Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- What is Secondary Research? | Definition, Types, & Examples

What is Secondary Research? | Definition, Types, & Examples
Published on 20 January 2023 by Tegan George .
Secondary research is a research method that uses data that was collected by someone else. In other words, whenever you conduct research using data that already exists, you are conducting secondary research. On the other hand, any type of research that you undertake yourself is called primary research .
Secondary research can be qualitative or quantitative in nature. It often uses data gathered from published peer-reviewed papers, meta-analyses, or government or private sector databases and datasets.
Table of contents
When to use secondary research, types of secondary research, examples of secondary research, advantages and disadvantages of secondary research, frequently asked questions.
Secondary research is a very common research method, used in lieu of collecting your own primary data. It is often used in research designs or as a way to start your research process if you plan to conduct primary research later on.
Since it is often inexpensive or free to access, secondary research is a low-stakes way to determine if further primary research is needed, as gaps in secondary research are a strong indication that primary research is necessary. For this reason, while secondary research can theoretically be exploratory or explanatory in nature, it is usually explanatory: aiming to explain the causes and consequences of a well-defined problem.
Prevent plagiarism, run a free check.
Secondary research can take many forms, but the most common types are:
Statistical analysis
Literature reviews, case studies, content analysis.
There is ample data available online from a variety of sources, often in the form of datasets. These datasets are often open-source or downloadable at a low cost, and are ideal for conducting statistical analyses such as hypothesis testing or regression analysis .
Credible sources for existing data include:
- The government
- Government agencies
- Non-governmental organizations
- Educational institutions
- Businesses or consultancies
- Libraries or archives
- Newspapers, academic journals, or magazines
A literature review is a survey of preexisting scholarly sources on your topic. It provides an overview of current knowledge, allowing you to identify relevant themes, debates, and gaps in the research you analyse. You can later apply these to your own work, or use them as a jumping-off point to conduct primary research of your own.
Structured much like a regular academic paper (with a clear introduction, body, and conclusion), a literature review is a great way to evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
A case study is a detailed study of a specific subject. It is usually qualitative in nature and can focus on a person, group, place, event, organisation, or phenomenon. A case study is a great way to utilise existing research to gain concrete, contextual, and in-depth knowledge about your real-world subject.
You can choose to focus on just one complex case, exploring a single subject in great detail, or examine multiple cases if you’d prefer to compare different aspects of your topic. Preexisting interviews , observational studies , or other sources of primary data make for great case studies.
Content analysis is a research method that studies patterns in recorded communication by utilizing existing texts. It can be either quantitative or qualitative in nature, depending on whether you choose to analyse countable or measurable patterns, or more interpretive ones. Content analysis is popular in communication studies, but it is also widely used in historical analysis, anthropology, and psychology to make more semantic qualitative inferences.
Secondary research is a broad research approach that can be pursued any way you’d like. Here are a few examples of different ways you can use secondary research to explore your research topic .
Secondary research is a very common research approach, but has distinct advantages and disadvantages.
Advantages of secondary research
Advantages include:
- Secondary data is very easy to source and readily available .
- It is also often free or accessible through your educational institution’s library or network, making it much cheaper to conduct than primary research .
- As you are relying on research that already exists, conducting secondary research is much less time consuming than primary research. Since your timeline is so much shorter, your research can be ready to publish sooner.
- Using data from others allows you to show reproducibility and replicability , bolstering prior research and situating your own work within your field.
Disadvantages of secondary research
Disadvantages include:
- Ease of access does not signify credibility . It’s important to be aware that secondary research is not always reliable , and can often be out of date. It’s critical to analyse any data you’re thinking of using prior to getting started, using a method like the CRAAP test .
- Secondary research often relies on primary research already conducted. If this original research is biased in any way, those research biases could creep into the secondary results.
Many researchers using the same secondary research to form similar conclusions can also take away from the uniqueness and reliability of your research. Many datasets become ‘kitchen-sink’ models, where too many variables are added in an attempt to draw increasingly niche conclusions from overused data . Data cleansing may be necessary to test the quality of the research.
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts, and meanings, use qualitative methods .
- If you want to analyse a large amount of readily available data, use secondary data. If you want data specific to your purposes with control over how they are generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to test a hypothesis by systematically collecting and analysing data, while qualitative methods allow you to explore ideas and experiences in depth.
Sources for this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
George, T. (2023, January 20). What is Secondary Research? | Definition, Types, & Examples. Scribbr. Retrieved 22 April 2024, from https://www.scribbr.co.uk/research-methods/secondary-research-explained/
Largan, C., & Morris, T. M. (2019). Qualitative Secondary Research: A Step-By-Step Guide (1st ed.). SAGE Publications Ltd.
Peloquin, D., DiMaio, M., Bierer, B., & Barnes, M. (2020). Disruptive and avoidable: GDPR challenges to secondary research uses of data. European Journal of Human Genetics , 28 (6), 697–705. https://doi.org/10.1038/s41431-020-0596-x
Is this article helpful?
Tegan George
Other students also liked, primary research | definition, types, & examples, what is a literature review | guide, template, & examples, case study | definition, examples & methods.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Supplements
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 8, Issue 10
- Multiple modes of data sharing can facilitate secondary use of sensitive health data for research
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Tsaone Tamuhla 1 ,
- Eddie T Lulamba 1 ,
- Themba Mutemaringa 2 , 3 ,
- http://orcid.org/0000-0001-5083-2735 Nicki Tiffin 1
- 1 South African National Bioinformatics Institute, University of the Western Cape , Bellville , South Africa
- 2 Provincial Health Data Centre, Health Intelligence Directorate , Western Cape Department of Health and Wellness , Cape Town , Western Cape , South Africa
- 3 Computational Biology Division, Department of Integrative Biomedical Sciences , University of Cape Town , Rondebosch , Western Cape , South Africa
- Correspondence to Professor Nicki Tiffin; ntiffin{at}sanbi.ac.za
Evidence-based healthcare relies on health data from diverse sources to inform decision-making across different domains, including disease prevention, aetiology, diagnostics, therapeutics and prognosis. Increasing volumes of highly granular data provide opportunities to leverage the evidence base, with growing recognition that health data are highly sensitive and onward research use may create privacy issues for individuals providing data. Concerns are heightened for data without explicit informed consent for secondary research use. Additionally, researchers—especially from under-resourced environments and the global South—may wish to participate in onward analysis of resources they collected or retain oversight of onward use to ensure ethical constraints are respected. Different data-sharing approaches may be adopted according to data sensitivity and secondary use restrictions, moving beyond the traditional Open Access model of unidirectional data transfer from generator to secondary user. We describe collaborative data sharing, facilitating research by combining datasets and undertaking meta-analysis involving collaborating partners; federated data analysis, where partners undertake synchronous, harmonised analyses on their independent datasets and then combine their results in a coauthored report, and trusted research environments where data are analysed in a controlled environment and only aggregate results are exported. We review how deidentification and anonymisation methods, including data perturbation, can reduce risks specifically associated with health data secondary use. In addition, we present an innovative modularised approach for building data sharing agreements incorporating a more nuanced approach to data sharing to protect privacy, and provide a framework for building the agreements for each of these data-sharing scenarios.
- Public Health
- Health policies and all other topics
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjgh-2023-013092
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Summary box
Data sharing can ensure maximal ethical use of data resources to inform evidence-based health care.
Different models of data sharing that move beyond direct open access sharing can be used to address challenges arising from ethical and equity constraints on data re-use.
Data anonymisation and perturbation can increase protection of privacy and data security for sensitive data.
A framework using modularised data sharing elements can facilitate creating fit-for-purpose data sharing agreements.
Introduction
Data about health is the fundamental base on which evidence-based healthcare is constructed and underpins progress and innovation in health sciences and healthcare towards improved patient outcomes. 1–3 Traditionally, however, competitive research practices have discouraged data sharing, 4 and researchers may withhold research datasets they have generated in order to protect their career interests and retain the capacity to publish innovative and high impact research. In addition, concerns about intellectual property (IP) and commercial applications have also created barriers to secondary use of health data beyond the primary purpose for which they were collected. 5
There has been a growing recognition of the need to use and re-use health data for as many diverse and future analyses as possible, within the scope of permissions for use provided by research participants through their informed consent. This need reflects an ethical imperative to maximise the benefits for evidence-based healthcare from the use of health data as an offset against the risks or discomfits faced by participants contributing those data, 6 7 and has led to increasing pressure on researchers to make data and research outputs Open 8 9 meaning that access to scientific resources should be unrestricted and free of charge wherever this is possible. The prioritisation of Open Access has resulted in requirements to adhere to Open data access principles in order to receive research funding and to publish research findings in academic journals. 10 The Open Access principles are also reflected in FAIR principles, which provide guidelines for ensuring data and resources are Findable, Accessible, Interoperable and Reusable. 11
While there is widespread support for the Open principles, more recently it has become evident that not all data—and especially sensitive personal data such as health or genomic data—are appropriate for Open Access and unrestricted re-use. Furthermore, depending on the national privacy legislation and Health Act in place, reusing certain kinds of sensitive data without participant consent or moving these data across borders may be illegal. Not all data can be made Open in line with Open Access principles, for example some large datasets and real-world data are generated without informed consent from individuals and yet are granular enough to potentially be used to reidentify individuals if combined with other identified data resources. 12 A prominent example is the use of anonymised health datasets generated from routine health data or electronic medical records, which carry the potential risk of reidentification of health clients who have not consented to participate in research, have not been informed of the risk of reidentification, and have not had an opportunity to choose whether their sensitive health data are used for research. 13 The epidemiological and health systems value of mining these data is undisputed, but the risks posed to unwitting participants should be absolutely minimised given the circumstances under which the data are generated and used.
Another scenario in which Open secondary use cannot always be implemented occurs with legacy datasets which were collected in the past at a time when legislation and general research practices were much more permissive about data collection without detailed informed consent, during which time uniquely identifying data such as genomic data may have been generated without the knowledge or agreement of those individuals or without their explicit consent for secondary data use. These data cannot ethically be handed on to additional researchers without participant informed consent for secondary use, as this would expose participants through the use of their data to associated risks—to which they have not agreed.
With the rapid growth of genomic data generated from global populations there is increasing recognition of the potential for additional family and community harms that might arise from the analysis of these data. 14 Although individual informed consent might be in place, consideration must equally be given to the risks of onward data use for relatives, associated communities and identifiable population groups. If an individual’s genomic data are Open and identifiable, what might be the implications for their offspring or relatives who did not consent to the use of those data? How are communities affected when their population-level genetic or epidemiological data become open information, for example stigma that might arise when high risk genetic variants or particular diseases are associated with a specific population group? The community-level impact of genomic studies with San participants in Southern Africa clearly illustrates these risks. 15 16 Responsible data governance, sharing, analysis and reporting are particularly important to support the inclusion of underrepresented populations in health research, in order to ensure that innovations and new therapeutic approaches are equitable and effective for all populations groups; and equitable and appropriate sharing of data from under-represented groups can contribute to addressing the existing bias in health research. 17–19
Fortunately, together with the growing availability of granular and identifying datasets and a concomitant growing recognition of the need to protect the interests of individuals, communities and researchers, there has been rapid growth in the development of data governance and ethical data use to address these challenges. Traditionally, Open data sharing has been viewed as a unidirectional process whereby researchers who collect and generate data pass them onward for secondary use, either directly or via centralised repositories. In the process they must usually cede any control over how the data are used further. Recently, more nuanced approaches are being developed to ensure maximal ethical secondary use of data resources while minimising risks and respecting the level of informed consent provided by participants.
Here, we provide an overview of four different approaches to data sharing that can be adopted according to data sensitivity and/or restrictions on secondary use. We discuss direct data sharing in a traditional model; collaborative data sharing which facilitates research by combining datasets and undertaking meta-analysis involving all collaborating partners; federated data analysis, in which partners undertake synchronous and harmonised analyses on their independent datasets and then combine the results of their analyses in a final coauthored report; and the use of trusted research environments (TREs) in which data may be analysed in a controlled environment from which only aggregate research results may be exported. We also review how deidentification and anonymisation methods can reduce the risks associated with secondary use of health data. In addition, we present a modularised approach for building data-sharing agreements (DSAs), with a framework that can be used to build such an agreement for each of these scenarios. This provides a new approach to building such agreements that is accessible and manageable for researchers without prior experience in drafting such memoranda.
We have focused here primarily on sharing of data which have been generated directly from individuals and/or biospecimens collected from individual participants. Many of the principles we outline here can similarly be applied to secondary use of biospecimen collections, and while the re-use of biospecimens is not covered exhaustively we have noted some areas where these data-sharing principles may also relate to the sharing of biospecimens.
Modes of data sharing
Here we discuss four modes of secondary data sharing that may accommodate some of the challenges associated with sharing data and conducting meta-analyses, as outlined in table 1 .
- View inline
Key advantages and challenges for different modes of secondary data sharing
Direct sharing
Direct data sharing is the traditional model of data sharing which has been most commonly in use and is routinely required by funders and peer-reviewed journals. In this model, the researcher who has generated data, the data producer, provides a full set of the data to other users, data consumers, for all types of secondary use ( figure 1 ). This may be done via a specific centralised repository—for example, the H3Africa programme ( www.h3africa.org ) funders require submission of genomic data from the programme to the European Genome-Phenome Archive ( https://ega-archive.org/ ) and submission of biospecimens to centralised H3Africa biorepositories. 20 Controlled access for secondary use occurs under the oversight of a Data and Biospecimen Access Committee, 21 and an embargo period formalises a time period for which the data generators have protected access to the data for analysis and publication to ensure they are not scooped by secondary users. Another example is the Research Resource for Complex Physiologic Signals (PhysioNet, https://physionet.org/about/ 22 ), which offers free access to large collections of physiological and clinical data, while facilitating a level of control over the data by resource generators and promoting collaboration and data sharing.
- Download figure
- Open in new tab
- Download powerpoint
Direct sharing. Unidirectional transfer of resources from generator to consumer. The consumer performs data analysis and generates the research output.
A central challenge for direct sharing of health data is how to ensure that the privacy of participants is maintained. 23 While it remains impossible to anonymise genomic data, which is by its intrinsic nature identifying, 24–26 participant safe-guarding for the use of highly granular clinical and epidemiological data can be achieved through data deidentification, anonymisation and also data perturbation. As large datasets become increasingly granular and health metrics become increasingly precise, the opportunity to reidentify individuals through cross-reference with other records does become higher—for example, given the dates and locations of sequential health facility visits together with participant age and comorbidity profile it becomes feasible to reidentify a study participant through cross reference to identified routine health facility records. Legislation that protects privacy increasingly recognises the sensitivity of health data and may offer specific protections in addition to national Health Acts that enshrine healthcare client confidentiality. An example of this is the Protection of Personal Information Act in South Africa which categorises health data as ‘special’ data, requiring additional considerations and protections. 27
Collaborative meta-analysis
Whereas direct data sharing results in a unidirectional transfer of data without collaborative opportunities, growing use of data standards has provided greater opportunities to harmonise datasets and combine them for collaborative meta-analysis. 28 In this model, data generators work together to combine their anonymised datasets and then conduct analyses that provide more statistical power and generalisable findings than when analysing the individual datasets ( figure 2 ). Sometimes in these studies it is also possible to have discovery and validation dataset analysis to measure the generalisability of findings from particular analyses. A significant advantage of collaborative meta-analyses is that the data generators have oversight of the onward use of the data they have generated and can ensure that ethical and informed consent constraints are respected. In addition, they are able to receive recognition for the ongoing analysis of the data they have generated, which can contribute to ensuring sustainability of their research and avoid their work being ‘scooped’ before they have brought it to publication. 29 This attribution is particularly important in under-resourced research environments where securing research grants is both difficult and also essential for sustainability.
Collaborative meta-analysis. Generators combine their resources and do a joint meta-analysis on the combined dataset. The generators do a joint analysis and generate a collaborative research output.
One of the major challenges for performing meta-analyses is the combination of large datasets which may have different data structures and captured variables, even when they are addressing similar primary research questions. The process of data harmonisation and associated data quality checking can be extremely time and resource-consuming, and requires the development of common data models. For example, The International Epidemiology Databases to Evaluate AIDS consortium developed these tools, and used the OMOP common data model ( https://ohdsi.github.io/CommonDataModel/index.html ) in order to combine international datasets from studies of populations living with HIV/AIDS across multiple countries for meta-analysis and comparative studies. 30 Large consortia such as the Global Genomic Medicine Collaboration 31 ( https://g2mc.org/ ), Global Alliance for Genomics and Health 32 ( https://www.ga4gh.org/ ) and International HundredK+Cohorts Consortium 33 ( https://ihccglobal.org/ ) now contribute significant resources into developing data standards for wider use, to enable such meta-analyses using health, epidemiological and genomic data without requiring retrofitting and retrospective harmonisation of data for meta-analysis.
Federated analysis
In some cases, data use permissions, ethical constraints or informed consent limitations mean that data may not be shared with other parties in direct transfer or collaborative meta-analysis agreements. In addition, for very large datasets their size may also prohibit routine transfer of datasets for secondary use. In these cases, federated analysis is another approach that may be used to optimise secondary knowledge generation from datasets that cannot be shared. For the federated data analysis model, datasets are held separately by collaborating parties but are analysed locally in the same way, and then aggregate data and/or findings are combined and reported jointly ( figure 3 ). The complete, granular datasets are never shared and never combined, and the analyses are run by the data generators only on their local dataset. This approach is used increasingly because it can circumnavigate some of the more difficult logistical, procedural and practical challenges that can hinder meta-analyses 34 35 —as described in oncology research using routine health data, 36 and pharmaco-epidemiology networks, 37 by way of example.
Federated analysis. Researchers independently conduct the same analysis on their own datasets and then combine their analysis outputs. Only the independently generated analysis results are combined in a joint research output.
Similar to collaborative meta-analysis, datasets for federated analysis still need to be comparable so that aggregate results and analysis outputs may be compared and/or combined, but the requirement for an exact replication of data structure and coding is less rigorous, even though federated analysis still requires common data elements and care needs to be taken to ensure that analysis outputs are comparable. 38 Standardised univariate data exploration of the data from each collaborator can help to flag existing biases in any of the contributory datasets. An additional application of this approach for multicentre collaborative studies is for a single data infrastructure to be created, but with partitioning that allows each centre control of and access to only their own data in the database. 39 An implementation example for federated database access is the assignment of Data Access Groups in REDCap databases, 40 ensuring user groups are only able to see certain records in the database that are entered by members of their own user group although the common data structure is used by all. Increasingly, researchers are also practicing federated learning whereby only algorithm weightings are shared and can be integrated by all collaborators in order to build a final model. 41
A Trusted Research ecosystem
As data-sharing models evolve, it has become evident that many researchers wishing to undertake secondary analysis on shared datasets will do so in a responsible and considered way, and that trusted and validated users may be able to run their own analyses directly on large datasets under controlled conditions.
A Trusted Research Environment
Generating and managing datasets for sharing can be a time-consuming, labour-intensive task that is often not recognised in assignment of budgets and personnel time. As datasets become ever larger, the number of data consumers wishing to use those data are also rapidly increasing, and many of these are repeatedly requesting related datasets. Organisations holding such large datasets have begun creating platforms where trusted users are able to directly query the complete dataset without visualising the personalised data or extracting any sections of the dataset for download. 42 In this online environment, the data consumer can run their required data analyses and export only the output and aggregated results ( figure 4 ). This provides the data provider with full control over the access and use of the shared data, while enabling secure access to data for appropriate research purposes. Ongoing query logging tracks the user activities on the platform to ensure accountability. Some examples of TREs include the UK’s Secure Data Environment server ( https://digital.nhs.uk/services/secure-data-environment-service ) for research access to anonymised health service patient data; the UK Biobank Research Analysis Platform, 43 the Terra platform developed by the Broad Institute ( https://terra.bio/about/mission/ ) and the Seven Bridges Platform ( https://www.sevenbridges.com/platform/ ).
Trusted Research Environment. Researchers register for an account that allows them access to a dataset on a secure platform where they can run analyses and generate outputs, but can only download and take away the outputs of the analyses without copying, downloading or retaining the raw data. Researchers generate independent analyses and research outputs from a common data source.
Trusted third party for data linkage, anonymisation and perturbation
Linking datasets from disparate data sources but for the same group of individuals can provide important epidemiological and health insights. In some legislative circumstances, this kind of analysis can only be done using anonymised data which creates the paradox in which identifying data fields are required to perform the linkage, but should not be revealed to the researchers using the linked dataset. In these circumstances, data linkage and subsequent anonymisation and perturbation of the linked dataset may be undertaken by a trusted third party who provides the linkage facility but has no further investment or involvement in the provided and output datasets. This third party will sign a non-disclosure agreement or memorandum of understanding regarding confidentiality, data protection and non-use of the data, as well as committing to deleting all data related to the linkage process within a specified time frame.
Additional considerations for data sharing
Considerations for commercial use of data.
Use of data for commercial use is a specific use case that comes with many additional and specific complexities. These include IP and potential licensable and/or patentable output. Because of the complexity and the difficulty in generalising these kinds of DSAs, in this study we have focused on sharing for academic research, recognising that the legal and ethical complications of data sharing for commercial purposes require an in-depth review that is beyond the scope of the current analysis.
Data deidentification and anonymisation
Data deidentification and data anonymisation refer to the processes of preparing, managing and distributing datasets removed of personally identifiable information. This is important in multicentre health research studies, for example, to provide a scalable and secure way for sharing medical information from health service records while safeguarding the privacy of patients. 44 These approaches can also alleviate concerns that consent requirements for the use of identified data negatively impact research cost, recruitment rates, research duration and outcomes and may also exacerbate recruitment bias (reviewed in 45–47 ).
Data deidentification is a process used to remove or replace the patient identifiers, such as name and identity number, from private records to prevent the relinking of the personally identifiable data to the data subject. 44 At the earliest opportunity, personal identifiers of the medical data are encrypted and the deidentified dataset is stored in a separate database. An internal anonymous key is used to link the deidentified data with the attribute data, and the dataset is always differentially perturbated for each dataset release to prevent linkage of independent datasets released leading to reidentification. Necessary access to databases with personally identifiable information, for example by database developers or analysts undertaking data linkage or curation, is tightly managed and restricted to absolute instances and subjected to both specific approvals and governance undertakings. Such data deidentification still allows for the future reassociation between the personally identifiable data and the individual, and similarly pseudonymisation replaces personally identifiable information with pseudonyms with a separate lookup table that can map pseudonyms to personally identifiable information. True data anonymisation, however, removes this association while preserving the utility of the information as much as possible.
Data perturbation is the addition of alterations or noise to the data to prevent the reidentification of the study participants and can be applied on different types of datasets to protect both privacy and confidentiality, including analysis extracts, research extracts without informed consent, and data in databases that are used for maintenance and development work within data storage environments. 48 Some examples of simple types of data perturbation include using only year of birth rather than date of birth; adding an undisclosed integer to all event dates so that times between key dates remain unchanged but reidentification through date-defined events is minimised, and using age in years at an event rather than providing birth year or event dates. More advanced statistical approaches may also be used to ensure privacy, providing a framework for ensuring that it is very difficult to infer information about individuals from a dataset while ensuring results of analyses remain true to the underlying dataset. 49 50 Anonymisation techniques are vulnerable to reidentification attacks using auxiliary datasets to compromise the privacy of data subjects, and it is advisable to apply perturbation to as many fields as possible for all requests, with perturbation techniques varying per request depending on the intended research questions and ethical concerns. Techniques and metrics such as k-anonymisation and metrics such as l-diversity can be used to reduce these risks. 44 51
Data-sharing agreements
A DSA is a formal document which allows for the regulation of data exchange between data generators and consumers in a controlled manner. This is done by defining a priori specific guidelines and procedures agreed on by both parties on what is required, permissible or denied with respect to data covered by the agreement. 52 While there are multiple clauses in a DSA ( table 2 ), in this paper we have focused on informed consent, benefit sharing, IP, intended outputs and authorship, attributions and acknowledgements as they are central to ensuring ethical and equitable data sharing among researchers, and can often be focal points for contention if they are not established up front. Online supplemental table S1 provides descriptions of some of the most commonly included DSA elements.
Supplemental material
Data sharing agreement modules for four types of data sharing
Informed consent
Consent protocols and documents must be robust, and those conducting the primary study need to ensure that relevant consent that allows for secondary use of the data is in place, and that the consent documents are aligned with the intended onward use of the data and/or biospecimens. 53 This is essential for direct sharing where control over secondary use is completely relinquished and the primary data generators lose oversight of the onward use of the data. In addition, if any commercial onward use is intended, participants need to have specifically consented for the use of their data for commercial purposes, and any share in profits or benefits from such onward use, or lack thereof, needs to be clearly identified in the consent information for the participants.
Benefit sharing
Generating primary data in under-resourced settings is often a challenging and expensive undertaking for researchers, and participation in research may itself be challenging in under-resourced environments. There is, therefore, an ethical imperative to ensure maximal return of benefits from onward sharing of these data. Such benefit sharing is often overlooked, especially for secondary use of data. While collaborative and federated sharing and the use of TREs ensure that the primary data generators are still involved in the secondary use of data, in the case of direct sharing consideration should be given to ensuring both the data generators and research participants might benefit from the secondary use of their data. While benefit sharing is not yet commonly inculcated in research planning, there is increasing recognition of the need to plan for benefit sharing, and available frameworks provide guidance for implementation. 54 For non-human genetic resources, such benefit sharing is governed by the access and benefit-sharing provisions of the United Nations’ Convention on Biological Diversity and its supplementary Nagoya Protocol. These agreements recognise that countries have the sovereign right to regulate access to their genetic resources. It is uncertain whether digital sequence information (DSI) associated with those genetic resources are included in these agreements, but since December 2022, processes are underway to incorporate DSI in the benefit-sharing accords. 55 It is also important to recognise that benefit-sharing protocols may need to be tailored according to the specific requirements of under-represented population groups. 15 56
Intellectual property
For all modes of data sharing, ownership of the current and future IP rights associated with the shared data must be clearly assigned, and this should be done a priori to avoid problems arising in the future. In addition, as this has legal implications this element of DSAs should be compiled with the input of legal and/or technology transfer departments at the institutions of the parties entering into the DSA.
Intended output
A detailed description of the intended or anticipated outputs from the shared data, such as manuscripts, training materials, tools and products, needs to be described in detail a priori to ensure that they align with the consent provided by participants, especially in the case of direct sharing where control of the data is relinquished by the data producers and they lose oversight of onward data use. Having clearly defined outputs can also strengthen collaborative and federated analysis, by ensuring that the parties involved are working towards explicit common goals, and that they also do not accidentally infringe on each other’s independent research agendas.
Authorship, attribution and acknowledgements
Agreement on the authorship and attribution plan for outputs generated from the data and/or biospecimens should be reached and recorded in DSAs. For academic output, documenting first, senior and corresponding authorship in future publications arising from the agreed data sharing can focus collaborators’ roles and prevent disputes down the line. For direct sharing, the data generators should also be adequately acknowledged in research outputs emanating from the secondary use of the data that they have made available. In collaborative and federated DSAs, this can ensure that such agreements do not disadvantage any of the partners, and ensuring this kind of equity is especially important where partnerships occur between more established and early career researchers, between highly resourced and poorly resourced research groups, and between researchers in the global North and global South. 57 To foster equity in research, there is increasing recognition of the need to encourage primary data generators from under-resourced environments and the global South to take active roles in subsequent research using the datasets they generated, and to take on senior author roles in subsequent publications.
The value to be derived from secondary use of data and biospecimens is undisputed. It is more complex, however, to ensure that such secondary use of resources is done in a way that is ethically sound and respects the preferences and privacy of the participants who donated those resources for research. In addition, there is increasing awareness of the need for equitable research agreements that do not reinforce the inequitable research dynamics that have been common to date.
Here, we have described four different modes of data sharing that may provide ways for ethical secondary use of data, including ways of sharing that might be used where data cannot be directly shared onwards to third parties. This need arises most frequently in situations where data are particularly sensitive, where informed consent for secondary analysis has not been provided by research participants, and for legacy datasets for which terms of consent were insufficient or not documented. While direct, unidirectional sharing has been the most common mode of sharing to date, with increasingly granular health and personal data the risks to individual participants of reidentification and breach of privacy are also increasing. We have outlined here some of the approaches used for data deidentification, anonymisation and perturbation, which all increase the security of participants when their data are shared onward for secondary analyses. As the global repositories of granular personal data rapidly expand, the availability of data to triangulate for reidentification of individuals also increases along with the risk of data breach, with the consequence that these approaches to prevent reidentification by anonymisation and perturbation are more important than ever before.
We anticipate that the development of more nuanced data-sharing models such as those described here may facilitate DSAs which might not have previously been possible. For example, a common concern of data generators is that they do not wish to lose oversight of how the data they have collected from participants are being used by other researchers; and another is that data generators, especially those with fewer resources for data analysis, might be scooped by better resourced research groups as soon as they make their data resources available. The options for collaborative meta-analysis and federated analysis both provide models in which these concerns are fully addressed without hindering the possibility of onward use of data resources. Concerns about data privacy, the potential for misuse of sensitive data and risks to participant privacy may also be taken into consideration through federated analysis or the use of a TRE. These data-sharing solutions do not require centralisation of data and provide opportunities to negotiate collaborative secondary research and benefit-sharing, and we have also provided an overview of the types of clauses which should be included in DSAs using these approaches.
We have, in this way, approached the challenges for secondary sharing of sensitive health data with a solutions-based lens, proposing different models of data sharing that can overcome common barriers to secondary analysis. While the approaches we have described are not exhaustive, we hope to encourage creative thinking that moves beyond direct, unidirectional sharing for secondary use, and to facilitate collaborative and equitable data sharing that can effectively advance and support a growing evidence base for the provision of optimal healthcare.
Ethics statements
Patient consent for publication.
Not applicable.
- Bittencourt MS
- Churchill D
- Bloomrosen M ,
- ↵ Afraid of Scooping – Case Study on Researcher Strategies against Fear of Scooping in the Context of Open Science , Available : https://datascience.codata.org/articles/10.5334/dsj-2017-029/ [Accessed 27 Apr 2018 ].
- Centre for Open Science
- ↵ Budapest Open Access Initiative , Available : http://www.budapestopenaccessinitiative.org/ [Accessed 28 Jan 2018 ].
- Zahuranec AJ ,
- Verhulst SG
- McKiernan EC ,
- Bourne PE ,
- Brown CT , et al
- The FAIR Data Principles
- Panagiotakos D
- Hendrickx JM ,
- de Montjoye Y-A
- Diallo AA ,
- Dearden PK , et al
- Schroeder D ,
- Hirsch F , et al
- Osuafor CN ,
- Golubic R ,
- Cellini J ,
- Charpignon M-L , et al
- Martínez-García M ,
- Villegas Camacho JM ,
- Hernández-Lemus E
- Croxton T ,
- Abimiku A , et al
- Beiswanger CM ,
- Abimiku A ,
- Carstens N , et al
- Goldberger AL ,
- Amaral LAN ,
- Glass L , et al
- Culnane C ,
- Rubinstein BIP ,
- Pe’er I , et al
- McGuire AL ,
- Golan D , et al
- Narayanan A
- Information Regulator South Africa
- Schmidt B-M ,
- Colvin CJ ,
- Hohlfeld A , et al
- Gomes DGE ,
- Pottier P ,
- Crystal-Ornelas R , et al
- Stephens J ,
- Musick B , et al
- Ginsburg GS
- Knoppers BM
- Manolio TA ,
- Goodhand P ,
- Jochems A ,
- van Soest J , et al
- Holmes JH ,
- Shah K , et al
- Bardenheuer K ,
- Passey A , et al
- Brown JS , et al
- Dawoud D , et al
- Ganzinger M ,
- Blumenstock M ,
- Fürstberger A , et al
- Van Bulck L ,
- Wampers M ,
- Li W , et al
- UK Health Data Research Alliance
- Kushida CA ,
- Nichols DA ,
- Jadrnicek R , et al
- El Emam K ,
- Dankar FK ,
- Issa R , et al
- Kosseim P ,
- Tiffin N , et al
- Bangash AH ,
- Alraoui O , et al
- Tamuhla T ,
- Bedeker A ,
- Nichols M ,
- Allie T , et al
- Scholz AH ,
- Freitag J ,
- Lyal CHC , et al
- Liggins L ,
- Burgess RA , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Handling editor Seye Abimbola
Contributors NT designed the study. TT compiled datasharing agreement section, ETL and TM compiled data anonymisation section. All authors contributed to writing, editing and reviewing the manuscript.
Funding This work is supported by the African Data and Biospecimen Exchange Project, funded by a Calestous Juma Fellowship (PI: Nicki Tiffin, INV-037558) from the Bill & Melinda Gates Foundation. NT, ETL and TT are supported by funding from the Bill & Melinda Gates Foundation (The African Data and Biospecimen Exchange, INV-037558), NT, TT and TM are funded by the UKRI/MRC (award number MC_PC_22007).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Policy and Social Care Move Fast: How…
Policy and Social Care Move Fast: How Rapid Qualitative Methods Can Help Researchers Match Their Pace
Date posted:.

The field of social care integration, which refers to the study and implementation of clinically based programs to address the social needs of patients and families, is advancing at an increasingly rapid pace. This acceleration, driven by heightened need post-pandemic as well as mandates at the state and federal levels for health systems to implement screening and referral programs, has increased the urgency for high-quality evidence to support policy decisions about the delivery of social care—in other words, how health systems identify and address social needs, like access to healthy food and safe housing.
Qualitative research is particularly useful in guiding social care integration as it can shed light on the patient or caregiver experience of participating in social care interventions, barriers to getting help that should be addressed, and appropriate next steps from the perspective of those directly impacted.
However, traditional qualitative data analysis can be time consuming, and evidence-based solutions for addressing families’ social needs from the clinical setting are needed in the short term. In this post, I’ll share how we adapted and applied rapid qualitative methods to a social care-focused study as an example of how this approach can be used to inform social care integration in real time.
Integrating a Rapid Research Approach
The Socially Equitable Care by Understanding Resource Engagement ( SECURE ) study is a mixed method pragmatic trial aimed at understanding how best to increase family-level engagement with social resources from the pediatric health care setting. Caregivers in the study were randomized to complete one of three different social assessments (surveys asking about their social circumstances and/or desire for social resources) before receiving a resource map on their personal smartphone where, if interested, they could search for community resources in their neighborhood. Caregivers also had the option of talking to our study-specific resource navigator to receive additional support finding resources.
The overall goal of the qualitative component of the study is to capture caregivers’ preferences and experiences receiving social care through SECURE. Our traditional qualitative protocol involved transcribing caregiver interviews verbatim, coding transcripts and conducting thematic analysis. Recognizing the need for implementation-oriented results on a fast timeline, our team explored rapid qualitative methodologies to supplement the traditional approach. The rapid methods we chose were derived from existing literature on rapid qualitative approaches, which were then adapted to suit our study’s protocol and the social care field in general.
In our rapid approach, interviewers took notes using a structured template during or immediately after each caregiver interview. The template was designed to capture the data most salient to social care integration efforts such as caregiver’s likes, dislikes and preferences about receiving social care at their child’s doctor’s office. Then, content from the templates was transposed onto an analytic matrix, where we compared data across participants to identify themes. While we explored the full range of themes that emerged from our caregiver interviews in traditional qualitative analysis, we wanted to be sure that rapid analysis focused on findings that would be most applicable to social care integration efforts so the results could inform social care policy at Children’s Hospital of Philadelphia (CHOP) and elsewhere in real time. For example, what parts of participating in SECURE were helpful for caregivers? Did anything make them uncomfortable?
To ensure that our rapid approach produced results in line with those generated through traditional methods, we analyzed ten of our interviews using both traditional and rapid methods and compared the results. This analysis yielded a 92.8% theme match—meaning the two qualitative methods yielded largely the same themes. This builds upon previous literature, indicating that rapid analysis can be an effective tool in capturing implementation-oriented themes from qualitative data.
How the SECURE Study Can Inform Future Research Efforts
Our rapid qualitative methods allowed us to effectively adapt and respond to the quickly evolving landscape of social care integration, even before we had the full study results. I personally saw this first-hand while working with the SECURE team in 2023 conducting caregiver interviews. For example, we were able to inform hospital efforts in response to a recent insurance requirement of health systems to share caregivers' responses to social screening questions. We successfully gathered patients’ feedback on this new requirement and shared this information and suggestions for what CHOP could do to make caregivers feel more comfortable answering social assessment questions.
While not intended to replace traditional qualitative analysis, being able to produce actionable qualitative findings in a timely manner through rapid methods has allowed SECURE findings to help shape social care interventions at CHOP and beyond in real time.
Our hope is that other researchers in social care who face time pressures may find similar rapid qualitative methods as a useful and effective approach to adapt to the dynamic nature of the field and generate family-centered solutions faster than would otherwise be possible.
Latest Blog Posts

The Heat Is Turned Up: Focus on Climate Solutions for Kids
2023 was the hottest year on record and 2024 is predicted to rank among the top five warmest years. Extreme heat at this scale can be a danger to not…
A Sense of Purpose Can Support Teen Mental Health
Adolescence–the years between about 10 to about 25–is an essential time to help young people proactively build positive mental health and well-being…

Q&A: Engaging Communities to Alleviate Period Poverty with Lynette Medley
There are 16.9 million women, girls and all other people who experience a menstrual cycle in the United States living in poverty, many of whom are…
Check out our new Publications View Publications
Adolescent Health & Well-Being
Behavioral health, population health sciences, health equity, family & community health.

Qualitative Research – a practical guide for health and social care researchers and practitioners
(0 reviews)
Darshini Ayton, Monash University
Tess Tsindos, Monash University
Danielle Berkovic, Monash University
Copyright Year: 2023
Last Update: 2024
ISBN 13: 9780645755404
Publisher: Monash University
Language: English
Formats Available
Conditions of use.
Table of Contents
- Acknowledgement of Country
- About the authors
- Accessibility statement
- Introduction to research
- Research design
- Data collection
- Data analysis
- Writing qualitative research
- Peer review statement
- Licensing and attribution information
- Version history
Ancillary Material
About the book.
This guide is designed to support health and social care researchers and practitioners to integrate qualitative research into the evidence base of health and social care research. Qualitative research designs are diverse and each design has a different focus that will inform the approach undertaken and the results that are generated. The aim is to move beyond the “what” of qualitative research to the “how”, by (1) outlining key qualitative research designs for health and social care research – descriptive, phenomenology, action research, case study, ethnography, and grounded theory; (2) a decision tool of how to select the appropriate design based on a guiding prompting question, the research question and available resources, time and expertise; (3) an overview of mixed methods research and qualitative research in evaluation studies; (4) a practical guide to data collection and analysis; (5) providing examples of qualitative research to illustrate the scope and opportunities; and (6) tips on communicating qualitative research.
About the Contributors
Associate Professor Darshini Ayton is the Deputy Head of the Health and Social Care Unit at Monash University in Melbourne, Australia. She is a transdisciplinary implementation researcher with a focus on improving health and social care for older Australians and operates at the nexus of implementation science, health and social care policies, public health and consumer engagement. She has led qualitative research studies in hospitals, aged care, not-for-profit organisations and for government and utilises a range of data collection methods. Associate Professor Ayton established and is the director of the highly successful Qualitative Research Methods for Public Health short course which has been running since 2014.
Dr Tess Tsindos is a Research Fellow with the Health and Social Care Unit at Monash University in Melbourne, Australia. She is a public health researcher and lecturer with strong qualitative and mixed methods research experience conducting research studies in hospital and community health settings, not-for-profit organisations and for government. Prior to working in academia, Dr Tsindos worked in community care for government and not-for-profit organisations for more than 25 years. Dr Tsindos has a strong evaluation background having conducted numerous evaluations for a range of health and social care organisations. Based on this experience she coordinated the Bachelor of Health Science/Public Health Evaluation unit and the Master of Public Health Evaluation unit and developed the Evaluating Public Health Programs short course in 2022. Dr Tsindos is the Unit Coordinator of the Master of Public Health Qualitative Research Methods Unit which was established in 2022.
Dr Danielle Berkovic is a Research Fellow in the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. She is a public health and consumer-led researcher with strong qualitative and mixed-methods research experience focused on improving health services and clinical guidelines for people with arthritis and other musculoskeletal conditions. She has conducted qualitative research studies in hospitals and community health settings. Dr Berkovic currently provides qualitative input into Australia’s first Living Guideline for the pharmacological management of inflammatory arthritis. Dr Berkovic is passionate about incorporating qualitative research methods into traditionally clinical and quantitative spaces and enjoys teaching clinicians and up-and-coming researchers about the benefits of qualitative research.
Contribute to this Page
Secondary Research Methods
- Secondary research methods involve using data that has already been collected by others.
- This type of research is useful for gaining a broader understanding of a topic.
- It can validate primary research findings and provide context for new research.
Existing Statistical Data
- Existing statistical data refers to numerical data that has already been collected.
- This data often comes from government databases , research studies, or organisational records.
- Pros: large amounts of data available, often from reliable sources.
- Cons: may not be tailored to your specific research question, and the quality or relevance of data may vary.
Literature Reviews
- Literature reviews involve a detailed exploration of existing academic literature related to a topic.
- This can include academic journal articles , books, and conference papers.
- Pros: can identify gaps in existing research and provide context for your study.
- Cons: can be time-consuming to find and analyse relevant literature.
Media and Document Analysis
- Media and document analysis involves critiquing media sources such as newspapers, films, and online content, or organisational documents like policies, minutes of meetings etc.
- It can provide insight into public opinion, societal trends, or internal company perspectives.
- Pros: easy to access and may provide a cultural or societal perspective.
- Cons: may contain bias and may not be directly applicable to your research question.
Internet Research
- Internet research involves accessing online information related to your topic.
- This can include websites , online publications, blogs, social media sites, forums, and digital archives.
- Pros: can access a vast amount of information quickly, and often free.
- Cons: quality and credibility of information varies greatly, and outdated content is common.
When using secondary research, it is crucial to evaluate the source’s credibility , check for potential bias , and understand the context in which the data was collected. Keeping ethical considerations in mind such as intellectual property rights and data privacy is also essential.
Cookies on the NHS England website
We’ve put some small files called cookies on your device to make our site work.
We’d also like to use analytics cookies. These send information about how our site is used to a service called Google Analytics. We use this information to improve our site.
Let us know if this is OK. We’ll use a cookie to save your choice. You can read more about our cookies before you choose.
Change my preferences I'm OK with analytics cookies
Embedding research in the NHS
Research is vital in providing the evidence we need to transform services and improve outcomes, such as in developing new models of care, redesigning urgent and emergency care, strengthening primary care and transforming mental health and cancer services. Research is the attempt to derive generalisable or transferable new knowledge .
The NHS benefits greatly from delivering research directly, not only in terms of breakthroughs enabling earlier diagnosis, more effective treatments and improved system design, all of which improve patient care and health outcomes, but also increased workforce satisfaction and retention and patient and carer experience. Mortality is lower in research active hospitals. The NHS also benefits financially from delivering research.
The purpose of the Embedding Research team in NHS England is to enable the NHS to increase the scale, pace and diversity of those taking part in research and to provide system guidance and assurance.
Guidance is available to help integrated care systems to maximise the benefits of research for their diverse populations. This guidance sets out what good research practice looks like and supports integrated care boards in fulfilling their research duties. Guidance is also available to help NHS organisations manage research finance in the NHS . This guide provides practical information on costing research and the use of income generated by research to support building research capacity and capability.
NHS England a key research delivery partner
NHS England is a proud partner of The Future of UK Clinical Research Delivery – a collective vision to realise the full potential of clinical research to make the UK one of the best places in the world to conduct clinical research.
Through a cross-sector, collaborative approach, NHS England works closely with the rest of the UK’s clinical research system on a coordinated and coherent programme of work that has been developed to ensure the resilience and growth of the UK’s clinical research sector.
Using The Future of UK Clinical Research Delivery as the collective vision, this continuous improvement programme aims to deliver faster, more efficient and more innovative clinical research through 5 overarching themes that underpin the programme of work.
- A sustainable and supported research workforce to ensure that healthcare staff of all backgrounds and roles are given the right support to deliver clinical research as an essential part of care.
- Clinical research embedded in the NHS so that research is increasingly seen as an essential part of healthcare to generate evidence about effective diagnosis, treatment and prevention.
- People-centred research to make it easier for patients, service users and members of the public across the UK to access research and be involved in the design of research, and to have the opportunity to participate.
- Streamlined, efficient and innovative research so that the UK is seen as one of the best places in the world to conduct cutting-edge clinical research, driving innovation in healthcare.
- Research enabled by data and digital tools to ensure the best use of resources, leveraging the strength of UK health data assets to allow for more high-quality research to be delivered.
Key activity undertaken in support of this vision includes guidance for health professionals. Many health professionals combine research and providing care in the NHS. The following publications have been published by NHS England to support this:
- The guidance Making research matter sets out a policy framework for developing and investing in nursing related research activity across the NHS.
- The Allied health professions’ research and innovation strategy for England contains a definitive collective national reference statement that encompasses and supports the existing research and innovation strategies of all the allied health professional associations.
- NHS England has published the self-assessment of organisational readiness tool: a guide to improve nursing research capacity in health and care . The tool helps organisations to assess their preparedness for supporting the Chief Nursing Officer for England’s strategic plan for research.
- Guidance has been published to support the involvement of NHS workforce in health and social care research. The Multi-professional practice-based research capabilities framework highlights and promotes active involvement in research as an integral component of practice for practice-based health and care professionals.
- In addition, a UK survey of pharmacy professionals’ involvement in research has resulted in this report with recommendations to inform a clinical academic career pathway for pharmacy, which supports embedding research at all stages of a pharmacy professional’s career.
NHS England works closely with the National Institute for Health and Care Research (NIHR), which provides funding for research studies as well as academic training, facilities, career development and research capability development. In addition, through Be Part of Research NIHR supports participation on research in a wide range of long-term conditions, diseases and disabilities. See the NIHR website for more information on NIHR’s support offer.
Developing treatments for all: Increasing diversity in research participation
Health research plays an integral part in how the NHS develops services and continues to provide high quality healthcare for our population. However, NIHR data has revealed that UK geographies that experience high rates of disease also have the lowest number of patients taking part in research. The areas where there are the lowest levels of research participation also align closely to areas where incomes are lowest, and indices of deprivation are highest. This means that research is often conducted with individuals who are healthier and wealthier, and lacks representation from our diverse society.
It is important that people from different communities have the opportunity to participate in research to ensure that treatments, technologies and services reflect the needs of our diverse population. NHS England has committed to increasing participation in the research taking place in the NHS.
The Research Engagement Network Development Programme aims to increase diversity in research participation through the development of research engagement networks with communities who are often underserved by research, and by ensuring diversity in research is considered by integrated care systems (ICSs).
Launched in 2022, NHS England and the Department of Health and Social Care have funded all 42 ICSs in England to grow their local research engagement networks by working with local voluntary, community and social enterprises to engage underserved communities. In addition, a further 9 teams have been funded to plan how to address specific existing barriers to inclusion in research such as language, cultural barriers and/or age limitations and/or restrictions across a range of conditions and clinical or care settings.
NHS England has published Increasing diversity in research participation: a good practice guide for engaging with underrepresented groups , which provides practical insights for researchers on how to engage more diverse participants in health research. More diverse participation will help ensure that the health service continues to serve and be available to all.
Professional/Short course Health and Social Care Research: Methods and Methodology
15 or 20 credit level 7 module (online learning option available).
Due to the places required from our partnership organisations outweighing the actual places available on this module, it will not be opened up to general applications until six weeks before the start date. Please contact your employer to see if you are eligible to apply, they will supply you with the relevant links to undertake this process.
Page last updated 30 November 2023
Introduction
The Health and Social Care Research: Methods and Methodology level 7 (Masters level) module is available at both 15 (UZWSPX-15-M) or 20 (UZWRGQ-20-M) credits. There is also a 15 (UZWSRV-15-M) or 20 (UZWYGP-20-M) credit online learning module option available.
These 15 or 20 credit level 7 module, Health and Social Care Research: Methods and Methodology, will give you an overview of:
- the current state of research in health and social care, including, for example issues of funding, the formulation of research questions, the relationship between evidence and practice and the implementation of research findings in different settings
- the access, use and the development of information systems: databases; libraries; bibliographic searching; the Internet
- evaluating intervention research (experimental and quasi-experimental research; randomised controlled trials; action research; descriptive and inferential statistics including both parametric and non-parametric approaches)
- evaluating survey research
- evaluating qualitative research (open interviews, discourse and content analysis, observational research)
- evaluation criteria: reliability, validity; issues of corroboration; triangulation
- the main research methodologies and strategies
- Health Service evaluation
- ethical issues in research
- innovations in research and the development of new methodologies.
There is also a 15 credit (UZWSRV-15-M) or 20 credit (UZWYGP-20-M) online learning module option available.
Careers / Further study
This course can contribute towards the following Programmes subject to relevant credit to be undertaken:
- MSc Specialist Practice (District Nursing)
- MSc Advanced Practice
- Professional Development Awards
The module syllabus typically includes:
Knowledge and understanding
- Critically analyse the rationale for particular qualitative and quantitative research methodologies and methods.
- Interpret the stages of the research process and the meaning and significance of data generation and analysis in qualitative and quantitative research.
- Apply the critical knowledge of the relationships between sampling and theory generation.
- Demonstrate a critical awareness of the need for and the process of research governance.
Intellectual skills
- Make evaluative judgements on the relevance of qualitative and quantitative approaches to the investigation of research issues/questions.
- Justify the appropriate uses of primary and secondary sources of data.
Subject/professional/practical skills
- Critically appraise published research relating to their discipline area and its implications for policy and practice.
- Critically appraise a selection of appropriate tools for data collections and analysis.
- Demonstrate an ability to deal effectively with the ethical issues arising in the conduct of research.
Transferable skills
- Demonstrate a reflective approach to research.
- Demonstrate a critical insight into ethical issues, intellectual property rights and other legal considerations arising in the conduct of research.
Learning and Teaching
A variety of learning approaches will be used in conjunction with weekly face-to-face seminars and self-directed study.
You will require easy access to a computer and the internet for the duration of the module.
- For the 15 credit level 7 (Masters level) module: a 3,000 word assignment.
- For the 20 credit level 7 (Masters level) module: a 4,000 word assignment.
Study facilities
The College of Health, Science and Society has an excellent reputation for the quality of its teaching and the facilities it provides.
Get a feel for the Health Professions facilities we have on offer here from wherever you are.

Prices and dates
There is currently no published fee data for this course.
Supplementary fee information
Please visit full fee information to see the price brackets for our modules.
Please click on the 'Apply Now' button to view dates.
How to apply
Please click on the Apply Now button on this page to apply online for this course, which you can take as a stand-alone course or as part of a postgraduate (Masters level) programme.
Extra information
If the course you are applying for is fully online or blended learning, please note that you are expected to provide your own headsets/microphones.
For further information
- Email: [email protected]
You can also follow us on Twitter @UWEhasCPD .
- Open access
- Published: 27 April 2024
Exploring health care providers’ engagement in prevention and management of multidrug resistant Tuberculosis and its factors in Hadiya Zone health care facilities: qualitative study
- Bereket Aberham Lajore 1 na1 nAff5 ,
- Yitagesu Habtu Aweke 2 na1 nAff6 ,
- Samuel Yohannes Ayanto 3 na1 nAff7 &
- Menen Ayele 4 nAff5
BMC Health Services Research volume 24 , Article number: 542 ( 2024 ) Cite this article
Metrics details
Engagement of healthcare providers is one of the World Health Organization strategies devised for prevention and provision of patient centered care for multidrug resistant tuberculosis. The need for current research question rose because of the gaps in evidence on health professional’s engagement and its factors in multidrug resistant tuberculosis service delivery as per the protocol in the prevention and management of multidrug resistant tuberculosis.
The purpose of this study was to explore the level of health care providers’ engagement in multidrug resistant tuberculosis prevention and management and influencing factors in Hadiya Zone health facilities, Southern Ethiopia.
Descriptive phenomenological qualitative study design was employed between 02 May and 09 May, 2019. We conducted a key informant interview and focus group discussions using purposely selected healthcare experts working as directly observed treatment short course providers in multidrug resistant tuberculosis treatment initiation centers, program managers, and focal persons. Verbatim transcripts were translated to English and exported to open code 4.02 for line-by-line coding and categorization of meanings into same emergent themes. Thematic analysis was conducted based on predefined themes for multidrug resistant tuberculosis prevention and management and core findings under each theme were supported by domain summaries in our final interpretation of the results. To maintain the rigors, Lincoln and Guba’s parallel quality criteria of trustworthiness was used particularly, credibility, dependability, transferability, confirmability and reflexivity.
Total of 26 service providers, program managers, and focal persons were participated through four focus group discussion and five key informant interviews. The study explored factors for engagement of health care providers in the prevention and management of multidrug resistant tuberculosis in five emergent themes such as patients’ causes, perceived susceptibility, seeking support, professional incompetence and poor linkage of the health care facilities. Our findings also suggest that service providers require additional training, particularly in programmatic management of drug-resistant tuberculosis.
The study explored five emergent themes: patient’s underlying causes, seeking support, perceived susceptibility, professionals’ incompetence and health facilities poor linkage. Community awareness creation to avoid fear of discrimination through provision of support for those with multidrug resistant tuberculosis is expected from health care providers using social behavioral change communication strategies. Furthermore, program managers need to follow the recommendations of World Health Organization for engaging healthcare professionals in the prevention and management of multidrug resistant tuberculosis and cascade trainings in clinical programmatic management of the disease for healthcare professionals.
Peer Review reports
Introduction
Mycobacterium tuberculosis, the infectious agent that causes multi-drug resistant tuberculosis (MDR-TB), is resistant to at least rifampicin and isoniazid. Direct infection can cause the disease to spread, or it can develop secondary to improper management of tuberculosis among drug susceptible tuberculosis cases and associated poor adherence [ 1 ].
Multidrug-resistant strains of mycobacterium tuberculosis have recently emerged, which makes achieving “End TB Strategy” more difficult [ 2 ]. Multi drug resistant tuberculosis (MDR-TB) has been found to increasingly pose a serious threat to global and Ethiopian public health sector. Despite the fact that a number of risk factors for MDR-TB have been identified through various research designs, the epidemiology of this disease is complex, contextual, and multifaceted [ 1 ]. Quantitative studies demonstrate that prior treatment history [ 3 , 4 , 5 , 6 , 7 ], interrupted drug supply [ 8 ], inappropriate treatments and poor patient compliance [ 3 , 7 , 9 ], poor quality directly observed treatment short course (DOTS), poor treatment adherence [ 10 ], age [ 5 ], and malnutrition [ 11 ] were factors associated with multi drug resistant TB.
Globally, an estimated 20% of previously treated cases and 3.3% of new cases are thought to have MDR-TB; these levels have essentially not changed in recent years. Globally, 160,684 cases of multidrug-resistant TB and rifampicin-resistant TB (MDR/RR-TB) were notified in 2017, and 139,114 cases were enrolled into treatment in 2017 [ 12 ]. A systematic review in Ethiopia reported 2% prevalence of MDR-TB [ 3 ] that is higher than what is observed in Sub-Saharan Africa, 1.5% [ 13 ]. The prevalence of MDR-TB, according to the national drug-resistant tuberculosis (DR-TB) sentinel report, was 2.3% among newly diagnosed cases of TB and 17.8% among cases of TB who had already received treatment,. This suggests a rising trend in the prevalence of TB drug resistance compared to the results of the initial drug-resistant TB survey carried out in Ethiopia from 2003 to 2005 [ 14 ].
Ethiopia has placed strategies into place that emphasize political commitment, case finding, appropriate treatment, a continuous supply of second-line anti-TB medications of high quality, and a recording system. Due to other competing health priorities, the nation is having difficulty accelerating the scale-up of the detection, enrollment and treatment of drug-resistant TB patients [ 15 , 16 ]. To address these issues, the nation switched from a hospital-based to a clinic-based ambulatory model of care, which has allowed MDR-TB services to quickly decentralize and become more accessible. Accordingly, the nation has set up health facilities to act as either treatment initiating centers (TIC) or treatment follow-up centers (TFC) or both for improved referral and communication methods [ 15 ].
One of the key components of the “End TB strategy” is engagement of health care professionals in the prevention and management of multidrug resistant tuberculosis [ 17 ]. Inadequate engagement of healthcare providers is one aspect of the healthcare system that negatively influences MDR-TB prevention and control efforts [ 17 ]. This may be manifested in a number of ways, including inadequate understanding of drug-resistant tuberculosis, improper case identification, failure to initiate treatment again, placement of the wrong regimens, improper management of side effects and poor infection prevention [ 1 ]. These contributing factors are currently being observed in Ethiopia [ 18 ], Nigeria [ 7 , 19 , 20 ] and other countries [ 21 , 22 ]. According to a study conducted in Ethiopia, MDR-TB was linked to drug side effects from first-line treatments, being not directly observed, stopping treatment for at least a day, and retreating with a category II regimen [ 17 ].
This may be the result of a synergy between previously investigated and other contextual factors that have not yet been fully explored, such as professional engagement, beliefs, and poor preventive practices. The engagement of health professionals in MDR-TB prevention and control is assessed using a number of composite indicators. Health professionals may interact primarily inside the healthcare facilities. Typically, they play a significant role in connecting healthcare services with neighborhood-based activities [ 17 ]. One of the main research areas that have not sufficiently addressed is evidence indicating the status of healthcare professionals’ engagement and contextual factors in MDR-TB prevention and management.
It is increasingly urgent to identify additional and existing factors operating in a particular context that contribute to the development of the disease in light of the epidemic of drug resistance, including multi-drug resistance (MDR-TB) and extensively drug resistant TB (XDR-TB) in both new and previously treated cases of the disease [ 23 ]. In order to develop and implement control measures, it is therefore essential to operationally identify a number of contextual factors operating at the individual, community, and health system level.
Therefore, the overall purpose of this study was to explore the level of engagement of health care providers and contextual factors hindering/enabling the prevention and provision of patient-centered care for MDR-TB in health facilities, DOTS services centers and MDR-TB treatment initiation center [TIC], in Hadiya Zone, Southern Ethiopia.
Qualitative approach and research paradigm
Descriptive phenomenological qualitative study design was employed to explore factors influencing engagement of health professionals in MDR-TB prevention and management and thematic technique was employed for the analysis of the data.
Researchers’ characteristics and reflexivity
Three Principal investigators conducted this study. Two of them had Masters of public health in Epidemiology and Reproductive health and PhD candidates and the third one had Bachelor’s degree in public health with clinical experience in the area of Tuberculosis prevention and management and MPH in Biostatistics. The principal investigators have research experience with published articles in different reputable journals. There were no prior contacts between researchers and participants before the study whereas researchers have built positive rapport with study participants during data collection to foster open communication and trust and had no any assumptions and presuppositions about the research topic and result.
Context/ study setting and period
The study was conducted between 2 and 9 May, 2019 in Hadiya Zone with more than 1.7 million people residing in the Zone. There are 300 health posts, 63 health centers, 3 functional primary hospitals and 1 comprehensive specialized hospital in the Zone. Also, there are more than 350 private clinics and 1 private hospital in the Zone. All of the public health facilities and some private health facilities provide directly observed short course treatment (DOTS) service for tuberculosis patients. There are more than eight treatment initiation centers (TICs) for MDR-TB patients in Hadiya Zone. MDR-TB (Multidrug-resistant tuberculosis) treatment initiation centers are specialized facilities that provide comprehensive care, diagnosis and treatment initiation, psychosocial support, and follow up services to individuals with MDR-TB. The linkage between MDR-TB treatment initiation centers and other healthcare facilities lies in the coordination of care, referral pathways, and collaboration to ensure comprehensive and integrated care for individuals with MDR-TB. Overall, healthcare providers play a crucial role in the management of MDR-TB by providing specialized care, ensuring treatment adherence, monitoring progress and outcomes, and supporting individuals in achieving successful treatment outcomes and improved health.
Units of study and sampling strategy
Our study participants were health care professionals working in MDR-TB TICs in both private and public health facilities, and providing DOTS services, MDR-TB program leaders in treatment initiation centers, as well as TB focal persons, disease prevention and health promotion focal person, and project partners from district health offices. The study involved four focus group discussion (FGDs) and five key informants’ interview (KII) with a total of 26 participants to gather the necessary information. Expert purposive sampling technique was employed and sample size was determined based on the saturation of idea required during data collection process.
Data collection methods and instruments
Focus group discussion and face to face key informants’ interviews were employed to collect the data. We conducted a total of four FGD and five key informants’ interviews with participants chosen from DOTS providing health facilities and MDR-TB program leaders in treatment initiation centers, as well as TB focal persons and project partners from district health offices and disease prevention and health promotion focal person. One of the FGDs was conducted among health professionals from the public MDR-TB treatment initiation centers. Three FGDs were conducted among disease prevention and health promotion focal persons, TB focal persons and DOTS providers in public health facilities (health centers).
An observation checklist was developed to assess the general infection prevention and control measures used by specific healthcare facilities in the study area. We used unstructured FGD guide, key informant interview guide, observation checklist and audio recorders to collect primary data and it was collected using local language called Amharic. Prior to data collection, three people who are not among principal investigators with at least a master’s degree in public health and prior experience with qualitative research were trained by principal investigators. Three of them acts as a tape recorder, a moderator, and as a note taker alternatively. The length of FGD ranged from 58 to 82 min and that of key informants’ interview lasted from 38 to 56 min.
Data processing and data analysis
Memos were written immediately after interviews followed by initial analysis. Transcription of audio records was performed by principal investigators. The audio recordings and notes were refined, cleaned and matched at the end of each data collection day to check for inconsistencies, correct errors, and modify the procedures in response to evolving study findings for subsequent data collection. Transcribed interviews, memos, and notes from investigator’s observation were translated to English and imported to Open Code 4.02 [ 2 ] for line by line coding of data, and categorizing important codes (sub theming). The pre-defined themes for MDR-TB prevention and control engagement were used to thematize the line-by-line codes, categories, and meanings using thematic analysis. Finally, the phenomenon being studied was explained by emerging categories and themes. Explanations in themes were substantiated by participants’ direct quotations when necessary.
Trustworthiness
Phone calls and face to face briefing were requested from study participants when some expressions in the audio seems confusing while transcripts were performed. To ensure the credibility of the study, prolonged engagement was conducted, including peer debriefing with colleagues of similar status during data analysis and inviting available study participants to review findings to ensure as it is in line with their view or not. Memos of interviews and observation were crosschecked while investigator was transcribing to ensure credibility of data as well as to triangulate investigator’s categorizing and theming procedures. For transferability, clear outlines of research design and processes were provided, along with a detailed study context for reader judgment. Dependability was ensured through careful recording and transcription of verbal and non-verbal data, and to minimize personal bias, scientific procedures were followed in all research stages. Conformability was maintained by conducting data transcription, translation, and interpretation using scientific methods. Researchers did all the best to show a range of realities, fairly and faithfully. Finally, an expert was invited to put sample of codes and categories to emerged corresponding categories and themes respectively.
Demographic characteristics of study participants
Four focus group discussions and five key informants’ interviews were conducted successfully. There were 26 participants in four focus group discussions, and key informants’ interview. Ages of participants ranges from 20 to 50 years with an average age of 33.4 ± 6.24 SD years. Participants have five to ten years of professional experience with DOTS services (Table 1 ).
Emergent themes and subthemes
The study explored how health care providers’ engagement in MDR-TB prevention and management was influenced. The investigation uncovered five major themes. These themes were the patient’s underlying causes, seeking support, perceived susceptibility, healthcare providers’ incompetence, and poor linkage between health facilities. Weak community TB prevention, health system support, and support from colleagues were identified subthemes in the search for help by health professionals whereas socioeconomic constraints, lack of awareness, and fear of discrimination were subthemes under patients underlying factors (Fig. 1 ).
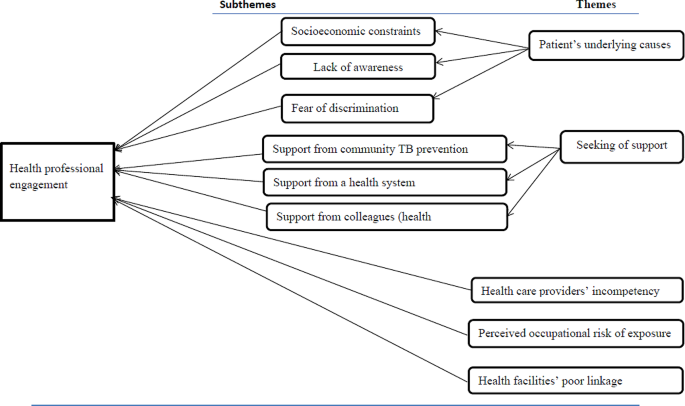
Themes and subthemes emerged from the analysis of health professionals’ engagement in MDR-TB prevention and management study in Hadiya zone’s health facilities, 2019
The patient’s underlying causes
This revealed why TB/MDR-TB treatment providers believe health professionals are unable to provide standard MDR-TB services. The subthemes include TB/MDR-TB awareness, fear of discrimination, and patients’ socioeconomic constraints.
Socioeconomic constraints
According to our research, the majority of healthcare professionals who provided directly observed short-course treatment services mentioned socioeconomic constraints as barriers to engage per standard and provide MDR-TB prevention and management service. More than half of the participants stated that patients’ primary reasons include lack of money for house rental close to the treatment centers, inability to afford food and other expenses, and financial constraints to cover transportation costs.
In addition to this, patients might have additional responsibilities to provide food and cover other costs for their families’ need. The majority of health care professionals thought that these restrictions led to their poor engagement in MDR-TB prevention and management. One of the focus groups’ discussants provided description of the scenario in the following way:
“…. I have many conversations with my TB/MDR-TB patients. They fail to complete DOTS or treatment intensive care primarily as a result of the requirement of prolonged family separation. They might provide most of the family needs, including food and other expenses” (FGD-P01).
Lack of awareness about MDR-TB
This subtheme explains how MDR-TB patients’ knowledge of the illness can make it more difficult for health professionals to provide DOTS or TICs services. The majority of DOTS providers stated that few TB or MDR-TB patients were aware of how MDR-TB spreads, how it is treated, and how much medication is required. Additionally, despite the fact that they had been educated for the disease, majority of patients did not want to stop contact with their families or caregivers. A health care provider stated,
“…. I provided health education for MDR-TB patients on how the disease is transmitted and how they should care for their family members. They don’t care; however, give a damn about their families .” (FGD-P05).
Some healthcare professionals reported that some patients thought that MDR-TB could not be cured by modern medication. One medical professional described the circumstance as follows:
“…. I noticed an MDR-TB patient who was unwilling to be screened. He concluded that modern medication is not effective and he went to spiritual and traditional healers” (FGD-P02).
As a result, almost all participants agreed on the extent to which patient knowledge of TB and MDR-TB can influence a provider’s engagement to MDR-TB services. The majority suggested that in order to improve treatment outcomes and preventive measures, the media, community leaders, health development armies, one-to-five networks, non-governmental organizations, treatment supporters, and other bodies with access to information need to put a lot of efforts.
Fear of discrimination
According to our research, about a quarter of healthcare professionals recognized that patients’ fear of discrimination prevents them from offering MDR-TB patients the DOTS services they need, including counseling index cases and tracing contact histories.
HEWs, HDAs, and 1-to-5 network members allegedly failed to monitor and counsel the index cases after their immediate return to their homes, according to the opinions from eight out of twenty-six healthcare professionals. The patients began to engage in routine social and political activities with neighbors while hiding their disease status. A healthcare professional described this situation as follows:
“…. I understood from my MDR-TB patient’s words that he kept to himself and avoided social interaction. He made this decision as a result of stigmatization by locals, including health extension workers. As a result, the patient can’t attend social gatherings. …. In addition, medical professionals exclude MDR-TB patients due to fear of exposures. As a result, patients are unwilling to undergo early screening” (FGD-P04).
Professionals’ perceived risk of occupational exposure
This theme highlights the anxiety that healthcare workers experience because of MDR-TB exposure when providing patient care. Our research shows that the majority of health professionals viewed participation as “taking coupons of death.” They believed that regardless of how and where they engaged in most healthcare facilities, the risk of exposure would remain the same. According to our discussion and interview, lack of health facility’s readiness takes paramount shares for the providers’ risk of exposures and their susceptibility.
According to the opinion from the majority of FGD discussants and in-depth interviewees, participants’ self-judgment score and our observation, the majority of healthcare facilities that offer DOTS for DS-TB and MDR-TB did not create or uphold standards in infection prevention in the way that could promote better engagement. These include poor maintenance of care facilities, lack of personal protective equipment, unsuitable facility design for service provision, lack of patient knowledge regarding the method of MDR-TB transmission, and lack of dedication on the part of health care staff.
As one of our key informant interviewees [District Disease Prevention Head], described health professionals’ low engagement has been due to fear of perceived susceptibility. He shared with us what he learned from a community forum he moderated.
Community forum participant stated that “… There was a moment a health professional run-away from the TB unit when MDR-TB patient arrived. At least they must provide the necessary service, even though they are not willing to demonstrate respectful, compassionate, or caring attitude to MDR-TB patients” (KII-P01). Besides , one of the FGD discussants described the circumstance as follows:
“…. Emm…. Because most health facilities or MDR-TB TIC are not standardized, I am concerned about the risk of transmission. They are crammed together and poor ventilation is evident as well as their configuration is improper. Other medical services are causing the TICs to become overcrowded. Most patients and some medical professionals are unconcerned with disease prevention ” (FGD-P19).
Participants’ general fear of susceptibility may be a normal psychological reaction and may serve as a motivation for taking preventative actions. However, almost all participants were concerned that the main reasons for their fear were brought up by the improper application of programmatic management and MDR-TB treatment standards and infection prevention protocols in healthcare facilities.
Health care providers’ incompetence
This theme illustrates how professionalism and dedication impact participation in MDR-TB prevention and management. The use of DS-TB prevention and management by health professionals was also taken into account because it is a major factor in the development of MDR-TB. This theme includes the participants’ perspectives towards other healthcare workers involved in and connected to MDR-TB.
Nearly all of the participants were aware of the causes and danger signs of MDR-TB. The majority of the defined participants fit to the current guidelines. However, participants in focus groups and key informant interviews have brought up shortcomings in MDR-TB service delivery practice and attitude. We looked at gaps among healthcare professionals’ knowledge, how they use the national recommendations for programmatic management and prevention of MDR-TB, prevent infections, take part in community MDR-TB screenings, and collaborate with other healthcare professionals for better engagement.
More than half of the participants voiced concerns about their attitudes and skill sets when using MDR-TB prevention and management guideline. When asked about his prior experiences, one of the focus group participants said:
“…. Ok, let me tell you my experience, I was new before I attended a training on MDR-TB. I was unfamiliar with the MDR-TB definition given in the recommendations. When I was hired, the health center’s director assigned me in the TB unit. I faced difficulties until I received training” (FGD-P24). Furthermore , one of the key informant interview participants shared a story: “…. In my experience, the majority of newly graduated health professionals lack the required skill. I propose that pre-service education curricula to include TB/MDR-TB prevention and management guideline trainings” (KII-P01).
The majority of participants mentioned the skill gap that was seen among health extension workers and laboratory technicians in the majority of healthcare facilities. Some of the participants in the in-depth interviews and FGD described the gaps as follows:
“…. According to repeated quality assurance feedbacks, there are many discordant cases in our [ District TB Focal Person ] case. Laboratory technicians who received a discrepant result (KII-P01) are not given training which is augmented by shared story from FGD discussants, “According to the quality assurance system, laboratory technicians lack skill and inconsistent results are typical necessitating training for newly joining laboratory technicians” (FGD-P20).
Through our discussions, we explored the level of DOTS providers’ adherence to the current TB/MDR-TB guideline. As a result, the majority of participants pointed out ineffective anti-TB management and follow-up care. One of the participants remembered her practical experience as follows:
“…. In my experience, the majority of health professionals fail to inform patients about the drug’s side effects, follow-up procedures, and other techniques for managing the burden of treatment. Only the anti-TB drug is provided, and the patient is left alone. The national treatment recommendation is not properly implemented by them” (FGD-P04).
Many barriers have been cited as reasons that might have hindered competencies for better engagement of health professionals. Training shortage is one of the major reasons mentioned by many of the study participants. One of discussants from private health facility described the problem as
“…. We are incompetent, in my opinion. Considering that we don’t attend update trainings. Many patients who were diagnosed negative at private medical facilities turned out to be positive, and vice versa which would be risky for drug resistance” (FGD-P14) which was supported by idea from a participant in our in-depth interview: “…. We [Program managers] are running short of training for our health care providers at different health centers and revealed that four out of every five healthcare professionals who work in various health centers are unaware of the TB/MDR-TB new guideline” (KII-P02).
Seeking support
This theme focuses on the significance and effects of workplace support in the engagement of MDR-TB prevention and control. This also explains the enabling and impeding elements in the engagement condition of health professionals. Three elements make up the theme: coworkers (other health professionals) in the workplace, support from community TB prevention actors, and a healthcare system.
Support from community TB prevention actors
This subtheme includes the assistance provided to study participants by important parties such as community leaders, the health development army, and other stakeholders who were involved in a community-based TB case notification, treatment adherence, and improved patient outcomes.
Many of the study participants reported that health extension workers have been poorly participating in MDR-TB and TB-related community-based activities like contact tracing, defaulter tracing, community forums, health promotion, and treatment support. One study participant described their gap as follows:
“…. I understood that people in the community were unaware of MDR-TB. The majority of health extension workers do not prioritize raising community awareness of MDR-TB” (FGD-P13). This was supported by idea from a district disease prevention head and stated as: “…. There is no active system for contacts tracing. Health educators send us information if they find suspected cases. However, some patients might not show up as expected. We have data on three family members who tested positive for MDR-TB” (KII-P3).
Support from a health system
The prime focus of this subtheme is on the enabling elements that DOTS providers require assistance from the current healthcare system for better engagement. All study participants expressed at least two needs to be met from the health system in order for them to effectively participate in MDR-TB prevention, treatment, and management. All study participants agreed that issues with the health system had a negative impact on their engagement in the prevention, treatment, diagnosis, and management of MDR-TB in almost all healthcare facilities. Poor conditions in infrastructure, resources (supplies, equipment, guidelines, and other logistics), capacity building (training), supportive supervision, establishment of public-private partnerships, and assignment of motivated and trained health professionals are some of the barriers that needs to be worked out in order to make them engage better. One of the participants pronounces supplies and logistics problems as:
“…. The health center I worked in is listed as a DOTS provider. However, it lacks constant electricity, a working microscope, lab supplies, medications, etc, and we refer suspected cases to nearby health centers or district hospitals for AFB-examination and, “Sometimes we use a single kit for many patients and wait for the medication supply for three or more weeks and patients stops a course of therapy that might induce drug resistance” (FGD-PI04) which was augmented by statement from FGD participant who works at a treatment initiation center: “…. We faced critical shortage of supplies and hospital administrators don’t care about funding essential supplies for patient care. For instance, this hospital (the hospital in which this FGD was conducted) can easily handle N-95 masks. Why then they (hospital administrators working in some TIC) can’t do it?” (FGD-P18).”
Regarding in-service training on MDR-TB, almost all participants pointed out shortage of on-job training mechanisms. One of our FGD participants said:
“…. I missed the new training on MDRTB programmatic management guidelines. I’ve heard that new updates are available. I still work using the old standard” (FGD-PI05). A health professional working in private clinic heightens the severity of training shortage as: “…. We have not participated in TB/MDR-TB guidelines training. You know, most of for-profit healthcare facilities do not provide any training for their staff. I’m not sure if I’m following the (TB/MDR-TB) guideline” (FGD-P14). One of our key informant interview participants; MDR-TB center focal person suggested the need for training as: “…. I’ve received training on the MDR-TB services and public-private partnership strategy. It was crucial in my opinion for better engagement. It is provided for our staff [MDRTB center focal person]. However, this has not yet been expanded to other health facilities” (KII-P04).
Concerning infrastructures, transportation problem was one of the frequently mentioned obstacles by many participants that hinder engagement in MDR-TB/TB service. This factor had a negative impact to both sides (health professionals and patients). One of discussants said:
“…. I face obstacles such as transport cost to perform effective TB/MDR-TB outreach activities like health education, tracing family contacts and defaulters and community mobilization. Rural kebeles are far apart from each other. How can I support 6 rural Kebeles?” (FGD-P01). One of the participants; MDR-TB treatment centers supervisor/program partner seconded the above idea as: “…. I suggest government must establish a system to support health professionals working in remote health care facilities in addition to MDR-TB centers. I guess there are more than 30 government health centers and additional private clinics. We can’t reach them all due to transportation challenges” (KII-P05). One of the participants , a district disease prevention head added: “…. Our laboratory technicians take sample from MDR-TB suspects to the post office and then, the post office sends to MDR-TB site. Sometimes, feedback may not reach timely. There is no any system to cover transportation cost. That would make case detection challenging” (FGD-P02).
Support from colleagues
Study participants stated the importance of having coworker with whom they could interconnect. However, eight participants reported that they were discriminated by their workmates for various reasons, such as their perceived fear of exposure to infection and their perception as if health professionals working in TB/MDR-TB unit get more training opportunities and other incentives. One of the focus group discussants said:
“…. My colleagues [health professional working out of MDR-TB TICs] stigmatize us only due to our work assignment in MDR-TB clinic. I remember that one of my friends who borrowed my headscarf preferred to throw it through a window than handing-over it back safely. Look, how much other health professionals are scared of working in MDR-TB unit. This makes me very upset. I am asking myself that why have I received such training on MDR-TB?” (FGD-P04).
Some of the participants also perceived that, health professionals working in MDR-TB/TB unit are the only responsible experts regarding MDR-TB care and treatment. Because, other health professionals consider training as if it is an incentive to work in such units. One of the FGD discussants described:
“… Health professionals who work in other service units are not volunteer to provide DOTS if TB focal person/previously trained staffs are not available. Patients wait for longer time” (FGD-P11).
Health facilities’ poor linkage
This theme demonstrates how various healthcare facilities, including private and public healthcare facilities such as, health posts, health care centers and hospitals, and healthcare professionals working at various levels of the healthcare system in relation to TB/MDR-TB service, are inter-linked or communicating with one another.
Many study participants noted a lack of coordination between higher referral hospitals, TB clinics, health posts, and health centers. Additionally, the majority of the assigned healthcare professionals had trouble communicating with patients and their coworkers. A focus group discussant also supported this idea as
“…. There is a lack of communication between us [DOTS providers at treatment initiation centers] and health posts, health centers, and private clinics. We are expected to support about 30 public health facilities. It’s of too much number, you know. They are out of our reach. We only took action when a problem arose” (FGD-P16).
Significant number of participants had raised the problem of poor communication between health facilities and treatment initiation centers. One of the interviewees [program manager] said:
“…. I see that one of our challenges is the weak referral connections between treatment initiation centers and health centers. As a result, improper sample transfer to Gene- Xpert sites and irregular postal delivery are frequent” . “Our; DOTS staff at the MDR-TB center, DOTS staff at the health center, and health extension workers are not well connected to one another. Many patients I encountered came to this center [MDR-TB center] after bypassing both health post and health center. Poor linkage and communication, in my opinion, could be one of the causes. The same holds true for medical facilities that are both public and private ” (KII-P02).
Engagement of individual healthcare providers is one of the peculiar interventions to achieve the goal of universal access to drug resistance tuberculosis care and services [ 17 ]. Healthcare providers engagement in detecting cases, treating and caring for multidrug resistant tuberculosis (MDR-TB) may be influenced by various intrinsic (individual provider factors) and extrinsic (peer, health system, political and other factors) [ 15 ]. Our study explored engagement of individual DOTS providers and factors that influence their engagement in MDR-TB prevention and management service. This is addressed through five emergent themes and subthemes as clearly specified in our results section.
The findings showed patients’ socioeconomic constraints were important challenges that influence health professionals’ engagement, and provision of MDR-TB prevention and management services. Although approaches differ, studies in Ethiopia [ 24 ], South Africa [ 25 ] and India [ 26 , 27 ] documented that such factors influence health providers’ engagement in the prevention and management of multi drug resistant tuberculosis. Again, the alleviation of these factors demands the effort from patients, stakeholders working on TB, others sectors, and the healthcare system so that healthcare providers can deliver the service more effectively in their day-to-day activities and will be more receptive to the other key factors.
We explored participants’ experiences on how patients’ awareness about drug sensitive or multi drug resistant tuberculosis influenced their engagement. Accordingly, participants encountered numerous gaps that restricted their interactions with TB/MDR-TB patients. In fact, our study design and purposes vary, studies [ 28 , 29 , 30 ] indicated that patients awareness influenced providers decision in relation to MDR-TB services and patients’ awareness status is among factors influencing healthcare providers’ decision making about the care the MDR-TB patient receives. As to our knowledge, patients’ perceived fear of discrimination was not documented whether it had direct negative impact on reducing providers’ engagement. Therefore, patients’ awareness creation is an important responsibility that needs to be addressed by the community health development army, health extension workers, all other healthcare providers and stakeholder for better MDR-TB services and patient outcomes.
Our study indicates that healthcare providers perceived that they would be exposed to MDR-TB while they are engaged. Some of the participants were more concerned about the disadvantages of engagement in providing care to MDR-TB patients which were predominantly psychological and physical pressure. In this context, the participants emphasized that engagement in MDR-TB patient care is “always being at risk” and expressed a negative attitude. This finding is similar to what has been demonstrated in a cross-sectional study conducted in South Africa in which majority of healthcare providers believed their engagement in MDR-TB services would risk their health [ 21 ].
However, majority of the healthcare providers demonstrated perceived fear of exposures mainly due to poor infection prevention practices and substandard organization of work environment in most TB/MDR-TB units. This is essentially reasonable fear, and needs urgent intervention to protect healthcare providers from worsening/reducing their effective engagement in MDR-TB patient care. On the other side of the coin, perceived risk of occupational exposure to infection could be source for taking care of oneself to combat the spread of the infection.
In our study, healthcare provider’s capability (competence) also had an impact on their ability to engage in prevention and management of MDR-TB. Here, participants had frequently raised their and other healthcare providers’ experience regarding skill gaps, negative attitude towards the service unit they were working in, ineffective use of MDR-TB guideline, poor infection prevention practices and commitment. In addition, many health professionals report serious problems regarding case identification and screening, drug administration, and side effect management. These findings were supported by other studies in Ethiopia [ 7 ] and in Nigeria [ 19 , 20 ]. This implies an urgent need for training of health care worker on how to engage in prevention and management of multidrug resistant TB.
Moreover, our findings provide insights into the role of community TB prevention actors, currently functioning health system, and colleagues and other stakeholders’ regarding healthcare providers’ engagement. Participants emphasized that support from community TB prevention actors is a key motivation to effectively engage on management and prevention roles towards MDR-TB. Evidence shows that community TB prevention is one of the prominent interventions that study participants would expect in DOTS provision as community is the closest source of information regarding the patients [ 31 , 32 ].
Similarly, all participants had pointed out the importance of support from a health system directly or indirectly influence their engagement in the prevention, diagnosis, treatment, and management of MDR-TB. Researches indicated that health system supports are enabling factors for healthcare providers in decision making regarding TB/MDR-TB prevention and treatment [ 33 ]. This problem is documented by the study done in Ethiopia [ 22 ]. In addition, support from colleagues and other stakeholders was also a felt need to engage in MDR-TB which was supported by the World Health Organization guideline which put engagement in preventing MDR-TB and providing patients centered care needs collaborative endeavor among healthcare providers, patients, and other stakeholders [ 17 ].
Participants showed that there were poor linkage among/within DOTS providers working in health post (extension workers), health centers, hospitals and MDRTB treatment initiation centers. Our finding is consistent with a research in South Africa which shows poor health care attitude is linked to poor treatment adherence [ 34 ]. Our study implies the need for further familiarization especially on clinical programmatic management of drug resistant tuberculosis. Moreover, program managers need to follow health professionals’ engagement approaches recommended by the World Health Organization: End TB strategy [ 17 ].
Limitations of the study
There are some limitations that must be explicitly acknowledged. First, participants from private health facilities were very few, which might have restricted the acquisition and incorporation of perspectives from health care providers from private health care facilities. Second, healthcare providers’ engagement was not measured from patient side given that factors for engagement may differ from what has been said by the healthcare provides. Third, power relationships especially among focus group discussant in MDR-TB treatment initiation centers might have influenced open disclosures of some sensitive issues.
The study showed how healthcare provider’s engagement in MDR-TB management and prevention was influenced. Accordingly, patient’s underlying causes, seeking support, perceived occupational exposure, healthcare provider’s incompetence and health facilities poor linkage were identified from the analysis. Weak community TB prevention efforts, poor health system support and support from colleagues, health care providers’ incompetence and health facilities poor linkage were among identified factors influencing engagement in MDR – TB prevention and management. Therefore, measures need to be in place that avert the observed obstacles to health professionals’ engagement including further quantitative studies to determine the effects of the identified reasons and potential factors in their engagement status.
Furthermore, our findings pointed out the need for additional training of service providers, particularly in clinical programmatic management of drug-resistant tuberculosis. Besides, program managers must adhere to the World Health Organization’s recommendations for health professional engagement. Higher officials in the health sector needs to strengthen the linkage between health facilities and service providers at different levels. Community awareness creation to avoid fear of discrimination including provision of support for those with MDR-TB is expected from health experts through implementation of social behavioral change communication activities.
Abbreviations
Directly observed treatment short-course
Drug susceptible tuberculosis
Millennium development goals
Multidrug resistant tuberculosis
Sustainable development goals
Tuberculosis
Treatment initiation center
World Health Organization
Extensively drug resistant TB
WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO; Geneva, Switherland. 2014.
WHO. Implementing the end TB Strategy: The Essentials. WHO, Geneva. Switherland; 2015.
Eshetie S, Gizaw M, Dagnew M, Kumera G, Woldie H, Fikadu A. et.al. Multidrug-resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis Ethiopia: efforts to expand diagnostic services, treatment and care. BMC Antimicrobial Resist Infect Control. 2014;3:31.
Biadglegne F, Sack U, Rodloff AC. Multidrug-resistant tuberculosis in Ethiopia: efforts to expand diagnostic services, treatment and care. BMC Antimicro Resist Infect Control. 2014;3:31.
Lema NA, Majigo M, Mbelele PM, Abade AMIM. Risk factors associated with multidrug resistant tuberculosis among patients referred to Kibong’oto Infectious Disease Hospital in northern Tanzania. Tanzania J Health Res. 2016;18:4.
Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S. G. K. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15:461.
Selamawit H, Girmay M, Belaineh G, Muluken M, Alemayehu M, et al. PS. Determinants of multidrug-resistant tuberculosis in patients who underwent first-line treatment in Addis Ababa: a case control study. BMC Public Health 2013. 2013;13:782.
Google Scholar
Safwat TM, Elmasry AA, Mohamed A. Prevalence of multi-drug resistant tuberculosis in abbassia Chest hospital from july 2006 to december 2009. Egyptian J Bronchol. 2011;5(2):124–30.
World Health Organization. WHO Global tuberculosis report 2015. Geneva: WHO. 2015.
Rifat M, Hall J, Oldmeadow C, Husain A, Hinderaker SG. AH. M. Factors related to previous tuberculosis treatment of patients with multidrug resistant tuberculosis in Bangladesh. BMJ Open. 2015;5.
Gupta KB, Gupta R, Atreja A, Verma MSV. Tuberculosis and nutrition. Lung India: Offic Organ of Indian Chest Soc. 2009;26(1):9–16. https://doi.org/10.4103/0970-2113.45198 . 2009.
World Health Organization. WHO Global tuberculosis report 2018. Geneva: WHO. 2018.
Deus L, Willy S, Kenneth M, George WK, Frank JCMJ. et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health 2015;15.
Federal Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, TB/HIV and Leprosy in Ethiopia. Fifth Edition. Addis Ababa. March, 2013.
FDRE/MOH. Guidelines on programmatic management of drug resistant tuberculosis in Ethiopia. 2ND ED.2014 ADDIS ABABA. 2014.
CSIS. Center for Strategic and International Studies. As Ethiopia moves toward tuberculosis elimination, success requires higher investment: A report of CSIS global health policy center. CSIS, Washinton DC, America. 2016.
World Health organization. Framework for the engagement of all health care providers in the management of drug resistant tuberculosis. Geneva; Switherland. WHO 2015.
Vries G, Tsolova S, Anderson LF, Gebhard AC, Helda E, Hollo V. Health system factors influencing management of multidrug-resistant tuberculosis in four European Union countries - learning from country experiences. BMC Public Health. 2017;17:334.
Isara AR, Akpodiete A. Concerns about the knowledge and attitude of multidrug resistant tuberculosis among health care workers and patients in Delta State, Nigeria. Nigerian J Clin Pract. 2015;18:5.
Luka MI, Idris SH, Patrick N, Ndadilnasiya EW, Moses OA, Phillip P., et al. Health care workers’ knowledge and attitude towards TB patients under Direct Observation of Treatment in Plateau state Nigeria, 2011. Pan Afr Med J. 2014;18 (Supp1):8.
Malangu N, Adebanjo OD. Knowledge and practices about multidrug-resistant tuberculosis amongst healthcare workers in Maseru. Afr J Prm Health Care Fam Med. 2015;7:1.
Molla Y, Jerene D, Jema I, Nigussie G, Kebede T, Kassie Y. et.al. The experience of scaling up a decentralized, ambulatory model of care for management of multidrug-resistant tuberculosis in two regions of Ethiopia. J Clinic Tubercul Other Mycobacter Dis. 2017;7:28–33.
Merza MA, Farnia P, Tabarsi P, Khazampour M, Masjedi MR. AA. V. Anti-tuberculosis drug resistance and associated risk factors in a tertiary level TB centre in Iran: a retrospective analysis. J Infect Dev Ctries. 2011;5(7):511–19.
Gebremariam MK, Bjune GA. FJ. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC Public Health. 2010;10:651.
Suri AGK. S. C. Voices from the Field: Perspectives from Community Health Workers on Health Care Delivery in Rural KwaZulu-Natal, South Africa. J Infect Dis. 2007;196:S505–11.
Deshmukh RD, Dhande DJ, Sachdeva KS, Sreenivas AN, Kumar AMV. M. P. Social support a key factor for adherence to multidrug-resistant tuberculosis treatment. Indian J Tubercul. 2017.
Samuel B, Volkmann T, Cornelius S, Mukhopadhay S, Mitra K, Kumar AM., et al. Relationship between Nutritional Support and Tuberculosis Treatment Outcomes in West Bengal, India. J Tuberc Res. 2016;4(4):213–19.
NC. E. The making of a public health problem: multi-drug resistant tuberculosis in India. Health Policy Plan 2013;28:375–85.
Gler MT, Podewils LJ, Munez N, Galipot M, Quelapio MID, Tupasi TE. Impact of patient and program factors on default during treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16(7):955–60. https://doi.org/10.5588/ijtld.11.0502 .
Article CAS Google Scholar
Horter S, Stringer B, Greig J, Amangeldiev A, Tillashaikhov MN, Parpieva N., et al. Where there is hope: a qualitative study examining patients’ adherence to multidrug resistant tuberculosis treatment in Karakalpakstan, Uzbekistan. BMC Infect Dis. 2016;16:362.
Naidoo P, Niekerk MV, Toit ED, Beyers N, Leon N. Pathways to multidrug-resistant tuberculosis diagnosis and treatment initiation: a qualitative comparison of patients’ experiences in the era of rapid molecular diagnostic tests. BMC Health Serv Res. 2015;15:488.
WHO. ENGAGE-TB. Training of community health workers and community volunteers. Integrating community-based tuberculosis activities into the work of nongovernmental and other civil society organizations. 2015.
Gerard de Vries S, Tsolova FL, Anderson AC, Gebhard E, Heldal et al. Health system factors influencing management of multidrug-resistant tuberculosis in four European Union countries - learning from country experiences BMC Public Health. 2017;17:334. https://doi.org/10.1186/s12889-017-4216-9 .
Finlay A, Lancaster JHT, Weyer K, Miranda A. M. W. Patient- and Provider level risk factors associated with default from tuberculosis treatment, South Africa: a case control study. BMC Publich Health. 2012;12:56.
Download references
Acknowledgements
We would like to acknowledge Hosanna College of Health Sciences Research and community service directorate for providing us the opportunity and necessary fund to conduct this research. Our appreciation also goes to heads of various health centers, hospitals, district health and Hadiya Zone Health office for unreserved cooperation throughout data collection.
The authors declare that this study received funding from Hosanna College of Health Sciences. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author information
Bereket Aberham Lajore & Menen Ayele
Present address: Hossana College of Health Sciences, Hosanna, SNNPR, Ethiopia
Yitagesu Habtu Aweke
Present address: College of Health Sciences, School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia
Samuel Yohannes Ayanto
Present address: College of Health Sciences, Institute of Public Health, Department of -Population and Family Health, Jimma University, Jimma, Ethiopia
Bereket Aberham Lajore, Yitagesu Habtu Aweke and Samuel Yohannes Ayanto contributed equally to this work.
Authors and Affiliations
Department of Family Health, Hossana College of health sciences, Hossana, Ethiopia
Bereket Aberham Lajore
Department of Health informatics, Hossana College of Health Sciences, Hossana, Ethiopia
Department of Midwifery, Hossana College of Health Sciences, Hossana, Ethiopia
Department of Clinical Nursing, Hossana College of Health Sciences, Hossana, Ethiopia
Menen Ayele
You can also search for this author in PubMed Google Scholar
Contributions
Bereket Aberham Lajore, Yitagesu Habtu Aweke, and Samuel Yohannes Ayanto conceived the idea and wrote the proposal, participated in data management, analyzed the data and drafted the paper and revised the analysis and subsequent draft of the paper. Menen Ayele revised and approved the proposal, revised the analysis and subsequent draft of the paper. Yitagesu Habtu and Bereket Aberham Lajore wrote the main manuscript text and prepared all tables. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Bereket Aberham Lajore .
Ethics declarations
All methods and report contents were performed in accordance with the standards for reporting qualitative research.
Ethics approval and consent to participate
Ethical approval was obtained from Institutional review board [IRB] of Hossana College of health sciences after reviewing the protocol for ethical issues and provided a formal letter of permission to concerned bodies in the health system. Accordingly, permission to conduct this study was granted by respective health facilities in Hadiya zone. Confidentiality of the information was assured and participants’ autonomy not to participate or to opt-out at any stage of the interview were addressed. Finally, informed consent was obtained from the study participants after detailed information.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lajore, B.A., Aweke, Y.H., Ayanto, S.Y. et al. Exploring health care providers’ engagement in prevention and management of multidrug resistant Tuberculosis and its factors in Hadiya Zone health care facilities: qualitative study. BMC Health Serv Res 24 , 542 (2024). https://doi.org/10.1186/s12913-024-10911-6
Download citation
Received : 27 February 2023
Accepted : 27 March 2024
Published : 27 April 2024
DOI : https://doi.org/10.1186/s12913-024-10911-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Healthcare providers
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Insights & Events
About McDermott
- Firm Overview
- Vision & Values
- Our Approach
- Justice, Diversity, Equity & Inclusion
- Pro Bono & Community Service
ICO Publishes New Transparency Guidance for Health and Social Care Sector
- ICO Publishes New Transparency Guidance for Health and Socia...
Healthcare sector organisations are increasingly deploying new technologies that use large amounts of personal information to support both direct care and secondary purposes, such as planning and research. Although these data-driven solutions offer many benefits to the public, often people are reluctant to agree to organisations sharing and using their information, especially where it is not clear how it will be used.
Following a consultation last year, the Information Commissioner’s Office (ICO) has published transparency guidance for organisations which deliver health and social care services or process health and social care information, including for secondary purposes such as research and planning. The guidance incorporates feedback from health and social care organisations across the UK.
What Are the Transparency-Related Requirements Under the UK GDPR?
Under the UK GDPR, organisations must (i) operate transparently (the transparency principle) and (ii) provide specific privacy information to individuals (the right to be informed). Since the transparency principle is less prescriptive than the right to be informed, the guidance correspondingly distinguishes between “privacy information” (which is required under Articles 13 and 14 of the UK GDPR) and “transparency information” which should be provided as a matter of best practice.
What Are Some of the Recommendations in the New Guidance?
The guidance states that to increase transparency and trust, in addition to providing the privacy information required by Articles 13 and 14, organisations should consider providing extra information, such as:
- confirmation of what the organisation will not do with people’s information;
- lists of information disclosed to researchers and the reasoning behind this; and
- information that challenges or proactively deals with contentious issues, for example when addressing misconceptions relating to third-party access to sensitive health information.
Further, given the co-existence of consents for different purposes in the healthcare and research sector (for example for data protection, common law duty of confidentiality, clinical trial participation and other purposes), the guidance emphasizes that it is important to set out the position in respect of consents and choice clearly. For example, an organisation must make clear for what purposes they are using consent. Is it as a lawful basis to process personal information, or for other purposes, such as consent to research participation?
The guidance also encourages organisations to consult with the public throughout the process of designing or updating transparency information, as it will improve the organisation’s understanding of data subjects’ needs, concerns and expectations, and to consider the most effective means to communicate transparency information.
What Are the Next Steps?
Although some sections of the guidance are written with the NHS organisations in mind, the guidance is highly relevant to life sciences and research organisations, and those providing services to NHS organisations. The next steps for organisations to consider include the following:
- Review what personal information they use and plan to use and why.
- Identify transparency issues and make improvements in response. For example, do they have any additional transparency material beyond privacy notice? Does it contain more than what is strictly required?
- Design an evaluation strategy to ascertain whether their communications raised awareness and understanding of their practice by patients/data subjects.
Stay Connected
Sharon lamb.
Partner | London
Michaela Novakova
Associate | London
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Future Healthc J
- v.5(3); 2018 Oct

Using primary care data for health research in England – an overview
Stephen h bradley.
A York Street Practice, Leeds, UK
B Academic Unit of Primary Care, University of Leeds, Leeds, UK
Neil R Lawrence
C NHS Digital, UK
Paul Carder
D West Yorkshire Research & Development, Bradford, UK
In contrast to secondary care, where handwritten records remain widespread, electronic patient records have long been a key feature of UK general practice. By 1996, 96% of general practices were computerised and now almost every primary care consultation in the UK is recorded on a computerised clinical system. Consequently, we now have a vast repository of patient health data that spans decades, which could be used to address a range of important research questions. Unfortunately, accessing primary care data for health researchers can be a burdensome, confusing and time-consuming process. Understanding the way in which primary care data are recorded and ‘coded’ is not intuitive to those unfamiliar with general practice. The requirements of information governance mean that some data, or data presented in particular ways, are not available at all. This review provides a practical overview of the types of data recorded in primary care, the bodies responsible for them and how they can be accessed.
Introduction
Electronic patient records (EPRs) have long been a key feature of English general practice. By 1996, 96% of general practices were computerised 1 and now almost every primary care consultation in the UK is recorded on a computerised clinical system. This has resulted in a vast repository of patient health data that spans decades, which can be used to address a range of important research questions.
The process of accessing primary care data for health researchers can be burdensome, confusing and time-consuming. Understanding the way in which primary care data are recorded and ‘coded’ is not intuitive to those unfamiliar with general practice. Legislation around the Data Protection Act and the requirements this produces for information governance effectively results in some data being unavailable to researchers. While significant barriers remain to accessing primary care data for research, many important research findings have been accomplished through utilising this data, some examples of significance are given in Box 1. Here, we present a practical overview of the types of data recorded in primary care (simplified representation in Fig Fig1), 1 ), the bodies responsible for controlling them and the methods available to access them (Fig (Fig2 2 and Box 2).

Pathway of data from collection through to application in research. Please note: data may only flow between organisations with a legal instruction from the data controller to the data processor. a Research databases (eg Clinical Practice Research Datalink).
CCG = Clinical Commissioning Group; CQRS = Calculating Quality Reporting Service; CSU = Commissioning Support Unit; DSCROs = Data services for commissioners regional offices; EBM DL = Evidence-based Medicine DataLab; NHSBSA = NHS Business Services Authority; NHS LA = NHS England local area team

Flow chart to direct researchers towards appropriate sources of data for research in general practice. a Research databases include (not exhaustive): Clinical Practice Research Datalink, QResearch and Research One.
FOI = freedom of information; CCG = Clinical Commissioning Group; CSU = Commissioning Support Unit
Data sources in primary care
Most GPs use one of four commercially provided software platforms to manage their EPRs. These are EMISWeb (EMIS), SystmOne (TPP/Pheonix partnership), Vision (In Practice Systems) and Evolution (Microtest). 12
GPs generally enter narrative information regarding the consultation, in a free-text format. In some systems, this might be entered in a defined format, for example with fields for history, examination, diagnosis and plan. Data entered as ‘free text’, for example a medical diagnosis entered as text with no corresponding code, can often only be retrieved by inspection of the individual patient record, although free-text search functionality is a feature of some systems.
Free text is navigable for direct patient care, but inappropriate for large-scale data analysis. Clinical coding is the conversion of discrete items of information within the narrative mapped to a standardised thesaurus of clinical terms. Until recently ‘Read’ has been the system used but this is being succeeded by SNOMED CT which has been introduced across general practice in phases since April 2018. Clinicians or administrative staff can use this system to improve the patient record and are incentivised to do so through payments linked to coded entries. SNOMED CT is more comprehensive than diagnostic coding systems, incorporating items such as symptoms, procedures, body structures and so on. However, diagnoses in SNOMED CT map directly to those found in the International Classification of Diseases (ICD). The aim in the NHS is for all coding across the entire health system in England to use SNOMED CT by 2020. 13
It is important to recognise that the practice of coding is highly variable among clinicians. 14 It is not possible to assume that all diagnoses are coded accurately, or at all: patients frequently attend with multiple presentations; a clinician might not be able to find a sufficiently appropriate code for the presentation; codes might be entered in error; or the clinician might rely on free-text data entry only.
Quality of data recording
The quality of coding is related to the presence of incentives for accurate recording. These can be financial (eg the Quality Outputs Framework [QOF]) or safety and quality driven (eg a diagnosis of type 1 diabetes mellitus). Payment regimens, such as QOF, have been criticised for creating ‘perverse incentives’ for the ways in which data are coded and the effect this has on clinician behaviour. 15 This can distort the numbers of patients with recorded diagnoses, as manifested by the fluctuating numbers of patients diagnosed with depression, proven to be influenced by the existence of a financial incentive. 16
Many primary care computer systems facilitate the entry of discrete values where appropriate, such as gender, weight, height, blood pressure and peak expiratory flow. The ability to perform searches based on these values depends on them having been entered in the appropriate format.
Data sharing
Sharing of anonymised data for research, for example with Clinical Practice Research Datalink (CPRD) or with the research divisions of clinical systems, is determined via a voluntary ‘opt in’ for each practice. The decision to opt in or out of data sharing is usually the responsibility of the Caldicott guardian or information governance-responsible officer of each GP practice. GP practices that have opted in to data sharing can still exercise judgement to exclude individual patient records. However, many organisations lack the capacity to decide whether to include individual patient records.
Patients maintain the right to opt out of their data being shared for purposes other than direct care, regardless of whether their practice has opted in. Opt-outs currently take two forms, and prevent the use of data beyond direct patient care. A ‘type 1 opt-out’ prevents information that identifies a patient from leaving the GP practice (except when required by law for public health reasons). A ‘type 2 opt-out’ prevents information that identifies a patient from leaving the central repository in NHS Digital (formerly the Health and Social Care Information Centre; HSCIC). 17 Following Dame Fiona Caldicott’s review of data security, consent and opt-outs, 18 an online national system for patients to opt out of sharing of their data was launched in May 2018, with the aim of giving patients a more convenient way of expressing their preferences. 19
Legislation
All data processing must be conducted in line with the Data Protection Act 1998, the incoming General Data Protection Regulation (GDPR) and all other relevant legislation. However, section 251 of the NHS Act 2006 allows ‘important’ medical research to make use of identifiable patient information without explicit patient consent, 20 while adhering to data protection principles. Application for Section 251 approval for research purposes is made through the Confidentiality Advisory Group (CAG), which is administered by the Health Research Authority (HRA). Section 251 approval grants access to data for valued research where obtaining patient consent would be impractical. The HRA provides comprehensive guidelines, and maintains a register of approved CAG applications, which is accessible online. 21
Prescribing data
In England, primary care drugs are prescribed almost entirely through practice IT systems. Handwritten prescriptions account for a small minority, often only used in the case of system failure and home visits. This produces high-quality prescribing data from primary care. Information about the volumes and types of drug prescribed at the level of individual GP practices has been made available through NHS Digital, 22 while a search interface for these data has been created by EBM Datalab’s (University of Oxford) OpenPrescribing projects. 23 More individualised prescribing information is recorded and can be retrieved through the individual primary care clinical systems. Prescribing information is also available through the NHS Business Services Authority (NHS BSA). 22
Processing of data
Primary care clinical systems providers.
The data entered through the four dominant primary care clinical systems are processed under instruction by each corresponding contractor. Procurement of the systems are managed by NHS digital, with each practice choosing which to use through ‘GP Systems of Choice’. 24 Some contractors have developed research divisions, including QResearch (the University of Nottingham and EMIS) and ResearchOne (SystmOne), which engage with clinical researchers.
Distributions of practices using each system vary heterogeneously across England, with more than one system commonly in use within each region. Therefore, it is important for researchers to determine whether patterns of usage for each individual system correspond well with the population under study if the method of access is to be through single contractors. In West Yorkshire, for example, where the market is dominated by SystmOne and EMISWeb, researchers wishing to achieve comprehensive access to patient data through clinical systems would need to engage with both QResearch and ResearchOne.
Linking data sets
Linking different data sets from multiple sources that relate to an individual patient offers great opportunities for clinical research. For instance, linking primary care records documenting different diagnoses to secondary care pathology reports could reveal possible associations and insights into how certain diseases could be diagnosed earlier. For linking to take place, data cannot be anonymised, but they could be pseudonymised to protect patient-identifiable data (PID). In this process, all identifying fields within a patient record are replaced with anonymous identifiers. A reidentification key that unlocks pseudonymisation to maintain an access pathway to patient-level data can then be restricted to designated persons, in defined organisations. This allows the reidentification of a patient in special circumstances if there is a safety issue or direct care of the patient would benefit from the insight gained from the research. The NHS Business Service Authority’s pseudonymisation and anonymisation of data policy outlines definitions of these terms as well as a series of actions that must be taken to pseudonymise data. 25
Despite adequate control of reidentification keys, linking of data still has the potential to undermine the pseudonymisation of data and could lead to individuals becoming identifiable. Reidentification is particularly problematic when dealing with small data sets or rare diseases. For this reason, interrogation that generates numeric data relating to fewer than five patients cannot be released, although it can be expressed as percentages. This can have important consequences for the interpretation of data that are aggregated from several sources, each representing small numbers.
The failure of the NHS England Care.Data programme was a significant setback to ambitious large-scale data sharing. 26,27 This has prompted organisations to exercise significant caution in consenting to the use of data in the creation of linked data sets.
Research databases
The CPRD is a large anonymised primary care database comprising data that includes 6.9% of the UK population and is broadly representative in terms of age, sex and ethnicity. 28 Developed from the General Practice Research Database (GPRD) that collated patient information from over 500 GP practices, the CPRD now links pseudonymised general practice data to national data sets and data from other health providers. 29 The CPRD is based at the Medicines and Healthcare Products Regulatory Agency (MHRA), is run jointly with the National Institute of Health Research (NIHR), and has been used in over 1,700 published studies. 30
Researchers can apply for access to CPRD data that include demographics, symptoms and signs, tests, diagnoses, prescriptions, lifestyle information, and referrals to secondary care. 10 The cost to researchers for data extracts from CPRD can be considerable but can be reduced if accessed through organisations that have institutional membership.
The Health Improvement Network (THIN), based at University College London, contains data from over 550 GP practices across the UK. 31 The Royal College of General Practitioners’ Research and Surveillance Centre (RSC) collects data from around 200 practices. 32 Data are extracted twice weekly and have proved particularly fruitful for monitoring the spread of disease such as influenza. Historic data from the past 10 years are stored and managed by the University of Surrey.
As with other sources, the utility of data from research databases can be limited by the original quality and consistency of data entry by clinicians and administrators in individual practices. The size of data sets will also be affected by data-sharing opt-outs as exercised by GP practices on behalf of their patients or by individual patients themselves.
Accessing primary care data for research
Individual general practices and gps.
The HRA has determined that each individual general practice is considered a distinct entity with the ability to enter into data-sharing agreements. 33 Practices have nominated Caldicott guardians or information governance-responsible officers whose role involves safeguarding patient data. The data controller can be either an individual or the GP practice as a legal entity. Collaboration can then occur by researchers acting as data processors and agreeing a data-sharing agreement directly with the data controller. Once there is an instruction from the data controller to the data processor, data can flow in compliance with the Data Protection Act. 34 GP practices can also access research and development support from their Clinical Commissioning Group (CCG) or Commissioning Support Unit (CSU), but a similar direction must always be in place for data to flow legally.
Historically, GPs have been pioneers in establishing approaches to information management because of being under moral, professional and legal obligation to protect their patients’ privacy. Revelations around the use of NHS data by the Home Office has highlighted ways in which information sharing could undermine patients’ access to healthcare. 35
CCGs and CSUs
Following the Health and Social Care Act 2012, administration of primary care was divided into CCGs with regional-level support functions offered by CSUs. This has had important consequences for information governance, because it has subsequently been considered that CCGs should not be allowed to access PID, unless the CCG attains ‘Accredited Safe Haven’ (ASH) status. 36 ASH status is obtained from NHS Digital through Data Access Request Service (DARS), which must stipulate the data requested, why they are required and how they will be processed. Applications must then be approved by Independent Group Advising on the Release of Data (IGARD) and, in practice, take several months. Maintaining ASH status is subject to audit and entails maintaining necessary information governance. These governance procedures create significant barriers to accessing data directly from CCGs.
Receipt and processing of PID are managed by CSUs in collaboration with NHS Digital. By setting up ‘Data Services for Commissioners Regional Offices’ inside NHS Digital, into which staff from CSUs are seconded, fair processing is maintained by only staff within NHS Digital having access to PID. 37
Following the NHS England lead provider framework (2015), 38 private-sector organisations have been allowed to tender to provide CSU service for CCGs, provided they have ASH status. CSUs can charge a service fee for each episode of access to data. The institution of fees for access to data could become more prevalent as CSUs are tendered to private-sector providers. It is vital in maintaining public trust that such transactions are not perceived as ‘selling’ personal data and that charges are understood to be for the service of transacting data rather than for the data themselves.
NHS Digital
NHS Digital, which succeeded the HSCIC, is a non-departmental body of the Department of Health, and acts as the provider and overseer of data and IT systems for the NHS in England. Specific information for defined purposes is extracted automatically from GP practices to the systems of NHS Digital over a specific period of time, through the General Practice Extraction Service (GPES). 39 Data are in turn processed and presented by the Calculating Quality Reporting Service (CQRS). However, some mandated data submissions are not automated through GPES, and require manual entry by GP practice staff onto the CQRS. Data within the CQRS are then used to calculate payments to GP practices.
To access data held centrally at NHS Digital, one must submit a DARS application and have this approved by IGARD. The charges for such requests can be found on the NHS Digital website. 11 In seeking central approval to link data sets, it is crucial to demonstrate that linkage is necessary to address the research question. Before formally applying for data linkage through DARS or via CAG, it is good practice to have received advice from information governance and research and development leads within one’s own organisation, and to have constructed a privacy impact assessment document.
Access to data through NHS Digital has been perceived as slow and costly, 40 with detailed attention to legal basis, information governance and fair processing. In our experience, many researchers opt to develop separate data-sharing agreements with providers outside of NHS Digital to avoid the DARS process. The DARS team at NHS Digital offers support through email content and webinars for researchers, and is working to streamline the data-access process. 11 Several other sources of data derived from or pertinent to primary care are available in addition to those described in this article. Some examples of these are listed in Box 3.
Conclusions
To earn the trust of the public and the confidence of the doctors to whom they have entrusted their information, research using patient data must proceed in an open and transparent manner, demonstrating clear benefits for patients.
For researchers to utilise primary care data effectively, it is vital to understand the ways in which data are entered in practice and the limitations imposed by variation in the consistency and quality of data entry. Similarly, an understanding of the strengths and limitations of the different repositories of primary care data and what can be expected from each will benefit study design.
Currently, the ability of researchers to access primary care data for research frequently requires navigating burdensome and complex approval processes. Linked data sets could generate a rich resource for researchers to address important questions in clinical research, particularly between primary and secondary care. Approval to achieve this through separate data-sharing agreements between each body responsible for recording or processing data is challenging, and highlights the value of the centrally held data repositories within NHS Digital.
Primary care data represent a rich resource for researchers that can help to create a health system that learns from everyone who is treated. Such a ‘learning health system’ can help to address a range of clinical and research challenges. 41–43 Navigating the complex infrastructure and information governance arrangements represents a significant challenge and can consume substantial additional time and resources. We recommend that researchers make contact early with relevant bodies and set aside sufficient time to navigate these processes. These resource costs should be included in grant applications. Researchers should also understand the pattern of usage of the different primary care clinical systems within the areas included in the study.
The difficulty in accessing primary data for research is not only a result of legitimate concerns around data security, but also a consequence of the fragmenting of information governance oversight following the Health and Social Care Act 2012. To unleash the potential of primary care, and particularly linked data sets, it might be necessary to rationalise and harmonise information governance procedures while adhering to the principles of data security and patient consent.
Author contributions SB prepared the manuscript based on an oral presentation prepared by PC. NL added further content, including the two figures and text boxes and made other corrections to the text.
Examples of studies based on primary care data
Example research study proposals and appropriate route of data access guided by flow chart (Fig (Fig2 2 )
Additional sources of data pertinent to primary care studies
Acknowledgments
The authors would like to thank Dr Peter Short, Dr Tom Foley, Ms Rosemary Dewey and Professor Richard Neal for their information and advice when writing this paper.

IMAGES
VIDEO
COMMENTS
Secondary research is a very common research method, used in lieu of collecting your own primary data. It is often used in research designs or as a way to start your research process if you plan to conduct primary research later on. Since it is often inexpensive or free to access, secondary research is a low-stakes way to determine if further ...
Secondary analysis of data collected by another researcher for a different purpose, or SDA, is increasing in the medical and social sciences. This is not surprising, given the immense body of health care-related research performed worldwide and the potential beneficial clinical implications of the timely expansion of primary research (Johnston, 2014; Tripathy, 2013).
Additionally, the use of secondary data, particularly open data, is progressively preferred to increase efficiency and gain geographical breadth. ... the self-resistance to accepting other research methods and interrogating the definitions of "truth" or "fact" proved to be an obstacle to reaching the stage of understanding social and ...
Introduction. Data about health is the fundamental base on which evidence-based healthcare is constructed and underpins progress and innovation in health sciences and healthcare towards improved patient outcomes. 1-3 Traditionally, however, competitive research practices have discouraged data sharing, 4 and researchers may withhold research datasets they have generated in order to protect ...
Significance of Qualitative Research. The qualitative method of inquiry examines the 'how' and 'why' of decision making, rather than the 'when,' 'what,' and 'where.'[] Unlike quantitative methods, the objective of qualitative inquiry is to explore, narrate, and explain the phenomena and make sense of the complex reality.Health interventions, explanatory health models, and medical-social ...
The earliest reference to the use of secondary data analysis in the nursing literature can be found as far back as the 1980's, when Polit & Hungler (1983), in the second edition of their classic nursing research methods textbook, discussed this emerging approach to analysis.At that time, this method was rarely used by nursing researchers.
Evidence-based healthcare relies on health data from diverse sources to inform decision-making across different domains, including disease prevention, aetiology, diagnostics, therapeutics and prognosis. Increasing volumes of highly granular data provide opportunities to leverage the evidence base, with growing recognition that health data are highly sensitive and onward research use may create ...
In both sectors, these are important drivers to facilitate and promote research as being an integral part of routine clinical care. The NIHR CRN, through its 15 constituent Local Clinical Research Networks (LCRNs), engages widely with health and social care with both secondary and primary care colleagues. In 2022-2023, 100% of acute Trusts ...
As secondary qualitative data analysis continues to evolve, more methodological guidance is needed. This book outlines three approaches to secondary data analysis and addresses the key issues that researchers need to wrestle with, such as ethical considerations, voice, and representation. Intellectual and interpretive hazards that can ...
ABSTRACT. This is the first textbook to show how research using a range of qualitative and quantitative methods relates to improving health and social care practice. The book shows how different research approaches are undertaken in practice and the challenges and strengths of different methodologies, thus facilitating students to make informed ...
Qualitative research is particularly useful in guiding social care integration as it can shed light on the patient or caregiver experience of participating in social care interventions, barriers to getting help that should be addressed, and appropriate next steps from the perspective of those directly impacted.However, traditional qualitative ...
Evidence from trials without randomisation or from single before-and-after studies, cohort, time series or matched case-controlled studies or observational studies. Evidence from well-designed descriptive studies or qualitative research. Opinions from expert committees or formal consensus methods. Expert opinion.
Health and Social Care Important concepts in research Primary and secondary sources and data The sources used and the data or information obtained are described as either primary or secondary. Primary data is information which has been generated by the researcher as a result of research which he or she has personally undertaken (either alone or
Background Integrated care has the potential to ease the increasing pressures faced by health and social care systems, however, challenges around measuring the benefits for providers, patients, and service users remain. This paper explores stakeholders' views on the benefits of integrated care and approaches to measuring the integration of health and social care. Methods Twenty-five semi ...
1 INTRODUCTION. Over the past decade, an increased focus on the way that integrated health and social care (IHSC) services are delivered and a growing demand for improved service user experience have driven forward improvements in worldwide health and social care (HSC; World Health Organization, 2016a).Person-centred IHSC systems aim to follow principles of participatory care and governance ...
Annex 2 - Public health and social care research. Public health research investigates issues that impact at a population rather than an individual level. This can be done within the NHS with system-level studies, such as secondary prevention of cardiovascular disease and examining the impact on health inequalities of changes to the NHS ...
This guide is designed to support health and social care researchers and practitioners to integrate qualitative research into the evidence base of health and social care research. Qualitative research designs are diverse and each design has a different focus that will inform the approach undertaken and the results that are generated. The aim is to move beyond the "what" of qualitative ...
Secondary Research Methods Secondary Research Methods Definition. Secondary research methods involve using data that has already been collected by others. This type of research is useful for gaining a broader understanding of a topic. It can validate primary research findings and provide context for new research. Existing Statistical Data
Research is vital in providing the evidence we need to transform services and improve outcomes, such as in developing new models of care, redesigning urgent and emergency care, strengthening primary care and transforming mental health and cancer services. Research is the attempt to derive generalisable or transferable new knowledge.
Despite a long history in quantitative research, it is only recently that enthusiasm for secondary analysis of qualitative data has gained momentum across health and social science disciplines. Given that researchers have long known the inordinate amount of time and energy invested in conducting qualitative research, the appeal of secondary analysis of qualitative data is clear. Involving the ...
The Health and Social Care Research: Methods and Methodology level 7 (Masters level) module is available at both 15 (UZWSPX-15-M) or 20 (UZWRGQ-20-M) credits. There is also a 15 (UZWSRV-15-M) or 20 (UZWYGP-20-M) credit online learning module option available. ... Justify the appropriate uses of primary and secondary sources of data.
Activity indicators measure how frequently an event happens, such as the rate of influenza immunisation. In contrast, quality indicators infer a judgment about the quality of care provided, 6 and performance indicators 8 are statistical devices for monitoring performance (such as use of resources) without any necessary inference about quality. . Indicators do not provide definitive answers but ...
Engagement of healthcare providers is one of the World Health Organization strategies devised for prevention and provision of patient centered care for multidrug resistant tuberculosis. The need for current research question rose because of the gaps in evidence on health professional's engagement and its factors in multidrug resistant tuberculosis service delivery as per the protocol in the ...
Healthcare sector organisations are increasingly deploying new technologies that use large amounts of personal information to support both direct care and secondary purposes, such as planning and research. Although these data-driven solutions offer many benefits to the public, often people are reluctant to agree to organisations sharing and using their information, especially where it is not ...
Electronic patient records (EPRs) have long been a key feature of English general practice. By 1996, 96% of general practices were computerised 1 and now almost every primary care consultation in the UK is recorded on a computerised clinical system. This has resulted in a vast repository of patient health data that spans decades, which can be ...