Advances in Kidney Cancer Research

About 5% to 8% of kidney cancers are caused by inherited genetic changes.
NCI-funded researchers are working to advance our understanding of how to detect and treat kidney cancer. Much progress has been made over the last few decades, especially in identifying genes that can drive the development of kidney cancer. This knowledge has led to more effective treatments. Today, about 75% of people with kidney cancer will be alive 5 years after diagnosis.
This page highlights some of the latest research in kidney cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.

Early Detection of Kidney Cancer
Liquid biopsies to detect small kidney cancers.
There is no screening test that can diagnose kidney cancer early, before symptoms develop. Researchers are trying to develop blood or urine tests—sometimes called liquid biopsy tests—to detect small kidney cancers.
A 2020 study found that one of these tests, which analyzes DNA shed by kidney tumors, has promise to identify even early stage kidney tumors .
More research is needed to confirm these findings, and to improve the test. Scientists hope that tests of this type could eventually be used to screen people who are at high risk of developing kidney cancer, such as those with certain inherited conditions . They might also be used for tracking response to treatment and monitoring for cancer recurrence.
Genetic Testing for Kidney Cancer Risk
About 5% to 8% of kidney cancers are caused by inherited genetic changes. Many different hereditary syndromes increase the risk of kidney cancer (and sometimes other cancers). The gene changes that cause these syndromes have been identified, and people who have a history of kidney cancer in their family can now undergo genetic testing to see if they carry any of these changes.
This information from genetic testing can help health care providers develop a personalized plan for monitoring kidney health. Genetic testing and counseling may also be recommended based on factors such as age at diagnosis and what type of kidney cancer a person has.
Kidney Cancer Treatment
Until a few decades ago, kidney cancer was considered to be a single disease. But that changed after the first gene linked to kidney cancer , called the VHL gene , was discovered at NCI in the 1990s. Alterations in this gene can be inherited (in people with Von Hippel-Lindau disease ), or they can arise during someone’s lifetime.
Since this discovery, researchers have come to recognize that kidney cancer is many different diseases, each driven by distinct genetic features. This work has led to the development of many targeted therapies for kidney cancer. Ongoing research is working to further personalize targeted treatments and to tease out the role of immunotherapy in kidney cancer treatment.
Targeted Therapies for Advanced Kidney Cancer
Clear-cell renal cancer.
The most common type of kidney cancer is clear-cell renal cancer. It is also called clear-cell renal cell carcinoma or clear-cell RCC. VHL is the most commonly altered gene in that cancer type. The VHL protein normally blocks tumor development. However, when it is altered or missing, tumors can grow. Several drugs that target the VHL gene pathway have been approved by the FDA to treat clear-cell renal cancer.
Researchers are continuing to study new treatments that target the VHL pathway. For example, clinical trials are testing drugs that shut down a protein in the VHL pathway called HIF -2α.
- One study found that a drug called belzutifan (Welireg) , which blocks HIF-2α, shrank clear-cell renal tumors in people with Von Hippel-Lindau disease . These responses to treatment were long-lasting.
- Based on the above study, the FDA has approved belzutifan for people with Von Hippel-Lindau disease who have clear-cell renal cancer. It is the first drug approved to treat cancers associated with this hereditary condition.
- Results from a recent large study that compared belzutifan with another targeted drug, everolimus (Afinitor) , led to the FDA approval of belzutifan for people with advanced clear-cell renal cancer and no family history of the disease , who have already received two prior treatments.
- Other studies are also testing belzutifan in combination with other targeted therapies and with immunotherapy drugs.
Other types of drugs are also being tested in kidney cancer. For example, a new NCI-sponsored study is testing a combination of targeted drugs to help reduce the symptoms of kidney cancer that has spread to the bone.
Rare Kidney Cancer Types
About 15% of people with kidney cancer have papillary renal cell carcinoma , or papillary RCC. It is thought to start in a different kind of cell than clear-cell renal cancer. Data from The Cancer Genome Atlas and other research efforts have shown that some cases of papillary RCC are driven by changes in a gene called MET . A number of studies are underway to improve treatment for people with this rare kidney cancer. Examples include:
- Researchers compared cabozantinib (Cabometyx) , which targets the MET protein, with sunitinib (Sutent) in the first large randomized trial ever completed in this rare cancer type. People with advanced papillary RCC who were given cabozantinib lived about 50% longer without their disease progressing than people who received sunitinib.
- In 2020, an NCI research team found that some people with papillary RCC that had spread to other organs ( metastasized ) had strong responses to treatment with the drugs bevacizumab (Avastin) and erlotinib (Tarceva) , which target changes specific to that subtype. In that small clinical trial, those patients lived for a median of almost 2 years without their disease progressing. Work is ongoing to better personalize treatment in this rare disease.
- An ongoing NCI-sponsored trial is now testing the combination of cabozantinib and the immunotherapy atezolizumab (Tecentriq) to treat metastatic papillary RCC.
Immunotherapy for Advanced Kidney Cancer
Immunotherapies are treatments that help the body’s immune system fight cancer more effectively. Immunotherapy has become a major focus of kidney cancer treatment research. Today, most people with advanced kidney cancer will receive a type of immunotherapy drug called an immune checkpoint inhibitor at some point during their treatment.
Nivolumab Injections Could Make Cancer Treatment Easier
In a clinical trial, an injectable form of the immunotherapy drug worked as well against tumors as the IV form.
A small minority of people with clear-cell renal cancer and other, rarer types of kidney cancer have their tumors disappear entirely during treatment with these drugs. Studies are under way to uncover characteristics of patients or tumors that make immunotherapy more likely to work. And combinations of immunotherapies or of immunotherapies plus targeted therapies have been approved or are being studied in trials.
- A combination of two immune checkpoint inhibitors— ipilimumab (Yervoy) and nivolumab (Opdivo) — has been approved for the treatment of advanced kidney cancer.
- Pembrolizumab (Keytruda), plus the targeted drug axitinib (Inlyta)
- Nivolumab plus cabozantinib
- Pembrolizumab plus lenvatinib (Lenvima)
- Avelumab plus axitinib (Inlyta)
- Ongoing trials are testing other combinations of immune checkpoint inhibitors and targeted therapies, in clear-cell renal cancer as well as papillary RCC and other rare types of kidney cancer. Such trials include adding cabozantinib to the combination of ipilimumab and nivolumab .
Once cancer has spread from the kidney to other parts of the body, it’s not clear whether using surgery or radiation therapy to treat the initial kidney tumor helps patients live longer than treatment with immunotherapy alone. Ongoing NCI-sponsored trials are testing:
- Adding surgery to immunotherapy for advanced kidney cancer
- Adding radiation therapy to immunotherapy for advanced kidney cancer
- Adding radiation therapy to other types of standard treatment for advanced kidney cancer
To date, studies have not compared existing immunotherapy combinations directly, or tested whether these drugs work better when given together than given sequentially.
Treatment of Kidney Tumors in Children
Although rare, kidney cancer can develop in children and adolescents. The most common type of kidney cancer in children is called Wilms tumor . Although screening for kidney cancer in adults hasn't been shown to be effective to date, screening ultrasounds of the kidneys may benefit children with high genetic risk for Wilms tumor .
Treatment with the combination of surgery, radiation therapy, and chemotherapy has increased 5-year survival rates for children with all stages of Wilms tumor from 40% in the 1950s to nearly 90% today.
But this intensive treatment can have serious or even fatal long-term side effects, including second cancers and scarring of the lungs. So researchers are now testing whether less-intensive treatment regimens can maintain high survival rates while reducing side effects. For example:
- An NCI-sponsored clinical trial, led by the NCI-funded Children’s Oncology Group (COG), found that some children with advanced Wilms tumor may be able to skip radiation therapy to the lungs.
- Another study led by COG enrolled children with a genetic predisposition to develop Wilms tumor who had developed cancer in one kidney. About two-thirds of these children who had chemotherapy before surgery did not need to have the entire kidney removed .
- Another COG trial found that some young children with small Wilms tumors at low risk of recurrence can safely have surgery alone , without chemotherapy.
The COG also conducts studies of rarer types of childhood kidney cancer. One COG study is currently analyzing data collected on the combination of targeted therapy and immunotherapy for a rare type of kidney cancer in children, adolescents, and young adults called translocation renal cell carcinoma (tRCC). This study also enrolled adult patients with this rare cancer.
NCI-Supported Research Programs
Many NCI-funded researchers working at the NIH campus, as well as across the United States and throughout the world, are seeking ways to address kidney cancer more effectively. Some research is basic, exploring questions such as the biological underpinnings of cancer. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in kidney cancer.
- NCI’s Kidney Cancer Specialized Programs of Research Excellence (SPOREs) promote collaborative, interdisciplinary research. SPORE grants involve both basic and clinical/applied scientists working together. They support the efficient movement of basic scientific findings into clinical settings, as well as studies to determine the biological basis for observations made in individuals with cancer or in populations at risk for cancer.
- NCI's National Clinical Trials Network (NCTN) is a collection of organizations and clinicians that coordinates and supports cancer clinical trials at more than 3,000 sites across the United States and Canada. NCTN currently has a variety of trials testing treatments for kidney and other genitourinary cancers .
- NCI has also formed partnerships with the pharmaceutical industry, academic institutions, and individual investigators for the early clinical evaluation of innovative cancer therapies. The Experimental Therapeutics Clinical Trials Network (ETCTN) was created to evaluate these therapies using a coordinated, collaborative approach to early-phase clinical trials. The ETCTN is currently running early-stage trials in kidney and other genitourinary cancers .
- NCI’s Division of Cancer Epidemiology and Genetics (DCEG) conducts studies on kidney cancer to learn about risk factors for the disease. Some of their areas of study are described in these kidney cancer research studies .
- As part of the Cancer Moonshot℠, the Approaches to Identify and Care for Individuals with Inherited Cancer Syndromes initiative seeks the best approaches to identify people with an inherited cancer syndrome—including syndromes that can increase the risk of kidney cancer—and provide appropriate follow-up care.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for kidney cancer diagnosis and treatment .
Kidney Cancer Research Results
The following are some of our latest news articles on kidney cancer research:
- SBRT Emerging as an Important Treatment for Early-Stage Kidney Cancer
- Belzutifan Approved to Treat Tumors Linked to Inherited Disorder VHL
- Targeted Therapy Cabozantinib Slows Progression of Rare Kidney Cancer
- Liquid Biopsy Detects Brain Cancer and Early-Stage Kidney Cancer
- Targeted Therapy–Immunotherapy Combinations Effective for Advanced Kidney Cancer
- Some Children with Wilms Tumor Can Receive Less Therapy, Study Suggests
View the full list of Kidney Cancer Research Results and Study Updates .
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Kidney Cancer: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about kidney cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about kidney cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials. Always talk with your doctor about the best diagnostic and treatment options for you.
Because most types of kidney cancer do not respond well to traditional chemotherapy, medication research for kidney cancer focuses on using immunotherapy and targeted therapy (see Types of Treatment ).
Targeted therapy. Several recently discovered drugs that affect the process of blood vessel development and/or cancer cell growth are being tested as targeted therapies for kidney cancer. The results from these clinical trials show that these types of drugs may be effective treatments for kidney cancer, and this is an area of rapid scientific change.
Many targeted therapies and immunotherapies are being studied for use as adjuvant therapies, which are treatments given after the main treatment(s) to lower the risk of recurrence and to get rid of any remaining cancer cells. One targeted therapy, sunitinib, slowed the cancer from coming back in patients with localized kidney cancer at high risk for recurrence after having a nephrectomy. Other studies have not shown this effect, so using this type of targeted therapy as adjuvant treatment still needs to be studied.
Cancer vaccines. Cancer vaccines are treatments that help a person’s immune system fight cancer. Doctors are testing the use of several cancer vaccines to treat kidney cancer and to prevent recurrence for people with later-stage renal cell carcinoma. One vaccine being studied is made from a person's tumor and given after surgery, while others are made from proteins found on the surface of kidney cancer cells or blood vessel cells found in the tumor. There is currently no cancer vaccine that is approved for kidney cancer.
Modified cytokines. Interleukin (IL)-2 is a proven treatment for metastatic kidney cancer but has serious side effects (see "Immunotherapy" in Types of Treatment ). There is a new treatment that chemically modifies IL-2 (bempegaldesleukin), and it is associated with less frequent serious side effects. Clinical trials continue to study this treatment for kidney cancer.
Immune checkpoint inhibitors . As explained in Types of Treatment , this type of immunotherapy works by taking the brakes off the immune system so it is better able to destroy the cancer. These drugs use antibodies directed at specific molecules found on the surface of immune cells, such as PD-1 and CTLA-4. These drugs appear to work in kidney cancer, and many clinical trials are currently ongoing.
Palliative and supportive care. Clinical trials are underway to find better ways of reducing symptoms and side effects of current kidney cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in kidney cancer, explore these related items that will take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to review news and information about kidney cancer, including podcasts from ASCO experts discussing current clinical trials in kidney cancer , as well as highlights from recent scientific meetings.
Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Kidney Cancer Guide
Cancer.Net Guide Kidney Cancer
- Introduction
- Medical Illustrations
- Risk Factors and Prevention
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
The Latest Breakthroughs That Could Improve Kidney Cancer Treatment
D r. David McDermott started treating people with kidney cancer in the 1990s. Back then, he says the prognosis for most of his patients with advanced disease was dispiritingly grim. “We had very few treatment options, and the survival for patients was a year or less,” he recalls. “Radiation and chemotherapy were tried, but they didn’t work.”
Things began to change when researchers discovered that kidney cancers were highly “angiogenic” compared to most other forms of cancer, meaning that kidney tumors are rich in blood vessels. This insight supported the development of angiogenesis inhibitors, a type of drug that cuts off the blood supply to these tumors. “These drugs were very effective because of the biology that drives most kidney cancers,” says McDermott, a professor of medicine at Harvard Medical School and a cancer specialist at Beth Israel Deaconess Medical Center in Boston. Life expectancies doubled—a great leap forward, but one that still left plenty of room for additional improvement.
A more significant breakthrough—one that some cancer researchers say has revolutionized the treatment of kidney cancer—arrived just a decade ago. “The big innovation that changed things was immune checkpoint inhibitors,” McDermott says. Many cancers, including kidney cancers, have built-in defenses that allow them to repel the human immune system’s attacks. Immune checkpoint inhibitors help thwart these defenses. “If you think of a tumor’s defense against an immune attack as barbed wire, this class of immunotherapy drugs aims to cover the barbed wire and allow the immune system to do its job,” he explains.
Kidney cancers are among the top 10 most common cancers in both men and women: about 79,000 new cases will be diagnosed in 2022, according to the American Cancer Society. When the cancer is first diagnosed, more than 20% of patients already have advanced disease, meaning the cancer has spread beyond the kidneys. Even among those whose cancer is confined to the kidney and who undergo surgery, 30% will eventually develop metastatic disease.
Immune checkpoint inhibitors remain one of the primary treatments for kidney cancers , and recent developments in the science of these drugs remain a big story in treatment advancements. But it’s not the whole story. Here, McDermott and other experts described the current care landscape, including the latest innovations.
Unshackling the immune system
Thirty years ago, the American immunologist Jim Allison led research efforts that revealed the existence of immune checkpoints. Specifically, he and colleagues found that a protein on the surface of immune system T cells acts as a checkpoint, or brake, to prevent an overzealous immune system response.
Allison’s work led to the discovery that cancer cells take advantage of these checkpoints in ways that allow them to limit or evade the human body’s built-in protections. These insights have led to the creation of medicines that have changed the face of cancer care, including for kidney cancers. “The work of professor Jim Allison opened the field for immune checkpoint inhibitors and other immune therapies, which has led to the golden era of immunotherapy that we’re now seeing,” says Dr. Nizar Tannir, an oncologist and cancer researcher at the University of Texas MD Anderson Cancer Center in Houston. The U.S. Food and Drug Administration (FDA) approved the first immune checkpoint inhibitor for the treatment of kidney cancer in 2015. Since then, more of these drugs have received FDA approval. They target CTLA-4, PD-1, and PD-L1—proteins on immune cells that can limit the immune system’s attacks against cancer cells.
Even among people who don’t have metastatic kidney cancer, immune checkpoint inhibitors are now being tested as a therapy after kidney surgery. “Patients with kidney cancer that is confined to the organ usually undergo nephrectomy,” Tannir says, referring to a surgery that involves the removal of the kidney. “Just last November, the FDA approved pembrolizumab in patients who have a high risk of relapse after nephrectomy.” That approval came after a clinical trial showed that patients who got pembrolizumab were significantly more likely to be alive and disease-free two years after surgery compared to those who got a placebo.
While immune checkpoint inhibitors began as “second-line” therapies, to be used only after other treatments have failed, they’re increasingly being used as first-line therapies. “This is probably the biggest innovation of the last five years,” McDermott says. One of the interesting things about immune checkpoint therapy, he explains, is that some of the best-responding patients are the ones with the most aggressive tumors. “This is the opposite of what you would see with chemotherapy, where patients with more indolent tumors often benefit more,” he says. Why does this happen? One of the theories is that aggressive cancers may look very different than normal tissues, and so the immune system, once its brakes are released, is better able to locate and attack these tumors. Because some kidney cancers are fast-developing, starting immune checkpoint therapy as soon as possible can be preferable. “By giving immunotherapy early, as first-line treatment, more patients are not dying early,” he says.
Another advancement in immunotherapy involves using combinations of these drugs—either with one another or with other kidney cancer medications—instead of deploying them alone. Right now, McDermott says it’s more common to combine a single immune checkpoint inhibitor with the older class of kidney cancer drugs (the angiogenesis inhibitors). “This is a fusion of the old first-line therapy—the blood vessel-targeting drugs—with the new first-line therapy,” he says. “When you put them together, most of the benefit is additive, but in some patients it can also be synergistic.” In other words, the drugs may work better together than either would be when used independently.
While using two or more immune checkpoint inhibitors in combination is less common, some combinations are approved for use in both the U.S. and Europe. McDermott says he’s a proponent of this approach despite the greater potential for adverse reactions. “Blocking two of these immune checkpoints instead of one can dramatically increase the immune response to both the tumor and to normal tissues,” he says. “So it appears to increase the chances of disease remission, but it also increases the chances of toxicities.”
How much does that risk of toxicity increase? When taking one immune checkpoint inhibitor, he estimates that roughly 1 in 10 patients is forced to stop the treatment due to side effects, which can include joint pain, gut dysfunction, and other debilitating symptoms. This rate doubles to 2 in 10 when a second immunotherapy is added. Even short of having to stop treatment, the side effects tend to be more severe when someone is taking multiple immunotherapies. “It really comes down to philosophy of the appropriate goals of therapy,” he says. “Most oncologists don’t think kidney cancer is a curable disease.” As a result, he says they tend to opt for drug combinations with a milder side-effect profile and good near-term results. “I would argue that the longer-term outcomes are better with a combination of immune checkpoint inhibitors, but comparative trials are warranted to formally resolve this important debate,” he says.
Other experts share his view that combinations of immune therapies may provide the best chance for long-term survival. “If you look at median survival 10 years before the first immune checkpoint inhibitor was approved, clinical trials reported median overall survival of 20 to 30 months for patients with newly diagnosed metastatic cancer,” says Dr. Martin Voss, a clinical director and kidney cancer specialist at Memorial Sloan Kettering Cancer Center in New York City. Citing the latest research on combination treatments using immune checkpoint inhibitors, he says median survival is estimated to be 50 months or more for most patients. “So that’s almost double what it was, and a much higher percentage of patients are able to achieve complete remission,” he says.
This last point hints at a compelling area of kidney-cancer research. Why do some patients respond so well to the current drugs—in some cases, the cancer is eradicated—while others don’t? Advancements in tumor profiling and kidney cancer biomarkers—a catchall term for the cancer’s traits or characteristics—may help solve this puzzle.
Read More: Coping With the Side Effects of Kidney-Cancer Treatment
Unmasking the enemy
A major theme in cancer research—and not just for kidney cancers—is the recognition that the disease is highly variable. Voss says that kidney cancer comes in different “flavors,” or molecular subtypes, that help experts understand how the cancer evolves and why it may respond to different types of treatment. By studying kidney cancer biomarkers, he and other experts hope they’ll be able to better predict which patients do best on specific drugs or drug combinations.
“Some tumors seem to be more dependent on metabolism being changed in certain ways, while others are greatly dependent on tumor vasculature,” he says. Understanding these sorts of variations and their treatment ramifications, and also identifying ways to assess the presence of these variations in people with kidney cancer, is critical to improving outcomes. Scientists are studying how to better address these variations “to match people with the right treatment,” he says.
Kidney biomarkers and subtyping can also help improve the science of targeted therapies (including immunotherapies ) for kidney cancer. “If you understand what’s happening on the surface of the cancer cells, you can deliver much more directed therapies and achieve a much more potent immune response,” Voss says. For example, chimeric antigen receptors, or CARs, are molecularly engineered proteins that are designed to bind to a cancer cell (but not healthy cells) and summon an immune response. While these have been used successfully for other cancers, they’ve only recently made their way into clinical kidney cancer trials. “The whole field is holding its breath for those results, which we should have in the next year or two,” he says. These could provide another big leap forward in kidney cancer treatment.
Read More: 4 Important Steps to Take After a Cancer Diagnosis
More reason for optimism
There’s a lot more going on in kidney cancer care. McDermott says another promising advancement involves a class of drug known as hypoxia inducible factor (HIF)-2α inhibitors. HIF-2α helps cancerous tumors develop new blood vessels, use nutrients more efficiently, and otherwise adjust in ways that support their spread and survival. HIF inhibitors are drugs that can block all of these adjustments. “Early results of these HIF-2α agents are encouraging,” he says. Meanwhile, he says that many novel immunotherapies and targeted therapies are also in development.
Taking a 10,000-foot view, it appears that the treatment foundations have been laid—and in some cases, impressively built upon—that will eventually lead to reliable remission for most people with advanced kidney cancers. Already, once-deadly cancers are being succesfully treated. Looking ahead, the evolution of cancer subtyping and biomarker mapping should help ensure that patients are given the most effective drugs with the lowest risk of side effects. Considering how dramatically the treatment picture has changed in just the last five or 10 years, there’s reason to expect more significant advancements in the near future.
As MD Anderson’s Tannir says, “There is more hope than ever for patients to have improved survival, and even a complete and durable remission with the potential for cure.”
More Must-Reads From TIME
- Jane Fonda Champions Climate Action for Every Generation
- Biden’s Campaign Is In Trouble. Will the Turnaround Plan Work?
- Why We're Spending So Much Money Now
- The Financial Influencers Women Actually Want to Listen To
- Breaker Sunny Choi Is Heading to Paris
- Why TV Can’t Stop Making Silly Shows About Lady Journalists
- The Case for Wearing Shoes in the House
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]
You May Also Like
- Find a Doctor
- Appointments and Second Opinions
- Find a Location
- Patient Portals
FDA approves kidney cancer therapy after Dana-Farber-led research shows improved outcomes for patients with advanced disease
Belzutifan, a HIF-2α inhibitor, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with advanced kidney cancer previously treated with immune checkpoint inhibitors and anti-angiogenic therapies.
The FDA approval was based on results of the phase 3 LITESPARK-005 trial , a study led by Toni K. Choueiri, MD , director of the Lank Center for Genitourinary Cancer at Dana-Farber Cancer Institute.
Findings from LITESPARK-005 were presented at the European Society of Medical Oncology annual meeting in October 2023. Researchers reported belzutifan significantly reduced the risk of progression of clear cell renal cell carcinoma (ccRCC), the most common type of kidney cancer, in this patient population. The trial compared belzutifan to everolimus and the data showed the risk of progression was reduced by 26%. Responses were 6 times higher with belzutifan compared to everolimus.
"This approval is exciting news for our patients as it gives us a new option for refractory patients with kidney cancer," said Choueiri. "Belzutifan is an oral drug with a novel mechanism of action that reduced risk of disease progression or death and had favorable quality of life in this patient population, when compared to everolimus."
Previously, the FDA approved belzutifan for patients with Von Hippel-Landau (VHL) disease-associated renal cell carcinoma, a rare form of kidney cancer. The drug was originally investigated and approved for kidney cancer patients with VHL disease because they have inherited a mutation that inactivates the VHL gene, which results in an overabundance of HIF-2α in cells.
When overabundant in cells, HIF-2α is associated with increased cancer-driving activity, such as cell proliferation, immune evasion, low oxygen levels (called hypoxia), and blood vessel formation (called angiogenesis). Dana-Farber’s William G. Kaelin, Jr., MD, was awarded a Nobel Prize in Physiology or Medicine in 2019 for the discovery of the role HIF-2α in cancer and other diseases.
LITESPARK-005, enrolled 746 patients with metastatic ccRCC who had progressed after treatment with both an immune checkpoint inhibitor (ICI), such as a PD-1 or PD-L1 inhibitor, and an anti-angiogenic therapy. ICIs and anti-angiogenic medicines have become a standard part of first- and second-line therapies for metastatic ccRCC, though most patients eventually experience disease progression and need additional treatment options.
Patients were randomized to receive treatment with either belzutifan or everolimus. At the second interim analysis, after a median of 25.7 months, patients taking belzutifan were 26% less likely to have progressed compared with those taking everolimus.
The overall response rate was also higher with belzutifan, at 22% versus 3.5%, and 13 patients experienced a complete response with belzutifan compared to none with everolimus.
Regarding safety, findings for belzutifan were consistent with data from previously reported studies and patients taking belzutifan were less likely to discontinue therapy due to side effects.
The trial is sponsored by Merck Sharp & Dohme LLC.
Media Contacts
If you are a journalist and have a question about this story, please call 617-632-4090 and ask to speak to a member of the media team, or email [email protected] .
The Media Team cannot respond to patient inquiries. For more information, please see Contact Us .

A CT scan of a healthy kidney. Kidneys filter waste from the bloodstream and control the level of certain helpful chemical substances.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Renal cancer articles within Nature Reviews Nephrology
Consensus Statement | 05 March 2024
Clinical practice recommendations for kidney involvement in tuberous sclerosis complex: a consensus statement by the ERKNet Working Group for Autosomal Dominant Structural Kidney Disorders and the ERA Genes & Kidney Working Group
Care for patients with tuberous sclerosis complex (TSC) should be coordinated by a multidisciplinary team. This Consensus Statement, which involved input from nephrologists, urologists, geneticists, radiologists, interventional radiologists and pathologists as well as patient representatives, provides an overview of TSC-associated kidney manifestations and outlines current recommendations for the management of kidney involvement in TSC.
- Djalila Mekahli
- , Roman-Ulrich Müller
- & John Bissler
Research Highlight | 26 February 2024
Pericyte–stem cell crosstalk in ccRCC
- Susan J. Allison
Review Article | 22 January 2024
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Renal cell carcinoma is a metabolic disease linked to a variety of alterations in genes that regulate cellular metabolism. Here, the authors examine cell-intrinsic metabolic alterations in hereditary and sporadic renal cell carcinoma, and how they can be exploited to develop novel therapeutic interventions.
- Nathan J. Coffey
- & M. Celeste Simon
Review Article | 27 March 2023
Immunogenicity in renal cell carcinoma: shifting focus to alternative sources of tumour-specific antigens
Renal cell carcinoma is sensitive to immune checkpoint blockade despite having a moderate traditional tumour mutational burden profile. Here, the authors discuss how the high prevalence of frameshift insertion or deletions in renal cell carcinoma, as well as the reactivation of endogenous retroviral gene expression, might provide alternative neoantigens that potentiate responses to immunotherapy.
- Melissa M. Wolf
- , W. Kimryn Rathmell
- & Aguirre A. de Cubas
Research Highlight | 24 January 2023
Targeting glutamine use in RCC
- Monica Wang
Review Article | 31 May 2022
Extracellular vesicles in kidney disease
In this Review, the authors discuss the roles of extracellular vesicles in kidney physiology and disease as well as the beneficial effects of stem cell-derived extracellular vesicles in preclinical models of acute kidney injury and chronic kidney disease. They also highlight current and future clinical applications of extracellular vesicles in kidney diseases.
- Cristina Grange
- & Benedetta Bussolati
In Brief | 25 March 2022
Anti-tumour antibody-producing plasma cells disseminate from tertiary lymphoid structures
- Ellen F. Carney
News & Views | 07 February 2022
Belzutifan: a novel therapy for von Hippel–Lindau disease
A recent clinical trial reports promising efficacy and safety data for belzutifan in patients with von Hippel–Lindau (VHL) disease–associated renal cell carcinoma. On the basis of these results, belzutifan became the first therapeutic agent to be approved for the systemic treatment of cancer associated with VHL disease.
- Jingcheng Zhou
- & Kan Gong
In Brief | 18 October 2021
ccRCC–adipose crosstalk in disease pathogenesis
- Susan Allison
Review Article | 29 June 2021
Non-invasive molecular imaging of kidney diseases
In vivo non-invasive molecular imaging techniques have potential to improve clinical research and practices in nephrology. Here, the authors discuss the benefits and challenges of preclinical and clinical applications of molecular imaging to acute kidney injury and chronic kidney disease, transplantation and kidney cancer.
- Barbara M. Klinkhammer
- , Twan Lammers
- & Peter Boor
In Brief | 22 June 2021
New insights into the ccRCC microenvironment
In Brief | 30 April 2021
Preferential glutamine uptake in cancer cells
Review Article | 03 November 2020
Clear cell renal cell carcinoma ontogeny and mechanisms of lethality
The molecular features that define the initiation and progression of clear cell renal cell carcinoma (ccRCC) are being increasingly defined. This Review summarizes common genomic and chromosomal copy number abnormalities in ccRCC, providing a mechanistic framework with which to organize these features into initiating events, drivers of progression and factors that confer lethality.
- Eric Jonasch
- , Cheryl Lyn Walker
- & W. Kimryn Rathmell
Review Article | 30 July 2020
The immunology of renal cell carcinoma
Here, the authors describe the effector cell populations and immunosuppressive networks that are present in renal cell carcinoma (RCC) tumours. They also discuss the use of immune checkpoint inhibitors and novel approaches such as adoptive cell therapy in patients with RCC.
- C. Marcela Díaz-Montero
- , Brian I. Rini
- & James H. Finke
Review Article | 19 June 2020
Genomic profiling in renal cell carcinoma
Genomic profiling of renal cell carcinoma has demonstrated the clinical relevance of several genetic alterations in different disease subtypes. Pal and colleagues discuss the prognostic and predictive value of these alterations, and how they might help to improve treatment selection and patient outcomes.
- Nazli Dizman
- , Errol J. Philip
- & Sumanta K. Pal
Research Highlight | 06 April 2020
Epigenetic control of inflammatory cells
News & Views | 02 March 2020
New insights into the obesity paradox in renal cell carcinoma
Paradoxically, elevated BMI is a recognized positive prognostic factor in renal cell carcinoma (RCC). A recent investigation of the transcriptomic signatures of RCC tumours and peritumoural tissues suggests potential biological mechanisms underlying this effect. However, the clinical utility of BMI in the context of RCC remains uncertain.
- Chun Loo Gan
- & Daniel Y. C. Heng
News & Views | 28 November 2019
Proteomic signatures of clear cell renal cell carcinoma
In recent years, the molecular view of clear cell renal cell carcinoma (ccRCC) has been based primarily on gene transcription data with limited information on protein features. A new study led by the Clinical Proteomic Tumor Analysis Consortium now offers a comprehensive view of the ccRCC proteome.
- Chad J. Creighton
Review Article | 21 October 2019
Oncometabolites in renal cancer
Oncometabolites — conventional metabolites that, when aberrantly accumulated, have pro-oncogenic capabilities — have been implicated in renal cell carcinoma (RCC). Here, the authors review the role of oncometabolites in RCC, their origins and downstream effects and their potential applications as novel therapeutic targets and biomarkers.
- , Grant D. Stewart
- & Christian Frezza
Research Highlight | 18 October 2019
Synthetic lethality between loss of CDK4/6 activity and VHL inactivation
Research Highlight | 03 September 2019
PAX8 : a candidate oncogene in RCC
News & Views | 16 April 2019
Immune-based combination therapy for metastatic kidney cancer
New data from the JAVELIN Renal 101 and KEYNOTE-426 trials provide evidence that immune-based combination therapy has superior efficacy to sunitinib monotherapy in patients with advanced renal cell carcinoma. The new findings raise important questions regarding the optimum choice of combination therapy for these patients.
- Camillo Porta
- & Mimma Rizzo
Research Highlight | 15 April 2019
Unique metabolic traits identify CCPAP
Review Article | 26 March 2019
The adjuvant treatment of kidney cancer: a multidisciplinary outlook
Effective adjuvant therapies are needed to reduce the risk of recurrence of kidney cancer. Here, the authors discuss the results of adjuvant therapy trials, the potential of immune checkpoint inhibitors as adjuvant therapies and the need for multidisciplinary management of patients with resected kidney cancer.
- , Laura Cosmai
- & Axel Bex
Review Article | 31 January 2019
The genetic changes of Wilms tumour
Wilms tumour is the most common renal malignancy of childhood. Here, the authors review the genetic landscape of Wilms tumour and discuss how precision medicine guided by genomic information might lead to new therapeutic approaches and improve patient survival.
- Taryn Dora Treger
- , Tanzina Chowdhury
- & Sam Behjati
Consensus Statement 03 December 2018 | Open Access
The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group
This Expert Consensus Document from the International Kidney and Monoclonal Gammopathy Research Group includes an updated definition of monoclonal gammopathy of renal significance (MGRS) and recommendations for the use of kidney biopsy and other modalities for evaluating suspected MGRS
- Nelson Leung
- , Frank Bridoux
- & Samih H. Nasr
Research Highlight | 14 November 2018
A central anti-oncogenic pathway in ccRCC
Research Highlight | 19 October 2018
Arginine auxotrophy in PKD
Research Highlight | 12 September 2018
Sweet success for ccRCC isotope tracing
- Caroline Barranco
News & Views | 04 July 2018
The role of nephrectomy in metastatic renal cell carcinoma
Cytoreductive nephrectomy is the current treatment paradigm for metastatic renal cell carcinoma (RCC). However, the introduction of targeted therapies has dramatically changed the treatment landscape and may limit the role of nephrectomy in this disease. The recent CARMENA trial supports initial medical treatment of patients with RCC and synchronous metastases.
- Viktor Grünwald
News & Views | 06 June 2018
The origin, evolution and route to metastasis of clear cell RCC
Three reports from the TRACERx Renal study delineate the precise origin and evolution of clear cell renal cell carcinoma in minute detail. The insights gained from these studies might provide improved disease prognostics and identify novel therapeutic targets.
- Christopher J. Ricketts
- & W. Marston Linehan
Research Highlight | 24 May 2018
Altered ammonia metabolism in ccRCC
News & Views | 05 March 2018
A link between stemness and tumorigenesis in the kidney
The cellular origins of angiomyolipoma and other tuberous sclerosis complex-associated neoplasms are unknown. Now, two studies show that these neoplasms derive from cancer stem cells that originate from multipotent renal epithelial cells. The new findings provide a link between stemness and tumorigenesis in the kidney.
- Francesca Becherucci
- & Paola Romagnani
Comment | 05 February 2018
The role of nephrologists in the management of small renal masses
Renal cell carcinoma (RCC) is the most common malignancy seen in the nephrology clinic, yet most nephrologists have inadequate knowledge of current treatment options. Here we discuss RCC presentation and therapies, including potential renal adverse effects, and highlight the need for involvement of nephrologists in the multidisciplinary management of this disease.
- Susie L. Hu
- & Robert H. Weiss
Research Highlight | 22 January 2018
PBRM1 loss promotes tumour response to immunotherapy
Research Highlight | 30 October 2017
Renewal of NPCs requires MYC and β-catenin
- Jack M. Heintze
Research Highlight | 11 September 2017
Targeting Wilms tumour
Review Article | 31 July 2017
Mechanisms and consequences of carbamoylation
Patients with chronic kidney disease have elevated levels of carbamoylated proteins. Here the authors review the mechanisms of carbamoylation, the effects of this post-translational modification on renal function and strategies to reduce the carbamoylation load.
- Sigurd Delanghe
- , Joris R. Delanghe
- & Marijn M. Speeckaert
Review Article | 10 July 2017
Targeted therapies for renal cell carcinoma
This Review provides an overview of the molecular determinants of renal cell carcinoma, how understanding the underlying mechanisms of disease has fuelled the development of targeted therapies, and tools to assess the value of these agents.
- Edwin M. Posadas
- , Suwicha Limvorasak
- & Robert A. Figlin
In Brief | 12 June 2017
New mouse model of clear cell renal cell carcinoma
Review Article | 08 May 2017
Metabolic reprogramming in clear cell renal cell carcinoma
Clear cell renal cell carcinoma is associated with reprogramming of metabolic pathways including glucose and fatty acid metabolism and the tricarboxylic acid cycle. Here, the authors discuss these reprogrammed pathways and the opportunities they provide for new therapies, imaging modalities and biomarkers.
- Hiromi I. Wettersten
- , Omran Abu Aboud
Research Highlight | 19 April 2017
Targeting metabolism in RCC
- Susan. J. Allison
Research Highlight | 13 February 2017
CCR4: a new target for RCC
Year in Review | 19 January 2017
The evolution of anti-angiogenic therapy for kidney cancer
Tyrosine kinase inhibitors that target pro-angiogenic pathways improve progression-free and overall survival in patients with metastatic kidney cancer and were thus tested in the adjuvant setting in studies published this past year. 2016 also saw the emergence of new inhibitors of pro-angiogenic pathways that might represent the next step in kidney cancer therapy.
- Chung-Han Lee
- & Robert J. Motzer
Review Article | 28 November 2016
The epigenetic landscape of renal cancer
New data suggests that, in addition to mutations in tumour-suppressor genes, renal cancer is associated with epigenetic aberrations. Here, the authors discuss the mechanisms by which epigenetically silenced genes and mutations in genes that are involved in histone modification or chromatin remodelling dysregulate crucial cellular pathways in renal cancer.
- Mark R. Morris
- & Farida Latif
Review Article | 31 October 2016
Nanomedicines for renal disease: current status and future applications
The use of nanoparticles has great potential for targeted delivery of therapeutics to specific cell types in the kidney. Here, the authors discuss the characteristics of nanoparticles and of renal physiology that must be considered when developing nanomedicines to treat kidney disease, as well as the remaining challenges in clinical translation of this technology.
- Nazila Kamaly
- , John C. He
- & Omid C. Farokhzad
In Brief | 17 October 2016
IMPRINT: no survival benefit of IMA901 in RCC
Review Article | 03 October 2016
Precision medicine from the renal cancer genome
Epigenetic machinery and chromatin remodelling complexes are disrupted in >80% of clear cell renal cell carcinoma tumours. Here, the authors discuss the impact of genomics in identifying genes that affect susceptibility to renal cell carcinoma as well as the opportunities for a precision medicine approach to diagnosis and treatment.
- Yasser Riazalhosseini
- & Mark Lathrop
Research Highlight | 08 August 2016
OCT2 demethylation cracks open oxaliplatin resistance
- Andrea Aguilar
News & Views | 25 April 2016
Rest ASSUREd, much can be learned from adjuvant studies in renal cancer
The first, highly anticipated randomized trial of adjuvant antiangiogenic therapy in renal cancer was recently reported. Although far from assuring, data from the adjuvant sorafenib or sunitinib for unfavorable renal carcinoma (ASSURE) trial offer a wealth of insights into the disease, treatments, and biological considerations for studies aimed at risk reduction.
- David D. Chism
Browse broader subjects
- Urogenital diseases
- Urological cancer
- Kidney diseases
Browse narrower subjects
- Renal cell carcinoma
- Wilms tumour
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Exp Clin Cancer Res

Current status of research on exosomes in general, and for the diagnosis and treatment of kidney cancer in particular
1 Department of Urology, Shidong Hospital of Yangpu District, No. 999 Shiguang Road, Yangpu District, Shanghai, 200438 China
2 Department of Urology, Affiliated Zhongda Hospital of Southeast University, No. 87 Dingjiaqiao, Hunan Road, Gulou District, Nanjing, 210009 China
Associated Data
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Kidney cancer is a common urological tumour. Owing to its high prevalence and mortality rate, it is the third most malignant tumour of the urinary system, followed by prostate and bladder cancers. It exerts a high degree of malignancy, and most of the distant metastasis occurs at an early stage; it is insensitive to chemoradiotherapy and easily develops drug resistance. The current treatment for kidney cancer mainly includes surgery, interventional embolization and targeted therapy; however, the treatment efficacy is poor. In recent years, the role of exosomes as mediators of intercellular communication and information exchange in the tumour microenvironment in tumour pathogenesis has attracted much attention. Exosomes are rich in bioactive substances such as nucleic acids, proteins and lipids and are involved in angiogenesis, immune regulation, drug resistance, formation of pre-metastatic niche, invasion and metastasis. This article reviews the ongoing research and applications of exosomes for the diagnosis and treatment of kidney cancer.
Kidney cancer, also known as renal cell carcinoma (RCC), is one of the most common malignancies of the urinary tract, and its incidence has increased at a rapid rate of 2% per year over the past two decades [ 1 , 2 ]. In 2018, approximately 400,000 new cases and 170,000 deaths owing to kidney cancer were reported worldwide [ 3 ]. In 2015, approximately 74,000 new cases and 27,000 deaths owing to kidney cancer were reported in China [ 4 ]. Kidney cancer is insensitive to radiotherapy and chemotherapy, and surgery remains the mainstay of treatment for kidney cancer. However, approximately 30% of patients with kidney cancer develop metastasis on initial diagnosis, and approximately 25% of patients with localized kidney cancer may develop local recurrence or distant metastasis after surgery [ 5 , 6 ]. Owing to recurrence or distant metastasis, the 5-year survival rate of patients with advanced kidney cancer is extremely low, approximately 5–10% [ 7 , 8 ].
Exosomes are small extracellular vesicles composed of a lipid bilayer membrane structure; they are actively secreted by normal and cancer cells in the body and contain proteins, nucleic acids, lipids and other bioactive substances [ 9 , 10 ]. Exosomes play an important role in the exchange of information between cells by releasing bioactive substances that fuse with receptor cell membranes or bind to cell surface receptors [ 11 , 12 ]. Studies have demonstrated that exosomes play an important role in the development, diagnosis and treatment of kidney, prostate, bladder and breast cancers and serve potential clinical applications as tumour markers, therapeutic targets and drug nanocarriers in clinical settings [ 13 – 15 ].
This article reviews the ongoing research and applications of exosomes for the diagnosis and treatment of kidney cancer.
Overview of exosomes
Exosomes are nanoscale biological vesicles released into surrounding body fluids upon fusion of multivesicular bodies and the plasma membrane; they are produced and secreted autonomously by living cells in vivo and are the smallest extracellular vesicles [ 16 , 17 ]. Exosomes are subgroups of extracellular vesicles with an average diameter of about 30–100 nm [ 18 , 19 ]. Exosomes originate from the intracellular body structure, which influences the composition of exosome contents after interaction with other intracellular vesicles and organelles [ 20 ]. Exosomes were previously considered non-functional substances until 2007, when it was discovered that exosomes may act as ‘messengers’ that carry genetic material for the exchange of intercellular information and act within the recipient cells, suggesting that exosomes can be involved in intercellular information exchange [ 21 – 23 ]. The membrane structure of exosomes is resistant to exogenous proteases and RNA enzymes, thus resulting in more stable intracellular functional proteins, messenger RNAs (mRNAs) and microRNAs (miRNAs) that make exosomes a sensitive marker for disease diagnosis [ 24 , 25 ]. In many diseases, exosomes can function by altering cellular or tissue states, and exosome-related assays can be used as effective and non-invasive methods for disease diagnosis and monitoring [ 9 , 10 ]. In addition, the study of molecular mechanisms related to exosome-mediated intercellular material exchange will also provide a theoretical basis for the development of exosome-related therapies [ 26 , 27 ].
Composition of exosomes
As observed under the electron microscope, exosomes are hemispherical structures with a lipid bilayer membrane [ 28 ]. Exosomes are composed of various components, mainly including proteins, lipids and nucleic acids (Table 1 ), and are abundantly present in body fluids, including blood, tears, urine, saliva, milk and ascites [ 38 ] (Fig. 1 ). Proteins mainly include tetraspanin, heat shock proteins, MVB formation, membrane transport and fusion proteins, antigen presentation, adhesion molecules, lipid raft and cytoskeletal proteins, which participate in the fusion of cell membranes and release of exosomes [ 29 – 31 ]. Lipids mainly include cholesterol, ceramide, phosphatidylserine, phosphatidylinositol, phosphatidylcholine, sphingomyelin and ganglioside, which are involved in the biological activity of exosomes [ 32 – 34 ]. Nucleic acids mainly include DNA, mRNA, miRNA, long non-coding RNA (lncRNA) and circular RNA (circRNA), which participate in the transmission of genetic information and diagnosis of diseases [ 35 – 37 ]. The specific components of exosomes are displayed in Table Table1 1 .

The hallmarks and cargos of exosomes. Exosomes are hemispherical structures with lipid bilayer membrane under electron microscope. Exosomes are composed of various components, mainly including proteins, lipids and nucleic acids
Formation of exosomes
The exact mechanism of exosome formation remains poorly understood, and the endosomal sorting complex required for transport (ESCRT) is a classical pathway [ 39 , 40 ] (Fig. 2 ). The two main steps in the formation of exosomes are as follows: First, the cell membrane sags inward to form the early endosomes with accumulated luminal vesicles (ILV), and the endosomes are wrapped with proteins, lipids and nucleic acids synthesised by the cells; the endosomal membrane is depressed to bud inward to form tubular vesicles (intraluminal vesicles), that is, early endosomes (EEs) [ 41 , 42 ]. Subsequently, the depressed membrane matures into multivesicular bodies (MVBs) with dynamic subcellular structures, that is, late endosomes (LEs), which can expose the transmembrane protein domain of the cytoplasm and release multiple vesicle structures into the extracellular environment upon fusion with the plasma membrane to form exosomes. Rab27a and Rab27b direct the movement of LEs/MVBs toward the cell periphery, the SNARE complex helps LEs/MVBs fuse with the plasma membrane to release exosomes, and the rest of LEs/MVBs are degraded by lysosomes [ 43 , 44 ].

Exosome biogenesis and secretion within endosomal system by the endosomal sorting complex required for transport (ESCRT) pathway. Early endosomes (EEs) are formed by the fusion of endsomes. Subsequently, EEs depend on ESCRT to form multivesicular late endosomes (LEs)/bodies (MVBs). Rab27a and Rab27b direct the movement of LEs/MVBs toward the cell periphery, the SNARE complex helps LEs/MVBs fuse with the plasma membrane to release exosomes, and the rest of LEs/MVBs are degraded by lysosomes

Secretion of exosomes
Exosomes are secreted extracellularly through exocytosis upon the fusion of intercalated compartments with plasma membrane, which is the most basic and common process in cells [ 45 ]. However, in T cells and mast cells, this fusion is dependent on calcium ions for activation [ 46 ]. Most intracellular membrane fusions occur through specific protein mechanisms, such as N-ethylmaleimide-sensitive factor (NSF) for soluble factors and soluble NSF adhesion protein (SNAP) and SNAP adhesion protein receptor (SNARE) for membrane complex factors [ 47 ]. The two membranes in which fusion occurs should contain the corresponding SNAREs, namely vesicular SNARE (v-SNARE) and target SNARE (t-SNARE) [ 48 , 49 ]. In addition, exosome secretion is controlled by Ras-associated GTP-binding protein 27a (Rab27a) and Rab27b [ 50 , 51 ]. Synaptic binding protein-like 4 (SYTL4) and exophilin 5 (EXPH5) can inhibit Rab27a and Rab27b, leading to exosome secretion [ 51 ]. The exact mechanism of regulation of exosome secretion remains unclear, and the role of the above-mentioned molecules in exosome secretion requires further investigation.
Function of exosomes
Exosomes are released by different cell types and can regulate the biological activity of target cells by transporting proteins, lipids and nucleic acids. They play a role in various biological processes such as angiogenesis, antigen presentation, apoptosis and inflammation [ 17 ]. They act by transferring informative substances, thus influencing physiological and pathological processes involved in cancer, neurodegenerative diseases, infections and autoimmune diseases [ 52 – 56 ]. Exosomes affect the recipient cells through two pathways [ 57 ]. The first pathway involves ligand–receptor interactions between exosomes and recipient cells, without internalising the exosome or its contents into the target cell. This pathway can regulate the activation or inhibition of target cell signalling pathways. The second pathway involves the entry of exosomes into cells through membrane fusion or endocytosis, wherein their components are taken up and released into the cytoplasm, thus affecting the host cells by regulating specific gene expression and signalling pathways and ultimately leading to changes in the cell function or phenotype.
Detection of exosomes
In recent years, with the progress of research on exosomes in tumours, various technologies for exosome detection have been introduced that focus on the following three aspects: isolation and enrichment, identification and content analysis [ 58 – 62 ]. In addition, some researchers have developed various kits for the diagnosis and prognostic risk assessment of tumours based on the composition of exosomes [ 56 , 63 – 66 ]. Development of such detection kits is a major clinical breakthrough in the field of early tumour diagnosis and provides an effective test for clinical diagnosis and the assessment of efficacy. However, there is a lack of a unified gold-standard method for exosome detection, which makes it difficult to be widely promoted in clinical settings. Therefore, it is necessary to discover a uniform and clinically recognised exosome detection technology.
Exosomes and kidney cancer
Involvement in the formation of tumour microenvironment.
The tumour microenvironment is a key factor in the formation of tumours, and tumour cells can interact with their microenvironment to promote tumorigenesis and progression [ 67 ]. Exosomes exhibit certain characteristics of tissue and organ cellophilia, and the expression of this tendency is related to the expression of integrins on the surface of exosomes [ 68 ]. The establishment of pre-metastatic ecological niche is a complex process that involves the binding of exosomes secreted by cancer cells to the stromal cells of target organs, leading to the reprogramming of target cells, activation of signalling pathways and ultimately the establishment of a pre-metastatic microenvironment in target organs, thus providing the prerequisite for tumour metastasis [ 69 , 70 ]. Exosomes are considered the main mediators of cell–cell interactions in the tumour microenvironment and are involved in promoting tumour cell invasion, angiogenesis and immunosuppression [ 71 – 75 ]. The role of exosomal constituents in kidney cancer are shown in Fig. 3 .

Role of exosomal constituents in kidney cancer. Exosomal component sare involved in the proliferation, migration and invasion, metastasis, angiogenesis, drug resistance, and epithelial mesenchymal transition (EMT) of kidney cancer
Contribution to angiogenesis
During tumorigenesis, tumour cells require a large supply of nutrients and oxygen to maintain rapid cell growth and reproduction. The formation of new blood vessels in the primary tumour foci provides more nutrients for the growth and spread of tumour cells [ 76 , 77 ]. Tumour cells promote angiogenesis by activating endothelial cells [ 78 ]. Endothelial cells secrete exosomes rich in vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), angiopoietin-1 (ANGPT1), ephrin A3 (EFNA3), matrix metallopeptidase 2 (MMP2), matrix metallopeptidase 9 (MMP9) and azurocidin 1 (AZU1), which can stimulate the production of adjacent tumour blood vessels [ 78 – 82 ]. Grange et al. [ 70 ] verified that a subset of CD105-expressing tumour-initiating cells in human kidney cancer released microvesicles, which triggered angiogenesis and promoted the formation of pre-metastatic niches. Hou et al. [ 72 ] demonstrated that oncogenic miR-27a delivered by exosomes can bind to secreted frizzled-related protein 1 (SFRP1) and promote angiogenesis in kidney cancer. Tyrosine kinase inhibitor (TKI)-resistant kidney cancer can secrete low levels of exosomal miR-549a to induce vascular permeability and angiogenesis to promote kidney cancer metastasis [ 83 ]. Li et al. [ 84 ] found that ApoC1 transfer from kidney cancer cells to vascular endothelial cells through exosomes promoted angiogenesis and enhanced the migration and invasion of human umbilical vein endothelial cells (HUVEC) cells by activating signal transducer and activator of transcription 3 (STAT3). In addition, exosomes with high expression of carbonic anhydrase IX (CA IX) are associated with kidney cancer revascularisation [ 85 ]. The establishment of a vascular network is not only essential for the normal growth of tumour tissues but also provides an important channel for tumour invasion [ 86 ].
Contribution to immune escape
Myeloid-derived suppressor cells (MDSCs) exert potent inhibitory effects on several immune cells, and their high concentration aggregation in the tumour microenvironment is one of the reasons for the formation of tumour immune escape [ 87 , 88 ]. It was found that Hsp70 was abundantly present in exosomes secreted by mouse kidney cancer cells (Renca cells), upregulated the expression of arginase 1 (ARG-1), iNOS, interleukin 6 (IL-6) and VEGF and induced the expression of MDSCs by phosphorylating STAT3 (p-STAT3) pathway, thus promoting tumour growth [ 75 , 89 ].
Natural killer (NK) cells are the main host defence factors against kidney cancer cells and can exert anti-tumour effects by either directly mediating cytotoxic activity through degranulation or promoting anti-tumour activity and producing immunomodulatory cytokines [ 90 – 92 ]. Xia et al. [ 93 ] found that exosomes of kidney cancer origin induced defective NK cell function through transforming growth factor-beta (TGF-β)/SMAD signalling pathway to evade natural immunity.
Exosomes secreted by kidney cancer cells can induce immune responses in T cells to trigger apoptosis of activated T lymphocytes by activating the caspase pathway. They can diminish the cytotoxicity of NK cells and reduce the production of IL-2, interferon gamma (IFN-γ), IL-6 and IL-10, which contribute to the immune escape and promote the development of kidney cancer [ 77 , 94 , 95 ]. In addition, exosomes isolated from human renal adenocarcinoma ACHN cells contain Fas ligands, which inhibit the action of the human immune system by inducing apoptosis of CD8+ T cells and ultimately help cancer cells in achieving immune escape [ 96 ].
Involvement in cancer cell invasion and metastasis
A key molecular event in the development of target organ/tissue metastasis by tumours is the formation of tumour pre-metastatic niches [ 97 ]. Tumour pre-metastatic niches are defined as some molecular and cellular changes in metastatic-designated organs/tissues that can facilitate the colonisation of target organs/tissues by circulating tumour cells and promoting distant tumour metastasis [ 98 ]. In recent years, secretory components and cells found in distant metastatic tissues of different tumour animal models, including soluble factors, exosomes, vesicles and MDSCs, have confirmed the presence of tumour pre-metastatic niches in most types of malignancies [ 99 , 100 ]. Exosomes can alter the target cell function through substances they carry; tumour-derived exosomes that act on epithelial cells lead to epithelial–mesenchymal transition (EMT), which is important in tumour metastasis [ 101 ].
Role of exosomes in the diagnosis of kidney cancer
Raimondo et al. [ 73 ] identified 261 and 186 proteins by isolating urinary exosomes from normal patients and patients with kidney cancer, respectively, and most proteins were membrane-associated or cytoplasmic. Among these proteins, the expression of MMP9, ceruloplasmin (CP), podocalyxin like (PODXL), carbonic anhydrase IX (CAIX) and dickkopf 4 (DKK4) in urinary exosomes was higher in patients with kidney cancer than that in normal patients, and the expression of CD10, extracellular matrix metalloproteinase inducer (EMMPRIN), dipeptidase 1 (DPEP1), syntenin 1 and aquaporin 1 (AQP1) in urinary exosomes was higher in normal patients than that in patients with kidney cancer. These proteins may serve as potential markers of kidney cancer. Wang et al. [ 68 ] investigated the effect of exosomes isolated from cancer stem cells (CSCs) of 76 patients with metastatic RCC and 133 patients with localised RCC and found that CD103+ played a role in directing CSC exosomes to target cancer cells and organs. In addition, Tsuruda et al. [ 102 ] found that Rab27b protein can play an oncogenic role in renal cancer and sunitinib resistance through exosome-independent function.
mRNAs are a class of single-stranded RNAs that carry genetic information and can direct protein synthesis; they are transcribed from a strand of DNA as a template [ 103 , 104 ]. Exosomes can carry and transport large amounts of mRNA to function in the recipient cells [ 23 ]. Grange et al. [ 70 ] identified mRNAs implicated in tumour progression and metastasis through molecular characterisation of microvesicles, including VEGF, FGF2, ANGPT1, EFNA3, MMP2 and MMP9. In addition, Palma et al. [ 105 ] reported that the mRNA levels of glutathione s-transferase alpha 1 (GSTA1), CCAAT enhancer binding protein alpha (CEBPA) and pterin-4 alpha-carbinolamine dehydratase 1 (PCBD1) in urinary extracellular vesicles were lower in patients with RCC than those in controls, and the mRNA levels of these three genes returned to normal 1 month after nephrectomy. This demonstrates that mRNA levels in urinary extracellular vesicles serve as potential molecular markers for the diagnosis of RCC.
miRNAs are smaller endogenous non-coding RNAs (18–24 nucleotides) that regulate protein translation after gene transcription [ 106 , 107 ]. They can act as oncogenes or tumour suppressors involved in tumorigenesis [ 108 , 109 ]. Several exosomal miRNAs have been identified to be differentially expressed in patients with renal cancer and normal patients. Grange et al. [ 70 ] found that 24 miRNAs, including miR-200c and miR-650, were significantly upregulated in CD105+ microvesicles, and 33 miRNAs, including miR-100 and miR-296, were significantly downregulated, and several miRNAs such as miR-29a, miR-650, and miR-151 were associated with tumour invasion and metastasis. Zhang et al. [ 110 ] found that the expression levels of miR-210 and miR-1233 in blood exosomes were significantly higher in patients with RCC than those in healthy subjects, and the expression levels were significantly decreased after surgical removal of the tumour. Xiao et al. [ 111 ] sequenced exosomal miRNAs from plasma samples and found that the expression level of miR-149-3p and miR-424-3p was upregulated, whereas that of miR-92a-1-5p was significantly decreased. In addition, other miRNAs were reported to be potential diagnostic biomarkers of kidney cancer [ 68 , 83 , 112 – 118 ].
lncRNAs are RNAs that are longer than 200 nucleotides and cannot code for proteins [ 119 ]. They can control cellular transcription and protein translation by interacting with proteins, mRNAs or miRNAs [ 120 ]. Malignant tumour cells can express specific lncRNA markers, indicating that lncRNAs can be used as disease-specific markers that are important for cancer diagnosis [ 121 ]. lncRNAs are abundantly expressed in exosomes and can be protected by the exosomal tegument with higher stability [ 122 , 123 ]. Similar to miRNAs, lncRNAs play an important role in the growth, proliferation, invasion and metastasis of cancer cells [ 124 ]. Qu et al. [ 125 ] demonstrated that exosome-transmitted lncARSR promoted AXL and c-MET expression in RCC cells by competitively binding to miR-34/miR-449, thereby promoting sunitinib resistance. Exosomal lncRNAs are important in tumour biology, and further studies are required to understand the role of exosomal lncRNAs in renal cancer.
CircRNAs are a newly discovered type of non-coding RNAs that form a covalently closed continuous loop structure that originates from exons or introns by specific selective shearing [ 126 – 128 ]. It has been found that a large number of circRNAs can be detected in exosomes. circRNAs function as miRNA sponges during gene regulation [ 129 , 130 ]. Based on the circRNA expression array data, Xiao et al. [ 131 ] found that circ_400068 was significantly upregulated in exosomes derived from RCC. At present, circRNAs in exosomes derived from renal cancer cells have been investigated in a relatively small number of studies, and therefore, further investigation is required. Potential biomarkers derived from exosomes that have been validated in kidney cancer are listed in Table 2 . Figure 3 demonstrates the role of exosomal constituents in kidney cancer.
Exosomes derived potential biomarker for kidney cancer
The role of exosomes in kidney cancer treatment
Tumour drug resistance.
Tumour drug resistance is one of the main reasons for the failure of clinical treatment of tumours. Drug-resistant tumour cells can secrete exosomes that contain the genetic information of multiple drug resistance-associated proteins, which in turn cause other tumour cells to acquire drug resistance [ 132 , 133 ]. Several receptor tyrosine kinases associated with angiogenesis and tumour microenvironment are overexpressed mainly owing to the inactivation of Von Hippel–Lindau (VHL) tumour suppressor genes in renal cancer; therefore, TKIs, including sunitinib, have become one of the first-line therapies for renal cancer [ 134 ]. However, sunitinib resistance has made the clinical benefit of sunitinib treatment limited at present [ 135 ]. Qu et al. [ 125 ] found that drug-resistant cells in nephropathy transmitted lncARSR to other cells through exosomes, causing them to develop drug resistance, and lncARSR promoted AXL/c-MET expression by competitively binding to miR-34/miR-449. MET expression, which in turn promoted lncARSR expression as positive feedback, further promoted drug resistance in renal cancer cells. In addition, Tsuruda et al. [ 102 ] found that Rab27b can play an oncogenic role in sunitinib resistance in renal cancer through exosome-independent function. The above-mentioned study demonstrates that exosomes mediate the development of drug resistance in tumour cells, which can not only provide novel therapeutic targets for patients but also predict the responsiveness of patients to anti-tumour drugs through the detection of exosomal markers, thus providing an important reference for individualised treatment of kidney cancer [ 44 , 136 ].
Drug carriers
Owing to their lipid bilayer membrane structure, exosomes can protect RNA present inside the membranes from degradation by RNA enzymes, and owing to their smaller particle size and deformability, they can cross the biological membranes more easily, thus facilitating precise delivery of therapeutic genes to the target cells [ 137 , 138 ]. Exosomes can mediate the transfer of genetic material, thus altering the biological activity of recipient cells [ 139 ]. Exosomes can carry various therapeutic substances, including RNAs and antisense oligonucleotides [ 24 ]. Exosomes can deliver therapeutic substances directly to target organs through different biological barriers, for example, macrophage-derived exosomes can effectively cross the blood–brain barrier to deliver protein-like substances [ 140 ]. Ligand enrichment on engineered exosomes can also be used to induce or inhibit signalling in the receptor cells for targeting exosomes to specific cells [ 141 ]. In addition, exosomes can be effectively loaded with chemotherapeutic drugs with low toxic side effects. Therefore, they can serve as well-tolerated and promising drug carriers [ 142 , 143 ]. Currently, exosomes are considered important drug delivery carriers for the treatment of cardiovascular diseases and pancreatic cancer [ 35 , 144 ]; however, their role in kidney cancer requires further investigation [ 145 ].
Tumour vaccines
Compared with conventional vaccines, the vaccines developed using exosomes derived from tumour cell secretion may exert incomparable effects with higher affinity [ 146 ]. Exosomes secreted by tumour cells can present tumour-associated antigens and induce the development of immunity against tumours [ 94 ]. Zhang et al. found that IL-12-anchored kidney cancer cell-derived exosomes induced the production of more cytotoxic T lymphocytes specific for kidney cancer antigens and improved anti-tumour effects [ 147 ]. They further constructed an enhanced immunogenic EXO-IL-12 vaccine capable of stably expressing kidney cancer-specific antigen G250, immune-associated protein and GPI-IL-12, which can significantly enhance the proliferation and activation of T lymphocytes in vitro and exert an induced antigen-specific killing effect [ 74 , 148 ]. Another study found that mice with kidney cancer vaccinated with tumour exosome-loaded dendritic cell (DC-TEX) vaccine had a longer survival period than that of mice vaccinated with tumour cell lysate-loaded dendritic cell vaccine [ 149 ]. Exosomes that are loaded and delivered with tumour suppressor genes that inhibit tumour cell growth provide necessary conditions for the development of exosomal tumour vaccines [ 150 – 152 ].
Early diagnosis of kidney cancer is one of the key factors in improving the survival rate of patients. Exosomes may benefit early diagnosis. Exosomes secreted by kidney cancer cells are abundantly present in blood, urine and other body fluids, thus providing advantages such as easy availability, non-invasive examination and tumour specificity. Owing to their small size, high mobility and lipid bilayer structure, they can easily passthrough biological membranes and protect rich bioactive substances present inside the membranes from degradation; therefore, exosomes have become a prime focus of research. Tumour-derived exosomes carry a large number of substances, including proteins, nucleic acids and lipids, which can alter the biological behaviour of target cells and participate in the development of kidney cancer. Numerous studies have found that the expression of exosomes is significantly different in patients with kidney cancer and normal subjects. Exosomes play an important role in the infiltration and metastasis of kidney cancer and also participate in tumour drug resistance and immune escape. Studies related to exosomes provide new ideas for the diagnosis and treatment of kidney cancer and offer adequate developmental prospects. However, studies on exosomes derived from renal cancer cells are mostly retrospective, and the tissue types mostly include renal clear cell carcinoma. To promote the application of exosomes in clinical settings, more extensive studies combined with clinical trials are required, and future studies should include increased sample sizes and different tissue types and adopt a prospective study design, which will be more convincing and provide substantial medical data support for clinical translation. In addition, the study of exosomes in kidney cancer is relatively independent and none of the molecules identified seem to have been repeatedly validated in different studies, which requires more prospective clinical trials leading to more reproducible biomarkers. Moreover, further investigation is required for developing exosome-mediated tumour vaccines and understanding the effect and mechanism of drug resistance on targeted therapy for kidney cancer.
Acknowledgments
We thank Home for Researchers ( www.home-for-researchers.com ) optimizing the figures and Bullet Edits ( http://www.bulletedits.cn/ ) for editing this manuscript.
Abbreviations
Authors’ contributions.
WM, KW and ZW contributed equally to this work and share first authorship. Conceptualization, WM, BX and MC; writing—original draft preparation, WM; writing—review and editing, BX and MC; visualization, KW and ZW; supervision, KW and ZW; funding acquisition, WM and MC. All authors read and approved the final manuscript.
This study was supported by the Scientific Research Foundation of Graduate School of Southeast University (YBPY2173), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0156), Jiangsu Provincial Key Research and Development Program (BE2019751), Innovative Team of Jiangsu Provincial (2017XKJQW07), and The National Key Research and Development Program of China (SQ2017YFSF090096).
Availability of data and materials
Declarations.
Not applicable.
We have obtained consents to publish this paper from all the participants of this study.
We declare that there are no conflicts of interest between authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weipu Mao, Keyi Wang and Zonglin Wu contributed equally to this work.
Contributor Information
Weipu Mao, Email: moc.361@88upiewoam .
Keyi Wang, Email: moc.361@0190iyekgnaw .
Zonglin Wu, Email: moc.621@liaswnc .
Bin Xu, Email: moc.621@2891bxjn .
Ming Chen, Email: moc.621@uesnehcgnim .
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
A new drug candidate can shrink kidney cysts
Press contact :, media download.

*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."

Previous image Next image
Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease, can lead to kidney enlargement and eventual loss of function. The disease affects more than 12 million people worldwide, and many patients end up needing dialysis or a kidney transplant by the time they reach their 60s.
Researchers at MIT and Yale University School of Medicine have now found that a compound originally developed as a potential cancer treatment holds promise for treating ADPKD. The drug works by exploiting kidney cyst cells’ vulnerability to oxidative stress — a state of imbalance between damaging free radicals and beneficial antioxidants.
In a study employing two mouse models of the disease, the researchers found that the drug dramatically shrank kidney cysts without harming healthy kidney cells.
“We really believe this has potential to impact the field and provide a different treatment paradigm for this important disease,” says Bogdan Fedeles, a research scientist and program manager in MIT’s Center for Environmental Health Sciences and the lead author of the study, which appears this week in the Proceedings of the National Academy of Sciences .
John Essigmann, the William R. and Betsy P. Leitch Professor of Biological Engineering and Chemistry at MIT; Sorin Fedeles, executive director of the Polycystic Kidney Disease Outcomes Consortium and assistant professor (adjunct) at Yale University School of Medicine; and Stefan Somlo, the C.N.H. Long Professor of Medicine and Genetics and chief of nephrology at Yale University School of Medicine, are the senior authors of the paper .
Cells under stress
ADPKD typically progresses slowly. Often diagnosed when patients are in their 30s, it usually doesn’t cause serious impairment of kidney function until patients reach their 60s. The only drug that is FDA-approved to treat the disease, tolvaptan, slows growth of the cysts but has side effects that include frequent urination and possible liver damage.
Essigmann’s lab did not originally set out to study PKD; the new study grew out of work on potential new drugs for cancer. Nearly 25 years ago, MIT research scientist Robert Croy, also an author of the new PNAS study, designed compounds that contain a DNA-damaging agent known as an aniline mustard, which can induce cell death in cancer cells.
In the mid 2000s, Fedeles, then a grad student in Essigmann’s lab, along with Essigmann and Croy, discovered that in addition to damaging DNA, these compounds also induce oxidative stress by interfering with mitochondria — the organelles that generate energy for cells.
Tumor cells are already under oxidative stress because of their abnormal metabolism. When they are treated with these compounds, known as 11beta compounds, the additional disruption helps to kill the cells. In a study published in 2011, Fedeles reported that treatment with 11beta compounds significantly suppressed the growth of prostate tumors implanted in mice.
A conversation with his brother, Sorin Fedeles, who studies polycystic kidney disease, led the pair to theorize that these compounds might also be good candidates for treating kidney cysts. At the time, research in ADPKD was beginning to suggest that kidney cyst cells also experience oxidative stress, due to an abnormal metabolism that resembles that of cancer cells.
“We were talking about a mechanism of what would be a good drug for polycystic kidney disease, and we had this intuition that the compounds that I was working with might actually have an impact in ADPKD,” Bogdan Fedeles says.
The 11beta compounds work by disrupting the mitochondria’s ability to generate ATP (the molecules that cells use to store energy), as well as a cofactor known as NADPH, which can act as an antioxidant to help cells neutralize damaging free radicals. Tumor cells and kidney cyst cells tend to produce increased levels of free radicals because of the oxidative stress they’re under. When these cells are treated with 11beta compounds, the extra oxidative stress, including the further depletion of NADPH, pushes the cells over the edge.
“A little bit of oxidative stress is OK, but the cystic cells have a low threshold for tolerating it. Whereas normal cells survive treatment, the cystic cells will die because they exceed the threshold,” Essigmann says.
Shrinking cysts
Using two different mouse models of ADPKD, the researchers showed that 11beta-dichloro could significantly reduce the size of kidney cysts and improve kidney function.
The researchers also synthesized a “defanged” version of the compound called 11beta-dipropyl, which does not include any direct DNA-damaging ability and could potentially be safer for use in humans. They tested this compound in the early-onset model of PKD and found that it was as effective as 11beta-dichloro.
In all of the experiments, healthy kidney cells did not appear to be affected by the treatment. That’s because healthy cells are able to withstand a small increase in oxidative stress, unlike the diseased cells, which are highly susceptible to any new disturbances, the researchers say. In addition to restoring kidney function, the treatment also ameliorated other clinical features of ADPKD; biomarkers for tissue inflammation and fibrosis were decreased in the treated mice compared to the control animals.
The results also suggest that in patients, treatment with 11beta compounds once every few months, or even once a year, could significantly delay disease progression, and thus avoid the need for continuous, burdensome antiproliferative therapies such as tolvaptan.
“Based on what we know about the cyst growth paradigm, you could in theory treat patients in a pulsatile manner — once a year, or perhaps even less often — and have a meaningful impact on total kidney volume and kidney function,” Sorin Fedeles says.
The researchers now hope to run further tests on 11beta-dipropyl, as well as develop ways to produce it on a larger scale. They also plan to explore related compounds that could be good drug candidates for PKD.
Other MIT authors who contributed to this work include Research Scientist Nina Gubina, former postdoc Sakunchai Khumsubdee, former postdoc Denise Andrade, and former undergraduates Sally S. Liu ’20 and co-op student Jake Campolo. The research was funded by the PKD Foundation, the U.S. Department of Defense, the National Institutes of Health, and the National Institute of Environmental Health Sciences through the Center for Environmental Health Sciences at MIT.
Share this news article on:
Related links.
- John Essigmann
- Center for Environmental Health Sciences
- Department of Biological Engineering
- Department of Chemistry
Related Topics
- Biological engineering
- Center for Environmental Health Sciences (CEHS)
- National Institutes of Health (NIH)
Related Articles

Detecting mutations could lead to earlier liver cancer diagnosis

New evidence for how a rare form of liver cancer arises
Forced mutations doom HIV
Previous item Next item
More MIT News
Most work is new work, long-term study of U.S. census data shows
Read full story →
Does technology help or hurt employment?
A first-ever complete map for elastic strain engineering

“Life is short, so aim high”

Shining a light on oil fields to make them more sustainable

MIT launches Working Group on Generative AI and the Work of the Future
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Wilms tumor: Kidney cancer in children
Share this:.
By Mayo Clinic staff
While kidney cancer is rare in children, Wilms tumor is the most common type found in kids, with between 500 and 600 children diagnosed annually in the U.S. The disease most often affects kids ages 3 to 4.
Thanks to advances in treatments over the past several decades, survival rates from this rare cancer have improved significantly. Developing a treatment plan customized for each child with Wilms tumor has also helped to improve the prognosis for children with this disease. "Every Wilms tumor is different — in size, location, complexity, and whether it has spread to other organs," says Stephanie Polites, M.D. , a Mayo Clinic pediatric surgeon. "Having a multidisciplinary team of healthcare professionals who can develop an individualized care plan for each child is key."
Here's what families should know about treatment for this rare childhood cancer:
A team approach is best.
At Mayo Clinic, treatment for Wilms tumor is planned by a team of healthcare professionals from multiple specialties. "From the moment a child is diagnosed with a kidney tumor, our team meets to discuss the plan of attack," says Candace Granberg, M.D. , a Mayo Clinic pediatric urologist.
Dr. Granberg is part of the pediatric solid tumor team at Mayo Clinic, which includes:
- Pediatric oncologists: Doctors who specialize in treating children and young adults who have cancer.
- Radiation oncologists: Doctors who specialize in treating cancer with radiation therapy , a type of cancer treatment that uses beams of intense energy — X-rays or protons — to kill cancer cells.
- Pediatric radiologists: Doctors who specialize in using imaging technology to diagnose and treat children with various conditions.
- Pediatric surgeons: Doctors who specialize in performing surgery in children.
- Pediatric urologists : Doctors who specialize in diagnosing and treating problems involving the male and female urinary tract and the male reproductive organs in children.
This team meets to review the needs of each child with Wilms tumor and determines the first step for treatment. "We coordinate care to streamline appointments and procedures to minimize the frequency of trips families must take," says Dr. Granberg.
Before starting treatment, your child's care team will determine if cancer treatment could affect the child's future fertility. If so, they offer the family options for fertility preservation . These options will depend on a child's age, puberty stage, diagnosis and proposed treatment plan. At Mayo Clinic, procedures to preserve fertility are integrated with other treatment procedures or imaging scans whenever possible to minimize a child's exposure to anesthesia.
Surgeons need expertise in complex procedures.
Treatment for Wilms tumor may begin with surgery to remove all or part of a kidney. At Mayo Clinic, pediatric radiologists construct life-size, anatomic 3D models of the Wilms tumor and surrounding structures to help with surgical planning. This approach is useful in challenging circumstances, such as removing only the part of the kidney containing the tumor (partial nephrectomy) or removing multiple tumors from one kidney.

Treatment often involves the surgical removal of the entire kidney and nearby lymph nodes through an incision in the abdomen. Given the substantial size of many Wilms tumors and their proximity to vital structures and organs, your child's pediatric surgical team needs to specialize in complex abdominal operations for children to ensure the best outcomes.
Sometimes, a tumor will extend into major blood vessels, and the approach requires the assistance of vascular surgeons who specialize in treating circulatory system diseases. "We are blessed at Mayo Clinic to have these specialists right down the hall from our pediatric operating rooms. They bring their entire team to our room and either reconstruct the major vessels or place grafts if needed," says Dr. Granberg. "These are procedures they do all the time, so having them there for these rare cases in kids is necessary for a successful surgery."
Chemotherapy can be used before surgery to shrink tumors and make them easier to remove. After surgery, it can kill cancer cells left in the body. Chemotherapy may also be an option for children whose cancers are too far along to be removed completely with surgery.
New technology and research continue to improve treatment.
Radiation therapy may also be used to treat Wilms tumor. Radiation therapy most often uses X-rays to beam intense energy to kill cancer cells. Proton beam therapy , another important advancement in caring for children with Wilms tumor and other childhood cancers, may also be used. This highly targeted precision beam therapy destroys cancer while sparing healthy tissue. It's an important component of the treatment plan for children, as it prevents damage to developing organs and reduces long-term complications, side effects and secondary malignancies.
Research on Wilms tumor is ongoing, and Mayo Clinic researchers are studying new ways to treat this rare cancer. "Though a cure is possible in the majority of cases, we need to do better — we want to cure everyone," says Patricio Gargollo, M.D. , a Mayo Clinic pediatric urologist.
Dr. Gargollo and his colleagues recommend seeking a children's cancer center with experience treating Wilms tumors. With the right care team, children with Wilms tumor can have excellent outcomes.
Learn more about Wilms tumor and find a clinical trial at Mayo Clinic.
Join the Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- Planning for tomorrow: Fertility preservation and childhood cancer
- What do you know about these 3 rare childhood cancers?
Related Posts

Learn about three rare types of cancer that affect children: retinoblastoma, rhabdomyosarcoma and Wilms tumor.

Pediatric hematologist and oncologist Dr. Asmaa Ferdjallah shares what families need to know about the most common form of childhood cancer.

Dr. Stephanie Polites explains how neuroblastoma, a cancer commonly affecting children, is diagnosed and treated.
- Patient Care & Health Information
- Diseases & Conditions
- Kidney cancer
- Kidney cancer FAQs
Urologic oncologist Bradley Leibovich, M.D., answers the most frequently asked questions about kidney cancer.
I'm Dr. Brad Leibovich, a urologic oncologist at Mayo Clinic, and I'm here to answer some questions patients may have about kidney cancer.
Patients diagnosed with kidney cancer often want to know what could they have done differently to prevent this from happening in the first place. In most cases, kidney cancer is completely unrelated to how you've lived your life. And there's really nothing you could have done differently to have prevented this.
Prognosis for kidney cancer depends upon the stage at which the kidney cancer is discovered. For patients with early stage disease, the prognosis is excellent and the expectation is typically that somebody will be cured of their kidney cancer. For later stage disease, thankfully, we have many new treatments. And even if it's not possible to cure a patient, the expectation is we will significantly extend their life.
Patients that have been diagnosed with kidney cancer often want to know if it's necessary to remove the entire kidney. In some cases, the kidney can be preserved and only the tumor needs to be removed. In other cases, it's necessary to remove the entire kidney. Thankfully, most patients have a second kidney and have good enough kidney function with just one kidney, that this is not a problem.
Since most patients have relatively normal kidney function after having a kidney removed, in the majority of circumstances, you do not have to change your lifestyle. Most important is that you have a healthy lifestyle overall. Get good sleep, regular exercise, and have a healthy balanced diet. If you do need to change something about your lifestyle, your doctor will tell you.
Many patients want to know if they need to alter their diet after treatment for kidney cancer. In the majority of circumstances, people have normal enough kidney function that no special diet is required, and people can eat and drink however they did previously.
In my opinion, being the best partner to your medical team means learning as much as you can about your diagnosis and about your options. This will empower you to make the best decisions that are right for you. Never hesitate to ask your medical team any questions or inform them of any concerns you may have. Being informed makes all the difference. Thank you for your time. We wish you well.
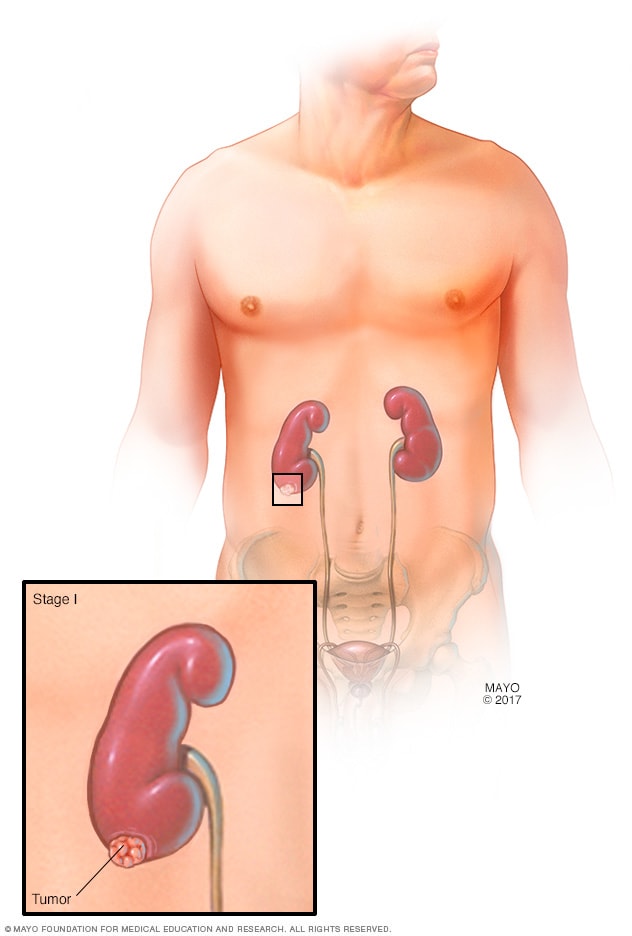
- Stage I kidney tumor
The tumor can be up to 2 3/4 inches (7 centimeters) in diameter. The cancer is only in one kidney and completely contained within it.
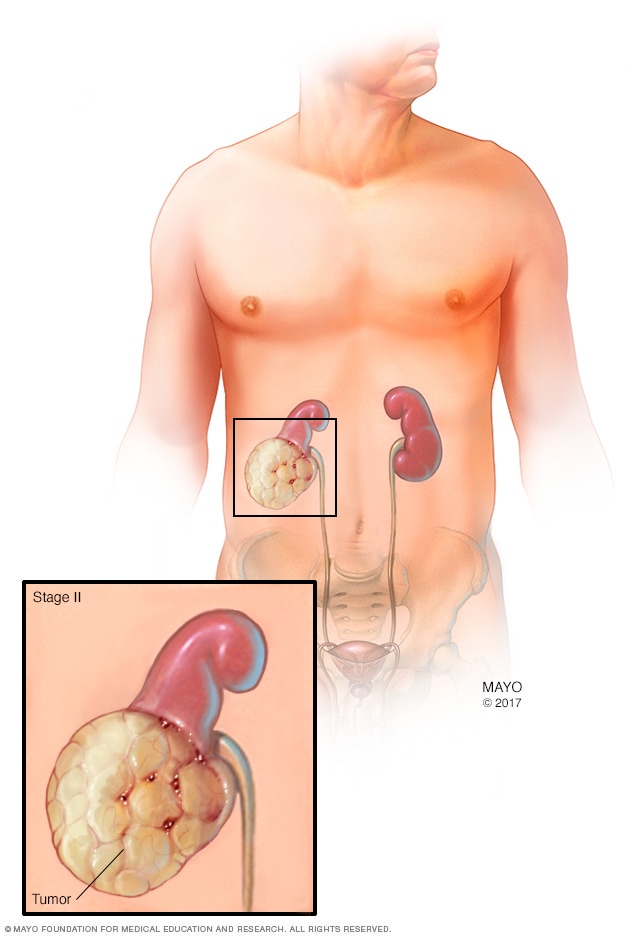
- Stage II kidney tumor
The tumor is larger than 2 3/4 inches (7 centimeters) in diameter, but it's still confined to the kidney.
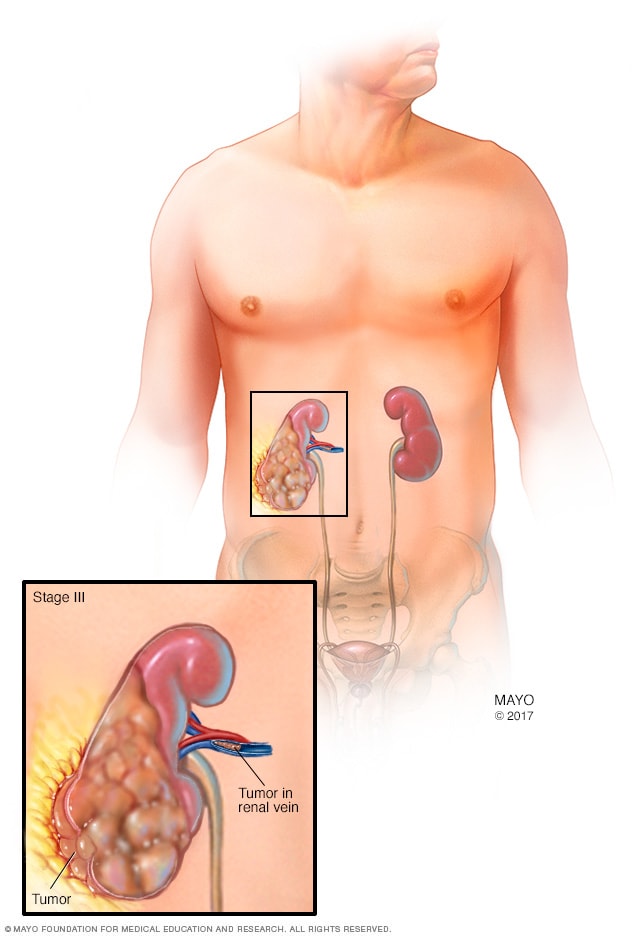
- Stage III kidney tumor
The tumor extends beyond the kidney to the surrounding tissue and may also have spread to nearby lymph nodes.
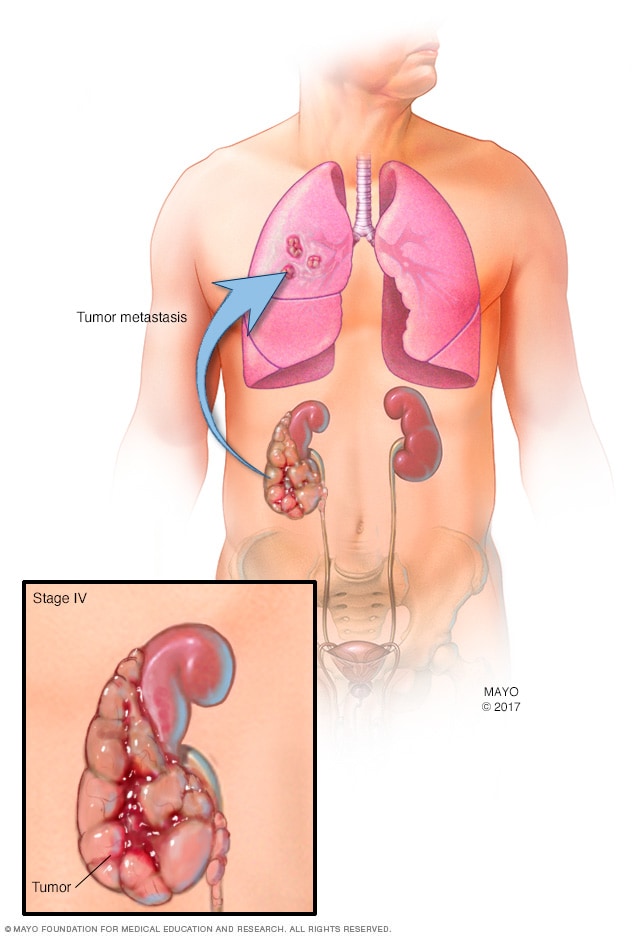
- Stage IV kidney tumor
Cancer spreads outside the kidney, to multiple lymph nodes or to distant parts of the body, such as the bones, liver or lungs.
Tests and procedures used to diagnose kidney cancer include:
- Blood and urine tests. Tests of your blood and your urine may give your doctor clues about what's causing your signs and symptoms.
- Imaging tests. Imaging tests allow your doctor to visualize a kidney tumor or abnormality. Imaging tests might include ultrasound, X-ray, CT or MRI .
- Removing a sample of kidney tissue (biopsy). In some situations, your doctor may recommend a procedure to remove a small sample of cells (biopsy) from a suspicious area of your kidney. The sample is tested in a lab to look for signs of cancer. This procedure isn't always needed.
Kidney cancer staging
Once your doctor identifies a kidney lesion that might be kidney cancer, the next step is to determine the extent (stage) of the cancer. Staging tests for kidney cancer may include additional CT scans or other imaging tests your doctor feels are appropriate.
The stages of kidney cancer are indicated by Roman numerals that range from I to IV, with the lowest stages indicating cancer that is confined to the kidney. By stage IV, the cancer is considered advanced and may have spread to the lymph nodes or to other areas of the body.
More Information
Kidney cancer care at Mayo Clinic
- Computerized tomography (CT) urogram
Kidney cancer treatment usually begins with surgery to remove the cancer. For cancers confined to the kidney, this may be the only treatment needed. If the cancer has spread beyond the kidney, additional treatments may be recommended.
Together, you and your treatment team can discuss your kidney cancer treatment options. The best approach for you may depend on a number of factors, including your general health, the kind of kidney cancer you have, whether the cancer has spread and your preferences for treatment.
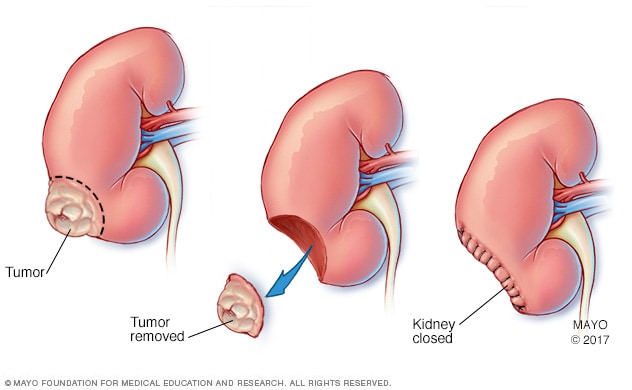
Partial nephrectomy
During a partial nephrectomy, only the cancerous tumor or diseased tissue is removed (center), leaving in place as much healthy kidney tissue as possible. Partial nephrectomy is also called kidney-sparing surgery.
For most kidney cancers, surgery is the initial treatment. The goal of surgery is to remove the cancer while preserving normal kidney function, when possible. Operations used to treat kidney cancer include:
Removing the affected kidney (nephrectomy). A complete (radical) nephrectomy involves removing the entire kidney, a border of healthy tissue and occasionally additional nearby tissues such as the lymph nodes, adrenal gland or other structures.
The surgeon may perform a nephrectomy through a single incision in the abdomen or side (open nephrectomy) or through a series of small incisions in the abdomen (laparoscopic or robotic-assisted laparoscopic nephrectomy).
Removing the tumor from the kidney (partial nephrectomy). Also called kidney-sparing or nephron-sparing surgery, the surgeon removes the cancer and a small margin of healthy tissue that surrounds it rather than the entire kidney. It can be done as an open procedure, or laparoscopically or with robotic assistance.
Kidney-sparing surgery is a common treatment for small kidney cancers and it may be an option if you have only one kidney. When possible, kidney-sparing surgery is generally preferred over a complete nephrectomy to preserve kidney function and reduce the risk of later complications, such as kidney disease and the need for dialysis.
The type of surgery your doctor recommends will be based on your cancer and its stage, as well as your overall health.
Nonsurgical treatments
Small kidney cancers are sometimes destroyed using nonsurgical treatments, such as heat and cold. These procedures may be an option in certain situations, such as in people with other health problems that make surgery risky.
Options may include:
- Treatment to freeze cancer cells (cryoablation). During cryoablation, a special hollow needle is inserted through your skin and into the kidney tumor using ultrasound or other image guidance. Cold gas in the needle is used to freeze the cancer cells.
- Treatment to heat cancer cells (radiofrequency ablation). During radiofrequency ablation, a special probe is inserted through your skin and into the kidney tumor using ultrasound or other imaging to guide placement of the probe. An electrical current is run through the needle and into the cancer cells, causing the cells to heat up or burn.
Treatments for advanced and recurrent kidney cancer
Kidney cancer that comes back after treatment and kidney cancer that spreads to other parts of the body may not be curable. Treatments may help control the cancer and keep you comfortable. In these situations, treatments may include:
- Surgery to remove as much of the kidney cancer as possible. If the cancer can't be removed completely during an operation, surgeons may work to remove as much of the cancer as possible. Surgery may also be used to remove cancer that has spread to another area of the body.
- Targeted therapy. Targeted drug treatments focus on specific abnormalities present within cancer cells. By blocking these abnormalities, targeted drug treatments can cause cancer cells to die. Your doctor may recommend testing your cancer cells to see which targeted drugs may be most likely to be effective.
- Immunotherapy. Immunotherapy uses your immune system to fight cancer. Your body's disease-fighting immune system may not attack your cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process.
- Radiation therapy. Radiation therapy uses high-powered energy beams from sources such as X-rays and protons to kill cancer cells. Radiation therapy is sometimes used to control or reduce symptoms of kidney cancer that has spread to other areas of the body, such as the bones and brain.
- Clinical trials. Clinical trials are research studies that give you a chance to try the latest innovations in kidney cancer treatment. Some clinical trials assess the safety and effectiveness of potential treatments. Other clinical trials try to find new ways to prevent or detect disease. If you're interested in trying a clinical trial, discuss the benefits and risks with your doctor.
- Ablation therapy
- Biological therapy for cancer
- Nephrectomy (kidney removal)
- Radiation therapy
- Radiofrequency ablation for cancer
- Grateful patient talks about his Mayo Clinic experience
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Alternative medicine
No alternative medicine therapies have been proved to cure kidney cancer. But some integrative treatments can be combined with standard medical therapies to help you cope with side effects of cancer and its treatment, such as distress.
People with cancer often experience distress. If you're distressed, you may have difficulty sleeping and find yourself constantly thinking about your cancer. You may feel angry or sad.
Discuss your feelings with your doctor. Specialists can help you sort through your feelings and help you devise strategies for coping. In some cases, medications may help.
Integrative medicine treatments may also help you feel better, including:
- Art therapy
- Massage therapy
- Music therapy
- Relaxation exercises
- Spirituality
Talk with your doctor if you're interested in these treatment options.
Coping and support
Each person copes with a cancer diagnosis in his or her own way. Once the fear that comes with a diagnosis begins to lessen, you can find ways to help you cope with the daily challenges of cancer treatment and recovery. These coping strategies may help:
- Learn enough about kidney cancer to feel comfortable making treatment decisions. Ask your doctor for details of your diagnosis, such as what type of cancer you have and the stage. This information can help you learn about the treatment options. Good sources of information include the National Cancer Institute and the American Cancer Society.
- Take care of yourself. Take care of yourself during cancer treatment. Eat a healthy diet full of fruits and vegetables, be physically active when you feel up to it, and get enough sleep so that you wake feeling rested each day.
- Take time for yourself. Set aside time for yourself each day. Time spent reading, relaxing or listening to music can help you relieve stress. Write your feelings down in a journal.
- Gather a support network. Your friends and family are concerned about your health, so let them help you when they offer. Let them take care of everyday tasks — running errands, preparing meals and providing transportation — so that you can focus on your recovery. Talking about your feelings with close friends and family also can help you relieve stress and tension.
- Get mental health counseling if needed. If you feel overwhelmed, depressed or so anxious that it's difficult to function, consider getting mental health counseling. Talk with your doctor or someone else from your health care team about getting a referral to a mental health professional, such as a certified social worker, psychologist or psychiatrist.
Preparing for your appointment
Start by making an appointment with your primary care doctor if you have signs or symptoms that worry you. If your doctor suspects you may have kidney cancer, you may be referred to a doctor who specializes in urinary tract diseases and conditions (urologist) or to a doctor who treats cancer (oncologist).
Consider taking a family member or friend along. Sometimes it can be hard to remember all the information provided during an appointment. Someone who accompanies you may remember something that you missed or forgot.
What you can do
At the time you make the appointment, ask if there's anything you need to do in advance, such as restrict your diet. Then make a list of:
- Symptoms you're experiencing, including any that may seem unrelated to the reason for your appointment
- Key personal information, including any major stresses or recent life changes
- All medications (prescription and over-the-counter), vitamins, herbs or other supplements that you're taking
- Questions to ask your doctor
List your questions from most to least important in case time runs out. Some basic questions to ask your doctor include:
- Do I have kidney cancer?
- If so, has my cancer spread beyond my kidney?
- Will I need more tests?
- What are my treatment options?
- What are the potential side effects of each treatment?
- Can my kidney cancer be cured?
- How will cancer treatment affect my daily life?
- Is there one treatment option you feel is best for me?
- I have these other health conditions. How can I best manage them together?
- Should I see a specialist?
- Are there brochures or other printed material that I can have? What websites do you recommend?
Don't hesitate to ask additional questions that may occur to you during your appointment.
What to expect from your doctor
Your doctor is likely to ask you a number of questions. Be ready to answer them so that you'll have time to cover any points you want to focus on. Your doctor may ask:
- When did you first begin experiencing symptoms?
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?

- Kidney cancer. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed May 8, 2020.
- Partin AW, et al., eds. Malignant renal tumors. In: Campbell-Walsh-Wein Urology. 12th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed May 8, 2020.
- Niederhuber JE, et al., eds. Cancer of the kidney. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed May 8, 2020.
- Renal cell cancer treatment (PDQ). National Cancer Institute. https://www.cancer.gov/types/kidney/patient/kidney-treatment-pdq. Accessed May 8, 2020.
- Distress management. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed May 8, 2020.
- Alt AL, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011; doi:10.1002/cncr.25836.
- Lyon TD, et al. Complete surgical metastasectomy of renal cell carcinoma in the post-cytokine era. The Journal of Urology. 2020; doi:10.1097/JU.0000000000000488.
- Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Medicine. 1999;5:1365.
- Peyronnet B, et al. Impact of hospital volume and surgeon volume on robot-assisted partial nephrectomy outcomes: A multicenter study. BJU International. 2018; doi:10.1111/bju.14175.
- Hsu RCJ, et al. Impact of hospital nephrectomy volume on intermediate- to long-term survival in renal cell carcinoma. BJU International. 2020; doi:10.1111/bju.14848.
- NCCN member institutions. National Comprehensive Cancer Network. https://www.nccn.org/members/network.aspx. Accessed May 20, 2020.
- Locations. Children's Oncology Group. https://www.childrensoncologygroup.org/index.php/locations. Accessed May 20, 2020.
- Kidney Cancer
- What is kidney cancer? An expert explains
Associated Procedures
News from mayo clinic.
- Mayo Clinic Minute: How is kidney cancer treated? March 20, 2023, 02:27 p.m. CDT
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Mayo Clinic Comprehensive Cancer Center
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Jacksonville, Florida, and Mayo Clinic in Phoenix/Scottsdale, Arizona, have been recognized among the top Urology and Cancer hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.
Patient-Centered Research and Outcomes in Cancer and Kidney Transplantation
Affiliations.
- 1 Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Centre for Kidney Research, Kids Research Institute, The Children's Hospital at Westmead, Westmead, Australia. Electronic address: [email protected].
- 2 Centre for Kidney Research, Kids Research Institute, The Children's Hospital at Westmead, Westmead, Australia; Rural and Remote Health NT, Flinders University, Alice Springs, Australia.
- 3 Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Centre for Kidney Research, Kids Research Institute, The Children's Hospital at Westmead, Westmead, Australia.
- 4 Centre for Kidney Research, Kids Research Institute, The Children's Hospital at Westmead, Westmead, Australia; Menzies Centre for Health Policy and Economics, The University of Sydney, Sydney, Australia.
- 5 Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Centre for Kidney Research, Kids Research Institute, The Children's Hospital at Westmead, Westmead, Australia; Centre for Transplant and Renal Research, Westmead Hospital, Westmead, Australia.
- PMID: 38538454
- DOI: 10.1016/j.semnephrol.2024.151499
Cancer has been identified by kidney transplant recipients as a critically important outcome. The co-occurrence of cancer and kidney transplantation represents a complex intersection of diseases, symptoms, and competing priorities for treatments. Research that focuses on biochemical parameters and clinical events may not capture the priorities of patients. Patient-centered research can improve the relevance and efficiency of research and is particularly pertinent in the setting of cancer and kidney transplantation to facilitate shared decision-making in complex clinical situations. In addition, patient-reported outcomes can facilitate the assessment of patients' experiences, symptom burden, treatment side effects, and quality of life. This review discusses patient-centered research in the context of kidney transplantation and cancer, including consumer involvement in research and patient-centered outcomes and their measures and inclusion in core outcome sets.
Keywords: Cancer; kidney transplantation; patient-centered research; patient-reported outcome measures; patient-reported outcomes.
Copyright © 2024 Elsevier Inc. All rights reserved.
Publication types
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 27, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers create new tool for assessing risk of kidney injury after chemotherapy
by Brigham and Women’s Hospital
Using patient data from six major U.S. cancer centers, Brigham researchers and collaborators developed a risk prediction model for moderate-to-severe kidney injury after receiving the chemotherapy drug cisplatin in the largest, first generalizable study of its kind
Cisplatin is a highly effective chemotherapy that has been used to treat cancer for decades, but it can cause kidney injury that can potentially lead to the discontinuation of life-saving cancer treatments. Investigators from Brigham and Women's Hospital (BWH), a founding member of the Mass General Brigham health care system, with researchers from the Dana-Farber Cancer Institute and other institutions, developed a comprehensive tool to predict which patients are at highest risk of moderate-to-severe kidney injury after cisplatin.
They found that the highest-risk patients had as much as a 20-fold higher risk of developing kidney injury after cisplatin than those in the lowest-risk group.
"Patients receiving treatment for cancer are increasingly affected by kidney injury, which is associated with higher mortality and can jeopardize eligibility for other therapies," said first author Shruti Gupta, MD, MPH, director of Onco-nephrology at BWH and Dana-Farber and a physician in BWH's Division of Renal Medicine.
"Cisplatin is a well-known kidney toxin, and even though there are newer treatments available, it remains a cornerstone of therapy for patients with cancer globally. This large, multicenter collaboration and resulting risk prediction model is an important step in the care of patients who are getting cisplatin."
The researchers examined data from over 24,000 patients across six major U.S. cancer centers, including Dana-Farber Brigham Cancer Center, Mass General Cancer Center, Memorial Sloan Kettering Cancer Center, MD Anderson Cancer Center, University of Colorado, and Northwell Health, and analyzed the risk of moderate-to-severe acute kidney injury within the first 14 days following a single, first IV dose of cisplatin.
The model developed by the research team included several important risk factors for kidney injury, including age, high blood pressure, diabetes, laboratory findings from routinely available bloodwork, and higher doses of cisplatin. They found that patients who developed kidney injury from cisplatin had a considerably higher risk of death compared to those who did not.
Another key finding was that lower levels of magnesium were an important risk factor for acute kidney injury . The researchers plan to use the same rich database to try to identify therapies that might prevent kidney injury, including magnesium.
Using the risk score, the research team created a simple online calculator that will be made available for use at MDCalc.com . A patient or physician can use this calculator to quantify the risk of kidney injury by inputting information, including whether the patient has high blood pressure , diabetes, or other diseases or medical conditions, along with results from their bloodwork.
"This new tool can help an oncologist and a patient have more informed conversations about the risks and benefits of cisplatin. If a patient is at high risk, their clinical team can consider preventative measures such as administering more IV fluids before receiving cisplatin or monitoring their kidney function more closely afterward," said senior author David E. Leaf, MD, MMSc, director of clinical and translational research in acute kidney injury at BWH's Division of Renal Medicine.
"The clinical characteristics and lab values that are incorporated in our model are readily available and easily obtainable from medical records , so our hope is that this tool can be implemented anywhere cisplatin is given."
The research is published in the BMJ .
Explore further
Feedback to editors
Study documents safety, improvements from stem cell therapy after spinal cord injury
35 minutes ago
Researchers produce grafts that replicate the human ear
Mar 30, 2024
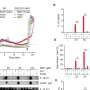
An infamous 'inflammasome'—a rogue protein complex—appears to underlie a rare and disabling autoimmune disorder
Mar 29, 2024

Researchers discover skin biomarkers in infants that predict early development of food allergies

Veterans help provide greater insight into Klinefelter and Jacobs syndromes
High-resolution images reveal similarities in protein structures between Alzheimer's disease and Down syndrome
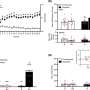
How blocking a neural receptor responsible for addiction could reduce alcohol use

Study finds few hospitals promoting potentially predatory medical payment products

COVID-19 research: Study reveals new details about potentially deadly inflammation
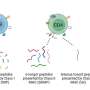
Enhanced melanoma vaccine offers improved survival for men
Related stories.

Scientists seek to shift treatment of kidney damage caused by cancer drug
Dec 7, 2021
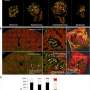
Unresolved injury, not fibrosis, contributes to cisplatin-induced CKD
Mar 27, 2019

Commercial AI tool moderately successful at predicting hospitalization-related kidney injury
Feb 18, 2024
Are sodium-glucose cotransporter-2 inhibitors safe for patients with diabetes and cancer?
Nov 2, 2023
Low follow-up kidney testing after hospital discharge with moderate to severe AKI, requires improvement
Sep 21, 2023

Combined tests can predict kidney injury risk in critically ill children
Nov 10, 2019
Recommended for you

Researchers develop AI-based tool paving the way for personalized cancer treatments

Researchers demonstrate technique for identifying single cancer cells in blood for the first time

Private and secure generative AI tool supports operations and research in a cancer center
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Today, about 75% of people with kidney cancer will be alive 5 years after diagnosis. This page highlights some of the latest research in kidney cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
December 22, 2021. Radha Chitale. Research. 2021 was a bumper year for kidney cancer developments, including several new treatment approvals by the FDA and a much better understanding of how kidney cancer is impacted by COVID-19 infections and vaccines. "All are very important topics that are affecting patients as we speak," said Nancy ...
Cancer vaccines are treatments that help a person's immune system fight cancer. Doctors are testing the use of several cancer vaccines to treat kidney cancer and to prevent recurrence for people with later-stage renal cell carcinoma. One vaccine being studied is made from a person's tumor and given after surgery, while others are made from ...
Kidney cancers are among the top 10 most common cancers in both men and women: about 79,000 new cases will be diagnosed in 2022, according to the American Cancer Society. When the cancer is first ...
Research into the causes, detection, diagnosis, and treatment of kidney cancer (renal cell carcinoma) is being done at many medical centers, university hospitals, and other institutions across the nation.. Treatment options for non-clear cell renal cell carcinoma. The most common type of renal cell carcinoma (RCC) is called clear cell.
"This approval is exciting news for our patients as it gives us a new option for refractory patients with kidney cancer," said Choueiri. "Belzutifan is an oral drug with a novel mechanism of action that reduced risk of disease progression or death and had favorable quality of life in this patient population, when compared to everolimus."
Focusing on Kidney Cancer. The research was led by cancer biologist Joan Massagué, Chair of the Sloan Kettering Institute's Cancer Biology and Genetics Program and Director of the Metastasis Research Center. In recent years, his laboratory has uncovered basic causes of metastasis in a number of cancer types, including breast and lung cancers.
Kidney Cancer. Researchers at The University of Texas MD Anderson Cancer have engineered a new model of aggressive renal cell carcinoma (RCC), highlighting molecular targets and genomic events that trigger chromosomal instability and drive metastatic progression. The study, published in Nature Cancer, demonstrates that the loss of a cluster of ...
Choueiri is supported in part by the Dana-Farber/Harvard Cancer Center Kidney Specialized Program of Research Excellence (2P50CA101942-16), by a grant (5P30CA006516-56) from the National Cancer ...
Several newly approved therapies have substantially altered the treatment paradigm for multiple genitourinary cancers. Considering the existence of numerous possible treatment approaches ...
New hope for kidney cancer treatment using existing drugs. The most comprehensive study of kidney cancer at single-cell level has discovered a potential drug target to treat renal cell carcinoma ...
Core Tip: Kidney cancer therapeutics is a fast-changing field, and the outcome of metastatic renal cell carcinoma (mRCC) has thus improved considerably in recent years with the introduction of different combinations of immune checkpoint and vascular endothelial growth factors inhibitors.State-of-the-art systemic therapy regimens must be addressed to be in a position to offer patients the best ...
The work of J.U.S., G.D.S. and S.H.R. on screening in RCC is funded by a research grant from Kidney Cancer UK. J.U.S. is supported by a Cancer Research UK Cancer Prevention Fellowship (C55650/A21464).
Read the latest Research articles in Renal cancer from Nature Reviews Nephrology ... that target pro-angiogenic pathways improve progression-free and overall survival in patients with metastatic ...
Renal cell carcinoma (RCC) is a typical malignant kidney lesion which represents 3% of all malignant tumors and has a high rate of relapse and a mortality rate of over 40%. In the last 20 years, there has been an annual increase of 2% in RCC incidence both worldwide and in Europe, leading to approximately 99 200 new RCC cases and 39 100 kidney ...
Recent research has shown that stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy (SABR), can be an effective treatment for people whose cancer remains in the kidney (localized). This type of radiation therapy uses highly focused X-ray radiation beams to send high doses of radiation to the area to be treated.
Cancer is a diagnosis that is feared by most patients with kidney failure and rated by patients and physicians as an important end point for clinical trials in transplantation. 1 Much is now known about the increased risk of cancer in transplant recipients, patients on dialysis, and, to a lesser extent, patients with chronic kidney disease (CKD ...
Background. Kidney cancer, also known as renal cell carcinoma (RCC), is one of the most common malignancies of the urinary tract, and its incidence has increased at a rapid rate of 2% per year over the past two decades [1, 2].In 2018, approximately 400,000 new cases and 170,000 deaths owing to kidney cancer were reported worldwide [].In 2015, approximately 74,000 new cases and 27,000 deaths ...
The drug works by exploiting kidney cyst cells' vulnerability to oxidative stress — a state of imbalance between damaging free radicals and beneficial antioxidants. In a study employing two mouse models of the disease, the researchers found that the drug dramatically shrank kidney cysts without harming healthy kidney cells.
ChRCC is a rare type of kidney cancer, making up approximately 5% of cases diagnosed each year. While it's typically less likely to spread than other types of kidney cancer, it does become metastatic in about 5% of cases. ... Recent research, however, has identified potential new pieces (targetable pathways) with edges that match the ChRCC ...
While kidney cancer is rare in children, Wilms tumor is the most common type found in kids, with between 500 and 600 children diagnosed annually in the U.S. The disease most often affects kids ages 3 to 4. ... New technology and research continue to improve treatment. Radiation therapy may also be used to treat Wilms tumor. Radiation therapy ...
Clinical trials are research studies that give you a chance to try the latest innovations in kidney cancer treatment. Some clinical trials assess the safety and effectiveness of potential treatments. Other clinical trials try to find new ways to prevent or detect disease. If you're interested in trying a clinical trial, discuss the benefits and ...
Abstract. Cancer has been identified by kidney transplant recipients as a critically important outcome. The co-occurrence of cancer and kidney transplantation represents a complex intersection of diseases, symptoms, and competing priorities for treatments. Research that focuses on biochemical parameters and c ….
Men are almost twice as likely as women to be diagnosed with kidney cancer. The kidneys are a pair of organs located at the back of the abdomen on either side of the spine. They filter waste products and water from the blood, producing urine. AICR'S latest report on kidney cancer found that maintaining a healthy weight can lower your risk.
Using patient data from six major U.S. cancer centers, Brigham researchers and collaborators developed a risk prediction model for moderate-to-severe kidney injury after receiving the chemotherapy ...
It's considered a rare disease affecting about 60,000 people in the U.S., but it's common among kidney conditions — 1 in 10 kidney biopsies shows signs of the condition, according to the ...
Carcinogenesis often involves significant alterations in the cancer genome architecture, marked by large structural and copy number variations (SVs and CNVs) that are difficult to capture with short-read sequencing. Traditionally, cytogenetic techniques are applied to detect such aberrations, but they are limited in resolution and do not cover features smaller than several hundred kilobases ...
Healthcare staff caring for patients with cancer must have access to high quality and generalizable data regarding the toxicities and repercussions of cancer treatments Onconephrology, the intersection between oncology and nephrology, is a rapidly evolving field that has gained considerable interest over the past 15 years. Cancer treatments are often highly effective at targeting cancer cells ...
In 2024, the American Cancer Society estimates that 81,610 new cases of kidney cancer will be diagnosed and there will be an estimated 14,390 deaths as a result. The most common type of kidney cancer is renal cell carcinoma (RCC), which accounts for 90% of diagnoses. Some groups of people are at higher risk for developing RCC.