- Fact sheets
Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /

HIV and AIDS
- HIV remains a major global public health issue, having claimed an estimated 42.3 million lives to date. Transmission is ongoing in all countries globally.
- There were an estimated 39.9 million people living with HIV at the end of 2023, 65% of whom are in the WHO African Region.
- In 2023, an estimated 630 000 people died from HIV-related causes and an estimated 1.3 million people acquired HIV.
- There is no cure for HIV infection. However, with access to effective HIV prevention, diagnosis, treatment and care, including for opportunistic infections, HIV infection has become a manageable chronic health condition, enabling people living with HIV to lead long and healthy lives.
- WHO, the Global Fund and UNAIDS all have global HIV strategies that are aligned with the SDG target 3.3 of ending the HIV epidemic by 2030.
- By 2025, 95% of all people living with HIV should have a diagnosis, 95% of whom should be taking lifesaving antiretroviral treatment, and 95% of people living with HIV on treatment should achieve a suppressed viral load for the benefit of the person’s health and for reducing onward HIV transmission. In 2023, these percentages were 86%, 89%, and 93% respectively.
- In 2023, of all people living with HIV, 86% knew their status, 77% were receiving antiretroviral therapy and 72% had suppressed viral loads.
Human immunodeficiency virus (HIV) is a virus that attacks the body’s immune system. Acquired immunodeficiency syndrome (AIDS) occurs at the most advanced stage of infection.
HIV targets the body’s white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
HIV is spread from the body fluids of an infected person, including blood, breast milk, semen and vaginal fluids. It is not spread by kisses, hugs or sharing food. It can also spread from a mother to her baby.
HIV can be prevented and treated with antiretroviral therapy (ART). Untreated HIV can progress to AIDS, often after many years.
WHO now defines Advanced HIV Disease (AHD) as CD4 cell count less than 200 cells/mm3 or WHO stage 3 or 4 in adults and adolescents. All children younger than 5 years of age living with HIV are considered to have advanced HIV disease.
Signs and symptoms
The symptoms of HIV vary depending on the stage of infection.
HIV spreads more easily in the first few months after a person is infected, but many are unaware of their status until the later stages. In the first few weeks after being infected people may not experience symptoms. Others may have an influenza-like illness including:
- sore throat.
The infection progressively weakens the immune system. This can cause other signs and symptoms:
- swollen lymph nodes
- weight loss
Without treatment, people living with HIV infection can also develop severe illnesses:
- tuberculosis (TB)
- cryptococcal meningitis
- severe bacterial infections
- cancers such as lymphomas and Kaposi's sarcoma.
HIV causes other infections to get worse, such as hepatitis C, hepatitis B and mpox.
Transmission
HIV can be transmitted via the exchange of body fluids from people living with HIV, including blood, breast milk, semen, and vaginal secretions. HIV can also be transmitted to a child during pregnancy and delivery. People cannot become infected with HIV through ordinary day-to-day contact such as kissing, hugging, shaking hands, or sharing personal objects, food or water.
People living with HIV who are taking ART and have an undetectable viral load will not transmit HIV to their sexual partners. Early access to ART and support to remain on treatment is therefore critical not only to improve the health of people living with HIV but also to prevent HIV transmission.
Risk factors
Behaviours and conditions that put people at greater risk of contracting HIV include:
- having anal or vaginal sex without a condom;
- having another sexually transmitted infection (STI) such as syphilis, herpes, chlamydia, gonorrhoea and bacterial vaginosis;
- harmful use of alcohol or drugs in the context of sexual behaviour;
- sharing contaminated needles, syringes and other injecting equipment, or drug solutions when injecting drugs;
- receiving unsafe injections, blood transfusions, or tissue transplantation; and
- medical procedures that involve unsterile cutting or piercing; or accidental needle stick injuries, including among health workers.
HIV can be diagnosed through rapid diagnostic tests that provide same-day results. This greatly facilitates early diagnosis and linkage with treatment and prevention. People can also use HIV self-tests to test themselves. However, no single test can provide a full HIV positive diagnosis; confirmatory testing is required, conducted by a qualified and trained health worker or community worker. HIV infection can be detected with great accuracy using WHO prequalified tests within a nationally approved testing strategy and algorithm.
Most widely used HIV diagnostic tests detect antibodies produced by a person as part of their immune response to fight HIV. In most cases, people develop antibodies to HIV within 28 days of infection. During this time, people are in the so-called “window period” when they have low levels of antibodies which cannot be detected by many rapid tests, but they may still transmit HIV to others. People who have had a recent high-risk exposure and test negative can have a further test after 28 days.
Following a positive diagnosis, people should be retested before they are enrolled in treatment and care to rule out any potential testing or reporting error. While testing for adolescents and adults has been made simple and efficient, this is not the case for babies born to HIV-positive mothers. For children less than 18 months of age, rapid antibody testing is not sufficient to identify HIV infection – virological testing must be provided as early as birth or at 6 weeks of age. New technologies are now available to perform this test at the point of care and enable same-day results, which will accelerate appropriate linkage with treatment and care.
HIV is a preventable disease. Reduce the risk of HIV infection by:
- using a male or female condom during sex
- being tested for HIV and sexually transmitted infections
- having a voluntary medical male circumcision
- using harm reduction services for people who inject and use drugs.
Doctors may suggest medicines and medical devices to help prevent HIV infection, including:
- antiretroviral drugs (ARVs), including oral Pre-Exposure Prophylaxis (PrEP) and long acting products
- dapivirine vaginal rings
- injectable long acting cabotegravir.
ARVs can also be used to prevent mothers from passing HIV to their children.
People taking antiretroviral therapy (ART) and who have no evidence of virus in the blood will not pass HIV to their sexual partners. Access to testing and ART is an important part of preventing HIV.
Antiretroviral drugs given to people without HIV can prevent infection
When given before possible exposures to HIV it is called pre-exposure prophylaxis (PrEP) and when given after an exposure it is called post-exposure prophylaxis (PEP). People can use PrEP or PEP when the risk of contracting HIV is high; people should seek advice from a clinician when thinking about using PrEP or PEP.
There is no cure for HIV infection. It is treated with antiretroviral drugs, which stop the virus from replicating in the body.
Current antiretroviral therapy (ART) does not cure HIV infection but allows a person’s immune system to get stronger. This helps them to fight other infections.
Currently, ART must be taken every day for the rest of a person’s life.
ART lowers the amount of the virus in a person’s body. This stops symptoms and allows people to live full and healthy lives. People living with HIV who are taking ART and who have no evidence of virus in the blood will not spread the virus to their sexual partners.
Pregnant women with HIV should have access to, and take, ART as soon as possible. This protects the health of the mother and will help prevent HIV transmission to the fetus before birth, or through breast milk.
Advanced HIV disease remains a persistent problem in the HIV response. WHO is supporting countries to implement the advanced HIV disease package of care to reduce illness and death. Newer HIV medicines and short course treatments for opportunistic infections like cryptococcal meningitis are being developed that may change the way people take ART and prevention medicines, including access to injectable formulations, in the future.
More information on HIV treatments
WHO response
Global health sector strategies on HIV, viral hepatitis, and sexually transmitted infections for the period 2022–2030 ( GHSSs ) guide strategic responses to achieve the goals of ending AIDS, viral hepatitis B and C, and sexually transmitted infections by 2030.
WHO’s Global HIV, Hepatitis and STIs Programmes recommend shared and disease-specific country actions supported by WHO and partners. They consider the epidemiological, technological, and contextual shifts of previous years, foster learning, and create opportunities to leverage innovation and new knowledge.
WHO’s programmes call to reach the people most affected and most at risk for each disease, and to address inequities. Under a framework of universal health coverage and primary health care, WHO’s programmes contribute to achieving the goals of the 2030 Agenda for Sustainable Development.
- Global HIV, Hepatitis and STIs Programmes
- Global Health Sector Strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030 (GHSS)
- GHSS report on progress and gaps 2024
- HIV country profiles
- HIV statistics, globally and by WHO region, 2024
What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medicine as Prescribed
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Nutrition and People with HIV
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 25
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
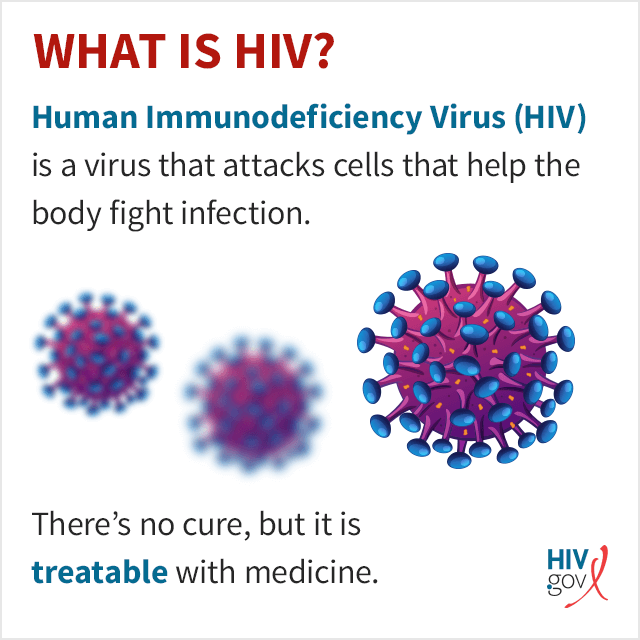
What Is HIV?
HIV ( human immunodeficiency virus ) is a virus that attacks cells that help the body fight infection, making a person more vulnerable to other infections and diseases. It is spread by contact with certain bodily fluids of a person with HIV, most commonly during unprotected sex (sex without a condom or HIV medicine to prevent or treat HIV), or through sharing injection drug equipment.
If left untreated, HIV can lead to the disease AIDS ( acquired immunodeficiency syndrome ).
The human body can’t get rid of HIV and no effective HIV cure exists. So, once you have HIV, you have it for life. Luckily, however, effective treatment with HIV medicine (called antiretroviral therapy or ART) is available. If taken as prescribed, HIV medicine can reduce the amount of HIV in the blood (also called the viral load) to a very low level. This is called viral suppression. If a person’s viral load is so low that a standard lab can’t detect it, this is called having an undetectable viral load. People with HIV who take HIV medicine as prescribed and get and keep an undetectable viral load can live long and healthy lives and will not transmit HIV to their HIV-negative partners through sex .
In addition, there are effective methods to prevent getting HIV through sex or drug use, including pre-exposure prophylaxis (PrEP) , medicine people at risk for HIV take to prevent getting HIV from sex or injection drug use, and post-exposure prophylaxis (PEP) , HIV medicine taken within 72 hours after a possible exposure to prevent the virus from taking hold. Learn about other ways to prevent getting or transmitting HIV .
What Is AIDS?
AIDS is the late stage of HIV infection that occurs when the body’s immune system is badly damaged because of the virus.
In the U.S., most people with HIV do not develop AIDS because taking HIV medicine as prescribed stops the progression of the disease.
A person with HIV is considered to have progressed to AIDS when:
- the number of their CD4 cells falls below 200 cells per cubic millimeter of blood (200 cells/mm3). (In someone with a healthy immune system, CD4 counts are between 500 and 1,600 cells/mm3.) OR
- they develop one or more opportunistic infections regardless of their CD4 count.
Without HIV medicine, people with AIDS typically survive about 3 years. Once someone has a dangerous opportunistic illness, life expectancy without treatment falls to about 1 year. HIV medicine can still help people at this stage of HIV infection, and it can even be lifesaving. But people who start HIV medicine soon after they get HIV experience more benefits—that’s why HIV testing is so important.
How Do I Know If I Have HIV?
The only way to know for sure if you have HIV is to get tested . Testing is relatively simple. You can ask your health care provider for an HIV test. Many medical clinics, substance abuse programs, community health centers, and hospitals offer them too. If you test positive, you can be connected to HIV care to start treatment as soon as possible. If you test negative, you have the information you need to take steps to prevent getting HIV in the future.
To find an HIV testing location near you, use the HIV Services Locator .
HIV self-testing is also an option. Self-testing allows people to take an HIV test and find out their result in their own home or other private location. With an HIV self-test, you can get your test results within 20 minutes. You can buy an HIV self-test kit at a pharmacy or online. Some health departments or community-based organizations also provide HIV self-test kits for a reduced cost or for free. You can call your local health department or use the HIV Testing and Care Services Locator to find organizations that offer HIV self-test kits near you. (Contact the organization for eligibility requirements.)
Note: State laws regarding self-testing vary and may limit availability. Check with a health care provider or health department Exit Disclaimer for additional testing options.
Learn more about HIV self-testing and which test might be right for you .
Related HIV.gov Blogs
On national hiv testing day, level up your self-love by checking your status, june 27 is national hiv testing day, leaders’ reflections: the ongoing importance of national hiv testing day.
- HIV Testing Day National HIV Testing Day
- World AIDS Day
- HIVinfo.NIH.gov – HIV and AIDS: The Basics
- CDC – HIV Basics
- NIH – HIV/AIDS
- OWH – HIV and AIDS Basics
- VA – HIV/AIDS Basics
- Español (Spanish)
Access presentations from different HIV-related organizations.
| Webpage (HTML) | Centers for Disease Control and Prevention | |
| Webpage (HTML) | UNAIDS | |
| Webpage (HTML) | AIDS Education and Training Center Program National Coordinating Resource Center | |
| Webpage (HTML) | Health Resources and Services Administration Ryan White HIV/AIDS Program | |
| Webpage (HTML) | Florida Health |
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

fall pumpkin
56 templates

fall background
24 templates

rosh hashanah
19 templates

cute halloween
17 templates

122 templates

indigenous canada
48 templates
HIV Disease
It seems that you like this template, hiv disease presentation, free google slides theme, powerpoint template, and canva presentation template.
The human immunodeficiency virus (HIV) affects more than 35 million people in the world. Since the first cases were known in the 1980s, great advances have been made, which continue to develop today. If you need to create a presentation about HIV, download this template with a light background and bright colours with which you can provide all the information about this disease: description, how it is diagnosed, recommendations and prevention measures, what is its treatment and conclusions about new findings.
Features of this template
- 100% editable and easy to modify
- 26 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Combines with:
This template can be combined with this other one to create the perfect presentation:

Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?

Register for free and start downloading now
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

- Patient Care & Health Information
- Diseases & Conditions
Acquired immunodeficiency syndrome (AIDS), is an ongoing, also called chronic, condition. It's caused by the human immunodeficiency virus, also called HIV. HIV damages the immune system so that the body is less able to fight infection and disease. If HIV isn't treated, it can take years before it weakens the immune system enough to become AIDS . Thanks to treatment, most people in the U.S. don't get AIDS .
HIV is spread through contact with genitals, such as during sex without a condom. This type of infection is called a sexually transmitted infection, also called an STI. HIV also is spread through contact with blood, such as when people share needles or syringes. It is also possible for a person with untreated HIV to spread the virus to a child during pregnancy, childbirth or breastfeeding.
There's no cure for HIV / AIDS . But medicines can control the infection and keep the disease from getting worse. Antiviral treatments for HIV have reduced AIDS deaths around the world. There's an ongoing effort to make ways to prevent and treat HIV / AIDS more available in resource-poor countries.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Assortment of Pill Aids from Mayo Clinic Store
The symptoms of HIV and AIDS vary depending on the person and the phase of infection.
Primary infection, also called acute HIV
Some people infected by HIV get a flu-like illness within 2 to 4 weeks after the virus enters the body. This stage may last a few days to several weeks. Some people have no symptoms during this stage.
Possible symptoms include:
- Muscle aches and joint pain.
- Sore throat and painful mouth sores.
- Swollen lymph glands, also called nodes, mainly on the neck.
- Weight loss.
- Night sweats.
These symptoms can be so mild that you might not notice them. However, the amount of virus in your bloodstream, called viral load, is high at this time. As a result, the infection spreads to others more easily during primary infection than during the next stage.
Clinical latent infection, also called chronic HIV
In this stage of infection, HIV is still in the body and cells of the immune system, called white blood cells. But during this time, many people don't have symptoms or the infections that HIV can cause.
This stage can last for many years for people who aren't getting antiretroviral therapy, also called ART. Some people get more-severe disease much sooner.
Symptomatic HIV infection
As the virus continues to multiply and destroy immune cells, you may get mild infections or long-term symptoms such as:
- Swollen lymph glands, which are often one of the first symptoms of HIV infection.
- Oral yeast infection, also called thrush.
- Shingles, also called herpes zoster.
Progression to AIDS
Better antiviral treatments have greatly decreased deaths from AIDS worldwide. Thanks to these lifesaving treatments, most people with HIV in the U.S. today don't get AIDS . Untreated, HIV most often turns into AIDS in about 8 to 10 years.
Having AIDS means your immune system is very damaged. People with AIDS are more likely to develop diseases they wouldn't get if they had healthy immune systems. These are called opportunistic infections or opportunistic cancers. Some people get opportunistic infections during the acute stage of the disease.
The symptoms of some of these infections may include:
- Fever that keeps coming back.
- Ongoing diarrhea.
- Swollen lymph glands.
- Constant white spots or lesions on the tongue or in the mouth.
- Constant fatigue.
- Rapid weight loss.
- Skin rashes or bumps.
When to see a doctor
If you think you may have been infected with HIV or are at risk of contracting the virus, see a healthcare professional as soon as you can.
More Information
- Early HIV symptoms: What are they?
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
HIV is caused by a virus. It can spread through sexual contact, shooting of illicit drugs or use of shared needles, and contact with infected blood. It also can spread from parent to child during pregnancy, childbirth or breastfeeding.
HIV destroys white blood cells called CD4 T cells. These cells play a large role in helping the body fight disease. The fewer CD4 T cells you have, the weaker your immune system becomes.
How does HIV become AIDS?
You can have an HIV infection with few or no symptoms for years before it turns into AIDS . AIDS is diagnosed when the CD4 T cell count falls below 200 or you have a complication you get only if you have AIDS , such as a serious infection or cancer.
How HIV spreads
You can get infected with HIV if infected blood, semen or fluids from a vagina enter your body. This can happen when you:
- Have sex. You may become infected if you have vaginal or anal sex with an infected partner. Oral sex carries less risk. The virus can enter your body through mouth sores or small tears that can happen in the rectum or vagina during sex.
- Share needles to inject illicit drugs. Sharing needles and syringes that have been infected puts you at high risk of HIV and other infectious diseases, such as hepatitis.
- Have a blood transfusion. Sometimes the virus may be transmitted through blood from a donor. Hospitals and blood banks screen the blood supply for HIV . So this risk is small in places where these precautions are taken. The risk may be higher in resource-poor countries that are not able to screen all donated blood.
- Have a pregnancy, give birth or breastfeed. Pregnant people who have HIV can pass the virus to their babies. People who are HIV positive and get treatment for the infection during pregnancy can greatly lower the risk to their babies.
How HIV doesn't spread
You can't become infected with HIV through casual contact. That means you can't catch HIV or get AIDS by hugging, kissing, dancing or shaking hands with someone who has the infection.
HIV isn't spread through air, water or insect bites. You can't get HIV by donating blood.
Risk factors
Anyone of any age, race, sex or sexual orientation can have HIV / AIDS . However, you're at greatest risk of HIV / AIDS if you:
- Have unprotected sex. Use a new latex or polyurethane condom every time you have sex. Anal sex is riskier than is vaginal sex. Your risk of HIV increases if you have more than one sexual partner.
- Have an STI . Many STIs cause open sores on the genitals. These sores allow HIV to enter the body.
- Inject illicit drugs. If you share needles and syringes, you can be exposed to infected blood.
Complications
HIV infection weakens your immune system. The infection makes you much more likely to get many infections and certain types of cancers.
Infections common to HIV/AIDS
- Pneumocystis pneumonia, also called PCP. This fungal infection can cause severe illness. It doesn't happen as often in the U.S. because of treatments for HIV / AIDS . But PCP is still the most common cause of pneumonia in people infected with HIV .
- Candidiasis, also called thrush. Candidiasis is a common HIV -related infection. It causes a thick, white coating on the mouth, tongue, esophagus or vagina.
- Tuberculosis, also called TB. TB is a common opportunistic infection linked to HIV . Worldwide, TB is a leading cause of death among people with AIDS . It's less common in the U.S. thanks to the wide use of HIV medicines.
- Cytomegalovirus. This common herpes virus is passed in body fluids such as saliva, blood, urine, semen and breast milk. A healthy immune system makes the virus inactive, but it stays in the body. If the immune system weakens, the virus becomes active, causing damage to the eyes, digestive system, lungs or other organs.
- Cryptococcal meningitis. Meningitis is swelling and irritation, called inflammation, of the membranes and fluid around the brain and spinal cord, called meninges. Cryptococcal meningitis is a common central nervous system infection linked to HIV . A fungus found in soil causes it.
Toxoplasmosis. This infection is caused by Toxoplasma gondii, a parasite spread primarily by cats. Infected cats pass the parasites in their stools. The parasites then can spread to other animals and humans.
Toxoplasmosis can cause heart disease. Seizures happen when it spreads to the brain. And it can be fatal.
Cancers common to HIV/AIDS
- Lymphoma. This cancer starts in the white blood cells. The most common early sign is painless swelling of the lymph nodes most often in the neck, armpit or groin.
- Kaposi sarcoma. This is a tumor of the blood vessel walls. Kaposi sarcoma most often appears as pink, red or purple sores called lesions on the skin and in the mouth in people with white skin. In people with Black or brown skin, the lesions may look dark brown or black. Kaposi sarcoma also can affect the internal organs, including the lungs and organs in the digestive system.
- Human papillomavirus (HPV)-related cancers. These are cancers caused by HPV infection. They include anal, oral and cervical cancers.
Other complications
- Wasting syndrome. Untreated HIV / AIDS can cause a great deal of weight loss. Diarrhea, weakness and fever often happen with the weight loss.
- Brain and nervous system, called neurological, complications. HIV can cause neurological symptoms such as confusion, forgetfulness, depression, anxiety and difficulty walking. HIV -associated neurological conditions can range from mild symptoms of behavior changes and reduced mental functioning to severe dementia causing weakness and not being able to function.
- Kidney disease. HIV -associated nephropathy (HIVAN) is swelling and irritation, called inflammation, of the tiny filters in the kidneys. These filters remove excess fluid and waste from the blood and pass them to the urine. Kidney disease most often affects Black and Hispanic people.
- Liver disease. Liver disease also is a major complication, mainly in people who also have hepatitis B or hepatitis C.
There's no vaccine to prevent HIV infection and no cure for HIV / AIDS . But you can protect yourself and others from infection.
To help prevent the spread of HIV :
Consider preexposure prophylaxis, also called PrEP. There are two PrEP medicines taken by mouth, also called oral, and one PrEP medicine given in the form of a shot, called injectable. The oral medicines are emtricitabine-tenofovir disoproxil fumarate (Truvada) and emtricitabine-tenofovir alafenamide fumarate (Descovy). The injectable medicine is called cabotegravir (Apretude). PrEP can reduce the risk of sexually transmitted HIV infection in people at very high risk.
PrEP can reduce the risk of getting HIV from sex by about 99% and from injecting drugs by at least 74%, according to the Centers for Disease Control and Prevention. Descovy hasn't been studied in people who have sex by having a penis put into their vaginas, called receptive vaginal sex.
Cabotegravir (Apretude) is the first U.S. Food and Drug Administration-approved PrEP that can be given as a shot to reduce the risk of sexually transmitted HIV infection in people at very high risk. A healthcare professional gives the shot. After two once-monthly shots, Apretude is given every two months. The shot is an option in place of a daily PrEP pill.
Your healthcare professional prescribes these medicines to prevent HIV only to people who don't already have HIV infection. You need an HIV test before you start taking any PrEP . You need to take the test every three months for the pills or before each shot for as long as you take PrEP .
You need to take the pills every day or closely follow the shot schedule. You still need to practice safe sex to protect against other STIs . If you have hepatitis B, you should see an infectious disease or liver specialist before beginning PrEP therapy.
Use treatment as prevention, also called TasP. If you have HIV , taking HIV medicines can keep your partner from getting infected with the virus. If your blood tests show no virus, that means your viral load can't be detected. Then you won't transmit the virus to anyone else through sex.
If you use TasP , you must take your medicines exactly as prescribed and get regular checkups.
- Use post-exposure prophylaxis, also called PEP, if you've been exposed to HIV . If you think you've been exposed through sex, through needles or in the workplace, contact your healthcare professional or go to an emergency room. Taking PEP as soon as you can within the first 72 hours can greatly reduce your risk of getting HIV . You need to take the medicine for 28 days.
Use a new condom every time you have anal or vaginal sex. Both male and female condoms are available. If you use a lubricant, make sure it's water based. Oil-based lubricants can weaken condoms and cause them to break.
During oral sex, use a cut-open condom or a piece of medical-grade latex called a dental dam without a lubricant.
- Tell your sexual partners you have HIV . It's important to tell all your current and past sexual partners that you're HIV positive. They need to be tested.
- Use clean needles. If you use needles to inject illicit drugs, make sure the needles are sterile. Don't share them. Use needle-exchange programs in your community. Seek help for your drug use.
- If you're pregnant, get medical care right away. You can pass HIV to your baby. But if you get treatment during pregnancy, you can lessen your baby's risk greatly.
- Consider male circumcision. Studies show that removing the foreskin from the penis, called circumcision, can help reduce the risk of getting HIV infection.
- About HIV and AIDS . HIV.gov. https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/what-are-hiv-and-aids. Accessed Oct. 18, 2023.
- Sax PE. Acute and early HIV infection: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/search. Accessed Oct. 18, 2023.
- Ferri FF. Human immunodeficiency virus. In: Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV . HIV.gov. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations. Accessed Oct. 18, 2023.
- AskMayoExpert. Human immunodeficiency virus (HIV) infection. Mayo Clinic; 2023.
- Elsevier Point of Care. Clinical Overview: HIV infection and AIDS in adults. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Male circumcision for HIV prevention fact sheet. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/male-circumcision-HIV-prevention-factsheet.html. Accessed Oct. 19, 2023.
- Acetyl-L-carnitine. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Whey protein. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Saccharomyces boulardii. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Vitamin A. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Red yeast rice. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
Associated Procedures
- Liver function tests
News from Mayo Clinic
- Mayo Clinic Minute: Know your status -- the importance of HIV testing June 25, 2024, 04:00 p.m. CDT
- Unlocking the mechanisms of HIV in preclinical research Jan. 10, 2024, 03:30 p.m. CDT
- Mayo Clinic expert on future of HIV on World AIDS Day Dec. 01, 2023, 05:15 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

Slide sets from talks and workshops
9 July 2024. Related: Advocates , Resources .
The following selected slide sets from over 60 community workshops and other talks were produced by i-Base advocates.
These are free to use for any non-commercial application.
When third-party slides are used these remain the copyright of the original author.
Most talks are by Simon Collins, unless specified otherwise.
2024 changes in the UK Guide to PrEP: a community perspective

It is a community perspective on the upcoming changes in the upcoming joint BHIVA/BASHH PrEP Guidelines expected later in 2024.]
A technical presentation of the key studies and data supporting these changes was given by Dan Clutterbuck at the BHIVA annual conference this year and also by Sheena McCormack to the HIV GUM training course several week earlier.
The non-techincal talk also explains the background to both the UK Community Guide and the timeline for the UK Guidelines. The slideset includes the data slides relating to oral PrEP from Dan Clutterbuck’s talk at BHIVA.
C&W PrEP – PDF (4.4 MB)
C&W PrEP – PowerPoint file (9.5 MB)
500 sexual partners: Including community in HIV research

The session was about approaches to statistical modelling.
This talk was abiout the importance of including community to make sure the best and most relevant data is available for these models. It includes examples of where community input changed the design of research studies.
It also highlighted examples where the lack of data becomes a block to effective services, for example over transgender health, support services for chemsex and PrEP for trans men.
The title was based on the example of partner numbers asked for in recent survey, where the upper limit was just >10. This ignored an important percentage of people where numbers could easily be much higher. The importance of risk ir perhaps more important than partner number.
BASHH modelling 2024 FINAL (PDF)
Treatment and prevention update 2024

They were run by Simon Collins and Angelina Namiba at the 6th UK Conference for people living with HIV.
The workshops included first-hand experience from someone using injectable ART (CAB/RPV-LA), important new options on for taking PrEP, and the importance of solidarity and support for trans and non-binary people during 2024.
HIV+ UK 2024 FINAL 010624 (15 MB)
4MNet: Women, treatment and our wellbeing

It included co-morbidities that affect us and about living well with HIV.
This was for women living in Kenya, Uganda and the UK.
The talk is also posted to Vimeo online . https://vimeo.com/907478359/ceb50db723
IAS 2023 – UK-CAB Feedback
Community feedback talk from the IAS 2023 conference held in Brisbane, Australia in July 2023.
The slides were based on virtual reporting from the meetings.
Highlights included new cure-related studies, including the Geneva patient. Also lots about HIV complications including the REPRIEVE study that used a statin to reduce cardiovascular risk.
IAS 2023 feedback – PDF talk IAS 2023 feedback – PowerPoint slides
cliniQ conference: PrEP for trans people
These few slides were used as an introduction to aa panel discussion at the 9th CliniQ Conference held on 20 April 2023.
The discussion wanted to cover important issues including the lack of data on trans people in the UK who use PrEP, but also to emphasise that PrEP is just as safe and effective as for cisgender people.
Also that there are no drug interactions between PrEP and gender affirming hormones.
CliniQ PrEP apr 23 – PowerPoint slides CliniQ PrEP apr 23 – PDF slides.
Aidsfonds Stakeholder meeting on cure-related research
This was research workshop held on 10 January 2023 brought together different stakeholders involved in cure-related research.
The couple of slides here highlighted three headline points that were used in some of the group discussions.
AIDSFONDS slides – jan 23 – PowerPoint AIDSFONDS slides – jan 23 – PDF
HIV activism and future HIV drug research
This talk was given as part of the Turkish Civil Society #HIV2022 Conference held from 4 to 6 November 2022 in Istanbul.
Although the main conference was aa face-to-face meeting, this talk was given virtually.
It looks at the role of activism in developing effective HIV drugs and includes information of drugs currently in the pipeline.
Turkey #HIV2022 – PowerPoint Turkey #HIV2022 – PDF
HIV late diagnosis: Article and interview
These links are to an article in a special supplement the medical journal HIV Medicine about late HIV diagnosis from a community perspective.
The authors are all members of the UK-CAB.
The interview is for Medscape about this and other papers from the supplement.
Late diagnosis of HIV in 2022: Why so little change? Collins S, Namiba A, Sparrowhawk A, Strachan S, Thompson M, Nakamura H. HIV Medicine. 2022; 23(11): 1118–1126. doi:10.1111/hiv.13444. https://onlinelibrary.wiley.com/doi/10.1111/hiv.13444
Medscape. Late HIV tied to misclassifications in European surveillance efforts (21 December 2022). https://www.medscape.com/viewarticle/985966
BMJ podcast on mpox
This links to an interview and discussion about the mpox outbreak.
EATG/STEPS cure workshop
This short talk was given on 23 October 2022 as part of a workshop on cure research organised by the EATG at the Glasgow Congress.
It recommends the IAS review of cure-related research, and talks about community issues in that document. This includes the importance of have greater diversity of participants in these studies – including by sex, gender, race, ethnicity and geographic region.
Includes new data from TAG on sex and ethnicity in cure research and the personal impact of joining a cure study.
Download slides – PDF file
HIV science for the community
This slide set covers two European workshops in September 2022 organised by Africa AIDS Foundation (AAF).
They give an introduction to HIV treatment for health workers who have clients who are living with HIV,
Download PowerPoint slides (5 MB) Download PDF (2 MB)
Research priorities for an HIV cure: the science in context: a community perspective
This talk was given in July 2022 as part of the IAS cure research workshop held in Montreal just before the AIDS 2022 conference.
It included talking about how community advocates are involved in this research, but that this needs to be expanded, Community representation needs to include a wider group of people globally just as cure-related research also needs to be international.
Download PDF file (12 MB) Link to all talks at the workshop .
HIV treatment and innovations in care
Talk given jointly in June 2022 with Angelina Namiba from the 4MM network. This was for a workshop as part of the 5th UK Conference of people living with HIV.
This covered latest HIV drugs and those expected in the near future.
This included long-acting drugs and the potential for different formulations.
It also talked about standards of care in the UK and the issues linked to HIV and ageing.
Download PowerPoint slides PowerPoint slides (5 MB) Download PDF file (1.8 MB)
The RIO study
This is a very short talk given to participants on the UK RIO study.
This study uses two bNAbs to see whether viral load can remain undetectable after infusions of bNAbs and a treatment interruption.
Download PDF (300 KB)
Community involvement in the IAS cure strategy
Talk given in May 2022 as part of an IAS webinar.
This focussed on the IAS strategy for cure research, especially the latest 5-year review on recent and future research.
This included input of community advocates in this process.
Download PowerPoint slides (3.4 MB) Download PDF file (3 MB)
Feedback from CROI 2022
This is a talk to the UK-CAB in April 2022 about the main new from the recent CROI 2022 conference.
This included new drugs like lenacapavir, bNAbs, long-acting injectable ART, cure-related research, the ANCHOR study and more.
Download PDF (6 MB)
Women and ageing
This talk in March 2022 looked at issues that are important for women getting older with HIV.
This includes being actively involved in care and treatment decisions, medical issues that need monitoring and screening, side effects of ART and the importance of good mental health.
Download PDF (3 MB)
Women, treatment and well-being
Talk on 23 March 2022 for workshops organised by the 4MM project.
This is a general overview about being engaged and active in your own treatment decisions.
It was an introduction to HIV including CD4 counts and viral load, adherence and starting and changing HIV meds.
Download PowerPoint slides (5 MB) Download PDF file (1 MB)
Caution on CAB-LA PrEP and population drug resistance
This was a short talk for the AfroCAB about long-acting cabotegravir injections (CAB-LA) and PrEP.
It is essential that CAB-LA becomes rapidly available as an option to protect against HIV all people globally. Modelling studies show that access can safely provided with only a small risk of drug resistance.
This talk highlights some of these areas with a caution that models should include the possibility that larger percentages of people discontinue CAB-LA, as they have done with oral PrEP.
Download PowerPoint slides (2 MB) Download PDF (2 MB)
Healthy Living with HIV: what do COVID vaccines mean for people living with HIV
Talk given to a medical workshop on the 2 October 2021 about what vaccines mean for people living with HIV.
PowerPoint slides
Use of generic HIV medicines in the UK
Slides from a short talk on 1 July 2021 at a regional meeting in on Access, Quality and Pricing of HIV Drugs in SEE Countries (Bosnia and Herzegovina, Montenegro, North Macedonia, and Serbia).
i-Base was included to give a perspective from the UK, including that generic HIV drugs are not only widely used, but are an essential strategy to retaining free health care on the NHS.
Generics in the UK – (PowerPoint slides) Generics in the UK (PDF)
HIV 40 Years On: perspective of being an active patient
Webinar on 23 June 2021, jointly organised by the Fast Track Cities London and the Worshipful Society of Apothecaries.
This was both to recognise the medical advances for the 40th anniversary of the first publication of HIV cases in the US and to look forward to the goals of “Getting to Zero” by 2030.
Download slides (PDF) Webcast and info on Fast Track Cities website Webcast on You Tube
Community panel webinar on COVID-19
Roundtable discussion organised by Jim Pickett from AIDS Foundation Chicago and IAS-USA with six community advocates on difference aspects of COVID-19. No slides but maybe of interest to hear about different international perspectives.
Watch on YouTube .
Introduction to U=U for Trans and MSM HIV support group in Nepal
These slides were for a virtual workshop on 22 May 2021 about U=U for a Trans and MSM HIV support group in Nepal.
Many of the slides are similar to other U=U presentations, but this started with general questions and discussions.
The science showing U=U is a fact came after everyone had a good understanding of what U=U meant in practice.
U=U peer support Nepal – Slides with notes (PDF 3 MB) U=U peer support Nepal – 22 May 2021 (PDF 8MB)
History of U=U: getting wider awareness
This talk was part of a peer support virtual workshop on 1 May 2021 on U=U.
It was held with 50 community advocates in countries in South East Asia.
Download PDF file . (7 MB)
Treatment interruption in cure research during COVID-19
This talk on 4 March 2021 was part of the always excellent Community Cure Workshops organised by TAG, DARE and other US activist groups before CROI every year.
The talk looked at whether HIV cure research should continue to use analytic treatment interruptions (ATIs) during COVID-19. This is because of concerns that it might increase risks for study participants.
The talk looks at two discussion papers (on mainly from the US and the other mainly from the UK). It includes way to reduces risks (new entry criteria, fewer hospital visits, use of vaccines) and of course the importance of informed consent.
Powerpoint slides (6 MB) – Please contact i-Base PDF file (6 MB) – Please contact i-Base Webcast (with discussion)

Q&A on COVID-19: vaccines and variants
Slides for a zoom talk on 15 February 2021 to Positive Action Foundation Philippines (PAPFI).
This included a short introduction to COVID-19 and potential treatments, but was mainly an update on the safety and efficacy of the new vaccines.
Plus a range of frequent questions over safety of COVID-19 vaccines for HIV positive people.
Powerpoint slides (.PPT 3MB) PDF slides (PDF 3MB)
Non-technical review of vaccines for COVID-19

This includes that more than 300 candidate vaccines are in studies, 40 in humans, 10 in phase 3, and two approved or almost approved (Pfizer/BioNTech and Moderna/NIH).
It briefly describes the main approaches with summary results on the Pfizer, Moderna and Oxford/Astra-Zeneca vaccines – and the importance of future studies using an active rather than placebo control.
Also, the plans for fair and equitable access globally. And the challenges given that high income countries have already bought or optioned nearly all the first vaccines to be manufactured next year.
Finally, examples of many questions HIV positive people have about the chance of using these vaccines ourselves.
Powerpoint slides .pptx and PDF slides (both about 8 MB)
Link to webcast – STILL TO COME
Lest we forget – Early community involvement in UK HIV response

The community talk included examples of the first reports in the community newspaper Capital Gay and the response from Lesbian and Gay pubs and clubs to collect money for HIV projects. It covers the diversity of community organisations to provide support to different communities of people living with HIV.
This included THT, Body Positive, Birchgrove, Positively Women, Blackliners, Mainliners and many more – and many publications that provided important information and also generated a stronger community network.
PowerPoint slides – (9 MB) Webcast link to both talks . The community talk starts at 16.50.
COVID-19 and upcoming HIV drugs
Workshop on 21 October to the long-standing and dynamic community organisation Positive EAST.
This included this 30-minute talk with Greg Leonard who runs many of the support groups at this project in East London and who recently launched a programme of virtual workshops linked to their FaceBook page.
The talk was given as the second wave of COVID-19 had just become establish in London.
Download PDF slides – (3 MB) – PDF file Download Powerpoint slides – (6 MB) – PowerPoint file Watch talk on FaceBook
Introduction to science and HIV research
Workshop as part of EATG STEP course – an introduction to HIV research.
The talk was on 25 September 2020.
It includes research examples that will surprise most people – and therefore show how science can change out mind,
It also includes references to other online resources on treatment literacy.
Download PDF slides . (4.7 MB) Download PowerPoint slides. (4.8 MB)
History of Treatment as Prevention (TasP) and U=U
Workshop as part of EATG STEP course on use of ART as HIV prevention.
The talk highlights key research over the last 22 years that steadily accumulated enough evidence to establish that effective HIV treatment prevents HIV transmission.
Download PDF slides. (5MB) Download PowerPoint slides. (5MB)
Introduction to science (via COVID-19)
This virtual zoom workshop for the EATG general assembly (GA) on 19 September 2020 was about understanding, monitoring, and discussing scientific data for advocacy.
It was a refresher course on different types of research studies using examples from the last nine months of COVID-19 – compressing the equivalent of 40 years of HIV research. This includes data sites (epidemiology), how COVID-19 develops (pathogenesis) and a review of effective treatments and those that should not be used.
In the search for effective treatment, and among thousands of studies, it shows why randomised controlled trials (RCTs) are still needed. Actually, that they are the only way to really know whether or not a promising drug is effective.
Webcast link .
Download PDF of slides . (4.5 MB)
Readability: importance of information that is easy to read for most people
This talk is avoid the importance of good readability scores when producing patient information material – especially in documents for clinical studies.
The talk was given on 3 September 2020 to a working group at Gilead Sciences in the UK and US.
It was based on a talk given at the BHIVA conference in April 2015 (see below).
Download slides (PDF) – 9 MB Readabilty study Gilead Sep 2020 – (PowerPoint – 9.3 MB)
AIDS 2020 feedback (and COVID-19 update no.3)
Feedback to UK-CAB on 28 July 2020 on treatment news from the AIDS 2020 virtual conference and another update on COVID-19.
These studies included positive news on dolutegravir and neural tube defects, longer follow-up on continued weight gain in the ADVANCE study, results using cabotegravir long-acting injections for PrEP and a case reporting HIV remission.
It also included problems navigating the AIDS 2020 website.
The COVID-19 updated summarised important studies reported in the HIV and COVID-19 bulletins from i-Base.
Watch talk on YouTube .
COVID-19 and HIV coinfection: Update 2
A second talk to UK-CAB on 5 June 2020 about advances in research on COVID-19 over the last month.
It also includes statisticians from Public Health England to talk a new report highlighting risk factors for COVID-19, including race, ethnicity and employment.
The main talk includes six new studies on HIV/COVID-19 coinfection, US approval and UK access to remdesivir, reviews of UK research and of promising results with anakinra, anticoagulants, ACE inhibitors, interferon and convalescent plasma. Also controversial results with hydroxychloroquine. Plus updates from BHIVA and EACS statements.
Watch zoom talk on YouTube (via UK-CAB)
UK-CAB COVID19 june 2020 – (PDF – 12MB) UK-CAB COVID-19 june 2020 – (PowerPoint) – please email as large file
COVID-19: summary of current research
Workshop with the European AIDS Treatment Group (EATG) on 15 May 2020.
This was a 30 minute talk about rapidly changing knowledge about COVID-19.
It was also, for EATG members to be able to talk about COVID-19 responses in our different countries and how this has been affecting people living with HIV.
The slides in this talk are similar to the UK-CAB meeting below.
Watch zoom talk on YouTube (via EATG) Powerpoint slides (.PPT 2.6 MB) PDF slides (PDF, 2.2 MB)
COVID-19 and HIV coinfection: key research
Talk to virtual UK-CAB Zoom meeting on 1 May 2020. Mainly links to recent research from recent HTB coverage about HIV and COVID-19.
UK-CAB COVID-19 apr 2020 – (PDF – 2MB) UK-CAB COVID-19 apr 2020 – (PowerPoint – 2.2 MB)
CROI 2020 feedback – virtual meeting
Talk as part of virtual UK-CAB Zoom meeting on 30 April 2020.
The talk mainly focused on studies involving new HIV drugs for treatment or prevention (PrEP).
UK-CAB feedback from CROI 2020 (April 2020) – PDF (17 MB)
Please email for Powerpoint slides as these are too large to post online
Future PrEP: next generation drugs

It reviews a range of new drugs that are being developed both as HIV treatment and PrEP. This might include:
- Long-acting injections (every two months).
- A removable implant that would provide PrEP for over a year, and
- A small oral pill that you might only need to take once a month.
Watch to video on YouTube (15 minute talk) HIV PrEP pipeline feb 2020 – PDF (8MB) HIV PrEP pipeline feb 2020 – PowerPoint (8MB)
U=U: approaches by community and professional organisations in the UK
This talk in January 2020 was given at a U=U conference in Tokyo organised by the Japanese HIV Society.

This talk focused on the ways that BHIVA has shown leadership to publicise U=U. It also includes many examples of how individuals and organisations in the UK have also widened awareness of how HIV treatment prevents sexual transmission.
UK approach to putting U=U in practice This notes file shows slides in colour with written talk below on each page – PDF (5 MB)
The PowerPoint file is too large to post here but we can send an individual weblink to download if anyone would like this. Please email: [email protected] ).
Community cure meeting: bNAbs and the RIO study
Talk from November 2019 included in the programme of the European AIDS Conference (EACS 2019) held in Basel.
The workshop looked at advances and issues related to HIV cure-related research.
It included information about a study called RIO that will be using two long-acting bNAbs with a treatment interruption. The RIO study is due to start in early 2020.
This workshop was organised by the European Community Advisory Board (ECAB) which is part of the European AIDS Treatment Group (EATG).
RIO Study – EATG cure meeting EACS – Powerpoint slides (3 MB)
RIO Study – EATG cure meeting EACS – PDF (2.5 MB)
Community perspectives of new research
Talk from June 2019 as part of HMRG European HIV Seminar held in Dublin.
The talk looked at advances in treatment over three decades and yet new drugs are still approved with limited data in women, during pregnancy and with TB drugs. Also the delay in access for children.
HMRG 2019 – PDF slides (7 MB)
CROI 2019 feedback
Set of approximately 60 slides compiled from key studies presented at the recent CROI 2019 medical conference in Seattle.
This presentation was to the UK-Community Advisory Board (UK-CAB) meeting on 6 April 2019.
CROI 2019 feedback UK-CAB – PDF slides (5.6 MB)
Is rapid ART right for all?
Talk given on the last day of the 25th BHIVA conference held in Bournemouth (BHIVA 2019).
The talk includes reports from two clinics where people are offered ART when they have their first appointment after diagnosis – often on the same day. All other services and tests and services are reorganised to enable this.
Webcast link – (At approx 1hr2mins if doesn’t load automatically)
Rapid ART for all? – PDF slides. (3 MB)
Rapid ART for all? – notes – PDF with notes. (3 MB)
Ethical issues and informed consent in HIV cure research studies: EATG workshop, Glasgow 2018
A talk looking at a common disconnect between researchers and participants in HIV cure-related studies.
More researchers expect no or little clinical benefit. Many participants still think they had a slight or more significant hope that they might be cured.
ECAB cure – Glasgow 2018 – PDF (100 Mb)

20th National HIV Nurses Association (NHIVNA) conference, July, Brighton
Evidence for U=U: the PARTNER study and Prevention Access Campaign
Talk as part of the pre-conference workshop about the evidence over twenty years for the U=U campaign.

This included the London version: A=A: Ain’t no viral load, ain’t no risk of HIV.
NHIVNA U=U talk 27 June 2018 (PDF – (7.2 MB)
NHIVNA U=U talk with NOTES (PDF – 3.4 MB)
4th National Conference of People Living with HIV (October 2017)
Treatment update for London HIV positive conference.
Topics cover evidence for U=U and treatment updates for new drugs.
This talk was largely based on the talks given at the HIV Scotland conference below.
UK HIV+ conference 2017 Treatment update (PDF – 8.5 MB)
Paediatric HIV pipeline drugs (October 2017)
Talk on pipeline HIV drugs for children given by Polly Clayden to a closed meeting of the IMPAACT trial network.
This is a focus on the paediatric pipeline, that usually takes several years (at least) after a drug is approved for adults.
This also usually occurs in age bands – first adolescents, then children and finally infants and babies. Many HIV drugs, however, are never approved for the youngest children.
Paediatric ART pipeline IMPAACT – (PDF – 950 Kb)

Positive Peoples Forum – HIV Scotland (July 2017)
Two talks for the annual HIV positive conference held in Glasgow this year.
Undetectable = untransmittable (U=U) – PDF (3.3 MB)
A talk about HIV treatment as prevention (TasP) that reviews the evidence for why so many doctors, scientist and community groups say that an undetectable viral load means you cannot transmit HIV to partners.
This was included as an idea in US guidelines in 1998 and has been supported by accumulating evidence from different studies over the last 20 years.
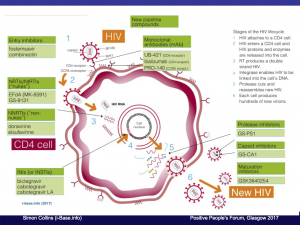
Short update about new HIV meds in development, drug pricing in the UK, cure research and PrEP.
This includes the HIV lifecycle to show how current HIV drugs work at different sites and the new targets for the most promising upcoming drugs.
A couple of slides are included on drug pricing in the UK and how generics affect this.
CROI 2017: feedback to UK-CAB (April 2017)
Short talk to feedback key studies for the UK-CAB.
Mainly to cover cure research, new drugs and treatment strategies.
CROI 2017 feedback UK-CAB – PDF (5 MB)
Review of i-Base treatment information services (April 2016)
Talk given by Robin Jakob to BHIVA conference about the phoneline and Q&A services.
http://www.bhiva.org/160420RobinJakob.aspx
Reference: HIV treatment information and advocacy 2014/15: continued demand for community support services . R Jakob, R Trevelion, J Dunworth, M Sachikonye and S Collins. 22nd Annual BHIVA Conference, 19-22 April 2016, Manchester. Oral abstract O5. HIV Medicine, 17 (Suppl. 1), 3–13. HIV Medicine 17, 4, 2016. Treatment advocacy – 2014-15 – Robin Jakob – PDF slides. http://www.bhiva.org/documents/Conferences/2016Manchester/AbstractBook2016.pdf
HIV testing: who, how and why? (September 2015)

Martin was one of the key doctors whose skills, energy and determination over the last 20 years were responsible for ensuring that HIV positive people in the UK received such high standards of care.
He was also a scientist and researcher, activist and friend. Martin was an incredibly kind, passionate, popular and modest man and his partner Adrian together with his family, friends and colleagues have launched this foundation to celebrate and continue his work.
HIV Testing Martin Fisher foundation talk – PowerPoint (2 Mb) HIV testing MFF – PDF (2 Mb)
Watch webcast on Vimeo .
2015 treatment update: good time for a change (September 2015)

- Impact of START study on BHIVA and WHO guidelines.
- Absolute and relative benefits from earlier treatment.
- TasP and PrEP.
- Immune inflammation.
- ART in acute infection and cure research.
UK HIV conference 2015 – PDF (900 Kb) UK PWA conference 2015 – PowerPoint (1.1 Mb)
START study results and new BHIVA guidelines – UK-CAB (July 2015)

- The question of when to start HIV treatment.
- The differences between observational studies and randomised controlled trials (RCTs).
- Design of the START study.
- Top-line results and implications.
- Draft UK BHIVA treatment guidelines: key changes and how to comment.
PowerPoint slides UK-CAB july 2015 (250 Kb) PDF file UK-CAB july 2015 (160 Kb)
Treatment Q&A at Bloomsbury Clinic (May 2015)

The talk included a doctor, a study researcher and an advocate.
PowerPoint – (4.5 MB) PDF – (4.8 Mb)
Are patient information leaflets for research studies too difficult to read? (April 2015)
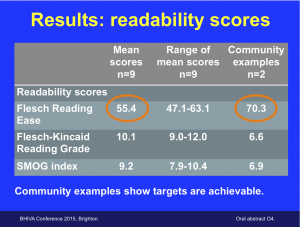
It was a small study lead by advocates at i-Base and was selected by the conference for an oral presentation (Abstract O_4).
The study calculated readability scores from nine ongoing studies and compared them to two community-produced examples.
The presentation was also awarded one of two prizes from BHIVA and Mediscript for the best oral or poster research on social science or community-based work.
PowerPoint slides (1.6 Mb) PDF version – (1.1 Mb)
Community feedback from CROI 2015 – UK-CAB (April 2015)

- The PROUD and IPERGAY studies
- Other PrEP studies including PARTNERS PrEP and FACTS 001.
- TAF – new version of TDF
- Attachment inhibitors and maturation inhibitors.
PowerPoint file – (5.3 Mb) PDF slides file – PDF (3MB) 6-up handout – PDF (1.2 Mb)
Seminar on PrEP and chemsex – Copenhagen (March 2015)

Talk about recent and current experiences in the UK about PrEP.
This included community involvement in the PROUD study, the IDMC recommendation in October 2014 to offer PrEP to all participants, and community responses since.
Plus a short update on PrEP studies at CROI 2015.
The meeting was organised by the Danish HIV organisation AIDSFONDNET and included HIV positive and HIV negative community activists and health professionals.
PrEP talk copenhagen – PDF (5.9 Mb)
Imperial College BSc Global Health course (Jan 2015)

PDF file (1.5 MB)
Please email for higher resolution or PowerPoint files.
Imperial College medical students (Dec 2014)
Talk to 300 first-year medical students at Imperial College about role of patient/doctor partnership and communication. Also how treatment literacy can support people to take an active role in their healthcare.
Includes references to early US activism, including the Denver Principles (1983) and personal perspectives as a patient activist in the UK.
PowerPoint with notes (6.1 MB) PDF file (6.1 MB) – please email for higher resolution files .
PrEP: a community perspective (November 2014)

Talk about PrEP (Pre-Exposure Prophylaxis) from the HIV Conference in Glasgow. The talk looks at the history of PrEP and a few myths.
Although optimal use will be in situations where HIV risk is high, PrEP – just like TasP (Treatment as Prevention) – may help reduce stigma against HIV.
It may also help improve quality of life by reducing fear and anxiety about HIV.
Powerpoint with notes (7.5 MB) PDF file (2.4 MB) PrEP talk slide notes Word.doc (50 Kb)
This talk is available as a webcast. https://vimeo.com/244353226
Collins S. Journal of the International AIDS Society 2014, 17(Suppl 3) :19522 Conference abstract .

Option to use ART in early HIV infection (Oct 2014)
Talk about the importance for people who are diagnosed within 6 months of infection to be given the choice of using ART, irrespective of CD4 count. This short window may be very different to starting at any time after 6 months and yet many people do not have this discussion. Talk for BHIVA autumn conference 2014.
BHIVA 2014 early ART talk – PDF (200 Kb) BHIVA 2014 early ART talk – PowerPoint (400 Kb)
UK-CAB advocates training (Sep 2014)
- Introduction to science and importance of evidence-based medicine. Powerpoint slides (1 MB) and PDF (1.1 MB)
- Tips for writing patient-friendly information – Powerpoint slides (200 Kb) and PDF file (280 Kb)
Two talks for this 4-day UK-CAB advocacy training .
Health service constraints on HIV care – the research agenda: a UK perspective (March 2014)
Short presentation to European CHAIN research network to mark the completion of this five-year research programme. PDF and PowerPoint (500 Mb)
Community views on long-acting ARVs (March 2014)
Short presentation to US NIH mixed stakeholder meeting (researchers, regulators, industry and community) on the development of long-acting HIV meds, especially injections. PowerPoint (600 Mb) and PDF (400 Mb),
Talk to medical students (Nov 2013)
Talk to 300 first-year medical students at Imperial College about how HIV activism has affected patent involvement in care. Powerpoint (with notes) (6 MB) and PDF (slides only) (6 MB)
Hot topics in HIV treatment (Oct 2013)
Talk to UK HIV positive conference on six important topics that could change HIV care in the next year: Access to ARVs and generics in the new NHS/When to start and why guidelines differ… /Treatment as Prevention/New HIV drugs: Stribild, dolutegravir, TAF, GSK-744/Hepatitis C and sexual transmission/Hepatitis C: new HCV drugs (DAAs) – PDF (98 Kb)
UK-CAB advocates training (Oct 2013)
- Introduction to science and importance of evidence-based medicine. Powerpoint slides (1 MB) and PDF (1.1 MB)
- Tips for writing patient-friendly information – Powerpoint slides (200 Kb) and PDF file (280 Kb)
HIV and cancer (April 2013)

This talk highlights that it is mainly because HIV treatment is so effective that we are living long enough for cancer to be a complication. For nearly all cancers, older age is one of the key risk factors.
HIV makes the risk of some cancers slightly higher than the general population. These risks are still relatively unlikely events. To reduce these risks, the same lifestyle changes as the general population are just as important for HIV positive people – perhaps more so.
From a personal perspective, managing a cancer diagnosis is serious and meant you have to make your health a main priority to give yourself the best chance. This gives many people a new chance to make the most of their time, irrespective of the outcome.
PowerPoint (135 Kb) PDF (70 Kb)
HIV cure research: pieces in the puzzle (January 2013)

This non technical talk explains the science behind four key approaches to cure research. This is a puzzle and all four are likely to be needed on the way to finding a cure.
The context includes the context for the research, background on why a cure is difficult and ethical issues related to this research.
PowerPoint (2.5 Mb) PDF (3.4 Mb)
Budget issues affecting treatment choice: the London tender process (November 2012)

PowerPoint slides November 2012 – (180 Kb) PDF file (140 Kb) Text notes (Word.docx)
This talk is available as a webcast on the conference website: MAC webcast – PC webcast .
IAS July 2012 – Washington
Feedback from the International AIDS conference held in Washington in July 2012: PDF (400 Kb) PowerPoint (975 Kb)
Slides for a talk at a feedback meeting organised by the UK Consortium .
CROI February 2010 – San Francisco
A talk on community perspectives from the Retroviruses Conference (CROI) given to the feedback meetings organised by BHIVA.
- BHIVA CROI community slides reduced PDF (4MB)
Treatment Action Campaign
- Factsheet: measures used for CD4 and viral load (millilitre, microlitre, mL, mm3, μL) PDF Jan 2010
Showing the different measures for CD4 counts and viral load including millilitre, microlitre, mL, mm3, μL). Also, showing why you still have many millions of CD4 cells in your body even at very low CD4 counts.
- Pharmacology: how drugs are absorbed in your body PDF – Jan 2010
Set of graphs showing how drugs are absorbed and then leave your body and how missing a dose leads to low levels and the risk of resistance. Explains the pharmocology terms including Cmax, Tmax, half-life (T½), MEC (minimum effective concentration) etc.
- Key studies and new issues from 2009 research PDF – 2009
Set of 12 slides listing important studies and issues from 2010.
- Introduction to drug resistanceTAC PDF – 2008
Trials and Research
- Community involvement in Data & Safety Monitoring Boards (DSMB)
What is a DSMB, why should community members be involved, what is required. Training for the European Community Advisory Board.
UK-CAB presentations
- Presentations from more than 75 UK-CAB meetings
Slides from all UK-CAB meetings are posted online as an open access training resource.
Conference slides (archive)
- Retrovirus conference (CROI) 2008: ARVs – PDF
- IAS conference 2007 feedback (Sydney) – PDF ,
- IAS conference 2006 feedback (Toronto) – PowerPoint
- Drug development timelines (2006) TAC – PDF , PowerPoint
- Importance of treatment literacy (2006) Moscow – PowerPoint
- Community outreach in national and international trials (2006) MRC – PowerPoint
- Life experience: self empowerment through treatment advocacy (2005) Taiwan – PowerPoint

Global AIDS Update 2024: Core Epidemiology Slides
These factsheet slides from Joint United Nations Programme on HIV/AIDS (UNAIDS) provide a global snapshot of the state of the world’s HIV/AIDS epidemic. The slides present a high-level summary of the most current global estimates on the prevalence, transmission, and mortality of HIV/AIDS. The slides provide HIV/AIDS statistics for children and adults, by global region, and for specific age groups and vulnerable populations over time. The slides may be downloaded in PowerPoint format.
Core Epidemiology Slides. Joint United Nations Programme on HIV/AIDS 2024. https://www.unaids.org/en/resources/documents/2024/core-epidemiology-slides .
Learn more with these Related Resources
This article from The Lancet Global Health estimates mortality trends in youth aged 15 to …
This article from The Lancet Global Health estimates mortality trends in youth aged 15 to 24 using data from the Global Burden of Disease Study (GBD) 2019. According to the authors, the probability of an individual dying between 15 to 24 years was 11.2 deaths per 1000 youths in 2019. This demonstrates a 34% decrease between 1990 and 2019, from 17.1 deaths per 1000 in 1990. However, this progress is considerably slower than declines in…
This resource portal from Save the Children offers free online access to more than 5,000 …
This resource portal from Save the Children offers free online access to more than 5,000 resources related to the organization’s thematic priorities: child protection, child rights governance, health and nutrition, education, and child poverty. Materials include briefs and fact sheets, case studies, child-friendly resources, classroom and training materials, guides and manuals, reports, research articles, international legal documents, and more. Individuals can filter by numerous categories, including topic, publisher, language, region or country, content type, or…
This webcast seminar from The Forum at the T.H. Chan School of Public Health examines …
This webcast seminar from The Forum at the T.H. Chan School of Public Health examines the safekeeping of American drinking water. Although Flint, Michigan has been the most visible example of polluted drinking water in the past several years, many American cities have histories of unsafe levels of lead and other toxins in their drinking water as a result of old infrastructure and other environmental issues, and recent polls show that Americans are very worried…

HIV Infection and AIDS Clinical Presentation
- Author: Shelley A Gilroy, MD, FACP, FIDSA; Chief Editor: Michael Stuart Bronze, MD more...
- Sections HIV Infection and AIDS
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Approach Considerations
- Screening for HIV Infection
- CD4 T-cell Count
- Secondary HIV Testing
- Baseline Studies
- Histologic Findings
- HAART Studies and DHHS Guidelines
- Prophylaxis for Opportunistic Infections
- Treatment of Opportunistic Infections
- Treatment of HIV-Associated Lipodystrophy
- Suppressive Therapy for Herpes Simplex Virus 2 Infection
- Treatment of HIV-Associated Diarrhea
- Deterrence and Prevention of HIV Infection
- Consultations
- Long-Term Monitoring
- CDC Guidelines on HIV Screening in Men Who Have Sex with Men
- European AIDS Clinical Society Guidelines
- Department of Health and Human Services
- International Antiviral Society-USA Panel
- Medication Summary
- Antiretroviral agent, nucleoside reverse-transcriptase inhibitor
- Antiretroviral Agent, Protease Inhibitor
- Antiretroviral agent, non-nucleoside reverse-transcriptase inhibitor
- Antiretroviral Agent, Integrase Inhibitor
- Antiretroviral agent, CCR5 antagonist
- HIV, Entry Inhibitors
- HIV, Attachment Inhibitors
- Capsid Inhibitors
- Complete Regimen Combinations
- Antiretroviral Combinations
- CYP3A4 Inhibitors
- Antibiotic, Sulfonamide Derivative
- Growth hormone releasing factor
- Questions & Answers
- Media Gallery
The history should be carefully taken to elicit possible exposures to human immunodeficiency virus (HIV). Risk factors include the following:
Unprotected sexual intercourse, especially receptive anal intercourse (8-fold higher risk for transmission)
A large number of sexual partners
Prior or current sexually transmitted diseases (STDs): Gonorrhea and chlamydia infections increase the HIV transmission risk 3-fold, syphilis raises the transmission risk 7-fold, and herpes genitalis raises the transmission risk up to 25-fold during an outbreak
Sharing of intravenous drug paraphernalia
Receipt of blood products (before 1985 in the United States)
Mucosal contact with infected blood or needle-stick injuries
Maternal HIV infection (for newborns, infants, and children): Steps taken to reduce the risk of transmission at birth include cesarean delivery and prenatal antiretroviral therapy in the mother and antiretroviral therapy in the newborn immediately after birth.
The patient may present with signs and symptoms of any of the stages of HIV infection. Acute seroconversion manifests as a flulike illness, consisting of fever, malaise, and a generalized rash. The asymptomatic phase generally is benign. Generalized lymphadenopathy is common and may be a presenting symptom.
AIDS manifests as recurrent, severe, and occasionally life-threatening infections and/or opportunistic malignancies. The signs and symptoms are those of the presenting illness, meaning that HIV infection should be suspected as an underlying illness when unusual infections present in apparently healthy individuals.
HIV infection itself does cause some sequelae, including AIDS-associated dementia/encephalopathy and HIV wasting syndrome (chronic diarrhea and weight loss with no identifiable cause).
No physical findings are specific to HIV infection. The physical findings are those of the presenting infection or illness. Generalized lymphadenopathy is common. Weight loss may be apparent.
Evidence for risk factors or minor concurrent opportunistic infections (eg, herpetic lesions on the groin, widespread oral candidiasis) may be clues to HIV infection.
Justiz Vaillant AA, Naik R. HIV-1 Associated Opportunistic Infections. StatPearls . 2022 Jan. [QxMD MEDLINE Link] . [Full Text] .
Waymack JR, Sundareshan V. Acquired Immune Deficiency Syndrome. StatPearls . 2022 Jan. [QxMD MEDLINE Link] . [Full Text] .
US Preventive Services Task Force., Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA . 2019 Jun 18. 321 (23):2326-2336. [QxMD MEDLINE Link] . [Full Text] .
Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep . 2006 Sep 22. 55:1-17; quiz CE1-4. [QxMD MEDLINE Link] . [Full Text] .
Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med . 2009 Jan 20. 150(2):125-31. [QxMD MEDLINE Link] . [Full Text] .
Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection : updated recommendations. Centers for Disease Control and Prevention. Available at https://stacks.cdc.gov/view/cdc/23447 . June 27, 2014; Accessed: January 9, 2023.
World Health Organization (WHO). HIV/AIDS. World Health Organization (WHO). Available at https://www.who.int/health-topics/hiv-aids/#tab=tab_1 .
Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis . 2012 Jun. 12(6):441-8. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. October 17, 2017. U.S. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf . September 21, 2022; Accessed: January 9, 2023.
US Food and Drug Administration. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies. US Department of Health and Human Services, US Food and Drug Administration. Available at https://www.hiv.gov/blog/fda-approves-first-rapid-diagnostic-test-to-detect-both-hiv-1-antigen-and-hiv-12-antibodies . August 8, 2013; Accessed: January 9, 2023.
Lowes R. FDA OKs First Rapid Test for HIV-1/2 Antibodies, HIV-1 Antigen. Medscape [serial online]. Available at https://www.medscape.com/viewarticle/809183 . Accessed: August 15, 2013.
1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep . 1992 Dec 18. 41:1-19. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. DHHS. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf . Last update: Sep. 21, 2022; Accessed: December 29, 2022.
Talal AH, Monard S, Vesanen M, et al. Virologic and immunologic effect of antiretroviral therapy on HIV-1 in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr . 2001 Jan 1. 26(1):1-7. [QxMD MEDLINE Link] . [Full Text] .
Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol . 2003 Nov. 77(21):11708-17. [QxMD MEDLINE Link] . [Full Text] .
Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol . 2003 May. 77(10):5621-31. [QxMD MEDLINE Link] . [Full Text] .
Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol . 2006 Aug. 80(16):8236-47. [QxMD MEDLINE Link] . [Full Text] .
Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol . 1995 Nov. 69(11):6859-64. [QxMD MEDLINE Link] . [Full Text] .
Keating SM, Golub ET, Nowicki M, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS . 2011 Sep 24. 25(15):1823-32. [QxMD MEDLINE Link] . [Full Text] .
Landires I, Bugault F, Lambotte O, et al. HIV infection perturbs interleukin-7 signaling at the step of STAT5 nuclear relocalization. AIDS . 2011 Sep 24. 25(15):1843-53. [QxMD MEDLINE Link] . [Full Text] .
Lederman MM. Immune restoration and CD4+ T-cell function with antiretroviral therapies. AIDS . 2001 Feb. 15 Suppl 2:S11-5. [QxMD MEDLINE Link] . [Full Text] .
Finkel TH, Tudor-Williams G, Banda NK, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med . 1995 Feb. 1(2):129-34. [QxMD MEDLINE Link] . [Full Text] .
Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell . 1996 Aug 9. 86(3):367-77. [QxMD MEDLINE Link] . [Full Text] .
U.S. Department of Health and Human Services. AIDSInfo. AIDSInfo. Available at https://www.hiv.gov/authors/aidsinfo . Accessed: January 9, 2023.
Jeffrey S. FDA approves first antidiarrheal drug for HIV/AIDS. Medscape Medical News . Dec 31, 2012. [Full Text] .
Gao F, Bailes E, Robertson DL, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature . 1999 Feb 4. 397(6718):436-41. [QxMD MEDLINE Link] . [Full Text] .
Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature . 1989 Jun 1. 339(6223):389-92. [QxMD MEDLINE Link] . [Full Text] .
Popper SJ, Sarr AD, Gueye-Ndiaye A, Mboup S, Essex ME, Kanki PJ. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J Virol . 2000 Feb. 74(3):1554-7. [QxMD MEDLINE Link] . [Full Text] .
Popper SJ, Sarr AD, Travers KU, et al. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis . 1999 Oct. 180(4):1116-21. [QxMD MEDLINE Link] . [Full Text] .
Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med . 1997 Jun 15. 126(12):946-54. [QxMD MEDLINE Link] . [Full Text] .
Rodríguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA . 2006 Sep 27. 296(12):1498-506. [QxMD MEDLINE Link] . [Full Text] .
Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep . 1981 Jul 3. 30(25):305-8. [QxMD MEDLINE Link] . [Full Text] .
Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science . 1983 May 20. 220(4599):868-71. [QxMD MEDLINE Link] . [Full Text] .
Ascher MS, Sheppard HW, Winkelstein W Jr, Vittinghoff E. Does drug use cause AIDS?. Nature . 1993 Mar 11. 362(6416):103-4. [QxMD MEDLINE Link] . [Full Text] .
Korber B, Muldoon M, Theiler J, et al. Timing the ancestor of the HIV-1 pandemic strains. Science . 2000 Jun 9. 288(5472):1789-96. [QxMD MEDLINE Link] . [Full Text] .
Koopman G, Haaksma AG, ten Velden J, Hack CE, Heeney JL. The relative resistance of HIV type 1-infected chimpanzees to AIDS correlates with the maintenance of follicular architecture and the absence of infiltration by CD8+ cytotoxic T lymphocytes. AIDS Res Hum Retroviruses . 1999 Mar 1. 15(4):365-73. [QxMD MEDLINE Link] . [Full Text] .
Birch MR, Learmont JC, Dyer WB, et al. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J Clin Virol . 2001 Oct. 22(3):263-70. [QxMD MEDLINE Link] . [Full Text] .
Dyer WB, Geczy AF, Kent SJ, et al. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS . 1997 Nov. 11(13):1565-74. [QxMD MEDLINE Link] .
Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med . 1993 Feb 4. 328(5):327-35. [QxMD MEDLINE Link] .
Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol . 1995. 13:487-512. [QxMD MEDLINE Link] .
Weber J. The pathogenesis of HIV-1 infection. Br Med Bull . 2001. 58:61-72. [QxMD MEDLINE Link] .
Frazer IH, Mackay IR, Crapper RM, et al. Immunological abnormalities in asymptomatic homosexual men: correlation with antibody to HTLV-III and sequential changes over two years. Q J Med . 1986 Oct. 61(234):921-33. [QxMD MEDLINE Link] .
Schechter MT, Boyko WJ, Craib KJ, et al. Effects of long-term seropositivity to human immunodeficiency virus in a cohort of homosexual men. AIDS . 1987 Jul. 1(2):77-82. [QxMD MEDLINE Link] .
Talal AH, Irwin CE, Dieterich DT, Yee H, Zhang L. Effect of HIV-1 infection on lymphocyte proliferation in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr . 2001 Mar 1. 26(3):208-17. [QxMD MEDLINE Link] .
Poles MA, Boscardin WJ, Elliott J, et al. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr . 2006 Sep. 43(1):65-8. [QxMD MEDLINE Link] .
van Marle G, Gill MJ, Kolodka D, McManus L, Grant T, Church DL. Compartmentalization of the gut viral reservoir in HIV-1 infected patients. Retrovirology . 2007 Dec 4. 4:87. [QxMD MEDLINE Link] . [Full Text] .
Al-Harthi L, Marchetti G, Steffens CM, Poulin J, Sékaly R, Landay A. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA). J Immunol Methods . 2000 Apr 3. 237(1-2):187-97. [QxMD MEDLINE Link] .
Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med . 1999 Jan. 5(1):83-9. [QxMD MEDLINE Link] .
Bandera A, Ferrario G, Saresella M, et al. CD4+ T cell depletion, immune activation and increased production of regulatory T cells in the thymus of HIV-infected individuals. PLoS One . 2010 May 24. 5(5):e10788. [QxMD MEDLINE Link] . [Full Text] .
Franco JM, Rubio A, Martínez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood . 2002 May 15. 99(10):3702-6. [QxMD MEDLINE Link] .
Mohri H, Perelson AS, Tung K, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med . 2001 Nov 5. 194(9):1277-87. [QxMD MEDLINE Link] . [Full Text] .
Bird JJ, Brown DR, Mullen AC, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity . 1998 Aug. 9(2):229-37. [QxMD MEDLINE Link] .
Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature . 1996 Aug 22. 382(6593):722-5. [QxMD MEDLINE Link] .
Poropatich K, Sullivan DJ Jr. Human immunodeficiency virus type 1 long-term non-progressors: the viral, genetic and immunological basis for disease non-progression. J Gen Virol . 2011 Feb. 92:247-68. [QxMD MEDLINE Link] .
van der Ende ME, Schutten M, Raschdorff B, et al. CD4 T cells remain the major source of HIV-1 during end stage disease. AIDS . 1999 Jun 18. 13(9):1015-9. [QxMD MEDLINE Link] .
Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5?32/?32 stem cell transplantation. Blood . 2011 Mar 10. 117(10):2791-9. [QxMD MEDLINE Link] .
Kostense S, Raaphorst FM, Notermans DW, et al. Diversity of the T-cell receptor BV repertoire in HIV-1-infected patients reflects the biphasic CD4+ T-cell repopulation kinetics during highly active antiretroviral therapy. AIDS . 1998 Dec 24. 12(18):F235-40. [QxMD MEDLINE Link] .
McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature . 2001 Apr 19. 410(6831):974-9. [QxMD MEDLINE Link] .
Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS . 2001 Sep 28. 15(14):1749-56. [QxMD MEDLINE Link] .
Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature . 1993 Mar 25. 362(6418):355-8. [QxMD MEDLINE Link] .
Vago L, Antonacci MC, Cristina S, et al. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl Pathol . 1989. 7(5):298-309. [QxMD MEDLINE Link] .
Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis . 2010 Dec 15. 202(12):1819-25. [QxMD MEDLINE Link] . [Full Text] .
Karris MY, Anderson CM, Morris SR, Smith DM, Little SJ. Cost savings associated with testing of antibodies, antigens, and nucleic acids for diagnosis of acute HIV infection. J Clin Microbiol . 2012 Jun. 50(6):1874-8. [QxMD MEDLINE Link] . [Full Text] .
Pruss D, Bushman FD, Wolffe AP. Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. Proc Natl Acad Sci U S A . 1994 Jun 21. 91(13):5913-7. [QxMD MEDLINE Link] .
Schröder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell . 2002 Aug 23. 110(4):521-9. [QxMD MEDLINE Link] .
Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med . 2002. 53:557-93. [QxMD MEDLINE Link] .
Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A . 1998 Jul 21. 95(15):8869-73. [QxMD MEDLINE Link] .
Ho DD, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med . 1989 Dec 14. 321(24):1621-5. [QxMD MEDLINE Link] .
Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A . 2007 Apr 17. 104(16):6776-81. [QxMD MEDLINE Link] . [Full Text] .
Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol . 2000 Feb 1. 164(3):1602-11. [QxMD MEDLINE Link] .
Alimonti JB, Kimani J, Matu L, et al. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol Cell Biol . 2006 Oct. 84(5):482-5. [QxMD MEDLINE Link] .
Alter G, Heckerman D, Schneidewind A, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature . 2011 Aug 3. 476(7358):96-100. [QxMD MEDLINE Link] . [Full Text] .
Zoufaly A, an der Heiden M, Kollan C, et al. Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis . 2011 Feb 1. 203(3):364-71. [QxMD MEDLINE Link] . [Full Text] .
Mills EJ, Bakanda C, Birungi J, Yaya S, Ford N. The prognostic value of baseline CD4 cell count beyond 6 months of antiretroviral therapy in HIV positive patients in Uganda. AIDS . 2012 Apr 21. [QxMD MEDLINE Link] .
Ezeamama AE, Spiegelman D, Hertzmark E, et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis . 2012 May 15. 205(10):1486-94. [QxMD MEDLINE Link] . [Full Text] .
Lambert-Niclot S, Tubiana R, Beaudoux C, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002-2011 survey. AIDS . 2012 May 15. 26(8):971-5. [QxMD MEDLINE Link] .
Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS . 2012 Apr 24. 26(7):867-75. [QxMD MEDLINE Link] .
CDC. Diagnoses of HIV Infection in the United States and Dependent Areas, 2018 (Preliminary), Volume 30. CDC. Available at https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-preliminary-vol-30.pdf . November 2019; Accessed: January 9, 2023.
Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2018 (Preliminary). Available at https://www.cdc.gov/hiv/topics/surveillance/resources/reports/ . December 2018; Accessed: March 5, 2020.
Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men --- 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep . 2010 Sep 24. 59(37):1201-7. [QxMD MEDLINE Link] .
Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. National vital statistics reports; vol 58 no 19. Hyattsville, MD: National Center for Health Statistics. 2010. Available at https://www.cdc.gov/NCHS/data/nvsr/nvsr58/nvsr58_19.pdf . Accessed: June 21, 2011.
Vital Signs: HIV Infection, Testing, and Risk Behaviors Among Youths - United States. MMWR Morb Mortal Wkly Rep . 2012 Nov 30. 61(47):971-6. [QxMD MEDLINE Link] . [Full Text] .
UNAIDS. Global AIDS update 2019 — Communities at the centre. UNAIDS. Available at https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf . December 10, 2019; Accessed: March 5, 2020.
Ng M, Gakidou E, Levin-Rector A, Khera A, Murray CJ, Dandona L. Assessment of population-level effect of Avahan, an HIV-prevention initiative in India. Lancet . 2011 Nov 5. 378(9803):1643-52. [QxMD MEDLINE Link] .
Greenhalgh H. Men With Same-Sex Partners 28 Times More Likely to Get HIV - U.N. Medscape News. Available at https://www.medscape.com/viewarticle/899482 . July 19, 2018; Accessed: July 24, 2018.
Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain Behav Immun . 2009 Feb. 23(2):184-8. [QxMD MEDLINE Link] . [Full Text] .
Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med . 1995 Jan 26. 332(4):201-8. [QxMD MEDLINE Link] .
Levy JA. HIV pathogenesis and long-term survival. AIDS . 1993 Nov. 7(11):1401-10. [QxMD MEDLINE Link] .
Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med . 1995 Jan 26. 332(4):209-16. [QxMD MEDLINE Link] .
Paroli M, Propato A, Accapezzato D, Francavilla V, Schiaffella E, Barnaba V. The immunology of HIV-infected long-term non-progressors--a current view. Immunol Lett . 2001 Nov 1. 79(1-2):127-9. [QxMD MEDLINE Link] .
Nesheim SR, Kapogiannis BG, Soe MM, et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986-2004. Pediatrics . 2007 Jul. 120(1):100-9. [QxMD MEDLINE Link] .
Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med . 2006 Sep 19. 145(6):397-406. [QxMD MEDLINE Link] . [Full Text] .
Palella FJ Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr . 2006 Sep. 43(1):27-34. [QxMD MEDLINE Link] .
Brooks M. Low Level HIV Viremia a Modifiable Risk Factor for Non-Hodgkin Lymphoma. Medscape Medical News. Available at https://www.medscape.com/viewarticle/821277 . Accessed: March 12, 2014.
Achenbach CJ, Buchanan AL, Cole SR, Hou L, Mugavero MJ, Crane HM, et al. HIV viremia and incidence of non-Hodgkin lymphoma in patients successfully treated with antiretroviral therapy. Clin Infect Dis . 2014 Feb 12. [QxMD MEDLINE Link] .
[Guideline] Brooks M. New CDC HIV Testing Recommendations Offer Faster Diagnosis. Medscape Medical News . Jun 26 2014. [Full Text] .
[Guideline] CDC. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/hiv/pdf/HIVtestingAlgorithmRecommendation-Final.pdf . Accessed: Jul 7 2014.
Barclay L. ACOG Guidelines Recommend Repeat HIV Screening, Prophylaxis. Medscape Medical News. Available at https://www.medscape.com/viewarticle/824112 . Accessed: May 3, 2014.
[Guideline] Committee Opinion No 596: Routine Human Immunodeficiency Virus Screening. Obstet Gynecol . 2014 May. 123(5):1137-1139. [QxMD MEDLINE Link] .
Skwarecki B. ACOG updates recommendations for prenatal HIV testing. Available at:. Medscape Medical News. WebMD Inc. Available at https://www.medscape.com/viewarticle/845416 . May 27, 2015;
[Guideline] American College of Obstetricians and Gynecologists. Committee opinion no: 635: prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol . 2015 Jun. 125 (6):1544-7. [QxMD MEDLINE Link] .
Torian LV, Eavey JJ, Punsalang AP, et al. HIV type 2 in New York City, 2000-2008. Clin Infect Dis . 2010 Dec 1. 51(11):1334-42. [QxMD MEDLINE Link] .
Claassen CW, Diener-West M, Mehta SH, Thomas DL, Kirk GD. Discordance Between CD4+ T-Lymphocyte Counts and Percentages in HIV-Infected Persons With Liver Fibrosis. Clin Infect Dis . 2012 Jun. 54(12):1806-13. [QxMD MEDLINE Link] .
Detection of Acute HIV Infection in Two Evaluations of a New HIV Diagnostic Testing Algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep . 2013 Jun 21. 62(24):489-94. [QxMD MEDLINE Link] .
Hull MW, Rollet K, Odueyungbo A, et al. Factors associated with discordance between absolute CD4 cell count and CD4 cell percentage in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis . 2012 Jun. 54(12):1798-805. [QxMD MEDLINE Link] .
Hoffmann CJ, Brown TT. Thyroid function abnormalities in HIV-infected patients. Clin Infect Dis . 2007 Aug 15. 45(4):488-94. [QxMD MEDLINE Link] .
Lee PL, Yiannoutsos CT, Ernst T, et al. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging . 2003 Jun. 17(6):625-33. [QxMD MEDLINE Link] .
Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest . 1999 May 15. 103(10):1391-8. [QxMD MEDLINE Link] . [Full Text] .
Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med . 1998 Feb. 4(2):208-14. [QxMD MEDLINE Link] .
Masia M, Padilla S, Alvarez D, et al. Risk, predictors, and mortality associated with non-AIDS events in newly diagnosed HIV-infected patients: role of antiretroviral therapy. AIDS . 2013 Jan 14. 27(2):181-9. [QxMD MEDLINE Link] .
Thompson MA, Horberg MA, Agwu AL, Colasanti JA, Jain MK, Short WR, et al. Primary Care Guidance for Persons With Human Immunodeficiency Virus: 2020 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis . 2021 Dec 6. 73 (11):e3572-e3605. [QxMD MEDLINE Link] .
Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis . 2010 Oct 15. 51(8):957-62. [QxMD MEDLINE Link] . [Full Text] .
Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst . 2011 May 4. 103(9):753-62. [QxMD MEDLINE Link] . [Full Text] .
Puhan MA, Van Natta ML, Palella FJ, Addessi A, Meinert C. Excess mortality in patients with AIDS in the era of highly active antiretroviral therapy: temporal changes and risk factors. Clin Infect Dis . 2010 Oct 15. 51(8):947-56. [QxMD MEDLINE Link] . [Full Text] .
Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet . 2009 Apr 18. 373(9672):1352-63. [QxMD MEDLINE Link] . [Full Text] .
Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med . 2010 Jul 15. 363(3):257-65. [QxMD MEDLINE Link] .
Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med . 2011 Apr 19. 154(8):509-15. [QxMD MEDLINE Link] .
US Department of Health and Human Services. National Institutes of Health. NIH News. Starting Antiretroviral Therapy Earlier Yields Better Clinical Outcomes . June 8, 2009.
Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis . 2006 Sep 15. 194(6):725-33. [QxMD MEDLINE Link] .
Hecht FM, Wellman R, Busch MP, et al. Identifying the early post-HIV antibody seroconversion period. J Infect Dis . 2011 Aug 15. 204(4):526-33. [QxMD MEDLINE Link] . [Full Text] .
Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA . 2020 Oct 27. 324 (16):1651-1669. [QxMD MEDLINE Link] .
Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA . 2023 Jan 3. 329 (1):63-84. [QxMD MEDLINE Link] .
Rhee SY, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother . 2010 Oct. 54(10):4253-61. [QxMD MEDLINE Link] . [Full Text] .
Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet . 2009 Sep 5. 374(9692):796-806. [QxMD MEDLINE Link] .
Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr . 2013 May 1. 63(1):77-85. [QxMD MEDLINE Link] .
Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet . 2013 Mar 2. 381(9868):735-43. [QxMD MEDLINE Link] .
Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG; S/GSK1349572) + Abacavir/Lamivudine Once Daily Statistically Superior to Tenofovir/Emtricitabine/Efavirenz: 48-Week Results - SINGLE (ING114467). Abstract presented at: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) . Sept 2012. Abstract H-556b:
Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet . 2013 Jul 2. [QxMD MEDLINE Link] .
Nichols G, Mills A, Grossberg R, et al. Antiviral Activity of Dolutegravir in Subjects With Failure on an Integrase Inhibitor–Based Regimen: Week 24 Phase 3 Results From VIKING-3. Poster presented at: 11th International Congress on Drug Therapy in HIV Infection . Nov 2012. Poster O232:
Ndembi N, Goodall RL, Dunn DT, et al. Viral rebound and emergence of drug resistance in the absence of viral load testing: a randomized comparison between zidovudine-lamivudine plus Nevirapine and zidovudine-lamivudine plus Abacavir. J Infect Dis . 2010 Jan 1. 201(1):106-13. [QxMD MEDLINE Link] .
Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet . 2012 Jun 30. 379(9835):2439-48. [QxMD MEDLINE Link] .
DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet . 2012 Jun 30. 379(9835):2429-38. [QxMD MEDLINE Link] .
McMahon DK, Zheng L, Hitti J, Chan ES, Halvas EK, Hong F, et al. Greater Suppression of Nevirapine Resistance With 21- vs 7-Day Antiretroviral Regimens After Intrapartum Single-Dose Nevirapine for Prevention of Mother-to-Child Transmission of HIV. Clin Infect Dis . 2013 Apr. 56(7):1044-51. [QxMD MEDLINE Link] . [Full Text] .
Spector SA, McKinley GF, Lalezari JP, et al. Oral ganciclovir for the prevention of cytomegalovirus disease in persons with AIDS. Roche Cooperative Oral Ganciclovir Study Group. N Engl J Med . 1996 Jun 6. 334(23):1491-7. [QxMD MEDLINE Link] .
Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis . 2012 May. 54(9):1364-72. [QxMD MEDLINE Link] .
Falutz J, Potvin D, Mamputu JC, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr . 2010 Mar 1. 53(3):311-22. [QxMD MEDLINE Link] .
Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med . 2007 Dec 6. 357(23):2359-70. [QxMD MEDLINE Link] .
Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Loughrey H, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab . 2010 Sep. 95(9):4291-304. [QxMD MEDLINE Link] .
Stanley TL, Falutz J, Marsolais C, et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis . 2012 Jun. 54(11):1642-51. [QxMD MEDLINE Link] . [Full Text] .
Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet . 2010 Mar 6. 375(9717):824-33. [QxMD MEDLINE Link] . [Full Text] .
Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis . 2012 Apr. 54(7):1001-9. [QxMD MEDLINE Link] . [Full Text] .
Gaps in HIV Testing and Treatment Hinder Efforts to Stop New Infections. CDC. Available at https://www.cdc.gov/media/releases/2019/p0315-gaps-hinder-hiv-testing.html . March 18, 2019; Accessed: March 20, 2019.
Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis . 2011 Oct 3. [QxMD MEDLINE Link] .
Marazzi MC, Palombi L, Nielsen-Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS . 2011 Aug 24. 25(13):1611-8. [QxMD MEDLINE Link] .
Burgard M, Jasseron C, Matheron S, et al. Mother-to-child transmission of HIV-2 infection from 1986 to 2007 in the ANRS French Perinatal Cohort EPF-CO1. Clin Infect Dis . 2010 Oct 1. 51(7):833-43. [QxMD MEDLINE Link] .
Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med . 2009 Dec 3. 361(23):2209-20. [QxMD MEDLINE Link] .
Robb ML, Rerks-Ngarm S, Nitayaphan S, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis . 2012 Jul. 12(7):531-7. [QxMD MEDLINE Link] . [Full Text] .
Brooks M. CDC updates HIV preexposure prophylaxis guidelines. Medscape Medical News . May 14, 2014. [Full Text] .
Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014 Clinical Practice Guideline. Centers for Disease Control and Prevention . May 2014. Available at https://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf .
Descovy (emtricitabine/tenofovir AF) [package insert]. Foster City, CA: Gilead Sciences. October, 2019. Available at [Full Text] .
Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med . 2010 Dec 30. 363(27):2587-99. [QxMD MEDLINE Link] . [Full Text] .
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med . 2011 Aug 11. 365(6):493-505. [QxMD MEDLINE Link] . [Full Text] .
Choopanya K, Martin M, Suntharasam P, Sangkum U, Mock P, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet . 2013. 2083-90.
Leibowitz AA, Parker KB, Rotheram-Borus MJ. A US Policy Perspective on Oral Preexposure Prophylaxis for HIV. Am J Public Health . 2011 Jun. 101(6):982-5. [QxMD MEDLINE Link] .
Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep . 2011 Jan 28. 60(3):65-8. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. Last updated February 22, 2011. HIV/AIDS - Pre-Exposure Prophylaxis (PrEP). [Full Text] .
[Guideline] Marrazzo JM, del Rio C, Holtgrave DR, et al, for the International Antiviral Society-USA Panel. HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA . 2014 Jul 23-30. 312(4):390-409. [QxMD MEDLINE Link] . [Full Text] .
Smith M. Antiviral group: 'Biomedical' tx could slow HIV. MedPage Today . July 21, 2014. [Full Text] .
[Guideline] Günthard HF, Aberg JA, Eron JJ, for the International Antiviral Society-USA Panel. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA . 2014 Jul 23-30. 312(4):410-25. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] DiNenno EA, Prejean J, Irwin K, Delaney KP, Bowles K, Martin T, et al. Recommendations for HIV Screening of Gay, Bisexual, and Other Men Who Have Sex with Men - United States, 2017. MMWR Morb Mortal Wkly Rep . 2017 Aug 11. 66 (31):830-832. [QxMD MEDLINE Link] .
EACS. European AIDS Clinical Society Guidelines Version 10.0. European AIDS Clinical Society. Available at https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf . November 2019; Accessed: December 2, 2019.
[Guideline] Department of Health and Human Services. Panel on Clinical Practices for Treatment of HIV Infection., Henry J. Kaiser Family Foundation. Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents, January 28, 2000 by the Panel on Clinical Practices for Treatment of HIV Infection. HIV Clin Trials . 2000 Jul-Aug. 1 (1):60-110. [QxMD MEDLINE Link] . [Full Text] .
Mounzer K, Palella F, Slim J, et al. SPIRIT: Simplifying to rilpivirine/emtricitabine/tenofovir Df single-tablet regimen from boosted protease inhibitor regimen maintains HIV suppression in the black subgroup [abstract H-656]. Presented at: The 53rd Interscience Conference onAntimicrobial Agents and Chemotherapy (ICAAC); September 11, 2013; Denver, Colorado. [Full Text] .
Kling J. Single-tablet HIV regimen effective. Medscape Medical News . September 19, 2013. [Full Text] .
Tucker ME. FDA OKs New Triple-Combination Pill (Triumeq) for HIV. Medscape Medical News . Aug 22 2014. [Full Text] .
Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV . 2018 May. 5 (5):e211-e220. [QxMD MEDLINE Link] .
Orkin C, Squires KE, Molina JM, Sax PE, Wong WW, Sussmann O, et al. Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate is Non-inferior to Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate in Treatment-naive Adults With Human Immunodeficiency Virus-1 Infection: Week 48 Results of the DRIVE-AHEAD Trial. Clin Infect Dis . 2018 Aug 31. [QxMD MEDLINE Link] .
Lewis S, Fessel J, Emu B, Schrader S, Kumar P, Richmond GJ, et al. Long-acting ibalizumab in patients with multi-drug resistant HIV-1: A 24-week study. Presented at the 2017 Conference on Retroviruses and Opportunistic Infections. February 13-16, 2017. Seattle, WA. Abstracts from the 2017 Conference on Retroviruses and Opportunistic Infections. February 13-16, 2017. Seattle, Washington. Top Antivir Med . 2017 Apr. 25 (1s):4s-446s. [QxMD MEDLINE Link] . [Full Text] .
Segal-Maurer S, DeJesus E, Stellbrink HJ, and the, CAPELLA Study Investigators. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N Engl J Med . 2022 May 12. 386 (19):1793-1803. [QxMD MEDLINE Link] . [Full Text] .
Barclay L. HIV: guidelines stress role of primary care in management. Medscape Medical News . November 14, 2013. [Full Text] .
Mocellin LP, Ziegelmann PK, Kuchenbecker R. A systematic review and meta-analysis assessing antiretroviral therapy for treatment-experienced HIV adult patients using an optimized background therapy approach: is there evidence enough for a standardized third-line strategy?. Syst Rev . 2022 Nov 17. 11 (1):243. [QxMD MEDLINE Link] .
- Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
- Genome layout of human immunodeficiency virus (HIV)–1 and HIV-2.
- Timeline of CD4 T-cell and viral-load changes over time in untreated human immunodeficiency virus (HIV) infection. Courtesy of Wikipedia (based on an original from Pantaleo et al (1993)).
- Incidence of HIV infection by risk group. Courtesy of CDC (derived from the revised 2006 estimated figures).
- Changes in survival of people infected with HIV. As therapies have become more aggressive, they have been more effective, although survival with HIV infection is not yet equivalent to that in uninfected people. Modified from Lohse N et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87-95.
- Table 1. Antiretroviral Drug Classes and Agents
- Table 2. Antiretroviral Combination Products
Nucleoside reverse transcriptase inhibitors (NRTIs) | Abacavir (Ziagen, ABC) Didanosine (Videx, Videx EC, ddI) Emtricitabine (Emtriva, FTC) Lamivudine (Epivir, 3TC) Stavudine (Zerit, Zerit XR, d4T)* Tenofovir DF (Viread, TDF) Tenofovir AF (TAF) Zalcitabine (Hivid, ddC)* Zidovudine (Retrovir, ZDV, AZT) |
Protease inhibitors (PIs) | Amprenavir (Agenerase, AVP)* Atazanavir (Reyataz , ATV) Darunavir (Prezista, DRV) Fosamprenavir (Lexiva, f-APV) Indinavir (Crixivan, IDV) Lopinavir and ritonavir (Kaletra, LPV/r) Nelfinavir (Viracept, NFV) Ritonavir (Norvir, RTV) Saquinavir (Invirase [hard gel] capsule, SQV)* Tipranavir (Aptivus, TPV) |
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | Delavirdine (Rescriptor, DLV) Efavirenz (Sustiva, EFV) Etravirine (Intelence, ETR) Nevirapine (Viramune, NVP) Rilpivirine (Edurant) Doravirine (Pifeltro, DOR) Cabotegravir (CAB) |
Fusion inhibitors | Enfuvirtide (Fuzeon, T-20)* |
Cellular chemokine receptor (CCR5) antagonists | Maraviroc (Selzentry, MVC) |
Integrase inhibitors | Raltegravir (Isentress, RAL) Dolutegravir (Tivicay, DTG) Elvitegravir (Vitekta, EVG) |
| Entry inhibitors (CD4 post-attachment inhibitors) | Ibalizumab (Trogarzo, IBA) |
| gp120 Attachment inhibitors | Fostemsavir (Rukobia, FTR) |
| Capsid inhibitors | Lenacapavir (Sunlenca, LEN) |
*No longer available on market | |
|
|
|
Darunavir 800 mg Cobicistat 150 mg Emtricitabine 200 mg Tenofovir AF 10 mg | Symtuza | 1 tab PO qd with food |
Bictegravir 50 mg Emtricitabine 200 mg Tenofovir AF 25 mg | Biktarvy | 1 tab PO qd |
Efavirenz 400 mg Lamivudine 300 mg Tenofovir DF 300 mg | Symfi Lo | 1 tab PO qHS on an empty stomach |
Efavirenz 600 mg Lamivudine 300 mg Tenofovir DF 300 mg | Symfi | 1 tab PO qHS on an empty stomach |
Dolutegravir 50 mg Rilpivirine 25 mg | Juluca | 1 tab PO qd with a meal Note: This is a complete once-daily regimen in adults who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable ART regimen for ≥6 months with no history of treatment failure and no known substitutions associated with resistance |
Dolutegravir 50 mg Lamivudine 300 mg | Dovato | 1 tab PO qd with or without food Note: This a complete once-daily regimen in treatment-naïve adults or adolescents with no known substitutions associated with resistance to dolutegravir or lamivudine or to replace a stable regimen |
Elvitegravir 150 mg Cobicistat 150 mg Emtricitabine 200 mg Tenofovir AF 10 mg | Genvoya | 1 tab PO qd |
Elvitegravir 150 mg Cobicistat 150 mg Emtricitabine 200 mg Tenofovir DF 300 mg | Stribild | 1 tab PO qd |
Cabotegravir IM
copackaged with
Rilpivirine IM | Cabenuva | One-time initiating injection (on last day of oral lead-in dosing): Cabotegravir 600 mg IM plus rilpivirine 900 mg IM, THEN
Once-monthly continuation injections: Cabotegravir 400 mg IM plus rilpivirine 600 mg IM |
Abacavir 600 mg Dolutegravir 50 mg Lamivudine 300 mg | Triumeq | 1 tab PO qd |
Efavirenz 600 mg Emtricitabine 200 mg Tenofovir DF 300 mg | Atripla | 1 tab PO qd on empty stomach Note: May be used alone as a complete regimen or in combination with other ARTs |
Emtricitabine 200 mg Rilpivirine 25 mg Tenofovir DF 300 mg | Complera | 1 tab PO qd with a meal |
Emtricitabine 200 mg Rilpivirine 25 mg Tenofovir AF 25 mg | Odefsey | 1 tab PO qd with a meal |
Emtricitabine 200 mg Tenofovir DF 300 mg | Truvada | 1 tab PO qd CrCl 30-49 mL/min: 1 tab PO q48h CrCl < 30 mL/min: Do not administer |
Emtricitabine 200 mg Tenofovir AF 300 mg | Descovy | 1 tab PO qd CrCl < 30 mL/min: Do not administer |
Lamivudine 300 mg Tenofovir DF 300 mg | Cimduo | 1 tab PO qd |
Doravirine 100 mg Lamivudine 300 mg Tenofovir DF 300 mg | Delstrigo | 1 tab PO qd with or without food |
*Not indicated for patients requiring dosage adjustments (eg, weight < 40 kg, renal impairment, hepatic impairment, dose-limiting adverse effects) unless otherwise stated. Complete once-daily regimen | ||

Contributor Information and Disclosures
Shelley A Gilroy, MD, FACP, FIDSA Associate Professor of Medicine, Infectious Disease and HIV Medicine, Albany Medical College; Associate Chief of Staff for Education/DEO, Lead Physician for HIV Medicine, Division of Infectious Diseases, Albany Stratton VA Medical Center Shelley A Gilroy, MD, FACP, FIDSA is a member of the following medical societies: American College of Physicians , American Medical Association , Infectious Diseases Society of America Disclosure: Nothing to disclose.
John J Faragon, PharmD, BCPS, AAHIVP Pharmacist, HIV/HCV Medicine, Division of HIV Medicine and Department of Pharmacy, Albany Medical Center; Regional Pharmacy Director, Northeast Caribbean AIDS Education and Training Center; HIV and HCV Education Consultant, VirologyEd Consultants Disclosure: Serve(d) as a speaker or a member of a speakers bureau for: Gilead; Janssen; Merck.
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , American Medical Association , Association of Professors of Medicine , Infectious Diseases Society of America , Oklahoma State Medical Association , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Nicholas John Bennett, MBBCh, PhD, FAAP, MA(Cantab) Nicholas John Bennett, MBBCh, PhD, FAAP, MA(Cantab) is a member of the following medical societies: Alpha Omega Alpha , American Academy of Pediatrics Disclosure: Nothing to disclose.
Aaron Glatt, MD Professor of Clinical Medicine, New York Medical College; President and CEO, Former Chief Medical Officer, Departments of Medicine and Infectious Diseases, St Joseph Hospital (formerly New Island Hospital)
Aaron Glatt, MD is a member of the following medical societies: American College of Chest Physicians , American College of Physician Executives , American College of Physicians , American College of Physicians-American Society of Internal Medicine , American Medical Association , American Society for Microbiology , American Thoracic Society , American Venereal Disease Association, Infectious Diseases Society of America , International AIDS Society , and Society forHealthcare Epidemiology of America
Disclosure: Nothing to disclose.
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Laboratory Assays for Diagnosis and Monitoring of HIV Infection
- Early Symptomatic HIV Infection
- Pediatric HIV Infection
- HIV Infection and AIDS
- HIV Treatment Regimens CDC Guidelines, Adult/Adolescent
- Primary Care of Patients With HIV Infection
- Ocular Manifestations of HIV Infection
- US, Mexican Border Restrictions Did Not Reduce HIV Infection
- Diagnosing Infection in Tocilizumab Users: What's Helpful?
- Antipsychotics Tied to Severe Respiratory Infection Risk

- Treatment Failure in Patient With Severe Mpox and Untreated HIV, Maryland, USA
- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2010biktarvy-bictegravir-emtricitabine-tenofovir-af-1000199Drugs Drugs bictegravir/emtricitabine/tenofovir AF

- 20022172322-overviewTables & Protocols Tables & Protocols HIV Treatment Regimens CDC Guidelines, Adult/Adolescent
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Hiv and aids.
Helena M. Swinkels ; Angel A. Justiz Vaillant ; Andrew D. Nguyen ; Peter G. Gulick .
Affiliations
Last Update: July 27, 2024 .
- Continuing Education Activity
Clinical prevention of HIV and AIDS is the cornerstone of controlling the global HIV pandemic, which has claimed over 40.4 million lives worldwide, including 1.5 million children. Although a cure remains out of reach, HIV is a chronic illness due to the effectiveness of antiretroviral therapy. Combined with significant advancements in prevention, the goal of halting the global HIV pandemic is now feasible. Current interventions acknowledge the complexity of clinical management, considering socio-economic factors, patient-centered care, continuous quality improvement, and the importance of social and regulatory environments to achieve optimal patient and population outcomes.
This activity comprehensively reviews HIV transmission, pathophysiology, clinical presentations, evaluation, up-to-date treatment, reporting, and implementation considerations for specific population groups to prevent the diagnosis of AIDS. Clinicians increase their knowledge, skills, and competence in managing HIV, improving patient outcomes, reducing transmissions, and contributing to global efforts of disease eradication. The role of the interprofessional team is highlighted, emphasizing collaboration among clinical, public health, and interdisciplinary team members.
- Determine the stage of HIV and related comorbidities to ensure timely diagnosis and treatment for patients.
- Apply the principles of antiretroviral therapy and prophylaxis for opportunistic infections to prevent and manage adverse effects.
- Interpret HIV treatment guidelines to ensure cultural sensitivity and maintain confidentiality for individual patients and affected populations.
- Collaborate with interprofessional team members to ensure continuity of care, improve patient outcomes for HIV prevention and treatment, and mitigate the risk of AIDS.
- Introduction
HIV was first identified in 1983 and has since claimed approximately 40.4 million lives worldwide as of 2022. This number is staggering, and if left unchecked, HIV could become a global health crisis. However, the research, development, and widespread availability of highly active antiretroviral therapies (ARTs) have helped control the HIV pandemic. Likewise, advances in the treatment of HIV and opportunistic infections have rendered the disease a manageable chronic illness. Patients with HIV can live long and healthy lives. Preventing chronic diseases is a top health priority for this population due to the underlying immunodeficiency.
Adequate resources and advances in prevention, treatment, and implementation science make the United Nations General Assembly's 95-95-95 goals attainable. By 2025, the goal is to ensure that 95% of patients with HIV are diagnosed, 95% of diagnosed patients receive ART, and 95% of those prescribed ART achieve viral load suppression.[WHO. Global Health Sector HIV Strategies 2022 ] Globally, HIV and mortality rates show a steady decrease. However, some countries report an uptrend in the rate of infections, mostly where political or other turmoil is occurring or where HIV is highly stigmatized.[UNAIDS. Global Report 2023 ] With improvements in treatment, the number of patients with HIV is also increasing, with approximately 37.7 million patients diagnosed in 2020 and 39 million patients diagnosed in 2022—two-thirds of whom live in Africa.
HIV imposes high costs on both patients and the healthcare system. Infection with HIV increases the risk of chronic disease, particularly cardiac and neurological. Although ART delays disease progress, treatment does not cure HIV, causes adverse effects, and requires consistent, prolonged connection to the healthcare system. Several barriers to universal treatment exist, including public- and self-stigma, lack of adequate access to care, inappropriate care, and costs. Using local clinical guidelines for managing HIV improves patient outcomes and prevents HIV transmission. Clinical guidelines promote quality programming for prompt diagnosis, treatment, and connection to care for patients with or at risk of acquiring HIV. Increasing the involvement of community-led organizations in HIV testing and treatment and integrating medical services for related health issues extend the reach of precise services, improve linkage to care, and improve overall health. Supportive social and policy environments regarding access to services, screening, reporting test results, and discrimination can safeguard patients and the community.
This clinical reference focuses primarily on HIV-1 and is designed to review the pathophysiology, clinical manifestations, and recommended treatment options for patients with HIV, providing clinicians with concise and up-to-date guidance for managing HIV. The optimal social and policy environments to support the HIV response, as recommended by the World Health Organization (WHO), the Joint United Nations Programme for HIV/AIDS (UNAIDS), the United States Centers for Disease Control (CDC), and state legislatures, with Florida legislation provided as an example, are discussed. Please see StatPearls' companion resource, " HIV-2 Infection ," for more information. [1]
HIV is part of the Retroviridae family in the Lentivirus genus. The virus mainly targets CD4+ T-lymphocyte helper cells, leading to extreme immune suppression with a continuous loss of cells. This suppression weakens the immune system and causes many clinical manifestations. Untreated HIV eventually progresses to AIDS. At this stage, the immune system cannot prevent infections, resulting in death due to opportunistic infections. Two main types of HIV include HIV-1 and HIV-2. Although their genomes are structurally similar, they diverge significantly at the amino acid level. The 2 viruses result from 2 different zoonotic transmissions of simian immunodeficiency viruses and, as a result, have substantial differences in their severity, transmissibility, and prognosis. Note that HIV-1 and HIV-2 are only 60% identical at the amino acid level and have a mere 48% identity similarity at the nucleotide level.
HIV-1 and HIV-2 particles comprise a lipid membrane surrounding a protein capsid. The capsid holds a nucleoprotein complex or core consisting of 2 identical copies of RNA and nucleocapsid, integrase, and reverse transcriptase proteins. The capsid protein organizes into a lattice structure, giving the capsid a characteristic conical shape. HIV is transmitted through various body fluids, such as blood, amniotic fluid, breast milk, semen, pre-ejaculate, rectal fluids, and vaginal fluids. HIV can be transmitted through sexual contact, during pregnancy and delivery, and through fomites, such as reusable medical equipment or syringes. Please see StatPearls' companion resource, " HIV Prevention ," for more information. [1] [2]
- Epidemiology
HIV is a significant public health issue worldwide. HIV-1 causes most infections, with HIV-2 accounting for only 1 to 2 million infections. The prevalence of HIV-2 exceeds 1% of infections only in West Africa, although infections occur less commonly on all continents, particularly in cases with colonial or other ties to the area. According to the WHO HIV factsheet, there were 39 million patients with HIV at the end of 2022, with the majority (25.6 million) living in Sub-Saharan Africa.[WHO. HIV Data and Statistics 2023. ] In 2022, 1.3 million new cases of HIV were reported worldwide, with 630,000 deaths related to HIV in the same year.
Although some countries report an increase in new infections, the overall global trend in HIV incidence has decreased. In particular, significant gains prevent and treat HIV in eastern and southern Africa, where the virus is most prevalent. From 2010 to 2022, 57% fewer new infections and 58% fewer AIDS-related deaths occurred in the region. Progress is slower in other areas, mainly where marked inequities and low prioritization of the HIV response exist. A study of global trends in HIV among adolescents and young adults showed a decrease in incidence from 34.5 per 100,000 population in 1990 to 22.7 per 100,000 population in 2019. However, between 2010 and 2022 in Asia and the Pacific, a quarter of new infections affected people aged between 15 and 24 and their partners, with some countries reporting nearly half of all new infections in this population. In total, new HIV cases have decreased by only 14% in the region.
HIV incidence is increasing in some regions. The WHO Middle East and North African regions had a 61% increase in the incidence of HIV between 2010 and 2022, the largest in the world. Due to the low prevalence of HIV in this region, the number of people infected (about 16,000 people in 2022) is small relative to the 160,000 people estimated to be infected in Eastern Europe and Central Asia in 2022. New HIV cases in this region increased by 49% between 2010 and 2022. Challenging legal environments, human rights violations, and military conflicts have hindered the HIV response.
HIV diagnosis, treatment, and viral suppression rates vary across and within countries and regions. Globally, women accounted for 65.8% of new HIV cases in 2019. However, diagnosis, treatment, and viral suppression rates are lower among adolescent and adult men. For example, a phylogenetic study in Uganda estimated that men are 1.5 to 1 times less likely to be virally suppressed compared to women. Interventions that increase viral suppression rates among men similar to women close the gender disparity in incident HIV cases. UNAIDS and WHO identify 5 key populations who are disproportionately affected by HIV and warrant specific care and support to reduce global transmissions—men who have sex with men, sex workers, people in prisons and other closed settings, people who inject drugs, and transgender and gender-diverse people.[UNAIDS. Global Report 2023. ]
The vast majority of new HIV cases worldwide occur from sexual contact. Most of these infections are transmitted through heterosexual contact due to the high number of infections in Africa, where this mode of transmission is dominant. Most new HIV diagnoses in most other regions of the world occur in men who have sex with men.[WHO. HIV Data and Statistics 2023. ] In the United States, in 2021, 70% of all new infections were in men who have sex with men, whereas only 22% occurred through heterosexual contact.[CDC. Basic Statistics 2023. ] Drug use is another significant risk factor for HIV. According to a meta-analysis from 2008, approximately 3 million people who inject drugs are living with HIV worldwide. According to the CDC HIV Factsheet, 1 in 10 new HIV cases in the United States can be attributed to injection drug use either alone or in men who have sex with men who report injection drug use. A systematic review evaluating the effectiveness of needle and syringe exchange programs reported that adequate needle and syringe exchange programs are effective in reducing the sharing and reuse of needles and syringes and HIV transmission among people who inject drugs. Please see StatPearls' companion resource, " HIV Prevention ," for more information. [4] [5] [6] [7] [8]
- Pathophysiology
The Retroviridae family is unique among viruses, with the RNA viral genome reverse-transcribed into DNA before being integrated into the host DNA, resulting in lifelong infection. The pathophysiological process is best known for HIV-1, with more recent research contributing to the understanding of differences in HIV-2 and HTLV viral replication and biology. As the primary host cellular receptor for HIV-1 and HIV-2 is the CD4+ antigen, T cells and macrophages that express CD4+ are the primary viral targets. Envelope glycoprotein on the surface of the viral particle facilitates viral entry into the host cell. Chemokine coreceptors 5 (CCR5) and 4 (CXCR4) on the host cell surface trigger a conformational change in the envelop protein, which results in the fusion of the viral and host cellular membranes. The viral capsid is released into the host cell when membrane fusion occurs.
The HIV-1 capsid remains intact or nearly intact until reaching the nuclear pore complexes on the nuclear envelope of the host cell. Previously believed to occur in the cytoplasm, reverse transcription is now believed to occur during or shortly after the capsid is imported into the nucleus; the nuclear capsid is essential in the efficiency of reverse transcription. The precise location and mechanism of reverse transcription and the role of the capsid have not been fully elucidated. Some molecular studies suggest uncoating and reverse transcription begins in the nucleus. In contrast, others suggest a partial uncoating of the viral capsid at the nuclear pore complexes, with early stages of viral DNA production occurring near the nuclear envelope of the host cell.
The viral reverse transcriptase enzyme initiates reverse transcription using host transfer RNA as primers, which bind at the 5' ends of the 2 identical RNA strands and progress in a 5' to 3' direction. Negative-sense single-stranded DNA is initially produced, with doubling of the strand beginning part way along the viral genome. Elongation continues to the end of the genome, after which each strand is synthesized using the other as a template. The viral integrase protein then somewhat randomly integrates the double-stranded DNA into the host DNA. When viral particles are formed, they can infect other host cells to propagate the infection. Within 2 days of the initial mucosal exposure, HIV can be detected in the regional lymph node tissue. From here, the virus only requires 3 days to be detected in the plasma.
Genomic diversification is an important aspect of the pathogenesis of HIV, leading to changes in the severity of the disease and the response to ART. One of the primary driving factors for HIV-1 mutagenesis is the error rate of the reverse transcriptase encoded by the virus. According to results from several studies, the error rate of HIV-1 group M reverse transcriptase (subtype B) is 100 to 1000 times higher than that of the cellular DNA polymerases. These errors are incorporated into the viral genome and contribute to viral diversification. Other intrinsic and extrinsic factors, such as recombination errors, host restriction factors, and depletion of host deoxynucleoside triphosphates, can lead to viral mutagenesis and diversification, leading to ART failure. [1]
In the initial phase of the infection, viral replication is rampant, with an exponential increase in the plasma HIV RNA level due to the large population of susceptible CD4+ T cells without any host immune response. Subsequently, a significant decline from the peak viremia level occurs due to the HIV-specific immune response from the cytotoxic CD8+ T cells. After this decline, the HIV replication settles when replication and infection continue, but the initial intense immune response with associated symptoms resolves. The exact mechanisms involving the failure of humoral immunity are not fully understood. T cells within B-cell follicles, particularly the follicular T-helper cells and follicular regulatory T cells, are believed to be involved in poor humoral immunity and HIV persistence in patients treated with ART. In addition, follicular CD8+ cytotoxic T cells are relatively less abundant compared to their extrafollicular counterparts in patients with HIV, likewise believed to contribute to disrupted immunogenesis. [2] [3]
- Histopathology
Lymphadenopathy exhibiting distinctive morphological changes is evident in patients with HIV. In untreated cases, pronounced follicular hyperplasia emerges initially, marked by enlarged, irregularly shaped follicles that occupy a substantial portion of the lymph node's cross-sectional area. The mantle cell zones are absent or dramatically decreased. Centroblasts are the dominant cell types; the germinal centers have a starry-sky appearance. Follicle lysis or fragmentation is present when small lymphocytes infiltrate the follicle. Sinusoidal monocytoid B-cell hyperplasia is also present. Mixed follicular hyperplasia is the intermediate stage between florid and follicular involution. Here, the interfollicular area is relatively large compared to florid follicular hyperplasia, and the follicles and interfollicular area are more cellular compared to those observed in follicular involution.
In follicular involution, small, atrophic, and hypocellular follicles are observed. The germinal centers contain hyalinized follicular dendritic cell meshworks with few germinal center B cells. Hyalinized blood vessels are observed penetrating the follicles. Due to the scarcity of lymphocytes, the interfollicular area is expanded with a washed-out appearance. Histiocytes and polytypic plasma cells are abundantly present. Eventually, the lymph nodes enter the lymphocyte depletion stage, characterized by the loss of germinal centers and the near absence of lymphocytes. These lymph nodes contain medullary cords and sinusoids. The interfollicular area primarily consists of histiocytes, plasma cells, and a few immunoblasts. Focal hyaline deposits may be present with subcapsular and sinusoidal fibrosis. [4]
- History and Physical
A medical history and physical examination include a review of systems indicated for patients with confirmed or suspected HIV. Attention to signs and symptoms rule out other conditions in the differential diagnosis that signal opportunistic infections or HIV-associated sequelae. The clinical presentation stages the HIV infection and ensures that any concurrent conditions are addressed. In addition to characterizing symptoms, the history identifies risk factors for HIV transmission in a nonjudgmental manner, including sexual contact and behavior, drug use, and blood transfusions. The sexual history should elucidate information regarding the number of partners, sexual practices, frequency and type of barrier protection, and previous history of sexually transmitted infections (STIs). Information about the HIV status of current and past partners should also be obtained.
The drug use history should include the type and frequency of substances used, means of administration, and source and sharing of equipment. A mental health assessment is essential for identifying conditions such as depression, other mental illnesses, or substance use that may result in barriers to care or contribute to the development of chronic diseases. Obtaining an immunization history is crucial to determine which vaccines could offer future protection against vaccine-preventable illnesses. For instance, Streptococcus pneumoniae , associated with adverse outcomes in HIV-positive individuals, and hepatitis B, linked to a heightened risk of hepatocellular carcinoma progression in patients with HIV, underscore the importance of such proactive measures.
Social history is an integral part of any medical evaluation. In the context of HIV and AIDS, this history provides insights into the patient's perceptions of and ability to adhere to treatment and potential barriers to connection to healthcare services. Identifying resources of support assesses living situation, income, insurance, social support, experiences of stigma, coping strategies, and exposure to sexual or other violence. The United States National Institutes of Health (NIH) describes 3 broad stages of HIV that develop over time—acute HIV, chronic or asymptomatic HIV, and AIDS.[NIH. Stages of HIV Fact Sheet 2021. ] The CDC maintains a staging system based on CD4+ and AIDS-defining illnesses intended for surveillance. [10] The WHO has a staging system based on clinical presentation for clinical or surveillance purposes in areas with limited or no availability of CD4+ testing.[WHO. HIV Case Definitions 2007. ] [5] [6] [7]
Approximately 90% of patients with acute HIV experience at least 1 symptom within the first 4 weeks after primary HIV infection. These symptoms are typically mild, nonspecific, and self-limited. Some patients present with more severe symptoms, known as acute retroviral syndrome or seroconversion illness. These symptoms are listed below in order of decreasing frequency.
- Muscle pain
- Sore throat
- Swollen lymph nodes
- Night sweats
Symptom onset occurs acutely around 2 to 4 weeks (with a range of 4 days to 8 weeks) after viral infection, just before the peak of viremia. Lasting an average of 18 days, the resolution of symptoms coincides with the setting of a viral replication set-point approximately 30 days after the initial viremia. Without treatment, higher viral load and increased severity and duration of conversion illness are early predictors of a poor prognosis. Mucocutaneous ulceration is a characteristic feature of acute HIV characterized by shallow, sharply demarcated ulcers that have a white base surrounded by a thin area of erythema. Depending on the mode of transmission, ulcers may be located on the oral, anal, penile, or esophageal mucosa. Acute aseptic meningoencephalitis is reported as a clinical presentation of acute HIV-1. [2] [8] [9] [10] [11]
Chronic HIV
After HIV acquisition and subsequent setting of the viral set point, patients enter the chronic phase of the infection. Most patients with chronic HIV remain asymptomatic before developing AIDS. However, nonspecific fatigue may present, and persistent generalized lymphadenopathy is usual. Generalized lymphadenopathy is characterized by at least 2 noncontiguous sites other than inguinal nodes exhibiting enlarged lymph nodes for more than 3 to 6 months, not explained by other lymphoproliferative or infectious causes. [4] [12] Patients with chronic HIV without AIDS can develop oropharyngeal candidiasis, recurrent vulvovaginal candidiasis, oral hairy leukoplakia, disseminated cutaneous herpes simplex virus, and cervical dysplasia or cervical carcinoma in situ. [13] [14] [15] Cutaneous manifestations such as seborrheic dermatitis, bacillary angiomatosis, varicella-zoster virus reactivations, and molluscum contagiosum infections are common and tend to be severe in patients with HIV. [16] [17] [18]
Eventually, HIV progresses to advanced disease, particularly if left untreated or inadequately treated. AIDS is diagnosed when specific AIDS-defining conditions are noted, regardless of the CD4+ count. These AIDS-defining conditions, outlined by the CDC, include:
- Candidiasis of the digestive tract (other than thrush)
- Candidiasis of the pulmonary tract
- Invasive cervical cancer
- Extrapulmonary or disseminated coccidioidomycosis, histoplasmosis, or cryptococcosis, including cryptococcal meningitis
- Chronic intestinal cryptosporidiosis or isosporiasis
- Cytomegalovirus retinitis
- Kaposi sarcoma
- HIV encephalitis and HIV-associated neurocognitive disorder
- Tuberculosis
- Primary lymphoma of the brain
- Non-Hodgkin lymphoma
- Burkitt lymphoma
- Mycobacterial infections
- Pneumocystis jirovecii pneumonia
- Progressive multifocal leukoencephalopathy
- Salmonella septicemia
- HIV-associated wasting syndrome [19]
These AIDS-defining illnesses tend to occur most frequently with low CD4+ counts <200 cells/mm 3 , which correlates with untreated advanced HIV as the total lymphocyte count depletes over time. [20] [21]
Testing is essential to confirm a diagnosis of HIV. Antibody, antigen-antibody, and nucleic acid amplification tests are available for screening or confirming HIV in symptomatic illness. No currently available testing technology can detect HIV during the initial viremic phase of the infection, known as the window or eclipse period, which lasts up to 20 days. The following tests are used to detect viral proteins:
- Nucleic acid amplification tests: Detects HIV RNA in the blood 6 to 8 days after infection, up to 33 days.
- Antigen tests: Detect viral proteins such as p24 antigen as early as 13 to 20 days after infection.
- Antigen-antibody tests: Detect viral proteins as with other antigen tests, plus anti-HIV immunoglobulin M (IgM) and IgG antibodies around 20 and 30 days, respectively, after infection.[CDC. HIV testing 2024 .]
In clinical settings, combination antigen/antibody (Ag/Ab) tests are recommended to identify patients with HIV. These tests are available in most commercial laboratories and hospitals in developed nations and are increasingly available worldwide. Ideally, the Ag/Ab test should be a fourth-generation test capable of detecting HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen. If the initial positive test cannot cannot distinguish between HIV-1 and HIV-2 this distinction, a supplemental antibody immunoassay is required. All initial positive test results are followed by a second HIV test, preferably a test that is laboratory-based, to confirm a diagnosis of HIV. The false positive rate of third- and fourth-generation tests is very low.
If the combination assay result is negative, no further testing is indicated unless HIV exposure is too recent for detectable p24 antigen levels to have developed. If the initial Ag/Ab test result is negative and early HIV is suspected, an HIV-1 nucleic acid amplification test to detect HIV RNA should be performed. Specimens indeterminate on the initial Ag/Ab test or nonreactive or indeterminate with the HIV-1– and HIV-2–specific antibody assay are followed up with an HIV-1 nucleic acid amplification test. [24]
An acute HIV case is diagnosed with a positive nucleic acid amplification test result in the following settings:
- A recent negative screening immunoassay result
- A positive antigen-antibody immunoassay result, with a negative antibody-only immunoassay
- A nonreactive or indeterminate HIV-1– and HIV-2–specific antibody assay result following a positive screening assay
A negative HIV-1 nucleic acid amplification test result in the last settings indicates a false-positive HIV-1 test. When clinical suspicion for HIV is high and initial test results are negative, testing should be repeated in 1 to 3 weeks. Home-based, point-of-care, or rapid tests are essential to increase testing frequency or extend testing into populations that may otherwise not get tested. As with other HIV tests, positive results from point-of-care testing should be followed with standard laboratory, instrument-based immunoassays to confirm the diagnosis. [22]
When the diagnosis of HIV is established, a baseline laboratory evaluation facilitates the staging of HIV progression, selection of ART, and identification of comorbidities.[NIH. Guidelines for HIV 2023. ]
- Quantitative CD4+ T-lymphocyte cell count
- Quantitative plasma HIV-1 RNA viral load
- Complete blood count, glucose, blood urea nitrogen, creatinine, liver enzymes, bilirubin, urinalysis, serum lipids, and serology for hepatitis A, B, and C
- HLAb*5701 test (if abacavir is being considered)
- Genotypic drug-resistance assessment focusing on genes for reverse transcriptase and protease in treatment-naive people, with the addition of integrase strand transfer inhibitor (INSTI) for patients who have been treated with ARTs
- Other tests may be indicated based on the history and physical examination, such as testing for sexually transmitted infections, including Chlamydia trachomatis , Neisseria gonorrhoeae , syphilis serology, opportunistic infections, or cancer
Viral load is the most important indicator of initial and sustained response to ART and aids in ART selection. Some treatment regimes are ineffective in patients with high baseline viral loads. Viral load should be monitored at entry into care, initiation of therapy, and periodically afterward. CD4+ is the best indicator of immune function, disease progression, and survival and determines the need to start prophylaxis for opportunistic infections. Blood must be drawn for CD4+ testing before starting ART, but ART should not be delayed pending results. If CD4+ is unavailable, the WHO staging system should be used. Monitoring of other CD subsets is not recommended due to costs and lack of clinical utility. Routine testing for herpes simplex IgG, cytomegalovirus IgG, and toxoplasma IgG is not recommended, given that these tests do not delineate active disease versus previous exposure. Testing for serum cryptococcal antigen should be considered in patients with a CD4+ count of 100 cells/mm 3 or less. Please see StatPearls' companion resource, " HIV Testing ," for more information. [23]
- Treatment / Management
The goal of HIV-1 therapy with antiretroviral medications is to achieve sustained virologic suppression. According to current WHO and CDC guidelines, ART should begin after the diagnosis is confirmed and an initial assessment is completed for all patients with HIV, regardless of their immune status or clinical stage, unless a severe opportunistic infection is present.[NIH. HIV Guidelines 2023 ] Management of HIV must include identifying specific support required by the patient to maintain medication adherence, particularly for marginalized patients. Treatment choice is initiated based on patient preferences and ability to comply with a medication regimen.[WHO. Global HIV Strategies 2022. ]
Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), INSTIs, and protease inhibitors are used in treatment-naive patients. NRTIs inhibit viral replication by binding the viral reverse transcriptase and terminating DNA prolongation in HIV-1 and HIV-2 infections. NNRTIs block DNA prolongation by binding the viral reverse transcriptase at a separate site but are only active against HIV-1. INSTIs inhibit the transcribed viral DNA from integrating into the host genome. Protease inhibitors block the last step in the viral maturation process, rendering assembled viral particles immature and noninfectious. The most common drugs within each class in the initial treatment of HIV are listed below.[NIH. HIV Guidelines. 2023] [23]
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
- Abacavir (contraindicated for patients who are HLA-B*5701 positive)
- Emtricitabine
- Tenofovir (tenofovir alafenamide or tenofovir disoproxil fumarate)
Non-Nucleoside Reverse Transcriptase Inhibitors
- Rilpivirine
Integrase Strand Transfer Inhibitors
- Raltegravir
- Elvitegravir with boosting agent cobicistat
- Dolutegravir
- Bictegravir
- Cabotegravir
Protease Inhibitors
- Atazanavir
- Fosamprenavir
- Ritonavir-boosted lopinavir
Other drug classes, such as CCR5 antagonists, fusion inhibitors, attachment inhibitors, capsid inhibitors, and post-attachment inhibitors, are reserved for patients with multidrug-resistant HIV.
Recommended Therapy for Treatment-Naive Patients
Current guidelines recommend initiating INSTI-based therapy with a dual NRTI backbone before conducting further laboratory testing for most patients with HIV. In select cases where the HIV RNA level is <500,000 copies/mL, no coinfection with hepatitis B occurs, and no genotypic resistance is present, a 2-drug regimen with dolutegravir and lamivudine can be considered first-line. Tenofovir plus lamivudine or emtricitabine are the preferred NRTIs, and bictegravir and dolutegravir are the preferred INSTIs due to their effectiveness, adverse effect profiles, shorter duration to virological suppression, and lower propensity to develop resistance. INSTI-based regimens achieve faster viral suppression compared to protease inhibitor or NNRTI-containing regimens. The addition of other agents in the setting of advanced HIV on presentation does not improve clinical outcomes at the onset of treatment and is not recommended. Abacavir is no longer recommended as initial therapy due to the association with cardiovascular disease, the risk of hypersensitivity, and the need for HLA B*5701 testing. Antiretroviral treatment should be started regardless of the CD4+ count to reduce a combination of serious AIDS- and non–AIDS-related events and death from any cause if started at diagnosis compared to a deferred initiation of treatment. [24] [25] [26] [27] [28]
Comorbid conditions affect HIV ART selection due to contraindications or co-benefits of treatment, including the following:
- Hepatitis B virus coinfection: Tenofovir-containing ART regimens are preferred as tenofovir suppresses hepatitis B virus replication. ART regimens that use lamivudine or emtricitabine without tenofovir should be avoided as they can result in the rapid emergence of resistant hepatitis B virus.
- Renal dysfunction: Tenofovir disoproxil fumarate is associated with proximal tubular dysfunction and should be avoided in patients with an estimated glomerular filtration rate of less than 60 mL/min/1.73 m 2 . Tenofovir alafenamide can be used unless the patient has reduced renal function (estimated glomerular filtration rate <30 mL/min/1.73 m 2 ) and is not on dialysis, in which case no tenofovir formulation is indicated. The preferred regimen is dolutegravir plus lamivudine, adjusted for renal function. Atazanavir should also be avoided in patients with reduced kidney function.
- Osteoporosis: Tenofovir alafenamide is associated with less bone loss and is preferred over tenofovir disoproxil fumarate–containing regimens.
- Pregnancy: Bictegravir, doravirine, cabotegravir, dolutegravir/lamivudine combination, and dolutegravir/rilpivirine combination should not be initiated during pregnancy due to limited data to support their safety. Cobicistat-containing regimens should not be used during pregnancy due to inadequate drug levels.[NIH. HIV Guidelines 2023. ]
When laboratory test results are available, the initial regime may be adjusted. A dual NRTI coformulation plus an antiretroviral from another class is prescribed for patients unable to take an INSTI-based regimen. For example, the protease inhibitor darunavir is boosted with cobicistat, ritonavir, or an NNRTI, either efavirenz or rilpivirine. [15]
Recommended Therapy for Treatment-Experienced Patients
Treatment-experienced patients require a wider range of antiviral options compared to initial treatment due to an increased probability of multidrug-resistant strains of HIV. Notably, the barrier to resistance is higher with combination antiretroviral therapy containing later-generation integrase inhibitors, including dolutegravir and bictegravir. However, emerging reports of increasing resistance to this class are apparent. Common resistance mutations identified at the initiation of antiretroviral therapy in treatment-experienced patients include M184V and KS65R with tenofovir, K103N/S in NNRTIs, and thymidine-analogue mutations in NRTIs. [29] [30] [31] [32]
Initial therapy remains the same for treatment-naive patients with the goals of maintaining long-term virological suppression and limiting the progression of the disease. Patients still require 2 to 3 active agents against HIV to reduce the risk of virological failure and drug resistance. The regimens typically include an NRTI backbone with NNRTI and an integrase or protease inhibitor. The NADIA and REVAMP trials, among others, indicated that resistance to first-line antiretroviral agents did not indicate failures in virological suppression with second-line agents incorporating classes from the first-line agents, suggesting that drug-resistance assay may not always be required. However, given that these results were not primary endpoints, these findings should be interpreted cautiously, and drug-resistance assays may still yield better choices in well-resourced clinical settings. A genotypic drug-resistance assay is indicated when the patient is still taking the ART or as soon as possible after ART discontinuation. [24] [33] [34]
The challenge with antiretroviral agent resistance is that resistance may not predict clinical outcomes accurately. [35] In cases of complex drug resistance, phenotypic assays may be considered to test drug susceptibility against HIV. [36] When possible, drug resistance therapies can guide alternatives to first-line NNRTIs, integrase, or protease inhibitors. The therapies may include second-generation NNRTIs, capsid inhibitors, pharmacologically boosted protease inhibitors, and later-generation integrase inhibitors. Policies should be instituted nationally or regionally for presentations requiring third-line therapy. In cases of resistance to all available therapy, continuing the regimen that the patient best tolerates and maintains some level of virological suppression is important. Novel antiretroviral agents approved by the United States Food and Drug Administration, such as capsid or attachment inhibitors, may be considered when conventional treatment options result in virological and clinical failure. Future drugs based on monoclonal antibodies are being developed, informing treatment for treatment-experienced populations. [37] [38] [39] [40] [41]
Recommended Therapy for Patients on Preexposure Prophylaxis
Patients on preexposure prophylaxis with tenofovir alafenamide or tenofovir disoproxil fumarate with emtricitabine who subsequently acquire HIV require resistance testing before initiating therapy. They can be initiated on INSTI-containing regimens outlined above while genotype or resistance testing is pending. Patients who acquire HIV after receiving cabotegravir for preexposure prophylaxis require INSTI genotyping before beginning therapy with an INSTI-based regimen. Although the results are pending, a boosted protease inhibitor regimen containing darunavir and a tenofovir-based dual NRTI coformulation should be used. Please see StatPearls' companion resource, " Preexposure Prophylaxis for HIV Prevention ," for more information. [24]
Therapy Considerations for Patients With HIV-2 Infections
Current recommendations for treating HIV-2 are primarily based on single-arm or observational studies, as most research and treatment efforts have focused on HIV-1. The initial regimen for managing HIV-2 should include 2 NRTIs plus a second-generation INSTI or a ritonavir-boosted protease inhibitor.[NIH. HIV Guidelines 2023. ] Tenofovir disoproxil fumarate and emtricitabine are the preferred NRTIs. Dolutegravir is recommended as the preferred INSTI. Darunavir and lopinavir are more active against HIV-2 compared to other protease inhibitors and are the preferred agents. Two-drug regimens to treat HIV-1 and any regimen containing NNRTIs are ineffective against HIV-2 and should not be used in these infections. [42]
Drug-resistance assays for HIV-2 are limited to research laboratories, complicating the management of these infections. Current data suggest that HIV-2 is susceptible to NRTIs; however, HIV-2 is more likely to develop resistance compared to HIV-1. Patients with HIV-2 mutations may exhibit complex patterns of protease inhibitor cross-resistance, making sequential regimens ineffective for patients with this infection. In vitro studies report strong efficacy for INSTIs against HIV-2. Clinical data regarding mutations and efficacy of the most commonly used INSTI, such as dolutegravir, are limited, especially in those previously exposed to other INSTIs. Further research is needed to understand resistance patterns for antiretrovirals in patients with HIV-2. [43] [44]
Opportunistic Infections
In addition to rapid ART initiation, prophylaxis for opportunistic infections should be started based on the level of immunosuppression.
- Pneumocystis jirovecii (previously P carinii ): Prophylaxis is indicated for patients with thrush on presentation, a CD4+ count of less than 200 cells/mm 3 or a CD4+ count <14%.
- Cryptococcus neoformans and Cryptococcus gattii : Patients with a CD4+ count of less than 100 cells/mm 3 and a positive serum cryptococcal antigen result require prophylaxis.
- Histoplasma capsulatum : Prophylaxis is recommended in areas where histoplasmosis is endemic and the patient's CD4+ count is less than 150 cells/mm 3 .
- Mycobacterium avium complex infection: If patients with HIV are rapidly initiated on ART, prophylaxis for Mycobacterium avium complex is not required. Patients with a CD4+ count of less than 50 cells/mm 3 without ART should receive prophylaxis.
- Toxoplasma gondii : Patients with CD4+ counts less than 100 cells/mm 3 who have positive test results for toxoplasma antibodies require chemoprophylaxis . [26]
In patients with a severe opportunistic infection, rapid initiation of ART can result in immune reconstitution inflammatory syndrome. [23] The opportunistic infection should be treated before initiating ART to decrease the risk. Initiate ART within two weeks of initiating treatment for most acute opportunistic infections, except acute cryptococcal meningitis. [45] Patients with cryptococcal meningitis may commence HIV therapy within 2 to 4 weeks of antifungal therapy. HIV treatment should be initiated within 2 weeks of tuberculosis treatment in patients who have active tuberculosis, especially if they have severe immunosuppression (CD4+ count <50 cells/mm 3 ); however, if evidence of tuberculous meningitis is detected, ART should be given with high-dose corticosteroid treatment. [24]
Recommended Therapy for Prophylaxis of Opportunistic Infections
Prophylaxis and treatment in HIV-infected patients with opportunistic infections is dependent on the level of immunosuppression for the patient and the isolation of any causative pathogens. Improving the underlying cause of the immunosuppression by treating HIV is recommended when managing opportunistic infections. [46] Given the high risk of P jirovecii with CD4+ counts <200 cells/mm 3 or CD4+ count <200 cells/μL or a CD4+ count <14%, low-dose trimethoprim/sulfamethoxazole (co-trimoxazole) prophylaxis is recommended, which protects against cerebral toxoplasmosis, bacterial infections, and malaria in endemic settings. [47] [48] Alternatives for sulfur allergy where desensitization is not feasible include inhaled pentamidine, dapsone, or atovaquone. [49] Hypoglycemia must be monitored with the administration of pentamidine, whereas G6PD deficiency needs to be assessed before starting dapsone. [50] [51] Atovaquone can be considered but may not be as efficacious. Prophylaxis is often discontinued after the CD4+ count returns to >200 CD4+ cells/mm 3 for 3 or more months in patients on antiretroviral therapy. [52] [53]
For the treatment of P jirovecii , weight-based dosing of trimethoprim/sulfamethoxazole (co-trimoxazole) for 21 days is recommended in addition to high-dose steroids to manage the progressive respiratory complications of acute pneumonia and reduce mortality risk. Alternatives include primaquine with clindamycin and intravenous pentamidine for 21 days. Primary prophylaxis of toxoplasmosis, where the risk is greatest with a CD4+ count <100 cells/mm 3 , is also covered by low trimethoprim/sulfamethoxazole (co-trimoxazole). Alternatives in the event of a non-severe allergy to sulfur include dapsone with pyrimethamine and calcium folinate. Discontinuation of primary prophylaxis may be considered if the CD4+ count is >200 cells/mm 3 for 3 or more months in individuals on antiretroviral therapy. [54] [55] [56] [57] Vaccinations should be encouraged for patients with HIV, as these may reduce the risk of mortality from influenza, pneumococcal, and meningococcal pneumonia and reduce the risk of other blood-borne viral infections. [58] [59] [60] [61] Given the higher risk of human papillomavirus–related cancers in patients with HIV, human papillomavirus vaccination is recommended. [62] Please see StatPearls' companion resource, " Prevention of Opportunistic Infections in HIV/AIDS ," for more information.
Monitoring Following Initiation of Treatment
When treatment is initiated, the patient's HIV viral load should be evaluated in 2 to 4 weeks and no later than 8 weeks. The viral load can be rechecked every 4 to 8 weeks to ensure the levels decline. Virologic suppression to undetectable levels (defined as an HIV RNA level of <200 copies/mL) may take up to 24 weeks of continuous therapy. If the HIV RNA level has not declined by 2 log10 copies/mL within 12 weeks and adherence is confirmed, evaluation for resistance with genotype testing for the patient's regimen is recommended. Genotype resistance testing should also be obtained if virologic suppression is not achieved. [13] When viral suppression is established, the viral load should be monitored every 3 to 4 months. [7] An HIV RNA level that remains persistently below this lower limit of detection demonstrates sustained virologic suppression—the primary goal of HIV therapy. Please see StatPearls' companion resource, " HIV Antiretroviral Therapy, " for more information.
Inadequate Viral Suppression and Development of Resistance
When inadequate viral suppression occurs, the HIV viral load and selection pressure increase, inducing mutations favorable to resistance against active antiretroviral treatments. Virological failure occurs when HIV viral is above 1000 copies/mL on 2 separate and consecutive measurements over 3 months while on current antiretroviral treatment for 6 months or longer. This condition can result in clinical failure, described as a new event or recurrence of WHO stage 4 severe immunodeficiency after 6 months of current effective antiretroviral treatment. Clinical failure suggests concern for antiretroviral resistance while a patient adheres to effective antiretroviral treatment for 6 months or longer. [63] Given HIV has an RNA genome prone to mutations, selection pressure from active antiretroviral agents during reverse transcriptase can encourage drug-resistant mutants that lead to virological failure, clinical failure, and eventually immunological failure, defined as a CD4+ count of 250 cells/mm 3 or less. [64] [65] Thus, while trials such as NADIA and REVAMP indicated treatment regimens incorporating antiretroviral agents with prior resistance is feasible, the theoretical basis of how HIV develops new mutations with inadequate viral suppression should be considered by the treating clinician in managing treatment-experienced patients. [33] [34]
Immunological Recovery
In patients who had a baseline CD4+ count of 250 cells/mm 3 or less at diagnosis or immunological failure while on antiretroviral treatment but then subsequently recovered a CD4+ count at or above 500 cells/mm 3 , immunological recovery constitutes only a minority of all patients on effective antiretroviral treatment. The reasons are often multifactorial, including adherence, baseline CD4+ count at diagnosis, the age and sex of the patient, the type of initial antiretroviral agents initiated at diagnosis and any delays, and the patient's baseline functional status. [66] [67] [68] When immunological recovery does occur, the timeline is typically over many months or years but depends on multiple factors, including the class of antiretroviral therapy, the baseline functional status of the patient, and treatment adherence. Conversely, inadequate immunological responses are associated with an increased risk of serious non-AIDS events and mortality in patients with HIV. Therefore, the treating clinician needs to monitor the CD4+ count in patients undergoing treatment with antiretroviral agents for potential sequelae. [69] [70]
- Differential Diagnosis
HIV should be considered in any patient with recurrent serious infections. Other conditions that may have similar effects on the patient's immune system include:
- Severe malnutrition
- Severe combined immune deficiency syndrome
- Chemotherapy-induced immunosuppression
The differential diagnosis in patients who present with acute HIV includes:
- Mononucleosis
- Toxoplasmosis
- Viral hepatitis
- Systemic lupus erythematosus
- Toxicity and Adverse Effect Management
When prescribing antiretrovirals, a wide variety of potential drug-drug interactions, toxicities, and other adverse effects must be considered. Consultation with a pharmacologist is recommended. Some common reactions are listed below.[NIH. HIV Guidelines 2023. ]
- Abacavir is contraindicated for patients who are positive for the HLA-B*5701 allele due to the risk of hypersensitivity reactions. Pretesting is required before prescribing any regimen containing abacavir. Some studies have also demonstrated increased cardiovascular risk with abacavir.
- Tenofovir alafenamide is associated with higher lipid levels and weight gain but is associated with less renal toxicity compared to tenofovir disoproxil fumarate.
- Tenofovir disoproxil fumarate is associated with renal toxicity; proximal tubulopathies, such as Fanconi syndrome; and acute or chronic renal insufficiency, particularly when combined with boosters. Tubulopathy can cause osteomalacia, and tenofovir disoproxil fumarate can also cause decreased bone density.
- Emtricitabine has been associated with hyperpigmentation of the palms and soles.
- Didanosine and stavudine are no longer used due to severe adverse reactions and toxicity, such as fatal lactic acidosis, pancreatitis, and peripheral neuropathy. Lamivudine can rarely be associated with pancreatitis. [71] [72] [73] [74]
- Doravirine, efavirenz, and rilpivirine have the potential for cytochrome P450 (CYP) enzyme drug interactions.
- Efavirenz can also cause dyslipidemia, rash, and QTc interval prolongations. The drug has the potential for short- and long-term psychiatric complications, suicidality, catatonia, and late-onset ataxia and encephalopathy.
- Rilpivirine can cause QTc interval prolongation but does not appear to be increased beyond 48 weeks compared to efavirenz. The drug is less commonly associated with depression, suicidality, and rash compared to efavirenz. [75] [76] [77] [78] [79]
- All drugs of this class can cause weight gain compared to other antiretroviral classes.
- Several drugs inhibit creatinine excretion without affecting glomerular filtration, including bictegravir, dolutegravir, and cobicistat.
- Bictegravir is a substrate for the enzymes CYP3A4 and UGT1A1 (uridine diphosphate glucuronosyltransferase 1A1) and has the potential for drug-drug interactions. Examples include aluminum- and magnesium-based antacids, where bictegravir should be administered 2 hours before or 6 hours after, and rifampicin, a potent CYP3A inducer.
- Early studies suggested that dolutegravir exposure during conception may be associated with neural tube defects. However, a large cohort study of over 4 million pregnancies in the United States in 2023 did not identify an increased risk of neural tube defects among infants. Clinicians should discuss the current evidence when prescribing medications to people of childbearing potential.
- Raltegravir can increase creatinine kinase and less frequently cause myopathy and rhabdomyolysis. The drug is known to cause severe hypersensitivity reactions, including Stevens-Johnson syndrome and toxic epidermal necrosis. As a UGT1A1 substrate, the potential for drug-drug interactions is recognized. Rarely, raltegravir has been associated with depression and suicidal ideation in patients with preexisting psychiatric conditions.
- Cobicistat is an INSTI used exclusively as a pharmacokinetic enhancer of certain protease inhibitors and INSTIs. The drug strongly inhibits CYP3A4, resulting in significant potential for drug-drug interactions. Cobicistat must be discontinued in severe hepatic impairment. Similar to other INSTIs, the drug can increase creatinine excretion without impacting glomerular filtration. [80] [81] [82] [83] [84]
- Protease inhibitors other than atazanavir are associated with an increased risk of cardiovascular events.
- Atazanavir and darunavir are both CYP3A4 inhibitors and substrates with the potential for many drug interactions. Both are co-formulated with cobicistat or ritonavir due to their ability to boost therapeutic levels of atazanavir and darunavir.
- Atazanavir coformulations can cause indirect hyperbilirubinemia, nephrolithiasis, cholelithiasis, nephrotoxicity, and gastrointestinal adverse effects.
- Darunavir coformulations can cause skin rash, gastrointestinal adverse effects, and hepatotoxicity, particularly in patients with preexisting liver disease. [85] [86] [87] [88]
CCR5 Inhibitor
Maraviroc adverse effects are uncommon, but when they occur, they are typically constitutional and include gastrointestinal upset and fever. [89]
Fusion Inhibitors
Enfurvitide, an injectable antiretroviral agent, can be associated with injection site reactions, including granuloma annulare and other symptomatic lesions. [90]
Capsid Inhibitors
The phase III clinical trial of lenacapavir involving 72 patients showed no serious adverse effects (grade 3 or above), with only injection site reactions and gastrointestinal symptoms identified. However, given the small numbers involved, post-marketing reports should be reviewed. [39]
Attachment Inhibitors
The phase III trial of fostemavir involving 371 patients showed mild gastrointestinal adverse effects that led to treatment discontinuation. However, given the small cohort, post-marketing reports should be reviewed. Various drug interaction tools exist online, allowing clinicians to access bedside information. When managing these potential adverse effects, the treating clinician should balance the competing priorities of maintaining virological suppression and reducing future antiretroviral class resistance with patient tolerance and concerns of long-term adverse effects. Considering the individual's circumstances strengthens the therapeutic relationship and encourages treatment adherence. [40] [91]
Staging systems can be used for clinical or surveillance purposes to assess the rate of progression to more advanced stages, assist in monitoring the HIV burden at a population level, plan for prevention and care, and evaluate interventions. The CDC published the current definitions of HIV surveillance cases in 2014. The definitions of surveillance cases are not intended for clinical decision-making. HIV testing confirms the diagnosis and determines acute infection in stage 0, and the CD4+ count determines stages 1 to 3. All surveillance cases are assumed to be HIV-1 unless laboratory evidence indicates HIV-2. The criteria for stage 0 supersede and are independent of the criteria used for other stages. [19]
Confirmed HIV Case
For all adults, adolescents, and children aged 18 months and older:
- A second, different HIV test confirms a positive result from an initial HIV antibody or antibody-antigen test.
- Clinical criteria can be used where HIV test results have not been recorded, but other presumptive evidence exists, such as the history of therapy for HIV or an AIDS-defining illness.
This stage is defined by a positive test result within 180 days of:
- A negative or indeterminate test result
- A negative initial immunoassay result followed by a positive nucleic acid amplification test result to confirm acute infection
- A positive nucleic acid amplification test result following a positive antigen or antigen-antibody test result but unconfirmed by a second test
Stages 1 to 3
These stages are determined based on the CD4+ count for all people aged 6 and older (separate criteria exist for infants aged younger than 1 and children aged 1 to 5):
- Stage 1: 500 or more cells/μL
- Stage 2: 200 to 499 cells/μL
- Stage 3: less than 200 cells/μL
Globally, case definitions for HIV vary by country based on the testing technologies most appropriate for use in the local context. The WHO recommends test-based confirmation in adults and children aged 18 months or older to require a positive result from a rapid or laboratory-based HIV antibody, antigen, or virological test, confirmed by a second positive test result relying on different antigens or different test operating characteristics. Advanced HIV is confirmed by the diagnosis of a condition associated with advanced disease or a CD4+ count of less than 200 cells/mm 3 in an adult or child with HIV. The WHO's clinical staging for HIV can be used for clinical and surveillance purposes in resource-limited areas where CD4+ testing may be unavailable.[WHO. HIV Case Definition 2007 .] Advanced disease is defined as stage 3 or 4.
Stage 0 or Primary HIV Infection
Patients have 1 or more symptoms associated with acute HIV or a constellation of symptoms consistent with acute retroviral syndrome.
In stage 1, patients are asymptomatic or have persistent generalized lymphadenopathy, defined as enlarged lymph nodes (>1 cm) in 2 or more non-contiguous sites (excluding inguinal nodes) not explained by any other cause.
In this stage, patients show unexplained weight loss (moderate degree, <10% of body weight), recurrent respiratory tract infections, herpes zoster exacerbations (mild-to-moderate severity), angular cheilitis, recurrent oral ulcerations, papular pruritic eruptions, seborrhoeic dermatitis, or fungal fingernail infections.
In stage 3, patients experience severe weight loss (>10% of body weight); unexplained chronic diarrhea; persistent fever; oral candidiasis; oral hairy leukoplakia; pulmonary tuberculosis; severe invasive bacterial infections, such as pneumonia, empyema, osteomyelitis, meningitis, and bacteremia; acute necrotizing ulcerative stomatitis; gingivitis or periodontitis; or unexplained anemia, neutropenia, or thrombocytopenia for more than 1 month.
Stage 4 or AIDS
In this stage, patients develop HIV wasting syndrome; pneumocystis pneumonia; chronic herpes simplex infection; esophageal candidiasis; extrapulmonary tuberculosis; Kaposi sarcoma; toxoplasmosis; HIV encephalopathy; extrapulmonary cryptococcosis infections; disseminated nontuberculous mycobacterial infections; progressive multifocal leukoencephalopathy; pulmonary candidiasis; cryptosporidiosis; isosporiasis; cytomegalovirus retinitis (or in an organ other than liver, spleen, or lymph nodes); disseminated mycoses, such as histoplasmosis, coccidioidomycosis, and penicilliosis; recurrent salmonella septicemia; lymphoma (cerebral or B-cell non-Hodgkin); invasive cervical carcinoma; or visceral leishmaniasis.
The WHO defines HIV wasting syndrome as the presence of unexplained weight loss greater than 10% of the body weight, with the presence of either unexplained chronic diarrhea or unexplained fever for 1 month or more. WHO clinical stage 4 and the CDC criteria for AIDS are almost identical. [13] A wide variety of studies in resource-limited settings have evaluated the utility of the WHO staging system for predicting immunological status defined by the CD4+ count. The findings indicate highly variable sensitivity and specificity across various CD4+ cut-off levels, questioning the utility for clinical decision-making. This issue underscores the importance of expanding the availability of CD4+ and viral load testing and the WHO and CDC recommendations to offer treatment to all patients who are HIV positive.[WHO. Global HIV Strategies 2022-2030. ] [16] [14] [15] [35] [43] [44]
Without therapy, HIV infections are invariably fatal. However, effective ART with sustained virologic suppression dramatically improves the clinical outcomes for patients with HIV. According to a meta-analysis from 2017, life expectancy in high-income countries is estimated to be 43.3 years if ART begins at 20, and 32.2 years if ART begins at 35. In low- to middle-income countries, life expectancy is estimated to be 28.3 years and 25.6 years if ART starts at 20 and 35, respectively. In all regions, regardless of income, life expectancy after starting ART has improved, reflecting improvements in therapy and management such as improved ART regimens, earlier initiation of ART, and better socioeconomic and adherence support. [92]
Viral suppression is the key determinant of prognosis. Patients who achieve virologic suppression for at least 3 years without full immunologic recovery (CD4+ count <200 cells/mm 3 ) have 2.6 times greater all-cause mortality compared to those who achieve immunologic recovery (CD4+ >200 cells/mm 3 ). Treatment at diagnosis is associated with improved outcomes and better immune system recovery. Delaying ART until the patient's CD4+ count is less than 200 cells/mm 3 decreases the likelihood of the CD4+ count normalizing after multiple years of otherwise effective antiretroviral therapy, thereby increasing the patient's risk of AIDS and non-AIDS-related morbidity and mortality. Other factors correlating with poor immunologic recovery include older age, lower nadir CD4+ count, and extended ART initiation to viral suppression time. Hepatitis C, hepatitis B, and active injection drug use are identified as important factors contributing to higher morbidity and mortality among patients with HIV-1. For people who inject drugs, socioeconomic and adherence support should be offered, mainly if treatment is unavailable. [93] [94] [95]
- Complications
Complications Related to HIV
The advent of ART has dramatically decreased the incidence of opportunistic infections and HIV-associated malignancies. However, progression to AIDS remains a significant complication of HIV. Screening and monitoring are warranted for AIDS-defining illnesses as appropriate to the patient's clinical status. In addition, screening and monitoring for specific HIV- and ART-related complications are listed below:
- HIV-associated neurocognitive disorders and psychiatric complications, with the use of efavirenz and, less frequently, rilpivirine and other INSTIs
- HIV-associated distal symmetric polyneuropathy
- HIV-associated lipodystrophy
- Mitochondrial toxicity of HIV NRTIs
- HIV-associated Kaposi sarcoma inflammatory cytokine syndrome and multicentric Castleman disease
- Hematological malignancies, including primary effusion, follicular, non-Hodgkin, Burkitt, and diffuse large B-cell lymphomas [96] [97] [98]
Please see StatPearls' companion resources, " Acquired Immune Deficiency Syndrome ," " HIV Neurocognitive Disorders ," and " HIV-Associated Lipodystrophy " for more information.
ART-Related Complications
The advent of ART has also raised the risk of cardiovascular disease morbidity and mortality. Patients with HIV experience age-related comorbidities such as cardiovascular disease more frequently, and much of the long-term care for patients with HIV focuses on minimizing cardiovascular risks. Multiple different factors contribute to increased cardiovascular risk for patients with HIV, including:
- The prevalence of dyslipidemia among patients with HIV, with and without ART, is high.
- Glucose intolerance or diabetes frequently occurs in patients receiving ART; this may be due to specific ART drugs, such as earlier-generation protease inhibitors.
- Multiple ART regimens are associated with weight gain.
Weight gain is common among patients on ART and is 1 of the significant contributors to cardiovascular risk for patients with HIV. The exact mechanisms involved are unknown. Some ART agents contribute more to weight gain compared to others. INSTIs are associated with more weight gain compared to protease inhibitors or NNRTIs, and tenofovir alafenamide is associated with more weight gain compared to tenofovir disoproxil fumarate, abacavir, or zidovudine. Current guidelines recommend lifestyle modification counseling from the onset of ART to mitigate weight gain and metabolic complications. Routine screening for glucose intolerance, diabetes, and hyperlipidemia is recommended. [5] [99]
- Deterrence and Patient Education
Education, support, and counseling from the interdisciplinary care team are essential for patients with HIV. Patients can be referred to organizations such as the CDC or NIH, which offer freely accessible education.[NIH. Fact-sheets. ]
HIV Treatment
Patients should be informed about the need for regular labwork and follow-up. They should also be encouraged to keep all medical appointments and speak freely and openly with their clinician to ensure that adverse effects, potential barriers to treatment, and other health concerns can be addressed effectively. Patients undergoing HIV treatment require consistent education and counseling to promote medication adherence, including the importance of starting ART as soon as possible after diagnosis and the need to take the prescribed medications in compliance.
Medication adherence is essential to achieve HIV viral load suppression. Viral load increases within weeks of stopping HIV medications and enhances the risk of developing resistant organisms, complications from HIV, and transmission. Overcoming the challenges with medication adherence includes seeking support through counseling, support groups, or consistent communication with the treating teams. Patients should be offered home nurse visits, blister packs, or automated reminders to support adherence.
Identifying the signs and symptoms that indicate toxicity from ART and the recommended next steps should be relayed when educating patients. Minor adverse effects, such as nausea upon initiation of therapy, can be managed with over-the-counter medications. Signs and symptoms of liver or kidney injury should lead patients to seek immediate medical care. Patients should be aware of the potential for drug-drug interactions and the importance of the pharmacist in managing their medications. They should be encouraged to document their medication regimen to maintain self-efficacy in treatment. Patients with HIV are at elevated risk of cardiac and metabolic complications, may face complications that affect nutrition, and may need to avoid certain foods due to the immunocompromised state. Lifestyle modifications to encourage healthy eating, regular exercise, and the management of other risk factors, such as smoking, are recommended.
Prevention of HIV Transmission
Patients with HIV are often highly motivated to prevent transmission to others, particularly when they have a partner who is not infected with HIV. Recent data in the United States reveal that every 10% increase in viral suppression on a population level is associated with a 4% decline in the incidence of HIV in the subsequent year. Clinical education and emphasis on undetectable equals untransmittable are crucial elements of HIV treatment, benefiting both the patient's health and that of their partner or partners. People of child-bearing age may be concerned about the transmission of HIV during pregnancy and should be aware of the options for treatment and the benefits of planning.
People with an undetectable viral load may continue to make proactive decisions to protect people within immediate contact. The person can correctly and consistently use condoms, choose sexual activities with lower risk, encourage their partners to take preexposure prophylaxis, and avoid sharing needles, syringes, and other drug injection equipment. People may be required to disclose their HIV or other communicable disease status, for example, to clinicians in jurisdictions with these requirements or to healthcare regulatory authorities if they provide healthcare services. [19]
Stigma, Discrimination, and Mental Health
Diagnosis with a chronic illness can be a significant source of stress for anyone, given the medical burden on lifestyle. The diagnosis can challenge one's sense of well-being or complicate existing mental health or other conditions. Individuals may feel sadness, hopelessness, or anger. In addition to the challenges of a new diagnosis of a serious chronic illness, patients with HIV face further difficulties due to stigma and discrimination, including self-stigma. Self-stigma occurs when patients with HIV internalize the negative opinions of others, such as believing only certain kinds of people acquire HIV or that they deserve to contract the disease of their behaviors.
The need to disclose HIV status to sexual or injection partners before sex or drug use can be uncomfortable and provoke anxiety, especially where punitive laws exist. Patients with HIV may have fears associated with protecting others, which limit their interactions with other people and lead to isolation. Referrals to a psychologist, social worker, specialized nurse, public health, other interdisciplinary team member, or support groups can assist patients in coping with social issues. Likewise, encouraging patients to share their HIV status with certain friends and family can lead to practical and emotional benefits. Please see StatPearls' companion resources, " HIV Antiretroviral Therapy ," and " HIV Prevention ," for more information.
- Pearls and Other Issues
Clinicians must be aware of any rules and regulations regarding HIV screening, testing, reporting, and managing HIV in the jurisdiction where they practice. These rules and regulations vary widely. In some jurisdictions, rules and regulations may exist as public health acts and follow a progressive enforcement framework; in others, they may be incorporated into criminal law and carry significant penalties.
Relevant State Laws in the United States
Most states in the United States have defined guidelines in their administrative codes. For example, Florida Administrative Code Rule 64B8-13.005 states that every physician licensee must complete 1 hour of Category I American Medical Association CME that covers HIV and AIDS every 2 years. This requirement includes information regarding Florida State Law for HIV testing and test result reporting provided in statutes 381.004 and 384.25 mandating the following:
- Before HIV testing, the patient must be notified orally or in writing that the test is planned. The patient must be given the right to refuse testing, which is documented in the medical record.
- If the patient or their legal guardian signs a medical consent form for medical care in a healthcare setting, such as a clinic, emergency setting, or hospital, a separate consent form for an HIV test during the period in which the general consent is in effect is not required.
- In a nonhealthcare setting, such as community outreach programs, informed consent with the option to decline testing and information regarding sites that provide anonymous testing in the community must be obtained.
- In the event of a positive test result, the patient must receive appropriate care and medical support services, information on the importance of notifying partners who may have been exposed, and ways to prevent transmission.
- In the event of a negative test result, every effort should be made to notify the patient and provide information regarding ways to prevent the acquisition of HIV.
- In a healthcare setting, if the patient is discharged before the results are available, the county health department must notify the test subject to fulfill the responsibility after a positive test result.
- The person tested and clinicians responsible for the medical care and decision-making for the tested individual.
- The person tested and clinicians responsible for the care and decision-making of a newborn who may be affected by these results.
- Healthcare personnel subject to significant exposure from the person whose results were positive.
- Individuals tested using rapid testing technologies under manufacturers' instructions approved by the United States Food and Drug Administration.
- Unconfirmed test results must not be presented to the patient as an HIV diagnosis. The rationale for releasing unconfirmed results must be documented in the chart. Confirmatory testing must be obtained, and the results must be communicated to the individual tested.
- Positive HIV test results, after confirmatory testing, may only be released to the tested individual or their designated legally authorized representative.
- Results must be reported to the State Health Department following rules for reporting and controlling disease. Results may also be shared with clinicians who use semen or body parts from an infected individual, health facility committees for purposes of program monitoring and evaluation, and authorized researchers and epidemiologists, as proscribed under appropriate statutes and procedures.
- The patient must provide written authorization for the release of such testing to any other individual or third-party payor for HIV test results to be released. In this scenario, consent beyond general consent is required to release medical records. A specific authorization for the release of HIV test results must be provided.
- If HIV testing is conducted due to medical personnel exposure, the occurrence should be documented and recorded only in the medical personnel's personnel records. In addition, the cost of the initial HIV test should be borne by the medical personnel or their employer.
- If the source of the exposure is unavailable or not voluntarily present for testing, the medical personnel or the employer may seek a court order for HIV testing from the source individual. The test results should be released to the source and the person who experienced the exposure.
- Clinicians, laboratories, and healthcare facilities that diagnose or treat individuals with HIV/AIDS must report the result no later than 2 weeks following the diagnosis or treatment as outlined above.
- Violation of these rules is subject to penalties, fines, and disciplinary actions.
Impact of Legislation on HIV Testing in the United States
Laws and regulations concerning HIV and AIDS are developed considering a variety of parameters that impact patients' willingness to test for HIV. For example, to provide patients with privacy, confidentiality, and dignity, the Florida legislature considered the need for informed consent and privacy in designing laws regarding HIV and other sexually transmitted diseases, including laws for reporting.
Informed consent
- Informed consent includes an explanation to the patient regarding confidentiality, mandatory reporting, and the opportunity for anonymous testing.
- This consent maintains that in a healthcare setting, a patient must be notified of a planned HIV test, and they have the right to refuse the test.
- Informed consent allows a legal guardian to provide informed consent if a person is incompetent, incapacitated, or a legal minor.
Confidentiality
- The patient gives consent.
- The data are provided for statistical purposes and exclude identifying information.
- The clinician or facility must disclose the result for mandatory reporting to medical personnel, state agencies, or mandated court jurisdiction.
- The information needs to be disclosed during a medical emergency; only relevant information for the patient's care can be disclosed.
- The person commits a misdemeanor of the first degree, which is punishable by a fine of up to $1000 and up to 1 year in prison.
- A person who spreads information about a patient with HIV or another sexually transmitted disease for monetary gain or with malicious intent commits a felony in the third degree, which is punishable by a fine of up to $5,000 or imprisonment of up to 5 years.
- Reporting ensures results are promptly and confidentially reported to the Florida Department of Health to allow for contact follow-up and other public health activities, such as surveillance. A positive test result or other diagnosis of HIV or AIDS must be reported within 2 weeks using the system developed by the CDC or an equivalent system to ensure confidentiality.
- The Department of Health may fine anyone who fails to report HIV or AIDS up to $500 for each offense, and a regulatory agency is informed of the violation.
Similar laws exist on these ethical principles in other settings worldwide, which are important to reference when treating 1 or more patients with HIV.
- Enhancing Healthcare Team Outcomes
Almost 21 million lives have been saved with antiretroviral therapy worldwide. However, current prevention and treatment services miss or inadequately serve millions. Key indicators of the quality of HIV care for optimal patient and population health outcomes include the linkage of HIV-positive individuals to care, documentation of treatment by patients linked to care, and attainment of viral suppression among patients who are treated. For example, in the United States in 2021, 80% of patients with HIV were linked to care within 30 days of diagnosis, defined as at least 1 viral load or CD4+ test. However, of all people with an HIV diagnosis in 2021, only 54% were retained in care (defined as ≥2 viral loads or CD4+ tests ≥3 months apart in 2021), and only 66% were virally suppressed (defined as an HIV RNA level of <200 copies/mL).
Within countries, variability in key quality care indicators exists across populations. Although the disparities are greatest in low- and middle-income countries, significant inequities continue to exist in prevalence and incidence across population groups in countries with well-developed HIV responses. For example, compared to the general population in the United States, HIV disproportionately affects people who inject drugs, patients who live in the United States South, and patients who are Black, Hispanic, Latino, transgender, or men who have sex with men. Across the European Economic Area, migrants accounted for 44% of new HIV diagnoses in 2019.
A successful HIV response depends on multiple factors, including utilizing a team-based and patient-centered care approach, a focus on prevention in the community and healthcare settings, robust monitoring and surveillance systems, continuous program quality improvement, and supportive social and legal environments. An integrated interprofessional team including physicians, nurses, pharmacists, social workers, public health officials, and community partners is essential to improve clinical outcomes for patients with HIV, improve population health, and ultimately change the course of the global HIV pandemic. [24]
Patient-Centered, Team-Based Care
Patient-centered care facilitates the maximum benefit from prevention services, improving clinical and public health outcomes. As HIV is an epidemic that disproportionately affects marginalized populations, programming that places the individual at the center of care ensures equality, minimizes stigma, and overcomes socioeconomic barriers that limit care access. For example, patients with HIV or who are at risk for HIV acquisition and have underlying socioeconomic disadvantages or substance use disorders are at high risk for medical nonadherence. Physicians, nurse practitioners, and physician assistants all provide primary care to patients with HIV. Clinician use of current HIV guidelines, genotype resistance testing, and measures to support adherence to therapy ensures patients receive adequate medical treatment. Systematic monitoring for complications minimizes the risk and consequences of the disease and ART.
Nurses are essential in achieving care and public health goals by supporting patients in understanding, for example, the risk of transmission, complications, and the need for medication adherence. Home-visiting nurses can help patients remain compliant with medical care and laboratory testing. According to a study from sub-Saharan Africa, immediate ART at the time of home-based positive test results increased clinical follow-up and linkage to care. Nurses and nutritionists can monitor weight and promote adequate nutrition and hydration to promote overall health, minimize cardiovascular risks, and minimize the risk of HIV wasting syndrome.
Pharmacists augment the patient's understanding by discussing potential adverse effects of therapy, ways to minimize drug interactions, and ensuring patients know when to seek help should any treatment complications occur. Measures such as blister-packed medications can ease the burden of setting up pills, minimize the risk of incorrect dosing, and improve medication adherence. Public health nurses and physicians are important in connecting and following up with contacts to prevent further transmission. They have a primary role in preventing HIV in the community, including identifying and responding rapidly to HIV outbreaks in the community. They also support the patient care team in unusual instances where patients are persistently unwilling or unable to follow recommendations to prevent transmission.
Community health, peer support, and social workers are vital in promoting patient well-being and improving linkage to care. Social workers can allocate resources for transportation and childcare support, provide assistance with mental health services, or make referrals to finance medications. Community health and peer support workers, for example, assist in the development of stigma-free services or increase the availability of home-based services. Please see StatPearls' companion resource, " HIV Antiretroviral Therapy ," for more information. [10]
Community HIV Prevention
Primordial, primary, and secondary prevention are all important in the context of improving outcomes for HIV. The percentage of new transmissions and the number of HIV-positive people who are aware of their HIV status are key quality indicators for prevention efforts. Worldwide, 86% of the 39 million people estimated to be living with HIV knew their status in 2022. In the United States, 87% of the estimated 1.2 million people living with HIV knew their status in 2021. Primordial prevention includes addressing the determinants of health that lead to increased risks for acquiring HIV, including poverty, discrimination, stigma, or other mechanisms that marginalize groups of people. Beneficial social and policy environments are essential in primordial prevention and the prevention and care spectrum.
Primary prevention targets patients who have risk factors, including public awareness and education regarding safe sexual practices and ways to reduce the risk of HIV transmission among people who inject drugs. The promotion of safer sexual practices, treatment of opioid use disorder, and widespread access to clean syringe services and other harm reduction approaches are effective prevention strategies that should be implemented across the globe. [19] Voluntary medical circumcision for heterosexual males is an effective prevention approach in southern and eastern areas of Africa where HIV is highly prevalent. Preexposure prophylaxis is a highly effective strategy for patients at high risk, for example, due to a sexual partner who is HIV positive, having multiple sexual partners without using condoms consistently, or the use of injectable drugs.
Developing cost-effective recommendations and education to screen for HIV is a critical component of prevention. For example, the United States Preventive Services Task Force (USPSTF) recommends that clinicians in the United States screen all pregnant women and at-risk persons aged 15 to 65, with particular emphasis on individuals at high risk of infection. [20] Maternal HIV screening has resulted in a significant decline in mother-to-child HIV transmission, preventing nearly 22,000 perinatal infections between 1994 and 2010. [21] Treatment as prevention recognizes that HIV is not sexually transmitted if the viral load is undetectable (defined as <200 copies of HIV-1 RNA/mL of plasma). [22] [23] Otherwise known as undetectable=utransmittable or U=U, evidence is increasing of the effectiveness for those who share drug injection equipment. Emphasis on U=U is a strong motivator for patient adherence to medication. However, with undetectable viral loads, HIV transmission occurs perinatally and through breast milk. Please see StatPearls' companion resource, " Prevention of HIV ," for more information.
HIV Prevention in Healthcare Settings
In the healthcare context, the use of rigorous infection prevention and control procedures has led to widespread decreases in occupationally and iatrogenically transmitted HIV. The elimination of the reuse of needles, syringes, and other medical equipment that can transmit HIV has been a global success story in many countries. The widespread implementation of standard precautions, previously called universal precautions, is another key preventive strategy. Initially developed to prevent HIV transmission, standard precautions are utilized to avoid the transmission of all infectious agents. The first line of defense to break the chain of transmission of infectious agents in healthcare settings is the application of standard precautions, irrespective of their known or suspected infection status. The type of infection control practice necessary is based on the level of anticipated contact with the patient and assumes that any patient's blood or body fluids may contain an infectious agent.
Hand hygiene is the most important measure to prevent transmission of disease, encompassing washing hands with soap and water for at least 40 to 60 seconds when visibly soiled, after restroom use, or potential exposure to spore-forming organisms. Alcohol-based hand rubs can otherwise be used. Clinicians should wash their hands between patients immediately after gloves are removed and before and after any direct patient contact or contact with invasive devices, blood or body fluids, secretions, mucous membranes, or nonintact skin if gloves are worn. Gloves, masks, goggles, eye visors, face shields, or gowns are used when blood, body fluids, secretions, or excretions could cause contamination. Needles and sharps should be discarded immediately in appropriate puncture-resistant containers.
Occupational post-exposure prophylaxis is crucial for preventing infections in healthcare settings, as it is challenging to avoid accidental exposures to potentially infectious body fluids. Post-exposure prophylaxis involves the provision of a minimum of 3 antiretroviral drugs for 28 days for all occupational exposures to HIV after the exposure and within 72 hours. Counseling, HIV testing at baseline and follow-up, and monitoring for toxicity are also provided. Using the post-exposure prophylaxis consultation service for clinicians is recommended in all cases. Please see StatPearls' companion resource, " Universal Precautions ," for more information. [24] [27]
Monitoring, Surveillance, and Continuous Quality Improvement
Collecting and using reliable, granular, and timely data are essential for improving team performance and population health. Using data means identifying at-risk clients, setting targets, and developing strategies. UNAIDS first proposed a set of global 90-90-90 HIV prevention targets for HIV in 2014 by countries of the United Nations General Assembly. Although these goals were not reached, many countries made significant progress. Setting 95-95-95 goals for 2025, the goals were updated in 2020 and adopted by the United Nations General Assembly in 2021 and include:
- 95% of all people with HIV who know their status
- 95% of all people who have HIV and receive antiretroviral therapy
- 95% of all patients on ART who achieve viral suppression
The projected impact is fewer than 370,000 people acquiring HIV and fewer than 250,000 people dying from HIV worldwide in 2025. The WHO and country and state disease control and prevention centers, such as the United States CDC, are primary data sources for monitoring HIV trends and progress toward goals. Behavioral and clinical characteristics of adults with HIV are followed in the United States by the Medical Monitoring Project. Instituted in 2005, this is a cross-sectional, nationally representative, complex sample survey. The CDC's AtlasPlus allows clinicians to explore national HIV, hepatitis, sexually transmitted infections, and tuberculosis data relevant to their population.[CDC. NCCHHSTP AtlasPlus 2023. ] All data from 2020 and 2021 must be interpreted in the context of decreased case surveillance and services during the COVID-19 pandemic. Consequently, clinicians can improve care and contribute to population health improvements by completing required documentation, participating in initiatives to streamline surveillance, and engaging in continuous program quality improvement within their practice setting utilizing public health data.
Social and Policy Environments
Local legislative bodies, policy-making organizations, and institutions can improve patient-centered care and population health outcomes for patients with HIV by basing legislative and policy frameworks that influence the effectiveness of HIV and AIDS programming on recommendations developed by organizations such as the UNAIDS, WHO, and the NIH. The WHO recommends specific HIV policies that promote optimal HIV testing and care and track any country's progression. These recommendations include the use of preexposure prophylaxis, dual HIV and syphilis rapid diagnostic tests, self-testing, optimal first- and second-line treatments, routine viral load testing, and same-day ART initiation, amongst other measures, in national policies and guidelines. UNAIDS, WHO, and national HIV strategies outline key social, legal, and policy factors that lead to better patient and population health outcomes for HIV prevention and care.
Political commitment and adequate resources are fundamental building blocks for an adequate public health response. The setting of targets can garner political commitment. The United States National HIV/AIDS Strategy and the Ending the HIV Epidemic in the United States initiative aims to reduce new HIV transmissions in the United States by 90% by 2030, prioritizing the reduction of HIV-related health disparities and inequities and improving the well-being of patients with HIV.[CDC. Ending the HIV epidemic 2023. ] A renewed global commitment to funding is necessary. The gap between actual and required funding is widening in low- and middle-income countries, mainly due to a decrease in the proportion of funding provided by high-income nations. Fully funded, resilient, integrated, and accessible public and community health systems lead to increased uptake of both HIV and other health services. Social and structural inequalities to HIV-related services, resources, and tools can be addressed through universal health care, shared service delivery models, and measures to improve access to medicines and health technologies for patients experiencing marginalization, including uninsured or underinsured individuals in the United States.
The removal, or at a minimum, the nonenforcement of harmful laws must be a priority. For example, laws criminalizing HIV exposure, non-disclosure, and transmission discourage people from getting tested, increasing transmission and creating a barrier to early treatment. Likewise, the criminalization of particular groups of people undermines an effective HIV response by discouraging sex workers, men who have sex with men, and those who are transgender or inject drugs from using prevention and treatment programs due to fear of arrest and prosecution. A 2015 study estimated that 33% to 46% of all new HIV cases over a decade could be avoided by decriminalizing sex work worldwide.
Many countries have amended laws to remove barriers to the HIV response, including decriminalizing vertical HIV transmission in Belize in 2023, sex work in Belgium in 2022, and same-sex sexual relations in several countries in 2022 and 2023. However, concerning setbacks occurred in many countries, including Indonesia. Strengthening laws, policies, and systems that realize and protect human rights is also necessary. For example, the promotion of gender equity and safe work environments leads to lower vulnerability for women and girls. Modeling studies from Canada and Kenya show that the elimination of violence by police, clients, and strangers could avert 17% to 20% of new HIV cases among women who are sex workers and their clients in a decade.
Strengthened policies and laws augment the education and community action necessary to address the stigma and discrimination that impact the health and well-being of patients with HIV. According to UNAIDS, 1 in 3 countries reporting on HIV policies indicate that 10% of men who have sex with men and more than 50% of sex workers, people who inject drugs, and transgender individuals avoid healthcare due to fears of stigma, discrimination, or confidentiality. Stigma and discrimination, rooted in outdated fears of HIV from the 1980s, must be addressed through open discussion about HIV using non-stigmatizing language both within and outside of health care. Discrimination can manifest in healthcare settings as the refusal of service to patients with HIV, the use of stigmatizing language, or the absence of appropriate services, resources, or tools.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Helena Swinkels declares no relevant financial relationships with ineligible companies.
Disclosure: Angel Justiz Vaillant declares no relevant financial relationships with ineligible companies.
Disclosure: Andrew Nguyen declares no relevant financial relationships with ineligible companies.
Disclosure: Peter Gulick declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Swinkels HM, Justiz Vaillant AA, Nguyen AD, et al. HIV and AIDS. [Updated 2024 Jul 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Tuberculosis. [Major Infectious Diseases. 2017] Review Tuberculosis. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, et al. Major Infectious Diseases. 2017 Nov 3
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Preexposure Prophylaxis for HIV Prevention. [StatPearls. 2024] Preexposure Prophylaxis for HIV Prevention. Sundareshan V, Swinkels HM, Nguyen AD, Mangat R, Koirala J. StatPearls. 2024 Jan
- HIV Prevention. [StatPearls. 2024] HIV Prevention. Huynh K, Vaqar S, Gulick PG. StatPearls. 2024 Jan
- Review The Minderoo-Monaco Commission on Plastics and Human Health. [Ann Glob Health. 2023] Review The Minderoo-Monaco Commission on Plastics and Human Health. Landrigan PJ, Raps H, Cropper M, Bald C, Brunner M, Canonizado EM, Charles D, Chiles TC, Donohue MJ, Enck J, et al. Ann Glob Health. 2023; 89(1):23. Epub 2023 Mar 21.
Recent Activity
- HIV and AIDS - StatPearls HIV and AIDS - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Skip to main content
- Keyboard shortcuts for audio player
Goats and Soda
- Infectious Disease
- Development
- Women & Girls
- Coronavirus FAQ
'America's Nobel' goes to a power couple who made a startling discovery about HIV
Kate Bartlett

Professors Salim Abdool Karim and Quarraisha Abdool Karim, married for more than 40 years, are respected around the world for their research on HIV. In September they were named winners of the Lasker Award for public service in recognition for their groundbreaking medical research. Phill Magakoe/AFP/Getty Images hide caption
In the early days of the AIDS epidemic, back in the 1980s, the virus was seen as a threat mainly to gay men.
South African husband and wife researchers Salim and Quarraisha Abdool-Karim changed that narrative with their ground-breaking research. They discovered that in South Africa, young women had a high rate of HIV. And then they did something about it.
The couple met at university over a temperature-controlled ultracentrifuge and fell in love. It was a whirlwind romance: A week later Salim was headed to Columbia University in New York. They persisted with a long-distance relationship and just months later were married, with Quarraisha going on to join Salim at Columbia.
Last week, after decades of partnership, in and out of the lab, the epidemiologists received the prestigious Lasker Prize — often referred to as “America’s Nobel” — for their life-saving HIV research.
The power couple won the prize in the public service category “for illuminating key drivers of heterosexual HIV transmission; introducing life-saving approaches to prevent and treat HIV; and statesmanship in public health policy and advocacy,” the Lasker Foundation said in a statement. The honor comes with a $250,000 award.
Growing up as South African Indians under the brutal apartheid system of white-minority rule, the Abdool-Karims faced discrimination and segregation and had limited educational opportunities. Despite this, Salim and Quarraisha — who were both active in the anti-apartheid movement — went on to become two of the foremost scientists of their generation.
One of their major findings came in the early days of the HIV pandemic, in the 1980s, when the disease was seen in the U.S. and Western countries as predominantly affecting gay men. The Abdool-Karims found that in their home country it was predominantly women in their teens getting infected by older men.
The couple set out to find a way for these women to protect themselves — a challenging task. After almost two decades of research, the Abdool-Karims had their breakthrough: their trials of a drug called Tenofovir showed that when used as a vaginal gel, it could help prevent HIV transmission to women.
South Africa has one of the world’s highest number of people living with HIV — an estimated 8 million — but almost 80% of them are now on anti-retroviral drugs (ARVs). New infections have dropped significantly from a peak of around half a million in 2000 to 150,000 last year. The Abdool-Karim's work has contributed in no small part to these successes, according to the Lasker Foundation.
NPR spoke to the Abdool-Karims hours before they attended the prize ceremony in New York on Friday. The interview has been edited for length and clarity.
What barriers did you face entering the sciences in apartheid South Africa?
Salim: Growing up in apartheid South Africa with hindsight is somewhat of a surreal experience.
We lived in an area where only Indian people lived. When we went to the post office, we entered by the door that said non-whites. When we went to the parks, we sat on a bench that said non-whites.
Living in that kind of situation, the message you get over and over and over is that you're not good enough and you are inferior because you are not white. And having grown up in that kind of environment [it] was almost a challenge for us to say, actually, we are good enough.
And eventually, I went to medical school at the University of Natal, which at that stage was the only medical school for Black students. And became an anti-apartheid activist and joined the struggle for freedom. And having graduated as a young doctor, I decided to specialize in virology.
Quarraisha: And maybe I'll add a little bit because I was growing up on the north coast, north of Durban. And it's in the middle of the sugarcane plantations. My great-grandparents came as part of the Indians from India brought to South Africa in 1860 to work on the sugarcane plantations or the coal mines.
But [my family] really valued education. When I finished high school in 1976, I knew I wanted to be a scientist. So I went to the University of Durban and did my BSc [bachelor of science] degree. And during that time , [as I was] studying biochemistry and microbiology, there was an emerging understanding of immunology.
You both studied in the States, then returned to South Africa in the early days of HIV/AIDS. What differences did you see between the two countries?
Salim: What became very clear to us in New York is that HIV is going to be a big problem in Africa. When we came back to South Africa at the end of 1988, we decided that we will pursue research in HIV. And that pretty much defined the rest of our 35 years of research together.
And that’s when you made a startling discovery about HIV.
Salim: Our study that we did back in 1989-1990 produced data that took us quite by surprise. When we looked at the information from this large community survey, we found that in teenage boys, the prevalence of HIV was quite low. But if you looked at teenage girls, they had very high rates of infection [6 to 8% of women between ages 15 and 24 compared to 2% or less of males the same age. However, the rate among men in the 1990 study rises as they get into their mid-to-late 20s.]
That was a signal that these young women were not acquiring HIV from teenage boys; they were acquiring HIV from men who were about 10 years older. And so that started for us a whole research program to understand this age-disparate sex, and essentially laid the basis for research that we focused on, which is, how can we develop a technology that would empower women to protect themselves from HIV? Because, you know, condoms are under the control of men.
Describe what your breakthrough with Tenofovir gel was and how that research progressed since?
Salim: It took 18 years of research before we produced a successful outcome. And when we announced in 2010 that we had shown that this drug called Tenofovir, invented in the 1970s, protected women against HIV when used in a gel [applied vaginally before and after sex]. It provided new hope to the field of HIV at the time. The issue was that the gel was more expensive to make and didn't have the same level of protection as the tablets of Tenofovir. So it turned out that taking Tenofovir pills was as good and sometimes better than the gel and cheaper, and that's what led eventually in 2015 to the World Health Organization recommendation that Tenofovir-containing pre-exposure prophylaxis (PrEP) be offered to all individuals at high risk of infection.
Were there any challenges in getting these young women to take the pill?
Salim: Our problem became that young women were not overly keen to go and stand in a long queue at a clinic to collect tablets to take to prevent a disease they didn't have. And it became a real challenge for us because the uptake of Tenofovir PrEP was quite low. And even those who did start taking Tenofovir tablets for pre-exposure prophylaxis, after a while they just stopped taking it. And so we started a whole new research program to look at developing technologies that were long - acting.
South Africa’s second democratic president, Thabo Mbeki, engaged in AIDS-denialism while the country was in the middle of an epidemic. That must have been disappointing to see?
Salim: It came as a mortal blow in a way that when Mbeki took over from President Mandela, he dallied with these kinds of denialists and was influenced by their thinking and started espousing these falsehoods and this disinformation that HIV was not the cause of AIDS. And you know, we as scientists certainly couldn't just let that lie. We had to ensure that the public was aware of what the factual situation was. I was the head of AIDS research at the Medical Research Council at the time, and so I was often quoted in the media challenging the president and so were many others.
Quarraisha: It was a particularly painful time in that when we first started doing AIDS research in South Africa, we were dealing with a silent epidemic. And at the time Mbeki comes in, we're starting to see the face of AIDS, there were people dying all around us and in large numbers. And in 2000, when we hosted the International AIDS Conference, this is coinciding with the president, the democratically elected president, challenging this.
This year, you’ve been working on a new study with another drug, Lenacapovir, that’s shown impressive results.
Salim: That study showed that if you take the injectable of Lenacapovir [every six months], it was highly effective. In fact, there were no infections in women who took this injection. Young women could come in just once every six months, get an injection, and then they don't need to be concerned about the risk of HIV until they are due for their next injection six months later.
There is a heated debate over the presumed high cost of the injectable.
Salim: What we hope will be done is when it's licensed, it's not yet licensed, is that there will be a kind of access program that will enable poor countries to access lenacapovir at low cost.
What are you going to do with the prize money, and what’s next for you both?
Salim: The prize money will be used for research and/or training of students. We are now working on an annual long-acting prevention technology so that women will only need to have it once a year. (This could be in the form of a matchstick-size implant that has enough Tenofovir in it enough to release slowly over an entire year so that young girls don't need to think about prophylaxis.)
And to end on a lighter note: Salim, you’re widely known by your nickname, “Slim.” Where does it come from?
Salim: I acquired this nickname in high school when my Afrikaans teacher said to me “Jy dink jy is slim” — you think you are clever? — in response to some cheeky comment I must have made. Since my name was Salim, dropping the “a” made it a single syllable, I was the top student in class and I was quite rotund — so, for multiple reasons, her referring to me as “slim” just stuck.
Kate Bartlett is a freelance journalist based in Johannesburg, South Africa.
- South Africa
- Lasker prize
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- All Summits
- STATUS List
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Gilead strikes voluntary licensing deals in poor countries for its pricey HIV drug
Agreements allow companies to make generic versions of lenacapavir for 120 countries.
- Manage alerts for this article
- Email this article
- Share this article

By Ed Silverman
Oct. 2, 2024
Pharmalot Columnist, Senior Writer
In response to increased criticism of its pricing, Gilead Sciences has reached voluntary licensing deals with companies to make generic versions of its twice-yearly HIV medicine, lenacapavir, in 120 mostly low- and lower-middle-income countries.
The move comes after a pair of late-stage clinical trials found the injectable medicine was highly effective in preventing HIV, paving the way for the company to seek regulatory approval for pre-exposure prophylaxis, or PrEP. The results generated considerable excitement since a daily pill is nearly 100% effective but compliance is spotty and there is a stigma attached to regularly taking a drug for HIV.
advertisement
But its impact on addressing an epidemic that, as of 2022, still led to more than 1 million new infections each year is uncertain. And the reason is pricing. Lenacapavir is already approved for treating HIV, but has a hefty price tag of $42,250. So patient advocates have been urging the company to reach a licensing deal but also lower its price.
STAT+ Exclusive Story
Already have an account? Log in

This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts..
Totals $468 per year
for 3 months, then $399/year
Then $399/year
Savings start at 25%!
Annually per user
$300 Annually per user
To read the rest of this story subscribe to STAT+.
About the reporting
STAT’s investigation is based on interviews with nearly 100 people around the country, including incarcerated patients and grieving families, prison officials, and legal and medical experts. Reporter Nicholas Florko also filed more than 225 public records requests and combed through thousands of pages of legal filings to tell these stories. His analysis of deaths in custody is based on a special data use agreement between STAT and the Department of Justice.
You can read more about the reporting for this project and the methodology behind our calculations.
The series is the culmination of a reporting fellowship sponsored by the Association of Health Care Journalists and supported by The Commonwealth Fund.
Ed Silverman, a senior writer and Pharmalot columnist at STAT, has been covering the pharmaceutical industry for nearly three decades.
More on Long Covid

STAT Plus: As Humira biosimilars take over the market, CVS has created a new ploy: the drug ‘rebate credit’

Understand how science, health policy, and medicine shape the world every day
Your data will be processed in accordance with our Privacy Policy and Terms of Service . You may opt out of receiving STAT communications at any time.
Recommended

STAT Plus: Lawmakers want FTC to probe big PBMs over private-label biosimilar deals

STAT Plus: Pharmalittle: We’re reading about a Blue Cross California deal for Humira, Gilead licensing an HIV drug, and more
Stat plus: pharmalittle: we’re reading about cvs exploring a breakup, obesity drug coverage, and more.

STAT Plus: U.S. lawmaker urges FDA to clarify drug patent listings in a key government registry

STAT Plus: J&J backs down on plan to switch payments to some 340B hospitals
Subscriber picks, stat plus: what the withdrawal of its sickle cell drug means for pfizer, patients, and the fda, stat plus: can anne wojcicki save 23andme, stat plus: pfizer pulls sickle cell treatment oxbryta off global markets, stat plus: the little-known chinese biotech whose cancer drug beat keytruda has global ambitions, stat plus: health care ceos dialed back their pay in 2023. they still made $3.5 billion.
September 27, 2024
Related Announcements
May 07, 2020 - NIH Exploratory/Developmental Research Grant Program (Parent R21 Clinical Trial Not Allowed). See NOFO PA-20-195 .
May 05, 2020 - NIH Research Project Grant (Parent R01 Clinical Trial Not Allowed). See NOFO PA-20-185 .
May 05, 2020 - Research Project Grant (Parent R01 Basic Experimental Studies with Humans Required). See NOFO PA-20-184 .
The National Institute of Dental and Craniofacial Research (NIDCR) is issuing this Notice of Special Interest (NOSI) to encourage innovative and multi-disciplinary basic and translational research to elucidate the mechanisms of, and strategies for, addressing HIV pathogenesis, HIV-associated comorbidities, co-infections, and complications in the oropharyngeal cavity.
Background:
It is estimated that 1.2 million persons in the United States were living with diagnosed and undiagnosed HIV at the end of 2022. New HIV infections decreased by 12% from 36,300 in 2018 to 31,800 in 2022, most likely driven by the increased use of pre-exposure prophylaxis (PrEP) among young people. Well-tolerated, long-acting antiretroviral therapy (ART) has enhanced health and quality of life and reduced incidence and mortality in people living with HIV (PLWH). The perinatal ART prophylaxis also helped mother-to-child transmission (MTCT) significantly, yet approximately 10% of children 14 years and younger acquire HIV during gestation, childbirth, or breastfeeding through the oral mucosa and tonsillar tissues.
Oropharyngeal tissues are key sources of HIV-related comorbidities, coinfections, and complications (CCCs). It is known that oral mucosal immune homeostasis is essential in protecting HIV-associated opportunistic infections and comorbidities in PLWH. Studies suggest that behavioral and biological risk factors such as smoking, risky sexual behaviors, aging, ART, and oral diseases such as dental caries, periodontal diseases and HIV-associated salivary gland disease (HIV-SGD) may contribute to the dysbiosis of the oral microbial ecosystem and further disrupt the oral mucosal epithelial immune barrier in PLWH. In some circumstances, these co-infections lead to other severe comorbidities such as oral and oropharyngeal cancers.
Some oral malignancies in PLWH are reportedly associated with enhanced local and systemic inflammatory states and closely linked to other viruses, such as Epstein-Barr virus and Kaposi sarcoma-associated herpesvirus. It has been reported that PLWH are at two- to four-fold increased risk for oral human papillomavirus associated oropharyngeal cancers (HPV-OPC) compared with HIV-negative individuals. However, the mechanisms and pathogenic progression, such as how oral mucosal immunity and biology impact HIV and its relation to CCCs in oropharyngeal tissues, have not been fully elucidated. Moreover, untreated oral diseases in PLWH can impact their nutritional uptake, social relationships, and quality of life and, in turn, worsen overall health and HIV outcomes. To advance whole person health approaches in HIV health care and support services, there is an urgent need to better understand the relationships between oral health, overall health, and HIV status in the context of PLWH.
Scientific Areas of Interest:
Applicants responding to this NOSI should propose statistically valid, hypothesis-driven projects aimed at elucidating biological, behavioral, and/or multi-level mechanisms that facilitate HIV infection, HIV-associated comorbidities, co-infection, and complications in the oropharyngeal cavity. Multidisciplinary research is encouraged as appropriate for the proposed research.
The scope of this NOSI includes but is not limited to, the following research areas:
- Biologic mechanisms of oral HIV transmission, persistence, and latency within the oropharyngeal tissue
- Natural history and factors that may impact persistence and progression of HIV-associated oral CCCs in the context of PLWH
- Biomarkers to predict and measure HIV-related oral co-infections and comorbidities
- Mechanisms in which other comorbidities and/or medical conditions interplay with HIV-associated oral CCCs in the context of PLWH
- Oral mucosal vaccination research aiming at developing novel prophylactic oral mucosal vaccines against HIV infection
- Developing combinations of antiretroviral drugs and compounds that can be used in sustained-release formulations, in the oral cavity, for potential new PrEP strategies
- The impact of HIV treatment regimens on oral health and conditions, e.g., oral transmission in perinatal HIV, oral microbiome and HIV-associated CCCs in adolescent and adult HIV populations
Application and Submission Information
This notice applies to due dates on or after January 7, 2025, and subsequent receipt dates through January 8, 2028.
Submit applications for this initiative using one of the following notices of funding opportunity (NOFOs) or any reissues of these announcements through the expiration date of this notice.
PA-20-195 NIH Exploratory/Developmental Research Grant Program (Parent R21 Clinical Trial Not Allowed)
PA-20-185 NIH Research Project Grant (Parent R01 Clinical Trial Not Allowed)
PA-20-184 NIH Research Project Grant (Parent R01 Basic Experimental Studies with Humans Required)
Applications proposing a clinical trial that does not meet the Basic Experimental Studies with Human (BESH) definition (please see NIH’s definition of a clinical trial ) must use a NOFO that allows clinical trials (please see NOT-DE-21-014 NIDCR Guidance on Applications for Investigator-Initiated Clinical Trials ).
All instructions in the How to Apply - Application Guide and the notice of funding opportunity used for submission must be followed, with the following additions:
- For funding consideration, applicants must include “NOT-DE-25-038” (without quotation marks) in the Agency Routing Identifier field (box 4B) of the SF424 R&R form. Applications without this information in box 4B will not be considered for this initiative.
Please direct all inquiries to the Scientific/Research, Peer Review, and Financial/Grants Management contacts in Section VII of the listed notice of funding opportunity.
Scientific/Research Contact(s)
Hiroko Iida, DDS, MPH National Institute of Dental and Craniofacial Research (NIDCR) Phone: 301-594-7404 E-mail: [email protected]
Peer Review Contact(s)
Examine your eRA Commons account for review assignment and contact information (information appears two weeks after the submission due date).
Financial/Grants Management Contact(s)
Gabriel Hidalgo, M.B.A. National Institute of Dental and Craniofacial Research (NIDCR)Phone: 301-827-4630 Email: [email protected]


IMAGES
VIDEO
COMMENTS
The human immunodeficiency virus (HIV) is the virus that causes HIV infection. If untreated, HIV may cause acquired immunodeficiency syndrome (AIDS), the most advanced stage of HIV infection.; People with HIV who are not on medication and do not have consistent control of their HIV can transmit HIV through vaginal or anal sex, sharing of needles, pregnancy, and/or breastfeeding.
HIV causes other infections to get worse, such as hepatitis C, hepatitis B and mpox. Transmission. HIV can be transmitted via the exchange of body fluids from people living with HIV, including blood, breast milk, semen, and vaginal secretions. HIV can also be transmitted to a child during pregnancy and delivery.
What Is HIV? HIV (human immunodeficiency virus) is a virus that attacks cells that help the body fight infection, making a person more vulnerable to other infections and diseases.It is spread by contact with certain bodily fluids of a person with HIV, most commonly during unprotected sex (sex without a condom or HIV medicine to prevent or treat HIV), or through sharing injection drug equipment.
Access presentations from different HIV-related organizations. Skip to main content Menu 1-800-448-0440 (1 p.m. to 4 p.m. ET) [email protected]. Search Site ... Health Resources and Services Administration Ryan White HIV/AIDS Program : Slide Decks on HIV Screening, Prevention, and Treatment and Care : Webpage (HTML)
Slides (PPT) - Fast-Track: Ending the AIDS epidemic by 2030. 24 November 2014. The new set of targets that would need to be reached by 2020 include achieving 90-90-90: 90% of people living with HIV knowing their HIV status; 90% of people who know their HIV-positive status on treatment; and 90% of people on treatment with suppressed viral loads. ...
HIV Disease Presentation . Medical . Free Google Slides theme, PowerPoint template, and Canva presentation template . The human immunodeficiency virus (HIV) affects more than 35 million people in the world. Since the first cases were known in the 1980s, great advances have been made, which continue to develop today.
Symptoms. The symptoms of HIV and AIDS vary depending on the person and the phase of infection.. Primary infection, also called acute HIV. Some people infected by HIV get a flu-like illness within 2 to 4 weeks after the virus enters the body. This stage may last a few days to several weeks. Some people have no symptoms during this stage.
1 The new consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations bring together all existing World Health Organization (WHO) guidance relevant to five key populations: men who have sex with men, people who inject drugs, people in prisons and other closed settings, sex workers and transgender people.It includes a number of new recommendations and updates ...
This slide set covers two European workshops in September 2022 organised by Africa AIDS Foundation (AAF). They give an introduction to HIV treatment for health workers who have clients who are living with HIV, ... This talk in January 2020 was given at a U=U conference in Tokyo organised by the Japanese HIV Society. As well as my presentation ...
North Dakota HIV/AIDS Statistics As of December 31, 2008 • 441 Cumulative cases since 1984 • 37% had AIDS at HIV diagnosis • 84% are male • 77% are white, 11% are black, and 10% are American Indian • 51% are men who have sex with men (MSM), 18% had heterosexual relations, and 13% used injectable drugs (IDU)
Explore the Joint United Nations Programme on HIV/AIDS, focusing on prevention, harm reduction, and progress towards ending AIDS as a public health threat.
These factsheet slides from Joint United Nations Programme on HIV/AIDS (UNAIDS) provide a global snapshot of the state of the world’s HIV/AIDS epidemic. The slides present a high-level summary of the most current global estimates on the prevalence, transmission, and mortality of HIV/AIDS. The…
AIDS is a disease that can develop in people who have the HIV virus. Treatment with antiretroviral drugs can typically prevent AIDS from developing in people with HIV.
Human immunodeficiency virus (HIV) is a blood-borne virus typically transmitted via sexual intercourse, shared intravenous drug paraphernalia, and mother-to-child transmission (MTCT), which can occur during the birth process or during breastfeeding. HIV disease is caused by infection with HIV-1 or HIV-2, which are retroviruses in the Retrovir...
HIV was first identified in 1983 and has since claimed approximately 40.4 million lives worldwide as of 2022. This number is staggering, and if left unchecked, HIV could become a global health crisis. However, the research, development, and widespread availability of highly active antiretroviral therapies (ARTs) have helped control the HIV pandemic. Likewise, advances in the treatment of HIV ...
In 2018 HIV incidence and mortality ranged from 2.8 to 1585.9/100,000 people and 0.8 to 676.5/100,000 people, respectively, highlighting the need for renewed approaches for HIV control. 3 In 2014, the United Nations Programme on HIV/AIDS (UNAIDS) 2020 "90-90-90" goal was established whereby 90% of people with HIV were to be diagnosed, 90% ...
South Africa has one of the world's highest number of people living with HIV — an estimated 8 million — but almost 80% of them are now on anti-retroviral drugs (ARVs). New infections have ...
Lenacapavir is already approved for treating HIV, but has a hefty price tag of $42,250. So patient advocates have been urging the company to reach a licensing deal but also lower its price.
The National Institute of Dental and Craniofacial Research (NIDCR) is issuing this Notice of Special Interest (NOSI) to encourage innovative and multi-disciplinary basic and translational research to elucidate the mechanisms of, and strategies for, addressing HIV pathogenesis, HIV-associated comorbidities, co-infections, and complications in the oropharyngeal cavity.