- Get new issue alerts Get alerts

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Case report of long-term survival with metastatic triple-negative breast carcinoma
Treatment possibilities for metastatic disease.
Editor(s): NA.,
Lifespring Cancer Treatment Center, Seattle, WA.
∗Correspondence: Bryce Douglas La Course, Lifespring Cancer Treatment Center, 510A Rainier Avenue South, Seattle, WA (e-mail: [email protected] ).
Abbreviations: ALL = acute lymphoblastic leukemia, CA = cancer antigen, CT = computed tomography, DC = dendritic cell, ER = estrogen receptor, GM-CSF = granulocyte-macrophage-colony-stimulating factor, HER2 = human epidermal growth factor receptor 2, MTD = maximum tolerated dose, PET = positron emission tomography, PR = progesterone receptor, TNBC = triple-negative breast cancer, Tregs = regulatory T cells.
Written informed consent was obtained from the patient in the study for publication of this case report and any accompanying images.
The authors have no conflicts of interest to disclose.
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. http://creativecommons.org/licenses/by/4.0
Rationale:
Breast cancer is the most common as well as one of the most devastating cancers among women in the United States. Prognosis is poor for patients with metastatic breast cancer, especially for patients with so-called “triple-negative” disease. The lack of effective therapies for metastatic triple-negative breast cancer outlines the need for novel and innovative treatment strategies.
Patient concerns:
A 58-year-old underwent a mastectomy which revealed a recurrent triple-negative breast carcinoma. Afterward, she presented with a growing mass in her left axilla and chest wall. A computed tomography scan showed axillary and supraclavicular adenopathy, nodules in the left upper and lower lobe of the lungs, and 2 areas of disease in the liver. A bone scan showed lesions in the ribs.
Diagnosis:
The patient was diagnosed with a recurrent metastatic triple-negative breast carcinoma that spread to the lung, liver, and bones.
Interventions:
The patient was treated with metronomic chemotherapy, sequential chemotherapy regimens, and immunotherapy.
Outcomes:
The patient is now over 15 years out from her diagnosis of metastatic disease without any evidence of recurrent disease, likely due to the patient's treatment strategy which included sequential metronomic chemotherapy regimens and immunotherapy.
Lessons:
Sequential metronomic chemotherapy regimens in combination with immunotherapy might be an effective treatment option for patients with metastatic triple-negative breast cancer. We hope that this case can provide some guidance for the treatment of metastatic triple-negative breast cancer and motivate research that can potentially lead to more cases of long-term survival for patients who develop this dismal disease.
1 Introduction
Breast cancer remains the most common cancer diagnosed among women in the United States and is the second leading cause of cancer-related deaths, with approximately 41,000 patients projected to die from this disease in 2018 alone. [1] The prognosis for patients with metastatic breast cancer varies based on many factors including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status. Tumors that do not express the ER, PR, or HER2 receptors are known as “triple-negative” breast cancers (TNBCs) and represent approximately 11% of all breast cancers. [2] This subtype of breast cancer is known for being aggressive, having a high probability of distant recurrence after adjuvant therapy, and progressing quickly on palliative chemotherapy treatment in the metastatic setting. [2,3] Patients with metastatic TNBC have a poor prognosis, with a median overall survival of 13.3 months with treatment. [3] Continuing chemotherapy treatment until disease progression is currently the standard of care for patients with metastatic TNBC, with no preferred chemotherapy regimens established at this time. The lack of effective therapies for this aggressive disease highlights the need for the development of novel treatment strategies. Here we report the case of a patient with metastatic TNBC that metastasized to the lungs, liver, and bones who achieved long-term remission without evidence of disease recurrence after 15 years. Her case is followed by a discussion of the treatment strategy which likely has led to her remarkable survival.
2 Case report
A Caucasian female initially presented with a nodule in her left breast in March 2001 at the age of 56. Before this, the patient was a homemaker with a relatively unremarkable past medical history aside from some mild arthritis. Her father and brother had prostate cancer, and her mother apparently had uterine cancer. Her social history was negative for tobacco, alcohol as well as illicit drug use. A core biopsy performed in mid-April 2001 showed a high-grade infiltrating ductal carcinoma, with a Bloom–Richardson score of 9/9. Immunohistochemistry showed that the tumor was positive for ER expression and negative for PR expression and HER2 overexpression. The patient underwent a left lumpectomy with an axillary lymph node dissection shortly after the biopsy. Pathology revealed a 2.0 × 1.8 × 1.3 cm high-grade invasive ductal carcinoma. 0 of the 15 left axillary lymph nodes examined were positive for carcinoma, and no lymphatic invasion was identified. The patient declined adjuvant chemotherapy and radiation therapy.
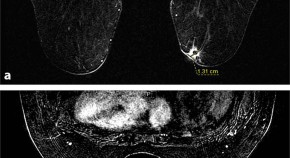
In the spring of 2003, the patient noticed another mass under the nipple of her left breast. An ultrasound-guided left breast biopsy was performed in early May 2003 which revealed a high-grade ductal carcinoma. The patient then underwent bilateral simple mastectomies to remove the left breast tumor in late May 2003. Pathology of the left breast mass showed a poorly-differentiated carcinoma that measured 3.0 × 3.5 × 2.0 cm with a Bloom–Richardson score of 9/9. The tumor was negative for ER, PR, HER2 by immunohistochemistry, and for amplification of the HER2 gene by fluorescence in situ hybridization, indicating she had triple-negative recurrent disease ( Fig. 1 ). The patient again declined any treatment.

In early September 2003, the patient noticed a growing mass in her left axilla and chest wall. A computed tomography (CT) scan of the chest showed a 3.8 cm necrotic left axillary lymph node with axillary and supraclavicular adenopathy. Nodules were also seen in the left upper and lower lobe of the lungs along with 2 subtle areas of low attenuation in the liver. A bone scan performed in December 2003 revealed bone lesions along the left 3rd and 6th ribs posterolaterally. The patient was concluded to have metastatic disease to the lungs, liver, and bones. Unfortunately, these images have been destroyed by the imaging facility and cannot be used in this publication. The details of the scans were obtained from the official written scan reports.
After consulting with the patient, she decided to proceed with chemotherapy treatment. She was given 4 sequential chemotherapy regimens ( Table 1 ) to try and control her cancer along with monthly zoledronic acid to try to prevent bone complications due to her osseous metastases. Granulocyte-macrophage-colony-stimulating factor (GM-CSF) was used throughout the treatment to prevent or treat chemotherapy-induced neutropenia as well as stimulate the immune system. After 14 doses of weekly paclitaxel and carboplatin, there was remarkable tumor shrinkage in the left chest wall and axillary area on her physical examinations. Her cancer antigen (CA) 27.29 (normal range: <38 U/mL) also decreased from a pretreatment level of 52.1 to 19.8 in mid-April 2004 which was consistent with the patient's physical exam findings. The patient's CA 27.29 then remained within normal range from this point onward. The patient was then switched to weekly doxorubicin liposome in June 2004. Twelve doses were planned, but she only received 8 doses due to developing palmar-plantar erythrodysesthesia. Her symptoms resolved after the drug was withdrawn in late July 2004. The patient then continued chemotherapy treatment and received 6 doses of weekly gemcitabine and cisplatin from August 2004 to October 2004. The patient developed thrombocytopenia and her second to the last dose of this regimen was given with a reduced dose of cisplatin. Gemcitabine was dose reduced during her last infusion of this regimen. She was then switched to weekly vinorelbine in early December 2004 and completed 12 doses of chemotherapy treatment in late February 2005.

Aside from developing neutropenia and thrombocytopenia secondary to chemotherapy treatment and palmar-plantar erythrodysesthesia secondary to doxorubicin liposome, the patient tolerated her treatment relatively well. A CT scan performed in March 2005 showed no evidence of disease in the lung and liver. The patient was taken off of chemotherapy treatment but continued on monthly zoledronic acid and pursued a watchful waiting approach from February 2005 to June 2005.
In June 2005 the patient developed several small skin nodules in her left axillary chest wall. A positron emission tomography (PET) and CT scan performed shortly after showed minimal thickening of the left chest wall, suspicious for recurrent disease, although no biopsy was performed. There was no other evidence of metastatic disease. She was started on imiquimod cream, which was applied on the skin nodules, for immune stimulation. The patient reported pruritus and erythema in the applied area, but she did not complain of having any pain. These nodules resolved after a few weeks. In October 2005, the patient noticed several pea-sized nodules in her left axilla which were also suspicious for disease recurrence. The patient continued applying imiquimod cream to the area. She developed erythema, ulceration, and skin breakdown in that area, but this resolved after stopping imiquimod. The skin nodules resolved by January 2006. The patient's condition continued to improve, and the frequency of her zoledronic acid infusions was reduced to every other month in May 2006, and then quarterly in September 2007. In December 2008 she continued with biannual infusions of zoledronic acid. A germline BRCA1 and BRCA2 analysis performed in December 2009 did not detect any mutations. Routine PET/CT and CT scans continue to show no evidence of recurrent or persistent metastatic disease. The patient is now 73 years old and is enjoying a good quality of life. She is currently over 15 years out from her diagnosis of recurrent metastatic TNBC.
3 Discussion
The prognosis of this patient before starting treatment was particularly poor, not only because her tumor did not express the ER, PR, or HER2 receptors, but also because she had stage IV disease with multiple visceral metastases. With the typical prognosis of metastatic TNBC being slightly over 1 year, the 15-year survival of this patient is quite remarkable, especially given that she is currently free of disease and has not received any chemotherapy treatment since late February 2005. To our knowledge, this patient is the longest reported survivor of metastatic TNBC. Her long-term survival without recurrence suggests that this patient may be cured of a cancer that is not thought to be curable. We believe that our treatment methodology, which included using metronomic chemotherapy, switching chemotherapy regimens before anticipated disease progression, and utilizing immune therapies, all contributed to her outstanding survival. Below, we will describe each of these treatment strategies in detail.
3.1 Metronomic chemotherapy
The dosing of a standard chemotherapy regimen is based on a maximum tolerated dose (MTD). This dose is typically the highest possible dose that is not lethal to the patient. The idea of an MTD was originally developed with the logic that “more is better” to try and maximize the amount of cancer cell death. A high dose of chemotherapy can kill cancer cells, but due to the relatively nonspecific mechanism of action of most chemotherapy agents, this high dose of chemotherapy can also result in clinical toxicities which is why standard chemotherapy regimens are often administered every 3 weeks. The breaks in between standard chemotherapy doses are crucial for the recovery of normal tissues, but logically this can also give time for cancer cells to grow and progress as well. Thus, the dosing of chemotherapy agents and the frequency of chemotherapy administration may play an important role in the efficacy of treatment as well as the patient's quality of life.
This patient received lower doses of chemotherapy on a more frequent basis, also known as “metronomic chemotherapy.” Although lower doses of chemotherapy agents were given to this patient during each administration, the overall dose intensity (the total dose of chemotherapy administered per unit time) of her chemotherapy agents was a similar, if not higher, dose intensity, compared to the standard dosing of each respective chemotherapy agent. Studies have shown that reducing dose intensity, most commonly due to myelosuppression, correlates with poorer disease-free survival and overall survival, while maintaining a relatively high planned dose intensity is associated with better clinical outcomes. [4] Due to the lower doses used during each administration, metronomic chemotherapy regimens can minimize severe adverse events and prolonged drug-free breaks. In addition, a more steady dosing schedule may actually kill more cancer cells by maintaining a more constant drug concentration in the body. This logic may explain why dose-dense regimens (chemotherapy regimens with an increased frequency of administration) have been shown to be more effective in the treatment of several kinds of cancer, including breast cancer, when compared to standard treatment. [5] We have also treated pancreatic cancer patients using a similar dosing strategy, which has yielded exciting results. [6,7]
The main mechanism of action of metronomic chemotherapy was initially thought to be its effects on endothelial cells resulting in antiangiogenic effects. There is a fair amount of literature that suggests that paclitaxel has antiangiogenic effects when administered in lower doses more frequently. [8] Likely due to their aggressive nature, TNBCs are known to have enhanced angiogenesis. [9] The proangiogenic tumor microenvironment creates an abnormal vascular network that can result in increased interstitial pressure and decrease drug penetration, ultimately decreasing the efficacy of systemic treatment. [10] By blocking angiogenesis and normalizing the tumor vasculature, more chemotherapy can reach the tumor, potentially improving the efficacy of treatment.
In addition to having direct cytotoxic and antiangiogenic effects, it has been discovered that metronomic chemotherapy can also have antistromal and immunostimulatory effects. [11–13] There are even some thoughts that the effects that metronomic chemotherapy has on the tumor microenvironment can decrease the rate of acquired chemotherapy resistance. [11,12] Targeting both cancer cells and the tumor microenvironment may play an important role in the future of cancer treatment.
3.2 Sequential chemotherapy regimens
The standard way to administer chemotherapy treatment is to continue a single chemotherapy regimen until noticeable disease progression. Patients with metastatic TNBC tend to relapse quickly on chemotherapy treatment, likely due to acquired disease resistance. [3] Drug resistance is seen as the primary cause of failure of chemotherapy treatment for cancer and continuing a chemotherapy regimen until disease progression will inevitably breed chemotherapy-resistant disease. Switching chemotherapy regimens before disease progression, as we did for this patient, may prevent the development of disease resistance, allowing for a continual decrease in the number of cancer cells, and perhaps a better chance of achieving long-term survival.
Tumors are known to have a large amount of genetic diversity, even within a single mass, and chemotherapy treatment can induce strong selective pressure for cells that have intrinsic or acquired mutations which can resist treatment. [14] Switching chemotherapy agents may eradicate cancer cells that developed resistance to the cytotoxic agents in the previous regimen, especially if the new chemotherapy agents have a different mechanism of action. There are even some suggestions that cancer cells can become dependent on certain therapies after long-term drug exposure, and switching the drugs used may increase treatment efficacy by inducing cell death of the cancer cells that have become “addicted” to the previous therapy. [12,13] The idea of switching chemotherapy treatments before the development of disease resistance has broad implications and could transform the idea of cancer being an acute disease to more of a chronic illness. In addition to potentially increasing treatment efficacy and preventing the development of disease resistance, switching treatment regimens can also help prevent the accumulation of chemotoxicity from a single chemotherapy regimen, which can improve the quality of life of patients receiving treatment.
This idea of sequential chemotherapy regimens has been successfully introduced in the treatment of metastatic non-small cell lung carcinoma. Patients who responded to first-line chemotherapy and pursued a switch maintenance therapy were found to have improved overall survival compared to placebo or observation, and switch maintenance therapy was also less toxic compared to continuous maintenance therapy. [15] A similar treatment strategy has also been applied and has found major success in the treatment of pediatric acute lymphoblastic leukemia (ALL). A diagnosis of ALL was fatal for children in the 1950s. Currently, this disease has a cure rate of more than 80% in children. The current treatment for ALL involves several combination chemotherapy regimens that are given sequentially to eliminate any remaining disease. [16] Sequential chemotherapy regimens in ALL has been considered one of the greatest achievements in the field of oncology to date.
This patient received several different chemotherapy regimens sequentially ( Table 1 ). Her first regimen consisted of paclitaxel and carboplatin. Paclitaxel that is administered on a weekly basis has been shown to be superior to paclitaxel that is administered every 3 weeks in the treatment of metastatic breast cancer, with increased response rates, time to progression, as well as survival. [17] Moreover, there is some evidence that platinum-based chemotherapy regimens improve the overall survival of patients with metastatic TNBC. [18] The addition of platinum agents to treatment regimens has likely been slow to catch on due to the significant toxicity of the standard doses of these agents. However, the carboplatin dose of AUC 2.25 and cisplatin dose of 15 to 20 mg/m 2 that was given on a weekly basis were tolerated relatively well by this patient.
After she received 14 doses of paclitaxel and carboplatin, she was switched to weekly doxorubicin liposome. Anthracyclines are known to be very effective in the treatment of breast cancer, but carry a risk of cardiotoxicity and significant myelosuppression. We prefer to use doxorubicin liposome instead of doxorubicin because doxorubicin liposome has a favorable toxicity profile, less hematological toxicity, and has been found to cause less cardiotoxicity compared to doxorubicin. [19] This is an important consideration to reduce potential comorbidities in the future, especially if patients have the potential to achieve long-term survival. The patient tolerated treatment with weekly doxorubicin liposome well aside from developing palmar-plantar erythrodysesthesia, but perhaps this treatment might be more tolerable if it was administered every 2 weeks due to doxorubicin liposome's relatively long half-life. This treatment might also be more effective if it was combined with weekly paclitaxel.
The patient's next regimen, gemcitabine, and cisplatin, has been shown to improve outcomes in patients with metastatic TNBC compared to patients without metastatic TNBC. [20] The patient also tolerated this regimen well aside from developing thrombocytopenia, but maybe this could be lessened by starting with a dose of gemcitabine 600 mg/m 2 and cisplatin 15 mg/m 2 instead of gemcitabine 750 mg/m 2 and cisplatin 20 mg/m 2 , which is what we have done subsequently with other patients. Her final regimen consisted of vinorelbine, which is another effective drug for the treatment of metastatic breast cancer patients who have been exposed to anthracyclines and taxanes in previous treatments. [21] Since the patient's diagnosis 15 years ago, there are several new treatments available that may be more effective than vinorelbine, such as eribulin or irinotecan.
By giving this series of effective treatments sequentially, we believe that this prevented disease resistance and allowed the patient to achieve complete remission after approximately 1 year of treatment. The combination of agents was chosen to avoid overly additive side effects, such as myelosuppression, so that the patient could receive continuous treatment without interruption and also maintain a relatively high overall dose intensity. In regards to the order of these regimens, it is unknown whether or not this is the most optimum sequence of regimens and this should be further investigated.
3.3 Immunotherapy
More evidence is accumulating suggesting that some subtypes of metastatic TNBC can be particularly responsive to immunotherapy, with some studies showing promising results. [22] However, cancers can escape an antitumor immune response in several ways such through the upregulation of regulatory T cells (Tregs) and secretion of immunosuppressive cytokines into the tumor microenvironment as well as through the expression of immunosuppressive proteins, such as programmed death-ligand 1, on the cell surface. [22–24] The situation is further exacerbated by the immunosuppressive effect of standard dose chemotherapy. [25] When the immunosuppressive activity of the tumor outweighs the body's antitumor immune response, this is thought to promote tumor progression.
Metronomic chemotherapy, in addition to having a lesser impact on blood counts, is thought to have immunomodulatory properties. In preclinical studies, low-dose paclitaxel and gemcitabine have been shown to decrease the number and viability of Tregs as well as myeloid-derived suppressor cells in the tumor microenvironment, which could potentially allow for a more potent antitumor immune response. [26] Moreover, the antiangiogenic effects of metronomic chemotherapy may be synergistic with its immunostimulatory properties. Normalizing the tumor vasculature could allow the immune system to better reach the tumor bed, just as it is thought to allow more chemotherapy treatment to reach the tumor. Interestingly, the patient also received zoledronic acid due to her bone metastases, which may also have immunomodulatory properties. This effect seems to be more prevalent in ER-positive breast cancer cells, but more research will need to be conducted to assess the immunomodulatory properties in ER-negative breast cancer. [27]
The patient also received several immunostimulatory agents which may have played a role in her long disease-free remission. While receiving chemotherapy treatment, the patient received GM-CSF to prevent or treat chemotherapy-induced neutropenia. We prefer the use of GM-CSF compared to other colony-stimulating factors because GM-CSF stimulates both the granulocyte as well as the monocyte/macrophage and dendritic cell (DC) lines, while other colony-stimulating factors only stimulate the granulocyte cell line. DCs are crucial for antigen presentation and activation of the adaptive immune system and macrophages can remove dead tumor cells via phagocytosis. Moreover, preclinical evidence suggests that low-dose paclitaxel can stimulate DC maturation and the anthracyclines can promote the phagocytosis of tumor cells by DCs, suggesting a synergistic role of GM-CSF in combination with 2 of the patient's chemotherapy regimens. [28]
The patient also applied imiquimod topically to small superficial lesions in her left axillary region that were likely secondary to recurrent disease. Imiquimod has known antitumor activity in superficial basal cell carcinoma and it is thought to work by stimulating the innate and adaptive immune system in the applied area via toll-like receptor 7. [29] The patient's left axillary lesions resolved after she applied imiquimod cream to the area, suggesting that imiquimod may be able to be used to treat superficial metastases from TNBC. One small study also had similar findings, and it would be interesting to investigate whether metastatic TNBC was more responsive to local immunotherapy than other breast cancer types. [30] Perhaps the success of imiquimod in this patient was due to the minimal tumor burden the patient had at the time, especially considering that a smaller tumor size in basal cell carcinoma is correlated with a more favorable prognosis after treatment with imiquimod.
4 Conclusion
The patient described in this case report is now 15 years out from her diagnosis of recurrent metastatic TNBC without evidence of persistent or recurrent metastatic disease. Her treatment, which included the use of sequential metronomic chemotherapy regimens as well as several immunotherapies, was tolerated relatively well and likely contributed to her remarkable survival. Although this is only 1 case, we have treated another patient with metastatic TNBC with a similar strategy who is now over 6 years out and free of disease as well as a few other patients who achieved longer than average survivals. We are planning to publish this data in a small case series in the future. We hope that this encouraging case can offer hope to those who are suffering from this debilitating disease and spark the formation of larger clinical trials to further evaluate this treatment strategy due to the potential significant medical, psychological, and economic implications. These ideas not only have the possibility to shift the paradigm of treating metastatic TNBC, but also potentially other metastatic cancers as well.
Acknowledgments
We would like to thank Max Tse and Jeffrey Wang for drafting earlier versions of this paper, and Emmanuel De Dios, without whom none of this work could have been done.
Author contributions
Conceptualization: Ben Man-Fai Chue, Bryce Douglas La Course.
Formal analysis: Ben Man-Fai Chue, Bryce Douglas La Course.
Writing – original draft: Ben Man-Fai Chue, Bryce Douglas La Course.
Writing – review and editing: Ben Man-Fai Chue, Bryce Douglas La Course.
- Cited Here |
- Google Scholar
immunotherapy; long-term survival; metastatic triple-negative breast cancer; metronomic chemotherapy; sequential chemotherapy regimens
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Can we cure stage iv triple-negative breast carcinoma: another case report of....
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Case Reports
Solid-basaloid variant of adenoid cystic carcinoma of the breast with near complete response to neoadjuvant chemotherapy
- Anne Grabenstetter
- Hannah Y. Wen
Clinical consequences of BRCA2 hypomorphism
- Laia Castells-Roca
- Sara Gutiérrez-Enríquez
- Jordi Surrallés
Radiation recall dermatitis following letrozole administration in patient with a remote history of radiation therapy
- Evan Sweren
- Pathik Aravind
- Michelle L. Kerns
Potential of ultra-high-resolution photon-counting CT of bone metastases: initial experiences in breast cancer patients
- L. T. Rotkopf
Semaphorin 7a is a biomarker for recurrence in postpartum breast cancer
- Virginia F. Borges
- Traci R. Lyons

Olaparib for metastatic breast cancer in a patient with a germline PALB2 variant
- Sherko Kuemmel
- Hakima Harrach
- Mattea Reinisch
Response to Olaparib in a Patient with Germline BRCA2 Mutation and Breast Cancer Leptomeningeal Carcinomatosis
- Pedro Exman
- Robert M. Mallery
- Heather A. Parsons
A case report of vanishing bile duct syndrome after exposure to pexidartinib (PLX3397) and paclitaxel
- Sorbarikor Piawah
- Colby Hyland
- A. Jo Chien
Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18]fluoroestradiol
- Ella F. Jones
- Kimberly M. Ray
- Nola M. Hylton
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Introduction
- Conclusions
- Article Information
Patients from SGM groups experienced a delay in diagnosis compared with Cis-Het patients (median time to diagnosis, 64 vs 34 days). Cis-Het indicates cisgender heterosexual; SGM, sex and gender minority.
Patients from SGM groups experienced a higher rate of breast cancer recurrence compared with Cis-Het patients (32% vs 13%). Cis-Het indicates cisgender heterosexual; SGM, sex and gender minority.
eTable 1. Percentage of Patients From SGM Groups With Breast Cancer Identified by Search Term
eTable 2. Medical History of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 3. Hormonal Risk Factors of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 4. Breast Cancer Screening and Diagnosis of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 5. Genetic Testing and Clinical Trial Enrollment of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 6. Breast Cancer Treatment and Recurrence of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 7. Benjamini-Hochberg Corrected P Values for Prespecified Metrics
eTable 8. Adjusted Association Between Race and Sex and Gender Minority Outcomes
Data Sharing Statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Eckhert E , Lansinger O , Ritter V, et al. Breast Cancer Diagnosis, Treatment, and Outcomes of Patients From Sex and Gender Minority Groups. JAMA Oncol. 2023;9(4):473–480. doi:10.1001/jamaoncol.2022.7146
Manage citations:
© 2024
- Permissions
Breast Cancer Diagnosis, Treatment, and Outcomes of Patients From Sex and Gender Minority Groups
- 1 Department of Medicine, Stanford University School of Medicine, Stanford, California
- 2 Qualitatitive Statistical Unit, Department of Medicine, Stanford University School of Medicine, Stanford, California
- 3 Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, California
- 4 Department of Epidemiology and Biostatistics, University of California, San Francisco
Question Are there disparities in breast cancer treatment and outcomes of patients from sex and gender minority (SGM) groups compared with cisgender heterosexual patients?
Findings In this exposure-matched case-control study of 92 patients from SGM groups matched to cisgender heterosexual patients by year of breast cancer diagnosis, age, tumor stage, estrogen receptor status, and ERBB2 (HER2) status, those from SGM groups had delays in diagnosis, declined oncologist-recommended therapies more often, and experienced a 3-fold higher rate of breast cancer recurrence compared with cisgender heterosexual patients.
Meaning Findings suggest several potential health care disparities among patients from SGM groups with breast cancer, necessitating further evaluation to inform interventions.
Importance Sexual orientation and gender identity data are not collected by most hospitals or cancer registries; thus, little is known about the quality of breast cancer treatment for patients from sex and gender minority (SGM) groups.
Objective To evaluate the quality of breast cancer treatment and recurrence outcomes for patients from SGM groups compared with cisgender heterosexual patients.
Design, Setting, and Participants Exposure-matched retrospective case-control study of 92 patients from SGM groups treated at an academic medical center from January 1, 2008, to January 1, 2022, matched to cisgender heterosexual patients with breast cancer by year of diagnosis, age, tumor stage, estrogen receptor status, and ERBB2 (HER2) status.
Main Outcomes and Measures Patient demographic and clinical characteristics, as well as treatment quality, as measured by missed guideline-based breast cancer screening, appropriate referral for genetic counseling and testing, mastectomy vs lumpectomy, receipt of chest reconstruction, adjuvant radiation therapy after lumpectomy, neoadjuvant chemotherapy for stage III disease, antiestrogen therapy for at least 5 years for estrogen receptor–positive disease, ERBB2-directed therapy for ERBB2-positive disease, patient refusal of an oncologist-recommended treatment, time from symptom onset to tissue diagnosis, time from diagnosis to first treatment, and time from breast cancer diagnosis to first recurrence. Results were adjusted for multiple hypothesis testing. Compared with cisgender heterosexual patients, those from SGM groups were hypothesized to have disparities in 1 or more of these quality metrics.
Results Ninety-two patients from SGM groups were matched to 92 cisgender heterosexual patients (n = 184). The median age at diagnosis for all patients was 49 years (IQR, 43-56 years); 74 were lesbian (80%), 12 were bisexual (13%), and 6 were transgender (6%). Compared with cisgender heterosexual patients, those from SGM groups experienced a delay in time from symptom onset to diagnosis (median time to diagnosis, 34 vs 64 days; multivariable adjusted hazard ratio, 0.65; 95% CI, 0.42-0.99; P = .04), were more likely to decline an oncologist-recommended treatment modality (35 [38%] vs 18 [20%]; multivariable adjusted odds ratio, 2.27; 95% CI, 1.09-4.74; P = .03), and were more likely to experience a breast cancer recurrence (multivariable adjusted hazard ratio, 3.07; 95% CI, 1.56-6.03; P = .001).
Conclusions and Relevance This study found that among patients with breast cancer, those from SGM groups experienced delayed diagnosis, with faster recurrence at a 3-fold higher rate compared with cisgender heterosexual patients. These results suggest disparities in the care of patients from SGM groups and warrant further study to inform interventions.
Little is known about the quality of breast cancer care among patients from sex and gender minority (SGM) groups. Only 24% of National Cancer Institute Community Oncology Research Program practice groups capture data on sexual orientation, and only 10% capture data on gender identity. 1 A 2022 survey of American Society of Clinical Oncology members found that although more than 75% of respondents agreed that knowing sexual orientation and gender identity (SOGI) data is important to providing high-quality care, less than half of them reported collection of SOGI data at their institution. 2 , 3 Even within the SGM literature, women who have sex with women (WSW) and transgender patients are understudied. Of studies funded by the National Institutes of Health that focus on patients from SGM groups, only 13.5% include data on WSW, and just 6.8% include data on transgender patients. 4 In the clinic, physicians rarely ask about SOGI status in the context of providing breast cancer care, 5 and insufficient sexual history taking is further compounded by the fact that many patients from SGM groups do not reveal their sexual orientation to their care team. Survey estimates of the percentage of WSW who disclose their sexual orientation to their physicians range from 28% to 84%, proportions influenced by a patient’s perception of the safety of the clinic space, the extent to which they disclose their sexual orientation in other circumstances, and certain demographic characteristics (such as patient age, race, and ethnicity). 5
Women who have sex with women have a higher theoretical risk of breast cancer because of increased prevalence of risk factors, including nulliparity, alcohol and tobacco use, and higher body mass index. 6 , 7 However, the incidence of breast cancer among WSW—and whether it is higher than that among heterosexual women—remains unclear. 7 - 9 Less is known about breast cancer risk for transgender patients, although there is a suggestion of a lower risk of breast cancer among transgender women and transgender men compared with cisgender women. 10 , 11 The 58 000-patient National Health Interview Survey from 2013 to 2017 confirmed for the first time that WSW had lower rates of screening mammography compared with heterosexual patients 7 , 12 , 13 and that the lower rates of screening mammography exemplified disengagement from primary care. Women who have sex with women were more likely to report difficulty finding a primary care physician with whom they are comfortable, less likely to have consulted a health care professional in the past year, and less likely to have received any form of preventive health care. These differences were independent of reported income or health care insurance status, suggesting that the disparity in preventive care stems from social factors (ie, stigma and difficulty finding a physician with the requisite knowledge to provide high-level primary care to patients from SGM groups) rather than financial ones. This hypothesis is supported by qualitative studies, including cancer research, on patients from SGM groups, that highlight themes of being subjected to systemic homophobia, feeling out of place in heteronormative health care systems, and having to educate their own health care team about caring for SGM patients. 14 - 17
To our knowledge, there are no data in the literature on treatment quality and patient outcomes for SGM patients with breast cancer. To address the need for data on SGM populations, a 2020 National Academies report called for adding measures of SOGI to ongoing data collection efforts, including clinical settings, and linking data sets that contain SOGI data to existing databases. 18 We used an integrated breast cancer research database, Oncoshare, that incorporates electronic medical records (EMRs) of patients treated at Stanford University and the community-based Sutter Medical Network, linked to the statewide California Cancer Registry. We tested the hypothesis that patients from SGM groups with breast cancer experience treatment and outcome disparities compared with matched cisgender heterosexual patients.
In this case-control study, a key word search algorithm was used to identify patients from SGM groups treated at Stanford University between January 1, 2008, and January 1, 2022, with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis of a breast neoplasm and the presence of a SOGI identifier, as has been described previously for cohort identification of SGM patients with cancer. 19 eTable 1 in Supplement 1 describes the search terms used to identify patients from SGM groups. This initial cohort identification tool was designed to maximize sensitivity and identified 686 patients’ EMRs that were subsequently manually reviewed, after which the final cohort comprised 92 patients.
Using the 92 identified patients from SGM groups, we selected matched cisgender heterosexual patients with breast cancer from the Oncoshare database (n = 184). Oncoshare 20 - 23 integrates information from the EMRs of 2 San Francisco Bay Area health care systems, Stanford University Health Care and Sutter Medical Network, with patient-level data from the California Cancer Registry, which comprise registries that are also part of the Surveillance, Epidemiology, and End Results Program. Matching variables were age, year of diagnosis, tumor stage, estrogen receptor (ER) status, and ERBB2 (HER2) status. Exact matching was required for tumor stage, ER status, and ERBB2 status (patients with unknown ERBB2 status were allowed to be matched to those with positive, negative, or unknown ERBB2 status). Age was matched within 5 years, and year of diagnosis was matched within 7 years.
After identification of all patients, information was collected from the Stanford University EMR about demographic characteristics (including race and ethnicity, defined by patients), medical history, social history, history of breast cancer screening, breast cancer diagnosis, genetic counseling and testing, breast cancer treatment, clinical trial participation, treatment adherence, and breast cancer recurrence. This study was approved and a waiver for informed consent was granted by the institutional review board at Stanford University. A waiver of authorization was approved by the Stanford University institutional review board, based on the conclusion that disclosure of protected health information involved minimal risk to the participant and the research could not be conducted without access to it. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
For the following prespecified quality metrics of the National Comprehensive Cancer Network 24 - 26 and American Society of Clinical Oncology, 27 we applied both unadjusted and adjusted conditional logistic regression to evaluate the association between each outcome and SGM status, controlling for matched pair: missed guideline-based screening, receipt of appropriate genetic testing, receipt of mastectomy (vs lumpectomy), receipt of chest reconstruction, receipt of adjuvant radiation therapy after lumpectomy, receipt of neoadjuvant chemotherapy for stage III disease, receipt of antiestrogen therapy for at least 5 years for ER-positive disease, receipt of ERBB2-directed therapy for ERBB2-positive disease, and patient refusal of an oncologist’s recommended treatment modality. In addition to SGM status, analyses were adjusted for race and ethnicity, neighborhood-level socioeconomic status, 28 and private insurance status. In sensitivity analysis, we further adjusted for tumor stage, age at diagnosis, year of diagnosis, ER status, ERBB2 status, and any variables found to be unbalanced by the exposure or associated with the outcome in bivariate analyses. Odds ratios with 95% CIs and 2-sided Wald tests were reported. All statistical analyses were performed with R, version 4.1 (R Foundation for Statistical Computing). P < .05 was deemed statistically significant.
For analyzing the following 3 time-to-event outcomes, we applied unadjusted and adjusted Cox proportional hazards models stratified by matched pair for time from symptom onset to diagnosis, time from diagnosis to treatment, and time from diagnosis to recurrence. Analyses of residuals were performed to verify the modeling assumptions. Hazard ratios and 95% CIs were reported.
The cohort was described with median values, IQRs, counts, and proportions where appropriate. Differences in baseline demographic and clinical characteristics by SGM status are reported as median differences or differences in proportions with 95% CIs.
We adjusted for multiple testing by applying the Benjamini-Hochberg correction assuming a false discovery rate of 20%. 29 For the findings that remained statistically significant after this correction, we conducted a set of sensitivity analyses; we further adjusted for tumor stage, age at diagnosis, year of diagnosis, ER status, ERBB2 status, and any variables found to be imbalanced by the exposure or associated with the outcome in bivariate analyses. An additional sensitivity analysis was conducted to determine whether race and ethnicity were differential regarding the association between SGM status and the outcomes. Data were analyzed from April 1, 2022, through October 27, 2022.
Demographic characteristics are summarized in Table 1 . The median age at diagnosis for all 92 patients was 49 years (IQR, 43-56 years); 74 were lesbians (80%), 12 were bisexual (13%), and 6 were transgender men (6%, including 4 who were heterosexual [4%], 1 who was gay [1%], and 1 who was asexual [1%]). The patients from the SGM group and the cisgender heterosexual patients were not matched by race and ethnicity a priori, and the patients from the SGM group had more non-Hispanic White patients (72 [78.3%] vs 58 [63.0%]; difference, 15.1%; 95% CI, 1.2%-29.3%) and Hispanic patients (13 [14.1%] vs 7 [7.6%]; difference, 6.5%; 95% CI, –3.5% to 16.6%) and fewer Asian or Pacific Islander patients (3 [3.3%] vs 23 [25.0%]; difference, −21.7%; 95% CI, –32.4% to –11.1%) than the cisgender heterosexual patients. No differences in socioeconomic status or insurance type were observed between the 2 groups of patients.
Medical history and health-associated behaviors are summarized in eTable 2 in Supplement 1 . Rates of diabetes, coronary artery disease, and obesity were similar between the 2 groups of patients. Among the 92 patients from SGM groups compared with cisgender heterosexual patients, there was a suggestion of higher rates of at-risk alcohol use (12 [13.0%] vs 4 [4.3%]; difference, 8.7%; 95% CI, –0.4% to 17.8%) 30 and cannabis use (23 [25.0%] vs 7 [7.6%]; difference, 17.4%; 95% CI, 5.9%-28.8%).
Hormonal risk factors are summarized in eTable 3 in Supplement 1 . The patient groups were well balanced with respect to median age at menarche, age at first delivery, menopausal status, use of oral contraceptives, and use of hormone replacement therapy. Compared with cisgender heterosexual patients, the 92 patients from SGM groups were more often nulligravid (59 [64%] vs 16 [17%]; difference, 47%; 95% CI, 33%-60%) and had fewer children (median [IQR], 0 [0-0] vs 2 [0-2]).
Data summarizing breast cancer screening and diagnosis are presented in Table 2 and eTable 4 in Supplement 1 . There was no difference in use of appropriate guideline-based screening mammography between the 2 groups of patients. 24 - 27 The percentage of patients who presented with symptomatic breast masses was similar between cohorts. However, patients from SGM groups experienced a delay in diagnosis compared with cisgender heterosexual patients, with a median time to diagnosis of 64 days (IQR, 32-118 days) and 34 days (IQR, 16-75 days), respectively. This delay in diagnosis was independent of race and ethnicity, socioeconomic status, or insurance type in multivariable analysis (adjusted hazard ratio, 0.65; 95% CI, 0.42-0.99; P = .04). Figure 1 shows a time-to-event analysis from symptom onset to tissue diagnosis.
Genetic counseling and testing and clinical trial enrollment are summarized in eTable 5 in Supplement 1 . There were no discrepancies in the rates of appropriate genetic testing referrals or clinical trial engagement (whether that was clinical trial offering, trial enrollment, or type of trial offered or enrolled in). 25
Data on breast cancer treatment and cancer recurrence are presented in Table 2 and eTable 6 in Supplement 1 . Once patients received a diagnosis, there was no difference in time to first treatment between the 2 patient groups. There was no difference in receipt of lumpectomy vs mastectomy for treatment of localized disease, receipt of adjuvant radiation therapy after lumpectomy, or receipt of neoadjuvant systemic therapy among patients with stage III disease. The point estimate suggested that patients from SGM groups with ER-positive disease may be less likely to receive at least 5 years of antiestrogen treatment compared with cisgender heterosexual patients, although the 95% CIs were wide (adjusted odds ratio, 0.46; 95% CI, 0.15-1.44; P = .18). Reasons for this potential difference included both patient choice and intolerance to therapy. Patients with ERBB2-positive disease received ERBB2-directed therapy at equivalent rates between the 2 patient groups. Patients from SGM groups had a higher rate of documented refusal of an oncologist-recommended cancer-directed therapy (35 [38%] vs 18 [20%]; adjusted odds ratio, 2.27; 95% CI, 1.09-4.74; P = .03), with antiestrogens being the most declined treatment. Patients from SGM groups were also more likely to use alternative medicines compared with cisgender heterosexual patients (42 [46%] vs 28 [30%]; difference, 16%; 95% CI, 0.3%-30.2%).
Patients from SGM groups had higher rates of cancer recurrence overall compared with cisgender heterosexual patients (29 [32.2%] vs 12 [13.3%]; difference, 18.9%; 95% CI, 5.8%-32.0%). Two patients in each group had stage IV cancer at diagnosis and so by definition could not have a cancer recurrence. Rates of local cancer recurrence were 17.3% among patients from SGM groups compared with 2.5% among cisgender heterosexual patients, whereas rates of metastatic recurrence were 24.7% among patients from SGM groups compared with 13.6% among cisgender heterosexual patients. Figure 2 presents a Cox proportional hazards model of the rate of recurrence, which shows that patients from SGM groups experienced a 3-fold higher rate than cisgender heterosexual patients, which persisted in multivariable analysis after controlling for race and ethnicity, socioeconomic status, and insurance type (adjusted hazard ratio, 3.07; 95% CI, 1.56-6.03; P = .001).
The reported adjusted effect estimates for SGM groups remained significant after accounting for multiple testing using the Benjamini-Hochberg correction with a false discovery rate of 20% (eTable 7 in Supplement 1 ). Adjustments for factors that were imbalanced between groups, including race and ethnicity, did not affect these estimates for SGM groups significantly in a sensitivity analysis (eTable 8 in Supplement 1 ).
To our knowledge, this is the first study to examine the quality of breast cancer treatment and breast cancer outcomes for patients from SGM groups. In our study, SGM patients had a near doubling of the time from symptom onset to tissue diagnosis, as well as nearly 3 times the recurrence rate compared with matched cisgender heterosexual patients.
Historically, identification of patients from SGM groups has hampered health care disparities research aimed at improving the outcomes of this patient population. This study validates a search term–based method to identify patients from SGM groups in the EMR, but it contrasts with prior studies 19 , 31 in also using behavioral search terms, which resulted in a substantially higher yield than ICD-10 codes and SGM identity categories. 32
Health-associated behaviors in patients from SGM groups were consistent with those described in the medical literature. The SGM patients had higher rates of substance use, but apart from having fewer children than cisgender heterosexual patients, they had similar hormonal risk factors (ages at menarche and menopause; oral contraceptive and hormone replacement therapy use). 33 These small differences in risk factors do not explain the magnitude of the difference in recurrence rates between the 2 patient groups. In the absence of a clear biological rationale for this difference in outcomes, the reasons for it appear to be associated with structural or social factors.
Patients from SGM groups received breast cancer screening, genetic testing, and clinical trial enrollment at rates similar to those of cisgender heterosexual patients. The equivalent rates of breast cancer screening before breast cancer diagnosis between the 2 patient groups in the present study are unique within the SGM cancer literature; to our knowledge, there is no prior reference for genetic counseling and testing or for clinical trial enrollment. We speculate that these results speak to the well-resourced nature of the population in the San Francisco Bay Area and to the highly motivated and self-advocating nature of patients from SGM groups who have the social capital to know when to disclose their sexual orientation to their medical team.
With respect to diagnostic delay, among patients who presented with a symptomatic breast mass, time from symptom onset to tissue diagnosis was significantly delayed among patients from SGM groups vs cisgender heterosexual patients. The retrospective nature of this study precludes root cause analysis into the causes of this delay; however, within the confines of EMR review, the reasons for the delay appear to stem from both later presentation to care on the part of the patient and longer time to evaluation on the part of the patient’s care team. Clinic-based studies of patients from SGM groups suggest that the reasons for these delays could include patient distrust of health care professionals owing to prior discriminatory experiences or failure on the part of health care professionals to evaluate signs and symptoms reported by SGM patients. 34
Patients from SGM groups declined recommended oncologic treatment and used alternative medicine more often than cisgender heterosexual patients; a point estimate was also suggestive of less antiestrogen therapy use by ER-positive SGM patients, although this was not statistically significant. Although we found no association between diagnostic delay and subsequent refusal of oncologist-recommended care among patients from SGM groups, numerous studies underscore that discrimination and mistreatment of SGM patients in health care settings remain common. 35 , 36 Among WSW, those who have previously experienced health care discrimination are less likely to trust health care professionals in the future and often turn to the internet instead of physicians for future health care concerns. 37 In addition, patients who use alternative medicine and refuse conventional cancer treatment are known to have worse outcomes, including increased risk of death, 38 although given the retrospective nature of this study, it was not possible to establish a causal relationship between alternative medicine use and refusal of conventional cancer treatment. Nevertheless, these findings speak to an opportunity to better align the goals and values of patients from SGM groups with those of their oncologists through targeted education and culturally appropriate supportive care programs.
Overall, this study underscores that patients from SGM groups with breast cancer represent a high-risk population because of delays in diagnosis, noncompliance with recommended therapy, and a higher breast cancer recurrence rate. To address this structural health care disparity in the clinic, physicians must ask about and record the SOGI status of their patients so that they can pay special attention to those from SGM groups, evaluate concerning symptoms quickly, and provide ongoing education to patients about the importance of guideline-based treatment modalities.
This study has several limitations. Given the observational and retrospective design, we cannot draw conclusions about causation and note the potential for residual confounding by unknown factors. The 2 patient groups were imbalanced by race and ethnicity, although a sensitivity analysis of race and ethnicity did not demonstrate that this imbalance altered our conclusions; we were unable to evaluate the association of Asian and Pacific Islander status owing to the small number of these patients in the SGM cohort. Sex and gender minority research is always subject to selection bias because patients who choose to disclose their sexual orientation to their care teams and to researchers tend to have greater social resources than those who do not. 5 This relative exclusion of the most vulnerable SGM groups from SGM research likely leads to an underestimation of the true extent of disparities affecting the SGM population at large. 39 Because this study retrospectively identified patients according to both stated sexual orientation and sexual behavior, those who were not sexually active were less likely to be included; this selection bias is also a known factor that may influence patient decisions to disclose their SGM status to their physician. 5 This was a study of patients treated at Stanford University, which serves a high percentage of patients in the top socioeconomic brackets and is located in the San Francisco Bay Area, which tends to be more accepting of people from SGM groups. Although these factors hamper generalizability to all patients from SGM groups with breast cancer, the health care disparities identified here are all the more notable because they persisted despite a more favorable set of social conditions for the SGM population in our hospital’s catchment area.
The results of this case-control study suggest that health care disparities in breast cancer treatment and outcomes of patients from SGM groups should be evaluated by adding SOGI data to large cancer databases and should also be investigated in prospective population-based studies, both of which have the potential to inform health care interventions aimed at improving the quality of care for SGM patients with breast cancer.
Accepted for Publication: October 31, 2022.
Published Online: February 2, 2023. doi:10.1001/jamaoncol.2022.7146
Corresponding Author: Erik Eckhert, MD, MS, Department of Medicine, Stanford University School of Medicine, 875 Blake Wilbur Dr, Stanford, CA 94305 ( [email protected] ).
Author Contributions: Drs Eckhert and Ritter had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Eckhert, Lansinger, Ritter, Han, Schapira, Gomez, Sledge, Kurian.
Acquisition, analysis, or interpretation of data: Eckhert, Lansinger, Ritter, Liu, Han, John, Kurian.
Drafting of the manuscript: Eckhert, Ritter, Liu, Schapira, Kurian.
Critical revision of the manuscript for important intellectual content: Eckhert, Lansinger, Ritter, Han, Schapira, John, Gomez, Sledge.
Statistical analysis: Eckhert, Lansinger, Ritter.
Obtained funding: Kurian.
Administrative, technical, or material support: Eckhert, Liu.
Supervision: Han, Schapira, Sledge, Kurian.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by the Breast Cancer Research Foundation, the Susan and Richard Levy Gift Fund, the Suzanne Pride Bryan Fund for Breast Cancer Research, the Jan Weimer Junior Faculty Chair in Breast Oncology, the Regents of the University of California’s California Breast Cancer Research Program (grants 16OB-0149 and 19IB-0124), the BRCA Foundation, and the Biostatistics Shared Resource of the National Institutes of Health–funded Stanford Cancer Institute (grant P30CA124435). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code section 103885; the Centers for Disease Control and Prevention’s National Program of Cancer Registries under cooperative agreement 5NU58DP006344; and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California.
Role of the Funder/Sponsor: The role of each of these funding organizations was to support development of the Oncoshare data resource. No funder had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclaimer: The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- In-person and virtual events just for HCPs

- Conferences
- Publications
- Biomarker Consortium
Case Study: Treating HR+ and HER2- Breast Cancer
For High-Definition, Click
In the second case study of the series, moderator Adam M. Brufsky, MD, PhD, describes a 63 year-old woman presenting with thickening of the outer left breast and an enlarging mass. The thickening began a year earlier and the enlargement began within the past month, Brufsky notes.
The patient experiences slight dyspnea on exertion but does not have bone pain. Upon further examination, a 6 cm mass with slight dimpling and underlying skin contraction was noted on the left breast. A PET-CT scan was performed which confirmed the presence of the left breast mass and found multiple areas of metastatic disease in her thoracic/lumbar spine and hips. However, there were not any areas that suggested impending fractures of her long bones.
A biopsy was performed and found the patient was strongly ER-positive, with an H-score of 300, indicating that 100% of the cells were intensity 3+ for ER, notes Brufsky. Additionally, the patient was PR-positive and HER2-negative by both IHC and FISH with a ratio of 1.02.
For this patient, Sara Hurvitz, MD, would initially begin treatment with a bone-stabilizing agent. Following this, if a physical examination does not indicate a local issue, treatment with an endocrine therapy would be appropriate. If the examination showed the potential for the breast mass to break through the skin or if there was severe pain, Hurvitz recommends a more aggressive treatment with radiation along with an aromatase inhibitor and fulvestrant.
In a similar situation, Hope S. Rugo, MD, notes seeing a pathological complete response with an aromatase inhibitor in a patient whose tumor had eroded through the skin. Erring on the side of caution, Joyce A. O'Shaughnessy, MD, would administer the 500 mg dose of fulvestrant in combination with an aromatase inhibitor, regardless of local issues.
Another option for this patient would be to seek a clinical trial investigating the CDK 4/6 inhibitor palbociclib, notes Rugo. This trial is enrolling patients who have not had prior systemic treatments for ER-positive breast cancer. In this phase III trial, patients with ER-positive, HER2-negative advanced breast cancer are randomized to either letrozole alone or in combination with palbociclib.

First-Line Toripalimab Plus Chemo Wins Approval in China for Advanced TNBC

Oncology Experts Preview Top Abstracts From the 2024 ASCO Annual Meeting

Patient Selection Takes Priority as Standard of Cares Shift in HR+ and HER2+ Breast Cancer

Revisit the OncLive On Air Episodes From February 2024

European Commission Approves Capivasertib Plus Fulvestrant for ER+ Breast Cancer

The Pursuit of Targeted Agents Broadens the HR+ Breast Cancer Treatment Paradigm
Latest Conference Coverage

Dostarlimab/Chemo/Niraparib Generates PFS Benefit in Primary/Recurrent Endometrial Cancer

Rucaparib Maintenance Elicits Continued PFS Benefit Across Newly Diagnosed Ovarian Cancer Subgroups

Lenvatinib/Pembrolizumab Misses OS End Points, But Still Shows Activity, in Advanced Endometrial Cancer

Frontline Durvalumab/Chemo, Then Maintenance Durvalumab ± Olaparib, Improves ORR in Endometrial Cancer
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Case Presentation
Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn’t reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left breast as the patient raises her arms over her head. When the NP mentions it to Mrs. B.C. she is surprised and denies noticing it before today. A firm, non-tender, immobile nodule is palpated in the upper quadrant of her breast . The NP then asks Mrs. B.C. how frequently she is performing breast self-exams, she admits to only doing them randomly when she remembers, which is about every few months. She reports no recent or abnormal drainage from her breast. Further examination reveals palpable axillary lymph nodes.
Mrs. B.C. is about 30 pounds overweight and walks her dog around her neighborhood every morning before work and every evening when she gets home. She reports drinking a glass of white wine before bed each night. She denies any history of tobacco use. She reports use of a combination birth control pill on and off for 25 years until she reached menopause. She is not currently taking any prescription medications.
Past Medical History
- Menarche (Age 10)
- Post-menopausal (Age 53)
- No other pertinent medical history
Family History:
- Father George- deceased from stroke (75 years old), history of hypertension, CAD, HLD
- Mother Maryanne alive- 76 years old, history of dementia, osteoporosis
- Brother Michael- alive, 57 years old, history of hypertension, CAD and cardiac stent placement (54 years old)
- Sister, Michelle- alive 53 years old, history of GERD, Asthma
- Brother- Jimmy- alive 50 years old, no past medical history
Social History:
Mrs. B.C. works Monday-Friday 8am-5pm at the local dentist’s office at the front desk as a schedule coordinator. She is planning to retire in a few years. In her spare time, she is involved in various community efforts to feed the homeless and helps to prepare dinners at her local church one night a week. She also enjoys cooking and baking at home, gardening, and nature photography.
Mrs. B.C. has two children. Her oldest son, Patrick, is 21 years old and is in his final year of pre-med. He is attending a public university about 2 hours away from home where he lives year-round. As an infant, Patrick was breastfed until 18 months when he self-weaned. Her daughter, Veronica, is 19 years old and lives at home while attending the local branch campus of a state university. She is in her second year of a business degree and then plans to transfer to the main campus next year. When Veronica was an infant she had difficulty latching onto the breast due to an undiagnosed tongue and lip ties resulting in Mrs. BC exclusively pumping and bottle feeding for six months. After six months, Mrs. B.C. was having a hard time keeping up while working and her found her supply diminished. Veronica had begun eating solid foods so Mrs. B.C. switched to supplemental formula, which was a big relief.
Mrs. B.C. was married to her now ex-husband Kent for 26 years. They divorced two years ago when Veronica was a senior in high school. They have remained friends and Kent lives 25 minutes away in a condo with his girlfriend. She also has two brothers who live nearby and a sister who lives out of state. Her 7 nieces and nephews range in age from 9 years old to 26 years old. Her father, George, passed away from a sudden stroke 4 years ago. Her mother, Maryanne, has dementia and is living in a nearby memory care facility. She also has many close friends.

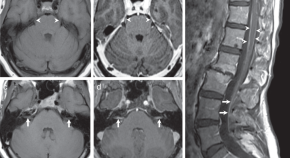
Breast cancer brain metastases
Citation, doi, disclosures and case data.
At the time the case was submitted for publication Bahman Rasuli had no financial relationships to ineligible companies to disclose.
Presentation
Headache and vertigo. Background of metastatic breast carcinoma.
Patient Data
Abnormal signal intra-axial enhancing mass lesions with water restriction on DWI images are seen in the right cerebral and cerebellar hemispheres surrounded by vasogenic edematous changes.
Midline shift to the left side measuring about 5 mm and subfalcine herniation also is noted.
No transtentorial or uncal herniation.
No ventricular entrapment.
Case Discussion
The brain MRI results show that the patient, who is known to have breast cancer , has developed brain metastases .
1 public playlist includes this case
- neuro tumors 2 by Emil Michalski
Related Radiopaedia articles
- Brain metastases
- Breast cancer metastases
- Breast neoplasms
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

Case Study in Breast Cancer: Primary Treatment of HR-positive, HER2-negative Advanced Breast Cancer
—this case illustrates the current treatment paradigm for postmenopausal, hr-positive, her2-negative, advanced breast cancer that has not been previously treated..
By Pooja Murthy, MD Reviewed by Francisco J. Esteva, MD, PhD
Patient history and assessment
A 65-year-old woman with no previous medical history of breast cancer was referred to the medical oncology clinic for newly diagnosed metastatic breast cancer. Three months ago, she developed left breast pain and a palpable breast mass. Mammogram and ultrasound revealed a 5.2-cm left breast mass with an enlarged ipsilateral axillary lymph node. Core biopsy of the mass showed invasive ductal carcinoma, estrogen receptor (ER) 95% positive, progesterone receptor 85% positive, and HER2 negative. Fine needle aspiration of the axillary lymph node was positive for adenocarcinoma. Positron emission tomography/computed tomography was obtained, and revealed multiple 1- to 2-cm, positron emission tomography-avid pulmonary nodules and enlarged mediastinal and hilar lymph nodes, suspicious for metastases. Interventional radiology was consulted for core biopsy of one of the pulmonary nodules. The biopsy confirmed metastatic breast cancer, ER 95% positive, progesterone receptor 90% positive, and HER2 negative.

The patient presents to clinic feeling well today. She denies shortness of breath, pain, or fatigue. She works as a high school teacher and has good energy at work. There is no family history of breast, ovarian, or other cancers. She has no medical problems and takes no medications. Menarche was at age 11 and menopause was at age 50. There is no history of hormone replacement therapy. She has two children, with her first pregnancy at age 29.
On physical exam, her height is 62 inches (157 cm), and her weight is 148 lbs (67 kg). Her body mass index is 27. A large, 6-cm left breast mass is palpable with some overlying skin puckering. Nipples are everted bilaterally with no nipple discharge. Enlarged left axillary lymph nodes are also palpable. Lungs are clear to auscultation. Otherwise, results of the exam are unremarkable.
Laboratory results include an unremarkable comprehensive metabolic panel and complete blood count.
In summary, the patient is a 65-year-old postmenopausal woman with newly diagnosed, de novo, HR-positive, HER2-negative metastatic breast cancer. Metastatic sites include pulmonary nodules and lymph nodes, and metastatic disease has been biopsy proven. She has an excellent performance status and no comorbidities.
Treatment recommendations
At this initial medical oncology visit, the patient was placed on the combination regimen of letrozole 2.5 mg by mouth daily and palbociclib 125 mg by mouth daily for 21 days followed by a 7 day rest period (to complete a 28-day cycle). This regimen is supported by the National Comprehensive Cancer Network guidelines as first-line therapy for postmenopausal women with HR-positive metastatic breast cancer. Letrozole is an aromatase inhibitor, and palbociclib is a small-molecule inhibitor of the CDKs 4 and 6. The combination of these medications was evaluated in a randomized phase II trial (PALOMA-1), in which letrozole plus palbociclib was compared with letrozole plus placebo as first-line treatment for postmenopausal, HR-positive, HER2-negative, advanced breast cancer. Results showed a significantly improved progression-free survival (PFS) in the palbociclib group (20.2 months versus 10.2 months). There was a trend towards improved overall survival in this group, but it was not statistically significant. 1
Prior to prescribing letrozole plus palbociclib, the physician discussed the diagnosis of metastatic breast cancer, and that the goal of treatment is to slow the progression of disease, improve quality of life, and prolong survival. The patient was informed that metastatic breast cancers are rarely cured, but rather are managed like chronic disease with sequential therapy as the mainstay of treatment.
Common adverse effects of letrozole, including joint pains, hot flashes, and increased risk of osteoporosis, were discussed with the patient. Adequate vitamin D and calcium supplementation, as well as regular exercise, were strongly recommended as measures to protect against osteoporosis and improve overall health.
The common adverse effects of palbociclib include neutropenia, leucopenia, and fatigue. The medication is also associated with an increased risk of venous thromboembolism (1%–5% of patients treated with the medication). The patient was made aware of these potential adverse effects, and that regular monitoring of blood counts is necessary (every 2 weeks for the first two cycles, then prior to each cycle).
More than 1.5 million new cases of breast cancer are reported worldwide each year, of which 60% are HR-positive. Hormonal therapy has been the mainstay of treatment for HR-positive, HER2-negative advanced breast cancers. However, resistance to hormonal therapy eventually develops, which has prompted increasing interest in modulating the mechanisms of resistance. Disruptions in cell cycle regulation are one of the possible mechanisms of resistance to hormonal therapy, with orderly progression through the cell cycle controlled by a group of proteins that include CDKs.
Palbociclib is a reversible, oral, small molecule inhibitor of CDK 4 and 6. CDK 4 and 6 have a critical role in the regulation of the G1 to S transition, therefore inhibition of their activity can cause cell cycle arrest. 2 Preclinical data suggested that palbociclib has activity in HR-positive breast cancers in conjunction with antiestrogen medications. The PALOMA-1 trial was a randomized phase II study evaluating the safety and efficacy of palbociclib in combination with letrozole as first-line treatment of patients with advanced, ER-positive, HER2-negative breast cancer. 1 165 patients were randomized to palbociclib plus letrozole versus letrozole alone (plus placebo). Median PFS was 20.2 months in the palbociclib plus letrozole group and 10.2 months in the letrozole alone group (hazard ratio 0.49, P = .0004). There was a trend towards improved overall survival in the palbociclib group, but it was not statistically significant. Grade 3 to 4 neutropenia was reported in 54% of the patients in the palbociclib group versus 1% in the letrozole group, but no patients treated with palbociclib developed febrile neutropenia. Other significant adverse effects in the palbociclib group include pulmonary embolism (4%), back pain (2%), and diarrhea (2%). 1
Previous to the PALOMA-1 results, the preferred first-line treatment for advanced, HR-positive, HER2-negative breast cancer had been a nonsteroidal aromatase inhibitor or fulvestrant. Now, with the compelling PFS results from PALOMA-1, we would treat first line with palbociclib plus letrozole instead. As before, chemotherapy instead of hormonal therapy is recommended for patients with HR-positive, HER2-negative advanced breast cancer who have rapidly progressive disease and/or visceral crisis. 3
Palbociclib was also evaluated in the setting of endocrine-resistant advanced breast cancer (PALOMA-3). Postmenopausal patients with advanced HR-positive, HER2-negative breast cancer who had progressed on prior endocrine therapy were treated with palbociclib plus fulvestrant versus fulvestrant plus placebo. Similar to the PALOMA-1 results, the palbociclib group had a significantly improved PFS compared with the fulvestrant-alone group. This data therefore support the use of palbociclib plus fulvestrant for patients who have not yet been treated with palbociclib and have progressed on an aromatase inhibitor. 4
Investigational agents
Other CDK 4/6 inhibitors are also in development, including ribociclib and abemaciclib, with promising results so far. MONALEESA-2 is a phase III randomized, double-blind, multicenter trial involving 668 postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had received no prior therapy for their advanced breast cancer. 5 The patients were randomized to ribociclib (600 mg daily, 3 weeks on and 1 week off), or placebo, in combination with letrozole 2.5 mg daily. 5 Results from a preplanned interim analysis showed a significantly improved PFS for the ribociclib group; therefore the Independent Data Monitoring Committee recommended stopping the trial early. Full results are pending. 6 MONALEESA-3 is an ongoing trial evaluating ribociclib in combination with fulvestrant compared with fulvestrant alone in men and postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had received no or a maximum of one prior line of endocrine therapy. 6,7 Abemaciclib is another investigational CDK 4/6 inhibitor which is being evaluated in the MONARCH-1 trial trial as a single agent (in contrast to the PALOMA and MONALEESA-2 trials) in patients with HR-positive, HER2-negative metastatic breast cancer. 8 This is a phase II trial involving patients who were endocrine resistant and heavily pretreated for advanced disease. Abemaciclib induced a response rate of nearly 20% with a median PFS of 6 months in these patients. 9
Treatment outcomes
A follow-up complete blood count 4 weeks after treatment initiation was remarkable for an absolute neutrophil count (ANC) of 1200/mm 3 (grade 2 neutropenia). The patient was feeling well and was afebrile, so palbociclib was continued. Four weeks later, ANC had decreased to 800/mm 3 , and the patient remained afebrile. Since this was grade 3 neutropenia, palbociclib was held for 1 week and was resumed when ANC was >1000/mm 3 . Otherwise, she tolerated the treatment well with no other adverse effects. Computed tomography scans of the chest, abdomen, and pelvis were done 3 months after treatment initiation, and showed significant decrease in size of the breast mass, lymph nodes, and pulmonary nodules.
CDK 4/6 inhibitors, including palbociclib, ribociclib, and abemaciclib, are emerging as promising therapies for advanced, HR-positive, HER2-negative breast cancer, possibly through mediation of endocrine resistance. Palbociclib plus letrozole is approved for use in the first-line setting for advanced, postmenopausal, HR-positive breast cancer, backed by the PALOMA-1 trial results showing a significantly improved PFS in the palbociclib group. The combination of ribociclib and letrozole was also evaluated as first-line treatment in a similar patient population of postmenopausal women with advanced HR-positive, HER2-negative breast cancer, and showed a significantly improved PFS based on a preplanned interim analysis; final results from this trial are still pending.
Patient education
For patient education materials, visit:
- Metastatic Breast Cancer Network: Guide for the Newly Diagnosed
- Susan G. Komen’s Facts for Life: Clinical Trials
Published: December 20, 2016
- 1. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Onco l. 2015;16:25-35.
- 2. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res . 2016;18:17.
- 3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer, Version 2.2016. 5/6/16.
- 4. Turner NC, Ro J, André F, et al; PALOMA3 Study Group. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med . 2015;373:209-219.
- 5. ClinicalTrials.gov. Study of efficacy and safety of LEE011 in postmenopausal women with advanced breast cancer (MONALEESA-2).
- 7. ClinicalTrials.gov. Study of efficacy and safety of LEE011 in men and postmenopausal women with advanced breast cancer. (MONALEESA-3).
- 8. ClinicalTrials.gov. A study of abemaciclib (LY2835219) in participants with previously treated breast cancer that has spread (MONARCH 1).
- 9. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH1: results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. In: 2016 ASCO Annual Meeting; abstract number 510.
Preterm delivery and early maternal cardiac disease, sex hormones only part of the equation in modulating hsdd, 10 more questions to challenge your women’s health knowledge.

Menopause: What You Need to Know
Dysmenorrhea: what you need to know, 10 questions to challenge your women’s health knowledge, abnormal/excessive vaginal bleeding: what you cannot afford to miss, risk of stroke increased by pregnancy among younger, but not older women.

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals
You are here
Breast cancer.
- Erosive adenomatosis of the nipple (adenoma of the nipple) Shikha Mandloi , Bhushan Madke , Samarth Shukla , Naga Nitya BMJ Case Reports CP May 2024, 17 (5) e260896; DOI: 10.1136/bcr-2024-260896
- Clinical, electrophysiologic and serologic evidence of cancer associated retinopathy preceding a diagnosis of breast cancer Jordan E Ball , Brian M Smith , Kent Zocchi , Jennifer Doyle BMJ Case Reports CP Apr 2024, 17 (4) e257911; DOI: 10.1136/bcr-2023-257911
- Breast cryoablation for the palliative treatment of indolent subtype of multicentric triple-negative breast cancer Monica L Huang , Deanna L Lane , Hannah Bomar , Henry Kuerer BMJ Case Reports CP Apr 2024, 17 (4) e259465; DOI: 10.1136/bcr-2023-259465
- Malignant phyllodes tumour of the breast with early recurrence in a young patient Andrea Klooz , Terrence Kumar , Jessica Maxwell BMJ Case Reports CP Mar 2024, 17 (3) e258352; DOI: 10.1136/bcr-2023-258352
- Male ductal carcinoma in situ: diagnosis and management of a rare disease in men Changtai Tian , Rosalinda Alvarado , Thomas Kim , Jessica Slostad BMJ Case Reports CP Mar 2024, 17 (3) e256608; DOI: 10.1136/bcr-2023-256608
- Thoracic splenosis: an important consideration in oncology patients Katherine Julian , Monali Vasekar BMJ Case Reports CP Nov 2023, 16 (11) e257091; DOI: 10.1136/bcr-2023-257091
- Klinefelter syndrome presenting as metastatic bilateral breast cancer: missed diagnostic opportunities Roxana Maria Tudor , Elsheikh Mohammed Ali , Salman Ullah Khan , John McDermott BMJ Case Reports CP Nov 2023, 16 (11) e255703; DOI: 10.1136/bcr-2023-255703
- Partial hippocampal avoidance whole brain radiotherapy in a patient with metastatic infiltration of the left hippocampus Sandra Leskinen , Harshal A. Shah , Randy S. D' Amico , A. Gabriella Wernicke BMJ Case Reports CP Nov 2023, 16 (11) e257988; DOI: 10.1136/bcr-2023-257988
- Right breast ulceration and dystrophic calcification as a late sequela following postmastectomy radiotherapy Puo Nen Lim , Simon Tilston BMJ Case Reports CP Feb 2023, 16 (2) e254086; DOI: 10.1136/bcr-2022-254086
- Benign phyllodes tumour in a transgender woman receiving hormonal therapy Jacquelyn Dillon , Stephanie Bernik , Nebras Zeizafoun , Tara Balija BMJ Case Reports CP Mar 2024, 17 (3) e258616; DOI: 10.1136/bcr-2023-258616
- Anaesthesia (213)
- Cardiovascular medicine (992)
- Complementary medicine (6)
- Dentistry and oral medicine (216)
- Dermatology (545)
- Clinical diagnostic tests (50)
- Radiology (diagnostics) (146)
- Surgical diagnostic tests (23)
- Diagnostics
- Drugs and medicines (1781)
- Ear, nose and throat/otolaryngology (464)
- Disaster response
- Poisoning (49)
- Resuscitation (57)
- Trauma (160)
- Emergency medicine (481)
- Adrenal disorders (118)
- Calcium and bone (97)
- Diabetes (127)
- Drugs: endocrine system (25)
- Lipid disorders (26)
- Metabolic disorders (89)
- Pituitary disorders (57)
- Thyroid disease (118)
- Endocrinology (660)
- Changing physician behavior
- Effectiveness of care
- Gastroenterology (920)
- Drugs: gastrointestinal system (44)
- Endoscopy (165)
- GI bleeding (99)
- Infection (gastroenterology) (107)
- Inflammatory bowel disease (72)
- Liver disease (186)
- Neurogastroenterology
- Oesophagus (55)
- Pancreas and biliary tract (100)
- Pancreatitis (61)
- Portal hypertension (16)
- Small intestine (76)
- Stomach and duodenum (85)
- General practice / family medicine (245)
- Clinical genetics
- Cytogenetics
- Genetic screening / counselling (92)
- Molecular genetics
- Genetics (293)
- Elder abuse
- End of life decisions (geriatric medicine) (2)
- Long term care (18)
- Psychogeriatrics
- Geriatric medicine (65)
- Haematology (incl blood transfusion) (538)
- HIV/AIDS (80)
- Immunology (including allergy) (57)
- Infectious diseases (1329)
- Adult intensive care (301)
- Mechanical ventilation (19)
- Neonatal intensive care (90)
- Paediatric intensive care (69)
- Intensive care (564)
- Brain stem / cerebellum (85)
- Cerebral palsy (6)
- Clinical neurophysiology (40)
- Coma and raised intracranial pressure (29)
- Cranial nerves (107)
- Dementia (7)
- Drugs: CNS (not psychiatric) (33)
- Epilepsy and seizures (116)
- Headache (including migraine) (9)
- Hydrocephalus (22)
- Infection (neurology) (166)
- Motor neurone disease (14)
- Movement disorders (other than Parkinsons) (63)
- Multiple sclerosis (19)
- Muscle disease (40)
- Neuroimaging (396)
- Neurological injury (71)
- Neuromuscular disease (77)
- Neurooncology (116)
- Neuroophthalmology (8)
- Pain (neurology) (47)
- Parkinson's disease (7)
- Peripheral nerve disease (84)
- Sleep disorders (neurology) (8)
- Spinal cord (105)
- Stroke (239)
- Trauma CNS / PNS (40)
- Variant Creutzfeld-Jakob Disease (2)
- Neurology (1617)
- Nursing (36)
- Childhood nutrition (5)
- Malnutrition (24)
- Obesity (nutrition) (17)
- Vitamins and supplements (46)
- Nutrition and metabolism (118)
- Obstetrics and gynaecology (601)
- Oncology (1486)
- Ophthalmology (770)
- Orthopaedics (378)
- Adolescent health
- Bilirubin disorders (9)
- Child abuse (6)
- Child and adolescent psychiatry (paedatrics) (9)
- Child health
- Childhood nutrition (paediatrics) (7)
- Congenital disorders (280)
- Developmental paediatrics (4)
- Failure to thrive (19)
- Infant health (32)
- Infant nutrition (including breastfeeding) (5)
- Materno-fetal medicine (72)
- Neonatal and paediatric intensive care (96)
- Neonatal health (93)
- Paediatrics (899)
- End of life decisions (palliative care) (14)
- Pain (palliative care) (8)
- Palliative care (37)
- Chemical pathology
- Cytopathology
- Histopathology
- Inflammation
- Microbiology
- Molecular biology
- Morbid anatomy / surgical pathology
- Neuropathology
- Pathology (572)
- Drug misuse (including addiction) (33)
- Medicines regulation
- Toxicology (56)
- Unwanted effects / adverse reactions (311)
- Pharmacology and therapeutics (477)
- Prison medicine (1)
- Adjustment disorders
- Alcohol-related disorders (4)
- Anxiety disorders (including OCD and PTSD) (18)
- Child and adolescent psychiatry (11)
- Delirium (17)
- Drugs misuse (including addiction) (27)
- Drugs: psychiatry (29)
- Eating disorders (7)
- Impulse control disorders (6)
- Memory disorders (psychiatry) (22)
- Mood disorders (including depression) (33)
- Personality disorders (2)
- Psychiatry of old age (8)
- Psychotherapy (14)
- Psychotic disorders (incl schizophrenia) (37)
- Sexual and gender disorders (2)
- Sleep disorders (2)
- Somatoform disorders (6)
- Suicide (psychiatry) (7)
- Psychiatry (221)
- Radiology (879)
- Continuing medical education
- Disability (17)
- Other rehabilitative therapies
- Physiotherapy (41)
- Rehabilitation medicine (85)
- Acute renal failure (107)
- Chronic renal failure (49)
- Dialysis (61)
- Fluid electrolyte and acid-base disturbances (78)
- Nephrotic syndrome (29)
- Proteinurea (29)
- Renal transplantation (48)
- Renal medicine (373)
- Respiratory medicine (620)
- Ankylosing spondylitis (2)
- Biological agents (37)
- Connective tissue disease (97)
- Degenerative joint disease (9)
- Drugs: musculoskeletal and joint diseases (26)
- Fibromyalgia (2)
- Musculoskeletal syndromes (71)
- Osteoarthritis (13)
- Osteoporosis (11)
- Rheumatoid arthritis (40)
- Sjogren's syndrome (3)
- Systemic lupus erythematosus (60)
- Vascularitis (161)
- Rheumatology (576)
- Sexual health (102)
- Sports and exercise medicine (124)
- Breast surgery (69)
- Cardiothoracic surgery (205)
- Gastrointestinal surgery (432)
- General surgery (457)
- Neurosurgery (204)
- Oral and maxillofacial surgery (142)
- Orthopaedic and trauma surgery (235)
- Otolaryngology / ENT (246)
- Plastic and reconstructive surgery (177)
- Surgical oncology (230)
- Transplantation (61)
- Urological surgery (183)
- Vascular surgery (192)
- Surgery (2593)
- Urology (276)
- Communication
- Clinical trials (epidemiology)
- Epidemiologic studies
- Population trends
- Screening (epidemiology)
- Survey techniques
- Therapeutic trials
- Time-to-event methods
- Artificial and donated transplantation
- Assisted dying
- Clinical ethics
- Codes of professional ethics
- Competing interests (ethics)
- Confidentiality
- Drug issues
- End of life decisions (ethics)
- Ethics of abortion
- Ethics of reproduction
- Experiments in vivo
- Informed consent
- Research and publication ethics
- Sexuality / gender
- Ethics (16)
- Ethnic studies (2)
- Health economics (7)
- Information management
- Telemedicine
- Health policy
- Health service research
- Journalology
- Legal and forensic medicine
- Choosing / changing career
- Combining life and work
- Continuous professional development
- Flexible training
- Getting and changing jobs
- Getting and changing jobs (including job descriptions and contracts)
- Leaving medicine
- Medical careers
- Organise and prepare your CV
- Organise working abroad
- Pay, working conditions, and health
- Personal development
- Portfolio development
- Prepare for appraisal
- Prepare for interview
- Professional conduct and regulation
- Professional regulation and standards
- History taking and the physical examination
- Postgraduate
- Undergraduate
- Medical education (108)
- Doctors' morale and well being
- Medical error/ patient safety
- Quality improvement
- Medical management (208)
- Occupational and environmental medicine (141)
- Abuse (child, partner, elder)
- Air pollution
- Drug misuse
- Environmental issues
- Health education
- Health of indigenous peoples
- Health promotion
- Housing and health
- Human rights
- Obesity (public health) (5)
- Screening (public health)
- Sexual health (public health)
- Social conditions and disease
- Suicide (public health)
- Urban health
- Vaccination programs
- Violence (other)
- Violence against women
- Public health (68)
- Smoking and tobacco (16)
- Sociology (1)
- Comparative effectiveness research
- Qualitative research
- Quantitative research
- Statistics and research methods (1)
- 10-Minute Consultation
- Assessment of older adults
- BMJ clinical evidence summaries
- Care of older people
- Change page
- Clinical evidence
- Clinical governance in primary care
- Clinical guidelines (the theory)
- Clinical Update
- Competent novice
- Complexity science
- Conflict and health
- Coping with loss
- Data campaign
- Diagnosis in general practice
- Digital theme issue: Overdiagnosis
- Drug points
- E-cigarettes
- Easily missed
- Economics notes
- Ethical debates
- Evidence base of clinical diagnosis
- Evidence based case report
- Evidence based management of hypertension
- Evidence based paediatrics
- Extracts from BestTreatments
- For and against (controversies in management)
- Getting research findings into practice
- Guideline summaries
- Health needs assessment
- How does it work?
- If I ruled the NHS
- Improving the quality of health care
- Interactive case reports
- Investigation
- Lesson of the week
- Making a difference
- Managing demand
- Maternal, child, and adolescent health
- Measuring quality of life
- Methods in health services research
- Mid Staffordshire Inquiry
- Modernising the NHS
- Multimorbidity
- Narrative based medicine
- Non-communicable diseases
- Patient authored
- Patient journeys
- Pharmacology
- Practice pointer
- Pregnancy plus
- Primary care groups
- Qualitative research in health care
- Quality improvement report
- Rapid Recommendation
- Rational imaging
- Rational testing
- Regulating nursing homes
- Research pointers
- Safety alerts
- Science, medicine, and the future
- Scrap the Cap
- Seven day NHS
- Shared decision making
- Spotlight on climate change
- State of the Art
- Statistics notes
- Systematic reviews in health care
- Teaching rounds
- The BMJ Awards 2015
- The World Bank and world health
- Theories in health care and research
- Therapeutics
- Too much medicine
- UK general election 2015
- Uncertainties page
- Understanding controlled trials
- What the educators are saying
- What your patient is thinking
- What's new this month in BMJ Journals
- When I use a word
- Access to health care (10)
- Adverse drug reactions and complications (328)
- Corrections
- Determinants of health (2)
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect (259)
- Global health (72)
- Health in vulnerable communities (12)
- Images in... (2283)
- Learning from errors (76)
- Learning from unexpected outcome (positive or negative) (147)
- Medical student electives
- Medicine in the humanitarian sector
- Myth exploded
- New disease (44)
- Novel diagnostic procedure (55)
- Novel treatment (new drug/intervention; established drug/procedure in new situation) (396)
- Other full case
- Rare disease (2621)
- Reminder of important clinical lesson (715)
- Rural medicine
- Unexpected outcome (positive or negative) including adverse drug reactions (219)
- Unusual association of diseases/symptoms (708)
- Unusual presentation of more common disease/injury (795)
- Video reports
- COVID-19 (550)
- Editor's choice (66)
- Anaesthetics and ITU
- Anatomy quiz
- Case report
- Case Review
- Case scenario
- Drugs and therapeutics
- Emergency medicine
- Endocrinology
- Gastroenterology
- General practice
- Haematology
- Histopathology/cytology/pathology/autopsy
- Infectious diseases
- Law and ethics
- Learning disabled
- Obstetrics and gynaecology
- onExamination questions
- Ophthalmology
- Orthopaedics and trauma
- Paediatrics
- Picture quiz
- Renal medicine
- Respiratory
- Rheumatology
- Spot Diagnosis
- GUT Recent advances in clinical practice
- GUT Snapshot
- IP Cochrane corners
- JCP Education
- JECH Gallery
- JMG Online mutation reports
- Open access (805)
- Animal Models
- Biomarker Studies
- Childhood Lupus
- Clinical Trials and Drug Discovery
- Co-morbidities
- Cutaneous Lupus
- Epidemiology and Outcomes
- Immunology and Inflammation
- Lupus Nephritis
- Reproductive Health and APS
- Quality Improvement Reports
- Open access
- Published: 27 June 2024
Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution
- Serena Mazzucchelli ORCID: orcid.org/0000-0001-6904-8895 1 ,
- Lorena Signati ORCID: orcid.org/0000-0001-7695-3471 1 ,
- Letizia Messa ORCID: orcid.org/0000-0003-1718-2229 2 , 4 , 5 ,
- Alma Franceschini ORCID: orcid.org/0000-0003-1670-3598 6 ,
- Arianna Bonizzi ORCID: orcid.org/0000-0002-5948-8747 3 ,
- Lorenzo Castagnoli ORCID: orcid.org/0000-0002-0220-6497 6 ,
- Patrizia Gasparini ORCID: orcid.org/0000-0002-9548-3724 7 ,
- Clarissa Consolandi ORCID: orcid.org/0000-0002-9328-9557 8 ,
- Eleonora Mangano ORCID: orcid.org/0000-0002-4040-4654 8 ,
- Paride Pelucchi ORCID: orcid.org/0000-0001-5415-1515 8 ,
- Ingrid Cifola ORCID: orcid.org/0000-0003-2819-1171 8 ,
- Tania Camboni ORCID: orcid.org/0000-0002-3430-9984 8 ,
- Marco Severgnini ORCID: orcid.org/0000-0001-8655-1206 8 ,
- Laura Villani ORCID: orcid.org/0000-0001-6284-0468 3 ,
- Barbara Tagliaferri ORCID: orcid.org/0000-0002-4889-5574 3 ,
- Stephana Carelli ORCID: orcid.org/0000-0003-4603-396X 4 , 5 ,
- Serenella M. Pupa ORCID: orcid.org/0000-0002-4592-6830 6 ,
- Cristina Cereda ORCID: orcid.org/0000-0001-9571-0862 5 &
- Fabio Corsi ORCID: orcid.org/0000-0002-6469-4086 1 , 3
Cancer Cell International volume 24 , Article number: 220 ( 2024 ) Cite this article
Metrics details
A reliable preclinical model of patient-derived organoids (PDOs) was developed in a case study of a 69-year-old woman diagnosed with breast cancer (BC) to investigate the tumour evolution before and after neoadjuvant chemotherapy and surgery. The results were achieved due to the development of PDOs from tissues collected before (O-PRE) and after (O-POST) treatment.
PDO cultures were characterized by histology, immunohistochemistry (IHC), transmission electron microscopy (TEM), scanning electron microscopy (SEM), confocal microscopy, flow cytometry, real-time PCR, bulk RNA-seq, single-cell RNA sequencing (scRNA-seq) and drug screening.
Both PDO cultures recapitulated the histological and molecular profiles of the original tissues, and they showed typical mammary gland organization, confirming their reliability as a personalized in vitro model. Compared with O-PRE, O-POST had a greater proliferation rate with a significant increase in the Ki67 proliferation index. Moreover O-POST exhibited a more stem-like and aggressive phenotype, with increases in the CD24 low /CD44 low and EPCAM low /CD49f high cell populations characterized by increased tumour initiation potential and multipotency and metastatic potential in invasive lobular carcinoma. Analysis of ErbB receptor expression indicated a decrease in HER-2 expression coupled with an increase in EGFR expression in O-POST. In this context, deregulation of the PI3K/Akt signalling pathway was assessed by transcriptomic analysis, confirming the altered transcriptional profile. Finally, transcriptomic single-cell analysis identified 11 cell type clusters, highlighting the selection of the luminal component and the decrease in the number of Epithelial–mesenchymal transition cell types in O-POST.
Neoadjuvant treatment contributed to the enrichment of cell populations with luminal phenotypes that were more resistant to chemotherapy in O-POST. PDOs represent an excellent 3D cell model for assessing disease evolution.
Breast cancer (BC) is the most commonly diagnosed type of tumour and the principal cause of death among women worldwide [ 1 ]. This type of cancer is divided into different histological and molecular subtypes [ 2 , 3 ]. Although the biomolecular classification generally indicates the sensitivity of different tumours to distinct therapies and is a valid tool for defining prognosis, many tumours escape this categorization and have unexpected responses to treatments and a prognosis that is inconsistent with their biomolecular characterization.
Inter- and intra-individual tumour heterogeneity is the major cause of patients’ partial response to anticancer treatments and represents the main obstacle to successful therapy. Indeed, certain treatments are effective for some patients but do not show promising results for others [ 4 ]. Although breakthroughs have been made in elucidating the pathobiological complexity of BC, novel molecular and pharmacogenomic markers for predicting drug responses in patients are needed. Therefore, a reliable in vitro preclinical model that closely reflects the original in vivo tumour context may be valuable for assessing the complex biological conditions of BC.
In recent years, three-dimensional (3D) cell cultures have been widely used as preclinical models of numerous diseases, including cancer, to bridge the gap between two-dimensional (2D) cell cultures and animal models [ 5 ]. Initially, tumour spheroids were developed and consisted of cell aggregates generated from clones of a single cancer cell, with a specific genetic identity, that grew in suspension. These models had various challenges, mainly due to the lack of tumour cell interactions with the surrounding stromal compartment, which limited their applications as reliable preclinical tumour models. More recently, the tumour patient-derived organoid (PDO) approach, in which the cellular complexity and internal genetic heterogeneity of original cancerous tissue are preserved, has emerged as a very promising tool in translational cancer research and personalized cancer medicine [ 6 ]. Organoid cultures consist of clusters of organ-specific cells (stem or progenitor cells) directly derived from fragments of the original tumour. Research has been widely demonstrated that the organoid partially reconstructs the tissue of origin, with a similar structural and functional organization [ 7 ]. In BC, PDOs can be successfully derived from all subtypes and show concordance with the corresponding tumour tissue of origin [ 8 ]. Indeed, PDO tissue culture has been proposed not only as a model for studying tumour biology but also as a platform for testing different drug therapies for personalized medicine [ 9 , 10 ]. However, despite the relevant results achieved with colon cancer and other cancer subtypes [ 11 , 12 ], it is still too early to propose BC-PDOs as a screening platform to predict patient response to therapeutic strategies due to the low success rate and low growth of this type of organoid. In the case of BC, PDOs may be much more useful for studying tumour evolution to resolve the molecular and cellular complexity of the tumour and identify more effective therapies.
In this study, we report the case of a 69-year-old BC patient from whom two PDO cultures were successfully established from specimens collected before (O-PRE) and after (O-POST) neoadjuvant chemotherapy (NACT). Through in-depth characterization of both PDO cultures, we generated a model that was able to recapitulate in vitro patient tumour evolution following NACT, revealing specific cell type gene expression changes and biological and molecular profiles focused on the expression profiles of several biomarkers associated with tumour proliferation and metastasis.
Patient information and sample collection
The patient enrolled at the Breast Unit of the ICS Maugeri IRCCS (Pavia, Italy) according to the protocol of the Bruno Boerci Oncological Biobank (approved by the ethical committee of the ICS Maugeri IRCCS on 27 July 2009) was a 69-year-old woman with a 6 cm ulcerated lesion of left mammary gland. The patient didn’t have a family history for breast or ovarian neoplasia. The biopsy sample was classified as a lobular carcinoma, luminal B molecular subtype (oestrogen 90%, progesterone < 5%, Ki67 60%, HER2 2+ without amplification). At the time of biopsy, after the patient signed the informed consent form, another biopsy specimen was obtained during clip positioning.
Establishment of PDO culture from biopsy and surgical samples
The biopsy sample obtained from the second biopsy was collected in Ad-DF + + + medium (HyClone DMEM-F/12 1:1 supplemented with 10 mM HEPES, 1% penicillin/streptomycin and 1% L-glutamine) and stored at 4 °C until it was processed within 1 h. The specimen was transferred to a Petri dish and finely minced with a scalpel. Then, the sample was collected in a 15 mL tube and digested in 2 mL of Ad-DF + + + medium supplemented with 100 µL of 20 mg/mL collagenase (Sigma, C9407) and 2 µL of 10 mM Y27632 (ForLab, M1817) for 3 h at 37 °C. For removal of undigested fragments, the sample was filtered through a 100 µm cell strainer, collected in a 15 mL tube and then centrifuged at 500 × g for 5 min at 8 °C. The supernatant was removed, and the pellet was washed twice. Finally, the pellet was resuspended in 35 µL of cold Cultrex ® Ultimatrix Reduced Growth Factor Basement Membrane Matrix (BME) (Bio-Techne, BME0010 [ 13 ]), seeded in a prewarmed 24-well plate and transferred to an incubator. After approximately 40 min, the BME-PDOs were solidified, and 250 µL of culture medium (CM; DMEM/F12, 1 × ; L-glutamine, 1%; penicillin/streptomycin, 1%; HEPES, 10 mM; Noggin-conditioned medium, 25 × ; B27 supplement, 1 × ; N-acetyl-cysteine, 1.25 mM; nicotinamide, 0.2 mM; 83–01, 500 nM; Y-27632, 5 µM; R-spondin1-conditioned medium, 10%; Primocin, 50 µg/mL; human EGF, 5 ng/mL; FGF-10, human recombinant, 20 ng/mL; KGF/FGF-7, human recombinant, 5 ng/mL; Heregulin-beta-1, human recombinant, 37.5 ng/mL; and SB, 202190, 500 nM) was added and changed every 2–3 days.
BC surgical tissues were cut into 1–3 mm 3 pieces, and two random pieces were frozen and stored at −80 °C in the Bruno Boerci Oncological Biobank at the ICS Maugeri IRCCS (Pavia, Italy) for DNA/RNA isolation. Two other random pieces were fixed in formalin and embedded in paraffin for haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) labelling via routine procedures. Primary O-POST cultures were obtained following the procedure described in a consolidated protocol [ 13 ]. Briefly, the collected tissue was removed from the adipose tissue and mechanically and enzymatically digested in 10 mL of Ad-DF + + + medium supplemented with 500 µL of 20 mg/mL collagenase and 10 µL of 10 mM Y27632 for 1–2 h at 37 °C. The sample was filtered to remove the undigested tissue, collected in a 15 mL tube and centrifuged. The pellet was washed twice, resuspended in the appropriate amount of BME and seeded in a prewarmed multiwell plate. Once the BME-PDO drops were solidified, the appropriate amount of CM, depending on the multiwell size, was gently added. Every 7–10 days when confluence was achieved, O-PRE and O-POST cultures were collected and passaged.
Each organoid culture was frozen in cell culture freezing medium (Gibco, 12,648–010). Briefly, it is important to dissolve the BME by adding 1 µg/mL dispase (Gibco, 17,105–041) to each well, collect the organoids in a 15 mL tube and centrifuge them at 8 °C and 500 × g for 5 min. After two washes with 10 mL of cold Ad-DF + + + , the supernatants were removed, and the pellet was resuspended in freezing medium. First, the vials were transferred to −80 °C, and after approximately 24 h, they were kept in liquid nitrogen for long-term storage.
Histology and IHC
BME-organoid drops were removed with a sterile cell lifter from the plate and transferred into a mould containing a layer of optimal cutting temperature compound (OCT). Once the drops were included in the OCT, the mould was kept at -80 °C until processing. The OCT-embedded PDOs were sectioned to obtain histological slices of approximately 3 µm thickness. After fixation, the histological slides were stained with H&E and labelled with VENTANA BenchMark ULTRA following automated IHC protocols for oestrogen receptor, progesterone receptor, HER2 (c-ErbB2) and Ki67 [ 14 ]. For comparisons between the organoids and the tumour of origin, two random surgical tissue samples were fixed in formalin and embedded in paraffin for H&E and IHC labelling via routine procedures. For HER2 amplification assessment, FISH was performed on OCT-embedded sections using the PathVision HER2 DNA probe kit (Abbott Molecular) according to the manufacturer’s protocol.
TEM and SEM
For morphological transmission electron microscopy (TEM) analysis, one BME-organoid drop was removed from the plate with a cell lifter and transferred to a 1.5 mL tube containing 1 mL of 2.5% glutaraldehyde in cacodylate buffer for 2 h. The samples were postfixed with 1.5% osmium tetroxide in cacodylate buffer, dehydrated on an ethyl alcohol ascending scale and then incubated in Epon. The procedure is described in a previous protocol [ 13 ]. The slices were analysed by a Tecnai Spirit Biotween electron microscope (FEI).
For scanning electron microscopy (SEM) analysis, O-PRE and O-POST were treated with 1 µg/mL dispase (Gibco, 17105–041) to dissolve the BME, fixed in 2.5% glutaraldehyde in cacodylate buffer, washed three times and dehydrated on an ascending ethanol scale from 10 to 100% by centrifuging them at each step. Eventually, they were immersed in hexamethyldisilazane, and two drops of the suspension were placed on a coverslip. Once the hexamethyldisilazane was evaporated, the PDOs were coated with palladium gold and analysed by a Leica S-420 scanning electron microscope.
Confocal microscopy
For immunofluorescence analysis, 3 × 10 6 organoids were isolated from the BME by incubating them with 1 µg/mL dispase at 37 °C for 1–2 h. PDOs were collected, washed in phosphate buffer (PBS) three times and fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature (RT). After fixation, the PDOs were washed in PBS three times and then permeabilized using 0.1% Triton X-100 for 10 min at RT. After three washes, the PDO pellets were resuspended in 500 µL of blocking solution containing 2% goat serum/2% bovine serum albumin (BSA) in 1 × PBS for 1 h at RT. PDOs were incubated with primary antibodies in blocking solution for 2 h at RT. We used primary rabbit antibodies against Ki67 (Abcam ab243878, 1:500), EGFR (Genetex GTX35199, 1:200), Vimentin (Genetex GTX100619, 1:500) and HER2 (Cell Signalling Technologies #2165, 1:200). PDOs were washed three times in PBS and incubated with the secondary antibodies anti-rabbit Alexa Fluor 546 (1:300), wheat germ agglutinin (FITC) (1:300), and DAPI (1:10000) in blocking solution overnight at 4 °C. After staining, the PDOs were washed three times in 1 × PBS and seeded on specimen slides in ProLong ™ Gold mounting medium (Invitrogen, P36935) for acquisition with a Leica SP8 confocal microscope equipped with 405, 488 and 513 nm lasers. Acquisition was performed at 1024 × 1024 dpi resolution.
Flow cytometry
For CD24, CD44, CD49f and EPCAM staining, organoids bearing approximately 3 × 10 6 cells were isolated from the BME by incubating them with dispase (1 µg/mL). After collection, PDOs were reduced to single cells through the shearing procedure using TrypLe ™ Select (1 × ; Gibco, 12563–029). After three washes with Hank’s balanced salt solution (HBSS from HyClone) (SH30268.02), the cells were fixed with 4% PFA for 5–10 min on ice. The fixed cells were washed three times with HBSS supplemented with 2% FBS and aliquoted into four tubes containing approximately 7.5 × 10 5 cells each. The first tube was labelled with a lineage PE cocktail of antibodies (PE mouse anti-human CD2, Cod. 555327, 1:100; PE mouse anti-human CD3, Cod. 555333, 1:00; PE mouse anti-human CD10, Cod. 555375, 1:100; PE mouse anti-human CD16, Cod.555407, 1:100; PE mouse anti-human CD18, Cod. 555924, 1:100; PE mouse anti-human CD31, Cod. 555446, 1:100; PE mouse anti-human CD64, Cod. 558592, 1:100; PE mouse anti-human CD140b, Cod. 558821, 1:100; BD Biosciences) for 15 min at RT to gate the lineage-positive cells, which were excluded from the analysis. The second tube contained only unstained cells to acquire negative signals. The third tube was labelled for 15 min at RT with a lineage cocktail, FITC mouse anti-human CD24 (Cod. 555427, 1:50, BD Biosciences) and APC mouse anti-human CD44 (Cod. 559942, 1:50, BD Biosciences) to identify the CD24/CD44 cell population, while the fourth tube was labelled with lineage cocktail supplemented with FITC rat anti-human CD49f (Cod. 555735, 1:50, BD Biosciences) and APC mouse anti-human EPCAM (Cod. 347200, 1:100, BD Biosciences) to identify CD49f/EPCAM populations. After staining, the labelled cells were washed three times with HBSS supplemented with 2% FBS and analysed using a CytoFLEX flow cytometer (Beckman Coulter). Acquisition was performed on 20,000 events within the selected region of singlets of viable cells.
For EGFR evaluation, 1.5 × 10 6 organoids were isolated from the BME, reduced into single cells and fixed as described in the previous paragraph. The fixed cells were washed three times with HBSS supplemented with 2% FBS and transferred to two tubes containing approximately 7.5 × 10 5 cells each. The tube was labelled with the primary chimeric monoclonal antibody cetuximab (CTX, 1:200) for 15 min at RT. The labelled cells were washed three times with HBSS supplemented with 2% FBS. Both tubes were labelled with the Alexa Fluor 488 (AF488) goat anti-human secondary antibody (Thermo Fisher, 1:300). A tube containing cells labelled with only the secondary antibody was used to determine the region of positivity. After staining, the labelled cells were washed three times with HBSS supplemented with 2% FBS and analysed as described above.
RNA-Seq and bioinformatic analysis
Three different human samples (one healthy breast tissue, one O-POST organoid, three O-PRE organoids and three FFPE tissues) were subjected to RNA-Seq analysis. PDOs were isolated from BME by adding 1 µg/mL dispase and incubating at 37 °C for 1–2 h. The PDOs were collected in a 15 mL tube and centrifuged at 500 × g and 8 °C for 5 min. The pellet was washed twice, and then, an aliquot (1 mL) of PDO suspension was treated with TrypLe™ Select, reduced to single cells and quantified. A PDO suspension containing approximately 30,000 cells was centrifuged, the supernatant was removed and the pellet was maintained at −80 °C until RNA extraction. The O-PRE pellet (N = 3) was collected for RNA-Seq at two different times, at cell passages 3 and 14, while O-POST (N = 1) has been collected at cell passage 19. RNA was extracted using TRIzol ® (Invitrogen) following the manufacturer’s instructions. Nanodrop One C (Thermo Fisher) was used for RNA quantification and quality control. RNA-Seq libraries were prepared with the CORALL Total RNA-Seq Library Prep Kit (Lexogen, Vienna, Austria) using 150 ng total RNAs. The RiboCop rRNA Depletion Kit (Lexogen, Vienna, Austria) was used to remove rRNA. The qualities of the sequencing libraries were assessed with D1000 ScreenTape Assay using the 4200 TapeStation System (Agilent Technologies, Santa Clara, CA, USA) to account for variability in library quality and quantified with a Qubit ™ dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA). RNA-Seq processing was performed via Illumina NextSeq 500 Sequencing. Raw FastQ files were generated via Illumina bcl2fastq2, version 2.17.1.14 ( http://support.illumina.com/downloads/bcl-2fastq-conversion-software-v217.html ). The bioinformatic data analysis pipeline processed FASTQ data generated by the Illumina NextSeq sequencer through Unique Molecular unique molecular identifier (UMI) extraction, trimming, alignment and quality control steps. As CORALL libraries contain N12 UMI at the start of Read 1, in the first step, UMI were removed through UMI tools. Then, adapter sequences, poly(A) sequences at the 3′ end of Read 1 and poly(T) sequences the 5′ end of Read 2 were trimmed through Cutadapt software. After UMI extraction and trimming, trimmed reads were aligned through STAR using GENCODE Release 38 (GRCh38.p13) as a reference human genome. Gene and transcript abundance were assessed using FeatureCounts software, with the “stranded forward” option. Differential expression analysis was performed using R package DESeq2. Genes were considered differentially expressed and retained for further analysis with |log2(condition sample/control sample) |≥ 1 and a False Discovery false discovery rate (FDR) ≤ 0.05. The R software was used to generate heatmaps (heatmap.2 function from the R ggplots package) and Volcano plots (EnhancedVolcano function from the R EnhancedVolcano package). Gene set enrichment analysis (GSEA) and overrepresentation analysis (ORA) were conducted using KEGG pathway analyses with the clusterProfiler R package (version 4.2.2).
Quantitative real-time PCR (qRT‒PCR)
Total RNA was extracted from 1 × 10 6 of O-PRE and O-POST cells using QIAzol (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was reverse-transcribed from 1 µg of total RNA in a 20 µL volume using the High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific) and subjected to qRT‒PCR using the Applied Biosystems SYBR Green dye-based PCR assay on the ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). HER2, Notch3, Notch4, Vimentin and Ki67 mRNA transcripts were amplified using 200 nM primers. The primer sequences are reported in Table S1. The data were normalized to the GAPDH data using the comparative 2-ΔCt method. Only the HER2 data were normalized to the β-actin data.
For RNA-Seq validation, total RNA was extracted from 1 × 10 6 cells of O-PRE (cell passage 9) and O-POST (cell passage 12) using TRIzol Reagent ™ (Invitrogen) in accordance with manufacturer's instructions. Then, 2000 ng of RNA was reverse transcribed using the iScript ™ Reverse Transcription Supermix for qRT-PCR (Bio-Rad). Real Time PCR was performed with the CFX Connect Real-Time System (Bio-Rad) using Sso SYBR Green Supermix (Bio-Rad). Genes were selected among the most interesting BC-related pathways highlighted by the clusterProfiler analysis. Primers were designed using human gene sequences available from NCBI ( www.ncbi.nlm.nih.gov/nucleotide ) and selected using NCBI's Primer- BLAST tool at the exon junction level to optimize amplification from RNA templates and avoiding nonspecific amplification products. Primers were designed to have a sequence of approximately 20 bp and generate a PCR product size of maximum 250 bp. The primers used are listed in Table S2. Data were normalized to GAPDH using the comparative 2 −ΔΔCt method.
Drug treatment
For establishment of a cell viability assay, the organoids were sheared 2–3 days before seeding to obtain smaller and more uniform PDOs. The organoids were isolated from the BME by adding 1 µg/mL dispase to each well, and the plate was transferred to an incubator at 37 °C for 1–2 h. Once the BME was dissolved, the organoids were collected in 15 mL tubes and washed twice with Ad-DF + + + . An aliquot (1 mL) of PDO suspension was treated with TrypLe™ Select, reduced to single cells and used for the cell count. The PDO suspension was diluted in CM containing 10% BME and seeded at 10,000 cells/well in a 96-well spheroid microplate (Corning, 4520) at a concentration of 200 cells/µL. After 24 h, 4 different concentrations of cetuximab (Erbitux ® 5 mg/mL, Merck) and the humanized anti-HER2 monoclonal antibody trastuzumab (Ontruzant ® 150 mg, Samsung Bioepis) (both ranging from 0.5 nM to 200 nM) were added to 10 replicates. Untreated cells were used as a negative control. After 3 days of expansion at 37 °C and 5% CO 2 , the Cell Titer Glo 3D Kit (Promega, G9682) was used, according to the manufacturer’s instructions, to measure the ATP content as an indicator of cell viability. Emitted luminescence was read in a microplate reader (PerkinElmer, Victor Nivo Multimode), and the data were analysed using GraphPad Prism 8. This experiment has been performed twice.
scRNA-seq library preparation and sequencing
After PDO shearing and dissociation at the single-cell level using TrypLe ™ Select, as previously described, 16,500 cells from each of the two PDOs, O-PRE and O-POST, were processed as recommended in the 10X Genomics ® Single Cell protocol (v3.1 chemistry) (10X Genomics, Pleasanton, CA, USA). In detail, for each sample, by individually partitioning thousands of cells into nanoliter-scale gel bead-in-emulsion (GEM) products, cDNAs sharing a common 10X Genomics barcode were generated after 11 PCR cycles. scRNA-seq libraries were prepared starting from the cDNAs by 13 PCR cycles, and 10X Genomics barcodes were used to associate individual reads back to each partition. scRNA-seq libraries were diluted 1:10 and run on TapeStation High Sensitivity D5000 screen tape (Agilent Technologies, Santa Clara, CA, USA) for quality assessment. Finally, the two scRNA-seq libraries were sequenced on an Illumina HiSeq2500 instrument (Illumina, San Diego, CA, USA) in a paired-end run (28 cycles for read1, 91 cycles for read2) to obtain at least 40,000 reads per cell for a total of 400 M reads/sample (considering approximately 10,000 cells recovered for each sample).
scRNA-seq bioinformatics data analysis
Cell Ranger software (v.7.0.0, 10X Genomics) was used to process the obtained reads files. In detail, FASTQ files were generated from demultiplexed raw base call (.BCL) files through the Cell Ranger mkfastq pipeline. The Cell Ranger count pipeline was applied to FASTQ to perform alignment against the GRCh38 human reference genome, quality filtering, barcode processing and single-cell gene UMI counting. The Cell Ranger aggr tool was used to aggregate outputs from the two samples from the Cell Ranger count, normalize to the same sequencing depth and then recompute the feature-barcode matrices. Cell filtering, data normalization and unsupervised clustering were carried out using the Seurat R package (v.4.1.1) [ 15 , 16 ]. Based on the principal component (PC) vs. variance plots, the top 10 PCs were retained for further analysis. Genes expressed in fewer than three cells were filtered out, as well as genes with fewer than 200 genes and genes with more than 25% mitochondrial gene counts, since mitochondrial RNAs are markers of cell apoptosis. Single cells were filtered based on the following criteria: nCount_RNA [1000–50000] and nFeature_RNA [200–10000]. The scDblFinder Bioconductor/R package (v.1.8.0, [ 17 ]) was used for identifying and removing doublets in the dataset. Finally, the Seurat LogNormalize function was used to normalize genes by relying on library sequencing depth followed by log transformation. Furthermore, the data were scaled by regressing on different confounding factors, such as the expression of cell cycle genes, the number of UMIs and the percentage of mitochondrial genes.
Pseudobulk analysis
A pseudobulk approach starting from scRNA-seq data was used for gene set enrichment analysis (GSEA) to fully account for biological variation due to therapeutic treatments. Read counts from cells with the same PDO (O-PRE or O-POST) combination were summed together to form a pseudobulk sample. GSEA was conducted using KEGG pathway analyses via the clusterProfiler R package (version 4.2.2) with the default parameters [ 18 ].
Cluster identification and refinement
After filtering and regression, the Seurat R package (v.4.1.1) was used to project all the cells onto two dimensions by using the uniform manifold approximation and projection (UMAP) method. For exclusion of batch effects, the two biological samples were processed in parallel, and cells from each sample were transcriptionally profiled. Then, the original Louvain graph-based clustering algorithm was used to cluster cells at a resolution of 0.6. Next, we leveraged a nonlinear dimensional reduction technique to aggregate transcriptionally similar cells, and we removed clusters likely to be of low quality resulting from debris, doublets/multiplets and dead cells. Marker genes for each cell cluster were identified using the Seurat FindMarkers function with default parameters.
For determination of the cell cluster identity, known cell type-specific markers were selected from previous scRNA-seq studies described in the literature. A cluster showing distinct high expression levels of known marker genes specific for a particular cell type was considered to carry the identity of that cell type. These markers were sufficient to define all major cell types. All marker gene annotations are provided in Table 2 .
Statistical analysis
Statistical data were evaluated using GraphPad Prism version 8.0a (GraphPad Software Inc., La Jolla, USA). The data are reported as the mean ± standard error of the mean (SEM). The level of statistical significance was set at p = 0.05. For confocal image quantification, flow cytometry and qRT‒PCR data were assessed by two-tailed unpaired Student’s t test. The drug screening results were analysed by two-way ANOVA to determine the effects of both the drug and organoid type. The linear regression slopes of the growth curves were calculated using GraphPad Prism version 8.0a (GraphPad Software, Inc., La Jolla, USA), while the doubling times were determined by nonlinear fitting.
Establishment and characterization of PDO cultures
A 69-year-old woman (Supplementary Material 1 : Figure S1, A-E) presented with ulcerated left breast lobular invasive cancer (6 cm) with stage cT2c, 90% oestrogen receptor, < 5% progesterone receptor, 60% Ki67, HER2 2 + (c-erbB2) without gene amplification, cN + (clinical nodes), luminal B-like molecular subtype, and stage III. At this time, a biopsy sample was used to establish O-PRE (Supplementary Material 1 : Figure S2). According to tumour histological and molecular characterization, the patient underwent NACT with anthracyclines and taxanes (90 mg/m 2 epirubicin and 600 mg/m 2 cyclophosphamide once every 3 weeks (EC) for four cycles followed by 12 cycles of weekly paclitaxel at 80 mg/m 2 ). Radiological evaluation revealed > 50% pathological complete response (pCR) in the breast, as observed in Figure S1 (Supplementary Material 1 : Figure S1, Panel F). No axillary node response was observed in the preoperative evaluation post-NACT. The patient therefore underwent a left radical mastectomy with complete axillary lymphadenectomy. Pathologic assessment revealed that 8 of 10 lymph nodes were metastatic, with extension to the perinodal adipose tissue. From a surgical sample 3.3 cm in length, a fragment of tissue of approximately 0.5 × 0.5 × 0.5 cm 3 was used to establish the O-POST PDO (Supplementary Material 1 : Figure S2). The biomolecular characteristics of the surgical sample displayed a locally advanced primitive tumour at stage T4b (pTy4b) with involvement of regional lymph nodes (pNy2a) and absence of distant metastasis (pM0); oestrogen receptor 90%, progesterone receptor < 2%, Ki67 20%, and HER2 1+. After the surgery, the patient was treated with locoregional radiotherapy to the left chest wall and ipsilateral clavicular region (dose of 50.4 Gray in 28 fractions with 3D conformal technique). After surgery patient started therapy with aromatase inhibitors (anastrozole). The patient is currently free of disease (Fig. 1 A).
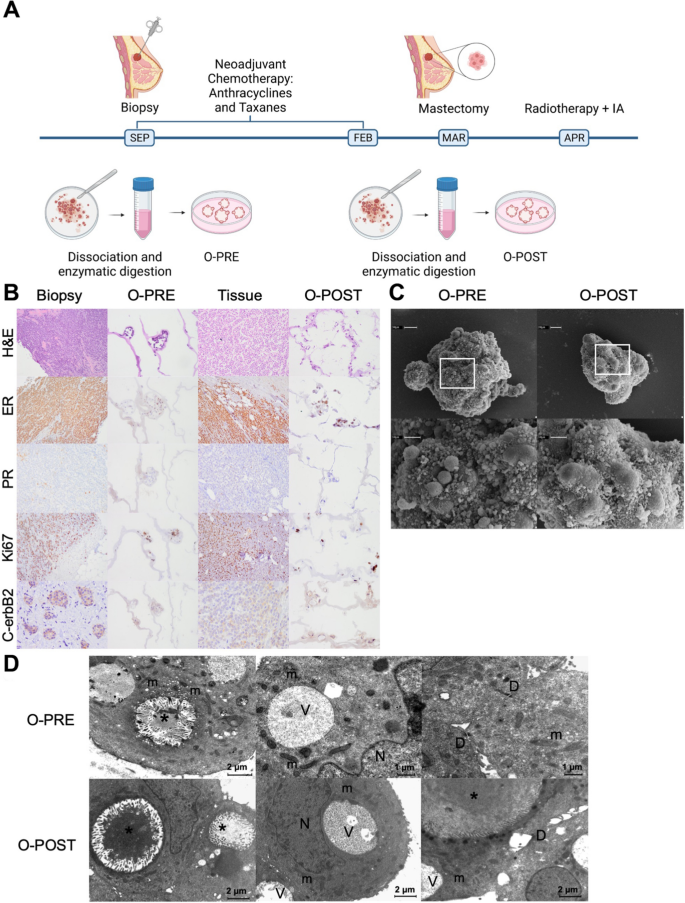
Patient clinical history and characterization of the two tumour tissue samples and matched organoids derived before and after NACT. A Timeline of the establishment of the BC PDOs O-PRE and O-POST. B Histological and molecular characterization of O-PRE, tumour tissues and O-POST through H&E staining and IHC for oestrogen (ER) and progesterone (PR) receptors, the Ki67 proliferation index and the HER2 (c-erbB2) receptor. O-PRE and O-POST were compared with respect to the histological and molecular features of the tissues of origin. C SEM images of O-PRE and O-POST. Both types of organoids showed an almost spheroidal shape. At higher magnification, microvilli and debris are in close proximity to the cell junctions. D TEM images of O-PRE and O-POST. They share the same morphological characteristics: numerous intracellular lumens (*) rich in microvilli and containing amorphous material of probable protein origin; elongated mitochondria (m) rich in ridges; and small desmosomes (D) and vacuoles (V). N = nucleus
To evaluate the effect of NACT on the patient, we generated PDO cultures from PRE- and POST-conditioned samples, as described in the Methods section (O-PRE and O-POST; Supplementary Material 1 : Figure S2).
O-PRE and O-POST cultures recapitulated the histological and molecular profiles of the original tissues (Fig. 1 B). Indeed, the morphology revealed the features of the original invasive lobular carcinoma (ILC), and the IHC analysis of oestrogen, progesterone, Ki67 and HER2 (c-erbB2) confirmed the molecular characteristics of the biopsy and surgical original tissue. Specifically, O-PRE displayed 40% oestrogen expression, 0% progesterone expression, 60% Ki67 expression and 1% HER2 1+ expression, while O-POST showed 80% oestrogen expression, 0% progesterone expression, 30% Ki67 expression and HER2 2 + expression without gene amplification Fig. 1 and Supplementary Material 1 : Figure S3).
We further characterized O-PRE and O-POST morphology by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) to study the ability of PDOs to reproduce the spatial organization of tumour tissue, highlighting how both organoids display typical mammary gland organization. Indeed, the apical secretory portion of the cell faces the interior of the organoid, recapitulating the features of the mammary epithelium [ 13 ]. These PDOs are characterized by a 3D well-organized structure with ovoidal cells that are rich in desmosomes. Moreover, they display intercellular lumens and vacuoles that suggest intense secretory activity and are mitochondria-rich in cristae, suggesting that they are involved in functional energy metabolism (Fig. 1 C–D). The extensive cell debris extruded at cell junctions on both organoids (Fig. 1 D) indicated high secretory activity and cell turnover.
In summary, all these evaluations allowed us to confirm the consistency of O-PRE and O-POST with the tissue of origin.
O-POST organoids display increased proliferative potential, stemness and aggressiveness
After tissue dissociation and organoid formation, the O-PRE and O-POST cultures exhibited different proliferation rates when expanded in vitro, as shown in Fig. 2 A and Supplementary Material 1 : Figure S2; moreover, the O-PRE and O-POST cultures exhibited distinct growth patterns even when cultured under the same conditions. In particular, O-POST displayed a 6.5-fold greater slope (0.2851 vs. 1.858) and a sixfold lower doubling time (2.388 vs. 0.369 days) than O-PRE. Since these PDOs exhibited diverse tumour growth patterns, although cultured under the same conditions, we evaluated their Ki67 proliferation index by qRT‒PCR and confocal microscopy analyses (Fig. 2 B‒D). Indeed, O-POST, which exhibited faster growth kinetics, showed a significant increase in Ki67 expression compared with O-PRE (Fig. 2 B-D).

Differences in the proliferation rate and EMT potential between O-PRE and O-POST. A Growth curves of O-PRE and O-POST cultures (N = 2–3). ( B - D ) Characterization of O-PRE and O-POST by qRT‒PCR ( B ) and confocal microscopy analyses ( C , D) of Ki67 expression. Box plot analysis showing the Ki67 expression levels, evaluated as the relative expression of Ki67 ( B ) and as the mean fluorescence intensity (MFI) ( C - D ), obtained from image quantification of O-PRE and O-POST cultures. ****p < 0.0001. Representative images of Ki67 immunofluorescence staining are shown in Panel C. Nuclei (blue, DAPI), membrane (green, WGA FITC) and Ki67 (pink, Anti-Rb AF546) are labelled. Scale bar = 10 µm. E O-PRE and O-POST were evaluated by multiparametric flow cytometry for the cell surface markers CD24 and CD44. F O-PRE and O-POST were analysed by multiparametric flow cytometry for the expression of EPCAM and CD49f markers
The different proliferation rates and, thus, the accelerated in vitro growth capacity shown by O-POST were supported by the greater stemness of the cells that constitute the organoid [ 19 ]. Therefore, we evaluated the cell populations linked with the stemness phenotype, i.e. , associated with CD24 and CD44 marker expression (Fig. 2 E and Supplementary Material 1 : Figure S4). Although most studies have reported that the CD24 low /CD44 high population is associated with multipotency, tumour-initiating potential and metastatic properties [ 20 ], in the ILC subtype, these features were attributed to the CD24 low /CD44 low cell subset [ 21 ], which showed greater representation in O-POST (99.37% ± 0.184) than in O-PRE (93.57% ± 0.368). Moreover, we analysed the expression levels of key markers associated with increased tumour aggressiveness and metastatic potential, such as EPCAM [ 22 , 23 ]. Specifically, the EPCAM low /CD49f high cell population, linked with an elevated probability of metastasis, was enriched among the neoplastic cells that formed O-POSTs (72.11%) (Fig. 2 F and Supplementary Material 1 : Figure S4).
Expression of specific surface cancer biomarkers
We analysed the main surface BC biomarkers, particularly HER2 and EGFR receptors. As shown by a pathologist, by comparing O-PRE and O-POST, we observed a three-fold reduction in O-POST HER2 protein and mRNA expression (Figs. 1 B and 3 A), along with an approximately six-fold increase in the relative expression of Notch3 and Notch4 (Fig. 3 B, C ).
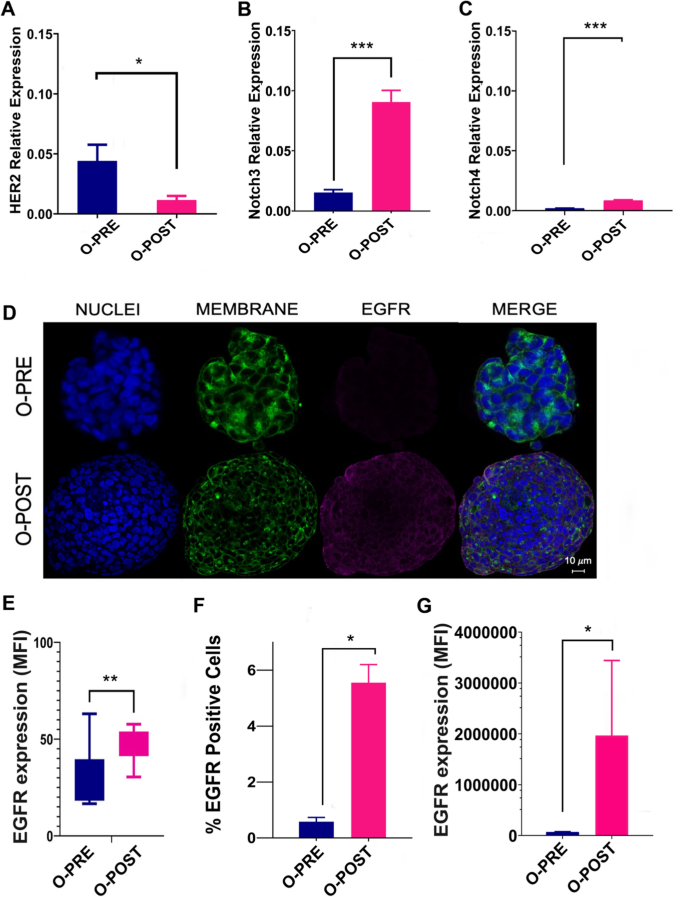
Differential expression of surface cancer biomarkers. A qRT‒PCR analysis to evaluate HER2 gene expression was performed with mRNA extracted from O-PRE and O-POST cultures. The data are the means of three independent experiments. The transcript expression levels are presented as the normalized expression. B - C qRT‒PCR analysis to evaluate Notch3 and Notch4 gene expression was performed with mRNA extracted from O-PRE and O-POST cultures. The data are presented as the means of three independent experiments ± s.e. The transcript expression levels are presented as the normalized expression of β-actin (for HER2) or GAPDH (for Notch3 and Notch4). *p < 0.05; ***p < 0.005. D Representative images of EGFR immunofluorescence staining (pink, anti-Rb AF546) in O-PRE and O-POST cultures. Nuclei (blue, DAPI), membrane (green, WGA FITC), and EGFR. Scale bar = 10 µm. E Quantification of the EGFR fluorescence signal detected by confocal microscopy in terms of the MFI. **p < 0.01 (p value = 0.004). F , G EGFR expression in the O-PRE and O-POST groups was evaluated by flow cytometry analysis, using untreated cells to determine the region of positivity and the singlets gate. *p < 0.05
We also observed an increase in EGFR expression in O-POST cells (Fig. 3 D–F and Supplementary Material 1 : Figure S5), which was confirmed by confocal microscopy and flow cytometry analyses. In this case, a significant difference in EGFR expression between the two organoids was observed. In fact, O-POST resulted in increased EGFR expression on the surface, not only in terms of the mean fluorescence intensity (MFI) but also in terms of the percentage (%) of EGFR-positive cells (Fig. 3 E–G and Supplementary Material 1 : Figure S5).
Deregulation of transcriptomic profiles in organoids obtained following neoadjuvant chemotherapy confirms drug responsiveness to specific inhibitors
To further investigate the potential effect of NACT on gene expression in cultured organoids, we focused on O-PRE and O-POST PDO samples. Genes were considered differentially expressed (DE) RNAs and retained for further analysis with |log2(O-PRE/O-POST)|≥ 1 and an FDR ≤ 0.05. Heatmap of the DE RNAs showed different expression profiles (Fig. 4 A), as the samples grouped separately. The volcano plot shows the DE RNAs (Fig. 4 B), and 3671 DE RNAs were identified; 2260 (61.5%) were found to be upregulated, whereas 1411 (39.5%) were downregulated. Moreover, 90% of the DE RNAs were annotated as coding genes, whereas 10% were noncoding DE RNAs (Table 1 ). The list of coding DE RNAs, ranked by their fold change (FC), is reported in Supplementary Material as Table S3.
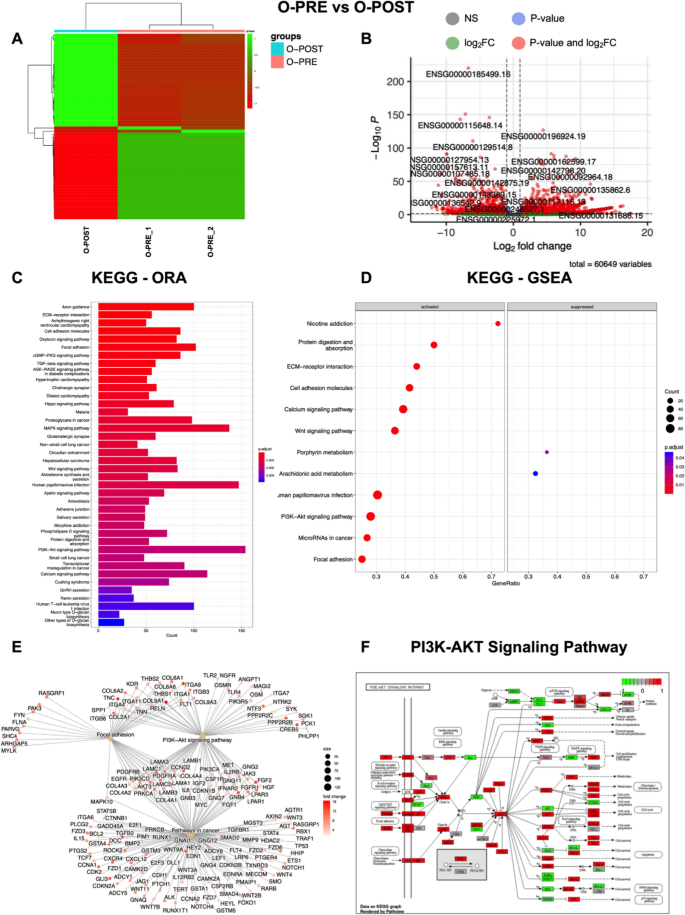
Transcriptome analysis highlights different expression profiles in O-PRE vs. O-POST cultures. We considered as differentially expressed only genes showing |log2(samples/control samples)|≥ 1 and a false discovery rate ≤ 0.05. A Heatmap of differentially expressed genes (DE RNAs) in O-PRE vs. O-POST organoid cultures. B Volcano plot showing DE RNAs between O-PRE and O-POST. The x-axis shows the log2FC. The p value is shown on a logarithmic scale on the y-axis. Genes that respected the conditions in terms of log2FC and FDR are reported in red, non-DEGs are reported in grey, and genes that respected only one condition are reported either in blue or in green. Considering the 0 on the x-axis, upregulated genes are on the right, while downregulated genes are on the left. C Bar plot of the top 40 KEGG pathways in O-PRE vs. O-POST obtained by performing the overrepresentation analysis via clusterProfiler. The y-axis represents the name of the pathway, the x-axis represents the number of DE RNAs in the pathway, and the colour indicates the adjusted p value. D Dot plot of KEGG pathways in O-PRE vs. O-POST organoid cultures obtained by performing GSEA via clusterProfiler. The figure shows the significantly activated pathways and inhibited pathways. Dot size refers to the number of genes associated with each pathway. The gene ratio is the ratio between the enriched genes and the total genes in the relative pathway database. E Cnet plot of specific KEGG pathways in O-PRE vs. O-POST organoid cultures obtained by performing GSEA via clusterProfiler. The plot shows the principal node with the name of the specific pathway and the gene of the GSEA core enrichment coloured by log2FC. F KEGG pathway analysis of the PI3K-Akt signalling pathway in O-PRE vs. O-POST. The upregulated genes are represented in red, whereas the downregulated genes are represented in green
To investigate the differences in molecular interactions, we subjected the genes for the O-PRE vs. O-POST comparison to overrepresentation analysis with KEGG via clusterProfiler, and the top 40 pathways are shown in Fig. 4 C. KEGG analysis demonstrated the deregulation of pathways involved in cytoskeleton regulation, such as “Adherens junction”, “Focal Adhesion” and “Axon guidance”. Moreover, pathways associated with cancer in general (e.g., “Transcriptional misregulation in cancer”, “Small cell lung cancer” and “Proteoglycans in cancer”) and pathways mainly involved in BC, such as the “PI3K-Akt signalling pathway” and “Hippo signalling pathway”, were deregulated (Fig. 4 C). Furthermore, the KEGG GSEA performed on clusterProfiler highlighted the activation of “Focal adhesion”, “PI3K-Akt signalling pathway”, “Wnt signalling pathway” and “Pathways in cancer” in patient-derived O-PRE vs. O-POST organoid cultures (Fig. 4 D, E). The results obtained via RNA-Seq indicated a strong perturbation in the gene expression of central components of the cytoskeleton as well as in pathways involved in BC. Via qRT‒PCR, we analysed and confirmed the expression of 9 genes involved in BC-related pathways, CTNNB, FGF2, GNB4, CDH2, FGFR1, CHRM3, ITGA9, SGK1 and COL4A2 (Supplementary material 1 , Supplemental data, Figure S6, Panels A‒I), validating the results of the RNA‒Seq analysis. Moreover, the identification of pathways found to be deregulated in O-PRE vs. O-POST by transcriptomic analysis was further corroborated by performing a pseudobulk approach on the scRNA-Seq data, where GSEA confirmed the activation of the “PI3K-Akt signalling pathway”, “Wnt signalling pathway”, “Focal adhesion”, “Pathways in cancer” and “ECM-receptor interaction” pathways (Supplementary Material 1 : Figure S7).
Since these data highlighted deregulation of the PI3K/Akt pathway (Fig. 4 F), we performed a cell viability assay to test the effect of two specific inhibitors that target this pathway and are the most commonly employed in the first-line treatment of cancer, despite not being clinically relevant in this specific subset. O-PRE and O-POST were tested with 4 different concentrations (from 0.5 to 200 nM) of trastuzumab and cetuximab, which, respectively target HER2 and EGFR tyrosine kinase receptors upstream of the PI3Ks signalling pathway [ 24 , 25 , 26 , 27 ]. These drugs mainly displayed cytostatic activity in vitro, preventing the generation of a clear dose‒response curve in vitro since their efficacy is dependent mainly on antibody-dependent cellular cytotoxicity (ADCC). Both organoids were not responsive to treatment directed at the HER2 receptor, and O-POST seemed to be even less sensitive (Fig. 5 A). Similarly, we observed a significant increase in EGFR expression in the O-POST group (Fig. 3 C-E). In this case, both O-PRE and O-POST displayed sensitivity to cetuximab, and O-POST, characterized by higher EGFR expression, was significantly less sensitive to cetuximab than was O-PRE, characterized by low EGFR expression, as shown by a cell viability assay (Fig. 5 B).

Cell viability assay to assess O-PRE and O-POST drug sensitivity. A Drug response of O-PRE and O-POST cultures to treatment with trastuzumab at four different concentrations (0.5 nM, 50 nM, 100 nM, and 200 nM). Ten replicates for each condition were used. B Drug response of O-PRE and O-POST to treatment with cetuximab at four different concentrations (0.5 nM, 50 nM, 100 nM, and 200 nM). Ten replicates for each condition were used. The data are reported as the means ± SDs; *p < 0.0332; **p < 0.0021; ***p < 0.0002; ****p < 0.0001
Single-cell transcriptomics of breast cancer patient-derived organoids
For further characterization of the transcriptome profile of PDOs at the single-cell level, a total of 16,500 isolated cells were obtained and counted from each single-cell suspension derived from O-PRE and O-POST. The numbers of recovered cells, reads, genes and transcripts/cells are reported in Table S4. After filtering, the total number of cells obtained from the two samples was 9115, and the cells were separated into 11 cell clusters (Fig. 6 A). The genes most highly expressed in each cell cluster compared to all the others were identified using the Seurat FindMarkers function (Table S5; Fig. 6 B). Cell type identification was carried out by using marker genes selected from the literature and reported in Table 2 . Clusters were categorized into seven cell types or subtypes typical of BC. Given the data available in the literature, we identified the two main BC epithelial ( EPCAM -positive) cell types: luminal epithelial (KRT18-positive) and basal/myoepithelial cells ( KRT14 -positive) (Supplementary Material 1 : Fig. S8). Specifically, we identified Clusters 1, 3, 6, and 7 as luminal epithelial cells, as they expressed typical markers, such as the KRT18 and KRT8 genes, while Clusters 2, 5, 8 and 9 were identified as basal/myoepithelial cells, marked by the KRT14, KRT5 and KRT17 genes. Moreover, among the luminal epithelial cells, we highlighted specific luminal cell subtypes, such as the luminal hormone responsive (L2) subtype (Cluster 1), which expresses the ESR1, PIP, AGR2 , and ANKR30A marker genes, and the luminal progenitor (LP) subtype (Clusters 3 and 6), which is marked by the CLDN4, S100A8 and S100A9 genes. Interestingly, Clusters 7 and 8 were highly proliferative with increased expression of the MKI67 gene, a typical marker of proliferation (Fig. 6 C). In addition to luminal and basal BC cell types, we also identified other cell types. Cluster 10 expressed the LAMA4, NRP 2, BDP1 and CLIC4 genes, which are typical of endothelial cells but are negative for EPCAM , KRT14 and KRT18 (Supplementary Material 1 : Fig. S8). Cluster 4 showed the expression of the TCF4 , DNE R , and GPC6 genes, which promote epithelial–mesenchymal transition (EMT) and are associated with an early state of EMT.

Single-cell RNA-seq analysis of O-PRE and O-POST to characterize cellular populations. A UMAP visualization of the 11 identified cell clusters. B Heatmap of the top 10 most highly expressed genes in each cluster. C UMAP visualization of MKI67 expression. D UMAP representation of principal cell types and related clusters in O-PRE and O-POST organoids. E Histogram showing the number of cells in each cluster
Cluster 0 was defined as the late-EMT cell type with a mesenchymal phenotype since it expressed typical marker genes such as VIM, S100A4, and CTNN B1.
By comparing clusters in the O-PRE and O-POST groups, we observed markedly different cellular compositions (Fig. 6 D, E ) . O-PRE strongly enriched the basal/myoepithelial cell type, while in O-POST, there was a predominance of the luminal component, suggesting that NACT selects the luminal component, which is less sensitive to chemotherapy. We highlighted a dramatic decrease in the number of EMT cell types in O-POST organoids (Fig. 6 D, E ).
In this case study, we successfully established two PDO lines (O-PRE and O-POST) derived from a BC patient. They represent matched organoids derived at different time points during the timeline of the BC patient’s clinical history, i.e., before (O-PRE) and after (O-POST) NACT; thus, they appear to be a reliable tool for studying the biological and genetic cellular evolution of neoplastic disease following therapy. The histology and molecular profiles of O-PRE and O-POST cultures were found to recapitulate the main characteristics of the original tumour tissues, supporting the feasibility of using PDOs as a personalized in vitro 3D tumour model. The molecular comparison between O-PRE and O-POST cultures revealed a markedly aggressive phenotype in O-POST. In fact, the two matched organoids grown under the same conditions, i.e. , subjected to the same environmental stimuli, displayed different growth kinetics. This in vitro biological behaviour is directly related to the expression level of the proliferation marker Ki67, which was greater in O-POST than in O-PRE (Fig. 2 B–D), although this finding deviated from what was estimated by the pathologists on original tissues and on O-PRE and O-POST optical cutting temperature (OCT) compound inclusions (60% vs. 30%; Fig. 1 ). This discrepancy of Ki67 determined in tissues could be most likely due to the effects of the unavoidable selection that occurred when tissue-derived cells were cultured. However, if we consider the scRNA-seq results of the corresponding MKI67 gene (Fig. 6 C), we can see that in O-PRE, the global number of Ki67-overexpressing cells is greater than that in O-POST, despite lower levels of expression, which is consistent with the findings of the pathologist's assessment that revealed a reduction in the percentage of Ki-67-positive cells. Moreover, if we focus only on clusters with high expression of MKI67 (e.g., Clusters 7 and 8; Fig. 6 E), the presence of Cluster 7 in O-POST and of Cluster 8 in O-PRE, both characterized by increased expression of the MKI67 gene, once again confirms the finding of increased proliferative potential of O-POST.
Moreover, the increased proliferative potential coupled with the expression of other biomarkers associated with stemness features and metastasis highlights the increased neoplastic aggressiveness observed in O-POST. Indeed, we found consistent downregulation of the epithelial marker EPCAM in O-POST (Fig. 2 F), indicated by the disappearance of the EPCAM high /CD49f low cell population and the relevant reduction in the EPCAM high /CD49f high cell population. Notably, the greater percentage of EPCAM low /CD49f high cells in O-POST, which is associated with a greater probability of distant metastasis after surgery and shorter disease-free survival (DFS) and overall survival (OS), supports its aggressive phenotype [ 27 , 28 ]. Furthermore, the expression of the CD24 low /CD44 low population, which reflects the main phenotype of luminal BC and is characterized by tumorigenic and metastatic properties, also supports the inherent multipotency and invasive potential of O-POST [ 21 ]. These data are in accordance with the in vivo findings of 8 metastatic lymph nodes out of the 10 examined, despite the scRNA-seq results and clinical assessment of lymph node positivity at diagnosis, suggesting that they probably did not develop during NACT. Indeed, Cluster 4, which included cells that overexpress markers of early EMT, and Cluster 0, which was defined as a late-EMT cell type, drastically decreased and disappeared in O-POST (Fig. 6 D, E ). Moreover, Cluster 10, which likely contains noncancerous stromal cells with a crucial role in supporting the immunosuppressive microenvironment, was completely removed by NACT.
Given the scRNA-seq data, we could argue that NACT is mainly effective against basal/myoepithelial cells in Clusters 2, 5, 8 and 9, as already demonstrated [ 29 ].
This phenomenon results in the selection and enrichment of the luminal component due to NACT, as observed in O-POST. These luminal clusters (1, 3, 6 and 7) were effectively treated by adjuvant therapy, allowing the patient to achieve a disease-free interval (DFI) of 40 months. Indeed, Cluster 1, which contains hormone-responsive luminal cells, is the ideal target of hormone therapy administered to patients in combination with radiotherapy. The enrichment of the luminal progenitor (Clusters 3 and 6) observed in O-POST is in agreement with the increase in multipotency and aggressiveness observed in O-POST.
In this specific context, we can assume that O-POST arises from a tissue in which a more aggressive phenotype has been selected due to the administration of NACT. Therefore, the higher expression of invasiveness markers could explain the lower responsiveness of O-POST to in vitro treatments (Fig. 5 ), as this is characterized by more aggressive cells.
Since PDOs are also reliable platforms for testing drug efficacy and predicting patient drug response [ 30 , 31 ], we evaluated the expression of HER-2 and EGFR as cell surface-associated tumour biomarkers to identify the most suitable candidate therapeutic targets in this specific case. More importantly, O-POST was associated with increased expression of EGFR and an increased percentage of EGFR-positive cells, suggesting that NACT upregulates EGFR expression.
Furthermore, based on the identification of different deregulated gene pathways, including the PI3K/Akt pathway, a pro-oncogenic signalling axis acting downstream of HER-2 and EGFR whose activation is heavily involved in the regulation of cell survival, cell cycle progression and cellular growth [ 32 , 33 ] we tested the ability of trastuzumab and cetuximab to target HER-2 and EGFR, respectively. Although both organoids were similarly and barely susceptible to trastuzumab activity, O-PRE showed significantly greater sensitivity to cetuximab than O-POST. RNA-Seq analysis highlighted global transcriptional deregulation when considering O-PRE with respect to O-POST organoid cultures, suggesting that O-PRE could be a good model for evaluating both disease progression and therapeutic sensitivity. Moreover, GSEA revealed the downregulation of PI3K/Akt pathway components in O-POST, indicating that organoid growth is not dependent on EGFR; rather, proliferation could be driven by another proliferative signalling pathway. This observation could explain why O-POST organoids are less sensitive to pharmacological treatments that are able to block the PI3K/Akt pathway by targeting upstream EGFR. Taken together, these results clearly indicate that in O-POST, a more aggressive phenotype is selected. Despite the major efforts made in understanding the complexity of BC, there still remains a need to study the biology of the tumour and to monitor and predict the patient's response to therapy. The case study reported here supports the reliability of PDO development as a preclinical tool to study in vitro the tumour biology and changes resulting from its evolution. In fact, due to the possibility of obtaining PDOs from the same patient but at different times in her clinical history, we could highlight some differences in proliferative capacity, aggressiveness, and propensity for invasion. These differences could be a consequence of the patient’s NACT, which contributed to the selection of more treatment-resistant cell populations that triggered alternative growth strategies and allowed the development of a more aggressive phenotype. To date, these results further confirm how therapeutic scheduling established by current guidelines is fully suitable to what truly occurs in tumour evolution. Indeed, the adjuvant treatment proposed for that patient after NACT and surgery are fully reliable with the luminal enrichment observed in the molecular landscape depicted by scRNA-seq in O-POST.
Availability of data and materials
The data are available in a publicly accessible repository ( https://doi.org/10.13130/RD_UNIMI/XSYQJQ ) after publication upon request. The additional experimental data are available in the supplementary material.
Abbreviations
- Breast cancer
Basement Membrane Matrix
Culture medium
Clinical nodes
Differentially expressed RNAs
Epithelial–mesenchymal transition
Gene set enrichment analysis
Immunohistochemistry
Invasive lobular carcinoma
Mean fluorescence intensity
Neoadjuvant chemotherapy
Optimal cutting temperature compound
Paraformaldehyde
Room temperature
Transmission electron microscopy
Scanning electron microscopy
Unique molecular identifiers
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Article PubMed Google Scholar
Denkert C, Loibl S. Response-based molecular subtyping—emergence of the third generation of breast cancer subtypes. Cancer Cell. 2022;40:592–4.
Article CAS PubMed Google Scholar
Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397:1750–69.
Pasha N, Turner NC. Understanding and overcoming tumor heterogeneity in metastatic breast cancer treatment. Nat Cancer. 2021;2(7):680–92.
Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839–45.
Papaccio F, et al. Personalized medicine opinion will organoids fill the gap towards functional precision medicine? J Pers Med. 2022. https://doi.org/10.3390/jpm12111939 .
Article PubMed PubMed Central Google Scholar
Gunti S, Hoke ATK, Vu KP, London NR. Organoid and spheroid Tumor models: techniques and applications. Cancers. 2021;13:874.
Article CAS PubMed PubMed Central Google Scholar
Sachs N, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373-386.e10.
Driehuis E, Kretzschmar K, Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc. 2020;15(10):3380–409.
Ooft SN, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aay2574 .
Drost J, Clevers H, Es HA, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 2018;36:358–71.
Article Google Scholar
Papaccio F, et al. Proteotranscriptomic analysis of advanced colorectal cancer patient derived organoids for drug sensitivity prediction. J Exp clin Cancer Res. 2023. https://doi.org/10.1186/s13046-022-02591-z .
Signati L, et al. Ultrastructural analysis of breast cancer patient-derived organoids. Cancer Cell Int. 2021;21:1–13.
Mazzucchelli S, et al. Establishment and morphological characterization of patient-derived organoids from breast cancer. Biol Proced Online. 2019. https://doi.org/10.1186/s12575-019-0099-8 .
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20.
Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–14.
Germain PL, Robinson MD, Lun A, Garcia Meixide C, Macnair W. Doublet identification in single cell sequencing data using scDblFinder. F1000Res. 2021. https://doi.org/10.12688/f1000research.73600.1 .
Wu T, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation. 2021;2:100141.
CAS PubMed PubMed Central Google Scholar
Wang X. Stem cells in tissues, organoids, and cancers. Cell Mol Life Sci. 2019;76:4043–70.
Pupa SM, et al. HER2 signaling and breast cancer stem cells: the bridge behind her2-positive breast cancer aggressiveness and therapy refractoriness. Cancers. 2021;13:4778.
Vikram R, Chou WC, Hung SC, Shen CY. Tumorigenic and metastatic role of CD44−/low/CD24−/low cells in luminal breast cancer. Cancers. 2020. https://doi.org/10.3390/cancers12051239 .
Ye F, et al. The presence of EpCAM-/CD49f+ cells in breast cancer is associated with a poor clinical outcome. J Breast Cancer. 2015;18:242–8.
Liu C-Y, et al. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6:15966–83.
Smith I, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. The Lancet. 2007;369:29–36.
Article CAS Google Scholar
Albanell J, Codony J, Rovira A, Mellado B, Gascón P. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. Adv Exp Med Biol. 2003;532:253–68.
Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chimeric human-murine monoclonal antibody. Drugs Today. 2005;41:107–27.
Ye F, et al. CD49f can act as a biomarker for local or distant recurrence in breast cancer. J Breast Cancer. 2017;20:142–9.
Hyun K-A, et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7:24677–87.
Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med. 2012;12:96.
Driehuis E, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci U S A. 2019;116:26580–90.
Lee SH, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515-528.e17.
Hoxhaj G, Manning BD. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Na Rev Cancer. 2019;20(2):74–88.
Miricescu D, et al. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int J Mol Sci. 2021;22:1–24.
Google Scholar
Peng S, Hebert LL, Eschbacher JM, Kim S. Single-cell RNA sequencing of a postmenopausal normal breast tissue identifies multiple cell types that contribute to breast cancer. Cancers. 2020;12:3639.
Nguyen QH, et al. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat Commun. 2018;9(1):1–12.
Bhat-Nakshatri P, et al. A single-cell atlas of the healthy breast tissues reveals clinically relevant clusters of breast epithelial cells. Cell Rep Med. 2021;2: 100219.
Zeng C, Yan R, Yang G, Xiang S, Zhao F. Hedgehog signaling activation required for glypican-6-mediated regulation of invasion, migration, and epithelial-mesenchymal transition of gastric cancer cells. Biosci Rep. 2020;40:20193181.
Wang Z, et al. DNER promotes epithelial–mesenchymal transition and prevents chemosensitivity through the Wnt/β-catenin pathway in breast cancer. Cell Death Dis. 2020;11(8):1–16.
Sánchez-Tilló E, et al. β-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011;108:19204–9.
Liu F, Gu LN, Shan BE, Geng CZ, Sang MX. Biomarkers for EMT and MET in breast cancer: an update. Oncol Lett. 2016;12:4869.
Cabarcas-Petroski S, Schramm L. BDP1 alterations correlate with clinical outcomes in breast cancer. Cancers. 2022;14:1658.
Sanchez VC, et al. Host CLIC4 expression in the tumor microenvironment is essential for breast cancer metastatic competence. PLoS Genet. 2022;18: e1010271.
Ross JB, Huh D, Noble LB, Tavazoie SF. Identification of molecular determinants of primary and metastatic tumour re-initiation in breast cancer. Nat Cell Biol. 2015;17(5):651–64.
Yasuoka H, et al. Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer. 2009;9:220.
Download references

Acknowledgements
The authors acknowledge support from the University of Milan through the APC initiative. The research leading to this work has been supported by MUR in the framework PRIN 2017, ID2017EA2NR project.
We acknowledge the ICS Maugeri IRCCS oncological biobank B. Boerci for providing us with tumour samples for organoid development, the University of Milan and ICS Maugeri for the L. S. fellowship.
Author information
Authors and affiliations.
Dipartimento di Scienze Biomediche e Cliniche, Università di Milano, Via G. B. Grassi 74, 20157, Milan, Italy
Serena Mazzucchelli, Lorena Signati & Fabio Corsi
Department of Electronics, Information and Bioengineering (DEIB), Politecnico di Milano, 20133, Milan, Italy
Letizia Messa
Istituti Clinici Scientifici Maugeri IRCCS, 27100, Pavia, Italy
Arianna Bonizzi, Laura Villani, Barbara Tagliaferri & Fabio Corsi
Pediatric Research Center “Romeo and Enrica Invernizzi”, Università di Milano, 20157, Milan, Italy
Letizia Messa & Stephana Carelli
Center of Functional Genomics and Rare Diseases, Buzzi Children’s Hospital, 20154, Milan, Italy
Letizia Messa, Stephana Carelli & Cristina Cereda
Microenvironment and Biomarkers of Solid Tumors, Department of Experimental Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, 20133, Milan, Italy
Alma Franceschini, Lorenzo Castagnoli & Serenella M. Pupa
Epigenomics and Biomarkers of Solid Tumors, Department of Experimental Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, 20133, Milan, Italy
Patrizia Gasparini
Institute for Biomedical Technologies, National Research Council (ITB-CNR), Via F. lli Cervi 93, 20054, Segrate, Italy
Clarissa Consolandi, Eleonora Mangano, Paride Pelucchi, Ingrid Cifola, Tania Camboni & Marco Severgnini
You can also search for this author in PubMed Google Scholar
Contributions
L.S., A.B., P.G. and S.M.: PDO processing and culture, drug screening, flow cytometry, immunofluorescence; A. F., L. C., L. M. and S. C.: rt-PCR; L. M., C. C. and S. C.: conception and execution of RNAseq and bioinformatic analysis; E. M., T. C., P. P., M. S., I. C. and C. C.: scRNAseq experiment and bioinformatic analysis; L. V.: and processing and analysis of patient’s tissues; F.C. and B. T.: patient recruitment; L.S., S.C., L. M., and S.M.: writing and original draft preparation; writing─review and editing, C. C., S. P., S. C., B. T., F.C. and S.M; S. M. and F. C.: conception of the study. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Serena Mazzucchelli or Fabio Corsi .
Ethics declarations
Ethics approval and content to participate.
All experimental procedures were conducted in accordance with the guidelines of the Declaration of Helsinki for the use of patient biological samples. The sample collection activity of the Bruno Boerci Oncological Biobank of the ICS Maugeri was approved on 27 July 2009 by the ethical committee of the ICS Maugeri IRCCS.
Consent for publication
Informed consent was obtained from the patients involved in sample collection from the ICS Maugeri IRCCS Biobank according to the ethical guidelines of the ICS Maugeri IRCCS.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary materials 1., supplementary materials 2., supplementary materials 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mazzucchelli, S., Signati, L., Messa, L. et al. Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution. Cancer Cell Int 24 , 220 (2024). https://doi.org/10.1186/s12935-024-03375-5
Download citation
Received : 14 December 2023
Accepted : 16 May 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s12935-024-03375-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient-derived organoids
- Tumour evolution
Cancer Cell International
ISSN: 1475-2867
- General enquiries: [email protected]
- Open access
- Published: 21 June 2024
Factors influencing U.S. women’s interest and preferences for breast cancer risk communication: a cross-sectional study from a large tertiary care breast imaging center
- Jessica D. Austin 1 ,
- Emily James 2 ,
- Rachel L Perez 2 ,
- Gina L. Mazza 3 ,
- Juliana M. Kling 4 ,
- Jessica Fraker 4 ,
- Lida Mina 5 ,
- Imon Banerjee 6 ,
- Richard Sharpe 6 &
- Bhavika K. Patel 6
BMC Women's Health volume 24 , Article number: 359 ( 2024 ) Cite this article
172 Accesses
1 Altmetric
Metrics details
Breast imaging clinics in the United States (U.S.) are increasingly implementing breast cancer risk assessment (BCRA) to align with evolving guideline recommendations but with limited uptake of risk-reduction care. Effectively communicating risk information to women is central to implementation efforts, but remains understudied in the U.S. This study aims to characterize, and identify factors associated with women’s interest in and preferences for breast cancer risk communication.
This is a cross-sectional survey study of U.S. women presenting for a mammogram between January and March of 2021 at a large, tertiary breast imaging clinic. Survey items assessed women’s interest in knowing their risk and preferences for risk communication if considered to be at high risk in hypothetical situations. Multivariable logistic regression modeling assessed factors associated with women’s interest in knowing their personal risk and preferences for details around exact risk estimates.
Among 1119 women, 72.7% were interested in knowing their breast cancer risk. If at high risk, 77% preferred to receive their exact risk estimate and preferred verbal (52.9% phone/47% in-person) vs. written (26.5% online/19.5% letter) communications. Adjusted regression analyses found that those with a primary family history of breast cancer were significantly more interested in knowing their risk (OR 1.5, 95% CI 1.0, 2.1, p = 0.04), while those categorized as “more than one race or other” were significantly less interested in knowing their risk (OR 0.4, 95% CI 0.2, 0.9, p = 0.02). Women 60 + years of age were significantly less likely to prefer exact estimates of their risk (OR 0.6, 95% CI 0.5, 0.98, p < 0.01), while women with greater than a high school education were significantly more likely to prefer exact risk estimates (OR 2.5, 95% CI 1.5, 4.2, p < 0.001).
U.S. women in this study expressed strong interest in knowing their risk and preferred to receive exact risk estimates verbally if found to be at high risk. Sociodemographic and family history influenced women’s interest and preferences for risk communication. Breast imaging centers implementing risk assessment should consider strategies tailored to women’s preferences to increase interest in risk estimates and improve risk communication.
Peer Review reports
Breast cancer remains the most common malignancy in the United States (U.S.) with wide variation in incidence and mortality. This variation is attributable to a myriad of factors such as biological sex, age, reproductive history, hormone use, family history, genetic mutations, breast density, obesity, and alcohol intake, each alone explaining a modest proportion in variation in risk [ 1 , 2 , 3 , 4 ]. Evolving guideline organizations [ 5 , 6 , 7 ], including the National Comprehensive Cancer Network, American College of Radiology, and American Cancer Society, recommend formal breast cancer risk assessment (BCRA) starting between 25 and 30 years of age to guide those at increased risk to appropriate risk-reduction care. Risk-based approaches to breast cancer screening have the potential to decrease harms (i.e., false-positive, overdiagnosis) associated with current age-based approaches and may improve early detection in high-risk women leading to improvements in survival rates and quality of life [ 8 , 9 , 10 ].
Several validated models exist that utilize a combination of women’s patient-reported information and medical records data to accurately quantify a woman’s lifetime, 10-year or 5-year risk of developing breast cancer [ 11 ], and are increasingly used at mammography screening facilities to facilitate guideline-recommended risk-reduction care. Implementation of these models in clinical settings has shown to significantly improve identification of individuals at high-risk for breast cancer [ 12 , 13 , 14 , 15 ]; but have not led to the uptake guideline recommended preventive care including supplemental screening, genetic testing/counseling, or risk reducing medications for those at increased risk [ 13 , 15 , 16 , 17 , 18 , 19 , 20 ] While multifaceted, these findings may be partially explained by ineffective risk communication. Integration of BCRAs into electronic health records lend itself to delivering written communication to clinicians and women via clinical reports or patient portals. Yet, these communications of breast cancer risk results have not led to changes to women’s risk perceptions or uptake of recommended care [ 21 , 22 ] Moreover, implementation of risk-based approaches should be accepted and supported by women [ 23 ]. Limited prior studies suggest that women welcome risk-based screening [ 24 , 25 ], but few include perspectives of women in the U.S.
As efforts to implement BCRAs increase, several questions remain regarding women’s interest in knowing their risk and how best to communicate breast cancer risk estimates in a manner that aligns with their preferences. Effective risk communication is essential for helping women understand their vulnerability to a disease [ 26 , 27 ]. Yet, most women are unaware of or misperceive their breast cancer risk [ 28 , 29 ]. These misperceptions can have harmful consequences, including emotional distress and missed opportunities to utilize guideline recommended preventive care for those at increased risk [ 30 , 31 ] Prior efforts to improve risk communication have largely been tasked to clinicians to address gaps in clinician and organizational barriers to implementation [ 32 , 33 , 34 ]. While important, successful implementation of BCRA requires an understanding of women’s preferences for communication to optimize transfer of risk knowledge and recommendations. This study aims to characterize and identify factors associated interest in and preferences for breast cancer risk communication among a large cohort of U.S. women undergoing mammography screening at a large breast imaging clinic.
This is a cross-sectional, quality improvement, survey study on a convenient sample of 1221 women presenting for screening mammography at the Mayo Clinic in Arizona (MCA) Breast Imaging Clinic between January 2021 and March 2021. The Breast Imaging Clinic provides approximately 14,000 screening mammograms annually, including no-cost screening to underserved populations through community-based partnerships. Informed consent was not required for this study as it was deemed exempt from ethics approval by the Mayo Clinic Institutional Review Board.
Data collection
A paper survey was administered at the time of women’s mammography screening appointment. Adapted from prior assessments [ 35 , 36 ], the 18-item survey (see Appendix) assessed sociodemographic characteristics, known breast cancer risk factors, and included items assessing if the women were ever provided a personal risk estimate, interest in knowing personal risk, and preferences for receiving and communicating risk information.
Interest in knowing breast Cancer risk
Interest in knowing one’s personal risk for breast cancer is the primary outcome of this analysis. Women indicated their level of agreement on a 5-point scale (‘Strongly agree’ to ‘Strongly disagree’) to the following item: “I am interested in knowing my risk for breast cancer”. For this analysis, responses were dichotomized as ‘interested’ (‘strongly agree’/’agree’) and ‘neutral’/’uninterested’ (‘neither agree nor disagree’/’disagree’/ ‘strongly disagree’). Those who did not respond to this item were excluded from the analysis ( n = 10).
Preferred mode of risk communicatio
Women were presented with a hypothetical scenario in which they were at high risk for breast cancer and asked how they prefer to receive this information and in how much detail [ 35 , 36 ]. Women’s preferences for receiving information about their breast cancer risk was assessed using a single item: “If you are found to be at high risk of breast cancer, how would you prefer to receive the result of your estimated breast cancer risk?”. Women had the ability to select multiple options including ‘Face-to-face meeting with the health professional who ordered the mammogram’, ‘Telephone call from the health professional who ordered the mammogram’, ‘Face-to-face meeting with the radiologist who interpreted your mammogram’, ‘Telephone call from the radiologist who interpreted your mammogram’, ‘Face-to-face meeting with a breast risk practitioner’, ‘Telephone call from a breast risk practitioner’, ‘Mailed letter accompanying your annual mammogram result’, ‘Mailed letter separate from your mammogram result’, ‘E-mailed copy separate from your mammogram result’, ‘View the result through Patient Online Services (MyChart)’, and ‘Referral to a high-risk breast center’. For this analysis, responses were categorized as face-to-face with a health care professional, telephone call from a healthcare professional, mailed letter, electronic communication, or referral.
Preferred level of detail for risk communication
Women were also asked how much detail they prefer to receive if they were considered high risk for breast cancer. Response options were ‘less detailed (for example, “your calculated breast cancer risk was high and you may need further testing”)’, ‘moderate detail (for example, “your calculated breast cancer risk was greater than 20% and you may need further testing”)’, ‘very detailed (for example, “your calculated breast cancer risk was 26%, which is considered high risk, and you may need further testing”)’, and ‘I would not like my risk to appear in my radiology report’. Responses were dichotomized as ‘yes’ (‘Very detailed’) or ‘no’ (‘Moderate detail’, ‘Less detail’, ‘No detail’) to wanting their exact risk estimate.
To align with guideline recommendations for initiating breast cancer screening for women at average risk, we excluded women under the age of 40 ( n = 34). We also excluded women with a history or unknown history of breast cancer ( n = 53) or a known genetic mutation for breast cancer ( n = 5) since their interest and preferences for risk communication likely differ from those without a cancer diagnosis. The final sample size for this analysis was 1119 women. Summary statistics were calculated to describe the distribution of key variables. We examined differences in interest in knowing one’s breast cancer risk estimate (interested vs. neutral/not interested) by sociodemographic, breast cancer risk factors, and mammography screening history using Fisher’s exact test. We estimated two multivariable logistic regression models to identify sociodemographic and breast cancer risk factors predictive of one’s interest in (1) knowing their breast cancer risk and (2) knowing their exact breast cancer risk estimate. To provide supplemental information, these multivariable logistic regression models were also estimated while excluding women who reported ever having received an estimate of their breast cancer risk. Results from the multivariable logistic regression models are reported using adjusted odds ratios (OR) and 95% Wald confidence intervals (CI). All analyses were performed using SAS 9.4 with p-values < 0.05 considered statistically significant.
A summary of patient characteristics is provided in Table 1 . Most women were 60 years of age or older (51.2%), self-identified as white (86.8%) and non-Hispanic (92.7%), with greater than a high school education (93.1%). In addition, 81.1% reported no primary family history of breast cancer, 77.2% reported no prior breast biopsy, and 83.5% report receiving a mammogram annually.

Summary of participant characteristics ( N =1119)
Interest in knowing breast cancer risk
Overall, 72.7% of women were interested in knowing their risk for breast cancer though only 13.2% reported ever being provided their personal breast cancer risk. Interest in knowing one’s personal risk differed by mammography frequency, with women receiving mammograms annually reporting higher interest in knowing their risk (Fisher’s exact p = 0.01; Table 2 ). Results from the multivariable logistic regression analysis for our entire sample (see Fig. 1 ) show that women with a primary family history of breast cancer were significantly more interested in knowing their risk compared to women without a primary family history (OR 1.5; 95% CI 1.0, 2.1; p = 0.04), while women categorized as “more than one race or other” were significantly less interested in knowing their risk compared to women identifying as White (OR 0.4; 95% CI 0.2, 0.9; p = 0.02). Mammography frequency was not significantly associated with interest in knowing one’s breast cancer risk when controlling for all other variables in the model. These findings remained in supplementary analyses excluding women who were ever provided their risk estimates. Additionally, women 60 years of age and older were significantly less interested in knowing their breast cancer risk compared to women under the age of 60 (OR 0.7; 95% CI 0.5, 0.9; p = 0.02) after excluding women ever provided their risk estimate.

Differences in interest in knowing breast cancer risk by participant characteristics.

Forest plot of the odds ratio and 95% confidence intervals of factors predicting interest in knowing one’s personal risk for breast cancer for the entire sample ( N =1058). Abbreviations: FHx, Family History; AA, African American; PI, Pacific Islander; AI/AN, American Indian or Alaskan Native; HS, High School
Preferred mechanism for risk communication
Figure 2 describes preferences for risk communication for our entire sample. If considered to be at high risk for breast cancer, 52.9% would prefer to receive the results by telephone with a healthcare professional, followed by 47.1% preferring a face-to-face meeting with a healthcare professional. Some form of verbal communication—whether face-to-face or by telephone—was preferred by 83.4% of women (85.0% when additionally considering that referral to a high-risk breast cancer center may lead to a face-to-face discussion). Of the 402 women who preferred to receive results by mailed letter or electronic communication, 245 (60.9%) also wanted some form of verbal communication (i.e., face-to-face or by telephone; 255 [63.4%] when additionally considering referral to a high-risk breast cancer center). Moreover, 77.2% of women preferred having detailed information about their exact risk estimate.

UpSet plot showing the number of women endorsing each combination of preferences for receivingbreast cancer risk estimates. Notes: 21 patients made no selection regarding their preferences. F2F=face-to-face.
Preferred Level of Detail for Risk Communication
Results from the multivariable logistic regression analyses (see Fig. 3 ) show that women 60 years of age and older were significantly less likely to prefer exact estimates of their risk compared to women under the age of 60 (OR 0, 95% CI 0.5, 0.9; p = 0.003). Women with greater than a high school education were significantly more likely to prefer exact risk estimates, compared to women with a high school degree or less (OR 2.5; 95% CI 1.5, 4, p < 0.001). Based on the full sample, we did not observe significant differences in preferences for detailed risk estimates by race, ethnicity, prior breast biopsy, primary family history of breast cancer, or mammography frequency. Results from the supplementary analysis excluding women who were ever provided their risk estimates show similar patterns by age and education, as well as women with a history of breast biopsy being significantly more likely to prefer detailed risk information compared to those with no history of breast biopsy (OR 1.5; 95% CI 1.0, 2.3; p = 0.05).

Forest plot of the odds ratio and 95% confidence intervals of factors predicting for knowing exact breast cancer for the entire sample ( N =1037). Abbreviations: FHx, Family History; AA, African American; PI, Pacific Islander; AI/AN, American Indian or Alaskan Native; HS, High School
This cross-sectional, quality improvement, survey study of women receiving a screening mammogram adds to the growing empirical evidence supporting women’s interest in and preferences for risk communication. Despite evolving guideline recommendations, only 13.2% of women in our study reported ever being told their personal breast cancer risk though nearly 73% of women were interested in knowing their breast cancer risk. Differences in interest in knowing breast cancer risk were observed by family history and race. We also found that women, if identified as increased risk, would prefer to receive their exact risk estimate verbally (i.e., phone or face-to-face) from a health care professional, though differences in preferences were observed by age and education.
Though multifactorial, women’s interest in knowing their breast cancer risk is key to successful implementation of BCRA programs [ 23 ]. Similar to prior studies [ 37 ], the majority of women in our study expressed a strong interest in knowing their estimated lifetime risk of breast cancer, aligning with the growing emphasis on shared decision-making and women’s autonomy in modern medicine [ 38 , 39 ]. However, we also identified groups who may be less interested in their breast cancer risk including women identifying as “more than one race or other race” in our study. Not much exists in the current literature to explain this phenomenon. One potential explanation could be the low proportion of our sample identifying as “more than once race or other race” ( n = 47/1119, 4.3%). However, we did not see differences among other race groups with similarly lower proportions. Higher levels of perceived risk have been associated with a higher degree of willingness to undergo BCRA in prior studies and is consistent with health behavior theories including the Health Belief Model and Protection Motivation Theory [ 24 , 26 , 40 ]. This could also explain increased interest among women with a primary family history of breast cancer, where their experiences and knowledge might influence their perceived breast cancer risk.
Contrary a prior study [ 41 ], we found that women 60 years of age or older were less likely to prefer exact information about their cancer risk. While breast cancer risk increases with age so do complications from other chronic conditions, which may explain lower preferences for exact breast cancer risk estimates. Additionally, we found that women with a high school degree or less were less likely to prefer exact information about their risk. This finding may be attributed to how the response options were presented, showing numeric risk estimates only. Low numeracy is pervasive, particularly among lower educated populations, and can impair risk communication as it is associated with difficulty in understanding and assessing risk-related information [ 42 , 43 ]. Existing recommendations for risk communication suggest presenting information using a variety of formats including lay language, numerical (e.g., 20%; 1 out of 10) and pictorial information [ 44 ]. Additionally, decision support tools combining these recommendations with experience-based dynamic interfaces, such as games, has shown to significantly improve accuracy of breast cancer risk perceptions among high and low numeracy women [ 45 ]. However, qualitative analyses suggest that accurate risk perceptions alone are insufficient in the adoption of risk-appropriate breast cancer prevention strategies [ 45 , 46 ].
Healthcare systems are encouraged to allow women to view and download their personal health records via electronic health record portals [ 47 ]. While leveraging such systems can support access to and personalization of care, our results support that women prefer verbal rather than written communications about their breast cancer risk [ 35 , 37 ]. Combining written with verbal communication by clinicians has shown to be associated with greater awareness of one’s individual risk and greater adherence to guideline recommended care [ 48 ]. Yet, tailoring and communicating breast cancer risk to each women’s abilities and preferences can present significant challenges for clinicians and organizations, particularly in an era of increasing work volumes. Moreover, a recent study found that tailoring risk presentation formats to women’s preferences does not necessarily translate to improvements in risk comprehension [ 49 ]. Further research is needed to explore the feasibility, workflow challenges, and most effective formats for improving risk comprehension while aligning with women’s preferences [ 50 ].
This study has limitations. The cross-sectional nature of our study limits our ability to determine temporal causality of factors influencing interest and preferences for risk communication. Our study population was also limited to a single academic imaging center serving predominately educated, non-Hispanic, White women, thus limiting our ability to generalize findings to other settings. Despite the relative homogeneity of our sample, our large sample size allowed for detecting differences in interest and risk communication preferences by sociodemographic and clinical factors, critical for hypothesis generation. Specifically, we were able to detect lower levels of interest among women identifying as “more than one race or other race”, emphasizing the need for future studies to understand experiences and preferences of diverse populations. It is also important to note that the level of interest and preferences for knowing exact risk estimates may be overestimations since all women were recruited at the time of their screening mammogram and women demonstrating high levels of routine screening mammography. Future studies should assess acceptability of BCRA, including interest and willingness, among populations who lack access to or have not initiated screening, but may benefit from risk assessment and earlier screening.
Risk assessment at the time of mammography screening has the potential to reach a wide audience, but ineffective risk communication may continue to undermine effective implementation and uptake of guideline recommended care. Our study adds to the growing empirical literature demonstrating that women are interested in and prefer to receive detailed breast cancer risk estimates verbally, though these preferences may differ by sociodemographic characteristics [ 35 , 37 ]. These findings suggest that clinicians and organizations implementing risk assessment as part of routine mammography screening carefully consider methods for incorporating women’s communication preferences as part of an integrated, standardized workflow [ 15 ]. While combining written with verbal communication by clinicians was shown to be associated with greater awareness of one’s individual risk and greater adherence to supplemental MRI screening [ 48 ], presenting and discussing risk alone may not improve uptake of guideline recommended care [ 50 , 51 ]. Providing risk information in conjunction with education on how to reduce risk has shown promising results [ 52 ], but education does not address other barriers that hinder uptake of recommended care including psychological distress, cost, transportation, and time [ 34 , 53 ]. To this end, more research is needed to identify effective approaches to risk communication that also improve uptake on guideline recommended care. Additionally, our findings emphasize the need for more research to understand interest and preferences for risk communication among racially/ethnically diverse populations under the age of 40 who are not eligible for routine mammography screening but should undergo risk assessment in primary care settings.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Mayo Clinic Arizona
Confidence Interval
Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54.
Article PubMed Google Scholar
Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87(22):1681–5.
Article CAS PubMed Google Scholar
Tamimi RM, Spiegelman D, Smith-Warner SA, et al. Population attributable risk of modifiable and nonmodifiable breast Cancer risk factors in postmenopausal breast Cancer. Am J Epidemiol. 2016;184(12):884–93.
Article PubMed PubMed Central Google Scholar
Cohen SY, Stoll CR, Anandarajah A, Doering M, Colditz GA. Modifiable risk factors in women at high risk of breast cancer: a systematic review. Breast Cancer Res. 2023;25(1):45.
Daly MB, Pal T, Berry MP, et al. Genetic/Familial High-Risk Assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102.
Nelson HD, Pappas M, Cantor A, Haney E, Holmes R, Risk, Assessment. Genetic counseling, and genetic testing for BRCA-Related Cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(7):666–85.
Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408–14.
Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–37.
Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–76.
Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–25.
Kim G, Bahl M. Assessing risk of breast Cancer: a review of risk prediction models. J Breast Imaging. 2021;3(2):144–55.
Allweis TM, Hermann N, Berenstein-Molho R, Guindy M. Personalized screening for breast Cancer: Rationale, Present practices, and future directions. Ann Surg Oncol. 2021;28(8):4306–17.
Weiss A, Grossmith S, Cutts D, et al. Customized breast cancer risk assessment in an ambulatory clinic: a portal for identifying women at risk. Breast Cancer Res Treat. 2019;175(1):229–37.
Shah C, Berry S, Dekhne N, Lanni T, Lowry H, Vicini F. Implementation and outcomes of a multidisciplinary high-risk breast Cancer Program: the William Beaumont Hospital Experience. Clin Breast Cancer. 2012;12(3):215–8.
Laws A, Mulvey TM. Implementation of a High-Risk Breast Clinic for Comprehensive Care of Women with elevated breast Cancer Risk identified by Risk Assessment models in the community. JCO Oncol Pract. 2021;17(2):e217–25.
Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large Health System of a program to identify women at high risk for breast Cancer. J Oncol Pract. 2011;7(2):85–8.
Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575–90.
Wehbe A, Gonte MR, O’Neill SC, et al. Predictors of nonadherence to breast cancer screening guidelines in a United States urban comprehensive cancer center. Cancer Med. 2023;12(14):15482–91.
Hill DA, Haas JS, Wellman R, et al. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33(3):275–83.
Evans DG, Harvie M, Bundred N, Howell A. Uptake of breast cancer prevention and screening trials. J Med Genet. 2010;47(12):853–5.
Morman NA, Byrne L, Collins C, Reynolds K, Bell JG. Breast Cancer Risk Assessment at the Time of Screening Mammography: perceptions and clinical management outcomes for women at high risk. J Genet Couns. 2017;26(4):776–84.
Vaidya AM, Chetlen AL, Schetter SE. Does a high-risk recommendation in Mammography reports increase attendance at a breast Cancer Risk Assessment Clinic? J Am Coll Radiol. 2015;12(9):923–9.
Haas JS. The complexity of achieving the Promise of Precision breast Cancer Screening. J Natl Cancer Inst 2017;109(5).
Mbuya Bienge C, Pashayan N, Brooks JD et al. Women’s Views on Multifactorial Breast Cancer Risk Assessment and Risk-Stratified Screening: A Population-Based Survey from Four Provinces in Canada. J Pers Med 2021;11(2).
Koitsalu M, Sprangers MA, Eklund M, et al. Public interest in and acceptability of the prospect of risk-stratified screening for breast and prostate cancer. Acta Oncol. 2016;55(1):45–51.
Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47.
Vernon SW. Risk perception and risk communication for cancer screening behaviors: a review. J Natl Cancer Inst Monogr. 1999;25:101–19.
Fehniger J, Livaudais-Toman J, Karliner L, et al. Perceived versus objective breast cancer risk in diverse women. J Womens Health (Larchmt). 2014;23(5):420–7.
Orom H, Kiviniemi MT, Underwood W 3rd, Ross L, Shavers VL. Perceived cancer risk: why is it lower among nonwhites than whites? Cancer Epidemiol Biomarkers Prev. 2010;19(3):746–54.
Trask PC, Paterson AG, Wang C, et al. Cancer-specific worry interference in women attending a breast and ovarian cancer risk evaluation program: impact on emotional distress and health functioning. Psychooncology. 2001;10(5):349–60.
Andersen MR, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12(4):314–20.
CAS PubMed Google Scholar
Rainey L, van der Waal D, Jervaeus A, et al. Are we ready for the challenge of implementing risk-based breast cancer screening and primary prevention? Breast. 2018;39:24–32.
French DP, Woof VG, Ruane H, Evans DG, Ulph F, Donnelly LS. The feasibility of implementing risk stratification into a national breast cancer screening programme: a focus group study investigating the perspectives of healthcare personnel responsible for delivery. BMC Womens Health. 2022;22(1):142.
Spalluto LB, Bonnet K, Sonubi C, et al. Barriers to implementation of breast Cancer Risk Assessment: the Health Care Team Perspective. J Am Coll Radiol. 2023;20(3):342–51.
Amornsiripanitch N, Mangano M, Niell BL. Screening Mammography: patient perceptions and preferences regarding communication of estimated breast Cancer risk. Am J Roentgenol. 2017;208(5):1163–70.
Article Google Scholar
Mangano MD, Rahman A, Choy G, Sahani DV, Boland GW, Gunn AJ. Radiologists’ role in the communication of imaging examination results to patients: perceptions and preferences of patients. AJR Am J Roentgenol. 2014;203(5):1034–9.
Amornsiripanitch N, Ameri SM, Goldberg RJ. Impact of Age, Race, and socioeconomic status on women’s perceptions and preferences regarding communication of estimated breast Cancer risk. Acad Radiol. 2021;28(5):655–63.
Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18.
Mühlbacher AC, Juhnke C. Patient preferences Versus Physicians’ judgement: does it make a difference in Healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–80.
Rogers RW. A Protection Motivation Theory of Fear appeals and attitude Change1. J Psychol. 1975;91(1):93–114.
Henneman L, van Asperen CJ, Oosterwijk JC, Menko FH, Claassen L, Timmermans DRM. Do Preferred Risk Formats lead to better understanding? A Multicenter Controlled Trial on communicating familial breast Cancer risks using different risk formats. Patient Prefer Adherence. 2020;14(null):333–42.
Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135(6):943–73.
Ancker JS, Kaufman D. Rethinking health numeracy: a multidisciplinary literature review. J Am Med Inf Assoc. 2007;14(6):713–21.
Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103(19):1436–43.
Kukafka R, Yi H, Xiao T, et al. Why breast Cancer risk by the numbers is not enough: evaluation of a decision aid in multi-ethnic, low-numerate women. J Med Internet Res. 2015;17(7):e165.
Henneman L, Timmermans DR, Bouwman CM, Cornel MC, Meijers-Heijboer H. A low risk is still a risk’: exploring women’s attitudes towards genetic testing for breast cancer susceptibility in order to target disease prevention. Public Health Genomics. 2011;14(4–5):238–47.
Colicchio TK, Cimino JJ, Del Fiol G. Unintended Consequences of Nationwide Electronic Health Record Adoption: challenges and opportunities in the Post-meaningful Use Era. J Med Internet Res. 2019;21(6):e13313.
Brinton JT, Barke LD, Freivogel ME, et al. Informing women and their Physicians about recommendations for adjunct breast MRI screening: a Cohort Study. Health Commun. 2018;33(4):489–95.
Barnes AJ, Hanoch Y, Miron-Shatz T, Ozanne EM. Tailoring risk communication to improve comprehension: do patient preferences help or hurt? Health Psychol. 2016;35(9):1007–16.
Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161(4):270–80.
French DP, Cameron E, Benton JS, Deaton C, Harvie M. Can communicating personalised Disease Risk promote healthy Behaviour Change? A systematic review of systematic reviews. Ann Behav Med. 2017;51(5):718–29.
Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–43.
Guo F, Hirth JM, Fuchs EL, et al. Knowledge, attitudes, willingness to pay, and patient preferences about genetic testing and subsequent risk management for Cancer Prevention. J Cancer Educ. 2022;37(2):362–9.
Download references
Acknowledgements
We would like to thank Jhenitza P. Raygoza Tapia for the preparation of this manuscript for submission to BMC Women’s Health.
This study is funded by the Mayo Clinic Transform the Practice Award.
Author information
Authors and affiliations.
Department of Quantitative Health Sciences, Division of Epidemiology, Mayo Clinic, 13400 E. Shea Blvd, Scottsdale, AZ, 85259, USA
Jessica D. Austin
Mayo Clinic College of Medicine of Medicine and Science, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ, 85054, USA
Emily James & Rachel L Perez
Department of Quantitative Health Sciences, Division of Clinical Trials and Biostatistics, Mayo Clinic, 13400 E. Shea Blvd, Scottsdale, AZ, 85259, USA
Gina L. Mazza
Women’s Health Internal Medicine, Department of Internal Medicine, Mayo Clinic, 13400 E. Shea Blvd, Scottsdale, AZ, 85259, USA
Juliana M. Kling & Jessica Fraker
Department of Internal Medicine, Division of Medical Oncology, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ, 85054, USA
Department of Diagnostic Radiology, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ, 85054, USA
Imon Banerjee, Richard Sharpe & Bhavika K. Patel
You can also search for this author in PubMed Google Scholar
Contributions
J.D.A. and B.K.P. conceived the study. J.D.A., G.M., B.K.P. contributed to data collection and analysis. J.D.A., E.J., and R.L.P. drafted the manuscript. G.L.M., J.M.K., J.F., L.M., I.B., R.S., and B.K.P. edited and reviewed various versions of the manuscript. All authors read and approved the final manuscript and are accountable for all aspects of the work. Funding for this study was received by B.K.P.
Corresponding author
Correspondence to Jessica D. Austin .
Ethics declarations
Ethics approval and consent to participate.
This study was submitted to, approved, and deemed exempt by the Mayo Clinic Institutional Review Board. Informed consent was not required for this study as it was deemed exempt from ethics approval by the Mayo Clinic Institutional Review Board.
Consent for Publication
Not applicable.
Consent to Participate
Participants were not required to complete informed consent for participation in research as it was deemed exempt from ethics approval by the Mayo Clinic Institutional Review Board. Prospective participants were told the purpose of the study when approached for recruitment.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Austin, J.D., James, E., Perez, R.L. et al. Factors influencing U.S. women’s interest and preferences for breast cancer risk communication: a cross-sectional study from a large tertiary care breast imaging center. BMC Women's Health 24 , 359 (2024). https://doi.org/10.1186/s12905-024-03197-7
Download citation
Received : 17 April 2024
Accepted : 10 June 2024
Published : 21 June 2024
DOI : https://doi.org/10.1186/s12905-024-03197-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer risk assessment
- Women’s preferences
- Health communication
- Mammography
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Journal of Hematology & Thromboembolic Diseases Open Access
ISSN: 2329-8790
Case Report - (2021)Volume 9, Issue 12
A Case Report of 58-Year-Old Female with Breast Cancer after Mastectomy of Left Breast and Axillary Lymph Node Dissection
Author info »
Breast cancer is Malignment cell growth in the breast. If the cancer isn't treated, it will spread to other parts of the body. Except for skin cancer, the most frequent type of cancer among women is breast cancer. Mammograms can detect breast cancer early, possibly before it has spread. Main symptoms and/or important clinical findings: A 58-year-old female patient registered to the surgery department on 05/10/2021 with the main complaint of pain in the left breast for 1 year and a lump in the left breast for 9 months. Now she came to AVBRH for further treatment of that. The main Diagnosis, therapeutic intervention, and outcomes: The doctor identified a case of cancer of the left breast after a physical examination and all investigations such as blood, FNAC, biopsy, Mammography Colour Doppler USG. For that Doctor advice the surgery and 22/10/2021 mastectomy of the left breast and axillary lymph node dissection was done. Dressing of suturing site. Inj Cefixime 1 Gm BD, Tab. Pan 40 Mg OD, Tab. Dolo 650 Mg Sos, Syp. Orofer Xt 2tsf Bd, Syp. Lupizyme 2tsf BD, Protein Powder 2 Spun TDS, Syp Duphalac 20 Ml Hs, Steam Inhalation TDS, Nebulization with Normal Saline TDS, Tab. Diclomol SP Bd, Tab. Limcee Od, Cap. Because Od, Spirometer for Breathing Exercise, Chest Physiotherapy, all the treatment was taken and the result was fine. Conclusion: She responded to both medicine and physician counseling.
Carcinoma in breast; Ductal carcinoma in Situ, Mammogram; Axillary lymph node dissection
Introduction
The most frequent type of cancer among women is breast cancer. [ 1 , 2 ] after skin cancer most frequent cancer among women is breast cancer. 66 % of breast cancer patients identified beyond the age of 55, becoming older is the commonest risk factor for developing this disease [ 3 ]. Cancers developing from the ducts that called ductal carcinomas. [ 4 , 5 ].
a breast cancer diagnosis is confirmed by taking a biopsy of the concerning tissue. Following the diagnosis, more tests are performed to see if cancer has spread beyond the breast and to identify which treatments are most likely to be effective. For prognostic and curative goals, axillary lymph node dissection is the therapy of choice in locally advanced breast cancer [ 6 ].
Breast cancer is the most frequent cancer in women around the world, accounting for over a quarter (23%) of all malignancies in women. In 2008, about 1.4 million women were diagnosed with breast cancer worldwide, with 459,000 deaths reported. The worldwide breast cancer burden is predicted to exceed 2 million by 2030, with rising proportions from developing countries [ 7 , 8 ]. In India, 145,000 women were diagnosed with breast cancer in 2012, with an age-standardized incidence rate of 25.8 per 100,000 women. In the year 2012, India's death toll was expected to be around 70,000.7 Breast cancer incidence rates in India vary by 3–4 times across the country, with the highest rates found in the Northeast and big metropolises like Mumbai and New Delhi [ 9 ].
Patient Information: A 58 years old female patient was admitted to the female surgery ward on 05/10/2021 with a complaint of pain in the left breast for 1 year and a lump in the left breast for 9 months after the history & physical examination and all investigation done and diagnosed the carcinoma of the left breast. For further treatment of that doctor advice the surgery and on 22/10/2021 left-sided mastectomy with axillary lymph node dissection surgery was done.
Past medical and surgical history, and relevant outcomes from interventions : 1 month before they visited the private hospital for the same complaint, after investigation report such as left breast FNAC and mammography showing breast cancer. Now she came to further treatment of that. Other than that, she was not having any history of DM, HTN, TB. Her Tubal ligation operation was done for 25 years. She was belonging to a nuclear family. Not having any significant history of this disease. All family members are healthy. She maintained a good interpersonal relationship with the family members except for her husband and not having any bad habits like tobacco chewing etc. The sleeping pattern was normal bowel and a bladder habit is normal.
Menstrual history : She has P3L3 and FTND, 1st childbirth was at the age of 22 years. Menarche starts at 13 years and Menopause at the age of 47 years.
Clinical Findings: On arrival, she was Afebrile, pulse was 96 beats/ min, Respiratory rate was 18 breath /min and Blood pressure 130 / 80 MMHG, the patient was conscious, cooperative, well oriented to time, place, and person. She looks anxious, febrile, & all vital parameters are normal and thin body built, and hygiene is not maintained properly. Her weight was 63 kg and her height was 1.55 m with a body mass index (BMI) of 26.3 kg/m2. Her neurological, chest examination - left breast –a lump of size 7 × 6 cm behind NAC, lump feel hard, non-tender, mobile. Left axilla – single lymph node of size 1X1 cm in the central group. Right breast normal and abdominal findings were normal. Pain present and restricted movement of the right shoulder.
Timeline: She is alright 1 year back when she started getting pain in her left breast. The pain was insidious in onset, intermittent and dull aching type no aggravating or relieving factors for the same. The patient also complained of a lump in the left breast for 9 months. It was insidious in onset and initially of a smaller size and has progressed to its present size. 1 month before their visit to a private hospital for the same complaint, after investigation report such as left breast FNAC and mammography showing breast cancer. Now she came to further treatment of that and was admitted female surgery ward in AVBRH on 05/10/2021 after investigation doctor advise her surgery and on 22/10/2021 her left side mastectomy and axillary lymph node dissection surgery was done. Now she taking further treatment at our hospital.
Diagnostic Assessments: After General history, physical examination, Blood investigations were also done hemoglobin 13.8gm, WBC Count 13800cu.mm is increased, total platelet count was is 3.61 RBS- glucose plasma random 96%, total protein was 7.5 g/DL, albumin was 4.3 g/DL, LDH level was 209 U/L, Kidney Function Test –urea 19 mg/dl, creatinine 0.7 mg/dl, Potassium 4.9 mmol/L, Sodium 144 mmol/L, urine exam. (Routing) urine albumin was nil, pus cell 0-1 cells/hpf & epithelial cell was 1-2 cells/hpf, sugar nil, HBcAg negative, HCV & HIV also negative. Coagulation profile – APTT –control was 29.50, APTT –patient 29.70, prothrombin time – control & patient was 11.90. ECG was normal. Colour Doppler USG Per Breast with Bilateral Axilla - the report is well defined heterogeneously hypoechoic lesion measuring 3.5 × 3.3 cm noted in left breast posterior to nipple-areola complex with irregular margins Calcification, mild to moderate vascularity. S/o neoplastic etiology, BIRADS - 4. USG per abdomen report was no obvious abnormality noted in the present scan. Digital Laser X-Ray Mammography & Mammography of Both Breast- shows evidence of hyper dense, irregular, nodular mass lesion is seen at 1to 2 o'clock inner location of left breast, measuring 3.6 cm × 2.8 cm × 3.2 cm in dimension. Margins are irregular. Micro calcific foci are seen within. Appears moderately vascular on color Doppler examination. Features suggestive of? Malignant etiology (Figure 1).
FNAC from lump in the left breast (microscopic finding) smear: Are cellular revealing scattered cells as well as non-cohesive clusters of moderately pleomorphic cells. Few cells show moderate to severe nucleomegaly. Few cells show nuclear irregularity with prominent nucleoli. The cytoplasm is scant to moderate. The background shows granular debris and blood.
Impression : - Breast lesion appears to be malignant,
Cytopathology examination: Smear show sheet of dyscohesive epithelial cell, few of the cell sheet shows the formation of ducts and dissociated cell population. Cell carry enlarged moderately to marked pleomorphic nuclei. A few nuclei with standout nucleomegaly, nucleation, and rare mitosis. Chromatin is granular but uneven, cytoplasm is modes to ample. A rare tumor giant cell is evident. The background shows sparse lymphocytes, atypical, smudged nuclei, and tumor necrosis. Present cytomorphological features suggestive of ductal carcinoma-poorly differentiated.
Histopathology examination: TRU CUT Biopsy from left breast lump- section from given tissue pieces shows histopathology features suggestive of invasive ductal carcinoma of the breast.
Diagnostic challenges: No challenging during diagnostic evaluation.
Diagnosis : After a physical examination and investigation doctor diagnosed a case of carcinoma of the left breast for that left side mastectomy and axillary lymph node dissection surgery was done.
Prognosis: After 10 years, 81.2 percent of women who had a double mastectomy& 79.9% of women who had a single mastectomy were still living.
Therapeutic interventions : Medical and surgical management was provided to the patient. The initial care of the patient was with intravenous normal saline, to correct dehydration. Surgical sidedressing did, Inj Cefixime 1 Gm Bd, Tab. Pan 40 Mg Od, Tab Dolo 650 Mg Sos. Syp. Orofer Xt 2tsf Bd, Syp. Lupizyme 2tsf Bd, Protein Powder 2 Tsf, Tds, Syp Duphalac 20 Ml Hs, Steam Inhalation Tds , Nebulization With Normal Saline Tds ,Tab. Diclomol Sp Bd, Tab. Limcee Od, Cap. Becosule Od, Spirometer, Chest Physiotherapy, Strict input, and output chart monitoring, TPR charting 6 hourly, blood pressure monitoring of the patient.
A 58 years old female patient was admitted to the female surgery ward with a complaint of pain in the left breast for 1 year and a lump in the left breast for 9 months after all the history & physical examination and all necessary investigation carried out and diagnosed the carcinoma of the left breast. For further treatment of that doctor advice the surgery and on 22/10/2021 left-sided mastectomy with axillary lymph node dissection surgery was done in this case report Early detection of breast cancer and necessary treatment was taken for better outcome Early detection of breast cancer remains the best defense for preventing the development of this life-threatening disease. Tumors that are smaller and nonpalpable are more treatable and have a better prognosis. [ 10 - 12 ].
Breast cancer risk has been associated with age at menarche, menopause, and first pregnancy in multiple studies. Menarche before the age of 14 years raises the risk of breast cancer by a factor of 1.1 to 1.9 when compared to menarche after the age of 14 [ 14 , 15 ]. Similarly, late menopause raises the risk of breast cancer, with women who go through menopause before the age of 45 having roughly half the breast cancer rate of women who go through menopause beyond the age of 55 [ 16 - 18 ].
Informed consent: Before taking this case, information was given to the patients and theirs, and informed consent was obtained from the patient as well as relatives.
Breast cancer is a disease in which cells in the breast grow out of control, and invasive ductal carcinoma is a type of cancer in which cancer cells start in the ducts and then spread to other sections of the breast tissue. In this case, the patient have breast carcinoma of the left breast after mastectomy of the left breast and axillary lymph node dissection was done and after taking treatment patient's condition was improved.
Conflict of Interest
No conflict of Interest
- Kamià ?ska M, Ciszewski T, Kukieà ?ka-Budny B, Kubiatowski T, Baczewska B, Makara-Studzià ?ska M, et al. Life quality of women with breast cancer after mastectomy or breast conserving therapy treated with adjuvant chemotherapy. Ann Agric Environ Med. 2015;22(4).
- Grunfeld EA, Ramirez AJ, Hunter MS, Richards MA. Women's knowledge and beliefs regarding breast cancer. Br J Cancer. 2002; 86(9):1373-8.
- Selvy PT, Palanisamy V, Purusothaman T. An Improved GA-MILSVM classification approach for diagnosis of breast lesions from stain images. Int J Adv Eng Technol. 2012; 4(2):216.
- Patlak M, Nass SJ, Henderson IC, Lashof J. Mammography and beyond: developing technologies for the early detection of breast cancer. NAP.
- Gambardella C, Clarizia G, Patrone R, Offi C, Mauriello C, Romano R, et al. Advanced hemostasis in axillary lymph node dissection for locally advanced breast cancer: new technology devices compared in the prevention of seroma formation. BMC Surgery. 2019;18(1):1-9.
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237-48.
- Manoharan N, Nair O, Shukla NK, Rath GK. Descriptive epidemiology of female breast cancer in Delhi, India. Asian Pacific journal of cancer prevention: APJCP. 2017;18(4):1015.
- Gupta A, Shridhar K, Dhillon PK. A review of breast cancer awareness among women in India: Cancer literate or awareness deficit?. European J Cancer. 2015;51(14):2058-66.
- Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clinical breast cancer. 2008;8(4):334-42.
- Aditi G., Samarth S., Sunita V., Sourya A., Miheer J., Kumbhare J.M. Utility of microvessel density and it’s correlation with nottingham prognostic index in carcinoma breast. J Pharm Res Int. 2019;11:2013–2017.
- Daga, S., Phatak, S., Khan, S., Rawekar, S., 2018. Axillary galactocele of ectopic breast: Ultrasound and mammography correlation. Medical J Dr. D.Y. Patil Vidyapeeth. 242–244.
- Dawande P, Bhatt N, Noman O, Bahadure S, Bhake A, Bhatt, N. Corelation between cytological and histological grading of breast cancer and its utility in patient’s management. Int J Curr Res. 12, 71–76.
- Domkunti R, Lamture YR, Rinait A, Gode D. Breast carcinoma in axillary tail of spence: A rare case report. Int J Curr Res. 2020; 12:92–95.
- Hemant, L.H., Keshao, H., Sunita, V., Gode, C.S., 2019. Correlation of total serum LDH levels with differentiation of breast carcinoma. Int J Curr Res. 11, 1211–1215.
- Kishor T.A, Madhavrao, H.K, Sunit V, Palsodkar P. Study of androgen receptor as a quadruple marker in breast carcinoma. Int J Curr Res. 2019;11:1173–1178.
- Kumar S, Jena L, Sahoo M, Mohod K, Daf S, Varma AK. Effect of single amino acid mutations on C-terminal domain of breast cancer susceptible protein 1. Int J Biosci Biochem Bioinforma. 2019;15:305–323.
- Madurwar K.A., Phatak S.V. Benign fibrous histiocytoma of male breast: Ultrasonography, doppler, and elastography imaging with pathological correlation. J Datta Meghe Inst Med Sci. 2019;14:103–105.
Author Info
Citation: Umare H, Ganeshpur B, Umate R, (2021) A Case Report of 58-Year-Old Female with Breast Cancer after Mastectomy of Left Breast and Axillary Lymph Node Dissection. J Hematol Thrombo Dis 9:469. DOI: 10.24105/2329-8790.2021.9.469
Received: 30-Nov-2021 Accepted: 14-Dec-2021 Published: 21-Dec-2021
Copyright: © 2021 Umare H, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
- Search Menu
Sign in through your institution
- Advance articles
- Editor's Choice
- 100 years of the AJE
- Collections
- Author Guidelines
- Submission Site
- Open Access Options
- About American Journal of Epidemiology
- About the Johns Hopkins Bloomberg School of Public Health
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

The Healthy Participant Effect: insights and results from a population-based case-control study on breast cancer
- Article contents
- Figures & tables
- Supplementary Data
Kevin Maldonado-Cañón, Annika Möhl, Nadia Obi, Sabine Behrens, Fabian Flaßkamp, Petra Seibold, Jenny Chang-Claude, Heiko Becher, The Healthy Participant Effect: insights and results from a population-based case-control study on breast cancer, American Journal of Epidemiology , 2024;, kwae155, https://doi.org/10.1093/aje/kwae155
- Permissions Icon Permissions
Agreement to participate in case-control studies has become low. Healthy participant bias resulting from differential response proportions in cases and controls can distort results; however, the magnitude of bias is difficult to assess. We investigated the effect in a large population-based case-control study on breast cancer, with a participation rate of 43.4% and 64.1% for controls and cases.
We performed a mortality follow-up in 2020 for 3,813 cases and 7,335 controls recruited between 2002-2005. Standardized mortality ratios (SMR) for overall mortality and selected causes of death were estimated.
The mean age at recruitment was 63.1 years. The overall mortality for controls was 0.66 times lower (95%CI 0.62–0.69) than for the reference population. For causes of death other than breast cancer, SMRs were similar in cases and controls (0.70 and 0.64). Higher education was associated with lower SMRs in both cases and controls.
Options for adjusting the healthy participant bias are limited if the true risk factor distribution in the underlying population is unknown. However, a relevant bias in this particular case-control study is considered unlikely since a similar healthy participant effect was observed for both controls and cases.

Society for Epidemiologic Research members
Personal account.
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Short-term Access
To purchase short-term access, please sign in to your personal account above.
Don't already have a personal account? Register
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1476-6256
- Print ISSN 0002-9262
- Copyright © 2024 Johns Hopkins Bloomberg School of Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
- Career Center
Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer
- Vijayakrishna K. Gadi, MD, PhD
- Sara A. Hurvitz, MD
Adam Brufsky, MD, PhD, presents the case of a 37-year-old woman with HER2+/HR- metastatic breast cancer and polls the audience about screening for brain metastases.
EP: 1 . Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer
EP: 2 . Management Approaches for Patients with HER2+ BC at Risk for Brain Metastases
EP: 3 . The Role of Prophylactic Therapy in HER2+ BC
Ep: 4 . decision making in selecting treatment in her2+ bc, ep: 5 . her2+ bc: considerations for sequencing treatment, ep: 6 . screening and recognition of ild in her2+ bc, ep: 7 . monitoring symptoms of ild in her2+ breast cancer, ep: 8 . management of ild in her2+ bc, ep: 9 . the evolving space of her2+ breast cancer.

EP: 10 . HER2-Positive Breast Cancer: Special Challenges and Expert Insight
Adam Brufsky, MD, PhD: Welcome to this CancerNetwork ® Around the Practice presentation, “HER2+ Breast Cancer Special Challenges and Expert Insight.” I am your host, Dr Adam Brufsky, from UPMC [University of Pittsburgh Medical Center] Hillman Cancer Center in Pittsburgh, Pennsylvania. Tonight we have a great panel of very enthusiastic investigators, all of whom are very experienced in this field and have lots to add to this discussion. We have Dr VK Gadi from University of Illinois Cancer Center in Chicago; Dr Sara Hurvitz from UCLA [University of California, Los Angeles] Jonsson Comprehensive Cancer Center in Los Angeles, California; and finally, Dr Neil Iyengar from Memorial Sloan Kettering Cancer Center in New York City.
Tonight, we’re going to talk about 3 challenges we face in our practices. The first is how to optimally treat patients with HER2-positive breast cancer and brain metastases. We’ll also discuss considerations for sequencing therapies and how we make sequencing decisions in second-, third-, and fourth-line HER2+ metastatic breast cancer. I’ll add parenthetically that it’s a comment on how far this field has come that we’re now debating third-, fourth-, and fifth-line therapy in HER2+ metastatic disease. Finally, we’ll talk about the identification and management of interstitial lung disease and how it impacts the next steps for therapy. During this time, we’ll review a single patient case. Instead of 3 cases, we’re going to do 1 case and add to it as we go along during this hour. We’re going to ask the audience several polling questions using an interactive polling platform.
Let’s start. We’d appreciate if people answer this question. How often do you screen asymptomatic patients with metastatic HER2+ breast cancer for brain metastases? Always, sometimes, rarely, or never?
The results show 20% are always screening, 60% sometimes, 20% rarely, and 0% never.
How often do you treat prophylactically for brain metastases in asymptomatic patients with HER2+ breast cancer? Always, sometimes, rarely, or never? Please fill out the poll.
This is interesting stuff. No one chose always, 25% do sometimes, 25% do rarely, and 2 votes for never. Interesting. Let’s move on and talk about a case. I’ll fill in the details of this case. It is hard to put it all in 1 or 2 slides. This is a 37-year-old woman who 3 or 4 years ago presented with a 5-cm lump in her right breast. She has the typical HER2+, IHC [immunohistochemistry] score of 3+, hormone receptor-negative breast cancer, and no other metastatic disease or LVF [left ventricular failure], and she feels well otherwise. We gave her neoadjuvant TCHP [docetaxel, carboplatin, trastuzumab, pertuzumab] for 6 cycles, and she did pretty well with it. Then she had a lumpectomy after being on radiation, had a pCR [pathologic complete response], and then was given trastuzumab and pertuzumab for the remainder of her therapy, which we can debate back and forth.
She did well for about 2.5 years and then came to the clinic complaining of increased fatigue and persistent cough. She had a chest CT scan that showed a 1.5-cm nodule in the left upper—not superior—lobe. She got a lung biopsy, which showed she has adenocarcinoma persistent with the breast primary that is IHC 3+ for HER2 and negative for ER [estrogen receptor] and PR [progesterone receptor]. She had a PET [positron emission tomography]/CT scan that showed no other bone or liver metastases, but did show several lung metastases, not just 1. Had she just had 1, we would have probably taken it out and called it a day. That’s a whole other question. She had 3 or 4 lung metastases, each of which is about 2 to 3 cm.
At this point, she was given a typical first-line regimen. She was given THP [docetaxel, trastuzumab, pertuzumab], and started on bisphosphonates every 3 months. She was given the docetaxel for probably 8 cycles and then complained of neuropathy. At that point, we discontinued that and just continued her on HP [trastuzumab, pertuzumab]. But it’s now 2 years later and a routine CT showed 3 liver metastases, each of which is about 1 or 2 cm in diameter. There is 1 that’s about 3 cm. She has 4 liver metastases, none of which are incredibly damaging to her organs, but she does clearly have visceral disease progression in her liver after THP [docetaxel, trastuzumab, pertuzumab] in the first line.
Here is the first polling question for the audience. You have a woman who has been on THP [docetaxel, trastuzumab, pertuzumab] for 2 years for metastatic disease. Would you screen her for brain metastases at that point? Obviously, she has had PET/CT, but would you actually screen an asymptomatic patient for brain metastases?
Let’s see what we’ve got. Fifty-fifty, right down the middle. This is going to be an interesting discussion with our esteemed audience and our esteemed group of investigators to see what they think.
If you did not know whether she had brain metastases, and you decide not to screen her, what would you give this patient in the absence of an MRI? She has progressive disease in her liver. She has been on HP [trastuzumab, pertuzumab] alone for about 18 months. Would you rechallenge her with THP [docetaxel, trastuzumab, pertuzumab], give her T-DM1 [trastuzumab emtansine], give her trastuzumab deruxtecan, or something else? The results show that 14% would rechallenge with THP [docetaxel, trastuzumab, pertuzumab], which is not a bad idea, 57% would give T-DM1 [trastuzumab emtansine], 29% would give trastuzumab deruxtecan, and 0% would give other.
Transcript edited for clarity.

36 MONARCH 3: Final Overall Survival Results of Abemaciclib Plus a Nonsteroidal Aromatase Inhibitor as First-Line Therapy for HR+/HER2– Advanced Breast Cancer

Applying Updated Breast Cancer Findings From ASCO to Clinical Practice
Neil M. Iyengar, MD, and Paolo Tarantino, MD, discuss updated data on agents such as T-DXd and abemaciclib in breast cancer presented at 2024 ASCO.
Capivasertib Combo Earns European Approval in ER+/HER2– Breast Cancer
Data from CAPItello-291 support the approval of capivasertib/fulvestrant for ER-positive, HER2-negative breast cancer in the European Union.

Finding a Place for Exercise Oncology in the Treatment of Breast Cancer
Neil M. Iyengar, MD, spoke about the potential impact of exercise on patient-reported outcomes in cancer and achieving work-life balance.

Applying Updated Findings From SABCS 2023 to HER2+ Breast Cancer Practice
Carey K. Anders, MD, spoke about updated findings in the HER2-positive breast cancer space, based on data from 2023 SABCS.

Plant-Based Diet/Exercise Tied to Weight Loss in HR+ Breast Cancer
Patients with hormone receptor-positive breast cancer experienced weight loss when given a plant-based diet plus exercise.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Help | Advanced Search
Computer Science > Artificial Intelligence
Title: accelerating complex disease treatment through network medicine and genai: a case study on drug repurposing for breast cancer.
Abstract: The objective of this research is to introduce a network specialized in predicting drugs that can be repurposed by investigating real-world evidence sources, such as clinical trials and biomedical literature. Specifically, it aims to generate drug combination therapies for complex diseases (e.g., cancer, Alzheimer's). We present a multilayered network medicine approach, empowered by a highly configured ChatGPT prompt engineering system, which is constructed on the fly to extract drug mentions in clinical trials. Additionally, we introduce a novel algorithm that connects real-world evidence with disease-specific signaling pathways (e.g., KEGG database). This sheds light on the repurposability of drugs if they are found to bind with one or more protein constituents of a signaling pathway. To demonstrate, we instantiated the framework for breast cancer and found that, out of 46 breast cancer signaling pathways, the framework identified 38 pathways that were covered by at least two drugs. This evidence signals the potential for combining those drugs. Specifically, the most covered signaling pathway, ID hsa:2064, was covered by 108 drugs, some of which can be combined. Conversely, the signaling pathway ID hsa:1499 was covered by only two drugs, indicating a significant gap for further research. Our network medicine framework, empowered by GenAI, shows promise in identifying drug combinations with a high degree of specificity, knowing the exact signaling pathways and proteins that serve as targets. It is noteworthy that ChatGPT successfully accelerated the process of identifying drug mentions in clinical trials, though further investigations are required to determine the relationships among the drug mentions.
| Comments: | 9 pages double columns, 5 figures, 3 algorithms, 3 tables, and 1 listing, Submitted to IEEE MedAI'24 Conference, to be held November 15-17, Chongqing, China |
| Subjects: | Artificial Intelligence (cs.AI); Computation and Language (cs.CL); Information Retrieval (cs.IR) |
| classes: | I.2; I.2.6 |
| Cite as: | [cs.AI] |
| (or [cs.AI] for this version) | |
| Focus to learn more arXiv-issued DOI via DataCite |
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Rep Pract Oncol Radiother
- v.26(3); 2021

A clinical case of diagnosis of breast cancer in patients with family history of BRCA mutations 1
Marzhan aitmagambetova.
1 West Kazakhstan Marat Ospanov Medical University, Kazakhstan
Gaziza Smagulova
Yerbol bekmukhambetov.
2 National Chamber of Health, Esil District, Kazakhstan
Oksana Zavalyonnaya
Anar tulyaeva.
The incidence of breast cancer is growing rapidly worldwide (1.7 million new cases and 600,000 deaths per year). Moreover, about 10% of breast cancer cases occur in young women under the age of 45. The aim of the study was to report a rare case of BRCA 1-mutated breast cancer in a young patient with multiple affected relatives. Breast cancer is due to a genetic predisposition with BRCA1 and BRCA2 representing a significant proportion of families with a very high risk of developing the disease over a lifetime of up to 50–80%.
Case presentation
In this paper we report a case of a 29-year-old woman with a confirmed diagnosis of left breast carcinoma.
Conclusions
Mutations of the BRCA1 gene were revealed in the patient, in two of her sisters, brother and brother’s daughter.
Introduction
Germline mutations in BRCA1 and BRCA2 genes account for genetic predisposition and increased risk of breast and ovarian cancers [ 1 ]. Most hereditary breast and ovarian cancers are due to highly penetrant germline BRCA mutations which are inherited in an autosomal dominant fashion. In these patients, there are frequently several generations of women affected with breast cancer (often premenopausal) and in some families’ ovarian cancer as well. In addition, other BRCA-associated malignancies, such as prostate, male breast, and pancreatic cancer may also be observed [ 2 ]. The Hereditary Breast and Ovarian Cancer (HBOC) syndrome is caused by loss-of-function mutations in the BRCA1 and BRCA2 genes and explains approximately 16% of inherited breast cancers [ 3 ]. Germline deleterious mutations in BRCA1 (17q11) and BRCA2 (13q12–q13) are associated with a substantially increased risk of breast cancer as compared with the general population, with a cumulative lifetime breast cancer risk of 46–60% in BRCA1 carriers and 43–55% in BRCA2 carriers [ 4 – 6 ]. These mutations have a prevalence of 5–10% in the general breast cancer population and up to 10–20% in those patients with triple-negative breast cancer (TNBC) [ 7 – 9 ], and they have been suggested to be responsible for approximately half of all hereditary breast and ovarian cancer cases [ 10 ]. Couch et al. 1996 reported a total of 254 BRCA1 mutations, 132 (52%) of which were unique. These represented mutations entered into a database established by the Breast Cancer Information Core (BIC). A total of 221 (87%) of all mutations or 107 (81%) of the unique mutations are small deletions, insertions, nonsense point mutations, splice variants, and regulatory mutations that result in truncation or absence of the BRCA1 protein. A total of 11 disease-associated missense mutations (5 unique) and 21 variants (19 unique) as yet unclassified as missense mutations or polymorphisms had been detected. Thirty-five independent benign polymorphisms had been described. The most common mutations were 185delAG (113705.0003) and 5382insC (113705.0018), which accounted for 30 (11.7%) and 26 (10.1%), respectively, of all the mutations [ 11 ]. Consistent with the role of BRCA1 and BRCA2 in DNA repair, germline mutations in these genes lead to impaired homologous repair of chromosomal double strand breaks, with at least a five-fold reduction in DNA double strand break repair [ 12 ]. This, subsequently, predisposes patients to chromosomal instability and leads to unique treatment opportunities, including sensitivity to DNA-damaging agents, ionizing radiation, and poly (ADP-ribose) polymerase (PARP) inhibitors [ 13 – 15 ]. Multiple studies have evaluated neoadjuvant chemotherapy in BRCA-mutated breast cancers, specifically focusing on pathologic complete response (pCR) rates, a known surrogate for clinical efficacy and outcome. A larger observational study evaluating pCR rates in BRCA1-positive breast cancer patients found high rates of pCR in patients receiving neoadjuvant cisplatin (pCR in 10 of 12 patients, 83%), substantially higher than pCR rates in BRCA1-mutated breast cancer patients treated with cyclophosphamide, methotrexate, and fluorouracil (CMF, 7%); doxorubicin and docetaxel (AT, 8%); or doxorubicin and cyclophosphamide with and without fluorouracil (FAC, 22%) [ 16 ]. Patients with invasive breast cancer caused by hereditary breast cancer syndromes constitute a unique patient population with individualized, rationally targeted systemic treatment options. The most common syndrome leading to an increased risk of breast cancer, BRCA-related breast cancer syndrome, involves harmful germline mutations in BRCA1 and BRCA2. These patients are particularly sensitive to DNA damaging agents such as platinum agents, as well as to PARP inhibitors, which, by blocking the formation of ADP-ribose polymers at the site of single-strand breaks, prevent recruitment of DNA damage repair [ 17 ]. Other unique therapies tested in BRCA-mutated invasive breast cancer patients include trabectin, given preclinical evidence suggesting specific activity against intact metastatic breast cancer with excisional nucleotide repair or homologous repair by recombination [ 18 ], and lurbinectin, which induces double-strand breaks resulting in activity against platinum-resistant tumors and cell lines deficient in homologous recombination [ 19 ].
We report a rare case of BRCA1-mutated breast cancer in a young patient with multiple affected relatives.
Patient A., 29 years old, Asian (Kazakh), housewife. Diagnosed with carcinoma of the left breast STIIIAT2N2M0. Condition after neoadjuvant chemotherapy (neo-PCT) + bilateral radical mastectomy + radiation therapy.
In September 2016, while breast-feeding the fourth child, she independently found a mass in the left breast. Ultrasonography of the mammary glands in one month revealed nodulation with size 26 × 22 × 17 mm. Further PET-CT ( Fig. 2 ) was performed and a conclusion was: Abnormal growth of metabolic activities observed in the lower part of the left breast and lymph nodes of the left axillary region compatible with malignant structure. Local examination at the time of diagnosis: In the left breast in the lower quadrant — a dense, inactive tumor with the size 3 × 3 cm and in the left axillary region lymph node 1 × 1 cm. Right breast and lymph nodes without pathology. Trepan biopsy of the left breast lump was performed. The result of histology was invasive ductal carcinoma of nonspecific type ( Fig. 1 ).

PET-CT of the mammary glands

The pedigree of patient under study
The result of IGH — RE-0B, RP — 2B, HER2Neo — negative, Ki 67 — 60%. The patient received 4 courses of neoadjuvant polichemotherapy (NPCh) with the FEC scheme (dexamethasone 12 mg I/V drip to 100 ml physiological saline solution on day 1, epirubicin 138 mg I/V drip to 100 ml physiological saline solution on day 1, fluorouracil 690 mg I/V drip on day 1, cyclophosphamide 690 mg I/V drip physiological saline solution on day 1). Then the patient received 12 courses of paclitaxel 110 mg I/V per 500 ml of physiological saline solution. After treatment, there was regression. Heredity is burdened. Her older sister died of ovarian cancer at the age of 43. Her father died of lung cancer at the age of 51. Pregnancy — 4; childbirth — 4; abortion — 0.
Considering the family history, the patient underwent medical genetic counseling with informed consent. Peripheral whole blood was taken in a volume of 10 ml. Extraction of DNA from 100 μl of peripheral blood was performed using a set of reagents “Proba-GS-Genetics” (NPO DNA-Tekhnologiya, Russia), according to the manufacturer’s instructions.
Genotyping was performed by real-time polymerase chain reaction (PCR) using the BRCA OncoGenetics kit (including 8 mutations in the BRCA1 genes (mutations 185delAG, 4153delA, 5382insC, 3819delGTAAA, 3875delGTCT, 300T> G (Cysdel-61Gly), 2080 BRCA2 (mutation 6174delT) according to the manufacturer’s instructions. PCR was performed using a DTprime detecting amplifier (NPO DNA-Tekhnologiya, Russia).
The study was carried out in a scientific molecular genetic laboratory at the Marat Ospanov West Kazakhstan Medical University. Conclusion: the study of the DNA sample revealed: heterozygous replacement in the BRCA1 gene mutation 5382incS. The patient after chemotherapy treatment had bilateral subcutaneous mastectomy with axillary lymph node dissection. The result of the final histology — fibrosis in both mammary glands, metastases of the axillary lymph nodes were revealed. Then, a postoperative course of radiation therapy on the linear accelerator “Trubeam” on the left breast and the zone of the regional lymph node was given SOD-46 Gr. when the ROD was 2.0 Gr. Considering the presence of a gene mutation (5382incS) in the patient and her family history, medical and genetic counseling of relatives (mother, brother, sisters and their adult children) was carried out, informed consent was obtained from all members of her family. 8 relatives were examined, in whom 3 mutations were identified. The analysis revealed a similar mutation 5382incS in her own older brother (42 years old) and her own daughter (17 years old), her own second older sister (37 years old). The eldest sister, J., 1975 year of birth. In October of 2015 she was diagnosed with carcinoma of the ovary STIIIT3N1M0. In November 2018 molecular genetic study of mutation of BRCA1 , BRCA2 genes was carried out. Conclusion: in the studied sample in exon 20 of the BRCA1 gene detected 5382incS mutation in a heterozygous state. Despite the treatment, the therapy was unsuccessful, the patient died on 28.11.2018 at the age of 43 years.
Native second eldest sister, A. born in 1981. Discovered BRCA1 mutation 5382incS. She was examined. According to mammography — BI-RADS M2-L2, breast ultrasound — the lump of the left breast, the size of 1.5–2 cm. A puncture of the mass was done, the result of cytology without atypia. Ultrasound of the pelvis — without pathology. The patient is under the supervision of oncologists.
Older brother (G.) born in 1976. BRCA1 mutation 5382 incS was detected. At the time of the medical examination he had no complaints. Tumor marker PSA — 0.3 ng\ml. MRI of the prostate — MRI picture is typical for nodulation of the right prostate. The patient is under observation.
Niece (brother’s daughter) — born in 2002, was similarly examined in January 2019. BRCA1 mutation 5382 insc was detected. According to ultrasound of the pelvis: no structural changes. Breast ultrasound — in the right breast of the upper outer quadrant hypoallergenic formation of 2.0 × 1.2 cm, similar to the left breast 2.9 × 1.9 cm. Fibroadenoma of both mammary glands. A puncture of the formation of mammary glands was performed. The result of cytology without atypia. The patient is under observation.
The pedigree was based on the interview of the patient under study; it is impossible to determine the genetic status of deceased ancestors ( Fig. 2 ).
In the presented case, the patient has luminal B type breast cancer. According to TCGA, breast cancer luminal B is characterized by a high TP53 mutation rate (29%) and a slightly lower mutation rate of the PIK3CA catalytic subunit alpha (29%). The main characteristics of genomic, clinical and proteomic subtypes are as follows: mRNA expression — lower ER cluster; high proliferation; DNA methylation — hypermethylated phenotype for a subset; protein expression — less estrogen signaling; high FOXM1 and MYC; reactive RPPA subtypes; DNA mutations — TP53 (32%); PIK3CA (32%); MAP3K1 (5%) [ 20 ].
Unlike sporadic cancers, up to 10% of all cancers are due to hereditary genetic defects. Hereditary cancer was first described by doctors-researchers A. Warthin and H. Lynch, who detected families predisposed to cancer, which, in turn, led to the identification of hereditary cancer syndromes [ 20 ]. A number of affected families will inherit one allele of the mutated predisposition gene, called the “germ line mutation”, which is hidden in every cell of the body. As a result, hereditary cancers are characterized by 1) early onset cancer, often younger than 50 years at diagnosis compared to an average age of 60 years in the general population, 2) family history of cancer across generations in which cancer types correspond to the tumor spectrum of the syndrome, as confirmed in this clinical case [ 22 ]. Our data are consistent with the research of A. Rathore who showed that mutated BRCA carcinoma is found in young people with a family history of the disease [ 23 ]. In our case, four out of six children were found to have a mutation in the BRCA1 gene, and two generations were found to have this mutation. One family member died of ovarian cancer. This patient developed the disease at early age. Other family members have a benign formation. However, at the insistence of the family members themselves, we are not talking about the preventive removal of organs. Therefore, they are under careful supervision of oncologists.
Only a relatively small proportion of breast cancers can be explained by the presence of genetic mutations with high penetrance, such as the BRCA1 and BRCA2 genes [ 24 ]. Together with mutations in genes of intermediate penetrance, such as ATM , BARD1 , PALB2 , and CHECK2 , they explain 20–25% of the risk of breast cancer, leaving a significant proportion of heredity that remains to be explained by variants with low penetrance [ 25 ]. Undoubtedly, ongoing research into families with multiple cancer-affected members will lead to the identification of other variants in these genes that also predispose to breast cancer [ 26 ].
Many genes for different populations have been thoroughly studied [ 27 ]. The identified BRCA1 5382insC mutation in our patient was identified at a frequency of 0.13% in the Jewish population [ 28 ], also found in the population of Austria, Slovenia, Germany, Czech Republic, Greece, Denmark, Poland, Latvia, Lithuania, Belarus and Russia [ 10 ]. In Kazakhstan, according to the work of the author B. Apsalikov, out of 250 women, a similar mutation was found in 23. This indicates the occurrence of this mutation in our population [ 29 ]. But all this still requires further large-scale research.
In addition, molecular genetic testing is becoming an important tool for predicting drug reactions, as new target therapeutic agents, such as poly inhibitors, appear and platinum-based sensitivity is reported [ 30 ]. In our case, the patient was prescribed 12 courses of paclitaxel and there was a significant regression of the tumor, which was confirmed by the instrumental study.
Given that BRCA1 and BRCA2 were identified more than 20 years ago, preventive mastectomy remains the gold standard, mutation carriers have strong preferences for chemoprophylaxis [ 31 ]. The patient underwent bilateral subcutaneous mastectomy with lymph node dissection.
The Cancer Genome Atlas (TCGA), a landmark program in the genomics of cancer, has molecularly characterized over 20,000 primary cancers and matched normal samples covering 33 cancers. This collaborative effort between the National Cancer Institute and the National Human Genome Research Institute began in 2006 and brought together researchers from different disciplines and multiple institutions.
Over the next ten years, TCGA generated over 2.5 petabytes of genomic, epigenomic, transcriptomic, and proteomic data. Data that have already improved our ability to diagnose, treat, and prevent cancer [ 32 ]. We compared the results of the presented case with the TCGA data. TCGA identified PIK3CA, the alpha catalytic subunit of PI3K, as the most common SMG in breast lumen cancer, occurring at 45% and 29% rates in the lumen A and lumen B subtypes, respectively. Often these are missense mutations that are grouped in the helical domain and the kinase domain of PI3 kinase and are capable of causing cellular transformation when introduced into mammary epithelial cells [ 33 ].
The TP53 gene product is a potent tumor suppressor that induces apoptosis or cell cycle arrest in response to cellular stress. A mutation in the TP53 gene is a characteristic feature of basal-like breast cancer. In ER+ breast cancer, TP53 mutation is less common (30% in lumen B and 12% in lumen A) [ 34 , 35 ].
The national integrated cancer control network (NCCN) has established guidelines for testing BRCA mutations and for treating people who have a mutation in the BRCA1\2 genes. As our patient has the early age of developing the disease, family history, we used the principles of this guide. NCCN has published recommendations for observing women with positive test for one of the BRCA mutations [ 31 ]. Guided by the principle of NCCN, we will observe the relatives of our patient with detected mutations carefully, under strict control.
Acknowledgments
The authors thank the staff of the Medical Center of the West Kazakhstan Medical University named after Marat Ospanov for providing samples of human blood and the Scientific and Practical Center for the technical assistance provided. The article was written as part of grant funding for a scientific and technical project No. 0118РК01065, the Ministry of Education and Science of the Republic of Kazakhstan and the NAO of the West Kazakhstan Medical University Marat Ospanov.
Conflict of interest
None declared.
The article was written as part of grant funding for a scientific and technical project No. 0118РК01065, the Ministry of Education and Science of the Republic of Kazakhstan and the NAO of the West Kazakhstan Medical University Marat Ospanov.
Author contributions
Conceptualization, M.A. and G.S.; methodology, Y.B.; software, O.Z.; validation, A.T., O.Z. and Y.B.; formal analysis, G.S.; investigation, M.A.; resources, A.T.; data curation, M.A.; writing — original draft preparation, O.Z.; writing — review and editing, A.T.; visualization, Y.B.; supervision, M.A.; project administration, G.S.; funding acquisition, Y.B. All authors have read and agreed to the published version of the manuscript.
Information pertaining to writing assistance
Not applicable.
Ethical disclosure
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Consent for publication
The patients provided written informed consent for the publication of any associated data and accompanying images.
Data sharing statement
Data will be available on request.

IMAGES
VIDEO
COMMENTS
Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study ...
Background. Worldwide, male breast cancer is extremely rare, accounting for <1% of all breast tumors and <1% of all malignancies in men [1-3].Recently, the incidence of male breast cancer has increased from 1.0 per 100,000 men in the late 1970s to 1.2 per 100,000 men from 2000 to 2004 [4-7].The American Cancer Society reported a similar trend in the incidence of breast cancer in men from ...
Carol Edwards, a 39 year-old premenopausal woman, had a screening mammogram which revealed an abnormality in the right breast. She had no palpable masses on breast exam. A mammographically localized surgical biopsy was done and revealed a small (0.9 cm) grade III infiltrating ductal carcinoma with some associated ductal carcinoma-in-situ (DCIS ...
Primary Objective. Objective BR2.7: Factors Affecting Response and Prognosis of Breast Cancer.Explain the prognosis and likelihood of recurrence and response to therapy for breast cancer patients based on knowledge of molecular classification and/or gene expression profiling, morphologic classification, grade, prognostic marker studies, and other predictive factors.
Breast cancer is the most common cancer and the one that associates the highest mortality rates among Spanish women, with 32,953 new cases estimated to be diagnosed in Spain in 2020 [ 1 ]. Thanks to early diagnosis and therapeutic advances, survival has increased in recent years [ 2 ]. The 5-year survival rate is currently around 85% [ 3, 4 ].
1 Introduction. Breast cancer remains the most common cancer diagnosed among women in the United States and is the second leading cause of cancer-related deaths, with approximately 41,000 patients projected to die from this disease in 2018 alone. The prognosis for patients with metastatic breast cancer varies based on many factors including estrogen receptor (ER), progesterone receptor (PR ...
Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18]fluoroestradiol. Ella F. Jones. Kimberly M. Ray. Nola M. Hylton. Case Report Open Access 16 Apr 2019. Browse ...
Key Points. Question Are there disparities in breast cancer treatment and outcomes of patients from sex and gender minority (SGM) groups compared with cisgender heterosexual patients?. Findings In this exposure-matched case-control study of 92 patients from SGM groups matched to cisgender heterosexual patients by year of breast cancer diagnosis, age, tumor stage, estrogen receptor status, and ...
Case Studies in Social Medicine. ... neighborhoods of concentrated poverty without the same health care resources as affluent neighborhoods. 4 In the case of breast cancer mortality, these social ...
In the second case study of the series, moderator Adam M. Brufsky, MD, PhD, describes a 63 year-old woman presenting with thickening of the outer left breast and an enlarging mass. The thickening ...
Presentation of Case Dr. Kevin S. Oh (Radiation Oncology): A 45-year-old woman was evaluated in the oncology clinic of this hospital because of metastatic triple-negative breast cancer.
Pathophysiology of Breast Cancer. Except for skin cancer, breast cancer is the most common cancer in American women. Most breast cancer occurs in women older than 50 years. The major risk factors for breast cancer are classified as reproductive, such as nulliparity and pregnancy-associated breast cancer; familial, such as inherited gene ...
Patient Case Presentation. Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn't reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left ...
If rounding is necessary, round to the whole number.) 250 x 15/60 minutes = 62.5 rounded to 63 drops/minute. After receiving vancomycin for 7 days, the client complains that her mouth is painful when she swallows. When assessing her mouth, the nurse visualizes white, patchy lesions.
Adam M. Brufsky, MD, PhD: Let's talk about this case. This is a 48-year-old woman who presented to her primary care physician a number of years ago with a lump in her breast. She had a 4.4-cm left breast mass and 3 palpable axillary lymph nodes. Her ultrasound and mammogram confirmed these physical findings.
Rasuli B, Breast cancer brain metastases. Case study, Radiopaedia.org (Accessed on 27 Jun 2024) https://doi.org/10.53347/rID-191046
The Bottom Line. More than 1.5 million new cases of breast cancer are reported worldwide each year, of which 60% are HR-positive. Hormonal therapy has been the mainstay of treatment for advanced ...
Breast cryoablation for the palliative treatment of indolent subtype of multicentric triple-negative breast cancer. Monica L Huang, Deanna L Lane, Hannah Bomar, Henry Kuerer. BMJ Case Reports CP Apr 2024, 17 (4) e259465; DOI: 10.1136/bcr-2023-259465. Malignant phyllodes tumour of the breast with early recurrence in a young patient.
A reliable preclinical model of patient-derived organoids (PDOs) was developed in a case study of a 69-year-old woman diagnosed with breast cancer (BC) to investigate the tumour evolution before and after neoadjuvant chemotherapy and surgery. The results were achieved due to the development of PDOs from tissues collected before (O-PRE) and after (O-POST) treatment.
Schematic representation of the proposed framework. In stage 1, multimodal datasets from cancer patients (e.g. BC) were sourced from a published study [].This dataset comprises clinical features, DNA mutations, and gene expression from pre-treatment tumors, alongside post-treatment response classes (pCR, RCB-I to III).
In this study, we compared breast cancer patients in the BCSC top 2.5% of risk for their age to patients from the remaining 97.5%. We found that women in the top 2.5% of risk for their age, who have double the risk of getting breast cancer relative to the average women, had more than six-fold higher odds of presenting with interval cancers.
Breast imaging clinics in the United States (U.S.) are increasingly implementing breast cancer risk assessment (BCRA) to align with evolving guideline recommendations but with limited uptake of risk-reduction care. Effectively communicating risk information to women is central to implementation efforts, but remains understudied in the U.S. This study aims to characterize, and identify factors ...
Abstract. Breast cancer is Malignment cell growth in the breast. If the cancer isn't treated, it will spread to other parts of the body. Except for skin cancer, the most frequent type of cancer among women is breast cancer. Mammograms can detect breast cancer early, possibly before it has spread. Main symptoms and/or important clinical findings: A 58-year-old female patient registered to the ...
Healthy participant bias resulting from differential response proportions in cases and controls can distort results; however, the magnitude of bias is difficult to assess. We investigated the effect in a large population-based case-control study on breast cancer, with a participation rate of 43.4% and 64.1% for controls and cases.
It is hard to put it all in 1 or 2 slides. This is a 37-year-old woman who 3 or 4 years ago presented with a 5-cm lump in her right breast. She has the typical HER2+, IHC [immunohistochemistry] score of 3+, hormone receptor-negative breast cancer, and no other metastatic disease or LVF [left ventricular failure], and she feels well otherwise.
Study with Quizlet and memorize flashcards containing terms like The client attended a community education program promoting breast cancer awareness with her daughter., At the community education program, the client learns that breast self-exam (BSE) is best performed 2-3 days after the start of the menstrual flow when the breasts are least engorged and tender. Since the client had a ...
Breast Cancer Case Study Group one. Patient Profile R M. is a 68-year-old white female who went to her healthcare provider with a complaint of "feeling tightness" around a lump in her right breast. She has a history of hypertension and smoking (25 pack- year history). Subjective Data Has a family history of breast cancer-one sister recently had lumpectomy and radiation therapy.
The objective of this research is to introduce a network specialized in predicting drugs that can be repurposed by investigating real-world evidence sources, such as clinical trials and biomedical literature. Specifically, it aims to generate drug combination therapies for complex diseases (e.g., cancer, Alzheimer's). We present a multilayered network medicine approach, empowered by a highly ...
Background Women with dense breasts benefit from supplemental cancer screening with US, but US has low specificity. Purpose To evaluate the performance of breast US tomography (UST) combined with full-field digital mammography (FFDM) compared with FFDM alone for breast cancer screening in women with dense breasts. Materials and Methods This retrospective multireader multicase study included ...
The incidence of breast cancer is growing rapidly worldwide (1.7 million new cases and 600,000 deaths per year). Moreover, about 10% of breast cancer cases occur in young women under the age of 45. The aim of the study was to report a rare case of BRCA 1-mutated breast cancer in a young patient with multiple affected relatives.