- Enroll & Pay
- KU School of Pharmacy
- Doctor of Pharmacy (Pharm.D.)
- Medicinal Chemistry
- Pharmacology & Toxicology
- Pharmacy Practice
- Neuroscience

Doctor of Philosophy (Ph.D.) in Pharmaceutical Chemistry
Developing new medicines and vaccines
Apply for Ph.D.
Program Overview
Standard ku graduate admission requirements —.
Students must meet all requirements for Graduate Admissions .
Prerequisites —
Previous degree requirement —.
B.S. or M.S. degree in chemistry, pharmacy, the biological sciences, material sciences, chemical engineering or related disciplines.
Grade Point Average (GPA) —
- The department does not have a minimum GPA for admissions, but it is important that potential applicants have shown mastery of their undergraduate coursework, especially those relevant to furthering their education in Pharmaceutical Chemistry.
- We view each applicant carefully and consider the combination of GPA (with emphasis on chemistry courses), GRE scores, letters of recommendation and the personal statement.
Graduate Record Exam (GRE) Scores —
- GRE scores are no longer required to be included with your application.
- If you have already taken the exam and would like to include your report with your application, please request your score report be sent directly to KU Graduate Admissions using the institution code: R6871
English Proficiency Requirements —
- Non-native English speakers must demonstrate proficiency in reading, writing and listening via English Proficiency Scores from the TOEFL, IELTS or PTE test.
- See KU's English Proficiency Requirements for detailed information, including minimum score requirements.
- Request that the testing agency send your official scores directly to KU (code: KU-6871).
Applicant Background —
Incoming students are expected to be competent in basic principles of physical/organic chemistry and mathematics. These requirements are typically satisfied with most degrees in the STEM disciplines.
Time to Complete —
- From the point a student joins the department until the time they defend their dissertation is approximately 5 - 6 years.
- The university confers degrees at the end of each semester. Therefore, it is possible that the thesis or dissertation defense may occur several months prior to the time the actual degree is conferred.
Departmental Graduate Handbook —
KU Pharmaceutical Chemistry Graduate Handbook 2021-22 (pdf)
Coursework and Research —
See Ph.D. - Coursework and Research for details about coursework and research requirements for the Ph.D. program
Seminar Presentations —
All graduate students are required to attend the weekly departmental seminar. Seminars consist of presentations by guest speakers, faculty members, and students. Typically, graduate students are required to present at least two departmental seminars during their time in the program. The seminar may be based on the progress achieved in their research or on a literature review of work related to their research.
Dissertation Defense —
- Each Ph.D. candidate is required to submit and defend a dissertation resulting from research of sufficient originality and quality for publication in peer-reviewed scientific journals.
- The research is conducted under the supervision and guidance from the student's advisor, with input from the dissertation committee as needed.
- The median time for students to complete their Ph.D. degree in the Department is 5.3 years.
Stipend, Tuition and Insurance —
Those accepted to the program will receive a competitive stipend, tuition, and basic health insurance.
Fellowships —
- The Department of Pharmaceutical Chemistry has been named as a Madison and Lila Self Graduate Fellowship Program partner at The University of Kansas.
- The program provides a generous stipend and tuition for four years of graduate study to outstanding students.
- Honors Fellowship - Awarded on a competitive basis to incoming graduate students throughout the university.
- Dissertation Fellowship - Awarded to outstanding students during their last year of doctoral study.
- Graduate Minority Opportunity Fund Fellowship - Awarded to outstanding ethnic minority students for work toward the doctoral degree.
- Takeru Higuchi Fellowship
- Siegfried Lindenbaum Scholarship
- Howard Rytting Fellowships
- Stella Family Predoctoral Fellowship
- Valentino Stella Students and Friends Predoctoral Fellowship
- Wanda Waugh Predoctoral Fellowship
- See Fellowships/Scholarships for more info.
Affiliated Programs —
While studying in the Department of Pharmaceutical Chemistry, students are eligible to participate in the following affiliated programs.
Takeru Higuchi Intersearch Program
- Students are also eligible to participate in the Takeru Higuchi Intersearch Program, which allows them to conduct a portion of their research at the Victorian College of Pharmacy, which is a part of Monash University located in Melbourne Australia.
- Ideally, the students select a surrogate advisor who specializes in an area of research outside that of the primary advisor, allowing for more breadth to their overall research project.
- The department is a full member of the Globalization of Pharmaceutical Education Network (GPEN) a professional organization that meets bi-annually at other member institutions.
- Senior students are typically selected to participate in these events.
- The GPEN meeting, now attended by 43 academic institutions across the world, was founded at KU in 1996 by Professor Ronald Borchardt.
- Participants in these programs have generally found the experiences to be extremely beneficial to their overall experience at KU.
- Learn more about GPEN
John Stobaugh Director of Graduate Studies [email protected] 785-864-3996
Graduate Student Application Michelle Huslig Program Coordinator [email protected] 785-864-4495
KU Graduate Admissions [email protected] 785-864-3140
PhD in Medicinal Chemistry
Application deadline for incoming class of 2024: December 16th, 2024 Prospective student visits (by invitation only): Late Feb 2025
The Department
We have 8 faculty running active research labs tackling health-related problems at the interface of chemistry and biology. Our department is internationally recognized for work on the mechanism and kinetics of xenobiotic metabolism by cytochrome P450s (CYPs) and other detoxification enzymes. CYPs metabolize most drugs used in the clinic, and their dysfunction is linked to harmful drug-drug interactions, inflammation, cancer, heart disease, and impaired neurodevelopment. Recently, our research has diversified into other areas of biochemistry, pharmaceutical chemistry, biophysics and chemical biology. Some faculty study therapeutic antibodies, peptides and other biologics, which are revolutionizing clinical practice. Other faculty study virology with the goal of developing better vaccines and treatments for diseases like HIV/AIDS and influenza, or neurodegeneration with the goal of improving the diagnosis and treatment of dementias like Alzheimer’s disease. Our faculty also develop new analytical techniques, such as mass spectrometry methods to characterize lipids, metabolites and glycoproteins more quickly and sensitively than ever before. Learn more about each lab’s research here .
The PhD Experience

Coursework requires students to become proficient in organic, medicinal and physical chemistry, pharmacology, biochemistry, and molecular biology. (See a typical Program of Study , and course descriptions in the course catalog .) The curriculum is adaptable to individual interests and needs, and most didactic coursework is completed in the first two years. For more information on the program please refer to our most recent Med Chem student handbook .
Professional development outside the laboratory and classroom is a major point of emphasis. Students build communication skills through regular presentations to their labs, and in departmental journal clubs and research seminars. Many students interested in biotech/pharma careers have benefited from our innovative industry mentorship and internship programs.
Financial Support
Incoming graduate students are generally supported by a research assistantship from the department for the first year of study, allowing students to dedicate their time to study and work in the lab. It currently covers tuition (excluding a $265 per quarter student fee) and an additional stipend of $3259 /month. In subsequent years, support is provided either by the department or by research or training grants. Outstanding applicants are considered for an ARCS Scholarship that provides an additional stipend of $7,500 for the first year and $5,000 for the next two years of graduate school. The research assistantship also provides health insurance at no charge for students; coverage is available for spouses and dependents for an additional fee. (You can find more information on the Graduate Appointee Insurance Program and other benefits through UW Human Resources .)
Career Opportunities
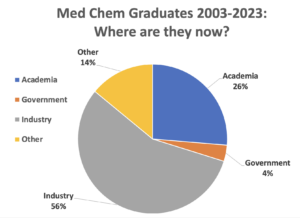
Our graduates also excel in academia. Recent graduates have done post-doctoral work at institutes such as Yale University, University of California San Francisco, University of California San Diego, Lawrence Berkeley National Lab, Queen Mary University of London, and Children’s Hospital Research Center. Subsequently, alums have gone on to tenure-track faculty positions at top-tier research universities, colleges of pharmacy, and liberal arts colleges.
More Application Info & FAQs

Pharmaceutical Chemistry, Ph.D.
Among Public National Universities, U.S. News & World Report
Featured in the Fiske Guide to Colleges
Nationally, Best Online Bachelor's Programs, U.S. News & World Report
Our Alumni Have Careers As:
Work as a researcher in laboratories to discover and develop new drugs and therapies to treat human disease. Ph.D.-level scientists have a wide range of career opportunities including research and development (R&D), manufacturing, quality assurance, patents, and regulation.
A range of companies that support the healthcare industry need scientists with a Ph.D. Some areas of work include sample and data analysis, biomedical engineering, diagnostics, pharmacology, health educator, and science writer.

Demonstrate the ability to locate, evaluate, and effectively use scientific information from accepted sources
Demonstrate knowledge of concepts in chemistry (organic, analytical, biochemistry, physical, inorganic, and chemical analysis)
Apply knowledge in chemistry to real-world problems
Conduct chemistry experiments, use various analytical instruments, collect and analyze data
Engage in rigorous scientific inquiry and communicate ideas in writing
Program Objectives
The Department of Chemistry and Biochemistry offers research-centered Master and Ph.D. programs in Chemistry and Pharmaceutical Chemistry. The objectives of these programs are to give students who have an undergraduate foundation in chemistry the opportunity to engage in advanced course work, in-depth study and independent research. Students work on cutting-edge research projects in Pharmaceutical Chemistry, Marine and Atmospheric Science, Biochemistry, Organic and Inorganic Synthesis, Analytical Chemistry, Computational and Physical Chemistry, and Chemical Education. Much of this research results in publications in peer reviewed journals or presentations at professional meetings, most of which have student co-authors.
In response to regional economic needs, we added a track in pharmaceutical chemistry to the M.S. degree program. We offer graduate students a diversity of research areas from within the traditional bounds of chemistry as well as non-traditional research programs. Graduate students have many opportunities to teach undergraduate labs and mentor undergraduates and new graduate students in research.
The Pharmaceutical Chemistry, Ph.D. program ensures that UNCW students are prepared for a flexible career in pharmaceutical chemistry. The Chemistry & Biochemistry faculty enable this goal by offering a broad range of pharmaceutical chemistry courses and research foci, from traditional and non-traditional natural products drug discovery and medicinal chemistry to formulation of therapeutic antibodies. In addition to employment opportunities in the pharmaceutical industry, graduates of this program will be ideally positioned to work in the rapidly expanding contract research (CRO) and biotechnology sectors, pursue academic careers, or establish innovative start-up companies.
Bolstered by a generous gift from Dr. Yousry and Linda Sayed, former UNCW faculty members and founders of Quality Chemical Laboratories, this program is one of the most rapidly growing initiatives at UNCW.

Sample Courses
| CHM 505 and 506 | Pharmaceutical Chemistry 1 and 2 |
| CHM 512 | Structure Elucidation |
| CHM 545 | Advanced Inorganic Chemistry |
| CHM 516 | Advanced Organic Chemistry |
| CHM 575 | Chemical Oceanography |
| CHM 576 | Chemical and Physical Analysis of Seawater |
| CHM 568 | Advanced Biochemistry |
| CHM 536 | Advanced Analytical Chemistry |
Admission Deadlines & Requirements
Information: pharmaceutical chemistry ph.d..
- Complete applications are reviewed for admission by the program as a group after the priority deadline.
- All application and supporting documents must be received by the published deadline.
Coordinator
- Dr. Thomas Coombs 910-962-7250 [email protected]
Location/Delivery Method
- Main Campus
Deadlines (11:59 p.m. EST)
- Priority Consideration: January 15, 2024
- Space Available Deadline: April 15, 2024
Transcripts
- One official transcript is required from each U.S. post-secondary institution attended. Refer to the Getting Started page for international transcript instructions.
Test Scores
- GRE General Test
- WAIVED - Test scores are waived for this program during 2024 due to the pandemic.
- No additional action required. Test scores will be waived during application processing
Recommendations
- 3 letters of recommendation from individuals who are knowledgeable of the applicant's academic acumen and ability to complete the Ph.D. in Pharmaceutical Chemistry.
Additional Requirements
- Upload Supplemental Documents After Application Submission Upload Supplemental Documents
- Curriculum Vitae
- Personal Statement: A personal statement describing educational, professional and research experiences, career goals and reasons for pursuing the Ph.D. degree
- Abstract: Abstract from the student’s master’s thesis research (optional)
- Publications: Copies of publications (optional).
- Note: Contact the program coordinator prior to application submission to determine your eligibility.
See everything happening in the
Are You an International Student?
This is a U.S. Department of Homeland Security STEM designated program that allows eligible F1 international students to apply for a 24-month optional practical training extension.
Related Programs
Chemistry, m.s..
The Chemistry M.S. is a research-focused program that will prepare you for further graduate school or a career in chemistry through mentored independent research and advanced coursework.
Chemistry (Online), M.S.
UNCW's online program deepens your knowledge of chemistry with extensive coursework, while also providing tracks in business, education, or clinical research to advance career goals.

The PhD Program in Medicinal Chemistry educates and trains students in the design and synthesis of novel, biologically active compounds and in delineating their mechanisms of action using biochemical, biophysical, and pharmacological approaches. Research specializations are available in synthetic, biochemical/pharmacological, and biophysical aspects of medicinal chemistry. Doctoral research in these specializations will relate to faculty areas of research, which currently include substance use disorders and addiction; neuropathic pain; obesity and metabolic disorders; neuropsychiatric disorders (psychoses, ADHD, depression, anxiety, eating disorders); and neurodegenerative diseases.
In The News

Cannabis Will Transform Medicine—Once We Figure Out How to Get Rid of Its Side Effects

Drug Discovery Spurs Innnovation, Collaboration

Groundbreaking Cancer Gene Therapy
This specialization trains students in the design and synthesis of novel, biologically active small molecules and study of their mechanisms of action using biochemical, biophysical, and pharmacological approaches. Concentrations are available in synthetic, biochemical/pharmacological, and biophysical medicinal chemistry, and nanomedicine. New chemical matter is targeted to treat drug abuse, addiction, neuropathic pain, obesity, neuropsychiatric disorders (psychoses, ADHD, depression, anxiety, eating disorders), neurodegenerative disorders, and glaucoma. Students may be supported by a training grant from the National Institute on Drug Abuse.
Where They Work
- Novartis Institutes for Biomedical Research
What They Do
- Healthcare Services
- Business Development
What They’re Skilled At
- High-Performance Liquid Chromatography
- Pharmaceutical Industry
- Cell Culture
- Biotechnology
Application Materials
Application.
- Application fee – US $50
- Three letters of recommendation
- Transcripts from all institutions attended
- Personal Statement
- Official GRE scores
- TOEFL score for applicants who do not hold a degree from a U.S. institution and whose native language is not English
- Please note all international applicants will need to provide a WES evaluation. Link to WES: https://www.wes.org/ https://www.wes.org/
Admissions deadline for Fall term: December 6
- Program Website
Request Information for PhD in Medicinal Chemistry and Drug Discovery
- Prospective Student Inquiries [email protected]
- General Inquiries [email protected]
- Job Posting Request
- Building Hours & Maps
- Prospective Students
- Current Students
What's Happening
Student resources, quick links.
- Alumni & Friends
Update Your Information
- Academic Departments
- Clinical Pharmacy
- Medicinal Chemistry
- Pharmaceutical Sciences
Research in Clinical Pharmacy
Research in medicinal chemistry, research in pharmaceutical sciences, research cores and services, biointerfaces institute, michigan drug discovery.
- Michigan Institute for Clinical & Health Research
Translational Oncology Program
- Faculty Publications
- Research Opportunities
- Research Collaborations
UM Pharmacy Professor Research Outreach (PRO)
- About the College
Message from the Dean
- Dean Search
Accreditation
New cop building, our history, our leadership.
- Our Mission, Vision & Organization
Teaching Excellence Awards
Job openings.
- Diversity, Equity & Inclusion
Department Contact Info
Emergency information.
- COVID-19 Updates
Sexual and Gender-Based Misconduct
- Alternative and Complementary Medicines
- Diagnosis and Health Conditions
- Healthy Choices
- Information for Caregivers
- Medication Information
- Other Resources
- COP Directory
Search form
- Alumni & Friends
Why U-M Pharmacy?
Recruitment events, student blogs, career potential, program overview.

- Information Request Form
PharmD Program
- Experiential Education
- Pharmacy Phamilies
- PharmD Curriculum
- Assessments
- Pharmacy Student Ambassadors
- Pre-Pharmacy Student Organization (PPSO)
PhD in Clinical Pharmacy
Phd in medicinal chemistry, phd in pharmaceutical sciences, ms in integrated pharmaceutical sciences.
- Career Flexibility
BS in Pharmaceutical Sciences
- Program Goals
- Fast Track to PharmD
- Student Services
- Research and Honors Program
- Student Outcomes
Dual Programs
- Dual PharmD and MBA Program
- Dual PharmD and MPH Program
- Dual PharmD and PhD Program
Post-Doc in Clinical Pharmacy
Residency program, ambulatory care enrichment program, research experiences for undergraduates program.
- Eligibility
- Program Schedule
- Application
- Pharmacy Scholars Program
- Frequently Asked Questions
- Income Guidelines
Assignment of Credit Hours
Pharmacy community college connect, postdoctoral collegiate fellows program.
- Expectations of Faculty Mentors
- Faculty Mentor List
- Review & Selection
PharmD Program Admissions
- Application Overview
- PharmD Prerequisites
- Preferred Admission Programs
- Applicant Characteristics
PhD Program Admissions
Ms program admissions, bachelors program admissions, funding your education, financial aid brochure.
- Tuition and Fees
- PharmD Scholarships
- Graduate Support
Student Organizations
- Student News
Course Descriptions
Student affairs.
- Financial Aid & Scholarship
- Advising & Registration
- Career Counseling
- Personal Counseling
- Campus Resources
- Student Affairs Directory
Career Connections
Student handbook, access pharmacy, campus groups, cornerstone learning, outlook in the cloud, rx preceptor, taubman library, wolverine access.
- Leaders and Legacy
Alumni Awards
- Alumni News
Job Opportunities
Submit personal news, meet the advancement team, update your alumni record.
- News & Events
- Previous Faculty Spotlights
- Department Metrics
- Research Laboratories
- CPTS PhD Program
- CPTS Fellowship Program
- Post-Graduate Residency
- REACH Fellowship
- Infectious Diseases Fellowship
- ACE Program
- Vision and Mission
- Department Directory
- Simulated Patient Program
- Biochemical NMR Core
- Clinical Pharmacogenomics Laboratory
- Pharmacokinetic and Mass Spectrometry Core
- Vahlteich Medicinal Chemistry Core
Michigan Institute for Clinical & Health Research
- Previous Seminars
- Newly Awarded
- Available Funding
- Grant Tools "Coming Soon"
- Giving Tuesday
- Faculty News
- Research News
- Annual Report Archive
- Upcoming Events
- Newsletter Archive
Our Mission, Vision & Organization
- COP Organization Chart
- COP Strategic Plan
Diversity, Equity & Inclusion
- Dean's Vision Statement
- DEI Strategic Plan
- Upcoming News & Events
- REU Program
- Education & Training
- McKesson Foundation Health Equity Speaker Series
- Resources & Support
- Concern Reporting
- Emergency FAQs
- Stay Informed!
MedChem Banner

TOP RESEARCH EXPENDITURE IN THE U.S.
Medicinal chemistry involves the application of a number of specialized disciplinary approaches all focused on the ultimate goal of drug discovery. Drug target identification and validation, rational (target-based) drug design, structural biology, computational-based drug design, methods development (chemical, biochemical, and computational), and “Hit-to-lead” development are all aspects of medicinal chemistry. The techniques and approaches of chemical biology, synthetic organic chemistry, combinatorial (bio)chemistry, mechanistic enzymology, computational chemistry, chemical genomics, and high-throughput screening are all used applied by medicinal chemists towards drug discovery.
For our Pharm.D. students, medicinal chemistry is integrated with pharmacology to present a coherent picture of the principles of drug action. Pharmacology mainly deals with drug action at the cellular, tissue/organ and organism levels. Medicinal chemistry focuses on the molecular aspects of drug action: interactions with the drug targets from both the drug and the target point of view, the relationship of drug chemical structure to drug action and the effects of metabolism on the drug structure and hence its action.
The Department of Medicinal Chemistry and the Interdepartmental Program in Medicinal Chemistry (Med Chem IDP), while inextricably linked, are distinct with related but separate missions. Historically, the Med Chem IDP is ~60 years old, while the Department of Medicinal Chemistry is ~25 years old. Other key differences are the composition of the respective faculties and the responsibilities for the programs as briefly summarized below.
As a discipline, Medicinal Chemistry in the United States started at the University of Michigan, College of Pharmacy with Professor F. F. Blicke in 1926. Prof. Blicke initiated the first graduate education program in Pharmaceutical Chemistry, focusing on synthetic organic chemistry. The program expanded in the 1950s to include analytical aspects and pharmaceutics. After Prof. Blicke’s retirement in 1960, his former student, Prof. J. H. Burkhalter returned to the College and argued for an independent graduate education program in Medicinal Chemistry. Until that time, Ph.D. degrees in the College were in Pharmaceutical Chemistry, Pharmacy and Pharmacognosy. Since there were so few Medicinal Chemistry faculty in the College (2), Rackham would not approve it. However, in 1967, under the direction of Graduate School Dean Alfred Sussman and with the participation of a core group of interdepartmental faculty, the Interdepartmental Program in Medicinal Chemistry (Med Chem IDP) was established. The College then applied for a Pharmacological Sciences Training Program Grant (PSTP) at the same time as the Department of Pharmacology. NIH had a rule that no institution could have duplicate training grants and asked that the two applications be merged. This was done and Dean Sussman was made the first director of the PSTP. The directorship has alternated between Medicinal Chemistry and Pharmacology ever since. The mission of the Med Chem IDP was, and still is to train students in a broad range of chemistry-based disciplines so that its graduates are able to apply the rigor and methods of the physical sciences to drug discovery research. This was the first such formal program in the country and involved the College of Pharmacy, the College of Literature, Science and the Arts, the Medical School, and the School of Dentistry.
The Med Chem IDP is currently administered by the Horace H. Rackham School of Graduate Studies with direct oversight by the College of Pharmacy. The Med Chem IDP includes all faculty from the Department of Medicinal Chemistry and faculty with appropriate interests from the Department of Pharmaceutical Sciences in the College of Pharmacy as well as select faculty from a variety of schools (e.g., Literature, Science and the Arts, Medical School) and departments at Michigan (e.g., Biological Chemistry, Biophysics, Chemistry, Pathology, Pharmacology, Radiology). The Med Chem IDP serves to administer the Med Chem PhD program, with responsibility for graduate student recruitment, training, progression and graduation. Approximately half of the Med Chem IDP faculty have their primary appointments outside of the Department of Medicinal Chemistry. These faculty currently mentor ~20% of the Med Chem PhD students and are fully engaged in the Med Chem PhD program in many other ways including seminar attendance, recruitment of students, teaching in our graduate courses, and serving on candidacy and dissertation committees. There is an annual meeting of the Med Chem IDP faculty to review the status of the IDP and the students.
In 1999, in response to the significant growth of the College of Pharmacy under previous Dean Ara G. Paul, then Dean George L. Kenyon initiated a process of departmentalization of the College of Pharmacy. The rationale included the recognition that the expertise and research areas of the College faculty had grown to be so diverse that it was impossible for a single person to adequately mentor and administer all faculty. Additionally, the administration of the professional PharmD and clinical programs and the research enterprise of the College was so complex that a departmental structure was needed to efficiently carry out our mission. Prof. James K. Coward was the first Chair of the Department of Medicinal Chemistry and Director of the Med Chem IDP. The Department of Medicinal Chemistry is the administrative component of the College of Pharmacy that oversees the Medicinal Chemistry faculty, research scientists and postdoctoral fellows (e.g., recruitment, mentoring, evaluation), has responsibility for the medicinal chemistry PharmD and PhD courses and seminar program, and coordinates the participation of medicinal chemistry faculty in College-level committees and other administrative duties. The Department of Medicinal Chemistry also administers the Med Chem IDP activities (e.g., student recruitment and progression). In 2017, the position of Director of Education was established. In 2022, the positions of department chair and IDP director were separated. In 2023-24, a national search was conducted to recruit a new department chair, the first time a chair was recruited from outside of the department.
Chairs/Directors of Medicinal Chemistry at Michigan
Fredrick F. Blicke (Director, IDP) 1926 - 1960
Joseph H. Burckhalter (Director, IDP) 1960 - 1972
Raymond Counsell (Director, IDP) 1973 - 1976
Leroy B. Townsend (Director, IDP) 1977 - 1997
James K. Coward (Chair and Director, IDP) 1998 - 2004
Ronald W. Woodard (Chair and Director, IDP) 2005 - 2011
George A. Garcia (Chair and Director, IDP) 2012 - 2020
Mustapha Beleh (Director, Education) 2017 - present
Heather A. Carlson (Chair and Director, IDP) 2020 - 2021
George A. Garcia (Interim Chair) 2022 - 2024
Amanda L. Garner (Director, IDP) 2022 - present
Robert H. Cichewicz (Chair) 2024 - present


Academic Catalog 2024-2025
Medicinal chemistry and drug discovery, phd, journal club participation, colloquium attendance, internship requirements and regulations for department of pharmaceutical sciences, qualifying examination, doctoral candidacy status, doctoral dissertation committee, dissertation proposal defense, registration for dissertation, publications and presentations, phd dissertation preparation, pharmaceutical sciences colloquium, sopps professional code of conduct .
The PhD Program in Medicinal Chemistry and Drug Discovery educates and trains students in the design and synthesis of novel, biologically active compounds and in delineating their mechanisms of action using biochemical, biophysical, and pharmacological approaches. Research specializations are available in synthetic, biochemical/pharmacological, and biophysical aspects of medicinal chemistry. Doctoral research in these specializations will relate to faculty areas of research, which currently include substance use disorders and addiction; neuropathic pain; obesity and metabolic disorders; neuropsychiatric disorders (psychoses, ADHD, depression, anxiety, eating disorders); and neurodegenerative diseases.
The Department of Pharmaceutical Sciences sponsors weekly journal clubs, Pharmaceutical Science Seminar ( PHSC 6300 ) , at which students present and evaluate current scientific literature in their fields of study. Students must attend one of these journal clubs (Pharmaceutics & Drug Delivery Journal Club, Pharmacology Journal Club, or Medicinal Chemistry & Drug Discovery Journal Club), chosen in consultation with their advisors.
Attendance at one of these journal clubs is required each and every academic semester, as an integral part of the PhD curriculum, with the exception of the last year (year four) in the program. All PhD students must participate full-time in journal club for course credit, Pharmaceutical Science Seminar ( PHSC 6300 ) , for six semesters. Failure to attend journal club regularly may result in sanctions such as probation or dismissal from the PhD program. Any student who does not comply with these (or any other) conditions required in the PhD program faces potential dismissal.
All PhD students, regardless of program, are required to attend the weekly Pharmaceutical Science Colloquium series. Announcements of times and locations will be distributed weekly to students by email to their university email addresses. Attendance is recorded by sign-up sheet. One excused absence is permitted per semester. Failure to attend colloquia may result in sanctions such as probation or dismissal from the PhD program.
Internships provide an experiential component of the graduate curriculum that fosters professional development through work in the pharmaceutical and biotechnology industries.
After PhD candidates have completed their dissertation research and are working on their dissertations, they are able, with the express permission of their PhD advisor, to participate in an internship if they choose. They are never allowed to intern while they are serving as teaching assistants.
- Students are responsible for finding their own internship and must be honest and accurate representing their experiences on their resumés. Students are responsible for tracking this experience on their resumés as there will be no detailed record on students’ transcripts of these opportunities.
- In order to be eligible for internship, students must take Professional Development for Pharmaceutical Sciences ( PHSC 5305 ) a semester before internship.
- Students must not accept more than one position. They must honor the first offer accepted. Any student not adhering to this requirement will not be allowed to participate.
- International students must register for Pharmaceutical Science Internship ( PHSC 6401 ) and follow instructions to receive Curricular Practical Training authorization from the Office of Global Services every semester they work. This applies to part-time jobs and volunteer opportunities. International students cannot engage in full-time CPT authorization totaling more than 52 weeks. Doing so will eliminate the possibility of engaging in the postgraduation benefit of Post-Completion Optional Practical Training.
- In order to receive a grade for the course, students must write at least two learning goals within the first two weeks of the internship and a one- to two-page paper describing what they learned, mid- and end of semester. Supervisors for internships will reply to a questionnaire about students’ performance.
- Taking internship must not extend international students’ visas.
- There are no vacations on co-op/internships. Companies’ sick time policies may vary. Students should check with their employers. For all other matters, please see the Universitywide Academic Policies and Procedures and/or Bouvé College of Health Sciences Academic Policies and Procedures .
The PhD qualifying examination is required for students in all four programs under the auspices of the Department of Pharmaceutical Sciences: pharmacology, medicinal chemistry and drug discovery, biomedical sciences, and pharmaceutics and drug delivery. Students from each of the four programs will take the exams within the same time frame (below), regardless of specialty-area program focus.
Doctoral students should have selected a dissertation advisor by the end of their first year in the program and are expected to have begun research and demonstrated initial proficiency in the laboratory before taking the PhD qualifying examination.
The PhD qualifying examination tests the candidates’ knowledge and skills in core courses and program content areas. The overall PhD qualifying examination consists of two written exams and one oral exam. The qualifying examination is taken as a course, Doctoral Training and Research ( PHSC 8940 ) , no later than during the fall semester of the student's second year, after having successfully completed all the core courses of their respective programs.
At least two departmental faculty will contribute questions for the written exams, and no one faculty member will write more than the equivalent of one entire exam. All students qualified to sit for the exams are expected to take them at the times announced.
The format for the written exams may vary (e.g., faculty may ask a series of comprehensive essay questions or provide research publications(s) from the biomedical literature and ask questions based upon the publications’ content). The first exam is given in the first week of fall semester, with the written portion of the second exam (i.e., the F31 written document) to be submitted to the student’s exam committee by the end of October, with the oral presentation to be completed by mid-November and graded by the providers of the question(s).
- For example, if the student is in the pharmaceutics and drug delivery PhD program, part 1 will be about pharmaceutics and drug delivery, and part 2 can focus either on pharmacology or medicinal chemistry and drug discovery.
- Written exam 2 requires that students write an NIH F31 grant proposal and have the proposal signed off as passing by their examination committee after an oral defense.
A score of at least 70% is required to pass the first written exam (two parts). Students must pass all written portions of the PhD qualifying examination prior to the oral defense of the F31 proposal. Students who fail one written exam will have one opportunity to retake and pass that examination. A student who fails the first exam twice will be required to withdraw from the PhD program.
During the oral exam, students defend their NIH F31 grant proposal before an examination committee of, minimally, four faculty members: the dissertation advisor, at least two other Department of Pharmaceutical Sciences faculty members, and at least one member from outside the department. This committee is convened only for the oral exam and does not need to be the same committee as the student's dissertation committee.
Members of the oral examination committee are selected by the student, after consultation with the dissertation advisor and/or the director of graduate studies. The oral exam is graded on a pass/fail basis. Students who fail the oral exam on the first attempt may retake the exam within a time period designated by the examination committee not to exceed two months from the first oral exam. Those who fail twice will be dismissed from the program.
Doctoral students who have completed satisfactorily and thereby earned the credits for all required core courses (including those for their specialized area) and who have passed the written and oral qualifying examinations shall be admitted to candidacy status for the PhD degree.
Doctoral students must complete a dissertation that embodies the results of extended research and makes an original contribution to their field. This work should give evidence of candidates’ abilities to conduct independent investigation and interpret the results of their research in a professional manner. The doctoral dissertation advisor serves as chairperson of the Doctoral Dissertation Committee, which consists of no fewer than five members. Selection of an advisor is by mutual consent of the student and a member of the faculty, with approval by the director of graduate studies in the Department of Pharmaceutical Sciences. At least two members of the Doctoral Dissertation Committee must be faculty members in the Department of Pharmaceutical Sciences. At least one member is to be selected from outside the department. Committee members are chosen for their expertise in students’ research areas.
Within a year after successful completion of the PhD qualifying examination, but no later than the beginning of the fall semester of the third year, students must prepare and defend a written proposal detailing their planned dissertation project. Failure to do so will be regarded as a failure to progress in the PhD program and will result in a warning from the director of graduate studies of the Department of Pharmaceutical Sciences.
Students who do not correct this deficiency within one semester will be placed on academic probation. Students on academic probation must complete the dissertation proposal defense and return to nonprobationary status within one semester or be dismissed from the PhD program.
The dissertation proposal should be no more than 50 double-spaced pages (12-point font minimum and one-half-inch margins on all sides). This page limit excludes references but includes figures, figure legends, and tables. Aside from these exceptions, the proposal should otherwise conform to the format and structure of an NIH grant proposal with four main sections: specific aims, background and significance, preliminary studies, and experimental design and methods. The Department of Pharmaceutical Sciences Dissertation Proposal document provides detailed instructions on the preparation of a dissertation proposal. Associated required forms may be found on the SOPPS Student Portal Canvas site.
The dissertation proposal must be defended orally before the student's dissertation committee and signed by all dissertation committee members in approval of the student's planned dissertation research. Upon dissertation approval, the copies of the signed proposal approval cover sheet must be submitted to the department’s director of graduate studies and to the Bouvé College of Health Sciences Graduate Office.
Biannual Review
Dissertation committees meet routinely at six-month intervals, but no less than once a year, to evaluate students’ research progress and to be presented with written and oral progress reports on the direction and status of the research. Progress reports should be written in a brief format, identical to that described for the formal dissertation (see instructions listed on the SOPPS Student Portal Canvas site). Unsatisfactory productivity provides the basis for a warning by the dissertation committee and/or the Graduate Committee. Two such warnings will result in a student’s dismissal from the program.
Advisor consent and completion of all coursework (with the exception of the colloquium course) must be documented before students register for the first dissertation course. Students must register for Dissertation Term 1 ( PHSC 9990 ) and Dissertation Term 2 ( PHSC 9991 ) . Students must register for Dissertation Continuation ( PHSC 9996 ) each semester thereafter until the dissertation has been successfully defended. The department strongly encourages PhD students to complete the program within five years after acceptance, i.e., by three years after establishing degree candidacy. According to university policy, no PhD students may remain in the program for more than seven years.
Prior to completion of PhD training, candidates must present their research either as a poster or podium presentation at a regional or national scientific conference. Also prior to completion, the student must have submitted (preferably, published) at least one manuscript in a peer-reviewed journal that reflects original findings and laboratory work from the candidate's dissertation research.
Detailed guidelines for the format and content of the written dissertation are given in Instructions for Preparation of the Dissertation found on the SOPPS Student Portal Canvas site. The completed dissertation document should be reviewed first by the dissertation advisor. Feedback from the advisor should be incorporated into the dissertation draft before its distribution to the dissertation committee. The completed dissertation should be delivered to all dissertation committee members no later than two weeks before the scheduled oral defense.
All PhD candidates nearing completion of their research are required to present their dissertation findings at the department’s Pharmaceutical Sciences Colloquium. These presentations should be scheduled at least six months before anticipated completion of the dissertation. In turn, the dissertation should be completed no later than one year after the colloquium presentation. Students must register for Pharmaceutical Science Colloquium ( PHSC 6810 ) during the semester that the colloquium presentation is to be given.
Oral Dissertation Defense
The oral dissertation defense takes place after students complete their PhD dissertation research and all other requirements for the PhD degree. The oral defense deals with the subject matter of the dissertation, significant developments in the field, and students’ background knowledge in their field of concentration.
The dissertation committee conducts the final defense. The committee may recommend that the student clarify, amplify, or rewrite portions of the dissertation before the final defense is scheduled. Once the committee concurs that that written dissertation document is acceptable, a date is chosen for the final oral examination.
At least two weeks prior to the defense, students should inform the director of graduate studies in the Department of Pharmaceutical Sciences of the date of defense, so that advance announcement may be distributed. The final defense is open to anyone who wishes to attend and typically lasts at least two hours. After presentation of the work by the student in a seminar format, and responses to audience and committee questions, the committee meets first with the student for any follow-up discussion and then in executive session to decide whether the student has defended the dissertation successfully.
The committee’s decision is then announced to the student. If the committee’s vote is favorable, the student incorporates committee suggestions and corrections, if applicable, and the dissertation is signed and passed on to the department’s director of graduate studies. Requests for a second defense are highly irregular but may be permitted in the event that the previous oral defense was judged by the committee to be highly promising but inadequate in one critical aspect.
The final dissertation must be written, defended, and approved at least two weeks before the university commencement deadline. Students must submit signed copies of their dissertations to the website designated by the university and must abide by any embargo sanctioned by the student’s principal dissertation advisor and/or dissertation committee. The students should apply for graduation before the final dissertation defense, on the assumption that the dissertation will be approved. If the dissertation committee decides that more time is required to complete the dissertation beyond the commencement date, then the application for graduation can be withdrawn and a new one submitted pending final dissertation approval.
All SOPPS students (BSPS, Preprofessional, MS, and PhD) are expected to adhere to the Code of Conduct .
Please visit Bouvé College of Health Sciences Program Learning Outcomes for the specific student learning outcomes for this program.
- Concentrations and course offerings may vary by campus and/or by program modality. Please consult with your advisor or admissions coach for the course availability each term at your campus or within your program modality.
- Certain options within the program may be required at certain campuses or for certain program modalities. Please consult with your advisor or admissions coach for requirements at your campus or for your program modality.
Complete all courses and requirements listed below unless otherwise indicated.
Qualifying examination Doctoral candidacy status Doctoral dissertation committee Dissertation proposal Biannual review Pharmaceutical Sciences Colloquium Oral dissertation defense
Core Requirements
A grade of C– or higher is required in each course.
| Code | Title | Hours |
|---|---|---|
| Seminar | ||
| Complete the following repeatable course for six semesters: | 6 | |
| Pharmaceutical Science Seminar | ||
| Required Core | ||
| Concepts in Pharmaceutical Science | 2 | |
| Concepts in Pharmaceutical Science 2 | 2 | |
| Research Skills and Ethics | 2 | |
| Professional Development for Pharmaceutical Sciences | 1 | |
| Ethical Problems in Health Sciences Research | 2 | |
| Experimental Design and Biostatistics | 2 | |
| Medicinal Chemistry and Drug Discovery | ||
| Organic Synthesis 1 | 3 | |
| Principles of Spectroscopy of Organic Compounds | 3 | |
| Contemporary Approaches to Drug Design | 3 | |
Research and Dissertation
| Code | Title | Hours |
|---|---|---|
| Pre-Qualifying Exam Course | ||
| Scientific Writing: Thesis Proposal | 2 | |
| Qualifying Exam | ||
| Doctoral Training and Research | 1 | |
| Proposal Preparation | ||
| Doctoral Proposal | 2 | |
| Dissertation | ||
| Dissertation Term 1 | ||
| Dissertation Term 2 | ||
| Colloquium | ||
| Pharmaceutical Science Colloquium | 1 | |
Program Credit/GPA Requirements
32 total semester hours required Minimum 3.000 GPA required
Plan of Study
| Year 1 | |||||
|---|---|---|---|---|---|
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 3 | 2 | 2 | |||
| 3 | 3 | ||||
| 2 | 2 | ||||
| 2 | 1 | ||||
| 1 | |||||
| 11 | 8 | 2 | |||
| Year 2 | |||||
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 1 | 1 | 2 | |||
| 1 | 0 | ||||
| 2 | 1 | 2 | |||
| Year 3 | |||||
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 1 | 1 | 0 | |||
| 0 | 0 | ||||
| 1 | 1 | 0 | |||
| Year 4 | |||||
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 1 | 2 | 0 | |||
| 1 | 0 | ||||
| 2 | 2 | 0 | |||
| Total Hours: 32 | |||||
Scientific Writing: Thesis Proposal ( PHSC 7020 ) must be taken the summer before the qualifying exams.
Doctoral Proposal ( PHSC 9681 ) should be taken in summer of second year, but no later than fall of third year.
Pharmaceutical Science Colloquium ( PHSC 6810 ) must be taken six months before dissertation defense.
PHSC 5305 & PHSC 6213 is suggested to be taken in the fourth year, but can be taken at any point before graduation.
Plan of Study - Advanced Entry
| Year 1 | |||||
|---|---|---|---|---|---|
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 1 | 1 | 2 | |||
| 1 | or | 0 | |||
| 2 | 1 | 2 | |||
| Year 2 | |||||
| Fall | Hours | Spring | Hours | Summer Full Semester | Hours |
| 1 | 1 | 0 | |||
| 0 | 0 | ||||
| 1 | 1 | 0 | |||
| Year 3 | |||||
| Fall | Hours | Spring | Hours | ||
| 1 | 2 | ||||
| 0 | |||||
| 1 | 2 | ||||
| Total Hours: 10 | |||||
Doctoral Proposal ( PHSC 9681 ) may be taken in spring of first year but must be taken before fall of second year.
Pharmaceutical Science Colloquium ( PHSC 6810 ) must be taken six months before dissertation defense.
Advanced entry into the Medicinal Chemistry and Drug Discovery PhD program requires a master's degree in pharmaceutical sciences or a related area and focuses on various advanced research courses and successful defense of the dissertation. An applicant's transcripts are required to be reviewed by the admissions committee to ensure they are eligible to be in the advanced entry program.
Annual review Qualifying examination Dissertation committee Dissertation proposal Dissertation defense
| Code | Title | Hours |
|---|---|---|
| Required | ||
| Ethical Problems in Health Sciences Research | 2 | |
| Seminar | ||
| Complete the following repeatable course four times: | 4 | |
| Pharmaceutical Science Seminar | ||
| Colloquium | ||
| Pharmaceutical Science Colloquium | 1 | |
| Code | Title | Hours |
|---|---|---|
| Qualifying Examination | ||
| Doctoral Training and Research | 1 | |
| Proposal Preparation | ||
| Doctoral Proposal | 2 | |
| Dissertation | ||
| Dissertation Term 1 | ||
| Dissertation Term 2 | ||
10 total semester hours required Minimum 3.000 GPA required
Print Options
Send Page to Printer
Print this page.
Download Page (PDF)
The PDF will include all information unique to this page.
2023-24 Undergraduate Day PDF
2023-24 CPS Undergraduate PDF
2023-24 Graduate/Law PDF
2023-24 Course Descriptions PDF
College of Pharmacy
- Our Department
- DEI Efforts
- About the PhD Graduate Program
- PhD Requirements
- Research Facilities and Equipment
- Faculty Research Foci
- Postdocs & Research Staff
- Abul-Hajj and Hanna Award for Exceptional Graduate Student in Medicinal Chemistry
- Ole Gisvold Lecture
- Philip S. Portoghese Distinguished Lecture
- Taito O. Soine Lecture
- Distinguished Lectures
- Medicinal Chemistry Seminars
- Epigenetics Consortium Seminars
- Chemical Biology Initiative Seminars
- Epigenetics Consortium
- From Digitalis to Ziagen
PhD Graduate Program

Our students receive an education in chemistry and biology that prepares them for the evolving multidisciplinary research of the pharmaceutical industry and academia.
- Research Interests
- University Collaboration
- Career Outcomes
Our students' learning and development within the program is enhanced by diverse research interests within our department:
- Antiviral and Anticancer Drug Design
- Cancer Chemoprevention
- Chemical Biology
- Chemical Carcinogenesis
- Drug Metabolism
- Gene Therapy
- Molecular Recognition
- Natural Products Chemistry
- Neuroscience
- NMR Crystallography
- Peptidomimetics
- Receptor Modeling
- X-Ray Crystallography
The University of Minnesota has one of the largest Academic Health Centers in the United States, which provides our students with the opportunity to collaborate with nationally recognized programs in:
- Pharmacology
- Biochemistry
- Public Health
Recent alumni have gone on to succeed in industry or academic positions with the following organizations:
- Aligos Therapeutics
- Beckman Coulter
- Foundation Medicine
- Harvard University
- Intellia Therapeutics
- Mayo Clinic
- UC Berkeley
- VMLY&R Health
... and more!
- About Our College
- Message from the Dean
- Our Campuses Overview
- Duluth Campus
- Twin Cities Campus
- Living in MN
- Our Faculty
- Faculty by Department
- Administration
- Our Commitment to Equity, Diversity & Inclusion
- Job Opportunities
- Degrees and Programs Overview
- Doctor of Pharmacy
- Dual Degree Programs
- Graduate Programs
- Medical Laboratory Sciences Program
- Occupational Therapy Program
- Postgraduate Pharmacy Residency Program Overview
- Choosing the University of Minnesota
- Mission and Vision
- Comprehensive Medication Management
- Learning Experiences
- Program Administration
- Admissions Overview
- Brochures and Other Materials
- Recruitment Events
- Virtual Open House
- Residency Sites & Emphasis Areas
- Meet Our Current Residents
- News, Events and Publications
- Summer Undergraduate Experience Program
- Undergraduate Electives Overview
- Available Courses Overview
- PHAR 1001: Orientation to Pharmacy
- PHAR 1002: Medical Terminology
- PHAR 1003: Non-Prescription Medications and Self-Care
- PHAR 1004: Common Prescription Drugs and Diseases
- Phar 2001: Health sciences calculations and patient safety
- PHAR 2002: Precision Medicine and Health: Understanding the Personal Genome
- PHAR 3206: Foundations of Health Literacy
- PHAR 3700/5700: Fundamentals of Pharmacotherapy
- PHAR 4204W/5204: Drugs and the U.S. Healthcare System
- PHAR 5201: Applied Medical Terminology
- PHAR 5205: Obesity: Issues, Interventions, Innovations
- PHAR 5310: Topics in Pharmacy Ethics (Pandemics)
- Continuing Professional Development
- Departments & Divisions Overview
- Experimental and Clinical Pharmacology
- Medical Laboratory Sciences
- Medicinal Chemistry
- Occupational Therapy
- Office of Professional & Clinical Affairs
- Pharmaceutical Care & Health Systems
- Pharmaceutics
- Pharmacy Practice and Pharmaceutical Sciences
- Professional Education Division
- Centers and Institutes Overview
- Brain Barriers Research Center
- Center for Allied Health Programs
- Center for Clinical and Cognitive Neuropharmacology
- Center for Drug Design
- Center for Forecasting Drug Response
- Center for Leading Healthcare Change
- Center for Orphan Drug Research
- Epilepsy Research and Education Program
- The Institute for Therapeutics Discovery and Development
- Institute of Personalized Medicine (IPM)
- PRIME Institute
- Wulling Center for Innovation & Scholarship in Pharmacy Education
- Research Overview
- Research Service Labs
- Endowed Chairs/Professors
- Expertise Guide
- Office of the Associate Dean for Research
- Medical Laboratory Sciences Alumni
- Occupational Therapy Alumni
- Pharmacy Alumni
- Events and Awards
- Celebrating Our Alumni
- MNovationRX
- Covid-19 Stories
- Publications & Newsletters
- Mental Health First Aid Training

PhD Program
The graduate programs in the College of Pharmacy offer advanced education in all aspects of pharmaceutical sciences including drug discovery, development and application.
The PhD program in pharmaceutical sciences includes coursework as well as cutting-edge research focused on topics like discovery and evaluation of novel drugs, determination of a drug's effects on the body, delivery methods to improve drug treatment, and how medication is used and applied to enhance patient outcomes.
Because the scope of pharmaceutical sciences is so broad, our graduate program has a number of specialty disciplines:
- Medicinal Chemistry and Pharmacognosy focuses on the interdisciplinary application of chemical, biochemical and molecular principles to the identification and development of therapeutic agents. This includes both synthesis of new chemical entities and isolation of medicinal agents from natural sources (pharmacognosy).
- Pharmaceutics and Pharmacology focuses on pharmacodynamics and pharmacokinetics, with a special emphasis on drug delivery and targeting systems and on determination of biochemical and physiological mechanisms by which drugs exert their effects.
- Outcomes and Translational Science conducts research across the interface from the laboratory bench to the patient bedside.
Admission to the PhD program does not first require application to the MS program.
In this section

Medicinal Chemistry & Pharmacognosy Graduate Studies

Pharmaceutics & Pharmacology Graduate Studies

Translational Science Graduate Studies
PhD Program Overview
The Pharmaceutical Sciences PhD Program at the University of Wisconsin–Madison provides a rigorous background in scientific disciplines that are critical to the preparation of the next generation of pharmaceutical scientists. With approximately 30 faculty trainers and approximately 65 graduate students, the program’s interdisciplinary training combines pharmaceutically relevant aspects of classical disciplines such as chemistry, biology, and engineering. This training allows our graduates to pursue careers in academia, industry, government, and other sectors.
Students earn a PhD in Pharmaceutical Sciences, concentrating in one of three research cores: Drug Discovery , Drug Action , or Drug Delivery .
Research in Drug Discovery focuses on areas related to medicinal chemistry such as small molecule development, natural products isolation and characterization, organic synthesis, chemical biology, and rational drug design.
Drug Action research focuses on areas related to pharmacology, toxicology, cellular differentiation, development, and disease. Interests include the impact of drugs and toxins on biological systems, mechanisms of normal biology, and mechanisms of disease. These are studied at the cellular, genetic, molecular, and biochemical levels using diverse model systems.
Drug Delivery research emphasizes principles in physical chemistry and drug transport, aiming for advances in formulation, drug targeting, and multi-modal therapy. This includes research involving biomaterials, cell engineering, immunotherapy, liquid biopsy, molecular recognition, molecular imaging, nanomedicine, pharmacokinetics, and solid-state chemistry.

We invite you to explore our webpages to learn more about the Pharmaceutical Sciences Division, our PhD program, and life in Madison.
Lara Collier, PhD Director of Graduate Studies Pharmaceutical Sciences PhD program
Contact us at: [email protected]
Other Degrees in the School of Pharmacy
Bs pharmacology – toxicology.
An interdisciplinary, research-driven, biomedical health focused undergraduate major
Doctor of Pharmacy
4-year program that trains students to become a Doctor of Pharmacy (PharmD)
Psychoactive Pharmaceutical Investigation, MS
An interdisciplinary Master’s program focused on the psychoactive pharmaceutical and biopharmaceutical industries (psychedelic, entheogen, and cannabinoid research/application)
Applied Drug Development, MS
An accelerated master’s program focused on developing practical and professional skills needed across the lifecycle of drug development, manufacturing, and ongoing safety management
Health System Pharmacy Administration, MS/Residency
A combined Master’s degree and residency that provides a solid background in academics and the administration of exemplary pharmacy services across an integrated health system
Health Services Research in Pharmacy, PhD
Prepares health services researchers to best meet the needs of patients and the communities in which they live, with a focus on improving medication outcomes
Questions about our program?
Check our FAQ page for detailed answers to common questions
Visit FAQ page
We’re here to help – send us your questions at any time!
Send us an email
PhD in Pharmacological Sciences

Fall 2025 Admission Deadline: December 2, 2024
UC Irvine’s PhD in Pharmacological Sciences program provides a unique opportunity for those interested in any scientific discipline represented by the Pharmaceutical Sciences faculty to have a year of broad, interdisciplinary training and self-selected lab rotations followed by focused doctoral research in the Pharmaceutical Sciences research group of their choice.
Students can choose from one of three tracks within the program: Pharmaceutical Sciences, Pharmacology or Medicinal Chemistry.
The current areas of study in the Pharmaceutical Sciences Department include:
- Structural and chemical biology
- Medicinal chemistry
- Structure-based drug design
- Molecular neuropharmacology
- Pharmacology of aging
- Molecular evolution
- Synthetic biology
- Natural product biosynthesis and synthase engineering
- Cancer prevention and therapy
- Gene regulation and intercellular signaling
- Computational biology and bioinformatics
- Nanomedicine for targeted drug and gene delivery
“The school has rotations that are longer than most departments – lasting a full quarter as opposed to a few weeks – which allows you to get a taste for what research is like in the industry and to really figure out what environments are best suitable for you.” David Wych, PhD ’21
About Our PhD Program
The Pharmacological Sciences PhD program is flexible and tailored to the needs of each individual student. Students are actively engaged in research throughout their training: In the first year, laboratory rotations ensure exposure to a variety of techniques and research problems. By the end of their first year students have worked with several faculty members and selected a lab to join. During their third year, students are considered for advancement to PhD candidacy on the basis of academic standing, laboratory performance, and a qualifying examination. After advancement to candidacy, students devote their time to completion of an original research dissertation.
CLICK HERE to view the sample curriculum for the Pharmaceutical Sciences Track.
CLICK HERE to view the sample curriculum for the Pharmacology Track.
CLICK HERE to view the sample curriculum for the Medicinal Chemistry Track.
For more details regarding the required course work, please visit our program’s section in the UCI General Catalogue .
Application Instructions
Complete the Online Application which is submitted to the UCI Graduate Division. When completing the “Degree Program” section of the online application for admission, please make the following selections:
- School/Department: Pharmaceutical Sciences
- Major/Degree: Pharmacological Sciences-PhD
Students are admitted to the Pharmacological Sciences PhD program on an annual basis in the fall quarter only. The admissions committee screens applications immediately after the application deadline. First round applicants selected to interview will be notified by early January. Admitted applicants can expect to receive an offer of admission in late January through mid-March.
Submit applications by December 1 for full consideration
The online application and supporting materials should be received by December 1, 2023.
Applicants are required to submit:
- An official online application including the application fee ($135 for domestic applicants, i.e. US citizens and permanent residents and $155 for international applicants)
- For application review purposes only, scan and upload copies of transcripts for all institutions attended since high school. In the online application, you will be prompted to upload your scanned documents. Please upload both the front and back sides of the transcript. Uploaded transcripts should be recent and include the following: your name, dates of attendance, grades/marks received, credits and grading legend. Official transcripts will be requested by the Graduate Division if and when you are admitted and decide to attend UCI. Do not send official transcripts until this time.
- A Statement of Purpose – must include your specific research interest and three possible research advisors you would be interested in working with. You can describe your research interests, career goals, and other related information.
- A Personal History Statement – this can discuss how your personal background– including any relevant educational, familial, cultural, economic, or social experiences, challenges or opportunities– informs your decision to pursue a Ph.D. in Pharmacological Sciences. If you have overcome socioeconomic or educational challenges, please indicate that you are a diversity candidate and describe your experience in detail.
- Three letters of recommendation – uploaded to the online application by your recommender.
- UCI no longer requires the GRE.
- International students are also required to submit TOEFL scores (Code: 4859)
Applicants are encouraged to upload the following in their application:
- Current curriculum vitae or resume
- List of publications
For additional details about applying to the PhD in Pharmacological Sciences program, view our information sheet here .
Prerequisites
- An MS degree is not required for consideration. However, research experience (laboratory or fieldwork) is a primary criterion for acceptance into our graduate programs.
- Some biology and chemistry courses are required. However, because we are an interdisciplinary program, we admit students from various academic backgrounds, so there are no specific course requirements. Applicants recently admitted to our program have undergraduate degrees in a wide range of disciplines, including molecular biology, psychology, and chemical engineering, as well as chemistry and biology.
- Minimum cumulative undergraduate GPA of 3.0.
Admission Statistics
The acceptance rate for the Pharmacological Sciences program is approximately 23%, and admitted applicants from previous cycles had the below characteristics:
- Average undergraduate GPA: 3.32
Student Funding
Admitted applicants receive funding for tuition, health insurance, and a monthly stipend/salary through a combination departmental or university fellowships and/or teaching assistant (TA) and graduate student researcher (GSR) positions in their first year. In years two through five, students are generally funding by their faculty advisor as GSRs, as well as through a combination of university fellowships, extramural grants/fellowships, and/or TA positions.
Diversity Fellowships
UCI is committed to the recruitment, admission, and retention of a high quality and diverse graduate student population and has several diversity fellowships for new and returning students who qualify. If you have overcome socioeconomic or educational challenges, please indicate that you are a diversity candidate and describe your experience in detail within the Personal History section of the application.
English Language Proficiency Requirements
TOEFL or IELTS
All graduate applicants are required to demonstrate English proficiency for admissions consideration. Applicants are waived from the English Language Proficiency requirement if they have earned an undergraduate degree from an institution at which English was the sole language of instruction according to the World Higher Education Database (WHED) . Please see WHED’s instructions on how to search for your institution. If English is not the sole language of instruction listed or if no language is listed at all, the waiver does not apply and the applicant is required to take and pass an approved English proficiency test. Approved tests and minimum scores are outlined in the next section.
Please note: Test results that are two years old or older are not acceptable.
IMPORTANT NOTE: If a student will be supported as a Teaching Assistant (TA), please read the English proficiency summary chart for teaching assistants . Students who have not earned an undergraduate degree from an institution at which English was the sole language of instruction according to WHED are required to demonstrate English language proficiency to serve as a TA when they apply to the program.
The TOEFL is administered by the Educational Testing Service (ETS).
- Please select institution code 4859 to have your official score sent to UCI. No department code is needed.
- We only accept scores submitted electronically by ETS.
- Test results that are two years old or older are not acceptable .
- We do not accept MyBest scores; you must submit all individual test scores.
- Results of institutional (non-ETS) administrations of the TOEFL are not acceptable .
- We will accept the TOEFL iBT Special Home Edition test. The same minimum score applies.
- We do NOT accept the TOEFL ITP Plus test for China or the TOEFL Essentials test.
- For more information, please visit their website at www.ets.org/toefl
TOEFL Score Requirements for Admission Consideration:
- An overall minimum score of 80
- A minimum score of 26 on the speaking section to be eligible for a Teaching Assistant position
As an alternative to the TOEFL, you may submit scores from the Academic Modules of the International English Language Testing System (IELTS).
- We only accept scores submitted electronically by the IELTS test center. No paper Test Report Forms will be accepted.
- We will accept the IELTS Indicator test. The same minimum score applies.
- An institutional code is NOT required. Please contact the test center directly where you took the IELTS test and request that your test scores be sent electronically using the IELTS system. All IELTS test centers worldwide are able to send scores electronically to our institution.
- For more information, please visit their website at www.ielts.org
IELTS Score Requirements for Admission Consideration:
- An overall minimum score of 7 for admission, with a score of no less than 6 on any individual module.
- A Minimum score of 8 on the speaking module to be eligible for a Teaching Assistant position.

Your browser is unsupported
We recommend using the latest version of IE11, Edge, Chrome, Firefox or Safari.
College of Pharmacy - Chicago | Rockford
Phd in pharmaceutical sciences.
We enable students with backgrounds in fundamental sciences to become leaders in pharmaceutical sciences
Located in the vibrant and multicultural city of Chicago, UIC's PhD Program in Pharmaceutical Sciences is one of the strongest and largest of its type in the United States. Our college is consistently ranked in the top ten in terms of funds secured annually from the National Institutes of Health and by US News and World Report. We pride ourselves on giving students from all types of backgrounds the tools they need to become independent researchers. Students in the program select one of the program concentrations, described below.
Important dates Heading link Copy link

We are so pleased you are considering graduate studies in Pharmaceutical Sciences at the University of Illinois Chicago! Although Pharmaceutical Sciences is one of the best graduate programs of its kind in the country, our real pride is mentoring students into independent researchers who become leaders in our field. The program has some unique strengths, including providing flexibility to carry out internships in your later years. Have a look around our website. If you have questions, feel free to reach out to us at [email protected] . We look forward to reading your application! Debra Tonetti, PhD | Professor, Pharmaceutical Sciences
Program Coursework Heading link Copy link
All students in the Pharmaceutical Sciences program take the following courses. Additional concentration coursework is also required and is shown in each of the concentration tabs.
- Drug Discovery, Design, and Development (PSCI 501, 3 credit hours)
- Training in Research Presentation (PSCI 502, 1 credit hour)
- PSCI 503: Biostatistics for Pharmaceutical Scientists (1 credit hour)
- BSTT 400: Biostatistics I (4 credit hours) [Note: BSTT 400 is required for the Pharmaceutics and Drug Delivery concentration]
- Scientific Ethics and the Responsible Conduct of Research (GC 501, 1 credit hour)
- Research Rotation (PSCI 592; 3-4 credit hours)
- PSCI PhD Course Requirements
- PSCI Department Course Descriptions
Program Concentrations Heading link Copy link
Five concentrations comprise the PhD program in Pharmaceutical Sciences. Click on the tabs below to learn more about each of them. To see the faculty mentors for each concentration, visit the Faculty Mentors page .
Chemistry in Drug Discovery
Concentration description.
Faculty in the Chemistry in Drug Discovery concentration use the tools and techniques of chemistry to discover and develop new chemical probes and potential therapeutics. Students in this concentration learn how to design, synthesize, characterize and analyze small molecules, peptides, and proteins.
Concentration Coursework
Students in the Chemistry in Drug Discovery Concentration take the following courses:
- Fundamental of Drug Action I (PHAR 422, 4 credit hours)
- Principles of Medicinal Chemistry (PSCI 530, 5 credit hours)
- Electives (9 credit hours)
Concentration Coordinator
Prof. Terry Moore ([email protected])
Molecular Mechanisms and Therapeutics
The Molecular Mechanisms and Therapeutics concentration is designed to provide advanced understanding of fundamental causes of diseases, strategies that identify new drug targets, and mechanistic explanations of how drugs work (or fail) from the perspective of the target and systems they impact. Faculty affiliated with MMT integrate a wide variety of molecular, biochemical, genetic, bioinformatic, and bioengineering approaches to study mechanisms of pathogenesis ranging from infectious diseases to cancer. Students will enroll in fundamental molecular and cellular biology courses and select elective courses in areas of their focused research.
Students in the Molecular Mechanisms and Therapeutics Concentration take the following courses:
- Biochemistry (e.g., GEMS 501 or equivalent graduate-level biochemistry course, 3 credit hours)
- Molecular Biology (e.g., GEMS 502 or equivalent molecular biology course, 3 credit hours)
- Biostatistics I (BSTT 400, 4 credit hours)
- Molecular Genetics (GEMS 511, 3 credit hours)
- Receptor Pharmacology and Cell Signaling (GEMS 515, 3 credit hours)
- Microbial Pathogenesis (MIM 560, 3 credit hours)
- Cancer Biology and Therapeutics (PSCI 540, 3 credit hours)
Prof. Alessandra Eustaquio ( [email protected] )
Pharmaceutics and Drug Delivery
Faculty in the Pharmaceutics and Drug Delivery concentration use the tools and techniques of physical and biologic sciences and engineering to understand and develop delivery systems and formulations for therapeutic molecules and control the biodistribution of therapeutic molecules. Students in this concentration learn how to design, synthesize, characterize and analyze novel materials and drug delivery systems and design and develop technologies related to therapeutic distribution in the body.
Students in the Pharmaceutics and Drug Delivery Concentration take the following courses:
- *This 4 credit hour course will count 1 hour toward the program core statistics requirement and 3 hours toward the Pharmaceutics and Drug Delivery concentration requirements. Students will not receive credit for two introductory statistics courses.
- Essentials for Animal Research (GC 470, 1 credit hour)
- Experimental Animal Techniques (GC 471, 2 credit hours)
- Principles of Pharmaceutics and Drug Delivery (PSCI 510, 3 credit hours)
Prof. Richard Gemeinhart ([email protected])
Pharmacognosy
Faculty research programs in the Pharmacognosy concentration aim to develop therapeutics from natural products and to study the mechanisms of pain, cancers, and a wide array of infectious and tropical diseases. Students of this concentration are trained in a combination of bioinformatics, synthetic biology, genetic engineering, chromatography, and spectroscopy to achieve these goals.
Students in the Pharmacognosy Concentration take the following courses:
- Research Techniques in Pharmacognosy (PSCI 520 or equivalent; 3 credit hours)
- Structure Elucidation of Natural Products (PSCI 521 or equivalent; 3 credit hours)
- Advanced Pharmacognosy (PSCI 522 or equivalent; 3 credit hours)
Prof. Brian Murphy ([email protected])
PharmD/PhD Joint Program Heading link Copy link
Pharmaceutical Sciences participates in the joint PharmD/PhD program, which trains students for careers in academic pharmacy and bench science research. Students admitted to this joint program participate in the PharmD curriculum and pursue original doctoral research projects in the laboratories of the university’s graduate faculty in the Department of Pharmaceutical Sciences.
The joint program offers the potential of reducing the time of earning both degrees in sequence (9 or more years) by approximately two years. The trade-off is that both degrees are awarded at the end of the training period and neither degree can be received before the other is completed.
The PharmD/PhD program is for exceptional, highly motivated and achieving students ready to meet the challenge of increased academic load and independent research project.
Program coordinator: Dr. Lindsey McQuade ( [email protected] )
- Joint PharmD/PhD Course Requirements
- Joint PharmD/PhD Program Page
PSCI Slideshow Heading link Copy link
- Go to slide 1
- Go to slide 2
- Go to slide 3
- Go to slide 4
- Go to slide 5
- Go to slide 6
- Go to slide 7
- Go to slide 8
- Go to slide 9

- Go to the next slide
- Go to the previous slide

Pride Points for PhD PSCI Heading link Copy link
$ 37,500 annual graduate stipend for students on teaching assistantship or research assistantship
33 internships completed by department graduate students in the last five years
19 students currently on training grant or fellowship
# 7 nationally ranked College of Pharmacy according to US News
# 7 nationally ranked total research funding among Colleges of Pharmacy according to AACP

Start your application Heading link Copy link
The Pharmaceutical Sciences Program at UIC offers a supportive, inclusive environment and rigorous academic preparation for students who are interested in careers in pharmaceutical sciences. If you have any questions about the program or about your application, please contact [email protected].
Get in touch: Contact Us

Where discoveries are delivered. SM
Ph.D. Program in 'Pharmaceutical Sciences and Drug Development'
Graduate education in Pharmaceutical Sciences and Drug Development (PSDD) provides training in research strategies in the design and development of novel therapeutic agents to improve human life in disease and health. The PSDD training area will provide translational sciences research training that bridges basic sciences and clinical research for the purpose of addressing the world’s challenges in unmet therapeutic needs. Research in pharmaceutical sciences encompasses multi-faceted, interdisciplinary drug development research.
Training in PSDD for the Ph.D degree with the Biomedical Sciences Graduate Program is described at: https://biomedsci.ucsd.edu/training-areas/molecular-pharmacology.html
This web site includes information for student applications to the PSDD Ph.D program.
Ph.D Training in ‘Pharmaceutical Sciences and Drug Development’ (PSDD)
Faculty Leader Contact:
Vivian Hook ([email protected])
Summary of PSDD Research Training
The Pharmaceutical Sciences and Drug Development (PSDD) training area is a unique joint effort between the Skaggs School of Pharmacy and Pharmaceutical Sciences (SSPPS), the Scripps Institution of Oceanography (SIO), Center for Drug Discovery Innovation (cDDI), the UCSD Drug Development Pipeline , the Center for Compound Resources, the Center for Computer-Aided Drug Design, with programs of the School of Medicine , School of Engineering , and UC BRAID . The overall goal of this training area is to provide students with a visionary perspective on the drug discovery and development process.
Graduate education in “Pharmaceutical Sciences and Drug Development” (PSDD) provides training in research strategies in the design and development of novel therapeutic agents to improve human life in disease and health. The PSDD training area will provide translational sciences research training that bridges basic sciences and clinical research for the purpose of addressing the world’s challenges in unmet therapeutic needs to improve human lives. Research in pharmaceutical sciences encompasses multi-faceted, interdisciplinary drug development research in (a) design and discovery of drug molecules targeted to regulators of disease processes, including marine natural products, (b) in vitro and in vivo efficacy of candidate drug therapies, (c) chemical optimization by medicinal chemistry approaches, (d) drug pharmacodynamics, pharmacokinetics in ADME research based on drug delivery strategies, (e) safety and toxicity of drug molecules, and (f) advanced analytical technologies of drug molecule properties. Graduate students will be trained in these disciplines through a complete curriculum and state-of-the-art research strategies for drug development. Faculty of the Skaggs School of Pharmacy and Pharmaceutical Sciences (SSPPS) and the BMS program will train students in the area of PSDD. PSDD training will provide students with exciting opportunities in the professional field to become leaders in academic, government, private industry, biotechnology, and related areas to advance innovative drug development via pharmaceutical sciences research.
PhD degree:
Students apply for admissions to the UCSD ‘Biomedical Graduate Program’ (BMS) for training by faculty in ‘Pharmaceutical Sciences and Drug Development (PSDD). Research training in PSDD is associated with the BMS areas of ‘Molecular Pharmacology and Drug Discovery’. Graduate students of the BMS program deveop their Individual Development Plans (IDP) with faculty advisors of the program. The IDP plans the research, coursework, and degree requirements for the student.
How to Apply
Training Areas
Individual Development Plans
PharmD/PhD degree:
First year pharmacy students can pursue the PharmD/PhD degree by conducting 3 research rotations with faculty on research topics of PSDD during years 1-2 of the pharmacy curriculum. Pharmacy students in their 2nd year can apply for admissions for the PhD program of the Biomedical Sciences graduate program at UCSD (see previous paragraph). See information about the dual PharmD/Ph.D degree at
https://pharmacy.ucsd.edu/degree-programs/dual-pharmd-phd-program
Core Graduate Courses in ‘Pharmaceutical Sciences and Drug Development’
SPPS 263A Principles in Pharmaceutical Sciences and Drug Development: Pre-Clinical Drug Discovery and Development
PPS 263B Principles in Pharmaceutical Sciences and Drug Development: Pre-Clinical to Clinical Drug Development
Courses in Selected Areas of Pharmaceutical Sciences and Drug Development
SPPS 226 Pharmacokinetics/Pharmacodynamics
SPPS 219 Pharmacogenomics
SPPS 222 Pharmaceutical and Physical Chemistry
SPPS 223 Pharmaceutical Biochemistry
SPPS 224 Biopharmaceutics
SPPS 225 Dosage Forms and Drug Delivery Systems
SPPS 268 Systems Mass Spectrometry
SPPS 281 Medicinal Aspects of Natural Products
Faculty in Drug Discovery & Development in Pharmaceutical Sciences

PhD Program
- Frequently Asked Questions (FAQ)
- Financial Aid
- Interview/Recruitment Days (BY INVITATION ONLY)
- PhD Program Requirements
- Progression to PhD
- Is a Career in the Pharmaceutical and Translational Sciences Right for Me?
- Scholarly Achievement and Placement of Alumni
- Student Involvement

The Pharmaceutical & Translational Sciences (PHTS) Program brings together, under one umbrella, the school’s three laboratory-based PhD programs—CXPT, MPTX, PSCI. This promotes a more cohesive interdisciplinary experience advancing education and offering opportunities to sample the entire range of innovative research options. This doctoral training program prepares students for careers in the pharmaceutical industry, academia and advanced scientific research in a broad range of settings. The training encompasses a unique scientific framework from drug discovery, delivery and development to application of genetics and genomics to experimental and clinical translational research.
PHTS Umbrella
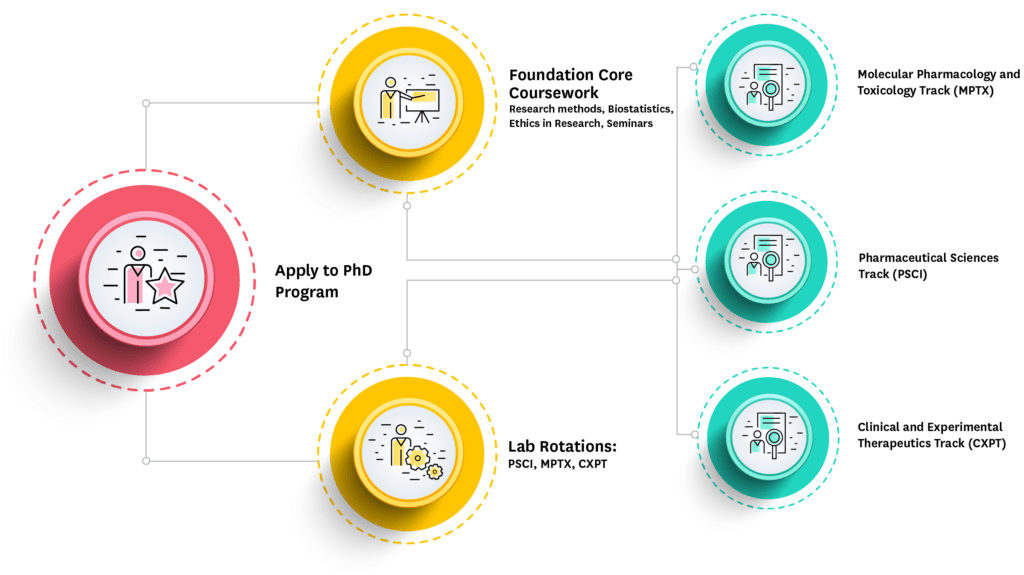
The umbrella structure allows students to attend courses and seminars together, and rotate through laboratories across programs during their first year. This fosters interdisciplinary crosstalk among students and faculty, helping students find an ideal laboratory and faculty mentor as well as a specialized track of study they want to pursue. Upon successful completion of the first year, students will select an area of study from one of the three listed below, in which they will earn the Doctor of Philosophy degree following successful completion of the PhD Program.
Molecular Pharmacology and Toxicology (MPTX)
The MPTX PhD Program provides training in molecular mechanisms of disease as well as disease and drug interaction. Coursework emphasizes molecular pharmacology and the interaction of drugs with cell physiology. Research areas are laboratory-based and include drug design and development, receptor pharmacology, pharmacodynamics, medicinal chemistry, cancer biology and pharmacology, immunology, metabolism and biochemistry, molecular- and neuro-pharmacology, and oxidant and environmental toxicology. The MPTX PhD program and the PSCI PhD program are both administered under the umbrella of the Department of Pharmacology and Pharmaceutical Sciences.
Pharmaceutical Sciences (PSCI)
The PSCI PhD Program provides training that emphasizes basic as well as applied research through advanced coursework in contemporary pharmaceutical sciences. Independent laboratory research areas include drug design, development, targeting and delivery; medicinal chemistry; computational chemistry; pharmaceutics; pharmacokinetics; pharmacodynamics; immunology; and molecular and cell biology. The PSCI PhD program and the MPTX PhD program are both administered under the umbrella of the Department of Pharmacology and Pharmaceutical Sciences.
Clinical and Experimental Therapeutics (CXPT)
The CXPT track provides cross-training between clinical and basic sciences—focusing on the investigation of disease processes, drug development, and the efficacy and toxicity of therapeutic regimens. Course requirements and research opportunities offer both experimental (basic) and disease-focused experiences. The emphasis in this track is clinical translational, using molecular and translational science techniques to address clinically relevant research questions.

How did your time at the USC Mann School impact you?
“The rigorous training I received through the USC Pharmaceutical Sciences PhD program equipped me with a strong foundation in natural product chemistry, microbial genetics and analytical chemistry. This comprehensive background has proven invaluable in my research on unraveling the molecular mechanisms underlying microbe-host interactions.”
Chun-Jun (CJ) Guo
Phd pharmaceutical sciences ’14, assistant professor, weill cornell graduate school of medical sciences.

“My experience at USC has been instrumental in my current success. The health economics and outcomes research curriculum provided me with the relevant technical expertise to work in the field. I was fortunate as a student to get two internships, at Amgen and AbbVie, in addition to working with my professors on industry projects.”
Darshan Mehta
Phd health economics ’18, ms pharmaceutical economics and policy ’14, director, global health economics and outcomes research, moderna.

“My USC experiences and mentors enhanced both my technical and soft skills, priming me for my professional career. Their impact on me, professionally and personally, is priceless.”
Meleeneh Derhartunian
Phd, molecular and cellular biology ’10, certificate in clinical trial design and management, regulatory science, regulatory documentation team leader, product development, genetech.

“There is something about the culture of the school at USC that motivates people to strike out and be creative in how things get done. Since graduating I’ve been a postdoctoral researcher contributing new and exciting lines of study to an existing research program, I’ve provided research support in fields outside of my primary training (e.g., cancer therapy and early detection), to now serving in leadership roles focused on enhancing institutional equity in graduate programs and training the next generation of scientists.”
Letisha R. Wyatt
Phd, molecular pharmacology and toxicology ’13, assistant professor of neurology at oregon health and science university.

Why did you choose USC?
“Faculty and staff are able to help you with whatever path you choose: academics, industry or government. There is always someone who can help you. USC makes a promise and can keep that promise.”
Samuel Garza
Phd molecular pharmacology and toxicology ‘23, ms regulatory science ‘23.

Why was USC a good fit for you?
“USC was a good fit for me because it had provided me with opportunities to develop necessary scientific skills and grow a professional network for a postgrad career in the pharmaceutical industry.”
Amy Tran-Guzman
Ms, regulatory science ’17, phd, molecular pharmacology and toxicology ’22, scientist at bristol myers squibb.

Why was this program attractive to you?
“I chose the Clinical and Experimental Therapeutics program because it afforded me a foot in the biomedical research doors of both clinical and foundational science. It’s truly translational in that it involves going to the clinic and then pursuing solutions in the lab.”
Timothy Bensman
Pharmd ’11, phd clinical and experimental therapeutics ’16.

What was your experience like at USC?
“Overall, my experience at USC was excellent and it really did set me up for my career. The alumni connection was strong which helped my transition to the industry a smooth one. The ‘Trojan family’ is really a great one and I see that whenever I visit USC as an adjunct faculty or when I attend USC receptions at conferences or when I get in touch with faculty at USC who are always willing to listen and are always looking to get the alumni involved in bettering the various programs at the school.”
Ashutosh (Ash) Kulkarni
Phd pharmaceutical and translational sciences ’04, head of clinical pharmacology and dmpk at stealth biotech startup.

What inspired you to pursue a PharmD/PhD at USC?
“I only applied to pharmacy schools that advertised a dual degree program; plus USC is the number-one private pharmacy school in the country. While it was a struggle to take PharmD and PhD classes at the same time, the School’s dual degree program allowed me to complete my clinical and research training two years faster than if I had pursued the degrees separately.”
PharmD ’17, PhD Molecular Pharmacology and Toxicology ’21
Field application scientist at agilent technologies.

Why did you choose USC Mann?
“One of the appealing factors that sold me was the famous USC alumni network, the focus on translational science at various levels, and the option to earn a masters in either regulatory science or drug development. Taken together I believe they contribute to making me more competitive in the job market after my PhD.”
PhD, Pharmaceutical and Translational Sciences ’21
Advisor, eli lilly and company.

What is your advice to new or prospective students?
“The Pharmaceutical & Translational Sciences (PHTS) Program offers students a broad range of research labs. The school is constantly expanding its research capability in both personnel and hardware. If you intend to join the field of pharmaceutical and translational research, this is the program you will love.”
Zhefu (Jeff) Dai
Phd, pharmaceutical sciences ’21, scientist at igm biosciences, inc..

Why did you choose USC School of Pharmacy?
“I chose USC Mann because the program is geared towards developing researchers who are prepared for highly competitive industry spots after graduation. PhD students are also eligible to enroll in a regulatory science masters degree program to compliment their research and coursework. This program is part of what helps PhD graduates from USC Mann to successfully move into industry positions.”
Alicia Warnecke
Phd, clinical and experimental therapeutics ’20, clinical project manager at iqvia.

M.S. and Ph.D. Programs
Choose pharmacy at the university of texas at austin.
The University of Texas at Austin College of Pharmacy operates longstanding and well-developed graduate training programs in pharmaceutical sciences and translational sciences.
The goal of graduate study at The University of Texas at Austin College of Pharmacy is to develop the intellectual breadth and specialized training necessary for a career in teaching, research or advanced professional practice.
Emphasis is placed on the knowledge, methods and skills needed for scholarly teaching; original research and problem solving; intellectual leadership; creative expression; and professional prominence and recognition.
We are committed to eliminating disparities, raising awareness and fostering a culture where everyone has equal opportunities and a sense of belonging in our graduate training program.
The graduate training programs are dedicated to nurturing a culture of acceptance and support and are committed to ensuring that aspiring Pharmacy Longhorns from all backgrounds have equal access to the graduate programs through intentional outreach and engagement efforts.
Ready to apply?
Let's Get Started
Interested in learning more? Download the UT Austin College of Pharmacy graduate brochure .
Pathway Programs
The UT Austin College of Pharmacy is committed to ensuring that aspiring Pharmacy Longhorns from all backgrounds have equal access to the college through intentional outreach and engagement efforts. The college has multiple programs that demonstrate a commitment to providing a supportive and collaborative approach to student and trainee success.
- LEADER - A Summer Undergraduate Research Program
- BOOT Program - Building Our Own Talent
Visit our Pathway Programs page to learn more.
Pharmaceutical Science Degrees
Pharmaceutical Science is an interdisciplinary field that includes areas of study in chemical biology and medicinal chemistry, pharmaceutics, pharmacology, toxicology, pharmacotherapy, and health outcomes. Students applying to the graduate program in pharmaceutical sciences will select a division-specific training track that most closely matches their interests. The University of Texas College of Pharmacy offers a Ph.D. program with specialization in each of these areas of pharmaceutical sciences and a M.S. degree program that is limited to the pharmacotherapy and health outcomes areas of research.
Graduate training in the pharmaceutical sciences program is housed within five different divisions. Students applying to pharmaceutical sciences select a division-specific training track that most closely matches their interests. Visit each of the division homepages to learn more about faculty research and to get details on the division specific graduate program objectives and curriculum.
Courses of study leading to a master of science degree are only offered in pharmacotherapy and in health outcomes.
Each graduate student’s course of study may be uniquely tailored to meet the specific learning objectives of the student.
- Health Outcomes
- Pharmacotherapy
Graduate education, research, and scholarly work leading to a Ph.D. are developed uniquely for each student as part of his/her plan of work.
Students applying to the graduate program in pharmaceutical sciences will select a division-specific training track that most closely matches their interests.
Each of the five division specific tracks, listed below, have course work and research training specifically tailored for training in the track-specific field.
- Chemical Biology and Medicinal Chemistry
- Molecular Pharmaceutics and Drug Delivery
- Pharmacology & Toxicology
Translational Science Degree
The Translational Science program is designed to provide training in the area of translational science toward the goal of applying basic biomedical scientific discoveries into strategies that will improve healthcare delivery, patient outcomes, and community health.
For many years, extensive national resources have been devoted to basic biomedical (bench) and clinical (human subject) research; however, significant barriers continue to exist in moving knowledge bi-directionally between basic research, clinical research, and applications to improve health outcomes in individuals and the community. These barriers make it difficult to efficiently and effectively translate new knowledge into improved patient care.
To remedy these problems, Translational Science has emerged as an academic and scientific discipline. Translational Science (TS) is a scientific discipline that investigates methods to move discovery more efficiently and effectively into application. TS investigators must respond, interact, facilitate change, and conduct research within and among varied organizational behaviors, structures, and cultures. TS conducts rigorous studies that close the knowledge gaps between different levels of the research process – the basic science laboratory, clinical research discoveries, and application of these research findings toward disease prevention and management, and strategies to improve human, community, and global health.
The doctoral degree program in Translational Science is offered as a multi-institutional joint degree program between The University of Texas at Austin, The University of Texas Health Science Center at San Antonio (UT Health San Antonio), and The University of Texas at San Antonio, in collaboration with The University of Texas School of Public Health regional campus in San Antonio. Graduate students complete their research on the campus of the university of their faculty mentor.
The Translational Science graduate program is designed to leverage the existing resources and expertise in specific key areas of each university to offer a strong, diverse, and competitive Ph.D. training program. This program is designed to prepare the next generation of scientists to lead multi-disciplinary biomedical research teams in increasingly complex research environments. These scientists will advance knowledge in the area of translational research toward the goal of applying basic biomedical scientific discoveries into strategies that will improve healthcare delivery, patient outcomes, and community health.
A complete description of the objectives of this graduate training program, course curriculum and milestones, eligibility for admission, and application process can be found on the UTHSCSA Translational Science site .
Graduate Research Tracks at the College of Pharmacy
| Research Track | Degrees Offered |
|---|---|
Academic Advising
Dr. Maria Croyle , the Graduate Advisor in the College of Pharmacy has overall responsibility for counseling and academic advising of graduate students in pharmaceutical sciences. However, each division within the College of Pharmacy appoints a Division Graduate Adviser, (DGA) who aids the students with course selection and programmatic progress. Each student must be advised by their DGA each semester before registering until they enter candidacy, to ensure the student is on track to graduate.
Contact the Pharmacy Graduate Coordinator in PHR 4.220 (512-471-6590) for an appointment or contact your supervising professor.

Give to the School

Drug Discovery: PhD Program
Uniquely positioned to bridge the chemical and biological worlds.

Our program seamlessly blends chemistry and biology, which distinguishes it from traditional graduate programs. We develop and exploit novel chemical tools relevant to the fields of biochemistry, biology, pharmacology, and medicine. Research is directed toward biomedical and pharmaceutical discovery by applying both chemical and biological principles to interactions between molecular structure and biological activity.
All of our graduates have been successful in finding desirable positions. An important measure of the success of our program is whether students are obtaining their first-choice postdoctoral positions. To a large extent, our recent graduates are receiving multiple offers from top labs. Our recent graduates have taken postdoctoral positions in prestigious labs at Harvard, Duke, Scripps, and MIT, to name a few.
PhD Requirements
Students enter the CBMC program through application to either the Pharmaceutical Sciences program or the Biological and Biomedical Sciences Program, a large umbrella program offering an entry to 16 PhD programs on the UNC-Chapel Hill campus. The Figure below shows the overview of the CBMC program. To complete the CBMC graduate program, a student needs to pass 8 courses, cumulative exams, qualifying exam and thesis defense. In addition, students will participate in the weekly seminar series throughout the training. Over the last 10 years, more than 50 PhD students have graduated from the CBMC program and the average time to completion is approximately 5 years. More details are described in the Student Handbook .
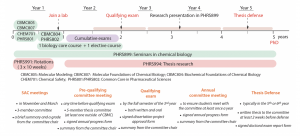
PhD Curriculum
CBMC 807: Molecular Foundations in Chemical Biology Prerequisites: Students are expected to have a solid understanding of introductory organic chemistry as taught at the undergraduate level.
This course provides a review of important concepts in organic chemistry as they apply to biological research. Topics include a review of intermolecular interactions as they apply to biological structures and function, a discussion of how small molecules interact with their targets, an overview of synthetic methods that relate particularly to drug molecules, and basic strategies of drug design.
CBMC 805: Molecular Modeling Prerequisites: None
This course provides a general introduction to the field of Molecular Modeling while providing relevant applications of theory to both academic and industrial research endeavors.
PHRS 801: Common Core in Pharmaceutical Sciences Prerequisites: None.
This course provides an interdisciplinary environment for students from each of the four Divisional PhD programs in UNC Eshelman School of Pharmacy. Students will learn about and develop skills in topics related to responsible conduct of research, pharmaceutical development, professional development, and independent development.
PHRS 899: Seminars in Chemical Biology Students must register for PHRS 899 each semester of their graduate program. However, only 4 credits of PHRS 899 (2 credits for MS) may count toward requirement for the PhD degree. Seminars are conducted jointly with the Division of Chemical Biology and Bioorganic Chemistry in the Department of Chemistry. Attendance at all Division seminars is mandatory and two unexcused absences will result in a grade of F. As an additional component of the seminar requirements of the graduate curriculum, attendance by all students is required during a student’s Doctoral Defense. These Defense seminars are held outside of the normal seminar series.
Each student is required to present a seminar in PHRS 899 either in the student’s third year or in the beginning of his/her fourth year and will be considered in assigning the grade in PHRS 899. In this seminar, the student critically reviews the area pertinent to his/her thesis topic making sure that s/he discusses studies that include his/her group’s contributions and those of other laboratories and includes a description of the student’s ongoing studies that add to this body of research. Faculty members will evaluate the student seminar. Students receiving an overall failing evaluation on the presentation will receive an “incomplete” grade in PHRS 899 for that semester and must consult with the seminar coordinator before giving a make-up seminar at a later date. Additionally, students receiving “incomplete” grades may be advised to seek further training in presentations. In those cases where the student’s research contains intellectual property (IP) and where disclosure risks the IP, a student can provide a comprehensive review of a different subject in medicinal chemistry. Permission to do so will require approval of the thesis adviser and the seminar coordinator. Each seminar topic, title, summary, and research article must be approved by the student’s research adviser and seminar coordinator. A student presenter should send the title of their seminar talk to the Graduate Program Coordinator upon request.
PHRS 991: Research in Pharmaceutical Sciences (Research Rotations) During the first two semesters, the student conducts three ~10-week research rotation projects, each under the supervision of a different faculty member. These rotation projects are considered as course work for PHRS 991. Students select rotations from any of the CBMC Faculty. With approval of the DDGS, students may also perform rotation projects with faculty outside the labs of the CBMC Faculty, especially when the rotation will provide training in an area that is outside of the expertise of the CBMC Faculty.
To select an adviser for each research rotation, the student should interview members of the CBMC faculty about possible projects. Prior to each rotation, the student will turn in a RESEARCH ROTATION LAB SELECTION FORM (Appendix A) to the Graduate Program Coordinator. Over the course of the first year each student is encouraged to schedule individual interviews with all members of the CBMC faculty. Although varied slightly from year to year, the rotations usually start in the late August and end in the late April in the next year. The schedule for the 2022-2023 academic year is as follows:
Fall : Monday, August 22 – Friday, October 28 (OK to start before August 22)
Winter : Monday, November 7-Friday, January 20
Spring : Monday, January 30 – Friday, April 7
Students admitted in the fall semesters can begin their rotations the summer before. In that scenario, the student must contact the Graduate Program Coordinator at least four weeks prior to the start of the rotation. The summer rotation will be considered as one of their three required rotations. A waiver for one research rotation may be granted if a student has previously completed substantial independent research. Students seeking such a waiver must petition the DDGS, and provide information (e.g. reports, manuscripts, grant proposals, and/or letters from research advisers) about their previous research. If a student has obtained a specific fellowship to work with a CBMC faculty member, then research rotations may be optional.
During lab rotations, students are expected to work in the laboratory at least 20 hours per week. Students are fully integrated into the laboratory during their rotation projects and are involved in lab meetings and journal clubs. At the beginning and end of each rotation, the rotation adviser and student review a written or oral statement of expectations for the student’s performance in the laboratory. These discussions provide the student with the advisers’ expectations and critical comments on areas of excellence and weakness. Guidelines for the research rotations are described in the PHRS 991 syllabus.
At the end of each rotation, students will submit a written report using the Research in Pharmaceutical Sciences – Student Evaluation Form (Appendix B) to the Graduate Program Coordinator. Students will also present the results of their rotation projects to their Student Advisory Committee (SAC). The SAC committee, in consultation with the rotation adviser, will provide a brief summary and evaluation and submit a grade to the Graduate Program Coordinator and the DDGS to be entered at the end of the semester. After the third rotation, students will present the work of their rotation in the CBMC End of Year Mini-Symposium.
The DDGS serves as the temporary advisor for the first-year students who enroll in PHRS 991. The SAC committee provides additional mentoring and consists of three CBMC faculty. The SAC is formed at the beginning of the first semester based on a student’s request and availability of faculty members.
CHEM 701 (Introduction to Laboratory Safety) Prerequisites: first year graduate student status or permission of instructor
This course provides an overview of safety rules and regulations, guidance in safe laboratory practice, and creates a culture of laboratory safety.
CBMC 804A: Biochemical Foundations of Chemical Biology . Prerequisites: CHEM 466, BIOC 505, 601, or PHCO 643; or permission of instructors.
This course covers core biochemical and molecular biology techniques, concepts, and tools used to conduct research at the interface of chemistry and biology. Topics include enzymology, characterization of drug-target interactions, mechanisms-based inhibitor design, assay design and development, targeting kinases and GPCRs, biopharmaceuticals, gene therapy, nucleic-acid binding agents, information-based drugs, chemical tools to study epigenetics, harnessing biosynthetic pathways for chemical diversity, and other recent advances and techniques in drug discovery.
CBMC 804B: Foundations of Chemical Biology Journal Club . Prerequisites: Enrollment in CBMC 804A.
This course is a series of presentations by students that run in concert with CBMC 804A.
Biology Core Course Each student has the option to choose one 3- or 4-credit hour course on campus that is focused on biological systems or techniques. A good starting point to find such a course is the BBSP website. Some examples include PHCO701 (Introduction to Molecular Pharmacology), BIOC706 (Biochemistry of Human Disease), GNET631 ( Advanced Molecular Biology), CBIO643 (Cell Structure and Function) and CBIO893 (Advanced Cell Biology).
PHRS 802: Drug Development and Professional Skills Development Prerequisites: None.
This course provides an interdisciplinary environment for students from each of the four Divisional PhD programs in UNC Eshelman School of Pharmacy. Students will learn about the general process of drug development and develop associated professional skills.
Elective Course: Students have the option to take one elective course of their interest. There is no requirement on the number of credit hours of the course. Students typically choose a course that provides specific skills and knowledge their thesis work needs.
PHRS 994: Research in Pharmaceutical Sciences (Thesis Research) The students begin to register 3 credit hours PHRS 994 each semester once they have chosen the thesis adviser. Guidelines for the thesis research are described in the PHRS 994 syllabus.
Meet Our Faculty and Staff
Cbmc faculty, katelyn arnold.

Katelyn Arnold is a Research Assistant Professor in the Division of Chemical Biology and Medicinal Chemistry (CBMC). Dr. Arnold’s research interest is in therapeutic development of synthetic heparin. She uses chemoenzymatically synthetized oligosaccharides in various animal models to investigate the relationship between oligosaccharide structure and function to understand anti-inflammatory mechanisms. This work also involves pharmacokinetic studies using methods and standards specific for synthetic heparin.

ACCEPTING DOCTORAL STUDENTS
The Aubé laboratory uses synthetic chemistry to enable the study of biological pathways and as starting points for drug discovery. Current efforts in the group include the study of new opioids lacking side effects, new approaches for the treatment of tuberculosis, androgen biosynthesis inhibitor discovery, the search for RNA-protein interaction inhibitors, and the development of new synthetic methods.
Alison Axtman

The Axtman lab is devoted to characterization of the dark proteome, especially those proteins with underexplored roles in the brain. Our interests lie at the interface of chemistry and biology, with a focus on using small molecules, specifically potent and selective chemical probes, to explore and impact disease-propagating pathways. Active projects are aimed at finding pre-clinical small molecule candidates that, after further optimization, can help address the need for new therapeutics in human diseases. Our scientists are working to design and synthesize chemical modulators and develop screening assays. All data and reagents are openly shared to facilitate and expedite scientific advancement.
Albert Bowers

Albert Bowers received his PhD in organic chemistry (synthetic methods) from the University of Illinois at Chicago. He carried out postdoctoral research (total synthesis) at Colorado State University before moving as an NIH sponsored fellow to Harvard Medical School (biosynthesis). He is a member of the UNC Lineberger Comprehensive Cancer Center and affiliate member of the Center for Integrative Chemical Biology and Drug Discovery.
Rafael Couñago

Rafael M. Couñago, PhD, is a Research Associate Professor in the Division of Chemical Biology and Medicinal Chemistry Department in the UNC Eshelman School of Pharmacy and a Principal Investigator at the Structural Genomics Consortium (SGC) at UNC. Rafael´s research group at SGC-UNC uses protein biochemistry, structural biology and cell-based assays to illuminate protein function and explore new therapeutic strategies for human diseases.
David Drewry

The Drewry lab in focused on designing, synthesizing, evaluating, and sharing small molecule chemical probes for protein kinases. These tools are used to build a deeper understanding of disease pathways and facilitate identification of important targets for drug discovery. Through wide ranging partnerships with academic and industrial groups, the Drewry lab is building a Kinase Chemogenomic Set (KCGS) that is available to the community for screening.
Kevin Frankowski

Research in the Frankowski lab uses synthetic chemistry to develop new approaches for the treatment of unmet medical needs. Our current efforts focus on programs to treat metastatic cancer, hepatitis C virus infection and the development of chemical tools for studying dopamine and sigma receptors.
Stephen Frye

Dr. Stephen Frye is currently a Professor in the Center for Integrative Chemical Biology and Drug Discovery (CICBDD) which he previously directed at the University of North Carolina at Chapel Hill (UNC). Prior to joining UNC to create the CICBDD in 2007, Dr. Frye was the world-wide vice president of Discovery Medicinal Chemistry (DMC) at GlaxoSmithKline (GSK). Dr. Frye led DMC for seven years, overseeing five departments and more than 200 chemists in the U.S. and U.K. developing global protein target-class chemical science for GSK. During his 20-year career at GSK, the teams led by Dr. Frye successfully developed three FDA approved drugs: Avodart, a dual 5a-reductase inhibitor for treatment of benign prostatic hyperplasia, Tykerb, a dual erbB2/EGFR inhibitor for the treatment of metastatic breast cancer, and Pazopanib, a multi-targeted receptor tyrosine kinase inhibitor for the treatment of renal cell carcinoma and soft tissue sarcoma. As founding director and current faculty member in the CICBDD at UNC, Dr. Frye plays a key role in translational research through collaborative drug discovery projects with other UNC faculty. A clinical candidate from one of these projects created in the Center is now progressing through multiple human trials. In addition, his lab has established a leading program in the area of chemical biology of chromatin regulation with an emphasis on protein-protein interactions dependent upon lysine methylation. Dr. Frye has published more than 130 papers in the fields of organic and medicinal chemistry.
Ryan Gumpper , Ph.D.

ACCEPTING DOCTORAL STUDENTS The Gumpper lab utilizes structural biology (cryoEM), biophysical/biochemical assays, and computational approaches (MD simulations, molecular modeling, and docking) to study the molecular mechanisms controlling G-protein-coupled receptor (GPCR) signaling. We are interested in revealing how molecular interactions influence the larger downstream biological and therapeutic outcomes. As a model system we focus on studying psychedelic compounds and their receptor targets (namely 5-HT2A and 5-HT2C receptors) to produce novel chemical matter to treat pain and neuropsychiatric disorders.
Lab website: https://tarheels.live/gumpperlab/
Lauren Haar

Our projects focus on investigating the role that refined spatial and temporal control of intracellular signaling cascades can play in the progression of cardiovascular injury. We use a research strategy involving plasmid and optogenetic protein engineering, high content screening, high resolution microscopy and physiologically based cell analysis. With this approach we hope to uncover new targets for therapeutic development by better defining signaling cascades that drive cardiovascular disease response.
Nate Hathaway

The Hathaway lab was established at UNC with a founding idea that the group could make a contribution to understanding dynamic epigenetic processes by using unique chemical biology approaches they pioneered. Through the combination of protein bioengineering, synthetic organic chemistry, and mammalian cell-based model systems, they have created platforms that use chemically tethered enzymatic recruitment to specific chromatin loci to produce a host of mechanistic insights. The Hathaway group also has drug discovery programs to identify new small molecules that inhibit disease relevant epigenetic pathways both for research purposes and as potential future therapeutics.
Lindsey Ingerman James

The James lab is interested in modulating the activity of chromatin reader proteins with small-molecule ligands, specifically potent and selective chemical probes, in order to open new avenues of research in the field of chromatin biology and potentially translate to compounds of therapeutic value. They are also interested in applying novel probe-based techniques, such as affinity labeling technologies, to the study of epigenetic regulators.
Michael Bruce Jarstfer

Michael Jarstfer, PhD, is an Associate Professor within the Division of Chemical Biology and Medicinal Chemistry and the Associate Dean for Graduate Education. He is also the Director of Graduate Studies for the Pharmaceutical Sciences PhD program. Dr. Jarstfer has expertise in drug target identification, high-throughput-screening, medicinal chemistry, and compound optimization for drug discovery as well as pharmacology in preclinical animal models.
David S. Lawrence

NOT ACCEPTING GRADUATE STUDENTS
The Lawrence lab works to understand the biochemical processes of the cell by studying them as they happen in the cell as opposed to studying them in vitro. He currently focuses on applying his discoveries to cancer detection and treatment and, to a more limited extent, inflammatory diseases.
Andrew L Lee

Andrew Lee studies the role of conformational dynamics in protein function, conformational changes, enzyme catalysis, drug binding, and allostery. His laboratory uses a variety of biophysical and biochemical tools, especially NMR spectroscopy. NMR spectroscopy is a powerful approach that yields atomic-resolution molecular information and is uniquely sensitive to molecular fluctuations over a broad range of timescales.

Research in the Jian Liu group is focused on glycobiology and glycobiochemistry, an emerging field that emphasizes the biological functions of carbohydrates. We are particularly interested in understanding the biosynthetic mechanism of sulfated polysaccharides known as heparan sulfate and heparin.
Rihe Liu , PhD

The Liu laboratory’s research interests focus on the development and application of novel drug target-binding affinity molecules by integrating directed molecular selection and evolution, ligand design and engineering, in vitro cellular and signaling characterization, and in vivo therapeutic efficacy studies in tumor mouse models. The Liu laboratory has extensive experiences in the design, synthesis, characterization, and delivery of diagnostic and therapeutic agents based on both polypeptides and polynucleotides.
Robert McGinty

The McGinty lab studies molecular mechanisms of chromatin signaling. By pairing atomic precision protein chemistry with high resolution structural biology, they aim to understand how the nucleosome functions as a signaling hub for gene expression, DNA replication, and DNA damage repair in development and disease.
Eugene Muratov

Dr. Muratov served as a corresponding author on an approach used by regulators to initially screen new chemical products for toxic effects. They have proposed an improvement that could increase the accuracy of toxicity estimation to as much as 85 percent, saving millions of dollars and years of development time for new drugs and other products while improving safety.
Samantha Pattenden

The Pattenden lab develops innovative techniques in chromatin-based therapeutic target discovery and cancer diagnostics. Our research program enables discovery of novel molecular targets, pathways and mechanisms. Our central strategy exploits tumor-specific changes in chromatin accessibility, a universal feature that is directly linked with transcriptional activation, DNA damage repair, replication, RNA processing, and nuclear organization.
Kenneth Pearce, Jr

Ken Pearce, Ph D is the director of the Center for Integrative Chemical Biology and Drug Discovery. Pearce’s primary expertise and interests are fundamentals of protein methods, biochemical and cell assay development, medium- and high-throughput screening, hit validation and mode-of-action, biophysical methods for characterizing protein-protein and small molecule-protein interactions, and structure-activity relationships for early drug discovery. He joined the center as director of lead discovery and characterization in mid-2015 after spending over 18 years at GlaxoSmithKline and legacy companies in the Molecular Discovery Research organization.
Konstantin Popov

The Popov Lab develops inventive, cutting-edge approaches to solve problems in modern computational structural biology and drug discovery. Their computational research, in collaboration with experimental screening and medicinal chemistry efforts in the Center for Integrative Chemical Biology and Drug Discovery enables the identification of novel chemical probes and drug candidates to advance understanding of biological processes. Some of their recent projects include: • Identification and characterization of allosteric and cryptic binding sites • Development of AI-driven methods for accelerated virtual screening (VS) • AI approaches for DNA-encoded library (DEL)-guided virtual screening (VS) and new-generation DEL library designs • Collaborative hit discovery and optimization projects

We are actively engaged in drug discovery efforts via the shared resources of the National Institute of Mental Health’s Psychoactive Drug Screening Program. Our goals are to discover and develop novel small molecule probes for in vitro and in vivo validation of molecular targets for therapeutic drug discovery. We have particular strengths with GPCR and ion-channels and have expanded our capabilities to enable screening of the entire GPCR-ome in massively parallel screening campaigns (see Keiser et al, Nature 2009; Huang et al, Nature in press and Kroeze et al, Nature Structural Biology 2015 for recent papers).
Paul Sapienza

Paul Sapienza is a research assistant professor in the Division of Chemical Biology and Medicinal Chemistry at the UNC Eshelman School of Pharmacy. His research aims to further understanding of the role of dynamics in biomolecular recognition, enzymatic catalysis, and allostery. He uses nuclear magnetic resonance spectroscopy to study protein dynamics on multiple timescales, while other tools such as calorimetry, crystallography, and kinetics serve to link dynamics with function. He is focusing on thymidylate synthase as it is an enzyme with a multistep catalytic cycle, is a cancer drug target, and exhibits negative cooperativity (allostery).
Scott Singleton

Scott Singleton is engaged in educational innovation and research. His work attempts to build on what is understood about memory and attention to devise, test, and implement effective teaching and learning strategies. His current work focuses on teaching that positively affects student engagement in the classroom, identifying core basic science concepts that serve as threshold concepts for pharmacy students, and evaluating the transfer of learning between courses in the professional PharmD program.
Junjiang Sun

Junjiang Sun is a Research Assistant Professor in the Division of Chemical Biology and Medicinal Chemistry (CBMC). Sun’s research interests focus on gene therapy for hemophilia, hemophilia associated joint disease (Hemophilia Arthropathy), by expressing bypassing agents (activated coagulation factor V, IX) via AAV vectors. This technology platform provides novel therapeutic approaches for rheumatoid arthritis (RA) and osteoarthritis (OA).
Alexander Tropsha , PhD

Alex Tropsha, Ph.D., is an expert in the fields of computational chemistry, cheminformatics and data science. His laboratory develops new methodologies, software tools and applications in the areas of computer-assisted drug design, chemical toxicology, materials informatics, text mining, and health care informatics.
Xiaodong Wang

The Wang lab is interested in developing drug leads/candidates for kinase, phosphate kinase and protein targets identified by UNC faculty and external investigators. We have successfully used the structure- and/or ligand-based drug design approaches to deliver compounds to clinic (MerTK inhibitors such as MRX-2843) or licensing (IDH1 inhibitor, co-developed with NCATs). We will continue to apply the similar approaches for drug discovery towards new targets.
Tim Willson

The Willson laboratory is home to the US site of the SGC, an open science consortium that accelerates research on the lesser studied regions of the genome. The laboratory works closely with pharma companies and academic investigators to develop small molecule chemical probes for hundreds of dark kinases that are openly shared with the scientific community. Current research has led to the development of the Kinase Chemogenomic Set (KCGS) that contains selective inhibitors of more than 200 kinases as well as high quality chemical probes for several of the dark kinases.

Dr. Xu co-authored the Heparin study with Dr. Jian Liu and Dr. Lindhardt. Heparin is a naturally occurring polysaccharide that prevents blood clotting, or coagulation, and has been in use since the late 1930s. A polysaccharide is a long chain of carbohydrate molecules.
Qisheng Zhang

The Zhang lab studies lipid signaling pathways that are involved in human disease by developing novel chemical probes and technologies. They currently focus on discovering new bioactive lipids, developing small molecule modulators and biosensors for lipid metabolizing enzymes, and applying their research results to novel diagnosis and treatment of cancer, Parkinson’s disease, and antimicrobial resistance.
CBMC Joint Appointments
Cbmc emeriti faculty, cbmc adjunct faculty, yuriy abramov , ph.d.
Nikolay Dokholyan, PhD, MS
Sean ekins , ph.d., denis fourches, phd, clark d jeffries , ph.d., jian jin , ph.d., kyoko nakagawa-goto , ph.d., david nichols , ph.d., lars pedersen, ph.d..
[email protected] Adjunct Associate Professor, UNC Eshelman School of Pharmacy Staff Scientist, Head of Collaborative Crystallography Lab, NIEHS
Dr. Tang is a collaborating member from ViiV Healthcare with the UNC-Chapel Hill HIV Cure Center. His research is focused on discovering novel HIV latency reversal agents with better tolerability and exploring new ways to clear the latent T cells upon activation and viral particles.
Lan Xie , Ph.D.
Weifan zheng , ph.d..

Sara Leslie

Ain G. Mason

Jacqueline Norris-Drouin

Michael Stashko


Meet our PhD Students and Fellows

Our students receive full financial support, including a competitive stipend, paid tuition and health insurance. They complete the Ph.D. program in approximately five years, and our graduates have taken postdocs in prestigious labs (at Harvard, Duke, Scripps and MIT to name a few) and found desirable positions in academia and pharmaceutical and biotechnology companies. We meet many of our students for the first time during the School’s Recruitment Weekend each January.
Download Brochure
- Graduate Students

- Andrew Perkowski

- Feijun Wang

Ready to take the next step?
ABOUT THE DIVISION APPLY NOW CONTACT US DOWNLOAD OUR BROCHURE

UC Davis Graduate Studies
Pharmaceutical chemistry, about the program, learn more about the program.
Students in the M.S. degree program in Pharmaceutical Chemistry will gain an in-depth understanding of the experimental and computational processes and societal issues that surround the discovery and design of modern pharmaceuticals. The program is intended for students seeking to be employed as research chemists in the pharmaceutical and biotechnology industries.
College of Letters and Science
Admissions and Fellowship Information
UC Davis General Admission Requirements Program Admissions Requirements
Admissions Actions
Program contact information, primary program contacts.
Program Coordinator Katie Danyo [email protected]
Graduate Program Chair Sheila David (530) 752-4280 | [email protected]
Department Chair David Goodin [email protected]
Additional Contacts
Advisor: Admissions Only David Olson Pharmaceutical Chemistry [email protected]
Advisor: Admissions and Primary Contact Dean Tantillo Pharmaceutical Chemistry [email protected]
Internal Fellowship Analyst Heidi West (530) 754-9473 | [email protected]
Senior Academic Advisor Samantha Duesdieker (530) 752-4928 | [email protected]
External Fellowship Analyst Yvette Garcia [email protected]

Pharmaceutical Chemistry
College of Letters and Science
- Bachelor of Science
Do you want to be on the forefront of modern pharmaceuticals? The demand for pharmaceutical chemists is high and is anticipated to grow as modern chemistry and biology provide us with increasingly accurate tools to develop unique therapies. The pharmaceutical chemistry major provides students with an in-depth understanding of the processes and societal issues surrounding the design and development of modern pharmaceuticals. Students in this major will learn how drugs work and how to synthesize, design and deliver them, as well as the ethical issues surrounding pharmaceutical development.
Major Requirements
The pharmaceutical chemistry program at UC Davis provides a strong foundation in biology, chemistry, mathematics and physics. The major focuses on design principles and experimental methods used in pharmaceutical and medical chemistry. Undergraduate research and study abroad are strongly encouraged and may apply to major requirements.
Contact Information
- Research Associate
- Facilities TCM Engineer
- STEM Educator
- Junior Specialist of Pathology and Lab Medicine
Graduate Study
- Biochemistry
- Medicinal chemistry
- Pharmaceutical sciences
- Chemical physics
Alumni Employers
- Origin Materials
- Terracon Consultants Incorporated
- Gilead Sciences
- Genentech, Inc.
- California Department of Justice, Santa Rosa
Related Degrees

Biochemical Engineering

Chemical Engineering

Internship opportunities
Explore internships and jobs on campus and beyond.

Your course roadmap
Find the detailed course requirements for your program and map out your path to graduation.

The faculty you will work with
Our undergraduates work directly with our faculty through research projects and labs.

Global learning programs
Expand your horizons by studying abroad or pursuing global learning on campus.

Undergraduate research
Get hands on with your interests. Participate in one of our hundreds of research opportunities.

What can I do with my pharmaceutical chemistry major?
Learn how to connect your pharmaceutical chemistry major to career opportunities.

IMAGES
COMMENTS
The Ph.D. in Pharmaceutical Chemistry is a full-time program offered in a face-to-face format at our Glassboro, NJ campus. It requires the completion of 63 graduate semester hours (21 courses). The following courses make up the Ph.D. in Pharmaceutical Chemistry. The courses listed above are not official and are subject to change.
The graduate program in Pharmaceutical Sciences spans the entire life cycle of a drug, from bench to bedside. The Medicinal Chemistry concentration focuses on drug discovery and development, part of the pre-clinical studies phase of the cycle. Pharmaceutical Sciences Ph.D. Programs Distinction through five interrelated training opportunities involving the entire life cycle of a drug.…
The Department of Pharmaceutical Chemistry has been named as a Madison and Lila Self Graduate Fellowship Program partner at The University of Kansas. The program provides a generous stipend and tuition for four years of graduate study to outstanding students. Other university-level awards include: Honors Fellowship - Awarded on a competitive ...
The department offers a PhD degree with no terminal MS option. In the first year, students typically conduct two to four 10-week rotations in different faculty labs to sample the breadth of research in the department. They then decide on a faculty mentor who will guide their dissertation research. Coursework requires students to become ...
The Department of Chemistry and Biochemistry offers research-centered Master and Ph.D. programs in Chemistry and Pharmaceutical Chemistry. The objectives of these programs are to give students who have an undergraduate foundation in chemistry the opportunity to engage in advanced course work, in-depth study and independent research.
The PhD in Pharmaceutical Sciences at Temple University's School of Pharmacy is a rigorous, research-based graduate course of study that prepares you for advanced scientific research. The curriculum integrates biochemistry, organic chemistry, pharmaceutics and pharmacology. You will acquire expertise on drug synthesis, action, delivery and ...
Medicinal Chemistry and Drug Discovery. The PhD Program in Medicinal Chemistry educates and trains students in the design and synthesis of novel, biologically active compounds and in delineating their mechanisms of action using biochemical, biophysical, and pharmacological approaches. Research specializations are available in synthetic ...
The program expanded in the 1950s to include analytical aspects and pharmaceutics. After Prof. Blicke's retirement in 1960, his former student, Prof. J. H. Burkhalter returned to the College and argued for an independent graduate education program in Medicinal Chemistry. Until that time, Ph.D. degrees in the College were in Pharmaceutical ...
Part 2 will test students' overall knowledge in another program focus covered by the pharmaceutical sciences curriculum. For example, if the student is in the pharmaceutics and drug delivery PhD program, part 1 will be about pharmaceutics and drug delivery, and part 2 can focus either on pharmacology or medicinal chemistry and drug discovery.
PhD Graduate Program. Our students receive an education in chemistry and biology that prepares them for the evolving multidisciplinary research of the pharmaceutical industry and academia. Our graduate program has been awarding PhD degrees to students for over seventy-five years and has a long and distinguished history of being among the top ...
The PhD program in pharmaceutical sciences includes coursework as well as cutting-edge research focused on topics like discovery and evaluation of novel drugs, determination of a drug's effects on the body, delivery methods to improve drug treatment, and how medication is used and applied to enhance patient outcomes. ... Medicinal Chemistry and ...
The Pharmaceutical Sciences PhD Program at the University of Wisconsin-Madison provides a rigorous background in scientific disciplines that are critical to the preparation of the next generation of pharmaceutical scientists. With approximately 30 faculty trainers and approximately 65 graduate students, the program's interdisciplinary training combines pharmaceutically relevant aspects of ...
Fall 2025 Admission Deadline: December 2, 2024 PhD in Pharmacological Sciences UC Irvine's PhD in Pharmacological Sciences program provides a unique opportunity for those interested in any scientific discipline represented by the Pharmaceutical Sciences faculty to have a year of broad, interdisciplinary training and self-selected lab rotations followed by focused doctoral research in the ...
Five concentrations comprise the PhD program in Pharmaceutical Sciences. Click on the tabs below to learn more about each of them. ... Faculty in the Chemistry in Drug Discovery concentration use the tools and techniques of chemistry to discover and develop new chemical probes and potential therapeutics. Students in this concentration learn how ...
First year pharmacy students can pursue the PharmD/PhD degree by conducting 3 research rotations with faculty on research topics of PSDD during years 1-2 of the pharmacy curriculum. Pharmacy students in their 2nd year can apply for admissions for the PhD program of the Biomedical Sciences graduate program at UCSD (see previous paragraph).
PhD Program. The Pharmaceutical & Translational Sciences (PHTS) Program brings together, under one umbrella, the school's three laboratory-based PhD programs—CXPT, MPTX, PSCI. This promotes a more cohesive interdisciplinary experience advancing education and offering opportunities to sample the entire range of innovative research options.
Department of Pharmaceutical Chemistry faculty members also teach in the Doctor of Medicine (MD) degree program, which is administered by the School of Medicine Dean's Office. The PharmD professional program prepares pharmacists to lead and innovate in pharmacy practice, policy, and science; to remain lifelong experts in the safe and ...
The PSPG graduate program, and before that the Pharmaceutics pathway of the Pharmaceutical Chemistry graduate program, is internationally recognized for training in the drug development sciences. Recently, we have added a new area of research, regulatory sciences, to drug development sciences through procurement of a large center grant in ...
The UT Austin College of Pharmacy operates longstanding and well-developed graduate training programs in pharmaceutical sciences and translational sciences. ... Pharmaceutical Science is an interdisciplinary field that includes areas of study in chemical biology and medicinal chemistry, pharmaceutics, pharmacology, toxicology, pharmacotherapy ...
Chemical Biology and Medicinal Chemistry (CBMC) is a dynamic, multifaceted graduate program dedicated to improving human health through research leading to new concepts for the design and development of therapeutic and diagnostic agents. Our program seamlessly blends chemistry and biology, which distinguishes it from traditional graduate ...
We explore fundamental biological mechanisms and molecules of therapeutic relevance for better health, empowered by novel technologies at the interface of chemistry, physics, and computational sciences. Elisabeth Fall.
Students in the M.S. degree program in Pharmaceutical Chemistry will gain an in-depth understanding of the experimental and computational processes and societal issues that surround the discovery and design of modern pharmaceuticals. The program is intended for students seeking to be employed as research chemists in the pharmaceutical and biotechnology industries.
Major Requirements. The pharmaceutical chemistry program at UC Davis provides a strong foundation in biology, chemistry, mathematics and physics. The major focuses on design principles and experimental methods used in pharmaceutical and medical chemistry. Undergraduate research and study abroad are strongly encouraged and may apply to major ...
The program is designed for completion in four years and requires a strong pre-pharmacy background, including coursework in biology, chemistry, physics, and mathematics. Program Length: 4 years; Tracks/concentrations: Doctorate in Pharmacy; Cost per Credit: $1,233; Required Credits to Graduate: 151; Accreditation: Accreditation Council for ...