Novel aerial observations of a possible newborn white shark ( Carcharodon carcharias ) in Southern California
- Open access
- Published: 29 January 2024
- Volume 107 , pages 249–254, ( 2024 )

Cite this article
You have full access to this open access article

- Carlos Gauna 1 &
- Phillip C. Sternes ORCID: orcid.org/0000-0001-7223-3725 2
37k Accesses
2143 Altmetric
411 Mentions
Explore all metrics
The white shark ( Carcharodon carcharias ) is the largest macropredatory fish in the world. Yet, there remains a paucity of data on the early life history and reproduction of this iconic shark. Here, we present aerial observations of an individual white shark that appears to be sloughing a white film from its body. We propose two possibilities for the possession of the white film: (1) this is a newly born white shark with intrauterine substances still adhered to its body, or (2) this white shark has an unknown skin disorder resulting in shedding, discharge, or possibly a microbial growth over the dermal layer. We discuss the possibility that this individual is a newborn and its implications for the Southern California region as a critical birthing location.
Avoid common mistakes on your manuscript.
The white shark ( Carcharodon carcharias ) is an iconic, large predatory shark with a worldwide distribution that attracts considerable interest from both the scientific community and the public (Klimley and Ainley 1996 ; Domeier 2012a ; Huveneers et al. 2018 ; Ebert et al. 2021 ). Despite the high level of interest, there remain some major gaps in white shark life history such as information on breeding and newborns (Klimley 1985 ; Klimley and Ainley 1996 ; Domeier 2012a ; Huveneers et al. 2018 ; Santana-Morales et al. 2020 ; Anderson et al. 2021 ). For example, Klimley ( 1985 ) suggested that large adult female sharks may use the area from Santa Barbara, California to north Baja California, Mexico to give birth. Recent evidence has supported this as Sebastian Vizcaino Bay (Orñate-González et al. 2017 ) and Cedros Island near Baja California, Mexico (Tamburin et al. 2020 ) as well as the coast of Southern California, USA (Anderson et al. 2021 ) had an abundance of young of the year white sharks that displayed high residency and restricted movements at these areas over the course of a year (Fig. 2 ). Furthermore, Santana-Morales et al. ( 2020 ) reported the smallest known free-living white shark was caught off the Pacific coast near the USA-Mexico international border, measuring 106.6 cm total length (TL) with morphological features similar to that of white shark embryos.
On July 9, 2023, a pale white shark (Fig. 1 ; Supplemental Video which is available by request/contact authors directly) was spotted via aerial drone (Mavic 3 Pro) 0.4 km off the coast of Carpinteria, CA, USA (Fig. 2 ). Upon close examination of video and photos, the individual’s pale color appears to be a thin white film covering the shark. We noted that as the shark was swimming, the whitish film was being sloughed off. During post-video analysis, we estimated the size of the individual to be 1.5 ± 0.2 m TL, although with some uncertainty ( Supplementary Information ). We also wish to point out that, both days prior and the day of, large likely mature sharks were also recorded in the same area (Fig. 3 ). We propose two possibilities for this shark’s interesting pale color: (1) the whitish film is left over intrauterine substances being sloughed off the shark due to it being a newborn shark, or (2) the whitish film is due to an unknown skin disorder that has not been reported in white sharks before in the published literature.

Images of white shark with a white film covering its body observed 0.4 km off the coast of Carpinteria, CA, USA (Photo Credits: Carlos Gauna)
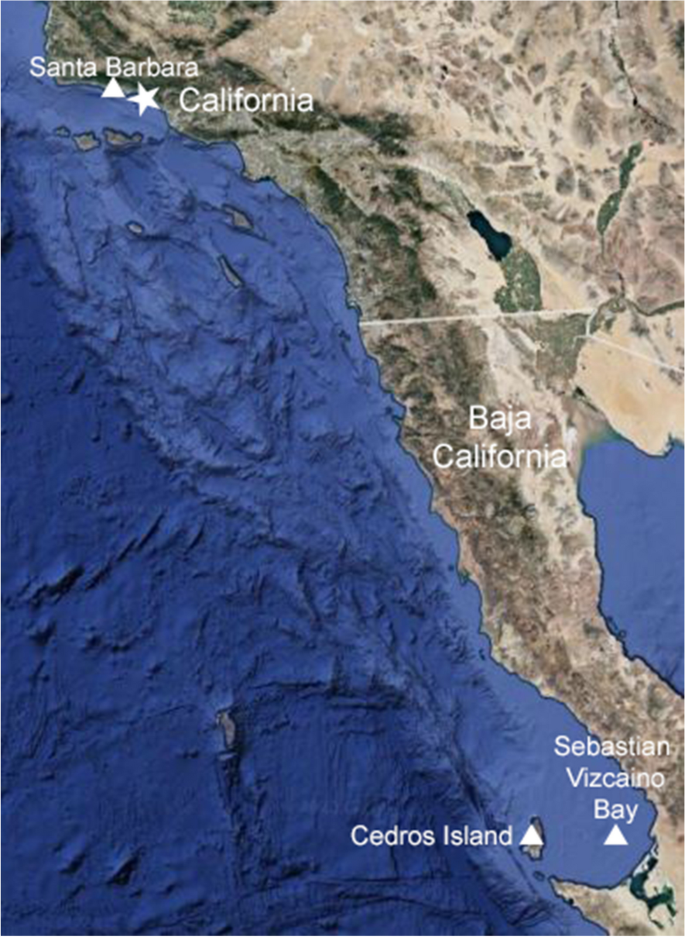
Locations with high residency of young of the year white sharks = ▲. Location of the white shark with white film =★

Images of one of several large likely mature sharks filmed the days prior and the day of in the same area (0.4 km off the coast of Carpinteria, CA, USA) as the pale white shark being spotted (Photo Credits: Carlos Gauna)
First, like other lamniform sharks, white sharks are viviparous, and embryonic sharks perform the unique behavior of oophagy (Gilmore 1993 ; Francis 1996 ; Uchida et al. 1996 ; Conrath and Musick 2012 ; Sato et al. 2016 ; Ebert et al. 2021 ). Oophagous sharks will consume eggs and store yolk in their stomachs as a source of nutrition (Gilmore 1993 ; Francis 1996 ; Uchida et al. 1996 ; Sato et al. 2016 ). In addition, pregnant female white sharks will also produce “uterine milk,” a yellowish and milky fluid, for the embryos to also consume (Sato et al. 2016 ). However, it remains unclear on the duration of this fluid being produced during the estimated 12-month gestation period (Sato et al. 2016 ). After the gestation period, an estimated 2–17 pups may be birthed (Ebert et al. 2021 ). In terms of morphology, the pups are very similar in appearance to near-term embryos as they are notable for being 1–1.6 m TL and with more rounded fin apexes (Francis 1996 ; Uchida et al. 1996 ; Tomita et al. 2018 ; Santana-Morales et al. 2020 ; Ebert et al. 2021 ). In the Northeast Pacific, Klimley ( 1985 ) proposed that from Santa Barbara to Baja California, during late summer and early fall, pregnant female white sharks would give birth to pups. Recent studies have supported this claim as vast amounts of young of the year white sharks have been tagged and monitored in this geographical range and time period (Orñate-González et al. 2017 ; White et al. 2019 ; Santana-Morales et al. 2020 ; Tamburin et al. 2020 ; Anderson et al. 2021 ).
Here, we offer one hypothetical scenario for this individual white shark and its whitish film. First, this individual white shark is an estimated 1.5 ± 0.2 m TL ( Supplementary Information ), well within the size range of newborn sharks (i.e., 1–1.6 m TL), and its overall appearance (i.e., rounded fin apexes) is similar to that of near-term embryos and the smallest reported free-living white shark (Francis 1996 ; Uchida et al. 1996 ; Tomita et al. 2018 ; Santana-Morales et al. 2020 ; Ebert et al. 2021 ). Second, this white shark was filmed in both the proposed area and specific time when pups are birthed (Klimley 1985 ). Third, large likely mature sharks were also recorded in the area with our drone surveillance (Fig. 3 ) and were also sighted by other researchers in the area (C. Lowe, personal communication). Taking these factors into consideration, a parsimonious interpretation is that the larger sharks may be mature females known to frequent this region (Klimley 1985 ) and that the pale subject is a newly born individual, possibly being days or even hours old. We propose that the whitish film being sloughed off the body is composed of intrauterine substances that had adhered to the shark while still in its mother’s uterus. Given that white sharks produce “uterine milk,” it is within the realm of possibility either this fluid or another fluid could have adhered to the shark right before birth.
In the alternative scenario, this individual white shark has a unique unknown skin disorder. Skin disorders are known to occur in sharks and rays, but it is rare (Gervais et al. 2016 ; Rodrigues et al. 2023 ). For example, albinism and leucism have been reported in only 62 (including one reported case of albinism in the white shark) of the 1200 species of elasmobranchs (Smale and Heemstra 1997 ; Bigman et al. 2016 ; Arronte et al. 2022 ; Becker et al. 2023 ; Rodrigues et al. 2023 ). It has also been noted that pollution and temperature may affect normal pigmentation development leading to irregular color patterns (Gervais et al. 2016 ; Bruckner and Coward 2018 ). However, the evident sloughing of a semi-opaque layer from the small shark reveals normal pigmentation below; therefore, albinism and leucism are not supported (Fig. 1 ; Supplemental Video which is available by request/contact authors directly). Skin diseases such as dermatitis and other bacterial infections have been reported in sharks (Leibovitz and Lebouitz 1985 ; Garner 2013 ; Pogoreutz et al. 2019 ; Newton and Ritchie 2022 ), but none of these reported conditions are similar to the one observed in this individual white shark. Therefore, this may be an unknown skin disorder that has not been reported in the literature before.
Here, we have presented evidence of an individual white shark with a whitish film covering its body, observed off the coast of Southern California. We propose two explanations for this individual’s white milky appearance: the first being it is a newborn shark with embryonic substance forming a layer on the shark and the second being the shark has an unknown skin disorder. For the first proposal, previous studies have demonstrated that this region of Southern California, USA, is a critical nursery habitat for white sharks in the Eastern Pacific (Klimley 1985 ; Domeier 2012b ; White et al. 2019 ; Anderson et al. 2021 ). If this is indeed a newborn individual, this demonstrates the critical importance of this area in Southern California to Eastern Pacific white sharks. White sharks are highly protected off California (Heneman and Glazer 1996 ), but incidental catches by fishermen do occur (Lowe et al. 2012 ; Benson et al. 2018 ). Although individuals are released, some may retain hooks and fishing leaders that cause stress, damage, and possible subsequent death to the individuals (Fig. 4 ; Lowe et al. 2012 ; Benson et al. 2018 ). Therefore, more effort and stricter management might be required to protect not only this area but the entire coastline extending down to Baja California as well for white shark conservation. In terms of the second proposal, this would represent a previously unknown skin disorder that has not been observed in white sharks or sharks and rays in general. Both hypotheses will require further investigation and additional evidence for support or refutation. Nevertheless, in either case, the use of the aerial drone has provided shark science with another interesting set of information (Butcher et al. 2021 ). Therefore, future drone observations of sharks in this area will greatly improve our knowledge and understanding of white shark life history.

Images of a white shark that has suffered abrasions due to a fishing leader that it retains after being released by fishermen (Photo Credits: Carlos Gauna)
Data availability
Data is available upon request.
Anderson JM, Burns ES, Meese EN, Farrugia TJ, Stirling BS, White CF, Logan RK, O’Sullivan JO, Winkler C, Lowe CG (2021) Interannual nearshore habitat use of young of the year white sharks off Southern California. Front Mar Sci 8:64512
Article Google Scholar
Arronte JC, Antolínez A, Bañon R, Rodríguez-Gutiérrez J, Ortíz JJ, Martinez JM (2022) First recorded case of leucism in the velvet belly lantern shark Etmopterus spinax (Squaliformes: Etmopteridae). J Appl Ichthyol 38:455–461
Becker MA, Kline CG, Maisch HM IV, Sternes PC, Shimada K (2023) First-hand observations of rare piebaldism in the nurse shark, Ginglymostoma cirratum , near East Bahia Honda, Florida Keys, Florida. Florida Scientist 86:13–16
Google Scholar
Benson JF, Jorgensen SJ, O’Sullivan JB, Winkler C, White CF, Garcia-Rodgriguez E, Sosa-Nishizaki O, Lowe CG (2018) Juvenile survival, competing risks, and spatial variation in mortality risk of a marine apex predator. J Appl Ecol 55:2888–2897
Bigman JS, Knuckley JDS, Ebert DA (2016) Color aberrations in chondrichthyan fishes: first records in the genus Bathyraja (Chondrichthyes: Rajiformes: Arhynchobatidae). Mar Biodivers 46:579–587
Bruckner AW, Coward G (2018) Unusual occurrence of abnormal skin pigmentation in blacktip reef sharks ( Carcharhinus melanopterus ). Coral Reefs 37:389
Article ADS Google Scholar
Butcher PA, Colefax AP, Gorkin RA, Kajiura SM, Lόpez NA, Mourier J, Purcell CR, Skomal GB, Tucker JP, Walsh AJ, Williamson JE, Raoult V (2021) The drone revolution of shark science: a review. Drones 5:8
Conrath CL, Musick JA (2012) Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, second edn. CRC Press, Boca Raton, pp 291–311
Domeier ML (2012a) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton
Book Google Scholar
Domeier ML (2012b) A new life-history hypothesis for white sharks, Carcharodon carcharias , in the Northeastern Pacific. In: Domeier ML (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton, pp 199–224
Chapter Google Scholar
Ebert DA, Dando M, Fowler S (2021) Sharks of the world: a complete guide. Princeton University Press, Princeton
Francis MP (1996) Observations on a pregnant white with a review of reproductive biology. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias . Academic Press, San Diego, pp 157–172
Garner MM (2013) A retrospective study of disease in elasmobranchs. Vet Pathol 50:377–389
Article MathSciNet PubMed CAS Google Scholar
Gervais C, Mourier J, Rummer J (2016) Developing in warm water: irregular colouration and patterns of a neonate elasmobranch. Mar Biodiversity 4:743–744
Gilmore RG (1993) Reproductive biology of lamnoid sharks. Environ Biol Fishes 38:95–114
Heneman B, Glazer M (1996) More rare than dangerous: a case study of the white shark conservation in California. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias . Academic Press, San Diego, pp 481–491
Huveneers C, Apps K, Becerril-García EE et al (2018) Future research directions on the “elusive” white shark. Front Mar Sci 5:455
Klimley AP (1985) Areal distribution and autoecology of the white shark off the western coast of North America. Mem Calif Acad Sci 9:15–40
Klimley AP, Ainley DG (1996) Great white sharks: the biology of Carcharodon carcharias . Academic Press, San Diego
Leibovitz L, Lebouitz SS (1985) A viral dermatitis of the smooth dogfish sharks, Mustelus canis (Mitchell). J Fish Dis 8:273–279
Lowe CG, Blasius ME, Jarvis ET, Mason TJ, Goodmanlowe GD, O’Sullivan JB (2012) Historic fishery interactions with white sharks in the Southern California Bight. In: Domeier ML (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton, pp 169–185
Newton AL, Ritchie KB (2022) Elasmobranch health, pathology, and the host microbiome. In: Carrier JC, Simpfendorfer CA, Heithaus MR, Yopak KE (eds) Biology of sharks and their relatives, third edn. CRC Press, Boca Raton, pp 421–485
Orñate-González EC, Sosa-Nishizaki O, Herzka SZ, Lowe CG, Lyons K, Santana-Morales O, Sepulveda C, Guerrero-Avila C, García-Rodríguez E, O’Sullivan JB (2017) Importance of Bahia Sebasatian Vizcaino as a nursery area for white sharks ( Carcharodon carcharias ) in the Northeastern Pacific: a fishery dependent analysis. Fish Res 188:125–137
Pogoreutz C, Gore MA, Perna G, Millar C, Nestler R, Ormond RF, Clarke CR, Voolstra CR (2019) Similar bacterial communities on healthy and injured skin of black tip reef sharks. Anim Microbiome 1:9
Article PubMed PubMed Central Google Scholar
Rodrigues A, Shkola W, Ranel BS (2023) Extending records of albinism and skin disorders in American cownose rays to southeastern Brazil. J Fish Biol 103:453–456
Article PubMed Google Scholar
Santana-Morales O, Abadía-Cardoso A, Hoyos-Padilla M, Naylor GJP, Corrgian S, Malpica-Cruz L, Aquino-Baleytό M, Beas-Luna R, Sepulveda CA, Castillo-Géniz JL (2020) The smallest known free-living white shark Carcharodon carcharias (Lamniformes: Lamnidae): ecological and management implications. Copeia 108:39–46
Sato K, Nakumura M, Tomita T, Toda M, Miyamot J, Nozu R (2016) How great white sharks nourish their embryos to a large size: evidence of lipid histrotrophy lamnoid shark reproduction. Biol Open 5:1211–1215
Article PubMed PubMed Central CAS Google Scholar
Smale MJ, Heemstra PC (1997) First record of albinism in the great white shark, Carcharodon carcharias (Linnaeus, 1758). S Afr J Sci 93:243–245
Tamburin E, Hoyos-Padilla M, Sánchez-González A, Hernández-Herrera A, Elorriaga-Verplancken FR, Galván-Magaña F (2020) New nursery area for white sharks ( Carcharodon carcharias ) in the Eastern Pacific Ocean. Turk J Fish Aquat Sci 20:325–329
Tomita T, Toda M, Miyamoto K, Oka S, Ueada K, Sato K (2018) Development of the lunate-shaped caudal fin in white shark embryos. Anat Rec 301:1068–1073
Uchida S, Toda M, Teshima K, Yano K (1996) Pregnant white sharks and full-term embryos from Japan. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias . Academic Press, San Diego, pp 139–155
White CF, Lyons K, Jorgensen SJ, O’Sullivan J, Winkler C, Weng KC, Lowe CG (2019) Quantifying habitat selection and variability in habitat suitability for juvenile white sharks. PLoS ONE 14:e0214642
Download references
Acknowledgements
We deeply thank J. Worthington (UCSD) for their help in estimating the size of sharks. We also thank A. Shultz (LACM) for providing useful information on pelican size ranges. Lastly, we thank the four anonymous reviewers and the editor for their comments and suggestions that greatly improved the quality of this paper.
Author information
Authors and affiliations.
Malibu Artists Inc., Camarillo, CA, USA
Carlos Gauna
Department of Evolution, Ecology, and Organismal Biology, University of California, Riverside, CA, USA
Phillip C. Sternes
You can also search for this author in PubMed Google Scholar
Contributions
CG flew the drone and collected video and pictures of the sharks. CG and PCS both analyzed video and pictures of the sharks. PCS wrote the manuscript. CG and PCS reviewed and edited the text.
Corresponding author
Correspondence to Phillip C. Sternes .
Ethics declarations
Ethics approval.
Sharks were viewed by drone requiring no protocol approval.
Consent to participate
All authors agreed to participate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 121 KB)
Supplementary file2 (xlsx 18.1 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Gauna, C., Sternes, P.C. Novel aerial observations of a possible newborn white shark ( Carcharodon carcharias ) in Southern California. Environ Biol Fish 107 , 249–254 (2024). https://doi.org/10.1007/s10641-024-01512-7
Download citation
Received : 09 August 2023
Accepted : 05 January 2024
Published : 29 January 2024
Issue Date : February 2024
DOI : https://doi.org/10.1007/s10641-024-01512-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lamniform shark
- Find a journal
- Publish with us
- Track your research
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, three-dimensional movements and habitat selection of young white sharks ( carcharodon carcharias ) across a temperate continental shelf ecosystem.

- 1 Fisheries Ecology and Conservation Laboratory, Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
- 2 Atlantic Highly Migratory Species Management Division, National Oceanic and Atmospheric Administration (NOAA) National Marine Fisheries Service, Gloucester, MA, United States
- 3 South Fork Natural History Museum, Bridgehampton, NY, United States
- 4 Wildlife Conservation Society, Bronx, NY, United States
- 5 OCEARCH, Park City, UT, United States
As highly mobile predators with extensive home ranges, some shark species often utilize a continuum of habitats across the continental shelf ranging from the surf zone to the open ocean. For many species, these cross-shelf distributions can change depending on ontogeny or seasonal conditions. Recent research has confirmed a white shark ( Carcharodon carcharias ) summer nursery off Long Island, New York; however, habitat characterization of this nursery has not yet been conducted nor has fine-scale analysis of vertical behavior. Between 2016 and 2019, 21 young-of-the-year and juvenile white sharks were fitted with satellite and acoustic tags to examine distribution and selection for a suite of oceanographic variables during their late summertime (i.e., August to October) residence in the New York Bight. Horizontal position estimates were used to extract a suite of environmental measurements via remote sensing platforms and were linked with vertical profiles to produce three-dimensional movements for a subset of individuals also fitted with pop-up satellite archival tags ( n = 7). Sharks exhibited horizontal movements parallel to Long Island’s southern shoreline and coastal New Jersey, with distances from 0.1 to 131.5 km from shore. Log-likelihood chi-square analyses determined selection for waters with underlying bathymetry of 20–30 m, sea surface temperatures between 20.0 and 22.0°C, sea surface salinities between 31.0 and 32.0 ppt, and chlorophyll-a concentrations between 2.0 and 8.0 mg⋅m –3 . Multiple individuals also traversed the mid- to outer shelf region after leaving the Montauk tagging area. Vertical depth profiles illustrated oscillations between the surface and 199 m of water, with an average swimming depth of 9.2 ± 8.9 m. Water column temperatures during these oscillations ranged between 7.9 and 26.2°C (mean = 19.5 ± 2.0°C) with several individuals traversing highly stratified regions presumably associated with a mid-shelf cold pool adjacent to the Hudson Shelf Valley. These results suggest young white sharks exhibit connectivity between the immediate shoreline and mid-continental shelf region, where they play important ecological roles as predators on a variety of species. Our study improves characterization of essential fish habitat for young white sharks and provides new insights into their reliance on this productive continental shelf ecosystem.
Introduction
Shark nursery habitats are areas that can disproportionately contribute to the productivity of a population ( Beck et al., 2001 ; Heupel et al., 2007 ). These areas typically provide an appropriate food supply (both quality and quantity), ideal physical conditions (temperature, salinity, etc.), and reduced biological interactions (predation, competition, etc.) for immature individuals thereby increasing survival rates compared to other habitats ( Heupel et al., 2007 ; Parsons et al., 2008 ). Coastal shark nursery habitats may include habitat types such as mud flats, coral reefs, mangrove forests, and seagrass beds that are found in enclosed embayments or nearshore areas ( Heupel et al., 2018 ). These habitats perform a nursery role as they limit entry of large predators (as a result of the shallow depth), and offer abundant food resources critical to rapid growth during early life stages ( Heupel et al., 2018 ). However, the current definition is somewhat biased due to a historical focus on tropical and subtropical regions where nurseries are primarily located within semi-enclosed estuaries and lagoons ( Heupel et al., 2018 ). There is considerably less information on coastal and offshore nursery areas ( Knip et al., 2010 ; Heupel et al., 2018 ).
Temperate continental shelves are among some of the most dynamic and productive marine ecosystems in the world, particularly during spring and summertime when primary and secondary production is at its highest ( Friedland et al., 2015 ). For example, the mid-Atlantic Bight, located within the NE shelf large marine ecosystem of the US, seasonally supports large mobile predators such as sea turtles ( Murray and Orphanides, 2013 ; Dodge et al., 2014 ), marine mammals ( Stepanuk et al., 2018 ), and sharks ( Simpfendorfer et al., 2002 ; Kohler and Turner, 2019 ; Latour and Gartland, 2020 ). While the importance of these areas to sharks has been known for some time, the finer-scale use of shelf systems by these animals has been limited, and impacts our understanding of potential shark “hotspots” within these large ecosystems ( Bangley et al., 2020a ). The mid-Atlantic Bight has characteristic habitat heterogeneity and dynamic features that arise from the immediate shoreline out to the shelf-edge. These include Gulf Stream eddies ( Churchill et al., 1993 ), bathymetric breaks, valleys, and canyons ( Knebel, 1979 ), as well as major riverine discharges ( Hossler and Bauer, 2013 ). Should sharks exhibit preferences for or aggregate in any of these ephemeral or spatially restricted habitats, these can increase their vulnerability to overexploitation or other anthropogenic disturbances in these regions. As such, understanding the use of these shelf ecosystems by predators is important for species conservation and identifying potential threats to sustainability. This is particularly relevant in the mid-Atlantic Bight where numerous shark species are exposed to commercial and recreational fisheries ( Kohler and Turner, 2019 ; NMFS, 2020), expanding offshore wind energy infrastructure ( Methratta, 2020 ), and climate change ( Saba et al., 2016 ). This region is among the most rapidly warming large marine ecosystems in the world ( Saba et al., 2016 ), with variable consequences likely for species that rely on its habitats including potential distribution shifts and declines in productivity ( Hare et al., 2016 ; Kleisner et al., 2016 ).
Habitat use and movement dynamics of sharks along and across shelf habitats has been historically challenging to study, although research on young shark movements is growing in such areas ( Curtis et al., 2018 ; White et al., 2019 ; Bangley et al., 2020a ; Logan et al., 2020 ). This is particularly true for most pelagic shark species, which remain offshore for much of their life history and rarely captured in fishery-independent surveys. These characteristics have limited horizontal and vertical habitat data for these widely ranging species. Fortunately, such information can be revealed using individual-based biotelemetry (i.e., electronic tagging) techniques. For example, through the use of active acoustic tracking along with depth-sensing transmitters, Klimley et al. (2002) and Cartamil et al. (2010) characterized both horizontal and vertical movement patterns of young shortfin mako ( Isurus oxyrinchus ), blue ( Prionace glauca ), white ( Carcharodon carcharias ), and common thresher sharks ( Alopias vulpinus ) in the southern California Bight. However, due to the limitations of acoustic tracking, which requires being within the detection range of the receiver, tracks only lasted maximally 3.1 days and thus were both temporally and spatially restricted. While passive acoustic receiver networks can facilitate extended tracking of movements ( Bangley et al., 2020b ), these aren’t always feasible in exploratory studies of offshore animal movement and behavior around short-lived features. More recently, researchers have begun combining archival and satellite-transmitting tag technologies to support horizontal and vertical tracking of free-ranging individuals and the dynamic habitats they experience. Combined with other remote sensing environmental observations, this approach has been recently utilized to explore how temperate shark species such as basking sharks ( Cetorhinus maximus ), adult white sharks, and blue sharks use ephemeral features such as primary production hotspots along the continental shelf and mesoscale eddies in the open ocean ( Curtis et al., 2014 ; Gaube et al., 2018 ; Braun et al., 2019 ). Such coupled information permits analyses of how individual sharks interact with oceanography, transfer nutrients across ecosystem gradients, and advance our understanding of the overall movement dynamics of these highly mobile species.
Here, we utilize a similar three-dimensional approach to examine young-of-the-year (YOY) and young juvenile (age 1–2) white shark habitat use and cross-shelf connectivity in the New York Bight, an established white shark nursery ( Curtis et al., 2018 ). The summer distribution of large juvenile and adult white sharks in the northwest Atlantic generally ranges from New Jersey to Nova Scotia, with aggregations occurring adjacent to burgeoning pinniped colonies ( Casey and Pratt, 1985 ; Curtis et al., 2014 ; Skomal et al., 2017 ), but there is still little data on the movements and habitat of YOY and small juvenile sharks ( Curtis et al., 2018 ). All life stages migrate out of northern latitudes during the fall and overwinter off the southeastern U.S. ( Curtis et al., 2014 , 2018 ; Skomal et al., 2017 ). While the northwest Atlantic white shark population appears to be recovering from historical overfishing ( Curtis et al., 2014 ), there remains considerable uncertainty in their population dynamics, seasonal habitat preferences, ecological roles, and exposure to anthropogenic impacts ( Skomal et al., 2017 ; Curtis et al., 2018 ; Bastien et al., 2020 ; Bowlby and Gibson, 2020 ). Improved understanding of habitat selection within the only known northwest Atlantic nursery area will inform ongoing conservation strategies for this vulnerable white shark population.
Materials and Methods
Study location, animal collection, and tagging.
This study was conducted in the New York Bight between 2016 and 2019. The New York Bight is the coastal region between Montauk, New York and Cape May, New Jersey. Sharks were collected, sampled, tagged, and released following methods described by Curtis et al. (2018) . Briefly, sharks were caught via hook and line using live baitfish. In 2016 and 2017, sharks were tagged using a boatlift platform on the 42 m long M/V OCEARCH, whereas in 2018 and 2019, sharks were tagged in the water while being secured alongside a 7 m fishing vessel.
Sharks were fitted with either a FastGPS Argos transmitter (Sirtrack F6F) alone, or a combination of a Smart Position or Temperature transmitting (SPOT) tag (Wildlife Computers SPOT-258A) and an acoustic transmitter (Vemco V16-6H). The SPOT tags started uplinks after being dry for <0.25 s and sent 10 uplinks per message. Minimum uplink interval was 45 s with a maximum of 160 transmissions per day. The tags were fitted onto the first dorsal fin using nylon bolts and transmitted when the fin was above the sea surface as described by Curtis et al. (2018) .
FastGPS Argos transmitters were fitted using the same procedure as the SPOT tags. These tags are designed to transmit to the Argos Satellite System similarly to SPOT tags, but with additional capabilities to also receive radio signals from a GPS satellite when the tag is above the surface for a sufficient period of time. After the transmitter retrieved the signal from the GPS satellite, it then transmitted the retrieved location to the Argos Satellite System. In general, FastGPS tags are capable of producing positions with a lower estimated error (<100 m) than SPOT tags ( Dujon et al., 2014 ). For the first 28 days of deployment, FastGPS transmitters were programmed to transmit to the Argos Satellite System every 45 s, with a GPS fix interval every 120 min. After 28 days, the transmitters were then programmed to continue transmitting to the Argos Satellite System every 45 s, but to increase the GPS fix interval to 180 min to balance battery life throughout the duration of the study. With these settings, tags were expected to receive an average of 35 messages per day and have an expected battery life of 472 days. For both SPOT and FastGPS tags, all Argos position estimates classified as Class Z were removed from the analysis due to the large estimated error associated with that location class ( Boyd and Brightsmith, 2013 ).
Acoustic transmitters (Vemco V16-6H) were cold sterilized with benzalkonium chloride (Benz-all), and surgically placed into the coelomic cavity of each shark and the 4 cm incision was closed in a simple interrupted pattern with 2-O polydioxinone suture (Ethicon PDS II). The implanted acoustic transmitters randomly transmitted a unique signal every 60–90 s, and had a battery life of approximately 10 years. These tags were detected by Vemco acoustic receivers from collaborative acoustic monitoring arrays distributed across the Atlantic coast prior to this study (refer to Bangley et al., 2020b for an explanation of receiver coverage). Given the uncertainty in reporting across the collaborative networks, all acoustic telemetry-based position estimates were considered presence-only data (no absences).
During 2017–2019, a subset of white sharks were also fitted with high-rate pop-up satellite archival tags (Model PSAT LIFE, Lotek Wireless, Inc.). These tags archived light level, temperature, and pressure measurements at 10 s intervals for up to 28 days post-release after which they detached from the shark, floated to the surface, and transmitted data to the Argos satellite system. The transmitted data were aggregated into 5 min bins, with the full 10 s resolution data available only if the tag was physically recovered. Temperature-depth time-series were generated for each tag and summary statistics were compiled.
Movements and Habitat Selection
Horizontal movements were analyzed by downsampling the position estimates from the satellite tags (location classes A, B, 0, 1, 2, and 3) and acoustic transmitters to find daily mean position estimates for each of the 20 individuals with SPOT/FastGPS tags. No horizontal positions were estimated from PSAT data. Following Curtis et al. (2018) , gaps between days were linearly estimated and these daily position estimates were then plotted in ArcGIS (version 10.3) and movements faster than 10 km⋅h –1 were filtered out using Movement Ecology Tools for ArcGIS (ArcMET version 10.2.2 v3; Wall, 2014 ). Any position estimates found on land were also removed.
Environmental data (sea surface temperature, sea surface salinity, chlorophyll a) located at the horizontal position estimates were extracted from NOAA’s ERDDAP server using the Xtractomatic and rerddapXtracto packages in R (version 3.6.0) to characterize habitat use. The resolution of the environmental data was coarser than the expected accuracy of most tag positions (<5 km), so horizontal positions were only matched to a single underlying environmental grid cell. Sea surface temperature (SST) was gathered from the GHRSST Level 4 MUR Global Foundation Sea Surface Temperature Analysis dataset (v4.1), which provided daily SSTs with a resolution of 0.1°. The Sea Surface Salinity, Near Real Time, Soil Moisture Active Passive (SMAP) Daily Composite dataset was used to compile daily sea surface salinity with a resolution of 0.25°. Chlorophyll a was used as a proxy for productivity, or areas with high amounts of phytoplankton ( Trujillo and Thurman, 2016 ), as satellites are able to calculate the color of the water to determine relative amounts of phytoplankton on the surface of the ocean ( National Aeronautics and Space Administration (NASA), 2019 ). Daily chlorophyll-a amounts were collected from the Chlorophyll-a Aqua MODIS dataset (0.05° resolution).
A gridded bathymetric dataset (global 30 arc-s interval grid) from the General Bathymetric Chart of the Oceans (GEBCO) was used to analyze the bottom depth and features of the benthos below horizontal position estimates [ General Bathymetric Chart of the Oceans (GEBCO), 2019 ]. Additionally, a 1 km by 1 km grid was calculated in ArcGIS. The corresponding latitudes and longitudes were imported into R Studio in order to identify the available environmental variables throughout the entire New York Bight (coastal waters bound between 41.367°N, 70.296°W and 37.902°N, 75.327°W). The Xtractomatic and rerddapXtracto packages were used to find available environmental data located at the 1 km by 1 km intervals for the entire New York Bight. Environmental data that coincided with the time frame of each individual’s track was collected.
A log-likelihood chi-squared test was then conducted to assess habitat preferences of tagged individuals. A log-likelihood chi-squared test compares the goodness-of-fit of the hypothesized model against the observed model and can be used to compute a p-value. For this exercise we assumed all habitat data extracted from the continental shelf of the New York Bight were “available” to sharks. Following Rogers and White (2007) , three log-likelihood chi-square statistics were calculated. The first chi-square statistic was used to determine if the sharks were using the various habitats in a similar fashion. The null hypothesis states that all sampled individuals are using the habitats in the same proportions as each other. The following bin widths (i.e., categories) were created for each parameter based on the distribution of the data and to facilitate interpretation: 10 m (bathymetry), 2°C (temperature), 2 mg⋅m –3 (chlorophyll a), and 1 ppt (salinity). A p < 0.05 indicates evidence for heterogeneity, signifying individuals were using the various habitats in different proportions. A second chi-square statistic was calculated to examine if selection was occurring for individual habitat types (i.e., particular ranges) by some of the sharks. The null hypothesis states selection is not occurring in at least some of the sharks. The final chi-square statistic was calculated by taking the difference between the first two. This statistic describes whether, on average, sharks were using the various habitat types in proportion to their availability, regardless of which ones were selected. A p < 0.05 indicates strong selection for certain habitat types.
In order to determine if there was a preference for specific habitats or environmental variable ranges, selection ratios were calculated. A selection ratio greater than one indicates preference for that habitat, with a selection ratio less than one indicating avoidance for that particular habitat. All statistical tests were conducted in R Studio (version 1.1.453). Selection ratios were plotted for all four parameters to assess which had mean and confidence intervals that were clearly above or below one, indicating habitat selection or avoidance, respectively.
Vertical Activity and Three-Dimensional Movement
For double-tagged sharks (i.e., SPOT/FastGPS + PSAT), the PSAT temperature and depth logs were chronologically integrated with the geopositional data from the SPOT/FastGPS and acoustic transmitters. The horizontal position estimates were then filtered to meet the PSAT time frame of approximately 28 days. With the aim of matching the horizontal position estimates to the vertical log provided by the PSAT, the horizontal position estimates were linearly interpolated to match the interval of the PSAT log at 5 min or 10 s intervals, depending on whether or not the individual’s PSAT had been physically recovered. The resulting three-dimensional tracks were plotted in ArcScene 10.3 and overlaid onto bathymetry for visualization of movements with respect to bottom features, and reflect the best possible tracks given the availability of horizontal positions.
Horizontal Movements and Habitat Selection
Movement data from 21 white sharks (11 males, 10 females) were collected between 2016 and 2019. A total of 880 positions were received from SPOT/FastGPS transmitters and 4,478 detections were received from 49 unique ACT acoustic receivers, which were subsequently downsampled to daily positions. Individual sizes ranged from 138.0 to 166.4 cm total length (TL; Table 1 ). Horizontal position estimates from the satellite tags and acoustic detections demonstrated movement parallel to Long Island’s southern shoreline and along the New Jersey coastline ( Figure 1 ). During this time frame, individuals traveled 0.1 to 131.5 km away from shore, with an average (±1 SD ) distance from shore of 12.7 ± 0.2 km. Total track distances between 57.4 and 2,089.0 km were observed, with an average of 616.1 ± 126.7 km. Individual track durations during the study period ranged from 8 to 170 days, with an average track duration of 58 ± 10 days. All daily position estimates were located along the continental shelf except one; WS-11 had one daily position estimate on the continental slope.
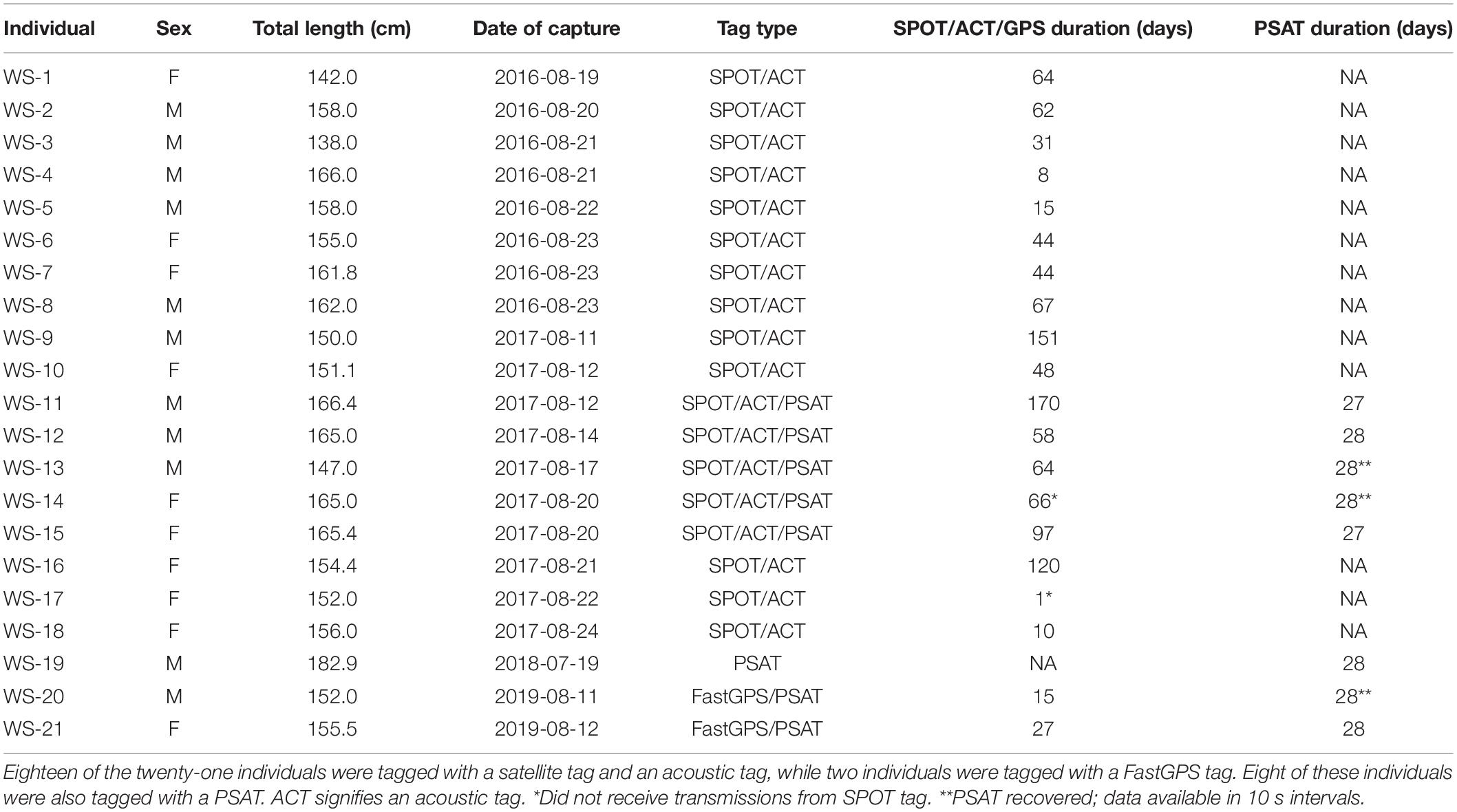
Table 1. Biological information of tagged sharks in the study.
Figure 1. Horizontal tracks of the 21 white sharks tagged in the study. Yellow arrow depicts capture and release site off Montauk, NY. Dots represent daily locations via downsampling of Smart Position or Temperature (SPOT) transmitting tags, FastGPS, and/or acoustic detections.
The average available bathymetry in the New York Bight was 52.1 ± 33.3 m, with a maximum depth of 294.3 m. The average underlying bathymetry (i.e., depths that tagged individuals swam over) was 27.7 ± 13.4 m, with a maximum depth of 338.2 m, and a minimum depth of 7.2 m. While the most commonly available bathymetry bin in the New York Bight was 30–40 m (available in 15% of the area), individuals were most frequently observed in shallower areas, between 20 and 30 m (48% of the time). Individuals only swam over depths between 30.0 and 40.0 m 27% of the time. Chi-square statistical results confirmed heterogeneity in underlying bathymetry use among sampled sharks (χ L 12 = 852.1356, df = 551, P = 0.00001; Table 2 ), and that some individuals were selective in underlying bathymetry (χ L 22 = 2,743.34, df = 580, P = 0.00001). On average, individuals were not using the bathymetry in proportion to its availability, regardless of which depths were selected (χ L 22 – χ L 12 = 1,891.201, df = 29, P = 0.00001). Selection ratio results showed a preference for underlying depths between 20 and 30 m, avoidance of depths shallower than 10 m and deeper than 40 m ( Figure 2A ). Mean selection ratios were above 1 for the 10–20 and 30–40 m intervals; however, confidence intervals were below the threshold value. As such, these habitats were considered to be neither selected nor avoided.
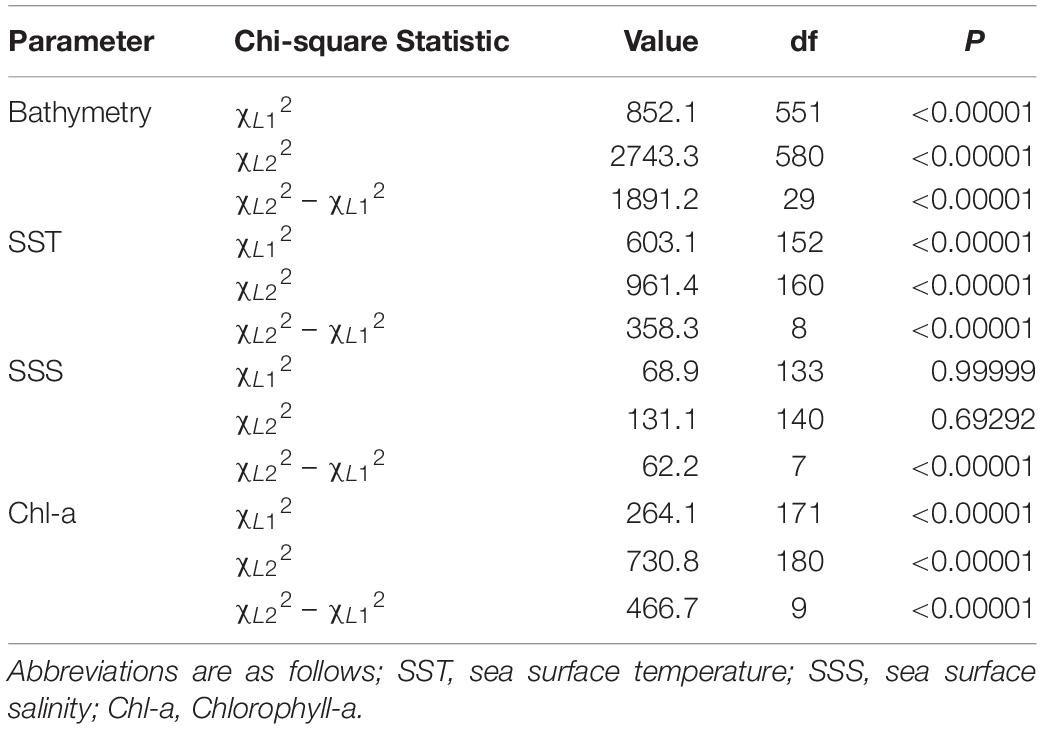
Table 2. Table of chi-square test statistics and associated values for each of the parameters analyzed in with respect to habitat preferences.
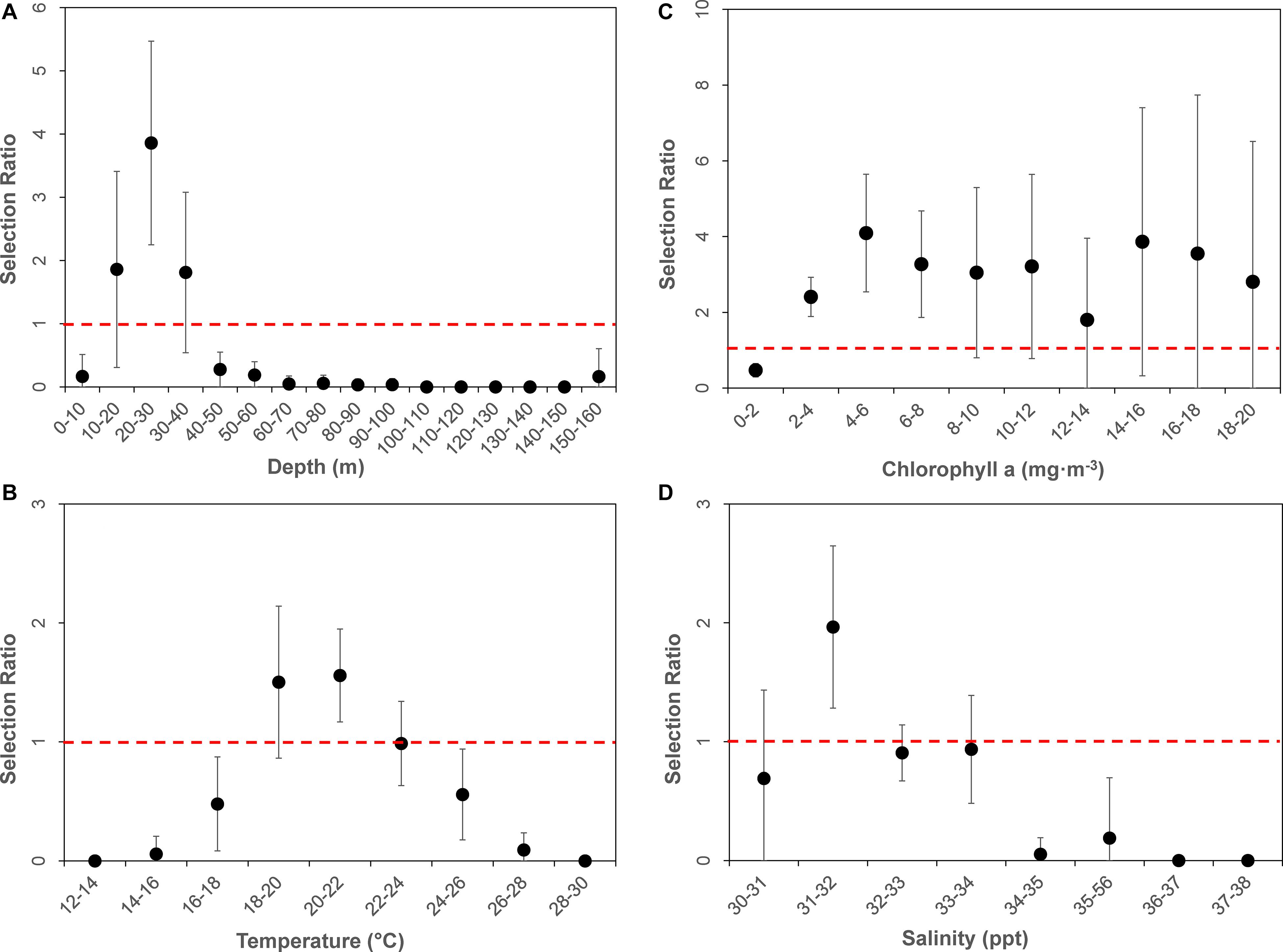
Figure 2. Dot plot of mean selection ratios (and confidence intervals) for various environmental parameters by bins: (A) Bathymetry, (B) Sea Surface Temperature, (C) Chlorophyll-a, and (D) Sea Surface Salinity. All environmental data were derived from remote sensing at daily horizontal position estimates of tagged animals. Dashed horizontal red line represents selection ratio of 1, above which values are “selected” for, and below which are considered “avoided.”
Sea Surface Temperature
Throughout the study period SST in the New York Bight ranged from 12.4 to 29.4°C (mean = 21.9 ± 2.8°C). Individuals swam in mean SSTs of 21.3 ± 2.0°C, with a maximum SST of 26.4°C, and a minimum SST of 15.7°C. All three chi-square statistics for SST analysis were found to be significant ( P < 0.0001; Table 2 ), suggesting tagged individuals were using the SSTs differently and selecting for specific ranges of temperature. Chi-square results show sampled sharks exhibited heterogeneous use of available SSTs (χ L 12 = 603.08, df = 152, P < 0.0001), and individuals were demonstrating selection (χ L 22 = 961.379, df = 160, P < 0.0001). Additionally, there was evidence that the average selection was not in proportion to the availability of resources (χ L 22 – χ L 12 = 358.299, df = 8, P < 0.0001). Selection ratios results suggested a preference for SST between 20.0 and 22.0°C, and potentially between 18.0 and 20.0°C; confidence intervals extended slightly below the selection ratio threshold, so preference at the latter range was unclear ( Figure 2B ). Results also showed an avoidance of SSTs below 18.0°C and above 24.0°C, with no evidence of selection or avoidance for the 22.0–24.0°C temperature bin.
Chlorophyll-a
The mean chlorophyll-a concentration in the New York Bight ranged from 0.1 to 20.0 mg⋅m –3 (mean = 1.7 ± 2.3 mg⋅m –3 ). Individuals swam in an average of 3.6 mg⋅m –3 (±3.2 mg⋅m –3 ), with a maximum of 19.6 mg⋅m –3 , and a minimum of 0.3 mg⋅m –3 . Chlorophyll-a was used heterogeneously (χ L 12 = 264.0624, df = 170, P = < 0.00001; Table 2 ). At least some of the sharks were selective in the chlorophyll-a concentrations that were swam in compared to that chlorophyll-a concentration’s availability (χ L 22 = 730.8104, df = 180, P = 0.00001). On average, the sampled sharks were not using chlorophyll-a concentrations in proportion to their availability, regardless of which concentrations that were being selected for (χ L 22 – χ L 12 = 466.748, df = 9, P = 0.00001). Selection ratio results found avoidance for lower concentrations of chlorophyll a between 0.0 and 2.0 mg⋅m –3 , and preference for concentrations between 2.0 and 8.0 mg⋅m –3 ( Figure 2C ). There was also potential for preference above concentrations of 8.0 mg⋅m –3 ; however, confidence intervals extended below the selection ratio threshold. Due to this, a preference for concentrations greater than 8.0 mg⋅m –3 was unresolved.
Sea Surface Salinity
The average available sea surface salinity in the New York Bight was 32.8 ± 1.2 ppt, with a maximum of 37.5 ppt, and a minimum of 30.0 ppt. The average sea surface salinity that individuals swam in was 32.3 ppt (±0.9), with a maximum of 35.7 ppt, and a minimum of 30.5 ppt. The first chi-square statistic was not significant (χ L 12 = 68.897, df = 133, P = 0.99), which suggests all sampled sharks were using sea surface salinities in the same proportions as the other sampled sharks ( Table 2 ). Selection was not occurring in at least some of the sharks; some of the sharks were using the sea surface salinities in proportion to their availability (χ L 22 = 131.0885, df = 140, P = 0.69). On average, there was strong selection for certain sea surface salinities (χ L 22 – χ L 12 = 62.19167, df = 7, P = 0.00001), as was demonstrated on an individual basis. For example, WS-3, WS-10, and WS-12 had a strong selection for sea surface salinities between 31.0 and 32.0 ppt, while WS-7 selected for 32.0–33.0 ppt, and WS-21 had a strong selection for 30.0–32.0 ppt. Overall, selection ratio results illustrated a preference for sea surface salinities between 31.0 and 32.0 ppt, with neutral responses to salinity ranges of 30.0–31.0 and 32.0–34.0 and avoidance for anything >34.0 ( Figure 2D ).
Vertical Movements
White shark depth ranges from the 8 individuals fitted with PSAT tags spanned the surface to 199 m, with individual means between 6.6 and 11.7 m ( Table 3 ). These mean depths were positioned at roughly half of the available water column based on underlying bathymetry estimated from horizontal positions. Temperatures recorded by PSAT tags ranged from 7.9 to 26.2°C with individual means from 19.2 to 20.7°C ( Table 3 ). Mean temperatures recorded by PSAT tags approximated those extracted from remote sensing of SSTs (i.e., within 1°C) based on horizontal positions, although the latter recorded warmer temperatures. The smallest individual tagged with a PSAT (WS-13) exhibited the shallowest max depth (24.3 m) and warmest minimum temperature (17.3°C), as well as the narrowest depth and temperature ranges ( Table 3 ).
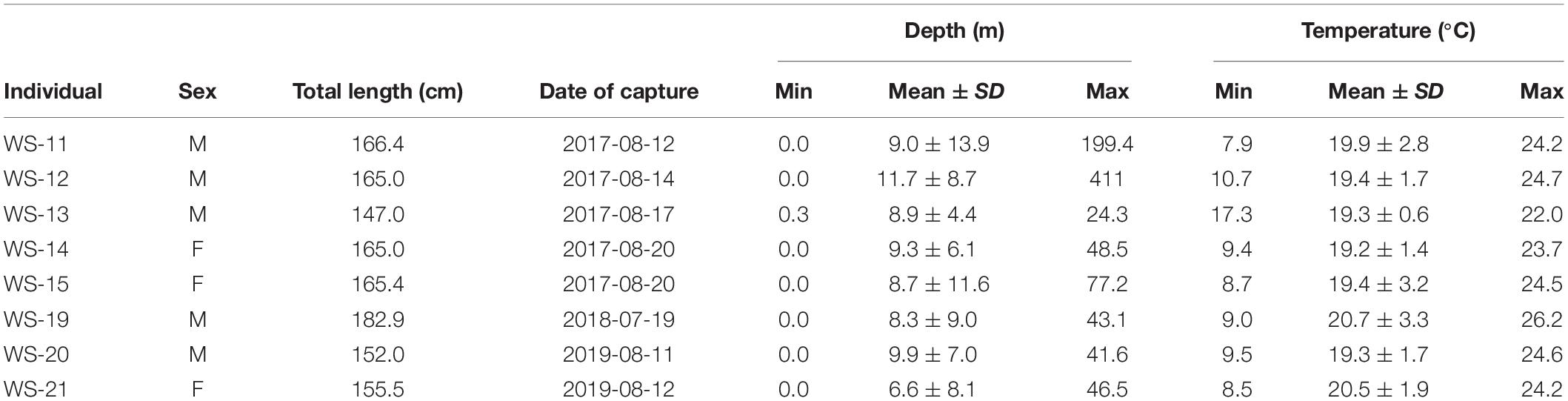
Table 3. Descriptive statistics for temperature and depth from the eight individuals fitted with pop-off satellite archival tags.
Three-dimensional interpolation of vertical and horizontal positions was possible for 7 individuals. Analyses of these data found that individuals swam over benthos between 4 and 424 m deep, mainly on the continental shelf, with one dive recorded off the continental slope ( Figures 3 , 4 ). There were multiple instances of sharks traversing and presumably interacting with large bathymetric and/or oceanographic features as they moved across the continental shelf, although this varied across individuals ( Supplementary Figures 1 – 7 ). For example, several individuals (WS-11, WS-12, WS-15, and WS-21) crossed the Hudson Shelf Valley during southward movements across the New York Bight ( Figure 4A ; S1, S2, S5, S7). One individual, WS-15, also appeared to interact with relatively cold water (<10°C) when traversing the Hudson Shelf Valley (S5), which was apparent from 20 to 80 m depth and in stark contrast with surface water that approached 25°C during this period in late August 2017 ( Figures 3F , 4B ). Other instances of considerable thermal stratification (i.e., 10°C difference between surface water and deepest dives) were evident in dive profiles of WS-14 ( Figure 3D ), WS-19 ( Figure 3G ), WS-20 ( Figure 3H ), and WS-21 ( Figure 3I ). Unfortunately, WS-14 had limited horizontal position estimates due to a lack of transmissions from its SPOT tag (S4). However, the depth profiles from this recovered tag showed extensive oscillations between the surface and depth (i.e., 30–50 m) ( Figure 3E ). Other portions of temperature-depth profiles for these individuals were more homogeneous, with temperatures around 20°C and more limited depths <30 m. For example, WS-13 stayed close to Montauk, NY ( Figure 4A ; S3) and did not travel as far south as the other tagged sharks during the 28 days period.
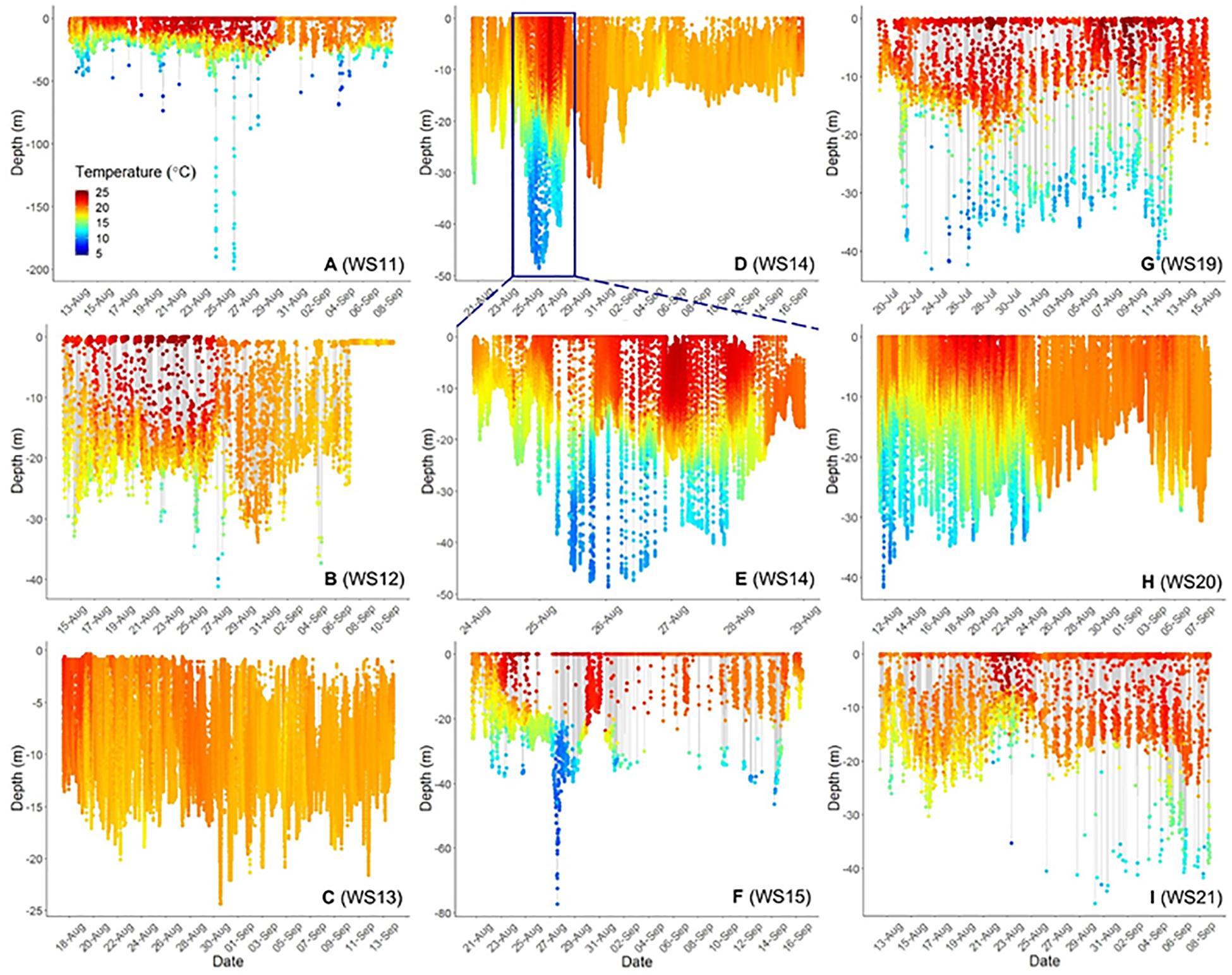
Figure 3. Time-series scatterplots of vertical depth and temperature profiles (color bar on bottom left of first plot) for 8. White Sharks 11–15 (A–F) were tagged in 2017, whereas 19 was tagged in 2018 (G) and 20 and 21 (H,I) are from 2019. (E) Represents an expansion of the blue box in (D) to demonstrate diel patterns in depth and temperature use. (C,D,H) Represent complete dive profiles from recovered PSAT tags.
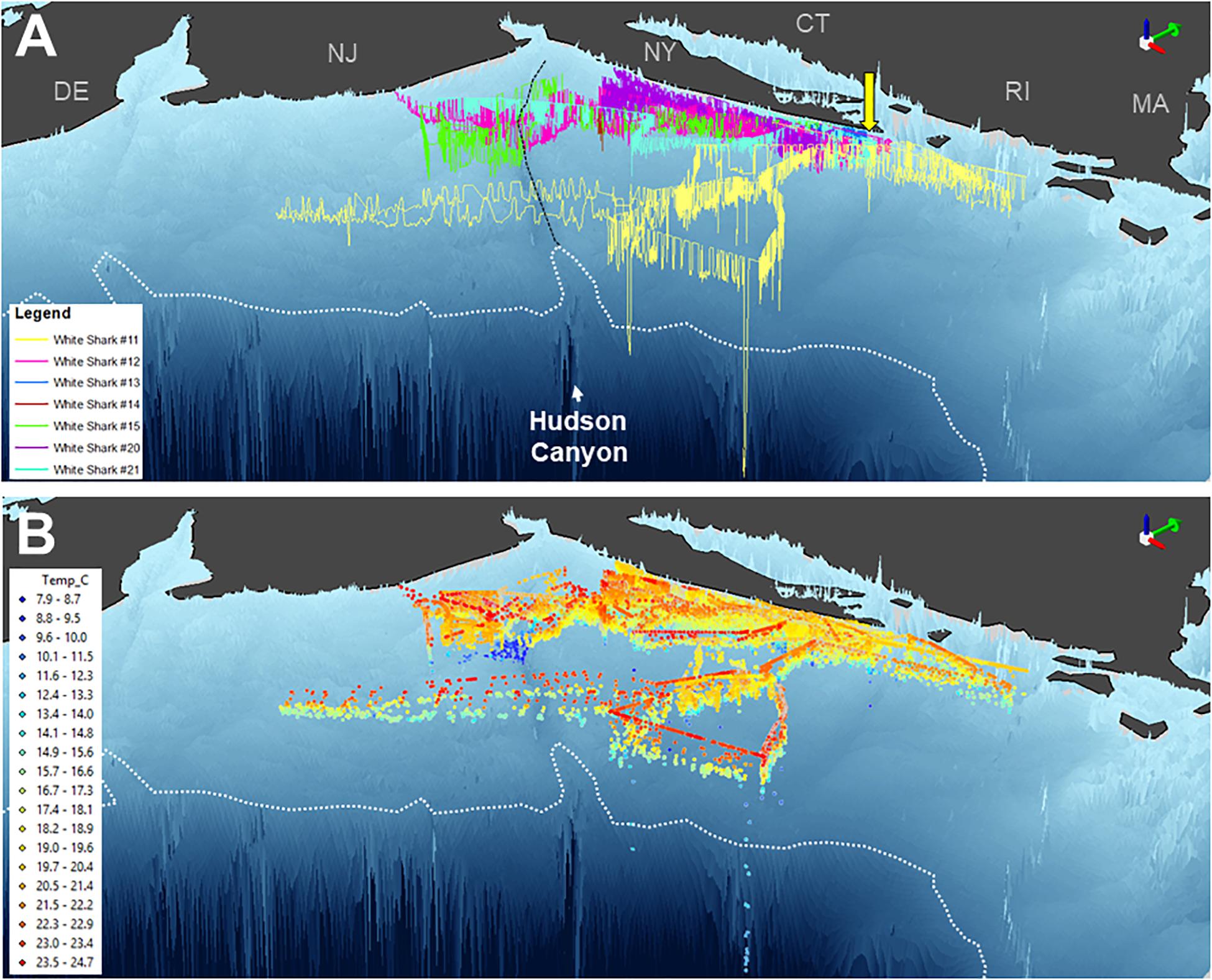
Figure 4. Three-dimensional position estimates for 7 double or triple-tagged sharks in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
This study significantly expands our understanding of fine-scale vertical movement patterns and habitat selection of YOY and small juvenile white sharks in the New York Bight, the only confirmed nursery area for the northwest Atlantic white shark population ( Curtis et al., 2018 ). Furthermore, it contributes to the growing body of information on the general life history and ecology of white sharks in this comparatively under-studied region ( Curtis et al., 2014 , 2018 ; Skomal et al., 2017 ; Huveneers et al., 2018 ; Bastien et al., 2020 ). The residency and consistent selection of continental shelf habitat in the New York Bight by young white sharks results in a striking size-based segregation of the population during summer and autumn months (July through November) when larger white sharks (>2.5 m) mainly occupy more northern waters from Massachusetts to Newfoundland, Canada ( Casey and Pratt, 1985 ; Curtis et al., 2014 ; Skomal et al., 2017 ; Bastien et al., 2020 ). The relative scarcity of large white sharks in the nursery area during this period provides young sharks a refuge from natural mortality and risk effects associated with predation, and permits them to play a role as apex predators in the system. Thus, their habitat selection patterns can result in important direct and indirect effects on ecosystem structure and nutrient pathways from the coastal zone to offshore habitats.
Habitat Selection
Across the multiple years examined in this study, tagged individuals consistently displayed horizontal movements parallel to Long Island’s southern shoreline and the New Jersey coast. Consistent with the preliminary observations of Curtis et al. (2018) , horizontal movements ranged from the surf zone to over 130 km from shore; however, over 90% of the horizontal movements were within 20 km of Long Island’s southern shoreline. Juvenile white sharks in the Southern California Bight were also found to stay close to shore, with an average distance of 7.2 ± 5.7 km ( White et al., 2019 ). White et al. (2019) attempted to model suitable juvenile white shark habitat along the U.S. Atlantic coast using observations from the U.S. Pacific coast, including distance from shore as a key variable. Our results suggest that distance from shore may not be as important as bathymetry, given the sharks in the present study occupied waters further from shore than predicted by White et al. (2019) , and tended to select waters >10 km from shore overlying bottom depths from 20 to 30 m. This is likely due to significant differences in the width of the continental shelf in southern California compared to the New York Bight (<10 vs. >100 km, respectively). The reasons for the apparent avoidance of nearshore shallow waters (<10 m deep) by young white sharks tracked in this study requires further exploration, but could be due to lower prey availability, higher wave energy and related higher turbidity, and/or competition from other co-occurring predators (e.g., sand tiger shark Carcharias taurus , sandbar shark Carcharhinus plumbeus , or dusky shark C. obscurus ). Based on the available observations globally, YOY and juvenile white sharks appear to be primarily coastal and shelf-oriented, consistently occurring over depths of less than 200 m, but utilizing habitats across the breadth of the shelf ( Weng et al., 2007 ; Bruce and Bradford, 2012 ; White et al., 2019 ; Spaet et al., 2020 ). Occasional forays beyond the shelf edge, particularly in the northeastern Pacific where the continental shelf is very narrow, results in juvenile white sharks displaying epipelagic behaviors ( Dewar et al., 2004 ; Weng et al., 2007 , 2012 ). Selection of focal areas within shelf ecosystems are likely influenced by other environmental conditions including temperature, productivity, and prey availability.
The sharks in this study selected waters with SSTs between 18.0 and 22.0°C. Juvenile white sharks in the northeastern Pacific Ocean were found in similar temperatures between 14.0 and 24.0°C ( White et al., 2019 ). The highest catch rates of juveniles in eastern Australia occurred in SSTs between 17.0 and 18.0°C ( Bruce et al., 2019 ). White sharks exhibit regional endothermy, and as such, are able to tolerate a wider range of temperatures than most ectothermic fish providing a variety of predatory advantages ( Carey et al., 1982 ; Watanabe et al., 2019 ). Summer-autumn water temperatures in the New York Bight may span the optimal physiological temperatures for young white sharks, making the region ideal from a thermal perspective. However, YOY and juvenile white sharks appear to occupy a narrower range of temperatures than larger individuals ( Curtis et al., 2014 ; Skomal et al., 2017 ), and the most restricted temperature range recorded from PSATs was from the smallest individual tagged in the study. These ontogenetic differences could be due to the smaller body mass, less developed heat exchange mechanisms (i.e., less red muscle, smaller retia), and higher surface area to volume ratios of young sharks, making it more physiologically costly to defend an elevated core temperature over as wide a range of temperatures as adults. This has important implications for the future of young white shark habitats given the effects of climate change and variability, especially in the mid-Atlantic Bight which is warming at a much faster rate than most of the global ocean ( Saba et al., 2016 ; Huveneers et al., 2018 ).
Young white sharks in the New York Bight also selected areas with relatively high levels of productivity (i.e., mesotrophic waters) as reflected by salinity and chlorophyll-a concentration. Tagged individuals selected sea surface salinities between 31.0 and 32.0 ppt (i.e., slightly less saline than oceanic waters), and chlorophyll-a concentrations >2.0 mg m –3 . White sharks are not considered euryhaline, although they do occasionally occur within estuarine water bodies ( Harasti et al., 2017 ). Sea surface salinity preferences have not been studied previously for white sharks, but are commonly used in habitat suitability models for other species. Shallow areas close to land tend to have lower salinities due to proximity to coastal runoff and freshwater flow, and may contribute to a decreased predation risk to young sharks as larger individuals avoid these areas ( Simpfendorfer et al., 2005 ; Wetherbee et al., 2007 ; Knip et al., 2011; Trujillo and Thurman , 2016 ). Freshwater inputs and longshore currents also contribute to increased primary productivity and phytoplankton blooms nearshore, as indicated by the shoreward increase in chlorophyll-a concentrations in this region ( Xu et al., 2011 ). Phytoplankton make up the base of the food web, and as such, high concentrations in an area can support an abundance of life, including higher-order predators like sharks ( Trujillo and Thurman, 2016 ). Due to upwelling (the flow of deep nutrient rich water to the surface), coastal areas are generally high in nutrients and phytoplankton ( Trujillo and Thurman, 2016 ). Similarly, phytoplankton concentrations along Long Island’s southern shoreline are affected by groundwater upwelling, which is the occurrence of groundwater high in nutrients seeping through sediment on the seafloor ( Gobler and Sañudo-Wilhelmy, 2001 ). Additionally, this area is home to several rivers, including the Hudson River, and as such, nutrient runoff may cause an increase of primary productivity. Thus, it is not unexpected that YOY and juvenile white sharks select areas with high productivity to be used as a foraging ground where they prey on a variety of fishes and invertebrates ( Casey and Pratt, 1985 ). Our results differ from those found in the northeastern Pacific Ocean as White et al. (2019) noted chlorophyll-a was found to not be a significant variable in habitat selection for juvenile white sharks in the Southern California Bight. However, in the Mediterranean Sea, studies have suggested high productivity in the Adriatic Sea and the Sicilian Channel may be the reason behind the higher occurrence of white sharks in the area ( Coll et al., 2007 ; Boldrocchi et al., 2017 ). In nursery areas off eastern Australia, seasonal upwelling and therefore, nutrient enrichment, are believed to coincide with suitable prey aggregations of various teleosts, providing the nurseries with an abundance of prey for immature white sharks ( Bruce and Bradford, 2012 ).
Three-Dimensional Movements
Few studies have explored vertical behavior of YOY or juvenile white sharks ( Klimley et al., 2002 ; Dewar et al., 2004 ; Weng et al., 2007 , 2012 ), and this is the first such study in the Atlantic Ocean basin. Vertical behavior of the PSAT-tagged sharks varied between individuals and locations within the New York Bight. The drivers of shark vertical behavior have long been a subject of inquiry, with a variety of physical and biological variables suggested to hold influence (e.g., Carey et al., 1990 ; Klimley et al., 2002 ; Gaube et al., 2018 ). While SSTs ranging between 15.7 and 26.4°C were found at the locations of the tagged individuals, further research is needed to determine if vertical behavior is influenced by SST (e.g., Andrzejaczek et al., 2018 ).
The vertical diving behavior and accompanying measurements of temperature identified several areas across the shelf with thermally stratified water column structure. This was most evident around the Hudson Shelf Valley region where some of the coldest temperatures were recorded (<10°C) during the summertime tracking period from WS-14 and WS-15. Interestingly, these 50–80 m waters were colder than those recorded during the deepest dives of WS-11 to 200 m, which occurred seaward of this region off the continental shelf. Previous physical measurements in the mid-Atlantic Bight have shown that a “cold pool” of water commonly develops along the bottom of the mid-shelf region through the summertime ( Houghton et al., 1982 ; Falkowski et al., 1983 ; Rona et al., 2015 ). Although primary production decreases at the surface of this region relative to the coastline, chlorophyll-a levels at depth (i.e., 20–40 m) approximate those measured nearshore ( Falkowski et al., 1983 ). The presence of multiple white sharks in these areas for several days suggests that the edge of the mid-Atlantic Cold Pool may provide suitable subsurface habitat for these predators, as has been recently suggested for juvenile dusky sharks ( Bangley et al., 2020a ). Indeed, the waters surrounding the Hudson Shelf Valley as well as shelf-edge waters of the mid-Atlantic Bight are targeted by several fisheries ( Rona et al., 2015 ), and have high levels of habitat and biological diversity ( Pierdomenico et al., 2015 , 2017 ). Young white sharks may therefore be exploiting more abundant food resources that accompany this unique subsurface feature, which apparently facilitates oceanographic conditions that support high levels of prey productivity. Additional tagging, including high-resolution biologging, accelerometry, and animal-borne video systems, to observe young white shark behavior is needed from this area. Further, what drives these individuals to move offshore from protected and productive waters along Long Island’s southern shorelines is still uncertain and should be explored.
Prey availability also likely influences young white shark vertical activity. Juvenile white sharks are documented to feed mainly on smaller demersal elasmobranchs ( Hypanus spp., Myliobatis spp., Leucoraja spp., Mustelus canis ), and teleosts such as searobins ( Prionotus spp.), hakes ( Urophycis spp.), and flounders (Pleuronectidae and Paralichthyidae), which may influence bottom-oriented behavior ( Casey and Pratt, 1985 ; Santana-Morales et al., 2012 ; Onate-Gonzalez et al., 2017 ). Likewise, locally abundant pelagic prey species including squids ( Illex spp. and Doryteuthis spp.), mackerel ( Scomber spp.), and menhaden ( Brevoortia tyrannus ) may drive surface-oriented or diel vertical migration behaviors in certain locations. In the New York Bight, demersal species increase in abundance from the continental shelf edge shoreward ( National Oceanic and Atmospheric Administration (NOAA), 2018 ), and the highly productive nearshore waters are vastly diverse and home to over 300 species of fish ( Briggs and Waldman, 2002 ). However, pelagic prey may also become locally aggregated, particularly in thermally stratified waters where we observed extensive diving in upper layers ( Gaube et al., 2018 ).
Undoubtedly, our work shows that young white sharks traverse variable oceanographic features across the continental shelf in the New York Bight, and these may be influenced by the underlying bathymetry. Further efforts to integrate multiple complementary tag technologies on each tagged individual will provide more complete characterizations of movements and the drivers of habitat selection in an inherently three-dimensional environment.
Implications for Management
There is growing recognition of the importance of nursery areas to the overall sustainability of shark populations ( Heupel et al., 2007 , 2018 ). In order to inform conservation and management efforts in these areas, however, they must first be accurately characterized with an understanding of where, when, and how a given species uses the habitat. The study of juvenile white shark habitats and potential anthropogenic impacts on those areas was recently considered to be a high research priority amongst white shark scientists around the world ( Huveneers et al., 2018 ). As the New York Bight, a relatively small and discrete region, remains the only confirmed white shark nursery area in the entire North Atlantic Ocean, it may be of critical importance to the long-term maintenance of the regional white shark population. The results from this study may help improve the characterization of Essential Fish Habitat (EFH) for YOY and juvenile white sharks for NOAA National Marine Fisheries Service (NMFS) fishery management plans (e.g., National Oceanic and Atmospheric Administration (NOAA), 2017a ). NMFS has considered designating a Habitat Area of Particular Concern (HAPC) in the northern mid-Atlantic Bight and the shoreline off southern New England for YOY and juvenile white sharks; however, the agency determined that an insufficient amount of data was available at the time to support this ( National Oceanic and Atmospheric Administration (NOAA), 2017a , b ). The improvement of EFH characterization and the potential designation of HAPCs for young white sharks using the data presented herein could benefit the ongoing assessment and mitigation of habitat impacts from fisheries, offshore energy development, habitat degradation, and other human activities.
Fisheries bycatch remains a primary threat to white sharks in the northwest Atlantic ( Curtis et al., 2014 ; Huveneers et al., 2018 ; Bowlby and Gibson, 2020 ) and tracking data from the present study can also be used to assess bycatch susceptibility and potentially inform spatial management by NMFS ( Lyons et al., 2013 ; Queiroz et al., 2019 ). Finally, understanding species-habitat relationships are critical for predicting the potential impacts of long-term environmental changes including climate change (e.g., Kleisner et al., 2017 ; Crear et al., 2020 ). White shark coastal nursery areas may be comparatively vulnerable to the effects of global warming ( Huveneers et al., 2018 ) and given the importance of water temperature in habitat selection and seasonal movements of young white sharks ( Weng et al., 2007 ; Curtis et al., 2018 ; this study), climate change impacts on the mid-Atlantic continental shelf ecosystem ( Saba et al., 2016 ) could pose viable threats to the survival of juveniles, negatively affecting sustained recruitment to the adult population. Telemetry and biologging tools continue to provide the information necessary to simultaneously address numerous questions on the ecology, behavior, and conservation of highly mobile marine species that have traditionally been challenging to explore.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Florida Atlantic University. All individuals collected in this animal study were collected and tagged using university-approved animal use protocols (FAU AUP# 16-07, 19-19). All activities were undertaken with permits or letters of acknowledgment from both state (New York State Department of Environmental Conservation LPSCI-1634) and federal (NMFS SHK-EFP-16-04, SHKLOA-17-02, SHK-LOA-18-11, SHK-LOA-19-06) agencies. All efforts were made to minimize animal pain and suffering during capture and handling procedures. All surgeries were overseen by a licensed veterinarian specializing in aquatic veterinary medicine.
Author Contributions
RS, TC, and MA designed the study. TC, GM, GF, and MA secured funding for the project. RS led the data analysis with assistance from TC and MA and led the writing of manuscript with assistance from all authors. All authors participated in fieldwork.
Funding and support was provided by the OCEARCH, Harbor Branch Oceanographic Institute Foundation, Florida Atlantic University Foundation, South Fork Natural History Museum, Andrew F. Sabin Family Foundation, NMFS Atlantic Highly Migratory Species Management Division, Southampton Public Schools, and a number of private donors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all of the organizations and individuals that contributed to the field work and data collection associated with this study including OCEARCH, South Fork Natural History Museum, Reel Science Charters, Harbor Branch Oceanographic Institute, Wildlife Conservation Society, and the New York Aquarium, with special thanks to B. McBride, B. Eyre, D. J. Lettieri, T. Goggins, A. Meite, R. Hueter, L. Winn, J. Quinlan, M. Hyatt, H. Walters, J. Labelle, M. Camhi, C. Eddy, M. Drymon, G. Roskar, L. Hoopes, F. Quevedo, C. Paparo, W. Zublionis, J. Metzger, and M. Berkhout. We thank M. Frisk and his lab at Stony Brook University, Wildlife Conservation Society, and other members of the ACT Network for providing acoustic tag detections in the New York Bight. For analytical assistance we also thank C. Bangley, B. Galuardi, D. Crear, B. DeGroot, and L. Brewster. The views or opinions expressed herein are those of the authors and do not necessarily reflect those of NOAA, the Department of Commerce, or any other institution.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.643831/full#supplementary-material
Supplementary Figure 1 | Three-dimensional position estimates for WS-11 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 2 | Three-dimensional position estimates for WS-12 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 3 | Three-dimensional position estimates for WS-13 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 4 | Three-dimensional position estimates for WS-14 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 5 | Three-dimensional position estimates for WS-15 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 6 | Three-dimensional position estimates for WS-20 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Supplementary Figure 7 | Three-dimensional position estimates for WS-21 in the New York Bight region. Positions are colored by individual shark in (A) (as per Figure 1 ) and temperature in (B) . Bathymetry is presented based on GEBCO data, with the continental shelf break (i.e., 200 m isobath) indicated by a white dotted line and the Hudson Shelf Valley identified by black dashed line. Green 3D arrows (top right of panels) point north. Yellow arrow depicts capture and release site off Montauk, NY.
Andrzejaczek, S., Gleiss, A. C., Jordan, L. K. B., Pattiaratchi, C. B., Howey, L. A., Brooks, E. J., et al. (2018). Temperature and the vertical movements of oceanic whitetip sharks, Carcharhinus longimanus . Sci. Rep. 8:8351. doi: 10.1038/s41598-018-26485-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Bangley, C. W., Curtis, T. H., Secor, D. H., Latour, R. J., and Ogburn, M. B. (2020a). Identifying important juvenile Dusky Shark habitat in the northwest Atlantic Ocean using acoustic telemetry and spatial modeling. Mar. Coast. Fish. 12, 348–363. doi: 10.1002/mcf2.10120
CrossRef Full Text | Google Scholar
Bangley, C. W., Whoriskey, F. G., Young, J. M., and Ogburn, M. B. (2020b). Networked animal telemetry in the Northwest Atlantic and Caribbean Waters. Mar. Coast. Fish. 12, 339–347. doi: 10.1002/mcf2.10128
Bastien, G., Barkley, A., Chappus, J., Heath, V., Popov, S., Smith, R., et al. (2020). Inconspicuous, recovering, or northward shift: status and management of the white shark ( Carcharodon carcharias ) in Atlantic Canada. Can. J. Fish. Aquat. Sci . 77, 1666–1677. doi: 10.1139/cjfas-2020-0055
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., et al. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 51, 633–641. doi: 10.1641/0006-3568(2001)051[0633:ticamo]2.0.co;2
Boldrocchi, G., Kiszka, J., Purkis, S., Storai, T., Zinzula, L., and Burkholder, D. (2017). Distribution, ecology, and status of the white shark, Carcharodon carcharias , in the Mediterranean Sea. Rev. Fish Biol. Fish. 27, 515–534. doi: 10.1007/s11160-017-9470-5
Bowlby, H. D., and Gibson, A. J. F. (2020). Implications of life history uncertainty when evaluating status in the Northwest Atlantic population of white shark ( Carcharodon carcharias) . Ecol. Evol. 10, 4990–5000. doi: 10.1002/ece3.6252
Boyd, J. D., and Brightsmith, D. J. (2013). Error properties of Argos satellite telemetry locations using least squares and Kalman filtering. PLoS One 8:e63051. doi: 10.1371/journal.pone.0063051
Braun, C. D., Gaube, P., Sinclair-Taylor, T. H., Skomal, G. B., and Thorrold, S. R. (2019). Mesoscale eddies release pelagic sharks from thermal constraints to foraging in the ocean twilight zone. Proc. Natl. Acad. Sci. 116, 17187–17192. doi: 10.1073/pnas.1903067116
Briggs, P. T., and Waldman, J. R. (2002). Annotated list of fishes reported from the marine waters of New York. Northeastern Nat. 9:35.
Google Scholar
Bruce, B., Harasti, D., Lee, K., Gallen, C., and Bradford, R. (2019). Broad-scale movements of juvenile white sharks Carcharodon carcharias in eastern Australia from acoustic and satellite telemetry. Mar. Ecol. Prog. Ser. 619, 1–15. doi: 10.3354/meps12969
Bruce, B. D., and Bradford, R. W. (2012). “Habitat use and spatial dynamics of juvenile white sharks, Carcharodon carcharias , in eastern Australia,” in Global Perspectives on the Biology and life History of the White Shark , ed. M. L. Domeier, (Boca Raton, FL: CRC Press), 225–254.
Carey, F. G., Kanwisher, J. W., Brazier, O., Gabrielson, G., Casey, J. G., and Pratt, H. L. Jr. (1982). Temperature and activities of a white shark. Carcharodon carcharias . Copeia 2, 254–260. doi: 10.2307/1444603
Carey, F. G., Scharold, J. V., and Kalmijn, Ad. J (1990). Movements of blue sharks ( Prionace glauca ) in depth and course. Mar. Biol. 106, 329–342. doi: 10.1007/BF01344309
Cartamil, D., Wegner, N. C., Aalbers, S., Sepulveda, C. A., Baquero, A., and Graham, J. B. (2010). Diel movement patterns and habitat preferences of the common thresher shark ( Alopias vulpinus ) in the Southern California Bight. Mar. Freshw. Res. 61:596. doi: 10.1071/MF09153
Casey, J. G., and Pratt, H. P. (1985). Distribution of the white shark, Carcharodon carcharias , in the Western North Atlantic. Mem. South. Calif. Acad. Sci. 9, 2–14.
Churchill, J. H., Levine, E. R., Connors, D. N., and Cornillon, P. C. (1993). Mixing of shelf, slope and Gulf Stream water over the continental slope of the Middle Atlantic Bight. Deep Sea Res. I Oceanogr. Res. Pap. 40, 1063–1085. doi: 10.1016/0967-0637(93)90090-p
Coll, M., Santojanni, A., Palomera, I., Tudela, S., and Arneri, E. (2007). An ecosystem model of the Northern and Central Adriatic Sea: analysis of ecosystem structure and fishing impacts. J. Mar. Syst. 67, 119–154. doi: 10.1016/j.jmarsys.2006.10.002
Crear, D. P., Latour, R. J., Friedrichs, M. A., St-Laurent, P., and Weng, K. C. (2020). Sensitivity of a shark nursery habitat to a changing climate. Mar. Ecol. Prog. Ser. 652, 123–136. doi: 10.3354/meps13483
Curtis, T. H., McCandless, C. T., Carlson, J. K., Skomal, G. B., Kohler, N. E., Natanson, L. J., et al. (2014). Seasonal distribution and historic trends in abundance of white sharks, Carcharodon carcharias , in the western North Atlantic Ocean. PLoS One 9:e99240. doi: 10.1371/journal.pone.0099240
Curtis, T. H., Metzger, G., Fischer, C., McBride, B., McCallister, M., Winn, L. J., et al. (2018). First insights into the movements of young-of-the-year white sharks ( Carcharodon carcharias ) in the western North Atlantic Ocean. Sci. Rep. 8:10794.
Dewar, H., Domeier, M., and Nasby-Lucas, N. (2004). Insights into young of the year white shark, Carcharodon carcharias , behavior in the Southern California Bight. Environ. Biol. Fish. 70, 133–143. doi: 10.1023/b:ebfi.0000029343.54027.6a
Dodge, K. L., Galuardi, B., Miller, T. J., and Lutcavage, M. E. (2014). Leatherback turtle movements, dive behavior, and habitat characteristics in ecoregions of the Northwest Atlantic Ocean. PLoS One 9:e91726. doi: 10.1371/journal.pone.0091726
Dujon, A. M., Lindstrom, R. T., and Hays, G. C. (2014). The accuracy of Fastloc-GPS locations and implications for animal tracking. Methods Ecol. Evol. 5, 1162–1169. doi: 10.1111/2041-210x.12286
Falkowski, P. G., Vidal, J., Hopkins, T. S., Rowe, G. T., Whitledge, T. E., and Harrison, W. G. (1983). Summer nutrient dynamics in the Middle Atlantic Bight: primary production and utilization of phytoplankton carbon. J. Plankton Res. 5, 515–537.
Friedland, K. D., Leaf, R. T., Kane, J., Tommasi, D., Asch, R. G., Rebuck, N., et al. (2015). Spring bloom dynamics and zooplankton biomass response on the US Northeast Continental Shelf. Cont. Shelf Res. 102, 47–61. doi: 10.1016/j.csr.2015.04.005
Gaube, P., Braun, C. D., Lawson, G. L., McGillicuddy, D. J., Della Penna, A., Skomal, G. B., et al. (2018). Mesoscale eddies influence the movements of mature female white sharks in the Gulf Stream and Sargasso Sea. Sci. Rep. 8, 1–8. doi: 10.1016/j.dsr.2017.02.006
General Bathymetric Chart of the Oceans (GEBCO), (2019). Global Ocean & Land Terrain Models. Available online at: https://www.gebco.net/data_and_products/gridded_bathymetry_data/#a1 (accessed December 1, 2020).
Gobler, C., and Sañudo-Wilhelmy, S. (2001). Temporal variability of groundwater seepage and brown tide blooms in a Long Island embayment. Mar. Ecol. Prog. Ser. 217, 299–309. doi: 10.3354/meps217299
Harasti, D., Lee, K., Bruce, B., Gallen, C., and Bradford, R. (2017). Juvenile white sharks Carcharodon carcharias use estuarine environments in south-eastern Australia. Mar. Biol. 164:58.
Hare, J. A., Morrison, W. E., Nelson, M. W., Stachura, M. M., Teeters, E. J., Griffis, R. B., et al. (2016). A vulnerability assessment of fish and invertebrates to climate change on the Northeast US Continental Shelf. PLoS One 11:e0146756. doi: 10.1371/journal.pone.0146756
Heupel, M. R., Carlson, J. K., and Simpiendorfer, C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel, M. R., Kanno, S., Martins, A. P. B., and Simpfendorfer, C. A. (2018). Advances in understanding the roles and benefits of nursery areas for elasmobranch populations. Mar. Freshw. Res. 70, 897–907. doi: 10.1071/MF18081
Hossler, K., and Bauer, J. E. (2013). Amounts, isotopic character, and ages of organic and inorganic carbon exported from rivers to ocean margins: 1. Estimates of terrestrial losses and inputs to the Middle Atlantic Bight. Glob. Biogeochem. Cycles 27, 331–346. doi: 10.1002/gbc.20033
Houghton, R. W., Schlitz, R., Beardsley, R. C., Butman, B., and Chamberlin, J. L. (1982). The Middle Atlantic Bight cold pool: evolution of the temperature structure during summer 1979. J. Phys. Oceanogr. 12, 1019–1029. doi: 10.1175/1520-0485(1982)012<1019:tmabcp>2.0.co;2
Huveneers, C., Apps, K., Becerril-García, E. E., Bruce, B., Butcher, P. A., Carlisle, A. B., et al. (2018). Future research directions on the “Elusive” White Shark. Front. Mar. Sci. 5:455. doi: 10.3389/fmars.2018.00455
Kleisner, K. M., Fogarty, M. J., McGee, S., Barnett, A., Fratantoni, P., Greene, J., et al. (2016). The effects of sub-regional climate velocity on the distribution and spatial extent of marine species assemblages. PLoS one 11:e0149220. doi: 10.1371/journal.pone.0149220
Kleisner, K. M., Fogarty, M. J., McGee, S., Hare, J. A., Moret, S., Perretti, C. T., et al. (2017). Marine species distribution shifts on the US Northeast Continental Shelf under continued ocean warming. Prog. Oceanogr. 153, 24–36. doi: 10.1016/j.pocean.2017.04.001
Klimley, A. P., Beavers, S. C., Curtis, T. H., and Jorgensen, S. J. (2002). Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environ. Biol. Fish. 63, 117–135. doi: 10.1023/a:1014200301213
Knebel, H. J. (1979). Anomalous topography on the continental shelf around Hudson Canyon. Mar. Geol. 33, 67–75. doi: 10.1016/0025-3227(79)90074-4
Knip, D. M., Heupel, M. R., and Simpfendorfer, C. A. (2010). Sharks in nearshore environments: models, importance, and consequences. Mar. Ecology Prog. Ser. 402, 1–11. doi: 10.3354/meps08498
Knip, D. M., Heupel, M. R., Simpfendorfer, C. A., Tobin, A. J., and Moloney, J. (2011). Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar. Ecology Prog. Ser. 425, 233–246. doi: 10.3354/meps09006
Kohler, N. E., and Turner, P. A. (2019). Distributions and movements of atlantic shark species: A 52-year retrospective atlas of mark and recapture data. Mar. Fish. Rev. 81, 1–94. doi: 10.7755/mfr.81.2.1
Latour, R. J., and Gartland, J. (2020). Dynamics of the shark community in the Mid-Atlantic Bight. Mar. Biol. 16:100.
Logan, R. K., Vaudo, J. J., Sousa, L. L., Sampson, M., Wetherbee, B. M., and Shivji, M. S. (2020). Seasonal movements and habitat use of juvenile smooth hammerhead sharks in the western North Atlantic Ocean and significance for management. Front. Mar. Sci. 7:731. doi: 10.3389/fmars.2020.566364
Lyons, K., Jarvis, E. T., Jorgensen, S. J., Weng, K., O’Sullivan, J., Winkler, C., et al. (2013). The degree and result of gillnet fishery interactions with juvenile white sharks in southern California assessed by fisheries-independent and -dependent methods. Fish. Res. 147, 370–380. doi: 10.1016/j.fishres.2013.07.009
Methratta, E. T. (2020). Monitoring fisheries resources at offshore wind farms: BACI vs. BAG designs. ICES J. Mar. Sci. 77, 890–900. doi: 10.1093/icesjms/fsaa026
Murray, K. T., and Orphanides, C. D. (2013). Estimating the risk of loggerhead turtle Caretta caretta bycatch in the US mid-Atlantic using fishery-independent and-dependent data. Mar. Ecol. Prog. Ser. 477, 259–270. doi: 10.3354/meps10173
National Aeronautics and Space Administration (NASA), (2019). Chlorophyll. Available online at: https://earthobservatory.nasa.gov/global-maps/MY1DMM_CHLORA (accessed December 1, 2020).
National Oceanic and Atmospheric Administration (NOAA), (2017a). Atlantic Highly Migratory Species; Essential Fish Habitat. Available online at: https://www.federalregister.gov/documents/2017/09/07/2017-18961/atlantic-highly-migratory-species-essential-fish-habitat (accessed December 1, 2020).
National Oceanic and Atmospheric Administration (NOAA), (2017b). Final Amendment 10 to the 2006 Consolidated Atlantic Highly Migratory Species Fishery Management Plan: Essential Fish Habitat and Environmental Assessment. Available online at: https://www.habitat.noaa.gov/application/efhinventory/docs/a10_hms_efh.pdf#page=419 (accessed December 1, 2020).
National Oceanic and Atmospheric Administration (NOAA), (2018). Ecology of the Northeast US Continental Shelf. Available online at: https://www.nefsc.noaa.gov/ecosys/ecosystem-ecology/fish-squid.html (accessed December 1, 2020).
Onate-Gonzalez, E. C., Sosa-Nishizaki, O., Herska, S. Z., Lowe, C. G., Lyons, K., Santana-Morales, O., et al. (2017). Importance of Bahia Sebastian Vizcaino of a nursery area for white sharks ( Carcharodon carcharias ) in the Northeastern Pacific: a fishery dependent analysis. Fish. Res . 188, 125–137. doi: 10.1016/j.fishres.2016.12.014
Parsons, G. R., Hoffmayer, E. R., Frank, J., and Bet-Sayed, W. (2008). “A review of shark reproductive ecology: life history and evolutionary implications,” in Fish Reproduction , Edn. 1 Edn, eds M. J. Rocha, A. Aruke, and B. G. Kapoor, (Abingdon: Taylor and Francis), 435–469.
Pierdomenico, M., Gori, A., Guida, V. G., and Gili, J. M. (2017). Megabenthic assemblages at the Hudson Canyon head (NW Atlantic margin): Habitat-faunal relationships. Prog. Oceanogr. 157, 12–26. doi: 10.1016/j.pocean.2017.08.001
Pierdomenico, M., Guida, V. G., Macelloni, L., Chiocci, F. L., Rona, P. A., Scranton, M. I., et al. (2015). Sedimentary facies, geomorphic features and habitat distribution at the Hudson Canyon head from AUV multibeam data. Deep Sea Res. II Top. Stud. Oceanogr. 121, 112–125. doi: 10.1016/j.dsr2.2015.04.016
Queiroz, N., Humphries, N. E., Couto, A., Vedor, M., da Costa, I., Sequeira, A. M. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466. doi: 10.1038/s41586-019-1444-4
Rogers, K. B., and White, G. C. (2007). “Analysis of movement and habitat use from telemetry data,” in Analysis and Interpretation of Freshwater Fisheries Data , eds C. S. Guy and M. L. Brown, (Bethesda, MA: American Fisheries Society), 625–676.
Rona, P., Guida, V., Scranton, M., Gong, D., Macelloni, L., Pierdomenico, M., et al. (2015). Hudson submarine canyon head offshore New York and New Jersey: a physical and geochemical investigation. Deep Sea Res. II Top. Stud. Oceanogr. 121, 213–232. doi: 10.1016/j.dsr2.2015.07.019
Saba, V. S., Griffies, S. M., Winton, M., Anderson, W., Alexander, M. A., Hare, J. A., et al. (2016). Enhanced warming of the US Northeast Continental Shelf under climate change: implications for fisheries. AGUOS 2016: PC14B–2067.
Santana-Morales, O., Sosa-Nishizaki, O., Escobedo-Olvera, M. A., Onate-González, E. C., O’Sullivan, J. B., and Cartamil, D. (2012). “Incidental catch and ecological observations of juvenile white sharks, Carcharodon carcharias , in western Baja California, Mexico. Conservation implications,” in Global Perspectives on the Biology and Life History of the White Shark , ed. M. L. Domeier, (New York, NY: CRC Press), 187–198.
Simpfendorfer, C. A., Freitas, G. G., Wiley, T. R., and Heupel, M. R. (2005). Distribution and habitat partitioning of immature bull sharks ( Carcharhinus leucas ) in a southwest Florida estuary. Estuaries 28, 78–85. doi: 10.1007/bf02732755
Simpfendorfer, C. A., Hueter, R. E., Bergman, U., and Connett, S. M. (2002). Results of a fishery-independent survey for pelagic sharks in the western North Atlantic, 1977–1994. Fish. Res. 55, 175–192. doi: 10.1016/s0165-7836(01)00288-0
Skomal, G. B., Braun, C. D., Chisholm, J. H., and Thorrold, S. R. (2017). Movement of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser . 580, 1–16. doi: 10.3354/meps12306
Spaet, J. L., Patterson, T. A., Bradford, R. W., and Butcher, P. A. (2020). Spatiotemporal distribution patterns of immature Australasian white sharks ( Carcharodon carcharias ). Sci. Rep. 10, 1–13.
Stepanuk, J. E., Read, A. J., Baird, R. W., Webster, D. L., and Thorne, L. H. (2018). Spatiotemporal patterns of overlap between short-finned pilot whales and the US pelagic longline fishery in the Mid-Atlantic Bight: An assessment to inform the management of fisheries bycatch. Fish. Res. 208, 309–320. doi: 10.1016/j.fishres.2018.07.008
Trujillo, A. P., and Thurman, H. V. (2016). “Biological productivity and energy transfer,” in Essentials of Oceanography: Twelfth Edition , (Boston, MA: Pearson), 403–441.
Wall, J. (2014). Movement Ecology Tools for ArcGIS (ArcMET). Available online at: http://www.movementecology.net/arcmet_manual.html (accessed December 1, 2020).
Watanabe, Y. Y., Payne, N. L., Semmens, J. M., Fox, A., and Huveneers, C. (2019). Swimming strategies and energetics of endothermic white sharks during foraging. J. Exp. Biol. 222:jeb185603. doi: 10.1242/jeb.185603
Weng, K. C., O’Sullivan, J. B., Lowe, C. G., Winker, C. E., Blasius, M. E., Loke-Smith, K. A., et al. (2012). “Back to the Wild: release of juvenile white sharks from the Monterey Bay Aquarium,” in Global Perspectives on the Biology and Life History of the White Shark , ed. M. L. Domeier, (Boca Raton, FL: CRC Press), 419–446.
Weng, K. C., O’Sullivan, J. B., Lowe, C. G., Winkler, C. E., Dewar, H., and Block, B. A. (2007). Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar. Ecol. Prog. Ser . 338, 211–224. doi: 10.3354/meps338211
Wetherbee, B., Gruber, S., and Rosa, R. (2007). Movement patterns of juvenile lemon sharks Negaprion brevirostris within Atol das Rocas, Brazil: a nursery characterized by tidal extremes. Mar. Ecol. Prog. Ser. 343, 283–293. doi: 10.3354/meps06920
White, C. F., Lyons, K., Jorgensen, S. J., O’Sullivan, J., Winkler, C., Weng, K. C., et al. (2019). Quantifying habitat selection and variability in habitat suitability for juvenile white sharks. PLoS One 14:e0214642. doi: 10.1371/journal.pone
Xu, Y., Chant, R., Gong, D., Castelao, R., Glenn, S., and Schofield, O. (2011). Seasonal variability of chlorophyll a in the Mid-Atlantic Bight. Cont. Shelf Res. 31, 1640–1650. doi: 10.1016/j.csr.2011.05.019
Keywords : white shark ( Carcharodon carcharias ), Atlantic Ocean (North), New York Bight, telemetry, habitat use, diving behavior
Citation: Shaw RL, Curtis TH, Metzger G, McCallister MP, Newton A, Fischer GC and Ajemian MJ (2021) Three-Dimensional Movements and Habitat Selection of Young White Sharks ( Carcharodon carcharias ) Across a Temperate Continental Shelf Ecosystem. Front. Mar. Sci. 8:643831. doi: 10.3389/fmars.2021.643831
Received: 18 December 2020; Accepted: 05 February 2021; Published: 22 March 2021.
Reviewed by:
Copyright © 2021 Shaw, Curtis, Metzger, McCallister, Newton, Fischer and Ajemian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Ajemian, [email protected]
This article is part of the Research Topic
Movement and Connectivity of Large Pelagic Sharks
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Seasonal Distribution and Historic Trends in Abundance of White Sharks, Carcharodon carcharias , in the Western North Atlantic Ocean
* E-mail: [email protected]
Affiliation National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Greater Atlantic Regional Fisheries Office, Gloucester, Massachusetts, United States of America
Affiliation National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Northeast Fisheries Science Center, Narragansett, Rhode Island, United States of America
Affiliation National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Fisheries Science Center, Panama City, Florida, United States of America
Affiliation Massachusetts Division of Marine Fisheries, New Bedford, Massachusetts, United States of America
Affiliation Florida Program for Shark Research, Florida Museum of Natural History, University of Florida, Gainesville, Florida, United States of America
Affiliations National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Northeast Fisheries Science Center, Narragansett, Rhode Island, United States of America, Mote Marine Laboratory, Summerland Key, Florida, United States of America
- Tobey H. Curtis,
- Camilla T. McCandless,
- John K. Carlson,
- Gregory B. Skomal,
- Nancy E. Kohler,
- Lisa J. Natanson,
- George H. Burgess,
- John J. Hoey,
- Harold L. Pratt Jr

- Published: June 11, 2014
- https://doi.org/10.1371/journal.pone.0099240
- Reader Comments
Despite recent advances in field research on white sharks ( Carcharodon carcharias ) in several regions around the world, opportunistic capture and sighting records remain the primary source of information on this species in the northwest Atlantic Ocean (NWA). Previous studies using limited datasets have suggested a precipitous decline in the abundance of white sharks from this region, but considerable uncertainty in these studies warrants additional investigation. This study builds upon previously published data combined with recent unpublished records and presents a synthesis of 649 confirmed white shark records from the NWA compiled over a 210-year period (1800-2010), resulting in the largest white shark dataset yet compiled from this region. These comprehensive records were used to update our understanding of their seasonal distribution, relative abundance trends, habitat use, and fisheries interactions. All life stages were present in continental shelf waters year-round, but median latitude of white shark occurrence varied seasonally. White sharks primarily occurred between Massachusetts and New Jersey during summer and off Florida during winter, with broad distribution along the coast during spring and fall. The majority of fishing gear interactions occurred with rod and reel, longline, and gillnet gears. Historic abundance trends from multiple sources support a significant decline in white shark abundance in the 1970s and 1980s, but there have been apparent increases in abundance since the 1990s when a variety of conservation measures were implemented. Though the white shark's inherent vulnerability to exploitation warrants continued protections, our results suggest a more optimistic outlook for the recovery of this iconic predator in the Atlantic.
Citation: Curtis TH, McCandless CT, Carlson JK, Skomal GB, Kohler NE, Natanson LJ, et al. (2014) Seasonal Distribution and Historic Trends in Abundance of White Sharks, Carcharodon carcharias , in the Western North Atlantic Ocean. PLoS ONE 9(6): e99240. https://doi.org/10.1371/journal.pone.0099240
Editor: A. Peter Klimley, University of California Davis, United States of America
Received: February 27, 2014; Accepted: May 12, 2014; Published: June 11, 2014
This is an open-access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Funding: The authors have no support or funding to report.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The white shark Carcharodon carcharias is one of the largest, most widespread ocean predators distributed in sub-polar to tropical seas of both hemispheres [1] . White sharks are important apex predators that occupy trophic levels similar to that of carnivorous marine mammals (trophic level = 4.5) [2] – [3] . While white shark productivity (expressed as intrinsic rates of increase or population rebound potentials) falls along the midpoint of a continuum of productivity values calculated for a suite of shark species [4] – [5] , they may have naturally low abundance [6] and possess general life history traits that make them vulnerable to exploitation [7] – [9] . Although white sharks have not historically been subjected to directed fisheries, there are numerous accounts of incidental captures in commercial fisheries worldwide [1] , [10] – [14] . Moreover, their iconic status and highly valued jaws and fins have subjected them to targeted recreational and trophy fisheries where or when their populations have been unprotected [1] , [11] .
To date, only Baum et al. [15] and McPherson and Myers [16] have attempted any quantitative assessment of the status of the white shark population in the northwest Atlantic Ocean (NWA). While some of these results have been criticized as unreliable and overly pessimistic [17] , analysis of pelagic longline fishery logbook data from the NWA suggested a sharp decline (between 59 and 89%) in white shark numbers between 1986 and 2000 [15] . Similarly, using sparse sightings data (N = 31) from Atlantic Canada, McPherson and Myers [16] estimated a 3-950 fold decrease in white shark population size between 1926 and 1988. Due to studies such as these, evidence of population declines in other regions around the world (e.g., [18] – [19] ), and their iconic and charismatic nature, white sharks have been afforded some of the highest level of protection of any elasmobranch. For example, they have been listed on the appendices of The United Nations Convention on Law of the Sea (UNCLOS), the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and the Convention for the Conservation of Migratory Species (CMS). The World Conservation Union (IUCN) currently lists the white shark globally as ‘Vulnerable’ [20] . In the NWA, The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) has recommended that white sharks be listed as “Endangered,” and they have been listed as a prohibited species (i.e., no commercial or recreational harvest) in US waters since 1997 [21] . Due to these conservation concerns, and the high uncertainty associated with previous studies [15] – [16] , there is a need to better understand the historic and current status of white sharks in the NWA, incorporating as much reliable data as possible.
Despite recent advances in field research on white sharks in several regions around the world (e.g., [22] – [23] ), opportunistic capture and sighting records remain the primary source of information on this species in the NWA [14] , [16] , [24] – [25] . This is due to their sparse distribution and a historic lack of discrete coastal aggregation sites in this region. Casey and Pratt [24] provided a qualitative assessment of the distribution of NWA white sharks, but this study took place before the significant expansion in the 1980s of directed large coastal shark fisheries in the US Atlantic (e.g., [26] – [27] ). White sharks were found to range from Newfoundland, Canada to the Gulf of Mexico and northern Caribbean Sea, but were most frequently encountered from the Gulf of Maine south to Cape Hatteras, North Carolina [24] . They have been considered only occasional visitors to the warmer waters off the southeastern US and Gulf of Mexico [24] , [28] – [31] .
Herein, we report on the patterns of distribution and relative abundance of white sharks in the NWA region based on a comprehensive compilation of historic and recent white shark capture and sighting records. A variety of fishery-dependent and -independent sources were synthesized, resulting in the largest white shark dataset yet compiled from this region. We provide a robust description of their historical abundance trends, spatio-temporal distribution, fishery interactions, and essential habitats. This updated information will improve the conservation and management of white sharks regionally and internationally, and provide a new baseline for future studies.
White shark occurrence records were collected from numerous sources, including landings data, commercial fishery observer programs, recreational tournament information, scientific research surveys, commercial and recreational fishermen, collaborating scientists, newspaper articles, personal communications, and the scientific literature ( [14] , [24] and references therein, [25] , [31] – [33] ). Due to species misreporting problems in the pelagic longline fishery [17] , logbook records from this fishery were considered unreliable and excluded. The data in each record typically included date, location, measured or estimated shark total length (TL), and capture gear (unless a visual observation). Lengths estimated at greater than 6 m were considered unreliable [34] . Where lengths were reported in fork length, conversion to TL was performed using the formula in Kohler et al. [35] . Based on published length-at-age and length-at-maturity estimates [7] – [8] , [36] , sharks were classified as neonate (<1.5 m TL), young-of-the-year (YOY, <1.75 m TL), juvenile (1.75–3.79 and 1.75–4.5 m TL for males and females, respectively), or mature (>3.79 and >4.5 m TL for males and females, respectively). Some records had more complete data including shark weight, sex, stomach contents, photographs, water temperature, depth, or other observations. All records were given a subjective reliability ranking of A, B, C, or F similar to that described by Casey and Pratt [24] and Skomal et al. [14] . Records receiving a low ranking of C or F, in which the identification of the white shark seemed suspect, could not be corroborated, and/or lacked photographic evidence, were excluded from the analysis.
Distribution analysis
All records were analyzed with reference to spatial and temporal patterns of presence, as well as bottom depth and sea surface temperature (SST), when recorded. If not reported, white shark sighting locations (latitude and longitude) were assigned where possible. Data were plotted using Geographic Information System (GIS) software (ArcGIS v. 10.0, ESRI, Redlands, California). Bottom depth was subsequently assigned to each observation by matching the position to ETOPO1 Ocean Relief Model bathymetry in ArcGIS. To investigate seasonal changes in distribution, year was divided into four seasons: winter (January through March), spring (April through June), summer (July through September), and fall (October through December). Due to the inherent limitations of using presence-only information where observation effort and detectability are unknown, raw positions were simply mapped in their corresponding season, and no quantitative species distribution models were applied. In order to visualize shark distribution relative to typical SST conditions in the region, seasonal shark positions were overlaid on satellite-based 4 km Advanced Very High Resolution Radiometer (AVHRR) Pathfinder v.5.0 Seasonal Climatologies, averaged from 1985–2001 (National Oceanographic Data Center/University of Miami).
Trends in abundance
Multiple historic and current data sources were examined for the presence of white sharks. Of those examined, we determined that only four data sources contained adequate information to estimate white shark trends in abundance for the NWA. Longline catch data were obtained from two sources: fishery-independent longline surveys conducted by the NMFS Northeast Fisheries Science Center (NEFSC) and its predecessor agencies between 1961 and 2009 [37] – [38] and the observer program of the directed shark bottom longline fishery from 1994–2010 [26] , [39] . Data collected by the NMFS NEFSC at five recreational fishing tournaments from 1965 to 1996 (white sharks were listed as a NMFS prohibited species in 1997) were also used in this study. The tournaments were based out of New York (Bayshore Mako Tournament, Montauk Marine Basin Shark Tag Tournament, and Freeport Hudson Anglers, Inc. Shark Tournament) and New Jersey (Jersey Coast Shark Anglers Invitational Shark Tournament and South Jersey Shark Tournament). The final data source included sightings and capture records of white sharks in the NWA from 1800–2010 [14] , [24] , excluding records from the previous three time series, recent directed sightings effort, and accounting for historical directed effort leading up to and directly following the publication of the first comprehensive NWA white shark distribution paper [24] . Historical directed sightings effort was removed from the sightings time series during the late 1970s through the1980s based on the original datasheet notations and knowledge of the persons collecting the data during that time, resulting in an 80% reduction in these sightings records ( Figure S1 ). Following initial analyses of the sightings data, additional sightings records in the vicinity of Monomoy Island, Massachusetts were removed in recent years for trend comparisons with respect to the increase in sightings near a growing population of gray seals ( Halichoerus grypus ) in that area [14] .
Due to excess zero observations in the observer data, the fishery-independent longline surveys, and the tournament data, we used a mixture of a Bernoulli distribution (with a point mass of one at zero) for presence/absence data and a Poisson distribution for count data (including zeros) in a zero-inflated Poisson (ZIP) mixture model [40] – [41] to develop standardized indices of abundance. A number of parameters were considered as potential covariates affecting the presence/absence of white sharks and/or the white shark catch per set or tournament. For the NEFSC longline surveys, the variables available for consideration were year, season, depth, SST (<10°C, 10–14°C, 15–19°C, 20–24°C, >25°C), latitude, target (coastal shark, pelagic shark, pelagic inshore), bait type (teleost, elasmobranch, mixed), gear fishing on the bottom or up in the water column, leader type (wire, monofilament, mixed), hook number, and soak time. Variables available for the NEFSC tournament database were year, tournament, number of boats, number of days fished, and area (NY, NJ). For the observer program, the variables available for consideration were year, season, time of day, depth, area (Gulf of Mexico, southern Atlantic), hook type (small, medium, large, other), bait type (clupeid, elasmobranch, teleost, tuna, other), hook number, and research fishery participation (Amendment 2 to the 2006 Consolidated Highly Migratory Species Fishery Management Plan established a scientific research fishery in 2008 to gather information on Carcharhinus plumbeus ). Stepwise forward model selection was used to determine which variables to retain in all final models based on the Akaike information criterion (AIC) and given a likelihood ratio test between the chosen model and the null model (intercept only) produced a test statistic value close to zero (≤0.01) [42] – [43 . All models retained “year” in order to develop annual indices of abundance. Residual plots were used to determine the adequacy of model fits [43] .
These standardized indices of abundance were then analyzed using a hierarchical framework to estimate a single time series of relative abundance [44] . This approach allows for the combination of multiple time series with differing lengths that do not all overlap in time [44] . The hierarchical approach developed by Conn [44] assumes that each index is measuring relative abundance and is subject to both process error and sampling error, the latter of which is presumably captured by the standardization process used to develop the indices of abundance. The indices (standardized to their means) and coefficients of variation were used in the hierarchical analysis to estimate individual index process error, assuming a lognormal error structure, and a hierarchical index of abundance [44] . The hierarchical analysis was conducted in a Bayesian framework using the same set of prior distributions as described by Conn [44] and used for other shark species for stock assessment purposes [45] .
Annual white shark sightings were modeled using the approach developed by McPherson and Myers [16] to examine population trends from observational data. This method extracts the abundance trend in relative terms by fitting a series of generalized linear models to the difference in the count data between two points in time (difference between the most recent time point and any reference date) using a Poisson distribution and guards against sensitivity to unusually high or low counts by varying the reference period used to derive the count differences [16] . The estimated trend in relative abundance can then be viewed by plotting the magnitude of change in the number of reported sightings by year in log-space. Resulting values larger than 1 suggest an overall declining trend in abundance, values of 1 suggest a stable population, and values less than 1 suggest an overall increasing trend in abundance. This approach was used on the sightings data for multiple time frames. The sightings data were analyzed given any reference year from 1800 to 2008, 1950 to 2008, 1960 to 1986, and 1990 to 2008. Sensitivity analyses were conducted assuming changes in observation effort had either increased or decreased by 25% and 50% [16] . All analyses were conducted using the R programming environment [46] .
We compiled a total of 649 verified white shark records from the NWA during the period 1800–2010. While the records date as far back as 1800, 94% occurred since 1950. Of these, 596 records had sufficient data (i.e., date and location) for seasonal distribution analysis and 433 were included in relative abundance time series runs (excluding directed effort, N = 200, and sightings with no associated year, N = 5).
Sex of the shark was confirmed in 297 records and included 148 males and 149 females. Sharks that were accurately measured (N = 279) ranged in length from 1.22–5.63 m TL. An additional 259 records included estimated lengths, which we rounded down to the nearest m TL (1–9 m TL) ( Figure 1 ). The records collectively included 124 YOY, 310 juveniles, and 104 mature sharks. While some white sharks were reported at estimated lengths exceeding 9 m, these estimations were considered unreliable. The largest shark considered accurately measured was a female specimen landed on Prince Edward Island, Canada in August 1983, which measured 5.26 m fork length (5.63 m TL).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Length frequency of white sharks from the western North Atlantic (N = 538). These data include lengths from accurately measured specimens (N = 279), as well as estimated lengths, rounded down to the nearest m.
https://doi.org/10.1371/journal.pone.0099240.g001
Gear interactions
Confirmed gear interactions represented 66% (404) of the white shark records compiled, including both targeted and incidental catches. Forty-one percent of these records were derived from recreational rod and reel fishing ( Figure 2 ). Amongst the remaining gear types, white sharks were most frequently captured by fishery-dependent (13%) and -independent (11%) longline gear (bottom and pelagic), harpoon (11%), and gillnet (11%, sink and drift), with fewer numbers caught in trawls (8%) and fish weirs/traps (4%, Figure 2 ). The practice of harpooning large white sharks, responsible for the majority (33%) of mature white shark captures, was more prevalent prior to 1980, and has been uncommon since 1997 when white sharks were prohibited from commercial and recreational harvest. Since 1985, fishery-dependent longline gear (40%) dominated reported white shark captures with rod and reel captures dropping to 35%. Within commercial fisheries (1985–2009), longline (60%) and gillnet (17%) have been the primary sources of incidental captures reported, and these gears predominantly catch immature sharks ( Figure 2 ). Recreational rod and reel fishing accounted for 28% of the mature white sharks landed, with 72% of these captured between 1960 and 1990. Most of these landings occurred between Long Island, New York, and Massachusetts. However, juvenile white sharks (including YOY) were also frequently caught by rod and reel fishermen ( Figure 2 ) targeting other large gamefish along the US coast.
Reported fishery-dependent and fishery-independent gear interactions with white sharks by life stage in the NWA, 1800–2009 (N = 390).
https://doi.org/10.1371/journal.pone.0099240.g002
Seasonal distribution
The range of white shark occurrence extended from the north coast of Newfoundland (51° N) to as far south as the British Virgin Islands (18° N), as far east as the Grand Banks (50° W) and Bermuda (65° W), to as far west as the coast of Texas in the Gulf of Mexico (97° W, Figure 3 ). While this overall distribution is quite broad, 90% of white sharks occurred along the US coast between 22° 00’ and 45° 30’ N (100% YOY, 86% juvenile, 89% mature). The center of distribution was in southern New England and the Mid-Atlantic Bight (between 35° 00’ and 42° 00’ N), where 66% of white sharks occurred (97% YOY, 54% juvenile, 70% mature).
Distribution of white shark presence records (white circles) in the NWA during (a) winter, (b) spring, (c) summer, and (d) fall. Positions are overlaid on seasonal average SST conditions (1985–2001). The 200 m bathymetric contour is displayed to delineate the edge of the continental shelf. CC = Cape Cod, NYB = New York Bight, CH = Cape Hatteras, FL = Florida, GOM = Gulf of Mexico, and CS = Caribbean Sea.
https://doi.org/10.1371/journal.pone.0099240.g003
White sharks of all size/age classes were present in continental shelf waters throughout the year. However, there were considerable differences in distribution across seasons ( Figure 3 ). During winter months, white sharks (2% YOY, 75% juvenile, 27% mature) were primarily distributed off the southeastern US and in the Gulf of Mexico ( Figure 3a ). Only one YOY white shark was captured during the winter months. This shark measured 1.64 m TL and was captured off North Carolina in January 1996. The median latitude of occurrence during winter months ranged from ∼28–31° N ( Figure 4 ). No white sharks were reported north of Cape Hatteras (∼35° N) during winter ( Figure 3a ). Focal areas of winter occurrence were identified off the northeast coast of Florida (smaller juvenile through mature-sized individuals), off the Florida Keys (larger juvenile and mature sharks), and offshore of Tampa Bay (smaller juvenile through mature sharks) in the eastern Gulf of Mexico ( Figure 3a ).
Box plots of latitudinal distribution of white shark presence by month in the NWA. The sample size in each month is given above the x-axis.
https://doi.org/10.1371/journal.pone.0099240.g004
During spring months, there was a clear expansion northward ( Figure 3b , Figure 4 ). White sharks (28% YOY, 50% juvenile, 22% mature) occurred widely along the coast, mostly between the eastern Gulf of Mexico and the New York Bight (waters off the US Atlantic coast from Cape May Inlet in New Jersey to Montauk Point in Long Island, New York, Figure 3b ). Median latitude of occurrence shifted dramatically across spring months, from 28° N in April to 40° N in June ( Figure 4 ). The northernmost occurrences during this period typically occurred in late spring (May and June) ( Figure 4 ) and the majority were large juvenile and mature sharks.
By summer, white sharks (23% YOY, 47% juvenile, 30% mature) appeared largely absent from southern coastal waters, occurring primarily in the Mid-Atlantic Bight, New England, and Canadian waters ( Figure 3c ). Only a few white sharks (mature) have been reported from south of Cape Hatteras during summer ( Figure 3c ). Most records were centered from the New York Bight eastward and north to Cape Cod. White sharks, predominately large juvenile and mature individuals, appear to reach the most northern portions of their NWA range (Newfoundland, Gulf of St. Lawrence) during August ( Figure 4 ), but the median latitude of occurrence for all life stages remains around 40–41° N throughout the summer ( Figure 4 ).
YOY sharks were most frequently encountered during summer between the central coast of New Jersey and Massachusetts Bay. However, most YOY shark observations (64%) were concentrated in the New York Bight between Great Bay, New Jersey, and Shinnecock Inlet, Long Island, New York. Neonate-sized white sharks (N = 46) were documented in this area between June and October (85% in June-August). Mature-sized female white sharks were also documented from this region during summer months, but no gravid or post-partum females were examined.
White sharks (15% YOY, 64% juvenile, 21% mature) remained in northern latitudes into the fall ( Figure 3d ), but appeared to begin a southward transition in November and December ( Figure 4 ). Similar to spring months, white shark occurrence was broadly distributed along the coast between New England and the east coast of Florida ( Figure 3d ). The largest shift in median latitude occurred between November (42° N) and December (34° N, Figure 4 ).
Habitat Use
While environmental observations were limited throughout this data set, some patterns of habitat use were identified. Depth distribution data (N = 564) indicated that white sharks were predominantly encountered over continental shelf waters (200 m, Figures 3 and 5a ). Over 92% of observations occurred in waters 100 m deep, and the median reported depth at occurrence was 30 m (mean ±1 SD = 69±235 m). Only 23 observations occurred in deeper waters off the continental shelf, however, many of these were still relatively close to shore (e.g., off the Florida Keys, Figure 3 ). For YOY (N = 102), juvenile (N = 265), and mature (N = 125) sharks, the median depth at occurrence was 32 m (mean ±1 SD = 32±19 m), 26 m (mean ±1 SD = 45±74 m), and 50 m (mean ±1 SD = 89±190 m), respectively; indicating a potential increase in the use of deeper waters by white sharks with increased size/age.
Distribution of (a) bottom depths (N = 564) and (b) SST (N = 124) associated with NWA white shark captures/sightings.
https://doi.org/10.1371/journal.pone.0099240.g005
White sharks were captured in SSTs (N = 124) of 9–28°C (mean ±1 SD = 18.3±3.5°C). For YOY (N = 26), juvenile (N = 68), and mature (N = 21) sharks, the median reported SST at occurrence was 19.5°C (mean ±1 SD = 19.0±1.9°C), 18°C (mean ±1 SD = 18.1±3.5°C), and 16°C (mean ±1 SD = 17.7±4.6°C), respectively. Over 80% of observations with temperature information were between 14 and 23°C ( Figure 5b ). Additionally, analysis of the NEFSC longline survey database suggested a preference for a similar SST range (see Trends in Abundance section).
Trends in Abundance
The best fit model for the NEFSC longline surveys indicated that both the presence/absence and number of sharks per set were primarily influenced by soak time. There was a higher likelihood of catch with longer soak times, but within the positive catch sets, the longest soak times produced fewer white sharks, possibly due to bite offs (observed severed leaders) and/or predation. The presence/absence of white sharks in the NEFSC longline surveys was also influenced by SST with a higher likelihood of catch in the 15–19°C and 20–24°C temperature categories. Depth also influenced catch per set with higher catch rates in shallower depths. The presence/absence of a white shark at sampled tournaments was influenced by tournament location, with a higher likelihood of catching a white shark during one of the tournaments based out of New Jersey during the reported sampling time frame. For the observer program, the presence/absence of white sharks was primarily influenced by area fished and effort (number of hooks); catch per set was also influenced by area fished as well as season (highest catches off the Atlantic coast of Florida during the winter).
Both standardized indices of relative abundance for the NEFSC longline surveys and the tournament data show decreasing estimates over time until the end of tournament time series, when white sharks were prohibited. Then the NEFSC longline index appears to increase based on best fit regression models of the data ( Figure 6 ). The second order polynomial trend line estimated for this time series fits with our knowledge of the survey data in that the ZIP model could not provide estimates for several zero catch years during the mid to late 1990s and into the early 2000s. The observer index, which started after the implementation of the first shark fishery management plan in 1993, has an overall increasing trend in relative abundance throughout the time series, despite the large peak in the early 2000s, which the standardization process could not account for ( Figure 6 ).
White shark indices of abundance (index/mean) standardized using a zero-inflated Poisson model plotted by year for three time series: NEFSC LL = Northeast Fisheries Science Center fishery-independent longline surveys, TOURN = NEFSC tournament database, and OBS LL = observer program of the directed shark longline fishery. Trend lines are best fit regression models of the standardized data (second order polynomial for NEFSC LL and exponential for TOURN and OBS), using R 2 values and considering the biology of the white shark. The dashed red line indicates the year of the first fishery management plan (FMP) for Atlantic sharks in 1993 [77] and the solid red line indicates the year that white sharks were listed as a NMFS prohibited species in 1997 [21] .
https://doi.org/10.1371/journal.pone.0099240.g006
The hierarchical trend combining all three indices, although slightly masked by the large credible intervals for the index, shows historically higher abundances during the 1960s and into the mid-1970s with a declining trend into the late 1980s and then begins a gradual increasing trend through the remainder of the time series ( Figure 7 ). During the mid-1970s and throughout the 1980s, white shark relative abundance had declined between 27 and 86%, with a median value of 73%. The most recent year in the time series (2010) shows only a 31% decline in white shark abundance from its historical abundance estimate in 1961. Estimates of process error show the three indices performed reasonably well for white shark abundance and values were similar across indices (indices process standard deviation estimates ranged from 0.405–0.457, Figure S2 ).
Time series of white shark relative abundance in the NWA as estimated from hierarchical analysis. The continuous black line gives the posterior mean, and the shaded area represents a 95% credible interval about the time series. The red line is the estimated trend based on locally weighted polynomial regression using the LOWESS smoother.
https://doi.org/10.1371/journal.pone.0099240.g007
Excluding the time series analyzed separately and directed effort, a total of 346 white sharks were sighted between 1800 and 2009 ( Figure 8a ), with over 86% (299) of the sightings occurring between 1950 and 2009 ( Figure 8c ). Under the assumption of no change in observational effort, the sightings model estimated that there was an overall increasing trend (all estimated values less than 1) in the NWA white shark population since the 1800s, most notably during the beginning of the time series through the 1950s and during more recent years ( Figure 8b ). A closer look at the relative abundance trend starting in the 1950s, reveals that even though the change in magnitude from any reference year between 1950 and 2008 to the terminal year in 2009 results in an increase in relative abundance (magnitude of change 1), there still appears to be a declining trend during the 1970s into the mid 1980s ( Figure 8d ). Sensitivity analyses estimating 25 and 50% increases and decreases in observation effort clearly increases the uncertainty surrounding the estimates of change in abundance, but the overall trend remains the same. Analysis of the sightings data with a terminal year of 1987 reveals an estimated 2–4-fold (median estimate = 2.71, 63% decline) decrease in the population since any reference year between 1970 and 1986 ( Figure 9 ). If we reduce the observational effort by 25% and 50%, it reduces the estimated decline during the 1970s into the mid 1980s to 51% and 26%, respectively (median estimates = 2.02 and 1.36, respectively, Figure 10 ). A 98% reduction in observational effort is needed to avoid a decline in abundance during that time frame (model estimates and confidence bounds consistently drop below 1). During the 1990s, the relative abundance trend appears to stabilize and then begins an increasing trend during the 2000s until the end of the time series ( Figures 8b, 8d ). This overall increasing trend in relative abundance during the end of the time series is retained when assuming 25 and 50% increases and decreases in observation effort ( Figures 8b, 8d ).
(a) Number of annual white shark sightings reported in the NWA from 1800 to 2009. (b) Estimates of relative change in abundance (filled circles) with 95% credible intervals (dashed lines) for any reference year between 1800 and 2008 assuming no change (black plot), a 50% increase (red plot), and a 50% decrease in observation effort. (c) Number of annual white shark sightings reported in the NWA from 1950 to 2009. (d) Estimates of relative change in abundance (filled circles) with 95% credible intervals (dashed lines) for any reference year between 1950 and 2009 assuming no change in observation effort (black plot), a 25% and 50% increase in observation effort (green and red plots, respectively), and a 25% and 50% decrease in observation effort (blue and purple plots, respectively).
https://doi.org/10.1371/journal.pone.0099240.g008
Estimates of relative decline in abundance (filled circles) with 95% credible intervals (dashed lines) for any reference year between 1960 and 1986 assuming no change in observation effort (black plot), a 25% and 50% increase in observation effort (green and red plots, respectively), and a 25% and 50% decrease in observation effort (blue and purple plots, respectively). Note that the scale for the y-axis has been reversed when compared to Figure 8 to visualize the declining trend in abundance during this time period.
https://doi.org/10.1371/journal.pone.0099240.g009
(a) Estimated trend from the hierarchical analysis, and (b) estimated trend from the sightings analysis.
https://doi.org/10.1371/journal.pone.0099240.g010
A comparison of abundance trends between the hierarchical and sightings methods reveals a strikingly similar pattern except at the end of the time series, where the sightings time series has a much steeper increase in abundance ( Figure 10 ). Removal of white shark sightings from the sightings data during the 1990s and 2000s around a growing gray seal colony on Monomoy Island still provides an increasing trend, but an overall smaller magnitude of change and results in a more gradual slope that is more in line with the trend estimated for the hierarchical index ( Figures 10 , 11 ).
Estimates of relative change in abundance (filled circles) with 95% credible intervals (dashed lines) for any reference year between 1990 and 2008 assuming no change in observation effort (black plot), a 25% and 50% increase in observation effort (green and red plots, respectively), and a 25% and 50% decrease in observation effort (blue and purple plots, respectively) for the original sightings time series from 1990 to 2009 (a) and the time series with sightings that occurred near Monomoy Island during that time frame removed (b).
https://doi.org/10.1371/journal.pone.0099240.g011
This study represents the most comprehensive synthesis of data on NWA white sharks to date, and significantly updates previous reviews [24] – [25] . In general, the white shark remains an uncommon and sparsely distributed predator in the NWA. However, by combining over two centuries worth of observations the results have provided new insights into population and distribution trends along the east coast of North America.
Seasonal Distribution and Habitat
The use of presence-only data for describing species distributions has inherent limitations (e.g., [47] ). Results may be biased by spatial and temporal variability in observation effort, detectability, and catchability [16] , [47] . However, presence records from captures and sightings are often the best source of baseline information on comparatively uncommon marine species like the white shark [10] , [24] , [48] – [49] . Since the majority of our records were derived from fisheries interactions, patterns in fishing effort and gear over space and time should partially account for the patterns we have described. One important bias is that the occurrence of adult white sharks in our dataset is likely underestimated due to the fact that these large individuals can more easily escape entanglements/hooking in fishing gear.
Since most fishing effort and boating activity in the NWA occurs over continental shelf waters, encounter rates with white sharks may be biased toward the coasts. Therefore, white shark occurrence in offshore waters may be underrepresented in this analysis. The only fishery likely to encounter white sharks in offshore waters is the pelagic longline fishery, which targets tunas and swordfish, but regularly incidentally captures pelagic shark species including silky ( Carcharhinus falciformis ), dusky ( C. obscurus ), oceanic whitetip ( C. longimanus ), and blue ( Prionace glauca ) sharks [15] , [50] . However, the occurrence of white sharks in this offshore fishery appears to be extremely low [24] , [50] – [51] . We agree with the assertions of Burgess et al. [17] that the 6,087 white sharks reported in pelagic longline fishery logbooks according to Baum et al. [15] were probably not in fact Carcharodon carcharias , and these records should not be used to infer distribution or abundance patterns for this species. Given the occasional reports of white sharks from offshore waters beyond the continental shelf, including their documented occurrence in Bermuda waters [33] and recent satellite tracking data (GBS, unpublished data), further observations, stable isotope analyses, and/or advanced technology tagging studies are needed to provide a greater understanding of their use of offshore habitats in this region.
In the absence of seasonal shifts in shark distribution, fisheries would be expected to have fairly equal probability of encountering white sharks across the year throughout their range. However, this was not the case for several fisheries, as encounters were unevenly distributed across seasons. For example, despite observer coverage for the majority of the year in the shark bottom longline fishery [26] , [39] , no white sharks were encountered during summer months off the southeast US. Likewise, catch and observer records in commercial trawl and gillnet fisheries off New England and Canada primarily documented white sharks during summer months, despite year-round fishing activity and observer coverage (NMFS Northeast Fisheries Observer Program, unpublished data). These trends appear to support the seasonal north-south distribution shift of the NWA white shark population, despite the limitations of using presence-only information. This north-in-summer, south-in-winter distributional pattern is typical of numerous temperate, coastal, migratory fishes in the northern hemisphere (e.g., [52] – [53] ) and white shark migrations from temperate to subtropical waters have also been documented off the west coast of the United States and Mexico [54] – [55] and off the Pacific coasts of Australia and New Zealand [56] – [57] .
Consistent with previous studies on white sharks (e.g., [18] , [24] , [31] ), temperature appears to exert a significant influence on distribution, and is likely a key migratory cue in the region. The seasonal movement of the white shark population up and down the Atlantic coast of North America, an average shift of approximately 12° of latitude (28–40° N, Figure 4 ), allows white sharks to remain within an apparently preferred SST range of ∼14–23°C. Given their comparatively large body mass and endothermic capabilities [58] , this relatively narrow temperature range does not define the white sharks thermal tolerance which extends from at least 3–28°C [55] , [59] – [60] , but it does appear to largely define the bounds of their seasonal latitudinal range in this region. Therefore, while temperature may drive seasonal distribution shifts, the selection of specific summer and winter habitats is likely based upon environmental characteristics secondary to temperature (e.g., prey availability).
The relatively broad summer focal area for white sharks between the coasts of New Jersey and Massachusetts likely include important foraging areas across life stages. YOY and juvenile white sharks, which were more prevalent in the New York Bight region during summer, would have access to a wide variety of demersal and pelagic teleosts and elasmobranchs for prey [24] . The waters less than 50 m deep on the broad continental shelf in the New York Bight area may represent primary nursery habitat for YOY white sharks [24] . The seasonal peak in the presence of neonate-sized sharks suggests that parturition may occur near this area between May and August. White shark nursery habitat has also been identified in other regions along continents where larger expanses of shelf habitat exist [56] , [61] .
Large white sharks (3.0 m) tend to preferentially feed upon marine mammals including pinnipeds, small cetaceans, and large whale carcasses [10] , [18] , [62] – [63] . Since pinniped populations in the NWA have been severely depressed throughout most of the last century [64] , confirmed predations on seals ( Phoca vitulina , Halichoerus grypus ) have been rare until very recently [14] , [32] , [65] . Whale carcasses are thought to be one of the most important sources of food for large white sharks in this region [66] . White sharks have been observed scavenging dead whales off New England and Long Island, New York on numerous occasions [24,63, 66–67, NMFS unpublished data, JKC personal observation), but they also supplement their diet with odontocete whales such as the harbor porpoise ( Phocoena phocoena ) [68] – [69] and fishes including tunas (Thunnus spp.), sea robins ( Prionotus spp.), menhaden ( Brevoortia tyrannus ), hakes ( Urophycis spp.), skates (Rajidae), bluefish ( Pomatomus saltatrix ), smooth dogfish ( Mustelus canis ), and other shark species ( [24] , NMFS unpublished data).
Due to the dynamic and broad distribution of prey (i.e., teleosts, marine mammals) in this region, white sharks must forage over a broad area, rather than at discrete aggregation sites like those off California, Australia, or South Africa (e.g., [70] – [72] ). However, the recovery of NWA gray seal populations over the last decade [64] and their increasing concentrations at specific sites along Cape Cod, Massachusetts, appears to be producing new localized summer feeding aggregations for white sharks [14] .
Although the summer distribution of white sharks in the NWA has been described in previous studies [24] – [25] , there has been very limited information on the focal areas for white shark occurrence during winter months. White sharks have long been thought to be rare and occasional visitors to coastal waters off the southeast US, Gulf of Mexico, and the northern Caribbean Sea [24] , [28] – [31] . However, the current results indicate that white sharks visit these subtropical waters on a regular basis during the winter. The most notable areas of repeated occurrence during winter months are the Atlantic shelf waters between southern Georgia and Cape Canaveral, Florida and Gulf of Mexico shelf waters west of Tampa Bay, Florida for small juvenile through mature sized individuals, and Atlantic coastal waters along the Florida Keys for larger juvenile and mature white sharks.
The reasons why white sharks are drawn to particular subtropical areas during winter months are unclear, but they likely include important foraging grounds. Analysis of white shark stomach contents from this region are extremely limited, however, documented prey items include dolphins (Delphinidae), sharks (Carcharhinidae), red drum ( Sciaenops ocellatus ), sea turtles, and squid ( [31] , Authors unpublished stomach contents data). Historically, white sharks that occurred along the Florida Keys and northern Caribbean islands may have also preyed upon the now extinct Caribbean monk seal ( Monachus tropicalis ) [73] . Juvenile and adult white sharks have also been observed scavenging upon the carcasses of North Atlantic right whales ( Eubalaena glacialis ) in the waters off Georgia and northern Florida on several occasions [74] . This area is designated as critical habitat for the right whale, and includes their primary (December-March) calving grounds [75] . White sharks are not known to actively prey upon healthy adult mysticete whales [63] , [76] , but it is possible that they are drawn to this area during the right whale calving season in order to attempt to prey upon calves [74] , or scavenge upon occasional carcasses of adults or calves and/or whale placentas. Seasonal movement of white sharks to subtropical calving grounds of humpback whales ( Megaptera novaengliae ) has been documented in the North and South Pacific Oceans (e.g., [49] , [54] , [57] ). Despite the unpredictable availability of large whale carcasses, white sharks may regularly migrate to whale aggregation areas for foraging/scavenging. The particularly high caloric value of whale blubber tissue [66] makes it an optimal food choice to help meet the high energetic demands of the endothermic white shark [58] , [63] .
In summary, given the available information on white shark distribution, feeding habits, and habitat use, it appears that the annual north-south distribution shift of the white shark population is driven by a combination of environmental preferences and prey availability. White sharks move into summer feeding areas off the northeast US when SST rises above approximately 14°C. They feed on a wide variety of prey over a broad area, but large white sharks have been increasingly associated with emerging gray seal colonies off Massachusetts in recent years [14] . As temperatures decline during the fall, the shark population shifts southward, eventually reaching putative foraging grounds off Georgia and Florida. White sharks have been documented to occur on continental shelf waters throughout the year, and may migrate along the Atlantic coast rather than regularly moving into offshore pelagic waters, as they do in the eastern North Pacific (e.g., [54] – [55] ). The sparse observations in Mid-Atlantic waters between Maryland and South Carolina for all life stages suggest this stretch of coast may be a migratory corridor, connecting northern and southern feeding areas. However, preliminary satellite tracking data from this region suggest that some individuals may also spend considerable amounts of time beyond the continental shelf (GBS, unpublished data). More observations, tagging, and telemetry studies are necessary to shed more light on these patterns.
Abundance Trends and the Status of NWA White Sharks
The results of our relative abundance analyses offer a more optimistic outlook for NWA white sharks than previous reports [15] , [16] . Consistent with previous analyses, significant declines (63–73%) through the 1970s and 1980s were identified, but previously undocumented positive trends were present in available time series since the early 1990s. The hierarchical method, allowing the combination of multiple time series that did not all overlap in time, had the largest amount of uncertainty associated with its estimated trend of relative abundance. During simulation testing of the hierarchical method, Conn [44] reported that the credible intervals for the hierarchical index were frequently wider than nominal for all simulation scenarios, suggesting that the estimation procedure was overly conservative. Although there is uncertainty in all trends used in this study, the concordance of multiple data sources in the timing of population changes lends credence to the observed patterns. The population declines of the 1970s and 1980s and the increases during the 1990s are also parsimonious with our understanding of the expansion and eventual regulation of shark fisheries during this period [21] , [27] , [77] .
Though no real trend can be inferred, an additional source of historic and contemporary relative abundance comes from the shark bottom longline fishery off Florida [24] , [26] , [51] . From 1935–1950, prior to widespread commercial shark fishing and purported population declines, white sharks represented approximately 1 out of every 3,704 sharks captured in this fishery [24] , [51] . Despite some likely changes to gear and effort over time, Morgan et al. [26] reported that white sharks represented approximately 1 out of every 3,443 sharks captured in the same fishery between 1994 and 2003, a remarkably small difference between observations separated by over 40 years. Though these are just two points in time, the similarity in relative occurrence may indicate that white shark abundance in this region is currently comparable to what it was in the 1930s and 1940s. Had the stock collapsed and remained at decimated levels, the relative occurrence ratio in Morgan et al. [26] would likely have been significantly lower than that reported by Springer [51] .
There is evidence suggestive of recent increases in white shark abundance in other regions, similar to what is documented here for the NWA. Catch per unit effort from protective beach nets show an apparent increasing trend in relative abundance for white sharks during the 2000s in South Africa [78] and during the mid 1990s through the 2000s in New Zealand [79] . Catches of white sharks from southern California fisheries have also increased in recent years despite significant reductions in fishing effort [12] . Similar to the US Atlantic, all of these regions have legally protected white sharks from harvest since the 1990s. Though data remain comparatively sparse for white sharks, and significant uncertainty remains in all abundance trend estimates ( [12] , [16] , [78] – [79] , this study), there is growing evidence that legal protections for white sharks in the NWA and elsewhere around the world have been effective. Population declines appear to have been halted and populations may now be stabilized or growing in several regions. However, given the white sharks inherent sensitivity to exploitation and low productivity [4] , [9] , fishery bycatch mortality remains a concern to the long-term sustainability of their populations.
Despite some recent progress in our understanding of the biology of white sharks in the NWA ( [9] , [14] , [74] , [80] , this study), there are still considerable knowledge gaps in this region compared to other areas [23] . Significant questions remain on life history, population structure and size, behavior, habitat preferences, feeding habits, movements, and migration. Other than the possible presence of a summer nursery area in the New York Bight, virtually nothing is known about the location and timing of mating or parturition. It is not known if the timing and extent of white shark migrations in the NWA are similar to those described in recent satellite tracking studies in the Pacific and Indian Oceans [54] – [55] , [59] , [81] . Further research will help fill in many of these information gaps, and continued compilation of opportunistic sightings, fishery captures, and examination of occasional specimens will, over time, help to further expand our knowledge and improve conservation strategies.
Supporting Information
Time series of white shark sightings. (a) Number of annual white shark sightings reported in the NWA from 1800 to 2009, excluding the time series used in the hierarchical analysis and recent directed effort. The vertical red line indicates the year the first comprehensive NWA white shark distribution paper was published [24] . (b) Number of annual white shark sightings used to model trends in abundance, contains an 80% reduction in records leading up to and directly following the Casey and Pratt [24] publication (red line) to account for directed effort during that time.
https://doi.org/10.1371/journal.pone.0099240.s001
Process errors for white shark relative abundance indices. Posterior means and 95% credible intervals for the standard deviation (SD) of process error for the three indices used in the hierarchical analysis. NEFSC LL = Northeast Fisheries Science Center fishery-independent longline surveys, TOURN = NEFSC tournament database, and OBS LL = observer program of the directed shark longline fishery.
https://doi.org/10.1371/journal.pone.0099240.s002
Acknowledgments
We are grateful to the numerous individuals and organizations who contributed white shark records and technical assistance for this project, including Jack Casey, John Chisholm, Steve Correia, Doug Adams, Joe Mello, Ken Goldman, John Morrissey, Robert Hueter, John Tyminski, Franklin Snelson, Josh Loefer, Samuel Gruber, Walter Anoushian, Jessica Taylor, Gordon Hubbell, Jason Seitz, Brad McHale, Larry Beerkircher, Mark Grace, William Driggers, Lori Hale, Daniel Marrone, Peter Duley, Jose Castro, NMFS fishery observers, numerous fishing vessel captains, New England Aquarium, and the IFAW Marine Mammal Rescue and Research Team. Assistance with GIS was provided by Dean Szumylo and Chris Orphanides. Thanks to Paul Conn for helpful discussions on the hierarchical analysis and for providing the R code to run the analysis. We greatly appreciate comments by Wendy Gabriel on an early draft, and Christopher Lowe and an anonymous reviewer on the submitted draft that helped to improve the final manuscript. This is Massachusetts Division of Marine Fisheries Contribution No. 49.
Author Contributions
Conceived and designed the experiments: THC CTM JKC GBS. Performed the experiments: THC CTM JKC NEK. Analyzed the data: THC CTM JKC GBS NEK. Contributed reagents/materials/analysis tools: THC CTM GBS NEK LJN GHB JJH HLP. Wrote the paper: THC CTM JKC. Provided intellectual and editorial comments: THC CTM JKC GBS NEK LJN HLP.
- 1. Compagno LJV (2001) Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Vol. 2. Bullhead, mackerel, and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO Species Catalogue for Fishery Purposes, No. 1, Vol. 2. Rome, FAO. 269 pp.
- View Article
- Google Scholar
- 6. Chapple TK, Jorgensen SJ, Anderson SD, Kanive PE, Klimley AP (2011) A first estimate of white shark, Carcharodon carcharias , abundance off Central California. Biol Lett doi: https://doi.org/10.1098/rsbl.2011.0124 .
- 7. Francis MP (1996) Observations on a pregnant white shark with a review of reproductive biology. In: Klimley AP, Ainley DG, editors. Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. pp. 157–172.
- 11. Ellis R, McCosker JE (1991) Great white shark. New York: Harper Collins/Stanford University Press. 270 p.
- 12. Lowe CG, Blasius ME, Jarvis ET, Mason TJ, Goodmanlowe GD (2012) Historic fishery interactions with white sharks in the Southern California Bight. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 169–185.
- 13. Santana-Morales O, Sosa-Nishizaka O, Escobedo-Olvera MA, Onate-Gonzalez EC, OSullivan JB (2012) Incidental catch and ecological observations of juvenile white sharks, Carcharodon carcharias , in western Baja California, Mexico: Conservation implications. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 187–198.
- 14. Skomal GB, Chisholm J, Correia SJ (2012) Implications of increasing pinniped populations on the diet and abundance of white sharks off the coast of Massachusetts. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 405–417.
- 20. IUCN (2012) IUCN Red List of Threatened Species. Version 2013.1. URL http://www.iucnredlist.org .
- 21. National Marine Fisheries Service (1997) Framework seasonal adjustment of management measures under the fishery management plan for sharks, final environmental assessment and regulatory impact review/final regulatory flexibility analysis.United States Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Office of Sustainable Fisheries, Silver Spring, Maryland .
- 22. Klimley AP, Ainley DG, editors (1996) Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. 517 p.
- 23. Domeier ML, editor (2012) Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. 543 p.
- 25. COSEWIC (2006) COSEWIC assessment and status report on the white shark Carcharodon carcharias (Atlantic and Pacific populations) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa, vii ( www.sararegistry.gc.ca/status/status_e.cfm ).
- 33. Smith-Vaniz WF, Collette BB, Luckhurst BE (1999) Fishes of Bermuda: History, zoogeography, annotated checklist, and identification keys. American Society of Ichthyologists and Herpetologists Special Publication No. 4.
- 34. Mollet HF, Cailliet GM, Klimley AP, Ebert DA, Testi AD (1996) A review of length validation methods and protocols to measure large white sharks. In: Klimley AP, Ainley DG, editors. Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. pp. 91–108.
- 35. Kohler NE, Casey JG, Turner PA (1996) Length-length and length-weight relationships for 13 shark species from the western North Atlantic. NOAA Tech Memo NMFS-NE-110.
- 36. Pratt Jr HL (1996) Reproduction in the male white shark. In: Klimley AP, Ainley DG, editors. Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. pp. 131–138.
- 37. Hoey JJ, Aires-da-Silva A, Turner PA, Syc T, Kohler NE (2005) A review of exploratory longline surveys and biological sampling of sharks from the Sandy Hook, NJ and Narragansett, RI labs: 1961–1991. Southeast Data, Assessment, and Review Data Workshop for Large Coastal Sharks LCS05/06-DW-23.
- 38. McCandless CT, Natanson NJ (2010) Standardized catch rates for sandbar and dusky sharks caught during the NEFSC coastal shark bottom longline survey. Southeast Data Assessment and Review 21 Data Workshop for blacknose, sandbar, and dusky shark, SEDAR 21-DW-28.
- 39. Hale LF, Gulak SJB, Napier AM, Carlson JK (2011) Characterization of the shark bottom longline fishery: 2010. NOAA Tech Memo NMFS-SEFSC-611.
- 45. Conn PB (2010b) Hierarchical analysis of blacknose, sandbar, and dusky shark CPUE indices. Southeast Data Assessment and Review 21 Assessment Workshop for blacknose, sandbar, and dusky shark, SEDAR 21-AP-01.
- 46. R Development Core Team (2012). R: A language and environment for statistical computing, reference index version 2.15.2. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org .
- 48. Fergusson IK (1996) Distribution and autecology of the white shark in the eastern North Atlantic Ocean and the Mediterranean Sea. In: Klimley AP, Ainley DG, editors. Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. pp. 321–345.
- 49. Clua E, Seret B (2012) New Caledonia (South Pacific) as a potential tropical wintering ground for the white shark, Carcharodon carcharias . In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 343–353.
- 56. Bruce BD, Bradford RW (2012) Habitat use and spatial dynamics of juvenile white sharks, Carcharodon carcharias , in eastern Australia. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 225–254.
- 57. Duffy CAJ, Francis MP, Manning MJ, Bonfil R (2012) Regional population connectivity, oceanic habitat, and return migration revealed by satellite tagging of white sharks, Carcharodon carcharias , at New Zealand aggregation sites. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 301–318.
- 60. Francis MP, Duffy CAJ, Bonfil R, Manning MJ (2012) The third dimension: Vertical habitat use by white sharks, Carcharodon carcharias , in New Zealand and in oceanic and tropical waters of the southwest Pacific Ocean. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 319–342.
- 64. Wood-Lafond SA (2009) Dynamics of recolonization: A study of the gray seal ( Halichoerus grypus ) in the northeast U.S. Ph.D. dissertation, University of Massachusetts, Boston.
- 75. Garrison LP (2007) Defining the North Atlantic right whale calving habitat in the southeastern United States: An application of a habitat model. NOAA Tech Memo NOAA-NMFS-SEFSC-553.
- 76. Long DJ, Jones RE (1996) White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. In: Klimley AP, Ainley DG, editors. Great white sharks: The biology of Carcharodon carcharias . San Diego: Academic Press. pp. 293–307.
- 77. National Marine Fisheries Service (1993) Fishery management plan for sharks of the Atlantic Ocean. United States Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Silver Spring, Maryland.
- 80. Gubili C, Duffy CAJ, Cliff G, Wintner SP, Shivji M (2012) Application of molecular genetics for conservation of the white shark, Carcharodon carcharias , L. 1758. In: Domeier ML, editor. Global perspectives on the biology and life history of the great white shark. Boca Raton: CRC Press. pp. 357–380.

Peer-Reviewed Journal Publications
Gallagher, A.J., Brownscombe, J.W., Alsudairy, N.A., Casagrande, A.B., Fu, C., Harding, L., Harris, S.D., Hammerschlag, N., Howe, W., & Huertas, A.D., et al. (2022). Tiger sharks support the characterization of the world’s largest seagrass ecosystem. Nat Commun 13, 6328. https://doi.org/10.1038/s41467-022-33926-1; https://www.nature.com/articles/s41467-022-33926-1?fbclid=PAAaZFRW4hLDbC1OP-Lkitw3ld2labXK913Bd-sjMuYcdxHi4zG0SOiu78ajk
Bowlby HD, Hammerschlag N, Irion DT and Gennari E (2022) How continuing mortality affects recovery potential for prohibited sharks: The case of white sharks in South Africa. Frontiers in Conservation Science 3:988693. doi: 10.3389/fcosc.2022.988693; https://www.frontiersin.org/articles/10.3389/fcosc.2022.988693/full
Irschick DJ, Christiansen F, Hammerschlag N, Martin J, Madsen P, Wyneken J, Brooks A, Gleiss A, Fossette S, Siler C, Gamble T, et al. (2022) 3D Visualization Processes for Recreating and Studying Organismal Form. iScience ; 25(9): 104867; https://www.cell.com/iscience/fulltext/S2589-0042(22)01139-7
Andrzejaczek S, Lucas TC, Goodman MC, Hussey NE, Armstrong AJ, Carlisle A, Coffey DM, Gleiss AC, Huveneers C, Jacoby DM, Meekan MG…Hammerschlag N, et al. (2022) Diving into the vertical dimension of elasmobranch movement ecology. Science Advances ; 8(33):eabo1754. https://www.science.org/doi/full/10.1126/sciadv.abo1754
Lubitz N, Bradley M, Sheaves M, Hammerschlag N, Daly R, Barnett A. (2022). The role of context in elucidating drivers of animal movement. Ecology and Evolution , 12, e9128. https://doi.org/10.1002/ece3.9128
McClain MA, Hammerschlag N, Gallagher AJ, Drymon JM, Grubbs RD, Guttridge TL, Smukall MJ, Frazier BS, Daly-Engel TS (2022) Age-Dependent Dispersal and Relatedness in Tiger Sharks ( Galeocerdo cuvier ). Front. Mar. Sci. 9:900107. doi: 10.3389/fmars.2022.900107
Hammerschlag N, Gutowsky LFG, Rider MJ, Roemer R, Gallagher AJ (2022) Urban sharks: residency patterns of marine top predators in relation to a coastal metropolis. Marine Ecology Progress Series 691:1-17. https://doi.org/10.3354/meps14086
Griffin LP, Casselberry GA, Lowerre‐Barbieri SK, Acosta A, Adams AJ, Cooke SJ, Filous A, Friess C, Guttridge TL, Hammerschlag N, Morley D, Rider MJ, Skomal GB, Smukall MJ, Danylchuk AJ, Brownscombe JW. (2022) Predator–prey landscapes of large sharks and game fishes in the Florida Keys. Ecological Applications :e2584. https://esajournals.onlinelibrary.wiley.com/doi/abs/10.1002/eap.2584
Talwar BS, Bradley D, Berry C, Bond ME, Bouyoucos IA, Brooks AML, Fields KYA, Gallagher AJ, Guttridge TL, Guttridge AE, Hammerschlag N, et al. (2022) Estimated life-history traits and movements of the Caribbean reef shark ( Carcharhinus perezi ) in The Bahamas based on tag-recapture data. Marine Biology 169, 55: https://doi.org/10.1007/s00227-022-04044-9
Rangel BS, Hammerschlag N, Martinelli LA, Moreira RG. (2022) Effects of urbanization on the nutritional ecology of a highly active coastal shark: Preliminary insights from trophic markers and body condition. Science of The Total Environment . https://doi.org/10.1016/j.scitotenv.2022.154082
Jorgensen SJ, Micheli F, White TD, Van Houtan KS, Alfaro-Shigueto J, Andrzejaczek S, Arnoldi NS, Baum JK, Block B, Britten GL, Butner C, Cardeñosa D, Chapple TK, Clarke D, Cortés E, Dulvy NK, Fowler S, Gallagher AJ, Gilman E, Godley BJ, Graham RT, Hammerschlag N, et al. (2022) Emergent research and priorities for shark and ray conservation. Endangered Species Research . 47:171-203. https://www.int-res.com/abstracts/esr/v47/p171-203/
Verkamp H, Hammerschlag N, Quinlan J, Langan J, Sulikowski J. (2022) Preliminary investigation of reproductive hormone profiles in the blacktip shark ( Carcharhinus limbatus) , a placental viviparous species, in southern Florida. Marine and Freshwater Research . doi:10.1071/MF21235
Hammerschlag N, McDonnell LH, Rider MJ, Street GM, Hazen EL, Natanson LJ, McCandless CT, Boudreau MR, Gallagher A J, Pinsky ML, Kirtman B (2022). Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark ( Galeocerdo cuvier ). Global Change Biology , 00, 1–16. https://doi.org/10.1111/gcb.16045
Black C, Merly L, Hammerschlag N. (2021) Bacterial communities in multiple tissues across the body surface of three coastal shark species. Zoological Studies 60:69. doi:10.6620/ZS.2021.60-69 .
Williams LH, Anstett A, Bach Muñoz V, Chisholm J, Fallows C, Green JR, Higuera Rivas JE, Skomal G., Winton M, Hammerschlag N. (2021) Sharks as exfoliators: widespread chafing between marine organisms suggests an unexplored ecological role. Ecology, e03570. https://doi.org/10.1002/ecy.3570
Bates AE, Primack RB, Duarte CM, and PAN-Environment Working Group (including Hammerschlag N, Rider MJ, Albano PS) (2021). Global COVID-19 lockdown highlights humans as both threats and custodians of the environment. Biological Conservation , (263). 109175. https://doi.org/10.1016/j.biocon.2021.109175.
Rider MJ, Kirsebom OS, Gallagher AJ, Staaterman E, Ault JS, Sasso CR, Jackson T, Browder JA, Hammerschlag N. (2021) Space use patterns of sharks in relation to boat activity in an urbanized coastal waterway. Marine Environmental Research . 172, 105489 https://doi.org/10.1016/j.marenvres.2021.105489 .
Calich HJ, Rodríguez JP, Eguíluz VM, Hammerschlag N, Pattiaratchi C, Duarte CM, Sequeira AM. (2021) Comprehensive analytical approaches reveal species-specific search strategies in sympatric apex predatory sharks. Ecography https://doi.org/10.1111/ecog.05953
Jacoby DM, Fairbairn BS, Frazier BS, Gallagher AJ, Heithaus MR, Cooke SJ, Hammerschlag N. (2021) Social network analysis reveals the subtle impacts of tourist provisioning on the social behaviour of a generalist marine apex predator. Frontiers in Marine Science, 1202. https://doi.org/10.3389/fmars.2021.665726
Albano PS, Fallows C, Fallows M, Sedgwick O, Schuitema O, Bernard ATF, Hammerschlag N. (2021) Successful parks for for sharks: no-take marine reserve provides conservation benefits to endemic and threatened sharks off South Africa ; Biological Conservation; https://doi.org/10.1016/j.biocon.2021.109302
Lowerre‐Barbieri SK, Friess C, Griffin LP, Morley D, Skomal GB, Bickford JW, Hammerschlag N, Rider MJ, Smukall MJ, van Zinnicq Bergmann MP, Guttridge TL, t al. (2021) Movescapes and eco‐evolutionary movement strategies in marine fish: Assessing a connectivity hotspot. Fish and Fisheries; https://doi.org/10.1111/faf.12589
Rangel BS, Hammerschlag N, Sulikowski JA, Moreira RG. (2021) Physiological markers suggest energetic and nutritional adjustments in male sharks linked to reproduction. Oecologia. https://doi.org/10.1007/s00442-021-04999-4
Queiroz N, et al. (2021) Reply to: Caution over use of ecological big-data for conservation. Nature 595, E20-E28. https://www.nature.com/articles/s41586-021-03464-9
Queiroz N, et al. (2021) Reply to: Shark mortality cannot be assessed by fishery overlap alone. Nature 595, E8-E16. https://www.nature.com/articles/s41586-021-03397-3
Tinari AM, Hammerschlag N. (2021) An ecological assessment of large coastal shark communities in South Florida. Ocean & Coastal Management, 211(1):105772. https://doi.org/10.1016/j.ocecoaman.2021.105772 .
Gutowsky LF, Rider M, Roemer RP, Gallagher AJ, Heithaus MR, Cooke SJ, Hammerschlag N. (2021) Large sharks exhibit varying behavioral responses to major hurricanes. Estuarine, Coastal and Shelf Science, 256(9):107373. https://doi.org/10.1016/j.ecss.2021.107373
Sequeira AM, O’Toole M, Keates TR, McDonnell LH, Braun CD, Hoenner X, Jaine FR, Jonsen ID, Newman P, Pye J, Bograd SJ, et al. (2021) A standardisation framework for bio‐logging data to advance ecological research and conservation. Methods in Ecology and Evolution, 12(6):996-1007. https://doi.org/10.1111/2041-210X.13593
Wosnick N, Niella Y, Hammerschlag N, Chaves AP, Hauser-Davis RA, da Rocha RC, Jorge MB, de Oliveira RW, Nunes JL. (2021) Negative metal bioaccumulation impacts on systemic shark health and homeostatic balance. Marine Pollution Bulletin, 168(3):112398. https://doi.org/10.1016/j.marpolbul.2021.112398
Rangel BS, Hammerschlag N, Sulikowski JA, Moreira RG (2021) Dietary and reproductive biomarkers in a generalist apex predator reveal differences in nutritional ecology across life stages. Marine Ecology Progress Series, 664:149-163 . https://doi.org/10.3354/meps13640
Friess C, Lowerre-Barbieri SK, Poulakis GR, Hammerschlag N, et al. (2021) Regional-scale variability in the movement ecology of marine fishes revealed by an integrative acoustic tracking network. Friess et al. 2021_egional-scale variability in the movement ecology of marine fishes . Marine Ecology Progress Series, 663:157-177. https://doi.org/10.3354/meps13637
de Sousa Rangel B, Moreira RG, Niella YV, Sulikowski JA, Hammerschlag N. (2021) Metabolic and nutritional condition of juvenile tiger sharks exposed to regional differences in coastal urbanization . Science of The Total Environment, 780:146548. https://doi.org/10.1016/j.scitotenv.2021.146548
Rider MJ, McDonnell LH, Hammerschlag N. (2021) Multi-year movements of adult and subadult bull sharks (Carcharhinus leucas): philopatry, connectivity, and environmental influences. Aquatic Ecology, 55(3). https://doi.org/10.1007/s10452-021-09845-6
de Sousa Rangel B, Hammerschlag N, Moreira RG (2021) Urban living influences the nutritional quality of a juvenile shark species. Science of The Total Environment, 776:146025. https://doi.org/10.1016/j.scitotenv.2021.146025
Gallagher AJ, Shipley ON, van Zinnicq Bergmann MPM, Brownscombe JW, Dahlgren CP, Frisk MG, Griffin LP, Hammerschlag N, Kattan S, Papastamatiou YP, Shea BD, Kessel ST, Duarte CM (2021) Spatial Connectivity and Drivers of Shark Habitat Use Within a Large Marine Protected Area in the Caribbean, The Bahamas Shark Sanctuary. Frontiers in Marine Science, 7:608848. https://doi.org/10.3389/fmars.2020.608848
Shipley ON, Lee CS, Fisher NS, Sternlicht JK, Kattan S, Staaterman ER, Hammerschlag N, Gallagher AJ (2021) Metal concentrations in coastal sharks from The Bahamas with a focus on the Caribbean Reef shark. Scientific Reports, 11(218). https://doi.org/10.1038/s41598-020-79973-w
McDonnell LH, Jackson TL, Burgess GH, Phenix L, Gallagher AJ, Albertson H, Hammerschlag N, Browder JA. (2020) Saws and the city: smalltooth sawfish Pristis pectinata encounters, recovery potential, and research priorities in urbanized coastal waters off Miami, Florida, USA. Endangered Species Research, 43:543-53. https://doi.org/10.3354/esr01085
Moorhead SG, Gallagher AJ, Merly L, Hammerschlag N. (2020) Variation of body condition and plasma energy substrates with life stage, sex, and season in wild‐sampled nurse sharks Ginglymostoma cirratum. Journal of Fish Biology, 98(3):680-693. https://doi.org/10.1111/jfb.14612
Morgan A, Calich C, Sulikowski J, Hammerschlag N. (2020) Evaluating spatial management options for tiger shark (Galeocerdo cuvier) conservation in US Atlantic Waters. ICES Journal of Marine Science , 77(7-8):3095-3109. https://doi.org/10.1093/icesjms/fsaa193
Skubel RA, Wilson K, Papastamatiou YP, Verkamp HJ, Sulikowski JA, Benetti D, Hammerschlag N. (2020) A scalable, satellite-transmitted data product for monitoring high-activity events in mobile aquatic animals. Animal Biotelemetry, 8(1). https://doi.org/10.1186/s40317-020-00220-0
Ajemian MJ, Drymon JM, Hammerschlag N, Wells RJD, Street G, Falterman B, et al. (2020) Movement patterns and habitat use of tiger sharks ( Galeocerdo cuvier ) across ontogeny in the Gulf of Mexico. PLoS ONE, 15(7):e0234868. https://doi:10.1371/journal.pone.0234868
Cartolano MC, Berenshtein I, Heuer RM, Pasparakis C, Rider M, Hammerschlag N, Paris CB, Grosell M, McDonald MD. (2020) Impacts of a local music festival on fish stress hormone levels and the adjacent underwater soundscape. Environmental Pollution, 265(Pt A):114925. https://doi.org/10.1016/j.envpol.2020.114925
Cooke SJ, J, Bergman JN, Nyboer EA, Reid AJ, Gallagher AJ, Hammerschlag N, Van de Riet K, Vermaire JC (2020) Overcoming the concrete conquest of aquatic ecosystems. Biological Conservation, 247:108589. https://doi.org/10.1016/j.biocon.2020.108589
Gallagher AJ, Amon DJ, Bervoets T, Shipley ON, Hammerschlag N, Sims DW, (2020) The Caribbean needs big marine protected areas . Science, 367(6479):749.
AtallahBenson L, Merly L, Cray C, Hammerschlag N (2020) Serum protein analysis of nurse sharks. Journal of Aquatic Animal Health, 32(2):77-82. https://doi.org/10.1002/aah.10100
Bohaboy EC, Guttridge TL, Hammerschlag N, Van Zinnicq Bergmann MP, Patterson WF. (2019) Application of three-dimensional acoustic telemetry to assess the effects of rapid recompression on reef fish discard mortality. ICES Journal of Marine Science, 77(1):83-96. https://doi.org/10.1093/icesjms/fsz202
Flecker AS, Twining CW, Schmitz OJ, Cooke SJ, Hammerschlag N. (2019) Aquatic Predators Influence Micronutrients: Important but Understudied. Trends in Ecology & Evolution , 34(10):882-883. https://doi.org/10.1016/j.tree.2019.07.006
Shipley ON, Gallagher AJ, Shiffman DS, Kaufman L, Hammerschlag N. (2019) Diverse resource-use strategies in a large-bodied marine Predator guild: evidence from differential use of resource subsidies and intraspecific isotopic variation. Marine Ecology Progress Series , 623:71-83. https://doi.org/10.3354/meps12982
Queiroz N, et al. (2019) Global spatial risk assessment of sharks under the footprint of fisheries. Nature , 572:461-466. https://doi.org/10.1038/s41586-019-1444-4
Hammerschlag N (2019) Quantifying shark predation effects on prey: dietary data limitations and study approaches. Endangered Species Research, 38:147–151. https://doi.org/10.3354/esr00950
Shiffman DS, Heithaus MR, Kaufman L, Hammerschlag N (2019) Intraspecific differences in relative isotopic niche area and overlap of co-occurring sharks. Aquatic Ecology, 53(2):233-250. doi: 10.1007/s10452-019-09685-5
Martin KL, Abel DC, Crane D, Hammerschlag N, Burge EJ. (2019) Blacktip shark Carcharhinus limbatus presence at fishing piers in South Carolina: fidelity and environmental drivers. Journal of Fish Biology , 94(3):469-480. https://doi.org/10.1111/faf.12358
Merly L, Lange L, Meÿer M, Hewitt AM, Koen P, Fischer C, Muller J, Schilack V, Wentzel M, Hammerschlag N. (2019) Blood plasma levels of heavy metals and trace elements in white sharks (Carcharodon carcharias) and potential health consequences. Marine Pollution Bulletin , 142(4):85-92. https://doi.org/10.1016/j.marpolbul.2019.03.018
Hays GC, Bailey H, Bograd SJ, Bowen WD, Campagna C, Carmichael RH, Casale P, Chiaradia A, Costa DP, Cuevas E, Bruyn PJND, Dias MP, Duarte CM, Dunn DC, Dutton PH, Esteban N, Friedlaender A, Goetz KT, Godley BJ, Halpin PN, Hamann M, Hammerschlag N, et al. (2019) Translating Marine Animal Tracking Data into Conservation Policy and Management. Trends in Ecology & Evolution, 34(5):459-473. https://doi.org/10.1016/j.tree.2019.01.009
Hammerschlag N, Schmitz OJ, Flecker AJ, Lafferty KD, Sih A, Atwood T, Gallagher AJ, Irschick DJ, Skubel R, Cooke SJ. (2019) Ecosystem Function and Services of Aquatic Predators in the Anthropocene. Trends in Ecology & Evolution , 34(4):369-383. https://doi.org/10.1016/j.tree.2019.01.005
Hammerschlag N, Williams L, Fallows M, Fallows C. (2019) Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the sevengill shark. Scientific Reports, 9(1):1908. https://doi.org/10.1038/s41598-018-37576-6
Rooker JR, Dance MA, Wells RJD, Ajemian MJ, Block BA, Castelton MR, Drymon JM, Falterman BJ, Franks JS, Hammerschlag N, Hoffmaer ER, Kraus RT, McKinney JA, Secor DH, Stunz GW, Walter JF. (2019) Population connectivity of pelagic megafauna in the Cuba-Mexico-U.S. triangle. Scientific Reports, 9(1):1663. https://doi.org/10.1038/s41598-018-38144-8
Wosnick N, Niella YV, Navas CA, Monteiro-Fiho ELA, Freire CA, Hammerschlag N. (2019) Multispecies thermal dynamics of air-exposed ectothermic sharks and its implications for fisheries conservation. Journal of Experimental Marine Biology & Ecology , 513(1):1-9. https://doi.org/10.1016/j.jembe.2019.01.002
Enchelmaier AC, Babcock EA, Hammerschlag N. (2018) Survey of fishes within a restored mangrove habitat of a subtropical bay . Estuarine Coastal and Shelf Science, 244:106021. https://doi.org/10.1016/j.ecss.2018.11.009
Calich H, Estevanez M, Hammerschlag N. (2018) Overlap between highly suitable habitats and longline gear management areas reveals vulnerable and protected regions for highly migratory sharks. Marine Ecology Progress Series , 602:183-195. https://doi.org/10.3354/meps12671
Wosnick N, Navas CA, Niella YV, Monteiro-Filho ELA, Freire CA, Hammerschlag N. (2018) Thermal Imaging Reveals Changes in Body Surface Temperatures of Blacktip Sharks (Carcharhinus limbatus) during Air Exposure. Physiological and Biochemical Zoology , 91(5):1005-1012. https://doi.org/10.1086/699484
Glynn, P.W., Coffman, B., Primov, K.D., Moorhead, S.G., Vanderwoude, J., Barrales, R.N., Williams, M.K. and Roemer , R.P., (2018) Benthic ctenophores (Platyctenida: Coeloplanidae) in South Florida: predator–prey interactions. Invertebrate Biology .
Hammerschlag N, Skubel RA, Sulikowski J, Irschick DJ, Gallagher AJ. (2018) A Comparison of Reproductive and Energetic States in a Marine Apex Predator (the Tiger Shark, Galeocerdo cuvier). Physiological and Biochemical Zoology , 91(4):933-942. https://10.1086/698496
Sequeira AMM, Rodríguez JPP, Eguíluz VM, Harcourt R, Hindell M, Sims DW, Duarte CW, Costa DP, Fernández-Gracia J, Ferreira LC, Hays GC, Heupel MR, Meekan MG, Aven A, Bailleul F, Baylis AMM, Berumen ML, Braun CD, Burns J, Caley MJ, Campbell R, Carmichael RH, Clua E, Einoder LD, Friedlaender A, Goebel ME, Goldsworthy SD, Guinet C, Gunn J, Hamer D, Hammerschlag N, Hammill M, Hückstädt LA, Humphries NE, Lea MA, Lowther A, Mackay A, McHuron E, McKenzie J, McLeay L, McMahon CR, Mengersen K, Muelbert MMC, Pagano AM, Page B, Queiroz N, Robinson PW, Shaffer SA, Shivji M, Skomal GB, Thorrold SR, Villegas-Amtmann S, Weise M, Wells R, Wetherbee B, Wiebkin A, Wienecke B, Thums M. (2018). Convergence of marine megafauna movement patterns in coastal and open oceans. Proceedings of the National Academy of Sciences , 115(12). https://doi.org/10.1073/pnas.1716137115
Skubel RA, Kirtman BP, Fallows C, Hammerschlag N (2018) Patterns of long-term climate variability and predation rates by a marine apex predator, the white shark Carcharodon carcharias . Marine Ecology Progress Series , 587:129-139.
Hammerschlag N, Barley SC, Irschick DJ, Meeuwig JJ, Nelson ER, Meekan MG (2018) Predator declines and morphological changes in prey: evidence from coral reefs depleted of sharks. Marine Ecology Progress Series , 586:127-139. https://doi.org/10.3354/meps12426
Hammerschlag N, Meÿer M, Seakamela S M, Kirkman S, Fallows C, Creel S. (2017) Physiological stress responses to natural variation in predation risk: evidence from white sharks and seals. Ecology , 98(12):3199–3210. https://doi.org/10.1002/ecy.2049
Jerome JM, Gallagher AJ, Cooke SJ, Hammerschlag N. (2017) Integrating reflexes with physiological measures to evaluate coastal shark stress response to capture. ICES Journal of Marine Science , 75(2):796-804. https://doi.org/10.1093/icesjms/fsx191
Macdonald C, Gallagher AJ, Barnett A, Brunnschweiler J, Shiffman DS, Hammerschlag N. (2017) Conservation potential of apex predator tourism. Biological Conservation , 215: 132-141. https://doi.org/10.1016/j.biocon.2017.07.013
Klimley AP, Flagg M, Hammerschlag N, Hearn A. (2017) The value of using measurements of geomagnetic field in addition to irradiance and sea surface temperature to estimate geolocations of tagged aquatic animals. Animal Biotelemetry , 5:19. DOI: 10.1186/s40317-017-0134-y
Glynn PW, Coffman B, Fuller MPC, Moorhead SG, Williams MK, Primov KD, Fortson TN, Barrales RN, Glynn PJ. (2017) Benthic ctenophores (Platyctenida: Coeloplanidae) in south Florida: environmental conditions, habitats, abundances, and behaviors. Invertebrate Biology x(x): 1-15. https://doi.org/10.1111/ivb.12189
Acuña-Marrero D, Smith ANH, Hammerschlag N, Hearn A, Anderson MJ, Calich H, et al. (2017) Residency and movement patterns of an apex predatory shark (Galeocerdo cuvier) at the Galapagos Marine Reserve. PLoS ONE , 12(8). https://doi.org/10.1371/journal.pone.0183669
Irschick DJ, Fu A, Lauder G, Wilga C, Kuo C-Y, Hammerschlag N. (2017) A comparative morphological analysis of body and fin shape for eight shark species. Biological Journal of the Linnean Society , 122(3):1-16. https://doi.org/10.1093/biolinnean/blx088
Shiffman DS, Macdonald C, Ganz HY, Hammerschlag N. (2017) Fishing practices and representations of shark conservation issues among users of a land-based shark angling online forum. Fisheries Research , 196: 13-26. https://doi.org/10.1016/j.fishres.2017.07.031
Kough AS, Cronin H, Skubel R, Belak CA, Stoner AW. (2017) Efficacy of an established marine protected area at sustaining a queen conch Lobatus gigaspopulation during three decades of monitoring. Mar Ecol Prog Ser 573:177-189. https://doi.org/10.3354/meps12163
Hammerschlag N, Gutowsky LFG, Gallagher AJ, Matich P, Cooke SJ. (2017) Diel habitat use patterns of a marine apex predator (tiger shark, Galeocerdo cuvier) at a high use area exposed to dive tourism. Journal of Experimental Marine Biology and Ecology , 495(2): 24-34. https://doi.org/10.1016.j.jembe.2017.05.010
Hammerschlag N, Gallagher AJ. (2017) Extinction Risk and Conservation of the Earth’s National Animal Symbols. BioScience , 67(8): 744-749. https://doi.org/10.1093/biosci/bix054
Gallagher AJ, Shiffman DS, Byrnes EE, Hammerschlag-Peyer CM, Hammerschlag N. (2017) Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquatic Ecology , 51(1):435-448. DOI: 10.1007/s10452-017-9627-2
Cooke SJ, Gallagher AJ, Sopinka NM, Nguyen VM, Skubel RA, Hammerschlag N, Boon S, Young N, and Danylchuk AJ (2017) Considerations for effective science communication. FACETS , 2(1):233-248. https://doi.org/10.1139/facets-2016-0055
Gallagher AJ, Skubel RA, Pethybridge HR, Hammerschlag N. (2017) Energy metabolism in mobile, wild-sampled sharks inferred by plasma lipids. Conservation Physiology , 5(1). https://doi.org/10.1093/conphys/cox002
Matulik AG, Kerstetter DW, Hammerschlag N, Divoll T, Hammerschmidt CR, Evers DC. (2017) Bioaccumulation and biomagnification of mercury and methylmercury in four sympatric coastal sharks in a protected subtropical lagoon. Marine Pollution Bulletin , 116(1):357-364. https://doi.org/10.1016/j.marpolbul.2017.01.033
Hammerschlag N, Meyer C, Grace M, Kessel S, Sutton T, Harvey E, Paris C, Kerstetter D, Cooke SJ. (2017) Shining a Light on Fish at Night: an overview of patterns and processes operating in fish and fisheries at night and in the perpetual darkness of deep and polar seas. Bulletin of Marine Science -Miami- , 93(2): 253-284. https://doi.org/10.5345/bms.2016.1082
Hammerschlag N, Skubel R, Calich H, Nelson ER, Shiffman DS, Wester J, Macdonald C, Cain S, Jennings L, Enchaelmaier A, Gallagher AJ. (2017) Nocturnal and crepuscular behavior in elasmobranchs: a review of movement, habitat use, foraging, and reproduction in the dark. Bulletin of Marine Science -Miami- , 93(2):355-374.
Rangel BdS, Wosnick N, Hammerschlag N, Ciena AP, Kfoury Junior JR. Rici REG (2016) A preliminary investigation into the morphology of oral papillae and denticles of blue sharks (Prionace glauca) with inferences about its functional significance across life stages. Journal of Anatomy , 230(3):389-397. https://doi.org/10.1111/joa.12574
Gallagher AJ, Hammerschlag N, Danylchuk AJ, Cooke SJ. (2016) Shark recreational fisheries: Status, challenges, and research needs. Ambio; 1-14.
Gallagher AJ, Staaterman ER, Cooke SJ, Hammerschlag N. (2016) Behavioral responses to fisheries capture among various shark species reveal mechanisms of species-specific sensitivity. Canadian Journal of Fisheries and Aquatic Sciences; 74(1): 1-7.
Fallows C, Fallows M, Hammerschlag N. (2016) Effects of lunar phase on predator-prey interactions between white shark (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus). Environmental Biology of Fishes; 99(11): 805-812.
Hammerschlag N, Davis DA, Mondo K, Seely MS, Murch SJ, Glover WB, Divoll T, Evers DC, Mash DC. (2016) Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins 2016, 8, 238.
Hammerschlag N, Bell I, Fitzpatrick I, Gallagher AJ, Hawkes LA, Meekan MG, Stevens JD, Thums M, Witt MJ, Barnett A. (2016) Behavioral evidence suggests facultative scavenging by a marine apex predator during a food pulse. Behavioral Ecology and Sociobiology; 70(1): 1777-1788.
Roemer RP, Gallagher AJ, Hammerschlag N. (2016) Shallow water tidal flat use and associated specialized foraging behavior of the great hammerhead shark (Sphyrna mokarran). Marine and Freshwater Behaviour and Physiology; 49(4): 235-249.
Cooke SJ, Nguyen VM, Wilson AD, Donaldson MR, Gallagher A, Hammerschlag N, Haddaway NR. (2016) The need for speed in a crisis discipline: perspectives on peer review duration and implications for conservation science. Endangered Species Research 30: 11-19
Shiffman, D.S. and Hammerschlag, N. (2016) Shark Conservation and Management Policy: A Review and Primer for Non-Specialists. Animal Conservation; 19(5): 401-412.
Fu A, Lauder G, Wilga C, Kuo C, Hammerschlag N, Irschick DJ. (2016) Ontogeny of head and caudal fin shape of an apex marine predator: the tiger shark (Galeocerdo cuvier). Journal of Morphology; 277(5): 556-564.
Shiffman, DS and Hammerschlag, N. (2016) Preferred conservation policies of shark researchers. Conservation Biology; 30(4): 805-815.
Graham F, Rynne P, Estevanez M, Luo J, Ault JS, Hammerschlag N. (2016) Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Diversity and Distributions; 22(5): 534-546.
Queiroz N, Humphries N.E., Mucientes G., Hammerschlag N, Lima F.P, Scales K.L, Miller P.I., Sousa L.L., Seabra R., Sims D.W. (2016) Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proceedings of the National Academy of Sciences; 113(6): 1582-1587.
Sulikowski J, Wheeler CR, Gallagher AJ, Prohaska BK, Langan JA, Hammerschlag N. (2016) Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female dominated site. Aquatic Biology, 24: 175-184
Creel S, Becker M, Christianson D, Dröge E, Hammerschlag N, Haward MW, Karanth U, Loveridge A, Macdonald DW, Wigganson M, M’soka J, Murray D, Rosenblatt E, Schuette P. (2015) Questionable policy for large carnivore hunting . Science, 350(6267): 1473-1475
Gallagher AJ, Cooke SJ, Hammerschlag N. (2015) Risk perceptions and conservation ethic among recreational anglers targeting threatened sharks in the subtropical Atlantic. Endangered Species Research, 29(1): 81-93.
Thaler, A.D.T. and Shiffman, D.S. (2015) Fish tales: Combating fake science in popular media. Ocean and Coastal Management
Nguyen VM, Haddaway NR, Gutowsky LFG, Wilson ADM, Gallagher AJ, Donaldson MR, Hammerschlag N, Cooke SJ. (2015) How Long Is Too Long in Contemporary Peer Review? Perspectives from Authors Publishing in Conservation Biology Journals. PLoS ONE 10(8): e0132557.
Gallagher AJ, Hammerschlag N, Cooke SJ, Costa DP, Irschick DJ (2015) One size does not always fit all: a reply to Stroud and Feeley. Trends in Ecology and Evolution; 30(6): 297-298.
Hammerschlag N, Broderick AC, Coker JW, Coyne MS, Dodd M, Frick MG, Godfrey MH, Godley BJ, Griffin DB, Hartog K, Murphy SR, Murphy TM, Nelson ER, Williams KL, Witt MJ, Hawkes LA (2015) Evaluating the landscape of fear between apex predatory sharks and mobile sea turtles across a large dynamic seascape. Ecology, 96(8): 2117-2126.
Gallagher AJ, Vianna GMS, Papastamatiou YP, Macdonald C, Guttridge TL, Hammerschlag N. (2015) Biological effects, conservation potential, and research priorities of shark diving tourism. Biological Conservation, 184: 365-379
Gallagher AJ, Hammerschlag N, Cooke SJ, Costa DP, Irschick DJ (2015) Evolutionary theory as a tool for predicting extinction risk. Trends in Ecology and Evolution, 30(2): 61-65
Fallows C, Benoit HP, Hammerschlag N. (2015) Intraguild predation and partial consumption of blue sharks (Prionace glauca) by Cape fur seals (Arctocephalus pusillus pusillus). African Journal of Marine Science; 37(1): 125-128.
D.S. Shiffman, A.J. Gallagher, J. Wester C.C. Macdonald, A.D. Thaler, S.J. Cooke, and N. Hammerschlag. (2015) A letter of clarification from the authors of “trophy fishing for species threatened with extinction”. Marine Policy 53, 213-214.
Ferry, L., and Shiffman, D.S. (2014) The Value of Taxon-focused Science: 30 Years of Elasmobranchs in Biological Research and Outreach. Copeia 743-746.
Hammerschlag N, Cooke SJ, Gallagher AJ, Godley BJ. (2013) Considering the fate of electronic tags: user responsibility and interactions when encountering tagged marine animals; Methods in Ecology and Evolution; 11(5): 1147-1153.
Shiffman DS, Hammerschlag N. (2014) An assessment of the scale, practices, and conservation implications of Florida’s charterboat-based recreational shark fishery. Fisheries, 39(9): 395-407
Irschick, DJ, Hammerschlag N. (2014) Morphological scaling of body form in four shark species differing in ecology and life-history. Biological Journal of the Linnean Society; 114(1): 126-135.
Shiffman, D.S, Gallagher AJ, Wester, Macdonald C, Thaler AD, Cooke SJ, Hammerschlag N (2014) Trophy fishing for species threatened with extinction: a way forward building on a history of conservation Marine Policy, 50: 318-322
Shiffman, D.S. (2014) Commentary: Keeping swimmers safe without killing sharks is a revolution in shark control. Animal Conservation DOI: 10.1111/acv.12155
Gallagher AJ, Orbesen ES, Hammerschlag N, Serafy JE (2014) Survival and vulnerability of pelagic shark species to pelagic longline bycatch. Global Ecology and Conservation; 1: 50-59.
Rumbold D, Wasno B, Hammerschlag N, Volety A. (2014) Mercury accumulation in sharks from the coastal waters of Southwest Florida. Archives of Environmental Contamination and Toxicology; 67(3): 402-412.
Irschick DI., Hammerschlag N. (2014) A new metric for measuring condition in large predatory sharks. Journal of Fish Biology; 85(3), 917-926.
Gallagher, AJ, Wagner, DN, Irschick, DJ, Hammerschlag, N. (2014) Body condition predicts energy stores in apex predatory sharks. Conservation Physiology; 2 (1): cou022
Shiffman, DS, Frazier, B, Kucklick, J, Abel, D, Brandes, J and Sancho, G. (2014) Feeding ecology of the sandbar shark (Carcharhinus plumbeus) in South Carolina estuaries revealed through δ13C and δ15N stable isotope analysis. Marine and Coastal Fisheries; 6(1): 156-169
Gallagher AJ, Hammerschlag N, Shiffman DS, Giery ST (2014) Evolved for extinction: the cost and conservation implications of extreme specialization in hammerhead sharks. BioScience; 64(7): 619-624.
Staaterman ER, Bhandiwad AA, Gravinese PM, Moeller PM, Reichenbach ZC, Shantz AA, Shiffman DS, Toth LT, Warneke AM, Gallagher AJ. (2014) Lights, camera, science: The utility and growing popularity of film festivals at scientific meetings. Ideas in Ecology and Evolution 7: 11-16.
Cooke SJ, Hogan ZS, Butcher PA, Stokesbury MJW, Raghavan R, Gallagher AJ, Hammerschlag N, Danylchuk AJ. (2014) Angling for endangered fish: Conservation problem or conservation action? Fish and Fisheries; 17(1): 249-265.
Gallagher AJ, Romeiro J, Canabal D, Canabal V, Hammerschlag N (2014) Novel social behaviors in a threatened apex marine predator, the oceanic whitetip shark Carcharhinus longimanus. Ethology Ecology & Evolution; 26(4), 413-417.
Gallagher, AJ, Serafy, JE, Cooke, SJ, Hammerschlag, N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Marine Ecology Progress Series; 494: 207-218
ECM Parsons, DS Shiffman, ES Darling, N Spillman, and AJ Wright. (2013) How Twitter Literacy Can Benefit Conservation Scientists. Conservation Biology.
Darling E, Shiffman D, Drew J, and Cote Isabelle (2013) The role of twitter in the life cycle of a scientific publication. Ideas in Ecology and Evolution 6, 32-43.
Hammerschlag N, Gallagher AJ, Carlson JK. (2013) A revised estimate of daily ration in the tiger shark ( Galeocerdo cuvier ) with implications for assessing ecosystem impacts of apex predators. Functional Ecology 27 (5): 1273-1274.
Fallows C, Gallagher AJ, Hammerschlag N (2013) White Sharks (Carcharodon carcharias) Scavenging on Whales and Its Potential Role in Further Shaping the Ecology of an Apex Predator. PLoS ONE 8(4): e60797. doi:10.1371/journal.pone.0060797.
Carr, LA, Stier, AC, Fietz, K, Montero, I, Gallagher, AJ, Bruno, JF. (2013) Illegal shark fishing in the Galapagos Marine Reserve Marine Policy 39: 317-321.
Hammerschlag N, Luo J, Irschick DJ, Ault JS (2012) A Comparison of Spatial and Movement Patterns between Sympatric Predators: Bull Sharks ( Carcharhinus leucas ) and Atlantic Tarpon ( Megalops atlanticus ). PLoS ONE 7(9): e45958. doi:10.1371/journal.pone.0045958.
Shifman DS. (2012) Twitter as a tool for conservation education and outreach: what scientific conferences can do to promote live-tweeting. Journal of Environmental Studies and Sciences DOI 10.1007/s13412-012-0080-1.
Shiffman DS, Gallagher AJ, Boyle MD, Hammerschlag-Peyer CM, Hammerschlag N. (2012, Cover) Stable Isotope Analysis as a Tool for Elasmobranch Conservation Research: A Primer for Non-Specialists. Marine and Freshwater Research 63:635-643.
Hammerschlag N, Gallagher AJ, Wester J, Luo J, Ault JS. (2012, Cover) Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Functional Ecology 26(3): 567-576
Gallagher AJ, Kyne PM, Hammerschlag N. (2012) Ecological risk assessment and its application to elasmobranch conservation and management. Journal of Fish Biology 85(5): 1727-1748
Martin RA, Hammerschlag N. (2012) Marine predator—prey contests: Ambush and speed versus vigilance and agility . Marine Biology Research 8:1, 90-94.
Mondo K, Hammerschlag N, Basile M, Pablo J, Banack SA, Mash DC. (2012) Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins . Marine Drugs 10(2), 509-520; doi:10.3390/md10020509
Thaler AD, Zelnio KA, Freitag A, MacPherson R, Shiffman DS, Bik H, Goldstein MC, McClain C. (2012) Digital environmentalism: tools and strategies for the evolving online ecosystem. SAGE Reference — Environmental Leadership: A Reference Handbook D. Gallagher (Ed.).
Fallows C, Martin RA, Hammerschlag N. (2012) Comparisons between white shark-pinniped interactions at Seal Island (South Africa) with other sites in California (United States). Global Perspectives on the Biology and Life History of the Great White Shark, ed. Michael L. Domeier, CRC Press, Boca Raton, FL.
Hammerschlag N, Martin RA, Fallows C, Collier R, Lawrence R. (2012) Investigatory Behavior towards surface objects and Non-consumptive Strikes on Seabirds by White Sharks ( Carcharodon carcharias ) at Seal Island, South Africa. Global Perspectives on the Biology and Life History of the Great White Shark, ed. Michael L. Domeier, CRC Press, Boca Raton, FL: 91-103.
Hammerschlag N, Trussell G. (2011) Beyond the Body Count: Behavioral Downgrading of Planet Earth. Science. (E-Letter, 11 November 2011).
Gallagher AJ, Jackson T, Hammerschlag N. (2011) Evidence of tiger shark ( Galeocerdo cuvier ) scavenging on avian prey and its possible connection between large-scale bird die-offs in the Florida Keys. Florida Scientist, 74(4):264-269.
Staaterman ER, Clark CW, Gallagher AJ, deVries, MS, Claverie T, Patek SN. (2011) Rumbling in the benthos: the acoustic behavior of the California mantis shrimp and the presence anthropogenic noise. Aquatic Biology, 13(2):97–105.
Hammerschlag N, Sulikowski J. (2011) Killing for Conservation: The Need for Alternatives to Lethal Sampling of Apex Predatory Sharks. Endangered Species Research, 14(2):135–140.
Gallagher AJ, Hammerschlag N. (2011) Global Shark Currency: The Distribution, Frequency and Economic Value of Shark Eco-tourism. Current Issues in Tourism, 14(8):1–16. DOI: 10.1080/13683500.2011.585227.
Hammerschlag N, Gallagher AJ, Lazarre DM. (2011) A Review of Shark Satellite Tagging Studies. Journal of Experimental Marine Biology and Ecology, 398(1):1–8.
Hammerschlag N, Gallagher AJ, Lazarre DM, Slonim C. (2011) Range extension of the endangered great hammerhead shark Sphyrna mokarran in the Northwest Atlantic: Preliminary data and significance for conservation. Endangered Species Research, 13(2):111–116.
Serrano X, Grosell M, Serafy JE. (2011) Osmoregulatory capabilities of the gray snapper, Lutjanus griseus : salinity challenges and field observations. Marine and Freshwater Behaviour and Physiology , 44(3):1-12.
Brand LE, Pablo J, Compton A, Hammerschlag N, Mash DC. (2010) Cyanobacterial Blooms and the Occurrence of the neurotoxin beta-N-methylamino-L-alanine (BMAA) in South Florida Aquatic Food Webs. Harmful Algae , 9(6):620–635.
Hammerschlag N, Heithaus MR, Serafy JE. (2010) The influence of predation risk and food supply on nocturnal fish foraging distributions along a subtropical mangrove-seagrass ecotone. Marine Ecology Progress Series , 414:223-235.
Hammerschlag N, Ovando D, Serafy, JE. (2010) Seasonal diet and feeding habits of juvenile fishes foraging along a subtropical marine ecotone. Aquatic Biology, 9(3):279–290.
Hammerschlag N, Morgan A, Serafy JE. (2010) Relative predation risk for fishes along a subtropical mangrove-seagrass ecotone. Marine Ecology Progress Series, 401: 259–267.
Hammerschlag N, Serafy JE. (2010) Nocturnal fish utilization of a subtropical mangrove-seagrass ectone. Marine Ecology, 31(2):364–374.
Gallagher AJ, Lorenz FH, Bushnell PG, Brill RW, Mandelman JW. (2010) Blood Gas, Oxygen Saturation, pH, and Lactate Values in Elasmobranch Blood Measured with a Commercially Available Portable Clinical Analyzer and Standard Laboratory Instruments , Journal of Aquatic Animal Health, 22(4) 229–234.
Serrano X, Grosell M, Serafy JE. (2010) Salinity selection and preference of the grey snapper Lutjanus griseus : field and laboratory observations. Journal of Fish Biology, 76(7): 1592–1608.
Martin RA, Rossmo DK, Hammerschlag N. (2009) Hunting patterns and geographic profiling of white shark predation. Journal of Zoology, 279(2): 111–118.
Milano GR, Hammerschlag N, Barimo J, Serafy JE. (2007) Restoring essential fish habitat in southeast Florida: Mangrove and seagrass habitat design components and successful monitoring. Bulletin of Marine Science, 80(3): 928-929.
Hammerschlag N, Martin RA, Fallows C. (2006) Effects of environmental conditions on predator-prey interactions between white sharks ( Carcharodon carcharias ) and Cape fur seals ( Arctocephalus pusillus pusillus ) at Seal Island, South Africa. Environmental Biology of Fishes , 76(2): 341–350.
Hammerschlag N. (2006) Osmoregulation in Elasmobranchs: A review for fish biologists, behaviourists and ecologists. Marine and Freshwater Behaviour and Physiology, 39(3): 209–228.
Martin RA, Hammerschlag N, Collier R, Fallows C. (2005) Predatory Behaviour of White Sharks ( Carcharodon carcharias ) at Seal Island, South Africa. Journal of the Marine Biological Association of the UK , 85(5): 1121–1135.
Hammerschlag N, Fallows C. (2005) Galapagos sharks ( Carcharhinus galapagensis ) at the Bassas da India atoll: first record from the Mozambique Channel and possible significance as a nursery area. South African Journal of Science , 101: 375–377.
Hammerschlag N. (2004) A review of osmoregulation in freshwater and marine elasmobranchs. In: R.A. Martin and D. MacKinlay (ed.) Extended Abstract in Proceedings of the American Fisheries Society, fourth International Congress on the Biology of Fish, Manaus, Brazil: 35-41.

Privacy Statement and Legal Notices Copyright © 2018, University of Miami All rights reserved.
4600 Rickenbacker Causeway Miami, Fl 33149-1098 +1 305 421 4000
This site uses cookies. By continuing to browse the site, you are agreeing to our use of cookies.
Cookie and Privacy Settings
We may request cookies to be set on your device. We use cookies to let us know when you visit our websites, how you interact with us, to enrich your user experience, and to customize your relationship with our website.
Click on the different category headings to find out more. You can also change some of your preferences. Note that blocking some types of cookies may impact your experience on our websites and the services we are able to offer.
These cookies are strictly necessary to provide you with services available through our website and to use some of its features.
Because these cookies are strictly necessary to deliver the website, refuseing them will have impact how our site functions. You always can block or delete cookies by changing your browser settings and force blocking all cookies on this website. But this will always prompt you to accept/refuse cookies when revisiting our site.
We fully respect if you want to refuse cookies but to avoid asking you again and again kindly allow us to store a cookie for that. You are free to opt out any time or opt in for other cookies to get a better experience. If you refuse cookies we will remove all set cookies in our domain.
We provide you with a list of stored cookies on your computer in our domain so you can check what we stored. Due to security reasons we are not able to show or modify cookies from other domains. You can check these in your browser security settings.
We also use different external services like Google Webfonts, Google Maps, and external Video providers. Since these providers may collect personal data like your IP address we allow you to block them here. Please be aware that this might heavily reduce the functionality and appearance of our site. Changes will take effect once you reload the page.
Google Webfont Settings:
Google Map Settings:
Google reCaptcha Settings:
Vimeo and Youtube video embeds:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

A biologging database of juvenile white sharks from the northeast Pacific
John o’sullivan.
1 Monterey Bay Aquarium, Monterey, California 93940 USA
Christopher G. Lowe
2 Department of Biological Sciences, California State University, Long Beach, California 90815 USA
Oscar Sosa-Nishizaki
3 Department of Biological Oceanography, CICESE, Ensenada, Mexico
Salvador J. Jorgensen
9 Present Address: Institute of Marine Sciences, University of California, Santa Cruz, California 95064 USA
James M. Anderson
Thomas j. farrugia.
10 Present Address: Alaska Ocean Observing System, Anchorage, Alaska 99501 USA
Emiliano García-Rodríguez
11 Present Address: Georgia Aquarium, Atlanta, GA 30313 USA
Megan K. McKinzie
4 Monterey Bay Aquarium Research Institute, Moss Landing, California 95039 USA
Erick C. Oñate-González
5 Biological Sciences, Autonomous University of Nuevo León, San Nicolás de los Garza, 66450 Mexico
6 Hopkins Marine Station, Stanford University, Stanford, California 93950 USA
12 Present Address: Virginia Institute of Marine Science, College of William & Mary, Gloucester Point, Virginia USA
Connor F. White
13 Present Address: Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts USA
Chuck Winkler
7 Aquatic Research Consultants, San Pedro, California USA
Kyle S. Van Houtan
8 Nicholas School of the Environment, Duke University, Durham, North Carolina 27708 USA
14 Present Address: Loggerhead Marinelife Center, Juno Beach, Florida 33408 USA
Associated Data
- O’Sullivan J, 2022. A biologging database of juvenile white sharks from the Northeast Pacific. Animal Telemetry Network. [ CrossRef ]
All the data and code used in this study are available open access from the ATN DAC Data Portal ( https://portal.atn.ioos.us ) and the Research Workspace DataONE member node ( https://search.dataone.org/portals/RW ) as well as at GitHub ( https://bit.ly/3noJCJD ). As we are providing the raw telemetry data and metadata from the platform manufacturer, the code we provide is for data visualization used to make the figures in this manuscript.
Species occurrence records are vital data streams in marine conservation with a wide range of important applications. From 2001–2020, the Monterey Bay Aquarium led an international research collaboration to understand the life cycle, ecology, and behavior of white sharks ( Carcharodon carcharias ) in the southern California Current. The collaboration was devoted to tagging juveniles with animal-borne sensors, also known as biologging. Here we report the full data records from 59 pop-up archival (PAT) and 20 smart position and temperature transmitting (SPOT) tags that variously recorded pressure, temperature, and light-level data, and computed depth and geolocations for 63 individuals. Whether transmitted or from recovered devices, raw data files from successful deployments ( n = 70) were auto-ingested from the manufacturer into the United States (US) Animal Telemetry Network’s (ATN) Data Assembly Center (DAC). There they have attributed a full suite of metadata, visualized within their public-facing data portal, compiled for permanent archive under the DataONE Research Workspace member node, and are accessible for download from the ATN data portal.
Background & Summary
The biologging and biotelemetry revolution that began with isolated, descriptive case studies 1 – 5 has since grown into wide-ranging research disciplines with applications across marine science 6 – 8 . The Monterey Bay Aquarium (“Aquarium”) has been active in biologging research, helping to pioneer studies across diverse marine taxa, including tunas, sharks, devil rays, sunfish, sea otters, and jellyfish 3 , 4 , 9 – 11 . Over two decades ago, the Aquarium initiated the Juvenile White Shark Project (“Project”), spurred on by the first successful deployment of a satellite transmitting tag on a juvenile white shark ( Carcharodon carcharias ) in 2000 12 . At this time there were several active research programs devoted to understanding adult white sharks, however, none were focused on the juvenile demographic of these apex ocean predators during this important developmental phase. As a result, the Aquarium began an international collaboration devoted to the comprehensive study of juvenile white sharks in the northeastern Pacific.
This collaboration to date has resulted in many scientific publications and graduate student projects 13 – 22 , each study arising from the analysis of a subset of the overall dataset presented here. The published findings about the biology and ecology of juvenile white sharks initially provided vital insights to the Aquarium’s husbandry team that enabled their successful white shark exhibition (2004–2011). The Aquarium’s exhibitions of juvenile white sharks aimed to inspire ocean conservation through promoting public awareness of the important ecological role played by white sharks as well as the threats sharks face in the wild. Through 2020, the Project deployed 79 electronic tags on 63 juvenile white sharks that have helped document their seasonal migrations and oceanographic preferences, fisheries interactions, nursery locations, ontogenetic shifts, and habitat shifts arising from ocean warming.
Beyond its own programmatic and scientific achievements, through knowledge sharing and funding, the Project helped launch additional juvenile white shark research programs in Australia (Commonwealth Scientific and Industrial Research Organization, principal investigator Barry Bruce), Mexico (Centro de Investigación Científica y de Educación Superior de Ensenada, principal investigator Oscar Sosa-Nishizaki), and southern California (California State University Long Beach, principal investigator Chris Lowe). This collaborative network, within the region and beyond, contributed significantly to the Project’s success and longevity. Beyond the scientific community, the trust and support of countless commercial fisherman was a key element that contributed immeasurably to the Project, particularly in the program’s early development. Throughout, this Project was a privately funded research program, whose present and future status was adversely affected by the economic hardships arising from the COVID-19 pandemic and resulting budgetary reductions.
Here, we provide an extensively curated dataset from electronic tags affixed to juvenile white sharks in the northeast Pacific Ocean. These data were collected from two biotelemetry platforms (details below) that potentially recorded pressure, temperature, and light-level data and additionally computed depth and geolocation information. These data were auto-ingested, post-processed, annotated, and hosted on the United States (US) Animal Telemetry Network (ATN) Data Assembly Center (DAC), where they are publicly accessible. The ATN DAC is an essential part the Integrated Ocean Observing System (IOOS) network of the National Oceanic and Atmospheric Administration (NOAA) National Ocean Service (NOS).
Tagging deployments and study subjects
Table 1 contains an overview of the fields in the metadata file (JWS_metadata.xlsx) providing extensive background details on each of the 79 tag deployments and 63 study subjects. The data in this file give essential contextual information needed to understand the methodological, environmental, and demographic factors surrounding the deployments, which are critical for further examination and hypothesis testing of the sensor data. These metadata fall into several specific categories, but are not limited to, (i) information on the deployed electronic devices (platform, model, Platform Transmitter Terminal identifications), (ii) sharks (unique identifying numbers, sex, length), (iii) capture event (date, location, duration, methodology, interaction type), and (iv) the reporting period (duration, linear surface travel distance).
Metadata descriptions of the sharks, tagging operations, and deployments for all tags included in the database.
This describes the fields in the supplemental file JWS_metadata.xlsx.
Figure 1 illustrates a typical C. carcharias tagging operation. This involves a contracted commercial fishing vessel with purpose-built gears to capture sharks (Fig. 1a ) and a research crew to handle animals, monitor health (Fig. 1b ) and attach electronic tags (Fig. 1c ). More details on the tagging program and its methodologies are provided elsewhere 14 , 19 , 20 . Figure. 2 provides summaries of the deployment schedule, geographic locations, devices, and capture operations. Of note, 39.7% (25/64) of all tagging operations involved collaborations with commercial fishery operators (Fig. 2f–h ), whose engagement was temporarily impacted (Fig. 2a ) during the scientific review process when the population was under consideration for US Endangered Species Act listing. Figure 3 displays the demographic focus on small juvenile C. carcharias , with modest deployment durations and travel distances.

Depiction of a typical research operation for capturing and tagging juvenile White Sharks in the Southern California Bight. ( a ) Aquarium research vessel (RV Lucile) with crew approaching a contracted purse seine vessel containing a captured juvenile white shark. ( b ) Research crew on the RV Lucile leading the shark into a sling, where it is subsequently transferred to the vessel’s deck for tagging. ( c ) Successfully applied PAT and acoustic tags each positioned lateral of the dorsal fin, anchored via leaders, and affixed with titanium darts (yellow arrows). All images taken by Steve McNicholas (Great White Shark 3D) for the Monterey Bay Aquarium and used with permission.

Metadata summaries of the field program that deployed biologging tags on juvenile white sharks in the southern California Current. ( a ) Deployment schedule for 72 electronic tags released on 64 White Sharks from 2001–2020 ( b ) Tagging activity peaked in the late summer months when the population is most locally abundant. Field operations decreased from 2011–2013 when the population was being considered for listing under the U.S. Endangered Species Act (ESA). ( c ) Deployments focused on opportunities in the Southern California Bight coastline and included deployments in the nursery area of Bahía Sebastian Vizcaíno, Mexico and releases after exhibition at the Monterey Bay Aquarium. ( d ) Researchers released a variety of pop-up archival transmitting (PAT, 58 sharks), acoustic (21 sharks), and smart position and temperature (SPOT, 20 sharks) tags. This manuscript only reports the geolocation, temperature and depth data from the PAT and SPOT platforms. ( e ) Half (35 of 64, 54.7%) of all sharks received multiple tags, primarily to compare their relative performance. ( f ) Most tags (38 of 64, 60.3%) were deployed during focused scientific research operations. ( g ) The remainder were joint operations resulting from opportunistic bycatch in commercial fisheries using various gears and ( h ) Targeting various species. “Jab” gear refers to research operations that uses pole extensions to apply tags to sharks without capturing and handling.

Demographic and deployment summaries from the juvenile white shark tagging program. ( a ) Total body length (TL) histogram indicates that most individuals tagged were either neonates (<1.50 m total body length “TBL”), young-of-year (YOY, <1.75 m TBL), or other juveniles (<2.5 m TBL) which typically inhabit the geographic region of focus 18 , 20 , 21 , 25 . This plot excludes one 396 cm TBL female that was opportunistically tagged off Santa Rosa Island. ( b ) Over half of the individuals (33 of 64, 51.6%) were females, with the sex undetermined for 4 individuals (6.3%) that were jab-tagged and not landed. ( c ) A majority of tags had a short deployment length which is the duration when the tag is logging data on the shark’s activity. ( d ) For 50 of 64 sharks (78.1%) this was less than 6 months, and less than 3 months for half of all sharks averaged for each deployment year represented as boxplots with the raw observations. ( e ) Histogram of straight linear distance between the release site and the location of first tag reporting. While many juvenile White Sharks did not travel far during tag deployments, there are notable exceptions. ( f ) Two juvenile sharks, for example, swam a linear distance of nearly 2,000 km, each in under 200 days. Solid orange line is a locally weighted regression (shaded area is standard error) which is influenced by location of release and annual migration cycles.
SPOT5 platform sensors and configuration
Smart position and temperature transmitting (SPOT) tag data included in this dataset were obtained from SPOT5 tags (Wildlife computers, Redmond, WA) deployed on juvenile White Sharks between 2006 and 2009. These tags generated the locations of tagged sharks when the fin-mounted tag broke the surface of the water and there were Argos satellites overhead ( http://www.argos-system.org ). Several factors will influence the frequency of Argos locations from SPOT tags (hourly to weekly), as well as the accuracy of the positions (<250 m to >10 km), including: the sea state, the shark’s surface-oriented behavior, and the satellite coverage 23 . These factors determine how many messages from the tag reach the Argos satellite system, and therefore the location quality class. For instance, location class Z does not allow for a valid location to be estimated, classes B and A are assigned when only 2 and 3 messages are received, respectively and cannot estimate the accuracy of the location. Class 0, 1, 2 and 3 all require 4 messages to be received and have estimated errors of, respectively, over 1500 m (sometimes much greater), between 500 m and 1500 m, between 250 m and 500 m, and below 500 m. For additional technical details on location classes and position accuracy, see the CLS Argos User Manual (available at https://bit.ly/3uuMbzt ).
The SPOT tags were programmed to only transmit location (and not time at temperature histograms or haul out statistics). They were also programmed to check if the wet/dry sensor is dry (and therefore the tag is out of the water and able to transmit) every 0.25 seconds. Messages sent by the tag were received by the Argos satellite system and transferred to the Wildlife Computers data portal from which data files were downloaded. The file formats included the proprietary .DIAG and .PRV file formats as well as a series of .CSV files. Location data are available within the locations.csv file for each tag.
PAT platform sensors and configuration
Pop-up archival transmitting (PAT) tags deployed on juvenile White Sharks used to collect data for this dataset included MK10 (deployed 2003–2016), MiniPAT (deployed 2010–2020), PAT2 (deployed 2001–2003) or PAT4 (deployed 2004–2005) tags from Wildlife Computers (Redmond, WA). All PAT tag models included wet/dry, light level, pressure, and temperature sensors. These tags were programmed to collect light level, depth and temperature data while deployed on White Sharks, and at a pre-determined date, release from their anchor and float to the surface where they could transmit a subset of their data to the Argos satellite system.
Once at the surface, a final (pop-up) location of the tag is calculated by the Argos satellite system by measuring the “Doppler shift” of repeated transmissions received by the satellite as it moves over the tag. Multiple Argos locations are calculated in this way, and just as with SPOT tags described above, Argos quality classes are associated with each location. If tags reported on schedule, the first high quality location (class 1, 2 or 3) of the tag as determined by the Argos satellite is considered the final known location of the tagged shark.
While the battery remains sufficiently charged, the tag transmits packets of information to the Argos satellite system with the archived data from the light, pressure, and temperature sensors. Due to the combination of deployment length, battery life and satellite availability over the location of the tag, only a small percentage of the archived data will be able to be transmitted. For this reason, PAT tags can be programmed by the user to prioritize which data to transmit and in what format (e.g., full time series of depth or temperature vs. binned histograms of time spent at depth and temperature). Table 2 contains an overview of the fields in the metadata file (PAT_programming.xlsx) that reveals how the PAT tags used in this study were programmed. When a tag that has popped up could be recovered, the full archived data set was downloaded. This provided fine scale data on the depth, temperature and light level experienced by the tag, which were then uploaded to the Wildlife Computers data portal.
Metadata descriptions of how the deployed tags were programmed.
This describes the fields in the supplemental file PAT_programming.xlsx.
One of the most useful outputs of the PAT tag platforms are estimates of the location of the tagged shark while at liberty (between the known position at the time of release, and the known position at pop-up directly through the Argos satellite system). To estimate position while on the shark, the tag records light levels and produces two light-level curves each day of deployment. Using the onboard clock set to UTC time, the times of the local dawn and dusk are compared to UTC, which provides an estimate of the longitude of the tag on that day. The time between dawn and dusk (i.e., the length of the day) is used to estimate the latitude of the tag based on the day of the year. Both the latitude and longitude estimates are very much dependent on the quality of the light curves, which in turn are very dependent on the environmental conditions experienced by the tag (cloud cover, depth water turbidity, etc.). Higher quality light curves will produce more accurate geolocation estimates. This approach has been used for decades 19 and has been independently validated 20 .
To further refine these geolocation estimates, the light-level data are processed through a proprietary geolocation algorithm on the Wildlife Computer portal called GPE3. The user provides an estimate of the average swimming speed of the tagged animal, and the GPE3 process employs a discretized Hidden Markov model that uses light levels, sea surface temperatures from satellites to compare with the onboard temperature recordings, and any known locations (such as the deployment and pop-up locations) to reduce the uncertainty around each daily geolocation estimate. More information about the GPE3 can be obtained from Wildlife Computers ( www.wildlifecomputers.com ).
Data transmission and processing
Data from successful SPOT ( n = 19) and PAT ( n = 51) tag deployments were transmitted through Argos Services directly to the manufacturer and then decoded using their data analysis program (DAP; Wildlife Computers). Data from recovered archival tags ( n = 26) were manually uploaded directly to the Wildlife Computers (WC) data portal by participating researchers and then decoded using DAP. Decoded raw telemetry data and when applicable processed GPE3 files (PAT tags only, see above) were then downloaded from the Wildlife Computers data portal to the ATN DAC via the Wildlife Computers API as .CSV files and in some cases in the proprietary WC file format using the unique manufacturer assigned deployment ids (Table 1 ). Downloaded data were zipped and maintained as is.
Data Records
Researcher-assigned unique deploy identification numbers (i.e., Shark ID_PTT) were used to label each zip file (see Table 1 ). The subset of files included within each deployment folder are contingent on tag model, programming selections and whether a tag was successfully recovered. Individual data files, from successfully transmitted tags, regardless of tag type were prefixed by a tag’s assigned Platform Transmitter Terminal (PTT) id followed by the specific WC file type. Recovered archival PAT tag data files were prefaced with ‘out’ followed by the file type. Processed GPE3 files were prefixed with the deployments unique deploy identification number and the numerical suffix indicates researcher selected GPE3 file run. Unique deploy id, PTT id and tag type were provided within each individual data file to assist with future merging and reuse of these data. Blank cells contained within any of the provided data files signify attributes were not collected or determined. While data file and attribute descriptions are separately provided by the manufacturer, the full suite of animal and tag deployment metadata are fully described with an accompanying ISO 19115 metadata record for geospatial data.
Public access to the full data records and metadata from these 70 successfully transmitted and/or recovered electronic tags deployed on juvenile C. carcharias from 2001–2020 are available through the ATN data portal ( https://bit.ly/2ZTvbFS ) as well as the Research Workspace (RW) Data Observation Network for Earth (DataONE) member node ( https://search.dataone.org/portals/RW ). These data have a standard CC-BY license and a standalone, upstream Digital Objective Identifier (10.24431/rw1k6c3) specific to the dataset itself 23 . These deployment location files (i.e., location.csv or GPE3-X.csv) are also visualized within the ATN DAC data portal (project page, https://bit.ly/2YUlJ4P ).
These data are publicly accessible and free to use without restriction, but we request future users of these data acknowledge the ATN as well as cite this data manuscript from which the data were obtained in any future publications and/or representations of these data.
Technical Validation
Post-processing of raw data.
Raw data files were harvested directly from the tag manufacturer by the ATN DAC and preserved as is. Files were reviewed for completeness, and to ensure proper ids were provided and correct folder and file labels applied. However, it is strongly encouraged that users carefully review provided data files as well as read and fully comprehend associated metadata prior to use. The accuracy and precision of Argos derived location estimates are known to vary and device sensors can drift over time or even report erroneous results (outliers) due to data transmission errors. It is essential that prudent actions are taken to ensure data used in any future analyses are biologically sound and only include data from within each tags reported deployment window (see Table 1 ). This applies to both individual use of these data and aggregated use with other data.
Usage Notes
Seven juvenile white sharks that were captured and tagged, were not immediately released, and their transmissions should be interpreted accordingly. Five of these sharks (6_10, 07_05, 08_11, 09_11B, 11_06) were displayed on exhibit at the Aquarium for a period of 22–198 days 24 . Three of these sharks were released back into the ocean in Monterey Bay (6_10, 07_05, 09_11B), which at the time, was significantly north of their natural habitat 18 , 20 , 25 . The remaining two exhibited sharks were released near Goleta, California at the northern edge of their historical range. Sharks 04_02 and 07_03 were kept in floating coastal pens (see Fig. 1a ) for 6–8 days before being released. Shark 09_11 and 09_11B are the same individual, but only the tag from 09_11B reports data from the wild environment. The tag deployed on shark 09_11 while in the pen was not programmed to transmit and acted as a control. No exhibited sharks carried tags while on display. Shark 09_09 and 09_09B are also the same individual. Shark was 09_09 was recaptured and tagged with a new PAT tag at which point it was also given an updated shark id (09_09B). Additional context on the future application of these data to understand animal movements, migrations, habitat preferences, niche modeling, mortality, and climate change impacts are provided in studies published with partial subsets of the present data 13 – 22 .
Supplementary information
Acknowledgements.
K. Peterson, A. Copenhaver and two anonymous reviewers improved earlier versions of this manuscript, J. Packard provided continual support of the research and development of this program over two decades. This work would not have been accomplished without the many students, staff, and interns who assisted with the field programs and tag deployments, and the commercial fishery operators who partnered to safely tag and release bycaught sharks. Axiom Data Science (Anchorage, AK) designed and maintains the cyberinfrastructure components of the ATN DAC and assisted in the data archival and visualization process. This study was supported by the generous contributions of members, visitors, and donors to the Monterey Bay Aquarium.
Author contrubtions
J.O., C.L. and O.S. designed the research program and with K.V. conceived of the manuscript. J.O., C.L. and O.S. supervised the data collection and program administration, C.W. administered the commercial fishery partnership and in-water animal care infrastructure. All authors contributed, analyzed, and curated data. M.M. prepped dataset for archive and public release. K.V. generated the figures and drafted the manuscript with contributions from J.O., C.L., T.F. and M.M. All authors reviewed the manuscript.
Code availability
Competing interests.
The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
John O’Sullivan, Email: gro.qayabm@navillusoj .
Kyle S. Van Houtan, Email: gro.efileniram@elyk .
The online version contains supplementary material available at 10.1038/s41597-022-01235-3.
July 2, 2023
Great White Sharks Are Surging off Cape Cod
Cape Cod has quickly become one of the largest white shark hotspots in the world and the first ever in the North Atlantic
By Jim Behnke

Researchers photograph a white shark moments after it made a seal kill in shallow water near an inlet in North Chatham, Mass.
Wayne Davis
They’re here. Once rare in this area, great white sharks —hundreds of them—are hunting in the shallow waters along the beaches of Cape Cod in Massachusetts. The upsurge over the past decade has caught just about everyone by surprise, including marine scientists. Renowned shark expert Greg Skomal, who has studied white sharks off the coast of New England since the early 1980s, says he didn’t encounter one anywhere near Cape Cod until 2004 and didn’t tag his first one there until 2009.
A new study shows that this peninsula’s eastern shoreline now hosts one of the largest seasonal white shark gatherings in the world and is the first such hotspot documented in the North Atlantic. Cape Cod joins established hotspots in South Africa, central California, Mexico’s Guadalupe Island and Australia’s Neptune Islands. The sharks are most concentrated by Massachusetts during June through October—the same time of year when more than three million vacationers regularly flock to the cape.
The public safety implications are obvious. The sharks hunt in the swells along some 40 miles of beachfront managed by the Cape Cod National Seashore. Leslie Reynolds, deputy superintendent for the organization, says 2012 was the first time a shark injured a person along this beachfront area. Two incidents followed, including a fatal encounter in 2018. Although there have been no attacks since then, the danger is ever present. A working group convened by Reynolds that included municipal beach safety directors, along with representatives of the Massachusetts Department of Marine Fisheries and The Atlantic White Shark Conservancy, has implemented safety measures such as new beach signs, a flag warning system, training on how to stop a victim’s bleeding, and public outreach and education about shark threats. An iPhone app called Sharktivity gives the latest sightings.
The shark conservancy brought in white shark experts from other parts of the world to advise the safety planners. “Understanding shark population trends, movement and behavior is crucial for shark safety and conservation,” says Alison Kock, a marine biologist for the South African National Parks, who visited Cape Cod in 2016.

A 15-foot female white shark hunts at the edge of a surf break along Cape Cod’s popular Coast Guard Beach in Eastham, Mass. Credit: Pamela King
Sharks are convening on the cape because the gray seal population there is rebounding, experts say. The seals had been extirpated from New England by the early 1960s, largely a result of culling by commercial fishers. The Marine Mammal Protection Act of 1972 made it safe for the seals to return from Canadian waters, but they didn’t establish pupping colonies until the early 1990s. With no natural predators and plenty of fish to eat, the seals thrived. By one estimate, more than 30,000 seals were on the cape by 2017. The sharks were likely drawn to them by scent trails. Seals are a favorite fat-rich prey for white sharks as the fish make their annual northern migration.
White sharks are protected as well. The U.S. government designated them as a “prohibited species” in 1997, making it illegal to catch the sharks between three and 200 nautical miles out to sea. Massachusetts followed suit in 2005, prohibiting capture between shore and three miles out.
Many environmentalists view what’s happening on Cape Cod as a conservation success story. Public safety concerns about both seals and sharks could have led to draconian measures, such as culling, but instead nature was left to take its course. “What we’re seeing at Cape Cod is a reestablishment of the trophic (food) web and what it may have been like before overfishing and the slaughter of many of the animals at the top of the food chain,” says Chris Lowe, director of the Shark Lab at California State University, Long Beach.

A tagged shark called Mr. Spot Claw is followed by Atlantic White Shark Conservancy researchers on the Aleutian Dream as dozens of beachgoers watch from Cape Cod’s Nauset Beach in Orleans, Mass. Credit: Wayne Davis
As white shark sightings rose in the early 2010s, people ranging from National Park officials to conservation organization leaders to cape residents wanted to know how big the population was getting. That prompted a four-year study that included Skomal, a senior fisheries biologist at Massachusetts’s Division of Marine Fisheries. The researchers estimate that about 800 individual white sharks visited the sampling area from 2015 to 2018.
Over the past decade population studies have been conducted at white shark hotspots along California’s central coast, in South Africa and at Guadalupe Island; the estimated populations were 300, 908 and 78, respectively. Like those studies, the Cape Cod project identified sharks by their unique coloration and dorsal fin profiles. But the other studies all used baits or artificial attractants to lure sharks to a research vessel for observation. Sharks that are willing to approach a boat are likely a subset of all sharks in the area, and this introduces uncertainty in the data.
“No decoys or baits were used to attract sharks” in the Cape Cod study, says Megan Winton, lead researcher and a staff scientist at the Atlantic White Shark Conservancy , the nonprofit organization that provided most of the research funding. “Instead we relied on a spotter plane to find free-swimming sharks and radioed their locations to a research vessel,” the Aleutian Dream . “Once we located a shark, we shot underwater videos with a GoPro camera attached to a fiberglass painter’s pole.” The sheer volume of data compiled also distinguishes the study: the researchers conducted 137 survey trips, encountered sharks 2,295 times and collected a total of 2,803 videos.
But does the Cape Cod shark population represent an increase in the overall North Atlantic population? It’s hard to know. Those sharks were somewhere else before they started visiting the cape. “Just because we’re seeing them or we’re not seeing them doesn’t mean that their numbers have changed,” says Taylor Chapple, an assistant professor at Oregon State University’s marine, fishing and wildlife department, who was involved with the central California studies. “It may just mean that their behavior or distribution has changed or our ability to find them has changed.”
Skomal agrees but adds, “Our population estimates increased with each year of our study. This general trend is indicative of growing population, at the very least at Cape Cod and possibly beyond.”
The study has stimulated other questions. Is the shark population off Cape Cod still growing? What impact would this have on the seal population? What is the size of the broader Northwest Atlantic white shark population?
To pursue answers, the Cape Cod research team is studying predator-prey relationships, the fine-scale movement of sharks near the shore and other phenomena that will provide greater insight into the biology, ecology and natural history of the species. As John King, captain of the Aleutian Dream , puts it, “The population study is just the end of the beginning.”
Premium Content
Why great white sharks are still a mystery to us
Thanks to Jaws, they're the ocean's most iconic and feared fish. But we know surprisingly little about them.
Meeting a great white shark in the wild is nothing like you expect it would be. At first glance it’s not the malevolent beast we’ve come to expect from a thousand TV shows. It’s portly, bordering on fat, like an overstuffed sausage. Flabby jowls tremble down its body when it opens its mouth, which otherwise is a chubby, slightly parted smirk. From the side, one of the world’s greatest predators is little more than a slack-jawed buffoon.
It’s only when the underwater clown turns to face you that you understand why it’s the most feared animal on Earth. From the front its head is no longer soft and jowly but tapers to an arrow that draws its black eyes into a sinister-looking V. The bemused smile is gone, and all you see are rows of two-inch teeth capable of crunching down with almost two tons of force. Slowly, confidently, it approaches you. It turns its head, first to one side and then the other, evaluating you, deciding whether you’re worth its time. Then if you’re lucky, it turns away, becoming the buffoon again, and glides lazily into the gloom.

There are more than 500 species of sharks, but in popular imagination there’s really only one. When Pixar needed an underwater villain for its animated film Finding Nemo, it didn’t look to the affable nurse shark or the aggressive bull shark. Not even the tiger shark, which would be more appropriate in Nemo’s coral-reef home. It was the great white shark—with its wide, toothy grin—that was plastered on thousands of movie billboards across the world.
The great white shark is the ocean’s iconic fish, yet we know little about it—and much of what we think we know simply isn’t true. White sharks aren’t merciless hunters (if anything, attacks are cautious), they aren’t always loners, and they may be smarter than experts have thought. Even the 1916 Jersey Shore attacks famously mentioned in Jaws may have been perpetrated by a bull shark, not a great white.
We don’t know for sure how long they live, how many months they gestate, when they reach maturity. No one has seen great whites mate or give birth. We don’t really know how many there are or where, exactly, they spend most of their lives. Imagine that a land animal the size of a pickup truck hunted along the coasts of California, South Africa, and Australia. Scientists would know every detail of its mating habits, migrations, and behavior after observing it in zoos, research facilities, perhaps even circuses. But the rules are different underwater. Great whites appear and disappear at will, making it nearly impossible to follow them in deep water. They refuse to live behind glass—in captivity some have starved themselves or slammed their heads against walls. (Several aquariums have released them for their own safety or because they were attacking tank-mates.)

Yet scientists today, using state-of-the-art technologies, may be on the verge of answering two of the most vexing mysteries: How many are there, and where do they go? Unraveling these mysteries could be critical to deciding how to protect ourselves from them and them from us. When we finally see the great white clearly from all angles, will the world’s most fearsome killer deserve our fear or our pity?
A 24-foot fishing boat sits just off the southern tip of Cape Cod, Massachusetts, on a perfect summer afternoon. The passengers—three scientists, two paying customers, two journalists, and the boat’s captain—lounge on the seats, looking off toward Nantucket. The voice of a spotter pilot flying 1,000 feet above breaks out over the radio in a sharp New England accent. “We’ve got a wicked nice shark over here to the south!”
Fisheries biologist Greg Skomal perks up. He’s standing five feet off the bow on the pulpit, a fenced-in walkway resembling a pirate’s plank. If this were a Hollywood movie, he’d have a harpoon and a peg leg. Instead he carries a GoPro camera attached to a 10-foot pole. He grins like a little kid as the captain guns the engine.
Cape Cod's Great Whites

Before 2004 hardly anyone in modern times saw great white sharks in the waters off the East Coast. Occasionally one would appear near a beach or in a fishing net, but they were anomalies. Elsewhere, great whites congregate seasonally around five “hubs” or territories, including California’s coast down to Mexico’s Baja California, South Africa’s southern shores, and Australia’s southern coast, where they gather to feed on seals. But there’s been no hub on the East Coast, nor have there been many seals. Sharks here were wanderers without a home. Then, in 2004, a single female found her way into shallow inlets and shoals near Woods Hole, Massachusetts.
For Skomal, who’d been tagging other sharks for 20 years, this was the chance of a lifetime—a great white in his own backyard. “I thought it was a fluke. This will never happen again,” he says with his broad, boyish grin under ruffled salt-and-pepper hair. Over the next two weeks Skomal and his colleagues followed the shark, which they named Gretel after the lost girl in the fairy tale, and affixed an electronic tracker on her. Tracking a white shark across the Atlantic Ocean offered a chance to solve so many riddles. But 45 minutes into the journey, Gretel’s tag malfunctioned and popped off. “I went from this superhigh to this really deep low, because I was convinced that this was the shot in my career to study a white shark,” Skomal says.
It wasn’t. Over the next few years he thought a lot about Gretel and wondered whether she was indeed alone. Then, on Labor Day, 2009, everything changed. A pilot saw five great whites off the cape. Over that weekend Skomal tagged them all. “I absolutely freaked out. My adrenaline was pumping. My heart—I could feel it just pounding in my chest. This was everything I was dreaming of.”
White sharks have returned every summer since, leading some to call Cape Cod the sixth hub. How many great whites are there? For that we turn to the hub running from California to Baja California. The effort to count sharks there was pioneered by Scot Anderson while he was a volunteer seabird scientist in the mid-1980s on an island west of San Francisco’s Golden Gate Bridge. Anderson and others have tracked the sharks—at first by sight, then by acoustic tags, and most recently with satellites. During the past 30 years, teams have assembled thousands of observations of individual sharks recognized by the shape and marks of their dorsal fins, while others have used the distinctive line between their gray bodies and white underbellies. Scientists know where the sharks congregate and how they feed. And each year most sharks they see are the ones they saw in previous years.
This raised an intriguing question: With enough observations, could you use the sharks you see to estimate how many you can’t see? In 2011 a team in California did just that and came up with just 219 adults in California’s most shark-rich region. Even among top predators, generally less abundant than their prey, that’s a tiny number. The study shocked the public and came under immediate attack from other experts.
Of course, counting great whites is a lot harder than counting land animals or even marine mammals. So scientists make massive assumptions about shark movements and then extrapolate. In California the biggest assumption was that a few feeding grounds were representative of the entire hub. Other teams crunched the same data using different assumptions, and one study estimated about 10 times more sharks. (That count was bolstered by adding juveniles, which the first excluded because so little is known about them.) Pretty soon scientists began quantifying white sharks in the other hubs. A team in South Africa estimated the population there at around 900, while another team put Mexico’s Guadalupe Island population, part of the California hub, at just 120 or so.
Are these large numbers or small? Are great whites thriving or dwindling? The world has about 4,000 tigers and 25,000 African lions. Using the lowest estimates, global great white numbers resemble the estimate for tigers, an endangered species. Using the highest estimate, the population is closer to that of the lions, which are classified as vulnerable. Several experts see them heading toward extinction; others see a positive trend. Some say rising seal populations are a sign that great whites are nearly gone, while others say more seals mean more sharks. Aaron MacNeil, an Australian statistician who crunches shark data, says the appearance of sharks around Cape Cod and the increased activity in the Southern Hemisphere suggest the latter. “I haven’t seen any evidence in the last decade that white sharks are declining,” says MacNeil. “Yes, there is a historical depletion of white sharks. But the story is not that they are going extinct. The story is that they are probably increasing very, very slowly.”

There’s reason to be hopeful. Few if any fishermen target great whites today, yet a global pact, the Convention on International Trade in Endangered Species, gives white sharks its second strongest conservation rating because fishermen catch them unintentionally. With numbers so low, even accidental catches can play havoc with the species, which, as a top predator, has an ecologically important role in managing the oceans.
To understand whether great white sharks need our protection, we must know not only how many there are but also where they go. Their migrations aren’t neat, like a bird’s or a butterfly’s. They’re messy, with one hugging the coast while another zigzags hundreds of miles out to sea. Many, but not all, seem to seasonally move between warm and cold water. And the paths seem different for males, females, and juveniles.
Today, with long-term, long-distance tags that can communicate via satellite, scientists are finally getting some clarity. For years scientists have noticed that adult great whites in California and Mexico quit the coast in late fall. Now we know where they go: deep water in the middle of the Pacific Ocean. Why they visit this great white shark “café” remains unclear. “I call it Burning Man for white sharks,” says Salvador Jorgensen, a biologist who studies factors that drive great white migration and ecology. “They are heading out to what some people call the desert of the ocean, and what the hell are they doing out there?”
One possible answer is mating, which might explain why no one has ever observed it. The area is roughly the size of California and thousands of feet deep, which makes it hard to monitor sharks there. But satellite tags tell us that the females swim predictable straight patterns while the males swim up and down in the water column, possibly searching for mates. Thus a rough sketch of the lives of California white sharks is forming. After spending the summer and fall gorging on seals, they head out to the deep ocean to breed, relying on energy stores to live. The males then swim back to the coast while the females wander to unknown places, where they remain for another year or so, perhaps to birth their young. Newborn sharks then show up at feeding grounds—say, the waters off Southern California—devouring fish until they are big enough to join their elders in the north or south hunting seals.
You May Also Like
Single orca seen killing great white shark for first time ever

This might be the first newborn great white shark ever recorded

Why did these shark hunters bury their dead with extra limbs?
It’s not a perfect picture. Females and males aren’t in the café together for long, and we don’t know where the babies are born. But it explains a lot. For example, as a population rebounds, its young become plentiful, which is likely why Southern Californians have encountered a lot of sharks lately. Yet it’s tougher to figure out elsewhere. Australian sharks forage along the southern coast but don’t seem to have a pattern or café. And in the Atlantic we know even less. “We’ve got wanderers, and we’ve got coastal sharks. And what dictates which, I have no idea,” Skomal says.

Even though he doesn’t understand their migrations yet, Skomal is sure that white sharks have a long history here. At his office in New Bedford, just west of Cape Cod, he opens a document that compiled studies of seal bones from Native American archaeological sites along the eastern seaboard. The discarded bones suggest that seal populations crashed from overhunting perhaps a century before the Declaration of Independence. In other words, we’ve had very few Atlantic gray seals throughout the United States’ 240-year history. Today, thanks to the Marine Mammal Protection Act, seal colonies now populate New England. And when the seals returned, the sharks came home as well.
One bright August morning I board a two-seater plane with Wayne Davis, a veteran spotter pilot for tuna and swordfish who now helps scientists track down white sharks. Unlike the hubs, the water here is so shallow that sharp eyes can spot them from the air. In just 30 minutes of flying we see seven, all patrolling beaches where gray seals are foraging in open waters. On the way back Davis and I fly past several beaches a mile or so to the north packed with vacationers.
So far locals have embraced their new neighbors. There are stuffed animals, T-shirts, posters, and a community art exhibit called “Sharks in the Park.” Even the new high school’s mascot is a great white. Most of the time the sharks are shown from the side—cheerful, buffoonish. Experts warn, though, that at some point someone here will meet the other version—the one with teeth.
Attacks on people are incredibly rare. In waters off California, the chances of a surfer being bitten by a great white shark are one in 17 million; for swimmers, it’s even rarer—one attack in every 738 million beach visits, according to a recent Stanford University study . On Cape Cod, fatalities may not be a question of if, but when. The last lethal shark attack off New England was in 1936, but there have been several close calls recently. A swimmer there was bitten on both legs in 2012, and two paddlers in Plymouth were knocked from their kayaks in 2014, although they escaped unscathed.
If a more serious attack happens, Massachusetts will join the other hubs in weighing the benefits versus the dangers of sharks in their waters.
It may be that great white sharks are rebounding across the world: following the bigger seal and sea lion populations, re-establishing themselves in their old hunting grounds, reclaiming the coasts they nearly lost.
Then again, it may be that great whites today are hanging over the abyss of extinction, clutching the edge by the skin of their jagged teeth. Will we look past our fear and reach out a hand to this creature? Can we take pity on the pitiless eyes of a monster?
Related Topics

Want to see great white sharks? Consider Cape Cod.

Sharks are still being killed at high rates—despite bans on finning

500 baby sharks to be released: An exclusive look at an unprecedented mission

Cape Cod may have the highest density of great white sharks in the world

Scared of sharks? This photographer aims to turn shark fear into fascination
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Articles on Great white sharks
Displaying 1 - 20 of 32 articles.

Who would win in a fight between a great white shark and a blue whale?
Chandra Salgado Kent , Edith Cowan University

Killer whales are hunting great white sharks in South Africa’s waters
Ozayr Patel, The Conversation

Millions of years ago, the megalodon ruled the oceans – why did it disappear?
Michael Heithaus , Florida International University

How great white sharks outsmarted the massive megalodon to first rule the oceans, 3 million years ago
Nicholas Ray , Nottingham Trent University

Great white sharks occasionally hunt in pairs – new research sheds light on social behavior of these mysterious predators
Yannis Papastamatiou , Florida International University


White sharks can easily mistake swimmers or surfers for seals. Our research aims to reduce the risk
Laura Ryan , Macquarie University and Charlie Huveneers , Flinders University

South Africa’s plan to protect sharks needs an urgent update
Alison Kock , South African Institute for Aquatic Biodiversity

Oceanic sharks and rays have declined by 71% since 1970 – a global solution is needed
David Sims , University of Southampton

Fatal shark attacks are at a record high. ‘Deterrent’ devices can help, but some may be nothing but snake oil
Daryl McPhee , Bond University

FactFile: the facts on shark bites and shark numbers
Jane Williamson , Macquarie University and Vincent Raoult , University of Newcastle

World-first genetic analysis reveals Aussie white shark numbers
Rich Hillary , CSIRO ; Russ Bradford , CSIRO , and Toby Patterson , CSIRO

Why do shark bites seem to be more deadly in Australia than elsewhere?
Blake Chapman , The University of Queensland

Curious Kids: Do sharks sneeze?
Jane Williamson , Macquarie University

How drones can help fight the war on shark attacks
Brendan Kelaher , Southern Cross University ; Andrew Colefax , Southern Cross University ; Bob Creese , Southern Cross University ; Paul Butcher , Southern Cross University , and Vic Peddemors , Sydney Institute of Marine Science

Not just nets: how to stop shark attacks without killing sharks
Nathan Hart , Macquarie University and Charlie Huveneers , Flinders University

Stop freaking out about sharks in British waters – let’s welcome them
Christopher Bird , University of Southampton

Finally, a proven way to keep great white sharks at arm’s length
Ryan Kempster , The University of Western Australia and Shaun Collin , The University of Western Australia

Relax, shark numbers aren’t booming, but more research can make us safer
Jessica Meeuwig , The University of Western Australia ; Laurie Laurenson , Deakin University , and Shanta Barley , The University of Western Australia

Giant monster Megalodon sharks lurking in our oceans: be serious!
John Long , Flinders University

The science behind that ‘Jaws’ sighting – and why it’s cause for celebration
Related topics.
- Bull sharks
- Marine conservation
- New South Wales
- Shark attacks
- Shark conservation
- WA shark cull
- Western Australia
- White sharks
Top contributors
Lecturer in Public Policy, University of Sydney
Doctoral School Programmes Manager, Nottingham Trent University
Marine Biologist, South African National Parks (SANParks); Honorary Research Associate, South African Institute for Aquatic Biodiversity (SAIAB), South African Institute for Aquatic Biodiversity
Shark biologist, The University of Western Australia
Wen Family Chair in Conservation, The University of Western Australia
Professor in Marine Fisheries Ecology, Macquarie University
PhD graduate, University of Southampton
Associate professor, Flinders University
Associate Professor, School of Life and Environmental Sciences, Deakin University
Director, Florida Program for Shark Research and Coordinator of Museum Operations, Florida Museum of Natural History, University of Florida
Principal Research Scientist, CSIRO
Professor of Aquatic Biosciences, Nord University
Winthrop Professor/WA Premier's Research Fellow, School of Biological Sciences and the Oceans Institute, The University of Western Australia
Strategic Professor in Palaeontology, Flinders University
Senior Lecturer in Geography, University of Wollongong
- X (Twitter)
- Unfollow topic Follow topic
Local News | Sharks ‘adapting their movements and routines,’…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to print (Opens in new window)
- Your Tax Dollars
- Massachusetts
Breaking News
Local news | nfl draft 2024: patriots add south carolina cb marcellas dial in sixth round, local news | sharks ‘adapting their movements and routines,’ great white researchers discover, white sharks will be returning to cape cod over the next few months.

Do great white sharks change their behaviors in different environments, or do the apex predators follow the same routines regardless of location?
Researchers recently set out to solve this shark puzzle, as they reportedly discovered great white behaviors by attaching smart tags and cameras to their fins.
Great whites adapt their movements and routines based on the specific habitat they’re hunting in, according to the shark scientists.
“… The sharks are adapting their movements and routines to suit their local environment, rather than behaving the same way everywhere they’re found,” said shark researcher Oliver Jewell.
In the study, 21 white sharks from small juveniles to large adults were fitted with motion-sensitive biologging tags along the California coast in different environments — offshore islands, coastal headlands, and an inshore cove.
The tags were attached for up to six days at a time and measured swimming depths and body movements, before they detached and floated to the surface.
The California coast provided a unique space to conduct the work, not only because researchers had been studying this specific population for decades, but the area is home to both juvenile and adult white sharks.
“White sharks visit the same areas of central California year after year, with some seen in the same spot for 30 years or more,” said Jewell, a researcher at the University of Western Australia.
“We were looking to see what shapes their movements and routines while they are there,” Jewell added.
During the day, sharks at all sites were generally more active — swimming up and down the water column, suggesting they were actively searching for prey.
However, sharks showed more active behavior at both dawn and dusk in places where they were thought to feed on fish rather than marine mammals.
“We found the greatest differences in movements were from sharks from different areas, while the size of the shark and time of day were also important,” Jewell said.
It’s important to review the location of a shark when considering why it might be behaving a certain way, the scientist added.
“Hopefully, we can apply the research in a number of ways going forward — we’ve already been tagging more sharks in more areas, and will follow this up with further studies,” Jewell said.
Great white sharks will be returning to Cape Cod’s waters over the next few months, where they feed on seals throughout the summer and fall. Many sharks also head farther north to Maine and Canada.
This recent research was a part of Monterey Bay Aquarium’s “Project White Shark” and led by Jewell, a former Murdoch University PhD candidate, with Harry Butler Institute and the School of Environmental and Conservation Sciences — in collaboration with Oregon State University, Stanford University and California State University Monterey Bay.
More in Local News

SUBSCRIBER ONLY
Crime & public safety | boston police department releases names of protesters arrested at emerson college encampment.

Local News | Former Boston police commissioner knocks Mayor Wu in ‘war’ over North End outdoor dining

Politics | Boston commission that criticized Mayor Wu accused of overreach in push to landmark private property

Politics | Pols & Politics: Get ready for Beacon Hill’s crazy sprint to the end of July
Shark conservation has been so successful that researchers are finding ways to curb human-shark interaction
Sharks off the Gulf of Mexico are increasingly engaging in depredation.
Shark conservation efforts in the last two decades have been so successful that researchers are testing ways to mitigate human-shark conflict as populations continue to rise.
Sharks are increasingly becoming a nuisance to fishers as they engage in depredation -- or the act of attacking or plundering -- of catch, said Demian Chapman, senior scientist and director for the Center for Shark Research at the Mote Marine Laboratory & Aquarium in Sarasota.
MORE: Warmer sea surface temperatures have led to a bull shark population increase, scientists say
A device about the size of a roll of quarters that shocks sharks that attempt to bite on fishing lines may be the answer to reducing shark depredation as well as sharks ending up as bycatch, Chapman told ABC News.

The SharkGuard electrical pulse deterrent attaches to the fishing line in place of the sinker, about 10 inches away from the bait. The device sends out shock waves with about the strength of a prank pen, which aggravate the sensitive pores sharks have to detect prey.
"It's a sense we don't have," Chapman said. "It doesn't seem to hurt them, but they don't enjoy the sensation."
MORE: 10 people killed in unprovoked shark attacks last year, report finds
The device is manufactured by a U.K.-based company called Fishtek Marine, which published a paper in 2022 that showed promising results in bycatch mitigation in tuna longline fishery.
Now, researchers at the Mote Marine Laboratory & Aquarium in Sarasota, Florida, are conducting their own tests in its research laboratory equipped with tanks that can hold 60,000 gallons of water -- a much less expensive process than running tests in the field, Chapman said.

When the device is placed in the tank, it both delays and deters the sharks from attempting to get the bait, Chapman said. After the initial shock, the sharks sometimes won't take the bait at all and will "sulk" at the end of the tank, Chapman said.
For the sharks that try again, it takes them more than double the amount of time to get to the bait, the tests show.
MORE: Global warming could increase risk of human-elephant conflict, researchers say
The delay could prove to be helpful to the fishers, who then have more time to retrieve the fish before the shark makes another attempt of depredation, Chapman said.
So far, the researchers have tested small hammerhead and bonnet head sharks. But they are aiming to test a wider variety of species, including sandbar sharks, which are known to steal fish, Chapman said.

An estimated 100 million sharks are killed per year by humans, a study published in Science in 2019 found. Much of those deaths are as a result of fishing bycatch.
Related Stories
Golden retriever steals the show at wedding
- Apr 26, 11:42 AM

Time capsule found in school during demolition
- Apr 25, 10:24 PM

Southwest Airlines CEO may reevaluate open seating
- Apr 26, 2:58 PM
Management practices put in place in the last two decades have allowed shark populations to bounce back, Chapman said.
MORE: As tiger populations increase, so do conflicts with humans
Mote has been conducting shark surveys off the coast of Sarasota since 2003. Every quarter, researchers venture into the Gulf of Mexico to tag sharks in an effort to understand better the rate of population rise.
In 2021, researchers caught a record of 104 sharks -- eight different species -- in four days, the most ever caught in a summer quarter.

The number of sharks tagged per hour has been increasing over the past 20 years, but Chapman wouldn't character the rise in numbers as an overpopulation or infestation.
"We don't know if they're at the level where they need to be to be important for the ecosystem, " he said. "But we do know there's more of them, and so we have to learn how to live with them again."
MORE: Scientists have an explanation why there is an increase of shark attacks off East Coast
Related topics.

Fossil footprints unearth new 'megaraptor'
- Apr 26, 12:16 PM

Matthew McConaughey steps out with family at gala
- Apr 26, 9:48 AM
ABC News Live
24/7 coverage of breaking news and live events

Sharks Seen 'Adapting' to Different Environments for First Time
Great white sharks might have more flexible behavior than scientists first thought, according to new research.
After hooking great white sharks up with smart tags, researchers caught a glimpse of the true behaviors of these marine monsters. Rather than stiffly behaving in the same way regardless of context, it turns out that great white sharks are able to adapt their behavior based on different hunting scenarios, according to a new paper in the journal Ecosphere .
Great white sharks are the largest predatory fish in the world, growing up to lengths of 20 feet. They are found in cooler coastal waters off several countries worldwide , including the United States, Canada, South Africa, Australia, and New Zealand. Their diet consists mainly of marine mammals such as seals, sea lions, and small whales , as well as large fish like tuna.
Researchers from Murdoch University, Oregon State University, Stanford University , and California State University Monterey Bay tagged a total of 21 great white sharks of all ages with trackers. These sharks were found in a variety of environments along the coast of California, ranging from islands to shallow waters near the shore. These tags, which measured the location, depths and movements of the sharks, were designed to stay attached to the animals for six days before detaching and floating to the surface
The researchers then collected the trackers and analyzed the data to find out if sharks behaved the same in all locations or varied their behavior in different environments.
They discovered that sharks were more active during the day than the night at all sites, swimming up and down and twisting and turning about, suggesting that they were hunting for prey. During dawn and dusk, however, they were more active only in places where there was more fish prey than mammal prey present.
"We found the greatest differences in movements were from sharks from different areas, while the size of the shark and time of day were also important," study author Oliver Jewell, a researcher at the University of Western Australia and previous Murdoch University Ph.D. candidate, said in a statement. "This means the sharks are adapting their movements and routines to suit their local environment, rather than behaving the same way everywhere they're found."
Adapting their behavioral patterns allows great white sharks to exploit local feeding opportunities and prey behaviors, the researchers explain in their paper. In some regions of South Africa, Cape fur seals are more vulnerable at dawn, leading to increased shark activity during this time. Conversely, in areas where seals take shelter within kelp forests, sharks adjust by being active throughout the day to optimize their chances of finding prey.
Meanwhile, California made for an ideal location for this study, as it is home to adult and juvenile great whites , and has a vast number of possible habitats for the sharks to pass through.
"White sharks visit the same areas of central California year after year, with some seen in the same spot for 30 years or more," Jewell said. "We were looking to see what shapes their movements and routines while they are there."
The researchers hope that their results and their tagging technology will help shark scientists to better understand these behemoth creatures, and also pave the way for further research into shark behavior.
"Modern technology provides us with unprecedented views into the lives of some of the hardest to study species, in turn offering us novel insights into the functioning of our oceans," study author Adrian Gleiss, a behavioral ecologist at Murdoch University, said in the statement.
Jewell agreed, adding that "hopefully, we can apply the research in a number of ways going forward—we've already been tagging more sharks in more areas, and will follow this up with further studies."
Do you have an animal or nature story to share with Newsweek ? Do you have a question about great white sharks? Let us know via [email protected].
Related Articles
- Ferret Once Thought Extinct Has Been Cloned
- Venus Is Leaking Carbon and Oxygen—and Scientists Don't Know Why
- 'Wildly Different' Shark That Can Live for Centuries at Risk
- Space 'Sat-Nav' Maps Routes Between Worlds
- Toxic Chemicals in Everyday Products 'Enter the Human Body' via Touch
Start your unlimited Newsweek trial

Ancient, 30-foot relative of great white shark unearthed in Mexico quarry
"Exceptionally preserved" fossils of an ancient shark that lived alongside the dinosaurs has finally revealed what the predator looked like — and why it may have gone extinct.

Complete fossils from an enormous shark that lived alongside the dinosaurs reveal crucial information about this enigmatic predator — including it being an ancient relative of the great white shark .
The sharks, from the genus Ptychodus , were first discovered in the mid-eighteenth century. Descriptions of this genus were largely based on their teeth — which could be nearly 22 inches (55 centimeters) long and 18 inches (45 cm) wide, and were adapted for crushing shells — found in numerous marine deposits dating to the Cretaceous period (145 million to 66 million years ago).
Without the ability to examine a fully intact specimen, researchers had hotly debated what the shark's body shape might look like — until now.
"The discovery of complete Ptychodus specimens is really exciting because it solves one of the most striking enigmas in vertebrate paleontology," lead author Romain Vullo , a researcher at Géosciences Rennes, told Live Science in an email.
In a study published Wednesday (April 24) in the journal Proceedings of the Royal Society B: Biological Sciences , researchers have described complete fossils of the shark discovered in limestone quarries in Nuevo León, northeastern Mexico. Its outline was still fully preserved, and its body shape suggests it hunted sea turtles — which could explain its extinction around 76 million years ago as it was competing with other animals that ate the same prey.

The specimens "show an exquisite preservation," because they were deposited in a quiet area with no scavengers, Vullo said. "The carcasses of animals were rapidly buried in a soft lime mud before being entirely disarticulated."
Related: 325 million-year-old shark graveyard discovered deep within Mammoth Cave harbors new fossilized species
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Analysis of the fossils reveals this large predator belonged to the mackerel shark group (Lamniformes), which includes great whites ( Carcharodon carcharias ), mako, and salmon sharks. It grew to around 33 feet (10 meters) long and is known for its massive, grinding teeth, which are unlike those we see in sharks today.

It was widely believed that Ptychodus fed on invertebrates from the seabed — the ancient relatives of clams and mussels. But the new fossils challenge that, revealing that this ancient shark had a streamlined body shape, indicating it was a fast-swimming pelagic predator. "The newly discovered fossils from Mexico indicate that Ptychodus looked like the living porbeagle shark," Vullo said, but with "unique grinding dentition."
— Terror beast fossils unearthed in Greenland are more than half a billion years old
— 390 million-year-old fossilized forest is the oldest ever discovered
— Massive graveyard of fossilized shark teeth found deep in the Indian Ocean
This new information has led the researchers to believe it preyed on large ammonites — a type of crustacean with a hard shell — and sea turtles.
" Ptychodus occupied a special ecological niche in Late Cretaceous seas," Vullo said, because it was the only pelagic shark that was adapted to eating hard-shelled prey such as turtles. This may explain why it died out around 10 million years before the extinction event that ended the Cretaceous period. "Toward the end of the Cretaceous, these large sharks were likely in direct competition with some marine reptiles (mosasaurs) targeting the same prey," he said.
Editor's note: The headline of a previous version of this article said Ptychodus was an ancestor of great white sharks . This was corrected to say it was a relative at 6:24 ET on April 24.

Melissa Hobson is a freelance writer who specializes in marine science, conservation and sustainability, and particularly loves writing about the bizarre behaviors of marine creatures. Melissa has worked for several marine conservation organizations where she soaked up their knowledge and passion for protecting the ocean. A certified Rescue Diver, she gets her scuba fix wherever possible but is too much of a wimp to dive in the UK these days so tends to stick to tropical waters. Her writing has also appeared in National Geographic, the Guardian, the Sunday Times, New Scientist, VICE and more.
Watch hammerhead sharks swim in 'cyclones' around ancient volcano in rare footage
There's 5 times as many bull sharks off Alabama now — but don't worry about shark bites
Eclipse from space: Paths of 2024 and 2017 eclipses collide over US in new satellite image
Most Popular
- 2 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 3 George Washington's stash of centuries-old cherries found hidden under Mount Vernon floor
- 4 Scientists find one of the oldest stars in the universe in a galaxy right next to ours
- 5 DNA analysis spanning 9 generations of people reveals marriage practices of mysterious warrior culture
- 2 Tweak to Schrödinger's cat equation could unite Einstein's relativity and quantum mechanics, study hints
- 3 New UTI vaccine wards off infection for years, early studies suggest
- 4 Lasers reveal prehistoric Irish monuments that may have been 'pathways for the dead'
- 5 Plato's burial place finally revealed after AI deciphers ancient scroll carbonized in Mount Vesuvius eruption

- Colleges & Degrees
- Academic Calendar
- International Education
- Graduate Studies
- Accreditation
- Tuition and Fees
- Parking & Maps
- Careers with CSULB
- Alumni Home
- Alumni Volunteering
- Alumni Giving
Campus Life
- Centers & Organizations
- Commencement
- Student Life
- Office of the President
- Office of the Provost
- Administration & Finance
- Student Affairs
- University Relations & Development
- Information Technology
- Beach Shops
- Campus Directory
- Enrollment Services
- Financial Aid
- Schedule of Classes
- Student Records
- 49er Foundation
- Research Foundation

1250 BELLFLOWER BOULEVARD LONG BEACH, CALIFORNIA 90840 562.985.4111
Professor Remembers the "Shark Whisperer" at CSULB
Professor Don Nelson, who passed away in 1977, was the founder of Cal State Long Beach's Shark Lab and recently featured in the online journal Nautilus as the "Shark Whisperer." The current director of the Shark Lab, Professor Chris Lowe, provided insight to Dr. Nelson's research and drive to understand the behavior and biology of white sharks.

- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- Health & Well-being
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
Shaping Sustainable Research "Shark"-style
"shark"-style research competition.
Leadership at Yale Urology has committed again to back its new tradition of “Shark Tank,” a departmental research competition patterned after the television show of the same name, which aims to promote innovation that can include even risky ideas.
During this, the competition’s second year, two teams have received funding to work on original projects.
Urinary Tract Infections and Spina Bifida
A team led by Adam Hittelman, MD, PhD, and Darryl Martin, PhD, will use its funding to try to identify new treatments for urinary tract infections in the pediatric spina bifida population.
“We are excited to receive the urology pilot grant,” says Hittelman. “Recurrent urinary tract infections are an especially challenging problem with this group [of patients]. We hope to collect enough initial data to apply for an NIH grant.”
Chair Isaac Y. Kim, MD, PhD, MBA, started the program in 2023 to serve as seed money – encouraging faculty to take calculated risks and try new approaches across divisions that may lead to innovative solutions. With enough data, it is possible these groups may receive sustainable backing and move the dial on patient care.
Bladder Cancer, Chemo, and Surgery
Fed Ghali, MD, and John Onofrey, PhD, lead the other team awarded in this year’s competition. They are set on answering the question asked by a subset of bladder cancer patients having surgery after chemo: "If there was no cancer in the bladder, why did I need to have this big surgery?”
“It turns out at least part of the answer is that we do not have a reliable way to identify chemo-responders that have been made cancer-free until their bladder is already removed and assessed by a pathologist,” says Ghali.
Through their research, titled "Integrating machine learning and radiomics for automated assessment of pathologic response following neoadjuvant chemotherapy in muscle-invasive bladder cancer,” Drs. Ghali and Onofrey are eager to see where it will lead patient care.
They have already begun to gather data and organize the next steps of their study, with a particular focus on technology.
“Dr. Onofrey and his associates specialize in machine learning and artificial intelligence for evaluating imaging studies. For instance, he and his lab use CT scans to glean all kinds of information that humans can’t extract by standard methods of interpretation,” says Ghali. “It’s possible this technology could help us identify those super-responders to chemotherapy and perhaps avoid radical surgery in up to 40% of patients.”
Needle Tract Circulating Tumor Cells
The Department of Urology’s inaugural “Shark Tank” competition in 2023 had several winners, including Darryl Martin, PhD, and Ralph Devito, MD.
Their research aims to determine the prevalence of needle tract circulating tumor cells, which are cancer cells that break away from the primary tumor and enter the bloodstream.
“We are still in the project's data collection phase but are very excited about its direction,” says Martin. “Dr. Devito and I have established a strong collaboration and are working well together. The project's next phase will be data analysis and manuscript preparation, and then we will seek additional funding opportunities.”
Yale Urology’s new vice chair of research, Michael Leapman, MD, MHS, says he is pleased with the overall progress of the competition. “The pilot awards represent an exciting investment, allowing investigators to ask innovative and provocative questions that may translate into new discoveries in the future.”
- Bladder Cancer
Featured in this article
- Ralph J. Devito, MD Assistant Professor of Urology
- Fed Ghali, MD Assistant Professor
- Adam Benjamin Hittelman, MD, PhD Associate Professor in Urology; Section Chief of Pediatric Urology, Urology
- Isaac Y. Kim, MD, PhD, MBA Professor of Urology; Chair, Urology; Chief, Urology; Co-Leader, Cancer Signaling Networks, Yale Cancer Center
- Michael S. Leapman, MD, MHS Associate Professor of Urology; Clinical Program Leader, Prostate & Urologic Cancers Program, Yale Cancer Center; Assistant Professor, Chronic Disease Epidemiology
- Darryl T. Martin, PhD Assistant Professor
- John Onofrey, PhD Assistant Professor of Radiology & Biomedical Imaging and of Urology
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
MIT announces 2024 Bose Grants
Press contact :.

Previous image Next image
MIT Provost Cynthia Barnhart announced four Professor Amar G. Bose Research Grants to support bold research projects across diverse areas of study, including a way to generate clean hydrogen from deep in the Earth, build an environmentally friendly house of basalt, design maternity clothing that monitors fetal health, and recruit sharks as ocean oxygen monitors.
This year's recipients are Iwnetim Abate, assistant professor of materials science and engineering; Andrew Babbin, the Cecil and Ida Green Associate Professor in Earth, Atmospheric and Planetary Sciences; Yoel Fink, professor of materials science and engineering and of electrical engineering and computer science; and Skylar Tibbits, associate professor of design research in the Department of Architecture.
The program was named for the visionary founder of the Bose Corporation and MIT alumnus Amar G. Bose ’51, SM ’52, ScD ’56. After gaining admission to MIT, Bose became a top math student and a Fulbright Scholarship recipient. He spent 46 years as a professor at MIT, led innovations in sound design, and founded the Bose Corp. in 1964. MIT launched the Bose grant program 11 years ago to provide funding over a three-year period to MIT faculty who propose original, cross-disciplinary, and often risky research projects that would likely not be funded by conventional sources.
“The promise of the Bose Fellowship is to help bold, daring ideas become realities, an approach that honors Amar Bose’s legacy,” says Barnhart. “Thanks to support from this program, these talented faculty members have the freedom to explore their bold and innovative ideas.”
Deep and clean hydrogen futures
A green energy future will depend on harnessing hydrogen as a clean energy source, sequestering polluting carbon dioxide, and mining the minerals essential to building clean energy technologies such as advanced batteries. Iwnetim Abate thinks he has a solution for all three challenges: an innovative hydrogen reactor.
He plans to build a reactor that will create natural hydrogen from ultramafic mineral rocks in the crust. “The Earth is literally a giant hydrogen factory waiting to be tapped,” Abate explains. “A back-of-the-envelope calculation for the first seven kilometers of the Earth’s crust estimates that there is enough ultramafic rock to produce hydrogen for 250,000 years.”
The reactor envisioned by Abate injects water to create a reaction that releases hydrogen, while also supporting the injection of climate-altering carbon dioxide into the rock, providing a global carbon capacity of 100 trillion tons. At the same time, the reactor process could provide essential elements such as lithium, nickel, and cobalt — some of the most important raw materials used in advanced batteries and electronics.
“Ultimately, our goal is to design and develop a scalable reactor for simultaneously tapping into the trifecta from the Earth's subsurface,” Abate says.
Sharks as oceanographers
If we want to understand more about how oxygen levels in the world’s seas are disturbed by human activities and climate change, we should turn to a sensing platform “that has been honed by 400 million years of evolution to perfectly sample the ocean: sharks,” says Andrew Babbin.
As the planet warms, oceans are projected to contain less dissolved oxygen, with impacts on the productivity of global fisheries, natural carbon sequestration, and the flux of climate-altering greenhouse gasses from the ocean to the air. While scientists know dissolved oxygen is important, it has proved difficult to track over seasons, decades, and underexplored regions both shallow and deep.
Babbin’s goal is to develop a low-cost sensor for dissolved oxygen that can be integrated with preexisting electronic shark tags used by marine biologists. “This fleet of sharks … will finally enable us to measure the extent of the low-oxygen zones of the ocean, how they change seasonally and with El Niño/La Niña oscillation, and how they expand or contract into the future.”
The partnership with sharks will also spotlight the importance of these often-maligned animals for global marine and fisheries health, Babbin says. “We hope in pursuing this work marrying microscopic and macroscopic life we will inspire future oceanographers and conservationists, and lead to a better appreciation for the chemistry that underlies global habitability.”
Maternity wear that monitors fetal health
There are 2 million stillbirths around the world each year, and in the United States alone, 21,000 families suffer this terrible loss. In many cases, mothers and their doctors had no warning of any abnormalities or changes in fetal health leading up to these deaths. Yoel Fink and colleagues are looking for a better way to monitor fetal health and provide proactive treatment.
Fink is building on years of research on acoustic fabrics to design an affordable shirt for mothers that would monitor and communicate important details of fetal health. His team’s original research drew inspiration from the function of the eardrum, designing a fiber that could be woven into other fabrics to create a kind of fabric microphone.
“Given the sensitivity of the acoustic fabrics in sensing these nanometer-scale vibrations, could a mother's clothing transcend its conventional role and become a health monitor, picking up on the acoustic signals and subsequent vibrations that arise from her unborn baby's heartbeat and motion?” Fink says. “Could a simple and affordable worn fabric allow an expecting mom to sleep better, knowing that her fetus is being listened to continuously?”
The proposed maternity shirt could measure fetal heart and breathing rate, and might be able to give an indication of the fetal body position, he says. In the final stages of development, he and his colleagues hope to develop machine learning approaches that would identify abnormal fetal heart rate and motion and deliver real-time alerts.
A basalt house in Iceland
In the land of volcanoes, Skylar Tibbits wants to build a case-study home almost entirely from the basalt rock that makes up the Icelandic landscape.
Architects are increasingly interested in building using one natural material — creating a monomaterial structure — that can be easily recycled. At the moment, the building industry represents 40 percent of carbon emissions worldwide, and consists of many materials and structures, from metal to plastics to concrete, that can’t be easily disassembled or reused.
The proposed basalt house in Iceland, a project co-led by J. Jih, associate professor of the practice in the Department of Architecture, is “an architecture that would be fully composed of the surrounding earth, that melts back into that surrounding earth at the end of its lifespan, and that can be recycled infinitely,” Tibbits explains.
Basalt, the most common rock form in the Earth’s crust, can be spun into fibers for insulation and rebar. Basalt fiber performs as well as glass and carbon fibers at a lower cost in some applications, although it is not widely used in architecture. In cast form, it can make corrosion- and heat-resistant plumbing, cladding and flooring.
“A monomaterial architecture is both a simple and radical proposal that unfortunately falls outside of traditional funding avenues,” says Tibbits. “The Bose grant is the perfect and perhaps the only option for our research, which we see as a uniquely achievable moonshot with transformative potential for the entire built environment.”
Share this news article on:
Related links.
- Bose Fellows
- Office of the Provost
- Iwnetim Abate
- Andrew Babbin
- Skylar Tibbits
- Department of Earth, Atmospheric and Planetary Sciences
- School of Architecture and Planning
Related Topics
- Climate change
- Alternative energy
- Construction
- Carbon sequestration
- Oceanography and ocean engineering
- Electrical Engineering & Computer Science (eecs)
Related Articles

MIT announces 2023 Bose Grants for daring new research

MIT announces 2022 Bose grants for ambitious ideas

A fabric that “hears” your heart's sounds
Scientists build new atlas of ocean’s oxygen-starved waters
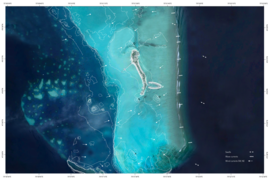
Transformation by design
Previous item Next item
More MIT News

Three from MIT awarded 2024 Guggenheim Fellowships
Read full story →

A musical life: Carlos Prieto ’59 in conversation and concert

Two from MIT awarded 2024 Paul and Daisy Soros Fellowships for New Americans

MIT Emerging Talent opens pathways for underserved global learners

The MIT Edgerton Center’s third annual showcase dazzles onlookers

Seven from MIT elected to American Academy of Arts and Sciences for 2024
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2021
The role and value of science in shark conservation advocacy
- David S. Shiffman 1 nAff4 ,
- Catherine C. Macdonald 2 , 5 ,
- S. Scott Wallace 3 &
- Nicholas K. Dulvy 1
Scientific Reports volume 11 , Article number: 16626 ( 2021 ) Cite this article
9114 Accesses
6 Citations
292 Altmetric
Metrics details
- Environmental impact
- Ichthyology
- Marine biology
- Sustainability
Many species of sharks are threatened with extinction, and there has been a longstanding debate in scientific and environmental circles over the most effective and appropriate strategy to conserve and protect them. Should we allow for sustainable fisheries exploitation of species which can withstand fishing pressure, or ban all fisheries for sharks and trade in shark products? In the developing world, exploitation of fisheries resources can be essential to food security and poverty alleviation, and global management efforts are typically focused on sustainably maximizing economic benefits. This approach aligns with traditional fisheries management and the perspectives of most surveyed scientific researchers who study sharks. However, in Europe and North America, sharks are increasingly venerated as wildlife to be preserved irrespective of conservation status, resulting in growing pressure to prohibit exploitation of sharks and trade in shark products. To understand the causes and significance of this divergence in goals, we surveyed 155 shark conservation focused environmental advocates from 78 environmental non-profits, and asked three key questions: (1) where do advocates get scientific information? (2) Does all policy-relevant scientific information reach advocates? and (3) Do advocates work towards the same policy goals identified by scientific researchers? Findings suggest many environmental advocates are aware of key scientific results and use science-based arguments in their advocacy, but a small but vocal subset of advocates report that they never read the scientific literature or speak to scientists. Engagement with science appears to be a key predictor of whether advocates support sustainable management of shark fisheries or bans on shark fishing and trade in shark products. Conservation is a normative discipline, and this analysis more clearly articulates two distinct perspectives in shark conservation. Most advocates support the same evidence-based policies as academic and government scientists, while a smaller percentage are driven more by moral and ethical beliefs and may not find scientific research relevant or persuasive. We also find possible evidence that a small group of non-profits may be misrepresenting the state of the science while claiming to use science-based arguments, a concern that has been raised by surveyed scientists about the environmental community. This analysis suggests possible alternative avenues for engaging diverse stakeholders in productive discussions about shark conservation.
Similar content being viewed by others

Novel data show expert wildlife agencies are important to endangered species protection

Conservation prioritization can resolve the flagship species conundrum
A global analysis of coral reef conservation preferences
Introduction.
The most effective and appropriate way to conserve and manage the environment has long been debated 1 , exemplified in the United States by the rational utilization approach of Gifford Pinchot (i.e., active management of resources with the goal of exploiting them with minimal impacts on the environment) versus the preservationist approach of John Muir (i.e., fully protect areas of pristine wilderness and associated wildlife). There is broad public agreement about which of these approaches to apply to some environmental problems; few Americans would dispute that the great whales are “wildlife” to be preserved from exploitation while anchovies are “natural resources” to be sustainably exploited (for more on the framing and language around these issues, see 2 ). Conflicts within the environmental community, and between the environmental community and other stakeholder groups, can occur when there is a dispute over which philosophical approach applies to specific environmental issues 3 . The debate over the best approach to take when conserving and managing chondrichthyan fishes (sharks and their relatives, though in this paper we focus exclusively on sharks) is an interesting case study of such a conflict. Some stakeholders assert that sharks are natural resources to be sustainably exploited, and others assert that sharks are wildlife to be preserved.
Chondrichthyan fishes (sharks, rays, skates and chimaeras) comprise over 1200 species, and include some of the most threatened vertebrates on Earth, due largely to overfishing 4 . However, one quarter of species are considered Least Concern by the IUCN Red List 4 , and many are sustainably exploited 5 . Since sharks can be ecologically important [e.g., 6 ] and are a popular encounter for scubadivers and other marine tourists [e.g., 7 ], issues surrounding their conservation have resulted in significant public interest and concern 8 .
Possible conservation solutions for threatened sharks can be broadly categorized into two main policy families: “target-based” solutions (e.g., traditional fisheries management) which are intended to maximize sustainable fisheries exploitation, and “limit-based” solutions (e.g., bans on shark fishing within a country’s entire EEZ termed “Shark Sanctuaries,” or bans on the sale of shark fins), which are intended to ban all fisheries exploitation and trade of all sharks regardless of the sustainability of a particular fishery or the health of a particular stock 9 . While conservationists often generally agree on broad principles and goals 10 , mutually exclusive goals (i.e., a nation cannot simultaneously promote sustainable fisheries while banning all fishing at least in the same place and time) can create disagreement or even conflict between different environmental groups or between stakeholders 11 . While a country’s management strategy can certainly contain a mixture of target-based policies and limit-based policies (e.g., sustainable fisheries exploitation for species whose populations can withstand fishing pressure and no-take marine protected areas,) it is notable that some limit-based policies are intended to be nationwide in nature and preclude the inclusion of target-based policies into a management framework. Generally, the goal of most industrialized nations’ fisheries management strategies is sustainable fisheries management for species that can withstand fishing pressure, and this has been true for a long time 9 .
Support for limit-based policies is sometimes tied to a belief that sustainable shark fisheries cannot or do not exist 12 and that bans are therefore the only possible solution. Support for target-based policies is likely to correlate with beliefs that sustainable shark fisheries can and do exist, and offer social, economic, or conservation benefits not available under limit-based bans, including contributions to food security and local livelihoods, as well as fisheries-dependent data-gathering. The scientific literature is clear that shark fisheries can 13 and do 5 , 14 exist, though the majority of the world’s shark fisheries are currently unsustainable and many likely cannot be made sustainable under current management regimes. While proponents of limit-based policies often point to genuine evidence-based concerns about the practicality of implementing sustainable fisheries for sharks, some limit-based proponents sometimes appear to misunderstand or misrepresent the science 9 , 11 .
Conservation and management planning has long been dependent on expert opinions, especially those of professional scientists [see 15 for how this system works for shark management in the United States]. A recent survey of members of the American Elasmobranch Society, the oldest and largest professional society focusing on the scientific study and management of chondricthyan fishes, found that majorities of surveyed scientists agree that sustainable shark fisheries are possible (84%), currently exist in the world today (83%,) and should be the goal of conservation advocacy over bans when possible (90%) 11 . These AES scientists also showed significantly greater support for target-based policies versus limit-based policies. Additionally, surveyed experts expressed concern that some environmental non-profit groups working in the shark conservation space were focusing on problems that the data showed were not the most critical problems, advocating for ineffective solutions, and/or in some cases miscommunicating or misunderstanding information related to shark conservation threats and policy solutions 11 . While values-based approaches to conservation have contributed significantly to numerous successful advocacy campaigns (e.g., early anti-shark-finning advocacy focused on both the sustainability concerns and the animal welfare concerns bringing together multiple stakeholder groups), issues can arise when proponents of such an approach use misleading or inaccurate appeals to scientific authority to support their values-based advocacy 16 .
This paper represents a case study on sharks which illuminates philosophical divisions over exploitation versus preservation more broadly, and discusses how these divisions have contributed to difficulties in communication around conservation issues between scientific experts and some members of the shark conservation advocacy community. Here we report on the results of a survey sent to employees of environmental non-profit organizations who work on shark conservation advocacy and public education. The survey focused on three general questions: (1) Where do environmental advocates get their scientific information? (2) Does all relevant scientific information reach environmental advocates? and (3) Do environmental advocates report working towards the stated policy preferences of scientists?
Identification of shark conservation NGO employees
We identified 78 environmental non-profits that participate in shark conservation advocacy or public education in the English-speaking world (Supplementary materials Table S1 ) using a combination of internet search engine searches for shark conservation advocacy, our own records from combined decades working on ocean conservation science issues, and snowball sampling (i.e., asking contacted advocates to suggest other organizations we should be sure to include).
Representatives of each non-profit were contacted via e-mail and asked to provide a list of contact information for anyone at their organization or partner organizations who works directly on these issues. We compiled a base list of 155 names of employees of environmental non-profit groups whose job focuses on shark conservation advocacy and/or public education (henceforth “environmental advocates”). Shark conservation advocacy and public education was defined broadly to capture as much of the diversity of thought and action in this space as possible, but did not include employees of non-profit groups whose primary duties included scientific research, as the intended focus of this study is individuals engaged in advocacy, outreach, and education.
These 155 individual environmental advocates were sent an individualized but anonymized link to participate in a voluntary online survey hosted through SurveyMonkey.com. The survey included 49 questions, focusing on environmental advocates’ perspectives on shark conservation policies and relevant science (Supplementary Materials Table S2 ). Respondents were promised no compensation for completing the survey and were permitted to stop answering questions at any time.
We included questions relating to demographic background (age, gender, education), NGO background (size, scope, role in the advocacy process), science knowledge and attitudes (questions concerning advocates’ past experience working with scientists, their perspectives on the state of scientific research, and how well-versed they are in the current scientific literature) conservation background questions (thoughts on threats facing sharks and the general issues surrounding shark conservation), and policy preferences (support for and opposition to certain specific shark conservation policies).We also asked about awareness of rebuttals (scientific papers disputing the results of previous scientific papers), which were used as a proxy for awareness of technical information related to shark conservation and management. All respondents were offered the opportunity to participate in 30-min follow-up interviews over Skype, and eight respondents chose to participate in a follow-up interview. These respondents included representatives from four countries, employed by large ocean focused non-profits as well as small regional shark-focused non-profits. Responses from follow-up interviews were used only to provide representative examples of certain attitudes and no statistical analysis was performed on these results.
Average completion time of the survey was 43 min. Respondents who did not complete the entire survey had the answers they did provide analyzed, and the blank responses were not counted. This research is approved under Simon Fraser University’s Office of Research Ethics permit # 2017-S0524. All research was performed in accordance with all relevant human subjects research ethics guidelines and regulations, and as explicitly stated in the recruitment materials, participation in the survey constituted informed consent.
Statistical analysis
Analyses focused on determining patterns in support of or in opposition to certain conservation policies, as well as patterns in awareness of scientific background information. Variables analyzed included demographic background of the respondent and information about their employer (e.g., are people that work for large non-profits with easy access to scientific expertise more likely to support the policy goals identified by scientists than employees of smaller non-profits without easy access to scientific expertise, etc.) All statistical analysis was performed using R software (R version 3.5.2 2018-12-20—"Eggshell Igloo").
We used conditional inference trees to determine the primary partitioning variables (demographic or NGO background) responsible for particular outcomes (awareness of scientific research and support for certain policies) using the PartyKit package in R 17 . These trees highlight which variable is responsible for the greatest difference in output by fitting models to each combination of variables, and splitting the dataset at the variable associated with the greatest divergence in output. Conditional inference trees were run to determine the primary partitioning variables associated with the following outputs: general preference for sustainable fisheries or for total bans, support for or opposition to shark fin trade bans, awareness that sustainable shark fisheries exist, and technical understanding of the state of published scientific evidence concerning sustainable shark fisheries.
Demographics of survey respondents
More than half of contacted environmental advocates completed the survey (54.2% response rate, N = 84). However, many questions were optional and only applicable for respondents who had worked on particular issues. The response rate on optional questions ranged from 26.2 to 67.8% While this is a low N overall, we believe that it represents a major segments of the entire English-speaking world’s population of shark conservation advocates and educators, as our method for identifying non-profits to approach for this survey included extensive personal records, search engine searches, and the opportunity for non-profits we contacted to suggest collaborators working for other groups. However, this approach may have missed smaller regional non-profits outside of North America and Europe who do not have a large online presence and have not worked extensively with international collaborators. Fifty-seven percent of respondents identified as female, and one respondent preferred not to answer this question. Respondents ranged in age from 24 to 70, with a mean age of 41 years old. Age or gender were not significantly correlated with any variables measuring understanding of current science or support for any management policy and were not analyzed further. Thirty-seven respondents have a Master’s degree, seventeen have a Ph.D, and the remainder have undergraduate degrees or did not answer this question.
Non-profits were classified by scope (shark-focused, ocean-focused including but not limited to sharks, or focused on all environmental issues including but not limited to sharks), area of geographic focus, and size (number of employees). Forty-six percent of respondents worked for an ocean-focused conservation NGO that included a shark portfolio (e.g., Oceana), while thirty-two percent worked for an exclusively shark-focused NGO (e.g., the Shark Trust). The remaining twenty-two percent of respondents worked for large NGOs that focus on a variety of land and sea conservation issues (e.g., World Wildlife Fund). Of those who did not claim that the scope of their work was global, the largest proportion of respondents worked in North America and Europe, followed by the Caribbean, South Africa, and Southeast Asia. Some respondents also worked in Central America, Brazil, the South Pacific, and Australia (Fig. 1 A).
( A ) Where respondents primarily work, excluding those who responded with “worldwide,” with bubbles scaled using PowerPoint ( B ) distribution of the size of non-profits (by number of employees) that respondents self-report working for.
Respondents reported that the size of their non-profit ranged from zero paid employees (i.e., all volunteer) to over 5000, with a median of 14 employees (Fig. 1 B). Twenty-four respondents reported working for a non-profit with more than 100 employees, 36 reported working for a non-profit with fewer than 5 employees.
Where do environmental advocates get their scientific information?
Two-thirds of respondents indicated that they regularly read several peer-reviewed primary scientific literature articles each month, and only eight percent of respondents indicated that they had never read a peer-reviewed primary literature article. All respondents who reported having never read the literature worked for a non-profit with fewer than 10 employees. Fifty-five percent of respondents indicated that they had been a coauthor or lead author on at least one peer-reviewed primary scientific literature article.
Fifty-six percent of respondents reported that scientists were directly employed by their non-profit, and 16% reported that their non-profit had a formal scientific advisory board composed of independent scientists who were available for technical consultation. Only 12% of respondents indicated that their NGO did not work with any scientists in any capacity (each of the 8% of respondents who reported never having read a scientific article also reported never working with scientists in any capacity). Of the respondents who did not work with scientists in any capacity, 75% reported working for very small (less than five employees) shark-focused NGOs in Europe and North America.
Respondents reported that science-based arguments were by far the most commonly advanced arguments for shark conservation used by their NGO (Fig. 2 , Supplementary Materials Table S3 ), especially the idea that shark population declines can cause negative ecosystem-wide effects. Respondents reported that moral or values-based arguments were much less frequently employed than science-based arguments.
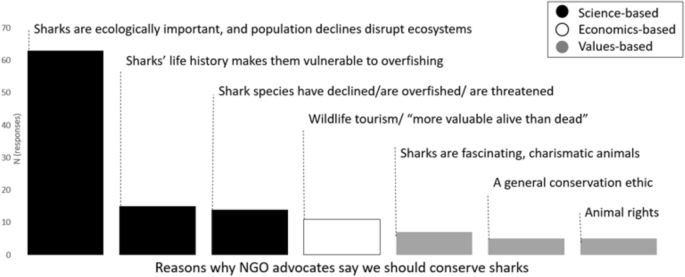
Categorized responses to the question “What arguments does your NGO make to conserve sharks,” with science-based, economics-based, or emotional arguments as possible categories.
Does all relevant scientific information reach environmental advocates?
Surveyed environmental advocates were most aware of scientific papers showing severe shark population declines and papers showing negative ecological consequences of those declines (97% and 100% awareness respectively). While rebuttals to those papers (72.7% awareness) and papers showing that that sustainable shark fisheries are possible (82.6% awareness) were still widely known, they had lower awareness among respondents compared with papers showing population declines and ecological consequences of those declines. Some widely-publicized papers about shark conservation have been considered somewhat controversial by other scientists in the field resulting in rebuttals. Rebuttals can be seen as an attempt to correct the scientific record by pointing out an error in the first paper, therefore awareness of the rebuttals was considered to be a proxy for technical knowledge related to the current state of scientific evidence of shark conservation and management issues.
In follow-up interviews, respondents reported actively looking for rebuttals whenever they found a paper that appeared to support their perspective. One explained “ We look for it all, and we’re always open to using new science that comes along and tells us something different. ” Another said, “ We always try and include rebuttals and contradictory data to provide the whole picture. ” A third told interviewers that “ It’s too easy to see a paper that justifies your claim that you can jump on and use, but how valid is that? ” Interviewees consistently stressed the importance of seeking out data that not only supported their arguments but was scientifically valid.
Some of the environmental non-profit advocates surveyed here also raised concerns about the focus of most shark research produced by academic scientists. Several respondents suggested that research could be made more useful by broadening the current focus (a few well-studied species in a few well-studied regions) to include less charismatic species and less-visited study sites, especially those in the developing world. Respondents also raised concerns about scientists claiming to do conservation-relevant research without consulting managers, local colleagues, or members of the impacted community to see what kind of data would be most useful. One follow-up Skype interview participant said that if scientists want to do policy-relevant research, “ The first step is to try and identify information needs of policymakers; they know what they don’t know and what they need to know- talk to them as early as possible when starting a research project! ”.
Respondents also suggested new roles for scientists, such as serving as public educators or advocates for conservation by communicating their research to the public. Several respondents from the developing world suggested the scientists from wealthy institutions or nations should cease engaging in “helicopter science” or “parachute science” 18 by visiting faraway places and leaving as soon as their research was done (while this term can also refer to exploitative relationships with geographically proximate indigenous communities, we note that our respondents were clear that they meant international applications of this termInstead, respondents request that visiting scholars provide training and opportunities to colleagues and students in the developing world to develop local capacity. There were also calls for more research on the human dimensions of shark conservation, including socioeconomic studies of shark fishers.
Do environmental advocates work towards the stated policy preferences of scientist?
Over half (56%) respondents correctly identified that published scientific evidence shows that sustainable shark fisheries are possible, and nearly half (46%) correctly identified that published scientific evidence shows that sustainable shark fisheries exist in the world today (there is no factual or scientific doubt that such fisheries can and do exist; while preferring bans based on personal values is a valid approach, claiming that bans are universally necessary because sustainable fisheries are scientifically impossible is a misrepresentation of the science). More than three-quarters (78%) of respondents agreed with the statement that the goal of shark conservation advocacy should be to promote sustainable exploitation instead of complete bans on exploitation and trade (Fig. 3 , Supplementary Materials Table S4 ). In each case, significantly fewer NGO employees than previously-surveyed AES scientists agreed with these statements.
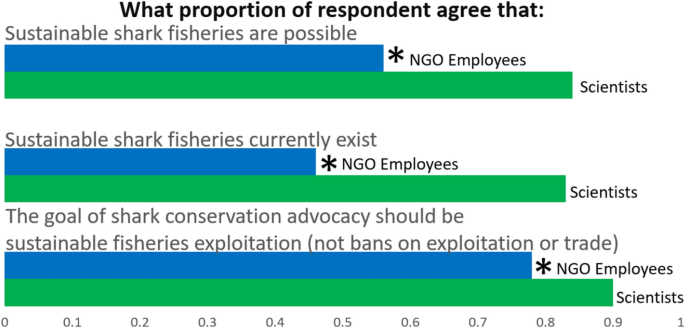
Proportion of environmental advocate respondents from this study (blue) and scientists respondents from Shiffman and Hammerschlag 2016B (green) who agree with these three questions, with * indicating a significant difference in responses to each question between the two groups using a 2-tailed Z test of independent proportions (for Question 1, Z = − 3.66, P = 0.0003, for Question 2, Z = − 4.48, P < 0.00001, for Question 3, Z = − 1.87, P = 0.03).
Results show that in general, the environmental advocates who most strongly supported bans on fisheries and trade were the least familiar with the current state of scientific knowledge on sustainable shark fisheries. While many of these respondents reported that science was important to advocacy and that their arguments were based on science, many arguments misrepresented the state of the science. A conditional inference tree found that the primary partitioning variable associated with general support for bans on trade was self-reported regularity of reading the scientific literature; 100% of respondents who report never reading the scientific literature (N = 4 of 4) supported bans over sustainable fisheries, compared with just 10.5% of respondents who reported regularly reading the scientific literature (N = 4 of 38), (Figs. 4 , 5 ). There was also a clear geographic bias with respondents who worked in the developed world more likely to support bans than those working in the developing world (Fig. 4 ).
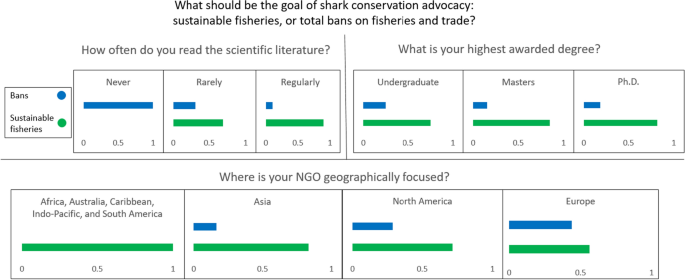
Proportion of environmental advocate respondents who agree with the statement that sustainable fisheries (as compared with bans on all fishing and trade) should be the goal of shark conservation, broken down by regularity of reading the literature, highest degree earned, and geographic area of focus.
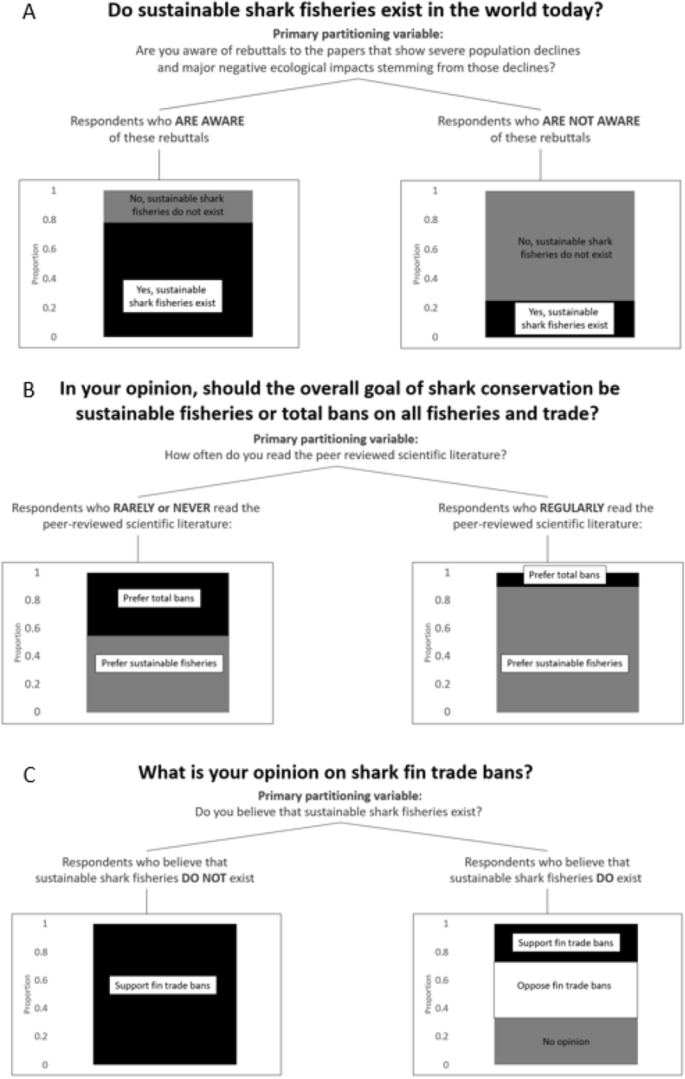
Results of conditional inference trees for the questions “should the goal of shark conservation be sustainable fisheries or total bans on fisheries and trade,” “do you support shark fin trade bans,” and “do sustainable shark fisheries exist in the world today,” showing primary partitioning variables for each and the results of separating the data by those partitioning variables.
Of respondents who supported a total ban on all exploitation and trade over sustainable fisheries, 86% work in the US or Europe, while 100% of respondents who work in South America (N = 3), the Indo Pacific (N = 1), the Caribbean (N = 1), or Africa (N = 1) who answered this question preferred sustainable fishing over bans (Fig. 4 ). One-third (N = 7) of respondents who work at shark-specific environmental non-profits support bans over sustainable fisheries, compared to just 12% (N = 3) of those who work in ocean-focused environmental non-profits. Fifty percent of all stated preferences for bans over sustainable fisheries came from advocates working for very small (less than five employees) non-profits.
Respondents who did not agree that sustainable shark fisheries are possible or exist cited a variety of reasons, ranging from not having personally seen evidence of sustainability to technical concerns to a general overarching belief that sustainable fisheries in general cannot and do not exist (Table 1 ).
The individual who reported never having personally seen any evidence of sustainable shark fisheries also reported never having read the scientific literature and never interacting with professional scientists. Those respondents who did agree that sustainable shark fisheries are possible and exist mostly cited peer-reviewed published technical literature to support their opinion (Table 2 ), though several noted that while these fisheries can and do exist, there are not very many of them, and not all shark fisheries are potentially sustainable.
This suggests that while some advocates are not aware of the current state of science on this topic or are misinformed about it, for some, the issue is less about knowledge of whether sustainable shark fisheries are possible in theory than about whether they personally believed successful implementation of sustainable management practices was probable or practical in the complex real world—there are certainly many examples of poorly managed shark fisheries, a point broadly understood both by those advocating for improving fisheries sustainability and those advocating for bans.
One respondent explicitly mentioned misinformation from other non-profits as a possible cause of public misunderstanding on the issue of sustainable shark fisheries. A conditional inference tree indicated that the primary partitioning variable driving agreement with the statement that sustainable shark fisheries exist was awareness of rebuttals to high-profile shark conservation papers, which was used as a proxy for awareness of the current state of technical literature (Fig. 5 ). Only 24% of respondents who were unaware of those rebuttals agreed that sustainable shark fisheries exist, compared to 78% of those who were aware of these rebuttals. Of respondents who report regularly reading the scientific literature, 88.6% (N = 39) agree that there are current examples of sustainable shark fisheries, compared with just 50% (N = 2) of those who never read the literature and 62% (N = 10) of those who rarely read the literature. Sixty-five percent of all respondents who agree that sustainable fisheries do not exist come from the US or Europe, and no respondents in the Caribbean, South America, or Africa agreed with the statement that sustainable shark fisheries do not exist.
A plurality of respondents (45.8%) agreed with the statement that the science concerning the sustainability of shark fisheries is currently uncertain, and 15.2% of respondents agreed with the statement that the science is clear that sustainable shark fisheries cannot and do not exist. Sixty-five percent of respondents with a Masters or Ph.D. degree correctly identified that the science is clear that sustainable shark fisheries can and do exist (and 0 respondents with a Masters of Ph.D. degree indicated that the science is clear that sustainable shark fisheries cannot and do not exist). There was also a divide by familiarity with the scientific literature, none of the respondents who never read scientific papers correctly identified that the science is clear that sustainable shark fisheries can and do exist, compared with 26.6% of those who rarely read the literature and 45.2% of those who regularly read the literature. Fifty percent of respondents who never read the literature inaccurately reported that they agree that the science is clear that sustainable shark fisheries cannot and do not exist, compared with 26.6% of those who rarely read the literature and 9.5% of those who regularly read the literature. Nine percent of respondents reported that their opinions about shark fisheries come from their personal ethical values, and therefore scientific measures of sustainability are not relevant to their decision-making on this topic.
Fifty-four percent of respondents reported that their environmental non-profit organization has worked on fisheries management tools in the last five years, compared with lower numbers of those whose employer worked on no-take marine protected areas (45.2%), fin bans (25%), and Shark Sanctuaries (16.7%). Many respondents noted that different contexts (cultural, political, and economic) require different kinds of solutions, that there is no one “silver bullet” policy for shark conservation, and that enforcement of existing management rules is critical no matter which policy strategy is selected. Significantly more respondents support traditional fisheries management tools (73.3%) than support either Shark Sanctuaries (49.1%) or shark fin trade bans (41%), and significantly more respondents oppose Shark Sanctuaries (30.1%) and shark fin trade bans (32.7%) than oppose traditional fisheries management tools (5%) (Fig. 6 ). There was no difference in support for or opposition to no-take marine protected areas versus traditional fisheries management tools. The only respondent who strongly disagreed with traditional science-based fisheries management tools also reported never reading the literature or interacting with a scientist.
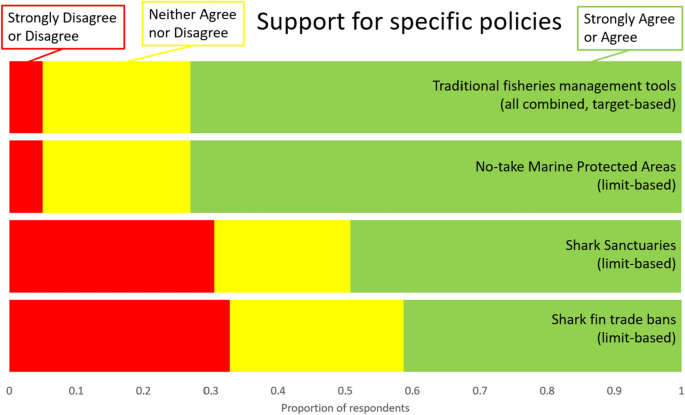
Proportion of respondents who support, oppose, or have no opinion concerning a variety of conservation policies. All target-based traditional fisheries management tools were grouped together because there were no differences in responses.
A conditional inference tree indicated that the primary partitioning variable associated with support for shark fin trade bans was agreement with the statement that sustainable shark fisheries cannot exist (Fig. 5 ); 100% of respondents who agree that sustainable shark fisheries cannot exist support shark fin trade bans, compared with 24% of respondents who agree that sustainable shark fisheries can exist. Respondents with a Ph.D. showed the least support for shark fin bans (18.1%, compared to 43.7% support from respondents with a Bachelor’s degree and 54.1% support from respondents with a Masters). Respondents who regularly read the literature had the lowest support for fin bans (31.5% support, compared with 57.1% support from those who rarely read the literature and 75% support from those who never read the literature) (Fig. 7 ). The only two geographic regions where more respondents supported fin bans than opposed them were Europe (56.2% support) and North America (60% support). In Asia, 50% of respondents opposed fin bans compared to 33.3% who support them. No respondents from the Caribbean supported fin bans. The Indo-Pacific and South America had equal numbers of supporters and opponents.
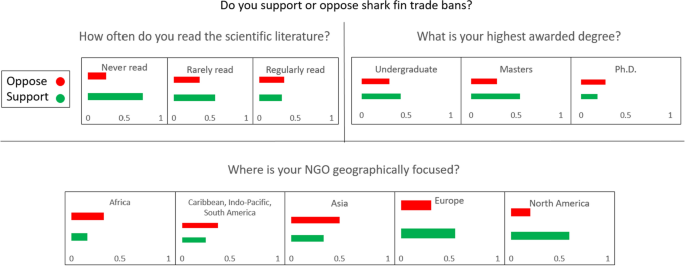
Proportion of respondents who support or oppose shark fin bans (neutral responses/no opinion removed), broken down by regularity of reading the literature, highest degree earned, and geographic area of focus.
We reveal here some of the possible drivers of poor communication and misunderstanding between stakeholder groups concerned about shark conservation. Our results provide important context about factors shaping policy preferences for conservation groups, showing further evidence of a target-based versus limit-based schism in shark conservation advocacy. We also provide information that may help explain how that schism formed, and potential strategies for improving communications.
Many environmental advocates in the shark conservation space work for scientifically informed and scientifically engaged non-profits—these environmental advocates regularly read the scientific literature, regularly engage with scientists, and work towards some of the policy preferences supported by scientists. This suggests that science can indeed influence and assist with advocacy and policy change, heartening information for scientists who want their research to make a difference 19 .
However, a number of smaller shark conservation non-profits are not scientifically informed, even as some of these non-profits claim to base their arguments on scientific facts. Employees of these non-profits were not aware of relevant scientific information, and in some cases mischaracterized relevant scientific information while supporting policies that are the least supported (and most opposed) by surveyed scientists. There is no doubt that conservation is a normative discipline and values can play an important role; animal welfare concerns rather than sustainability concerns drove some of the earliest restrictions on shark finning, for example. However, ignoring relevant science can lead to suboptimal policy outcomes 16 , and evidence suggests that focusing only on the perceived cruelty of shark finning and not on the unsustainability of associated overfishing of sharks did nothing to reduce overall shark mortality in the Pacific Ocean 20 . Environmental advocacy based on moral or ethical beliefs is entirely valid and can play an important role in driving policy change, but interviewees repeatedly noted that it is problematic to offer false or misleading information as part of “science-based” arguments for conservation. In other words, approaching conservation discussions with arguments that do not involve scientific facts is a valid and important approach, but when claiming to use scientific facts it is important that those facts be accurate.
It is also worth noting that many of the concerns about the current focus of scientific research raised by surveyed environmental advocates are legitimate, and any scientist concerned about misrepresentation of science by advocates should also be concerned with improving the quality and diversity of conservation-relevant scientific data. Calls for scientists to focus our efforts on species that have not already been well-studied or on places where there is little scientific infrastructure in place (and to develop local capacity while doing so) point to a genuine problem with the state of shark research. Requests that scientists learn how policymaking works so they can generate more policy relevant research illustrates one way that science could provide greater value to managers. Increased engagement with the public is another worthy avenue to pursue, especially in a field where many scientific experts hope to use their data to improve management and conservation outcomes 11 , 21 .
While many advocates report regularly reading the literature, not all literature is equally well-known. Research showing severe population declines and the negative ecosystem consequences of those declines is better known than rebuttals to those papers, supporting the assertion by 22 that rebuttals have value, but may not substantially adjust future usage of a rebutted paper. It should also be noted that smaller non-profits who do not read the scientific literature may simply not have the resources to afford access to scientific journals and databases, which can be quite expensive [see 23 ]. Though several employees of non-profits surveyed here explicitly stated that this was not the case for them, it is possible that easier, more affordable access to published literature and expert scientific advice for smaller NGOs could help address some of this divide.
Advocates who regularly read the literature, advocates based in the developing world, and advocates with advanced degrees supported sustainable fisheries more than those who never or rarely read the literature, those in North America and Europe, and those without advanced degrees, respectively. While it is important to note that skeptics of sustainable shark fisheries management raised valid concerns regarding the historical unsustainability of shark fisheries and difficulties with implementing sustainable management in some nations, it is demonstrably true that sustainable shark fisheries can and do exist 5 , and claims to the contrary misrepresent the state of scientific knowledge. It is also noteworthy that supporters of the goal of sustainable fisheries are more likely to come from the developing world where such fisheries are relatively rare, and supporters of total bans are more likely to come from developed nations where successful sustainable fisheries management is more common. This may be because a ban on fishing is less feasible in less prosperous nations where fishing is an economically critical activity vital to local food security. Additionally, some types of animal welfare concerns (e.g., animal cruelty in food production) may be more common in developed nations.
While a majority of advocates work towards target-based fisheries management policies, some work towards the policies least supported by scientists. Interestingly, while of course some shark fin trade ban proponents are scientifically informed and engaged, it is noteworthy that more environmental non-profit employees than scientists 11 oppose shark fin bans, for essentially the same reasons given by scientists 14 . It is also noteworthy that support for fin trade bans is higher among those who never or rarely read the literature than among those who read it regularly.
Conclusions
Surveyed scientists in 11 raised concerns that some non-profit groups are not focusing on what scientific experts perceive as the most important problems, are not accurately describing the state of shark conservation threats, and are advocating for solutions not supported by the best available scientific data. Instances of those concerns were represented here primarily, but not exclusively, by employees of NGOs with certain shared characteristics: very small (less than 5 employees), based in North America or Europe, and employing advocates who report never reading the scientific literature or communicating with scientists. However, it should be noted that there are some very small NGOs who regularly work with scientists in support of science-based policies (e.g., Shark Advocates International), and it should be noted that some large NGOs who employ scientists have supported limit-based policies (e.g., the Pew Charitable Trusts with Shark Sanctuaries, and Oceana with shark fin trade bans), so size and interaction with scientists are clearly not the only predictors of policy preferences.
Typically (but not always), environmental policy change involves science and advocacy working together towards the same goal 16 , 24 and therefore it is concerning to see stark differences in messaging between some high-profile shark advocacy campaigns and the policy recommendations of scientific experts—especially when some messaging uses demonstrably false information. Our results illustrate that despite notable instances of misinformation, many environmental non-profit employees work regularly with scientists, read the scientific literature, and support science-driven policy goals. This survey’s results also point to an opportunity to bring multiple forms of expertise [see 25 ] and multiple perspectives to bear on conservation problems. Additionally, while scientists have previously identified scientifically inaccurate claims by advocates as a problem, advocates also point to meaningful, actionable ways scientists can increase the real-world relevance of their supposedly policy-relevant research: by engaging with the public, focusing on species and locations that are understudied, and committing long-term to research sites, local communities, and the development local scientific capacity. Advocates’ expertise and experience also includes and recognizes practical dimensions sometimes underrepresented in scientific research. For example, while it is a scientific fact that sustainable shark fisheries can and do exist and claims to the contrary are inaccurate, some advocates accurately point out that sustainable shark fisheries are uncommon, difficult to ensure, and present significant practical management challenges. While misrepresenting science is a problem, insights from stakeholders with diverse values, perspectives, and forms of expertise, including from those engaged in values-based approaches to advocacy, should be represented in discussions about shark conservation. The addition of these perspectives would make some conversations about conservation and management and associated trade-offs more nuanced and useful. This survey identified a communications problem in which some advocates may not have access to or be aware of certain scientific data; however, our results also highlight potential opportunities to more effectively address conservation problems through increased engagement between groups that too often talk past one another.
Data availability
In keeping with the terms of our human subjects research ethics permits, an anonymized version of the dataset used in this study with any possible identifying information removed is available upon request.
Callicott, J. B. A brief history of American conservation philosophy. in Sustainable Ecological Systems: Implementing an Ecological Approach to Management (eds Covington, W. W. & DeBano, L.F.) 10–14. Gen. Tech Re RM-247 (Rocky Mountain Forest and Range Experiment Station, 1993).
Scott, J. C. Seeing Like a State: How Certain Schemes to Improve the Human Condition have Failed (Yale University Press, 1998).
Google Scholar
Miller, T. R., Minteer, B. A. & Malan, L. C. The new conservation debate: The view from practical ethics. Biol. Conserv. 144 (3), 948–957 (2011).
Article Google Scholar
Dulvy, N. K. et al. Extinction risk and conservation of the world’s sharks and rays. elife 3 , e00590 (2014).
Simpfendorfer, C. A. & Dulvy, N. K. Bright spots of sustainable shark fishing. Curr. Biol. 27 (3), R97–R98 (2017).
Article CAS Google Scholar
Heithaus, M. R., Frid, A., Wirsing, A. J. & Worm, B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23 (4), 202–210 (2008).
Gallagher, A. J. & Hammerschlag, N. Global shark currency: The distribution, frequency, and economic value of shark ecotourism. Curr. Issues Tour. 14 (8), 797–812 (2011).
Simpfendorfer, C. A., Heupel, M. R., White, W. T. & Dulvy, N. K. The importance of research and public opinion to conservation management of sharks and rays: A synthesis. Mar. Fresh. Res. 62 (6), 518–527 (2011).
Shiffman, D. S. & Hammerschlag, N. Shark conservation and management policy: A review and primer for non-specialists. Anim. Conserv. 19 (5), 401–412 (2016).
Sandbrook, C., Fisher, J. A., Holmes, G., Luque-Lora, R. & Keane, A. The global conservation movement is diverse but not divided. Nat. Sust. 2 (4), 316–323 (2019).
Shiffman, D. S. & Hammerschlag, N. Preferred conservation policies of shark researchers. Conserv. Biol. 30 (4), 805–815 (2016).
Lawrence, A. N. I. S. S. A. Collaborations for conservation. In Sharks: Conservation, Governance and Management (eds Techera, E. J. & Klein, N.) 135–156 (Routledge, 2014).
Walker, T. I. Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Mar. Fresh. Res. 49 (7), 553–572 (1998).
Shiffman, D. S. & Hueter, R. E. A United States shark fin ban would undermine sustainable shark fisheries. Mar. Pol. 85 , 138–140 (2017).
Stone, R. B., Bailey, C. M., McLaughlin, S. A., Mace, P. M. & Schultze, M. B. Federal management of US Atlantic shark fisheries. Fish. Res. 39 (2), 215–221 (1998).
Ford, A. T. et al. Understanding and avoiding misplaced efforts in conservation. FACETS 6 , 252–271 (2021).
Hothorn, T. & Zeileis, A. partykit: A Modular toolkit for recursive partitioning in R. J. Mach. Learn. Res. 16 , 3905–3909 (2015).
MathSciNet MATH Google Scholar
Stefanoudis, P. V. et al. Turning the tide of parachute science. Curr. Biol. 31 (4), R184–R185 (2021).
Parsons, E. C. M. “Advocacy” and “Activism” are not dirty words-how activists can better help conservation scientists. Front. Mar. Sci. 3 , 229 (2016).
Clarke, S. C., Harley, S. J., Hoyle, S. D. & Rice, J. S. Population trends in Pacific Oceanic sharks and the utility of regulations on shark finning. Conserv. Biol. 27 (1), 197–209 (2013).
Ferry, L. A. & Shiffman, D. S. The value of taxon-focused science: 30 years of elasmobranchs in biological research and outreach. Copeia 2014 (4), 743–746 (2014).
Banobi, J. A., Branch, T. A. & Hilborn, R. Do rebuttals affect future science?. Ecosphere 2 (3), 1–11 (2011).
Cvitanovic, C. et al. Utility of primary scientific literature to environmental managers: An international case study on coral-dominated marine protected areas. Ocean Coast. Manag. 102 , 72–78 (2014).
Phillis, C. C. et al. Multiple pathways to conservation success. Conserv. Lett. 6 (2), 98–106 (2013).
Martin, T. G. et al. Eliciting expert knowledge in conservation science. Conserv. Biol. 26 (1), 29–38 (2012).
Download references
Acknowledgements
The authors would like to thank attendees at a 2018 presentation of this research at Sharks International, as well as attendees of several departmental seminars about this research, for their helpful and constructive advice. We would also like to thank the participants who took this survey and participated in follow-up interviews for sharing their time and expertise.
Author information
David S. Shiffman
Present address: New College of Interdisciplinary Arts and Sciences, Arizona State University, 4701 W Thunderbird Road, Glendale, AZ, 85306, USA
Authors and Affiliations
Earth to Oceans Group, Department of Biological Sciences, Simon Fraser University, 8888 University Drive, Burnaby, BC, V5A 1S6, Canada
David S. Shiffman & Nicholas K. Dulvy
Field School Scientific Consulting, Miami, FL, USA
Catherine C. Macdonald
David Suzuki Foundation, 2211 West 4th Avenue, Vancouver, BC, V6K 4S2, Canada
S. Scott Wallace
Rosenstiel School of Marine and Atmospheric Science, University of Miami, 4600 Rickenbacker Causeway, Miami, FL, 33149, USA
You can also search for this author in PubMed Google Scholar
Contributions
This research was carried out as part of lead author DSS’s Postdoctoral Fellowship in Conservation Biology, funded by the Liber Ero Fellowship and hosted at Simon Fraser University. Authors S.S.W. and N.K.D. were his co-supervisors, and provided advice and guidance throughout the design, analysis, and writeup of this project. Author C.C.M. assisted intensively with survey design and analysis, as well as guidance throughout the rest of the project. The authors with to thank two anonymous referees whose feedback contributed significantly to improving the manuscript. All authors reviewed the manuscript.
Corresponding authors
Correspondence to David S. Shiffman or Nicholas K. Dulvy .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shiffman, D.S., Macdonald, C.C., Wallace, S.S. et al. The role and value of science in shark conservation advocacy. Sci Rep 11 , 16626 (2021). https://doi.org/10.1038/s41598-021-96020-4
Download citation
Received : 10 April 2021
Accepted : 27 July 2021
Published : 17 August 2021
DOI : https://doi.org/10.1038/s41598-021-96020-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Shark leaves teenage boy with 'big gashes' after he was bitten on boat off West Beach
A 16-year-old boy who was bitten in the leg by a shark off Adelaide's coast has been left with "big gashes" but is doing "fine", his family says.
Nathan Ness was on a fishing boat with his brother and sister when they caught the shark, believed to be a young great white, about 1:40pm.
His father Michael Ness, who was also on board, said he removed the hook from the animal's mouth and that they were about to throw it back when it suddenly turned around and snapped.
"We were out fishing and Nathan hooked up to a big shark. He reeled it up to the boat and we hauled it into the boat just to get the hook out and I grabbed a pair of pliers, ripped the hook out of it," Mr Ness said.
"Then he picked it up to throw it back into the water and take a picture of it and as he picked it up, it swung around and grabbed hold."
The teenager was bitten in the lower leg by the shark, which was estimated to be about five feet in length.
"I reached down then and opened its mouth and the shark let go and he hauled it up and threw it back in the water," Mr Ness said.
He expressed relief that the outcome wasn't worse, saying his son was doing well, considering what had occurred.
"He's fine, he just needs stitches. He's got big gashes in his leg," he said.
'The kid is lucky'
Paramedics were called to the West Beach boat ramp about 1:40pm, and the SA Ambulance Service (SAAS) said the boy would be treated in hospital.
Nathan Ness's sister Megan said her brother had been left with minor flesh wounds, and the shark had taken a small "chunk" of his skin.
"He thought it would be cool if he held the tail so he could get a photo with it, and then the shark turned around and bit his leg," she said.
"There were only about three cuts, he's going to need to get stitches though.
"First he probably got shock, he was like 'get it off, get it off'."
She said they initially thought it was a Mako shark, but later found out it was a great white.
Fishermen Brodie and Colin North said it was unusual to catch sharks in waters off West Beach, and said the incident could have been much worse.
"They're lucky they got away with a few stitches considering the size of the shark and how aggressive they are when they're pulled onto a boat, especially given there were three children on board," Brodie said.
"The kid is lucky he didn't get bitten on a main artery."
"I've never seen anyone pull up a shark of any kind here," Colin North said.
"My word he was very fortunate."
There have been several shark attacks — three of them fatal — in South Australian waters in the past 12 months.
- X (formerly Twitter)
Related Stories
Mitigation measures in spotlight after third fatal shark attack in south australia.
- Animal Attacks
- Shark Attacks

IMAGES
VIDEO
COMMENTS
White sharks are highly migratory apex predators, globally distributed in temperate, sub-tropical, and tropical waters. Knowledge of white shark biology and ecology has increased recently based on research at known aggregation sites in the Indian, Atlantic, and Northeast Pacific Oceans; however, few data are available for the Northwest Pacific Ocean. This study provides a meta-analysis of 240 ...
Shark Research Institute (SRI) conducts and sponsors rigorous, peer-reviewed field research about sharks and uses science-based information to educate and advocate for shark conservation policies and protections by the world's governing bodies, including CITES. ... Unprovoked Attacks by White Sharks Off the South African Coast. (1996) Marie ...
Figure 1. Annual number of white shark publications (1980-2018) based on a Web of Science search (12 June 2018) for items with titles including "white shark(s)" and/or "Carcharodon carcharias".The large numbers of publications in 1985, 1996, and 2012 are due to the publication of books or symposium proceedings about white sharks.
In recent years, white sharks (Carcharodon carcharias) have become more accessible to researchers off the northeastern U.S. as feeding aggregation sites have emerged and the population has increased.
We note that the white shark historically declined by an estimated 70% worldwide over the past half-century, ... George Chen Shark Research Center, National Taiwan Ocean University, Center of ...
From 2001-2020, the Monterey Bay Aquarium led an international research collaboration to understand the life cycle, ecology, and behavior of white sharks ( Carcharodon carcharias) in the ...
We found some white sharks to be highly active at depth, sometimes greater than 300 m deep, while others were more active in shallow water (e.g. the interactions with the sea lion was at 10 m depth). White shark hunting behaviour appears very different from other areas with murkier water where pinnipeds are ambushed on the surface [12,15-17].
The white shark (Carcharodon carcharias) is the largest macropredatory fish in the world. ... Apps K, Becerril-García EE et al (2018) Future research directions on the "elusive" white shark. Front Mar Sci 5:455. Article Google Scholar Klimley AP (1985) Areal distribution and autoecology of the white shark off the western coast of North ...
As highly mobile predators with extensive home ranges, some shark species often utilize a continuum of habitats across the continental shelf ranging from the surf zone to the open ocean. For many species, these cross-shelf distributions can change depending on ontogeny or seasonal conditions. Recent research has confirmed a white shark (Carcharodon carcharias) summer nursery off Long Island ...
White sharks are highly migratory and segregate by sex, age and size. Unlike marine mammals, they neither surface to breathe nor frequent haul-out sites, hindering generation of abundance data required to estimate population size. A recent tag-recapture study used photographic identifications of white sharks at two aggregation sites to estimate abundance in "central California" at 219 ...
Methods. White shark occurrence records were collected from numerous sources, including landings data, commercial fishery observer programs, recreational tournament information, scientific research surveys, commercial and recreational fishermen, collaborating scientists, newspaper articles, personal communications, and the scientific literature (, and references therein, , -).
2.1. Study site. Guadalupe Island (29.0528°N, 118.3041°W) is located in the southern region of the California Current, 240 km off the western coast of the Baja California peninsula, Mexico (Figure (Figure1). 1).It is a volcanic cone rising 1300 m above sea level with a length of 36 km (oriented in the N-S direction) and width of 12 km (W-E) (García‐Gutiérrez et al., 2005).
Fallows C, Martin RA, Hammerschlag N. (2012) Comparisons between white shark-pinniped interactions at Seal Island (South Africa) with other sites in California (United States). Global Perspectives on the Biology and Life History of the Great White Shark, ed. Michael L. Domeier, CRC Press, Boca Raton, FL.
235 Orleans Road. North Chatham, MA, 02650. (508) [email protected]. Hours. Atlantic White Shark Conservancy researchers continue to research the white shark on Cape Cod and sharks' behaviors, movements, and management.
The Monterey Bay Aquarium ("Aquarium") has been active in biologging research, helping to pioneer studies across diverse marine taxa, including tunas, sharks, devil rays, sunfish, sea otters, and jellyfish 3, 4, 9 - 11. Over two decades ago, the Aquarium initiated the Juvenile White Shark Project ("Project"), spurred on by the first ...
The torpedo shape of the great white is built for speed: up to 35 miles per hour (50 kilometers per hour). And then there are the teeth -- 300 total in up to seven rows. But more than brawn, the great white shark has a tremendous brain that coordinates all the highly-developed senses of this efficient hunter. Its prey, including seals and ...
The upsurge over the past decade has caught just about everyone by surprise, including marine scientists. Renowned shark expert Greg Skomal, who has studied white sharks off the coast of New ...
Cape Cod's Great Whites. A great white shark near Cape Cod (left) investigates a seal decoy. The waters off the cape, unlike other places inhabited by great whites, are shallow enough to spot ...
White shark tagging. Between 2006 and 2013, we tagged 165 white sharks (Carcharodon carcharias) with acoustic tags (V16-4H-A69, Vemco; transmitting at 158 Db every 60-180 s for >1400 days) in ...
New research has used genetic analysis in a world-first effort to accurately estimate Australian and New Zealand white shark numbers. White sharks' ability to stay warm in cold water makes them ...
This recent research was a part of Monterey Bay Aquarium's "Project White Shark" and led by Jewell, a former Murdoch University PhD candidate, with Harry Butler Institute and the School of ...
On 16 May 2022, between 14:00 and 15:00 PM, D. Archer of Mossel Bay Helicopters flew a series of short tourist flights over the same area of Hartenbos (34°07′ S, 22°07′ E). The pilot witnessed two white sharks being killed by killer whales, but neither was fully captured on film. However, using a Samsung S21 cell phone, the pilot captured ...
Shark conservation efforts in the last two decades have been so successful that researchers are testing ways to mitigate human-shark conflict as populations rise.
Great white sharks might have more flexible behavior than scientists first thought, according to new research. After hooking great white sharks up with smart tags, researchers caught a glimpse of ...
In a study published Wednesday (April 24) in the journal Proceedings of the Royal Society B: Biological Sciences, researchers have described complete fossils of the shark discovered in limestone ...
Professor Don Nelson, who passed away in 1977, was the founder of Cal State Long Beach's Shark Lab and recently featured in the online journal Nautilus as the "Shark Whisperer." The current director of the Shark Lab, Professor Chris Lowe, provided insight to Dr. Nelson's research and drive to understand the behavior and biology of white sharks.
Leadership at Yale Urology has committed again to back its new tradition of "Shark Tank," a departmental research competition patterned after the television show of the same name, which aims to promote innovation that can include even risky ideas. During this, the competition's second year, two teams have received funding to work on ...
Publication Date. April 24, 2024. Press Inquiries. Caption. Left to right: Iwnetim Abate, Yoel Fink, Andrew Babbin, Skylar Tibbits. Credits. Photo credits: Jason Sparapani, Adam Glanzman. MIT Provost Cynthia Barnhart announced four Professor Amar G. Bose Research Grants to support bold research projects across diverse areas of study, including ...
A plurality of respondents (45.8%) agreed with the statement that the science concerning the sustainability of shark fisheries is currently uncertain, and 15.2% of respondents agreed with the ...
A 16-year-old boy who was bitten in the leg by a shark off Adelaide's coast has been left with "big gashes" but is doing "fine", his family says. Nathan Ness was on a fishing boat with his brother ...