Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Clinical Presentation
Clinical considerations for care of children and adults with confirmed COVID-19
‹ View Table of Contents
- The clinical presentation of COVID-19 ranges from asymptomatic to critical illness.
- An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity.
- Uncommon presentations of COVID-19 can occur, might vary by the age of the patient, and are a challenge to recognize.
- In adults, age is the strongest risk factor for severe COVID-19. The risk of severe COVID-19 increases with increasing age especially for persons over 65 years and with increasing number of certain underlying medical conditions .
Incubation Period
Data suggest that incubation periods may differ by SARS-CoV-2 variant. Meta-analyses of studies published in 2020 identified a pooled mean incubation period of 6.5 days from exposure to symptom onset. (1) A study conducted during high levels of Delta variant transmission reported an incubation period of 4.3 days, (2) and studies performed during high levels of Omicron variant transmission reported a median incubation period of 3–4 days. (3,4)
Presentation
People with COVID-19 may be asymptomatic or may commonly experience one or more of the following symptoms (not a comprehensive list) (5) :
- Fever or chills
- Shortness of breath or difficulty breathing
- Myalgia (Muscle or body aches)
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
The clinical presentation of COVID-19 ranges from asymptomatic to severe illness, and COVID-19 symptoms may change over the course of illness. COVID-19 symptoms can be difficult to differentiate from and can overlap with other viral respiratory illnesses such as influenza(flu) and respiratory syncytial virus (RSV) . Because symptoms may progress quickly, close follow-up is needed, especially for:
- older adults
- people with disabilities
- people with immunocompromising conditions, and
- people with medical conditions that place them at greater risk for severe illness or death.
The NIH COVID-19 Treatment Guidelines group SARS-CoV-2 infection into five categories based on severity of illness:
- Asymptomatic or pre-symptomatic infection : people who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test [NAAT] or an antigen test) but who have no symptoms that are consistent with COVID-19.
- Mild illness : people who may have any of the various signs and symptoms of COVID-19 but who do not have shortness of breath, dyspnea, or abnormal chest imaging.
- Moderate illness : people who have evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO 2 ) ≥94% on room air at sea level.
- Severe illness : people who have oxygen saturation <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO 2 /FiO 2 ) <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%
- Critical illness : people who have respiratory failure, septic shock, or multiple organ dysfunction.
Asymptomatic and presymptomatic presentation
Studies have documented SARS-CoV-2 infection in people who never develop symptoms (asymptomatic presentation) and in people who are asymptomatic when tested but develop symptoms later (presymptomatic presentation). ( 6,7 ) It is unclear what percentage of people who initially appear asymptomatic progress to clinical disease. Multiple publications have reported cases of people with abnormalities on chest imaging that are consistent with COVID-19 very early in the course of illness, even before the onset of symptoms or a positive COVID-19 test. (9)
Radiographic Considerations and Findings
Chest radiographs of patients with severe COVID-19 may demonstrate bilateral air-space consolidation. (23) Chest computed tomography (CT) images from patients with COVID-19 may demonstrate bilateral, peripheral ground glass opacities and consolidation. (24,25) Less common CT findings can include intra- or interlobular septal thickening with ground glass opacities (hazy opacity) or focal and rounded areas of ground glass opacity surrounded by a ring or arc of denser consolidation (reverse halo sign). (24)
Multiple studies suggest that abnormalities on CT or chest radiograph may be present in people who are asymptomatic, pre-symptomatic, or before RT-PCR detection of SARS-CoV-2 RNA in nasopharyngeal specimens. (25)
Common COVID-19 symptoms
Fever, cough, shortness of breath, fatigue, headache, and myalgia are among the most commonly reported symptoms in people with COVID-19. (5) Some people with COVID-19 have gastrointestinal symptoms such as nausea, vomiting, or diarrhea, sometimes prior to having fever or lower respiratory tract signs and symptoms. (10) Loss of smell and taste can occur, although these symptoms are reported to be less common since Omicron began circulating, as compared to earlier during the COVID-19 pandemic. (11,19-21) People can experience SARS-CoV-2 infection (asymptomatic or symptomatic), even if they are up to date with their COVID-19 vaccines or were previously infected. (8)
Several studies have reported ocular symptoms associated with SARS-CoV-2 infection, including redness, tearing, dry eye or foreign body sensation, discharge or increased secretions, and eye itching or pain. (13)
A wide range of dermatologic manifestations have been associated with COVID-19; timing of skin manifestations in relation to other COVID-19 symptoms and signs is variable. (14) Some skin manifestations may be associated with increased disease severity. (15) Images of cutaneous findings in COVID-19 are available from the American Academy of Dermatology .
Uncommon COVID-19 symptoms
Less common presentations of COVID-19 can occur. Older adults may present with different symptoms than children and younger adults. Some older adults can experience SARS-CoV-2 infection accompanied by delirium, falls, reduced mobility or generalized weakness, and glycemic changes. ( 12)
Transmission
People infected with SARS-CoV-2 can transmit the virus even if they are asymptomatic or presymptomatic. ( 16) Peak transmissibility appears to occur early during the infectious period (prior to symptom onset until a few days after), but infected persons can shed infectious virus up to 10 days following infection. (22 ) Both vaccinated and unvaccinated people can transmit SARS-CoV-2. ( 17,18) Clinicians should consider encouraging all people to take the following prevention actions to limit SARS-CoV-2 transmission:
- stay up to date with COVID-19 vaccines,
- test for COVID-19 when symptomatic or exposed to someone with COVID-19, as recommended by CDC,
- wear a high-quality mask when recommended,
- avoiding contact with individuals who have suspected or confirmed COVID-19,
- improving ventilation when possible,
- and follow basic health and hand hygiene guidance .
Clinicians should also recommend that people who are infected with SARS-CoV-2, follow CDC guidelines for isolation.
Table of Contents
- › Clinical Presentation
- Clinical Progression, Management, and Treatment
- Special Clinical Considerations
- Bhaskaran K, Bacon S, Evans SJ, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. Jul 2021;6:100109. doi:10.1016/j.lanepe.2021.100109
- Kim L, Garg S, O'Halloran A, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. Jul 16 2020;doi:10.1093/cid/ciaa1012
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Preventing chronic disease. Jul 1 2021;18:E66. doi:10.5888/pcd18.210123
- Ko JY, Danielson ML, Town M, et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. Sep 18 2020;doi:10.1093/cid/ciaa1419
- Wortham JM, Lee JT, Althomsons S, et al. Characteristics of Persons Who Died with COVID-19 - United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. Jul 17 2020;69(28):923-929. doi:10.15585/mmwr.mm6928e1
- Yang X, Zhang J, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. Open Forum Infect Dis. Sep 2021;8(9):ofab428. doi:10.1093/ofid/ofab428
- Rader B.; Gertz AL, D.; Gilmer, M.; Wronski, L.; Astley, C.; Sewalk, K.; Varrelman, T.; Cohen, J.; Parikh, R.; Reese, H.; Reed, C.; Brownstein J. Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep. April 1, 2022;71(13):489–494. doi:http://dx.doi.org/10.15585/mmwr.mm7113e1
- Pingali C, Meghani M, Razzaghi H, et al. COVID-19 Vaccination Coverage Among Insured Persons Aged >/=16 Years, by Race/Ethnicity and Other Selected Characteristics - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021. MMWR Morb Mortal Wkly Rep. Jul 16 2021;70(28):985-990. doi:10.15585/mmwr.mm7028a1
- Wiltz JL, Feehan AK, Molinari NM, et al. Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. Jan 21 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
- Murthy NC, Zell E, Fast HE, et al. Disparities in First Dose COVID-19 Vaccination Coverage among Children 5-11 Years of Age, United States. Emerg Infect Dis. May 2022;28(5):986-989. doi:10.3201/eid2805.220166
- Saelee R, Zell E, Murthy BP, et al. Disparities in COVID-19 Vaccination Coverage Between Urban and Rural Counties - United States, December 14, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. Mar 4 2022;71(9):335-340. doi:10.15585/mmwr.mm7109a2
- Burki TK. The role of antiviral treatment in the COVID-19 pandemic. Lancet Respir Med. Feb 2022;10(2):e18. doi:10.1016/S2213-2600(22)00011-X
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. Feb 10 2022;386(6):509-520. doi:10.1056/NEJMoa2116044
- Bai Y, Du Z, Wang L, et al. Public Health Impact of Paxlovid as Treatment for COVID-19, United States. Emerg Infect Dis 2024;30(2) (In eng). DOI: 10.3201/eid3002.230835.
- Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin Infect Dis 2023;76(3):e342-e349. (In eng). DOI: 10.1093/cid/ciac443.
- Shah MM, Joyce B, Plumb ID, et al. Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 - United States, April-September 2022. MMWR Morb Mortal Wkly Rep 2022;71(48):1531-1537. (In eng). DOI: 10.15585/mmwr.mm7148e2.
- Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study. Ann Intern Med 2023;176(1):77-84. (In eng). DOI: 10.7326/m22-2141.
- Lewnard JA, McLaughlin JM, Malden D, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis 2023;23(7):806-815. (In eng). DOI: 10.1016/s1473-3099(23)00118-4.
- Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022;386(15):1397-1408. (In eng). DOI: 10.1056/NEJMoa2118542.
- Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. N Engl J Med 2022;387(9):790-798. (In eng). DOI: 10.1056/NEJMoa2204919.
- Skarbinski J, Wood M, Chervo T, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: A retrospective cohort study. Lancet Reg Health Am 2022;12:100297. https://dx.doi.org/10.1016/j.lan.
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med. Dec 17 2020;383(25):2477-2478. doi:10.1056/NEJMc2029240
- Jordan TB, Meyers CL, Schrading WA, Donnelly JP. The utility of iPhone oximetry apps: A comparison with standard pulse oximetry measurement in the emergency department. Am J Emerg Med. May 2020;38(5):925-928. doi:10.1016/j.ajem.2019.07.020
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):146-152. doi:10.15585/mmwr.mm7104e4
- Taylor CA, Whitaker M, Anglin O, et al. COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Delta and Omicron Variant Predominance, by Race/Ethnicity and Vaccination Status - COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. Mar 25 2022;71(12):466-473. doi:10.15585/mmwr.mm7112e2
- Johnson AG, Amin AB, Ali AR, et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):132-138. doi:10.15585/mmwr.mm7104e2
- Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 Infection and Hospitalization Among Adults Aged >/=18 Years, by Vaccination Status, Before and During SARS-CoV-2 B.1.1.529 (Omicron) Variant Predominance - Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep. Feb 4 2022;71(5):177-181. doi:10.15585/mmwr.mm7105e1
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
An official website of the United States government
Here’s how you know
The final update of the NIH COVID-19 Treatment Guidelines was on February 29, 2024. PDFs of the Guidelines can be downloaded until August 16, 2024, when the website will be shut down.
Guideline PDFs
- Section Only (PDF | 259 KB)
- Full Guideline (PDF | 6.1 MB)
Sign Up For Updates
Related Content
- Guidelines Archive
- How to Cite These Guidelines
Clinical Spectrum of SARS-CoV-2 Infection
Last Updated: February 29, 2024
Patients with SARS-CoV-2 infection can experience a range of clinical manifestations, from no symptoms to critical illness. In general, adults with SARS-CoV-2 infection can be grouped into the following severity of illness categories; however, the criteria for each category may overlap or vary across clinical guidelines and clinical trials, and a patient’s clinical status may change over time.
- Asymptomatic or presymptomatic infection: Individuals who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test [NAAT] or an antigen test) but have no symptoms consistent with COVID-19.
- Mild illness: Individuals who have any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but do not have shortness of breath, dyspnea, or abnormal chest imaging.
- Moderate illness: Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation measured by pulse oximetry (SpO 2 ) ≥94% on room air at sea level.
- Severe illness: Individuals who have an SpO 2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO 2 /FiO 2 ) <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%.
- Critical illness: Individuals who have respiratory failure, septic shock, or multiple organ dysfunction.
SpO 2 is a key parameter for defining the illness categories listed above. However, pulse oximetry has important limitations (discussed in more detail below). Clinicians who use SpO 2 when assessing a patient must be aware of those limitations and conduct the assessment in the context of that patient’s clinical status.
The risk of progressing to severe disease increases with age and the number of underlying conditions. Patients aged ≥50 years, especially those aged ≥65 years, and patients who are immunosuppressed, unvaccinated, or not up to date with COVID-19 vaccinations are at a higher risk of progressing to severe COVID-19. 1,2 Certain underlying conditions are also associated with a higher risk of severe COVID-19, including cancer, cardiovascular disease, chronic kidney disease, chronic liver disease, chronic lung disease, diabetes, advanced or untreated HIV infection, obesity, pregnancy, cigarette smoking, and being a recipient of immunosuppressive therapy or a transplant. 3 Health care providers should closely monitor patients who have COVID-19 and any of these conditions until clinical recovery is achieved.
The initial evaluation for patients may include chest imaging (e.g., X-ray, ultrasound or computed tomography scan) and an electrocardiogram, if indicated. Laboratory testing should include a complete blood count with differential and a metabolic profile, including liver and renal function tests. Although inflammatory markers such as C-reactive protein (CRP), D-dimer, and ferritin are not routinely measured as part of standard care, results from such measurements may have prognostic value. 4-7
The definitions for the severity of illness categories also apply to pregnant patients. However, the threshold for certain interventions is different for pregnant and nonpregnant patients. For example, oxygen supplementation for pregnant patients is generally used when SpO 2 falls below 95% on room air at sea level to accommodate the physiologic changes in oxygen demand during pregnancy and to ensure adequate oxygen delivery to the fetus. 8
If laboratory parameters are used for monitoring pregnant patients and making decisions about interventions, clinicians should be aware that normal physiologic changes during pregnancy can alter several laboratory values. In general, leukocyte cell count increases throughout gestation and delivery and peaks during the immediate postpartum period. This increase is mainly due to neutrophilia. 9 D-dimer and CRP levels also increase during pregnancy and are often higher in pregnant patients than in nonpregnant patients. 10 Detailed information on treating COVID-19 in pregnant patients can be found in Special Considerations During Pregnancy and After Delivery and in the pregnancy considerations subsections in the Guidelines.
In children with COVID-19, radiographic abnormalities are common and, for the most part, should not be the only criteria used to determine the severity of illness. The normal values for respiratory rate also vary with age in children. Therefore, hypoxemia should be the primary criterion used to define severe COVID-19, especially in younger children. In a small subset of children and young adults, SARS-CoV-2 infection may be followed by the severe inflammatory condition multisystem inflammatory syndrome in children (MIS-C). 11,12 This syndrome is discussed in detail in Special Considerations in Children .
Clinical Considerations for the Use of Pulse Oximetry
During the COVID-19 pandemic, the use of pulse oximetry to assess and monitor patients’ oxygenation status increased in hospital, outpatient health care facility, and home settings. Although pulse oximetry is useful for estimating blood oxygen levels, pulse oximeters may not accurately detect hypoxemia under certain circumstances. To avoid delays in recognizing hypoxemia, clinicians who use pulse oximetry to assist with clinical decisions should keep these limitations in mind.
Pulse oximetry results can be affected by skin pigmentation, thickness, or temperature. Poor blood circulation or the use of tobacco or fingernail polish also may affect results. The Food and Drug Administration (FDA) advises clinicians to refer to the label or manufacturer website of a pulse oximeter or sensor to ascertain its accuracy. 13 The FDA also advises using pulse oximetry only as an estimate of blood oxygen saturation, because an SpO 2 reading represents a range of arterial oxygen saturation (SaO 2 ). For example, an SpO 2 reading of 90% may represent a range of SaO 2 from 86% to 94%. Studies that compared SpO 2 and SaO 2 levels measured before the pandemic found that pulse oximeters overestimated oxygen saturation in people who were classified as having darker skin pigmentation and in people whose race or ethnic origin was reported as non-Hispanic Black, Black, or African American. 14,15
Several published reports have compared SpO 2 and SaO 2 measurements in patients with COVID-19, including children. 14,16-18 The studies demonstrated that occult hypoxemia (defined as an SaO 2 <88% despite an SpO 2 >92%) was more common in patients with darker skin pigmentation, which may result in adverse consequences. The likelihood of error was greater in the lower ranges of SpO 2 . In 1 of these studies, occult hypoxemia was associated with more organ dysfunction and hospital mortality. 17 These studies did not specify the specific devices used to assess SpO 2 levels. The FDA has recognized the need for better real-world evidence to address ongoing concerns about the accuracy of pulse oximeters when they are used to measure oxygen saturation in people with darker skin pigmentation. 19
A 5-hospital registry study of patients evaluated in the emergency department or hospitalized for COVID-19 found that 24% were not identified as eligible for treatment due to overestimation of SaO 2 . 20 The majority of patients (55%) who were not identified as eligible were Black. The study also examined the amount of time delay patients experienced before being identified as eligible for treatment. The median delay for patients who were Black was 1 hour longer than the delay for patients who were White.
In pulse oximetry, skin tone is an important variable, but accurately measuring oxygen saturation is a complex process. One observational study in adults was unable to identify a consistently predictable difference between SaO 2 and SpO 2 over time for individual patients. 16 Factors other than skin pigmentation (e.g., peripheral perfusion, pulse oximeter sensor placement) are likely involved.
Despite the limitations of pulse oximetry, an FDA-cleared pulse oximeter for home use can contribute to an assessment of a patient’s overall clinical status. Practitioners should advise patients to follow the manufacturer’s instructions for use, place the oximeter on the index or ring finger, and ensure the hand is warm, relaxed, and held below the level of the heart. Fingernail polish should be removed before testing. Patients should be at rest, indoors, and breathing quietly without talking for several minutes before testing. Rather than accepting the first reading, patients or caretakers should observe the readings on the pulse oximeter for ≥30 seconds until a steady number is displayed and inform their health care provider if the reading is repeatedly below a previously specified value (generally 95% on room air at sea level). 13,21 Pulse oximetry has been widely adopted as a remote patient monitoring tool, but when the use of pulse oximeters is compared with close monitoring of clinical progress via video consultation, telephone calls, text messaging, or home visits, there is insufficient evidence that it improves clinical outcomes. 22,23
Not all commercially available pulse oximeters have been cleared by the FDA. SpO 2 readings obtained through devices not cleared by the FDA, such as over-the-counter sports oximeters or mobile phone applications, lack sufficient accuracy for clinical use. Abnormal readings on these devices should be confirmed with an FDA-cleared device or an arterial blood gas analysis. 24,25
Regardless of the setting, SpO 2 should always be interpreted within the context of a patient’s entire clinical presentation. Regardless of a pulse oximeter reading, a patient’s signs and symptoms (e.g., dyspnea, tachypnea, chest pain, changes in cognition or attentional state, cyanosis) should be evaluated.
Asymptomatic or Presymptomatic Infection
Asymptomatic SARS-CoV-2 infection can occur, although the percentage of patients who remain truly asymptomatic throughout the course of infection is variable and incompletely defined. The percentage of individuals who present with asymptomatic infection and progress to clinical disease is unclear. Some asymptomatic individuals have been reported to have objective radiographic findings consistent with COVID-19 pneumonia. 26,27
Mild Illness
Patients with mild illness may exhibit a variety of signs and symptoms (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell). They do not have shortness of breath, dyspnea on exertion, or abnormal imaging. Most patients who are mildly ill can be managed in an ambulatory setting or at home. No imaging or specific laboratory evaluations are routinely indicated in otherwise healthy patients with mild COVID-19. Patients aged ≥50 years, especially those aged ≥65 years, patients with certain underlying comorbidities, and patients who are immunosuppressed, unvaccinated, or not up to date with COVID-19 vaccinations are at higher risk of disease progression and are candidates for antiviral therapy. 1,2 See Therapeutic Management of Nonhospitalized Adults With COVID-19 for recommendations regarding anti-SARS-CoV-2 therapies.
Moderate Illness
Moderate illness is defined as evidence of lower respiratory disease during clinical assessment or imaging, with an SpO 2 ≥94% on room air at sea level. Given that pulmonary disease can progress rapidly in patients with COVID-19, patients with moderate disease should be closely monitored. See Therapeutic Management of Nonhospitalized Adults With COVID-19 for recommendations regarding anti-SARS-CoV-2 therapies in patients at high risk of progression to severe disease.
Severe Illness
Patients with COVID-19 are considered to have severe illness if they have an SpO 2 <94% on room air at sea level, PaO 2 /FiO 2 <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%. These patients may experience rapid clinical deterioration and should be given oxygen therapy and hospitalized. See Therapeutic Management of Hospitalized Adults With COVID-19 for treatment recommendations.
Critical Illness
SARS-CoV-2 infection can cause acute respiratory distress syndrome, virus-induced distributive (septic) shock, cardiac shock, an exaggerated inflammatory response, thrombotic disease, and exacerbation of underlying comorbidities.
The clinical management of patients with COVID-19 who are in the intensive care unit should include treatment with immunomodulators and, in some cases, the addition of remdesivir. These patients should also receive treatment for any comorbid conditions and nosocomial complications. For more information, see Critical Care for Adults and Therapeutic Management of Hospitalized Adults With COVID-19 .
Infectious Complications in Patients With COVID-19
Some patients with COVID-19 may have additional infections when they present for care or that develop during the course of treatment. These coinfections may complicate treatment and recovery. Older patients or those with certain comorbidities or immunocompromising conditions may be at higher risk for these infections. The use of immunomodulators such as dexamethasone, Janus kinase inhibitors (e.g., baricitinib, tofacitinib), interleukin-6 inhibitors (e.g., tocilizumab, sarilumab), tumor necrosis factor inhibitors (e.g., infliximab), or abatacept to treat COVID-19 may also increase the risk of infectious complications. However, when these therapies are used appropriately, the benefits outweigh the risks.
Infectious complications in patients with COVID-19 can be categorized as follows:
- Coinfections at presentation: Although most individuals present with only SARS-CoV-2 infection, concomitant viral infections, including influenza and other respiratory viruses, have been reported. 28-30 Community-acquired bacterial pneumonia has also been reported, but it is uncommon. 28,31,32 Antibacterial therapy is generally not recommended unless additional evidence for bacterial pneumonia is present (e.g., leukocytosis, the presence of a focal infiltrate on imaging).
- Reactivation of latent infections: There are case reports of underlying chronic hepatitis B virus and latent tuberculosis infections reactivating in patients with COVID-19 who receive immunomodulators as treatment, although the data are currently limited. 33-35 Reactivation of herpes simplex virus and varicella zoster virus infections have also been reported. 36 Cases of severe and disseminated strongyloidiasis have been reported in patients with COVID-19 during treatment with tocilizumab and corticosteroids. 37,38 Many clinicians would initiate empiric treatment (e.g., with the antiparasitic drug ivermectin), with or without serologic testing, in patients who require immunomodulators for the treatment of COVID-19 and have come from areas where Strongyloides is endemic (i.e., tropical, subtropical, or warm temperate areas). 39,40
- Nosocomial infections: Hospitalized patients with COVID-19 may acquire common nosocomial infections, such as hospital-acquired pneumonia (including ventilator-associated pneumonia), line-related bacteremia or fungemia, catheter-associated urinary tract infection, and diarrhea associated with Clostridioides difficile . 41,42 Early diagnosis and treatment of these infections are important for improving outcomes in these patients.
- Opportunistic fungal infections: Invasive fungal infections, including aspergillosis and mucormycosis, have been reported in hospitalized patients with COVID-19. 43-46 Although these infections are relatively rare, they can be fatal, and they may be seen more commonly in patients who are immunocompromised or receiving mechanical ventilation. The majority of mucormycosis cases have been reported in India and are associated with diabetes mellitus or the use of corticosteroids. 47,48 The approach for managing these fungal infections should be the same as the approach for managing invasive fungal infections in other settings.
SARS-CoV-2 Reinfection and Breakthrough Infection
As seen with other respiratory viral infections, reinfection after recovery from prior infection has been reported for SARS-CoV-2. 49 Reinfection may occur as initial immune responses to the primary infection wane over time. Data regarding the prevalence, risk factors, timing, and severity of reinfection are evolving and vary depending on the circulating variants. Breakthrough SARS-CoV-2 infections (i.e., infection in people who are up to date with COVID-19 vaccinations) also occur. 50 When compared with infection in people who are unvaccinated, breakthrough infections in vaccinated individuals appear less likely to lead to severe illness or symptoms that persist ≥28 days. 50-53 The time to breakthrough infection has been reported to be shorter for patients with immunocompromising conditions (i.e., solid organ or bone marrow transplant recipients or people with HIV) than for those with no immunocompromising conditions. 50
Although data are limited, no evidence suggests that the treatment of suspected or documented SARS-CoV-2 reinfection or breakthrough infection should be different from the treatment used during the initial infection, as outlined in Therapeutic Management of Nonhospitalized Adults With COVID-19 and Therapeutic Management of Hospitalized Adults With COVID-19 .
Prolonged Viral Shedding, Persistent Symptoms, and Other Conditions After SARS-CoV-2 Infection
Symptomatic SARS-CoV-2 infection is typically associated with a decline in viral shedding and resolution of COVID-19 symptoms over days to weeks. However, in some cases, reduced viral shedding and symptom resolution are followed by viral or symptom rebound. People who are immunocompromised may experience viral shedding for many weeks. Some people experience symptoms that develop or persist for more than 4 weeks after the initial COVID-19 diagnosis.
Viral or Symptom Rebound Soon After COVID-19
Observational studies and results from clinical trials of therapeutic agents have described SARS-CoV-2 viral or COVID-19 symptom rebound in patients who have completed treatment for COVID-19. 54-56 Viral and symptom rebounds have also occurred when anti-SARS-CoV-2 therapies were not used. 56,57 Typically, this phenomenon has not been associated with progression to severe COVID-19.
Prolonged Viral Shedding in Patients Who Are Immunocompromised
Patients who are immunocompromised may experience prolonged shedding of SARS-CoV-2 with or without COVID-19 symptoms. 58,59 Prolonged viral shedding may affect SARS-CoV-2 testing strategies and isolation duration for these patients. In some cases, the prolonged shedding may be associated with persistent COVID-19 symptoms. See Special Considerations in People Who Are Immunocompromised for more information on the clinical management of people who are immunocompromised.
Persistent, New, or Recurrent Symptoms More Than 4 Weeks After SARS-CoV-2 Infection
Some patients report persistent, new, or recurrent symptoms and conditions (e.g., cardiopulmonary injury, neurocognitive impairment, new-onset diabetes, gastrointestinal and dermatologic manifestations) more than 4 weeks after the initial COVID-19 diagnosis. 60 The nomenclature for this phenomenon is evolving; no clinical terminology has been established. The terminology used includes long COVID, post-COVID condition, post–COVID-19 syndrome, and post-acute sequelae of SARS-CoV-2. Patients who have these symptoms or conditions have been called “long haulers.”
Data on the incidence, natural history, and etiology of these symptoms are emerging. However, reports on these syndromes have several limitations, such as differing case definitions, a lack of comparator groups, and overlap between the reported symptoms and the symptoms of post-intensive care syndrome that have been described in patients without COVID-19. In addition, many reports only included patients who attended post-COVID clinics. Details on the pathogenesis, clinical presentation, and treatment for these conditions are beyond the scope of these Guidelines. The Centers for Disease Control and Prevention provides information about the timeframes, presentation of symptoms, and management strategies for post-COVID conditions. Research on the prevalence, characteristics, and pathophysiology of persistent symptoms and conditions after COVID-19 is ongoing, including research through the National Institutes of Health’s RECOVER Initiative , which aims to inform potential therapeutic strategies.
MIS-C and multisystem inflammatory syndrome in adults (MIS-A) are serious postinfectious complications of SARS-CoV-2 infection. For more information on these syndromes, see Therapeutic Management of Hospitalized Children With MIS-C, Plus a Discussion on MIS-A .
- Skarbinski J, Wood MS, Chervo TC, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am . 2022;12:100297. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35756977 .
- Taylor CA, Patel K, Patton ME, et al. COVID-19–associated hospitalizations among U.S. adults aged ≥65 Years—COVID-NET, 13 states, January–August 2023. MMWR Morb Mortal Wkly Rep . 2023;72(40):1089-1094. Available at: https://www.ncbi.nlm.nih.gov/pubmed/37796744 .
- Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. 2023. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html . Accessed January 10, 2024.
- Tan C, Huang Y, Shi F, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol . 2020;92(7):856-862. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32281668 .
- Berger JS, Kunichoff D, Adhikari S, et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol . 2020;40(10):2539-2547. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32840379 .
- Casas-Rojo JM, Antón-Santos JM, Millán-Núñez-Cortés J, et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 registry. Rev Clin Esp (Barc) . 2020;220(8):480-494. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32762922 .
- Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J . 2021;42(23):2270-2279. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33448289 .
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. 2021. Available at: https://s3.amazonaws.com/cdn.smfm.org/media/2734/SMFM_COVID_Management_of_COVID_pos_preg_patients_2-2-21_(final).pdf .
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326-1331. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19935037 .
- Anderson BL, Mendez-Figueroa H, Dahlke JD, et al. Pregnancy-induced changes in immune protection of the genital tract: defining normal. Am J Obstet Gynecol . 2013;208(4):321.e1-321.e9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23313311 .
- Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32386565 .
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32410760 .
- Food and Drug Administration. Pulse oximeter accuracy and limitations: FDA safety communication. 2023. Available at: https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication . Accessed January 10, 2024.
- Valbuena VSM, Seelye S, Sjoding MW, et al. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013–19: multicenter, retrospective cohort study. BMJ. 2022;378:e069775. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35793817 .
- Shi C, Goodall M, Dumville J, et al. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: a systematic review and meta-analysis. BMC Med . 2022;20(1):267. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35971142 .
- Chesley CF, Lane-Fall MB, Panchanadam V, et al. Racial disparities in occult hypoxemia and clinically based mitigation strategies to apply in advance of technological advancements. Respir Care . 2022;67(12):1499-1507. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35679133 .
- Wong AI, Charpignon M, Kim H, et al. Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open . 2021;4(11):e2131674. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34730820 .
- Savorgnan F, Hassan A, Borges N, Acosta S. Pulse oximetry and arterial saturation difference in pediatric COVID-19 patients: retrospective analysis by race. Pediatr Crit Care Med . 2023;24(6):458-462. Available at: https://www.ncbi.nlm.nih.gov/pubmed/36825900 .
- Food and Drug Administration. Approach for improving the performance evaluation of pulse oximeter devices taking into consideration skin pigmentation, race and ethnicity. 2023. Available at: https://www.fda.gov/media/173905/download .
- Fawzy A, Wu TD, Wang K, et al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med . 2022;182(7):730-738. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35639368 .
- Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc . 2020;17(9):1040-1046. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32521167 .
- Alboksmaty A, Beaney T, Elkin S, et al. Effectiveness and safety of pulse oximetry in remote patient monitoring of patients with COVID-19: a systematic review. Lancet Digit Health . 2022;4(4):e279-e289. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35337644 .
- Lee KC, Morgan AU, Chaiyachati KH, et al. Pulse oximetry for monitoring patients with COVID-19 at home—a pragmatic, randomized trial. N Engl J Med . 2022;386(19):1857-1859. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35385625 .
- Harskamp RE, Bekker L, Himmelreich JCL, et al. Performance of popular pulse oximeters compared with simultaneous arterial oxygen saturation or clinical-grade pulse oximetry: a cross-sectional validation study in intensive care patients. BMJ Open Respir Res . 2021;8(1):e000939. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34489238 .
- Lipnick MS, Feiner JR, Au P, Bernstein M, Bickler PE. The accuracy of 6 inexpensive pulse oximeters not cleared by the Food and Drug Administration: the possible global public health implications. Anesth Analg . 2016;123(2):338-345. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27089002 .
- Zhang R, Ouyang H, Fu L, et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol . 2020;30(8):4417-4426. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32279115 .
- Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship Diamond Princess with coronavirus disease 2019 (COVID-19). Radiol Cardiothorac Imaging . 2020;2(2):e200110. Available at: https://pubmed.ncbi.nlm.nih.gov/33778566 .
- Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085-2086. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32293646 .
- Krumbein H, Kümmel LS, Fragkou PC, et al. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: a systematic review and meta-analysis. Rev Med Virol . 2023;33(1):e2365. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35686619 .
- Alosaimi B, Naeem A, Hamed ME, et al. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol J . 2021;18(1):127. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34127006 .
- Kubin CJ, McConville TH, Dietz D, et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with health care-associated infections. Open Forum Infect Dis . 2021;8(6):ofab201. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34099978 .
- Hedberg P, Johansson N, Ternhag A, et al. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect Dis . 2022;22(1):108. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35100984 .
- Garg N, Lee YI. Reactivation TB with severe COVID-19. Chest . 2020;158(4):A777. Available at: https://journal.chestnet.org/article/S0012-3692(20)32910-X/fulltext .
- Rodríguez-Tajes S, Miralpeix A, Costa J, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat . 2021;28(1):89-94. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32969557 .
- Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID-19 induced hepatitis B virus reactivation: a novel case from the United Arab Emirates. Cureus . 2020;12(6):e8645. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32550096 .
- Xu R, Zhou Y, Cai L, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol . 2020;183(6):1145-1147. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32790074 .
- Lier AJ, Tuan JJ, Davis MW, et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg . 2020;103(4):1590-1592. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32830642 .
- Marchese V, Crosato V, Gulletta M, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection . 2021;49(3):539-542. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32910321 .
- Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related Strongyloides hyperinfection. JAMA. 2020;324(7):623-624. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32761166 .
- Mohareb AM, Rosenberg JM, Bhattacharyya RP, et al. Preventing infectious complications of immunomodulation in COVID-19 in foreign-born patients. J Immigr Minor Health . 2021;23(6):1343-1347. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34159495 .
- Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis . 2021;40(3):495-502. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33389263 .
- Lingscheid T, Lippert LJ, Hillus D, et al. Characterization of antimicrobial use and co-infections among hospitalized patients with COVID-19: a prospective observational cohort study. Infection . 2022;50(6):1441-1452. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35420370 .
- Salmanton-García J, Sprute R, Stemler J, et al. COVID-19-associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis . 2021;27(4):1077-1086. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33539721 .
- Chong WH, Neu KP. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect . 2021;113:115-129. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33891985 .
- Machado M, Valerio M, Álvarez-Uría A, et al. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses . 2021;64(2):132-143. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33210776 .
- Yusuf E, Seghers L, Hoek RAS, et al. Aspergillus in critically ill COVID-19 patients: a scoping review. J Clin Med . 2021;10(11):2469. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34199528 .
- Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr . 2021;15(4):102146. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34192610 .
- Pal R, Singh B, Bhadada SK, et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses . 2021;64(12):1452-1459. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34133798 .
- Cohen JI, Burbelo PD. Reinfection with SARS-CoV-2: implications for vaccines. Clin Infect Dis . 2021;73(11):e4223-e4228. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33338197 .
- Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med . 2022;182(2):153-162. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34962505 .
- Link-Gelles R, Ciesla AA, Roper LE, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults—increasing community access to testing program, United States, December 2022–January 2023. MMWR Morb Mortal Wkly Rep . 2023;72(5):119-124. Available at: https://pubmed.ncbi.nlm.nih.gov/36730051 .
- Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis . 2022;22(1):43-55. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34480857 .
- Kuodi P, Gorelik Y, Zayyad H, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020–21, Israel. NPJ Vaccines . 2022;7(1):101. Available at: https://pubmed.ncbi.nlm.nih.gov/36028498 .
- Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med . 2022;387(11):1045-1047. Available at: https://www.ncbi.nlm.nih.gov/pubmed/36069968 .
- Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for coronavirus disease 2019 (COVID-19). Clin Infect Dis . 2023;76(3):e526-e529. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35737946 .
- Food and Drug Administration. Fact sheet for healthcare providers: Emergency Use Authorization for Lagevrio (molnupiravir) capsules. 2023. Available at: https://www.fda.gov/media/155054/download .
- Ritonavir-boosted nirmatrelvir (Paxlovid) [package insert]. Food and Drug Administration. 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf .
- Qutub M, Aldabbagh Y, Mehdawi F, et al. Duration of viable SARS-CoV-2 shedding from respiratory tract in different human hosts and its impact on isolation discontinuation polices revision; a narrative review. Clin Infect Pract . 2022;13:100140. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35190799 .
- Tarhini H, Recoing A, Bridier-Nahmias A, et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis . 2021;223(9):1522-1527. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33556961 .
- Centers for Disease Control and Prevention. Post-COVID conditions: information for healthcare providers. 2023. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html . Accessed January 2, 2024.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 November 2020
Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis
- Carlos K. H. Wong 1 , 2 na1 ,
- Janet Y. H. Wong 3 na1 ,
- Eric H. M. Tang 1 ,
- C. H. Au 1 &
- Abraham K. C. Wai 4
Scientific Reports volume 10 , Article number: 19765 ( 2020 ) Cite this article
6069 Accesses
54 Citations
3 Altmetric
Metrics details
- Health care
- Medical research
- Microbiology
- Risk factors
This systematic review and meta-analysis investigated the comorbidities, symptoms, clinical characteristics and treatment of COVID-19 patients. Epidemiological studies published in 2020 (from January–March) on the clinical presentation, laboratory findings and treatments of COVID-19 patients were identified from PubMed/MEDLINE and Embase databases. Studies published in English by 27th March, 2020 with original data were included. Primary outcomes included comorbidities of COVID-19 patients, their symptoms presented on hospital admission, laboratory results, radiological outcomes, and pharmacological and in-patient treatments. 76 studies were included in this meta-analysis, accounting for a total of 11,028 COVID-19 patients in multiple countries. A random-effects model was used to aggregate estimates across eligible studies and produce meta-analytic estimates. The most common comorbidities were hypertension (18.1%, 95% CI 15.4–20.8%). The most frequently identified symptoms were fever (72.4%, 95% CI 67.2–77.7%) and cough (55.5%, 95% CI 50.7–60.3%). For pharmacological treatment, 63.9% (95% CI 52.5–75.3%), 62.4% (95% CI 47.9–76.8%) and 29.7% (95% CI 21.8–37.6%) of patients were given antibiotics, antiviral, and corticosteroid, respectively. Notably, 62.6% (95% CI 39.9–85.4%) and 20.2% (95% CI 14.6–25.9%) of in-patients received oxygen therapy and non-invasive mechanical ventilation, respectively. This meta-analysis informed healthcare providers about the timely status of characteristics and treatments of COVID-19 patients across different countries.
PROSPERO Registration Number: CRD42020176589
Similar content being viewed by others
Global prevalence and effect of comorbidities and smoking status on severity and mortality of COVID-19 in association with age and gender: a systematic review, meta-analysis and meta-regression
Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients
Risk factors for severe COVID-19 differ by age for hospitalized adults
Introduction.
Following the possible patient zero of coronavirus infection identified in early December 2019 1 , the Coronavirus Disease 2019 (COVID-19) has been recognized as a pandemic in mid-March 2020 2 , after the increasing global attention to the exponential growth of confirmed cases 3 . As on 29th March, 2020, around 690 thousand persons were confirmed infected, affecting 199 countries and territories around the world, in addition to 2 international conveyances: the Diamond Princess cruise ship harbored in Yokohama, Japan, and the Holland America's MS Zaandam cruise ship. Overall, more than 32 thousand died and about 146 thousand have recovered 4 .
A novel bat-origin virus, 2019 novel coronavirus, was identified by means of deep sequencing analysis. SARS-CoV-2 was closely related (with 88% identity) to two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21, but were more distant from SARS-CoV (about 79%) and MERS-CoV (about 50%) 5 , both of which were respectively responsible for two zoonotic human coronavirus epidemics in the early twenty-first century. Following a few initial human infections 6 , the disease could easily be transmitted to a substantial number of individuals with increased social gathering 7 and population mobility during holidays in December and January 8 . An early report has described its high infectivity 9 even before the infected becomes symptomatic 10 . These natural and social factors have potentially influenced the general progression and trajectory of the COVID-19 epidemiology.
By the end of March 2020, there have been approximately 3000 reports about COVID-19 11 . The number of COVID-19-related reports keeps growing everyday, yet it is still far from a clear picture on the spectrum of clinical conditions, transmissibility and mortality, alongside the limitation of medical reports associated with reporting in real time the evolution of an emerging pathogen in its early phase. Previous reports covered mostly the COVID-19 patients in China. With the spread of the virus to other continents, there is an imminent need to review the current knowledge on the clinical features and outcomes of the early patients, so that further research and measures on epidemic control could be developed in this epoch of the pandemic.
Search strategy and selection criteria
The systematic review was conducted according to the protocol registered in the PROSPERO database (CRD42020176589). Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline throughout this review, data were identified by searches of MEDLINE, Embase and references from relevant articles using the search terms "COVID", “SARS-CoV-2”, and “novel coronavirus” (Supplementary material 1 ). Articles published in English up to 27th March, 2020 were included. National containment measures have been implemented at many countries, irrespective of lockdown, curfew, or stay-at-home orders, since the mid of March 2020 12 , except for China where imposed Hubei province lockdown at 23th January 2020, Studies with original data including original articles, short and brief communication, letters, correspondences were included. Editorials, viewpoints, infographics, commentaries, reviews, or studies without original data were excluded. Studies were also excluded if they were animal studies, modelling studies, or did not measure symptoms presentation, laboratory findings, treatment and therapeutics during hospitalization.
After the removal of duplicate records, two reviewers (CW and CHA) independently screened the eligibility criteria of study titles, abstracts and full-texts, and reference lists of the studies retrieved by the literature search. Disagreements regarding the procedures of database search, study selection and eligibility were resolved by discussion. The second and the last authors (JW and AW) verified the eligibility of included studies.
Outcomes definitions
Signs and symptoms were defined as the presentation of fever, cough, sore throat, headache, dyspnea, muscle pain, diarrhea, rhinorrhea, anosmia, and ageusia at the hospital admission 13 .
Laboratory findings included a complete blood count (white blood count, neutrophil, lymphocyte, platelet count), procalcitonin, prothrombin time, urea, and serum biochemical measurements (including electrolytes, renal-function and liver-function values, creatine kinase, lactate dehydrogenase, C-reactive protein, Erythrocyte sedimentation rate), and treatment measures (i.e. antiviral therapy, antibiotics, corticosteroid therapy, mechanical ventilation, intubation, respiratory support, and renal replacement therapy). Radiological outcomes included bilateral involvement identified and pneumonia identified by chest radiograph.
Comorbidities of patients evaluated in this study were hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, chronic kidney disease, liver disease and cancer.
In-patient treatment included intensive care unit admission, oxygen therapy, non-invasive ventilation, mechanical ventilation, Extracorporeal membrane oxygenation (ECMO), renal replacement therapy, and pharmacological treatment. Use of antiviral and interferon drugs (Lopinavir/ritonavir, Ribavirin, Umifenovir, Interferon-alpha, or Interferon-beta), antibiotic drugs, corticosteroid, and inotropes (Nor-adrenaline, Adrenaline, Vasopressin, Phenylephrine, Dopamine, or Dobutamine) were considered.
Data analysis
Three authors (CW, EHMT and CHA) extracted data using a standardized spreadsheet to record the article type, country of origin, surname of first author, year of publications, sample size, demographics, comorbidities, symptoms, laboratory and radiology results, pharmacological and non-pharmacological treatments.
We aggregated estimates across 90 eligible studies to produce meta-analytic estimates using a random-effects model. For dichotomous outcomes, we estimated the proportion and its respective 95% confidence interval. For laboratory parameters as continuous outcomes, we estimated the mean and standard deviation from the median and interquartile range if the mean and standard deviation were not available from the study 14 , and calculated the mean and its respective 95% confidence intervals. Random-effect models on DerSimonian and Laird method were adopted due to the significant heterogeneity, checked by the I 2 statistics and the p values. I 2 statistic of < 25%, 25–75% and ≥ 75% is considered as low, moderate, high likelihood of heterogeneity. Pooled estimates were calculated and presented by using forest plots. Publication bias was estimated by Egger’s regression test. Funnel plots of outcomes were also presented to assess publication bias.
All statistical analyses were conducted using the STATA Version 13.0 (Statacorp, College Station, TX). The random effects model was generated by the Stata packages ‘Metaprop’ for proportions 15 and ‘Metan’ for continuous variables 16 .
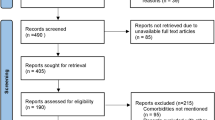
The selection and screen process are presented in Fig. 1 . A total of 241 studies were found by our searching strategy (71 in PubMed and 170 in Embase). 46 records were excluded due to duplication. After screening the abstracts and titles, 100 English studies were with original data and included in full-text screening. By further excluding 10 studies with not reporting symptoms presentation, laboratory findings, treatment and therapeutics, 90 studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 and 76 studies with more than one COVID-19 case 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 34 , 35 , 36 , 37 , 38 , 39 , 42 , 43 , 44 , 45 , 49 , 50 , 51 , 53 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 67 , 69 , 70 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 98 , 100 , 101 , 102 , 103 , 104 , 105 were included in the current systematic review and meta-analysis respectively. 73.3% 66 studies were conducted in China. Newcastle–Ottawa Quality Assessment Scale has been used to assess study quality of each included cohort study 107 . 30% (27/90) of included studies had satisfactory or good quality. The summary of the included study is shown in Table 1 .
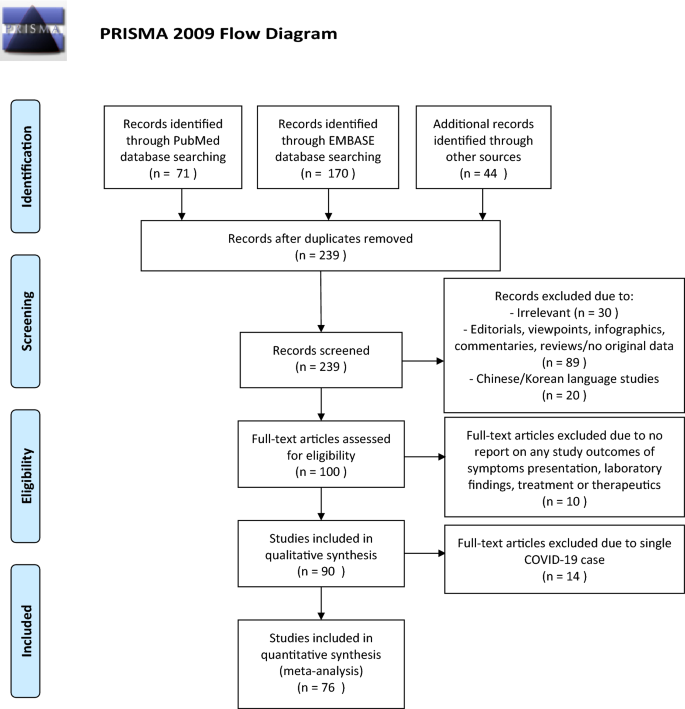
PRISMA flowchart reporting identification, searching and selection processes.
Of those 90 eligible studies, 11,028 COVID-19 patients were identified and included in the systematic review. More than half of patients (6336, 57.5%) were from mainland China. The pooled mean age was 45.8 (95% CI 38.6–52.5) years and 49.3% (pooled 95% CI 45.6–53.0%) of them were male.
For specific comorbidity status, the most prevalent comorbidity was hypertension (18.1%, 95% CI 15.4–20.8%), followed by cardiovascular disease (11.8%, 95% CI 9.4–14.2%) and diabetes (10.4%, 95% CI 8.7–12.1%). The pooled prevalence (95% CI) of COPD, chronic kidney disease, liver disease and cancer were 2.0% (1.3–2.7%), 5.2% (1.7–8.8%), 2.5% (1.7–3.4%) and 2.1% (1.3–2.8%) respectively. Moderate to substantial heterogeneity between reviewed studies were found, with I 2 statistics ranging from 39.4 to 95.9% ( p values between < 0.001–0.041), except for liver disease (I 2 statistics: 1.7%, p = 0.433). Detailed results for comorbidity status are displayed in Fig. 2 .
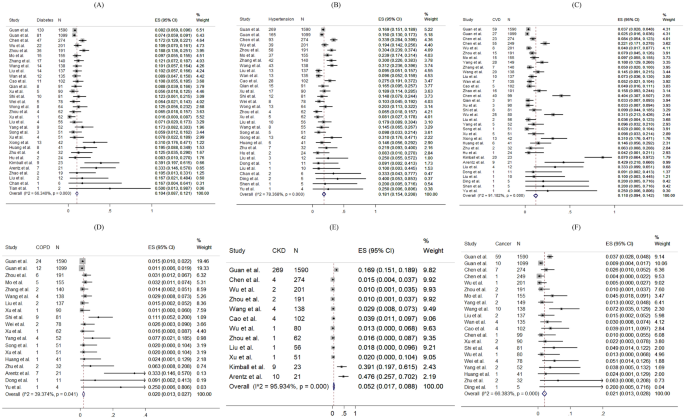
Random-effects meta-analytic estimates for comorbidities. ( A ) Diabetes mellitus, ( B ) Hypertension, ( C ) Cardiovascular disease, ( D ) Chronic obstructive pulmonary disease, ( E ) Chronic kidney disease, ( F ) Cancer.
Regarding the symptoms presented at hospital admission, the most frequent symptoms were fever (pooled prevalence: 72.4%, 95% CI 67.2–77.7%) and cough (pooled prevalence: 55.5%, 95% CI 50.7–60.3%). Sore throat (pooled prevalence: 16.2%, 95% CI 12.7–19.7%), dyspnoea (pooled prevalence: 18.8%, 95% CI 14.7–22.8%) and muscle pain (pooled prevalence: 22.1%, 95% CI 18.6–25.5%) were also common symptoms found in COVID-19 patients, but headache (pooled prevalence: 10.5%, 95% CI 8.7–12.4%), diarrhoea (pooled prevalence: 7.9%, 95% CI 6.3–9.6%), rhinorrhoea (pooled prevalence: 9.2%, 95% CI 5.6–12.8%) were less common. However, none of the included papers reported prevalence of anosmia and ageusia. The I 2 statistics varied from 68.5 to 97.1% (all p values < 0.001), indicating a high heterogeneity exists across studies. Figure 3 shows the pooled proportion of symptoms of patients presented at hospital.

Random-effects meta-analytic estimates for presenting symptoms. ( A ) Fever, ( B ) Cough, ( C ) Dyspnoea, ( D ) Sore throat, ( E ) Muscle pain, ( F ) Headache.
For laboratory parameters, white blood cell (pooled mean: 5.31 × 10 9 /L, 95% CI 5.03–5.58 × 10 9 /L), neutrophil (pooled mean: 3.60 × 10 9 /L, 95% CI 3.31–3.89 × 10 9 /L), lymphocyte (pooled mean: 1.11 × 10 9 /L, 95% CI 1.04–1.17 × 10 9 /L), platelet count (pooled mean: 179.5 U/L, 95% CI 172.6–186.3 U/L), aspartate aminotransferase (pooled mean: 30.3 U/L, 95% CI 27.9–32.7 U/L), alanine aminotransferase (pooled mean: 27.0 U/L, 95% CI 24.4–29.6 U/L) and C-reactive protein (CRP) (pooled mean: 22.0 mg/L, 95% CI 18.3–25.8 mg/L) and D-dimer (0.93 mg/L, 95% CI 0.68–1.18 mg/L) were the common laboratory test taken for COVID-19 patients. Above results and other clinical factors are depicted in Fig. 4 . Same with the comorbidity status and symptoms, high likelihood of heterogeneity was detected by I 2 statistics for a majority of clinical parameters.
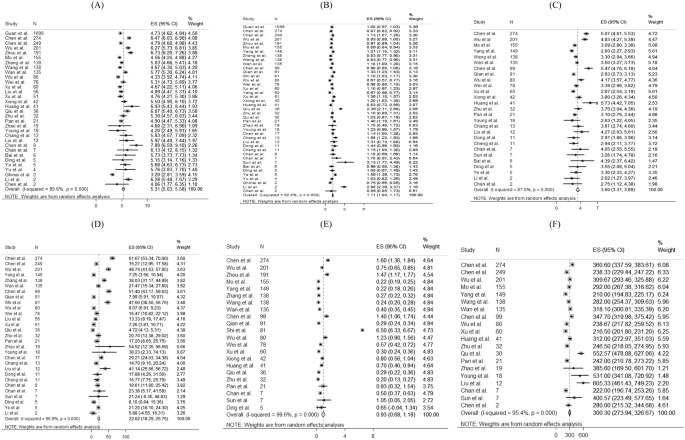
Random-effects meta-analytic estimates for laboratory parameters. ( A ) White blood cell, ( B ) Lymphocyte, ( C ) Neutrophil, ( D ) C-creative protein, ( E ) D-dimer, ( F ) Lactate dehydrogenase.
Figure 5 presents the distribution of the pharmacological treatments received for COVID-19 patients. 10.6% of patients admitted to intensive care units (pooled 95% CI 8.1–13.2%). For drug treatment, 63.9% (pooled 95% CI 52.5–75.3%), 62.4% (pooled 95% CI 47.9–76.8%) and 29.7% (pooled 95% CI 21.8–37.6%) patients used antibiotics, antiviral, and corticosteroid, respectively. 41.3% (pooled 95% CI 14.3–68.3%) and 50.7% (pooled 95% CI 9.2–92.3%) reported using Lopinavir/Ritonavir and interferon-alpha as antiviral drug treatment, respectively. Among 14 studies reporting proportion of corticosteroid used, 7 studies (50%) specified the formulation of corticosteroid as systemic corticosteroid. The remaining one specified the use of methylprednisolone. No reviewed studies reported the proportion of patients receiving Ribavirin, Interferon-beta, or inotropes.
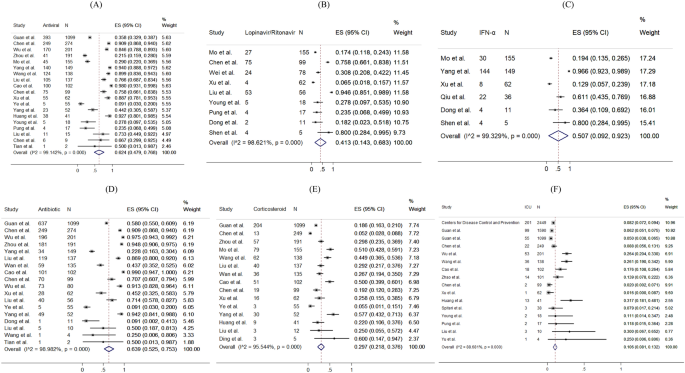
Random-effects meta-analytic estimates for pharmacological treatments and intensive unit care at hospital. ( A ) Antiviral or interferon drugs, ( B ) Lopinavir/Ritonavir, ( C ) Interferon alpha (IFN-α), ( D ) Antibiotic drugs, ( E ) Corticosteroid, ( F ) Admission to Intensive care unit.
The prevalence of radiological outcomes and non-pharmacological treatments were presented in Fig. 6 . Radiology findings detected chest X-ray abnormalities, with 74.4% (95% CI 67.6–81.1%) of patients with bilateral involvement and 74.9% (95% CI 68.0–81.8%) of patients with viral pneumonia. 62.6% (pooled 95% CI 39.9–85.4%), 20.2% (pooled 95% CI 14.6–25.9%), 15.3% (pooled 95% CI 11.0–19.7%), 1.1% (pooled 95% CI 0.4–1.8%) and 4.7% (pooled 95% CI 2.1–7.4%) took oxygen therapy, non-invasive ventilation, mechanical ventilation, ECMO and dialysis respectively.
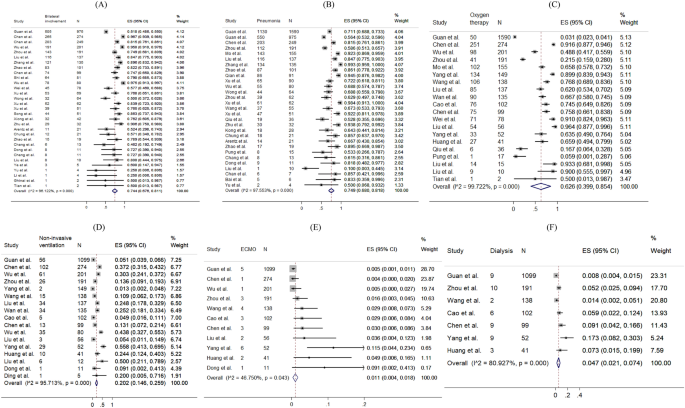
Random-effects meta-analytic estimates for radiological findings and non-pharmacological treatments at hospital. ( A ) Bilateral involvement, ( B ) Pneumonia, ( C ) Oxygen therapy, ( D ) Non-invasive ventilation, ( E ) Extracorporeal membrane oxygenation (ECMO), ( F ) Dialysis.
The funnel plots and results Egger’s test of comorbidity status, symptoms presented, laboratory test and treatment were presented in eFigure 1 – S5 in the Supplement. 63% (19/30) of the funnel plots (eFigure 1 – S5 ) showed significance in the Egger’s test for asymmetry, suggesting the possibility of publication bias or small-study effects caused by clinical heterogeneity.
This meta-analysis reveals the condition of global medical community responding to COVID-19 in the early phase. During the past 4 months, a new major epidemic focus of COVID-19, some without traceable origin, has been identified. Following its first identification in Wuhan, China, the virus has been rapidly spreading to Europe, North America, Asia, and the Middle East, in addition to African and Latin American countries. Three months since Wuhan CDC admitted that there was a cluster of unknown pneumonia cases related to Huanan Seafood Market and a new coronavirus was identified as the cause of the pneumonia 108 , as on 1 April, 2020, there have been 858,371 persons confirmed infected with COVID-19, affecting 202 countries and territories around the world. Although this rapid review is limited by the domination of reports from patients in China, and the patient population is of relative male dominance reflecting the gender imbalance of the Chinese population 109 , it provides essential information.
In this review, the pooled mean age was 45.8 years. Similar to the MERS-CoV pandemic 110 , middle-aged adults were the at-risk group for COVID-19 infections in the initial phase, which was different from the H1N1 influenza pandemic where children and adolescents were more frequently affected 111 . Biological differences may affect the clinical presentations of infections; however, in this review, studies examining the asymptomatic COVID-19 infections or reporting any previous infections were not included. It is suggested that another systematic review should be conducted to compare the age-specific incidence rates between the pre-pandemic and post-pandemic periods, so as to understand the pattern and spread of the disease, and tailor specific strategies in infection control.
Both sexes exhibited clinical presentations similar in symptomatology and frequency to those noted in other severe acute respiratory infections, namely influenza A H1N1 112 and SARS 113 , 114 . These generally included fever, new onset or exacerbation of cough, breathing difficulty, sore throat and muscle pain. Among critically ill patients usually presented with dyspnoea and chest tightness 22 , 29 , 39 , 72 , 141 (4.6%) of them with persistent or progressive hypoxia resulted in the requirement of intubation and mechanical ventilation 115 , while 194 (6.4%) of them required non-invasive ventilation, yielding a total of 11% of patients requiring ventilatory support, which was similar to SARS 116 .
The major comorbidities identified in this review included hypertension, cardiovascular diseases and diabetes mellitus. Meanwhile, the percentages of patients with chronic renal diseases and cancer were relatively low. These chronic conditions influencing the severity of COVID-19 had also been noted to have similar effects in other respiratory illnesses such as SARS, MERS-CoV and influenza 117 , 118 . Higher mortality had been observed among older patients and those with comorbidities.
Early diagnosis of COVID-19 was based on recognition of epidemiological linkages; the presence of typical clinical, laboratory, and radiographic features; and the exclusion of other respiratory pathogens. The case definition had initially been narrow, but was gradually broadened to allow for the detection of more cases, as milder cases and those without epidemiological links to Wuhan or other known cases had been identified 119 , 120 . Laboratory investigations among COVID-19 patients did not reveal specific characteristics—lymphopenia and elevated inflammatory markers such as CRP are some of the most common haematological and biochemical abnormalities, which had also been noticed in SARS 121 . None of these features were specific to COVID-19. Therefore, diagnosis should be confirmed by SARS-CoV–2 specific microbiological and serological studies, although initial management will continue to be based on a clinical and epidemiological assessment of the likelihood of a COVID-19 infection.
Radiology imaging often plays an important role in evaluating patients with acute respiratory distress; however, in this review, radiological findings of SARS-CoV-2 pneumonia were non-specific. Despite chest radiograph usually revealed bilateral involvement and Computed Tomography usually showed bilateral multiple ground-glass opacities or consolidation, there were also patients with normal chest radiograph, implying that chest radiograph might not have high specificity to rule out pneumonia in COVID-19.
Limited clinical data were available for asymptomatic COVID-19 infected persons. Nevertheless, asymptomatic infection could be unknowingly contagious 122 . From some of the official figures, 6.4% of 150 non-travel-related COVID-19 infections in Singapore 123 , 39.9% of cases from the Diamond Princess cruise ship in Japan 124 , and up to 78% of cases in China as extracted on April 1st, 2020, were found to be asymptomatic 122 . 76% (68/90) studies based on hospital setting which provided care and disease management to symptomatic patients had limited number of asymptomatic cases of COVID-19 infection. This review calls for further studies about clinical data of asymptomatic cases. Asymptomatic infection intensifies the challenges of isolation measures. More global reports are crucially needed to give a better picture of the spectrum of presentations among all COVID-19 infected persons. Also, public health policies including social and physical distancing, monitoring and surveillance, as well as contact tracing, are necessary to reduce the spread of COVID-19.
Concerning potential treatment regime, 62.4% of patients received antivirals or interferons (including oseltamivir, lopinavir-ritonavir, interferon alfa), while 63.9% received antibiotics (such as moxifloxacin, and ceftriaxone). In this review, around one-third of patients were given steroid, suggestive as an adjunct to IFN, or sepsis management. Interferon and antiviral agents such as ribavirin, and lopinavir-ritonavir were used during SARS, and the initial uncontrolled reports then noted resolution of fever and improvement in oxygenation and radiographic appearance 113 , 125 , 126 , without further evidence on its effectiveness. At the time of manuscript preparation, there has been no clear evidence guiding the use of antivirals 127 . Further research is needed to inform clinicians of the appropriate use of antivirals for specific groups of infected patients.
Limitations of this meta-analysis should be considered. First, a high statistical heterogeneity was found, which could be related to the highly varied sample sizes (9 to 4226 patients) and study designs. Second, variations of follow-up period may miss the event leading to heterogeneity. In fact, some patients were still hospitalized in the included studies. Third, since only a few studies had compared the comorbidities of severe and non-severe patients, sensitivity analysis and subgroup analysis were not conducted. Fourthly, the frequency and severity of signs and symptoms reported in included studies, primarily based on hospitalized COVID-19 patients were over-estimated. Moreover, different cutoffs for abnormal laboratory findings were applied across countries, and counties within the same countries. Lastly, this meta-analysis reviewed only a limited number of reports written in English, with a predominant patient population from China. This review is expected to inform clinicians of the epidemiology of COVID-19 at this early stage. A recent report estimated the number of confirmed cases in China could reach as high as 232,000 (95% CI 161,000, 359,000) with the case definition adopted in 5th Edition. In this connection, further evidence on the epidemiology is in imminent need.
Oliveira N. Shrimp vendor identified as possible coronavirus ‘patient zero,’ leaked document says. 27 March 2020. New York Daily News. 2020.
World Health Organization. Basic protective measures against the new coronavirus (2020). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public . Accessed 7 Oct 2020.
Google Trend. When will coronavirus end (2020). https://trends.google.com/trends/explore?date=today%203-m&q=when%20will%20coronavirus%20end,%2Fm%2F01cpyy . Accessed 10 Oct 2020.
Worldometer. COVID-19 Coronavirus Pandemic (2020). https://www.worldometers.info/coronavirus/ . Accessed 13 Oct 2020.
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224), 565–574 (2020).
Article CAS PubMed PubMed Central Google Scholar
Ralph, R. et al. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J. Infect. Dev. Ctries. 14 (1), 3–17 (2020).
Article CAS PubMed Google Scholar
Sun, Z., Thilakavathy, K., Kumar, S. S., He, G. & Liu, S. V. Potential factors influencing repeated SARS outbreaks in China. Int. J. Environ. Res. Public Health 17 (5), 1633 (2020).
Article CAS PubMed Central Google Scholar
Zhao, S. et al. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: a data-driven correlational report. Travel Med. Infect. Dis. 33 , 101568 (2020).
Article PubMed PubMed Central Google Scholar
Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382 (13), 1199–1207 (2020).
Chen, J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 22 (2), 69–71 (2020).
World Health Organization. Database of publications on coronavirus disease (COVID-19) (2020). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov . Accessed 30 Mar 2020.
Wong, C. K. H. et al. Impact of national containment measures on decelerating the increase in daily new cases of COVID-19 in 54 countries and 4 epicenters of the pandemic: comparative observational study. J. Med. Internet Res. 22 (7), e19904 (2020).
Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Symptoms of Coronavirus (2020).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 (1), 135 (2014).
Nyaga, V. N., Arbyn, M. & Aerts, M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health 72 (1), 39 (2014).
Harris, R. J. et al. metan: fixed- and random-effects meta-analysis. Stata J. 8 (1), 3–28 (2008).
Article Google Scholar
Xu, X. et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 47 (5), 1275–1280 (2020).
Cao, J. et al. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 46 (5), 851–853 (2020).
Xiong, Y. et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest. Radiol. 55 (6), 332–339 (2020).
Arentz, M. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 323 (16), 1612–1614 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506 (2020).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720 (2020).
Zhao, D. et al. A comparative study on the clinical features of coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin. Infect. Dis. 71 (15), 756–761 (2020).
Xu, X. W. et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 19 (368), m606 (2020).
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 (10223), 514–523 (2020).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 (10223), 507–513 (2020).
Pung, R. et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet 395 (10229), 1039–1046 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323 (11), 1061–1069 (2020).
Young, B. E. et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323 (15), 1488–1494 (2020).
Chen, H. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395 (10226), 809–815 (2020).
Huang, W. H. et al. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J. Microbiol. Immunol. Infect. 53 (3), 481–484 (2020).
Cheng, S. C. et al. First case of coronavirus disease 2019 (COVID-19) pneumonia in Taiwan. J. Formos. Med. Assoc. 119 (3), 747–751 (2020).
Holshue, M. L. et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382 (10), 929–936 (2020).
Wei, M. et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 323 (13), 1313–1314 (2020).
Bernard Stoecklin, S. et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 25 (6), 20–26 (2020).
Shi, H. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20 (4), 425–434 (2020).
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382 (8), 727–733 (2020).
Ghinai, I. et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395 (10230), 1137–1144 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (10229), 1054–1062 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8 (5), 475–481 (2020).
Kim, J. Y. et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J. Korean Med. Sci. 35 (5), e61 (2020).
Okada, P. et al . Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Euro Surveill . 25 (8), 6–10 (2020).
Arashiro, T., Furukawa, K. & Nakamura, A. COVID-19 in 2 persons with mild upper respiratory tract symptoms on a cruise ship, Japan. Emerg. Infect. Dis. 26 (6), 1345–1348 (2020).
Lillie, P. J. et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J. Infect. 80 (5), 578–606 (2020).
Tian, S. et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 15 (5), 700–704 (2020).
Haveri, A. et al . Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill . 25 (11), 16–21 (2020).
Nicastri, E. et al . Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill . 25 (11) (2020).
Van Cuong, L. et al. The first Vietnamese case of COVID-19 acquired from China. Lancet Infect Dis. 20 (4), 408–409 (2020).
Spiteri, G. et al . First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill . 25 (9), 2–7 (2020).
Rothe, C. et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 382 (10), 970–971 (2020).
Tong, Z. D. et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg. Infect. Dis. 26 (5), 1052–1054 (2020).
Bai, Y. et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 323 (14), 1406–1407 (2020).
Yu, P., Zhu, J., Zhang, Z. & Han, Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 221 (11), 1757–1761 (2020).
Li, P. et al. Transmission of COVID-19 in the terminal stages of the incubation period: a familial cluster. Int. J. Infect. Dis. 96 , 452–453 (2020).
Tang, A. et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 26 (6), 1337–1339 (2020).
Kam, K. Q. et al. A well infant with coronavirus disease 2019 with high viral load. Clin. Infect. Dis. 71 (15), 847–849 (2020).
Zhou, S., Wang, Y., Zhu, T. & Xia, L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan. China. AJR Am J Roentgenol. 214 (6), 1287–1294 (2020).
Article PubMed Google Scholar
Zhao, W., Zhong, Z., Xie, X., Yu, Q. & Liu, J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am. J. Roentgenol. 214 (5), 1072–1077 (2020).
Cheng, Z. et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am. J. Roentgenol. 215 (1), 121–126 (2020).
Chung, M. et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295 (1), 202–207 (2020).
Liu, K. et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 133 (9), 1025–1031 (2020).
Chang, L. M. et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 323 (11), 1092–1093 (2020).
Team C-NIRS. COVID-19, Australia: Epidemiology Report 7 (Reporting week ending 19:00 AEDT 14 March 2020). Commun. Dis. Intell. 44 (2018).
Pan, F. et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 295 (3), 715–721 (2020).
Wang, S. et al. A case report of neonatal 2019 coronavirus disease in China. Clin. Infect. Dis. 71 (15), 853–857 (2020).
Bastola, A. et al. The first 2019 novel coronavirus case in Nepal. Lancet Infect. Dis. 20 (3), 279–280 (2020).
Qiu, H. et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 20 (6), 689–696 (2020).
Zhang, J. J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75 (7), 1730–1741 (2020).
Ye, G. et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 80 (5), e14–e17 (2020).
Liu, Y. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 63 (3), 364–374 (2020).
Chen, T. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 26 (368), m1091 (2020).
Guan, W. J. et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 55 (5), 2000547 (2020).
Wong, H. Y. F. et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 296 (2), E72–E78 (2020).
Xu, T. et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 94 , 68–71 (2020).
Shen, C. et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323 (16), 1582–1589 (2020).
Kimball, A. et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. Morb. Mortal. Wkly. Rep. 69 (13), 377–381 (2020).
Article CAS Google Scholar
Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. Morb. Mortal. Wkly. Rep. 69 (12), 343–346 (2020).
Wu, J. et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 71 (15), 706–712 (2020).
Yang, W. et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 80 (4), 388–393 (2020).
Zhu, L. et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 20 (7), 1859–1863 (2020).
Zhu, W. et al. Initial clinical features of suspected coronavirus disease in two emergency departments outside of Hubei, China. J. Med. Virol. 92 , 1525–1532 (2019).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180 (7), 934–943 (2020).
Wang, Z., Chen, X., Lu, Y., Chen, F. & Zhang, W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 14 (1), 64–68 (2020).
Wang, Y. et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J. Infect. Dis. 221 (11), 1770–1774 (2020).
Wan, S. et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med.. Virol. 92 (7), 797–806 (2020).
Tian, S. et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 80 (4), 401–406 (2020).
Sun, D. et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 16 (3), 251–259 (2020).
Song, F. et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295 (1), 210–217 (2020).
Hu, Z. et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 63 (5), 706–711 (2020).
Qu, R. et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 92 , 1533–1541 (2020).
Qian, G. Q. et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 113 (7), 474–481 (2020).
Mo, P. et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis . (2020).
Liu, W. et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med. J. (Engl) 133 (9), 1032–1038 (2020).
Liu, K., Chen, Y., Lin, R. & Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 80 (6), e14–e18 (2020).
Liu, F. et al. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 95 , 183–191 (2020).
Liu, D. et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am. J. Roentgenol. 215 (1), 127–132 (2020).
Guillen, E. et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation?. Am. J. Transplant. 20 (7), 1875–1878 (2020).
Dong, X. et al. Eleven faces of coronavirus disease 2019. Allergy 75 (7), 1699–1709 (2020).
Fan, C. et al . Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. (2020).
Chen, R. et al. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can. J. Anaesth. 67 (6), 655–663 (2020).
Chen, L. et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes Infect. 9 (1), 313–319 (2020).
Chen, J. et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 80 (5), e1–e6 (2020).
Ding, Q., Lu, P., Fan, Y., Xia, Y. & Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 92 , 1549–1555 (2020).
Covid-19 National Emergency Response Center E & Case Management Team KCfDC, Prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res. Perspect. 11 (1), 8–14 (2020).
Li, Y., Guo, F., Cao, Y., Li, L. & Guo, Y. Insight into COVID-2019 for pediatricians. Pediatr. Pulmonol. 55 (5), E1–E4 (2020).
Ai, J. W., Zhang, Y., Zhang, H. C., Xu, T. & Zhang, W. H. Era of molecular diagnosis for pathogen identification of unexplained pneumonia, lessons to be learned. Emerg Microbes Infect. 9 (1), 597–600 (2020).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605 (2010).
Khan N. New virus discovered by Chinese scientists investigating pneumonia outbreak. Wall Street J . (2020).
国家统计局 (National Bureau of Statistics). 2019 年国民经济运行总体平稳 发展主要预期目标较好实现 (In 2019, the overall stable development of the national economic operation is expected to achieve the main goals (2020). http://www.stats.gov.cn/tjsj/zxfb/202001/t20200117_1723383.html . Accessed 30 Mar 2020.
Park, J. E., Jung, S., Kim, A. & Park, J. E. MERS transmission and risk factors: a systematic review. BMC Public Health 18 (1), 574 (2018).
Van Kerkhove, M. D. et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 8 (7), e1001053 (2011).
Wang, C. et al. Epidemiological and clinical characteristics of the outbreak of 2009 pandemic influenza A (H1N1) at a middle school in Luoyang, China. Public Health 126 (4), 289–294 (2012).
Lee, N. et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348 (20), 1986–1994 (2003).
Booth, C. M. et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289 (21), 2801–2809 (2003).
Fowler, R. A. et al. Critically ill patients with severe acute respiratory syndrome. JAMA 290 (3), 367–373 (2003).
Christian, M. D., Poutanen, S. M., Loutfy, M. R., Muller, M. P. & Low, D. E. Severe acute respiratory syndrome. Clin Infect Dis. 38 (10), 1420–1427 (2004).
Mertz, D. et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 23 (347), f5061 (2013).
Badawi, A. & Ryoo, S. G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 49 , 129–133 (2016).
Tsang, T. K. et al. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: a modelling study. Lancet Public Health. 5 (5), e289–e296 (2020).
国家卫生健康委办公厅 (Office of National Health Comission). 新型冠状病毒肺炎诊疗方案 (试行第七版) (Clinical Guideline for Novel Coronavirus Pneumonia—Interim 7th Edition) (2020).
File, T. M. Jr. & Tsang, K. W. Severe acute respiratory syndrome: pertinent clinical characteristics and therapy. Treat. Respir. Med. 4 (2), 95–106 (2005).
Day, M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ 2 (369), m1375 (2020).
Wei, W. E. et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. Morb. Mortal. Wkly. Rep. 69 (14), 411–415 (2020).
Mizumoto, K., Kagaya, K., Zarebski, A. & Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 25 (10), 2000180 (2020).
Article PubMed Central Google Scholar
Poutanen, S. M. et al. Identification of severe acute respiratory syndrome in Canada. N. Engl. J .Med. 348 (20), 1995–2005 (2003).
Tsang, K. W. et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348 (20), 1977–1985 (2003).
Cao, B. et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 382 (19), 1787–1799 (2020).
Download references
Acknowledgements
There was no funding source for this study.
Author information
These authors contributed equally: Carlos K. H. Wong and Janet Y. H. Wong.
Authors and Affiliations
Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Carlos K. H. Wong, Eric H. M. Tang & C. H. Au
Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Carlos K. H. Wong
School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Janet Y. H. Wong
Emergency Medicine Unit, Li Ka Shing, Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Abraham K. C. Wai
You can also search for this author in PubMed Google Scholar
Contributions
C.W., J.W. and A.W. contributed equally to all aspects of study design, conduct, data interpretation, and the writing of the manuscript. C.W., E.T. and C.H.A. contributed to eligibility screening, data extraction from eligible studies, and data analysis and interpretation.
Corresponding author
Correspondence to Abraham K. C. Wai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary figure 1., supplementary figure 2., supplementary figure 3., supplementary figure 4., supplementary figure 5., supplementary material 6., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wong, C.K.H., Wong, J.Y., Tang, E.H.M. et al. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep 10 , 19765 (2020). https://doi.org/10.1038/s41598-020-74988-9
Download citation
Received : 04 May 2020
Accepted : 25 September 2020
Published : 13 November 2020
DOI : https://doi.org/10.1038/s41598-020-74988-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Comorbidity genetic risk and pathways impact sars-cov-2 infection outcomes.
- Rachel K. Jaros
- Tayaza Fadason
- Justin M. O’Sullivan
Scientific Reports (2023)
Adrenal function in relation to cytokines and outcome in non-critically ill patients with COVID-19
- N. Athanasiou
- A. Diamantopoulos
- D. A. Vassiliadi
Journal of Endocrinological Investigation (2023)
The Role of Multidimensional Prognostic Index to Identify Hospitalized Older Adults with COVID-19 Who Can Benefit from Remdesivir Treatment: An Observational, Prospective, Multicenter Study
- Carlo Custodero
- Nicola Veronese
- Julia Schlotmann
Drugs & Aging (2023)
Prevalence of hypertension and associated risks in hospitalized patients with COVID-19: a meta-analysis of meta-analyses with 1468 studies and 1,281,510 patients
- Yousof Khairy
- Deniz Naghibi
- Saber Azami-Aghdash
Systematic Reviews (2022)
An alternative approach to determination of Covid-19 personal risk index by using fuzzy logic
- Hakan Şimşek
- Elifnaz Yangın
Health and Technology (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
REVIEW article
Coronavirus disease (covid-19): comprehensive review of clinical presentation.

- 1 Department of Medicine, King Edward Medical University/ Mayo Hospital, Lahore, Pakistan
- 2 Department of Anesthesia and Intensive Care, Post-Graduate Medical Institute/LGH, Lahore, Pakistan
- 3 Rajarshee Chhatrapati Shahu Maharaj Government Medical College, Kolhapur, India
- 4 Department of Medicine, Faculty of Medicine, University of Tlemcen, Tlemcen, Algeria
- 5 School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan
- 6 Institute of Research and Development, Duy Tan University, Da Nang, Vietnam
COVID-19 is a rapidly growing pandemic with its first case identified during December 2019 in Wuhan, Hubei Province, China. Due to the rampant rise in the number of cases in China and globally, WHO declared COVID-19 as a pandemic on 11th March 2020. The disease is transmitted via respiratory droplets of infected patients during coughing or sneezing and affects primarily the lung parenchyma. The spectrum of clinical manifestations can be seen in COVID-19 patients ranging from asymptomatic infections to severe disease resulting in mortality. Although respiratory involvement is most common in COVID-19 patients, the virus can affect other organ systems as well. The systemic inflammation induced by the disease along with multisystem expression of Angiotensin Converting Enzyme 2 (ACE2), a receptor which allows viral entry into cells, explains the manifestation of extra-pulmonary symptoms affecting the gastrointestinal, cardiovascular, hematological, renal, musculoskeletal, and endocrine system. Here, we have reviewed the extensive literature available on COVID-19 about various clinical presentations based on the organ system involved as well as clinical presentation in specific population including children, pregnant women, and immunocompromised patients. We have also briefly discussed about the Multisystemic Inflammatory Syndrome occurring in children and adults with COVID-19. Understanding the various clinical presentations can help clinicians diagnose COVID-19 in an early stage and ensure appropriate measures to be undertaken in order to prevent further spread of the disease.
Introduction
COVID-19 is a growing pandemic with initial cases identified in Wuhan, Hubei province, China toward the end of December 2019. Labeled as Novel Coronavirus 2019 (2019-nCoV) initially by the Chinese Center for Disease Control and Prevention (CDC) which was subsequently renamed as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) due to its homology with SARS-CoV by the International Committee on Taxonomy of Viruses (ICTV) ( 1 , 2 ). The World Health Organization (WHO) later renamed the disease caused by SARS-CoV-2 as Coronavirus Disease-2019 (COVID-19) ( 3 ). COVID-19 is primarily a disease of the respiratory system affecting lung parenchyma with fever, cough, and shortness of breath as the predominant symptoms. Recent studies have shown that it can affect multiple organ systems and cause development of extra-pulmonary symptoms. Presence of extra-pulmonary symptoms can often lead to late diagnosis and sometimes even mis-diagnosis of COVID-19 which can be detrimental to patients. As researchers globally continue to understand COVID-19 and its implications on the human body, knowledge about the various clinical presentations of COVID-19 is paramount in early diagnosing and treatment in order to decrease the morbidity and mortality caused by the disease.
Epidemiology and Pathophysiology
While studying the early transmission dynamics of COVID-19 outbreak in Wuhan, many cases were found to be linked to the Huanan wholesale seafood market. Further investigation revealed <10% of the total cases could be linked to the market which led to the conclusion of human-to-human transmission of the virus occurring through respiratory droplets and contact transmission contributing to the rise in the number of affected individuals ( 4 ). The exponential rise in the number of cases in China and reporting of cases outside China in multiple countries led WHO to declare COVID-19 as a pandemic on 11th March 2020 ( 5 ).
SARS-CoV-2 tends to infect all age groups and is transmitted via direct contact or respiratory droplets generated during coughing or sneezing by the infected patient during both symptomatic or pre-symptomatic phase of infection. Other routes of transmission include fecal-oral route and fomites along with small risk of vertical transmission from mother to child if infection occurs during third trimester of pregnancy ( 6 , 7 ). There has also been evidence of asymptomatic transmission of COVID-19 ( 8 ). The concept of super spreaders in relation to COVID-19 is emerging where a single individual either symptomatic or asymptomatic can infect a disproportionately large number of individuals in an appropriate super spreading conditions such as mass gathering due to production of large number of infectious agent for prolonged duration of time ( 9 ). As per the literature, the incubation period of COVID-19 ranges from 2 to 14 days with a mean incubation period of 3 days ( 10 ). The basic Reproduction number (Ro) of SARS-CoV-2 is 2–2.5. Each individual infected with COVID-19 can infect 2–2.5 other individuals in a naïve population which also explains the exponential growth in the number of cases ( 10 ). The disease tends to be of mild to moderate severity in roughly 80% of patients, and severe disease is associated with infants, elderly patients above 65 years, and patients with other comorbidities such as diabetes mellitus, hypertension, coronary artery disease, and other chronic conditions ( 1 , 2 ). COVID-19 has also been found to be more severe in males than in females with a case fatality rate of 2.8% in males and 1.7% in females ( 11 ). The major organ system affected by the virus is the respiratory system, but it can affect other organ systems either directly or by the effect of host immune response. SARS-CoV-2, the causative agent of COVID-19, after entering the human host initially replicates in the epithelial mucosa of the upper respiratory tract (nose and pharynx) followed by migration to the lungs where further replication of virus occurs causing transient viraemia. The virus uses Angiotensin Converting Enzyme 2 (ACE2) receptor as a primary entry to cells. ACE2 is found abundantly in the mucosal lining of the respiratory tract, vascular endothelial cells, heart, intestine, and kidney. Thus, the virus has potential for replication in all these organs. After entry into cells, the virus undergoes further rapid replication within the target cells and induces extensive epithelial and endothelial dysfunction leading to exponential inflammatory response with the production of a large amount of proinflammatory cytokines and chemokines. Activation of proinflammatory cytokines and chemokines leads to neutrophil activation and migrations and results in the characteristic cytokine storm. The immunological downregulation of ACE2 by the virus contributes to acute lung injury in COVID-19. ACE2 also regulates the renin angiotensin system (RAS); thus, downregulation of ACE2 also causes dysfunction of RAS which contributes to enhanced inflammation ( 2 , 11 – 15 ). These entire factors contribute to symptoms of COVID-19 with sepsis, multi-organ dysfunction, acute respiratory distress syndrome (ARDS), and prothrombotic state leading to an exacerbation of organ dysfunction.
Clinical Manifestation
We review here the system based clinical features of COVID-19.
Respiratory
According to report from WHO-China-Joint Mission on COVID-19, 55,924 laboratories confirmed cases of COVID-19 had fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), difficulty breathing (18.6%), sore throat (13.9%), chills (11.4%), nasal congestion (4.8%), and hemoptysis (0.9%) ( 1 ).
Some patients may rapidly progress to acute lung injury and ARDS with septic shock. The median interval between the onset of initial symptoms to development of dyspnea, hospital admission, and ARDS was 5, 7, and 8 days respectively ( 10 ). Some patients with COVID-19 may have reduced oxygen saturation in blood (≤ 93%) with oxygen saturation down to 50 or 60% but remained stable without significant distress, and as such, were termed as salient hypoxia or happy hypoxia ( 16 , 17 ). Trial of oxygen therapy, prone positioning, high flow continuous positive airway pressure, non-re-breathable mask alongside trial of anticoagulation are often used to manage these patients ( 16 , 17 ). However, further study is required to define the role of these strategies in management.
The most frequent radiological abnormality among 975 patients with COVID-19 in computed tomography (CT) scan of chest was ground glass opacity (56.4%) and bilateral patchy shadowing (51.8%) ( 18 ). A scientific review of 2,814 patients have shown that the most common chest CT finding in COVID-19 patients was ground glass opacity followed by consolidation. However, the findings can vary in different patients and at various stages of diseases. Other CT findings include interlobular septal thickening, reticular pattern, crazy paving, etc. Atypical findings like air bronchogram, bronchial wall thickening, nodule, pleural effusion, and lymphadenopathy have also been noted in some studies ( 19 ). A study showed that among 877 patients with non-severe diseases and 173 patients with severe diseases, 17.9 and 2.9% of the patients did not have any detectable radiological abnormalities, respectively ( 18 ).
ENT (Ear, Nose, and Throat)
ENT manifestations are one of the most frequent symptoms encountered by physicians in COVID-19. A peculiar clinical presentation in some COVID-19 patients includes the deterioration of sense, taste (dysgeusia), and loss of smell (anosmia). A systematic review and meta-analysis of 10 studies with 1,627 participants surveyed for olfactory deterioration and 9 studies with 1,390 participants examined for gustatory symptoms demonstrated prevalence of 52.73 and 43.93% of these symptoms among COVID-19 patients, respectively. These clinical features may often present at earlier stages of the disease ( 20 ). Additionally, sore throat, rhinorrhea, nasal congestion, tonsil edema, and enlarged cervical lymph nodes are commonly seen among otolaryngological dysfunctions in patients ( 21 ). A large observational study of 1,099 COVID-19 patients reported tonsils swelling in 23 patients (2.1%), throat congestion in 19 patients (1.7%) and enlarged lymph nodes in 2 patients (0.2%) ( 18 ). This can be explained by the fact that there is a high expression of ACE2 receptors on the epithelial cells of the oral and nasal mucosa including the tongue. It has been known that the novel coronavirus has a strong binding affinity to ACE2 receptors through which it invades host cells ( 22 ). This theory may explain the exhibition of extra-respiratory symptoms including ENT manifestations as part of COVID-19 symptoms.
Cardiovascular
Cardiac manifestation in patient with COVID-19 can occur due to cardiac strain secondary to hypoxia and respiratory failure, direct effect of SARS-CoV-2 on heart or secondary to inflammation and cytokine storm, metabolic derangements, rupture of plaque and coronary occlusion by thrombus, and consequences of drugs used for treatment ( 23 – 25 ). The need for intensive care admission, non-invasive ventilation (46.3 vs. 3.9%), and invasive mechanical ventilation (22 vs. 4.2%) were higher among patients with cardiac ailments as compared to those without cardiac involvement as well as higher hospital mortality than those without myocardial involvement (51.2 vs. 4.5%) ( 26 ). These patients tend to have electrocardiographic (ECG) changes as well as elevations in high sensitivity cardiac troponin (hsCTn) and N- terminal pro-B-type natriuretic peptide (NT proBNP) which corresponded to raised inflammatory markers. Hypertension, acute and fulminant myocarditis, ventricular arrhythmias, atrial fibrillation, stress cardiomyopathy, hypotension and heart failure, acute coronary syndrome (ACS) with ST elevation or depression MI with normal coronaries have been reported ( 23 , 27 ). In a Chinese cohort of 138 patients, 16.7% had arrhythmias with risk higher among those needing ICU care with no mention of the type of arrhythmia that was present ( 28 ). Less frequently, cardiac symptoms like chest pain or tightness and palpitation can be the initial presenting features without fever producing a diagnostic dilemma. Some of these patients eventually go on to develop respiratory symptoms as diseases progress ( 29 ). Patients who have recovered from acute illness may develop arrhythmias as a result of myocardial scar and need future monitoring ( 27 ). One important point to note is use of Renin Angiotensin Aldosterone System (RAAS) modulators in patients with COVID-19. Guidelines from ACC/AHA/HFSA recommends continuing them in high risk patient based on goal directed therapy approach supported by a recent systematic review and meta-analysis conducted by Hasan et. Al. which demonstrated use of ACEI/ARB in COVID-19 patients is associated with lower odds/ hazards of mortality and development of severe/critical diseases as compared to no use of ACEI/ARB ( 30 , 31 ).
Gastrointestinal
In the initial cohort of patients from China, nausea or vomiting and diarrhea were present in 5 and 3.7% of patients ( 1 ). Review of data from 2,023 patients showed anorexia to be the most frequently occurring gastrointestinal symptom in adults. Diarrhea was the most common presenting gastrointestinal symptom in both adults and children while vomiting was found to be more common in children ( 32 ). Other rare symptoms included nausea, abdominal pain, and gastrointestinal bleeding. There have been few instances where COVID-19 patients presented with only gastrointestinal symptoms without the development of fever or respiratory symptoms at the onset and during disease progression ( 33 ). In a smaller cohort of 204 patients, 50.5% had some form of intestinal symptoms and of those, 5.8% had only intestinal symptoms while the remaining patients developed respiratory symptoms subsequently. The most common symptoms reported among them was anorexia (78.64%), non-dehydrating diarrhea (34%), vomiting (3.9%), and abdominal pain (1.94%) ( 34 ). In addition, those with GI symptoms tend to have a longer interval between symptom onset and hospital admission (9 vs. 7.3 days) possibly due to lack of clinical suspicion and delay in diagnosis. Patients with gastrointestinal symptoms tend to have higher elevation in AST and ALT indicating coexistent liver injury ( 34 ). The mechanism behind GI illness is not clearly known but could be due to direct invasion of virus via ACE2 receptor in the intestinal mucosa. This can be supported by the fact that viral RNA can be detected in stool samples of COVID-19 patients which may also hint toward possible fecal-oral transmission ( 35 ). Liver dysfunction is likely secondary to the use of hepatotoxic drugs, hypoxia induced liver injury, systemic inflammation, and multi organ failure ( 36 ).
Renal manifestation in patients with COVID-19 can occur due to direct invasion of podocytes and proximal tubular cells by SARS-CoV-2 virus, secondary endothelial dysfunction causing effacement of foot process with vacuolation and detachment of podocytes, and acute proximal tubular dysfunction ( 37 ). Furthermore, hypoxia, cytokine storm, rhabdomyolysis, nephrotoxic drugs, and overlying infections can all exacerbate renal injury ( 38 ). Based on initial reports, prevalence of Acute Kidney Injury (AKI) among COVID-19 hospitalized patients range from 0.5 to 29%. In a cohort of 701 patients, proteinuria (43.9%), hematuria (26.7%), elevated creatinine (14.4%), elevated blood urea nitrogen (13.1%), and low glomerular filtration rate (≤ 60 ml/min/1.73 m 2 ) (13.1%) were present at the time of hospital admission with 5.1% developing AKI during the illness. AKI was more prevalent among those with baseline renal impairment ( 39 ). In another large cohort of 5,449 patients, 36.6% had AKI with prevalence higher among mechanically ventilated patients compared to non-ventilated patients (89.7 vs. 21.7%) ( 40 ). Patients developing renal impairment are prone to have higher mortality within the hospital. Another point to highlight is the presentation of COVID-19 in renal transplant recipients. Due to immunosuppression, these patients are likely to have low fever at presentation with swift clinical decline and requirement for mechanical ventilation with high mortality as compared to the general population ( 41 ).
Neurological
Most patients with COVID-19 develop neurological symptoms along with respiratory symptoms during the course of illness; however, several case reports in review of literature document patient presentation of neurological dysfunction without typical symptoms of fever, cough, and difficulty breathing ( 42 ). There is a 2.5-fold enhanced risk of severe illness and increased death in patients with a history of previous stroke with similar findings among those with Parkinson's diseases. The prevalence of neurological features ranges from 6 to 36% along with hypoxic ischemic encephalopathy up to 20% in some series of patients ( 43 ). Neurological symptoms tend to occur early in the course of illness (median 1–2 days) with most common neurological features being headache, confusion, delirium, anosmia or hyposmia, dysgeusia or ageusia, altered mental status, ataxia, and seizures ( 44 ). Among patients admitted with COVID-19, the prevalence of ischemic stroke ranges from 2.5 to 5% despite receiving prophylaxis for venous thromboembolism. Patients prone to have established cardiovascular risk factors are likely to have a more severe diseases ( 43 ). Other presentations include viral encephalitis, acute necrotizing encephalopathy (ANE), infectious toxic encephalopathy, meningitis, Guillain Barre Syndrome (GBS), Miller Fisher syndrome, and polyneuritis cranialis with GBS being the first feature of COVID-19 in few cases ( 42 , 43 , 45 ). In COVID-19 patients, CNS features are possibly due to direct invasion of neurons and glial cells by SARS-CoV-2 as well as by endothelial dysfunction of blood brain barrier (BBB). Virus can gain access to CNS via hematogenous spread or retrograde movement across the olfactory bulb. The virus can be detected in CSF by RT-PCR and on brain parenchyma during autopsy. The fact that most patients develop anosmia or hyposmia during illness support this theory ( 45 ). After entry, the virus can cause reactive gliosis with activation of the inflammatory cascade. The combination of systemic inflammation, cytokine storm, and coagulation dysfunction can impair BBB function and alter brain equilibrium causing neuronal death ( 42 ).
Ocular manifestations can vary from conjunctival injection to frank conjunctivitis. In a Chinese cohort of 38 patients, 31.6% had ocular symptoms consisting primarily of conjunctivitis while conjunctival hyperemia, foreign body sensation in eye, chemosis, tearing or epiphora were more common among severe COVID-19 patients. Among them SARS-CoV-2 can be demonstrated in conjunctival as well as nasopharyngeal swab in 5.2% of patients, indicating a potential route for viral transmission ( 46 ). Conjunctivitis or tearing can be the initial presenting symptoms of COVID-19. Despite this fact, there is no documented case of severe ocular features relating to COVID-19.
Similar to other viral infections, SARS-CoV-2 can also produce varied dermatological features. A study of 88 patients from Italy showed that about 20.4% had some form of skin manifestations with 44.4% developing features at onset and duration of the disease progression ( 47 ). Maculopapular exanthem (36.1%) was identified as most common dermatological features followed by papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis (2.8%), and petechiae (1.4%) ( 48 ). A study of 375 COVID-19 cases in Spain identified five different patterns of cutaneous manifestations in patients: acral areas of erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedo or necrosis (6%) ( 49 ). Majority of patients had lesions on the trunk with some experiencing lesions on hands and feet. There are case reports of COVID-19 associated with erythema multiforme and Kawasaki Disease in children ( 50 , 51 ). Pathogenesis behind skin involvement remains unclear with some features explained by small vessel vasculitis, thrombotic events like DIC, hyaline thrombus formation, acral ischemia, or the direct effect of the virus like other viral illnesses ( 52 ).
Musculoskeletal
The initial report from China revealed 14.8% of patients had myalgia or arthralgia among 55,924 COVID-19 patients. A review article reports that of 12,046 patients, fatigue was identified in 25.6% and myalgia and/or arthralgia in 15.5% with most patients reporting symptoms from the start of illness ( 53 ). There are reports suggesting myositis and rhabdomyolysis with markedly elevated creatinine kinase can occur during COVID-19 illness especially in patients with severe diseases and multi organ failure. Additionally, in some patients, rhabdomyolysis has been documented as the initial presentation of COVID-19 illness without typical respiratory symptoms ( 54 , 55 ). A case series of four patients developing acute arthritis during hospital admission for COVID-19 has been reported with exacerbation of crystal arthropathy (gout and calcium pyrophosphate diseases) but negative for SARS-CoV-2 RT-PCR in synovial fluid ( 56 ). Treatment with steroids and colchicine was used in all four cases. An important consideration to note was that all four patients developed arthritis despite previous treatment with immunomodulatory therapy (hydroxychloroquine, tocilizumab, and pulse methylprednisolone).
Hematological
As stated, COVID-19 is a systemic disease inducing systemic inflammation and occasionally cytokine storm. This can significantly impact the process of hematopoiesis and hemostasis. During early disease, normal or decreased leukocyte and lymphocyte counts were documented with marked lymphopenia as the diseases progressed, especially in those with cytokine storms and severe disease. In a study of 1,099 patients, lymphopenia, thrombocytopenia, and leukopenia were present in 83.2, 36.2, and 33.7%, respectively, with findings more marked in those with severe diseases ( 18 ). Leukocytosis in COVID-19 patients might suggest a bacterial infection or a superinfection with leukocytosis found more commonly in severe cases (11.4%) as compared to mild and moderate cases (4.8%) ( 18 ). Similarly, thrombocytopenia has been found to be more common (57.7%) in severe cases in contrast to mild and moderate cases (31.6%) ( 18 ). Lymphopenia was also linked with an increased necessity for ICU admission and the risk of ARDS. Thrombocytosis with elevated platelet to lymphocyte ratio may indicate a more marked cytokine storm ( 57 ).
Also, coagulation abnormality can manifest in the form of thrombocytopenia, prolonged prothrombin time (PT), low serum fibrinogen level, and raised D-dimer suggesting Disseminated Intravascular Coagulation (DIC) with these changes more marked in those with severe diseases ( 58 ). Raised lactate dehydrogenase (LDH) and serum ferritin were also present and correlated with the degree of systemic inflammation. In a study of 426 COVID-19 patients, C-Reactive Protein (CRP) was noted to be increased in 75–93% of patients, more commonly in patients with severe disease. Serum procalcitonin levels might not be altered at admission, but progressive increase in its value can suggest a worsening prognosis. Severe disease is linked to increased ALT, bilirubin, serum urea, creatinine, and lowered serum albumin ( 59 ). A study of 1,426 patients showed that Interleukin-6 (IL-6) were raised more in patients with severe COVID-19 than non-severe COVID-19 with progressive rise indicating an increased risk of mortality. Thus, its levels could be regarded as an important prognostic indicator for the extensive inflammation and cytokine storm in COVID-19 patients ( 60 ). Other plasma cytokines and chemokines like IL1B, IFNγ, IP10, MCP, etc. have also been found to be elevated in patients with COVID-19 both in severe and non-severe diseases. Additionally, GCSF, IP10, IL2, IL7, IL10, MCP1, MIP1A, and TNFα were increased in patients who require ICU admission which indicates that cytokine storm is associated with a severe disease ( 61 ).
Endocrine and Reproductive
From the available literature there is no doubt that diabetes mellitus is an important risk factor for COVID-19 illness and is associated with increased risk of development of severe disease. Additionally, there are case reports of subacute thyroiditis linked to SARS-CoV-2 infection ( 62 , 63 ). Based on the statement released from European Society of Endocrinology, patients with primary adrenal failure and congenital adrenal hyperplasia may have theoretically increased susceptibility to infection with higher risk of complications and ultimately mortality but there is no current evidence to support this ( 64 ). The dose of steroids may need to be doubled if there is a clinical suspicion of infection in these patients.
Several claims have been made regarding the impact of COVID-19 on male reproductive function, hypothesizing that COVID-19 can cause potential testicular damage either by binding directly to testicular ACE2 receptors, which are highly expressed in the testicles or by damaging the testis indirectly by exciting local immune system ( 65 ). A study comparing 81 male COVID-19 patients with 100 age matched healthy adults highlighted the presence of low testosterone levels, high levels of luteinizing hormone (LH), low testosterone/LH ratios, low Follicle stimulating hormone (FSH) to LH ratio, and raised serum prolactin. This may suggest a potential COVID-19 testicular damage affecting the Leydig cells in the testis ( 66 ). COVID-19 infected male patients may have reduced sperm count and decreased motility leading to diminished male fertility for 3 months post-infection ( 67 ).
Clinical Presentation in Specific Population
In children.
A case series of 72,314 cases published by the Chinese Center for Disease Control and Prevention reported that 0.9% of the total patients were between 0 and 9 years of age, and 1.2% of the total patients were between 10 and 19 years of age ( 68 ). The most common symptoms found in children are fever, (59%), cough (46%), few cases (12%) of gastrointestinal symptoms, and some cases (26%) showed no specific symptoms initially with patchy consolidation and ground glass opacities in CT chest findings ( 69 ). Chilblain-like acral eruptions, purpuric, and erythema multiforme-like lesions have been found to be more common in children and young adult patients mainly with asymptomatic or mild disease ( 70 ). Lymphopenia in children is relatively less common which is in direct contrast in cases of SARS in children where lymphopenia was more commonly noted ( 69 ).
Multisystem inflammatory syndrome (MIS) is another feared complication of Covid-19 seen in children. Abrams et al. systematically summarized the clinical evidence of 8 studies reporting MIS in 440 children. The median age of patients ranged from 7.3 to 10 years with 59% of all patients being male. The greatest proportion of patients had gastrointestinal symptoms (87%) followed by mucocutaneous symptoms (73%) and cardiovascular symptoms (71%) while fewer patients reported respiratory (47%), neurologic (22%), and musculoskeletal (21%) symptoms. Ferritin and d-dimer were elevated in 50% of patients, and C-reactive protein, interleukin-6, and fibrinogen were elevated in at least 75% of patients. Additionally, 100% of children with cardiovascular involvement reported elevated cardiac-damage markers such as Troponin. Although respiratory manifestation is most frequently expressed in adults, children with MIS exhibited less pulmonary symptoms and more of the other manifestations ( 71 ).
In Pregnant Women
The most common symptoms reported in pregnant women are fever (61.96%), cough (38.04%), malaise (30.49%), myalgia (21.43%), sore throat (12%), and dyspnea (12.05%). Other symptoms found in pregnant women are diarrhea and nasal congestion ( 72 ). In a systematic review including 92 patients, 67.4% manifested diseases at presentation with 31.7% having negative RT-PCR though they had features of viral pneumonia. Only one patient required admission to intensive care and 0% mortality. Fetal outcomes were reported as: 63.8% preterm delivery, 61.1% fetal distress, 80% Cesarean section delivery, 76.92% neonatal intensive care admission, 42.8% low birth weight, and 66.67% had lymphopenia ( 72 ). There was no evidence of vertical transmission. A study of 41 pregnant women with COVID-19 showed that consolidation was more commonly found in CT of pregnant women in contrast to ground-glass opacities in CT of non-pregnant adults ( 73 ). WHO also recommends encouraging lactating mothers with confirmed or suspected COVID-19 to begin or continue breastfeeding including 24-h rooming in, skin to skin contact, and kangaroo mother care especially in immediate postnatal period ( 74 ). On July 14th, 2020, Vivanti et al. published the first case of transplacental transmission of COVID-19 from a 23-years-old pregnant woman to her baby ( 75 ). Thereafter, more studies reported the possibility of the vertical transmission of COVID-19. In this context, Kotlayer et al. published a systematic review of 38 studies. Out of 936 neonates from COVID-19 mothers, 27 tested positive for the virus indicating a pooled proportion of 3.2% (2.2–4.3) for vertical transmission ( 7 ).
In Immuno-Compromised Population
Due to their impaired immune response, it is not surprising that immunocompromised patients with COVID-19 infection might be at greater risk of developing severe forms of the disease and co-infections in comparison to normal populations. Nevertheless, recent studies showed the association between cytokine storm syndrome and the overreaction of the immune system with COVID-19 raising the possibility that immunodeficient states might alleviate the overexpression of the host immune system and thereby prevent deadly forms of the disease ( 76 ). After the RECOVERY trial ( 77 ) that showed the efficacy of dexamethasone in lowering the mortality in severe forms of the disease, many questions were raised regarding whether immunocompromised patients have a greater or lower risk of developing severe forms of the disease. In order to address these questions, Minotti et al. recently published a systematic review that included 16 studies with 110 patients presenting mostly with cancer along with transplantation and immunodeficiency. Out of the 110 patients, 72 (65.5%) recovered without being admitted to the intensive care unit while 23 (20.9%) died ( 76 ). The authors concluded that immunosuppression in both children and adults seem to have a better disease course in comparison to normal population. One of the limitations of this study is that the conclusion was made only based on qualitative synthesis and no meta-analysis was performed. On the other hand, Gao et al. performed a meta-analysis on 8 relevant studies with 4,007 patients. The study showed that immunosuppression and immunodeficiency were associated with non-statistically significant increased risk of severe COVID-19 disease ( 78 ). Additionally, Mirzaei et al. summarized the clinical evidence of 252 HIV positive patients co-infected with COVID-19. The clinical manifestation did not differ from that of the general population. However, out of the 252 patients, 204 (80.9%) were male. Low CD4 count (<200 cells/mm 3 ) were reported for 23 of 176 patients (13.1%). COVID-19 symptoms were present in 223 patients with the most common symptoms of fever in 165 (74.0%) patients, cough in 130 (58.3%), headache in 44 (19.7%), arthralgia and myalgia in 33 (14.8%), gastrointestinal symptoms in 29 (13.0%) followed by sore throat in 18 (8.1%) patients ( 79 ). The number of deaths accounted for 36 (14.3%). Similar to the general population, immunocompromised, and HIV patients were no different in terms of clinical manifestation or severity. However, the results from these studies should be interpreted with caution and more studies are recommended to establish the link between this particular group of patients with severity of the disease.
Multisystem Involvement in COVID-19
As evident from the discussion above, SARS-CoV-2 can affect multiple organ systems and produce a wide array of clinical presentation of COVID-19. Certain studies conducted in Europe and United States have shown that COVID-19 can also have a multi-systemic presentation in individuals in form of a multi-system inflammatory syndrome (MIS) which has been found in both children and adults and is known as MIS-C and MIS-A, respectively ( 80 – 83 ).
According to a recent CDC report about MIS-A, it was found that only half of the patients with MIS-A had preceding respiratory symptoms of COVID-19 ~2–5 weeks before ( 80 ). The most common clinical signs and symptoms included fever, chest pain, palpitations, diarrhea, abdominal pain, vomiting, skin rash, etc. Nearly all patients had electro-cardiological abnormalities like arrythmias, elevated troponin levels, and electrocardiography evidence of left or right ventricular dysfunction. Even though most patients had minimal respiratory symptoms, chest imaging had features of ground glass opacity and pleural effusion. All patients had signs of elevated laboratory markers of inflammation, coagulation markers, and lymphopenia ( 80 ).
MIS-C can clinically mimic Kawasaki Disease ( 81 ). By the end of July, about 570 cases of MIS-C with COVID-19 were found in the United States ( 81 ). In MIS-C, there is involvement of at least four organ systems, most commonly the gastrointestinal system followed by cardiovascular and dermatological systems ( 81 ). Prominent signs and symptoms found in children with MIS-C were abdominal pain, vomiting, skin rash, diarrhea, hypotension, and conjunctival injection. The majority of the children needed ICU admission due to the development of severe complications including cardiac dysfunction, shock, myocarditis, coronary artery aneurysm, and acute kidney injury ( 81 ).
Association Between Clinical Presentations, COVID-19 Severity and Prognosis
Evaluation of 55,924 laboratory confirmed COVID-19 cases in China, the presence of dyspnea, respiratory rate ≥ 30/min, blood saturation levels ≤ 93%, PaO2/FiO2 ratio ≤ 300, lung infiltrates ≥ 50% of the lung fields between 12 and 48 h were associated with severe COVID-19 infection ( 1 ). Clinical signs suggestive of respiratory failure, septic shock, or multiple organ dysfunction/failure were associated with critical disease and poor prognosis ( 1 ). Individuals at highest risk of severe disease and deaths were patients with age > 80 years and associated co-morbidities such as underlying cardiovascular disease, diabetes, hypertension, chronic respiratory disease, and cancer ( 1 ). Another study done with 418 patients in Catalonia (Spain) showed that dyspnea was an important predictor of severe disease while confusion was an important predictor of death, and the presence of cough was strongly associated with good prognosis ( 84 ). Advanced age, male sex, and obesity were independent markers of poor prognosis while eosinophilia was a marker of less severe disease ( 84 ). The mortality was lower in patients with symptoms of diarrhea, arthromyalgia, headache, and loss of smell and taste sensations while low oxygen saturation, high CRP levels, and higher number of lung quadrants affected on Xray were found to be associated with severe disease and death ( 84 ).
COVID-19 is a viral illness which can cause multi-systemic manifestations. Review of existing literature concludes that SARS-CoV-2 can affect any organ system either directly or indirectly leading to a myriad of clinical presentation. The most commonly affected system is the respiratory system with presenting symptoms of fever, cough, and shortness of breath, etc. Other systems which can be affected in COVID-19 include ENT (sore throat, loss of taste, smell, and sensations, and rhinorrhea), cardiovascular system (chest pain, chest tightness, palpitations, and arrhythmias), gastrointestinal system (anorexia, diarrhea, vomiting, nausea, and abdominal pain), renal (proteinuria, hematuria, and acute kidney injury), neurological (headache, confusion, delirium, and altered mental status), ocular (conjunctival hyperemia, foreign body sensation in the eye, chemosis, and tearing), cutaneous (rash, papules, and urticaria), musculoskeletal system (myalgia and arthralgia), hematological (lymphopenia, thrombocytopenia, leukopenia, elevated inflammatory markers, and elevated coagulation markers), endocrine (low testosterone, low FSH, and high LH) and reproductive system (decreased sperm count and decreased sperm motility). Clinical presentation in specific populations like children, pregnant women, and immunocompromised people may vary which emphasizes the importance of further investigation in order to avoid late diagnosis of COVID-19. Severe multi-systemic involvement in COVID-19 in the form of MIS-C and MIS-A can cause significant morbidity and mortality if undiagnosed. The clinical presentations of respiratory failure, acute kidney injury, septic shock, cardiovascular arrest is associated with severe COVID-19 disease and can result in poor prognosis. In the light of exponentially growing pandemic, every patient presenting to hospital must be tested for SARS-CoV-2 by RT-PCR if resources are available to detect early presentations of diseases even if the features are atypical. Understanding of the various clinical presentations of COVID-19 will help the clinicians in early detection, treatment, and isolation of patients in order to contain the virus and slow down the pandemic.
Author Contributions
All authors have contributed equally to the work, and all agreed to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ms. Sairah Zia (American University of Caribbean, School of Medicine, Sint Maarten), a native speaker of English, for proofreading the manuscript.
Abbreviations
ACC/AHA/HFSA, American College of Cardiology/American Heart Association/Heart Failure Society of America; IL1B, Interleukin 1B; IFNγ, Interferon Gamma; IP10, Interferon-inducible Protein 10; MCP1, Monocyte Chemoattractant Protein 1; GCSF, Granulocyte Colony Stimulating Factor; IL2, Interleukin 2; IL7, Interleukin 7; IL10, Interleukin 10; MIP1A, Macrophage Inflammatory Protein-1 alpha; TNFα, Tumor Necrosis Factor alpha.
1. Who-China-Joint-Mission-on-Covid-19-Final-Report.pdf . Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed June 1, 2020).
2. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. (2020) 12:372. doi: 10.3390/v12040372
CrossRef Full Text | Google Scholar
3. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes it . (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed June 7, 2020).
Google Scholar
4. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020.03.019
PubMed Abstract | CrossRef Full Text | Google Scholar
5. WHO Director-General's opening remarks at the media briefing on COVID-19 . (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (accessed June 7, 2020).
6. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
7. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.07.049. [Epub ahead of print].
8. Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018
9. Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioeth Rev. (2020) 12:235–42. doi: 10.1007/s41649-020-00118-2
10. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. (2020) 12:e7560. doi: 10.7759/cureus.7560
11. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
PubMed Abstract | CrossRef Full Text
12. Veerdonk F, van de Netea MG, Deuren M, van Meer JWM, van der Mast Q, de Bruggemann RJ, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. eLife. (2020) 9:e57555. doi: 10.7554/eLife.57555
13. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
14. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220, 1–13. doi: 10.1016/j.trsl.2020.04.007
15. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
16. Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Den NorLegeforening. (2020) 140. doi: 10.4045/tidsskr.20.0299
17. Couzin-Frankel J. The mystery of the pandemic's “happy hypoxia.” Science . (2020) 368:455–6. doi: 10.1126/science.368.6490.455
18. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
19. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. (2020) 30:4381–9. doi: 10.1007/s00330-020-06801-0
20. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Neck Surg. (2020) 163:3–11. doi: 10.1177/0194599820926473
CrossRef Full Text
21. Krajewska J, Krajewski W, Zub K, Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol. (2020) 277:1885–97. doi: 10.1007/s00405-020-05968-y
22. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
23. Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
24. Clerkin Kevin J, Fried Justin A, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
25. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013
26. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
27. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. (2020) 31:1003–8. doi: 10.1111/jce.14479
28. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
29. Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
30. Patients Taking ACE-i and ARBs who Contract COVID-19 Should Continue Treatment Unless Otherwise Advised by Their Physician . American Heart Association (2020). Available online at: https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician (accessed June 27, 2020).
31. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020). doi: 10.22541/au.158880148.84250526. [Epub ahead of print].
32. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. doi: 10.1111/apt.15731
33. An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, et al. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset . Rochester, NY: Social Science Research Network (2020). Available online at: https://papers.ssrn.com/abstract=3532530 (accessed June 27, 2020). doi: 10.2139/ssrn.3532530
34. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of covid-19 patients with digestive symptoms in hubei, china: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. (2020) 115, 766–73. doi: 10.14309/ajg.0000000000000620
35. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. (2020) 158:1831–3.e3. doi: 10.1053/j.gastro.2020.02.055
36. Feng G, Zheng KI, Yan Q-Q, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. (2020) 8:18–24. doi: 10.14218/JCTH.2020.00018
37. Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
38. Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. (2020) 16:308–10. doi: 10.1038/s41581-020-0284-7
39. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
40. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
41. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med. (2020) 382:2475–7. doi: 10.1056/NEJMc2011117
42. Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. (2020) 12:e8192. doi: 10.7759/cureus.8192
43. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. (2020) 38:1549.e3–7. doi: 10.1016/j.ajem.2020.05.024
44. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
45. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. (2020). doi: 10.21203/rs.3.rs-31183/v1. [Epub ahead of print].
46. Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The ocular manifestations and transmission of COVID-19; recommendations for prevention. J Emerg Med. (2020) 59:137–40. doi: 10.1016/j.jemermed.2020.04.060
47. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. (2020) 34:e212–3. doi: 10.1111/jdv.16387
48. Sachdeva M, Gianotti R, Shah M, Lucia B, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. (2020) 98:75–81. doi: 10.1016/j.jdermsci.2020.04.011
49. Casas CG, Català A, Hernández GC, Rodríguez-Jiménez P, Fernández-Nieto D, Lario AR-V, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. (2020) 183:71–7. doi: 10.1111/bjd.19163
50. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) 10:537–40. doi: 10.1542/hpeds.2020-0123
51. Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Saïd PB, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. (2020) 34:e539–41. doi: 10.1111/jdv.16666
52. Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID-19): a brief review. Dermatol Ther. (2020) 33:e13528. doi: 10.1111/dth.13528
53. Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg. (2020) 15:178. doi: 10.1186/s13018-020-01702-w
54. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. (2020) 12:e7561. doi: 10.7759/cureus.7561
55. Chan KH, Farouji I, Hanoud AA, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am J Emerg Med. (2020) 38:1548.e1–3. doi: 10.1016/j.ajem.2020.05.015
56. López-González M-C, Peral-Garrido ML, Calabuig I, Tovar-Sugrañes E, Jovani V, Bernabeu P, et al. Case series of acute arthritis during COVID-19 admission. Ann Rheum Dis. (2020) doi: 10.1136/annrheumdis-2020-217914. [Epub ahead of print].
57. Qu R, Ling Y, Zhang Y, Wei L, Chen X, Li X, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. (2020) 92:1533–41. doi: 10.1002/jmv.25767
58. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. (2020) 7:e438–40. doi: 10.1016/S2352-3026(20)30145-9
59. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med CCLM. (2020) 58:1131–4. doi: 10.1515/cclm-2020-0198
60. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. (2020) 92:2283–5. doi: 10.1002/jmv.25948
61. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
62. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. (2020) 105:dgaa276. doi: 10.1210/clinem/dgaa276
63. AsfurogluKalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. (2020) 43:1173–4. doi: 10.1007/s40618-020-01316-3
64. Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases a statement from the European society of endocrinology. Endocrine. (2020) 68:2–5. doi: 10.1007/s12020-020-02294-5
65. Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. (2020) 52:e13654. doi: 10.1111/and.13654
66. Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.21.20037267
67. Segars J, Katler Q, McQueen DB, Kotlyar A, Glenn T, Knight Z, et al. Prior and novel coronaviruses, Coronavirus disease 2019 (COVID-19), and human reproduction: what is known? FertilSteril. (2020) 113:1140–9. doi: 10.1016/j.fertnstert.2020.04.025
68. CDC Weekly C, The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly . (2020) 2:113–22. doi: 10.46234/ccdcw2020.032
69. Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. (2020) 119:982–9. doi: 10.1016/j.jfma.2020.04.007
70. Wollina U, Karadag AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. (2020) 33:e13549. doi: 10.1111/dth.13549
71. Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. (2020) 226:45–54.e1. doi: 10.1016/j.jpeds.2020.08.003
72. Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS ONE. (2020) 15:e0234187. doi: 10.1371/journal.pone.0234187
73. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. (2020) 80:e7–13. doi: 10.1016/j.jinf.2020.03.007
74. Breastfeeding and COVID-19 . (2020). Available online at: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed Jun 25, 2020).
75. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11:3572. doi: 10.1038/s41467-020-17436-6
76. Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? a systematic review. J Infect. (2020) 81:e61–6. doi: 10.1016/j.jinf.2020.04.026
77. Europe PMC . (2020). Available online at: https://europepmc.org/articles/pmc7383595/bin/nejmoa2021436_appendix.pdf (accessed November 20, 2020).
78. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. (2020) 81:e93–5. doi: 10.1016/j.jinf.2020.05.017
79. Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. (2020) 1–8. doi: 10.1007/s10461-020-02983-2. [Epub ahead of print].
80. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. Morb Mortal Wkly Rep. (2020) 69:1450–6. doi: 10.15585/mmwr.mm6940e1
81. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children — United States, March–July 2020. Morb Mortal Wkly Rep. (2020) 69:1074–80. doi: 10.15585/mmwr.mm6932e2
82. Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May (2020. Eurosurveillance. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
83. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259. doi: 10.1001/jama.2020.10369
84. Rodríguez-Molinero A, Gálvez-Barrón C, Miñarro A, Macho O, López GF, Robles MT, et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLoS ONE. (2020) 15:e0239571. doi: 10.1371/journal.pone.0239571
Keywords: SARS-CoV-2, Covid-19, symptomatology, clinical presentation, signs and symptoms, clinical features, coronavirus
Citation: Mehta OP, Bhandari P, Raut A, Kacimi SEO and Huy NT (2021) Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 8:582932. doi: 10.3389/fpubh.2020.582932
Received: 13 July 2020; Accepted: 15 December 2020; Published: 15 January 2021.
Reviewed by:
Copyright © 2021 Mehta, Bhandari, Raut, Kacimi and Huy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nguyen Tien Huy, tienhuy@nagasaki-u.ac.jp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Coronavirus Disease 2019 (COVID-19) Clinical Presentation
- Author: David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS; Chief Editor: Michael Stuart Bronze, MD more...
- Sections Coronavirus Disease 2019 (COVID-19)
- Practice Essentials
- Route of Transmission
- Epidemiology
- Physical Examination
- Complications
- Approach Considerations
- Laboratory Studies
- CT Scanning
- Chest Radiography
- Medical Care
- Antiviral Agents
- Immunomodulators and Other Investigational Therapies
- Investigational Antibody-Directed Therapies
- Antithrombotics
- Renin Angiotensin System Blockade and COVID-19
- Diabetes and COVID-19
- Therapies Determined Ineffective
- QT Prolongation with Potential COVID-19 Pharmacotherapies
- Investigational Devices
- Guidelines Summary
- CDC Evaluating and Testing Persons Under Investigation (PUI) for COVID-19 Clinical Guidelines
- CDC Sample Collection and Testing Guidelines for COVID-19
- Guidance for Hospitals on Containing Spread of COVID-19
- American Academy of Pediatrics Guidance on Management of Infants Born to Mothers with COVID-19
- NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
- Infectious Diseases Society of America (IDSA) Management Guidelines
- Thromboembolism Prevention and Treatment
- Medication Summary
- Corticosteroids
- Immunomodulators
- Complement Inhibitors
- COVID-19, Monoclonal Antibodies
- Questions & Answers
- Media Gallery
Presentations of COVID-19 range from asymptomatic/mild symptoms to severe illness and mortality. Common symptoms include fever, cough, and shortness of breath. [ 100 ] Other symptoms, such as malaise and respiratory distress, also have been described. [ 90 ]
Symptoms may develop 2 days to 2 weeks after exposure to the virus. [ 100 ] A pooled analysis of 181 confirmed cases of COVID-19 outside Wuhan, China, found the mean incubation period was 5.1 days, and that 97.5% of individuals who developed symptoms did so within 11.5 days of infection. [ 101 ]
Symptom rebound and viral rebound have been described in patients (with or without antiviral treatment). In untreated patients, those (n = 563) receiving placebo in the ACTIV-2/A5401 (Adaptive Platform Treatment Trial for Outpatients with COIVD-19) platform trial recorded 13 symptoms daily between days 1 and 28. Symptom rebound was identified in 26% of participants at a median of 11 days after initial symptom onset. Viral rebound was detected in 31% and high-level viral rebound in 13% of participants. [ 102 ]
The following symptoms may indicate COVID-19 [ 100 ] :
- Fever or chills
- Shortness of breath or difficulty breathing
- Muscle or body aches
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
Other reported symptoms include the following:
- Sputum production
- Respiratory distress
- Neurologic (eg, headache, altered mentality)
Wu and McGoogan reported that, among 72,314 COVID-19 cases reported to the CCDC, 81% were mild (absent or mild pneumonia), 14% were severe (hypoxia, dyspnea, >50% lung involvement within 24-48 hours), 5% were critical (shock, respiratory failure, multiorgan dysfunction), and 2.3% were fatal. [ 103 ] These general symptom distributions have been reconfirmed across multiple observations. [ 104 , 105 ]
Clinicians evaluating patients with fever and acute respiratory illness should obtain information regarding travel history or exposure to an individual who recently returned from a country or US state experiencing active local transmission. [ 106 ]
Williamson and colleagues, in an analysis of 17 million patients, reaffirmed that severe COVID-19 and mortality was more common in males, older individuals, individuals in poverty, Black persons, and patients with medical conditions such as diabetes and severe asthma, among others. [ 107 ]
A multicenter observational cohort study conducted in Europe found frailty was a greater predictor of mortality than age or comorbidities. [ 108 ]
Type A blood has been suggested as a potential factor that predisposes to severe COVID-19, specifically in terms of increasing the risk for respiratory failure. Blood type O appears to confer a protective effect. [ 109 , 110 ]
Patients with suspected COVID-19 should be reported immediately to infection-control personnel at their healthcare facility and the local or state health department. CDC guidance calls for the patient to be cared for with airborne and contact precautions (including eye shield) in place. [ 22 ] Patient candidates for such reporting include those with fever and symptoms of lower respiratory illness who have travelled from Wuhan City, China, within the preceding 14 days or who have been in contact with an individual under investigation for COVID-19 or a patient with laboratory-confirmed COVID-19 in the preceding 14 days. [ 106 ]
A complete or partial loss of the sense of smell (anosmia) has been reported as a potential history finding in patients eventually diagnosed with COVID-19. [ 20 ] A phone survey of outpatients with mildly symptomatic COVID-19 found that 64.4% (130 of 202) reported any altered sense of smell or taste. [ 111 ] In a European study of 72 patients with PCR results positive for COVID-19, 53 patients (74%) reported reduced olfaction, whereas 50 patients (69%) reported a reduced sense of taste. Forty-nine patients (68%) reported both symptoms. [ 112 ]
Patients who are under investigation for COVID-19 should be evaluated in a private room with the door closed (an airborne infection isolation room is ideal) and asked to wear a surgical mask. All other standard contact and airborne precautions should be observed, and treating healthcare personnel should wear eye protection. [ 22 ]
The most common serious manifestation of COVID-19 upon initial presentation is pneumonia. Fever, cough, dyspnea, and abnormalities on chest imaging are common in these cases. [ 113 , 114 , 115 , 116 ]
Huang and colleagues found that, among patients with pneumonia, 99% had fever, 70% reported fatigue, 59% had dry cough, 40% had anorexia, 35% experienced myalgias, 31% had dyspnea, and 27% had sputum production. [ 113 ]
Complications of COVID-19 include pneumonia , acute respiratory distress syndrome , cardiac injury, arrhythmia, septic shock , liver dysfunction, acute kidney injury , and multi-organ failure, among others.
Approximately 5% of patients with COVID-19, and 20% of those hospitalized, experience severe symptoms necessitating intensive care. The common complications among hospitalized patients include pneumonia (75%), ARDS (15%), AKI (9%), and acute liver injury (19%). Cardiac injury has been increasingly noted, including troponin elevation, acute heart failure, dysrhythmias, and myocarditis. Ten percent to 25 percent of hospitalized patients with COVID-19 experience prothrombotic coagulopathy resulting in venous and arterial thromboembolic events. Neurologic manifestations include impaired consciousness and stroke.
ICU case fatality is reported up to 40%. [ 104 ]
As the COVID-19 pandemic has matured, more patients have reported long-term, post-infection sequelae. Most patients recover fully, but those who do not have reported adverse symptoms such as fatigue, dyspnea, cough, anxiety, depression, inability to focus (ie, “brain fog”), gastrointestinal problems, sleep difficulties, joint pain, and chest pain lasting weeks to months after the acute illness. Long-term studies are underway to understand the nature of these complaints. [ 117 ]
Post-acute sequelae of SARS-CoV-2 (PASC) infection is the medical term for what is commonly called long COVID or "long haulers". The NIH includes discussion of persistent symptoms or organ dysfunction after acute COVID-19 within guidelines that discuss the clinical spectrum of the disease. [ 118 ]
The UK National Institute for Health and Care Excellence (NICE) issued guidelines on care of long COVID that define the syndrome as: signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis. [ 119 ]
Please see Long COVID-19 .
Future public health implications
Public health implications for long COVID need to be examined, as reviewed by Datta et al. As with other infections (eg, Lyme disease, syphilis, Ebola), late inflammatory and virologic sequelae may emerge. Accumulation of evidence beyond the acute infection and postacute hyperinflammatory illness is important to evaluate to gain a better understanding of the full spectrum of the disease. [ 120 ]
Thrombotic manifestations of severe COVID-19 are caused by the ability of SARS-CoV-2 to invade endothelial cells via angiotensin-converting enzyme-2 (ACE-2), which is expressed on the surface of endothelial cells. Subsequent endothelial inflammation, complement activation, thrombin generation, platelet and leukocyte recruitment, and the initiation of innate and adaptive immune responses culminate in immunothrombosis, and can ultimately cause microthrombotic complications (eg, DVT, PE, stroke). [ 121 ]
Kotecha et al describe patterns of myocardial injury in hospitalized patients with severe COVID-19 who had elevated troponin levels. During convalescence, myocarditis-like injury was observed, with limited extent and minimal functional consequence. However, in a proportion of patients, there was evidence of possible ongoing localized inflammation. Roughly 25% of patients had ischemic heart disease, of which two thirds had no previous history. [ 122 ]

Reinfection
COVID-19 reinfection is defined as an infected person who has undergone full vaccination, whether they have had a booster or boosters. According to the CDC, reinfection is COVID-19 infection of an individual with 2 different viral strains that occurs at least 45 days apart. It also may occur when an individual has 2 positive CoV-2 RT-PCR tests with negative tests between the 2 positive tests. [ 123 ]
It is essential to determine reinfection rates to establish the effectiveness of current vaccine prophylaxis. Reinfection in vaccinated and non-vaccinated persons probably is due to a variant. [ 123 , 124 ]
It is important to differentiate reinfection from reactivation or relapse of the virus, which occurs in a clinically recovered person within the first 4 weeks of infection, during which viral RNA testing has remained positive. During relapse, a tiny viral load of dormant virus reactivates, the reason of which often is unclear.
The only way to prove this state is to show that genetic samples taken at the beginning and at the time of reactivation differ genetically; such testing is unusual at the beginning of a person’s illness.
For more information, see COVID-19 Reinfections
CDC. 2019 Novel Coronavirus, Wuhan, China. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/about/index.html . Aug. 11, 2022; Accessed: February 20, 2024.
Gallegos A. WHO Declares Public Health Emergency for Novel Coronavirus. Medscape Medical News. Available at https://www.medscape.com/viewarticle/924596 . January 30, 2020; Accessed: February 29, 2024.
Ramzy A, McNeil DG. W.H.O. Declares Global Emergency as Wuhan Coronavirus Spreads. The New York Times. Available at https://nyti.ms/2RER70M . January 30, 2020; Accessed: February 29, 2024.
The New York Times. Coronavirus Live Updates: W.H.O. Declares Pandemic as Number of Infected Countries Grows. The New York Times. Available at https://www.nytimes.com/2020/03/11/world/coronavirus-news.html#link-682e5b06 . March 11, 2020; Accessed: February 29, 2024.
Coronavirus Updates: The Illness Now Has a Name: COVID-19. The New York Times. Available at https://www.nytimes.com/2020/02/11/world/asia/coronavirus-china.html . February 11, 2020; Accessed: February 29, 2024.
WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Available at https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 . February 11, 2020; Accessed: February 29, 2024.
Gorbalenya AE. Severe acute respiratory syndrome-related coronavirus – The species and its viruses, a statement of the Coronavirus Study Group. Available at https://read.qxmd.com/doi/10.1101/2020.02.07.937862 . February 11, 2020; Accessed: February 29, 2024.
CDC COVID-19 Response Team, Jorden MA, Rudman SL, et al. Evidence for Limited Early Spread of COVID-19 Within the United States, January-February 2020. MMWR. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments . May 29, 2020; Accessed: February 29, 2024.
End of the Federal COVID-19 Public Health Emergency (PHE) Declaration. CDC Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html . 2023 May 05; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019 (COVID-19): Recommendations for Cloth Face Covers. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html . April 3, 2020; Accessed: February 29, 2024.
Ferguson NM, Laydon D, Nedjati-Gilani G, Imai N, Ainslie K, Baguelin M, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College COVID-19 Response Team. Available at https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf . 2020 Mar 16; Accessed: February 29, 2024.
Christie A, Brooks JT, Hicks LA, et al. Guidance for Implementing COVID-19 Prevention Strategies in the Context of Varying Community Transmission Levels and Vaccination Coverage. MMWR Morb Mortal Wkly Rep . 2021. 70:1044–1047. [Full Text] .
Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep . 2020 Dec 04. [Full Text] .
Centers for Disease Control and Prevention. How to Protect Yourself & Others. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html . November 29, 2021; Accessed: February 29, 2024.
CDC. Underlying medical conditions associated with high risk for severe COVID-19: Information for healthcare providers. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html . 2021 Mar 29; updated Feb. 9, 2023; Accessed: February 29, 2024.
Tanaka H, Ogata T, Shibata T, Nagai H, Takahashi Y, Kinoshita M, et al. Shorter Incubation Period among COVID-19 Cases with the BA.1 Omicron Variant. Int J Environ Res Public Health . 2022 May 23. 19 (10): [QxMD MEDLINE Link] .
Clinical characteristics of COVID-19. European Centre for Disease Prevention and Control. Available at https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical . Updated: 30 May 2023; Accessed: February 20, 2024.
Hentsch L, Cocetta S, Allali G, Santana I, Eason R, Adam E, et al. Breathlessness and COVID-19: A Call for Research. Respiration . 2021. 100 (10):1016-1026. [QxMD MEDLINE Link] .
Zoghi G, Moosavy SH, Yavarian S, HasaniAzad M, Khorrami F, Sharegi Brojeni M, et al. Gastrointestinal implications in COVID-19. BMC Infect Dis . 2021 Nov 4. 21 (1):1135. [QxMD MEDLINE Link] .
Rabin RC. Lost Sense of Smell May Be Peculiar Clue to Coronavirus Infection. The New York Times. Available at https://www.nytimes.com/2020/03/22/health/coronavirus-symptoms-smell-taste.html . March 22, 2020; Accessed: February 29, 2024.
Coelho DH, Reiter ER, French E, Costanzo RM. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol Head Neck Surg . 2022 May 3. 1945998221097656. [QxMD MEDLINE Link] .
CDC. 2019 Novel Coronavirus, Wuhan, China: Interim Healthcare Infection Prevention and Control Recommendations for Patients Under Investigation for 2019 Novel Coronavirus. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/infection-control.html . January 18, 2020; Accessed: December 1, 2021.
Rizk JG, Forthal DN, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, et al. Expanded Access Programs, compassionate drug use, and Emergency Use Authorizations during the COVID-19 pandemic. Drug Discov Today . 2020 Nov 27. [QxMD MEDLINE Link] . [Full Text] .
Abutaleb Y. How the new coronavirus differs from SARS, measles and Ebola. The Washington Post. Available at https://www.washingtonpost.com/health/how-the-new-coronavirus-differs-from-sars-measles-and-ebola/2020/01/23/aac6bb06-3e1b-11ea-b90d-5652806c3b3a_story.html . January 23, 2020; Accessed: February 20, 2024.
World Health Organization. MERS situation update, February 2022. WHO Regional Office for the Eastern Mediterranean. Available at http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html . February 2022; Accessed: May 17, 2022.
WHO. Leading health agencies outline updated terminology for pathogens that transmit through the air. News Release. World Health Organization. Available at https://www.who.int/news/item/18-04-2024-leading-health-agencies-outline-updated-terminology-for-pathogens-that-transmit-through-the-air . April 18, 2024; Accessed: April 25, 2024.
Handiso TB, Jifar MS, Nuriye Hagisso S. Coronavirus's (SARS-CoV-2) airborne transmission. SAGE Open Med . 2022. 10:20503121221094185. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. COVID-19 Overview and Infection Prevention and Control Priorities in non-U.S. Healthcare Settings. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html . Updated Oct 27, 2023; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019: Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html . 2020 Oct 05; Accessed: December 1, 2021.
Morawska L, Milton DK. It is Time to Address Airborne Transmission of COVID-19. Clin Infect Dis . 2020 Jul 6. [QxMD MEDLINE Link] .
Mask use in the context of COVID-19 (1 December 2020). World Health Organization. Available at https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak . December 1, 2020; Accessed: February 29, 2024.
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med . 2020 Mar 17. [QxMD MEDLINE Link] .
Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe . April 2, 2020. [Full Text] .
Rahmani A, Dini G, Leso V, Montecucco A, Kusznir Vitturi B, Iavicoli I, et al. Duration of SARS-CoV-2 shedding and infectivity in the working age population: a systematic review and meta-analysis. Med Lav . 2022 Apr 26. 113 (2):e2022014. [QxMD MEDLINE Link] .
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020 Mar 11. [QxMD MEDLINE Link] .
Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis . 2020 Mar 19. [QxMD MEDLINE Link] .
Zhou B, She J, Wang Y, Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis . 2020 Apr 17. [QxMD MEDLINE Link] .
Mole B. Recovered COVID-19 patients test positive but not infectious, data finds. Ars Technica. Available at https://arstechnica.com/science/2020/05/feared-reactivation-of-covid-19-infections-disputed-by-new-data/ . May 19, 2020; Accessed: February 29, 2024.
Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med . 2020 Dec 24. 383 (26):2586-2588. [QxMD MEDLINE Link] . [Full Text] .
Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bachman M, et al. Prolonged SARS-CoV-2 replication in an immunocompromised patient. J Infect Dis . 2020 Oct 22. [QxMD MEDLINE Link] . [Full Text] .
Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open . 2020 May 1. 3 (5):e208292. [QxMD MEDLINE Link] .
Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection : A Narrative Review. Ann Intern Med . 2020 Sep 1. 173 (5):362-367. [QxMD MEDLINE Link] . [Full Text] .
Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open . 2021 Jan 4. 4 (1):e2035057. [QxMD MEDLINE Link] . [Full Text] .
Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med . 2020 Feb 19. [QxMD MEDLINE Link] .
WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. Available at https://covid19.who.int/ . 2023 Apr 05; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019 (COVID-19) – Cases in the U.S. Centers for Disease Control and Prevention (CDC). Available at https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days . 2023 Mar 29; Accessed: April 5, 2023.
Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional Mortality Data - United States, 2020. MMWR Morb Mortal Wkly Rep . 2021 Apr 9. 70 (14):519-522. [QxMD MEDLINE Link] . [Full Text] .
Ahmad FB, Cisewski JA, Anderson RN. Provisional Mortality Data - United States, 2021. MMWR Morb Mortal Wkly Rep . 2022 Apr 29. 71 (17):597-600. [QxMD MEDLINE Link] . [Full Text] .
Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med . 2020 Jun 25. 382 (26):2534-2543. [QxMD MEDLINE Link] . [Full Text] .
Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health . 2021 Nov 24. 20 (1):248. [QxMD MEDLINE Link] .
Zelner J, Trangucci R, Naraharisetti R, Cao A, Malosh R, Broen K, et al. Racial disparities in COVID-19 mortality are driven by unequal infection risks. Clin Infect Dis . 2020 Nov 21. [QxMD MEDLINE Link] . [Full Text] .
Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin Infect Dis . 2020 Jun 20. [QxMD MEDLINE Link] . [Full Text] .
Millet C, Racoosin E, Narvar S, Horani G, et al. The Racial Divide: A Follow Up Study on Racial Disparity Amongst COVID-19 Survivors in an Urban Community. J Community Hosp Intern Med Perspect . 2022 May 2. 12(3):66-70. [QxMD MEDLINE Link] . [Full Text] .
Hobbs CV, Kim SS, Vemula P, Inagaki K, et al. Active Surveillance With Seroprevalence-based Infection Rates Indicates Racial Disparities With Pediatric SARS-CoV-2 Requiring Hospitalization in Mississippi, March 2020-February 2021. Pediatr Infect Dis J . 2022 Jun 7. [QxMD MEDLINE Link] .
Ward LA, Black KP, Britton CL, Tompkins ML, Provost EM. COVID-19 Cases, Hospitalizations, and Deaths Among American Indian or Alaska Native Persons - Alaska, 2020-2021. MMWR Morb Mortal Wkly Rep . 2022 Jun 3. 71 (22):730-733. [QxMD MEDLINE Link] .
Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical Outcomes in Young US Adults Hospitalized With COVID-19. JAMA Intern Med . 2020 Sep 9. [QxMD MEDLINE Link] . [Full Text] .
Park YJ, Choe YJ, Park O, and the, COVID-19 National Emergency Response Center, Epidemiology and Case Management Team. Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg Infect Dis . 2020 Oct. 26 (10):2465-2468. [QxMD MEDLINE Link] . [Full Text] .
Schwartz NG, Moorman AC, Makaretz A, Chang KT, Chu VT, Szablewski CM, et al. Adolescent with COVID-19 as the Source of an Outbreak at a 3-Week Family Gathering - Four States, June-July 2020. MMWR Morb Mortal Wkly Rep . 2020 Oct 9. 69 (40):1457-1459. [QxMD MEDLINE Link] . [Full Text] .
Zhu F, Ang JY. COVID-19 Infection in Children: Diagnosis and Management. Curr Infect Dis Rep . 2022 Apr 11. 1-12. [QxMD MEDLINE Link] .
Waghmare A, Hijano DR. SARS-CoV-2 Infection and COVID-19 in Children. Clin Chest Med . 2023 Jun. 44 (2):359-371. [QxMD MEDLINE Link] .
Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr . 2020 Apr 22. [QxMD MEDLINE Link] .
Aronoff SC, Hall A, Del Vecchio MT. The Natural History of SARS-Cov-2 Related Multisystem Inflammatory Syndrome in Children (MIS-C): A Systematic Review. J Pediatric Infect Dis Soc . 2020 Sep 14. [QxMD MEDLINE Link] . [Full Text] .
Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, et al. COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine . 2020 Jul. 24:100433. [QxMD MEDLINE Link] . [Full Text] .
Ahmed M, Advani S, Moreira A, Zoretic S, Martinez J, Chorath K, et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine . 2020 Sep 4. 100527. [QxMD MEDLINE Link] . [Full Text] .
Children and COVID-19: State-Level Data Report. American Academy of Pediatrics. Available at https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ . 2022 Oct 27; Accessed: November 7, 2022.
Hillis SD, Blenkinsop A, Villaveces A, Annor FB, Liburd L, Massetti GM, et al. COVID-19-Associated Orphanhood and Caregiver Death in the United States. Pediatrics . 2021 Oct 7. [QxMD MEDLINE Link] . [Full Text] .
CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Available at https://covid.cdc.gov/covid-data-tracker/#datatracker-home . May 11, 2023; Accessed: March 6, 2024.
Bixler D, Miller AD, Mattison CP, and the, Pediatric Mortality Investigation Team. SARS-CoV-2-Associated Deaths Among Persons Aged MMWR Morb Mortal Wkly Rep</i>. 2020 Sep 18. 69 (37):1324-1329. [QxMD MEDLINE Link] . [Full Text] .
Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr . 2020 Aug. 223:14-19.e2. [QxMD MEDLINE Link] . [Full Text] .
Han MS, Choi EH, Chang SH, Jin BL, Lee EJ, Kim BN, et al. Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea. JAMA Pediatr . 2020 Aug 28. [QxMD MEDLINE Link] . [Full Text] .
Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis . 2020 Nov. 20 (11):e276-e288. [QxMD MEDLINE Link] .
Feldstein LR, Tenforde MW, Friedman KG, and the, Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA . 2021 Feb 24. [QxMD MEDLINE Link] . [Full Text] .
Ko JY, DeSisto CL, Simeone RM, Ellington S, Galang RR, Oduyebo T, et al. Adverse Pregnancy Outcomes, Maternal Complications, and Severe Illness Among US Delivery Hospitalizations With and Without a Coronavirus Disease 2019 (COVID-19) Diagnosis. Clin Infect Dis . 2021 Jul 15. 73 (Suppl 1):S24-S31. [QxMD MEDLINE Link] . [Full Text] .
Wang H, Li N, Sun C, Guo X, Su W, Song Q, et al. The association between pregnancy and COVID-19: A systematic review and meta-analysis. Am J Emerg Med . 2022 Apr 6. 56:188-195. [QxMD MEDLINE Link] .
Ayala-Ramírez P, González M, Escudero C, Quintero-Arciniegas L, Giachini FR, Alves de Freitas R, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy. A Non-systematic Review of Clinical Presentation, Potential Effects of Physiological Adaptations in Pregnancy, and Placental Vascular Alterations. Front Physiol . 2022. 13:785274. [QxMD MEDLINE Link] .
DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, et al. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep . 2021 Nov 26. 70 (47):1640-1645. [QxMD MEDLINE Link] . [Full Text] .
Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, Perinatal and Neonatal Outcomes With COVID-19: A Multicenter Study of 242 Pregnancies and Their 248 Infant Newborns During Their First Month of Life. Pediatr Infect Dis J . 2020 Sep 11. [QxMD MEDLINE Link] .
Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM . 2020 Aug. 2 (3):100134. [QxMD MEDLINE Link] . [Full Text] .
Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy Outcomes Among Women With and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw Open . 2020 Nov 2. 3 (11):e2029256. [QxMD MEDLINE Link] .
Chambers C, Krogstad P, Bertrand K, Contreras D, Tobin NH, Bode L, et al. Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women. JAMA . 2020 Aug 19. [QxMD MEDLINE Link] . [Full Text] .
Pace RM, Williams JE, Järvinen KM, Meehan CL, Martin MA, Ley SH, et al. Milk From Women Diagnosed With COVID-19 Does Not Contain SARS-CoV-2 RNA but Has Persistent Levels of SARS-CoV-2-Specific IgA Antibodies. Front Immunol . 2021. 12:801797. [QxMD MEDLINE Link] . [Full Text] .
Dandachi D, Geiger G, Montgomery MW, and the, HIV-COVID-19 consortium. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin Infect Dis . 2020 Sep 9. [QxMD MEDLINE Link] . [Full Text] .
Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. COVID-19 Pneumonia in Patients With HIV: A Case Series. J Acquir Immune Defic Syndr . 2020 Sep 1. 85 (1):e4-e5. [QxMD MEDLINE Link] . [Full Text] .
Karmen-Tuohy S, Carlucci PM, Zervou FN, Zacharioudakis IM, Rebick G, Klein E, et al. Outcomes Among HIV-Positive Patients Hospitalized With COVID-19. J Acquir Immune Defic Syndr . 2020 Sep 1. 85 (1):6-10. [QxMD MEDLINE Link] . [Full Text] .
Ho HE, Peluso MJ, Margus C, Matias Lopes JP, He C, Gaisa MM, et al. Clinical outcomes and immunologic characteristics of Covid-19 in people with HIV. J Infect Dis . 2020 Jun 30. [QxMD MEDLINE Link] . [Full Text] .
Oyelade T, Alqahtani JS, Hjazi AM, Li A, Kamila A, Raya RP. Global and Regional Prevalence and Outcomes of COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis. Trop Med Infect Dis . 2022 Feb 3. 7 (2): [QxMD MEDLINE Link] .
Self WH, Tenforde MW, Stubblefield WB, and the, CDC COVID-19 Response Team IVY Network. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network – 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep . 2020 Aug 31. 69: [Full Text] .
Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus Disease 2019 Hospitalizations Attributable to Cardiometabolic Conditions in the United States: A Comparative Risk Assessment Analysis. J Am Heart Assoc . 2021 Feb. 10 (5):e019259. [QxMD MEDLINE Link] . [Full Text] .
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis . 2020 Jan 14. 91:264-266. [QxMD MEDLINE Link] .
Vangeel L, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. bioRxiv . 2021 Dec 28. [Full Text] .
Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv . 2021 Dec 02. [Full Text] .
Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv . 2021 Dec 13. [Full Text] .
Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv . 2021 May 03. [Full Text] .
Edara VV, Lai L, Sahoo MK, Floyd K, Sibai M, Solis D, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv . 2021 May 10. [Full Text] .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med . 2021 Jul 21. [QxMD MEDLINE Link] . [Full Text] .
Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Report 42 - Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. Imperial College London, MRC Centre for Global Infectious Disease Analysis. Available at https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-12-31-COVID19-Report-42-Preprint-VOC.pdf . 2020 Dec 31; Accessed: December 1, 2021.
Nelson G, Oleksandr B, Spilman P, Niazi K, Rabizadeh S, Soon-Shiong P. Molecular dynamic simulation reveals E484K mutation enhances spike RBD-ACE2 affinity and the combination of E484K, K417N and N401Y mutations (501Y.V2 variant) induces conformational change greater than N401Y mutant alone, potentially resulting in an escape mutant. bioRxiv . 2021 Jan 13. [Full Text] .
Sabino EC, Buss LF, Carvalho MPS, Prete CA, Crispim MAE, Fraiji NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet . 2021 Jan 27. [Full Text] .
CDC. Symptoms of Coronavirus. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html . May 13, 2020 (updated March 22, 2022); Accessed: April 29, 2022.
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med . 2020 Mar 10. [QxMD MEDLINE Link] .
Deo R, Choudhary MC, Moser C, and the, ACTIV-2/A5401 Study Team†. Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. Ann Intern Med . 2023 Feb 21. [QxMD MEDLINE Link] . [Full Text] .
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA . 2020 Feb 24. [QxMD MEDLINE Link] .
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA . 2020 Aug 25. 324 (8):782-793. [QxMD MEDLINE Link] .
Burke RM, Killerby ME, Newton S, Ashworth CE, Berns AL, Brennan S, et al. Symptom Profiles of a Convenience Sample of Patients with COVID-19 - United States, January-April 2020. MMWR Morb Mortal Wkly Rep . 2020 Jul 17. 69 (28):904-908. [QxMD MEDLINE Link] .
CDC. Healthcare Workers: Information on COVID-19. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html . March 31, 2021 (updated); Accessed: December 1, 2021.
Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature . 2020 Jul 8. [QxMD MEDLINE Link] .
Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health . 2020 Jun 30. [QxMD MEDLINE Link] .
Ellinghaus D, Degenhardt F, Bujanda L, et al. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS-CoV-2 respiratory failure in an Italian-Spanish genome-wide association analysis. medRxiv. Available at https://www.medrxiv.org/content/10.1101/2020.05.31.20114991v1 . June 2, 2020; Accessed: December 1, 2021.
Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide Association Study of Severe Covid-19 With Respiratory Failure. NEJM . 2020 Jun 17. [QxMD MEDLINE Link] . [Full Text] .
Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA . 2020 Apr 22. [QxMD MEDLINE Link] .
Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F, et al. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19). Clin Infect Dis . 2020 May 1. [QxMD MEDLINE Link] .
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020 Jan 24. [QxMD MEDLINE Link] .
Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med . 2020 Feb 28. [QxMD MEDLINE Link] .
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet . 2020 Feb 15. 395 (10223):507-513. [QxMD MEDLINE Link] .
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA . 2020 Feb 7. [QxMD MEDLINE Link] .
CDC. Long-Term Effects of COVID-19. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html . 2020 Nov 13; Accessed: December 1, 2021.
[Guideline] COVID-19 Treatment Guidelines Panel. Clinical Spectrum of SARS-CoV-2 Infection. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ . March 1 2024 (updated); Accessed: February 29, 2024.
[Guideline] COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE). Available at https://www.nice.org.uk/guidance/NG188 . 2020 Dec 18; Accessed: December 1, 2021.
Datta SD, Talwar A, Lee JT. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. JAMA . 2020 Dec 8. 324 (22):2251-2252. [QxMD MEDLINE Link] . [Full Text] .
McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res . 2020 Jul 31. 127 (4):571-587. [QxMD MEDLINE Link] . [Full Text] .
Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J . 2021 Feb 18. [QxMD MEDLINE Link] . [Full Text] .
Sciscent BY, Eisele CD, Ho L, King SD, Jain R, Golamari RR. COVID-19 reinfection: the role of natural immunity, vaccines, and variants. J Community Hosp Intern Med Perspect . 2021. 11 (6):733-739. [QxMD MEDLINE Link] .
Pérez-Lago L, Kestler M, Sola-Campoy PJ, et al. SARS-CoV-2 superinfection and reinfection with three different strains. Transbound Emerg Dis . 2021 Oct 23. [QxMD MEDLINE Link] .
CDC. 2019 Novel Coronavirus, Wuhan, China: Frequently Asked Questions and Answers. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/faq.html . October 19, 2021; Accessed: December 1, 2021.
FDA. Coronavirus (COVID-19) Update: Daily Roundup. fda.gov. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup . March 23, 2020; Accessed: December 1, 2021.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med . 2020 Mar 13. [QxMD MEDLINE Link] .
Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis . 2020 Feb 24. [QxMD MEDLINE Link] .
Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol . 2020 Mar 3. 1-6. [QxMD MEDLINE Link] .
Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology . 2020 Mar 10. 200823. [QxMD MEDLINE Link] .
ACR. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. American College of Radiology. Available at https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection . March 22, 2020; Accessed: December 1, 2021.
Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci . 2020 Mar 4. [QxMD MEDLINE Link] .
Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J Infect Dis . 2020 Mar 17. [QxMD MEDLINE Link] .
Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, et al. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease. Acad Radiol . 2020 Mar 20. [QxMD MEDLINE Link] .
Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients. Radiology . 2019 Mar 27. 201160. [QxMD MEDLINE Link] .
Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of Unknown Etiology in Wuhan, China: Potential for International Spread Via Commercial Air Travel. J Travel Med . 2020 Jan 14. [QxMD MEDLINE Link] .
Veklury (remdesivir) [package insert]. Foster City, CA: Gilead Sciences, Inc. February 2024. Available at [Full Text] .
RECOVERY Collaborative Group., Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med . 2021 Feb 25. 384 (8):693-704. [QxMD MEDLINE Link] . [Full Text] .
WHO. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with COVID-19. WHO. Available at https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19 . April 19, 2020; Accessed: December 1, 2021.
[Guideline] Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. IDSA. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ . 2021 Oct 01; updated 6/26/2023; Accessed: February 29, 2024.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O’Mera MJ, et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv. Available at https://www.biorxiv.org/content/10.1101/2020.03.22.002386v1 . 2020 Mar 22; Accessed: December 1, 2021.
Arshad U, Pertinez H, Box H, Tatham L, Rajoki RKR, Curley P, et al. Prioritisation of potential anti-SARS-CoV-2 drug repurposing opportunities based on ability to achieve adequate target site concentrations derived from their established human pharmacokinetics. medRxiv . 2020 Apr 22. [Full Text] .
World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. WHO. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments . 2020 Oct 16; Accessed: December 1, 2021.
WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med . 2020 Dec 2. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA . 2020 Mar 24. [QxMD MEDLINE Link] . [Full Text] .
Rome BN, Avorn J. Drug Evaluation during the Covid-19 Pandemic. N Engl J Med . 2020 Apr 14. [QxMD MEDLINE Link] . [Full Text] .
Frellick M. Key Drugs Join PPEs on List of Front-Line Shortages. Medscape Medical News . 2020 Apr 02. Available at https://www.medscape.com/viewarticle/928039 .
Live Updates: Which Drugs Are In Shortage Because of COVID-19?. GoodRx. Available at https://www.goodrx.com/blog/covid-19-drug-shortages-updates/ . July 13, 2020; Accessed: December 1, 2021.
Wilson FP. Hydroxychloroquine for COVID-19: What’s the Evidence? (Commentary). Medscape Medical News and Perspectives . 2020 Mar 25. Available at https://www.medscape.com/viewarticle/927342 .
CDC. Coronavirus Disease 2019 (COVID-19): Community-Related Exposures. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html . March 1, 2021 (updated); Accessed: December 1, 2021.
CDC. Coronavirus Disease 2019 (COVID-19): Travel-Associated Exposures. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html . November 5, 2021 (updated); Accessed: December 1, 2021.
Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med . 2020. [Full Text] .
Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ . 2016 May 17. 188 (8):567-574. [QxMD MEDLINE Link] .
TLR 2/6/9 agonist PUL-042. NCI Drug Dictionary. Available at https://www.cancer.gov/publications/dictionaries/cancer-drug/def/tlr-2-6-9-agonist-pul-042 . Accessed: December 1, 2021.
The Use PUL-042 Inhalation Solution to Prevent COVID-19 in Adults Exposed to SARS-CoV-2. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT04313023 . 2020 Mar 24; Accessed: December 1, 2021.
The Use of PUL-042 Inhalation Solution to Reduce the Severity of COVID-19 in Adults Positive for SARS-CoV-2 Infection. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT04312997?term=PUL-042&cond=COVID&cntry=US&draw=2&rank=2 . 2020 May 07; Accessed: December 1, 2021.
Holshue ML, DeBolt C, Lindquist S, and, the Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med . 2020 Mar 5. 382 (10):929-936. [QxMD MEDLINE Link] .
National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. U.S. Department of Health and Human Services. Available at https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins . 2020 Feb 25; Accessed: March 24, 2020.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med . 2020 Oct 8. [QxMD MEDLINE Link] . [Full Text] .
Harrington DP, Baden LR, Hogan JW. A Large, Simple Trial Leading to Complex Questions. N Engl J Med . 2020 Dec 2. [QxMD MEDLINE Link] . [Full Text] .
Ader F, Bouscambert-Duchamp M, Hites M, and the, DisCoVeRy Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis . 2021 Sep 14. [QxMD MEDLINE Link] . [Full Text] .
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med . November 5, 2020. 383:1827-1837. [QxMD MEDLINE Link] . [Full Text] .
Spinner CD, Gottlieb RL, Criner GJ, and the, GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2020 Aug 21. [QxMD MEDLINE Link] . [Full Text] .
Gottlieb RL, Vaca CE, Paredes R, and the, GS-US-540-9012 (PINETREE) Investigators. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med . 2021 Dec 22. [QxMD MEDLINE Link] . [Full Text] .
Veklury (remdesivir) reduced risk of mortality in hospitalized COVID-19 patients across all variant time periods in a real world study of more than 500,000 patients. Gilead . 2023 Jan 21. Available at https://www.gilead.com/news-and-press/press-room/press-releases/2023/2/veklury-remdesivir-reduced-risk-of-mortality-in-hospitalized-covid19-patients-across-all-variant-time-periods-in-a-real-world-study-of-more-than-5 .
Amstutz A, et al. Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomized controlled trials. Lancet Respir Med . 2023 Feb 21. [Full Text] .
Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) (CARAVAN). ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT04431453 . 2020 Jun 01; Accessed: December 1, 2021.
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter interim guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc . 2020 Sep 12. [QxMD MEDLINE Link] . [Full Text] .
Burwick RM, Yawetz S, Stephenson KE, Collier AY, Sen P, Blackburn BG, et al. Compassionate Use of Remdesivir in Pregnant Women with Severe Covid-19. Clin Infect Dis . 2020 Oct 8. [QxMD MEDLINE Link] . [Full Text] .
McCoy JA, Short WR, Srinivas SK, Levine LD, Hirshberg A. Compassionate use of remdesivir for treatment of severe coronavirus disease 2019 in pregnant women at a United States academic center. Am J Obstet Gynecol MFM . 2020 Aug. 2 (3):100164. [QxMD MEDLINE Link] . [Full Text] .
Hammond J, Leister-Tebbe H, Gardner A, and the, EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med . 2022 Feb 16. [QxMD MEDLINE Link] . [Full Text] .
Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. Pfizer, Inc . 2021 Dec 14. Available at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results .
A post-exposure prophylaxis study of PF-07321332/ritonavir in adult household contacts of an individual with symptomatic COVID-19. ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT05047601 . 2021 Dec 01; Accessed: December 14, 2021.
Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv . November 5, 2022. [Full Text] .
Liu J, Pan X, Zhang S, Li M, Ma K, Fan C, et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac . 2023 Feb 6. 100694. [QxMD MEDLINE Link] . [Full Text] .
Wong CKH, Lau KTK, Au ICH, Lau EHY, Poon LLM, Hung IFN, et al. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect Dis . 2023 Feb 13. [QxMD MEDLINE Link] . [Full Text] .
Zheng Q, Ma P, Wang M, Cheng Y, Zhou M, Ye L, et al. Efficacy and safety of Paxlovid for COVID-19:a meta-analysis. J Infect . 2023 Jan. 86 (1):66-117. [QxMD MEDLINE Link] . [Full Text] .
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, and the, MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med . 2022 Feb 10. 386:509-520. [QxMD MEDLINE Link] . [Full Text] .
Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ . 2023 Mar 7. 380:e072705. [QxMD MEDLINE Link] . [Full Text] .
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis. Clin Infect Dis . 2023 Feb 8. 76 (3):453-460. [QxMD MEDLINE Link] . [Full Text] .
Merck provides update on phase 3 MOVe-AHEAD trial evaluating Lagevrio (molnupiravir) for post-exposure prophylaxis for prevention of COVID-19. Merck . 2023 Feb 21. Available at https://www.merck.com/news/merck-provides-update-on-phase-3-move-ahead-trial-evaluating-lagevrio-molnupiravir-for-post-exposure-prophylaxis-for-prevention-of-covid-19/ .
Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in COVID-19. Lancet Respir Med . 2020 May 4. [QxMD MEDLINE Link] . [Full Text] .
Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs . 2020 Sep. 80 (13):1267-1292. [QxMD MEDLINE Link] . [Full Text] .
Peterson JH, Paranjape NS, Grundlingh N, Priestley JL. Outcomes and Adverse Effects of Baricitinib Versus Tocilizumab in the Management of Severe COVID-19. Crit Care Med . 2023 Mar 1. 51 (3):337-346. [QxMD MEDLINE Link] . [Full Text] .
Cawcutt KA, Kalil AC. Baricitinib or Tocilizumab? Treatment of Patients Hospitalized With Severe COVID-19. Crit Care Med . 2023 Mar 1. 51 (3):413-415. [QxMD MEDLINE Link] .
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis . 2020 Apr. 20 (4):400-402. [QxMD MEDLINE Link] . [Full Text] .
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet . 2020 Feb 15. 395 (10223):e30-e31. [QxMD MEDLINE Link] . [Full Text] .
Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richarson PJ, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. 2020 Apr 15. [Full Text] .
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med . 2020 Dec 11. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC, Stebbing J. Baricitinib: the first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial. Lancet Respir Med . 2021 Aug 31. [QxMD MEDLINE Link] . [Full Text] .
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med . 2021 Aug 31. [QxMD MEDLINE Link] . [Full Text] .
Lilly and Incyte’s baricitinib reduced deaths among patients with COVID-19 receiving invasive mechanical ventilation. Lilly. Available at https://investor.lilly.com/news-releases/news-release-details/lilly-and-incytes-baricitinib-reduced-deaths-among-patients . 2021 Aug 03; Accessed: December 1, 2021.
Guimarães PO, Quirk D, Furtado RH, and the STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med . 2021 Jun 16. [QxMD MEDLINE Link] . [Full Text] .
Wagner JL, Veve MP, Barber KE. Using IL-6 inhibitors to treat COVID-19. ContagionLive. Available at https://www.contagionlive.com/publications/contagion/2020/august/using-il6-inhibitors-to-treat-covid19?ekey=c2NiZXJnbWFuQG5lYnJhc2thbWVkLmNvbQ . 2020 Aug 17; Accessed: December 1, 2021.
[Guideline] NIH. The COVID-19 Treatment Guidelines Panel’s Statement on the Use of Tocilizumab (and Other Interleukin-6 Inhibitors) for the Treatment of COVID-19. COVID-19 Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-6-inhibitors/ . 2021 Mar 05; Accessed: December 1, 2021.
Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med . 2021 Jan 7. 384 (1):20-30. [QxMD MEDLINE Link] . [Full Text] .
Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med . 2021 Nov. 47 (11):1258-1270. [QxMD MEDLINE Link] . [Full Text] .
REMAP-CAP Investigators., Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med . 2021 Apr 22. 384 (16):1491-1502. [QxMD MEDLINE Link] . [Full Text] .
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet . 2021 May 1. 397 (10285):1637-1645. [QxMD MEDLINE Link] . [Full Text] .
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med . 2021 Apr 22. 384 (16):1503-1516. [QxMD MEDLINE Link] . [Full Text] .
Rubin EJ, Longo DL, Baden LR. Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup. N Engl J Med . 2021 Apr 22. 384 (16):1564-1565. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] NIH. Interleukin-1 Inhibitors. COVID-19 Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-1-inhibitors/ . 2020 Jul 17; Accessed: September 8, 2021.
[Guideline] Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. World Health Organization. Available at https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected . 2020 Mar 13; Accessed: March 24, 2020.
Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet . 2020 Feb 15. 395 (10223):473-475. [QxMD MEDLINE Link] .
[Guideline] Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med . 2021 Mar 1. 49 (3):e219-e234. [QxMD MEDLINE Link] . [Full Text] .
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA . 2020 Sep 2. [QxMD MEDLINE Link] . [Full Text] .
Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA . 2020 Sep 2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] WHO. Corticosteroids for COVID-19 – Living Guidance. World Health Organization. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 . 2020 Sep 02; Accessed: December 1, 2021.
Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol . 2022 Feb. 22 (2):77-84. [QxMD MEDLINE Link] . [Full Text] .
Carvelli J, Demaria O, Vély F, Batista L, Chouaki Benmansour N, Fares J, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature . 2020 Dec. 588 (7836):146-150. [QxMD MEDLINE Link] . [Full Text] .
Aiello S, Gastoldi S, Galbusera M, Ruggenenti P, Portalupi V, Rota S, et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv . 2022 Jan 8. 6 (3):866-881. [QxMD MEDLINE Link] . [Full Text] .
Vlaar APJ, Witzenrath M, van Paassen P, and the, PANAMO study group. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med . 2022 Dec. 10 (12):1137-1146. [QxMD MEDLINE Link] . [Full Text] .
Strich JR, Tian X, Samour M, King CS, Shlobin O, Reger R, et al. Fostamatinib for the treatment of hospitalized adults with COVD-19 A randomized trial. Clin Infect Dis . 2021 Sep 1. [QxMD MEDLINE Link] . [Full Text] .
Reis G, Moreira Silva EAS, Medeiros Silva DC, and the, TOGETHER Investigators. Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med . 2023 Feb 9. 388 (6):518-528. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC, Mehta AK, Patterson TF, and the, ACTT-3 study group members. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med . 2021 Oct 18. [QxMD MEDLINE Link] . [Full Text] .
Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents?. Circulation . 2004 Jun 1. 109 (21 Suppl 1):II18-26. [QxMD MEDLINE Link] . [Full Text] .
Hariyanto TI, Kurniawan A. Statin therapy did not improved the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr . 2020 Aug 26. 14(6):1613-1615. [QxMD MEDLINE Link] . [Full Text] .
Kow CS, Hasan SS. Meta-analysis of Effect of Statins in Patients with COVID-19. Am J Cardiol . 2020 Aug 12. [QxMD MEDLINE Link] . [Full Text] .
Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother . 2020 Jul 1. 6 (4):258-259. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] NIH. Covid-19 Treatment Guidelines - Adjunctive Therapy. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/ . 2020 Jul 17; Accessed: December 1, 2021.
Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, et al. The minimal effect of zinc on the survival of hospitalized patients with Covid-19: an observational study. Chest . 2020 Jul 22. [QxMD MEDLINE Link] . [Full Text] .
Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open . 2020 Sep 1. 3 (9):e2019722. [QxMD MEDLINE Link] . [Full Text] .
Sahota O. Understanding vitamin D deficiency. Age Ageing . 2014 Sep. 43 (5):589-91. [QxMD MEDLINE Link] . [Full Text] .
Bishop CW, Ashfaq A, Melnick JZ, Vazquez-Escarpanter E, Fialkow JA, Strugnell SA, et al. Results from the REsCUe trial: a randomized controlled trial with extended-release calcifediol in symptomatic outpatients with COVID-19. medRXiv . 2022 Feb 05. [Full Text] .
NIH COVID-19 Treatment Guidelines Panel. Statement on the emergency use authorization of convalescent plasma for the treatment of COVID-19. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/statement-on-convalescent-plasma-eua/ . 2022 Dec 01; Accessed: February 1, 2023.
Senefeld JW, Franchini M, Mengoli C, Cruciani M, Zani M, Gorman EK, et al. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw Open . 2023 Jan 3. 6 (1):e2250647. [QxMD MEDLINE Link] . [Full Text] .
Writing Committee for the REMAP-CAP Investigators, Estcourt LJ, Turgeon AF, McQuilten ZK, et al. Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA . 2021 Oct 4. [QxMD MEDLINE Link] .
A Study to Investigate the Prevention of COVID-19 with VYD222 in Adults With Immune Compromise and in Participants Aged 12 Years or Older Who Are at Risk of Exposure to SARS-CoV-2. ClinicalTrials.gov. Available at https://classic.clinicaltrials.gov/ct2/show/NCT06039449 . 2024 January 18; Accessed: April 1, 2024.
Levin MJ, Ustianowski A, De Wit S, and the, PROVENT Study Group. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med . 2022 Jun 9. 386 (23):2188-2200. [QxMD MEDLINE Link] . [Full Text] .
Young-Xu Y, Epstein L, Marconi VC, Davey V, Zwain G, Smith J, et al. Tixagevimab/cilagavimab for prevention of COVID-19 during the omicron surge: Retrospective analysis of national VA electronic data. medRxiv . 2022 May 29. [Full Text] .
Westendorf K, Žentelis S, Wang L, Foster D, Vaillancourt P, Wiggin M, et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep . 2022 May 17. 39 (7):110812. [QxMD MEDLINE Link] . [Full Text] .
Gupta A, Gonzalez-Rojas Y, Juarez E, and the, COMET-ICE Investigators. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med . 2021 Nov 18. 385 (21):1941-1950. [QxMD MEDLINE Link] . [Full Text] .
GSK and Vir submit emergency use authorization application to FDA for intramuscular administration of sotrovimab for the early treatment of COVID-19. GlaxoSmithKline . 2022 Jan 13. Available at https://us.gsk.com/en-us/media/press-releases/gsk-and-vir-submit-emergency-use-authorization-application-to-fda-for-intramuscular-administration-of-sotrovimab-for-the-early-treatment-of-covid-19/ .
O'Brien MP, Forleo-Neto E, Musser BJ, and the, Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med . 2020 Dec 17. [QxMD MEDLINE Link] . [Full Text] .
RECOVERY Collaborative Group, Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. medRxiv . 2021 Jun 16. [Full Text] .
Dougan M, Nirula A, Azizad M, and the, BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med . 2021 Oct 7. 385 (15):1382-1392. [QxMD MEDLINE Link] . [Full Text] .
Cohen MS, Nirula A, Mulligan MJ, and the, BLAZE-2 Investigators. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA . 2021 Jul 6. 326 (1):46-55. [QxMD MEDLINE Link] . [Full Text] .
Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med . 2020 May 21. 382 (21):1969-1973. [QxMD MEDLINE Link] . [Full Text] .
Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev . 2020 Sep. 35:43-49. [QxMD MEDLINE Link] . [Full Text] .
Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med . 2020 Sep. 48 (9):1358-1364. [QxMD MEDLINE Link] . [Full Text] .
Katneni UK, Alexaki A, Hunt RC, Schiller T, DiCuccio M, Buehler PW, et al. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. Thromb Haemost . 2020 Aug 24. [QxMD MEDLINE Link] . [Full Text] .
Ferguson J, Volk S, Vondracek T, Flanigan J, Chernaik A. Empiric therapeutic anticoagulation and mortality in critically ill patients with respiratory failure from SARS-CoV-2: A retrospective cohort study. J Clin Pharmacol . 2020 Sep 3. [QxMD MEDLINE Link] . [Full Text] .
Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol . 2020 Jul 7. 76 (1):122-124. [QxMD MEDLINE Link] . [Full Text] .
NIH ACTIV-4B COVID-19 outpatient thrombosis prevention trial ends early. Brigham Health . 2021 Jun 22. Available at https://www.brighamandwomens.org/about-bwh/newsroom/press-releases-detail?id=3925 .
REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
Assessing Safety, Hospitalization and Efficacy of rNAPc2 in COVID-19 (ASPEN). ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT04655586 . 2021 Nov 03; Accessed: December 6, 2021.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature . 2020 Mar. 579 (7798):270-273. [QxMD MEDLINE Link] . [Full Text] .
Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll-Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial. Am Heart J . 2020 Aug. 226:49-59. [QxMD MEDLINE Link] . [Full Text] .
Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation . 2020 Mar 21. [QxMD MEDLINE Link] . [Full Text] .
Fang L, Karakiulakis G, Roth M. Correction to Lancet Respir Med 2020; 8: e21. Lancet Respir Med . 2020 Jun. 8 (6):e54. [QxMD MEDLINE Link] . [Full Text] .
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med . 2005 Aug. 11 (8):875-9. [QxMD MEDLINE Link] . [Full Text] .
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon S. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med . 2020 March 30. [QxMD MEDLINE Link] . [Full Text] .
Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension . 2004 May. 43 (5):970-6. [QxMD MEDLINE Link] . [Full Text] .
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation . 2005 May 24. 111 (20):2605-10. [QxMD MEDLINE Link] . [Full Text] .
Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med . 2015 Aug. 19 (8):1965-74. [QxMD MEDLINE Link] . [Full Text] .
Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace . 2017 Aug 1. 19 (8):1280-1287. [QxMD MEDLINE Link] . [Full Text] .
Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) . 2012 Dec. 123 (11):649-58. [QxMD MEDLINE Link] .
Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J Card Fail . 2020 May. 26 (5):370. [QxMD MEDLINE Link] . [Full Text] .
Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J . 2020 May 14. 41 (19):1810-1817. [QxMD MEDLINE Link] . [Full Text] .
Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, et al. Association Between Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis. JAMA Netw Open . 2021 Mar 1. 4 (3):e213594. [QxMD MEDLINE Link] . [Full Text] .
Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax . 2015 Oct. 70 (10):984-9. [QxMD MEDLINE Link] . [Full Text] .
Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev . 2020 Apr 15. [QxMD MEDLINE Link] . [Full Text] .
Muniyappa R, Gubbi S. COVID-19 Pandemic, Corona Viruses, and Diabetes Mellitus. Am J Physiol Endocrinol Metab . 2020 Mar 31. [QxMD MEDLINE Link] . [Full Text] .
DPP4 dipeptidyl peptidase 4. National Center for Biotechnology Information (NCBI). Available at https://www.ncbi.nlm.nih.gov/gene/1803 . 2020 Apr 20; Accessed: 2020 Apr 21.
RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. The Lancet . 2020 Oct 05. [Full Text] .
Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnology & Biotechnological Equipment. 2020 . 2020. 34(1):469-74. [Full Text] .
Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and Novel Coronavirus Disease (COVID-19): Keeping Rigor in Times of Urgency. Am J Trop Med Hyg . 2020 Apr 16. [QxMD MEDLINE Link] . [Full Text] .
López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA . 2021 Apr 13. 325 (14):1426-1435. [QxMD MEDLINE Link] . [Full Text] .
Lim SCL, Hor CP, Tay KH, and the, I-TECH Study Group. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern Med . 2022 Feb 18. [QxMD MEDLINE Link] . [Full Text] .
Reis G, Silva EASM, Silva DCM, and the, TOGETHER Investigators. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med . 2022 May 5. 386 (18):1721-1731. [QxMD MEDLINE Link] . [Full Text] .
Naggie S, Boulware DR, Lindsell CJ, for the, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2022 Oct 25. 328 (16):1595-1603. [QxMD MEDLINE Link] . [Full Text] .
Naggie S, Boulware DR, Lindsell CJ, for the, Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA . 2023 Feb 20. [QxMD MEDLINE Link] . [Full Text] .
Bramante CT, Huling JD, Tignanelli CJ, for the COVID-OUT Trial Team. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Eng J Med . 2022. 387:599-610. [Full Text] .
Reis G, Dos Santos Moreira-Silva EA, Silva DCM, and the, TOGETHER investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health . 2022 Jan. 10 (1):e42-e51. [QxMD MEDLINE Link] . [Full Text] .
McCarthy MW, Naggie S, Boulware DR, and the, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators. Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2023 Jan 24. 329 (4):296-305. [QxMD MEDLINE Link] .
Golan Y, Campos JAS, Woolson R, Cilla D, Hanabergh R, Gonzales-Rojas Y, et al. Favipiravir in Patients With Early Mild-to-moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial. Clin Infect Dis . 2023 Feb 8. 76 (3):e10-e17. [QxMD MEDLINE Link] . [Full Text] .
Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med . 2012 May 17. 366 (20):1881-90. [QxMD MEDLINE Link] . [Full Text] .
Simpson TF, Kovacs RJ, Stecker EC. Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment For COVID-19. American College of Cardiology; Cardiology Magazine. Available at https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 . 2020 Mar 29; Accessed: April 1, 2020.
[Guideline] Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment. Circulation . 2020 Apr 8. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. Mayo Clin Proc . 2020 Apr 07. [Full Text] .
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol . 2020 May 1. [QxMD MEDLINE Link] . [Full Text] .
Chorin E, Dai M, Shulman E, Wadhwani L, et al. The QT Interval in Patients with SARS-CoV-2 Infection Treated with Hydroxychloroquine/Azithromycin. medRxiv . 2020 Apr 03. [Full Text] .
Borba M, de Almeida Val F, Sampaio VS, Alexandre MA, Melo GC, Brito M, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). MedRxiv . 2020 Apr 11. [Full Text] .
Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv . 2020 Apr 10. [Full Text] .
Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med . 2021 Jul. 9 (7):755-762. [QxMD MEDLINE Link] . [Full Text] .
Matson J, Lange P, Honore PM, Chung KK. Adverse outcomes with extracorporeal adsorbent blood treatments in toxic systemic inflammation: a perspective on possible mechanisms. Ann Intensive Care . 2022 Nov 12. 12 (1):105. [QxMD MEDLINE Link] . [Full Text] .
Zhang Q, et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett . 2020 Jun 17. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19). CDC. Available at https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html . 2020 Nov 05; Accessed: November 16, 2020.
[Guideline] Center for Clinical Standards and Quality/Quality, Safety & Oversight Group. Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge. Available at https://www.cms.gov/files/document/qso-20-13-hospitalspdf.pdf-2 . March 4, 2020; Accessed: March 4, 2020.
Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Management of Infants Born to Mothers with COVID-19. American Academy of Pediatrics. Available at https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf . April 2, 2020; Accessed: April 3, 2020.
[Guideline] AAP. FAQs: Management of Infants Born to Mothers with Suspected or Confirmed COVID-19. American Academy of Pediatrics. Available at https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/faqs-management-of-infants-born-to-covid-19-mothers/ . May 21, 2020; Accessed: May 27, 2020.
[Guideline] COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/ . 2021 Mar 05; Accessed: March 12, 2021.
[Guideline] Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID-19: CHEST Guideline and Expert Panel Report. Chest . 2020 Jun 2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost . 2020 May. 18 (5):1023-1026. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] COVID-19 Treatment Guidelines Panel. Antithrombotic Therapy in Patients with COVID-19. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ . 2021 Feb 11; Accessed: June 28, 2021.
World Health Organization. Roadmap to improve and ensure good indoor ventilation in COVID-19 context (1 March 2021). World Health Organization. Available at https://www.who.int/publications/i/item/9789240021280 . March 1, 2021; Accessed: February 20, 2024.
Lim EHT, Vlaar APJ, Bos LDJ, and the, Amsterdam UMC COVID-19 Biobank Investigators. Anti-C5a antibody vilobelimab treatment and the effect on biomarkers of inflammation and coagulation in patients with severe COVID-19: a substudy of the phase 2 PANAMO trial. Respir Res . 2022 Dec 24. 23 (1):375. [QxMD MEDLINE Link] . [Full Text] .
Management Strategies in Children and Adolescents with Mild to Moderate COVID-19. American Academy of Pediatri cs. Available at https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/outpatient-covid-19-management-strategies-in-children-and-adolescents/ . Updated February 8, 2023; Accessed: March 6, 2024.
Huang YH, Kuo HC. Kawasaki Disease, MIS-C and COVID-19. Children (Basel) . 2023 Sep 22. 10 (10): [QxMD MEDLINE Link] . [Full Text] .
Simbar M, Nazarpour S, Sheidaei A. Evaluation of pregnancy outcomes in mothers with COVID-19 infection: a systematic review and meta-analysis. J Obstet Gynaecol . 2023 Dec. 43 (1):2162867. [QxMD MEDLINE Link] .
Rojas-Suarez J, Miranda J. Coronavirus Disease-2019 in Pregnancy. Clin Chest Med . 2023 Jun. 44 (2):373-384. [QxMD MEDLINE Link] .
He YF, Liu JQ, Hu XD, Li HM, Wu N, Wang J, et al. Breastfeeding vs. breast milk transmission during COVID-19 pandemic, which is more important?. Front Pediatr . 2023. 11:1253333. [QxMD MEDLINE Link] . [Full Text] .
Weiss A, Donnachie E, Beyerlein A, Ziegler AG, Bonifacio E. Type 1 Diabetes Incidence and Risk in Children With a Diagnosis of COVID-19. JAMA . 2023 Jun 20. 329 (23):2089-2091. [QxMD MEDLINE Link] .
Lippi G, Sanchis-Gomar F, Henry BM. COVID-19 and its long-term sequelae: what do we know in 2023?. Pol Arch Intern Med . 2023. 133: [Full Text] .
Brolly J, Chadwick DR. COVID-19 infection in people living with HIV. Br Med Bull . 2023 Sep 12. 147 (1):20-30. [QxMD MEDLINE Link] . [Full Text] .
- The heart is normal in size. There are diffuse, patchy opacities throughout both lungs, which may represent multifocal viral/bacterial pneumonia versus pulmonary edema. These opacities are particularly confluent along the periphery of the right lung. There is left midlung platelike atelectasis. Obscuration of the left costophrenic angle may represent consolidation versus a pleural effusion with atelectasis. There is no pneumothorax.
- The heart is normal in size. There are bilateral hazy opacities, with lower lobe predominance. These findings are consistent with multifocal/viral pneumonia. No pleural effusion or pneumothorax are seen.
- The heart is normal in size. Patchy opacities are seen throughout the lung fields. Patchy areas of consolidation at the right lung base partially silhouettes the right diaphragm. There is no effusion or pneumothorax. Degenerative changes of the thoracic spine are noted.
- The same patient as above 10 days later.
- The trachea is in midline. The cardiomediastinal silhouette is normal in size. There are diffuse hazy reticulonodular opacities in both lungs. Differential diagnoses include viral pneumonia, multifocal bacterial pneumonia or ARDS. There is no pleural effusion or pneumothorax.
- Axial chest CT demonstrates patchy ground-glass opacities with peripheral distribution.
- Coronal reconstruction chest CT of the same patient above, showing patchy ground-glass opacities.
- Axial chest CT shows bilateral patchy consolidations (arrows), some with peripheral ground-glass opacity. Findings are in peripheral and subpleural distribution.
- Table 1. SARS-CoV-2 Monoclonal Antibodies – inactive EUAs
| Antibody | Description |
|---|---|
| Evusheld (tixagevimab/cilgavimab) | EUA for preexposure prophylaxis halted in January 2023 owing to Omicron XBB VOCs. Initial authorization was based on the phase 3 PROVENT in unvaccinated individuals with comorbidities and a retrospective cohort study of veterans who were immunosuppressed. , ] |
| Bebtelovimab | Data supporting the treatment EUA were primarily based on analyses from the phase 2 BLAZE-4 trial conducted before the emergence of the Omicron BQ.1 and BQ.1.1 VOCs. Most participants were infected with the Delta (49.8%) or Alpha (28.6%) VOCs. ] |
| Sotrovimab | EUA stopped owing to resistance to Omicron BA.2 subvariant. Initial IV and IM authorization based on COMET-ICE and COMET-TAIL studies. , ] |
| Casirivimab/imdevimab | EUA stopped in January 2022, as the Omicron variant is not susceptible. The EUA for treatment was supported by US trials and the UK RECOVERY trial. , , ] |
| Bamlanivimab/etesevimab | EUA revoked in April 2021 as the Delta VOC emerged. Initial EUA was supported by Phase 3 BLAZE-1 trial for treatment and the BLAZE-2 trial for postexposure prophylaxis. , ] |
Contributor Information and Disclosures
David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS Associate Professor of Medicine and Pediatrics, Adult and Pediatric Infectious Diseases, Rutgers New Jersey Medical School David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS is a member of the following medical societies: American Academy of HIV Medicine , American Academy of Pediatrics , American College of Physicians , American Medical Association , HIV Medicine Association , Infectious Diseases Society of America , Medical Society of New Jersey , Pediatric Infectious Diseases Society Disclosure: Nothing to disclose.
Scott J Bergman, PharmD, FCCP, FIDSA, BCPS, BCIDP Antimicrobial Stewardship Program Coordinator, Infectious Diseases Pharmacy Residency Program Director, Department of Pharmaceutical and Nutrition Care, Division of Infectious Diseases, Nebraska Medicine; Clinical Associate Professor, Department of Pharmacy Practice, College of Pharmacy, University of Nebraska Medical Center Scott J Bergman, PharmD, FCCP, FIDSA, BCPS, BCIDP is a member of the following medical societies: American Association of Colleges of Pharmacy , American College of Clinical Pharmacy , American Pharmacists Association , American Society for Microbiology , American Society of Health-System Pharmacists , Infectious Diseases Society of America , Society of Infectious Diseases Pharmacists Disclosure: Received research grant from: Merck & Co., Inc.
Keith M Olsen, PharmD, FCCP, FCCM Dean and Professor, College of Pharmacy, University of Nebraska Medical Center Disclosure: Nothing to disclose.
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Nothing to disclose.
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , American Medical Association , Association of Professors of Medicine , Infectious Diseases Society of America , Oklahoma State Medical Association , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Molly Marie Miller, PharmD Clinical Infectious Diseases Pharmacist Practitioner, Nebraska Medicine Molly Marie Miller, PharmD is a member of the following medical societies: Society of Infectious Diseases Pharmacists Disclosure: Nothing to disclose.
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- COVID-19 Variants
- COVID-19 Pulmonary Management
- COVID-19 Reinfections
- Long Coronavirus 2019 (COVID-19)
- Symptoms and Management of Coronavirus Disease 2019 (COVID-19) FAQ
- Coronavirus Disease 2019 (COVID-19)
- Coronavirus Disease 2019 (COVID-19) Triage Precautions FAQ
- Picture of COVID-19 in Europe Is Complex
- Global Consensus Reached on COVID-19 Vaccine Updates
- Beyond COVID-19: building Europe’s pandemic preparedness

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2010comirnaty-covid-19-vaccine-mRNA-pfizer-4000140Drugs Drugs COVID-19 vaccine, mRNA-Pfizer

- 2010covid-19-vaccine-adjuvanted-novavax-4000146Drugs Drugs COVID-19 vaccine, adjuvanted-Novavax
- 2010spikevax-covid-19-vaccine-mRNA-moderna-4000149Drugs Drugs COVID-19 vaccine, mRNA-Moderna
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The differences of clinical manifestations and laboratory findings in children and adults with dengue virus infection
Affiliation.
- 1 Department of Microbiology, Faculty of Public Health, Mahidol University, Bangkok, Thailand. [email protected]
- PMID: 17507286
- DOI: 10.1016/j.jcv.2007.04.006
Background: Dengue haemorrhagic fever is an important public health problem and mainly occurs in children less than 15 years of age. Recently, the incidence of the disease have increased in adults but data on clinical and laboratory presentations of those affected are limited.
Objectives: To assess and compare clinical manifestations and laboratory findings of dengue virus infected children and adults in Thailand.
Study design: A 1-year study was conducted from September 2003 to August 2004 for dengue virus infected patients admitted to Phetchabun Provincial Hospital, Thailand. Physical signs, symptoms, and laboratory features were recorded. All dengue patients were confirmed using immunochromatographic test on convalescent sera.
Results: Based on serology-confirmed dengue virus infection, there was 286 dengue patients including 15 (5.3%) dengue fever and 271 (94.7%) dengue haemorrhagic fever (DHF). Among DHF cases, clinical classifications were DHF I, 40.9%; DHF II, 43%; and DHF III or dengue shock syndrome (DSS), 10.8%. Of all dengue patients, 231 cases (80.8%) were children aged less than 15 years and 55 cases (19.2%) were adults. The highest proportion of child cases was DHF I (42.9%), whereas that of adults was DHF II (51%). Some clinical manifestations were more common in adult patients, such as petechiae, melena, headache, retro-orbital pain, joint pain, myalgia, nausea and vomiting (p-value<0.05). Signs found commonly in children were epistaxis, oliguria, and liver enlargement (p-value<0.05). Haemoconcentration, thrombocytopenia, increased alanine aminotransferase, and longer prothrombin time were found to be significantly higher in adults than in children (p-value<0.05).
Conclusions: Some clinical presentations of dengue disease and laboratory findings in adults are different from those in children. Therefore, adults as well as pediatric cases of DHF need appropriate and prompt case management to reduce the mortality rate of DHF.
PubMed Disclaimer
Similar articles
- Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection. Noisakran S, Perng GC. Noisakran S, et al. Exp Biol Med (Maywood). 2008 Apr;233(4):401-8. doi: 10.3181/0707-MR-198. Exp Biol Med (Maywood). 2008. PMID: 18367628 Review.
- Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP. Lee MS, et al. J Microbiol Immunol Infect. 2006 Apr;39(2):121-9. J Microbiol Immunol Infect. 2006. PMID: 16604244
- Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Wichmann O, et al. Trop Med Int Health. 2004 Sep;9(9):1022-9. doi: 10.1111/j.1365-3156.2004.01295.x. Trop Med Int Health. 2004. PMID: 15361117
- Clinical presentations of dengue hemorrhagic fever in infants compared to children. Kalayanarooj S, Nimmannitya S. Kalayanarooj S, et al. J Med Assoc Thai. 2003 Aug;86 Suppl 3:S673-80. J Med Assoc Thai. 2003. PMID: 14700166
- [Hemorrhagic fever and the dengue shock syndrome]. Ramos C, García H, Villaseca JM. Ramos C, et al. Salud Publica Mex. 1993 Jan-Feb;35(1):39-55. Salud Publica Mex. 1993. PMID: 8470020 Review. Spanish.
- Identifying citrus limonoids as a potential fusion inhibitor of DENV-2 virus through its in silico study and FTIR analysis. Das S, Sarma G, Panicker NJ, Sahu PP. Das S, et al. In Silico Pharmacol. 2024 Apr 25;12(1):35. doi: 10.1007/s40203-024-00207-2. eCollection 2024. In Silico Pharmacol. 2024. PMID: 38680655
- Potential Pathways and Pathophysiological Implications of Viral Infection-Driven Activation of Kallikrein-Kinin System (KKS). Coelho SVA, Augusto FM, Arruda LB. Coelho SVA, et al. Viruses. 2024 Feb 3;16(2):245. doi: 10.3390/v16020245. Viruses. 2024. PMID: 38400022 Free PMC article. Review.
- The Effect of Age on Dengue Presentation and the Diagnostic Accuracy of the 2015 Pan American Health Organization Case Criteria in a Puerto Rican Cohort. Odio CD, Sánchez-González L, Delorey M, Adams LE, Jones ES, Lorenzi O, Munoz-Jordan J, Rivera-Amill V, Paz-Bailey G. Odio CD, et al. Open Forum Infect Dis. 2023 Jul 19;10(8):ofad373. doi: 10.1093/ofid/ofad373. eCollection 2023 Aug. Open Forum Infect Dis. 2023. PMID: 37663092
- Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Zerfu B, Kassa T, Legesse M. Zerfu B, et al. Trop Med Health. 2023 Feb 24;51(1):11. doi: 10.1186/s41182-023-00504-0. Trop Med Health. 2023. PMID: 36829222 Free PMC article. Review.
- Predictive Factors for the Complications of Dengue Fever in Children: A Retrospective Analysis. Uthraraj NS, Sriraam LM, Hiriyur Prakash M, Kumar M, Palanisamy U, Chettiakkapalayam Venkatachalam KU. Uthraraj NS, et al. Cureus. 2022 Dec 27;14(12):e33027. doi: 10.7759/cureus.33027. eCollection 2022 Dec. Cureus. 2022. PMID: 36721616 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound
- PubChem Substance
LinkOut - more resources
Full text sources.
- Elsevier Science
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Get new issue alerts Get alerts
- Submit your manuscript
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Clinical manifestation and disease progression in COVID-19 infection
Tsai, Ping-Hsing a ; Lai, Wei-Yi a ; Lin, Yi-Ying a ; Luo, Yung-Hung b,c ; Lin, Yi-Tsung c,d ; Chen, Hsiao-Kang c,e ; Chen, Yuh-Min b,c ; Lai, Yi-Chun c,e ; Kuo, Li-Chiao c,e ; Chen, Shew-Dan c,e ; Chang, Kao-Jung a,c ; Liu, Cheng-Hsuan a,c ; Chang, Shih-Chieh c,e,* ; Wang, Fu-Der c,d,* ; Yang, Yi-Ping a,c,f,*
a Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
b Department of Chest Medicine, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
c School of Medicine, National Yang-Ming Medical University, Taipei, Taiwan, ROC
d Division of Infectious Diseases, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
e Department of Medicine, National Yang-Ming University Hospital, Yilan, Taiwan, ROC
f Institute of Food Safety and Health Risk Assessment, School of Pharmaceutical Sciences, National Yang-Ming University, Taipei, Taiwan, ROC
Received July 10, 2020; accepted August 4, 2020.
Author Contributions: Dr. Ping-Hsing Tsai, Dr. Yi-Ying Lin, and Dr. Wei-Yi Lai contributed equally to this study.
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
* Address correspondence. Dr. Shih-Chieh Chang, Department of Medicine, National Yang-Ming University Hospital, 152, Xinmin Road, Yilan 260, Taiwan, ROC. E-mail address: [email protected] (S.-C. Chang); Dr. Fu-Der Wang, Division of Infectious Disease, Department of Medicine, Taipei Veterans General Hospital, 201, Section 2, Shi-Pai Road, Taipei 112, Taiwan, ROC. E-mail address: [email protected] (F.-D. Wang); Dr. Yi-Ping Yang, Department of Medical Research, Taipei Veterans General Hospital, 201, Section 2, Shi-Pai Road, Taipei 112, Taiwan, ROC. E-mail address: [email protected] (Y.-P. Yang).
This is an open access article under the CC BY-NC-ND license ( http://creativecommons.org/licenses/by-nc-nd/4.0/ )
Coronavirus disease 2019 (COVID-19) is mainly an infectious disease of the respiratory system transmitted through air droplets, and pulmonary symptoms constitute main presentations of this disease. However, COVID-19 demonstrates a clinically diverse manifestation ranging from asymptomatic presentation to critically illness with severe pneumonia, acute respiratory distress syndrome, respiratory failure, or multiple organ failure. Accumulating evidences demonstrated that COVID-19 has extrapulmonary involvement, including neurological, smelling sensation, cardiovascular, digestive, hepatobiliary, renal, endocrinologic, dermatologic system, and others. Over a third of COVID-19 patients manifest a wide range of neurological symptoms involving the central/peripheral nervous system. Underlying cardiovascular comorbidities were associated with detrimental outcomes, meanwhile the occurrence of cardiovascular complications correlate to poor survival. Gastrointestinal symptoms frequently occur and have been associated with a longer period of illness. Impaired hepatic functions were associated with the severity of the disease. Higher rate of acute kidney injury was reported in critically ill patients with COVID-19. Endocrinologic presentations of COVID-19 include exacerbating hyperglycemia, euglycemic ketosis, and diabetic ketoacidosis. The most common cutaneous manifestation was acro-cutaneous (pernio or chilblain-like) lesions, and other skin lesions consist of maculopapular rash, vesicular lesions, livedoid/necrotic lesions, exanthematous rashes, and petechiae. This review article summarized the general clinical signs and symptoms, radiologic features, and disease manifestation with progression in patients with COVID-19.
1. INTRODUCTION
Coronaviruses with RNA club-shaped spikes on the viral surface are one of the largest among RNA viruses. 1 The structure of coronaviruses consists of enveloped surface, a nucleocapsid, and single-stranded RNA genome. In the 1930s, the first coronaviruses were discovered when infectious bronchitis virus caused an acute respiratory tract infection. 2 In the 1940s, mouse hepatitis virus and transmissible gastroenteritis virus were identified as the first coronaviruses in mammals. The first human coronaviruses were identified in the 1960s. 3 Two human coronaviruses, coronavirus 229E and OC43, cause upper respiratory tract infections, nasal symptoms, cough, and pharyngitis. According to previous reports, human coronavirus usually results in a low mortality rate and rarely causes critical illness. 4 , 5 Nonetheless, the severe acute respiratory syndrome coronavirus (SARS-CoV) may cause more severe symptoms which are not usually caused by known coronavirus. In 2003, SARS-CoV caused pulmonary infection, and the mortality rate reached approximately 10%. 4 Therefore, the World Health Organization (WHO) reported that SARS-CoV might cause severe acute infectious disease in the world. In 2012, another severe coronavirus infection was discovered in the Middle East. The discovery process was similar to SARS. A patient had severe unexplained pneumonia and was later found to be caused by a new type of coronavirus. The novel virus, Middle East respiratory syndrome coronavirus (MERS-CoV), was first found in a patient from Saudi Arabia with severe unexplained pneumonia. 4 The MERS-CoV outbreak in 2012-2015 affected 25 countries (mostly Saudi Arabia and South Korea) with 2506 cases and 862 deaths (34% fatality rate). 5 In December 2019, a new type of pneumonia with unknown cause occurred in Wuhan, China and started to spread rapidly. The Chinese government identified an extreme pathogenic coronavirus on January 9, 2020 when WHO named such novel SARS-CoV. People also called this disease of SARS-CoV-2 as COVID-19 or Wuhan pneumonia. 6 The number of cases increased significantly in January 2020, and the global statistics as of August 4 had 18 166 298 confirmed cases, and the death toll had reached 690 953 all of which exceeded the SARS record of that year. Although COVID-19 was initially limited to China, it has rapidly spread to >180 countries because of its highly contagious pathogen. This pandemic infection is continuously spreading across the world with exponentially increasing death toll. It is important to clarify and understand the clinical manifestation and disease progression in COVID-19 pandemic infection.
2. CLINICAL SYMPTOM AND DISEASED MANIFESTATION IN COVID-19
In recent decades, two previous coronavirus outbreaks have been reported, and the clinical manifestations of SARS-CoV-2, in comparison with SARS-CoV and MERS-CoV, are summarized in the Table 1 . 7–9 Pulmonary infectious diseases, such as SARS and MERS, present a major threat to public health. 10 In late December 2019, several new SARS-like pneumonia cases were reported in a Chinese city of Wuhan. A new coronavirus SARS-CoV-2 was rapidly identified as the etiologic pathogen leading to the outbreaks of COVID-19. 10 This novel coronavirus infection demonstrated human-to-human transmission of SARS-CoV-2 among healthcare practitioners in a Wuhan hospital. 11 , 12 The global spread and lethality of SARS-CoV-2 lead to primary challenges for the worldwide healthcare system. SARS-CoV-2 is highly contagious, and the incubation period of COVID-19 has been reported around 1 to 14 days (interquartile range [IQR], 2-7 days). 11 , 13–15
| SARS-CoV-2 | SARS-CoV | MERS-CoV | |
|---|---|---|---|
| Start time | December 2019 | November 2002 | June 2012 |
| Initial area | Wuhan, China | Guangdong, China | Jedda, Saudi Arabia |
| Confirmed patients | 214 894 | 8096 | 2494 |
| Mean age (range) | 47-56 (0.5-92) | 39.9 (1-91) | 56 |
| Male | 58%-75% | 44% | 76.70% |
| HCWs | 2%-29% | 23.10% | 9.80% |
| Symptoms | |||
| Fever | 83%-98% | 99%-100% | 98% |
| Dry cough | 59%-78% | 29%-75% | 47% |
| Dyspnea | 19%-55% | 40%-42% | 72% |
| Diarrhea | 2%-10% | 20%-25% | ... |
| Sore throat | 5%-17% | 13%-25% | ... |
| Ventilator support | 2%-12% | 14%-20% | 80% |
| ARDS | 3%-29% | 20%-30% | Case reports |
| Mortality | 690 953 (3.8%) | 744 (10%) | 858 (37%) |
The common symptoms of SARS-CoV-2 infection are fever (83%-98%), cough (50%-82%), fatigue (25%-44%), shortness of breath (19%-55%), and muscle soreness (11%-44%). 14 , 15 Some patients may suffer from sputum production, rhinorrhea, chest tightness, sore throat, nausea, vomiting, diarrhea, headache, ageusia, and anosmia a few days before the occurrence of fever, suggesting that fever is critical but not the only initial symptom of infection. 14 Some patients only had a mild fever, mild fatigue, or even no symptoms. 13 , 15–17
About 80% of SARS-CoV-2 infections in ambulatory patients manifest as a mild respiratory illness and could usually be managed by outpatient care. About 15% of patients need inpatient care for moderate to severe pneumonia. 18 Among the hospitalized patients, the median time from initial symptoms to the occurrence of dyspnea is five days (IQR, 1-10 days), and the median time to be hospitalized is 5 days (IQR, 4-8 days). 13 Disease course may show rapid progression to multiple organ failure and even death in severely ill patients. 11 , 13 Patients (3%-29%) may require admission to an intensive care unit (ICU) for the management of complications, including hypoxemic respiratory failure or hypotension. Overall mortality rate appears to be approximately 3.8% 11 , 13 , 14 , 16 , 19 ( Table ). Some patients with dyspnea and hypoxemia could quickly progress into acute respiratory distress syndrome (ARDS), septic shock, blood clotting dysfunction, and even multiple organ failure in 1 week. 16 , 20 The median time to ARDS is 8 days (IQR, 6-12 days). The high incidence of multiple organ failure is one of the features of COVID-19. 15 Most of the critically ill patients are related to comorbidities, including cardiovascular disease, hypertension, diabetes, and renal disease. Moreover, the mortality rates are relatively high in COVID-19 patients with these comorbidities. 21 The severity of COVID-19 patients is also related to age, and the death toll was concentrated among those aged ≥40. Studies have shown that morbidity rate is lower in children and infants than in adults. 22 , 23
Infected patients may have lymphopenia which is the most common laboratory manifestation, normal or lower white blood cell counts, or thrombocytopenia, with elevated C-reactive protein level. 11 , 13 , 14 , 16 Fever, lymphopenia, or leukopenia with the symptoms of upper respiratory tract is highly suspected to be the manifestations of patients with COVID-19, which is supported by the traveling history to the endemic area or close exposure history. The asymptomatic individuals in incubation period are critical sources of infection, which results in difficulties in the epidemic prevention and disease control. 24 , 25
Currently, respiratory droplets are believed to be the primary route of transmission; however, transmission via the ocular surface should also be carefully prevented because the conjunctival epithelium is vulnerable to the infectious droplets and body fluid. 26 The fecal-oral route is suspected due to the identification of SARS-CoV-2 nucleic acids in the stool specimens from COVID-19 patients with pneumonia and abdominal symptoms. 27 Additionally, vertical transmission between mothers and infants has been reported as a potential transmission route according to the finding of a 30-hour-old newborn tested positive for SARS-CoV-2 infection. Despite tremendous efforts on investigating this pathogen, the contagious period of SARS-CoV-2 still varies in different reports. 19 Furthermore, the incidental hosts of SARS-CoV-2 are not yet clear to researcher. The unconfirmed incidental hosts may lead to repeated zoonotic transmission and the potentially underestimated contagious period also constitutes a significant challenge to the epidemic prevention. Additionally, the human-to-human transmission of SARS-CoV-2 occurs mainly in communities and between family members, suggesting that this pathogen could rapidly spread before the occurrence of symptoms. Another report showed that patients who have recovered from COVID-19 after two consecutive real-time reverse transcriptase-polymerase chain reaction tests turned out to demonstrate positive results a few days later. 28 Therefore, infected patients could be contagious before the onset of symptoms and after treatment of COVID-19. Besides, the optimal strategy for hospital discharge and cessation of quarantine remains to be elucidated in order to achieve superior disease control.
3. RADIOLOGICAL FEATURES IN COVID-19
Chest X-ray (CXR) and computed tomography (CT) scan are necessary radiological examinations for early identification of COVID-19. 29 The radiological features of COVID-19 pneumonia are similar to influenza, SARS-CoV, and MERS-CoV pneumonia. 30–34 CXR of patients with COVID-19 pneumonia may reveal unilateral, bilateral, peripheral, and patchy opacities. In the early stage of COVID-19 pneumonia, CXR may not be able to detect abnormal findings, because CXR is not sensitive for ground-glass opacity (GGO). 35
Severe COVID-19 cases may show the bilateral multiple consolidative lesion in the CXR radiograph. Besides, CT-scan radiograph can provide more information to assist the COVID-19 diagnosis. According to the experience on CT scan, COVID-19 patients exhibit bilateral pulmonary GGOs and consolidative lesions in the lung parenchyma. In addition, CT scan detects the feature of multiple pulmonary nodules, pleural effusion, lymphadenopathy, and absence of pulmonary cavitary lesion. Clinical doctors can efficiently catch pneumonia from early stage of COVID-19 patient according to CT scans. 15 Hence, CT scan provides a rapid evaluation of progression and severity of COVID-19. However, COVID-19 pneumonia and other pulmonary infectious diseases are sometimes difficult to distinguish from CT findings. Moreover, the early stage of COVID-19 patients might present no significant difference in the pulmonary image. Even nucleic acid detection sometimes show a false negative result due to the low virus abundance or inefficient sampling. As a result, the combination of nucleic acid detection assay and CT imaging is useful for the precise identification of COVID-19. 36–40
4. EXTRAPULMONARY MANIFESTATIONS OF COVID-19
Although many COVID-19 patients suffered from respiratory symptoms, SARS-CoV-2 can also lead to several extrapulmonary manifestations, including thromboembolic complications, cardiac injury and arrhythmia, acute coronary syndromes, acute renal injury, gastrointestinal (GI) symptoms, liver function impairment, hyperglycemia and diabetic ketosis, neurologic deficits, and dermatologic complications. 41 We summarized the extrapulmonary organ-specific manifestation and pathophysiology for patients with COVID-19 to facilitate the understanding and monitoring of various manifestations in COVID-19 patients.
4.1. Neurologic manifestations
Neurologic presentations in COVID-19 patients are shown in the Fig. 1 . Neurologic symptoms were reported in 36% of the patients with severe COVID-19. 42 The mild neurological symptoms in COVID-19 patients include headache, dizziness, anorexia, anosmia, myalgia/fatigue, and ageusia. 11 , 13 , 14 More-severe manifestations consist of acute stroke, 43 , 44 confusion or impaired consciousness, 42 , 45 acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome), 46 , 47 meningoencephalitis, acute necrotizing encephalopathy including the brainstem and basal ganglia. 48 , 49

4.2. Thromboembolic manifestations
The thromboembolic manifestations of COVID-19 are shown in the Fig. 1 . Thromboembolic complications were reported in up to 30% of patients from ICUs. 50 , 51 Emerging evidence revealed the occurrence of thrombosis in intravenous catheters and extracorporeal circuits, acute myocardial infarction, acute limb ischemia, and cerebral vascular events, in patients with severe disease. 43 , 52–55 High rates of thromboembolic events were reported in severely ill patients with COVID-19 (17%-22%) who had received prophylactic anticoagulant therapies. 54 , 56–58 The rates of pulmonary emboli were reported to be notably higher in ICU patients with COVID-19 than those without (20.6% vs 6.1%, respectively). 59 Moreover, several studies demonstrated high rates of thromboembolic complications in critically ill patients with COVID-19 who were routinely screened for the thrombotic disease, ranging from 69% to 85%, despite prophylactic anticoagulant therapies. 41
4.3. Cardiovascular manifestations
Clinical manifestations of COVID-19 pertaining to the cardiovascular system are shown in the Figure . COVID-19 can lead to direct and indirect cardiovascular injury, including acute coronary syndrome, myocardial injury, cardiomyopathy, cardiac arrhythmias, cor pulmonale, cardiogenic shock, and thromboembolic complications. 60 , 61 Around 20% to 30% of inpatients with COVID-19 were reported to have myocardial injury, 62 and higher degree of troponin elevations was associated with more severe complications and worse outcomes. 62 , 63 Biventricular cardiomyopathy occurred in approximately 7% to 33% of critically ill patients. 64 , 65 Hospitalized patients (17%-44%) with COVID-19 were reported to have cardiac arrhythmias, including atrial fibrillation, ventricular arrhythmias, and heart block. 13 Prolonged QTc was found in 6% of COVID-19 patients in a multicenter study. In an Italian cohort, the rate of out-of-hospital cardiac arrest increased by around 60% during COVID-19 pandemic compared with the similar period in 2019.
4.4. Renal manifestations
Clinical features pertaining to the kidney damage are shown in the Figure. The incidence of acute kidney injury (AKI) in hospitalized patients ranged from 0.5% to 37% with a median onset time of 7 to 14 days during admission. 16 , 66–69 Higher rate of AKI, ranging from 78% to 90%, was reported in critically ill patients in New York City; an 31% of ICU patients with COVID-19 required renal replacement therapy. 69–73 Up to 87% of critically ill patients had proteinuria in study. 71 Higher mortality rate of COVID-19 was also reported in patients with end-stage renal disease and kidney transplant recipients compared with those without. 74–76
4.5. GI manifestations
GI symptoms occur in some patients with COVID-19 ( Figure ). GI symptoms in COVID-19 patients have been associated with a longer duration of illness, with the incidence of 12% to 61%. 77–80 The reported GI manifestations include anorexia (21%-35%), nausea/vomiting (7%-26%), diarrhea (9%-34%), and abdominal pain (3%). 79 , 80 The occurrence of GI symptoms has been associated with a 70% increased risk of identification of COVID-19 in a research, 81 and GI bleeding was rare in patients with prolonged mechanical ventilation or thrombocytopenia. 81
4.6. Hepatobiliary manifestations
Clinical presentations of COVID-19 regarding the hepatobiliary system are shown in the Figure. In a systemic review, the prevalence of liver function impairment is around 19% (95% CI, 9%-32%) and associated with the severity of disease. 79 Patients usually have elevated transaminases which are less than five times the upper limit of normal and severe acute hepatitis is rarely reported. 82 , 83 Critically ill patients (14%-53%) with COVID-19 was reported to have hepatocellular injury. 16 , 65–67 , 84 Some studies reported that elevated bilirubin was associated with the severity of disease and deterioration to critical illness. 85 , 86
4.7. Endocrinologic manifestations
Endocrinologic presentations in COVID-19 patients are shown in the Figure. The abnormalities of glycemic metabolism in hospitalized patients with COVID-19 include exacerbating hyperglycemia, euglycemic ketosis, and diabetic ketoacidosis. 16 , 18 , 87 A study in China reported that 6.4% of hospitalized patients had ketosis without the presence of fever or diarrhea. 88
4.8. Dermatologic manifestations
Dermatologic manifestations of COVID-19 are shown in the Figure. A single-center study reported that 20% of hospitalized patients presented with dermatologic symptoms, including erythematous rash, urticaria, and chickenpox-like vesicles, and around 44% of dermatological findings occurred at disease onset. 89 The most common cutaneous manifestation was acro-cutaneous (pernio or chilblain-like) lesions in a systemic review, and other skin lesions consist of maculopapular rash, vesicular lesions, livedoid/necrotic lesions, exanthematous rashes, and petechiae in patients with COVID-19. 90–93
In conclusion, this review introduces the current status of knowledge on the global pandemic and clinical features of COVID-19. Given that SARS-CoV-2 is the third introduction of a deadly coronavirus into human society, after SARS-CoV in 2003 and MERS-CoV in 2012, respectively, various clinical manifestations of these three viruses were compared. Whereas SARS-CoV-2 is featured by relatively lower lethality compared with SARS-CoV and MERS-CoV, it has demonstrated significantly broader range of clinical presentation and apparent higher contagiousness. The COVID-19 outbreak started from China and then spread to other countries all over the world. Currently, the persistent pandemic still locates in Europe and North America. We also summarized the image findings for COVID-19.
Beside the deadly pulmonary complications of SARS-CoV-2, extrapulmonary spread, including neurological, smelling sensation, cardiovascular, digestive, hepatobiliary, renal, endocrinologic, and dermatologic system, are increasingly being appreciated. SARS-CoV-2 has undoubtedly catched the world’s attention at the beginning of 2020 by posing a significant challenge toward the public health system. Still, despite the rapid development of countermeasures looming on the horizon, further investigation and development of drugs and vaccines are in urgent need, as according to our current knowledge of coronaviruses, where and when the next outbreak would take place is unpredictable. Therefore, only by equipping ourselves with adequate understanding and diverse treatment modalities can we devise appropriate strategies against the constantly changing nature of novel coronaviruses.
ACKNOWLEDGMENTS
This study was funded by Ministry of Science and Technology (106-3114-B-010-002, 107-2633-B-009-003, and 109-2320-B-075-008), Taipei Veterans General Hospital (V107E-002-2 and V108D46-004-MY2-1), and Yen Tjing Ling Medical Foundation (CI-109-26).
- Cited Here |
- Google Scholar
Coronavirus; COVID-19; Severe acute respiratory syndrome coronavirus 2
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Otoprotection against aminoglycoside- and cisplatin-induced ototoxicity..., leber’s hereditary optic neuropathy: update on the novel genes and therapeutic..., lymphovenous shunts in the treatment of lymphedema, connecting the dots: sarcopenia’s roles on chronic condition management toward..., winners of the 2022 honor awards for excellence at the annual meeting of the....
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Signs and Symptoms
From the Scientific Publications Division, American Medical Association, Chicago.
Most physicians, if asked to distinguish between signs and symptoms, would reply in a fashion something like this:
A symptom is a manifestation of disease apparent to the patient himself, while a sign is a manifestation of disease that the physician perceives. The sign is objective evidence of disease; a symptom, subjective. Symptoms represent the complaints of the patient, and if severe, they drive him to the doctor's office. If not severe, they may come to light only after suitable questions. The patient perceives, for example, subjective pains and discomforts [Doctor, I have a bad headache], or disturbances of function [Doctor, I can't move my arm the way I used to], or some simple appearance [Doctor, I have had this rash for the past ten days and I'm worried about it].
But the physician, as a skilled observer, can discern what the patient cannot. He can look, palpate and percuss,
King LS. Signs and Symptoms. JAMA. 1968;206(5):1063–1065. doi:10.1001/jama.1968.03150050051011
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 72, Issue 10
- The multifaceted clinical presentations and manifestations of Erdheim–Chester disease: comprehensive review of the literature and of 10 new cases
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Giulio Cavalli 1 ,
- Barbara Guglielmi 1 ,
- Alvise Berti 1 ,
- Corrado Campochiaro 1 ,
- Maria Grazia Sabbadini 1 , 2 ,
- Lorenzo Dagna 1 , 2
- 1 Vita-Salute San Raffaele University, Milan , Italy
- 2 The Unit of Medicine and Clinical Immunology , San Raffaele Scientific Institute, Milan , Italy
- Correspondence to Professor Lorenzo Dagna, Unit of Medicine and Clinical Immunology, Vita-Salute San Raffaele University, San Raffaele Scientific Institute, Via Olgettina, 60, Milano I-20132, Italy; lorenzo.dagna{at}unisr.it
Objectives Erdheim–Chester disease (ECD) is a rare inflammatory disorder characterised by organ infiltration by non-Langerhans’ histiocytes. Although rare, ECD is clearly an overlooked diagnosis. No data specifically addressing the most frequent presentations of ECD at the time of onset in a large cohort of patients are currently available.
Methods We reviewed all the published cases in the English literature of histologically-confirmed ECD. We excluded reports in which data regarding onset and diagnosis were not univocal, as well as repeated reports of the same case(s). We also included in the analysis 10 new unpublished patients from our cohort. We analysed the disease presentation with particular regard to the manifestations that induced patients to seek medical attention and their subsequent evolution.
Results In the cumulative cohort of 259 cases, ECD predominantly presented with skeletal symptoms, diabetes insipidus, neurological and constitutional symptoms. Diabetes insipidus and constitutional symptoms, if not present at onset, seemed to only seldom develop. There were differences in ECD presentation and course among different age groups of patients.
Conclusions Physicians should be aware of the extraordinarily heterogeneous clinical presentations and manifestations of ECD in order to include ECD in the differential diagnosis of several conditions.
- Epidemiology
- Inflammation
- Autoimmune Diseases
https://doi.org/10.1136/annrheumdis-2012-202542
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:
- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Collections
- Supplements
- InSight Papers
- BSR Registers Papers
- Virtual Roundtables
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About Rheumatology
- About the British Society for Rheumatology
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, conclusions, supplementary material, data availability.
- < Previous
Myocarditis in anti-synthetase syndrome: clinical features and diagnostic modalities
G.D.L. and C.C. contributed equally.
- Article contents
- Figures & tables
- Supplementary Data
Giacomo De Luca, Corrado Campochiaro, Anna Palmisano, Elisa Bruno, Davide Vignale, Giovanni Peretto, Simone Sala, Arianna Ferlito, Maria Bernardette Cilona, Antonio Esposito, Marco Matucci-Cerinic, Lorenzo Dagna, Myocarditis in anti-synthetase syndrome: clinical features and diagnostic modalities, Rheumatology , Volume 63, Issue 7, July 2024, Pages 1902–1910, https://doi.org/10.1093/rheumatology/kead541
- Permissions Icon Permissions
Myocarditis is an overlooked manifestation of anti-synthetase syndrome (ASS). Our study describes the clinical and instrumental features of ASS myocarditis and evaluates the performance of cardiac MRI (CMRI) with mapping techniques in assisting diagnosis of ASS myocarditis.
Data from patients with ASS were retrospectively analysed. CMRI data for patients diagnosed with myocarditis, including late gadolinium enhancement (LGE), T2 ratio, T1 mapping, extracellular volume (ECV) and T2 mapping, were reviewed. Myocarditis was defined by the presence of symptoms and/or signs suggestive for heart involvement, including increased high-sensitive troponin T (hs-TnT) and/or N-terminal pro-brain natriuretic peptide (NT-proBNP), and at least an instrumental abnormality. The clinical features of patients with ASS with and without myocarditis were compared. A P -value of <0.05 was considered statistically significant.
Among a cohort of 43 patients with ASS [median age 58 (48.0–66.0) years; females 74.4%; anti-Jo1 53.5%], 13 (30%) were diagnosed with myocarditis. In 54% of those 13 patients, myocarditis was diagnosed at clinical onset. All patients with ASS with myocarditis had at least one CMRI abnormality: increased ECV in all cases, presence of LGE in 91%, and increased T1 and T2 mapping in 91%. The 2009 Lake Louise criteria (LLC) were satisfied by 6 patients, and the 2018 LLC by 10 patients. With the updated LLC, the sensitivity for myocarditis improved from 54.6% to 91.0%. Patients with ASS with myocarditis were more frequently males (53% vs 13%; P = 0.009) with fever (69% vs 17%; P = 0.001), and had higher hs-TnT [88.0 (23.55–311.5) vs 9.80 (5.0–23.0) ng/l; P < 0.001], NT-proBNP [525.5 (243.5–1575.25) vs 59.0 (32.0–165.5; P = 0.013) pg/ml; P = 0.013] and CRP [7.0 (1.7–15.75) vs 1.85 (0.5–2.86) mg/l; P = 0.011] compared with those without myocarditis.
In ASS, myocarditis is frequent, even at clinical onset. Patients with ASS with myocarditis frequently presented with fever and increased CRP, suggesting the existence of an inflammatory phenotype. The use of novel CMRI mapping techniques may increase diagnostic sensitivity for myocarditis in ASS.
Myocarditis is frequent in anti-synthetase syndrome (ASS), even at clinical onset, and is associated with peculiar clinical features.
Detection of myocarditis in ASS even at the subclinical stage is crucial to allow prompt therapeutic intervention.
Cardiac MRI mapping techniques can improve our ability to detect myocardial inflammation in patients with ASS with myocarditis.
Anti-synthetase syndrome (ASS) is a rare immune-mediated systemic disease that belongs to the idiopathic inflammatory myopathies (IIMs). ASS is characterized by the presence of any one of a number of mutually exclusive autoantibodies directed against amino-acyl transfer RNA-synthetases [ 1 ]. The syndrome has a broad spectrum of clinical features, including interstitial lung disease (ILD), myositis, arthritis/arthralgia, RP, fever, mechanic’s hands, and skin rashes [ 1 ]. In patients with ASS, myocarditis is seldom diagnosed [ 2 ]. In patients with autoimmune diseases, the diagnosis of myocarditis is a challenge. This is due to the clinical presentation, which is frequently subclinical and not specific, and the absence of a definite diagnostic algorithm [ 3–6 ]. An early diagnosis of myocarditis is essential for prompt commencement of immunosuppressive therapy, avoiding early casualties and late-stage cardiac complications. Thus, in patients with ASS, a comprehensive characterization of clinical and instrumental features is crucial for the early and even subclinical recognition of myocarditis.
Cardiac MRI (CMRI) is the tool of choice for the non-invasive diagnosis of myocarditis and for the morpho-functional and structural characterization of the myocardium [ 7–9 ]. It is widely used for diagnosis and monitoring inflammatory cardiomyopathies and for the characterization of myocardial inflammation and myocardial fibrosis [ 7–11 ]. The presence of at least two out of three ‘Lake Louise criteria‘ (LLC) is required for the CMRI diagnosis of myocarditis [ 8 ]. However, in CTDs traditional CMRI has limitations in detecting myocarditis, and there is a risk of false-negative results in patients with coexisting myositis [ 12 ]. Novel CMRI techniques, which include T1 mapping, T2 mapping, and extracellular volume (ECV) quantification, can, however, be incorporated into the revised LLC and may overcome these limitations [ 8 ].
The aims of the present study were: (1) to comprehensively describe the clinical and instrumental features of ASS myocarditis, (2) to investigate the clinical features associated with myocarditis in patients with ASS, and (3) to investigate the performance of CMRI with mapping techniques in detecting myocardial inflammation.
Data from patients diagnosed with ASS according to Solomon’s criteria [ 13 ] and followed at the IRCCS San Raffaele Hospital (Milan, Italy) between December 2016 and December 2022 were retrospectively reviewed.
The study is in agreement with the recommendations of Declaration of Helsinki. The Institutional Review Board at San Raffaele Hospital specifically approved the study, and written informed consent to participation was obtained from each patient.
All enrolled patients underwent a comprehensive evaluation of their disease characteristics, including: disease onset and duration, autoantibody profile, inflammatory markers (ESR, CRP), presence of skeletal myositis, arthritis, fever, ILD and/or skin rashes, and traditional cardiovascular risk factors. All patients with ASS underwent the same evaluation, which is described in the Supplementary Material , available at Rheumatology online.
ASS with myocarditis was defined by ASS plus the presence of symptoms and/or signs of cardiac involvement (dyspnea, palpitations, chest pain, signs of congestive heart failure) associated with increased serum levels of high-sensitivity troponin T (hs-TnT) and/or N-terminal pro-brain natriuretic peptide (NT-proBNP) and at least an instrumental sign of cardiac involvement from the 24 h-ECG holter and/or echocardiography and/or CMRI.
The predominant signs or symptoms that led to the suspicion of myocarditis were considered for the definition of the clinical presentation pattern (see Supplementary Material , available at Rheumatology online) [ 14 ].
All patients with ASS diagnosed with myocarditis, as previously defined, underwent a further comprehensive non-invasive cardiologic evaluation with CMRI with mapping techniques. CMRI was performed on 1.5-T systems (Achieva dStream, Philips Medical Systems, Eindhoven, The Netherlands) equipped with a 32-channel phased-array coil [ 7 , 11 ]. CMRI analysis was performed as previously described [ 7 , 11 ] and included: volume and function, early and late gadolinium enhancement (EGE and LGE, respectively), T2-weighted Short-tau Inversion Recovery(STIR) oedema-sensitive images, T1 mapping , T2 mapping and ECV (see Supplementary Material , available at Rheumatology online).
In selected cases, an endomyocardial biopsy (EMB) was performed, according to current guidelines [ 5 , 6 , 15–20 ], to confirm the diagnosis and to rule out a viral aetiology (see Supplementary Material , available at Rheumatology online).
During the follow-up, myocarditis-related complications, such as cardiac death, end-stage heart failure, malignant arrhythmias, or need for an implantable cardioverter defibrillator (ICD), according to current guidelines [ 21 ], were recorded.
Statistical analysis
SPSS22.0 (IBM Corp., Armonk, NY, USA) was employed in analysing the data. Non-parametric tests were employed in the statistical analysis. Categorical variables were analysed using the Fisher’s exact test, and continuous variables were analysed using the Mann–Whitney U test. Continuous variables were reported as median and interquartile (IQR) range (25th–75th percentiles), while categorical variables were expressed as numbers and percentages. Spearman’s rank correlation was utilized to correlate the various disease parameters. Univariable and multivariable logistic regression analyses [expressed in terms of odds ratio (OR) and 95% CI] were applied to analyse the predictive ability of baseline variables for myocardial involvement. A P -value of <0.05 was accepted as indicating statistical significance.
Patient demographics and clinical characteristics
Overall, 43 AAS patients were identified (females 74.4%, median age 58 [48.0–66.0] years). Their demographic, clinical and laboratory characteristics are reported in Table 1 .
Demographic and clinical characteristics of patients with ASS
| . | Patients with ASS ( = 43) . |
|---|---|
| Age (years), median [IQR] | 58 [48–66] |
| Females, (%) | 32 (74.4) |
| Disease duration (months), median [IQR] | 6 [4–13] |
| Autoantibodies | |
| ANA positivity, (%) | 28 (65.2) |
| Anti-Jo1, (%) | 23 (53.5) |
| Anti-PL-7, (%) | 7 (16.3) |
| Anti-PL-12, (%) | 6 (14.0) |
| Anti-EJ, (%) | 3 (7.0) |
| Anti-SSA, (%) | 20 (46.5) |
| Anti-Ro52, (%) | 9 (20.9) |
| Anti-Ro60, (%) | 4 (9.3) |
| Anti-SSB, (%) | 1 (2.3) |
| Clinical features | |
| Overall skeletal involvement , (%) | 43 (100) |
| Interstitial lung disease , (%) | 31 (72.1) |
| Myositis , (%) | 23 (53.5) |
| Muscle weakness, (%) | 23 (53.5) |
| Dyspnea, (%) | 21(48.8) |
| Arthralgias, (%) | 16 (37.2) |
| Fever, (%) | 16 (37.2) |
| Raynaud phenomenon, (%) | 15 (34.9) |
| Skin rashes, (%) | 15 (34.9) |
| Myocarditis , (%) | 13 (30.2) |
| Myalgias, (%) | 13 (30.2) |
| Arthritis , (%) | 13 (30.2) |
| Scleroderma pattern at NVC, (%) | 10 (23.2) |
| Dysphagia, (%) | 9 (20.9) |
| Mechanic hands, (%) | 9 (20.9) |
| Palpitations, (%) | 4 (9.3) |
| Weight loss, (%) | 4 (9.3) |
| Calcinosis, (%) | 1 (2.3) |
| MMT score, median [IQR] | 76 [70–80] |
| Laboratory features | |
| Increased CPK, (%) | 23 (53.5) |
| CPK serum level, median [IQR] (UI/L) | 283 [85–1668] |
| CPK serum level in patients with myositis, median [IQR] (UI/L) | 1169 [329.75–4506.75] |
| LDH serum level (mU/ml), median [IQR] | 280.5 [244.25–584] |
| Increased aldolase, (%) | 13 (30.2) |
| Aldolase serum level (UI/L), median [IQR] | 7.80 [5.15–34.6] |
| Aldolase serum level (UI/L), in patients with myositis, median [IQR] | 9.80 [7.60–41.0] |
| Increased hs-TnT, (%) | 17 (39.5) |
| Hs-TnT serum levels, median [IQR] (ng/l) | 23.55 [7.95– 172.75] |
| Hs-TnT serum levels in patients with myocarditis, median [IQR] (ng/l) | 88.0 [23.55– 311.5] |
| Increased NT-proBNP, (%) | 15 (34.9) |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 102.0 [42.0– 407.0] |
| NT-proBNP serum levels in patients with myocarditis, median [IQR] (pg/ml) | 525.5 [243.5– 1575.25] |
| Increased CPR, (%) | 12 (27.9) |
| Increased ESR, (%) | 15 (34.9) |
| CRP serum levels, median [IQR] (mg/l) | 2.0 [1.0–8.5] |
| ESR serum levels, median [IQR] (mm/1h) | 26 [8.0–39.5] |
| . | Patients with ASS ( = 43) . |
|---|---|
| Age (years), median [IQR] | 58 [48–66] |
| Females, (%) | 32 (74.4) |
| Disease duration (months), median [IQR] | 6 [4–13] |
| Autoantibodies | |
| ANA positivity, (%) | 28 (65.2) |
| Anti-Jo1, (%) | 23 (53.5) |
| Anti-PL-7, (%) | 7 (16.3) |
| Anti-PL-12, (%) | 6 (14.0) |
| Anti-EJ, (%) | 3 (7.0) |
| Anti-SSA, (%) | 20 (46.5) |
| Anti-Ro52, (%) | 9 (20.9) |
| Anti-Ro60, (%) | 4 (9.3) |
| Anti-SSB, (%) | 1 (2.3) |
| Clinical features | |
| Overall skeletal involvement , (%) | 43 (100) |
| Interstitial lung disease , (%) | 31 (72.1) |
| Myositis , (%) | 23 (53.5) |
| Muscle weakness, (%) | 23 (53.5) |
| Dyspnea, (%) | 21(48.8) |
| Arthralgias, (%) | 16 (37.2) |
| Fever, (%) | 16 (37.2) |
| Raynaud phenomenon, (%) | 15 (34.9) |
| Skin rashes, (%) | 15 (34.9) |
| Myocarditis , (%) | 13 (30.2) |
| Myalgias, (%) | 13 (30.2) |
| Arthritis , (%) | 13 (30.2) |
| Scleroderma pattern at NVC, (%) | 10 (23.2) |
| Dysphagia, (%) | 9 (20.9) |
| Mechanic hands, (%) | 9 (20.9) |
| Palpitations, (%) | 4 (9.3) |
| Weight loss, (%) | 4 (9.3) |
| Calcinosis, (%) | 1 (2.3) |
| MMT score, median [IQR] | 76 [70–80] |
| Laboratory features | |
| Increased CPK, (%) | 23 (53.5) |
| CPK serum level, median [IQR] (UI/L) | 283 [85–1668] |
| CPK serum level in patients with myositis, median [IQR] (UI/L) | 1169 [329.75–4506.75] |
| LDH serum level (mU/ml), median [IQR] | 280.5 [244.25–584] |
| Increased aldolase, (%) | 13 (30.2) |
| Aldolase serum level (UI/L), median [IQR] | 7.80 [5.15–34.6] |
| Aldolase serum level (UI/L), in patients with myositis, median [IQR] | 9.80 [7.60–41.0] |
| Increased hs-TnT, (%) | 17 (39.5) |
| Hs-TnT serum levels, median [IQR] (ng/l) | 23.55 [7.95– 172.75] |
| Hs-TnT serum levels in patients with myocarditis, median [IQR] (ng/l) | 88.0 [23.55– 311.5] |
| Increased NT-proBNP, (%) | 15 (34.9) |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 102.0 [42.0– 407.0] |
| NT-proBNP serum levels in patients with myocarditis, median [IQR] (pg/ml) | 525.5 [243.5– 1575.25] |
| Increased CPR, (%) | 12 (27.9) |
| Increased ESR, (%) | 15 (34.9) |
| CRP serum levels, median [IQR] (mg/l) | 2.0 [1.0–8.5] |
| ESR serum levels, median [IQR] (mm/1h) | 26 [8.0–39.5] |
Overall skeletal involvement includes: myositis, arthritis, arthralgias, mechanic hands.
Interstitial lung disease evaluated by pulmonary function tests and confirmed by high-resolution CT.
Myositis diagnosed in the presence of clinical symptoms associated with increase total CPK and/or aldolase plus at least one abnormality at electromyography and/or magnetic resonance.
Myocarditis considered as specified in the Methods section.
Arthritis defined as clinical evidence of swollen and tender joints. MMT: Manual Muscle Testing; CPK: creatine phosphokinase; LDH: lactate dehydrogenase; hs-TnT: high-sensitivity troponin T; ASS: anti-synthetase syndrome; n : number.
The anti-Jo1 was the most commonly found disease-specific antibody. It was positive in 23 cases (53.5%), followed by anti-PL7 in 7 (16.3%). Anti-PL-12 and anti-EJ were more rarely found (14% and 7%, respectively). Anti-SSA antibody positivity was found in 20 patients (46.5%), together with other disease-specific antibodies in 16 cases.
Muskoloskeletal involvement refers to any signs/symptom related to ASS, as inflammatory arthralgias, arthritis and/or mechanic hands. Myositis was diagnosed in 23 cases (53.5%) and confirmed by muscle biopsy in 10 cases (23.3%). In the remaining 13 cases, the diagnosis of myositis was confirmed by means of electromyography and/or proximal muscle MRI. In 72.1% of the patients, ILD was found through high-resolution chest CT. At baseline, almost half of the patients complained of some degree of dyspnea (48.8%), while palpitations were present in only 4 patients (9.3%). At ASS clinical onset, fever was recorded in 16 patients (37.2%).
Total creatine phosphokinase (CPK) serum levels were increased in 23 patients (53.5%), and aldolase in 13 patients (30.2%). Increased ESR and CRP were observed in 15 (34.9%) and 12 patients (27.9%), respectively. Elevated hs-TnT and NT-proBNP serum levels were found in 17 (39.5%) and 15 patients (34.9%), respectively.
At the time of the first evaluation, the majority of the patients were on steroids (86.0%), at a median (IQR) dose of 5.0 (5.0–15.0) mg of prednisone (or equivalent), for the treatment of musculoskeletal involvement. Overall, based on retrospective records from the entire cohort, the most commonly used immunosuppressant was MMF [26 patients (60.5%)], followed by MTX in 11 cases (25.6%). Concomitant therapy with rituximab, IVIGs or anakinra was prescribed in 15 (34.9%), 6 (13.9%) and 3 patients (7.0%), respectively. Only 7 patients (16.3%) had been previously treated—before ASS with myocarditis diagnosis—with i.v. CYC.
Patients with ASS diagnosed with myocarditis: clinical features
Among our cohort of 43 patients with ASS, 13 patients (30%) with myocarditis were identified. The diagnosis was made at ASS clinical onset or within the first 3 months of ASS diagnosis in 7 out of the 13 patients (54%). In the remaining cases, myocarditis was diagnosed after a median (IQR) time of 18 (10.5–29.25) months.
In all cases, myocarditis was diagnosed as defined in the methods section. In 5 patients (38.5%), an EMB was performed, including in 2 patients without available CMRI: in all biopsied patients, a virus-negative myocarditis was confirmed.
Clinical, laboratory and instrumental data for patients with ASS diagnosed with myocarditis are reported in Table 2 .
Clinical and instrumental features of patients with ASS with myocarditis
| . | Patients with ASS with myocarditis ( = 13) . |
|---|---|
| Age, median [IQR] | 58 [54.25–64.25] |
| Females, (%) | 6 (46.1) |
| Clinical and biochemical findings | |
| Subclinical onset, (%) | 7 (53.8) |
| Infarct-like onset, (%) | 2 (15.4) |
| Arrhythmic onset, (%) | 2 (15.4) |
| Congestive heart failure onset, (%) | 2 (15.4) |
| Concomitant myositis, (%) | 8 (61.5) |
| Increased hs-TnT, (%) | 12 (92.3) |
| Hs-TnT serum levels, median [IQR] (ng/l) | 88.0 [23.55–311.5] |
| Increased NT-proBNP, (%) | 11 (84.6) |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 525.5 [243.5–1575.25] |
| Increased CRP, (%) | 7 (53.8) |
| Increased ESR, (%) | 7 (53.8) |
| CRP serum levels, median [IQR] (mg/l) | 7.0 [1.7–15.75] |
| ESR serum levels, median [IQR] (mm/1h) | 32.0 [8.0–45.0] |
| Echocardiogaphy | |
| Echocardiogaphic abnormalities, (%) | 7 (53.8) |
| Reduced LVEF, (%) | 2 (15.4) |
| LVEF (%), median [IQR] | 58.0 [55.0–60.0] |
| Hypokinesia, (%) | 3 (23) |
| Diastolic dysfunction, (%) | 4 (31) |
| 24-h ECG holter analysis | |
| 24-h ECG holter abnormalities, (%) | 2 (15.4) |
| Frequent VEBs, (%) | 4 (31) |
| VEBs number, median [IQR] | 1027 [123–1782] |
| CMRI findings | |
| Any abnormality, (%) | 11 (100) |
| LGE areas, (%) | 10 (91.0) |
| LGE burden (%), median [IQR] | 2.50 [1.75–3.50] |
| Positive LGE segments ( ), median [IQR] | 2 [1-3] |
| Pericardial effusion, (%) | 9 (81.1) |
| Reduced LVEF, (%) | 4 (36.4) |
| LVEF (%), median [IQR] | 58.0 [51.25–64.25] |
| EDV-LV (ml), median [IQR] | 123.25 [105.30–141.10] |
| EDV-RV (ml), median [IQR] | 137.40 [105.15–150.10] |
| RVEF (%), median [IQR] | 62.0 [56.5–71.0] |
| Patients with positive T2 ratio on STIR, (%) | 6 (54.5) |
| Mean T2 ratio, median [IQR] | 1.90 [1.70–1.95] |
| Global native T1 relaxation time (ms), median [IQR] | 1071 [1042.0–1084.0] |
| Increased native T1 mapping (ms) (normal value ≤1045 ms), (%) | 10 (91.0) |
| ECV (%; global), median [IQR] | 30.5 [27.5–31.0] |
| Increased ECV (normal value ≤27%), (%) | 11 (100) |
| Increased T2 mapping (normal value ≤50 ms), (%) | 10 (91.0) |
| Global T2 relaxation time (ms), median [IQR] | 52.7 [51.25–57.20] |
| Global T2 relaxation time in patients with increased T2 relaxation time (ms), median [IQR] | 53.2 [52.0–58.5] |
| Lake Louise criteria | |
| 2009 LLC | |
| Patients with positive regional or global STIR (%) | 6 (54.5) |
| Patients with positive EGE, (%) | 0 0 |
| Patients with positive LGE, (%) | 10 (91.0) |
| 2009 LLC criteria satisfied , (%) | 6 (54.5) |
| 2018 LLC | |
| Patients with positive T1 criteria, (%) | 10 (91.0) |
| Patients with positive T2 criteria, (%) | 10 (91.0) |
| 2018 LLC criteria satisfied, (%) | 10 (91.0) |
| . | Patients with ASS with myocarditis ( = 13) . |
|---|---|
| Age, median [IQR] | 58 [54.25–64.25] |
| Females, (%) | 6 (46.1) |
| Clinical and biochemical findings | |
| Subclinical onset, (%) | 7 (53.8) |
| Infarct-like onset, (%) | 2 (15.4) |
| Arrhythmic onset, (%) | 2 (15.4) |
| Congestive heart failure onset, (%) | 2 (15.4) |
| Concomitant myositis, (%) | 8 (61.5) |
| Increased hs-TnT, (%) | 12 (92.3) |
| Hs-TnT serum levels, median [IQR] (ng/l) | 88.0 [23.55–311.5] |
| Increased NT-proBNP, (%) | 11 (84.6) |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 525.5 [243.5–1575.25] |
| Increased CRP, (%) | 7 (53.8) |
| Increased ESR, (%) | 7 (53.8) |
| CRP serum levels, median [IQR] (mg/l) | 7.0 [1.7–15.75] |
| ESR serum levels, median [IQR] (mm/1h) | 32.0 [8.0–45.0] |
| Echocardiogaphy | |
| Echocardiogaphic abnormalities, (%) | 7 (53.8) |
| Reduced LVEF, (%) | 2 (15.4) |
| LVEF (%), median [IQR] | 58.0 [55.0–60.0] |
| Hypokinesia, (%) | 3 (23) |
| Diastolic dysfunction, (%) | 4 (31) |
| 24-h ECG holter analysis | |
| 24-h ECG holter abnormalities, (%) | 2 (15.4) |
| Frequent VEBs, (%) | 4 (31) |
| VEBs number, median [IQR] | 1027 [123–1782] |
| CMRI findings | |
| Any abnormality, (%) | 11 (100) |
| LGE areas, (%) | 10 (91.0) |
| LGE burden (%), median [IQR] | 2.50 [1.75–3.50] |
| Positive LGE segments ( ), median [IQR] | 2 [1-3] |
| Pericardial effusion, (%) | 9 (81.1) |
| Reduced LVEF, (%) | 4 (36.4) |
| LVEF (%), median [IQR] | 58.0 [51.25–64.25] |
| EDV-LV (ml), median [IQR] | 123.25 [105.30–141.10] |
| EDV-RV (ml), median [IQR] | 137.40 [105.15–150.10] |
| RVEF (%), median [IQR] | 62.0 [56.5–71.0] |
| Patients with positive T2 ratio on STIR, (%) | 6 (54.5) |
| Mean T2 ratio, median [IQR] | 1.90 [1.70–1.95] |
| Global native T1 relaxation time (ms), median [IQR] | 1071 [1042.0–1084.0] |
| Increased native T1 mapping (ms) (normal value ≤1045 ms), (%) | 10 (91.0) |
| ECV (%; global), median [IQR] | 30.5 [27.5–31.0] |
| Increased ECV (normal value ≤27%), (%) | 11 (100) |
| Increased T2 mapping (normal value ≤50 ms), (%) | 10 (91.0) |
| Global T2 relaxation time (ms), median [IQR] | 52.7 [51.25–57.20] |
| Global T2 relaxation time in patients with increased T2 relaxation time (ms), median [IQR] | 53.2 [52.0–58.5] |
| Lake Louise criteria | |
| 2009 LLC | |
| Patients with positive regional or global STIR (%) | 6 (54.5) |
| Patients with positive EGE, (%) | 0 0 |
| Patients with positive LGE, (%) | 10 (91.0) |
| 2009 LLC criteria satisfied , (%) | 6 (54.5) |
| 2018 LLC | |
| Patients with positive T1 criteria, (%) | 10 (91.0) |
| Patients with positive T2 criteria, (%) | 10 (91.0) |
| 2018 LLC criteria satisfied, (%) | 10 (91.0) |
LGE burden evaluated using the ±3 S.D. method.
LLC suggestive for active myocardial inflammation. n : number; ASS: anti-synthetase syndrome; hs-TnT: high-sensitivity troponin T; LV-EF: left-ventricular ejection fraction; VEBs: ventricular ectopic beats (frequent if higher than 720/24 h); NS-VT: non-sustained ventricular tachycardia; CMRI: cardiac MRI; LV-EDV: left-ventricular end diastolic volume; RV-EDV: right-ventriclular end diastolic volume; BSA: body surface area; ECV: extracellular volume; RV-EF: right-ventricular ejection fraction; STIR: Short-Tau Inversion Recovery; LLC: Lake Louise criteria; EGE: early gadolinium enhancement.
The most common clinical symptom in patients with myocarditis was dyspnea, which was reported by 7 patients (54.4%), while palpitations were reported by 4 patients. All but one patient (92.3%) had increased hs-TnT levels, and 11 patients (84.6%) had increased NT-proBNP serum levels.
Subclinical onset was the most common myocarditis clinical presentation (53.8%). In 2 patients each (15.4% each), myocarditis onset was classified as ‘infarct-like’, ‘arrhythmic’ or ‘congestive heart failure’ (see Supplementary Material , available at Rheumatology online). A concomitant skeletal myositis was disclosed in 8 of these cases (61.5%).
In analysing our cohort of patients with ASS with clinically diagnosed myocarditis, 11 patients (91%) underwent CMRI, and in all cases, a diagnosis of myocarditis was confirmed by the presence of at least one CMRI abnormality. In particular, the detected abnormalities in the context of a clinically suspected myocarditis, were: LGE areas, T2 areas of myocardial oedema on STIR images, increased T1 mapping, increased T2 mapping, increased ECV or increased T2 ratio. In the remaining 2 patients who did not perform CMRI, myocarditis was confirmed by EMB. The most common CMRI abnormalities were: increased ECV, present in all cases, areas of LGE (91%), and increased T1 mapping (91%) and T2 mapping (91%). STIR images suggestive of myocardial oedema were detected in 6 patients (54.5%). A concomitant pericardial effusion was present in 9 cases (81.1%), and only 4 patients (36.4%) had a depressed left-ventricular ejection fraction (LV-EF).
The 2009 Lake Louise criteria (LLC) were satisfied by 6 patients, whereas the 2018 LLC were satisfied by 10 patients. Thus, with the updated CMRI criteria for myocarditis diagnosis, which include T1 mapping and T2 mapping, the sensitivity improved from 54.6% to 91.0% ( Fig. 1 ).

Cardiac MRI in patient with anti-synthetase syndrome. Cardiac MRI shows ventricles with normal volumes and function. STIR images showed (A) absent focal oedema, but subtle diffuse oedema as showed by (B) T2 mapping and (F) AHA bullseye (global value 58 ms; normal value ≤50 ms). (E) Late gadolinium enhancement showed mid-wall hyperintensity in the basal posterior septum, associated with normal native T1 mapping (C and G) (1037 ms; normal value <1045 ms) but increased ECV values (D and H) (29%, normal values <27%), as for mild increase in interstitial fibrosis. Moreover, mild pericardial effusion was evident without significant increase in the pericardium thickness. AHA: American Heart Association; STIR: Short-tau Inversion Recovery; ECV: extracellular volume
In patients with ASS diagnosed with myocarditis who underwent CMRI, no statistically significant correlation emerged between CMRI parameters (LVEF, T2 ratio, T1 mapping, T2 mapping, and ECV) and disease parameters (hs-TnT, NTproBNP, ESR, CRP and CPK serum levels) ( P -value non-significant).
At the time of myocarditis diagnosis, 11 out of 13 patients (84.6%) were on steroids, at a median (IQR) dose of 5.0 (7.5–18.75) mg of prednisone (or equivalent). Only 5 out of 13 patients (38.5%) were already being treated with immunosuppressants, mainly for ILD, arthritis and/or myositis. Specifically, 2 patients were on MTX, 2 patients with severe disease complicated by myositis and ILD received i.v. CYC (combined with IVIG in one case), and 1 patient was on AZA.
Once myocarditis was diagnosed, all patients were treated with steroids 1 mg/kg per day (then tapered within 4 months), preceded by i.v. methylprednisolone (1 g for 3 consecutive days) in 6 patients (46.1%) with concomitant severe myositis.
Twelve patients (92.3%) were treated with a combination of CSs and MMF [up to 1500 mg per day in 3 patients (25%), up to 2 g per day in 4 patients (33.3%), and up to 3 g per day 5 patients (41.7%)]. The remaining patient (7.7%) received AZA. In 3 cases (23.1%) with severe or refractory disease, rituximab and anakinra were administered as additional second-line therapy.
During follow-up, 3 patients presented a myocarditis relapse, which was managed with an increase in the CS dose combined with anakinra in 1 case and with rituximab in 2 patients with concomitant myositis relapse. One patient (7.7%) with myocarditis and signs and symptoms of inflammation (high CRP and fever), already on steroids, MMF and anakinra, died of sudden cardiac death upon anakinra suspension. Two (15.4%) patients required ICD insertion for arrhythmic complications.
Clinical features of patients with ASS with and without myocarditis
To identify the clinical features of patients with ASS that were associated with myocardial involvement, clinical, immunological, laboratory and instrumental characteristics of patients with ASS with myocarditis were compared with those of patients with ASS without myocarditis.
Laboratory data for the patients with myocarditis were collected at clinical onset, before the initiation of specific immunosuppressive therapy. The laboratory data for the patients without myocarditis were obtained at the first clinical evaluation.
Patients with ASS with myocarditis were more frequently males (53%) compared with those without myocarditis (13%) ( P = 0.009), while age and disease duration were similar when comparing patients with ASS with and without myocarditis ( P -value non-significant for both comparisons). No significant differences emerged when comparing the presence of traditional cardiovascular risk factors or autoantibody profiles in patients with ASS with and without myocarditis.
As expected, patients with ASS with myocarditis had higher levels of hs-TnT [88.0 (23.55–311.5) vs 9.80 (5.0–23.0) ng/l] and NTproBNP [525.5 (243.5–1575.25) vs 59.0 (32.0–165.5) pg/ml] ( P < 0.001 and P = 0.013, respectively), while CPK levels did not differ between groups ( P -value non-significant). Liver enzymes (AST and ALT) and CRP were markedly increased in ASS patients with myocarditis compared with those without myocarditis [97.5 (40.0–194.0) vs 27.5 (21.0–34.25) U/l, 136.5 (32.25–194.0) vs 212.5 (12.0–33.25) U/l and 7.0 (1.7–15.75) vs 1.85 (0.5–2.86) mg/l, respectively ( P = 0.007, P = 0.006 and P = 0.011, respectively).
No statically significant differences emerged for dyspnea, muscle involvement, ILD, or arthritis between the two groups of patients, while fever at ASS presentation was more frequently observed in patients with ASS with a diagnosis of myocarditis (69% vs 17%, P = 0.001). No significant differences in terms of echocardiographic abnormalities between the two groups emerged. At multivariate logistic regression analysis, only the presence of fever at ASS clinical onset (OR 7.707, 95% CI 1.026–57.917, P = 0.047) was associated with myocarditis ( Table 3 ).
| . | Patients with ASS with myocarditis ( = 13) . | Patients with ASS without myocarditis ( = 30) . | . |
|---|---|---|---|
| Age, median [IQR] | 58 [54.25–64-25] | 0.641 | |
| Females, (%) | 6 (46) | 26 (87) | |
| CV risk factors | |||
| Smoking, (%) | 6 (46) | 7 (23) | 0.173 |
| Arterial hypertension, (%) | 4 (31) | 11 (37) | 0.739 |
| Obesity, (%) | 2 (15) | 2 (7) | 0.589 |
| Diabetes, (%) | 1 (8) | 2 (7) | 0.999 |
| Ischemic heart disease, (%) | 2 (15) | 3 (10) | 0.637 |
| Autoantibodies | |||
| Anti-Jo1, (%) | 7 (54) | 16 (53) | 0.999 |
| Anti-PL7, (%) | 3 (23) | 4 (13) | 0.655 |
| Anti-PL12, (%) | 1 (8) | 5 (17) | 0.649 |
| Anti-EJ, (%) | 1 (8) | 2 (7) | 0.999 |
| Anti-SSA, (%) | 7 (54) | 13 (43) | 0.740 |
| Clinical features | |||
| Fever, (%) | 9 (69) | 5 (17) | |
| Muscle weakness, (%) | 8 (61) | 15 (50) | 0.526 |
| Myalgias, (%) | 6 (46) | 7 (23) | 0.163 |
| RP, (%) | 5 (38) | 10 (33) | 0.742 |
| ILD, (%) | 10 (77) | 21 (70) | 0.727 |
| Dyspnea, (%) | 7 (54) | 14 (47) | 0.747 |
| Dysphagia, (%) | 3 (23) | 6 (20) | 0.999 |
| Mechanical hands, (%) | 3 (23) | 6 (20) | 0.999 |
| Arthritis, (%) | 5 (38) | 8 (27) | 0.485 |
| MMT score, median [IQR] | 78.0 [73.0–80.0] | 75.5 [70.0–80.0] | 0.564 |
| Biochemistry | |||
| CPK serum levels, median [IQR] (UI/l) | 337.0 [110.0–3959.75] | 243.0 [80.25–1268.0] | 0.249 |
| LDH serum levels, median [IQR] (UI/l) | 609.0 [267.0–747.75] | 265.5 [251.0–392.75] | 0.249 |
| CRP serum levels, median [IQR] (mg/l) | 7.0 [1.7–15.75] | 1.85 [0.5–2.86] | |
| ESR, median [IQR] (mm/h) | 32.0 [8.0–45.0] | 23.0 [8.75–37.0] | 0.584 |
| AST (U/l), median [IQR] | 97.5 [40.0–194.0] | 27.5 [21.0–34.25] | |
| ALT (U/l), median [IQR] | 136.5 [32.25–194.0] | 212.5 [12.0–33.25] | |
| Cardiac enzymes | |||
| Hs-TnT serum levels, median [IQR] (ng/l) | 88.0 [23.55–311.5] | 9.80 [5.0–23.0] | |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 525.5 [243.5–1575.25] | 59.0 [32.0–165.5] | |
| Echocardiogaphy | |||
| LV-EF (%), median [IQR] | 58.0 [55.0–60.0] | 63.5 [60.0–65.0] | 0.076 |
| LV-EF reduced, (%) | 2 (15) | 2 (9) | 0.586 |
| Hypokinesia, (%) | 3 (23) | 1 (4) | 0.106 |
| Diastolic dysfunction, (%) | 4 (31) | 2 (9) | 0.148 |
| . | Patients with ASS with myocarditis ( = 13) . | Patients with ASS without myocarditis ( = 30) . | . |
|---|---|---|---|
| Age, median [IQR] | 58 [54.25–64-25] | 0.641 | |
| Females, (%) | 6 (46) | 26 (87) | |
| CV risk factors | |||
| Smoking, (%) | 6 (46) | 7 (23) | 0.173 |
| Arterial hypertension, (%) | 4 (31) | 11 (37) | 0.739 |
| Obesity, (%) | 2 (15) | 2 (7) | 0.589 |
| Diabetes, (%) | 1 (8) | 2 (7) | 0.999 |
| Ischemic heart disease, (%) | 2 (15) | 3 (10) | 0.637 |
| Autoantibodies | |||
| Anti-Jo1, (%) | 7 (54) | 16 (53) | 0.999 |
| Anti-PL7, (%) | 3 (23) | 4 (13) | 0.655 |
| Anti-PL12, (%) | 1 (8) | 5 (17) | 0.649 |
| Anti-EJ, (%) | 1 (8) | 2 (7) | 0.999 |
| Anti-SSA, (%) | 7 (54) | 13 (43) | 0.740 |
| Clinical features | |||
| Fever, (%) | 9 (69) | 5 (17) | |
| Muscle weakness, (%) | 8 (61) | 15 (50) | 0.526 |
| Myalgias, (%) | 6 (46) | 7 (23) | 0.163 |
| RP, (%) | 5 (38) | 10 (33) | 0.742 |
| ILD, (%) | 10 (77) | 21 (70) | 0.727 |
| Dyspnea, (%) | 7 (54) | 14 (47) | 0.747 |
| Dysphagia, (%) | 3 (23) | 6 (20) | 0.999 |
| Mechanical hands, (%) | 3 (23) | 6 (20) | 0.999 |
| Arthritis, (%) | 5 (38) | 8 (27) | 0.485 |
| MMT score, median [IQR] | 78.0 [73.0–80.0] | 75.5 [70.0–80.0] | 0.564 |
| Biochemistry | |||
| CPK serum levels, median [IQR] (UI/l) | 337.0 [110.0–3959.75] | 243.0 [80.25–1268.0] | 0.249 |
| LDH serum levels, median [IQR] (UI/l) | 609.0 [267.0–747.75] | 265.5 [251.0–392.75] | 0.249 |
| CRP serum levels, median [IQR] (mg/l) | 7.0 [1.7–15.75] | 1.85 [0.5–2.86] | |
| ESR, median [IQR] (mm/h) | 32.0 [8.0–45.0] | 23.0 [8.75–37.0] | 0.584 |
| AST (U/l), median [IQR] | 97.5 [40.0–194.0] | 27.5 [21.0–34.25] | |
| ALT (U/l), median [IQR] | 136.5 [32.25–194.0] | 212.5 [12.0–33.25] | |
| Cardiac enzymes | |||
| Hs-TnT serum levels, median [IQR] (ng/l) | 88.0 [23.55–311.5] | 9.80 [5.0–23.0] | |
| NT-proBNP serum levels, median [IQR] (pg/ml) | 525.5 [243.5–1575.25] | 59.0 [32.0–165.5] | |
| Echocardiogaphy | |||
| LV-EF (%), median [IQR] | 58.0 [55.0–60.0] | 63.5 [60.0–65.0] | 0.076 |
| LV-EF reduced, (%) | 2 (15) | 2 (9) | 0.586 |
| Hypokinesia, (%) | 3 (23) | 1 (4) | 0.106 |
| Diastolic dysfunction, (%) | 4 (31) | 2 (9) | 0.148 |
ASS: anti-synthetase syndrome; n : number; CV: cardiovascular; ILD: interstitial lung disease; MMT: Manual Muscle Testing; CPK: creatine phosphokinase; LDH: lactate dehydrogenase; hs-TnT: high-sensitivity troponin T; AST:aspartate aminotransferase; ALT: alanine aminotransferase; LV-EF: left-ventricular ejection fraction.
Bold typeface refers to P values that are statistically significant.
Our study shows that myocarditis is a frequent and likely overlooked manifestation in patients with ASS and that it can be one of the presenting, even isolated, features associated with ASS. We also highlighted that CMRI mapping techniques are fundamental to improving the sensitivity to detect myocardial inflammation in patients with ASS. Moreover, among disease features, the presence of fever and raised CRP were associated in our cohort with myocardial involvement at disease onset.
In contrast to previous studies that reported a low prevalence of myocarditis in patients with ASS, we observed a significant frequency of myocarditis in our cohort (30%).
A large retrospective series of patients with ASS (a cohort of 300 patients belonging to the French National Registry) reported a prevalence of myocarditis of 3.4%, as only 12 cases of myocarditis were identified from 2000 to 2014 [ 2 ]. Similarly, within the Johns Hopkins Myositis Center Research Registry, of 3082 adult patients with IIM, 14 patients were identified as having myocarditis, when applying an encounter code of myocarditis from 2004 to 2021 [ 22 ].
In our cohort, the significantly high prevalence of myocarditis may be due to several reasons. First, our centre is a tertiary regional referral centre for myocarditis, and a Myocarditis Disease Unit is operating at our centre. Therefore, an over-referral of patients with ASS with suspected myocarditis may be expected. Second, our standard of care includes a thorough myocardial evaluation for all patients with CTDs. This includes evaluation of signs and symptoms suggestive for cardiac involvement, assessment of cardiac enzymes, standard 12-leads ECG, echocardiography and monitoring with a 24-h ECG holter for all patients. CMRI is also performed in all patients with suspicion of myocardial involvement. Thus, early and/or subclinical myocarditis could be identified in most cases. Finally, the more extensive use of CMRI as a non-invasive diagnostic tool for detecting myocarditis, as well as the inclusion of novel CMRI techniques from 2000–2014 vs 2016–2022, may explain the higher prevalence of myocarditis in our recent ASS cohort.
Importantly, myocarditis was diagnosed at the clinical onset of ASS in >50% of patients, thus representing a potential early manifestation of the disease. This is in agreement with the results from the French cohort, in whom myocarditis was the first disease manifestation in 42% of cases [ 2 ].
Diagnosing myocarditis remains a challenge in patients with autoimmune diseases, however, since clinical signs could be absent or not specific, or concealed by other clinical features. This suggests that a comprehensive evaluation of cardiac involvement is needed in all patients with ASS. Indeed, myocarditis was rarely detectable by echocardiography. Echocardiographic abnormalities suggestive for myocardial involvement were only apparent in approximately half of the patients with ASS who were eventually diagnosed by CMRI. Importantly, a decreased LV-EF was only rarely detected and, consistently, clinical onset with heart failure was not recorded in our cohort. In the French cohort, left or right ventricular dysfunction at echocardiography was present in the vast majority of myocarditis patients, and these findings were paralleled by the presence of congestive heart failure in 75% of cases, leading to admission to the intensive care unit in 50% of cases [ 2 ]. Similarly, in the study by Chung, the vast majority of patients had a clinically severe myocarditis, requiring hospitalization in 93% of cases due to depressed LV-EF in 71% of cases [ 22 ]. These results underline again the different clinical profiles of the patients with ASS with myocarditis in the previous studies compared with the clinical profiles of our patients with ASS with myocarditis. This discrepancy is at least partially due to the method used for identifying myocardial involvement. In both the French and American cohorts, patients were retrospectively identified from registries, whereas in our study we systematically evaluated the presence of myocardial involvement in all patients with ASS. As a consequence, an earlier diagnosis of even subclinical myocarditis was possible in our cohort. Unfortunately, though, while EMB is the diagnostic gold standard for diagnosing myocarditis, the limited sensitivity and the risks associated with the procedure limit its regular use. For this reason, CMRI is an alternative non-invasive diagnostic tool for myocarditis [ 7–10 , 23 ]. In CTDs, CMRI has been widely used to evaluate cardiac involvement and has progressively become a key diagnostic and prognostic tool [ 5–12 , 15 , 23–28 ]. Historically, the CMRI diagnosis of myocarditis relies on the LLC [ 7 ], which include only non-parametric techniques depending on a qualitative or semi-quantitative analysis of signal intensities on STIR, EGE and LGE images. In suspected acute myocarditis, the CMRI with the LLC has a diagnostic accuracy close to 80%. However, these criteria have important limitations when applied to patients with CTDs [ 12 ]. STIR images have a modest sensitivity for detecting diffuse myocardial oedema, and this is further reduced by the need for muscle signal intensity as reference tissue, with subsequent risk of false-negative results in patients with coexistent skeletal myositis [ 12 ].
T1 mapping, T2 mapping, and ECV have emerged as novel techniques that can overcome these limitations [ 8 , 23 , 24 ]. In particular, T1 and T2 mapping are more sensitive than STIR in detecting diffuse myocardial oedema [ 7 , 23 , 24 ]. This was also shown in our cohort, in whom CMRI sensitivity for diagnosing myocarditis improved with the inclusion of mapping techniques, specifically T2 mapping, based on the updated LLC. When the native T1 mapping, T2 mapping, and ECV were included in the 2018 revised LLC [ 8 ] for our patients, the sensitivity of CMRI increased from 54.6% to 91.0%. This improvement was mainly driven by the additional value of T2 mapping in detecting subtle oedema. This notion is of great clinical importance, since the early identification of myocarditis is mandatory to establishing prompt therapeutic intervention and improving the outcomes for patients. The timely use of immunosuppressive drugs may be a successful approach in ASS with myocarditis [ 2 ], and, in particular in the early phases of the disease, the treatment can prevent the progression to late-stage cardiac damage.
In our cohort, myocarditis was more common in males and clinically manifested with dyspnea in almost all cases, or palpitations, which were reported by a minority of patients. These non-specific symptoms were almost always associated with increased troponin serum levels, which were elevated in all but one patient, whereas NT-proBNP was raised in 85% of cases. Therefore, cardiac enzymes should be routinely measured in all patients with ASS, either at baseline or during follow-up.
The greater occurrence of myocarditis in males is in keeping with previous studies reporting a sex ratio for myocarditis of 1:2–4 females to males [ 29 ]. Toll-like receptor (TLR)2 and TLR4 signalling pathways, which are elevated in male individuals, are considered responsible for this imbalance, as they play a central role in increasing inflammation during myocarditis and in promoting remodelling and fibrosis [ 29 ].
The finding that a significantly higher percentage of patients with ASS with myocarditis, compared with those without myocarditis, had fever and increased CRP levels at ASS clinical onset suggests that an inflammatory phenotype does exist and that myocarditis is one of the primary clinical features. In ASS, the presence of an inflammatory phenotype and its association with a more aggressive disease has already been reported in a large number of Chinese patients [ 30 ]. Based on these findings, a high suspicion of myocarditis should always be borne in mind when dealing with patients with ASS with such inflammatory features. Of note, in our cohort, two patients were fully responsive to the IL-1 blocking agent anakinra. Consistently, the efficacy of anakinra in treating both patients with refractory ASS and those with different types of myocarditis has been recently reported [ 31–35 ]. This evidence may indicate a possible pathogenic role of IL-1–mediated inflammation in ASS with myocarditis.
Our data show that myocarditis in patients with ASS could be diagnosed using a comprehensive algorithm that includes analysis of clinical signs and disease characteristics, cardiac biomarkers, and instrumental evaluation, including CMRI with mapping techniques.
To the best of our knowledge, this is the first study that has comprehensively evaluated the presence and the clinical and instrumental features of myocarditis in patients with ASS. Similarly, we assessed for the first time the additional value of CMRI mapping techniques in the detection of myocardial inflammation in patients with ASS with suspected myocarditis. Refining the diagnostic approach for detecting myocardial inflammation, taking into account the phenotype of patients with ASS with myocarditis, and by using CMRI with mapping techniques could pave the way to novel and earlier therapeutic perspectives.
An additional strength of our study is the comprehensive characterization of ASS with myocarditis in all patients at a dedicated multidisciplinary Myocarditis Disease Unit, using a rigorous algorithm.
Nevertheless, our study has some limitations. The high rate of myocarditis we observed was at least partially due to the routine assessment of myocardial involvement in a dedicated Myocarditis Disease Unit with readily available CMRI. The differences found between patients with and without myocarditis were determined after the diagnosis of myocarditis (and most of them are part of the diagnostic criteria used to define myocarditis, as cardiac biomarkers). As a consequence, even considering the finding at multivariate analysis of fever at ASS diagnosis as associated with the occurrence of myocarditis, our results need to be confirmed. The low number of patients may be considered a limitation for drawing solid conclusions, even though the rarity of the disease (and of this specific complication) needs to be taken into account. Moreover, EMB was performed in only five patients, thus precluding the possibility of correlating histological features with CMRI parameters. The consensus reading of CMRI represents a methodological limitation. To date, the segmentation is performed using advanced dedicated cardiac MRI software, which allows automatic segmentation based on artificial intelligence algorithms, further checked and manually corrected by an experienced operator. Moreover, the qualitative assessment of the signal intensities was supported by semi-quantitative analysis performed automatically on the same software, thus limiting subjectivity in the image interpretation. Finally, due to the retrospective nature of our study, we could not assess the prognostic role of the clinical and CMRI features in ASS with myocarditis.
Myocarditis is frequent in patients with ASS, especially in males, and could be one of the presenting features of the disease. Patients with ASS with myocarditis more frequently had fever and increased inflammatory markers compared with those without myocarditis, suggesting that a more inflammatory phenotype may be associated with myocarditis. Novel CMRI mapping techniques with the inclusion of T2 mapping increase the diagnostic sensitivity for ASS-myocarditis.
Supplementary material is available at Rheumatology online.
The data underlying this article will be shared on reasonable request to the corresponding author.
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Witt LJ , Curran JJ , Strek ME. The diagnosis and treatment of antisynthetase syndrome . Clin Pulm Med 2016 ; 23 : 218 – 26 .
Google Scholar
Dieval C , Deligny C , Meyer A et al. Myocarditis in patients with antisynthetase syndrome: prevalence, presentation, and outcomes . Medicine (United States) 2015 ; 94 : e798 .
Brady S , Melath S , Scalco RS , Penn H. Fatal cardiac involvement complicating antisynthetase syndrome . BMJ Case Rep 2014 ; 2014 : 1 – 3 .
Sharma K , Orbai AM , Desai D et al. Brief report: antisynthetase syndrome-associated myocarditis . J Card Fail 2014 ; 20 : 939 – 45 .
Peretto G , Sala S , De Luca G et al. Impact of systemic immune-mediated diseases on clinical features and prognosis of patients with biopsy-proved myocarditis . Int J Cardiol 2019 ; 280 : 110 – 6 .
De Luca G , Campochiaro C , De Santis M et al. Systemic sclerosis myocarditis has unique clinical, histological and prognostic features: a comparative histological analysis . Rheumatology (Oxford) 2020 ; 59 : 2523 – 33 .
Friedrich MG , Sechtem U , Schulz-Menger J et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC White Paper . J Am Coll Cardiol 2009 ; 53 : 1475 – 87 .
Ferreira VM , Schulz-Menger J , Holmvang G et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations . J Am Coll Cardiol 2018 ; 72 : 3158 – 76 .
Palmisano A , Benedetti G , Faletti R et al. Early T1 myocardial MRI mapping: value in detecting myocardial hyperemia in acute myocarditis . Radiology 2020 ; 295 : 316 – 25 .
De Luca G , Bombace S , Monti L. Heart involvement in systemic sclerosis: the role of magnetic resonance imaging . Clin Rev Allergy Immunol 2023 ; 64 : 343 – 57 .
De Luca G , Palmisano A , Campochiaro C et al. Cardiac magnetic resonance in systemic sclerosis myocarditis: the value of T2 mapping to detect myocardial inflammation . Rheumatology (Oxford) 2022 ; 61 : 4409 – 19 .
Mavrogeni S , Schwitter J , van Rossum A et al. Cardiac magnetic resonance imaging in myocardial inflammation in autoimmune rheumatic diseases: an appraisal of the diagnostic strengths and limitations of the Lake Louise criteria . Int J Cardiol 2018 ; 252 : 216 – 9 .
Solomon J , Swigris JJ , Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome . J Brasil Pneumol 2011 ; 37 : 100 – 9 .
Harvey RM , Doyle EF , Ellis K et al. ; The Criteria Committee of the New York Heart Association . Major changes made by Criteria Committee of the New York Heart Association . Circulation 1974 ; 49 : 390 .
Pieroni M , De Santis M , Zizzo G et al. Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression . Semin Arthritis Rheum 2014 ; 43 : 526 – 35 .
De Luca G , Bosello SL , Gabrielli FA et al. Prognostic role of ventricular ectopic beats in systemic sclerosis: a prospective cohort study shows ECG indexes predicting the worse outcome . PLoS One 2016 ; 11 : e0153012 .
De Luca G , Campochiaro C , Sartorelli S et al. Unexpected acute lymphocytic virus-negative myocarditis in a patient with limited cutaneous systemic sclerosis: a case report . Scand J Rheumatol 2018 ; 30 : 1 – 2 .
Bosello S , De Luca G , Ferraccioli G. Troponin in stable ischemic heart disease and diabetes . N Engl J Med 2015 ; 373 : 1977 – 8 .
De Luca G , Bosello S , Leone AM et al. Life-threatening arrhythmias in a scleroderma patient: the role of myocardial inflammation in arrhythmic outburst . Scand J Rheumatol 2017 ; 46 : 78 – 80 .
Leone O , Veinot JP , Angelini A et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology . Cardiovasc Pathol 2012 ; 21 : 245 – 74 .
Zeppenfeld K , Tfelt-Hansen J , de Riva M et al. ; ESC Scientific Document Group . 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death . Eur Heart J 2022 ; 43 : 3997 – 4126 .
Chung MP , Lovell J , Kelly W et al. Myocarditis in patients with idiopathic inflammatory myopathies: clinical presentation and outcomes . J Rheumatol 2023 ; 50 : 1039 – 46 .
Palmisano A , Vignale D , Bruno E et al. Cardiac magnetic resonance findings in acute and post-acute COVID-19 patients with suspected myocarditis . J Clin Ultrasound 2022 ; 51 : 613 – 21 .
Esposito A , Francone M , Faletti R et al. ; Working Group of the Italian College of Cardiac Radiology by SIRM . Lights and shadows of cardiac magnetic resonance imaging in acute myocarditis . Insights Imaging 2016 ; 7 : 99 – 110 .
Bohnen S , Radunski UK , Lund GK et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis . Eur Heart J Cardiovasc Imaging 2017 ; 18 : 744 – 51 .
Ntusi NA , Piechnik SK , Francis JM et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis - a clinical study using myocardial T1-mapping and extracellular volume quantification . J Cardiovasc Magn Reson 2014 ; 16 : 21 .
Galea N , Rosato E , Gigante A et al. Early myocardial damage and microvascular dysfunction in asymptomatic patients with systemic sclerosis: a cardiovascular magnetic resonance study with cold pressor test . PLoS One 2020 ; 15 : e0244282 .
Haaf P , Garg P , Messroghli DR et al. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review . J Cardiovasc Magn Reson 2017 ; 18 : 1 – 12 .
Fairweather D , Beetler DJ , Musigk N et al. Sex and gender differences in myocarditis and dilated cardiomyopathy: an update . Front Cardiovasc Med 2023 ; 10 : 1129348 .
Sun S , Chen Z , Zhang D et al. Description and analysis of a novel subtype of the anti-synthetase syndrome characterized by frequent attacks of fever and systemic inflammation in a single-center cohort study . Front Immunol 2021 ; 12 : 729602 .
Campochiaro C , Farina N , De Luca G et al. Anakinra for the treatment of antisynthetase syndrome: a monocentric case series and a systematic literature review . J Rheumatol 2023 ; 50 : 151 – 3 .
De Luca G , Cavalli G , Campochiaro C et al. Interleukin-1 and systemic sclerosis: getting to the heart of cardiac involvement . Front Immunol 2021 ; 12 : 653950 .
De Luca G , Cavalli G , Campochiaro C , Tresoldi M , Dagna L. Myocarditis: an interleukin-1- mediated disease? Front Immunol 2018 ; 9 : 1335 .
De Luca G , Campochiaro C , Dinarello CA , Dagna L , Cavalli G. Treatment of dilated cardiomyopathy with interleukin-1 inhibition . Ann Intern Med 2018 ; 169 : 819 – 20 .
De Luca G , Campochiaro C , Sartorelli S , Peretto G , Dagna L. Therapeutic strategies for virus-negative myocarditis: a comprehensive review . Eur J Intern Med 2020 ; 77 : 9 – 17 .
Author notes
Supplementary data.
| Month: | Total Views: |
|---|---|
| October 2023 | 52 |
| November 2023 | 37 |
| December 2023 | 38 |
| January 2024 | 111 |
| February 2024 | 93 |
| March 2024 | 59 |
| April 2024 | 85 |
| May 2024 | 83 |
| June 2024 | 58 |
Email alerts
Citing articles via.
- Rheumatology Twitter
- BSR Twitter
- BSR Facebook
- Recommend to Your Librarian
Affiliations
- Online ISSN 1462-0332
- Print ISSN 1462-0324
- Copyright © 2024 British Society for Rheumatology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Register to get your text revised right away for FREE ⚡
Today more than 1001 people got their English checked.

By continuing to use this website, you agree to our Terms of Service .
Get a FREE revision 🎁
Register a new account, welcome back, confirm your email.
Please click the link that we've sent to this address to post your question to our experts. Ok, I'll check my email
not your email? Change it now
Set a new email
Here you can set your new address email. Remember to use a valid email address. We will send you an email to confirm your account.
Facebook Login Discontinued
Unfortunately, the Facebook login method has been discontinued.
To access your TextRanch account, please click the "Reset Password" button below and input your Facebook Email. Our team will send you an email with further instructions.
If you don't remember your email, please fill out this form .
Your text is being reviewed by one of our Experts. We will notify you when your revision is ready.
Or wait in this page
Leave this page open, and your corrected text will appear as soon as it's ready!

You need to add a payment method to get our special promo ⚡
Enter your email below to get instant access to the first Chapter of our Ebook
Downloaded more than 1320 times today.
Add payment method
NOTE: Credits are valid for one year.
We're so happy that you liked your revision! Your feedback helps us improve our service. Want more FREE revisions ? 🎁
Step 1 out of 2!
Like us on Facebook by clicking the like button below:
Almost there!
Last step (2/2)
Share TextRanch on Facebook by clicking on the button below.
Congrats! You've just earned 3 credits!
Closing your account will prevent you from accessing your past revisions, and you will no longer be eligible for a FREE daily revision.
There is no cost to keep your TextRanch account, and we store all of your past revisions in a secure and private manner.
Help us understand
If we didn't meet your expectations, we'd really like to know more. Please tell us why you are closing your account:
Which one is correct? "clinical manifestation" or "clinical presentation"?
TextRanch: The best way to perfect your writing.
Discover why 1,062,726 users count on TextRanch to get their English corrected!
One of our experts will correct your English.

100% Human-Powered Editing!
- clinical manifestation
This phrase is correct and commonly used in the medical field to describe the signs and symptoms of a disease or condition.
- The clinical manifestation of the flu includes fever, cough, and body aches.
- One of the clinical manifestations of diabetes is increased thirst and frequent urination.
Alternatives:
clinical presentation
- symptoms and signs
- disease indicators
- medical symptoms
- clinical features
- The patient's clinical presentation suggested a possible case of pneumonia.
- The clinical presentation of the disease varied among the study participants.
Last Updated: April 03, 2024
Thanks to TextRanch, I was able to score above 950 on TOEIC, and I got a good grade on ACTFL OPIC as well. + Read the full interview
I love TextRanch because of the reliable feedback. The editors' comments are helpful and the customer service is amazing. + Read the full interview
TextRanch has helped me to improve my written skills as well as to communicate more naturally, like a local English speaker. + Read the full interview
TextRanch is amazingly responsive and really cares about the client. It's the best online service that I have ever used! + Read the full interview
I started to use TextRanch when I began to learn English. It has been an awesome way to improve my English skills. + Read the full interview
I love that TextRanch editors are real people who revise the text and provide feedback – it makes it so personal. + Read the full interview
I sometimes wonder if my English expressions make sense clearly and TextRanch helps me a lot in such cases. + Read the full interview
TextRanch has been really helpful in improving the flow and repairing the structure of my sentences. + Read the full interview
“Faster than AI"
“This was very helpful and I personally think this site is the best."
“It was extremely thorough and very helpful!"
“7 years without any disappointment. Always 100% satisfied. You guys are the best in the world at what you do. Thank you so much :)"
“In a world of text messages and online communication, this is great to have as a live tool. Thank you."
“Without textranch I would be stuck!"
“Accuracy and fast response. Personal comments from editor. Thank you."
“I wasn't aware of this service, it's fascinating and more reliable than standard IA tools available on the internet"
“The fact that you can get reliable fast feedback on your texts."
“you guys are better than grammarly i'm being honest here"
“OMG! This is really good than any other text correction tools I've used so far. Highly recommend this."
“Very fast and accurate. thank you."
“I love this app because it's help to writing skills all of students ♥️"
“This was exactly the mistake I was looking for, the wording dind´t sound right at first. Better than grammarly!"
“The immediate help that I received was reassuring and very satisfactory. Thanks."
“this helps A LOT for my studies."
“Woow!! I would never have expected such precision! Thank you soooo much!!"
“Real Time Editor and not AI. Many Thanks."
“The very first thing excites me about Textranch is how much your editors care."
“The fact that texts are checked by human editors rather than by AI, etc. I appreciate this!"
“Feel welcome, immediate response, high quality feedback"
“This is the best app that I have ever seen"
“Quick response and got what I intend to say. Grammar correction is excellent because the meaning is retained."
“Excellent, I truly loved this textRanch for quick revision. This textRanch for quick revision is a 10/10 for me."
⚡️Ask our Editor now.
Fresh content for your texts, so you can be more professional.
estimated time: 30 minutes , directly in your inbox
📝 ️Notes for your editor
Let our editor help you, include background information, explanations of unusual words and special terms, or instructions about specific improvements you want.

Want to improve your English business writing?
More than 150,000 people like you receive our weekly newsletter to master their English skills!
Why choose TextRanch?
Lowest prices Up to 50% lower than other online editing sites.
Fastest Times Our team of editors is working for you 24/7.
Qualified Editors Native English experts for UK or US English.
Top Customer Service We are here to help. Satisfaction guaranteed!
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Med (Lond)
- v.20(4); 2020 Jul

Clinical presentation and diagnosis of multiple sclerosis
Leeds Centre for Neurosciences, Leeds, UK
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. MS is a disease of young adults with a female predominance. There are characteristic clinical presentations based on the areas of the central nervous system involved, for example optic nerve, brainstem and spinal cord. The main pattern of MS at onset is relapsing–remitting with clinical attacks of neurological dysfunction lasting at least 24 hours. The differential diagnosis includes other inflammatory central nervous system disorders. Magnetic resonance imaging of the brain and lumbar puncture are the key investigations. New diagnostic criteria have been developed to allow an earlier diagnosis and thus access to effective disease modifying treatments.
- The diagnosis of multiple sclerosis is a clinical diagnosis supported by investigation findings.
- There is no single sensitive and specific diagnostic test for multiple sclerosis.
- The principle of dissemination of lesions in time and space underpins the diagnosis.
- Eighty-five per cent of people with multiple sclerosis have a relapsing-remitting course at onset.
- New diagnostic criteria aim to allow an earlier, accurate diagnosis.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating central nervous system (CNS) disease. Its onset is typically in adults with peak age at onset between 20–40 years. There is a female predominance of up to 3:1. The course of MS is relapsing–remitting (RRMS) at onset in 85% with episodes of neurological dysfunction followed by complete or incomplete recovery. Fifteen per cent of people present with a gradually progressive disease course from onset known as primary progressive MS (PPMS). A single episode in isolation with no previous clinical attacks in someone who does not fulfil the diagnostic criteria for MS is known as clinically isolated syndrome (CIS). Over time, people with RRMS can develop gradually progressive disability called secondary progressive MS (SPMS). This usually occurs at least 10–15 years after disease onset. These descriptions of clinical disease course are still used in practice (Fig (Fig1). 1 ). However, increased understanding of MS and its pathology has led to new definitions focused on disease activity (based on clinical or magnetic resonance imaging (MRI) findings) and disease progression. 1

Multiple sclerosis disease course.
Clinical presentation
MS is a CNS disease characterised by demyelinating lesions in regions including the optic nerves, brainstem, cerebellum, periventricular and spinal cord. Histopathology also shows widespread involvement of the cerebral grey matter, although this is not well appreciated on conventional MRI. The clinical features of an MS attack depend on the areas of the brain or spinal cord involved. As this is an inflammatory condition, the onset of symptoms of an attack in RRMS is usually gradual and can evolve over days. Sudden onset with symptoms maximal at onset would be much more suggestive of a vascular event. A clinical attack must last at least 24 hours in the absence of fever or infection. In primary progressive MS, symptoms would be expected to have a gradual and insidious onset over at least 12 months by the time of diagnosis.
A common first presentation of RRMS is with unilateral optic neuritis characterised by gradual onset monocular visual loss, pain on moving the eye and altered colour vision. Visual loss rarely progresses beyond 2 weeks from the onset. Visual recovery usually takes longer than 2 weeks and may not recover to baseline. On examination, visual acuity is typically reduced, there may be a relative afferent pupillary defect, a central scotoma or impaired colour vision. On funduscopy, the optic disc may appear normal (retrobulbar neuritis) or swollen acutely, and may become pale and atrophic over time following the attack.
An inflammatory lesion in the spinal cord causes a myelitis that is usually partial and presents with gradual onset sensory and motor symptoms of the limbs. Evolution is over hours to days. The severity of myelitis can vary from a mild sensory syndrome to a severe disabling attack causing tetraparesis. A lesion in the cervical cord can cause Lhermitte's phenomenon with an electric shock-like sensation down the neck and back on flexing the neck. This can be a useful clue to the diagnosis. Thoracic cord lesions can cause a tight band-like sensation around the trunk or abdomen often described as the ‘MS hug’. In severe cases this has been misinterpreted as being due to a cardiac event. On examination, signs can include sensory signs of reduced fine touch, vibration sense and joint position sense. There may be a sensory level. Motor signs are typical of an upper motor neuron lesion with increased tone or spasticity, pyramidal weakness and hyperreflexia with extensor plantar responses. Myelitis may be partial causing a hemi-cord syndrome or partial Brown-Séquard.
Brainstem syndromes can present with diplopia, oscillopsia, facial sensory loss, vertigo and dysarthria. Typical findings include an isolated sixth nerve palsy, gaze evoked nystagmus or an internuclear ophthalmoplegia. Bilateral internuclear ophthalmoplegia is pathognomonic of MS.
The diagnosis of MS is based on the clinical features of the attacks including the history and examination findings. The guiding principle of the diagnosis is that of dissemination in time (DIT) and dissemination in space (DIS). There is no single diagnostic laboratory test for MS. The diagnosis is based on the clinical findings supported by investigations.
Investigations
Magnetic resonance imaging.
MRI has been increasingly used to support the diagnosis of MS and to look for any atypical features suggesting an alternative diagnosis. Brain and spinal cord MRI are used to determine dissemination in space (DIS) and for evidence of dissemination in time (DIT) in patients with a typical CIS. DIS can be demonstrated by one or more MRI T2-hyperintense lesions that are characteristic of MS in two or more of four areas of the CNS: periventricular; cortical or juxtacortical; infratentorial; and the spinal cord. DIT can be demonstrated by the simultaneous presence of gadolinium-enhancing and non-enhancing lesions at any time or a new T2 lesion or gadolinium-enhancing lesion on follow-up MRI. 2
Cerebrospinal fluid
Cerebrospinal fluid (CSF) examination remains a valuable diagnostic test, particularly when clinical and MRI evidence is insufficient to confirm the diagnosis of MS. There has been a major change in the most recent MS diagnostic criteria in that oligoclonal bands in the CSF can be used as a surrogate marker of DIT to confirm the diagnosis of RRMS in people with CIS and MRI evidence of DIS. 3 CSF findings are also important when there is a progressive course from onset (PPMS) and when there are any atypical clinical or imaging findings. Evidence of intrathecal antibody synthesis (ie oligoclonal bands in the CSF but not in a paired serum sample) supports the diagnosis of MS. An elevated CSF protein >1.0 g/L or significant pleocytosis >50 cells/mm³ or the presence of neutrophils would suggest an alternative diagnosis.
Visually evoked potentials and optical coherence tomography
Visually evoked potentials (VEPs) were historically included in MS diagnostic criteria with an abnormal VEP (delayed but with a well preserved waveform) being used as objective evidence of a second lesion if the clinical presentation did not include the visual pathway. 4,5 In the 2017 criteria, it was recommended that further studies are needed to determine the role of VEPs and optical coherence tomography (OCT) in supporting the diagnosis of MS and VEPs are not included in the criteria. 3 In clinical practice VEPs can be useful, for example in a patient with a progressive spinal cord syndrome and normal brain MRI.
Differential diagnosis
The differential diagnosis of MS is wide and varies depending on the site of presentation eg optic nerve or spinal cord. It is important for the clinician to be vigilant for atypical clinical findings or investigation results. 6,7 Non-specific symptoms with white matter lesions on the MRI can be a common cause of misdiagnosis of common disorders, such as migraine or small vessel vascular disease in the elderly.
Other CNS inflammatory diseases including neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease and acute disseminated encephalomyelitis (ADEM) are important differential diagnoses as the treatment approaches are different. Several other rarer inflammatory, infective and metabolic conditions should also be considered (Table (Table1 1 ).
Differential diagnosis of multiple sclerosis
| Autoimmune/inflammatory | CNS infections | Metabolic | Vascular conditions | Other |
|---|---|---|---|---|
| Neuromyelitis optica spectrum disorder (NMOSD) | CNS syphilis | Vitamin B deficiency | Small vessel disease | CNS lymphoma |
| Acute disseminated encephalomyelitis (ADEM) | Lyme disease | Copper deficiency | Stroke | Paraneoplastic |
| Myelin oligodendrocyte glycoprotein (MOG) antibody disease | Human T-lymphotropic virus (HTLV) | Mitochondrial disease | CADASIL | |
| Sjögren's syndrome | HIV | Leukodystrophies | Susac's syndrome | |
| CNS lupus | Anti-phospholipid antibody syndrome | |||
| Sarcoidosis | ||||
| Behçet's | ||||
| CNS vasculitis |
CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CNS = central nervous system.
NMOSD is an inflammatory CNS syndrome distinct from MS that can be associated with serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG). 8 NMOSD is stratified by serologic testing into NMOSD with or without AQP4-IgG. NMOSD presents with severe episodes of complete transverse myelitis and/or severe episodes of optic neuritis with incomplete recovery or a brainstem syndrome of the area postrema causing nausea and vomiting or hiccups. More stringent clinical criteria with additional neuroimaging findings, are required for diagnosis of NMOSD without AQP4-IgG or when serologic testing is unavailable. The myelitis is usually extensive on MRI with a T2 spinal cord lesion extending over three or more spinal segments (longitudinally extensive transverse myelitis (LETM)). Brain lesions in NMO are located in areas of high expression of aquaporin 4, including the hypothalamus, medulla, and other brainstem areas. Oligoclonal bands in the CSF are detected in 10–20% of patients with NMO.
MOG antibody disease is associated with pathogenic serum antibodies against myelin oligodendrocyte glycoprotein. 9 Most are AQP4-IgG-seronegative. The presentation is varied ranging from isolated optic neuritis to classic NMO to ADEM. It affects both children and adults and there is no sex predominance. The disease can relapse, but medium-term immunosuppression is usually effective. Permanent disability is less frequent than in NMOSD associated with positive AQP4 antibodies. Sphincter and sexual dysfunction are common as the transverse myelitis involves the conus.
ADEM tends to present with a subacute encephalopathy with altered level of consciousness, behaviour or cognitive function. It often follows an infectious illness and is most common in children. It was initially thought to be a monophasic condition, but some patients experience recurrence of their initial ADEM symptoms. Around 60% of children with ADEM have MOG antibodies. MRI typically shows symmetrical multifocal or diffuse brain lesions (Table (Table1 1 ).
Diagnostic criteria
Diagnostic criteria for MS have been developed since the first description of MS as ‘La sclérose en plaques disséminées’ by Charcot in 1868. He described a triad of nystagmus, intention tremor and scanning speech. Clinical criteria were supplemented by CSF, MRI and evoked potentials in the Poser criteria in 1983. 4 With the more widespread availability of MRI, the McDonald criteria were developed by the International Panel on Diagnosis of Multiple Sclerosis in 2001 giving increasing weight to MRI in the diagnosis of MS. 5 There have been subsequent revisions of these criteria in 2005 and 2010 with the most recent revision in 2017 (Table (Table2 2 ). 3 The new criteria allow for an earlier diagnosis of MS in patients experiencing a typical clinically isolated syndrome. Earlier diagnosis of MS has become much more important with the availability of highly effective disease modifying treatments (DMTs) for MS. However, the benefits of earlier diagnosis have to be balanced with the risks of misdiagnosis. 10 The 2017 position paper addresses concerns about the potential for misdiagnosis, particularly with misinterpretation of non-specific symptoms and non-specific MRI findings. The development of the 2017 McDonald criteria was informed by the new MRI criteria for diagnosing MS proposed by the European Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) network. 1,2
2017 McDonald criteria for the diagnosis of multiple sclerosis in patients with an attack at onset 3
| Number of attacks at clinical presentation | Number of lesions with objective clinical evidence | Additional data needed for diagnosis of multiple sclerosis |
|---|---|---|
| ≥2 | ≥2 | None |
| ≥2 | 1 (as well as clear-cut historical evidence of a previous attack involving a lesion in a distinct anatomical location) | None |
| ≥2 | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site |
| Or by MRI | ||
| 1 | ≥2 | Dissemination in time demonstrated by an additional clinical attack |
| Or by MRI | ||
| Or demonstration of CSF-specific oligoclonal bands | ||
| 1 | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site |
| Or by MRI | ||
| And dissemination in time demonstrated by an additional clinical attack | ||
| Or by MRI | ||
| Or demonstration of CSF-specific oligoclonal bands |
a = no additional tests are required to demonstrate dissemination in space and time. However, unless MRI is not possible, brain MRI should be obtained in all patients in whom the diagnosis of multiple sclerosis is being considered. In addition, spinal cord MRI or CSF examination should be considered in patients with insufficient clinical and MRI evidence supporting multiple sclerosis, with a presentation other than a typical clinically isolated syndrome, or with atypical features. If imaging or other tests (eg CSF) are undertaken and are negative, caution needs to be taken before making a diagnosis of multiple sclerosis, and alternative diagnoses should be considered. CNS = central nervous system; CSF = cerebrospinal fluid; MRI = magnetic resonance imaging.
The diagnosis of MS is still based on a combination of clinical, MRI and laboratory (eg CSF) findings. The diagnostic criteria are designed to be used for patients with typical clinical presentations and should not be applied in cases where MRI changes are incidentally identified in asymptomatic individuals. In that situation, cases are referred to as radiologically isolated syndrome (RIS). The risk of misdiagnosis may have harmful consequences if patients are started on DMTs inappropriately, for example, some MS DMTs can worsen outcomes for patients with NMO spectrum disorders.
The aim of the criteria is to make an earlier and accurate diagnosis of MS and lessen the period of uncertainty for the patient and clinician. This enables appropriate management including confirmation of the diagnosis for the patient and access to effective disease modifying treatments.
The clinical diagnosis of MS is based on history and examination providing evidence of typical neurological dysfunction. Dissemination in time and space can be demonstrated clinically but MRI is now routinely used to confirm the diagnosis. With a single episode, or clinically isolated syndrome, MRI evidence can allow an earlier diagnosis using new diagnostic criteria. Over-interpretation of non-specific symptoms and non-specific white matter lesions on MRI can lead to misdiagnosis. The differential diagnosis of MS includes other CNS inflammatory conditions such as NMOSD, ADEM and MOG antibody-related disease. It is important to differentiate these conditions as the treatment approach is different. CNS infections, metabolic conditions and vascular disease also need to be considered. There are now highly effective DMTs for MS and an accurate, timely diagnosis is crucial to ensure appropriate access to treatment.
Clinical analysis of 23 cases of epidermoid cyst of testis in children
- Original Article
- Published: 02 July 2024
- Volume 40 , article number 165 , ( 2024 )
Cite this article

- Jie Tao 1 , 2 , 3 ,
- Fei Chen 1 , 2 , 3 ,
- Xia Chen 1 , 2 , 3 &
- Junhong Liu 1 , 2 , 3
This study aims to examine the clinical characteristics and surgical management of pediatric testicular epidermoid cysts, thereby contributing to the existing body of knowledge pertinent to the diagnosis and therapeutic intervention 你 s for this condition.
A retrospective analysis was conducted on the clinical records of 23 pediatric patients diagnosed with testicular epidermoid cysts, who were admitted to our institution between April 2013 and February 2024. Concurrently, a comprehensive review and analysis of pertinent literature were undertaken to augment the findings.
The mean age at which the onset of epidermoid cysts was observed was 6.0 years. All cases were singular and unilateral. B-ultrasound diagnosis categorized 6 cases as epidermoid cysts, 11 as teratomas, and 6 as indeterminate, yielding a diagnostic sensitivity of 26.1%. All patients underwent testicle-sparing mass resection, and nine patients underwent rapid intraoperative frozen section analysis, revealing eight cases of testicular epidermoid cysts and one teratoma, with a diagnostic sensitivity of 88.89%. Postoperative histopathological examination confirmed the diagnosis of testicular epidermoid cyst.
Conclusions
Pediatric testicular epidermoid cysts are an uncommon occurrence, primarily presenting as a painless scrotal mass, which can mimic the clinical features of malignant testicular tumors. Imaging modalities and histopathological assessment are pivotal in the diagnostic process for pediatric testicular epidermoid cysts. For cases where B-ultrasound is inconclusive, rapid intraoperative pathological examination should be considered.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Shah KH, Maxted WC, Chun B (1981) Epidermoid cysts of the testis: a report of three cases and an analysis of 141 cases from the world literature. Cancer 47(3):577–582
Article CAS PubMed Google Scholar
Loya AG, Said JW, Grant EG (2004) Epidermoid cyst of the testis: radiologic-pathologic correlation. Radiographics 24(Suppl 1):S243–S246
Article PubMed Google Scholar
Liu Y, Pan Y, Wang Z (2022) MRI diagnostic value of epidermoid cyst of testis. J Med Imaging 32(11):2025–2029
Google Scholar
Arellano CM et al (2011) Testicular epidermoid cysts in children: sonographic characteristics with pathological correlation. Pediatr Radiol 41(6):683–689
Maulana R, Siregar S, Hernowo BS (2022) Testicular epidermoid cyst: a rare case report. Int J Surg Case Rep 94:107167
Article PubMed PubMed Central Google Scholar
Dogra VS et al (2001) Testicular epidermoid cysts: sonographic features with histopathologic correlation. J Clin Ultrasound 29(3):192–196
Li Y et al (2022) Research progress of epidermoid cyst of testis. Cancer Prog 20(07):656–658
Win ML et al (2020) Bilateral testicular epidermoid cysts in a man with klinefelter syndrome: a case report. Cureus 12(12):e11834
PubMed PubMed Central Google Scholar
Zhang J, Li Y (2022) Value of high frequency ultrasonography in diagnosing epidermoid cyst of testis. J Shandong Med College 44(04):309–310
Rong H, Gao N, Wang D (2022) The value of high frequency ultrasound combined with alpha-fetoprotein detection in the accurate and qualitative diagnosis of testicular tumors in children. Basic Clin Oncol 35(06):525–527
Price EJ (1969) Epidermoid cysts of the testis: a clinical and pathologic analysis of 69 cases from the testicular tumor registry. J Urol 102(6):708–713
Changli Xu et al (2019) Epidermoid cyst of testis: report of 5 cases and literature review. Chin J Androl 33(05):60–62
Download references
Acknowledgements
The authors would like to thank the parents and children who enrolled in the study and the health professionals from the department of ultrasonography. Their outstanding support and contributions are gratefully appreciated.
No funding.
Author information
Authors and affiliations.
Department of Day Surgery, National Clinical Research Center for Child Health and Disorders, Ministry of Education, Key Laboratory of Child Development and Disorder, Children’s Hospital of Chongqing Medical University, Chongqing, China
Jie Tao, Fei Chen, Xia Chen & Junhong Liu
China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing, China
Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing, China
You can also search for this author in PubMed Google Scholar
Contributions
All authors have worked on the manuscript. Jie Tao wrote the manuscript under the supervision of Junhong Liu. Fei Chen and Xia Chen followed up the patient.
Corresponding author
Correspondence to Junhong Liu .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Tao, J., Chen, F., Chen, X. et al. Clinical analysis of 23 cases of epidermoid cyst of testis in children. Pediatr Surg Int 40 , 165 (2024). https://doi.org/10.1007/s00383-024-05750-9
Download citation
Accepted : 24 June 2024
Published : 02 July 2024
DOI : https://doi.org/10.1007/s00383-024-05750-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidermoid cyst
- Testicular cancer
- Find a journal
- Publish with us
- Track your research

COMMENTS
The clinical presentation of COVID-19 ranges from asymptomatic to critical illness. An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity. Uncommon presentations of COVID-19 can occur, might vary by the age of the ...
(See "Chronic kidney disease in children: Clinical manifestations and evaluation".) CLINICAL PRESENTATION — Patients with chronic kidney disease (CKD) may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no clinical symptoms, and kidney disease is often ...
Clinical manifestations Patients with COVID-19 experience varying degrees of severity, and 80% of them have mild infection [ 102 ]. Approximately 15% of cases develop severe disease characterized by dyspnea, hypoxia, and lung changes on imaging; 5% are critically ill, with respiratory failure from ARDS, shock, and/or multi-organ dysfunction [ 3 ...
Patients with SARS-CoV-2 infection can experience a range of clinical manifestations, from no symptoms to critical illness. In general, adults with SARS-CoV-2 infection can be grouped into the following severity of illness categories; however, the criteria for each category may overlap or vary across clinical guidelines and clinical trials, and a patient's clinical status may change over time.
While we found differences in clinical features of COVID-19 compared to other acute respiratory illnesses, there was significant overlap in presentation and comorbidities. Patients with COVID-19 were more likely to be admitted to the hospital, have longer hospitalizations and develop ARDS, and were unlikely to have co-existent viral infections.
Although some of the clinical manifestations of SARS, MERS, and COVID appears to be similar, distinct symptoms have also been reported in some patients, so faster differential diagnosis is required for treatment.[3,11,12,13] Laboratory findings of patients infected by COVID-19 showed lymphopenia and leukopenia. Furthermore, findings of chest ...
This review provides an updated framework and essential knowledge to help radiologists better understand the etiology, epidemiology, clinical manifestations, and pathology of COVID-19. Most importantly, it highlights the radiological findings and the potential effects of chest CT on the management of suspected and confirmed patients, with ...
Cheng, Z. et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am. J. Roentgenol. 215 (1), 121-126 (2020).
ENT manifestations are one of the most frequent symptoms encountered by physicians in COVID-19. A peculiar clinical presentation in some COVID-19 patients includes the deterioration of sense, taste (dysgeusia), and loss of smell (anosmia).
Thrombotic manifestations of severe COVID-19 are caused by the ability of SARS-CoV-2 to invade endothelial cells via angiotensin-converting enzyme-2 (ACE-2), which is expressed on the surface of endothelial cells. ... A Non-systematic Review of Clinical Presentation, Potential Effects of Physiological Adaptations in Pregnancy, and Placental ...
In February 2020, the World Health Organization designated the disease COVID-19, which stands for coronavirus disease 2019 [ 1 ]. The virus that causes COVID-19 is designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); previously, it was referred to as 2019-nCoV. Understanding of COVID-19 is evolving.
Objectives: To assess and compare clinical manifestations and laboratory findings of dengue virus infected children and adults in Thailand. Study design: A 1-year study was conducted from September 2003 to August 2004 for dengue virus infected patients admitted to Phetchabun Provincial Hospital, Thailand. Physical signs, symptoms, and ...
The clinical presentation of lupus nephritis is highly variable, ranging from asymptomatic hematuria and/or proteinuria to nephrotic syndrome and rapidly progressive glomerulonephritis with loss of kidney function. ... Clinical manifestations - Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with a wide range of ...
In conclusion, this review introduces the current status of knowledge on the global pandemic and clinical features of COVID-19. Given that SARS-CoV-2 is the third introduction of a deadly coronavirus into human society, after SARS-CoV in 2003 and MERS-CoV in 2012, respectively, various clinical manifestations of these three viruses were compared.
Abstract. Most physicians, if asked to distinguish between signs and symptoms, would reply in a fashion something like this: A symptom is a manifestation of disease apparent to the patient himself, while a sign is a manifestation of disease that the physician perceives. The sign is objective evidence of disease; a symptom, subjective.
Global collaboration and sequencing technologies have helped to uncover the pathogenic mechanisms behind SARS-CoV-2 infection and its associated clinical manifestations, collectively called coronavirus disease 2019 (COVID-19). Nevertheless, COVID-19 remains a novel disease and its complete pathogenesis is yet to be elucidated.
Its clinical spectrum at presentation is broad, since the infiltration of different organs leads to the development of protean manifestations. Current literature identified bone, central nervous system and retroperitoneal as the most common localisations of ECD. 1 , 6 Still, the clinical picture at the time of onset may often be substantially ...
The symptoms of RA can affect patients' capacity to perform the activities of daily living (eg, walking, stairs, dressing, use of a toilet, getting up from a chair, opening jars, doors, typing) and those required in their occupation. Systemic symptoms may also be present in these patients, particularly those with disease onset after age 60 ...
Myocarditis is an overlooked manifestation of anti-synthetase syndrome (ASS). Our study describes the clinical and instrumental feature. ... This is due to the clinical presentation, which is frequently subclinical and not specific, and the absence of a definite diagnostic algorithm . An early diagnosis of myocarditis is essential for prompt ...
clinical manifestation. This phrase is correct and commonly used in the medical field to describe the signs and symptoms of a disease or condition. It refers to the observable or measurable signs and symptoms that indicate the presence of a particular disease or condition. The clinical manifestation of the flu includes fever, cough, and body ...
Bilateral internuclear ophthalmoplegia is pathognomonic of MS. The diagnosis of MS is based on the clinical features of the attacks including the history and examination findings. The guiding principle of the diagnosis is that of dissemination in time (DIT) and dissemination in space (DIS). There is no single diagnostic laboratory test for MS.
underlying comorbidities at presentation • Children (< 18 years), regardless of illness severity or underlying comorbidities at presentation 3. Patients with protracted or life -threatening manifestations of mpox at presentation as defined by one of the following: • Lesions affecting ≥ 25% of body surface that may be confluent,
One study noted that the most common symptoms at presentation were cough (55 percent), dyspnea (45 percent), pain (38 percent), and weight loss (36 percent) ( table 1) [ 3 ]. This discussion will present the clinical manifestations of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Screening and risk factors for lung ...
An expert opinion/approach: clinical presentations, diagnostic considerations, and therapeutic options for gastrointestinal manifestations of common variable immune deficiency. Am J Gastroenterol ...
HF is a complex clinical syndrome identified by presence of current or prior characteristic symptoms, such as dyspnea and fatigue, and evidence of cardiac dysfunction as a cause of these symptoms (eg, abnormal left ventricular [LV] and/or right ventricular [RV] filling and elevated filling pressures) [ 1-5 ]. From a hemodynamic perspective, HF ...
The mean duration from mass detection to clinical presentation was 9.4 months (range, 1 day to 84 months). Lesion location was in the middle pole of the testis in 13 patients, upper pole in 8, and lower pole in 2. ... which can easily lead to misdiagnosis, while the ultrasonic manifestations of testicular epidermoid cysts in young people are ...
CLINICAL PRESENTATION. Pneumothorax should be suspected in patients who present with acute dyspnea and chest pain (classically pleuritic), particularly in those with an underlying risk factor ( table 1 ). The major competing diagnoses include acute pulmonary embolism, pleuritis, pneumonia, myocardial ischemia or infarction, pericarditis, and ...
The epidemiology, clinical manifestations, pathologic features, diagnosis, and classification of MDS are reviewed in this topic. ... CLINICAL PRESENTATION — Clinical manifestations of MDS are variable. Some patients present with fatigue, infections, bruising, or other manifestations of cytopenias, while others are asymptomatic and come to ...