CBSE NCERT Solutions
NCERT and CBSE Solutions for free

Case Study Chapter 3 Metals and Non-Metals
Please refer to Chapter 3 Metals and Non-Metals Case Study Questions with answers provided below. We have provided Case Study Questions for Class 10 Science for all chapters as per CBSE, NCERT and KVS examination guidelines. These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations.
Case Study Questions Chapter 3 Metals and Non-Metals
Case/Passage – 1
Metals are electropositive elements. They can easily lose electrons to form ions. Metals show distinguished physical as well as chemical properties. Generally most of the metals are ductile and malleable with exception such as mercury. These properties make them valuable for commercial as well as domestic uses. Reaction of a metal with water is one of important chemical property. Metals like sodium and potassium reacts with cold water while magnesium reacts with hot water. Metals like aluminium, zinc do not react with hot/cold water but they easily react with steam. When a metal react with hot/cold water the products are metal hydroxide and hydrogen,and when it react with steam, the product are metal oxide and hydrogen. Some metals like sodium, potassium react violently with water.
Question: When zinc reacts with steam it produces: (a) Zn(OH) 2 (b) ZnO (c) O 2 (d) ZnO 2
Question: During the reaction of calcium with water, pieces of metal start floating due to the formation of: (a) Ca(OH) 2 (b) CO2 (c) H 2 (d) none of these
Question: Consider the reactions: Na(s) + H 2 O (l) → NaOH (aq) + H2 (g) ……….(i) Ca(s) + H 2 O (l) → Ca(OH) 2 (aq) + H 2 (g) ………(ii) (a) Reaction (i) is endothermic reaction. (b) Reaction (ii) is endothermic reaction. (c) Reaction (ii) is more exothermic than reaction (i). (d) Reaction (i) is more exothermic than reaction (ii).
Question: Most ductile metal among the following is: (a) Au (b) Ag (c) Cu (d) Al
Question: Metals can be converted into thin sheet by hammering.This property is known as: (a) Ductility (b) Sonorous (c) Malleability (d) Both (a) and (c)
Case/Passage – 2
Elements can be classified as metals or non-metals on the basis of their properties. The easiest way to start grouping substances is by comparing their physical properties. Metals, in their pure state, have a shining surface. This property is called metallic luster. metals are generally hard. The hardness varies from metal to metal. some metals are used for making cooking vessels.
Question: The most abundant metal in the earth’s crust is – (a) iron (b) copper (c) aluminium (d) mercury
Question: The metal that reacts with cold water is – (a) mercury (b) sodium (c) zinc (d)tungsten
Question: Metal present in chloroplast is (a) Iron (b) Copper (c) Magnesium (d) Cobalt
Question: Metals generally are (a) reducing agents (b) oxidising agent (c) both oxidising and reducing agents (d) None of these
Question: Which of the following metal(s) catch fire on reaction with water? (a) Sodium (b) Potassium (c) Magnesium (d) both (a) and (b)
Case/Passage – 3
The huge annual loss due to corrosion is a national waste and should be minimized. Following are some methods which are helpful to prevent corrosion
(i) Coating the iron surface with paint or oil or grease prevents moist oxygen from coming in contact with the metal and thus effectively prevents rusting of iron. (ii) Galvanisation : Iron is blasted with fine sand to make the surface rough dipped in molten zinc and then cooled. A thin layer of zinc forms on the iron surface. Since zinc is more reactive than iron, it acts as a sacrificial metal and is preferentially oxidised thus preventing oxidation of iron. (iii) Electroplating with tin, nickel or chromium also prevents rusting. (iv) Alloying (mixing iron in its molten state with other metals) prevents rusting. Stainless steel is an alloy of iron with Cr or Ni.
Question: The most convenient method to protect the bottom of ship made of iron is : (a) coating it with red lead oxide. (b) white tin plating. (c) connecting it with Mg block. (d) connecting it with Pb block.
Question: The best way to prevent rusting of iron is : (a) making it cathode (b) putting in saline water (c) both of these (d) none of these
Question: The most durable metal plating on iron to protect against corrosion is : (a) nickel plating (b) copper plating (c) tin plating (d) zinc plating
Case/Passage – 4
Some metals are chemically very reactive, whereas others are less reactive or unreactive. On the basis of vigourness of reactions of various metals with oxygen, water and acids, as well as displacement reactions, the metals have been arranged in a group or series according to their chemical reactivity. The arrangement of metals in a vertical column in the order of decreasing reactivities is called reactivity series of metals (or activity series of metals). In reactivity series, the most reactive metal is placed at the top whereas the least reactive metal is placed at the bottom. As we come down in the series, the chemical reactivity of metals decreases. Since the metals placed at the bottom of the reactivity series (like silver and gold) are less reactive, so they are usually found in free state (native state) in nature.
Question: Copper sulphate solution can be safely kept in a container made of : (a) aluminium (b) lead (c) silver (d) zinc
Question: When metal Z is added to dilute HCl solution, there is no evolution of gas. Metal is : (a) K (b) Na (c) Ag (d) Zn
Question: Metal always found in free state is : (a) gold (b) silver (c) copper (d) sodium

Related Posts

Class 10 English Notes and Questions

Old Man at the Bridge Summary by Ernest Miller Hemingway

Minerals and Energy Resources Class 10 Social Science Notes and Questions
- Bihar Board
TBSE Result
Cfa institute, srm university.
- MBOSE Result 2024
- TBSE Result 2024
- Maharashtra SSC Result
- Odisha Board Result
- RBSE 10th Result 2024
- CBSE Board Result 2024
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
- CBSE Class 10
CBSE Class 10 Science Chapter Wise Important Case Study Questions
Chapter wise important case study questions cbse class 10 science: cbse class 10 science board exam 2024 is just around the corner and students are working hard to score maximum marks. check these case study questions from class 10 science to ace your examination this year also download the solutions from the pdf attached towards the end. .

CBSE Class 10 Science Chapter Wise Important Case Study Questions: While the CBSE Board exam for Class 10 students are ongoing, the CBSE Class 10 Science board exam 2024 is to be held on March 2, 2024. With the exams just a few days away, CBSE Class 10th Board exam candidates are rushing to prepare the remaining syllabus, practising their weak portions, trying to revise the important questions from the past year papers, practise questions, etc.
Why are CBSE Class 10 Science Case Study Questions Important?
- Section A : 20 Multiple Choice Questions (MCQs) carrying 1 mark each.
- Section B : 6 Very Short Answer type questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C : 7 Short Answer type questions carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D : 3 Long Answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E : 3 Case Based/ Source Based units of assessment (4 marks each) with sub-parts.
How to solve case study questions in CBSE Class 10 Science?
- Read the case given and the associated questions carefully.
- Read the questions attentively and analyse what they are asking.
- Apply your subject knowledge and theories in the given case to decide what the correct answers should be.
1.A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The massive force that pushes the rocket forward through space is generated due to the
(a) combination reaction
(b) decomposition reaction
(c) displacement reaction
(d) double displacement reaction
(ii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of
(a) lead nitrate
(b) nitrogen oxide
(c) lead oxide
(d) oxygen gas
(iii) Which of the following reactions represents a combination reaction?
(a) CaO (s) + H2O (l) → Ca (OH)2 (aq)
(b) CaCO3 (s) → CaO (s) + CO2(g)
(c) Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu(s)
(d) 2FeSO4(s) → Fe2O3 (s) +SO2(g) + SO3(g)
(iv) Complete the following statements by choosing correct type of reaction for X and Y.
Statement 1: The heating of lead nitrate is an example of ‘X’ reaction.
Statement 2: The burning of magnesium is an example of ‘Y’ reaction.
(a)X-Combination,Y-Decomposition
(b)X-Decomposition,Y-Combination
(c)X-Combination,Y-Displacement
(d) X- Displacement, Y-Decomposition
2.The earlier concept of oxidation and reduction is based on the addition or removal of oxygen or hydrogen elements so, in terms of oxygen and hydrogen, oxidation is addition of oxygen to a substance and removal of hydrogen from a substance. On the other hand, reduction is addition of hydrogen to a substance and removal of oxygen from a substance. The substance which gives oxygen to another substance or removes hydrogen from another substance in an oxidation reaction is known as oxidising agent, while the substance which gives hydrogen to another substance or removes oxygen from another substance in a reduction reaction is known as reducing agent. For example,
(i) A redox reaction is one in which
(a) both the substances are reduced
(b) both the substances are oxidised
(c) an acid is neutralised by the base
(d) one substance is oxidised while the other is reduced.
(ii) In the reaction, H2S+Cl2⟶S+2HCl
(a) H2S is the reducing agent.
(b) HCl is the oxidising agent.
(c) H2S is the oxidising agent.
(d) Cl2 is the reducing agent.
(iii) Which of the following processes does not involve either oxidation or reduction?
(a) Formation of slaked lime from quicklime.
(b) Heating mercuric oxide.
(c) Formation of manganese chloride from manganese oxide (MnO2).
(d) Formation of zinc from zinc blende.
(iv) Mg+CuO⟶MgO+Cu
Which of the following is wrong relating to the above reaction?
(a) CuO gets reduced
(b) Mg gets oxidised.
(c) CuO gets oxidised.
(d) It is a redox reaction.
3.A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lemon on the vessel, the surface is cleaned, and the vessel begins to shine again. This is due to the fact that which reacts with the acid present in lemon to form a salt which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed.
1.Which of the following acids is present in lemon?
(a) Formic acid
(b) Acetic acid
(c) Citric acid
(d) Hydrochloric acid
2.The nature of copper oxide is
d) amphoteric
3.Name the salt formed in the above reaction
a) copper carbonate
b) copper chloride
c)copper citrate
d) copper citrate
4.The phenomenon of copper getting tarnished is
a) corrosion
b) rancidity
c) displacement
d)none of these
4.Metals as we know, are very useful in all fields, industries in particular. Non-metals are no less in any way. Oxygen present in air is essential for breathing as well as for combustion. Non-metals form a large number of compounds which are extremely useful, e.g., ammonia, nitric acid, sulphuric acid, etc. Non-metals are found to exist in three states of matter. Only solid non-metals are expected to be hard however, they have low density and are brittle. They usually have low melting and boiling points and are poor conductors of electricity.
i.____________ is a non-metal but is lustrous
A.Phosphorus
ii.Which of the following is known as 'King of chemicals'?
C. Sulphuric acid
D. Nitric acid
iii.Which of the following non-metals is a liquid?
iv.Hydrogen is used
A.for the synthesis of ammonia
B. for the synthesis of methyl alcohol
C.nitrogenous fertilizers
D. all of these
5.Nisha observed that the bottoms of cooking utensils were turning black in colour while the flame of her stove was yellow in colour. Her daughter suggested cleaning the air holes of the stove to get a clean, blue flame. She also told her mother that this would prevent the fuel from getting wasted.
a) Identify the reasons behind the sooty flame arising from the stove.
b) Can you distinguish between saturated and unsaturated compounds by burning them? Justify your answer.
c) Why do you think the colour of the flame turns blue once the air holes of the stove are cleaned?
6.Blood transport food, Oxygen and waste materials in our bodies. It consists of plasma as a fluid medium. A pumping organ [heart] is required to push the blood around the body. The blood flows through the chambers of the heart in a specific manner and direction. While flowing throughout the body, blood exerts a pressure against the wall or a vessel.
- Pulmonary artery
- Pulmonary vein
- Very narrow and have high resistance
- Much wide and have low resistance
- Very narrow and have low resistance
- Much wide and have high resistance
- It is a hollow muscular organ
- It is four chambered having three auricles and one ventricle.
- It has different chambers to prevent O2 rich blood from mixing with the blood containing CO2
- Both A & C
- Blood = Plasma + RBC + WBC + Platelets
- Plasma = Blood – RBC
- Lymph = Plasma + RBC
- Serum = Plasma + RBC + WBC
7.A brain is displayed at the Allen Institute for Brain Science. The human brain is a 3-pound (1.4-kilogram) mass of jelly-like fats and tissues—yet it's the most complex of all known living structures The human brain is more complex than any other known structure in the universe. Weighing in at three pounds, on average, this spongy mass of fat and protein is made up of two overarching types of cells—called glia and neurons— and it contains many billions of each. Neurons are notable for their branch-like projections called axons and dendrites, which gather and transmit electrochemical signals. Different types of glial cells provide physical protection to neurons and help keep them, and the brain, healthy. Together, this complex network of cells gives rise to every aspect of our shared humanity. We could not breathe, play, love, or remember without the brain.
1)Animals such as elephants, dolphins, and whales actually have larger brains, but humans have the most developed cerebrum. It's packed to capacity inside our skulls and is highly folded. Why our brain is highly folded?
- b) Learning
3)Which among these protects our brain?
a)Neurotransmitter
b) Cerebrospinal fluid
d) Grey matter
4.Ram was studying in his room. Suddenly he smells something burning and sees smoke in the room. He rushes out of the room immediately. Was Ram’s action voluntary or involuntary? Why?
8.Preeti is very fond of gardening. She has different flowering plants in her garden. One day a few naughty children entered her garden and plucked many leaves of Bryophyllum plant and threw them here and there in the garden. After few days, Preeti observed that new Bryophyllum plants were coming out from the leaves which fell on the ground.
1.What does the incident sited in the paragraph indicate?
(a). Bryophyllum leaves have special buds that germinate to give rise to new plant.
(b). Bryophyllum can propagate vegetatively through leaves.
(c). Bryophyllum is a flowering plant that reproduces only asexually
(d). Both (a) and (b).
2.Which of the following plants can propagate vegetatively through leaves like Bryophyllum?
3.Do you think any other vegetative part of Bryophyllum can help in propagation? If yes, then which part?
(c) Flowers
4.Which of the following plant is artificially propagated (vegetatively) by stem cuttings in horticultural practices?
(b)Snakeplant
(d)Water hyacinth
9.The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of the population.
1) What are common signs of sexual maturation in boys?
a) Broadening of shoulders
b) Development of mammary glands
c) Broadening of waist
d) High pitch of voice
2) Common sign of sexual maturation in girls is
a) Low pitch voice
b) Appearance of moustache and beard
c) Development of mammary glands
d) Broadening of shoulders
3) Which contraceptive method changes the hormonal balance of the body?
b) Diaphragms
c) Oral pills
d) Both a) and b)
4) What should be maintained for healthy society?
a) Rate of birth and death rate
b) Male and female sex ratio
c) Child sex ratio
d) None of these
10.Pea plants can have smooth seeds or wrinkled seeds. One of the phenotypes is completely dominant over the other. A farmer decides to pollinate one flower of a plant with smooth seeds using pollen from a plant with wrinkled seeds. The resulting pea pod has all smooth seeds.
i) Which of the following conclusions can be drawn?
(1) The allele for smooth seeds is dominated over that of wrinkled seeds.
(2) The plant with smooth seeds is heterozygous.
(3) The plant with wrinkled seeds is homozygous.
(b) 1 and 2 only
(c) 1 and 3 only
(d) 1, 2 and 3
ii) Which of the following crosses will give smooth and wrinkled seeds in same proportion?
(a) RR X rr
(b) Rr X rr
(d) rr X rr
iii) Which of the following cross can be used to determine the genotype of a plant with dominant phenotype?
(a) RR X RR
(b) Rr X Rr
(c) Rr X RR
(d) RR X rr
iv) On crossing of two heterozygous smooth seeded plants (Rr), a total of 1000 plants were obtained in F1 generation. What will be the respective number of smooth and wrinkled seeds obtained in F1 generation?
(a) 750, 250
(b) 500, 500
(C) 800, 200
(d) 950, 50
11.Food chains are very important for the survival of most species.When only one element is removed from the food chain it can result in extinction of a species in some cases.The foundation of the food chain consists of primary producers.Primary producers or autotrophs,can use either solar energy or chemical energy to create complex organic compounds,whereas species at higher trophic levels cannot and so must consume producers or other life that itself consumes producers. Because the sun’s light is necessary for photosynthesis,most life could not exist if the sun disappeared.Even so,it has recently been discovered that there are some forms of life,chemotrophs,that appear to gain all their metabolic energy from chemosynthesis driven by hydrothermal vents,thus showing that some life may not require solar energy to thrive.
1.If 10,000 J solar energy falls on green plants in a terrestrial ecosystem,what percentage of solar energy will be converted into food energy?
(d)It will depend on the type of the terrestrial plant
2.Matter and energy are two fundamental inputs of an ecosystem. Movement of
(a)Energy is by directional and matter is repeatedly circulating
(b)Energy is repeatedly circulating and matter is unidirectional
(c)Energy is unidirectional and matter is repeatedly circulating
(d)Energy is multidirectional and matter is bidirectional
3.Raj is eating curd/yoghurt. For this food intake in a food chain he should be considered as occupying
(a)First trophic level
(b)Second trophic level
(c)Third trophic level
(d)Fourth trophic level
4.Which of the following, limits the number of trophic levels in a food chain
(a)Decrease in energy at higher trophic levels
(b)Less availability of food
(c)Polluted air
5.The decomposers are not included in the food chain. The correct reason for the same is because decomposers
(a) Act at every trophic level at the food chain
(b) Do not breakdown organic compounds
(c) Convert organic material to inorganic forms
(d) Release enzymes outside their body to convert organic material to inorganic forms
12.Shyam participated in a group discussion in his inter school competition on the practical application of light and was very happy to win an award for his school. That very evening his father gave treat to celebrate Shyam’s win. Shyam while sitting saw an image of a person sitting at his backside in his curved plate and could see that person’s mobile drop in the flower bed. Person was not aware until Shyam went and informed him. He thanked Shyam for his clever move.
a)From which side of his plate Shyam observed the incident –
i)outward curved
ii)inward curved
iii)plane surface
b)Part of plate from which Shyam observed the incident acted like a-
i)concave mirror
ii)convex mirror
iii)plane mirror
c)The nature of the size of the image formed in above situation is –
i)real, inverted and magnified
ii)same size , laterally inverted
iii)virtual, erect and diminished
iv)real , inverted and diminished
d)Magnification of the image formed by convex mirror is –
more than 1
iii)equal to 1
iv)less than 1
- The location of image formed by a convex lens when the object is placed at infinity is
(a) at focus
(c) at optical center
- When the object is placed at the focus of concave lens, the image formed is
(a)real and smaller
(b) virtual and smaller
(c) virtual and inverted
- The size of image formed by a convex lens when the object is placed at the focus ofconvex lens is
(a) highly magnified
(b) point in size
- When the object is placed at 2F in front of convex lens, the location of image is
(b) between F and optical center
(c) at infinity
(d) none of the above
14.One of the wires in domestic circuits supply, usually with a red insulation cover, is called live wire. with black insulation is called neutral wire. The earth wire, which has insulation of green colour, is usually connected to a metal plate deep in the earth near the house appliances that has a metallic body. Overloading contact, in such a situation the current in the circuit abruptly increases. circuit prevents damage to the appliances and the circuit due to overloading.
1 When do we say that an electrical appliance
2 Mention the function of earth wire in electrical line
3 How is an electric fuse connected in a domestic circuit?
4 When overloading and short circuiting are said to occur?
5 What is a live wire?
15.Light of all the colours travel at the same speed in vacuum for all wavelengths. But in any transparent medium(glass or water), the light of different colours travels at different speeds for different wavelengths, which means that the refractive index of a particular medium is different for different wavelengths. As there is a difference in their speeds, the light of different colours bend through different angles. The speed of violet colour is maximum and the speed of red colour is minimum in glass so, the red light deviates least and violet colour deviates most. Hence, higher the wavelength of a colour of light, smaller the refractive index and less is the bending of light.
(i)Which of the following statements is correct regarding the propagation of Light of different colours of white light in air?
(a) Red light moves fastest.
(b) Blue light moves faster than green light.
(c) All the colours of the white light move with the same speed.
(d) Yellow light moves with the mean speed as that of the red and the violet light.
(ii)Which of the following is the correct order of wavelength?
(a) Red> Green> Yellow
(b) Red> Violet> Green
(c) Yellow> Green> Violet
(d) Red> Yellow> Orange
(iii)Which of the following is the correct order of speed of light in glass?
(a) Red> Green> Blue
(b) Blue> Green> Red
(c) Violet> Red> Green
(d) Green> Red> Blue
(iv)Which colour has maximum frequency?
16.The region around a magnet where magnetism acts is represented by the magnetic field.The force of magnetism is due to moving charge or some magnetic material. Like stationary charges produce an electric field proportional to the magnitude of charge, moving charges produce magnetic fields proportional to the current. In other words, a current carrying conductor produces a magnetic field around it. The subatomic particles in the conductor, like the electrons moving in atomic orbitals, are responsible for the production of magnetic fields. The magnetic field lines around a straight conductor (straight wire) carrying current are concentric circles whose centres lie on the wire.
1)The magnetic field associated with a current carrying straight conductor is in anti- clockwise direction. If the conductor was held horizontally along east west direction,what is the direction of current through it?
2)Name and state the rule applied to determine the direction of magnetic field in a straight current carrying conductor.
3)Ramus performs an experiment to study the magnetic effect of current around a current carrying straight conductor with the help of a magnetic compass. He reports that
a)The degree of deflection of magnetic compass increases when the compass is moved away from the conductor.
b)The degree of deflection of the magnetic compass increases when the current through the conductor is increased.
Which of the above observations of the student appears to be wrong and why?
Case Study Questions Class 10 Science CBSE Chapter Wise PDF
Related resources to prepare for cbse 10th science board exam 2024.
- CBSE class 10 Science syllabus 2024
- NCERT Book for Class 10th Science 2023-2024 (PDF)
- NCERT Solutions for Class 10 Science
- CBSE Class 10 Science sample paper
- Previous Year Questions of CBSE Class 10 Science
- CBSE Class 10 Science Important Questions and Answers
- CBSE Class 10 Physics Chapter Wise Important Questions and Answers
- CBSE Class 10 Chemistry Chapter Wise Important Questions and Answers
- CBSE Class 10 Biology Chapter Wise Important Questions and Answers
- CBSE Class 10 Science Topper Answer Sheet
- CBSE Class 10 Science Practice Paper 2023 with Answers
- Class 10 CBSE Admit Card 2023-24
- CBSE Class 10 Date Sheet 2023
- CBSE Class 10 Syllabus 2023 - 2024
- CBSE Class 10 DELETED Syllabus 2023-24
- CBSE Class 10th Sample Paper 2022-23: Download Sample Question Papers and Marking Scheme
- CBSE Class 10 Previous Year Question Papers for 2022-23
- CBSE Class 10 Important Questions and Answers for 2023-24 of ALL Chapters
- CBSE Class 10 Practice Papers: All Subjects
- CBSE Topper Answer Sheet Class 10: Model Answer Paper Download PDF
- CBSE Class 10 Mock Tests: All Subjects
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- Tripura Board 10th, 12th Result 2024
- Meghalaya SSLC Result 2024 Roll Number
- MBOSE Meghalaya 10th, 12th Arts Result 2024
- megresults.nic.in Result 2024
- MBOSE HSSLC Arts Result 2024
- MBOSE SSLC Toppers List 2024
- megresults.nic.in Arts Result 2024
- tbse.tripura.gov.in Result 2024
- TBSE 10th, 12th Toppers List 2024
- CBSE Class 10 QnA
Latest Education News
Find 3 Differences In 33 Seconds In This Windy Scene
CHSE Odisha 12th Result 2024 On May 26, Check Odisha 12th Results at orissaresults.nic.in
Purple Cap in IPL 2024: Top Players List with Most Wickets in TATA IPL
Orange Cap in IPL 2024: Top Players List with Most Runs in TATA IPL
IPL Final 2024: KKR vs SRH Match Date, Stadium, Venue, Tickets Price and How to Book Ticket Online
INMA Elects 12 Media Executives to Board of Directors | CEO JNM Bharat Gupta Among Board Members
Who Won Yesterday IPL Match: SRH vs RR, Qualifier 2, Check All Details and Latest Points Table
IPL 2024 Full Schedule: आईपीएल 2024 का फाइनल चेन्नई में, KKR और SRH के बीच IPL 2024 का Final
IPL Points Table 2024: आईपीएल 2024 Updated पॉइंट टेबल यहां देखें, KKR और SRH के बीच IPL 2024 का Final
(Updated) T20 World Cup 2024 All Team Squads and Players List
Pakistan T20 World Cup Squad 2024: Complete List of Team Players and Name
Pakistan T20 World Cup Squad 2024: पाक की विश्व कप टीम में किसे मिला मौका कौन हुआ बाहर देखें यहां
Brain Teaser IQ Test: You have high IQ if you can find the fake millionaire in 5 seconds!
SSC JE Application Status 2024: जारी हुआ जेई परीक्षा का एप्लीकेशन स्टेटस, ssc.gov.in से करें डाउनलोड
UGC NET Syllabus 2024: Download Paper 1 & Paper 2 Syllabus PDF
SSC JE Application Status 2024 OUT: Check Admit Card Updates Here
Dr RML Recruitment 2024 for 255 Junior Resident Posts, Download Notification
Brain Teaser: Can You Find the Imposter Number in this 9 Seconds Challenge?
Postal Ballot: पोस्टल वोटिंग के लिए न्यूनतम आयु सीमा क्या है?
Lok Sabha Election 2024 Dates: अबकी बार 97 करोड़ वोटर्स करेंगे मतदान, फेज-वाइज फुल शेड्यूल यहां देखें

Case Study Questions Class 10 Science Chapter 3 Metals And Non-Metals
- Post author: studyrate
- Post published:
- Post category: class 10th
- Post comments: 0 Comments
CBSE Board Exam is on the way, so you must practice some good Case Study Questions Class 10 Science to boost your preparation to score 95+% on Boards. In this post, you will get Case Study and Passage Based Questions that will come in CBSE Class 10 Science Board Exams.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Re a son . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Metals And Non-Metals Case Study Questions With answers
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 3 Metals And Non-Metals
Case Study/Passage-Based Questions
Question 1:
The arrangement of metals in a vertical column in the decreasing order of their reactivities is called the reactivity series or activity series of metals. The most reactive metal is at the top position of the reactivity series. The least reactive metal is at the bottom of the reactivity series. Hydrogen, though non-metal, has been included in the activity series of metals only for comparison. Apart from it, the hydrogen atom also has a tendency to lose its valence electron and form a cation that behaves like a metal. H→H++e−H→H++e− (i) Which metal can be displaced by copper from its salt solution?
Answer: (b) Silver
(ii) An element ‘X after reacting with acids liberates hydrogen gas and can displace lead and mercury from their salt solutions. The metal ‘X is
Answer: (c) calcium
(iii) the most reactive metal is
Answer: (a) potassium
(iv) The metal which does not liberate hydrogen gas after reacting with acid is
Answer: (d) gold
(v) Which of the following metals does not react with water at all? (I) Sodium (II) Copper (III) Aluminium (IV) Lead
Answer: (c) II and IV only
Case Study 2: Metals and non-metals are two broad categories of elements that exhibit distinct physical and chemical properties. Metals are generally hard, shiny, malleable, and good conductors of heat and electricity. They tend to lose electrons and form positive ions. Common examples of metals include iron, copper, aluminum, and gold. Non-metals, on the other hand, are generally dull, brittle, and poor conductors of heat and electricity. They tend to gain or share electrons and form negative ions. Non-metals include elements such as carbon, sulfur, oxygen, and nitrogen. The periodic table provides a systematic arrangement of elements based on their properties. Metals and non-metals react with each other to form compounds, such as metal oxides and non-metallic chlorides. Understanding the properties and reactivity of metals and non-metals is important in various applications, such as in the construction industry, electrical conductors, and chemical reactions.
What are metals characterized by? a) Dull appearance and poor conductivity b) Shiny appearance and good conductivity c) Brittle nature and negative ions d) Gain or share electrons Answer: b) Shiny appearance and good conductivity
What are non-metals characterized by? a) Dull appearance and poor conductivity b) Shiny appearance and good conductivity c) Brittle nature and negative ions d) Loss of electrons and positive ions Answer: a) Dull appearance and poor conductivity
Which of the following is an example of a metal? a) Carbon b) Sulfur c) Iron d) Oxygen Answer: c) Iron
What property of metals allows them to be shaped into different forms? a) Malleability b) Brittleness c) Ductility d) Conductivity Answer: a) Malleability
How do non-metals generally react with metals? a) Gain or share electrons b) Lose electrons c) Form positive ions d) Have good conductivity Answer: a) Gain or share electrons
Hope the information shed above regarding Case Study and Passage Based Questions for Class 10 Science Chapter 3 Metals And Non-Metals with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 10 Science Metals And Non-Metals Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible. By Team Study Rate
You Might Also Like
Extra questions of class 10 science chapter 8 how do organisms reproduce pdf download, case study questions class 10 science chapter 10 light reflection and refraction, mcq class 10 social science economics consumer rights quiz with answers, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
myCBSEguide
- Case Study Questions Class...
Case Study Questions Class 10 Science
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Download Case study questions for CBSE class 10 Science in PDF format from the myCBSEguide App . We have the new pattern case study-based questions for free download. Class 10 Science case study questions
This article will guide you through:
What are case study questions?
- Sample Papers with Case Study questions
- Class 10 Science Case Study question examples
- How to get case-based questions for free?
- How to attempt the case-based questions in Science?
Questions based on case studies are some real-life examples. The questions are asked based on a given paragraph i.e. Case Study. Usually, 4-5 questions are asked on the basis of the given passage. In most cases, these are either MCQs or assertion & reason type questions. Let’s take an example to understand. There is one paragraph on how nitrogen is generated in the atmosphere. On the basis of this paragraph, the board asks a few objective-type questions. In other words, it is very similar to the unseen passages given in language papers. But the real cases may be different. So, read this article till the end to understand it thoroughly.
What is CBE?
CBSE stands for competency-based education. The case study questions are part of this CBE. The purpose of CBE is to demonstrate the learning outcomes and attain proficiency in particular competencies.
Questions on Real-life Situations
As discussed the case study questions are based on real-life situations. Especially for grade 10 science, it is very essential to have the practical knowledge to solve such questions. Here on the myCBSEguide app, we have given many such case study paragraphs that are directly related to real-life implications of the knowledge.
Sample Papers with Case Study Questions
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App . There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions. In some cases, you will find that the question is not asked directly from the passage but is based on the concept that is discussed there. That’s why it is very much important to understand the background of the case study paragraph.
CBSE Case Study Sample Papers
You can download CBSE case study sample papers from the myCBSEguide App or Student Dashboard. Here is the direct link to access it.
Case Study Question Bank
As we mentioned that case study questions are coming in your exams for the last few years. You can get them in all previous year question papers issued by CBSE for class 1o Science. Here is the direct link to get them too.
Class 10 Science Case Study Question Examples
As you have already gone through the four questions provided in the CBSE model question paper , we are proving you with other examples of the case-based questions in the CBSE class 10 Science. If you wish to get similar questions, you can download the myCBSEguide App and access the Sample question papers with case study-type questions.
Case-based Question -1
Read the following and answer any four questions: Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. This is the salt that is used in food. Some salt is called rock salt bed of rack salt was formed when seas of bygone ages dried up. The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, and bleaching powder.
- Phosphoric acid
- Carbonic acid
- Hydrochloric acid
- Sulphuric acid
- Blue vitriol
- Washing soda
- Baking soda
- Bleaching powder
Case-based Question -2
- V 1 + V 2 + V 3
- V 1 – V 2 +V 2
- None of these
- same at every point of the circuit
- different at every point of the circuit
- can not be determined
- 20 3 Ω 203Ω
- 15 2 Ω 152Ω
Case-based Question -3
- pure strips
- impure copper
- refined copper
- none of these
- insoluble impurities
- soluble impurities
- impure metal
- bottom of cathode
- bottom of anode
How to Attempt the Case-Based Questions in Science?
Before answering this question, let’s read the text given in question number 17 of the CBSE Model Question Paper.
All living cells require energy for various activities. This energy is available by the breakdown of simple carbohydrates either using oxygen or without using oxygen.
See, there are only two sentences and CBSE is asking you 5 questions based on these two sentences. Now let’s check the first questions given there.
Energy in the case of higher plants and animals is obtained by a) Breathing b) Tissue respiration c) Organ respiration d) Digestion of food
Now let us know if you can relate the question to the paragraph directly. The two sentences are about energy and how it is obtained. But neither the question nor the options have any similar text in the paragraph.
So the conclusion is, in most cases, you will not get direct answers from the passage. You will get only an idea about the concept. If you know it, you can answer it but reading the paragraph even 100 times is not going to help you.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- CBSE Practice Papers 2023
- Class 10 Science Sample Papers 2024
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
2 thoughts on “Case Study Questions Class 10 Science”
Where is the answer
Class 10 Science MCQ
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
CBSE Expert
CBSE Class 10 Science Case Study Questions Download Free PDF
If you are looking for the CBSE Class 10 Science Case Study Questions in PDF, then you are in the right place. CBSE 10th Class Case Study for the Science Subject is available here. These Case studies can help the students to solve the different types of questions that are based on the case study.

CBSE Board will be asking case study questions based on Science subjects in the upcoming board exams. Thus, it becomes an essential resource to study.
The Science Subject case study for class 10th covers a wide range of chapters from the Science. Students willing to score good marks in their board exams can use it. The questions are highly interactive and it allows students to use their thoughts and skills to solve such kinds of questions.
Case Study Questions Class 10 Science
In board exams, students will find the questions based on assertion and reasoning . Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
- Case Study Questions for Chapter 1 Chemical Reactions and Equations
- Case Study Questions for Chapter 2 Acids, Bases, and Salts
- Case Study Questions for Chapter 3 Metals and Non-Metals
- Case Study Questions for Chapter 4 Carbon and Its Compounds
- Case Study Questions for Chapter 5 Periodic Classification of elements
- Case Study Questions for Chapter 6 Life Processes
- Case Study Questions for Chapter 7 Control and Coordination
- Case Study Questions for Chapter 8 How do organisms reproduce?
- Case Study Questions for Chapter 9 Heredity and Evolution
- Case Study Questions for Chapter 10 Light reflection and refraction
- Case Study Questions for Chapter 11 Human eye and colorful world
- Case Study Questions for Chapter 12 Electricity
- Case Study Questions for Chapter 13 Magnetic effects of current
- Case Study Questions for Chapter 15 Our Environment
The above Case studies for CBSE Class 10 Science will help you to score good marks in the Case Study questions that have been coming in your examinations. These CBSE Class 10 Science Case Study have been developed by experts of cbseexperts.com for benefit of Class 10 students.
Class 10 Science Assertion and Reason Questions
Case Study Type Questions in Science Class 10
Case Study Type Questions in Science Class 10 include the information or data. Students willing to solve them are required to read the passage carefully and then solve them. While solving the paragraph the ideal way is to highlight the key information or given data.
Because later it will ease them to write the final answers. Science Case study type questions consist of 4 to 5 questions that should be answered in an MCQ manner.
While reading the paragraph students will get the clue in between about the possible answer of the question. They should definitely highlight those questions. This is the best way to solve such kind of Case study Type Questions.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
Talk to our experts
1800-120-456-456
- Important Questions for CBSE Class 10 Science Chapter 3 - Metals and Non-metals 2024-25

CBSE Class 10 Science Chapter-3 Important Questions with Answers - Free PDF Download
The important questions on CBSE Class 10 Science Chapter 3 - Metals and Non-metals have been curated by our expert teachers in a manner that will allow students to explore the different kinds of problems that can be looked into based on the topics under this chapter. Students will get good practice before their exams and will be able to solve most of the problems related to the chapter. The questions and their answers are easy to comprehend and even easier to remember. These questions are most likely to be asked in their Class 10 Science exam since they follow the strict CBSE guidelines and are absolutely aligned with the Class 10 Science syllabus .
Here we discuss the third chapter of science, metals and mon-metals. The chapter discusses different types of metals, non-metals, their differences, reactions with other elements, symbols, and position on the periodic table. important questions for class 10 science chapter 3 metals and non-metals will easily get through the exam.
Vedantu is a platform that provides free CBSE Solutions (NCERT) and other study materials for students. You can download Class 10 Science and Class 10 Maths NCERT Solutions to help you to revise the complete syllabus and score more marks in your examinations.
Download CBSE Class 10 Science Important Questions 2024-25 PDF
Also, check CBSE Class 10 Science Important Questions for other chapters:

Related Chapters
Important Topics under CBSE Class 10 Science Chapter 3 - Metals and Non-metals
Following are the important topics that are covered under the chapter on Metals and Non-metals:
What are Metals?
What are Non-metals?
Properties of Metals
Properties of Non-metals
Difference between Metals and Nonmetals
Study Important Questions for Class 10 Science Chapter 3 – Metals and Non-Metals
Very Short Answer Questions (1 Mark)
1. A mineral is known as ore if metal
Cannot be produced from it
Can be produced from it
Can be extracted from it profitably
Is very costly
Ans: (c) Can be extracted from it profitably
2. The earthy impurities associated with minerals used in metallurgy are called
Ans: (c) Gangue
3. A basic lining is given to a furnace by using
Calcined dolomite
Copper sulphate
Ans: (a) Calcined dolomite
4. Malachite is an ore of:
Ans: (b) Copper
5. Metal always found in free state is:
Ans: (a) Gold
6. A process employed for the concentration of sulphide ore is
Froth floatation
Electrolysis
Bessemerisation
Ans: (a) Froth floatation
7. The slag obtained during the extraction of copper pyrites is composed mainly of
\[C{{u}_{2}}S\]
$FeSi{{O}_{3}}$
$CuSi{{O}_{3}}$
$Si{{O}_{2}}$
Ans: (b) $FeSi{{O}_{3}}$
8. The common method for extraction of metals from the oxide ore is
Reduction with carbon
Reduction with hydrogen
Reduction with aluminium
Electrolytic method
Ans: (a) Reduction with carbon
9. An iron nail was suspended in $CuS{{O}_{4}}$ solution and kept for a while the solution is
Remained blue and coating was found on the nail.
Turned green and a coating was formed on the nail
Remained blue and no coating was formed on the nail
Turned green and no coating was formed on the nail
Ans: (b) Turned green and a coating was formed on the nail
10. The sulphide ore among the following is
Zinc blende
Ans: (d) Zinc blende
11. Chemically rust is
Hydrated ferrous oxide
Hydrated ferric oxide
Only ferric oxide
None of these
Ans: (b) Hydrated ferric oxide
12. Heating pyrites to remove sulphur is called
Calcination
Ans: (d) Roasting
13. Setting of Plaster of Paris takes place due to
Dehydration
Ans: (d) Hydration
14. Some crystals of $CuS{{O}_{4}}$ were dissolved in water. The color of the solution obtained would be
Ans: (c) Blue
15. Most abundant metal on the surface of the earth
Ans: (b) Aluminium
16. Zone refining is used for the
Concentration of an ore
Reduction of metal oxide
Purification of metal
Purification of an ore
Ans: (c) Purification of metal
17. Which of the following processes is used for the concentration of Bauxite $(A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O)$
Magnetic separation
Ans: (b) Leaching
18. During smelting, an additional substance is added which combines with impurities to form a fusible product. It is known as –
Ans: (d) Flux
19. The luster of a metal is due to
Its high density
Its high polishing
Its chemical inertness
Presence of free electrons.
Ans: (d) Presence of free electrons.
20. In the thermite process, the reducing agent is
Ans: (d) Aluminium
21. In addition to iron, stainless steel contains:
Nickel and Chromium
Copper and Tin
Aluminium and Magnesium
Carbon and Magnesium
Ans: (a) Nickel and Chromium
22. The correct decreasing order of the metals in the activity series is:
$Ca,Mg,Ni,Fe$
$Ni,Ca,Mg,Fe$
$Ca,Mg,Fe,Ni$
$Mg,Ca,Fe,Ni$
Ans: (c) $Ca,Mg,Fe,Ni$
23. Which of the following oxides is amphoteric in nature?
$N{{a}_{2}}O$
$A{{l}_{2}}{{O}_{3}}$
Ans: (d) $A{{l}_{2}}{{O}_{3}}$
24. A student adds one big iron nail each in four test tubes containing a solution of zinc sulphate, aluminium sulphate, copper sulphate, and iron sulphate. A reddish-brown coating was observed only on the surface of the iron nail which was added in the solution of:
Zinc Sulphate
Iron Sulphate
Copper Sulphate
Aluminium Sulphate
Ans: (c) Copper Sulphate
25. An iron nail was kept in a solution kept in a test tube. After half an hour it was observed that the color of the solution was changed. The solution in the test tube was that of:
26. Name two metals that are found in nature in the free state.
Ans: Gold and Platinum
27. What chemical process is used for obtaining a metal from its oxide?
Ans: The chemical process that is used for obtaining a metal from its oxide is called reduction. Here, the oxide of the metal will be reduced to the metal with the help of reducing agents or with substitution reactions with another highly reactive metal.
28. Which of the following pairs will give displacement reactions?
$NaCl$ solution and copper metal
$MgC{{l}_{2}}$ solution and aluminum metal
$FeS{{O}_{4}}$ solution and silver metal
$AgN{{O}_{3}}$ solution and copper metal
Ans: $AgN{{O}_{3}}$ solution and copper metal
29. Which of the following method is suitable for preventing an iron fry pan from rusting?
Applying grease
Applying paint
Applying a coating of zinc
All of the above
Ans: (c) Applying a coating of zinc
30. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
Ans: (a) Calcium
31. Food cans are coated with tin and not zinc because
Zinc is costlier than tin
Zinc has higher melting point
Zinc is more reactive than tin
Zinc is less reactive than tin
Ans: (c) Zinc is more reactive than tin
32. What types of oxides are formed when non-metals combine with oxygen?
Ans: When non-metals combine with oxygen they result in the formation of acidic oxides.
33. Royal water is prepared by mixing two acids ‘A’ and ‘B’. It can dissolve gold and platinum. It is a highly corrosive and fuming liquid. Identify ‘A’ and ‘B’. What is the ratio in which ‘A’ and ‘B’ are mixed?
Ans: Acid ‘A’ is Hydrochloric Acid $(HCl)$ and acid ‘B’ is Nitric Acid $(HN{{O}_{3}})$. The ratio in which ‘A’$(HCl)$and ‘B’$(HN{{O}_{3}})$ are mixed is $3:1$.
Short Answer Questions (2 Marks)
1. Which gas is produced when a metal reacts with dilute hydrochloric acid? Write the chemical reaction when iron reacts with dilute ${{H}_{2}}S{{O}_{4}}$.
Ans: When a metal reacts with dilute hydrochloric acid, hydrogen gas $({{H}_{2}})$ is produced.
The chemical reaction when iron reacts with dilute ${{H}_{2}}S{{O}_{4}}$ is as follows:
$Fe(s)+2HCl(dil)\to FeC{{l}_{2}}(aq)+{{H}_{2}}(g)$
This reaction involves the formation of ferric chloride salt $(FeC{{l}_{2}})$ and the liberation of hydrogen gas $({{H}_{2}})$.
2. What would you observe when Zinc is added to a solution of Iron (II) sulphate? Write chemical reaction that takes place.
Ans: When Zinc is added to a solution of Iron (II) sulphate, the color of the solution changes from green to colorless. This happens because, zinc being more reactive than iron, displaces iron from iron sulphate and form the colorless zinc sulphate solution, and the iron gets precipitated at the bottom, in the form of a grey-colored precipitate.
The chemical reaction when Zinc is added to a solution of Iron (II) sulphate is:
$Zn(s)+FeS{{O}_{4}}(aq)\to ZnS{{O}_{4}}(aq)+Fe(s)\downarrow $
3. Why do ionic compounds have high melting points?
Ionic compounds have a high melting point because of the strong forces of attraction between the oppositely charged cations and anions.
Typically, ionic compounds have an equal number of cations and anions that are tightly packed and arranged in a three-dimensional lattice to form their crystalline structures, which also attributes to their high melting points.
4. Why sodium is kept immersed in kerosene oil?
Ans: Sodium is highly reactive in nature. It reacts vigorously with both air and water and burns. So it is kept immersed in kerosene oil to avoid contact with air and water.
5. State two ways to prevent the rusting of iron.
Ans:
(i). By coating the surface of iron with oil, grease, or paint
(ii). By depositing a layer of zinc on the surface of iron, through the process of galvanization.
6. What type of oxide ore is formed when non-metals combine with oxygen?
Ans: The type of oxide formed when non-metals combine with oxygen is acidic in nature. When these oxides are dissolved in water, the resulting solution turns blue litmus red.
Example – $C+{{O}_{2}}\xrightarrow{heat}C{{O}_{2}}$
7. What are amphoteric oxides? Give examples?
Ans: Amphoteric oxides are oxides that behave as both acidic and basic oxides. They can neutralize both acids and bases.
They undergo neutralization reaction to form water and salt when reacting with acid and form complex salts and water when reacting with base.
Examples – Aluminium oxide $(A{{l}_{2}}{{O}_{3}})$ and Zinc Oxide $(ZnO)$
8. Name two metals that can displace hydrogen from dilute acids and two metals which cannot do so?
Metals that can displace hydrogen from dilute acids are Sodium and Calcium.
Metals that cannot displace hydrogen from dilute acids are Copper and Silver.
9. Give the reason why platinum, gold, and silver are used to make jewelry.
Platinum, gold, and silver are used to make jewelry due to their low reactivity. These metals are placed at the bottom of the activity series and are also called noble metals.
They do not corrode when exposed to air, water, or chemicals. Thus they do not lose their shine and have a bright luster, which makes them suitable for making jewelry.
These metals are also highly malleable and ductile, so they can be shaped and designed as required.
10. Why copper is used to make hot water tanks and not steel?
Ans: Copper is used to make hot water tanks, rather than steel because it is a good conductor of heat than steel. Also copper does not react with cold or hot water, or even with steam, while the iron component of steel (steel is an alloy of iron), reacts with steam and forms ferrous oxide. This makes the steel tank weak. Thus copper is used to make hot water tanks and not steel.
11. Can all minerals of a metal act as ores? Justify.
Ans: Ores are minerals that have a higher concentration of certain elements, while a mineral is a naturally occurring inorganic solid with definite chemical composition and crystalline structure.
A mineral can be considered as an ore when a metal can be extracted commercially from that mineral. But not all minerals are ores, because they can contain unwanted substances as well. Thus we can say that all ores are minerals, but not all minerals are ores.
12. How does Galvanization check rusting of iron?
Ans: Galvanization is the method of coating the surface of the iron with a thin layer of zinc preventing iron from coming in contact with moisture.
In the activity series zinc lies above iron, so it acts as a sacrificial metal, i.e. it oxidizes instead of iron when exposed to the moisture, thus prevents iron from rusting.
13. Metals are arranged in the reactivity series. Why hydrogen is kept in the series though it is not a metal?
Ans: Hydrogen being a non-metal is placed in the reactivity series because its reactivity is similar to that of an electropositive element, say metals i.e. can lose electrons to form positive ions. Since hydrogen has one electron in its valance shell, it can lose its electron and become electropositive $({{H}^{+}})$.
14. Why are metals generally lustrous?
Ans: Metals are generally lustrous because of the flow of free electrons. These electrons can move freely through the metal, which can absorb the photons from light falling on their surface. After absorption, these electrons then release the energy as light, making the metal lustrous.
Also, the free electrons can reflect the light falling on them in the form of diffuse reflection, making the metal surface appear shiny.
15. Corrosion of metals is not always harmful. Illustrate.
Ans: Corrosion of metals is a harmful process that results in the destruction of metal. But in certain cases, corrosion is actually advantageous.
Consider the case of corrosion of aluminium. When aluminium corrodes, it forms a layer of aluminium oxide \[(A{{l}_{2}}{{O}_{3}})\] over the metal. This oxide layer deposited on its surface acts as a protective coating and prevents further corrosion of the metal from the attacks of water, air, acids, or alkalis.
16. Why does copper not liberate hydrogen on reacting with dilute sulphuric acid?
Ans: Copper does not liberate hydrogen on reaching with dilute sulphuric acid because copper lies below hydrogen in the activity series. Due to this, copper cannot displace hydrogen from the acid, i.e. it cannot lose electrons to ${{H}^{+}}$ions and liberate hydrogen gas as other metals do.
17. Why are non-metals gaseous at room temperature?
Ans: The reason why non-metals are gaseous at room temperature is because of their mostly filled electronic structure. In their molecular form, non-metals exist as monoatomic or diatomic molecules like ${{H}_{2}},{{O}_{2}},{{N}_{2}},C{{l}_{2}},C{{O}_{2}}$, etc. Their atoms are covalently bonded in a molecule, while the intermolecular forces of attraction are weak that can be easily overcome at room temperature. Therefore, they exist as gases at room temperature.
18. Both calcium and magnesium are heavier than water but still float over them. Explain.
Ans: The density of Calcium is $1.74g/cc$ and that of Magnesium is $1.55g/cc$ while the density of water is $1.0g/cc$ at room temperature. And yet, both metals float over the water surface. It is because they react with water to produce metal-hydroxide, $Ca{{(OH)}_{2}},Mg{{(OH)}_{2}}$ respectively, and hydrogen gas $({{H}_{2}})$. The hydrogen gas, in the form of bubbles, sticks on the metal surface and makes them float above water.
19. What is thermit reaction?
Ans: Thermit reactions are highly exothermic displacement reactions between a metal and metal oxide. The heat released in this exothermic reaction is so large, that the metal is obtained in its molten state.
The most common thermit reaction is between ferric oxide with aluminium metal, which is used to join railway tracks or cracked machine parts.
Reaction – $F{{e}_{2}}{{O}_{3}}(s)+2Al(s)\xrightarrow{heat}2Fe(l)+A{{l}_{2}}{{O}_{3}}(s)+heat$
20. Write the equation for the reaction of
(i). Iron with steam
(ii). Calcium and potassium with water
(i). Iron with steam – $3Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+{{H}_{2}}$
(ii). Calcium with water – \[Ca+2{{H}_{2}}O\to Ca{{(OH)}_{2}}+{{H}_{2}}\]
Potassium with water – \[2K+2{{H}_{2}}O\to 2KOH+{{H}_{2}}\]
21. Which gas is produced when dilute hydrochloric acid is added to reactive metal?
Ans: Hydrogen gas is produced when dilute hydrochloric acid is added to a reactive metal.
Example – Iron reacts with dilute hydrochloric acid to produce iron sulphate and hydrogen gas.
$Fe+2HCl\to FeC{{l}_{2}}+{{H}_{2}}\uparrow $
22. Which metals do not corrode easily?
Ans: Noble metals which are at the bottom of activity series like silver, gold, and platinum do not corrode easily.
23. What are alloys?
Ans: An alloy is a homogeneous mixture of two or more metals, or a metal and a nonmetal. It is prepared by first melting the primary metal, and then, dissolving the other elements in it in definite proportions. It is then cooled to room temperature.
Examples – Brass is an alloy of copper and zinc (Cu and Zn), and bronze is an alloy of copper and tin (Cu and Sn).
24. In the electrolytic refining of a metal M, what would you take as the anode, the cathode, and the electrolyte?
Anode – a rod of impure metal M.
Cathode – thin strips of pure metal M.
Electrolyte – the salt solution of metal M.
25. Metal acts as a good reducing agent. It reduces $F{{e}_{2}}{{O}_{3}}$and $Mn{{O}_{2}}$. The reaction with $F{{e}_{2}}{{O}_{3}}$ is used for welding broke railway tracks. Identify & the metal and write all the chemical reactions.
Ans: The metal that acts as a good reducing agent, reducing $F{{e}_{2}}{{O}_{3}}$and $Mn{{O}_{2}}$ is Aluminum.
The thermite reaction between ferric oxide $F{{e}_{2}}{{O}_{3}}$ with aluminium metal is used for welding broke railway tracks.
Reactions –
$F{{e}_{2}}{{O}_{3}}(s)+2Al(s)\xrightarrow{heat}2Fe(l)+A{{l}_{2}}{{O}_{3}}(s)+heat$
$3Mn{{O}_{2}}(s)+4Al(s)\xrightarrow{heat}Mn(l)+2A{{l}_{2}}{{O}_{3}}(s)+heat$
26. A yellow-colored powder ‘X’ is soluble in carbon disulfide. It burns with a blue flame, forming suffocating smelling gas which turns moist blue litmus red. Identify ‘X’ and gives a chemical reaction. Identify whether it is a metal or non-metal.
Ans: The yellow-colored powder ‘X’ is sulfur.
Sulfur is soluble in carbon disulfide.
Sulfur burns in oxygen with a blue flame to form sulfur dioxide, which has a suffocating smell.
Reaction – $S(s)+{{O}_{2}}(g)\to S{{O}_{2}}(g)$
$S{{O}_{2}}$ turns moist blue litmus red, as it is acidic in nature.
Sulfur is a non-metal.
27. A student set up an electric circuit as shown in Fig. He placed the metal to be tested in the circuit between terminals A and B as shown.
(Image will be uploaded soon)
(i). Does the bulb glow? What does this indicate?
(ii). Why are electric wires coated with rubber-like materials?
(i). Yes, the bulb glows when a metal is placed between terminals A and B. This indicates that metal is a good conductor of electricity.
(ii). Electric wires are coated with rubber-like materials because rubber is a poor conductor of electricity/ it acts as an insulator, which protects the person from getting electric shock due to current flow.
28. A, B and C are 3 elements that undergo chemical reactions according to the following equations:
(a) ${{A}_{2}}{{O}_{3}}+2B\to {{B}_{2}}{{O}_{3}}+2A$
(b) $3CS{{O}_{4}}+2B\to {{B}_{2}}{{(S{{O}_{4}})}_{3}}+3C$
(c) $3CO+2A\to {{A}_{2}}{{O}_{3}}+3C$
The answer of the following:
(i). Which element is most reactive?
(ii). Which element is least reactive?
Ans: Hint – a more reactive element will replace a lesser reactive element.
(i). From reactions (a) and (b), we can say that the most reactive element is B because it can replace both A and C from their compounds.
(ii). From reactions (b) and (c), we can say that element C is the least reactive as it has been replaced both by A and B.
29. An element X on reacting with ${{O}_{2}}$ forms the oxide$(X{{O}_{2}})$. This oxide dissolves in water and turns blue litmus paper red. Predict the nature of the element whether it is a metal or a non-metal.
Ans: The oxide is acidic in nature because it turns blue litmus to red.
Thus element X is a non-metal.
30. An element E combines with ${{O}_{2}}$ to form an oxide${{E}_{2}}O$, which is a good conductor of electricity. Answer the following:
(i). How many electrons will be present in the outermost shell of E?
(ii). Write the formula of the compound formed when it combines with Chlorine.
(i) The number of electrons in the outermost shell of element E is $1$. Here, the valency of oxygen is $2$. Since the oxide formed is ${{E}_{2}}O$, it means that the valency of E is$1$, i.e. E has only one electron in the valence shell.
(ii) It is known that the valency of Chlorine is $1$and since the valency of E is also $1$, the resultant compound is $ECl$.
Reaction – ${{E}^{+}}+C{{l}^{-}}\to ECl$
Short Answer Questions (3 Marks)
1. Arrange the following metals in decreasing order of their reactivity:
(1) $Cu,Ca,Mg,Na,Zn$
(2) You are provided with three metals: sodium, magnesium, and copper. Using only water as the reactant, how will you identify each of them?
(3) Which metal listed in (1) is most likely to occur in the native state?
(1) The decreasing order of reactivity is – Na > Ca > Mg > Zn > Cu
(2) With water as the only reactant we can distinguish Sodium, Magnesium, and Copper:
Sodium reacts vigorously with cold water and burns.
Magnesium is not reactive with cold water, but reacts with hot water and produces hydrogen gas bubbles, and floats to the surface.
Copper remains unaffected or unreactive with neither cold nor hot water.
(3) Copper metal is the one that is most likely to occur in the native state, because it is at the bottom of the activity series, meaning it is least reactive.
2. Which method of concentration of ore is preferred in the following cases and why?
(1) The ore has higher density particles mixed with a large bulk of low-density impurities.
(2) The ore consists of copper sulphide intermixed with clay particles.
(3) Give an example of amalgam.
(1) The concentration of ore can be done by gravity separation method or hydraulic washing, because of the difference in densities, the low-density impurities can be washed away, while the high-density ore particles would settle down.
(2) The concentration of ore is done by the froth floatation process because of the difference in the wetting characteristic of the metal and clay particles with oil and water.
(3) An alloy, with one of the metals as mercury, is called amalgam. An example of amalgam is the mixture of mercury with silver which is used to fill dental cavities.
(a) Why is $ZnO$ called an amphoteric oxide? Name another amphoteric oxide.
(b) What are alkalis? Give one example of alkali.
(a) Amphoteric oxides are oxides that behave as both acidic and basic oxides. They can neutralize both acids and bases.
Zinc oxide (ZnO) is an amphoteric oxide because it behaves both as acidic and basic oxide.
As acidic oxide –
$ ZnO(s)+2NaOH(aq)\to N{{a}_{2}}Zn{{O}_{2}}(aq)+{{H}_{2}}O(l) $
$ ZincOxide(acid)+SodiumHydroxide(alkali)\to SodiumZincate(salt)+water $
As basic oxide –
$ ZnO(s)+2HCl(aq)\to ZnC{{l}_{2}}(aq)+{{H}_{2}}O(l) $
$ ZincOxide(alkali)+HydrochloricAcid(acid)\to ZincChloride(salt)+water $
(b) Alkalis are bases that are completely soluble in water or they are the water-soluble hydroxides of metals.
Examples – Sodium Hydroxide $(NaOH)$
4. You are given a hammer, a battery, a bulb, wires, and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests to distinguish between metals and non-metals.
(a) To distinguish between metals and non-metals –
With a hammer: Checking for malleability.
By beating the sample with a hammer we can find if the sample is a metal or non-metal. Metals exhibit the property of malleability, where they can be beaten into thin sheets, while non-metals being brittle, tend to break.
With a battery, bulb, wires, and switch: Checking for conductivity.
Set up the circuit below.
By placing the sample between terminals A and B, we can find if the sample is metal or non-metal. Metals are good conductors of electricity, while non-metals are not. So if by placing the sample between the terminals the bulb glows, it is a metal; if the bulb does not glow, then it is a non-metal.
(b) These tests are useful in distinguishing between metals and non-metals:
Beating the sample with a hammer, we can check for its malleability, i.e. checking the physical property of the sample. Metals exhibit the property of malleability, where they can be beaten into thin sheets, while non-metals being brittle, tend to break.
Using an electric circuit we can check for the conductivity of the sample to determine whether it is a metal or a non-metal. Metals are good conductors of electricity, while non-metals are not.
5. Name an alloy of
(i). Aluminium is used in the construction of aircraft.
(ii). Lead is used in joining metals for electric work.
(iii). Copper is used in household vessels.
(i). Duralumin is a strong, hard, lightweight alloy of aluminum that is widely used in aircraft construction.
Composition of Duralumin – $Al(95\%),Cu(4\%),Mg(0.5\%),Mn(0.5\%)$
(ii). Solder is an alloy of lead and tin that has a low melting point and is used for welding electrical wires together.
Composition of Solder – $Pb(50\%),Sn(50\%)$
(iii). Brass is an alloy of copper and zinc that is used for making household vessels.
Composition of Brass – $Cu(80\%),Zn(20\%)$
6. What are the three important properties of aluminium which are responsible for its great demand in the industry?
(i). Aluminium is a good conductor of electricity.
(ii). It is not attacked by water, i.e. it is resistant to corrosion.
(iii). It is a powerful reducing agent.
(iv). It is malleable and ductile.
7. Which of the following metals would give hydrogen when added to dilute HCl?
(2) Copper
(3) Magnesium
Iron and magnesium would liberate hydrogen gas on reacting with dilute HCl. These are active metals that are placed above hydrogen in the activity series.
Reaction –
$Mg+2HCl\to MgC{{l}_{2}}+{{H}_{2}}\uparrow $
Since copper is placed below hydrogen in the activity series, it has lower reactivity and will not evolve hydrogen gas when added to dilute HCl.
\[Cu+HCl\to (No-reaction)\]
8. Define an alloy and an amalgam. State the main constituents of the following alloys – Stainless steel, Bronze. In which property, each of them is different from its main constituent?
An alloy is a homogeneous mixture of two or more metals, or a metal and a nonmetal. It is prepared by first melting the primary metal, and then, dissolving the other elements in it in definite proportions. It is then cooled to room temperature.
Examples of Alloys – Brass is an alloy of copper and zinc (Cu and Zn), and bronze is an alloy of copper and tin (Cu and Sn).
An alloy, with one of the metals as mercury, is called amalgam.
An example of amalgam is the mixture of mercury with silver which is used to fill dental cavities.
Main constituents of:
Stainless steel – Stainless steel is an alloy of iron, chromium, carbon, and nickel. It exhibits a higher level of resistance to corrosion by rust formation than compared to its major constituent iron.
Bronze – Bronze is an alloy of copper and tin (Cu and Sn). It has a lower electrical conductivity than its constituents and is less malleable than copper.
9. A group of students looked at different metals and metal sulphate solutions are given in a tabular form. From the data, answer the following:
(a) Which metal reacted with all other sulphate solutions?
(b) Which metal did not react with any other metal sulphate solution?
(c) Arrange the metals in decreasing order of reactivity.
(a) Magnesium (Mg) reacted with all other sulphate solutions.
(b) Copper (Cu) did not react with any other metal sulphate solution.
(c) The decreasing order of reactivity of metals is Mg > Cr > Co > Cu.
10. Choose the appropriate element from the following:
(1) A metal that gets covered with a protective film of its oxide (Al, Cu, Ag).
(2) A metal that burns in the air with golden flame (Zn, K, Na).
(3) A metal that can displace hydrogen from boiling water as well as steam (K, Zn, Fe).
(1) Aluminium (Al)
(2) Sodium (Na)
(3) Zinc (Zn)
11. Write one point of difference between electrolytic reduction and reduction with carbon. Give one example of each.
Ans: In the case of electrolytic reduction, electrolysis is used for reduction, i.e. the reduction takes place at the cathode by the gain of electrons during electrolysis.
Example –
$ NaCl(molten)\xrightarrow{electrolysis}N{{a}^{+}}+C{{l}^{-}} $
$ Cathode-N{{a}^{+}}+{{e}^{-}}\to Na(reduction) $
While reduction with carbon, carbon acts as the reducing agent, i.e. reduction is carried out by heating a metal oxide with coke.
$ZnO+C(coke)\xrightarrow{heat}Zn+CO$
12. Write the equation for the reaction of
(a) Iron with steam.
(b) Calcium with water.
(c) Potassium with water.
Ans:
$3Fe(s)+4{{H}_{2}}O(g)\to F{{e}_{3}}{{O}_{4}}(s)+{{H}_{2}}(g)$
$Ca(s)+2{{H}_{2}}O(l)\to Ca{{(OH)}_{2}}(aq)+{{H}_{2}}(g)$
$2K(s)+2{{H}_{2}}O(l)\to 2KOH(aq)+{{H}_{2}}(g)+heat$
13. Define the following terms:
(a) Minerals – Minerals are substances that are formed naturally on Earth. They are usually solid and inorganic with a crystal structure and are formed naturally by geological processes. These are combined states of metals with other materials like soil, sand, rocks, etc.
(b) Ores – Ores are minerals that have a higher concentration of a certain element. A mineral can be considered as an ore when a metal can be extracted commercially and profitably from that mineral.
(c) Gangue – Gangue is the earthy impurities such as mud, sand, clay, rock, or any other material that is associated with ores.
14. Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it. What will be the action of gas in –
(1) Dry litmus paper?
(2) Moist litmus paper?
Write a balanced chemical equation for the reaction taking place.
Sulfur burns in oxygen with a blue flame to form sulfur dioxide $S{{O}_{2}}$, which has a suffocating smell.
Balanced Chemical Equation – $S(s)+{{O}_{2}}(g)\to S{{O}_{2}}(g)$
(1) $S{{O}_{2}}$ with dry litmus paper – $S{{O}_{2}}$in its gaseous state does not change the color of dry litmus.
(2) $S{{O}_{2}}$ with moist litmus paper –$S{{O}_{2}}$ turns moist blue litmus red, as it forms sulfurous acid with water, which is acidic in nature.
Balanced Chemical Equation – $S{{O}_{2}}(g)+{{H}_{2}}O(aq)\to {{H}_{2}}S{{O}_{3}}(aq)$
15. Write any three differences between metals and non-metals on the basis of chemical properties?
16. Why is titanium metal called strategic metal? Mention two of its properties that make it so special.
Ans: Titanium metal is called strategic metal because it is used in the production of spacecraft, aircraft and missiles, and other war equipment.
Properties that make titanium special are:
(i) It is light in weight but at the same time stronger than the other metals.
(ii) It is not affected by corrosion even after being exposed to the atmosphere for a long duration.
17.
(a) What is corrosion?
(b) How is corrosion caused?
(c) Complete the reaction – $2Fe+\dfrac{3}{2}{{O}_{2}}+x{{H}_{2}}O\to $
(a) Corrosion is the process that causes damage to the metal, due to the eating up of the surface of the metal when kept exposed to air and moisture for a long time. It is a natural process that results in the transformation of pure metals into undesirable substances when they react with air and water.
(b) Corrosion is mainly caused by a chemical or electrochemical reaction of the metal with its environment that results in its gradual destruction. It converts the metal into a more chemically stable oxide or hydroxide or sulphide.
Example – Copper metal reacts with moist carbon dioxide in the air and results in the formation of a green coat of copper carbonate over the metal surface.
$ 2Cu+{{H}_{2}}O+C{{O}_{2}}+{{O}_{2}}\to Cu{{(OH)}_{2}}.CuC{{O}_{3}} $
$ Copper+AtmosphericGases\to CopperCarbonate(green) $
(c) $2Fe+\dfrac{3}{2}{{O}_{2}}+x{{H}_{2}}O\to F{{e}_{2}}{{O}_{3}}.x{{H}_{2}}O$
Here, $F{{e}_{2}}{{O}_{3}}.x{{H}_{2}}O$ is hydrated ferric oxide, i.e. rust.
(1) Choose metal from the reactivity series which will not react with steam.
(2) Choose one metal that will safely react with dilute sulphuric acid.
(3) Name the salt formed when metal chosen in (2) reacts with sulphuric acid.
(1) Gold (Au)
(2) Zinc (Zn)
(3) The salt formed is zinc sulphate $ZnS{{O}_{4}}$and it is colorless.
19. A copper plate was dipped into a solution of $AgN{{O}_{3}}$. After some time a black layer was deposited on the copper plate. State the reason for it. Write the chemical equation for the reaction involved.
Ans: When a copper plate is dipped in a solution of silver nitrate $AgN{{O}_{3}}$, a black layer of silver will be deposited over the copper plate. This is because copper is above silver in the activity series, meaning copper is more reactive than silver. Thus, a displacement reaction occurs between copper (more reactive) and silver (less reactive), that copper displaces silver from its solution $AgN{{O}_{3}}$ and forms copper nitrate $Cu{{(N{{O}_{3}})}_{2}}$. This results in the deposition of silver over the plate that can be seen as a black layer.
Reaction – $Cu(s)+2AgN{{O}_{3}}(aq)\to Cu{{(N{{O}_{3}})}_{2}}(aq)+2Ag(s)$
20. Give an example of metal which
(i). is a liquid at room temperature.
(ii). can be easily cut with a knife.
(iii). is the best conductor of heat.
(iv). is a poor conductor of heat.
(i). Mercury
(ii). Sodium
(iii). Silver
21. Explain the meaning of malleable and ductile.
Malleable – The ability of metals to be beaten into thin sheets is called malleability.
Example: Iron, Copper.
Ductile – The ability of metals to be drawn into thin wires is called ductility.
Example: Gold, Silver.
(i). Write the electron-dot structures for sodium, oxygen, and magnesium.
(ii). Show the formation of $N{{a}_{2}}O$and $MgO$ by the transfer of electrons.
(iii). What are the ions present in these compounds?
(iii) Ions present in $N{{a}_{2}}O$ are $N{{a}^{+}}$ and ${{O}^{2-}}$
Ions present in $MgO$ are $M{{g}^{2+}}$ and ${{O}^{2-}}$
23. Metallic oxides of zinc, magnesium, and copper were heated with the following metals:
In which case will you find displacement reactions taking place?
Ans: Based on the activity series of metals, the displacement reactions will take place as below:
24. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Ans: Copper metal reacts with moist carbon dioxide in the air and results in the formation of a green coat of copper carbonate over the metal surface. Thus it loses its shiny brown appearance.
$2Cu+{{H}_{2}}O+C{{O}_{2}}+{{O}_{2}}\to Cu{{(OH)}_{2}}.CuC{{O}_{3}} $
To remove the green layer of copper carbonate, lemon juice or tamarind juice is used. The citric acid and/or tartaric acid present in these juices dissolves the copper carbonate and converts it into soluble copper citrate or copper tartrate, which can be easily removed from the surface and hence restoring the shiny brown appearance of copper.
25. A man went door to door posing as a goldsmith. He promised to bring back the glitter of the old and dull ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument, the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Ans: The man used the Aqua-regia solution, which is a $3:1$ ratio mixture of concentrated hydrochloric acid and concentrated nitric acid. This solution has the ability to dissolve gold. Thus, when the man dipped the gold bangles in this solution, it had dissolved a significant amount of gold from the bangles, which explains the drastic loss in weight of the bangles. But by dissolving the outer layer, the inner shiny layer appears that gives off the appearance that the bangles were cleaned.
26. Differentiate between metal and non-metal on the basis of their chemical properties.
Ans: The differences are:
27. An element reacts with oxygen to form an oxide which dissolves in dilute hydrochloric acid. The oxide formed also turns a solution of red litmus blue. Is the element metal or non-metal? Explain with the help of a suitable example.
Ans: The element is metal because the oxides of metals are basic in nature, which can turn red litmus solution blue. Also since the oxide seems to have dissolved in dilute hydrochloric acid, this also suggests that the oxide is basic.
Since metallic oxides are typically basic in nature, we can conclude that the element is a metal.
Example – Consider the metal to be Magnesium.
Reactions –
With oxygen to form an oxide: $2Mg(s)+{{O}_{2}}(g)\to 2MgO(s)$
Oxide dissolving in hydrochloric acid: $MgO(s)+2HCl(aq)\to MgC{{l}_{2}}(aq)+{{H}_{2}}O(l)$
In aqueous conditions, it forms a base that turns red litmus blue:$MgO(s)+{{H}_{2}}O(l)\to Mg{{(OH)}_{2}}$
28. Nikita took Zn, Al, Cu, Fe, Mg, and Na metals, and put each metal in cold water and then hot water. She then reacted the metal with steam.
(i) Name the metal which reacts with cold water.
(ii) Which of the above metals react with steam?
(iii) Name the metal which reacts with hot water
(iv) Arrange these metals in order of increasing reactivity
(ii) Zn, Al, Fe
(iv) Na > Mg > Al > Zn > Fe > Cu
29. A student was given Mg, Zn, Fe, and Cu metals. He put each of them in dilute HCl contained in different test tubes. Identify which of them
(i) will not displace ${{H}_{2}}$ from dilute HCl.
(ii) forms a pale green substance.
(iii) will give ${{H}_{2}}$ with 5% $HN{{O}_{3}}$.
(iv) will be displaced from its salt solution by all other metals.
(i) Cu, because it is less reactive than hydrogen in the activity series.
(ii) Fe, because it forms ferrous chloride in hydrochloric acid.
(iii) Mg gives off hydrogen gas upon reacting with nitric acid.
(iv) Cu, because it has lower activity than compared to the other three metals.
30. A metal ‘X’ is found in the form of filings which burns vigorously when sprinkled on flame. When these filings are treated with sulphur, a black-colored compound ‘Y’ is formed which is not attracted by a magnet. ‘X’ reacts with dilute HCl to liberate hydrogen gas. ‘X’ reacts with steam to form ‘Z’ along with hydrogen gas. Identify ‘X’, ‘Y’, and ‘Z’. Write the reactions involved.
Ans: We can say that X is a metal that can react with steam and with hydrochloric acid to liberate hydrogen gas. Since ‘X’ is magnetic but its sulphide is non-magnetic, we can say that ‘X’ is iron and ‘Y’ is iron sulphide.
When iron reacts with steam it forms ‘Z’, which is iron (ii, iii) oxide.
Iron filings with sulphur: $Fe+S\to FeS$
Iron filings with hydrochloric acid: $Fe+2HCl\to FeC{{l}_{2}}+{{H}_{2}}$
Iron filings with steam: $3Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}$
Thus – X is$Fe$, Y is $FeS$ and Z is $F{{e}_{3}}{{O}_{4}}$.
Long Answer Questions (5 Marks)
(a) Name a metal that does not stick to glass?
(b) Name a non-metal which is a good conductor of electricity?
(c) Name the metal which is commonly used in thermit welding?
(d) What gets deposited at the cathode, a pure or impure metal?
(e) What is the nature of Zinc oxide?
(a) Mercury
(b) Graphite
(c) Aluminum
(d) A pure metal is always deposited at the cathode
(e) Zinc oxide (ZnO) is an amphoteric oxide.
2. Name three common forms in which metals occur in nature. Explain the interaction between metals and dilute acid?
Ans: The three common forms in which metals occur in nature are:
Sulphide form – e.g. copper pyrite $(CuFe{{S}_{2}})$
Oxide form – e.g. Bauxite $(A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O)$
Carbonate form – e.g. Calamine $(ZnC{{O}_{3}})$
Active metals generally interact with dilute hydrochloric acid or dilute sulphuric acid and liberate hydrogen gas. Also, those metals that are below hydrogen in the activity series neither liberate hydrogen gas nor react with the dilute acid.
Example reactions of metals with dilute hydrochloric acid –
3. Sample pieces of five metals A, B, C, D, and E were added to the tabulated solutions separately. The results observed are shown in the table:
Based on the observations recorded in the table, answer the following:
(1) Which is the most reactive metal?
(2) Which is the least reactive metal?
(3) What would be observed if metal D were added to a solution of copper (II) sulphate?
(4) What would be observed if metal E were added to a solution of iron (II) sulphate?
(5) Arrange the metals A, B, C, D, and E in decreasing order to their reactivity?
(1) E is the most reactive because it displaces almost all of the elements from their solutions
(2) C is the least reactive because it does not react with any of the solutions to undergo displacement.
(3) It is clear that D is more reactive than silver but less reactive than iron. Thus it can displace copper from $CuS{{O}_{4}}$
(4) Metal E is more reactive than zinc because it can displace zinc from its solution. Since iron is less reactive than zinc, we can conclude that E would displace iron from $FeS{{O}_{4}}$
(5) The decreasing order of reactivity – E > B > D > A > C.
4. Hydrogen gas is evolved by reacting a piece of magnesium ribbon with water:
(1) Describe how it could be shown that the gas collected is hydrogen.
(2) Write a chemical equation for the reaction taking place between magnesium and water using symbols.
(3) Suggest how the appearance of magnesium would change after a week.
(4) A few drops of universal indicator solution were added to water in the beaker. What color would expect to see and what pH would this color indicate?
(1) If a lighted splint is brought near the collected gas, it will burn very brightly, along with a ‘pop’ sound. This shows that the gas evolved by a magnesium ribbon upon its reaction with water is hydrogen.
(2) The chemical equation for the reaction is: $Mg(s)+2{{H}_{2}}O(l)\to Mg{{(OH)}_{2}}(aq)+{{H}_{2}}(g)\uparrow $
(3) After a week’s time, magnesium will lose all its shine and a deposit of magnesium hydroxide will be formed on the surface of the metal.
(4) The indicator will become blue, indicating that the solution is basic. The pH of the solution would be more than seven.
5. Samples of four metals A, B, C, and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows:
Use the table given above to answer the following questions about metals A, B, C, and D.
(i). Which is the most reactive metal?
(ii). What would you observe if B is added to a solution of Copper(II) sulphate?
(iii). Arrange the metals A, B, C, and D in order of decreasing reactivity.
(i). B is the most reactive metal because it displaces the iron from its solution, which is the most reactive of all the elements.
(ii). It is clear that B is more reactive than iron, and since copper is less reactive than iron, B can displace copper from $CuS{{O}_{4}}$ .
(iii). The decreasing order of reactivity is B > A > C > D.
6. Give reasons:
(a) Platinum, gold, and silver are used to make jewelry.
(b) Sodium, potassium, and lithium are stored under oil.
(c) Aluminum is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
(a) Platinum, gold, and silver are used to make jewelry due to their low reactivity. These metals are placed at the bottom of the activity series and are also called noble metals. They do not corrode when exposed to air, water, or chemicals. Thus they do not lose their shine and have a bright luster, which makes them suitable for making jewelry. These metals are also highly malleable and ductile, so they can be shaped and designed as required.
(b) Sodium, potassium, and lithium are highly reactive in nature. They can react vigorously with both air and water and burn. So they are kept immersed in oil to avoid contact with air and water.
(c) Aluminium, despite being a highly reactive metal is typically used to make cooking utensils because the corrosion of aluminium has an advantage. When aluminium corrodes, it forms a layer of aluminium oxide \[(A{{l}_{2}}{{O}_{3}})\] over the metal. This oxide layer deposited on its surface acts as a protective coating and prevents further corrosion of the metal from the attacks of water, air, acids or alkalis, or even from heat. Also, aluminium is a good conductor of heat, which helps in the cooking process. It is easily available, malleable, and ductile as well. These factors make it suitable for cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because it is easier to extract the metal from its oxide than compared to carbonates and sulphides. This is also economically feasible and profitable.
7. Four metals A, B, C, and D are, in turn, added to the following solutions one by one. The observations made are tabulated below:
Answer the following questions based on the above information.
(i) Which is the most active metal and why?
(ii) What would be observed if B is added to a solution of copper (II) sulfate and Why?
(iii) Arrange the metals A, B, C, and D in order of increasing reactivity.
(iv) Container of which metal can be used to store both zinc sulfate solution and silver nitrate solution.
(v) Which of the above solutions can be easily stored in a container made up of any of these metals?
(i) B is the most reactive metal because it displaces the iron from its solution, which is the most reactive of all the elements.
(ii) It is clear that B is more reactive than iron, and since copper is less reactive than iron, B can displace copper from $CuS{{O}_{4}}$ .
(iii) The decreasing order of reactivity is B > A > C > D.
(iv) Metal D can be used to make containers because out of all four elements, it is the least reactive. Also, it shows no displacement reactions with both zinc sulfate solution and silver nitrate solution.
(v) Zinc sulfate can be easily stored in a container made up of any of these metals, because it shows no reaction with these metals.
Important Questions of Class 10 Science Chapter 3: Download Free PDF
To pass the board test in science, you must have a great deal of insight and information. Because it is a challenging subject, acquiring a thorough comprehension of the chapter is critical. Metals and nonmetals is an important scientific chapter that will help you comprehend the different metals and nonmetals, as well as their physical and chemical properties and distinctions. Students will also be familiar with ionic substances and their characteristics. The chapter also discusses ores, alloys, and their characteristics.
Vedantu provides free key questions and answers on metals and nonmetals in Science chapter three. Students may easily view the document on the Vedantu website and obtain the key questions. Class 10 Chapter 3 is a superior resource for pupils studying for the board test.
Metal And Non-Metal Class 10 Important Questions
Metals and non-metals is an important chapter. It helps the students get a better idea about the periodic table, valency, the physical and chemical properties that help differentiate between the metals and non-metals on. Here, we have a brief description of the various topics of the chapter and the important sections.
There are various types of materials present around us, and these elements are classified as metals and non-metals. To distinguish an element as metal or non-metal, it is crucial to know about their physical and chemical properties. Metals are good conductors of electricity and heat like aluminium and copper. Non-metals, on the other hand, are insulators like sulphur and phosphorus. We have assembled chapter 3 science class 10 important questions for all students.
Definition of Metals
The periodic table consists of elements of which most of them are metals, including transition metals, alkali metals, alkaline earth metals, actinides, and lanthanides. Starting from carbon to radon, a zigzag line separates metals from non-metals. Iodine, phosphorus, and selenium are the elements that fall in between metals and non-metals. The elements right to these, including these, are known as non-metals. The metals left to the line are known as metalloids or semimetals. Metalloids have a combination of the properties of both metals and non-metals. The elements like metals lose electrons and form positive ions which is why it is known as electropositive elements.
Physical Properties of Metals
Except for soft metals like alkali metals like lithium, sodium, potassium, and more, most metals are solid and hard. Mercury, on the other hand, is the only metal in a liquid state and at room temperature.
Most of the metals on the periodic table have high tensile strength and are powerful. Iron and copper are used to make solid and big structures.
Except for mercury, metals are solid and at room temperature.
Metals are known as resonant as they make a ringing sound. These sounds are identified as metallic tones. Metallic wires are used in creating musical devices.
Electric cables are made of either aluminium or copper because metals are good conductors of electricity and heat.
Metals can be beaten to form thin sheets which makes them malleable. This is the reason; ships are built on iron sheets.
Metal wires are often used in electric boxes and for other purposes. These are ductile, strong and can be beaten to thin wires.
Except for sodium and potassium, which have low melting and boiling points, metals usually have high boiling and melting points.
The density of metals is high.
Except for gold and copper, metals are usually grey.
Chemical Properties of Metals
All metals when combines with oxygen from metal oxides. For example, copper, when reacts with oxygen forms copper oxides. Aluminium, when reacts with oxygen forms aluminium oxides.
Metal oxides are basic. However, metal oxides like zinc oxide, aluminium oxides show both basic and acidic behaviour. Such metal oxides react with both bases and acids to form water and salt. These are known as amphoteric oxides.
Most of the metal oxides are not soluble in water. However, some of the metal oxides dissolve in water and form alkali.
All metals do not react similar way with oxygen. Metals like sodium and potassium when reacts with oxygen can catch fire if kept in the open. Therefore, these metals are dipped in kerosene oil for protection against fire.
Magnesium, zinc, lead, etc. are covered with a thin layer of oxide at ordinary temperature to protect them from further oxidation.
Copper does not burn under the burner because of its covering layer of copper oxide. Even at high temperatures, Gold and silver do not react with oxygen.
Metals are great reducing agents.
Metals have fewer electronegativities.
What are Non Metals?
There are only a few non-metals present on the right side of the periodic table. Hydrogen, carbon, phosphorus, sulphur, oxygen, nitrogen, selenium, and all halogen and noble gases are non-metals. Non-metals are soft and dull in appearance. If tapered with a hammer, the non-metals break down into a thin powder. The non-metals are poor conductors of electricity and heat.
Physical Properties of Non-Metals
The hard non-metals are brittle and dull in colour. They can be easily broken down into powder with one tap of hammer-like sulphur and copper. However, the hardest non-metal among them is a Diamond.
Non-metals are present in the three states of matter, either solid, gaseous, or liquid.
Except for Graphite, non-metals are poor conductors of electricity and heat.
The metallic lustre is absent in non-metals.
Non-metals do not make any ringing sound and are not sonorous.
Non-metals cannot be beaten to thin sheets and are not malleable.
Non-metals cannot be beaten to thin wires and are non-ductile.
Chemical Properties of Non-Metals
Non-metals form non-metallic oxide when reacts with oxygen.
Carbon monoxide is formed when carbon is burned in less supply of air. However, it is a toxic substance and can turn fatal.
Sulphur can catch fire when reacts with oxygen.
The non-metallic oxides are acidic and turn blue litmus red in a la liquid state.
Non-metals produce chloride when in contact with chlorine gas.
The outer shell generally had four to eight electrons.
It can easily lose it to gain valance electrons.
Non-metals have high electronegative elements.
Class 10 Chapter 3 Important Questions
A set of metal and non-metals important questions will help the students study the chapter thoroughly, and get through various mock tests and competitive exams. The expert team of Vedantu has assembled a set of ten important questions which will help the students to cover the base and prepare for their board's exams.
Give one example each:
A hardest non-metal
A soft metal that you can cut with sharp objects
A metal at room temperature in the liquid state
A non-metal in a liquid state at room temperature
Name the metals:
Two metals to make stainless steel
Two metals used in jewellery making
Write three reasons for the following with a supportive chemical reaction.
Magnesium is a metal.
Sulphur is a non-metal
What is cinnabar? Explain how metal is extracted from it.
With the transfer of electrons, show the formation of KCL.
Write the electron structure of sulphur and calcium. With the transfer of electrons, show the formation of CaS. With an atomic number of calcium as 20 and sulphur as 16, lead the atoms present on CaS.
With the help of magnesium ribbon and sulphur powder, show that non-metals are acidic in nature and metal oxides are basic.
Give reasons:
Electric wires are made of wires.
School bells are made of metals.
Why and which metals are dipped in kerosene.
Name a metal that melts when kept on the palm.
Benefits of Ch 3 Science Class 10 Important Questions
Class 10 Science Metals and Non-metals important questions will help the students prepare for their final exams and are beneficial for the following reasons.
The metals and non-metals important questions will help the students to practice the chapter instantly while revising it. It is the best last-minute revision set before the exams.
The important question set consists of all the necessary topics that the students must know about the chapter. It is the best example of a group of questions that will give the students an idea about the upcoming paper.
Extra questions for class 10 Science chapter 3 are available for free at the Vedantu website, helping the students gain confidence before sitting for their finals.
Class 10 Science chapter 3 crucial questions are intended for students who are preparing to take competitive examinations or who are going to take their board exam. Science is a difficult subject that requires a lot of hard study and review before examinations. The experienced team's preparation of these class 10 science ch 3 crucial questions will assist students in covering all areas and becoming confident before examinations.
Important Related Links for CBSE Class 10 Science

FAQs on Important Questions for CBSE Class 10 Science Chapter 3 - Metals and Non-metals 2024-25
1. What are Metals? What are its Properties?
Metals are opaque, lustrous elements that are good conductors of heat and electricity. Most metals are malleable and ductile and are, in general, denser than the other elemental substances.
The Physical and Chemical Properties of Metals are as follows:
Some of the physical properties of metals are listed below.
Shiny (lustrous) in nature
Metal is a good conductor of electricity and heat
Density and melting point is high
Mouldable (Malleable)
At room temperature, it is in solid form except for mercury
Some of the chemical properties of metals are listed below.
Easily corrodible
Can lose electrons
Form basic oxides
Have low electronegativities
Good reducing agents
2. What are Non-metals? What are its properties?
Non-metal is a chemical element that does not have metal’s properties. They occur in liquid or gaseous states. There are very few non metals in nature and all of them are present in the periodic table.
The physical and chemical properties of Non-Metals are as follows:
Some of the physical properties of non-metals are listed below.
Poor conductors of electricity and heat
Non-Ductile metals
Brittle solids
Maybe solids, liquids or gases at room temperature
These are not sonorous
Transparent
Some of the chemical properties of non-metals are listed below.
The number of electrons in the outer shell is generally 4-8
Easily gain or lose valence electrons
Form acidic oxides whenever they come in contact with oxygen
High electronegative elements
Great oxidizing agents
3. What is an Alkaline metal? What is the electronic configuration of alkali metals?
Alkali metals are any of the elements found in Group IA of the periodic table (the first column). Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. In general ‘alkali’ refers to the basic or alkaline nature of their metal hydroxides.
The electronic Configuration of Alkali Metals are as follows:
Alkali metals have only one electron in their valence shell.
The electronic configuration is given by ns 1 . For example, the electronic configuration of lithium is given by 1ns 1 2ns 1 .
They tend to lose the outer shell electron to form cations with charge +1 (monovalent ions).
This makes them the most electropositive elements and due to the same reason, they are not found in the pure state.
They readily give away their valence electrons to form strongly bonded compounds with non-metals.
4. Why should students refer to the NCERT Solutions of class 10 chapter 3 - Metals and Non-metals?
Students should refer to the NCERT Solutions of class 10 chapter 3 - Metals and Non-metals for the follows reasons:
The answers and solutions provided here are absolutely correct, easy to understand and easy to remember.
This NCERT solution ensures that it provides all the answers to all the questions in all the exercises.
These solutions also ensure that they even provide answers to all the exercises even in between the lesson
The answers given in the NCERT Solutions are curated in a way as to make students understand the correct method of answering a question in the exam. The answers in these solutions are written by subject matter experts making the solutions all the more reliable.
5. Name some chemical properties of metals according to Chapter 3 of Class 10 Science.
As discussed in Chapter 3 of Class 10 Science, some chemical properties observed in metals include:
Usually, metals have a high density.
All metals are ductile and malleable.
All metals are generally in a solid-state at room temperature. However, mercury is an exception as it is in a liquid state at room temperature.
While lead is an exception, all other metals are good conductors of electricity and heat.
6. What is meant by corrosion of metals?
Corrosion refers to a natural phenomenon that causes damage to metals. This process leads to the accumulation of rust on the metal surfaces, which is usually a result of the reactions that occur due to oxygen and moisture present in the air. Corrosion leads to the formation of many new products like oxide, sulphide, or hydroxide. The equations of corrosion differ depending upon the different metals and the chemical reactions being involved.
7. What are the important questions for Chapter 3 of Class 10 Science?
Chapter 3 in Class 10 Science includes many important questions. Some examples of these important questions include:
“Name two metals that are found in nature in their free state.”
“What types of oxides are formed when nonmetals combine with oxygen?”
“Which gas is produced when a metal reacts with dilute hydrochloric acid? Write the chemical reaction for iron reacting with dilute H₂SO₄.”
8. What are the most important topics covered in Chapter 3 of Class 10 Science?
While it is essential to prepare all topics that are part of Class 10 Science Chapter 3, students must make sure that they put in extra focus and practice for the following topics as they hold higher importance from the exam’s point of view:
The Reactivity Series
Extraction of Metals
Refining of Metals
Students can also find these topics covered in the Important Questions for CBSE Class 10 Science Chapter 3 - Metals and Non-metals.
9. How are important questions for Class 10 Science Chapter 3 beneficial?
Referring to Important Questions for CBSE Class 10 Science Chapter 3 - Metals and Nonmetals are meant to help students by providing ready-made study material that they use for practice and revisions as well. These important questions can also be beneficial by saving time and helping in last-minute quick revisions of the chapter. These questions cover all the important topics that students must prepare well before their exams and these are available free of cost on Vedantu’s e-platform.
CBSE Class 10 Science Important Questions
Cbse study materials.

NCERT Solutions for Class 10 Science Chapter 3 Metals and Non Metals

Chapter 3 Metals and Non Metals Class 10 NCERT Solutions
Ncert solutions for class 10 science chapters:, why do silver articles become black after sometime when exposed to air, they get tarnished by reacting with atmospheric air to form silver sulphide. which gas is usually liberated when an acid reacts with a metal, name the metal which react with a very dilute hno 3 to evolve hydrogen gas, why does calcium floats on water, in the formation of a compound x, yatom 'x' gives one electron to an atom of 1'. what is the nature of bond in xy, contact form.
NCERT Solutions for Class 10 Science Chapter 3 Metal and Non-Metals
The Class 10 NCERT Solutions for Science Chapter 3 Metal and Non-Metals includes all the intext and exercise questions. Class 10 Science Chapter 3 Metal and Non-Metals NCERT questions and answers help students to clear their doubts and to obtain good marks in Class 10 board exam. All the solutions provided in this article are strictly based on the CBSE syllabus and curriculum.
Class 10 Science Chapter 3 NCERT Questions and Answers
Class 10 Science Chapter 3 Metal and Non-Metals NCERT Questions and Answers are prepared by experts with a detailed explanation that will help students complete their assignments & homework. Having a good grasp over CBSE NCERT Solutions for Class 10 Science will further help the students in their preparation for board exams and other competitive exams such as NTSE, Olympiad, etc.
NCERT Solutions for Class 10 Science Chapter 3 Intext Questions
Intext Questions (Page No. 40)
Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor conductor of heat.
Answer: (i) Metal that exists in liquid state at room temperature → Mercury
(ii) Metal that can be easily cut with a knife → Sodium, Potassium
(iii) Metal that is the best conductor of heat → Silver, Gold
(iv) Metals that are poor conductors of heat → Mercury and lead
Question 2: Explain the meanings of malleable and ductile.
Answer: Malleable: Substances that can be converted into thin sheets by beating are called malleable. Most of the metals are malleable. Gold and Silver are most malleable metals.
Ductile: Substances that can be drawn into thin wires are called ductile. Most of the metals are ductile. Gold is the most ductile metal.
Intext Questions (Page No. 46)
Question 1: Why is sodium kept immersed in kerosene oil?
Answer: Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
Question 2: Write equations for the reactions of (i) iron with steam (ii) calcium and potassium with water
Answer: (i) Iron react with steam to form the metal oxide and hydrogen.
3Fe( s ) + 4H 2 O( g ) → Fe 3 O 4 ( s ) + 4H 2 ( g )
(ii) The reaction of calcium with water is exothermic but the heat evolved is not sufficient for the hydrogen to catch fire.
Ca(s) + 2H 2 O(l) → Ca(OH) 2 (aq) + H 2 (g)
Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
Potassium react violently with cold water and its reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
2K(s) + 2H 2 O(l) → 2KOH(aq) + H 2 (g) + heat energy
Question 3: Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
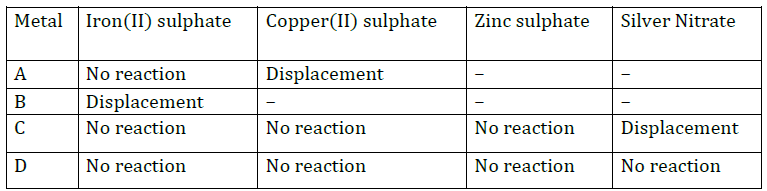
Use the Table above to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of Copper (II) sulphate?
Arrange the metals A, B, C and D in the order of decreasing reactivity
Answer: (i) As per reactivity series, Iron is most reactive metal among Iron, Silver and Copper. Since B can displace Iron from its sulphate, so B is the most reactive metal.
(ii) As B is more reactive than Iron (As discussed in answer (i)), so it will displace Copper from its Copper Sulphate solution.
(iii) B is most reactive as discussed in part (i) and D is the least reactive metal as unable to displace any of the solutions. Copper is more reactive than Silver and metal A can displace Copper, so A is more reactive than C.
Hence, the order of decreasing reactivity is B > A > C > D.
Question 4: Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H 2 SO 4 .
Answer: When reactive metals react with dilute hydrochloric acids, gives a salt and hydrogen gas
Metal + Dilute acid → Salt + Hydrogen
Reaction between Fe and H 2 SO 4 : Fe + H 2 SO 4 → FeSO 4 + H 2
Question 5: What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Answer: Zinc is more reactive than Iron. When Zn is added to Iron (II) Sulphate, Zinc displaces Iron from its solutions and Zinc sulphate is formed.
Zn(s) + FeSO 4 (aq) → ZnSO 4 (aq) + Cu(s)
Intext Questions (Page No. 49)
Question 1: (i) Write the electron-dot structures for sodium, oxygen and magnesium. (ii) Show the formation of Na 2 O and MgO by the transfer of electrons. (iii) What are the ions present in these compounds?
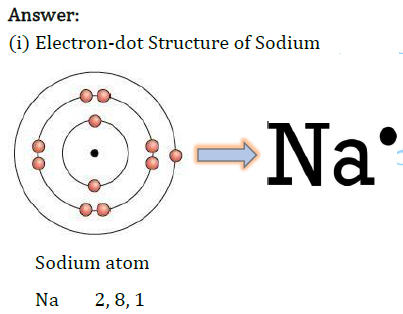
(ii) Formation of by transfer of electron
Two sodium atoms transfer their 2 outermost electrons to an oxygen atom. By losing two electrons, the two sodium atoms form tow sodium ions (2Na + ). And by gaining two electrons, the oxygen atom forms an oxide ion (O 2- ).
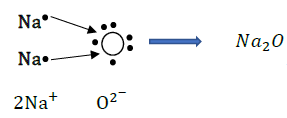
Formation of by transfer of electron
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing two electrons, the magnesium atoms form a magnesium ion (Mg 2+ ) and by gaining two electrons, the oxygen atom forms an oxide ion (O 2- ).
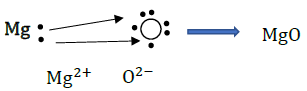
(iii) The ions present in sodium oxide compound (Na 2 O) are sodium ions (2Na + ) and oxide ions (O 2- ).
The ions present in Magnesium oxide compound (MgO) are magnesium ions Mg 2+ and oxide ions (O 2- ).
Question 2: Why do ionic compounds have high melting points?
Answer: Ionic compounds have high melting and boiling points. Because ionic compounds are formed by the attraction force of two opposite ions and a considerable amount of energy is required to break this strong inter-ionic attraction.
Intext Questions (Page No. 53)
Question 1: Define the following terms. (i) Mineral (ii) Ore (iii) Gangue
Answer: (i) Mineral: The elements or compounds, which occur naturally in the earth’s crust, are known as minerals.
(ii) Ore: If minerals contain a very high percentage of a particular metal and the metal can be profitably extracted from it. These minerals are called ores.
(iii) Gangue: Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue.
Question 2: Name two metals which are found in nature in the free state.
Answer: The metals which are the least reactive, they are often found in a free state. For example: Gold, silver, platinum and copper are found in the free state.
Question 3: What chemical process is used for obtaining a metal from its oxide?
Answer: Metals in the low of the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone.
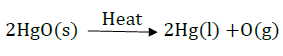
The metals in the middle of the activity series such as iron, zinc, lead, copper, etc., are moderately reactive. These metal oxides are reduced to the corresponding metals by using suitable reducing agents
ZnO(s) + C(s) → Zn(s) + CO(g)
The metals high up in the reactivity series are very reactive. They are separated from their oxides by electrolysis process.
Intext Questions (Page No. 55)
Question 1: Metallic oxides of zinc, magnesium and copper were heated with the following metals.
Answer: Magnesium is the most reactive among these three metals and Zinc is more reactive than Copper. So, Magnesium will displace Zinc oxide and Copper oxide whereas Zinc will displace Copper oxide only.
Question 2: Which metals do not corrode easily?
Answer: The metals which are the least reactive, do not corrode easily.
For example: Gold, silver, platinum and copper.
Question 3: What are alloys?
Answer: An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal.
For example:
- Stainless steel is an alloy of Nickel and Chromium.
- Amalgam is an alloy of Mercury.
- Brass is an alloy of Copper and Zinc.
- Bronze is an alloy of Copper and Tin.
- Solder is an alloy of Lead and Tin.
NCERT Solutions for Class 10 Science Chapter 3 Exercise Questions
Question 1: Which of the following pairs will give displacement reactions? (a) NaCl solution and copper metal (b) MgCl 2 solution and aluminium metal (c) FeSO 4 solution and silver metal (d) AgNO 3 solution and copper metal
Answer: (d) AgNO 3 solution and copper metal
Question 2: Which of the following methods is suitable for preventing an iron frying pan from rusting? (a) Applying grease (b) Applying paint (c) Applying a coating of zinc (d) all of the above.
Answer: (c) Applying a coating of zinc
Question 3: An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be (a) calcium (b) carbon (c) silicon (d) iron
Answer: (a) The element is likely to be calcium.
Question 4: Food cans are coated with tin and not with zinc because (a) zinc is costlier than tin. (b) zinc has a higher melting point than tin. (c) zinc is more reactive than tin. (d) zinc is less reactive than tin.
Answer: (c) zinc is more reactive than tin
Question 5: You are given a hammer, a battery, a bulb, wires and a switch. (a) How could you use them to distinguish between samples of metals and non-metals? (b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer: (a) With the hammer, we can beat the sample and if it can be beaten into thin sheets (that is, it is malleable), then it is a metal otherwise a non-metal. Similarly, we can use the battery, bulb, wires, and a switch to set up a circuit with the sample. If the sample conducts electricity, then it is a metal otherwise a non-metal.
(b) The above tests are useful in distinguishing between metals and non-metals as these are based on the physical properties. No chemical reactions are involved in these tests.
Question 6: What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer: Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Examples: PbO and Al 2 O 3 .
Amphoteric oxides are the one which reacts with both acids and bases to form salt and water. Examples: Lead oxide – PbO and Aluminium oxide – Al 2 O 3 .
Question 7: Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer: Metals that are more reactive than hydrogen displace it from dilute acids. For example: sodium and potassium.
Metals that are less reactive than hydrogen do not displace it. For example: copper and silver.
Question 8: In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer: In the process of electrolytic refining of metal called ‘M’, An impure and thick block of metal M. is considered as anode, Thin strip or wire of pure metal M is taken as anode A suitable salt solution of metal M is considered as the electrolyte
Question 9: Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
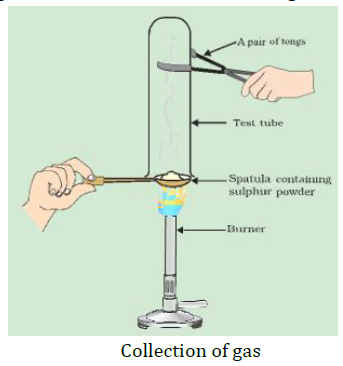
(a) What will be the action of gas on (i) dry litmus paper? (ii) moist litmus paper? (b) Write a balanced chemical equation for the reaction taking place.
Answer: (a) (i) There will be no action on dry litmus paper.
(ii) Since the gas is sulphur dioxide (SO 2 ), it turns moist blue litmus paper to red because sulphur dioxide reacts with moisture to form sulphurous acid.
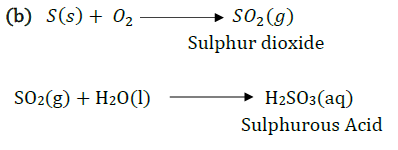
Question 10: State two ways to prevent the rusting of iron.
Answer: Two ways to prevent the rusting of iron are:
Oiling, greasing, or painting: By applying oil, grease, or paint, the surface becomes water proof and the moisture and oxygen present in the air cannot come into direct contact with iron. Hence, rusting is prevented.
Galvanization: An iron article is coated with a layer of zinc metal, which prevents the iron to come in contact with oxygen and moisture. Hence, rusting is prevented
Question 11: What type of oxides is formed when non-metals combine with oxygen?
Answer: When non-metals combine with oxygen it forms either acidic or neutral oxides. Ex: N 2 O 5 or N 2 O 3 is an acidic oxide; CO is a neutral oxide.
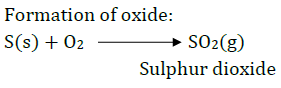
Question 12: Give reasons?
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer: (a) Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily.
(b) Sodium, potassium, and lithium are very reactive metals and react very vigorously with air as well as water. Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion. This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide. This oxide layer is very stable and prevents further reaction of aluminium with oxygen. Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulphides.
Question 13: You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer: Copper reacts with moist carbon dioxide in air to form copper carbonate and as a result, copper vessel loses its shiny brown surface forming a green layer of copper carbonate. The citric acid present in the lemon or tamarind neutralises the basis copper carbonate and dissolves the layer. That is why, tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
Question 14: Differentiate between metal and non-metal on the basis of their chemical properties. Answer:
Question 15: A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer: He must have dipped the gold in the solution of aqua regia − a 3:1 mixture of conc. HCl and conc. HNO 3 . Aqua regia is a fuming, highly corrosive liquid. It dissolves gold in it. After dipping the gold ornaments in aqua regia, the outer layer of gold gets dissolved and the inner shiny layer appears. That is why the weight of gold ornament reduced.
Question 16: Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer: Copper does not react with cold water, hot water, or steam. However, iron reacts with steam. If the hot water tanks are made of steel (an alloy of iron), then iron would react vigorously with the steam formed from hot water.
Topics covered under Class 10 Science Chapter 3 Metal and Non-Metals
Below we have listed the topics discussed in NCERT Solutions for Class 10 Science Chapter 3. The list gives you a quick look at the different topics and subtopics of this chapter.
NCERT Solutions for Class 10 Science Chapter 3 – A Brief Discussion
Chapter Overview: In this chapter, you will learn about the physical and chemical properties metals and non-metals. Physical properties mainly include physical state, luster, hardness, ductility, conductance towards heat and electricity, and sonority. Chemical properties mainly include reactivity of metals with air, water, acids, and salts of other metals. This chapter further discusses the reactivity series of metals. Moreover, in this chapter, you will also learn about metallurgy and corrosion of metals.
- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

CBSE 10th Standard Science Subject Chemical Reactions and Equations Chapter Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 10 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams

QB365 - Question Bank Software
Cbse 10th standard science subject chemical reactions and equations case study questions with solution 2021.
10th Standard CBSE
Final Semester - June 2015
Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it. In a chemical equation, the substances which combine or react are called reactants and new substances produced are called products. A chemical equation is a short hand method of representing a chemical reaction. A balanced chemical equation has equal number of atoms of different elements in the reactants and products side. An unbalanced chemical equation has unequal number of atoms of one or more elements in reactants and products. Formulae of elements and compounds are not changed to balance an equation. (i) Consider the following reaction: pMg 3 N 2 + qH 2 O ⇾ rMg(OH) 2 + sNH 3 When the equation is balanced, the coefficients p, q, r, s respectively are
(ii) Which of the following information is not conveyed by a balanced chemical equation?
(iii) The balancing of chemical equations is in accordance with
(iv) Which of the following chemical equations is an unbalanced one?
(v) Which of the following statements is/are correct?
In decomposition reactions, a single reactant breaks down to form two or more products. A decomposition reaction is opposite to combination reaction. Thermal decomposition reactions use the energy in form of heat for the decomposition of reactants. Electrolytic decomposition reactions involve the use of electrical energy for the decomposition of reactant molecules. Photolysis or photochemical decomposition involves the use of light energy for the purpose of decomposition. (i) Which of the following reactions is a decomposition reaction?
\({ (ii) \ } 2 \mathrm{~Pb}\left(\mathrm{NO}_{3}\right)_{2} \longrightarrow 2 \mathrm{PbO}+n A+\mathrm{O}_{2}\) What is nA in the given reaction?
(iii) Amino acid is formed by the decomposition of which component of our diet?
(iv) Silver chloride on exposure to sunlight for a long duration turns grey due to (I) the formation of silver by decomposition of silver chloride (II) sublimation of silver chloride (III) decomposition of chlorine gas from silver chloride (IV) oxidation of silver chloride The correct statement(s) is/are
(v) What type of chemical reaction takes place when electricity is passed through water?
Redox reactions are those reactions in which oxidation and reduction occur Simultaneously. A redox reaction is made up of two half reactions. In the first half reaction, oxidation takes place and in second half reaction, reduction occurs. Oxidation is a process in which a substance loses electrons and in reduction, a substance gains electrons. The substance which gains electrons is reduced and acts as an oxidising agent. On the other hand, a substance which loses electrons is oxidised and acts as a reducing agent. (i) Which of the following is a redox reaction?
(ii) Identify the reaction in which H2 02 is acting as a reducing agent.
(iii) For the following reactions, identify the one in which H 2 S acts as a reducing agent.
(iv) For the following reaction, identify the correct statement. \(\mathrm{ZnO}+\mathrm{CO} \longrightarrow \mathrm{Zn}+\mathrm{CO}_{2}\)
(v) In the following reaction, which substance is reduced? \(\mathrm{PbS}+4 \mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{PbSO}_{4}+4 \mathrm{H}_{2} \mathrm{O}\)
In a balanced chemical reaction, equal number of atoms are present on both sides of reaction. A balanced chemical reaction is based on law of conservation of mass which means that total mass of reactants and products participating in a reaction must be equal. For example, a balanced chemical equation of burning of magnesium in oxygen to form magnesium oxide is written as : \(2 \mathrm{Mg}+\mathrm{O}_{2} \longrightarrow 2 \mathrm{MgO}\) The mass of reactants (2 x 24 + 32 = 80) is equal to the mass of products [2 x (24 + 16) = 80] (i) In a reaction, 35 g of reactant, PQ breaks down into 20 g of product, P and an unknown amount of product, Q. Using the law of conservation of mass, weight of products, Q will be
(ii) When solid mercury (II) oxide is heated, liquid mercury and oxygen gas are produced. Which of the following statements is true regarding the balanced chemical equation for this process? (a) 1 mole of mercury (II) oxide produces two moles of mercury and one mole of oxygen gas (b) 2 moles of mercury (II) oxide produce one mole of mercury and one mole of oxygen gas (c) 1 mole of mercury (II) oxide produces half mole of mercury and half mole of oxygen gas (d) 2 moles of mercury (II) oxide produce 2 moles of mercury and one mole of oxygen gas (iii) Which of the following laws is satisfied by a balanced chemical equation?
(iv) In the given chemical reaction \(\mathrm{C}_{6} \mathrm{H}_{6(l)}+15 \mathrm{O}_{2(g)} \longrightarrow m \mathrm{CO}_{2(g)}+n \mathrm{H}_{2} \mathrm{O}_{(l)}\) The values of m and n are respectively
(v) Sulphur dioxide reacts with oxygen to form sulphur trioxide. What would be the molar ratio of sulphur dioxide to sulphur trioxide?
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reaction are known as characteristics of chemicals reactions. Some important characteristics of chemical reactions are: (I) Evolution of heat (II) Formation of precipitate (III) Change in colour (IV) Change in temperature (V) Change in state Anyone of these general characteristics can tell us whether a chemical reaction has taken place or not. (i) Reaction of magnesium with air is a/an
(ii) In the following reaction \(\mathrm{Ca}_{(a q)}^{2+}+2 \mathrm{OH}_{(a q)}^{-} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2(s)}\) precipitate of calcium hydroxide will be of
(iii) In the given reaction, \(\mathrm{S}_{(s)}+\mathrm{O}_{2(g)} \longrightarrow \mathrm{SO}_{2}\) the physical state of SO 2 is
(iv) Which one of the following processes involve chemical reactions?
(v) In which of the following reactions, high amount of heat energy will be evolved?
*****************************************
Cbse 10th standard science subject chemical reactions and equations case study questions with solution 2021 answer keys.
\({ (i) }(\mathrm{b}): \mathrm{Mg}_{3} \mathrm{~N}_{2}+6 \mathrm{H}_{2} \mathrm{O} \longrightarrow 3 \mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{NH}_{3}\) (ii) (d) (iii) (c): In a balanced chemical equation, total mass of reactants must be equal to the total mass of products. This is the statement of law of conservation of mass. (iv) (b) (v) (d)
(i) (c) \({ (ii) \ }(b): \ 2 \mathrm{~Pb}\left(\mathrm{NO}_{3}\right)_{2} \longrightarrow 2 \mathrm{PbO}+4 \mathrm{NO}_{2}+\mathrm{O}_{2}\) (iii) (c) : Proteins in our diet get broken down into amino acids. \({ (iv) }(\mathrm{a}): 2 \mathrm{AgCl}_{(\mathbf{s})} \stackrel{\text { Sunlight }}{\longrightarrow} 2 \mathrm{Ag}_{(s)}+\mathrm{Cl}_{2(g)}\) (v) (b): Electrolysis of water is electrolytic decomposition. \(\mathrm{H}_{2} \mathrm{O} \stackrel{\text { Current }}{\longrightarrow} 2 \mathrm{H}_{2}+\mathrm{O}_{2}\)
(I) (b) : H 2 is oxidised to HCI while Cl 2 is reduced to HCl. (ii) (c) \((iii) (c): 2 \mathrm{Fe} \mathrm{Cl}_{3}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{s}\) H 2 Sitself gets oxidised to Sand reduces FeCl 3 to FeCI 2 (iv) (a ): ZnO is reduced to Zn and CO is oxidised to CO 2 (v) (b) : H 2 O 2 is reduced to water by removal of oxygen.
\(\text { (i) }(\mathbf{d}): P Q \longrightarrow P+Q\) \(35 \mathrm{~g} \quad \ \ \ 20 \mathrm{~g}+\text { ? }\) According to law of conservation of mass, Mass of PQ = Mass of P + Mass of Q \(\therefore\) Mass of Q = (35 - 20)g = 15 g \({ (ii) \ }(\mathbf{d}): 2 \mathrm{HgO}_{(s)} \longrightarrow 2 \mathrm{Hg}_{(l)}+\mathrm{O}_{2(g)}\) (iii) (b) (iv) (b) (v) (b)
(i) (a) (ii) (d) : Calcium hydroxide is a white colour solid. (iii) (c) : SO 2 is gaseous in nature. (iv) (d) : When copper is heated in the presence of air in a very high temperature, a chemical reaction takes place. Copper reacts with oxygen of the air to form a thin layer of copper oxide on the surface of metallic copper (v) (c) : On burning ofL.P.G., heat is evolved.
Related 10th Standard CBSE Science Materials
10th standard cbse syllabus & materials, cbse 10th maths probability chapter case study question with answers, cbse 10th maths statistics chapter case study question with answers, cbse 10th maths surface areas and volumes chapter case study question with answers, cbse 10th maths areas related to circles chapter case study question with answers, cbse 10th maths circles chapter case study question with answers, cbse 10th maths some applications of trigonometry chapter case study question with answers, cbse 10th maths introduction to trigonometry chapter case study question with answers, cbse 10th maths coordinate geometry chapter case study question with answers, cbse 10th maths triangles chapter case study question with answers, cbse 10th maths arithmetic progressions chapter case study questions with answers, cbse 10th maths quadratic equations chapter case study questions with answers, cbse 10th social science the making of a global world chapter case study question with answers, cbse 10th social science nationalism in india chapter case study question with answers, cbse 10th social science the rise of nationalism in europe chapter case study question with answers, cbse 10th maths pair of linear equation in two variables chapter case study question with answers.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

10th Standard CBSE Study Materials

10th Standard CBSE Subjects

- Book Solutions
- State Boards
Case Study Questions Class 10 Science Acids, Bases and Salts
Case study questions class 10 science chapter 2 acids, bases and salts.

CBSE Class 10 Case Study Questions Science Acids, Bases and Salts. Important Case Study Questions for Class 10 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Acids, Bases and Salts.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 10 Science Acids, Bases and Salts
Case study : 1.
The reaction between carbon dioxide and calcium hydroxide (lime water), Calcium hydroxide, which is a base, reacts with carbon dioxide to produce a salt and water. Since this is similar to the reaction between a base and an acid, we can conclude that nonmetallic oxides are acidic in nature.
Based on the above paragraph answer the following questions:
i) What is the nature of Carbon dioxide?
Ans: It is a non- metallic oxide as carbon belongs to non- metals group i.e P – Block elements group 6.
ii) Give another reaction of non- metallic oxide and a base?
Ans: CO2(g) + 2NaOH(aq)→ Na2CO3 (aq) + H2O(aq)
iii) Arrange the following bases in increasing order: NaOH, Ca(OH)2 & Mg(OH)2 .
Ans: Mg(OH)2 < Ca(OH)2 < NaOH.
iv) Write the complete reaction between calcium hydroxide and carbon dioxide with physical states?
Ans: Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O(l)
v) What is the nature of non- metallic oxide?
Ans: The non- metallic oxide are acidic in nature because when they dissolved in water, they form acidic substance turning blue litmus into red.
CASE STUDY : 2
Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc. n Fix two nails on a cork, and place the cork in a 100 mL beaker. Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch, as shown in. Now pour some dilute HCl in the beaker and switch on the current. Repeat with dilute sulphuric acid. What do you observe? Repeat the experiment separately with glucose and alcohol solutions. What do you observe now? Does the bulb glow in all cases?
Following the above paragraph, answer the following questions;
i) What was the changes occur in case of acids i.e HCl, H2SO4?
Ans: The bulbs will start glowing as it contains hydrogen ions H+ ions (aq) as cation and Cl- or SO4^2- as anion.
ii) Why do glucose and alcohol do not conduct electricity?
Ans: They do not contains free ions neither cation nor anion. To conduct electricity, free ions are required.
iii) Why do acids do not show acidic behaviour in absence of water?
Ans: Acidic behaviour are shown by releasing of H+ ions from acids. To dissociate into H+ ions, the acids need medium i.e water.
iv) Does rain water or distilled water will conduct electricity?
Ans: Rain water will conduct electricity as it contains both positive and negative ions of different salts in it.
V) Why do aqueous solution of acids conduct electricity?
Ans: The acid contains Hydrogen ions in solutions as well as anions. Due to the presence of free ions they conduct electricity.
CASE STUDY : 3
Plaster of Paris
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate ( CaSO 4 .½ H 2 O ). This is called Plaster of Paris.Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. Chemical formula for hydrated copper sulphate is Cu SO4 . 5H2O. Now you would be able to answer the question whether the molecule of Na2CO3 .10H2O is wet.
Answer the following questions on the basis of the above paragraph:
i) What is the molecular formula of gypsum?
Ans: CaSO4. 2H2O
ii) Write the equation of formation of plaster of paris by heating gypsum?
Ans: CaSO4. 2H2O + heat👉 CaSO4. 1/2 H2O + 1^1/2 H2O
iii) What are the uses of Plaster of Paris?
Ans: It is used by doctor for supporting of fractured bones, to make toys etc.
iv) Give the equation when POP is mixed with water?
Ans: CaSO4. 1/2H2O + 1^1/2 H2O 👉 CaSO4. 2H2O
v) What does this 2 denotes in CaSO4. 2 H2O?
Ans: 2 denotes the two water molecules as water of crystallisation.
CASE STUDY : 4
Sodium hydroxide When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
Based on the above given information, answer the following questions:
i) Write the chemical equation involved in this process?
Ans: 2NaCl (aq)+ 2H2O(l) → 2 NaOH(aq) + Cl2 (g) + H2(g)
ii) What are the substance that are formed at anode and cathode on chlor- alkali process?
Ans: At anode Chlorine gas & at cathode hydrogen gas are formed.
iii) What are the uses of chlorine?
Ans: •Used for water treatment
Disinfectants
pesticides.
iv) Where does the sodium hydroxide solution is formed?
Ans: It is formed near the cathode.
V) What are the uses of Sodium hydroxide?
Ans: • uses in making soaps and detergents
artificial fibres
paper making
CASE STUDY : 5 (Acids Bases and Salts)
Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7.
Answer the following in reference to the above paragraph:
i) Classify the following as strong bases and weak bases: KOH, NaOH, CsOH, NH4OH
Ans: Strong bases: KOH, NaOH, CsOH
Weak base: NH4OH
ii) Write a reaction of a strong acid and a weak base?
Ans- HCl(aq) + NH4OH(aq) → NH4Cl(aq) + H2O(aq)
iii) What happens when strong acids and bases react to each other? Explain by giving example.
Ans: HCl + NaOH → NaCl + H2O
i.e neutral salt is formed.
iv) Identify the following as strong acid: CH3COOH, H2SO4, HNO3, H3PO4, H2CO3, HCl.
Ans: Strong acid: HCl, HNO3, H2SO4.
v) Classify the following acis as strong or weak acid: acetic acid, citric acid, tartaric acid, oxalic acid.
Ans: All are weak acid present in fruits and vegetables.
CASE STUDY : 6
A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
Answer the following on the basis of above paragraph:
i) What does the scale represent when pH value increases from 7 to 14?
Ans: It represents an increase in OH- ions concentration in the solution i.e the increment in the strength of alkali.
ii) What is the pH value of milk of magnesia?
Ans: It is 10.
iii) What are the important of pH in everyday life?
Ans: We human beings, plants and animals all are sensitive to pH i.e their body work on normal pH such as plants grow between the pH range of 6 to 8. Our human body work within the pH range of 7 to 7.8.
iv) What happens when the pH of mouth is lower than 5.5?
Ans: Tooth decay starts in which the enamel gets corroded due to the much production of acids in mouth by bacteria.
V) Two solutions X&Y. The pH of X is 4 and the pH of Y is 7. What is the nature of two solution?
Ans: Solution X is acidic in nature and the solution Y is neutral in nature.
For more update follow net explanations page
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Rs aggarwal class 5 solutions chapter 11, 2025 solved icse specimen paper class 10 english paper 1, sikkim scert class 4 evs chapter 2 games we play solution, sikkim scert class 4 evs chapter 1 changing families solution.
Sign in to your account
Username or Email Address
Remember Me

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
- Last modified on: 2 weeks ago
- Reading Time: 11 Minutes
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Here, we have provided case based/passage based questions for Class 10 Science Chapter 1 Chemical Reactions and Equations.

Table of Contents
Case Study/Passage Based Questions on Chemical Reactions and Equations
Case Study/Passage Based Questions
Question 1:
Corrosion is the phenomenon of deterioration of surface of metal in presence of air and moisture. It is a natural process and in the presence of a moist atmosphere, chemically active metals get corroded. This is an oxidation reaction. Rusting is the process where iron corrodes due to exposure to the atmosphere. The main circumstance of corrosion occurs with iron because it is a structural material in construction, bridges, buildings, rail transport, ships, etc. Aluminium is also an important structural metal, but even aluminium undergoes oxidation reactions. However, aluminium doesn’t corrode or oxidize as rapidly as its reactivity suggests. Copper (Cu) corrodes and forms a basic green carbonate.
(i) What is rusting?
(ii) Which two metals do not corrode easily?
(iii) Write the chemical name of the compound formed on corrosion of silver.
(iv) Corrosion is (a) a redox reaction (b) a reduction reaction (c) a displacement reaction (d) an oxidation reaction
Also read: Assertion Reason Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 2:
Oxidation is the process of gaining of oxygen, or losing of hydrogen. Reduction is the process of losing of oxygen or gaining of hydrogen. The substance which undergoes oxidation is the reducing agent while the substance which undergoes reduction is known as the oxidising agent. Oxidation and reduction always take place together and these type of reactions are known as redox reactions. Some of the examples of redox reactions are given below:

(i) Give two examples of oxidation reaction from your everyday life.
(ii) Write the oxidising agent in the reaction III and VI.
(iii) Which of the following is an oxidising agent? (a) LiAlH 4 (b) Alkaline KMnO 4 (c) Acidified K 2 Cr 2 O 7 (d) Both (b) and (c)
(iv) Out of oxidation and reduction, which reaction takes place at anode?
Also read: Extra Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 3:
A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The chemical reaction in which a single substance breaks down into two or more simpler substances upon heating is known as (a) thermal decomposition reaction (b) photo decomposition reaction (c) electric decomposition reaction (d) both (a) and (c)
(ii) The massive force that pushes the rocket forward through space is generated due to the (a) combination reaction (b) decomposition reaction (c) displacement reaction (d) double displacement reaction
(iii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of (a) lead nitrate (b) nitrogen oxide (c) lead oxide (d) oxygen gas
(iv) Which of the following reactions represents a combination reaction? (a) CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) (b) CaCO 3 (s) → CaO (s) + CO 2 (g) (c) Zn(s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu(s) (d) 2FeSO 4 (s) → Fe 2 O 3 (s) +SO 2 (g) + SO 3 (g)
(v) Complete the following statements by choosing correct type of reaction for X and Y. Statement 1: The heating of lead nitrate is an example of ‘X’ reaction. Statement 2: The burning of magnesium is an example of ‘Y’ reaction. (a) X- Combination, Y- Decomposition (b) X- Decomposition, Y-Combination (c) X- Combination, Y-Displacement (d) X- Displacement, Y-Decomposition
Related Posts
Download cbse books.
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
3 thoughts on “ Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations ”
Good examples! But can you please available practical types and equations type of case based questions which we can read and learn an then they help us to solve the Boards examm. Pleaseeww🙂🙂🙂
would love to see more equation based questions. nevertheless, it proved quite useful in my revision!
after going through the above content child should develops ideas to answer based on knowledge acquired.
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
- CBSE Notes For Class 10
- Class 10 Science Notes
- Chapter 3: Metals And Non Metals
CBSE Class 10 Chapter 3 Metals and Non-metals Notes
Cbse class 10 science notes chapter 3 metals and non-metals, for more information on metals and non-metals, watch the below video.

Physical Properties
Physical properties of metals.
● Hard and have a high tensile strength – Carbon is the only non-metal with very high tensile strength. ● Solid at room temperature – One non-metal, bromine, is a liquid at room temperature. The other non-metals are solids at room temperature, including carbon and sulfur. ● Sonorous – Metals produce a typical ringing sound when hit by something. ● Good conductors of heat and electricity – Graphite is a good conductor of heat and electricity. ● Malleable, i.e., can be beaten into thin sheets ● Ductile, i.e., can be drawn into thin wires ● High melting and boiling points (except Caesium (Cs) and Gallium (Ga)) – Graphite, a form of carbon (a non-metal), has a high boiling point and exists in a solid state at room temperature. ● Dense (except alkali metals). Osmium – highest density, and lithium – least density ● Lustrous – Metals have the quality of reflecting light from their surface and can be polished, e.g., gold, silver and copper. Iodine and carbon are non-metals which are lustrous. Note that carbon is lustrous only in certain forms like diamond and graphite. ● Silver-grey in colour (except gold and copper) – Metals usually have a silver or grey colour.
Nonmetals are those elements which do not exhibit the properties of metals.
Physical Properties of Non-metals
- Occur as solids, liquids and gases at room temperature
- Non-malleable
- Non-ductile
- Non-sonorous
- Bad conductors of heat and electricity
Exceptions in Physical Properties
- Alkali metals (Na, K, Li) can be cut using a knife.
- Mercury is a liquid metal.
- Lead and mercury are poor conductors of heat.
- Mercury expands significantly for the slightest change in temperature.
- Gallium and caesium have very low melting points.
- Iodine is non-metal, but it has lustre.
- Graphite conducts electricity.
- Diamond conducts heat and has a very high melting point.
Examples of Non-metals
- Hydrogen – Gas
- Nitrogen – Gas
- Oxygen – Gas
- Fluorine – Gas
- Chlorine – Gas
- Bromine – Liquid
- Iodine – Solid
- Carbon – Solid
- Sulphur – Solid
- Phosphorous – Solid
- Silicon – Solid
For more information on the Physical Properties of Metals and Non-Metals, watch the below video

To know more about the Properties of Metals and Non-Metals, visit here .
Chemical Properties
Chemical properties of metals.
● Alkali metals (Li, Na, K, etc.) react vigorously with water and oxygen or air. ● Mg reacts with hot water. ● Al, Fe and Zn react with steam. ● Cu, Ag, Pt, and Au do not react with water or dilute acids.
Reaction of Metals with Oxygen (Burnt in Air)
A metal oxide is formed when metals are burned in the air and react with oxygen in the air. Metal oxides are a type of basic material found in nature. They change the colour of red litmus to blue. To avoid reactions with oxygen, moisture, and carbon dioxide in the air, sodium and potassium metals are kept in kerosene oil.
Metal + Oxygen→ Metal oxide (basic) ● Na and K are kept immersed in kerosene oil as they react vigorously with air and catch fire. 4K(s)+O 2 (g)→2K 2 O(s) (vigorous reaction) ● Mg, Al, Zn, and Pb react slowly with air and form a protective layer that prevents corrosion. 2Mg(s)+O 2 (g)→2MgO(s) (Mg burns with white dazzling light) 4Al(s)+3O 2 (g)→2Al 2 O 3 (s) ● Silver, platinum and gold don’t burn or react with air.
Basic Oxides of Metals
Metal oxides are crystalline solids that contain a metal cation and an oxide anion. They typically react with water to form bases or with acids to form salts. MO + H 2 O → M(OH) 2 (where M = group 2 metal). Thus, these compounds are often called basic oxides.
Some metallic oxides get dissolved in water and form alkalis. Their aqueous solution turns red litmus blue.
Na 2 O(s)+H 2 O(l)→2NaOH(aq) K 2 O(s)+H 2 O(l)→2KOH(aq)
Amphoteric Oxides of Metals
Amphoteric oxides are metal oxides which react with both acids as well as bases to form salt and water. For example – Al 2 O 3 , ZnO, PbO, SnO Al 2 O 3 (s)+6HCl(aq)→2AlCl 3 (aq)+3H 2 O(l) Al 2 O 3 (s)+2NaOH(aq)→2NaAlO 2 (aq)+H 2 O(l) ZnO(s)+2HCl(aq)→ZnCl 2 (aq)+H 2 O(l) ZnO(s)+2NaOH(aq)→Na 2 ZnO 2 (aq)+H 2 O(l)
For more information on Chemical Properties of Metals and Non-Metals, watch the below video

To know more about the Chemical Properties of Metals, visit here .
Reactivity Series
The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities.
The below table illustrates the reactivity of metals from high order to low order.
To know more about Reactivity Series, visit here .
Converts sulphide ores into oxides on heating strongly in the presence of excess air. It also removes volatile impurities. 2ZnS(s)+3O 2 (g)+Heat→2ZnO(s)+2SO 2 (g)
Calcination
Converts carbonate and hydrated ores into oxides on heating strongly in the presence of limited air. It also removes volatile impurities.
ZnCO 3 (s)+heat→ZnO(s)+CO 2 (g) CaCO 3 (s)+heat→CaO(s)+CO 2 (g) Al 2 O 3 .2H 2 O(s)+heat→2Al 2 O 3 (s)+2H 2 O(l) 2Fe 2 O 3 .3H 2 O(s)+heat→2Fe 2 O 3 (s)+3H 2 O(l)
Reaction of Metals with Water or Steam
Aluminium, iron, and zinc are metals that do not react with either cold or hot water. However, when they come into contact with steam, they produce metal oxide and hydrogen. Lead, copper, silver, and gold are metals that do not react with water.
Metal+Water→Metalhydroxide or Metaloxide+Hydrogen
2Na+2H 2 O(cold)→2NaOH+H 2 +heat Ca+2H 2 O(cold)→Ca(OH) 2 +H 2 Mg+2H 2 O(hot)→Mg(OH) 2 +H 2 2Al+3H 2 O(steam)→Al 2 O 3 +3H 2 Zn+H 2 O(steam)→ZnO+H 2 3Fe+4H 2 O(steam)→Fe 3 O 4 +4H 2
Reaction of Metals with Acid
Metal+diluteacid→Salt+Hydrogengas 2Na(s)+2HCl(dilute)→2NaCl(aq)+H 2 (g) 2K(s)+H 2 SO 4 (dilute)→K 2 SO 4 (aq)+H 2 (g)
Only Mg and Mn, react with very dilute nitric acid to liberate hydrogen gas. Mg(s)+2HNO 3 (dilute)→Mg(NO 3 ) 2 (aq)+H 2 (g) Mn(s)+2HNO 3 (dilute)→Mn(NO 3 )2(aq)+H 2 (g)
Displacement Reaction
A more reactive element displaces a less reactive element from its compound or solution.
How Do Metals React with the Solution of Other Metal Salts
A more reactive metal can displace a less reactive metal from its salt solution in a displacement reaction. Metal displacement reaction is a common name for this reaction. The reactivity of certain regularly used metals has been ordered in decreasing order. This is referred to as the reactivity or activity series.
Metal A+Salt of metal B → Salt of metal A + Metal B
Fe(s)+CuSO 4 (aq)→FeSO 4 (aq)+Cu(s) Cu(s)+2AgNO 3 (aq)→Cu(NO 3 )(aq)+2Ag(s)
- It’s a component of thermite welding. Aluminium displaces iron from its oxide in this process.
- It is used in the production of steel. In which iron is displaced from its oxide by carbon.
- It is mostly utilised in metal extraction.
Reaction of Metals with Bases
The base has a bitter taste and a slippery texture. A base dissolved in water is called an alkali. When chemically reacting with acids, such compounds produce salts. Bases are known to turn blue on red litmus paper.
Base+metal → salt+hydrogen 2NaOH(aq)+Zn(s) → Na 2 ZnO 2 (aq)+H 2 (g) 2NaOH(aq)+2Al(s)+2H 2 O(l) → 2NaAlO 2 (aq)+2H 2 (g)
Extraction of Metals and Non-Metals
Applications of displacement reaction.
Uses of displacement reaction
- Extraction of metals
- Manufacturing of steel
- Thermite reaction: Al(s)+Fe 2 O 3 (s) → Al 2 O 3 +Fe(molten)
The thermite reaction is used in the welding of railway tracks, cracked machine parts, etc.
Occurrence of Metals
Most elements, especially metals, occur in nature in a combined state with other elements. All these compounds of metals are known as minerals . But out of them, only a few are viable sources of that metal. Such sources are called ores . Au, Pt – exists in the native or free state.
Extraction of Metals
The process of extracting metal ores buried deep underground is called Mining. The metal ores are found in the earth’s crust in varying abundance. The extraction of metals from ores is what allows us to use the minerals in the ground! The ores are very different from the finished metals that we see in buildings and bridges. Ores consist of the desired metal compound and the impurities and earthly substances called Gangue.
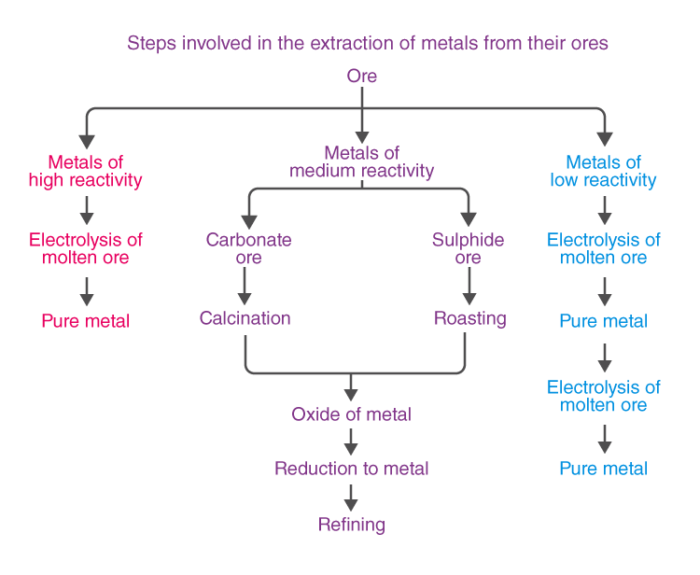
Metals of high reactivity – Na, K, Mg, Al. Metals of medium reactivity – Fe, Zn, Pb, Sn. Metals of low reactivity – Cu, Ag, Hg
To know more about the Extraction of Metals, visit here .
Enrichment of Ores
It means the removal of impurities or gangue from ore through various physical and chemical processes. The technique used for a particular ore depends on the difference in the properties of the ore and the gangue.
In chemistry, a gangue is an undesirable substance or impurity that surrounds the mineral in an ore deposit, such as sand, rock, or any other material. When it comes to mining, this mineral is very frequent.
Extracting Metals Low in Reactivity Series
By self-reduction- when the sulphide ores of less electropositive metals like Hg, Pb, Cu etc., are heated in air, a part of the ore gets converted to oxide, which then reacts with the remaining sulphide ore to give the crude metal and sulphur dioxide. In this process, no external reducing agent is used.
1. 2HgS(Cinnabar)+3O 2 (g)+heat→2HgO(crude metal)+2SO 2 (g) 2HgO(s)+heat→2Hg(l)+O 2 (g)
2. Cu 2 S(Copper pyrite)+3O 2 (g)+heat→2Cu 2 O(s)+2SO 2 (g) 2Cu 2 O(s)+Cu 2 S(s)+heat→6Cu(crude metal)+SO 2 (g)
3. 2PbS(Galena)+3O 2 (g)+heat→2PbO(s)+2SO 2 (g) PbS(s)+2PbO(s)→2Pb(crudemetal)+SO 2 (g)
Extracting Metals in the Middle of Reactivity Series
Calcination is a process in which ore is heated in the absence of air, or air might be supplied in limited quantity. Roasting involves heating ore lower than its melting point in the presence of air or oxygen. Calcination involves the thermal decomposition of carbonate ores.
Smelting – it involves heating the roasted or calcined ore (metal oxide) to a high temperature with a suitable reducing agent. The crude metal is obtained in its molten state. Fe 2 O 3 +3C(coke)→2Fe+3CO 2
Aluminothermic reaction – also known as the Goldschmidt reaction, is a highly exothermic reaction in which metal oxides, usually of Fe and Cr, are heated to a high temperature with aluminium. Fe 2 O 3 +2Al→Al 2 O 3 +2Fe+heat Cr 2 O 3 +2Al→Al 2 O 3 +2Cr+heat
Extraction of Metals Towards the Top of the Reactivity Series
Electrolytic reduction:
1. Down’s process: Molten NaCl is electrolysed in a special apparatus.
At the cathode (reduction): Na + (molten)+e − →Na(s) Metal is deposited.
At the anode (oxidation): 2Cl − (molten)→Cl 2 (g)+2e – Chlorine gas is liberated.
2. Hall’s process: A mixture of molten alumina and a fluoride solvent, usually cryolite (Na 3 AlF 6 ), is electrolysed.
At the cathode (reduction): 2Al 3+ +6e – → 2Al(s) Metal is deposited.
At the anode (oxidation): 6O 2 – → 3O 2 (g)+12e – Oxygen gas is liberated.
The metals at the top of the reactivity series are highly reactive. They cannot be obtained from their compounds by heating with carbon, because these metals have more affinity for oxygen than carbon. Hence, for the extraction of such metals electrolytic reduction method is used.
Refining of Metals
Refining of metals – removing impurities or gangue from crude metal. It is the last step in metallurgy and is based on the difference between the properties of metal and gangue.
Electrolytic Refining
Metals like copper, zinc, nickel, silver, tin, gold etc., are refined electrolytically. Anode: impure or crude metal Cathode: a thin strip of pure metal Electrolyte: aqueous solution of metal salt
From anode (oxidation): metal ions are released into the solution At cathode (reduction): the equivalent amount of metal from the solution is deposited Impurities deposit at the bottom of the anode.
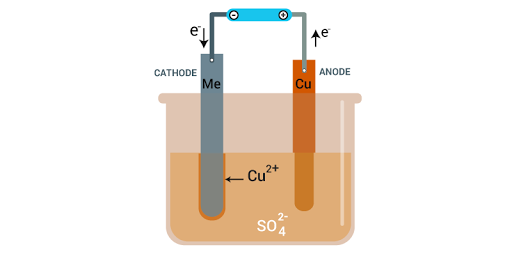
To know more about Electrolytic Refining, visit here .
The Why Questions
Electronic configuration.
Group 1 elements – Alkali metals
Group 2 elements – Alkaline earth metals
How Do Metals and Non-Metals React?
Metals lose valence electron(s) and form cations. Non-metals gain those electrons in their valence shell and form anions. The cation and the anion are attracted to each other by strong electrostatic force, thus forming an ionic bond. For example: In calcium chloride, the ionic bond is formed by opposite-charged calcium and chloride ions. The calcium atom loses 2 electrons and attains the electronic configuration of the nearest noble gas (Ar). By doing so, it gains a net charge of +2.
The two Chlorine atoms take one electron each, thus gaining a charge of -1 (each) and attain the electronic configuration of the nearest noble gas (Ar).
Ionic Compounds
Ionic compounds are neutral compounds that are made up of positively charged cations and negatively charged anions. Binary ionic compounds (ionic compounds containing only two types of elements) are named by first writing the name of the cation, then the name of the anion.
The electrostatic attractions between the opposite-charged ions hold the compound together. Example: MgCl 2 , CaO, MgO, NaCl etc.
Properties of Ionic Compound
Ionic compounds
- Are usually crystalline solids (made of ions).
- Have high melting and boiling points.
- Conduct electricity when in aqueous solution and when melted.
- Are mostly soluble in water and polar solvents.
To know more about Ionic Compounds, visit here .
Physical Nature
Ionic solids usually exist in regular, well-defined crystal structures.
Electric Conduction of Ionic Compounds
Ionic compounds conduct electricity in the molten or aqueous state when ions become free and act as charge carriers. In solid form, ions are strongly held by electrostatic forces of attraction and are not free to move; hence do not conduct electricity.
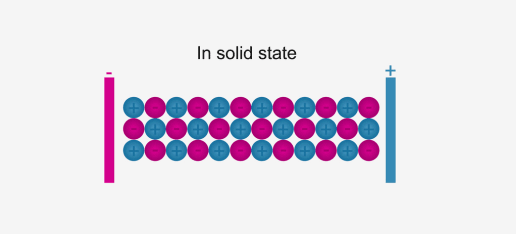
Melting and Boiling Points of Ionic Compounds
In ionic compounds, the strong electrostatic forces between ions require a high amount of energy to break. Thus, the melting point and boiling point of an ionic compound are usually very high.
Solubility of Ionic Compounds
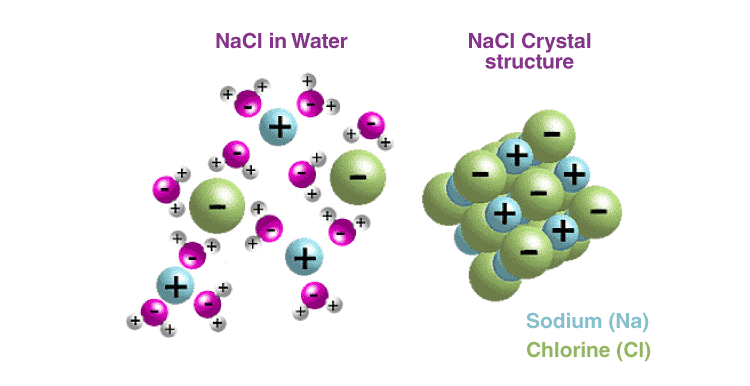
Most ionic compounds are soluble in water due to the separation of ions by water. This occurs due to the polar nature of water. For example, NaCl is a 3-D salt crystal composed of Na + and Cl − ions bound together through electrostatic forces of attraction. When a crystal of NaCl comes into contact with water, the partially positive-charged ends of water molecules interact with the Cl − ions, while the negatively charged end of the water molecules interacts with the Na + ions. This ion-dipole interaction between ions and water molecules assists in the breaking of the strong electrostatic forces of attraction within the crystal and, ultimately, in the solubility of the crystal.
Alloys are homogeneous mixtures of a metal with other metals or nonmetals. Alloy formation enhances the desirable properties of the material, such as hardness, tensile strength and resistance to corrosion.
Examples of a few alloys: Brass: copper and zinc Bronze: copper and tin Solder: lead and tin Amalgam: mercury and other metal
To know more about Alloys, visit here .
Gradual deterioration of a material, usually a metal, by the action of moisture, air or chemicals in the surrounding environment. Rusting: 4Fe(s)+3O 2 (from air)+ x H 2 O(moisture)→2Fe 2 O 3 . x H2O(rust)
Corrosion of copper: Cu(s)+H 2 O(moisture)+CO 2 (from air)→CuCO 3 .Cu(OH) 2 (green)
Corrosion of silver: Ag(s)+H 2 S(from air)→Ag 2 S(black)+H 2 (g)
For more information on Corrosion of Metals, watch the below video

To know more about Corrosion, visit here .
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:
- Metals and Non-Metals Short Notes
- Metals and Non-Metals MCQ Practice Questions
- Metals and Non-Metals MCQ Practice Solutions
Prevention of Corrosion
Prevention : 1. Coating with paints or oil or grease: The application of paint or oil or grease on metal surfaces keep out air and moisture.
2. Alloying: Alloyed metal is more resistant to corrosion. Example: stainless steel.
3. Galvanization: This is a process of coating molten zinc on iron articles. Zinc forms a protective layer and prevents corrosion.
4. Electroplating: It is a method of coating one metal with another by the use of an electric current. This method not only lends protection but also enhances the metallic appearance. Examples: silver plating, and nickel plating.
5. Sacrificial protection: Magnesium is more reactive than iron. When it is coated on articles made of iron or steel, it acts as the cathode undergoes a reaction (sacrifice) instead of iron and protects the articles.
- NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals
- Important Questions for Class 10 Science Chapter 3 – Metals and Non-metals
- Carbon and its Compounds Class 10 Chapter 4 Notes
- NCERT Exemplar Class 10 Science Solutions for Chapter 3 – Metals and Non-metals
- Metals and Non-Metals
- Maths Notes For Class 10
- CBSE Class 10 Social Science Notes
Frequently Asked Questions on Metals and Non-Metals
A student performs an experiment in which he dipped a copper coil into the silver nitrate solution. what will be observed from this experiment.
A grey-coloured layer of silver appears on the surface of the copper coil.
A student performs an experiment of burning magnesium ribbon in the air. A chemical reaction takes place, and as a result, a white powder X forms along with a bright white light. The aqueous solution of changes the colour of litmus paper to?
Oxides of metals like magnesium are basic in nature. Therefore, the aqueous solution will change the red litmus to blue.
The atomic number of two elements, A and B, are 12 and 8, respectively. What type of compound is formed when they combine?
The compound formed is AB which is ionic in nature. As we know, an ionic compound is a chemical compound in which ions are held together by electrostatic force. The electronic configuration of two elements, A and B, are 2, 8, 2 and 2, 6, respectively. From their electronic configuration, we see that A (magnesium) is a metal and B (oxygen) is a non-metal; thereby, A loses its valence electrons and forms a cation, while B accepts those electrons and forms an anion. These oppositely charged ions are drawn closer due to electrostatic forces, and an ionic compound (MgO) is formed.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam

SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
CBSE Class 10th - SCIENCE : Chapterwise Case Study Question & Solution
In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. For Science subjects, there would be 5 case-based sub-parts questions, wherein a student has to attempt 4 sub-part questions.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences
- CBSE Class 10 Revaluation Application 2024 Process Begins : How to Apply, Fees, Direct Link & Step-by-Step Guide 21 May, 2024, 4:02 pm
- CBSE Class 10 Result 2024 Latest Update : Verification of Marks, Revaluation & Photocopy of Answer Sheet; Check Complete Process 14 May, 2024, 11:55 am
- CBSE Class 10 Result 2024 Out: 93.60% Pass Percentage, JNV and Kendriya Vidyalaya shine with 99.09% score 13 May, 2024, 5:05 pm
- CBSE Class 10th Result 2024 Out : Supplementary, Re-verification & Re-evaluation Date Released; Check Details Here 13 May, 2024, 3:15 pm
- CBSE 10th Toppers List 2024: Check Toppers Name, Marks, Pass Percentage, Merit List, District & Result Full Statistics 13 May, 2024, 1:30 pm
- CBSE 10th Results 2024 Out : CBSE 10th Result Declared; Check Scorecard Here from Direct Link 13 May, 2024, 1:01 pm
- CBSE 10th 2024-25 : Science Official Competency Focused Practice Questions released by CBSE 7 May, 2024, 4:43 pm
- CBSE 10th 2024-25 : Mathematics Official Competency Focused Practice Questions released by CBSE 7 May, 2024, 4:11 pm
- CBSE Class 10 Result 2024: Not Released Yet! Board Clarifies Fake Notice; Details Here 2 May, 2024, 1:19 pm

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


IMAGES
VIDEO
COMMENTS
These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations. Case Study Questions Chapter 3 Metals and Non-Metals. Case/Passage - 1. Metals are electropositive elements. They can easily lose electrons to form ions.
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Here, we have provided case … Continue reading Case Study and Passage Based Questions for Class 10 Science Chapter 3 ...
CBSE Case Based Questions Class 10 Science Chemistry Chapter 3. CASE STUDY :1. Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue. The impurities must be removed from the ore prior to the extraction of the metal. The processes Several steps are involved in the extraction ...
The Chapter wise Important case study based questions with their solved answers in CBSE Class 10 Science can be accessed from the table below: CBSE Class 10 Science Chapter 1 Chemical Reactions ...
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 3 Metals And Non-Metals. Case Study/Passage-Based Questions. Question 1: The arrangement of metals in a vertical column in the decreasing order of their reactivities is called the reactivity series or activity series of metals.
Case Study Class 10 Science: Here, you will get class 10 Science case study questions and answers pdf at free of cost. Along with you can also download case study questions class 10 Science chapter wise for getting higher marks in board examinations. Sharda University Admission - 100% Scholarship upto - Limited Time Offer - Apply Now.
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 3 Metals And Non-Metals. Case Study/Passage-Based Questions. Question 1: The arrangement of metals in a vertical column in the decreasing order of their reactivities is called the reactivity series or activity series of metals.
Selfstudys provides case studies for the Class 10 Science chapter Metals and Non-Metals with solutions. The Solutions can be helpful for students to refer to if there is a doubt in any of the case studies problems. The solutions from the Selfstudys website are easily accessible and free of cost to download. This accessibility can help students ...
Such types of questions are solved by reading the given scenario in the paragraph. CBSE Class 10 Science Chapter Wise Case Study. Science Chapter 1 Chemical Reactions & Equations Case Study. Science Chapter 2 Acids, Bases & Salts Case Study. Science Chapter 3 Metals & Non-Metals Case Study. Science Chapter 4 Carbon & Its Compounds Case Study.
This solution contains questions, answers, images, and examples from the whole Chapter 3, Metals and Nonmetals of Science, which was taught in Class 10. Metals and Nonmetals is an important chapter that students should understand in 10th grade. These solutions are also prepared in accordance with the guidelines of the NCERT textbook.
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App. There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions.
The information provided in these NCERT Solutions for Class 10 Science Chapter 3 - Metals and Non-metals is authentic and easy to understand. These solutions provide answers to all the exercise questions present at the end of Chapter 3, Metals and Non-metals, from NCERT Class 10 Science textbook. The solutions to questions asked in between ...
Case Study Questions Class 10 Science. In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Case Study Questions for Chapter 1 Chemical Reactions and Equations.
As discussed in Chapter 3 of Class 10 Science, some chemical properties observed in metals include: Usually, metals have a high density. All metals are ductile and malleable. All metals are generally in a solid-state at room temperature. However, mercury is an exception as it is in a liquid state at room temperature.
Here, we have provided Chapter 3 Metals and Non-metals questions and answers which will help the students in learning basics of the lessons.Our experts have tried the best to prepare the questions and answers according to the latest pattern of CBSE. These Class 10 Science NCERT Solutions help in building a great foundation of concepts and make easy for the students to understand basics.
Class 10 Metals and Non Metals NCERT Book Page Number: 40. Question 1 Give an example of a metal which : (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor conductor of heat. Answer: (i) Mercury (ii) Sodium (iii) Silver (iv) Lead.
NCERT Solutions for Class 10 Science Chapter 3 Intext Questions. (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor conductor of heat. Answer: (i) Metal that exists in liquid state at room temperature → Mercury.
CBSE 10th Standard Science Subject Chemical Reactions and Equations Case Study Questions With Solution 2021. 10th Standard CBSE. Reg.No. : Science. Time : 00:30:00 Hrs. Total Marks : 20. Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it.
CBSE Case Based Questions Class 10 Science Chemistry Chapter 6. CASE STUDY : 1. Carbon and energy requirements of the autotrophic organism are fulfilled by photosynthesis. It is the process by which autotrophs take in substances from the outside and convert them into stored forms of energy. This material is taken in the form of carbon dioxide ...
CASE STUDY : 5 (Acids Bases and Salts) Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7. Answer the following in reference to the above ...
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Here, we have provided … Continue reading Case Study and Passage Based Questions for Class 10 Science Chapter 1 ...
CBSE Class 10 Science Notes Chapter 3 Metals And Non-Metals. Download PDF. In a periodic table, all elements found on the planet have been appropriately ordered based on their increasing atomic numbers. There are a total of 118 elements known to us, 92 of which are derived naturally, and the remaining 26 are created artificially in the laboratory.
CBSE Class 10th - SCIENCE : Chapterwise Case Study Question & Solution. In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.