
- Research Article
- Neuroscience

Neurophysiological trajectories in Alzheimer’s disease progression
- Kamalini G Ranasinghe
- Hirofumi Morise
- Faatimah Syed
- Kensuke Sekihara
- Katherine P Rankin
- Bruce L Miller
- Joel H Kramer
- Gil D Rabinovici
- Keith Vossel
- Heidi E Kirsch
- Biomagnetic Imaging Laboratory, Department of Radiology and Biomedical Imaging, University of California, San Francisco, United States ;
- Medical Imaging Business Center, Ricoh Company Ltd, Japan ;
- Memory and Aging Center,UCSF Weill Institute for Neurosciences, University of California, San Francisco, United States ;
- Signal Analysis Inc, Japan ;
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, United States ;
- Mary S. Easton Center for Alzheimer’s Research and Care, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, United States ;
- Open access
- Copyright information
Share this article
Cite this article.
- Kiwamu Kudo
- Srikantan S Nagarajan
- Copy to clipboard
- Download BibTeX
- Download .RIS
Reviewer #1 (Public Review):
Reviewer #2 (public review):, author response.
- Timothy E Behrens
- University of Oxford, United Kingdom
- Björn Herrmann
- Baycrest, Canada
The authors aimed to infer the trajectories of long range and local neuronal synchrony across the Alzheimer's disease continuum, relative to neurodegeneration and cognitive decline. The trajectories are inferred using event-based models, which infer a set of data-driven disease stages from a given dataset. The authors develop an adapted event-based modelling approach, in which they characterise each stage as a particular biomarker increasing by a particular z-score deviation from controls. Fitting infers the optimal set of z-scores to use for each biomarker and the order in which each biomarker reaches each z-score. The authors apply this approach to data from 148 individuals (70 cognitively unimpaired older adults and 78 individual with mild cognitive impairment or Alzheimer's disease), identifying trajectories in which long-range (amplitude-envolope correlation) and local (regional spectral power) neuronal synchrony in the alpha and beta bands becomes abnormal prior to neurodegeneration (measured as the volume of the parahippocampal gyrus) and cognitive decline (measured using the mini-mental state examination).
- The main strength is that the authors assess two models. In the first they derive a staging system based only on the volume of the parahippocampal gyrus and mini-mental state examination score. They then investigate how neuronal synchrony metrics change compared to this staging system. In the second they derive a staging system that also includes an average (combined long-range and local) neuronal synchrony metric and investigate how long-range and local synchrony metrics change relative to this staging system. This is a strength as the first model provides confidence that there is not overfitting to the neuronal synchrony data, and the second provides more detailed insights into the dynamics of the early neuronal synchrony changes.
- Another strength is that the authors automatically infer the optimal z-scores to choose, rather than having to pre-select them manually, as in previous approaches.
Weaknesses:
- The authors do not have a dataset for external validation.
Summary: This work presented by Kudo and colleagues is of great importance to strengthen our understanding of electrophysiological changes in the course of AD. Although the main conclusions regarding functional connectivity and spectral power change through the course of the disease are not new and have been largely studied and theorised on, this article offers an innovative approach that certainly consolidates previous knowledge on the topic. Not only that, this article also broadens our knowledge presenting useful and important details on the specificity of frequency and cortical distribution of these early alterations. The main take-home message of this work is the early disruption of electrophysiological signatures that precedes detectable alterations in other more commonly used pathology markers (i.e. gray matter atrophy and cognitive impairment). More specifically, these signatures include long-range connectivity in the alpha and beta bands, and local synchrony (spectral power) in the same frequency bands.
Strengths: The present work has some major strengths that make it paramount for the advance of our understanding of AD electrophysiology. It is a very well written manuscript that, despite the complexity of the analyses employed, runs the reader through the different steps of the analysis in a pedagogic and clever way, making the points raised by the results easy to grasp. The methodology itself is carefully chosen and appropriate to the nature of the question posed by the researchers, as event-based models are well-suited for cross-sectional data.
The quality of the figures is outstanding; not only are they aesthetic but, more importantly, the figures convey information exceptionally well and facilitate comprehension of the main results.
The conclusions of the paper are, in general, well described and discussed, and consider the state-of-the-art works of AD electrophysiology. Furthermore, even though the conclusions themselves are not groundbreaking at all (synaptic damage preceding structural and cognitive impairment is one of the epitomes of the pathological cascading model proposed by Jack in 2010), this article is innovative and groundbreaking in the way they address with clever analyses in a relatively large sample for neuroimaging standards.
Weaknesses: The authors increased the clarity of sample description after revisions (particularly control group characterization). However, even though it is true that a certain percentage of AB positivity is to be expected amongst cognitively healthy individuals, that doesn´t discard they are not expressing preclinical AD to some extent. I still feel that including only biomarker negative participants in the control group would increase the quality of the work. However, the sample is relatively well characterized as a whole and the results are interesting and in line with previous literature, thus limiting the apparent impact of these possible confounds.
The following is the authors’ response to the original reviews.
eLife assessment This work presents important findings for the field of Alzheimer's disease, especially for the electrophysiology subfield, by investigating the temporal evolution of different disease stages typically reported using M/EEG markers of resting-state brain activity. The evidence supporting the conclusions is solid and the methodology as well as the descriptions of the processes are of high quality, although a separation of individuals who are biomarker positive versus negative would have strengthened the interpretability of the results and the conclusions of the study.
Response: Thank you for the positive assessment of the paper.
Public Reviews: Reviewer #1 (Public Review): Summary: The authors aimed to infer the trajectories of long range and local neuronal synchrony across the Alzheimer's disease continuum, relative to neurodegeneration and cognitive decline. The trajectories are inferred using event-based models, which infer a set of data-driven disease stages from a given dataset. The authors develop an adapted event-based modelling approach, in which they characterise each stage as a particular biomarker increasing by a particular z-score deviation from controls. Fitting infers the optimal set of z-scores to use for each biomarker and the order in which each biomarker reaches each z-score. The authors apply this approach to data from 148 individuals (70 cognitively unimpaired older adults and 78 individual with mild cognitive impairment or Alzheimer's disease), identifying trajectories in which long-range (amplitude-envolope correlation) and local (regional spectral power) neuronal synchrony in the alpha and beta bands becomes abnormal prior to neurodegeneration (measured as the volume of the parahippocampal gyrus) and cognitive decline (measured using the mini-mental state examination). Strengths: The main strength is that the authors assess two models. In the first they derive a staging system based only on the volume of the parahippocampal gyrus and mini-mental state examination score. They then investigate how neuronal synchrony metrics change compared to this staging system. In the second they derive a staging system that also includes an average (combined long-range and local) neuronal synchrony metric and investigate how long-range and local synchrony metrics change relative to this staging system. This is a strength as the first model provides confidence that there is not overfitting to the neuronal synchrony data, and the second provides more detailed insights into the dynamics of the early neuronal synchrony changes. Another strength is that the authors automatically infer the optimal z-scores to choose, rather than having to pre-select them manually, as in previous approaches.
Response: Thank you for the positive comments and a succinct summary of the paper and its strengths.
Weaknesses: The dataset is small and no external validation is performed.
Response: We agree that future validation studies of the predictions are necessary. We now include the related sentences in the last paragraph of the limitations section in the revised manuscript.
A high proportion of the data is from controls (nearly 50%) with no biomarker evidence of Alzheimer's disease, and so the changes may be driven by aging or other non-Alzheimer's effects.
Response: We would like to clarify that the z-scores of the metrics used in the EBMs were computed using age-adjusted values. All our controls were recruited from an ongoing longitudinal study of healthy aging. Amongst the 70 controls, 39 have confirmed A-beta negative PET scans and 8 were confirmed A-beta positive PET scans, and in the rest of the 23 we do not have any biomarker data available. However, in all the controls, we have conducted comprehensive neuropsychological assessment (see Appendix 1—table 1 in the revised supplementary file) and based on this data we can be quite confident about their lack of clinical deficits, and we have a very high degree of confidence that none of the controls have any neurodegeneration (AD-related or otherwise). Consistent with this assessment, in our EBM analyses, most of the control participants were indeed categorized to the preclinical stages.
Inferring the optimal z-scores is a strength, however as different sets of z-scores are allowed per biomarker, there is a concern that the changes reflected are mainly driven by the choice of z-score, rather than the markers themselves (e.g. if lower z-scores are selected for one marker than another, then changes in that marker will appear to be detected earlier, even if both markers change at the same time).
Response: Indeed, the biomarker sequence depends on the choice of the z-scores per biomarker. However, please note that our choice of z-scores is based on maximizing the sequence likelihood. Therefore, other values of the z-scores will have by construction a smaller likelihood of sequence occurrence compared to the results shown.
In equation 2 it is unclear why the gaussian is measured based on a sum over I. The more obvious choice would be to use a multivariate gaussian with no covariance, which would mean taking the product rather than the sum over I.
Response: We thank the reviewer for pointing this out and we now clarify this point. In this revision, we do not use the term ‘multivariate’. Indeed, the model likelihood assumes independence for each metric’s priors, and hence is the product of each metric’s univariate gaussian probability distribution. This can be seen in equations 1 and 2 of the revision manuscript (Section titled “Event-based sequencing modeling’). The assumption about independent priors is similar to the one used in the original event-based model (see equation (2) in A .L. Young et al., Nature Comm. 9.1 (2018): 4273).
In the original event-based model, k is a hidden variable. Presumably that is also the case here, however the notation k=stage(j) makes it seem like each subject is assigned a stage during the sequence optimisation.
Response: We would like to clarify that the posterior probability of each stage for every subject is estimated during the sequence optimization. To clarify the notation, we have now deleted the term “stage” and use “tj” to denote stages for each subject j. The sequence optimization was performed with the assumption of a uniform prior distribution p(tj=k) = 1/(N+1) for each stage k. Then, the posterior probability p(tj=k|Zj,S), i.e., the probability that subject j belongs to stage k, given the metrics and the sequence, was computed during the sequence optimization procedure.
Typically for event-based modeling, positional variance diagrams are created from the markov chain monte carlo samples of the event sequence, enabling visualisation of the uncertainty in the sequence, but these are not included in the study.
Response: In the revised supplementary file, we have now included positional uncertainty diagrams for the optimal set of z-score events that were created from 50,000 MCMC samples. Please see Appendix 1—figure 2 for the AC-EBM and Appendix 1—figure 9 for the SAC-EBMs.
Many of the figures in the manuscript (e.g. Figure 1E/G, Figure 2A/B, Figure 3A/B/E/F/I/J, Figure 4 A/B/E/F/I/J) are based on averages in both the x and the y axis. In the x dimension, individuals have a weighted contribution to the value on the y axis, depending on their stage probability. In the y dimension, the values are averages across those individuals, and the error bars represent the standard error rather than the standard deviation. Whilst the trajectories themselves are interesting, they may not be discriminative at the individual level and may be more heterogeneous than it appears.
Response: In the current study, the predictions of trajectories are intended at the cohort level. Individual level investigations will be the topic of future investigations.
The bootstrapped statistical analyses comparing metrics between the stages do not consider the variability in the sequence.
Response: Please see the response above. The positional uncertainty diagrams are included in the revised supplementary file.
Reviewer #2 (Public Review): Summary: This work presented by Kudo and colleagues is of great importance to strengthen our understanding of electrophysiological changes in the course of AD. Although the main conclusions regarding functional connectivity and spectral power change through the course of the disease are not new and have been largely studied and theorised on, this article offers an innovative approach that certainly consolidates previous knowledge on the topic. Not only that, this article also broadens our knowledge presenting useful and important details on the specificity of frequency and cortical distribution of these early alterations. The main take-home message of this work is the early disruption of electrophysiological signatures that precedes detectable alterations in other more commonly used pathology markers (i.e. gray matter atrophy and cognitive impairment). More specifically, these signatures include long-range connectivity in the alpha and beta bands, and local synchrony (spectral power) in the same frequency bands.
Response: Thank you for the positive comments and for providing a nice succinct summary.
Strengths: The present work has some major strengths that make it paramount for the advance of our understanding of AD electrophysiology. It is a very well written manuscript that, despite the complexity of the analyses employed, runs the reader through the different steps of the analysis in a pedagogic and clever way, making the points raised by the results easy to grasp. The methodology itself is carefully chosen and appropriate to the nature of the question posed by the researchers, as event-based models are well-suited for cross-sectional data. The quality of the figures is outstanding; not only are they aesthetic but, more importantly, the figures convey information exceptionally well and facilitate comprehension of the main results. The conclusions of the paper are, in general, well described and discussed, and consider the state-of-the-art works of AD electrophysiology. Furthermore, even though the conclusions themselves are not groundbreaking at all (synaptic damage preceding structural and cognitive impairment is one of the epitomes of the pathological cascading model proposed by Jack in 2010), this article is innovative and groundbreaking in the way they address with clever analyses in a relatively large sample for neuroimaging standards.
Response: Thank you for the positive comments of the strengths of the paper.
Weaknesses: The main limitation of the work revolves around sample definition and inclusion criteria that are somewhat confusing obscuring some of the points of the analyses. Firstly it is not clear why the purely clinical approach is employed to diagnose the "probable Alzheimer´s Disease" for the 78 participants in the "AD group". In the same paragraph, it is stated that 67 out of the 78 participants show biomarker positivity, thus allowing a more biologically guided diagnosis that is preferred according to current NIA-AA criteria. This would avoid highly possible mixing of different subtypes of dementia etiologies. One might wonder, why would those 11 participants be included if we have strong indications that their symptoms are not due to AD? Furthermore, the real pathological status of the control group is somewhat questionable. The authors do not specify whether common AD biomarkers are available for this subgroup. In that case, it would have highly increased the clarity and interpretability of the results if this group was subdivided in a preclinical and completely healthy control group. This would be particularly interesting since a significant proportion of the control group is labeled as belonging to stages 2,3,4 (MCI) and even 5 (mild dementia). This raises the question of whether these participants are true healthy controls mislabeled by the EBM model, or actual cognitive controls with actual underlying AD pathology well identified by the model proposed.
Response: Please see responses above to a similar comment from R1. To clarify, all our controls were recruited from an ongoing longitudinal study of healthy aging. Amongst the 70 controls, 39 have confirmed A-beta negative PET scans and 8 were confirmed A-beta positive PET scans, and in the rest of the 23 we do not have any biomarker data available. The biomarker positivity rates in our control cohort are completely consistent with the prevalence of A-beta positivity in cognitively healthy individuals and are within a normal biological continuum for amyloid beta (Jansen WJ et al. 2015). In all the controls, we have conducted comprehensive neuropsychological assessment (see Appendix 1—table 1 in the revised supplementary file) and based on this data we can be quite confident about their lack of clinical deficits, and we have a high degree of confidence that none of the controls have any neurodegeneration (AD-related or otherwise). We include these details in the revision (see the revised ‘Participants’ section in the Materials and methods.).
Jansen WJ et al., 2015 JAMA; 667 313(19):1924-1938.
On this note, Figure 2 (C and D) and Figure 3 (C, G and K) show a cortical surface depicting the mean difference of each stage vs the control group, which again, is formed by subjects that can be included (and in fact, are included) in all those stages, obscuring the meaning and interpretability of these cortical distributions.
Response: We would like to clarify that these figures depict the regional maps of each metric for each stage of AD progression, not the contrast against a control group.
Reviewer #1 (Recommendations For The Authors): If possible, perform independent validation of the results.
Response: This is something we indeed intend to examine in our future investigations.
Repeat the analysis in the subset of individuals that are amyloid positive.
Response: Amongst the 78 AD patients, 20 had autopsy confirmed AD neuropathology, an additional 41 patients had molecular pathology identified by Abeta-PET, and another additional 9 had fluid biomarker (CSF) confirmation of amyloid and tau levels consistent with AD diagnosis. Eight remaining patients had a diagnosis of AD with high certainty, based on clinical presentation, neurological assessment, and cortical atrophy on MRI. Given that there are only eight patients who had clinical diagnosis of AD (with no biomarkers), and the comprehensive clinical characterization of all the AD patients in our cohort (Appendix 1—table 1), we do not believe that any subgroup analysis is warranted.
When inferring the optimal z-scores, select the same set of z-scores per biomarker, or include diagrams of stage vs z-score that include all of the markers so that it is easy to see how one marker changes relative to the others (overlay Figure 1G on Figure 2A and 2B).
Response: How the neural synchrony metrics, PHG volume and MMSE scores change relative to each other is exactly what we show in Figures 3 B/F/J and 4 B/F/J. Since each EBM model optimizes the z-score thresholds, sequence likelihood and posterior probability of each stage for each subject, the EBM framework provides the most likely estimate for each metric at every stage. Therefore, the SAC-EBM model gives the most accurate description of the relative differences in these metrics over the AD progression stages. The reviewer’s suggestion to overlay Figure 1G (now figure 1F, based on optimized z-scores for PHG volume and MMSE scores) on Figures 2A and 2B will be inaccurate, as the neural synchrony measures plotted in figures 2A and 2B are not for optimized z-scores.
Change equation 2 to use a multivariate gaussian.
Response: We now clarify that we use a factorized multivariate form that reflects independent priors for each metric which are Gaussian.
Clarify whether k is a hidden variable and possibly change the notation.
Response: We now clarify that in our notation, k is a label for the stage [k=1,..,7 (when I=2) or k=1,...,10 (when I = 3)] and is indeed a hidden variable and not observed (but inferred from the EBM). Specifically, the posterior probability for each subject j belonging to stage k was estimated as part of the sequence optimization procedure.
Generate positional variance diagrams of the MCMC samples.
Response: We are doing the MCMC to obtain the most likely sequence. We have now included positional variance diagrams of the optimal set of z-score events in Appendix 1—figure 2 and Appendix 1—figure 9 in the revised supplementary file.
It would be interesting to study whether the stages are predictive of conversion or look at longitudinal data, if available.
Also look at statistics across MCMC samples of the sequence.
Response: Thank you for this suggestion. In the Appendix 1—figure 10, we now include an example of the MCMC samples for an SAC-EBM including the alpha-band AEC. We then derived the positional variances for each metric that are now shown in Appendix 1—figure 2 and Appendix 1—figure 9.
Reviewer #2 (Recommendations For The Authors): Some really minor changes are suggested on two specific points that somewhat confused me as a reader and got me stuck in the reading process to try to get the meaning of what I was seeing/reading: 1. It is not specified (or at least I was unable to find it) what are you comparing exactly for the group comparison in the long-range synchrony metric (AEC) before creating your scalar metric. Are you comparing individual links (in which case you would have 93 link values for each ROI to compare)? Or are you comparing the strength for each ROI (thus, one value -the individual links sum- for each ROI)? I guess it should be the latter for what I see in the figures but it could be useful to specify it.
Response: The reviewer is correct. We compare the strength of each ROI, i.e., averaging over edges of the symmetric AEC matrix of functional connectivity. We now clarify this in the Amplitude-envelope correlation section and the caption of the revised Appendix 1—figure 6.
1. In Figure 1 (which, by the way, is exceptionally aesthetic, congratulations for that!) I got stuck for a relatively long time in a really small detail and I am not completely sure if I came to the right conclusion. It is regarding the X axis of the histograms in panels B and D. They are expressed as "PHG volume loss" and "MMSE decline". So I supposed those histograms were showing some kind of subtraction, (maybe from stage X to stage Y, or from group X to group Y). I was trying to understand the histogram and rereading methods to see if I overlooked any description of that graphic and then just realized they might be just the Z-score itself for each group (control and AD) with respect to the whole population. If that is the case I would suggest changing the X-label to "PHG z-score" and "MMSE z-score" avoiding the reference to "loss and "decline" as they are just reflecting the direct transformation to z-score.
Response: Thank you. We would like to clarify that the z-score for PHG volume and MMSE scores were sign-inverted so that higher values denote “PHG Volume loss” and “MMSE decline”, respectively. We now clarify this point in the revised text and legend for the revised figure 1.
Lastly, regarding the point I raised in the limitations section of the public review, I understand it might fall out of the scope of eLife reviewing process as it would require a more extensive change of the current manuscript, which is great as it is. But as a reader and researcher in the field, I would have recommended using biomarkers to divide the control group (if available) thus including in the models only those belonging to the AD continuum according to their biomarker status, and leaving those control without any biomarker positivity as the reference group for the figures I mention in that section (those showing differences for each stage in the cortical surface with respect to the control group).
Response: Please see a similar comment from R1. Amongst the 70 controls, 39 have confirmed A-beta negative PET scans and only 8 were confirmed A-beta positive PET scans, and in the rest of the 23 we do not have any biomarker data available. In all the controls, we have conducted comprehensive neuropsychological assessment (see Appendix 1—table 1 in the revised supplementary file) and based on this data we can be quite confident about their lack of clinical deficits, and we have a high degree of confidence that none of the controls have any neurodegeneration (AD-related or otherwise). Since only 8 participants were confirmed as amyloid positive in the control group and this sample size is small, we do not conduct this recommended re-analysis in this manuscript.
Download links
Downloads (link to download the article as pdf).
- Article PDF
- Figures PDF
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools), be the first to read new articles from elife.

A systematic literature review of automatic Alzheimer's disease detection from speech and language
Affiliation.
- 1 Department of Theoretical and Applied Linguistics, University of Cambridge, Language Technology Lab, Cambridge, UK.
- PMID: 32929494
- PMCID: PMC7671617
- DOI: 10.1093/jamia/ocaa174
Objective: In recent years numerous studies have achieved promising results in Alzheimer's Disease (AD) detection using automatic language processing. We systematically review these articles to understand the effectiveness of this approach, identify any issues and report the main findings that can guide further research.
Materials and methods: We searched PubMed, Ovid, and Web of Science for articles published in English between 2013 and 2019. We performed a systematic literature review to answer 5 key questions: (1) What were the characteristics of participant groups? (2) What language data were collected? (3) What features of speech and language were the most informative? (4) What methods were used to classify between groups? (5) What classification performance was achieved?
Results and discussion: We identified 33 eligible studies and 5 main findings: participants' demographic variables (especially age ) were often unbalanced between AD and control group; spontaneous speech data were collected most often; informative language features were related to word retrieval and semantic, syntactic, and acoustic impairment; neural nets, support vector machines, and decision trees performed well in AD detection, and support vector machines and decision trees performed well in decline detection; and average classification accuracy was 89% in AD and 82% in mild cognitive impairment detection versus healthy control groups.
Conclusion: The systematic literature review supported the argument that language and speech could successfully be used to detect dementia automatically. Future studies should aim for larger and more balanced datasets, combine data collection methods and the type of information analyzed, focus on the early stages of the disease, and report performance using standardized metrics.
Keywords: Alzheimer’s disease; dementia; language; natural language processing; speech.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Alzheimer Disease / complications
- Alzheimer Disease / diagnosis*
- Artificial Intelligence*
- Decision Trees
- Language Disorders / diagnosis*
- Language Disorders / etiology
- Language Tests*
- Machine Learning
- Natural Language Processing

An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of the Assistant Secretary for Planning and Evaluation
Racial and Ethnic Disparities in Alzheimer's Disease: A Literature Review
This study aimed to describe and characterize the published literature on disparities between racial and ethnic groups among individuals with Alzheimer’s disease and related dementias. To identify relevant studies, we searched electronic sources for peer-reviewed articles and research reports published through 2014 related to the Alzheimer’s population and their caregivers that provided evidence of racial and ethnic disparities, discussed reasons for disparities, or described interventions to address disparities. The literature shows consistent and adverse disparities among blacks and Hispanics compared to non-Hispanic whites in the prevalence and incidence of Alzheimer’s disease, mortality, participation in clinical trials, use of medications and other interventions, use of long-term services and supports, health care expenditures, quality of care, and caregiving. The literature suggests numerous underlying causes, including factors related to measurement of the disease, genetics, socioeconomic factors, cultural differences, lack of culturally competent providers, and discrimination. Although these disparities are well known, little is known about the effectiveness of various strategies, such as cultural competence training, to address these differences within the context of Alzheimer’s disease, with almost no studies available that evaluate possible interventions. [34 PDF pages]
This study aimed to describe and characterize the published literature on disparities between racial and ethnic groups among individuals with Alzheimer's disease and related dementias. To identify relevant studies, we searched electronic sources for peer-reviewed articles and research reports published through 2014 related to the Alzheimer's population and their caregivers that provided evidence of racial and ethnic disparities, discussed reasons for disparities, or described interventions to address disparities. The literature shows consistent and adverse disparities among Blacks and Hispanics compared to non-Hispanic Whites in the prevalence and incidence of Alzheimer's disease, mortality, participation in clinical trials, use of medications and other interventions, use of long-term services and supports, health care expenditures, quality of care, and caregiving. The literature suggests numerous underlying causes, including factors related to measurement of the disease, genetics, socioeconomic factors, cultural differences, lack of culturally competent providers, and discrimination. Although these disparities are well known, little is known about the effectiveness of various strategies, such as cultural competence training, to address these differences within the context of Alzheimer's disease, with almost no studies available that evaluate possible interventions.
About the Authors
Lisa M. Lines, PhD, MPH, is a health services researcher in the Aging, Disability, and Long-term Care group at RTI International, Waltham, MA.
Joshua M. Wiener, PhD, is a Distinguished Fellow and Program Director of the Aging, Disability and Long-Term Care group at RTI International, Washington, DC.
The following acronyms are mentioned in this report.
Introduction
Existing evidence for Alzheimer's disease and related dementias suggests that there are significant differences in prevalence, incidence, treatment, and mortality of Alzheimer's disease across racial and ethnic groups. There are also substantial differences in participation in clinical trials, the use of services, and disease-related expenditures. Disparities across racial and ethnic groups in diagnosis and treatment rates and in the use of services are concerns from an ethical and policy perspective. In an ideal world, the burden of disease and access to services would not vary based on a person's race or ethnic background.
As the population ages and minorities become a higher proportion of the older population, a higher percentage of people with Alzheimer's disease will be minorities. Among the population aged 85 and older, which is the age group most likely to have Alzheimer's disease, the proportion of the population that is White is projected to decline from 90% in 2010 to 81% in 2050 (Vincent & Velkoff, 2010). The proportion of the 85 and older population that is non-Hispanic White is projected to decline from 85% in 2010 to 67% in 2050. During the same time period, the proprtion of the population aged 85 and older that is Hispanic is projected to increase from 5% to 15%.
This research report describes evidence from the literature of racial and ethnic disparities in Alzheimer's disease, explores possible reasons for these disparities, and reviews interventions that have attempted to reduce disparities in this population.
To identify relevant studies, we searched PubMed, Google Scholar, and our in-house electronic library for peer-reviewed articles and research reports published in English through 2014 related to the Alzheimer's population and their caregivers. We also searched the Internet for gray literature using similar search terms. We included studies and reports that provided evidence of racial and ethnic disparities, discussed reasons for disparities, or described interventions to address disparities, including other reviews, books, government and non-profit research reports, and research studies published in peer-reviewed journals. Our searches of the peer-reviewed literature combined free text and Medical Subject Heading (MeSH) terms, such as "disparities," "disparity," and "discrimination," with free text and MeSH terms for Alzheimer's disease and dementia ("dementia" OR "alzheimer disease" OR "cognitive impairment"). Journal articles were required to have an abstract and pertain to human subjects. Our initial search located 151 article abstracts. After excluding articles that were clearly irrelevant based on their title or abstract, we reviewed the full texts of 35 refereed articles and 15 other sources (books, reports, web pages, etc.).
Evidence of Racial and Ethnic Disparities
Incidence estimates also suggest higher risk of Alzheimer's disease among non-White populations. In a 7-year study in the Washington Heights and Inwood communities of New York City, overall adjusted incidence rates for probable and possible Alzheimer's disease (excluding vascular and other dementias) among Whites were 0.4% per person-year for ages 65-74, 2.6% for ages 75-84, and 4.2% for ages 85+ (Tang et al., 2001). Incidence was higher among African Americans (1.7%, 4.4%, and 11.4%) and Caribbean Hispanics (1.4%, 4.4%, and 8.8%) in the same community. One recent study found that the unadjusted hazard ratio for developing dementia over 12 years was 1.44 for Black participants compared to White, but after adjusting for demographics, apolipoprotein (APOE) E4, comorbidities, lifestyle factors, and socioeconomic status, the Black-White difference was reduced to 1.09 and was no longer significant (Yaffe et al., 2013).
TABLE 1. Use of and Medicare Payments for Health Care Services among Medicare Beneficiaries with a Dementia Diagnosis, by Race/Ethnicity, 2006
SOURCE : Adapted from Alzheimer's Association, 2011.
Possible Reasons for Racial and Ethnic Disparities
Possible reasons for racial and ethnic disparities include factors related to measurement of Alzheimer's disease, genetics, cardiovascular and cerebrovascular disease, socioeconomic factors, cultural differences, and racial and ethnic discrimination. A range of pathways have been suggested that link race and ethnicity with cognitive impairment, some of which have implications for disparities ( Figure 3 ) (Glymour & Manley, 2008). In this section, we describe the evidence underlying selected factors, mediators, and pathways associated with disparities in this population.
FIGURE 3. Pathways Linking Race/Ethnicity and Cognitive Impairment
SOURCE : Adapted from Glymour and Manly, 2008.
Factors Related to Measurement of Alzheimer's Disease Prevalence and Incidence
The historically higher prevalence rates for community-dwelling African Americans than for Whites may be partially attributable to the measurement of disease prevalence and incidence. For example, historically, lower institutionalization rates among African Americans in the past left more people with dementia in the community, whereas comparable Whites were in nursing homes (Froehlich et al., 2001). The institutionalization rate of people with dementia would affect estimated prevalence rates because most studies are of the community-based population.
The effects of education on cognitive test results, and cultural biases in reporting of cognitive impairment are likely to substantially affect prevalence estimates. The number of years of education may not be equivalent between racial/ethnic groups because of disparities in school quality, particularly in older cohorts. Therefore, even prevalence studies that control for education may not be able to adequately control for differences in educational quality (Glymour & Manley, 2008).
Cognitive testing instruments--including the most widely used instrument, the MMSE--perform differently among individuals of different educational levels and racial/ethnic groups (Teresi et al., 2002). Instrument bias can result from lower literacy and education, lack of test-taking experience and stress related to test-taking, varying degrees of acculturation, and language issues such as poor translation. In addition, greater variability in scores for African Americans on standard cognitive tests makes it difficult to establish cutpoints for abnormal results (Froehlich et al., 2001).
Minorities seeking care are often more impaired at the time of their diagnosis, which suggests that they may be accessing services later in the disease process (Cooper et al., 2010). In addition, many community physicians are reluctant to diagnose Alzheimer's disease for reasons such as inadequate reimbursement for evaluation and management, lack of time to provide appropriate follow-up care, lack of knowledge of when and to whom to refer, lack of information about diagnostic criteria, cultural resistance, and belief that there is no treatment. Anecdotally, this reluctance is more prevalent in minority communities (National Institute on Aging, 2010). Moreover, there is some evidence that caregivers of Black elders with cognitive impairment report less cognitive decline in the care receiver than do their White peers, despite no significant differences in impairment, suggesting that Black caregivers may perceive changes in cognition differently (Rovner et al., 2012).
The issues of cultural framework, perception, and understanding of disability are important when interpreting scales that include items with different cultural connotations. Diagnosis is clearly problematic when physicians and patients do not speak the same language or do not share the same cultural perspective. Moreover, some diagnostic instruments contain items that may carry substantial cultural implications that vary by group. For example, a hangman's noose, one of the items on the Boston Naming Test, may have very different implications for elderly African American and White respondents (Jett, 2006).
APOE is a gene that comes in a normal or neutral form (allele), known as E3, and two variants, E2 and E4, which have been implicated in vascular and Alzheimer's diseases. The E4 variant is the most important known genetic risk factor for Alzheimer's disease.
In a recent review and meta-analysis, E4 has been shown to be associated with about 65%-75% of cases of sporadic (not genetically inherited) Alzheimer's disease and up to 20% of all dementias (Crean et al., 2011). The meta-analysis found that 39% of Alzheimer's disease patients from Asian countries carried the E4 allele; 43% of patients from Southern Europe and the Mediterranean; 54% from Central Europe; 59% from North America; and 64% from Northern Europe.
Having one or two alleles, or forms, of the APOE gene is a major predictor of Alzheimer's disease in Whites but a weak or inconsistent predictor in African Americans and Hispanics (Crean et al., 2011). In an earlier meta-analysis, the prevalence of E4 among those with Alzheimer's disease was highest in Whites (37%), followed by African Americans (32%), Japanese (28%), and Hispanics (19%) (Farrer et al., 1997). Two genetic studies have found that the E4/E4 genotype is more likely to be associated with dementia in African Americans than in Whites (Green et al., 2002; Maestre et al., 1995), but a third found that one or more E4 alleles was not associated with increased risk in African Americans or Hispanics, but was in Whites (Maestre et al., 1995). The E2/E4 and E3/E4 genotypes have been shown to be associated with an increased risk of Alzheimer's in Whites, but not African Americans, in one study (Green et al., 2002). However, an earlier study found an increased risk associated with the E2/E3 genotype among African Americans, but not Whites (Maestre et al., 1995).
Cardiovascular and Cerebrovascular Disease
Vascular factors and conditions that may be associated with cognitive decline and dementia include stroke, diabetes, hypertension, congestive heart failure, high fat intake, high cholesterol, smoking, alcohol misuse, atrial fibrillation, low folate, and obesity (Glymour & Manley, 2008). Although there is no definitive evidence linking cardiovascular disease and Alzheimer's disease, studies have found associations between cognitive impairment and cardiovascular disease (Purnell et al., 2009).
Most of the cardiovascular disease risk factors are more common in African Americans and Hispanics (Glymour & Manley, 2008). Geographic variation in the prevalence of cardiovascular disease risk factors may be related to differences in regional dietary patterns. More than 80% of Blacks aged 65+ in 2000 were born in the South, and Southern-born individuals have significantly higher rates of circulatory disease mortality (Glymour & Manley, 2008).
Vascular dementia accounts for a larger proportion of cases of related dementias in African Americans than in Whites (Froehlich et al., 2001). However, as with other dementia disorders, it is unclear whether differences in the prevalence of vascular dementia reflect true differences or are a result of measurement bias because of differences in education, socioeconomic status, or other cultural factors. One recent study found that reducing ethnic and racial disparities in the incidence of Type 2 diabetes could reduce the incidence of cognitive impairment and dementia by 17% (Noble et al., 2012).
A few studies suggest that differences in Alzheimer's disease prevalence cannot be attributed to differences in underlying cardiovascular disease rates. In one study, Black Alzheimer's disease patients had higher crude rates of hypertension than did Whites, but differences in other rates of cardiovascular disease (heart disease, stroke, diabetes) were not significantly different between the groups (Hargrave et al., 1998). In another study, although the cumulative incidence rate of Alzheimer's disease was twice as high among African Americans and Caribbean Hispanics, the presence of cardiovascular or cerebrovascular disease did not contribute to increased risk (Tang et al., 2001).
Socioeconomic Factors
Parental or early life socioeconomic position, childhood IQ, measures of early growth (such as infant head circumference and childhood height), educational attainment, occupational characteristics, and various measures of social integration have all been linked to cognitive function and neurocognitive disorders in adulthood and old age (Glymour & Manley, 2008). Individual socioeconomic position may affect cognitive status or diagnoses through: (1) material conditions; (2) psychosocial conditions (such as status); (3) direct cognitive stimulation; or (4) test-taking skills (Glymour & Manley, 2008) .
Geronimus et al. (2006) attributed many racial and ethnic disparities in health to "weathering," the accumulated consequences of exposure to economic and social adversity. Having financial resources leads to health-enhancing conditions such as healthy housing, high-quality food, safe working conditions, and access to high-quality medical care (and the reverse is true as well). Occupation influences health both through stress and material deprivation, and through toxic work conditions (Glymour & Manley, 2008).
Poverty often reduces access to educational opportunities or is associated with poor-quality education, thereby increasing the likelihood of adult poverty, which may increase the risk of depression and cognitive impairment (Glymour & Manley, 2008). If the effect of education on cognitive aging is primarily through material advantages, then credentials may be more important than quality. But if education's effect is because of cognitive skills or engagement, then school quality is more important. Resources available to parents are also very important to a child's cognitive development, and differences in parental socioeconomic status predict dementia. Extra schooling appears to have substantial benefits for memory function in the elderly (Glymour & Manley, 2008).
Additional evidence for the link between cognitive impairment and low income, less education, and having lived in a rural area comes from the 2006 Health and Retirement Study. All of these socioeconomic characteristics are more common among people with cognitive impairment, as shown in Figure 4 (Alzheimer's Association, 2011). For example, 89% of Hispanics over age 55 with cognitive impairment possess less than 12 years of education, compared to 49% of those with normal cognition.
FIGURE 4. Socioeconomic Characteristics by Cognitive Impairment and Race/Ethnicity, 2006 Health and Retirement Study
SOURCE : Alzheimer's Association, 2011.
Cultural Differences
Culture has been defined as a group's values, beliefs, traditions, symbols, language, and social organization (Harwood & Ownby, 2000). The United States model of health care, which values autonomy in medical decision making, contrasts with preferences for more family-based, physician-based, or shared physician and family-based decision making in other cultures. Moreover, although United States culture emphasizes full disclosure by providers, it is common for health care professionals in other countries to conceal serious diagnoses from patients because disclosure can be viewed as disrespectful, impolite, or even harmful to the patient (Searight & Gafford, 2005).
Cultural influences on African Americans that may affect disparities in treatment or access to medical care include the legacy of slavery and Jim Crow laws, the Tuskegee syphilis study, the interaction of religion with health care, the use of home remedies, distrust of the medical system, being of a different or the same race as one's medical provider, and health literacy (Eiser & Ellis, 2010). Some African Americans have strong religious beliefs, including the belief that illness can be cured or is controlled by God. Strong spirituality has been correlated with lower medication adherence and later stage cancer diagnosis (Eiser & Ellis, 2010). Similarly, some elderly African Americans and other non-White patients are more likely to use traditional or herbal medicines instead of, prior to, or alongside allopathic medicines.
Many individuals and cultures perceive dementia-related symptoms as a natural part of aging (Ayalon & Arean, 2004; Eiser & Ellis, 2010; Gelman, 2010; Gray et al., 2009; Jett, 2006). For example, one study asked participants whether the following statement is true: "Significant loss of memory/mental ability, commonly known as senility, is a normal part of aging." Of Whites, 23% agreed, compared to 55% of Hispanics and 33% of Chinese (Gray et al., 2009). An earlier study used different wording and found very different results: "Alzheimer's disease is a normal process of aging, like graying of hair or wrinkles." In that study, 66% of Whites, 50% of African Americans, 24% of Latinos, and 17% of Asians agreed with the statement (Ayalon & Arean, 2004).
Numerous cultural differences around caregiving for patients with Alzheimer's disease may also contribute to racial and ethnic disparities (Gray et al., 2009; Napoles et al., 2010). The most frequently documented cultural differences for both African American and Hispanic caregivers (compared to White caregivers) are more positive views of caregiving, greater spirituality, a stronger sense of duty to family, and higher value placed on extended family networks (Napoles et al., 2010). In a review of the literature, seven studies found evidence of worse mental health among Hispanic caregivers compared to Whites, whereas among African American caregivers, 11 studies found evidence of better mental health compared to Whites (Napoles et al., 2010). African American caregivers of people with Alzheimer's disease also appear to have more social support than White caregivers.
Research focused on Asian American caregivers is more limited, but there is evidence of a strong sense of filial responsibility in those communities (Napoles et al., 2010). Confucian cultures have a tradition of first-born sons and their wives being responsible for elder care (Janevic & Connell, 2001); people from these cultures may also be less likely to seek outside help in dealing with their family member and be less affected by the stress of caregiving.
Racial and Ethnic Discrimination
Although discrimination by providers against racial and ethnic minorities in the United States is commonly asserted as the cause of racial and ethnic disparities, we were unable to find any empirical studies on this topic that focused on people with Alzheimer's disease. Indeed, there appear to be few empirical studies on this topic in health care.
A rare study of discrimination in health care used the implicit association test to assess the degree to which implicit racial bias affected physicians' decisions on thrombosis (Green et al., 2007). A total of 287 internal and emergency medicine residents from four hospitals in Boston and Atlanta participated in an online study. Half of the physicians received information about a White patient, and the other half received the same information but were told that the patient was African American. Although physicians self-reported that they did not prefer treating one group or the other and did not see either group as more cooperative, the test found a preference for treating Whites and the perception that African Americans were less cooperative. Similarly, Blanchard and Lurie (2004) found that minorities were more likely to report being looked down upon or treated with disrespect than Whites. Specifically, adjusting for sex, language, income, insurance coverage, and education, 20% of Asians, 19% of Hispanics, and 14% of Blacks reporting being treated disrespectfully or looked down upon by their provider compared to 9% of Whites.
Although not specifically about Alzheimer's disease, the Institute of Medicine's (IOM's) Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (Smedley et al., 2003) identified racial bias and prejudice (conscious and unconscious) as obvious potential reasons for differences in treatment for a number of diseases. Provider lack of empathy and limited contact with people of other races/ethnicities were identified as potential factors. The IOM report also cites the theory that the pressures of the health care environment can lead to providers stereotyping patients as a cognitive shortcut, which reduces the amount of time they need to spend with patients. The common tendency to see the patient-provider relationship as one of a lower-status person coming to a higher-status person for assistance, rather than a relationship between collaborators--regardless of race or ethnicity--could be a barrier to quality care.
Interventions to Reduce Racial and Ethnic Disparities
Although there is extensive evidence documenting disparities among different racial and ethnic groups with Alzheimer's disease, there are few studies evaluating interventions to address disparities in this population. Almost all interventions designed to explicitly address racial and ethnic disparities focus on cultural competency on the part of the health care provider and/or health system. Interventions not specific to the Alzheimer's disease population include care coordination, care management, community health workers, and culturally tailored education interventions (Quinones et al., 2011).
Cultural Competence
Cultural competency is especially important in the health care setting, where barriers to communication and access to quality care can result in delayed diagnosis, inappropriate treatment, or worse. Cultural competence involves both awareness and knowledge about other cultures and skill in relating to people of other cultures. Cultural competence requires acknowledging that culture and ethnicity guide and affect behavior, and that all people are cultural beings (Betancourt et al., 2003).
A culturally competent health care environment includes the following characteristics (Anderson et al., 2003): a culturally diverse staff that reflects the communities served; bilingual staff or interpreters for the clients' languages; culture-specific and language-specific training for providers; and signs and materials in the clients' languages that are sensitive to cultural norms.
One example of a policy designed to address disparities at the systems level is the Culturally and Linguistically Appropriate Services standards issued in 2000 (Office of Minority Health, 2001). This policy mandates certain activities (e.g., competent language assistance services and signage/materials in different languages) and encourages others (such as culturally competent care and staff diversity). The evidence for improved outcomes as a result of interpreter services is mixed, but a few studies suggest that patients with limited English proficiency who receive language assistance are more satisfied with their care and have better clinical outcomes (Smedley et al., 2003).
Cultural competence education is mandated as part of medical licensure or continuing medical education requirements in New Jersey, Washington, and California, and other states are debating similar rules (Eiser & Ellis, 2010). The accrediting authority for medical schools in the United States also has standards for cross-cultural curricula as part of undergraduate medical education (Liaison Committee on Medical Education, 2012).
Many studies of cultural competence training, not specific to Alzheimer's disease, have found that it has a beneficial effect on the attitudes, knowledge, and skills of physicians and on patient satisfaction (Beach et al., 2005). For example, 17 of 19 studies of cultural competence training for health professionals found a beneficial effect in terms of knowledge; 21 of 25 studies found that such training improved attitudes; and 14 of 14 studies showed benefits of training in improving skills. In addition, three studies found that cultural competence training of health professionals improved patient satisfaction. No definitive evidence has yet linked this training to improved health outcomes (Beach et al., 2005; Betancourt et al., 2003). One study of counseling of Black female patients showed that cultural competence improved patient adherence (Beach et al., 2005) .
The goal of the Administration for Community Living-funded Alzheimer's Disease Supportive Services Program (previously called the Alzheimer's Disease Demonstration Grants to States program) is to provide or expand support services for traditionally underserved or hard-to-serve Alzheimer's patients and their caregivers, especially minorities and rural residents. The Illinois intervention focused on older persons who spoke Arabic, Assyrian, Bosnian, Hindi, and Urdu (Wiener & Mitchell, 2007). The grant worked with the Coalition of Limited English Speaking Elderly (CLESE), an organization representing 45 Chicago-area ethnic organizations that provide services to older people. Under the grant, CLESE organizations translated materials into the appropriate languages, identified home care clients with memory loss, made home visits to try to enroll elderly people into the program, conducted caregiver training, and trained providers to screen for dementia.
A recent comprehensive review of interventions focused on Alzheimer's disease and related disorders caregivers found 18 studies, of which 11 considered cultural factors in their design; eight of those 11 studies were from the REACH initiative (Napoles et al., 2010). Cultural tailoring in these studies addressed familism (i.e., the primacy of the family over individual interests), language, bicultural and bilingual staffing, health literacy, the need for advocacy, protecting elders, and logistical barriers.
Quality of care is related to cultural competence and diversity among providers (Daker-White et al., 2002; Means, 2002). Where service providers match clients with staff according to ethnicity and language, patient outcomes may be improved (Braun & Browne, 1998). Similarly, when staff receive training in cultural awareness and sensitivity and cognitive testing is language-appropriate, this may help address some disparities (Daker-White et al., 2002).
Caregiver interventions may be more effective if delivered by staff who are not only bilingual but also bicultural (Napoles et al., 2010). For example, the REACH and REACH II projects--multisite studies to evaluate culturally tailored interventions--were effective at reducing depression and improving quality of life among caregivers (Belle et al., 2006). One of REACH's strengths is the tailoring of the intervention materials individual caregivers, and the bilingual/bicultural staff.
Outreach to Minority Communities
Another approach to addressing disparities involves targeting programs and outreach to minority populations. For example, the Alzheimer's Disease Demonstration Grants to States program provided demonstration grants to Florida, Kentucky, and the District of Columbia, that focused on African Americans (Wiener & Mitchell, 2007). For example, in the District of Columbia, the intervention focused on educational efforts conducted within church communities. They also held awareness events and developed caregiver respite programs. More recently, several of the current Alzheimer's Disease Supportive Services Program grantees, including South Carolina, California, Florida, North Carolina and Puerto Rico, are targeting ethnic or racial groups.
Another intervention aimed at reaching minority populations is the Alzheimer's Disease Research Center Satellite Diagnostic and Treatment Clinics program (National Institute on Aging, 2010). Begun in 1990, the program established satellite clinics linked with one of the 30 existing Alzheimer's Disease Centers. In recent years, satellite clinics have been established on the Choctaw nation reservation, in Harlem, at Grady Hospital in Atlanta, and in St. Louis. The Alzheimer's Disease Centers are actively involved in formulating strategies and plans to recruit diverse populations to their clinics. Strategies that have met success include improved patient coordination, increasing the personal attention patients receive, home visits, and support groups (National Institute on Aging, 2010).
Conclusions
This paper reviews the research literature on ethnic and racial disparities as it relates to Alzheimer's disease. The literature shows consistent and adverse disparities among Blacks and Hispanics compared to non-Hispanic Whites in the prevalence and incidence of Alzheimer's disease, mortality, participation in clinical trials, use of medications and other interventions, use of long-term services and supports, health care expenditures, quality of care, and caregivers.
The reasons for these disparities are not well understood, but include possible genetic differences, prevalence of other diseases that may increase the risk of Alzheimer's disease, higher rates of poverty, and lower levels of education. In addition, differences in the use of services and expenditures may be related to cultural differences and racial and ethnic discrimination. Although these disparities are well known, little is known about the effectiveness of various strategies, such as cultural competence training, to address these differences within the context of Alzheimer's disease, with almost no studies available that evaluate possible interventions.
In seeking to alleviate these disparities, one of the important points made in the IOM's Unequal Treatment report is that matching needs to services is a more important goal than trying to provide equal amounts of services to different groups (Smedley et al., 2003). Both undertreatment and overtreatment can be a problem, and it would be undesirable to insist on all patients being equally overtreated. Instead, the goal should be the right care, delivered to the right patient, at the right time, in the right setting (Fowler et al., 2011).
Alzheimer's Association. (2011). "2010 Alzheimer's Disease Facts and Figures." Retrieved May 11, 2012, from http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf .
Alzheimer's Association. (2012). "2011 Alzheimer's Disease Facts and Figures." Retrieved May 11, 2012, from http://www.alz.org/downloads/facts_figures_2011.pdf .
Alzheimer's Association. (2014). "2013 Alzheimer's Disease Facts and Figures." Retrieved January 21, 2014, from http://www.alz.org/alzheimers_disease_facts_and_figures.asp .
Anderson, L.M., S.C. Scrimshaw, M.T. Fullilove, J.E. Fielding, & J. Normand. (2003). "Culturally competent healthcare systems: A systematic review." Am J Preventive Medicine , 24(3 Suppl), 68-79.
Ayalon, L., & P.A. Arean. (2004). "Knowledge of Alzheimer's disease in four ethnic groups of older adults." Int J Geriatr Psychiatry , 19(1), 51-57; doi: 10.1002/gps.1037.
Beach, M.C., E.G. Price, T.L. Gary, K.A. Robinson, A. Gozu, A. Palacio, et al. (2005). "Cultural competency: A systematic review of health care provider educational interventions." Med Care , 43(4), 356-373.
Belgrave, L.L., M.L. Wykle, & J.M. Choi. (1993). "Health, double jeopardy, and culture: The use of institutionalization by African-Americans." Gerontologist , 33(3), 379-385.
Belle, S.H., L. Burgio, R. Burns, D. Coon, S.J. Czaja, D. Gallagher-Thompson, et al. (2006). "Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial." Ann Intern Med , 145(10), 727-738.
Betancourt, J.R., A.R. Green, J.E. Carrillo, & O. Ananeh-Firempong. (2003). "Defining cultural competence : A practical framework for addressing racial/ethnic disparities in health and health care." Pub Health Rep , 118(August), 293-302.
Connolly, A., E.L. Sampson, & N. Purandare. (2012). "End-of-life care for people with dementia from ethnic minority groups: A systematic review." J Am Geriatr Soc , 60(2), 351-360; doi: 10.1111/j.1532-5415.2011.03754.x.
Cooper, C., A.R. Tandy, T. Balamurali, & G. Livingston. (2010). "A Systematic Review and Meta-Analysis of Ethnic Differences in Use of Dementia Treatment, Care, and Research." Am J Geriatr Psychiatry , 18(3), 193-203.
Crean, S., A. Ward, C.J. Mercaldi, J.M. Collins, M.N. Cook, N.L. Baker, et al. (2011). "Apolipoprotein E4 prevalence in Alzheimer's disease patients varies across global populations: A systematic literature review and meta-analysis." Dement Geriatr Cogn Disord , 31(1), 20-30.
Eiser, A.R., & G. Ellis. (2010). "Cultural competence and the African American experience with health care: The case for specific content in cross-cultural education." Academic Med , 82(2), 176-183.
Farrer, L.A., L.A. Cupples, J.L. Haines, B. Hyman, W.A. Kukull, R. Mayeux, et al. (1997). "Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis." APOE and Alzheimer Disease Meta Analysis Consortium. JAMA , 278(16), 1349-1356.
Feng, Z., M.L. Fennell, D.A. Tyler, M. Clark, & V. Mor. (2011). "Growth of racial and ethnic minorities in US nursing homes driven by demographics and possible disparities in options." Health Aff , 30(7), 1358-1365.
Fowler, F.J., C.A. Levin, & K.R. Sepucha. (2011). "Informing and involving patients to improve the quality of medical decisions." Health Aff , 30(4), 699-706.
Froehlich, T.E., S.T. Bogardus, & S.K. Inouye. (2001). "Dementia and race: Are there differences between African Americans and Caucasians?" J Am Geriatr Soc , 49(4), 477-484.
Gelman, C.R. (2010). "Learning from recruitment challenges: Barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer's disease." J Gerontol Social Work , 53(1), 94-113.
Gilligan, A.M., D.C. Malone, T.L. Warholak, & E.P. Armstrong. (2012). "Racial and ethnic disparities in Alzheimer's disease pharmacotherapy exposure: An analysis across four state Medicaid populations." American Journal of Geriatric Pharmacotherapy , 10(5), 303-312; doi: 10.1016/j.amjopharm.2012.09.002.
Gilligan, A.M., D.C. Malone, T.L. Warholak, & E.P. Armstrong. (2013). "Health disparities in cost of care in patients with Alzheimer's disease: An analysis across 4 state Medicaid populations." American Journal of Alzheimer's Disease and Other Dementias , 28(1), 84-92.
Gillum, R.F., & T.O. Obisesan. (2011). "Differences in mortality associated with dementia in U.S. Blacks and Whites." J Am Geriatr Soc , 59(10), 1823-1828.
Glymour, M.M., A. Kosheleva, V.G. Wadley, C. Weiss, & J.J. Manly. (2011). "Geographic distribution of dementia mortality: Elevated mortality rates for Black and White Americans by place of birth." Alzheimer Disease and Associated Disorders , 25(3), 196-202.
Glymour, M.M., & J.J. Manly. (2008). "Lifecourse social conditions and racial and ethnic patterns of cognitive aging." Neuropsychology Review , 18(3), 223-254.
Gray, H.L., D.E. Jimenez, M.A. Cucciare, H.Q. Tong, & D. Gallagher-Thompson. (2009). "Ethnic differences in beliefs regarding Alzheimer disease among dementia family caregivers." Am J Geriatr Psychiatry , 17(11), 925-933.
Green, A.R., D.R. Carney, D.J. Pallin, L.H. Ngo, K.L. Raymond, L.I. Iezzoni, et al. (2007). "Implicit bias among physicians and its prediction of thrombolysis decisions for Black and White patients." J Gen Intern Med , 22(9), 1231-1238.
Green, R.C., L.A. Cupples, R. Go, K.S. Benke, T. Edeki, P.A. Griffith, et al. (2002). "Risk of dementia among White and African American relatives of patients with Alzheimer disease." JAMA , 287(3), 329-336.
Gruber-Baldini, A.L., B. Stuart, I.H. Zuckerman, L. Simoni-Wastila, & R. Miller. (2007). "Treatment of dementia in community-dwelling and institutionalized Medicare beneficiaries." J Am Geriatr Soc , 55(10), 1508-1516.
Gruneir, A., S.C. Miller, Z. Feng, O. Intrator, & V. Mor. (2008). "Relationship between state Medicaid policies, nursing home racial composition, and the risk of hospitalization for Black and White residents." Health Serv Res , 43(3), 869-881.
Gurland, B.J., D.E. Wilder, R. Lantigua, Y. Stern, J. Chen, E.H. Killeffer, et al. (1999). "Rates of dementia in three ethnoracial groups." International Journal of Geriatric Psychiatry , 14(6), 481-493.
Hargrave, R., M. Stoeklin, M. Haan, & B. Reed. (1998). "Clinical aspects of Alzheimer's disease in Black and White patients." Journal of the National Medical Association , 90(2), 78-84.
Harwood, D.G., & R.L. Ownby. (2000). "Ethnicity and dementia." Current Psychiatry Reports , 2(1), 40-45.
Hernandez, S., M.J. McClendon, X-H.A. Zhou, M. Sachs, & A.J. Lerner. (2010). "Pharmacological treatment of Alzheimer's disease: Effect of race and demographic variables." Journal of Alzheimer's Disease , 19(2), 665-672.
Husaini, B.A., D.E. Sherkat, M. Moonis, R. Levine, C. Holzer, & V.A. Cain. (2003). "Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services." Psychiatric Services , 54(1), 92-96.
Janevic, M.R., & C.M. Connell. (2001). "Racial, ethnic, and cultural differences in the dementia caregiving experience: Recent findings." Gerontologist , 41(3), 334-347.
Jett, K. (2006). "Mind-loss in the African American community: Dementia as a normal part of aging." Journal of Aging Studies , 20(1), 1-10.
Liaison Committee on Medical Education. (2012). "Accreditation Standards." Retrieved May 12, 2012 from http://www.lcme.org/ .
Maestre, G., R. Ottman, Y. Stern, B. Gurland, M. Chun, M.X. Tang, et al. (1995). "Apolipoprotein E and Alzheimer's disease: Ethnic variation in genotypic risks." Annals of Neurology , 37(2), 254-259.
McClendon, M.J., S. Hernandez, K.A. Smyth, & A.J. Lerner. (2009). "Memantine and acetylcholinesterase inhibitor treatment in cases of CDR 0.5 or questionable impairment." Journal of Alzheimer's Disease , 16(3), 577-583.
Mehta, K.M., K. Yaffe, E.J. Pérez-Stable, A. Stewart, D. Barnes, B.F. Kurland, et al. (2008). "Race/ethnic differences in AD survival in US Alzheimer's Disease Centers." Neurology , 70(14), 1163-1170.
Miller, S.C., J.C. Lima, & S.L. Mitchell. (2010). "Hospice care for persons with dementia: The growth of access in US nursing homes." American Journal of Alzheimer's Disease and Other Dementias , 25(8), 666-673.
Napoles, A.M., L. Chadiha, R. Eversley, & G. Moreno-John. (2010). "Developing culturally sensitive dementia caregiver interventions: Are we there yet? Am J Alzheimers Dis Other Demen , 25(5), 389-406.
National Institute on Aging. (2010). "Health Disparities Strategic Plan: Fiscal Years 2009-2013." Retrieved May 12, 2012 from http://www.nia.nih.gov/about/health-disparities-strategic-plan-fiscal-years-2009-2013/40-areas-emphasis-integration .
Noble, J.M., J.J. Manly, N. Schupf, M.X. Tang, & J.A. Luchsinger. (2012). "Type 2 diabetes and ethnic disparities in cognitive impairment." Ethnicity and Disease , 22(1), 38-44.
Office of Minority Health. (2001). "National Standards on Culturally and Linguistically Appropriate Services (CLAS)." Retrieved from http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15 .
Perryman, M., M. Lewis, & P.A. Rivers. (2009). "Treatment disparities in medication prescribing for Alzheimer's: disease among ethnic groups." Journal of Health Care Finance , 35(4), 64-73.
Purnell, C., S. Gao, C.M. Callahan, & H.C. Hendrie. (2009). "Cardiovascular risk factors and incident Alzheimer disease: A systematic review of the literature." Alzheimer Dis Assoc Disord , 23(1), 1-10; doi: 10.1097/WAD.0b013e318187541c.
Quiñones, A.R., M. O'Neil, S. Saha, M. Freeman, S.R. Henry, & D. Kansagara. (2011). "Interventions to Improve Minority Health Care and Reduce Racial and Ethnic Disparities Health Care." VA-ESP Project #05-225. Available at http://www.hsrd.research.va.gov/publications/esp/healthcare-disparities.pdf .
Roff, L.L., L.D. Burgio, L. Gitlin, L. Nichols, W. Chaplin, & J.M. Hardin. (2004). "Positive aspects of Alzheimer's caregiving: The role of race." J Gerontol B Psychol Sci Soc Sci , 59(4), P185-190.
Rovner, B.W., R.J. Casten, C. Arenson, B. Salzman, & E.B. Kornsey. (2012). "Racial differences in the recognition of cognitive dysfunction in older persons." Alzheimer Dis Assoc Disord , 26(1), 44-49; doi: 10.1097/WAD.0b013e3182135f09.
Samper-Ternent, R., Y.F. Kuo, L.A. Ray, K.J. Ottenbacher, K.S. Markides, & S. Al Snih. (2012). "Prevalence of health conditions and predictors of mortality in oldest old Mexican Americans and non-Hispanic Whites." Journal of the American Medical Directors Association , 13(3), 254-259; doi: 10.1016/j.jamda.2010.07.010.
Searight, H.R., & J. Gafford. (2005). "Cultural diversity at the end of life: Issues and guidelines for family physicians." Am Fam Physician , 71(3), 515-522.
Sink, K.M., K.E. Covinsky, R. Newcomer, & K. Yaffe. (2004). "Ethnic differences in the prevalence and pattern of dementia-related behaviors." J Am Geriatr Soc , 52(8), 1277-1283.
Smedley, B.D., A.Y. Stith, & A.R. Nelson. (2003). "Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care." Washington, DC: National Academies Press.
Tang, M.X., P. Cross, H. Andrews, D.M. Jacobs, S. Small, K. Bell, et al. (2001). "Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan." Neurology , 56(1), 49-56.
Teresi, J.A., D. Holmes, M. Ramirez, B.J. Gurland, & R. Lantigua. (2002). "Performance of Cognitive Tests Among Different Racial/Ethnic and Education Groups: Findings of Differential Item Functioning and Possible Item Bias Multicultural Measurement In Older Populations." New York, NY: Springer.
Vincent, G.K., & V.A. Velkoff. (2010). "The Next Four Decades--The Older Population in the United States: 2010 to 2050. U.S. Department of Commerce, Economics and Statistics Administration, Bureau of the Census.
Wendler, D., R. Kington, J. Madans, G. Van Wye, H. Christ-Schmidt, L.A. Pratt, et al. (2006). "Are racial and ethnic minorities less willing to participate in health research?" PLoS Medicine , 3(2), e19-e19.
Yaffe, K., C. Falvey, T.B. Harris, A. Newman, S. Satterfield, A. Koster, et al. (2013). "Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study." BMJ , 347, f7051; doi: 10.1136/bmj.f7051.
The Effects of Different Exercise Interventions on Patients with Subjective Cognitive Decline: A Systematic Review and Network Meta-Analysis
- Original Research
- Open access
- Published: 26 March 2024
Cite this article
You have full access to this open access article
- R. Chen 1 ,
- B. Zhao 2 ,
- J. Huang 1 ,
- M. Zhang 1 ,
- Y. Wang 1 ,
- H. Liang 1 &
- Hongrui Zhan 1
172 Accesses
4 Altmetric
Explore all metrics
Background and Objective
Exercise is a promising non-pharmacological therapy for subjective cognitive decline, but it is unclear which type of exercise is most effective. The objective was to assess the comparative effects and ranks of all exercise-based interventions on cognitive function in patients with subjective cognitive decline (SCD).
In this network meta-analysis, Online databases for Web of Science, PubMed, Embase, Medline, Cochrane Library and PsycINFO were searched from inception to April 30, 2023. The included studies are randomized controlled trials assessing the efficacy of exercise interventions for individuals with SCD. The primary outcome measure is memory, while secondary outcome measures encompass executive function, attention, verbal fluency, and global cognitive function. Represented using Standardized Mean Differences (SMDs) along with their 95% Confidence Intervals (CIs). Bias assessment was conducted in accordance with the ‘Cochrane Risk of Bias Assessment Tool, 2nd Edition’ (RoB 2). Pairwise meta-analysis was carried out using the ‘meta-analysis’ module within STATA 14.0, and network meta-analysis was performed using the ‘mvmeta’ and ‘network’ packages available in STATA 14.0. Registration number CRD42023289687.
This study included a total of 11 randomized controlled trials, encompassing 1,166 patients. Mind-body exercise was found to be efficacious in enhancing or sustaining memory (SMD: 0.58, 95%CI: 0.06 ∼ 1.10) and executive function (SMD: 0.41, 95%CI: 0.09 ∼ 0.73) in individuals with subjective cognitive decline. Furthermore, mind-body exercise exhibited the highest probability of being the most effective measures for improving or preventing the decline in memory (surface under cumulative ranking curve (SUCRA) value: 90.4) and executive function (SUCRA value: 91.8). The second-ranked moderate-intensity aerobic exercise has also shown a positive effect on the improvement of executive function in patients with subjective cognitive decline (SMD: 0.23, 95%CI: 0.03 ∼ 0.43, SUCRA value: 68.2). However, we did not observe a significant effectiveness of exercise interventions on verbal fluency, attention, and overall cognitive function in subjective cognitive decline.
Mind-body exercise may potentially be the optimal strategies for enhancing memory and executive function in individuals with subjective cognitive decline. Additionally, moderate-intensity aerobic exercise has shown a modest positive effect on executive function in subjective cognitive decline. When resources permit, practical application of these findings may be considered. Nevertheless, further support for the conclusions of this study is warranted through larger sample sizes and well-designed multicenter trials.
Similar content being viewed by others

The effect of combined cognitive intervention and physical exercise on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials
Qiuyan Meng, Huiru Yin, … Li Chen
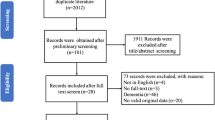
Association of nonpharmacological interventions for cognitive function in older adults with mild cognitive impairment: a systematic review and network meta-analysis
Xueyan Liu, Guangpeng Wang & Yingjuan Cao

Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis
Feng-Tzu Chen, Jennifer L. Etnier, … Yu-Kai Chang
Avoid common mistakes on your manuscript.
Introduction
Subjective Cognitive Decline (SCD) refers to a decline in subjective memory or cognitive function without apparent cognitive impairments in objective cognitive assessments and without impairment in activities of daily living ( 1 ). SCD stands as an intermediate state between normal aging and Mild Cognitive Impairment (MCI), acknowledged as one of the earliest phases and initial cognitive alterations in the onset of Alzheimer’s disease (AD) ( 2 ), carrying a heightened risk of progression to MCI or AD ( 3 ). Epidemiological investigations indicate that among individuals aged 50 and older, the prevalence of SCD is 26.6% ( 4 ). SCD is associated with a 1.73-fold increased risk of progression to MCI in older adults and a 1.9-fold increased risk of progression to AD ( 5 ).
In the early stages of the AD progression, the brain can functionally compensate for neuropathological changes, thereby enabling individuals with SCD to maintain objective cognitive scores within the normal range on neuropsychological tests ( 6 ). Recent research ( 7 ) reports that individuals with SCD, despite not demonstrating significant declines in objective neuropsychological assessments, exhibit subtle impairments in global cognitive function, memory, executive function, and language abilities when compared to healthy controls. Notably, the most pronounced deterioration is observed within the domain of memory ( 7 ). While SCD does not meet the diagnostic criteria for MCI, it does share analogous patterns of cerebral alterations with patients diagnosed with MCI and those suffering from dementia attributed to AD ( 8 ).
Neuroimaging research ( 9 ) reveals that AD has undergone progressive neurofunctional deterioration and incurs irreversible cognitive impairment. Hence, the genuine promise in treating AD may lie in early intervention ( 10 ). As a preclinical stage of AD and MCI, SCD potentially represents a critical therapeutic window for slowing or preventing cognitive deterioration ( 11 ). Early intervention holds the promise of reversing cognitive decline and mitigating the risk of developing AD( 11 ). Given the limited efficacy of pharmacological interventions in enhancing cognitive function in patients with SCD, along with the potential for adverse effects, non-pharmacological interventions have garnered considerable attention for their impact on cognitive rehabilitation in SCD ( 12 ).
Exercise, as one of the non-pharmacological interventions, can modulate neuronal electrical activity associated with cognitive function, enhance brain structural plasticity ( 13 ), stimulate the generation of new neurons and synapses related to learning and memory ( 14 , 15 ), promote the secretion of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1) ( 16 , 17 ), to subsequently enhance cognitive function. Exercise has a positive impact on the cognitive function of individuals with SCD ( 18 , 19 ). The «Physical Activity Guidelines for Americans» ( 20 )also explicitly recommend that older individuals can enhance their cognitive function and reduce the risk of developing AD through regular physical activity.
Previous pertinent research shows that diverse forms of exercise, such as aerobic exercise (AE), resistance exercise (RE) and mind-body exercise (MBE), may potentially exert cognitive enhancement effects through the modulation of distinct molecular mechanisms outlined above, resulting in variable magnitudes of impact (e.g., MBE: standard mean difference (SMD) =0.38 0.52 0.72 ( 18 , 21 , 22 ), AE: SMD= 0.14 0.24 0.31 ( 18 , 21 , 23 ), RE: SMD=0.22 0.29 0.39 ( 18 , 21 , 24 )). Hence, the choice of exercise modality constitutes a pivotal consideration for clinical professionals when devising exercise prescriptions aimed at preventing or mitigating cognitive decline ( 25 ).
Nonetheless, the absence of clinical studies concurrently comparing various types of exercise interventions, coupled with the scarcity of available direct evidence (head-to-head randomized clinical trials) within the literature incorporated in traditional pairwise meta-analyses, poses a formidable challenge in assessing the comparative efficacy of different exercise therapies. Therefore, the most efficacious exercise treatment modality for preventing or mitigating cognitive decline in SCD patients remains unclear at present. This ambiguity makes it challenging for healthcare professionals to draw definitive conclusions regarding the “most effective”exercise types and to formulate exercise intervention measures that are most effective in treating SCD patients.
Network Meta-Analysis (NMA) serves as a robust quantitative framework that amalgamates both direct and indirect evidence stemming from clinical trial networks ( 26 ). It facilitates the assessment of the effectiveness of different clinical interventions based on clinical evidence, effectively surmounting the limitations inherent in traditional meta-analyses ( 27 ). Furthermore, NMA also enables the ranking of intervention measures, yielding a hierarchy of all exercise therapies. Understanding which exercise selection is deemed the “most effective” can assist physicians in making clinical decisions, informing clinical practice, and integrating the optimal type of exercise into the patient’s rehabilitation objectives.
This systematic review was conducted following the PRISMA ( 28 ) and the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses ( 29 ). The protocol for this study was registered with PROSPERO (registration number CRD42023289687).
Search strategy
Online databases for Web of Science, PubMed, Embase, Medline, Cochrane Library and PsycINFO were searched from inception to April 30, 2023. Referring to the study by Huang et al ( 30 ), the search strategy was initially devised for PubMed and subsequently adapted for other databases. The details of the retrieval strategy formulated for PubMed can be found in the appendix 1. The retrieval process was independently conducted by two researchers. In case of any disagreements, consensus was reached through discussions between the two researchers.
Study selection
The literature search records will be uploaded to Endnote X9, and a deduplication process will be conducted within the software. In the first stage, two researchers (BZ and JH) independently conducted a screening of the titles and abstracts of relevant articles, followed by a meticulous full-text assessment in strict accordance with the inclusion and exclusion criteria. Any discrepancies were resolved through consensus reached through discussions between the two researchers or, if necessary, adjudicated by a third researcher.
The detailed inclusion criteria as follows: (1) The study design must adhere to a randomized controlled trial (RCT) methodology. (2) The Participants must be diagnosed with SCD (meet the SCD conceptual framework proposed by Jessen et al. in 2014( 1 ) and China AD Preclinical Alliance( 11 )). (3) Intervention measures may encompass any form of exercise training. (4) The control group must fall into one of the following categories: standard care, health education, blank control (without administering any treatment or implementing any specific interventions) or treatment as usual. (5) The study must report at least one of the following outcomes: global cognitive function, memory function, executive function, attention, and verbal fluency. (6) The research must be documented in the English language. The SCD conceptual framework as follows: 1) subjective decline of memory rather than other domains of cognition; 2) onset of SCD within the last 5 years; 3) worries associated with SCD; and 4) worse self-perceived memory than others in the same age group; 5) absence of objective clinical impairment of MCI(the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA) or the modified Mini Mental Status examination (3MS) are within the normal range after years of education correction, and have not been clinically diagnosed as MCI as determined by clinical doctors).
The detailed exclusion criteria are as follows: 1) Studies specifically examining cognitive impairment in patients with various types of cancer, Parkinson’s disease, Huntington’s disease, epilepsy, multiple sclerosis, Psychiatric illnesses (e.g., major depression, schizophrenia and bipolar disorder) (these diseases, aside from being associated with cognitive dysfunction, typically exhibit diverse pathological changes; consequently, they may interfere the effects of exercise on cognitive function). 2) Significant cerebrovascular lesions (e.g., evident cerebral infarction or evident cerebral hemorrhage) ( 11 ). 3) Studies specifically investigating the effects of acute exercise (the effects generated after a single instance of acute exercise). 4) Comprehensive intervention measures in which exercise is not the primary component (e.g., exercise combined with cognitive training or exercise combined with physical therapy).
Outcomes: The core symptom of SCD is a decline in memory function ( 31 ), followed by impairments in executive function, attention and verbal fluency domains ( 32 ). Therefore, we used memory function as the primary outcome measure and analyzed executive function, attention, verbal fluency and global cognitive function as secondary outcome measures. The assessment scales with different outcome measures, arranged in order based on their frequency of use and psychometric characteristics, are listed sequentially in Appendix 2. When utilizing multiple instruments to assess a particular cognitive dimension, we select the most appropriate tool based on predefined criteria.
Data extraction
Two researchers (JF and HL) independently established data extraction forms according to the Cochrane Handbook’s guidelines ( 33 ), then proceeded to independently extract data, cross-referencing their findings. The extracted data encompassed general information (authors, publication year), participant characteristics (population, gender, average age), study features (number of patients, intervention measures, control measures, intervention duration, frequency, duration of each session), and outcomes (means, standard deviations (SDs), respective measurement tools). In instances where relevant statistical metrics were reported incompletely, we employed estimation methods for mean and SD based on sample size, median, range, and p-values, in accordance with the Cochrane Handbook guidelines ( 33 ). Additionally, we initiated email correspondence with the authors to procure any missing or incomplete data.
During the data extraction process, in order to assess the efficacy of various types of exercise interventions, we categorized exercise interventions following the American College of Sports Medicine Exercise Testing and Prescription Guidelines ( 20 ) and previous systematic reviews ( 34 , 35 ). These categories included Moderate-Intensity aerobic exercise (MI, such as walking, running, and cycling, etc), High-Intensity aerobic exercise (HI, such as Boxing and Track and Field, etc), Resistance exercise (RE, aimed at increasing muscle strength, e.g., using elastic tubes, elastic bands, and weight machines), Multicomponent exercise (ME, combining two or more types of exercise, such as MI combine RE and balance training) and Mind-body exercise (MBE, emphasizing the integration of movement with breathing, mindfulness and memory, including practices like baduanjin, yoga, and mind-motor exercise). To investigate the moderating variables of exercise effects, we categorized and coded exercise frequency, intensity, duration per session, and intervention duration (Appendix 3).
Risk of bias assessment
Two researchers (MZ and JH) independently conducted methodological quality assessments of the included RCTs using the “Cochrane Risk of Bias Assessment Tool, 2nd Edition” (RoB 2) ( 36 ). Divergences were resolved through consensus discussions, and in cases where differences couldn’t be reconciled, consultation with a senior researcher was sought. Studies were categorized into low, high, or some concerns of bias based on the following criteria: Randomization process; Deviations from intended interventions; Missing outcome data; Measurement of the outcome; Selection of the reported result.
Data synthesis and statistical analysis
Pairwise meta-analyses.
We utilized the “meta-analysis” module in Stata 14.0 (Verson 14.0; StataCorp, College Station, TX, USA) to perform pairwise analyses for all direct comparisons, thereby elucidating the effects of various exercise interventions compared to the control group individually. Depending on the magnitude of heterogeneity in the data, we employed the random-effects model (I-V heterogeneity method) or the fixed-effects model (inverse variance method) to calculate the standardized mean difference (SMD) and its corresponding 95% confidence interval (95% CI) for the differences in scores before and after the intervention. Using the I 2 statistic to estimate the proportion of total variance attributed to heterogeneity between studies in each pairwise comparison.
Network meta-analysis
Network meta-analyses (NMA) were conducted using the “mvmeta” ( 37 , 38 ) and “network” ( 39 , 40 ) packages in Stata 14.0 (Verson 14.0; StataCorp, College Station, TX, USA), based on a frequentist analysis framework, for both primary and secondary outcome measures. NMA integrates the results from individual studies, and each treatment effect of the intervention/control group can be obtained through direct or indirect comparison ( 41 , 42 ). When there is no direct connection between two treatment arms, the results are based on indirect evidence ( 41 , 42 ). To visualize the network geometry and connectivity of nodes, we created network diagrams for each cognitive outcome measure. Each node represents an intervention, and the connecting lines between two nodes represent one or more direct comparisons ( 43 ). The size of each node is proportional to the number of participants receiving that intervention, with larger nodes indicating a higher number of participants who received the intervention ( 43 ). The thickness of the connecting lines is related to the number of studies that directly compared these two interventions, with thicker lines representing a greater number of studies ( 43 ).
We initially employ an inconsistency model for global inconsistency analysis. A p-value below 0.05 in the inconsistency test indicates the presence of global inconsistency ( 44 ). The local inconsistency analysis was conducted using the node-splitting method. The presence of local incongruity is signified when the p-value of the incongruity test falls below 0.05 ( 44 ). When evidence closed loops are present in the network diagram, we use the loop-specific method for loop inconsistency analysis. When the incongruity factor approaches zero, it signifies the concordance between two sources of evidence ( 44 ).
Fitting with multivariate random-effects (restricted maximum likelihood estimation) meta-analysis model in the framework of frequentism. This model takes into consideration the heterogeneity between studies caused by clinical and other factors, providing more conservative confidence intervals (CI) for the combined point estimates, in order to account for the interrelation of effect sizes among trials involving more than two groups ( 44 , 45 ). After combining direct and/or indirect comparisons for any pair of interventions, we computed pooled effect sizes represented as SMDs with corresponding 95% CIs. The effect sizes were categorized as small (SMD <0.40), moderate (0.40 ≤ SMD ≤ 0.70), or large (SMD >0.70) following the Cochrane handbook guidelines( 33 ).
To rank exercise interventions, we utilized a parameter-guided bootstrapping procedure with 10,000 resamples to assess the effectiveness of each intervention and calculated the Surface Under the Cumulative Ranking curve (SUCRA) values. SUCRA is a precise estimation of the cumulative ranking probability for the top i treatments. For each treatment j among the n compared treatments, the cumulative probability of treatment j being ranked within the top i is calculated using the following formula: \(\text{SUCRA}_{j}=(\sum\nolimits_{\rm{i}=1}^{\rm{n}-1}\ cum_{j,i})/(\rm{n}-1)\) ( 46 ). The range of SUCRA values spans from 0% to 100%, and the closer the value approaches 100%, the higher the likelihood that the intervention is more effective.
Regression and sensitivity analysis
To further explore the sources of heterogeneity and inconsistency, we conducted a Meta-regression analysis on the primary outcome measures, using the frequency, intensity, duration, and duration of exercise interventions as covariates.
A sensitivity analysis was conducted by excluding RCTs with at least one domain assessed as high risk of bias in pairwise meta-analyses, aiming to explore the robustness of the study outcomes.
Small study effects
Egger’s test was employed to assess the presence of small study effects. Additionally, funnel plots were generated for visual inspection of publication bias for each comparison of outcomes ( 47 ).
Literature search and selection
After deduplication, a total of 1316 records were identified. Among these records, 58 were considered potentially relevant following the initial screening of titles and abstracts. Following the application of inclusion and exclusion criteria, a total of 11 randomized controlled trials, comprising 1166 participants, were included in the network meta-analysis (Fig 1 ). The appendix 4 provide detailed characteristics of the studies included in the analysis, and the appendix 5 contain all citations of the studies included in the NMA.
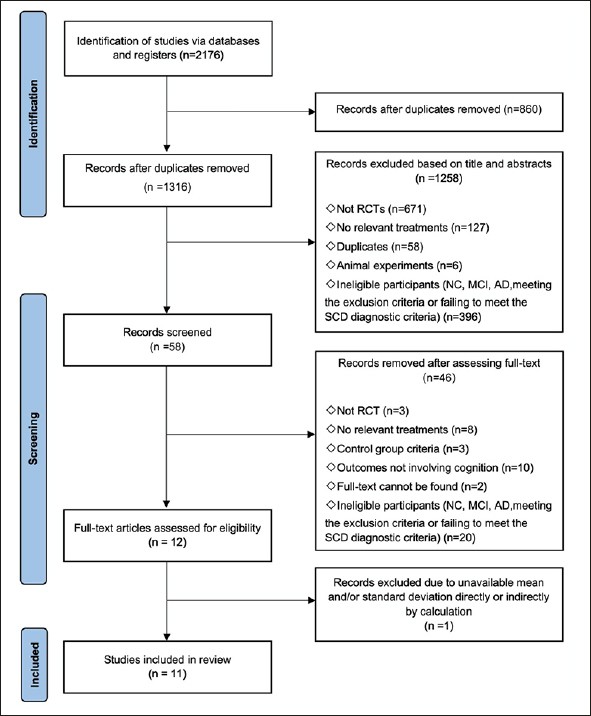
Flow of studies in the review
Note: RCT: Randomized controlled trial, NC: Normal cognitive, SCD: Subjective cognitive decline, MCI: Mild cognitive impairment, AD: Alzheimer’s disease.
Characteristics of included studies
Among the included studies, the sample sizes ranged from 31 to 415, with a mean age of 62.87 years. Eight studies (1079 participants) investigated the impact of exercise on memory function ( 48 – 55 ), eight studies (921 participants) examined the influence of exercise on executive function ( 48 , 49 , 51 – 54 , 56 , 57 ), while three studies (573 participants) explored the effects of exercise on attention ( 48 , 51 , 53 ), three studies (610 participants) investigated the influence of exercise on verbal fluency ( 49 , 53 , 58 ), and six studies (729 participants) assessed the impact of exercise on global cognitive function ( 48 , 49 , 52 , 53 , 56 , 57 ). There are a total of five intervention categories, which include Moderate-Intensity aerobic exercise (MI, N = 8) ( 49 – 53 , 55 , 57 , 58 ), High-Intensity aerobic exercise (HI, N = 2) ( 52 , 56 ), Resistance exercise (RE, N = 2) ( 48 , 53 ), Multicomponent exercise (ME, N = 3) ( 50 , 53 , 56 ), and Mind-body exercise (MBE, N = 3) ( 52 , 54 , 58 ) (Appendix 4, Appendix 5).
Risk of bias
The summary of bias risk can be found in the supplementary materials. All included studies were RCTs, but 31% of the trials did not adequately report the implementation methods of randomization. Out of the 11 trials, 6 trials (54.5%) were rated as having a low risk of bias. For these 6 trials, a significant proportion of “Some concerns” (36.4%) arose due to the lack of detailed descriptions regarding group concealment or the handling of missing outcome data. One trial (9.1%) received a high risk of bias assessment due to the absence of researcher blinding (Appendix 6).
Appendix 7 presents the outcomes of pairwise meta-analysis and estimates of heterogeneity. In brief, exercise interventions were found to be more efficacious than control groups in the domains of memory (Combine SMD=0.20, 95 % CI: 0.07∼0.34, I 2 =33.8%), executive function (−0.15, 95 % CI: −0.29 ∼ −0.01, I 2 =25.0%), and verbal fluency (−0.21, 95%CI: −0.35 ∼ −0.06, I 2 =9.3 %) in SCD. Nevertheless, exercise interventions did not exhibit significant differences in improving global cognitive function ( P =0.72, I 2 =0.0%) and attention ( P =0.88, I 2 =0.0%) in SCD compared to the control groups.
A total of five different interventions and control arms were included, comprising 1166 patients with SCD in our network meta-analysis. The inconsistency test based on network analysis showed no statistically significant differences in global inconsistency (memory: P =0.07, executive function: P =0.39, verbal fluency: P =0.10, attention: P =0.61, global cognitive function: P =0.22), detailed results are provided in the appendix 8. When evaluating closed-loop networks, no statistically significant differences in inconsistency between direct and indirect outcomes were observed. Detailed results can be found in the appendix 8. The network diagram (Fig 2 ) displays the weights of all available comparisons included in this network meta-analysis. Comparative effects between exercise interventions can be found in the league table (Fig 3 ). Cumulative probability plots for different exercise interventions and the ranking of SUCRAs are presented in Fig 4 and Table 1 , respectively.
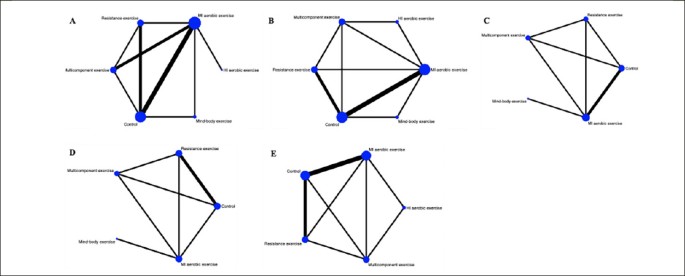
Network plot of available treatment comparisons
Note: Each node represents an intervention, and the connecting lines between two nodes represent one or more direct comparisons. The size of each node is weighted based on the number of participants receiving that intervention, while the thickness of the connecting lines is weighted based on the number of studies directly comparing those two interventions. A: memory, B: executive function, C: verbal fluency, D: attention, E: global cognitive function. MI aerobic exercise: Moderate-Intensity aerobic exercise, HI aerobic exercise: High-Intensity aerobic exercise.
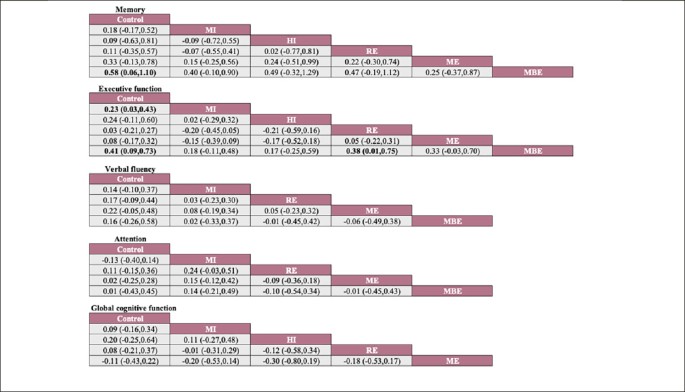
Network meta-analysis of effectiveness comparison
Note: Each cell displays the SMD with a 95% confidence interval. For any cell, a negative SMD favors interventions in the upper-left corner, while a positive SMD favors interventions in the lower-right corner. Significant results are highlighted in bold. MI: Moderate-Intensity aerobic exercise, HI: High -Intensity aerobic exercise, RE: Resistance exercise, ME: Multicomponent exercise, MBE: Mind-body exercise.
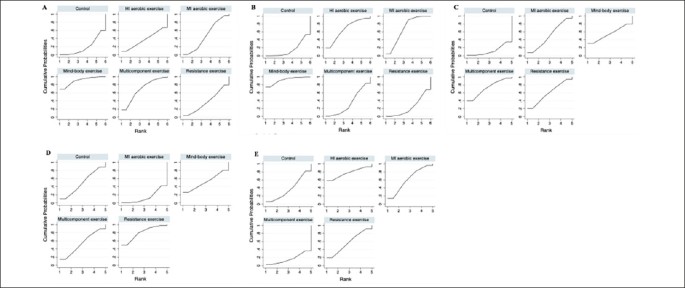
Cumulative ranking probability plot
Note: A: memory, B: executive function, C: verbal fluency, D: attention, E: global cognitive function. MI aerobic exercise: Moderate-Intensity aerobic exercise, HI aerobic exercise: High-Intensity aerobic exercise.
The network meta-analysis results indicate a significant impact of mind-body exercise on memory function in SCD patients when compared to the control group (combined SMD: 0.58, 95%CI: 0.06 ∼ 1.10). There were no significant differences between different types of exercises. Mind-body exercise (MBE) had the highest probability (70.6%) of being the most effective exercise modality, with a SUCRA value of 90.4. Followed by multicomponent exercise (ME, SUCRA=68.5, P=17.3%), moderate-intensity aerobic exercise (MI, SUCRA=47.1, P=7.8%), resistance exercise (RE, SUCRA=37.3, P=3.4%), and high-intensity aerobic exercise (HI, SUCRA=37.3, P=0.8%). Refer to Fig 3 , Fig 4 and table 1 for details.
For executive function in SCD patients, MBE (combined SMD: 0.41, 95%CI: 0.09 ∼ 0.73) and moderate-intensity aerobic exercise (combined SMD: 0.23, 95%CI: 0.03 ∼ 0.43) demonstrated significantly greater improvements compared to the control group. Furthermore, mind-body exercise significantly outperformed resistance exercise in improving executive function (combined SMD: 0.38, 95%CI: 0.01 ∼ 0.75). MBE has the highest probability (74.0%) of being the most effective exercise type for preserving executive function, with a SUCRA value of 91.8. Following that are MI (SUCRA=68.2, P=19.7%), HI (SUCRA=67.1, P=4.9%), ME (SUCRA=33.8, P=1%), and RE (SUCRA=23.5, P=0.4%).
Refer to Fig 3 , Fig 4 and table 1 for details.
However, we did not observe significant differences between different types of exercise interventions compared to the control group or in comparisons between different types of exercise interventions in the remaining cognitive dimensions. Network plots, cumulative ranking plots, and SUCRA values for exercise interventions in the remaining cognitive dimensions are presented in Fig 2 , Fig 3 , Fig 4 , as well as Appendix 8.
Meta-regression analyses were separately conducted for different types of exercise interventions (primarily focusing on the main outcome measure: memory), with exercise intervention frequency, intensity, duration per session, and intervention duration were included as covariates. The results show that the intensity of each session serves as a moderating factor affecting the effectiveness of exercise interventions on memory in individuals with SCD (see the appendix 9 for details).
We excluded the study ( 56 ) with a high risk of bias in at least one domain, which encompassed cognitive domains such as global cognitive function and executive function. Sensitivity analysis revealed that the outcomes of the interventions remained unchanged (see the appendix 10 for details).
Overall, we did not find compelling evidence of small study effects among the outcomes. The points on the funnel plots for each study domain are visually symmetrically distributed around the mean estimated treatment effect (see the appendix 11 for details). The p-values for Egger’s test were as follows: 0.39 for memory, 0.84 for executive function, 0.14 for verbal fluency, 0.87 for attention, and 0.36 for global cognition (see the appendix 11 for details).
This systematic review and network meta-analysis on exercise interventions for patients with Subjective Cognitive Decline (SCD) included data from 11 clinical trials involving a total of 1166 participants. To our knowledge, this review is the first network meta-analysis aimed at exploring the relative efficacy of different types of exercise on cognitive function in SCD. Our study results corroborate the beneficial impact of exercise interventions on cognitive function in SCD and highlight Mind-Body Exercise (MBE) as the most promising exercise therapy for attenuating memory and executive function decline in SCD patients.
Over the past few decades, the beneficial effects of MBE on cognitive function have gradually become a research focus. MBE represents a multimodal form of exercise that emphasizes the harmonious integration of mind, body, and spirit ( 59 ). In addition to aiding in balance control, flexibility, and muscle strength, MBE also places emphasis on mental focus, procedural memory, physical equilibrium, and relaxation ( 60 ). Compared to aerobic and resistance exercises that focus primarily on cardiovascular fitness and strength, MBE integrate movement sequences with breath control and attention regulation. Additionally, they have been shown to increase oxygenated hemoglobin levels in the prefrontal cortex ( 61 ). The combination of these physical and neural resources can offer an explanation for the observed differences in the effects of exercise therapy.
Furthermore, the combined nature of ME involving two or more modalities presents challenges in ensuring that each exercise component meets optimal durations, frequencies, and intensities during training. This can lead to a diminished practical efficacy of ME in real-world applications. It is worth noting that we observed exercise intensity as a moderating variable influencing the magnitude of exercise effects, with very high-intensity exercise not necessarily resulting in improved memory function for SCD individuals. MBE typically involves slow-paced and low-intensity activities, making it a more suitable option for older adults compared to other forms of exercise ( 62 , 63 ). This may potentially serve as another explanation for why MBE demonstrates the greatest potential in mitigating cognitive decline in SCD. Researchers should also take these factors into consideration in future studies.
Recent research findings have supported the potential relationship between physical activity and neural changes, indicating that MBE can modulate brain structures ( 64 ) and induce alterations in brain neural activity and functional connectivity ( 64 , 65 ), including regions such as the hippocampus ( 66 ) and prefrontal cortex ( 64 ), which play a crucial role in cognitive function. Currently, consistent observations indicate reduced structural integrity in the hippocampus and prefrontal gray matter in individuals with SCD ( 67 – 70 ), as well as a decrease in the functional connectivity between the hippocampus and prefrontal regions ( 71 , 72 ). The hippocampus and prefrontal cortex are the core regions responsible for memory and executive function processes, respectively ( 73 , 74 ). The modulation of brain structure, neural activity, and functional connectivity by MBE may serve as the foundation for the beneficial effects of such exercise on memory and executive function in SCD.
MBE is also highly likely to synergize its benefits in enhancing memory and executive function through other neurobiological mechanisms that may initiate favorable biochemical alterations. For instance, MBE may enhance cognitive function by upregulating levels of brain-derived neurotrophic factor (BDNF) in the plasma, which is a crucial mediator of central nervous system neuroplasticity ( 75 ). Additionally, it may also exert beneficial effects on the brain and cognition by modulating inflammatory cytokines (levels of which are associated with cognitive impairment ( 76 – 78 )) such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-10, and IL-1β, with studies ( 79 – 81 ) reporting that, levels of inflammatory cytokines are associated with cognitive impairment. These mechanisms contribute to its potential to enhance cognitive function. Overall, our research findings suggest that MBE may have unexpected benefits for memory and executive function in SCD patients, consistent with previous meta-analytic ( 82 ) results.
For executive function, while the efficacy is lower compared to MBE, we also observed a facilitative effect on executive function from moderate-intensity aerobic exercise (MI). Prior research ( 83 ) has indicated that executive function is the cognitive domain most sensitive to the beneficial effects of aerobic exercise. Potential underlying mechanisms may involve aerobic exercise’s modulation of cerebral vascular health, enhancement of cerebral blood flow, and regional cortical thickness, which induce cortical activation in the prefrontal subregions ( 84 – 86 ). In previous research ( 87 – 89 ), it has been observed that the intensity of aerobic exercise selectively impacts executive function, with high-intensity aerobic exercise appearing to have less pronounced effects on the improvement of executive function, which aligns with our findings. They propose that the positive impact of aerobic exercise on executive function follows an inverted U-shaped curve, increasing from no intensity to moderate intensity and then declining with further increases in intensity ( 87 – 89 ). Additionally, the release of various neurochemical substances related to cognitive function (e.g., catecholamines, cortisol, BDNF) induced by exercise also depends on factors such as exercise intensity ( 90 ).
Consistent with the study by Coelho et al. ( 91 ), we cannot provide evidence suggesting a significant enhancement of executive function in SCD through combined exercise. This may be related to the substantial disparities in exercise intensity and frequency used in some studies compared to the activity standards recommended for older adults. The American College of Sports Medicine ( 20 ) recommends that older adults should engage in at least 5 days of moderate-intensity exercise for 30–60 minutes per day each week. However, within the articles included in this meta-analysis, the highest intervention frequency observed for studies focusing on executive function was 3 times, and it only involved one study ( 52 ). The insufficient exercise dosage may potentially attenuate the positive effects of ME.
It is noteworthy that within our network meta-analysis, no significant efficacy of exercise on attention, verbal fluency, and global cognitive function in individuals with SCD was observed. In the preclinical stage of Alzheimer’s disease, SCD typically exhibits the earliest cognitive impairment in the domain of memory, followed by executive functions ( 92 ). We speculate that the relatively limited effects of exercise on attention and verbal fluency cognitive functions may be attributed to the less severe impairment of these cognitive domains in individuals with SCD. However, Venegas-Sanabria et al. ( 93 ), when assessing the impact of exercise on individuals with Alzheimer’s disease or mild cognitive impairment, also found that exercise did not yield significant beneficial effects on verbal fluency and attention. The emergence of such results may be related to the insensitivity of the domains of attention and language fluency to exercise, as cognitive domains exhibit varying levels of sensitivity to exercise (e.g., aerobic exercise having the most pronounced therapeutic effect on executive functions ( 83 )). Interestingly, in our conventional pairwise meta-analysis, we observed a positive effect of exercise on verbal fluency in SCD. However, this finding was based on the inclusion of only one study ( 53 ). Given the limited number of clinical trials within the studies included in the analysis that have investigated the impact of exercise on attention and verbal fluency in SCD, the findings warrant cautious interpretation and necessitate further experiments in the future to augment the required evidence.
In addition, one of the diagnostic criteria for SCD is the absence of objectively demonstrable clinical impairment associated with MCI. Based on this diagnostic criterion, the neurobehavioral scores for global cognitive function in the SCD patients included in the analysis were within the normal range, and baseline data demonstrated preserved cognitive performance. This suggests that there is only minimal decline in global cognitive function, leaving limited room for exercise to improve global cognitive function in SCD. It has been reported that exercise interventions require a sufficiently long duration to exert an impact on specific cognitive domains ( 94 , 95 ). The majority of studies included in the analysis had a duration of 6 months or less, which impedes the long-term investigation of exercise effects on attention, verbal fluency, and global cognitive function in SCD. As for the mechanisms involved in this, more clinical trials are needed to fill this gap.
We anticipate that our research findings will hold significant implications for both clinical decision-making and scientific investigation. In the context of clinical rehabilitation, MBE deserves heightened attention and can be recommended as an adjunct therapy for SCD patients due to its significant benefits in improving memory and executive function. MI can also be considered as a routine non-pharmacological treatment for SCD patients to enhance their executive function. Certainly, future clinical trials should undertake more investigations into elucidating the mechanisms by which exercise influences SCD cognitive functions to explain the reasons behind the benefits it confers. For clinical researchers, regular updates to NMAs on this topic are indispensable as new data continues to emerge.
While our meta-analysis demonstrated the beneficial impact of exercise on SCD cognition, there are still some limitations to consider. Firstly, the number of studies included in our review was limited, and to validate our findings, large-scale trials will be needed in the future. Secondly, only a small number of studies have reported long-term follow-up data after the conclusion of interventions. Consequently, we extracted data only from the included studies for the period after the interventions were completed, resulting the sustained benefits of exercise on various aspects of SCD cognition unverified. Furthermore, among the included studies, only one study ( 53 ) employed a multi-arm design, directly comparing the effects of different types of exercise on SCD cognition. Many effect size estimates were reliant on indirect comparisons. Considering that evidence from direct comparisons is more robust than indirect comparisons, it is imperative to conduct more multi-arm designed studies in the future.
Our NMA also has other limitations, such as a lack of exploration into the most effective exercise dosages (intensity, frequency, session duration, and length of intervention). This is another critical factor influencing rehabilitation outcomes. The dose-response relationship of exercise interventions on SCD cognitive function needs further investigation. In addition, due to the unavailability of complete data, we did not conduct subgroup analysis. Future research endeavors may explore whether different forms of exercise are beneficial for distinct types of SCD (e.g., the impact of aerobic or resistance exercise on cognitive function in SCD with cardiovascular risk). This would be an intriguing topic, as research ( 96 ) suggests a novel direction in dementia prevention by offering diverse intervention measures tailored to individual prevention needs, varying with lifestyle factors and identified risks of cognitive decline. Lastly, our NMA only included articles written in English, which could potentially result in the omission of information from eligible research reports conducted in other languages.
This network meta-analysis has synthesized the existing evidence from clinical studies, offering clinical professionals and researchers some important findings regarding exercise therapy. Our research reveals that among individuals experiencing SCD, MBE emerges as the most effective exercise modality in slowing down the decline in memory and executive function. Additionally, MI demonstrates a favorable capacity for improving executive function in SCD patients. Nevertheless, in light of the limitations of our aforementioned meta-analysis and the paucity of studies in the current literature, the results should be interpreted with caution. To bolster the findings of this NMA, further investigation is warranted, including well-designed, large-scale, multi-arm, multicenter trials.
Jessen F, Amariglio RE, Boxtel MV, et al. A Conceptual Framework for Research on Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Alzheimer’s & Dementia, 2014, 10.
Jessen F, Amariglio RE, Buckley RF, et al. The Characterisation of Subjective Cognitive Decline. The Lancet Neurology, 2020, 19(3): 271–8. https://doi.org/10.1016/S1474-4422(19)30368-0
Article PubMed PubMed Central Google Scholar
Pichet Binette A, Vachon-Presseau É, Morris J, et al. Amyloid and Tau Pathology Associations with Personality Traits, Neuropsychiatric Symptoms, and Cognitive Lifestyle in the Preclinical Phases of Sporadic and Autosomal Dominant Alzheimer’s Disease. Biol Psychiatry, 2021, 89(8): 776–85. https://doi.org/10.1016/j.biopsych.2020.01.023
Article CAS PubMed Google Scholar
Liew TM. Depression, Subjective Cognitive Decline, and the Risk of Neurocognitive Disorders. Alzheimer’s research & therapy, 2019, 11(1): 70-. https://doi.org/10.1186/s13195-019-0527-7
Article Google Scholar
Pike KE, Cavuoto MG, Li L, et al. Subjective Cognitive Decline: Level of Risk for Future Dementia and Mild Cognitive Impairment, a Meta-Analysis of Longitudinal Studies. Neuropsychology review, 2021. https://doi.org/10.1007/s11065-021-09522-3
Jessen F, Amariglio RE, Buckley RF, et al. The Characterisation of Subjective Cognitive Decline. Lancet Neurol, 2020, 19(3): 271–8. https://doi.org/10.1016/s1474-4422(19)30368-0
Wolfsgruber S, Kleineidam L, Guski J, et al. Minor Neuropsychological Deficits in Patients with Subjective Cognitive Decline. Neurology, 2020, 95(9): e1134–e43. https://doi.org/10.1212/wnl.0000000000010142
Li K, Luo X, Zeng Q, et al. Aberrant Functional Connectivity Network in Subjective Memory Complaint Individuals Relates to Pathological Biomarkers. Transl Neurodegener, 2018, 7: 27. https://doi.org/10.1186/s40035-018-0130-z
Livingston G, Sommerlad A, Orgeta V, et al. Dementia Prevention, Intervention, and Care. The Lancet, 2017, 390(10113).
Crous-Bou M, Minguillón C, Gramunt N, et al. Alzheimer’s Disease Prevention: From Risk Factors to Early Intervention. Alzheimer”s Research & Therapy, 2017, 9(1): 71.
Ying Han. Recommendations for Diagnosis and Treatment of Subjective Cognitive Decline Due to Preclinical Alzheimer Disease in China. Journal of China Clinic Medical Imaging, 2018, 29(8): 5-.
Google Scholar
Sheng C, Yang K, Wang X, et al. Advances in Non-Pharmacological Interventions for Subjective Cognitive Decline: A Systematic Review and Meta-Analysis. Journal of Alzheimer’s disease: JAD, 2020, 77(2).
Hamilton GF, Rhodes JS. Exercise Regulation of Cognitive Function and Neuroplasticity in the Healthy and Diseased Brain. Prog Mol Biol Transl, 2015, 135: 381–406.
Cassilhas R, Tufik S, Mello M. Physical Exercise, Neuroplasticity, Spatial Learning and Memory. Cellular & Molecular Life Sciences, 2015, 73(5): 1–9.
Hötting K, Röder B. Beneficial Effects of Physical Exercise on Neuroplasticity and Cognition. Neurosci Biobehav Rev, 2013, 37(9 Pt B): 2243–57. https://doi.org/10.1016/j.neubiorev.2013.04.005
Article PubMed Google Scholar
Ding Q, Vaynman S, Akhavan MM, et al. Insulin-Like Growth Factor I Interfaces with Brain-Derived Neurotrophic Factor-Mediated Synaptic Plasticity to Modulate Aspects of Exercise-Induced Cognitive Function. Neuroscience, 2006, 140(3): 823–33.
Trejo JL, Carro E, Torres-Aleman I. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J Neurosci, 2001, 21(5): 1628–34. https://doi.org/10.1523/jneurosci.21-05-01628.2001
Article CAS PubMed PubMed Central Google Scholar
Northey JM, Cherbuin N, Pumpa KL, et al. Exercise Interventions for Cognitive Function in Adults Older Than 50: A Systematic Review with Meta-Analysis. Br J Sports Med, 2018, 52(3): 154–60. https://doi.org/10.1136/bjsports-2016-096587
Lü J, Fu W, Liu Y. Physical Activity and Cognitive Function among Older Adults in China: A Systematic Review. J Sport Health Sci, 2016, 5(3): 287–96. https://doi.org/10.1016/j.jshs.2016.07.003
MEDICINE ACOS. Acsm’s Guidelines for Exercise Testing and Prescription. 10th ed. New York: Lippincott Williams & Wilkins, 2018.
Chen FT, Hopman RJ, Huang CJ, et al. The Effect of Exercise Training on Brain Structure and Function in Older Adults: A Systematic Review Based on Evidence from Randomized Control Trials. J Clin Med, 2020, 9(4). https://doi.org/10.3390/jcm9040914
Wu C, Yi Q, Zheng X, et al. Effects of Mind-Body Exercises on Cognitive Function in Older Adults: A Meta-Analysis. J Am Geriatr Soc, 2019, 67(4): 749–58. https://doi.org/10.1111/jgs.15714
Shu Y, He Q, Xie Y, et al. Cognitive Gains of Aerobic Exercise in Patients with Ischemic Cerebrovascular Disorder: A Systematic Review and Meta-Analysis. Front Cell Dev Biol, 2020, 8: 582380. https://doi.org/10.3389/fcell.2020.582380
Landrigan JF, Bell T, Crowe M, et al. Lifting Cognition: A Meta-Analysis of Effects of Resistance Exercise on Cognition. Psychol Res, 2020, 84(5): 1167–83. https://doi.org/10.1007/s00426-019-01145-x
Barha CK, Galea LA, Nagamatsu LS, et al. Personalising Exercise Recommendations for Brain Health: Considerations and Future Directions. Br J Sports Med, 2017, 51(8): 636–9. https://doi.org/10.1136/bjsports-2016-096710
Yang A, Pechlivanoglou P, Aoyama K. Interpreting and Assessing Confidence in Network Meta-Analysis Results: An Introduction for Clinicians. J Anesth, 2022, 36(4): 524–31. https://doi.org/10.1007/s00540-022-03072-5
Dias S, Ades AE, Welton NJ, et al. Network Meta-Analysis for Decision Making, 2018.
Page MJ, McKenzie JE, Bossuyt PM, et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj, 2021, 372: n71. https://doi.org/10.1136/bmj.n71
Hutton B, Salanti G, Caldwell DM, et al. The Prisma Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med, 2015, 162(11): 777–84. https://doi.org/10.7326/m14-2385
Huang X, Zhao X, Li B, et al. Comparative Efficacy of Various Exercise Interventions on Cognitive Function in Patients with Mild Cognitive Impairment or Dementia: A Systematic Review and Network Meta-Analysis. J Sport Health Sci, 2022, 11(2): 212–23. https://doi.org/10.1016/j.jshs.2021.05.003
Si T, Xing G, Han Y. Subjective Cognitive Decline and Related Cognitive Deficits. Front Neurol, 2020, 11: 247. https://doi.org/10.3389/fneur.2020.00247
Schütz H, Caspers S, Moebus S, et al. Prevalence and Psychosocial Correlates of Subjectively Perceived Decline in Five Cognitive Domains: Results from a Population-Based Cohort Study in Germany. Int J Geriatr Psychiatry, 2020, 35(10): 1219–27. https://doi.org/10.1002/gps.5359
Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane, 2023. Available from www.training.cochrane.org/handbook .
Chen FT, Etnier JL, Chan KH, et al. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med, 2020, 50(8): 1451–67. https://doi.org/10.1007/s40279-020-01292-x
Wang S, Yin H, Wang X, et al. Efficacy of Different Types of Exercises on Global Cognition in Adults with Mild Cognitive Impairment: A Network Meta-Analysis. Aging Clin Exp Res, 2019, 31(10): 1391–400. https://doi.org/10.1007/s40520-019-01142-5
Sterne JAC, Savovi J, Page MJ, et al. Rob 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ Clinical Research, 2019, 366: l4898.
White IR. Multivariate Random-Effects Meta-Analysis. Stata Journal, 2009, 9(1): 40–56.
White IR. Multivariate Random-Effects Meta-Regression: Updates to Mvmeta. Stata Journal, 2011, 11(2): 255–70.
White IR. A Suite of Stata Programs for Network Meta-Analysis; proceedings of the Stata Users Group, 2013.
Mutz J, Vipulananthan V, Carter B, et al. Comparative Efficacy and Acceptability of Non-Surgical Brain Stimulation for the Acute Treatment of Major Depressive Episodes in Adults: Systematic Review and Network Meta-Analysis. Bmj, 2019, 364: l1079. https://doi.org/10.1136/bmj.l1079
Salanti G. Indirect and Mixed-Treatment Comparison, Network, or Multiple-Treatments Meta-Analysis: Many Names, Many Benefits, Many Concerns for the Next Generation Evidence Synthesis Tool. Res Synth Methods, 2012, 3(2): 80–97. https://doi.org/10.1002/jrsm.1037
Bhatnagar N, Lakshmi PV, Jeyashree K. Multiple Treatment and Indirect Treatment Comparisons: An Overview of Network Meta-Analysis. Perspect Clin Res, 2014, 5(4): 154–8. https://doi.org/10.4103/2229-3485.140550
Shim S, Yoon BH, Shin IS, et al. Network Meta-Analysis: Application and Practice Using Stata. Epidemiol Health, 2017, 39: e2017047. https://doi.org/10.4178/epih.e2017047
White IR, Barrett JK, Jackson D, et al. Consistency and Inconsistency in Network Meta-Analysis: Model Estimation Using Multivariate Meta-Regression. Res Synth Methods, 2012, 3(2): 111–25. https://doi.org/10.1002/jrsm.1045
Higgins JP, Jackson D, Barrett JK, et al. Consistency and Inconsistency in Network Meta-Analysis: Concepts and Models for Multi-Arm Studies. Res Synth Methods, 2012, 3(2): 98–110. https://doi.org/10.1002/jrsm.1044
Salanti G, Ades AE, Ioannidis JP. Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J Clin Epidemiol, 2011, 64(2): 163–71. https://doi.org/10.1016/j.jclinepi.2010.03.016
Chaimani A, Higgins JP, Mavridis D, et al. Graphical Tools for Network Meta-Analysis in Stata. PLoS One, 2013, 8(10): e76654. https://doi.org/10.1371/journal.pone.0076654
Busse AL, Filho WJ, Magaldi RM, et al. Effects of Resistance Training Exercise on Cognitive Performance in Elderly Individuals with Memory Impairment: Results of a Controlled Trial. Einstein, 2008, 6(4).
Lautenschlager NT, Cox KL, Flicker L, et al. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. Jama, 2008, 300(9): 1027.
Zuniga KE, Mackenzie MJ, Kramer A, et al. Subjective Memory Impairment and Well-Being in Community-Dwelling Older Adults. Psychogeriatrics, 2016, 16(1): 20–6. https://doi.org/10.1111/psyg.12112
Boa Sorte Silva NC, Nagamatsu LS, Gill DP, et al. Memory Function and Brain Functional Connectivity Adaptations Following Multiple-Modality Exercise and Mind-Motor Training in Older Adults at Risk of Dementia: An Exploratory Sub-Study. Front Aging Neurosci, 2020, 12: 22. https://doi.org/10.3389/fnagi.2020.00022
Boa Sorte Silva NC, Petrella AFM, Christopher N, et al. The Benefits of High-Intensity Interval Training on Cognition and Blood Pressure in Older Adults with Hypertension and Subjective Cognitive Decline: Results from the Heart & Mind Study. Front Aging Neurosci, 2021, 13: 643809. https://doi.org/10.3389/fnagi.2021.643809
Makino T, Umegaki H, Ando M, et al. Effects of Aerobic, Resistance, or Combined Exercise Training among Older Adults with Subjective Memory Complaints: A Randomized Controlled Trial. J Alzheimers Dis, 2021, 82(2): 701–17. https://doi.org/10.3233/jad-210047
Su H, Wang H, Meng L. The Effects of Baduanjin Exercise on the Subjective Memory Complaint of Older Adults: A Randomized Controlled Trial. Medicine (Baltimore), 2021, 100(30): e25442. https://doi.org/10.1097/md.0000000000025442
Chou CC, Chien LY, Lin MF, et al. Effects of Aerobic Walking on Memory, Subjective Cognitive Complaints, and Brain-Derived Neurotrophic Factor among Older Hypertensive Women. Biol Res Nurs, 2022, 24(4): 484–92. https://doi.org/10.1177/10998004221098974
Ramnath U, Rauch L, Lambert EV, et al. Efficacy of Interactive Video Gaming in Older Adults with Memory Complaints: A Cluster-Randomized Exercise Intervention. PLoS One, 2021, 16(5): e0252016. https://doi.org/10.1371/journal.pone.0252016
Stroehlein JK, Vieluf S, Zimmer P, et al. Learning to Play Golf for Elderly People with Subjective Memory Complaints: Feasibility of a Single-Blinded Randomized Pilot Trial. BMC Neurology, 2021, 21(1).
Boa Sorte Silva NC, Gill DP, Gregory MA, et al. Multiple-Modality Exercise and Mind-Motor Training to Improve Mobility in Older Adults: A Randomized Controlled Trial. Exp Gerontol, 2018, 103: 17–26. https://doi.org/10.1016/j.exger.2017.12.011
Laird KT, Paholpak P, Roman M, et al. Mind-Body Therapies for Late-Life Mental and Cognitive Health. Curr Psychiatry Rep, 2018, 20(1): 2. https://doi.org/10.1007/s11920-018-0864-4
Chan JSY, Deng K, Wu J, et al. Effects of Meditation and Mind-Body Exercises on Older Adults’ Cognitive Performance: A Meta-Analysis. Gerontologist, 2019, 59(6): e782–e90. https://doi.org/10.1093/geront/gnz022
Yang Y, Chen T, Shao M, et al. Effects of Tai Chi Chuan on Inhibitory Control in Elderly Women: An Fnirs Study. Front Hum Neurosci, 2019, 13: 476. https://doi.org/10.3389/fnhum.2019.00476
Guo Y, Shi H, Yu D, et al. Health Benefits of Traditional Chinese Sports and Physical Activity for Older Adults: A Systematic Review of Evidence. J Sport Health Sci, 2016, 5(3): 270–80. https://doi.org/10.1016/j.jshs.2016.07.002
Taylor-Piliae RE, Newell KA, Cherin R, et al. Effects of Tai Chi and Western Exercise on Physical and Cognitive Functioning in Healthy Community-Dwelling Older Adults. J Aging Phys Act, 2010, 18(3): 261–79. https://doi.org/10.1123/japa.18.3.261
Zhang X, Zong B, Zhao W, et al. Effects of Mind-Body Exercise on Brain Structure and Function: A Systematic Review on Mri Studies. Brain Sci, 2021, 11(2). https://doi.org/10.3390/brainsci11020205
Jiang J, Guo W, Wang B. Effects of Exergaming on Executive Function of Older Adults: A Systematic Review and Meta-Analysis. PeerJ, 2022, 10: e13194. https://doi.org/10.7717/peerj.13194
Tao J, Liu J, Chen X, et al. Mind-Body Exercise Improves Cognitive Function and Modulates the Function and Structure of the Hippocampus and Anterior Cingulate Cortex in Patients with Mild Cognitive Impairment. Neuroimage Clin, 2019, 23: 101834. https://doi.org/10.1016/j.nicl.2019.101834
Chen B, Wang Q, Zhong X, et al. Structural and Functional Abnormalities of Olfactory-Related Regions in Subjective Cognitive Decline, Mild Cognitive Impairment and Alzheimer’s Disease. The international journal of neuropsychopharmacology, 2021. https://doi.org/10.1093/ijnp/pyab091
Jessen F, Wolfsgruber S, Kleineindam L, et al. Subjective Cognitive Decline and Stage 2 of Alzheimer Disease in Patients from Memory Centers. Alzheimers Dement, 2023, 19(2): 487–97. https://doi.org/10.1002/alz.12674
Pini L, Wennberg AM. Structural Imaging Outcomes in Subjective Cognitive Decline: Community Vs. Clinical-Based Samples. Exp Gerontol, 2021, 145: 111216. https://doi.org/10.1016/j.exger.2020.111216
Teipel SJ, Cavedo E, Weschke S, et al. Cortical Amyloid Accumulation Is Associated with Alterations of Structural Integrity in Older People with Subjective Memory Complaints. Neurobiol Aging, 2017, 57: 143–52. https://doi.org/10.1016/j.neurobiolaging.2017.05.016
Ribarič S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines, 2023, 11(2). https://doi.org/10.3390/biomedicines11020355
Fu Z, Zhao M, He Y, et al. Aberrant Topological Organization and Age-Related Differences in the Human Connectome in Subjective Cognitive Decline By using Regional Morphology from Magnetic Resonance Imaging. Brain Struct Funct, 2022, 227(6): 2015–33. https://doi.org/10.1007/s00429-022-02488-9
Bettio LEB, Rajendran L, Gil-Mohapel J. The Effects of Aging in the Hippocampus and Cognitive Decline. Neurosci Biobehav Rev, 2017, 79: 66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030
Friedman NP, Robbins TW. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology, 2022, 47(1): 72–89. https://doi.org/10.1038/s41386-021-01132-0
Sungkarat S, Boripuntakul S, Kumfu S, et al. Tai Chi Improves Cognition and Plasma Bdnf in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Neurorehabil Neural Repair, 2018, 32(2): 142–9. https://doi.org/10.1177/1545968317753682
Tegeler C, O’Sullivan J, Bucholtz N, et al. The Inflammatory Markers Crp, Il-6, and Il-10 Are Associated with Cognitive Function—Data from the Berlin Aging Study Ii. Neurobiology of Aging, 2016, 38.
Lecca D, Jung YJ, Scerba MT, et al. Role of Chronic Neuroinflammation in Neuroplasticity and Cognitive Function: A Hypothesis. Alzheimers Dement, 2022, 18(11): 2327–40. https://doi.org/10.1002/alz.12610
Lopez-Rodriguez AB, Hennessy E, Murray CL, et al. Acute Systemic Inflammation Exacerbates Neuroinflammation in Alzheimer’s Disease: Il-1β Drives Amplified Responses in Primed Astrocytes and Neuronal Network Dysfunction. Alzheimers Dement, 2021, 17(10): 1735–55. https://doi.org/10.1002/alz.12341
Black DS, Cole SW, Irwin MR, et al. Yogic Meditation Reverses Nf-Kb and Irf-Related Transcriptome Dynamics in Leukocytes of Family Dementia Caregivers in a Randomized Controlled Trial. Psychoneuroendocrinology, 2013, 38(3): 348–55. https://doi.org/10.1016/j.psyneuen.2012.06.011
Cahn BR, Goodman MS, Peterson CT, et al. Yoga, Meditation and Mind-Body Health: Increased Bdnf, Cortisol Awakening Response, and Altered Inflammatory Marker Expression after a 3-Month Yoga and Meditation Retreat. Front Hum Neurosci, 2017, 11: 315. https://doi.org/10.3389/fnhum.2017.00315
Verdone L, Marson F, Caserta M, et al. Quadrato Motor Training (Qmt) Influences Il-1β Expression and Creativity: Implications for Inflammatory State Reduction and Cognitive Enhancement. Prog Brain Res, 2023, 277: 63–83. https://doi.org/10.1016/bs.pbr.2022.12.008
Blomstrand P, Tesan D, Nylander EM, et al. Mind Body Exercise Improves Cognitive Function More Than Aerobic- and Resistance Exercise in Healthy Adults Aged 55 years and Older - an Umbrella Review. Eur Rev Aging Phys Act, 2023, 20(1): 15. https://doi.org/10.1186/s11556-023-00325-4
Yu F, Vock DM, Barclay TR. Executive Function: Responses to Aerobic Exercise in Alzheimer’s Disease. Geriatr Nurs, 2018, 39(2): 219–24. https://doi.org/10.1016/j.gerinurse.2017.09.005
Guadagni V, Drogos LL, Tyndall AV, et al. Aerobic Exercise Improves Cognition and Cerebrovascular Regulation in Older Adults. Neurology, 2020, 94(21): e2245–e57. https://doi.org/10.1212/wnl.0000000000009478
Tarumi T, Patel NR, Tomoto T, et al. Aerobic Exercise Training and Neurocognitive Function in Cognitively Normal Older Adults: A One-Year Randomized Controlled Trial. J Intern Med, 2022, 292(5): 788–803. https://doi.org/10.1111/joim.13534
Damrongthai C, Kuwamizu R, Suwabe K, et al. Benefit of Human Moderate Running Boosting Mood and Executive Function Coinciding with Bilateral Prefrontal Activation. Sci Rep, 2021, 11(1): 22657. https://doi.org/10.1038/s41598-021-01654-z
Möller F, Hoffmann U, Dalecki M, et al. Physical Exercise Intensity During Submersion Selectively Affects Executive Functions. Hum Factors, 2021, 63(2): 227–39. https://doi.org/10.1177/0018720819879313
McMorris T, Hale BJ. Differential Effects of Differing Intensities of Acute Exercise on Speed and Accuracy of Cognition: A Meta-Analytical Investigation. Brain Cogn, 2012, 80(3): 338–51. https://doi.org/10.1016/j.bandc.2012.09.001
Steinberg F, Pixa NH, Fregni F. A Review of Acute Aerobic Exercise and Transcranial Direct Current Stimulation Effects on Cognitive Functions and Their Potential Synergies. Front Hum Neurosci, 2018, 12: 534. https://doi.org/10.3389/fnhum.2018.00534
Mcmorris T, Turner A, Hale BJ, et al. Beyond the Catecholamines Hypothesis for an Acute Exercise-Cognition Interaction: A Neurochemical Perspective. Exercise-Cognition Interaction, 2016.
Coelho Junior HJ, Callado Sanches I, Doro M, et al. Multicomponent Exercise Improves Hemodynamic Parameters and Mobility, but Not Maximal Walking Speed, Transfer Capacity, and Executive Function of Older Type Ii Diabetic Patients. Biomed Res Int, 2018, 2018: 4832851. https://doi.org/10.1155/2018/4832851
Wasef S, Laksono I, Kapoor P, et al. Screening for Subjective Cognitive Decline in the Elderly Via Subjective Cognitive Complaints and Informant-Reported Questionnaires: A Systematic Review. BMC Anesthesiol, 2021, 21(1): 277. https://doi.org/10.1186/s12871-021-01493-5
Venegas-Sanabria LC, Martinez-Vizcaino V, Cavero-Redondo I, et al. Effect of Physical Activity on Cognitive Domains in Dementia and Mild Cognitive Impairment: Overview of Systematic Reviews and Meta-Analyses. Aging Ment Health, 2021, 25(11): 1977–85. https://doi.org/10.1080/13607863.2020.1839862
Yu F, Vock DM, Zhang L, et al. Cognitive Effects of Aerobic Exercise in Alzheimer’s Disease: A Pilot Randomized Controlled Trial. J Alzheimers Dis, 2021, 80(1): 233–44. https://doi.org/10.3233/jad-201100
Müller P, Rehfeld K, Schmicker M, et al. Evolution of Neuroplasticity in Response to Physical Activity in Old Age: The Case for Dancing. Front Aging Neurosci, 2017, 9: 56. https://doi.org/10.3389/fnagi.2017.00056
Livingston G, Huntley J, Sommerlad A, et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet, 2020, 396(10248): 413–46. https://doi.org/10.1016/s0140-6736(20)30367-6
Download references
Acknowledgments
We extend our gratitude to the authors of the original manuscripts for providing the requisite data for this network meta-analysis.
Funding: This research was supported by the Project of Administration of Traditional Chinese Medicine of Guangdong Province, China [grant numbers 20201066]; Guangdong Basic and Applied Basic Research Foundation, China [grant number 2021A1515010135]; and the IIT Research Project of The Fifth Affiliated Hospital of Sun Yat-sen University [YNZZ 2021-09.
Author information
Authors and affiliations.
Department of Rehabilitation Medicine, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai City, Guangdong Province, China
R. Chen, J. Huang, M. Zhang, Y. Wang, J. Fu, H. Liang & Hongrui Zhan
College of Rehabilitation Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou City, Fujian Province, China
You can also search for this author in PubMed Google Scholar
Contributions
Authors’ contributions: HZ conceived and oversaw this study. BZ and JH made significant contributions to the literature search and screening. MZ and YW provided substantial contributions to bias risk assessment. JF and HL performed data extraction and conducted cross-verification. RC analyzed and interpreted the extracted data and drafted the initial version of the manuscript. All listed authors meet the authorship eligibility criteria, and no additional eligible authors have been omitted. Furthermore, all authors have reviewed and approved the final version of the manuscript and have reached a consensus on the order of authorship.
Corresponding author
Correspondence to Hongrui Zhan .
Ethics declarations
Competing interests: The authors declare that this network meta-analysis was conducted without any discernible commercial or financial associations that could be construed as potential conflicts of interest.
Ethical standards: Not required.
Electronic Supplementary Material
Supplementary material, approximately 374 kb., rights and permissions.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
Reprints and permissions
About this article
Chen, R., Zhao, B., Huang, J. et al. The Effects of Different Exercise Interventions on Patients with Subjective Cognitive Decline: A Systematic Review and Network Meta-Analysis. J Prev Alzheimers Dis (2024). https://doi.org/10.14283/jpad.2024.65
Download citation
Received : 28 November 2023
Accepted : 18 February 2024
Published : 26 March 2024
DOI : https://doi.org/10.14283/jpad.2024.65
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Subjective cognitive decline
- cognitive function
- network meta-analysis
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 March 2024
The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease
- Jordi Manuello ORCID: orcid.org/0000-0002-9928-0924 1 , 2 ,
- Joosung Min ORCID: orcid.org/0000-0002-5541-5014 3 ,
- Paul McCarthy 1 ,
- Fidel Alfaro-Almagro 1 ,
- Soojin Lee 1 , 4 ,
- Stephen Smith 1 ,
- Lloyd T. Elliott 3 na1 ,
- Anderson M. Winkler 5 , 6 na1 &
- Gwenaëlle Douaud ORCID: orcid.org/0000-0003-1981-391X 1
Nature Communications volume 15 , Article number: 2576 ( 2024 ) Cite this article
9803 Accesses
2049 Altmetric
Metrics details
- Genetics research
- Neuroscience
- Risk factors
We have previously identified a network of higher-order brain regions particularly vulnerable to the ageing process, schizophrenia and Alzheimer’s disease. However, it remains unknown what the genetic influences on this fragile brain network are, and whether it can be altered by the most common modifiable risk factors for dementia. Here, in ~40,000 UK Biobank participants, we first show significant genome-wide associations between this brain network and seven genetic clusters implicated in cardiovascular deaths, schizophrenia, Alzheimer’s and Parkinson’s disease, and with the two antigens of the XG blood group located in the pseudoautosomal region of the sex chromosomes. We further reveal that the most deleterious modifiable risk factors for this vulnerable brain network are diabetes, nitrogen dioxide – a proxy for traffic-related air pollution – and alcohol intake frequency. The extent of these associations was uncovered by examining these modifiable risk factors in a single model to assess the unique contribution of each on the vulnerable brain network, above and beyond the dominating effects of age and sex. These results provide a comprehensive picture of the role played by genetic and modifiable risk factors on these fragile parts of the brain.
Similar content being viewed by others
The causes and consequences of Alzheimer’s disease: phenome-wide evidence from Mendelian randomization
Roxanna Korologou-Linden, Laxmi Bhatta, … Neil M. Davies

Heterogeneous effects of genetic risk for Alzheimer’s disease on the phenome
Hei Man Wu, Alison M. Goate & Paul F. O’Reilly
A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease
Douglas P. Wightman, Iris E. Jansen, … Danielle Posthuma
Introduction
The development of preventative strategies based on modifying risk factors might prove to be a successful approach in ensuring healthy ageing. Factors particularly scrutinised in dementia and unhealthy ageing have included cerebrovascular factors such as high blood pressure, diabetes and obesity, but also lifestyle ones such as alcohol consumption, and protective factors such as exercise 1 . Assessing these modifiable risk factors together makes it possible to identify the unique contribution of each of these factors on the brain or on cognitive decline. A Lancet commission, updated in 2020 to include, e.g., pollution for its possible role in the incidence of dementia 2 , examined the relative impact of 12 modifiable risk factors for dementia, and showed that these 12 factors may account for 40% of the cases worldwide 3 . Conversely, genetic factors are non-modifiable in nature, but can inform us about the mechanisms underlying the phenotypes of interest. These mechanisms sometimes can be shared across these phenotypes. For instance, genetic overlap has been found for Alzheimer’s and Parkinson’s diseases at a locus in the MAPT region 4 . Likewise, one of the most pleiotropic variants, in the SLC39A8 / ZIP8 gene, shows genome-wide associations with both schizophrenia and fluid intelligence, amongst many other phenotypes 5 , 6 .
One way to objectively and robustly assess susceptibility for unhealthy ageing is to look non-invasively at brain imaging markers 7 . Using a data-driven approach on a lifespan cohort, we previously identified an ensemble of higher-order, ‘transmodal’ brain regions that degenerates earlier and faster than the rest of the brain 8 . The very same areas also develop relatively late during adolescence, thus supporting the ‘last in, first out’ (LIFO) hypothesis, which posits that the process of age-related brain decline mirrors developmental maturation. Importantly, this network of brain regions further demonstrated heightened vulnerability to schizophrenia and Alzheimer’s disease, two disorders that impact on brain structure during adolescence and ageing respectively. Accordingly, this LIFO network was strongly associated with cognitive traits whose impairment is specifically related to these two disorders, namely fluid intelligence and long-term memory 8 .
Here, our main objective was to assess both the genetic and modifiable risk factors’ contributions to the vulnerability of these most fragile parts of the brain. We conducted a genome-wide association study on a prospective cohort of nearly 40,000 participants of the UK Biobank study who had received brain imaging, and in total evaluated the association between the LIFO brain network and 161 modifiable risk factors, classified according to 15 broad categories: blood pressure, cholesterol, diabetes, weight, alcohol consumption, smoking, depressive mood, inflammation, pollution, hearing, sleep, socialisation, diet, physical activity and education.
The vulnerable LIFO brain network in UK Biobank
Similar to our previously observed results 8 , the loadings of the LIFO brain network, i.e., the normalised grey matter volume in the network after regressing out the effects of all the other brain maps (see Methods), demonstrated a strong quadratic association with age in the UK Biobank cohort of 39,676 participants ( R 2 = 0.30, P < 2.23 × 10 −308 , Fig. 1 ). These higher-order regions thus show an accelerated decrease of grey matter volume compared with the rest of the brain. Furthermore, these areas define a network mainly involved in behavioural tasks related to execution, working memory, and attention (Fig. 1 , Supplementary Information ).
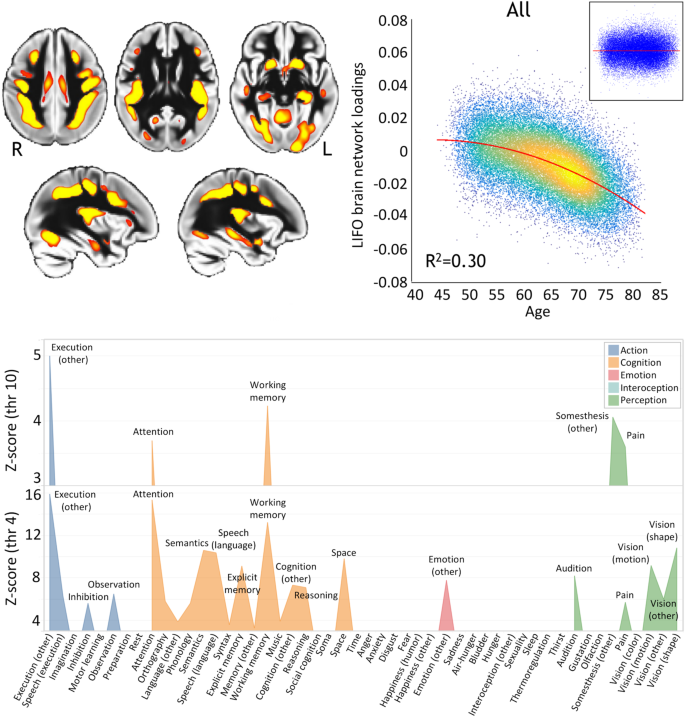
Top left, spatial map of the LIFO network (in red-yellow, thresholded at Z > 4 for visualisation) used to extract the loadings from every scanned participant from UK Biobank ( n = 39,676). Top right, these LIFO loadings (in arbitrary units) show a strong quadratic association with age in the UK Biobank cohort, i.e. grey matter volume decreases quadratically with older age in these specific regions ( R 2 = 0.30, P < 2.23 × 10 −308 ; inset: residual scatterplot). Bottom, the vulnerable network appears to encompass areas mainly involved in execution, working memory, and attention (using the BrainMap taxonomy 60 , and with the LIFO brain network thresholded at both Z = 4 and Z = 10, see Supplementary Information ).
Genetic influences over the vulnerable LIFO brain network
Using a minor allele frequency filter of 1% and a –log 10 (P) threshold of 7.5, we found, in the 39,676 participants, genome-wide associations between the LIFO brain network and seven genetic clusters whose top variants were all replicated (Table 1 /Supplementary Data 1 , Fig. 2 ).
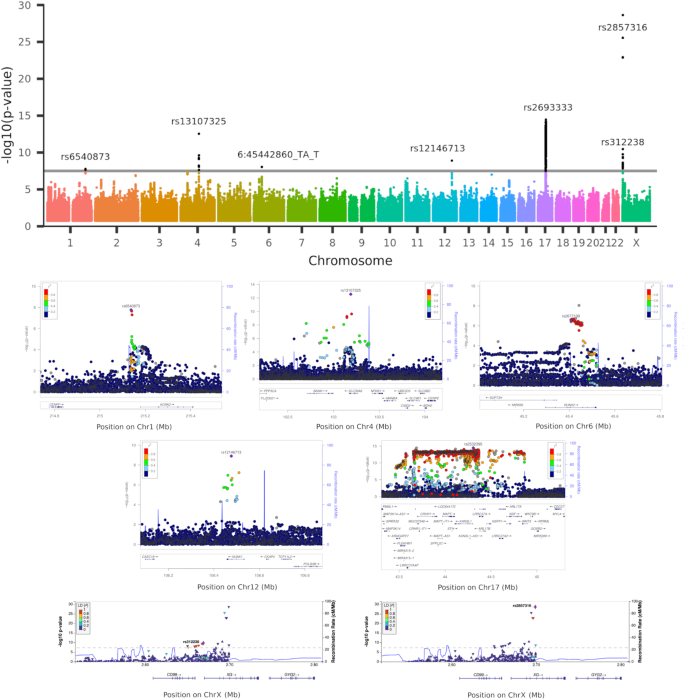
Top row, Manhattan plot showing the 7 significant genetic clusters associated with the LIFO brain network (–log 10 ( P ) > 7.5). Second and third rows, regional association plots of the top variants for each of the 5 autosomal genetic clusters: rs6540873 on chromosome (Chr) 1 ( KCNK2 ), rs13107325 on Chr4 ( SLC39A8 ), rs2677109 on Chr6 ( RUNX2 ) (as a proxy in high LD R 2 = 0.86 with indel 6:45442860_TA_T), rs12146713 on Chr12 ( NUAK1 ), and rs2532395 on Chr17 ( MAPT , KANSL1 )(highest variant after tri-allelic rs2693333; see Supplementary Data 4 for a complete list of significant variants in this 5th MAPT genetic cluster). Bottom row, regional association plots of the top variants for the two genetic clusters in the pseudo-autosomal region PAR1 of the X chromosome: rs312238 ( XG , CD99 ) and rs2857316 ( XG )(UK Biobank has no genotyped variants on the 3’ side). Based on Human Genome build hg19. P -values are derived from a two-sided linear association test.
The first autosomal genetic cluster, on chromosome 1, included two variants (lead variant: rs6540873, β = 0.06, P = 1.71 × 10 −8 , and rs1452628, with posterior probabilities of inclusion in the causal variant set of 0.56 and 0.45, respectively) close to, and eQTL of, KCNK2 ( TREK1 ). This gene regulates immune-cell trafficking into the central nervous system, controls inflammation, and plays a major role in the neuroprotection against ischemia. Of relevance, these two loci are in particular related in UK Biobank participants with the amount of alcohol consumed, insulin levels, inflammation with interleukin-8 levels, as well as, crucially, with late-onset Alzheimer’s disease (Table 1 /Supplementary Data 1 ).
The second autosomal genetic cluster on chromosome 4 was made of 7 loci, with the lead variant rs13107325 in an exon of SLC39A8/ZIP8 ( β = 0.14, P = 2.82 × 10 −13 , posterior probability: 0.99). This locus is one of the most pleiotropic SNPs identified in GWAS, and is, amongst many other associations, related in UK Biobank with cholesterol, blood pressure, weight, inflammation with C-reactive proteins levels, diabetes with insuline-like growth factor 1 levels, alcohol intake, sleep duration, and cognitive performance/impairment, including prospective memory (Table 1 /Supplementary Data 1 ).
The third locus was an indel in chromosome 6 in an intron, and eQTL, of RUNX2 (rs35187443, β = 0.06, P = 9.03 × 10 −9 ), which plays a key role in differentiating osteoblasts, and has been very recently shown to limit neurogenesis and oligodendrogenesis in a cellular model of Alzheimer’s disease 9 .
The fourth locus was a SNP in chromosome 12, in an intron of NUAK1 (rs12146713, β = −0.10, P = 1.26 × 10 −9 ), and remarkably its top association in UK Biobank was with the contrast between schizophrenia and major depressive disorder 10 , and it was also associated with insulin-like growth factor 1 levels (Table 1 /Supplementary Data 1 ).
The final genetic autosomal genetic cluster was made of 3,906 variants in the MAPT region. Its lead non-triallelic variant, rs2532395 ( β = −0.09, P = 3.56 × 10 −15 ) was more specifically <10 kb from KANSL1 and an eQTL of KANSL1 , MAPT and other genes in brain tissues (Table 1 /Supplementary Data 1 , Supplementary Data 4 ). This locus was also associated in UK Biobank with tiredness and alcohol intake. MAPT is in 17q21.31, a chromosomal band involved with a common chromosome 17 inversion 11 . Adding chromosome 17 inversion status as a confounder reduced the significance of the association ( β = −0.15, P = 8.45 × 10 −3 ). Since the genotype for rs2532395 was also strongly correlated with chromosome 17 inversion in our dataset (Pearson correlation r = 0.98, P < 2 × 10 −16 ), this would suggest that the association between MAPT and the LIFO network is not independent from chromosome 17 inversion. As this extended genetic region is known for its pathological association with many neurodegenerative disorders including Alzheimer’s disease, we investigated whether the LIFO brain regions mediated the effect of the MAPT genetic cluster (using the lead bi-allelic variant rs2532395) on Alzheimer’s disease (see Methods). Despite small average causal mediated effect (ACME) sizes, we found a significant effect for both the dominant model (ACME β = 1.16 × 10 −4 ; 95% CI = [5.19 × 10 −5 , 1.99 × 10 −4 ]; P = 4 × 10 −5 ) and the recessive model (ACME β = 1.55 × 10 −4 ; 95% CI = [3.96 × 10 −5 , 3.74 × 10 −4 ]; P = 4 × 10 −5 ; full output of the mediation package on the dominant and recessive models in Supplementary Information ).
The two last genetic clusters of 8 and 9 variants respectively were found on the X chromosome, notably in a pseudo-autosomal region (PAR1), which is interestingly hit at a higher rate than the rest of the genome ( P = 1.56 × 10 −5 , see Supplementary Information ). The top variants for these clusters were related to two homologous genes coding for the two antigens of the XG blood group: rs312238 ( β = −0.05, P = 1.77 × 10 −10 ) ~ 10 kb from, and an eQTL of, CD99/MIC2 , and rs2857316 ( β = −0.08, P = 2.27 × 10 −29 ) in an intron and eQTL of XG (Table 1 /Supplementary Data 1 ). Since chromosome X has hardly been explored, we carried out our own association analyses between these two top variants and non-imaging variables in UK Biobank. Intriguingly, the first of these two PAR1 loci, rs312238, was found to be significantly associated in the genotyped participants who had not been scanned (out-of-sample analysis in n = 374,230 UK Biobank participants) with nitrogen dioxide air pollution, our ‘best’ MRF for pollution (see below), and many other environmental, socioeconomic, and early life factors (such as urban or rural setting, distance from the coast, place of birth, number of siblings, breastfed as a baby, maternal smoking around birth), as well as health outcomes (Supplementary Data 2 ). In particular, amongst the more easily interpretable findings of the most associated variables with rs312238, the T allele of this locus was associated with two increased measures of deprivation and/or disability (worse socioeconomic status), the ‘Townsend deprivation index’ and the ‘Health score’, but also with ‘Nitrogen dioxide air pollution’, ‘Maternal smoking around birth’, as well as ‘Number of full brothers’ and ‘Number of full sisters’, thus showing consistent signs of association between this variant and these phenotypes.
We found that the heritability of the LIFO network was significant, with h 2 = 0.15 (se = 0.01). The genetic co-heritability between the LIFO network and Alzheimer’s disease or schizophrenia was not statistically significant (coefficient of co-heritability = −0.12, se = 0.10; P = 0.23; coefficient of co-heritability = −0.16, se = 0.04, P = 0.07, respectively).
Modifiable risk factors’ associations with the vulnerable LIFO brain network
Including the modifiable risk factors (MRFs) in a single general linear model allows us to assess the unique contribution of each factor on the LIFO brain network. Not all UK Biobank participants have data available for all of the MRF variables however. An analysis limited to those with complete data for all MRFs would be biased, and based on a relatively small, low-powered sample. We addressed this issue via a two-stage analysis in which: (i) we first identified which variable within each of the 15 MRF categories best represented associations of that category with the LIFO brain network loadings (based on two criteria: significance and <5% missing values), (ii) we investigated the unique contribution of that MRF category, over and above all other categories and the dominating effects of age and sex, to the LIFO loadings.
From the first stage of our analysis, 12 of the 15 categories of MRFs had at least one ‘best’ MRF, i.e., with a significant effect on the LIFO brain network and enough non-missing values across all scanned participants to be investigated further (Table 2 /Supplementary Data 3 ). The contribution of the MRFs on the vulnerable brain network differed vastly depending on whether confounding effects of age, sex and head size were taken into account. The effect size and significance of some MRFs diminished because of some clear collinearity with the confounders. For instance, for the category of blood pressure, the most significant MRF was first “systolic blood pressure, automatic (second) reading” ( r = −0.20, P < 2.23 × 10 −308 ), but after regressing out the confounders, the ‘best’ MRF for this category was “medication for blood pressure” ( r = −0.05, P = 7.55 × 10 −22 ). Conversely, regressing out the effects of age served to unmask the significant deleterious effects of pollution on the vulnerable brain regions, such as nitrogen dioxide air pollution or particulate matter air pollution (Table 2 /Supplementary Data 3 ).
When considered together in a single model in the second stage of the analysis, 3 best MRFs had an effect on the LIFO brain network that remained significant beyond the dominating effects of age and sex, and of the 9 other best MRFs: diabetes (“diabetes diagnosed by doctor”, r = −0.05, P = 1.13 × 10 −24 ), pollution (“nitrogen dioxide air pollution in 2005”, r = −0.05, P = 5.39 × 10 −20 ) and alcohol (“alcohol intake frequency”, r = −0.04, P = 3.81 × 10 −17 ) (Table 3 ). No MRFs showed any bias in their sub-sampling distribution, i.e., any significant difference between the original sample and the reduced sample of 35,527 participants who had values for all 18 variables considered (the 12 best MRFs and 6 confounders: age, sex, age 2 , age × sex, age 2 × sex, head size; Supplementary Information ). In total, the 12 best MRFs explained 1.5% of the effect on the vulnerable brain network ( F 12;35509 = 43.5).
While 6 out of the 7 genetic clusters associated with the LIFO network were correlated with many variables related to each of the 15 MRF categories, including diabetes, alcohol consumption and traffic pollution (Supplementary Data 1 ), we also found some genetic overlap between the very specific best MRF of “alcohol intake frequency” and the LIFO network in the pleiotropic rs13107325 variant (cluster 2), as well as rs17690703, part of the large genetic cluster 5 in MAPT (Supplementary Data 4 ). No genetic overlap was found for the precise “nitrogen dioxide air pollution in 2005” or “diabetes diagnosed by doctor”, nor for approximate variables.
This study reveals, in a cohort of nearly 40,000 UK Biobank participants, the genetic and modifiable risk factors’ associations with brain regions in a ‘last in, first out’ (LIFO) network that show earlier and accelerated ageing and are particularly vulnerable to disease processes such as that of Alzheimer’s disease 8 . Seven genetic clusters, two of which in the pseudo-autosomal region of the sex chromosomes coding for two antigens of the XG blood system, were found significantly associated and replicated genome-wide. In addition, after accounting for age and sex effects, diabetes, traffic-related pollution and alcohol were the most deleterious modifiable risk factors (MRFs) on these particularly vulnerable brain regions.
Three lead variants for our significant genetic clusters have been previously associated with ageing-related brain imaging measures in recent studies: one, in cluster 1, an eQTL of KCNK2 ( TREK1 ) 12 , 13 , whose increase in expression mediates neuroprotection during ischemia 14 , the ubiquitous rs13107325 (cluster 2), and one, in cluster 4, in an intron of NUAK1 ( ARK5 ) 15 , 16 , 17 , which has been associated with tau pathology 18 (Table 1 /Supplementary Data 1 ). On the other hand, of the seven genetic clusters, three were entirely novel (clusters 3, 6 and 7), and not found in other brain imaging studies, including our most recent work that expanded on our previous GWAS of all of the brain IDPs available in UK Biobank 19 by including more participants—in fact, the same number of participants as analysed in this present work—and, crucially, by also including the X chromosome 20 (Table 1 /Supplementary Data 1 ). This suggests that, beyond the genetic hits that were meaningfully associated with the LIFO brain network and an array of relevant risk factors, lifestyle variables and brain disorders, and found in a few other imaging GWAS, some of the genetic underpinnings of the LIFO network are intrinsically specific to it and to no other pre-existing imaging phenotype.
All five autosomal genetic clusters identified through the GWAS of the LIFO phenotype had relevant associations with risk factors for dementia (Results; Supplementary Data 1 ), including precisely two of the best MRFs (for clusters 2 and 5), and three of them directly related in UK Biobank to the two diseases showing a pattern of brain abnormalities following the LIFO network: schizophrenia (clusters 2 and 4) and Alzheimer’s disease (cluster 1) (Supplementary Data 1 ). In particular, cluster 2 has its lead variant rs13107325 in an exon of one of the most pleiotropic genes ZIP8 , which codes for a zinc and metal transporter. Considering the vulnerability of the LIFO brain network to adolescent-onset schizophrenia and its significant association with fluid intelligence that we previously demonstrated 8 , it is notable that this variant has been associated genome-wide with schizophrenia 6 , as well as intelligence, educational attainment and mathematics ability 5 , 21 . In line with the LIFO brain network being both prone to accelerated ageing and susceptible to Alzheimer’s disease, this genetic locus has also been associated genome-wide with well-known risk factors for dementia. These comprise alcohol—including the exact same variable of “alcohol intake frequency” as identified as one of the best MRFs—cholesterol, weight, sleep—including “sleep duration”—and blood pressure 22 , 23 , 24 , 25 , 26 , all of which significantly contribute to modulating the LIFO brain network when considered separately (Table 2 /Supplementary Data 3 ). Of relevance, this genetic locus is also associated to an increased risk of cardiovascular death 27 . Cluster 5, a large genetic cluster in the MAPT region (Microtubule-Associated Protein Tau), comprised in total 3906 significant variants (Supplementary Data 4 ). This genetic region plays a role in various neurodegenerative disorders related to mutations of the protein tau, such as frontotemporal dementia 28 and progressive supranuclear palsy 29 , but also, of particular pertinence to the LIFO brain network, Alzheimer’s and Parkinson’s disease, with a genetic overlap between these two diseases in a locus included in our significant cluster 5 (rs393152, β = −0.09, P = 6.35 × 10 −14 ) 4 . Despite the relatively low number of people with diagnosed Alzheimer’s disease in the genetic discovery cohort, we were able to establish—albeit with small effect sizes—a significant mediation role for the LIFO brain regions between the lead bi-allelic variant for cluster 5 and this Alzheimer’s diagnosis, suggesting once more the importance played by these vulnerable brain areas in unhealthy ageing.
Finally, of the seven clusters, two were located in the pseudo-autosomal region (PAR1) of the sex chromosomes corresponding to the genes XG and CD99 , coding for the two antigens of the XG blood group. This blood group system has been largely neglected, its main contribution related to the mapping of the X chromosome itself, and its clinical role remains elusive 30 . In order to investigate further the possible role of these two variants of the XG blood group, we examined out-of-sample their associations with thousands of non-imaging phenotypes. This analysis revealed that the first of these two loci was significantly and consistently associated with early life factors, environmental factors and health outcomes, including particulate matter and nitrogen dioxide air pollution, the second most deleterious MRF to the LIFO brain network (Supplementary Data 2 ). Whether these associations are due to stratification or genotyping artefacts, or to the fact that this specific variant, which is inherited from a parent, has a parental impact that modulates the effect of early life environment of the UK Biobank participants, the so-called “nature of nurture”, will need further investigation 31 .
Intriguingly, an analysis revealed that the genes involved in the loci associated with the LIFO network (Table 1 /Supplementary Data 1 ) are enriched for the gene ontology terms of leucocyte extravasation, namely “positive regulation of neutrophil extravasation” ( P = 4.75 × 10 −6 ) and “T cell extravasation” ( P = 4.75 × 10 −6 ). This result held when removing the genes included in the MAPT extended region (with P = 2.54 × 10 −6 and P = 2.54 × 10 −6 , respectively). Leucocyte extravasation facilitates the immune and inflammatory response, and there has been renewed focus on the fact that a breakdown of the blood-brain barrier together with leukocyte extravasation might contribute to both Alzheimer’s disease and schizophrenia 32 , 33 . In line with the enrichment findings, 4 out of the 7 genetic clusters associated with the LIFO network are correlated in UK Biobank blood assays with percentage or count of immune cells (neutrophil, lymphocyte, platelet, monocyte, etc.; Supplementary Data 1 ).
Regarding MRFs’ effects on the LIFO brain network, diabetes and alcohol consumption have been consistently shown to be associated with both cerebral and cognitive decline 34 , 35 . On the other hand, pollution—and notably that of nitrogen oxides—has emerged more recently as a potential MRF for dementia 2 , 36 . In particular, the increase of dementia risk due to nitrogen oxide pollution, a proxy for traffic-related air pollution, seems to be enhanced by cardiovascular disease 37 . In this study, we found that nitrogen dioxide pollution has one of the most deleterious effects onto the fragile LIFO brain regions. This effect could only be unmasked by regressing out the effects of age and sex, as traffic-related air pollution is modestly inversely-correlated with age (Supplementary Data 5 ). It is also worth noting that including age and sex as confounding variables in the first stage of our analysis reduced considerably the contribution of what had appeared at first—before regression—as the most harmful risk factors: blood pressure, cholesterol and weight (Table 2 /Supplementary Data 3 ). Furthermore, the benefit of examining these MRFs in a single model in the second stage of our analysis is that we can assess the unique contribution of each of these factors on the LIFO brain network; in doing so, blood pressure, cholesterol and weight were no longer significant (Table 3 ).
One defining characteristic of the LIFO brain network is how much age explains its variance. Indeed, in the dataset covering most of the lifespan that was initially used to identify the LIFO and spatially define it 8 , age explained 50%. In the UK Biobank imaging project, where imaged participants are over 45 years old, age explained 30% (Fig. 1 ). It is thus perhaps unsurprising that, while the explained variance by each of the MRFs varies widely (Table 2 /Supplementary Data 3 ), it reduces notably once the effect of age and other confounders has been regressed out (without confounders included in the model: maximum 8.4%; with confounders: maximum 0.5%). Combined, the 12 best MRFs explained a significant 1.5% of the effect on the vulnerable brain network after regressing out age, head size and sex effects. Regarding the genetic hits, we found a significant heritability with h 2 = 0.15, in keeping with our results for structural brain phenotypes (except for subcortical and global brain volumes, which demonstrate higher heritability 19 ).
The uniqueness of this study relies on the fact that we combined the strengths of two different cohorts: the first, which revealed the LIFO grey matter network, is lifespan, demonstrating the mirroring of developmental and ageing processes in the LIFO brain areas, something that could never be achieved with UK Biobank because of its limited age range. Of note, for this initial work with the lifespan cohort 8 , we not only included grey matter partial volume images, as done in this current study, but also Freesurfer information of cortical thickness and surface area. The LIFO network showed no contribution from Freesurfer cortical thickness or area. This might hint at processes that only partial volume maps are able to detect due to the LIFO network’s specific localisation, including in the cerebellum and subcortical structures, which are not included in the area and thickness surface methods from Freesurfer.
Limitations of our study pertain to the nature of the data itself and the way each variable is encoded in the UK Biobank (binary, ordinal, categorical, continuous), the number of missing values, what is offered as variables for each modifiable risk factor category (e.g. we chose not to create any compound variables, such as the ratio of cholesterol levels or systolic and diastolic blood pressures), and the curation of each of these variables. Some of the factors might be proxies for another category, but including the ‘best’ ones in a single model alleviate these issues to some extent. Another limitation is the assumption in our models that each risk factor has a linear, additive effect on the vulnerable LIFO brain network. It is also important to note that cross-sectional and longitudinal patterns of brain ageing can differ, as has been shown for instance for adult span trajectories of episodic and semantic memory, especially in younger adults 38 . A recent study has also demonstrated a specific ‘brain age’ imaging measure to be more related to early life influences on brain structure than within-person rates of change in the ageing brain 39 . Further work will be needed to establish how the LIFO network data changes in terms of within-person trends, for instance by investigating the growing UK Biobank longitudinal imaging database. While we took care of assessing the replicability of our genetic results by randomly assigning a third of our dataset for such purposes (all our significant genetic hits were replicated), this was performed within the UK Biobank cohort that exhibits well-documented biases, being well-educated, less deprived, and healthier than the general population, especially for its imaging arm 40 . Independent replications will be needed to confirm the existence of the LIFO-associated genetic loci.
In conclusion, our study reveals the modifiable and non-modifiable factors associated with some of the most fragile parts of the brain particularly vulnerable to ageing and disease process. It shows that, above and beyond the effect of age and sex, the most deleterious modifiable risk factors to this brain network of higher-order regions are diabetes, pollution and alcohol intake. Genetic factors are related to immune and inflammatory response, tau pathology, metal transport and vascular dysfunction, as well as to the XG blood group system from the pseudo-autosomal region of the sex chromosomes, and meaningfully associated with relevant modifiable risk factors for dementia. The unprecedented genome-wide discovery of the two variants on the sex chromosomes in this relatively unexplored blood group opens the way for further investigation into its possible role in underlying unhealthy ageing.
Supplementary Information is available for this paper.
For the present work the imaging cohort of UK Biobank was used and we included 39,676 subjects who had been scanned and for whom the brain scans had been preprocessed at the time of the final set of analyses (M/F 47–53%; 44–82 years, mean age 64 ± 7 years; as of October 2020) 41 , 42 . Structural T1-weighted scans for each participant were processed using the FSL-VBM automated tool to extract their grey matter map 43 , 44 . The ‘last in, first out’ (LIFO) network of mainly higher-order brain regions was initially identified by performing a linked independent component analysis on the grey matter images of another, lifespan observational cohort of 484 subjects 8 , 45 , 46 . This map of interest, along with the other 69 generated by the analysis, was first realigned to the UK Biobank ‘standard’ space defined by the grey matter average across the first 15,000 participants, then regressed into the UK Biobank participants’ grey matter data, to extract weighted average values of grey matter normalised volume inside each of the z-maps, using the z-score as weighting factor. This made it possible to assess the unique contribution of this specific LIFO map, above and beyond all the rest of the brain represented in the other 69 maps. At the end of this process, we obtained a single imaging measure for each of the 39,676 participants, i.e. a ‘loading’ corresponding to their amount of grey matter normalised volume in the LIFO brain network.
Human participants: UK Biobank has approval from the North West Multi-Centre Research Ethics Committee (MREC) to obtain and disseminate data and samples from the participants ( http://www.ukbiobank.ac.uk/ethics/ ), and these ethical regulations cover the work in this study. Written informed consent was obtained from all of the participants.
Modifiable risk factors selection
The following 15 categories of modifiable risk factors (MRFs) for dementia were investigated based on previous literature: blood pressure, diabetes, cholesterol, weight, alcohol, smoking, depression, hearing, inflammation, pollution, sleep, exercise, diet/supplementation, socialisation, and education. These included well-documented cerebrovascular risk factors, and in particular included all of the 12 modifiable risk factors considered in the updated Lancet commission on dementia, with the sole exception of traumatic brain injury 3 . For each category, several MRF variables from UK Biobank were very minimally pre-processed ( Supplementary Information ). In total, 161 MRF variables were obtained. To optimise the interpretability of the results, and to be able to relate them to previous findings, we did not carry out any data reduction, which would have prevented us from identifying exactly which variable—and subsequently, which genetic component for this specific variable—contribute to the effect. For these same reasons, we did not create any compound variable.
Statistical analyses
Genome-wide association study.
We followed the same protocol we had developed for the first genome-wide association study (GWAS) with imaging carried out on UK Biobank 19 . Briefly, we examined imputed UK Biobank genotype data 47 , and restricted the analysis to samples that were unrelated (thereby setting aside only ~450 participants), without aneuploidy and with recent UK ancestry. To account for population stratification, 40 genetic principal components were used in the genetic association tests as is recommended for UK Biobank genetic studies 19 , 20 , 47 . We excluded genetic variants with minor allele frequency <0.01 or INFO score <0.03 or Hardy-Weinberg equilibrium –log 10 ( P ) > 7. We then randomly split the samples into a discovery set with 2/3 of the samples ( n = 22,128) and a replication set with 1/3 of the samples ( n = 11,083). We also examined the X chromosome with the same filters, additionally excluding participants with sex chromosome aneuploidy: 12 in non-pseudoautosomal region (PAR) and 9 in PAR for the discovery set, 3 in non-PAR and 6 in PAR for the replication set. Variants were considered significant at –log 10 ( P ) > 7.5, and replicated at P < 0.05.
Modifiable risk factor study
In the first stage, the general linear model was used to investigate, separately, the association between each of these 161 MRFs and the LIFO network loadings in all the scanned UK Biobank participants ( n = 39,676). We ran each model twice: once as is, and once adding 6 confounders: age, age 2 , sex, age × sex, age 2 × sex, and head size, to estimate the contribution of these MRFs on the LIFO network above and beyond the dominating effects of age and sex. Sex was based on the population characteristics entry of UK Biobank. This is a mixture of the sex the NHS had recorded for the participant at recruitment, and updated self-reported sex. For the GWAS, both sex and genetic sex were used (the sample was excluded in case of a mismatch). In total, 32 variables tailored to structural imaging had been considered as possible confounders, and we retained those with the strongest association ( R 2 ≥ 0.01; see Supplementary Information ). Socioeconomic status via the Townsend deprivation index was also considered as a possible confounding variable but explained little variance ( R 2 < 0.001) and thus was not included as a confounder.
MRFs were not considered further if they were not significant—not surviving Bonferroni-correction, i.e., P > 1.55 × 10 −4 —and if more than 5% of the subjects had their MRF values missing. For each category, a single ‘best’ MRF was then selected as the variable with the highest R 2 among those remaining, after regressing out the confounding effects of age and sex.
In the second stage, all these best MRFs were then included in a single general linear model, together with the same 6 confounders used in the first stage, to assess the unique contribution of each factor on the LIFO brain network loadings. A prerequisite to carry out this single general linear model analysis was to only include participants who would have values for all best MRFs and confounders. This explains the additional criterion of only including MRFs that had no more than 5% of values missing, to ensure that the final sample of participants who had values for all these best and confounding factors would not be biased compared with the original sample—something we formally tested (see Supplementary Information )—especially as data are not missing at random in UK Biobank, and exhibit some genetic structure 48 . The sample was therefore reduced to a total of 35,527 participants for this second stage analysis (M/F 17,290–18,237; 45–82 years, mean 64 ± 7 years). The effect of these best MRFs taken altogether was considered significant with a very conservative Bonferroni correction for multiple comparisons across all combinations of every possible MRF from each of the initial 15 MRF categories ( P < 4.62 × 10 −17 , see Supplementary Information for more details). In addition, both full and partial correlations were computed for the same set of best MRFs and confounders, in order to assess possible relationships between variables.
Post hoc genetic analyses
Chromosome 17 inversion.
We investigated chromosome 17 inversion status of the participants in the discovery cohort by considering their genotype on 32 variants that tag chromosome 17 inversion according to Steinberg et al. 11 . Of these 32 variants, 24 were present in our genetic data. We labelled the participants homozygous inverted, heterozygous, or homozygous direct (not inverted) when all 24 of these alleles indicated the same zygosity. This yielded an unambiguous inversion status for 21,969 participants (99% of the discovery cohort). To examine if the association between the non-triallelic lead variant of the MAPT genetic cluster (rs2532395, Table 1 /Supplementary Data 1 ) and the LIFO network was independent from this common inversion, we determined inversion/direct status of the discovery cohort and: 1. repeated the association test between rs2532395 and the LIFO phenotype, with chromosome 17 inversion status added as a confounder; and 2. correlated the genotype for rs2532395 with chromosome 17 inversion.
Causality within each genetic cluster
We used CAVIAR (Causal Variants Identification in Associated Regions 49 ) to assess causality of variants that passed the genome-wide significance threshold in each of the genetic clusters we report. CAVIAR uses a Bayesian model and the local linkage disequilibrium structure to assign posterior probabilities of causality to each variant in a region, given summary statistics for an association. We did not perform CAVIAR analysis on the genetic cluster on chromosome 17, as its non-triallelic lead variant (rs2532395) was strongly correlated with chromosome 17 inversion, and the LD matrix was large and low rank. We excluded the X chromosome loci from this analysis due to the difficulty in assessing LD in this chromosome.
Enrichment analysis
Based on the genes listed in the ‘Genes’ column of Table 1 /Supplementary Data 1 , we performed an enrichment analysis for the genes associated with the LIFO brain network using PANTHER 50 . PANTHER determines whether a gene function is overrepresented in a set of genes, according to the gene ontology consortium 51 , 52 .
Mediation analysis between MAPT top variant and Alzheimer’s disease, via the LIFO brain network
As the gene MAPT is associated with Alzheimer’s disease, and as we found a significant association between MAPT and the LIFO brain network, we examined to what extent the effect of MAPT is mediated by the LIFO brain regions. We conducted a mediation analysis using the counterfactual framework in which the average indirect effect of the treatment on the outcome through the mediator is nonparametrically identified (version 4.5.0 of the R package ‘mediation' 53 ). This is a general approach that encompasses the classical linear structural equation modelling framework for causal mediation, allowing both linear and non-linear relationships. In this analysis, the genotype for the lead bi-allelic variant of the MAPT association was used as the treatment, the LIFO loadings as the mediator, and Alzheimer’s disease diagnosis as the outcome.
From the ~43 K UK Biobank participants who had been scanned, we searched for those who had been diagnosed with Alzheimer’s disease specifically, regardless of whether this diagnosis occurred before, or after their brain scans. Based on hospital inpatient records (ICD10: F000, F001, F002, F009, G300, G301, G308, and G309 and ICD9: 3310) and primary care (GP) data (Eu00., Eu000, Eu001, Eu002, Eu00z, F110., F1100, F1101, Fyu30, X002x, X002y, X002z, X0030, X0031, X0032, X0033, XaIKB, XaIKC, and XE17j), we identified 65 such cases— UK Biobank being healthier than the general population, and those scanned showing an even stronger healthy bias—of which 34 were included in the discovery set after QC.
We considered two conditions for the effect of the treatment on the outcome. First, a dominant condition in which the minor allele is assumed to be dominant and for which at least one copy of the minor allele is considered treated. Second, a recessive condition in which the minor allele is assumed to be recessive. We considered that either condition was nominally significant if the confidence interval of the average causal mediated effect did not intersect zero, and had an associated P < 0.05 ÷ 2 (correcting for the two conditions). We assessed confidence intervals and P -values using 50,000 bootstrapped samples.
Associations between the LIFO brain network’s genetic hits and the MRFs
First, we reported in Table 1 / Supplementary Data 1 the significant associations between the LIFO genetic hits and UK Biobank variables related to the 15 categories listed for the MRFs. For this, we used the Open Targets Genetics website, which reports the GWAS carried out in UK Biobank ( https://genetics .opentargets.org/ ). Second, we assessed whether there was any genetic overlap between the known genetic components of the 3 best MRFs and the LIFO phenotype. Again, we used the Open Targets Genetics website outputs for these 3 very specific UK Biobank variables, and compared the significant hits for these 3 best MRFs within ±250 kbp of, or in high LD (>0.8) with, our own LIFO variants. If reported hits were limited, we also searched online for GWAS done on similar variables. Finally, we also included the list of significant hits for diabetes 54 , which focused on a potential genetic overlap between diabetes and Alzheimer’s disease.
Post hoc association for the sex chromosomes variants
The allele counts of each participant for two specific significant variants of the sex chromosomes not—or hardly—available in open databases such as https://genetics.opentargets.org/ 55 were further associated out-of-sample with all non-imaging phenotypes of UK Biobank ( n = 16,924). This analysis was carried out in the entire genotyped, quality-controlled sample where participants who had been scanned were removed (final sample: 374,230 participants), taking into account the population structure (40 genetic principal components), as well as the confounding effects of age, sex, age x sex, age 2 and age 2 x sex. Results were corrected for multiple comparisons across all non-imaging phenotypes and the two variants.
Heritability
We examined the heritability of the LIFO phenotype, and the coheritability between the LIFO network and Alzheimer’s disease or schizophrenia using LDSC 56 . This method uses regression on summary statistics to determine narrow sense heritability h 2 of a trait, or the shared genetic architecture between two traits. LDSC corrects for bias LD structure using LD calculated from a reference panel (we used LD from the Thousand Genomes Project Phase 1 57 ). We obtained summary statistics for a meta-analysis of Alzheimer’s disease involving 71,880 cases and 383,378 controls 58 . The number of genetic variants in the intersection between the summary statistics was 1,122,435. For schizophrenia, the summary statistics were obtained from a meta-analysis involving 53,386 cases and 77,258 controls 59 . A total of 1,171,319 genetic variants were in the intersection with the summary statistics for LIFO. For both Alzheimer’s and schizophrenia, the X chromosome was not included in the heritability calculation, as it was excluded from the meta-analysis that we sourced the summary statistics from.
Reproducibility
No data was excluded for the MRF analyses. For the genetic analyses, these were restricted to samples that were unrelated, without aneuploidy and with recent UK ancestry (see above).
No statistical method was used to predetermine sample size. The experiments were not randomised. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.

Data availability
All the FLICA decomposition maps − including the LIFO grey matter network − in UK Biobank standard space, the UK Biobank grey matter template, scripts, and the LIFO loadings for all of the participants are freely available on a dedicated webpage: open.win.ox.ac.uk/pages/douaud/ukb-lifo-flica/ .
Baumgart, M. et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11 , 718–726 (2015).
Article PubMed Google Scholar
Chen, H. et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ. Int. 108 , 271–277 (2017).
Article CAS PubMed Google Scholar
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396 , 413–446 (2020).
Article PubMed PubMed Central Google Scholar
Desikan, R. S. et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol. Psychiatry 20 , 1588–1595 (2015).
Article CAS PubMed PubMed Central Google Scholar
Hill, W. D. et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 24 , 169–181 (2019).
Pardinas, A. F. et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 50 , 381–389 (2018).
Douaud, G. et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl Acad. Sci. USA 110 , 9523–9528 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Douaud, G. et al. A common brain network links development, aging, and vulnerability to disease. Proc. Natl Acad. Sci. USA 111 , 17648–17653 (2014).
Nakatsu, D. et al. BMP4-SMAD1/5/9-RUNX2 pathway activation inhibits neurogenesis and oligodendrogenesis in Alzheimer’s patients’ iPSCs in senescence-related conditions. Stem Cell Rep. 18 , 1246 (2023).
Article CAS Google Scholar
Peyrot, W. J. & Price, A. L. Identifying loci with different allele frequencies among cases of eight psychiatric disorders using CC-GWAS. Nat. Genet. 53 , 445–454 (2021).
Steinberg, K. M. et al. Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat. Genet. 44 , 872–880 (2012).
Le Guen, Y. et al. eQTL of KCNK2 regionally influences the brain sulcal widening: evidence from 15,597 UK Biobank participants with neuroimaging data. Brain Struct. Funct. 224 , 847–857 (2019).
Jonsson, B. A. et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 10 , 5409 (2019).
Heurteaux, C. et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 23 , 2684–2695 (2004).
Vojinovic, D. et al. Genome-wide association study of 23,500 individuals identifies 7 loci associated with brain ventricular volume. Nat. Commun. 9 , 3945 (2018).
Article ADS PubMed PubMed Central Google Scholar
Zhao, B. et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat. Genet. 51 , 1637–1644 (2019).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits ( n = 17,706). Mol. Psychiatry https://doi.org/10.1038/s41380-019-0569-z (2019).
Lasagna-Reeves, C. A. et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92 , 407–418 (2016).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562 , 210–216 (2018).
Smith, S. M. et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci. 24 , 737–745 (2021).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50 , 1112–1121 (2018).
Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 , 707–713 (2010).
Sanchez-Roige, S. et al. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. Am. J. Psychiatry 176 , 107–118 (2019).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10 , 1100 (2019).
International Consortium for Blood Pressure Genome-Wide Association, S. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 , 103–109 (2011).
Article ADS Google Scholar
Johansson, A. et al. Genome-wide association and Mendelian randomization study of NT-proBNP in patients with acute coronary syndrome. Hum. Mol. Genet. 25 , 1447–1456 (2016).
Poorkaj, P. et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43 , 815–825 (1998).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8 , 711–715 (1999).
Johnson, N. C. XG: the forgotten blood group system. Immunohematology 27 , 68–71 (2011).
Kong, A. et al. The nature of nurture: Effects of parental genotypes. Science 359 , 424–428 (2018).
Zenaro, E., Piacentino, G. & Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 107 , 41–56 (2017).
Pong, S., Karmacharya, R., Sofman, M., Bishop, J. R. & Lizano, P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry 6 , 30–46 (2020).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Veldsman, M. et al. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat. Commun. 11 , 4340 (2020).
Power, M. C., Adar, S. D., Yanosky, J. D. & Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56 , 235–253 (2016).
Grande, G., Ljungman, P. L. S., Eneroth, K., Bellander, T. & Rizzuto, D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 77 , 801–809, (2020).
Ronnlund, M., Nyberg, L., Backman, L. & Nilsson, L. G. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol. Aging 20 , 3–18 (2005).
Vidal-Pineiro, D. et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. Elife https://doi.org/10.7554/eLife.69995 (2021).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186 , 1026–1034 (2017).
Alfaro-Almagro, F. et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166 , 400–424 (2018).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19 , 1523–1536 (2016).
Good, C. D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14 , 21–36 (2001).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130 , 2375–2386 (2007).
Groves, A. R. et al. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. NeuroImage 63 , 365–380 (2012).
Smith, S. et al. Structural variability in the human brain reflects fine-grained functional architecture at the population level. J. Neurosci. 39 , 6136–6149 (2019).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562 , 203–209 (2018).
Mignogna, G. et al. Patterns of item nonresponse behaviour to survey questionnaires are systematic and associated with genetic loci. Nat. Hum. Behav . https://doi.org/10.1038/s41562-023-01632-7 (2023).
Hormozdiari, F., Kostem, E., Kang, E. Y., Pasaniuc, B. & Eskin, E. Identifying causal variants at loci with multiple signals of association. Genetics 198 , 497–508 (2014).
Thomas, P. D. et al. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 31 , 8–22 (2022).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25 , 25–29 (2000).
Gene Ontology, C. et al. The Gene Ontology knowledgebase in 2023. Genetics https://doi.org/10.1093/genetics/iyad031 (2023).
Imai, K., Keele, L. & Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 15 , 309–334 (2010).
Hardy, J., de Strooper, B. & Escott-Price, V. Diabetes and Alzheimer’s disease: shared genetic susceptibility? Lancet Neurol. 21 , 962–964 (2022).
Carvalho-Silva, D. et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 47 , D1056–D1065 (2019).
Gazal, S., Marquez-Luna, C., Finucane, H. K. & Price, A. L. Reconciling S-LDSC and LDAK functional enrichment estimates. Nat. Genet. 51 , 1202–1204 (2019).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Jansen, I. E. et al. Author Correction: Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 52 , 354 (2020).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604 , 502–508 (2022).
Laird, A. R. et al. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinform. 3 , 23 (2009).
Download references
Acknowledgements
We are grateful to Profs Christian K. Tamnes, Lars T. Westlye, Kristine B. Walhovd and Anders M. Fjell, and Dr Andreas Engvig for providing the lifespan cohort which was used to initially derive the original ‘last in, first out’ brain network map, and to Prof Augustine Kong for helpful discussion on the associations between the PAR hit and early life and environmental factors. G.D. was supported by a UK MRC Career Development Fellowship (MR/K006673/1) and a Wellcome Collaborative Award (215573/Z/19/Z). S.S. was supported by Wellcome (203139/Z/16/Z; 215573/Z/19/Z). L.E. was funded by NSERC grants (RGPIN/05484-2019; DGECR/00118-2019) and a Michael Smith Health Research BC Scholar Award. A.M.W. received support through the NIH Intramural Research Program (ZIA-MH002781; ZIA-MH002782). This research was funded in whole, or in part, by the Wellcome Trust (215573/Z/19/Z; 203139/Z/16/Z; 203139/A/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This research was also supported by the NIHR Oxford Health Biomedical Research Centre (NIHR203316). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z and 203139/A/16/Z).
Author information
These authors contributed equally: Lloyd T. Elliott, Anderson M. Winkler.
Authors and Affiliations
FMRIB Centre, Wellcome Centre for Integrative Neuroimaging (WIN), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
Jordi Manuello, Paul McCarthy, Fidel Alfaro-Almagro, Soojin Lee, Stephen Smith & Gwenaëlle Douaud
FOCUS Lab, Department of Psychology, University of Turin, Turin, Italy
Jordi Manuello
Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby, Canada
Joosung Min & Lloyd T. Elliott
Pacific Parkinson’s Research Centre, The University of British Columbia, Vancouver, BC, Canada
National Institutes of Mental Health, National Institutes of Health, Bethesda, MD, USA
Anderson M. Winkler
Department of Human Genetics, University of Texas Rio Grande Valley, Brownsville, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
G.D. conceived and supervised the work, and carried out some of the genetic and modifiable risk factors analyses. J.Ma. carried out most of the genetic and modifiable risk factors analyses. J.Mi., S.L., A.M.W., and L.T.E. carried out additional genetics analyses. G.D., P. McC., F.A.-A., S.S., and L.T.E. created/extracted the imaging and genetics data, and organised the non-imaging data and confound variables. L.T.E. co-supervised the genetic analyses. A.M.W. co-supervised the modifiable risk factor analyses. G.D. interpreted the results and wrote the paper. J.Ma., S.S., L.T.E., and A.M.W. revised the paper.
Corresponding author
Correspondence to Gwenaëlle Douaud .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Xavier Caseras and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1-5, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Manuello, J., Min, J., McCarthy, P. et al. The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease. Nat Commun 15 , 2576 (2024). https://doi.org/10.1038/s41467-024-46344-2
Download citation
Received : 15 February 2023
Accepted : 22 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1038/s41467-024-46344-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
ORIGINAL RESEARCH article
Clinical utility of plasma aβ42/40 ratio by lc-ms/ms in alzheimer’s disease assessment.

- 1 Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, United States
- 2 Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL, United States
- 3 Wien Center for Alzheimer's Disease and Memory Disorders, Mount Sinai Medical Center, Miami Beach, FL, United States
- 4 Department of Neurology, Center for Translational Research in Neurodegenerative Disease, 1Florida Alzheimer’s Disease Research Center (ADRC), University of Florida, Gainesville, FL, United States
- 5 Department of Pharmacology and Chemical Biology, Department of Neurology, Emory Center for Neurodegenerative Disease, Emory University, Atlanta, GA, United States
Introduction: Plasma Aβ42/40 ratio can help predict amyloid PET status, but its clinical utility in Alzheimer’s disease (AD) assessment is unclear.
Methods: Aβ42/40 ratio was measured by LC-MS/MS for 250 specimens with associated amyloid PET imaging, diagnosis, and demographic data, and for 6,192 consecutive clinical specimens submitted for Aβ42/40 testing.
Results: High diagnostic sensitivity and negative predictive value (NPV) for Aβ-PET positivity were observed, consistent with the clinical performance of other plasma LC-MS/MS assays, but with greater separation between Aβ42/40 values for individuals with positive vs. negative Aβ-PET results. Assuming a moderate prevalence of Aβ-PET positivity, a cutpoint was identified with 99% NPV, which could help predict that AD is likely not the cause of patients’ cognitive impairment and help reduce PET evaluation by about 40%.
Conclusion: High-throughput plasma Aβ42/40 LC-MS/MS assays can help identify patients with low likelihood of AD pathology, which can reduce PET evaluations, allowing for cost savings.
1 Introduction
Alzheimer’s disease (AD) pathophysiology is characterized by cognitive impairment and the accumulation of extracellular beta-amyloid (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein in brain tissue ( 1 – 3 ). Because Aβ aggregation and deposition occurs 10–20 years prior to clinical presentation, plaque detection reflects an underlying pathophysiologic process at a prodromal or pre-clinical disease stage ( 4 – 6 ) and a potential opportunity for early intervention and treatment. In addition, ruling out AD can prompt investigation of non-AD causes of dementia and cognitive decline ( 7 ).
Recently, the United States Food and Drug Administration (FDA) approved the first disease-modifying treatments for AD, using monoclonal antibodies targeting Aβ aggregates and removing them from the brain. Administering monoclonal antibodies that target Aβ slowed the rate of functional and cognitive decline among patients with AD in the mild cognitive impairment (MCI) or mild dementia stage. Presence of Aβ protein was assessed using positron emission tomography (PET) ( 8 , 9 ). PET and measurement of cerebrospinal fluid (CSF) beta-amyloid 42 (Aβ42) ( 10 , 11 ) are methods that have been used as entry criteria for clinical trials and/or as outcome measures for disease-modifying AD treatments ( 12 ).
Blood-based biomarkers, including the plasma beta-amyloid ratio (Aβ42/40), may guide, complement, or be alternatives to PET and CSF testing. However, despite its lower cost and decreased invasive nature, routine clinical use of plasma Aβ42/40 testing has been suggested to have substantial challenges ( 13 ).
Accurately determining plasma Aβ42/40 ratio depends on a high degree of assay robustness, which is characterized by minimal preanalytical errors, imprecision, and bias. Lack of robustness can potentially lead to a high risk for misclassification, particularly when differences in the Aβ42/40 ratio are small between amyloid PET-positive and PET-negative individuals. Recently, the Elecsys electrochemiluminescence (ECL) immunoassay was evaluated for robustness using specimens from a cohort with about a 10% difference in Aβ42/40 means between amyloid PET-positive and PET-negative individuals. A simulated 10% imprecision lead to a misclassification rate of 26% and a simulated 10% bias (22% bias in total if Aβ42 and Aβ40 values drifted in opposite directions) led to almost all PET-positive individuals being reclassified as PET-negative ( 13 ). Despite these concerns, no liquid chromatography–tandem mass spectrometry (LC-MS/MS) platform has been fully evaluated for assay robustness ( 13 ), even though head-to-head comparisons have shown superior diagnostic accuracy for the best performing LC-MS/MS assays vs. immunoassays in identifying amyloid PET status ( 13 , 14 ).
We developed and validated a protein immunoprecipitation (IP)-LC-MS/MS assay for the detection of plasma Aβ40 and Aβ42 ( 15 ). Here we assessed the clinical performance of using the Aβ42/40 ratio to identify amyloid PET status in a well-characterized cohort that included age-matched Aβ PET-positive and Aβ PET-negative individuals characterized as AD, MCI, or healthy controls with APOE genotype inferred from apolipoprotein E (ApoE) proteoform. Part of the assessment included examining the effect of imprecision and bias estimates on classifying associated risk for PET positivity ( 13 ). Based on these performance characteristics, we evaluated incorporating Aβ42/Aβ40 results into AD assessment using a limited data set from 6,192 specimens submitted for Aβ42/Aβ40 ratio testing to Quest Diagnostics.
2.1 Study design and clinical assessments
This cross-sectional study involved 250 participants who provided plasma specimens at the 1Florida Alzheimer’s Disease Research Center (ADRC cohort). The study was approved by the Mount Sinai Medical Center IRB, and all participants provided informed consent. Participants were between the ages of 50 and 95 years, had a minimum 6th grade reading comprehension level, and spoke English or Spanish as their primary language. Participants were excluded from the study if they had significant visual or auditory deficits or non-AD medical or psychiatric illness.
Information regarding concurrent medications for AD participants during initial and annual follow-up visits was obtained in accordance with the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set version 3 (UDSv3) protocol. 1 Most were taking a cholinesterase inhibitor, namely donepezil, galantamine or rivastigmine. A few AD participants were also taking memantine. Anti-amyloid therapies were not available at time of specimen collection.
In the ADRC cohort, all participants underwent clinical assessments including an extensive medical, neurological, psychiatric, and neuropsychological evaluation. Assessments included the clinical dementia rating (CDR) ( 16 ) and the mini-mental state exam (MMSE) ( 17 ), and cognitive diagnoses were carried out using a previously reported algorithmic procedure ( 18 ). Participants were categorized as healthy controls (HC), having mild cognitive impairment (MCI), or AD ( 19 ). The cohort included 72 HC (ADRC-HC; 5 Aβ positive by PET [Aβ-PET+] and 67 negative [Aβ-PET-] by PET), 124 with MCI (ADRC-MCI; 42 Aβ-PET+ and 82 Aβ-PET-), and 54 with AD (ADRC-AD; all Aβ-PET+). Participant demographics are summarized in Table 1 ; Supplementary Table S1 . AD participants were further subclassified using CDR (4.5 to 9, mild dementia, 9.5 to 15.5, moderate dementia; ≥ 16, severe dementia) ( 16 ) and MMSE (≥ 25, cognitively unimpaired; 20 to 24, mild cognitive impairment; 10 to 19, moderate cognitive impairment; 0 to 9, severe cognitive impairment) ( 17 ) score-based cutoffs for severity of cognitive dysfunction.
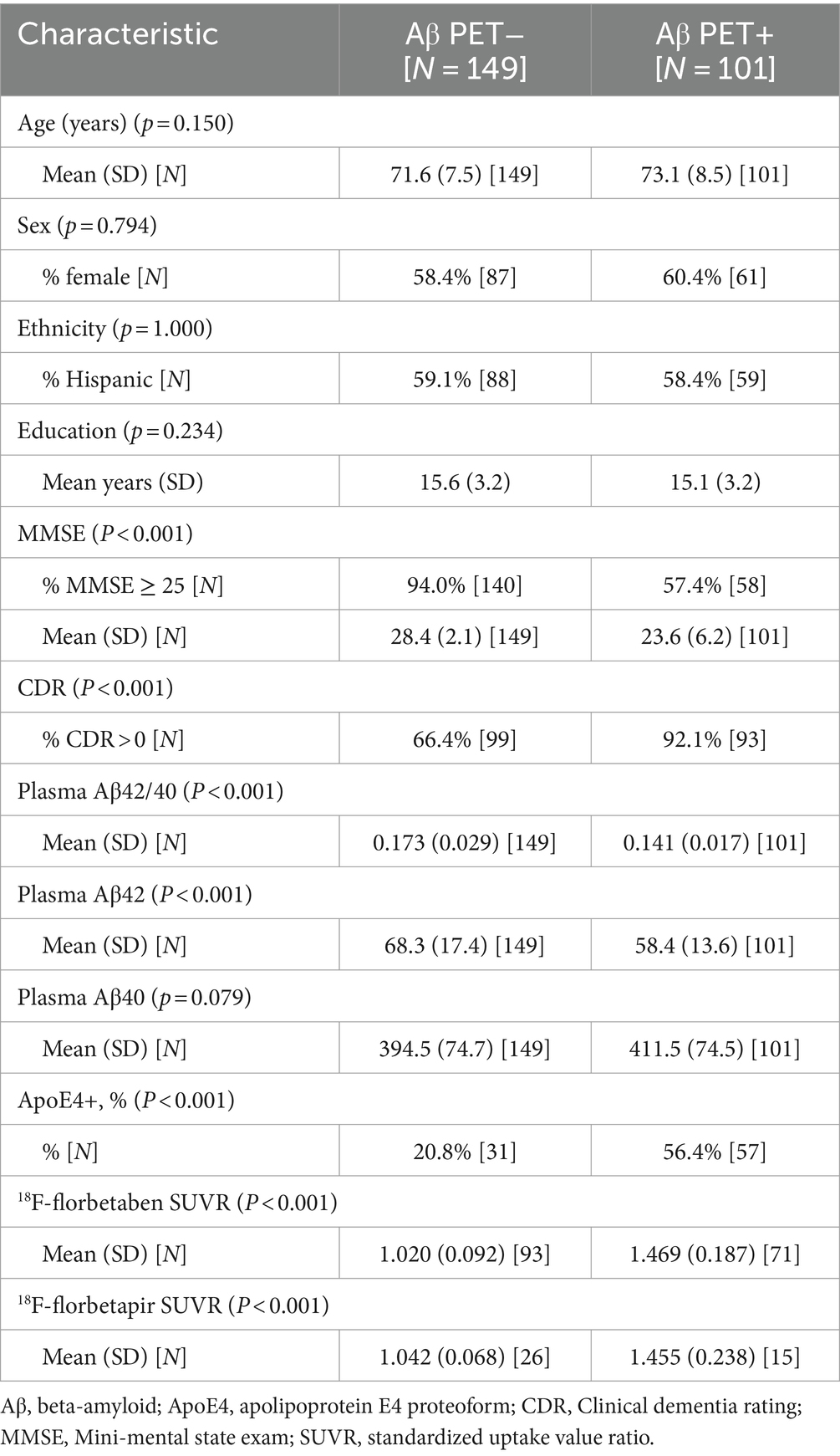
Table 1 . Alzheimer’s Disease Research Center (ADRC) cohort participant characteristics by amyloid positron emission tomography (Aβ PET) status.
In addition, we performed a retrospective analysis of 6,192 consecutive plasma specimens submitted to Quest Diagnostics for Aβ42/40 ratio testing. This was a limited data set 2 with only patient sex and age information retained. Participant demographics are summarized in Table 2 .
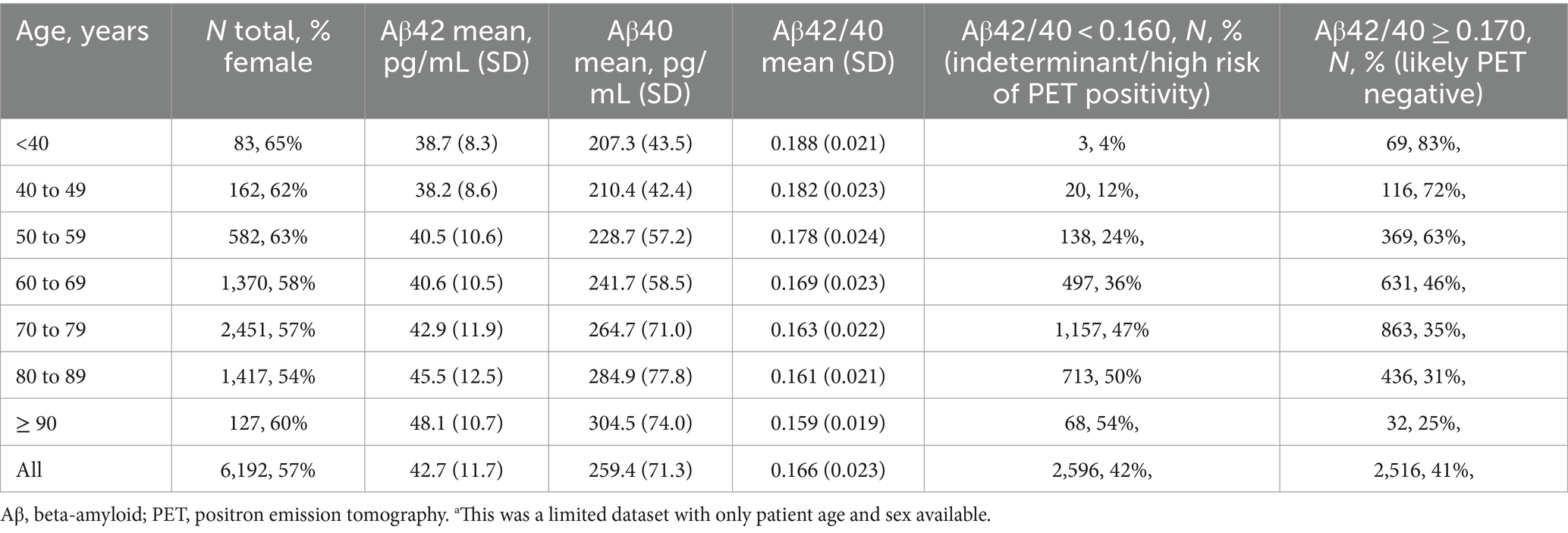
Table 2 . Demographics for 6,192 clinical specimens a submitted for Aβ42/40 testing.
2.2 Assessment of amyloid positivity from amyloid PET scans
Qualitative and quantitative analysis of amyloid positivity by visual reads and standardized uptake value ratio (SUVR) data have previously been described ( 19 ). All ADRC participants underwent an amyloid PET scan within 12 months of plasma collection. Of these individuals, 205 had SUVR computed using 2 different tracers: [ 18 F]-florbetapir ( n = 41, cutoff >1.11) and [ 18 F]-florbetaben ( n = 164, cutoff > 1.40). SUVR values were transformed into a binary scale (Aβ-PET+ or Aβ-PET−) based on SUVR cutoffs. However, visual reads were used as the gold standard for amyloid positivity designations ( 19 ) whenever there were conflicting data between SUVR cutoffs and visual reads. Amyloid PET statuses for the remaining 45 individuals were determined by visual reads alone ( 19 , 20 ).
2.3 Plasma beta-amyloid assay
Blood specimens were collected by venipuncture into 10-mL tubes containing EDTA as anticoagulant, kept on ice (<1 h) until centrifugation at 1,200 relative centrifuge force for 12 min at room temperature. Aliquots of plasma (0.5 mL) were transferred into polypropylene tubes and stored at −80°C until analysis. All plasma specimens were deidentified, and results were blinded during the analysis.
A detailed description of the development and validation will be reported elsewhere ( 15 ). Briefly, 0.5 mL of calibrators, quality control (QC) samples, and plasma specimens were diluted with 0.5 mL of PBS and 0.1 mL of 1% Tween-20/CHAPS (v/v). Internal standard for Aβ40 (uniformly 13 C/ 15 N labeled) and Aβ42 (uniformly 15 N labeled) were added to each sample, and Aβ40 and Aβ42 were simultaneously immunoprecipitated, proteolytically digested using the enzyme Lys-C, and desalted and concentrated using a mixed-mode anion exchange solid-phase extraction (SPE) plate (Waters, Milford, MA). All sample preparation steps were automated using a Hamilton Star liquid handler (Hamilton, Reno, NV). Digested samples (70 μL) were injected onto a XBridge Protein BEH 300 Å C4 column (Waters, Milford, MA) and separated using a Transcend Vanquish TLX-4 TurboFlow UPLC (ThermoFisher Scientific, Waltham, MA) using a staggered 4-column configuration to facilitate high throughput. Both peptides were chromatographically separated using a 16-min gradient at a flow rate of 0.6 mL/min of solvent A (water with 0.15% formic acid) and solvent B (acetonitrile with 0.15% formic acid) with a 2-min acquisition window. Detection was achieved using a TSQ Altis Plus Triple Quadrupole MS (ThermoFisher Scientific, Waltham, MA) operated in multiple-reaction monitoring (MRM) mode, and data was collected every 4 min using the described staggered 4-column configuration. Each 96-well plate consisted of an 8-point calibration curve for Aβ40 and Aβ42, two sets of 4 quality control samples, and 79 patient samples. The ratio of the peak area of the analyte to the internal standard was used to calculate the concentrations from the standard curve using TraceFinder Clinical Research v5.1 software (ThermoFisher Scientific). The ratio of Aβ42 to Aβ40 was determined by taking the back-calculated value for Aβ42 and dividing it by the back-calculated value for Aβ40.
Analytical validation studies, including assay precision (within-run and between-run), analytical measurement range (AMR), analytical sensitivity (limit-of-blank [LOB], limit-of-detection [LOD], and limit-of-quantification [LOQ]), interference testing, and stability were conducted according to CLSI guidelines ( 21 – 25 ).
For this study, we determined average inter-assay (between-run) imprecision by taking the average of 4 quality control (QC) samples run in duplicate over 25 consecutive days (Weber et al. submitted; ranges were 163–619 pg/mL Aβ40, 40–188 pg/mL Aβ42, and 0.124–0.304 Aβ42/40; a non-physiological high QC value was excluded) ( 15 ).
Analytical robustness and the effects of reclassification bias were experimentally determined by reanalyzing previously tested samples using a different operator and a different lot of reagents and calibrators. A total of 196 plasma specimens were randomly selected and reanalyzed over the course of 1 month. Both freeze/thaw and storage stability were considered when selecting specimens for reanalysis. Values for Aβ40, Aβ42, and the Aβ42/40 ratio were compared as a percentile difference calculated between the mean values of the original results and the mean values of the retest results to establish “measured bias.”
2.4 Statistical analysis
Two-sample t -tests were used to evaluate differences in baseline continuous measures across PET status. Differences in categorical variables were evaluated by Fisher’s exact test. Comparisons between groups with more than two outcomes were performed using one-way analysis of variance (ANOVA) for continuous variables and Fisher’s exact tests for categorical variables. Effect size for means were determined by eta-square measurements. Statistically significant results from ANOVA were followed by post-hoc analysis using Tukey multiple pairwise-comparisons between group means. The performance of the Aβ42/40 ratio on classification of amyloid PET status was evaluated by logistic regression and ROC curve analysis. ROC-AUC 95% confidence intervals (CI) and comparisons between ROC curves were determined using the DeLong method ( 26 ). Optimal cutoffs for sensitivity and specificity from ROC analyses were determined by Youden’s ( 27 ). Correlation of the Aβ42/40 ratio and PET SUVR were assessed by Spearman’s rho. Robustness simulations were based on the methodology described in Rabe et al. ( 13 ).
To account for the potential underestimation of variability in the measure of the Aβ42/40 ratio in this study, 10,000 simulations of the Aβ42 and Aβ40 responses were generated with an added 10% CV [per Rabe et al. ( 13 )] and 6% measured CV from a scaled standard normal distribution. For each simulated pair of markers with added noise, an Aβ42/40 ratio was calculated. The rate of reclassification around the 0.160 Aβ42/40 threshold from the original observed Aβ42/40 ratio to the simulated noise-added ratio was calculated. The average reclassification rate was estimated as the mean of the 10,000 simulated reclassification rates. A 95% CI for the mean reclassification rate was estimated by the 2.5th and 97.5th percentiles of the simulated reclassification rates.
To estimate the effects of potential bias in the measure of Aβ42 and Aβ40 on the performance characteristics of the Aβ42/40 ratio, per Rabe et al. ( 13 ), we evaluated a 10% increase in Aβ42 response and a 10% decrease in the Aβ40 response for a total Aβ42/40 bias of 22% (1.1/0.9 = 1.22). The measured bias was similarly assessed. Performance characteristics were calculated for both the observed Aβ42/40 ratio and the biased Aβ42/40 ratio. Joint 95% CI were obtained for sensitivity/specificity and PPV/NPV on classification of PET status ( 28 ). All CI for performance characteristics were obtained by non-parametric bootstrap. All analyses were conducted using R (version 4.2.1).
3.1 ADRC cohort participant demographics: PET status and cognitive outcomes
Compared to ADRC Aβ-PET− individuals, Aβ-PET+ individuals were more likely to be APOE4 carriers, have lower MMSE scores, and more likely to have CDR scores greater than 0 ( p < 0.001 for all; Table 1 ). No statistically significant differences ( p > 0.05) between the Aβ-PET− and the Aβ-PET+ group were observed in age, sex, percentage of individuals identifying as Hispanic, or years of education ( Table 1 ).
When categorized by a cognitive diagnosis, from HC to MCI to AD ( Supplementary Table S1 ; Supplementary Figure S1A ), we observed lower MMSE scores among MCI and AD patients compared to HC ( p < 0.001; Supplementary Table S1 ). In addition, the frequency of APOE4 carriers was greater in the ADRC HC Aβ-PET+, MCI Aβ-PET+, and AD Aβ-PET+ groups compared to the HC Aβ-PET− and MCI Aβ-PET− group ( p < 0.001). The ADRC-MCI Aβ-PET− group had a lower percentage of female participants relative to the other groups ( p = 0.039; Supplementary Table S1 ). There were no statistically significant differences in PET status based on age, patient education, or percentage of individuals identifying as Hispanic.
In distinguishing a cognitive diagnosis of AD vs. HC, the AUC for the Aβ42/40 ratio was 0.91 (95% CI = 0.86 to 0.97). At the Aβ42/40 ratio classification threshold of 0.160, sensitivity was 96% (95% CI = 91 to 100%) and specificity was 83% (95% CI = 75 to 92%; Supplementary Figure S1B ).
Among the 54 AD participants, MMSE scores increased as the Aβ42/40 ratio increased, and CDR scores increased as Aβ42/40 decreased; however, the trend did not reach statistical significance ( p > 0.05; Supplementary Figure S2 ). To establish a stronger relationship between the Aβ42/40 ratio and cognitive dysfunction, a more uniform distribution of AD subjects across the cognitive dysfunction classes, particularly those with severe dementia (represented by only 4 individuals), is required.
3.2 Performance and robustness of plasma Aβ42/40 ratio for identifying amyloid PET status
Overall, for the 250 individuals with PET data (quantitative and qualitative reads), plasma Aβ42 concentrations and Aβ42/40 ratios were significantly lower ( p < 0.001) in ADRC Aβ-PET+ individuals compared with Aβ-PET− individuals, with no significant differences in Aβ40 concentrations ( Table 1 ; Figure 1A ). Using ROC analysis and the maximum of Youden’s J index, a plasma Aβ42/40 cutoff ratio of 0.160 had an AUC of 0.84 (95% CI = 0.79 to 0.89, Figure 1B ) with 91% sensitivity, 76% specificity, and overall accuracy of 82% ( Table 3 ). Based on a 40% prevalence of amyloid positivity in the ADRC cohort (40.4% observed), we found a positive predictive value (PPV) of 72% and a negative predictive value (NPV) of 93% at the 0.160 cutpoint ( Table 3 ). Based on a 34% prevalence of amyloid positivity in the patients in the ADRC cohort with MCI, which may be more reflective of the target population, PPV decreased to 56% and NPV decreased to 90% at the 0.160 cutpoint ( Table 3 ) when HC and AD patients were excluded.
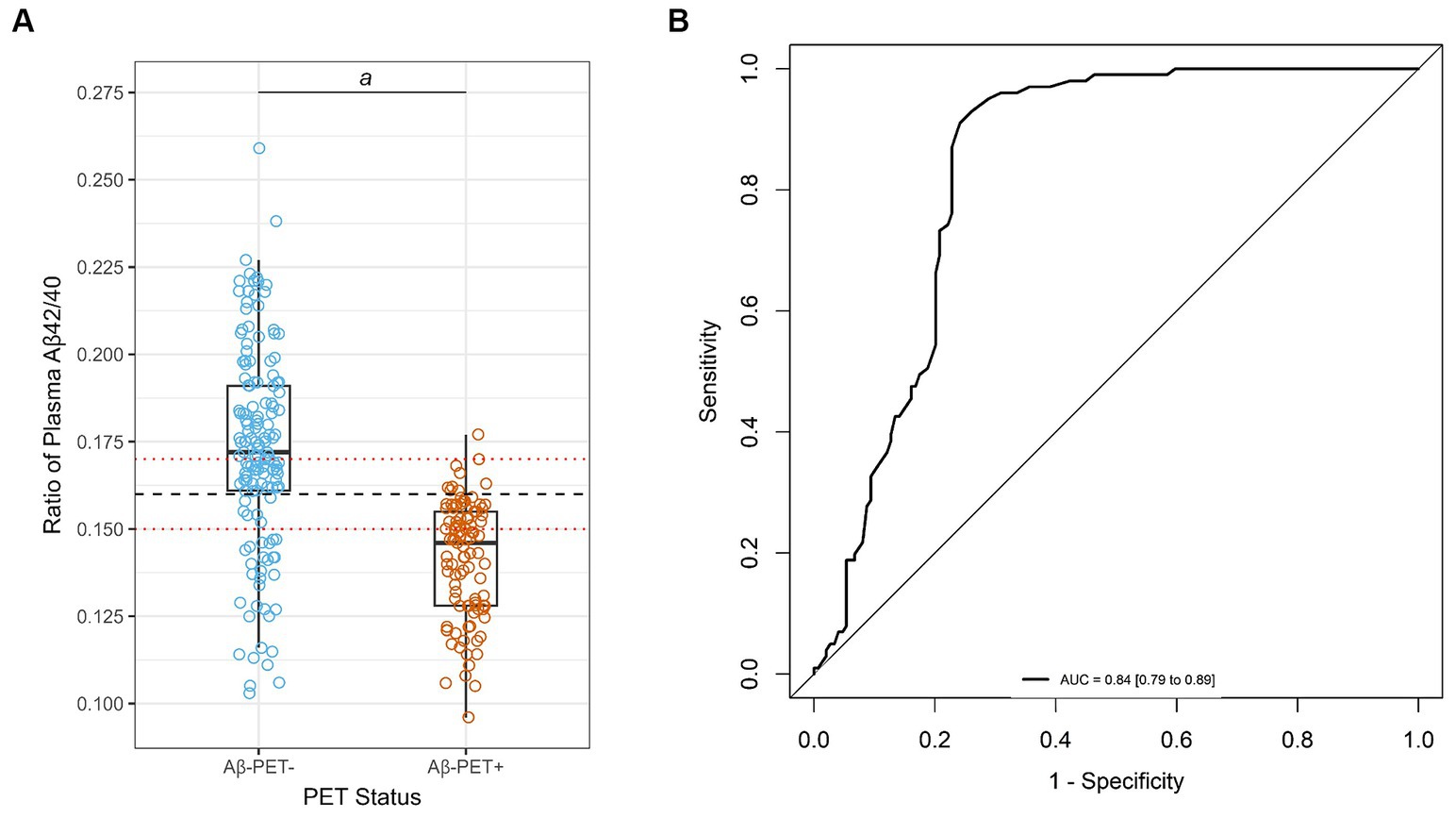
Figure 1 . Correlation and diagnostic performance of the Aβ42/40 ratio and amyloid PET imaging. (A) Plasma Aβ42/40 ratio compared with amyloid PET status (Aβ-PET− and Aβ-PET+). Black dashed line denotes optimal Aβ42/40 ratio cutoff of 0.160. Red dotted lines denote Aβ42/40 ratio indeterminant risk cutoffs (0.150 and 0.170); a = significant at p < 0.001; (B) ROC-AUC of the plasma Aβ42/40 ratio for prediction of amyloid PET positivity. AUC, area under the curve; PET, positron emission tomography; ROC, receiver operating characteristic.
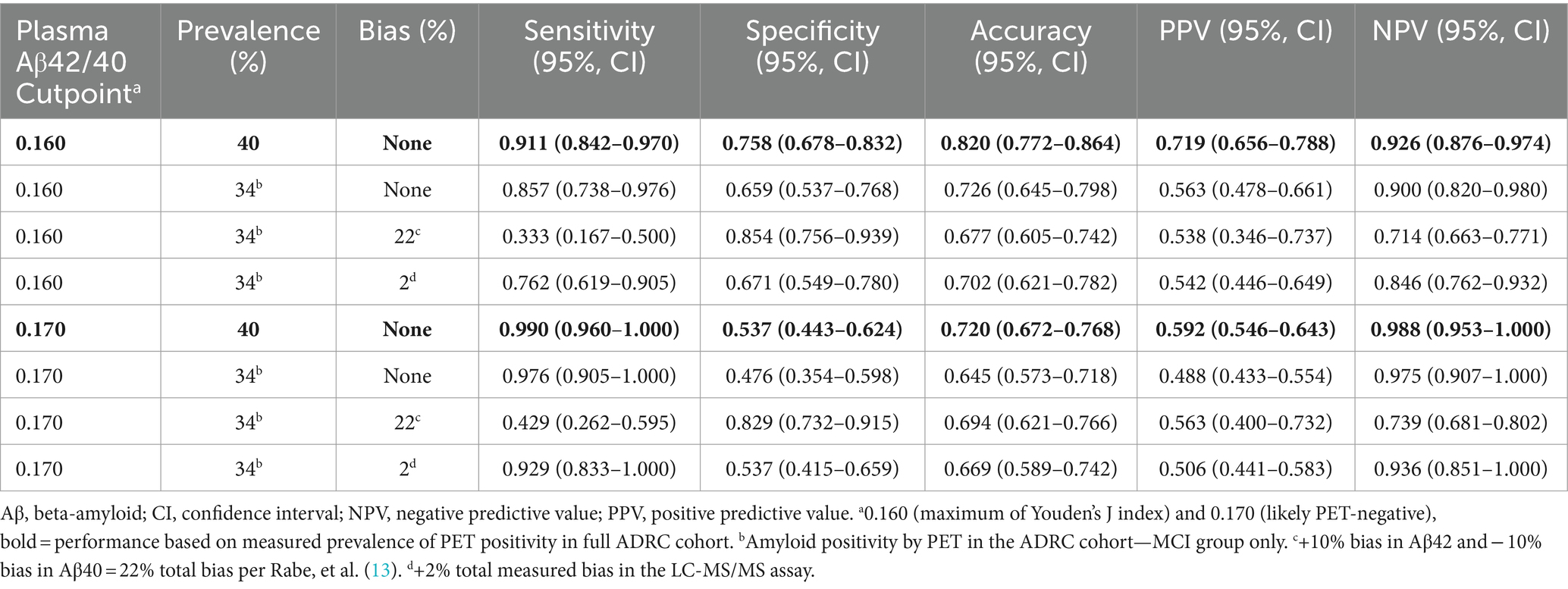
Table 3 . Performance characteristics and the effects of prevalence and bias for detecting positron emission tomography (PET) status in the Alzheimer’s Disease Research Center (ADRC) cohort.
The difference in means between the PET+ and PET− Aβ42/40 ratio was about 18% with substantial shift of Aβ PET+ to PET− status after applying 10% bias (22% total bias if Aβ42 and bias in Aβ40 shifted in opposite directions, Figure 2A ) per Rabe et al. ( 13 ). This level of bias would substantially diminish the PPVs and NPVs of this assay ( Table 3 ). However, the measured bias in this LC-MS/MS assay was 11.5% (95% CI = 9.5% to 13.6%) for Aβ42, 10.6% (95% CI = 9.0% to 12.6%) for Aβ40 (both in the same direction), and 0.7% (95% CI = −0.3% to 1.8%) for Aβ42/40 ratio. Accordingly, we used a higher estimate (upper 95% limit of the ratio CI rounded to 2%) to reflect worst-case effects of bias ( Figure 2A ). PPVs and NPVs are much less affected under these conditions ( Table 3 ).
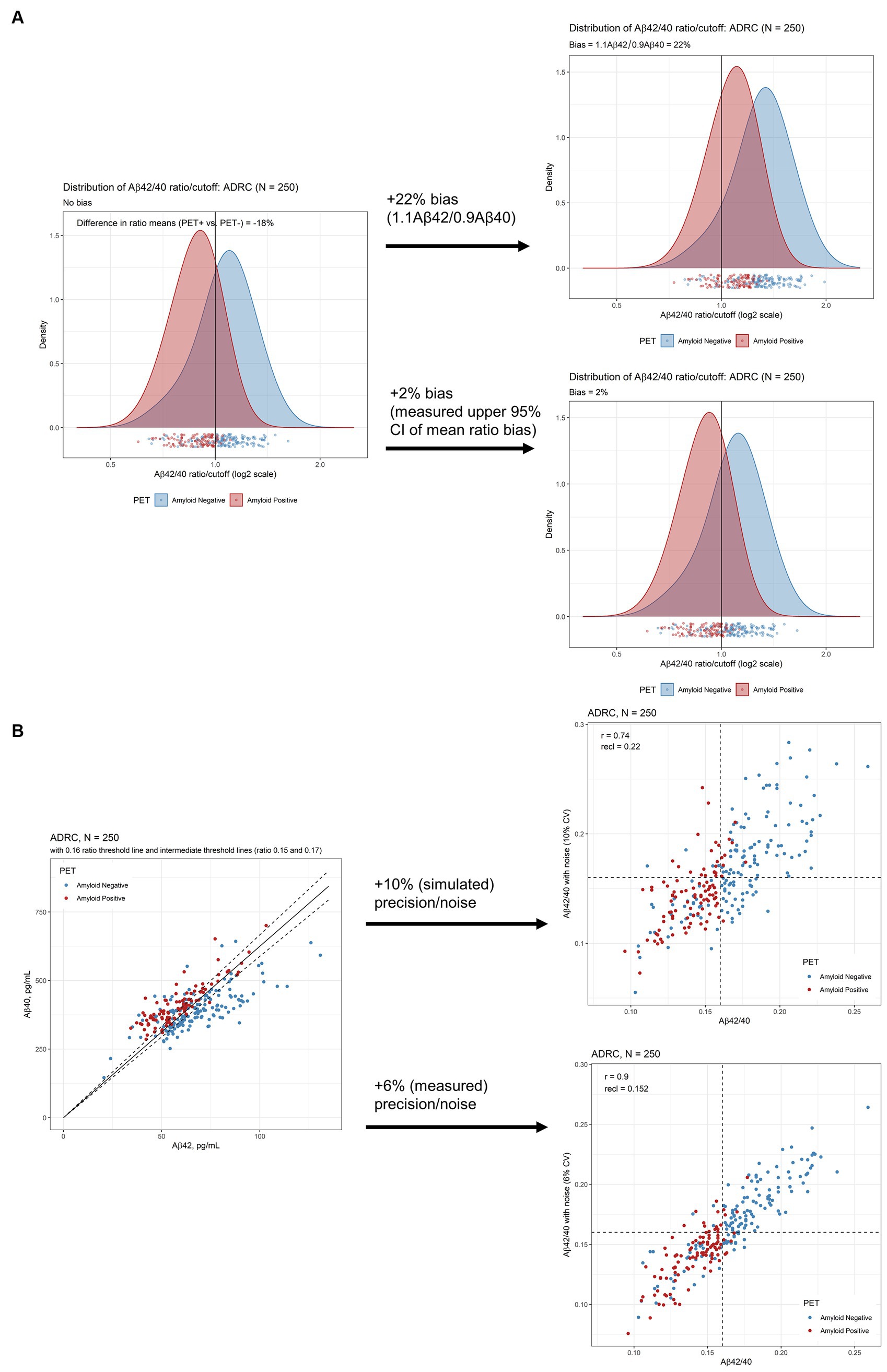
Figure 2 . Robustness assessment of Aβ42/40 ratio for predicting amyloid PET imaging results. (A) Densities of plasma Aβ42/40 ratio by LC-MS/MS in ADRC cohort with and without 10% bias (22% total, upper plot) and 2% measured bias (lower plot) added. (B) Scatterplots of Aβ42 and Aβ40 to illustrate proximity to cutoffs defining indeterminate risk and scatterplots of Aβ42/40 ratio with and without 10% CV added noise (upper plot) and 6% added CV (measured) noise (lower plot). Aβ, beta-amyloid; ADRC, Alzheimer’s Disease Research Center; CI, confidence interval; PET, positron emission tomography.
Assuming an estimated imprecision of 10% per Rabe et al. ( 13 ), we simulated a reclassification rate of 22% (95% CI = 18% to 27%) in the ADRC cohort at a cutpoint of 0.160 ( Figure 2B , upper scatterplot). However, measured mean inter-assay imprecision, calculated by taking the average imprecision across 5 study samples analyzed in duplicate over 25 days, was closer to 6% ( 15 ). Using this measured precision, the reclassification rate by simulation is 15% (95% CI = 11% to 19%; Figure 2B lower scatter plot).
Based on 6% assay imprecision, we propose cutpoints of 0.150 and 0.170 as defining indeterminate risk of amyloid PET positivity, constituting 30% of the ADRC cohort ( Figure 2B ; Table 4 ). The 0.170 cutpoint has an NPV of 99% in the ADRC cohort for ruling out amyloid positivity (98% in the target population with MCI, 94% when adding measured bias, Table 3 ). Large differences were observed within the ranges defined by indeterminate risk in the ADRC cohort including about 4-fold higher PET positivity and ApoE4 proteotype ( APOE4 genotype) and 9-fold higher AD diagnosis, in the 0.150–0.159 range vs. the 0.160–0.169 range ( Table 4 ).
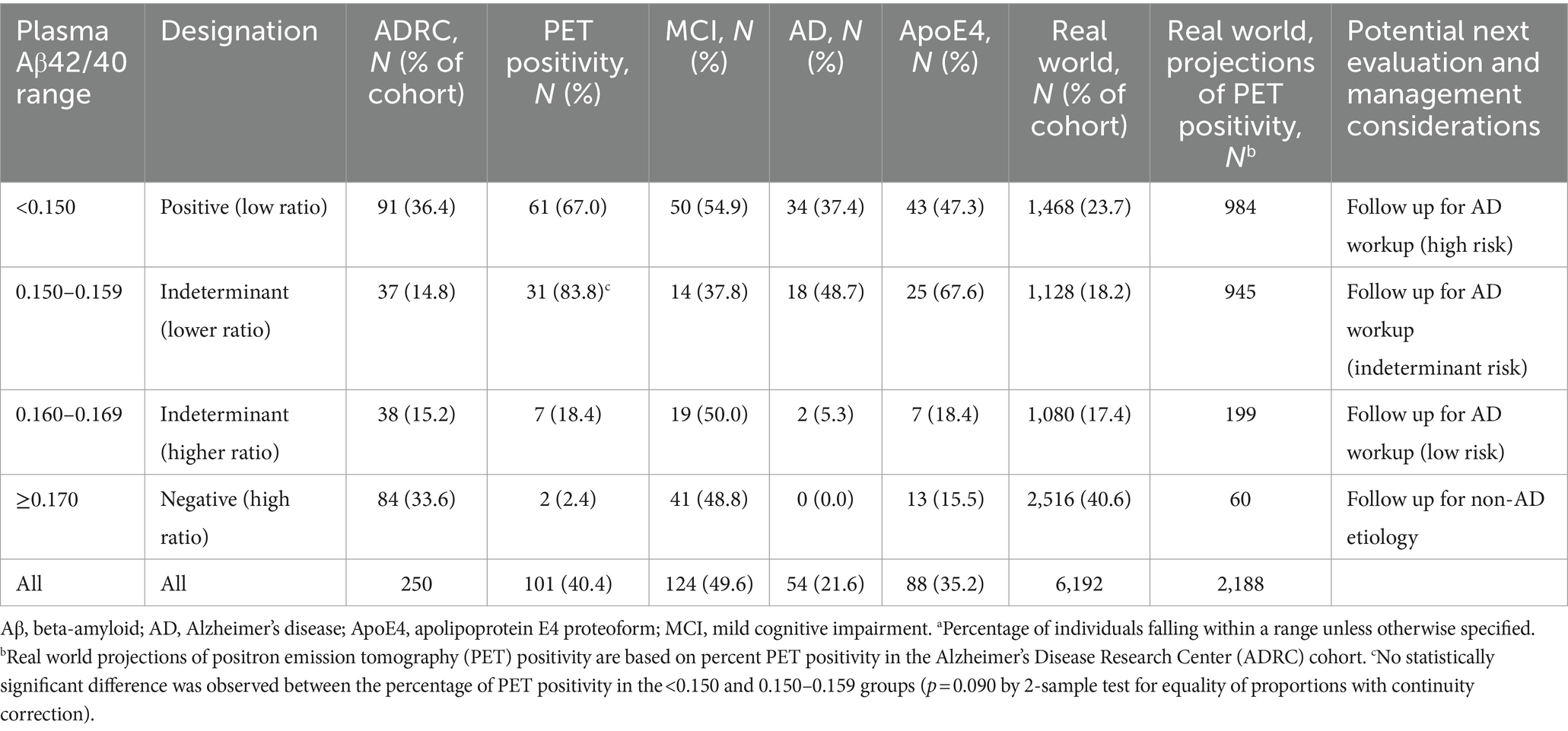
Table 4 . Proposed plasma Aβ42/40 ranges for clinical decision making a .
For the 205 individuals for whom we had quantitative PET data, 5 [ 18 F]-florbetapir positive results (range 1.11 to 1.15) were reclassified as negative and 25 [ 18 F]-florbetaben negative results (range 1.09 to 1.39) were reclassified as positive based on visual reads. After reclassification, the concordance of the Aβ42/40 ratio increased from 80 to 88% for [ 18 F]-florbetapir and from 74 to 83% [ 18 F]-florbetaben. Overall concordance between Aβ42/40 ratio and SUVR data changed from 75% before to 84% after reclassification based on visual reads.
Both tracers showed similar results when plotted against the Aβ42/40 ratio ( Figures 3A , B ). We observed a significant inverse relationship between the Aβ42/40 ratio and quantitative SUVR values for [ 18 F]-florbetaben PET (Spearman’s rho of −0.54 [95% CI = −0.65 to −0.42, p < 0.001], Figure 3A ) and [ 18 F]-florbetapir PET (Spearman’s rho of −0.58 [95% CI = −0.76 to −0.32, p < 0.001], Figure 3B ).
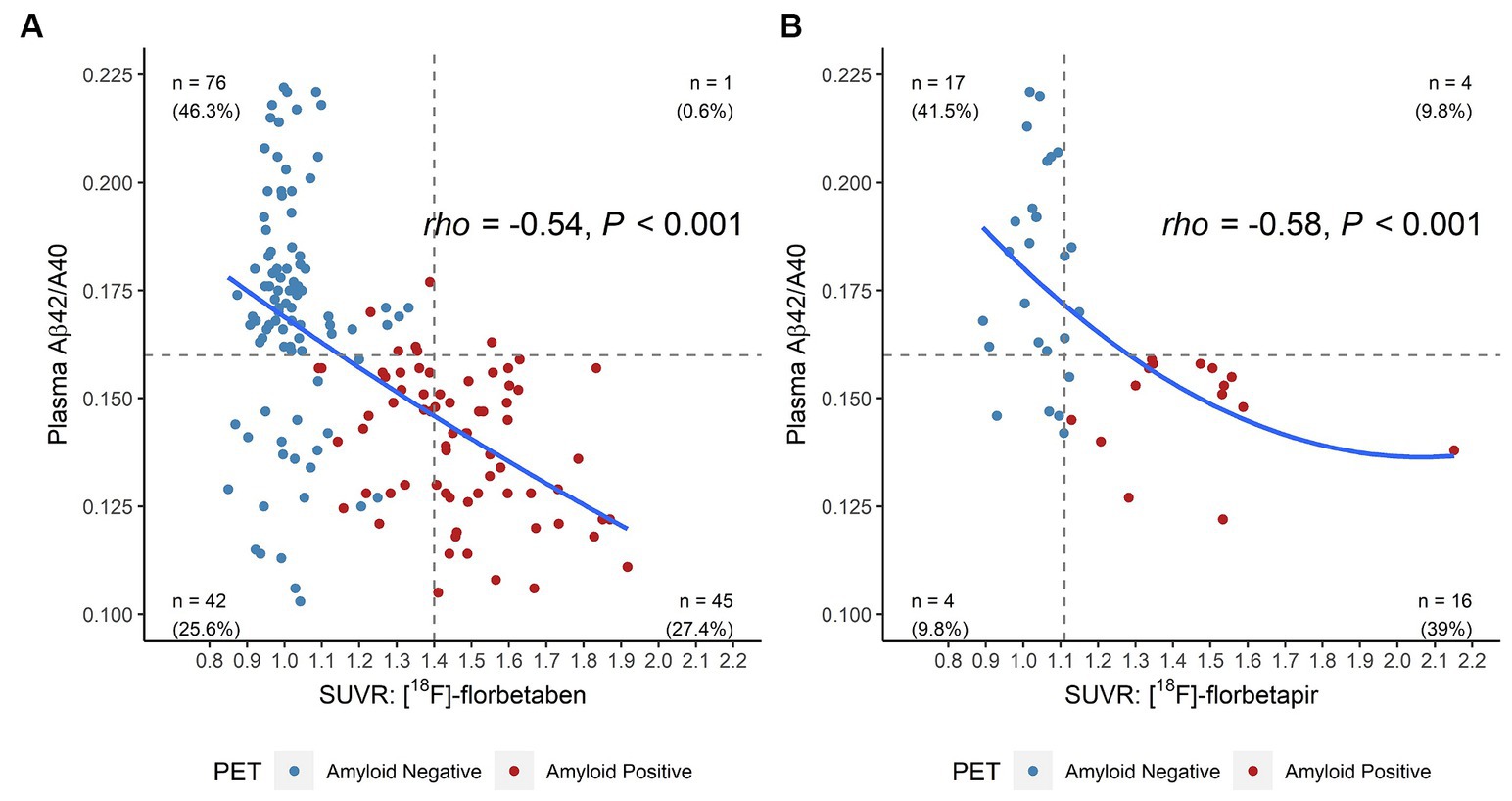
Figure 3 . Four-quadrant plot illustrating the relationship between the plasma Aβ42/40 ratio and (A) [ 18 F]-florbetaben SUVR values and (B) [ 18 F]-florbetapir SUVR values in the ADRC cohort. Horizontal dashed line = optimal plasma Aβ42/40 ratio cutoff (0.160). Vertical dashed lines = optimized SUVR cutoff values for each tracer. Color coding for amyloid positivity and negativity is based SUVR cutoffs or gold-standard visuals reads. Aβ, beta-amyloid; ADRC, Alzheimer’s Disease Research Center; PET, positron emission tomography; SUVR, standardized uptake value ratio.
3.3 Plasma Aβ42/40 ratio and clinical specimens
A significant inverse relationship between age and Aβ42/40 ratio was observed for the 6,192 clinical specimens (Spearman’s rho = −0.25, 95% CI = −0.27 to −0.23, p < 0.001; Figure 4A ; Table 2 ), contrasting with the increases of plasma Aβ42 and Aβ40 concentrations with age ( Figures 4B , C ; Table 2 ). Indeterminate results defined by cutpoints of 0.150 and 0.170 define 2,208 (35.7%) almost evenly split between individuals with ratios from 0.150 to 0.159 and individuals with ratios from 0.160 to 0.169, but with the former group potentially representing a much higher percentage of PET-positivity based on the ADRC cohort ( Table 4 ). Using a 0.170 cutpoint to indicate the likelihood of PET negativity, we identified 2,516 individuals for whom a PET scan/CSF would be potentially unnecessary. As expected, among these individuals, the percentage who are likely PET negative decreases with age from <40 years (83%) to ≥90 years (25%; Table 2 ).
Figure 4 . Distribution (upper) and scatterplots by age for the 6,192 clinical specimens submitted for Aβ42/40 testing. (A) Aβ42/40 ratio, Spearman’s rho = −0.25, 95%CI, −0.27 to −0.22. (B) Aβ42 concentrations, Spearman’s rho = 0.22, 95%CI 0.20 to 0.25; and (C) Aβ40 concentrations, Spearman’s rho = 0.41, 95%CI 0.39 to 0.43. Black dashed line denotes Aβ42/40 ratio cutoff of 0.160. Red dotted lines denote Aβ42/40 ratio indeterminant risk cutoffs (0.150 and 0.170). Aβ, beta-amyloid.
4 Discussion
4.1 assay performance, robustness, and clinical decision making.
Our data support the use of plasma Aβ42/40 ratio by LC-MS/MS to help predict a low likelihood of PET-positivity in AD assessment. In the ADRC group, with an Aβ42/40 ratio cutpoint of 0.170, we observed a 99% NPV. Although previous studies have cast doubt on the clinical utility of plasma Aβ42/40 assays based on misclassification potential, the only detailed analysis was provided for the Elecsys Aβ42 and Aβ40 electrochemiluminescence assay. LC-MS/MS assays, although acknowledged to have better discriminatory performance as assessed by ROC-AUC analysis, were said to have similar issues in terms of narrow analytical ranges and fold changes between Aβ-PET+ and PET− individuals yielding insufficient robustness for clinical decision making ( 13 ).
We followed the example of Rabe et al. ( 13 ) by applying these tools to the results for the ADRC cohort obtained using our new LC-MS/MS assay. The ADRC cohort has a moderate prevalence of amyloid PET positivity (40%), within the range of 18% to 65% ( Supplementary Table S2 ) previously reported for cohorts studied by LC-MS/MS ( 29 – 32 ).
Applying a 10% bias [in opposite directions, total 22% per Rabe et al. ( 13 )] negatively impacted assay sensitivity, specificity, accuracy, NPV, and PPV ( Table 3 ). However, applying a measured mean Aβ42/40 ratio bias (0.7%, worst case 2%) had a much lesser effect ( Figure 2A ), despite mean bias for each analyte being close in magnitude to the simulations for Aβ42 (~12%) and Aβ40 (~11%). Under real-word conditions, bias for each analyte drifted in the same direction, unlike the simulations, effectively canceling out its effect on the ratio.
Sources of bias that affect accurate determination of the Aβ42/40 ratio are those that negatively impact one analyte over the other, effectively skewing the ratio over time. For example, specimen storage at room temperature can result in differential loss of Aβ42 relative to Aβ40 ( 33 ), suggesting that Aβ42 is more susceptible to proteolytic cleavage, aggregation, or absorptive loss under these conditions. However, proper handling and storage mitigates these risks in the clinical laboratory. Immunoassays may be more susceptible to matrix effects that affect one analyte over the other compared with LC-MS/MS assays ( 14 ), wherein analytical losses and suppression are corrected for using isotopically labeled internal standards. Errors in calibrator concentrations based on inaccurate supplier-provided peptide content can also cause bias, which we avoid by employing independent quantitative amino acid analysis of peptide calibrators ( 34 ). All these factors are impediments to standardization of these assays and may explain the high variability of Aβ42/40 ratio across assays and laboratories ( 14 ).
Applying a simulated 10% imprecision per Rabe et al. ( 13 ) yielded a relatively high reclassification rate for individuals in our ADRC cohort (22%), similar to their immunoassay and the BioFINDER cohort (26%). However, measured imprecision was closer to 6%, yielding a reclassification rate of 15%. Although this still represents a substantial group of individuals, it should not unduly affect the clinical utility of this assay for identifying those with a low likelihood of PET-positivity.
Our clinical data set was obtained from 6,192 consecutively run Aβ42/40 ratio specimens, presumably submitted by healthcare providers to help understand the cause of memory decline and dementia, and potentially help diagnose AD. The trends we observed in our data set, namely that plasma Aβ42/40 decreased as plasma Aβ42 and Aβ40 concentrations increased with age, generally aligned with those recently reported in China for ~200 specimens obtained from apparently healthy, older (50–89 years) individuals ( 35 ). Age-dependent trends suggest that NPV could be influenced by age, especially for individuals under 60 years of age who tend to have higher Aβ42/40 ratios (see below).
Based on cutpoints defining high, low, and indeterminant risk in the ADRC cohort, we suggest that plasma Aβ42/40 ratio results can help guide clinical decision making ( Table 4 ). Notably, the high cutpoint of 0.170 almost rules out PET-positivity with an NPV of almost 99% (acknowledging potential variations caused by prevalence and bias in Table 3 ). Projecting percentages of PET-positivity at this cutpoint in the ADRC cohort onto the 6,192 clinical specimens, the assay could potentially negate the need for PET testing in 2,516 patients ( Table 4 ). Assuming a PET scan cost of $5,000 in the United States ( 36 ), this approach saves $12,580,000 before accounting for the cost of 6,192 LC-MS/MS analyses (at current list pricing ~$550 USD) to identify these patients and 60 PET scans for the projected 2.4% patients with false negative results who would eventually need a PET scan. This represents a total savings of $8.9 million or about $1,432 per patient. In addition to younger (<60 years) patients, exceptions to this clinical decision making may include some patients with autosomal dominant AD and both amyloid precursor protein and presenilin-1 mutations who may have an elevated Aβ42/40 ratio ( 37 ).
4.2 Assay performance comparisons
In keeping with previous studies, we found that the plasma Aβ42/40 ratio was significantly lower in individuals who were Aβ-PET+ compared to those who were Aβ-PET-. Analytical performance characteristics were comparable to other mass spectrometry-based assays but with higher throughput on a lower-cost MS instrument and with a relatively large difference (18%) between Aβ42/40 ratio means for PET+ and PET− individuals ( Figure 2A ) compared to most literature reports ( Supplementary Table S2 ) ( 14 , 30 , 32 , 38 , 39 ). Robustness was comparable to the best performing LC-MS/MS assays for which precision and bias estimates were available ( Supplementary Table S2 ) ( 13 , 29 ).
Interestingly, the 0.160 Aβ42/40 ratio cutoff optimized for maximum sensitivity and specificity for detecting PET positivity is identical to the optimized cutoff for differentiating HCs from individuals with AD, both in the current plasma study ( Supplementary Figure S1 ) and in CSF ( 40 ). This suggests that the proportion of Aβ40 and Aβ42 is similar in CSF and plasma. Unfortunately, PET data were not available for the CSF study, and while both studies differentiated HC vs. AD participants, the specimens came from different cohorts and locations making performance comparisons difficult.
ROC-AUC analysis provides a means to make assay comparisons of clinical performance with other studies but with some major caveats. Head-to-head comparison using the same specimens from the same cohorts using the different methods are rare because they employ precious clinically characterized cohort specimens ( 14 , 41 ). Otherwise, the cited performance is only relevant to the cohorts in which those assays were performed; differences in the prevalence of amyloid positivity, cognitive status, and PET-tracer cutoffs used among cohorts make these comparisons fraught as described by Brand et al.’s review of clinical performance of Aβ42/40 in 21 publications ( 31 ). In addition, age, the number of APOE4 alleles, and race/ethnicity, can all impact the AUCs. In fact, these characteristics have been incorporated in an algorithmic approach to improve performance above and beyond the Aβ42/40 ratio (discussed below).
Given the marked variation across cohorts in the literature, we compared our Aβ42/40 clinical performance with recent studies having a similar prevalence of PET-positivity (~40%; Supplementary Table S3 ) ( 14 , 30 , 42 ). Our AUC of 0.84 compares favorably with the IP-LC-MS/MS assay of West et al. with an AUC of 0.81 ( 30 ) (best performance of 0.83 in a head-to-head comparison with other assays also at ~40% prevalence of PET-positivity) ( 14 ). This assay is similar to ours but employs prohibitively expensive high-resolution MS instrumentation that may not be accessible to many clinical laboratories; in contrast, our assay employees a low-resolution and relatively low-cost MS platform ( Supplementary Table S2 ). In addition, concerns for assay robustness for the West et al. method comes from the small separation of Aβ42/40 values between Aβ PET+ and Aβ PET− individuals in most reports using this methodology (8%–11%) ( 13 , 14 , 29 , 30 , 41 ); in contrast, we observed a larger 18% separation in the current study ( Supplementary Table S2 ). However, measured bias (i.e., <1% “drift” Aβ42/40 ratio values) was low and similar to the current study ( 29 ). Another important difference was that we used age-matched subjects, whereas the average age difference between Aβ PET+ and PET− cohorts in their study was about 6 years ( 30 ).
Similarly, Bun et al. recently used a chemiluminescence enzyme immunoassay to achieve an AUC of 0.95 (37% prevalence of PET-positivity) but again with a 6-year age difference between PET+ and PET− participants ( Supplementary Table S3 ) ( 42 ). Age is a known risk factor for Alzheimer’s disease ( 43 ), and in our clinical specimen cohort, we observed a significant difference in the mean Aβ42/40 ratio between the 60- to 69-year age group and the 70- to 79-year age group (0.169 vs. 0.163, p < 0.001), irrespective of clinical outcomes or amyloid PET status ( Figure 4A ). While age is known to improve model predictors for PET-positivity, this covariate is not reflected in the clinical performance for our aged-matched populations or, interestingly, Bun et al.’s more age-disparate populations. In addition, although the Bun et al. study had good precision (<4% for each analyte), the effects of bias on reclassification rates were not evaluated; ( 42 ) these effects can be marked in immunoassays, where separate Aβ42 and Aβ40 values can drift in opposite directions ( 13 ).
Similar to Bun et al. ( 42 ) but in contrast to many studies ( 29 , 30 , 32 , 44 ), we found that the number of APOE4 alleles did not substantially improve classification accuracy based on ROC analysis, possibly due to the demographics of our cohort. Previous studies included predominantly White and/or Asian populations ( 29 , 30 , 32 , 44 ). Notably, the association between the APOE4 allele and AD risk has been reported to be lower in Hispanic and Black non-Hispanic individuals than in White non-Hispanic individuals ( 45 , 46 ). Our ADRC cohort had roughly 3-times the number of Hispanic individuals (59.1% of the Aβ PET+ and 58.4% of the Aβ PET− individuals) compared with similar studies that showed a significant improvement in AUC when APOE4 status was included ( 30 ). Our findings are a cautionary note that algorithms incorporating Aβ42/40 and APOE4 allele status may not be generally applicable for all races and ethnicities.
4.3 Study limitations
We did not perform a detailed comparison with other biomarkers in the current study; instead, we focused on the utility of the plasma Aβ42/40 ratio biomarker in isolation. However, in a separate investigation, we show that early AD-related pathological changes in the Aβ42/40 biomarker were associated with quantifiable changes in brain microstructure and connectivity in Aβ-PET negative patients preceding deviations in other plasma biomarkers including t-tau, p-tau, neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP), and cortical atrophy ( 47 ).
We did not examine adding an additional plasma biomarker such as p-tau or GFAP. Some studies have combined plasma biomarkers to enhance performance in predicting clinical outcome. Addition of plasma p-tau with plasma Aβ42/40 might enhance the diagnosis in patients with MCI, because plasma p-tau levels typically increase with disease progression ( 48 ). The combination of biomarkers could be used in patients with specific clinical or demographic characteristics to further help define individual outcomes ( 49 ). The combination of Aβ42/40 and p-tau may better predict cognitive decline, but performance of the individual assays used would also play a role in how well the combination aids with this prediction ( 50 ). In addition, the combination of Aβ42/40 and GFAP enhanced the likelihood of becoming p-tau positive, suggesting that this biomarker might distinguish AD patients with progressive features ( 51 ).
Other study limitations include a relatively small sample size for individuals with PET data and insufficient racial diversity (predominantly Hispanic) that may pose a potential bias to our results, especially when it comes to the effect of the APOE4 allele on AD status. An unequal distribution of subjects across the AD dysfunctional classes (especially those with severe MMSE or CDR scores) prevented us from establishing firm Aβ42/40 ratio cutpoints that may help identify patients who may benefit from disease-modifying therapeutics. The lack of longitudinal plasma specimens from individuals that either converted from being Aβ-PET− to Aβ-PET+, or individuals that transitioned from being cognitively normal to MCI and AD limit our current understanding of the prognostic utility of the Aβ42/40 ratio in monitoring disease progression.
5 Conclusion
Our IP-LC-MS/MS assay accurately identified individuals with positive amyloid PET and differentiated individuals diagnosed with AD from age-matched HC. These findings support the use of this blood-based assay for assessing presence of AD pathology in individuals with cognitive impairment and can help reduce PET evaluations of patients with low likelihood of AD pathology, allowing for more efficient allocation of limited imaging resources.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Mount Sinai Medical Center IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. ST: Writing – original draft, Writing – review & editing. RL: Formal analysis, Visualization, Writing – review & editing. JK: Methodology, Validation, Writing – review & editing. SG: Supervision, Writing – review & editing. NC: Conceptualization, Writing – review & editing. DV: Resources, Writing – review & editing. RD: Resources, Writing – review & editing. KM: Resources, Writing – review & editing. WW: Resources, Writing – review & editing. TG: Resources, Writing – review & editing. MR: Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by National Institutes of Health (NIH) Center Core Grant P30AG066506 (TG) and NIH Research Grants R01NS052318 and R01NS075012 (DV).
Acknowledgments
The authors acknowledge helpful comments from Lee Hilborne, Harvey Kaufman, Matt Stroh, and Andrew Hellman.
Conflict of interest
DW, ST, RL, JK, SG, NC, and MR were employees of Quest Diagnostics. DW and NC hold patents for the detection of beta-amyloid by mass spectrometry as well as for the detection of apolipoprotein E proteoforms by mass spectrometry.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1364658/full#supplementary-material
1. ^ https://naccdata.org/data-collection/forms-documentation/uds-3
2. ^ https://privacyruleandresearch.nih.gov/pr_08.asp
1. Lane, CA, Hardy, J, and Schott, JM. Alzheimer's disease. Eur J Neurol . (2018) 25:59–70. doi: 10.1111/ene.13439
Crossref Full Text | Google Scholar
2. Murphy, MP, and LeVine, H 3rd. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis . (2010) 19:311–23. doi: 10.3233/JAD-2010-1221
PubMed Abstract | Crossref Full Text | Google Scholar
3. Iqbal, K, Liu, F, Gong, CX, and Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res . (2010) 7:656–64. doi: 10.2174/156720510793611592
4. Caroli, A, and Frisoni, GB. Alzheimer's disease neuroimaging I. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's disease neuroimaging initiative cohort. Neurobiol Aging . (2010) 31:1263–74. doi: 10.1016/j.neurobiolaging.2010.04.024
5. Jack, CR Jr, Knopman, DS, Jagust, WJ, Petersen, RC, Weiner, MW, Aisen, PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol . (2013) 12:207–16. doi: 10.1016/S1474-4422(12)70291-0
6. Hadjichrysanthou, C, Evans, S, Bajaj, S, Siakallis, LC, McRae-McKee, K, de Wolf, F, et al. The dynamics of biomarkers across the clinical spectrum of Alzheimer's disease. Alzheimers Res Ther . (2020) 12:74. doi: 10.1186/s13195-020-00636-z
7. Morley, JE, Morris, JC, Berg-Weger, M, Borson, S, Carpenter, BD, Del Campo, N, et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc . (2015) 16:731–9. doi: 10.1016/j.jamda.2015.06.017
8. Dunn, B, Stein, P, and Cavazzoni, P. Approval of Aducanumab for Alzheimer disease-the FDA's perspective. JAMA Intern Med . (2021) 181:1276–8. doi: 10.1001/jamainternmed.2021.4607
9. van Dyck, CH, Swanson, CJ, Aisen, P, Bateman, RJ, Chen, C, Gee, M, et al. Lecanemab in early Alzheimer's disease. N Engl J Med . (2023) 388:9–21. doi: 10.1056/NEJMoa2212948
10. Johnson, KA, Minoshima, S, Bohnen, NI, Donohoe, KJ, Foster, NL, Herscovitch, P, et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. J Nucl Med . (2013) 54:476–90. doi: 10.2967/jnumed.113.120618
11. Shaw, LM, Arias, J, Blennow, K, Galasko, D, Molinuevo, JL, Salloway, S, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement . (2018) 14:1505–21. doi: 10.1016/j.jalz.2018.07.220
12. Cummings, J, Lee, G, Zhong, K, Fonseca, J, and Taghva, K. Alzheimer's disease drug development pipeline: 2021. Alzheimers Dement . (2021) 7:e12179. doi: 10.1002/trc2.12179
13. Rabe, C, Bittner, T, Jethwa, A, Suridjan, I, Manuilova, E, Friesenhahn, M, et al. Clinical performance and robustness evaluation of plasma amyloid-beta(42/40) prescreening. Alzheimers Dement . (2023) 19:1393–402. doi: 10.1002/alz.12801
14. Janelidze, S, Teunissen, CE, Zetterberg, H, Allue, JA, Sarasa, L, Eichenlaub, U, et al. Head-to-head comparison of 8 plasma amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol . (2021) 78:1375–82. doi: 10.1001/jamaneurol.2021.3180
15. Weber, DM, Kim, JC, Goldman, SM, Clarke, NJ, and Racke, MK. New plasma LC-MS/MS assays for the quantitation of beta-amyloid peptides and identification of apolipoprotein E proteoforms for Alzheimer’s disease risk assessment . Submitted for publication.
Google Scholar
16. Morris, JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology . (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
17. Folstein, MF, Folstein, SE, and McHugh, PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res . (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
18. Duara, R, Loewenstein, DA, Greig, M, Acevedo, A, Potter, E, Appel, J, et al. Reliability and validity of an algorithm for the diagnosis of normal cognition, mild cognitive impairment, and dementia: implications for multicenter research studies. Am J Geriatr Psychiatry . (2010) 18:363–70. doi: 10.1097/JGP.0b013e3181c534a0
19. Duara, R, Loewenstein, DA, Lizarraga, G, Adjouadi, M, Barker, WW, Greig-Custo, MT, et al. Effect of age, ethnicity, sex, cognitive status and APOE genotype on amyloid load and the threshold for amyloid positivity. Neuroimage Clin . (2019) 22:101800. doi: 10.1016/j.nicl.2019.101800
20. Landau, SM, Breault, C, Joshi, AD, Pontecorvo, M, Mathis, CA, Jagust, WJ, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med . (2013) 54:70–7. doi: 10.2967/jnumed.112.109009
21. CLSI. Evaluation of precision of quantitative measurement procedures; approved guideline—Third edition. CLSI document EP05-A3 . Wayne, PA: Clinical and Laboratory Standards Institute (2014).
22. CLSI. User verification of linearity implementation guide In: CLSI implementation guide EP06-Ed2-IG . 1st ed: Clinical and Laboratory Standards Institute (2022).
23. CLSI. Interference testing in clinical chemistry In: CLSI guideline EP07 . 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute (2018).
24. CLSI. Evaluation of detection capability implementation guide In: CLSI Implementation Guide EP17-Ed2-IG . 1st ed: Clinical and Laboratory Standards Institute (2021).
25. CLSI. Evaluation of stability of in vitro diagnostic reagents; approved guideline. CLSI document EP25-A . Wayne, PA: Clinical and Laboratory Standards Institute (2009).
26. DeLong, ER, DeLong, DM, and Clarke-Pearson, DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics . (1988) 44:837–45. doi: 10.2307/2531595
27. Youden, WJ. Index for rating diagnostic tests. Cancer . (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
28. Pepe, MS. The statistical evaluation of medical tests for classification and prediction . Oxford: Oxford University Press (2003).
29. Hu, Y, Kirmess, KM, Meyer, MR, Rabinovici, GD, Gatsonis, C, Siegel, BA, et al. Assessment of a plasma amyloid probability score to estimate amyloid positron emission tomography findings among adults with cognitive impairment. JAMA Netw Open . (2022) 5:e228392. doi: 10.1001/jamanetworkopen.2022.8392
30. West, T, Kirmess, KM, Meyer, MR, Holubasch, MS, Knapik, SS, Hu, Y, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener . (2021) 16:30. doi: 10.1186/s13024-021-00451-6
31. Brand, AL, Lawler, PE, Bollinger, JG, Li, Y, Schindler, SE, Li, M, et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer's disease: a literature review. Alzheimers Res Ther . (2022) 14:195. doi: 10.1186/s13195-022-01117-1
32. Pascual-Lucas, M, Allue, JA, Sarasa, L, Fandos, N, Castillo, S, Terencio, J, et al. Clinical performance of an antibody-free assay for plasma Abeta42/Abeta40 to detect early alterations of Alzheimer's disease in individuals with subjective cognitive decline. Alzheimers Res Ther . (2023) 15:2. doi: 10.1186/s13195-022-01143-z
33. Walter, M, Wiltfang, J, and Vogelgsang, J. Pre-analytical sampling and storage conditions of amyloid-beta peptides in venous and capillary blood. J Alzheimers Dis . (2020) 78:529–35. doi: 10.3233/JAD-200777
34. Taylor, SW, Clarke, NJ, and McPhaul, MJ. Quantitative amino acid analysis in insulin and C-peptide assays. Clin Chem . (2016) 62:1152–3. doi: 10.1373/clinchem.2016.256313
35. Chen, J, Zhao, X, Zhang, W, Zhang, T, Wu, S, Shao, J, et al. Reference intervals for plasma amyloid-beta, total tau, and phosphorylated tau181 in healthy elderly Chinese individuals without cognitive impairment. Alzheimers Res Ther . (2023) 15:100. doi: 10.1186/s13195-023-01246-1
36. Anderson, J, and Pierce, C. PrecivityAD for diagnosis of Alzheimer disease. Am Fam Physician . (2022) 105:79–81.
PubMed Abstract | Google Scholar
37. O'Connor, A, Pannee, J, Poole, T, Arber, C, Portelius, E, Swift, IJ, et al. Plasma amyloid-beta ratios in autosomal dominant Alzheimer's disease: the influence of genotype. Brain . (2021) 144:2964–70. doi: 10.1093/brain/awab166
38. Keshavan, A, Pannee, J, Karikari, TK, Rodriguez, JL, Ashton, NJ, Nicholas, JM, et al. Population-based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain . (2021) 144:434–49. doi: 10.1093/brain/awaa403
39. Nakamura, A, Kaneko, N, Villemagne, VL, Kato, T, Doecke, J, Dore, V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature . (2018) 554:249–54. doi: 10.1038/nature25456
40. Weber, DM, Tran, D, Goldman, SM, Taylor, SW, Ginns, EI, Lagier, RJ, et al. High-throughput mass spectrometry assay for quantifying beta-amyloid 40 and 42 in cerebrospinal fluid. Clin Chem . (2019) 65:1572–80. doi: 10.1373/clinchem.2018.300947
41. Zicha, S, Bateman, RJ, Shaw, LM, Zetterberg, H, Bannon, AW, Horton, WA, et al. Comparative analytical performance of multiple plasma Abeta42 and Abeta40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimers Dement . (2022) 19:956–66. doi: 10.1002/alz.12697
42. Bun, S, Ito, D, Tezuka, T, Kubota, M, Ueda, R, Takahata, K, et al. Performance of plasma Abeta42/40, measured using a fully automated immunoassay, across a broad patient population in identifying amyloid status. Alzheimers Res Ther . (2023) 15:149. doi: 10.1186/s13195-023-01296-5
43. Hou, Y, Dan, X, Babbar, M, Wei, Y, Hasselbalch, SG, Croteau, DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol . (2019) 15:565–81. doi: 10.1038/s41582-019-0244-7
44. Li, Y, Schindler, SE, Bollinger, JG, Ovod, V, Mawuenyega, KG, Weiner, MW, et al. Validation of plasma amyloid-beta 42/40 for detecting Alzheimer disease amyloid plaques. Neurology . (2021) 98:–13211. doi: 10.1212/WNL.0000000000013211
45. Campos, M, Edland, SD, and Peavy, GM. Exploratory study of apolipoprotein E epsilon4 genotype and risk of Alzheimer's disease in Mexican Hispanics. J Am Geriatr Soc . (2013) 61:1038–40. doi: 10.1111/jgs.12292
46. Weiss, J, Hossain, S, Maldonado, AI, Shen, B, Beydoun, HA, Kivimaki, M, et al. Associations between race, APOE genotype, cognition, and mortality among urban middle-aged white and African American adults. Sci Rep . (2021) 11:19849. doi: 10.1038/s41598-021-98117-2
47. DeSimone, JC, Wang, W, Loewenstein, DA, Duara, R, Smith, GE, KN, McFarland, et al. Diffusion MRI relates to plasma Aβ42/40 in PET negative participants without dementia. Alzheimer’s Dement . (2024). doi: 10.1002/alz.13693 (Online ahead of print).
48. Janelidze, S, Palmqvist, S, Leuzy, A, Stomrud, E, Verberk, IMW, Zetterberg, H, et al. Detecting amyloid positivity in early Alzheimer's disease using combinations of plasma Abeta42/Abeta40 and p-tau. Alzheimers Dement . (2022) 18:283–93. doi: 10.1002/alz.12395
49. Palmqvist, S, Tideman, P, Cullen, N, Zetterberg, H, and Blennow, Kthe Alzheimer’s Disease Neuroimaging Initiative, et al. Prediction of future Alzheimer's disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med . (2021) 27:1034–42. doi: 10.1038/s41591-021-01348-z
50. Cullen, NC, Leuzy, A, Palmqvist, S, Janelidze, S, Stomrud, E, Pesini, P, et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat Aging . (2021) 1:114–23. doi: 10.1038/s43587-020-00003-5
51. Bellaver, B, Povala, G, Ferreira, PCL, Ferrari-Souza, JP, Leffa, DT, Lussier, FZ, et al. Astrocyte reactivity influences amyloid-beta effects on tau pathology in preclinical Alzheimer's disease. Nat Med . (2023) 29:1775–81. doi: 10.1038/s41591-023-02380-x
Keywords: Alzheimer’s disease, beta-amyloid, blood biomarkers, LC-MS/MS, PET, prescreening
Citation: Weber DM, Taylor SW, Lagier RJ, Kim JC, Goldman SM, Clarke NJ, Vaillancourt DE, Duara R, McFarland KN, Wang W-e, Golde TE and Racke MK (2024) Clinical utility of plasma Aβ42/40 ratio by LC-MS/MS in Alzheimer’s disease assessment. Front. Neurol . 15:1364658. doi: 10.3389/fneur.2024.1364658
Received: 02 January 2024; Accepted: 08 March 2024; Published: 25 March 2024.
Reviewed by:
Copyright © 2024 Weber, Taylor, Lagier, Kim, Goldman, Clarke, Vaillancourt, Duara, McFarland, Wang, Golde and Racke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darren M. Weber, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Case report
- Open access
- Published: 27 March 2024
Donepezil-induced bradycardia in a schizophrenic patient with comorbid neurocognitive disorder: a case report and review of the literature
- Nkolika Odenigbo 1 ,
- Stanley Nkemjika 1 ,
- Ayodele Atolagbe 1 ,
- Christian Nwabueze 1 ,
- Connie Olwit 2 ,
- Jeffery Lawrence 1 &
- Tolulope Olupona 1
Journal of Medical Case Reports volume 18 , Article number: 129 ( 2024 ) Cite this article
109 Accesses
Metrics details
Trials of cholinergic and glutamatergic agents have improved cognition and memory for the geriatric schizophrenic population. Donepezil is an acetylcholinesterase inhibitor that improves cognition by preventing postsynaptic degradation of hippocampal acetylcholine in patients with mild-to-moderate dementia. Donepezil has been attributed to some adverse effects, especially gastrointestinal symptoms. However, cardiovascular adverse effects are not common as there remains a dearth of literature regarding donepezil-induced bradycardia.
Hence, we present the case of a 70-year-old Hispanic female with past psychiatry history of schizophrenia who developed bradycardia and syncope following the commencement of low-dose donepezil in the inpatient unit and subsequent resolution with cessation. She had no prior cardiovascular symptoms or diagnosis.
Considering there is no baseline cardiac monitoring requirement guideline for patients on Donepezil treatment, pre-assessment electrocardiogram is advised before the commencement of acetylcholinesterase inhibitors. Finally, routine monitoring of vital signs for at least the first 72 hours following the start of donepezil might be good proactive practice for all psychiatrists. Extending this practice to inpatient and outpatient service settings will be worthwhile.
Peer Review reports
Introduction
Acetylcholinesterase inhibitor (AI) is a class of medications that improves cognition by preventing post-synaptic degradation of hippocampal acetylcholine in patients with mild-to-moderate dementia [ 1 ]. AI has also been described in the literature as an adjunct agent in managing mood symptoms. Donepezil, the most common class of AI, is widely used as the mainstay treatment of mild-to-moderate Alzheimer’s disease (AD). Donepezil’s pharmacodynamics promotes acetylcholine binding to nicotinic acetylcholine receptors in the brain [ 2 ]. Hence, improving the ability to interact with people, memory function, and attention. As with most pharmacological agents, donepezil trials in randomized controlled trial studies described myriads of documented attributable adverse effects, which are cholinergic dependent [ 3 , 4 ]. Notable documented adverse effects are gastrointestinal symptoms, while other side effects are sparingly reported in the literature [ 4 ]. Cardiovascular adverse effects are uncommon, but they have been mentioned in hospital settings as syncope, bradycardia, atrioventricular block, and QT prolongation among patients with comorbid cardiac problems [ 5 ]. However, there remains a paucity of literature regarding donepezil-induced bradycardia (DIB) in patients without comorbid cardiovascular disease. Bradycardia owing solely to donepezil pharmacotherapy without concurrent antiarrhythmic therapy in geriatric patients is also unusual. Additionally, bradycardia has not been described among patients with Schizophrenia. Hence, we present a case of a patient who developed bradycardia and syncope following the commencement of low-dose donepezil in the inpatient psychiatric unit and subsequent resolution with cessation of donepezil.
A 70-year-old Hispanic female presented to the psychiatric emergency room with worsening confusion, grossly disorganized behavior, forgetfulness, impaired activities of daily living, and inattentiveness. The patient’s family reported that her symptoms started a month prior to her presentation. Her outpatient provider also reported that during patient’s recent outpatient follow up visit, she demonstrated grossly disorganized behavior and reported noncompliance with her psychiatric medication regimen. The patient’s history was notable for schizophrenia, hypertension, hypothyroidism, dyslipidemia, and mild dementia.
On evaluation, the patient appeared disheveled with poor grooming. Her affect was constricted. She endorsed auditory hallucinations. Paranoid and persecutory delusions were elicited. Her speech was also markedly disorganized. Her chest radiograph, urine studies, and serum electrolytes were unremarkable. Head computed tomography (CT) at presentation revealed bitemporal lobe microvascular changes and electrocardiogram (EKG) was normal. She was recommenced on her home medications of haloperidol 5 mg orally twice per day and olanzapine 7.5 mg orally at bedtime. Psychosis improved with recommencement of her home medications in the inpatient psychiatric unit. However, she remained confused, and short and long-term memory loss persisted. Other patients reported that she was very distressed. She was evaluated by the neurology team and commenced a trial of donepezil 5 mg PO daily on the 7th day of inpatient psychiatric stay.
Baseline vital signs prior to the commencement of donepezil indicated blood pressure of 118/73 mmHg, pulse rate of 73 bpm, respiratory rate of 16 cpm, temperature of 98.4 F, and oxygen saturation of 98%. Basal metabolic rate (BMI) was 19.5 kg/m 2 . The baseline thyroid stimulating hormone (TSH) at the time of admission was normal at 2.69 mIU/L, and free thyroxine (T4) was 1.23 ng/dL.
On her 9th day of inpatient psychiatric admission, she was observed to have fallen in the day room but was immediately arousable. Her heart rate was 42 bpm, and her blood pressure was 110/76 mmHg. There was no head trauma. She was transferred to the telemetry unit.
The EKG done in the telemetry unit showed marked sinus bradycardia of 40 bpm. Her corrected QT interval (QTc) was unremarkable at 430 ms. Her blood pressure was normal in the telemetry unit. Chest radiograph showed no infiltrates or acute cardiopulmonary disease. Her hemoglobin (10.8 g/dL) and hematocrit level (33%) remained unchanged from baseline. The iron panel was normal except for a slight elevation in ferritin of 158 mg/L. The echocardiogram showed an ejection fraction of 60% with mild tricuspid and mitral valve regurgitation. Cardiac pacing or atropine was not indicated as her vitals remained stable. The carotid Doppler study showed no stenosis and repeat head CT imaging showed no acute changes. Fasting blood glucose was 118 mg/dL. All medications were stopped with an improvement of her heart rate to the 60 seconds over a 48 hour period. The patient did not have any repeat episodes of syncope or dizziness. Haloperidol 5 mg orally twice daily and olanzapine 7.5 mg orally at bedtime were recommenced on the 12th day of admission without any reduction in heart rate. Recommencement of donepezil on the 14th day reduced her heart rate from baseline 60s to late 40s and was identified as the causal agent.
She was subsequently discharged home from the medical unit on haloperidol 5 mg orally twice daily and olanzapine 7.5 mg orally at bedtime on the 20th day of admission. Patient followed up with outpatient cardiology and psychiatry clinic and recorded clinical improvement with stable vital signs.
Donepezil is a commonly used AI in inpatient and outpatient medical and neurological settings [ 6 , 7 ]. There is also sparing use of donepezil in inpatient psychiatric settings to control mood and behavioral concerns. Though there is documented evidence of adverse gastrointestinal effects with donepezil pharmacotherapy [ 8 ], this was not evident in our patient. Instead, bradyarrhythmia was evident, which resolved following the termination of donepezil pharmacotherapy. Our patient did not have acute or chronic cardiovascular disease symptoms and was not on any cardiovascular medications with the potential for a drug-to-drug interaction that may manifest as bradycardia. Cases of drug-induced bradycardia attributable to donepezil therapy are rare in literature [ 9 ], especially among patients without history of antiarrhythmic medications.
We reviewed the literature on the EMBASE, PSYCHINFO, and PubMed databases regarding evidence on the adverse presentation of DIB. The search results showed a gap in the literature on the topic, especially regarding the psychiatric population without comorbid cardiovascular problems. Notably, evidence in the literature suggests synergistic pharmacodynamic effects of donepezil with other antiarrhythmic agents on heart rate [ 10 ]. Additionally, the literature’s findings also indicate that the role of genetics might be in play as inherited cardiac muscle defects have been reported as a risk factor [ 11 ]. Other reported risk factors are higher doses of antiarrhythmic, pharmacological naivety, and advanced in age [ 12 ]. On the basis of our case presentation, the only attributable factor present was the age of this patient. Considering that this was the initial exposure of donepezil for our patient, this also suggests that the notion of AI or psychotropic naivety may have been a probable cause of DIB. Hence, an incidental finding of bradycardia following 2 days of commencement of donepezil therapy, in the absence of any identifiable cause, is exceptional.
Bradycardia, a heart rate below 50–60 bpm, is potentially life-threatening, and its causality could be physiological or pathological in origin [ 8 , 9 , 12 , 13 ]. Though different causes have been postulated in patients with comorbid cardiac problems and age-related differences, possible genetic predispositions have also been documented. There was no conduction disorder of the heart before the commencement of medications in our patient, which started following the initiation of a low dose of donepezil. Hence, we concluded that donepezil induced the observed changes. Additionally, geriatric patients with bradycardia may present with syncopal episodes or dizziness. Similarly, there are reports of increased falls and head trauma owing to comorbidities, gait impairment, and musculoskeletal deconditioning [ 14 ]. However, there is not enough literature with regards to this incident.
Regarding pathophysiology, the documented evidence suggests that dizziness, syncope, and bradycardia are more familiar adverse effects with rivastigmine [ 15 ] (another AI class). However, donepezil may cause bradyarrhythmia by increasing acetylcholine availability peripherally with cardiac muscarinic M2 receptor activation [ 16 ]. Considering that bradycardia is a rarely reported adverse effect of donepezil, AIs can exert their pharmacodynamic properties via vagotonic effects on the sinoatrial (SA) node, resulting in bradyarrhythmia [ 16 ]. Similarly, elderly patients on antiarrhythmics, especially beta-blockers and calcium channel blockers with augmented treatment using AI, are at greater risk for bradycardia [ 10 ]. Regarding the severity of the patient’s symptoms, medication dose tapering might be considered in mild bradycardia as the overall benefit may outweigh the adverse effect. In this case report, considering the acuteness of the presentation in a patient without any comorbid cardiovascular concern, we halted the medication with a successful resolution. Other authors have reported successful resolution following a switch to another AI, such as rivastigmine [ 17 ].
Our case provides evidence of the dearth of literature on bradycardia resolved after the halt of medications. Thus, this case is also unique because the patient was not on any cardiovascular medication with the potential for drug-to-drug interaction nor the capability of reducing the heart rate. This patient did not have any baseline cardiac abnormality before admission and commencement of medication, as evidenced by the typical EKG report, but was elderly. Notably, age had been described as a risk factor for DIB, but there is little evidence in literature to support it. Our finding is bolstered by a study by Bordier et al . [ 13 ], which reported that, among patients receiving donepezil therapy, 69% of those with syncope had an identifiable organic etiology. Hence, the dearth of literature on this occurrence.
DIB is unusual in patients without background comorbid cardiovascular problems, and its incidence rate is unknown. Presently, there are no evidence-based alternative AI recommendations for incidental DIB. For the case presented here, we resolved DIB by stopping the medication and reappearance of symptoms following retrial confirmed causality. Hence, switching to oral or even a transdermal rivastigmine patch could be an alternate treatment plan for such a scenario. Alternatively, N -methyl- d -aspartate (NMDA) receptor antagonists, such as memantine, are also a safe alternative in patients with similar bradycardia events.
Presently, there is no baseline cardiac monitoring requirement guideline for patients on donepezil treatment. Hence, we infer that pre-assessment EKG should be considered before the commencement of AIs. Moreover, routine monitoring of a patient’s vital signs for at least the first 72 hours following the start of medication, irrespective of the baseline EKG findings, might be good practice for all psychiatrists. Extending this practice to inpatient and outpatient service settings will be worthwhile.
Data availability
Not applicable.
Availability of supporting data
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–15. https://doi.org/10.2174/1570159x13666150716165726 .
Article CAS PubMed PubMed Central Google Scholar
Di Angelantonio S, Bernardi G, Mercuri NB. Donepezil modulates nicotinic receptors of substantia nigra dopaminergic neurones. Br J Pharmacol. 2004;141(4):644–52. https://doi.org/10.1038/sj.bjp.0705660 .
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50(1):136–45. https://doi.org/10.1212/wnl.50.1.136 .
Article CAS PubMed Google Scholar
Greenberg SM, Tennis MK, Brown LB, et al . Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol. 2000;57(1):94–9. https://doi.org/10.1001/archneur.57.1.94 .
Kuwahata S, Takenaka T, Motoya T, et al . Effect of QT prolongation in patients taking cholinesterase inhibitors (donepezil) for Alzheimer’s disease. Circul Rep. 2021;3(3):115–21. https://doi.org/10.1253/circrep.CR-20-0115 .
Article Google Scholar
Howard RJ, Juszczak E, Ballard CG, et al . Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med. 2007;357(14):1382–92. https://doi.org/10.1056/NEJMoa066583 .
Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry. 2005;162(4):676–82. https://doi.org/10.1176/appi.ajp.162.4.676 .
Jackson S, Ham RJ, Wilkinson D. The safety and tolerability of donepezil in patients with Alzheimer’s disease. Br J Clin Pharmacol. 2004;58(Suppl 1):1–8. https://doi.org/10.1111/j.1365-2125.2004.01848.x .
Howes LG. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug safety. 2014;37(6):391–5. https://doi.org/10.1007/s40264-014-0161-z .
Rosenbloom MH, Finley R, Scheinman MM, Feldman MD, Miller BL, Rabinovici GD. Donepezil-associated bradyarrhythmia in a patient with dementia with Lewy bodies (DLB). Alzheimer Dis Assoc Disord. 2010;24(2):209–11. https://doi.org/10.1097/WAD.0b013e3181b7642b .
Article PubMed PubMed Central Google Scholar
Mehta AV, Chidambaram B, Garrett A. Familial symptomatic sinus bradycardia: autosomal dominant inheritance. Pediatr Cardiol. 1995;16(5):231–4. https://doi.org/10.1007/bf00795713 .
Kokras N, Stamouli E, Sotiropoulos I, et al . Acetyl cholinesterase inhibitors and cell-derived peripheral inflammatory cytokines in early stages of Alzheimer’s disease. J Clin Psychopharmacol. 2018;38(2):138–43. https://doi.org/10.1097/jcp.0000000000000840 .
Bordier P, Lanusse S, Garrigue S, et al . Causes of syncope in patients with Alzheimer’s disease treated with donepezil. Drugs Aging. 2005;22(8):687–94. https://doi.org/10.2165/00002512-200522080-00005 .
Montero-Odasso M, Speechley M, Chertkow H, et al . Donepezil for gait and falls in mild cognitive impairment: a randomized controlled trial. Eur J Neurol. 2019;26(4):651–9. https://doi.org/10.1111/ene.13872 .
Knudtzen FC, Christophersen TB. Third degree atrioventricular block associated with treatment with rivastigmine transdermal patch. J Geriatr Cardiol JGC. 2013;10(1):113–5. https://doi.org/10.3969/j.issn.1671-5411.2013.01.017 .
Article PubMed Google Scholar
Basselin M, Nguyen HN, Chang L, Bell JM, Rapoport SI. Acute but not chronic donepezil increases muscarinic receptor-mediated signaling via arachidonic acid in unanesthetized rats. J Alzheimer’s Dis JAD. 2009;17(2):369–82. https://doi.org/10.3233/jad-2009-1058 .
Sadowsky CH, Dengiz A, Olin JT, Koumaras B, Meng X, Brannan S. Switching from donepezil tablets to rivastigmine transdermal patch in Alzheimer’s disease. Am J Alzheimer’s Dis Dement. 2009;24(3):267–75. https://doi.org/10.1177/1533317509333037 .
Download references
Acknowledgements
Author information, authors and affiliations.
Department of Psychiatry, Interfaith Medical Center, Brooklyn, NY, USA
Nkolika Odenigbo, Stanley Nkemjika, Ayodele Atolagbe, Christian Nwabueze, Jeffery Lawrence & Tolulope Olupona
Department of Nursing, Makere University, Kampala, Uganda
Connie Olwit
You can also search for this author in PubMed Google Scholar
Contributions
NO, SN, AA, CN, CO, JL, and OO contributed equally to all sections of this manuscript. SN and AA conducted the literature review. SN, AA, and NO drafted the main manuscript text. SN and OO critically reviewed, discussed, and modified the intellectual content of the article. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Connie Olwit .
Ethics declarations
Ethics approval and consent to participate.
Since this study is a case report, the study did not require any ethical approval.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
All authors declare that they have no financial relationships at present or within the previous 3 years with any organizations that might have an interest in the submitted work. All authors declare that there are no other relationships or activities that could appear to have influenced the submitted work. The authors declare no conflicts of interest. All authors consent to this manuscript’s publication.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Odenigbo, N., Nkemjika, S., Atolagbe, A. et al. Donepezil-induced bradycardia in a schizophrenic patient with comorbid neurocognitive disorder: a case report and review of the literature. J Med Case Reports 18 , 129 (2024). https://doi.org/10.1186/s13256-024-04454-x
Download citation
Received : 23 May 2023
Accepted : 12 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1186/s13256-024-04454-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anticholinesterase inhibitor
- Schizophrenia
- Bradycardia
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.7(8); 2017

Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016
Fidelia bature.
1 Institute for Health Research, Putteridge Bury Campus, University of Bedfordshire, Putteridgebury, Luton, UK
Barbara-ann Guinn
2 School of Life Sciences, The University of Hull, Hull, UK
Yannis Pappas
Associated data.
bmjopen-2016-015746supp001.pdf
Late diagnosis of Alzheimer’s disease (AD) may be due to diagnostic uncertainties. We aimed to determine the sequence and timing of the appearance of established early signs and symptoms in people who are subsequently diagnosed with AD.
We used systematic review methodology to investigate the existing literature. Articles were reviewed in May 2016, using the following databases: MEDLINE, PsycINFO, CINAHL, British Nursing Index, PubMed central and the Cochrane library, with no language restriction. Data from the included articles were extracted independently by two authors and quality assessment was undertaken with the quality assessment and diagnostic accuracy tool-2 (QUADAS tool-2 quality assessment tool).
We found that depression and cognitive impairment were the first symptoms to appear in 98.5% and 99.1% of individuals in a study with late-onset AD (LOAD) and 9% and 80%, respectively, in early-onset AD (EOAD). Memory loss presented early and was experienced 12 years before the clinically defined AD dementia in the LOAD. However, the rapidly progressive late-onset AD presented predominantly with 35 non-established focal symptoms and signs including myoclonus (75%), disturbed gait (66%) and rigidity. These were misdiagnosed as symptoms of Creutzfeldt-Jacob disease (CJD) in all the cases. The participant with the lowest mini-mental state examination score of 25 remained stable for 2 years, which is consistent with the score of the healthy family members.
Conclusions
The findings of this review suggest that neurological and depressive behaviours are an early occurrence in EOAD with depressive and cognitive symptoms in the measure of semantic memory and conceptual formation in LOAD. Misdiagnosis of rapidly progressive AD as CJD and the familial memory score can be confounding factors while establishing a diagnosis. However, the study was limited by the fact that each one of the findings was based on a single study.
Strengths and limitations of this study
- The review indicates a paucity of data on the study objectives and heterogeneity in the timing of symptoms presentation in published studies.
- Comprehensive search strategy was used to identify articles for this review.
- This is the first review to identify the sequence and timing of the signs and symptoms in the early stage of Alzheimer’s disease.
- Dearth of data, heterogeneity in methodology and findings made it impossible to draw a definite conclusion.
- Several other potential sources of heterogeneity like age, gender and education could not be investigated with the dearth of data.
Introduction
Alzheimer’s disease (AD), the most common type of dementia, is a devastating disease with multiple presentations. While the disease is associated with old age, scientists 1 2 have discovered that disease can develop at any age and the reason for this is unclear. The disease could develop before the age of 65 years, known as early-onset AD (EOAD), which might be inherited or sporadic, or after the age of 65 years, known as late-onset AD (LOAD), that accounts for 90% of all AD cases. 3 In the UK, a prevalence of 520 000 has been reported in 2014 4–6 with high individual, healthcare and financial burden. 7 8 There are challenges in diagnosing the disease early, 9–11 which can result to non-reversible symptoms progression, that lead to institutionalisation and high mortality rate among this group. 12 There is also the emotional and physical burden to the caregivers 13 14 as well as emotional, physical and financial burden to the healthcare system. 15 Even though there is discourse in the meaning of the early diagnosis, here, it refers to the diagnosis at the lowest threshold of the disease or at the stage of mild cognitive impairment (MCI), with cluster of early signs and symptoms and the diagnosis of the pathology of the disease before dementia. This is because the disease has a preclinical stage with the clinical symptoms yet evident but with changes in the brain and the risk of progression unknown, intermediate stage with mild cognitive and functional changes and dementia due to AD stage with severe cognitive and functional decline.
Among the reasons for the late diagnosis is that the signs and symptoms, at the early stages of AD, are sometimes not recognised and/or mistaken for signs of old age or symptoms of other conditions. 5 16–18 The above may be partly due to the fact that the timing and sequence of the early presentation of signs and symptoms are not reported by current studies. 19–21 Delaying onset of the disease by 5 years through early diagnosis and intervention could reduce the mortality rate of dementia (advanced stage of AD) by 30 000 yearly. 22
This review attempts to answer the following research question: how far back from diagnosis and in what sequence do the first symptoms that warrant an AD diagnosis appear? Further understanding of the timing and the sequence of the presentation of signs and symptoms may enable practitioners to offer timely intervention.
Types of studies
All types of empirical studies were considered, excluding those of qualitative design.
Participants
Included participants were aged between 30 and 85 years and diagnosed with AD.
Primary care, memory clinics or secondary care settings.
Target condition
AD and any subtypes were diagnosed with the following tools: (a) National Institute of Neurological and Communicative Disorders and Stroke AD and Related Disorders Association (NINCDS-ADRDA, UK), a commonly used criteria for AD dementia; (b) National Institute on Aging-Alzheimer’s Association (USA), a more recent criteria that use biomarkers to support the diagnosis; (c) Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV) 23 ; and (d) DSM-5. 24
The outcomes of this review included (i) the sequence of presentation of the signs and symptoms that are indicative of AD prior to diagnosis, 20 (ii) the timing from the first reported symptom to diagnosis, 20 (iii) the timing from MCI to diagnosed dementia stage, 25 (iv) the timing of assessments leading up to a diagnosis of AD 26 and (v) the timing from clinical presentations to case fatality or death. 27
Index symptoms
We used an index of early symptoms as a reference to ascertain the timing and sequence of events prior to disease presentation. The index is based on previous studies, 28–32 which includes apathy, agitation, anxiety, anosognosia, aberrant motor behaviour, acalculia, alexia, anomia, disinhibition, depression, irritability, hallucination and olfactory disturbances and weight loss. 28–32
Exclusion criteria
- Participants with other dementia or other neurological conditions;
- Inaccurate diagnostic criteria;
- Single index symptom;
- Late-stage AD (AD dementia); a set of symptoms including memory loss, difficulty in thinking, problem solving or language difficulties. 33
Search criteria for identification of studies
We searched the literature via OvidSP MEDLINE (1950), PsycINFO (1887), British Nursing Index (1994), CINAHL (1937), PubMed central (2000) and the Cochrane register for diagnostic and intervention studies. We also used ‘snowballing’ and searched the references of relevant articles. Searches covered the period from 1937 to May 2016. No language or publication restrictions were applied. We used medical subject headings terms to standardise and improve the search; AD was the main term followed by the basic terms timing, onset and country, and the combination of terms. Details of the database search strategies are presented in online supplementary appendix 1 .
Data collection and analysis
Assessment of methodological quality.
The qualities of included studies were assessed using the QUADAS-2 tool, a methodological quality assessment tool used to assess diagnostic accuracy studies 34 ( table 1 ) and PRISMA checklist. The tool consists of 14 items that rates the risk of bias, source of variations (applicability and reporting of quality), with each item rated as ‘yes’, ‘no’ or ‘unclear’, tailored under four domains that includes: participants selection; index test (signs and symptoms interpretation), reference standard (diagnostic criteria that correctly classify the target condition) and flow and timing (time interval and intervention between index test and reference standard).
Quality assessment using the QUADAS tool
Results of the search
The process by which articles were identified, screened and selected for the review is described in figure 1 . A total of 3528 articles were identified in the databases including 318 duplicates. Nine others were identified through hand searching, and 3179 were excluded based on the review of titles and abstract alone. The full-text versions of 40 were assessed for eligibility, 13 were initially included but 9 later excluded (reasons stated below). Four articles were finally included in the review.

Flowchart indicating the process for the selection of studies. The flowchart indicates the articles identified through the search: those reviewed as title and abstract, those reviewed fully and the ones that met the inclusion criteria.
Reasons for exclusion
Although 13 studies were reviewed in full, 9 were excluded. The reasons for exclusion were: four studies were on unspecified dementia 30 35–37 ; one study was undertaken in a developing country 38 ; another on caregiver’s distress 31 ; one study was on a single case 22 ; one study had incomplete data, 39 while another did not have a reference point for the diagnosis of AD. 19
Summary of findings
Methodological quality of included studies.
The methodological quality in each domain was assessed individually.
The QUADAS-2 scores for each domain ( table 1 ) of the studies included in the review are shown in figures 2 and 3 . The reviewer included a nested case control with random sampling, 25 longitudinal follow-up of patients with MCI, 20 longitudinal prospective study of individuals at risk of autosomal dominant familial AD (FAD) 26 and a retrospective case study (postmortem). 27 For the case studies, 20 26 27 the exclusion criteria were appropriate and sample selection was consecutive, which reduced the risk of selection bias. ( table 2 , figure 2 )

Graph representing the risk of bias and applicability concerns. Each domain is represented as a percentage across included studies for the review; the red colour indicates high risk, while green indicates low risk. However, none of the studies were given an unclear risk of bias and applicability concerns (QUADAS-2 tool).

Summary of the risk of bias and applicability concerns. The reviewer’s judgement on each domain for the included studies is shown with a high risk of bias and applicability concerns on index test for. 32
Summary of study methodology and key findings
AD, Alzheimer’s disease; EOAD; early-onset AD; MMSE, mini-mental state examination.
The index test was not influenced by the reference standard in three studies. 20 25 26 However, the index test domain was judged as having a high risk of bias in a study 27 due to the fact that the index tests were interpreted based on the knowledge of the disease (postmortem). In the applicability concerns, the conduct and interpretation of the index symptoms were different from the review question in Fox et al 26 and Schmidt et al . 27 The Fox et al 26 study focused on the mean time from first assessment to the appearance of symptoms at reporting, while the study published by Schmidt et al 27 focused on identifying the median time span from clinical presentation of the disease to case fatality or death.
In the reference standard domain, all studies were undertaken using the diagnostic criteria for AD, recognised internationally that could correctly classify the condition with masking in all. The Schmidt et al study 27 on rapidly progressive AD (RPAD) was undertaken postmortem, the gold standard for the diagnosis of AD. However, none of the studies reported how the reference standard was operationalised or applied. They were assessed as being a low risk of concern about applicability.
In the flow and timing domain, there was an appropriate interval between the appearance of symptoms and signs and the reference standard. There was no mention of treatment in between the timings and all of the participants were diagnosed using the same reference standard. All participants were included in the analysis.
Of the 148 participants in the Devier et al study, 20 39 (26%) converted to AD and all of the converters were 55 years at baseline, indicating an EOAD. There were differences in the first symptom at presentation with memory decline reported as the first in 118 (80%) of the cases, depressed mood in 13 (9%), declined language in 6 (4%), change in performance of higher order/cognitive activities in 4 (3%), disorientation in 3 (2%), personality changes and behavioural changes in 2 (1%), with no group difference in symptoms reporting. Sequentially in the order of appearance of the signs and symptoms in all the participants, memory decline was the first followed by performance changes, changes in language, disorientation, personality changes, depressed mood, behavioural changes and psychosis, consecutively. However, for depression, reverse causality could be the case, as the history of depression with the first onset before the age of 60 years represents a risk of developing AD in later life 40 and all cause dementia. 41
Memory decline was experienced in 38.5 months before diagnosis, 20 depressed mood in 37.4 months, performance in 36.8 months, personality changes in 32.5 months, behavioural changes in 31.1 months, language difficulties at 29.2 months, disorientation in 29.1 months and psychosis at 14.0 months prior to the diagnosis.
Outcome III
Amieva et al 25 study reported cognitive decline 12 years before dementia in a measure of semantic memory and conceptual formation. Depressive symptoms appeared concomitantly with the cognitive decline and followed 2 years later with verbal memory decline. Two years later, visual disturbances were recorded and worsened until the dementia stage.
Of the 63 subjects in the Fox et al 26 study of autosomal dominant FAD, 10 converted to probably AD and the mean time (±SD) from first assessment to the appearance of symptoms was 2.6±1.4 years. Episodic memory loss was the most common and noticed on average 6 months before symptomatic assessment. The study suggests that cognitive decline is present 2–3 years before symptoms and 4–5 years before individuals fulfil the criteria for probably AD. There was no distinction in presentations with regards to age, gender and handedness. Verbal memory was superior to semantic memory in differentiating AD from normal ageing, with the lowest score in mini-mental state examination (MMSE) of 25 in a participant remaining stable for 2 years consistent with family members with the same score that remained healthy. This could help discriminate individuals at risk of conversion.
Thirty-five distinct neurological, psychiatric and autonomic symptoms and signs were identified in the Schmidt et al 27 study. The sequence and timing in months (averagely 26.4) of the presentation of the signs and symptoms were as follows: disinhibition, 51.1; spasticity, 31.1; dysphagia, 21.6; akinetic mutism, 20.0; significant weight loss, 20.0; apraxia, 19.5; apathy, 17.0; sleep disorder, 16.0; delusions, 15.0; myoclonus, hallucinations, seizures, 13.0; impaired concentration, 4.5; depression, 4.0; and disorientation, 2.0, with others following thereafter. A third of RPAD experienced rapid weight loss and sleep disorder, indicating their significance in discriminating the disease from other dementias.
Signs and symptoms
A pooled estimate was not possible to be reported due to the differences in participants, symptoms and types of AD, as well the scarcity of research that had reported on the sequence and timing of the early signs and symptoms. MCI was required at baseline in the Devier et al study, 20 with memory complaints 6 months to 10 years prior to enrolment. The study began long before the Petersen 42 MCI criteria definition. Prior to enrolment, memory loss was observed on average of 38.5; depressed mood, 37.4; performance, 36.8; personality, 32.1; behaviour deficits, 31.1; language deficits, 29.2; disorientation, 29.1 and psychosis, 14.0 months, before diagnosis.
For the 10 converters in the Fox et al study, 26 the mean time (±SD) from initial assessment to first symptomatic assessment was 3.1±1.5 years (range 1–5 years). The most common presentations were symptoms of very mild deficit in episodic memory. Two of the 10 subjects already had deficits in verbal memory and were the first to be symptomatic. Verbal memory deficit was observed 1–5 years during the symptomatic phase, indicating higher early sensitivity than the semantic memory and cognitive changes 2–3 years before the symptomatic phase. There was no difference observed between cases and non-converters in terms of age, gender, handedness or MMSE at initial assessment and symptomatic assessment.
In the Schmidt et al 27 study, the median disease duration was 26.4 months and the median age at clinical onset was 73 years. The authors were unable to obtain a summary of the data from the onset of the symptoms to disease diagnosis.
All the studies were diagnosed with the NINCDS-ADRDA diagnostic criteria and symptoms measured with the neuropsychiatry inventory score.
Four studies met the inclusion criteria, which had heterogeneous objectives, diagnosis and participants. The four studies had a total of 593 people who were followed for conversion to AD. All the studies assessed the timing of the signs and symptoms of AD prior to a formal diagnosis and/or case fatality, but with different participants and type of AD.
Studies were assessed methodologically with the QUADAS-2 tool. Three of the included studies 20 26 27 validated their results via NINCDS-ADRDA and one study 27 via postmortem examination.
Even though there were differences in timing, objectives, participants and type of AD, the Fox et al 26 study on FAD identified a participant with an MMSE score of 25/30, the lowest in the group, that remained the same for 2 years, similar to family members that remained well throughout. This supports the evidence that MMSE offers a reasonably good diagnosis and classification of AD, 43 especially the accuracy of the MMSE baseline score. However, critics advised that the measurement should be interpreted with caution. 44 45 Furthermore, Schmidt et al 27 discovered additional focal neurological symptoms consistent with Creutzfeldt-Jacob disease (CJD); AD was misdiagnosed as CJD until the postmortem study proved AD as the cause of the presentations. This finding is in line with Mega et al 29 and Zahodne et al , 46 who reported that there are measurable behavioural changes in AD and suggested that focal neurological symptoms are associated with poor prognosis. 46
Memory disturbances remain the predominant differentiating factor between early AD and normal ageing in all of the studies. Verbal memory was more vulnerable than non-verbal in the early-onset familial AD. 26 The memory test for words indicated significant differences in scores, 1–5 years before becoming symptomatic, against the notion of semantic memory vulnerability.
Depressed symptoms appeared at the same time as cognitive symptoms, and each of these was the first symptom to appear in some individuals with LOAD. However, memory loss presented early and frequently in this group too. 20 40 The rapidly progressive LOAD 42 presented predominantly with myoclonus (75%), disturbed gait (66%) and rigidity (50%). These symptoms were also early in the presentation process occurring before apathy. Neurological and depressive behavioural presentations are an early occurrence in EOAD. 20 This calls for further studies to identify the sequence and timing of the early signs and symptoms preceding the diagnosis, to aid the early detection and subsequent diagnosis of AD.
The main limitation of this systematic review was the dearth of data and heterogeneity in methodology and findings in the included studies. Moreover, pooled estimate or statistical analysis for the signs and symptoms was not possible to be calculated and several other potential sources of heterogeneity like the age of onset, gender and education could not be investigated given the paucity of relevant data.
We excluded studies on individual symptoms and signs, as well as other types of dementia, where it was not possible to isolate AD. Further and rigorous research is needed to understand the timing and sequence of the appearance of the signs and symptoms that elude to AD prior to diagnosis, with the aim of supporting as early an AD diagnosis as possible.
There is a proposition of multiple definitions including MCI and subjective cognitive decline to capture the intermediate stage between ageing and mild cognitive changes, which is in line with the effort to diagnose AD early, by recognising the signs and symptoms as reliable predictive markers of the disease. 46
There are currently insufficient published data on the sequence and timing of the early signs and symptoms of AD. We advocate that more research should be undertaken in this area.
This review is important to general practitioners, researchers, health policymakers, the pharmaceutical industry and the public. The review is also of importance to neurologists and other practitioners dealing with dementing disorders.
Supplementary Material
Contributors: FB and BG conceived the study and participated in the design and drafting of the manuscript. DP participated in the analysis and helped to draft the manuscript. YP suggested the design and participated in the selection of studies, design and drafting of the manuscript. FB developed the protocol of the study, conducted the analysis and drafted the manuscript. FB, DP, BG and YP read and approved the final manuscript for this publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.

IMAGES
COMMENTS
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Alzheimer disease is a neurodegenerative disorder that causes cognitive impairment. This Primer by Knopman et al. reviews the epidemiology of cognitive manifestations and risk factors, summarizes ...
Alzheimer's disease (AD) is prevalent throughout the world and is the leading cause of dementia in older individuals (aged ≥ 65 years). To gain a deeper understanding of the recent literature on the epidemiology of AD and its progression, we conducted a review of the PubMed-indexed literature (2014-2021) in North America, Europe, and Asia. The worldwide toll of AD is evidenced by rising ...
This Review summarizes recent advances in biomarkers and therapies for Alzheimer disease—the products of decades of research—and discusses the challenges, gaps and clinical implications.
Mr. Vaibhav. G. Kute. View. ... Alzheimer's disease is a progressive neurodegenerative disease that causes brain cells to waste away and die. It is characterized by progressive cognitive ...
Introduction Alzheimer's disease (AD) is a disease continuum from pathophysiologic, biomarker and clinical perspectives. With the advent of advanced technologies, diagnosing and managing patients is evolving. Methods A systematic literature review (SLR) of practice guidelines for mild cognitive impairment (MCI) and AD dementia was performed following the Preferred Reporting Items for ...
Background Alzheimer's disease (AD) is the most common neurodegenerative disease worldwide, and an updated quantification of its impact on morbidity, disability, and mortality is warranted. We conducted a systematic literature review, focusing on the past decade, to characterize AD and assess its impact on affected individuals. Methods Searches of Embase, MEDLINE, and the Cochrane Library ...
Alzheimer's disease (AD) is a progressive neurodegenerative disease that impairs memory and cognitive judgment. It is the leading cause of dementia in late adult life and is associated with a significant social burden and increased morbidity and mortality in the elderly. Because of mixed effectiveness of medications, exercise has been ...
Alzheimer's disease (AD) is a disorder that causes degeneration of the cells in the brain and it is the main cause of dementia, which is characterized by a decline in thinking and independence in personal daily activities. AD is considered a multifactorial disease: two main hypotheses were proposed as a cause for AD, cholinergic and amyloid hypotheses. Additionally, several risk factors such ...
Reviewer #1 (Public Review): Summary: The authors aimed to infer the trajectories of long range and local neuronal synchrony across the Alzheimer's disease continuum, relative to neurodegeneration and cognitive decline. The trajectories are inferred using event-based models, which infer a set of data-driven disease stages from a given dataset.
Antioxidant vitamins hold promise for lowering the risk of Alzheimer's disease and cognitive decline in older persons. In animal models and cell lines, these vitamins, such as vitamins E and C, prevent neuronal damage caused by free radicals, delaying brain aging and, perhaps, memory loss.
Conclusion: The systematic literature review supported the argument that language and speech could successfully be used to detect dementia automatically. Future studies should aim for larger and more balanced datasets, combine data collection methods and the type of information analyzed, focus on the early stages of the disease, and report ...
Alzheimer's disease (AD) is a neurodegenerative disorder with a complex pathology that is not completely understood. Over time, AD reduces one's cortical and subcortical functioning. ... Marcucci V, Kleiman J. Biomarkers and Their Implications in Alzheimer's Disease: A Literature Review. Explor Res Hypothesis Med. 2021;6(4):164-176. doi ...
This study aimed to describe and characterize the published literature on disparities between racial and ethnic groups among individuals with Alzheimer's disease and related dementias. To identify relevant studies, we searched electronic sources for peer-reviewed articles and research reports published through 2014 related to the Alzheimer's population and their caregivers that provided ...
Background and Objective Exercise is a promising non-pharmacological therapy for subjective cognitive decline, but it is unclear which type of exercise is most effective. The objective was to assess the comparative effects and ranks of all exercise-based interventions on cognitive function in patients with subjective cognitive decline (SCD). Method In this network meta-analysis, Online ...
As this extended genetic region is known for its pathological association with many neurodegenerative disorders including Alzheimer's disease, we investigated whether the LIFO brain regions ...
Alzheimer's disease (AD) is prevalent throughout the world and is the leading cause of dementia in older individuals (aged ≥ 65 years). To gain a deeper understanding of the recent literature on the epidemiology of AD and its progression, we conducted a review of the PubMed-indexed literature (2014-2021) in North America, Europe, and Asia.
1 Introduction. Alzheimer's disease (AD) pathophysiology is characterized by cognitive impairment and the accumulation of extracellular beta-amyloid (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein in brain tissue (1-3).Because Aβ aggregation and deposition occurs 10-20 years prior to clinical presentation, plaque detection reflects an ...
Background Trials of cholinergic and glutamatergic agents have improved cognition and memory for the geriatric schizophrenic population. Donepezil is an acetylcholinesterase inhibitor that improves cognition by preventing postsynaptic degradation of hippocampal acetylcholine in patients with mild-to-moderate dementia. Donepezil has been attributed to some adverse effects, especially ...
Epidemiological and economic burden of Alzheimer's disease: a systematic literature review of data across Europe and the United States of America. ... Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; ...