
- Free Resources
- Project Search
- Featured Projects
- Member Benefits
1059 Main Avenue, Clifton, NJ 07011
The most valuable resources for teachers and students

(973) 777 - 3113
1059 Main Avenue
Clifton, NJ 07011
07:30 - 19:00
Monday to Friday
123 456 789
Goldsmith Hall
New York, NY 90210

- Why We’re Unique

Water Filtration
Introduction: (initial observation).
Humans may live for a month or more without food, but only a few days without water; only oxygen is more important. Each day, the body loses up to three quarts of water. A loss of only 10-20% of the body’s water content could be fatal.
When we take untreated water from a river or reservoir, the water often contains natural wastes and pollutants, such as bacteria, solids ( like mud, sand, and debris), inorganic minerals, and decayed organic compounds, as well as trace amounts of certain other contaminants. Such water is not good for human consumption.
Water suppliers transfer this water to a treatment plant where water will be filtered and chemically treated to be ready for human use.
People who have a water well at home, do a similar process in smaller scale and filter their own water.
There are also companies who offer varieties of filtration systems for home.
This project guide contains information that you need in order to start your project. If you have any questions or need more support about this project, click on the “ Ask Question ” button on the top of this page to send me a message.
If you are new in doing science project, click on “ How to Start ” in the main page. There you will find helpful links that describe different types of science projects, scientific method, variables, hypothesis, graph, abstract and all other general basics that you need to know.
Project advisor
Information Gathering:
There are many government organizations who research, control and offer information about water and water treatment. Food and drug administration (FDA) and Department of environmental protection (DEP) are among the best sources for information. Also many universities continuously research and offer courses about water filtration and treatment.
Following links are recommended for additional information.
- US Environmental Protection Agency (Office of Water)
- History of drinking water treatment (PDF)
- Water testing Scams
- Encyclopedia of Water Terms
- Home water treatment options
- Available Filtration Technologies
What are the impurities in water?
Water impurities are:
- Solids (Like mud, sand, debris, pollen, mold, fungal spores, dust mites, algae, cockroach dust, ..).
- Inorganic Minerals dissolved in water such as Calcium and Magnesium Salts.
- Decayed organic compounds from dead plants and animals and animal waste. These are the impurities that give a bad taste and odor to the water.
What are the sources of impurities?
- Wildlife that inhabits the water and the surrounding lands are a main cause of water contamination. Small animals such as fish, frog and insects excrete waste material from their bodies to the water.
- Mines and exposed acid producing minerals.
How each impurity is removed?
- Paper or fabric filters separate solid impurities such as mud, sand and dust.
- Ceramic or sand filters separate much of organic impurities from plants and animals. In municipal water treatment plants this separation is done by settling the organic material using Alum or Aluminum Sulfate.
- Carbon block filter removes oils, benzene, herbicides, pesticides and other volatile organic hydrocarbons.
- Granular activated carbon removes chemicals that cause bad taste and odors.
What is water treatment?
Water treatment is disinfection of water by killing disease causing virus, bacteria and parasites.
Water treatment is done by adding chlorine, ozone or using Ultra Violet light.
- Chlorine gas kills bacteria with adequate contact time.
- Ozone units super-oxygenate water which kills bacteria with adequate contact time.
- Ultraviolet light systems make use of the ability of this portion of the light spectrum to kill bacteria. Such systems are only effective on bacteria, viruses and some algae.
Using sand, ceramics and charcoal to filter water is a slow process and is not economical for large amount of water, that is why all water suppliers are using methods other than filtration.
What do water treatment facilities do?
Water treatment facilities purify large amount of water in large tanks using the following three steps.
First material floated on the water will be separated (Using steel mesh) Then other impurities will be settled slowly and by adding chemicals Finally Chlorine will be added for disinfection.
Question/ Purpose:
The purpose of this project is to learn about filtration, what it does and how it works. This project will also help us to know the difference between filtration and treatment.
Some of the specific questions that can be studies for this project are:
- How does the height of charcoal layer in a filter affect the rate in which organic impurities will be absorbed?
- How does the size of charcoal pieces in a filter affect the rate in which organic impurities will be absorbed?
Identify Variables:
When you think you know what variables may be involved, think about ways to change one at a time. If you change more than one at a time, you will not know what variable is causing your observation. Sometimes variables are linked and work together to cause something. At first, try to choose variables that you think act independently of each other.
If you are doing this project as a display project, you can skip this section. However, if you are doing this as an experimental project, then you will need to come up with questions, write your hypothesis, identify variables and do experiments to test your hypothesis.
For the above two questions that we have proposed, this is how you define variables.
- The height of charcoal layer is an independent variable (manipulated variable). The rate in which organic substances are absorbed is the dependent variable. Controlled variables are: Size of filter, concentration and type of pollutants, order and amount of filter material.
- The size of charcoal pieces is an independent variable (manipulated variable). The rate in which organic substances are absorbed is the dependent variable. Controlled variables are: Size of filter, concentration and type of pollutants, order and amount of filter material.
Hypothesis:
Based on your gathered information, make an educated guess about what types of things affect the system you are working with. Identifying variables is necessary before you can make a hypothesis. Following are two sample hypothesis for two questions suggested above.
- By increase in the height of charcoal layer, the rate of filtration of organic material will increase.
- Smaller size charcoal can result a higher rate of filtration. Large piece of charcoals may have no filtering affects at all.
Experiment Design:
Design an experiment to test each hypothesis. Make a step-by-step list of what you will do to answer each question. This list is called an experimental procedure. For an experiment to give answers you can trust, it must have a “control.” A control is an additional experimental trial or run. It is a separate experiment, done exactly like the others. The only difference is that no experimental variables are changed. A control is a neutral “reference point” for comparison that allows you to see what changing a variable does by comparing it to not changing anything. Dependable controls are sometimes very hard to develop. They can be the hardest part of a project. Without a control you cannot be sure that changing the variable causes your observations. A series of experiments that includes a control is called a “controlled experiment.”
Experiment 1: Experiment with filters
Material Needed:
- 2-liter soda bottle, cut and dispose 2 inches from the bottom (by an adult)
- napkins or paper towels
- gravel, sand and cotton balls for your filter
- Charcoal (Cheapest one that don’t have liquid fuel added and have no odors)
- dirty water, (If you cant find any, make it yourself) Instructions:
- Put the the soda bottle upside-down (like a funnel) and secure it so it will remain that way. You may do it by making a stand for that or hang it from some place.
- Layer the filter materials inside the soda bottle. Think about what each material might remove from the dirty water and in what order you should layer the materials. For an added challenge, use one additional materials to build your filter.
- Pour the dirty water through the filter. What does the filtered water look like?
- Take the filter apart and look at the different layers. Can you tell what each material removed from the water?
- Wipe the bottle clean and try again. Try putting materials in different layers or using different amounts of materials.
Exact measurements of the layers are optional; however, they affect the quality and the price of your filter. For example you may use a lot of activated carbon in your filter. In this case your filter will be very good, but it will also become expensive. On the other hand if you use a lot of sand with no or little activated carbon, then your filter will not be good and it will be cheap.
Now it’s time to experiment. Think of a question you want answered. Like, are there better materials for cleaning water? Be sure to predict what you think is going to happen. Then, test it out using different materials and record the results for your report.
Other samples:
- Water Filter
- Water Plant tour
Following are sample images of water filtration experiment. Material used for filtration in these images are Fish tank charcoal, Sand, Gravel and Coffee Filter

Experiment 2:
Test the effect of charcoal layer on filtration of organic compounds.
- Mix some food coloring with water to simulate water polluted with organic material.
- Make 3 identical filters with different heights of charcoal layer.
- Filter two cups of the colored water that you have made with each of your three filters.
- Compare the color of three filtered waters and record the results. Visually determine the rate of filtration (or the rate of color loss) and write that in your results table.
Your results table may look like this:
Height of charcoal layer Rate of filtration
You may also use the above results table to draw a bar graph.
Experiment 3: (Permittivity)
Compare the filtration time of different filter materials
Introduction:
In a multi-layer water filter, it takes certain amount of time for water to travel each layer. The total filtration time is the sum of the individual travel times for different layers. Travel time of water in each layer depends on how pores the materials are and the attraction forces between the molecules of water and the molecules of filter materials. In this experiment we compare some of the filtration materials for their speed of filtration also known as permittivity.
- Get 5 identical bottles. Fill each bottle with 100ml water and mark the water level.
- Get 5 identical funnels and place them on the bottles. Write the name of filter materials on the funnels or bottles.
- At the bottom of each funnel place a piece of plastic mesh or steel mesh (like those used in making sieves). This is used to hold the filter materials in place.
- Fill each funnel to half with different filtration materials. (Filtration materials may include sand, clay, activated carbon, or any other substance that may be used in filtration).
- To each funnel add some water to saturate the filtration materials. Wait about 5 minutes. Empty any water that are entered in bottles.
- Add 200ml water to each funnel.
- Record the time it takes for the first 100ml of water that leave the funnel and enter the bottle. Your data table may look like this:
Materials and Equipment:
Multi-layer filters are normally a cylindrical container with layers of filter material. Top layers usually separate large solids and debris from the water. Middle layers separate much finer impurities to create a clear water. Lower levels are usually activated carbon to separate hydrocarbons, gasoline, insecticides and impurities that cause bad taste and bad odor.
A typical filter may start with a layer of gravel at the bottom, and then large sand, fine sand, clay, activated carbon, fine sand again, and large sand on the top.
These filters can not separate all viruses, bacteria and other micro organisms. Micro organisms later must be destroyed using chlorine or other disinfectant material.
Since filter absorbs some micro organisms such as bacteria and viruses, these micro organisms will reproduce inside the filter and exit the filter in later uses. That’s why filter material must be renewed so often.
Some filters are made only from different types of sand.
Results of Experiment (Observation):
Experiments are often done in series. A series of experiments can be done by changing one variable a different amount each time. A series of experiments is made up of separate experimental “runs.” During each run you make a measurement of how much the variable affected the system under study. For each run, a different amount of change in the variable is used. This produces a different amount of response in the system. You measure this response, or record data, in a table for this purpose. This is considered “raw data” since it has not been processed or interpreted yet. When raw data gets processed mathematically, for example, it becomes results.
Calculations:
If you do any calculations for your project, make sure to write your calculations in this section of your report.
Summery of Results:
Summarize what happened. This can be in the form of a table of processed numerical data, or graphs. It could also be a written statement of what occurred during experiments.
It is from calculations using recorded data that tables and graphs are made. Studying tables and graphs, we can see trends that tell us how different variables cause our observations. Based on these trends, we can draw conclusions about the system under study. These conclusions help us confirm or deny our original hypothesis. Often, mathematical equations can be made from graphs. These equations allow us to predict how a change will affect the system without the need to do additional experiments. Advanced levels of experimental science rely heavily on graphical and mathematical analysis of data. At this level, science becomes even more interesting and powerful.
Conclusion:
Using the trends in your experimental data and your experimental observations, try to answer your original questions. Is your hypothesis correct? Now is the time to pull together what happened, and assess the experiments you did.
Related Questions & Answers:
What you have learned may allow you to answer other questions. Many questions are related. Several new questions may have occurred to you while doing experiments. You may now be able to understand or verify things that you discovered when gathering information for the project. Questions lead to more questions, which lead to additional hypothesis that need to be tested.
Possible Errors:
If you did not observe anything different than what happened with your control, the variable you changed may not affect the system you are investigating. If you did not observe a consistent, reproducible trend in your series of experimental runs there may be experimental errors affecting your results. The first thing to check is how you are making your measurements. Is the measurement method questionable or unreliable? Maybe you are reading a scale incorrectly, or maybe the measuring instrument is working erratically.If you determine that experimental errors are influencing your results, carefully rethink the design of your experiments. Review each step of the procedure to find sources of potential errors. If possible, have a scientist review the procedure with you. Sometimes the designer of an experiment can miss the obvious.
References:
List of Related links. http://poolplaza.com/pool-filters.shtml
http://phys4.harvard.edu/~wilson/Feroze_Ahmed/Sec_3.htm
http://www.cc.cc.ca.us/pfp/Pfpfilter.htm
http://www.orival.com/water.shtml
Related information
How does a water softener work.
We call water “hard” if it contains a lot of calcium or magnesium impurities dissolved in it. Hard water causes two problems: It can cause “scale” to form on the inside of pipes, water heaters, tea kettles and so on. The calcium and magnesium precipitate out of the water and stick to things. The scale doesn’t conduct heat well and it also reduces the flow through pipes. Eventually pipes can become completely clogged. It reacts with soap to form a sticky scum, and also reduces the soap’s ability to lather. Since most of us like to wash with soap, hard water makes bath-time or shower-time less enjoyable. The solution to hard water is either to filter the water by distillation or reverse osmosis to remove the calcium and magnesium, or to use a water softener. Filtration would be extremely expensive to use for all the water in a house, so a water softener is usually a less costly solution. The idea behind a water softener is simple. The calcium and magnesium ions in the water are replaced with sodium ions. Since sodium does not precipitate out in pipes or react badly with soap, both of the problems of hard water are eliminated. To do the ion replacement, the water in the house runs through a bed of small plastic beads or a chemical matrix called zeolite. The beads or zeolite are covered with sodium ions. As the water flows past the sodium ions, they swap places with the calcium and magnesium ions. Eventually the beads or zeolite contain nothing but calcium and magnesium and no sodium, and at this point they stop softening the water. It is now time to regenerate the beads or zeolite.Regeneration involves soaking the beads or zeolite in a stream of sodium ions. Salt is Sodium Chloride, so the water softener mixes up a very strong brine solution and flushes it through the zeolite or beads (this is why you load up a water softener with salt). The strong brine displaces all of the calcium and magnesium that has built up in the zeolite and replaces it again with sodium. The remaining brine plus all of the calcium and magnesium is flushed out through a drain pipe. Regeneration can create a lot of salty water.
What is “activated charcoal” and why is it used in filters?
Charcoal is carbon. Activated charcoal is charcoal that has millions of tiny pores between the carbon atoms. According to Encyclopedia Britannica, “The use of special manufacturing techniques results in highly porous charcoals that have surface areas of 300-2,000 square meters per gram. These so-called active, or activated, charcoals are widely used to adsorb odorous or colored substances from gases or liquids.” The word adsorb is important here. When a material adsorbs something, it means that it attaches to it by chemical attraction. The huge surface area of activated charcoal gives it countless bonding sites. When certain chemicals pass next to the carbon surface they attach to the surface and are trapped.
Activated charcoal is good at trapping other carbon-based impurities (“organic” chemicals), as well as things like chlorine. Many other chemicals are not attracted to carbon at all – sodium, nitrates, etc. – so they pass right through. This means that an activated charcoal filter will remove certain impurities while ignoring others. It also means that, once all of the bonding sites are filled, an activated charcoal filter stops working. At that point you must replace the filter.
Water Filters General Information Activated charcoal (activated carbon) filters have been used in homes to remove taste and odor. Taste and odor, although undesirable, are generally not considered unhealthy. In recent years, however, activated charcoal filters have been used to remove some of the contaminants that have been discovered in water supplies.
Activated charcoal is most effective at removing organic compounds such as volatile organic compounds, pesticides and benzene. It can also remove some metals, chlorine and radon. As with any treatment system, it cannot remove all possible drinking water contaminants.
Because activated charcoal systems are limited in the types of compounds they can effectively remove, it is essential that the homeowner determine which water contaminants are present before purchasing such a system. Anyone who suspects they have a water quality problem should first have their water analyzed by their local health department or a reputable laboratory. These analyses are costly, but worth the expense since they are necessary to determine the appropriate home treatment system and how best to operate such a system. A state or local health official can interpret water analysis results. Some laboratories may also provide this service.
Note that home water treatment is considered only a temporary solution. The best solutions to a contaminated drinking water problem are to either end the practices causing the contamination or change water sources. Activated charcoal is a black solid substance resembling granular or powdered charcoal. It is extremely porous with a very large surface area. Certain contaminants accumulate on the surface of the activated charcoal in a process called adsorption. The two main reasons that chemicals adsorb onto activated charcoal are a “dislike” of the water, and attraction to the activated charcoal. Many organic compounds, such as chlorinated and non- chlorinated solvents, gasoline, pesticides and tri-halo-methane can be adsorbed by activated charcoal. Activated charcoal is effective in removing chlorine and moderately effective in removing some heavy metals. Activated charcoal will also remove metals that are bound to organic molecules. It is important to note that charcoal is not necessarily the same as activated charcoal. Activated charcoal removes vastly more contaminants from water than does ordinary charcoal.
Home activated charcoal treatment systems are quite simple. The activated charcoal is normally packaged in filter cartridges that are inserted into the purification device. Water needing treatment passes through the cartridge, contacting the activated charcoal on its way to the faucet. Activated charcoal filters eventually become fouled with contaminants and lose their ability to adsorb pollutants. At this time, they need to be replaced. Activated charcoal treatment systems are typically point of use installed where they typically treat water used for drinking and cooking only. Activated charcoal filters can be placed on the end of the faucet, on the countertop, or under the sink. Point of use systems often have a bypass so that water for purposes other than drinking and cooking can also be dispensed at the tap without being treated. This increases the life of the activated charcoal, reducing the time between filter replacements.
A point of entry system is more appropriate if a contaminant is present that poses a health threat from general use as well as from consumption. Volatile organic compounds and radon are examples of this type of contaminant. These contaminants may get into the indoor air when water is used for showering and washing. In this case, it is more economical to have a large pint of entry system that treats water as it enters the home than to have point of use systems at each tap.
Activated charcoal filters used for home water treatment contain either granular activated charcoal or powdered block charcoal. The amount of activated charcoal in a filter is one of the most important characteristics affecting the amount and rate of pollutant removal. More charcoal in a cartridge means more capacity for chemical removal, resulting in longer cartridge lifetime. This means fewer cartridge changes and less chance of drinking contaminated water. Particle size will also affect the rate of removal; smaller activated charcoal particles generally show higher adsorption rates. Rust, scale, sand or other sediments can clog any activated charcoal filter. A solution to this problem is to place foam or cotton filters (often called sediment or fiber filters) between the cartridge and incoming water. When sediment filters become clogged, they need to be replaced or they will cause water pressure to drop.
An activated charcoal filter must be deep enough so that the pollutants will adsorb to the activated charcoal in the time it takes the water to move through the filter. The appropriate filter depth depends on the flow rate of water through the filter. The slower the flow rate, the better the removal. The poor performance of some end of faucet devices is probably due to improper filter depth.
Physical and chemical characteristics of the water will also affect performance. The acidity and temperature can be important. Greater acidity and lower water temperatures tend to improve the performance of activated charcoal filters. Activated charcoal filters have a limited lifetime. Eventually, the surface of the activated charcoal will be saturated with adsorbed pollutants and no further purification will occur. This is called breakthrough; the pollutants have broken through the filter to emerge in the treated water. When this occurs, it is possible that the contaminant concentrations in the treated water will be even higher than those in the untreated water. At this time, the cartridge needs to be replaced. Knowing when breakthrough will occur and thus when to replace the cartridge is a major problem with activated charcoal treatment.
Some cartridges are sold with predictions about their longevity. These are generally only crude estimates since they do not take into consideration factors that are characteristic to a specific water source, such as pollutant concentration. The retailer you purchase the treatment device from can make better estimates of the filter’s useful lifetime based on water usage (flow rate) and pollutant concentrations shown in the chemical analysis. Hence, to get the most accurate estimates, you should learn what these amounts are before purchasing the system. Note that if pollutant concentrations increase over time and testing is not performed to reveal this change, such estimates may turn out to be not very practical or useful.
Unfortunately, activated charcoal filters can be excellent places for bacteria to grow. Conditions for bacterial growth are best when the filter is saturated with organic contaminants, which supply the food source for the bacteria, and when the filter has not been used for a long period of time. It is still unclear whether the bacteria growing on the charcoal poses a health threat. Some manufacturers have placed silver in the activated charcoal in order to prevent the growth of bacteria. The effectiveness of this procedure has not been independently verified. In addition, silver may contaminate the drinking water.
The above considerations have led public health officials to consider activated charcoal home treatment a temporary solution to be used only until the source of contamination can be eliminated and the water supply is safe. Even with proper installation, maintenance and operation, malfunction of home water treatment systems can occur.
Activated Charcoal Filter Guidelines
Make sure the filter contains activated charcoal. Know the quantity of activated charcoal in the filter since this will determine the amount and rate of pollutant removal. Use pre-filter to add life to activated charcoal filters. Replace pre-filters and activated charcoal filters regularly. Determine appropriate intervals for replacement of activated charcoal filters based on contaminant concentration, water characteristics, water flow rate, depth of filter, type and amount of activated charcoal and prefilter. Retailers can help in this analysis.
It is always important for students, parents and teachers to know a good source for science related equipment and supplies they need for their science activities. Please note that many online stores for science supplies are managed by MiniScience.
Testimonials
" I called School Time and my husband and son came with me for the tour. We felt the magic immediately."
- Robby Robinson
" My husband and son came with me for the tour. We felt the magic immediately."
- Zoe Ranson
LET THE MAGIC OF PURE WATER TOUCH YOUR LIFE...
The science behind water filtration: how it really works, promo video, introduction.
Have you ever considered the intricate path a single droplet of water takes, navigating through the labyrinth of filtration systems? In today’s fast-paced and ever-changing world, grasping the science behind water filtration is more than satisfying curiosity; it’s about recognizing a vital process integral to our everyday existence. This exploration goes beyond just providing safe drinking water; it delves into the transformation of unfiltered water laden with harmful contaminants and unpleasant odors into something pure and safe. From the complexities of different types of filters to the essential removal of impurities, this article unravels the mysteries of water filtration.
Join us as we simplify and illuminate the science behind water filtration, offering clear explanations and captivating insights into a process that ensures the water in our bottles and from our taps is clean and wholesome.
What is Water Filtration?
Water filtration, in its essence, is the guardian of our water quality. Imagine a complex, multi-layered defense system designed to capture and eliminate various contaminants – that’s what water filtration does. It’s not merely a physical barrier. It incorporates a blend of physical, chemical, and biological processes to remove everything from visible particles to microscopic threats. The goal? To transform water from its raw, uncertain state into a clear, safe, and drinkable resource.
Tracing the Roots: The History of Filtration
Embarking on a historical journey – The science of water filtration is as old as civilization itself. Ancient civilizations like the Greeks, Egyptians, and Indians were pioneers in early water purification techniques. Their rudimentary methods, ranging from boiling water to using sand and gravel as filters, laid the groundwork for today’s sophisticated systems. This historical perspective is not just about acknowledging their ingenuity but understanding how our current practices are rooted in these ancient wisdoms.
The Invisible Foes: Understanding Contaminants
Unveiling the hidden enemies – Water, in its journey from source to tap, encounters various contaminants. These include physical particles like dirt and rust, biological threats like bacteria and viruses, and chemical pollutants like pesticides and heavy metals. Each type of contaminant presents a unique challenge and requires a specific approach to filtration. Recognizing these adversaries is crucial in selecting the appropriate filtration method to ensure safe, clean water.
Activated Carbon: The Silent Purifier
The unsung hero in our faucets – Activated carbon filters, found in many household water purifiers, are akin to a dense forest trapping unwanted travelers. These filters use a process called adsorption, where contaminants are trapped inside the pore structure of the carbon substrate. Activated carbon is exceptionally adept at removing chlorine, improving taste and odor, and reducing certain harmful chemicals. It’s the first line of defense in many filtration systems, providing an essential barrier against a range of impurities.
Reverse Osmosis: The Fine Sieve of Filtration
A deeper dive into molecular purity – Reverse osmosis (RO) takes filtration to a microscopic level. It involves forcing water through a semi-permeable membrane that acts like an ultra-fine sieve, allowing only water molecules to pass through. This process is effective at removing a wide range of contaminants, including dissolved salts, bacteria, and viruses. Think of it as a meticulous editor, ensuring that only the purest water gets through.
UV Purification: Nature’s Disinfectant
Emulating the sun’s sterilizing power – UV purification is a marvel of mimicking nature. By exposing water to ultraviolet light, this method effectively neutralizes bacteria and viruses without adding chemicals or altering the water’s taste and odor. It’s a swift, effective, and environmentally friendly way to disinfect water, akin to bathing it in a beam of pure sunlight.
The Gravity of Sedimentation
Letting gravity do the work – Sedimentation is a simple yet effective process where gravity helps in the filtration journey. Here, heavier particles in the water settle to the bottom over time, akin to leaves settling at the bottom of a still pond. While not a complete filtration method on its own, sedimentation is a crucial preliminary step that prepares water for further purification.
Filtration vs. Purification: The Nuances
Deciphering the subtle differences – Filtration and purification, while often used interchangeably, have distinct roles. Filtration primarily removes physical impurities, while purification goes a step further to eliminate microscopic contaminants, including bacteria and chemicals. It’s the difference between cleaning the surface and performing a deep, thorough cleanse.
Environmental Footprints of Filtration
Treading lightly on the planet – Water filtration isn’t just about human health; it has significant environmental implications too. By reducing our reliance on bottled water, we diminish plastic waste and conserve natural resources. Moreover, advancements in filtration technology are continuously seeking more sustainable and energy-efficient methods, underscoring the importance of environmental stewardship in water filtration.
Home Filtration: Myths and Realities
Navigating the DIY landscape – While DIY water filtration can be an interesting home project, it’s important to recognize its limitations. Homemade filters might not be effective against all contaminants and cannot replace professionally designed systems. It’s vital to approach home filtration with a blend of curiosity and caution, ensuring that safety and effectiveness aren’t compromised.
The Next Wave in Water Filtration
Innovations shaping our future – The future of water filtration is a canvas of innovation and improvement. Emerging technologies promise more efficient, effective, and environmentally-friendly filtration systems. From nanotechnology to bio-filtration, the horizon is bustling with possibilities that could revolutionize how we access clean water.
Selecting Your Shield: Filtration Systems
Tailoring your defense against contaminants – Choosing the right water filtration system is a personalized decision. It requires understanding the specific contaminants in your water and selecting a system that effectively targets those concerns. With a range of options available, from under-sink filters to whole-house systems, finding the right fit is about matching your needs with the right technology.
The Lifeline: Maintenance and Care
Ensuring enduring purity – Regular maintenance is the heartbeat of any water filtration system. Neglecting it can lead to decreased efficiency, potential contamination, and a shortened lifespan of the system. Adhering to maintenance schedules and understanding the care requirements of your system is crucial for ensuring its ongoing effectiveness.
Debunking Filtration Myths
Separating fact from fiction – In the realm of water filtration, myths and misconceptions abound. From overestimating the capabilities of certain filters to misunderstanding the nature of contaminants, it’s essential to be well-informed. Debunking these myths not only enhances our understanding but also guides us towards making better choices for our water needs.
How exactly does a water filter improve the taste of water?
Water filters remove contaminants like chlorine and heavy metals, which can cause unpleasant tastes and odors, resulting in cleaner and more pleasant-tasting water.
Are there any health risks associated with not changing a water filter on time?
Yes, a clogged or old filter can harbor bacteria and let contaminants back into the water, posing health risks.
Can water filtration systems remove beneficial minerals from water?
Some systems, like reverse osmosis, can remove minerals, but many are designed to retain beneficial minerals while removing harmful substances.
Is filtered water as effective as boiling water for purification?
Filtered water is effective for removing contaminants, but boiling is recommended for killing pathogens in areas with unsafe water supplies.
How do I know which water filtration system is right for my home?
It depends on your water source, the specific contaminants present, and your household needs. Testing your water and consulting with a filtration expert can help determine the best system for you.
The Crystal Clear Conclusion
In conclusion, understanding the science behind water filtration is more than a deep dive into a technical subject; it’s about embarking on a journey towards purity and responsibility. As we explore the various ways to remove impurities from water, including biological contaminants and substances that cause bad tasting water, we become better equipped to protect ourselves from the dangers of contaminated water . This knowledge empowers us to make well-informed choices about the water we drink, whether it comes from municipal water supplies or other sources. By familiarizing ourselves with different filtration types and their roles in purifying water, we not only enhance our own health but also contribute to environmental conservation and responsible living. Ultimately, as we unravel and understand the complexities of water quality and the science behind its filtration, we step up as guardians of one of Earth’s most vital resources.
Take the Step Towards Pure, Healthy Water Today!
Are you ready to transform the way you drink water? Our advanced water filtration systems offer a seamless solution for ensuring every sip you take is pure, safe, and refreshing. Don’t wait for a healthier lifestyle – make the change now.
🌊 Click [ here ] to contact us about our cutting-edge water filtration systems.
Discover the difference of clean water right in your home. For any queries or assistance, our team of experts is just a message away.
Join the movement towards a healthier, happier planet. Hydrate the smart way with The Good Water Guy.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- (915) 321-3820
- [email protected]
- 8935 Gateway S Blvd Suite B5, El Paso, TX 79904
Weekly Newsletter
Automated page speed optimizations for fast site performance

Charcoal Water Purifying Experiment
Sharing is caring!

Do you use some sort of charcoal water filter in your home? You might have a pitcher with a charcoal filter r or a filter that attaches to your faucet . Have you ever wondered how that process actually works? Try this charcoal water purifying experiment to learn all about it!
Science Experiments For Independent Learning
From start to finish, she is responsible for the experiment. Of course, she can ask questions or get help if she needs it, but I have found that the more she does the experiments, the less help she needs.
During the experiment, my daughter keeps notes on an experiment sheet. The rest of this blog post is her lab report as recorded on her experiment worksheet.
This charcoal water purifying experiment is a great experiment for middle school students to try on their own.
Can Charcoal Remove Molecules From Water?

My pre-experiment hypothesis is yes, charcoal can remove molecules from water. This is based on my knowledge of using charcoal filters to clean water.
- a measuring cup
- 2 baby food jars with lids
- red food coloring
Fill a measuring cup with 1/2 cup of water. Add 8 drops of red food coloring to the water and stir until mixed. Now, add 1/4 cup of colored water to each of the baby food jars.
To one jar, add 2 teaspoons of the activated carbon. Put the lids on both jars and set the jars in a place where they won’t be disturbed for a couple days.
Note the color of the jars when the carbon was added, 4 hours later, 24 hours later, and each day for 3 days.
Observations and Results
At the beginning of the experiment, the color of the water in the jars was the same.

By the next day, the water was almost totally clear in the activated carbon jar and the other jar was just as red as the beginning. On the second day after the experiment began, the water in the activated carbon jar was totally clear.
My results conclude that my hypothesis was correct. Charcoal can remove molecules from the water.
What Happened?
Activated charcoal is charcoal that has been specially treated to create cracks and holes in the charcoal. This treatment process is called activation and involves heating the charcoal to a high temperature. The high temperature changes the structure of the charcoal and makes it more porous and causes cracking. The cracking creates a greater surface area and more bonding sites. It is these binding sites that attract many chemicals in liquid and chemical form.
The food coloring is made of molecules. These molecules are bigger than water molecules.
When the food coloring, water, and charcoal are mixed together, the food coloring molecules break their bond to the water and attach to the carbon molecules of the activated carbon.
When the food coloring molecules (a liquid) “unstick” from the water (another liquid) and attach to the activated carbon (a solid), this is called adsorption. Adsorption happens when a liquid molecule separates from another liquid molecule and attaches to the molecule of a solid.
This is how the activated charcoal is able to remove the food coloring from the water. The food coloring molecules bind with the charcoal and come out of the water solution. This property of activated charcoal is what makes it a great filter/purifier.
Try this at home with your kids or let them try it for themselves!
For More Chemistry Activities
Check out The Homeschool Scientist’s Chemistry Resource Page!
Check out our printable unit on The Periodic Table of the Elements
Studying the properties of water? Check out these activities that demonstrate polarity, adhesion, cohesion, and more .

I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
Homemade Water Filter Experiment
July 6, 2019 By Emma Vanstone 11 Comments
Today we are looking at filtering and how it can be used to clean dirty water. This water filter science project is very simplified but gives kids a great overview of how water purification is carried out to give us lovely clean drinking water.
Do not drink the water in the activity

Water Filter Science Project
Filtering water – what you need.
Dirty water ( or mud and clean water )
Coffee filter/paper towel/muslin
An empty bottle or other containers
Filtering Water Experiment
The great thing about this experiment is that you can design it however you like.
We set up coffee filters containing sand, stones and then just plain filters.

We carefully poured the same amount of water through each filter and observed the results.

More ideas to try – Water Filter Science Project
Set up an experiment where the conditions are:
1 coffee filter
2 coffee filters
3 coffee filters
Is kitchen roll better than a coffee filter, would just a sieve work?
You could also work in stages, so try a colander, then a sieve and then a paper towel. Each stage should trap smaller and smaller particles.
How do filters work?
A filter is a porous material which a liquid can be passed though to separate the liquid from solids suspended in it.

More Filtering Investigations
Make a toy filter .
Try filtering potions using a sieve and colander.
Can you filter water using sand and stones ?

More Science for Kids
I’ve got a fun collection of water science experiments , including a water cycle activity, dissolving experiment, ice investigation and density trick!
Try one of our easy science experiments for kids to do at home ! We’ve got egg experiments, ice experiments, paper helicopters, STEM challenges and lots more science fun for kids of all ages!

Suitable for Key stage 2 Science
Properties and Changes of Materials
Use knowledge of solids, liquids and gases to decide how mixtures might be separated, including through filtering, sieving and evaporating.

Last Updated on May 31, 2021 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
May 15, 2012 at 11:49 pm
This is such a neat experiment. I bet it would be fun to let the kids think of better ways to filter the water. It will definitely give them a better appreciation for our clean water!
May 18, 2012 at 8:22 pm
So simple, and yet so fun! A very good way to learn about filters and clean water. We will definitely have to try this one, thanks 🙂
May 22, 2012 at 8:21 pm
We did that once too and it’s amazing how much gets filtered out and how gross that water still is. I think yours was more easily visble, we have very clay like dirt, so it didn’t quite dissolve right. Thanks for linking up to Science Sunday!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org .
- TeachEngineering
- Water Filtration Project: Make Your Own Water Filters
Hands-on Activity Water Filtration Project: Make Your Own Water Filters
Grade Level: 4 (3-5)
Time Required: 1 hour
This activity also requires some non-expendable items; see the Materials List for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Number and Operations, Science and Technology
NGSS Performance Expectations:

TE Newsletter
Engineering connection, learning objectives, materials list, worksheets and attachments, more curriculum like this, introduction/motivation, investigating questions, user comments & tips.

Clean water is not available in all parts of the world. Many people live with polluted water that is unhealthy to drink and bathe in. Civil, environmental , materials and mechanical engineers all contribute to developing technologies and systems to purify unclean water. Purifying water can be done easily if it is a small amount that is fairly clean, but larger amounts that are very polluted are much more complicated. Typical steps for full water treatment include aeration, coagulation, sedimentation, filtration and disinfection.
After this activity, students should be able to:
- Understand how filtration works.
- Create creative design methods.
- Problem solve given a design challenge.
- Apply mathematics (multiplication) reinforcement.
- Engage in teamwork to solve a challenge.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
Ngss: next generation science standards - science, common core state standards - math.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
International Technology and Engineering Educators Association - Technology
State standards, massachusetts - math, massachusetts - science.
- 1 liter of water prepared in advance with soil and sand in it until it is thin but relatively opaque
- 3 test tubes prepared with the water standards "A," "B" and "C" (C is filtered through some grass, B is filtered through a coffee filter, and A is filtered through 2 coffee filters with a paper towel in the middle)
- cotton balls
- gauze squares
- tulle/netting
- paper towels
- coffee filters
- gravel (aquarium gravel works great)
- 3 test tubes per student
- test tube racks
- graduated cylinders
- Design Components Worksheet
One of our most valuable and often overlooked resources is water. We can survive for a few weeks without food, but only a few days without water. Having clean water to drink is a luxury. The water that eventually comes out of our faucets sometimes does not start off being safe to drink. In most cases, it has gone through a water treatment plant designed by engineers prior to reaching our faucets.
This is a great activity for students to experience some "real-life" engineering.
Most any materials in a typical classroom can be adapted as filtration materials.
Water from lakes and rivers often has contaminants that make it unfit for drinking. The water may contain dirt, rocks and other objects that can be easily identified. Water may also contain bacteria and other microscopic organisms that cannot be seen easily. For these reasons, water that is delivered to our homes must go through a water treatment process. This is typically a five-part process that consists of aeration, coagulation, sedimentation, filtration and disinfection. This activity is only concerned with filtration, which removes most but not all of the impurities from the water. Make sure students know that in this activity the filtered water is still unfit to drink.
Recommended Resources:
Information on the water treatment process and drinking water standards: https://www.epa.gov/ground-water-and-drinking-water .
Information on different types of filters and filtration processes: https://www.thoughtco.com/filtration-definition-4144961 .
Before the Activity
- Gather materials and make copies of the Design Components Worksheet .
- Make the liter of dirty water and the "A," "B" and "C" tubes.
With the Students
- Engineering Challenge : Tell the students they have been hired by (your last name) Water Supply Company. With the ongoing drought, not enough water is available for all the things we need to supply – people, animals and plants. Tell them that they will each be given a sample of the dirty water they have remaining, and show them the tubes "A," "B" and "C." A is nearly ready for human use, B is nearly ready for animal use, and C is nearly ready to feed the plants. Remind them that no one must taste anything in the lab. They will be paid for their supply of filtered water: A gets $10 per ml, B gets $5 per ml, and C gets $1 per ml.
- Have students complete the worksheet to make sure they understand the activity purpose, and to help them think about the components of engineering design.
- Put trays of materials in front of the students. Let them decide in teams what materials they would like to use to filter their water. To challenge students, include one of the following constraints:
- Limit the amount of materials allowed for the design.
- Assign a price per unit of material and give students a budget to work within.
- Have students draw schematics of the layers. Once completed, give each team 25 ml of the dirty water to begin to filter in their test tubes.
- Once filtering is complete, have them bring the test tubes to you for observation. Decide if the water is A, B or C grade and help them measure their sample in a graduated cylinder. They must return to their desks and do the math to come up with their $ value. Have students put their $ values on the board.
Activity Embedded Assessment
Have students complete the Design Components Worksheet to assess their understanding of the activity and to encourage them to consider the design components involved in design a water filtration system. Sample answers are provided on the Design Components Worksheet Answer Key .
Post-Activity Assessment
Use the attached Rubric for Performance Assessment to evaluate students' design projects using criteria for the final filtering system and teamwork effort.
- What was the best filtering agent and why?
- What are other ways we purify our water?
- Design a package for your "clean" water.
Safety Issues
Make sure students know that in this activity the filtered water is still unfit to drink.

Students learn about water quality testing and basic water treatment processes and technology options. Biological, physical and chemical treatment processes are addressed, as well as physical and biological water quality testing, including testing for bacteria such as E. coli.
Students learn about the various methods developed by environmental engineers for treating drinking water in the United States.

Contributors
Supporting program.
Last modified: October 24, 2019
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Design and analysis of water quality monitoring and filtration system for different types of water in Malaysia
N. a. razman.
Advanced Device and System, Faculty of Engineering and Built Environment, Universiti Sains Islam Malaysia, 71800 Nilai, Negeri Sembilan Malaysia
W. Z. Wan Ismail
M. h. abd razak, j. jamaludin, associated data.
All data are presented in the manuscript.
The coding will be provided if needed.
Water pollution always occurs in Malaysia due to industrial, construction, agriculture, and household activities. River pollution can disturb water supply that eventually affects business and household activities. Thus, water quality monitoring system is needed to detect contaminated water. We developed a water quality monitoring and filtration system controlled by Arduino. The proposed system was designed in Proteus software and ThingSpeak platform was used for real-time monitoring. The main objective of the study was to compare water quality of river, lake and tap water in terms of pH, temperature, turbidity, electrical conductivity and oxidation–reduction potential. If the water quality was not satisfied, the water sample would be filtered through filtration system. Water turbidity level, pH, temperature, electrical conductivity, and oxidation–reduction potential for filtered and nonfiltered water were compared and analyzed according to international and national water quality standard. Besides that, statistical analysis such as box plot and one-way analysis of variance test was applied to validate data from the system. The real-time water quality monitoring system was implemented through data storage, data transfer, and data processing. The system was connected to wireless fidelity whereas the output data was sent to the user and monitored by ThingSpeak. The system can be further upgraded and scaled up to be applied in the main tank at our home or factory. The outcome of this research can be used as a reference for further study on lake and river pollution monitoring system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13762-022-04192-x.
Introduction
Surface water and groundwater are the main water supplies for Malaysia. Although Malaysia has abundant water resources, the accelerating pace of industrial development and population growth in recent decades has affected the quality of water (Bao 2010 ). Pandemic issue, Covid-19 also affects water quality when the non-essential services such as work for development project are ceased (Najah 2021 ). Domestic sewage, waste, and discharge from agriculture and manufacturing industries are the main sources of river pollution. Groundwater sources near agricultural areas, radioactive waste landfills, municipal water supply sources, and waste dumps are mostly polluted with arsenic, iron, lead and others (Khalit 2008 ; Bilal et al. 2016 ). Water pollution increases in parallel with the country development that shows the requirement to detect water pollutants. Detection system was built to recognize metal ions in water using colorimetric sensors (Alberti et al. 2020 ). Raril and Manjunatha ( 2020 ) developed a system that traced mercury and lead ions in water through the usage of polyglycine-modified graphene paste electrode. Each pollutant has distinct characteristics that can be traced. Thus, the detection system needs to be fast, accurate, and continuously up to date.
Monitoring water quality was previously done based on temporal and spatial scales which used multi-metric indices and operational indicators (Sany 2018 ). The quality of water in canals, drains and irrigation wells were analyzed and compared using MODFLOW software which could forecast the effects of surface water on groundwater in long-term period (Galal et al. 2020 ). It is essential to measure and monitor physical, chemical, and biological parameters of water in order to identify its quality. Then, types of water treatment can be identified before it can be supplied to consumers as tap water and drinking water (Huang et al. 2015 ; Alsulaili et al. 2015 ). Elsheikh et al. ( 2018 ) compared three different types of water filtration systems and allowed assessment and evaluation to be made towards the execution and fulfillment of each water filtration systems. Delgado et al. ( 2020 ) determined the effect of illegal mining on the water quality by applying grey system. By applying grey clustering method, water quality was grouped into three categories: good, moderate, and low water quality. Supriyono et al. ( 2020 ) developed a telemonitoring system that automatically measured and collected variables of water and air quality in coastal fishponds and continuously displayed the data to the user.
Water quality management and monitoring networks are also crucial. The allowed wastewater discharge rates for contaminant resources depend on two factors: treatment costs and water quality standard (Aghasian et al. 2017 ). Aghasian et al. integrated water quality simulation model with particle swarm optimization model and developed various pollution loadings discharge policies using bankruptcy method. Results showed that the proposed model reduced the salinity of the allocated water demands leading to a decrease in salinity discharged into the river. Genetic algorithm was developed to minimize wastewater treatment costs and dissolved oxygen violation from the standard level. This method was estimated to reduce waste load from 145.5 to 79 and treatment cost from the range of ($160,000–180,000) to ($100,000–130,000). Thus, the proposed models can satisfy water quality with low treatment cost (Farjoudi et. al. 2020 ). Meanwhile, monitoring network of groundwater was designed using DRASTIC method and capture zone analysis where the design was developed according to monitoring wells priority. Differential Evolution (DE) algorithm was used to optimize the DRASTIC model in order to find the highest correlation between high and vulnerable areas of electrical conductivity. The authors claimed that by proper estimating vulnerability of existing wells and the capture zones, the monitoring wells could be prioritized (Yousefi et. al. 2021 ). Thus, proper water quality monitoring, filtering, and treatment methods are needed.
Most previous studies on water quality monitoring system used different microcontroller unit such as Raspberry Pi, Arduino, and Waspmote. The studies mainly focused on the capability of the system to monitor water quality by detecting water quality parameter using various electronics sensors. The previous research focused more on the system design without doing any analysis.
This study proposes a real-time water quality monitoring system using Arduino and five types of sensors, completed with data analysis. ThingSpeak open-source software based on IoT technology was applied to analyze live data streams. Analysis was done for different types of water where one-way Analysis of Variance (ANOVA) test was used to observe the significance of the experimental results. The quality of filtered and unfiltered water was also compared according to water quality standard established by World Health Organization (Jabatan Alam Sekitar 2019 ; Engineering Services Division 2016 ; WHO 2011 ). Due to ongoing Restricted Movement Control Order from Malaysian Government because of Covid-19 Pandemic, water samples are limited to sites situated in Nilai, Negeri Sembilan. The water samples consisted of tap water taken from a local household situated in Nilai, river water from Mahang River and lake water from a lake in Universiti Sains Islam Malaysia. The sampling process was done in the month of November 2020 for ten hours (from 9:00 a.m. until 6:00 p.m) a day.
Materials and methods
Water samples were collected from Mahang River, a lake in Universiti Sains Islam Malaysia, and tap water. We monitored pH, turbidity, temperature, electrical conductivity (EC), and oxidation–reduction potential (ORP) of the samples from 9:00 a.m. until 6:00 p.m. for 10 h. Water samples were also transferred into a tank to undergo filtration process. Readings from the sensors were collected, translated and processed by the microcontroller before being displayed using LCD and uploaded into cloud storage for monitoring.
We designed a water monitoring and filtration system using sensors to detect temperature, pH value, oxidation–reduction potential (ORP), electrical conductivity (EC), and turbidity. A microcontroller unit consisted of Arduino Uno and Mega, was used as the main controller. We also used a data transmission block, ESP8266 Wi-Fi module and a water filtration unit which consisted of a water pump and a water filter.
NodeMCU software and open-source framework were used to construct Internet of Things (IoT) devices. The data processed by the microcontroller unit was uploaded and updated into Cloud storage and ThingSpeak application through data communication unit, ESP8266 Wi-Fi module. After logging into the ThingSpeak application with our unique user ID and password, we could access data from the monitoring system in real-time provided that the system was connected to the internet. Water quality for filtered and unfiltered water was also compared. Figure 1 shows the flowchart of the system.

Flow Chart of the Water Quality Monitoring System
The Arduino Uno processed the turbidity, pH, temperature, electrical conductivity (EC), and oxidation–reduction potential (ORP) of the water samples and sent to the ThingSpeak application using internet network. ThingSpeak application displayed the temperature, turbidity, pH, electrical conductivity (EC) and oxidation–reduction potential (ORP) value of water in real-time. Based on Fig. 1 , we determined the bad quality of water if the sensor readings exceed range of the threshold values. Water would be automatically pumped into a water filter for the filtration process. The threshold values for the sensors were set according to the limit set in the national water standard and World Health Organization (WHO) (Jabatan Alam Sekitar 2019 ; Engineering Services Division 2016 ; WHO 2011 ). The sensors were easy to use, cost-effective, and portable. Figure 2 shows the circuit design of the system in Proteus software. Simulation was done using the Proteus software to ensure the system could run properly before assembling the real circuit on board.

Circuit design drawn in Proteus Software. The design was translated into hardware system in Fig. 3
Figure 3 shows the electronic circuit of the proposed water quality monitoring system. All water quality parameter sensors, LCD display, Wi-Fi module and water pump were connected to the main microcontroller board. Figure 4 shows water quality system with filtration unit. Lake and river water samples were collected, stored and monitored in a tank connected to a filtration system. The sensor readings of the filtered water were observed and compared with the unfiltered water.

Electronic circuit of the proposed water quality monitoring system

The proposed technique of monitoring water with water filtration system
- Analysis Methods
We used boxplot analysis to observe the range of data and to identify any abnormal data. Boxplot is a technique to describe and summarize data. Boxplot graph shows the data distribution, range, minimum value, maximum value, mean, and skewness by showing five-number summary of a set of data which includes minimum and maximum range values, lower quartile, upper quartile, and median (Potter 2006 ).
Analysis of variance (ANOVA) is a statistical method that is useful to give important information like interpreting outcomes of an experiment and identifying the influence of a factor on the processing parameters by comparing the mean values of some samples (Ostertagova 2013 ). One-way ANOVA is used for data that are divided into several groups associated with only one factor. The mean values are compared to determine the significant level of differences in the associated population of samples. One-way ANOVA test produces F value (Eq. 1 ) (Sullivan 2021 ) and p-value .
n j is the size of j th group, X ¯ j is the sample mean in j th group, X ¯ is the overall mean, k is the number of independent groups and N is the total number of observations in the analysis (Sullivan 2021 ). MS represents mean squares, thus MS between is the mean sum of squares between sample groups and MS within is the mean sum of squares within the sample groups. F value is used to determine p-value where the p-value is the probability of F statistical values that measures the evidence to accept or reject hypothesis. To evaluate the null hypothesis, the differences between means are identified by comparing the p-value with the standard significance level, 0.05 (Ostertagova 2013).
Results and discussion
In this section, we depict output from water quality monitoring system and analyze the output based on five water quality parameters: pH, temperature, turbidity, oxidation–reduction potential and electrical conductivity. Real-time values of the parameters are observed through Thingspeak application which indirectly can assist users to monitor the system continuously. Figure 5 shows the real-time water quality monitoring system for users of ThingSpeak application.

The display of the ThingSpeak application shows the turbidity of the river water. Date and time are also displayed for real-time monitoring
(a) Physical parameters of water quality with box plot analysis
Temperature is an important parameter for water monitoring system as the taste, smells, viscosity, solubility and chemical reactions are influenced by temperature (APHA 2005 ; Alley 2007 ). Turbidity describes the cloudiness of water due to the presence of suspended particles like clay, silts, and precipitation of chemical and organic particles (APHA 2005 ; WHO 2017 ). Turbidity can be used as a good indicator to detect the presence of dangerous particles and contaminants (WHO 2017 ). Turbidity is expressed in terms of nephelometric turbidity unit (NTU). Turbidity above 5 NTU is visible to eyes (WHO 2017 ). The pH is an important water quality parameter to determine the basicity of an aqueous solution. The pH value from 0 to 14 indicates the acidity, neutrality or alkalinity of the water (Tomar 1999 ). Acidic solution has pH less than 7 and pH ~ 7 indicates a neutral solution. Meanwhile, pH greater than 7 indicates an alkalic solution (WHO 2011 ). Unreasonably high or low pH of water is not safe for household usage and drinking water (DeZuane 1997 ). The acceptable pH for consuming and drinking water is from 6.5 to 9 (Engineering Services Division 2016 ) whereas water temperature can be influenced by surrounding environment.
Electrical conductivity (EC) refers to the ability of electric to flow in the water. Conductivity value of water is normally constant throughout the times. Thus, any changes in conductivity depict the possibility of water pollution due to chemical reaction with water. Conductivity will increase due to the presence of chemicals like chloride, phosphate and nitrate ions (EPA 2012 ). Meanwhile, oxidation–reduction potential (ORP) can be defined as the capability of a water source to break down waste products like dead plants and animals. Unpolluted lakes and rivers have high value of oxidation–reduction potential, showing that the water has high amount of dissolved oxygen (Kremer 2018 ).
Monitoring water quality parameters is done by taking the readings of the sensors for ten hours from 9:00 a.m. until 6:00 p.m. All water quality parameters detected in the system are compared with the National Water Quality Standard and Drinking Water Quality Standard (WHO 2011 ; Engineering Services Division 2016 ).
Figure 6 (a) shows temperature values for tap, lake and river water and Fig. 6 (b) depicts the range of temperature in boxplot. There are two abnormal temperature values for tap water and river water shows higher fluctuation compared to others. The fluctuation in temperature is due to sensitivity of sensors. Figure 6 (a) and (b) show that river water has the lowest temperature with the range from 27.87 °C to 28.91 °C with the average, 28.19 °C and the standard deviation, 0.349. Lake water has the highest range of temperature, from 30.18 to 30.76 °C with the average, 30.32 °C and the standard deviation, 0.170. The temperature of tap water fluctuates from 27.6 to 30 °C. The mean temperature is 29.55 °C and the standard deviation value is 0.754. Lake water has the highest temperature as the surface of the water is exposed to sunlight and has zero or low motion water. Lake water also shows stable temperature, proved by the small standard deviation whereas temperature of tap water fluctuates resulting in the highest standard deviation. Tap water comes from tank and pipe, can be easily affected by the temperature of the day. Water takes longer time to warm up and cool down compared to air. River water has the lowest temperature because it is continuously flowing resulting in slow heat transfer.

a Temperature values for three water samples; b Temperature boxplot for three water samples. Ten readings are used for the analysis
Figure 7 (a) and (b) show the pH variations of water samples and the boxplot for pH values. Normal tap water has neutral pH from 7.16 to 8.33 with the average pH, 7.551 and the standard deviation, 0.477. High fluctuation of pH in tap water (Fig. 7 (b)) is attributed to chemicals in the water as pH is related to the amount of hydrogen and hydroxyl ions. (Tomar 1999 ). River water has higher pH compared to tap water with the range from 8.04 to 8.16, the average pH, 8.09 and standard deviation, 0.062. Lake water has the highest pH ranging from 9.04 to 9.18. The mean pH for lake water sample is 9.09 with standard deviation, 0.051. The lake water has the highest pH due to the presence of carbonated-rich soils like limestone. Besides that, algae and aquatic plants in the lake water apply photosynthesis process that uses hydrogen which also can contribute to the high pH in the lake water (Fondriest Environmental 2013 ). The pH of river and lake can also be affected by the activities at the shoreline.

a pH variation for three water samples; b pH boxplot for three water samples. Ten readings are used for the analysis
Figure 8 (a) and (b) show the turbidity variations and the boxplot for all samples. Lake water depicts the highest fluctuation of turbidity in 10 h (Fig. 8 (b)), due to variation of organic materials. Tap water shows the lowest turbidity level whereas river depicts the highest turbidity level. Tap water has turbidity level from 0 to 2 NTU. The mean value is 0.24 NTU and the standard deviation value is 0.622. River water depicts turbidity level from 48.05 NTU to 98.43 NTU while lake water sample shows the highest turbidity values, from 136.92 NTU to 223.72 NTU. The mean turbidity value for river and lake samples are 77.004 NTU and 181.292 NTU respectively. The standard deviation of the turbidity for river and lake water are 16.860 and 39.07, showing higher fluctuation compared to tap water. Tap water has the lowest turbidity level because the water is transparent and clear. The river water is turbid due to the presence of suspended and colloidal matter like clay, silt, finely divided organic and inorganic matter, plankton and other microscopic organisms (Allen et al. 2008 ). Lake has the highest range of turbidity, attributed to the clays, silts, suspended bottom sediments, phytoplankton and algae. According to Malaysia National Water Standard, based on the turbidity, pH and temperature of all water samples, tap water is safe to be consumed but lake and river water need to be treated and filtered before we can consume it as drinking water (Jabatan Alam Sekitar 2019 ).

a Turbidity values for the three water samples; b Turbidity boxplot for three water samples. Ten readings are used for the analysis
Figure 9 shows the electrical conductivity values of the water samples. Due to the use of chlorine that contains electrons in water treatment process, tap water shows the highest range of electrical conductivity values and highest fluctuation (Kremer 2018 ). Electrical conductivity of tap water has maximum value ~ 1.81mS/cm and minimum value ~ 0.88mS/cm with standard deviation ~ 0.33 and mean ~ 1.2mS/cm. The range of electrical conductivity of river water is from 0.22 to 0.33mS/cm, the lowest range compared to other samples. The mean and standard deviation of electrical conductivity for river water are 0.25 and 0.04 respectively. Charged ions in river and lake water come from the presence of dissolved minerals like clay soils or limestone (Huron River Watershed Council 2013 ).

a Electrical conductivity values for three water samples; b Electrical conductivity boxplot for three water samples. Ten readings are used for the analysis
Figure 10 shows the readings of oxidation–reduction potential of the water samples. The oxidation–reduction potential values of tap water are in the highest range, from 280 to 289 mV with standard deviation ~ 3.14 while river water has the lowest oxidation–reduction potential readings with range from 153 to 173 mV and standard deviation ~ 6.15. Tap water has the highest range of oxidation–reduction potential due to water treatment that involves chlorine. Chlorine is used in water treatment to kill bacteria, parasites, viruses and microbes in water by neutralizing and oxidizing bacteria, parasites, viruses and microbes. This oxidation process increases the level of oxidation–reduction potential values of water (Kremer 2018 ). The oxidation–reduction potential values of river and lake water samples fall slightly under the healthy range of freshwater oxidation–reduction potential value, from 300 to 500 millivolts. Lower oxidation–reduction value is also expected at the bottom of the lake and river. Oxygen level at the bottom of the lake and river is low due to the usage of the oxygen by bacteria (Wetzel 2001 ).

a Oxidation–reduction potential variation of three water samples; b Oxidation–reduction potential boxplot for three water samples. Ten readings are used for the analysis
(b) Statistical analysis—One-way ANOVA
One-way Analysis of variance (ANOVA) model is done to test the null hypothesis that states all parameters have similar population mean. If the variability of the sample mean is large, the null hypothesis is rejected. The alternate hypothesis that states at least one population mean is different from the others will be accepted (Ostertagova 2013 ). When there are statistically significant differences between the means, p-value is less than or equal to α value (0.05). Null hypothesis is rejected as there is at least one different population mean. Table Table1 1 shows one-way ANOVA test results done using OriginPro software. The one-way ANOVA test revealed that there was a statistically significant difference in the means of pH, temperature, turbidity, electrical conductivity and oxidation–reduction potential between at least two groups with ( F (3,10) = [78.1138], p = 0.0000), ( F (3,10) = [48.6633], p = 0.0000), ( F (3,10) = [136.7881], p = 0.0000), ( F (3,10) = [62.3271], p = 0.0000) and ( F (3,10) = [466.1869], p = 0.0000) respectively. From the table, the p-value of all parameters is less than 0.05. Thus, null hypothesis is rejected. For all water parameters tested, at least one group of water samples has a significant difference from overall mean of the water samples. The statistical significance shows that data collected are probably true and is due to factors of interest. Thus, we strongly suppose that pH, temperature, turbidity, electrical conductivity, and oxidation–reduction potential can give effect the analysis of water quality.
One-way ANOVA test for different water parameters
(c) Comparison of filtered and unfiltered water
Figure 11 shows the turbidity, pH and temperature of normal tap water from the same source for two days. As normal tap water is commonly safe to be used, the water does not need to undergo filtration process. The tap water sample from same source is compared for two different days. The mean value for turbidity, pH value and temperature of the tap water sample in the first and second day are 0.1 NTU, 7.501 and 29.21 °C respectively. The standard deviation values for tap water in both days are 0.208 for turbidity, 0.429 for pH values and 1.026 for temperature. The mean and standard deviation depicts that the system is reliable due to less fluctuation especially for turbidity sensing system. The turbidity readings for tap water are lower than 1 NTU, in the safe range of water quality (WHO 2017 ). The pH of the tap water shows that the water is in the neutral and light alkalic range. The temperature of tap water fluctuates depending on the weather and surrounding environment. Figure 12 shows the turbidity, temperature, and pH value of lake water before and after filtration process. The average of turbidity, temperature, and pH for lake water before filtration is 181.29 NTU, 30.32 °C and 9.09 respectively while the average of turbidity, temperature, and pH for lake water after filtration are 16.91 NTU, 29.43 °C and 7.34 respectively. Figure 13 shows the turbidity, temperature, and pH of river water before and after filtration process. The average of turbidity, temperature, and pH for river water before filtration is 77.00 NTU, 28.18 °C and 8.09 respectively while the mean values of turbidity, temperature, and pH for river water after filtration are 24.22 NTU, 29.5 °C and 7.52 respectively.

Turbidity levels, pH value and temperature of tap water for day one and day two

Comparison of water quality parameters for unfiltered and filtered lake water

Comparison of water quality parameters for unfiltered and filtered river water
For both lake and river water samples, the turbidity level decreases after the water samples go through filtration process. It proves that the water filtration process successfully provides better quality of water. Meanwhile, the pH values for lake and river water after filtration system are neutral. The system filters unwanted particles in the sample such as mud and soil which are the main contributors for high turbidity and pH values. Some abnormal pH values of river sample after filtration are detected due to sensor sensitivity as the electrode of the pH sensor is very susceptible to interference. The temperature of the lake sample after filtration is slightly lower than the unfiltered samples with small fluctuation. Higher temperature of the filtered river water than the unfiltered river water is attributed to the less mud and soil in the samples and surrounding temperature of the filter.
Many studies have been done to improve existing water quality monitoring systems. Kumar et al. ( 2020 ) developed a water quality system measuring pH, conductivity, temperature and turbidity which allowed real time monitoring. Demetillo et al. ( 2019 ) developed a system for monitoring water quality in a large aquatic area highlighting the usage of the wireless sensor network (WSN). Pasika et al. ( 2020 ) applied arduino microcontroller unit (MCU) and interfaced with several physical water parameter sensors to develop a smart water quality monitoring system with cost-effective using IoT. User can monitor the water quality using ThingSpeak platform where data obtained from the system is sent to cloud. However, most of the studies do not analyze the data and do comparison with the national and international standard. Thus, our study is important due to reliable data that can become a good reference to the Malaysia government.
In conclusion, water pollution is a detrimental issue that leads to water cut, health problem and a threat to aquatic life. Monitoring water quality is important to control water pollution. Here, water quality monitoring and filtration system was designed using Proteus software. Arduino was used as the main controller and five sensors were applied to detect physical parameters of water to monitor water quality. The data is analyzed based on three types of water samples, tap water, lake and river in Nilai, Negeri Sembilan, Malaysia. The purpose of this research is to investigate and monitor the turbidity level, pH value, temperature, electrical conductivity and oxidation–reduction potential of three different types of water resources in Malaysia. The study shows that tap water that is supplied to the household, has the safe range of turbidity and pH. The filtered lake and river water which give lower turbidity and neutral pH values than the unfiltered samples prove that the filtration system is crucially needed for the household use. Tap water shows the highest electrical conductivity and oxidation–reduction potential due to chlorine that is used for treating the water. The data analysis is done using box plot and one-way ANOVA test. The ANOVA test shows that the sensing parameters can be used to monitor water quality. The ThingSpeak application gives real-time and continuous water quality monitoring system. The research outcome is important to analyze water quality in Malaysia before it can be safely used by consumers. The system can be upgraded using high endurance and capacity of microcontroller and sensors. Then, it can be applied in the groundwater source or tank at home. We believe the proposed system can assist Malaysia government in closely monitor physical parameters of water quality and indirectly mitigate water pollution issues.
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge Ministry of Education Malaysia under FRGS grant (FRGS/1/2021/WAB02/USIM/02/1), Universiti Sains Islam Malaysia (USIM) under matching grant (USIM/MG/MMU-PPKMT-ZDA/FKAB/SEPADAN-S/70822) and Faculty of Engineering and Built Environment (FKAB) for the funding and support.
Author contributions
The experiment was done by Nur Afiqah with help from Muhammad Hakim. Wan Zakiah, Irneza and Juliza contribute to literature review and discussion.
Funding for this project is provided by Ministry of Higher Education, Malaysia under Fundamental Research Grant Scheme (FRGS) (grant code: FRGS/1/2021/WAB02/USIM/02/1) and Universiti Sains Islam Malaysia (matching grant:USIM/MG/MMU-PPKMT-ZDA/FKAB/SEPADAN-S/70822).
Data availability
Code availability, declarations.
The authors declare that they have no competing interests.
Not applicable.
Not appplicable.
- Aghasian K, Moridi A, Mirbagheri A, Abbaspour A. A conflict resolution method for waste load reallocation in river syatems. Int J of Environ Sci Tech. 2017 doi: 10.1007/s13762-018-1993-3. [ CrossRef ] [ Google Scholar ]
- Alberti G, Zanoni C, Magnaghi LR, Biesuz R. Low-cost, disposable colourimetric sensors for metal ions detection. J Analyt Sci Tech. 2020; 11 :30. doi: 10.1186/s40543-020-00221-x. [ CrossRef ] [ Google Scholar ]
- Allen M J, Brecher R W, Copes R, Hrudey S E, Payment P (2008) Turbidity and microbial risk in drinking water. Ministerial technical advisory committee
- Alley E. Water quality control handbook. New York: McGraw Hill; 2007. [ Google Scholar ]
- Alsulaili A, Al-Harbi M, Al-Tawari K. Physical and chemical characteristics of drinking water quality in kuwait: tap vs bottled water. J Eng Res. 2015; 3 (1):25–50. doi: 10.7603/s40632-015-0002-y. [ CrossRef ] [ Google Scholar ]
- APHA . Standard methods for the examination of water and wastewater. 21. Washington: American Public Health Association; 2005. [ Google Scholar ]
- Bao JL. Water quality study and its relationship with high tide and low tide at kuantan river. Kuantan: Universiti Malaysia Pahang; 2010. [ Google Scholar ]
- Bilal H, Ghafoor A, Munir A. Desalination of brackish water using dual acting solar still. J Eng Res. 2016; 4 (4):178–193. [ Google Scholar ]
- Delgado A, Jiminez J, Ochoa L, Mendiola L, Alvarado A, Huamani EL. Applying grey systems to analyze water quality on the river. Int J Emerging Trends in Eng Res. 2020; 8 (7):3880–3886. doi: 10.30534/ijeter/2020/156872020. [ CrossRef ] [ Google Scholar ]
- Demetillo AT, Japitana MV, Taboada EB. A System for monitoring water quality in a large aquatic area using wireless sensor network technology. Sustain Environ Res. 2019; 29 :12. doi: 10.1186/s42834-019-0009-4. [ CrossRef ] [ Google Scholar ]
- DeZuane J. Handbook of drinking water quality. 2. New York: John Wiley & Sons; 1997. [ Google Scholar ]
- Elsheikh MA, Saleh HI, Elmala Y. Performance of filtration systems to enhance treated water quality. J Eng App Sc. 2018; 65 (2):125–137. [ Google Scholar ]
- Engineering Services Division . Drinking water quality standard. Malaysia: Ministry of Health Malaysia; 2016. [ Google Scholar ]
- Fondriest Environmental (2013) Fondriest environmental learning centre - pH of Water. Fondriest environmental inc. retrieved December 10, 2020, from https://www.fondriest.com/environmental-measurements/parameters/water-quality/ph/
- EPA (2012) 5.9 Conductivity In water: monitoring and assessment. Retrieved July 09, 2021, from http://water.epa.gov/type/rsl/monitoring/vms59.cfm
- Galal MM, Soliman A, Kamel G, Zaher K, El-Fakharany Z. Prediction and assessment of surface water quality effect on groundwater in El-Qaluybia. Egypt J Eng App Sc. 2020; 67 (8):2129–2148. [ Google Scholar ]
- Huang Y F, Ang S Y, Lee K M, Lee T S (2015) Quality of water resources in Malaysia. In:research and practices in water quality. Rijeka: IntechOpen. 10.5772/58969
- Huron river watershed council (2013) conductivity the activity. Retrieved July 09, 2021, from http://www.hrwc.org/wp-content/uploads/2013/02/Conductivity-v2.pdf
- Jabatan Alam Sekitar . Malaysia environmental quality report 2017 - river water quality. Malaysia: Ministry of Environment and Water; 2018. [ Google Scholar ]
- Jabatan Alam Sekitar . National water quality standard. Malaysia: Ministry of Environment and Water; 2019. [ Google Scholar ]
- Khalit AR. Current state of water environment in Malaysia. Malaysia: Conservation and Environment Management Division KeTSA; 2008. [ Google Scholar ]
- Kremer M (2018) Water quality 101: what is ORP in water testing? Jenco. Retrieved Aug 02, 2021, from https://blog.jencoi.com/what-is-orp-in-water-testing
- Kumar SS, Subba Rao BV, Prasad JR. Design and development of a water quality monitoring system by using IoT. Int J Emerging Trends in Eng Res (IJRTE) 2020; 8 (9):6441–6447. doi: 10.30534/ijeter/2020/245892020. [ CrossRef ] [ Google Scholar ]
- Matin M A, Islam M M (2012) Overview of wireless sensor network. In: wireless sensor networks - technology and protocols Malaysia. IntechOpen 10.5772/49376.
- Najah A, Teo FY, Chow MF, Huang YF, Latif SD, Abdullah S, Ismail M, El-Shafie A. Surface water quality status and prediction during movement control operation order under COVID-19 pandemic: case studies in Malaysia. Int J Environ Sc Technol. 2021; 18 :1009–1018. doi: 10.1007/s13762-021-03139-y. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ostertagova E, Oskar O. Methodology and Application of One-way ANOVA. American J Mechanical Eng. 2013; 1 (7):256–261. doi: 10.12691/ajme-1-7-21. [ CrossRef ] [ Google Scholar ]
- Pasika S, Gandla ST. Smart water quality monitoring system with cost-effective using IoT. Heliyon. 2020; 6 (7):1–9. doi: 10.1016/j.heliyon.2020.e04096. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Potter K (2006) Methods for presenting statistical information: the box plot. Retrieved 2 Aug 2021, from https://www.sci.utah.edu/~kpotter/publications/potter-2006-MPSI.pdf
- Raril C, Manjunatha JG. Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of mercury and lead ions in water and biological samples. J Analytical Sc Tech. 2020; 11 :3. doi: 10.1186/s40543-019-0194-0. [ CrossRef ] [ Google Scholar ]
- Sullivan L (2021) The ANOVA approach. hypothesis testing – analysis of variance (ANOVA). Boston university school of public health. Retrieved August 02, 2021, from https://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704_HypothesisTesting-ANOVA/BS704_HypothesisTesting-Anova_print.html
- Supriyono H, Majid A, Harismah K. Portable system for coastal fishpond water quality telemonitoring continuously. Int J Emerging Trends Eng Res. 2020; 8 (9):5163–5170. doi: 10.30534/ijeter/2020/45892020. [ CrossRef ] [ Google Scholar ]
- Tavakoly Sany SB, Monazami G, Rezayi M, Borgheipour H. Application of water quality indices for evaluating water quality and anthropogenic impact assessment. Int J Environ Sc Technol. 2018; 16 :3001–3012. doi: 10.1007/s13762-018-1894-5. [ CrossRef ] [ Google Scholar ]
- Tomar M. Water and wastewater. Boca Raton: CRC Press; 1999. [ Google Scholar ]
- Wetzel RG. Limnology: lake and river ecosystems. 3. San Diego: Academic Press; 2001. [ Google Scholar ]
- WHO . Guidelines for drinking water quality. Geneva: World Health Organisation; 2011. [ Google Scholar ]
- WHO. (2017). Water quality and health - review of turbidity: information for regulators and water suppliers. World Health Organisation
- Yousefi H, Omidi M, Moridi A, Sarang A. Groundwater monitoring network design using optimized DRASTIC method and capture zone analysis. Int J of Environ Res. 2021 doi: 10.1007/s41742-021-00348-8. [ CrossRef ] [ Google Scholar ]
- Zare Farjoudi S, Moridi A, Sarang A. Multi-objective waste load allocation in river system under inflow uncertainty. Int J of Environ Sc and Tech. 2020 doi: 10.1007/s13762-020-02897-5. [ CrossRef ] [ Google Scholar ]
Design and analysis of water quality monitoring and filtration system for different types of water in Malaysia
- Original Paper
- Published: 11 June 2022
- Volume 20 , pages 3789–3800, ( 2023 )
Cite this article
- N. A. Razman 1 ,
- W. Z. Wan Ismail ORCID: orcid.org/0000-0003-2298-1535 1 ,
- M. H. Abd Razak 1 ,
- I. Ismail 1 &
- J. Jamaludin 1
9791 Accesses
6 Citations
Explore all metrics
Water pollution always occurs in Malaysia due to industrial, construction, agriculture, and household activities. River pollution can disturb water supply that eventually affects business and household activities. Thus, water quality monitoring system is needed to detect contaminated water. We developed a water quality monitoring and filtration system controlled by Arduino. The proposed system was designed in Proteus software and ThingSpeak platform was used for real-time monitoring. The main objective of the study was to compare water quality of river, lake and tap water in terms of pH, temperature, turbidity, electrical conductivity and oxidation–reduction potential. If the water quality was not satisfied, the water sample would be filtered through filtration system. Water turbidity level, pH, temperature, electrical conductivity, and oxidation–reduction potential for filtered and nonfiltered water were compared and analyzed according to international and national water quality standard. Besides that, statistical analysis such as box plot and one-way analysis of variance test was applied to validate data from the system. The real-time water quality monitoring system was implemented through data storage, data transfer, and data processing. The system was connected to wireless fidelity whereas the output data was sent to the user and monitored by ThingSpeak. The system can be further upgraded and scaled up to be applied in the main tank at our home or factory. The outcome of this research can be used as a reference for further study on lake and river pollution monitoring system.
Similar content being viewed by others

In-situ Measurement in Water Quality Status—Udalka Uttarakhand, India
Internet of things enabled real time water quality monitoring system.
S. Geetha & S. Gouthami

Toward Sustainable Smart Cities: Smart Water Quality Monitoring System Based on IoT Technology
Avoid common mistakes on your manuscript.
Introduction
Surface water and groundwater are the main water supplies for Malaysia. Although Malaysia has abundant water resources, the accelerating pace of industrial development and population growth in recent decades has affected the quality of water (Bao 2010 ). Pandemic issue, Covid-19 also affects water quality when the non-essential services such as work for development project are ceased (Najah 2021 ). Domestic sewage, waste, and discharge from agriculture and manufacturing industries are the main sources of river pollution. Groundwater sources near agricultural areas, radioactive waste landfills, municipal water supply sources, and waste dumps are mostly polluted with arsenic, iron, lead and others (Khalit 2008 ; Bilal et al. 2016 ). Water pollution increases in parallel with the country development that shows the requirement to detect water pollutants. Detection system was built to recognize metal ions in water using colorimetric sensors (Alberti et al. 2020 ). Raril and Manjunatha ( 2020 ) developed a system that traced mercury and lead ions in water through the usage of polyglycine-modified graphene paste electrode. Each pollutant has distinct characteristics that can be traced. Thus, the detection system needs to be fast, accurate, and continuously up to date.
Monitoring water quality was previously done based on temporal and spatial scales which used multi-metric indices and operational indicators (Sany 2018 ). The quality of water in canals, drains and irrigation wells were analyzed and compared using MODFLOW software which could forecast the effects of surface water on groundwater in long-term period (Galal et al. 2020 ). It is essential to measure and monitor physical, chemical, and biological parameters of water in order to identify its quality. Then, types of water treatment can be identified before it can be supplied to consumers as tap water and drinking water (Huang et al. 2015 ; Alsulaili et al. 2015 ). Elsheikh et al. ( 2018 ) compared three different types of water filtration systems and allowed assessment and evaluation to be made towards the execution and fulfillment of each water filtration systems. Delgado et al. ( 2020 ) determined the effect of illegal mining on the water quality by applying grey system. By applying grey clustering method, water quality was grouped into three categories: good, moderate, and low water quality. Supriyono et al. ( 2020 ) developed a telemonitoring system that automatically measured and collected variables of water and air quality in coastal fishponds and continuously displayed the data to the user.
Water quality management and monitoring networks are also crucial. The allowed wastewater discharge rates for contaminant resources depend on two factors: treatment costs and water quality standard (Aghasian et al. 2017 ). Aghasian et al. integrated water quality simulation model with particle swarm optimization model and developed various pollution loadings discharge policies using bankruptcy method. Results showed that the proposed model reduced the salinity of the allocated water demands leading to a decrease in salinity discharged into the river. Genetic algorithm was developed to minimize wastewater treatment costs and dissolved oxygen violation from the standard level. This method was estimated to reduce waste load from 145.5 to 79 and treatment cost from the range of ($160,000–180,000) to ($100,000–130,000). Thus, the proposed models can satisfy water quality with low treatment cost (Farjoudi et. al. 2020 ). Meanwhile, monitoring network of groundwater was designed using DRASTIC method and capture zone analysis where the design was developed according to monitoring wells priority. Differential Evolution (DE) algorithm was used to optimize the DRASTIC model in order to find the highest correlation between high and vulnerable areas of electrical conductivity. The authors claimed that by proper estimating vulnerability of existing wells and the capture zones, the monitoring wells could be prioritized (Yousefi et. al. 2021 ). Thus, proper water quality monitoring, filtering, and treatment methods are needed.
Most previous studies on water quality monitoring system used different microcontroller unit such as Raspberry Pi, Arduino, and Waspmote. The studies mainly focused on the capability of the system to monitor water quality by detecting water quality parameter using various electronics sensors. The previous research focused more on the system design without doing any analysis.
This study proposes a real-time water quality monitoring system using Arduino and five types of sensors, completed with data analysis. ThingSpeak open-source software based on IoT technology was applied to analyze live data streams. Analysis was done for different types of water where one-way Analysis of Variance (ANOVA) test was used to observe the significance of the experimental results. The quality of filtered and unfiltered water was also compared according to water quality standard established by World Health Organization (Jabatan Alam Sekitar 2019 ; Engineering Services Division 2016 ; WHO 2011 ). Due to ongoing Restricted Movement Control Order from Malaysian Government because of Covid-19 Pandemic, water samples are limited to sites situated in Nilai, Negeri Sembilan. The water samples consisted of tap water taken from a local household situated in Nilai, river water from Mahang River and lake water from a lake in Universiti Sains Islam Malaysia. The sampling process was done in the month of November 2020 for ten hours (from 9:00 a.m. until 6:00 p.m) a day.
Materials and methods
Water sampling method
Water samples were collected from Mahang River, a lake in Universiti Sains Islam Malaysia, and tap water. We monitored pH, turbidity, temperature, electrical conductivity (EC), and oxidation–reduction potential (ORP) of the samples from 9:00 a.m. until 6:00 p.m. for 10 h. Water samples were also transferred into a tank to undergo filtration process. Readings from the sensors were collected, translated and processed by the microcontroller before being displayed using LCD and uploaded into cloud storage for monitoring.
System design
We designed a water monitoring and filtration system using sensors to detect temperature, pH value, oxidation–reduction potential (ORP), electrical conductivity (EC), and turbidity. A microcontroller unit consisted of Arduino Uno and Mega, was used as the main controller. We also used a data transmission block, ESP8266 Wi-Fi module and a water filtration unit which consisted of a water pump and a water filter.
NodeMCU software and open-source framework were used to construct Internet of Things (IoT) devices. The data processed by the microcontroller unit was uploaded and updated into Cloud storage and ThingSpeak application through data communication unit, ESP8266 Wi-Fi module. After logging into the ThingSpeak application with our unique user ID and password, we could access data from the monitoring system in real-time provided that the system was connected to the internet. Water quality for filtered and unfiltered water was also compared. Figure 1 shows the flowchart of the system.
The Arduino Uno processed the turbidity, pH, temperature, electrical conductivity (EC), and oxidation–reduction potential (ORP) of the water samples and sent to the ThingSpeak application using internet network. ThingSpeak application displayed the temperature, turbidity, pH, electrical conductivity (EC) and oxidation–reduction potential (ORP) value of water in real-time. Based on Fig. 1 , we determined the bad quality of water if the sensor readings exceed range of the threshold values. Water would be automatically pumped into a water filter for the filtration process. The threshold values for the sensors were set according to the limit set in the national water standard and World Health Organization (WHO) (Jabatan Alam Sekitar 2019 ; Engineering Services Division 2016 ; WHO 2011 ). The sensors were easy to use, cost-effective, and portable. Figure 2 shows the circuit design of the system in Proteus software. Simulation was done using the Proteus software to ensure the system could run properly before assembling the real circuit on board.
Figure 3 shows the electronic circuit of the proposed water quality monitoring system. All water quality parameter sensors, LCD display, Wi-Fi module and water pump were connected to the main microcontroller board. Figure 4 shows water quality system with filtration unit. Lake and river water samples were collected, stored and monitored in a tank connected to a filtration system. The sensor readings of the filtered water were observed and compared with the unfiltered water.
Analysis Methods
Boxplot analysis
We used boxplot analysis to observe the range of data and to identify any abnormal data. Boxplot is a technique to describe and summarize data. Boxplot graph shows the data distribution, range, minimum value, maximum value, mean, and skewness by showing five-number summary of a set of data which includes minimum and maximum range values, lower quartile, upper quartile, and median (Potter 2006 ).
One-way ANOVA
Analysis of variance (ANOVA) is a statistical method that is useful to give important information like interpreting outcomes of an experiment and identifying the influence of a factor on the processing parameters by comparing the mean values of some samples (Ostertagova 2013 ). One-way ANOVA is used for data that are divided into several groups associated with only one factor. The mean values are compared to determine the significant level of differences in the associated population of samples. One-way ANOVA test produces F value (Eq. 1 ) (Sullivan 2021 ) and p-value .
\({n}_{j}\) is the size of j th group, \({\overline{X} }_{j}\) is the sample mean in j th group, \(\overline{X }\) is the overall mean, \(k\) is the number of independent groups and \(N\) is the total number of observations in the analysis (Sullivan 2021 ). \(MS\) represents mean squares, thus \({MS}_{between}\) is the mean sum of squares between sample groups and \({MS}_{within}\) is the mean sum of squares within the sample groups. F value is used to determine p-value where the p-value is the probability of F statistical values that measures the evidence to accept or reject hypothesis. To evaluate the null hypothesis, the differences between means are identified by comparing the p-value with the standard significance level, 0.05 (Ostertagova 2013).
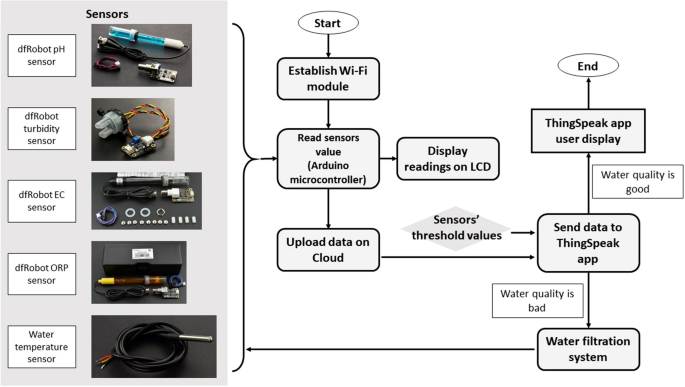
Flow Chart of the Water Quality Monitoring System
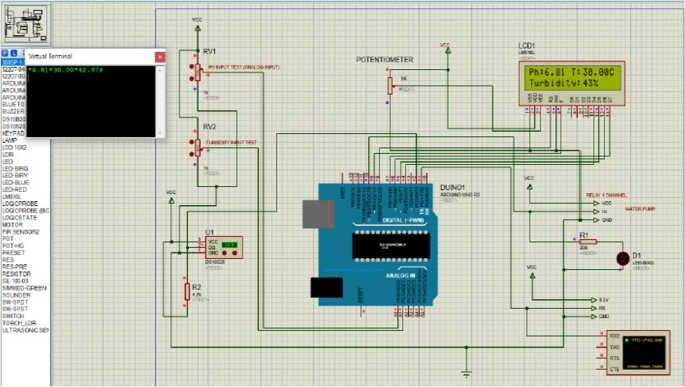
Circuit design drawn in Proteus Software. The design was translated into hardware system in Fig. 3
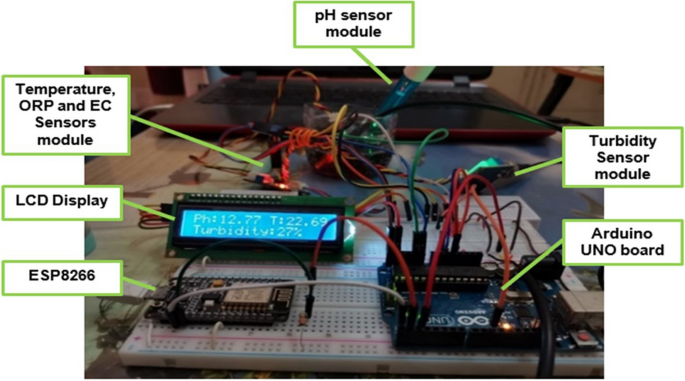
Electronic circuit of the proposed water quality monitoring system
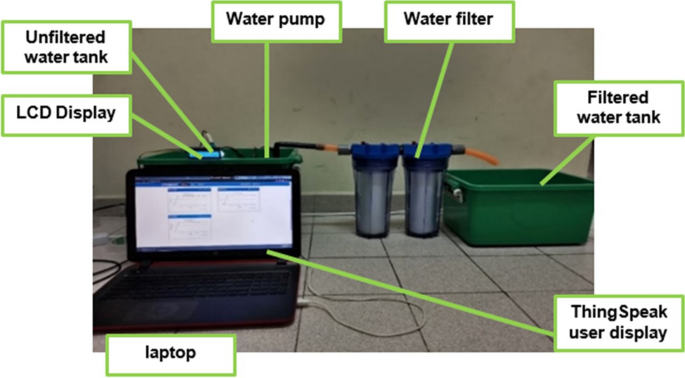
The proposed technique of monitoring water with water filtration system
Results and discussion
In this section, we depict output from water quality monitoring system and analyze the output based on five water quality parameters: pH, temperature, turbidity, oxidation–reduction potential and electrical conductivity. Real-time values of the parameters are observed through Thingspeak application which indirectly can assist users to monitor the system continuously. Figure 5 shows the real-time water quality monitoring system for users of ThingSpeak application.
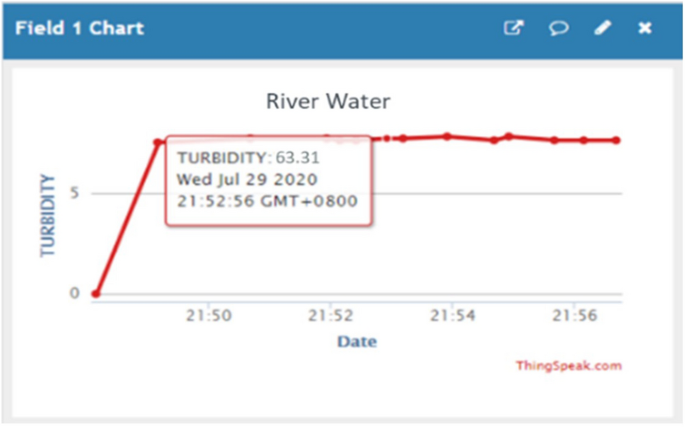
The display of the ThingSpeak application shows the turbidity of the river water. Date and time are also displayed for real-time monitoring
(a) Physical parameters of water quality with box plot analysis
Temperature is an important parameter for water monitoring system as the taste, smells, viscosity, solubility and chemical reactions are influenced by temperature (APHA 2005 ; Alley 2007 ). Turbidity describes the cloudiness of water due to the presence of suspended particles like clay, silts, and precipitation of chemical and organic particles (APHA 2005 ; WHO 2017 ). Turbidity can be used as a good indicator to detect the presence of dangerous particles and contaminants (WHO 2017 ). Turbidity is expressed in terms of nephelometric turbidity unit (NTU). Turbidity above 5 NTU is visible to eyes (WHO 2017 ). The pH is an important water quality parameter to determine the basicity of an aqueous solution. The pH value from 0 to 14 indicates the acidity, neutrality or alkalinity of the water (Tomar 1999 ). Acidic solution has pH less than 7 and pH ~ 7 indicates a neutral solution. Meanwhile, pH greater than 7 indicates an alkalic solution (WHO 2011 ). Unreasonably high or low pH of water is not safe for household usage and drinking water (DeZuane 1997 ). The acceptable pH for consuming and drinking water is from 6.5 to 9 (Engineering Services Division 2016 ) whereas water temperature can be influenced by surrounding environment.
Electrical conductivity (EC) refers to the ability of electric to flow in the water. Conductivity value of water is normally constant throughout the times. Thus, any changes in conductivity depict the possibility of water pollution due to chemical reaction with water. Conductivity will increase due to the presence of chemicals like chloride, phosphate and nitrate ions (EPA 2012 ). Meanwhile, oxidation–reduction potential (ORP) can be defined as the capability of a water source to break down waste products like dead plants and animals. Unpolluted lakes and rivers have high value of oxidation–reduction potential, showing that the water has high amount of dissolved oxygen (Kremer 2018 ).
Monitoring water quality parameters is done by taking the readings of the sensors for ten hours from 9:00 a.m. until 6:00 p.m. All water quality parameters detected in the system are compared with the National Water Quality Standard and Drinking Water Quality Standard (WHO 2011 ; Engineering Services Division 2016 ).
Figure 6 (a) shows temperature values for tap, lake and river water and Fig. 6 (b) depicts the range of temperature in boxplot. There are two abnormal temperature values for tap water and river water shows higher fluctuation compared to others. The fluctuation in temperature is due to sensitivity of sensors. Figure 6 (a) and (b) show that river water has the lowest temperature with the range from 27.87 °C to 28.91 °C with the average, 28.19 °C and the standard deviation, 0.349. Lake water has the highest range of temperature, from 30.18 to 30.76 °C with the average, 30.32 °C and the standard deviation, 0.170. The temperature of tap water fluctuates from 27.6 to 30 °C. The mean temperature is 29.55 °C and the standard deviation value is 0.754. Lake water has the highest temperature as the surface of the water is exposed to sunlight and has zero or low motion water. Lake water also shows stable temperature, proved by the small standard deviation whereas temperature of tap water fluctuates resulting in the highest standard deviation. Tap water comes from tank and pipe, can be easily affected by the temperature of the day. Water takes longer time to warm up and cool down compared to air. River water has the lowest temperature because it is continuously flowing resulting in slow heat transfer.
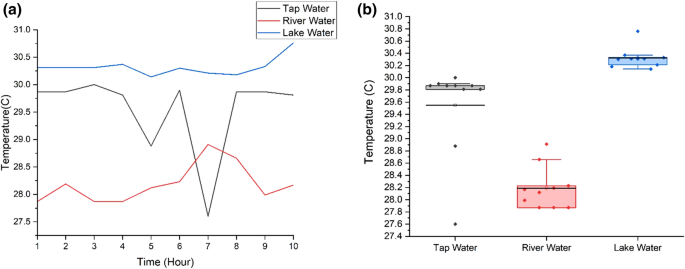
a Temperature values for three water samples; b Temperature boxplot for three water samples. Ten readings are used for the analysis
Figure 7 (a) and (b) show the pH variations of water samples and the boxplot for pH values. Normal tap water has neutral pH from 7.16 to 8.33 with the average pH, 7.551 and the standard deviation, 0.477. High fluctuation of pH in tap water (Fig. 7 (b)) is attributed to chemicals in the water as pH is related to the amount of hydrogen and hydroxyl ions. (Tomar 1999 ). River water has higher pH compared to tap water with the range from 8.04 to 8.16, the average pH, 8.09 and standard deviation, 0.062. Lake water has the highest pH ranging from 9.04 to 9.18. The mean pH for lake water sample is 9.09 with standard deviation, 0.051. The lake water has the highest pH due to the presence of carbonated-rich soils like limestone. Besides that, algae and aquatic plants in the lake water apply photosynthesis process that uses hydrogen which also can contribute to the high pH in the lake water (Fondriest Environmental 2013 ). The pH of river and lake can also be affected by the activities at the shoreline.
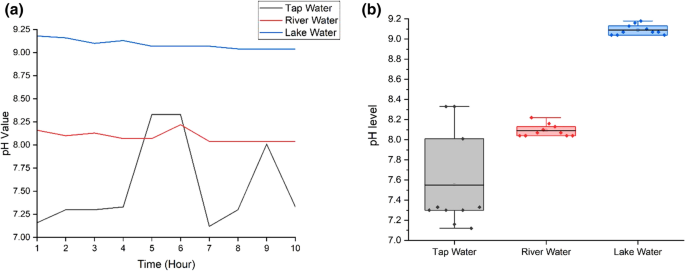
a pH variation for three water samples; b pH boxplot for three water samples. Ten readings are used for the analysis
Figure 8 (a) and (b) show the turbidity variations and the boxplot for all samples. Lake water depicts the highest fluctuation of turbidity in 10 h (Fig. 8 (b)), due to variation of organic materials. Tap water shows the lowest turbidity level whereas river depicts the highest turbidity level. Tap water has turbidity level from 0 to 2 NTU. The mean value is 0.24 NTU and the standard deviation value is 0.622. River water depicts turbidity level from 48.05 NTU to 98.43 NTU while lake water sample shows the highest turbidity values, from 136.92 NTU to 223.72 NTU. The mean turbidity value for river and lake samples are 77.004 NTU and 181.292 NTU respectively. The standard deviation of the turbidity for river and lake water are 16.860 and 39.07, showing higher fluctuation compared to tap water. Tap water has the lowest turbidity level because the water is transparent and clear. The river water is turbid due to the presence of suspended and colloidal matter like clay, silt, finely divided organic and inorganic matter, plankton and other microscopic organisms (Allen et al. 2008 ). Lake has the highest range of turbidity, attributed to the clays, silts, suspended bottom sediments, phytoplankton and algae. According to Malaysia National Water Standard, based on the turbidity, pH and temperature of all water samples, tap water is safe to be consumed but lake and river water need to be treated and filtered before we can consume it as drinking water (Jabatan Alam Sekitar 2019 ).
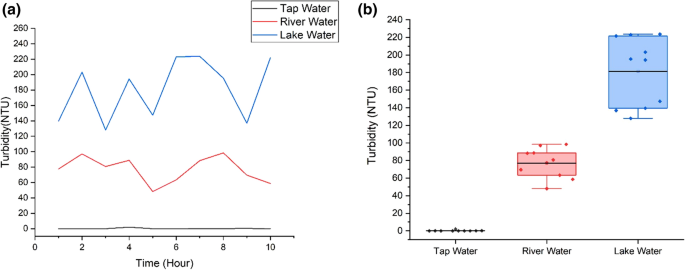
a Turbidity values for the three water samples; b Turbidity boxplot for three water samples. Ten readings are used for the analysis
Figure 9 shows the electrical conductivity values of the water samples. Due to the use of chlorine that contains electrons in water treatment process, tap water shows the highest range of electrical conductivity values and highest fluctuation (Kremer 2018 ). Electrical conductivity of tap water has maximum value ~ 1.81mS/cm and minimum value ~ 0.88mS/cm with standard deviation ~ 0.33 and mean ~ 1.2mS/cm. The range of electrical conductivity of river water is from 0.22 to 0.33mS/cm, the lowest range compared to other samples. The mean and standard deviation of electrical conductivity for river water are 0.25 and 0.04 respectively. Charged ions in river and lake water come from the presence of dissolved minerals like clay soils or limestone (Huron River Watershed Council 2013 ).
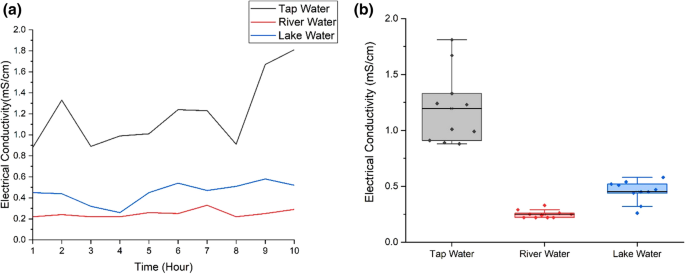
a Electrical conductivity values for three water samples; b Electrical conductivity boxplot for three water samples. Ten readings are used for the analysis
Figure 10 shows the readings of oxidation–reduction potential of the water samples. The oxidation–reduction potential values of tap water are in the highest range, from 280 to 289 mV with standard deviation ~ 3.14 while river water has the lowest oxidation–reduction potential readings with range from 153 to 173 mV and standard deviation ~ 6.15. Tap water has the highest range of oxidation–reduction potential due to water treatment that involves chlorine. Chlorine is used in water treatment to kill bacteria, parasites, viruses and microbes in water by neutralizing and oxidizing bacteria, parasites, viruses and microbes. This oxidation process increases the level of oxidation–reduction potential values of water (Kremer 2018 ). The oxidation–reduction potential values of river and lake water samples fall slightly under the healthy range of freshwater oxidation–reduction potential value, from 300 to 500 millivolts. Lower oxidation–reduction value is also expected at the bottom of the lake and river. Oxygen level at the bottom of the lake and river is low due to the usage of the oxygen by bacteria (Wetzel 2001 ).
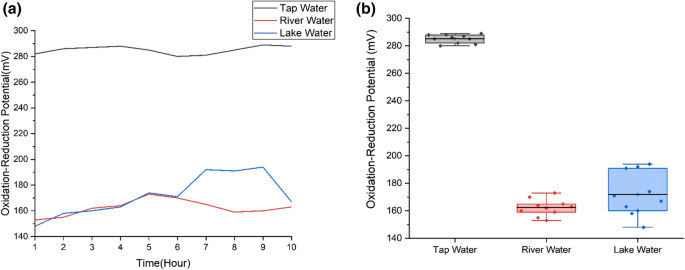
a Oxidation–reduction potential variation of three water samples; b Oxidation–reduction potential boxplot for three water samples. Ten readings are used for the analysis
(b) Statistical analysis—One-way ANOVA
One-way Analysis of variance (ANOVA) model is done to test the null hypothesis that states all parameters have similar population mean. If the variability of the sample mean is large, the null hypothesis is rejected. The alternate hypothesis that states at least one population mean is different from the others will be accepted (Ostertagova 2013 ). When there are statistically significant differences between the means, p-value is less than or equal to α value (0.05). Null hypothesis is rejected as there is at least one different population mean. Table 1 shows one-way ANOVA test results done using OriginPro software. The one-way ANOVA test revealed that there was a statistically significant difference in the means of pH, temperature, turbidity, electrical conductivity and oxidation–reduction potential between at least two groups with ( F (3,10) = [78.1138], p = 0.0000), ( F (3,10) = [48.6633], p = 0.0000), ( F (3,10) = [136.7881], p = 0.0000), ( F (3,10) = [62.3271], p = 0.0000) and ( F (3,10) = [466.1869], p = 0.0000) respectively. From the table, the p-value of all parameters is less than 0.05. Thus, null hypothesis is rejected. For all water parameters tested, at least one group of water samples has a significant difference from overall mean of the water samples. The statistical significance shows that data collected are probably true and is due to factors of interest. Thus, we strongly suppose that pH, temperature, turbidity, electrical conductivity, and oxidation–reduction potential can give effect the analysis of water quality.
(c) Comparison of filtered and unfiltered water
Figure 11 shows the turbidity, pH and temperature of normal tap water from the same source for two days. As normal tap water is commonly safe to be used, the water does not need to undergo filtration process. The tap water sample from same source is compared for two different days. The mean value for turbidity, pH value and temperature of the tap water sample in the first and second day are 0.1 NTU, 7.501 and 29.21 °C respectively. The standard deviation values for tap water in both days are 0.208 for turbidity, 0.429 for pH values and 1.026 for temperature. The mean and standard deviation depicts that the system is reliable due to less fluctuation especially for turbidity sensing system. The turbidity readings for tap water are lower than 1 NTU, in the safe range of water quality (WHO 2017 ). The pH of the tap water shows that the water is in the neutral and light alkalic range. The temperature of tap water fluctuates depending on the weather and surrounding environment. Figure 12 shows the turbidity, temperature, and pH value of lake water before and after filtration process. The average of turbidity, temperature, and pH for lake water before filtration is 181.29 NTU, 30.32 °C and 9.09 respectively while the average of turbidity, temperature, and pH for lake water after filtration are 16.91 NTU, 29.43 °C and 7.34 respectively. Figure 13 shows the turbidity, temperature, and pH of river water before and after filtration process. The average of turbidity, temperature, and pH for river water before filtration is 77.00 NTU, 28.18 °C and 8.09 respectively while the mean values of turbidity, temperature, and pH for river water after filtration are 24.22 NTU, 29.5 °C and 7.52 respectively.
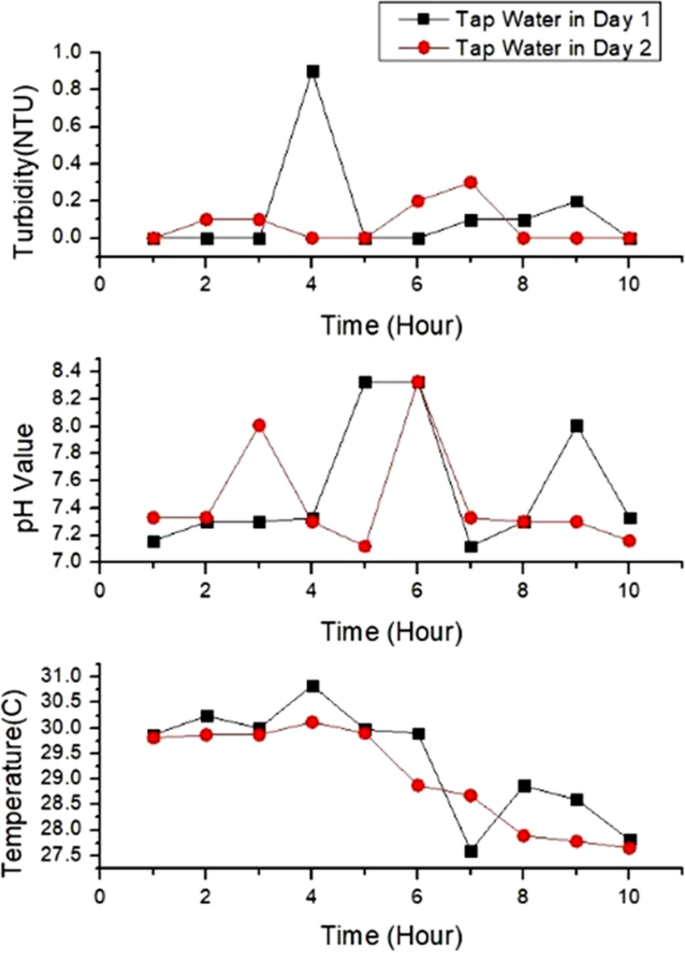
Turbidity levels, pH value and temperature of tap water for day one and day two
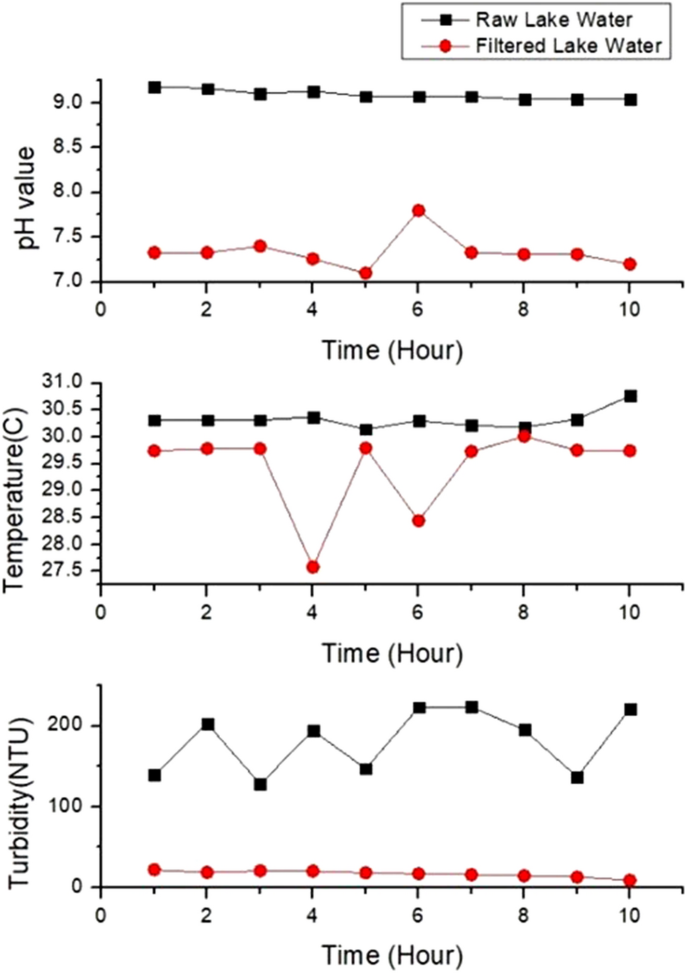
Comparison of water quality parameters for unfiltered and filtered lake water
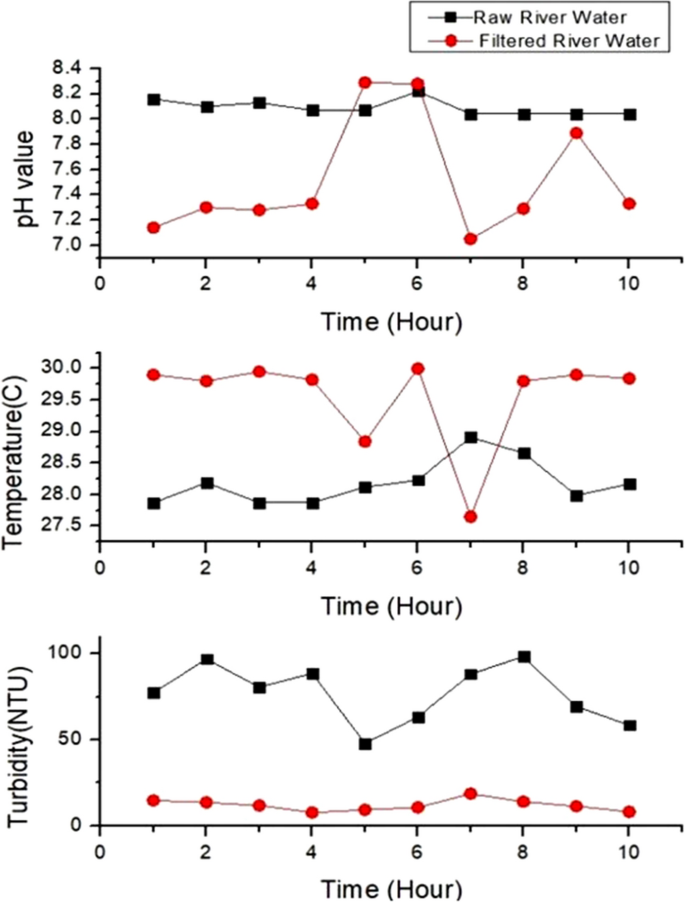
Comparison of water quality parameters for unfiltered and filtered river water
For both lake and river water samples, the turbidity level decreases after the water samples go through filtration process. It proves that the water filtration process successfully provides better quality of water. Meanwhile, the pH values for lake and river water after filtration system are neutral. The system filters unwanted particles in the sample such as mud and soil which are the main contributors for high turbidity and pH values. Some abnormal pH values of river sample after filtration are detected due to sensor sensitivity as the electrode of the pH sensor is very susceptible to interference. The temperature of the lake sample after filtration is slightly lower than the unfiltered samples with small fluctuation. Higher temperature of the filtered river water than the unfiltered river water is attributed to the less mud and soil in the samples and surrounding temperature of the filter.
Many studies have been done to improve existing water quality monitoring systems. Kumar et al. ( 2020 ) developed a water quality system measuring pH, conductivity, temperature and turbidity which allowed real time monitoring. Demetillo et al. ( 2019 ) developed a system for monitoring water quality in a large aquatic area highlighting the usage of the wireless sensor network (WSN). Pasika et al. ( 2020 ) applied arduino microcontroller unit (MCU) and interfaced with several physical water parameter sensors to develop a smart water quality monitoring system with cost-effective using IoT. User can monitor the water quality using ThingSpeak platform where data obtained from the system is sent to cloud. However, most of the studies do not analyze the data and do comparison with the national and international standard. Thus, our study is important due to reliable data that can become a good reference to the Malaysia government.
In conclusion, water pollution is a detrimental issue that leads to water cut, health problem and a threat to aquatic life. Monitoring water quality is important to control water pollution. Here, water quality monitoring and filtration system was designed using Proteus software. Arduino was used as the main controller and five sensors were applied to detect physical parameters of water to monitor water quality. The data is analyzed based on three types of water samples, tap water, lake and river in Nilai, Negeri Sembilan, Malaysia. The purpose of this research is to investigate and monitor the turbidity level, pH value, temperature, electrical conductivity and oxidation–reduction potential of three different types of water resources in Malaysia. The study shows that tap water that is supplied to the household, has the safe range of turbidity and pH. The filtered lake and river water which give lower turbidity and neutral pH values than the unfiltered samples prove that the filtration system is crucially needed for the household use. Tap water shows the highest electrical conductivity and oxidation–reduction potential due to chlorine that is used for treating the water. The data analysis is done using box plot and one-way ANOVA test. The ANOVA test shows that the sensing parameters can be used to monitor water quality. The ThingSpeak application gives real-time and continuous water quality monitoring system. The research outcome is important to analyze water quality in Malaysia before it can be safely used by consumers. The system can be upgraded using high endurance and capacity of microcontroller and sensors. Then, it can be applied in the groundwater source or tank at home. We believe the proposed system can assist Malaysia government in closely monitor physical parameters of water quality and indirectly mitigate water pollution issues.
Data availability
All data are presented in the manuscript.
Code availability
The coding will be provided if needed.
Aghasian K, Moridi A, Mirbagheri A, Abbaspour A (2017) A conflict resolution method for waste load reallocation in river syatems. Int J of Environ Sci Tech. https://doi.org/10.1007/s13762-018-1993-3
Article Google Scholar
Alberti G, Zanoni C, Magnaghi LR, Biesuz R (2020) Low-cost, disposable colourimetric sensors for metal ions detection. J Analyt Sci Tech 11:30. https://doi.org/10.1186/s40543-020-00221-x
Article CAS Google Scholar
Allen M J, Brecher R W, Copes R, Hrudey S E, Payment P (2008) Turbidity and microbial risk in drinking water. Ministerial technical advisory committee
Alley E (2007) Water quality control handbook, vol 2. McGraw Hill, New York
Google Scholar
Alsulaili A, Al-Harbi M, Al-Tawari K (2015) Physical and chemical characteristics of drinking water quality in kuwait: tap vs bottled water. J Eng Res 3(1):25–50. https://doi.org/10.7603/s40632-015-0002-y
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Bao JL (2010) Water quality study and its relationship with high tide and low tide at kuantan river. Universiti Malaysia Pahang, Kuantan
Bilal H, Ghafoor A, Munir A (2016) Desalination of brackish water using dual acting solar still. J Eng Res 4(4):178–193
CAS Google Scholar
Delgado A, Jiminez J, Ochoa L, Mendiola L, Alvarado A, Huamani EL (2020) Applying grey systems to analyze water quality on the river. Int J Emerging Trends in Eng Res 8(7):3880–3886. https://doi.org/10.30534/ijeter/2020/156872020
Demetillo AT, Japitana MV, Taboada EB (2019) A System for monitoring water quality in a large aquatic area using wireless sensor network technology. Sustain Environ Res 29:12. https://doi.org/10.1186/s42834-019-0009-4
DeZuane J (1997) Handbook of drinking water quality, 2nd edn. John Wiley & Sons, New York
Elsheikh MA, Saleh HI, Elmala Y (2018) Performance of filtration systems to enhance treated water quality. J Eng App Sc 65(2):125–137
Engineering Services Division (2016) Drinking water quality standard. Ministry of Health Malaysia, Malaysia
Fondriest Environmental (2013) Fondriest environmental learning centre - pH of Water. Fondriest environmental inc. retrieved December 10, 2020, from https://www.fondriest.com/environmental-measurements/parameters/water-quality/ph/
EPA (2012) 5.9 Conductivity In water: monitoring and assessment. Retrieved July 09, 2021, from http://water.epa.gov/type/rsl/monitoring/vms59.cfm
Galal MM, Soliman A, Kamel G, Zaher K, El-Fakharany Z (2020) Prediction and assessment of surface water quality effect on groundwater in El-Qaluybia. Egypt J Eng App Sc 67(8):2129–2148
Huang Y F, Ang S Y, Lee K M, Lee T S (2015) Quality of water resources in Malaysia. In:research and practices in water quality. Rijeka: IntechOpen. https://doi.org/10.5772/58969
Huron river watershed council (2013) conductivity the activity. Retrieved July 09, 2021, from http://www.hrwc.org/wp-content/uploads/2013/02/Conductivity-v2.pdf
Jabatan Alam Sekitar (2018) Malaysia environmental quality report 2017 - river water quality. Ministry of Environment and Water, Malaysia
Jabatan Alam Sekitar (2019) National water quality standard. Ministry of Environment and Water, Malaysia
Khalit AR (2008) Current state of water environment in Malaysia. Conservation and Environment Management Division KeTSA, Malaysia
Kremer M (2018) Water quality 101: what is ORP in water testing? Jenco. Retrieved Aug 02, 2021, from https://blog.jencoi.com/what-is-orp-in-water-testing
Kumar SS, Subba Rao BV, Prasad JR (2020) Design and development of a water quality monitoring system by using IoT. Int J Emerging Trends in Eng Res (IJRTE) 8(9):6441–6447. https://doi.org/10.30534/ijeter/2020/245892020
Matin M A, Islam M M (2012) Overview of wireless sensor network. In: wireless sensor networks - technology and protocols Malaysia. IntechOpen https://doi.org/10.5772/49376.
Najah A, Teo FY, Chow MF, Huang YF, Latif SD, Abdullah S, Ismail M, El-Shafie A (2021) Surface water quality status and prediction during movement control operation order under COVID-19 pandemic: case studies in Malaysia. Int J Environ Sc Technol 18:1009–1018. https://doi.org/10.1007/s13762-021-03139-y
Ostertagova E, Oskar O (2013) Methodology and Application of One-way ANOVA. American J Mechanical Eng 1(7):256–261. https://doi.org/10.12691/ajme-1-7-21
Pasika S, Gandla ST (2020) Smart water quality monitoring system with cost-effective using IoT. Heliyon 6(7):1–9. https://doi.org/10.1016/j.heliyon.2020.e04096
Potter K (2006) Methods for presenting statistical information: the box plot. Retrieved 2 Aug 2021, from https://www.sci.utah.edu/~kpotter/publications/potter-2006-MPSI.pdf
Raril C, Manjunatha JG (2020) Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of mercury and lead ions in water and biological samples. J Analytical Sc Tech 11:3. https://doi.org/10.1186/s40543-019-0194-0
Sullivan L (2021) The ANOVA approach. hypothesis testing – analysis of variance (ANOVA). Boston university school of public health. Retrieved August 02, 2021, from https://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704_HypothesisTesting-ANOVA/BS704_HypothesisTesting-Anova_print.html
Supriyono H, Majid A, Harismah K (2020) Portable system for coastal fishpond water quality telemonitoring continuously. Int J Emerging Trends Eng Res 8(9):5163–5170. https://doi.org/10.30534/ijeter/2020/45892020
Tavakoly Sany SB, Monazami G, Rezayi M, Borgheipour H (2018) Application of water quality indices for evaluating water quality and anthropogenic impact assessment. Int J Environ Sc Technol 16:3001–3012. https://doi.org/10.1007/s13762-018-1894-5
Tomar M (1999) Water and wastewater. CRC Press, Boca Raton
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, San Diego
WHO (2011) Guidelines for drinking water quality. World Health Organisation, Geneva
WHO. (2017). Water quality and health - review of turbidity: information for regulators and water suppliers. World Health Organisation
Yousefi H, Omidi M, Moridi A, Sarang A (2021) Groundwater monitoring network design using optimized DRASTIC method and capture zone analysis. Int J of Environ Res. https://doi.org/10.1007/s41742-021-00348-8
Zare Farjoudi S, Moridi A, Sarang A (2020) Multi-objective waste load allocation in river system under inflow uncertainty. Int J of Environ Sc and Tech. https://doi.org/10.1007/s13762-020-02897-5
Download references
Acknowledgements
We acknowledge Ministry of Education Malaysia under FRGS grant (FRGS/1/2021/WAB02/USIM/02/1), Universiti Sains Islam Malaysia (USIM) under matching grant (USIM/MG/MMU-PPKMT-ZDA/FKAB/SEPADAN-S/70822) and Faculty of Engineering and Built Environment (FKAB) for the funding and support.
Funding for this project is provided by Ministry of Higher Education, Malaysia under Fundamental Research Grant Scheme (FRGS) (grant code: FRGS/1/2021/WAB02/USIM/02/1) and Universiti Sains Islam Malaysia (matching grant:USIM/MG/MMU-PPKMT-ZDA/FKAB/SEPADAN-S/70822).
Author information
Authors and affiliations.
Advanced Device and System, Faculty of Engineering and Built Environment, Universiti Sains Islam Malaysia, 71800, Nilai, Negeri Sembilan, Malaysia
N. A. Razman, W. Z. Wan Ismail, M. H. Abd Razak, I. Ismail & J. Jamaludin
You can also search for this author in PubMed Google Scholar
Contributions
The experiment was done by Nur Afiqah with help from Muhammad Hakim. Wan Zakiah, Irneza and Juliza contribute to literature review and discussion.
Corresponding author
Correspondence to W. Z. Wan Ismail .
Ethics declarations
Conflict of interests.
The authors declare that they have no competing interests.
Consent to participate
Not applicable.
Consent for publication
Not appplicable.
Ethics approval
Additional information.
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSX 91 KB)
Rights and permissions.
Reprints and permissions
About this article
Razman, N.A., Wan Ismail, W.Z., Abd Razak, M.H. et al. Design and analysis of water quality monitoring and filtration system for different types of water in Malaysia. Int. J. Environ. Sci. Technol. 20 , 3789–3800 (2023). https://doi.org/10.1007/s13762-022-04192-x
Download citation
Received : 27 May 2021
Revised : 31 December 2021
Accepted : 10 April 2022
Published : 11 June 2022
Issue Date : April 2023
DOI : https://doi.org/10.1007/s13762-022-04192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Temperature
- Electrical conductivity
- Oxidation–reduction potential
- Analysis of variance
- Find a journal
- Publish with us
- Track your research
- Mobile Site
- Staff Directory
- Advertise with Ars
Filter by topic
- Biz & IT
- Gaming & Culture
Front page layout
It's in the genes —
Studies reveal new clues to how tardigrades can survive intense radiation, radiation damages their dna; they're just able to repair that damage very quickly..
Jennifer Ouellette - Apr 16, 2024 8:55 pm UTC

Since the 1960s, scientists have known that the tiny tardigrade can withstand very intense radiation blasts 1,000 times stronger than what most other animals could endure. According to a new paper published in the journal Current Biology, it's not that such ionizing radiation doesn't damage tardigrades' DNA; rather, the tardigrades are able to rapidly repair any such damage. The findings complement those of a separate study published in January that also explored tardigrades' response to radiation.
“These animals are mounting an incredible response to radiation, and that seems to be a secret to their extreme survival abilities,” said co-author Courtney Clark-Hachtel , who was a postdoc in Bob Goldstein's lab at the University of North Carolina at Chapel Hill, which has been conducting research into tardigrades for 25 years. “What we are learning about how tardigrades overcome radiation stress can lead to new ideas about how we might try to protect other animals and microorganisms from damaging radiation.”
As reported previously , tardigrades are micro-animals that can survive in the harshest conditions: extreme pressure, extreme temperature, radiation, dehydration, starvation—even exposure to the vacuum of outer space. The creatures were first described by German zoologist Johann Goeze in 1773. They were dubbed tardigrada ("slow steppers" or "slow walkers") four years later by Lazzaro Spallanzani, an Italian biologist. That's because tardigrades tend to lumber along like a bear. Since they can survive almost anywhere, they can be found in lots of places: deep-sea trenches, salt and freshwater sediments, tropical rain forests, the Antarctic, mud volcanoes, sand dunes, beaches, and lichen and moss. (Another name for them is "moss piglets.")
Further Reading
When their moist habitat dries up, however, tardigrades go into a state known as "tun"—a kind of suspended animation, which the animals can remain in for as long as 10 years. When water begins to flow again, water bears absorb it to rehydrate and return to life. They're not technically members of the extremophile class of organisms since they don't so much thrive in extreme conditions as endure; technically, they belong to the class of extremotolerant organisms. But their hardiness makes tardigrades a favorite research subject for scientists.
For instance, a 2017 study demonstrated that tardigrades use a special kind of disordered protein to literally suspend their cells in a glass-like matrix that prevents damage. The researchers dubbed this a "tardigrade-specific intrinsically disordered protein" (TDP). In other words, the cells become vitrified. The more TDP genes a tardigrade species has, the more quickly and efficiently it goes into the tun state.
In 2021, another team of Japanese scientists called this " vitrification " hypothesis into question , citing experimental data suggesting that the 2017 findings could be attributed to water retention of the proteins. The following year, researchers at the University of Tokyo identified the mechanism to explain how tardigrades can survive extreme dehydration: cytoplasmic-abundant heat soluble (CAHS) proteins that form a protective gel-like network of filaments to protect dried-out cells. When the tardigrade rehydrates, the filaments gradually recede, ensuring that the cell isn't stressed or damaged as it regains water.
When it comes to withstanding ionizing radiation, a 2016 study identified a DNA damage suppressor protein dubbed "Dsup" that seemed to shield tardigrade genes implanted into human cells from radiation damage. However, according to Clark-Hatchel et al., it still wasn't clear whether this kind of protective mechanism was sufficient to fully account for tardigrades' ability to withstand extreme radiation. Other species of tardigrade seem to lack Dsup proteins, yet still have the same high radiation tolerance, which suggests there could be other factors at play.
A team of French researchers at the French National Museum of Natural History in Paris ran a series of experiments in which they zapped water bear specimens with powerful gamma rays that would be lethal to humans. They published their results earlier this year in the journal eLife. The French team found that gamma rays did actually damage the tardigrade DNA, much like they would damage human cells. Since the tardigrades survived, this suggested the tardigrades were able to quickly repair the damaged DNA.
Further experiments with three different species (including one that lacks Dsup proteins) revealed the tardigrades were producing very high amounts of DNA repair proteins. They also found a similar uptick of proteins unique to tardigrades, most notably tardigrade DNA damage response protein 1 (TDR1), which seems to protect DNA from radiation. "We found that TDR1 protein interacts with DNA and forms aggregates at high concentration suggesting it may condensate DNA and act by preserving chromosome organization until DNA repair is accomplished," the authors wrote.
Clark-Hatchel et al. independently arrived at similar conclusions from their own experiments. Taken together, the two studies confirm that this extremely rapid up-regulation of many DNA repair genes in response to exposure to ionizing radiation should be sufficient to explain the creatures' impressive resistance to that radiation. It's possible that there is a "synergy between protective and repair mechanisms" when it comes to tardigrade tolerance of ionizing radiation.
That said, "Why tardigrades have evolved a strong IR tolerance is enigmatic given that it is unlikely that tardigrades were exposed to high doses of ionizing radiation in their evolutionary history," Clark-Hatchel et al. wrote. They thought there could be a link to the mechanisms that enable tardigrades to survive extreme dehydration, which can also result in damaged DNA. Revisiting data from desiccation experiments did not show nearly as strong an increase in DNA repair transcripts, but the authors suggest that the uptick could occur later in the process, upon rehydration—an intriguing topic for future research.
Current Biology, 2024. DOI: 10.1016/j.cub.2024.03.019 ( About DOIs ).
eLife, 2024. DOI: 10.7554/eLife.92621.1
reader comments
Channel ars technica.

IMAGES
VIDEO
COMMENTS
A water filter is a device that removes impurities (such as dirt) from water using a physical barrier, a chemical process, or a biological process. In this environmental engineering science project, you will experiment with a water filter column kit and see how it works to find out how effective it is at filtering color and visible particles ...
Following are two sample hypothesis for two questions suggested above. By increase in the height of charcoal layer, the rate of filtration of organic material will increase. ... In a multi-layer water filter, it takes certain amount of time for water to travel each layer. The total filtration time is the sum of the individual travel times for ...
White bars show the initial pure water permeability, and grey bars show the pure water permeability after 24 h of dead-end filtration of 1,000 mg per litre of bovine serum albumin in phosphate ...
After 10 minutes pour the water and powdered carbon mixture onto the filters that you prepared in step 3 of "Preparing Your Samples and Filters" and let the water seep through the filters. Make sure that no water is staying inside the filters. For the filtration experiment using no activated carbon, pour each of the prepared water samples onto ...
Hypothesis: Based on our prior knowledge of the subject, which is that there are several ways to purify water other than the conventional, $100-dollar Brita filters, such as boiling water, we hypothesize that we will be able to find an efficient method of purifying water. We know, that from our research, filtering water can be done with very ...
It involves forcing water through a semi-permeable membrane that acts like an ultra-fine sieve, allowing only water molecules to pass through. This process is effective at removing a wide range of contaminants, including dissolved salts, bacteria, and viruses. Think of it as a meticulous editor, ensuring that only the purest water gets through.
Membrane filtration is an advanced drinking water treatment that is widely used nowadays in water treatment processes, mainly for drinking water. Examples of types of membranes include microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), electrodialysis (ED), forward osmosis (FO), and membrane distillation (MD).
H A conceptual model for water and waste water filtration processes is presented and compared with the results of laboratory experiments. Efficient filtration involves both particle destabilization and particle transport. Destabilization in filtration is similar to destabilization in coagulation; effective coagulants are observed to be effective "filter aids."
This article relates the fundamental concepts of granular media filtration to the major factors influencing the overall quality of filtered water. In present-day theory, the particle removal mechanisms of transport, attachment, and detachment are described in two different modeling approaches: (1) phenomenological theories, and (2) trajectory ...
Abstract. This article briefly summarizes certain aspects of filtration theory, and outlines the framework for a conceptual model for the filtration process. Water filtration is generally considered to include both physical and chemical phenomena. Experimental evidence related to the chemical aspects of water filtration is presented and discussed.
The hypothesis of the present study is that plant xylem, from a variety of trees, can be used to remove key contaminants from natural waters, providing safe drinking water for at least one person per day. ... During surface water filtration, xylem was quickly clogged by suspended solids and organic matter, whereas filtration of groundwater ...
The filtration of water significantly decreased its fluoride concentration. The fluoride content of purified water was approximately as much as zero in some cases. Keywords: Water Purification, Fluoride, Spectrophotometry. Introduction. Fluoride is a natural element branched from Fluorine. This element can be found in all sorts of water and soil.
Last modified: November 11, 2020. In this hands-on activity, students investigate different methods—aeration and filtering—for removing pollutants from water. Working in teams, they design, build and test their own water filters—essentially conducting their own "dirty water projects." A guiding data collection worksheet is provided.
water purification, process by which undesired chemical compounds, organic and inorganic materials, and biological contaminants are removed from water.That process also includes distillation (the conversion of a liquid into vapour to condense it back to liquid form) and deionization (ion removal through the extraction of dissolved salts). One major purpose of water purification is to provide ...
However, the results support the hypothesis that SBAC filters can be effective for reducing levels of Pb and Cu (as well as many other elements) in drinking water with moderately elevated concentrations, as was the case in Flint. The Flint filter study also demonstrates that moderately scaled panel studies can provide a model for assessing ...
Procedure. Fill a measuring cup with 1/2 cup of water. Add 8 drops of red food coloring to the water and stir until mixed. Now, add 1/4 cup of colored water to each of the baby food jars. To one jar, add 2 teaspoons of the activated carbon. Put the lids on both jars and set the jars in a place where they won't be disturbed for a couple days.
More ideas to try - Water Filter Science Project. Set up an experiment where the conditions are: 1 coffee filter. 2 coffee filters. 3 coffee filters. Is kitchen roll better than a coffee filter, would just a sieve work? You could also work in stages, so try a colander, then a sieve and then a paper towel. Each stage should trap smaller and ...
Hypothesis: To filtrate out the salt crystals, a filter with holes large enough for water molecules but smaller then salt crystals must be used. It is therefore they hypothesis of this experiment that using items collected from around the house, a filter with these qualifications will not be found, and therefore the filters will not work.
This is typically a five-part process that consists of aeration, coagulation, sedimentation, filtration and disinfection. This activity is only concerned with filtration, which removes most but not all of the impurities from the water. Make sure students know that in this activity the filtered water is still unfit to drink.
Thus, null hypothesis is rejected. For all water parameters tested, at least one group of water samples has a significant difference from overall mean of the water samples. The statistical significance shows that data collected are probably true and is due to factors of interest. ... It proves that the water filtration process successfully ...
Water pollution always occurs in Malaysia due to industrial, construction, agriculture, and household activities. River pollution can disturb water supply that eventually affects business and household activities. Thus, water quality monitoring system is needed to detect contaminated water. We developed a water quality monitoring and filtration system controlled by Arduino. The proposed system ...
Set your timer to 10 minutes and promptly stir each solution with the activation carbon uses a clean spoon. After 10 minutes, pour the water and carbon mixture into the filters that you prepared in step 3 of "Preparing Your Example additionally Filters," and let the water seep through the cleans.
Water Filter Experiment Water Filter experiment by: Gabriel Gonzalez and Juan Diego Luengo 10-4 Biology Hypothesis Materials Used If water containing bacteria and dirt goes through the water filter then the water will be clearer after filtration because materials like sand, dirt
In 2021, another team of Japanese scientists called this "vitrification" hypothesis into question, citing experimental data suggesting that the 2017 findings could be attributed to water retention ...