FDA approves groundbreaking treatment for advanced melanoma
The Food and Drug Administration on Friday approved a new cancer therapy that could one day transform the way a majority of aggressive and advanced tumors are treated.
The treatment, called Amtagvi, from Iovance Biotherapeutics , is for metastatic melanoma patients who have already tried and failed other drugs. It’s known as TIL therapy and involves boosting the number of immune cells inside tumors, harnessing their power to fight the cancer.
It’s the first time a cellular therapy has been approved to treat solid tumors. The drug was given a fast-track approval based on the results of a phase 2 clinical trial. The company is conducting a larger phase 3 trial to confirm the treatment’s benefits. The therapy’s list price — the price before insurance and other potential discounts — is $515,000 per patient.
“This is going to be huge,” said Dr. Elizabeth Buchbinder, a senior physician at Dana-Farber Cancer Institute in Boston. Melanoma is “not one of those cancers where there’s like 20 different” possible treatments, she said. “You start running out of options fast.”

Friday’s approval is only for melanoma, the deadliest form of skin cancer , but experts say it holds promise for treating other solid tumors, which account for 90% of all cancers.
“It is our hope that future iterations of TIL therapy will be important for lung cancer, colon cancer , head and neck cancer, bladder cancer and many other cancer types,” said Dr. Patrick Hwu, chief executive of the Moffitt Cancer Center in Tampa, Florida. Moffitt has been involved with Iovance’s clinical trials of TIL therapy.
TIL stands for tumor-infiltrating lymphocytes, which are immune cells that exist within tumors . But there are nowhere nearly enough of those cells to effectively fight off cancer cells. TIL therapy involves, in part, extracting some of those immune cells from the patient’s tumor and replicating them billions of times in a lab, then reinfusing them back into the patient.
It’s similar to CAR-T cell therapy, where healthy cells are taken out of a person’s body and then modified in a lab to fight cancers. That’s usually used for hard-to-treat blood cancers such as leukemia and lymphoma. With TIL therapy, the cells used are already programmed to recognize cancer — no lab modifications needed — they just need a boost in numbers to fight it.
Like CAR-T, TIL therapy is a one-time treatment, though the entire process can take up to eight weeks. The TIL cells are first harvested from the tumor through a minimally invasive procedure and then grown and multiplied in the lab, a process that takes 22 days, according to Iovance.
While that’s happening, patients are given chemotherapy to clear out their immune cells to make room for the billions of new melanoma-fighting TIL cells. Once the TIL cells are reinfused back into the body, patients get a drug called interleukin-2 to further stimulate those cells.
Hwu said that most side effects in patients undergoing TIL therapy are not from the reinfusion of cells, but from the chemotherapy and the interleukin-2. These can include nausea and extreme fatigue, and patients are also vulnerable to other illnesses because the body is depleted of disease-fighting white blood cells.
Putting billions of cells back into the body is not entirely risk-free, however, said Dr. William Dahut, chief scientific officer of the American Cancer Society. It’s possible that the body’s immune system could overreact in what’s known as a cytokine storm, which can cause flu-like symptoms, low blood pressure and organ damage. “There are risks for immune-related side effects, which could be serious,” he said.
Common side effects associated with Amtagvi can include abnormally fast heart rate, fluid buildup, rash, hair loss and feeling short of breath, the FDA said.
Those side effects can be managed, said Dr. Steven Rosenberg, chief of the surgery branch at the National Cancer Institute. “They’re a small price to pay for a growing cancer that would otherwise be lethal.”
Overall, Dahut said the approval of TIL therapy is “meaningful.”
“What’s nice about this is that patients will receive a wide variety of tumor fighting lymphocytes that will be able to have the capacity to overcome resistance and actually be a living therapy over time, too, to target additional cancer cells should they develop,” Dahut said.
In addition to melanoma, Dahut said that TIL therapy is most likely to be useful in cancers that respond to drugs that “take the brakes off the immune system,” called checkpoint inhibitors .
“Those would be things like non-small cell lung cancer, kidney cancer, maybe bladder cancer, that we know are responsive to immune-based therapies to begin with,” he said. “Many of those patients relapse, so another immune-based therapy that works in a different way, seems to me, the most likely way for this to be effective.”
Much more research is needed, and it may be years before TIL therapy is approved for other types of cancer.
One of Iovance’s clinical trials investigating TIL therapy for non-small cell lung cancer was forced to pause when a participant died. While the death is under investigation, the company said it may have been the result of either chemotherapy or interleukin 2 — therapies meant to knock down each patients’ immune system before they can get the reinfusion of their TIL cells.
The therapy is not expected to work for every metastatic melanoma patient. Clinical trial data that Iovance submitted to the FDA showed that tumors shrank in about a third of patients who received TIL therapy.
Of those patients, about half saw their tumors shrink for at least one year, Dr. Friedrich Graf Finckenstein, chief medical officer of Iovance Biotherapeutics. “Some of these patients even had their tumor completely disappear,” he said.
Another study, conducted in the Netherlands , did a head-to-head analysis of TIL therapy and another form of immunotherapy, called ipilimumab. Twenty percent of the patients who received TIL had complete remissions, compared with 7% of patients who got ipilimumab. Iovance was not involved with the Dutch trial.
The goal of the therapy, Hwu said, “is to get rid of the cancer and have it stay away. These immune cells stay in the body and live in the body for decades.”
The technology has been in development and studied for nearly 40 years. It was Rosenberg who pioneered TIL therapy — first describing how it could shrink melanoma tumors in the New England Journal of Medicine in 1988 .
“I’ve been waiting for a very long time to see this given to patients, because I know that it can cure some patients that have metastatic melanoma that cannot be affected by any other treatment,” Rosenberg said.
It’s worked so far for Dan Bennett, 59, of Clermont, Florida. Bennett was diagnosed with melanoma in 2011 after his daughter noticed a suspicious mole on his neck that had changed color.
Despite surgery, chemotherapy and radiation, his cancer kept returning. In 2014, his doctors at Moffitt recommended he try TIL therapy.
“At first, we were pretty leery about it because it was unproven,” Bennett said. Ten years later, Bennett is convinced the TIL therapy is the reason he has survived so long with stage 4 melanoma, which usually has a five-year survival rate of 22.5% .
“I would recommend any experimental drug if it’s your last opportunity,” he said. “You owe it to yourself and your family to do whatever you can to stay alive and to be a productive member of society.”
Buchbinder, the Dana-Faber doctor, was not involved with Iovance’s TIL therapy trial for melanoma, but she is scheduled to begin similar trials with other drugmakers.
“We literally have patients right now waiting for approval because they are hoping they’ll be able to go on it,” Buchbinder said. “It is definitely a practice-changing therapy.”
Erika Edwards is a health and medical news writer and reporter for NBC News and "TODAY."
Anne Thompson is NBC News’ chief environmental affairs correspondent.
Marina Kopf is an associate producer with the NBC News Health and Medical Unit.

Melanoma and Other Skin Cancers Research Results and Study Updates
See Advances in Melanoma and Other Skin Cancers Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
In an event more than three decades in the making, FDA has approved lifileucel (Amtagvi), the first cancer treatment that uses immune cells called tumor-infiltrating lymphocytes, or TILs.
People with desmoplastic melanoma, a rare form of skin cancer, are likely to benefit from treatment with a single immunotherapy drug, pembrolizumab (Keytruda), according to new results from a small clinical trial.
For melanoma that can be treated with surgery, a few doses of pembrolizumab (Keytruda) beforehand looks to be a good choice. In a clinical trial, people who got the presurgical immunotherapy were much less likely to have their cancer come back than those who only received it after surgery.
Male patients with metastatic melanoma don’t live as long as females, and their tumors are more likely to become resistant to commonly used treatments. A new study may help explain why: the androgen receptor.
Regular skin cancer screening leads to many diagnoses of very early-stage melanomas, results from a new study suggest. The results add to a debate about whether screening is fueling an overdiagnosis of melanoma in the United States.
The immunotherapy treatment, which combines the LAG-3 inhibitor relatlimab and PD-1 inhibitor nivolumab, becomes the first new immune checkpoint inhibitor approved in 8 years. Both drugs are given to patients via a single infusion to treat advanced melanoma.
Melanoma cells that travel to the brain produce their own amyloid beta, helping the cells survive and form metastases, a new study in mice shows. The Alzheimer’s-linked proteins appear to tamp down the brain’s immune response to the cancer cells.
NCI researchers have found that a diet rich in fiber may help some people being treated for melanoma respond to immunotherapy treatment by influencing the gut microbiome. The new findings come from an analysis of people with melanoma and mouse models of the disease.
Clinical trial finds that ipilimumab (Yervoy) and nivolumab (Opdivo) combo is superior to a combination of the targeted therapies dabrafenib (Tafinlar) and trametinib (Mekinist) as the first treatment for metastatic BRAF-positive melanoma.
Trial results show patients who received the immunotherapy pembrolizumab (Keytruda) after surgery to remove high-risk stage II melanomas were less likely to have the cancer come back than those who received no treatment after surgery.
People with advanced melanoma treated with two immunotherapy drugs—nivolumab (Opdivo) and a new drug called relatlimab—lived longer without their cancer getting worse than those treated only with nivolumab, results from a large clinical trial show.
While doctors are familiar with the short-term side effects of immune checkpoint inhibitors, less is known about potential long-term side effects. A new study details the chronic side effects of these drugs in people who received them as part of treatment for melanoma.
In a large trial, tebentafusp helped patients with uveal melanoma live longer than patients who received other treatments for the disease. Uveal melanoma is an aggressive cancer of the eye, and many patients do not survive for a year once it has spread.
For patients with cancers that do not respond to immunotherapy drugs, the use of fecal transplants to modify the gut microbiome may help some of these patients respond to the immunotherapy drugs.
Melanoma cells that pass through the lymphatic system before entering the bloodstream are more resistant to cell death and spread more readily than cells that enter the bloodstream directly. The finding could lead to new treatment approaches.
After rising steadily for decades, the number of people in the United States who die each year from the skin cancer melanoma has dramatically dropped in recent years, results from a new study show. Learn what has contributed to the dramatic decline.
Melanoma cells that metastasize to other parts of the body produce high levels of a protein called MCT1, a new study in mice has found. Blocking MCT1 with an investigational drug, AZD3965, led to fewer and smaller metastatic tumors.
Researchers have developed a device that uses lasers and sound waves to scan circulating blood for melanoma cells. In a small study, the device accurately detected and reduced the amount of cancer cells in participants’ blood.
FDA has approved pembrolizumab (Keytruda) to treat people with Merkel cell carcinoma, a rare and deadly form of skin cancer. The approval covers use of the drug to treat locally advanced or metastatic forms of the disease.
The Food and Drug Administration approved the immunotherapy drug cemiplimab (Libtayo) for an advanced form of cutaneous squamous cell carcinoma (SCC), a common type of skin cancer. It is the first agent to be approved specifically for advanced SCC.
Results from a clinical trial show that the combination of nivolumab (Opdivo) and ipilimumab (Yervoy) halted the growth of or shrank metastatic brain tumors in more than half of participants with melanoma that had spread to the brain.
In a new study, NCI-led researchers developed a gene expression predictor that can indicate whether melanoma in a specific patient is likely to respond to treatment with immune checkpoint inhibitors, a type of immunotherapy.
A new study has linked age with how well patients with melanoma responded to treatment with immune checkpoint inhibitors. Experiments in mice suggested that the response pattern may be due to an age-related shift in the kinds of immune cells in tumors.
FDA recently approved the targeted-drug combination to treat patients with advanced melanoma and a subset of patients with a rare and aggressive form of thyroid cancer whose tumors have a specific mutation in the BRAF gene.
A new study suggests that patients with a rare form of melanoma, called desmoplastic melanoma, may be particularly likely to benefit from treatments known as immune checkpoint inhibitors. An NCI-sponsored clinical trial is already testing one such drug in patients with this cancer.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
AI improves accuracy of skin cancer diagnoses in Stanford Medicine-led study
Artificial intelligence algorithms powered by deep learning improve skin cancer diagnostic accuracy for doctors, nurse practitioners and medical students in a study led by the Stanford Center for Digital Health.
April 11, 2024 - By Krista Conger

Artificial intelligence helped clinicians diagnose skin cancer more accurately, a Stanford Medicine-led study found. Chanelle Malambo/peopleimages.com - stock.adobe.com
A new study led by researchers at Stanford Medicine finds that computer algorithms powered by artificial intelligence based on deep learning can help health care practitioners to diagnose skin cancers more accurately. Even dermatologists benefit from AI guidance, although their improvement is less than that seen for non-dermatologists.
“This is a clear demonstration of how AI can be used in collaboration with a physician to improve patient care,” said professor of dermatology and of epidemiology Eleni Linos , MD. Linos leads the Stanford Center for Digital Health , which was launched to tackle some of the most pressing research questions at the intersection of technology and health by promoting collaboration between engineering, computer science, medicine and the humanities.
Linos, associate dean of research and the Ben Davenport and Lucy Zhang Professor in Medicine, is the senior author of the study , which was published on April 9 in npj Digital Medicine . Postdoctoral scholar Jiyeong Kim , PhD, and visiting researcher Isabelle Krakowski, MD, are the lead authors of the research.
“Previous studies have focused on how AI performs when compared with physicians,” Kim said. “Our study compared physicians working without AI assistance with physicians using AI when diagnosing skin cancers.”
AI algorithms are increasingly used in clinical settings, including dermatology. They are created by feeding a computer hundreds of thousands or even millions of images of skin conditions labeled with information such as diagnosis and patient outcome. Through a process called deep learning, the computer eventually learns to recognize telltale patterns in the images that correlate with specific skin diseases including cancers. Once trained, an algorithm written by the computer can be used to suggest possible diagnoses based on an image of a patient’s skin that it has not been exposed to.

Eleni Linos
These diagnostic algorithms aren’t used alone, however. They are overseen by clinicians who also assess the patient, come to their own conclusions about a patient’s diagnosis and choose whether to accept the algorithm’s suggestion.
An accuracy boost
Kim and Linos’ team reviewed 12 studies detailing more than 67,000 evaluations of potential skin cancers by a variety of practitioners with and without AI assistance. They found that, overall, health care practitioners working without aid from artificial intelligence were able to accurately diagnose about 75% of people with skin cancer — a statistical measurement known as sensitivity. Conversely, the workers correctly diagnosed about 81.5% of people with cancer-like skin conditions but who did not have cancer — a companion measurement known as specificity.
Health care practitiones who used AI to guide their diagnoses did better. Their diagnoses were about 81.1% sensitive and 86.1% specific. The improvement may seem small, but the differences are critical for people told they don’t have cancer, but do, or for those who do have cancer but are told they are healthy.
When the researchers split the health care practitioners by specialty or level of training, they saw that medical students, nurse practitioners and primary care doctors benefited the most from AI guidance — improving on average about 13 points in sensitivity and 11 points in specificity. Dermatologists and dermatology residents performed better overall, but the sensitivity and specificity of their diagnoses also improved with AI.
“I was surprised to see everyone’s accuracy improve with AI assistance, regardless of their level of training,” Linos said. “This makes me very optimistic about the use of AI in clinical care. Soon our patients will not just be accepting, but expecting, that we use AI assistance to provide them with the best possible care.”

Jiyeong Kim
Researchers at the Stanford Center for Digital Health, including Kim, are interested in learning more about the promise of and barriers to integrating AI-based tools into health care. In particular, they are planning to investigate how the perceptions and attitudes of physicians and patients to AI will influence its implementation.
“We want to better understand how humans interact with and use AI to make clinical decisions,” Kim said.
Previous studies have indicated that a clinician’s degree of confidence in their own clinical decision, the degree of confidence of the AI, and whether the clinician and the AI agree on the diagnosis all influence whether the clinician incorporates the algorithm’s advice when making clinical decisions for a patient.
Medical specialties like dermatology and radiology, which rely heavily on images — visual inspection, pictures, X-rays, MRIs and CT scans, among others — for diagnoses are low-hanging fruit for computers that can pick out levels of detail beyond what a human eye (or brain) can reasonably process. But even other more symptom-based specialties, or prediction modeling, are likely to benefit from AI intervention, Linos and Kim feel. And it’s not just patients who stand to benefit.
“If this technology can simultaneously improve a doctor’s diagnostic accuracy and save them time, it’s really a win-win. In addition to helping patients, it could help reduce physician burnout and improve the human interpersonal relationships between doctors and their patients,” Linos said. “I have no doubt that AI assistance will eventually be used in all medical specialties. The key question is how we make sure it is used in a way that helps all patients regardless of their background and simultaneously supports physician well-being.”
Researchers from the Karolinska Institute, the Karolinska University Hospital and the University of Nicosia contributed to the research.
The study was funded by the National Institutes of Health (grants K24AR075060 and R01AR082109), Radiumhemmet Research, the Swedish Cancer Society and the Swedish Research Council.
For more news about responsible AI in health and medicine, sign up for the RAISE Health newsletter.
Register for the RAISE Health Symposium on May 14.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Melanoma articles from across Nature Portfolio
Melanoma is the most serious type of skin cancer. It develops when skin cells multiply rapidly as a consequence of mutations in their DNA caused by UV exposure. Melanomas originate in the pigment-producing melanocytes in the basal layer of the epidermis; they often resemble moles and are generally black or dark brown.
Latest Research and Reviews
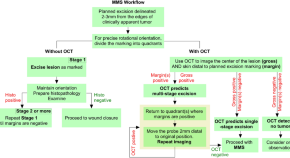
Using optical coherence tomography to optimize Mohs micrographic surgery
- Sruti S. Akella
- Kamran Avanaki
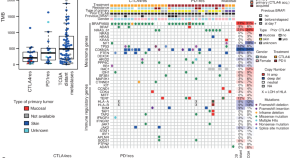
Molecular patterns of resistance to immune checkpoint blockade in melanoma
A large fraction of patients with melanoma still does not benefit from immune checkpoint blockade, associated with both primary and acquired resistance. Here the authors report genetic and immunological patterns of resistance in patients with melanoma after progression on anti-CTLA4 or anti-PD1 monotherapy.
- Martin Lauss
- Bengt Phung
- Göran Jönsson

Stroma-infiltrating T cell spatiotypes define immunotherapy outcomes in adolescent and young adult patients with melanoma
Therapeutic resistance to immune checkpoint inhibitor treatment is incompletely understood in adolescent and young-adult (AYA) patients with melanoma. Here, the authors demonstrate that AYA patients exhibit a unique stroma-infiltrating T cell immunogenomic profile compared with adults, which impacts on their responsiveness to immunotherapy.
- Grace H. Attrill
- Camelia Quek
Genetic predisposition to childhood obesity does not influence the risk of developing skin cancer in adulthood
- Jay Keatley
- Matthew H. Law
- Jean Claude Dusingize
Orthotopic model for the analysis of melanoma circulating tumor cells
- Markéta Pícková
- Zuzana Kahounová
- Karel Souček

Inactivation of kindlin-3 increases human melanoma aggressiveness through the collagen-activated tyrosine kinase receptor DDR1
- Coralie Reger De Moura
- Baptiste Louveau
- Samia Mourah
News and Comment
Personalized neoantigen mrna vaccine mitigates melanoma recurrence.
- David Killock
Explaining counterfactual images
Leveraging the expertise of physicians to identify medically meaningful features in ‘counterfactual’ images produced via generative machine learning facilitates the auditing of the inference process of medical-image classifiers, as shown for dermatology images.
- Ilana Traynis
Diverse routes to melanoma metastasis and ICI resistance

Computing brain metastasis impact
In a recent study, Sanchez-Aguilera, Masmudi-Martín et al. find that a molecular program explains the cognitive impairment often seen in patients with brain metastasis, challenging the prevailing paradigm of the tumour mass being the sole cause of altered brain function.
- Daniela Senft

Synaptic activity promotes melanoma formation
In a recent study, Tagore et al. find that the formation of synapse-like structures that serve to transfer GABA between premalignant melanocytes and keratinocytes promotes melanoma initiation by the BRAF V600E oncogene.
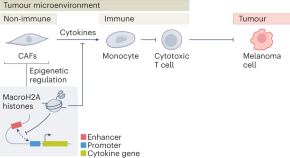
Epigenetic control of cancer inflammation
The interaction of non-immune and immune cells in the tumour microenvironment (TME) determines the quality of the immune attack on nascent tumour cells. A new study in melanoma cells shows that specific histone variants dampen the expression of cytokine genes in cancer-associated fibroblasts, leading to an immunosuppressive TME.
- David Corujo
- Marcus Buschbeck
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)
- PMC10417305

Recent Advances in Clinical Research for Skin Cancer Chemoprevention
1 Department of Pharmaceutical Sciences, College of Pharmacy, Western University of Health Sciences, Pomona, CA 91766, USA; [email protected] (R.T.); [email protected] (S.H.); ude.unretsew@neserdnab (B.A.); ude.unretsew@dihahsa (A.S.); ude.unretsew@gnawj (J.W.)
Samuel Hanoun
Bradley andresen, ayaz shahid, jeffrey wang, kristen m. kelly.
2 Department of Dermatology, University of California, Irvine, CA 92697, USA; ude.icu.sh@yllekmk
Frank L. Meyskens, Jr.
3 Departments of Medicine and Biological Chemistry, Chao Family Comprehensive Cancer Center, University of California, Irvine, CA 92868, USA; ude.icu.sh@eksyemlf
Simple Summary
Skin cancer is the most common cancer type in the United States and the world. Both non-melanoma and melanoma skin cancer show a clear association with overexposure to solar ultraviolet radiation. Chemoprevention is an appealing strategy to control the increasing rate of skin cancer. Since the target population for cancer chemoprevention is healthy individuals with high cancer risk, pharmacological agents that can be used for preventive purposes should be both effective and safe. The present review outlines the current state of skin cancer chemoprevention clinical trials, in terms of study populations, agents, outcomes (including cancer risk reduction), predictive biomarkers, and adverse reactions. The most studied agents include non-steroidal anti-inflammatory drugs, retinoids, 5-fluorouracil, and nicotinamide. The route of administration can be oral or topical. Since the trial outcomes for most of these agents are inconsistent, there is a need for additional research in this area.
Neoplasm arising from the keratinocytes or melanocytes in the skin is the most prevalent type of cancer in the United States and worldwide. Since ultraviolet (UV) radiation may be a causing factor for several types of skin cancer, effective strategies to manage skin cancer include preventive measures such as minimizing exposure to UV and applying sunscreens. However, the effect of sunscreen in reducing skin cancer incidence remains uncertain. An alternative approach to prevent skin cancer is chemoprevention, which is defined as using either natural products or synthetic compounds to inhibit, delay, or reverse the development of cancer. Preclinical studies have demonstrated the effectiveness of multiple pharmacological agents and dietary supplements. However, whether preclinical findings can be translated into clinical application is unknown. This review evaluates the state of recent clinical trials investigating chemopreventive agents focusing on skin cancer to compare the target populations, interventions, endpoints, and outcomes of these trials. The ClinicalTrials and PubMed databases were searched for their available literature using the key words “skin cancer” and “chemoprevention”. The objective of this review is to provide updated information on the effectiveness and side effects of promising chemopreventive agents in human subjects and to identify research gaps.
1. Introduction
Skin cancer is one of the most common types of malignancy in the United States and the world, with rising incidence [ 1 , 2 ]. It is estimated that one out of five Americans will develop skin cancer by the age of 70, with Caucasians displaying the highest incidence [ 3 ]. Non-melanoma skin cancer (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is the most common. Merkel cell carcinoma (MCC) less common, while malignant melanoma is one of the types of skin cancer with a higher potential for mortality. Skin cells can undergo neoplastic transformation due to DNA damage caused by environmental factors like exposure to ultraviolet (UV) radiation from sunlight or artificial sources like tanning beds and sunlamps [ 3 ]. UV not only induces DNA damage, but also creates an inflammatory and immunosuppressive microenvironment in which premalignant cells may grow into tumors [ 4 ].
BCC is the most common form of skin cancer, with an estimated 3.6 million cases yearly in the United States [ 5 ]. BCC is an uncontrolled and abnormal growth of skin cells in the epidermis, specifically the basal cell layer ( Figure 1 ). BCCs are usually found on skin areas exposed to the sun, including the face, neck, ears, scalp, shoulders, and back. Besides UV radiation, risk factors for BCC include older age, male gender, fair skin, and outdoor careers [ 5 ]. Genetic mutations in the genes of the hedgehog pathway contribute to the majority of BCCs [ 5 ]. BCCs grow slowly, cause minimal damage, and rarely metastasize when detected and treated early. However, some lesions can be highly destructive if left untreated.

Risk factors for the most common types of skin cancer. BCC: basal cell carcinoma; SCC: squamous cell carcinoma.
SCC is the second most prevalent type of skin cancer, with an estimated annual occurrence of 1.8 million cases within the United States [ 6 ]. SCC is an uncontrolled and abnormal growth of squamous cells that are in the epidermis layer ( Figure 1 ). Like BCC, SCC is also found in similar places exposed to the sun, such as the face, ears, scalp, neck, and hands. SCCs share similar risk factors to BCC but with the addition of a weakened immune system, sun-sensitive conditions such as Xeroderma pigmentosum , skin precancers such as actinic keratosis (AK), and a history of human papillomavirus infection [ 7 ]. In particular, organ transplant recipients who receive long-term immunosuppressive treatment are at greater risk for skin cancer: for BCC, there is a 10-fold increased risk, while the risk of developing SCC is greater by 65–250 times [ 8 ]. Compared to BCC, SCC may grow relatively more rapidly and metastasize more quickly if not detected or treated early.
Melanoma or malignant melanoma develops from melanocytes, which are specialized cells responsible for producing pigment in the skin ( Figure 1 ). Melanoma is a potentially lethal form of skin cancer. Over the past few decades, the incidence of melanoma is increasing in a rate more than any other malignancy in the United States [ 9 ]. Unlike other types of skin cancer, melanoma can grow in existing moles or may develop in various skin regions, even in areas not typically exposed to the sunlight. Risk factors for melanoma include UV exposure, weakened immune system, atypical moles, fair skin, skin cancer history, and family history of melanoma [ 10 ].
A rare type of skin cancer is MCC, which is an aggressive neuroendocrine carcinoma of the skin. The risk factors for MCC include older age, fair skin, extensive UV exposure, history of multiple skin cancers, and chronic immunosuppression due to HIV or solid organ transplantation [ 11 ]. About 80% of MCC is caused by Merkel cell polyomavirus infection, while 20% is caused by UV-mediated skin damage.
Other than immunosuppressants, recently, concerns have been raised about increased skin cancer risks associated with some commonly prescribed drugs: tumor necrosis factor alpha inhibitors, angiotensin-receptor blockers, phosphodiesterase type 5 inhibitors, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA)-reductase inhibitors [ 12 ]. These drugs may interact with UV radiation, leading to photosensitivity responses in susceptible individuals [ 13 ].
In contrast to many other types of cancers, skin cancers emerge on the surface of the body, are usually visible, and thus, can be detected early by regular skin examinations at home and by dermatologists. Although the majority cases of NMSC can be cured with surgical excision, due to the high incidence, treatment of these diseases leads to a huge burden on health care systems [ 14 ]. Aside from early detection, managing the known risk factors contributing to skin cancer development is important, e.g., using UV-protective strategies such as avoiding excessive sun exposure and applying sunscreen. However, the implementation of UV-protective strategies is inconsistent; there is inadequate evidence as to whether sunscreen use can reduce the risk of skin cancer [ 14 , 15 , 16 ]. Cancer prevention has become one area of priority in “The War on Cancer” and represents a recently renewed goal aiming for reducing the cancer death rate by at least 50% over the next 25 years [ 17 ]. Chemoprevention, which is defined as the use of natural products or pharmacological agents to inhibit, block, or reverse cancer initiation, promotion, and progression, has also been investigated for skin cancer prevention [ 18 , 19 ]. The number of preclinical studies on skin cancer chemoprevention has been growing. However, there are few clinical studies able to provide sufficient evidence for recommending the use of chemopreventive agents for high-risk individuals.
This review aims to examine the status of recent skin cancer chemoprevention clinical trials regarding target populations, interventions, mechanisms of action, biomarkers, and outcomes. The ClinicalTrials and PubMed databases were systematically searched to identify relevant trials. By investigating these trials, the present review provides updated efficacy and side effect data for promising chemopreventive agents and identifies critical research gaps.
A systematic search was conducted in the ClinicalTrials.gov database using the search terms “skin cancer” and “chemoprevention”. A total of 72 studies were identified, which were then filtered by registration date from 1995 to 2022, resulting in 65 studies. Exclusion criteria were then applied to eliminate studies involving other cancer types, resulting in a final analysis of 18 studies that were specific to skin cancer ( Table 1 ).
Search results from the ClinicalTrials.gov database.
NMSC: non-melanoma skin cancer; BCC: basal cell carcinoma; SCC: squamous cell carcinoma; AK: actinic keratosis; XP: Xeroderma pigmentosum ; DFMO: difluoromethylornithine; PUFA: polyunsaturated fatty acid; 5-FU: 5-fluorouracil.
The PubMed database was also searched using the keywords “skin cancer” and “chemoprevention”, which generated 115 results. Further filtering was performed to select only clinical trials and randomized controlled trials with a registration date from 2010 to 2023, resulting in 55 studies. Exclusion criteria were applied to eliminate studies involving arsenical skin lesions, colorectal, advanced premalignant lesions, lung cancer prevention, subcutaneous pocket infection, surgical site infection, prevention of surgical site infection, gynecological cancer, periocular actinic keratinocytes, breast cancer, second primary tumor, pre-engraftment bloodstream infection, and hepatocellular carcinoma. Studies that did not evaluate drug effects or adverse drug reactions (ADRs), such as those assessing quality of life or predictors of toxicity, were also excluded. The remaining studies were screened, resulting in 21 studies that were considered relevant to skin cancer chemoprevention ( Table 2 ).
PubMed database outcomes.
3. Cancer Types or Conditions of the Clinical Trials
The results of the clinical trial search reveal that most studies focused on NMSC, with a few studies specifically focusing on BCC or SCC. Some studies did not specify cancer types as they utilized precancerous biomarkers or focused on pharmacokinetics or intervention safety. Out of the 18 studies from the ClinicalTrials.gov site, 10 were centered on NMSC prevention, with one each focused on SCC, BCC, AK, and skin immunity, and four were unspecified. No study was focused on melanoma prevention.
The PubMed search results generated more outcomes than ClinicalTrials.gov, and similarly, most studies (10) targeted NMSC, followed by specified BCC (4) and melanoma (3). One study focused on examining how intervention impacted skin immunity. All studies retrieved from PubMed had explicitly stated the targeted conditions.
When the results from both ClinicalTrials.gov and PubMed were combined, NMSC was still the most widely studied cancer type. Fewer trials were conducted for melanoma. Skin immunity was also included as a condition, as it may be a predictor of future skin cancer risk.
4. Interventions
Many of the studies found in the ClinicalTrials and PubMed databases were focused on the efficacy of preventive interventions for skin cancers and the adverse effects associated with preventive agents. Most of the studies were conducted with monotherapy, and commonly tested therapeutic agents were nicotinamide, retinoids, non-retinoid topical agents, nonsteroidal anti-inflammatory agents (NSAIDs), and nicotinamide ( Table 3 ). The most frequently studied agent was oral nicotinamide 500 mg, with study durations ranging from 6 to 12 months. Topical 5-fluorouracil 5% strength and topical diclofenac 3% strength were the next most frequently studied agents, with study durations ranging from 2 to 4 weeks and 1 to 9 months, respectively. Celecoxib 200 mg twice daily and aspirin 81 to 100 mg were other agents that appeared multiple times in the search results. Statins, anti-diabetic medications, and dietary supplements were less commonly evaluated but were present in the filtered results ( Table 3 ).
Summary of chemopreventive agents identified from the ClinicalTrials and PubMed databases.
PAR: retinoic acid receptors.
Both topical and systemic (oral) agents were studied. Although a considerable number of studies showed notably positive outcomes, certain studies did not exhibit any significant positive results or were not published. The lack of significant results may suggest the need for a larger sample size, a longer duration of treatment, or a different combination of agents. Overall, further clinical research is required to confirm the effectiveness of these agents in skin cancer prevention and the identification of potential adverse effects.
5. Populations
In the search results from the PubMed and ClinicalTrials databases, different target populations were assessed, including healthy individuals and those with an increased risk of skin cancer. The latter included patients with actinic keratoses, a history of NMSC, as well as organ transplant recipients taking immunosuppressants. People at high risk for skin cancer also include patients with the hereditary conditions such as Xeroderma pigmentosum . Healthy individuals with sun-damaged skin were included in some trials. Due to the high-impact Veterans Affairs (VA) Keratinocyte Carcinoma Chemoprevention Trials (VAKCCT), elderly individuals with a history of NMSC have received the most attention in identified clinical studies. The higher incidence of skin cancer and the potential to gain benefit from preventive interventions may be reasons that the elderly with a history of NMSC or actinic keratoses have been the most studied populations. Immunosuppressed organ transplant recipients represent a special population that have increased risk for skin cancer, particularly NMSC. It is very common for patients to develop multiple NMSC [ 24 ]. Once the first cancer is diagnosed, the patient has a higher risk of developing additional cancer. In contrast, research has paid the least attention to healthy individuals. It is equally important to investigate chemoprevention in healthy individuals for agents with proven safety because the agents can apply to the prevention of future development of skin cancer and to supplement the use of sunscreen in general population.
6. Endpoints
A variety of outcomes were measured in the identified trials. The incidence or rate of NMSC was the primary endpoint. Another interesting endpoint that was evaluated in some trials was cost-effectiveness or the ability to save money by using a particular drug. With current research, cost-effectiveness studies may not be the most beneficial due to limited FDA-approved options. To enable early intervention and halt disease progression, crucial endpoints for future research should include biological and immune function markers indicative of future cancer development. Moreover, endpoints such as improved quality of life, symptom relief, and prevention of tumor regression demand further investigation.
7. ClinicalTrials.Gov Outcomes
Out of the 18 search results, 9 clinical trials have published their findings ( Table 1 ). For example, topical 5-fluorouracil (5-FU) reduced the risk of SCC for up to one year, but no benefit was observed for BCC ( {"type":"clinical-trial","attrs":{"text":"NCT00847912","term_id":"NCT00847912"}} NCT00847912 ) [ 25 ]. Oral celecoxib (200 mg) significantly reduced BCC burden ( {"type":"clinical-trial","attrs":{"text":"NCT00023621","term_id":"NCT00023621"}} NCT00023621 ) [ 20 ]. Another trial found that a low dose of eflornithine (or difluoromethylornithine, DFMO) effectively reduced skin biopsy nuclear abnormality in patients with AK ( {"type":"clinical-trial","attrs":{"text":"NCT00021294","term_id":"NCT00021294"}} NCT00021294 ) [ 26 ]. The results from some studies retrieved from the ClinicalTrials.gov database were insignificant, such as interventions with high-dose topical tretinoin and oral acitretin on NMSC ( Table 1 ). Some trials have not been completed or did not report data.
8. PubMed Database Outcomes
Table 2 lists 20 studies identified from the PubMed database, most of which were focused on agents with anti-inflammatory activities. These agents can be classified into chemotherapy, retinoids, NSAIDs, vitamins, dietary supplements, or agents used for other disorders that can be repurposed for cancer chemoprevention. Their proposed mechanisms of action are shown in Table 3 . These agents can be administered orally, topically, or both. The topical delivery provides advantages of higher skin targeting effects and fewer systemic side effects. For studies found in both ClinicalTrials and PubMed, the trials are only listed in Table 1 .
9. Examples of Chemoprevention Trials
9.1. nicotinamide.
Multiple studies evaluated nicotinamide, a form of vitamin B3 or niacin, as a preventative measure for skin cancer. The mechanisms of action for nicotinamide possibly involve increasing DNA repair by blocking UV-induced cellular ATP loss and reducing UV-induced immunosuppression [ 35 , 52 ]. Due to the chemopreventive effects of nicotinamide in preclinical models and earlier small-scale studies in human subjects, a phase III randomized controlled trial was conducted to assess the efficacy of nicotinamide as a chemopreventive agent for NMSC in the Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) trial, published in 2015 [ 32 ]. This trial, with a large sample size of 386 immunocompetent participants, was able to conclude statistically significant differences between nicotinamide use and placebo for decreasing rates of new-onset AK and NMSC [ 32 ]. Based on the evidence, nicotinamide is recommended by up to 76.9% of Mohs surgeons for NMSC prevention [ 53 ].
Later, at least two clinical trials evaluated the effects of oral nicotinamide in immunocompromised individuals. One phase II randomized controlled trial of nicotinamide was conducted to evaluate the skin cancer chemoprevention in renal transplant recipients [ 33 ]. This study could not conclude statistically significant results due to a small sample size ( n = 22), although they reported reductions in AKs in the nicotinamide group compared to the placebo group [ 33 ]. More recently, given nicotinamide’s potential activity against immunosuppression, the Oral Nicotinamide to Reduce Actinic Cancer after Transplant (ONTRANS) trial was conducted on solid-organ transplant recipients with a history of multiple NMSC who received nicotinamide for 12 months [ 34 ]. The incidence of NMSC was nearly identical in nicotinamide and placebo groups. Therefore, the use of nicotinamide as a preventative measure for NMSC produced negative results in immunosuppressed solid-organ transplant recipients [ 32 ]. Although most studies supported the safety of nicotinamide as a chemopreventive agent with little to no side effects at doses as high as three grams daily [ 32 ], in the ONTRAC trial, patients who received nicotinamide, compared to the patients who received placebo, had significantly more mucocutaneous infections (lip, mucosal, nail, skin, and wound infections, as well as paronychia and sinusitis) [ 54 ].
The potential efficacy of nicotinamide was also shown in individuals living in arsenic-contaminated areas based on preclinical studies [ 55 ].
Nicotinamide is a substrate of nicotinamide N-methyltransferase (NNMT), which catalyzes the N-methylation of nicotinamide and regulates its level. In the past two decades, NNMT has been shown involved in carcinogenesis and tumor progression, including skin cancer [ 56 ]. The interaction of NNMT and nicotinamide needs further investigations.
9.2. NSAIDs
Cyclooxygenase-2 (COX-2) and its metabolic product prostaglandin E 2 (PGE 2 ) can be induced by UV radiation and play important roles in skin inflammation and carcinogenesis [ 57 ]. Nonsteroidal anti-inflammatory drugs (NSAIDs) exhibit anti-inflammatory effects by inhibiting COX-2 selectively or non-selectively and inhibiting the production of PGE 2 and have been investigated in multiple trials. NSAIDs include celecoxib, diclofenac, etodolac, rofecoxib, ibuprofen, naproxen, indomethacin, MF-tricyclic, sulindac, piroxicam, and aspirin. Since the anti-inflammatory activity of NSAIDs is believed to be mediated by the inhibition of COX-2, while gastrointestinal toxicity is due to COX-1 inhibition, several COX-2 selective inhibitors, such as celecoxib, were developed to avoid gastrointestinal adverse reactions [ 58 ]. None of the clinical studies could confirm that NSAIDs have significant effects as skin cancer chemopreventive agents, but there were other findings to note. One study focused on NSAIDs’ effects on BCC chemoprevention in mice and humans that are carriers of the mutant PTCH1, a receptor of the hedgehog pathway [ 20 ]. Oral celecoxib, a selective COX-2 inhibitor, was given to the human participants at a dose of 200 mg twice daily. Although celecoxib showed a 75% decrease in BCC tumor burden in mice, in the human trial, the effects of oral celecoxib in reducing BCC burden in all subjects were insignificant. However, when considering only 60% of the patients with less severe diseases (<15 BCCs at study entry), celecoxib significantly reduced the BCC numbers and burden. In another study, research on celecoxib for the chemoprevention of NMSC was conducted [ 36 ]. Participants were randomized and began treatment with either celecoxib or placebo and then evaluated at 3, 6, 9, and 11 months after randomization. No difference in AKs was found at nine months of treatment, but at 11 months, there was a significant reduction in the mean NMSCs per patient in the celecoxib group. After adjusting for age, sex, skin cancer history, etc., the results were still found to be statistically significant. However, the results were inconclusive due to the early termination of the study by the FDA based on another finding of an association between a COX-2 inhibitor and an increased risk of cardiovascular adverse events [ 36 ]. The efficacy results are consistent with previous observational studies, however, due to potential increased cardiovascular risks, celecoxib’s use as a chemopreventive agent could be limited.
A prospective cohort study examined the association between NSAIDs and NMSC in veterans with a higher risk of skin cancer [ 38 ]. The investigators hypothesized that NSAIDs and COX-2-selective inhibitors would provide transient protection against keratinocyte carcinoma, with COX-2-selective inhibitors having greater effects. The study participants were all from the Veterans Affairs Topical Tretinoin Chemoprevention Trial (VATTC), with 1131 veterans recruited. During a median follow-up time of 2 years for BCC and 2.5 years for SCC, 472 occurrences of BCC and 309 occurrences of SCC were observed. Time-fixed analyses and time-varying analyses were performed to avoid potential confounding bias. The time-fixed analyses produced a negative association but were determined not to be valid, and the time-varying analyses produced null results. Overall, this study did not prove a negative association between the use of NSAIDs and the risk of NMSC. The study concluded that the inverse dose response observed in the current study and in prior studies may be an artifact of analytic method.
Since regular use of the non-selective COX-1/COX-2 inhibitor aspirin (acetylsalicylic acid) has been associated with the risk reduction of multiple cancer types [ 57 ], the chemopreventive properties of aspirin were studied for skin cancer. One study tested the effect of aspirin on melanoma in elderly patients, but the results did not provide strong evidence that aspirin was associated with a reduced incidence of melanoma [ 37 ]. Another study examined the effects of aspirin alone or combined with folic acid in a randomized, double-blind, placebo-controlled clinical trial [ 39 ]. A total of 1121 patients were enrolled in the trial of prevention of colorectal adenomas, which was repurposed for BCC. BCC was confirmed by a blinded review of the pathology reports. Although aspirin and folic acid failed to show statistically significant effects on reducing the risk of BCC, subgroup analysis indicates that BCC risk was lower with aspirin use in those with previous skin cancer, while folic acid was unrelated to BCC incidence. Consistently, a retrospective study (2010–2018) conducted using the Humana Health Insurance database concluded that aspirin use was associated with a significantly decreased risk of BCC [ 59 ]. Therefore, given the high incidence and cost of BCC treatment, the low cost of aspirin and its widely accepted use may promote its preventive use for this type or other types of skin cancer.
Given the systemic adverse effects observed for most NSAIDs, topical delivery has been investigated for achieving a local preventive activity. Diclofenac sodium 3%, in combination with hyaluronic acid 2.5% (diclofenac 3%/HA 2.5%; Solaraze ® , Fougera Pharms) is the only NSAID approved in the United States for the topical treatment of AK lesions (for review, see [ 60 ]). The topical Solaraze ® proved to be effective for patients with existing AK. It is well tolerated, with skin irritation as the main side effect [ 57 ]. Another topical COX-2 inhibitor is piroxicam, which is a nonspecific COX-1 and COX-2 inhibitor, with higher inhibitory activity (10-fold) for COX-1. Finally, 1% piroxicam gel, topically applied daily for 12 weeks, was shown to effectively induce the complete regression of 48% of evaluated AKs, with an adverse effect of only skin irritation [ 40 ]. Thus, the topical application of NSAIDs is promising for providing cancer preventive efficacy with minor side effects.
9.3. Retinoids
Since the retinoid signaling pathway plays an important role in organ homeostasis and carcinogenesis, the natural and synthetic vitamin A derivatives, retinoids, may be effective for the prevention and the treatment of several types of cancer, including skin cancer (for review, see [ 61 ]). Initially, oral retinoids demonstrated efficacy as chemopreventive agents against NMSC and other types of cancer [ 24 , 48 ]. Therefore, there was a trend during the 1960s and 1970s to develop synthetic retinoids for cancer prevention and treatment [ 62 ]. Oral isotretinoin, acitretin, and etretinate have been reported to reduce BCCs in patients with Xeroderma pigmentosum , organ transplantation recipients, and individuals with basal cell nevus (Gorlin) syndrome (BCNS) [ 48 ]. However, the protective effects were lost after the therapy was discontinued. Lower doses with fewer side effects were ineffective [ 24 ]. Long-term and high-dose use of systemic retinoids has been associated with significant dose-dependent side effects [ 62 ]. For example, published in 2012, systematic use of acitretin for 2 years in nontransplantation patients at high risk for NMSC did not show statistically significant reduction in the rate of new NMSC, while the patients who received acitretin reported significantly more mucositis and skin toxicities compared to the placebo group [ 21 ]. Therefore, systematic use of retinoids is limited in the general population with no or few skin cancers.
Topical use of retinoids, e.g., tretinoin, has been used for decades for the treatment of acne and photoaging, without systematic side effects [ 24 ]. Thus, topical retinoids have been investigated in the Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial [ 24 ]. In this trial, 1131 patients were given topical 0.1% tretinoin or a matching vehicle control for 1.5–5.5 years. Reported in 2012, the primary outcomes, the rates of new BCC and SCC, did not differ significantly for the treatment. The tretinoin group showed worse cutaneous symptoms. This trial concluded that in high-risk patients, high-dose topical tretinoin was ineffective at reducing risk of NMSC.
Tazarotene (Tazorac ® , Allergan, Irvine, CA) is a topical retinoid with relative specificity for retinoic acid receptor (RAR)-β and RAR-γ receptors. A randomized, double-blind, vehicle-controlled study in patients with basal cell naevus syndrome (BCNS) evaluated the efficacy of topically applied tazarotene for BCC chemoprevention ( n = 34 subjects), along with an open-label trial evaluating tazarotene’s efficacy for chemotherapy of BCC lesions ( n = 36 subjects) for a maximum follow-up period of 3 years. Only 6% of patients had a chemopreventive response, and only 6% of treated BCC target lesions were clinically cured. Thus, this study provides no evidence for either chemopreventive or chemotherapeutic effect of tazarotene against BCCs in patients with BCNS. Therefore, despite the robust effects of topical retinoids in preclinical studies, they failed to demonstrate the effects in clinical studies.
9.4. 5-Fluorouracil (5-FU)
5-FU is a chemotherapy agent that belongs to a class of antimetabolites. It is used topically to treat skin cancer and AK. An earlier study that is frequently referenced in prevalent skin cancer publications is Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial [ 41 ]. The study followed participants, mostly men with a median age of 72 with a history of heavily sun damaged skin. The subjects were required to have at least two forms of NMSC in the five years prior to their enrollment and had a follow up period of approximately four years. The purpose of this study was to find alternative preventive measures other than systemic retinoids, which have significant toxicity. A total of 1131 participants were screened, and of those participants, 23% developed at least one new SCC. The most important predictors of new SCC were identified as the number of prior carcinomas, the number of prior in situ carcinomas, the number of AKs prior to the study, the amount of sun exposure, and a history of 5-FU use. Participants that fell in the category of having many predictors had a significantly higher hazard ratio than those in the category of least predictors. Through a univariable analysis, all predictors were found to be statistically significant, while total sun exposure was found to have a greater association with newly developed SCC. The study concluded that a history of 5-FU use was strongly associated with an increased risk of future SCCs. On the other hand, using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers reduced risk of SCC development. Furthermore, the study concluded that the administration of high-dose topical tretinoin was ineffective in diminishing the risk of NMSC.
Topical 5-FU (5%) has been investigated in multiple clinical trials of skin cancer chemoprevention, and most produced positive results. In the randomized Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial of 932 veterans at high risk for NMSC, a 2–4-week duration of topical 5-FU reduced the risk of SCC for 1 year, but no effects were seen on BCC incidence in the first year [ 25 ]. There were no effects on SCC or BCC incidence at 4 years. Due to a potent chemopreventive effect in immunocompetent patients, a recent phase II open-label randomized controlled trial compared topical 5-FU, 5% imiquimod, and sunscreen in organ transplant recipients [ 45 ]. The pilot feasibility study suggested that topical 5-FU may be superior to imiquimod and sunscreen in AK clearance and prevention. Thus, 5-FU topical chemoprevention should be further investigated in SCC/AK prevention for immunocompromised patients.
The oral prodrug of 5-FU, capecitabine, was examined for the prevention and treatment of AK and NMSC. A systemic review indicated that capecitabine treatment may be associated with a decrease in the incidence of SCCs in organ transplant recipients [ 63 ]. ADRs, including fatigue, nausea, vomiting, diarrhea, elevated creatinine level, hand–foot syndrome, hyperuricemia, weight loss, anemia, and cardiomyopathy, limited the duration of chemoprevention in several patients.
9.5. Difluoromethylornithine (DFMO)
Difluoromethylornithine (DFMO), an inhibitor of ornithine decarboxylase, inhibits polyamine synthesis, which can be increased in UV-induced skin cancer [ 64 ]. A number of clinical trials have evaluated the effects of systemic DFMO, alone or in combination with other agents, for preventing skin cancer. Although the benefit of DFMO is promising, it has been reported to cause some side effects, such as hearing loss [ 64 ]. Therefore, topical application of DFMO is an option for reduced systemic effects. A previous study ( {"type":"clinical-trial","attrs":{"text":"NCT00021294","term_id":"NCT00021294"}} NCT00021294 ) investigated the efficacy of topical administration of DFMO (10%), triamcinolone (1%), and the combination of DFMO plus triamcinolone for the reduction of cell nuclear abnormality in moderately sun-damaged skin [ 26 ]. Eucerin ® (Beiersdorf Inc., Hamburg, Germany), a commercially available cream, was used as a vehicle. A total of 102 participants with sun-damaged skin on their posterolateral forearms were recruited for this study, and 185 skin biopsies were collected, with 16,395 nuclei recorded. High-resolution imagery of nuclei was utilized to assess the reduction of nuclei post-treatment and to compare them to baseline levels. Four treatment groups were established, including applying DFMO + Eucerin ® , DFMO + triamcinolone, triamcinolone + Eucerin ® , and Eucerin ® + Eucerin ® as a placebo. Participants applied 1 inch of cream to their forearms once daily throughout the study. The study found that applying these treatments resulted in a significant reduction in cell nuclear abnormality by 15–20%. These findings suggest that low-dose topical applications of DFMO, triamcinolone, and the combination of DFMO plus triamcinolone may effectively improve nuclear abnormality in moderately sun-damaged skin.
Since topical DFMO and topical diclofenac (an NSAID) as monotherapy have demonstrated chemopreventive activity against SCC, a phase IIB randomized trial of topical DFMO and diclofenac was conducted to evaluate the effects on sun-damaged skin in 136 patients who completed the study over three months. The goal was to examine whether the combination was more effective than monotherapy in reversing karyometric average nuclear abnormality, a predictor for SCC. However, the nuclear abnormalities increased in all three groups. The addition of topical DFMO to topical diclofenac did not enhance its activity but induced cutaneous inflammation as a side effect [ 27 ]. Based on this study, questions were raised regarding the efficacy of these agents, the status of cancer risk in the study population, and the validity of nuclear abnormalities as a marker [ 65 ].
9.6. Polyunsaturated Fatty Acids (PUFAs)
A study investigated the potential protective effect of the dietary supplement omega-3 polyunsaturated fatty acids (PUFAs) against photo-immunosuppression caused by solar UV radiation [ 30 ]. A total of 79 healthy female participants between the ages of 18 and 60 years were randomly assigned to either receive an oral placebo control lipid supplement or oral omega-3 PUFA (70% EPA and 10% DHA). The participants received nickel contact hypersensitivity patches to assess changes in photo-immunosuppression. After supplementation with either the control or PUFA, nickel was applied to the participants’ skin sites pre-exposed to three consecutive days of solar-simulated radiation (SSR) at a dose of 3.8 J/cm 3 and three unexposed control sites. The study found that omega-3 PUFAs were protective against photo-immunosuppression by lowering 50% of immunosuppression, measured by nickel contact hypersensitivity. The significance of this study lies in providing a potential solution to minimize skin damage caused by sunlight that can ultimately lead to skin cancer. This is particularly important because conventional sunscreens are inadequate in protecting against photo-immunosuppression compared to UV-induced erythema and are often misused or underutilized. Future well-designed human studies are needed to evaluate the effects of PUFAs.
9.7. Antidiabetic Drugs
Patients with type II diabetes show a higher risk of NMSC. These patients may benefit from the use of the commonly prescribed antidiabetic drug metformin to reduce the risk of NMSC and other types of cancer [ 47 ]. A secondary analysis of patients enrolled in the VAKCC trial was conducted to compare the risk for NMSC development between metformin users and non-users ( {"type":"clinical-trial","attrs":{"text":"NCT00847912","term_id":"NCT00847912"}} NCT00847912 ). Metformin-users had a significantly lower risk for SCC and BCC compared to non-users [ 66 ]. Another antidiabetic drug pioglitazone showed robust efficacy in preventing SCC in preclinical models [ 51 ], but no clinical trial data have been published yet. Future clinical trials are needed to answer the question of whether antidiabetic drugs are effective for skin cancer chemoprevention.
10. Safety Issues
Since the target population for cancer chemoprevention is healthy individuals with increased cancer risk, pharmacological agents that can be used for preventive purposes should be both effective and safe. Although the primary endpoints are usually efficacy, many clinical studies have reported side effects besides chemopreventive efficacy. Although some agents have demonstrated statistically significant efficacy, safety issues emerged after long-term and/or high-dose administration of these agents.
Among the chemopreventive interventions tested, trials using COX-2 inhibitors seemed to have the greatest risk to patients. Nearly all articles reported general adverse effects of these medications as well as serious cardiovascular effects. Common side effects associated with COX-2 inhibitors include infections, gastrointestinal disorders, musculoskeletal effects, and skin disorders. Cardiovascular effects included hypertension, myocardial infarction, stroke, congestive heart failure, or cardiovascular deaths. To better evaluate the safety profile, one trial performed a safety analysis that incorporated all randomly assigned patients that took at least one dose of the trial medication, placebo, or celecoxib [ 36 ]. The adverse events were compared by using either a chi-squared or Fisher exact test. Of the 183 subjects (87 in the celecoxib group and 96 in the placebo group), a total of 16 subjects reported experiencing severe adverse events, including 9 from the celecoxib group and 7 from the placebo group, with no deaths reported among all the subjects. The analysis concluded no significant differences between the placebo and the experimental groups.
Numerous studies have demonstrated toxicity associated with retinoids, particularly with systemic use [ 62 ]. Long-term retinoid therapy, especially at high doses, has been associated with teratogenicity [ 67 ], skeletal toxicity, such as the calcification of tendons and ligaments around joints, and hyperostosis of the spine, as well as osteoporosis [ 62 ]. Topical forms of retinoids can also induce toxicity, such as skin irritation. The safety issues may be related to a trend of less recent clinical trials on retinoids.
On the other hand, vitamins or dietary supplements, e.g., nicotinamide, are safe for long term use. Future studies should aim for proving the efficacy of these natural products.
11. Limitation of the Current Review
The limitations of this review include a narrow range of database searches using only two keywords. Additionally, this review could have included a more in-depth analysis of the studies’ methodologies to assess the quality of evidence. This review does not include intensive mechanisms of action or molecular targets for chemopreventive agents. Furthermore, this review’s focus on English-language studies may have resulted in the exclusion of relevant studies published in other languages.
12. Conclusions
This review provides an overview of the current state of the clinical research on the chemoprevention of skin cancer. While a significant proportion of the studies included in this review yielded positive efficacy results with statistical significance, the findings are not consistent when similar studies were conducted in different populations. For example, the results in the clinical trials for nicotinamide were not totally reproducible, and the data so far cannot provide sufficient evidence for drug efficacy due to doubts in the data analyses [ 68 ]. Further research is required to confirm the effectiveness of these agents in skin cancer prevention in various patient populations and to identify the complete profiles of adverse effects. The available evidence from multiple trials, including those found in the PubMed and ClinicalTrials databases, indicate that skin cancer chemoprevention is a largely under-researched area. Despite the high prevalence of skin cancer and the limited treatment options available, there has been a lack of attention given to this critical area of research, since cancer chemoprevention trials require relatively longer follow-up time compared to cancer treatment trials. Although certain skin cancer conditions, such as BCC, have been evaluated in several studies, others, including melanoma, have not received adequate attention. There is a significant need for future research studies to address the need for skin cancer chemoprevention particularly in subgroups of patients who are immunocompromised. Since systemic use of chemopreventive agents, such as COX-2 inhibitors and retinoids, can cause side effects, topical drug delivery shows advantages and should receive more attention. Systemic use of vitamins and dietary supplement should be a safer option. To address the safety issues of cancer chemopreventive agents, the effort should be directed to identify chemopreventive dietary supplements and to repurpose FDA-approved agents.
Funding Statement
This work was partly supported by the National Cancer Institute of the National Institutes of Health under the award number R01CA269653 (Y.H.). This work was also supported by the Western University of Health Sciences, College of Pharmacy, PharmD Student Summer Research Fellowship (R.T. and S.H.).
Author Contributions
Conceptualization, Y.H.; methodology, R.T. and S.H.; writing—original draft preparation, R.T. and S.H.; writing—review and editing, J.W., K.M.K., F.L.M.J. and B.A.; visualization, A.S.; supervision, Y.H.; funding acquisition, R.T., S.H. and Y.H. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Medscape: Skin adverse events rare after immunotherapy to treat skin cancers

Medscape highlighted University of Cincinnati research published in JAMA Dermatology that found certain skin adverse events were rare following immunotherapy treatments for certain skin cancers.
"Programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors are commonly used drugs for the treatment of various cancers," said Pushkar Aggarwal, MD, corresponding author and medical resident/fellow in UC's College of Medicine. "Having knowledge regarding possible associated skin reactions is vital for clinicians to guide management of the skin reaction and whether an adjustment is needed for the PD-1/PD-L1 inhibitors."
The researchers reviewed adverse event reports for patients treated with PD-1 and PD-L1 treatments between January 2004 and May 2023 to identify whether significant signals exist between PD-1/PD-L1 inhibitors and skin tumors keratoacanthoma and cutaneous squamous cell carcinoma (cSCC).
"For both keratoacanthoma and cSCC, significant signals were found with PD-1/PD-L1 inhibitors," Aggarwal said. But, out of 158,000 cases in the review, the data showed only 43 patients developing keratocanthoma and 83 developing cSCC, which highlights the conditions are "relatively rare adverse events."
Aggarwal said case reports, case-control trials and randomized clinical trials are needed to confirm the results of this study.
Read the Medscape article.
Featured photo at top of metastatic melanoma cells. Photo/National Cancer Institute.
- Faculty Staff
- In The News
- College of Medicine
- Dermatology
- Academic Health Center
- UC Cancer Institute
Related Stories
April 17, 2024
Medscape highlighted University of Cincinnati research published in JAMA Dermatology that found skin adverse events were rare following immunotherapy treatments for certain skin cancers.
Physics World names UC, Cincinnati Children's study among top 10 Breakthroughs of 2022
December 8, 2022
Physics World recognized the University of Cincinnati's first-in-human trial of FLASH radiotherapy as one of the Top 10 Breakthroughs of the Year for 2022.
GEN News: Biomarker discovery to help predict breast cancer outcomes
September 7, 2022
GEN News highlighted recent University of Cincinnati and Cincinnati Children's Hospital Medical Center research on metabolic signatures that help predict breast cancer outcomes and could open avenues for new treatments.
- Share full article
Advertisement
Supported by

How to Avoid One of the Deadliest Forms of Skin Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention.

By Ted Alcorn
When spring turns to summer and warm weather lures more people outside, skin cancer may be at most a distant concern. But experts said it’s important to take the risk seriously.
The ultraviolet rays in sunlight are a leading risk factor for skin cancer, which will affect one in five Americans over their lifetime. That includes melanoma, among the deadliest types. About 100,000 people are diagnosed with melanoma each year in the United States, and about 8,000 die from it annually, according to the American Cancer Society .
Fortunately, there are simple ways to reduce your risk, and to detect possible cases early while they are most curable. Therapies approved over the past 15 years have also transformed the treatment of melanoma, extending and improving the lives of patients even with late-stage cases.
Here’s what to know about melanoma, its treatments and how to protect yourself.
What is melanoma?
Melanoma is a cancer that typically starts in skin cells known as melanocytes that make the skin’s pigment. Compared with more common skin cancers that begin in squamous or basal cells, melanoma is more likely to spread to other parts of the body.
“It truly has a very aggressive behavior and biology behind it,” said Dr. Michael Davies, chairman of the melanoma medical oncology department at the University of Texas MD Anderson Cancer Center.
Most melanomas appear as flat or slightly elevated blotches of dark color on skin that has been frequently exposed to ultraviolet light, such as the scalp and face, arms, back and legs (though they can occur on areas that have never been exposed to the sun, too). In a smaller share of cases, the growth may appear as a dark- or red-colored bump and grow down into the skin, which can make it more difficult to detect.
A less common form, lentigo maligna melanoma, mostly afflicts older adults who have had significant sun exposure, and often appears as abnormally-shaped tan or brown spots on their heads or necks. An even rarer type, called acral lentiginous melanoma, occurs on the hands and feet (specifically the soles, palms, fingers, toes or nail beds) and accounts for more than half of melanoma cases in those who are not white. (The musician Bob Marley died from this type of melanoma, which was at first misidentified as a bruise.)
Melanoma can also occur in the eyes or mucosal membranes such as inside the nose or throat, but these cases are rare.
Who’s at risk? And can melanoma be prevented?
Melanoma is thought to be caused by a mix of genetic and environmental factors. One of the leading hazards is exposure to ultraviolet light, including from the sun and indoor tanning. A history of severe, blistering sunburns can raise your risk; as can living close to the Equator or at a high elevation, where the sun’s rays are more intense.
The best way to reduce your risk is to avoid unnecessary exposure to UV light. The sun’s rays are strongest between 10 a.m. and 4 p.m., so limit your time outside during those hours. Wear protective clothing and eyewear and regularly apply sunscreen of at least SPF 30 .
Dr. Shanthi Sivendran, a medical oncologist and senior vice president at the American Cancer Society, also warned against using tanning lamps and beds , which significantly increase melanoma risk. Twenty states and the District of Columbia have barred minors from using tanning beds, in part because of this concern, according to the Skin Cancer Foundation. But six states (Alaska, Colorado, Iowa, Montana, New Mexico and South Dakota) do not restrict them from doing so.
People with lighter skin are more vulnerable to damage from UV light. But Dr. Sivendran said that doesn’t mean those with darker skin shouldn’t also remain vigilant. “You can get melanoma regardless of what your skin color is,” she said.
It’s also important to know if melanoma runs in your family, which can heighten your risk. And people with compromised immune systems are also more likely to develop melanoma. While about half of cases occur in people aged 66 and over, younger people can also develop melanoma.
How can I detect melanoma?
Spotting melanoma early is vital, because nearly all cases that have not spread to other parts of the body are curable. However once the disease reaches the lymph nodes or more distant organs, five-year survival rates decrease significantly.
There are no standard guidelines for skin cancer screening, but clinicians can examine your skin for abnormalities during annual checkups. Dr. Kelly Nelson, a dermatologist at the MD Anderson Cancer Center, also recommended that patients conduct their own regular head-to-toe self exams .
To recognize changes in your skin, it’s helpful to be familiar with it, Dr. Nelson said. “People who are more aware of what the skin on their backs looks like are less likely to die of melanoma than people who have no clue at all.”
“It’s this fine line of balancing between having some degree of skin awareness, but also not being worried that every mole on your body is a ticking time bomb,” she added.
To distinguish melanomas from ordinary moles or irritations, dermatologists suggest checking for the “ABCDEs”: spots that have an asymmetrical shape, a notched or scalloped border, an unusual color pattern, a diameter larger than six millimeters, or a spot that has evolved over time.
In practice, however, Dr. Nelson said that patients often have trouble making these distinctions. She recommended looking out for “ugly ducklings,” meaning abnormalities that stand out for any reason .
How is melanoma treated?
For cancers that haven’t spread, a doctor will likely cut out the growth along with a margin of surrounding skin. They also may conduct a biopsy of nearby lymph nodes to assess the risk of the cancer spreading and help the doctor decide if further treatment is needed.
While melanoma is more likely to be deadly if it reaches distant parts of the body, major advances in treatment have improved the outlook, even for those who didn’t catch their cancer early.
These include treatments that harness the immune system to fight tumors, and targeted therapies that directly attack cancer cells.
There are more innovations on the way. In February, the Food and Drug Administration approved the first cancer treatment using tumor-infiltrating lymphocytes , known as TIL therapy, for use against melanomas that have not responded to other treatments. And scientists are also testing a vaccine tailored to the specific genetic makeup of a patient’s cancer in a late-stage clinical trial .
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.
- International edition
- Australia edition
- Europe edition

Hope for new skin cancer therapy as UK cases soar
Researchers discover a technique that can kill melanoma cells with few toxic side effects, but warn there is still no cure
Skin cancer rates are rocketing. Thanks to over-enthusiastic sunbathing in previous decades, melanoma cases have tripled in numbers in the UK since the early 1990s – and scientists predict worse is to come.
The type of cancer once relatively rare in Britain is now its fifth most common. Broadcaster Chris Evans recently revealed he had been diagnosed with the disease .
Scientists say skin cancer is likely to continue to rise up the nation’s tumour prevalence charts: most recent estimates suggest cases will increase a further 50% in the next 20 years .
The impact has been particularly marked among adults aged 55 and over. Case rates in this age group have increased by 195% since the 1990s, according to the latest figures from Cancer Research UK. A grim price is being paid for those cheap package holidays and tanned skins that were enjoyed in the 60s and 70s.
Early diagnoses and treatments for skin cancer are keeping down deaths but scientists say it is becoming urgent that new therapies are developed, particularly techniques for neutralising melanomas that have metastasized and spread from a patient’s skin to other parts of their body.
“We are making progress but we are still not yet at the stage where we have a cure,” said cell biologist Prof Dot Bennett of the Molecular and Clinical Sciences Research Institute, St George’s, University of London. “However, we recently uncovered a very promising process which we hope could become an effective way of dealing with metastatic skin cancer.”
The new technique has evolved the creation of a chemical – called a cell-penetrating peptide – that can kill melanoma cells efficiently and selectively, while having few toxic side effects. “We were very surprised at its effectiveness and although a great deal of extra work needs to be done before we can think of using this on human patients, it is very encouraging.”
Melanomas are linked to skin cell division. Sometimes, after a single mutation, this can produce moles. “However, after a certain number of divisions, a defence mechanism called cell senescence halts this process and the mole’s growth halts,” added Bennett. “So you can think of a mole as an aborted cancer. If it had not been terminated it would have grown and spread.”
The problem is that on occasions further mutations occur in a cell and the process of shutting down its division stops working. The result is melanoma and crucially all of the mutations involved can be triggered by ultraviolet (UV) radiation. The sun is a key source of UV radiation and bathing in its rays without proper protection not only produces sunburnt skin, it can trigger a melanoma that can take many years to materialise.
In many cases surgery or radiotherapy can halt the disease.

However, in some cases skin cancer can metastasize and spread to other organs before it is spotted. It is this form that has been the focus of research by Bennett and her team. Jointly funded by Cancer Research UK and the British Skin Foundation, they have identified worked on a protein known as p16, which was already known to be able to suppress tumour development, and on a small active part of p16 called a peptide.
“We made a series of changes to the peptide that previous research by other scientists showed might be effective and would allow it to enter cells, then added the peptide to melanoma cell lines in the laboratory,” Bennett said last week.
“The results were surprising. We expected the peptide might stop the cancer cells dividing. In fact it killed them. Moreover it did not kill normal cells.
This deadly impact is important. Like many other cancers, the stage at which melanoma is identified is crucial to a patient’s prospects of survival. The earlier the diagnosis, the better the outcome, as Michelle Mitchell, chief executive of Cancer Research UK, told the Observer .
She said: “More people than ever are surviving melanoma skin cancer, but there remains a critical gap in survival between people diagnosed at early stages compared with those diagnosed later. The p16-related peptide offers a promising new approach for those patients diagnosed with metastatic disease.”
Adil Sheraz, of the British Skin Foundation, said:. “The protein p16-related peptide preferentially targets melanoma cells, preserving fibroblasts – which play a vital part in skin repair and regeneration – and causing minimal damage to other cells. While further research is needed, this has the potential for much more favourable outcomes for melanoma patients.”
Bennett added: “We tried the p16 peptide on three lines made up of metastatic melanoma cells. All of those were nicely wiped out, which is very promising. Obviously we have to do a lot more research before using this technique on humans and that will take several years but it is a very good start.”
- Skin cancer
- The Observer
- Cancer research
- Chris Evans
Most viewed
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Charity News
- Health & Medicine
Soaring skin cancer cases hit a record high

7 July 2023
Melanoma skin cancer cases in the UK have reached an all-time high.
Our latest analysis shows there are 17,500 cases being diagnosed per year and projections reveal that these high numbers could continue to increase by around 50% over the next 20 years.
What’s causing these increases ?
Temperatures are still set to rise this summer and the UK public is keen to make the most of the good weather. But this comes with risks . It’s well proven that too much sun exposure is linked to skin cancer. And it’s believed that this is partially causing the rapid rise of cases.
Almost 9 out of 10 skin cancer cases in the UK are caused by overexposure to ultraviolet (UV) radiation from the sun and sunbeds. It damages the DNA in our skin cells and this damage can build up over time and lead to skin cancer.
Sunbathing has been popular since the 1970s, before people became more aware of the links to skin cancer, and now we’re seeing the consequences of the tanning trend. In people aged 55 and older the probability of getting skin cancer has almost tripled since the 1990s.
But the sun may not be the only factor leading to these high numbers. Older age is one of the main risk factors for cancer, and as the UK’s population grows, we’re also living longer.
More people are also noticing their skin changes and getting them checked by their GP.
But there is some good news too. Despite the record increase, death rates from skin cancer have started to decline. Now more people than ever are surviving skin cancer thanks to the incredible research and improvements in early diagnosis and treatments.
Our new analysis paints a mixed picture for cancer patients and the staff who care for them. While it’s promising that more people are seeking treatment for skin cancer earlier and survival is improving, it’s alarming that cases of the disease could soar over the coming years.
Justine’s story
Tanning was a regular part of Justine’s routine. As a teenager, she would often use sunbeds and admits she rarely spent time in the shade or used sunscreen when on holiday. But there is no such thing as safe tanning.
“I didn’t even consider skin cancer,” says Justine, now 52 years old.
“It was just when I got into the doctor’s surgery that I saw a poster on the wall about it and thought ‘you know what, that’s what I’ve got.’”
Justine was first diagnosed with skin cancer in 2006. She had surgery to remove the mole but over the years since she has had more cancerous moles removed.
Justine now hopes to use her experience to encourage others to stay safe in the sun.
“I’m now extremely careful in the sun and very alert to the signs. I wish I’d done that when I was younger,” she explains. “These days I leave nothing to chance, if a mole doesn’t seem quite right, I’ll go to my GP.”
To help everyone enjoy the sun safely this summer, Cancer Research UK experts are joining forces with NIVEA SUN and have created some easy-to-follow advice:
- Seek shade : especially between the hours of 11am-3pm in the UK, when the sun is strongest
- Cover up with clothing : wear a shirt with sleeves that cover your shoulders, a wide-brimmed hat and UV protection sunglasses
- Apply sunscreen : regularly and generously, choose one with at least SPF 15 and 4 or more stars
Protecting your skin
“Whether you’re holidaying abroad or enjoying the good weather closer to home, it’s important to take steps to reduce your risk of skin cancer, especially if you burn easily,” says Dr Julie Sharp, head of health and patient information at Cancer Research UK.
Anyone can develop skin cancer and getting sunburnt just once every two years can triple the risk.
The damage to skin cells caused by sunburn can last beyond the moments of pain and irritation. It can build up over time and increase cancer risk. So, protecting your skin, even if you’ve been sunburnt before, can make a big difference.
Looking at the weather’s UV index can tell us how strong the sun’s UV rays are – which can be seen on any weather website or app. The higher the value, the greater risk of sunburn and the less time it can take to damage your skin.
Sunburn doesn’t just happen abroad or during summer holidays. The sun in the UK is often strong enough to burn or damage skin even when it’s cloudy.
“It’s important to take care in the sun and to contact your GP if you notice any unusual changes to your skin – it’s not just changes to a mole that matter, it could be a sore that doesn’t heal or any unusual changes to an area of your skin. Spotting cancer early can make all the difference,” says Mitchell.
In the editorial of The Lancet( https://doi.org/10.1016/S1470-2045(23)00348-0 ) you write about skin cancer. It’s perhaps a mistake or not ?
Thanks for your comment. Whilst Cancer Research UK is referenced in that article, it was written by the team at the Lancet Oncology, not by us.
I hope that clears things up! Jacob, Cancer Research UK
I was diagnosed with Melanoma last year on my front shin, i did catch it early, but i am very surprised by my diagnosis as i do NOT expose myself to the sun, my legs never see the light of day, i have them covered all year round, i also never sunbathe as a child/teens as i used to suffer from terrible heat rash so i always kept in the shade. So how do i really stop myself from any recurrence when i’m not sure how or why i got it in the first place.
Hi can i see a graph if uk skin cancer case numbers please. would be v useful. i do a weekly email of interesting charts for clients and this would be a good one. Thanks Nick
Thanks for your comment.
You can find more detailed statistics on melanoma skin cancer, including graphs, here Please note the citation guidance at the bottom of the page if you do want to include any in your newsletter.
I hope that helps, Jacob, Cancer Research UK
Highlighted content
Are ultra-processed foods linked to cancer, making cancer screening work for you, clearing the smoke on age of sale: the hidden tactics of the tobacco industry, that cancer conversation podcast – longer, better lives: ep.2 why did a doctor have to wait for her cancer treatment, what's it like to be diagnosed with cancer as a teenager, can vaping cause changes in our cells, flame of hope awards 2024: spotlight on our inspiring volunteers, related topics.
- Sun & UV
- Real stories
Artificial human skin paves the way to new skin cancer therapy
By using artificial human skin, a research group from the University of Copenhagen have managed to block invasive growth in a skin cancer model.
The study has been published in Science Signaling and looks at what actually happens when a cell turns into a cancer cell.
"We have been studying one of the cells' signalling pathways, the so-called TGF beta pathway. This pathway plays a critical role in the cell's communication with its surroundings, and it controls e.g. cell growth and cell division. If these mechanisms are damaged, the cell may turn into a cancer cell and invade the surrounding tissue," explains Professor and Team Lead Hans Wandall from the Department of Cellular and Molecular Medicine at the University of Copenhagen.
Under normal circumstances, your skin cells will not just start to invade the hypodermis and wreak havoc. Instead, they will produce a new layer of skin. But when cancer cells emerge, the cells no longer respect the boundaries between skin layers, and they start to invade each other. This is called invasive growth.
Hans Wandall and his colleagues have been studying the TGF beta pathway and applied methods for blocking invasive growth and thus curbing the invasive growth in skin cancer.
"We already have various drugs that can block these signalling pathways and which may be used in tests. We have used some of them in this study," explains Associate Professor and co-author of the study Sally Dabelsteen from the School of Dentistry.
Hans Wandall and Sally Dabelsteen have worked together with Dr. Zilu Ye and Professor Jesper V. Olsen from the Novo Nordisk Foundation Center for Protein Research at the Faculty of Health and Medical Sciences.
"Some of these drugs have already been tested on humans, and some are in the process of being tested in connection with other types of cancer. They could also be tested on skin cancer specifically," she says.
Artificial skin is the closest we get to real human skin
The artificial skin used by the researchers in the new study consists of artificial, genetically manipulated human skin cells. Skin cells are produced on subcutaneous tissue made of collagen. This makes the cells grow in layers, just like real human skin.
Unlike mice models, the skin model, which is another word for artificial skin, allows researchers to introduce artificial genetic changes relatively quickly, which provide insight into the systems that support skin development and renewal.
This way they are also able to reproduce and follow the development of other skin disorders, not just skin cancer.
"By using artificial human skin we are past the potentially problematic obstacle of whether results from tests on mice models can be transferred to human tissue. Previously, we used mice models in most studies of this kind. Instead, we can now conclude that these substances probably are not harmful and could work in practice, because the artificial skin means that we are closer to human reality," says Hans Wandall.
The artificial skin used by the researchers resembles the skin used to test cosmetics in the EU, which banned animal testing in 2004. However, artificial skin does not allow the researchers to test the effect of a drug on the entire organism, Hans Wandall points out. Skin models like the one used here have been used by cosmetics companies since the mid-1980s.
"We can study the effect focussing on the individual organ -- the skin -- and then we reap experiences with regard to how molecules work, while we seek to determine whether they damage the structure of the skin and the healthy skin cells," he says.
- Skin Cancer
- Biotechnology
- Biochemistry
- Engineering
- Robotics Research
- Human skin color
- Ultraviolet
- Skin grafting
- Dog skin disorders
Story Source:
Materials provided by University of Copenhagen - The Faculty of Health and Medical Sciences . Note: Content may be edited for style and length.
Related Multimedia :
- Artificial skin
Journal Reference :
- Zilu Ye, Gülcan Kilic, Sally Dabelsteen, Irina N. Marinova, Jens F. B. Thøfner, Ming Song, Asha M. Rudjord-Levann, Ieva Bagdonaite, Sergey Y. Vakhrushev, Cord H. Brakebusch, Jesper V. Olsen, Hans H. Wandall. Characterization of TGF-β signaling in a human organotypic skin model reveals that loss of TGF-βRII induces invasive tissue growth . Science Signaling , 2022; 15 (761) DOI: 10.1126/scisignal.abo2206
Cite This Page :
Explore More
- Warming Antarctic Deep-Sea and Sea Level Rise
- Octopus Inspires New Suction Mechanism for ...
- Cities Sinking: Urban Populations at Risk
- Puzzle Solved About Ancient Galaxy
- How 3D Printers Can Give Robots a Soft Touch
- Combo of Multiple Health Stressors Harming Bees
- Methane Emission On a Cold Brown Dwarf
- Remarkable Memories of Mountain Chickadees
- Predicting Future Marine Extinctions
- Drain On Economy Due to Climate Change
Trending Topics
Strange & offbeat.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 16, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New insights could unlock immunotherapy for rare, deadly eye cancer
by University of Pittsburgh
New research from the University of Pittsburgh explains why metastatic uveal melanoma is resistant to conventional immunotherapies and how adoptive therapy, which involves growing a patient's T cells outside the body before reinfusing them, can successfully treat this rare and aggressive cancer.
In a paper published in Nature Communications , the Pitt researchers also explain how they developed a new clinical tool that predicts which patients will respond to adoptive therapy . The work is helping improve personalized therapies and avoid futile treatments for metastatic uveal melanoma .
"The dogma was that uveal melanoma is a 'cold' cancer, meaning that T cells can't get into these tumors," said senior author Udai Kammula, M.D., associate professor of surgery at Pitt and director of the Solid Tumor Cell Therapy Program at UPMC Hillman Cancer Center.
"We show that T cells are in fact infiltrating metastases and they're getting activated, but they're just sitting there in a dormant state because something in the tumor is suppressing them. Adoptive therapy allows us to rescue these cells from the suppressive tumor microenvironment and successfully treat some patients."
Uveal melanoma originates in the uveal tract of the eye but has a tendency to aggressively spread throughout the body, often to the liver. When metastasis occurs, this cancer is very difficult to treat and the prognosis for patients is almost always grim.
"Cutaneous melanoma, which affects the skin, is the poster child of immunotherapy. It responds incredibly well to immune checkpoint inhibitor drugs," said Kammula. "None of these conventional immunotherapies work for uveal melanoma, but we hadn't known why—until now."
In a previous Lancet Oncology study, Kammula and his team used adoptive therapy to surgically extract metastatic tumors from 19 uveal melanoma patients and grow T cells from these tumors in the laboratory. When they infused the cells back, 35% of patients had either partial or complete regression of their cancer, evidence against the assumption that cancer-fighting cells called tumor-infiltrating lymphocytes (TILs) aren't found in uveal melanoma. But it was still a mystery why immune checkpoint inhibitors, which rev up the activity of these T cells, are ineffective in treating this disease.
Kammula saw an opportunity to answer this question using a unique resource that he and his team have been building for the last decade: the largest known repository of uveal melanoma samples, corresponding tissues and clinical information.
When the researchers analyzed 100 metastases from 84 patients, they found that over half of these tumors were chock-full of T cells. Next, they performed single cell RNA sequencing to measure gene expression in almost 100,000 cells from six metastases. They found that the TILs in some of these tumors were activated and capable of attacking tumor cells in a dish, but they weren't proliferating to high numbers in the tumor.
"We found that TILs from metastatic uveal melanoma have the potential to attack the tumor, but something in the tumor microenvironment is shutting them down, so they're in a dormant, or quiescent, state," explained Kammula. "By liberating these cells from the suppressive environment and growing them in the lab, we can rescue their tumor-fighting capacity when infused back into the patient."
But TIL therapy doesn't work for everyone, as the researchers found in their earlier study. To predict which patients will respond and which will not, Kammula and lead author Shravan Leonard-Murali, M.D., a post-doctoral fellow in the lab, developed a clinical tool called Uveal Melanoma Immunogenic Score (UMIS), a holistic measure of the tumor that reflects the activity of more than 2,000 genes expressed by tumor cells, immune cells and other cells that form the tumor microenvironment. UMIS ranged from 0.114 to 0.347 across 100 metastases, with higher values indicating tumors with more potent TILs.
When the researchers looked at patients who received adoptive therapy in the earlier study, they found that patients with higher UMIS scores had better tumor regression, suggesting that this biomarker could predict which patients are likely to respond.
They also found that patients with metastases scoring above 0.246 had significantly improved progression-free survival and overall survival than those with UMIS below this cutoff.
"If a patient's UMIS level is below this threshold, we think that adoptive therapy is not appropriate. Using a biopsy to calculate a patient's UMIS could help avoid futile therapies and unnecessarily subjecting patients to invasive operations," said Kammula.
"But the immune system is not static. UMIS offers a window into the tumor that could also help us find the optimal time to treat a patient with adoptive therapy, like picking a fruit when it's at its ripest."
Kammula is now evaluating the score prospectively in an ongoing TIL therapy clinical trial at Pitt for patients with metastatic uveal melanoma.
He and his team are also taking what they've learned from uveal melanoma to tackle other difficult-to-treat tumors such as pancreatic cancer , and they are developing a pan-cancer version of UMIS that will predict how well a patient with any type of cancer is likely to respond to adoptive therapy.
Explore further
Feedback to editors

Occupations that are cognitively stimulating may be protective against later-life dementia
14 hours ago

Researchers develop a new way to safely boost immune cells to fight cancer
Apr 19, 2024

New compound from blessed thistle may promote functional nerve regeneration

New research defines specific genomic changes associated with the transmissibility of the mpox virus

New study confirms community pharmacies can help people quit smoking

Researchers discover glial hyper-drive for triggering epileptic seizures

Deeper dive into the gut microbiome shows changes linked to body weight

A new therapeutic target for traumatic brain injury

Dozens of COVID virus mutations arose in man with longest known case, research finds

Researchers explore causal machine learning, a new advancement for AI in health care
Related stories.
Determinants of overall survival in metastatic uveal melanoma identified
Jul 13, 2023
Clinical trial: New combination treatment for eye melanoma increased patient survival
Aug 27, 2021

Immune networks in tumors found to prime responses to personalized immunotherapy
Feb 6, 2024

Researchers identify promising drug combination for melanoma
Dec 3, 2020
New approach combines oncolytic virotherapy and adoptive T cell therapy for cancer treatment
Feb 12, 2024

New cell-based immunotherapy offered for melanoma
Feb 24, 2024
Recommended for you

Signs of multiple sclerosis show up in blood years before symptoms, study finds

Using AI to trace the origins of metastatic cancer cells

Engineered peptides open new avenue for immunotherapy drug development

Study of cancer-induced liver inflammation finds a promising therapeutic target
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
North Dakota State University
Extension and ag research news.
Accessibility
- Prairie Fare
Prairie Fare: Are you taking care of your skin?
- Dakota Gardener: Growing cucumbers on a trellis
- Prairie Fare: What you eat may affect your vision
- Dakota Gardener: How to plant potatoes
- Prairie Fare: Move more in April
“I thought you were wearing white knee socks,” someone said.
Then the person laughed when she was close enough to see me better.
I was not wearing white socks. I had bare legs.
That comment certainly built my confidence when I was a young girl.
Then self-tanning lotion came along. These early self-tanning lotions made my legs turn orange.
Thankfully, formulations have improved.
Actually, the older you get, the less you care about insensitive comments.
Self-tanning lotions usually contain DHA (dihydroxyacetone), which is non-toxic for use on our skin. Self-tanning lotions are much less hazardous than exposure to the ultraviolet rays of the sun. You still need to practice sun safety practices.
Some people use tanning beds to get a golden tan. Tanning beds, by the way, are among the “known carcinogens” according to the Food and Drug Administration.
Those of us with light skin are at higher risk of something more hazardous than embarrassment. Besides being fair-skinned, consider several risk factors for skin cancer. However, even if you naturally have darker skin or become tan easily, you still can be at risk for skin cancer.
If you answer “yes” to some of these questions you could be at greater risk of skin cancer.
- Do you have a history of previous skin cancer diagnosis?
- Do you have a family history of skin cancer?
- Do you have birthmarks, moles and/or light tans spots, which often are called “congenital nevi”?
- Do you have freckles?
- Are you male?
- Are you a tanning bed user?
- Are you a smoker?
- Do you have a tendency to get a sunburn?
Although we may hear more about prostate, colon, lung or breast cancer, skin cancer is the most common type of cancer, with 5.6 million cases occurring annually.
In fact, May is National Skin Cancer and Melanoma Awareness Month.
Basal cell carcinoma, squamous cell carcinoma and melanoma are the three main types of skin cancer. Melanoma is the deadliest form of skin cancer. Twenty people in the U.S. die of melanoma every day.
When caught early, skin cancer is treatable. By doing skin self-checks, you can spot the beginnings of skin cancer.
In particular, look for moles or spots that have changed color, have irregular borders, are larger than the size of a pencil eraser (1/4 inch) and appear after age 21. If you have spots that are itchy and do not heal, they can be a warning sign. Let your healthcare provider know these things, because you may need further testing.
As we move toward sunny, warmer months, many people spend more time in the sun gardening, golfing, fishing and doing other outdoor activities. When possible, seek shade when the sun is at its most intense (between 10 a.m. and 2 p.m.). Be sure to use a broad-spectrum sunscreen with a sun protection factor (SPF) of at least 30.
Wear a hat that covers your ears and the back of your neck. Ball caps are very popular, but they do not serve as good protection for the tops of your ears or the back of your neck.
Be a trend setter and wear a floppy cap that provides better protection. Wear long sleeves and long pants to protect your skin. Some sun-protection fabrics have been invented, which can block UVA and UVB rays.
Skin cancer awareness is among the priority topics of the North Dakota Cancer Coalition. This group has more information for adults and children. Check out the resources at www.ndcancercoalition.org/ priority-topics/skin-cancer/ . Children might enjoy listening to the Parker the Penguin video.
Be sure to stay hydrated in addition to protecting your skin. Your skin, like the rest of your body needs nourishing food to repair itself. This recipe is courtesy of Iowa State’s Spend Smart Eat Smart program. It is rich in vitamin C from the fruit, potassium from the banana, and protein and healthful fats from the peanut butter. Drink up!
Peanut Butter Berry Smoothie
1 cup strawberries, cut in half 1 banana, cut into four pieces 3 tablespoons milk 2 tablespoons smooth peanut butter 6 ice cubes
Place all ingredients in a blender. Blend until smooth and serve immediately.
Makes two servings. Each serving has 170 calories, 8 grams (g) fat, 5 g protein, 24 g carbohydrate, 4 g fiber and 65 milligrams sodium.
(Julie Garden-Robinson, Ph.D., R.D., L.R.D., is a North Dakota State University Extension food and nutrition specialist and professor in the Department of Health, Nutrition and Exercise Sciences.)
NDSU Agriculture Communication – April 18, 2024
Source: Julie Garden-Robinson, 701-231-7187, [email protected]
Editor: Elizabeth Cronin, 701-231-7006, [email protected]
Attachments
- esp - Praire Fare graphic identifier - (173.5927734375 kb)

Student Focused. Land Grant. Research Institution.
NDSU Dept. 7000
311 Morrill Hall, P.O. Box 6050
Fargo, ND 58108-6050
Theresa Tobin's Battle with Breast Cancer Inspires Revolutionary Science-Based Skincare Line
In 2021, Theresa Tobin's life took an unexpected turn when she received a breast cancer diagnosis.
Sydney, Australia - April 19, 2024 —

In 2021, Theresa Tobin's life took an unexpected turn when she received a breast cancer diagnosis. As a personal care formulation chemist with over 25 years of experience in the beauty industry, Tobin possessed a unique perspective that would eventually transform her personal battle into a pioneering skincare solution.
Radiation Therapy's Toll on Skin Health
Radiation therapy, essential for treating various cancers, inadvertently affects surrounding healthy tissues, leading to radiation dermatitis. This condition ranges from mild redness and dryness to severe blistering, significantly impacting the skin's health and patients' comfort.
Effective skincare emphasizing hydration and moisturization is vital in managing these side effects. Hydration and moisturization soothe discomfort, prevent infection, and facilitate skin recovery.
However, Tobin discovered that the standard moisturizing creams provided to patients, including those undergoing radiation therapy, often fell short. Many such products lack the specialized formulation needed to address the unique challenges of radiation-affected skin, such as severe dryness, irritation, and compromised barrier function.
"I immediately knew there were alternative ingredients that could provide more potent efficacy while being gentler and less irritating to the skin," Tobin stated.
Birth of a Barrier-Restoring Breakthrough
Driven by her scientific expertise and personal experience, Tobin embarked on extensive research focused on natural ingredients that could address the hydration needs of radiated skin while also bolstering the skin's barrier, minimizing transepidermal water loss, and easing irritation.
Her research and development resulted in the B-Restore™ Barrier Cream, uniquely featuring a pioneering combination of three ingredients - lactobacillus ferment lysate (postbiotics), bilberry seed oil, and Tasmanian pepper berry, marking the inception of CelRevive .
"Most skin moisturizers or creams that address the skin barrier to relieve symptoms of eczema, sensitive skin, and other skin disorders are offered by pharmaceutical companies and use traditional, unsustainable petroleum as an occlusive agent," Tobin explained. "We offer a natural, sustainable alternative."
CelRevive's commitment to sustainability extends beyond its formulations. The brand uses responsibly sourced, upcycled, and environmentally friendly ingredients, supporting farmers in sustainable practices. Additionally, all CelRevive products are packaged in post-consumer recycled materials and are 100% recyclable.
Tobin emphasized, "We believe that taking care of yourself should go hand in hand with caring for the planet."
Early Success and Global Ambitions
Since its recent launch in Australia, CelRevive has captured consumers' attention, garnering five-star ratings and glowing reviews from customers with eczema-prone, sensitive, and dry skin types. Buoyed by this early success, the brand is expanding to the U.S. market by 2025 to bring its sustainable skincare solutions to an international audience.
"Our aim is clear: to shake up the pharmaceutical skincare industry with our focus on natural, high-quality alternatives," Tobin stated confidently.
Scientific Approach Meets Human Touch
While CelRevive's formulations are rooted in clinical research and rigorous testing, the brand's approach is inherently human-centric. From purchase to disposal, every aspect of the customer experience positively impacts lives, offering solace and practical assistance beyond skincare.
"Through my personal journey with cancer, I understand the daily challenges that cancer patients are going through and appreciate the difference that effective and considered skin care can offer – mitigating side effects and providing peace of mind," Tobin shared.
With CelRevive, Theresa Tobin has transformed her personal struggle into a revolutionary skincare line that challenges industry norms. It also prioritizes sustainability and offers a human-centric approach to skin health – a testament to the power of perseverance and scientific innovation.
About CelRevive:
CelRevive is a pioneering Australian skincare brand founded by Theresa Tobin, a cancer survivor and personal care formulation chemist. Inspired by her journey battling breast cancer, Tobin developed an innovative line of products focused on restoring and strengthening the skin barrier. The brand's hero product, B-Restore Barrier Cream, utilizes a unique blend of natural ingredients to address issues like eczema, sensitivity, and dryness without using harsh petroleums.
Contact Info: Name: Theresa Tobin Email: Send Email Organization: Effiderma Pty Ltd Website: https://celrevive.com
Source: Baden Bower
Release ID: 89127636
In case of identifying any problems, concerns, or inaccuracies in the content shared in this press release, or if a press release needs to be taken down, we urge you to notify us immediately by contacting [email protected]. Our dedicated team will be readily accessible to address your concerns and take swift action within 8 hours to rectify any issues identified or assist with the removal process. We are committed to delivering high-quality content and ensuring accuracy for our valued readers.

IMAGES
VIDEO
COMMENTS
Friday's approval is only for melanoma, the deadliest form of skin cancer, but experts say it holds promise for treating other solid tumors, which account for 90% of all cancers.
The Skin SPORE program's main focus is on melanoma research activities, but it also includes projects in other skin cancer types, such as cutaneous T-cell lymphoma. NCI's National Clinical Trials Network (NCTN) is a collection of organizations and clinicians that coordinates and supports cancer clinical trials at more than 3,000 sites across ...
Study Adds to Debate about Screening for Melanoma. Posted: May 4, 2022. Regular skin cancer screening leads to many diagnoses of very early-stage melanomas, results from a new study suggest. The results add to a debate about whether screening is fueling an overdiagnosis of melanoma in the United States. Opdualag Becomes First FDA-Approved ...
It includes the latest information on risk factors, early detection, treatment, and current research for the most common types of cancer. Skin cancer is the most commonly diagnosed cancer in the US, with more than 5 million new cases diagnosed each year. Here are some other key facts about skin cancer for 2022:
The study was funded by the National Institutes of Health (grants K24AR075060 and R01AR082109), Radiumhemmet Research, the Swedish Cancer Society and the Swedish Research Council. For more news about responsible AI in health and medicine, sign up for the RAISE Health newsletter. Register for the RAISE Health Symposium on May 14.
Terahertz biosensor detects skin cancer with remarkable accuracy, ushering in new era of early detection. ScienceDaily . Retrieved April 16, 2024 from www.sciencedaily.com / releases / 2024 / 02 ...
Skin cancer can involve melanocytes, leading to melanoma, but there are other non-melanoma skin cancers. ... Research Highlights Open Access 01 Apr 2024 Signal Transduction and Targeted Therapy ...
Squamous-Cell Carcinoma of the Skin. A. WysongN Engl J Med 2023;388:2262-2273. Squamous-cell carcinoma is one of the most common cancers in humans. Most cases are localized and treatable; the ...
The U.S. Food and Drug Administration has approved a novel therapy for patients with metastatic or inoperable melanoma, an aggressive type of skin cancer.The treatment, developed based on original ...
Melanoma is the most serious type of skin cancer. It develops when skin cells multiply rapidly as a consequence of mutations in their DNA caused by UV exposure. Melanomas originate in the pigment ...
At 12 months, the rate of new nonmelanoma skin cancers was lower by 23% (95% confidence interval [CI], 4 to 38) in the nicotinamide group than in the placebo group (P=0.02).
Queen Mary University of London. Summary: New research has identified a protein that makes melanoma, the most serious type of skin cancer, more aggressive by giving cancer cells the ability to ...
Learn about skin cancer signs, symptoms and prevention. Read the latest medical research on skin cancer types, skin care and skin cancer treatment options. ... Jan. 9, 2023 — New research has ...
Melanoma arises from melanocytes, the pigment (melanin) producing cells in the skin. Melanoma is less common than the other types of skin cancer but is far more dangerous. The reason behind its ...
1. Introduction. Skin cancer is one of the most common types of malignancy in the United States and the world, with rising incidence [1,2].It is estimated that one out of five Americans will develop skin cancer by the age of 70, with Caucasians displaying the highest incidence [].Non-melanoma skin cancer (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is the ...
Research into the causes, prevention, detection, and treatment of basal and squamous cell skin cancer is going on in many medical centers throughout the world. Basic skin cancer research Scientists have made a great deal of progress in recent years in learning how ultraviolet (UV) light damages the DNA (genes) inside normal skin cells, and how ...
New Research in Skin Cancer: Melanoma, Merkel Cell Carcinoma and Basal Cell Carcinoma. ... Skin cancer is the most common cancer that kidney transplant patients develop. Typically, these cancers occur many years after transplant, are detected early and are easily treatable. However, some transplant recipients develop advanced skin cancers that ...
Medscape highlighted University of Cincinnati research published in JAMA Dermatology that found certain skin adverse events were rare following immunotherapy treatments for certain skin cancers. "Programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors are commonly used drugs for the treatment of various cancers," said Pushkar Aggarwal, MD, corresponding author and ...
This early sun exposure may damage the DNA (genes) in skin cells called melanocytes, which starts them on a path to becoming melanoma cells many years later. This might help explain why melanomas often occur on the thighs (in women) and trunk (in men), areas that generally aren't exposed to the sun as much in adulthood.
The best way to reduce your risk is to avoid unnecessary exposure to UV light. The sun's rays are strongest between 10 a.m. and 4 p.m., so limit your time outside during those hours.
The type of cancer once relatively rare in Britain is now its fifth most common. Broadcaster Chris Evans recently revealed he had been diagnosed with the disease. Scientists say skin cancer is ...
Gaining Momentum in Research. The human papillomavirus, or HPV, may play a role in the development of some squamous cell carcinomas of the skin (SCCs). Anna Nichols, MD, PhD, is determined to find out how and why.
Burn damage to skin cell (credit: LRI EM Unit) Melanoma skin cancer cases in the UK have reached an all-time high. Our latest analysis shows there are 17,500 cases being diagnosed per year and projections reveal that these high numbers could continue to increase by around 50% over the next 20 years.
FULL STORY. By using artificial human skin, a research group from the University of Copenhagen have managed to block invasive growth in a skin cancer model. The study has been published in Science ...
For more than 40 years, the Foundation has supported early career skin cancer researchers through its Research Grants program. In 2023, we were thrilled to award three investigators a total of $125,000 in grants to help them pursue promising research related to skin cancer detection and treatment. This year's award recipients included:
New research from the University of Pittsburgh explains why metastatic uveal melanoma is resistant to conventional immunotherapies and how adoptive therapy, which involves growing a patient's T ...
More than 5.4 million cases of nonmelanoma skin cancer were treated in over 3.3 million people in the U.S. in 2012, the most recent year new statistics were available. 1. More people are diagnosed with skin cancer each year in the U.S. than all other cancers combined. 2. At least one in five Americans will develop skin cancer by the age of 70. 3.
Although we may hear more about prostate, colon, lung or breast cancer, skin cancer is the most common type of cancer, with 5.6 million cases occurring annually. In fact, May is National Skin Cancer and Melanoma Awareness Month. Basal cell carcinoma, squamous cell carcinoma and melanoma are the three main types of skin cancer.
In a disturbing worldwide trend, new cancer cases among young people have been increasing sharply. Early-onset cancers, defined as cancer cases diagnosed in people under 50, increased globally by ...
In 2021, Theresa Tobin's life took an unexpected turn when she received a breast cancer diagnosis. Sydney, Australia - April 19, 2024 — In 2021, Theresa Tobin's life took an unexpected turn when ...