Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- How to Write a Research Proposal | Examples & Templates

How to Write a Research Proposal | Examples & Templates
Published on October 12, 2022 by Shona McCombes and Tegan George. Revised on November 21, 2023.
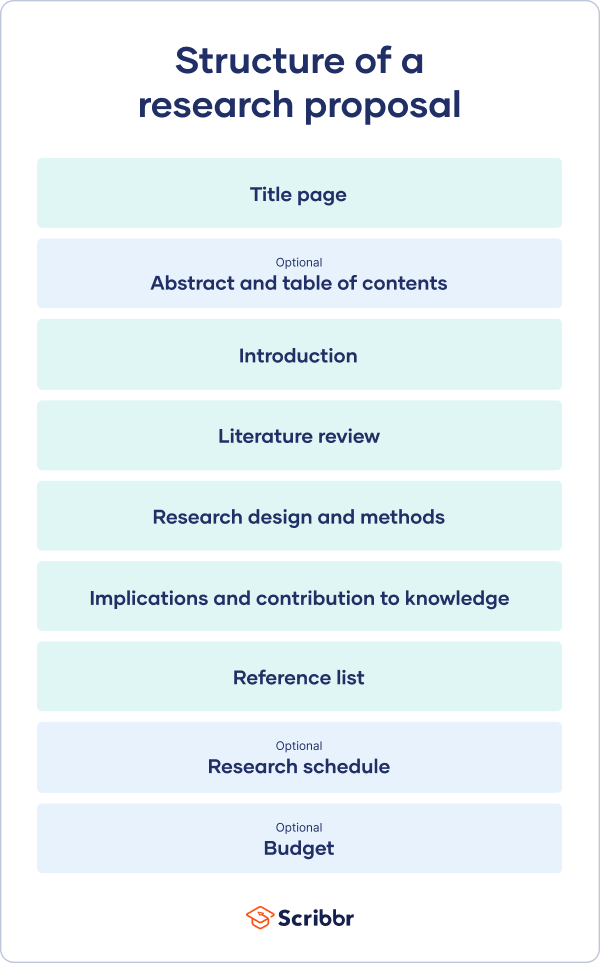
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:
Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organized and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, other interesting articles, frequently asked questions about research proposals.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: “A Conceptual Framework for Scheduling Constraint Management”
- Example research proposal #2: “Medical Students as Mediators of Change in Tobacco Use”
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
Prevent plagiarism. Run a free check.
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesize prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasize again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement .
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a dissertation committee) that your research topic is relevant and worthy of being conducted.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. & George, T. (2023, November 21). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved April 1, 2024, from https://www.scribbr.com/research-process/research-proposal/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a problem statement | guide & examples, writing strong research questions | criteria & examples, how to write a literature review | guide, examples, & templates, what is your plagiarism score.

Basic Methods Handbook for Clinical Orthopaedic Research pp 249–254 Cite as
How to Write a Winning Clinical Research Proposal?
- Christian Lattermann 8 &
- Janey D. Whalen 9
- First Online: 02 February 2019
2302 Accesses
This chapter addresses general approaches towards writing a clinical research proposal. The landscape in research has changed in the last decade and has become more translational in nature. Therefore, research proposals are read and evaluated by scientists from various fields that may not be intricately familiar with specific techniques and technique-related jargon. This chapter is designed to address the need for more translational and reviewer-friendly proposal writing and is built on errors and mistakes commonly seen in research proposals.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Shapiro FR. The Yale book of quotations, section: Blaise Pascal. New Haven: Yale University Press; 2006. p. 583.
Google Scholar
Download references
Author information
Authors and affiliations.
Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Christian Lattermann
Icartilage.com, Boston, MA, USA
Janey D. Whalen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian Lattermann .
Editor information
Editors and affiliations.
UPMC Rooney Sports Complex, University of Pittsburgh, Pittsburgh, PA, USA
Volker Musahl
Department of Orthopaedics, Sahlgrenska Academy, Gothenburg University, Sahlgrenska University Hospital, Gothenburg, Sweden
Jón Karlsson
Department of Orthopaedic Surgery and Traumatology, Kantonsspital Baselland (Bruderholz, Laufen und Liestal), Bruderholz, Switzerland
Michael T. Hirschmann
McMaster University, Hamilton, ON, Canada
Olufemi R. Ayeni
Hospital for Special Surgery, New York, NY, USA
Robert G. Marx
Department of Orthopaedic Surgery, NorthShore University HealthSystem, Evanston, IL, USA
Jason L. Koh
Institute for Medical Science in Sports, Osaka Health Science University, Osaka, Japan
Norimasa Nakamura
Rights and permissions
Reprints and permissions
Copyright information
© 2019 ISAKOS
About this chapter
Cite this chapter.
Lattermann, C., Whalen, J.D. (2019). How to Write a Winning Clinical Research Proposal?. In: Musahl, V., et al. Basic Methods Handbook for Clinical Orthopaedic Research. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-58254-1_27
Download citation
DOI : https://doi.org/10.1007/978-3-662-58254-1_27
Published : 02 February 2019
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-662-58253-4
Online ISBN : 978-3-662-58254-1
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical Literature
- Classical Reception
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Archaeology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Papyrology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Agriculture
- History of Education
- History of Emotions
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Variation
- Language Families
- Language Acquisition
- Language Evolution
- Language Reference
- Lexicography
- Linguistic Theories
- Linguistic Typology
- Linguistic Anthropology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Modernism)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Culture
- Music and Religion
- Music and Media
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Science
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Society
- Law and Politics
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Oncology
- Medical Toxicology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Medical Ethics
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Games
- Computer Security
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Neuroscience
- Cognitive Psychology
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business History
- Business Strategy
- Business Ethics
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Methodology
- Economic Systems
- Economic History
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Theory
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Behaviour
- Political Economy
- Political Institutions
- Politics and Law
- Public Administration
- Public Policy
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
87Chapter 4 Writing your research proposal
- Published: November 2011
- Cite Icon Cite
- Permissions Icon Permissions
In many ways it’s a reflection of yourself as a researcher and an insight into your proposed work. A poorly written proposal has the ability to wreck a project and embarrass the researcher before it has even begun. Similarly, a well-constructed proposal bodes well for the success of the project and displays the researcher in a good light amongst their peers and supervisors. The research proposal identifies: • What the topic is, both in terms of background and the individual area of interest. • What you plan to accomplish and why it needs doing. • What in particular you are trying to find out, i.e. the research question. • How you will get the answer to your question, i.e. your methodology. • What others will learn from it and why it is worth learning. • How long it will take. • How much money it will cost. Through your research proposal you are attempting to convince potential supporters that your project is worth doing, you are scientifically competent to run it, and are in possession of the necessary management skills to ensure its completion. The proposal concisely describes the key elements of the study process, although in sufficient depth to permit evaluation. It is a stand-alone document that must contain evidence of an answerable question, demonstrate your grasp of the literature, and also clearly show that your methodology is sound. A research time-table is required to demonstrate a realistic appreciation of how the study will progress through time. The research proposal serves many purposes to many different parties. Amongst these purposes, some of the key ones are: • Acting as a route map and timetable for all involved in your project. • Giving a clear overview of your planned work to ensure favourable decision at ethical review. • Gaining funding to carry out your proposed study. • Securing a place to undertake a higher scientific degree. • Being an opportunity to ‘blow your own trumpet’ on paper. Although there are several bodies who will be obliged to see your proposal, there is a reasonable chance it will end up being wider read than this, so a coherent piece of work will reflect well on you.
Signed in as
Institutional accounts.
- GoogleCrawler [DO NOT DELETE]
- Google Scholar Indexing
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Writing a research proposal
The format of a research proposal varies depending on what or who it is required by. They can vary in length, ie. be very concise or quite long and detailed. Also the headings for the different sections can vary. Therefore, this guide deals with the research proposal in its most generic form, which should be easily modifiable to fit the criteria for any research body.
The ultimate aim of any research proposal is to convince people that your research is important, has not been done before, is worthwhile and is feasible. Hence you have to make a strong argument for your research. The language used should be clear and easy to understand, as often non-experts will assess it. Some funders may, and the Research Ethics Committee application form will, want a ‘lay’ summary in addition to your basic proposal document. It is usually only in the background and methodology sections that writers tend to assume that the intended audience has a particular knowledge of their research area.
Additionally, it is crucial that different sections of your research proposal should link or follow on from each other, eg. the research question should link with the methodology. This may sound obvious, but revisions of one section can lead to mis-matches. Check this before submitting your proposal!
Typical stages in a research proposal
1. The purpose of a research proposal is:
- To help to focus on a relevant and current topic.
- To identify a gap or inadequacy in the research literature.
- To make sure that these are your ideas, and to help you to focus and crystallise your ideas.
- To help you to focus on what the actual stages involved in the research process will be, eg. the exact methodology and data analysis that will be adopted.
- To justify a proposed research project to a particular audience, eg. supervisor, departmental or faculty committee, external funding body etc.
2. Some strategies before you start:
- Search through literature for topic related articles and books, ie. search through databases/catalogues/journals etc.
- Look at what is already being done in the area i.e. existing data and research.
- Read critically, ie. look for interesting and suitable gaps – areas for research.
- Talk to your employer for approval – there is no point in starting research that you will not be allowed to complete.
- Talk to your local research and development teams. They will be able to tell you the specific criteria for any research proposal and may highlight some issues that you have overlooked.
- Talk to experts or supervisors in the field – in person, phone, letters, e-mail.
- If it is helpful, use concept maps to link ideas, and or formulate questions that the literature review should address.
3. Identifying your research question:
Any research proposal needs to have a clear research question for it to succeed. Without a clear question research will become confused and lack direction. Subsequent analysis will be difficult because the research question is key to forming your hypothesis or aims, and later analysis.
Do start by writing a question, not a statement. This will help clarify exactly what the issue is that you are trying to find a solution to. Hypotheses, aims etc can then follow from this.
Your research question should:
- Be as clear and concise as you can make it. Don’t use multi-barrelled questions if you can avoid them.
- Be informative – state your population of interest, locality etc.
- Avoid technical jargon – this is the golden rule in most areas of research proposals. Remember that your research question is what will capture the interest of the reader / assessor.
- Relate to the proposal title – often the research question is quoted as the title of the proposal.
- Relate to the aim of the research – again, the research question is often quoted as the research aim.
It should be obvious from your question alone what the project will aim to do, and on who.
4. Project title:
The title should be brief but informative. It is important that it is clear and easy to understand, and describes what your proposed research is. As previously stated, this is often the research question.
5. Abstract or summary:
This is a very important section which bears a disproportionate share of responsibility for success or failure of a proposal, as it may act as the initial ‘hook’.
It needs to be written for a wider audience, so technical vocabulary has to be limited. The abstract also needs to come quickly to the proposed research. Abstracts for grant proposals usually begin with the objective or purpose of the study, move on to methodology (procedures and design), and close with a modest but precise statement of the projects’ significance.
The significance should:
- Be about one paragraph – if it needs any longer it is advisable to rethink your research or break it down into more manageable chunks.
- Explain to the reader why the study is “significant”, in the sense of advancing general knowledge.
- Explain what the benefits to the patient / health community are.
- Encourage funding.
Although you present this first in the document, write it last so that its content accurately reflects the whole proposal.
6. Introduction:
The introduction is also written so that a more general audience can easily obtain a general idea of what the project is about, and the major concepts involved. It will also typically begin with the purpose of the proposed research. The introduction will typically be quite short, leaving the detail to the background and methodology sections.
7. Background:
It is only in the background and methodology sections that writers tend to assume that their intended audience is a specialist in their research area, and so use more technical language.
This section will include the literature review.
The purpose of a literature review is as follows:
- To become familiar with the research area and keep up to date with the current research in your area of interest.
- Identify an appropriate research question.
- Establish a theoretical framework for the research.
- Justify the need for the research.
Through the actual process of writing the literature review you, the researcher, can explore the relevant literature, formulate a problem, defend the value of the research, and compare the findings and ideas with your own. The literature review establishes a context and orientates the reader to your research topic.
The common structure of the literature review is likened to a “funnel effect”, which goes from general to more specific studies etc directly relating your intended project, ending with your research question, problem or objective.
In summary the stages of a literature review are as follows:
- General statement(s) about the field of research – the setting.
- More specific statements about the previous research.
- Statements that indicate the need for more investigation.
- Very specific statement(s) of the research question, problem or objective.
Your Trust librarians will be able to help with appropriate literature searching techniques if required.
8. Methodology:
The method or methodology section describes the steps you will follow in conducting your research. It is a very important section as assessors will scrutinise it to evaluate the feasibility and likelihood of successful completion of your proposed research.
Examine methodology sections of research articles in your research area. Arrange to discuss your research with a statistical and/or methodological specialist (Trust and other local research clinics / groups). Discuss with other researchers in your discipline the methodologies they have adopted. Consult methodology texts and statistical packages.
Overview of research:
Population/sample to be studied, including:
- How you have arrived at the sample size.
- How they will be recruited.
- Location of the research.
- Restrictions/limiting conditions.
- Sampling technique.
- Procedures.
- Analysis tools and methods.
9. Timescale:
10. Budget:
11. Ethical considerations:
- Benefits vs risks of the involvement in the project.
- Receipt of informed consent.
- Protection of participants (including data protection and storage issues).
- Privacy, minimising discomfort etc.
- Community values.
12. Dissemination strategy:
- The targets of your research eg. staff, patients, service users or carers,
- services locally and/or nationally,
- policies that drive the above services.
13. Bibliography and references:
Good luck with your project!
Useful reading: Bowling A (2002), Research Methods in Health: Investigating Health and Health Services 2Rev Ed edition, Open University Press Polgar S and Thomas SA. (4 th Edition 2004) Introduction to Research in the Health Sciences. Churchill Livingstone. MRC Good Research Practice Guidance

UCL Doctorate In Clinical Psychology
Guidelines for the Research Proposal

The purpose of the research proposal is to help you organise your ideas about your major research project, and to enable you to get feedback on what you are planning to do. It is worth putting in careful thought at this stage: it will mean that the project is more likely to run smoothly in the long run, and much of what you write in it can eventually be recycled into the final thesis write-up. The proposal is also needed for NHS ethics applications.
The proposal is a course requirement, but is not an assessed piece of work. It is due early in Term 1 of Year 2 (the date will be announced). Please submit an electronic copy to the Research Administrator (following the procedure detailed on the Project Support Moodle site).
There is no formal word limit (but conciseness is essential): we suggest that you aim for around 2500 words, plus references and any necessary appendices. Format it double-spaced, and include page numbers so that reviewers can easily refer back to specific points. Since it is not assessed work, it does not need your code number; please put your name on it.
Some sample proposals from previous years are available on the 'Proposal' (Topic 4) section of the Research Project Support Moodle.
The structure and content of the proposal is similar to that of the introduction and method sections of a journal article:
A title page with (1) the provisional title of the project (this can be modified later on), (2) your name, (3) your internal and external supervisors, (4) the setting where the study is likely to take place and (5) the date. If you are doing a joint project with other trainees, this should be stated here and the other trainees should be named. (Including all of this information on the title page is very helpful for the course's administrative purposes.)
The introduction (3 or 4 pages) states what the research topic is and why it is important. It succinctly reviews previous research in the area and relevant psychological theory, and summarises the rationale for the intended study. The introduction should end with one or more clearly stated research questions or hypotheses.
The method section (3 or 4 pages) describes in detail the proposed research methods: the setting, participants, sample size, research design, measures, ethical considerations, and data analysis procedures. For quantitative research, the sample size needs to be determined by a power calculation, which should be reported here (a separate document on power calculations is on the Project Support Moodle site). Measures that are not well known should be included as an appendix. For qualitative research, describe your interview schedule (append a draft) and your proposed method of analysis, including the types of "credibility checks" that you propose to use.
The service user involvement section (one or two or paragraphs) describes how the needs and views of service users or other relevant members of the public have shaped or will shape your project. This could include examples of service users influencing: (1) the choice of topic to be researched; (2) decisions about methodology; (3) the design of materials such as invitation letters and participant information sheets; (4) the design of a qualitative interview schedule, and (5) the ethics of the research. Please outline any plans for service user involvement later in the project. Remember, whilst there are formal ways of eliciting service user views, such as the use of focus groups and services such as FAST-R ( Feasibility And Support to Timely recruitment for Research ), informal sources of information are also valuable, and can be described here. This might include conversations with individual service users, experiences from clinical work, or interactions that take place on-line.
Whilst we strongly encourage trainees to use service user input when developing their research, this is not obligatory. Sometimes consultation with service users and other members of the public is not necessary, for example in some studies of healthy volunteers. If there has been no input from service users or members of the public, please use this section to state this, and briefly (a couple of sentences) explain why.
The feasibility section has a brief appraisal of how realistic your project is in practical terms, particularly with regard to recruiting participants. Many trainees (and their supervisors!) tend to be over-optimistic at this stage of the project, and it is a good idea to address potential recruitment problems at the outset. You should also include a fallback plan in case things go pear-shaped (which, sadly, in clinical research they often do). It would be helpful if you provided an estimate of what the smallest viable sample size would be, so that we (and you) have an idea of what a worst-case scenario might look like. A general timetable for the project is given in the guidelines for the major research project . If you anticipate any major departures from this, give details and a rationale.
The joint working section is, of course, only required if you are proposing a joint project. In this section provide a brief outline of what your anticipated contribution to the overall study will be, and what will be done by others. There should be a statement of how your research question(s) and analyses will be distinct from those of other students involved in the project. It will be helpful to consult the course guidelines on joint projects when planning any joint study.
The institutional arrangements , e.g., the setting, and who has agreed to be your internal and external supervisors.
The costings section sets out any substantial expenses that the project may entail. Note that the Department has limited funds and does not normally fund projects costing more than £250 over two years (see the course document on research funding ). If your project is likely to cost more than this, the course may possibly be able to provide some additional funding up to £400, although this cannot be guaranteed. It is your responsibility to secure additional funding for expenses beyond that allocated by the course.
The reference list gives all cited works. (It is important to check that this is complete, because reviewers may consult some of your references to understand the background to your study.)
Appendices include measures not in common use, draft qualitative interview schedules, etc.
Supervisors' input
Research proposals usually need to go through several drafts. Show your internal and external supervisors a draft early enough so that you can incorporate their comments into a revised draft before submission.
Review of the proposal
The proposal will be read by one of the academic staff, and will be discussed at a proposals review meeting in October. The resultant written feedback that you receive (towards the end of October) will give you a clear indication of the general feasibility of your project, and suggest any changes that will need to be made before it goes ahead.
This process counts as the "peer review" that is required for all NHS ethics applications. Therefore, once your proposal has passed the review stage, those of you applying for NHS ethics should contact Will Mandy to ask for a letter confirming that your project has been successfully peer reviewed.
Printable version of this page
Center for Clinical and Translational Science (CCaTS)
Postdoctoral master's degree program, research proposal.
The mentored research experience is a key component of the Postdoctoral Master's Degree Program in clinical and translational science.
Students develop their research proposals in CTSC 5010: Clinical Research Proposal Development, with a deadline based on the academic cycle listed below. Members of the CCaTS Scientific Review Group review research proposals, and the Master's and Certificate Programs Executive Committee approves them.
Include these items in your proposal packet:
- Research Proposal Packet Cover Page (PDF) .
- The proposal. Research proposals are approved by the CCaTS Master's and Certificate Programs Executive Committee and Mayo Clinic Graduate School of Biomedical Sciences (MCGSBS). Follow the Research Proposal Guidelines (PDF) , and construct your specific aims in a way that clearly defines the two or three publications resulting from the proposal.
- Research Proposal Project Timeline form .
- Translational Impact Statement. In one paragraph or a maximum of one page, describe the translational gap that your project will fill. You may include key literature that helped you identify that gap.
- Names. Your name should be first.
- Member expertise. Stick to the expertise that has value for this specific proposal.
- Help collect data?
- Train the student leader on necessary skills?
- Provide mentorship for the student leader for their research?
- Communication. How will the team meet, for example, in person or online? How often? How will the team resolve differences of opinion? What is the structure of meetings? Be clear about how you are situated in this structure.
- Final statement. Include a statement such as, "This team brings together fields of expertise that uniquely allow us to achieve [define a positive outcome]."
- Audience. Remember that you want to be writing for a broad readership. Limit your use of jargon and strive for clarity whenever possible
- Current curricula vitae for you and your mentor.
Thesis Advisory Committee (TAC) form. Complete the Proposed Master Thesis Advisory Committee form (PDF) . All TAC members are expected to provide scientific peer review and approve your proposal prior to submission. After your TAC has been approved by the CCaTS Master’s and Certificate Programs Executive Committee, the program staff will submit the MCGSBS TAC e-form on your behalf.
Read more about the Thesis Advisory Committee .
Verification statement from your department or division. This statement must document that peer review of the proposal has taken place and that the proposed work is scientifically sound and clinically meaningful. The minute excerpt from your department or division research committee or minute excerpt used for submission to the Mayo Clinic Institutional Review Board (IRB) may be used.
Note: If your project is deemed nontherapeutic and minimal risk — for example, a retrospective chart review — the IRB does not require divisional review and the CCaTS Minimal Risk Study form (PDF) is used in lieu of department or division verification.
If the proposal is part of a larger project, submit the abstract and documentation of scientific peer review of the larger project (that is, the minute excerpt from the appropriate research committee or funding agency).
- IRB approval email notification. This email notification documents that IRB review of your proposal was completed and approved. If the IRB has not yet approved your proposal, submit the email notification when approved. If your proposal is part of a larger project, please submit the IRB approval for the larger project.
- Research Proposal Potential Reviewers (PDF) .
Research proposal review criteria
The CCaTS Master's and Certificate Programs Executive Committee considers whether the thesis that will result from the proposal has the potential to meet the prescribed thesis standards and is feasible within the time frame identified. Proposals are reviewed by the CCaTS Scientific Review Group using these criteria:
- Clinical or translational research. Does the project meet the definition of clinical or translational research? If an animal model is being studied, has the scholar justified its significance to human disease?
- Scientific peer review. Is the hypothesis significant? Is the study design valid? Is the data collection and analysis plan consistent with the study goal? Is there statistical justification?
- IRB review. Has IRB approval for the project been obtained, or is it pending? If the scholar's project is part of a larger project, has documentation been provided showing IRB approval for the larger project?
- Authorship. Will the scholar be the first author on publications resulting from this project? If the scholar's project is part of a larger, multiauthor study, has the scholar explained the contribution to authorship? Will the project result in at least one publication?
- Feasibility. Is there sufficient funding for the project? Is there sufficient time to collect data and recruit participants? Does the project align with the scholar's research interests?
- Recruitment plan. Has a satisfactory subject recruitment plan, including a timeline, been provided?
- Current status. Has the status of the project been provided? For example, "Fifty percent of the data are collected, with data collection expected to be complete in six months."
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Advertisement

- Previous Issue
- Previous Article
- Next Article
Preparation of the Investigator for a Proposal
The research proposal, insights into the reviewer's perspective, conclusions, writing successful research proposals for medical science .
(Schwinn) Professor of Anesthesiology and Surgery; Associate Professor of Pharmacology/Cancer Biology, Duke University Medical Center; Senior Fellow, Duke Pepper Aging Center.
(DeLong) Associate Professor, Division of Biometry and Medical Informatics, Duke University Medical Center.
(Shafer) Staff Anesthesiologist, Palo Alto VA Health Care System; Associate Professor of Anesthesia, Stanford University.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
- Search Site
Debra A. Schwinn , Elizabeth R. DeLong , Steven L. Shafer; Writing Successful Research Proposals for Medical Science . Anesthesiology 1998; 88:1660–1666 doi: https://doi.org/10.1097/00000542-199806000-00031
Download citation file:
- Ris (Zotero)
- Reference Manager
HIGH-QUALITY research proposals are required to obtain funds for the basic and clinical sciences. In this era of diminishing revenues, the ability to compete successfully for peer-reviewed research money is essential to create and maintain scientific programs. Ideally, the essentials of “grantsmanship” are learned through observation and participation in grant preparation, but the training environment experienced by most physicians typically focuses on clinical skills. Most physicians are never exposed to a research environment and therefore do not learn how to write grants. The result is that many clinical studies, even when designed by skilled clinicians and those that address important clinical questions, often do not compete successfully with proposals written by basic scientists. This creates a perception that clinical studies are not favorably viewed by research review committees. The opposite is probably closer to the truth; research review committees are very keen to fund excellent clinical research. Although greater numbers of researchers with Ph.D. degrees have applied for National Institutes of Health (NIH) grants compared with researchers with M.D. degrees over the last 10 yr, funding rates (percent applications funded) have remained approximately the same for these investigators ( Figure 1 ; 1995 success rates: all degrees, 6,759 [26.8%]; M.D. - Ph.D., 370 [23.1%]; M.D., 1,518 [28.1%]; Ph.D., 4,746 [26.8%]; other degree, 125 [23.1%]).[section]
![proposal for clinical research Figure 1. Overall success rates for NIH funding of scientific applications, 1986 - 1995. No difference in funding rate is observed between applicants holding M.D. versus Ph.D. degrees. As the success rate for first-time applications was 11.3% in 1993, it is apparent that resubmission of a revised application significantly increases the overall chance of having research proposal ultimately funded.[section]](https://asa2.silverchair-cdn.com/asa2/content_public/journal/anesthesiology/88/6/10.1097_00000542-199806000-00031/4/m_31ff1.png?Expires=1714478590&Signature=DF7-UG0u~UJKg~wyOGmJ4waYoPjUxVIrlVBxQ0~ghz-Jf-oIsRlCqxm4Qt3wH4M-l6wWrzOwEaqCekVUFcbGhitKEhM8DIDKCOI~6XmRDAUa~phZZz2tt9Pl7RaAw2tnyOQTOVU-cDkRhxGNGTz1BeeAZ9Tg4v73jLJ6ltntapoy6Sp0kQBf0iHxYPBO3RDE~ds4jxXNEMLjNbzXSIWapjuJyb1rSYj0j9tRPeFq6UHlMddud6m4R67UnrD5KsRECAkYP14B9KZFv06k7IiCdsDfrxfswUJ2jY3gDlsQDLYqKaaXRWiCHHeE4fNLtu5jR-DXfly1vlt4igxeVh8sjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure 1. Overall success rates for NIH funding of scientific applications, 1986 - 1995. No difference in funding rate is observed between applicants holding M.D. versus Ph.D. degrees. As the success rate for first-time applications was 11.3% in 1993, it is apparent that resubmission of a revised application significantly increases the overall chance of having research proposal ultimately funded.[section]
Capable medical researchers ultimately write research proposals for funding by the NIH. Standards of excellence for NIH grants are high (only the top [almost equal to] 20% of grants are funded). Research questions posed must be hypothesis driven; the investigator must be qualified to perform the study; and preliminary evidence should be presented demonstrating that the research is feasible and will answer the questions posed. The goal of this article is to review important elements of successful research proposals, with emphasis on funding sources available to the anesthesiology community. Two important anesthesia-specific organizations exist to support anesthesia research - The Foundation for Anesthesia Education and Research (FAER, an organization under the auspices of the American Society of Anesthesiologists) and the International Anesthesiology Research Society (IARS).
Successful applications for research support from FAER and IARS have many of the characteristics of grants funded by the NIH and other peer-reviewed funding sources. These characteristics include (1) a highly qualified investigator(s);(2) for junior investigators, a mentor with a successful track record in scientific investigation, peer-reviewed funding, and mentorship of fellows and faculty;(3) a supportive academic environment; and (4) a scientifically sound proposal. Each of these characteristics is discussed in the subsequent sections.
Training of the Investigator
One of the most important components of a successful research proposal is a well-trained investigator. Training in clinical anesthesia is not training in research methodology or scientific thinking; it does not prepare an individual for a career in investigation. Although obvious for basic science research, clinical research also requires commitment of a minimum of 1 yr of dedicated training with a good mentor, and more typically 2 - 3 yr in the field of the proposed research. The applicant also needs to demonstrate commitment to a career in investigation. Several years of scientific training is the first demonstration of such commitment. Research proposals must document institutional support for nonclinical time, and the investigator must provide evidence that this time has been used wisely and will continue to be dedicated to the proposed research.
The research proposal must document a track record of productivity by the investigator. This expectation increases as the training and career of the investigator progresses. Fellowship awards do not have an expectation of prior research training, so publications from prior research are not expected. At the fellowship level, outstanding letters of recommendation, undergraduate and medical school performance, and related accomplishments are most important. Because previous training is not required of the fellowship applicant, prior success of the mentor (publications and track record with previous trainees) weighs heavily in the fellowship review. For junior faculty, peer-reviewed publications are expected from the fellowship period. Young Investigator Annoucements (from FAER) and several new IARS awards require several years as a successful junior faculty member, so expectations of demonstrated research success are further increased. The investigator must demonstrate (1) rigorous training, (2) commitment to research, (3) an appropriate career path, and (4) a track record of productive work. None of these are trivial issues, and none can be easily accomplished without making a commitment to research early in the academic career.
The quality of the mentor is another important aspect of awards granted to fellows and junior faculty. Identification of a mentor is explicitly required for FAER and certain junior level NIH grant applications. First and foremost, the mentor must be a successful investigator. Criteria for this include a track record of publication in the area of the proposed research, continued peer-reviewed funding, and a history of successfully training young investigators. Although mentorship is not considered heavily in more senior grant applications, input from a more experienced investigator often remains beneficial throughout one's career (as we can personally attest to). In addition to the mentor, high-quality coinvestigators, collaborators, and consultants also play important roles in strengthening a research proposal.
Environment
Good research is best accomplished in a supportive, cooperative environment. Because of the changing climate of clinical medicine, researchers (both clinical and basic science) face increasing pressure to minimize research time. It is not possible to become a successful investigator in one's spare time. Documentation of adequate nonclinical time for research (not for committee meetings or other unrelated tasks) is essential. Receiving funding at a junior level often enables the department to match funds or to guarantee nonclinical time to the budding investigator. In general, the more non-clinical time available to an investigator, the more competitive the application.
Other important elements of the environment include people, space, and institutional resources. People include mentors, consultants who can help with specific methodologies, statistical support, helpful colleagues, experienced technicians, a clinical research team, and a dedicated chairperson. There must be adequate space for performing the proposed studies, office space for research personnel, and storage space for equipment and supplies. Institutional resources include related departmental and interdepartmental seminar series, a critical mass of investigators in a related area, instrument development and repair shops, and necessary laboratory space and common facilities.
Criteria for a sound research proposal are the same whether the proposal is submitted to NIH, FAER, IARS, or other funding sources. In crafting a proposal, it is essential to consider the perspective of the reviewer; therefore, items of interest to the reviewer are listed after general definition of the grant proposal.
Review committees receive dozens of grants. NIH study sections may review as many as 150 proposals during one session. Typically, only two or three reviewers are assigned to read each grant in detail, but everyone is expected to read each abstract. Hence, the abstract is often one of the most important parts of the research proposal. The abstract should address the significance of the question and the overall topic, state the hypothesis, and point out key preliminary data. Additionally, the abstract should provide a synopsis of methodologies planned. In the end, the reviewer must be convinced that the applicant is uniquely (or ideally) suited to undertake this important study by the end of this concise paragraph.
Body of the Grant
Specific Aims. The specific aims section is critically important in a scientific proposal. It is here that the investigator crystallizes the overall goal of the research and states specific hypotheses.
Beginning with the specific aims, the proposal must be well written and logically organized. A poorly organized grant application is difficult to review, even if the science is otherwise excellent. Typically, the specific aims begin with a short introduction (one paragraph), followed by a formally stated hypothesis. The hypothesis must be answerable by the research methods proposed. Generally, two or three specific aims are outlined with subheadings where appropriate. Organization of the specific aims is often temporal, starting with a proposed mechanism or the first set of studies in a clinical project. In general, the specific aims section should be no longer than one page.
Background and Significance. The background section provides an opportunity to bring reviewers up to date on current research in the area of the proposal. This section should summarize succinctly studies from the literature and related work published by the investigator. The most crucial aspect of the background is to build a case for significance of the proposed research regarding the ultimate clinical application or mechanistic understanding. Ideally, the background section should demonstrate that the current proposal is a logical extension of previous studies in the field and will provide new information and novel insights. In general, the background section should be about one fourth of the length of the grant proposal.
Preliminary Data. Preliminary data provide the opportunity for the investigator to demonstrate his or her ability to perform the proposed research. The goal in presenting preliminary data is to convince the reviewer that the investigator is capable of performing the proposed studies and that the mechanisms proposed are plausible. Good preliminary data support novel (or even unlikely) hypotheses. Each experimental method proposed should be accompanied by preliminary data demonstrating facility and expertise with related preparations. For example, if the investigator proposes using a specific electrophysiologic technique to study an ion channel, evidence demonstrating that this technique has been used by the investigator with other ion channels and a Figure showingresults from pilot experiments on the channel of interest would suffice. In clinical studies, demonstration of a working investigative team and the ability to enroll a given number of patients per week is helpful. Figures or tables help to convey the message in a succinct manner. They also conserve space in the proposal and create a more impressive effect. Although it is best if the applicant has generated his or her own preliminary data, for training awards, preliminary data from the mentor's laboratory is entirely appropriate. An effective way to organize preliminary data is to present it in the same order as the specific aims (e.g., C.1 preliminary data corresponds to A.1 specific aims, C.2 preliminary data corresponds to A.2 specific aims, etc.). Presentation of preliminary data usually takes about one fourth to one third of the length of the grant application.
Methods. The methods are the guts of the research proposal. Unfortunately, many investigators run out of steam by the time they reach the methods, leaving reviewers unconvinced by the proposed methodology. Ideally, the model being investigated should be broken down into simple, logical components, each accompanied by a description of specific experiments/interventions to be performed. The investigator should assume that at least one reviewer is an expert in each method presented. Therefore, enough detail should be provided to convince an expert that the experiment or technique is being performed properly. Methods presented as a list of recipes, requiring the reviewer to guess which method applies to each study, are recipes for disaster. Individual experimental techniques should be state of the art. In addition, approaching a problem from several angles is often helpful. “Lingo” of the field should be avoided; it is very annoying to reviewers to have to look up unexplained abbreviations or to have models alluded to rather than described. For training grants, methods should involve techniques currently being performed in the laboratory of the mentor. An effective way to organize the methods section is to follow the same order as the preliminary data and specific aims sections (e.g., D.1 methods corresponds to C.1 preliminary data and A.1 specific aims, etc.).
The methods sections should include a description of the design, conduct, and analysis of each study being proposed. Common errors in design include lack of specification of primary outcome, lack of randomization or blinding in clinical trials, inadequate justification of sample size, failure to adjust the total study number for expected dropouts/failed experiments or patient refusal, and use of single drug doses or concentrations rather than development of dose - response or concentration - response relations. Common errors in conducting research include lack of confirmation of drug concentrations, inadequate reproducibility of final results, lack of standardization of procedures, inadequate follow-up, incomplete data recording, and overall lack of organization.
Inadequate or inappropriate statistical methods can be a major weakness of a grant proposal. Many investigators feel confident with all aspects of their methods except the statistical section. Because statistical issues underlie the design and analysis strategy for every study, the input of a biostatistician is essential in planning the research and writing the grant application. Statistical considerations include specification of the primary end points that drive power calculations. Common statistical errors in research proposals include lack of sample size/power calculations, treating continuous variables as dichotomous, repeated t tests when a more comprehensive modeling approach should be taken, application of statistical tests that assume normality without verifying assumptions, failure to consider covariate effects, and failure to distinguish between interindividual and intraindividual variability. The investigator should be familiar with the concept of statistical power and be prepared to estimate some of the quantities needed to formulate an alternative hypothesis appropriately. The statistical analysis should be clearly outlined with specific methodology directed toward the hypotheses of the study. A statistical reviewer is unlikely to be convinced by a statement that “appropriate statistical methodology will be used” or by a barrage of nonspecific statistical jargon. At least one full paragraph (and sometimes an entire page) of the research proposal should be devoted to statistical analysis. Often several smaller statistics sections are appropriately included after each method is presented.
Even the best methods have potential problems and weaknesses. It is critical that the methods section discuss potential problems that may be encountered during the study and state how the investigator proposes to deal with these problems creatively. Reviewers tend to be impressed when the investigator presents potential problems that never occurred to them, because it suggests that the investigator is an expert in this area of research. A time line and organizational plan (who will be responsible for what) should also be included in the methods section so the reviewers can determine whether the investigator is being realistic in his or her approach. The methods section is typically one third to one half of the length of the entire grant proposal.
Introduction to Revised Application. Because so few grant applications are funded on their first submission (11.5% in 1993), the new investigator should not be unduly alarmed if his or her application is not funded. When a grant application has been unsuccessful, an investigator should revise the application and reapply, even if the original score was “noncompetitive”(meaning the grant was in the lower 50% of applications). Often the reviewers suggest key changes that will improve the application significantly. When submitting a revised application, an introduction (placed before the specific aims section) is used to discuss how criticisms of the original grant have been addressed in the revised proposal. Because the reviewer's comments are intended to be helpful, it is important to address each concern carefully in the revised proposal (changed text should be highlighted in the revised application by italic, bold, or identifying lines in the margin), with changes outlined in the introduction section. Angry responses to reviewers do not facilitate funding of the revised application. Remember that reviewers usually have a copy of the prior review, and they expect corrections or, when appropriate, an explanation of why you have chosen not to incorporate some suggestions from a prior review. Time taken to revise an application is well spent; as Figure 1 demonstrates, investigators who persist in revising and resubmitting their applications have an increased chance ([almost equal to] 20% with no previous NIH support, [almost equal to] 35% if previously funded) of ultimately being funded.[section]
In writing a research grant, it is helpful to consider the reviewer's perspective. Key features considered by reviewers include significance, approach, and feasibility. It is wise for the investigator to reread his or her application before submission with these features in mind. The NIH recently has published two documents on-line that discuss review criteria; examination of these documents before submission of a research proposal may prove helpful. These include the Report of the Committee on Rating Grant Applications[double vertical bar] and Review Criteria for Rating Unsolicited Research Grants.#
Significance
First and foremost, is the investigator asking an important question? There are two general ways research studies can be significant. The first is to demonstrate clinical significance. The litmus test for clinical significance is whether the proposed research will improve patient care. The second is elucidation of fundamental mechanisms underlying disease or biologic processes. The ideal research question succeeds in being significant in both areas.
The reviewer assesses whether the research plan can support or refute the stated hypothesis. In addition, the reviewer assesses whether the methodologies used provide adequate or, better yet, elegant approaches to the problem. Recently, the NIH has mandated an increasing emphasis on innovation in research. [1] **
Review committees generally are composed of individuals with expertise in many scientific areas. Additionally, study sections often retain outside reviewers with expertise in the proposed research area. The investigator should assume that his or her methods will be critiqued by at least one expert. Therefore, the investigator should not propose a method that would strike the world's expert in the field as being simplistic, inappropriate, or nonsensical, because the world's expert just might be one of the reviewers. Conversely, some reviewers do not have expertise in the proposed area of research. To ensure that the nonexpert is convinced of the validity and importance of proposed methodologies, the overall proposal should be written with a logical flow of ideas that build from basic to sophisticated concepts. Beginning each portion of the methods section with a short introduction for the nonexpert, followed by a more detailed description of the proposed methods, is an effective strategy to address the needs of both expert and nonexpert reviewers.
Feasibility
The investigator must convince reviewers that the chosen approach is feasible. Preliminary data provide the best demonstration of feasibility. Feasibility is often demonstrated by a track record of publications or peer-reviewed grant support for the applicant or mentor using the proposed experimental approach. Feasibility also can be demonstrated by appropriate statistical analysis of the proposal. For example, a power analysis and corresponding data on the number of patients with the required characteristics at the investigator's institution helps convince reviewers that a clinical study is feasible.
Anesthesiology Funding Sources
Funding for research performed by anesthesiologists is available from many sources. Because the discipline of anesthesiology overlaps many other fields, anesthesiologists have the opportunity to apply for research funds from agencies as diverse as the American Academy of Pediatrics, American Cancer Society, American Heart Association (national and local), American Thoracic Society, American Society for Regional Anesthesiology, critical care societies, Department of Veterans Affairs, National Science Foundation, Shriners, Society for Cardiovascular Anesthesiology, Society for Obstetrics and Perinatology, National Aeronautics and Space Aviation, NIH, and many other private foundations. Grants from FAER and IARS are available specifically to the anesthesiology community.
It is important that anesthesiologists continue to apply for NIH grants. For fiscal year 1996, the NIH awarded 149 research grants (including career development grants, R29, R01, and program project grants) to departments of anesthesiology, totaling $21 million in direct costs ([almost equal to]$31 million in total costs). Because of the diversity of research projects in anesthesiology, these grants were awarded by 14 different institutes, centers, and divisions within the NIH. In analyzing data for three recent review sessions (June 1996, October 1996, and February 1997) from the surgery, anesthesiology, and trauma study section, 26% of anesthesiology applications scored in the top 20th percentile, and 31% scored in the top 25th percentile; clearly no bias exists against anesthesiology in this predominantly surgical study section, at least in this limited sample (Alison Cole, anesthesiology representative for the National Institute of General Medicine Science at the NIH, personal communication, December, 1997). Table 1
Table 1. Number of Recipients of NIH Research Project Annoucements

A brief list of funding opportunities available to anesthesiologists early in their career is shown in Table 2 . Several sites are available on the World Wide Web ( Table 3 ) to facilitate access to grant/training resources for anesthesiologists. We have created an additional website ( http://pkpd.icon.palo-alto.med.va.gov/grants/grants.htm ), which provides access to more comprehensive lists of funding agencies and direct links to funding sources. This website also contains example grants designed to illustrate the grant writing principles discussed in this article.
Table 2. Potential Funding Sources

Table 3. Grant/Training Resources on the WWW

Successful grant applications require a well-trained investigator who carefully outlines a hypothesis-driven research proposal. Unique to FAER and IARS research committees is that the reviewers are mostly investigators and practicing anesthesiologists. These reviewers fully appreciate the importance of clinical research and enthusiastically support high-quality clinical studies. Although descriptive clinical studies are interesting to practicing clinicians, from a scientific perspective, clinical research must be driven by testable hypotheses. Without a testable hypothesis, clinical research cannot pass the test of adequate significance required for funding.
It is our hope that by demystifying the grant writing and review process that more anesthesiologists will be encouraged to submit proposals for research funding. As part of this effort, we strongly encourage residents and fellows interested in research careers to obtain adequate research training and to apply for appropriate fellowship/junior faculty awards early in their careers.
[section] NIH Extramural Data and Trends, Fiscal Years 1986 - 1995. Bethesda, Office of Reports and Analysis (component of the Office of Extramural Research), National Institutes of Health. (Published on-line and periodically updated. http://www.nih.gov/grants/award/award.htm ).
[double vertical bar] Report of the Committee on Rating Grant Applications. Revised 5/17/96. Bethesda, National Institutes of Health. (Published on-line. http://www.nih.gov/grants/peer/rga.pdf ).
# Review Criteria for Rating Unsolicited Research Grants. NIH Guide, Vol. 26, No. 22, 6/27/97. Bethesda, National Institutes of Health. (Published on-line. http://www.nih.gov/grants/guide/1997/97.06.27/notice-review-criter9.html ).
** Brown KS: A winning strategy for grant application: Focus on impact. The Scientist 1997; April 8:13–4
Citing articles via
Most viewed, email alerts, related articles, social media, affiliations.
- ASA Practice Parameters
- Online First
- Author Resource Center
- About the Journal
- Editorial Board
- Rights & Permissions
- Online ISSN 1528-1175
- Print ISSN 0003-3022
- Anesthesiology
- ASA Monitor

- Terms & Conditions Privacy Policy
- Manage Cookie Preferences
- © Copyright 2024 American Society of Anesthesiologists
This Feature Is Available To Subscribers Only
Sign In or Create an Account

Clinical Research Proposal

In the time when the sea was the avenue through which a nation can conquer the world, Britain believed that a strong naval fleet of almost 2,000 men could make a dent out of Spain’s stronghold in America. After surviving the storms and long days at sea, the people did not expect that they would fall prey to a silent killer amongst them. Sailors had been dying from the most common maritime disease that had claimed the lives of countless others—scurvy. It was only later learned that the disease could be cured with citrus fruits, thanks to the physician James Lind. Years after the treatment for scurvy was available, Britain became a naval superpower, and the English language started to be introduced to the rest of the world. Lind’s observation was the first of many clinical research that changed the world.
7+ Clinical Research Proposal Templates and Samples
However revolutionary, there are downsides to fresh fruits as the go-to cure for the disease. Citrus fruits generally do not last long, especially then when there was no refrigerator nor electricity. The extract contains a volatile compound in its natural form. Heat and long periods of storage destroy the therapeutic activity of the substance, and the compound itself. It was much later that vitamin C was synthetically produced as tablets and capsules. Before a drug reaches the patients, it undergoes a series of tests and clinical trials that ensure that the drug is safe and significantly effective. Clinical research deals with treatments and services for the benefit of society. Clinical researchers are working on several treatment and prevention options to improve and advance medicine and human health. Their revolutionary ideas start with a research proposal for succeeding tests and validations.
1. Research Proposal Template

- Google Docs
Size: A4 & US Letter Sizes
Clinical research covers studies that deal with the improvement of health products and services. Each study proposes news or reviews the effect of the present method or product aimed to better the health quality of people. For the research to proceed to the testing for efficacy and safety for human consumption, researchers first have to convince a board or committee concerned that the study is worth pursuing. The research proposal should sway the respective authority to approve and support the activity. It should show that you are competent and capable of conducting the desired research undertaking. To help you in preparing for your proposal, you can download the sample Research Proposal Template shown above.
2. Research Project Proposal Template

A research proposal is a declaration of intent to pursue a research direction, pending the approval and endorsement of proper channels. It is also a ticket for a financial grant by government agencies and private institutions. The document introduces the study, its objectives, and its significance to the scientific body and society. It also includes an overview of the research methods and techniques that the researchers plan to perform, the project timeline , as well as a breakdown of possible expenses that will be incurred during the conduct of the study. You don’t start from scratch when you get a copy of the Research Project Proposal Template we’ve included above.
3. Brief Research Proposal Template

The document is a summary of the study. The problem statement and the methodology used for the research must be clear. The researchers should have a general idea of how their study will be conducted. This will be evident in the methods proposed. The research methodology and the schedule of activities should be feasible and realistic. This means that the materials, equipment, and facilities are available for intended use, and the results are obtainable at an expected deadline. When needed, you can resort to a Brief Research Proposal Template with customizable content, as shown above.
4. One-Page Proposal Template

Size: A4, US
One of the essential elements in a clinical research proposal should be a well-written and timely research question that your study will focus on. Your objectives should be aligned with your thesis statement . In the same manner, your methods should closely follow the study’s objectives. The proposal is an outline of your strategy. And like any strategy, there should be thorough planning before the execution. The document will guide you while conducting research. You will be able to monitor your progress and if you have answered your problem statement and objectives. Gather all these important elements in one short research proposal. Luckily, you may use the One-Page Proposal Template we’ve embedded above. Try it out now!
5. Nursing Research Proposal Template

A research proposal should indicate the background of the study. It should also include a review of related literature about the problem that you plan on addressing. The literature review should point to previous significant works that the researchers would use as a reference citing in their study. This applies when creating a nursing research or case study too. With the help of the Nursing Research Proposal Template shown above, crafting an informative and accurate research proposal while in nursing school will be easier.
6. Free Clinical Research Proposal Sample

Size: 232 KB
A good research proposal , like this sample, provides the reader with a research problem that talks about a current issue, the realistic goal of your research, and a well-explained methodology that will allow other researchers to replicate and verify your study. The specific amount and measurements, for example, should be indicated in your procedure. You must report the statistical analysis that you will perform on your data. Your statistical analysis will influence your results. Therefore, the reader must be informed on how you will treat your data. If you do not include a literature review, present a list of credible references that you will be using throughout your study.
7. Research Proposal Example

Size: 102 KB
For your reference on what makes for a compelling research proposal, you can download the sample Research Proposal Example embedded above. It is made by a person who qualified for the research program of the Mississippi State Department of Health. The document features a title that catches the attention of the reader. Because a person reads the title first, it can create an urge for the reader to keep reading what the writer has to say. The more content is read, the higher the chance that the proposal can sway support to your side. The research problem is relevant to the medical field and society, and the writer explains why it should be addressed. The writer expressed his or her plan on how the study will be performed. You can model your research proposal after this sample if you intend to try for a research program. Take note of the proposal guidelines for other agencies, though, as the requirements may be different.
8. Blank Research Proposal Template

Size: 114 KB
Now that you are ready to prepare a convincing research proposal, you can use the Blank Research Proposal Template featured above. It guides you on what to include in the document. You can look for a current issue or problem in the news. Identify a field or a broad topic you wish to study, and read on scientific journals about it. As you read, you can find gaps in the literature. You can also refer to the recommendations in some research articles for help in your next research topic. You can create a flow chart along the way so you will have a guide on your project. Remember the tips you have learned in this article and incorporate them into your proposal.

Proposal Maker
Text prompt
- Instructive
- Professional
Generate a proposal for a new school recycling program
Compose a proposal for a school field trip to a science museum.
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
NCCIH Research Blog
Targeting and developing your clinical research proposal.
May 4, 2018

Wendy J. Weber , N.D., Ph.D., M.P.H.
Branch Chief
Clinical Research in Complementary and Integrative Health Branch
National Center for Complementary and Integrative Health
View biographical sketch
If you’re planning to apply for an NCCIH grant for research involving human participants and will be attending the International Congress on Integrative Medicine and Health (ICIMH) , this Pre-Congress Workshop may be for you: “Honing Your Clinical Research Proposal for NIH and NCCIH Funding Opportunities.” It’s scheduled for Tuesday, May 8 from 2 to 5 p.m.
The symposium will cover NCCIH’s human subjects research priorities, available funding opportunities available, and review the significant recent changes to the National Institutes of Health’s (NIH) clinical trial policies, application forms, and review criteria. (Blog posts about those changes were published in May and September , 2017.)
Speakers will include:
- Dr. Emmeline Edwards, Director of NCCIH’s Division of Extramural Research (DER), will provide an overview of NCCIH’s strategic priorities for human subjects research.
- Dr. Wendy Weber, Acting Deputy Director of NCCIH and Chief of the Clinical Research in Complementary and Integrative Health Branch in the Center’s Division of Extramural Research (DER) will speak on NIH’s recent policy changes for clinical trials.
- Dr. Dave Clark, a program director in NCCIH’s Clinical Research Branch, will talk about NCCIH research funding opportunities focused on clinical outcomes.
- Dr. Wen Chen, NCCIH’s Acting Branch Chief for Basic and Mechanistic Research, will talk about NCCIH’s mechanism-focused clinical research funding opportunities.
- Dr. Martina Schmidt, Chief of NCCIH’s Office of Scientific Review, will explain the changes in NIH’s grant application forms and how they affect the review process.
After the presentations, participants will split into small groups for discussion and Q&A on how to interact with NCCIH staff to find the best funding opportunity for the research you want to conduct, how to shape your aims to be responsive to NCCIH funding opportunities, and what to include in your application to address the review criteria. Small group themes will be (1) clinical outcomes research and (2) human basic and mechanistic research.
We hope you will join us! We think you’ll find this session very informative, and the panelists look forward to answering your questions.
Tags: Meetings
Comments are now closed for this post.
Subscribe to the Blog
Get New Blog Posts by Email
Search the Blog
Leveling Up: Using Multilevel Interventions To Address Whole Person Health Jennifer Baumgartner, Ph.D. March 25, 2024
Change Is in the Air for RPG and NRSA Grants Jessica McKlveen, Ph.D. March 1, 2024
NCCIH-Supported Research Advances Understanding of How the Brain Clears Waste Inna Belfer, M.D., Ph.D. February 28, 2024
Grant and Research Policies (33)
Grant Application Review (17)
Grant Writing (31)
Intramural Research Program (1)
Lectures (20)
Meetings (68)
Military and Veterans (5)
Mind and Body Practices (15)
Natural Products (27)
NCCIH Advisory Council (20)
NCCIH Research (72)
NCCIH Staff News (13)
NIH News (12)
Notice of Funding Opportunity (NOFO) (67)
Research Priorities (44)
Research Results (5)
Research Training (26)
Strategic Plans (13)
Video Blog Posts (3)
NIH Director's Blog
NIGMS Feedback Loop Blog
Inside NIA: A Blog for Researchers
NIMH Director’s Blog
NIDA Directors’ Blog
I Am Intramural
Frequently Asked Questions (FAQs)
Comment Policy
Privacy Policy
System Status:
- Sponsored Research Administration
- Preparing Proposals
- Agreement Types
Clinical Research and Trials
Find links to information on Clinical Research and Trials.
A clinical trial is most often used in conjunction with obtaining new drug (IND) or device (IDE) approval from the U.S. Food and Drug Administration, although they can be designed with the sole purpose of collecting and analyzing data about an approved drug or device in order to contribute to medical knowledge about the treatment of a disease or medical conditions.
UC Definition
The controlled, clinical testing in human subjects of investigational new drugs, devices, treatments, or diagnostics, or comparisons of approved drugs, devices, treatments, or diagnostics, to assess their safety, efficacy, benefits, costs, adverse reactions, and/or outcomes. Such studies may be conducted under an industry-developed protocol or an investigator-developed protocol. This clinical trial definition is used for calculating indirect cost (IDC) Rate for industry-funded Clinical Trials.
National Institutes of Health (NIH) Definition
A research study in which one or more human subjects are prospectively assigned 1 to one or more interventions 1 (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes . Refer to a detailed breakdown of this definition here: NIH's Definition of a Clinical Trial .
Download a copy of the Clinical Trials quick guide
Submitting Requests
Create a Kuali Research Record : For tips and guidance related to entering various agreements into the enterprise system of record.
Kuali Research Systems Training : To register for various eCourses and Virtual Instructor Led training related to the various Kuali modules and to access the Kuali Research Training Guides.
Additional Resources and Help : To get more information on various resources, search the knowledge base and how to contact a Research Administration Client Experience agent.
NIH Clinical Trials
Find information and links on the changes and clarifications by NIH regarding clinical trials definition and regulations.
The National Institutes of Health (NIH) launched a series of initiatives that rolled out in 2017 - 2018 to enhance the accountability and transparency of clinical research. These initiatives target key points along the whole clinical trial lifecycle from concept to reporting of results. These initiatives include:
- Clarification of the definition of clinical trials (effective January 1, 2018)
- Good Clinical Practices training for clinical trials (effective January 2017)
- Clinical trial specific funding opportunities (proposals due on or after January 25, 2018)
- New PHS Human Subjects and Clinical Trials Information form (proposals due on or after January 25, 2018)
- Single IRB for multi-site research (effective January 2018)
- ClinicalTrials.gov registration and reporting requirements (effective January 2017)
For additional information, see:
- NIH Clinical Trial Requirements for Grants and Contracts page
- NIH video Overview of New NIH Policies on Human Subjects Research and Clinical Trials .
- NIH Clinical Trials FAQs page
- NIH Clinical Trial-Specific Funding Opportunities page
- NIH Clinical Trial-Specific Review Criteria page
- NIH "Open Mike" article "4 Questions for researchers involved with Human Subjects Research"
- NIH "Open Mike" article "Continuing to Clarify the NIH Definition of a Clinical Trial"
- NIH "Open Mike" article "Be Careful to Pick the Right Funding Opportunity Announcement (FOA)"
UC PI Authored vs Sponsor Initiated Protocols
Clinical Trial studies may be conducted under a sponsor-initiated protocol or a UC principal investigator-initiated protocol .
In general, a clinical trial is considered PI-initiated when the protocol is authored by:
- UC investigators within the course and scope of their University employment, or
- UC investigators within the course and scope of their University employment jointly with an employee of another entity (e.g., an employee of another non-profit institution, an employee of the industry sponsor, etc.)
Reporting Requirements to ClinicalTrials.gov
The Research Integrity and Compliance (RCI) Office is responsible for ClinicalTrials.gov Registration and Reporting Requirements. Refer to Research Compliance and Integrity Resource information to learn more about the CT.gov public registry aimed at increased transparency and improved public awareness of research.
Who Do I Work With?
Compliance offices supporting clinical research and trials.
Office of IRB Administration (OIA)
The Office of IRB Administration serves as the advocate for the rights and welfare of persons who participate in research programs conducted by UCSD faculty, staff, and students. For more information, please visit the OIA page.
Health Data Oversight Committee (HDOC)
The UC Office of the President has established a formal process to actively manage requests to share UC health data with third parties. The policy requires a UC Health campus committee to handle data-sharing requests according to UCOP guidelines. The UCSD Vice Chancellor of Health Sciences has established the UCSD Health Data Oversight Committee (HDOC) to perform this function. Please see the HDOC page for more information.
Conflict of Interest (COI)
COI applies to clinical trials as it does to all sponsored research at UC San Diego.
For more information, go to the COI Office page.
PI Eligibility
The PI Exception process is also the same as it is for all sponsored research at UC San Diego.
For more information, go to the PI Exception page.
Office of Coverage Analysis Administration (OCAA)
Most clinical trials require a coverage analysis to determine what services are billed to insurance, billed to a sponsor, or billed directly to the patient. For more information, contact OCAA .
Export Control Office
Export Control identifies and manages export risks and provides export licenses in support of the research activities of university faculty, staff, and students. For more information, contact the Export Control Office .
Government Agency Resources
- Overview of New NIH Policies on Human Subjects Research and Clinical Trials (NIH.gov)
- NIH Clinical Trial Requirements for Grants and Contracts (NIH.gov)
- HIPAA - Health Information and Privacy Information (HHS.gov)
- Food and Drug Administration (FDA) Page on Running Clinical Trials (FDA.gov)
UC BRAID Tools
The University of California Biomedical Research Acceleration, Integration & Development (UC BRAID) developed tools to support clinical research at the University of California. For more information, please visit the UC Braid Developing Tools page.
Budgeting and IDC
Depending on the sponsor, clinical trial budgets can look very different. Budgets might be based on a (1) capitated, per-patient basis or (2) include major cost categories similar to a sponsored research project.
Please work with your business office and the sponsor to develop your clinical trial budget.
Budget Best Practice Questions
- Are your Per-Patient Costs sufficient?
- Will coverage analysis be required? Contact OCAA for more info.
- Are you receiving enough working capital up-front to cover the start-up costs of the study?
- Does the budget include all the necessary items to conduct the clinical trial (e.g. applicable IRB fees)?
- Does the Schedule of Events match the Payment Schedule in the budget?
- Is there a Hold-Back Provision for the final payment? Can this be prorated if the Payment Schedule is milestone-based?
IDC Rate for Clinical Trials
- Industry-funded clinical trials, both UC PI-initiated and Sponsor-initiated(initiated by a non-UC party), are subject to the clinical trial indirect cost rate of 33% on total direct costs (TDC), including the total cost of all subsites, for agreements effective January 1, 2024.
- Non-profit funded clinical trials are subject to the nonprofit sponsor policy for IDC. If a nonprofit sponsor policy does not exist, the federal negotiated rate applies.
- Federally funded clinical trials, including subawards, are subject to the applicable federal negotiated rate.
The applicable IDC rate is based on the primary funding source and such rate is not altered should UCSD be serving as a subsite. The University cannot subsidize budgets for industry-funded clinical trials and budgets must cover the entire cost of conducting the clinical trial, including the applicable institutional costs (e.g., IRB fees) and indirect costs.
For more information a list of UC San Diego’s IDC rates and a copy of UC San Diego’s Federally negotiated rate agreement please visit the Indirect Costs page.

ACTRI Services
The Altman Clinical and Translational Research Institute (ACTRI) provides clinical research services. Please see their Center and Services page for services provided by ACTRI and recharge rates.
Applicable IRB fees must be included in the budget. Please see the Office of IRB Administration (OIA) IRB Review Fees page for current fees to be included in the budget.
Contract Negotiation
Due to the complexity and high-risk nature of clinical trials, contracts must address a substantial number of areas such as intellectual property rights, publication and/or data rights, material transfer, indemnification, subject injury, confidentiality, and regulatory requirements. Review by other compliance offices are often required. As a result, contract review and negotiations can take additional time while the Sponsored Projects Office works with the sponsor to arrive at mutually agreeable terms.
For more information or questions email [email protected] .
APPLY FOR FUNDING
Examples of grant recipients, board of trustees, apply for funding: clinical research proposals, application process & checklist of required attachments.
- Grant Application Cover Sheet
- Proposal Narrative Guidelines
- Semi-Annual Report Form
- ›› Physician and Public Awareness Proposals
- ›› Community Philanthropy Proposals
- ›› Douglas/Schwartz Annual Emerging Investigators Research Award
- ›› Proposal Evaluation Criteria
- ›› Apply Online
- Complete the Clinical Research Proposal Cover Sheet , providing all requested information
- Write and provide a Proposal Narrative
- Provide the Curriculum Vitae of PI (NIH grant application format)
- Provide a list of current board members, officers and staff, as well as who will be involved with the project, and any conflict of interests
- Provide proposed program budget
- Proof of federal and state not-for-profit status (IRS tax exempt determination letter and EIN Federal tax ID number)
- If applying from outside of the United States, proof of not-for-profit status must be attached to proposal along with tax identification number
- Provide IRB approval information
Grant Proposal Deadlines
Grant proposals will be reviewed biannually, and should be submitted according to the following timeline:
Application Deadline: March 15 September 15 Decision Made By: April 30 October 31
GRANT REVIEW PROCESS
Proposals are reviewed and recommendations made to fund or decline by the Scientific Review Committee. They are then reviewed and a final decision to approve or decline made by the Board of Trustees. In reviewing a proposal, the Proposal Evaluation Criteria will be used as a guideline to determine the study's fundability.
Be sure to include all required documentation, including a detailed budget for the project. Incomplete applications will delay the approval process.
Funding Process
If the board approves funding, a formal Letter of Agreement (LOA) between the ResMed Foundation and grantee will be sent for signature. Upon receipt of two fully executed originals, the initial funding payment will be made. Subsequent payments will be made based on the progress of the study, as outlined in the Semi-Annual Reports. The Foundation reserves the right to discontinue funding if the study is not carried out in a timely manner, patient recruitments achieved and/or the study in compliance with the original grant proposal.
Semi-Annual Report Deadlines
June 15 December 15
PROPOSAL SUBMISSIONS
Kristi Burlingame Executive Director ResMed Foundation 7514 Girard Avenue Suite I-343 La Jolla, CA 92037
e: [email protected] t: 858-361-0755
Applications may be submitted via the web: Apply Online .
ABOUT US | APPLY FOR FUNDING | EXAMPLES OF GRANT RECIPIENTS | BOARD OF TRUSTEES | CONTACT US
© 2024 all rights reserved • resmed foundation • 7514 girard avenue, suite 1-343 • la jolla, ca 92037 • 858-361-0755.
Meet our latest partner - Scoopeya
19+ SAMPLE Clinical Research Proposal in PDF

Clinical Research Proposal
19+ sample clinical research proposal, what is a clinical research proposal, when writing a clinical research proposal, what should you keep in mind, elements of clinical research proposal, steps in writing a clinical research proposal, how long should a research proposal be, why write a research proposal, why must you submit a research proposal.

Clinical Research Project Proposal

Center For Clinical and Translational Science Research Proposal

Clinical Research Proposal Process Improvement Project

Clinical Research Project Proposal Form

Clinical Research Skills Proposal

Clinical Research Investigator Proposal

Clinical Research Proposal Example

Clinical Research Proposal in PDF

Clinical Research Certificate Program Proposal

Basic Clinical Research Proposal

Clinical Research Grant Proposal

Clinical Nursing Research Grant Proposal

Clinical Research Ethica Proposal

Printable Clinical Research Proposal

Simple Clinical Research Proposal

Clinical Research Grant Proposal Form

Clinical Trials Research Proposal

Clinical Research Grant Application Proposal
Clinical Research Proposal Template

Clinical Nursing Research Proposal
1. your target audience, 2. pitfalls that could occur, 3. data and research, 1. define the statement of the problem, 2. present your proposal, 3. extend your research, 4. create a schedule and a budget plan, 5. indicate conclusion, 6. verify that your proposal is error-free, share this post on your network, you may also like these articles, 21+ sample demolition proposals in pdf | ms word.

Moth and rust can destroy a building as the years go by. If we own a company and our building has stood for a long time, it will be…
13+ SAMPLE Web Hosting Proposal in PDF | MS Word

Web hosting is one of the fundamental aspects of web management for keeping a website up and running. A good, high-quality, and reliable web hosting service is necessary to…
browse by categories
- Questionnaire
- Description
- Reconciliation
- Certificate
- Spreadsheet
Information
- privacy policy
- Terms & Conditions

- Policy & Compliance
- Clinical Trials
- Protocol Templates For Clinical Trials
Protocol Templates for Clinical Trials
NIH applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:
- Phase 2 or 3 clinical trials that require Investigational New Drug applications (IND) or Investigational Device Exemption (IDE) applications
- Behavioral and social sciences research involving humans
These clinical protocol templates can be accessed via the secure web-based e-Protocol Writing Tool and as Word templates
- enables participation for multiple writers and reviewers
- allows assignments for writers and collaborators
- tracks progress and ensure document version control
Word Templates
Related notices.
NOT-OD-17-064 NIH and FDA Release Protocol Template for Phase 2 and 3 IND/IDE Clinical Trials
- NOT-OD-19-092 NIH Releases Protocol Template for Behavioral and Social Sciences Research Involving Humans
This page last updated on: July 12, 2021
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
- Open access
- Published: 27 March 2024
A database for oncological research and quality assurance: implementation and first experiences with the University Clinical Cancer Registry Regensburg
- Anna Saibold 1 , 2 ,
- Michael Koller 3 ,
- Karolina Mueller 3 ,
- Oliver Koelbl 4 ,
- Veronika Vielsmeier 5 ,
- Tobias Pukrop 6 ,
- Oliver Spies 1 ,
- Vivian Eilers 1 ,
- Cathleen Brese 7 ,
- Denise Amann 7 &
- Julia Maurer 2 , 7
BMC Medical Research Methodology volume 24 , Article number: 76 ( 2024 ) Cite this article
76 Accesses
1 Altmetric
Metrics details
Legal requirements, certification specifications, as well as the demand for real world data on cancer research and treatment led to the decision to establish the University Clinical Cancer Registry Regensburg. The first organizational step in the implementation process of this oncological data registry was the evaluation and acquisition of suitable tumor documentation and database software. For this purpose, an evaluation matrix comprising required database software criteria was designed and consented by a multidisciplinary group of experts. Next, a yearly report of the Institute for Cancer Center Certification (OnkoZert 2019) was considered to identify database software already in use. The identified systems were rated according to the established criteria matrix and other relevant aspects. Onkostar was the system considered most suited for building up an oncological data repository. In the second step, the central IT department implemented Onkostar on-premise and migrated digitally available data after an adaptation and verification process. In parallel, a uniformed process for handling emerging oncological research questions was established. For research requirements, a data analysis concept was established comprising a proposal for data extraction, procedural instructions, and statistical training materials. In the final step, the implemented software and the process for handling research requirements in practice were evaluated by using two exemplary use cases with the focus on clinic-wide analyses and currently relevant scientific topics. A 2-month test phase conducted by various user groups showed a preference for Onkostar tumor documentation software from IT-Choice, mainly because of its adjustability to support research and treatment. Newly added and migrated data can be used for certification and research purposes. This software also provides support in current tumor documentation by displaying the course of cancer disease for individual patients over time. Such oncological data registries can be a powerful tool for legally required cancer registration, the certification of medical centers, as well as for additional oncological research. Tumor databases can be helpful in projects on cancer treatment and scientific aims. The experiences made at the University Hospital Regensburg may be used as a guidance for implementing clinical databases in similar settings with interdisciplinary responsibilities.
Peer Review reports
While the number of annual primary cancer diagnoses has been constantly rising in past decades, survival rates have been drastically improved through new methods of early detection, diagnostics, and therapy [ 1 ]. The German National Cancer Plan (Nationaler Krebsplan) [ 2 ] has identified four fields for further improving the situation of patients with cancer that include early detection programs, further development of oncological structure supply, quality management, and assurance of efficient oncological treatment with a special focus on patient-centric approaches [ 1 , 3 ]. In each of these fields, the structured digitalization of oncological data is crucial. Therefore, the newly founded University Clinical Cancer Registry (Universitaeres Klinisches Krebsregister Regensburg, UKKR) set up a database to document all oncological patients treated at University Cancer Center (UCC-R) at the University Hospital Regensburg (UKR). The UCC-R forms the clinical arm of the Comprehensive Cancer Center Ostbayern (CCCO), which as a scientific and clinical facility is the central coordinating institution for interdisciplinary oncological patient care, research, education and training in Eastern Bavaria. As an oncological center of excellence according to the German Cancer Aid Society (DKH) and a maximum care hospital (> 800 beds, 31 departments and institutions), the UCC-R treats all cancer diagnoses (approx. 22,000 patients per year). The CCCO, as a partner in the networks CCCWERA (Comprehensive Cancer Center Allianz Würzburg, Erlangen, Regensburg, Augsburg) and BZKF (Bayerisches Zentrum für Krebsforschung) with its partner sites, is responsible for disseminating its expertise throughout the entire network and thus to peripheral hospitals and oncologists in private practice.
An oncological database has to adhere to pertinent legal requirements. The Bavarian Cancer Registry Law (BayKRegG) [ 4 ] requires clinical cancer sites to transmit the pre-defined oncological basis dataset (oBDS) [ 5 ] to the Bavarian State Office for Health and Food Safety (LGL) within 2 months after the diagnosis of a cancer disease. The nationwide implementation of clinical cancer registries is aimed at improving cancer treatment. Additionally, the Bavarian Cancer Registry Regulation (BayKRegV) [ 6 ] further specifies obligatory transmitted data as oncological basis data, discharge information, and organ-specific data. This regulation also states that the transferal needs to be done electronically or at least in digital format. On top, Book V of the Social Insurance Code (SGB V) § 65c [ 7 ] article one indicates that the mandatory registration of cancer disease and its modules has to be done in form of the oncological basis dataset [ 8 , 9 ].
For the purpose of certification, the oncological basis dataset can be expanded with additional information that is required in the annual certification process. Such required information may be newly diagnosed tumor disease, cancer recurrence, and metastasis. The Bavarian Hospital Law [ 10 ] regulates the local collection and analysis of the data of patients treated at a hospital. All additional parameters are transferred in anonymized form and aggregated at the certification institution OnkoZert.
Further data need to be stored for certain research purposes [ 9 ]. Any value obtained during cancer treatment may be correlated to treatment outcome in some way. Therefore, it is important to store all additional values together with the minimum dataset in the same database, although these additional values will not be transferred to any national institution. Such storage allows proper data extractions and analyzes to answer research questions and to potentially improve cancer treatment. Research is also legally covered by the Bavarian Hospital Law. The article 27(4) regulates the opportunity for employed practitioners in Bavarian hospitals to use the data available in local hospital databases for intern research projects. The researchers are responsible for the ethically correct and data-protection compliant handling of the data [ 10 ]. The necessity of additional data for research and treatment as well as for changing legal requirements led to the decision to establish a registry in Regensburg. The main objectives were (1) fulfilling the legal requirements of cancer registration and certification, (2) complying with the data protection regulation, (3) optimizing data quality through standardization, (4) accelerating documentation, (5) supporting analysis processes, and (6) improving oncological research in particular by improving data interoperability with other subsystems [ 3 , 8 , 11 ].
The aim of this paper is to describe the process of specifying criteria for an oncological database platform, the selection and implementation process, as well as the design of using cases together with a statistical analysis concept. This paper also includes a few recommendations for institutions interested in engaging in a similar process that are based on the made experiences.
Material and methods
Selection of appropriate database software.
First of all, a working group was established with selected persons from the fields of clinical cancer registration and information technology to lead the project. The project plan included the steps (1) selection of software, (2) implementation and data migration, and (3) development of a data analysis concept (Fig. 1 ).
Flow chart—implementation and first experiences with the University Clinical Cancer Registry Regensburg
The first organizational step in implementing an oncological data registry was the evaluation and acquisition of an appropriate database together with a software program suited to tumor documentation and registration. Many scientific aspects, for instance, standardization, analysis procedures and data quality, as well as technical aspects such as infrastructure compatibility and data security need to be considered from the very beginning [ 12 ].
The yearly report of the Institute for cancer center certification (OnkoZert 2019) was used as a source of potential database software for tumor documentation. To find a database and state of the art software with high usability, sustainability, as well as adaptability, establishment criteria were designed and consented to by an interdisciplinary group of experts (oncologists, information technologists, health care research scientists, data protectionists, data analysists, and UKKR staff) within the UKR. The considered software products were evaluated according to the above criteria.
The required criteria were divided into the following superordinate categories:
Content-related requirements regarding legally required tumor documentation and certification, i.e. interface of forms as well as data analysis and harmonization with cooperation partners,
Technical and functional requirements including hardware and software components as well as usability and security aspects, i.e. user management/access rights, interoperability, and data structures, and
Supportive and financial requirements referring to personnel and license, i.e. maintenance, training, and human resources.
All required evaluation criteria are described in detail in the evaluation matrix established specific for this project (Fig. 2 ) and structured into the above-mentioned content-related categories. Each criterion was rated regarding its priority (can = 1; should = 2; must = 3) and degree of fulfillment (not = 0; partly = 1; complete = 2). The product of priority multiplied by fulfilment was calculated for each criterion and a summary score across all criteria was determined. The maximum summary score to be achieved was 382.
Criteria for evaluating tumor documentation software and evaluation matrix
Implementation and data migration
After the most suitable product for building up an oncological database was found, the implementation of hardware and software was started. Virtualized servers were essential for establishing the tumor database and its software into the existing clinical system environment at the UKR (Fig. 3 ).
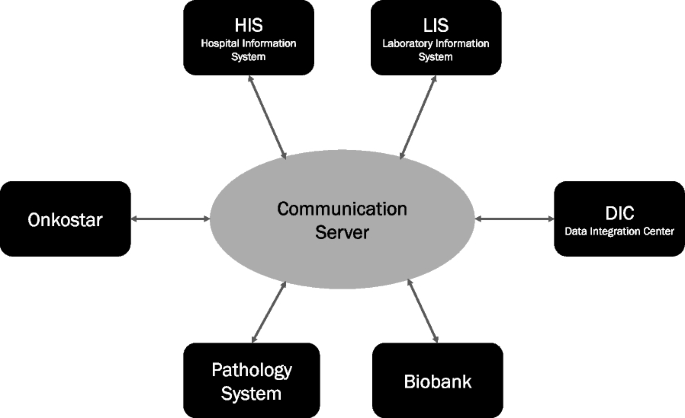
Clinical system environment at UKR
The IT department, which is located at the university hospital including specialized representatives for oncological projects, implemented all necessary components, assured the security and provided the application as a web-interface from every internal clinic computer.
For completeness, all digitally available data needed to be migrated into the newly implemented cancer registry database. The data migration process also needed to maintain the correctness of the information. A first set of patient data (approximately n = 60,000) was imported from an existing database that no longer fulfilled the requirements described in the introduction. All oncological data (as required for legal and certification purposes) of patients with a new diagnosis of cancer including therapy, follow-up, and further supplementary data has been documented since January 2021.
Data analysis concept and first use cases
To get an overview of required or requested projects and necessary adaptations at UKR, demands were informally assessed at all organ-specific cancer centers prior to 2021. Collected data included information about tumor documentation, tumor boards, and other clinic-specific requests, such as molecular pathological documentation beyond the oncological basis dataset.
During the finding period of possible projects, the necessity of a uniformed process for analyzing clinical oncological data of the comprehensive cancer center was recognized. This necessity was indicated on the one side through the rising number of research questions in the field of oncological data and analogously through the rising number of projects and scientific work in this field. Furthermore, the documentation process in general (including accountabilities and responsibilities) and the necessary entity-specific documentation [ 13 ] for legally required registration and certification are explicitly regulated by Standard Operating Procedures (SOPs) and are documented in a collaborative knowledge management system that should enhance quality. This system is not part of this paper.
To evaluate the previously elaborated steps and tools in practice, two exemplary use cases with the focus on clinic-wide analyses and currently relevant scientific topics were selected. The evaluation should show the usability of the selected software with its functionalities and adaptations, data migration, and the data extraction process to researching clinicians.
Use case 1 related to the impact of covid-19 pandemic on oncological treatment as part of a retrospective analysis of data of the University Cancer Center Regensburg (UCC-R) in Germany.
Use case 2 dealt with the topic of laryngectomy plus postoperative radio(system)therapy versus primary radio(system)therapy for the treatment of locally advanced laryngeal and hypopharyngeal cancer and presented the results from the University Clinical Cancer Registry Regensburg.
In March 2020, a set of nine available tumor documentation software systems (n = 9) was extracted from the yearly report (2019) of the Institute for Cancer Center Certification (OnkoZert). Of these nine systems, only commercial database software systems used in more than 50 organ centers in Germany in addition to the currently used software were considered (n = 6).
For the remaining six software systems, termed software A–F in the following text, the evaluation matrix was filled out by a group of representatives with a clinical, technical, or governance background (Fig. 2 ). Each company of the considered software systems was contacted, and the evaluation matrix was sent prior to the interviews. The interviews were conducted face-to-face in online meetings together with representatives of the software companies and employees specialized on IT applications and system-interfaces. All criteria were discussed one after the other along the evaluation matrix. In parallel, reviews on the different products and databases from other hospitals were obtained.
The software systems E–F did not meet all mandatory criteria and were thus excluded from further consideration. Although software C showed 341 points, it was not considered in the extended evaluation because it lacked the feature of creating own forms; therefore, adjustability was not given. Both, software A and B showed 343 points in total.
In May 2020, a decision was made to further investigate the two software tools A and B by installing test implementations. Additional relevant aspects were formulated.
The most relevant aspects in choosing between these two software systems were information on data input, highlighting of mandatory fields, and plausibility checks. Of similar importance was a clearly structured (especially in case of more than one tumor) and adaptable user interface as well as adaptable selection catalogues (i.e. therapies, physicians, etc.). Additionally the possibility of data input and analysis according to specifications from the DKG (OnkoZert) for all certifiable centers, modules, and specializations (Oncobox, certification figures, etc.) were considerable. A further relevant aspects was the fulfillment of legal requirements regarding the cancer registry law (documentation and registration of the oncological basis data set) including a technical link to the registry portal of LGL and pediatric cancer registry. On top of that, the focus was on the support of scientific analyses in form of structured and clear data extraction (with filters and adjustable to projects) and the possibility of exporting such data and import information such as entries from a previous tumor database. Important aspects were also the organization and accomplishment of tumor boards (together with external cooperation partners) as well as the possibility of archiving and deleting cases and storing consents. The system should also fulfill the technical requirements to be implemented in the existing IT infrastructure (e.g. web-based, industry standards, etc.) and should have user and access management, ideally via LDAP. Further considerations included the potential for on-going development in consultation with the users from UKR. A 2-month test phase by various user groups, mainly tumor documentation staff and IT employees, confirmed the preference for Onkostar tumor documentation software and database from IT-Choice. The main reasons for this preference in addition to the evaluation score were (1) the good user experience with the user interface, (2) the adjustability of own forms, and (3) the recommendations from other university hospitals and cancer registries in Germany.
Onkostar (software tool A) was considered the most suitable software for building up an oncological data repository at the UKR because of its high score in the evaluation matrix and the provision of the above-mentioned additional relevant aspects.
The first priority was the fulfillment of legal requirements and the registration of relevant data for the required tumor entities by enhancing accurate data input. Data necessary for legal purposes need to be exchanged with superordinate cancer registries in Germany. The structure of the dataset and the interface for exchange of XML files is nationwide standardized for full interoperability. This standardized interface allows direct exchange between different institutions as well as between different specialized software systems and allegorizes interoperability with regard to data protection regulations. These features are the basis for unified oncological documentation and comparable data collection and analysis [ 14 ].
Onkostar was implemented by central IT department as virtualized servers on-premise. The implementation included one testing and one productive environment, protected particularly by firewalls. The software is accessible to users as a web-interface from each clinic computer via secure hypertext transfer protocol (https). The underlying database structure is a common SQL database on the central database server.
The software interface allows (1) data input, (2) validation, (3) automation, and (4) adaptation. The graphical interface consists of various forms containing certain fields. The entered data can be automatically validated by a plausibility check or by a final validation of the XML file against the pre-defined schema. Such validation exposes missing mandatory fields, invalid entries, or unusual correlations. Automation in the documentation process can be achieved through digital interfaces with systems from outside; the clinical information system, for example, is directly connected to the oncological data repository via HL7 V2 technology. Basic patient information as well as operational procedures are transmitted and stored automatically. Getting information from a proprietary system imported to the database can be done in a semi-automated manner using the import functionality. If important information, for example for specific research, cannot be stored in the available forms, adaptations can be elaborated. Values and catalogues can be added and clustered in sub-categories, while existing forms can be supplemented with additional features. Completely new forms with individual fields can also be developed.
There are three main user groups: (1) documentation team, (2) selected clinical employees, and (3) administrational staff. In January 2021, the user groups were trained in using the software. Over time, minor modifications in authorizations were adapted. The software is primarily used by the documentation team at UKR for inserting and updating data in the data repository. Selected clinic employees can be authorized to access data records relevant to them, i.e. specific forms or projects, but modification of content is restricted to the first user group. The third group, administrational staff, supports the other user groups. Administrational staff may be employees of the IT department and the quality assurance team, who support with technical, content, and process-related input. Administrational staff have unrestricted access to the documentation system as well as to the database.
After the implementation of the database, the data stored in the database operated at the former Tumor Center Regensburg e.V. were migrated. Because of the similarity of the databases, certain data could be transferred automatically [ 15 ]. Reasons for data migration were (1) support of the certification process, (2) support of research requests, and general (3) quality assurance. Data were migrated in several migration rounds with structural and manual adaptions. Preparations for the migration process were the analysis of database structure. After final verification of the correct format, all records were loaded into the system.
Adjustments were done on a structural and a contextual level. Necessary structural adaptations were, for example, mapping medical departments and wards to the current prevalent naming conventions as well as assigning the various tumor boards to the different oncological entities according to the ICD Code. The assignments to the oncological centers were done on the basis of the date of foundation and the ICD code. Another necessary structural adaption was completion of missing patient IDs by a systematic search for the name, surname, and birth date in the clinical information system and partially by manual correction and the aggregation of cases. To achieve the highest possible quality of data, the content of the importable entries was reviewed and adjusted at some points. There were several mappings of items, for example, unifying names of substances, studies, chemotherapy protocols, and the assignment categories (chemotherapy, immunotherapy, targeted therapy, hormonotherapy, and others) of substances. Another example is the mapping of the type of pathological preparation to a new convention. Some data were not imported into the new system, either because of the high effort to map those data or due to the low quality of the existing data. For operations longer than 1 year ago, only relevant information was imported, to keep the database lean. Names of the surgeons as well as referring hospitals and physicians were therefore not imported. Only selected laboratory parameters (i.e. tumor markers) and therapies with connection to oncological treatment were imported. For a few data items, all entries had to be reviewed manually and edited carefully. Special therapies (i.e. RFA or TACE) were assigned to forms, and psycho-oncological and social service organizations needed to be allocated to the primary tumor for patients with recurrent cancer.
Since 1992, all digitally available tumor information has been stored and made accessible in the oncologic data registry. Comprehensive and complete data have become gradually available together with the development of various organ cancer centers over time.
A data analysis concept was developed for planning and conducting oncological research within the local and legislative regulations, including professional assistance in the field of data analysis. For that purpose, central documents were established in cooperation with the Center for Clinical Studies (Zentrum für Klinische Studien, ZKS) as guideline and support for clinical researchers.
The first established document is a proposal for scientific data extraction from the UKKR. The template of the proposal starts with general information such as the title, declaration of the responsible person/department, purpose of the proposal (retrospective/prospective), and the type of requested data extraction (aggregated/anonymized/pseudonymized). Followed by a detailed description of the requested data (i.e. selection criteria, data load, aggregation criteria, etc.) together with the description of necessary support for statistical analysis (e.g. estimation of number of cases, introduction into statistic software, specific analyses, and support in publishing). A central component of the proposal is a description of the planned project in form of a study synopsis with the following aspects: the primary aim of the study, secondary aim(s) of the study, the study type/design, the population of interest, inclusion/exclusion criteria, the number of patients, primary and secondary end points, biometry, timeline, planned publication, involved departments, and responsible persons from the clinic. The proposal informs the applicant about other relevant valid documents concerning data protection regulations. These documents need to be acknowledged by signature of the applicant. The last paragraph provides space for remarks and feedback from the (UCC-R and the ZKS towards the applicant. Each person involved as well as their tasks and responsibilities are written down together with the finally extracted dataset and any kind of agreed statistical support.
In parallel, a document with procedural instructions was introduced at UKR to support the completion of the above-mentioned data extraction proposal and to concisely display the entire process. The document implies the objective and purpose, normative references, responsibilities including contact information, and the process from the application to the response with the dataset and optional statistical supervision.
To enhance statistical quality of research, training material for descriptive statistics and ordinary analyses was prepared. If required, clinicians can consult a qualified statistician for statistical support.
The concrete steps from the perspective of health care researchers and employees of the cancer Registry and the ZKS are depictured in Fig. 4 .
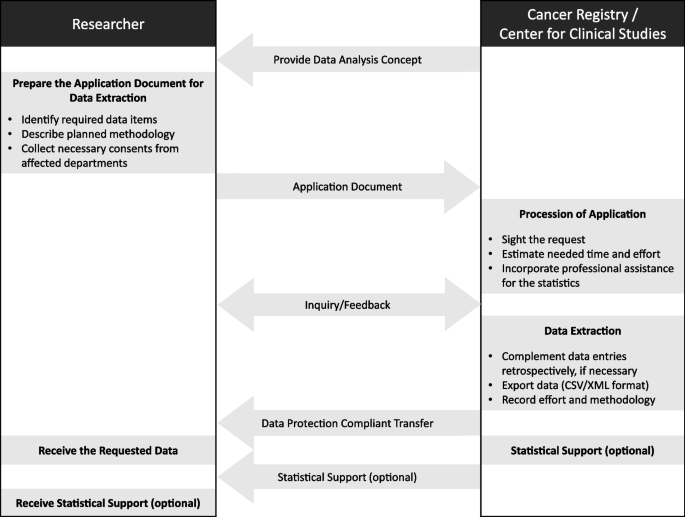
Utilization of the data analysis concept
The two use cases differed in data basis and period of time, which shows the broad range of possibilities of the available database and its contents. Tables 1 and 2 summarize the most important characteristics of the use cases and the associations to previously described topics (selection of database, implementation and migration, and data analysis concept).
First empirical experiences with the data extraction process have shown good comprehensibility of the developed documents without any major obstacles, although shortenings may be possible to avoid redundancies. First extractions revealed incomplete patient data from the years before the implementation of the new oncological cancer registry, which required retrospective manual completion, especially for patients who had not been subject to certification. In order to improve prospective data quality and transparency, the process of documentation is managed in great detail in a collaborative knowledge management system. For certain project-based requirements, forms in Onkostar can be extended in order to be able to record additional items prospectively.
First feedback from scientists using the process rated the high transparency as positive and considered the possibility of statistical support through training materials and contacts in the central study center a benefit.
A summary of the above-mentioned project plan in form of a resulting workflow over time is shown in Fig. 1 . This workflow can be used as a reference for project management in comparable implementations in respect of the necessary steps, their timeline, and the relevant specialists.
Starting point of the selection process was a self-created evaluation matrix that proved to be a very practical tool. Primarily, the classification of individual items into the above-mentioned item groups enabled the responsible groups to prepare and work through the individual items in a structured and targeted manner. Furthermore, this classification ensured the inclusion of all essential items in the selection process with high comparability. The matrix also served the software companies as relevant tool, particularly with regard to the preparation for the interviews. Another positive aspect of the matrix was the prioritization of the individual items that enabled compromise solutions for individual items (especially for optional and, in some cases, target criteria).
All those aspects not only allowed fulfilling the content-related requirements but also helped keeping to the scheduled timetable.
When deciding on the most appropriate database software, there was a special focus on the adaptation of the product to meet the needs of the oncologic center of excellence. Primarily, the possibility of adjusting existing selection catalogues and of adding new field catalogues as well as customized forms played an important role in decision-making with regard to future scientific requirements. Particularly one software company showed the potential and willingness to further develop their product in collaboration with users from UKR in a detailed and reliable manner.
Issues occurring during or after data migration were mainly structural differences between the databases. Therefore, it was not possible to incorporate all data items the same way, which would have led to a loss of data to a certain degree.
In this respect, some item packages needed to be migrated in an entity-specific manner in several migration rounds, hence in a very time-consuming process. Furthermore, such item packages often needed to be worked manually. The reasons for this problem were, for example, unidentifiable patients, difficulties to transform data (i.e. laboratory data), or the active decision to leave data out (e.g. external practitioners due to data protection issues). In summary, the migration process was highly complex and took almost 18 months, much longer than the initially expected 3–6 months.
To enhance data quality in the future, entries were validated manually, for example, samples from all organ centers were taken and monitored. For this reason, the processes for data collection and documentation were adjusted to improve available research data in the long-term. Concrete examples are the above-mentioned use cases, which required the retrospective documentation of missing data. The processes may be adjusted continuously to fulfill the aim of constantly increasing data quality.
The newly added and migrated data can be used for certification and research purposes. The data can also be supportive in current tumor documentation as they display the course of cancer diseases for one single patient over time. Making high quality tumor data available to researchers offers an invaluable benefit to research projects [ 13 ].
The implemented database as well as the collected and verified data in the cancer registry can be used for data analysis and research requests, for example, to better interpret data and improve clinical performance [ 16 ]. To improve oncological research supported by a database and professional assistance in the field of data analysis and statistics, a data analysis concept for planning and conducting oncological research was developed in Regensburg. A uniformed process is needed to ensure a valid data source and therefore valid statistics. This process should increase the analysis of oncological data in the hospital as well as in collaborations, leading to more publications with higher transparency regarding methods, participants, and data sources. Furthermore, a uniform process may promote the publication of study results and improve the transparency of project responsibilities and planned statistics. The quality of clinical epidemiological projects can benefit from statistical consultation.
As mentioned in the results section (Tables 1 and 2 ), certain data items were identified for both use cases. These data items needed to be emphasized in ordinary prospective tumor documentation, even though they are not part of legal cancer registration or required for certification of centers. For these two projects, it was necessary to retrospectively complement data entries. Data migration did not provide any obstacles in either study and can therefore be assessed positively. The application document for data extraction was usable in both cases, although minor changes were made for more user-friendliness. In respect to the required statistical support, the documents were sufficient because the focus was on descriptive statistics and analysis of correlation. Multivariate analysis was supported by the ZKS.
Future prospects
Implementation of further projects to enhance the processing and quality of patient data in cancer centers and hospital departments is intended.
As already mentioned, the first step of the process was to determine the requirements of the different departments with regard to improving tumor documentation and the interconnectivity between information systems. Therefore, content-related adaptations (e.g., the addition of specific items) are planned as well as the connection of systems, including structured data import from the clinical information system on the one hand and attachment of the tumor boards on the other hand. Tumor boards face both obligations as well as opportunities, i.e. data organization and documentation on a daily basis but also improved quality in patient care through the complementation of data entries by means of already available information [ 9 , 11 , 12 ].
To further improve the quality of the database, implementation of a module for patient-reported outcome (PRO) is initiated and its expansion arranged. Relevant examples of questionnaires are palliative screening (i.e. IPOS questionnaire) and quality of life based on a standardized set of questions (e.g., EORTC assessment tools). Naturally, synchronized feedback is transferred to the primary clinical information system. The interconnectivity of data together with clinical parameters not only results in tremendous opportunities for the treatment of patients in clinical routine but also for research [ 9 , 11 , 12 ].
High compatibility and interoperability of data can contribute to the success of internal and external research projects. One ongoing local project is the digitalization of genetic and molecular data used in oncology to gain insights into the course of diseases and potential prognoses [ 9 ]. There is a necessity for joint projects with interoperable (technically and content based) molecular pathological data items, not only locally but also among oncological centers for example in the scope of the German Network of Personalized Medicine (DNPM) [ 12 , 17 ].
At the same time, the connection to the newly established data integration center at UKR is in planning. This connection will allow direct exchange with other centers and national initiatives in the trending standard Fast Healthcare Interoperability Resources (FHIR) [ 18 ].
The interconnectedness of cancer databases enables the generalizability of research results and the sharing of a voluminous amount of oncological data as well as concepts and standards among different centers. Studies with larger data samples from a larger area will improve regional clinical performance and potentially even healthcare concepts [ 12 , 17 ]. Harmonization with cooperation partners can be contemplated because sharing the same database structure may lead to benefits when it comes to shared data analysis. In the local network of oncological centers, several institutions are using the same software. The same benefit applies to harmonizing processes and data structures with local partner hospitals. A monthly exchange with partners regarding questions about the actual documentation and quality aspects has been organized. The clinical cancer registry offers enormous opportunities for scientific research, given that harmonization as well as interoperability of clinical studies and screening programs are being continuously improved [ 9 , 16 , 19 ].
Lessons learned
In general, clinical databases are associated with advantages such as providing a structured format for reviewing patient information, although their development and implementation can be challenging and requires comprehensive considerations [ 13 ]. By describing this process exemplary for the UKR, insights may be gained for future implementations of oncological databases in similar settings.
Based on our experiences, it is highly recommendable to start off with a project plan, specify the aims by designing an evaluation matrix, think in terms of statistical analyses plans, and use cases at a very early stage of project development. It also needs to be pointed out that the migration of data from an existing database to a newly established documentation software is a very time-consuming process.
The software Onkostar was considered the most suitable system in terms of legal requirements as well as certification and research purposes. This highly adaptable and user-friendly tumor database supports research projects as substantiated in the first two use cases. By describing the process of selection and implementation at the University Hospital Regensburg, a guideline has been provided that may be useful for future implementations of oncological databases in similar settings.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Bundesministerium fuer Gesundheit, Referat 315. Nicht uebertragbare Krankheiten. Nationaler Krebsplan. Bonn: Hausdruckerei des BMAS; 2017.
Bundesministerium fuer Gesundheit. Nationaler Krebsplan; 2022. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Praevention/Broschueren/Broschuere_Nationaler_Krebsplan.pdf . Accessed 5 Jan 2023.
Hofstaedter F, Klinkhammer-Schalke M. Aufgaben und Strukturen fuer die klinische Krebsregistrierung. Onkologe. 2011;17:121–5.
Article Google Scholar
BayKRegG. Bayerisches Krebsregistergesetz vom 7. Maerz 2017 (GVBl. S. 26, BayRS 2126-12-G); 2017. https://www.gesetze-bayern.de/Content/Document/BayKRegG/true . Accessed 15 Jan 2023.
Bundesanzeiger. Bekanntmachung: Aktualisierter einheitlicher onkologischer Basisdatensatz (BAnz AT 12.07.2021 B4); 2021.
BayKRegV. Krebsregisterverordnung (BayKRegV) vom 26. Maerz 2018 (GVBl. S. 201, BayRS 2126-12-1-G); 2018. https://www.gesetze-bayern.de/Content/Document/BayKRegV/true . Accessed 15 Dec 2022.
§ 65c SGB V. Sozialgesetzbuch (SGB V) Fuenftes Buch Gesetzliche Krankenversicherung § 65c SGB V Klinische Krebsregister; 2022. https://www.sozialgesetzbuch-sgb.de/sgbv/65c.html . Accessed 2 Feb 2023.
Hoelzel D, et al. Klinisch-epidemiologische Krebsregistrierung in Deutschland. Onkologe. 2016;22:61–78.
Schubert-Fritschle G, et al. Use of Multicenter Data in a large cancer registry for evaluation of Outcome and implementation of novel concepts. Front Oncol. 2017;7:234.
Article PubMed PubMed Central Google Scholar
BayKrG. Bayerisches Krankenhausgesetz (BayKrG) in der Fassung der Bekanntmachung vom 28. Maerz 2007 (GVBl. S. 288) BayRS 2126-8-G Art.27 Abs. 4; 2007. https://www.gesetze-bayern.de/Content/Document/BayKrG-27 . Accessed 2 Feb 2023.
Engel J, Schubert-Fritschle G. Rueckmeldesysteme und Leistungsvergleiche mit Krebsregistern. Onkologe. 2011;17:126–34.
Kessel KA, Combs SE. Review of developments in electronic, clinical data collection, and documentation systems oder the last decade—are we ready for big data in routine health Care? Front Oncol. 2016;6:75.
Gallagher SA et al. Roadmap for the development of the University of North Carolina at Chapel Hill Genitourinary OncoLogy Database—UNC GOLD. Urol Oncol. 2014;32:1-9.
§ 65c Plattform. Bundeseinheitlicher onkologischer Basisdatensatz (oBDS). https://plattform65c.de/schnittstellen/ . Accessed 15 Dec 2022.
Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39:42–5.
Article PubMed Google Scholar
Anttila A, et al. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur J Cancer. 2014;51:241–51.
Potter D, et al. Summary of the 12 most common cancers in the CancerLinQ Discovery (CLQD) Database. JCO Clin Cancer Inform. 2021;5:658–67.
Setyawan R et al. Data integration and interoperability problems of HL7 FHIR implementation and potential solutions: a systematic literature review. In: 5th international conference on informatics and computational sciences (ICICoS); 2021.
Zahrieh D, et al. Successes and lessons learned in database development for national multi-site cancer care delivery research trials: the Alliance for Clinical Trials in Oncology experience. Trials. 2022;23(1):645.
Download references
Acknowledgements
We would like to thank Wolfgang Boerner, PhD, for his support regarding data protection regulation concerns and Monika Schoell for English language editing.
Open Access funding enabled and organized by Projekt DEAL. This study was supported by (Bavarian Cancer Research Center (BZKF), Germany).
Author information
Authors and affiliations.
Department of Information Technology, University Hospital Regensburg, Regensburg, Germany
Anna Saibold, Oliver Spies & Vivian Eilers
Bavarian Cancer Research Center (BZKF), Regensburg, Germany
Anna Saibold & Julia Maurer
Center for Clinical Studies, University Hospital Regensburg, Regensburg, Germany
Michael Koller & Karolina Mueller
Department of Radiation Oncology, University Hospital Regensburg, Regensburg, Germany
Oliver Koelbl
Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Regensburg, Regensburg, Germany
Veronika Vielsmeier
Department of Internal Medicine 3, University Hospital Regensburg, Regensburg, Germany
Tobias Pukrop
University Cancer Center Regensburg, University Hospital Regensburg, Franz-Josef-Strauss Allee 11, 93053, Regensburg, Germany
Cathleen Brese, Denise Amann & Julia Maurer
You can also search for this author in PubMed Google Scholar
Contributions
A.S. and J.M. wrote the main manuscript text and prepared all figures and tables. M.K., K. M. contributed the data analysis concept. O.K. was part of the executing team in use Case 1. O.K., T. P were supporting and advising the process of the manuscript. V.V. was part of the executing team in use Case 2. O.S., V.E., C.B., D.A. had a leading function of the evaluation for the tumor documentation software evaluation matrix. All authors reviewed the manuscript.
Corresponding author
Correspondence to Julia Maurer .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Saibold, A., Koller, M., Mueller, K. et al. A database for oncological research and quality assurance: implementation and first experiences with the University Clinical Cancer Registry Regensburg. BMC Med Res Methodol 24 , 76 (2024). https://doi.org/10.1186/s12874-024-02205-6
Download citation
Received : 19 May 2023
Accepted : 19 March 2024
Published : 27 March 2024
DOI : https://doi.org/10.1186/s12874-024-02205-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical cancer registry
- Database software selection
- Data migration
- Data analysis concept
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
- NIH Employee Intranet
- Staff Directory
- En Español
OCRECO Home > Clinical Research Education > Introduction to the Principles and Practice of Clinical Research (IPPCR)
OFFICE OF CLINICAL RESEARCH EDUCATION AND COLLABORATION OUTREACH
- Clinical Research Education
- Funding Opportunities

Introduction to the Principles and Practice of Clinical Research (IPPCR)
- Course Information
- Description
- Registration
- Course Login
Description Important Dates General Information Course Objectives Individual (Non-Registered) Lecture Option Texbook Contact --> Welcome
The Introduction to the Principles and Practice of Clinical Research (IPPCR) course trains registrants on how to effectively and safely conduct clinical research. The course focuses on the spectrum of clinical research and the research process by highlighting biostatistical and epidemiologic methods, study design, protocol preparation, patient monitoring, quality assurance, ethical and legal issues, and much more.
Course Objectives
Provide an overview of basic biostatistical and epidemiologic methods involved in conducting clinical research.
Describe the principles involved in the ethical, legal, and regulatory issues in clinical human subjects research, including the role of Institutional Review Boards (IRBs).
Describe principles and issues involved in monitoring patient-oriented research.
Describe the infrastructure required in performing clinical research and the steps involved in developing and funding research studies.
Intended Audience
This course will be of interest to physicians, scientists, medical and dental students, nurses, public health professionals, and others conducting or planning a career in clinical research.
Course Directors
Social media links.
- Bookmark & Share
- E-mail Updates
Page Footer
- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
- HHS Vulnerability Disclosure
National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
NIH…Turning Discovery Into Health®
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10250258

How to write a successful grant application: guidance provided by the European Society of Clinical Pharmacy
Anita e. weidmann.
1 Department of Clinical Pharmacy, Innsbruck University, Innsbruck, Austria
Cathal A. Cadogan
2 School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, Dublin, Ireland
Daniela Fialová
3 Department of Social and Clinical Pharmacy, Faculty of Pharmacy in Hradec Králové, Charles University, Hradec Králové, Czech Republic
4 Department of Geriatrics and Gerontology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic
Ankie Hazen
5 Julius Centre for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands
Martin Henman
6 Trinity College Dublin, Dublin, Ireland
Monika Lutters
7 Kantonsspital Aarau, Aarau, Switzerland
Betul Okuyan
8 Department of Clinical Pharmacy, Faculty of Pharmacy, Marmara University, Istanbul, Turkey
Vibhu Paudyal
9 University of Birmingham, Birmingham, United Kingdom
Francesca Wirth
10 Department of Pharmacy, University of Malta, Msida, Malta
Considering a rejection rate of 80–90%, the preparation of a research grant is often considered a daunting task since it is resource intensive and there is no guarantee of success, even for seasoned researchers. This commentary provides a summary of the key points a researcher needs to consider when writing a research grant proposal, outlining: (1) how to conceptualise the research idea; (2) how to find the right funding call; (3) the importance of planning; (4) how to write; (5) what to write, and (6) key questions for reflection during preparation. It attempts to explain the difficulties associated with finding calls in clinical pharmacy and advanced pharmacy practice, and how to overcome them. The commentary aims to assist all pharmacy practice and health services research colleagues new to the grant application process, as well as experienced researchers striving to improve their grant review scores. The guidance in this paper is part of ESCP’s commitment to stimulate “ innovative and high-quality research in all areas of clinical pharmacy ”.
Writing research grants is a central part of any good quality research. Once a detailed research proposal has been submitted, it is subjected to an expert peer review process. Such reviews are designed to reach a funding decision, with feedback provided to improve the study for this and any future submissions. Depending on the length of the proposal, complexity of the research and experience of the research team, a proposal can take between six to twelve months to write [ 1 ]. Ample time must be given to the writing of hypothesis/research aim, budgeting, discussion with colleagues and several rounds of feedback [ 2 ]. The draft research proposal should always be completed well before the deadline to allow for last minute delays. An application which is not fully developed should not be submitted since it will most likely be rejected [ 3 ].
Despite the large effort that goes into each grant application, success rates are low. Application success rates for Horizon 2020 were < 15% [ 4 ] and < 20% for the National Institute of Health (NIH) [ 5 – 8 ]. With these statistics in mind, it is evident that often repeated submissions are required before securing funding. Due to a paucity of specific clinical pharmacy grant awarding bodies, writing a grant application for a clinical pharmacy or pharmacy practice research project often involves multidisciplinary collaborations with other healthcare professions and focus on a specific patient population or condition. There is no guarantee of success when trying to secure funding for research. Even the most seasoned researchers will have applications rejected. The key is to never give up. This commentary provides useful pointers for the planning and execution of grant writing.
Conceptualising your research idea
Before writing a research grant proposal/application, consider what the research should achieve in the short, medium, and long term, and how the research goals will serve patients, science and society [ 9 , 10 ]. Practical implications of research, policy impact or positive impact on society and active patient/public involvement are highly valued by many research agencies as research should not be conducted “only for research”, serving the researchers’ interests. EU health policy and action strategies (CORDIS database) and other national strategies, such as national mental health strategy for grants within mental disorders, should be considered, as well as dissemination strategies, project deliverables, outcomes and lay public invitations to participate. The Science Community COMPASS has developed a useful “Message Box Tool” that can help in the identification of benefits and solutions, as well as the all-important “So What?” of the research [ 11 ]. Clearly determine what the lead researcher’s personal and professional strengths, expertise and past experiences are, and carefully select the research team to close these gaps [ 12 – 14 ].
How to find the right funding call
When trying to identify the right type of grant according to the research ambitions, one should be mindful that several types of grants exist, including small project grants (for equipment, imaging costs), personal fellowships (for salary costs, sometimes including project costs), project grants (for a combination of salary and project costs), programme grants (for comprehensive project costs and salary for several staff members), start-up grants and travel grants [ 15 ]. Types of grants include EU grants (e.g. Horizon, Norway Grant), commercial grants (e.g. healthcare agencies and insurance companies), New Health Program grants ideal for new, reimbursed clinical pharmacy service projects and national grants (e.g. FWF (Austria), ARRS (Slovenia), NKFIH (Hungary), NCN (Poland), FWO (Belgium), HRZZ (Croatia), GAČR (Czech Republic), SNSF (Switzerland), SSF (Sweden). It is worth remembering that early career researchers, normally within ten years of finishing a PhD, have a particular sub-category within most grants.
Many national agencies only have one “Pharmacy” category. This results in clinical pharmacy and advanced clinical pharmacy practice projects competing with pharmaceutical chemistry, pharmaceutical biology and pharmacy technology submissions, thereby reducing the success rate as these research areas can often be very advanced in most EU countries compared to clinical and advanced pharmacy practice. A second possible submission category is “Public Health”. Several essential factors can impact the grant selection, such as research field, budget capacity, leading researcher’s experience and bilateral grants. Examples of successful clinical pharmacy funded research studies can be found in the published literature [ 16 – 20 ].
Plan, plan, plan
One key element of successful grant writing is the ability to plan and organise time. In order to develop a realistic work plan and achieve milestones, it is imperative to note deadlines and to be well-informed about the details of what is required. The development of a table or Gantt Chart that notes milestones, outcomes and deliverables is useful [ 21 ].
All funders are quite specific about what they will and will not fund. Research your potential funders well in advance. It is vital to pay attention to the aims, ambitions and guidelines of the grant awarding bodies and focus your proposal accordingly. Submitting an application which does not adhere to the guidelines may lead to very early rejection. It is helpful to prepare the grant application in such a way that the reviewers can easily find the information they are looking for [ 15 , 22 ]. This includes checking the reviewers’ reports and adding “bolded” sentences into the application to allow immediate emphasis. Reviewers’ reports are often available on the agencies’ websites. It is extremely useful to read previously submitted and funded or rejected proposals to further help in the identification of what is required in each application. Most funding agencies publish a funded project list, and the ‘Centre for Open Science (COS) Database of Funded Research’ enables tracking of funding histories from leading agencies around the world [ 23 ]. Another useful recommendation is to talk to colleagues who have been successful when applying to that particular funder. Funding agency grant officers can provide advice on the suitability of the proposal and the application process.
It is important to pay particular attention to deadlines for the grant proposal and ensure that sufficient time is allocated for completion of all parts of the application, particularly those that are not fully within one’s own control, for example, gathering any required signatures/approvals. Funders will generally not review an application submitted beyond the deadline.
Lastly, it is important to obtain insight into the decision process of grants. Research applications are sent to several reviewers, who are either volunteers or receive a small compensation to judge the application on previously determined criteria. While the judging criteria may vary from funder to funder, the key considerations are:
- Is there a clear statement of the research aim(s)/research question(s)/research objective(s)?
- Is the proposed research “state-of-the-art” in its field and has all relevant literature been reviewed?
- Is the method likely to yield valid, reliable, trustworthy data to answer question 1.?
- If the answer to the second question is ’yes’, then what is the impact of financing this study on patient care, professional practice, society etc.?
- Is there sufficient confidence that the research team will deliver this study on time with expected quality outputs and on budget?
- Does the study provide value for money?
How to write
The key to good grant proposal writing is to be concise yet engaging. The use of colour and modern web-based tools such as #hashtags, webpage links, and links to YouTube presentations are becoming increasingly popular to improve the interest of a submission and facilitate a swift decision-making process. Ensure use of the exact section headings provided in the guidance, and use the keywords provided in the funding call documentation to reflect alignment with the funding bodies’ key interests. Attention to detail cannot be overstated; the quality and accuracy of the research proposal reflect the quality and accuracy of the research [ 24 ]. Try to adopt a clear, succinct, and simple writing style, making the grant easy to read. Having a clear focus can help to boost a grant to the top of a reviewer’s pile [ 25 , 26 ]. A clearly stated scientific question, hypothesis, and rationale are imperative. The reviewer should not have to work to understand the project [ 27 ]. Allow for plenty of time to incorporate feedback from trusted individuals with the appropriate expertise and consider having reviews for readability by non-experts.
What to write
Abstract, lay summary and background/rationale.
Take sufficient time to draft the scientific abstract and summary for the lay public. These should clearly state the long-term goal of the research, the aim and specific testable objectives, as well as the potential impact of the work. The research aim is a broad statement of research intent that sets out what the project hopes to achieve at the end. Research objectives are specific statements that define measurable outcomes of the project [ 28 , 29 ].
The lay summary is important for non-subject experts to quickly grasp the purpose and aims of the research. This is important in light of the increased emphasis on patient and public involvement in the design of the research. The abstract is often given little attention by the applicants, yet is essential. If reviewers have many applications to read, they may form a quick judgement when reading the abstract. The background should develop the argument for the study. It should flow and highlight the relevant literature and policy or society needs statements which support the argument, but at the same time must be balanced. It should focus on the need for the study at the local, national and international level, highlighting the knowledge gap the study addresses and what the proposed research adds. Ensure this section is well-referenced. The innovation section addresses the ‘‘So what?’’ question and should clearly explain how this research is important to develop an understanding in this field of practice and its potential impact. Will it change practice, or will it change the understanding of the disease process or its treatment? Will it generate new avenues for future scientific study? [ 30 ].
Hypothesis/aims and objectives
For the hypothesis, state the core idea of the grant in one or two sentences. It should be concise, and lead to testable specific aims. This section is fundamental; if it is unclear or poorly written, the reviewers may stop reading and reject the application. Do not attempt to make the aims overly complex. Well-written aims should be simply stated. Criteria such as PICO (population, intervention, comparison, outcomes) [ 31 ], and FINER (feasible, interesting, novel, ethical, relevant) [ 32 ], provide useful frameworks to help in writing aim(s), research question(s), objective(s) and hypotheses. Pay attention to the distinction between aim(s), research question(s), objective(s) and hypotheses. While it is tempting to want to claim that enormously complex problems can be solved in a single project, do not overreach. It is important to be realistic [ 25 ].
Experimental design, methods and expertise
The methodology is one of the most important parts of getting a grant proposal accepted. The reviewing board should be convinced that the relevant methodology is well within the research teams’ expertise. Any evidence of potential success, such as preliminary results or pilot studies strengthen the application significantly [ 33 ]. The methodology must relate directly to the aim. Structuring this section into specific activities/ set of activities that address each research question or objective should be considered. This clarifies how each question/ objective will be addressed. Each work-package should clearly define the title of the research question/objective to be addressed, the activities to be carried out including milestones and deliverables, and the overall duration of the proposed work-package. Deliverables should be presented in table format for ease of review. Each subsequent work-package should start once the previous one has been completed to provide a clear picture of timelines, milestones and deliverables which reflect stakeholder involvement and overall organisation of the proposed project. Using relevant EQUATOR Network reporting guidelines enhances the quality of detail included in the design [ 34 ]. Key elements of this methodology are detailed in Table 1 .
Summary of the key elements of the experimental design, methods and expertise
Proposed budget
The budget should be designed based on the needs of the project and the funding agency’s policies and instructions. Each aspect of the budget must be sufficiently justified to ensure accountability to the grant awarding body [ 35 ]. Costing and justification of the time of those involved, any equipment, consumables, travel, payment for participants, dissemination costs and other relevant costs are required. The funders will be looking for value for money and not necessarily a low-cost study. Ensure that the total budget is within the allocated funding frame.
Provide a breakdown of the key work packages and tasks to be completed, as well as an indication of the anticipated duration. Include a Gantt chart (A table detailing the most general project content milestones and activities) to demonstrate that all aspects of the proposal have been well thought through [ 21 ].
Critical appraisal, limitations, and impact of the proposed research
It is important to detail any strengths and limitations of the proposed project. Omitting these will present the reviewing board with sufficient grounds to reject the proposal [ 36 ]. Provide a clear statement about the short and long-term impact of the research [ 37 , 38 ]. The reviewers will pay particular attention to the differences the study can make and how potential impact aligns with the funding bodies goals as well as national policies. This statement is essential to make an informed decision whether or not to support the application. Useful diagrams summarise the different levels of impact [ 39 ].
Table 2 provides a summary of the key elements of project grants and key questions to ask oneself.
Summary of the key elements of project grants and key questions to ask oneself.
(Adapted from [ 5 ]: Koppelmann GH, Holloway JW. Successful grant writing. Paediatr Respir Rev. 2012; 13:63–66.)
Although the grant writing process is time-consuming and complex, support is widely available at each stage. It is important to involve colleagues and collaborators to improve the proposal as much as possible and invest time in the detailed planning and execution. Even if the grant is not awarded, do not be disheartened. Use the feedback for improvement and exercise resilience and persistence in pursuing your research ambition.
The guidance in this paper is part of ESCP’s commitment to stimulate “innovative and high-quality research in all areas of clinical pharmacy”. In a previous ESCP survey, it was found that few opportunities for collaboration (especially for grant applications) was one of the key barriers for members towards conducting research [ 40 ]. ESCP promotes networking, which is essential for multi-centre grant applications, both among ESCP members and with other organisations as it recognises the need for “multi-centre research in all areas of clinical pharmacy both within countries and between countries or differing healthcare delivery systems”. ESCP is planning to relaunch its own research grant which was paused during the pandemic, and it is also planning to provide ESCP members with information about the research grants offered by other organizations. ESCP is exploring partnering with other organisations to develop research proposals in areas of common interest and, in the near future, it will ask its members about their research priorities. Taken together, these initiatives will inform ESCP’s research strategy and help it to formulate policies to address the challenges its members face.
Acknowledgements
Research works of Assoc. Prof. Fialová were also supported by the institutional program Cooperation of the Faculty of Pharmacy, Charles University.
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. This work was conducted without external funding.
Conflicts of interest
The authors have not disclosed any competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
New Tumor Mapping Tech Shows Potential to Make ‘Major Impact’ on Cancer Research

Nicolas Chevrier in his lab, which develops interdisciplinary approaches and tools to study how the immune system functions across biological scales.
A new technology developed at the University of Chicago has been recognized for its potential to make a major impact on basic and clinical cancer research.
Nicolas Chevrier , assistant professor of molecular engineering at the University of Chicago’s Pritzker School of Molecular Engineering , recently received the Duckworth Family Commercial Promise Award for his proposal, Spatiomolecular mapping of the tumor microenvironment.
The proposal was selected from a competitive pool of proposals and judged by a team from the University of Chicago Medicine Comprehensive Cancer Center (UCCCC) and the Polsky Center for Entrepreneurship and Innovation , as well as external experts. The review criteria included commercialization potential, investigators, innovation, scientific merit, as well as feasibility of milestones and future plans.
“Dr. Chevrier’s proposal for a spatial profiling platform demonstrated potential to make a major impact on basic and clinical cancer research. It will enable the analysis of the tumor microenvironment to yield new biomarkers for diagnosis and prognosis, candidate therapeutic targets, and fundamental insights about cancer biology,” said Kunle Odunsi , director of UChicago Medicine’s Comprehensive Cancer Center, dean for oncology, and the AbbVie Foundation Distinguished Service Professor.
Combining DNA microarrays and next-generation sequencing, the technology enables the processing of small-to-large samples ranging from a standard biopsy to whole-mount human organs or model organisms. A prototype of the platform outperformed the currently available commercial platform in all metrics tested, including sensitivity, surface area, resolution, and cost.
“One of the immediate uses of this could be studying the spatial features of tumors across large cohorts of cancer patients for the first time,” said Chevrier. “In doing so, you can look for features that correlate with better or worse outcomes.”
Moving forward, one goal is to make the platform compatible with archived biospecimens stored at hospitals, so the researchers can retroactively study large cohorts of patients with known outcomes. This would help determine why a certain drug didn’t work in a specific group of patients while working in others.
“Or maybe we can discover spatial molecular features of cancer lesions that were not known before because we’d now be able to look at many, many samples in a format that also allows for the analysis of the whole tumor as opposed to small biopsies only,” added Chevrier. “It could also be used to come up with new therapeutic targets that could be tested or investigated.”
With any of these use cases, Chevrier knew the technology could have a much broader application beyond his work in the lab. “That’s how we got here,” said Chevrier, who also cofounded Flexomics in 2019. The startup last month was awarded a two-year, $2 million Small Business Innovation Research grant from the National Human Genome Research Institute to further develop its core single-cell screening technology for new applications.
In his lab , Chevrier said they typically start with very basic questions – applying fundamental findings to further translational work.
The funding from the Commercial Promise Award will help focus the work related to the proposal “from a more translational angle from the start,” noted Chevrier. “In the future, we hope to work more and more with specimens from patients or human donors so that we can tie our fundamental studies more closely to things that can benefit patients.”
As for next steps, Chevrier said the immediate goal is to establish robust protocols for the platform so that it can be ready to be used as a commercial kit or as a service platform. He has established a collaboration with a partner in industry, Agilent Technologies, in support of this, and also is benefitting from a great collaboration with Ben Shogan , a gastrointestinal surgeon at UChicago Medicine to benchmark the technology on human colon cancer sections at the whole-organ level.
// University of Chicago Medical Center Trustee Tom Duckworth and his wife Connie in 2019 committed more than $1 million to establish the Duckworth Family Cancer Fund at UChicago Medicine, expanding the partnership between UCCCC and the Polsky Center.
This site uses cookies and other tracking technologies to assist with navigation and your ability to provide feedback, analyze your use of products and services, assist with our promotional and marketing efforts.

IMAGES
VIDEO
COMMENTS
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management" Example research proposal #2: "Medical Students as Mediators of ...
steps. It begins with selecting a study topic, reviewing. the literature, setting goals, choosing a study design and. appropriate statistical tools, and formulating a research proposal. to obtain ...
This chapter addresses general approaches towards writing a clinical research proposal. The landscape in research has changed in the last decade and has become more translational in nature. Therefore, research proposals are read and evaluated by scientists from various fields that may not be intricately familiar with specific techniques and ...
A proposal needs to show how your work fits into what is already known about the topic and what new paradigm will it add to the literature, while specifying the question that the research will answer, establishing its significance, and the implications of the answer. [ 2] The proposal must be capable of convincing the evaluation committee about ...
clinical research proposal . What is a proposal •A document that presents the case for an idea and the action that one proposes to take with respect to it and aimed at eliciting a desired action from the reader (Fatusi, 2012) •An academic proposal is the first step in
The competition for funds to conduct clinical research is intense, and only a minority of grant proposals receive funding. In partic-ular, funding for patient-oriented research lags behind that allocated for basic science research. Grant writing is a skill of fundamental importance to the clinical researcher, and conducting
With your research mentor, develop a strategy for obtaining funding, a timeline for seeking funds, and the structure of your proposals. Arrange an in-house pre-review of your proposal prior its submission. Your department/center research administrator can help develop required forms, the budget, and budget justification.
The research proposal serves many purposes to many different parties. Amongst these purposes, some of the key ones are: • Acting as a route map and timetable for all involved in your project. • Giving a clear overview of your planned work to ensure favourable decision at ethical review.
It puts the proposal in context. 3. The introduction typically begins with a statement of the research problem in precise and clear terms. 1. The importance of the statement of the research problem 5: The statement of the problem is the essential basis for the construction of a research proposal (research objectives, hypotheses, methodology ...
Typical stages in a research proposal. 1. The purpose of a research proposal is: To help to focus on a relevant and current topic. To identify a gap or inadequacy in the research literature. To make sure that these are your ideas, and to help you to focus and crystallise your ideas.
Research proposals usually need to go through several drafts. Show your internal and external supervisors a draft early enough so that you can incorporate their comments into a revised draft before submission. Review of the proposal. The proposal will be read by one of the academic staff, and will be discussed at a proposals review meeting in ...
Research Proposal. The mentored research experience is a key component of the Postdoctoral Master's Degree Program in clinical and translational science. Students develop their research proposals in CTSC 5010: Clinical Research Proposal Development, with a deadline based on the academic cycle listed below.
HIGH-QUALITY research proposals are required to obtain funds for the basic and clinical sciences. In this era of diminishing revenues, the ability to compete successfully for peer-reviewed research money is essential to create and maintain scientific programs. ... Although obvious for basic science research, clinical research also requires ...
Clinical researchers are working on several treatment and prevention options to improve and advance medicine and human health. Their revolutionary ideas start with a research proposal for succeeding tests and validations. 1. Research Proposal Template. Details. File Format. Google Docs. MS Word. Pages.
For clinical research proposals, a composite case can bring the human element of the scientific rationale to light in a highly memorable way; clinical translational proposals can also benefit from a human health implication of the science. The hallmarks of excellent scientific writing deserve their own full manuscript discussion, but these ...
If you're planning to apply for an NCCIH grant for research involving human participants and will be attending the International Congress on Integrative Medicine and Health (ICIMH), this Pre-Congress Workshop may be for you: "Honing Your Clinical Research Proposal for NIH and NCCIH Funding Opportunities."It's scheduled for Tuesday, May 8 from 2 to 5 p.m.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on ...
Clinical trial specific funding opportunities (proposals due on or after January 25, 2018) New PHS Human Subjects and Clinical Trials Information form (proposals due on or after January 25, 2018) Single IRB for multi-site research (effective January 2018) ClinicalTrials.gov registration and reporting requirements (effective January 2017)
Complete the Clinical Research Proposal Cover Sheet, providing all requested information; Write and provide a Proposal Narrative; Provide the Curriculum Vitae of PI (NIH grant application format) Provide a list of current board members, officers and staff, as well as who will be involved with the project, and any conflict of interests
19+ SAMPLE Clinical Research Proposal in PDF. The vast majority of students and early-career researchers have a misconception of what a clinical research proposal is and how critical it is to the success of the project. Medical breakthroughs and treatments for illnesses and the like appeared out of nowhere in the early days of society.
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. ... Caution regarding the use of pilot studies to guide power calculations for study proposals. Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Arch ...
This protocol template aims to facilitate the development of two types of clinical trials involving human participants. The first type of trials are Phase 2 and 3 clinical trial protocols that require a Food and Drug Administration (FDA) Investigational New Drug (IND) or Investigational Device Exemption (IDE) application. IND/IDE Protocol Word ...
Proposal 1. Investigator desires to submit a proposal Initial pre-review by Clinical Research Admin Team 2. Investigator presents at the Clinical Research Meeting for feedback (optional) PASS Needs revisions Full Proposal 3. Investigator desires to proceed with the proposed research PASS Needs revisions Review by Clinical Research Committee ...
For research requirements, a data analysis concept was established comprising a proposal for data extraction, procedural instructions, and statistical training materials. ... Zahrieh D, et al. Successes and lessons learned in database development for national multi-site cancer care delivery research trials: the Alliance for Clinical Trials in ...
Describe the infrastructure required in performing clinical research and the steps involved in developing and funding research studies. Intended Audience. This course will be of interest to physicians, scientists, medical and dental students, nurses, public health professionals, and others conducting or planning a career in clinical research. ...
Conceptualising your research idea. Before writing a research grant proposal/application, consider what the research should achieve in the short, medium, and long term, and how the research goals will serve patients, science and society [9, 10].Practical implications of research, policy impact or positive impact on society and active patient/public involvement are highly valued by many ...
A new technology developed at the University of Chicago has been recognized for its potential to make a major impact on basic and clinical cancer research. Nicolas Chevrier, assistant professor of molecular engineering at the University of Chicago's Pritzker School of Molecular Engineering, recently received the Duckworth Family Commercial Promise Award for his proposal, Spatiomolecular