scholar]
- Hu FB , Willett WC . 62. 2002 . Optimal diets for prevention of coronary heart disease. JAMA 288 : 2569– 78 [Google Scholar]
- Hu T , Mills KT , Yao L , Demanelis K , Eloustaz M . 63. et al. 2012 . Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 176 : Suppl. 7 S44– 54 [Google Scholar]
- Hur IY , Reicks M . 64. 2012 . Relationship between whole-grain intake, chronic disease risk indicators, and weight status among adolescents in the National Health and Nutrition Examination Survey, 1999–2004. J. Acad. Nutr. Diet. 112 : 46– 55 [Google Scholar]
- Ibarrola-Jurado N , Bulló M , Guasch-Ferré M , Ros E , Martinez-González MA . 65. et al. 2013 . Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: the PREDIMED study. PLoS One 8 : e57367 [Google Scholar]
- Ingenbleek Y , McCully KS . 66. 2012 . Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition 28 : 148– 53 [Google Scholar]
- 67. Inst. Med 2013 . U.S. Health in International Perspective: Shorter Lives, Poorer Health. Washington, DC: Natl. Acad. [Google Scholar]
- Jacobson MF . 68. 2006 . Six Arguments for a Greener Diet Washington, DC: Cent. Sci. Public Interest [Google Scholar]
- Jenkins DJ , Kendall CW , Marchie A , Faulkner DA , Wong JM . 69. et al. 2003 . Effects of a dietary portfolio of cholesterol-lowering foods versus lovastatin on serum lipids and C-reactive protein. JAMA 290 : 502– 10 [Google Scholar]
- Jenkins DJ , Wong JM , Kendall CW , Esfahani A , Ng VW . 70. et al. 2009 . The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 169 : 1046– 54 [Google Scholar]
- Jeppesen C , Bjerregaard P , Jørgensen ME . 71. 2013 . Dietary patterns in Greenland and their relationship with type 2 diabetes mellitus and glucose intolerance. Public Health Nutr. Feb 11 : 1– 9 [Google Scholar]
- Jequier E , Bray GA . 72. 2002 . Low-fat diets are preferred. Am. J. Med. 113 : 41S– 46S [Google Scholar]
- Johnstone AM , Lobley GE , Horgan GW , Bremner DM , Fyfe CL . 73. et al. 2011 . Effects of a high-protein, low-carbohydrate v. high-protein, moderate-carbohydrate weight-loss diet on antioxidant status, endothelial markers and plasma indices of the cardiometabolic profile. Br. J. Nutr. 106 : 282– 91 [Google Scholar]
- Jönsson T , Granfeldt Y , Ahrén B , Branell UC , Pålsson G . 74. et al. 2009 . Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc. Diabetol. 8 : 35 [Google Scholar]
- Jönsson T , Granfeldt Y , Erlanson-Albertsson C , Ahrén B , Lindeberg S . 75. 2010 . A paleolithic diet is more satiating per calorie than a Mediterranean-like diet in individuals with ischemic heart disease. Nutr. Metab. 7 : 85 [Google Scholar]
- Katz DL . 76. 2005 . Competing dietary claims for weight loss: finding the forest through truculent trees. Annu. Rev. Public Health 26 : 61– 88 [Google Scholar]
- Katz DL . 77. 2008 . Clinically relevant carbohydrate metabolism. See Ref. 83 3– 11
- Katz DL . 78. 2008 . Culture, evolutionary biology, and the determinants of dietary preference. See Ref. 83 423– 33
- Katz DL . 79. 2008 . Diet, diabetes, and insulin resistance. See Ref. 83 102– 22
- Katz DL . 80. 2008 . Diet, weight regulation, and obesity. See Ref. 83 43– 101
- Katz DL . 81. 2008 . Dietary recommendations for health promotion and disease prevention. See Ref. 83 434– 47
- Katz DL . 82. 2008 . Medicine and media: state of the union?. Am. J. Prev. Med. 34 : 83– 84 [Google Scholar]
- Katz DL . 83. 2008 . Nutrition in Clinical Practice. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins [Google Scholar]
- Katz DL . 84. 2008 . Vegetarianism, veganism, and macrobiotic diets. See Ref. 83 414– 20
- Katz DL . 85. 2011 . The Paleo diet: Can we really eat like our ancestors did?. Huffington Post Jul. 6. http://www.huffingtonpost.com/david-katz-md/paleo-diet_b_889349.html [Google Scholar]
- Katz DL . 86. 2012 . Living (and dying) on a diet of unintended consequences. Huffington Post Sep. 11. http://www.huffingtonpost.com/david-katz-md/nutrition-advice_b_1874255.html [Google Scholar]
- Katz DL . 87. 2013 . Bamboozled: the follies of dietary history. US News World Rep. Feb. 25. http://health.usnews.com/health-news/blogs/eat-run/2013/02/25/nutrition-myths-and-common-sense [Google Scholar]
- Katz DL . 88. 2013 . Better diet? Bigger picture!. Huffington Post Feb. 26. http://www.huffingtonpost.com/david-katz-md/nutrition_b_2766942.html [Google Scholar]
- Katz DL . 89. 2013 . Fruits, nuts, and friends like these. Huffington Post Mar. 7. http://www.huffingtonpost.com/david-katz-md/diet-nutrition_b_2825049.html [Google Scholar]
- Katz DL . 90. 2013 . Lifestyle is medicine. Virtual Mentor 15 : 286– 92 [Google Scholar]
- Katz DL . 91. 2013 . Opinion stew. Huffington Post Apr. 12. http://www.huffingtonpost.com/david-katz-md/nutrition-advice_b_3061646.html [Google Scholar]
- Katz DL . 92. 2013 . Our comfortable affliction. Huffington Post Mar. 25. http://www.huffingtonpost.com/david-katz-md/media-health-coverage_b_2937624.html [Google Scholar]
- Katz DL , Njike VY , Faridi Z , Rhee LQ , Reeves RS . 93. et al. 2009 . The stratification of foods on the basis of overall nutritional quality: the Overall Nutritional Quality Index (ONQI). Am. J. Health Promot. 24 : 133– 43 [Google Scholar]
- Katz DL , Njike VY , Rhee LQ , Reingold A , Ayoob KT . 94. 2010 . Performance characteristics of NuVal and the Overall Nutritional Quality Index (ONQI). Am. J. Clin. Nutr. 91 : 1102S– 8S [Google Scholar]
- Klein S . 95. 2004 . Clinical trial experience with fat-restricted vs. carbohydrate-restricted weight-loss diets. Obes. Res. 12 : Suppl. 2 141S– 44S [Google Scholar]
- Knoops KT , deGroot LC , Kromhout D , Perrin AE , Moreiras-Varela O . 96. et al. 2004 . Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA 292 : 1433– 39 [Google Scholar]
- Knowler WC , Barrett-Connor E , Fowler SE , Hamman RF , Lachin JM . 97. et al. 2002 . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 : 393– 403 [Google Scholar]
- Kones R . 98. 2010 . Low-fat versus low-carbohydrate diets, weight loss, vascular health, and prevention of coronary artery disease: the evidence, the reality, the challenge, and the hope. Nutr. Clin. Pract. 25 : 528– 41 [Google Scholar]
- Kuipers RS , Joordens JCA , Muskiet FAJ . 99. 2012 . A multidisciplinary reconstruction of Palaeolithic nutrition that holds promise for the prevention and treatment of diseases of civilisation. Nutr. Res. Rev. 25 : 96– 129 [Google Scholar]
- Kvaavik E , Batty GD , Ursin G , Huxley R , Gale CR . 100. 2010 . Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom Health and Lifestyle Survey. Arch. Intern. Med. 170 : 711– 18 [Google Scholar]
- Lam TK , Cross AJ , Freedman N , Park Y , Hollenbeck AR . 101. et al. 2011 . Dietary fiber and grain consumption in relation to head and neck cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control 22 : 1405– 14 [Google Scholar]
- Lampe JW . 102. 2011 . Dairy products and cancer. J. Am. Coll. Nutr. 30 : 464S– 70S [Google Scholar]
- Lee IM , Shiroma EJ , Lobelo F , Puska P , Blair SN , Katzmarzyk PT . 103. 2012 . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380 : 219– 29 [Google Scholar]
- Liljeberg H , Akerberg A , Bjorck I . 104. 1999 . Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr. 69 : 647– 55 [Google Scholar]
- Lin DW , Neuhouser ML , Schenk JM , Coleman IM , Hawley S . 105. et al. 2007 . Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiol. Biomark. Prev. 16 : 2150– 54 [Google Scholar]
- Lindeberg S , Jönsson T , Granfeldt Y , Borgstrand E , Soffman J . 106. et al. 2007 . A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 50 : 1795– 807 [Google Scholar]
- Lopes HF , Martin KL , Nashar K , Morrow JD , Goodfriend TL , Egan BM . 107. 2003 . DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 41 : 422– 30 [Google Scholar]
- Lundin KE , Alaedini A . 108. 2012 . Non-celiac gluten sensitivity. Gastrointest. Endosc. Clin. N. Am. 22 : 723– 34 [Google Scholar]
- Ma XY , Liu JP , Song ZY . 109. 2012 . Glycemic load, glycemic index and risk of cardiovascular diseases: meta-analyses of prospective studies. Atherosclerosis 223 : 491– 96 [Google Scholar]
- Mann N . 110. 2000 . Dietary lean red meat and human evolution. Eur. J. Nutr. 39 : 71– 79 [Google Scholar]
- Martínez-Lapiscina EH , Clavero P , Toledo E , Estruch R , Salas-Salvadó J . 111. et al. 2013 . Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 84 : 12 1318– 25 [Google Scholar]
- McCarty MF . 112. 1999 . Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med. Hypotheses 53 : 459– 85 [Google Scholar]
- McCullough ML , Patel AV , Kushi LH , Patel R , Willett WC . 113. et al. 2011 . Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol. Biomark. Prev. 20 : 1089– 97 [Google Scholar]
- McGinnis JM , Foege WH . 114. 1993 . Actual causes of death in the United States. JAMA 270 : 18 2207– 12 [Google Scholar]
- McEvoy CT , Temple N , Woodside JV . 115. 2012 . Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 15 : 2287– 94 [Google Scholar]
- McMillan-Price J , Petocz P , Atkinson F , O'Neill K , Samman S . 116. et al. 2006 . Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch. Intern. Med. 166 : 1466– 75 [Google Scholar]
- Meckling KA , Sherfey R . 117. 2007 . A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Appl. Physiol. Nutr. Metab. 32 : 743– 52 [Google Scholar]
- Mirza NM , Palmer MG , Sinclair KB , McCarter R , He J . 118. et al. 2013 . Effects of a low glycemic load or a low-fat dietary intervention on body weight in obese Hispanic American children and adolescents: a randomized controlled trial. Am. J. Clin. Nutr. 97 : 276– 85 [Google Scholar]
- Mokdad AH , Marks JS , Stroup DF , Gerberding JL . 119. 2004 . Actual causes of death in the United States, 2000. JAMA 291 : 1238– 45 [Google Scholar]
- Montgomery KC , Chester J . 120. 2009 . Interactive food and beverage marketing: targeting adolescents in the digital age. J. Adolesc. Health 45 : S18– 29 [Google Scholar]
- 121. Natl. Diabetes Inf. Clearinghouse 2012 . Diabetes Prevention Program. Updated Sept. 9, 2013. NIDDK, Bethesda, Md. http://diabetes.niddk.nih.gov/dm/pubs/preventionprogram/ [Google Scholar]
- 122. Natl. Heart Lung Blood Inst 2012 . What is the DASH eating plan? Updated July 2. NHLBI, Bethesda, Md. http://www.nhlbi.nih.gov/health/health-topics/topics/dash/ [Google Scholar]
- Nilsson LM , Winkvist A , Johansson I , Lindahl B , Hallmans G . 123. et al. 2013 . Low-carbohydrate, high-protein diet score and risk of incident cancer: a prospective cohort study. Nutr. J. 12 : 58 [Google Scholar]
- Nordmann AJ , Suter-Zimmermann K , Bucher HC , Shai I , Tuttle KR . 124. et al. 2011 . Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 124 : 841– 51.e2 [Google Scholar]
- 125. NuVal 2013 . NuVal ® Community. Quincy, MA: NuVal http://www.nuval.com/community [Google Scholar]
- O'Neil CE , Nicklas TA , Zanovec M , Cho S . 126. 2010 . Whole-grain consumption is associated with diet quality and nutrient intake in adults: the National Health and Nutrition Examination Survey, 1999–2004. J. Am. Diet. Assoc. 110 : 1461– 68 [Google Scholar]
- Orchard TJ , Temprosa M , Barrett-Connor E , Fowler SE , Goldberg RB . 127. et al. 2013 . Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet. Med. 30 : 46– 55 [Google Scholar]
- Ornish D , Brown S , Scherwitz L . 128. 1990 . Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet 336 : 129– 33 [Google Scholar]
- Ornish D , Magbanua MJ , Weidner G , Weinberg V , Kemp C . 129. et al. 2008 . Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc. Natl. Acad. Sci. USA 105 : 8369– 74 [Google Scholar]
- Ornish D , Scherwitz LW , Billings JH , Brown SE , Gould KL . 130. et al. 1998 . Intensive lifestyle changes for reversal of coronary heart disease. JAMA 280 : 2001– 7 [Google Scholar]
- Osterdahl M , Kocturk T , Koochek A , Wandell PE . 131. 2008 . Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur. J. Clin. Nutr. 62 : 682– 85 [Google Scholar]
- Perreault L , Pan Q , Mather KJ , Watson KE , Hamman RF , Kahn SE . 132. 2013 . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 379 : 2243– 51 [Google Scholar]
- Pettersen BJ , Anousheh R , Fan J , Jaceldo-Siegl K , Fraser GE . 133. 2012 . Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 15 : 1909– 16 [Google Scholar]
- Phillips SA , Jurva JW , Syed AQ , Syed AQ , Kulinski JP . 134. et al. 2008 . Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension 51 : 376– 82 [Google Scholar]
- Pollan M . 135. 2007 . Unhappy meals. New York Times Mag Jan. 28. http://www.nytimes.com/2007/01/28/magazine/28nutritionism.t.html?pagewanted=all [Google Scholar]
- Pollan M . 136. 2013 . Some of my best friends are germs. New York Times Mag May 15. http://www.nytimes.com/2013/05/19/magazine/say-hello-to-the-100-trillion-bacteria-that-make-up-your-microbiome.html?pagewanted=1&_r=1&nl=todaysheadlines&emc=edit_th_20130519 [Google Scholar]
- 137. PRNewswire 2013 . Weight loss/obesity management market worth $361 billion by 2017. News Release, May 20. http://www.prnewswire.co.uk/news-releases/weight-lossobesity-management-market-worth-361-billion-by-2017-208129511.html
- Ryan MC , Itsiopoulos C , Thodis T , Ward G , Trost N . 138. et al. 2013 . The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 59 : 138– 43 [Google Scholar]
- Sacks FM , Katan M . 139. 2002 . Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 113 : Suppl. 9B 13S– 24S [Google Scholar]
- Schatzkin A , Mouw T , Park Y , Subar AF , Kipnis V . 140. et al. 2007 . Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 85 : 1353– 60 [Google Scholar]
- Serra-Majem L , Roman B , Estruch R . 141. 2006 . Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr. Rev. 64 : S27– 47 [Google Scholar]
- Sexton P , Black P , Metcalf P , Wall CR , Ley S . 142. et al. 2013 . Influence of Mediterranean diet on asthma symptoms, lung function, and systemic inflammation: a randomized controlled trial. J. Asthma 50 : 75– 81 [Google Scholar]
- Song Y , Chavarro JE , Cao Y , Qiu W , Mucci L . 143. et al. 2013 . Whole milk intake is associated with prostate cancer–specific mortality among U.S. male physicians. J. Nutr. 143 : 189– 96 [Google Scholar]
- Stern L , Iqbal N , Seshadri P , Chicano KL , Daily DA . 144. et al. 2004 . The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann. Intern. Med. 140 : 778– 85 [Google Scholar]
- Swain JF , McCarron PB , Hamilton EF , Sacks FM , Appel LJ . 145. 2008 . Characteristics of the diet patterns tested in the optimal macronutrient intake trial to prevent heart disease (OmniHeart): options for a heart-healthy diet. J. Am. Diet. Assoc. 108 : 257– 65 [Google Scholar]
- Tantamango-Bartley Y , Jaceldo-Siegl K , Fan J , Fraser G . 146. 2013 . Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol. Biomark. Prev. 22 : 286– 94 [Google Scholar]
- Tinker LF , Sarto GE , Howard BV , Huang Y , Neuhouser ML . 147. et al. 2011 . Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women's Health Initiative. Am. J. Clin. Nutr. 94 : 1600– 6 [Google Scholar]
- Tonstad S , Stewart K , Oda K , Batech M , Herring RP , Fraser GE . 148. 2013 . Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 23 : 292– 99 [Google Scholar]
- Trichopoulou A , Bamia C , Trichopoulos D . 149. 2009 . Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 338 : b2337 [Google Scholar]
- Turner-McGrievy GM , Barnard ND , Cohen J , Jenkins DJ , Gloede L , Green AA . 150. 2008 . Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J. Am. Diet. Assoc. 108 : 1636– 45 [Google Scholar]
- Turner-McGrievy GM , Barnard ND , Scialli AR . 151. 2007 . A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity 15 : 2276– 81 [Google Scholar]
- 152. US Dep. Agric. (USDA) 2010 . Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Press Release, June 15. http://www.cnpp.usda.gov/DGAs2010-Dgacreport.htm
- 153. US Dep. Agric. (USDA), US Dep. Health Hum. Serv 2011 . Dietary Guidelines for Americans, 2010. Washington, DC: US Gov. Print. Off http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm [Google Scholar]
- 154. US Dep. Agric. (USDA) 2013 . Dietary Reference Intakes Beltsville, MD: US Dep. Agric http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes [Google Scholar]
- 155. US News World Rep 2013 . Best Diets Overall. Washington, DC: US News World Rep http://health.usnews.com/best-diet/best-overall-diets [Google Scholar]
- van de Laar RJ , Stehouwer CD , van Bussel BC , te Velde SJ , Prins MH . 156. et al. 2012 . Lower lifetime dietary fiber intake is associated with carotid artery stiffness: the Amsterdam Growth and Health Longitudinal Study. Am. J. Clin. Nutr. 96 : 14– 23 [Google Scholar]
- Varady KA , Bhutani S , Klempel MC , Phillips SA . 157. 2011 . Improvements in vascular health by a low-fat diet, but not a high-fat diet, are mediated by changes in adipocyte biology. Nutr. J. 10 : 8 [Google Scholar]
- Venneria E , Fanasca S , Monastra G , Finotti E , Ambra R . 158. et al. 2008 . Assessment of the nutritional values of genetically modified wheat, corn, and tomato crops. J. Agric. Food Chem. 56 : 9206– 14 [Google Scholar]
- Volek JS , Fernandez ML , Feinman RD , Phinney SD . 159. 2008 . Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 47 : 307– 18 [Google Scholar]
- 160. Weight Watchers 2013 . Weight Watchers PointsPlus. New York: Weight Watch. Int http://www.weightwatchers.com/plan/eat/plan.aspx [Google Scholar]
- Weisburger JH . 161. 2002 . Lifestyle, health and disease prevention: the underlying mechanisms. Eur. J. Cancer Prev. 11 : Suppl. 2 S1– 7 [Google Scholar]
- 162. Wellmark 2013 . Blue Zones Project. Minneapolis, MN: Blue Zones http://www.bluezones.com/ [Google Scholar]
- Westman EC , Yancy WS Jr , Mavropoulos JC , Marquart M , McDuffie JR . 163. 2008 . The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 5 : 36 [Google Scholar]
- 164. World Health Organ. (WHO) 2011 . Nutrient Profiling Report of a WHO/IASO Technical Meeting London, UK, 4–6 October 2010. Geneva, Switz.: WHO/IASO [Google Scholar]
- Yancy WS Jr , Almirall D , Maciejewski ML , Kolotkin RL , McDuffie JR , Westman EC . 165. 2009 . Effects of two weight-loss diets on health-related quality of life. Qual. Life Res. 18 : 281– 89 [Google Scholar]
- Ye X , Scott T , Gao X , Maras JE , Bakun PJ , Tucker KL . 166. 2013 . Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J. Acad. Nutr. Diet. 113 : 276– 81.e3 [Google Scholar]
Data & Media loading...
- Article Type: Review Article
Most Read This Month
Most cited most cited rss feed, review of community-based research: assessing partnership approaches to improve public health, nature and health, measuring social class in us public health research: concepts, methodologies, and guidelines, the epidemiology of depression across cultures, acute respiratory effects of particulate air pollution, racism and health: evidence and needed research, the social determinants of health: coming of age, the role of behavioral science theory in development and implementation of public health interventions, mediation analysis: a practitioner's guide, the prescription opioid and heroin crisis: a public health approach to an epidemic of addiction.
Choosing the healthiest diet for a single day: A literature review

- Lamar State College Port Arthur
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Neeraj Singh Rajpurohit

- Shitij Arora
- Jawahar Lal Agarwal

- LIPIDS HEALTH DIS

- Kazunori Utsunomiya

- Nikki A. Ford

- Harpuneet Kaur
- Mohammad Emami
- Mehryar Soleimani

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Systematic review
- Open access
- Published: 20 January 2021
Urban poverty and nutrition challenges associated with accessibility to a healthy diet: a global systematic literature review
- Mireya Vilar-Compte ORCID: orcid.org/0000-0001-9047-1102 1 ,
- Soraya Burrola-Méndez 1 ,
- Annel Lozano-Marrufo 1 ,
- Isabel Ferré-Eguiluz 1 ,
- Diana Flores 1 ,
- Pablo Gaitán-Rossi 1 ,
- Graciela Teruel 1 &
- Rafael Pérez-Escamilla 2
International Journal for Equity in Health volume 20 , Article number: 40 ( 2021 ) Cite this article
53k Accesses
112 Citations
69 Altmetric
Metrics details
There is an increasing global trend towards urbanization. In general, there are less food access issues in urban than rural areas, but this “urban advantage” does not benefit the poorest who face disproportionate barriers to accessing healthy food and have an increased risk of malnutrition.
This systematic literature review aimed to assess urban poverty as a determinant of access to a healthy diet, and to examine the contribution of urban poverty to the nutritional status of individuals.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methodology, our review included quantitative and qualitative studies published in English or in Spanish between 2000 and 2019. The articles were eligible if they focused on nutrition access (i.e. access to a healthy diet) or nutrition outcomes (i.e., anemia, overweight and obesity, micronutrient deficiency, micronutrient malnutrition) among urban poor populations. Articles were excluded if they did not meet pre-established criteria. The quality of the quantitative studies was assessed by applying Khan et al.’s methodology. Similarly, we assessed the quality of qualitative articles through an adapted version of the National Institute for Health and Care Excellence (NICE) methodology checklist. Finally, we systematically analyzed all papers that met the inclusion criteria based on a qualitative content and thematic analysis.
Of the 68 papers included in the systematic review, 55 used quantitative and 13 used qualitative methods. Through the analysis of the literature we found four key themes: (i) elements that affect access to healthy eating in individuals in urban poverty, (ii) food insecurity and urban poverty, (iii) risk factors for the nutritional status of urban poor and (iv) coping strategies to limited access to food. Based on the systematization of the literature on these themes, we then proposed a conceptual framework of urban poverty and nutrition.
Conclusions
This systematic review identified distinct barriers posed by urban poverty in accessing healthy diets and its association with poorer nutrition outcomes, hence, questioning the “urban advantage”. A conceptual framework emerging from the existing literature is proposed to guide future studies and policies.
Systematic review registration
PROSPERO Registration number: CRD42018089788 .
Urbanization is a rising global phenomenon. Today 55% of the global population lives in urban areas, and it is estimated that by 2050 70% of the population will live in one of them [ 1 ]. Compared to rural areas, urban regions feature greater social and economic development, more labor opportunities, and access to more diverse and better essential services. However, urban areas also concentrate poverty [ 2 ]. The urban poor not only lack income and resources to ensure an adequate wellbeing, but frequently experience limited access to basic services, labor opportunities and to possibilities for social development. Prior studies highlight increasing trends in urban poverty, partially resulting from accelerating urbanization processes in low-and middle-income countries; it has been estimated that by 2035 the majority of individuals in extreme poverty (i.e. daily income less than US1.25) will live in urban areas [ 1 , 3 ].
These challenges have been addressed in the Sustainable Development Goals (SDG) [ 4 ]; specifically, SDG 11 establishes that countries need to have urban sustainable development plans to promote the wellbeing of people, especially the most socioeconomic vulnerable. Furthermore, SDG 1 states that all forms of poverty should be eradicated by 2030.
The SDGs are also strongly linked with food insecurity (FI) [ 5 ]. Urban environments imply a particular risk for FI and poor nutrition outcomes since access to food depends on the commercial supply that, in turn, is linked to income levels [ 6 , 7 ]. On the one hand, it has been previously recognized that the urban poor are particularly vulnerable to macroeconomic shocks that affect their capacity to generate income which in turn leads them to consume less healthy diets [ 8 , 9 ]. On the other hand, previous studies suggest that urban diets, on average, are better than rural diets because they are more diverse and, given the food distribution systems, there is greater access to products such as animal proteins [ 10 ]. However, this supposed urban advantage is not equally distributed as it does not extend to the poorest socioeconomic strata.
Previous research indicates that there are geographic differentials in access to food [ 11 ], which are linked to economic barriers in accessing healthy food options [ 12 ]. Hence, those with lower incomes do not have access to diets rich in heathy foods including fresh fruits and vegetables, tubers, and legumes. Instead they have relatively more access and consume higher amounts of sugars, fats, and highly processed or ultra-processed foods [ 13 ]. Although this phenomenon has been generically identified as part of the “nutritional transition”, it is important to emphasize that in urban centers, these outcomes are linked to socioeconomic inequities [ 6 ]. Ultra-processed products have a high energy density, have long shelf lives, many are ready-to-eat and they are relatively cheaper [ 14 , 15 ]. All these features make them convenient for urban and low-income individuals who may have limited resources such as household heating and cooking goods, safe drinking water supply, and sanitation, amongst other basic needs. A study of 74 countries from the Pan-American Health Organization conducted in 2015 found that sales of ultra-processed products were larger in more urbanized countries, and that the market is expanding to poorer sectors [ 16 ].
Food environments can influence the risk of malnutrition and corresponding infectious and non-communicable chronic diseases. In urban areas, food deserts and food swamps – understood as regions with very limited or difficult access to supermarkets and healthful food choices [ 17 ] – exemplify challenging food environments, which are generally more common in low-income urban areas [ 18 ]. These environments are in turn associated with unequal nutrition outcomes. For example, in Latin America, the risk of chronic malnutrition in urban children under 5 years of age is ten times higher among the poorest compared with their counterparts falling in the highest socioeconomic level [ 7 ].
Despite such compelling evidence, there are few studies that have attempted to document in detail the food access challenges and their relationship with different nutritional outcomes among poor urban populations. Therefore, the aim of this study was to conduct, from a global perspective, a systematic literature review (SLR) to assess urban poverty as a determinant of access to a healthy diet, and to document the association between urban poverty and the nutritional status of individuals.
The protocol for this systematic review was registered on PROSPERO prior to starting the literature search (CRD42018089788).
The review centered in nutrition outcomes related to: (i) access to a healthy diet as defined by the World Health Organization [ 19 ], which includes aspects of variety, quantity, balance and food safety, and (ii) nutrition outcomes related to the SDGs – anemia, overweight and obesity, micronutrient deficiency, and micronutrient malnutrition [ 20 ]. These outcomes were kept generic and subsequently categorized through the operationalizations used in the studies. The exposure variable of interest was urban poverty. Poverty was captured through different indicators such as income thresholds, poverty lines, multidimensional poverty measures, socioeconomic indexes (based on assets and services), wealth indexes, geographic areas considered highly vulnerable or lacking basic services (i.e. slums), or people participating in social programs targeted at the vulnerable/low income. Similarly, “urban” as a context where poverty happens was not defined through a unique criterion – as different countries used different criteria. Hence, “urban” was defined in terms of population size, population density, type of economic activity, level of infrastructure, or a combination of these criteria.
Inclusion and exclusion criteria
This systematic review followed the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 21 , 22 ]. We prepared a literature search protocol to define a priori inclusion criteria (see Table 1 ). Qualitative and quantitative studies were included if they focused on nutrition access or nutrition outcomes among urban poor populations (i.e. individuals, families, households). Quantitative studies could be observational or experimental.
Studies were excluded if they focused on the general population (i.e. without a specific focus on urban and poor settings) or if they were centered in populations with special conditions (i.e. refugees, prisoners). Only peer reviewed studies published in English or Spanish were included in the review.
Search strategy
Four bibliographical databases (PubMed, Web of Science, Scielo and EBSCO) were systematically searched for studies published between January 2000 and January 2019. The year 2000 was selected as a threshold because urbanization was recognized as key in the Millennium Development Goals (MDGs) linked to poverty and the health outcomes of individuals. Indeed, the MDGs led to specific research and interventions targeting the urban vulnerable populations [ 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 ]. Relevant literature was identified following the Boolean search algorithms summarized in Supplementary Table 1 . Free-text terms were used to generate search strategies for each database. Studies identified through each database were imported to Excel, and then duplicates were identified and removed. The studies were then imported to EndNote [ 31 ].
Study selection
In the first phase, abstracts were reviewed by three of the authors (DF, IF and SB) who were standardized to screen titles and abstracts of studies identified in the search. Articles were excluded if they did not meet the criteria established in Table 1 . They were included if there was an indication that access to healthful foods or any of the nutrition outcomes of interest were being described or analyzed, either through qualitative or quantitative approaches, in urban poor/vulnerable populations. In the next phase, articles were retrieved and independently assessed for eligibility (see criteria in Table 1 ). Consensus was reached in consultation with a fourth author (MVC) as needed.
Data extraction
The following information was extracted from each study: (i) methods (i.e. qualitative or quantitative study design, and corresponding details); (ii) territorial definition of the urban space (i.e. urban or semi-urban, large cities, slums, etc.); (iii) poverty definition; and (iv) operationalization of the food and nutrition variables (i.e. food access, nutrition outcomes). In addition, data were extracted to describe the study sample, confounding or mediating factors, statistical tests or data triangulation, and key findings.
Quality assessment
The studies’ quality assessment was conducted by reviewing each study according to specific guidelines. For quantitative studies, guidelines were adapted from those proposed by Khan [ 22 ] which focus on four aspects: (i) type of design; (ii) how exposure was operationalized; (iii) how outcome variables were ascertained; and (iv) if confounding variables were controlled for. Supplementary Table 2 provides further details on the definition of each of these elements. For qualitative studies a guideline was adapted from the National Institute for Health and Care Excellence (NICE) methodology checklist for qualitative studies [ 32 ]. Five quality domains were assessed for each study: (i) theoretical approach; (ii) study design; (iii) data collection; (iv) validity; and (v) analysis. Supplementary Table 3 defines how each of the areas were specifically assessed. Quality assessment was performed by two researchers (SB, IF); when there were conflicting results a third reviewer (ALM, MVC) provided input until consensus was reached. To estimate the agreement between reviewers, a Cohen’s Kappa statistic was computed.
Analysis of the systematized papers
The purpose of systematically examining the studies was to generate a common understanding about how urban poverty shapes nutrition (both in terms of access and outcomes). The analysis of the studies was based on a qualitative content and thematic analyses. The objective of such perspective was to analyze the textual data from the studies to elucidate themes [ 33 ]. Hence, a three folded analytical process was followed: (i) data from the studies was coded in NVivo 12 [ 34 ]; nodes were generated and significant information from the systematized papers was dropped in such nodes; (ii) meaning of the information in the different nodes was examined; and (iii) themes were generated. This analysis was performed by three of the authors (MVC, DF, SB) based on consensus about the nodes, meanings and themes. These findings led to proposing a conceptual framework about how urban poverty shapes nutrition.
Description of the studies
Figure 1 follows the PRISMA structure [ 22 ] and provides a detailed summary of the research results. After duplicated studies were removed, the abstracts of 717 records were screened, leading to 348 papers for full review. Sixty-eight studies met the eligibility criteria and quality assessment and were included in the review. Among these studies, the majority (81%) used quantitative methods, while fewer focused on qualitative approaches (19%). The average Cohen’s Kappa statistic between-reviewers for quantitative studies was 0.963 (an almost perfect agreement), and for qualitative studies 0.759 (a substantial level of agreement) [ 35 ].
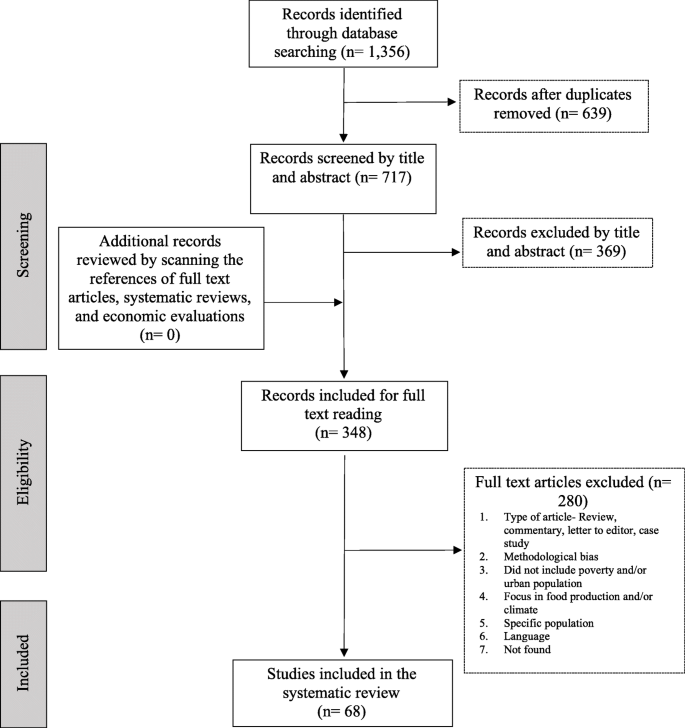
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Diagram
The geographical distribution of the included studies is presented in Table 2 . Based on the categorization by regions as classified by the World Bank [ 36 ], close to two thirds of the papers were based on studies conducted in the Americas (i.e. 39.7% in North America and 25% in Latin American & Caribbean), followed by 17.6% in Sub-Saharan Africa, and 17.6% in East Asia & Pacific. Only 8.8% were from South Asia, 5.9% from Europe & Central Asia, and 2.9% from Middle East & North Africa.
Tables 3 and 4 provide information on how studies operationalized the poverty construct. It was commonly defined through mainstream economic classifications such as: lower deciles or quintiles of income distribution (18.9%); low socioeconomic level, ascertained through education level, type of employment, or social class (17.6%); poverty lines or thresholds based on a minimum income to satisfy basic needs, or through more complex multidimensional measures of poverty (13.5%); composite measures such as assets indexes (5.4%) or social vulnerability indexes (2.7%); and relative household’s expenditure measures (1.4%) – which are commonly used in the economics literature due to their strong theoretical background. Together, these definitions of poverty or vulnerability were used in more than half of the studies (59.5%).
The second most common metrics used for determining poverty status was through geographical characteristics (27%). Based on community, municipality or other geographic units, the studies defined the poverty status based on access to services or gradients of human development, among others. The degree of specification of how “poor areas” were defined varied across studies. Finally, another subset of the studies included in the SLR defined poverty and vulnerability through specific unidimensional conditions such as poor housing conditions, FI or homelessness (13.5%).
Tables 3 and 4 also provide information about how the “urban” space was ascertained in the studies. More than half of the studies (54.4%) defined broadly the urban space as “cities” or “metropolitan areas”. Around one third of the studies (32.4%) centered in areas within a city, while 13.3% of the studies focused in specific peri-urban areas or slums.
Among the quantitative studies ( n = 55), 63% analyzed food access measures as dependent variables, 30% as nutrition outcomes, and 7% as both. As portrayed in Fig. 2 , the most common operationalization of access was through food security scales, dietary diversity indexes or scores, and through assessments of access to retail food stores. On the other hand, overweight and obesity and stunting were the most commonly assessed nutrition outcomes. Qualitative studies ( n = 13) focused in access to healthful choices from different perspectives: about half of the papers studied aspects of food security, around one quarter focused in understanding the food environment, close to one fifth addressed issues of affordability and food supply, and one study assessed coping strategies for lack of food access.
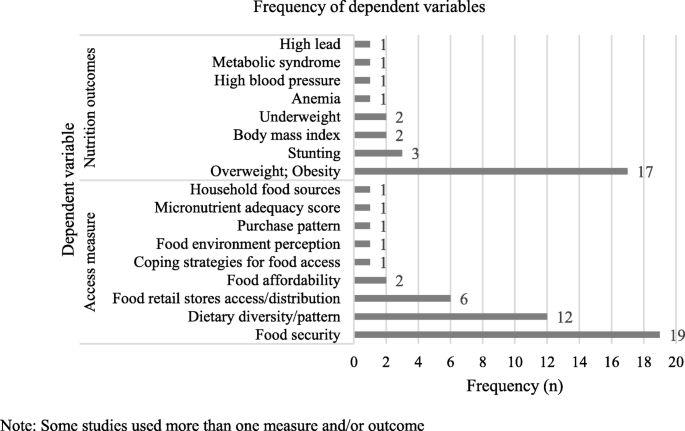
Access measures and nutrition outcomes used as dependent variables in quantitative studies. Note: Some studies used more than one measure and/or outcome
Assessment of the quality of research
For quantitative studies, quality was assessed through three dimensions: (i) type of design, (ii) comparison group or not, and (iii) control for potential confounders (i.e. adjusted models). As summarized in Table 3 , most studies relied on cross-sectional designs (80%). The rest of the studies were a mix of geospatial analyses (9.1%), cohort and longitudinal studies (9.1%), and only one study was based on a case-control design (1.8%). About 82% of the studies had a comparison group, which was commonly operationalized as urban non-poor populations, rural poor populations, or as comparisons between different subgroups of urban poor population (i.e. differences in income within poor groups, different levels of FI, amongst others). Among studies lacking a comparison group, they were mainly cross-sectional studies [ 38 , 39 , 42 , 43 , 47 , 52 , 77 , 81 , 88 , 89 ] that intended to provide descriptions of urban poverty in terms of nutrition outcomes. Close to 70% of all quantitative studies controlled for confounders and presented adjusted models. However, none of the geospatial analyses did so [ 42 , 52 , 78 , 80 , 91 ], neither the case-control study [ 48 ]. By contrast, 75% of the cross-sectional designs [ 37 , 39 , 40 , 45 , 46 , 49 , 50 , 51 , 54 , 55 , 56 , 59 , 61 , 62 , 63 , 64 , 65 , 66 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 82 , 83 , 84 , 86 , 87 , 89 , 90 ] and all the cohort and longitudinal studies controlled for confounders [ 44 , 53 , 60 , 69 , 88 ].
Among the 13 qualitative studies included in the SLR, all showed adequate research quality (see Table 4 ). All studies were found to have an adequate theoretical approach with clear aims, and a well-established study design including sample characteristics and qualitative sampling processes. Similarly, all the studies provided a description of the data collection process, recording and transcription of study materials, the study context and participants, and addressed some potential research biases. In terms of data triangulation, which is an important validity aspect of qualitative approaches, most studies reported collecting data through different sources and linking them for purposes of analysis; the only two exceptions were the studies by Dubowitz et al. [ 97 ] and Hammelman [ 99 ]. Despite their lack of triangulation, both studies were rated as having richness in data. In fact, all studies but one were rated as having dense and rich qualitative data; with the exception of a study focusing on FI among homeless and marginally housed adults in Sydney, Australia [ 104 ]. Qualitative studies applied different data collection techniques such as in-depth interviews [ 92 , 95 , 96 , 98 , 99 , 101 , 103 , 104 ], focus groups [ 93 , 94 , 97 , 101 ], participant observation [ 95 , 101 ], open-ended questionnaires [ 102 ] and photovoice [ 100 ].
Content and thematic analysis
Given the diversity of designs, methodological and measurement approaches, instead of summarizing effect sizes or aiming at a meta-analysis, we took a qualitative thematic approach to synthesize and analyze the literature. From such perspective, four broad categories emerged: (i) elements that affect access to healthy eating in individuals in urban poverty, (ii) FI and urban poverty, (iii) risk factors for the nutritional status of urban poor and (iv) coping strategies to limited access to food.

Elements of urban poverty that affect access to healthy eating
Urban poverty exerts different pressures which lead, in many cases, to problems of access to a healthy diet that are as serious as in rural areas (Supplementary Table 4 ). One of the risk factors documented in the literature for this lack of access are the economic barriers faced by the urban poor. These studies provide evidence that healthy diets are expensive, which leads to dose-response socioeconomic inequities in food choices. For example, in urban settings budgetary restrictions in the selection of food can lead to the consumption of diets that are very low in animal protein [ 51 ], or may disrupt requirements among populations with special dietary needs [ 92 , 101 ]. Urban dwellers in the lowest income deciles, allocate a higher proportion of their family income to food consumption [ 41 , 57 ], and may find restrictions to buying healthy foods [ 93 ].
In addition, low income urban neighborhoods, tend to have less access to healthful foods, thus, linking economic constrains of the population and place of living to a magnified lack of access to healthy foods [ 78 ]. There are effects of the market structure on access to food in urban poor areas, a common finding was a lower supply of supermarkets [ 42 , 78 , 91 ] that can lead to food deserts. In addition, supermarkets in urban poor areas tend to offer less variety of healthy products (i.e. fresh produce) and oftentimes products of lower quality [ 71 ]. Such fragmented market can lead to the establishment of informal arrangements, especially in low- and middle-income countries, such as street traders and house shops that are more likely to be unstable and deregulated [ 43 , 85 ]. Corner shops are another common source to meet food demand, but this has been associated with increased consumption of ultra-processed foods and inversely associated with home meal preparation, positive beliefs and self-efficacy toward healthy food [ 55 ].
Among poor urban dwellers accessing healthier choices commonly requires “out-shopping” defined as shopping outside of your residential area, but this is limited by transportation cost and lack of public transportation access [ 42 ]. In addition, this implies additional direct costs (i.e. transportation) and opportunity cost (i.e. time spent) in food purchasing [ 99 ]. This can be an even larger barrier to access when experiencing health conditions affecting physical mobility [ 92 ].
An additional barrier faced by the urban poor is the lack of social networks that allow them to access food during difficult times. Urban studies have documented less reciprocity with food exchanges than those observed in rural areas [ 68 ].
Food insecurity and urban poverty
An important body of literature emerged documenting the relationship between FI and urban poverty. FI is defined as “the limited or uncertain availability of nutritionally adequate and safe foods; or the limited and uncertain capacity to acquire adequate food in socially acceptable ways” [ 105 ]. This literature was grouped into: quantitative studies that address the determinants of FI, quantitative studies that analyze how FI is associated with unfavorable nutrition outcomes among the urban poor, and qualitative studies documenting experiences of FI among urban vulnerable populations.
Determinants of FI in poor urban settings
Studies from all regions of the world informed the literature on determinants of FI in poor urban settings. Almost all studies operationalized FI through experience-based scales. Most of the studies were based on cross-sectional designs and logistic regression analysis (see Supplementary Table 5 ).
One of the main FI risk factors identified in the literature was low household income; among those living on urban and peri-urban areas, low income increased risk of FI [ 38 , 44 , 45 , 46 , 50 , 53 , 58 , 59 , 65 , 72 , 76 , 82 , 84 , 89 ]. Similarly, a study found that lower socioeconomic status and higher levels of unemployment were associated with a higher prevalence of FI [ 37 ]. Few studies focused on assets-based measures and FI. A study documented that households with inconsistent access to utilities such as electricity or water, medical care, cooking fuel and cash had a significantly higher prevalence of severe FI [ 66 ]. Another study reported that access to a personal vehicle was inversely associated with FI [ 64 ].
In addition to experience-based FI scales, one study assessed dietary diversity finding similar associations with socioeconomic status. More specifically it documented that lower income adults in urban areas consumed less varied diets and lower amounts of vitamin C, calcium, iron, riboflavin, and zinc –even when compared with their low-income counterparts in rural areas [ 75 ].
Association between FI and nutrition outcomes among vulnerable urban groups
Studies that examined the association of FI and nutrition outcomes were mainly from the Americas and Africa, and were based on cross-sectional designs but used different data analysis approaches (see Supplementary Table 6 ). The literature found that FI is a risk factor for malnutrition of the urban poor. Few studies assessed the association between FI and stunting, and did not reach consensus. While a study documented that in poor urban settlements children under 5 years of age living in FI households were at greater risk of stunting [ 69 ], others reported that FI was not significantly associated with stunting among adolescents [ 62 ].
Most of the studies assessed the relationship between FI and overweight and obesity leading to mixed findings, partially because study populations were diverse. For example, among schoolchildren living in urban FI households a higher prevalence of overweight was documented [ 73 ]. But such associations could not be confirmed among adolescents [ 56 , 61 ] or preschool children [ 79 , 87 ]. Similarly, the association also depended on the severity of the FI [ 67 ] and the syndemic effect with other factors like parental stress [ 49 , 61 ].
Qualitative approaches to FI in poor urban settings
The qualitative studies included in the systematic review were conducted mostly in poor urban areas of high-income countries. Collectively, these studies exemplify the complexity of food access challenges in urban areas and emphasize that food availability is a necessary but not sufficient condition for adequate food access as de facto it depends on other elements as well. Among poor urban older adults living alone with physical and motor limitations, as well as lack of transportation, and social isolation increase the risk of FI [ 98 ]. Among the homeless FI was related to insufficient income from government welfare programs, low affordability of fresh food, transportation barriers, lack of safe shelter and housing, and limited food storage capacity [ 94 ] [ 95 ]. In fact, challenges with access to a kitchen and inadequate spaces to store food emerged in other studies as factors increasing FI [ 104 ].
Qualitative studies focusing on mothers living in poverty in urban areas revealed specific food access and healthy eating challenges. In large Metropolitan areas, the major limitations for adequate family nutrition were limited time for food shopping and cooking, as well as finding time for family activities, childcare and difficulties in transportation to and from the food stores [ 97 ]. Another factor that emerged is that mothers prioritize food pricing and optimization of food usage when making food selections, oftentimes sacrificing quality [ 96 , 101 ]. Mothers living in poor urban settlements also referred to an unhealthy food environment in their communities due to the abundance of street vendors and food stores selling junk food [ 102 ].
The qualitative studies also documented FI related challenges faced by people who live in urban areas, like increased feelings of anxiety, worry, shame, and uncertainty [ 103 ]; and limited self-control for chronic disease, since it prevents access to proper nutrition [ 92 ]. Moreover, while social protection and food assistance programs, such as community kitchens, help by providing access to basic nutrition, are insufficient to fully resolve their FI related challenges [ 104 ].
Risk factors of the nutritional status of the urban poor
Urban poverty poses major challenges for adequate food access and nutrition outcomes among the urban poor, exposing them to nutritional risks with long-term consequences. Our systematic review identified associations between food access barriers and increased risk for poor nutrition outcomes through three different pathways. First, urban poor have an increased risk of consuming unhealthy and energy dense foods associated with a higher prevalence of overweight and obesity [ 47 , 86 ]. Second, urban poverty was found to increase the chances of chronic undernutrition, leading to higher obesity prevalence in future stages of life [ 88 ]. And third, the review suggested that psycho-social factors are important determinants of obesity through plausible biological links with stress and feelings of despair commonly experienced by people living in urban poverty [ 49 , 76 , 104 ].
Coping strategies for limited food access
An aspect that emerged from the literature refers to strategies used by the urban poor to obtain food and, among them, the use of food banks [ 68 , 92 , 98 ] and community kitchens [ 92 ] stand out. These studies found that beneficiaries considered such support strategies valuable but insufficient to fully mitigate hunger and lack of access to food, hence, families and individuals need other coping mechanisms like selling food on the streets to generate income, while at the same time have more access to food [ 54 ]. Other strategies implied skipping meals or eating smaller portions [ 103 , 104 ]. These unhealthy coping mechanisms were more prevalent among mothers, who buffer their children against FI [ 53 , 103 ]. Finally, other strategies included buying stolen food at a lower price or eating food from garbage [ 104 ].
Conceptual framework
Figure 3 presents a conceptual framework that intends to graphically depict the key themes that emerged from our literature review. At the center two key themes shape the relationship between nutrition and urban poverty: access to food and household food security status. These elements are determined by the factors summarized in the left part of the Figure, which are grouped in different ecological levels: community, family and the individual. These themes and factors help explain nutritional and health outcomes in the context of urban poverty including overweight and obesity, short stature and stunting. The conceptual framework also highlights the coping strategies used among the urban poor to deal with food access challenges as well as FI.
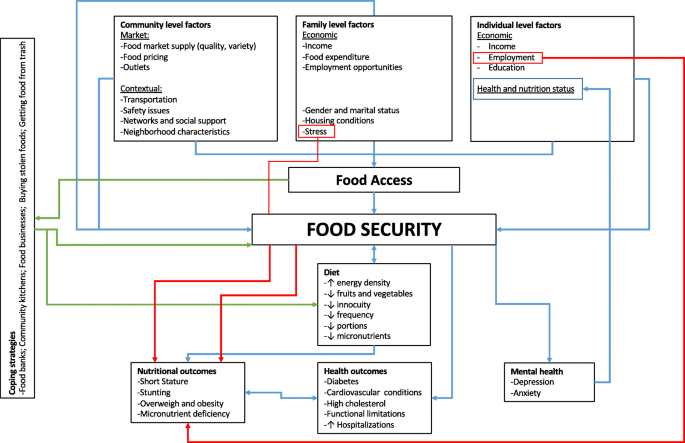
Conceptual Framework of nutrition and urban poverty
According to previous studies, in general, urban diets are likely to be more varied than rural diets [ 10 ]. However, this urban advantage strongly diminishes as a function of socioeconomic status representing a major social and health inequity in urban setting. In cities, food, for the most part, is bought and not grown for consumption. This implies that their access to healthy foods is strongly linked to income and to the structure of the food system, including its corresponding supply and access chains; i.e., “from farm to table”. These factors are two key determinants of the type of effective policies needed for urban populations to have access to a healthy diet [ 51 , 57 ].
The systematic literature review confirms that these determinants of food access in urban areas emerge in the context of poverty and high levels of FI of different countries [ 37 , 44 , 45 , 46 , 65 , 84 ], which are highly prevalent of poor nutrition and health outcomes [ 39 , 69 , 73 , 76 ]. Empirical evidence indeed supports the existence of a socioeconomic gradient in access to healthy food in urban areas [ 51 , 92 ]. The review emphasizes that access to food in urban areas is a complex process with multiple determinants and that it cannot be assumed that this access is always better for populations in urban vs. rural areas.
An important structural economic challenge for food access among the socioeconomically disadvantaged in urban areas is that the prices of healthy foods can be higher in poor neighborhoods, which at the same time also tend to have fewer food retail stores [ 41 , 42 ]. This is a strong structural barrier for families living in urban poverty. The structural challenges surrounding the food supply systems and markets in vulnerable urban areas means that sometimes individuals need to travel to other places to access healthy food, which increases costs (i.e. transportation) and mental stress due to the physical barriers to access food in their own communities. This adverse situation for the urban poor is compounded by problems of poor transport infrastructure as well as high community crime rates [ 42 ].
An interesting phenomenon that emerged from the literature –that in future studies may help compare challenges to food access among the urban and rural poor– is related to the nature of the social fabric and networks. Specifically, studies found that because urban networks tend to be weaker and, in the case of coping with FI, it may prevent families from “borrowing” or exchanging food with others [ 68 , 98 ].
Our review also found that urban poverty leads to increased risk of poor nutrition outcomes including stunting, overweight and obesity. Three themes that may help explain this finding emerged. First, the evidence indicates that urban environments foster a greater consumption of ultra-processed foods with high content of calories, fats, salt and sugars and very low nutritional value [ 47 , 86 ]. Likewise, studies show that lack of food-access may lead to skipping meals [ 53 , 103 , 104 ]. This is of public health concern, as it is known that prolonged fasting may predispose to unfavorable metabolic responses [ 106 , 107 ]. Finally, several articles pointed out how these experiences may be leading to mental health problems as a result of shame, and despair among those affected by FI without the ability to properly cope with it [ 76 , 104 ]. FI- related mental health stressors in turn can also increase the risk of cardiometabolic alterations and nutritional status [ 108 , 109 , 110 ]. Previous studies have established a strong plausibility for linking mental stress with the risk of overweight and obesity, mainly due to the increased release of hormones and neurotransmitters that can cause an increase in visceral adiposity and changes in the areas of the brain where hunger and satiety are regulated [ 108 , 109 , 110 ].
A substantive body of FI literature was identified. It is clear that FI in urban areas is strongly driven by income limitations. Specifically, low-income households need to allocate a high proportion of their total expenditure to food and are extremely vulnerable to any external shock including unemployment, health problems and food price inflation [ 45 , 46 , 65 , 84 ]. Similarly, the literature documented that the impact of FI on poor health is compounded by the fact that low-income urban households tend to have poor sanitation and other essential housing infrastructure and goods [ 46 ].
Given the findings from this review, it is not surprising that FI among the urban poor [ 49 , 73 , 76 ] has been associated with poor nutrition outcomes. This highlights the relevance of monitoring FI in urban populations. Food insecurity experience scales (FIES) are important in capturing this phenomenon among the urban poor, and efforts should be made to capture the different severity levels (i.e. mild, moderate, severe).
Another theme of great relevance is that social protection and food assistance programs designed to facilitate food access - such as monetary or in-kind transfer schemes, community kitchens and food banks - are insufficient by themselves to fully resolve the FI problem because they do not address barriers such as lack of cooking facilities or food storage, and competing health or housing expenses. Therefore is not surprising that socially unacceptable coping strategies, such as taking food from garbage, were reported, illustrating the depth of the negative effects of urban poverty on the right to food [ 104 ]. Interestingly, these FI coping behaviors contrast with those observed in rural areas, such as food exchanges and small family agriculture for self-consumption [ 44 , 68 ].
Urban poverty poses unique and diverse challenges and pathways to food access and the ability of families to consume healthy and nutritious diets that prevent access to healthy diets. It is possible that the nature of cities including unplanned built environments and challenging social network structures prevent low income individuals from finding strategies to cope with FI and lead to socially unacceptable behaviors to access foods.
In terms of the quality of the research examined, from a quantitative standpoint, most studies relied on cross-sectional designs, which do not allow to draw causal inferences, therefore there is a literature gap that requires further research with a longitudinal approach. While in the future more robust designs would be desirable, it should also be stressed that literature using different samples and conducted in a diverse set of countries is yielding similar conclusions in terms of the food access challenges and poor nutrition outcomes among the urban poor. However, further research needs to be conducted with more explicit comparison groups (such as urban population in very small, small, medium size cities, and metropolis) to answer the following questions: i) What is the role of social protection in terms of reducing FI for the vulnerable population? ii) Should it be continuous for some groups and intermittent for others? iii) What interventions should be put in place when food prices rise or economic conditions worsen to make sure the vulnerable are protected? iv) Should economic sanctions or incentives be put in place to induce away the demand of processed food consumption? v) What channels are more effective to assure quality access to food for the poor in urban settings? Finally, vi) What combination of policies could be recommended to be exerted together rather than in isolation?
Ideally, the proposed framework that emerge from the literature review should aid in the development of future research addressing food insecurity and nutrition outcomes in the context of urban poverty.
Furthermore, the operationalization of the definitions of “urban” and “poverty” were highly heterogenous across studies, hence, limiting the comparability of their findings. Future studies are needed to better harmonized definitions of poverty and the urban space, preferably studies should stratify samples according to the urban population size. The quality of qualitative studies was high overall, although there is room for improvement in terms of triangulation and reporting more explicit details on how data were retrieved, coded and analyzed.
In addition to the lack of uniform high quality across studies, this review has other important limitations when interpreting its findings. First, search algorithms were limited to specific nutrition outcomes that, despite being the more salient ones, might have excluded studies addressing other outcomes. Second, although FI is strongly linked to poverty, it is possible that some relevant studies that did not mention the word “poverty” but are related to disadvantages or inequalities, may have been left out from the review. Third, the review only included studies published in Spanish or English which may have led to excluding relevant literature published in other languages. Fourth, the search engines used retrieved studies in published academic journals, therefore the review may have excluded relevant studies only published in the grey literature. Fifth, the review did not conduct a meta-analysis to understand effect sizes of associations. This was not possible due to the strong heterogeneity across studies including the many different ways in which “poverty” and “urban” were defined. However, in recognition of such limitation, we performed a qualitative thematic analysis of the selected studies. Perhaps future reviews could narrow the search strategy to only studies that are more homogenous with regards to operational definitions of exposures and outcomes. Sixth, it is also important to note that mixed methods studies were excluded from the analysis due to the complexity of their systematization.
The systematic literature review evidenced the intricate link between urban poverty, food access, household food security, and nutrition. A contribution of this review is that it identified distinct barriers present in urban areas, questioned the supposedly “urban advantage” regarding access to healthful food, and developed a conceptual framework that focuses on the particular difficulties to achieve household food security among the urban poor through improved food access, which should inform future research. This systematic review provides consistent evidence that the right to food among those living in urban poverty is compromised; this is particularly worrisome considering that an urban setting is where the majority of the countries’ populations now live or will be living in the near future. It is essential that the social and public health sectors engage in addressing these issues jointly due to the complexity highlighted by the framework developed based on the available scientific evidence.
Availability of data and materials
Not applicable
UN Department of Economic and Social Affairs. 2018 revision of world urbanization prospects: United Nations; 2018. Available from: https://www.un.org/development/desa/publications/2018-revision-of-world-urbanization-prospects.html .
Google Scholar
UN Development Programme. Sustainable urbanization strategy: UNDP’S support to sustainable, inclusive and resilient cities in the developing world. New York City; 2016.
Davenport S, Carneiro Peixoto T. Governance for development: World Bank. 2015. [cited 2019]. Available from: https://blogs.worldbank.org/governance/more-voices-mean-smarter-cities .
UN. Transforming our world: The 2030 Agenda for Sustainable Development A/RES/70/1. 2015.
Pérez-Escamilla R. Food security and the 2015-2030 sustainable development goals: from human to planetary health: perspectives and opinions. Curr Dev Nutr. 2017;1(7):e000513.
Article PubMed PubMed Central Google Scholar
Dixon J, Omwega AM, Friel S, Burns C, Donati K, Carlisle R. The health equity dimensions of urban food systems. J Urban Health. 2007;84(3 Suppl):i118–29.
Article PubMed Google Scholar
Ruel M, Garrett J. Features of urban food and nutrition security and considerations for successful urban programming. Electron J Agric Dev Econ. 2004;1(2):242–71.
Arokiasamy P, Jain K, Goli S, Pradhan J. Health inequalities among urban children in India: a comparative assessment of empowered action group (EAG) and south Indian states. J Biosoc Sci. 2013;45(2):167–85.
Article CAS PubMed Google Scholar
Vilar-Compte M, Sandoval-Olascoaga S, Bernal-Stuart A, Shimoga S, Vargas-Bustamante A. The impact of the 2008 financial crisis on food security and food expenditures in Mexico: a disproportionate effect on the vulnerable. Public Health Nutr. 2015;18(16):2934–42.
Levin CE, Ruel MT, Morris SS, Maxwell DG, Armar-Klemesu M, Ahiadeke C. Working women in an urban setting: traders, vendors and food security in Accra. World Dev. 1999;27(11):1977–91.
Article Google Scholar
Horowitz CR, Colson KA, Hebert PL, Lancaster K. Barriers to buying healthy foods for people with diabetes: evidence of environmental disparities. Am J Public Health. 2004;94(9):1549–54.
Vilar-Compte M, Bernal-Stuart A, Sandoval-Olascoaga S, Pérez-Lizaur A. The effect of Mexican household food security status and income distribution on food access. Food Stud Interdiscip J. 2014;3:31.
Stamoulis KG, Pingali PL, Shetty P. Emerging challenges for food and nutrition policy in developing countries. Electron J Agric Dev Econ. 2004;01(2):154–67 Available from: http://ageconsearch.umn.edu/record/12000/files/01020154.pdf .
Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(Suppl 2):21–8.
Monteiro CA, Cannon G, Lawrence M, Costa-Louzada ML, Pereira-Machado P. Ultra-processed foods, diet quality, and health using the Nova classification system. Roma: FAO; 2019.
Pan American Health Organization. Ultra-processed food and drink products in Latin America: Sales, sources, nutrient profiles, and policy implications. Washington, D.C: PAHO; 2015. 2019-06-17.
Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place. 2010;16(5):876–84.
Glanz K, Sallis JF, Saelens BE, Frank LD. Healthy nutrition environments: concepts and measures. Am J Health Promot. 2005;19(5):330–3 ii.
WHO. Healthy diet. 2020. https://www.who.int/news-room/fact-sheets/detail/healthy-diet .
Fears R, ter Meulen V, von Braun J. Scientific opportunities for food and nutrition security. Lancet Planetary Health. 2018;2(1):e2–3.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Corburn J. Confronting the challenges in reconnecting urban planning and public health. Am J Public Health. 2004;94(4):541–6.
Freudenberg N. Time for a national agenda to improve the health of urban populations. Am J Public Health. 2000;90(6):837–40.
Article CAS PubMed PubMed Central Google Scholar
Geronimus AT. To mitigate, resist, or undo: addressing structural influences on the health of urban populations. Am J Public Health. 2000;90(6):867–72.
Institute of Medicine (US) Committee on Environmental Justice. Toward environmental justice: research, education, and health policy needs. 1999.
Institute of Medicine (US). Rebuilding the unity of health and the environment: a new vision of environmental health for the 21st century. 2001.
Duhl LJ, Sanchez AK. Healthy cities and the city planning process: a background document on links between health and urban planning. Copenhagen: WHO Regional Office for Europe; 1999.
National Center for Health Statistics. Healthy people 2010 final review. Hyattsville; 2012.
Speers MA, Lancaster B. Disease prevention and health promotion in urban areas: CDC’s perspective. Health Educ Behav. 1998;25(2):226–33.
Clarivate. EndNote. Online ed. 2019.
NICE. Methods for the development of NICE public health guidance: Appendix H Quality appraisal checklist - qualitative studies: National Institute for Health and Care Excellence; 2012. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-h-quality-appraisal-checklist-qualitative-studies#notes-on-the-use-of-the-qualitative-studies-checklist .
Vaismoradi M, Jones J, Turunen H, Snelgrove S. Theme development in qualitative content analysis and thematic analysis. J Nurs Educ Pract. 2016;6:100–10.
QSR International. NVivo. 12th ed; 2018. p. Qualitative data analysis software.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82.
World Bank. World Bank Country and Lending Groups: The World Bank Group; 2020. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups .
Agarwal S, Sethi V, Gupta P, Jha M, Agnihotri A, Nord M. Experiential household food insecurity in an urban underserved slum of North India. Food Secur. 2009;1(3):239–50.
Akinboade OA, Adeyefa SA. An analysis of variance of food security by its main determinants among the urban poor in the city of Tshwane, South Africa. Soc Indic Res. 2018;137(1):61–82.
Appelhans BM, Waring ME, Schneider KL, Pagoto SL. Food preparation supplies predict children's family meal and home-prepared dinner consumption in low-income households. Appetite. 2014;76:1–8.
Azambuja APO, Netto-Oliveira ER, Oliveira AAB, Azambuja MA, Rinaldi W. Prevalence of overweight/obesity and economical status of schoolchildren. Rev Paul Pediatr. 2013;31(2):166–71.
Barosh L, Friel S, Engelhardt K, Chan L. The cost of a healthy and sustainable diet – who can afford it? Aust N Z J Public Health. 2014;38(1):7–12.
Battersby J, Peyton S. The geography of supermarkets in Cape Town: supermarket expansion and food access. Urban Forum. 2014;25(2):153–64.
Battersby J. The food desert as a concept and policy tool in African cities: an opportunity and a risk. Sustainability. 2019;11(2):458.
Belachew T, Lindstrom D, Gebremariam A, Jira C, Hattori MK, Lachat C, et al. Predictors of chronic food insecurity among adolescents in Southwest Ethiopia: a longitudinal study. BMC Public Health. 2012;12(1):604.
Birhane T, Shiferaw S, Hagos S, Mohindra KS. Urban food insecurity in the context of high food prices: a community based cross sectional study in Addis Ababa, Ethiopia. BMC Public Health. 2014;14:680.
de Souza BL, Chaves dos Santos SM, de Jesus Pinto E, Aliaga MA, de Cássia Ribeiro-Silva R. Factors associated with food insecurity in households of public school students of Salvador City, Bahia, Brazil. J Health Popul Nutr. 2013;31(4):471–9.
Castañeda-Castaneira E, Ortiz-Pérez H, Robles-Pinto G, Molina-Frechero N. Consumo de alimentos chatarra y estado nutricio en escolares de la ciudad de México. Rev Mex Pediatr. 2016;83(1):15–9.
Cavanagh M, Jurkowski J, Bozlak C, Hastings J, Klein A. Veggie Rx: an outcome evaluation of a healthy food incentive programme. Public Health Nutr. 2017;20(14):2636–41.
Chambers EC, Duarte CS, Yang FM. Household instability, area poverty, and obesity in urban mothers and their children. J Health Care Poor Underserved. 2009;20(1):122–33.
Costa BVL, Horta PM, Ramos SA. Food insecurity and overweight among government-backed economy restaurant workers. Rev Nutr. 2019;32.
Cunha DB, Sichieri R, de Almeida RM, Pereira RA. Factors associated with dietary patterns among low-income adults. Public Health Nutr. 2011;14(9):1579–85.
Davies G, Frausin G, Parry L. Are there food deserts in rainforest cities? Ann Am Assoc Geographers. 2017;107(4):794–811.
Faye O, Baschieri A, Falkingham J, Muindi K. Hunger and food insecurity in Nairobi's slums: an assessment using IRT models. J Urban Health. 2011;88(Suppl 2):S235–55.
Floro MS, Bali SR. Food security, gender, and occupational choice among urban low-income households. World Dev. 2013;42:89–99.
Garcia MT, Sato PM, Trude ACB, Eckmann T, Steeves ETA, Hurley KM, et al. Factors associated with home meal preparation and fast-food sources use among low-income urban African American adults. Ecol Food Nutr. 2018;57(1):13–31.
Gundersen C, Lohman BJ, Eisenmann JC, Garasky S, Stewart SD. Child-specific food insecurity and overweight are not associated in a sample of 10- to 15-year-old low-income youth. J Nutr. 2008;138(2):371–8.
Jones HA, Charlton KE. A cross-sectional analysis of the cost and affordability of achieving recommended intakes of non-starchy fruits and vegetables in the capital of Vanuatu. BMC Public Health. 2015;15:301.
Kasper J, Gupta SK, Tran P, Cook JT, Meyers AF. Hunger in legal immigrants in California, Texas, and Illinois. Am J Public Health. 2000;90(10):1629–33.
Kirkpatrick SI, Tarasuk V. Housing circumstances are associated with household food access among low-income urban families. J Urban Health. 2011;88(2):284–96.
Lim S, Zoellner JM, Ajrouch KJ, Ismail AI. Overweight in childhood: the role of resilient parenting in African-American households. Am J Prev Med. 2011;40(3):329–33.
Lohman BJ, Stewart S, Gundersen C, Garasky S, Eisenmann JC. Adolescent overweight and obesity: links to food insecurity and individual, maternal, and family stressors. J Adolesc Health. 2009;45(3):230–7.
Lopes TS, Sichieri R, Salles-Costa R, Veiga GV, Pereira RA. Family food insecurity and nutritional risk in adolescents from a low-income area of Rio de Janeiro, Brazil. J Biosoc Sci. 2013;45(5):661–74.
Manyanga T, Tremblay MS, Chaput JP, Katzmarzyk PT, Fogelholm M, Hu G, et al. Socioeconomic status and dietary patterns in children from around the world: different associations by levels of country human development? BMC Public Health. 2017;17(1):457.
Martinez JC, Clark JM, Gudzune KA. Association of personal vehicle access with lifestyle habits and food insecurity among public housing residents. Prev Med Rep. 2019;13:341–5.
Martin-Fernandez J, Grillo F, Parizot I, Caillavet F, Chauvin P. Prevalence and socioeconomic and geographical inequalities of household food insecurity in the Paris region, France, 2010. BMC Public Health. 2013;13(1):486.
McCordic C, Abrahamo E. Family structure and severe food insecurity in Maputo and Matola, Mozambique. Sustainability. 2019;11(1):267.
Miller E, Wieneke KM, Murphy JM, Desmond S, Schiff A, Canenguez KM, et al. Child and parental poor health among families at risk for hunger attending a community health center. J Health Care Poor Underserved. 2008;19(2):550–61.
Morton LW, Bitto EA, Oakland MJ, Sand M. Accessing food resources: rural and urban patterns of giving and getting food. Agric Hum Values. 2008;25(1):107–19.
Mutisya M, Kandala NB, Ngware MW, Kabiru CW. Household food (in) security and nutritional status of urban poor children aged 6 to 23 months in Kenya. BMC Public Health. 2015;15:1052.
Article PubMed PubMed Central CAS Google Scholar
Nascimento-Ferreira MV, De Moraes AC, Carvalho HB, Moreno LA, Gomes Carneiro AL, dos Reis VM, et al. Prevalence of cardiovascular risk factors, the association with socioeconomic variables in adolescents from low-income region. Nutr Hosp. 2014;31(1):217–24.
PubMed Google Scholar
Odunitan-Wayas F, Okop K, Dover R, Alaba O, Micklesfield L, Puoane T, et al. Food purchasing characteristics and perceptions of neighborhood food environment of south Africans living in low-, middle- and high-socioeconomic neighborhoods. Sustainability. 2018;10(12):4801.
Omidvar N, Ghazi-Tabatabie M, Sadeghi R, Mohammadi F, Abbasi-Shavazi MJ. Food insecurity and its sociodemographic correlates among Afghan immigrants in Iran. J Health Popul Nutr. 2013;31(3):356–66.
Ortiz-Hernández L, Acosta-Gutiérrez MN, Núñez-Pérez AE, Peralta-Fonseca N, Ruiz-Gómez Y. Food insecurity and obesity are positively associated in Mexico City schoolchildren. Rev Investig Clin. 2007;59(1):32–41.
Park Y, Neckerman K, Quinn J, Weiss C, Jacobson J, Rundle A. Neighbourhood immigrant acculturation and diet among Hispanic female residents of New York City. Public Health Nutr. 2011;14(9):1593–600.
Ponce X, Ramirez E, Delisle H. A more diversified diet among Mexican men may also be more atherogenic. J Nutr. 2006;136(11):2921–7.
Ramsey R, Giskes K, Turrell G, Gallegos D. Food insecurity among adults residing in disadvantaged urban areas: potential health and dietary consequences. Public Health Nutr. 2012;15(2):227–37.
Rani D, Singh JK, Acharya D, Paudel R, Lee K, Singh SP. Household food insecurity and mental health among teenage girls living in urban slums in Varanasi, India: a cross-sectional study. Int J Environ Res Public Health. 2018;15(8).
Russell SE, Heidkamp CP. ‘Food desertification’: the loss of a major supermarket in New Haven, Connecticut. Appl Geogr. 2011;31(4):1197–209.
Sarki M, Robertson A, Parlesak A. Association between socioeconomic status of mothers, food security, food safety practices and the double burden of malnutrition in the Lalitpur district, Nepal. Arch Public Health. 2016;74(1):35.
Shaw H. Food access, diet and health in the UK: an empirical study of Birmingham. Br Food J. 2012;114(4):598–616.
Tsai J, Rosenheck RA. Obesity among chronically homeless adults: is it a problem? Public Health Rep. 2013;128(1):29–36.
Vedovato GM, Surkan PJ, Jones-Smith J, Steeves EA, Han E, Trude AC, et al. Food insecurity, overweight and obesity among low-income African-American families in Baltimore City: associations with food-related perceptions. Public Health Nutr. 2016;19(8):1405–16.
Villamor E, Finan CC, Ramirez-Zea M, Roman AV, Group NMCMSSN. Prevalence and sociodemographic correlates of metabolic syndrome in school-aged children and their parents in nine Mesoamerican countries. Public Health Nutr. 2017;20(2):255–65.
Vuong TN, Gallegos D, Ramsey R. Household food insecurity, diet, and weight status in a disadvantaged district of Ho Chi Minh City, Vietnam: a cross-sectional study. BMC Public Health. 2015;15:232.
Wagner J, Hinton L, McCordic C, Owuors O, Capron G, Gonzalez Arellano S. Do urban food deserts exist in the global south? An analysis of Nairobi and Mexico City. Sustainability. 2019;11:1963.
Wang H, Wang J, Liu MM, Wang D, Liu YQ, Zhao Y, et al. Epidemiology of general obesity, abdominal obesity and related risk factors in urban adults from 33 communities of Northeast China: the CHPSNE study. BMC Public Health. 2012;12:967.
Whitaker RC, Orzol SM. Obesity among US urban preschool children: relationships to race, ethnicity, and socioeconomic status. JAMA Pediatr. 2006;160(6):578–84.
Wrathall J. Linking obesity and malnutrition. Int J Sociol. 2014;44(2):63–86.
Yaemsiri S, Olson EC, He K, Kerker BD. Food concern and its associations with obesity and diabetes among lower-income new Yorkers. Public Health Nutr. 2012;15(1):39–47.
Zhai J, Xue H, Luo J, Zhang L, Cheng G. Associations between socioeconomic status and overweight among urban children aged 7-12 years in Chengdu, Southwest China. Asia Pac J Clin Nutr. 2018;27(3):617–23.
Zhang M, Debarchana G. Spatial supermarket redlining and neighborhood vulnerability: a case study of Hartford, Connecticut. Trans GIS. 2016;20(1):79–100.
Chan J, DeMelo M, Gingras J, Gucciardi E. Challenges of diabetes self-management in adults affected by food insecurity in a large urban centre of Ontario, Canada. Int J Endocrinol. 2015;2015:903468.
Cotter EW, Teixeira C, Bontrager A, Horton K, Soriano D. Low-income adults’ perceptions of farmers’ markets and community-supported agriculture programmes. Public Health Nutr. 2017;20(8):1452–60.
Crawford B, Yamazaki R, Franke E, Amanatidis S, Ravulo J, Steinbeck K, et al. Sustaining dignity? Food insecurity in homeless young people in urban Australia. Health Promot J Austr. 2014;25(2):71–8.
Dachner N, Tarasuk V. Homeless “squeegee kids”: food insecurity and daily survival. Soc Sci Med. 2002;54(7):1039–49.
de Morais SP, Unsain RF, Gittelsohn J, Sanches Tavares da Silva JG, Gonçalves Perez IC, Baeza Scagliusi F. Strategies used by overweight and obese low-income mothers to feed their families in urban Brazil. Appetite. 2017;111:63–70.
Dubowitz T, Acevedo-Garcia D, Salkeld J, Lindsay AC, Subramanian SV, Peterson KE. Lifecourse, immigrant status and acculturation in food purchasing and preparation among low-income mothers. Public Health Nutr. 2007;10(4):396–404.
Green-LaPierre RJ, Williams PL, Glanville NT, Norris D, Hunter HC, Watt CG. Learning from “knocks in life”: food insecurity among low-income lone senior women. J Aging Res. 2012;2012:11.
Hammelman C. Investigating connectivity in the urban food landscapes of migrant women facing food insecurity in Washington, DC. Health Place. 2018;50:89–97.
Leung MM, Agaronov A, Entwistle T, Harry L, Sharkey-Buckley J, Freudenberg N. Voices through cameras: using photovoice to explore food justice issues with minority youth in east Harlem, New York. Health Promot Pract. 2016;18.
Levay AV, Mumtaz Z, Faiz Rashid S, Willows N. Influence of gender roles and rising food prices on poor, pregnant women’s eating and food provisioning practices in Dhaka, Bangladesh. Reprod Health. 2013;10(1):53.
McInvale Trejo K, Shaw-Ridley M. Barriers and enablers to nutrition and physical activity in Lima, Peru: an application of the Pen-3 cultural model among families living in pueblos jóvenes. Ethn Health. 2019:1–11.
Piaseu N, Belza B, Shell-Duncan B. Less money less food: voices from women in urban poor families in Thailand. Health Care Women Int. 2004;25(7):604–19.
Wicks R, Trevena LJ, Quine S. Experiences of food insecurity among urban soup kitchen consumers: insights for improving nutrition and well-being. J Am Diet Assoc. 2006;106(6):921–4.
Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to measuring household food security, revised 2000. In: U. S. Dept. of Agriculture FaNS, editor. Alexandria; 2000.
Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev. 2007;65(6 Pt 1):268–81.
Toschke AM, Küchenhoff H, Koletzko B, Von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13(11):1932–8.
Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2(2):73–86.
Pervanidou P, Chrousos GP. Stress and obesity/metabolic syndrome in childhood and adolescence. Int J Pediatr Obes. 2011;6(sup1):21–8.
Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med (Maywood). 2010;235(10):1150–62.
Article CAS Google Scholar
Download references
Acknowledgements
The authors thank Ida Katerina García Appendini, Erika Germaine García Alberto, Alma Cecilia Pérez Navarro and Luis Alfredo Ortíz Vázquez for their thoughtful input during the developing of this manuscript. The authors also thank Marisol Silva Laya – one of the CO-PIs of the project – who always provided very useful critical insights.
This work was supported by the National Council of Science and Technology (CONACyT) and the Research Office of the Iberoamericana University.
Author information
Authors and affiliations.
Research Institute for Equitable Development EQUIDE, Universidad Iberoamericana, Prolongación Paseo de Reforma 880, Lomas de Santa Fé, 01219, Mexico City, Mexico
Mireya Vilar-Compte, Soraya Burrola-Méndez, Annel Lozano-Marrufo, Isabel Ferré-Eguiluz, Diana Flores, Pablo Gaitán-Rossi & Graciela Teruel
Yale School of Public Health, 60 College Street, New Haven, CT, 06510, USA
Rafael Pérez-Escamilla
You can also search for this author in PubMed Google Scholar
Contributions
MVC defined the scope of the research subject and developed the search strategy. SBM, ALM, IFE and DF undertook the search, reviewed the literature and summarized the search findings. MVC, SBM, ALM and DF drafted the manuscript. MVC, PGR, GT and RPE provided substantial input in the design stages of the review, critically reviewed the manuscript and helped shape the final version of the manuscript. All authors approved the final manuscript.
Corresponding author
Correspondence to Mireya Vilar-Compte .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vilar-Compte, M., Burrola-Méndez, S., Lozano-Marrufo, A. et al. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: a global systematic literature review. Int J Equity Health 20 , 40 (2021). https://doi.org/10.1186/s12939-020-01330-0
Download citation
Received : 15 May 2020
Accepted : 20 November 2020
Published : 20 January 2021
DOI : https://doi.org/10.1186/s12939-020-01330-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Urban poverty
- Social protection
- Public health
- Food security
International Journal for Equity in Health
ISSN: 1475-9276
- General enquiries: [email protected]
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Diet Quality Among Children
- 1 Department of Pediatrics, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2 Department of Obstetrics & Gynecology, University of Alberta, Edmonton, Alberta, Canada
Children’s transition from a liquid-based infant diet to a typical omnivorous diet establishes eating behaviors and dietary patterns, key determinants of food preferences and diet quality, contributing to lifelong nutritional habits and overall health. Previous studies focused on youth diet 1 or used older data and diet quality measures for infants and toddlers 2 (hereafter, children ). This study used data from 2005-2020 and the Healthy Eating Index (HEI) 3 to analyze contemporaneous temporal changes in diet quality among US children.
Read More About
Ding G , Wen C , Chen Y , Vinturache A , Zhang Y. Diet Quality Among Children. JAMA Pediatr. Published online July 08, 2024. doi:10.1001/jamapediatrics.2024.1880
Manage citations:
© 2024
Artificial Intelligence Resource Center
Pediatrics in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
REVIEW article
Examining nutrition strategies to influence dna methylation and epigenetic clocks: a systematic review of clinical trials.

- 1 Clinique la Prairie, Clarens-Montreux, Vaud, Switzerland
- 2 Genknowme SA, Lausanne, Switzerland
Nutrition has powerful impacts on our health and longevity. One of the mechanisms by which nutrition might influence our health is by inducing epigenetic modifications, modulating the molecular mechanisms that regulate aging. Observational studies have provided evidence of a relationship between nutrition and differences in DNA methylation. However, these studies are limited in that they might not provide an accurate control of the interactions between different nutrients, or between nutrition and other lifestyle behaviors. Here we systematically reviewed clinical studies examining the impact of nutrition strategies on DNA methylation. We examined clinical studies in community-dwelling adults testing the effects of nutrition interventions on i) global DNA methylation and its proxies, and ii) epigenetic clocks. We included 21 intervention studies that focused on the effects of healthy nutrition patterns, specific foods or nutrients, as well as the effect of multivitamin or multimineral supplements. In four studies on the methylation effects of healthy dietary patterns, as defined by being rich in vegetables, fruits, whole-grains, and nuts and reduced in the intake of added sugars, saturated fat, and alcohol, two of them suggested that a healthy diet, is associated with lower epigenetic age acceleration, one of them reported increases in global DNA methylation, while another one found no diet effects. Studies examining epigenetic effects of specific foods, nutrients, or mixtures of nutrients were scarce. For both folic acid and polyunsaturated fatty acids, the available independent studies produced conflicting findings. Although more evidence is still needed to draw firm conclusions, results begin to suggest that healthy dietary patterns have positive effects on DNA methylation. Additional evidence from large randomized-controlled clinical trials is needed to support the effects of healthy nutrition on the DNA methylome.
Introduction
Healthy nutrition patterns have powerful effects on general health and, more specifically, on cardio-vascular health ( Lichtenstein et al., 2021 ). In this regard, the Global Burden of Disease has reported that increases in diet quality could help prevent one in five deaths globally ( GBD, 2017 Diet Collaborators, 2019 ). One of the mechanisms that might mediate the effects of nutrition on general health is epigenetics.
Epigenetics comprise the study of genomic changes that do not modify the primary DNA nucleotide sequence. Their mechanisms include changes in methylation, histone modifications, and chromatin remodeling. Epigenetic factors alter gene expression, and in turn, they are modified by environmental factors, such as nutrition, and by internal factors, such as age ( Choi and Friso, 2010 ; Tammen et al., 2013 ; Pal and Tyler, 2016 ). DNA methylation is a stable and heritable epigenetic mechanism, and it refers to the addition of a methyl group to a CpG dinucleotide sequence. DNA methyltransferase catalyzes this methylation by using S-adenosylmethionine as the provider of methyl groups. DNA methylation at CpG sites influences gene expression by modifying DNA’s structure, thus affecting the transcription machinery’s access to DNA. This process can either enhance or suppress gene activity by regulating transcription factor binding ( Kim and Costello, 2017 ).
CpG methylation is fundamental during development, since it silences genes whose expression is no longer needed ( Pal and Tyler, 2016 ). With aging, changes in DNA methylation accumulate, and these changes have been associated with genomic instability and with a number of non-communicable disorders, including cardiovascular diseases and cancer ( Seale et al., 2022 ). Aging has been traditionally associated with general decreases in DNA methylation ( Lillycrop et al., 2014 ; Kane and Sinclair, 2019 ). At the same time, some CpG sites undergo hyper-methylation with age ( Horvath, 2013 ). All these aging-related changes in methylation may be stochastic, meaning that they are random variations in DNA methylation without an apparent cause, or environmentally-related, such as nutrition, stress, and pollution ( Lillycrop et al., 2014 ).
Some of the most common measures of epigenetics in relation with environmental factors, age, and age-related disorders are global methylation and epigenetic methylation clocks. These measures can be largely regarded as complementary. Global DNA methylation provides a comprehensive overview of the methylation state of the epigenome ( Li and Tollefsbol, 2021 ), and it has been associated with vulnerability to diseases such as cancer ( Boyne et al., 2018 ). Environmental factors can modify global DNA methylation, as shown by studies examining the effects of pollutants ( Ferrari, Carugno, and Bollati, 2019 ). Epigenetic methylation clocks are tightly associated with differences in the rates of biological aging ( Hannum et al., 2013 ; Horvath, 2013 ; Levine et al., 2018 ). High scores in epigenetic age acceleration are associated with mortality, as well as with the incidence of cancer and cardio-vascular disease ( Oblak et al., 2021 ), while low scores are associated with longer healthspan, or healthy longevity ( Jain et al., 2022 ).
In the specific context of nutrition, previous observational studies have suggested that diet is associated with differences in DNA methylation. A study examined nutrient patterns in the Molisani cohort from Italy, and reported that higher intake of zinc and vitamin B3 is associated with higher global DNA methylation ( Noro et al., 2022 ). Pooled results from the Women Health Initiative and the In-CHIANTI datasets show that higher fish intake is associated with lower age acceleration ( Quach et al., 2017 ). Other studies have also focused on global diet quality. Although the scores in some specific items may vary, a diet is generally considered to be high quality when it reflects a high intake of vegetables, fruits, whole-grains, and nuts, along with a reduced consumption of added sugars, saturated fats, red processed meat, and alcohol. Epigenetic studies have shown that healthy dietary patterns are associated with decelerations in biological aging, as measured with epigenetic clocks ( Kim et al., 2022 ; Kresovich et al., 2022 ).
Methodologically speaking, it is extremely difficult to examine the effects of single foods, or specific nutrients on biological outcomes, such as epigenetics. This is specially so because nutrients might interact with each other, with other lifestyle factors such as physical activity or pollution exposure, and with physiological processes such as aging or menopause. Although the previous observational studies had certain advantages to detect epigenetic changes associated with diet, such as large statistical power, clinical studies offer unique experimental settings to examine the effects of dietary patterns, macronutrients, or micronutrients in a controlled manner. In this context, in the present review we focus on clinical studies, both randomized and non-randomized. To obtain a more comprehensive view of the aging methylome, we focus on studies examining two different indexes of epigenetic methylation: i) global methylation indexes and its surrogates, such as LINE-1 methylation, and ii) epigenetic methylation clocks of biological aging.
The primary aim of our study was to determine what nutrition interventions are associated with global changes in DNA methylation as well as with more favorable outcomes in epigenetic aging. Our working hypothesis is that healthy nutrition patterns will be associated with increases in global methylation or with younger epigenetic aging trends. A secondary aim was to examine whether age and sex distribution affect the relationship between nutrition and DNA methylation.
We performed a systematic review of the literature to explore what nutrition interventions are associated with longitudinal changes in DNA methylation. The protocol of this review was preregistered in the following address: www.osf.io/89pzj and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( Moher et al., 2009 ).
We searched for peer-reviewed studies available on PubMed using the following query strings: (epigenetics or DNA methylation) and (nutrition or food or diet or lipid or carbohydrate or protein or vitamin or phytochemical or nutraceutical). Automatic filters from PubMed were applied in order to include the following types of papers: classical article, clinical studies, clinical trial, controlled clinical trial, multicenter study, observational study, RCTs. The references cited in relevant papers (e.g., systematic reviews that are close in scope ( ElGendy et al., 2018 ) and all of the studies included) were also searched for additional studies. Two authors working in parallel performed the literature search, and disagreements were solved by consensus. We used the PICO portal platform ( www.picoportal.net ) to assist with the literature screening. Figure 1 shows the results of the screening process.
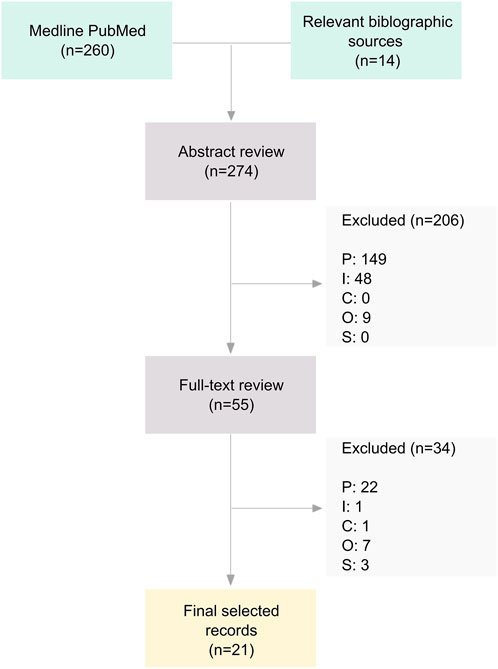
Figure 1 . Flowchart of the screening process. Notes: P, Participants; I, Intervention; C, Comparison; O, Outcome; S, Study type.
We included clinical studies in adults that administered a nutrition intervention and that provided measures of DNA methylation, either global methylation, proxies of global methylation (such as repetitive element methylation LINE-1), and epigenetic clocks based on methylation patterns and that were published until January 2024. The most important exclusion criteria were the following: a) prenatal studies, as well as studies on childhood or adolescence, since their results might not automatically generalize to adulthood; b) studies recruiting participants with medical comorbidities, since some of the results might be specific to the medical condition analyzed; c) animal studies; d) studies on the effects of caloric restriction or intermittent fasting, since they involve substantial changes in caloric intake and, as such, their results cannot properly inform about the effects of specific nutrients, foods, or food patterns; e) studies on alcohol intake; since we do not consider alcohol to be a nutrient; f) multimodal treatments (e.g., nutrition and meditation administered together) only if, in this last case, the study design does not allow to examine the effects of the nutrition intervention on its own (for the full list of inclusion and exclusion criteria see Table 1 ).
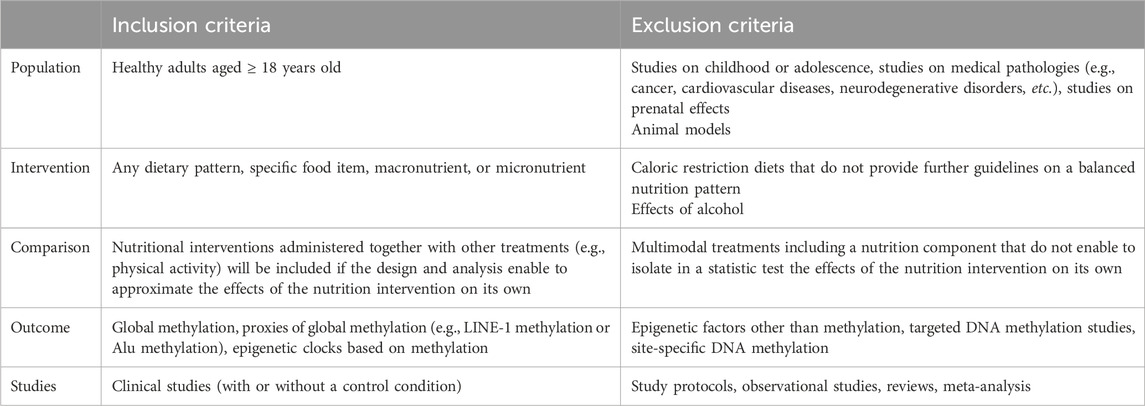
Table 1 . Inclusion and exclusion criteria used in this systematic review.
From the included studies, we extracted the following information: goal of the study, name of the study and country, study design, cross-over (Y/N), participants blind (Y/N), observer blind (Y/N), control condition, length of the intervention, sample size, average age, percentage of female participants, description of the nutrition intervention, description of other treatments administered, epigenetic markers, statistical analysis, control of confounders, main results. Two authors working sequentially extracted the data. If two or more studies had overlapping sample sizes and analyses, we cited both studies but extracted only the most comprehensive results.
Deviations from the preregistered protocol
In the preregistered protocol we specified that we would exclude studies on medical pathologies. This way, we aimed at focusing on those results that are relevant to the general population, easily generalizable, and to avoid potential biases associated with some medical diseases. This criterion has been kept mostly intact, and we have excluded studies focusing on patients with cancer, cardio-vascular disorders, or neurodegeneration. However, we have relaxed our inclusion criteria to include studies recruiting participants with overweight and obesity, participants who smoke, participants with suboptimal levels of vitamin D, participants with moderately elevated values of homocysteine, and participants with high triglycerides and/or low HDL cholesterol. This decision was taken after considering that these health problems are present in a significant portion of the population, and that by including them, we can provide a real-world representation of health as a continuum spanning from optimal to suboptimal.
Other clarifications regarding inclusion/exclusion criteria
In this review we have included a weight-loss intervention study, despite stating otherwise in the exclusion criteria ( Meir et al., 2021 ). The inclusion of this paper is because this study targets weight loss by providing nutrition guidelines that are well aligned with the Mediterranean diet (for further details on this diet, please refer to the results section). As such, we have considered that this study provides useful insights on the link between nutrition and DNA methylation changes.
We identified twenty-one clinical studies that examined the effects of nutrition on either global DNA methylation, proxies of global methylation (i.e., LINE-1), or on methylation-based epigenetic clocks. Among them, thirteen studies were randomized clinical trials, while seven studies were non-randomized ( Supplementary Table S1 , for a summary of the results see Figure 2 ). Since most studies examined methylation in blood tissues, in the text we have specified if DNA methylation is examined in body tissues other than blood.
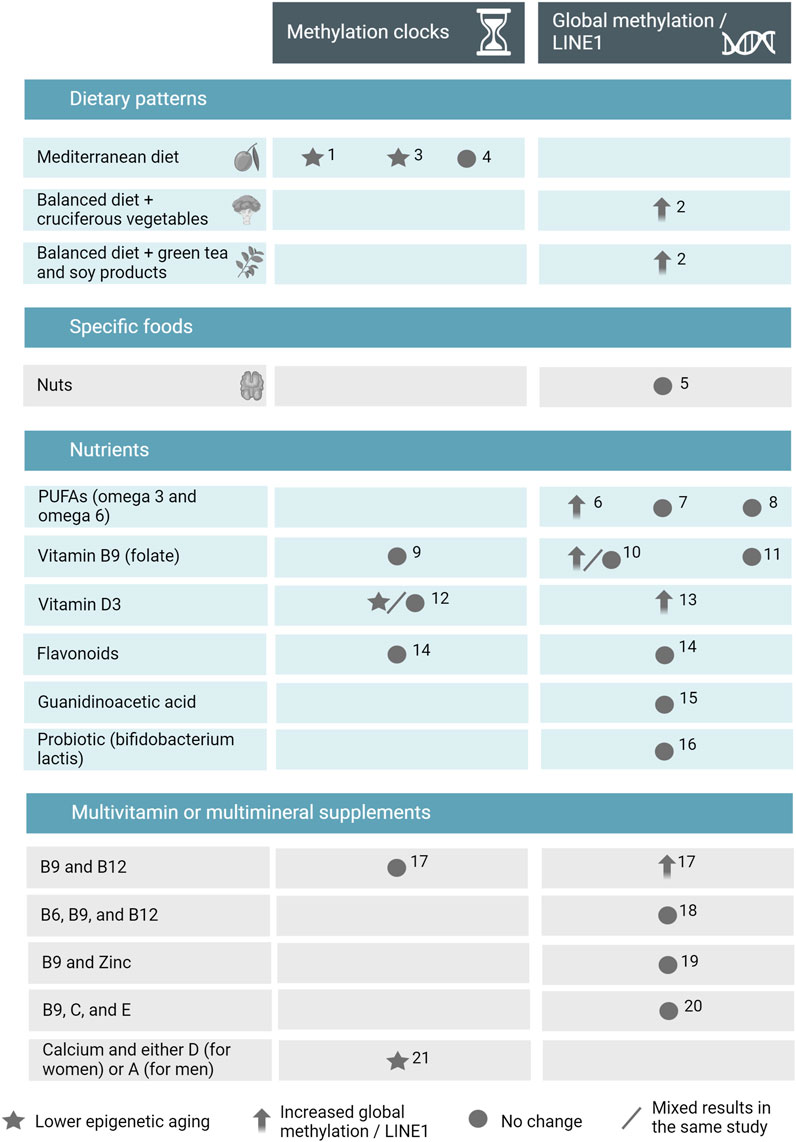
Figure 2 . Summary of the results of the systematic review on human intervention studies in adulthood examining the effects of nutrition on DNA methylation clocks and global DNA methylation. Abbreviations: LINE1, long interspersed elements 1; PUFAs, polyunsaturated fatty acids; 1, Gensous et al., 2020 ; 2, Scoccianti et al., 2011 ; 3, Fiorito et al., 2021 ; 4, Meir et al., 2021 ; 5, Salas-Huetos et al., 2021 ; 6, Perfilyev et al., 2017 ; 7, Frankhouser et al., 2022 ; 8, Hunter et al., 2019 ; 9, Michels and Binder 2024 ; 10, Crider et al., 2011 ; 11, Jung et al., 2011 ; 12, Chen et al., 2019 ; 13, Zhu et al., 2016 ; 14, flavonoids study in Sae-Lee et al., 2018 ; 15, Ostojic et al., 2018 ; 16, Worthley et al., 2009 ; 17, vitamins B9 and B12 study in Sae-Lee et al., 2018 ; 18, Hübner et al., 2013 ; 19, Jenkins et al., 2022 ; 20, Chan et al., 2017 ; 21, Demidenko et al., 2021 . Figure created with BioRender.
Studies on dietary patterns
We have included four intervention studies that focused on the effects of beneficial dietary patterns on DNA methylation. The NU-AGE study (n = 120, 58% women) evaluated the impact of 1-year adherence to a Mediterranean diet on epigenetic age, in an Italian and a Polish cohort aged on average 72 years old. In this study, the Mediterranean diet intervention promoted a high intake of wholegrains, fruits, vegetables, and legumes, a moderate increase of low-fat dairy, fish, low-fat meat, eggs, nuts, and olive oil, and a limited use of alcohol, salt, and sweets. Across both cohorts, higher adherence to the Mediterranean diet was associated with lower biological age acceleration, as measured with the Horvath clock ( Gensous et al., 2020 ).
Another study also conducted in an Italian cohort of males, with an average age of 52 years (n = 88) investigated the effects of three diets: i) an isocaloric diet with balanced fruits and vegetables, which constituted their “control” diet; ii) a regime based on their control diet that in addition included cruciferous vegetables and iii) a regime based on the control diet that included green tea and soy products. Participants were told to consume these diets for 4 weeks. The three diets were associated with small increases in LINE-1 methylation, spanning from 1% to 3% increases in methylation ( Scoccianti et al., 2011 ).
The DAMA (Diet Physical Activity and Mammography) study (n = 219) examined a group of Italian women with an average age of 59 years old that were randomly assigned to four groups during 2 years: i) dietary intervention, in which participants were counseled to adopt a dietary pattern characterized by being rich in whole grains, fruits, vegetables, legumes, and pulses, by the use of extra-virgin olive oil, and by the reduction in red meat, alcohol intake, cheese, and sweets; ii) a physical activity intervention; iii) a combination of dietary and the physical activity interventions; and iv) no interventions. They found that the dietary intervention was associated with a reduction of epigenetic aging measured using the GrimAge clock ( Fiorito et al., 2021 ).
Finally, the CENTRAL study (n = 120, Israel), a cohort of mostly men (92%) aged on average 49 years old, randomized participants with obesity and/or dyslipidemia to two hypocaloric diets: a low-fat diet, characterized by limiting their total fat intake to 30% of their caloric intake, and a Mediterranean diet with a low-carbohydrate pattern, characterized by being restrictive in the amount of carbohydrates and in red meat, and by being rich in vegetables, legumes, and nuts. Half of the participants in both conditions also received a physical activity intervention. The interventions lasted for 18 months. There were no effects of the nutrition intervention on epigenetic methylation age, as measured with a 240 CpGs algorithm. However, in a separate analysis examining the interaction between dietary intervention and weight loss, participants who achieved weight loss had a significantly lower increase in epigenetic age after the intervention (0.6 years) relative to participants who did not lose weight (1.1 years) ( Meir et al., 2021 ).
Studies on the effects of specific foods
One study has focused on the epigenetic effects of specific foods.
The FERTINUTS study, examined Spanish men aged on average 25 years old (n = 72), and investigated the effects of nuts in global methylation changes in sperm after a 14-weeks intervention study. The study reported a lack of differences in global methylation changes between participants assigned to the nut intervention and participants who did not include nuts in their diet ( Salas-Huetos et al., 2021 ).
Studies on specific macro- or micro-nutrients
We identified ten clinical studies that focused on the effects of specific macro- or micro-nutrients on global DNA methylation or on epigenetic clocks.
Regarding the effects of lipids, a study performed a secondary analysis of a larger randomized clinical trial performed in U.S. women, in which they selected a small subset of participants (n = 10, average age 51 years old), which showed good responses to omega-3 polyunsaturated fatty acids supplementation, taken for 6 months. This subset of participants was further analyzed for epigenetic changes. The authors reported no change in global DNA methylation after treatment with omega-3 polyunsaturated fatty acids ( Frankhouser et al., 2022 ). Similarly, a study conducted in men (n = 8, average age 40 years old) performed in the UK used a cross-over design and administered either omega-3 fatty polyunsaturated fatty acids or a supplement of extra virgin olive oil for 4 weeks. None of the fatty acid supplements altered global methylation ( Hunter et al., 2019 ). A third trial, The LIPOGAIN study, conducted in Sweden (n = 31, average age 27 years old, 36% women) evaluated methylation changes in the adipose tissue after a 7-weeks intervention study, in which participants were randomized to consume muffins with either added palm oil (rich in saturated fats) or added sunflower oil (rich in omega-6 polyunsaturated fatty acids). Both oils induced higher mean methylation in the adipose tissue ( Perfilyev et al., 2017 ).
The isolated effects of folic acid supplementation have been tested in three studies. A randomized cross-over study performed on Chinese women tested the effects of folic acid over 6 months in global methylation extracted from coagulated (n = 135, average age 30 years old) and uncoagulated (n = 76 subsample) blood analysis. While global methylation remained unchanged when extracted from uncoagulated blood, there was a reduction in global methylation after folic acid supplementation, when this measure was obtained from coagulated blood ( Crider et al., 2011 ). The FACIT trial (Folic Acid and Carotid Intima Media Thickness, the Netherlands) is a randomized double-blinded trial that recruited participants with moderately elevated homocysteine (n = 216, average age 61 years old, 45% women) and tested the effects of folic acid supplementation for 3 years on global DNA methylation. There was no difference in DNA methylation between placebo and treatment with folic acid, and no different effect was observed when stratifying the sample by age or by sex ( Jung et al., 2011 ). Finally, a randomized clinical trial conducted in Germany recruited male participants (n = 16, average age 39 years old) and tested the effect of supplementation of 400 μg/d vs. 800 μg/d of folic acid for 8 weeks on different methylation clocks (i.e., Horvath’s, Hannum’s, PhenoAge, GrimAge, GrimAge2, and DunedinPACE). None of the folic acid supplementation regimes elicited changes on the methylation clocks ( Michels and Binder, 2024 ).
Two studies examined the effects of vitamin D supplementation. A randomized clinical trial on overweight African-American participants with suboptimal levels of vitamin D (n = 51, average age 26 years old, 84% women) administered a supplement of D for 16 weeks using four conditions: placebo, 600 lU/d, 2000 lU/d, and 4,000 lU/d of vitamin D. They found that, in the intervention groups of 2000 and 4000 IU/d, epigenetic methylation age measured with the Horvath clock was decreased by 1.83 and 1.62 years compared to baseline. However, when they measured epigenetic age using another clock (Hannum’s) no results were found ( Chen et al., 2019 ). Using the same study design and sample, another paper already showed that increases in global DNA methylation associated with vitamin D supplementation were dose-dependent, that is, increases in global methylation were the highest after 4000 IU/d of vitamin D, followed by 2000 IU/d, and followed by 600 IU/d, relative to placebo ( Zhu et al., 2016 ).
Regarding flavonoids, one study looked at DNA methylation on 13 participants (average age 22 years old, 57% women) who, as part of a larger study, had been previously assigned to receive supplementation with monomeric and oligomeric flavonols derived from grape seeds for 8 weeks. They found no differences before and after supplementation neither in global methylation nor in the epigenetic age measured with the Horvath clock ( Sae-Lee et al., 2018 ). Part of these results (global DNA methylation) were also reported in another overlapping publication ( Milenkovic et al., 2014 ).
One study (n = 14) administered a supplement of guanidinoacetic acid, a precursor of creatine, for 12 weeks to young adults. They found no differences in global DNA methylation ( Ostojic et al., 2018 ).
Finally, a randomized clinical trial (n = 20, average age 60 years old, 35% women) examined whether a 4-week administration of either a probiotic ( bifidobacterium lactis ), a prebiotic (high amylose maize starch), or a synbiotic (administration of the prebiotic and the probiotic together) changed LINE-1 methylation in the rectal mucosa. After the dietary interventions, mean LINE-1 methylation remained unchanged ( Worthley et al., 2009 ).
Studies examining the effects of multivitamin or multicompound supplementation
Five studies have examined the effects of multivitamin or multicompound supplements on global methylation markers.
Among them, two studies have focused on sperm DNA methylation. A large randomized double-blind placebo-controlled trial named the FAZST (Folic Acid and Zinc Supplementation), performed in the U.S. examined the effects of a 6-month supplementation using folic acid and zinc on the DNA methylation sperm of men (n = 1,470, average age: 33 years old). They reported no effects of zinc and folic acid supplementation on global DNA methylation ( Jenkins et al., 2022 ). In another study conducted in the U.S., men (n = 8, 18–32 years old) were randomized to receive a multivitamin supplement (vitamin C, vitamin E, and folic acid) or placebo for 3 months. There were no significant changes in sperm DNA methylation after the multivitamin intervention ( Chan et al., 2017 ).
Another study (n = 42, average age 63 years old, 33.3% women) administered Rejuvant ® , a compound with two formulations: one for women, containing calcium-alpha-ketoglutarate and vitamin D, and one for men, containing calcium-alpha-ketoglutarate and vitamin A. They calculated epigenetic methylation age based on a proprietary algorithm which showed a moderate correlation with chronological age in the same sample (r = 0.77). They found that after 4–10 months of treatment with Rejuvant ® , epigenetic age was decreased by approximately 8 years, and that the effects were similar for men and for women ( Demidenko et al., 2021 ).
Two studies have examined the effect of combined B-complex vitamins. A randomized double-blind placebo-controlled trial (n = 44, 65–75 years old, 57% women) tested the effects of a folic acid and vitamin B12 supplement taken during 2 years on global methylation and on epigenetic age, as evaluated with the Horvath clock. While the analyses of epigenetic age had no significant results, the administration of folic acid and vitamin B12 was shown to increase global DNA methylation ( Sae-Lee et al., 2018 ). Another randomized double-blind trial (n = 50, average age 68 years old, 71% women) studied the effects of a 1-year administration of two food supplements: a control one, consisting of vitamin D and calcium, and one that contained B-vitamins (folic acid, vitamin B6, and vitamin B12) along with vitamin D and calcium. LINE-1 methylation remained unchanged before and after the supplementation, as analyzed with a paired-samples t-test ( Hübner et al., 2013 ). Of note, this same analysis was recycled by the same authors in a posterior publication with a different statistical test (linear regression). As in their first analysis, mean LINE-1 methylation remained unchanged after supplementation ( Pusceddu et al., 2016 ).
In this review, we have evaluated whether nutrition interventions can change global DNA methylation patterns. With regards to the effect of dietary patterns, we identified four independent studies that examined the effects of a “healthy” diet, characterized by being rich in vegetables, fruits, legumes, and nuts, and by aiming to reduce the intake of red meat, added sugars, and alcohol. Three of the studies concluded that this healthy dietary pattern is associated with DNA methylation changes. Among them, two studies lower accelerations in epigenetic aging ( Gensous et al., 2020 ; Fiorito et al., 2021 ), and one of them showed higher LINE-1 methylation measures ( Scoccianti et al., 2011 ). An additional study reported no significant effects on DNA methylation ( Meir et al., 2021 ). Although the evidence is still scarce to draw firm conclusions, results begin to point in the direction that a beneficial nutritional regime might have positive effects on DNA methylation. Healthy dietary patterns provide good sources of vitamins, such as vitamins A, C, E, or B-complex vitamins, minerals such as magnesium, selenium, and zinc, and other nutrients such as polyunsaturated fatty acids, flavonoids, and isoflavones. These nutrients constitute important components of the so-called anti-inflammatory diet , a dietary pattern associated with lower levels of circulating proinflammatory cytokines ( Shivappa et al., 2014 ). Adherence to anti-inflammatory dietary patterns has been associated with small reductions in the risk of developing inflammatory conditions such as rheumatoid arthritis ( Hu et al., 2017 ; Gill et al., 2022 ) as well as with improvements of chronic pain in such conditions ( Schönenberger et al., 2021 ). We interpret the findings of our systematic review as preliminary evidence suggesting that nutrition interventions that provide a balanced intake of different macro- and micro-nutrients might be particularly well-equipped to induce long-term changes in DNA stability and biological aging mechanisms. It has been suggested that particular nutrients, such as B-vitamins and choline, induce changes in DNA methylation by acting on the one-carbon metabolism pathway, a mechanism that provides methyl groups for reactions in methylation ( Choi and Friso, 2010 ; Amenyah et al., 2020 ). The intake of dietary polyphenols, such as flavonols or isoflavones, can change the composition of the gut microbiome, with potential implications for the promotion of gastrointestinal health. Moreover, polyphenol metabolites originating from the gut microbiome might act on epigenetic mechanisms by reducing inflammatory biomarkers and regulating oxidative stress ( Scott et al., 2022 ). In fact, polyphenols seem to influence methylation in genes that are critical for cancer ( Choi and Friso, 2010 ).
Studies on the effects of single foods, single nutrients, or multivitamin/multimineral supplements were generally scarce and produced conflictive findings. For instance, both in the case of folic acid and polyunsaturated fatty acids, independent studies yield nonconvergent results. Sometimes, in the same paper, different methods yielded different results, which cautions against an optimistic overinterpretation of the positive findings (e.g., the examination of coagulated vs. uncoagulated blood in Crider et al., 2011 ).
We did not find age or sex-related trends in the association between nutrition and methylation changes. At the same time, the relations between nutrients and methylation changes highlighted here might differ considerably at early developmental stages, which were not covered in this review. For example, although we found no solid evidence that folic acid has the capacity to modify the global methylation in adulthood, B-complex vitamins might be fundamental during the early embryonic period, since alterations in epigenetic reprogramming might explain neural tube defects caused by folate deficits in early embryonic stages ( Choi and Friso, 2010 ).
In our review, no study on the epigenetic effects of dietary polysaccharides met inclusion criteria. Dietary polysaccharides are complex carbohydrates composed of long chains of sugar units that are present in foods such as whole grains, vegetables, and fruits. Cell studies and research in rodents have shown that polysaccharides from natural sources such as resistant starch (RS4) and astragalus can induce epigenetic changes such as histone modification and DNA methylation ( Guo et al., 2023 ; Liu et al., 2023 ). Further research with human clinical trials is needed to confirm these epigenetic effects observed in these pre-clinical investigations.
We acknowledge several limitations in our review. First, all the studies were conducted in participants living in Europe and North America and Australia, and our results do not automatically generalize to populations in other parts of the world. Second, the validity of our conclusions is constrained by the quality of the original articles, and we have included some non-randomized studies and trials performed in a very small number of participants, which increases the risk of bias. Third, while global increases in DNA methylation or in its surrogates are interpreted in the original papers as a favorable epigenetic outcome, it is known that age also causes increases in DNA methylation at the CpG sites ( Seale et al., 2022 ). For this reason, the interpretation of results on higher global DNA methylation as positive can be oversimplistic and should ideally be confirmed with other global measures of the DNA methylation profile, such as epigenetic clocks. Fourth, it is still a matter of debate whether improvements in epigenetic age reflect ameliorations in biological aging. For instance, a study on chronic kidney disease found that a group of patients that underwent kidney transplantation had significant reductions in epigenetic age acceleration 1 year after transplantation. While this result is very encouraging, there is a caveat: the authors found no significant differences in epigenetic age acceleration between a control group of healthy individuals at baseline and the group patients with kidney transplantation 1 year after their surgery. This raises doubts about the validity of epigenetic clocks, especially since patients with kidney transplantation have a reduced life expectancy relative to controls, as it was acknowledged by the same authors ( Neytchev et al., 2024 ). The inclusion of different epigenetic clocks might partially overcome this issue since some epigenetic clocks have been specially designed to be more sensitive to the occurrence of adverse health effects. Despite this, only a few of the studies included here included different epigenetic clocks in their analyses (i.e., Chen et al., 2019 ; Michels and Binder, 2024 ).
In conclusion, we have examined what specific nutrition variables are able to induce DNA methylation changes in community-dwelling adults. Evidence from clinical studies is still scarce, and a higher number of well-powered randomized clinical studies are necessary to conclude upon the effects of nutrition on methylation and biological aging. Initial evidence starts to suggest the possibility of positive epigenetic outcomes related to healthy nutrition patterns, but these results should be regarded as preliminary. To draw definitive conclusions, we will need to wait for the results of robust clinical trials.
Author contributions
IG-G: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing–original draft. GG: Conceptualization, Data curation, Validation, Writing–review and editing. AH: Conceptualization, Validation, Writing–review and editing. SGi: Conceptualization, Validation, Writing–review and editing. SN: Writing–review and editing. SGo: Writing–review and editing. OD: Conceptualization, Project administration, Supervision, Validation, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
IG-G, GG, AH, SGi, and OD work at Clinique la Prairie, a private clinic specialized in longevity. In addition, SGi works for Holistic Health, a company that sells food supplements. SN and SGo are cofounders of Genknowme, a company active in the field of longevity and health prevention, and they are co-inventors of a pending patent on biological age by means of epigenetic biomarkers.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2024.1417625/full#supplementary-material
Amenyah, S. D., Ward, M., Strain, J. J., McNulty, H., Hughes, C. F., Dollin, C., et al. (2020). Nutritional epigenomics and age-related disease. Curr. Dev. Nutr. 6 (7), nzaa097. doi:10.1093/cdn/nzaa097
PubMed Abstract | CrossRef Full Text | Google Scholar
Boyne, D. J., O’Sullivan, D. E., Olij, B. F., King, W. D., Friedenreich, C. M., and Brenner, D. R. (2018). Physical activity, global DNA methylation, and breast cancer risk: a systematic literature review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored by Am. Soc. Prev. Oncol. 27 (11), 1320–1331. doi:10.1158/1055-9965.EPI-18-0175
CrossRef Full Text | Google Scholar
Chan, D., McGraw, S., Klein, K., Wallock, L. M., Konermann, C., Plass, C., et al. (2017). Stability of the human sperm DNA methylome to folic acid fortification and short-term supplementation. Hum. Reprod. 32 (2), 272–283. doi:10.1093/humrep/dew308
Chen, L., Dong, Y., Bhagatwala, J., Raed, A., Huang, Y., and Zhu, H. (2019). Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese african Americans with suboptimal vitamin D status: a randomized clinical trial. Journals Gerontology. Ser. A, Biol. Sci. Med. Sci. 74 (1), 91–98. doi:10.1093/gerona/gly223
Choi, S.-W., and Friso, S. (2010). Epigenetics: a new bridge between nutrition and health. Adv. Nutr. 1 (1), 8–16. doi:10.3945/an.110.1004
Crider, K. S., Quinlivan, E. P., Berry, R. J., Hao, L., Zhu, L., Maneval, D., et al. (2011). Genomic DNA methylation changes in response to folic acid supplementation in a population-based intervention study among women of reproductive age. PloS One 6 (12), e28144. doi:10.1371/journal.pone.0028144
Demidenko, O., Barardo, D., Budovskii, V., Finnemore, R., Palmer, F. R., Kennedy, B. K., et al. (2021). Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 Year reduction in biological aging, after an average of 7 Months of use, in the TruAge DNA methylation test. Aging 13 (22), 24485–24499. doi:10.18632/aging.203736
ElGendy, K., Malcomson, F. C., Lara, J. G., Bradburn, D. M., and Mathers, J. C. (2018). Effects of dietary interventions on DNA methylation in adult humans: systematic review and meta-analysis. Br. J. Nutr. 120 (9), 961–976. doi:10.1017/S000711451800243X
Ferrari, L., Carugno, M., and Bollati, V. (2019). Particulate matter exposure shapes DNA methylation through the lifespan. Clin. Epigenetics 11 (1), 129. doi:10.1186/s13148-019-0726-x
Fiorito, G., Caini, S., Palli, D., Bendinelli, B., Saieva, C., Ermini, I., et al. (2021). DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: the DAMA study. Aging Cell 20 (10), e13439. doi:10.1111/acel.13439
Frankhouser, D. E., Steck, S., Sovic, M. G., Belury, M. A., Wang, Q., Clinton, S. K., et al. (2022). Dietary omega-3 fatty acid intake impacts peripheral blood DNA methylation -Anti-Inflammatory effects and individual variability in a pilot study. J. Nutr. Biochem. 99 (January), 108839. doi:10.1016/j.jnutbio.2021.108839
GBD 2017 Diet Collaborators (2019). Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 393 (10184), 1958–1972. doi:10.1016/S0140-6736(19)30041-8
Gensous, N., Garagnani, P., Santoro, A., Giuliani, C., Ostan, R., Fabbri, C., et al. (2020). One-year mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: a pilot study from the NU-age Project. GeroScience 42 (2), 687–701. doi:10.1007/s11357-019-00149-0
Gill, P. A., Inniss, S., Kumagai, T., Rahman, F. Z., and Smith, A. M. (2022). The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front. Immunol. 13 (April), 866059. doi:10.3389/fimmu.2022.866059
Guo, T., Otobong, D. A., Luo, F., and Lin, Q. (2023). Dietary polysaccharides exert biological functions via epigenetic regulations: advance and prospectives. Crit. Rev. Food Sci. Nutr. 63 (1), 114–124. doi:10.1080/10408398.2021.1944974
Hannum, G., Guinney, J., Zhao, L., Zhang, L., Hughes, G., Sadda, S., et al. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49 (2), 359–367. doi:10.1016/j.molcel.2012.10.016
Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biol. 14, R115. doi:10.1186/gb-2013-14-10-r115
Hu, Y., Sparks, J. A., Malspeis, S., Costenbader, K. H., Hu, F. B., Karlson, E. W., et al. (2017). Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann. Rheumatic Dis. 76 (8), 1357–1364. doi:10.1136/annrheumdis-2016-210431
Hübner, U., Geisel, J., Kirsch, S. H., Kruse, V., Bodis, M., Klein, C., et al. (2013). Effect of 1 Year B and D vitamin supplementation on LINE-1 repetitive element methylation in older subjects. Clin. Chem. Laboratory Med. CCLM/FESCC 51 (3), 649–655. doi:10.1515/cclm-2012-0624
Hunter, D. J., James, L., Hussey, B., Wadley, A. J., Lindley, M. R., and Mastana, S. S. (2019). Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics Official J. DNA Methylation Soc. 14 (3), 294–309. doi:10.1080/15592294.2019.1582276
Jain, P., Binder, A. M., Chen, B., Parada, H., Gallo, L. C., Alcaraz, J., et al. (2022). Analysis of epigenetic age acceleration and healthy longevity among older US women. JAMA Netw. Open 5 (7), e2223285. doi:10.1001/jamanetworkopen.2022.23285
Jenkins, T., Aston, K., Carrell, D., DeVilbiss, E., Sjaarda, L., Perkins, N., et al. (2022). The impact of zinc and folic acid supplementation on sperm DNA methylation: results from the folic acid and zinc supplementation randomized clinical trial (FAZST). Fertil. Steril. 117 (1), 75–85. doi:10.1016/j.fertnstert.2021.09.009
Jung, A. Y., Yvo, S., Petra, V., Kok, F. J., Blom, H., Kok, R. M., et al. (2011). No effect of folic acid supplementation on global DNA methylation in men and women with moderately elevated homocysteine. PloS One 6 (9), e24976. doi:10.1371/journal.pone.0024976
Kane, A. E., and Sinclair, D. A. (2019). Epigenetic changes during aging and their reprogramming potential. Crit. Rev. Biochem. Mol. Biol. 54 (1), 61–83. doi:10.1080/10409238.2019.1570075
Kim, M., and Costello, J. (2017). DNA methylation: an epigenetic mark of cellular memory. Exp. Mol. Med. 49 (4), e322. doi:10.1038/emm.2017.10
Kim, Y., Huan, T., Joehanes, R., McKeown, N. M., Horvath, S., Levy, D., et al. (2022). Higher diet quality relates to decelerated epigenetic aging. Am. J. Clin. Nutr. 115 (1), 163–170. doi:10.1093/ajcn/nqab201
Kresovich, J. K., Park, Y.-M. M., Keller, J. A., Sandler, D. P., and Taylor, J. A. (2022). Healthy eating patterns and epigenetic measures of biological age. Am. J. Clin. Nutr. 115 (1), 171–179. doi:10.1093/ajcn/nqab307
Levine, M. E., Lu, A. T., Quach, A., Chen, B. H., Assimes, T. L., Bandinelli, S., et al. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging 10 (4), 573–591. doi:10.18632/aging.101414
Li, S., and Tollefsbol, T. O. (2021). DNA methylation methods: global DNA methylation and methylomic analyses. Methods 187 (March), 28–43. doi:10.1016/j.ymeth.2020.10.002
Lichtenstein, A. H., Appel, L. J., Vadiveloo, M., Hu, F. B., Kris-Etherton, P. M., Rebholz, C. M., et al. (2021). 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American heart association. Circulation 144 (23), e472–e487. doi:10.1161/CIR.0000000000001031
Lillycrop, K. A., Hoile, S. P., Grenfell, L., and Burdge, G. C. (2014). DNA methylation, ageing and the influence of early life nutrition. Proc. Nutr. Soc. 73 (3), 413–421. doi:10.1017/S0029665114000081
Liu, Q., Liu, X., Wang, G., Wu, F., Yuan, H., and Liu, H. (2023). Genome-wide DNA methylation analysis of Astragalus and danshen on the intervention of myofibroblast activation in idiopathic pulmonary fibrosis. BMC Pulm. Med. 23 (1), 325. doi:10.1186/s12890-023-02601-6
Meir, Y., Anat, M. K., Bernhart, S. H., Rinott, E., Tsaban, G., Zelicha, H., et al. (2021). Lifestyle weight-loss intervention may attenuate methylation aging: the CENTRAL MRI randomized controlled trial. Clin. Epigenetics 13 (1), 48. doi:10.1186/s13148-021-01038-0
Michels, K. B., and Binder, A. M. (2024). Impact of folic acid supplementation on the epigenetic profile in healthy unfortified individuals - a randomized intervention trial. Epigenetics 19 (1), 2293410. doi:10.1080/15592294.2023.2293410
Milenkovic, D., Berghe, W. V., Boby, C., Leroux, C., Declerck, K., Szarc vel Szic, K., et al. (2014). Dietary flavanols modulate the transcription of genes associated with cardiovascular pathology without changes in their DNA methylation state. PloS One 9 (4), e95527. doi:10.1371/journal.pone.0095527
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.for the PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Neytchev, O., Erlandsson, H., Witasp, A., Nordfors, L., Qureshi, A. R., Iseri, K., et al. (2024). Epigenetic clocks indicate that kidney transplantation and not dialysis mitigate the effects of renal ageing. J. Intern. Med. 295 (1), 79–90. doi:10.1111/joim.13724
Noro, F., Marotta, A., Bonaccio, M., Costanzo, S., Santonastaso, F., Orlandi, S., et al. (2022). Fine-grained investigation of the relationship between human nutrition and global DNA methylation patterns. Eur. J. Nutr. 61 (3), 1231–1243. doi:10.1007/s00394-021-02716-8
Oblak, L., van der Zaag, J., Higgins-Chen, A. T., Levine, M. E., and Boks, M. P. (2021). A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 69 (101348), 101348. doi:10.1016/j.arr.2021.101348
Ostojic, S. M., Mojsin, M., Drid, P., and Milan, V. (2018). Does dietary provision of guanidinoacetic acid induce global DNA hypomethylation in healthy men and women? Lifestyle Genomics 11 (1), 16–18. doi:10.1159/000487336
Pal, S., and Tyler, J. K. (2016). Epigenetics and aging. Sci. Adv. 2 (7), e1600584. doi:10.1126/sciadv.1600584
Perfilyev, A., Dahlman, I., Gillberg, L., Rosqvist, F., Iggman, D., Volkov, P., et al. (2017). Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am. J. Clin. Nutr. 105 (4), 991–1000. doi:10.3945/ajcn.116.143164
Pusceddu, I., Herrmann, M., Kirsch, S. H., Werner, C., Hübner, U., Bodis, M., et al. (2016). Prospective study of telomere length and LINE-1 methylation in peripheral blood cells: the role of B vitamins supplementation. Eur. J. Nutr. 55 (5), 1863–1873. doi:10.1007/s00394-015-1003-1
Quach, A., Levine, M. E., Tanaka, T., Lu, A. T., Chen, B. H., Ferrucci, L., et al. (2017). Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 9 (2), 419–446. doi:10.18632/aging.101168
Sae-Lee, C., Corsi, S., Barrow, T. M., Kuhnle, G. G. C., Bollati, V., Mathers, J. C., et al. (2018). Dietary intervention modifies DNA methylation age assessed by the epigenetic clock. Mol. Nutr. Food Res. 62 (23), e1800092. doi:10.1002/mnfr.201800092
Salas-Huetos, A., James, E. R., Salas-Salvadó, J., Bulló, M., Aston, K. I., Carrell, D. T., et al. (2021). Sperm DNA methylation changes after short-term nut supplementation in healthy men consuming a western-style diet. Andrology 9 (1), 260–268. doi:10.1111/andr.12911
Schönenberger, K. A., Schüpfer, A.-C., Gloy, V. L., Paul, H., Stanga, Z., Kaegi-Braun, N., et al. (2021). Effect of anti-inflammatory diets on pain in rheumatoid arthritis: a systematic review and meta-analysis. Nutrients 13 (12), 4221. doi:10.3390/nu13124221
Scoccianti, C., Ricceri, F., Ferrari, P., Cuenin, C., Sacerdote, C., Polidoro, S., et al. (2011). Methylation patterns in sentinel genes in peripheral blood cells of heavy smokers: influence of cruciferous vegetables in an intervention study. Epigenetics Official J. DNA Methylation Soc. 6 (9), 1114–1119. doi:10.4161/epi.6.9.16515
Scott, M. B., Styring, A. K., and McCullagh, J. S. O. (2022). Polyphenols: bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens 11 (7), 770. doi:10.3390/pathogens11070770
Seale, K., Horvath, S., Teschendorff, A., Eynon, N., and Voisin, S. (2022). Making sense of the ageing methylome. Nat. Rev. Genet. 23 (10), 585–605. doi:10.1038/s41576-022-00477-6
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., and Hébert, J. R. (2014). Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17 (8), 1689–1696. doi:10.1017/S1368980013002115
Tammen, S. A., Friso, S., and Choi, S.-W. (2013). Epigenetics: the link between nature and nurture. Mol. Aspects Med. 34 (4), 753–764. doi:10.1016/j.mam.2012.07.018
Worthley, D. L., Le Leu, R. K., Whitehall, V. L., Conlon, M., Christophersen, C., Belobrajdic, D., et al. (2009). A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am. J. Clin. Nutr. 90 (3), 578–586. doi:10.3945/ajcn.2009.28106
Zhu, H., Bhagatwala, J., Huang, Y., Pollock, N. K., Parikh, S., Raed, A., et al. (2016). Race/ethnicity-specific association of vitamin D and global DNA methylation: cross-sectional and interventional findings. PloS One 11 (4), e0152849. doi:10.1371/journal.pone.0152849
Keywords: nutrition, anti-inflammatory diet, DNA methylation, epigenetic clocks, mediterranean diet (MD)
Citation: García-García I, Grisotto G, Heini A, Gibertoni S, Nusslé S, Gonseth Nusslé S and Donica O (2024) Examining nutrition strategies to influence DNA methylation and epigenetic clocks: a systematic review of clinical trials. Front. Aging 5:1417625. doi: 10.3389/fragi.2024.1417625
Received: 15 April 2024; Accepted: 18 June 2024; Published: 15 July 2024.
Reviewed by:
Copyright © 2024 García-García, Grisotto, Heini, Gibertoni, Nusslé, Gonseth Nusslé and Donica. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabel García-García, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Dietary Interventions to Prevent Childhood Obesity: A Literature Review
Ana rita pereira.
1 Faculty of Health Sciences (Nutrition Sciences), University Fernando Pessoa, Rua Carlos da Maia 296, 4200-150 Porto, Portugal; tp.ude.pfu@06453
Andreia Oliveira
2 EPIUnit—Instituto de Saúde Pública da Universidade do Porto (Institute of Public Health of the University of Porto), Rua das Taipas 135, 4050-600 Porto, Portugal
3 Laboratory for Integrative and Translational Research in Population Health (ITR), Rua das Taipas 135, 4050-600 Porto, Portugal
4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, Alameda Professor Hernâni Monteiro, 4200-319 Porto, Portugal
Associated Data
Not applicable.
Several dietary interventions have been conducted to prevent/reduce childhood obesity, but most of them are known to have failed in tackling the obesity epidemic. This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness. A literature search was conducted using PubMed Central ® . Only articles published between 2009 and 2021, written in English, conducted in humans, and including children and/or adolescents (<18 years old) were considered. The majority of studies were school-based interventions, with some addressing the whole community, and including some interventions in the food sector (e.g., taxation of high fat/sugar foods, front-of-pack labelling) and through mass media (e.g., restrictions on food advertising for children) that directly or indirectly could help to manage childhood obesity. Most of the programs/interventions conducted focus mainly on person-based educational approaches, such as nutrition/diet education sessions, allied to the promotion of physical activity and lifestyles to students, parents, and school staff, and less on environmental changes to offer healthier food choices. Only a few trials have focused on capacity building and macro-policy changes, such as the adaptation of the built environment of the school, serving smaller portion sizes, and increasing the availability and accessibility of healthy foods and water in schools, and restricting the access to vending machines, for example. Overall, most of the intervention studies showed no consistent effects on changing the body mass index of children; they have only reported small weight reductions, clinically irrelevant, or no effects at all. Little is known about the sustainability of interventions over time.
1. Introduction
In the recent past, there was a shift from prevailing infectious diseases to a high prevalence of chronic and degenerative diseases associated with lifestyle choices [ 1 ]. Obesity is one of the conditions that has dramatically increased all over the world, and children, in particular, are a cause of public health concern [ 2 ].
The prevalence of overweight and obesity has increased substantially over the past four decades, and an epidemiological transition from underweight to overweight and obesity has been described throughout the world [ 3 ]. This alarming rise has been observed in all regions, including developing countries, with an increase of overweight and obesity prevalence from 1980 to 2013 of 8.1% to 12.9% (in boys) and 8.4% to 13.4% (in girls) [ 2 ]. These increases have been also reported in developed countries, among children and adolescents, with 23.8% of boys and 22.6% of girls having either overweight or obesity in 2013 [ 2 ]. Although the prevalence is clearly higher in developed countries at all ages, the differences between sexes are small. Nonetheless, the prevalence of childhood obesity in the United States and some European countries has apparently reached a plateau [ 4 ], but it continues at high rates.
Obesity is a complex, multifactorial disease. Although genetics may be an important etiological factor for obesity development, genes do not fully explain the huge and fast increase of obesity at the population level [ 4 , 5 ]. It is believed that this obesity epidemic may be due to gene–environment interactions [ 6 ], enhanced by an increasingly permissive obesogenic environment, with different levels of determinants [ 1 , 7 ]. There are micro-environmental settings, such as schools, workplaces, homes, and neighborhoods, and these are influenced by macro-environmental sectors, such as the health system and the food industry that may be key settings to tackle the obesity epidemic [ 7 ]. Now, more than ever, individuals are embedded in a more permissive environment with concern to eating habits and are more likely to adopt sedentary behaviors. It has been recognized many years ago in the Ottawa Charter that it is very important to promote supportive environments [ 8 ]. In the case of children, the family and school are included in a wider proximal context [ 7 ].
It is well known that diet and other habits are shaped at the earlier stages of life and maintained through adulthood [ 9 ]. With the current increasing rates of childhood obesity, there has been a growing amount of research focusing on the determinants of obesity in children and their families, and several studies have described possible dietary/nutritional interventions to prevent childhood obesity. It is known that interventions that are mostly based on educational, behavioral, or pharmacological measures are not very effective in preventing and treating obesity [ 10 , 11 ].
This study aims to review the existing literature on dietary interventions for the prevention of childhood obesity and to assess their effectiveness.
2. Materials and Methods
A literature search was conducted using the PubMed Central ® search engine, the most comprehensive dataset for biomedical literature. The search expression used for this search included the mesh terms “(pediatric obesity) OR (childhood obesity) AND (primary prevention) AND “diet”. Due to the extensive amount of published data, we limited the timeline to have only articles from 2009 up to 2019. An update search was then performed to include studies published between 2019 and 2021. Only articles written in English, conducted in humans, and including children and/or adolescents (<18 years old) were included. This search yielded 538 articles, of which we excluded 513, including 25 in this study. The literature search had three stages, the search for the titles, then abstracts, and finally the full-text papers were searched and retrieved (when deemed of interest). Some articles were discarded because they did not report measures to prevent obesity in children (n = 192) or because these measures were implemented only in adults (n = 321).
Additional papers (n = 27) were included in this review from a snowball process or searched to put into context the dietary interventions for the prevention of childhood obesity, totaling 52 references. Figure 1 presents the flowchart of the studies’ selection.

Flowchart of studies’ selection.
In this literature review, dietary interventions to prevent childhood obesity were grouped and described into four levels: school-based interventions, community-based interventions, interventions through mass media, and food sector interventions.
To prevent obesity in children there is a need to take multidimensional actions at different levels, including the individual, familial, institutional, and environmental levels. At the moment, these types of multilevel interventions seem to be the most promising ones to actually prevent/manage obesity. In particular, children are very influenced by social and environmental conditions, so at these ages, community-based interventions, changing the supportive environment, seem to play an especially important role [ 12 ].
Table 1 provides a descriptive summary of the dietary interventions to tackle childhood obesity, described in detail below.
Summary of dietary interventions on childhood obesity and their main characteristics and results.
| Author (Study Title) (Reference) | Country, Year | Type of Intervention | Intervention Description | Target Audience | Results |
|---|---|---|---|---|---|
| Niederer I, et al. (Ballabeina study) [ ] | Switzerland, 2009 | Cluster randomized controlled single-blinded trial | Lessons on nutrition (balanced nutrition and healthy nutritional behaviors in a didactic way), physical activity program, media use, and sleep, and adaptation of the built environment of the preschool. | Preschool children (mean 5.1 years) (n = 652), the parents, and the teachers | No differences in children’s BMI were found between groups. However, the intervention group had a reduction in body fat percentage, better motor agility, as well as benefits in reported physical activity, media use, and eating habits. |
| Singh AS, et al. [ , ] | Netherlands, 2006 | School-based trial | Educational component (classes in biology and physical education, and a computer-based information program); and an environmental component (e.g., serving smaller portion sizes in the canteen and healthier options, restricting access to vending machines, and food awareness by posters). | Students from the ages of 12–14 years (n = 1108) | With a twelve-month follow-up, a reduction in the skinfold thickness of the intervention groups was found, as well as lower consumption of sugar-containing beverages, and less screen time (but only in boys). |
| Kain J, et al. [ ] | Chile, 2004 | School-based obesity-prevention trial | Weekly classes on physical activity and healthy nutrition for parents and students. Some environmental changes were also made (e.g., school kiosks were instructed to offer healthier choices and at the same time remain lucrative). | Parents and students from 1st to 8th grade; 2141 schools in the intervention group and 945 in the control group. | After 6 months, there was a reduction of body mass index (BMI) z-scores in boys and better physical fitness in both genders. On the other hand, the modifications in the kiosk’s food availability did not seem to change the students’ food choices. |
| Hollar D, et al. (Healthier Options for Public Schoolchildren (HOPS)) [ ] | Florida, US, 2004–2006 | Randomized trial | Modifications in the school menu, school gardens, and physical activity; monthly newsletters with healthy nutrition and physical activity lessons for the students and parents. | 6 elementary schools (4588 children aged 6 to 13 years; 48% Hispanic) | After 2 years, a higher percentage of students who maintained a normal weight (<85th percentile of BMI-for-age) was found in the intervention group (52.1%) when comparing with the control group (40.7%). Students in the intervention group had improved academic performance. |
| Economos CD, et al. (Shape up Somerville) [ ] | United States, (September 2003–June 2005) | Non-randomized controlled trial | Dietary intervention (e.g., promotion of fresh fruit and vegetables and taste tests, posters with nutritional and health information, training of food staff, modification of food offers in restaurants according to the study guidelines); increase of physical activity opportunities around the school (e.g., information on safe routes); modifications inside the school space (e.g., new equipment). | 1178 children (average 7.92 years) attending public school in three different communities from Somerville, Massachusetts | After 1 year, the BMI z-scores were 0.06 lower in the intervention group than in the control group. There was a decrease in overweight and obesity and an increase in remission in both sexes in the intervention group. The study design did not include randomization of the intervention. |
| Bacardí-Gascon M, et al. [ ] | Mexico, 2012 | Randomized cluster controlled trial | Sessions discussing healthy lifestyles to the school board and the teachers; interactive lessons to the children to increase fruit and vegetables intake and physical activity practice, and reduce soda and high fat and salt-containing snacks intake, while simultaneously decreasing TV watching time; healthy eating sessions to parents. | 532 school-aged children from 2nd and 3rd grade | By the sixth month, there was a greater decrease in BMI in the intervention group than in the control group (difference of −0.82 kg/m in children BMI), although it was not sustained after 18 and 24 months of intervention. |
| Foster GD, et al. [ ] | USA, 2008 | Multicomponent School Nutrition Policy Initiative | School self-assessment (e.g., strategies like limiting the use of food as reward/punishment, promoting active recess, and serving breakfasts in classrooms); training of school staff and children in nutrition education; nutrition policies (e.g., changing sold foods); social marketing; school association meetings/workshops. | 1349 students in grades 4 through 6 from 10 schools | There were significantly fewer children in the intervention schools (7.5%) than in the control schools (14.9%) who became overweight after 2 years, but no differences after 2 years of follow-up. |
| Donnelly JE, et al. [ ] | Nebraska, USA, 1996 | 2-year trial | Nutrition education (basic nutrition, diet, and general health, nutrition for growth and development, healthy food choices, snack alternatives, food safety), modified school lunches (meals planned according to the Lunchpower! Program aiming to reduce energy, fat, and sodium lunches), and increased physical activity. | Students from grades 3 to 5 in two school districts in rural Nebraska (n = 338) | After 2 years of the intervention, both schools showed no significant changes in the body fat percentage, but a significant increase in the BMI. The control school showed significantly higher total energy, total fat and sodium intake, and lower fiber intake. |
| Liu Z, et al. (The DECIDE-Children study) [ ] | China, 2019 | Cluster-randomized controlled trial | Health education activities for parents and children; supervision and encouragement of children’s physical activity practice outside of school; school policies to prevent obesity. Development of an app called ‘Eat Wisely, Move Happily’ that aids in diffusing information, monitoring the children’s behavior, and managing their weight. | 4-grade primary schools (8–10 years old) (n = 1200) | No known results. |
| Xu H, et al. [ ] | China, 2020 | Multicenter randomized controlled trial | Development of a nutrition handbook that was given to all students; nutrition and health courses to students, parents, teachers, and health workers (e.g., meals proportion, how to choose healthy foods, reduce eating out and unhealthy foods); informative posters around the school; course on physical activity for parents, and physical activity classes for students. | 4846 school children aged 7–13 years | The effects on children’s BMI were studied. There were some improvements in the diversity of the foods consumed at breakfast and a decrease in the consumption of some unhealthy foods. |
| Hannon BA, et al. ( Program) [ ] | Illinois, California, Iowa, Texas, and Puerto Rico, 2019 | Randomized control trial | Workshop presentations and activities on nutrition education, family wellness, and physical activity. | Families of parents and 1 child aged 6–18 years (n = 500) | No known results. |
| Olsen NJ, et al. (Healthy Start) [ ] | Denmark, 2020 | Randomized controlled trial | Guidance on how to improve the child’s diet and physical activity, quantity and quality of sleep, and reduce their stress. Cooking classes, games focused on exercise and motor skills development, access to a website with recipes. | Children aged 2 to 6 years (n = 3722) | The clinical effects of this intervention in the children’s growth and body composition were small. |
| Homs C, et al. (FIVALIN project) [ ] | Barcelona, 2021 | Quasi-experimental design | Workshops on health education and sports educational sessions. | 810 children aged 8–12 years and 600 parents | No known results. |
| Li B, et al. (The CHIRPY DRAGON intervention) [ ] | China, 2019 | Cluster-randomized controlled trial | Workshops and family activities to promote physical activity and healthy eating behaviors; school support to improve physical activity and healthy food provision. | School children with a mean age of 6.15 years (n = 1641) | There was a decrease in the BMI z-scores of the children in the intervention group, along with an increase in the consumption of fruit and vegetables, and a decrease in the consumption of sugar-sweetened beverages and unhealthy snacks. There was also a decrease in screen time and an increase in physical activity in this group. |
| Anselma M, et al. (Kids in Action) [ ] | Amsterdam, 2019 | Controlled trial | Meetings with children to develop interventions that targeted their physical activity and healthy eating habits. These interventions consist of environmental changes, organizational changes, or educational approaches. | Children aged 9–12 years from four primary schools | No known results. |
| Hamulka J, et al. (The ABC of Healthy Eating Project) [ ] | Poland, 2018 | Education-based intervention study | Diet and lifestyle-related programs for the intervention group and school activities with the theme of nutrition and healthy lifestyles for both the intervention and the control group. | School children aged 11–13 years. (464 students) | No known results. |
| Elder JP, et al. (MOVE/me Muevo) [ ] | San Diego County, USA, 2014 | Randomized community trial | Activities and phone calls from health coaches on how to increase the consumption of fruit and vegetables; decrease the consumption of sugar-sweetened beverages; increase healthy food portions; reduce eating out and do healthier options when eating out; increase the availability and accessibility of healthy foods and beverages at home; reduce the screen time and avoid eating in front of the TV, and increase the number of family meals. | 541 families with children between the ages of 5 and 8 years old | After 2 years, there were no significant differences between the control and the intervention group concerning BMI or waist circumference. Some changes were observed in dietary intake, namely a reduction in fat and sugary beverages in the intervention group. |
| De Silva-Sanigorski A, et al. (Romp & Chomp) [ ] | Australia, 2020 | Community-based trial | Changes in the provision of water in childcare centers, childcare policies regarding healthy eating and physical activity; teaching of skills in physical activity and nutrition to the childcare professionals; production and distribution of promotional materials (balloons, stickers, posters, postcards). | Children aged 1–5 y (n = 12,000) and their families | After 3 years of intervention, the 3.5 years old subsample showed considerably lower mean weight, BMI, and z-score BMI, and the 2 and 3.5 years old children showed a considerably lower prevalence of overweight and obesity when compared with the baseline values. The intervention group also showed a considerably lower intake of packaged snacks and fruit juice. |
| Crespo NC, et al. (The Aventuras Para Niños Study) [ ] | Southern California, 2003 | Randomized Community-based trial | Three arms: family-only, community-only, or family+community intervention. In the family-only intervention, professionals call/make home visits to discuss how to maintain a healthy diet, prepare meals, and be physically active. The community-only intervention included improving the school’s playgrounds, implementing salad bars, as well as community parks, displaying water bottles in the classrooms for the students, better physical education equipment and healthy menus for the children, all of this combined with spreading media messages through posters, news and point-of-choice messages in grocery stores, with health messages. The family+community included all described. | 811 predominantly Mexican immigrant/Mexican-American mothers with children in kindergarten through second grade | No noteworthy main effects nor interactions for the family or community interventions were found, including on BMI z-scores. Despite the lack of significant effects on the children’s BMI z-score, there were multiple obesity-related behaviors in these children that were changed by the family intervention, like increased consumption of fruit and vegetables. |
| Borys JM, et al. (EPODE (Ensemble Prevenons l’Obesité Des Enfants/Together Let’s Prevent Childhood Obesity) [ ] | France, 2004 | Community-based intervention | Changes in local environments, childhood settings, and family norms to make them more supportive and aid the adoption of healthy lifestyles in children. | Children aged 1–12 years, and their families, as well as a wide variety of local stakeholders in 10 French pilot communities | No known results. |
| Swinburn BA, et al. and Schultz JT, et al. (Pacific Obesity Prevention in Communities (OPIC) project) [ , ] | Australia, Fiji, New Zealand, and Tonga, over 30 months, between 2004 and 2009 | Community-based intervention | Interventions aiming to reduce the consumption of high sugar content drinks and energy-dense snacks and increase physical activity. | 18 000 children 12–18 years, 300 stakeholders, 60 multi-professional research staff, 27 research students. | The authors state that the project can produce positive effects in diet and physical activity, but effects on childhood obesity are not clearly described. |
| World Health Organization and Assembly of the Republic (TV ban/restrictions of food commercials to kids in several countries [ ] and Portugal) [ ] | Sweden, Norway, Denmark, Austria, Ireland, Australia, Greece, and Portugal, 2019 | Mass-media based- intervention | Sweden has banned TV food commercials for children under the age of 12 and TV food advertising for children. Norway, Denmark, Austria, Ireland, Australia, and Greece have also made some restrictions on commercials for children. Portugal approved a law to restrict advertising to children for foodstuffs and beverages of high energy value, salt, sugar, and saturated fatty acids content. | Children | No efficacy results. However, energy-dense foods and drinks and fast-food companies often target children in their advertisements, since they are very easily influenced at this age, namely through TV commercials. |
| Goiana-da-Silva F, et al. (Taxation of sugar-sweetened beverages) [ ] | Portugal, 2017 | Food sector intervention | Taxation of sugar-sweetened beverages as an intervention to reduce its high consumption in the country. | Community | Decrease of 6.58 million liters per year, which translates into a decrease in consumption of 21% compared to the baseline consumption data of IAN-AF 2015–2016. The number of cases of obesity prevented had a higher impact in adolescents (0.012%), preventing 0.76 cases of obesity yearly, followed by an impact of 0.062% in adults aged 18 to <65 years, and the children showed an impact of 0.049%. These data show that Portugal achieved its goal, decreasing sales of sugar-sweetened beverages. |
| Young L, et al. (“Pick the Trick” program) [ ] | Australia and New Zealand | Food sector intervention | Providing foods with symbols for the consumers making it easier to identify the healthier choices. | Community | No known results |
| Kelly B, et al. and Nielsen S, et al. (WHO front-of-pack labeling system) [ ] | WHO-E Food and Nutrition Action Plan 2015–2020 | Food sector intervention | Among other future policies, there is the intention of application of a single front-of-pack labeling system in all countries. | Community | No known results |
3.1. School-Based Interventions
The Ballabeina study is a cluster-randomized controlled single-blinded trial that took place in some preschools in Switzerland, designed to study the effect of a multidimensional lifestyle intervention on aerobic fitness and adiposity, mainly in migrant preschoolers with the duration of over one school year [ 13 ]. This study included 652 preschool children with a mean age of 5.1 years. The interventions comprised a physical activity program, lessons on nutrition, media use, and sleep, and adaptation of the built environment of the preschool. The dietary intervention included weekly nutrition lessons given by a dietician; the students could learn about balanced nutrition and healthy nutritional behaviors in a didactic way. These lessons were centered on five messages: “drink water”, “eat fruit and vegetables”, “eat regularly”, “make clever choices”, and “turn your screen off when you eat”, which were developed in collaboration with the Swiss Society for Nutrition. These messages were also described on funny cards that children could get with a task to implement the message at home. After 4 months of intervention, the results showed no differences between the groups in the children’s body mass index (BMI). However, an increase in aerobic fitness by the end of the intervention was reported, and children in the intervention group also showed beneficial effects in the percentage of body fat (−1.1%), and their motor agility, when compared with the children in the control group. It was also possible to observe benefits in reported physical activity, media use (less screen time in boys), and eating habits, such as an increase in fruit and vegetable consumption in the intervention group [ 13 ].
In the Netherlands, a school-based trial was implemented including students from the ages of 12–14 years old (n = 1108), within a multidimensional health promotion intervention [ 14 , 15 ]. There were 10 intervention and eight control secondary schools included. The intervention included an educational component, with classes in biology and physical education, and a computer-based information program; and an environmental component, with propositions such as serving smaller portion sizes in the canteen and healthier food options, or restricting the access to vending machines. There were also posters affixed to create more awareness about which foods were healthier and which were not. With a twenty-month follow-up, it was observed in the intervention group a reduction in body composition measures, such as skinfold thickness, lower consumption of sugar-containing beverages at 12 months, and less screen time (but only in boys) [ 14 , 15 ].
A school-based obesity-prevention trial in Chile evaluated the effect of weekly physical activity classes and classes on healthy nutrition for parents and students from 1st to 8th grade; 2141 schools were in the intervention group and 945 in the control group [ 16 ]. Some environmental changes were also made, including instructing school kiosks to offer healthier options to students and still remain lucrative. The results showed a reduction in BMI z-scores in boys after 6 months of intervention and better physical fitness in both genders. On the other hand, the modifications in the kiosk’s food availability did not seem to change the students’ food choices [ 16 ].
The school-based Healthier Options for Public Schoolchildren (HOPS) is a randomized trial implemented over two school years (2004–2005 and 2005–2006) that included six elementary schools (4588 children aged 6 to 13 years; 48% Hispanic) in Osceola, Florida. Interventions implemented included modifications in the school menu, school gardens, and physical activity [ 17 ]. Complementarily, there were healthy nutrition and physical activity lessons for the students and parents through monthly newsletters. After 2 years, it was possible to observe a higher percentage of students who maintained a normal weight (under the 85th percentile of BMI-for-age) in the intervention group (52.1%) than in the control group (40.7%). Students in the intervention group had also improved academic performance compared to the control group [ 17 ].
The “Shape up Somerville” (SUS) is a non-randomized controlled trial conducted over two school years (September 2003–June 2005) in 1178 children in grades 1–3 (average of 8 years old) attending public school in three different communities from Somerville, Massachusetts, United States [ 18 ]. This intervention included more physical activity opportunities around the school, such as information on safe routes to school and walking to the school bus; modifications inside the school space, such as new equipment for physical activities; and a dietary intervention. This included taste tests of fruit and vegetables during lunchtime, where children could vote on whether they would like to see those fruits or vegetables on the monthly school menu; new vegetarian recipes and fresh fruit were made available every day for breakfast and lunch; colorful educational posters with nutrition and health information were displayed in the school cafeterias, and food service staff was trained. Additionally, there was an approval of restaurants according to SUS guidelines which offer low-fat dairy products, some dishes in smaller portion sizes, fruits and vegetables as side dishes, and have visible signs highlighting healthier options. After 1 year, results showed that the BMI z-scores were 0.06 lower in the intervention group than in the control group [ 18 ]. There was a decrease in overweight and obesity and an increase in remission in both sexes in the intervention group, but the comparison groups were not randomly assigned.
A randomized cluster controlled trial was performed in Mexico on 532 school-aged children from the 2nd and 3rd grades, with an average age of 8.5 ± 0.73 years at baseline (280 children in the intervention and 252 in the control group; each arm with one public and one private school, totaling four) [ 19 ]. It aimed to make these children and their parents reduce their sedentary behaviors, consumption of soft drinks and high-fat and salt-containing snack foods and increase their consumption of fruits and vegetables. The intervention consisted of sessions for discussing healthy lifestyles dedicated to the school board and teachers, conducted by nutritionists and physical activity professionals. There were also interactive lessons provided by nutrition graduated students for the children with the intent of increasing their fruit and vegetable intake, physical activity practice, and reducing their intake of soda and high-fat and salt-containing snacks, while simultaneously lowering their TV watching time. There were also nutrition sessions for parents run by nutrition professionals, with the intent of educating them about healthy eating. The results showed that by the sixth month of the intervention, there was a greater decrease in BMI in the intervention group than in the control group (difference of −0.82 kg/m 2 in children BMI), although this was not sustained in the long-term, after 18 and 24 months [ 19 ].
A Multicomponent School Nutrition Policy Initiative on the prevention of overweight and obesity among children was conducted in 1349 students in grades four through six from 10 schools in a US city [ 20 ]. This initiative included the following interventions: school self-assessment, in which the schools suggested strategies such as limiting the use of food as reward/punishment, promoting active recess, and serving breakfasts in the classrooms to ensure the students eat a healthy meal; training in nutrition education for the school staff; nutrition education classes for the children; nutrition policies in the intervention schools, such as changing the foods that were sold and served according to the Dietary Guidelines for Americans to meet the nutritional standards; social marketing, such as giving raffle tickets to students who purchased or brought from home healthy snacks and beverages; and parent outreach through nutrition educators in home and school association meetings, report card nights, parent education meetings, and weekly nutrition workshops [ 20 ]. The results of this intervention were a 50% reduction in the incidence of overweight. There were significantly fewer children in the intervention schools (7.5%) than in the control schools (14.9%) who had become overweight after 2 years. However, there were no differences in the incidence or prevalence of obesity, nor in the remission of being overweight or obesity after 2 years of follow-up [ 20 ].
Donnelly et al. [ 21 ] conducted a 2-year trial in students from grades three to five in two school districts in rural Nebraska aiming to reduce obesity and improve physical and metabolic fitness. The intervention consisted of nutrition education, modified school lunches, and increased physical activity. The meals were planned with the kitchen staff according to the Lunchpower! Program. This program consists of energy, fat, and sodium reduced lunches, in agreement with the Healthy People 2000 objectives [ 22 ]. According to this, the fat content is restricted to 30% of the total energy intake, the sodium is limited to 1000 mg, the cholesterol to 100 mg, and the dietary fiber is increased to 8 to 10 g per day. There were also nutrition classes given by the teacher, after being trained. These classes included basic nutrition, nutrition for proper growth and development, the relationship between diet and health, healthy food choices, how to reduce fat in the diet, snack alternatives, and food safety. After two years of intervention, the control school showed significantly higher total energy (9%) and total fat (25%). The control school also showed considerably greater values for sodium and smaller for fiber. After the first year of intervention, there were no significant differences between the control and intervention schools in nutrition knowledge. However, after two years of intervention, the intervention school reduced by 45% their wrong answers about nutrition knowledge. Concerning physical activity, the control school practiced significantly more sports outside school compared to the intervention school. After 2 years of the intervention, neither the control nor intervention schools showed significant increases in aerobic capacity. Both schools showed no significant changes in the percentage of body fat, but a significant increase in BMI [ 21 ].
The DECIDE-Children Study [ 23 ] is a cluster-randomized controlled trial conducted in 1200 Chinese students from four primary schools (8–10 years old). The intervention consisted of health education activities for the parents; supervision and encouragement of the children as a way of increasing their physical activity practice outside of school; school policies to prevent obesity and health education activities for the children. There was also the development of an app called ‘Eat Wisely, Move Happily’ that aids in diffusing information, monitoring the children’s behavior, managing their weight, and giving feedback for the teachers and parents. Since this study is ongoing, the results of this intervention are not yet available [ 23 ].
In 2020, a multicenter randomized controlled trial [ 24 ] was conducted in 4846 Chinese school children aged 7 to 13 years, in which the intervention consisted of the development of a nutrition handbook that was given to all students; nutrition and health courses to the students, parents, teachers, and health workers about the proportion of the meals, how to choose healthy foods, and how to reduce eating out, unhealthy fast food, sugar-sweetened beverages, and snacks; and displaying informative posters around the school. Courses on physical activity for the parents and physical activity classes for the students were also given. There were no significant improvements in the overall diversity of the food consumption in the intervention group; however, there were some improvements in the diversity of the foods consumed at breakfast and a decrease in the consumption of some unhealthy foods [ 24 ]. No effects on children’s BMI were studied.
The Abriendo Caminos Program [ 25 ] was implemented in several schools in Illinois, California, Iowa, Texas, and Puerto Rico targeting families of parents and one child aged 6−18 years old (n = 500). This randomized control trial consisted of workshops, presentations and activities on nutrition education, family wellness, and physical activity. There are still no known results from this study.
Another randomized control trial called Healthy Start [ 26 ] was conducted in Denmark and targeted school children aged 2 to 6 years (n = 3722) and consisted of guiding families on how to improve their children’s diet and physical activity practices, reduce stress, and improve sleep quantity and quality. Activities included cooking classes, games focused on exercise and motor skills development, and access to a website that provided recipe inspiration and ideas. The clinical effects of this intervention on children’s growth and body composition measures were small [ 26 ].
The FIVALIN Project [ 27 ] is a quasi-experimental study conducted in 810 children aged 8–12 years and 600 parents in Barcelona. This study consisted of workshops on health education and sports educational sessions. Educational materials, mobile messages to remind parents to attend the workshops, with the date and hour, and videos were sent to families to reinforce the health behaviors encouraged during the workshops and sports educational sessions. This study is ongoing; therefore, there are still no known results.
The CHIRPY DRAGON Intervention [ 28 ] was a cluster-randomized controlled trial led in Chinese school children with a mean age of 6.15 years (n = 1641). This school- and family-based obesity prevention program consisted of workshops and family activities to promote physical activity and healthy eating behaviors, and school support to improve physical activity and healthy food provision. After 12 months of intervention, the BMI z- scores of children in the intervention group decreased, along with an increase in the consumption of fruit and vegetables, and a decrease in the consumption of sugar-sweetened beverages and unhealthy snacks. Screen time also decreased and physical activity increased in this group [ 28 ].
The Kids in Action [ 29 ] was a controlled trial conducted with children aged 9–12 years from four primary schools in Amsterdam. The study consisted of meetings with the children to develop interventions that targeted their physical activity and healthy eating habits. This intervention consists of environmental changes, organizational changes, or educational approaches, and depending on the type of intervention, the executors could be dieticians, sports coaches, or supermarkets in the community. There are no results from this study yet.
In 2018, an education-based intervention study called The ABC of Healthy Eating Project (including 464 students) was conducted in Poland [ 30 ]. This study included students aged 11–13 years. The intervention group received a diet and lifestyle-related educational program and both the intervention and the control group partook in school activities with the theme of nutrition and healthy lifestyles. There are still no known results.
3.2. Community-Based Interventions
The MOVE/me Muevo was a randomized community trial implemented in 30 recreation centers in San Diego County in a total of 541 families with children between the ages of 5 and 8 years to prevent and control childhood obesity [ 31 ]. This program consisted of activities at the recreation centers and participants’ homes, as well as phone calls from health coaches and emailing tip sheets. The intervention families had “Family Health Coaches” who addressed the following nutrition behaviors: increase the consumption of fruit and vegetables through modifications in meal and snack purchases and preparation; decrease the consumption of sugar-sweetened beverages through changes in food purchases and setting limits; increase healthy food portions by modifying the food consumption behaviors; reduce eating out and when eating out, choosing healthier options; increase the availability and accessibility of healthy foods and beverages at home; reduce screen time and avoid eating in front of the television, and increase the number of meals eaten as a family. After 2 years, there were no significant differences between the control and intervention groups concerning BMI or waist circumference [ 31 ]. Some changes were observed in the dietary domain, namely a reduction in fat and sugary beverages, which means that it was easier for the participants to adopt healthier behaviors in this field, compared to the more complex and multidimensional attitudes of physical activity [ 31 ].
The “Romp & Chomp” is a community-based trial carried out in Australia in children aged 1–5 years old (n = 12,000) and their families [ 32 ]. There were changes regarding the provision of water in childcare centers, childcare policies regarding healthy eating and physical activity, and skills in physical activity and nutrition were taught to the childcare professionals. Amongst the nutrition interventions, there were the following: a collaboration with Dental Health Services Victoria, which provided some resources (lunch boxes and drink bottles, and some marketing material for the kindergarten children); training of the staff as a way to support nutrition messages and healthy eating choices for children aged 5 years; support from dental health professionals to the kindergartens, as a way to engage with parents on the topic of healthy eating and with the intent of providing support for the staff to implement health and nutrition policies; access to a dietitian and other allied health professionals through e-mails, phone calls, and site visits; production and distribution of promotional materials (balloons, stickers, posters, postcards). After 3 years of intervention, the 3.5 years old subsample showed considerably lower mean weight, BMI, and z-score BMI, and the 2 and 3.5 years old children showed a considerably lower prevalence of overweight and obesity when compared with baseline values. The intervention group also showed a considerably lower intake of packaged snacks and fruit juice [ 32 ].
The Aventuras Para Niños Study is a community-based intervention to promote healthy eating and physical activity and prevent excess weight gain in Latino children [ 33 ]. It was performed in thirteen elementary schools, with randomization to assign them to either a family-only intervention, a community-only, or a family+community intervention. In the family-only intervention, professionals would either call the families or make home visits as a way of discussing ways to pass through the difficulties of maintaining a healthy diet and being physically active, by showing them how to prepare healthy meals at home, as well as presenting them with the benefits of encouraging their children to eat healthily and practice physical activity. The community-only intervention included improving the schools’ playgrounds, implementing salad bars, as well as community parks, and displaying water bottles in classrooms for the students. It also included the implementation of better physical education equipment and healthy menus for the children, all of this combined with spreading media messages through posters, news, and point-of-choice messages in grocery stores with healthy messages. The family+community intervention included all of the interventions above. The results showed no noteworthy main effects for the family or community interventions. Therefore, it is possible that not any real effects for the family or community interventions were observed in the BMI z-scores of the children compared with either of those circumstances alone. Despite the lack of significant effects on children’s BMI z-scores, there were several obesity-related behaviors in these children that were changed by the family intervention, such as the increased consumption of fruits and vegetables [ 33 ].
The EPODE (Ensemble Prevenons l’Obesité Des Enfants/Together Let’s Prevent Childhood Obesity) aims to reduce childhood obesity through a societal process that consists of childhood settings, local environments, and family norms becoming more supportive and making it easier for children to adopt healthy lifestyles by enjoying healthy eating, active play, and recreation [ 34 ]. This program was launched in 2004 in 10 French pilot communities, and targeted children aged 1–12 years, their families, and various local stakeholders who have the power to initiate micro-changes in these children and their families through local initiatives focusing on better and balanced eating habits and the regular practice of physical activity. Recently, there have been some other programs, inspired by the EPODE methodology, such as the Healthy Weight Communities in Scotland or the JOGG program in the Netherlands.
The Pacific Obesity Prevention in Communities (OPIC) Project was carried out in four countries, Australia, Fiji, New Zealand, and Tonga, over 30 months, between 2004 and 2009 [ 35 ]. This was a complex community-based intervention that included 18,000 secondary-school children (aged 12–18 years) from eight ethnic and cultural groups, 60 multi-professional research staff, 300 stakeholders and partner organizations, and 27 higher degree research students. The interventions varied across sites, but all sites included targeting reductions in the consumption of high-sugar content drinks and energy-dense snacks and increasing physical activity. The authors state that the project may have positive effects on diet and physical activity, but the effects on childhood obesity are not clearly described [ 36 ].
3.3. Interventions through Mass Media
Some interventions to tackle childhood obesity through mass media have been based on restrictions on food advertising to children. It has been shown that restricting the number of hours spent watching television (TV) can be an effective approach to reduce the prevalence of childhood obesity, and reducing the meals in front of a TV has been shown to be as important as increasing physical activity [ 37 ]. Energy-dense foods and drinks and fast-food companies often target children in their advertisements, since they are very easily influenced at young ages, namely through TV commercials. Thus, reducing the time spent in front of the TV might be a useful strategy to try to reduce the childhood obesity prevalence. Sweden has banned TV commercials/advertisements to children under 12 and TV advertising to children. Norway, Denmark, Austria, Ireland, Australia, and Greece have also imposed some restrictions on advertising to children [ 38 ], as well as Portugal [ 39 ].
3.4. Food Sector Interventions
Food taxation is a primordial prevention measure taken that is currently being applied in several countries, such as some parts of the USA and Canada [ 40 ], to reduce the intake of unhealthy foods and, in the long term, their health effects such as obesity. Some examples are high-volume foods with low nutritional value, such as soft drinks, confectionery, and snack foods. Portugal has also adopted the taxation of sugar-sweetened beverages as an intervention to reduce its high consumption in the country [ 41 ]. There was a decrease of 6.58 million liters of sugar-sweetened beverages sold per year, which translates into a decrease in consumption of 21% compared to the baseline consumption data from the National Dietary Survey [ 41 ]. The number of cases of obesity prevented by taxing sugar-sweetened beverages was studied, concluding that there was a higher impact on adolescents (0.012%), preventing 0.76 cases of obesity yearly [ 41 ].
According to Teng et al. studies suggest that the implementation of sugar-sweetened beverages taxes worldwide has proven effective in reducing sugar-sweetened beverages purchases and intake [ 42 ]. Evidence also shows that the taxation of sugar-sweetened beverages might be an effective tool to reduce the consumption of sugar-sweetened beverages and an important component to prevent obesity [ 42 ]. Roberts et al. suggest that a fiscal strategy could very likely reduce the purchase of high-sugar content products, even if in the short term [ 43 ].
Another measure currently being taken is the addition of logos or some type of labeling to alert the consumers to the healthier products, making it easier for them to choose healthy foods. Although it is not directly focused on childhood obesity, it may have indirect effects. Anastasiou et al. reported that food labeling may affect the consumer’s dietary intake; however, results are inconclusive [ 44 ]. It is uncertain if using health-related claims is beneficial or damaging. Nonetheless, other than health-related claims, negative effects derived from food labeling seem highly unlikely according to the evidence. Therefore, food labeling should continue to be promoted in policies and education programs [ 44 ].
An example of this intervention is the “Pick the Trick” Program, conducted in Australia and New Zealand, providing foods with symbols for the consumers that make it easier to identify the healthier choices [ 45 ]. In Europe, the WHO European Food and Nutrition Action Plan 2015–2020 identifies the introduction of interpretative, consumer-friendly labeling on the front of packages as a priority policy issue [ 46 ]. Although the majority of countries in the region (n = 15) have some form of front-of-pack labeling, fewer countries have interpretive systems which provide judgments about the relative healthfulness of foods. Among other future policies, there is the intention of the application of a single front-of-pack labeling system in all countries. A WHO report summarizes the existing evidence on the development processes and effectiveness of front-of-pack food labeling policies in the WHO European region [ 47 ].
The portion sizes have also been getting increasingly larger over the past four decades in most high-income countries [ 48 , 49 ]. Despite this increase in portion sizes, few countries report measures to reduce them. Most measures are focused on information to consumers rather than changes in the food and drink environment [ 50 ].
4. Discussion
This study aimed to review the most recent literature on dietary interventions for the prevention of childhood obesity. It describes different levels of interventions: the school level, the community level, the mass media, and the food sector level, and provides an overview of their effectiveness (the ability to show consistent results overtime on decreasing children’s BMI), which stand out from previous reviews.
Given the complexity and multifactorial nature of obesity, it is consensual that there is a need to take actions at multidimensional levels, including individual, familial, institutional, and environmental. The majority of the studies included in this review aiming to reduce/manage childhood obesity were school-based interventions, with some addressing the whole community, and some including distal interventions through the food sector and mass media, which may have an indirect effect on childhood obesity by changing food behaviors.
Children are highly influenced by social and environmental conditions, so at these ages, the modification of the environment is expected to play an important role. However, most of the programs/interventions conducted focus mainly on person-based educational approaches, such as nutrition/diet education sessions combined with the promotion of physical activity and lifestyles to students, parents, and school staff, and less on environmental changes that facilitate healthier behavioral choices. Only some trials [ 13 , 14 , 16 , 17 , 18 , 20 , 21 , 30 , 31 ] have focused on capacity building and macro-policy changes, such as the adaptation of the built environment of the school, serving smaller portion sizes and increasing the availability and accessibility of healthy foods and water in schools, and restricting access to vending machines, for example.
Multidimensional intervention studies are usually difficult to evaluate and highly depend on the complexity of evaluation designs (e.g., only outcome evaluation vs. complex evaluation including process, impact, and outcome). Moreover, especially in the multidimensional community-based programs, it is hard to distinguish which part of the intervention was the most effective.
Overall, most of the intervention studies did not show consistent effects on changing children’s BMI. A large number of studies, mainly based on school interventions, did not show very effective results, which may be a reflection of the difficulties experienced trying to obtain significant results when relying only on school-based interventions. In fact, the small weight reductions described in most studies could be clinically irrelevant. It is difficult to figure why the interventions taken until now to prevent/reduce childhood obesity have failed to provide substantial results in terms of effectiveness. The ineffectiveness of some interventions may be due to insubstantial evaluation, or because studies were too short to detect appropriate outcomes, or, simply, because they do not work [ 51 ]. Another possible explanation is the lack of interventions at multiple levels of determinants, especially environmental changes (distal level). Importantly, little is known about the sustainability of interventions over time [ 51 ]. However, other positive results, such as the change of dietary behaviors or physical activity performance have been described and should not be discarded.
Actions to prevent childhood obesity need to be taken in multiple settings and incorporate a variety of approaches and involve a wide range of stakeholders [ 51 ]. Complex interventions focused on environmental changes and the strengthening of individuals and communities as well as macro-policy changes seem to be promising strategies to reduce childhood obesity without increasing socioeconomic inequalities [ 52 ]. The best approach should include the family context and contemplate early life determinants. An approach that could be much more effective to prevent obesity is a combination of interventions that promote healthier diets and increase physical activities through society, rather than an approach focused solely on school environments [ 52 ]. Focusing on mass media campaigns and political actions to prevent obesity by influencing people’s eating choices and the increase of physical activities might be an effective approach to this problem [ 52 ].
Overall, sustained interventions are likely to be required at several levels, at an individual level in schools and community settings to effect behavioral change, and in sector changes involving different stakeholders [ 51 ].
5. Conclusions
Most dietary interventions to tackle childhood obesity focus mainly on person-based educational approaches and less on environmental changes to offer healthier behavioral choices. Most of them failed to reduce childhood obesity.
The creation of environments supportive of healthier behaviors seems to be the best approach to mitigate the challenge that is childhood obesity. Complex and multilevel interventions focused on environmental changes and the strengthening of individuals and communities, including family, as well as macro-policy changes will have the potential to tackle childhood obesity without increasing socioeconomic inequalities.
Author Contributions
A.R.P. drafted the manuscript, played an important role in interpreting the result, and approved its final version. A.O. conceived the work that led to the submission, played an important role in interpreting the results, revised the manuscript, and approved its final version. All authors have read and agreed to the published version of the manuscript.
The authors acknowledge FEDER from the Operational Programme Factors of Competitiveness—COMPETE and national funding from the Foundation for Science and Technology—FCT (Portuguese Ministry of Education and Science) under the projects “Appetite regulation and obesity in childhood: a comprehensive approach towards understanding genetic and behavioral influences” (POCI-01-0145-FEDER-030334; PTDC/SAUEPI/30334/2017) and “Appetite and adiposity—evidence for gene-environment interplay in children” (IF/01350/2015), and the Investigator Contract (IF/01350/2015—Andreia Oliveira).
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Improvement of metabolic-associated fatty liver disease by magnetic resonance spectroscopy in morbidly obese women undergoing roux-en-y gastric bypass, following a postoperative mediterranean-like diet.

1. Introduction
2. materials and methods, 2.1. surgical technique, 2.2. magnetic resonance spectroscopy, 2.3. mediterranean diet, 2.4. assessment of adherence to the mediterranean diet, 2.5. variables, 2.6. statistical analysis, 3.1. adherence to the mediterranean diet, 3.2. anthropometric measurements, 3.3. mrs measurements, 3.4. biochemical parameters, 4. discussion, limitations, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Wilkins, T.; Tadkod, A.; Hepburn, I.; Schade, R.R. Nonalcoholic fatty liver disease: Diagnosis and management. Am. Fam. Physician 2013 , 88 , 35–42. [ Google Scholar ] [ PubMed ]
- Alves de Carvalho, M.S.; Coelho Cabral, P.; Kruze Grande de Arruda, I.; de Araújo Burgos, M.G.P.; da Silva Diniz, A.; Pernambuco, J.B.; de Lira, P.C. Risk factors associated with hepatic steatosis; a study in patients in the Northeast Brazil. Nutr. Hosp. 2012 , 27 , 1344–1350. [ Google Scholar ]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002 , 122 , 1649–1657. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Caballeria, L.; Torán, P. The fatty liver epidemic: An analysis from the primary care. Aten. Primaria 2019 , 51 , 525–526. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Speliotes, E.K.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Meigs, J.B.; Sahani, D.V.; Hirschhorn, J.N.; O’Donnell, C.J.; Fox, C.S. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham Heart Study. Hepatology 2010 , 51 , 1979–1987. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tai, C.-M.; Huang, C.-K.; Hwang, J.-C.; Chiang, H.; Chang, C.-Y.; Lee, C.-T.; Yu, M.-L.; Lin, J.-T. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 2012 , 22 , 1016–1021. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005 , 128 , 1898–1906. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ruiz-Tovar, J.; Zubiaga, L. Validation of biochemical scores for liver steatosis before and 1 year after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2019 , 15 , 1447–1453. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van Werven, J.R.; Marsman, H.A.; Nederveen, A.J.; Smits, N.J.; ten Kate, F.J.; van Gulik, T.M.; Stoker, J. Assessment of hepatic steatosis in patients undergoing liver resection: Comparison of US, CT, T1-weighted dual-echoMRimaging, and point-resolved 1H MR spectroscopy. Radiology 2010 , 256 , 159–168. [ Google Scholar ] [ CrossRef ]
- Alsina, M.E.; Ruiz-Tovar, J.; Bernabeu, A. Evolution of Liver Steatosis Quantified by MR Imaging and MR Spectroscopy, in Morbidly Obese Patients Undergoing Sleeve Gastrectomy: Short-Term Outcomes. Obes. Surg. 2017 , 27 , 1724–1728. [ Google Scholar ] [ CrossRef ]
- Costarelli, V.; Koretsi, E.; Georgitsogianni, E. Health-related quality of life of Greek adolescents: The role of the Mediterranean diet. Qual. Life Res. 2013 , 22 , 951–956. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mente, A.; de Koning, L.; Shannos, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009 , 169 , 659–669. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995 , 61 , 1402S–1406S. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of theMediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018 , 73 , 318–326. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017 , 20 , 1322–1330. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sánchez-Villegas, A.; Martinez, J.A.; De Irala I y Martinez-González, M.A. Determinants of the adherence to an “a priori” defined Mediterranean dietary pattern. Eur. J. Nutr. 2002 , 41 , 249–257. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lydakis, C.; Stefanaki, E.; Stefanaki, S.; Thalassinos, E.; Kavousanaki, M.; Lydaki, D. Correlation of blood pressure, obesity and adherence to the Mediterranean diet with indices of arterial stiffness in children. Eur. J. Pediatr. 2012 , 171 , 1373–1382. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ruiz-Tovar, J.; Boix, E.; Bozhychko, M.; Campo, J.M.D.; Martínez, R.; Bonete, J.M.; Calpena, R. Adherencia pre y postoperatoria a la dieta mediterránea y su efecto sobre la pérdida de peso y el perfil lipídico en pacientes obesos mórbidos sometidos a gastrectomía vertical como procedimiento bariátrico. Nutr. Hosp. 2014 , 30 , 756–762. [ Google Scholar ]
- Mokhtare, M.; Abdi, A.; Sadeghian, A.M.; Sotoudeheian, M.; Namazi, A.; Sikaroudi, M.K. Investigation about the correlation between the severity of metabolic-associated fatty liver disease and adherence to the Mediterranean diet. Clin. Nutr. ESPEN 2023 , 58 , 221–227. [ Google Scholar ] [ CrossRef ]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016 , 63 , 2032–2043. [ Google Scholar ] [ CrossRef ]
- Gastaldo, I.; Casas, R.; Moizé, V. Clinical Impact of Mediterranean Diet Adherence before and after Bariatric Surgery: A Narrative Review. Nutrients 2022 , 14 , 393. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Di Daniele, N.; Petramala, L.; Di Renzo, L.; Sarlo, F.; Della Rocca, D.G.; Rizzo, M.; Fondacaro, V.; Iacopino, L.; Pepine, C.J.; De Lorenzo, A. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013 , 50 , 409–416. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Routh and the mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004 , 7 , 931–935. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Duvnjak, M.; Lerotić, I.; Barsić, N.; Tomašić, V.; Jukić, L.V.; Velagić, V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J. Gastroenterol. 2007 , 13 , 4539–4550. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Musso, G.; Gambino, R.; de Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postpradial lipemia in non-alcoholic steatohepatitis. Hepatology 2003 , 37 , 909–916. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Toledo, F.G.; Sniderman, A.D.; Kelley, D.E. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care 2006 , 29 , 1845–1850. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vargas, V.; Allende, H.; Lecube, A.; Salcedo, M.T.; A Baena-Fustegueras, J.; Fort, J.M.; Rivero, J.; Ferrer, R.; Catalán, R.; Pardina, E.; et al. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J. Hepatol. 2012 , 4 , 382–388. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pontiroli, A.E.; Benetti, A.; Folini, L.; Merlotti, C.; Frige, F. Other aspects of bariatric surgery: Liver steatosis, ferritin and cholesterol metabolism. Nutr. Hosp. 2013 , 28 , 104–108. [ Google Scholar ] [ PubMed ]
- Cefalu, W.T.; Rubino, F.; Cummings, D.E. Metabolic surgery for type 2diabetes: Changing the landscape of diabetes care. Diabetes Care 2016 , 39 , 857–860. [ Google Scholar ] [ CrossRef ]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017 , 2 , 747–756. [ Google Scholar ] [ CrossRef ]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023 , 60 , 1007–1017. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ferrannini, E.; Camastra, S.; Astiarraga, B.; Nannipieri, M.; Castro-Perez, J.; Xie, D.; Wang, L.; Chakravarthy, M.; Haeusler, R.A. Increased bile acidsynthesis and deconjugation after biliopancreatic diversion. Diabetes 2015 , 64 , 3377–3385. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Le Roux, C.W.; Aylwin, S.J.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut hormone profilesfollowing bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 2006 , 243 , 108–114. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Libby, A.E.; Bales, E.; Orlicky, D.J.; McManaman, J.L. Perilipin-2deletion impairs hepatic lipid accumulation by interfering with sterol regula-tory element-binding protein (SREBP) activation and altering the hepaticlipidome. J. Biol. Chem 2016 , 291 , 24231–24246. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Motomura, W.; Inoue, M.; Ohtake, T.; Takahashi, N.; Nagamine, M.; Tanno, S.; Kohgo, Y.; Okumura, T. Upregulation of ADRP in fatty liver in humanand liver steatosis in mice fed with high fat diet. Biochem. Biophys. Res. Commun. 2006 , 340 , 1111–1118. [ Google Scholar ] [ CrossRef ]
- Angelini, G.; Castagneto-Gissey, L.; Casella-Mariolo, J.; Caristo, M.E.; Russo, M.F.; Lembo, E.; Verrastro, O.; Stefanizzi, G.; Marini, P.L.; Casella, G.; et al. Duodenal-jejunal bypass improves nonalcoholic fatty liver diseaseindependently of weight loss in rodents with diet-induced obesity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020 , 319 , G502–G511. [ Google Scholar ] [ CrossRef ]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013 , 59 , 138–143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Velasco, N.; Contreras, A.; Grassi, B. The Mediterranean diet, hepatic steatosis and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2014 , 17 , 453–457. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019 , 24 , 2001. [ Google Scholar ] [ CrossRef ]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018 , 84 , 1566–1574. [ Google Scholar ] [ CrossRef ]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive component oleuropein promotes β-cell insulin secretion and protects β-cells from amylin amyloid-induced cytotoxicity. Biochemistry 2017 , 56 , 5035–5039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017 , 68 , 18–27. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saavedra, Y.; Mena, V.; Priken, K. Effect of the Mediterranean diet on histological indicators and imaging tests in non-alcoholic fatty liver disease. Gastroenterol. Hepatol. 2022 , 45 , 350–360. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Vegetables | Spinach, chard, aubergines, watercress, endive, lettuce, cauliflower, mushrooms, leeks, asparagus, endive, cabbage, cucumber, peppers, tomatoes, green beans, beetroot, carrot, artichoke, or Brussels sprouts |
| Cereals | Pasta, semolina, rice, tapioca, potato, bread, or unsweetened biscuits |
| Legumes | Lentils, chickpeas, or beans |
| Fruits | Apple, pear, orange, peach, kiwi, peach, melon, watermelon, or strawberry |
| Protein sources | White fish, chicken, turkey, rabbit, beef, or eggs |
| Dairy products | Skimmed milk, skimmed yogurts, or fresh cheese |
| Fat source | Olive oil |
| Moderate Adherence | High Adherence | |
|---|---|---|
| 1. Have a fruit or fruit juice every day. | 100% | 100% |
| 2. You eat a second fruit every day. | 75% | 87.5% |
| 3. You eat fresh, raw, salad, or cooked vegetables regularly once a day. | 75% | 87.5% |
| 4. You eat fresh fish regularly (at least 2 or 3 times a week). | 50% | 75% |
| 5. You go once or more per week to a fast-food center (e.g., hamburger restaurant). | 16.7% | 0% |
| 6. Likes pulses and eats them more than once a week. | 33.3% | 50% |
| 7. Eats pasta or rice almost every day (5 days or more per week). | 25% | 25% |
| 8. Eats a cereal or cereal derivative (bread, toast, etc.) for breakfast. | 100% | 100% |
| 9. Eat nuts regularly (at least 2 or 3 times a week). | 16.7% | 37.5% |
| 10. Use olive oil at home. | 100% | 100% |
| 11. Do not eat breakfast. | 0% | 0% |
| 12. Have dairy for breakfast (milk or yogurt, etc.). | 25% | 50% |
| 13. Eats industrial pastries for breakfast. | 8.3% | 0% |
| 14. Eat 2 yogurts and/or 40 g of cheese every day. | 25% | 50% |
| 15. Eat sweets and candies every day. | 0% | 0% |
| Moderate Adherence (n = 12) | High Adherence (n = 8) | p | |
|---|---|---|---|
| Preoperative weight | 107.9 ± 17.9 | 106.5 ± 18.3 | NS |
| Preoperative BMI | 41.7 ± 3.5 | 41.1 ± 3.3 | NS |
| Postoperative weight | 78.1 ± 8.6 | 70.7 ± 7.4 | 0.048 |
| Postoperative BMI | 30.2 ± 2.7 | 27.3 ± 2.5 | 0.041 |
| Percentage of Excess weight loss | 68.9 ± 5.0 | 85.7 ± 5.4 | 0.027 |
| Moderate Adherence (n = 12) | High Adherence (n = 8) | p | |
|---|---|---|---|
| Preoperative PLC (%) | 14.5 ± 9.5 | 13.8 ± 9.3 | NS |
| Postoperative PLC (%) | 4.5 ± 1.8 | 3.3 ± 1.6 | 0.045 |
| D | <0.001 | <0.001 |
| Preoperative | Postoperative | p | |
|---|---|---|---|
| AST (U/L) | 26.3 ± 18.1 | 17.7 ± 9.2 | 0.025 |
| ALT (U/L) | 36.9 ± 19.6 | 19.1 ± 10.6 | 0.019 |
| Total cholesterol (mg/dL) | 221.2 ± 39.5 | 205.2 ± 22.7 | 0.16 |
| Triglyceride (mg/dL) | 178.2 ± 31.5 | 95.3 ± 16.3 | 0.001 |
| HDL-cholesterol (mg/dL) | 46.5 ± 12.3 | 61.4 ± 13.8 | 0.001 |
| LDL-cholesterol (mg/dL) | 128.7 ± 23.4 | 117 ± 21.1 | 0.216 |
| Fasting glucose (mg/dL) | 106.5 ± 34.2 | 84.9 ± 18.7 | 0.001 |
| Glycated hemoglobin (%) | 6.5 ± 1.7 | 5.1 ± 0.6 | 0.011 |
| Moderate Adherence | High Adherence | p | |
|---|---|---|---|
| AST (U/L) | 19.2 ± 9.5 | 16.3 ± 8.9 | 0.048 |
| ALT (U/L) | 21.0 ± 10.8 | 17.1 ± 10.4 | 0.042 |
| Total cholesterol (mg/dL) | 206.0 ± 22.7 | 204.6 ± 22.5 | 0.341 |
| Triglyceride (mg/dL) | 99.4 ± 16.4 | 91.3 ± 16.0 | 0.037 |
| HDL-cholesterol (mg/dL) | 60.2 ± 13.7 | 62.8 ± 13.9 | 0.089 |
| LDL-cholesterol (mg/dL) | 118.1 ± 21.1 | 116.1 ± 21.0 | 0.395 |
| Fasting glucose (mg/dL) | 87.8 ± 18.8 | 81.9 ± 18.5 | 0.044 |
| Glycated hemoglobin (%) | 5.2 ± 0.7 | 5.1 ± 0.6 | 0.412 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Ruiz-Tovar, J.; Llavero, C.; Rodriguez-Ortega, M.; De Castro, N.M.; Martín-Crespo, M.C.; Escobar-Aguilar, G.; Martin-Nieto, A.; Gonzalez, G. Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients 2024 , 16 , 2280. https://doi.org/10.3390/nu16142280
Ruiz-Tovar J, Llavero C, Rodriguez-Ortega M, De Castro NM, Martín-Crespo MC, Escobar-Aguilar G, Martin-Nieto A, Gonzalez G. Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients . 2024; 16(14):2280. https://doi.org/10.3390/nu16142280
Ruiz-Tovar, Jaime, Carolina Llavero, Maria Rodriguez-Ortega, Nuria M. De Castro, Maria Cristina Martín-Crespo, Gema Escobar-Aguilar, Ana Martin-Nieto, and Gilberto Gonzalez. 2024. "Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet" Nutrients 16, no. 14: 2280. https://doi.org/10.3390/nu16142280
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
The aim of this paper is to report a systematic review of the literature on young people and healthy eating. The objectives were (i) to undertake a 'systematic mapping' of research on the barriers to, and facilitators of, healthy eating among young people, especially those from socially excluded groups (e.g. low-income, ethnic minority—in ...
To better understand the current concept of a "healthy diet," this review describes the features and supporting clinical and epidemiologic data for diets that have been shown to prevent disease and/or positively influence health. ... Wirfalt E. Health effects associated with foods characteristic of the Nordic diet: A systematic literature ...
The ways in which particular variations in diet influence health and the course of disease remain largely undefined. The authors review the spectrum of types of diet and describe some attributes of...
A systematic review of the psychological literature on healthy diet. Results: Our findings suggest that consumers have a relatively poor understanding of a healthy diet. The literature also demonstrates that there is poor evidence on the health protective effects of single foods or nutrients.
Article PDF Available Literature Review. ... The definition of what constitutes a healthy diet is continually shifting to reflect the evolving understanding of the roles that different foods ...
A healthy diet during the primary age of children reduces the risk of immediate nutrition-related health problems of primary concern ... The item one of the results in this article presents the most general finding from the literature review, item two describes the variable found significant in two cases, while the remaining variables were ...
Introduction. A healthy diet is associated with physical 1 and mental health. 2,3 Yet only 1% of Australians consume enough fruits and vegetables per day to meet national dietary guidelines. 4 Processed foods high in salt, saturated fat and sugars are consumed in excess, with junk food accounting for over a third of the daily energy intake in both adolescents and adults. 5 Poor diet quality ...
The potential role of fruit and vegetables in aspects of psychological well-being: a review of the literature and future directions. Proceedings of the Nutrition Society 72 , 420-432 (2013).
Our review of the literature on determinants demonstrates that intentions, habits, self-regulatory skills, and the social and physical environment are the most important determinants of a healthy diet, which are in turn amenable to change by intervention strategies with varying levels of effectiveness. Educational interventions generally show a ...
Design: A systematic review of the psychological literature on healthy diet. Results: Our findings suggest that consumers have a relatively poor understanding of a healthy diet.
To support food security for current and future generations, there is a need to understand the relation between sustainable diets and the health of a population. In recent years, a number of studies have investigated and compared different dietary patterns to better understand which foods and eating patterns have less of an environmental impact while meeting nutritional needs and promoting health.
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on HEALTHY DIET. Find methods information, sources, references or conduct a literature review on HEALTHY ...
This systematic review was registered in the PROSPERO International Prospective Register of Systematic Reviews under the number CRD42017080579. ... To conclude, adhering to a healthy diet, in ...
Recognizing the key role of dietary change in reducing noncommunicable disease risk and addressing environmental degradation, it is crucial to understand how to shift individuals toward a sustainable and healthy diet (SHD). In this literature review, we introduced the concept of a SHD and outlined the dietary behaviors necessary to transition ...
In this Review, Stanton and colleagues examine the effect of different whole diets on the composition and function of the gut microbiome and explore how the diet-microbiome relationship ...
We focused on meat, fish, dairy, vegetables, fruit, coffee, tea, soft drinks, and dietary patterns. There was convincing evidence that a healthy dietary pattern may lower CKD risk. Plant-based foods, coffee, and dairy may be beneficial. Unhealthy diets and their components, such as red (processed) meat and sugar-sweetened beverages, may promote ...
A recent systematic review of longitudinal studies found no association between healthy dietary patterns, characterized by higher intakes of fruits, vegetables, fish, legumes, cereals, whole grains, and fiber and lower intakes of red meat, salt, and refined sugars, on end-stage renal disease but a lower rate of mortality (HR, 0.73; 95% CI, 0.63 ...
Conclusions: This systematic review identified distinct barriers posed by urban poverty in accessing healthy diets and its association with poorer nutrition outcomes, hence, questioning the "urban advantage". A conceptual framework emerging from the existing literature is proposed to guide future studies and policies.
Diet is established among the most important influences on health in modern societies. Injudicious diet figures among the leading causes of premature death and chronic disease. Optimal eating is associated with increased life expectancy, dramatic reduction in lifetime risk of all chronic disease, and amelioration of gene expression. In this context, claims abound for the competitive merits of ...
This review article demonstrates it is i mpossible to eat healthy. for a chosen day and onl y consume 50 g/day of dehydrated. protein. It is recommended we consume 720 ml of cow's. milk/day [1 ...
The protocol for this systematic review was registered on PROSPERO prior to starting the literature search (CRD42018089788). The review centered in nutrition outcomes related to: (i) access to a healthy diet as defined by the World Health Organization [], which includes aspects of variety, quantity, balance and food safety, and (ii) nutrition outcomes related to the SDGs - anemia, overweight ...
A total of 3,812 articles were identified in the databases, among which 3 RCTs and 12 cohort studies were included in this systematic review. The process of literature search and study selection is described in Figure 1.Studies excluded during full-text evaluation (n = 145) were summarized in Supplementary Table 6 with exclusion reasons.
Previous studies focused on youth diet 1 or used older data and diet quality measures for infants and toddlers 2 (hereafter, children). This study used data from 2005-2020 and the Healthy Eating Index (HEI) 3 to analyze contemporaneous temporal changes in diet quality among US children.
Ketogenic diet. Consumption of carbohydrates as < 10% of daily calories or < 50 mg/day 41. May decrease appetite, but long-term safety is unknown. High-protein diet. Increase protein intake to 30% of total daily calories or 1-1.2 g/kg of ideal body weight 43. Useful in maintaining weight loss and increasing satiety 47.
We performed a systematic review of the literature to explore what nutrition interventions are associated with longitudinal changes in DNA methylation. ... Carrell, D. T., et al. (2021). Sperm DNA methylation changes after short-term nut supplementation in healthy men consuming a western-style diet. Andrology 9 (1), 260-268. doi:10.1111/andr ...
This study aims to review the existing literature on dietary interventions for the prevention of childhood obesity and to assess their effectiveness. 2. Materials and Methods ... call the families or make home visits as a way of discussing ways to pass through the difficulties of maintaining a healthy diet and being physically active, by ...
A review paper, evaluating different types of diets, placed the Mediterranean diet as number one in the ranking of model diets for reducing cardiovascular risk . The traditional Mediterranean diet is characterized by a high intake of fruits, vegetables, legumes, nuts and cereals, a moderate ingestion of fish, and a low intake of meat and sweets ...