Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character

Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Spring 2024 | VOL. 36, NO. 2 CURRENT ISSUE pp.A4-174
- Winter 2024 | VOL. 36, NO. 1 pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Case Study 1: A 55-Year-Old Woman With Progressive Cognitive, Perceptual, and Motor Impairments
- Scott M. McGinnis , M.D. ,
- Andrew M. Stern , M.D., Ph.D. ,
- Jared K. Woods , M.D., Ph.D. ,
- Matthew Torre , M.D. ,
- Mel B. Feany , M.D., Ph.D. ,
- Michael B. Miller , M.D., Ph.D. ,
- David A. Silbersweig , M.D. ,
- Seth A. Gale , M.D. ,
- Kirk R. Daffner , M.D.
Search for more papers by this author
CASE PRESENTATION
A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties. Problems with depth perception affected her ability to back her car out of the driveway. When descending stairs, she had to locate her feet visually in order to place them correctly, such that when she carried her dog and her view was obscured, she had difficulty managing this activity. She struggled to execute relatively simple tasks, such as inserting a plug into an outlet. She lost the ability to type on a keyboard, despite being able to move her fingers quickly. Her symptoms worsened progressively for 3 years, over which time she developed a sad mood and anxiety. She was laid off from work as a nurse administrator. Her family members assumed responsibility for paying her bills, and she ceased driving.
Her past medical history included high blood pressure, Hashimoto’s thyroiditis with thyroid peroxidase antibodies, remote history of migraine, and anxiety. Medications included mirtazapine, levothyroxine, calcium, and vitamin D. She had no history of smoking, drinking alcohol, or recreational drug use. There was no known family history of neurologic diseases.
What Are Diagnostic Considerations Based on the History? How Might a Clinical Examination Help to Narrow the Differential Diagnosis?
Insidious onset and gradual progression of cognitive symptoms over the course of several years raise concern for a neurodegenerative disorder. It is helpful to consider whether or not the presentation fits with a recognized neurodegenerative clinical syndrome, a judgment based principally on familiarity with syndromes and pattern recognition. Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer’s disease (AD) ( 1 , 2 ). This patient’s symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in posterior cortical atrophy (PCA), although the history also includes mention of forgetfulness and word-retrieval difficulties. A chief goal of the cognitive examination would be to survey major domains of cognition—attention, executive functioning, memory, language, visual-spatial functioning, and higher somatosensory and motor functioning—to determine whether any domains stand out as more prominently affected. In addition to screening for evidence of focal signs, a neurological examination in this context should assess for evidence of parkinsonism or motor neuron disease, which can coexist with cognitive changes in neurodegenerative presentations.
The patient’s young age and history of Hashimoto’s thyroiditis might also prompt consideration of Hashimoto’s encephalopathy (HE; also known as steroid-responsive encephalopathy), associated with autoimmune thyroiditis. This syndrome is most likely attributable to an autoimmune or inflammatory process affecting the central nervous system. The time course of HE is usually more subacute and rapidly progressive or relapsing-remitting, as opposed to the gradual progression over months to years observed in the present case ( 3 ).
The patient’s mental status examination included the Montreal Cognitive Assessment (MoCA), a brief global screen of cognition ( 4 ), on which she scored 12/30. There was evidence of dysfunction across multiple cognitive domains ( Figure 1 ). She was fully oriented to location, day, month, year, and exact date. When asked to describe a complex scene from a picture in a magazine, she had great difficulty doing so, focusing on different details but having trouble directing her saccades to pertinent visual information. She likewise had problems directing her gaze to specified objects in the room and problems reaching in front of her to touch target objects in either visual field. In terms of other symptoms of higher order motor and somatosensory functioning, she had difficulty demonstrating previously learned actions—for example, positioning her hand correctly to pantomime holding a brush and combing her hair. She was confused about which side of her body was the left and which was the right. She had difficulty with mental calculations, even relatively simple ones such as “18 minus 12.” In addition, she had problems writing a sentence in terms of both grammar and the appropriate spacing of words and letters on the page.
FIGURE 1. Selected elements of a 55-year-old patient’s cognitive examination at presentation a
a BNT-15=Boston Naming Test (15-Item); MoCA=Montreal Cognitive Assessment.
On elementary neurologic examination she had symmetrically brisk reflexes, with spread. She walked steadily with a narrow base, but when asked to pass through a doorway she had difficulty finding her way through it and bumped into the door jamb. Her elemental neurological examination was otherwise normal, including but not limited to brisk, full-amplitude vertical eye movements, normal visual fields, no evidence of peripheral neuropathy, and no parkinsonian signs such as slowness of movement, tremor, or rigidity.
How Does the Examination Contribute to Our Understanding of Diagnostic Considerations? What Additional Tests or Studies Are Indicated?
The most prominent early symptoms and signs localize predominantly to the parietal association cortex: The patient has impairments in visual construction, ability to judge spatial relationships, ability to synthesize component parts of a visual scene into a coherent whole (simultanagnosia or asimultagnosia), impaired visually guided reaching for objects (optic ataxia), and most likely impaired ability to shift her visual attention so as to direct saccades to targets in her field of view (oculomotor apraxia or ocular apraxia). The last three signs constitute Bálint syndrome, which localizes to disruption of dorsal visual networks (i.e., dorsal stream) with key nodes in the posterior parietal and prefrontal cortices bilaterally ( 5 ). She has additional salient symptoms and signs suggesting left inferior parietal dysfunction, including ideomotor limb apraxia and elements of Gerstmann syndrome, which comprises dysgraphia, acalculia, left-right confusion, and finger agnosia ( 6 ). Information was not included about whether she was explicitly examined for finger agnosia, but elements of her presentation suggested a more generalized disruption of body schema (i.e., her representation of the position and configuration of her body in space). Her less prominent impairment in lexical-semantic retrieval evidenced by impaired confrontation naming and category fluency likely localizes to the language network in the left hemisphere. Her impairments in attention and executive functions have less localizing value but would plausibly arise in the context of frontoparietal network dysfunction. At this point, it is unclear whether her impairment in episodic memory mostly reflects encoding and activation versus a rapid rate of forgetting (storage), as occurs in temporolimbic amnesia. Regardless, it does not appear to be the most salient feature of her presentation.
This localization, presenting with insidious onset and gradual progression, is characteristic of a PCA syndrome. If we apply consensus clinical diagnostic criteria proposed by a working group of experts, we find that our patient has many of the representative features of early disturbance of visual functions plus or minus other cognitive functions mediated by the posterior cerebral cortex ( Table 1 ) ( 7 ). Some functions such as limb apraxia also occur in corticobasal syndrome (CBS), a clinical syndrome defined initially in association with corticobasal degeneration (CBD) neuropathology, a 4-repeat tauopathy characterized by achromatic ballooned neurons, neuropil threads, and astrocytic plaques. However, our patient lacks other suggestive features of CBS, including extrapyramidal motor dysfunction (e.g., limb rigidity, bradykinesia, dystonia), myoclonus, and alien limb phenomenon ( Table 1 ) ( 8 ).
a Consensus diagnostic criteria for posterior cortical atrophy per Crutch et al. ( 7 ) require at least three cognitive features and relative sparing of anterograde memory, speech-nonvisual language functions, executive functions, behavior, and personality. Diagnostic criteria for probable corticobasal syndrome per Armstrong et al. ( 8 ) require asymmetric presentation of at least two motor features and at least two higher cortical features. AD=Alzheimer’s disease; CBD=corticobasal degeneration; FDG-PET=[ 18 ]F-fluorodexoxyglucose positron emission tomography; JCD=Jakob-Creutzfeldt disease; LBD=Lewy body disease; PSP=progressive supranuclear palsy; SPECT=single-photon emission computed tomography; TDP=TDP–43 proteinopathy.
TABLE 1. Clinical features and neuropathological associations of posterior cortical atrophy and corticobasal syndrome a
In addition to a standard laboratory work-up for cognitive impairment, it is important to determine whether imaging of the brain provides evidence of neurodegeneration in a topographical distribution consistent with the clinical presentation. A first step in most cases would be to obtain an MRI of the brain that includes a high-resolution T 1 -weighted MRI sequence to assess potential atrophy, a T 2 /fluid-attenuated inversion recovery (FLAIR) sequence to assess the burden of vascular disease and rule out less likely etiological considerations (e.g., infection, autoimmune-inflammatory, neoplasm), a diffusion-weighted sequence to rule out subacute infarcts and prion disease (more pertinent to subacute or rapidly progressive cases), and a T 2 *-gradient echo or susceptibility weighted sequence to examine for microhemorrhages and superficial siderosis.
A lumbar puncture would serve two purposes. First, it would allow for the assessment of inflammation that might occur in HE, as approximately 80% of cases have some abnormality of CSF (i.e., elevated protein, lymphocytic pleiocytosis, or oligoclonal bands) ( 9 ). Second, in selected circumstances—particularly in cases with atypical nonamnesic clinical presentations or early-onset dementia in which AD is in the neuropathological differential diagnosis—we frequently pursue AD biomarkers of molecular neuropathology ( 10 , 11 ). This is most frequently accomplished with CSF analysis of amyloid-β-42, total tau, and phosphorylated tau levels. Amyloid positron emission tomography (PET) imaging, and most recently tau PET imaging, represent additional options that are approved by the U.S. Food and Drug Administration for clinical use. However, insurance often does not cover amyloid PET and currently does not reimburse tau PET imaging. [ 18 ]-F-fluorodeoxyglucose (FDG) PET and perfusion single-photon emission computed tomography imaging may provide indirect evidence for AD neuropathology via a pattern of hypometabolism or hypoperfusion involving the temporoparietal and posterior cingulate regions, though without molecular specificity. Pertinent to this case, a syndromic diagnosis of PCA is most commonly associated with underlying AD neuropathology—that is, plaques containing amyloid-β and neurofibrillary tangles containing tau ( 12 – 15 ).
The patient underwent MRI, demonstrating a minimal burden of T 2 /FLAIR hyperintensities and some degree of bilateral parietal volume loss with a left greater than right predominance ( Figure 2A ). There was relatively minimal medial temporal volume loss. Her basic laboratory work-up, including thyroid function, vitamin B 12 level, and treponemal antibody, was normal. She underwent a lumbar puncture; CSF studies revealed normal cell counts, protein, and glucose levels and low amyloid-β-42 levels at 165.9 pg/ml [>500 pg/ml] and elevated total and phosphorylated tau levels at 1,553 pg/ml [<350 pg/ml] and 200.4 pg/ml [<61 pg/ml], respectively.
FIGURE 2. MRI brain scan of the patient at presentation and 4 years later a
a Arrows denote regions of significant atrophy.
Considering This Additional Data, What Would Be an Appropriate Diagnostic Formulation?
For optimal clarity, we aim to provide a three-tiered approach to diagnosis comprising neurodegenerative clinical syndrome (e.g., primary amnesic, mixed amnesic and dysexecutive, primary progressive aphasia), level of severity (i.e., mild cognitive impairment; mild, moderate or severe dementia), and predicted underlying neuropathology (e.g., AD, Lewy body disease [LBD], frontotemporal lobar degeneration) ( 16 ). This approach avoids problematic conflations that cause confusion, for example when people equate AD with memory loss or dementia, whereas AD most strictly describes the neuropathology of plaques and tangles, regardless of the patient’s clinical symptoms and severity. This framework is important because there is never an exclusive, one-to-one correspondence between syndromic and neuropathological diagnosis. Syndromes arise from neurodegeneration that starts focally and progresses along the anatomical lines of large-scale brain networks that can be defined on the basis of both structural and functional connectivity, a concept detailed in the network degeneration hypothesis ( 17 ). It is important to note that neuropathologies defined on the basis of specific misfolded protein inclusions can target more than one large-scale network, and any given large-scale network can degenerate in association with more than one neuropathology.
The MRI results in this case support a syndromic diagnosis of PCA, with a posteriorly predominant pattern of atrophy. Given the patient’s loss of independent functioning in instrumental activities of daily living (ADLs), including driving and managing her finances, the patient would be characterized as having a dementia (also known as major neurocognitive disorder). The preservation of basic ADLs would suggest that the dementia was of mild severity. The CSF results provide supportive evidence for AD amyloid plaque and tau neurofibrillary tangle (NFT) neuropathology over other pathologies potentially associated with PCA syndrome (i.e., CBD, LBD, TDP-43 proteinopathy, and Jakob-Creutzfeldt disease) ( 13 , 14 ). The patient’s formulation would thus be best summarized as PCA at a level of mild dementia, likely associated with underlying AD neuropathology.
The patient’s symptoms progressed. One year after initial presentation, she had difficulty locating the buttons on her clothing or the food on her plate. Her word-finding difficulties worsened. Others observed stiffness of her right arm, a new symptom that was not present initially. She also had decreased ability using her right hand to hold everyday objects such as a comb, a brush, or a pen. On exam, she was noted to have rigidity of her right arm, impaired dexterity with her right hand for fine motor tasks, and a symmetrical tremor of the arms, apparent when holding objects or reaching. Her right hand would also intermittently assume a flexed, dystonic posture and would sometime move in complex ways without her having a sense of volitional control.
Four to 5 years after initial presentation, her functional status declined to the point where she was unable to feed, bathe, or dress herself. She was unable to follow simple instructions. She developed neuropsychiatric symptoms, including compulsive behaviors, anxiety, and apathy. Her right-sided motor symptoms progressed; she spent much of the time with her right arm flexed in abnormal postures or moving abnormally. She developed myoclonus of both arms. Her speech became slurred and monosyllabic. Her gait became less steady. She underwent a second MRI of the brain, demonstrating progressive bilateral atrophy involving the frontal and occipital lobes in addition to the parietal lobes and with more left > right asymmetry than was previously apparent ( Figure 2B ). Over time, she exhibited increasing weight loss. She was enrolled in hospice and ultimately passed away 8 years from the onset of symptoms.
Does Information About the Longitudinal Course of Her Illness Alter the Formulation About the Most Likely Underlying Neuropathological Process?
This patient developed clinical features characteristic of corticobasal syndrome over the longitudinal course of her disease. With time, it became apparent that she had lost volitional control over her right arm (characteristic of an alien limb phenomenon), and she developed signs more suggestive of basal ganglionic involvement (i.e., limb rigidity and possible dystonia). This presentation highlights the frequent overlap between neurodegenerative clinical syndromes; any given person may have elements of more than one syndrome, especially later in the course of a disease. In many instances, symptomatic features that are less prominent at presentation but evolve and progress can provide clues regarding the underlying neuropathological diagnosis. For example, a patient with primary progressive apraxia of speech or nonfluent-agrammatic primary progressive aphasia could develop the motor features of a progressive supranuclear palsy (PSP) clinical syndrome (e.g., supranuclear gaze impairment, axial rigidity, postural instability), which would suggest underlying PSP neuropathology (4-repeat tauopathy characterized by globose neurofibrillary tangles, tufted astrocytes, and oligodendroglial coiled bodies).
If CSF biomarker data were not suggestive of AD, the secondary elements of CBS would substantially increase the likelihood of underlying CBD neuropathology presenting with a PCA syndrome and evolving to a mixed PCA-CBS. But the CSF amyloid and tau levels are unambiguously suggestive of AD (i.e., very low amyloid-β-42 and very high p-tau levels), the neuropathology of which accounts for not only a vast majority of PCA presentations but also roughly a quarter of cases presenting with CBS ( 18 , 19 ). Thus, underlying AD appears most likely.
NEUROPATHOLOGY
On gross examination, the brain weighed 1,150 g, slightly less than the lower end of normal at 1,200 g. External examination demonstrated mild cortical atrophy with widening of the sulci, relatively symmetrical and uniform throughout the brain ( Figure 3A ). There was no evidence of atrophy of the brainstem or cerebellum. On cut sections, the hippocampus was mildly atrophic. The substantia nigra in the midbrain was intact, showing appropriate dark pigmentation as would be seen in a relatively normal brain. The remainder of the gross examination was unremarkable.
FIGURE 3. Mild cortical atrophy with posterior predominance and neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body a
a Panel A shows the gross view of the brain, demonstrating mild cortical atrophy with posterior predominance (arrow). Panel B shows the hematoxylin and eosin of the hippocampus at high power, demonstrating neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body.
Histological examination confirmed that the neurons in the substantia nigra were appropriately pigmented, with occasional extraneuronal neuromelanin and moderate neuronal loss. In the nucleus basalis of Meynert, NFTs were apparent on hematoxylin and eosin staining as dense fibrillar eosinophilic structures in the neuronal cytoplasm, confirmed by tau immunohistochemistry (IHC; Figure 4 ). Low-power examination of the hippocampus revealed neuronal loss in the subiculum and in Ammon’s horn, most pronounced in the cornu ammonis 1 (CA1) subfield, with a relatively intact neuronal population in the dentate gyrus. Higher power examination with hematoxylin and eosin demonstrated numerous NFTs, neurons exhibiting granulovacuolar degeneration, and Hirano bodies ( Figure 3B ). Tau IHC confirmed numerous NFTs in the CA1 region and the subiculum. Amyloid-β IHC demonstrated occasional amyloid plaques in this region, less abundant than tau pathology. An α-synuclein stain revealed scattered Lewy bodies in the hippocampus and in the amygdala.
FIGURE 4. Tau immunohistochemistry demonstrating neurofibrillary tangles (staining brown) in the nucleus basalis of Meynert, in the hippocampus, and in the cerebral cortex of the frontal, temporal, parietal, and occipital lobes
In the neocortex, tau IHC highlighted the extent of the NFTs, which were very prominent in all of the lobes from which sections were taken: frontal, temporal, parietal and occipital. Numerous plaques on amyloid-β stain were likewise present in all cortical regions examined. The tau pathology was confined to the gray matter, sparing white matter. There were no ballooned neurons and no astrocytic plaques—two findings one would expect to see in CBD ( Table 2 ).
a AD=Alzheimer’s disease; CBD=corticobasal degeneration; CBS=corticobasal syndrome; PCA=posterior cortical atrophy.
TABLE 2. Neuropathological features of this case compared with a case of corticobasal degeneration a
The case was designated by the neuropathology division as Alzheimer’s-type pathology, Braak stage V–VI (of VI), due to the widespread neocortical tau pathology, with LBD primarily in the limbic areas.
Our patient had AD neuropathology presenting atypically with a young age at onset (52 years old) and a predominantly visual-spatial and corticobasal syndrome as opposed to prominent amnesia. Syndromic diversity is a well-recognized phenomenon in AD. Nonamnesic presentations include not only PCA and CBS but also the logopenic variant of primary progressive aphasia and a behavioral-dysexecutive syndrome ( 20 ). Converging lines of evidence link the topographical distribution of NFTs with syndromic presentations and the pattern of hypometabolism and cortical atrophy. Neuropathological case reports and case series suggest that atypical AD syndromes arise in the setting of higher than normal densities of NFTs in networks subserving the functions compromised, including visual association areas in PCA-AD ( 21 ), the language network in PPA-AD ( 22 ), and frontal regions in behavioral-dysexecutive AD ( 23 ). In a large sample of close to 900 cases of pathologically diagnosed AD employing quantitative assessment of NFT density and distribution in selected neocortical and hippocampal regions, 25% of cases did not conform to a typical distribution of NFTs characterized in the Braak staging scheme ( 24 ). A subset of cases classified as hippocampal sparing with higher density of NFTs in the neocortex and lower density of NFTs in the hippocampus had a younger mean age at onset, higher frequency of atypical (nonamnesic) presentations, and more rapid rate of longitudinal decline than subsets defined as typical or limbic-predominant.
Tau PET, which detects the spatial distribution of fibrillary tau present in NFTs, has corroborated postmortem work in demonstrating distinct patterns of tracer uptake in different subtypes of AD defined by clinical symptoms and topographical distributions of atrophy ( 25 – 28 ). Amyloid PET, which detects the spatial distribution of fibrillar amyloid- β found in amyloid plaques, does not distinguish between typical and atypical AD ( 29 , 30 ). In a longitudinal study of 32 patients at early symptomatic stages of AD, the baseline topography of tau PET signal predicted subsequent atrophy on MRI at the single patient level, independent of baseline cortical thickness ( 31 ). This correlation was strongest in early-onset AD patients, who also tended to have higher tau signal and more rapid progression of atrophy than late-onset AD patients.
Differential vulnerability of selected large-scale brain networks in AD and in neurodegenerative disease more broadly remains poorly understood. There is evidence to support multiple mechanisms that are not mutually exclusive, including metabolic stress to key network nodes, trophic failure, transneuronal spread of pathological proteins (i.e., prion-like mechanisms), and shared vulnerability within network regions based on genetic or developmental factors ( 32 ). In the case of AD, cortical hub regions with high intrinsic functional connectivity to other regions across the brain appear to have high metabolic rates across the lifespan and to be foci of convergence of amyloid-β and tau accumulation ( 33 , 34 ). Tau NFT pathology appears to spread temporally along connected networks within the brain ( 35 ). Patients with primary progressive aphasia are more likely to have a personal or family history of developmental language-based learning disability ( 36 ), and patients with PCA are more likely to have a personal history of mathematical or visuospatial learning disability ( 37 ).
This case highlights the symptomatic heterogeneity in AD and the value of a three-tiered approach to diagnostic formulation in neurodegenerative presentations. It is important to remember that not all AD presents with amnesia and that early-onset AD tends to be more atypical and to progress more rapidly than late-onset AD. Multiple lines of evidence support a relationship between the burden and topographical distribution of tau NFT neuropathology and clinical symptomatology in AD, instantiating network-based neurodegeneration via mechanisms under ongoing investigation.
The authors report no financial relationships with commercial interests.
Supported by NIH grants K08 AG065502 (to Dr. Miller) and T32 HL007627 (to Dr. Miller).
The authors have confirmed that details of the case have been disguised to protect patient privacy.
1 Balasa M, Gelpi E, Antonell A, et al. : Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease . Neurology 2011 ; 76:1720–1725 Crossref , Medline , Google Scholar
2 Mercy L, Hodges JR, Dawson K, et al. : Incidence of early-onset dementias in Cambridgeshire, United Kingdom . Neurology 2008 ; 71:1496–1499 Crossref , Medline , Google Scholar
3 Kothbauer-Margreiter I, Sturzenegger M, Komor J, et al. : Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment . J Neurol 1996 ; 243:585–593 Crossref , Medline , Google Scholar
4 Nasreddine ZS, Phillips NA, Bédirian V, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc 2005 ; 53:695–699 Crossref , Medline , Google Scholar
5 Rizzo M, Vecera SP : Psychoanatomical substrates of Bálint’s syndrome . J Neurol Neurosurg Psychiatry 2002 ; 72:162–178 Crossref , Medline , Google Scholar
6 Rusconi E : Gerstmann syndrome: historic and current perspectives . Handb Clin Neurol 2018 ; 151:395–411 Crossref , Medline , Google Scholar
7 Crutch SJ, Schott JM, Rabinovici GD, et al. : Consensus classification of posterior cortical atrophy . Alzheimers Dement 2017 ; 13:870–884 Crossref , Medline , Google Scholar
8 Armstrong MJ, Litvan I, Lang AE, et al. : Criteria for the diagnosis of corticobasal degeneration . Neurology 2013 ; 80:496–503 Crossref , Medline , Google Scholar
9 Marshall GA, Doyle JJ : Long-term treatment of Hashimoto’s encephalopathy . J Neuropsychiatry Clin Neurosci 2006 ; 18:14–20 Link , Google Scholar
10 Johnson KA, Minoshima S, Bohnen NI, et al. : Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association . Alzheimers Dement 2013 ; 9:e-1–e-16 Crossref , Medline , Google Scholar
11 Shaw LM, Arias J, Blennow K, et al. : Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease . Alzheimers Dement 2018 ; 14:1505–1521 Crossref , Medline , Google Scholar
12 Alladi S, Xuereb J, Bak T, et al. : Focal cortical presentations of Alzheimer’s disease . Brain 2007 ; 130:2636–2645 Crossref , Medline , Google Scholar
13 Renner JA, Burns JM, Hou CE, et al. : Progressive posterior cortical dysfunction: a clinicopathologic series . Neurology 2004 ; 63:1175–1180 Crossref , Medline , Google Scholar
14 Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. : Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy . Neurology 2004 ; 63:1168–1174 Crossref , Medline , Google Scholar
15 Victoroff J, Ross GW, Benson DF, et al. : Posterior cortical atrophy: neuropathologic correlations . Arch Neurol 1994 ; 51:269–274 Crossref , Medline , Google Scholar
16 Dickerson BC, McGinnis SM, Xia C, et al. : Approach to atypical Alzheimer’s disease and case studies of the major subtypes . CNS Spectr 2017 ; 22:439–449 Crossref , Medline , Google Scholar
17 Seeley WW, Crawford RK, Zhou J, et al. : Neurodegenerative diseases target large-scale human brain networks . Neuron 2009 ; 62:42–52 Crossref , Medline , Google Scholar
18 Lee SE, Rabinovici GD, Mayo MC, et al. : Clinicopathological correlations in corticobasal degeneration . Ann Neurol 2011 ; 70:327–340 Crossref , Medline , Google Scholar
19 Whitwell JL, Jack CR Jr, Boeve BF, et al. : Imaging correlates of pathology in corticobasal syndrome . Neurology 2010 ; 75:1879–1887 Crossref , Medline , Google Scholar
20 Warren JD, Fletcher PD, Golden HL : The paradox of syndromic diversity in Alzheimer disease . Nat Rev Neurol 2012 ; 8:451–464 Crossref , Medline , Google Scholar
21 Hof PR, Archin N, Osmand AP, et al. : Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report . Acta Neuropathol 1993 ; 86:215–223 Crossref , Medline , Google Scholar
22 Mesulam MM, Weintraub S, Rogalski EJ, et al. : Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia . Brain 2014 ; 137:1176–1192 Crossref , Medline , Google Scholar
23 Blennerhassett R, Lillo P, Halliday GM, et al. : Distribution of pathology in frontal variant Alzheimer’s disease . J Alzheimers Dis 2014 ; 39:63–70 Crossref , Medline , Google Scholar
24 Murray ME, Graff-Radford NR, Ross OA, et al. : Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study . Lancet Neurol 2011 ; 10:785–796 Crossref , Medline , Google Scholar
25 Ossenkoppele R, Lyoo CH, Sudre CH, et al. : Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease . Alzheimers Dement 2020 ; 16:335–344 Crossref , Medline , Google Scholar
26 Phillips JS, Das SR, McMillan CT, et al. : Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease . Hum Brain Mapp 2018 ; 39:691–708 Crossref , Medline , Google Scholar
27 Tetzloff KA, Graff-Radford J, Martin PR, et al. : Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease . J Alzheimers Dis 2018 ; 62:1713–1724 Crossref , Medline , Google Scholar
28 Xia C, Makaretz SJ, Caso C, et al. : Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease . JAMA Neurol 2017 ; 74:427–436 Crossref , Medline , Google Scholar
29 Formaglio M, Costes N, Seguin J, et al. : In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings . J Neurol 2011 ; 258:1841–1851 Crossref , Medline , Google Scholar
30 Lehmann M, Ghosh PM, Madison C, et al. : Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease . Brain 2013 ; 136:844–858 Crossref , Medline , Google Scholar
31 La Joie R, Visani AV, Baker SL, et al. : Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET . Sci Transl Med 2020 ; 12:12 Crossref , Google Scholar
32 Zhou J, Gennatas ED, Kramer JH, et al. : Predicting regional neurodegeneration from the healthy brain functional connectome . Neuron 2012 ; 73:1216–1227 Crossref , Medline , Google Scholar
33 Buckner RL, Sepulcre J, Talukdar T, et al. : Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease . J Neurosci 2009 ; 29:1860–1873 Crossref , Medline , Google Scholar
34 Hoenig MC, Bischof GN, Seemiller J, et al. : Networks of tau distribution in Alzheimer’s disease . Brain 2018 ; 141:568–581 Crossref , Medline , Google Scholar
35 Liu L, Drouet V, Wu JW, et al. : Trans-synaptic spread of tau pathology in vivo . PLoS One 2012 ; 7:e31302 Crossref , Medline , Google Scholar
36 Rogalski E, Johnson N, Weintraub S, et al. : Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives . Arch Neurol 2008 ; 65:244–248 Crossref , Medline , Google Scholar
37 Miller ZA, Rosenberg L, Santos-Santos MA, et al. : Prevalence of mathematical and visuospatial learning disabilities in patients with posterior cortical atrophy . JAMA Neurol 2018 ; 75:728–737 Crossref , Medline , Google Scholar
- Jeffrey Maneval , M.D. ,
- Kirk R. Daffner , M.D. ,
- Scott M. McGinnis , M.D.
- Seth A. Gale , M.A., M.D. ,
- C. Alan Anderson , M.D. ,
- David B. Arciniegas , M.D.

- Posterior Cortical Atrophy
- Corticobasal Syndrome
- Atypical Alzheimer Disease
- Network Degeneration
Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021
- Open access
- Published: 09 June 2021
- Volume 8 , pages 371–386, ( 2021 )
Cite this article
You have full access to this open access article
- A. P. Porsteinsson 1 ,
- R. S. Isaacson 2 ,
- Sean Knox 3 ,
- M. N. Sabbagh 4 &
- I. Rubino 5
69k Accesses
95 Citations
65 Altmetric
Explore all metrics
Alzheimer’s disease is a progressive, irreversible neurodegenerative disease impacting cognition, function, and behavior. Alzheimer’s disease progresses along a continuum from preclinical disease, to mild cognitive and/or behavioral impairment and then Alzheimer’s disease dementia. Recently, clinicians have been encouraged to diagnose Alzheimer’s earlier, before patients have progressed to Alzheimer’s disease dementia. The early and accurate detection of Alzheimer’s disease-associated symptoms and underlying disease pathology by clinicians is fundamental for the screening, diagnosis, and subsequent management of Alzheimer’s disease patients. It also enables patients and their caregivers to plan for the future and make appropriate lifestyle changes that could help maintain their quality of life for longer. Unfortunately, detecting early-stage Alzheimer’s disease in clinical practice can be challenging and is hindered by several barriers including constraints on clinicians’ time, difficulty accurately diagnosing Alzheimer’s pathology, and that patients and healthcare providers often dismiss symptoms as part of the normal aging process. As the prevalence of this disease continues to grow, the current model for Alzheimer’s disease diagnosis and patient management will need to evolve to integrate care across clinical disciplines and the disease continuum, beginning with primary care. This review summarizes the importance of establishing an early diagnosis of Alzheimer’s disease, related practical ‘how-to’ guidance and considerations, and tools that can be used by healthcare providers throughout the diagnostic journey.
Similar content being viewed by others

The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention
X.-X. Zhang, Y. Tian, … Jin-Tai Yu

Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease
Samantha Budd Haeberlein, P.S. Aisen, … A. Sandrock

Alzheimer’s Disease: Epidemiology and Clinical Progression
Amir Abbas Tahami Monfared, Michael J. Byrnes, … Quanwu Zhang
Avoid common mistakes on your manuscript.
Introduction
D ementia is among the greatest global health crises of the 21st century. Currently, more than 50 million people are living with dementia worldwide ( 1 ), with this number estimated to triple to 152 million by 2050 as the world’s population grows older ( 2 ). Alzheimer’s disease (AD) is the most common cause of dementia and is thought to account for 60–80% of dementia cases ( 3 ). Currently, the total annual cost for AD and other dementias in the USA is $305 billion and is predicted to increase to more than $1.1 trillion by 2050 ( 3 ). This substantial economic burden includes not only healthcare and hospice support for patients with AD ( 3 ) but also lost productivity from patients and caregivers ( 4 ).
AD is a progressive, neurodegenerative disease associated with cognitive, functional, and behavioral impairments, and characterized by two underlying pathological hallmarks: the progressive accumulation of extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) ( 3 ). In AD, aggregated Aβ plaques are deposited within the brain as a result of either reduced Aβ clearance or excessive production ( 5 ); plaque deposition typically occurs ∼20 years before the onset of cognitive impairment ( 6 , 7 ). NFTs are formed by the abnormal accumulation of hyperphosphorylated-tau protein ( 5 ); these can be detected 10–15 years before the onset of symptoms ( 6 , 7 ).
AD follows a progressive disease continuum that extends from an asymptomatic phase with biomarker evidence of AD (preclinical AD), through minor cognitive (mild cognitive impairment [MCI]) and/or neurobehavioral (mild behavioral impairment [MBI]) changes to, ultimately, AD dementia. A number of staging systems have been developed to categorize AD across this continuum ( 7 – 9 ). While these systems vary in terms of how each stage is defined, all encompass the presence/absence of pathologic Aβ and NFTs, as well as deficits in cognition, function, and behavior ( 7 – 9 ). As a result, subtle but important differences exist in the nomenclature for each stage of AD depending on the selected clinical and research classifications (Figure 1 ).
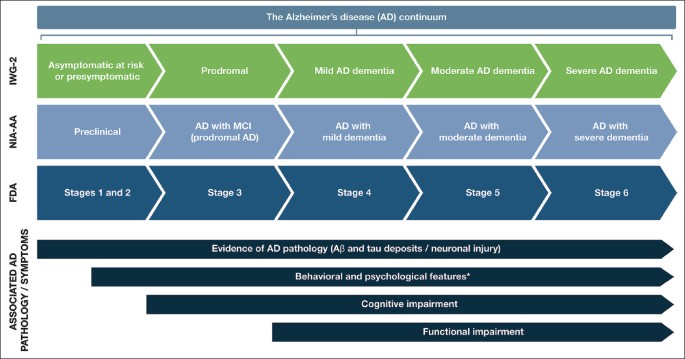
Stages within the Alzheimer’s disease continuum
The AD continuum can be classified into different stages from preclinical AD to severe AD dementia; the nomenclature associated with each stage varies between the different clinical and research classifications. This figure provides a summary of the different naming conventions that are used within the AD community and the symptoms associated with each stage of the continuum; *Mild behavioral impairment is a construct that describes the emergence of sustained and impactful neuropsychiatric symptoms that may occur in patients ≥50 years old prior to cognitive decline and dementia ( 112 ); Abbreviations: Aβ, amyloid beta. AD, Alzheimer’s disease. FDA, Food and Drug Administration. IWG, International Working Group. MCI, mild cognitive impairment. NIA-AA, National Institute on Aging—Alzheimer’s Association
Preclinical AD, as the earliest stage in the AD continuum, comprises a long asymptomatic phase, in which individuals have evidence of AD pathology but no evidence of cognitive or functional decline, and their daily life is unaffected ( 8 ) (Figure 1 ). The duration of preclinical AD can vary between individuals, but typically lasts 6–10 years depending on the age of onset ( 10 , 11 ). The risk of progression from preclinical AD to MCI due to AD (with/without MBI) depends on a number of factors, including age, sex, and apolipoprotein E (ApoE) status ( 11 , 12 ); however, not all individuals who have underlying AD pathology will go on to develop MCI or AD dementia ( 13 , 14 ). A recent meta-analysis of six longitudinal cohorts followed up for an average of 3.8 years found that 20% of patients with preclinical AD progressed to MCI due to AD ( 11 ). A further study by Cho et al., with an average follow-up rate of 4 years, found that 29.1% of patients with preclinical AD progressed to MCI due to AD ( 12 ).
For patients who do progress to MCI due to AD (with/without MBI), initial clinical symptoms typically include short-term memory impairment, followed by subsequent decline in additional cognitive domains ( 15 ) (Figure 1 ). On a day-to-day basis, an individual with MCI due to AD may struggle to find the right word (language), forget recent conversations (episodic memory), struggle with completing familiar tasks (executive function), or get lost in familiar surroundings (visuospatial function) ( 15 , 16 ). As individuals have varying coping mechanisms and levels of cognitive reserve, patients’ experiences and symptomology vary widely; however, patients tend to remain relatively independent at this stage, despite potential marginal deficits in function. The prognosis for patients with MCI due to AD can be uncertain; one study that followed up patients with MCI due to AD for an average of 4 years found that 43.4% progressed to AD dementia ( 12 ). Other studies reported 32.7% and 70.0% of individuals with MCI due to AD progress to AD dementia within 3.2 and 3.6 years of follow-up, respectively ( 17 , 18 ). Patients who do progress to AD dementia will develop severe cognitive deficits that interfere with social functioning and will require assistance with activities of daily living ( 7 ) (Figure 1 ). As the disease progresses further, increasingly severe behavioral symptoms will develop that significantly burden patients and their caregivers, and the disease ultimately results in severe loss of independence and the need for round-the-clock care ( 3 ).
An early diagnosis of AD can provide patients the opportunity to collaborate in the development of advanced care plans with their family, caregivers, clinicians, and other members of the wider support team. Importantly, it also enables patients to seek early intervention with symptomatic treatment, lifestyle changes to maintain quality of life, and risk-reduction strategies that can provide clinically meaningful reductions in cognitive, functional, and behavioral decline ( 19 – 22 ). It can also help reduce healthcare system costs and constraints: a study by the Alzheimer’s Association found that diagnosing AD in the early stages could save approximately $7 trillion. These savings were due to lower medical and long-term care costs for patients with managed MCI than for those with unmanaged MCI and dementia ( 3 ). Furthermore, an early diagnosis will be vital for patients when a therapy addressing the underlying pathology of AD becomes available; currently 19 biologic compounds are under Phase 2 or 3 investigation ( 23 ). Physicians will need to be prepared for the approval of these treatments, to optimize the potential benefit and prolong preservation of patients’ cognitive function and independence beyond that associated with current standard of care ( 19 ).
As the prevalence of AD continues to grow, the advancement of AD patient diagnosis will require an orchestrated effort, starting in the primary care setting and subsequently involving multiple healthcare provider (HCP) specialties (e.g., nurse practitioner [NP] or physician assistant [PA]) throughout the disease continuum. Galvin et al. recently highlighted the need for HCPs to work as an integrated, patient-centered care team to accommodate the growing and diverse population of patients with AD, beginning with diagnosis ( 24 ). For patients to receive a timely diagnosis, it is vital to implement an approach that minimizes the burden placed on the patient, clinician, and healthcare system ( 25 ). Here, we summarize the importance of establishing an early diagnosis of AD, related practical ‘how-to’ guidance and considerations, and tools that can be used by healthcare providers throughout the diagnostic journey.
The importance of an early diagnosis
Historically, a diagnosis of AD has been one of exclusion, and one only made in the latter stages of disease ( 26 ); however, the disease process can take years to play out, exacting a significant toll on the patient, caregiver, and healthcare system along the way ( 27 ).
To mitigate this burden, the early and accurate detection of AD-associated symptoms in clinical practice represents a critically needed but challenging advancement in AD care ( 19 , 28 – 30 ). Usually, a patient with early signs/symptoms of AD will initially present in a primary care setting ( 30 ). For some patients, minor changes in cognition and/or behavior may be detected during a routine wellness visit or an appointment to discuss other comorbidities ( 24 ). As the PCP is often the first to observe a patient’s initial symptomatology, it is vital they recognize the early signs and symptoms, and understand how to use the most appropriate assessment tools designed to detect these early clinical effects of the disease.
Because the neuropathologic hallmarks of AD (Aβ plaques and NFTs) can be detected decades prior to the onset of symptoms ( 6 , 7 ), biomarkers reflecting this underlying pathology represent an important opportunity for early identification of patients at greatest risk of developing MCI due to AD. Biomarkers support the diagnosis of AD (especially important early on when symptoms can be subtle), and the U.S. Food and Drug Administration (FDA) has recently published guidelines that endorse their use in this population ( 9 ). The National Institute on Aging—Alzheimer’s Association (NIA-AA) has recently created a research framework that acknowledges the use of biomarkers for diagnosing AD in vivo and monitoring disease progression ( 7 ).
Important biomarker information can be gathered from imaging modalities such as magnetic resonance imaging (MRI) and positive emission tomography (PET) that visualize early structural and molecular changes in the brain, respectively ( 25 , 30 ). Fluid biomarker testing, such as cerebrospinal fluid (CSF) can also be used; CSF biomarkers can directly reflect the presence of Aβ and aggregated tau within the brain ( 7 , 31 ). As will be discussed in more depth later in this article, a large number of clinical studies have shown that Aβ and tau biomarkers can contribute diagnostically important information in the early stages of disease ( 32 ). There is ongoing research to expand the current range of tests that can be used by clinicians as part of the multistage diagnostic process ( 25 ). For instance, once approved, blood-based biomarkers could be used to identify patients at risk of developing AD and for monitoring disease progression ( 33 , 34 ), which would also reduce the current capacity constraints associated with PET imaging ( 25 ).
Practical guide for an early diagnosis of Alzheimer’s disease in clinical practice
As already raised, recent recommendations for evolving AD care to a more patient-centric, transdisciplinary model include guidance on realizing an efficient diagnostic process—one in which HCPs, payers, and specialists are encouraged to combine their efforts to ensure the early warning signs of AD are not overlooked ( 24 ). The recommendations include dividing the diagnosis of AD into the following steps: detect, assess/differentiate, diagnose, and treat (Figure 2 ). We present here a practical guide for the early diagnosis of AD, based on this outlined approach, including a case study to highlight each of these key steps.
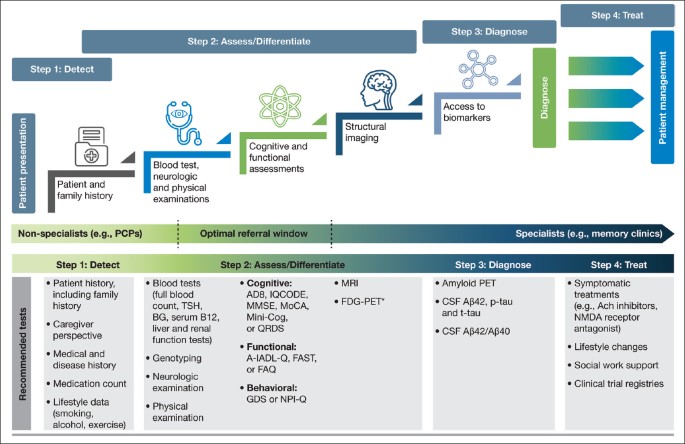
A stepwise infographic to highlight key stages within the diagnostic process, along with the recommended tests to support each step
The diagnostic process for AD can be divided into the following steps: detect, assess/differentiate, diagnose, and treat. It is important for clinicians to utilize appropriate tests when investigating a patient suspected of having AD in the early stages. Here, we highlight the most valuable tests for each step and which ones should be used in a primary care or specialist setting; *FDG-PET is usually considered after a diagnostic work-up; Abbreviations: A-IADL-Q, Amsterdam Instrumental Activities of Daily Living Questionnaire. Aβ, amyloid beta. Ach, acetylcholine. BG, blood glucose. CSF, cerebrospinal fluid. FAQ, Functional Activities Questionnaire. FAST, Functional Analysis Screening Tool. FDG-PET, fluorodeoxyglucose-PET. GDS, Geriatric Depression Scale. IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly. Mini-Cog, Mini Cognitive Assessment Instrument. MMSE, Mini-Mental State Examination. MoCA, Montreal Cognitive Assessment. MRI, magnetic resonance imaging. NMDA, N-Methyl-D-aspartic acid. NPI-Q, Neuropsychiatric Inventory Questionnaire. PCP, primary care physician. PET, positive emission tomography. p-tau, phosphorylated tau. QDRS, Quick Dementia Rating System. TSH, thyroid-stimulating hormone. t-tau, total tau depressive symptoms and anxiety, as well as irritability. Based on the patient’s symptoms, the PCP felt his presentation warranted further clinical assessment.
Step 1: Detect
The role of primary care in the early detection of ad.
The insidious and variable emergence of symptoms associated with AD and other dementias can make recognition extremely challenging, particularly in a primary care setting ( 30 , 35 ). Clinicians often have limited time with patients, so it is vital that they are able to quickly and accurately recognize the early signs and symptoms associated with AD ( Table 2 ) ( 3 , 30 , 36 ), and training for nurses, NPs, and PAs who may have more time to observe patients should provide substantial benefits. Although extremely variable, initial symptoms may include short-term memory loss or psychological concerns, including depressive symptoms and a loss of purpose ( 36 ).
Patients, family members, and even HCPs themselves may present barriers to the diagnosis of early-stage AD. Patients may hide their symptoms or even avoid making an appointment until their symptoms significantly affect their day-to-day life due to fear of the stigma associated with a diagnosis of AD ( 19 ). Additionally, patients, family members, and PCPs/HCPs may dismiss or misinterpret symptoms as simply part of the normal aging process ( 30 ). Retrieving information from a trusted family member or informant/caregiver is essential when trying to assess a patient for suspected AD, as this perspective can provide a more objective understanding of the daily routine, mood, and behavior of the patient, and how this may have changed over time ( 30 ). For patients presenting with even subtle symptoms associated with AD, it is important that the PCP/HCP conducts an initial assessment to confirm the presence of symptoms using a validated assessment for early-stage AD detection (Figure 2 ; Step 2: Assess/Differentiate).
Case study: Presentation
A 63-year-old Caucasian male (J.K.) presented to his PCP with short-term memory loss over the last 2 years ( Table 1A ). Accompanied by his wife, he acknowledged his job had been affected by issues with his short-term memory; however, he considered his memory similar to that of his peers. His wife reported that people at work had started to notice him struggling to keep up, and also that family had to remind him of his upcoming appointments. He admitted to having intermittent
Step 2: Assess and differentiate
Primary care: initial assessment when a patient presents.
When a patient initially presents with symptoms consistent with early stages of AD, a clinician must first conduct a comprehensive clinical assessment to rule out other potential non-AD causes of cognitive impairment (Figure 2 ). PCPs are well placed to conduct these initial assessments, as they may not require specialist input or hospital tests. During the initial assessment, the primary objective of the clinician should be to exclude possible reversible causes of cognitive impairment, such as depression, or vitamin, hormone, and electrolyte deficiencies ( 37 ). The initial assessment should include a thorough history to identify potential risk factors associated with AD, including a family history of AD or related dementias in first-degree relatives ( 31 , 38 ). Other known risk factors for AD that should be identified include age, female sex, ApoE ε4 status, physical inactivity, low education, diabetes, and obesity ( 3 ). It is also important to review for pre-existing medical conditions or prescribed medications that could be a cause of the patient’s cognitive impairment ( 36 ). Additionally, when conducting a thorough history, open-ended, probing questions should be directed to both the patient and the informant to ascertain how the patient’s cognition has changed over time and how the cognitive deficits affect their everyday activities; example questions for the initial assessment are detailed in Table 3 ( 30 ). Engaging with informants/caregivers is key to capturing additional information to help support all assessments. A routine differential diagnosis of AD begins with a detailed history, physical and neurologic examinations, and bloodwork analyses, followed by cognitive assessments and functional evaluation (Figure 2 ).
Primary care: Physical examination and blood analyses
A physical examination and blood tests can identify comorbid contributory medical conditions and reversible causes of cognitive impairment. A physical examination, including a mental status and neurological assessment, should be conducted to detect conditions such as depression and, for example, to look for signs such as issues with speaking or hearing as well as signs that could indicate a stroke ( 37 ). As part of the physical exam, a physician may ask the patient about diet and nutrition, review all medications (to see if these are the cause of any cognitive impairment, e.g. anti-cholinergics, analgesics, or sleep aids and anxiolytics), check blood pressure, temperature and pulse, and listen to the heart and lungs ( 36 , 39 ).
Blood tests can rule out potentially treatable illnesses as a cause of cognitive impairment, such as vitamin B 12 deficiency or thyroid disease ( 37 ). Suggested blood analyses include: 1) complete blood cell count; 2) blood glucose; 3) thyroid-stimulating hormone; 4) serum B 12 and folate; 5) serum electrolytes; 6) liver function; and 7) renal function tests ( 30 ). Although not routinely used in clinical practice, clinicians may request ApoE genotyping, as this can help assess the genetic risk of developing AD. ApoE is the dominant cholesterol carrier within the brain that supports lipid transport and injury repair ( 40 , 41 ), and the APOE gene exists as three polymorphic alleles: APOE ε2, ε3, and ε4. The ε4 allele of ApoE is associated with increased AD risk, whereas the ε2 allele is protective ( 40 , 42 ). The number of ApoE ε4 alleles a person carries increases their risk of developing AD and the age of disease onset ( 43 ). Homozygous ε4 carriers (those with two copies of the ε4 allele) have the greatest risk of developing AD and the lowest average age of onset ( 43 ). In some practice settings, ApoE genotyping can only be conducted by a genetic counselor; a referral for more comprehensive genetic testing may be considered by the HCP if there is a family history of early-onset AD or dementia. Consumer tests are also becoming more readily available for patients wanting to determine their risk of developing diseases such as AD based on genetic risk factors ( 44 ).
Primary care: Cognitive, functional, and behavioral assessments
Cognitive assessments.
If a patient is suspected of having AD following an initial assessment in primary care, and they are <65 years old, or if the case is complex, a referral to a dementia specialist such as a neurologist, geriatrician, or geriatric psychiatrist may be required for further evaluation. The specialist would then use an appropriate battery of cognitive, functional, and behavioral tests to assess the different aspects of disease, and ultimately to confirm diagnosis. However, not all patients with suspected cognitive deficits are immediately referred to a dementia specialist at this stage, which is only partly due to limited numbers of specialists ( 25 ) (Figure 2 ). In clinical practice, a two-stage process is often employed. This involves an initial ‘triage’ step conducted by non-specialists to clinically assess and select those patients who require further evaluation by a dementia specialist ( 45 ). During this ‘triage’ step, there are several clinical assessments available to non-specialists for assessing the presence of cognitive and functional impairments and behavioral symptoms (Table 4 ) ( 28 , 35 , 46 – 55 ).
Previous research has shown that clinicians have a tendency to choose one assessment over another due to their familiarity with the assessment, time constraints, or specific resources available to them within their clinic ( 30 ), but clinicians need to be aware of, and prepared to use, the most patient-appropriate assessments: the cultural, educational, and linguistic needs of the patient are important considerations ( 30 , 36 , 56 – 58 ). Some assessments have been translated into different languages or shortened, or have education-adjusted scoring classifications, where required ( 56 – 58 ).
Cognitive assessments that can be conducted quickly (<10 minutes), such as the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA), can be used by non-specialists to identify the presence and severity of cognitive impairment in patients before referring to a dementia specialist ( Table 4 ) ( 36 ). Both the MMSE and MoCA are used globally in clinical practice, particularly in primary care, but vary in terms of their sensitivity to identify AD in the early stages ( 28 , 59 ). The MMSE is sensitive and reliable for identifying memory and language deficits in general but has limitations in identifying impairments in executive functioning ( 59 ). MoCA was originally developed to improve the detection of MCI ( 28 ) and is more sensitive than the MMSE in its assessment of memory, visuospatial, executive, and language function, and orientation to time and place ( 59 ). Both tests are relatively easy to administer and take around 10 minutes to complete. Neither assessment requires extensive training by the clinician, although MoCA users do need to undergo a 1-hour certification as mandated by the MoCA Clinic and Institute ( 28 , 60 ).
For time-constrained clinicians, the Mini Cognitive Assessment Instrument (Mini-Cog) may be an appropriate tool to assess cognitive deficits that focus on memory, and components of visuospatial and executive function ( Table 4 ). The assessment includes the individual learning three items from a list, drawing a clock, and then recalling the three-item list. The Mini-Cog can be useful for clinicians in primary care, as it requires no training and the results are easy to interpret. As an alternative to these tests, PCPs might also consider using an informant-based structured questionnaire such as the AD8 or Informant Questionnaire on Cognitive Decline in the Elderly to help guide discussions with the patient and caregiver ( Table 4 ) ( 28 ).
Functional assessments
Functional assessments are valuable in identifying changes in a patient’s day-to-day functioning through the evaluation of their instrumental activities of daily living (IADLs). IADLs are complex activities that are necessary for the individual to function independently (e.g., cooking, shopping, and managing finances) and can be impaired during the early stages of cognitive impairment. While it is possible that functional decline may occur as a part of normal aging, a decline in a person’s IADL performance is strongly associated with neurodegenerative diseases such as AD ( 61 ). In the early stages of AD, patients may be functionally independent, and any impairment in IADLs may be subtle, such as difficulties paying bills or driving to new places. A patient’s functional independence is essential for their well-being and mental health ( 62 ), particularly in the early stages of the disease when the individual may still be working and socializing relatively independently ( 3 ). Consequently, functional independence is one of the most important clinical features for patients with AD. As the disease progresses, and patients have increasing functional impairment, this significantly impacts on their independence, and subsequently their and their family/caregiver’s quality of life.
Functional assessment is, therefore, an integral part of the diagnostic process for AD. The Functional Activities Questionnaire (FAQ) is an informant questionnaire that assesses the patient’s performance over a 4-week period and may take only a few minutes to complete ( Table 4 ). The questionnaire is scored from ‘normal’ to ‘dependent’, using numerical values assigned to categories, with higher scores indicative of increasing impairment ( 47 ). Previous research has shown that the FAQ has high sensitivity and reliability for detecting mild functional impairment in patients with MCI ( 47 ).
Determining an individual’s functional independence can be challenging and the clinician may require additional input from an informant to determine a patient’s functional decline and their ongoing ability to conduct activities of daily living ( 37 ). The clinician can gain greater insight through the informant into the patient’s day-to-day life and any issues the patient is having at home. This type of information is vital to the clinician, and when combined with other assessment tools, can help to narrow the differential diagnosis.
Behavioral assessments
Patients with suspected AD may experience several behavioral symptoms such as anxiety, disinhibition, apathy, and depression ( Table 2 ). In the early stages of disease, such symptoms are generally associated with poor long-term outcomes and caregiver burden, and are particularly distressing to both patients and their families ( 63 ). It is important for clinicians to use appropriate assessments to identify behavioral and psychiatric symptoms that are caused by neurodegenerative diseases, such as AD, rather than by alternative causes, such as a mood disorder.
The Geriatric Depression Scale (GDS) and Neuropsychiatric Inventory Questionnaire (NPI-Q) can be used by clinicians to assess neuropsychiatric symptoms in patients for whom early-stage AD is suspected ( Table 4 ). The GDS is a 15-item (or longer 30-item) questionnaire that assesses mood, has good reliability in older populations for detecting depression, and can be completed by the patient within 5–10 minutes ( 63 ). The NPI-Q can be used in conjunction with or as an alternative to the GDS. The NPI-Q is completed by a knowledgeable informant or caregiver who can report on the patient’s neuropsychiatric symptoms. The NPI-Q can be conducted in around 5 minutes to determine both the presence and severity of symptoms across several neuropsychiatric domains including depression, apathy, irritability, and disinhibition ( 49 ). Consequently, as it assesses depression, it can be used as an alternative to GDS if time constraints do not allow for both to be completed.
Behavioral symptoms can be non-specific, so it is important for clinicians to consider and rule out other potentially treatable causes of impairment when assessing this domain. For example, depression is associated with concentration and memory issues ( 64 ); apathy can occur in non-depressed elderly individuals and can impact cognitive function ( 65 ). Signs/symptoms such as social withdrawal, feelings of helplessness, or loss of purpose should be investigated closely, as these could be indicative of depression alone. It is important for clinicians to recognize that if changes over time in cognitive symptoms and mood symptoms match, then depression is most likely to be the root cause of subtle cognitive decline, rather than AD ( 28 ).
Primary care clinician checklist
If AD is still suspected following clinical assessment, referral to a specialist for further diagnostic testing, including imaging and fluid biomarkers, may be required. It is important the clinician confirms the following checks/assessments before the patient undergoes further evaluation:
Confirm medical and family history
Review the patient’s medications for any that could cause cognitive impairment
Perform blood tests to eliminate potential reversible causes of cognitive impairment
Conduct a quick clinical assessment to confirm the presence of cognitive impairment
Specialist role in assessment
Following the initial assessment in primary care, further cognitive, behavioral, functional, and imaging assessments can be carried out in a specialist setting. With their additional AD experience, access to other specialties, and possibly fewer time constraints than the PCP, the specialist is able to conduct a more comprehensive testing battery, using additional clinical assessments and biomarkers to determine causes of impairment and confirm diagnosis (Figure 2 ).
Because the cognitive impacts of early-stage AD may vary from patient to patient, it is important to consider which cognitive domains are affected in these early stages when considering which assessments to use. Specialists are able to conduct a full neuropsychological test battery that covers the major cognitive domains (executive function, social cognition/emotions, language, attention/concentration, visuospatial and motor function, learning and memory); preferably, a battery should contain more than one test per domain to ensure adequate sensitivity in capturing cognitive impairment ( 66 ). This step can help with obtaining an in-depth understanding of the subtle changes in cognition seen in the early stages of AD and enables the clinician to monitor subsequent changes over time.
Typically, episodic memory, executive function, visuospatial function, and language are the most affected cognitive domains in the early stages of AD ( 29 , 67 , 68 ). Currently, most cognitive assessment tools focus on a subset of the overall dimensions of cognition; it is therefore vital the clinician chooses the correct test to assess impairment in these specific cognitive domains that could be indicative of AD in the early stages. As cognitive impairment in the early stages of AD can be subtle and vary significantly between individuals ( 29 ), clinicians must choose appropriate, sensitive tests that can detect these changes and account for a patient’s level of activity and cognitive reserve ( 29 ). If there is large disparity in results across cognitive assessments, it is important for the clinician to shape their assessments based on the patient’s history. If the patient’s history is positive for neurodegenerative disease, but one assessment does not reflect this, it is important to conduct further tests to ascertain the cause of the cognitive impairment.
The Quick Dementia Rating System (QDRS) can be used by specialists to assess cognitive impairment ( Table 4 ). This short questionnaire (<5 minutes) is completed by a caregiver/informant and requires no training. The QDRS assesses several cognitive domains known to be affected by AD, including memory, language and communication abilities, and attention. The questionnaire can reliably discriminate between individuals with and without cognitive impairment and provides accurate staging for disease severity ( 28 ).
The Amsterdam IADL Questionnaire (A-IADL-Q) and Functional Assessment Screening Tool (FAST) can both be used to assess a patient’s functional ability ( Table 4 ) ( 53 ). The A-IADL-Q is a reliable computerized questionnaire that monitors a patient’s cognition, memory, and executive functioning over time. This questionnaire is completed by an informant of the patient and takes 10 minutes to complete ( 53 ). For patients with suspected early stage AD, the A-IADL-Q is a useful tool to monitor subtle changes in IADL independence over time and is less influenced by education, gender, and age than other functional assessments ( 53 ). The FAST is a useful assessment for clinicians to identify the occurrence of functional and behavioral problems in patients with suspected AD. The questionnaire is completed by informants who interact with the patient regularly; informants are required to answer Yes/No to a number of questions focusing on social and non-social scenarios ( 55 ).
Structural imaging
Structural imaging, such as MRI, provides clinically useful information when investigating causes of cognitive impairment ( 69 ) (Figure 2 ). MRI is routinely conducted to exclude alternative causes of cognitive impairment, rather than support a diagnosis of AD ( 37 , 70 ). It is well known that medial temporal lobe atrophy is the best MRI marker for identifying patients in the earliest stages of AD ( 70 , 71 ); however, specific patterns of atrophy may also be indicative of other neurodegenerative diseases. Atrophy alone is rarely sufficient to make a diagnosis. MRI findings can help to narrow the differential diagnosis, and the results should be considered in the context of the patient’s age and clinical examination ( 69 – 71 ).
Clinicians are advised to take a stepwise approach when reviewing structural imaging reports of a patient with suspected AD. These steps include: 1) excluding brain pathology that may be amenable to surgical intervention (e.g., the scan will show regions of hyper- or hypointensity rather than a uniform signal); 2) assessing for brain microbleeds (e.g., looking at signal changes within different areas of the brain can identify vascular comorbidities); and 3) assessing atrophy (e.g., medial temporal lobe atrophy is characteristic of AD) ( 69 ). Radiologists can conduct a quick and easy visual rating of any medial temporal lobe atrophy; these results can then be utilized by the specialist, in conjunction with a clinical assessment, to determine the likely cause of cognitive impairment. If the clinician is unable to determine a differential diagnosis, additional confirmatory tests can be requested.
Fluorodeoxyglucose-PET (FDG-PET) is a useful structural imaging biomarker that can support an early and differential diagnosis ( 72 ); however, specialists usually prefer to use this after their initial diagnostic work-up. As the brain relies almost exclusively on glucose as its source of energy, FDG (a glucose analog) can be combined with PET to identify regional patterns of reduced brain metabolism and neurodegeneration ( 70 , 72 ). FDG-PET is not recommended for diagnosing patients with preclinical AD, as there is no way to ascertain whether the hypometabolism is directly related to AD pathology ( 73 ); however, clinicians may refer patients with more established symptomatology for an FDG-PET scan to identify regions of glucose hypometabolism and neurodegeneration that could be indicative of AD ( 70 ).
Case study: Assess/differentiate
The initial assessment by the primary care clinician revealed that J.K.’s medical history was significant for hypertension, dyslipidemia, mild obesity, and glucose intolerance ( Table 1B ). There was no history of cerebrovascular events, significant head injuries, or focal findings on the neurologic exam. Besides the vascular risk factors, no medical conditions or current medications were found to be likely contributors to the cognitive deficit. The patient had a positive family history of dementia, where the onset typically occurred in the late 60s. Genotyping showed the patient to be a homozygous carrier of two ApoE ε4 alleles. Blood tests revealed elevated serum glucose and C-reactive protein but were otherwise normal. The patient had an unremarkable mental status examination, and his MoCA score was 21/30, with points lost on orientation, recall, and naming ( Table 1C ).
The patient was referred to a memory clinic for further assessment. The dementia specialist referred the patient for an MRI that predominantly showed mild small vessel disease and mild generalized atrophy with a significant reduction in hippocampal volume and ratio. Based on his medical and family history, cognitive assessments, and structural imaging results, the specialist deemed the severity of cognitive impairment to be in the mild range; consequently, the specialist referred the patient for biomarker assessment to determine the underlying cause.
Step 3: Diagnose
Historically, AD was only diagnosed postmortem until we developed the ability to ascertain the underlying pathology associated with the disease in new ways, namely imaging and fluid biomarkers. However, despite supportive results from single- and multicenter trials, the use and reimbursement of imaging and fluid biomarkers to support the diagnosis of AD still vary considerably between countries ( 70 ).
Imaging biomarkers
Recent advances have allowed physicians to visualize the proteins associated with AD, namely Aβ and tau, via PET scanning. Amyloid PET is currently the only imaging approach recommended by the Alzheimer’s Association and the Amyloid Imaging Task Force to support the diagnosis of AD ( 70 ). Amyloid PET utilizes tracers (florbetapir, flutemetamol, and florbetaben) that specifically bind to Aβ within amyloid plaques; a positive amyloid PET scan will show increased cortical retention of the tracer in regions of Aβ deposition within the brain ( 74 ), thus confirming the presence of Aβ plaques in the brain ( 74 , 75 ) and directly quantify brain amyloid pathology ( 76 ), thus making it a useful tool to supplement a clinical battery to diagnose AD ( 3 , 74 ). However, a positive amyloid PET scan alone does not definitively diagnose clinical AD, and these results must be combined with other clinical assessments, such as cognitive assessment, for an accurate diagnosis ( 74 ). It is also important to note that amyloid PET is expensive and not readily reimbursed by health insurance providers ( 70 ); if it is not possible to access amyloid PET, biomarker confirmation can be assessed using CSF.
Fluid biomarkers
An additional or alternative tool to amyloid PET is the collection and analysis of CSF for the presence of biomarkers associated with AD pathology. Patients who have symptoms suggestive of AD can be referred for a lumbar puncture to analyze their CSF for specific AD-associated biomarkers ( 3 ). CSF biomarkers are measures of the concentrations of proteins in CSF from the lumbar sac that reflect the rates of both protein production and clearance at a given timepoint ( 7 ). Lumbar punctures can be conducted safely and routinely in an outpatient setting or memory clinic ( 77 ). However, many patients still worry about the pain and possible side effects associated with the procedure and may require additional information and support from the clinician to undertake the procedure ( 77 ). Appropriate use criteria are available for HCPs to help identify suitable patients for lumbar puncture and CSF testing ( 78 ). For example, individuals presenting with persistent, progressing, and unexplained MCI, or those with symptoms suggestive of possible AD, should be referred for lumbar puncture and CSF testing ( 78 ). However, lumbar puncture and CSF testing are not recommended for determining disease severity in patients who have already received a diagnosis of AD or in lieu of genotyping for suspected autosomal dominant mutation carriers ( 78 ).
Because there is strong concordance between CSF biomarkers and amyloid PET, either can be used to confirm Aβ burden ( 79 ). As such, CSF biomarkers are widely accepted within the AD community to support a diagnosis ( 80 ). AD biomarkers from the brain can be detected in CSF well before the onset of overt clinical symptoms in early-stage AD ( 6 , 7 ). Core AD CSF biomarkers, such as Aβ42 (one of two main isoforms of Aβ and a major constituent of Aβ plaques) and phosphorylated tau (p-tau) and total tau (t-tau), can be measured to determine the presence of disease ( 80 ).
When interpreting CSF analyses for a patient with suspected AD, it is important to remember that AD is associated with decreased CSF Aβ42 and increased tau isoforms ( 32 ). Decreased CSF Aβ42 levels are a reflection of increased Aβ aggregation and deposition within the brain ( 32 ), and the concentration of CSF Aβ42 directly relates to the patient’s amyloid status (e.g., the presence or absence of significant amyloid pathology) and the total amount of Aβ peptides (e.g., Aβ42 and Aβ40) ( 32 ). Specialists’ use of ratios of these CSF biomarkers (e.g., Aβ42/40) rather than single CSF biomarkers alone has been shown to adjust for potential differences in Aβ production and provide a better index of the patient’s underlying amyloid-related pathology ( 81 ). The increase in CSF p-tau and t-tau associated with AD may directly reflect the aggregation of tau within the brain and neurodegeneration, respectively ( 32 ). P-tau in CSF provides a direct measure of the amount of hyperphosphorylated tau in the brain, which is strongly suggestive of the presence of NFTs, whereas CSF t-tau can predict the level of neurodegeneration in a patient with suspected AD; however, t-tau is also increased in other neurologic conditions ( 32 ).
Ultimately, the clinical decision to use amyloid PET or CSF to confirm amyloid and tau pathology can be affected by several practical factors (Table 5 ) ( 70 , 77 , 80 , 82 – 85 ).
Emerging diagnostic tools
Access constraints for amyloid PET have driven the need for alternative sensitive and specific CSF and blood-based biomarkers that can detect AD-associated pathology in the early stages ( 86 ). Significant efforts have been undertaken over the last decade to identify blood-based biomarkers to: 1) detect AD pathology; 2) identify those at risk of developing AD in the future; and 3) monitor disease progression ( 33 , 34 , 87 ). At present, only a limited number of approved blood-based assays are available to clinicians to detect AD pathology ( 88 ); however, several novel assays are currently under investigation, including those measuring various phosphorylated forms of tau, including p-tau181 and p-tau217 ( 89 ). Investigational use of plasma p-tau181 (an isoform of tau) has been shown to differentiate AD from other neurodegenerative diseases and predict cognitive decline in patients with AD ( 33 ). CSF p-tau217 (a different isoform of tau) is a promising biomarker under investigation for detecting preclinical and advanced AD ( 86 , 90 ). Given that blood testing is already a well-established part of clinical routines globally and can easily be performed in a variety of clinical settings, blood-based biomarkers could in future serve as the potential first step of a multistage diagnostic process. This would be a benefit to clinicians, particularly those in primary care, by helping to identify individuals requiring a referral to a specialist for diagnostic testing ( 87 ).
Case study: Diagnose
J.K. underwent a lumbar puncture for CSF analysis, which showed decreased Aβ42 and increased p-tau and t-tau protein ( Table 1D ). Based on the results from the genotyping, cognitive assessments, MRI, and CSF biomarkers, the clinician confirmed that the likely cause of the patient’s cognitive deficits was early-stage AD, especially in view of a positive family history of dementia with similar age of onset.
Step 4: Treat
The role of the clinician following a diagnosis of early-stage AD is to discuss the available management and treatment options while providing emotional and practical support to the patient, caregiver, and family where appropriate ( 37 ). Clinicians can also refer the patient and their caregiver(s) to social services for further support, as well as help connect them with reliable sources of information and even local research opportunities and clinical trials.
One important role for a clinician treating a patient diagnosed with early-stage AD is to closely monitor the patient’s disease progression through regular follow-up appointments (e.g., every 6–12 months); clinicians should encourage patients (and the caregiver) to make additional follow-up appointments, especially should symptoms worsen. Routine cognitive and functional assessments (Table 4 ) should be used to monitor disease progression; these tools can be used to identify unexpected trends, such as rapid decline, which could prompt the need for additional medical evaluation such as blood tests, imaging, or biomarker analyses. Results from such tests could help guide management and/or treatment decisions over the course of the patient’s disease.
Non-pharmacologic therapies (e.g., diet and exercise) may be employed for patients with early AD, with the goal to maintain or even improve cognitive function and retain their ability to perform activities of daily living. For patients in the early stages of disease, dietary changes (e.g., following a healthy diet high in green, leafy vegetables, fish, nuts, and berries), physical exercise, and cognitive training have demonstrated small but significant improvements in cognition ( 36 , 91 ). Nonpharmacologic therapies can have a positive impact on quality of life and are generally safe and inexpensive ( 36 ); however, compliance with these non-pharmacologic therapies should be monitored by the clinician. Research suggests that multimodal therapies, such as cognitive stimulation therapy, may also be more effective when used in combination with pharmacologic treatments ( 91 ).
Several pharmacologic treatments have received regulatory approval to treat the symptoms of mild to severe AD dementia. Acetylcholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and N-methyl-D-aspartate receptor antagonists (memantine) can be prescribed to patients to temporarily ameliorate the symptoms of AD dementia such as cognitive and functional decline ( 92 – 96 ). Meta-analyses of donepezil, rivastigmine, and galantamine have shown that patients with mild-to-moderate AD dementia experience some benefits in cognitive function, activities of daily living, and clinician-rated global clinical state ( 93 , 94 , 97 ). Furthermore, treatment with acetylcholinesterase inhibitors and/or memantine has also been shown to modestly improve measures of global function and temporarily stabilize measures of activities of daily living ( 96 ). However, it is important to note that these drugs provide only temporary, symptomatic benefit and that not all patients respond to treatment ( 36 , 98 ). Critically, none of the current drugs available address the underlying pathophysiology or alter the ultimate disease course.
Following AD diagnosis, a comprehensive approach toward clinical care can be individualized based on the patient’s specific AD risk factors ( 20 , 21 ). Clinicians should consider managing uncontrolled vascular risk factors (e.g., hypertension, hyperlipidemia, diabetes) with antithrombotics, antihypertensives, lipid-lowering, and/or antidiabetic agents, respectively, to reduce the risk of cerebrovascular ischemia and stroke, and subsequent cognitive decline ( 36 , 99 ). They should also consider the management of the patient’s behavioral symptoms. For most patients in the early stages of disease, behavioral symptoms will be relatively mild, and no pharmacologic management is required; however, pharmacologic treatment, such as a low-dose selective serotonin reuptake inhibitor, can be prescribed for patients with AD-associated depression and anxiety ( 100 , 101 ).
Specialist clinician checklist
The specialist’s role is critical to further evaluating the initial checks/assessments, providing the diagnosis, and developing the individualized patient management plan:
Identify deficits to specific cognitive domains using appropriate tests
Confirm functional performance, using patient and caregiver assessments
Perform structural imaging to complete assessment of the patient
Confirm diagnosis with imaging or fluid biomarkers
Develop a personalized management and follow-up plan
Direct the patient to additional support resources such as the Alzheimer’s Association
Case study: Treat
Following diagnosis, J.K. was advised on the available management options and research opportunities ( Table 1E ). The specialist emphasized the need to control his vascular risk factors and suggested lifestyle modifications to optimize the management of his other medical problems. The patient’s neuropsychiatric symptoms were considered mild and did not require pharmacologic intervention. The patient was also provided with details for a local social worker and directed toward further disease-specific information from the Alzheimer’s Association related to his disease. The patient was encouraged to return for additional follow-up visits so that his disease and associated symptoms could be appropriately monitored and managed.
Future perspectives
An early diagnosis of AD will become increasingly important as treatments that alter the underlying disease pathology become available—particularly given the expectation that such treatments will be more effective in preserving cognitive function, and thus prolonging independence, when given early in the course of the disease ( 19 ). The approval of such treatments will likely lead to an increased awareness of cognitive impairment and other AD-associated symptoms among both the public and non-specialists, such as those in primary care settings. This may encourage more patients/family members to seek help at an earlier stage of disease than is currently seen in community practice. Increased use of sensitive screening measures to proactively assess for the presence of AD symptoms will help identify patients suspected of having early AD. Assessment of cognitive impairment during a Medicare Annual Wellness Visit is inconsistent; the U.S. Preventative Services Task Force, whilst recognizing the importance of MCI, has maintained its decision that there is insufficient evidence to support the mandate of cognitive screening. However, sensitive screening procedures, along with the availability of disease-modifying treatments, are likely to change their recommendations. There is also a need for a mandated, standardized screening approach internationally. Together, this will result in an increase in patients requiring diagnosis, increasing the demand for specialists to evaluate and diagnose, the need for amyloid confirmation, and wait times for patients, which will collectively put further pressure on an already-stretched healthcare infrastructure ( 25 ).
Nevertheless, efforts continue within the AD field to streamline the diagnostic process. Planning for and implementing change will not only improve patient management now but also help prepare healthcare systems for an approved disease-modifying treatment for AD. A flexible, multidisciplinary team approach is recommended to integrate the care needed to detect, assess, differentiate, diagnose, treat, and monitor a diverse AD population ( 24 ). The development of tests that could be carried out routinely in a primary care setting, such as blood-based AD biomarkers, would help PCPs and non-specialists identify which patients may need further evaluation or referral to a specialist ( 25 ). Interest also remains high in advancing imaging techniques, such as amyloid and tau PET, to support a diagnosis of AD. Although amyloid and tau PET are not currently readily available, they may be useful for specialists in the future to determine disease staging or track progression, or as a surrogate marker of cognitive status ( 74 ). The introduction of new screening and diagnostic tools could ultimately help lower the burden on specialists and ensure patients are diagnosed in a timely manner.
Conclusions
Consensus within the AD community has recently shifted to encourage the diagnosis of AD as early as possible. This shift will enable patients to plan their future and consider symptomatic therapies and lifestyle changes that could reduce cognitive deficits and ultimately help preserve their quality of life. Promisingly, new, potentially disease-modifying therapeutic candidates are on the horizon that could be effective in early AD by targeting and ameliorating the underlying biological mechanisms ( 92 , 102 ). This paper has outlined a menu of practical tools for clinicians to use in the real world to support an early diagnosis of AD and how they may best be incorporated into current clinical practice. Ultimately, a coordinated, multidisciplinary approach that encompasses primary care and specialist expertise is required to ensure timely detection, assessment and differentiation, diagnosis, and management of patients with AD.
Hughes J. This is one of the biggest global health crises of the 21st century. World Econ Forum 2017. https://www.weforum.org/agenda/2017/09/dementia-trillion-dollar-global-crisis/#:~:text=This%20World%20Alzheimer’s%20Day%2C%20the,crises%20of%20the%2021st%20century (accessed May 18, 2020).
Alzheimer’s Disease International. World Alzheimer Report 2019. Attitudes to dementia 2019. https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf (accessed February 13, 2020).
Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 2020;16:391–460.
Article Google Scholar
Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res 2017;17:189–202.
Article PubMed PubMed Central Google Scholar
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011;1:a006189.
Article PubMed PubMed Central CAS Google Scholar
Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367:795–804.
Article CAS PubMed PubMed Central Google Scholar
Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–62.
Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010;9:1118–27.
Article PubMed Google Scholar
U.S. Food and Drug Administration (FDA). Early Alzheimer’s disease: developing drugs for treatment guidance for industry 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy (accessed August 11, 2020).
Insel PS, Weiner M, Mackin RS, et al. Determining clinically meaningful decline in preclinical Alzheimer disease. Neurology 2019;93:e322–33.
Vermunt L, Sikkes SA, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 2019;15:888–98.
Cho SH, Woo S, Kim C, et al. Disease progression modelling from preclinical Alzheimer’s disease (AD) to AD dementia. Sci Rep 2021;11:4168.
Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–95.
Article CAS PubMed Google Scholar
Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–44.
Kazim SF, Iqbal K. Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer’s disease. Mol Neurodegener 2016;11:50.
Tolbert S, Liu Y, Hellegers C, et al. Financial management skills in aging, MCI and dementia: cross sectional relationship to 18F-florbetapir PET cortical β-amyloid deposition. J Prev Alzheimers Dis 2019;6:274–82.
CAS PubMed Google Scholar
Ye BS, Kim HJ, Kim YJ, et al. Longitudinal outcomes of amyloid positive versus negative amnestic mild cognitive impairments: a three-year longitudinal study. Sci Rep 2018;8:5557.
Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol 2018;75:970–9.
Dubois B, Padovani A, Scheltens P, Rossi A, Dell’Agnello G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis 2016;49:617–31.
Isaacson RS, Ganzer CA, Hristov H, et al. The clinical practice of risk reduction for Alzheimer’s disease: a precision medicine approach. Alzheimers Dement 2018;14:1663–73.
Isaacson RS, Hristov H, Saif N, et al. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement 2019;15:1588–602.
Gauthier SG. Alzheimer’s disease: the benefits of early treatment. Eur J Neurol 2005;12:11–6.
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement NY 2020;6:e12050.
Google Scholar
Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s disease: evolving the care team for optimal patient management. Front Neurol 2021;11:592302.
Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the preparedness of the U.S. Health Care System infrastructure for an Alzheimer’s treatment 2017. https://www.rand.org/pubs/research_reports/RR2272.html (accessed May 5, 2018).
Sabbagh MN, Lue L-F, Fayard D, Shi J. Increasing precision of clinical diagnosis of Alzheimer’s disease using a combined algorithm incorporating clinical and novel biomarker data. Neurol Ther 2017;6:83–95.
Balasa M, Gelpi E, Antonell A, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology 2011;76:1720–5.
Galvin JE. Using informant and performance screening methods to detect mild cognitive impairment and dementia. Curr Geriatr Rep 2018;7:19–25.
Sabbagh MN, Boada M, Borson S, et al. Early detection of mild cognitive impairment (MCI) in primary care. J Prev Alzheimers Dis 2020;7:165–70.
Galvin JE, Sadowsky CH, NINCDS-ADRDA. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med 2012;25:367–82.
Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther 2017;9:60.
Blennow K, Zetterberg H. Biomarkers for Alzheimer disease - current status and prospects for the future. J Intern Med 2018;284:643–63.
Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–33.
Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020;26:379–86.
Iliffe S, Robinson L, Brayne C, et al. Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry 2009;24:895–901.
Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA 2019;322:1589–99.
Robinson L, Tang E, Taylor J-P. Dementia: timely diagnosis and early intervention. BMJ 2015;350:h3029.
Zucchella C, Bartolo M, Pasotti C, Chiapella L, Sinforiani E. Caregiver burden and coping in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord 2012;26:55–60.
Pfistermeister B, Tümena T, Gaßmann K-G, Maas R, Fromm MF. Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PLoS One 2017;12:e0171353.
Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol 2013;9:106–18.
Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract 2015;24:1–10.
Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 2015;77:43–51.
Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav 2014;8:262–73.
23andMe. What to know about health report test result - 23andMe. 23&Me® 2020. https://www.23andme.com/test-info/ (accessed October 15, 2020).
Hendry K, Green C, McShane R, et al. AD-8 for detection of dementia across a variety of healthcare settings. Cochrane Database Syst Rev 2019;3:CD011121.
PubMed Google Scholar
Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); Report No.: 19-05257-EF-1, 2020.
Teng E, Becker BW, Woo E, Cummings JL, Lu PH. Subtle deficits in instrumental activities of daily living in subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord 2010;30:189–97.
Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: where do they fit in the diagnosis of Alzheimer’s disease? Neurodegener Dis Manag 2012;2:483–91.
Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013;21:685–95.
Ismail Z, Emeremni CA, Houck PR, et al. A comparison of the E-BEHAVE-AD, NBRS and NPI in quantifying clinical improvement in the treatment of agitation and psychosis associated with dementia. Am J Geriatr Psychiatry 2013;21:78–87.
Bowden VM, Bowden CL. The Journal of Neuropsychiatry and Clinical Neurosciences. JAMA 1992;268:1473–4.
Galvin JE. The quick dementia rating system (QDRS): a rapid dementia staging tool. Alzheimers Dement Amst 2015;1:249–59.
Koster N, Knol DL, Uitdehaag BM, Scheltens P, Sikkes SA. The sensitivity to change over time of the Amsterdam IADL Questionnaire(©). Alzheimers Dement 2015;11:1231–40.
Sikkes SA, Pijnenburg YA, Knol DL, de Lange-de Klerk ES, Scheltens P, Uitdehaag BM. Assessment of instrumental activities of daily living in dementia: diagnostic value of the Amsterdam Instrumental Activities of Daily Living Questionnaire. J Geriatr Psychiatry Neurol 2013;26:244–50.
LaRue RH. Functional Assessment Screening Tool (FAST). In: Volkmar FR, editor. Encycl. Autism Spectr. Disord., New York, NY: Springer New York, 2018:1–2.
Lindgren N, Rinne JO, Palviainen T, Kaprio J, Vuoksimaa E. Prevalence and correlates of dementia and mild cognitive impairment classified with different versions of the modified Telephone Interview for Cognitive Status (TICS-m). Int J Geriatr Psychiatry 2019;34:1883–91.
Wang H, Fan Z, Shi C, et al. Consensus statement on the neurocognitive outcomes for early detection of mild cognitive impairment and Alzheimer dementia from the Chinese Neuropsychological Normative (CN-NORM) Project. J Glob Health 2019;9:020320.
Franzen S, van den Berg E, Goudsmit M, et al. A systematic review of neuropsychological tests for the assessment of dementia in non-western, low-educated or illiterate populations. J Int Neuropsychol Soc 2020;26:331–51.
Costa A, Bak T, Caffarra P, et al. The need for harmonisation and innovation of neuropsychological assessment in neurodegenerative dementias in Europe: consensus document of the Joint Program for Neurodegenerative Diseases Working Group. Alzheimers Res Ther 2017;9:27.
MoCA Test Inc. Upcoming mandatory training for MoCA testing. MoCA Montr - Cogn Assess 2021. https://www.mocatest.org/mandatory-moca-test-training/ (accessed February 3, 2021).
Tabira T, Hotta M, Murata M, et al. Age-related changes in instrumental and basic activities of daily living impairment in older adults with very mild Alzheimer’s disease. Dement Geriatr Cogn Disord Extra 2020;10:27–37.
Martyr A, Nelis SM, Quinn C, et al. The relationship between perceived functional difficulties and the ability to live well with mild-to-moderate dementia: findings from the IDEAL programme. Int J Geriatr Psychiatry 2019;34:1251–61.
Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 2016;12:195–202.
McAllister-Williams RH, Bones K, Goodwin GM, et al. Analysing UK clinicians’ understanding of cognitive symptoms in major depression: a survey of primary care physicians and psychiatrists. J Affect Disord 2017;207:346–52.
Richard E, Schmand B, Eikelenboom P, Yang SC, Ligthart SA. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimers disease in non-depressed subjects. Dement Geriatr Cogn Disord 2012;33:204–9.
Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2018;32:10–7.
Harrison JE, Hendrix S. Chapter 21 - The assessment of cognition in translational medicine: a contrast between the approaches used in Alzheimer’s disease and major depressive disorder. In: Nomikos GG, Feltner DE, editors. Handb. Behav. Neurosci., vol. 29, Elsevier, 2019:297–308.
Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013;29:753–72.
Harper L, Barkhof F, Scheltens P, Schott JM, Fox NC. An algorithmic approach to structural imaging in dementia. J Neurol Neurosurg Psychiatry 2014;85:692–8.
Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol 2017;16:661–76.
Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–29.
Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med 2012;2:a006213.
Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016;12:292–323.
Villemagne VL, Doré V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol 2018;14:225–36.
Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 2012;11:669–78.
Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir F 18). J Nucl Med 2010;51:913–20.
Duits FH, Martinez-Lage P, Paquet C, et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement 2016;12:154–63.
Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement 2018;14:1505–21.
Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–81.
Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement 2015;11:58–69.
Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther 2019;11:34.
Lee SA, Sposato LA, Hachinski V, Cipriano LE. Cost-effectiveness of cerebrospinal biomarkers for the diagnosis of Alzheimer’s disease. Alzheimers Res Ther 2017;9:18.
Dolgin E. Alzheimer’s disease is getting easier to spot. Nature 2018;559:S10–2.
Morris E, Chalkidou A, Hammers A, Peacock J, Summers J, Keevil S. Diagnostic accuracy of 18F amyloid PET tracers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2016;43:374–85.
Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement Amst 2017;8:111–26.
Barthélemy NR, Bateman RJ, Hirtz C, et al. Cerebrospinal fluid phosphotau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimers Res Ther 2020;12:26.
O’Bryant SE, Mielke MM, Rissman RA, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement 2017;13:45–58.
C2N Diagnostics. Press release. Alzheimer’s breakthrough: C 2 N first to offer a widely accessible blood test. C2N Diagn 2021. https://www.c2ndiagnostics.com/press/press/2020/10/28/alzheimers-breakthrough-cn-first-to-offer-a-widely-accessible-blood-test (accessed January 25, 2021).
Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylatedtau isoforms track CNS change in Alzheimer’s disease. J Exp Med 2020;217:e20200861.
Janelidze S, Stomrud E, Smith R, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun 2020;11:1683.
Chen J, Duan Y, Li H, Lu L, Liu J, Tang C. Different durations of cognitive stimulation therapy for Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging 2019;14:1243–54.
Cummings J, Fox N. Defining disease modifying therapy for Alzheimer’s disease. J Prev Alzheimers Dis 2017;4:109–15.
CAS PubMed PubMed Central Google Scholar
Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 2018;6:CD001190.
Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev 2015;9:CD001191.
Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004;291:317–24.
Cummings J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol Neurodegener 2021;16:2.
Loy C, Schneider L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev 2006;1:CD001747.
Atri A. The Alzheimer’s Disease Clinical Spectrum. Med Clin N Am 2019;103:263–93.
Berlowitz DR, Foy CG, Kazis LE, et al. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med 2017;377:733–44.
Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer’s disease - the citAD randomized controlled trial. JAMA 2014;311:682–91.
Sheline YI, Snider BJ, Beer JC, et al. Effect of escitalopram dose and treatment duration on CSF Aβ levels in healthy older adults: a controlled clinical trial. Neurology 2020;95:e2658–65.
Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord 2012;5:349–58.
Haubois G, Annweiler C, Launay C, et al. Development of a short form of Mini-Mental State Examination for the screening of dementia in older adults with a memory complaint: a case control study. BMC Geriatr 2011;11:59.
Horton DK, Hynan LS, Lacritz LH, Rossetti HC, Weiner MF, Cullum CM. An Abbreviated Montreal Cognitive Assessment (MoCA) for dementia screening. Clin Neuropsychol 2015;29:413–25.
Mini-Cog©. Mini-Cog© In other languages. Mini-Cog© Lang 2021. https://mini-cog.com/mini-cog-in-other-languages/ (accessed April 21, 2021).
Carnero Pardo C, de la Vega Cotarelo R, López Alcalde S, et al. Assessing the diagnostic accuracy (DA) of the Spanish version of the informant-based AD8 questionnaire. Neurologia 2013;28:88–94.
Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ. Informant questionnaire on cognitive decline in the elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Database Syst Rev 2015;3:CD010772.
Sanchez MA, Correa PC, Lourenço RA. Cross-cultural adaptation of the “Functional Activities Questionnaire - FAQ” for use in Brazil. Dement Neuropsychol 2011;5:322–7.
Kim G, DeCoster J, Huang C-H, Bryant AN. A meta-analysis of the factor structure of the Geriatric Depression Scale (GDS): the effects of language. Int Psychogeriatr 2013;25:71–81.
Mapi Research Trust. NPI - Officially distributed by Mapi Research Trust. Neuropsychiatr Inventory Quest NPI-Q 2021. https://eprovide.mapi-trust.org/instruments/neuropsychiatric-inventory-questionnaire (accessed April 21, 2021).
Mapi Research Trust. A-IADL-Q-SV - Amsterdam Instrumental Activity of Daily Living Questionnaire - Short version. Amst Instrum Act Dly Living Quest - Short Version -IADL-Q-SV n.d. https://eprovide.mapi-trust.org/instruments/amsterdam-instrumental-activity-of-daily-living-questionnaire-short-version (accessed April 21, 2021).
Ismail Z, Agüera-Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in predementia populations. J Alzheimers Dis 2017;56:929–38.
Download references
Acknowledgements
The authors would like to acknowledge and thank Dr. Giovanni Frisoni, Geneva University Neurocenter, for his contribution towards the development of this manuscript.
Funding: The authors developed this manuscript concept during an assessment of Alzheimer’s disease educational needs. The development of this manuscript was funded by Biogen. Editorial support was provided by Jodie Penney, MSc, PhD, Helios Medical Communications, Cheshire, UK, which was funded by Biogen.
Author information
Authors and affiliations.
University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
A. P. Porsteinsson
Weill Cornell Medical Center and New York-Presbyterian, New York, NY, USA
R. S. Isaacson
Biogen International GmbH, Neuhofstrasse 30, 6340, Baar, Switzerland
Cleveland Clinic Lou Ruvo Center for Brain Health s, Las Vegas, NV, USA
M. N. Sabbagh
Biogen Inc, Cambridge, MA, USA
You can also search for this author in PubMed Google Scholar
Contributions
Authors’ contributions: All authors participated in the review of the literature and in the drafting and reviewing of the manuscript. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Correspondence to Sean Knox .
Ethics declarations
Conflict of Interest: AP reports personal fees from Acadia Pharmaceuticals, Alzheon, Avanir, Biogen, Cadent Therapeutics, Eisai, Functional Neuromodulation, MapLight Therapeutics, Premier Healthcare Solutions, Sunovion, and Syneos; grants from Alector, Athira, Avanir, Biogen, Biohaven, Eisai, Eli Lilly, Genentech/Roche, and Vaccinex. RI has nothing to disclose. MS reports personal fees from Alzheon, Athira, Biogen, Cortexyme, Danone, Neurotrope, Regeneron, Roche-Genentech, and Stage 2 Innovations; stock options from Brain Health Inc, NeuroReserve, NeuroTau, Neurotrope, Optimal Cognitive Health Company, uMethod Health, and Versanum Inc. Additionally, he has intellectual property rights with Harper Collins. SK and IR report employment with Biogen.
Electronic supplementary material
Supplementary material, approximately 65.7 MB.
Rights and permissions
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
Reprints and permissions
About this article
Porsteinsson, A.P., Isaacson, R.S., Knox, S. et al. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J Prev Alzheimers Dis 8 , 371–386 (2021). https://doi.org/10.14283/jpad.2021.23
Download citation
Received : 22 February 2021
Accepted : 28 April 2021
Published : 09 June 2021
Issue Date : July 2021
DOI : https://doi.org/10.14283/jpad.2021.23
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alzheimer’s disease
- early diagnosis
- diagnostic work-up
- Find a journal
- Publish with us
- Track your research
- Clinical Trials
A Study of Early-onset Alzheimer's Disease
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
- Jacksonville, Florida: 18-003553
- Rochester, Minnesota: 18-003553
About this study
The purpose of this Longitudinal Early-onset Alzheimer's Disease Study (LEADS) is designed to look at disease progression in individuals with early onset cognitive impairment . Clinical/cognitive, imaging, biomarker, and genetic characteristics will be assessed across three cohorts: (1) early onset Alzheimer's Disease (EOAD) participants, (2) early onset non-Alzheimer's Disease (EO-nonAD) participants,and (3) cognitively normal (CN) control participants.
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Inclusion Criteria - Cognitively Impaired (EOAD and EO-nonAD) Cohorts Only:
- Meets NIA-AA criteria for MCI due to AD or probable AD dementia.
- Have a global CDR score ≤ 1.0.
- Have capacity to provide informed consent (IC) or has a legal authorized representative or guardian who provides IC.
- Age between 40-64 years (inclusive) at the time of consent .
- Must have a study partner (informant) who spends a minimum average of 10 hours per week with the participant (e.g., family member, significant other, friend, caregiver) who is generally aware of the participants' daily activities and can provide information about the participant's cognitive and functional performance. If the participant does not have a study partner who spends 10 face-to-face hours per week, other arrangements for identifying a viable study partner will be granted on a case-by-case basis by the Site PI .
- Willing and able to complete longitudinal study procedures aside from LP which is an optional procedure.
- Not pregnant or lactating. Women must be two years post-menopausal, be surgically sterile, or have a negative pregnancy test prior to each PET scan.
- Fluent in English.
Inclusion Criteria - Cognitively Normal (CN) Cohort Only:
- Meets criteria for cognitively normal, based on an absence of significant impairment in cognitive functions or activities of daily living.
- Have a global CDR score = 0.
- Have capacity to provide informed consent.
- Have a Mini-Mental State Exam score between 26-30 (inclusive). Exceptions may be made for participant with less than 8 years of education at the discretion of the Site PI.
- Age between 40-64 years (inclusive) at the time of consent.
- Must have a study partner (informant) who spends a minimum average of 10 hours per week with the participant (e.g., family member, significant other, friend, caregiver) who is generally aware of the participants' daily activities and can provide information about the participant's cognitive and functional performance. If the participant does not have a study partner who spends 10 face-to-face hours per week, other arrangements for identifying a viable study partner will be granted on a case-by-case basis by the Site PI.
Exclusion Criteria - All (EOAD, EO-nonAD and CN) Cohorts:
- Meets core clinical criteria for non-AD dementia.
- Two or more first degree relatives with a history of early-onset dementia suggestive of autosomal dominant transmission, unless known pathogenic mutations in APP, PSEN1, PSEN2 have been excluded.
- Known mutation in an ADAD gene (APP, PSEN1, PSEN2) or other autosomal dominant genes associated with other neurodegenerative disorders.
- Contraindications to 3T MRI (e.g., claustrophobia, pacemaker, select aneurismal clip, artificial heart valve, select ear implants, select stents incompatible with 3T MRI, metal fragments or foreign objects in the eyes, skin or body, etc.).
- Lifetime medical history of a brain disorder other than the disorder causing dementia except for headache (exceptions are allowed at the discretion of the Site PI - e.g., seizure disorder thought to be due to EOAD).
- MRI scan with evidence of infection or focal lesions, cortical strokes, multiple lacunes (single lacune is allowable unless it meets criteria for strategic lacune affecting cognition).
- Any significant systemic illness or unstable medical condition, which could lead to difficulty complying with the protocol (at the discretion of the Site PI).
- Medical radiation exposure will be assessed by the study physician. If the candidate participant has had more than one nuclear medicine study in the prior 12 months, study inclusion will require approval from the PET Core.
- Investigational agents are prohibited 30 days prior to entry.
- Previous enrollment in a therapeutic trial targeting amyloid or tau.
- Participation in other clinical studies with neuropsychological measures, with the exception of participants who are co-enrolled in the NACC Uniform Data Set (UDS) protocol (Note: This criterion is intended to reduce repeat measures effects during neuropsychological testing. Exceptions are allowed at the discretion of the Site PI).
- Lifetime history of schizophrenia spectrum disorders (DSM-5 criteria).
- Current history (in previous 12 months) of DSM-5 diagnosis of mania, bipolar disorder with or without psychotic features.
- Current history (in previous 6 months) of moderate or severe substance abuse (nicotine or caffeine is allowed).
- Suicidal behaviors in the past 12 months or active suicidal ideations.
- Residing in a 24-hour care skilled nursing facility (at the time of screening).
- History of torsades de pointes or taking medications known to prolong the QT interval.
- Corrected QT (QTc) interval ≥ 458 msec in males or ≥ 474 msec in females.
- Platelet count <100,000/µl;
- Abnormal PT or PTT at screening.
- Contraindications to the procedure, including but not limited to severe degenerative joint disease, deformity of the spine, history of a bleeding disorder.
- Suspected elevated intracranial pressure, Arnold Chiari malformation or mass lesion d. Use of the anticoagulant medications such as but not limited to warfarin, rivaroxaban, dabigatran.
- Deemed ineligible by the Site PI for any other reason.
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
- The Brain Health Assessment for Detecting and Diagnosing Neurocognitive Disorders. Possin KL. Katherine L; Moskowitz T. Tacie; Erlhoff SJ. Sabrina J; Rogers KM. Kirsten M; Johnson ET. Erica T; Steele NZR. Natasha Z R; Higgins JJ. Joseph J; Stiver J. Jordan; Alioto AG. Andrea G; Farias ST. Sarah T; Miller BL. Bruce L; Rankin KP. Katherine P. Journal of the American Geriatrics Society. 2018. Jan; 66(1):150-156 Brief cognitive screens lack the sensitivity to detect mild cognitive impairment (MCI) or support differential diagnoses. The objective of this study was to validate the 10-minute, tablet-based University of California, San Francisco (UCSF) Brain Health Assessment (BHA) to overcome these limitations. Read More on PubMed
- Effectiveness of Florbetapir PET Imaging in Changing Patient Management. Pontecorvo MJ. Michael J; Siderowf A. Andrew; Dubois B. Bruno; Doraiswamy PM. P Murali; Frisoni GB. Giovanni B; Grundman M. Michael; Nobili F. Flavio; Sadowsky CH. Carl H; Salloway S. Stephen; Arora AK. Anupa K; Chevrette A. Antoine; Deberdt W. Walter; Dell'Agnello G. Grazia; Flitter M. Matthew; Galante N. Nick; Lowrey MJ. Mark J; Lu M. Ming; McGeehan A. Anne; Devous MD. Michael D; Mintun MA. Mark A. Dementia and geriatric cognitive disorders. 2017. ; 44(3-4):129-143 To evaluate the impact of amyloid PET imaging on diagnosis and patient management in a multicenter, randomized, controlled study. Read More on PubMed
- Consensus guidelines for lumbar puncture in patients with neurological diseases. Engelborghs S. Sebastiaan; Niemantsverdriet E. Ellis; Struyfs H. Hanne; Blennow K. Kaj; Brouns R. Raf; Comabella M. Manuel; Dujmovic I. Irena; van der Flier W. Wiesje; Frölich L. Lutz; Galimberti D. Daniela; Gnanapavan S. Sharmilee; Hemmer B. Bernhard; Hoff E. Erik; Hort J. Jakub; Iacobaeus E. Ellen; Ingelsson M. Martin; Jan de Jong F. Frank; Jonsson M. Michael; Khalil M. Michael; Kuhle J. Jens; Lleó A. Alberto; de Mendonça A. Alexandre; Molinuevo JL. José Luis; Nagels G. Guy; Paquet C. Claire; Parnetti L. Lucilla; Roks G. Gerwin; Rosa-Neto P. Pedro; Scheltens P. Philip; Skårsgard C. Constance; Stomrud E. Erik; Tumani H. Hayrettin; Visser PJ. Pieter Jelle; Wallin A. Anders; Winblad B. Bengt; Zetterberg H. Henrik; Duits F. Flora; Teunissen CE. Charlotte E. Alzheimer's & dementia (Amsterdam, Netherlands). 2017. ; 8():111-126 Cerebrospinal fluid collection by lumbar puncture (LP) is performed in the diagnostic workup of several neurological brain diseases. Reluctance to perform the procedure is among others due to a lack of standards and guidelines to minimize the risk of complications, such as post-LP headache or back pain. Read More on PubMed
- Increased prevalence of autoimmune disease within C9 and FTD/MND cohorts: Completing the picture. Miller ZA. Zachary A; Sturm VE. Virginia E; Camsari GB. Gamze Balci; Karydas A. Anna; Yokoyama JS. Jennifer S; Grinberg LT. Lea T; Boxer AL. Adam L; Rosen HJ. Howard J; Rankin KP. Katherine P; Gorno-Tempini ML. Maria Luisa; Coppola G. Giovanni; Geschwind DH. Daniel H; Rademakers R. Rosa; Seeley WW. William W; Graff-Radford NR. Neill R; Miller BL. Bruce L. Neurology(R) neuroimmunology & neuroinflammation. 2016. Dec; 3(6):e301 To determine the prevalence of autoimmune disease in symptomatic (C9) mutation carriers and frontotemporal dementia with motor neuron disease (FTD/MND) cohorts. Read More on PubMed
- Neuropsychological and Neuroanatomical Correlates of the Social Norms Questionnaire in Frontotemporal Dementia Versus Alzheimer's Disease. Panchal H. Hemali; Paholpak P. Pongsatorn; Lee G. Grace; Carr A. Andrew; Barsuglia JP. Joseph P; Mather M. Michelle; Jimenez E. Elvira; Mendez MF. Mario F. American journal of Alzheimer's disease and other dementias. 2016. Jun; 31(4):326-32 Traditional neuropsychological batteries may not distinguish early behavioral variant frontotemporal dementia (bvFTD) from Alzheimer's disease (AD) without the inclusion of a social behavioral measure. We compared 33 participants, 15 bvFTD, and 18 matched patients with early-onset AD (eAD), on the Social Norms Questionnaire (SNQ), neuropsychological tests and 3-dimensional T1-weighted magnetic resonance imaging (MRI). The analyses included correlations of SNQ results (total score, overendorsement or "overadhere" errors, and violations or "break" errors) with neuropsychological results and tensor-based morphometry regions of interest. Patients with BvFTD had significantly lower SNQ total scores and higher overadhere errors than patients with eAD. On neuropsychological measures, the SNQ total scores correlated significantly with semantic knowledge and the overadhere subscores with executive dysfunction. On MRI analysis, the break subscores significantly correlated with lower volume of lateral anterior temporal lobes (aTL). The results also suggest that endorsement of social norm violations corresponds to the role of the right aTL in social semantic knowledge. Read More on PubMed
- Molecular genetics of early-onset Alzheimer's disease revisited. Cacace R. Rita; Sleegers K. Kristel; Van Broeckhoven C. Christine. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016. Jun; 12(6):733-48 As the discovery of the Alzheimer's disease (AD) genes, APP, PSEN1, and PSEN2, in families with autosomal dominant early-onset AD (EOAD), gene discovery in familial EOAD came more or less to a standstill. Only 5% of EOAD patients are carrying a pathogenic mutation in one of the AD genes or a apolipoprotein E (APOE) risk allele ε4, most of EOAD patients remain unexplained. Here, we aimed at summarizing the current knowledge of EOAD genetics and its role in ongoing approaches to understand the biology of AD and disease symptomatology as well as developing new therapeutics. Next, we explored the possible molecular mechanisms that might underlie the missing genetic etiology of EOAD and discussed how the use of massive parallel sequencing technologies triggered novel gene discoveries. To conclude, we commented on the relevance of reinvestigating EOAD patients as a means to explore potential new avenues for translational research and therapeutic discoveries. Read More on PubMed
- Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. Nicolas G. Gaël; Wallon D. David; Charbonnier C. Camille; Quenez O. Olivier; Rousseau S. Stéphane; Richard AC. Anne-Claire; Rovelet-Lecrux A. Anne; Coutant S. Sophie; Le Guennec K. Kilan; Bacq D. Delphine; Garnier JG. Jean-Guillaume; Olaso R. Robert; Boland A. Anne; Meyer V. Vincent; Deleuze JF. Jean-François; Munter HM. Hans Markus; Bourque G. Guillaume; Auld D. Daniel; Montpetit A. Alexandre; Lathrop M. Mark; Guyant-Maréchal L. Lucie; Martinaud O. Olivier; Pariente J. Jérémie; Rollin-Sillaire A. Adeline; Pasquier F. Florence; Le Ber I. Isabelle; Sarazin M. Marie; Croisile B. Bernard; Boutoleau-Bretonnière C. Claire; Thomas-Antérion C. Catherine; Paquet C. Claire; Sauvée M. Mathilde; Moreaud O. Olivier; Gabelle A. Audrey; Sellal F. François; Ceccaldi M. Mathieu; Chamard L. Ludivine; Blanc F. Frédéric; Frebourg T. Thierry; Campion D. Dominique; Hannequin D. Didier. European journal of human genetics : EJHG. 2016. May; 24(5):710-6 Causative variants in APP, PSEN1 or PSEN2 account for a majority of cases of autosomal dominant early-onset Alzheimer disease (ADEOAD, onset before 65 years). Variant detection rates in other EOAD patients, that is, with family history of late-onset AD (LOAD) (and no incidence of EOAD) and sporadic cases might be much lower. We analyzed the genomes from 264 patients using whole-exome sequencing (WES) with high depth of coverage: 90 EOAD patients with family history of LOAD and no incidence of EOAD in the family and 174 patients with sporadic AD starting between 51 and 65 years. We found three PSEN1 and one PSEN2 causative, probably or possibly causative variants in four patients (1.5%). Given the absence of PSEN1, PSEN2 and APP causative variants, we investigated whether these 260 patients might be burdened with protein-modifying variants in 20 genes that were previously shown to cause other types of dementia when mutated. For this analysis, we included an additional set of 160 patients who were previously shown to be free of causative variants in PSEN1, PSEN2 and APP: 107 ADEOAD patients and 53 sporadic EOAD patients with an age of onset before 51 years. In these 420 patients, we detected no variant that might modify the function of the 20 dementia-causing genes. We conclude that EOAD patients with family history of LOAD and no incidence of EOAD in the family or patients with sporadic AD starting between 51 and 65 years have a low variant-detection rate in AD genes. Read More on PubMed
- Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Duits FH. Flora H; Martinez-Lage P. Pablo; Paquet C. Claire; Engelborghs S. Sebastiaan; Lleó A. Alberto; Hausner L. Lucrezia; Molinuevo JL. José L; Stomrud E. Erik; Farotti L. Lucia; Ramakers IHGB. Inez H G B; Tsolaki M. Magda; Skarsgård C. Constance; Åstrand R. Ragnar; Wallin A. Anders; Vyhnalek M. Martin; Holmber-Clausen M. Marie; Forlenza OV. Orestes V; Ghezzi L. Laura; Ingelsson M. Martin; Hoff EI. Erik I; Roks G. Gerwin; de Mendonça A. Alexandre; Papma JM. Janne M; Izagirre A. Andrea; Taga M. Mariko; Struyfs H. Hanne; Alcolea DA. Daniel A; Frölich L. Lutz; Balasa M. Mircea; Minthon L. Lennart; Twisk JWR. Jos W R; Persson S. Staffan; Zetterberg H. Henrik; van der Flier WM. Wiesje M; Teunissen CE. Charlotte E; Scheltens P. Philip; Blennow K. Kaj. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016. Feb; 12(2):154-163 Lumbar puncture (LP) is increasingly performed in memory clinics. We investigated patient-acceptance of LP, incidence of and risk factors for post-LP complications in memory clinic populations. Read More on PubMed
- Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Monsell SE. Sarah E; Dodge HH. Hiroko H; Zhou XH. Xiao-Hua; Bu Y. Yunqi; Besser LM. Lilah M; Mock C. Charles; Hawes SE. Stephen E; Kukull WA. Walter A; Weintraub S. Sandra; . . Alzheimer disease and associated disorders. 2016. ; 30(2):134-9 Four new nonproprietary tests were recommended for use in the National Alzheimer's Coordinating Center's Uniform Data Set Neuropsychological Battery. These tests are similar to previous tests but also allow for continuity of longitudinal data collection and wide dissemination among research collaborators. Read More on PubMed
- Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer's disease. Ossenkoppele R. Rik; Cohn-Sheehy BI. Brendan I; La Joie R. Renaud; Vogel JW. Jacob W; Möller C. Christiane; Lehmann M. Manja; van Berckel BN. Bart N M; Seeley WW. William W; Pijnenburg YA. Yolande A; Gorno-Tempini ML. Maria L; Kramer JH. Joel H; Barkhof F. Frederik; Rosen HJ. Howard J; van der Flier WM. Wiesje M; Jagust WJ. William J; Miller BL. Bruce L; Scheltens P. Philip; Rabinovici GD. Gil D. Human brain mapping. 2015. Nov; 36(11):4421-37 Alzheimer's disease (AD) can present with distinct clinical variants. Identifying the earliest neurodegenerative changes associated with each variant has implications for early diagnosis, and for understanding the mechanisms that underlie regional vulnerability and disease progression in AD. We performed voxel-based morphometry to detect atrophy patterns in early clinical stages of four AD phenotypes: Posterior cortical atrophy (PCA, "visual variant," n=93), logopenic variant primary progressive aphasia (lvPPA, "language variant," n=74), and memory-predominant AD categorized as early age-of-onset (EOAD, 65 years, n=114). Patients with each syndrome were stratified based on: (1) degree of functional impairment, as measured by the clinical dementia rating (CDR) scale, and (2) overall extent of brain atrophy, as measured by a neuroimaging approach that sums the number of brain voxels showing significantly lower gray matter volume than cognitively normal controls (n=80). Even at the earliest clinical stage (CDR=0.5 or bottom quartile of overall atrophy), patients with each syndrome showed both common and variant-specific atrophy. Common atrophy across variants was found in temporoparietal regions that comprise the posterior default mode network (DMN). Early syndrome-specific atrophy mirrored functional brain networks underlying functions that are uniquely affected in each variant: Language network in lvPPA, posterior cingulate cortex-hippocampal circuit in amnestic EOAD and LOAD, and visual networks in PCA. At more advanced stages, atrophy patterns largely converged across AD variants. These findings support a model in which neurodegeneration selectively targets both the DMN and syndrome-specific vulnerable networks at the earliest clinical stages of AD. Read More on PubMed
- The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Ossenkoppele R. Rik; Pijnenburg YA. Yolande A L; Perry DC. David C; Cohn-Sheehy BI. Brendan I; Scheltens NM. Nienke M E; Vogel JW. Jacob W; Kramer JH. Joel H; van der Vlies AE. Annelies E; La Joie R. Renaud; Rosen HJ. Howard J; van der Flier WM. Wiesje M; Grinberg LT. Lea T; Rozemuller AJ. Annemieke J; Huang EJ. Eric J; van Berckel BN. Bart N M; Miller BL. Bruce L; Barkhof F. Frederik; Jagust WJ. William J; Scheltens P. Philip; Seeley WW. William W; Rabinovici GD. Gil D. Brain : a journal of neurology. 2015. Sep; 138(Pt 9):2732-49 A 'frontal variant of Alzheimer's disease' has been described in patients with predominant behavioural or dysexecutive deficits caused by Alzheimer's disease pathology. The description of this rare Alzheimer's disease phenotype has been limited to case reports and small series, and many clinical, neuroimaging and neuropathological characteristics are not well understood. In this retrospective study, we included 55 patients with Alzheimer's disease with a behavioural-predominant presentation (behavioural Alzheimer's disease) and a neuropathological diagnosis of high-likelihood Alzheimer's disease (n = 17) and/or biomarker evidence of Alzheimer's disease pathology (n = 44). In addition, we included 29 patients with autopsy/biomarker-defined Alzheimer's disease with a dysexecutive-predominant syndrome (dysexecutive Alzheimer's disease). We performed structured chart reviews to ascertain clinical features. First symptoms were more often cognitive (behavioural Alzheimer's disease: 53%; dysexecutive Alzheimer's disease: 83%) than behavioural (behavioural Alzheimer's disease: 25%; dysexecutive Alzheimer's disease: 3%). Apathy was the most common behavioural feature, while hyperorality and perseverative/compulsive behaviours were less prevalent. Fifty-two per cent of patients with behavioural Alzheimer's disease met diagnostic criteria for possible behavioural-variant frontotemporal dementia. Overlap between behavioural and dysexecutive Alzheimer's disease was modest (9/75 patients). Sixty per cent of patients with behavioural Alzheimer's disease and 40% of those with the dysexecutive syndrome carried at least one APOE ε4 allele. We also compared neuropsychological test performance and brain atrophy (applying voxel-based morphometry) with matched autopsy/biomarker-defined typical (amnestic-predominant) Alzheimer's disease (typical Alzheimer's disease, n = 58), autopsy-confirmed/Alzheimer's disease biomarker-negative behavioural variant frontotemporal dementia (n = 59), and controls (n = 61). Patients with behavioural Alzheimer's disease showed worse memory scores than behavioural variant frontotemporal dementia and did not differ from typical Alzheimer's disease, while executive function composite scores were lower compared to behavioural variant frontotemporal dementia and typical Alzheimer's disease. Voxel-wise contrasts between behavioural and dysexecutive Alzheimer's disease patients and controls revealed marked atrophy in bilateral temporoparietal regions and only limited atrophy in the frontal cortex. In direct comparison with behavioural and those with dysexecutive Alzheimer's disease, patients with behavioural variant frontotemporal dementia showed more frontal atrophy and less posterior involvement, whereas patients with typical Alzheimer's disease were slightly more affected posteriorly and showed less frontal atrophy (P Read More on PubMed
- Phonological facilitation effects on naming latencies and viewing times during noun and verb naming in agrammatic and anomic aphasia. Lee J. Jiyeon; Thompson CK. Cynthia K. Aphasiology. 2015. ; 29(10):1164-1188 Phonological priming has been shown to facilitate naming in individuals with aphasia as well as healthy speakers, resulting in faster naming latencies. However, the mechanisms of phonological facilitation (PF) in aphasia remain unclear. Read More on PubMed
- Common variants in psychiatric risk genes predict brain structure at birth. Knickmeyer RC. Rebecca C; Wang J. Jiaping; Zhu H. Hongtu; Geng X. Xiujuan; Woolson S. Sandra; Hamer RM. Robert M; Konneker T. Thomas; Lin W. Weili; Styner M. Martin; Gilmore JH. John H. Cerebral cortex (New York, N.Y. : 1991). 2014. May; 24(5):1230-46 Studies in adolescents and adults have demonstrated that polymorphisms in putative psychiatric risk genes are associated with differences in brain structure, but cannot address when in development these relationships arise. To determine if common genetic variants in disrupted-in-schizophrenia-1 (DISC1; rs821616 and rs6675281), catechol-O-methyltransferase (COMT; rs4680), neuregulin 1 (NRG1; rs35753505 and rs6994992), apolipoprotein E (APOE; ε3ε4 vs. ε3ε3), estrogen receptor alpha (ESR1; rs9340799 and rs2234693), brain-derived neurotrophic factor (BDNF; rs6265), and glutamate decarboxylase 1 (GAD1; rs2270335) are associated with individual differences in brain tissue volumes in neonates, we applied both automated region-of-interest volumetry and tensor-based morphometry to a sample of 272 neonates who had received high-resolution magnetic resonance imaging scans. ESR1 (rs9340799) predicted intracranial volume. Local variation in gray matter (GM) volume was significantly associated with polymorphisms in DISC1 (rs821616), COMT, NRG1, APOE, ESR1 (rs9340799), and BDNF. No associations were identified for DISC1 (rs6675281), ESR1 (rs2234693), or GAD1. Of note, neonates homozygous for the DISC1 (rs821616) serine allele exhibited numerous large clusters of reduced GM in the frontal lobes, and neonates homozygous for the COMT valine allele exhibited reduced GM in the temporal cortex and hippocampus, mirroring findings in adults. The results highlight the importance of prenatal brain development in mediating psychiatric risk. Read More on PubMed
- [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Xia CF. Chun-Fang; Arteaga J. Janna; Chen G. Gang; Gangadharmath U. Umesh; Gomez LF. Luis F; Kasi D. Dhanalakshmi; Lam C. Chung; Liang Q. Qianwa; Liu C. Changhui; Mocharla VP. Vani P; Mu F. Fanrong; Sinha A. Anjana; Su H. Helen; Szardenings AK. A Katrin; Walsh JC. Joseph C; Wang E. Eric; Yu C. Chul; Zhang W. Wei; Zhao T. Tieming; Kolb HC. Hartmuth C. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013. Nov; 9(6):666-76 We wished to develop a highly selective positron emission tomography (PET) imaging agent targeting PHF-tau in human Alzheimer's disease (AD) brains. Read More on PubMed
- Handedness and language learning disability differentially distribute in progressive aphasia variants. Miller ZA. Zachary A; Mandelli ML. Maria Luisa; Rankin KP. Katherine P; Henry ML. Maya L; Babiak MC. Miranda C; Frazier DT. Darvis T; Lobach IV. Iryna V; Bettcher BM. Brianne M; Wu TQ. Teresa Q; Rabinovici GD. Gil D; Graff-Radford NR. Neill R; Miller BL. Bruce L; Gorno-Tempini ML. Maria Luisa. Brain : a journal of neurology. 2013. Nov; 136(Pt 11):3461-73 Primary progressive aphasia is a neurodegenerative clinical syndrome that presents in adulthood with an isolated, progressive language disorder. Three main clinical/anatomical variants have been described, each associated with distinctive pathology. A high frequency of neurodevelopmental learning disability in primary progressive aphasia has been reported. Because the disorder is heterogeneous with different patterns of cognitive, anatomical and biological involvement, we sought to identify whether learning disability had a predilection for one or more of the primary progressive aphasia subtypes. We screened the University of California San Francisco Memory and Aging Center's primary progressive aphasia cohort (n = 198) for history of language-related learning disability as well as hand preference, which has associations with learning disability. The study included logopenic (n = 48), non-fluent (n = 54) and semantic (n = 96) variant primary progressive aphasias. We investigated whether the presence of learning disability or non-right-handedness was associated with differential effects on demographic, neuropsychological and neuroimaging features of primary progressive aphasia. We showed that a high frequency of learning disability was present only in the logopenic group (χ(2) = 15.17, P Read More on PubMed
- TDP-43 frontotemporal lobar degeneration and autoimmune disease. Miller ZA. Zachary A; Rankin KP. Katherine P; Graff-Radford NR. Neill R; Takada LT. Leonel T; Sturm VE. Virginia E; Cleveland CM. Clare M; Criswell LA. Lindsey A; Jaeger PA. Philipp A; Stan T. Trisha; Heggeli KA. Kristin A; Hsu SC. Sandy Chan; Karydas A. Anna; Khan BK. Baber K; Grinberg LT. Lea T; Gorno-Tempini ML. Maria Luisa; Boxer AL. Adam L; Rosen HJ. Howard J; Kramer JH. Joel H; Coppola G. Giovanni; Geschwind DH. Daniel H; Rademakers R. Rosa; Seeley WW. William W; Wyss-Coray T. Tony; Miller BL. Bruce L. Journal of neurology, neurosurgery, and psychiatry. 2013. Sep; 84(9):956-62 The aetiology and pathogenesis of non-genetic forms of frontotemporal dementia (FTD) is unknown and even with the genetic forms of FTD, pathogenesis remains elusive. Given the association between systemic inflammation and other neurodegenerative processes, links between autoimmunity and FTD need to be explored. Read More on PubMed
- Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Lehmann M. Manja; Ghosh PM. Pia M; Madison C. Cindee; Laforce R. Robert; Corbetta-Rastelli C. Chiara; Weiner MW. Michael W; Greicius MD. Michael D; Seeley WW. William W; Gorno-Tempini ML. Maria L; Rosen HJ. Howard J; Miller BL. Bruce L; Jagust WJ. William J; Rabinovici GD. Gil D. Brain : a journal of neurology. 2013. Mar; 136(Pt 3):844-58 The factors driving clinical heterogeneity in Alzheimer's disease are not well understood. This study assessed the relationship between amyloid deposition, glucose metabolism and clinical phenotype in Alzheimer's disease, and investigated how these relate to the involvement of functional networks. The study included 17 patients with early-onset Alzheimer's disease (age at onset Read More on PubMed
- Genetic insights in Alzheimer's disease. Bettens K. Karolien; Sleegers K. Kristel; Van Broeckhoven C. Christine. The Lancet. Neurology. 2013. Jan; 12(1):92-104 In the search for new genes in Alzheimer's disease, classic linkage-based and candidate-gene-based association studies have been supplanted by exome sequencing, genome-wide sequencing (for mendelian forms of Alzheimer's disease), and genome-wide association studies (for non-mendelian forms). The identification of new susceptibility genes has opened new avenues for exploration of the underlying disease mechanisms. In addition to detecting novel risk factors in large samples, next-generation sequencing approaches can deliver novel insights with even small numbers of patients. The shift in focus towards translational studies and sequencing of individual patients places each patient's biomaterials as the central unit of genetic studies. The notional shift needed to make the patient central to genetic studies will necessitate strong collaboration and input from clinical neurologists. Read More on PubMed
- Nonamnestic presentations of early-onset Alzheimer's disease. Mendez MF. Mario F; Lee AS. Albert S; Karve SJ. Simantini J.; Shapira JS. Jill S. American journal of Alzheimer's disease and other dementias. 2012. Sep; 27(6):413-20 Early-onset Alzheimer's disease (EOAD) beginning before the age of 65 may differ from late-onset AD (LOAD) in clinical course and frequency of nonamnestic presentations. In a 10-year retrospective review, 125 patients with EOAD, diagnosed clinically and verified by functional neuroimaging, were compared with 56 patients with LOAD and further classified depending on predominant cognitive difficulty on presentation. Eighty (64%) of the patients with EOAD had a nonamnestic presentation, compared with only 7 (12.5%) of the patients with LOAD. Compared with LOAD, the patients with EOAD had a shorter duration with lower Mini-Mental State Examination scores. The neuroimaging reports among the patients with EOAD showed more hippocampal atrophy with an amnestic presentation, more left parietal changes with impaired language presentations, and more right parietal and occipital changes with impaired visuospatial presentations. These findings indicate that EOAD differs from LOAD in a more aggressive course and in having predominantly nonamnestic presentations that vary in neuropathological location. Read More on PubMed
- Posterior cortical atrophy. Crutch SJ. Sebastian J; Lehmann M. Manja; Schott JM. Jonathan M; Rabinovici GD. Gil D; Rossor MN. Martin N; Fox NC. Nick C. The Lancet. Neurology. 2012. Feb; 11(2):170-8 Posterior cortical atrophy (PCA) is a neurodegenerative syndrome that is characterised by progressive decline in visuospatial, visuoperceptual, literacy, and praxic skills. The progressive neurodegeneration affecting parietal, occipital, and occipitotemporal cortices that underlies PCA is attributable to Alzheimer's disease in most patients. However, alternative underlying causes, including dementia with Lewy bodies, corticobasal degeneration, and prion disease, have also been identified, and not all patients with PCA have atrophy on clinical imaging. This heterogeneity has led to inconsistencies in diagnosis and terminology and difficulties in comparing studies from different centres, and has restricted the generalisability of findings from clinical trials and investigations of factors that drive phenotypic variability. Important challenges remain, including the identification of factors associated not only with the selective vulnerability of posterior cortical regions but also with the young age of onset of PCA. Greater awareness of the syndrome and agreement over the correspondence between syndrome-level and disease-level classifications are needed to improve diagnostic accuracy, clinical management, and the design of research studies. Read More on PubMed
- Autosomal recessive causes likely in early-onset Alzheimer disease. Wingo TS. Thomas S; Lah JJ. James J; Levey AI. Allan I; Cutler DJ. David J. Archives of neurology. 2012. Jan; 69(1):59-64 To determine the genetic contribution to non-autosomal dominant early-onset Alzheimer disease (EOAD) (onset age ≤60 years) cases and identify the likely mechanism of inheritance in those cases. Read More on PubMed
- Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. Leung L. Laura; Andrews-Zwilling Y. Yaisa; Yoon SY. Seo Yeon; Jain S. Sachi; Ring K. Karen; Dai J. Jessica; Wang MM. Max Mu; Tong L. Leslie; Walker D. David; Huang Y. Yadong. PloS one. 2012. ; 7(12):e53569 Apolipoprotein (apo) E4 is the major genetic risk factor for Alzheimer's disease (AD). ApoE4 has sex-dependent effects, whereby the risk of developing AD is higher in apoE4-expressing females than males. However, the mechanism underlying the sex difference, in relation to apoE4, is unknown. Previous findings indicate that apoE4 causes age-dependent impairments of hilar GABAergic interneurons in female mice, leading to learning and memory deficits. Here, we investigate whether the detrimental effects of apoE4 on hilar GABAergic interneurons are sex-dependent using apoE knock-in (KI) mice across different ages. We found that in female apoE-KI mice, there was an age-dependent depletion of hilar GABAergic interneurons, whereby GAD67- or somatostatin-positive--but not NPY- or parvalbumin-positive-interneuron loss was exacerbated by apoE4. Loss of these neuronal populations was correlated with the severity of spatial learning deficits at 16 months of age in female apoE4-KI mice; however, this effect was not observed in female apoE3-KI mice. In contrast, we found an increase in the numbers of hilar GABAergic interneurons with advancing age in male apoE-KI mice, regardless of apoE genotype. Moreover, male apoE-KI mice showed a consistent ratio of hilar inhibitory GABAergic interneurons to excitatory mossy cells approximating 1.5 that is independent of apoE genotype and age, whereas female apoE-KI mice exhibited an age-dependent decrease in this ratio, which was exacerbated by apoE4. Interestingly, there are no apoE genotype effects on GABAergic interneurons in the CA1 and CA3 subregions of the hippocampus as well as the entorhinal and auditory cortexes. These findings suggest that the sex-dependent effects of apoE4 on developing AD is in part attributable to inherent sex-based differences in the numbers of hilar GABAergic interneurons, which is further modulated by apoE genotype. Read More on PubMed
- Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Murray ME. Melissa E; Graff-Radford NR. Neill R; Ross OA. Owen A; Petersen RC. Ronald C; Duara R. Ranjan; Dickson DW. Dennis W. The Lancet. Neurology. 2011. Sep; 10(9):785-96 Neurofibrillary pathology has a stereotypical progression in Alzheimer's disease (AD) that is encapsulated in the Braak staging scheme; however, some AD cases are atypical and do not fit into this scheme. We aimed to compare clinical and neuropathological features between typical and atypical AD cases. Read More on PubMed
- Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE ɛ4 allele. van der Flier WM. Wiesje M; Pijnenburg YA. Yolande Al; Fox NC. Nick C; Scheltens P. Philip. The Lancet. Neurology. 2011. Mar; 10(3):280-8 Some patients with early-onset Alzheimer's disease (AD) present with a distinct phenotype. Typically, the first and most salient characteristic of AD is episodic memory impairment. A few patients, however, present with focal cortical, non-memory symptoms, such as difficulties with language, visuospatial, or executive functions. These presentations are associated with specific patterns of atrophy and frequently with a young age at onset. Age is not, however, the only determinant of phenotype; underlying factors, especially genetic factors, seem also to affect phenotype and predispose patients to younger or older age at onset. Importantly, patients with atypical early-onset disease seldom carry the APOE ɛ4 allele, which is the most important risk factor for lowering the age of onset in patients with AD. Additionally, theAPOE ɛ4 genotype seems to predispose patients to vulnerability in the medial temporal areas, which leads to memory loss. Conversely, patients negative for the APOE ɛ4 allele and with early-onset AD are more likely to be predisposed to vulnerability of cerebral networks beyond the medial temporal lobes. Other factors are probably involved in determining the pattern of atrophy, but these are currently unknown. Read More on PubMed
- Should EOAD patients be included in clinical trials? Szigeti K. Kinga; Doody RS. Rachelle S. Alzheimer's research & therapy. 2011. Feb; 3(1):4 Alzheimer disease (AD) is a devastating neurodegenerative disease affecting 1 in 68 in the population. An arbitrary cutoff 65 years as the age of onset to distinguish between early- and late-onset AD has been proposed and has been used in the literature for decades. As the majority of patients develop AD after 65 years of age, most clinical trials address this population. While the early-onset cases represent only 1% to 6% of AD cases, this population is the active working subset and thus contributes to a higher public health burden per individual, and early-onset cases are the most devastating at the level of the individual and their families. In this review, we compare and contrast the clinical, neuropsychological, imaging, genetic, biomarker, and pathological features of these two arbitrary groups. Finally, we discuss the ethical dilemma of non-abandonment and justice as it pertains to exclusion of the early-onset AD patients from clinical trials. Read More on PubMed
- Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. Andrews-Zwilling Y. Yaisa; Bien-Ly N. Nga; Xu Q. Qin; Li G. Gang; Bernardo A. Aubrey; Yoon SY. Seo Yeon; Zwilling D. Daniel; Yan TX. Tonya Xue; Chen L. Ligong; Huang Y. Yadong. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010. Oct; 30(41):13707-17 Apolipoprotein E4 (apoE4) is the major genetic risk factor for Alzheimer's disease. However, the underlying mechanisms are unclear. We found that female apoE4 knock-in (KI) mice had an age-dependent decrease in hilar GABAergic interneurons that correlated with the extent of learning and memory deficits, as determined in the Morris water maze, in aged mice. Treating apoE4-KI mice with daily peritoneal injections of the GABA(A) receptor potentiator pentobarbital at 20 mg/kg for 4 weeks rescued the learning and memory deficits. In neurotoxic apoE4 fragment transgenic mice, hilar GABAergic interneuron loss was even more pronounced and also correlated with the extent of learning and memory deficits. Neurodegeneration and tauopathy occurred earliest in hilar interneurons in apoE4 fragment transgenic mice; eliminating endogenous Tau prevented hilar GABAergic interneuron loss and the learning and memory deficits. The GABA(A) receptor antagonist picrotoxin abolished this rescue, while pentobarbital rescued learning deficits in the presence of endogenous Tau. Thus, apoE4 causes age- and Tau-dependent impairment of hilar GABAergic interneurons, leading to learning and memory deficits in mice. Consequently, reducing Tau and enhancing GABA signaling are potential strategies to treat or prevent apoE4-related Alzheimer's disease. Read More on PubMed
- Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Wolk DA. David A; Dickerson BC. Bradford C; . . Proceedings of the National Academy of Sciences of the United States of America. 2010. Jun; 107(22):10256-61 The epsilon4 allele of the apolipoprotein E (APOE) gene is the major genetic risk factor for Alzheimer's disease (AD), but limited work has suggested that APOE genotype may modulate disease phenotype. Carriers of the epsilon4 allele have been reported to have greater medial temporal lobe (MTL) pathology and poorer memory than noncarriers. Less attention has focused on whether there are domains of cognition and neuroanatomical regions more affected in noncarriers. Further, a major potential confound of prior in vivo studies is the possibility of different rates of clinical misdiagnosis for carriers vs. noncarriers. We compared phenotypic differences in cognition and topography of regional cortical atrophy of epsilon4 carriers (n = 67) vs. noncarriers (n = 24) with mild AD from the Alzheimer's Disease Neuroimaging Initiative, restricted to those with a cerebrospinal fluid (CSF) molecular profile consistent with AD. Between-group comparisons were made for psychometric tests and morphometric measures of cortical thickness and hippocampal volume. Carriers displayed significantly greater impairment on measures of memory retention, whereas noncarriers were more impaired on tests of working memory, executive control, and lexical access. Consistent with this cognitive dissociation, carriers exhibited greater MTL atrophy, whereas noncarriers had greater frontoparietal atrophy. Performance deficits in particular cognitive domains were associated with disproportionate regional brain atrophy within nodes of cortical networks thought to subserve these cognitive processes. These convergent cognitive and neuroanatomic findings in individuals with a CSF molecular profile consistent with AD support the hypothesis that APOE genotype modulates the clinical phenotype of AD through influence on specific large-scale brain networks. Read More on PubMed
- Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Rabinovici GD. Gil D; Furst AJ. Ansgar J; Alkalay A. Adi; Racine CA. Caroline A; O'Neil JP. James P; Janabi M. Mustafa; Baker SL. Suzanne L; Agarwal N. Neha; Bonasera SJ. Stephen J; Mormino EC. Elizabeth C; Weiner MW. Michael W; Gorno-Tempini ML. Maria L; Rosen HJ. Howard J; Miller BL. Bruce L; Jagust WJ. William J. Brain : a journal of neurology. 2010. Feb; 133(Pt 2):512-28 Patients with early age-of-onset Alzheimer's disease show more rapid progression, more generalized cognitive deficits and greater cortical atrophy and hypometabolism compared to late-onset patients at a similar disease stage. The biological mechanisms that underlie these differences are not well understood. The purpose of this study was to examine in vivo whether metabolic differences between early-onset and late-onset Alzheimer's disease are associated with differences in the distribution and burden of fibrillar amyloid-beta. Patients meeting criteria for probable Alzheimer's disease (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's; Disease and Related Disorders Association criteria) were divided based on estimated age at first symptom (less than or greater than 65 years) into early-onset (n = 21, mean age-at-onset 55.2 +/- 5.9 years) and late-onset (n = 18, 72.0 +/- 4.7 years) groups matched for disease duration and severity. Patients underwent positron emission tomography with the amyloid-beta-ligand [(11)C]-labelled Pittsburgh compound-B and the glucose analogue [(18)F]-labelled fluorodeoxyglucose. A group of cognitively normal controls (n = 30, mean age 73.7 +/- 6.4) was studied for comparison. [(11)C]-labelled Pittsburgh compound-B images were analysed using Logan graphical analysis (cerebellar reference) and [(18)F]-labelled fluorodeoxyglucose images were normalized to mean activity in the pons. Group differences in tracer uptake were assessed on a voxel-wise basis using statistical parametric mapping, and by comparing mean values in regions of interest. To account for brain atrophy, analyses were repeated after applying partial volume correction to positron emission tomography data. Compared to normal controls, both early-onset and late-onset Alzheimer's disease patient groups showed increased [(11)C]-labelled Pittsburgh compound-B uptake throughout frontal, parietal and lateral temporal cortices and striatum on voxel-wise and region of interest comparisons (P Read More on PubMed
- Early-versus late-onset Alzheimer's disease: more than age alone. Koedam EL. Esther L G E; Lauffer V. Vivian; van der Vlies AE. Annelies E; van der Flier WM. Wiesje M; Scheltens P. Philip; Pijnenburg YA. Yolande A L. Journal of Alzheimer's disease : JAD. 2010. ; 19(4):1401-8 Alzheimer's disease (AD) is the most common cause of dementia at older age. Although less prevalent before the age of 65 years, it is still the most frequent cause of early-onset dementia followed by frontotemporal dementia. The typical presentation of AD is memory dysfunction, however, presentations with prominent cognitive impairment in other domains besides memory, like prominent apraxia, language problems, or executive dysfunction, may occur and are relatively more common in early-onset AD. In this retrospective descriptive study, we determined the prevalence of non-memory presentations in a large sample of early-onset AD patients compared to late-onset AD. The clinical files of 270 patients with AD starting before the age of 65 years and 90 patients with late-onset AD ( 65 years) were reviewed to assess clinical characteristics. Patients were classified as memory presentation and non-memory presentation according to their clinical presentation. The mean age of the early-onset group was 56 +/- 5 years and 74 +/- 6 years for the late-onset group. A third of the early-onset AD group presented with non-memory symptoms compared to only 6% in the late-onset group (p Read More on PubMed
- Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Migliaccio R. R; Agosta F. F; Rascovsky K. K; Karydas A. A; Bonasera S. S; Rabinovici GD. G D; Miller BL. B L; Gorno-Tempini ML. M L. Neurology. 2009. Nov; 73(19):1571-8 Posterior cortical atrophy (PCA) and logopenic progressive aphasia (LPA) are clinical syndromes associated with posterior brain atrophy. We compared PCA and LPA to each other and to an age-matched group of patients with early age at onset of Alzheimer disease (EO-AD). We hypothesized that these 3 syndromes are part of a single clinical and biologic continuum. Read More on PubMed
- Age, neuropathology, and dementia. Savva GM. George M; Wharton SB. Stephen B; Ince PG. Paul G; Forster G. Gillian; Matthews FE. Fiona E; Brayne C. Carol; . . The New England journal of medicine. 2009. May; 360(22):2302-9 Research in Alzheimer's disease is focused mainly on younger old persons, whereas studies involving very old persons report attenuated relationships between the pathological features of Alzheimer's disease and dementia. Read More on PubMed
- Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer's disease. Filippini N. Nicola; Rao A. Anil; Wetten S. Sally; Gibson RA. Rachel A; Borrie M. Michael; Guzman D. Danilo; Kertesz A. Andrew; Loy-English I. Inge; Williams J. Julie; Nichols T. Thomas; Whitcher B. Brandon; Matthews PM. Paul M. NeuroImage. 2009. Feb; 44(3):724-8 APOE epsilon4 is the best-established genetic risk factor for sporadic Alzheimer's disease (AD). However, while homozygotes show greater disease susceptibility and earlier age of onset than heterozygotes, they may not show faster rates of clinical progression. We hypothesize that there are differential APOE epsilon4 allele-load dependent influences on neuropathology across the brain. Our aim was to define the relationship between APOE epsilon4 allele load and regionally-specific brain cortical atrophy in Alzheimer's Disease (AD). For this reason voxel-based morphometry (VBM) was performed using T1-weighted MR images from 83 AD patients, contrasting regional cortical grey matter by APOE epsilon4 load according to either dominant or genotypic models. Patients fulfilled NINCDS-ADRDA criteria and were genotyped for APOE epsilon4 (15 epsilon4/epsilon4, 39 epsilon4/- and 29-/-). We observed that grey matter volume (GMV) decreased additively with increasing allele load in the medial (MTL) and anterior temporal lobes bilaterally. By contrast, a 2 degree-of-freedom genotypic model suggested a dominant effect of the APOE epsilon4 allele in the left temporal lobe. Brain regions showing a significant APOE epsilon4 allele load effect on GMV in AD included only some of those typically described as having greatest amyloid plaque deposition and atrophy. Temporal regions appeared to show a dominant effect of APOE epsilon4 allele load instead of the additive effect previously strongly associated with age of onset. Regional variations with allele load may be related to different mechanisms for effects of APOE epsilon4 load on susceptibility and disease progression. Read More on PubMed
- The northwestern anagram test: measuring sentence production in primary progressive aphasia. Weintraub S. Sandra; Mesulam MM. M-Marsel; Wieneke C. Christina; Rademaker A. Alfred; Rogalski EJ. Emily J; Thompson CK. Cynthia K. American journal of Alzheimer's disease and other dementias. 2009. ; 24(5):408-16 Primary progressive aphasia (PPA) is a clinical dementia syndrome with early symptoms of language dysfunction. Postmortem findings are varied and include Alzheimer disease and frontotemporal lobar degeneration (FTLD), both tauopathies and TAR DNA binding protein (TDP-43) proteinopathies. Clinical-pathological correlations in PPA are complex but the presence in the clinical profile of agrammatism has a high association with tauopathy. Grammatical competence is difficult to assess in the clinical setting with available methods. This article describes the Northwestern Anagram Test (NAT), a new clinical measure of sentence production. A total of 16 patients with PPA and their controls assembled single printed words to create sentences describing pictures. Northwestern Anagram Test performance was significantly correlated with a measure of sentence production and with aphasia severity but not with measures of naming, single word comprehension, object recognition, or motor speech. The NAT can be used to assess syntax competence when patients cannot be tested with measures that require intact speech production. Read More on PubMed
- Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Rogalski E. Emily; Johnson N. Nancy; Weintraub S. Sandra; Mesulam M. Marsel. Archives of neurology. 2008. Feb; 65(2):244-8 Although risk factors for Alzheimer disease have been well studied, much less is known about risk factors for primary progressive aphasia (PPA). Read More on PubMed
- Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Schneider JA. Julie A; Arvanitakis Z. Zoe; Bang W. Woojeong; Bennett DA. David A. Neurology. 2007. Dec; 69(24):2197-204 To examine the spectrum of neuropathology in persons from the Rush Memory and Aging Project, a longitudinal community-based clinical-pathologic cohort study. Read More on PubMed
- Cognitive phenotypes in Alzheimer's disease and genetic risk. Snowden JS. Julie S; Stopford CL. Cheryl L; Julien CL. Camille L; Thompson JC. Jennifer C; Davidson Y. Yvonne; Gibbons L. Linda; Pritchard A. Antonia; Lendon CL. Corinne L; Richardson AM. Anna M; Varma A. Anoop; Neary D. David; Mann D. David. Cortex; a journal devoted to the study of the nervous system and behavior. 2007. Oct; 43(7):835-45 Variation in the clinical characteristics of patients with Alzheimer's disease (AD) is increasingly recognised, although the factors underlying variation are not fully understood. The study examined the cognitive characteristics of 523 AD patients at the time of their presentation to a neurological dementia clinic and explored the relationship to family history and apolipoprotein E (APOE) genotype. Distinct profiles were identified, which were mirrored by topographical differences on neuroimaging. Clinical distinctions were maintained over time. Two-thirds of patients showed a constellation of deficits at presentation which included memory, language, visuospatial and constructional difficulties. However, a quarter had circumscribed presentations of amnesia, aphasia, perceptuospatial disorder or apraxia. The rare presence of frontal lobe characteristics was associated with a younger age of onset, an increased incidence of myoclonus at presentation, a positive family history but not with possession of APOE epsilon4 allele. An amnestic presentation (severe, yet circumscribed amnesia) was strongly associated with an older age of onset, a positive family history and the presence of APOE epsilon4 allele. Posterior cortical presentations showed a female bias, were typically sporadic, and showed no association with APOE epsilon4. The findings support the notion of phenotypic variation in AD, and show that genetic risk factors can influence clinical presentation. The findings draw attention to the specific association between APOE epsilon4 allele and memory but challenge the commonly held notion that the presence of the epsilon4 allele inevitably reduces onset age. The findings indicate that risk factors other than APOE epsilon4 allele underlie the non-familial, early onset posterior hemisphere presentations of AD. Read More on PubMed
- Cognitive impairment in Alzheimer's disease is modified by APOE genotype. van der Vlies AE. Annelies E; Pijnenburg YA. Yolande A L; Koene T. Teddy; Klein M. Martin; Kok A. Astrid; Scheltens P. Philip; van der Flier WM. Wiesje M. Dementia and geriatric cognitive disorders. 2007. ; 24(2):98-103 We examined whether impairment in specific cognitive domains in Alzheimer's disease (AD) differed according to APOE genotype and age at onset. Read More on PubMed
- The effect of APOE genotype on clinical phenotype in Alzheimer disease. van der Flier WM. W M; Schoonenboom SN. S N M; Pijnenburg YA. Y A L; Fox NC. N C; Scheltens P. P. Neurology. 2006. Aug; 67(3):526-7 The authors classified 100 patients with Alzheimer disease (AD) as presenting with a memory or nonmemory phenotype. APOE genotype was determined. There was an association between APOE-epsilon4 and clinical phenotype (odds ratio = 3.0; 95% CI: 1.2 to 7.8), suggesting that two subtypes of AD can be identified. The typical amnestic phenotype seems to be promoted by the APOE-epsilon4 allele, whereas the atypical nonmemory phenotype occurs in the absence of the APOE-epsilon4 allele. Read More on PubMed
- Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Kim EJ. E J; Cho SS. S S; Jeong Y. Y; Park KC. K C; Kang SJ. S J; Kang E. E; Kim SE. S E; Lee KH. K H; Na DL. D L. Brain : a journal of neurology. 2005. Aug; 128(Pt 8):1790-801 The aims of this cross-sectional study were (i) to compare the overall glucose metabolism between early onset and late onset Alzheimer's disease in a large sample of patients; and (ii) to investigate the pattern of glucose metabolism as a function of dementia severity in early onset versus late onset Alzheimer's disease, using a statistical parametric mapping (SPM) analysis. Subjects consisted of four groups: 74 patients with early onset Alzheimer's disease, 46 patients with late onset of the disease, and two control groups age matched to each patient group. All the subjects underwent 2-[(18)F]fluoro-2-deoxy-d-glucose (FDG)-PET under the same scanning conditions. Severity of dementia was rated with the Clincial Dementia Rating (CDR). Voxel-based SPM99 was used for statistical analyses. Overall glucose hypometabolism of early onset Alzheimer's disease patients was much greater in magnitude and extent than that of late onset patients, though both groups were similar in dementia severity: the early onset group showed more severe hypometabolism in parietal, frontal and subcortical (basal ganglia and thalamus) areas. When the decline of glucose metabolism was compared as a function of CDR stage, the slope was steeper in early onset than in late onset Alzheimer's disease. The rapid decline occurred at CDR 0.5-1 in the early onset group, whereas similar changes occurred at CDR 2-3 in the late onset group. The greater hypometabolism in early onset than in late onset patients is required to reach the same severity of dementia, probably reflecting greater functional reserve in younger than in older subjects. Alternatively, the metabolic decline curve suggests that the early onset patients may take a more rapid course in the reduction of glucose metabolism than the late onset patients. Read More on PubMed
- The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Nasreddine ZS. Ziad S; Phillips NA. Natalie A; Bédirian V. Valérie; Charbonneau S. Simon; Whitehead V. Victor; Collin I. Isabelle; Cummings JL. Jeffrey L; Chertkow H. Howard. Journal of the American Geriatrics Society. 2005. Apr; 53(4):695-9 To develop a 10-minute cognitive screening tool (Montreal Cognitive Assessment, MoCA) to assist first-line physicians in detection of mild cognitive impairment (MCI), a clinical state that often progresses to dementia. Read More on PubMed
- Structural correlates of early and late onset Alzheimer's disease: voxel based morphometric study. Frisoni GB. G B; Testa C. C; Sabattoli F. F; Beltramello A. A; Soininen H. H; Laakso MP. M P. Journal of neurology, neurosurgery, and psychiatry. 2005. Jan; 76(1):112-4 To examine the brain structural correlates of age at onset in patients with Alzheimer's disease. Read More on PubMed
- The effect of APOE epsilon4 allele on cerebral glucose metabolism in AD is a function of age at onset. Hirono N. N; Hashimoto M. M; Yasuda M. M; Ishii K. K; Sakamoto S. S; Kazui H. H; Mori E. E. Neurology. 2002. Mar; 58(5):743-50 Although the APOE epsilon4 allele is a well-known risk factor for developing AD, the impact of the epsilon4 allele on clinical manifestations in patients with AD is still controversial. One possible reason for this controversy is that previous studies did not consider the effect of patient age at symptom onset. Read More on PubMed
- Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer's disease. Hashimoto M. M; Yasuda M. M; Tanimukai S. S; Matsui M. M; Hirono N. N; Kazui H. H; Mori E. E. Neurology. 2001. Oct; 57(8):1461-6 Although the APOE epsilon 4 allele increases the risk of developing AD, the effects of the epsilon 4 allele on brain atrophy in clinical AD patients are controversial. Read More on PubMed
- APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Geroldi C. C; Pihlajamäki M. M; Laakso MP. M P; DeCarli C. C; Beltramello A. A; Bianchetti A. A; Soininen H. H; Trabucchi M. M; Frisoni GB. G B. Neurology. 1999. Nov; 53(8):1825-32 To test the hypothesis that the e4 allele of APOE is associated with a region-specific pattern of brain atrophy in AD. Read More on PubMed
- ApoE-4 and age at onset of Alzheimer's disease: the NIMH genetics initiative. Blacker D. D; Haines JL. J L; Rodes L. L; Terwedow H. H; Go RC. R C; Harrell LE. L E; Perry RT. R T; Bassett SS. S S; Chase G. G; Meyers D. D; Albert MS. M S; Tanzi R. R. Neurology. 1997. Jan; 48(1):139-47 To explore the impact of apoE-4 on Alzheimer's disease (AD) and its age at onset. Read More on PubMed
- Clinical and neuropsychological characteristics in familial and sporadic Alzheimer's disease: relation to apolipoprotein E polymorphism. Lehtovirta M. M; Soininen H. H; Helisalmi S. S; Mannermaa A. A; Helkala EL. E L; Hartikainen P. P; Hänninen T. T; Ryynänen M. M; Riekkinen PJ. P J. Neurology. 1996. Feb; 46(2):413-9 Alzheimer's disease (AD) is a heterogeneous entity presenting as sporadic and familial disease. In familial AD, there is evidence for genetic linkage to a yet undefined gene on chromosome 14 in early-onset pedigrees and on chromosome 19 in late-onset pedigrees. In a few early-onset kindreds, there were mutations in the amyloid precursor gene on chromosome 21. There is an increased frequency of apolipoprotein E (ApoE) epsilon4 allele in patients with late-onset AD. We studied the clinical presentation and profile of cognitive deficits in 58 AD patients at the early stage of the disease. We divided the AD patients into subgroups of sporadic late-onset (SLO) (> or = 65 years), familial late-onset (FLO) (> or = 65 years), sporadic early-onset (SEO) ( Read More on PubMed
- Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer's disease: A CERAD analysis, Part XII. Koss E. E; Edland S. S; Fillenbaum G. G; Mohs R. R; Clark C. C; Galasko D. D; Morris JC. J C. Neurology. 1996. Jan; 46(1):136-41 To determine whether the age of the onset of Alzheimer's disease (AD) is related to the expression and rate of decline of this disorder, we examined the clinical and neuropsychological performance of 421 patients entered into the Consortium to Establish a Registry for Alzheimer's Disease and followed annually for up to 4 years. Statistical analyses were based on multivariable logistic regression analysis for dichotomous clinical measures and multivariable linear regression analysis for psychometric measures. All analyses examined the effect of age after controlling for gender, education, and stage of dementia. Clinical information obtained on entry into the study indicated that younger patients performed more poorly on measures of language and concentration, and older patients performed more poorly on measures of memory and orientation. On neuropsychological measures at entry, younger patients, performed more poorly on praxis and had significantly higher scores of confrontation naming. Younger age predicted a significantly faster rate of progression for all neuropsychological measures. These findings support the concept of age-related clinical subtypes of AD. Read More on PubMed
- The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Cummings JL. J L; Mega M. M; Gray K. K; Rosenberg-Thompson S. S; Carusi DA. D A; Gornbein J. J. Neurology. 1994. Dec; 44(12):2308-14 We developed a new instrument, the Neuropsychiatric Inventory (NPI), to assess 10 behavioral disturbances occurring in dementia patients: delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, and aberrant motor activity. The NPI uses a screening strategy to minimize administration time, examining and scoring only those behavioral domains with positive responses to screening questions. Both the frequency and the severity of each behavior are determined. Information for the NPI is obtained from a caregiver familiar with the patient's behavior. Studies reported here demonstrate the content and concurrent validity as well as between-rater, test-retest, and internal consistency reliability; the instrument is both valid and reliable. The NPI has the advantages of evaluating a wider range of psychopathology than existing instruments, soliciting information that may distinguish among different etiologies of dementia, differentiating between severity and frequency of behavioral changes, and minimizing administration time. Read More on PubMed
- Age at onset of Alzheimer's disease: relation to pattern of cognitive dysfunction and rate of decline. Jacobs D. D; Sano M. M; Marder K. K; Bell K. K; Bylsma F. F; Lafleche G. G; Albert M. M; Brandt J. J; Stern Y. Y. Neurology. 1994. Jul; 44(7):1215-20 We examined the pattern of cognitive impairment and rate of cognitive and functional decline as a function of age at symptom onset in 127 patients with probable Alzheimer's disease (AD). At baseline, early-onset (before age 65) and late-onset groups were mildly and comparably impaired on the modified Mini-Mental State Examination (mMMS) and the Blessed Dementia Rating Scale-Part 1 (BDRS). Repeated-measures analysis of variance revealed significantly more rapid decline in early-onset subjects over a 2-year follow-up period. Multivariate linear regression analyses indicated that age at symptom onset strongly predicted rate of decline on the mMMS and the BDRS, even after controlling for symptom duration, gender, family history of dementia, and baseline mMMS and BDRS scores. Early- and late-onset AD subjects also differed in terms of pattern of performance on the mMMS. Early-onset subjects scored significantly lower than late-onset subjects on attentional items of the mMMS at baseline and follow-up. Conversely, late-onset subjects scored significantly lower than early-onset subjects on memory and naming items at baseline, and the two groups were comparable on these tasks at follow-up. Results provide longitudinal evidence of more rapid cognitive and functional decline in subjects with early-onset AD and suggest that early-onset AD may be characterized by predominant impairment of attentional skills. Read More on PubMed
- Semantic associations and elaborative inference. McKoon G. G; Ratcliff R. R. Journal of experimental psychology. Learning, memory, and cognition. 1989. Mar; 15(2):326-38 In this article, a theoretical framework is proposed for the inference processes that occur during reading. According to the framework, inferences can vary in the degree to which they are encoded. This notion is supported by three experiments in this article that show that degree of encoding can depend on the amount of semantic-associative information available to support the inference processes. In the experiments, test words that express possible inferences from texts are presented for recognition. When testing is delayed, with other texts and test items intervening between a text and its test word, performance depends on the amount of semantic-associative information in the text. If the inferences represented by the test words are not supported by semantic associates in the text, they appear to be only minimally encoded (replicating McKoon & Ratcliff, 1986), but if they are supported by semantic associates, they are strongly encoded. With immediate testing, only 250 ms after the text, performance is shown to depend on semantic-associative information, not on textual information. This suggests that it is the fast availability of semantic information that allows it to support inference processes. Read More on PubMed
- A new rating scale for Alzheimer's disease. Rosen WG. W G; Mohs RC. R C; Davis KL. K L. The American journal of psychiatry. 1984. Nov; 141(11):1356-64 A new rating instrument, the Alzheimer's Disease Assessment Scale, was designed specifically to evaluate the severity of cognitive and noncognitive behavioral dysfunctions characteristic of persons with Alzheimer's disease. Item descriptions, administration procedures, and scoring are outlined. Twenty-seven subjects with Alzheimer's disease and 28 normal elderly subjects were rated on 40 items. Twenty-one items with significant intraclass correlation coefficients for interrater reliability (range, .650-.989) and significant Spearman rank-order correlation coefficients for test-retest reliability (range, .514-1) constitute the final scale. Subjects with Alzheimer's disease had significantly more cognitive and noncognitive dysfunction than the normal elderly subjects. Read More on PubMed
- Measurement of functional activities in older adults in the community. Pfeffer RI. R I; Kurosaki TT. T T; Harrah CH. C H; Chance JM. J M; Filos S. S. Journal of gerontology. 1982. May; 37(3):323-9 Two measures of social function designed for community studies of normal aging and mild senile dementia were evaluated in 195 older adults who underwent neurological, cognitive, and affective assessment. An examining and a reviewing neurologist and a neurologically trained nurse independently rated each on a Scale of Functional Capacity. Interrater reliability was high (examining vs. reviewing neurologist, r = .97; examining neurologist vs. nurse, tau b = .802; p less than .001 for both comparisons). Estimates correlated well with an established measure of social function and with results of cognitive tests. Alternate informants evaluated participants on the Functional Activities Questionnaire and the Instrumental Activities of Daily Living Scale. The Functional Activities Questionnaire was superior to the Instrumental Activities of Daily scores. Used alone as a diagnostic tool, the Functional Activities Questionnaire was more sensitive than distinguishing between normal and demented individuals. Read More on PubMed
- Development and validation of a geriatric depression screening scale: a preliminary report. Yesavage JA. J A; Brink TL. T L; Rose TL. T L; Lum O. O; Huang V. V; Adey M. M; Leirer VO. V O. Journal of psychiatric research. . ; 17(1):37-49 A new Geriatric Depression Scale (GDS) designed specifically for rating depression in the elderly was tested for reliability and validity and compared with the Hamilton Rating Scale for Depression (HRS-D) and the Zung Self-Rating Depression Scale (SDS). In constructing the GDS a 100-item questionnaire was administered to normal and severely depressed subjects. The 30 questions most highly correlated with the total scores were then selected and readministered to new groups of elderly subjects. These subjects were classified as normal, mildly depressed or severely depressed on the basis of Research Diagnostic Criteria (RDC) for depression. The GDS, HRS-D and SDS were all found to be internally consistent measures, and each of the scales was correlated with the subject's number of RDC symptoms. However, the GDS and the HRS-D were significantly better correlated with RDC symptoms than was the SDS. The authors suggest that the GDS represents a reliable and valid self-rating depression screening scale for elderly populations. Read More on PubMed
- Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Farrer LA. L A; Cupples LA. L A; Haines JL. J L; Hyman B. B; Kukull WA. W A; Mayeux R. R; Myers RH. R H; Pericak-Vance MA. M A; Risch N. N; van Duijn CM. C M. JAMA. . ; 278(16):1349-56 To examine more closely the association between apolipoprotein E (APOE) genotype and Alzheimer disease (AD) by age and sex in populations of various ethnic and racial denominations. Read More on PubMed
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 June 2021
Risk of early-onset dementia among persons with tinnitus: a retrospective case–control study
- Yen-Fu Cheng 1 , 2 , 3 , 4 , 5 ,
- Sudha Xirasagar 6 ,
- Tzong-Han Yang 3 , 7 ,
- Chuan-Song Wu 7 , 8 ,
- Yi-Wei Kao 9 , 10 &
- Herng-Ching Lin 11 , 12
Scientific Reports volume 11 , Article number: 13399 ( 2021 ) Cite this article
54k Accesses
4 Citations
4 Altmetric
Metrics details
- Risk factors
Higher rates of poor cognitive performance are known to prevail among persons with tinnitus in all age groups. However, no study has explored the association between tinnitus and early-onset dementia. We hypothesize that tinnitus may precede or occur concurrently with subclinical or early onset dementia in adults younger than 65 years of age. This case–control study used data from the Taiwan National Health Insurance Research Database, identifying 1308 patients with early-onset dementia (dementia diagnosed before 65 years of age) and 1308 matched controls. We used multivariable logistic regressions to estimate odds ratios (ORs) for prior tinnitus among patients with dementia versus controls. Among total 2616 sample participants, the prevalence of prior tinnitus was 18%, 21.5% among cases and 14.5% among controls ( p < 0.001). Multivariable logistic regression showed and adjusted OR for prior tinnitus of 1.6 for cases versus controls (95% CI: 1.3 ~ 2.0). After adjusting for sociodemographic characteristics and medical co-morbidities, patients with early-onset dementia had a 67% higher likelihood of having prior tinnitus (OR = 1.628; 95% CI = 1.321–2.006). Our findings showed that pre-existing tinnitus was associated with a 68% increased risk of developing early-onset dementia among young and middle-aged adults. The results call for greater awareness of tinnitus as a potential harbinger of future dementia in this population.
Similar content being viewed by others

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Joseph M. Rootman, Maggie Kiraga, … Zach Walsh

The serotonin theory of depression: a systematic umbrella review of the evidence
Joanna Moncrieff, Ruth E. Cooper, … Mark A. Horowitz

Neurofilaments as biomarkers in neurological disorders — towards clinical application
Michael Khalil, Charlotte E. Teunissen, … Jens Kuhle
Introduction
Dementia is a disorder characterized by a decline in cognitive abilities involving one or more cognitive domains. The development of dementia involves complex processes involving specific molecular pathways affecting multiple cellular functions of the central nervous system, leading to disruption of the functional networks underlying cognition, behavior and sensorimotor functions, eventually eroding autonomous functioning and decision-making abilities of affected individuals 1 , 2 . Although the prevalence of dementia increases with age, it may also affect younger individuals.
Early-onset dementia is defined as dementia diagnosed before the age of 65 3 , 4 The symptoms of early-onset dementia are similar to those observed among the elderly, behavioral changes, cognitive decline, and psychiatric manifestations. In addition to early-onset adult neurodegenerative disorders such as Alzheimer’s disease, vascular dementia and frontotemporal dementia, early-onset dementia may be caused by delayed onset of childhood neurodegenerative disorders caused by mitochondrial and lysosomal disorders 4 , 5 . The diagnosis of early-onset dementia is particularly challenging because the current diagnostic criteria require evidence of cognitive impairment and memory loss. Younger patients whose dementia is limited to progressive cognitive decline or focal neurological impairments may not be diagnosed with dementia or receive a delayed diagnosis. Further, studies show that patients with early-onset dementia take a longer time to seek their first consultation for dementia evaluation and for families to become aware of the dementia diagnosis 6 . Considering the newer treatment options that are becoming available to modify the course of dementia, the consequences of delayed or unrecognized early-onset dementia can be serious and unnecessary. Consequences that may be mitigated by early diagnosis include morbidity and stigma suffered by patients, and the economic and resource use burden borne by families and the healthcare system over the subsequent life span.
Tinnitus is a phantom auditory perception in the absence of an objective source of physical sound. Tinnitus is a common and disturbing phenomenon, with reported prevalence rates ranging from 7 to 20% in the general population 7 , 8 , 9 , 10 . One study even reported that the incidence of tinnitus was as high as 26.7% for people ages 65–84 years in the United States 11 . Tinnitus can occur due to pathologies occurring at any point between the cochlear apparatus and the auditory cortex. Increasing evidence shows tinnitus to be a disorder involving neuroplastic changes in the central auditory structures that occur when the brain is deprived of its normal input due to cochlear lesions 12 .
Studies show correlation between the presence of poor cognitive performance and tinnitus 13 , 14 , 15 , 16 , 17 , and a high rate of cognitive impairment is observed among in patients with tinnitus across all age groups 18 , 19 . Despite these suggestive associations, there are no documented studies that examined associations between tinnitus and early-onset dementia. We hypothesize that tinnitus may precede or occur parallel to subclinical or early dementia among the population younger than 65 years of age. We sought to examine whether tinnitus may represent an early sign preceding early-onset dementia using administrative claims data.
This retrospective case–control study used data from the Taiwan National Health Insurance (NHI) Research Database (NHIRD). The NHIRD comprises all medical claims data for approximately 99% of the Taiwanese population (about 24.02 million registered beneficiaries in December 2019) under Taiwan’s NHI program. Many researchers have used the NHIRD to track longitudinal use of medical care and diagnoses over follow-up for research purposes.
The study is based on de-identified administrative data provided by the NHIRD. It was approved and deemed exempt from informed consent requirement by the Institutional Review Board of Taipei Medical University (TMU-JIRB N202005074), and is compliant with the Declaration of Helsinki.
Study sample
To identify cases, we first identified 206,940 patients with a first-time diagnosis of dementia (ICD-9-CM codes 290.0 ~ 290.4, 294.1, 331.0 ~ 331.2, or 331.82 or ICD-10-CM code F03.90) in an outpatient setting (private clinics or hospital outpatient departments) between January 1, 2010 and December 31, 2016. We included only patients with a documented diagnosis of dementia at least two medical encounters during the study period to improve diagnostic validity. The date of the first-time dementia diagnosis during the study period was assigned as their index date. Next we selected patients aged between 30 and 64 years of age ( n = 11,361). Finally, we excluded patients with a history of major psychosis or a substance use-related disorder (ICD-9-CM codes 291 ~ 299, 303 ~ 305), stroke (ICD-9-CM codes 430 ~ 438), or traumatic brain injury (TBI) (ICD-9-CM codes 801 ~ 804 or 850 ~ 854) prior to the index date ( n = 10,053). The reason for excluding patients with a history of TBI was that TBI was reported as a potential risk factor for other neurodegenerative disorders that can be associated with dementia 20 . The remaining 1308 patients with early-onset dementia were included as cases in this study.
To select controls out of the remaining patients, we first excluded those who had ever received a diagnosis of dementia, major psychosis or a substance-related disorder, stroke, or traumatic brain injury, and those aged 65 years or over. We selected one propensity score-matched control per case, matching controls to cases using patient demographic variables (age, sex, monthly income, geographic location and urbanization level of the patient’s residence) and the co-morbidities relevant to dementia development hyperlipidemia, diabetes, coronary heart disease, hypertension, obesity, hearing loss, and alcohol abuse. We matched controls to a corresponding dementia case based on their utilization of any medical service in the index year of the case. For controls, we assigned the date of their first utilization of ambulatory care during the matched year as the index date. A total of 1,308 cases and 1308 controls were analyzed in the study.
Exposure assessment
Patients with a tinnitus diagnosis were identified based on ICD-9-CM code 388.3. We defined a patient as having tinnitus if they had at least one claim with a diagnosis of tinnitus prior to the index date during the study period.
Statistical analysis
Statistical analyses were carried out using the SAS system (SAS System for Windows, vers. 9.4, SAS Institute, Cary, NC). Chi-square test and t-tests were performed to examine differences in patient demographics and medical comorbidities between cases and controls. We used multivariable logistic regressions to estimate the odds ratios (ORs) of prior tinnitus among patients with dementia versus controls. We used two-sided p < 0.05 for statistical significance.
Study patients’ mean age was 59.5 years. Table 1 presents the sociodemographic characteristics and comorbidities among cases and controls, showing no significant differences in age, sex, monthly income, hypertension, and hyperlipidemia between cases and controls. However, there were significant differences in geographic region ( p = 0.002), and the prevalence of diabetes ( p = 0.047), coronary heart disease ( p < 0.001) and hearing loss ( p = 0.002).
Table 2 presents the prevalence of prior tinnitus among cases and controls. Among the total sample, the prevalence of prior tinnitus was 18%, 21.5% among cases and 14.5% among controls ( p < 0.001). Univariable logistic regression analysis showed an unadjusted OR for prior tinnitus of 1.610 among cases relative to controls (95% CI: 1.315 ~ 1.971, p < 0.001).
Multivariable logistic regression analysis showed that after adjusting for age, income, geographical location, urbanization level, hypertension, diabetes, coronary heart disease, hyperlipidemia, obesity, hearing loss, and alcohol abuse, patients with early-onset dementia were more likely to have had tinnitus before the index date, adjusted odds ratio1.628 (95% CI = 1.321–2.006; p < 0.001) (Table 3 ).
Table 4 presents the adjusted odds ratio for prior tinnitus of early-onset dementia patients versus controls stratified by sex, age group and the presence of co-morbidities. There was no statistically significant association between early-onset dementia and interaction terms of age * tinnitus, sex * tinnitus, hypertension * tinnitus, hyperlipidaemia * tinnitus, and hearing loss * tinnitus. In addition, we found that the association of early-onset dementia with prior tinnitus exists regardless whether there was the presence of hypertension, hyperlipidaemia or hearing loss.
To our knowledge, this study may represent the first population-based retrospective study to explore a possible association between tinnitus and subsequent early-onset dementia. We found that pre-existing tinnitus was significantly associated with dementia occurrence in the population aged 30–64 years of age, Tinnitus was associated with a 63% higher risk of early-onset dementia.
Dementia is generally regarded as a multifactorial disease, and its incidence increases with age. Several pathologies have been observed to contribute to the development of dementia, including neurodegenerative proteinopathies, vascular disease, dysregulated inflammation, etc. There is usually a considerable delay in the diagnosis of dementia, especially for early-onset dementia, which is estimated to take at least 2–4.4 years after the first onset of its various symptoms 21 , 22 , 23 . The delays may arise out of low prevalence causing a low index of suspicion among younger age groups, the large variety of etiologies, and confounding with neuropsychiatric symptoms and consequent misdiagnosis of early-onset dementia. Subtle neuro-pathologic changes usually precede a definitive diagnosis of dementia. If the associated neuropathology also involves the neural circuitry that triggers tinnitus, it appears plausible that tinnitus may precede or coexist with clinically detectable dementia symptoms such as impairment of memory, early signs of deterioration of executive functions, and impairments in visuoconstructional/ perceptual-motor functions, language functions, and social cognition which typically manifest in the later stages of dementia.
Cognitive impairment has been reported as a common occurrence among tinnitus patients 13 , 14 , 15 , 16 , 17 , 24 . Mild cognitive impairments (MCI), an intermediate state before dementia patients transition into clinically evident dementia, has been reported among patients with tinnitus. Other studies have shown that tinnitus is associated with cognitive deficits, and that tinnitus patients on the severe end of spectrum are at high risk of serous cognitive deficits 18 , 19 . However, the causal mechanism that links tinnitus and dementia remain elusive.
Both tinnitus and dementia represent clinical manifestations of heterogeneous pathologies involving complex neurological and functional processes. Tinnitus is linked to dysregulated neural synchrony across neural ensembles along the auditory pathway 25 . Accumulating evidence shows that tinnitus may occur concurrent with structural and functional disruptions of a diverse range of neuro-sensory structures, ranging from the peripheral and central auditory pathways to areas of the brain that are unrelated to normal hearing and processing of auditory stimuli. Animal and human neuroimaging studies have revealed neural tissue changes similar to those observed in tinnitus-associated areas of the brain in unrelated areas including those associated with cognition impairment and/or dementia, including the ventromedial prefrontal cortex 26 , parietal cortex 27 , anterior cingulate cortex 13 , 17 , 28 , prefrontal cortex 29 , amygdala 17 , 30 , hippocampus 13 , 17 , 30 , nucleus accumbens 31 , insula 13 , 17 , and thalamus 31 . Imaging studies have shown pathological changes in the hippocampus, amygdala and prefrontal cortex in the preclinical phase of dementia 32 , 33 , 34 , 35 , 36 , 37 . Autopsy studies of patients with tinnitus and cognitively normal brain function before death also show proteinopathy or accumulation of abnormal protein aggregates in the brain areas related to tinnitus 38 . These results suggest a shared neuronal pathology between tinnitus and dementia, and support the hypothesis that tinnitus may precede or occur concurrent with subclinical or early-onset dementia. One research implication of our study finding and the related hypothesis is to look for evidence of abnormal protein aggregates in tinnitus patients with normal cognition to explore the potential role of dementia-associated proteinopathy in the pathogenesis of tinnitus.
Our study may be the first and largest population-based study to examine the association between tinnitus and dementia in patients aged under 65 years. A key strength of the study is the ability to pool a large number of early-onset dementia cases from a nationwide medical care dataset. Another strength is the universal access, national health insurance system source of the data, enabling access to uninterrupted follow-up data of the entire population with comprehensive longitudinal data on comorbidities and demographic characteristics.
There are still some study limitations. First, an epidemiological association by itself does not imply biological causality. Our finding suggests a potential link between tinnitus and early-onset dementia which may serve as an early warning sign to elevate awareness of dementia risk among tinnitus patients to proactively watch for the early signs of dementia among young and middle-aged patients. Second, because of data privacy and confidentiality under the Personal Data Protection Law and related regulations of the NHIRD, it was not possible to validate or supplement the claims data by direct patient contact. Therefore, critical items of data on the risk factors for dementia and the severity of tinnitus that are not documented in medical claims could not be obtained. These data include, such as family history, laboratory data, genetic test data (such as Apolipoprotein E ), severity of dementia, imaging test results, hearing test results, and tinnitus severity scores. Further several known confounders involved in the development of dementia were not accounted for in the analyses, including smoking, educational level, occupation, specific environmental features obesity, hearing loss, and alcohol intake. To mitigate selection bias, we used the available data on the factors known to influence dementia occurrence to select propensity score–matched control patients. Third, this study used a case–control method which did not allow to establish causation due to its retrospective nature. Finally, the present study found that coronary heart disease was negatively associated with early dementia while no significant associations of early dementia with hypertension, diabetes or hyperlipidaemia were observed. We speculate that the medications such as statins or aspirin for the treatment of coronary heart disease could be one explanation for low odds of early-onset dementia on patients with coronary heart disease. Further studies are encouraged to explore the relationship between statins or aspirin and early-onset dementia.
Our findings showed that pre-existing tinnitus is associated with a 1.675-fold increase in the risk of early-onset dementia among the young and middle-aged population. Additional studies in other populations are encouraged to confirm the relationship between early-onset dementia and tinnitus and to explore the possible mechanisms behind this relationship. Further studies are also needed to clarify the shared underlying pathophysiology between tinnitus and dementia, and to explore whether early detection and treatment of tinnitus may prevent or delay early onset dementia.
Elahi, F. M. & Miller, B. L. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol 13 , 457–476 (2017).
Article Google Scholar
Miller, B. L. et al. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 57 , 817–821 (2001).
Article CAS Google Scholar
Masellis, M. et al. Early-onset dementias: diagnostic and etiological considerations. Alzheimers Res Ther 5 , S7-22 (2013).
Rossor, M. N. et al. The diagnosis of young-onset dementia. Lancet Neurol 9 , 793–806 (2010).
Kuruppu, D. & Matthews, B. Young-onset dementia. Semin Neurol 33 , 365–385 (2013).
Oyebode, J. R., Bradley, P. & Allen, J. L. Relatives’ experiences of frontal-variant frontotemporal dementia. Qual Health Res 23 , 156–166 (2013).
Nondahl, D. M. et al. Tinnitus and its risk factors in the Beaver Dam offspring study. Int J Audiol 50 , 313–320 (2011).
McCormack, A. et al. The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK population. J Psychosom Res 76 , 56–60 (2014).
Shargorodsky, J., Curhan, G. C. & Farwell, W. R. Prevalence and characteristics of tinnitus among US adults. Am J Med 123 , 711–718 (2010).
Kim, H. J. et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS ONE 10 , e0127578 (2015).
Kochkin, S., Tyler, R. & Born, J. MarkeTrak VIII: Prevalence of tinnitus and efficacy of treatments. Hearing Rev 18 , 10–26 (2011).
Google Scholar
Henry, J. A. et al. Underlying mechanisms of tinnitus: review and clinical implications. J Am Acad Audiol 25 , 5–22 (2014).
Vanneste, S. et al. The neural correlates of cognitive dysfunction in phantom sounds. Brain Res 1642 , 170–179 (2016).
Hallam, R. S., McKenna, L. & Shurlock, L. Tinnitus impairs cognitive efficiency. Int J Audiol 43 , 218–226 (2004).
Araneda, R. et al. Altered top-down cognitive control and auditory processing in tinnitus: evidences from auditory and visual spatial stroop. Restor Neurol Neurosci 33 , 67–80 (2015).
PubMed Google Scholar
Pierce, K. J. et al. Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J Clin Exp Neuropsychol 34 , 126–134 (2012).
Tyler, R. S., Aran, J. M. & Dauman, R. Recent advances in tinnitus. Am J Audiol 1 , 36–44 (1992).
Wang, Y. et al. The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav 8 , e00918-9 (2018).
Lee, S. Y. et al. Neurocognition of aged patients with chronic tinnitus: focus on mild cognitive impairment. Clin Exp Otorhinolaryngol 13 , 8–14 (2020).
Mendez, M. F. What is the relationship of traumatic brain injury to dementia?. J Alzheimers Dis 57 , 667–681 (2017).
Harris, P. B. & Keady, J. Living with early onset dementia: exploring the experience and developing evidence-based guidelines for practice. Alzheimer’s Care Today 5 , 111–122 (2004).
Rosness, T. A. et al. Frontotemporal dementia: a clinically complex diagnosis. Int J Geriatr Psychiatry 23 , 837–842 (2008).
van Vliet, D. et al. Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 43 , 423–432 (2013).
Tyler, R. et al. Development and validation of the tinnitus primary function questionnaire. Am J Audiol 23 , 260–272 (2014).
Weisz, N. et al. The neural code of auditory phantom perception. J Neurosci 27 , 1479–1484 (2007).
Leaver, A. M. et al. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 6 , 21 (2012).
Schlee, W. et al. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci 10 , 1169–1211 (2009).
Chen, Y. C. et al. Abnormal resting-state functional connectivity of the anterior cingulate cortex in unilateral chronic tinnitus patients. Front Neurosci 12 , 9 (2018).
Araneda, R. et al. A key role of the prefrontal cortex in the maintenance of chronic tinnitus: an fMRI study using a Stroop task. Neuroimage Clin 17 , 325–334 (2018).
De Ridder, D. et al. Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol Suppl 126 , 50–53 (2006).
Rauschecker, J. P., Leaver, A. M. & Mühlau, M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66 , 819–826 (2010).
Apostolova, L. G. et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging 31 , 1077–1088 (2010).
Coupé, P. et al. Detection of Alzheimer’s disease signature in MR images seven years before conversion to dementia: toward an early individual prognosis. Hum Brain Mapp 36 , 4758–4770 (2015).
Coupé, P. et al. Lifespan changes of the human brain in Alzheimer’s disease. Sci Rep 9 , 3998–4012 (2019).
Article ADS Google Scholar
den Heijer, T. et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 133 , 1163–1172 (2010).
Miller, M. I. et al. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer’s disease. Neuroimage Clin 3 , 352–360 (2013).
Younes, L., Albert, M. & Miller, M. I. Inferring changepoint times of medial temporal lobe morphometric change in preclinical Alzheimer’s disease. Neuroimage Clin 5 , 178–187 (2014).
Tsartsalis, S. et al. Early Alzheimer-type lesions in cognitively normal subjects. Neurobiol Aging 62 , 34–44 (2018).
Download references
No funding source.
Author information
Authors and affiliations.
Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan
Yen-Fu Cheng
Department of Otolaryngology-Head and Neck Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
Department of Speech, Language and Audiology, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
Yen-Fu Cheng & Tzong-Han Yang
Research Center of Sleep Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
Department of Otolaryngology-Head and Neck Surgery, School of Medicine, National Yang-Ming University, Taipei, Taiwan
Department of Health Services Policy and Management, Arnold School of Public Health, University of South Carolina, Columbia, USA
Sudha Xirasagar
Department of Otolaryngology, Taipei City Hospital, Taipei, Taiwan
Tzong-Han Yang & Chuan-Song Wu
College of Science and Engineering, Fu Jen University, New Taipei City, Taiwan
Chuan-Song Wu
Big Data Research Center, Taipei Medical University, Taipei, Taiwan
Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, New Taipei City, Taiwan
School of Health Care Administration, Taipei Medical University, 250 Wu-Hsing St., Taipei, 110, Taiwan
Herng-Ching Lin
Sleep Research Center, Taipei Medical University Hospital, Taipei, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
Y.F. and H.C. participated in the design of the study and helped to draft the manuscript. Y.W. performed the statistical analysis and helped to draft the manuscript. T.H., S. and C.S. conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Herng-Ching Lin .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cheng, YF., Xirasagar, S., Yang, TH. et al. Risk of early-onset dementia among persons with tinnitus: a retrospective case–control study. Sci Rep 11 , 13399 (2021). https://doi.org/10.1038/s41598-021-92802-y
Download citation
Received : 28 August 2020
Accepted : 16 June 2021
Published : 28 June 2021
DOI : https://doi.org/10.1038/s41598-021-92802-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Introduction
- Conclusions
- Article Information
The National Alzheimer's Coordinating Center database was frozen between 2005 and 2017, and all patients fulfilling criteria for dementia at first visit were included. These were divided by pathologically confirmed dementia diagnosis: Alzheimer disease (AD) or dementia with Lewy bodies (DLB). Patients who were younger than 65 years at both symptom onset and first assessment were considered to have early-onset dementia. Variables were compared between these groups. There was no loss to follow-up among the final selection of 542 patients, although missing data are detailed in eTable 1 in the Supplement. NIA-AA indicates National Institute on Aging–Alzheimer’s Association.
a Criteria included interference with ability to function, decline from previous function, not explained by delirium or major psychiatric disorder, cognitive impairment diagnosed through combination of history taking and objective assessment, and impairment in at least 1 domain (memory, reasoning/judgment, visuospatial, language, personality/behavior/comportment).
eTable 1. NACC uniform data set data elements with tabulation of number missing data for early-onset AD, early-onset DLB and late-onset DLB respectively
eTable 2. Level of agreement between final pathological diagnosis and clinical diagnosis at first visit in our patient groups
eTable 3. NACC neuropsychological testing results available for early-onset dementia with Lewy bodies (EODLB), compared to early-onset Alzheimer’s disease dementia (EOAD) and late-onset DLB (LODLB)
eTable 4. Distribution of Lewy bodies at post-mortem seen in limbic/transitional versus neocortical areas in each disease group
eTable 5. Comparison of amnestic clinical features in patients with late-onset and early-onset DLB who had Braak stage 0-2 (lower AD co-pathology) at autopsy
eFigure. Number of cases in early-onset AD and DLB groups that possessed at least three of the clinical features identified by multivariate analysis from Table 3 (first changes occurring in motor rather than behavioral or cognitive domains), motor slowing, presence of apathy, visual hallucinations and low agitation)
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sim J , Li H , Hameed S , Ting SKS. Clinical Manifestations of Early-Onset Dementia With Lewy Bodies Compared With Late-Onset Dementia With Lewy Bodies and Early-Onset Alzheimer Disease. JAMA Neurol. 2022;79(7):702–709. doi:10.1001/jamaneurol.2022.1133
Manage citations:
© 2024
- Permissions
Clinical Manifestations of Early-Onset Dementia With Lewy Bodies Compared With Late-Onset Dementia With Lewy Bodies and Early-Onset Alzheimer Disease
- 1 Department of Neurology, National Neuroscience Institute, Singapore General Hospital, Singapore
- 2 Health Services Research and Biostatistics Unit, Singapore General Hospital, Singapore
Question Do patients who develop dementia with Lewy bodies (DLB) younger than 65 years have unique clinical features?
Findings In this case-control study of 542 individuals, early-onset DLB was often misdiagnosed; certain motor features and, to a lesser extent, neuropsychiatric features were associated with a diagnosis of early-onset DLB over early-onset Alzheimer disease dementia. Late-onset DLB had more prominent amnestic features but lower rates of depression than early-onset DLB.
Meaning In evaluation of suspected early-onset DLB, one should assess for motor signs, apathy, depression, and determine if motor deterioration predated cognitive and behavioral changes; the amnestic features seen in late-onset DLB may be associated with Alzheimer disease copathology.
Importance Early-onset dementia, presenting in individuals younger than 65 years, is a diagnosis with significant social and financial implications. The early-onset form of dementia with Lewy bodies (DLB) is poorly understood.
Objective To investigate clinical features that distinguish early-onset DLB (onset and diagnosis at age <65 years) from late-onset DLB (onset at age ≥65 years) and from early-onset Alzheimer disease (AD) dementia.
Design, Setting, and Participants This is a retrospective case-control study on patients with pathologically confirmed DLB or AD enrolled in the National Alzheimer’s Coordinating Center database from January 2005 to July 2017. The National Alzheimer’s Coordinating Center Uniform Data Set comprised deidentified data collected by Alzheimer disease centers in the United States. Of patients fulfilling criteria for all-cause dementia at enrollment (n = 1152), those who at post mortem received a pathological diagnosis of either AD (n = 848) or Lewy body disease (n = 218) were selected. Excluding 52 patients owing to missing data and 12 diagnosed with Parkinson disease dementia, remaining patients were classified by age of symptom onset into early-onset AD, early-onset DLB, and late-onset DLB subgroups. Data were analyzed from June to December 2018 and from November to December 2021.
Exposures Demographics, cognitive, behavioral, and motor features recorded at first clinic visit and neuropathological characteristics at autopsy were analyzed by disease subgroup.
Main Outcomes and Measures Concordance between initial etiologic diagnosis of dementia and final pathological diagnosis was assessed, as was time to death.
Results A total of 542 individuals were categorized as having early-onset AD (n = 363; mean [SD] age, 53.0 [5.8] years; 208 [57.3%] male), early-onset DLB (n = 32; mean [SD] age, 57.9 [3.2] years; 23 [71.9%] male), and late-onset DLB (n = 147; mean [SD] age, 73.5 [5.5] years; 103 [70.1%] male). Early-onset DLB was clinically misdiagnosed in 16 individuals (50%). Features that predicted a diagnosis of early-onset DLB over early-onset AD included visual hallucinations (15 [46.9%] vs 42 [11.6%]), slowness (23 [71.9%] vs 95 [26.2%]), apathy (23 [71.9%] vs 189 [52.1%]), and motor deterioration that preceded cognitive and behavioral symptoms (7 [21.9%] vs 6 [1.7%]). Late-onset DLB had more amnestic features, but this was accounted for by a higher proportion of neocortical neuritic plaques and diffuse plaques (frequent in 79 [53.7%] vs 8 [25%]) than seen in early-onset DLB.
Conclusions and Relevance This study found that early-onset DLB has clinical features that distinguish it from early-onset AD, whereas features of late-onset DLB are associated with a higher burden of AD copathology.
Lewy body disease is the second most common neurodegenerative cause of dementia after Alzheimer disease (AD). 1 Lewy body disease pathology is associated with 2 clinical diagnoses: dementia with Lewy bodies (DLB) and Parkinson disease dementia. Core clinical features of DLB include parkinsonism, fluctuation of consciousness, visual hallucination, and rapid eye movement sleep behavior disorder, while supportive features include neuroleptic sensitivity, postural instability, falls, and autonomic dysfunction. 2 Interestingly, there is likely overlap in etiology of AD dementia and DLB. Overall, 66% to 80% of postmortem brain samples from patients had some evidence of AD copathology; conversely, 40% of those diagnosed with AD dementia had a degree of Lewy body pathology. 2 , 3
Dementia with onset in individuals younger than 65 years (early-onset dementia) poses a particular diagnostic challenge for the clinician. It is a rarer condition with many possible etiologies, each with different implications for prognosis. 4 Dementia that develops in individuals younger than 65 years leads to greater economic burden, caregiver stress, and mortality rates. 5 , 6 Early-onset and late-onset dementia have distinct clinical and neuroimaging findings. 7 For instance, one-third of early-onset AD dementia may present with nonamnestic symptoms including apraxia and visuospatial dysfunction, but this is the case for only 6% of late-onset AD dementia. 8
The mean age at onset of DLB is 75 years, 9 and incidence increases with age. 10 , 11 As with AD, Lewy body pathology can also cause dementia in individuals younger than 65 years. Case reports illustrate features of rapidly progressive dementia, myoclonus, cortical visual disturbance and neuropsychiatric changes, attention deficits, myoclonus, depression, visual construction issues, and apraxia. 12 - 14 There are no longitudinal studies on early-onset DLB, to our knowledge. It is unclear if the core clinical consensus criteria of DLB 15 are equally applicable to early-onset DLB or whether it is an entity distinct to late-onset DLB and prodromal DLB. 16 Based on comparative studies of DLB and AD dementia, 17 one might wonder how to distinguish early-onset DLB and early-onset AD dementia at presentation and how their prognosis compares.
This is a retrospective case-control study of patients with pathologically confirmed Lewy body disease and AD from the National Alzheimer’s Coordinating Center (NACC) database. Among individuals with dementia onset at younger than 65 years, we looked at how closely initial etiologic diagnoses agreed with these final pathological diagnoses. We identify factors that predicted a diagnosis of early-onset DLB over early-onset AD dementia based on information gathered at first clinic visit. We then compare the clinical features of early-onset DLB with late-onset DLB, with reference to differences identified on neuropathological data.
This was a retrospective case-control study based on the NACC Uniform Data Set, which comprises deidentified data provided with participants and informants’ written informed consent, collected by Alzheimer disease centers. The database is funded by the US National Institute on Aging (NIA). Data collection is in accordance with NIA policies. Database research is approved by the University of Washington institutional review board and data access complied with relevant data use agreements. 16 An NACC data set used for the current study was contributed by 17 centers in the United States and included 1152 patients evaluated from January 2005 to July 2017. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Data on self-reported race and ethnicity were collected by the NACC database, with categories of American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, or other. Patients who fulfilled clinical criteria for all-cause dementia on their first visit were included initially ( Figure ). Patients with more than 50% incomplete data were excluded. These were divided into 2 pathologically defined subgroups: (1) Lewy body disease: patients with Braak stage 4 and below, who either had Lewy body pathology at autopsy or were given a pathological diagnosis of DLB pathology. Patients whose only initial clinical diagnosis was Parkinson disease dementia were excluded. Patients were retrospectively classified as having early-onset DLB if onset of relevant symptoms and clinical diagnosis of dementia both occurred at younger than 65 years or late-onset DLB if symptom onset occurred at 65 years or older. (2) AD: patients who met NIA/Reagan Institute neuropathological criteria for AD pathology (either having a high NIA–Alzheimer’s Association AD neuropathologic change score or receiving a pathological diagnosis of AD, primary). Patients were classified as having early-onset AD dementia if symptom onset and clinical diagnosis of dementia both occurred at age younger than 65 years.
Based on these pathologically defined subgroups, data gathered at first clinic visit and at post mortem were then analyzed retrospectively. These included demographic, cognitive, and motor and behavioral variables, which were collected on different forms under the first visit packet in the NACC database (eTable 1 in the Supplement ). Formal test batteries in the NACC database used included the Mini-Mental State Examination (MMSE), Unified Parkinson’s Disease Rating Scale, Geriatric Depression Scale (GDS), Neuropsychiatric Inventory Questionnaire (NPI-Q), Logical Memory IA and IIA, Trails A and B, and Boston Naming test. Clinical variables were measured and recorded by the clinician, while the NPI-Q was administered through informants.
The etiologic diagnoses of dementia made by clinicians at first visit were also analyzed. The NACC recommended these diagnoses to be guided by consensus criteria for probable DLB, 15 and NIA–Alzheimer’s Association criteria for probable AD dementia. 18 A diagnosis was taken into account whether it was deemed a primary or contributing diagnosis for dementia.
The κ statistic was used to assess concordance between initial clinical and final pathological diagnoses of AD dementia and DLB. This is adjusted for agreement by chance and interpreted as such 19 : slight agreement, 0.21 to 0.40; fair agreement, 0.41 to 0.60; and moderate agreement, 0.61 to 0.80.
Mean (SD) were reported for continuous variables, while frequency and proportion were reported for categorical data. Two pairwise comparisons were performed for every variable: between early-onset AD and early-onset DLB and between early-onset DLB and late-onset DLB. Each pair was compared using a t test for continuous variables and Fisher exact tests for categorical variables. Univariate comparisons were adjusted for either MMSE score (early-onset DLB vs AD) or mood medication usage (early-onset DLB vs late-onset DLB). Missing values were not imputed; complete-case analysis was used.
Logistical regression was then performed to select factors that best distinguished early-onset DLB from early-onset AD. After variables with more than 40% missing values were excluded, all predictors with P value of .10 or less at univariate analysis were used to build a multivariable model. Reduced model selection was performed using a backward stepdown by applying the Akaike information criterion. All data analysis was performed in R version 3.5.3 (R Foundation), with 2-sided significance level set at .05. Data were analyzed from June to December 2018 and from November to December 2021.
After inclusion and exclusion criteria were applied, 363 patients with early-onset AD (mean [SD] age, 53.0 [5.8] years; 208 [57.3%] male), 32 patients with early-onset DLB (mean [SD] age, 57.9 [3.2] years; 23 [71.9%] male), and 147 patients with late-onset DLB remained (mean [SD] age, 73.5 [5.5] years; 103 [70.1%] male) ( Figure ). A tabulation of missing values is provided (eTable 1 in the Supplement ).
Among patients with early-onset dementia, the initial clinical diagnosis concurred with the pathologic diagnosis in 79.9% (290 of 363) for pathologically confirmed AD but only 50% (16 of 32) of patients with pathologically confirmed DLB (eTable 2 in the Supplement ). Based on κ statistics, there was only slight agreement in AD diagnoses and moderate agreement in DLB diagnoses. Interestingly, 12 of 32 patients (37.5%) with pathologically confirmed DLB and early-onset dementia received a clinical diagnosis of AD at their first clinic visit. This suggests that we can improve our ability to distinguish early-onset AD dementia and DLB clinically at presentation.
Patients with early-onset DLB in this cohort were older than those with early-onset AD at symptom onset and at first clinic visit but had shorter time to death and higher MMSE scores than patients with AD ( Table 1 ).
Even after correcting for baseline MMSE and age differences between these 2 groups, early-onset DLB was more likely to present with hallucinations, delusions, apathy, rapid eye movement sleep behavior disorder, and motor symptoms including altered gait, tremors, slowing, and increased falls than those with AD ( Table 2 ). They were more likely than patients with AD to report deterioration starting in the motor domain, rather than the cognitive or behavioral domains. Patients with early-onset DLB also had higher GDS scores, although the frequency of reporting low mood was similar in both groups. Although memory impairment was judged by clinicians to be present in more than 85% of patients with early-onset DLB at first visit, those with early-onset AD patients more likely to report cognitive changes as the first sign of deterioration and to do worse on Logical Memory tests but not Trails or Boston Naming ( Table 2 ). A comparison of other neuropsychological test battery results available in the NACC data set is also provided (eTable 3 in the Supplement ).
A multivariate regression was then performed ( Table 3 ) using all variables that distinguished early-onset DLB and AD at a significance level of P < .10 on univariate analysis, excluding variables with more than 40% missing values. This analysis identified 5 factors, combinations of which most specifically predicted a diagnosis of early-onset DLB over early-onset AD. These factors included slowness, visual hallucinations, apathy (on NPI-Q), absence of agitation (on NPI-Q), and changes being seen first in the motor domain (predating cognitive and behavioral changes).
Overall, 20 of 32 patients (63%) with early-onset DLB possessed at least 3 of these 5 factors at first clinic visit, compared with 57 of 363 patients (16%) with early-onset AD (eFigure in the Supplement ). The accurately diagnosed patients with DLB included 6 patients with early-onset DLB whose initial clinical diagnosis did not concur with their pathological diagnosis. Among 16 patients with early-onset DLB who were initially misdiagnosed, 8 showed motor slowing, 11 were not agitated, and 11 were apathetic (data not shown).
Patients with early-onset and late-onset DLB were largely similar, except for a few notable features. Although baseline MMSE scores were similar ( Table 1 ), late-onset DLB was characterized by more significant memory impairment and worse performance on neuropsychological test batteries than early-onset DLB ( Table 2 , with more complete neuropsychological testing data in eTable 3 in the Supplement ).
Patients with early-onset DLB also more frequently took mood-control medications than patients with late-onset DLB ( Table 1 ). Consistent with this, patients with early-onset DLB were more frequently reporting depressed mood and also higher GDS scores than patients with late-onset DLB ( Table 2 ). This association persisted even after correcting for prior use of mood medications.
Postmortem data from early and late-onset DLB subgroups were compared. There was no differential distribution of Lewy bodies across limbic and neocortical regions (eTable 4 in the Supplement ). However, there was a higher density of neocortical neuritic plaques and diffuse plaques in late-onset DLB brain samples, changes that are more classically seen in AD ( Table 4 ). After limiting analysis to patients with DLB in Braak stages 0 to 2, effectively excluding those with significant AD copathology, reported memory impairment and scores on selected neuropsychological tests became no different between patients with early- and late-onset DLB, yet differences in GDS scores remained (eTable 5 in the Supplement ). This strongly suggested that the amnestic features seen in the late-onset DLB group were driven by AD copathology.
Early-onset DLB was often misdiagnosed as AD dementia in this study, but we identify clear distinguishing characteristics that clinicians could focus on when confronted with an early-onset dementia of undifferentiated etiology. Patients with early-onset DLB showed more psychotic features, cognitive fluctuations, motor changes, and apathy than patients with early-onset AD. There was a predominance of motor features, more so than neuropsychiatric features. This has important implications, as it suggests a thorough motor examination is critical when assessing early-onset dementia. The limited range of neuropsychological tests used in this study could not consistently distinguish early-onset DLB from early-onset AD dementia. However, patients with late-onset DLB had more amnestic deficits than those with early onset, which we attribute to a higher burden of AD copathology.
The clinical features of early-onset DLB here are consistent both with those set forth in the 2017 DLB consortium criteria 15 and with those of prodromal DLB described elsewhere. 20 Visual hallucinations and parkinsonism are core features in consortium criteria, while apathy is a supportive feature. We highlight here that a focus on motor slowing, hallucinations, and the temporal sequence of motor changes relative to cognitive or behavioral changes improves our ability to discriminate early-onset DLB from early-onset AD. However, it was surprising that early-onset DLB was associated with lower agitation, whereas higher agitation levels are more commonly reported in DLB. 21
We also propose the importance of assessing for depression through different modalities as part of the workup of early-onset dementia. While subgroups were indistinguishable on clinician evaluation of low mood, patients with early-onset DLB had higher GDS scores. Early-onset DLB was also linked to more frequent prescriptions of mood medication compared with late-onset DLB; this is aligned with studies showing that depression predicts an earlier age of onset in DLB but not in other dementias, 22 perhaps highlighting a role for serotonergic pathways. 23
The findings of our comparison between early- and late-onset DLB are also consistent with current literature. Concomitant AD pathology is seen in up to 85% of DLB cases. 3 Individuals with mixed AD-DLB pathology are usually older and show more marked cognitive decline. 24 , 25 Braak stage 3 or higher copathology is associated with specific amnestic and nonamnestic features in DLB, compared with individuals with DLB without AD copathology. 26 Conversely early-onset DLB shows more divergence from the AD dementia phenotype, given the lower burden of AD copathology. In our data, comparing patients with early- and late-onset DLB in lower Braak stages (eTable 5 in the Supplement ), we show that AD copathology may account for the differences in amnestic features more so than the difference in GDS scores (nonamnestic features). We are unable to determine from this data set if AD copathology was present at disease onset or accumulated over time and whether vascular copathology contributed to the late-onset DLB phenotype. These should be explored in future studies.
This study would have benefited from a larger and newer data set with greater ethnic diversity. The small size of the early-onset DLB subgroup likely subjected it to greater bias from missing data than the other 2 subgroups. This work also lacked a statistical correction for multiple comparisons. Although cognitive fluctuations and rapid eye movement sleep behavior disorder are integral to DLB consensus criteria, they had to be excluded from our multivariate analysis owing to missing data (eTable 1 in the Supplement ), which likely limited the generalizability and accuracy of our predictions. Future work would involve applying the results of our multivariate analysis to a validation data set, without which the model remains largely descriptive. It would also be meaningful to do a longitudinal analysis of clinical features and diagnoses to determine if these changed over serial evaluations.
The evaluation of certain variables could also be improved. Rapid eye movement sleep disorder was assessed with a single question posed to clinicians and patients. Motor function evaluated by the Unified Parkinson’s Disease Rating Scale alone was not objectively measured but taken by report alone; we might instead consider using the Unified Parkinson’s Disease Rating Scale motor subscale for a more quantitative analysis. The use of NACC data has limitations for the study of DLB, as data collection was primarily focused on the AD spectrum, particularly in earlier database versions, and in the selection of neuropsychological tests available. Future study in DLB should include tests more specific to executive function, visuoperceptual abilities, and construction. 27
In this study, early-onset DLB was best distinguished from early-onset AD dementia by features largely found in DLB consortium criteria. Compared with late-onset DLB, early-onset DLB has a lower burden of AD copathology and saw an association with features of early depression. Further research into the neuropathological basis of these differences is warranted. Nevertheless, memory impairment was still the most common symptom in DLB regardless of age at onset. Thus, when approaching a new patient with early-onset dementia, careful history-taking to determine if motor changes preceded cognitive and behavioral impairment, asking caregivers about apathy and agitation levels, and examining for motor slowing might improve the accuracy of a DLB diagnosis. Finally, patients with early-onset DLB experienced a shorter time from dementia diagnosis to death than patients with early-onset AD dementia. This may reflect delays in presentation, delays in diagnosis, higher symptom burden, or worse prognosis in patients with early-onset DLB. We hope this study challenges clinicians to better understand the characteristics and needs of this disease subgroup.
Accepted for Publication: March 12, 2022.
Published Online: May 23, 2022. doi:10.1001/jamaneurol.2022.1133
Corresponding Author: Simon Kang Seng Ting, MD, Singapore General Hospital, Outram Road, Singapore 169608, Singapore ( [email protected] ).
Author Contributions : Drs Ting and Sim had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Hameed, Ting.
Acquisition, analysis, or interpretation of data: Sim, Li, Ting.
Drafting of the manuscript: Sim, Ting.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Sim, Li, Ting.
Administrative, technical, or material support: Sim, Ting.
Supervision: Ting.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was supported by Singhealth Foundation (grant NRS 15/001) and NNI Centre (grant NCG CS02). The National Alzheimer's Coordinating Center database is funded by the National Institute on Aging/National Institutes of Health (grant U24 AG072122). National Alzheimer's Coordinating Center data are contributed by the National Institute on Aging–funded Alzheimer disease research centers (grants P50 AG005131, P50 AG005133, P50 AG005134, P50 AG005136, P50 AG005138, P50 AG005142, P50 AG005146, P50 AG005681, P30 AG008017, P30 AG008051, P50 AG008702, P30 AG010124, P30 AG010129, P30 AG010133, P30 AG010161, P30 AG012300, P30 AG013846, P30 AG013854, P50 AG016573, P50 AG016574, P30 AG019610, P50 AG023501, P50 AG025688, P30 AG028383, P50 AG033514, P30 AG035982, P50 AG047266, P50 AG047270, P50 AG047366, P30 AG049638, P30 AG053760, P30 AG066546, P20 AG068024, P20 AG068053, P20 AG068077, P20 AG068082, P30 AG072958, and P30 AG072959.
Role of the Funder/Sponsor: Singhealth Foundation and the NNI Centre had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Case studies
These three case studies help you to consider different situations that people with dementia face. They are:
- Raj , a 52 year old with a job and family, who has early onset dementia
- Bob and Edith , an older married couple who both have dementia and are struggling to cope, along with their family
- Joan , an older woman, who lives alone and has just been diagnosed with dementia
Each case study contains:
- A vignette setting out the situation
- An ecogram showing who is involved
- An assessment which gives essential information about what is happening and the social worker’s conclusion
- A care and support plan which says what actions will be taken to achieve outcomes
You can use the practice guidance to think about how you would respond in these situations.

- Equal opportunities
- Complaints procedure
- Terms and conditions
- Privacy policy
- Cookie policy
- Accessibility

Needs in early onset dementia: A qualitative case from the NeedYD study
Affiliation.
- 1 Centre for Specialized Care in Early Onset Dementia, Rijswijk, The Netherlands. [email protected]
- PMID: 21131669
- PMCID: PMC10845426
- DOI: 10.1177/1533317510385811
Objectives: The aim was to explore the experiences of a caregiver of a patient with early onset dementia (EOD) and the needs of patient and caregiver.
Methods: A single case study design was used to explore (1) unmet needs of patient and caregiver and (2) caregiver's experiences of transitions in care and health care services. A qualitative analysis was used to examine the data.
Results: This study shows that a patient with EOD and the caregiver were confronted with specific issues during the care process, namely (1) prolonged time to diagnosis, (2) a lack of fit between needs and services, (3) the strain of dedication to care versus the caregiver's own future perspective, (4) the need for response of health care services to changing individual preferences.
Conclusion: This study illustrates specific issues related to early onset dementia that require specialized EOD care such as the differential and changing needs of patients and caregivers.
Publication types
- Case Reports
- Research Support, Non-U.S. Gov't
- Adaptation, Psychological
- Age of Onset
- Alzheimer Disease / psychology*
- Alzheimer Disease / therapy*
- Middle Aged
- Needs Assessment
- Qualitative Research
- Respite Care / psychology
- Social Support*
- Spouses / psychology*

CASE REPORT article
Case report: early-onset behavioral variant frontotemporal dementia in patient with retrotransposed full-length transcript of matrin-3 variant 5.

- 1 Department of Neurology, N. Bud Grossman Center for Memory Research and Care, University of Minnesota, Minneapolis, MN, United States
- 2 Department of Neurology, Mary S. Easton Center for Alzheimer's Disease Research at UCLA, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
- 3 Department of Clinical Genomics, Mayo Clinic Rochester, Rochester, MN, United States
- 4 Division of Genetics and Metabolism, University of Minnesota, Minneapolis, MN, United States
- 5 Molecular Diagnostics Laboratory, M Health-Fairview, University of Minnesota, Minneapolis, MN, United States
- 6 Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, United States
- 7 Department of Neurology, Mayo Clinic Rochester, Rochester, MN, United States
- 8 Institute for Translational Neuroscience, University of Minnesota Medical School, Minneapolis, MN, United States
Frontotemporal dementia (FTD) rarely occurs in individuals under the age of 30, and genetic causes of early-onset FTD are largely unknown. The current report follows a 27 year-old patient with no significant past medical history presenting with two years of progressive changes in behavior, rushed speech, verbal aggression, and social withdrawal. MRI and FDG-PET imaging of the brain revealed changes maximally in the frontal and temporal lobes, which along with the clinical features, are consistent with behavioral variant FTD. Next generation sequencing of a panel of 28 genes associated with dementia and amyotrophic lateral sclerosis (ALS) initially revealed a duplication of exon 15 in Matrin-3 ( MATR3 ). Whole genome sequencing determined that this genetic anomaly was, in fact, a sequence corresponding with full-length MATR3 variant 5 inserted into chromosome 12, indicating retrotransposition from a messenger RNA intermediate. To our knowledge, this is a novel mutation of MATR3 , as the majority of mutations in MATR3 linked to FTD-ALS are point mutations. Genomic DNA analysis revealed that this mutation is also present in one unaffected first-degree relative and one unaffected second-degree relative. This suggests that the mutation is either a disease-causing mutation with incomplete penetrance, which has been observed in heritable FTD, or a benign variant. Retrotransposons are not often implicated in neurodegenerative diseases; thus, it is crucial to clarify the potential role of this MATR3 variant 5 retrotransposition in early-onset FTD.
Introduction
Frontotemporal dementia (FTD) refers to a group of dementias characterized by degeneration in the frontal and temporal lobes of the brain ( 1 ). Rates of early-onset FTD are high with 13% of individuals with FTD being under the age of 50 ( 2 ). Younger cases of FTD, with onset before the age of 30, tend to exhibit frequent abrupt mood changes, increased aggression, behavioral disinhibition, lack of empathy, and deficits in working memory ( 3 ). There are three clinical presentations of FTD: behavioral variant (bvFTD) and two forms of primary progressive aphasia, wherein non-fluent or fluent aphasia are the key neurologic deficits ( 4 , 5 ). In addition, FTD can overlap with other neurodegenerative disease motor deficits including: corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis (ALS) ( 6 ). Approximately 30–50% of FTD cases have some family history of dementia, parkinsonism or ALS ( 7 ), and in 10–20% a genetic cause is found ( 8 ). Positive family history of dementia/parkinsonism/ALS typically shows an autosomal dominant pattern with high penetrance, with at least one first-degree relative of the proband being affected ( 7 ). Mutations in genes encoding microtubule associated protein tau ( MAPT ), progranulin ( GRN ), and chromosome 9 open reading frame 72 ( C9orf72 ) account for about half of all familial cases of FTD ( 9 , 10 ). Mutations in MATR3 , which encodes matrin-3, have been found in some cases of familial FTD as well ( 11 ). Matrin-3 is a DNA- and RNA-binding protein that is part of the nuclear matrix and has a wide variety of functions including transcriptional regulation, DNA binding, and RNA splicing and degradation ( 11 , 12 ). Matrin-3 interacts with pathologic markers, such as TAR DNA-binding protein 43 (TDP-43), which aggregates in neuronal cytoplasm in both FTD and ALS ( 13 ).
There are several pathological subtypes of FTD, categorized under frontotemporal lobar degeneration (FTLD) and classified by the presence of abnormal components of neuronal and glial inclusions. Some of the major subtypes include microtubule-associated protein tau in FTLD-tau, TDP-43 in FTLD-TDP, and fused in sarcoma (FUS) protein in FTLD-FUS ( 14 ). FTLD-TDP is the most common pathological subtype, occurring in ~50% of all cases. FTLD-tau accounts for ~45% of all cases, followed by FTLD-FUS in <5% of cases ( 13 ). The majority of individuals with FTLD-FUS are diagnosed with sporadic, early-onset bvFTD ( 6 ).
This report discusses the clinical presentation and genetic findings of an individual with early-onset FTD. Collectively, FTD is clinically heterogeneous, making it difficult to predict the underlying pathological or genetic processes. Thus, with these findings we aim to further understand the molecular pathology and systemic features of early-onset forms of FTD.
Case Report
The patient was in good baseline health until age 27 years, when her family noticed an increase in aggression and child-like behaviors. Initially, a family member brought her to the emergency department for behaving erratically. She was later discharged and diagnosed with adjustment disorder with mixed emotional features.
Four months later, the patient was brought into the emergency department (ED) with suspicion of a psychiatric disorder. On exam, she was noticeably withdrawn and inattentive. Due to apparent psychosis, she was placed on hold and hospitalized. Following admission, she was given a provisional diagnosis of bipolar disorder type 1. She was placed on oral olanzapine and observed over three days with slight improvements in symptoms allowing for voluntary discharge.
The patient was brought to the ED again two months later after a physical altercation. In the ED, her psychiatric exam was notable for disorganized thoughts, mood lability, poor insight, and rapid, tangential speech; however, she was not agitated or aggressive. She was admitted on a 72-h hold for mania and psychosis. Her drug screen was negative and she was admitted to the psychiatry service. She did not respond to multiple antipsychotic medications or neuroleptic drugs. For workup of medically refractory psychosis, she completed a brain MRI which showed bilateral medial frontal, anterior temporal, and caudate head atrophy ( Figure 1A ). Clinicians in the neurology department then evaluated her and transferred her to their service for further testing.
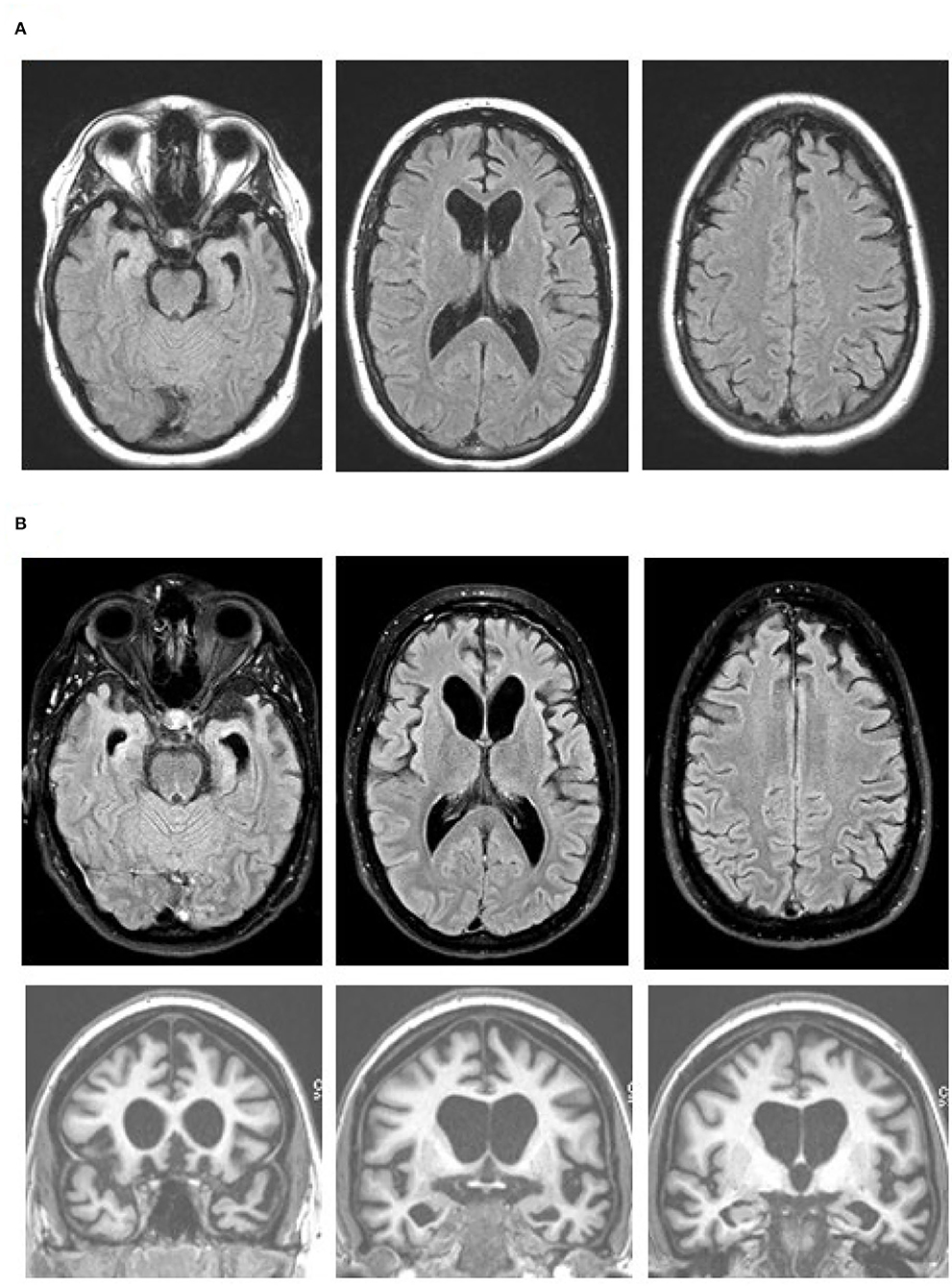
Figure 1 . Brain MR images at age 27 (A) and 19 months later (B) , with axial fluid attenuation inversion recovery (FLAIR) images (top and middle rows) and coronal T1-weighted images (bottom row). Note progression of the bilateral medial frontal, anterior temporal, and caudate head atrophy with the associated ventricular enlargement on the axial FLAIR images. The orbitofrontal as well as dorsomedial, anterior temporal, and caudate head atrophy are obvious on the coronal images. Images are in standard radiology orientation.
She completed an EEG, MRI, and lumbar puncture. The EEG was characterized by diffuse theta/delta slowing consistent with non-specific encephalopathy of unknown etiology. Her second MRI, two months after initial MRI, showed similar changes to the prior MRI. The cerebrospinal fluid workup for infectious and autoimmune conditions was unrevealing. The cerebrospinal fluid autoantibody panel inclued NMDA-R, CASPR2-lgG, GAD65, ANNA-3, AGNA-1, PCA-Tr, Amphiphysin, VGKC-Complex, LGI1-lgG, GABA-B-R, AMPA-R, ANNA-1, ANNA-2, PCA-1, PCA-2, and CRMP-5-IgG. The blood autoantibody panel included NMDA-R, CASPR2-lgG, GAD65, GABA-B-R, AMPA-R, ANNA-1, ANNA-2, ANNA-3, AGNA-1, PCA-Tr, Amphiphysin, ACh Receptor (Muscle) Binding, AChR Ganglionic Neuronal, Neuronal (V-G) K+ Channel, LGI1-lgG, PCA-1, PCA-2, N-Type Calcium Channel, P/Q-Type Calcium Channel, and CRMP-5-IgG. After completing these exams, she was readmitted to the psychiatry department.
Three weeks later, a fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan was performed, which showed hypometabolism that was severe in the orbitofrontal and caudate regions, moderate in the frontotemporal regions, and mild to moderate in the parietal and posterior cingulate regions ( Figure 2 ). FDG-PET from skull to thigh revealed no evidence of malignancy. In the following months, her behavioral disinhibition and psychosis did not improve on multiple antipsychotic medications. She was administered six courses of electroconvulsive therapy over two weeks but showed no improvement.
Figure 2 . Statistical z-score maps of brain FDG-PET at age 27, with the degree of hypometabolism reflected by the color bar on the left; dark blue is within normal limits and red is maximally abnormal. Note the degree of hypometabolism is severe in the orbitofrontal and caudate regions, moderate in the frontotemporal regions (left slightly more so than right), and mild to moderate in the parietal and posterior cingulate regions.
Although a primary psychiatric illness was considered on initial presentation, her clinical features, lack of response to antipsychotics, and prominent atrophy seen via brain imaging indicated a neurodegenerative condition. The brain MRI and FDG-PET findings, along with her clinical features were consistent with behavioral variant FTD (bvFTD) ( 14 ). Her presentation was notable for lack of insight, diminished empathy, aberrant motor behaviors, verbal aggression, stereotyped phrases, social withdrawal, disinhibition, and environmental dependence. There was no clinical evidence of motor or neuromuscular dysfunction. A five generation family history was reviewed and, with the exception of one second-degree relative with suspected mild cognitive impairment, two second-degree relatives with developmental delay, and one third-degree relative with arachnoid cyst, no other contributory or signficant family history was noted. Given the extremely young age and aggressive behavior with maintained verbal fluency, lack of family history of a neurodegenerative disease, and imaging findings particularly with prominent caudate atrophy/hypometabolsim, bvFTD with FUS proteinopathy was considered a strong possibility. MRI performed 19 months after the initial scan showed a progression of the medial frontal, anterior temporal, and caudate head atrophy with the associated ventricular enlargement ( Figure 1B ). No restricted diffusion was seen on this MRI or previous MRIs.
Genetic Analysis
Due to the unique presentation, we performed next generation sequencing (NGS) to analyze a panel of 28 genes associated with dementia and ALS. The panel included ALS2, APP, CHCHD10, CHMP2B, PCTN1, FUS, GRN, HNRNPA2B1, MAPT, MATR3, OPTN, PFN1, DRNP, PSEN1, PSEN2, PSG11, SETX, SIGMAR1, SNCA, SOD1, SQSTM1, TARDBP, TBK1, TFG, TREM2, UBQLN2, VAPB , and VCP . Genetic analysis initially indicated a duplication of exon 15 from the MATR3 gene in the patient. NGS of both parents' DNA revealed this same feature in one clinically unaffected parent. All other gene tests in this panel were negative. We also assessed for hexanucleotide repeat expansion in C9orf72 in the proband using a PCR based assay, and this test was normal (<20). In addition, we did clinical whole exome sequencing on the patient and the parents to evaluate for any other possible causes of FTD, which was performed using the Agilent SureSelect NovaSeq instrument with average coverage across the entire capture of 180x. The clinical whole exome sequence was negative for the patient and the parents.
We obtained blood samples from the patient and the parents to further characterize the MATR3 mutation that was identified through NGS. We performed whole genome sequencing (WGS) through the Genomics Center as follows. Three TruSeq unique dual-indexed DNA libraries were created. All libraries were pooled and sequenced on a NovaSeq 6000 system, with S2-type flow cell and run mode of 2 × 150 bp. ~300 million pass-filter reads or greater were generated for each sample. Mean quality scores for all libraries were ≥ Q30 indicating <0.1% error rate in base calling. WGS indicated that variant 5 MATR3 cDNA ( MATR3V5 ) had been inserted into chromosome 12 (CH12), flanked by 15 base-pair repeats, with an insertion site 20 kbp upstream of the LIM Homeobox 5 ( LHX5) gene. WGS results refuted the presence of an exon 15 duplication on chromosome 5, which was not detected. Huntington's disease was ruled out by WGS indicating that there was no evidence for HTT CAG repeat expansion in any of the samples.
We verified the results of WGS using Phusion High-Fidelity PCR (Thermo-Fisher) with primers crossing putative CH12/ MATR3V5 junctions. Two independent assays were completed: assay 1 [forward primer (CH12F1), TCTCTGCTGGCTCTACCTAAA; reverse primer (MATR3e15-16R1), AGTTCCTCGATCTTGTCCACC], and assay 2 [forward primer (MATR3e16F1), TGAGAACGCTGATGATCCCAA; reverse primer (CH12R1), AAAAAGGTGTTTCCTGGGAGCG], which targeted the 5′ and 3′ ends of the insertion, respectively ( Figure 3A ). Presence of DNA was confirmed and band size was measured using UV/ethidium bromide visualization in 1% agarose gel. The mutation was confirmed in the patient and one healthy parent ( Figure 3B ). To determine whether this mutation could have arisen de novo in the unaffected parent, blood and serum samples were collected from both grandparents biologically related to the healthy parent carrier. Targeted PCR revealed that one healthy grandparent also carries the MATR3V5 insertion in CH12 ( Figure 3B ). These results indicate that, despite the MATR3V5 mutation being present in multiple generations, only the patient showed clinical symptoms.
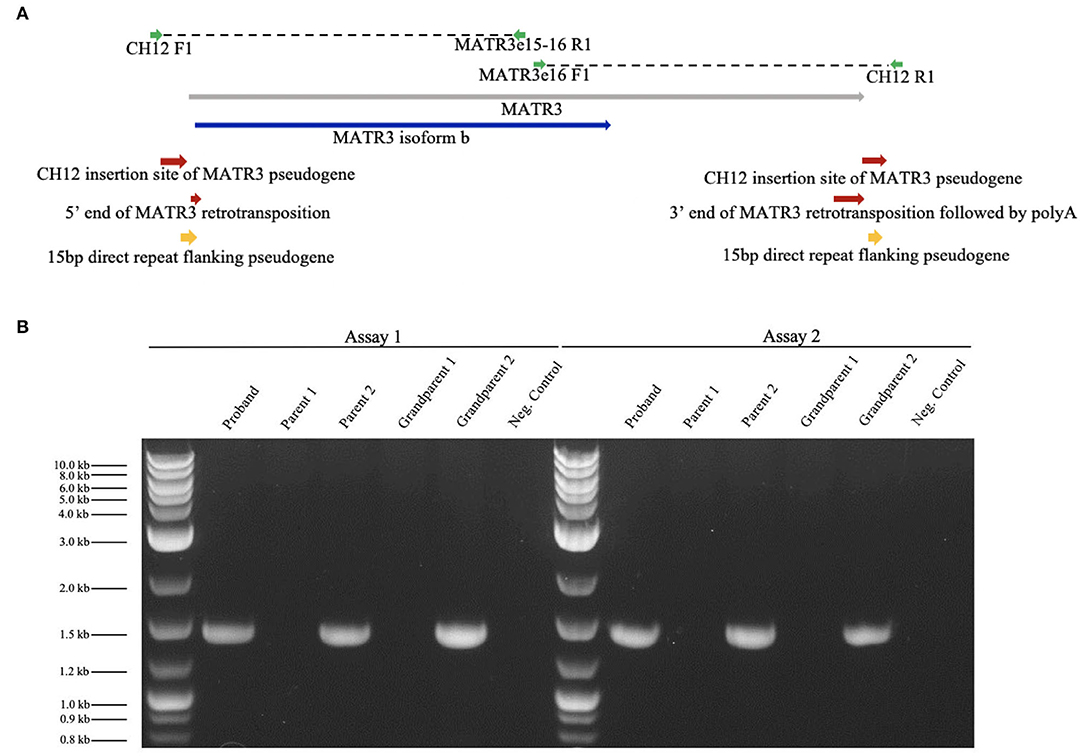
Figure 3 . Targeted PCR confirmed the MATR3V5 insertion in chromosome 12. (A) Diagram of chromosome 12 insertion site of MATR3 variant 5 and primers used for PCR. MATR3 gene sequence that codes for the matrin-3 protein isoform b is indicated in blue. (B) Patient (proband), parental, and grandparental genomic DNA were amplified by targeted PCR. Genomic DNA from the patient, parent 2, and grandparent 2 carried the mutation found in chromosome 12. A 1 kbp ladder was used in lanes 1 and 8. The expected product sizes were 1,457 bp for Assay 1 and 1,435 bp for Assay 2. Parent 1's genomic DNA was used for the negative control which lacked DNA polymerase.
To search for MATR3 transcripts in blood, total RNA was isolated using the PAXgene Blood RNA Kit for in vitro diagnostics (Qiagen, University of Minnesota Genomics Core). RNA integrity numbers were 7.4, 7.7, and 8.4 for the patient, parent, and grandparent, respectively, indicating minimally-degraded RNA. Using RT-PCR SuperScript III Reverse Transcriptase (Thermo-Fisher), a cDNA template was created for the proband, parents, and grandparents. First-strand synthesis was done using a primer near the 3′ end of MATR3V5 sequence (CCAAATGAAAGTCTGCAAGGCTCA). For PCR, primers were designed to detect MATR3 variants 1-5. However, no primer pairs produced detectable cDNA.
We present a case of an aggressive form of early-onset FTD. The patient exhibited many of the core features of bvFTD, including behavioral changes, verbal aggression, lack of empathy, stereotyped behaviors, and social withdrawal ( 15 ). Brain imaging showed significant atrophy in the frontal and anterior temporal lobes plus heads of the caudate, as well as hypometabolism maximal in bilateral frontal and temporal lobes and basal ganglia, consistent with bvFTD. This case presents a novel genetic mutation that may be linked with early-onset bvFTD. In the patient, one parent, and one grandparent, we discovered an insertion of MATR3V5 cDNA with flanking 15 bp repeats. However, second- and third-degree relatives were asymptomatic. The clinical symptoms observed in this case do not align with existing mutations in MATR3 associated with early-onset bvFTD, most of which are point mutations ( 16 ). This case is unique because of the patient's atypical clinical presentation as early-onset bvFTD associated with a mechanism of mutation that has not been linked to bvFTD.
The insertion into chromosome 12 of the MATR3V5 transcript likely occurred through retrotransposition. MATR3V5 is a rare splice variant of the MATR3 gene native to chromosome 5 ( 17 ). Some point mutations in MATR3 —S85C, F115C, and T622A—are associated with FTD-ALS spectrum pathology as well as distal myopathy and pharyngeal weakness ( 17 , 18 ), but retrotransposition of MATR3V5 has, to the best of our knowledge, never been associated with bvFTD. Since MATR3 mutations have previously been linked to FTD-ALS spectrum, it is possible that the novel MATR3 retrotransposition found in this case is causally linked to the observed neurodegeneration.
Often, retrotransposition is benign and associated with evolutionary genetic expansion and gene regulation ( 19 ). However, disruptive retrotransposition has been linked to neurological diseases such as Aicardi-Goutières syndrome, multiple sclerosis, ALS, and Alzheimer's disease ( 20 , 21 ). For example, increased levels of TDP-43, the most common pathological biomarker in FTD-ALS spectrum, leads to loss of post-transcriptional gene silencing responsible for retrotransposable element (RTE) repression ( 21 , 22 ). Increased activity of RTEs in TDP-43 mediated diseases leads to disruptive retrotransposition, indicated by DNA damage/DNA nicking, and subsequently results in apoptosis ( 21 ). Although disruptive retrotransposition has been observed in FTD-ALS, the MATR3 retrotransposition in the proband's case is unlikely to be a consequence of neurodegeneration since it is present in healthy carriers.
In most cases of FTD-ALS, TDP-43 is the primary component of ubiquitinated-positive cytoplasmic inclusions where the C-terminal fragments of TDP-43 are ubiquitinated and phosphorylated ( 5 , 21 , 22 ). In HEK293-FT cells, endogenous TDP-43 has been shown to co-immunoprecipitate with MATR3 protein ( 16 ). Further, the S85C mutant of MATR3 showed increased binding to TDP-43 compared to wild-type MATR3, but this effect was not seen in F115C and T622A mutants ( 16 ). Investigation of TDP-43's interaction with MATR3 may shed light on the mechanisms of FTD-ALS pathogenesis, though it remains unclear how retrotransposition of MATR3V5 into CH12, and potential aberrant expression of MATR3V5 , impacts cellular function.
In a small number of FTD-ALS cases, FUS, another DNA/RNA binding protein, is the primary pathological protein involved ( 23 ). A missense mutation in the FUS gene has recently been identified as a cause of familial ALS. A mutation in FUS has also been linked to tau-negative and TDP-43-negative FTLD subtypes ( 13 ). In addition, mutations in FUS have been associated with MATR3 and TDP-43 cytoplasmic aggregation ( 24 , 25 ). Complex interactions amongst proteins implicated in FTD-ALS indicate numerous pathways by which MATR3 dysfunction may cause cognitive deficits. Further study of MATR3's interactome is crucial to understanding its mechanism in FTD.
MATR3 modulation emulates aspects of FTD-ALS pathology in vitro . Elevated levels of wild-type MATR3 and MATR3 mutants (S85C, F115C, P154S, and T622A) elicit a dose-dependent toxicity in primary rat mixed cortical cultures. Similarly, siRNA knockdown of endogenous MATR3 also results in greater cell death compared to non-targeting siRNA. These results illustrate that both increased and decreased neuronal MATR3 leads to increased cell death, exemplifying that the mechanism of MATR3 in FTD-ALS pathology may be a gain or loss of function, or both ( 11 ).
It is unclear whether the MATR3V5 retrotransposition on CH12 encodes an mRNA or produces a protein. MATR3 expression in the blood is low, and attempts to isolate mRNA or protein from blood and serum samples have been largely unsuccessful. Transcription of MATR3V5 from CH12 could conceivably increase or decrease MATR3 protein levels by subsequent translation or RNA-silencing, respectively. MATR3V5 mRNA from CH5 codes for the MATR3 isoform b protein. Isoform a, translated from variants 1-4, is 847 amino acids in length while, isoform b translated from variant 5, is 559 amino acids and shorter on the N terminus. Both isoforms are DNA/RNA binding proteins and carry the same main domains ( 11 , 26 ). Determination of the functional outcome of CH12- MATR3V5 transcription requires protein-level analysis.
In future studies, it would be informative to culture forebrain neurons with this mutation using patient derived induced pluripotent stem cells (iPSCs), as this provides a more direct means to study differential localization, altered transcription and translation levels, and mechanisms of MATR3. Notably, MATR3V5 is located 20 kbp downstream of the LHX5 gene on CH12. LHX5 encodes a protein family that function as transcriptional regulators of neuronal differentiation, migration, and development of the forebrain. Because of its chromosomal proximity to a gene active in the cortex and hippocampus, it will be worth studying how the MATR3V5 insertion may affect—or be impacted by—the LHX5 locus. In addition, MATR3 cellular localization can be studied using iPSCs. MATR3 localization has been shown to play a role in neurotoxicity. Cytoplasmic MATR3 redistribution from the nucleus extends survival in neurons that overexpress MATR3, suggesting that nuclear MATR3 mediates neurotoxicity ( 11 ). Epigenetic differences between the patient and the unaffected carriers could also result in differences in expression of the MATR3 retrotransposed gene. Collectively, these analyses will allow a thorough investigation of the MATR3 protein and transcript and its role in the pathology of early-onset FTD.
This case study highlights a novel gene retrotransposition of MATR3V5 associated with a rare case of early-onset bvFTD. The results of this case study illustrate that point mutations may not be the only genetic mechanism by which MATR3 contributes to FTD. These findings also add to our knowledge of potentially active pseudogenes contributing to dementia pathologies. Future studies will investigate the significance of this gene retrotransposition, potential role of MATR3V5 in disease, and its association with the early-onset bvFTD pathology presented in this case. Moreover, a thorough understanding of this novel MATR3 mutation may help elucidate mechanisms of matrin-3 dysregulation in other neurodegenerative contexts.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the University of Minnesota's institutional review board. All procedures performed during this study involving human participants were in accordance with the ethical standards of the institutional review board at the University of Minnesota and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Surrogate consent was received for the participant. Informed consent was obtained from all other individuals able to independently consent. Written informed consent was obtained from the participant's authorized representative for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MC and KV obtained IRB approval. MC, NV, and KV drafted the manuscript. KV and BB examined the patient. DD, MB, and MK completed the genetic analysis. NV completed the PCR assays. SP provided guidance and technical support for PCR assays. All authors reviewed and revised the final manuscript.
This study was supported by funds from the N. Bud Grossman Center for Memory Research and Care and the Institute for Translational Neuroscience at the University of Minnesota.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our sincere thanks to the patient and her family members. We thank the University of Minnesota Genomics Center for whole genome sequencing. We thank Kasey Ah Pook for his contribution in obtaining IRB approval and editing the manuscript.
1. Merrilees J, Klapper J, Murphy J, Lomen-Hoerth C, Miller BL. Cognitive and behavioral challenges in caring for patients with frontotemporal dementia and amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2010) 11:298–302. doi: 10.3109/17482961003605788
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. (2013) 25:130–7. doi: 10.3109/09540261.2013.776523
3. Lanata SC, Miller BL. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry. (2016) 87:501–11. doi: 10.1136/jnnp-2015-310697
4. Goldman JS. Genetic testing and counseling in the diagnosis and management of young-onset dementias. Psychiatr Clin North Am. (2015) 38:295–308. doi: 10.1016/j.psc.2015.01.008
5. Neumann M, Tolnay M, Mackenzie IR. The molecular basis of frontotemporal dementia. Expert Rev Mol Med. (2009) 11:e23. doi: 10.1017/S1462399409001136
6. Liu MN, Lau CI, Lin CP. Precision medicine for frontotemporal dementia. Front Psychiatry. (2019) 10:75. doi: 10.3389/fpsyt.2019.00075
7. Goldman JS, Farmer JM, Van Deerlin VM, Wilhelmsen KC, Miller BL, Grossman M. Frontotemporal dementia: genetics and genetic counseling dilemmas. Neurologist. (2004) 10:227–34. doi: 10.1097/01.nrl.0000138735.48533.26
8. Goldman JS, Van Deerlin VM. Alzheimer's disease and frontotemporal dementia: the current state of genetics and genetic testing since the advent of next-generation sequencing. Mol Diagn Ther. (2018) 22:505–13. doi: 10.1007/s40291-018-0347-7
9. Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. (2011) 82:476–86. doi: 10.1136/jnnp.2010.212225
10. Ramos EM, Dokuru DR, Van Berlo V, Wojta K, Wang Q, Huang AY, et al. Genetic screening of a large series of North American sporadic and familial frontotemporal dementia cases. Alzheimers Dement. (2020) 16:118–30. doi: 10.1002/alz.12011
CrossRef Full Text | Google Scholar
11. Malik AM, Miguez RA, Li X, Ho YS, Feldman EL, Barmada SJ. Matrin 3-dependent neurotoxicity is modified by nucleic acid binding and nucleocytoplasmic localization. Elife. (2018) 7:e35977. doi: 10.7554/eLife.35977
12. Boehringer A, Garcia-Mansfield K, Singh G, Bakkar N, Pirrotte P, Bowser R. ALS associated mutations in matrin 3 alter protein-protein interactions and impede mrna nuclear export. Sci Rep. (2017) 7:14529. doi: 10.1038/s41598-017-14924-6
13. Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet. Neurol. (2010) 9:995–1007. doi: 10.1016/S1474-4422(10)70195-2
14. Bahia VS, Takada LT, Deramecourt V. Neuropathology of frontotemporal lobar degeneration: a review. Dement Neuropsychol. (2013) 7:19–26. doi: 10.1590/S1980-57642013DN70100004
15. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
16. Johnson JO, Pioro EP, Boehringer A, Chia R, Feit H, Renton AE, et al. Mutations in the matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat Neurosci. (2014) 17:664–6. doi: 10.1038/nn.3688
17. Senderek J, Garvey SM, Krieger M, Guergueltcheva V, Urtizberea A, Roos A, et al. Autosomal-dominant distal myopathy associated with a recurrent missense mutation in the gene encoding the nuclear matrix protein, matrin 3. Am J Hum Genet. (2009) 84:511–8. doi: 10.1016/j.ajhg.2009.03.006
18. Gallego-Iradi MC, Clare AM, Brown HH, Janus C, Lewis J, Borchelt DR. Subcellular localization of matrin 3 containing mutations associated with als and distal myopathy. PLoS ONE. (2015) 10:e0142144. doi: 10.1371/journal.pone.0142144
19. Janousek V, Laukaitis CM, Yanchukov A, Karn RC. The role of retrotransposons in gene family expansions in the human and mouse genomes. Genome Biol Evol. (2016) 8:2632–50. doi: 10.1093/gbe/evw192
20. Tam OH, Ostrow LW, Gale Hammell M. Diseases of the nERVous system: retrotransposon activity in neurodegenerative disease. Mob DNA. (2019) 10:32. doi: 10.1186/s13100-019-0176-1
21. Krug L, Chatterjee N, Borges-Monroy R, Hearn S, Liao WW, Morrill K, et al. Retrotransposon activation contributes to neurodegeneration in a drosophila TDP-43 model of ALS. PLoS Genet. (2017) 13:e1006635. doi: 10.1371/journal.pgen.1006635
22. Majumder V, Gregory JM, Barria MA, Green A, Pal S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. (2018) 18:90. doi: 10.1186/s12883-018-1091-7
23. Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. (2013) 79:416–38. doi: 10.1016/j.neuron.2013.07.033
24. Yamaguchi A, Takanashi K. FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription. Sci Rep. (2016) 6:35195. doi: 10.1038/srep35195
25. Kamelgarn M, Chen J, Kuang L, Arenas A, Zhai J, Zhu H, et al. Proteomic analysis of FUS interacting proteins provides insights into FUS function and its role in ALS. Biochim Biophys Acta. (2016) 1862:2004–14. doi: 10.1016/j.bbadis.2016.07.015
26. Belgrader P, Dey R, Berezney R. Molecular cloning of matrin 3. A 125-kilodalton protein of the nuclear matrix contains an extensive acidic domain. J Biol Chem. (1991) 266:9893–9.
PubMed Abstract | Google Scholar
Keywords: frontotemporal dementia, Matrin 3, case report, retrotransposons, whole genome sequencing
Citation: Castro M, Venkateswaran N, Peters ST, Deyle DR, Bower M, Koob MD, Boeve BF and Vossel K (2020) Case Report: Early-Onset Behavioral Variant Frontotemporal Dementia in Patient With Retrotransposed Full-Length Transcript of Matrin-3 Variant 5. Front. Neurol. 11:600468. doi: 10.3389/fneur.2020.600468
Received: 30 August 2020; Accepted: 19 November 2020; Published: 21 December 2020.
Reviewed by:
Copyright © 2020 Castro, Venkateswaran, Peters, Deyle, Bower, Koob, Boeve and Vossel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keith Vossel, kvossel@mednet.ucla.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 12 April 2024
Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis
- Zsolt Huszár 1 , 2 ,
- Marie Anne Engh 1 ,
- Márk Pavlekovics 1 , 3 ,
- Tomoya Sato 1 ,
- Yalea Steenkamp 1 ,
- Bernard Hanseeuw 4 , 5 ,
- Tamás Terebessy 1 ,
- Zsolt Molnár 1 , 6 , 7 ,
- Péter Hegyi 1 , 8 , 9 , 10 &
- Gábor Csukly 1 , 2
Alzheimer's Research & Therapy volume 16 , Article number: 81 ( 2024 ) Cite this article
587 Accesses
6 Altmetric
Metrics details
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the presence of these protein changes (A+ and T+) remains unclear. By performing a systematic review and meta-analysis, we investigated the risk of mild cognitive impairment (MCI) or dementia in the non-demented population with A+ and A- alone and in combination with T+ and T- as confirmed by PET or cerebrospinal fluid examination.
A systematic search of prospective and retrospective studies investigating the association of Aβ and p-tau with cognitive decline was performed in three databases (MEDLINE via PubMed, EMBASE, and CENTRAL) on January 9, 2024. The risk of bias was assessed using the Cochrane QUIPS tool. Odds ratios (OR) and Hazard Ratios (HR) were pooled using a random-effects model. The effect of neurodegeneration was not studied due to its non-specific nature.
A total of 18,162 records were found, and at the end of the selection process, data from 36 cohorts were pooled ( n = 7,793). Compared to the unexposed group, the odds ratio (OR) for conversion to dementia in A+ MCI patients was 5.18 [95% CI 3.93; 6.81]. In A+ CU subjects, the OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64]. Cerebrospinal fluid Aβ42 or Aβ42/40 analysis and amyloid PET imaging showed consistent results. The OR for conversion in A+T+ MCI subjects (11.60 [95% CI 7.96; 16.91]) was significantly higher than in A+T- subjects (2.73 [95% CI 1.65; 4.52]). The OR for A-T+ MCI subjects was non-significant (1.47 [95% CI 0.55; 3.92]). CU subjects with A+T+ status had a significantly higher OR for conversion (13.46 [95% CI 3.69; 49.11]) than A+T- subjects (2.04 [95% CI 0.70; 5.97]). Meta-regression showed that the ORs for Aβ exposure decreased with age in MCI. (beta = -0.04 [95% CI -0.03 to -0.083]).
Conclusions
Identifying Aβ-positive individuals, irrespective of the measurement technique employed (CSF or PET), enables the detection of the most at-risk population before disease onset, or at least at a mild stage. The inclusion of tau status in addition to Aβ, especially in A+T+ cases, further refines the risk assessment. Notably, the higher odds ratio associated with Aβ decreases with age.
Trial registration
The study was registered in PROSPERO (ID: CRD42021288100).
Affecting 55 million people worldwide, dementia is one of the leading causes of years spent with disability and one of the costliest long-term illnesses in society. The most common cause of dementia is Alzheimer's disease (AD), responsible for 60-80% of cases [ 1 , 2 ].
Two specific protein aggregates play a crucial role in the pathophysiology of AD. One is the amyloid plaque formation in the extracellular space, predominantly by Aβ aggregation. These plaques, among other pathological effects, inhibit the signaling function of neurons [ 3 ]. The other protein change is the appearance of neurofibrillary tangles within the neurons, which are formed by the phosphorylation of tau proteins (p-tau) and inhibit the axonal transport inside the cell [ 4 ]. Whereas the specific pathology could only be confirmed by autopsy in the past, in vivo tests are available today. Parallelly to this development, the diagnostic definitions of AD have evolved significantly over time, moving from purely clinical assessments and post-mortem examinations to the integration of in vivo amyloid and later p-tau biomarkers, emphasizing the role of preclinical stages [ 5 , 6 , 7 , 8 ]. Accordingly, researchers are increasingly trying to link the diagnosis of the disease to biological parameters. However, in general, the clinical practice only considers the quality of the symptoms of dementia and the fact of neurodegeneration confirmed by radiology when establishing an AD diagnosis.
The International Working Group (IWG) [ 5 ] emphasizes that diagnosis should align with clinical symptoms. However, for researchers in the field, the U.S. National Institute on Aging – Alzheimer’s Association (NIA-AA) has issued a new framework recommendation [ 6 ]. This recommendation defines AD purely in terms of specific biological changes based on the Aβ (A) and p-tau (T) protein status, while neurodegeneration (N) is considered a non-specific marker that can be used for staging. In the recommendation, the category ‘Alzheimer’s disease continuum’ is proposed for all A+ cases, ‘Alzheimer’s pathological changes’ for A+T- cases, and ‘Alzheimer’s disease’ for A+T+ cases. A-(TN)+ cases are classified as ‘non-Alzheimer pathological changes’.
Aβ and p-tau proteins have long been known to be associated with AD development, and their accumulation can begin up to 15-20 years before the onset of cognitive symptoms [ 9 ]. Pathological amyloid changes are highly prevalent in dementia: 88% of those clinically diagnosed with AD and between 12 and 51% of those with non-AD are A+, according to a meta-analysis [ 10 ]. At the same time, the specificity of the abnormal beta-amyloid level for AD and its central role in its pathomechanism have been questioned [ 11 ]. Their use as a preventive screening target is a subject of ongoing discourse [ 12 ]. Yet it is still unclear to what extent their presence accelerates cognitive decline. What are the predictive prospects for an individual with abnormal protein levels who is otherwise cognitively healthy or with only mild cognitive impairment (MCI), meaning cases where there is a detectable decline in cognitive ability with maintained ability to perform most activities of daily living independently? [ 13 ] Research on non-demented populations shows substantial variation; for example, studies have shown OR values for conversion to dementia ranging from 2.25 [95% CI 0.71; 7.09] [ 14 ] to 137.5 [95% CI 17.8; 1059.6] [ 15 ]. Comparing conversion data systematically is necessary to provide a clearer picture.
In the CU population over 50 years, the prevalence of being A+ ranges from 10 to 44%, while in MCI it ranges from 27 to 71%, depending on age. Taking this into consideration [ 16 ], we aim to investigate the effect of Aβ alone and in combination with p-tau on the conversion to MCI and dementia, through a systematic review and meta-analysis of the available literature. Knowing the prognostic effect can highlight the clinical potential of this current research framework, given that, at present, the therapy of MCI or dementia can only slow down the decline. Prevention starting at an early stage or even before symptoms appear, provides the best chance against the disease.
Study registration
Our study was registered in the PROSPERO database (ID: CRD42021288100), with a pre-defined research plan and detailed objectives, is reported strictly in accordance with the recommendation of the PRISMA 2020 guideline and was performed following the guidance of the Cochrane Handbook [ 17 ].
We aimed to determine the change in odds of progression to MCI or dementia among non-demented subjects based on abnormal Aβ levels alone, or in combination with abnormal p-tau levels.
Search and selection
We included longitudinal prospective and retrospective studies that used the NIA-AA 2018 recommended measurement of Aβ and p-tau (for Aβ: amyloid PET, CSF Aβ42, or Aβ42/40 ratio; for p-tau: tau PET, or CSF p-tau) and investigated the role of Aβ and +/- p-tau in CU and MCI subjects in progression to MCI or dementia. Case reports and case series were excluded. Overlapping populations were taken into account during the data extraction. Our search key was run in the Medline, Embase, and Central databases on 31 October 2021, and the search was updated on 9 January 2024 (see Supplementary Material, Appendix 1 ). After removing duplicates, we screened publications by title and abstract, and in the second round by full text. Two independent reviewers conducted the selection (ZH, MP), and a third reviewer (GC) resolved disagreements. The degree of the agreement was quantified using Cohen’s kappa statistics at each selection stage.
As part of the selection process, articles that only examined the ADNI database [ 18 ] were excluded, as patient-level data were used instead (see Supplementary Material Appendix 2 for details of the patient-level data analysis of the ADNI).
A standardized Excel (Microsoft Corporation, Redmond, Washington, USA) document sheet was used for data extraction (for one special case of data extraction see Supplementary Material Appendix 3 ). Where data were available in graphical form only, we used an online software (Plot Digitizer) [ 19 , 20 ]. The following data were extracted: source of data used in the studies (place of clinical trial or name of database), baseline characteristics of the population (age, gender, APOE status, and education level), type of exposure (Aβ, p-tau, and neurodegeneration), measurement technique of the exposure, data on cognitive impairment separately for the different exposure groups).
Data synthesis
Generally, where several studies used the same population sample or cohort, only data from the study with the largest sample size were used. Conversion to Alzheimer’s dementia and to unspecified dementia was assessed together, as the definition of Alzheimer’s dementia varied between the studies, and the diagnosis was based on neurocognitive tests. If conversion to both types of dementia was given, the value of the conversion to unspecified dementia was used. The population with subjective cognitive symptoms was scored jointly with the CU population, as these subpopulations could not be differentiated objectively.
Odds ratio and hazard ratio values were used or calculated based on the available information (for details on the methodology, see Supplementary Material Appendix 4 ). Considering that studies report their results on different age groups, a meta-regression analysis was performed to investigate how age affects the likelihood of developing dementia based on Aβ levels.
Studies applied different analysis methods to identify Aβ positivity. Where multiple amyloid categories were being considered, the preferred method was amyloid PET. When relying on CSF analysis, the Aβ42/40 ratio was given precedence over Aβ42 since the 42/40 ratio has a higher concordance with amyloid PET [ 21 ]. To estimate the confounding effect caused by different amyloid measurement techniques a subgroup analysis was performed. For the assessment of p-tau, studies measured p-tau181 levels from CSF samples, or employed tau PET. While there is also a limited number of tau PET measurements in the ADNI, in order to ensure consistency in the analyses, we used exclusively the CSF p-tau181 levels from the ADNI database.
For the OR analysis, studies with varying follow-up times were pooled. To estimate the resulting bias, a meta-regression analysis was performed to explore how follow-up time affected the results.
Statistical analysis
Statistical analyses were performed in the R programming environment (version 4.1.2) using the “meta” software package version 5.2-0. To visualize synthesized data, we used forest plots showing ORs or HRs and corresponding confidence intervals for each individual study and pooled effect sizes in terms of ORs and HRs. For dichotomous outcomes, odds ratios and hazard ratios with 95% confidence intervals (CI) were used as effect measures. To calculate odds ratios, the total number of patients in each study and the number of patients with the event of interest in each group were extracted from each study. Raw data from the selected studies were pooled using a random-effects model with the Mantel-Haenszel method [ 22 , 23 , 24 ]. The random-effects model was used as we assumed that the true effect would vary between studies due to differences in demographics and clinical measures, such as age or baseline cognitive impairment.
Heterogeneity was assessed by calculating I 2 , tau 2 , and the prediction interval. I 2 is defined as the percentage of variability in the effect size that is not caused by sampling error, whereas tau 2 is the square root of the standard deviation of the true effect size. As I 2 is heavily dependent on the precision of the studies and tau 2 is sometimes hard to interpret (as it is insensitive to the number of the studies and their precision), the prediction interval has also been calculated. The great advantage of the prediction interval is that this measure is easy to interpret: if the interval does not include zero, further studies are expected to show a similar result.
Sensitivity analysis
We performed outlier detection according to Viechtbauer et al. [ 25 ]. A study is considered an outlier if the confidence interval of the study does not overlap with the confidence interval of the pooled effect. The idea behind is to detect effect sizes that differ significantly from the overall effect. As a sensitivity analysis, we repeated the analyses after removing any outliers and then we compared the pooled effects before and after the exclusion, in order to detect if outliers would have a substiantial impact on the overall effect.
Risk of bias assement
The risk of bias was assessed according to the recommendation of the Cochrane Collaboration; using the QUIPS tool [ 26 ], two investigators (ZH and YS) independently assessed the quality of the studies, and a third author solved disagreements. Publication bias was examined using the Peter’s regression test [ 27 ] and visual inspection of the adjusted Funnel-plots.
Search results
During the systematic search (Fig. 1 ), 18,162 records were found, and finally, 46 eligible articles were obtained (Supplementary Material eTable 1 ); While some of the articles analyzed the same cohorts, we were able to pool data from 36 different cohorts or centres. The Cohens’s kappa was 0.91 for the title and abstract, and 0.86 for the full-text selection. Given the amount of data found, we decided to examine the targeted outcomes separately and focus only on the conversion data in this report.
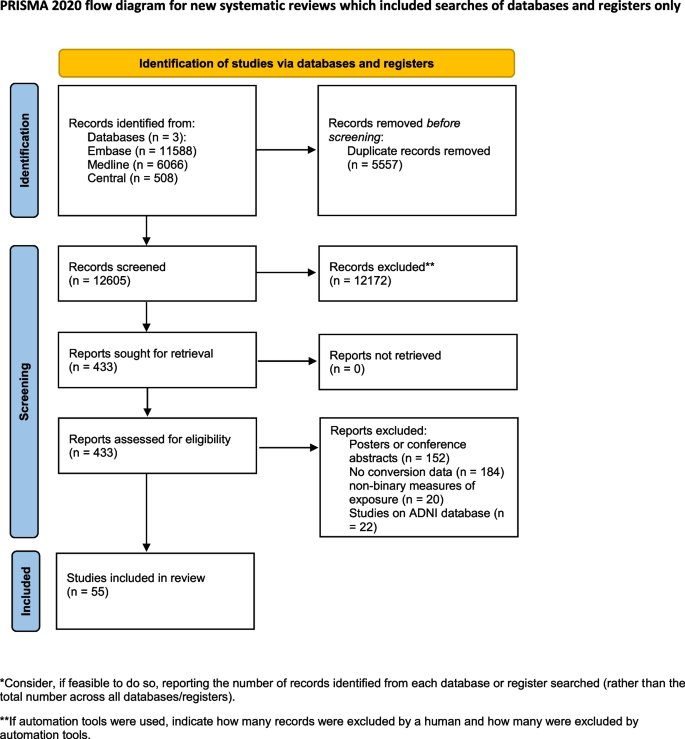
PRISMA flowchart of selection. Flowchart of the study screening process following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statement
The investigated studies expressed their results in different ways. They calculated unadjusted or adjusted hazard ratios or presented the number of conversions for the different follow-up periods. In the latter case, we calculated odds ratios for the defined time periods. The measured exposures also differed: data were given only for Aβ or in combination with p-tau or neurodegeneration. There were also differences in the techniques used to measure exposure, with CSF sample being used in some cases and PET scan in others.
During data extraction, one [ 28 ] article was excluded because of inconsistently reported conversion data, and four [ 15 , 29 , 30 , 31 ] were excluded from the A/T analysis because the definition of the pathologic Aβ and p-tau was based on Aβ/p-tau ratio, which did not comply with the NIA-AA 2018 recommendation.
The eligible studies investigated three groups: CU, MCI, and mixed - in which the results were collectively expressed for both the MCI and CU groups. The CU group comprised either cognitively healthy subjects or individuals with only subjective cognitive complaints. To define the MCI group, all studies followed the Petersen criteria [ 32 ]. Four studies examined mixed groups. Since all of them studied large samples ( n >180), it was considered more valuable to jointly analyze them with MCI, since the outcome was also the conversion to dementia. As a result of the joint analysis, our findings are based on a substantially larger sample. To support this decision, we performed a subgroup analysis comparing the Aβ positive MCI and mixed population studies. The OR differed significantly from the unexposed group in both the MCI (OR 5.83 [3.80; 8.93]) and the mixed (4.64 [95% CI 1.16; 18.61]) subgroups, and there was no significant difference between the two subgroups ( p =0.55) (Supplementary Material eFigure 1 ).
Conversion from MCI to dementia
Aβ exposition - in or.
Based on a mixed model meta-analysis of 3,576 subjects (Table 1 ), we observed a significant association between Aβ positivity and higher conversion rates. Compared to the unexposed, the OR for conversion to dementia in the amyloid positives were 5.18 [95% CI 3.93; 6.81]; t(21)=12.47; ( p <0.0001). The I 2 - test for heterogeneity revealed that 44.8% of the variance across studies was due to heterogeneity (Fig. 2 A). As a result of the outlier detection we excluded the Balassa study and found a very similar overall effect and a reduced heterogeneity (5.05 [95% CI 3.98; 6.40]; t(20) = 14.2; p < 0.0001; I 2 = 31.4%). Meta-regression analysis of mean age showed a statistically significant decrease in OR values with increasing age (R 2 = 59.05%, beta = -0.04, SE = 0.019, [95% CI = -0.03 to -0.083], df = 18, t = -2.27, p = 0.036) (Fig. 2 B). The Hartunk-Knapp method was applied to adjust test statistics and confidence intervals to reduce the risk of false positives.
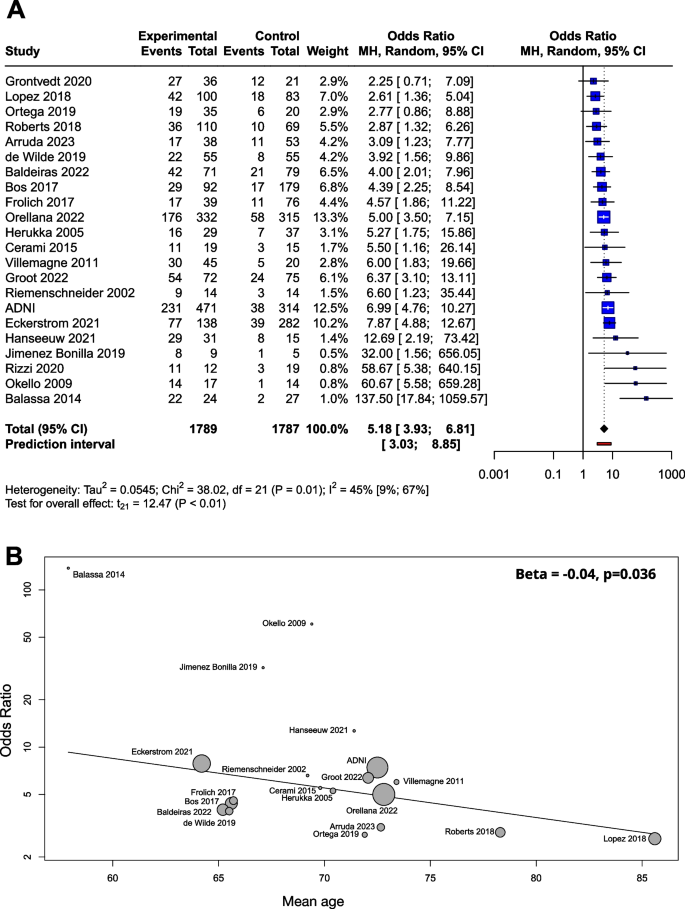
Conversion of Aβ exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A OR for Aβ exposition; B meta-regression of age and ORs for conversion regarding Aβ exposure. The size of the circle is proportional to the weight of each study in the meta-analysis. The line corresponds to meta-regression with age as covariate, and beta represents the slope of ORs by mean age
Beta-amyloid was determined by CSF Aβ42, CSF Aβ42/40 ratio or amyloid PET. When the three groups were compared in a subgroup analysis, the OR was 5.87 (2.83; 12.19) for CSF Aβ42, 5.00 (3.31; 7.55) for CSF Aβ42/40 ratio, and 5.32 (2.53; 11.18) for amyloid PET. The difference between the subgroups was not significant ( p =0.88) (Supplementary Material eFigure 2 ).
The meta-regression analysis performed to examine the role of follow-up time showed no association with respect to the ORs (R 2 = 0%, beta = -0.002, SE = 0.07, [95% CI = -0.02 - 0.01], df = 11, p = 0.77) (Supplementary Material eFigure 3 A).
We used a funnel plot to examine publication bias (Supplementary Material eFigure 4 A). Most of the studies with large sample sizes lie close to the midline, which confirms that the pooled effect size seems valid. However, the visual inspection of the plot raised the possibility of some publication bias in two ways: (1) Studies in the bottom right corner of the plot have significant results despite having large standard errors (2) The absence of studies in the bottom left corner (blank area in the figure) may indicate that studies with nonsignificant results were not published. In order to quantify funnel plot asymmetry, the Peter’s regression test was applied. The test results were not significant ( t = 1.7, df = 20, p = 0.11) so no asymmetry was proven in the funnel plot.
The effect of Aβ exposition in terms of HR
Several studies reported their results in HRs instead of or in addition to ORs (Supplementary Material eTable 2 ). The advantage of the HR value is that this measure is independent of the length of follow-up times of the studies. For these reasons, we also considered it important to analyze the results expressed in HR. Based on pooled data of patients studied ( n =1,888), the HR for conversion to dementia was 3.16 [95% CI 2.07; 4.83], p < 0.001 (Fig. 3 A).
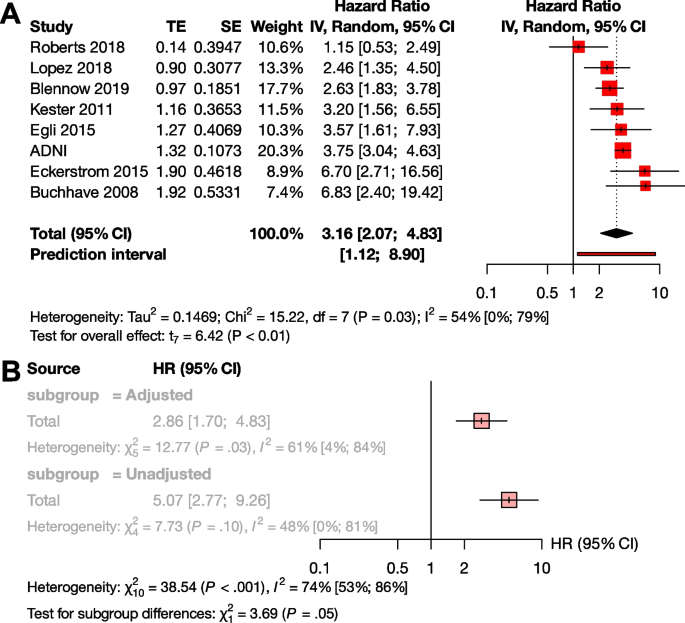
Conversion of Aβ exposed MCI groups to dementia in HR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A HR for Aβ exposition; B sub-group analysis of studies with adjusted and unadjusted HR values
To investigate the effect of adjustment, we conducted a subgroup analysis between the unadjusted and adjusted measurements. Although there was a trend for higher unadjusted HR values compared to the adjusted HRs, the difference did not reach statistical significance (unadjusted HR : 5.07 [95% CI 2.77 - 9.26], adjusted HR 2.86 [95% CI 1.70 - 4.83] p =0.055) (Fig. 3 B). We could not analyze HR in the A+T-, A+T+, and A-T+ subgroups, due to the low number of available studies.
The effect of Aβ and p-tau exposition in terms of OR
We examined the combined effect of p-tau and Aβ (Table 2 ), and compared A+T+, A+T-, and A-T+ exposures to A-T-. Based on pooled data for patients studied (n=1,327), the OR for conversion to dementia in A+T- was 2.73 [95% CI 1.65; 4.52], and the odds ratio was significantly higher in the presence of both exposures (A+T+) ( p <0.001), with an OR of 11.60 [95% CI 7.96; 16.91]. The effect of A-T+ exposure on conversion was not significant (OR: 1.47 [0.55; 3.92]) (Fig. 4 A).
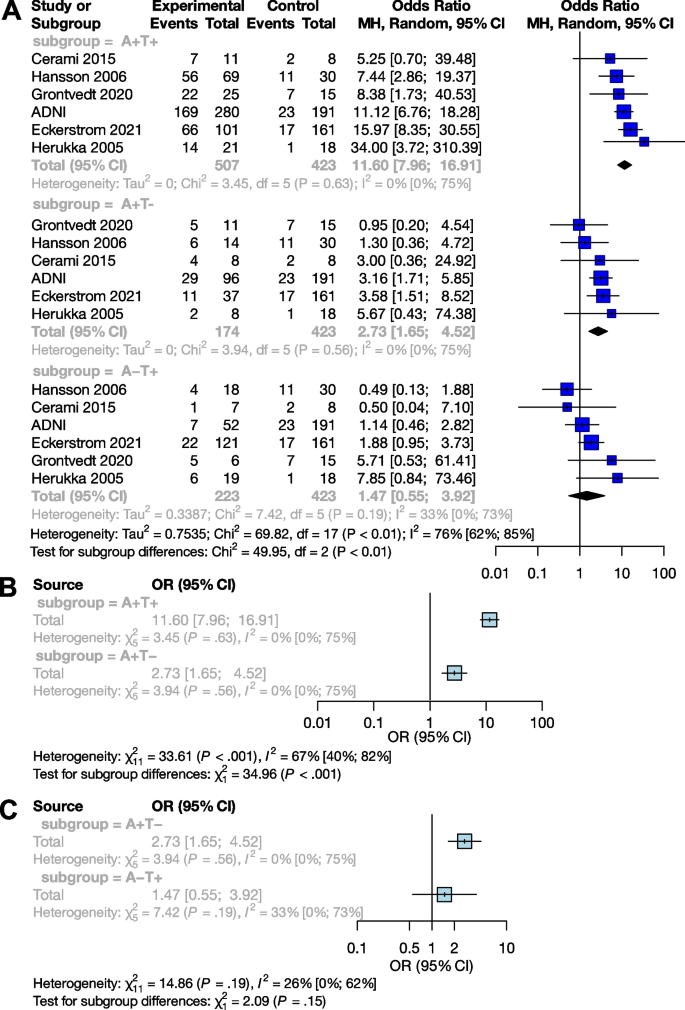
Conversion of Aβ and p-tau exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ and p-tau expositions in OR; B sub-group analysis of comparisons between the A+T+ and A+T- groups; C sub-group analysis of comparisons between the A+T- and A-T+ groups
Subgroup analyses showed that the A+T+ group had a significantly higher odds of conversion compared to the A+T- group ( p <0.001), while the A+T- and A-T+ groups did not differ significantly ( p =0.15) (Fig. 4 B and C).
Conversion from CU to MCI or dementia
The effect of aβ exposition in terms of or.
Analyses on the CU population ( n = 4,217) yielded very similar results to the MCI sample. The OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64] (t(13) = 5.43; p = 0.0001), the results of the studies did however show a high degree of heterogeneity (I 2 = 73% [55%; 84%]) (Table 3 , Fig. 5 A). As a result of the outlier detection we removed the Aruda study and found a very similar overall effect (6.33 [95% CI 3.42; 11.71]; t(12) = 6.54; p < 0.0001; I 2 = 72.1%).
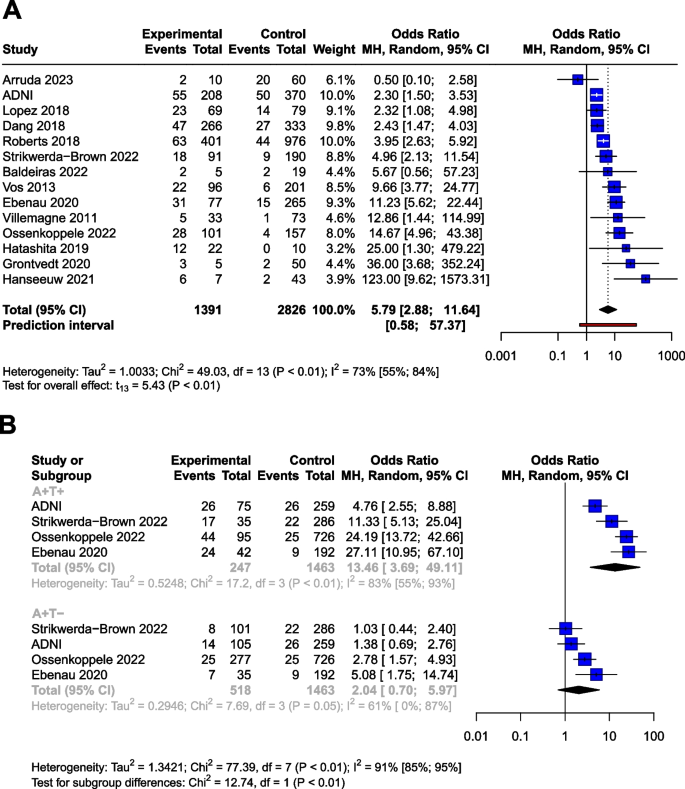
Conversion of Aβ and p-tau exposed CU groups to MCI or dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares' area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ exposition in OR. B Aβ and p-tau expositions in OR
Meta-regression analysis of mean age did not show a significant association with OR. (R 2 = 8.22%, beta = -0.05, SE = 0.05, [95% CI = -0.17 – 0.7], df = 11, t =, p = 0.37).
Meta-regression analysis also showed no association between follow-up time and ORs (R 2 = 0.35%, beta = -0.014, SE = 0.024, [95% CI = -0.07 - 0.04], df = 8, p = 0.58) (Supplementary Material eFigure 3 B).
We applied a funnel plot to examine publication bias (Supplementary Material eFigure 4 B).Most of the studies with large sample sizes lie close to the midline, which reaffirms the pooled effect size’s validity. In order to quantify funnel plot asymmetry, Peter’s regression test was applied. The test results were not significant ( t = 0.9, df = 12, p = 0.31) indicating that no asymmetry was demonstrated in the funnel plot.
Four cohorts provided HRs for the CU population ( n =2700) with one cohort (ADNI) representing the 55.3% of the total sample (weight: 78.5%) (Supplementary Material eTable 3 ). The pooled HR for conversion was 2.33 [95% CI 1.88; 2.88] ( p =0.001) (Supplementary Material eFigure 5 )
The combined effect of Aβ and p-tau exposition in terms of OR
Using data from a total of 2228 subjects, we investigated the effect of p-tau in combination with Aβ (Table 4 ) in the CU population. The OR for conversion is 2.04 [95% CI 0.70; 5.97] for A+T-, and 13.46 [95% CI 3.69; 49.11] for the A+T+, compared to the A-T- group The OR shows a trend level increased risk (t=2.1, P =0.12) for the A+T- group compared to the A-T- group.
Similarly to the MCI population, subgroup analyses showed that the A+T+ group had significantly higher OR for conversion compared to the A+T- group ( p <0.01). The analysis could not be performed for A-T+ due to the low number of these cases.
Risk of bias assessment
The risk of bias was assessed separately for the analyses discussed above. The overall risk of the studies ranged from low to moderate, except in three cases: twice we found a high risk of bias due to attrition of above 50% [ 59 , 60 ], and once due to a focus on monozygotic twins [ 61 ] (Supplementary Material, eFigure 6 ). These articles ( n =197) were excluded from all analyses.
Summary and context
A pathological Aβ state are strongly correlated with the risk of clinical progression. The odds ratio for conversion is 5.18 in the MCI population and 5.79 in the CU population. Therefore, measuring Aβ levels alone can identify a population at high risk. The OR for conversion to dementia differs significantly between the A+T+ and A+T- groups in both the MCI and CU populations: while the OR is 2.73 [95% CI 1.65; 4.52] for MCI and 2.04 [95% CI 0.70; 5.97] for CU subjects in the A+T- group, it increases to 11.60 [95% CI 7.96; 16.91] for MCI and 14.67 [95% CI 3.69; 49.11] for CU in the A+T+ group. Note that in the case of A+T- at CU population, only a trend-level statistical correlation is visible.
The results of the meta-regression show a decrease in OR with mean age (Fig. 2 B). Based on this result it seems that the impact of Amyloid positivity on conversion is decreasing with age. The fact that age is a risk factor for dementia and vascular and other neurodegenerative damage are more frequent in elderly age is a possible explanation to this finding. Our findings combined with the results of Rodrigue et al. [ 62 ] suggests that amyloid burden increases with age, while its impact on conversion rates slightly decreases with age.
The appearance of Aβ is assumed to be one of the earliest signs of AD [ 63 , 64 ]. Our results fit into this picture by showing that only the A+T+ and A+T- groups showed an increased risk for conversion compared to A-T-, the A-T+ group did not. Thus, Aβ alone is suitable for detecting the population at risk, while p-tau alone is not as effective in the prediction conversion. Our result is in line with previous studies showing that the A-T+ group has a weaker association with cognitive decline compared to the A+T- or A+T+ groups [ 65 , 66 ]. However, it is important to emphasize that previous results showing that T+ status is closely associated with neurodegeneration and the A-T+ group is related to frontotemporal dementia [ 67 ]. More research is needed to fully explain the significance of the A-T+ group.
The PET scan is known to be a more sensitive tool for detecting Amyloid positivity compared to CSF sampling [ 68 ]. However, from a prognostic point of view, our results did not show a significant difference ( p =0.73) between PET measurements (OR: 6.02) and the more cost-effective but invasive CSF Aβ42 measurements (OR: 5.11). It is important to note here that the present meta-analysis is underpowered for detecting prognostic differences between these methods. Due to the heterogeneity among studies, the impact of confounding factors, and standardised studies are required to evaluate the comparative prognostic value of these biomarkers accurately.
Our results based on ORs are further strengthened by the HR analyses giving similar results for Aβ exposure in the MCI (HR: 3.16) and CU (HR: 2.33) populations. It should be noted that in the HR analysis of the CU group, ADNI accounts for 78.5% of the weight, which is a limitation of this meta-analysis. This disproportionate representation may affect the overall result. Regarding the statistical trend-level association with a higher unadjusted HR, it should be noted that in the presence of a random distribution of other risk factors (e.g. baseline MMSE score or educational level), the unadjusted value may overestimate the HR. As in the case of a non-random distribution, the adjusted value underestimates the HR. With this in mind, we recommend reporting both values in the future.
Our analyses were performed on CU and MCI populations. Including mixed populations with the MCI population was a practical simplification, as several studies with a large number of cases gave their results combining MCI subjects with CU subjects, and we aimed to answer the set of questions based on the largest population. To investigate the potential bias of this method, we performed subgroup analysis comparing the mixed and MCI populations, and the result was not significant. The Aβ OR based on the mixed-only group is 4.64 [95% CI 1.16; 18.61], and the OR calculated on the MCI-only studies is 5.83 [95% CI 3.80; 8.93]. Thus, the inclusion of the mixed population in the pool decreases the OR of the main analysis (5.21 [95% CI 3.93; 6.90]) slightly (Supplementary Material eFigure 1 ).
Strengths and limitations
There are several limitations to consider when interpreting our results. The study populations differ in several aspects; for cognitive status, the population ranges from those with no cognitive symptoms through those with subjective cognitive symptoms (these two groups were considered CU) to MCI groups. Therefore, the distance from the cognitive state corresponding to MCI or dementia also varies. Due to the different cut-offs used in the studies, subjects with grey area scores may oscillate between A- and A+ groups, increasing heterogeneity. Our study could not examine the role of other risk factors such as education, cardiovascular status, obesity, diabetes, depression, social and physical activity [ 69 ], or genetic status [ 70 , 71 ], which may also contribute to heterogeneity. Furthermore, there is a considerable heterogeneity by mean age, and our meta-regression analysis of MCI group showed a significant decreasing effect of mean age on ORs.
In the OR analysis of Aβ in the CU group, in the context of the outlier value of the Arruda study, the possibility of a statistical extreme value can be assumed due to the small number of A+ subjects and the much larger A- group. Similarly, in the case of the Grontvedt [ 14 ] and Hanseeuw [ 41 ] studies, which show exceptionally high values, the A+ and A- groups show a similar uneven distribution. Similarly, the outliers in the MCI amyloid OR analysis are also associated with small sample sizes. For the Aβ HR analysis in the CU group, the interpretability of the result is strongly influenced by one specific cohort (ADNI), which accounts for 78% of the overall weight. In the A+T+/A+T-/A-T+ analyses, no outliers were found in either the MCI or CU groups.
Furthermore, we note that although the Aβ OR analyses could be confirmed by also calculating the HRs, the inability to analyze the effect of p-tau on HR due to the low number of studies limits the completeness of the A/T analysis.
We pooled studies reporting AD-type dementia conversion and studies reporting conversion to unspecified dementia. This simplification was necessary because different studies defined Alzheimer’s dementia differently, generally considering the amnestic clinical symptoms rather than biomarkers.
The fact that the studies used different neuropsychology tests to define MCI may contribute to the heterogeneity in the pooled sample. Another contributing factor would be the heterogeneity in the definition of MCI, however among the studies in our pool, only one, by Riemschneider et al. [ 48 ] (sample size = 28), precedes the 2003 ‘Key Symposium’ [ 72 ] that transformed the MCI concept. All other studies were published subsequent to it. While MCI subgroups were deifned after the 2003 Symposium, the definition of MCI (objective cognitive impairment, essentially preserved general cognitive functioning, preserved independence in functional abilities) did not change afterwards. Furthermore, most of the studies pooled in the analyses were published after 2010.
Another source of heterogeneity is the relatively small sample size of some studies, leading to a higher variability of results. However, we thought that including studies with lower sample sizes was also important to get a complete picture.
It is essential to discuss the difference in the follow-up times between studies. The follow-up times ranged from 20 months to more than 10 years. Follow-up times were given in different ways, either as mean, median or up to a certain point. While naturally, the odds of conversion increase over time, our meta-regression analysis suggests that there is no significant difference in the odds ratios over (follow-up) time. The moderate heterogeneity of the studies also points in this direction. We also note here that hazard ratios independent of follow-up time showed similar results to OR analyses. Finally, yet importantly, we would like to point out that pathological protein changes can begin up to 20 years before the appearance of symptoms [ 6 ]. Such an extended follow-up is very difficult to carry out; therefore, all studies were shorter than that.
The results for Aβ are based on 7,793 individuals, and the combined analyses of Aβ and p-tau are based on data of over 3,500 individuals. Studies using CSF sampling or amyloid/tau PET to detect Aβ and p-tau were pooled together, despite using different kits and thresholds for positivity, contributing to the heterogeneity of results. This variation is acknowledged in Tables 1 , 2 , 3 and 4 , where the cut-off values are provided. Previous large population studies have indicated that amyloid and tau PET scans exhibit slightly higher sensitivity compared to CSF sampling techniques [ 73 , 74 , 68 ]. Nonetheless, the concordance between these diagnostic methods remains substantial. Moreover, findings from prior research (Lee et al. [ 75 ], Toledo et al. [ 76 ], Palmqvist et al. [ 77 ]) demonstrating high concordance across different amyloid CSF and amyloid PET measurements suggest that the impact of methodological differences on heterogeneity may be limited, All techniques are recommended by the National Institute on Aging-Alzheimer’s Association (NIA-AA) [ 6 ] for measurement.
Future directions
Conversion to Alzheimer’s disease could not be analyzed specifically, as most of the articles examining conversion either did not define Alzheimer’s disease or the definition was based on neuropsychological testing but not on biomarkers (i.e., Aβ and p-tau status were assessed only at baseline). According to the NIA-AA guideline [ 6 ] and our results, we recommend biomarker-based studies to assess conversion rates to Alzheimer’s disease.
In view of the Aβ and p-tau status, the most endangered population can be identified before the appearance of cognitive symptoms or at least at a mild stage. While the significance of Aβ in conversion is clear, it appears that its ability to predict the onset decreases with age. If we consider the current therapeutic limitations and the importance of early prevention, we believe that the initiation of non-pharmacological and pharmacological treatments should be related to Aβ and p-tau status rather than cognitive status.
Identifying the most endangered population also makes research more effective. The efficacy of different dementia prevention approaches can be more accurately assessed by knowing the Aβ and p-tau status of the patient. As the population targeted by the interventions can be more homogeneous, the effectiveness can be measured more precisely by identifying the population most at risk of conversion.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Non-pathologic levels of beta-amyloid
Pathologic levels of beta-amyloid
- Beta-amyloid
- Alzheimer’s disease
Alzheimer’s Disease Neuroimaging Initiative
Confidance interval
Cognitively unimpaired
Cerebrospinal fluid
Hazard ratio
- Mild cognitive impairment
Absence of neurodegeneration
Presence of neurodegeneration
National Institute on Aging Alzheimer’s Association
Positron emission tomography
- Phosphorylated tau
Non-pathologic levels of phosphorylated tau
Pathologic levels of phosphorylated tau
Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542796/ .
Gauthier S, Rosa-Neto P, Morais JA, & Webster C. 2021. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer’s Disease International.
De Strooper B. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–15. https://doi.org/10.1016/j.cell.2015.12.056 .
Article CAS PubMed Google Scholar
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. The Lancet. 2021;397(10284):1577–90. https://doi.org/10.1016/s0140-6736(20)32205-4 .
Article CAS Google Scholar
Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 2021;20(6):484–96. https://doi.org/10.1016/s1474-4422(21)00066-1 .
Article CAS PubMed PubMed Central Google Scholar
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research framework: toward a biological definition of alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. https://doi.org/10.1016/j.jalz.2018.02.018 .
Article PubMed Google Scholar
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34(7):939–44. https://doi.org/10.1212/wnl.34.7.939 .
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005 .
Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–83. https://doi.org/10.1016/j.neurobiolaging.2010.04.007 .
Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes. JAMA. 2015;313(19):1939. https://doi.org/10.1001/jama.2015.4669 .
Article PubMed PubMed Central Google Scholar
Morris GP, Clark IA, Vissel B. Questions concerning the role of amyloid-β in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018;136(5):663–89. https://doi.org/10.1007/s00401-018-1918-8 .
Van Der Flier WM, Scheltens P. The ATN framework—moving preclinical Alzheimer disease to clinical relevance. JAMA Neurology. 2022;79(10):968. https://doi.org/10.1001/jamaneurol.2022.2967 .
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. https://doi.org/10.1001/archneur.56.3.303 .
Grøntvedt GR, Lauridsen C, Berge G, et al. The amyloid, tau, and neurodegeneration (A/T/N) classification applied to a clinical research cohort with long-term follow-up. J Alzheimers Dis. 2020;74(3):829–37. https://doi.org/10.3233/jad-191227 .
Balasa M, Sánchez-Valle R, Antonell A, et al. Usefulness of biomarkers in the diagnosis and prognosis of early-onset cognitive impairment. J Alzheimer’s Di. 2014;40(4):919–27. https://doi.org/10.3233/JAD-132195 .
Article Google Scholar
Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313(19):1924–38. https://doi.org/10.1001/jama.2015.4668 .
Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71. https://doi.org/10.1136/bmj.n71 .
Weiner MW. Alzheimer’s disease neuroimaging initiative. Available from: https://adni.loni.usc.edu/ .
Aydin O, Yassikaya MY. Validity and reliability analysis of the plotdigitizer software program for data extraction from single-case graphs. Perspect Behav Sci. 2022;45(1):239–57. https://doi.org/10.1007/s40614-021-00284-0 .
Huwaldt, J. A., & Steinhorst, S. (2020). Plot digitizer 2.6.9.PlotDigitizer-Software. http://plotdigitizer.sourceforge.net/ .
Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal Fluid Aβ42/40 Corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):813–22. https://doi.org/10.3233/jad-160722 .
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
CAS PubMed Google Scholar
Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124(5):719–23. https://doi.org/10.1093/oxfordjournals.aje.a114447 .
Thompson SG, Turner RM, Warn DE. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat Methods Med Res. 2001;10(6):375–92. https://doi.org/10.1177/096228020101000602 .
Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–25. https://doi.org/10.1002/jrsm.11 .
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009 .
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295(6):676–80. https://doi.org/10.1001/jama.295.6.676 .
Kemppainen NM, Scheinin NM, Koivunen J, et al. Five-year follow-up of 11C-PIB uptake in Alzheimer’s disease and MCI. Eur J Nucl Med Mol Imaging. 2014;41(2):283–9. https://doi.org/10.1007/s00259-013-2562-0 .
Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98–106. https://doi.org/10.1001/archgenpsychiatry.2011.155 .
Forlenza OV, Radanovic M, Talib LL, et al. Cerebrospinal fluid biomarkers in Alzheimer’s disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst). 2015;1(4):455–63. https://doi.org/10.1016/j.dadm.2015.09.003 .
Hansson O, Buchhave P, Zetterberg H, Blennow K, Minthon L, Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2009;30(2):165–73. https://doi.org/10.1016/j.neurobiolaging.2007.06.009 .
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x .
Arruda F, Rosselli M, Mejia Kurasz A, et al. Stability in cognitive classification as a function of severity of impairment and ethnicity: a longitudinal analysis. Article in Press. Appl Neuropsychol Adult. 2023:1-14. https://doi.org/10.1080/23279095.2023.2222861 .
Baldeiras I, Silva-Spínola A, Lima M, et al. Alzheimer’s disease diagnosis based on the amyloid, tau, and neurodegeneration scheme (ATN) in a real-life multicenter cohort of general neurological centers. J Alzheimer’s Dis. 2022;90(1):419–32. https://doi.org/10.3233/JAD-220587 .
Bos I, Verhey FR, Ramakers I, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther. 2017;9(1):101. https://doi.org/10.1186/s13195-017-0328-9 .
Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in Mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187–94. https://doi.org/10.1016/j.nicl.2014.12.004 .
de Wilde A, Reimand J, Teunissen CE, et al. Discordant amyloid-β PET and CSF biomarkers and its clinical consequences. Alzheimers Res Ther. 2019;11(1):78. https://doi.org/10.1186/s13195-019-0532-x .
Eckerström C, Svensson J, Kettunen P, Jonsson M, Eckerström M. Evaluation of the ATN model in a longitudinal memory clinic sample with different underlying disorders. Alzheimers Dement (Amst). 2021;13(1): e12031. https://doi.org/10.1002/dad2.12031 .
Frölich L, Peters O, Lewczuk P, et al. Incremental value of biomarker combinations to predict progression of mild cognitive impairment to Alzheimer’s dementia. Alzheimers Res Ther. 2017;9(1):84. https://doi.org/10.1186/s13195-017-0301-7 .
Groot C, Cicognola C, Bali D, et al. Diagnostic and prognostic performance to detect alzheimer’s disease and clinical progression of a novel assay for plasma p-tau217. Article Alzheimer’s Res Ther. 2022;14(1):67. https://doi.org/10.1186/s13195-022-01005-8 .
Hanseeuw BJ, Malotaux V, Dricot L, et al. Defining a Centiloid scale threshold predicting long-term progression to dementia in patients attending the memory clinic: an [(18)F] flutemetamol amyloid PET study. Eur J Nucl Med Mol Imaging. 2021;48(1):302–10. https://doi.org/10.1007/s00259-020-04942-4 .
Herukka SK, Hallikainen M, Soininen H, Pirttilä T. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Article Neurology. 2005;64(7):1294–7. https://doi.org/10.1212/01.WNL.0000156914.16988.56 .
Jiménez-Bonilla JF, Quirce R, De Arcocha-Torres M, et al. A 5-year longitudinal evaluation in patients with mild cognitive impairment by 11C-PIB PET/CT: a visual analysis. Nucl Med Commun. 2019;40(5):525–31. https://doi.org/10.1097/mnm.0000000000001004 .
Lopez OL, Becker JT, Chang Y, et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology. 2018;90(21):E1920–8. https://doi.org/10.1212/WNL.0000000000005549 .
Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–60. https://doi.org/10.1212/WNL.0b013e3181b23564 .
Orellana A, García-González P, Valero S, et al. Establishing in-house cutoffs of CSF Alzheimer’s disease biomarkers for the AT(N) stratification of the Alzheimer center barcelona cohort. Int J Mol Sci. 2022;23(13):6891. https://doi.org/10.3390/ijms23136891 .
Ortega RL, Dakterzada F, Arias A, et al. Usefulness of CSF biomarkers in predicting the progression of amnesic and nonamnesic mild cognitive impairment to Alzheimer’s disease. Curr Aging Sci. 2019;12(1):35–42. https://doi.org/10.2174/1874609812666190112095430 .
Riemenschneider M, Lautenschlager N, Wagenpfeil S, Diehl J, Drzezga A, Kurz A. Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol. 2002;59(11):1729–34. https://doi.org/10.1001/archneur.59.11.1729 .
Rizzi L, Missiaggia L, Schwartz IVD, Roriz-Cruz M. Value of CSF biomarkers in predicting risk of progression from aMCI to ADD in a 5-year follow-up cohort. SN Compr Clin Med. 2020;2(9):1543–50. https://doi.org/10.1007/s42399-020-00437-3 .
Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and Outcomes of amyloid positivity among persons without dementia in a longitudinal population-based setting. JAMA Neurol. 2018;75(8):970–9. https://doi.org/10.1001/jamaneurol.2018.0629 .
Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–92. https://doi.org/10.1002/ana.22248 .
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. https://doi.org/10.1016/s1474-4422(06)70355-6 .
Dang C, Harrington KD, Lim YY, et al. Relationship Between amyloid-β positivity and progression to mild cognitive impairment or dementia over 8 years in cognitively normal older adults. J Alzheimers Dis. 2018;65(4):1313–25. https://doi.org/10.3233/jad-180507 .
Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology. 2020;95(1):e46–58. https://doi.org/10.1212/wnl.0000000000009724 .
Hatashita S, Wakebe D. Amyloid β deposition and glucose metabolism on the long-term progression of preclinical Alzheimer’s disease. Future Sci OA. 2019;5(3):Fso356. https://doi.org/10.4155/fsoa-2018-0069 .
Ossenkoppele R, Pichet Binette A, Groot C, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nature Medicine. 2022;28(11):2381–7. https://doi.org/10.1038/s41591-022-02049-x .
Strikwerda-Brown C, Hobbs DA, Gonneaud J, et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurology. 2022;79(10):975. https://doi.org/10.1001/jamaneurol.2022.2379 .
Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–65. https://doi.org/10.1016/s1474-4422(13)70194-7 .
Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27(5):458–64. https://doi.org/10.1159/000216841 .
Hong YJ, Park JW, Lee SB, et al. The influence of amyloid burden on cognitive decline over 2 years in older adults with subjective cognitive decline: a prospective cohort study. Dement Geriatr Cogn Disord. 2021;50(5):437–45. https://doi.org/10.1159/000519766 .
Tomassen J, den Braber A, van der Landen SM, et al. Abnormal cerebrospinal fluid levels of amyloid and tau are associated with cognitive decline over time in cognitively normal older adults: A monozygotic twin study. Alzheimers Dement (N Y). 2022;8(1): e12346. https://doi.org/10.1002/trc2.12346 .
Rodrigue KM, Kennedy KM, Devous MD Sr, et al. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–95. https://doi.org/10.1212/WNL.0b013e318245d295 .
Donohue MC, Jacqmin-Gadda H, Le Goff M, et al. Estimating long-term multivariate progression from short-term data. Alzheimers Dement. 2014;10(5 Suppl):S400–10. https://doi.org/10.1016/j.jalz.2013.10.003 .
Young AL, Oxtoby NP, Daga P, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain. 2014;137(Pt 9):2564–77. https://doi.org/10.1093/brain/awu176 .
Oberstein TJ, Schmidt MA, Florvaag A, et al. Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain. 2022;145(11):4032–41. https://doi.org/10.1093/brain/awac297 .
Wisse LEM, Butala N, Das SR, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152–62. https://doi.org/10.1016/j.neurobiolaging.2015.08.029 .
Pouclet-Courtemanche H, Nguyen TB, Skrobala E, et al. Frontotemporal dementia is the leading cause of “true” A-/T+ profiles defined with Aβ(42/40) ratio. Alzheimers Dement (Amst). 2019;11:161–9. https://doi.org/10.1016/j.dadm.2019.01.001 .
Vos SJB, Gordon BA, Su Y, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. https://doi.org/10.1016/j.neurobiolaging.2016.03.025 .
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/s0140-6736(20)30367-6 .
Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–7. https://doi.org/10.1001/jama.2019.9879 .
Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–9. https://doi.org/10.1038/s41591-019-0547-7 .
Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–6. https://doi.org/10.1111/j.1365-2796.2004.01380.x .
La Joie R, Bejanin A, Fagan AM, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–90. https://doi.org/10.1212/wnl.0000000000004860 .
Wolters EE, Ossenkoppele R, Verfaillie SCJ, et al. Regional [(18)F]flortaucipir PET is more closely associated with disease severity than CSF p-tau in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2020;47(12):2866–78. https://doi.org/10.1007/s00259-020-04758-2 .
Lee J, Jang H, Kang SH, et al. Cerebrospinal fluid biomarkers for the diagnosis and classification of Alzheimer’s disease spectrum. J Korean Med Sci. 2020;35(44):361. https://doi.org/10.3346/jkms.2020.35.e361 .
Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23–35. https://doi.org/10.1007/s00401-012-0983-7 .
Palmqvist S, Zetterberg H, Mattsson N, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85(14):1240–9. https://doi.org/10.1212/wnl.0000000000001991 .
Download references
Acknowledgements
Not applicable.
Open access funding provided by Semmelweis University. 1. Supported by the GINOP-2.3.4-15-2020-00008 project. The project is co-financed by the European Union and the European Regional Development Fund.
2. This is an EU Joint Programme- Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organization under the aegis of JPND - www.jpnd.eu (National Research, Development and Innovation, Hungary, 2019-2.1.7-ERA-NET-2020-00006).
3. Supported by the National Research, Development and Innovation Office (NKFI/OTKA FK 138385).
Role of funding source: The sponsor(s), did not participate in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and affiliations.
Centre for Translational Medicine, Semmelweis University, Üllői út 26, Budapest, 1085, Hungary
Zsolt Huszár, Marie Anne Engh, Márk Pavlekovics, Tomoya Sato, Yalea Steenkamp, Tamás Terebessy, Zsolt Molnár, Péter Hegyi & Gábor Csukly
Department of Psychiatry and Psychotherapy, Semmelweis University, Balassa utca 6, Budapest, 1083, Hungary
Zsolt Huszár & Gábor Csukly
Department of Neurology, Jahn Ferenc Teaching Hospital, Köves utca 1, Budapest, 1204, Hungary
Márk Pavlekovics
Department of Neurology and Institute of Neuroscience, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, 1200, Belgium
Bernard Hanseeuw
Department of Radiology, Gordon Center for Medical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, 02155, USA
Department of Anesthesiology and Intensive Therapy, Semmelweis University, Üllői út 78/A, Budapest, Hungary
Zsolt Molnár
Department of Anesthesiology and Intensive Therapy, Poznan University of Medical Sciences, 49 Przybyszewskiego St, Poznan, Poland
Institute for Translational Medicine, Medical School, University of Pécs, Pécs, 7624, Hungary
Péter Hegyi
Institute of Pancreatic Diseases, Semmelweis University, Tömő 25-29, Budapest, 1083, Hungary
Translational Pancreatology Research Group, Interdisciplinary Centre of Excellence for Research Development and Innovation University of Szeged, Budapesti 9, Szeged, 6728, Hungary
You can also search for this author in PubMed Google Scholar
Contributions
ZH: conceptualisation, project administration, methodology, formal analysis, writing – original draft; ME: conceptualisation, methodology, formal analysis, writing – review and editing; MP: conceptualisation, formal analysis, writing - review and editing; TS formal analysis, writing – review and editing; YS: formal analysis, writing – review and editing; BH: writing - review and editing; TT: conceptualisation, writing – review and editing; ZM: conceptualisation, supervision, writing - review and editing; PH: conceptualisation, supervision, writing - review and editing; GCs: conceptualization, methodology, formal analysis, supervision, writing – original draft, visualization. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Gábor Csukly .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huszár, Z., Engh, M., Pavlekovics, M. et al. Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis. Alz Res Therapy 16 , 81 (2024). https://doi.org/10.1186/s13195-024-01455-2
Download citation
Received : 07 July 2023
Accepted : 08 April 2024
Published : 12 April 2024
DOI : https://doi.org/10.1186/s13195-024-01455-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My watchlist
- Stock market
- Biden economy
- Personal finance
- Stocks: most active
- Stocks: gainers
- Stocks: losers
- Trending tickers
- World indices
- US Treasury bonds
- Top mutual funds
- Highest open interest
- Highest implied volatility
- Currency converter
- Basic materials
- Communication services
- Consumer cyclical
- Consumer defensive
- Financial services
- Industrials
- Real estate
- Mutual funds
- Credit cards
- Credit card rates
- Balance transfer credit cards
- Business credit cards
- Cash back credit cards
- Rewards credit cards
- Travel credit cards
- Checking accounts
- Online checking accounts
- High-yield savings accounts
- Money market accounts
- Personal loans
- Student loans
- Car insurance
- Home buying
- Options pit
- Investment ideas
- Research reports
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Alzheimer's: I know I'm going to have early onset dementia
James Bexon was just 33 when he was told he had between 20 and 30 years before developing Alzheimer's.
His father was diagnosed at 58, and in 2018 James took an NHS test which said he had a 99.9% chance of getting a rare, genetic form of the condition.
He qualified for a test because of the strong familial link, which then left him wondering if he might pass it on to his own sons, Elijah and Jacob.
Neither son has inherited the gene which causes genetic Alzheimer's.
What is Alzheimer's and how common is it?
Doctor reveals how 'brutal' therapy tackled Rhod Gilbert's cancer
AI photos show people with cancer their lost future
James was living in Tan-lan, Gwynedd, when he started the testing process for genetic young-onset Alzheimer's.
His father died from the disease in late 2017, shortly before James' testing began.
Half of his family members have developed the condition over the years.
James had numerous counselling sessions before the blood test in March 2018, to prepare for the chance of bad news.
The results arrived six weeks later.
"The counsellor had a trainee with her, and I read it on her face when I went in," he said.
"You half expect it anyway, because it's prominent in the family. Obviously if it was a 'you haven't got it', that would be amazing.
"I just thought that was the hand I'd been dealt, I had that feeling, even though it's 50/50."
He added: "The build-up in the protein amyloid, which causes genetic dementia, stops your memory and slows everything down. That starts about 20 or 15 years before you have symptoms.
"So realistically it might have already started - I just don't know.
"Sometimes you can dwell on it too much. Elijah is five and Jacob is two. As they get older time does go fast.
"Suddenly you're 40, and actually then I'm not far off being 50.
"There's people who have their parents around into their 50s or 60s, and I want to have that. I want my kids to have a proper grandad. The kids have Gemma's stepdad, but on my side they don't have a grandad.
"It would be nice to live into my 70s, 80s, to be a grumpy old man."
James and his wife Gemma also had a tough decision to make - whether or not to test their two children for the gene while they were in the womb.
They decided to proceed with the testing, and both children tested negative.
You can only be tested on the NHS in Wales and England for rare inherited forms of dementia, such as frontotemporal dementia and some forms of young-onset Alzheimer's.
Dementia UK nurse Jules Knight said: "The stats at Dementia UK is 7% to 12% of cases of young-onset are a genetic form of dementia.
"In the UK at the moment, 70,800 people are living with young-onset dementia."
Drugs such as donanemab , which trials have shown slows cognitive decline, rely on early diagnosis, and private tests for other forms of dementia are not available on the NHS.
Ms Knight said there were some concerns over private tests, which only tell you the risk, rather than NHS tests, which can pinpoint a genetic mutation.
"The NHS test is definitive, in terms of you either have the gene mutation or you don't," she said.
"The genetic risk variant, some of us might have the genetic variant some of us might not, but it doesn't mean you are or aren't going to develop it."
She also said the NHS offered extensive emotional support.
"When someone is referred to genetic testing, they should have both pre and post counselling," Ms Knight said.
"The pre is support around whether you want to go ahead with the testing, the implications around that, and the support you might need."
For James, who now lives in Evesham, Worcestershire, the NHS test has given his family peace of mind.
"We're not going to have anymore children, so we know that's it for genetic Alzheimer's.
"For me, it was all about trying to stop it in its tracks in our family. There's only so much you can do, and we've done what we can."
Recommended Stories
Nba playoffs: knicks secure wild win over 76ers with frantic final minute to secure 2-0 series lead.
What a win for the Knicks at Madison Square Garden.
Chiefs make Andy Reid NFL's highest-paid coach, sign president Mark Donovan, GM Brett Veach to extensions
Reid's deal reportedly runs through 2029 and makes him the highest-paid coach in the NFL.
NFL Draft: Would QB-needy Patriots actually trade down from No. 3? Here's what the league thinks
There's bountiful recent precedent for trading the third overall pick. Question is, does New England fit the model of those teams?
NCAA officially ratifies new rules allowing athletes to transfer multiple times and still be immediately eligible
As long as they are academically eligible, the NCAA will no longer limit how many times athletes can transfer schools without penalty.
You're probably throwing away these food scraps. Here's why eating them is good for you and the environment.
Food scraps such as peels and root vegetable greens are densely packed with nutrients, and eating them can help reduce greenhouse gas-emitting landfills.
UnitedHealth says Change hackers stole health data on 'substantial proportion of people in America'
Health insurance giant UnitedHealth Group has confirmed that a ransomware attack on its health tech subsidiary Change Healthcare earlier this year resulted in a huge theft of Americans' private healthcare data. UnitedHealth said in a statement on Monday that a ransomware gang took files containing personal data and protected health information that it says may "cover a substantial proportion of people in America." Change Healthcare processes insurance and billing for hundreds of thousands of hospitals, pharmacies and medical practices across the U.S. healthcare sector; it has access to massive amounts of health information on about half of all Americans.
Report: Hornets interviewing ESPN analyst, former NBA, Duke guard JJ Redick for head-coaching vacancy
Redick currently works for ESPN as an NBA analyst.
TechCrunch Space: Engineering the future
Don't worry -- we'll be diving into the Mars Sample Return news shortly. This week's SOTW segment is dedicated to Mars Sample Return, NASA's troubled and ambitious plan to bring Martian rock and dust back to Earth. NASA administrator Bill Nelson has pronounced the agency’s $11 billion, 15-year plan to collect and return samples from Mars insufficient.
Eva Longoria says powder can settle into fine lines when you're over 40 — she uses this $12 creamy concealer instead
Blur blemishes and discoloration in seconds with this $12 formula by L'Oreal.
What is the Federal Reserve?
The Federal Reserve ensures the health and stability of the nation’s banks and economy through monetary policy and interbank lending.
Junkyard Gem: 1997 Geo Metro LSi
A 1997 Geo Metro LSi 3-door hatchback, found in a New Orleans self-service wrecking yard.
Zuckerberg wants more companies to build Meta-powered headsets
Mark Zuckerberg says Meta will open up its Quest operating system so that third-party companies can build new headsets.
28 Nordstrom new arrivals for April you don't want to miss, starting at just $15
I shop for a living and I'm lusting after these new Nordstrom finds from Ugg, Le Creuset, Adidas, Tory Burch, Spanx and more.
The 'luxury hotel'-quality queen sheet set 137,000+ Amazon shoppers snooze on is down to $16
No need to count sheep when your bedding gets an upgrade like this.
Arch Manning dominates in the Texas spring game, and Jaden Rashada enters the transfer portal
Dan Wetzel, Ross Dellenger & SI’s Pat Forde react to the huge performance this weekend by Texas QB Arch Manning, Michigan and Notre Dame's spring games, Jaden Rashada entering the transfer portal, and more
Stock market today: S&P 500 snaps 6-day losing streak ahead of Big Tech earnings rush
Big Techs are the highlight as hopes rest on this week's flood of earnings to reassure and reignite the market.
US government says security flaw in Chirp Systems' app lets anyone remotely control smart home locks
A vulnerability in a smart access control system used in thousands of U.S. rental homes allows anyone to remotely control any lock in an affected home. U.S. cybersecurity agency CISA went public with a security advisory last week saying that the phone apps developed by Chirp, which residents use in place of a key to access their homes, "improperly stores" hardcoded credentials that can be used to remotely control any Chirp-compatible smart lock. Apps that rely on passwords stored in its source code, known as hardcoding credentials, are a security risk because anyone can extract and use those credentials to perform actions that impersonate the app.
Nelly Korda, after historic fifth straight win, WDs from LA Championship: 'I am definitely feeling exhausted'
"I feel I need to listen to my body and get some rest, so I can be ready for the remainder of the season."
Anthony Davis fed up with not winning Defensive Player of the Year: 'The league doesn't like me'
Does Davis have a DPOY case? The Lakers' star definitely thinks so.
Report: Jets trading QB Zach Wilson to Broncos
Wilson's starting over in Denver.
- International edition
- Australia edition
- Europe edition

Mentally stimulating work plays key role in staving off dementia, study finds
People in routine and repetitive jobs found to have 31% greater risk of disease in later life, and 66% higher risk of mild cognitive problems
If work is a constant flurry of mind-straining challenges, bursts of creativity and delicate negotiations to keep the troops happy, consider yourself lucky.
Researchers have found that the more people use their brains at work, the better they seem to be protected against thinking and memory problems that come with older age.
In a study of more than 7,000 Norwegians in 305 occupations, those who held the least mentally demanding jobs had a 66% greater risk of mild cognitive impairment, and a 31% greater risk of dementia, after the age of 70 compared with those in the most mentally taxing roles.
“It really shows how important work is,” said Dr Trine Edwin, a geriatrician and postdoctoral fellow at Oslo university hospital. “It’s important to go to work and use your brain, and to use your brain to learn new things.”
Edwin and her colleagues examined the cognitive complexity of various jobs based on the amount of routine manual and mental work, and the degree of analytical and interpersonal tasks, they involved.
Most people worked jobs with similar degrees of cognitive demands throughout their 30s, 40s, 50s and 60s, meaning that those who started work in less mentally stimulating jobs tended to remain in them, as did those who took on cognitively challenging positions from the off.

After the age of 70, the volunteers took part in standard memory and thinking tests and were classified as having either no cognitive impairment, mild cognitive impairment or dementia. Of those who had worked in the least cognitively challenging jobs, 42% were diagnosed with mild cognitive impairment, compared with only 27% who had worked in the most cognitively stimulating roles.
Among the jobs ranked as most stimulating were teachers and university lecturers, according to the study, in Neurology . Some of the least cognitively demanding jobs were those that involved repetitive manual tasks, such as road work, cleaning and delivering the post.
Previous studies have shown that education has a significant protective effect against cognitive decline in old age. Part of the reason is that better educated people are more likely, and more able, to lead healthier lives. But education also appears to build “cognitive reserve” – the capacity to improvise and find alternative ways of doing things – which may help stave off mental decline, much as physical exercise delays frailty .
According to Trine, higher levels of education accounted for about 60% of the protective effect seen among people who did mentally stimulating jobs. “It means that education is very important, but it’s also what you do afterwards: it’s how you use your brain when you are working. You are building your cognitive reserve at work by being cognitively active,” she said.
The results suggest that people who spend their working lives in less mentally stimulating jobs might benefit from further education and pursuing more cognitively challenging pastimes outside work. “It’s not that you are doomed or you are not – we can empower people for their later cognitive health with education and tasks that are cognitively stimulating,” Trine said.
Prof Gill Livingston, professor of the psychiatry of older people at University College London, said the findings were in line with other studies on the impact of work. “It is not just that more educated people do more cognitively stimulating jobs – they do – but cognitive stimulation in work through problem solving and new situations has an effect by itself.
“This is a lot of cognitive stimulation, as most people work many hours for many years,” she said. But work may not have as big an impact as education, she added, because the brains of children and young adults may change more than those in adults to increase cognitive reserve.
- Work & careers
Hundreds of thousands face being denied revolutionary new dementia drugs in England
What are the symptoms of dementia and how do you get a diagnosis?
Thousands to be offered blood tests for dementia in UK trial

Air pollution could be significant cause of dementia – even for those not predisposed
Early blood test to predict dementia is step closer as biological markers identified.
Blood test could revolutionise diagnosis of Alzheimer’s, experts say

Alcohol misuse and loneliness ‘increase risk of early-onset dementia’

Inequality leaving 115,000 dementia cases ‘undiagnosed’ in England

Gabriel García Márquez’s last novel stands in tribute to his defiance of dementia

Dementia risk factors pose more danger for ethnic minorities, finds study
Most viewed.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Scientific Reports

Risk of early-onset dementia among persons with tinnitus: a retrospective case–control study
Yen-fu cheng.
1 Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan
2 Department of Otolaryngology-Head and Neck Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
3 Department of Speech, Language and Audiology, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
4 Research Center of Sleep Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
5 Department of Otolaryngology-Head and Neck Surgery, School of Medicine, National Yang-Ming University, Taipei, Taiwan
Sudha Xirasagar
6 Department of Health Services Policy and Management, Arnold School of Public Health, University of South Carolina, Columbia, USA
Tzong-Han Yang
7 Department of Otolaryngology, Taipei City Hospital, Taipei, Taiwan
Chuan-Song Wu
8 College of Science and Engineering, Fu Jen University, New Taipei City, Taiwan
9 Big Data Research Center, Taipei Medical University, Taipei, Taiwan
10 Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, New Taipei City, Taiwan
Herng-Ching Lin
11 School of Health Care Administration, Taipei Medical University, 250 Wu-Hsing St., Taipei, 110 Taiwan
12 Sleep Research Center, Taipei Medical University Hospital, Taipei, Taiwan
Higher rates of poor cognitive performance are known to prevail among persons with tinnitus in all age groups. However, no study has explored the association between tinnitus and early-onset dementia. We hypothesize that tinnitus may precede or occur concurrently with subclinical or early onset dementia in adults younger than 65 years of age. This case–control study used data from the Taiwan National Health Insurance Research Database, identifying 1308 patients with early-onset dementia (dementia diagnosed before 65 years of age) and 1308 matched controls. We used multivariable logistic regressions to estimate odds ratios (ORs) for prior tinnitus among patients with dementia versus controls. Among total 2616 sample participants, the prevalence of prior tinnitus was 18%, 21.5% among cases and 14.5% among controls ( p < 0.001). Multivariable logistic regression showed and adjusted OR for prior tinnitus of 1.6 for cases versus controls (95% CI: 1.3 ~ 2.0). After adjusting for sociodemographic characteristics and medical co-morbidities, patients with early-onset dementia had a 67% higher likelihood of having prior tinnitus (OR = 1.628; 95% CI = 1.321–2.006). Our findings showed that pre-existing tinnitus was associated with a 68% increased risk of developing early-onset dementia among young and middle-aged adults. The results call for greater awareness of tinnitus as a potential harbinger of future dementia in this population.
Introduction
Dementia is a disorder characterized by a decline in cognitive abilities involving one or more cognitive domains. The development of dementia involves complex processes involving specific molecular pathways affecting multiple cellular functions of the central nervous system, leading to disruption of the functional networks underlying cognition, behavior and sensorimotor functions, eventually eroding autonomous functioning and decision-making abilities of affected individuals 1 , 2 . Although the prevalence of dementia increases with age, it may also affect younger individuals.
Early-onset dementia is defined as dementia diagnosed before the age of 65 3 , 4 The symptoms of early-onset dementia are similar to those observed among the elderly, behavioral changes, cognitive decline, and psychiatric manifestations. In addition to early-onset adult neurodegenerative disorders such as Alzheimer’s disease, vascular dementia and frontotemporal dementia, early-onset dementia may be caused by delayed onset of childhood neurodegenerative disorders caused by mitochondrial and lysosomal disorders 4 , 5 . The diagnosis of early-onset dementia is particularly challenging because the current diagnostic criteria require evidence of cognitive impairment and memory loss. Younger patients whose dementia is limited to progressive cognitive decline or focal neurological impairments may not be diagnosed with dementia or receive a delayed diagnosis. Further, studies show that patients with early-onset dementia take a longer time to seek their first consultation for dementia evaluation and for families to become aware of the dementia diagnosis 6 . Considering the newer treatment options that are becoming available to modify the course of dementia, the consequences of delayed or unrecognized early-onset dementia can be serious and unnecessary. Consequences that may be mitigated by early diagnosis include morbidity and stigma suffered by patients, and the economic and resource use burden borne by families and the healthcare system over the subsequent life span.
Tinnitus is a phantom auditory perception in the absence of an objective source of physical sound. Tinnitus is a common and disturbing phenomenon, with reported prevalence rates ranging from 7 to 20% in the general population 7 – 10 . One study even reported that the incidence of tinnitus was as high as 26.7% for people ages 65–84 years in the United States 11 . Tinnitus can occur due to pathologies occurring at any point between the cochlear apparatus and the auditory cortex. Increasing evidence shows tinnitus to be a disorder involving neuroplastic changes in the central auditory structures that occur when the brain is deprived of its normal input due to cochlear lesions 12 .
Studies show correlation between the presence of poor cognitive performance and tinnitus 13 – 17 , and a high rate of cognitive impairment is observed among in patients with tinnitus across all age groups 18 , 19 . Despite these suggestive associations, there are no documented studies that examined associations between tinnitus and early-onset dementia. We hypothesize that tinnitus may precede or occur parallel to subclinical or early dementia among the population younger than 65 years of age. We sought to examine whether tinnitus may represent an early sign preceding early-onset dementia using administrative claims data.
This retrospective case–control study used data from the Taiwan National Health Insurance (NHI) Research Database (NHIRD). The NHIRD comprises all medical claims data for approximately 99% of the Taiwanese population (about 24.02 million registered beneficiaries in December 2019) under Taiwan’s NHI program. Many researchers have used the NHIRD to track longitudinal use of medical care and diagnoses over follow-up for research purposes.
The study is based on de-identified administrative data provided by the NHIRD. It was approved and deemed exempt from informed consent requirement by the Institutional Review Board of Taipei Medical University (TMU-JIRB N202005074), and is compliant with the Declaration of Helsinki.
Study sample
To identify cases, we first identified 206,940 patients with a first-time diagnosis of dementia (ICD-9-CM codes 290.0 ~ 290.4, 294.1, 331.0 ~ 331.2, or 331.82 or ICD-10-CM code F03.90) in an outpatient setting (private clinics or hospital outpatient departments) between January 1, 2010 and December 31, 2016. We included only patients with a documented diagnosis of dementia at least two medical encounters during the study period to improve diagnostic validity. The date of the first-time dementia diagnosis during the study period was assigned as their index date. Next we selected patients aged between 30 and 64 years of age ( n = 11,361). Finally, we excluded patients with a history of major psychosis or a substance use-related disorder (ICD-9-CM codes 291 ~ 299, 303 ~ 305), stroke (ICD-9-CM codes 430 ~ 438), or traumatic brain injury (TBI) (ICD-9-CM codes 801 ~ 804 or 850 ~ 854) prior to the index date ( n = 10,053). The reason for excluding patients with a history of TBI was that TBI was reported as a potential risk factor for other neurodegenerative disorders that can be associated with dementia 20 . The remaining 1308 patients with early-onset dementia were included as cases in this study.
To select controls out of the remaining patients, we first excluded those who had ever received a diagnosis of dementia, major psychosis or a substance-related disorder, stroke, or traumatic brain injury, and those aged 65 years or over. We selected one propensity score-matched control per case, matching controls to cases using patient demographic variables (age, sex, monthly income, geographic location and urbanization level of the patient’s residence) and the co-morbidities relevant to dementia development hyperlipidemia, diabetes, coronary heart disease, hypertension, obesity, hearing loss, and alcohol abuse. We matched controls to a corresponding dementia case based on their utilization of any medical service in the index year of the case. For controls, we assigned the date of their first utilization of ambulatory care during the matched year as the index date. A total of 1,308 cases and 1308 controls were analyzed in the study.
Exposure assessment
Patients with a tinnitus diagnosis were identified based on ICD-9-CM code 388.3. We defined a patient as having tinnitus if they had at least one claim with a diagnosis of tinnitus prior to the index date during the study period.
Statistical analysis
Statistical analyses were carried out using the SAS system (SAS System for Windows, vers. 9.4, SAS Institute, Cary, NC). Chi-square test and t-tests were performed to examine differences in patient demographics and medical comorbidities between cases and controls. We used multivariable logistic regressions to estimate the odds ratios (ORs) of prior tinnitus among patients with dementia versus controls. We used two-sided p < 0.05 for statistical significance.
Study patients’ mean age was 59.5 years. Table Table1 1 presents the sociodemographic characteristics and comorbidities among cases and controls, showing no significant differences in age, sex, monthly income, hypertension, and hyperlipidemia between cases and controls. However, there were significant differences in geographic region ( p = 0.002), and the prevalence of diabetes ( p = 0.047), coronary heart disease ( p < 0.001) and hearing loss ( p = 0.002).
Demographic characteristics and comorbidity status of patients with early-onset dementia and matched control patients in Taiwan (n = 2616).
Table Table2 2 presents the prevalence of prior tinnitus among cases and controls. Among the total sample, the prevalence of prior tinnitus was 18%, 21.5% among cases and 14.5% among controls ( p < 0.001). Univariable logistic regression analysis showed an unadjusted OR for prior tinnitus of 1.610 among cases relative to controls (95% CI: 1.315 ~ 1.971, p < 0.001).
Prevalence of prior tinnitus crude odds ratio (OR), and 95% confidence interval (CI) for prior tinnitus among early-onset dementia patients and controls.
*** p < 0.001.
OR = odds ratio.
Multivariable logistic regression analysis showed that after adjusting for age, income, geographical location, urbanization level, hypertension, diabetes, coronary heart disease, hyperlipidemia, obesity, hearing loss, and alcohol abuse, patients with early-onset dementia were more likely to have had tinnitus before the index date, adjusted odds ratio1.628 (95% CI = 1.321–2.006; p < 0.001) (Table (Table3 3 ).
Multiple regression analysis results showing the adjusted odds ratio (OR) for prior tinnitus of early-onset dementia patients vs. controls (n = 2616).
All variables listed in the table were included in the multiple logistic regression model.
Table Table4 4 presents the adjusted odds ratio for prior tinnitus of early-onset dementia patients versus controls stratified by sex, age group and the presence of co-morbidities. There was no statistically significant association between early-onset dementia and interaction terms of age * tinnitus, sex * tinnitus, hypertension * tinnitus, hyperlipidaemia * tinnitus, and hearing loss * tinnitus. In addition, we found that the association of early-onset dementia with prior tinnitus exists regardless whether there was the presence of hypertension, hyperlipidaemia or hearing loss.
The adjusted odds ratio (OR) for prior tinnitus of early-onset dementia patients vs. controls stratified by sex, age group and the presence of co-morbidities.
*Denotes p value for interaction term.
To our knowledge, this study may represent the first population-based retrospective study to explore a possible association between tinnitus and subsequent early-onset dementia. We found that pre-existing tinnitus was significantly associated with dementia occurrence in the population aged 30–64 years of age, Tinnitus was associated with a 63% higher risk of early-onset dementia.
Dementia is generally regarded as a multifactorial disease, and its incidence increases with age. Several pathologies have been observed to contribute to the development of dementia, including neurodegenerative proteinopathies, vascular disease, dysregulated inflammation, etc. There is usually a considerable delay in the diagnosis of dementia, especially for early-onset dementia, which is estimated to take at least 2–4.4 years after the first onset of its various symptoms 21 – 23 . The delays may arise out of low prevalence causing a low index of suspicion among younger age groups, the large variety of etiologies, and confounding with neuropsychiatric symptoms and consequent misdiagnosis of early-onset dementia. Subtle neuro-pathologic changes usually precede a definitive diagnosis of dementia. If the associated neuropathology also involves the neural circuitry that triggers tinnitus, it appears plausible that tinnitus may precede or coexist with clinically detectable dementia symptoms such as impairment of memory, early signs of deterioration of executive functions, and impairments in visuoconstructional/ perceptual-motor functions, language functions, and social cognition which typically manifest in the later stages of dementia.
Cognitive impairment has been reported as a common occurrence among tinnitus patients 13 – 17 , 24 . Mild cognitive impairments (MCI), an intermediate state before dementia patients transition into clinically evident dementia, has been reported among patients with tinnitus. Other studies have shown that tinnitus is associated with cognitive deficits, and that tinnitus patients on the severe end of spectrum are at high risk of serous cognitive deficits 18 , 19 . However, the causal mechanism that links tinnitus and dementia remain elusive.
Both tinnitus and dementia represent clinical manifestations of heterogeneous pathologies involving complex neurological and functional processes. Tinnitus is linked to dysregulated neural synchrony across neural ensembles along the auditory pathway 25 . Accumulating evidence shows that tinnitus may occur concurrent with structural and functional disruptions of a diverse range of neuro-sensory structures, ranging from the peripheral and central auditory pathways to areas of the brain that are unrelated to normal hearing and processing of auditory stimuli. Animal and human neuroimaging studies have revealed neural tissue changes similar to those observed in tinnitus-associated areas of the brain in unrelated areas including those associated with cognition impairment and/or dementia, including the ventromedial prefrontal cortex 26 , parietal cortex 27 , anterior cingulate cortex 13 , 17 , 28 , prefrontal cortex 29 , amygdala 17 , 30 , hippocampus 13 , 17 , 30 , nucleus accumbens 31 , insula 13 , 17 , and thalamus 31 . Imaging studies have shown pathological changes in the hippocampus, amygdala and prefrontal cortex in the preclinical phase of dementia 32 – 37 . Autopsy studies of patients with tinnitus and cognitively normal brain function before death also show proteinopathy or accumulation of abnormal protein aggregates in the brain areas related to tinnitus 38 . These results suggest a shared neuronal pathology between tinnitus and dementia, and support the hypothesis that tinnitus may precede or occur concurrent with subclinical or early-onset dementia. One research implication of our study finding and the related hypothesis is to look for evidence of abnormal protein aggregates in tinnitus patients with normal cognition to explore the potential role of dementia-associated proteinopathy in the pathogenesis of tinnitus.
Our study may be the first and largest population-based study to examine the association between tinnitus and dementia in patients aged under 65 years. A key strength of the study is the ability to pool a large number of early-onset dementia cases from a nationwide medical care dataset. Another strength is the universal access, national health insurance system source of the data, enabling access to uninterrupted follow-up data of the entire population with comprehensive longitudinal data on comorbidities and demographic characteristics.
There are still some study limitations. First, an epidemiological association by itself does not imply biological causality. Our finding suggests a potential link between tinnitus and early-onset dementia which may serve as an early warning sign to elevate awareness of dementia risk among tinnitus patients to proactively watch for the early signs of dementia among young and middle-aged patients. Second, because of data privacy and confidentiality under the Personal Data Protection Law and related regulations of the NHIRD, it was not possible to validate or supplement the claims data by direct patient contact. Therefore, critical items of data on the risk factors for dementia and the severity of tinnitus that are not documented in medical claims could not be obtained. These data include, such as family history, laboratory data, genetic test data (such as Apolipoprotein E ), severity of dementia, imaging test results, hearing test results, and tinnitus severity scores. Further several known confounders involved in the development of dementia were not accounted for in the analyses, including smoking, educational level, occupation, specific environmental features obesity, hearing loss, and alcohol intake. To mitigate selection bias, we used the available data on the factors known to influence dementia occurrence to select propensity score–matched control patients. Third, this study used a case–control method which did not allow to establish causation due to its retrospective nature. Finally, the present study found that coronary heart disease was negatively associated with early dementia while no significant associations of early dementia with hypertension, diabetes or hyperlipidaemia were observed. We speculate that the medications such as statins or aspirin for the treatment of coronary heart disease could be one explanation for low odds of early-onset dementia on patients with coronary heart disease. Further studies are encouraged to explore the relationship between statins or aspirin and early-onset dementia.
Our findings showed that pre-existing tinnitus is associated with a 1.675-fold increase in the risk of early-onset dementia among the young and middle-aged population. Additional studies in other populations are encouraged to confirm the relationship between early-onset dementia and tinnitus and to explore the possible mechanisms behind this relationship. Further studies are also needed to clarify the shared underlying pathophysiology between tinnitus and dementia, and to explore whether early detection and treatment of tinnitus may prevent or delay early onset dementia.
Author contributions
Y.F. and H.C. participated in the design of the study and helped to draft the manuscript. Y.W. performed the statistical analysis and helped to draft the manuscript. T.H., S. and C.S. conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors reviewed the manuscript.
No funding source.
Competing interests
The authors declare no competing interests.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Early onset Alzheimer disease (EOAD) is a neurodegenerative dementing disorder that is relatively rare (<1% of all Alzheimer cases). Various genetic mutations of the presenilin 1 (PSEN1) and presenilin 2 (PSEN2) as well as the amyloid precursor protein (APP) gene have been implicated.Mutations of PSEN1 and PSEN2 alter γ-secretase enzyme that cleaves APP resulting in increase in the relative ...
The patient was finally diagnosed with early-onset dementia and temporal lobe epileptiform abnormalities. This case emphasises the need for diagnostic consideration of dementia in cognitively impaired patients, even when they are not of an advanced age. Keywords: epilepsy and seizures, memory disorders (psychiatry)
Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer's disease (AD) (1, 2). This patient's symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in ...
Dementia in young individuals can be a diagnostic challenge given its often non-specific symptoms and findings. As a consequence, patients with early-onset dementia are more often misdiagnosed than those with late-onset dementia. 1 Although there is a set of known clinical criteria for early-onset dementia, misdiagnoses can occur even in ...
Presentation of Case. Dr. David L. Perez: A 62-year-old, left-handed man was seen in the memory disorders clinic of this hospital because of memory loss, personality changes, and odd behavior ...
A previous study conducted on nondemented young adults with Down syndrome compared their results with that of studies conducted on autosomal dominant early-onset AD patients, where two groups of subjects concordantly showed predominant striatal uptake. 8 Indeed, previous studies on autosomal early-onset AD patients consistently showed high ...
Frontotemporal dementia (FTD) is a neurodegenerative disease with behavioral and language variants [ 1 ]. One clinical feature of FTD is an early onset; most patients experience disease onset between the ages of 45 and 65 years [ 2, 3 ]. However, some cases of extremely early onset have been reported in which the initiation of neurodegeneration ...
Case study: Presentation. A 63-year-old Caucasian male (J.K.) presented to his PCP with short-term memory loss over the last 2 years ... a referral for more comprehensive genetic testing may be considered by the HCP if there is a family history of early-onset AD or dementia.
Abstract Objectives: The aim was to explore the experiences of a caregiver of a patient with early onset dementia (EOD) and the needs of patient and caregiver. Methods: A single case study design was used to explore (1) unmet needs of patient and caregiver and (2) caregiver's experiences of transitions in care and health care services.
The purpose of this Longitudinal Early-onset Alzheimer's Disease Study (LEADS) is designed to look at disease progression in individuals with early onset cognitive impairment . Clinical/cognitive, imaging, biomarker, and genetic characteristics will be assessed across three cohorts: (1) early onset Alzheimer's Disease (EOAD) participants, (2 ...
Short-term memory decline is the typical clinical manifestation of Alzheimer's disease (AD). However, early-onset AD usually has atypical symptoms and may get misdiagnosed. In the present case study, we reported a patient who experienced symptoms of memory loss with progressive non-fluent aphasia accompanied by gradual social withdrawal. He did not meet the diagnostic criteria of AD based on ...
This case-control study used data from the Taiwan National Health Insurance Research Database, identifying 1308 patients with early-onset dementia (dementia diagnosed before 65 years of age) and ...
Findings In this case-control study of 542 individuals, early-onset DLB was often misdiagnosed; certain motor features and, to a lesser extent, neuropsychiatric features were associated with a diagnosis of early-onset DLB over early-onset Alzheimer disease dementia. Late-onset DLB had more prominent amnestic features but lower rates of ...
Risk of early-onset dementia (EOD) might be modified by environmental factors and lifestyles, including diet. The aim of this study is to evaluate the association between dietary habits and EOD risk. We recruited 54 newly-diagnosed EOD patients in Modena (Northern Italy) and 54 caregivers as controls. We investigated dietary habits through a ...
These three case studies help you to consider different situations that people with dementia face. They are: Raj, a 52 year old with a job and family, who has early onset dementia. Bob and Edith, an older married couple who both have dementia and are struggling to cope, along with their family. Joan, an older woman, who lives alone and has just ...
Objectives: The aim was to explore the experiences of a caregiver of a patient with early onset dementia (EOD) and the needs of patient and caregiver. Methods: A single case study design was used to explore (1) unmet needs of patient and caregiver and (2) caregiver's experiences of transitions in care and health care services. A qualitative analysis was used to examine the data.
Introduction. Frontotemporal dementia (FTD) refers to a group of dementias characterized by degeneration in the frontal and temporal lobes of the brain ().Rates of early-onset FTD are high with 13% of individuals with FTD being under the age of 50 ().Younger cases of FTD, with onset before the age of 30, tend to exhibit frequent abrupt mood changes, increased aggression, behavioral ...
The Longitudinal Early-Onset Alzheimer's Disease Study (LEADS) is a two-year observational study designed to look at disease progression in adults with early-onset Alzheimer's disease. ... Two or more first-degree relatives with a history of early-onset dementia (unless known mutations in APP, PSEN1, PSEN2, MAPT, GRN, and C9ORF72 have been ...
Early-onset dementia (EOD) starts below the age of 65 years. It accounts for 4-10% of all cases of dementia. EOD has significant psychosocial consequences because it affects people in their most ...
Last modified on Mon 1 Apr 2024 17.21 EDT. A smartphone app could help detect a leading cause of early-onset dementia in people who are at high risk of developing it, data suggests. Scientists ...
Early-onset Alzheimer's disease (EOAD) with onset <65 years of age, while overshadowed by the more common late-onset AD (LOAD), differs significantly from LOAD. EOAD comprises about 5% of AD and is associated with delays in diagnosis, an aggressive course, and age-related psychosocial needs. One source of confusion is that a substantial ...
Both early-onset Alzheimer's disease (EOAD) and late-onset AD (LOAD) showed increased white matter hyperintensity (WMH) volume compared with their age-matched cognitively unimpaired participants. EOAD and LOAD exhibited distinct patterns of WMH distribution, with EOAD showing a posterior-focused pattern and LOAD displaying a wider distribution ...
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the presence of these protein changes (A+ and T+) remains unclear. By performing a systematic review and meta-analysis, we investigated the risk of mild cognitive impairment (MCI) or dementia ...
Researchers from Loughborough University and the University of Cambridge have found that a loss of visual sensitivity could predict dementia 12 years before it is diagnosed. The study, published in the journal Nature, suggests that visual problems may be an early indicator of cognitive decline. This is because amyloid plaques - proteins that ...
Case Outcome. The client is transferred to a long term care facility. His daughter visits him in the facility until his death, one year later. She has joined a support group to assist other caregivers experiencing the strain and loss of caring for a loved one with Alzheimer's disease. Study with Quizlet and memorize flashcards containing terms ...
Alzheimer's: I know I'm going to have early onset dementia. Alexandra Bánfi - BBC News. Sat, April 20, 2024, 11:04 PM PDT · 4 min read. James Bexon knows he will develop Alzheimer's between the ages of 53 and 63 [Family photo] James Bexon was just 33 when he was told he had between 20 and 30 years before developing Alzheimer's. His father was ...
A recent study suggests that people with poor vision sensitivity are more likely to go on and develop Alzheimer's disease, and that a simple eye test could help predict the onset of this condition.
Jonathan Schott, the chief medical officer at Alzheimer's Research UK, will lead a trial on the most promising blood biomarker in tests on 1,100 people across the UK. The second trial will test ...
In a study of more than 7,000 Norwegians in 305 occupations, those who held the least mentally demanding jobs had a 66% greater risk of mild cognitive impairment, and a 31% greater risk of ...
Early-onset dementia is defined as dementia diagnosed before the age of 65 3, 4 The symptoms of early-onset dementia are similar to those observed among the elderly, ... Third, this study used a case-control method which did not allow to establish causation due to its retrospective nature. Finally, the present study found that coronary heart ...