- Reference Manager
- Simple TEXT file

People also looked at
Mini review article, gas sensing performance of in 2 o 3 nanostructures: a mini review.

- 1 Hubei Key Laboratory for Processing and Application of Catalytic Materials, School of Physics and Electronic Information, Huanggang Normal University, Huanggang, China
- 2 Hubei Key Laboratory of Ferro and Piezoelectric Materials and Devices, Faculty of Physics and Electronic Sciences, Hubei University, Wuhan, China
Effective detection of toxic and hazardous gases is crucial for ensuring human safety, and high-performance metal oxide-based gas sensors play an important role in achieving this goal. In 2 O 3 is a widely used n-type metal oxide in gas sensors, and various In 2 O 3 nanostructures have been synthesized for detecting small gas molecules. In this review, we provide a brief summary of current research on In 2 O 3 -based gas sensors. We discuss methods for synthesizing In 2 O 3 nanostructures with various morphologies, and mainly review the sensing behaviors of these structures in order to better understand their potential in gas sensors. Additionally, the sensing mechanism of In 2 O 3 nanostructures is discussed. Our review further indicates that In 2 O 3 -based nanomaterials hold great promise for assembling high-performance gas sensors.
1 Introduction
In recent decades, there has been growing attention to the effective monitoring of air quality due to the increasingly serious environmental problems ( Ma et al., 2016 ; Zhang et al., 2018 ; Ge et al., 2019 ). Even toxic gases with low concentrations can be harmful to human health ( Park et al., 2016 ; Cordero et al., 2018 ; Zhou et al., 2022 ). For example, the toxic gas formaldehyde (HCHO) can cause serious blurred vision and vertigo when its concentration exceeds 0.1 mg/m 3 ( Peng and Huang, 2022 ). In the workplace, the concentration of n-butanol should be kept below 152 mg/m 3 to ensure the safety of human lives ( Zhao et al., 2021 ). In addition, a high risk of explosion may occur if the concentration of H 2 reaches 4%–75% in the air ( Phanichphant, 2014 ). It should be noted that many toxic, hazardous, or flammable gases are odorless, colorless, and tasteless, which means they cannot be detected by humans directly ( Wetchakun et al., 2011 ; Chi et al., 2014 ; Shi et al., 2018 ). Therefore, high-performance gas sensors are of great importance to effectively detect these gases and their concentrations in the air.
Metal oxide-based gas sensors have become a popular research topic in recent years due to their advantages of low cost, easy of fabrication, low power consumption and high sensor response to a wide range of gases ( Xu and Cheng, 2016 ; Lu et al., 2019 ; Nikolic et al., 2020 ). Nanostructured metal oxides always presented high specific surface areas and could provide abundant active sites on their surfaces ( Zhang et al., 2017 ; Srinivasan et al., 2019 ; Walker et al., 2019 ). This positive factor effectively promotes the adsorption and the diffusion of gas molecules in the sensing materials, resulting in the excellent gas sensing performances of nanostructured metal oxides. For instance, in the study conducted by Zhu et al., CuO nanoflowers demonstrated a significantly higher sensor response of 123.4 to 50 ppm H 2 S at 80°C compared to CuO-based microspheres, which only showed a sensor response of 4.36 ( Hu et al., 2017 ; Hu et al., 2018a ). Chen et al. also reported superior sensing performance of ZnO-based nanostructures with a sensor response as high as 6043 to 100 ppm triethylamine (TEA) at an optimal working temperature of 183.5°C, when compared to ZnO films (with a response of ∼22.5) or hierarchical ZnO microspheres (with a response of 242) ( Shen et al., 2018 ; Liu et al., 2021a ; Li et al., 2021 ). Furthermore, the net-like SnO 2 nanoarrays showed a response time of only 16.3 s to 10 ppm H 2 S at 350°C, which was approximately ten times lower than that of SnO 2 films (167.8 s) ( Ge et al., 2022 ). Thus, outstanding gas sensing properties could be expected through synthesizing nanostructured metal oxides.
In 2 O 3 is another popular n-type metal oxide that possesses a wide band gap of 3.5–3.7 eV ( Vuong et al., 2014 ; Han et al., 2015 ; Park, 2017 ). Its outstanding thermal stability, high conductivity, and excellent chemical/physical properties make it a promising candidate for gas detection ( Liang et al., 2015 ; Kumar et al., 2021 ; Meng et al., 2022 ). For example, Zhang et al. successfully prepared Ni-doped In 2 O 3 -based nanocubes through a hydrothermal method, achieving effective detection of 20 ppm HCHO with a response time of 76 s at room temperature ( Zhang et al., 2020 ). The research conducted by Han et al. demonstrated that the sensor response of In 2 O 3 nanorods doped with Co could be improved to 23.2 towards 10 ppm HCHO at 130 C ( Wang et al., 2018 ). Additionally, flower-like In 2 O 3 nanomaterials exhibited a sensor response and response time of 3.1 and 53 s, respectively, to 0.5 ppm isoprene at 190°C ( Han et al., 2020 ). A Google Scholar survey with keywords of “nano + In 2 O 3 +gas sensor” revealed that from 2017 to 2022, there were 826, 878, 919, 1060, 1210, and 1520 papers published on the topic. Although the data obtained may not be entirely accurate, the increasing number of published references highlights the growing attention given to In 2 O 3 -based gas sensors in recent years. Therefore, summarizing the recent developments in In 2 O 3 -based gas sensors would be meaningful to better understand their advantages in gas sensing.
In this paper, we have chosen several highly cited published references to conduct a mini review on typical In 2 O 3 -based gas sensors. Our focus was mainly on summarizing and comparing the high-performance characteristics of these gas sensors. Furthermore, we presented the methods used to prepare various In 2 O 3 -based materials. Additionally, we provided a brief review of the gas sensing mechanism for In 2 O 3 -based gas sensors.
2 Research status of gas sensing performances of recent In 2 O 3 nanostructures
2.1 pristine in 2 o 3 -based nanomaterials.
A novel self-heated gas sensor for detecting ethanol at room temperature was assembled by Nguyen et al. using In 2 O 3 nanowires ( Son et al., 2022 ). The sensor utilized the Joule effect generated by the In 2 O 3 nanowires under an operating voltage to achieve self-heating during operation. The In 2 O 3 nanowires were synthesized via a one-chip growth technique of thermal evaporation. The gap between the prepared electrodes was designed to be 10, 30 or 40 µm ( Figures 1A, B ), with the corresponding devices labeled as sensor-10, sensor-30, or sensor-40, respectively. Results showed that the well-crystallized In 2 O 3 nanowires were successful to bridge the gap of electrodes ( Figures 1C–E ). The In 2 O 3 nanowires had an average diameter of ∼100 nm and an average length of over 10 µm ( Figure 1F ). Sensor-40 exhibited better ethanol sensing performance compared to sensor-10 or sensor-30. Sensor-40 showed a superior sensing performance to 10–2000 ppm ethanol compared to NH 3 under a supplied power of 1.06 mW ( Figures 1G–J ). The sensor response of sensor-40–2000 ppm ethanol was ∼1.45. Meanwhile, the sensor response of sensor-40–1000 ppm ethanol was higher than that to 1000 ppm acetone, CO, H 2 S or NH 3 ( Figure 1K ), indicating good gas selectivity of the In 2 O 3 nanowires. Additionally, the sensor response of sensor-40 to ethanol was not significantly affected by humidity levels of 60%, 70%, or 80% ( Figure 1L ).
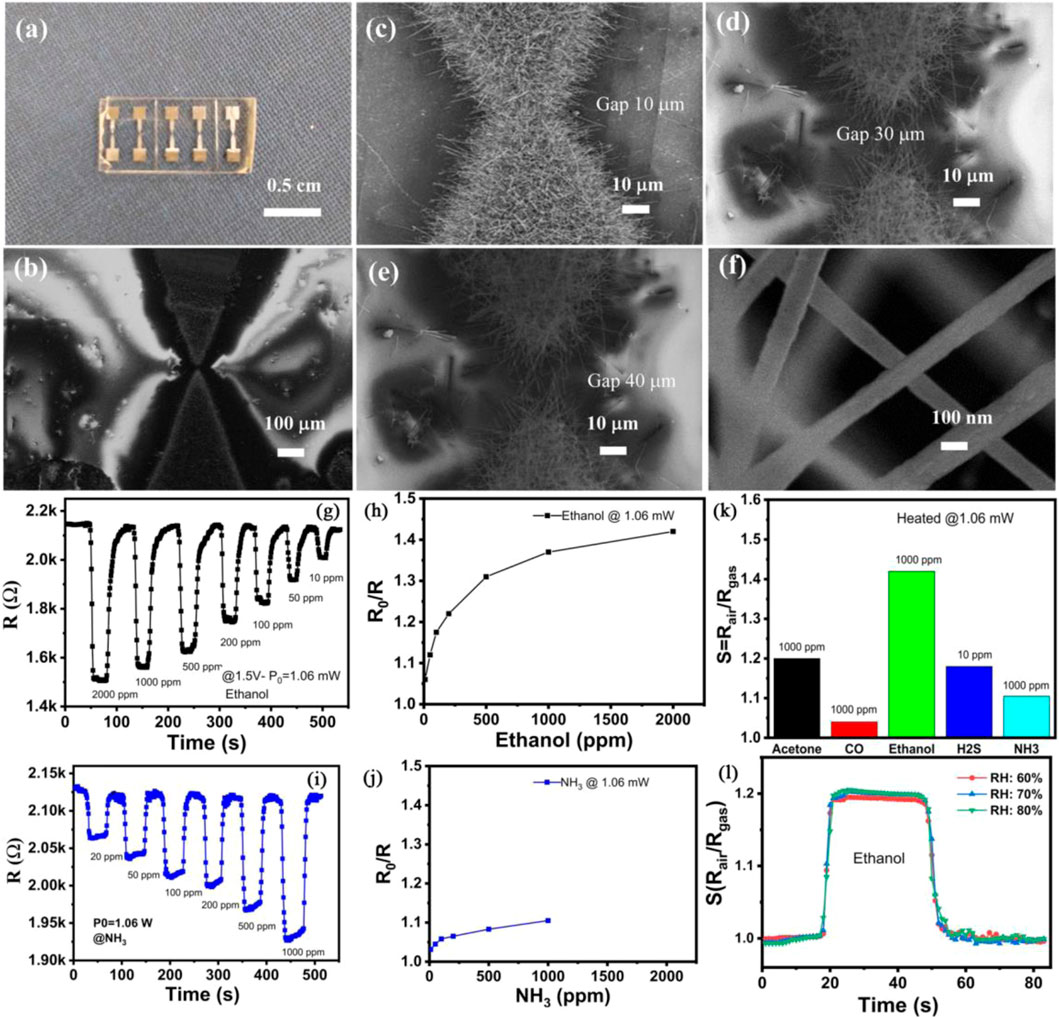
FIGURE 1 . (A) Digital image of the assembled gas sensor. (B) SEM image of a gas sensor. (C–E) SEM image of In 2 O 3 nanowires grown at electrodes with gasps of 10, 30 and 40 μm, respectively. (F) High magnification SEM of In 2 O 3 nanowires. (G, H) Dynamic gas sensing performance and sensor response (I, J) of sensor-40 to 10–2000 ppm ethanol or NH 3 at room temperature. (K) Selective gas sensing performance of sensor-40. (L) ethanol gas sensing behavior of sensor-40 under different humidity. Reprinted with permission from ref. ( Son et al., 2022 ). Copyright 2022, Elsevier.
Shboul et al. have developed a novel gas sensor mainly composed of solution-printed In2O3 nanoparticles ( Al Shboul and Izquierdo, 2021 ). In their study, In 2 O 3 nanoparticles, copper acetate (CuAc), graphite (Gt) flakes, and polystyrene (PS) were added to 10 mL of xylene to form a stable paste. The paste was then spread over a flexible PET substrate with prepared carbon electrodes to assemble the gas sensor. The In 2 O 3 -based sensor (SS), without CuAc, showed unresponsive sensing performance to H 2 S showed unresponsive sensing behavior towards H 2 S with concentrations below 100 ppb. The sensor response of the SS was only 2–100 ppb H 2 S, and the response time was as long as ∼25 min. The In 2 O 3 -based sensing material with 10 wt% CuAc (MS10) exhibited better sensing performance than sensing materials with 2 wt% CuAc (MS2), 25 wt% CuAc (MS25), or 50 wt% CuAc (MS50). The sensor response of the MS10 was found to be ∼18–100 ppm H 2 S at room temperature, with a relative humidity of ∼30%.
The study by Pham et al. also showed the potential of porous In 2 O 3 nanorods for detecting CO gas at 350°C ( Van Tong et al., 2021 ). The nanorods were synthesized via a hydrothermal method at 180°C for 10 h, and showed a sensor response of 3.46–400 ppm CO with a response and recovery time of 41/43 s. Similarly, Zhang et al. employed a surfactant-assisted co-precipitation method to prepare hierarchical branch-like In 2 O 3 nanomaterial for detecting ozone (O 3 ) ( Sui et al., 2021 ). The resulting hierarchical branch-like In 2 O 3 showed a high sensor response of 44–100 ppb O 3 at its optimum working temperature of 70°C.
2.2 In 2 O 3 -based composites
2.2.1 in 2 o 3 composited with noble metals.
Wang et al. have investigated the impacts of Au, Ag, Pt, and Pd on the ethanol gas sensing performance of long-range mesoporous In 2 O 3 ( Cheng et al., 2021 ). They synthesized the ordered mesoporous In 2 O 3 by replicating the structure of SBA-15, and then prepared Au, Ag, Pt, or Pd-doped In 2 O 3 through an in-situ doping routine. The mesoporous In 2 O 3 doped with Pd exhibited a specific surface area of 94.22 m 2 /g, significantly higher than that of pristine In 2 O 3 (64.55 m 2 /g), Au-doped In 2 O 3 (78.29 m 2 /g), Ag-doped In 2 O 3 (67.52 m 2 /g), or Pt-doped In 2 O 3 (76.41 m 2 /g). Similarly, the pore diameter for the Pd-doped In 2 O 3 was 3.6 nm, also larger than that for pristine In 2 O 3 (2.6 nm). The high specific surface area and large pore diameter are favorable for improving gas molecule adsorption and diffusion in the sensing material. Consequently, the concentration of chemisorbed oxygen species in Pd-doped In 2 O 3 was the highest among all samples, reaching 54.8%. The sensor based on Pd-doped In 2 O 3 also demonstrated the best performance for 100 ppm ethanol at an operating temperature of 200–350°C. The sensor response of Pd-doped In 2 O 3 was 39 at 250°C, higher than that of pristine In 2 O 3 (∼5), Au-doped In 2 O 3 (∼7.5), Ag-doped In 2 O 3 (∼15), or Pt-doped In 2 O 3 (∼17.5). Furthermore, Pd-doped In 2 O 3 exhibited higher sensor response to 100 ppm ethanol than to 100 ppm ammonia, methanol, toluene, benzene, acetone, formaldehyde, or ethanol, revealing excellent sensing selectivity.
The research conducted by Zhang et al. showed that an excellent hydrogen sensing performance could be achieved with the use of Tb-doped In 2 O 3 nanocomposites decorated with Ag (Ag-Tb-In 2 O 3 ) ( Bai et al., 2022 ). In their study, the Ag-modified Tb-doped In 2 O 3 nanocomposite was successfully synthesized through a hydrothermal process combined with a facile annealing method. Interestingly, the material exhibited two coexisting crystalline phases, including the hexagonal phase In 2 O 3 (h-In 2 O 3 ) and the cubic phase In 2 O 3 (c-In 2 O 3 ). Further analysis of the XRD patterns confirmed that the Tb was doped within the In 2 O 3 while the Ag was decorated on the surface of the nanocomposite. The Tb doping was found to reduce the grain sizes of both c-In 2 O 3 and h-In 2 O 3 , resulting in the generation of oxygen vacancies in the nanocomposite. Consequently, the Ag-Tb-In 2 O 3 nanocomposite exhibited a better sensing performance for 500 ppb H 2 at operating temperatures ranging from 120 to 200°C. The sensor response of the Ag-Tb-In 2 O 3 was found to be 4.63–500 ppb H 2 at its optimum operating temperature of 160°C, which is higher than that of the pristine In 2 O 3 (∼1.5), Tb-doped In 2 O 3 (∼2.5), or Ag-decorated In 2 O 3 (∼3.5).
2.2.2 In 2 O 3 composited with metal oxides
The study by Xie et al. demonstrated that the hydrogen sensing performance of In 2 O 3 nanotubes could be significantly enhanced by co-doping them with PdO and NiO ( Luo et al., 2021 ). Pristine In 2 O 3 and In 2 O 3 doped with NiO, PdO or NiO/PdO were synthesized using an electrospinning method. All four samples exhibited sensing performances to 5 ppm hydrogen gas at 160–300°C. Among them, the PdO/NiO-In 2 O 3 nanotubes showed the highest sensor response, with a value of 487.52 to 5 ppm H 2 at 160°C. In contrast, the sensor responses of pristine In 2 O 3 , NiO-In 2 O 3 , and PdO-In 2 O 3 were lower than 20. The response times of the pristine In 2 O 3 and NiO-In 2 O 3 were also relatively long, at 153 s and 97 s, respectively, which might not be suitable for rapid detection of hydrogen gas in practical applications. However, the addition of PdO significantly reduced the response time to only 1 s for both PdO-In 2 O 3 and PdO/NiO-In 2 O 3 , demonstrating the effectiveness of PdO in improving the response time of the In 2 O 3 -based material. Additionally, the incorporation of NiO reduced the recovery time of pristine In 2 O 3 (or PdO-In 2 O 3 ) from the original 232 s (or 674 s) to 168 s (or 336 s).
In a study by Wang et al., it was found that the ethanol gas sensing performance of In 2 O 3 nanoflowers could be significantly improved by combining them with metal-organic frameworks (MOF)-derived CO 3 O 4 ( Han et al., 2021 ). The In 2 O 3 nanoflowers were synthesized via a hydrothermal route at 150°C for 10 h. At the optimum operating temperature of 280°C, the CO 3 O 4 -In 2 O 3 nanoflowers exhibited a sensor response of over 5000 to 100 ppm ethanol. Similarly, the MOFs-derived porous Au@Cr 2 O 3 -In 2 O 3 nanorods were found to effectively detect 1 ppm isoprene with a sensor response of 6.4 at 180°C ( Wu et al., 2022 ).
2.2.3 In 2 O 3 composite with other materials
Song et al. reported an outstanding methanol sensing performance of In 2 O 3 nanocubes composited with Ti 3 C 2 T x MXene at room temperature ( Liu et al., 2021b ). The In 2 O 3 nanocubes were synthesized via a hydrothermal route at 140°C for 24 h, and the multilayer Ti 3 C 2 T x MXene was synthesized through etching the bulk MAX (Ti 3 AlC 2 ) phase with 10 mL HF solution (40 wt%). The In 2 O 3 nanocubes were modified with a cationic surfactant, (3-aminopropyl) triethoxysilane (APTES), to positively charge their surfaces. The positively charged In 2 O 3 nanocubes were then mixed with the Ti 3 C 2 T x MXene with negatively charged surfaces, and the mixture was treated under 120°C for a hydrothermal reaction. The In 2 O 3 /Ti 3 C 2 T x composite exhibited typical n-type gas sensing performance to ethanol at room temperature. However, the resistance of the composite after exposure to ethanol was unable to fully recover to its initial level in air, likely because residual methanol was not desorbed from the active site on the surface of the composite at room temperature. The sensor response of the composite to 5 ppm ethanol was ∼29.6 with a response/recovery time of 6.5/3.5 s. The composite also showed a promising gas sensing performance to 5–100 ppm ethanol and was not affected by relative humidity of ∼25–70%. The functional groups of the Ti 3 C 2 T x would be helpful in accelerating the adsorption of ethanol molecules, while the heterojunction between the In 2 O 3 and the Ti 3 C 2 T x could be another factor improving the ethanol gas sensing performance of the composite.
Song et al. also found that composting In 2 O 3 nanospheres with Ti 3 C 2 T x MXene nanosheets and Au could improve their HCHO sensing performance ( Liu et al., 2022 ). The Au-In 2 O 3 /Ti 3 C 2 T x composite exhibited a sensor response of approximately 31%, which is higher than that of the pristine Ti 3 C 2 T x (only ∼3.6%). Additionally, the response time and recovery time of the composite were as short as 5 s and 4 s, respectively, to 5 ppm HCHO at room temperature.
Zhu et al. reported an effective enhancement of the H 2 S sensing performance of In 2 O 3 nanocubes through the use of carbon canohorn (CNH) composites ( Zhou et al., 2022 ). The composite with a CNH mass concentration of 2 wt% (In 2 O 3 /CNH (2 wt%)) exhibited a high sensor response of 2906 to 2 ppm H 2 S at an optimum operating temperature of 70°C. Furthermore, the sensor response of the In 2 O 3 /CNH (2 wt%) to water vapor with 11%–95% humidity was not over 1.52, indicating that humidity did not significantly affect the sensing performance of the composite to H 2 S.
The use of In 2 O 3 -based nanomaterials as gas sensors has been well established, as discussed in the references above. The development of uniform two-dimensional In 2 O 3 nanomaterials may also lead to surprising gas sensing properties due to their large contact surface with air. Additionally, new materials can be explored to establish novel In 2 O 3 -based composites with heterostructure interfaces, leading to high-performance gas sensors. Careful investigation of the morphology and size effects of the second phase in the composite is essential to screen the best configuration for further improving gas sensing behavior. Machine learning algorithms can be applied to prepare In 2 O 3 -based gas sensors with excellent selectivity, and assembling several gas sensors in a gas sensor array can build a smart gas sensing system capable of simultaneously detecting several gases under mixed gas atmospheres.
3 Gas sensing mechanism of In 2 O 3 nanostructures
Understanding the gas sensing mechanism is crucial for the development of high-performance gas sensors based on In 2 O 3 . In general, the sensing performance of metal oxide-based sensors is attributed to the redox reaction between the adsorbed oxygen species (O 2 − , O − and O 2− ) and the target gas molecules ( Wang et al., 2018 ; Yang et al., 2018 ; Yang et al., 2019 ; Wang et al., 2020 ). For example, flower-like In 2 O 3 nanostructure have been shown to exhibit a promising sensor response of 3.1 towards 0.5 ppm isoprene at 190°C ( Han et al., 2020 ). In this case, oxygen gas is adsorbed on the active site, forming the adsorbed oxygen molecule in air (Eq. 1 ). Electrons are then transferred from the conductive bands of the flower-like In 2 O 3 nanostructure to the adsorbed oxygen molecule, forming adsorbed oxygen species (Eqs. 2–4 ; Figure 2A ). This leads to a bend in the band structure and the formation of a thick space-charge depletion layer at the surface region of the In 2 O 3 nanostructure ( Figure 2C ). Moreover, a high potential barrier is created between the contact flower-like In 2 O 3 nanostructure, resulting in a high resistance in air.
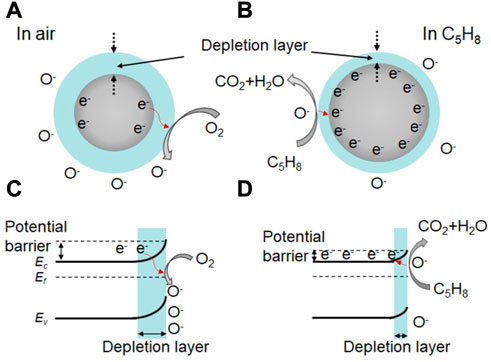
FIGURE 2 . (A, B) Schematic diagram of the isoprene sensing mechanism of In 2 O 3 nanostructure at 190°C, and the corresponding bending of band structures at the surface region of In 2 O 3 nanosturcture (C, D) .
When the target gas of isoprene is introduced into the testing chamber, the pre-adsorbed oxygen species react with the isoprene molecules (Eqs. 5, 6 ; Figure 2B ), releasing trapped electrons back to the In 2 O 3 nanostructure. As a result, the thickness of the space-charge depletion layer decreases ( Figure 2D ), and the potential barrier is also reduced between the contact flower-like In 2 O 3 nanostructure. This process leads to an effective reduction in the resistance of the sensor and a high sensor response to isoprene Similar theories apply to other In 2 O 3 -based sensors, including Ce-doped In 2 O 3 microspheres, CeO 2 -loaded In 2 O 3 hollow spheres, mesoporous In 2 O 3 , or Co-doped In 2 O 3 nanorods, which exhibit a promising sensing performance to glycol, H 2 , ethanol, or HCHO, respectively ( Hu et al., 2018b ; Liu et al., 2018 ; Wang et al., 2018 ; Cheng et al., 2021 ).
The high specific surface areas of the In 2 O 3 nanomaterials have been found to be beneficial in improving the adsorption of gas molecules ( Han et al., 2018 ; Tao et al., 2019 ; Cao et al., 2020 ). This increased surface area allows for more gas molecules to access the surface of In 2 O 3 nanomaterials, promoting the redox reaction between the adsorbed oxygen species and the target gas molecules. Additionally, the formation of a heterojunction between the main phase of In 2 O 3 and the introduced second phase in the composite can promote the transfer of electrons and holes across their surfaces, leading to the bending of their energy bands and the building of a high potential barrier ( Du et al., 2015 ; Ou et al., 2022 ). Modulating the height of this potential barrier can dramatically change the resistance of the composite, leading to improved sensing performance ( Feng et al., 2015 ). Overall, these two factors are commonly responsible for the high sensing performance of In 2 O 3 -based composites.
4 Conclusion
In this review, we provide a brief overview of current research on gas sensors based on In 2 O 3 nanostructures. Our analysis shows that uniform In 2 O 3 nanostructures with high specific surface areas generally exhibit superior gas sensing performance due to enhanced gas molecule adsorption and diffusion. Furthermore, the gas sensing properties of In 2 O 3 -based materials can be effectively enhanced by creating composites. Adding noble metals is a viable strategy for improving the interaction between gas molecules and In 2 O 3 , and metal oxides or Mxenes are widely used to further improve the gas sensing properties of In 2 O 3 nanostructures. The superior gas sensing performance of composites is primarily attributed to the high specific surface area and the formation of heterojunctions. Therefore, In 2 O 3 -based materials have immense potential for developing gas sensors with exceptional sensing capabilities.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 51802109, 51972102, 52072115, and U21A20500) and the Department of Science and Technology of Hubei Province (Grant No. 2022CFB525).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al Shboul, A. M., and Izquierdo, R. (2021). Printed chemiresistive In2O3 nanoparticle-based sensors with ppb detection of H2S gas for food packaging. ACS Appl. Nano Mater. 4, 9508–9517. doi:10.1021/acsanm.1c01970
CrossRef Full Text | Google Scholar
Bai, J., Kong, Y., Liu, Z., Yang, H., Li, M., Xu, D., et al. (2022). Ag modified Tb-doped double-phase In2O3 for ultrasensitive hydrogen gas sensor. Appl. Surf. Sci. 583, 152521. doi:10.1016/j.apsusc.2022.152521
Cao, J., Zhang, N., Wang, S., Chen, C., and Zhang, H. (2020). Researching the crystal phase effect on gas sensing performance in In2O3 nanofibers. Sensors Actuators B Chem. 305, 127475. doi:10.1016/j.snb.2019.127475
Cheng, P., Wang, Y., Wang, C., Ma, J., Xu, L., Lv, C., et al. (2021). Investigation of doping effects of different noble metals for ethanol gas sensors based on mesoporous In2O3. Nanotechnology 32, 305503. doi:10.1088/1361-6528/abf453
Chi, X., Liu, C., Liu, L., Li, S., Li, H., Zhang, X., et al. (2014). Enhanced formaldehyde-sensing properties of mixed Fe2O3–In2O3 nanotubes. Mat. Sci. Semicon Proc. 18, 160–164. doi:10.1016/j.mssp.2013.11.016
Cordero, J. M., Borge, R., and Narros, A. (2018). Using statistical methods to carry out in field calibrations of low cost air quality sensors. Sensors Actuators B Chem. 267, 245–254. doi:10.1016/j.snb.2018.04.021
Du, H., Wang, J., Sun, Y., Yao, P., Li, X., and Yu, N. (2015). Investigation of gas sensing properties of SnO2/In2O3 composite hetero-nanofibers treated by oxygen plasma. Sensors Actuators B Chem. 206, 753–763. doi:10.1016/j.snb.2014.09.010
Feng, C., Li, X., Ma, J., Sun, Y., Wang, C., Sun, P., et al. (2015). Facile synthesis and gas sensing properties of In2O3–WO3 heterojunction nanofibers. Sensors Actuators B Chem. 209, 622–629. doi:10.1016/j.snb.2014.12.019
Ge, C., Jin, C., Wang, M., Bai, L., Hussain, S., Qiao, G., et al. (2022). Template-derived net-like SnO2 nanoarrays for robust H2S sensing with broad-range linear response. Sensors Actuators B Chem. 366, 131991. doi:10.1016/j.snb.2022.131991
Ge, L., Mu, X., Tian, G., Huang, Q., Ahmed, J., and Hu, Z. (2019). Current applications of gas sensor based on 2-D nanomaterial: A mini review. Front. Chem. 7, 839. doi:10.3389/fchem.2019.00839
PubMed Abstract | CrossRef Full Text | Google Scholar
Han, B., Wang, J., Yang, W., Chen, X., Wang, H., Chen, J., et al. (2020). Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sensors Actuators B Chem. 309, 127788. doi:10.1016/j.snb.2020.127788
Han, D., Li, X., Zhang, F., Gu, F., and Wang, Z. (2021). Ultrahigh sensitivity and surface mechanism of gas sensing process in composite material of combining In2O3 with metal-organic frameworks derived Co3O4. Sensors Actuators B Chem. 340, 129990. doi:10.1016/j.snb.2021.129990
Han, D., Song, P., Zhang, S., Zhang, H., Xu, Q., and Wang, Q. (2015). Enhanced methanol gas-sensing performance of Ce-doped In2O3 porous nanospheres prepared by hydrothermal method. Sensors Actuators B Chem. 216, 488–496. doi:10.1016/j.snb.2015.04.083
Han, D., Zhai, L., Gu, F., and Wang, Z. (2018). Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sensors Actuators B Chem. 262, 655–663. doi:10.1016/j.snb.2018.02.052
Hu, J., Sun, Y., Xue, Y., Zhang, M., Li, P., Lian, K., et al. (2018). Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sensors Actuators B Chem. 257, 124–135. doi:10.1016/j.snb.2017.10.139
Hu, X., Zhu, Z., Chen, C., Wen, T., Zhao, X., and Xie, L. (2017). Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sensors Actuators B Chem. 253, 809–817. doi:10.1016/j.snb.2017.06.183
Hu, X., Zhu, Z., Li, Z., Xie, L., Wu, Y., and Zheng, L. (2018). Heterostructure of CuO microspheres modified with CuFe2O4 nanoparticles for highly sensitive H2S gas sensor. Sensors Actuators B Chem. 264, 139–149. doi:10.1016/j.snb.2018.02.110
Kumar, V., Majhi, S. M., Kim, K. H., Kim, H. W., and Kwon, E. E. (2021). Advances in In2O3-based materials for the development of hydrogen sulfide sensors. Chem. Eng. J. 404, 126472. doi:10.1016/j.cej.2020.126472
Li, Z., Liu, X., Zhou, M., Zhang, S., Cao, S., Lei, G., et al. (2021). Plasma-induced oxygen vacancies enabled ultrathin ZnO films for highly sensitive detection of triethylamine. J. Hazard Mater 415, 125757. doi:10.1016/j.jhazmat.2021.125757
Liang, X., Kim, T. H., Yoon, J. W., Kwak, C. H., and Lee, J. H. (2015). Ultrasensitive and ultraselective detection of H2S using electrospun CuO-loaded In2O3 nanofiber sensors assisted by pulse heating. Sensors Actuators B Chem. 209, 934–942. doi:10.1016/j.snb.2014.11.130
Liu, J., Zhang, L., Fan, J., Zhu, B., and Yu, J. (2021). Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sensors Actuators B Chem. 331, 129425. doi:10.1016/j.snb.2020.129425
Liu, M., Sun, R., Sima, Z., Song, P., Ding, Y., and Wang, Q. (2022). Au-decorated In2O3 nanospheres/exfoliated Ti3C2Tx MXene nanosheets for highly sensitive formaldehyde gas sensing at room temperature. Appl. Surf. Sci. 605, 154839. doi:10.1016/j.apsusc.2022.154839
Liu, M., Wang, Z., Song, P., Yang, Z., and Wang, Q. (2021). In2O3 nanocubes/Ti3C2Tx MXene composites for enhanced methanol gas sensing properties at room temperature. Ceram. Int. 47, 23028–23037. doi:10.1016/j.ceramint.2021.05.016
Liu, X., Jiang, L., Jiang, X., Tian, X., Sun, X., Wang, Y., et al. (2018). Synthesis of Ce-doped In2O3 nanostructure for gas sensor applications. Appl. Surf. Sci. 428, 478–484. doi:10.1016/j.apsusc.2017.09.177
Lu, Z., Zhou, Q., Wei, Z., Xu, L., Peng, S., and Zeng, W. (2019). Synthesis of hollow nanofibers and application on detecting SF6 decomposing products. Front. Mater. 6, 183. doi:10.3389/fmats.2019.00183
Luo, Y., An, B., Bai, J., Wang, Y., Cheng, X., Wang, Q., et al. (2021). Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interf. Sci. 599, 533–542. doi:10.1016/j.jcis.2021.04.125
Ma, L., Fan, H., Tian, H., Fang, J., and Qian, X. (2016). The n-ZnO/n-In2O3 heterojunction formed by a surface-modification and their potential barrier-control in methanal gas sensing. Sensors Actuators B Chem. 222, 508–516. doi:10.1016/j.snb.2015.08.085
Meng, D., Qiao, T., Wang, G., Shen, Y., San, X., Li, R., et al. (2022). Rational design of CuO/In2O3 heterostructures with flower-like structures for low temperature detection of formaldehyde. J. Alloys Compd. 896, 162959. doi:10.1016/j.jallcom.2021.162959
Nikolic, M. V., Milovanovic, V., Vasiljevic, Z. Z., and Stamenkovic, Z. (2020). Semiconductor gas sensors: Materials, technology, design, and application. Sensors 20, 6694. doi:10.3390/s20226694
Ou, Y., Zhu, G., Liu, P., Jia, Y., Zhu, L., Nie, J., et al. (2022). Anchoring platinum clusters onto oxygen vacancy-modified In2O3 for ultraefficient, low-temperature, highly sensitive, and stable detection of formaldehyde. ACS sensors 7, 1201–1212. doi:10.1021/acssensors.2c00334
Park, S. (2017). Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloys Compd. 696, 655–662. doi:10.1016/j.jallcom.2016.11.298
Park, S., Sun, G. J., Kheel, H., Lee, W. I., Lee, S., Choi, S. B., et al. (2016). Synergistic effects of codecoration of oxide nanoparticles on the gas sensing performance of In2O3 nanorods. Sensors Actuators B Chem. 227, 591–599. doi:10.1016/j.snb.2015.12.098
Peng, B., and Huang, X. (2022). Research status of gas sensing performance of Ti3C2Tx-based gas sensors: A mini review. Front. Chem. 10, 1037732. doi:10.3389/fchem.2022.1037732
Phanichphant, S. (2014). Semiconductor metal oxides as hydrogen gas sensors. Procedia Eng. 87, 795–802. doi:10.1016/j.proeng.2014.11.677
Shen, Z., Zhang, X., Mi, R., Liu, M., Chen, Y., Chen, C., et al. (2018). On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sensors Actuators B Chem. 270, 492–499. doi:10.1016/j.snb.2018.05.034
Shi, S., Zhang, F., Lin, H., Wang, Q., Shi, E., and Qu, F. (2018). Enhanced triethylamine-sensing properties of P-N heterojunction Co3O4/In2O3 hollow microtubes derived from metal–organic frameworks. Sensors Actuators B Chem. 262, 739–749. doi:10.1016/j.snb.2018.01.246
Son, D. N., Hung, C. M., Le, D. T. T., Xuan, C. T., Van Duy, N., Dich, N. Q., et al. (2022). A novel design and fabrication of self-heated In2O3 nanowire gas sensor on glass for ethanol detection. Sensors Actuators A Phys. 345, 113769. doi:10.1016/j.sna.2022.113769
Srinivasan, P., Ezhilan, M., Kulandaisamy, A. J., Babu, K. J., and Rayappan, J. B. B. (2019). Room temperature chemiresistive gas sensors: Challenges and strategies—a mini review. J. Mater. Sci. Mater. Electron. 30, 15825–15847. doi:10.1007/s10854-019-02025-1
Sui, N., Zhang, P., Zhou, T., and Zhang, T. (2021). Selective ppb-level ozone gas sensor based on hierarchical branch-like In2O3 nanostructure. Sensors Actuators B Chem. 336, 129612. doi:10.1016/j.snb.2021.129612
Tao, Z., Li, Y., Zhang, B., Sun, G., Xiao, M., Bala, H., et al. (2019). Synthesis of urchin-like In2O3 hollow spheres for selective and quantitative detection of formaldehyde. Sensors Actuators B Chem. 298, 126889. doi:10.1016/j.snb.2019.126889
Van Tong, P., Hoang Minh, L., Van Duy, N., and Manh Hung, C. (2021). Porous In2O3 nanorods fabricated by hydrothermal method for an effective CO gas sensor. Mater Res. Bull. 137, 111179. doi:10.1016/j.materresbull.2020.111179
Vuong, N. M., Hieu, N. M., Kim, D., Choi, B. I., and Kim, M. (2014). Ni2O3 decoration of In2O3 nanostructures for catalytically enhanced methane sensing. Appl. Surf. Sci. 317, 765–770. doi:10.1016/j.apsusc.2014.08.125
Walker, J. M., Akbar, S. A., and Morris, P. A. (2019). Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sensors Actuators B Chem. 286, 624–640. doi:10.1016/j.snb.2019.01.049
Wang, J., Zhou, Q., Peng, S., Xu, L., and Zeng, W. (2020). Volatile organic compounds gas sensors based on molybdenum oxides: A mini review. Front. Chem. 8, 339. doi:10.3389/fchem.2020.00339
Wang, Z., Hou, C., De, Q., Gu, F., and Han, D. (2018). One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS sensors 3, 468–475. doi:10.1021/acssensors.7b00896
Wetchakun, K., Samerjai, T., Tamaekong, N., Liewhiran, C., Siriwong, C., Kruefu, V., et al. (2011). Semiconducting metal oxides as sensors for environmentally hazardous gases. Sensors Actuators B Chem. 160, 580–591. doi:10.1016/j.snb.2011.08.032
Wu, X., Wang, H., Wang, J., Wang, D., Shi, L., Tian, X., et al. (2022). VOCs gas sensor based on MOFs derived porous Au@ Cr2O3-In2O3 nanorods for breath analysis. Colloids Surfaces A Physicochem. Eng. Aspects 632, 127752. doi:10.1016/j.colsurfa.2021.127752
Xu, J. M., and Cheng, J. P. (2016). The advances of Co 3 O 4 as gas sensing materials: A review. J. Alloys Compd. 686, 753–768. doi:10.1016/j.jallcom.2016.06.086
Yang, S., Song, Z., Gao, N., Hu, Z., Zhou, L., Liu, J., et al. (2019). Near room temperature operable H2S sensors based on In2O3 colloidal quantum dots. Sensors Actuators B Chem. 286, 22–31. doi:10.1016/j.snb.2019.01.110
Yang, W., Feng, L., He, S., Liu, L., and Liu, S. (2018). Density gradient strategy for preparation of broken In2O3 microtubes with remarkably selective detection of triethylamine vapor. ACS Appl. Mater. interfaces 10, 27131–27140. doi:10.1021/acsami.8b09375
Zhang, D., Cao, Y., Yang, Z., and Wu, J. (2020). Nanoheterostructure construction and DFT study of Ni-doped In2O3 nanocubes/WS2 hexagon nanosheets for formaldehyde sensing at room temperature. ACS Appl. Mater. interfaces 12, 11979–11989. doi:10.1021/acsami.9b15200
Zhang, H., Chen, W. G., Li, Y. Q., and Song, Z. H. (2018). Gas sensing performances of ZnO hierarchical structures for detecting dissolved gases in transformer oil: A mini review. Front. Chem. 6, 508. doi:10.3389/fchem.2018.00508
Zhang, Z., Zhu, L., Wen, Z., and Ye, Z. (2017). Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sensors Actuators B Chem. 238, 1052–1059. doi:10.1016/j.snb.2016.07.154
Zhao, R., Wei, Q., Ran, Y., Kong, Y., Ma, D., Su, L., et al. (2021). One-dimensional In2O3 nanorods as sensing material for ppb-level n-butanol detection. Nanotechnology 32, 375501. doi:10.1088/1361-6528/ac06f6
Zhou, M., Yao, Y., Han, Y., Xie, L., Zhao, X., Barsan, N., et al. (2022). Ultrasensitive gas sensor based on nanocube In2O3-CNH composite at low operating temperature. Sensors Actuators B Chem. 354, 131224. doi:10.1016/j.snb.2021.131224
Keywords: In 2 O 3 , nanostructrues, gas sensor, sensing mechanism, review
Citation: Yang S, Yin H, Wang Z, Lei G, Xu H, Lan Z and Gu H (2023) Gas sensing performance of In 2 O 3 nanostructures: A mini review. Front. Chem. 11:1174207. doi: 10.3389/fchem.2023.1174207
Received: 26 February 2023; Accepted: 31 March 2023; Published: 07 April 2023.
Reviewed by:
Copyright © 2023 Yang, Yin, Wang, Lei, Xu, Lan and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigao Lan, [email protected] ; Haoshuang Gu, [email protected]
This article is part of the Research Topic
Low-Dimensional Nanomaterials toward Gas Sensing Application
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Micromachines (Basel)

Recent Progress in Gas Sensor Based on Nanomaterials
Associated data.
Not applicable.
Nanomaterials-based gas sensors have great potential for substance detection. This paper first outlines the research of gas sensors composed of various dimensional nanomaterials. Secondly, nanomaterials may become the development direction of a new generation of gas sensors due to their high sensing efficiency, good detection capability and high sensitivity. Through their excellent characteristics, gas sensors also show high responsiveness and sensing ability, which also plays an increasingly important role in the field of electronic skin. We also reviewed the physical sensors formed from nanomaterials in terms of the methods used, the characteristics of each type of sensor, and the advantages and contributions of each study. According to the different kinds of signals they sense, we especially reviewed research on gas sensors composed of different nanomaterials. We also reviewed the different mechanisms, research processes, and advantages of the different ways of constituting gas sensors after sensing signals. According to the techniques used in each study, we reviewed the differences and advantages between traditional and modern methods in detail. We compared and analyzed the main characteristics of gas sensors with various dimensions of nanomaterials. Finally, we summarized and proposed the development direction of gas sensors based on various dimensions of nanomaterials.
1. Introduction
The twenty-first century is an era of electronic information technology, and the high development of electronic technology has completely changed human society’s way of life. At the same time, the application of high-efficiency gas sensors in various fields, such as substance detection, is extremely critical now and in the future. However, certain problems are being faced in the development of gas sensors. At present, the quality and working efficiency of gas sensors are relatively low, and there are problems such as the aging process and the unreasonable process. To produce efficient and stable gas sensors, it is essential to select quality materials. Nanomaterials make full use of their far lower scale and excellent characteristics than regular devices and ideally become the core of gas sensor manufacturing materials. Nano-sensors have advanced analysis to the atomic scale, which broadens the application fields of sensors and promotes their production level.
After introducing nanotechnology into the field of gas sensors, it has significantly enhanced the selectivity, improved the sensitivity, reduced the working temperature, and improved the detection performance of gas sensors, as well as promoted a new type of gas sensor. The sensor composed of nanomaterials plays a significant role in the development of sensors. Through Chen Ming’s research, it enhanced the gas sensing properties of carbon nanotube films through electrostatic self-assembly [ 1 ]. The recording sensitivity of carbon nanotubes-based gas sensors to nitrogen dioxide at room temperature is 1.97 times higher than that of random equipment of the same dimension, which is due to the use of the specific surface area of a carbon nanotube network, and it dramatically enhances the sensing performance. Ma Defu’s studies demonstrate a visible-light-driven room temperature gas sensor made of novel carbide nanocrystals [ 2 ]. The fluorescence emission of carbide nanocrystals has been attributed to light-driven sensing. The device also exhibits good selectivity and stability. Carbides have high adsorption energy in regards to specific gas molecules and low adsorption energy of other gases, which leads to the detection of certain gases.
This overview reviews the current development of gas sensors and introduces new gas sensors based on various nanomaterials, including their composition, types, principles, characteristics and corresponding applications. Nanotechnology has created great potential for manufacturing high sensitivity, low-cost and low power consumption gas sensors. It mainly introduces gas sensors based on various dimension nanomaterials. In each part, it has the methods we use, the characteristics of various categories and the contribution of each study. Herein, we will discuss how to solve the existing problems and combine their elements and applications to compare the main advantages and disadvantages of gas sensors with various dimensions of nanomaterials. Finally, we give a brief summary on nanomaterials with different dimensions in Table 1 and summarize the development direction of gas sensors based on various dimensions of nanomaterials.
A brief summary of nanomaterials with different dimensions.
2. Gas Sensors Based on Zero Dimensional Nanomaterials
2.1. gas sensors based on carbon dots.
Carbon dots have excellent optical properties, good water solubility, low toxicity, environmental friendliness, are a comprehensive source of raw materials and are low-cost with good biocompatibility [ 3 ]. As one of the zero-dimensional carbonaceous nanomaterials, carbon dots have many peculiar properties such as quantum size, abundant edges, functional groups, high conductivity and so on in physical chemistry, playing an essential role in the development of the nano-field, which is beneficial to the improvement of gas sensing performance, making it the perfect material for a gas sensor.
Jing Hu synthesized reduced graphene–oxide–carbon dots (rGO-CDs) hybrid materials via green one-pot method to situ generate ultra-small-sized surfaces on their surfaces while reducing GO [ 4 ]. The introduction of CDs significantly improves the gas sensing performance of rGO. Composite structures can detect extremely low NO 2 concentrations at room temperature. Prepared rGO–CDs exhibited high sensitivity and good selectivity for NO 2 at room temperature upon exposure. It attributed the improvement of rGO–CDs gas sensing performance to the increase of surface hole density rGO surface, which has few defects regarding residual nitrogen due to the introduction of CDs and the formation of an all-carbon nano heterojunction, which itself significantly promotes charge transfer and exhibits high sensitivity, high stability and high repeatability of an NO 2 gas sensor based on nanomaterials.
This fabrication method forms heterojunctions and a small amount of nitrogen doping in full-carbon nanoscale rGO–CDs, which effectively promotes charge transfer and perfectly demonstrates its good gas sensing performance. In observing the sensing performance of the sensor in the detection rGO-CDs, the sensor has the best performance. It exposed the sensor to air, and the adsorbed oxygen molecules captured electrons from the semiconductor, thereafter obtaining the chemical adsorbed oxygen species. This process resulted in the formation of a depletion layer on the rGO–CDs surface. There are grain boundaries at the same time in many active sites in rGO–CDs and the composite contact gas with NO 2 , and it used the whole carbon nanoscale for the selectivity of NO 2 heterojunction molecules. The nano-heterostructures and a small amount of n doping in carbon dots significantly promote the charge transfer in the depletion layer of nanomaterials. Because NO 2 has lone pair electrons and the interface electron exchange and gas will produce a very intense interaction, the sensor resistance changes dramatically. All of these factors affect the electrical characteristics of the system and significantly improve the sensing performance of the sensor.
Cheng Ming studies gas sensors based on carbon dots. He used simple environmentally friendly hydrothermal methods in the In 2 O 3 nanosphere to change the situation of a delamination situation to improve gas sensing performance. He also used a simple one-step hydrothermal method combined with subsequent annealing processes to prepare uniform and hierarchical In 2 O 3 /carbon-like nanospheres. As validation, it fabricated gas sensors based on In 2 O 3 /carbon dots and investigated their gas sensing properties.
This method makes use of the excellent properties of In 2 O 3 to make gas sensors. When exposed to the air, oxygen molecules will be adsorbed on the surface of In 2 O 3 . These adsorbed oxygen molecules will be ionized by trapping electrons in the In 2 O 3 conduction band to form chemically adsorbed oxygen species. The electron depletion layer will form indium oxide near the surface, which will reduce the electron concentration and increase the resistance [ 5 ]. When exposed to oxidized gases, the construction of nitrogen dioxide molecules that capture electrons from In 2 O 3 to NO 2 - increases resistance after combining with the carbon dots, the successful electron transfer, the formation of the heterostructure of In 2 O 3 and the carbon dots. The shielding effect is due to the introduction of carbon dots and the increased surface adsorption oxygen of the introduction of carbon dots. Carbon dots have several purposes, such as an active surface effect, and they show excellent sensing performance for NO 2 and other gases.
Compared to traditional gas sensors, In 2 O 3 /carbon point gas sensors have apparent advantages. They form heterojunctions at the interface between the In 2 O 3 and the carbon dots; electrons flow from the In 2 O 3 to the carbon dots until the electrical properties are equal; the electron depletion layer widens as the band bends, eventually leading to an increase in resistance. The introduction of carbon dots will produce a shielding effect to reduce the effective nuclear charge. Therefore, the released electrons will be absorbed by nitrogen dioxide molecules, further improving the sensing performance [ 6 ]. The percentage of surface adsorption oxygen increases after the introduction of carbon dots. Since the surface adsorption oxygen has high activity, the increase of surface adsorption oxygen percentage plays a vital role in improving the sensing ability. The rich crystal structure can provide more free electron adsorbed oxygen for atoms to react with NO 2 gas, promoting the sensing performance. The surface of carbon dots has ultra-high stable chemical activity, which is beneficial to the adsorption of NO 2 gas and oxygen so that the reaction can accelerate gas sensing. Because of its unique structure and characteristics, the gas sensor can significantly improve sensing performance.
Ziyang Yu studied the synthesis of ZnO and carbon dots (CDs) via the hydrothermal process [ 7 ]. ZnO/CDs composites were prepared by doping the CDs into the ZnO via the grinding method. X surface area of zinc oxide adsorbed gas can be provided via optical sheet diffraction and scanning electron microscope analysis. The ZnO/CDs composite has a high gas sensitivity response. The gas sensitivity test of the ZnO/CDs composite shows that the sensor has a high NO response. The reaction rate of ZnO/CDs composites to NO is much higher than that of traditional methods, and the active functional groups provided by CDs have a significant effect on the NO.
The most significant difference is that ZnO/CDs composites have enhanced gas response, and the doping CDs have an essential effect on NO. The spontaneous formation of free radicals is a distinctive feature of NO gases. The study on carbon dots introduces the surface of carbon dots with active functional groups, capturing free electrons in the ZnO conduction band in ZnO/CDs composites. When the concentration of the carrier decreases, the conductivity of the material decreases either. The measured resistance value increases, which shows that the gas sensing response of the ZnO/CDs composite is improved and enhanced.
The presence of carbon dots transforms the gas adsorption reaction into the solid-phase contact reaction in the composite, and the electron transfer in the solid-phase contact reaction is more likely to occur. Materials with a porous micromorphology have a large surface area; the larger surface of the material can absorb more oxygen molecules and test the target gas molecules, and more surface contact will occur at this time, resulting in better gas sensing reaction. The microsphere morphology of sheet assemblies with a large specific surface area in ZnO/CDs composites provides more contact with no gases [ 8 ]. The numerous active functional groups doped with CDs provide more non-gas-sensing reaction sites, so this method can increase the gas sensitivity of the material and improve the efficiency of the gas sensor.
2.2. Gas Sensors Based on Nanoclusters
In recent years, nanoclusters have become a new material in the field of nano-research. More and more researchers have paid attention to them, including, specifically, gold nanoclusters, as they are considered the most typical representative of metal nanoclusters, having more engagement. Gold nanoclusters are molecular level aggregates with fluorescence properties prepared from organic molecules as templates. Their size is similar to a fermi wavelength and can produce specific energy level separation. Therefore, it emitted fluorescence under excitation at a specific wavelength. Compared to traditional fluorescent materials, such as organic fluorescent dyes and nanoparticles, gold nanoclusters have become excellent materials for gas sensors because of their simple preparation methods and unique physicochemical properties. Nanoclusters have developed well in the research and manufacture of gas sensors because of their rich characteristics, such as light stability, excellent biocompatibility, light induced flourescence and outstanding sensing performance.
Hossain Khan studied a highly sensitive and selective nitrogen dioxide detection method [ 9 ], which itself functionalized Gallium Nitride (GaN) submicron wires with titanium dioxide (TiO 2 ). The nanoclusters fabricated dual-terminal gas/TiO 2 sensor devices using a top-down approach. Gas sensing makes it possible for the sensor to work at room temperature. After the study, it was found that the sensor had high selectivity to NO 2 and can resist other interfering substances. The sensor device had good long-term performance stability at room temperature and humidity, and is relatively stable and reliable in various climates.
This study uses metal oxide nanocluster functionalized GaN sensors to realize the sensing of NO 2 molecules. Under UV irradiation, metal oxide nanocluster photolysis water absorption and water in the GaN create oxygen-producing surface defect active sites and electron-hole pair frameworks; target analytes undergo chemisorption at these active sites; adsorption molecules dynamically capture and de-capture charge carriers at these active sites for GaN potential modifications of the main chains, leading to modulation of sensor currents, proportional to analyte concentrations [ 10 ]. Oxygen molecules are chemisorbed on Ti 3+ vacancies on the TiO 2 surface to obtain negative charges. Meanwhile, molecular adsorption or dissociation adsorption occurs on the surface of water molecules, and TiO 2 cluster surfaces produce oh substances at the Ti 3+ defect sites, which also have many advantages as a sensor. When the energy is higher than the bandgap energy of GaN and TiO 2 , it activates electron-hole pairs in GaN and TiO 2 clusters under UV excitation [ 11 ]. The carrier lifetime increases in the GaN submicron line due to the rise of photocurrent increases due to the bending of the surface energy band photogenic pores to the GaN surface. Chemisorbed oxygen and water are received TiO 2 molecules on nanoclusters and desorbed [ 12 ]. It adsorbs Nitrogen dioxide directly on these newly generated sites due to the high affinity of molecules. Some NO 2 molecules interact with and are adsorbed on the surface chemically adsorbed oxygen. TiO 2 nanoclusters and NO 2 molecules increase the depletion region width inside the GaN, thus reducing the sensitivity current of the sensor. The desorption of light-induced oxygen and subsequent charge transfer TiO 2 nanoclusters and NO 2 molecules to regulate the depletion region width within the GaN, thus contributing to high-performance NO 2 gas sensing.
Mingyuan Wang studied a gas nanosensor system combining silver nanoclusters with phosphorene [ 13 ]. The Ag N nanoclusters (1 ≤ N ≤ 13) can effectively reduce the degradation of phosphorene and hypophosphorous in the catalyst and exhibit various structures. Exposure to other active adsorbents can play a good adsorption role, which significantly improves the selectivity and sensitivity of the system to adsorbed molecules. Because of the participation of valence electrons, the modification of silver atoms from electron orbit can improve the sensitivity of phosphorene. It can also regulate the charge distribution between atoms to adsorb molecules and phosphorene. When the gas flows, the work function of the molecules adsorbed on Ag 1 phosphorene changes significantly, the adsorption amount of NO 2 molecules increases significantly, and NO 2 adsorption requires higher bias voltage than that of Ag 1 phosphorene. Then, the sensing of NO 2 gas is achieved.
This study proposes a silver-trimmed phosphorene composite system for gas sensing, which can prevent silver aggregation nanoclusters and reduce the degradation and passivation of phosphorene by metals through stronger interactions. It improved the selectivity and sensitivity by adjusting the adsorption energy and temperature [ 14 ]. In this study, the reagent has good selectivity and stability. The sensitivity of four molecules to phosphorene can significantly improve the modification of silver nanoclusters via surface modification. Single Ag decoration can improve the adsorption performance of NO 2 molecules, enhance the sensitivity and selectivity of NO 2 molecules and make it an ideal material for gas sensors.
The excellence of this experiment is that phosphorene is a suitable scattering substrate. Silver nanoclusters are efficiently sensed by increasing the binding of silver phosphorene and weakening the silver–silver bond. Single Ag and Ag 1 phosphorene show that Ag atoms cannot only increase the adsorption energy but also increase the energy difference, which means that the phosphorene modified by single silver can improve the sensitivity of the four gas molecules [ 15 ]. NO 2 adsorption requires higher bias voltage than single silver-modified phosphorene. With the increase of the number of Ag nanoclusters, the stability of Ag N nanoclusters to the adsorption energy of gas molecules is enhanced, which provides a variety of ways for the selective adsorption of gas molecules. Silver decoration can create new synergistic effects, improve efficiency by adjusting adsorption strength and temperature, and then enhance the sensitivity and selection characteristics of the phosphorene surface as a gas sensing element.
In YoungMin Byoun’s research, they synthesized heterostructured nanomaterials composed of p-type TeO 2 NWs and discrete n-type ZnO nanoclusters to detect NO 2 gas molecules [ 16 ]. These nanomaterials are synthesized via thermal evaporation and atomic layer deposition, and then the ability of NO 2 in terms of operating temperature, NO 2 response and selectivity was systematically investigated [ 17 ]. By forming discrete n–ZnO nanocrystals, it enhanced the reaction of the p-TeO 2 nanocrystalline sensor to NO 2 significantly. The synthesized sensors also show good NO 2 selectivity compared with SO 2 , C 2 H 5 OH and other interfering gases, forming discrete n–ZnO nanocrystals to improve the sensing ability of gas sensors to NO 2 significantly.
This study demonstrates an excellent reaction and results. ZnO-TeO 2 heterostructures exhibit good NO 2 gas sensing performance by electron sensitization. The heterojunction generated by n–ZnO functionalization can give the p–TeO 2 nanowires better resistance; besides, heterojunction nanowires are beneficial to the detection of oxidizing gases, and when p–TeO 2 contact n–ZnO, the flow of electrons from n-ZnO to p–TeO 2 —transferred to the n–ZnO to balance the Fermi level—results in a barrier with band bending [ 18 ]. As a result, the relative change of hal volume caused by adsorption and desorption of oxidant is more significant than that of reductive gas. Compared to SO 2 molecules, NO 2 molecules are more readily adsorbed on the surface ZnO–TeO 2 heterostructure nanocrystals. As a result, the response to the NO 2 is significantly enhanced.
The advantage of this study is the synthesis of heterostructures p–TeO 2 , the continuous functionalization of n–ZnO nanocrystals using thermal evaporation and ALD processes. It characterized and tested the synthetic products and systematically studied the SEM, XPS and NO 2 of the models. Fabricated sensors show significantly improved NO 2 sensing capabilities far better than the original sensors. Specifically, n–ZnO nanocrystals have a positive influence on the NO 2 response. The n–ZnO nanocrystals are functionalized on the surface of the p–TeO 2 nanocrystals, thus demonstrating good sensing performance for the gas.
2.3. Gas Sensors Based on Metal Nanoparticles
With a deepening of the research of nanomaterials, the huge application potential of gold nanoparticles in the field of gas detection technology has been widely recognized. Nano gas probes and their corresponding detection technology have been highly valued. In recent years, hybrid systems of gas molecules and spherical gold nanoparticles have been widely used in various biological analyses and have achieved encouraging results. Due to the characteristics of easy preparation, easy biochemical modification, high density and high dielectric constant, it used gold nanoparticles to prepare gas sensors to detect specific gases.
Sh.Nasresfahani investigated the effects of gold nanoparticles on the performance of CO gas sensing sensors [ 19 ]. It comprehensively studied room temperature properties of polyaniline and prepared Au/PAni nanocomposites. Also, it modified the fiber surface due to electrostatic interaction and hydrogen bonding PAni via emission scanning electron microscopy and x-ray spectroscopy analysis. Then, the study analyzed the gas sensitivity of each sensor to various carbon monoxide gases in a concise range. Experiments show that the Au/PAni sensor has high response and low noise, a very short response time, a wide dynamic range and good stability. The catalytic performance of gold nanoparticles determines their selectivity and the good sensing ability of the sensor.
The difference between this study and the traditional method is that it enhanced the sensitivity of p semiconductor PAni to CO gas after introducing au nanoparticles. The positive direction on the carbon atom transfers to the nitrogen on the amine, which increases the amount of the positive charge, so the conductivity of polyaniline increases. When it introduced gold nanoparticles into polyaniline, the Au–NPs can interact with CO molecules and transfer positive charges to polyaniline, which significantly improves the sensitivity of the sensor [ 20 ]. It prepared Au/PAni nano composites by ultrasonic mixing under appropriate conditions. The physical mixing of the two components resulted in negatively charged metal nanoparticles deposited on the positively charged polyaniline surface. Since the high surface energy of gold nanoparticles creates adsorption sites for CO gas molecules, the prepared sensors exhibit good gas sensing properties for various concentrations CO at room temperature.
Do Wan Kim proposed a highly sensitive and rapidly responsive nitrogen dioxide gas sensor based on gold nanoparticles modified zinc oxide nanowires. On the surface of zinc oxide nanowires, it modified gold nanoparticles on its surface by electrostatic force. The models without the aptes layers exhibit high NO 2 gas sensitivity due to the local surface plasmon resonance (LSPR), and, incredibly, the increase of the NO 2 gas response and response time was three-fold. Compared to the unmodified ZnO nanowires, the time was reduced by 80%. The presence of aptes layer improves the attachment of gold nanoparticles, and the LSPR effect can significantly improve the efficiency of gas sensors.
Figure 1 illustrates the machine-made strengthened NO 2 gas response of Au–ZnO and Au–ZnO/APTES through diagrams, especially the absorption and suction mechanisms in the dark and under green clearing luminary. We discussed two typical adsorption avenues here: the immediate chemisorption of NO 2 gas onto the ZnO surface by catching the electrons of the ZnO surface itself and the removement of the NO ions from the Au NPs where NO 2 gas catches the electrons of the Au NPs. The suction of the NO ions should only emerge on the ZnO surface. As shown in Figure 1 a, the Au NPs adherence should enhance the NO 2 gas adsorption under green lighting by engendering plasmon-mediated hot electrons from the Au NPs. In detail, both the afflux of hot electrons and the light stimulates electrons from the defect levels broaden the conducting channel of the ZnO NW under green light. Then, the NO 2 gas adsorption on the ZnO surface is extended by the increasing number of electrons, extending the depletion region by capturing the electrons. Then, the electric channel of the ZnO NW becomes narrower, as revealed by the red hollow cylinders in Figure 1 a. As a result, Au–ZnO achieved the maximum improvement ratio and NO 2 gas response. Even though the suction of the adsorbed NO 2 and O 2 ions was also enhanced by the generation of holes in the ZnO NW under green lighting, the hotelectrons-associated gas adsorption procedure appeared to be improved.

Schematic mechanisms for the enhanced NO 2 gas response of the Au nanoparticles-decorated ZnO nanowires, ( a ) without (Au–ZnO) and ( b ) with a (3-aminopropyl) triethoxysilane layer (Au–ZnO/APTES), in the dark ( left ) and under green illumination ( right ). The curved red arrows indicate the NO 2 gas adsorption and desorption processes, whose acceleration and diminishment are represented by the arrow thickness. The solid and dashed lines denote, respectively, the intrinsic conduction (EC) and valence (EV) bands and the Fermi (EF) and defect (Ed) levels. Reprinted with permission from Ref. [ 20 ].
On the other side, the APTES layer on the ZnO NW surface exerts an influence in the NO 2 gas absorption of Au–ZnO/APTES. The APTES layer hindered the NO 2 gas adsorption onto the ZnO NW, as certified by the PC decay results revealed in Figure 1 a,b and testified by the comparatively thin arrows of NO 2 in Figure 1 b. In contrast, once the NO 2 gas was adsorbed onto the ZnO NW surface of Au–ZnO/APTES, its suction was comparatively less hindered by the APTES layer compared to the absorption procedure. Au–ZnO/APTES showed a broadened electric channel of the ZnO NW under green lighting due to the afflux of hot electrons and the light stimulates electrons, similar to that of Au–ZnO. However, since the NO 2 gas adsorption on the ZnO NW surface was not abundantly expedited due to the hindering of the APTES layer, the narrowing in the electric channel of the ZnO NW was smaller compared to the Au–ZnO case. This could explain the comparatively small NO 2 gas response of Au–ZnO/APTES under green lighting with respect to Au–ZnO despite the LSPR effect of the Au NPs. Furthermore, for all three samples, both the red and green lighting obviously expedited the NO 2 gas suction procedure more than the adsorption one, except for the green lighting of Au–ZnO, where the LSPR effect was outstanding. Consequently, the NO 2 gas sensing mechanism of the samples could be directly proofed with the unity of the hot electron generation from the Au NPs via LSPR and the gas adsorption reduction by the APTES layer.
In the research of a gas sensor, there is a unique treatment. Plasma mediated enhanced the room temperature NO 2 gas sensing properties of gold, NPS modified ZnO nanowires, and proposed potential mechanisms for improved LSPR effects. The charge transfer resulting from the LSPR effect in Au–ZnO was characterized via light-irradiated (red and green, 650 and 532 nm, respectively) KPFM measurements. Compared with pristine ZnO nanowires, they have higher gas sensing performance, uniquely faster response time. Gas-sensitive properties of this hot electron increase sharply because it captured the hot electron by NO 2 gas easily and accelerates its chemisorption on the surface of zinc oxide [ 21 ]. Stimulated by hot electrons, it replaced the adsorption process of NO 2 gas by light-excited holes to understand the absorption process [ 22 ]. The LSPR effect of hot electrons can improve the response to NO 2 gases and significantly accelerate the response time of ZnO films. The performance of gas sensors can be significantly improved by using the LSPR effect.
Pu Li proposed in this study to fabricate microstructured gold nanoparticles functionalized gas sensors that are assembled and deposited between two electrodes. In response to volatile organic compounds, it determined the change of conductivity by interparticle properties such as a dielectric constant. The micro sensor shows the reaction of seven target analytes to o-xylene [ 23 ]. This micro-sensor exhibits a larger response to o-xylene than conventional sensors, improving sensitivity and shortening the response time to other volatile organic compounds because the larger surface volume results in better sensing performance than surface roughness and device miniaturization [ 24 ]. The gas sensor, composed of gold nanoparticles, produces an obvious reaction in a short reaction time.
An SEM image shows the surface morphology film of the sensor, which indicates that the microsensor has large surface roughness and surface volume ratio. The higher response is due to the enhanced surface effect, and the larger surface volume ratio can shorten the precipitation time of the gas. The miniaturization of gas sensors brings higher response speed and shorter response time. We compared the response time of various micro-sensors. For gas sensors based on gold nanoparticles, the response time of the VOC test is much shorter than that of other volatile gases. Compared to the laser-made micro-sensor by writing technology, the micro-sensor shows the excellent response to gas.
Electron transport between particles in this study is a unique method and there is no chemical reaction between nanoparticles and gas molecules. Nanoparticles are exposed to analytes, and the VOCs of the adsorption sensor surface can cause changes in physical parameters [ 25 ]. When the polar gas molecules diffuse in the air, the sensor, composed of gold nanoparticles and their binding nanoparticles, expands the distance particles between the particles. The diffusion analyte changes the permittivity constant and the interparticle distance. Using laser writing techniques, gold nanoparticles were successfully self-assembled at two electrodes. Gas sensors exhibit the selectivity of gold nanoparticles in 7 analytes [ 26 ]. Due to the excellent performance of the rough surface and high response and short response with the miniaturization of the device, the gas sensor, based on gold nanoparticles, shows a very high response-ability to gas matter.
Ahmad I. Ayesh investigated PbS nanocrystals, and this substance has recently shown room temperature sensing capability for specific gases (CH 4 ) [ 27 ]. Gold is another common noble metal used as an additive to improve device performance. The incorporation of gold nanoparticles (NPs) can improve methane sensing properties. PbS-NCs shows that adding appropriate amount of Au–NPs can improve PbS-NCs methane sensing characteristics, and studied and analyzed the conductivity, sensor response and sensor speed.
In this study, it studied the effect of gold nanoparticles on PbS electrical properties through various properties. Introducing Au–NPs into the PbS-NCs is feasible to reduce their electrical conductivity. The reaction of the Au–NPs sensor modified on the PbS-NCs surface improves its speed. The main reason is that Au–NPs can produce more oxygen adsorbed on the PbS–ncs surface, and methane molecules find that more adsorbed oxygen will affect the PbS–ncs surface [ 28 ]. In similar cases, for the same concentration of methane, it involved more oxygen ions by methane. This not only makes the sensor more sensitive but also more efficient than in the traditional way. The enhancement of gold to oxygen adsorption can improve the properties of the sensor by competing methane molecules and adsorbed oxygen interaction between gold and gold nuclei. With low methane concentrations, when we added PbS-NCs as more gold nuclear power sources changes, PbS–NCs occurred, and it adsorbed more oxygen by methane molecules and more essential conduction. At high methane concentration, there will be competition between gold nuclear power sources and interaction between CH 4 molecules and adsorbed oxygen, which will produce a better gas response. The effect of various gold content on PbS–NCs decoration affect NPs conductivity and the effect of methane sensing significantly improves its performance. The appropriate amount of gold nanoparticles enhanced the efficiency of the reaction and forms a more efficient gas sensor. (This section closely follows the topic to discuss. The knowledge of nano aspects is well used to analyze and solve the sensor problems. In this part, by reading a large number of data about gas sensors composed of nano materials, the experimental processes of different methods are analyzed in detail, and the analyzed data are compared. The advantages and disadvantages of different kinds of nano sensors are obtained, which shows the diversity of nano sensors.)
3. Gas Sensor Based on One Dimensional Nanomaterials
3.1. gas sensors based on nanowire.
The continuous progress of global industrialization not only improves production and living standards but also destroys the environment in varying degrees [ 29 ]. With the increasing diversity and complexity of harmful gas components in the background in recent years, human health and production safety are in crisis, and people’s awareness of self-protection is improved. Therefore, it is necessary to realize the real-time monitoring of toxic and harmful gases in the environment. Because of its advantages of high sensitivity, easy preparation and low cost, nanowire materials have developed well and play an essential role in the market, and the device performance has been gradually improved nanowire material as the carrier. By regulating its structure and morphology and exploring the synergistic recombination with other semiconductor metal oxides, we try to construct the correlation between material characteristics, gas sensing performance and sensing mechanism, and then improve the gas sensing performance of the sensor composed by it, which lays a foundation for further development in the future.
Zhicheng Cai synthesises SnO 2 nanowires modified by Pd nanoparticles to prepare highly selective and sensitive nanowire hydrogen sensors [ 30 ]. It prepared the SnO 2 nanowires by steam–liquid–solid process and modified the Pd nanoparticles by UV with a PdCl 2 solution to improve the hydrogen sensing performance of the SnO 2 nanowires. Pd nanoparticle-modified SnO 2 nanowires have good electrochemical performance, and various hydrogen-sensitive responses increase with an increasing number of Pd nanoparticles. Furthermore, the selectivity of this nanowire-based sensor also increases the nanoparticle with increasing Pd. SnO 2 and the sensing response of nanowires to several gases is similar, as they enhanced the hydrogen sensing response to other gases after various palladium nanoparticle modifications significantly.
Another special feature of this research is that when SnO 2 nanowires are exposed to the air, the oxygen in the air is adsorbed on the surface of the nanowires due to the attraction of static electricity. It converted the adsorbed oxygen into oxygen ions to adsorb electrons on the surface of SnO 2 nanowires, SnO 2 the surface depletion layer of nanowires expands and the resistance increases. When it exposed such nanowires to hydrogen, it adsorbed hydrogen on the surface of SnO 2 nanowires. Hydrogen reacts with oxygen ions adsorbed on the SnO 2 surface, and hydrogen is converted into H 2 O gas. Through this reaction, electrons absorbed by oxygen ions return to the SnO 2 nanowires, SnO 2 the carrier concentration, surface width and resistance of the nanowires return to the initial state. In this case, the palladium adsorbed nanoparticles, after the reaction with palladium nanoparticles, the electronic band structure changed and the initial dl was formed on the surface of SnO 2 nanowires when it exposed Pd nanoparticle-modified SnO 2 nanowires to air, it adsorbed oxygen by electrons of Pd nanoparticles. SnO 2 the surface of nanowires, it significantly reduced the resistance of such nanowires in the hydrogen environment due to the SnO 2 use of electrons as electrical carriers. After modifying Pd nanoparticles, the behavior of this nanowire in exposure to air and hydrogen and the change of gas sensing characteristics were more pronounced.
Tzu-Feng Weng used the vapor–liquid–solid growth method to grow high-density β-Ga 2 O 3 single crystal nanowires on silicon substrates, and studied the room temperature CO gas sensor of pure nanowires and gold-modified nanowires using multi-network arrays and single nanowire devices [ 31 ]. It studied the synthesized nanowires by field emission scanning electron microscopy. It fabricated single nanowire gas sensors by focusing ion beam technique. A single nanowire RT-CO gas sensing sensor using the proposed Au changed the β-Ga 2 O 3 nanowire to achieve remarkable sensitivity to CO gas at room temperature. It also compared the sensing characteristics β-Ga 2 O 3 RT-CO gas multi-network Au modified nanowires and single Au modified nanowires.
This study, due to the superior and stable RT gas sensing properties of gold, analyzed the effect of various gas concentrations on the performance of β–Ga 2 O 3 nanowire devices after modification. Various gas sensors have various measurement results in CO gas concentration. With the decrease of gas concentration, the response time and recovery time gradually decrease and increase. The activation energy of oxygen atoms pre-absorbed on the surface of gold nanoparticles adsorbed upon CO molecules decrease [ 32 ]. The length of response and recovery time depends on the availability of a large amount of oxygen trapped on the surface of the sample. If the number of oxygen vacancies increases, the pressure on the surface of the number of trapped oxygen molecules increases, which in turn enhances the efficiency of the sensor. It operated the CO-gas sensor by adsorption, the resistance decreases when the reducing agent reacts on the surface of the material. After it chemisorbed and absorbed the oxygen in the semiconductor by the reduced gas, the result is a free electron in the form of increased conductivity. The gas sensing structure diagram is shown in Figure 2 . Small fragments of the oxygen ion monolayer were absorded and formed additional deoxyribonucleic acids near the ion surface [ 33 ]. This is also the reason for the excellent gas sensing performance of Au modified β-Ga 2 O 3 .

An architecture diagram of a CO gas sensing measuring unit. Reprinted with permission from Ref. [ 31 ].
J.Y. Lin studied an SnO 2 nanowire hall-effect gas sensor for hydrogen detection [ 34 ]. It prepared SnO 2 nanowires on stainless steel mesh by horizontal electric furnace, and it analyzed the crystal structure, morphology and electron binding energy of SnO 2 nanowires by XRD, XPS. Gas response to H 2 at various operating temperatures and H 2 concentrations. It investigated a response mechanism of a SnO 2 -based hall-effect gas sensor. Hall effect gas sensors based on SnO 2 nanowires have super-high response and efficiency in preparing low-cost and high-performance gas sensors.
Compared to the experimental results of pure SnO 2 gas sensors, the response characteristics of hall-effect sensors are better. SnO 2 nanorods evaporation was synthesized via thermal method, which has a high response to H 2 . The sensing mechanism of target gas and hall effect principles with the surface reaction of hydrogen and oxygen was adsorbed on metal surface SnO 2 nanowires. It absorbed the electrons by atoms to adsorb oxygen molecules from the conduction band of electrons to form SnO 2 nanowires and oxygen ions on the surface, which leads to the decrease of electron density on the surface of SnO 2 nanowires [ 35 ]. The carrier concentration increased with time, and it exposed the gas sensor to hydrogen. With the growth of hydrogen concentration, the carrier concentration increased further. When it introduced hydrogen, hydrogen reacted with adsorbed oxygen. The absolute value of the hall coefficient increased with the increase of H 2 concentration, and the decrease of hall voltage decreased with the increase of H 2 concentration. Good sensing performance was therefore shown in response to a specific gas.
Chandan Samanta studied an NO gas sensor based on ZnO/Si nanowire heterojunction arrays operating at room temperature with extremely high response [ 36 ]. The sensor is highly selective for gas-free and confined gases due to the disturbance caused by moisture. Using economical and efficient chemical treatment compatible with wafer-level treatment, the sensor prepared vertically oriented nano-silica arrays by chemical corrosion and zinc oxide deposition, and prepared nanostructures by chemical solution deposition and spin-coating. The formation of heterostructures leads to a synergistic effect in which the sensing response is larger than the sum of individual components, zinc oxide and silicon nanowires. When it combined the n-ZnO nanostructures with the p-sinw interface, the reaction is powerful, which leads to a good sensing response to the gas.
In this way, it made gas sensors, and the response performance of the equipment increases with the decrease of moisture in the environment. It shows the variation of gas response with various values of temperature. With the increase of temperature, the reaction increases ZnO/Si nanowire devices respond to NO gases. Selectivity to a particular compound is a unique feature of the gas sensor. Checking the selectivity for NO gas, ZnO/p-Si nanowire sensor tested for contact with various types of gas. It showed the selectivity when exposed to various kinds of gases, and the gas sensors all had a good response [ 37 ]. The gas response of the device, such as nitrogen dioxide, was tested with other oxidants, the nanowire sensor has high selectivity to NO gas. ZnO/p-Si nanowire sensors also generated gas-sensitive responses to various gas lasers. ZnO/Si-NW heterostructures enhanced the performance in semiconductor materials, reflecting ZnO/Si role of nanowires. The room temperature conductive response nanostructured films and p-Si nanowires ZnO by measuring the heterostructure of NO gas showed the high response and sensing ability of the sensor to the gas.
Waldir Avansi Jr. studied the gas sensing properties of semiconductor nanomaterials with various energy bands, the chemical resistance sensing ability of titanium dioxide nanoparticles and the V 2 O 5 nanowires obtained by hydrothermal treatment of metal peroxide complexes [ 38 ]. The formation of V 2 O 5 /TiO 2 heterostructures was studied, characterized by X-ray diffraction (TEM) and X-ray photoelectron spectroscopy (XPS) measurement. This research also proposes an effective method for preparing one-dimensional vanadium pentoxide/titanium dioxide with good detection range and ozone sensing properties that are significantly related to repeatability and selectivity.
The difference in this study is that both V 2 O 5 and TiO 2 are n-type semiconductors with various structural electronic properties, such as electron affinity. When equilibrium occurs between semiconductors, electrons transfer from TiO 2 to the low-energy conduction band V 2 O 5 the semiconductor until their fermi levels become equal. This arrangement leads to the presence of more electron conduction bands in the TiO 2 . It attributed the ozone response of the heterostructure to the effect of the existence of carbon impurities on the active sites. For V 2 O 5 /TiO 2 , the heterostructure presents various kinetic curves, and it observed the response of the sensor, indicating that the formation of the heterojunction produces additional active sites. It connected the sensor response enhancement O 3 gas V 2 O 5 /TiO 2 heterostructure to the induced effect nanowires and titanium dioxide nanoparticles of vanadium pentoxide effective heterojunction at the two-phase interface, which leads to the change of resistance and then improves the response to the gas [ 39 ]. Synergistic effect promotes the chemical adsorption process of ozone. The gas sensor has good gas sensing performance and a strong sensor response to ozone gas.
3.2. Gas Sensors Based on Carbon Nanotubes
With the development of nanotechnology, it has created great potential for designing low energy consumption, high sensitivity, low cost and portable sensors. As emerging nanomaterials, carbon nanotubes’ excellent electrical conductivity, high surface area and unique hollow structure make them ideal materials for gas molecule adsorption. The commonly used semiconductor metal oxide gas sensing materials usually need to work typically at a higher temperature, showing semiconductor characteristics [ 40 ]. The incorporation of carbon nanotubes affects the gas sensing characteristics of semiconductor oxides, and its composite gas sensing materials show good gas sensing characteristics.
Sunil Kumar studied the chemical detection of toxic gases such as greenhouse gases by gas sensors based on single-walled carbon nanotubes (SWCNTs) to detect NO 2 gases with higher sensitivity [ 41 ]. In this study, the thin film sensor was fabricated on a SiO 2 substrate and functionalized with polyethylenimine (PEI) respectively. Confirmed that the PEI functionalized SWCNTs showed a high sensitivity for strong electron-absorbing solid. At room temperature, the sensitivity of SWCNTs that PEI functionalized gas sensing elements is nearly 50% higher than that of single-wall carbon nanotube gas sensing elements. The gas sensor shows a repeated response over the entire study concentration range. PEI functionalization improves the performance of single wall carbon nanotube gas sensing elements.
This study used the thermal CVD method to develop resistive SWCNTs-PEI functional gas sensors. PEI coated single-walled carbon nanotubes have higher electron-absorbing NO 2 adhesion coefficient than untreated single-walled carbon nanotubes. The resistive gas sensor with PEI–SWCNTs coating exhibits high sensing performance and rapid response to NO 2 at room temperature [ 42 ]. Due to the room temperature working characteristics of resistive gas sensors, more apparent results can be obtained in environmental observation. Through the accurate selection of the heat treatment process, it realized the complete recovery of the sensor and the sensitivity of SWCNTs increases with the extension of functionalization time. By controlling the alkalinity and functionalization concentration of single-walled carbon nanotube networks, PEI- SWCNTs sensors can be extended to various fields, and the selection of sensors can be configured according to various chemical environments. All of them show good response to gases [ 43 ].
Sukhananazerin Abdulla studied the formation of thin films with good polyaniline arrangements [ 44 ]. The high directional ordering of multi-walled carbon nanotubes is enhanced by Langmuir-Blodgett technology to improve the sensing characteristics of ammonia gas. In the process of interfacial assembly, polyaniline-multi-walled carbon nanotubes gradually form ordered small blocks at the gas-water interface and further organize into a globally well-defined oriented monolayer. The PANI@MWCNTs LB films were transferred at 25 °C onto the pre-cleaned gold sputtered double electrodes fabricated on SiO 2 substrates for gas sensor property analysis. Schematic illustration of the experimental setup used for gas sensing measurements is shown in Figure 3 . Direction and p-polyaniline multi-walled carbon nanotubes systematically study the Langmuir film at the air–water interface as the key to an ammonia gas sensor.

A schematic illustration of the experimental setup used for NH 3 gas sensing measurements.
The difference in this study is that the surface-functionalized polyaniline-modified multi-walled carbon nanotubes overcome the surface defects caused by the three-dimensional aggregation of carbon nanotubes. Formation stability of dense body single molecular membrane/multilayer structure polyaniline-multiwalled carbon nanotube LB based film. It used highly polyaniline functionalized multi-walled carbon nanotubes with oriented LB films for sensing at room temperature of ammonia gas. Sensitivity to NH 3 directional electron transport polyaniline multiwalled carbon nanotubes at room temperature compared to random networks [ 45 ]. Ultrathin LB membranes allow for fast analyte diffusion of active sensing layer assembly due to adequate molecular regulation. This study resulted in the formation of aligned assembled polyaniline-multi-walled carbon nanotubes, which formed remarkable monolayers at the air–water interface on large areas parallel to the barrier layer dense films. LB membrane-oriented polyaniline multi-walled carbon nanotubes exhibit high-performance gas sensing elements and sense ammonia gas [ 46 ].
Florin C. Loghin studied a transparent gas sensor on carbon nanotubes [ 47 ]. It deposited the sensing layer and electrode by jet deposited carbon nanotubes. High transmittance transparent sensor electrodes of the two sensing layers have characterized the properties of ammonia and carbon dioxide, and the sensitive reference sensor and transparent sensor for the NH 3 have shown a good response. In contract, the transparent device has higher sensitivity to carbon dioxide than the reference electrode. The effect electrodes with spacings between continuous data were also investigated with wider spacing in the results of fully carbon nanotubes-based sensors at higher sensitivity due to higher sensing resistance, whereas this effect was not observed in gold electrodes due to their negligible resistance relative to carbon nanotubes. The performance of a transparent sensor is better than that of other sensors, which shows good sensing characteristics of transparent gas.
Throughout this study, a unique point has been that the spacing plays an essential role in high resistance, and this effect will eventually affect the saturation distance of the electrode. Normalized response of the Carbon Nanotubes electrode will be concentrated to one of the Au electrodes if the resistance ratio of the sensing layer to the electrode is large enough. However, this studies a higher electrode distance, which will affect the total resistance of the sensor of the method, and the sensing performance of the translucent carbon dioxide sensor can be significantly improved. At high concentrations, the sensitivity of the sensor is better than that of some all-carbon nanotube gas sensors [ 48 ]. This effect may respond to the presence of electrodes in carbon-based sensors against gas molecules. However, they react variously to gases because of the various network density, plus the performance of carbon nanotube electrodes is superior to that of gold-contact carbon dioxide electrodes. At higher concentrations, carbon dioxide can cause a massive change in electrode resistance, resulting in an increasing in distance-insensitive normalized resistance, which then improves gas sensitivity.
Qian Rong used molecular imprinting technology to prepare high-performance acetone gas sensors ALFOMMIPs, studied the functional groups, grain size and surface morphology of synthetic materials, analyzed them through different characterization techniques and examined the gas response of the samples [ 49 ]. The CNTs and ALFOMMIPs nanocomposites (CNT/ALFOMMIP) showed higher thermal stability than the reaction of ALFOMMIPs models via an acetone gas sensing test and analysis. The sensor with carbon nanotubes has good gas stability sensing performance. The sensor shows a high response to acetone at various temperatures, and it has the best selectivity and sensing performance for acetone vapor due to molecular imprinting technology.
The porosity of the resistance CNT/ALFOMMIP is related to the number of holes [ 50 ]. When we placed CNT/ALFOMMIP gas-sensitive materials in air, chemisorbed oxygen molecules and physically adsorbed on the surface of objects CNT/ALFOMMIP gas-sensitive materials form depletion layers near the sensor surface with ALFOMMIP conductive electrons, reducing the adsorbed O 2 molecules to various forms of oxygen-containing anions and resulting in reduced electron density and increased sensor resistance [ 51 ]. When the acetone vapor in the air is in contact with the CNT/ALFOMMIP gas-sensitive material, the chemically adsorbed oxygen on the surface of the material will evaporate with acetone, producing the extracted electrons released into the CNT/ALFOMMIP conduction band. It significantly increased the resistance of the holes of the carbon nanotubes rapidly transferred to the ALFOMMIPs, CNT/ALFOMMIP sensor. The CNTs have good electrical conductivity, and the resistance of the sensor can be reduced due to the abundant groups such as -COOH or -OH surface CNTs. The electron passes through the percolation effect, which significantly improves the transmission rate. CNT/ALFOMMIP composites have a high response speed and low operating temperature. p-p homojunction is formed via close contact with metal alfomips carbon nanotubes that are prepared, which further improves the gas sensitivity of the sensor to acetone vapor.
Struzzi C investigated the modification of electronic properties on the surface of vertically aligned and randomly distributed films [ 52 ]. It used the hydrophobic properties of CNTs in Ar, F 2 and CF 4 plasma to optimize these sensing properties. By detecting the stability and responsiveness of fluorinated carbon nanotubes to water, reflecting the sensor sensing characteristics, fluorinated carbon nanotubes were revealed to detect two selected pollutants, such as nitrogen dioxide and ammonia (NO 2 and NH 3 ). By increasing the surface hydrophobic humidity level and the influence of sensing layer geometry on fluorination, resulting in response to reproducibility when used vertically and an enhanced sensor response of CNTs to NH 3 .
The unique feature of this gas sensor is that the fluorination reaction introduces a moderate induction reaction, which reduces the distance between ammonia and the predicted analyte and fluoride, promoting stronger interactions through the formation of hydrogen bonds, due to dipole electrostatic interactions providing enhanced charge transfer with the fluorinated carbon layer and confirming the significant role of CNTs forests in vertical geometry directly exposed to the tip playback of the target gas. The change of humidity concentration in the environment also has a great influence on the performance of nano-pipe network sensors. Fluorination uses CF 4 plasma, increasing the effect of humidity levels on the response, leading to the generation of an ionic current that promotes the generation of local space charges [ 53 ]. The original random CNTs models reacted to ammonia already in the dry state, and there was a hydrogen bond interaction on the surface of natural oxygen in the air, which was beneficial to charge transfer to the fluorinated CNTs sensor. The fluorination CNTs then showed good sensing characteristics for the gas (The topic selection has strong application value literature materials collected detailed, the knowledge learned has solved the problem, correct conclusions, innovative insights, experimental design is reasonable and feasible, can be carried out according to the experimental plan, and achieve the expected results).
3.3. Gas Sensors Based on Other One Dimensional Materials
Fei Yang studied a visible-light-driven room temperature gas sensor made of a novel carbon-acetylene nanocrystalline system [ 54 ]. It prepared nanocrystals via laser ablation. Scanning electron microscopy and transmission electron microscopy show that they are stacked flakes composed of rod-like crystals. Under illumination at 447 nm, a sensor containing nanocrystals detected NO 2 molecules with concentrations as low as 2 ppm at room temperature with a response and recovery time of less than 100 s. It attributed the light-driven sensing to the fluorescence emission of carbon acetylene nanocrystals. The device exhibits excellent selectivity and stability. The carbon nanocrystals have high adsorption energy NO 2 and the molecules have low adsorption energy to other gases, which leads to high NO 2 detection sensitivity.
The gas sensor fabricated in this way demonstrates the potential of carbon nanocrystals as a gas-sensing material. NO 2 adsorption is a non-dissociated process, is not determined by thermal energy. Because of the high absorption rate of the NO 2 , the NO 2 adsorption sites have limited barrier heights and a limited number of photogenerated electrons. This Au load effectively reduces the Au–C adsorption barrier Schottky junction, makes NO 2 − more easily adsorbed. In the presence of gold nanoparticles, energy barrier photogenerated electrons decrease the conduction band of NO 2 molecules from the bottom. Therefore, NO 2 − were adsorbed next to Au–C Schottky contact [ 55 ]. This behavior extends the width of the depletion region and increases the device resistance. In a dark nitrogen environment, the gas produced by carbyne nanocrystal has few electron-hole pairs, a very thin electron depletion layer on the surface and a high barrier height, resulting in high sensor resistance. When the sensor is exposed to light, carbyne nanocrystals produce electron-hole pairs, and the same electron depletion layer as in the N 2 environment appears in the atmosphere in the dark. When exposed to NO 2 gas, the photogenerated electrons react with NO 2 molecules to form NO 2 , which is adsorbed on the carbyne nanocrystal surface to form a thick electron elimination layer, high barrier height and high sensor resistance, and the sensor’s gas sensitivity to NO 2 is also further increased.
Zdenek Pytlicek studied anodized niobium oxide nanocrystalline films prepared by sputtering deposition [ 56 ]. The nanofilm consists of N 2 layers that maintain upright N 2 O 5 nanorods. It integrated each part into an advanced three-dimensional structure and multi-layer layout on a silicon wafer comprised of multiple micro-sensors, forming a top electrode and a multi-functional SiO 2 sandwich by combining Pt/NiCr via high-temperature vacuum or air annealing, sputtering deposition, and stripping lithography. The proposed on-chip sensor solution enables sensitive fast and highly selective hydrogen detection. This thin film formation and chip manufacturing technology can enhance the gas sensing ability and efficiency of one-dimensional metal oxide nanomaterials.
Other advantages of this study are that the nanorods annealed in air, with lower oxygen vacancy concentrations, extending deeper within the rods and resulting in a flat-band state, thus leaving the oxide wholly depleted nanorods, which is associated with a significant increase in resistance after patching. The thickness of the depletion layer on the Schottky junction at the top of the rod also increases but remains unchanged in the vacuum annealed nanostructures between vacuum or air-annealed nanorods, respectively, with conductive channels inside or completely depleted H 2 on the film resistance. It formed the track inside the rod, allowing a higher electronic conductivity than fully depleted nanorods in air. And the resistance decreases, resulting in a highly enhanced reaction to the H2. This will significantly enhance the sensing ability of the sensor on the high-capacity and low-power chip (In this part, through the analysis of the gas sensors composed of different kinds of one-dimensional nano materials, the principle, experimental steps, the differences from the traditional manufacturing method and the impact of this method are analyzed in detail. Finally, a detailed analysis, comparison and summary are provided for the researchers in the aspect of gas sensors composed of one-dimensional nano materials).
Hyoun Woo Kim and Sang Sub Kim synthesized SnO 2 -Cu 2 O C-S NWs and applied these to the detection of trace amounts of gases [ 57 ]. The resistance curves for the C-S NW sensors with different shell thicknesses were obtained upon exposure to 10 ppm C 7 H 8 , C 6 H 6 , and NO 2 . The sensor based on C-S NWs with a shell thickness of 30 nm exhibited the best response to reducing gases. The response of optimal gas sensor to 10 ppm C 7 H 8 , C 6 H 6 gases was 11.7, 12.5 at 300 °C. In addition, the response and recovery times were almost 4 s for both gases. The presence of the Cu 2 O shell decreased the NO 2 -sensing response of the C-S NW sensors. In ambient air, the concentration of holes can be divided into three regions considering the vacuum case because of oxygen adsorption onto the Cu 2 O shell and development of the C-S heterojunctions. The HAL (p+) is created by the extraction of electrons from the valence band of Cu 2 O by chemisorbed oxygen species. At a specifific temperature, the intrinsic hole concentration layer (po) remains at the equilibrium hole concentration in Cu 2 O, and the hole-defificient layer (p−) results from an electrostatic response to the hole layer by the electrons in the n-p heterojunction. An increase in the concentration of holes is observed in air. When the sensor is exposed to the reducing gas, the resistance of the p-Cu 2 O shell layer increases.
The profile of hole concentration (blue line) in air shifts toward the red line, which supports a decrease in the concentration of holes in the “p” shell layer. Therefore, the detection capability of pure Cu 2 O NWs was inferior to that of the C-S NWs because of the weaker hole-accumulation layer. The degree to which the resistance of the p+ layer is modulated varies inversely with the shell thickness. As a result, a thicker shell experiences less resistance modulation because it is in a state of partial hole accumulation. Considering the fraction of shell layers in the overall volume of the n-p C–S NWs (which is comparable to shell thickness), the response affords a bell-shaped curve as a function of shell thickness. The extension of the p+ layer is constrained owing to the existence of the p−|n− interface, which acts as a blocking layer for the expansion of the p+ layer, resulting in a slight resistance modulation to oxidizing NO 2 and low response to NO 2 .
4. Gas Sensors Based on Two Dimensional Nanomaterials
4.1. gas sensors based on graphene.
Graphene gas sensors are widely used and play an essential role in industrial production and environmental monitoring. Due to its unique physical, chemical and mechanical properties, graphene has become a hot topic for many scholars. The gas sensor based on graphene material can reduce the working temperature, improve the recovery, and cooperate with other organic polymer materials to prepare the gas sensor used at room temperature. Graphene sensors show good gas sensitivity, which will have a wide range of applications in gas sensors.
Shirong Huang studied a flavin monoclinic sodium salt for ammonia gas sensing materials in a chemically resistive gas sensor [ 58 ]. The detailed characterization shows that the graphene sheets exhibit good structural quality and the optimized ammonia sensors exhibit excellent performance: ultra-low detection limits and excellent sensitivity to gases. Regarding the role of FMNS in graphene preparation and NH 3 sensing, it studied the p-type doping of graphene-based sensing elements and the active adsorption site sensing for NH 3 gases via full atomic molecular dynamics simulation. Using FMNS-like molecules to design high-sensitivity graphene-based NH 3 gas sensors provides excellent sensing response and gas sensing capability.
A unique feature of the sensor is that the conductivity of the depleted graphene in graphene decreases, and the resistance increases when it rotates. Pure nitrogen, weak hydrogen bond breakage and NH 3 molecular release bring electrons back when rinsing the sensor. Donated electrons move from the graphene return FMNS of the Fermi level to the valence state. It returned this to the lower sensor resistance and sensor recovery. Few electron energies from NH 3 molecules are transferred to graphene, resulting in weak response sensors for graphene-based gases even when exposed to high ammonia concentrations. The specific modified hydrogen bond interaction of adsorbed NH 3 molecules on FMNS is much more fragile, giving G-FMNS sensor orientation NH 3 induction more than a covalent bond [ 59 ]. G-FMNS act as a graphene dispersion stabilizer as a P dopant element for graphene sensing and provide active adsorption sites for NH 3 gas sensing, which constitutes an efficient graphene NH 3 gas sensor and exhibits good gas sensing performance.
Muhanad. A. Ahmed studied the use of graphene nanosheets for ammonia gas detection [ 60 ]. The measurement is based on the measurement of electrical resistance before and after gas exposure. The resistance after gas exposure increased significantly. The graphene-based gas sensor was susceptible to NO 2 and NH 3 . It was also prudent to other factors of organic compounds. Based on the adsorption of gas molecules, the resistance changed. Graphene responds quickly to gases using ultraviolet or heating sensors to provide sufficient energy for desorption from graphene sheets [ 61 ]. Graphene proved to be a P semiconductor, so after the weak adsorption of gas molecules adsorbed on the surface of graphene, the carrier was mainly hole hybridized with coupled electrons on the surface of graphene, which led to the change of graphene conductivity. Then, it showed the excellent sensing characteristics of specific gases and the sensing performance of the sensor.
Ravi Kumar investigated the preparation of functionalized graphene oxide (GO) films via chemical deposition [ 62 ]. It used the evaporation measurement technology of film resistance deposited by the thermal deposition process for aluminum contact. It characterized the functionalized graphene oxide by X-ray diffraction, Fourier transform infrared spectroscopy and Raman spectroscopy. The sensor response of ammonia concentration in a particular range was studied. MTA, in higher concentrations, had a higher response to ammonia. Increased ester generation reactions on the surface of the sensing film eventually led to interactions with NH 3 gas molecules [ 63 ]. The selectivity of the sensor under various conditions was studied, and the sensor had strong selectivity to ammonia gas, which shows good sensing characteristics of ammonia gas.
The difference in this study is that carbon vacancies in oxygen species and FGO promote surface reactions with NH 3 , resulting in solid chemisorption and physical adsorption, and the adsorption and dissociation of ammonia on functionalized surfaces change structural and electronic properties. Functionalized GO and charge transfer occur from ammonia to functionalize the GO surface through the formation of surface hydrogen bonds. The presence of epoxy rings in the moving surface may break during functionalization. Moreover, the interaction process of the dissociation process of NH 3 molecules also uses the sensing film, which it decomposed into ammonia and hydrogen [ 64 ]. Free NH 2 and H molecules may react to obtain chemisorbed OH and NH 2 molecules on available carbon and oxygen sites. The sensor will show excellent selectivity and gas sensitivity to ammonia.
Vu Van Cat studied GO nanosheets (GO-NSs) which were applied to a quartz crystal microbalance sensor for a mass-type poisonous gas sensor [ 65 ]. The sensor is a QCM working electrode surface prepared via spray of GO-NS suspension and it studied the concentration of toxic gases, including NO 2 , SO 2 , CO and NH 3 via various methods of GO–NSs designed by the adsorbent which has good adsorption performance and becomes an efficient sensor for the detection of toxic gases.
In this study, the binding energy of NO 2 molecules and GO is stronger than SO 2 due to a reduction of oxygen-containing groups. The sensing result make known that the GO coated QCM sensor revealed a good sensitive factor (S-factor) with SO 2 and NO 2 gases, as exhibited in Figure 4 . QCM sensors exhibit good response, long-term cycling stability and reproducibility. GO is graphene form containing oxygen functional groups with a negatively charged surface and surface large aspect ratio nanosheet structures. The sensitivity values of the QCM sensor SO 2 of the go coating and the excellent sensitivity factor sensor of the NO 2 gas are shifted from the frequency of the sensor to a specific concentration [ 66 ]. The working mechanism of the mass gas sensor is based on the adsorption/desorption layer of gas molecules on the sensor coated with QCM active electrodes. Based on the adsorption of dipole–dipole interaction, the groups on the GO surface and the polar test gas molecules pass through hydrogen bonds [ 67 ]. The S coefficient of the sensor depends on the adsorption capacity and the molecular weight of the adsorbate. The sensor has high sensitivity to NO 2 gas and exists in the form of a dimer (N 2 O 4 ) at a high density. The S factor of the sensor for NO 2 gas is similar to that of SO 2 gas and is higher than that for CO gas. The complex of the quality sensor GO exhibits a long response time for toxic gases such as sulfur dioxide and nitrogen dioxide, and the GO-coated QCM sensor exhibits extremely high gas sensitivity.

The sensitive factors (S-factor) of SO 2 , NO 2 , CO and NH 3 at different concentrations.
4.2. Gas Sensors Based on MXenes
With the rapid development of the chemical industry, the air pollution caused by organic waste gas emission has endangered people’s health and living environment. Toxic volatile gases, such as ammonia, amides, sulfur and nitrogen compounds are hazardous gases in the atmospheric environment. Therefore, it is essential to monitor the composition and concentration of harmful gases in the atmosphere in real-time and efficiently. A MXenes gas sensor is a sort of material which can detect the existence of specific types of gas and the change of concentration in a limited range in real-time. It shows high sensitivity and accuracy in gas detection and has great room for development in the future.
Shoumya Nandy Shuvo studied the gas sensing properties of volatile organic compounds by the lead of S doping Ti 3 C 2 TX–MXene, and it shows unique selectivity to toluene [ 68 ]. The study synthesized and characterized the sulfur-doped raw materials as electrode materials, and it subjected the as-prepared sensors to dynamic impedance tests at room temperature for the presence of volatile organic compounds with various functional groups. P-toluene has unique selectivity synthesized by undoped and doped Ti 3 C 2 TX-Mxene and obtains the sulfur-doped Ti 3 C 2 TX–MXene sensor, which embodies excellent long-term stability. The study revealed the effect of S doping on a volatile organic compound analyte sensing and obtained the efficient gas sensor for specific gas detection.
This study further understood the unique gas sensing behavior of these materials, as well as the selective doping of natural gas and natural gas Ti 3 C 2 TX-Mxene sensors. The main contribution near the Fermi level comes from the functionalization of Ti atoms, which leads to the modified electronic structure of the material. It shows that functional groups near the Fermi level promote functionalization of the atom-resolved band structure of the non-functionalized and functionalized titanium carbide MXenes. It used the binding energy of horizontal and vertical toluene and titanium carbide MXenes at four various positions for adsorption to form atoms that can find MXenes priority functional groups [ 69 ]. The flat binding energy and −S of toluene and titanium carbide MXenes as surface end groups at four various positions. The electron MXenes surface obtained from air enhances the gas response and exhibits an efficient gas sensor.
Danxi Yang determined that by using density functional theory and the first-principles method, SC 2 CO 2 are very sensitive to NO molecules for their chemical interaction and large charge transfer, which will cause a change of current [ 70 ]. Also, it further enhanced the interaction by applying external strain, indicating the potential of SC 2 CO 2 as a gas trapping agent material. Additionally, it studied the adsorption manganese doping of CO on SC 2 CO 2 . Due to the vital adsorption energy, this can gain a pronounced effect. The excellent sensing performance of SC 2 CO 2 to CO in a gas sensor is reflected by the research.
This method differs from the traditional method. The interaction between molecules and gas sensing elements will detect the interaction between gas molecular materials and materials, resulting in a shift in material resistance. The adsorption of NH 3 molecules on the Ti 2 CO 2 is due to the change of charge carrier due to the transfer of charge between molecules based on the adsorption NH 3 molecules and Ti 2 CO 2 . The current–voltage relationship and the adsorption energy of no molecules on the SC 2 CO 2 is larger than that of NH 3 molecules on the Ti 2 CO 2 , and the charge transferred to no molecules is more than that of the molecules on Ti 2 CO 2 . The sensitivity of SC 2 CO 2 sensor is higher than that of Ti 2 CO 2 sensor [ 71 ]. Adsorbed no molecules can be achieved by removing the applied biaxial strain. SC 2 CO 2 shows good recognition and sensing ability of NO gas.
Shibin Sun studied the structure of W 18 O 49 /Ti 3 C 2 Tx composites based on the in situ growth of one-dimensional single crystals, using the solvothermal method to prepare W 18 O 49 nanorods on the surface of 2D-Ti 3 C 2 Tx-Mxene sheets [ 72 ]. W 18 O 49 /Ti 3 C 2 Tx composites have a high response to low concentrations of acetone. W 18 O 49 /Ti 3 C 2 Tx composites showed significantly improved acetone sensing performance. It treated the uniform distribution of W 18 O 49 on the Ti 3 C 2 Tx surface to remove the fluorinated group solvothermal process on the surface, as well as W 18 O 49 NRs and Ti 3 C 2 Tx sheets. W 18 O 49 /Ti 3 C 2 Tx-Mxene composites show excellent sensing properties for acetone [ 73 ].
A unique feature of this method is that the W 18 O 49 /Ti 3 C 2 Tx sensor has highly enhanced acetone sensing capability. When the W 18 O 49 /Ti 3 C 2 Tx sensor is exposed to air, the surface of W 18 O 49 /Ti 3 C 2 Tx composite can adsorb air and O 2 molecules. Oxygen molecules can capture electrons in oxide semiconductors and form oxygen species, reducing the electron concentration W 18 O 49 /Ti 3 C 2 Tx the resistance of the sensor. When exposed to acetone, acetone can react with ionic oxygen to release electrons [ 74 ]. The resistance of the W 18 O 49 /Ti 3 C 2 Tx sensor drops again. The oxygen vacancies of W 18 O 49 can promote the adsorption of oxygen and acetone molecules in air and acetone, respectively, which helps to increase the resistance change of the W 18 O 49 /Ti 3 C 2 Tx composite material, resulting in a high response of the sensor. Acetone molecules are easily adsorbed on the Ti 3 C 2 Tx surface, and the adsorbed acetone can reduce the number of carriers on the Ti 3 C 2 Tx surface, resulting in an enhanced resistance change of the W 18 O 49 /Ti 3 C 2 Tx composite [ 75 ]. Moreover, a barrier layer can be formed between the Ti 3 C 2 Tx sheet and the W 18 O 49 NRs, resulting in a decrease in resistance, and the sensor exhibits a greater resistance change; that is, a higher sensing response after contact with acetone [ 76 ] (This part discusses the most widely used two-dimensional nano materials in the field of gas sensors composed of nano materials, such as graphene, and performs a comprehensive and in-depth analysis and study of these materials, including their characteristics, specific action methods and their role in the development of sensors. This part also summarizes the gas sensor composed of two-dimensional nano materials, which is very comprehensive and perfect compared to the previous articles).
4.3. Gas Sensors Based on Other 2D Nanomaterials
Eunji Lee studied two-dimensional transition metal dihalides (TMDs) [ 77 ]. The inherent high specific surface area and their unique tunable band semiconductor characteristic gap make them attractive for sensing applications. In combination with two-dimensional nanomaterials, metal oxides are incorporated into two-dimensional transition metal dihalides, and the synergistic effect is used to improve the gas-sensing performance of these materials. The sensing mechanism and the synergistic effect of hybridization of 2D-TMDs and metal oxides were studied. 2D-tmd and its metal oxide hybrid material TMD have excellent capabilities in gas sensing.
The difference in this way is that the charge transfer process is between the gas molecule and the surface of the sensing material. 2D materials act as charge acceptors or donors and produce changes in electrical resistance. Exposed to reactive gases, gas molecules are electrostatically adsorbed on the surface of two-dimensional materials. The directional transfer of electron charges depends on the type of reductive or oxidizing reaction gas [ 78 ]. After resistance due to the desorption of gas molecules, the sensing material returns to its original value. Under the exposure of reducing gas, the resistance of the sensing material increases. At the working temperature, the target gas is introduced, and the electrons in the reducing gas are transferred to the conduction band of the metal oxide, resulting in a decrease in the resistance of the sensor. However, NH 3 acts as a reducing gas due to its lone electrons, and due to the leading role of oxygen ions, it exhibits the gas sensitivity and sensing performance of the gas sensor [ 79 ].
Bingshan Wang studied the synthesis of two-dimensional mesoporous ZnSnO 3 nanomaterials (TMZNS) by employing a template-free hydrothermal method [ 80 ]. The product was characterized by differential thermal analysis by TEM and SEM. The high-purity TMZNS was prepared by the hydrothermal method using a mixture of Zn 5 (OH) 6 (CO 3 ) 2 and ZnSnO 3 as the precursor. The two-dimensional mesoporous structure was studied through the process and mechanism of quasi-crystal growth. The gas-sensitivity characteristics of TMZNS were studied and characterized, and sensors based on TMZNS had excellent gas-sensitivity performance. When exposed to formaldehyde vapor, the sensor exhibited good gas sensitivity and sensing ability to formaldehyde gas [ 81 ].
In this research, the special feature is the surface of conductive material that occurs in the air, including a series of adsorption–oxidation–desorption. When TMZNS is in the air, a large number of oxygen molecules are adsorbed on the surface of the TMZNS sensor layer. The electrons on the surface of the TMZNS sensing layer are captured and adsorbed by a large amount of oxygen, which causes the trans-generation of oxygen species to oxygen ions to form a layer where the operating temperature and electrons are depleted. The barrier and resistance are relatively high. There is exposure to formaldehyde gas, formaldehyde gas interacts with the surface and it releases oxygen and electrons back into the air sensing materials. Chemically adsorbed oxygen and porous nanopores can improve sensing performance. A large specific surface area provides larger contact area and voids, more chemically active sites and less band gap energy helps the conduction band of the research ZnSnO 3 for the adsorption and capture of electrons on the surface [ 82 ]. Unique perforated nanopores are beneficial for adsorption to shorten the gas diffusion path. Therefore, TMZNS becomes an efficient gas sensor for HCHO detection.
Hanie Hashtroudi studied the gas sensing properties of two-dimensional hybrid nanomaterial conductance devices containing layered transition metals at room temperature and the effect of two-dimensional nanomaterials hybridization on the gas sensing properties [ 83 ]. By adding metals or polymers to the surface location, or by combining two or more different materials or by developing heterojunction layers, the sensing performance is improved. The gas is adsorbed on the active surface, which leads to a change in resistance, which in turn greatly improves its gas sensing capability [ 84 ].
The unique feature of this method is that the hybridization of various kinds of nanomaterials improves the sensitivity and acceleration response and recovery of the sensor. The interaction between gas molecules introduces new physical and electrical properties, thus improving the gas sensing performance. Surface engineering induced changes in the thickness and number of reaction sites for gas molecular interactions. The conductivity and charge transfer of the hybrid sensing, and the Fermi properties of the electrical and heterojunction layers at their interfaces change the directional energy differences of the current-carrying flow. Figure 5 demonstrates the dynamic response, sensitivity, and the selectivity of these two sensors for the NO 2 sensing at RT. Surface functionalization and unique treatment improve selectivity, sensitivity and specific sensing parameters and significantly improve the gas sensing characteristics and sensing performance of the sensor to gas in a natural environment [ 85 ].

( a ) The response of α-Fe 2 O 3 /rGO nanocomposite as a function of NO 2 concentration at RT, ( b ) dynamic responses of α-Fe 2 O 3 /rGO nanocomposite towards different NO 2 concentrations, and ( c ) selectivity of α-Fe 2 O 3 /rGO nanocomposite, rGO, and α-Fe 2 O 3 to different gases at RT. Adapted with permission.106 ( d ) The response of 12.2% α-Fe 2 O 3 /rGO as a function of NO 2 concentration at RT, ( e ) dynamic response of 12.2%α-Fe 2 O 3 /rGO at different NO 2 concentrations, and ( f ) selectivity of 12.2% α-Fe 2 O 3 /rGO and α-Fe 2 O 3 towards various gases. Adapted with permission.
5. Conclusions
The advantages of various dimension nanomaterials constitute gas sensors, which may become the development direction of a new generation of gas sensors due to their high sensing efficiency, good gas detection ability and high sensitivity. In this review, the development of gas sensors composed of nanomaterials is discussed from various dimensions of nanomaterials, sensing effect and sensing mechanism. At the same time, it reviewed the gas sensors composed of other dimension nanomaterials from the methods used, the characteristics of various categories and the advantages and contributions of each study. At present, most of the gas sensors have entered the nanometer field, which has a significant advantage over the traditional sensors, and will provide a new way to solve the problem of gas sensor sensing efficiency and sensing ability in gas detection. Future gas sensors composed of nanomaterials will have multiple functional characteristics, such as high gas sensing efficiency, high sensing sensitivity and a strong ability to detect specific gases, providing better development in the sensor field.
Acknowledgments
This work was supported by the National Natural Science Foundation of Liaoning (Grant Nos. 2020-MS-219).
Funding Statement
Author contributions.
Conceptualization, D.L. and K.X.; methodology, K.X.; software, D.L.; validation, K.X.; formal analysis, D.L.; investigation, D.L.; resources, K.X.; data curation, D.L.; writing—original draft preparation, D.L.; writing—review and editing, D.L.; visualization, D.L.; supervision, K.X.; project administration, K.X.; funding acquisition, K.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.

As a society-owned publisher with a legacy of serving scientific communities, we are committed to offering a home to all scientifically valid and rigorously reviewed research. In doing so, we aim to accelerate the dissemination of scientific knowledge and the advancement of scholarly communications to benefit all.
Nano Express supports this mission and actively demonstrates our core values of inclusive publishing and trusted science . To find out more about these values and how they can help you publish your next paper with us, visit our journal scope .
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Recent advances in g-C 3 N 4 based gas sensors for the detection of toxic and flammable gases: a review
Vijendra Singh Bhati 4,1 , Vishakha Takhar 2 , Ramesh Raliya 1 , Mahesh Kumar 4,3 and Rupak Banerjee 2
Published 4 March 2022 • © 2022 The Author(s). Published by IOP Publishing Ltd Nano Express , Volume 3 , Number 1 Focus on 2D Materials-Based Devices Citation Vijendra Singh Bhati et al 2022 Nano Ex. 3 014003 DOI 10.1088/2632-959X/ac477b
Article metrics
2846 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Nano Biotechnology Research Centre, Indian Farmers Fertilizers Cooperative Limited, Gandhinagar,-382423 India
2 Department of Physics Indian Institute of Technology Gandhinagar, Gandhinagar-382355 India
3 Department of Electrical Engineering, Indian Institute of Technology Jodhpur, Jodhpur-342011 India
Author notes
4 Authors to whom any correspondence should be addressed.
Vijendra Singh Bhati https://orcid.org/0000-0002-8894-8159
Vishakha Takhar https://orcid.org/0000-0003-0088-9383
Ramesh Raliya https://orcid.org/0000-0002-9534-4943
Mahesh Kumar https://orcid.org/0000-0002-5357-7300
Rupak Banerjee https://orcid.org/0000-0002-1189-1502
- Received 10 November 2021
- Revised 18 December 2021
- Accepted 30 December 2021
- Published 4 March 2022
Peer review information
Method : Double-anonymous Revisions: 1 Screened for originality? Yes
Buy this article in print
In recent years, many 2D nanomaterials like graphene, MoS 2 , phosphorene, and metal oxide nanosheets have been investigated for gas sensing applications due to their excellent properties. Amongst other 2D nanomaterials, graphitic carbon nitride (g-C 3 N 4 ) has attracted significant attention owing to its simple synthesis process, tunable electronic properties, and exceptional physicochemical properties. Such remarkable properties assert g-C 3 N 4 as a potential candidate for the next-generation high-performance gas sensors employed in the detection of toxic and flammable gases. Although several articles and reviews are available on g-C 3 N 4 for their synthesis, functionalities, and applications for the detection of humidity. Few of them have focused their attention on gas sensing using g-C 3 N 4 . Thus, in this review, we have methodically summed up the recent advances in g-C 3 N 4 and its composites-based gas sensor for the detection of toxic and flammable gases. Moreover, we have also incorporated the synthesis strategies and the comprehensive physics of g-C 3 N 4 based gas sensors. Additionally, different approaches are presented for the enhancement of gas sensing/detecting properties of g-C 3 N 4 based gas sensors. Finally, the challenges and future scope of g-C 3 N 4 based gas sensors for real-time monitoring of gases have been discussed.
Export citation and abstract BibTeX RIS
Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The area of 2D nanomaterials has expanded been enormous since the discovery of graphene. Several researchers have investigated various interesting physical phenomena when the dimension of materials transforms from 3D to 2D. The exciting properties of 2D nanomaterials include quantum confinement, good flexibility, high transparency, and excellent mechanical strength that make them suitable for wide industrial applications [ 1 – 4 ]. Mono-layer and few-layered nanomaterials exhibit some exciting physics because of the quantum confinement effect than conventional bulk materials [ 5 ]. Very interestingly, a small variation in the atomic layers of 2D nanomaterials will result in a change in its physical properties. Therefore, it is imperative to control the growth parameters of 2D nanomaterials for their specific applications. Nevertheless, their sustainability and low mass-production restrict their applications, but these bottlenecks can be circumvented by utilizing some modified synthesis techniques.
On the other hand, metal oxides (for example, ZnO, SnO 2 , TiO 2 , V 2 O 5 , CuO, and NiO) have been extensively employed as gas sensors because of their good sensitivity, fast response/recovery time, tunable transport properties, low cost, compatibility with semiconductor circuits, and good selectivity [ 6 – 16 ]. These sensors have drawn in critical consideration in different fields, for example, air quality investigation, breath analysis, and monitoring of chemical residues in industries. The basic working principles of these chemi-resistive sensors depend on the adsorption/desorption of target gas molecules on the surface of the sensor and the modifications in their resistance. In this direction, various types of chemi-resistive sensors have been explored to obtain excellent sensing properties. However, other carbon nanomaterials such as graphene oxide (GO), and carbon nanotubes (CNTs) have additionally been examined for gas sensing applications due to their large surface area, good mechanical strength, and high conductivity [ 17 ]. For example, Liu et al Noticed that Young's modulus of graphene oxides was varied from 380 to 470 GPa as the coverage of oxygen functional groups changed, respectively. However, the corresponding variations in Young's modulus of the amorphous graphene oxides with comparable coverage were smaller at 290–430 GPa. Moreover, the intrinsic thermal conductivity of graphene oxide is around 73W (mK) −1 with an oxidation degree of 0.35 at room temperature [ 18 ]. In contrast, CNTs are very strong in the axial direction, where Young's modulus is in the range of 270–950 GPa, and the tensile strength is in the range of 11–63 GPa. [ 19 ] Additionally, depending on the structure and order, CNTs exhibit different values of thermal conductivity, from the level of thermal insulation with the thermal conductivity of 0.1–6600 W mK −1 [ 20 ]. Mortazavi et al found that the elastic modulus and tensile strength of g-C 3 N 4 varied from 320-210 GPa, and 47-30 GPa, respectively for two different structures of g-C 3 N 4 sheets (s-triazine-based g-C 3 N 4 and tri-triazine-based g-C 3 N 4 ). Moreover, using equilibrium molecular dynamics simulations, the thermal conductivities of free-standing g-C 3 N 4 structures were also predicted to be around 7.6 W mK −1 and 3.5 W mK −1 [ 21 ]. However, metal oxide-based gas sensors have some disadvantages that include high operating temperature and higher consumption of power. Consequently, several studies have been done to improve the sensing response even at low operating temperatures. To improve the gas detecting execution, some of the techniques such as doping of transition metal elements, composite materials using two dissimilar nanomaterials (for example, combination of g-C 3 N 4 with metal oxide nanostructures), and functionalization of noble nanoparticles on the sensor surface have been successfully implemented [ 11 , 22 – 26 ].
The recent advancement in the synthesis and device fabrication of 2D g-C 3 N 4 has been widely explored due to its excellent optoelectronics and nanoelectronics application over the last two decades [ 27 ]. Moreover, the exceptional properties of g-C 3 N 4 make it a suitable candidate as a gas sensor for the detection of toxic and flammable gases. Carbon nitride (CN) consists of crystalline bonds of nitrogen and carbon atoms with seven phases [ 28 ]. Amidst all phases, graphitic carbon nitride (g-C 3 N 4 ) is the well-known stable phase of CN under atmospheric conditions. Carbon and nitrogen atoms of g-C 3 N 4 construct a tetrahedral geometry with multiple hybridizations (sp, sp 2 , and sp 3 ) [ 29 ]. Due to the existence of several C=N bonds, a few crystalline forms of C 3 N 4 possess a hardness similar to that of diamond [ 28 ]. An aromatic plane is formed by the heptazine or triazine units in the g-C 3 N 4 structures [ 30 ]. Interestingly, g-C 3 N 4 possesses defects containing polymeric g-CN, which act as an active site for incoming gas molecules. However, the electrical conductivity of g-C 3 N 4 can be modified by the self-doping method (carbon self-doping), which could change the electronic as well as the surface properties [ 31 ]. Few reports revealed that the replacement of N atoms with C atoms is possible, which results in the creation of delocalized π bonds [ 31 ]. Thus, the electrical conductivity of g-C 3 N 4 can be boosted by delocalized π bonds that facilitate electron transfer. Also, the carbon self-doping reduces the bandgap of g-C 3 N 4 and thus enhances the visible light absorption. The g-C 3 N 4 can be considered as a suitable material for gas sensing applications owing to its biocompatibility, favorable electronic band structure, simple synthesis, physicochemical properties, large surface active sites, tunable electronic structures, and thermal stability against the chemical reactions [ 31 ]. However, further improvement in the synthesis, as well as their gas sensing applications, can be performed via metal-doped g-C 3 N 4 , metal oxide/g-C 3 N 4 , and surface functionalization with g-C 3 N 4 approaches [ 32 – 35 ]. The gas sensing properties of different carbon nanomaterials-based sensors are provided in table 1 .
Table 1. Gas sensing properties of carbon nanomaterials and their composite-based gas sensors.
Here, we present a state-of-the-art of current developments in g-C 3 N 4 based gas sensors and have attempted to cover around two decades of research in this area. We begin with a short introduction of g-C 3 N 4, addressing its different properties and advantages. Thereafter, we discuss some of the current synthesis strategies of g-C 3 N 4 by introducing moderations into the conventional growth techniques. The sensing mechanism and related physics for the detection of target gas molecules are also explained in detail. Then, we introduce some of the techniques such as doping, surface functionalization, and the composite of different nanomaterials for enhancing the performance of g-C 3 N 4 based gas sensors. Lastly, this review summarizes the challenges and future scope of g-C 3 N 4 based gas sensors for practical applications.
2. Synthesis of 2D g-C 3 N 4 and its composites
Several synthesis techniques have been reported on g-C 3 N 4 and its composites based on their different applications. In the context of the present review, a short description of the synthesis process of g-C 3 N 4 is reported in this section for the development of sensors used to detect toxic and flammable gases. The most widespread synthesis techniques and strategies of g-C 3 N 4 are briefly discussed in the following sections:

2.1. Sonication method
The sonication process is the simplest route for the preparation of g-C 3 N 4 and its composites. It involves the mixing of g-C 3 N 4 powders (grown by nitrogen-containing precursors such as urea, melamine) with appropriate metal or metal oxides in water or an organic solvent solution. Thereby, the formation of composites is possible so that metal oxide could disperse over the surface of g-C 3 N 4 throughout the mixing or stirring process. The nanocomposite is then retrieved after the complete evaporation of solvent or water under the heat treatment. For example, Li et al developed g-C 3 N 4 @Cs x WO 3 nanocomposites via ultrasonic-assisted strategy [ 65 ]. Similarly, Konstas et al synthesized g-C 3 N 4 /SrTiO 3 heterojunction by the sonication method [ 66 ]. In another study, Wen et al synthesized MoS 2 /g-C 3 N 4 by using ultrasonic dispersion in ethanol and utilized visible light irradiation for the removal of nitric oxide [ 67 ]. Yuan et al prepared g-C 3 N 4 /ZnO nanosheets via a simple precipitation-calcination method for photocatalytic application [ 68 ]. Thus, a good intermixing between g-C 3 N 4 and metal oxide and consequently their heterojunctions formation could be achieved with the use of ultrasonic wave energy. Moreover, other factors such as the mechanical stirring and particle motion in the ultrasonic field can alter the reprecipitation and dissolution.
2.2. Calcination method
g-C 3 N 4 can be synthesized via thermal decomposition of several nitrogen- and carbon-rich organic precursors under a controlled environment [ 69 ]. Additionally, g-C 3 N 4 based composites can be obtained through the mixing of metal oxides with g-C 3 N 4 precursors in a crucible followed by heat treatment in the furnace. For example, Xiao et al synthesized g-C 3 N 4 nanosheets by calcination method and utilized them to detect CH 4 under UV- light irradiation [ 70 ]. For example, Ibrahim et al have grown g-C 3 N 4 using the calcination method for the detection of hydrogen gas [ 71 ]. They used urea as a precursor and then heated it to 550 °C to obtain irregular layers of g-C 3 N 4 . In another study, SnO 2 -g-C 3 N 4 composite was synthesized via calcination of stannous chloride pentahydrate and melamine for 3 h at 500 °C and utilized for acetone detection [ 30 ]. Recently, g-C 3 N 4 /ZnO composite was prepared through the precipitation-calcination technique for the detection of methane [ 60 ]. In this study, g-C 3 N 4 was synthesized by thermal reduction of urea in a tube furnace at different temperatures (250, 350, and 550 °C for 2 h). Thereafter, g-C 3 N 4 was incorporated into other precursors such as sodium hydroxide and zinc nitrate hexahydrate solution to prepare g-C 3 N 4 /ZnO.
2.3. Hydrothermal method
The hydrothermal method has become a promising technology for developing nanocomposite materials [ 72 ]. Using this method, the crystallite size, morphology, and crystallinity of the materials could be improved due to the process being executed at relatively lower temperatures. Additionally, there are some other parameters such as pressure, reaction temperature, pH, additives, types of solvent, aging time, and precursor composition that control the surface chemistry and play a vital role in the synthesis process. Importantly, this method utilizes simple instruments (autoclave) and processes that are environmentally friendly.
2.4. Sol-gel method
The sol-gel is a simple and extensively used technique for the synthesis of nanomaterials. In this method, the precursors are mixed in a solvent and followed by stirring at a low temperature. Thereafter, a sol is obtained in the form of suspended particles. An agglomeration of colloidal particles with time results in the formation of a gel. These gel-like structures further experience the hydrolysis and condensation reaction that lead to the development of metal hydroxide [ 73 ]. Consequently, the new bonds of oxygen bridges are formed via hydrolysis-polycondensation reactions and followed by the annealing process to form g-C 3 N 4 .
2.5. Microwave method
The synthesis of nanomaterials by utilizing the microwave method has become very popular due to the homogenous heating of precursors at a faster speed. This method has penetration properties that enable uniform heating of the reaction solution. As a result, the development of crystallites with narrow size distribution occurs attributed to the consistent nucleation and rapid crystal growth [ 74 ]. Moreover, the interface energy between the consisting phases decreases due to the thermal treatment of desired materials, which further allows the photogenerated electrons to flow easily through the interface [ 75 , 76 ]. Hence, a microwave method can provide good contact between the two semiconductor materials. The g-C 3 N 4 is unstable as an organic polymer in nature and decomposes at higher temperatures. Thus, the semiconductor which is coupled to the g-C 3 N 4 will activate oxygen and hence, increase the decomposition of g-C 3 N 4 [ 77 ]. For example, Shorie et al prepared g-C 3 N 4 quantum dots via microwave-assisted solvothermal technique for the removal of Hg 2+ ions from aqueous solutions [ 78 ]. In another study, a one-step microwave reduction technique was utilized for the synthesis of WO 3 /g-C 3 N 4 composite having WO 3 cluster particles distributed on g-C 3 N 4 for ethanol detection [ 79 ]. The ensuing advantages and disadvantages of all the above-mentioned synthesis techniques of g-C 3 N 4 are compared in table 2 .
Table 2. Advantages and disadvantages of different synthesis techniques for g-C 3 N 4 .
The combination of two basic structures like tri-s-triazine (C 6 N 7 ) rings and s-triazine (C 3 N 3 ) rings, for the most part, frames the precise engineering of C 3 N 4 [ 80 ]. Generally, the synthesis of g-C 3 N 4 nanomaterials can be achieved by thermal condensation with nitrogen-rich precursors (melamine, urea, cyanimide, thiourea, and dicyanamide) [ 71 , 81 ]. The formation of C 6 N 7 and C 3 N 3 rings mainly depends on the reaction process [ 82 ]. Zhang et al synthesized g-C 3 N 4 using thiourea or urea as a precursor and found that the synthesis has predominantly occurred via nucleophilic addition, polycondensation, and polymerization steps [ 83 ]. During the synthesis process, it was observed that the urea or thiourea molecules were initially transformed to melamine through additional and condensation reactions. However, the dicyandiamide molecules were converted to melamine [ 84 ]. Under the increasing temperature, the C 6 N 7 rings were formed by rearrangement of melamine, and that was further polymerized into g-C 3 N 4 nanomaterials. Hence, the thermal polymerization of urea, cyanamide, melamine, dicyandiamide, or thiourea at different temperatures (450 °C–550 °C) leads to the synthesis of g-C 3 N 4 [ 85 , 86 ].
Besides, noble nanoparticles such as Pd, Au, Pt can be coated with g-C 3 N 4 for gas sensing applications. For example, Li et al had grown Au/g-C 3 N 4 using a two-step process [ 87 ]. In this method, firstly, bulk g-C 3 N 4 was synthesized using melem hydrate fibers (as a template) via polycondensation, and thereafter, the bulk g-C 3 N 4 crystals were heated at 600 °C to form porous g-C 3 N 4 . Later on, the formation of Au nanoparticles@porous g-C 3 N 4 fibers towards the detection of NO 2 gas was achieved using the systematic addition of chloroauric acid, and sodium citrate dehydrates, under the irradiation with a halogen lamp. Similarly, Ibrahim et al have grown irregular layers of g-C 3 N 4 by the calcination of urea (as a precursor) at 550 °C [ 71 ]. After that, Pd nanoparticles (ammonium tetrachloropalladate as a precursor) were integrated with the g-C 3 N 4 matrix to improve the sensing response towards hydrogen gas.
Metal sulfide can be combined with g-C 3 N 4 to enhance the sensing response. Recently, Sun et al had developed g-C 3 N 4 using the thermal polymerization process of urea for 4 h at 550 °C and then ultrasonicated the solution for 30 min [ 64 ]. Thereafter, SnS 2 was synthesized on g-C 3 N 4 nanosheets by solvothermal technique for 12 h at 180 °C with the help of thioacetamide and stannous chloride (as a precursor) to acquire a g-C 3 N 4 /SnS 2 mixture for NO 2 gas sensing. During the synthesis process, the different concentrations of g-C 3 N 4 (5, 10, and 15 wt%) were taken in the SnS 2 matrix. The preparation process of g-C 3 N 4 /SnS 2 heterostructures can be seen in the schematic diagram (figure 1 (a)). It was noticed that the SnS 2 microsphere was well-dispersed on the loosely integrated hierarchical nanosheets, as shown in figure 1 (b).

Figure 1. (a) Schematic diagram of the synthesis process of g–C 3 N 4 /SnS 2 heterostructures (b) SEM image of g–C 3 N 4 /SnS 2 heterostructures. Reproduced from [ 64 ], © IOP Publishing Ltd. All rights reserved.
Download figure:
In another report, Meng et al reported g-C 3 N 4 nanosheets sensitized ZnO-rGO composites for ethanol sensing [ 88 ]. The electrostatic self-assembly and ultrasonic dispersion method were adopted to synthesize the 2D graphene oxide (GO)-hybridized g-C 3 N 4 composite. Afterward, a hydrothermal process was used to coat ZnO nanoparticles on GO/g-C 3 N 4 , and subsequently, GO was reduced into rGO (figure 2 (a)). The SEM image of the final product can be seen in figure 2 (b). Tian et al have grown Pt/ZnO/g-C 3 N 4 composite for detection towards NO 2 and ethanol [ 89 ]. The schematic diagram of the growth process for Pt/ZnO/g-C 3 N 4 composite is shown in figure 2 (c). Pyrolysis of dicyandiamide led to the growth of bulk g-C 3 N 4 after exposure for 4 h at 520 °C. Later on, bulk g-C 3 N 4 was thermally exfoliated and formed g-C 3 N 4 nanosheets after 8 h of heat treatment at 500 °C in a vacuum furnace. The SEM image of ZnO nanorods fabricated on g-C 3 N 4 nanosheets is shown in figure 2 (d).

Figure 2. (a) Schematic illustration of the synthesis of ZnO/rGO/g-C 3 N 4 composite and (b) its SEM image. Reproduced with permission [ 88 ]. Copyright 2019, ACS. (c) Schematic diagram of synthesis steps of Pt/ZnO/g-C 3 N 4 nanostructures, and (d) its SEM image. Reprinted from [ 89 ], © 2017 Elsevier B.V. All rights reserved.
The usage of metal oxide/g-C 3 N 4 composite-based gas sensors has been extensively investigated. For instance, Wang et al developed a ZnO/g-C 3 N 4 composite for the recognition of NO 2 gas [ 89 ]. In this process, the calcination (dicyandiamide at 550 °C) and hydrothermal methods were used to synthesize g-C 3 N 4 , and ZnO, respectively. They dispersed bulk g-C 3 N 4 in 30 ml of DI water and ultrasonicated for 2 h. Then, 100 mg of synthesized ZnO powder was incorporated into the above solution and appropriately dissolved. Eventually, the final product was calcined for 2 h at 400 °C to achieve ZnO/g-C 3 N 4 having 5, 10, and 15 wt% concentration of g-C 3 N 4 .
Additionally, the exfoliated g-C 3 N 4 /graphene composite can be synthesized via the probe sonication method, where graphene powder is a precursor. Ta et al constructed an Ag@rGO/g-C 3 N 4 layered structure through three stage-process for detecting NO 2 [ 90 ]. Firstly, melamine was heated at 550 °C for 4 h under an ambient atmosphere to produce g-C 3 N 4 . Then following the Hummers' method, the formed rGO and g-C 3 N 4 were combined in distilled water and subsequently stirred and ultrasonicated for 3 h. In the last stage, the addition of AgNO 3 (source of Ag) and NaBH 4 (a reducing agent) in the above solution produced Ag@rGO/g-C 3 N 4 composite.
3. Gas sensing mechanism of g-C 3 N 4 based sensors
This section highlights state of art in toxic and flammable gas detection using g-C 3 N 4 and its composite-based sensors. The g-C 3 N 4 nanosheets can be considered as a principle part of a gas sensor and surface modifier when incorporate with other nanomaterials.
Firstly, the sensing mechanism of a pure g-C 3 N 4 based sensor for the detection of toxic and flammable gases has been reviewed. Moreover, high adsorption energy and unique surface morphology of g-C 3 N 4 promote the high sensing response. The excellent charge transfer properties of g-C 3 N 4 during the interaction of different target gas molecules appreciably rely on the kind of conductivity. For example, Li et al investigated the sensing characteristics of nanoporous carbon nitride fibers towards the detection of NO 2 gas [ 87 ]. The gas sensing behavior towards reducing and neutral gas is elucidated in the schematic diagram in figure 3 . It is proposed that the sensing mechanism can be understood via the self-protonation of g-C 3 N 4 nanosheets. After self-protonation of g-C 3 N 4 occurs, the process of oxidation takes place. Very interestingly, after the pretreatment effect of g-C 3 N 4 with H 2 SO 4 , it is observed that the protonation of p-type g–C 3 N 4 increases drastically.

Figure 3. Schematic representation of sensing mechanism of g-C 3 N 4 for the detection of oxidizing (case 1), reducing (case 2), and neutral gas (case 3). Reproduced from [ 27 ], with permission of The Royal Society of Chemistry.
Further, high-temperature annealing of g-C 3 N 4 leads to changes from SO 4 2− to SO 3 . To acquire the electron acceptors like oxygen-containing groups (for example, C=O and O=C–OH) on the g-C 3 N 4 surface, an oxidation process is needed. Thus, more positive charges are accumulated on the surface due to electron acceptors. For example, the resistance of the surface decreases when p-type g-C 3 N 4 interacts with NO 2 or other similar gases, which withdraw electrons. The reduced resistance demonstrates enhanced p-doping of g-C 3 N 4 . Carbon nitrides, in general, contain amino groups that tend to attract oxygen molecules [ 33 ]. When NO 2 molecules interact with amino groups in g–C 3 N 4 , they capture more electrons because of higher electronegativity as compared to carbon atoms. Thereafter, the process of charge transfer takes place from the N atom of g-C 3 N 4 to that of NO 2 gas. Consequently, positive charge vacancy is created in g–C 3 N 4 , which leads to more p-type conductivity in g-C 3 N 4 . Afterward, NO 2 will react with the H 2 O molecules in the atmospheric air and form HNO 3 . As a consequence, this reaction could protonate g-C 3 N 4 in the course of the adsorption process. In contrast, the surface resistance increases because of diminished p-doping in g-C 3 N 4 during the interaction of reducing gases (acetone, ammonia) with g-C 3 N 4 . However, the charge scattering effect becomes dominant due to physisorbed molecules at the surface of g-C 3 N 4 under the exposure of neutral gas (hexane or ether). Therefore, the overall resistance of the surface of g-C 3 N 4 increases by the charge scattering effect. Hence, the large pore area, pore-volume, and surface area of pure g-C 3 N 4 are responsible factors for improving the sensing response [ 29 , 58 , 60 , 91 ]. Nevertheless, g-C 3 N 4 could be combined with metal and metal oxide to further enhance gas sensing properties. The gas sensing mechanism of g-C 3 N 4 /metal oxide/2D nanomaterials-based composite has been illustrated in the next section.
There are several reports on g-C 3 N 4 /metal oxide composite-based gas sensors. For instance, Meng et al developed a ternary nanocomposite based on ZnO/rGO/g-C 3 N 4 for ethanol sensing [ 88 ]. Their sensor exhibited an excellent response of about 178 (R a /R g ) towards 100 ppm ethanol at 300 °C along with a detection limit lower than 500 ppb, which was around 9-folds and 2-folds greater than pure ZnO and ZnO/rGO samples, respectively. Figure 4 (a) shows the repeatability measurement of ZnO/rGO/g-C 3 N 4 nanocomposites-based sensors and exhibited almost the same responses for three consecutive cycles towards 100 ppm ethanol at 300 °C. They also performed the selectivity test for various gases (100 ppm concentration for each gas) with the sensor at 300 °C, as shown in figure 4 (b). Surprisingly, the sensor displayed high selectivity to ethanol compared to other interfering gases. Their sensors (both pure ZnO and nanocomposite) were also tested for long-term stability (up to 14 days) under the same operating conditions and indicated that the sensor based on ZnO/rGO/g-C 3 N 4 nanocomposite revealed high stability with slight fluctuations at temperatures >150 °C (figure 4 (c)). These ternary composites-based sensors showed a higher gas sensing response to ethanol due to its small size, the high catalytic activity of g-C 3 N 4 , and excellent electron conductivity. The gas sensing mechanism of the ZnO/rGO/g-C 3 N 4 nanocomposite-based sensor has been explained in figure 4 (d). The small size effect of ZnO contributes to the sensitization. Interestingly, when the morphology of ZnO was transformed from bulk sheet to nanoparticles (less than 20 nm), the sensing response was increased drastically. The significant change in the resistance occurred for sensing film when the depletion layer enclosed the complete particle and formed fully depleted throughout the interaction with gas molecules. Besides, the excellent electron conductivity of rGO additionally assists with more detection capabilities. Moreover, the development of p-n heterojunctions at the interface of ZnO and rGO prompts the expulsion of more electrons from the conduction band of ZnO. Thus, the total resistance of nanocomposite is modified to a large extent. Apart from this, the sensitivity was enhanced by the homogenous coating and electronic sensitization mechanism of g-C 3 N 4 . They also described that the existence of N-atoms imparts more electrons for g-C 3 N 4 that increase an electron density in the sensing film. Under high-temperature operation, the electron-hole pair generation improved due to the wide band-gap of g-C 3 N 4 . As a consequence, electron movement from the conduction band transforms the O 2 molecules to oxygen ions species (O 2 − , O − , and O 2− ). On the other hand, the 2D/2D heterojunction interface between rGO and g-C 3 N 4 allows more electrons to drift to the ZnO and does not reduce the resistance instantly. Thus, an appreciable change in the resistance is registered during the interaction with the target gas resulting in more sensitivity. The enhanced sensing response of ZnO/rGO/g-C 3 N 4 nanocomposite-based sensor towards ethanol was mainly attributed to the combined effects of the small size of ZnO, excellent electron conductivity of rGO, p-n heterojunctions effect between ZnO and rGO, and improved electron-hole generation due to the wide band-gap of g-C 3 N 4 under high-temperature operation [ 88 , 92 ].

Figure 4. (a) Repeatability measurement, (b) selectivity test, and (c) long-term stability (up to 14 days) of ZnO/rGO/g-C 3 N 4 based sensor towards 100 ppm ethanol at 300 °C. (d) Schematic illustration of the interaction process between ethanol and ZnO/rGO/g-C 3 N 4 nanocomposites. Reprinted with permission from [ 88 ], Copyright (2019) American Chemical Society.
Recently, Sun et al developed a 2D/2D heterojunction based on a g-C 3 N 4 /SnS 2 sensor to support the detecting execution of SnS 2 towards the NO 2 detection at room temperature [ 64 ]. Their pristine SnS 2 sensor was inefficient while operating at room temperature.
Interestingly, the g-C 3 N 4 /SnS 2 -based sensor showed a high response (503%) and a small recovery time (166 s) to 1 ppm NO 2 at room temperature. For repeatability measurements, the sensor based on 10% g-C 3 N 4 /SnS 2 was studied for 1 ppm NO 2 gas up to 4 cycles (figure 5 (a)). Interestingly, the results for all cycles were almost the same, and the sensor exhibited identical sensing response and recovery time. A selectivity measurement was performed for several gases to investigate further the performance of the 10% g-C 3 N 4 /SnS 2 based gas sensor, as shown in figure 5 (b). Their sensor showed high selectivity towards NO 2 gas in comparison to other gases (C 2 H 5 OH, CH 4 , CO, NH 3 , and H 2 S) for 1 ppm concentration at room temperature. Moreover, the 10% g-C 3 N 4 /SnS 2 based sensor was investigated for long-term stability up to 3 months. Their sensor showed minor fluctuations in sensing response (526 ± 31%) as well as recovery time (161 ± 12 s) which means that the sensor had appreciable long-term stability (figure 5 (c)). The sensing mechanism was explained using the band diagram of g-C 3 N 4 and SnS 2 , displayed as the band alignments before and after contact between both nanomaterials in figure 5 (d). The work function of g-C 3 N 4 (4.34 eV) is smaller as compared to SnS 2 (5.02 eV) which means the Fermi level of g-C 3 N 4 is higher than SnS 2 . When both materials are combined together, the electron moves from g-C 3 N 4 to SnS 2 until the Fermi level aligns to the same energy state. As a consequence, the formation of 2D/2D heterojunctions occurs at the interface of g-C 3 N 4 and SnS 2 . Thus, the development of a large interfacial area at the interface of both nanomaterials makes it easier for electron movement from g-C 3 N 4 and SnS 2, which enhances electron transfer at the 2D/2D heterojunctions (due to face-to-face contact of heterojunctions). This enhanced electron transfer process in heterojunctions further boosts carrier mobility. On that account, the g-C 3 N 4 /SnS 2 based sensor showed good recovery during the exposure of NO 2 . Besides, the enhanced electron transfer process will lead to the accumulation of more electrons in SnS 2 and hence, decreases the resistance of SnS 2 . Thus, the reduction in the resistance of SnS 2 is more favorable for NO 2 sensing at room temperature with high sensing response [ 93 ]. Additionally, the presence of a large specific surface area in the g-C 3 N 4 /SnS 2 heterostructures provides more reaction sites for incoming NO 2 gas molecules during the adsorption/desorption process. Moreover, the combination of both these nanomaterials (g-C 3 N 4 and SnS 2 ) can diminish the activation energy needed in NO 2 desorption and decrease the recovery time. More prominently, the enhanced specific surface area of these 2D/2D heterostructured microflowers allows more adsorption of NO 2 gas. These combined effects improved the sensing response towards NO 2 gas at room temperature.

Figure 5. (a) Repeatability test, (b) selectivity test, and (c) long-term stability (up to 3 months) of 10% g-C 3 N 4 /SnS 2 to 1 ppm NO 2 at room-temperature (d) Band diagrams of g–C 3 N 4 /SnS 2 based sensor before and after equilibrium and their corresponding sensing mechanism. Reproduced from [ 64 ], © IOP Publishing Ltd. All rights reserved.
4. Techniques for improvement of the gas-sensing performance of g-C 3 N 4 based sensors
As discussed in the previous section, the 2D g-C 3 N 4 nanosheets have already registered their presence in the area of gas sensors by monitoring numerous toxic and hazardous environmental gases. These nanosheets can be considered reliable gas sensors attributed to their excellent structural as well as electronic properties. However, the fabrication of high-performance gas sensors using a pure form of g-C 3 N 4 still has some unresolved challenges due to its incomplete recovery and poor sensing response, which limits its practical uses. On that record, different methods have been adopted by researchers to boost the sensitivity and selectivity of g-C 3 N 4 based gas sensors. There are some efficient approaches such as doping, functionalization of noble nanoparticles, and nanocomposite of different material with g-C 3 N 4 to improve the gas sensing performance as listed below:
4.1. Doping
Introducing defects such as semiconductor, graphene, and metals into g-C 3 N 4 could restructure the morphology of g-C 3 N 4 in different forms (nanorods, nanosheets, mesoporous structure, and hierarchical structures). These morphologies and structures greatly influence the electronic, physical, and chemical properties of g-C 3 N 4 and its applications. Moreover, it has been observed that the doping of metallic and non-metallic elements in the bulk form of g-C 3 N 4 creates a defects state in the forbidden band which reduces the bandgap energy [ 94 ]. Additionally, g-C 3 N 4 nanosheets are extensively used as gas sensors owing to their high adsorption capability, non-toxicity, cost-effectiveness, and excellent electrical conductivity. It was noticed that the transition metal dopants substantially affect the adsorption capability of target gas molecules when embedded with g-C 3 N 4 . This effect is caused by the presence of more active sites on the g-C 3 N 4 surface and with high adsorption energies due to which, the gas sensing properties of g-C 3 N 4 can be improved.
For example, Zhang et al investigated metal-doped g-C 3 N 4 for highly sensitive NO 2 sensors by using density functional theory (DFT) calculations [ 95 ]. They considered several metal-doped (V-, Ag-, Ti-, Au-, Pd-, Co-, Pt-, Cr-, Mn-, Cu-, Na-, Fe-, Li-, K-) g-C 3 N 4 sheets. It was found that the NO 2 , CO, N 2 , CO 2 , NH 3 gas molecules adsorbed on metal-doped g-C 3 N 4 through chemical bonds. Charge transfer complexes arose when the chemisorbed gas molecules interacted with metal-doped g-C 3 N 4 . As a consequence, different charges were transferred from metal-doped g-C 3 N 4 to gas molecules. Later on, pure and metal-doped g-C 3 N 4 sheets were investigated as prime captures for specific gas molecules as per the adsorption energy, the density of states, and isosurface of electron density difference (displayed in figures 6 (a)–(e)). Among all metal-doped g-C 3 N 4 sheets, K-, Na-, Ag-, and Li-doped g-C 3 N 4 were considered sensitive towards NO 2 gas. Moreover, it was found that the adsorption energies between NO 2 gas and K-, Ag-, Li-, and Na-doped g-C 3 N 4 were remarkably higher than those of the other target gas molecules (CO 2 , CO, NH 3 , and N 2 ). The positive shifts of the density of state and formation of new electron states at Fermi level for NO 2 gas molecules/Ag-, K-, Na-, and Li-doped g-C 3 N 4 validated the outstanding selectivity of Ag-, K-, Na-, and Li-doped g-C 3 N 4 for NO 2 gas [ 95 ]. In addition to this, charge transfer results further indicate the presence of chemical interactions between NO 2 and K-, Ag-, Li-, Na-doped g-C 3 N 4 .

Figure 6. (a)–(e) Isosurface related to the electron density difference of different gas molecules adsorbing on Ag-doped C 3 N 4 sheet and their charge accumulation/depletion are displayed in yellow and teal, respectively. Reprinted from [ 95 ], © 2018 Elsevier B.V. All rights reserved.
Similarly, Zhou et al studied the adsorption behavior of CO 2 on the surface of g-C 3 N 4 with W–, Mo–, and Cr– elements using the DFT calculations [ 96 ]. Their results suggested that the conductivity of systems was highly enhanced by W–, Mo–, and Cr– doping. Thereafter, they established the E ads as: Cr (E ads = −0.18 eV) < W (E ads = −1.1 eV) < Mo (E ads = −1.2 eV). Thus, it can be summarized that the interaction energy between CO 2 and Mo–doped g–C 3 N 4 based system is quite negative (Mo (E ads = −1.2 eV)) as compared to other systems (Cr (E ads = −0.18 eV) and W(E ads = −1.1 eV)). Recently, Zhu et al investigated the adsorption of CO 2 gas molecules on pure g-C 3 N 4 and B-doped g-C 3 N 4 by utilizing DFT computations [ 97 ]. Their results revealed that the B-doped g-C 3 N 4 system is more selective towards the CO 2 gas molecules than the pure g-C 3 N 4 . In another study, Sethuraman et al synthesized Nb-doped urea blended melamine derived g-C 3 N 4 (200Nb-UMCN) nanosheets [ 98 ]. In this synthesis approach, they used a 5:1 ratio of urea and melamine doped with different amounts of Nb precursor (100, 200, and 300 mg) followed by pyrolysis at 550 °C for 3 h. Thereafter, the sensors based on Nb-doped UMCN were used for the detection of NH 3 at room temperature. However, they noticed that the sensor based on 200 Nb-UMCN showed higher sensitivity (76%), fast response (6 s), and recovery time (29 s) as compared to other fabricated sensors towards 50 ppm NH 3 at room temperature. Therefore, the metal-doped g-C 3 N 4 can be considered as a favorable candidate for the detection of particular gas molecules.
4.3. g-C 3 N 4 nanocomposites
It has been investigated that g-C 3 N 4 /metal oxide composite-based gas sensors exceptionally enhance the sensing response due to its fast charge transport, high catalytic activity, and combined effects of g-C 3 N 4 and metal oxide. For example, metal oxide coated on g-C 3 N 4 (e.g. Fe 2 O 3 /g-C 3 N 4 , and SnO 2 /g-C 3 N 4 ) has shown the creation of local heterojunctions that exhibited good sensing response towards gas molecules [ 29 , 57 , 58 , 61 , 62 , 101 ].
Hang et al prepared g-C 3 N 4 nanosheets to enhance the gas detecting capabilities of graphene [ 102 ]. They adopted a proton-enhanced liquid-phase exfoliation method to exfoliate the g-C 3 N 4 nanosheets (NS-CN) from bulk powder. The different amount (0%–90%) of as-prepared g-C 3 N 4 was incorporated in graphene solution and then ultrasonicated to form a graphene/g-C 3 N 4 nanocomposite (G/NS-CN). AFM images of exfoliated NS-CN on SiO 2 substrate and the height profile of the nanosheets are displayed in figure 9 (a). SEM images of G/20% NS-CN demonstrate the successful fabrication of exfoliated NS-CN (figure 9 (b)). Surprisingly, it was found that the G/NS-CN composite-based sensor exhibited a higher sensing response as compared to pure graphene sensor under similar operating conditions, as depicted in figures 9 (c)–(d). However, it was also observed that only an optimum amount as the weight ratio of NS-CN/graphene was responsible for increased sensing response. They noticed that the sensor based on 15 wt% of NS-CN showed a higher sensitivity to NO 2 gas. Figure 9 (e) displayed the schematic diagram of the sensing mechanism of G/NS-CN and their respective band diagram.

Figure 9. (a) AFM image of exfoliated NS-CN onto SiO 2 substrate and their height profile (b) SEM images of G/20% NS-CN (c) sensing response of NS-CN sensor to 5 ppm of NO 2 gas (d) dynamic sensing measurement of composite sensors for different concentrations of NO 2 gas (e) schematic illustration of sensing mechanism of G/NS-CN and their respective band diagram. Reprinted from [ 102 ], © 2017 Elsevier B.V. All rights reserved.
Very recently, Absalan et al have synthesized Pd/SnO 2 /porous g-C 3 N 4 with different content of g-C 3 N 4 and Pd using the hydrothermal technique for the detection of CO gas [ 100 ]. The FESEM and TEM results indicated that the porous g-C 3 N 4 nanosheets enabled the large surface area for the deposition of SnO 2 nanoparticles and the creation of a heterogeneous nanocomposite (figure 10 (a)). Only 5%Pd/SnO 2 /5%g-C 3 N 4 based composite sensor among different weight ratios of Pd/SnO 2 /g-C 3 N 4 showed the superior CO sensing performance like short response/recovery times and high response at 125 °C (figures 10 (b)–(c)). The long-term stability of 5%Pd/SnO 2 /5% g-C 3 N 4 based sensor was measured for 1000 ppm CO at 125 °C up to 15 days (figure 10 (d)). These results suggested a minor drop in the sensing response over a period of 15 days. Their sensor was further analyzed for repeatability test for 1000 ppm CO at 125 °C for 4 cycles as shown in figure 10 (e). Interestingly, the sensing response of the sensor for all 4 cycles was almost the same and revealed its repeatable characteristics. Furthermore, the effect of relative humidity (15%–85%) on sensing response was evaluated for 5%Pd/SnO 2 /5%g-C 3 N 4 sensor towards 1200 ppm CO at 125 °C (figure 10 (f)). It was noticed that the sensing response remained almost constant throughout the different ranges of relative humidity. However, a slight decrease in the response was observed due to the adsorption of a small amount of water molecules on the surface of the sensor, which further blocks the oxygen molecules [ 103 ]. In addition to these, their sensor was highly selective for CO as compared to other interfering gases (ethanol, acetone, CH 4 , CO 2 , HCHO, and NH 3 ) under the same operating conditions (figure 10 (g)). Additionally, The improved gas sensing performance of the nanocomposite-based sensor was described by the spill-over effect of Pd nanoparticles, the large surface area of porous g-C 3 N 4 , and the formation of heterojunctions between g-C 3 N 4 and SnO 2 , as shown in figures 10 (h)–(i).

Figure 10. (a) The FESEM images of 5%Pd/SnO 2 /5% g-C 3 N 4 (b) sensing response of Pd/SnO 2 /g-C 3 N 4 based sensors with various amounts of Pd and g-C 3 N 4 to 1000 ppm CO under different working temperature (c) normalized response of different sensors versus CO concentration at 125 °C (d) long-term stability, and (e) repeatability measurement of 5%Pd/SnO 2 /5%g-C 3 N 4 based sensors towards 1000 ppm CO at 125 °C (f) relative humidity test of 5%Pd/SnO 2 /5%g-C 3 N 4 based sensors towards 1200 ppm CO at 125 °C (g) selectivity histogram of 5%Pd/SnO 2 /5% g-C 3 N 4 based sensor for 1000 ppm CO and 4000 ppm concentration of different gases at 125 °C (h)–(i) schematic representation for energy band diagram of n-SnO 2 /n-C 3 N 4 heterojunction and CO sensing mechanism. Reprinted from [ 100 ], © 2019 Elsevier B.V. All rights reserved.
Very recently, Akhtar et al have synthesized pure CuO and g-C 3 N 4 /CuO nanocomposites (with a different weight ratio of g-C 3 N 4 and CuO) via hydrothermal route for the detection of various volatile organic compounds (VOCs) [ 104 ]. When different VOCs are exposed on the 4 wt% g–C 3 N 4 /CuO (S-3) nanocomposite based sensor, the highest sensing response (143.7 to 1000 ppm towards acetone) as well as selectivity (approx. 14.37 towards 1000 ppm acetone/1000 ppm ammonia) was achieved. Moreover, their sensor (S-3) showed a fast response (17 s) and recovery time (24 s) for 1000 ppm of acetone. They suggested that the decoration of CuO nanoparticles on g-C 3 N 4 sheets increased the specific surface area of the nanocomposite, which could be a possible reason for enhanced sensing response. Li et al prepared g-C 3 N 4 /ZnO composite via the precipitation-calcination method, where g-C 3 N 4 nanosheets were decorated on petals of ZnO flower-like structure [ 60 ]. Then their pure and composite sensor was investigated for the detection of CH 4 . Interestingly, it was noticed that g-C 3 N 4 /ZnO-based sensor (CNZ-3 contains 3wt% g-C 3 N 4 in ZnO) exhibited higher sensing response (11.9) as compared to pure ZnO sensor (5.3) towards 1000 ppm CH 4 at 320 °C. Moreover, their CNZ-3 sensor showed a fast response (15 s)/recovery time (28 s) and excellent long-term stability. The improved sensing response was due to the presence of pore structure, large surface area, and formation of g-C 3 N 4 /ZnO n-n junction. In another study, Chu et al prepared g-C 3 N 4 -WO 3 composite via hydrothermal route for the detection of acetone [ 29 ]. Their results suggested that the incorporation of optimum content of g-C 3 N 4 into WO 3 would improve the sensing response as well as selectivity to acetone. They found that the 2 wt% g-C 3 N 4 -WO 3 composite-based sensor showed a good sensing response (58.2) towards 1000 ppm acetone at 310 °C. A summary of some of the investigations on gas sensing properties based on g-C 3 N 4 based nanocomposites is listed in table 3 .
Table 3. Gas sensing properties of different sensors based on g-C 3 N 4 nanocomposite.
5. Conclusion and future perspectives
The current technological signs of progress have set up a new direction to develop high-performance g-C 3 N 4 based gas sensors. However, these advancements are as yet in their developing stage, and a lot of examination and research is expected to advance them to a stage where they can be deployed as commercial sensors. Regardless of the ideal properties of g-C 3 N 4 for developing superior gas sensors, the pre-eminent challenge to transform them into practical proof-of-concept applications still remains to be addressed. Therefore, an attentive expansion in synthesis techniques is needed to explore the entire potential of g-C 3 N 4 based gas sensors.
In this review, a brief summary of g-C 3 N 4 based gas sensors has been presented for the detection of toxic and flammable gases. The g-C 3 N 4 has a large specific surface area (provides more active sites to gas molecules), high porosity, excellent catalytic activity, and can combine easily with metal oxide. These properties enable improvement in gas sensing performance. It is noticed that most of the g-C 3 N 4 used for sensing application were prepared by the pyrolysis method. Moreover, several other techniques (arc deposition, laser ablation, ion beam deposition, chemical vapor deposition, sputtering, and microwave plasma) could be employed for the synthesis of g-C 3 N 4 . In addition to this, numerous surface modifications and composites with g-C 3 N 4 are still to be investigated for the detection of various gases. In order to improve the selective detection, limit of detection, and sensitivity of g-C 3 N 4 based gas sensor, the optimum amount of dopant concentrations/different composites/functionalized noble nanoparticles should be incorporated in the pure g-C 3 N 4 . There are various other materials (ZIFs, COFs, MOFs, conducting polymers, CNTs, and MoS 2 ) that can be combined with g-C 3 N 4 to form nanocomposite-based gas sensors. Moreover, gas sensing can be performed under the irradiation of visible light because of the semiconducting nature of g-C 3 N 4 , which is also a significant advantage of using it. Till now, there are only a few reports available on UV-activated gas sensing by g-C 3 N 4 based sensors. Very interestingly, g-C 3 N 4 can be deposited on flexible substrates such as polyimide or polyethylene terephthalate that may be used for the fabrication of wearable gas sensors for real-time monitoring of gas molecules in the ambient environment. Thus, such types of organic semiconductors like g-C 3 N 4 will have enormous possibilities for fabricating low-cost, portable, high-performance, and IoT integrated gas sensing devices for the detection of toxic and flammable gases.
Data availability statement
No new data were created or analyzed in this study.
A review of recent progress in sensing of gas concentration by impedance change
- Open access
- Published: 13 January 2011
- Volume 17 , pages 99–108, ( 2011 )
Cite this article
You have full access to this open access article

- Jonathan M. Rheaume 1 &
- Albert P. Pisano 1
5186 Accesses
61 Citations
6 Altmetric
Explore all metrics
The intent of this paper is to establish the state of the art of impedance-based gas sensors. This sensor type holds promise for accurate detection of gaseous species at single parts per million and below. Impedance-based sensors do not require reference air to function, but do require calibration. Progress in the development of impedancemetric sensors for the detection of NO x , H 2 O, hydrocarbons, and CO is reviewed. Sensing electrodes typically consist of a noble metal or a metal oxide. YSZ is the preferred electrolyte material. Counter electrodes of Pt were common in asymmetric cells. These sensors typically operate at 500–700 °C and are interrogated at 10 Hz or less. Selectivity of these sensors remains a challenge especially in lean environments. Stability is an infrequently discussed yet important concern. Equivalent circuit analysis has shed light on various detection mechanisms. The impedance changes due to analyte gases are exhibited in parameters that represent the low frequency behavior of the electrochemical system. Although the search for a detailed mechanism continues, the change in impedance due to a specific gas is generally attributed to transport processes such as adsorption and charge transfer.
Similar content being viewed by others

Metal oxide nanoparticles and their applications in nanotechnology

Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): a Powerful Analytical Technique for Elemental Analysis
Detection and removal of heavy metal ions: a review
Avoid common mistakes on your manuscript.
Introduction
Sensors that detect gaseous species by potentiometric, mixed potential, or amperometric signals have conventionally been developed for automobile exhaust gas detection. A new variety of sensors that operate on the basis of an impedance change hold promise for accurate gas detection on the single parts per million (ppm) level even in O 2 concentrations as high as 18.9% [ 1 ]. The simple, single cell design does not require an electrode for reference air however, selectivity, complicated supporting electronics, and long-term stability remain challenges [ 2 ], as discussed below.
Impedance-based sensors have a similar design as mixed potential type sensors. Instead of measuring the voltage, a sinusoidal voltage is applied and the resulting current is measured [ 3 ]. Impedance is then calculated as the ratio of voltage to current in the frequency domain. By using small amplitude sine wave perturbation, linearity in electrochemical systems can be assumed, allowing frequency analysis of a transfer function such as impedance. Analysis of impedance allows characterization of grain boundary resistance, mass transport rates, and chemical reaction rates [ 4 ]. Impedance analysis has also been further refined for the quantification of chemical species.
For almost two decades, the concept of sensing species by monitoring impedance has been known. Impedance sensors for gas detection are derived from original work on liquids. An early application of the impedance sensing technique involved monitoring blood glucose levels using a vascular prosthesis [ 5 ]. Gutiérrez et al. [ 6 ] investigated tin oxide (SnO 2 ) and noticed changes in the impedance spectra dependent on the surrounding gas atmosphere, whether it be humidified air, dry air, or blends of air and CO. They used a Voigt equivalent circuit to model their system. They stopped short of correlating changes in the impedance spectra to changes in species concentration.
The “theta” sensor concept pioneered by Weppner’s group proposed using the ellipsoidal “θ” shape of a current-voltage plot for detection of CO 2 [ 7 , 8 ]. They applied alternating perturbation signals to an electrochemical cell consisting of a fast solid electrolyte and various electrodes. Changes in partial pressure of analyte gas caused the shape of the plot to alter. Although impedance was not explicitly calculated, this analysis method was very similar to impedance spectroscopy. Phase angle was related to partial pressure [ 7 ]. An approximately linear relationship existed between phase angle and species in the window of 0–100 vpm. Curvature in this relationship could be seen over a larger window of partial pressure. The authors claimed several advantages of this type of sensor over potentiometric sensors: no reference electrode, better selectivity, more accurate detection, and improved response time [ 8 ]. Such "dynamic measurement" sensors were faster because thermoodynamic equilibria were not necessary [ 9 ].
Oelgeklaus and Baltruschat [ 10 ] proposed a hydrocarbon sensor with Pt electrodes and a sulfuric acid electrolyte. By continuously monitoring the impedance of their wet cell at a low frequency (10 Hz), they determined changes in capacitance of the electrode surface indicating the presence of hydrocarbon adsorbates. A follow-up paper by the same group reported a method to periodically strip the adsorbate from the surface to enable continuous monitoring of hydrocarbons. They also suggested a correction for O 2 cross-sensitivity. They found a linear dependence of the sensor signal on concentration. The sensor struggled, however, to distinguish between hydrocarbon species [ 11 ].
Miura et al. [ 12 ] proposed a novel, yttria-stabilized zirconia (YSZ)-based sensor that relates gas composition to an impedance signal. In specific, the sensor detects total nitrogen oxides (NO x ) concentration (both NO and NO 2 ) down to 50 ppm between 600 °C and 700 °C using a complex impedance signal. Sensitivity was measured by the difference in the magnitude of the impedance signal (modulus) measured at 0.1 Hz in air and in analyte gas. The change in modulus, ΔZ, was approximately linear with NO x concentration from 50 to 400 ppm. Sensing behavior was attributed to changes of resistance at the interface between the YSZ electrolyte and the oxide electrode; in specific, gas adsorption and electrochemical reactions affected this resistance. Although the sensing mechanism of impedance-based sensors was not perfectly understood [ 2 ], it was evident that physicochemical processes impacted the impedance spectrum in the low frequency range.
In the following, the operating principles of impedancemetric sensors are discussed. An overview of recent literature on impedance-based detection of various gaseous species follows. Sensor materials, equivalent circuit models, and proposed sensing mechanisms are presented.
Principle of operation
The presence of various gases reversibly alters the impedance of some electrochemical systems at low frequencies ( f < 100 Hz) in a repeatable fashion that can be correlated to concentration. This principle underlies impedance-based gas sensors. Generally these sensors have a solid state electrolyte consisting of YSZ. Reference to external air is not necessary however, the sensor must be calibrated in order to correlate the signal to known gas concentrations.
Figure 1 depicts the operation of a symmetric, single cell, impedance-type sensor with a zirconia-based electrolyte. In this example case, NO x is the analyte species. First, gases diffuse to the electrode surfaces, where they adsorb, dissociate, and diffuse along the surface to the electrode–electrolyte interface, followed by charge transfer (redox) reactions. Then ion transport through the electrolyte occurs with charge transfer across grain boundaries. At various frequencies, some of the processes may contribute to the impedance response, depending on the physical characteristics of the electrochemical cell and analyte species.
Impedance-based NO x sensor operation
The rate-limiting processes that occur at particular frequencies appear in the impedance spectra. Figure 2 shows a typical Nyquist plot of data recorded from a NO x sensor with an YSZ electrolyte, an Au working electrode, and a Pt counter electrode at 650 °C. The plot shows the results of two measurements made when the sensor was exposed to two different gases: (1) 10% O 2 in N 2 and (2) 10% O 2 in N 2 with 100 ppm NO. A Nyquist plot shows the resistive and capacitive contributions of impedance, but it does not explicitly show frequencies, so the six decades of frequency are indicated. Two impedance arcs labeled “high frequency” and “low frequency” resulted with both gas mixtures. The high frequency arc (approximately f > 10 4 Hz) provides information on kinetically controlled phenomena. Regardless of the gas, the high frequency arc remains invariant at a given temperature. In contrast, the low frequency arc (approximately f < 10 4 Hz) varies in the presence of various gas species; it is sensitive to transport events [ 13 ].
NO causes inward shift of low frequency arc on Nyquist plot
The effect of 100 ppm NO is to shift the low frequency arc inwards. Both the modulus, |Z|, and phase angle, Ө, change as a result. The modulus represents the length of the vector from the origin to an impedance measurement at a specific frequency (10 Hz). The phase angle is the arctangent of the ratio of the capacitive contribution of the impedance to the resistive component at a specific frequency. In Fig. 2 , the moduli at 10 Hz are depicted and the corresponding phase angles are labeled. Upon exposure to an analyte gas, the changes in modulus [ 12 ] and changes in phase angle [ 1 ] are linear over a specific range of gas concentration at a given frequency. A NO x sensor needs only to interrogate one frequency typically in the low frequency range (typically f < 100 Hz) [ 2 ]. The frequency is selected as a compromise between speed of response, which favors higher frequencies, and sensitivity, which favors lower frequencies.
For a sensor application, the shape of the low frequency impedance spectrum must change selectively in the presence of a specific gas. If the change is not selective, then a compensation scheme must be possible.
Review of literature on impedance-based gas sensing
Impedance-based gas sensors are discussed in several books in the larger context of solid state sensors including amperometric and potentiometric varieties. Coauthors of the seminal paper [ 12 ] that related NO x concentration to changes in modulus discuss their impedance sensor work in books by Zhuiykov and/or Miura [ 2 , 14 , 15 ].
The application of impedance techniques to sensors with an electrolyte of YSZ is particularly relevant to gas sensing. In 1969, Bauerle [ 16 ] first applied impedance spectroscopy techniques to solid electrolytes of YSZ thereby setting the stage for the impedance detection mechanism. Since then, researchers have used the impedance change principle in sensors that detect NO x , water vapor, hydrocarbons, and carbon monoxide. In the following sections, publications on impedance-based sensing are presented by the type of analyte gas and reviewed. A summary of impedance-type gas sensors appears in Table 1 .
Nitrogen oxides
Impedance-type NO x detection is the least developed of all types of NO x sensors. It has shown promise to detect NO x at lower ppm than is possible using conventional sensors [ 3 ]. By “NO x ”, one specifically refers to nitric oxide (NO) and nitrogen dioxide (NO 2 ), the two gaseous species that constitute virtually all NO x emissions from anthropogenic combustion processes [ 17 ]. For impedance-type NO x sensors, the response to NO and NO 2 has been demonstrated to be comparable in magnitude and of the same sign (in contrast to mixed potential sensors) at specific temperatures, thereby facilitating the measurement of total NO x concentration [ 1 , 12 , 18 – 23 ]. Impedance-based gas sensors for gas detection may help diesel vehicles meet future NO x OBD requirements.
Ho et al. [ 24 ] noticed a change in the electrical properties of a thick film of Nd 2 CuO 4-y in the presence of NO x gases. In specific, NO x gases caused changes in conductance that were related to species concentration.
Yoon et al. [ 25 ] noticed the effect of NO 2 on impedance spectra in their experiments with potentiometric sensors. They remarked that the low frequency impedance associated with the electrode/electrolyte interface changed in the presence of 100 ppm NO 2 . They suggested that the oxygen groups from the NO 2 decomposition caused a decrease in the overvoltage of the electrolyte/electrode interface. Other research groups built upon this finding by examining the impedance change over a wider range of partial pressures.
Miura et al. [ 12 ] originally proposed an impedance-based sensor with a ZnCr 2 O 4 sensing electrode by calibrating a low frequency modulus shift with NO x concentration in a linear fashion, as described above. Subsequently, Miura et al. [ 18 ] further proposed an equivalent circuit model for the NO x sensor in a follow-up publication. Their circuit consists of a Voigt circuit with two Voigt elements: R b -( R o C o )-( R i C i ). In their model, the lone resistor, R b , represents YSZ bulk resistance; the first Voigt element ( R o C o ) represents the resistance and capacitance of the bulk of the oxide electrode; finally, the second Voigt element ( R i C i ) represents the resistance and capacitance of the interface between the YSZ and the oxide electrode where adsorption and reactions occur. Only the resistance of the interface changed in the presence of NO x . It was suggested that this resistance or even the entire real component of impedance could be used to determine NO x concentration at low frequency however, the measurement should be taken by measuring complex impedance and by calculating the real component of resistance. In both these publications, Miura et al. called for further investigation in order to ascertain the sensing properties and detailed mechanism [ 12 , 18 ].
Miura et al. then experimented with the thickness of ZnCr 2 O 4 sensing electrodes in an impedance-based NO x sensor [ 19 ]. In some experiments, a platinum layer over the electrodes oxidized reducing gases that are known to evoke a cross-sensitive response (CO, H 2 , CH 4 , and C 3 H 8 ) at 700 °C. At high temperature, thick electrodes (39 μm) provide a large catalytic area for non-equilibrium mixtures of NO and NO 2 to equilibrate. An equilibrium NO x mixture at 700 °C consists of 95 vol.% NO and 5 vol.% NO 2 . Because NO 2 is mostly reduced to NO during equilibration, the impedance-based sensor shows essentially the same response to NO as to NO 2 at high temperature. They proposed that the sensing mechanism relies on the increased favorability of the electrochemical reaction involving oxygen in the presence of NO x . A similar equivalent circuit was proposed as in previous work except that the Voigt element representing the low frequencies is associated with electrode reactions: R b -( R o C o )-( R e C e ).
Martin et al. applied a NO x sensing technique using impedance to a planar sensor with a YSZ electrolyte and identical metal-oxide composite electrodes consisting of Cr 2 O 3 and YSZ (10:90% weight ratio) [ 20 ]. The NO x sensitivity was measured by the shift in phase angle at 10 Hz. The shift was linear with concentration between 8 and 50 ppm. The response to both NO and NO 2 was similar. They report different pathways for the NO and O 2 reactions which supports the parallel path model. Since the NO x sensor is cross-sensitive to O 2 , a compensation method was proposed. They suggest that the simultaneous measurement of NO x and O 2 can occur at different frequencies because the impedance response at f > 100 Hz was negligible for NO x but still significant for O 2 . The authors reported, however, that the response time of this sensor to O 2 would need to decrease.
Subsequently, Martin et al. demonstrated that the change in phase angle was evident over a larger frequency range than the change in modulus, and thus the phase angle shift became their preferred metric [ 1 ]. They further developed an equivalent circuit model for their work on composite electrodes. Instead of the standard Voigt model, this group’s model includes a fractal Gerischer element, which is used to model the diffusion of reacting gases. Their model consists of a series combination of a resistor, a fractal Gerischer (FG) element, and a Cole element: R s - FG -( R LF Q ). The Cole element models the low frequency behavior. The most significant parameter changes due to NO x exposure occur in this subcircuit element. This element corresponds to physical processes that occur at the electrode surface and at the interface of the electrode and electrolyte. They further broke down the resistance of the Cole element into contributions from simultaneous reactions that occur at the triple phase boundary (interface) that involve O 2 , NO x , and the ionic form of oxygen (O 2− ):
For an electrode to sense NO x , the values of R o 2 and R NOx must be within two orders of magnitude of each other otherwise NO x detection is not possible. They confirmed this statement by adding a porous gold layer or a dense Pt plate to their electrodes; these actions both had the effect of increasing the electrode surface area for which O 2 and NO x compete. The result was a decrease in R o 2 to such an extent that the presence of NO x no longer could be detected. R NOx was much greater than R o 2 ; since R NOx appears in the denominator, it did not contribute to the R of the Cole element. Martin et al. also provided a justification for their use of change in phase angle, ΔӨ, instead of change in modulus, |Z|, as the proxy for selectivity. The phase angle shift persists to higher frequency in the presence of NO x than does the modulus change. Increasing the frequency at which a sensor operates is desirable for a faster response. Since the sensor responds to both oxygen and NO x at 10 Hz, but at 1 kHz the sensor responds only to O 2 , compensation for O 2 was carried out at 1 kHz by measuring impedance. Phase angle varies linearly with O 2 concentration over the examined range of 2–18.9% at both frequencies, so phase angles can be calibrated to determine O 2 concentration at 1 kHz. Then the contribution of O 2 can be subtracted from the impedance at 10 Hz in order to determine NO x concentration. Lastly, some signal drift was detected that may have been due to aging. A detailed study of the precise NO x sensing mechanism did not lie within the scope of this work.
Martin’s coauthor, Murray, et al. investigated NO x sensors consisting of LaCr 0.95 Mg 0.05 O 3 overlaid with Ag–Pd on fully stabilized YSZ [ 26 ]. Phase angles were correlated to NO x concentration to serve as the measurement of sensitivity. An analysis of the effect of both O 2 and temperature on sensitivity led them to conclude that monitoring these variables would be necessary for accurate sensing. The signal from sensors was found to drift over several days of operation due to material instability. In addition, long response times were required with these sensors. They posited charge transfer to be the rate-limiting step.
Yet another coauthor of Martin, Woo, investigated planar, YSZ-based NO x sensors with dense gold electrodes [ 21 ]. A porous layer of electrolyte was present between the electrodes and the dense electrolyte. This layer enabled enhanced NO x sensing, but the mechanism was not understood. A simplified equivalent circuit analysis was performed in which the low frequency arc that is sensitive to NO x was modeled by a resistor in series with a Cole element: R s -( R LF Q ). R LF represents the low frequency resistance that is affected by O 2 and NO x , and R s is a series resistor that serves as a simplification for the higher frequency behavior of the rest of the circuit. Adsorption was suggested as the rate-limiting mechanism responsible for sensing behavior, however, no definitive mechanistic conclusions were possible.
In a follow-up paper, Woo et al. [ 22 ] expatiated upon possible mechanisms for the NO x response of the symmetrical Au/YSZ sensor. The resistor in the Cole element of the simplified equivalent circuit was proportional to the gas concentration according to a power law: R LF ~[gas] α . The determination of the exponent, α, suggested adsorption as the sensing mechanism. In addition, the activation energies obtained from Arrhenius plots of R 1 tended to support adsorption. Competition for adsorption sites may have been the rate-limiting step. The authors called for further studies to explain the exact mechanism.
In a subsequent publication, Woo et al. [ 27 ] applied EIS techniques to NO sensors with Au, Pt, or Ag electrodes on a mixture of solid and porous YSZ electrolyte. Only Au showed sensing activity as long as the electrode was dense; porous gold was insensitive. The insensitivity of Pt makes it an appropriate counter electrode material. Sensors that detected NO featured larger impedance values than those that did not; this suggested that a dearth of electrochemical reaction sites may play a role in NO sensing. An equivalent circuit model was used to investigate the low frequency arc of NO impedance spectra. It was similar to a Voigt circuit but with the capacitors replaced by constant phase elements (Q): R s -( R ′ LF Q ′)-( R ″ LF Q ″). In this work once again, R s serves as an approximation for the total cell resistance at high frequencies (>1 kHz). Two Cole elements were required to model one low frequency arc, implying that two physicochemical processes with similar time constants were taking place. Woo suggested that the rate-determining step for NO-sensing may depend on O 2 reactions that take place away from the triple-phase boundary. Although the exact sensing mechanism was outside of the scope of this work and therefore not determined, adsorption and diffusion of O 2 were offered as possible rate-limiting steps for the detection of NO.
Stranzenbach et al. [ 28 ] fabricated planar, impedance-based NO x sensors by sputtering sensing electrodes of the spinel NiCr 2 O 4 on both fully stabilized YSZ and partially stabilized zirconia (PSZ) electrolyte. They measured sensitivity by the change in magnitude of the impedance, ΔZ, on the addition of a sample gas. From 200 to 1,000 ppm, ΔZ varied linearly with NO concentration at 0.1 Hz. The impedance values and their associated magnitudes for the FSZ electrolyte were found to be much higher than those for PSZ. They analyzed the sensors for cross-sensitivity to various gases including O 2 (0–20%), CH 4 (0–500 ppm), CO (0–100 ppm), and CO 2 (0–500 ppm). They found that CO 2 does not affect sensing performance for either electrolyte. In lean atmospheres, CH 4 and CO are oxidized to CO 2 , so the sensor does not detect them either. In rich conditions, however, increases in both ohmic and capacitive resistance were observed and attributed to the reduction of the electrolyte. Typically electrolyte reduction is associated with a localized increase in electrons and a decrease in resistance [ 29 ]. No cross-sensitivity to NO 2 was found because at high temperature ( T > 600 °C), the NO 2 converts to NO at the electrodes due to thermodynamic equilibrium. They also identified an equivalent circuit model similar to a Voigt circuit, except in the last two elements, the capacitors are replaced with a constant phase element and a Warburg element, respectively: R 1 -( R 2 C 1 )-( R 3 C 2 )-( R 4 Q 1 )-( R 5 W 1 ). They found that changes in NO concentration affect these last two elements of the equivalent circuit. The second to last element, a Cole element, consists of a resistor and constant phase element in parallel. It represents changes in the double layer between the electrode and electrolyte which may be due to the reaction of NO over the catalytic sensing electrode. The last element, a resistor in parallel with a Warburg impedance represent a diffusion process, but did not conclusively specify which species.
Stranzenbach and Saruhan [ 30 ] then examined impedancemetric NO x sensors with NiO as the sensing electrode on YSZ and PSZ electrolytes. This sensor showed excellent selectivity for NO in both reducing and oxidizing conditions with low cross-sensitivity to O 2 and other combustible gases.
In a subsequent paper, Stranzenbach and Saruhan [ 31 ] performed EIS on NO x sensors with sputtered NiO or NiCr 2 O 4 sensing electrodes on pre-aged electrolytes of fully stabilized YSZ and PSZ deposited by EBPVD. They used the same equivalent circuit as in their previous work [ 28 ]. The resistor of the Cole element represents the “reaction-resistance” for adsorption, dissociation, and electrochemical reactions. It is an indicator of the oxygen sensitivity of the sensor. They also provided further discussion of the equivalent circuit and its relationship to the sensing mechanism. They postulated that the sensing mechanism was due to competitive molecular adsorption of NO and O 2 for both sensing electrode types. In the case of the NiCr 2 O 4 electrodes, NO dissociative adsorption controls the sensing behavior. They also posited that the impedancemetric NO x sensing behavior results from the charge transfer processes over the triple-phase boundary (TPB) and not from the anodic and cathodic reactions. The exact sensing mechanism and the factors that determine sensitivity were described as “open questions”.
Rheaume et al. [ 32 ] used statistical “Design of Experiments” techniques to perform a two level, half fractional factorial experimental design (2 III 3 − 1 ) on four single-cell impedancemetric NO x sensors (Au/YSZ/Pt) to determine the effect of design features on sensitivity. Three design factors were systematically varied: working electrode area (number of sensing wires), electrolyte thickness, and the distance between sensing wires. Sensing ability was evaluated by performing electrochemical impedance spectroscopy and calculating the change in the phase angle at 10 Hz upon the introduction of 50 ppm NO or NO 2 in 10% O 2 with balance N 2 . ANOVA indicated that the most significant effect was electrolyte thickness for sensing NO. A linear relationship between phase angle difference and NO concentration existed, insensitive to total flow rate. NO x sensitivity was several orders of magnitude larger than for O 2 . NO 2 evoked a larger response than NO at 650 °C. Self-compensation by the simultaneous determination of both O 2 and NO x concentrations was attempted by investigating the phase angle shifts at different frequencies (1 kHz for O 2 ). For this sensor chemistry, the phase angles did not exhibit dependence on gas concentration at frequencies of 1 kHz and greater as reported by Martin et al. [ 1 ]. At 10 5 Hz, phase angles were linearly related to temperature, independent of gas species. As a result, a stable sensor may be calibrated for use as a thermometer, as long as the materials are stable (i.e., do not change due to aging). The authors did not specify the sensing mechanism, but mentioned that it is related to a transport process.
Y. Shimizu et al. investigated a fundamentally different electrochemical cell consisting of a non-zirconia electrolyte with two Ag electrodes and various metal oxide receptors contiguous to the electrolyte [ 33 , 34 ]. They used a lithium solid electrolyte, Li 1.5 Al 0.5 Ti 1.5 (PO 4 ) 3 , for their investigations of impedance-based NO x sensors [ 33 ]. This electrolyte operates at a lower temperature (400–500 °C) than typical for zirconia electrolytes. They examined several different types of oxide receptors including numerous semiconductors and perovskites: TiO 2 , SnO 2 , WO 3 , NiO, Cr 2 O 3 , LaCrO 3 , LaMnO 3 , LaCoO 3 , LaFeO 3 , and LaNiO 3 . The role of the receptors was to exchange electrons with the electrolyte, which affected the impedance of the cell. They noticed different responses for receptors consisting of n -type versus p -type semiconducting oxides. The sensors with n -type receptors showed good responses to NO x at 400 °C. On the contrary, at 500 °C only the sensors with perovskite references LaCrO 3 and LaMnO 3 showed sensitivity to NO x , Sensors were interrogated over a frequency range of 50 Hz to 5 MHz; for most sensors, they investigated the response ΔZ at 1 kHz or at 10 kHz, which is two to three orders of magnitude higher than other research. In specific, they were interested in the change of resistance and capacitance at a specific frequency due to the exposure of NO x . They attributed the changes in these parameters to the interface of the electrode and electrolyte. They also examined the effect of N 2 O gas, but no electrode showed a response to it. They discussed a sensing mechanism that involved electron exchange with the metal oxide receptor, however, they declared that it requires further investigation.
Shimizu et al. [ 23 ] examined NO x sensors consisting of several metal oxides doped with platinum (WO 3 , TiO 2 , WO 3 /ZrO 2 , WO 3 /TiO 2 , MoO 3 /TiO 2 , V 2 O 5 /TiO 2 ) and equipped with interdigitated Au electrodes. This sensor type differs from other impedance-based ones in that they substitute platinum-impregnated metal oxide-sensing materials for the more commonly used YSZ. The metal oxides are n type semiconductors (TiO 2 and WO 3 ) unlike YSZ. As a result, a fundamentally different sensing mechanism is to be expected. The sensor with Pt-WO 3 /TiO 2 exhibited a nearly equal response to both NO and NO 2 at 500 °C. They attributed this feature to the Pt which promotes NO 2 decomposition to NO which is thermodynamically favored at the elevated temperature of these studies. The impedance at 4 Hz varied nearly linearly with the logarithm of NO x (NO or NO 2 ) concentration from 10 to 570 ppm. The logarithmic relationship may suggest a different mechanism than YSZ sensors, however, YSZ sensors might also exhibit logarithmic behavior over a wide range of analyte gas concentration. The low frequency impedance did not vary much with O 2 concentration, which is advantageous in comparison to sensors with YSZ electrolyte because they must be compensated. The authors proposed that the impedance change due to NO addition resulted from enhanced diffusion charge transport at the interface of the metal oxide electrolytes and the electrodes. In specific, NO adsorption on the surface of the doped metal oxide causes an enhancement of the diffusion of H + at the interface on account of the interaction of NO with the acidic W-OH site.
Water vapor
Gutiérez et al. studied changes in the impedance spectra of semiconductor SnO 2 due to humidity (H 2 O), CO, and air. The sensing mechanism results from changes of conductivity with respect dry air due to water vapor. In specific, OH − reacts with O 2 , thereby decreasing the depletion layer width. This group proposed a Voigt equivalent circuit model similar to the YSZ-based sensors. They did not, however, correlate the changes in impedance to changes in species concentration [ 6 ].
Miura et al. [ 12 ] did correlate impedance with gaseous species quantity in their original work on NO x detection. This work differed from that of Gutiérez et al. on account of the use of YSZ instead of a semiconductor. Nakatou and Miura [ 35 ] further applied the impedance-based sensing technique to detect water vapor (humidity) at high temperature. Several different single oxide sensing electrodes were evaluated with stabilized zirconia electrolyte. The maximum sensitivity was found using electrodes of In 2 O 3 operating at 900 °C. Sensitivity was measured by the change in the magnitude of the impedance, ΔZ, at 1 Hz. The impedance change was linear with respect to the logarithm of H 2 O concentration (70–30,000 ppm). Response times were measured in single seconds. The sensing mechanism is attributed to a change in impedance. In specific, the resistance of the electrode reaction at the interface of the oxide electrode and YSZ electrolyte decreases with increasing concentration of water vapor in the gas.
Hydrocarbons
Nakatou and Miura [ 35 ] found that the In 2 O 3 /YSZ impedancemetric sensor used to detect water vapor also indirectly senses hydrogen and hydrocarbons in dry gas at 900 °C [ 36 ]. They speculated that the sensing mechanism involves the working electrode serving as a catalyst for the oxidation of hydrocarbons that react to form CO 2 and H 2 O (water vapor). Whereas the CO 2 does not evoke a response, the In 2 O 3 /YSZ sensor detects the hydrocarbon indirectly by sensing the water vapor from combustion. Once again, the change in modulus, ΔZ, was used as the metric for sensitivity. The sensitivity, as measured by the change in impedance due to the analyte gas, is attributed to the change in resistance of the electrode reaction at the interface of the In 2 O 3 electrode and the YSZ electrolyte.
Nakatou and Miura developed a sensor that can selectively detect propene (C 3 H 6 ) in a humidified gas stream (1 vol.% H 2 O) at 600 °C by first oxidizing reducing species and by applying a bias of +50 mV to the sensing electrode versus the Pt counter electrode [ 37 ]. A sensing electrode of ZnO on YSZ shows negligible cross-sensitivity to CH 4 , where sensitivity is measured as the change in modulus upon exposure to a gas. Platinum (1.5 wt.%) was added to the sensing electrode in order to improve its catalytic activity for oxidation, resulting in the ability to detect propene even in the presence of CO, NO, NO 2 , H 2 , CH 4 , CO 2 , H 2 O, and O 2 . The sensing ability is attributed to the change in the resistance of electrochemical reaction taking place at the interface between the electrode and electrolyte, however, the authors stated that further study is required to understand the exact sensing mechanism.
A follow-up paper by Miura et al. [ 38 ] reported that their propene sensor with a ZnO sensing electrode and YSZ electrolyte detected the specific hydrocarbon at extremely low concentrations (0.05–0.8 ppm). Sensitivity was defined by the change in modulus; it varied linearly with propene concentration and was only insignificantly affected by H 2 O, NO 2 , NO, H 2 , CO, and CH 4 . In this study, an applied potential to the sensing electrode did not greatly affect sensitivity. The effect of propene on impedance is attributed to the change in resistance of the charge transfer reaction that occurs at the interface of the oxide electrode and YSZ electrolyte, which is a refinement of earlier work that suggested the cause was adsorption or electrode reactions for detection of NO x [ 12 , 18 ], water vapor [ 35 ], and hydrocarbons [ 36 , 37 ]. The authors cautioned that further examinations on the detailed sensing mechanism were necessary.
In the course of an investigation of NO x sensors, Stranzenbach et al. [ 28 , 30 ] examined cross-sensitivity to CH 4 . Reducing gases caused both ohmic and capacitive changes in the sensor that they associated with the changes in charge carriers in the electrolyte and not with electrode processes. Net oxidizing environments should result in the oxidation of CH 4 to CO 2 , which is not detected by the sensor. No investigation of the effect of water vapor on sensor response took place. The sensors were not calibrated to relate changes in impedance to the species quantity of CH 4 .
Carbon monoxide
As discussed above, Gutiérrez et al. [ 6 ] noticed changes in the impedance spectra of SnO 2 due to several gases including CO, but they did not perform the calibration necessary to make a sensor. They did, however, discuss a possible sensing mechanism. At elevated temperature, CO reacts with chemisorbed oxygen, which increases the number of carriers, decreases the voltage barriers, and results in increased conductivity.
Stranzenbach et al. [ 28 , 30 ] also looked at the cross-sensitivity of a NO x sensor to CO in addition to CH 4 . Since both are reducing gases, they drew the same conclusions for CO as they did for CH 4 .
Wu et al. [ 39 ] examined an electrochemical CO sensor with an electrode of Au-doped Ga 2 O 3 and an electrolyte of YSZ. They chose the Au-Ga 2 O 3 material because CO chemisorbs strongly on it. The change in modulus at 0.1 Hz was used for sensitivity measurements. An equivalent circuit was proposed: R c -( R b C b )-( R i Q ) where R c represents contact resistance, the Voigt element ( R b C b ) represents the YSZ bulk, and the Cole element ( R i Q ) represents the interface between the electrode and the electrolyte. The interpretation of R c is novel because this circuit element is usually associated with the bulk electrolyte whereas here it is the ohmic resistance of the electric connection. The value for C b was on the same order of magnitude as that for bulk YSZ found in a previous study, so the second element was identified as the bulk electrolyte. Neither R c , R b , nor C b changes with CO concentration. Rather the interfacial parameters R i and Q that represent the triple phase boundary respond to changes in CO concentration, where Q accounts for the non-ideal behavior of the double layer, and R i represents charge transfer. This conclusion agrees with other work [ 38 ]. Interfacial impedance depends on the electrolyte and the sensing electrode materials; any change in either could affect the impedance. The authors proposed that CO gas interacted with the sensor at the TPB in the following way: the alternating voltage induced electrochemical oxidation of CO thereby introducing charge carriers and changing the kinetics of charge transfer.
Impedance-based gas sensors have been surveyed for their materials, equivalent circuit models, and sensing mechanisms. This type of gas sensor is less developed than amperometric or mixed potential types [ 3 ]. Impedancemetric sensor designs consist of a simple, single electrochemical cell operating at temperature greater than 500 °C. Typically, the electrolyte consists of YSZ, the sensing electrode of a noble metal or a metal oxide, and the counter electrode of Pt. As a sensing material, dense Pt has been shown to be ineffective for NO x detection [ 27 ], making it appropriate for a counter electrode material. Symmetric electrode materials are also common. Some sensors consist of semiconducting materials whose electrical properties change in the presence of certain gases. The sensing mechanisms of electrolytic and semiconducting sensors are expected to be different, as would sensors with noble metal vs. metal oxide electrodes. Sensing mechanisms are poorly understood [ 2 ]. Further studies with isotopically marked gases are necessary to establish the exact sensing mechanism for the sensors. The rate-limiting step associated with sensing properties depends on sensor morphology, chemistry, and triple-phase boundary [ 20 ]. These characteristics differ across sensors, however, it is clear that the influence of the analyte gas is seen at low frequency ( f ≤ 100 Hz). Usually, the sensing property is attributed to a transport process at the interface of the electrode and the electrolyte.
Typically, a Voigt equivalent circuit model (sometimes with minor variations) is used to replicate impedance behavior over a wide range of frequencies. Most research groups use the change in modulus at a specific frequency (typically f ≤ 10 Hz) as the metric for sensitivity. This change in modulus can be linearly correlated to species concentration. Some groups report an equivalent response to NO and NO 2 alike allowing total NO x sensing at a specified temperature. It is unclear whether the equivocal sensitivity is due to the detection capability of the sensor or due to the natural thermal equilibrium shift of NO 2 to NO. At T > 600 °C, NO x in exhaust gases equilibrates so that the mixture consists primarily of NO (>90%) [ 2 ].
Impedancemetric sensors show several promising advantages over conventional sensors. Accurate detection of gaseous species on the single ppm level in high background O 2 concentration has been demonstrated [ 1 , 20 ]. These sensors do not require an air reference electrode. Some drawbacks exist, however, with impedance-based sensors. Selectivity remains a challenge. The partial pressure of O 2 in the sample gas must be known due to cross-sensitivity. In addition, the attendant electronics and signal processing equipment are more complicated than those of conventional sensors. Moreover, the long-term stability of the sensors must be further improved [ 8 ], perhaps by heat treatment or by a different fabrication method. In summary, numerous challenges remain to be overcome prior to the commercialization of impedancemetric sensors for gas detection.
Abbreviations
electrochemical impedance spectroscopy
Fractal Gerischer element
nitrogen oxides
parts per million
onboard diagnostics
partially stabilized zirconia
constant phase element
sensing electrode
volume parts per million
Warburg element
Zyttria-stabilized zirconia
phase angle shift
change in modulus
Martin LP, Woo LY, Glass RS (2007) Impedancemetric NO x sensing using YSZ electrolyte and YSZ/Cr 2 O 3 composite electrodes. J Electrochemical Soc 154(3):J97–J104
Article CAS Google Scholar
Zhuiykov S (2008) Electrochemistry of zirconia gas sensors. CRC Press, New York
Google Scholar
Fergus JW (2007) Materials for high temperature electrochemical NO x gas sensors. Sensor Actuator B Chem 121:652–663
Article Google Scholar
Orazem M, Tribollet B (2008) Electrochemical impedance spectroscopy. Wiley, New Jersey
Book Google Scholar
Preidel W, von Lucadou I, Lager W, Ruprecht L, Saeger S (1990) Glucose measurements by electrocatalytic sensor in the extracorporeal blood circulation of a sheep. Sensor Actuator B Chem 2:257–263
Gutiérrez J, Arés L, Horillo MC, Sayago I, Agapito J, López L (1991) Use of complex impedance spectroscopy in chemical sensor characterization. Sensor Actuator B Chem 4:359–363
Liu J, Weppner W (1992) θ-Sensors: a new concept for advanced solid-state ionic gas sensors. Appl Phys A55:250–257
CAS Google Scholar
Liaw BY, Liu J, Menne A, Weppner W (1992) Kinetic principles for new types of solid state ionic gas sensors. Sol Stat Ion 53–56(1):18–23
Steudel E, Weppner W (1996) Electrochemical gas detection based on dynamic measurements. Ionics 2:107–112
Oelgeklaus R, Baltruschat H (1997) Detection of hydrocarbons in air by adsorption on Pt-electrodes using continuous impedance measurements. Sensor Actuator B Chem 42:31–37
Ernst S, Herber R, Slavcheva E, Vogel I, Baltruschat H (2001) Continuous detection of volatile aromatic, unsaturated or halogenated hydrocarbons in air by adsorption on Pt-electrodes and subsequent oxidative desorption. Electroanalysis 13(14):1191–1197
Miura N, Nakatou M, Zhuiykov S (2002) Impedance-based total-NO x sensor using stabilized zirconia and ZnCr 2 O 4 sensing electrode operating at high temperature. Electrochem Comm 4:284–287
Bard AJ, Faulkner LR (1980) Electrochemical methods fundamentals and applications. Wiley, New York
Zhuiykov S, Miura N (2005) Solid-state electrochemical gas sensors for emission control. In: Sorrell CC, Nowotny J, Sugihara S (eds) Materials for energy conversion devices. Woodhead Publishing, Cambridge, pp 303–335
Miura N, Elumalai P, Plashnitsa VV, Ueda T, Wama R, Utiyama M (2009) Solid state gas sensing. In: Comini E, Faglia G, Sberveglieri G (eds) Solid state electrochemical gas sensing. Springer, New York, pp 200–203
Bauerle J (1969) Study of solid electrolyte polarization by a complex admittance method. J Phys Chem Solid 30:2657–2670
Vestreng V, Ntziachristos L, Semb A, Reis S, Isaksen ISA, Tarrasón L (2009) Evolution of NO x emissions in Europe with focus on road transport control measures. Atmos Chem Phys 9:1503–1520
Miura N, Nakatou M, Zhuiykov S (2003) Impedancemetric gas sensor based on zirconia solid electrolyte and oxide sensing electrode for detecting total NO x at high temperature. Sensor Actuator B Chem 93:221–228
Miura N, Koga T, Nakatou M, Elumalai P, Hasei M (2006) Electrochemical NO x sensors based on stabilized zirconia: comparison of sensing performances of mixed-potential-type and impedancemetric NO x sensors. J Electroceram 17:979–986
Martin LP, Woo LY, Glass RS (2007) Impedancemetric technique for NO x sensing using a YSZ-based electrochemical cell. Mater Res Soc Symp Proc 972:04–AA12
Woo LY, Martin LP, Glass RS, Gorte RJ (2007) Impedance analysis of electrochemical NO x sensor using a Au/yttria-stabilized zirconia (YSZ)/Au cell. Mater Res Soc Symp Proc 972:02–AA12
Woo LY, Martin LP, Glass RS, Gorte RJ (2007) Impedance characterization of a model Au/yttria-stabilized zirconia/Au electrochemical cell in varying oxygen and NO x concentrations. J Electrochem Soc 154(4):J129–J135
Shimizu K, Kashiwagi K, Nishiyama H, Kakimoto S, Sugaya S, Yokoi H, Satsuma A (2008) Impedancemetric gas sensor based on Pt and WO 3 co-loaded TiO 2 and ZrO 2 as total NO x sensing materials. Sensor Actuator B Chem 130:707–712
Ho KY, Miyayama M, Yanagida H (1997) NO x gas responding properties of Nd 2 Cu0 4-y , thick film. Mater Chem Phys 49:7–11
Yoon JW, Grilli ML, Di Bartolomeo E, Polini R, Traversa E (2001) The NO 2 response of solid electrolyte sensors made using nano-sized LaFeO 3 electrodes. Sensor Actuator B Chem 76:483–488
Murray EP, Novak RF, Kubinski DJ, Soltis RE, Visser JH, Woo LY, Martin LP, Glass RS (2008) Investigating the stability and accuracy of the phase response for NO x sensing 5% mg-modified LaCrO 3 electrodes. ECS Trans 6(20):43–62
Woo LY, Martin LP, Glass RS, Wang W, Jung S, Gorte RJ, Murray EP, Novak RF, Visser JH (2008) Effect of electrode composition and microstructure on impedancemetric nitric oxide sensors based on YSZ electrolyte. J Electrochem Soc 155(1):J32–J40
Stranzenbach M, Gramckow E, Saruhan B (2007) Planar, impedance-metric NO x -sensor with spinel-type SE for high temperature applications. Sensor Actuator B Chem 127:224–230
Luerßen B, Janek J, Günther S, Kiskinova M, Imbihl R (2002) Microspectroscopy at a moving reduction front in zirconia solid electrolyte. Phys Chem Chem Phys 4:2673–2679
Stranzenbach M, Saruhan B (June 10–14, 2007) Planar, impedance-metric NO x -sensor with NiO SE for high temperature applications. Solid-State Sensors Actuators and Microsystems Conference 2007:983–986
Stranzenbach M, Saruhan B (2009) Equivalent circuit analysis on NO x impedance-metric gas sensors. Sensor Actuator B Chem 137:154–163
Rheaume J, Pisano A (May 17–20, 2009) Fractional factorial experimental design applied to the development of an impedancemetric NO x sensor. 6th US National Combustion Meeting, Ann Arbor, Michigan 11A2
Koba D, Takase S, Shimizu Y (2006) A solid electrolyte impedancemetric NO x sensor using oxide receptor. ECS Trans 3(10):163–171
Shimizu Y, Takase S, Koba D (2008) A NOx sensor based on solid-electrolyte impedance transducer. Adv Mater Res 47–50:479–482
Nakatou N, Miura N (2004) Impedancemetric sensor based on YSZ and In 2 O 3 for detection of low concentrations of water vapor at high temperature. Electrochem Comm 6:995–998
Nakatou N, Miura N (2005) Detection of combustible hydrogen-containing gases by using impedancemetric zirconia-based water-vapor sensor. Sol Stat Ion 176:2411–2415
Nakatou N, Miura N (2006) Detection of propene by using new-type impedancemetric zirconia-based sensor attached with oxide sensing-electrode. Sensor Actuator B Chem 120:57–62
Wama R, Utiyama M, Plashnitsa VV, Miura N (2007) Highly sensitive impedance-based propene sensor using stabilized zirconia and zinc oxide sensing-electrode. Electrochem Comm 9:2774–2777
Wu N, Chen Z, Xu J, Chyu M, Maob SX (2005) Impedance-metric Pt/YSZ/Au–Ga 2 O 3 sensor. Sensor Actuator B Chem 110:49–53
Download references
Acknowledgments
The authors gratefully thank Lawrence Livermore National Laboratory for supporting this work. In particular, the authors acknowledge Dr. Robert Glass and Dr. Leta Y. Woo of Lawrence Livermore National Laboratory for their insights into sensor fabrication and evaluation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and affiliations.
University of California, Berkeley, CA, USA
Jonathan M. Rheaume & Albert P. Pisano
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jonathan M. Rheaume .
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Reprints and permissions
About this article
Rheaume, J.M., Pisano, A.P. A review of recent progress in sensing of gas concentration by impedance change. Ionics 17 , 99–108 (2011). https://doi.org/10.1007/s11581-010-0515-1
Download citation
Received : 16 August 2010
Revised : 16 November 2010
Accepted : 12 December 2010
Published : 13 January 2011
Issue Date : March 2011
DOI : https://doi.org/10.1007/s11581-010-0515-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Impedancemetric gas sensor
- Impedance sensing
- Electrochemical impedance spectroscopy (EIS)
- Yttria-stabilized zirconia (YSZ)
- Find a journal
- Publish with us
- Track your research
Gas sensor device creation
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

IMAGES
VIDEO
COMMENTS
In summaries, the research and fabrication of environmental smart sensors with high sensitivity, good selectivity, reliability, and fast response/low recovery time using advanced technology is necessary. The traditional metal oxide based sensors generally operate at 200 - 400° is now replacing with 2D/3D materials.
Further, the gas sensor market is project to reach $1,336.2 million by 2027, which is one of the biggest markets across sensor technology. (1) In order to create real-time multivariate data sources, gas sensors must be operated without any spatiotemporal limitations. Therefore, the gas sensor must be integrated with the Internet-of-Things (IoTs ...
Micro-electromechanical system (MEMS) gas sensors represent a system that is capable of detecting the existence and concentration of gasses in the atmosphere. These sensors have become incredibly popular and have been widely used in a variety of fields, including the biomedical field. Over the years, several gas sensors have been developed to detect and analyze a wide range of gases. As sensor ...
Abstract. This paper presents an overview of semiconductor materials used in gas sensors, their technology, design, and application. Semiconductor materials include metal oxides, conducting polymers, carbon nanotubes, and 2D materials. Metal oxides are most often the first choice due to their ease of fabrication, low cost, high sensitivity, and ...
This paper reveals a wide research review about the gas sensor structure as a sensing device in the field of the sensor. In the end, the current status, future perspectives as well as advantages of particular polymer-based sensors are summarized. ... Cite this paper. Saxena, P., Shukla, P. (2022). A Review on Gas Sensor Technology and Its ...
Flexible chemiresistive gas sensors have attracted growing interest due to their capability in real-time and rapid detection of gas. However, the performance of gas sensors has long been hindered by the poor charge transfer ability between the conventional metal electrode and gas sensing semiconductors. Herein, for the first time, a fully flexible paper-based gas sensor integrated with the ...
1. Introduction. Semiconductor-based gas sensor is playing an increasingly indispensable role in the application to detect harmful gases or gas-phase biomarkers, affording real-time, reliable response signals without the drawbacks of many conventional technologies [1], [2].The gas-induced electrical response on semiconductor surfaces is a very intriguing phenomenon.
Effective detection of toxic and hazardous gases is crucial for ensuring human safety, and high-performance metal oxide-based gas sensors play an important role in achieving this goal. In2O3 is a widely used n-type metal oxide in gas sensors, and various In2O3 nanostructures have been synthesized for detecting small gas molecules. In this review, we provide a brief summary of current research ...
The number of research papers has been investigated on the Web of Science on 22 March, 2022 (Fig. 1). Key words of the chemical formula of the sensor material and "gas sensor" are used for search. For instance, the keywords of "SnO 2 " and "gas sensor" are used to search for SnO 2 gas sensors.
This paper reveals a wide research review about the gas sensor structure as a sensing device in the field of the sensor. In the end, the current status, future perspectives as well as advantages of particular polymer-based sensors are summarized. 15.1 Introduction Gas sensors have extensive significant applications in the following areas which
1 Introduction. Chemical gas sensors have found a wide range of applications in defense, [1-3] safety, and disease diagnosis. [4-7] To enhance sensitivity, low-dimensional materials such as nanoparticles, [8-11] polycrystalline thin films, [12-14] chemical-vapor-deposited nanowires (NWs) [15-17] and 2D atomically thin materials [18-20] are often used as gas sensors due to their high surface-to ...
Nowadays, there is increasing interest in fast, accurate, and highly sensitive smart gas sensors with excellent selectivity boosted by the high demand for environmental safety and healthcare applications. Significant research has been conducted to develop sensors based on novel highly sensitive and selective materials. Computational and experimental studies have been explored in order to ...
Deepening our understanding of gas-sensing mechanisms is necessary to improve or optimize such sensors' performance. Only with more reliable performance will gas sensors draw increased attention and be applied to different areas. This Special Issue aims to collect research and review papers reporting on recent progress in materials utilized ...
Abstract: In this paper a review of different technologies for gas sensors is presented. The different types of gas. sensors technologies including catalytic gas sens or, electrochemical gas ...
Measurements of the properties of gas-sensitive materials are a subject of constant research, including continuous developments and improvements of measurement methods and, consequently, measurement set-ups. Preparation of the test set-up is a key aspect of research, and it has a significant impact on the tested sensor. This paper aims to review the current state of the art in the field of gas ...
Paper, as a flexible, low-cost, lightweight, tailorable, environmental-friendly, degradable, and renewable material, is emerging in electronic devices. Especially, many kinds of paper-based (PB) sensors have been reported for wearable applications in recent years. Among them, the PB gas, humidity, and strain sensors are widely studied for monitoring gas, humidity, and strain from the human ...
This paper first outlines the research of gas sensors composed of various dimensional nanomaterials. Secondly, nanomaterials may become the development direction of a new generation of gas sensors due to their high sensing efficiency, good detection capability and high sensitivity. ... In the research of a gas sensor, there is a unique ...
Paper • The following article is Open access. Recent advances in g-C 3 N 4 based gas sensors for the detection of toxic and flammable gases: a review. Vijendra Singh Bhati 4,1, Vishakha Takhar 2 ... and a lot of examination and research is expected to advance them to a stage where they can be deployed as commercial sensors.
The intent of this paper is to establish the state of the art of impedance-based gas sensors. This sensor type holds promise for accurate detection of gaseous species at single parts per million and below. Impedance-based sensors do not require reference air to function, but do require calibration. Progress in the development of impedancemetric sensors for the detection of NOx, H2O ...
The paper considers the structure of typical semiconductor gas sensor and describes the main stages of device production. Attention is focused on processes for creation of each device part. The influence of topology and geometry of heater element and measured electrodes is presented.
Developing high-performance chemiresistive gas sensors with mechanical compliance for environmental or health-related biomarker monitoring has recently drawn increasing research attention. Among them, two-dimensional MXene materials hold great potential for room-temperature hazardous gas (e.g., NH3) monitoring regardless of the complicated fabrication process, insufficient 2D/3D flexibilities ...
MQ-135 is a SnO2 semiconductor based gas sensor capable of MQ-135 such as CO, CO2, Ethanol, NH4, Toluene and Acetone in the ambient air [1]. Air pollution is increasing due to the number and ...
In this paper a low-cost advanced sensor-based gas leakage detector, alert and control system is. proposed and discussed. The system is very efficient, user friendly, portable, small in size and ...
The main contribution of this systematic paper is the synthesis of existing research, knowledge gaps, associated challenges, and future recommendations. Firstly, the IEEE database had the highest contribution to this research (48.51%). ... RH = 0-100%). The selection of gas sensors depends on their sensor range. Multi-gas sensors typically ...