
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

Synthesising the data

Synthesis is a stage in the systematic review process where extracted data, that is the findings of individual studies, are combined and evaluated.
The general purpose of extracting and synthesising data is to show the outcomes and effects of various studies, and to identify issues with methodology and quality. This means that your synthesis might reveal several elements, including:
- overall level of evidence
- the degree of consistency in the findings
- what the positive effects of a drug or treatment are , and what these effects are based on
- how many studies found a relationship or association between two components, e.g. the impact of disability-assistance animals on the psychological health of workplaces
There are two commonly accepted methods of synthesis in systematic reviews:
Qualitative data synthesis
- Quantitative data synthesis (i.e. meta-analysis)
The way the data is extracted from your studies, then synthesised and presented, depends on the type of data being handled.
In a qualitative systematic review, data can be presented in a number of different ways. A typical procedure in the health sciences is thematic analysis .
Thematic synthesis has three stages:
- the coding of text ‘line-by-line’
- the development of ‘descriptive themes’
- and the generation of ‘analytical themes’
If you have qualitative information, some of the more common tools used to summarise data include:
- textual descriptions, i.e. written words
- thematic or content analysis
Example qualitative systematic review
A good example of how to conduct a thematic analysis in a systematic review is the following journal article on cancer patients. In it, the authors go through the process of:
- identifying and coding information about the selected studies’ methodologies and findings on patient care
- organising these codes into subheadings and descriptive categories
- developing these categories into analytical themes
What Facilitates “Patient Empowerment” in Cancer Patients During Follow-Up: A Qualitative Systematic Review of the Literature
Quantitative data synthesis
In a quantitative systematic review, data is presented statistically. Typically, this is referred to as a meta-analysis .
The usual method is to combine and evaluate data from multiple studies. This is normally done in order to draw conclusions about outcomes, effects, shortcomings of studies and/or applicability of findings.
Remember, the data you synthesise should relate to your research question and protocol (plan). In the case of quantitative analysis, the data extracted and synthesised will relate to whatever method was used to generate the research question (e.g. PICO method), and whatever quality appraisals were undertaken in the analysis stage.
If you have quantitative information, some of the more common tools used to summarise data include:
- grouping of similar data, i.e. presenting the results in tables
- charts, e.g. pie-charts
- graphical displays, i.e. forest plots
Example of a quantitative systematic review
A quantitative systematic review is a combination of qualitative and quantitative, usually referred to as a meta-analysis.
Effectiveness of Acupuncturing at the Sphenopalatine Ganglion Acupoint Alone for Treatment of Allergic Rhinitis: A Systematic Review and Meta-Analysis
About meta-analyses

A systematic review may sometimes include a meta-analysis , although it is not a requirement of a systematic review. Whereas, a meta-analysis also includes a systematic review.
A meta-analysis is a statistical analysis that combines data from previous studies to calculate an overall result.
One way of accurately representing all the data is in the form of a forest plot . A forest plot is a way of combining the results of multiple studies in order to show point estimates arising from different studies of the same condition or treatment.
It is comprised of a graphical representation and often also a table. The graphical display shows the mean value for each study and often with a confidence interval (the horizontal bars). Each mean is plotted relative to the vertical line of no difference.
The following is an example of the graphical representation of a forest plot.

“File:The effect of zinc acetate lozenges on the duration of the common cold.svg” by Harri Hemilä is licensed under CC BY 3.0
Watch the following short video where a social health example is used to explain how to construct a forest plot graphic.
Forest Plots: Understanding a Meta-Analysis in 5 Minutes or Less (5:38 mins)
Forest Plots – Understanding a Meta-Analysis in 5 Minutes or Less (5:38 min) by The NCCMT ( YouTube )
Test your knowledge
Research and Writing Skills for Academic and Graduate Researchers Copyright © 2022 by RMIT University is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
Jump to navigation

Cochrane Training
Chapter 9: summarizing study characteristics and preparing for synthesis.
Joanne E McKenzie, Sue E Brennan, Rebecca E Ryan, Hilary J Thomson, Renea V Johnston
Key Points:
- Synthesis is a process of bringing together data from a set of included studies with the aim of drawing conclusions about a body of evidence. This will include synthesis of study characteristics and, potentially, statistical synthesis of study findings.
- A general framework for synthesis can be used to guide the process of planning the comparisons, preparing for synthesis, undertaking the synthesis, and interpreting and describing the results.
- Tabulation of study characteristics aids the examination and comparison of PICO elements across studies, facilitates synthesis of these characteristics and grouping of studies for statistical synthesis.
- Tabulation of extracted data from studies allows assessment of the number of studies contributing to a particular meta-analysis, and helps determine what other statistical synthesis methods might be used if meta-analysis is not possible.
Cite this chapter as: McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
9.1 Introduction
Synthesis is a process of bringing together data from a set of included studies with the aim of drawing conclusions about a body of evidence. Most Cochrane Reviews on the effects of interventions will include some type of statistical synthesis. Most commonly this is the statistical combination of results from two or more separate studies (henceforth referred to as meta-analysis) of effect estimates.
An examination of the included studies always precedes statistical synthesis in Cochrane Reviews. For example, examination of the interventions studied is often needed to itemize their content so as to determine which studies can be grouped in a single synthesis. More broadly, synthesis of the PICO (Population, Intervention, Comparator and Outcome) elements of the included studies underpins interpretation of review findings and is an important output of the review in its own right. This synthesis should encompass the characteristics of the interventions and comparators in included studies, the populations and settings in which the interventions were evaluated, the outcomes assessed, and the strengths and weaknesses of the body of evidence.
Chapter 2 defined three types of PICO criteria that may be helpful in understanding decisions that need to be made at different stages in the review:
- The review PICO (planned at the protocol stage) is the PICO on which eligibility of studies is based (what will be included and what excluded from the review).
- The PICO for each synthesis (also planned at the protocol stage) defines the question that the specific synthesis aims to answer, determining how the synthesis will be structured, specifying planned comparisons (including intervention and comparator groups, any grouping of outcome and population subgroups).
- The PICO of the included studies (determined at the review stage) is what was actually investigated in the included studies.
In this chapter, we focus on the PICO for each synthesis and the PICO of the included studies , as the basis for determining which studies can be grouped for statistical synthesis and for synthesizing study characteristics. We describe the preliminary steps undertaken before performing the statistical synthesis. Methods for the statistical synthesis are described in Chapter 10 , Chapter 11 and Chapter 12 .
9.2 A general framework for synthesis
Box 9.2.a A general framework for synthesis that can be applied irrespective of the methods used to synthesize results
Box 9.2.a provides a general framework for synthesis that can be applied irrespective of the methods used to synthesize results. Planning for the synthesis should start at protocol-writing stage, and Chapter 2 and Chapter 3 describe the steps involved in planning the review questions and comparisons between intervention groups. These steps included specifying which characteristics of the interventions, populations, outcomes and study design would be grouped together for synthesis (the PICO for each synthesis: stage 1 in Box 9.2.a ).
This chapter primarily concerns stage 2 of the general framework in Box 9.2.a . After deciding which studies will be included in the review and extracting data, review authors can start implementing their plan, working through steps 2.1 to 2.5 of the framework. This process begins with a detailed examination of the characteristics of each study (step 2.1), and then comparison of characteristics across studies in order to determine which studies are similar enough to be grouped for synthesis (step 2.2). Examination of the type of data available for synthesis follows (step 2.3). These three steps inform decisions about whether any modification to the planned comparisons or outcomes is necessary, or new comparisons are needed (step 2.4). The last step of the framework covered in this chapter involves synthesis of the characteristics of studies contributing to each comparison (step 2.5). The chapter concludes with practical tips for checking data before synthesis (Section 9.4 ).
Steps 2.1, 2.2 and 2.5 involve analysis and synthesis of mainly qualitative information about study characteristics. The process used to undertake these steps is rarely described in reviews, yet can require many subjective decisions about the nature and similarity of the PICO elements of the included studies. The examples described in this section illustrate approaches for making this process more transparent.
9.3 Preliminary steps of a synthesis
9.3.1 summarize the characteristics of each study (step 2.1).
A starting point for synthesis is to summarize the PICO characteristics of each study (i.e. the PICO of the included studies, see Chapter 3 ) and categorize these PICO elements in the groups (or domains) pre-specified in the protocol (i.e. the PICO for each synthesis). The resulting descriptions are reported in the ‘Characteristics of included studies’ table, and are used in step 2.2 to determine which studies can be grouped for synthesis.
In some reviews, the labels and terminology used in each study are retained when describing the PICO elements of the included studies. This may be sufficient in areas with consistent and widely understood terminology that matches the PICO for each synthesis. However, in most areas, terminology is variable, making it difficult to compare the PICO of each included study to the PICO for each synthesis, or to compare PICO elements across studies. Standardizing the description of PICO elements across studies facilitates these comparisons. This standardization includes applying the labels and terminology used to articulate the PICO for each synthesis ( Chapter 3 ), and structuring the description of PICO elements. The description of interventions can be structured using the Template for Intervention Description and Replication (TIDIeR) checklist, for example (see Chapter 3 and Table 9.3.a ).
Table 9.3.a illustrates the use of pre-specified groups to categorize and label interventions in a review of psychosocial interventions for smoking cessation in pregnancy (Chamberlain et al 2017). The main intervention strategy in each study was categorized into one of six groups: counselling, health education, feedback, incentive-based interventions, social support, and exercise. This categorization determined which studies were eligible for each comparison (e.g. counselling versus usual care; single or multi-component strategy). The extract from the ‘Characteristics of included studies’ table shows the diverse descriptions of interventions in three of the 54 studies for which the main intervention was categorized as ‘counselling’. Other intervention characteristics, such as duration and frequency, were coded in pre-specified categories to standardize description of the intervention intensity and facilitate meta-regression (not shown here).
Table 9.3.a Example of categorizing interventions into pre-defined groups
* The definition also specified eligible modes of delivery, intervention duration and personnel.
While this example focuses on categorizing and describing interventions according to groups pre-specified in the PICO for each synthesis, the same approach applies to other PICO elements.
9.3.2 Determine which studies are similar enough to be grouped within each comparison (step 2.2)
Once the PICO of included studies have been coded using labels and descriptions specified in the PICO for each synthesis, it will be possible to compare PICO elements across studies and determine which studies are similar enough to be grouped within each comparison.
Tabulating study characteristics can help to explore and compare PICO elements across studies, and is particularly important for reviews that are broad in scope, have diversity across one or more PICO elements, or include large numbers of studies. Data about study characteristics can be ordered in many different ways (e.g. by comparison or by specific PICO elements), and tables may include information about one or more PICO elements. Deciding on the best approach will depend on the purpose of the table and the stage of the review. A close examination of study characteristics will require detailed tables; for example, to identify differences in characteristics that were pre-specified as potentially important modifiers of the intervention effects. As the review progresses, this detail may be replaced by standardized description of PICO characteristics (e.g. the coding of counselling interventions presented in Table 9.3.a ).
Table 9.3.b illustrates one approach to tabulating study characteristics to enable comparison and analysis across studies. This table presents a high-level summary of the characteristics that are most important for determining which comparisons can be made. The table was adapted from tables presented in a review of self-management education programmes for osteoarthritis (Kroon et al 2014). The authors presented a structured summary of intervention and comparator groups for each study, and then categorized intervention components thought to be important for enabling patients to manage their own condition. Table 9.3.b shows selected intervention components, the comparator, and outcomes measured in a subset of studies (some details are fictitious). Outcomes have been grouped by the outcome domains ‘Pain’ and ‘Function’ (column ‘Outcome measure’ Table 9.3.b ). These pre-specified outcome domains are the chosen level for the synthesis as specified in the PICO for each synthesis. Authors will need to assess whether the measurement methods or tools used within each study provide an appropriate assessment of the domains ( Chapter 3, Section 3.2.4 ). A next step is to group each measure into the pre-specified time points. In this example, outcomes are grouped into short-term (<6 weeks) and long-term follow-up (≥6 weeks to 12 months) (column ‘Time points (time frame)’ Table 9.3.b ).
Variations on the format shown in Table 9.3.b can be presented within a review to summarize the characteristics of studies contributing to each synthesis, which is important for interpreting findings (step 2.5).
Table 9.3.b Table of study characteristics illustrating similarity of PICO elements across studies
BEH = health-directed behaviour; CON = constructive attitudes and approaches; EMO = emotional well-being; ENG = positive and active engagement in life; MON = self-monitoring and insight; NAV = health service navigation; SKL = skill and technique acquisition. ANCOVA = Analysis of covariance; CI = confidence interval; IQR = interquartile range; MD = mean difference; SD = standard deviation; SE = standard error, NS = non-significant. Pain and function measures: Dutch AIMS-SF = Dutch short form of the Arthritis Impact Measurement Scales; HAQ = Health Assessment Questionnaire; VAS = visual analogue scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index. 1 Ordered by type of comparator; 2 Short-term (denoted ‘immediate’ in the review Kroon et al (2014)) follow-up is defined as <6 weeks, long-term follow-up (denoted ‘intermediate’ in the review) is ≥6 weeks to 12 months; 3 For simplicity, in this example the available data are assumed to be the same for all outcomes within an outcome domain within a study. In practice, this is unlikely and the available data would likely vary by outcome; 4 Indicates that an effect estimate and its standard error may be computed through imputation of missing statistics, methods to convert between statistics (e.g. medians to means) or contact with study authors. *Indicates the selected outcome when there was multiplicity in the outcome domain and time frame.
9.3.3 Determine what data are available for synthesis (step 2.3)
Once the studies that are similar enough to be grouped together within each comparison have been determined, a next step is to examine what data are available for synthesis. Tabulating the measurement tools and time frames as shown in Table 9.3.b allows assessment of the potential for multiplicity (i.e. when multiple outcomes within a study and outcome domain are available for inclusion ( Chapter 3, Section 3.2.4.3 )). In this example, multiplicity arises in two ways. First, from multiple measurement instruments used to measure the same outcome domain within the same time frame (e.g. ‘Short-term Pain’ is measured using the ‘Pain VAS’ and ‘Pain on walking VAS’ scales in study 3). Second, from multiple time points measured within the same time frame (e.g. ‘Short-term Pain’ is measured using ‘Pain VAS’ at both 2 weeks and 1 month in study 6). Pre-specified methods to deal with the multiplicity can then be implemented (see Table 9.3.c for examples of approaches for dealing with multiplicity). In this review, the authors pre-specified a set of decision rules for selecting specific outcomes within the outcome domains. For example, for the outcome domain ‘Pain’, the selected outcome was the highest on the following list: global pain, pain on walking, WOMAC pain subscore, composite pain scores other than WOMAC, pain on activities other than walking, rest pain or pain during the night. The authors further specified that if there were multiple time points at which the outcome was measured within a time frame, they would select the longest time point. The selected outcomes from applying these rules to studies 3 and 6 are indicated by an asterisk in Table 9.3.b .
Table 9.3.b also illustrates an approach to tabulating the extracted data. The available statistics are tabulated in the column labelled ‘Data’, from which an assessment can be made as to whether the study contributes the required data for a meta-analysis (column ‘Effect & SE’) ( Chapter 10 ). For example, of the seven studies comparing health-directed behaviour (BEH) with usual care, six measured ‘Short-term Pain’, four of which contribute required data for meta-analysis. Reordering the table by comparison, outcome and time frame, will more readily show the number of studies that will contribute to a particular meta-analysis, and help determine what other synthesis methods might be used if the data available for meta-analysis are limited.
Table 9.3.c Examples of approaches for selecting one outcome (effect estimate) for inclusion in a synthesis.* Adapted from López-López et al (2018)
9.3.4 Determine if modification to the planned comparisons or outcomes is necessary, or new comparisons are needed (step 2.4)
The previous steps may reveal the need to modify the planned comparisons. Important variations in the intervention may be identified leading to different or modified intervention groups. Few studies or sparse data, or both, may lead to different groupings of interventions, populations or outcomes. Planning contingencies for anticipated scenarios is likely to lead to less post-hoc decision making ( Chapter 2 and Chapter 3 ); however, it is difficult to plan for all scenarios. In the latter circumstance, the rationale for any post-hoc changes should be reported. This approach was adopted in a review examining the effects of portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco (Hollands et al 2015). After preliminary examination of the outcome data, the review authors changed their planned intervention groups. They judged that intervention groups based on ‘size’ and those based on ‘shape’ of the products were not conceptually comparable, and therefore should form separate comparisons. The authors provided a rationale for the change and noted that it was a post-hoc decision.
9.3.5 Synthesize the characteristics of the studies contributing to each comparison (step 2.5)
A final step, and one that is essential for interpreting combined effects, is to synthesize the characteristics of studies contributing to each comparison. This description should integrate information about key PICO characteristics across studies, and identify any potentially important differences in characteristics that were pre-specified as possible effect modifiers. The synthesis of study characteristics is also needed for GRADE assessments, informing judgements about whether the evidence applies directly to the review question (indirectness) and analyses conducted to examine possible explanations for heterogeneity (inconsistency) (see Chapter 14 ).
Tabulating study characteristics is generally preferable to lengthy description in the text, since the structure imposed by a table can make it easier and faster for readers to scan and identify patterns in the information presented. Table 9.3.b illustrates one such approach. Tabulating characteristics of studies that contribute to each comparison can also help to improve the transparency of decisions made around grouping of studies, while also ensuring that studies that do not contribute to the combined effect are accounted for.
9.4 Checking data before synthesis
Before embarking on a synthesis, it is important to be confident that the findings from the individual studies have been collated correctly. Therefore, review authors must compare the magnitude and direction of effects reported by studies with how they are to be presented in the review. This is a reasonably straightforward way for authors to check a number of potential problems, including typographical errors in studies’ reports, accuracy of data collection and manipulation, and data entry into RevMan. For example, the direction of a standardized mean difference may accidentally be wrong in the review. A basic check is to ensure the same qualitative findings (e.g. direction of effect and statistical significance) between the data as presented in the review and the data as available from the original study.
Results in forest plots should agree with data in the original report (point estimate and confidence interval) if the same effect measure and statistical model is used. There are legitimate reasons for differences, however, including: using a different measure of intervention effect; making different choices between change-from-baseline measures, post-intervention measures alone or post-intervention measures adjusted for baseline values; grouping similar intervention groups; or making adjustments for unit-of-analysis errors in the reports of the primary studies.
9.5 Types of synthesis
The focus of this chapter has been describing the steps involved in implementing the planned comparisons between intervention groups (stage 2 of the general framework for synthesis ( Box 9.2.a )). The next step (stage 3) is often performing a statistical synthesis. Meta-analysis of effect estimates, and its extensions have many advantages. There are circumstances under which a meta-analysis is not possible, however, and other statistical synthesis methods might be considered, so as to make best use of the available data. Available summary and synthesis methods, along with the questions they address and examples of associated plots, are described in Table 9.5.a . Chapter 10 and Chapter 11 discuss meta-analysis (of effect estimate) methods, while Chapter 12 focuses on the other statistical synthesis methods, along with approaches to tabulating, visually displaying and providing a structured presentation of the findings. An important part of planning the analysis strategy is building in contingencies to use alternative methods when the desired method cannot be used.
Table 9.5.a Overview of available methods for summary and synthesis
9.6 Chapter information
Authors: Joanne E McKenzie, Sue E Brennan, Rebecca E Ryan, Hilary J Thomson, Renea V Johnston
Acknowledgements: Sections of this chapter build on Chapter 9 of version 5.1 of the Handbook , with editors Jonathan Deeks, Julian Higgins and Douglas Altman. We are grateful to Julian Higgins, James Thomas and Tianjing Li for commenting helpfully on earlier drafts.
Funding: JM is supported by an NHMRC Career Development Fellowship (1143429). SB and RR’s positions are supported by the NHMRC Cochrane Collaboration Funding Program. HT is funded by the UK Medical Research Council (MC_UU_12017-13 and MC_UU_12017-15) and Scottish Government Chief Scientist Office (SPHSU13 and SPHSU15). RJ’s position is supported by the NHMRC Cochrane Collaboration Funding Program and Cabrini Institute.
9.7 References
Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, McKenzie JE. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017; 2 : CD001055.
Hollands GJ, Shemilt I, Marteau TM, Jebb SA, Lewis HB, Wei Y, Higgins JPT, Ogilvie D. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database of Systematic Reviews 2015; 9 : CD011045.
Kroon FPB, van der Burg LRA, Buchbinder R, Osborne RH, Johnston RV, Pitt V. Self-management education programmes for osteoarthritis. Cochrane Database of Systematic Reviews 2014; 1 : CD008963.
López-López JA, Page MJ, Lipsey MW, Higgins JPT. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Research Synthesis Methods 2018; 9 : 336–351.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-.

Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet].
Quantitative synthesis—an update.
Investigators: Sally C. Morton , Ph.D., M.Sc., M. Hassan Murad , M.D., M.P.H., Elizabeth O’Connor , Ph.D., Christopher S. Lee , Ph.D., R.N., Marika Booth , M.S., Benjamin W. Vandermeer , M.Sc., Jonathan M. Snowden , Ph.D., Kristen E. D’Anci , Ph.D., Rongwei Fu , Ph.D., Gerald Gartlehner , M.D., M.P.H., Zhen Wang , Ph.D., and Dale W. Steele , M.D., M.S.
Affiliations
Published: February 23, 2018 .
Quantitative synthesis, or meta-analysis, is often essential for Comparative Effective Reviews (CERs) to provide scientifically rigorous summary information. Quantitative synthesis should be conducted in a transparent and consistent way with methodologies reported explicitly. This guide provides practical recommendations on conducting synthesis. The guide is not meant to be a textbook on meta-analysis nor is it a comprehensive review of methods, but rather it is intended to provide a consistent approach for situations and decisions that are commonly faced by AHRQ Evidence-based Practice Centers (EPCs). The goal is to describe choices as explicitly as possible, and in the context of EPC requirements, with an appropriate degree of confidence.
This guide addresses issues in the order that they are usually encountered in a synthesis, though we acknowledge that the process is not always linear. We first consider the decision of whether or not to combine studies quantitatively. The next chapter addresses how to extract and utilize data from individual studies to construct effect sizes, followed by a chapter on statistical model choice. The fourth chapter considers quantifying and exploring heterogeneity. The fifth describes an indirect evidence technique that has not been included in previous guidance – network meta-analysis, also known as mixed treatment comparisons. The final section in the report lays out future research suggestions.
The Agency for Healthcare Research and Quality (AHRQ), through its Evidence-based Practice Centers (EPCs), sponsors the development of evidence reports and technology assessments to assist public- and private-sector organizations in their efforts to improve the quality of health care in the United States. The reports and assessments provide organizations with comprehensive, science-based information on common, costly medical conditions and new health care technologies and strategies. The EPCs systematically review the relevant scientific literature on topics assigned to them by AHRQ and conduct additional analyses when appropriate prior to developing their reports and assessments.
Strong methodological approaches to systematic review improve the transparency, consistency, and scientific rigor of these reports. Through a collaborative effort of the Effective Health Care (EHC) Program, the Agency for Healthcare Research and Quality (AHRQ), the EHC Program Scientific Resource Center, and the AHRQ Evidence-based Practice Centers have developed a Methods Guide for Comparative Effectiveness Reviews. This Guide presents issues key to the development of Systematic Reviews and describes recommended approaches for addressing difficult, frequently encountered methodological issues.
The Methods Guide for Comparative Effectiveness Reviews is a living document, and will be updated as further empiric evidence develops and our understanding of better methods improves. We welcome comments on this Methods Guide paper. They may be sent by mail to the Task Order Officer named below at: Agency for Healthcare Research and Quality, 5600 Fishers Lane, Rockville, MD 20857, or by email to vog.shh.qrha@cpe .
- Gopal Khanna, M.B.A. Director Agency for Healthcare Research and Quality
- Arlene S. Bierman, M.D., M.S. Director Center for Evidence and Practice Improvement Agency for Healthcare Research and Quality
- Stephanie Chang, M.D., M.P.H. Director Evidence-based Practice Center Program Center for Evidence and Practice Improvement Agency for Healthcare Research and Quality
- Elisabeth Kato, M.D., M.R.P. Task Order Officer Evidence-based Practice Center Program Center for Evidence and Practice Improvement Agency for Healthcare Research and Quality
- Peer Reviewers
Prior to publication of the final evidence report, EPCs sought input from independent Peer Reviewers without financial conflicts of interest. However, the conclusions and synthesis of the scientific literature presented in this report does not necessarily represent the views of individual investigators.
Peer Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals with potential non-financial conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential non-financial conflicts of interest identified.
- Eric Bass, M.D., M.P.H Director, Johns Hopkins University Evidence-based Practice Center Professor of Medicine, and Health Policy and Management Johns Hopkins University Baltimore, MD
- Mary Butler, M.B.A., Ph.D. Co-Director, Minnesota Evidence-based Practice Center Assistant Professor, Health Policy & Management University of Minnesota Minneapolis, MN
- Roger Chou, M.D., FACP Director, Pacific Northwest Evidence-based Practice Center Portland, OR
- Lisa Hartling, M.S., Ph.D. Director, University of Alberta Evidence-Practice Center Edmonton, AB
- Susanne Hempel, Ph.D. Co-Director, Southern California Evidence-based Practice Center Professor, Pardee RAND Graduate School Senior Behavioral Scientist, RAND Corporation Santa Monica, CA
- Robert L. Kane, M.D. * Co-Director, Minnesota Evidence-based Practice Center School of Public Health University of Minnesota Minneapolis, MN
- Jennifer Lin, M.D., M.C.R. Director, Kaiser Permanente Research Affiliates Evidence-based Practice Center Investigator, The Center for Health Research, Kaiser Permanente Northwest Portland, OR
- Christopher Schmid, Ph.D. Co-Director, Center for Evidence Synthesis in Health Professor of Biostatistics School of Public Health Brown University Providence, RI
- Karen Schoelles, M.D., S.M., FACP Director, ECRI Evidence-based Practice Center Plymouth Meeting, PA
- Tibor Schuster, Ph.D. Assistant Professor Department of Family Medicine McGill University Montreal, QC
- Jonathan R. Treadwell, Ph.D. Associate Director, ECRI Institute Evidence-based Practice Center Plymouth Meeting, PA
- Tom Trikalinos, M.D. Director, Brown Evidence-based Practice Center Director, Center for Evidence-based Medicine Associate Professor, Health Services, Policy & Practice Brown University Providence, RI
- Meera Viswanathan, Ph.D. Director, RTI-UNC Evidence-based Practice Center Durham, NC RTI International Durham, NC
- C. Michael White, Pharm. D., FCP, FCCP Professor and Head, Pharmacy Practice School of Pharmacy University of Connecticut Storrs, CT
- Tim Wilt, M.D., M.P.H. Co-Director, Minnesota Evidence-based Practice Center Director, Minneapolis VA-Evidence Synthesis Program Professor of Medicine, University of Minnesota Staff Physician, Minneapolis VA Health Care System Minneapolis, MN
Deceased March 6, 2017
- Introduction
The purpose of this document is to consolidate and update quantitative synthesis guidance provided in three previous methods guides. 1 – 3 We focus primarily on comparative effectiveness reviews (CERs), which are systematic reviews that compare the effectiveness and harms of alternative clinical options, and aim to help clinicians, policy makers, and patients make informed treatment choices. We focus on interventional studies and do not address diagnostic studies, individual patient level analysis, or observational studies, which are addressed elsewhere. 4
Quantitative synthesis, or meta-analysis, is often essential for CERs to provide scientifically rigorous summary information. Quantitative synthesis should be conducted in a transparent and consistent way with methodologies reported explicitly. This guide provides practical recommendations on conducting synthesis. The guide is not meant to be a textbook on meta-analysis nor is it a comprehensive review of methods, but rather it is intended to provide a consistent approach for situations and decisions that are commonly faced by Evidence-based Practice Centers (EPCs). The goal is to describe choices as explicitly as possible and in the context of EPC requirements, with an appropriate degree of confidence.
EPC investigators are encouraged to follow these recommendations but may choose to use alternative methods if deemed necessary after discussion with their AHRQ project officer. If alternative methods are used, investigators are required to provide a rationale for their choices, and if appropriate, to state the strengths and limitations of the chosen methods in order to promote consistency, transparency, and learning. In addition, several steps in meta-analysis require subjective judgment, such as when combining studies or incorporating indirect evidence. For each subjective decision, investigators should fully explain how the decision was reached.
This guide was developed by a workgroup comprised of members from across the EPCs, as well as from the Scientific Resource Center (SRC) of the AHRQ Effective Healthcare Program. Through surveys and discussions among AHRQ, Directors of EPCs, the Scientific Resource Center, and the Methods Steering Committee, quantitative synthesis was identified as a high-priority methods topic and a need was identified to update the original guidance. 1 , 5 Once confirmed as a Methods Workgroup, the SRC solicited EPC workgroup volunteers, particularly those with quantitative methods expertise, including statisticians, librarians, thought leaders, and methodologists. Charged by AHRQ to update current guidance, the workgroup consisted of members from eight of 13 EPCs, the SRC, and AHRQ, and commenced in the fall of 2015. We conducted regular workgroup teleconference calls over the course of 14 months to discuss project direction and scope, assign and coordinate tasks, collect and analyze data, and discuss and edit draft documents. After constructing a draft table of contents, we surveyed all EPCs to ensure no topics of interest were missing.
The initial teleconference meeting was used to outline the draft, discuss the timeline, and agree upon a method for reaching consensus as described below. The larger workgroup then was split into subgroups each taking responsibility for a different chapter. The larger group participated in biweekly discussions via teleconference and email communication. Subgroups communicated separately (in addition to the larger meetings) to coordinate tasks, discuss the literature review results, and draft their respective chapters. Later, chapter drafts were combined into a larger document for workgroup review and discussion on the bi-weekly calls.
Literature Search and Review
A medical research librarian worked with each subgroup to identify a relevant search strategy for each chapter, and then combined these strategies into one overall search conducted for all chapters combined. The librarian conducted the search on the ARHQ SRC Methods Library, a bibliographic database curated by the SRC currently containing more than 16,000 citations of methodological works for systematic reviews and comparative effectiveness reviews, using descriptor and keyword strategies to identify quantitative synthesis methods research publications (descriptor search=all quantitative synthesis descriptors, and the keyword search=quantitative synthesis, meta-anal*, metaanal*, meta-regression in [anywhere field]). Search results were limited to English language and 2009 and later to capture citations published since AHRQ’s previous methods guidance on quantitative synthesis. Additional articles were identified from recent systematic reviews, reference lists of reviews and editorials, and through the expert review process.
The search yielded 1,358 titles and abstracts which were reviewed by all workgroup members using ABSTRACKR software (available at http://abstrackr.cebm.brown.edu ). Each subgroup separately identified articles relevant to their own chapter. Abstract review was done by single review, investigators included anything that could be potentially relevant. Each subgroup decided separately on final inclusion/exclusion based on full text articles.
Consensus and Recommendations
Reaching consensus if possible is of great importance for AHRQ methods guidance. The workgroup recognized this importance in its first meeting and agreed on a process for informal consensus and conflict resolution. Disagreements were thoroughly discussed and if possible, consensus was reached. If consensus was not reached, analytic options are discussed in the text. We did not employ a formal voting procedure to assess consensus.
A summary of the workgroup’s key conclusions and recommendations was circulated for comment by EPC Directors and AHRQ officers at a biannual EPC Director’s meeting in October 2016. In addition, a full draft was circulated to EPC Directors and AHRQ officers prior to peer review, and the manuscript was made available for public review. All comments have been considered by the team in the final preparation of this report.
Chapter 1. Decision to Combine Trials
1.1. goals of the meta-analysis.
Meta-analysis is a statistical method for synthesizing (also called combining or pooling) the benefits and/or harms of a treatment or intervention across multiple studies. The overarching goal of a meta-analysis is generally to provide the best estimate of the effect of an intervention. As part of that aspirational goal, results of a meta-analysis may inform a number of related questions, such as whether that best estimate represents something other than a null effect (is this intervention beneficial?), the range in which the true effect likely lies, whether it is appropriate to provide a single best estimate, and what study-level characteristics may influence the effect estimate. Before tackling these questions, it is necessary to answer a preliminary but fundamental question: Is it appropriate to pool the results of the identified studies? 6
Clinical, methodological, and statistical factors must all be considered when deciding whether to combine studies in a meta-analysis. Figure 1.1 depicts a decision tree to help investigators think through these important considerations, which are discussed below.
Pooling decision tree.
1.2. Clinical and Methodological Heterogeneity
Studies must be reasonably similar to be pooled in a meta-analysis. 1 Even when the review protocol identifies a coherent and fairly narrow body of literature, the actual included studies may represent a wide range of population, intervention, and study characteristics. Variations in these factors are referred to as clinical heterogeneity and methodological heterogeneity. 7 , 8 A third form of heterogeneity, statistical heterogeneity, will be discussed later.
The first step in the decision tree is to explore the clinical and methodological heterogeneity of the included studies (Step A, Figure 1.1 ). The goal is to identify groups of trials that are similar enough that an average effect would make a sensible summary. There is no objective measure or universally accepted standard for deciding whether studies are “similar enough” to pool; this decision is inherently a matter of judgment. 6 Verbeek and colleagues suggest working through key sources of variability in sequence, beginning with the clinical variables of intervention/exposure, control condition, and participants, before moving on to methodological areas such as study design, outcome, and follow-up time. When there is important variability in these areas, investigators should consider whether there are coherent subgroups of trials, rather than the full group, that can be pooled. 6
Clinical heterogeneity refers to characteristics related to the participants, interventions, types of outcomes, and study setting. Some have suggested that pooling may be acceptable when it is plausible that the underlying effects could be similar across subpopulations and variations in interventions and outcomes. 9 For example, in a review of a lipid-lowering medication, researchers might be comfortable combining studies that target younger and middle-aged adults, but expect different effects with older adults, who have high rates of comorbidities and other medication use. Others suggest that it may be acceptable to combine interventions with likely similar mechanisms of action. 6 For example, a researcher may combine studies of depression interventions that use a range of psychotherapeutic approaches, on the logic that they all aim to change a person’s thinking and behavior in order to improve mood, but not want to combine them with trials of antidepressants, whose mechanism of action is presumed to be biochemical.
Methodological heterogeneity refers to variations in study methods (e.g., study design, measures, and study conduct). A common question regarding study design, is whether it is acceptable to combine studies that randomize individual participants with those that randomize clusters (e.g., when clinics, clinicians, or classrooms are randomized and individuals are nested within these units). We believe this is generally acceptable, with appropriate adjustment for cluster randomization as needed. 10 However, closer examination may show that the cluster randomized trials also tend to systematically differ on population or intervention characteristics from the individually-randomized trials. If so, subgroup analyses may be considered.
Outcome measures are a common source of methodological heterogeneity. First, trials may have a wide array of specific instruments and cut-points for a common outcome. For example, a review considering pooling the binary outcome of depression prevalence may find measures that range from a depression diagnosis based on a clinical interview to scores above a cut-point on a screening instrument. One guiding principle is to consider pooling only when it is plausible that the underlying relative effects are consistent across specific definitions of an outcome. In addition, investigators should take steps to harmonize outcomes to the extent possible.
Second, there is also typically substantial variability in the statistics reported across studies (e.g., odds ratios, relative risks, hazard ratios, baseline and mean followup scores, change scores for each condition, between-group differences at followup, etc.). Methods to calculate or estimate missing statistics are available, 5 however the investigators must ultimately weigh the tradeoff of potentially less accurate results (due to assumptions required to estimate missing data) with the potential advantage of pooling a more complete set of studies. If a substantial proportion of the studies require calculations that involve assumptions or estimates (rather than straightforward calculations) in order to combine them, then it may be preferable to show results in a table or forest plot without a pooled estimate
1.3. Best Evidence Versus All Evidence
Sometimes the body of evidence comprises a single trial or small number of trials that clearly represent the best evidence, along with a number of additional trials that are much smaller or with other important limitations (Step B, Figure 1.1 ). The “best evidence” trials are generally very large trials with low risk of bias and with good generalizability to the population of interest. In this case, it may be appropriate to focus on the one or few “best” trials rather than combining them with the rest of the evidence, particularly when addressing rare events that small studies are underpowered to examine. 11 , 12 For example, an evidence base of one large, multi-center trial of an intervention to prevent stroke in patients with heart disease could be preferable to a pooled analysis of 4-5 small trials reporting few events, and combining the small trials with the large trial may introduce unnecessary uncertainty to the pooled estimate.
1.4. Assessing the Risk of Misleading Meta-analysis Results
Next, reviews should explore the risk that the meta-analysis will show results that do not accurately capture the true underlying effect (Step C, Figure 1.1 ). Tables, forest plots (without pooling), and some other preliminary statistical tests are useful tools for this stage. Several patterns can arise that should lead investigators to be cautious about combining studies.
Wide-Ranging Effect Sizes
Sometimes one study may show a large benefit and another study of the same intervention may show a small benefit. This may be due to random error, especially when the studies are small. However, this situation also raises the possibility that observed effects truly are widely variable in different subpopulations or situations. Another look at the population characteristics is warranted in this situation to see if the investigators can identify characteristics that are correlated with effect size and direction, potentially explaining clinical heterogeneity.
Even if no characteristic can be identified that explains why the intervention had such widely disparate effects, there could be unmeasured features that explain the difference. If the intervention really does have widely variable impact in different subpopulations, particularly if it is benefiting some patients and harming others, it would be misleading to report a single average effect.
Suspicion of Publication or Reporting Bias
Sometimes, due to lack of effect, trial results are never published (risking publication bias), or are only published in part (risking reporting bias). These missing results can introduce bias and reduce the precision of meta-analysis. 13 Investigators can explore the risk of reporting bias by comparing trials that do and do not report important outcomes to assess whether outcomes appear to be missing at random. 13 For example, investigators may have 30 trials of weight loss interventions with only 10 reporting blood pressure, which is considered an important outcome for the review. This pattern of results may indicate reporting bias as trials finding group differences in blood pressure were more likely to report blood pressure findings. On the other hand, perhaps most of the studies limited to patients with elevated cardiovascular disease (CVD) risk factors did report blood pressure. In this case, the investigators may decide to combine the studies reporting blood pressure that were conducted in high CVD risk populations. However, investigators should be clear about the applicable subpopulation. An examination of the clinical and methodological features of the subset of trials where blood pressure was reported is necessary to make an informed judgement about whether to conduct a meta-analysis.
Small Studies Effect
If small studies show larger effects than large studies, the pooled results may overestimate the true effect size, possibly due to publication or reporting bias. 14 When investigators have at least 10 trials to combine they should examine small studies effects using standard statistical tests such as the Egger test. 15 If there appears to be a small studies effect, the investigators may decide not to report pooled results since they could be misleading. On the other hand, small studies effects could be happening for other reasons, such as differences in sample characteristics, attrition, or assessment methods. These factors do not suggest bias, but should be explored to the degree possible. See Chapter 4 for more information about exploring heterogeneity.
1.5. Special Considerations When Pooling a Small Number of Studies
When pooling a small number of studies (e.g., <10 studies), a number of considerations arise (Step E, Figure 1.1 ):
Rare Outcomes
Meta-analyses of rare binary outcomes are frequently underpowered, and tend to overestimate the true effect size, so pooling should be undertaken with caution. 11 A small difference in absolute numbers of events can result in large relative differences, usually with low precision (i.e., wide confidence intervals). This could result in misleading effect estimates if the analysis is limited to trials that are underpowered for the rare outcomes. 12 One example is all-cause mortality, which is frequently provided as part of the participant flow results, but may not be a primary outcome, may not have adjudication methods described, and typically occurs very rarely. Studies are often underpowered to detect differences in mortality if it is not a primary outcome. Investigators should consider calculating an optimal information size (OIS) when events are rare to see if the combined group of studies has sufficient power to detect group differences. This could be a concern even for a relatively large number of studies, if the total sample size is not very large. 16 See Chapter 3 for more detail on handling rare binary outcomes.
Small Sample Sizes
When pooling a relatively small number of studies, pooling should be undertaken with caution if the body of evidence is limited only to small studies. Results from small trials are less likely to be reliable than results of large trials, even when the risk of bias is low. 17 First, in small trials it is difficult to balance the proportion of patients in potentially important subgroups across interventions, and a difference between interventions of just a few patients in a subgroup can result in a large proportional difference between interventions. Characteristics that are rare are particularly at risk of being unbalanced in trials with small samples. In such situations there is no way to know if trial effects are due to the intervention or to differences in the intervention groups. In addition, patients are generally drawn from a narrower geographic range in small trials, making replication in other trials more uncertain. Finally, although it is not always the case, large trials are more likely to involve a level of scrutiny and standardization to ensure lower risk of bias than are small trials. Therefore, when the trials have small sample sizes, pooled effects are less likely to reflect the true effects of the intervention. In this case, the required or optimal information size can help the investigators determine whether the sample size is sufficient to conclude that results are likely to be stable and not due to random heterogeneity (i.e., truly significant or truly null results; not a type I or type II error). 16 , 18 An option in this case would be to pool the studies and acknowledge imprecision or other limitations when rating the strength of evidence.
What would be considered a “small” trial varies for different fields and outcomes. For addressing an outcome that only happens in 10% of the population, a small trial might be 100 to 200 per intervention arm, whereas a trial addressing a continuous quality of life measure may be small with 20 to 30 per intervention. Looking carefully at what the studies were powered to detect and the credibility of the power calculations may help determine what constitutes a “small” trial. Investigators should also consider how variable the impact of an intervention may be over different settings and subpopulations when determining how to weigh the importance of small studies. For example, the effects of a counseling intervention that relies on patients to change their behavior in order to reap health benefits may be more strongly influenced by characteristics of the patients and setting than a mechanical or chemical agent.
When the number of trials to be pooled is small, there is a heightened risk that statistical heterogeneity will be substantially underestimated, resulting in 95% confidence intervals that are inappropriately narrow and do not have 95% coverage. This is especially concerning when the number of studies being pooled is fewer than five to seven. 19 – 21
Accounting for these factors should guide an evaluation of whether it is advisable to pool the relatively small group of studies. As with many steps in the multi-stage decision to pool, the conclusion that a given investigator arrives at is subjective, although such evaluations should be guided by the criteria above. If consideration of these factors reassures investigators that the risk of bias associated with pooling is sufficiently low, then pooling can proceed. The next step of pooling, whether for a small, moderate, or large body of studies, is to consider statistical heterogeneity.
1.6. Statistical Heterogeneity
Once clinical and methodological heterogeneity and other factors described above have been deemed acceptable for pooling, investigators should next consider statistical heterogeneity (Step F, Figure 1.1 ). We discuss statistical heterogeneity in general in this chapter, and provide a deeper methodological discussion in Chapter 4 . This initial consideration of statistical heterogeneity is accomplished by conducting a preliminary meta-analysis. Next the investigator must decide if the results of the meta-analysis are valid and should be presented, rather than simply showing tables or forest plots without pooled results. If statistical heterogeneity is very high, the investigators may question whether an “average” effect is really meaningful or useful. If there is a reasonably large number of trials, the investigators may shift to exploring effect modification with high heterogeneity, however this may not be possible if few trials are available. While many would likely agree that pooling (or reporting pooled results) should be avoided when there are few studies and statistical heterogeneity is high, what constitutes “few” studies and “high” heterogeneity is a matter of judgment.
While there are a variety of methods for characterizing statistical heterogeneity, one common method is the I 2 statistic, the proportion of total variance in the pooled trials that is due to inter-study variance, as opposed to random variation. 22 The Cochrane manual proposes ranges for interpreting I 2 : 10 statistical heterogeneity associated with I 2 values of 0-40% might not be important, 30-60% may represent moderate heterogeneity, 50-90% may represent substantial heterogeneity, and 75-100% is considerable heterogeneity. Ranges overlap to reflect that other factors—such as the number and size of the trials and the magnitude and direction of the effect—must be taken into consideration. Other measures of statistical heterogeneity include Cochrane’s Q and τ 2 , but these heterogeneity statistics do not have intrinsic standardized scales that allow specific values to be characterized as “small,” “medium,” or “large” in any meaningful way. 23 However, τ 2 can be interpreted on the scale of the pooled effect, as the variance of the true effect. All these measures are discussed in more detail in Chapter 4 .
Although widely used in quantitative synthesis, the I 2 statistic has come under criticism in recent years. One important issue with I 2 is that it can be an inaccurate reflection of statistical heterogeneity when there are few studies to pool and high statistical heterogeneity. 24 , 25 For example, in random effects models (but not fixed effects models), calculations demonstrate that I 2 tends to underestimate true statistical heterogeneity when there are fewer than about 10 studies and the I 2 is 50% or more. 26 In addition, I 2 is correlated with the sample size of the included studies, generally increasing with larger samples. 27 Complicating this, meta-analyses of continuous measures tend to have higher heterogeneity than those of binary outcomes, and I 2 tends to increase as the number of studies increases when analyzing continuous outcomes, but not binary outcomes. 28 , 29 This has prompted some authors to suggest that different standards may be considered for interpreting I 2 for meta-analyses of continuous and binary outcomes, but I 2 should only be considered reliable when there are a sufficient number of studies. 29 Unfortunately there is not clear consensus regarding what constitutes a sufficient number of studies for a given amount of statistical heterogeneity, nor is it possible to be entirely prescriptive, given the limits of I 2 as a measure of heterogeneity. Thus, I 2 is one piece of information that should be considered, but generally should not be the primary deciding factor for whether to pool.
1.7. Conclusion
In the end, the decision to pool boils down to the question: will the results of a meta-analysis help you find a scientifically valid answer to a meaningful question? That is, will the meta-analysis provide something in addition to what can be understood from looking at the studies individually? Further, do the clinical, methodological, and statistical features of the body of studies permit them to be quantitatively combined and summarized in a valid fashion? Each of these decisions can be broken down into specific considerations (outlined in Figure 1.1 ) There is broad guidance to inform investigators in making each of these decisions, but generally the choices involved are subjective. The investigators’ scientific goal might factor into the evaluation of these considerations: for example, if investigators seek a general summary of the combined effect (e.g., direction only) versus an estimated effect size, the consideration of whether to pool may be weighed differently. In the end, to provide a meaningful result, the trials must be similar enough in content, procedures, and implementation to represent a cohesive group that is relevant to real practice/decision-making.
Recommendations
- Use Figure 1.1 when deciding whether to pool studies
Chapter 2. Optimizing Use of Effect Size Data
2.1. introduction.
The employed methods for meta-analysis will depend upon the nature of the outcome data. The two most common data types encountered in trials are binary/dichotomous (e.g., dead or alive, patient admitted to hospital or not, treatment failure or success, etc.) and continuous (e.g., weight, systolic blood pressure, etc.). Some outcomes (e.g., heart rate, counts of common events) that are not strictly continuous, are often treated as continuous for the purposes of meta-analysis based on assumptions of normality and the belief that statistical methods that are applied to normal distributions can be applicable to other distributions (central limit theory). Continuous outcomes are also frequently analyzed as binary outcomes when there are clinically meaningful cut-points or thresholds (e.g., a patient’s systolic blood pressure may be classified as low or high based on whether it is under or over 130mmHG). While this type of dichotomization may be more clinically meaningful it reduces statistical information, so investigators should provide their rationale for taking this approach.
Other less common data types that do not fit into either the binary or continuous categories include ordinal, categorical, rate, and time to event to data. Meta-analyzing these types of data will usually require reporting of the relevant statistics (e.g., hazard ratio, proportional odds ratio, incident rate ratio) by the study authors.
2.2. Nuances of Binary Effect Sizes
Data needed for binary effect size computation.
Under ideal circumstances, the minimal data necessary for the computation of effect sizes of binary data would be available in published trial documents or from original sources. Specifically, risk difference (RD), relative risk (RR), and odds ratios (OR) can be computed when the number of events (technically the number of cases in whom there was an event) and sample sizes are known for treatment and control groups. A schematic of one common approach to assembling binary data from trials for effect size computation is presented in Table 2.1 . This approach will facilitate conversion to analysis using commercially-available software such as Stata (College Station, TX) or Comprehensive Meta-Analysis (Englewood, NJ).
Assembling binary data for effect size computation.
In many instances, a single study (or subset of studies) to be included in the meta-analysis provides only one measure of association (an odds ratio, for example), and the sample size and event counts are not available. In that case, the meta-analytic effect size will be dictated by the available data. However, choosing the appropriate effect size is important for integrity and transparency, and every effort should be made to obtain all the data presented in Table 2.1 . Note that CONSORT guidance requires that published trial data should include the number of events and sample sizes for both treatment and control groups. 30 And, PRISMA guidance supports describing any processes for obtaining and confirming data from investigators 31 – a frequently required step.
In the event that data are only available in an effect size from the original reports, it is important to extract both the mean effect sizes and the associated 95% confidence intervals. Having raw event data available as in Table 2.1 not only facilitates the computation of various effect sizes, but also allows for the application of either binomial (preferred) or normal likelihood approaches; 32 only normal likelihood can be applied to summary statistics (e.g., an odds ratio and confidence interval in the primary study report).
Choosing Among Effect Size Options
One absolute measure and two relative measures are commonly used in meta-analyses involving binary data. The RD (an absolute measure) is a simple metric that is easily understood by clinicians, patients, and other stakeholders. The relative measures, RR or OR, are also used frequently. All three metrics should be considered additive, just on different scales. That is, RD is additive on a raw scale, RR on a log scale, and OR on a logit scale.
Risk Difference
The RD is easily understood by clinicians and patients alike, and therefore most useful to aid decision making. However, the RD tends to be less consistent across studies compared with relative measures of effect size (RR and OR). Hence, the RD may be a preferred measure in meta-analyses when the proportions of events among control groups are relatively common and similar across studies. When events are rare and/or when event rates differ across studies, however, the RD is not the preferred effect size to be used in meta-analysis because combined estimates based on RD in such instances have more conservative confidence intervals and lower statistical power. The calculation of RD and other effect size metrics using binary data from clinical trials can be performed considering the following labeling ( Table 2.2 ).
Organizing binary data for effect size computation.
Equation Set 2.1. Risk Difference
- RD = risk difference
- V RD = variance of the risk difference
- SE RD = standard error of the risk difference
- LL RD = lower limit of the 95% confidence interval of the risk difference
- UL RD = upper limit of the 95% confidence interval of the risk difference
Number Needed To Treat Related to Risk Difference
- NNT = number needed to treat
In case of a negative RD, the number needed to harm (NNH) or number needed to treat for one patient to be harmed is = − 1/RD.
The Wald method 34 is commonly used to calculate confidence intervals for NNT. It is reasonably adequate for large samples and probabilities not close to either 0 or 1, however it can be less reliable for small samples, probabilities close to either 0 or 1, or unbalanced trial designs. 35 An adjustment to the Wald method (i.e., adding pseudo-observations) helps mitigate concern about its application in small samples, 36 but it doesn’t account for other sources of limitations to this method. The Wilson method of calculating confidence intervals for NNT, as described in detail by Newcome, 37 has better coverage properties irrespective of sample size, is free of implausible results, and is argued to be easier to calculate compared with Wald confidence intervals. 35 Therefore, the Wilson method is preferable to the Wald method for calculating confidence intervals for NNT. When considering using NNT as the effect size in meta-analysis, see commentary by Lesaffre and Pledger.38 When considering using NNT as the effect size in meta-analysis, see commentary on the superior performance of combined NNT on the RD scale as opposed to the NNT scale.
It is important to note that the RR and OR are effectively equivalent for event rates below about 10%. In such cases, the RR is chosen over the OR simply for interpretability (an important consideration) and not substantive differences. A potential drawback to the use of RR over OR (or RD) is that the RR of an event is not the reciprocal of the RR for the non-occurrence of that event (e.g., using survival as the outcome instead of death). In contrast, switching between events and non-occurrence of events is reciprocal in the metric of OR and only entails a change in the sign of OR. If switching between death and survival, for example, is central to the meta-analysis, then the RR is likely not the binary effect size metric of choice unless all raw data are available and re-computation is possible. Moreover, investigators should be particularly attentive to the definition of an outcome event when using a RR.
The calculation of RR using binary data can be performed considering the labeling listed in Table 2.2 . Of particular note, the metrics of dispersion related to the RR are first computed in a natural log metric and then converted to the metric of RR.
Equation Set 2.2. Risk Ratio
- RR = risk ratio
- ln RR = natural log of the risk ratio
- V lnRR = variance of the natural log of the risk ratio
- SE lnRR = standard error of the natural log of the risk ratio
- LLlnRR = lower limit of the 95% confidence interval of the natural log of the risk ratio
- UL lnRR = upper limit of the 95% confidence interval of the natural log of the risk ratio
- LL RR = lower limit of the 95% confidence interval of the risk ratio
- UL RR = upper limit of the 95% confidence interval of the risk ratio
Therefore, while the definition of the outcome event needs to be consistent among the included studies when using any measure, the investigators should be particularly attentive to the definition of an outcome event when using an RR.
Odds Ratios
An alternative relative metric for use with binary data is the OR. Given that ORs are frequently presented in models with covariates, it is important to note that the OR is ‘non-collapsible,’ meaning that effect modification varies depending on the covariates for which control has been made; this favors the reporting of RR over OR, particularly when outcomes are common and covariates are included. 39 The calculation of OR using binary data can be performed considering the labeling listed in Table 2.2 . Similar to the computation of RR, the metrics of dispersion related to the OR are first computed in a natural log metric and then converted to the metric of OR.
Equation Set 2.3. Odds ratios
- OR = odds ratio
- Ln OR = natural log of the odds ratio
- V lnOR = variance of the natural log of the odds ratio
- SE lnoR = standard error of the natural log of the odds ratio
- LLlnOR = lower limit of the 95% confidence interval of the natural log of the odds ratio
- UL lnOR = upper limit of the 95% confidence interval of the natural log of the odds ratio
- LL OR = lower limit of the 95% confidence interval of the odds ratio
- UL OR = upper limit of the 95% confidence interval of the odds ratio
A variation on the calculation of OR is the Peto OR that is commonly referred to as the assumption-free method of calculating OR. The two key differences between the standard OR and the Peto OR is that the latter takes into consideration the expected number of events in the treatment group and also incorporates a hypergeometric variance. Because of these difference, the Peto OR is preferred for binary studies with rare events, especially when event rates are less than 1%. But in contrast, the Peto OR is biased when treatment effects are large, due to centering around the null hypothesis, and in the instance of imbalanced treatment and control groups. 40
Equation Set 2.4. Peto odds ratios
ORpeto = exp [ { A − E ( A ) } / v ] where E(A) is the expected number of events in the treatment group calculated as: E ( A ) = n 1 ( A + E ) N and v is hypergeometric variance, calculated as: v = { n 1 n 2 ( A + C ) ( B + D ) } / { N 2 ( N − 1 ) }
There is no perfect effect size of binary data to choose because each has benefits and disadvantages. Criteria used to compare and contrast these measures include consistency over a set of studies, statistical properties, and interpretability. Key benefits and disadvantages of each are presented in Table 2.3 . In the table, the term “baseline risk” is the proportion of subjects in the control group who experienced the event. The term “control rate” is sometimes used for this measure as well.
Benefits and disadvantages of binary data effect sizes.
Time-to-Event and Count Outcomes
For time to event data, the effect size measure is a hazard ratio (HR), which is commonly estimated from the Cox proportional hazards model. In the best-case scenario, HR and associated 95% confidence intervals are available from all studies, the time horizon is similar across studies, and there is evidence that the proportional hazards assumption was met in each study to be included in a meta-analysis. When these conditions are not met, an HR and associated dispersion can still be extracted and meta-analyzed. However, this approach raises concerns about reproducibility due to observer variation. 44
Incident rate ratio (IRR) is used for count data and can be estimated from a Poisson or negative binomial regression model. The IRR is a relative metric based on counts of events (e.g., number of hospitalizations, or days of length of stay) over time (i.e., per person-year) compared between trial arms. It is important to consider how IRR estimates were derived in individual studies particularly with respect to adjustments for zero-inflation and/or over-dispersion as these modeling decisions can be sources of between-study heterogeneity. Moreover, studies that include count data may have zero counts in both groups, which may require less common and more nuanced approaches to meta-analysis like Poisson regression with random intervention effects. 45
2.3. Continuous Outcomes
Assembling data needed for effect size computation.
Meta-analysis of studies presenting continuous data requires both estimated differences between the two groups being compared and estimated standard errors of those differences. Estimating the between-group difference is easiest when the study provides the mean difference. While both a standardized mean difference and ratio of means could be given by the study authors, studies more often report means for each group. Thus, a mean difference or ratio of means often must be computed.
If estimates of the standard errors of the mean are not provided studies commonly provide confidence intervals, standard deviations, p-values, z-statistics, and/or t-statistics, which make it possible to compute the standard error of the mean difference. In the absence of any of these statistics, other methods are available to estimate standard error. 45
(Weighted) Mean Difference
The mean difference (formerly known as weighted mean difference) is the most common way of summarizing and pooling a continuous outcome in a meta-analysis. Pooled mean differences can be computed when every study in the analysis measures the outcome on the same scale or on scales that can be easily converted. For example, total weight can be pooled using mean difference even if different studies reported weights in kilograms and pounds; however it is not possible to pool quality of life measured in both Self Perceived Quality of Life scale (SPQL) and the 36-item Short Form Survey Instrument (SF-36), since these are not readily convertible to one format.
Computation of the mean difference is straightforward and explained elsewhere. 5 Most software programs will require the mean, standard deviation, and sample size from each intervention group and for each study in the meta-analysis, although as mentioned above, other pieces of data may also be used.
Some studies report values as change from baseline, or alternatively present both baseline and final values. In these cases, it is possible to pool differences in final values in some studies with differences in change from baseline values in other studies, since they will be estimating the same value in a randomized control trial. If baseline values are unbalanced it may be better to perform ANCOVA analysis (see below). 5
Standardized Mean Difference
Sometimes different studies will assess the same outcome using different scales or metrics that cannot be readily converted to a common measure. In such instances the most common response is to compute a standardized mean difference (SMD) for each study and then pool these across all studies in the meta-analysis. By dividing the mean difference by a pooled estimate of the standard deviation, we theoretically put all scales in the same unit (standard deviation), and are then able to statistically combine all the studies. While the standardized mean difference could be used even when studies use the same metric, it is generally preferred to use mean difference. Interpretation of results is easier when the final pooled estimate is given in the same units as the original studies.
Several methods can compute SMDs. The most frequently used are Cohen’s d and Hedges’ g .
Cohen’s d
Cohen’s d is the simplest S. computation; it is defined as the mean difference divided by the pooled standard deviation of the treatment and control groups. 5 For a given study, Cohen’s d can be computed as: d = m T − m C s p o o l e d
Where m T and m C are the treatment and control means and spooled is essentially the square root of the weighted average of the treatment and control variances.
It has been shown that this estimate is biased in estimating the true population SMD, and the bias decreases as the sample size increases (small sample bias). 46 For this reason, Hedges g is more often used.
Hedges’ g
Hedges’ g is a transformation of Cohen’s d that attempts to adjust for small sample bias. The transformation involves multiplying Cohen’s d by a function of the total sample size.5 This generally results in a slight decrease in value of Hedges’ g compared with Cohen’s d, but the reduction lessens as the total sample size increases. The formula is: d ( 1 − 3 4 N − 9 )
Where N is the total trial sample size.
For very large sample sizes the two estimates will be very similar.
Back Transformation of Pooled SMD
One disadvantages of reporting standardized mean difference is that units of standard deviation are difficult to interpret clinically. Guidelines do exist but are often thought to be arbitrary and not applicable to all situations.47 An alternative is to back transform the pooled SMD into a scale used in the one of the analyses. In theory, by multiplying the SMD (and its upper and lower confidence bounds) by the standard deviation of the original scale, one can obtain a pooled estimate in that original scale. The difficulty is that the true standard deviation is unknown and must be estimated from available data. Alternatives for estimation include using the standard deviation from the largest study or using a pooled estimate of the standard deviations across studies.5 One should include a sensitivity analysis and be transparent about the approach used.
Ratio of Means
Ratio of Means (RoM), also known as response ratio, has been presented as an alternative to the SMD when outcomes are reported in different non-convertible scales. As the name implies the RoM divides the treatment mean by the control mean rather than taking the difference between the two. The ratio can be interpreted as the percentage change in the mean value of the treatment group relative to the control group. By meta-analyzing across studies we are making the assumption that the relative change will be homogeneous across all studies, regardless of which scale was used to measure it. Similar to the risk ratio and odds ratio, the RoM is pooled on the log scale; computational formulas are readily available. 5
For the RoM to have any clinical meaning, it is required that in the scale being used, the values are always positive (or always negative) and that a value of “zero” truly means zero. For example, if the outcome were patient temperature, RoM would be a poor choice since a temperature of 0 degrees does not truly represent what we would think of as zero.
2.4. Special Topics
Crossover trials.
A crossover trial is one where all patients receive, in sequence, both the treatment and control interventions. This results in the final data having the same group of patients represented with both their outcome values while in the treatment and control groups. When computing the standard error of the mean difference of a crossover trial, one must consider the correlation between the two groups—a result of the two measurements on different within-person treatments. 5 For most variables, the correlation will be positive, resulting in a smaller standard error than would be seen with the same values in a parallel trial.
To compute the correct pooled standard error requires an estimate of the correlation between the two groups. If correlation is available, the pooled standard error can be computed using the following formula: S E P = S E T 2 + S E C 2 + 2 r S E T S E C
Where r is the within-patient correlation and SE P , SE T , and SE C are the pooled, treatment, and control standard errors respectively
Most studies do not give the correlation or enough information to compute it, and thus it often has to be estimated based on investigator knowledge or imputed. 5 An imputation of 0.5 has been suggested as a good conservative estimate of correlation in the absence of any other information. 48
If a cross-over study reports its data by period, investigators have sometimes used first period data only when including cross-over trials in their meta-analyses—essentially treating the study as if it were a parallel design. This eliminates correlation issues, but has the disadvantage of omitting half the data from the trial.
Cluster Randomized Trials
Cluster trials occur when patients are randomized to treatment and control in groups (or clusters) rather than individually. If the units/subjects within clusters are positively correlated (as they usually are), then there is a loss of precision compared to a standard (non-clustered) parallel design of the same size. The design effect (DE) of a cluster randomized trial is the multiplicative multiplier needed to adjust the standard error computed as if the trial were a standard parallel design. Reported results from cluster trials may not reflect the design effect, and thus it will need to be computed by the investigator. The formula for computing the design effect is: D E = 1 + ( M − 1 ) I C C
Where M is the average cluster size and ICC is the intra-class correlation coefficient (see below).
Computation of the design effect involves a quantity known as the intra-class correlation coefficient (ICC), which is defined as the proportion of the total variance (i.e., within cluster variance plus between cluster variance) that is due to between cluster variance. 5 ICC’s are often not reported by cluster trials and thus a value must be obtained from external literature or a plausible value must be assumed by the investigator.
Mean Difference and Baseline Imbalance
- Use followup data.
- Use change from baseline data.
- Use an ANCOVA model that adjusts for the effects of baseline imbalance. 49
As long as trials are balanced at baseline, all three methods will give similar unbiased estimates of mean difference. 5 When baseline imbalance is present, it can be shown that using ANCOVA will give the best estimate of the true mean difference; however the parameters required to perform this analysis (mean and standard deviations of baseline, follow-up and change from baseline values) are usually not provided by the study authors. 50 If it is not feasible to perform an ANCOVA analysis, the choice of whether to use follow up or change from baseline values depends on the amount of correlation between baseline and final values. If the correlation is less than or equal to 0.5, then using the follow up values will be less biased (with respect to the estimate in the ANCOVA model) than using the change from baseline values. If the correlation is greater than 0.5, then change from baseline values will be less biased than using the follow up values. 51 There is evidence that these correlations are more often greater than 0.5, so the change from baseline means will usually be preferred if estimates of correlation are totally unobtainable. 52 A recent study 51 showed that all approaches were unbiased when there were both few trials and small sample sizes within the trials.
- The analyst should consider carefully which binary measure to analyze.
- If conversion to NNT or NNH is sought, then the risk difference is the preferred measure.
- The risk ratio and odds ratio are likely to be more consistent than the risk difference when the studies differ in baseline risk.
- The risk difference is not the preferred measure when the event is rare.
- The risk ratio is not the preferred measure if switching between occurrence and non occurrence of the event is important to the meta-analysis.
- The odds ratio can be misleading.
- The mean difference is the preferred measure when studies use the same metric.
- When calculating standardized mean difference, Hedges’ g is preferred over Cohen’s d due to the reduction in bias.
- If baseline values are unbalanced, one should perform an ANCOVA analysis. If ANCOVA cannot be performed and the correlation is greater than 0.5, change from baseline values should be used to compute the mean difference. If the correlation less than or equal to 0.5, follow-up values should be used.
- Data from clustered randomized trials should be adjusted for the design effect.
Chapter 3. Choice of Statistical Model for Combining Studies
3.1. introduction.
Meta-analysis can be performed using either a fixed or a random effects model to provide a combined estimate of effect size. A fixed effects model assumes that there is one single treatment effect across studies and any differences between observed effect sizes are due to sampling error. Under a random effects model, the treatment effects across studies are assumed to vary from study and study and follow a random distribution. The differences between observed effect sizes are not only due to sampling error, but also to variation in the true treatment effects. A random effects model usually assumes that the treatment effects across studies follow a normal distribution, though the validity of this assumption may be difficult to verify, especially when the number of studies is small. Alternative distributions 53 or distribution free models 54 , 55 have also been proposed.
Recent advances in meta-analysis include the development of alternative models to the fixed or random effects models. For example, Doi et al. proposed an inverse variance heterogeneity model (the IVhet model) for the meta-analysis of heterogeneous clinical trials that uses an estimator under the fixed effect model assumption with a quasi-likelihood based variance structure. 56 Stanley and Doucouliagosb proposed an unrestricted weighted least squares (WLS) estimator with multiplicative error for meta-analysis and claimed superiority to both conventional fixed and random effects, 57 though Mawdsley et al. 58 found modest differences when compared with the random effects model. These methods have not been fully compared with the many estimators developed within the framework of the fixed and random effects models and are not readily available in most statistical packages; thus they will not be further considered here.
General Considerations for Model Choice
Considerations for model choice include but are not limited to heterogeneity across treatment effects, the number and size of included studies, the type of outcomes, and potential bias. We recommend against choosing a statistical model based on the significance level of a heterogeneity test, for example, picking a fixed effects model when the p-value for the test of heterogeneity is more than 0.10 and a random effects model when P < 0.10, since such an approach does not take the many factors for model choice into full consideration.
In practice, clinical and methodological heterogeneity are always present across a set of included studies. Variation among studies is inevitable whether or not the test of heterogeneity detects it. Therefore, we recommend random effects models, with special considerations for rare binary outcomes (discussed below in the section on combining rare binary outcomes). For a binary outcome, when the estimate of between-study heterogeneity is zero, a fixed effects model (e.g., the Mantel-Haenszel method, inverse variance method, Peto method (for OR), or fixed effects logistic regression) provides an effect estimate similar to that produced by a random effects model. The Peto method requires that no substantial imbalance exists between treatment and control group sizes within trials and treatment effects are not exceptionally large.
When a systematic review includes both small and large studies and the results of small studies are systematically different from those of the large ones, publication bias may be present and the assumption of a random distribution of effect sizes, in particular, a normal distribution, is not justified. In this case, neither the random effects model nor the fixed effects model provides an appropriate estimate and investigators may choose not to combine all studies. 10 Investigators can choose to combine only the large studies if they are well conducted with good quality and are expected to provide unbiased effect estimates. Other potential differences between small and large studies should also be examined.
Choice of Random Effects Model and Estimator
The most commonly used random effects model for combined effect estimates is based on an estimator developed by DerSimonian and Laird (DL) due to its simplicity and ease of implementation. 59 It is well recognized that the estimator does not adequately reflect the error associated with parameter estimation, in particular, when the number of studies is small, and between-study heterogeneity is high. 40 Refined estimators have been proposed by the original authors. 19 , 60 , 61 Other estimators have also been proposed to improve the DL estimator. Sidik and Jonkman (SJ) and Hartung and Knapp (HK) independently proposed a non-iterative variant of the DL estimator using the t-distribution and an adjusted confidence interval for the overall effect. 62 – 64 We refer to this as the HKSJ method. Biggerstaff–Tweedie (BT) proposed another variant of the DL method by incorporating error in the point estimate of between-study heterogeneity into the estimation of the overall effect. 65 There are also many other likelihood based estimators such as maximum likelihood estimate, restricted maximum likelihood estimate and profile likelihood (PL) methods, which better account for the uncertainty in the estimate of between-study variance. 19
Several simulation studies have been conducted to compare the performance of different estimators for combined effect size. 19 – 21 , 66 , 67 For example, Brockwell et al. showed the PL method provides an estimate with better coverage probability than the DL method. 19 Jackson et al. showed that with a small number of studies, the DL method did not provide adequate coverage probability, in particular, when there was moderate to large heterogeneity. 20 However, these results supported the usefulness of the DL method for larger samples. In contrast, the PL estimates resulted in coverage probability closer to nominal values. IntHout et al. compared the performance of the DL and HKSJ methods and showed that the HKSJ method consistently resulted in more adequate error rates than did the DL method, especially when the number of studies was small, though they did not evaluate coverage probability and power. 67 Kontopantelis and Reeves conducted the most comprehensive simulation studies to compare the performance of nine different methods and evaluated multiple performance measures including coverage probability, power, and overall effect estimation (accuracy of point estimates and error intervals). 21 When the goal is to obtain an accurate estimate of overall effect size and the associated error interval, they recommended using the DL method when heterogeneity is low and using the PL method when heterogeneity is high, where the definition of high heterogeneity varies by the number of studies. The PL method overestimated coverage probability in the absence of between-study heterogeneity. Methods like BT and HKSJ, despite being developed to address the limitations of the DL method, were frequently outperformed by the DL method. Encouragingly, Kontopantelis and Reeves also showed that regardless of the estimation method, results are highly robust against even very severe violations of the assumption of normally distributed effect sizes.
Recently there has been a call to use alternative random-effects estimators to replace the universal use of the Dersimonian-Laird random effects model. 68 Based on the results from the simulation studies, the PL method appears to generally perform best, and provides best performance across more scenarios than other methods, though it may overestimate the confidence intervals in small studies with low heterogeneity. 21 It is appropriate to use the DL method when the heterogeneity is low. Another disadvantage of the PL method is that it does not always converge. In those situations, investigators may choose the DL method with sensitivity analyses using other methods, such as the HKSJ method. If non-convergence is due to high heterogeneity, investigators should also reevaluate the appropriateness of combining studies. The PL method (and the DL method) could be used to combine measures for continuous, count, and time to event data, as well as binary data when events are common. Note that the confidence interval produced by the PL method may not be symmetric. It is also worth noting that OR, RR, HR, and incidence rate ratio statistics should be analyzed on the logarithmic scale when the PL, DL, or HKSJ method is used. Finally, a Bayesian approach can also be used since this approach takes the variations in all parameters into account (see the section on Bayesian methods, below).
Role of Generalized Linear Mixed Effects Models
The different methods and estimators discussed above are generally used to combine effect measures directly (for example, mean difference, SMD, OR, RR, HR, and incidence rate ratio). For study-level aggregated binary data and count data, we also recommend the use of the generalized linear mixed effects model assuming random treatment effects. For aggregated binary data, a combined OR can be generated by assuming the binomial distribution with a logit link. It is also possible to generate a combined RR with the binomial distribution and a log link, though the model does not always converge. For aggregated count data, a combined rate ratio can be generated by assuming the Poisson distribution with a log link. Results from using the generalized linear models and directly combining effect measures are similar when the number of studies and/or the sample sizes are large.
3.2. A Special Case: Combining Rare Binary Outcomes
When combining rare binary outcomes (such as adverse event data), few or zero events often occur in one or both arms in some of the studies. In this case, the binomial distribution is not well-approximated by the normal approximation and choosing an appropriate model becomes complicated. The DL method does not perform well with low-event rate binary data. 43 , 69 A fixed effects model often out performs the DL method even in the presence of heterogeneity. 70 When event rates are less than 1 percent, the Peto OR method has been shown to provide the least biased, most powerful combined estimates with the best confidence interval coverage, 43 if the included studies have moderate effect sizes and the treatment and control group are of relatively similar sizes. The Peto method does not perform well when either the studies are unbalanced or the studies have large ORs (outside the range of 0.2-5). 71 , 72 Otherwise, when treatment and control group sizes are very different, effect sizes are large, or when events become more frequent (5 percent to 10 percent), the Mantel-Haenszel method (without a correction factor) or a fixed effects logistic regression provide better combined estimates.
Within the past few years, many methods have been proposed to analyze sparse data from simple averaging, 73 exact methods, 74 , 75 Bayesian approaches 76 , 77 to various parametric models (e.g., generalized linear mixed effect models, beta-binomial model, Gamma-Poisson model, bivariate Binomial-Normal model etc.). Two dominating opinions are to not use continuity corrections, and to include studies with zero events in both arms in the meta-analysis. Great efforts have been made to develop methods that can include such studies.
Bhaumik et al. proposed the simple (unweighted) average (SA) treatment affect with the 0.5 continuity correction, and found that the bias of the SA estimate in the presence of even significant heterogeneity is minimal compared with the bias of MH estimates (with 0.5 correction). 73 A simple average was also advocated by Shuster. 78 However, potential confounding remains an issue for an unweighted estimator. Spittal et al. showed that Poisson regression works better than the inverse variance method for rare events. 79 Kuss et al. conducted a comprehensive simulation of eleven methods, and recommended the use of the beta-binomial model for the three common effect measures (OR, RR, and RD) as the preferred meta-analysis methods for rare binary events with studies of zero events in one or both arms. 80 The beta-binomial model assumes that the observed events follow a binomial distribution and the binomial probabilities follow a beta distribution. In Kuss’s simulation, using a generalized linear model framework to model the treatment effect, an OR was estimated using a logit link, and an RR, using a log link. Instead of using an identity link, RD was estimated based on the estimated event probabilities from the logit model. This comprehensive simulation examined methods that could incorporate data from studies with zero events from both arms and do not need any continuity correction, and only compared the Peto and MH methods as reference methods.
Given the development of new methods that can handle studies with zero events in both arms, we advise that older methods that use continuity corrections be avoided. Investigators should use valid methods that include studies with zero events in one or both arms. For studies with zero events in one arm, or studies with sparse binary data but no zero events, an estimate can be obtained using the Peto method, the Mantel-Haenszel method, or a logistic regression approach, without adding a correction factor, when the between-study heterogeneity is small. These methods are simple to use and more readily available in standard statistical packages. When the between-study heterogeneity is large and/or there are studies with zero events in both arms, the more recently developed methods, such as beta-binomial model, could be explored and used. However, investigators should note that no method gives completely unbiased estimates when events are rare. Statistical methods can never completely solve the issue of sparse data. Investigators should always conduct sensitivity analyses 81 using alternative methods to check the robustness of results to different methods, and acknowledge the inadequacy of data sources when presenting the meta-analysis results, in particular, when the proportion of studies with zero events in both arms are high. If double-zero studies are to be excluded, they should be qualitatively summarized, by providing information on the confidence intervals for the proportion of events in each arm.
A Note on an Exact Method for Sparse Binary Data
For rare binary events, the normal approximation and asymptotic theory for large sample size does not work satisfactorily and exact inference has been developed to overcome these limitations. Exact methods do not need continuity corrections. However, simulation analyses do not identify a clear advantage of early developed exact methods 75 , 82 over a logistic regression or the Mantel-Haenszel method even in situations where these exact methods would theoretically be advantageous. 43 Recent developments of exact methods include Tian et al.’s method of combining confidence intervals 83 and Liu et al.’s method of combining p-value functions. 84 Yang et al. 85 developed a general framework for meta-analysis of rare events by combining confidence distributions (CDs), and showed that Tian’s and Liu’s methods could be unified under the CD framework. Liu showed that exact methods performed better than the Peto method (except when studies are unbalanced) and the Mantel-Haenszel method, 84 though the comparative performance of these methods has not been thoroughly evaluated. Investigators may choose to use exact methods with considerations for the interpretation of effect measures, but we do not specifically recommend exact methods over other models discussed above.
3.3. Bayesian Methods
A Bayesian framework provides a unified and comprehensive approach to meta-analysis that accommodates a wide variety of outcomes, often, using generalized linear model (GLM) with normal, binomial, Poisson and multinomial likelihoods and various link functions. 86
It should be noted that while these GLM models are routinely implemented in the frequentist framework, and are not specific to the Bayesian framework, extensions to more complex situations are most approachable using the Bayesian framework, for example, allowing for mixed treatment comparisons involving repeated measurements of a continuous outcome that varies over time. 87
There are several specific advantages inherent to the Bayesian framework. First, the Bayesian posterior parameter distributions fully incorporate the uncertainty of all parameters. These posterior distributions need not be assumed to be normal. 88 In random-effects meta-analysis, standard methods use only the most likely value of the between-study variance, 59 rather than incorporating the full uncertainty of each parameter. Thus, Bayesian credible intervals will tend to be wider than confidence intervals produced by some classical random-effects analysis such as the DL method. 89 However, when the number of studies is small, the between-study variance will be poorly estimated by both frequentist and Bayesian methods, and the use of vague priors can lead to a marked variation in results, 90 particularly when the model is used to predict the treatment effect in a future study. 91 A natural alternative is to use an informative prior distribution, based on observed heterogeneity variances in other, similar meta-analyses. 92 – 94
Full posterior distributions can provide a more informative summary of the likely value of parameters than the frequentist approach. When communicating results of meta-analysis to clinicians, the Bayesian framework allows direct probability statements to be made and provides the rank probability that a given treatment is best, second best, or worst (see the section on interpreting ranking probabilities and clinically important results in Chapter 5 below). Another advantage is that posterior distributions of functions of model parameters can be easily obtained such as the NNT. 86 Finally, the Bayesian approach allows full incorporation of parameter uncertainty from meta-analysis into decision analyses. 95
Until recently, Bayesian meta-analysis required specialized software such as WinBUGS, 96 OpenBUGS, 97 and JAGS. 98 , 99 Newer open source software platforms such as Stan 100 and Nimble 101 , 102 provide additional functionality and use BUGS-like modeling languages. In addition, there are user written commands that allow data processing in a familiar environment which then can be passed to WinBUGS, or JAGS for model fitting. 103 For example, in R, the package bmeta currently generates JAGS code to implement 22 models. 104 The R package gemtc similarly automates generation of JAGS code and facilitates assessment of model convergence and inconsistency. 105 , 106 On the other hand, Bayesian meta-analysis can be implemented in commonly used statistical packages. For example, SAS PROC MCMC can now implement at least some Bayesian hierarchical models 107 directly, as can Stata, version 14, via the bayesmh command. 108
When vague prior distributions are used, Bayesian estimates are usually similar to estimates obtained from the above frequentist methods. 90 Use of informative priors requires considerations to avoid undue influence on the posterior estimates. Investigators should provide adequate justifications for the choice of priors and conduct sensitivity analyses. Bayesian methods currently require more work in programming, MCMC simulation and convergence diagnostics.
A Note on Using a Bayesian Approach for Sparse Binary Data
It has been suggested that using a Bayesian approach might be a valuable alternative for sparse event data since Bayesian inference does not depend on asymptotic theory and takes into account all uncertainty in the model parameters. 109 The Bayesian fixed effects model provides good estimates when events are rare for binary data. 70 However, the choice of prior distribution, even when non-informative, may impact results, in particular, when a large proportion of studies have zero events in one or two arms. 80 , 90 , 110 Nevertheless, other simulation studies found that when the overall baseline rate is very small and there is moderate or large heterogeneity, Bayesian hierarchical random effect models can provide less biased estimates for the effect measures and the heterogeneity parameters. 77 To reduce the impact of the prior distributions, objective Bayesian methods have been developed 76 , 111 with special attention paid to the coherence between the prior distributions of the study model parameters and the meta-parameter, 76 though the Bayesian model was developed outside the usual hierarchical normal random effects framework. Further evaluations of these methods are required before recommendations of these objective Bayesian methods might be made.
3.4. Recommendations
- The PL method appears to generally perform best. The DL method is also appropriate when the between-study heterogeneity is low.
- For study-level aggregated binary data and count data, the use of a generalized linear mixed effects model assuming random treatment effects is also recommended.
- Methods that use continuity corrections should be avoided.
- For studies with zero events in one arm, or studies with sparse binary data but no zero events, an estimate can be obtained using the Peto method, the Mantel-Haenszel method, or a logistic regression approach, without adding a correction factor, when the between-study heterogeneity is low.
- When the between-study heterogeneity is high, and/or there are studies with zero events in both arms, more recently developed methods such as a beta-binomial model could be explored and used.
- Sensitivity analyses should be conducted with acknowledgement of the inadequacy of data.
- If investigators choose Bayesian methods, use of vague priors is supported.
Chapter 4. Quantifying, Testing, and Exploring Statistical Heterogeneity
4.1. statistical heterogeneity in meta-analysis.
Statistical heterogeneity was explained in general in Chapter 1 . In this chapter, we provide a deeper discussion from a methodological perspective. Statistical heterogeneity must be expected, quantified and sufficiently addressed in meta-analyses. 112 We recommend performing graphic and quantitative exploration of heterogeneity in combination. 113 In this chapter, it is assumed that a well-specified research question has been posed, the relevant literature has been reviewed, and a set of trials meeting selection criteria have been identified. Even when trial selection criteria are aimed toward identifying studies that are adequately homogenous, it is common for trials included in a meta-analysis to differ considerably as a function of (clinical and/or methodological) heterogeneity that was reviewed in Chapter 1 . Even when these sources of heterogeneity have been accounted for, statistical heterogeneity often remains. Statistical heterogeneity refers to the situation where estimates across studies have greater variability than expected from chance variation alone. 113 , 114
4.2. Visually Inspecting Heterogeneity
Although simple histograms, box plots, and other related graphical methods of depicting effect estimates across studies may be helpful preliminarily, these approaches do not necessarily provide insight into statistical heterogeneity. However, forest and funnel plots can be helpful in the interpretation of heterogeneity particularly when examined in combination with quantitative results. 113 , 115
Forest Plots
Forest plots can help identify potential sources and the extent of statistical heterogeneity. Meta-analyses with limited heterogeneity will produce forest plots with grossly visual overlap of study confidence intervals and the summary estimate. In contrast, a crude sign of statistical heterogeneity would be poor overlap. 115 An important recommendation is to graphically present between-study variance on forest plots of random effects meta-analyses using prediction intervals, which are on the same scale as the outcome. 93 The 95% prediction interval estimates where true effects would be expected for 95% of future studies. 93 When between-study variance is greater than zero, the prediction interval will cover a wider range than the confidence interval of the summary effect. 116 As proposed by Guddat et al. 117 and endorsed by IntHout et al., 116 including the prediction interval as a rectangle at the bottom of forest plots helps differentiate between-study variation from the confidence interval of the summary effect that is commonly depicted as a diamond.
Funnel Plots
Funnel plots are often thought of as representing bias, but they also can aid in detecting sources of heterogeneity. Funnel plots are essentially the plotting of effect sizes observed in each study (x-axis) around the summary effect size versus the degree of precision of each study (typically by standard error, variance, or precision on the y-axis). A meta-analysis that includes studies that estimate the same underlying effect across a range of precision, and has limited bias and heterogeneity would result in a funnel plot that resembles a symmetrical inverted funnel shape with increasing dispersion ranging with less precise (i.e., smaller) studies. 115 In the event of heterogeneity and/or bias, funnel plots will take on an asymmetric pattern around the summary effect size and also provide evidence of scatter outside the bounds of the 95% confidence limits. 115 Asymmetry in funnel plots can be difficult to detect visually, 118 and can be misleading due to multiple contributing factors. 113 , 119 , 120 Formal tests for funnel plot asymmetry (such as Egger’s test 15 for continuous outcomes, or the arcsine test proposed by Rucker et al., 27 for binary data) are available but should not be used with a meta-analysis involving fewer than 10 studies because of limited power. 113 Given the above cautions and considerations, funnel plots should only be used to complement other approaches in the preliminary analysis of heterogeneity.
4.3. Quantifying Heterogeneity
The null hypothesis of homogeneity in meta-analysis is that all studies are evaluating the same effect, 22 (i.e., all studies have the same true effect parameter that may or may not be equivalent to zero) and the alternative hypothesis is that at least one study has an effect that is different from the summary effect.
- Where Q is the heterogeneity statistic,
- w is the study weight based on inverse variance weighting,
- x is the observed effect size in each trial, and
- x ^ w is the summary estimate in a fixed-effect meta-analysis.
The Q statistic is assumed to have an approximate χ 2 distribution with k – 1 degrees of freedom. When Q is in excess over k – 1 and the associated p-value is low (typically, a p-value of <0.10 is used as a cut-off), the null hypothesis of homogeneity can be rejected. 22 , 122 Interpretation of a Q statistic in isolation is not advisable however, because it has low statistical power in meta-analyses involving a limited number of studies 123 , 124 and may detect unimportant heterogeneity when the number of studies included in a meta-analysis is large. Importantly, since heterogeneity is expected in meta-analyses even without statistical tests to support that claim, non-significant Q statistics must not be interpreted as the absence of heterogeneity. Moreover, the interpretation of Q in meta-analyses is more complicated than typically represented, because the actual distribution of Q is dependent on the measure of effect 125 and only approximately χ 2 in large samples. 122 Even if the null distribution of Q were χ 2 , universally interpreting all values of Q greater than the mean of k − 1 as indicating heterogeneity would be an oversimplification. 122 There are expansions to approximate Q for meta-analyses of standardized mean difference, 125 risk difference, 125 and odds ratios 126 that should be used as alternatives to Q , particularly when sample sizes of studies included in a meta-analysis are small. 122 The Q statistic and expansions thereof must be interpreted along with other heterogeneity statistics and with full consideration of their limitations.
Graphical Options for Examining Contributions to Q
Hardy and Thompson proposed using probability plots to investigate the contribution that each study makes to Q . 127 When each study is labeled, those deviating from the normal distribution in a probability plot have the greatest influence on Q . 127 Baujat and colleagues proposed another graphical method to identify studies that have the greatest impact on Q . 128 Baujat proposed plotting the contribution to the heterogeneity statistic for each study on the horizontal axis, and the squared difference between meta-analytic estimates with and without the i th study divided by the estimated variance of the meta-analytic estimate without the i th study along the vertical axis. Because of the Baujat plot presentation, studies that have the greatest influence on Q are located in the upper right corner for easy visual identification. Smaller studies have been shown to contribute more to heterogeneity than larger studies, 129 which would be visually apparent in Baujat plots. We recommend using these graphical approaches only when there is significant heterogeneity, and only when it is important to identify specific studies that are contributing to heterogeneity.
Between-Study Variance
- Where τ 2 is the parameter of between-study variance of the true effects,
- DL is the DerSimonian and Laird approach to τ 2 ,
- Q is the heterogeneity statistic (as above),
- k -1 is the degrees of freedom, and
- w is the weight applied to each study based on inverse variance weighting.
Since variance cannot be less than zero, a τ 2 less than zero is set to zero. The value of τ 2 is integrated into the weights of random-effects meta-analysis as presented in Chapter 3 . Since the DerSimonian and Laird approach to τ 2 is derived in part from Q , the problems with Q described above apply to the τ 2 parameter. 122 There are many alternatives to DerSimonian and Laird when estimating between-study variance. In a recent simulation, Veroniki and colleagues 121 compared 16 estimators of between-study variance; they argued that the Paule and Mandel 130 method of estimating between-study variance is a better alternative to the DerSimonian and Laird parameter for continuous and binary data because it less biased (i.e., yields larger estimates) when between-study variance is moderate-to-large. 121 At the time of this guidance, the Paule and Mandel method of estimating between-study variance is only provisionally recommended as an alternative to DerSimonian and Laird. 129 , 131 Moreover, Veroniki and colleagues provided evidence that the restrictive maximum likelihood estimator 132 is a better alternative to the DerSimonian and Laird parameter of between-study variance for continuous data because it yields similar values for low-to-moderate between-study variance and larger estimates in conditions of high between-study variance. 121
Inconsistency Across Studies
Another statistic that should be generated and interpreted even when Q is not statistically significant is the proportion of variability in effect sizes across studies that is explained by heterogeneity vs. random error or I 2 that is related to Q . 22 , 133
- Where Q is the estimate of between-study variance, and
- k −1 is the degrees of freedom.
- Where τ 2 is the parameter of between-study variance, and
- σ 2 is the within-study variance.
I 2 is a metric of how much heterogeneity is influencing the meta-analysis. With a range from 0% (indicating no heterogeneity) to 100% (indicating that all of the observed variance is attributable to heterogeneity), the I 2 statistic has several advantages over other heterogeneity statistics including its relative simplicity as a signal-to-noise ratio, and focus on how heterogeneity may be influencing interpretation of the meta-analysis. 59 It is important to note that I 2 increases with increasing study precision and hence is dependent on sample size. 27 By various means, confidence/uncertainty intervals can be estimated for I 2 including Higgins’ test-based method. 22 , 23 the assumptions involved in the construction of 95% confidence intervals cannot be justified in all cases, but I 2 confidence intervals based on frequentist assumptions generally provide sufficient coverage of uncertainty in meta-analyses. 133 In small meta-analyses, it has even been proposed that confidence intervals supplement or replace biased point estimates of I 2 . 26 It is important to note that since I 2 is based on Q or τ 2 , any problems that influence Q or τ 2 (most notably the number of trials included in the meta-analysis) will also indirectly interfere with the computation of I 2 . It is also important to consider that I 2 also is dependent on which between-study variance estimator is used. For example, there is a high level of agreement comparing I 2 derived from DerSimonian and Laird vs. Paul and Mandel methods of estimating between-study variance. 131 In contrast, I 2 derived from other methods of estimating between-study variance have low levels of agreement. 131
Based primarily on the observed distributions of I 2 across meta-analyses, there are ranges that are commonly used to further categorize heterogeneity. That is, I 2 values of 25%, 50%, and 75% have been proposed as working definitions of what could be considered low, moderate, and high proportions, respectively, of variability in effect sizes across studies that is explained by heterogeneity. 59 Currently, the Cochrane manual also includes ranges for interpreting I 2 (0%-40% might not be important, 30%-60% may represent moderate heterogeneity, 50-90% may represent substantial heterogeneity and 75-100% may represent considerable heterogeneity). 10 Irrespective of which categorization of I 2 is used, this statistic must be interpreted with the understanding of several nuances, including issues related to a small number of studies (i.e., fewer than 10), 24 – 26 and inherent differences in I 2 comparing binary and continuous effect sizes. 28 , 29 Moreover, I 2 of zero is often misinterpreted in published reports as being synonymous with the absence of heterogeneity despite upper confidence interval limits that most often would exceed 33% when calculated. 134 Finally, a high I 2 does not necessarily mean that dispersion occurs across a wide range of effect sizes, and a low I 2 does not necessarily mean that dispersion occurs across a narrow range of effect sizes; the I 2 is a signal-to-noise metric, not a statistic about the magnitude of heterogeneity.
4.4. Exploring Heterogeneity
Meta-regression.
Meta-regression is a common approach employed to examine the degree to which study-level factors explain statistical heterogeneity. 135 Random effects meta-regression, as compared with fixed effect meta-regression, allows for residual heterogeneity (i.e., between-study variance that is not explained by study-level factors) to incorporated into the model. 136 Because of this feature, among other benefits described below and in Chapter 3 , random effects meta-regression is recommended over fixed effect meta-regression. 137 It is the default of several statistical packages to use a modified estimator of variance in random effects meta-regression that employs a t distribution in lieu of a standard normal distribution when calculating p-values and confidence intervals (i.e., the Knapp-Hartung modification). 138 This approach is recommended to help mitigate false-positive rates that are common in meta-regression. 137 Since the earliest papers on random effects meta-regression, there has been general caution about the inherent low statistical power in analyses when there are fewer than 10 studies for each study-level factor modelled. 136 Currently, the Cochrane manual recommends that there be at least 10 studies per characteristic modelled in meta-regression 10 over the enduring concern about inflated false-positive rates with too few studies. 137 Another consideration that is reasonable to endorse is adjusting the level of statistical significance to account for making multiple comparisons in cases where more than one characteristic is being investigated in meta-regression.
Beyond statistical considerations important in meta-regression, there are also several important conceptual considerations. First, study-level characteristics to be considered in meta-regression should be pre-specified, scientifically defensible and based on hypotheses. 8 , 10 This first consideration will allow investigators to focus on factors that are believed to modify the effect of intervention as opposed to clinically meaningless study-level characteristics. Arguably, it may not be possible to identify all study-level characteristics that may modify intervention effects. The focus of meta-regression should be on factors that are plausible. Second, meta-regression should be carried out under full consideration of ecological bias (i.e., the inherent problems associated with aggregating individual-level data). 139 As classic examples, the mean study age or the proportion of study participants who were female may result in different conclusions in meta-regression as opposed to how these modifying relationships functioned in each trial. 135
Multiple Meta-regression
It may be desirable to examine the influence of more than one study-level factor on the heterogeneity observed in meta-analyses. Recalling general cautions and specific recommendations about the inherent low statistical power in analyses wherein there are fewer than 10 studies for each study-level factors modelled, 10 , 136 , 137 multiple meta-regression (that is, a meta-regression model with more than one study-level factor included) should only be considered when study-level characteristics are pre-specified, scientifically defensible, and based on hypotheses, and when there are 10 or more studies for each study-level factor included in meta-regression.
Subgroup Analysis
Subgroup analysis is another common approach employed to examine the degree to which study-level factors explain statistical heterogeneity. Since subgroup analysis is a type of meta-regression that incorporates a categorical study-level factor as opposed to a continuous study-level factor, it is similarly important that the grouping of studies to be considered in subgroup analysis be pre-specified, scientifically defensible and based on hypotheses. 8 , 10 Like other forms of meta-regression, subgroup analyses have a high false-positive rate. 137 and may be misleading when few studies are included. There are two general approaches to handling subgroups in meta-analysis. First, a common use is to perform meta-analyses within subgroups without any statistical between-group comparisons. A central problem with this approach is the tendency to misinterpret results from within separate groups as being comparative. That is, identification of groups wherein there is a significant summary effect and/or limited heterogeneity and others wherein there is no significant summary effect and/or substantive heterogeneity does not necessarily indicate that the subgroup factor explains overall heterogeneity. 10 Second, it is recommended to incorporate the subgrouping factor into a meta-regression framework. 140 Doing so allows for quantification of both within and among subgroup heterogeneity as well as well as formal statistical testing that informs whether the summary estimates are different across subgroups. Moreover, subgroup analysis in a meta-regression framework will allow for formal testing of residual heterogeneity in a similar fashion to meta-regression using a continuous study-level factor.
Detecting Outlying Studies
Under consideration that removal of one or more studies from a meta-analysis may interject bias in the results, 10 identification of outlier studies may help build the evidence necessary to justify removal. Visual examination of forest, funnel, normal probability and Baujat plots (described in detail earlier in this chapter) alone may be helpful in identifying studies with inherent outlying characteristics. Additional procedures that may be helpful in interpreting the influence of single studies are quantifying the summary effect without each study (often called one study removed), and performing cumulative meta-analyses. One study removed procedures simply involve sequentially estimating the summary effect without each study to determine if single studies are having a large influence on model results. Using cumulative meta-analysis, 141 it is possible to graph the accumulation of evidence of trials reporting at treatment effect. Simply put, this approach integrates all information up to and including each trial into summary estimates. By looking at the graphical output (from Stata’s metacum command or the R metafor cumul() function), one can examine large shifts in the summary effect that may serve as evidence for study removal. Another benefit of cumulative meta-analysis is detecting shifts in practice (e.g., guideline changes, new treatment approval or discontinuation) that would foster subgroup analysis.
Viechtbauer and Chung proposed other methods that should be considered to help identify outliers. One option is to examine extensions of linear regression residual diagnostics by using studentized deleted residuals. 142 Other options are to examine the difference between the predicted average effect with and without each study (indicating by how many standard deviations the average effect changes) or to examine what effect the deletion of each study has on the fitted values of all studies simultaneously (in a metric similar to Cook’s distance). 142 Particularly in combination, there methods serve as diagnostics that are more formal than visual inspection and both one study removed and cumulative meta-analysis procedures.
4.5. Special Topics
Baseline risk (control-rate) meta-regression.
For studies with binary outcomes, the “control rate” refers to the proportion of subjects in the control group who experienced the event. The control rate can be viewed as a surrogate for covariate differences between studies because it is influenced by illness severity, concomitant treatment, duration of follow-up, and/or other factors that may differ across studies. 143 , 144 Groups of patients with higher underlying risk for poor outcomes may experience different benefits and/or harms from treatment compared with groups of patients who have lower underlying risk. 145 Hence, the control-rate can be used to test for interactions between underlying population risk at baseline and treatment benefit.
To examine for an interaction between underlying population risk and treatment benefit, we recommend a simplified approach. First, generate a scatter plot of treatment effect against control rate to visually assess whether there may be a relation between the two. Since the RD tends to be highly correlated with the control rate, 144 we recommend using an RR or OR when examining a treatment effect against the control rate in all steps. The purpose of generating a scatterplot is simply to give preliminary insight into how differences in baseline risk (control rate) may influence the amount of observed variability in effect sizes across studies. Second, use hierarchical meta-regression 144 or Bayesian meta-regression 146 models to formally test the interaction between underlying population risk and treatment benefit. Although a weighted regression has been proposed as an intermediary step between developing a scatter plot and meta-regression, this approach identifies a significant relation between control rate and treatment effect twice as often compared with more suitable approaches (above), 144 , 146 and a negative finding would likely need to be replicated using meta-regression. Hence, the simplified two-step approach may help streamline the process.
Multivariate Meta-analysis
There are both inherent benefits and disadvantages of using meta-analysis to examine multiple outcomes simultaneously (that is, “multivariate meta-analysis”), and much methodological work has been done in both frequentist and Bayesian frameworks in recent years. 147 – 156 . Some of these methods are readily available in statistical packages (for example, Stata mvmeta ).
One of the advantages of multivariate meta-analysis is being able to incorporate multiple outcomes into one model as opposed to the conduct of multiple univariate meta-analyses wherein the outcomes are handled as being independent. 150 Another advantage of multivariate meta-analysis is being able to gain insight into relationships among study outcomes. 150 , 157 An additional advantage of multivariate meta-analysis is that different clinical conclusions may be made; 150 it may be considered easier to present results from a single multivariate meta-analysis than from several univariate analyses that may make different assumptions. Further, multivariate methods may have the potential to reduce the impact of outcome reporting bias. 150 , 158 , 159
- the disconnect between how outcomes are handled within each trial (typically in a univariate fashion) compared with a multivariate meta-analysis;
- estimation difficulties particularly around correlations between outcomes (seldom reported; see Bland 160 for additional commentary);
- overcoming assumptions of normally-distributed random effects with joint outcomes (difficult to justify with joint distributions);
- marginal model improvement in the multivariate vs. univariate case (often not sufficient trade off in effort); and
- amplification of publication bias (e.g., secondary outcomes are not published as frequently). 150
Another potential challenge is the appropriate quantification of heterogeneity in multivariate meta-analysis; but, there are newer alternatives that seem to make this less of a concern. These methods include but are not limited to the multivariate H 2 statistic (the ratio of a generalization of Q and its degrees of freedom, with an accompanying generalization of I 2 ( I H 2 ) ). 163 Finally, limitations to existing software for broad implementation and access to multivariate meta-analysis has been a long-standing barrier to this approach. With currently available add-on or base statistical packages, however, multivariate meta-analysis can be more readily performed, 150 and emerging approaches to multivariate meta-analyses are available to be integrated into standard statistical output. 153 However, the gain in precision of parameter estimates is often modest, and the conclusions from the multivariate meta-analysis are often the same as those from the univariate meta-analysis for individual outcomes, 164 which may not justify the increased complexity and difficulty.
With the exception of diagnostic testing meta-analysis (which provides a natural situation to meta-analyze sensitivity and specificity simultaneously, but which is out of scope for this report) and network meta-analysis (a special case of multivariate meta-analysis with unique challenges, see Chapter 5 ), multivariate meta-analysis has not been widely used in practice. However, we are likely to see multivariate meta-analysis approaches become more accessible to stakeholders involved with systematic reviews. 160 In the interim, however, we do not recommend this approach be used routinely.
Dose-Response Meta-analysis
Considering different exposure or treatment levels has been a longstanding consideration in meta-analyses involving binary outcomes. 165 , 166 and new methods have been developed to extend this approach to differences in means. 167 Meta-regression is commonly employed to test the relationship between exposure or treatment level and the intervention effect (i.e., dose-response). The best-case scenario for testing dose-response using meta-regression is when there are several trials that compared the dose level versus control for each dosing level. That way, subgroup analysis can be performed to provide evidence of effect similarity within groups of study-by-dose in addition to a gradient of treatment effects across groups. 10 Although incorporating study-level average dose can be considered, it should only be conducted in circumstances where there was limited-to-no variation in dosing within intervention arms of the studies included. In many instances, exposure needs to be grouped for effective comparison (e.g., ever vs. never exposed), but doing so raises the issues of non-independence and covariance between estimates. 168 Hamling et al., developed a method of deriving relative effect and precision estimates for such alternative comparisons in meta-analysis that are more reasonable compared with methods that ignore interdependence of estimates by level. 168 In the case of trials involving differences in means, dose-response models are estimated within each study in a first stage and an overall curve is obtained by pooling study-specific dose-response coefficients in a second stage. 167 A key benefit to this emerging approach to differences in means is modeling non-linear dose-response curves in unspecified shapes (including the cubic spline described in the derivation study). 167 Considering the inherent low statistical power associated with meta-regression in general, results of dose-response meta-regression should generally not be used to indicate that a dose response does not exist. 10
- Statistical heterogeneity should be expected, visually inspected and quantified, and sufficiently addressed in all meta-analyses.
- Prediction intervals should be included in all forest plots.
- Investigators should be consider evaluating multiple metrics of heterogeneity, between-study variance, and inconsistency (i.e., Q , τ 2 and I 2 along with their respective confidence intervals when possible).
- A non-significant Q should not be interpreted as the absence of heterogeneity, and there are nuances to the interpretation of Q that carry over to the interpretation of τ 2 and I 2 .
- Random effects is the preferred method for meta-regression that should be used under consideration of low power associated with limited studies (i.e., <10 studies per study-level factor) and the potential for ecological bias.
- We recommend a simplified two-step approach to control-rate meta-regression that involves scatter plotting and then hierarchical or Bayesian meta-regression.
- Routine use of multivariate meta-analysis is not recommended.
Chapter 5. Network Meta-Analysis (Mixed Treatment Comparisons/Indirect Comparisons)
5.1. rationale and definition.
Decision makers, whether patients, providers or policymakers generally want head-to-head estimates of the comparative effectiveness of the different interventions from which they have to choose. However, head-to-head trials are relatively uncommon. The majority of trials compare active agents with placebo, which has left patients and clinicians unable to compare across treatment options with sufficient certainty.
Therefore, an approach has emerged to compare agents indirectly. If we know that intervention A is better than B by a certain amount, and we know how B compares with C; we can indirectly infer the magnitude of effect comparing A with C. Occasionally, a very limited number of head-to-head trials are available (i.e., there may be a small number of trials directly comparing A with C). Such trials will likely produce imprecise estimates due to the small sample size and number of events. In this case, the indirect comparisons of A with C can be pooled with the direct comparisons, to produce what is commonly called a network meta-analysis estimate (NMA). The rationale for producing such an aggregate estimate is to increase precision, and to utilize all the available evidence for decision making.
Frequently, more than two active interventions are available and stakeholders want to compare (rank) many interventions, creating a network of interventions with comparisons accounting for all the permutations of pairings within the network. The following guidance focuses on NMA of randomized controlled trials. NMA of nonrandomized studies is statistically possible; however, without randomization, NMA assumptions would likely not be satisfied and the results would not be reliable.
5.2. Assumptions
There are three key assumptions required for network meta-analysis to be valid:
I. Homogeneity of direct evidence
When important heterogeneity (unexplained differences in treatment effect) across trials is noted, confidence in a pooled estimate decreases. 169 This is true for any meta-analysis. In an NMA, direct evidence (within each pairwise comparison) should be sufficiently homogeneous. This can be evaluated using the standard methods for evaluating heterogeneity ( I 2 statistic, τ 2 , Cochran Q test, and visual inspection of forest plots for consistency of point estimates from individual trials and overlap of confidence intervals).
II. Transitivity, similarity or exchangeability
Patients enrolled in trials of different comparisons in a network need to be sufficiently similar in terms of the distribution of effect modifiers. In other words, patients should be similar to the extent that it is plausible that they were equally likely to have received any of the treatments in the network. 170 Similarly, active and placebo controlled interventions across trials need to be sufficiently similar in order to attribute the observed change in effect size to the change in interventions.
Transitivity cannot be assessed quantitatively. However, it can be evaluated conceptually. Researchers need to identify important effect modifiers in the network and assess whether differences reported by studies are large enough to affect the validity of the transitivity assumption.
III. Consistency (Between Direct and Indirect Evidence)
Comparing direct and indirect estimates in closed loops in a network demonstrates whether the network is consistent (previously called coherent). Important differences between direct and indirect evidence may invalidate combining them in a pooled NMA estimate.
Consistency refers to the agreement between indirect and direct comparison for the same treatment comparison. If a pooled effect size for a direct comparison is similar to the pooled effect size from indirect comparison, we say the network is consistent; otherwise, the network is inconsistent or incoherent. 171 , 172 Multiple causes have been proposed for inconsistency, such as differences in patients, treatments, settings, timing, and other factors.
Statistical models have been developed to assume consistency in the network (consistency models) or account for inconsistency between direct and indirect comparison (inconsistency models). Consistency is a key assumption/prerequisite for a valid network meta-analysis and should always be evaluated. If there is substantial inconsistency between direct and indirect evidence, a network meta-analysis should not be performed. Fortunately, inconsistency can be evaluated statistically.
5.3. Statistical Approaches
The simplest indirect comparison approach is to qualitatively compare the point estimates and the overlap of confidence intervals from two direct comparisons that use a common comparator. Two treatments are likely to have comparable effectiveness if their direct effects relative to a common comparator (e.g., placebo) have the same direction and magnitude, and if there is considerable overlap in their confidence intervals. However, such qualitative comparisons have to be interpreted cautiously because the degree to which confidence intervals overlap is not a reliable substitute for formal hypothesis testing. Formal testing methods adjust the comparison of the interventions by the results of their direct comparison with a common control group and at least partially preserve the advantages of randomization of the component trials. 173
Many statistical models for network meta-analysis have been developed and applied in the literature. These models range from simple indirect comparisons to more complex mixed effects and hierarchical models, developed in both Bayesian and frequentist frameworks, and using both contrast level and arm level data.
Simple Indirect Comparisons
Simple indirect comparisons apply when there is no closed loop in the evidence network. A closed loop means that each comparison in a particular loop has both direct and indirect evidence. At least three statistical methods are available to conduct simple indirect comparisons: (1) the adjusted indirect comparison method proposed by Bucher et al, 174 (2) logistic regression, and (3) random effects meta-regression.
When there are only two sets of trials, say, A vs. B and B vs. C, Bucher‘s method is sufficient to provide the indirect estimate of A vs. C as: log(OR AC )=log(OR AB )-log(OR BC ) and
Var(Log(OR AC )) = Var(Log(OR AB )) + Var(Log(OR BC )), where OR is the odds ratio. Bucher’s method is valid only under a normality assumption on the log scale.
Logistic regression uses arm-level dichotomous outcomes data and is limited to odds ratios as the measure of effect. By contrast, meta-regression and adjusted indirect comparisons typically use contrast-level data and can be extended to risk ratios, risk differences, mean difference and any other effect measures. Under ideal circumstances (i.e., no differences in prognostic factors exist among included studies), all three methods result in unbiased estimates of direct effects. 175 Meta-regression (as implemented in Stata, metareg ) and adjusted indirect comparisons are the most convenient approaches for comparing trials with two treatment arms. A simulation study supports the use of random effects for either of these approaches. 175
Mixed Effects and Hierarchical Models
More complex statistical models are required for more complex networks with closed loops where a treatment effect could be informed by both direct and indirect evidence. These models typically assume random treatment effects and take the complex data structure into account, and may be broadly categorized as mixed effects, or hierarchical models.
Frequentist Approach
Lumley proposed the term “network meta-analysis” and the first network meta-analysis model in the frequentist framework, and constructed a random-effects inconsistency model by incorporating sampling variability, heterogeneity, and inconsistency. 176 The inconsistency follows a common random-effects distribution with mean of 0. It can use arm-level and contrast-level data and can be easily implemented in statistical software, including R’s lme package. However, studies included in the meta-analysis cannot have more than two arms.
Further development of network meta-analysis models in the frequentist framework addressed how to handle multi-armed trials as well as new methods of assessing inconsistency. 171 , 177 – 179 Salanti et al. provided a general network meta-analysis formulation with either contrast-based data or arm-based data, and defined the inconsistency in a standard way as the difference between ‘direct’ evidence and ‘indirect’ evidence. 177 In contrast, White et al. and Higgins et al. proposed to use a treatment-by-design interaction to evaluate inconsistency of evidence, and developed consistency and inconsistency models based on contrast-based multivariate random effects meta-regression. 171 , 178 These models can be implemented using network , a suite of commands in Stata with input data being either arm-level or contrast level.
Bayesian Approach
Lu and Ades proposed the first Bayesian network meta-analysis model for multi-arm studies that included both direct and indirect evidence. 180 The treatment effects are represented by basic parameters and functional parameters. Basic parameters are effect parameters that are directly compared to the baseline treatment, and functional parameters are represented as functions of basic parameters. Evidence inconsistency is defined as a function of a functional parameter and at least two basic parameters. The Bayesian model has been extended to incorporate study-level covariates in an attempt to explain between-study heterogeneity and reduce inconsistency, 181 to allow for repeated measurements of a continuous endpoint that varies over time, 87 or to appraise novelty effects. 182 A Bayesian multinomial network meta-analysis model was also developed for unordered (nominal) categorical outcomes allowing for partially observed data in which exact event counts may not be known for each category. 183 Additionally, Dias et al. set out a generalized linear model framework for the synthesis of data from randomized controlled trials, which could be applied to binary outcomes, continuous outcomes, rate models, competing risks, or ordered category outcomes. 86
Commonly, a vague (flat) prior is chosen for the treatment effect and heterogeneity parameters in Bayesian network meta-analysis. A vague prior distribution for heterogeneity however may not be appropriate when the number of studies is small. 184 An informative prior for heterogeneity can be obtained from the empirically derived predictive distributions for the degree of heterogeneity as expected in various settings (depending on the outcomes assessed and comparisons made). 185 In the NMA framework, frequentist and Bayesian approaches often provide similar results; particularly because of the common practice to use non-informative priors in the Bayesian analysis. 186 – 188 Frequentist approaches, when implemented in a statistical package, are easily applied in real-life data analysis. Bayesian approaches are highly adaptable to complex evidence structures and provide a very flexible modeling framework, but need a better understanding of the model specification and specialized programing skills.
Arm-Based Versus Contrast-Based Models
It is important to differentiate arm-based/contrast-based models from arm-level/contrast-level data. Arm-level and contrast-level data describe how outcomes are reported in the original studies. Arm-level data represent raw data per study arm (e.g., the number of events from a trial per group); while contrast-level data show the difference in outcomes between arms in the form of absolute or relative effect size (e.g., mean difference or the odds ratio of events).
Contrast-based models resemble the traditional approaches used in meta-analysis of direct comparisons. Absolute or relative effect sizes and associated variances are first estimated (per study) and then pooled to produce an estimate of the treatment comparison. Contrast-based models preserve randomization and, largely, alleviate risk of observed and unobserved imbalance between arms within a study. They use effect sizes relative to the comparison group and reduce the variability of outcomes across studies. Contrast-based models are the dominant approach used in direct meta-analysis and network meta-analysis in current practice.
Arm-based models depend on directly combining the observed absolute effect size in individual arms across studies; thereby producing a pooled rate or mean of the outcome per arm. Estimates can be compared among arms to produce a comparative effect size. Arm-based models break randomization; therefore, the comparative estimate will likely be at an increased risk of bias. Following this approach, nonrandomized studies or even noncomparative studies can be included in the analysis. Multiple models have been proposed for the arm-based approach, especially in the Bayesian framework. 177 , 189 – 192 However, the validity of arm-based methods is under debate. 178 , 193 , 194
Assessing Consistency
Network meta-analysis generates results for all pairwise comparisons; however, consistency can only be evaluated when at least one closed loop exists in the network. In other words, the network must have at least one treatment comparison with direct evidence. Many statistical methods are available to assess consistency. 173 , 174 , 176 , 195 – 200
These methods can generally be categorized into two types: (1) an overall consistency measure for the whole network; and (2) a loop-based approach in which direct and indirect estimates are compared. In the following section, we will focus on a few widely used methods in the literature.
- Single Measure for Network Consistency : These approaches use a single measure that represents consistency for the whole network. Lumley assumes that, for each treatment comparison (with or without direct evidence), there is a different inconsistency factor; and the inconsistency factor varies for all treatment comparisons and follows a common random-effects distribution. The variance of the differences, ω, also called incoherence, measures the overall inconsistency of the network. 176 A ω above 0.25 suggests substantial inconsistency and in this case, network meta-analysis may be considered inappropriate. 201
- Global Wald Test : Another approach is to use global Wald test, which tests an inconsistency factor that follows a Χ 2 distribution under the null consistency assumption. 178 A p-value less than 0.10 can be used to determine statistical significance. Rejection of the null is evidence that the model is not consistent.
- Z-test : A simple z-test can be used to compare the difference of the pooled effect sizes between direct and indirect comparisons. 174 Benefits of this approach include simplicity, ease of application, and the ability to identify specific loops with large inconsistency. Limitations include the need for multiple correlated tests.
- Side-splitting: A “node” is a treatment and a “side” (or edge) is a comparison. Dias et al. suggests that each comparison can be assessed by comparing the difference of the pooled estimate from direct evidence to the pooled estimate without direct evidence. 196 Side-splitting (sometimes referred to as node-splitting) can be implemented using the Stata network sidesplit command or R gemtc package.
Several graphical tools have been developed to describe inconsistency. One is the inconsistency plot developed by Chaimani et al. 197 Similar to a forest plot, the inconsistency plot graphically presents an inconsistency factor (the absolute difference between the direct and indirect estimates) and related confidence interval for each of the triangular and quadratic loops in the network. The Stata ifplot command can be used for this purpose.
It is important to understand the limitations of these methods. Lack of statistical significance of an inconsistency test does not prove consistency in the network. Similar to Cochran’s Q test of heterogeneity testing in traditional meta-analysis (which is often underpowered), statistical tests for inconsistency in NMA are also commonly underpowered due to the limited number of studies in direct comparisons.
- Abandon NMA and only perform traditional meta-analysis;
- Present the results from inconsistency models (that incorporate inconsistency) and acknowledge the limited trustworthiness of the NMA estimates;
- Split the network to eliminate the inconsistent nodes;
- Attempt to explain the causes of inconsistency by conducting network meta-regression to test for possible covariates causing the inconsistency: and
- Use only direct estimates for the pairwise NMA comparisons that show inconsistency (i.e., use direct estimates for inconsistent comparisons and use NMA estimates for consistent comparisons).
5.4. Considerations of Model Choice and Software
Consideration of indirect evidence.
Empirical explorations suggest that direct and indirect comparisons often agree, 174 – 176 , 202 – 204 but with notable exceptions. 205 In principle, the validity of combining direct and indirect evidence relies on the transitivity assumption. However, in practice, trials can vary in numerous ways including population characteristics, interventions, and cointerventions, length of follow-up, loss to follow-up, study quality, etc. Given the limited information in many publications and the inclusion of multiple treatments, the validity of combining direct and indirect evidence is often unverifiable. The statistical methods to evaluate inconsistency generally have low power, and are confounded by the presence of statistical heterogeneity. They often fail to detect inconsistency in the evidence network.
Moreover, network meta-analysis, like all other meta-analytic approaches, constitutes an observational study, and residual confounding can always be present. Systematic differences in characteristics among trials in a network can bias network meta-analysis results. In addition, all other considerations for meta-analyses, such as the choice of effect measures or heterogeneity, also apply to network meta-analysis. Therefore, in general, investigators should compare competing interventions based on direct evidence from head-to-head RCTs whenever possible. When head-to-head RCT data are sparse or unavailable but indirect evidence is sufficient, investigators may consider incorporating indirect evidence and network meta-analysis as an additional analytical tool. If the investigators choose to ignore indirect evidence, they should explain why.
Choice of Method
Although the development of network meta-analysis models has exploded in the last 10 years, there has been no systematic evaluation of their comparative performance, and the validity of the model assumptions in practice is generally hard to verify.
Investigators may choose a frequentist or Bayesian mode of inference based on the research team expertise, the complexity of the evidence network, and/or the research question. If investigators believe that the use of prior information is needed and that the data are insufficient to capture all the information available, then they should use a Bayesian model. On the other hand, a frequentist model is appropriate if one wants inferences to be based only on the data that can be incorporated into a likelihood.
Whichever method the investigators choose, they should assess the consistency of the direct and indirect evidence, and the invariance of treatment effects across studies and the appropriateness of the chosen method on a case-by-case basis, paying special attention to comparability across different sets of trials. Investigators should explicitly state assumptions underlying indirect comparisons and conduct sensitivity analysis to check those assumptions. If the results are not robust, findings from indirect comparisons should be considered inconclusive. Interpretation of findings should explicitly address these limitations. Investigators should also note that simple adjusted indirect comparisons are generally underpowered, needing four times as many equally sized studies to achieve the same power as direct comparisons, and frequently lead to indeterminate results with wide confidence intervals. 174 , 175
When the evidence of a network of interventions is consistent, investigators can combine direct and indirect evidence using network meta-analysis models. Conversely, they should refrain from combining multiple sources of evidence from an inconsistent (i.e., incoherent) network where there are substantial differences between direct and indirect evidence that cannot be resolved by conditioning on the known covariates. Investigators should make efforts to explain the differences between direct and indirect evidence based upon study characteristics, though little guidance and consensus exists on how to interpret the results.
Lastly, the network geometry ( Figure 5.1 ) can also affect the choice of analysis method as demonstrated in Table 5.1 .
Common network geometry (simple indirect comparison, star, network with at least one closed loop).
Impact of network geometry on choice of analysis method.
Commonly Used Software
Many statistical packages are available to implement NMA. BUGS software (Bayesian inference Using Gibbs Sampling, WINBUGS, OPENBUGS) is a popular choice for conducting Bayesian NMA 206 that offers flexible model specification including NMA meta-regression. JAGS and STAN are alternative choices for Bayesian NMA. Stata provides user-written routines ( http://www.mtm.uoi.gr/index.php/stata-routines-for-network-meta-analysis ) that can be used to conduct frequentist NMA. In particular, the Stata command network is a suite of programs for importing data for network meta-analysis, running a contrast-based network meta-analysis, assessing inconsistency, and graphing the data and results. Further, in the R environment, three packages, gemtc ( http://cran.r-project.org/web/packages/gemtc/index.html ), pcnetmeta ( http://cran.r-project.org/web/packages/pcnetmeta/index.html ), and netmeta ( http://cran.r-project.org/web/packages/netmeta/index.html ), have been developed for Bayesian ( gemtc, pcnetmeta ) or frequestist ( netmeta ) NMA. The packages also include methods to assess heterogeneity and inconsistency, and data visualizations, and allow users to perform NMA with minimal programming. 207
5.5. Inference From Network Meta-analysis
Stakeholders (users of evidence) require a rating of the strength of a body of evidence. The strength of evidence demonstrates how much certainty we should have in the estimates.
The general framework for assessing the strength of evidence used by the EPC program is described elsewhere. However; for NMA, guidance is evolving and may require some additional computations; therefore, we briefly discuss the possible approaches to rating the strength of evidence. We also discuss inference from rankings and probabilities commonly presented with a network meta-analysis.
Approaches for Rating the Strength of Evidence
The original EPC and GRADE guidance was simple and involved rating down all evidence derived from indirect comparisons (or NMA with mostly indirect evidence) for indirectness. Therefore, following this original GRADE guidance, evidence derived from most NMAs would be rated to have moderate strength at best. 208 Subsequently, Salanti et al. evaluated the transitivity assumption and network inconsistency under the indirectness and inconsistency domains of GRADE respectively. They judged the risk of bias based on a ‘contribution matrix’ which gives the percentage contribution of each direct estimate to each network meta-analysis estimate. 209 A final global judgment of the strength of evidence is made for the overall rankings in a network.
More recently, GRADE published a new approach that is based on evaluating the strength of evidence for each comparison separately rather than making a judgment on the whole network. 210 The rationale for not making such an overarching judgment is that the strength of evidence (certainty in the estimates) is expected to be different for different comparisons. The approach requires presenting the three estimates for each comparison (direct, indirect, and network estimates), then rating the strength of evidence separately for each one.
In summary, researchers conducting NMA should present their best judgment on the strength of evidence to facilitate decision-making. Innovations and newer methodology are constantly evolving in this area.
Interpreting Ranking Probabilities and Clinical Importance of Results
Network meta-analysis results are commonly presented as probabilities of being most effective and as rankings of treatments. Results are also presented as the surface under the cumulative ranking curve (SUCRA). SUCRA is a simple transformation of the mean rank that is used to provide a hierarchy of the treatments accounting both for the location and the variance of all relative treatment effects. SUCRA would be 1 when a treatment is certain to be the best and 0 when a treatment is certain to be the worst. 211 Such presentations should be interpreted with caution since they can be quite misleading.
- Such estimates are usually very imprecise. An empirical evaluation of 58 NMAs showed that the median width of the 95% CIs of SUCRA estimates was 65% (the first quartile was 38%; the third quartile was 80%). In 28% of networks, there was a 50% or greater probability that the best-ranked treatment was actually not the best. No evidence showed a difference between the best-ranked intervention and the second or third best-ranked interventions in 90% and 71% of comparisons, respectively.
- When rankings suggest superiority of an agent over others, the absolute difference between this intervention and other active agents could be trivial. Converting the relative effect to an absolute effect is often needed to present results that are meaningful to clinical practice and relevant to decision making. 212 Such results can be presented for patient groups with varying baseline risks. The source of baseline risk can be obtained from observational studies judged to be most representative of the population of interest, from the average baseline risk of the control arms of the randomized trials included in meta-analysis, or from a risk stratification tool if one is known and commonly used in practice. 213
- Rankings hide the fact that each comparison may have its own risk of bias, limitations, and strength of evidence.
5.6. Presentation and Reporting
- Rationale for conducting an NMA, the mode of inference (e.g., Bayesian, Frequentist), and the model choice (random effects vs. fixed effects; consistency vs inconsistency model, common heterogeneity assumption, etc.);
- Software and syntax/commands used;
- Choice of priors for any Bayesian analyses;
- Graphical presentation of the network structure and geometry;
- Pairwise effect sizes to allow comparative effectiveness inference; and
- Assessment of the extent of consistency between the direct and indirect estimates.
- A network meta-analysis should always be based on a rigorous a rigorous systematic review.
- Homogeneity of direct evidence
- Transitivity, similarity, or exchangeability
- Consistency (between direct and indirect evidence)
- Investigators may choose a frequentist or Bayesian mode of inference based on the research team’s expertise, the complexity of the evidence network, and the research question.
- Evaluating inconsistency is a major and mandatory component of network meta-analysis.
- Evaluating inconsistency should not be only based on a conducting a global test. A loop-based approach can identify the comparisons that cause inconsistency.
- Inference based on the rankings and probabilities of treatments being most effective should be used cautiously. Rankings and probabilities can be misleading and should be interpreted based on the magnitude of pairwise effect sizes. Differences across interventions may not be clinically important despite such rankings.
- Future Research Suggestions
The following are suggestions for directions in future research for each of the topics by chapter.
Chapter 1. Decision To Combine Trials
- Guidance regarding the minimum number of trials one can validly pool at given levels of statistical heterogeneity
- Research on ratio of means—both clinical interpretability and mathematical consistency across studies compared with standardized mean difference
- Research on use of ANCOVA models for adjusting baseline imbalance
- Software packages that more easily enable use of different information
- Methods to handle zeros in the computation of binary outcomes
- Evidence on which metrics, and language used to describe these metrics, are most helpful in conveying meta-analysis results to multiple stakeholders
- Evaluate newly developed statistical models for combining typical effect measures (e.g., mean difference, OR, RR, and/or RD) and compare with current methods
- Heterogeneity statistics for meta-analyses involving a small number of studies
- Guidance on specification of hypotheses in meta-regression
- Guidance on reporting of relationships among study outcomes to facilitate multivariate meta-analysis
Chapter 5. Network Meta-analysis (Mixed Treatment Comparisons/Indirect Comparisons)
- Methods for combining individual patient data with aggregated data
- Methods for integrating evidence from RCTs and observational studies
- Models for time-to-event data
- User friendly software similar to that available for traditional meta-analysis
- Evidence to support model choice
This report is based on research conducted by the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers’ 2016 Methods Workgroup. The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report.
This research was funded through contracts from the Agency for Healthcare Research and Quality to the following Evidence-based Practice Centers: Mayo Clinic (290-2015-00013-I); Kaiser Permanente (290-2015-00007-I); RAND Corporation (290-2015-00010-I); Alberta (290-2015-00001-I); Pacific Northwest (290-2015-00009-I); RTI (290-2015-00011-I); Brown (290-2015-00002-I); and the Scientific Resource Center (290-2012-00004-C).
The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policy makers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients).
This report is made available to the public under the terms of a licensing agreement between the author and the Agency for Healthcare Research and Quality. This report may be used and reprinted without permission except those copyrighted materials that are clearly noted in the report. Further reproduction of those copyrighted materials is prohibited without the express permission of copyright holders.
AHRQ or U.S. Department of Health and Human Services endorsement of any derivative products that may be developed from this report, such as clinical practice guidelines, other quality enhancement tools, or reimbursement or coverage policies may not be stated or implied.
Persons using assistive technology may not be able to fully access information in this report. For assistance, contact vog.shh.qrha@cpe .
Suggested citation: Morton SC, Murad MH, O’Connor E, Lee CS, Booth M, Vandermeer BW, Snowden JM, D’Anci KE, Fu R, Gartlehner G, Wang Z, Steele DW. Quantitative Synthesis—An Update. Methods Guide for Comparative Effectiveness Reviews. (Prepared by the Scientific Resource Center under Contract No. 290-2012-0004-C). AHRQ Publication No. 18-EHC007-EF. Rockville, MD: Agency for Healthcare Research and Quality; February 2018. Posted final reports are located on the Effective Health Care Program search page . https://doi.org/ 10 .23970/AHRQEPCMETHGUIDE3 .
Prepared for: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services, 5600 Fishers Lane, Rockville, MD 20857, www.ahrq.gov Contract No.: 290-2012-00004-C . Prepared by: Scientific Resource Center, Portland, OR
- Cite this Page Morton SC, Murad MH, O’Connor E, et al. Quantitative Synthesis—An Update. 2018 Feb 23. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-.
- PDF version of this page (702K)
In this Page
- Decision to Combine Trials
- Optimizing Use of Effect Size Data
- Choice of Statistical Model for Combining Studies
- Quantifying, Testing, and Exploring Statistical Heterogeneity
- Network Meta-Analysis (Mixed Treatment Comparisons/Indirect Comparisons)
Other titles in these collections
- AHRQ Methods for Effective Health Care
- Health Services/Technology Assessment Text (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. [Methods Guide for Effectivenes...] Review Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2008
- Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. [J Clin Epidemiol. 2011] Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A, et al. J Clin Epidemiol. 2011 Nov; 64(11):1187-97. Epub 2011 Apr 7.
- The future of Cochrane Neonatal. [Early Hum Dev. 2020] The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Early Hum Dev. 2020 Nov; 150:105191. Epub 2020 Sep 12.
- Review Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. [Methods Guide for Effectivenes...] Review Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Berkman ND, Lohr KN, Ansari M, McDonagh M, Balk E, Whitlock E, Reston J, Bass E, Butler M, Gartlehner G, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2008
Recent Activity
- Quantitative Synthesis—An Update - Methods Guide for Effectiveness and Comparati... Quantitative Synthesis—An Update - Methods Guide for Effectiveness and Comparative Effectiveness Reviews
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Good review practice: a researcher guide to systematic review methodology in the sciences of food and health
- About this guide
- Part A: Systematic review method
- What are Good Practice points?
- Part C: The core steps of the SR process
- 1.1 Setting eligibility criteria
- 1.2 Identifying search terms
- 1.3 Protocol development
- 2. Searching for studies
- 3. Screening the results
- 4. Evaluation of included studies: quality assessment
- 5. Data extraction
- 6. Data synthesis and summary
- 7. Presenting results
- Links to current versions of the reference guidelines
- Download templates
- Food science databases
- Process management tools
- Screening tools
- Reference management tools
- Grey literature sources
- Links for access to protocol repository and platforms for registration
- Links for access to PRISMA frameworks
- Links for access to 'Risk of Bias' assessment tools for quantitative and qualitative studies
- Links for access to grading checklists
- Links for access to reporting checklists
- What questions are suitable for the systematic review methodology?
- How to assess feasibility of using the method?
- What is a scoping study and how to construct one?
- How to construct a systematic review protocol?
- How to construct a comprehensive search?
- Study designs and levels of evidence
- Download a pdf version This link opens in a new window
Data synthesis and summary
Data synthesis and summary .
Data synthesis includes synthesising the findings of primary studies and when possible or appropriate some forms of statistical analysis of numerical data. Synthesis methods vary depending on the nature of the evidence (e.g., quantitative, qualitative, or mixed), the aim of the review and the study types and designs. Reviewers have to decide and preselect a method of analysis based on the review question at the protocol development stage.
Synthesis Methods
Narrative summary : is a summary of the review results when meta-analysis is not possible. Narrative summaries describe the results of the review, but some can take a more interpretive approach in summarising the results . [8] These are known as " evidence statements " and can include the results of quality appraisal and weighting processes and provide the ratings of the studies.
Meta-analysis : is a quantitative synthesis of the results from included studies using statistical analysis methods that are extensions to those used in primary studies. [9] Meta-analysis can provide a more precise estimate of the outcomes by measuring and counting for uncertainty of outcomes from individual studies by means of statistical methods. However, it is not always feasible to conduct statistical analyses due to several reasons including inadequate data, heterogeneous data, poor quality of included studies and the level of complexity. [10]
Qualitative Data Synthesis (QDS) : is a method of identifying common themes across qualitative studies to create a great degree of conceptual development compared with narrative reviews. The key concepts are identified through a process that begins with interpretations of the primary findings reported to researchers which will then be interpreted to their views of the meaning in a second-order and finally interpreted by reviewers into explanations and generating hypotheses. [11]
Mixed methods synthesis: is an advanced method of data synthesis developed by EPPI-Centre to better understand the meanings of quantitative studies by conducting a parallel review of user evaluations to traditional systematic reviews and combining the findings of the syntheses to identify and provide clear directions in practice. [11]
- << Previous: 5. Data extraction
- Next: 7. Presenting results >>
- Last Updated: Sep 18, 2023 1:16 PM
- URL: https://ifis.libguides.com/systematic_reviews
- Top Articles
- Experiences
Analysis, Plus Synthesis: Turning Data into Insights
Conducting primary user research such as in-depth interviews or field studies can be fairly straightforward, when compared with what you face upon returning to the office with piles of notes, sketches, user journals, and audio and video recordings. You may ask, What should I do with all this data? and How do I turn it into something meaningful?
These are big questions that I cannot answer in just one article, and deciding what kind of documentation or design tool to develop—for example, personas, mental models, user scenarios, or usability test reports—depends on your goals for conducting the research in the first place. But regardless of the output, I believe, for most researchers, the overarching objective is to identify true insights, instead of just reporting facts. Research outputs that we build around a core insight or truth compel design teams to empathize with users, and thus, to design truly meaningful products and services.
In this article, I will outline an approach to gleaning insights from primary qualitative research data. This article is not a how-to for creating the design tools that are often the outputs of primary qualitative user research—such as personas, mental models, or user scenarios. Instead, it identifies an approach to generating overarching insights, regardless of the design tool you want to create.
Analysis + Synthesis
Great research involves analysis + synthesis .
Synthesis —“The composition or combination of parts or elements so as to form a whole.”— Webster’s
Too often, as Figure 1 represents, we focus purely on analysis—and the identification of facts—and ignore synthesis, which often occurs organically during analysis. As shown in Figure 2, synthesis involves detective work that lets us see the patterns in our data. Synthesis can present itself as a gut feeling that something is right or true when we examine our data and its patterns. (See Steve Baty’s article “ Patterns in User Research ” for an excellent overview.)

If you conducted the user research, remember this: You spent hours with people asking them questions, listening to them, having conversations, observing behaviors, and empathizing with them. To a certain degree, you know them. If you have a strong feeling about something a participant said or did and can connect that with something another participant said or did, it’s likely you’ve got a budding insight that is worth probing further. Allowing trust-your-gut synthesis can bring forth true insights.
Analysis and synthesis often occur at the same time. We plan and structure our analysis, allowing us to frame the problems, while synthesis is emergent and lets us make connections that identify breakthrough ideas and opportunities, as Figure 3 shows.

If you use the analysis + synthesis approach, you’ll make room for insights to emerge, taking your research beyond pure facts. Still, what do we do with all the data? How do we begin our process of analysis + synthesis? Here are some steps that have worked for me:
- Collect and organize the data. Make your data manageable.
- Mine the data. Identify what you see.
- Sort and cluster the data. Manipulate or reframe your data, as necessary.
- Identify insights. Discuss, articulate, incubate, and socialize your insights.
1. Collect and organize the data.
Either just before you set out to conduct your research or very soon after you start, it’s a good idea to set up a system for organizing the many files you’ll collect—including written notes, sketches, digital notes, photos, audio recordings, and video recordings. A system that works for me involves two levels of organization—one, digital; the other, physical.
Digital Organization
Plan to have the content of all audio, video, and hand-written files transcribed in digital format. Create folders for participants, session dates, or another category that works for your team. I have found organizing by participant to be the best approach.
In each folder, include a participant profile document that outlines who the participant is, including any relevant demographic or segmentation data. This profile should provide a quick scan of information about a participant, as a reminder to team members.
At the end of each day or session, store any and all files pertaining to specific participants in their respective folders, including scans or transcripts of hand-written notes, transcripts of audio files, plus the original audio file itself, photos, and video files.
Physical Organization
Designate a war room—a meeting room, a cluster of desks, or common space—to use over the course of your study. There should be a large wall or whiteboard available in this space.
Create your space. Stick your research goals on the wall, put up photos of the sessions, make your space feel like a physical representation of your study.
At the end of each day or session, conduct a team debrief to consolidate notes, talk about key findings, and discuss any interesting points of synthesis. Capture key findings and points of synthesis on large Post-it notes and stick them on a wall. Conduct a discussion about their meaning and importance.
2. Mine the data.
Once you have conducted your research and collected and organized all the data, you can begin to mine your data for facts and findings. This step involves a lot of analysis, or identifying what you see in the data. Here’s a basic process:
- Comb through all the files for each participant to identify findings.
- Depending on the goals and the desired output for your research, pay attention to key points such as behaviors and attitudes or needs and goals.
- Gather useful findings, which can come in the form of user quotations, rephrased points, or facts.
- Color-code Post-it notes by participant, type of finding, or whatever system supports your creating the desired output.
Mining the data and visually organizing it in a common physical space lets you more clearly see what’s there and easily reframe it to identify points of synthesis.
3. Sort and cluster the data.
Once you have mined all your research files to identify findings, you can use sorting and clustering techniques to reframe the data. This leads you on a path to creating meaningful outputs and tools from the data, but also allows synthesis to occur.
- open sorting —Group findings into undefined categories to see what connections emerge.

For example, you might have all of your findings for the category Goals and Needs sorted by participant on the wall. Using the closed-sorting technique, you can re-sort them by alikeness to see what goals and needs are duplicated across participants and which are most common and critical. This lets you discover patterns in your data, but what about identifying the true insights in all the patterns?
4. Identify insights.
You have organized your files, mined all the data, represented it visually in a physical space, sorted and re-sorted it. Throughout this process, you have likely found many patterns that give you a gut feeling they are important and even synthesized those patterns into some macro-patterns.
But, how do you take it to the next level and identify insights and core truths about your users from the patterns you’ve found? Follow these steps to identify insights:
- Discuss each pattern and point of synthesis as a team. Talk about why you think each is important and what it means. Recall exact quotations from participants, facial expressions, body language, feelings, and attitudes relating to the patterns.
- Articulate, in one simple statement, the insight that emerged out of each pattern or point of synthesis. Draft each insight on a Post-it note. But be flexible about changing them when you come back to them later.
- Incubate the insights. Leave them for 24 hours, do some other work, remove yourself from the war room. Let them sit with you, undisturbed, for an extended period of time.
- Return to and re-articulate the insights with the team. Think of a different way of expressing or articulating them. But if you got them right the first time, don’t change them.
- Socialize the insights. Show them to other people who were not involved in the research or analysis + synthesis process. Give them some context, show them the insights, and get their reactions. This will tell you whether the insights resonate. Do people get them? Do they speak to people as the truth? Do the insights compel the design team to create meaningful products and services?
Wrapping Up
It’s a good article, Lindsay. I think this is the most complex process on user experience research. Get significant insights and answer all your research goals. I like your sentence “I believe, for most researchers, the overarching objective is to identify true insights, instead of just reporting facts.”
The process described is great and will really help me with a small project I’m embarking on. Thank you for sharing.
Thank you. Your article was both helpful and relevant to my research.
Nice article. Now I’ll do my school project so easy.
Really well-written article, Lindsay. Thanks for sharing!
Join the Discussion
Lindsay ellerby.
Senior Design Director at Normative
Toronto, Ontario, Canada

Other Articles by Lindsay Ellerby
- Wireframing With Patterns
Other Articles on User Research
- Designing for the User: How Form Insights Shape UX Design Decisions
- Making Product Managers and UX Designers Wear Users’ Hats
- How Can UX Research Help Struggling SaaS Products for Businesses Become Successful?
- Adapting Top Tasks for Startups
New on UXmatters
- Misinformation and Disinformation Online: What Design Can Do to Remedy This Problem
- The Psychology Behind Successful User Onboarding: Leveraging Cognitive Biases
- 3 Crucial Steps in Designing Conversational AI
- Inclusive Digital Experiences: Redesign Strategies for Addressing Diverse Abilities and Accessibility Challenges, Part 1
- User Experience in the Era of AI: Enhancing Human-Machine Interactions
Share this article
Module 8: Analysis and Synthesis
Putting it together: analysis and synthesis.

The ability to analyze effectively is fundamental to success in college and the workplace, regardless of your major or your career plans. Now that you have an understanding of what analysis is, the keys to effective analysis, and the types of analytic assignments you may face, work on improving your analytic skills by keeping the following important concepts in mind:
- Recognize that analysis comes in many forms. Any assignment that asks how parts relate to the whole, how something works, what something means, or why something is important is asking for analysis.
- Suspend judgment before undertaking analysis.
- Craft analytical theses that address how, why, and so what.
- Support analytical interpretations with clear, explicitly cited evidence.
- Remember that all analytical tasks require you to break down or investigate something.
Analysis is the first step towards synthesis, which requires not only thinking critically and investigating a topic or source, but combining thoughts and ideas to create new ones. As you synthesize, you will draw inferences and make connections to broader themes and concepts. It’s this step that will really help add substance, complexity, and interest to your essays.
Contribute!
Improve this page Learn More
- Analysis. Provided by : University of Mississippi. License : CC BY: Attribution
- Putting It Together: Analysis and Synthesis. Provided by : Lumen Learning. License : CC BY: Attribution
- Image of a group in a workplace. Authored by : Free-Photos. Provided by : Pixabay. Located at : https://pixabay.com/photos/workplace-team-business-meeting-1245776/ . License : Other . License Terms : https://pixabay.com/service/terms/#license

How to synthesize user research data for more actionable insights
Last updated
12 October 2023
Reviewed by
Miroslav Damyanov
Data may be in the form of images, text, numbers, or checkboxes. The question is, how can you organize that valuable data to be useful and actionable?
Once you’ve collected user research data, the next critical step is synthesizing the data. This can help validate data from multiple sources, making it more reliable and actionable.
It’s only when you’ve organized the data in meaningful ways that real change can occur and boost decision-making. That’s all to provide better experiences for the end user.
Let’s learn more about how to synthesize data and why it’s important.
- What is data synthesis, and why does it matter?
Data synthesis is a step in the data review process. Once you’ve collected data, it needs analyzing for insights. Synthesis is an essential part of doing this accurately and effectively.
When you’re gathering data from multiple sources, you need to combine, integrate, and evaluate it. And it may come in many forms, like:
Focus groups
A/B testing
Call center notes
Social media comments
Usability testing
The process involves merging mixed data into a uniform sequence for simpler analysis.
Imagine several social media comments mention sluggish website processes. The call center may have also received this feedback. But which part of the website is sluggish? And where is critical investment needed?
That’s where combining data comes into play. Checking website analytics alongside this user feedback can help you validate this data. It also ensures your company can take relevant actions to radically change the user experience .
Data synthesis is essential for a few core reasons:
Holistic overview
Synthesizing data into one dataset means any insights are more holistic across different data collection methods . This provides a more comprehensive view of your users and more accurate insights.
Correlations
Combining data from multiple sources provides opportunities to see relationships between seemingly disparate items. This can boost your understanding of users and highlight issues you might not have noticed.
Hidden patterns
Patterns and trends may become clearer when you synthesize data. This can lead teams to draw deeper insights about users and continually stay ahead of the curve.
Boosted decision-making
Integrating data from multiple sources can unveil insights and boost decision-making across the business. This ensures that any business actions are data-backed and not based on assumptions.
Improved data quality
Synthesizing data can ensure high data quality . Data from multiple sources may be more robust, provide a greater degree of evidence, and show whether findings are consistent. Any outliers will also become more obvious and easier to exclude.
- What are valuable research insights?
Insights are key takeaways from rigorous data analysis . They provide a rich understanding of users, facilitating the design and development of better user experiences.
An insight may encompass a user’s pain points , motivations, preferences, or interests. It can also delve into specific metrics, such as wait times in a shopping cart, conversion rates, and overall customer sentiment .
However, an insight goes beyond merely what happened . It informs us about the underlying reasons driving particular behaviors in a specific situation. We can also uncover the subsequent implications.
Essentially, an insight reflects a user's pain point, motivation, preference, or interest, providing a comprehensive understanding of their experiences.
For insights to be actionable and valuable, they should be:
Your team’s insights need to be grounded in real, reliable data. Acting on assumptions can lead businesses in unhelpful directions. Synthesizing data can help boost the validity and reliability of data for decisions.
Understandable
All team members and stakeholders should be able to understand the insights and act on them accordingly. That means using simple sentences to ensure all teams can interpret insights, recognize their importance, and take action.
User-focused
To create user-centric experiences, consider your users at every step. User-centricity is essential for deeply understanding users and developing the best possible experience.
It can also boost customer satisfaction , loyalty, and retention.
Any insights should speak directly to your users’ wants, needs, and pain points. If you’re not solving a specific problem, perhaps it’s time to relook at what the data is telling you.
Communication
Insights are only useful with clear communication. Teams shouldn’t act in silos; instead, it’s crucial to communicate insights across the business. Taking ownership of the process can ensure teams act upon insights.
Retaining your customer data in a platform that acts as a single source of truth can improve collaboration. It also ensures that critical insights don’t go missing amid lots of data.
Actionable insights are findings that an organization can use to make positive changes. If insights are not actionable, they may be pretty useless.
Insights should not be broad statements or insufficient takeaways. They should be short sentences that speak directly to a problem and a solution. Actionability allows your team to act on the findings to improve user experience . Some examples of actionable insights could include:
Insight #1: 35% of shoppers are abandoning the shopping cart before payment because of the long, complex checkout process.
Action: Simplify the payment process to ensure users can quickly confirm payments.
Insight #2: Longer-form blog posts are performing 46% better than short-form. Google is favoring long-form content, which targets a large number of keywords and more backlinks.
Action: Assign more long-form blog content for the website.
Insight #3: Social media posts with video content receive 3x the engagement. They capture viewers’ interest in the first few seconds and are more likely to be shared online.
Action: Develop more video-based social posts.
- 6 common challenges with synthesizing UX research data
While synthesizing data is a cornerstone of accurate and reliable insights, it still has hurdles.
These are some of the most common challenges in data synthesis.
Large data sets
Synthesizing large datasets can be time-consuming. That’s why it’s crucial to manage data effectively. Prioritize the most critical research questions and synthesize data that speaks to those goals. This can significantly cut back on management time.
The right tool can also make all the difference. An all-in-one platform can house all your data to help you gain insights and act on them faster. And Dovetail is the perfect solution.
Nuanced interpretation of qualitative data
In user research, data synthesis is an art and a science. One of the recurrent challenges in this domain is the nuanced interpretation of qualitative data .
Qualitative insights from user interviews , observations, social media, and surveys often come in diverse forms. This requires a solid approach to categorization, labeling, and interpretation.
Striking a balance between preserving the richness of individual responses while identifying overarching patterns demands careful consideration.
Dovetail can come in handy for this. The platform uses natural language processing (NLP) to analyze and automatically uncover themes in your text.

Unclear goals
Synthesizing data can become time-consuming and problematic when your user experience (UX) research doesn’t have defined goals.
Data should link back to core goals to continually problem-solve for users. This ensures you create user-centric products and limit the data you collect to the most crucial areas.
Bias and assumptions
Teams may bring biases or rely on assumptions when synthesizing data. To avoid this, all team members must stay objective to ensure any insights are evidence-based.
Contradictory insights
Sometimes, throughout the synthesis process, you may discover conflicting findings. It’s helpful to cross-validate the data sources to discover why these contradictions may have occurred.
A deeper analysis may explain the discrepancies. Considering the context of different data sources can be insightful.
Insufficient reviews
When making investment decisions, it’s essential to review the data thoroughly. Mistakes can understandably occur when handling large amounts of data.
It’s helpful to use advanced tools, use AI to boost accuracy (but be wary of relying on it entirely), and have multiple people review the findings.
- How to synthesize data, analyze it, and gain insights
To get started on synthesizing your user research data, follow these best practice steps:
1. Define the research goals
All UX research projects should have clearly defined goals. This ensures data is relevant to improving your products’ UX and isn��’t speaking to issues your users don’t have.
In UX, all goals should keep the user front-of-mind, so any insights speak directly to their experiences.
Remember also that all goals should be SMART (specific, measurable, achievable, relevant, and time-based) to be effective.
Some examples of research goals could be:
Identify the pain points associated with the shopping cart to streamline the process and prevent drop-offs.
Understand user privacy concerns and define better ways of managing user data securely and communicating those measures to customers.
Identify core issues in customer sentiment to highlight how the business can improve across key areas.
2. Collect and organize data
Once you’ve defined your goals, collect data from a range of sources like:
Interviews
Focus groups
Website analytics
A/B testing
Diary studies
Chatbot conversations
Ideally, gather quantitative and qualitative data to gain a full understanding. These research types help you see how users think, feel, and behave in certain situations.
Researchers should collect all of the data with the project's core goals in mind.
3. Use a UX research repository
Gaining data from diverse sources is important, but it can make management tricky. To simplify things, gather and store data in one platform where all relevant team members can manage it. This will also allow your team to group the data in meaningful ways.
An all-in-one platform to store, manage, and analyze data is critical. Dovetail allows teams to bring all customer data into one streamlined platform to collaborate, discover insights, and act on them quickly. This can vastly speed up the process of managing disparate data sets.
4. Develop a UX research taxonomy
To synthesize data effectively, develop a categorization system known as a research taxonomy. This coding system can help your team quickly identify data trends and patterns.
A taxonomy ensures that you can organize and classify data from many sources. Creating a taxonomy means you can analyze the data as one group set to boost efficiency and draw deeper insights.
To develop a UX research taxonomy, keep these three best practice steps in mind:
Define clear terminology to understand how disparate pieces of data can logically and accurately fit together. This will keep the information uniform and legible to all team members.
Make it relevant : The best taxonomy for your organization’s project may differ from another’s. Ensure your taxonomy is relevant to the project’s aims.
Provide documentation to ensure all core stakeholders can access and understand the terminology. Documenting the process also enables teams to review and reuse the research in the future.
5. Look for patterns and trends
Group similar pieces of information into distinct categories or levels based on common characteristics, themes, or patterns. Use codes/tags that can be inductive (data-driven codes) or deductive (predefined codes).
Once you’ve synthesized the data into one coded set, it’s significantly easier for your team to discover patterns and trends. Common and specific pain points for customers will become more evident. And you’ll likely discover trends that you didn’t expect.
As you analyze the data, keep the core goals and end users in mind. The insights you gather throughout this process should link to the goals, which should always benefit the end user.
6. Share your findings
Once you’ve gathered insights, it’s essential to communicate them. Insights are wasted if core stakeholders can’t act on them. After all, they’re the ones who will drive change.
Collate all findings into an easy-to-understand report with critical insights, actions, and project owners highlighted. Consider different learning styles by adding color, images, and graphs to communicate the information simply and clearly.
Creating timelines can ensure that change happens in a timely way.
7. Ask “how might we” questions
Once you’ve shared your insights, it’s helpful to organize a design workshop to consider potential solutions. As part of this, “ how might we ” questions can help you ideate solutions to better deliver for customers.
These questions relate to the insights you’ve gathered.
Let’s take these examples:
Customers are dropping off at the shopping cart
Customers are avoiding sign-ups due to data privacy concerns
Customers are reviewing the business badly
Some “how might we” questions to address these problems could be:
How might we ensure users spend only a few minutes in the shopping cart?
How might we communicate data privacy when customers are signing up?
How might we ensure our customers have a better sentiment towards the company?
These questions can then lead to critical actions across the business.
- Gain actionable insights with data synthesizing
Data from multiple sources is more reliable and powerful. But organizing that data into a uniform pattern is key to making the most of it. That’s where data synthesis comes in.
Synthesizing data requires:
Bringing various sources and various formats into one platform
Creating a UX research taxonomy to make the data uniform
Drawing insights for action across the business
Deep analysis can take time, but it’s a critical aspect of user-centered design and development. Once you’ve organized the data meaningfully, you can draw vital insights to drive positive change for users.
Ultimately, synthesizing data can boost customer satisfaction, loyalty, and retention. And those can seriously boost your bottom line.
What is the difference between data analysis and data synthesis?
Synthesizing data is where you collate various data types and systematically categorize them.
User research data may come from:
These all deliver different data, so synthesis is crucial to make the most of it.
In contrast, data analysis examines data to uncover trends, patterns, and insights. You can turn these insights into actions, which can guide thoughtful design and development for better UX.
These complementary processes generate a comprehensive understanding of user behaviors, preferences, and needs.
How do you synthesize qualitative data?
It’s essential to bring qualitative data into one streamlined platform to synthesize it. You can organize the data by theme, sentiment, or trend. Multiple iterations and reviews may be necessary to ensure you’ve labeled and categorized all data pieces.
A taxonomy can help you organize the data into a uniform, easy-to-read dataset. It ensures researchers can more easily and accurately analyze the data.
Synthesize data meaning
Synthesizing data is bringing data from multiple sources into a meaningful and uniform pattern. This ensures researchers can review, evaluate, and analyze data for deeper insights and faster action.
Get started today
Go from raw data to valuable insights with a flexible research platform
Editor’s picks
Last updated: 21 December 2023
Last updated: 16 December 2023
Last updated: 6 October 2023
Last updated: 5 March 2024
Last updated: 25 November 2023
Last updated: 15 February 2024
Last updated: 11 March 2024
Last updated: 12 December 2023
Last updated: 6 March 2024
Last updated: 10 April 2023
Last updated: 20 December 2023
Latest articles
Related topics, log in or sign up.
Get started for free

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Digg
Latest Earthquakes | Chat Share Social Media
Collection of Data provided or contributed by SSAR programs. Selecting an item you'll find additional information and program point of contacts.
U.S. Geological Survey Data Citation Analysis, 2016-2022
Data release for an experimental translocation of invasive bigheaded carps and upstream passage success at a navigation lock, topographic and multispectral reflectance products, aerial imagery, ground spectra, vegetation, and associated gps data collected during uncrewed aircraft system operations - dog head marsh at south cape beach, mashpee, ma, october 7-8, 2021, microcosm experiment data of microcystin-degrading bacteria in lake erie source waters and drinking-water plants, 2015-18, update doi example, prism an81m spatial climate data extracted by usgs 12-digit hydrologic units of the conterminous united states (1895-2017), u.s. geological survey 2021 data management planning survey results and analyses, usgs state of the data project: rubric and assessment data, trad: thermal traits of anurans database for the southeastern united states, protected areas database of the united states (pad-us) 3.0 - world database on protected areas (wdpa) submission, ken pierce's legacy field notebooks and annotated aerial photographs, geological, and topographical maps from across the western united states 1963-2021 (ver. 2.0, january 2024).
- Business Advisory
- Human Capital
- Client Stories
- Sales Advisory
- Executive Search
Synthesis vs. Analysis: Breaking Down the Difference

Both synthesis and analysis play an important role in market and competitive intelligence (M/CI), but are two markedly different stages of a broader CI process. All too often, business leaders conflate synthesis and analysis, a mistake that can be very damaging to the overall success of M/CI efforts within an organization.
In this guide, we’ll break down the key differences between synthesis and analysis, discuss where you should focus the majority of your time, and explore ways to improve both your synthesis and analysis processes in the competitive intelligence infrastructure at your organization.
But first, let’s start with definitions for both synthesis and analysis as they relate to competitive intelligence activities.
What is synthesis?
Synthesis is the process of combining simple things into something more complex in order to understand their shared qualities.
What is analysis?
Analysis is the process of breaking down something into its basic parts to understand the nature, function or meaning of the relationships among the parts.
It is the understanding of the meaning that allows CI practitioners to create insights, intelligence, and knowledge.
It’s crucial to note that analysis can only be conducted by humans – not software. Too often, the makers of software programs or the latest technology claim that their product has the ability to conduct analysis. The reality is that today this just isn’t possible. Claims of non-human analysis are at best misleading and more likely fraudulent.
Synthesis vs. Analysis: Why Does It Matter?
To a layperson, these differences might seem trivial or a matter of semantics, but nothing could be further from the truth. Understanding this distinction is actually crucially important in helping competitive intelligence practitioners to educate their end customers. These end customers likely belong to a variety of departments or business units scattered around the organization, and consume M/CI insights to help them make better decisions. It’s fair to say that many end users’ understanding of M/CI is rudimentary at best.
Consumers of competitive intelligence should understand that analysis isn’t just something that happens with technology. Meaningful analysis requires a great deal of work to be performed by humans, and it’s important to recognize that this work takes time.
Yes, it’s possible to summarize information quickly. But analyzing information and transforming it into something valuable with context and meaning for your organization takes time.
Drawing a clear line between synthesis and analysis also helps to better align expectations across the organization. Many times, stakeholders might think they want a synthesis, but what they really want is an analysis. Let’s look at a quick example:
The leader of a sales organization reaches out to the M/CI team and asks for a report with the ten most recent deals that the sales team lost to a major competitor. This is a synthesis, and while the report does have value, it probably doesn’t provide any particularly meaningful information or helpful insights. In reality, what the sales leader likely wants to know is WHY their team lost those deals. Uncovering insights in this area requires an analysis of the data involved. This analysis report will certainly provide greater insights into the sales teams performance and will likely require more time to produce.
Making sure that the end users of competitive intelligence across the organization understand what they’re asking for, and the work involved in delivering it, enables the M/CI team to serve end users much more effectively.
Technology for Synthesis, Humans for Analysis
Another helpful way to think about the distinction between synthesis and analysis is the way in which the work is completed.
Today, the level of data that M/CI teams have access to continues to grow rapidly, and shows no signs of letting up. Compounding that issue is the increasing diversity of the requests that flow into the M/CI team from across the organization.
As a general rule, technology can perform synthesis much more effectively than humans can and M/CI teams should deploy technology to perform synthesis. Making sense of the information gathered and how it impacts certain areas of the business or markets (aka analysis) is work best performed by talented, well-qualified M/CI professionals. Take an example of an organization who want to track news about their competitors:
By setting up an automated monitoring tool that synthesizes competitor news, organizations can track news sources, social media platforms, and press releases for any news related to a set of pre-selected competitors. Any news will be pulled into a central platform, which will display all relevant news items in a real-time dashboard, email report, or some other format. This is far more efficient than relying on a human to track all these sources of information, and ensures key news items are never missed by the CI team.
Without software to assist M/CI teams overcome the deluge of data and inbound requests, it’s all too easy for even the most talented of M/CI professionals to get bogged down with low-value administrative tasks. . Organizations should look to incorporate sophisticated M/CI software platforms that employ technologies like Natural Language Processing (NLP) and Artificial Intelligence (AI) to effectively source, tag, and categorize competitive intelligence data. A lack of software and infrastructure around M/CI efforts often leads organizations to enter a cycle of competitive intelligence failure, where the CI function fails to prove their value to the wider organization and is eventually shut down.
Maximize the effectiveness of your competitive intelligence effort. Schedule a call with our experts today.
LEARN MORE ABOUT FAHRENHEIT’S BUSINESS ADVISORY CAPABILITIES.

MEET THE EXPERTS

Following service in the US Navy and as a counterterrorism analyst at a US government agency, Peter spent 8 years in the Strategy Practice of Deloitte Consulting. Peter then served as CEO of a PE-backed consulting and technology firm, leading the company through two successful exits. He’s helped middle market companies, Fortune 500 firms, and Federal agencies “see around the corner” and turn threats into opportunities.

Jennifer began her career fielding market research studies for clients in the Consumer Packaged Goods space before joining one of the largest grocery chains in the United States performing location intelligence and site analysis for their real estate division. After a period providing competitive intelligence services for a Fortune 100 infrastructure technology company, she joined a boutique firm offering strategic advice for clients in a variety of industries.
More Insights
- April 12, 2024 New California Employment Law Updates for 2024
- April 10, 2024 CASE STUDY: Operations, Employee & Assets Stabilization
- April 10, 2024 How Does Succession Planning Differ from Replacement Planning?
- March 18, 2024 The Business Disruption Risks of AI
Analysis vs. Synthesis
What's the difference.
Analysis and synthesis are two fundamental processes in problem-solving and decision-making. Analysis involves breaking down a complex problem or situation into its constituent parts, examining each part individually, and understanding their relationships and interactions. It focuses on understanding the components and their characteristics, identifying patterns and trends, and drawing conclusions based on evidence and data. On the other hand, synthesis involves combining different elements or ideas to create a new whole or solution. It involves integrating information from various sources, identifying commonalities and differences, and generating new insights or solutions. While analysis is more focused on understanding and deconstructing a problem, synthesis is about creating something new by combining different elements. Both processes are essential for effective problem-solving and decision-making, as they complement each other and provide a holistic approach to understanding and solving complex problems.

Further Detail
Introduction.
Analysis and synthesis are two fundamental processes in various fields of study, including science, philosophy, and problem-solving. While they are distinct approaches, they are often interconnected and complementary. Analysis involves breaking down complex ideas or systems into smaller components to understand their individual parts and relationships. On the other hand, synthesis involves combining separate elements or ideas to create a new whole or understanding. In this article, we will explore the attributes of analysis and synthesis, highlighting their differences and similarities.
Attributes of Analysis
1. Focus on details: Analysis involves a meticulous examination of individual components, details, or aspects of a subject. It aims to understand the specific characteristics, functions, and relationships of these elements. By breaking down complex ideas into smaller parts, analysis provides a deeper understanding of the subject matter.
2. Objective approach: Analysis is often driven by objectivity and relies on empirical evidence, data, or logical reasoning. It aims to uncover patterns, trends, or underlying principles through systematic observation and investigation. By employing a structured and logical approach, analysis helps in drawing accurate conclusions and making informed decisions.
3. Critical thinking: Analysis requires critical thinking skills to evaluate and interpret information. It involves questioning assumptions, identifying biases, and considering multiple perspectives. Through critical thinking, analysis helps in identifying strengths, weaknesses, opportunities, and threats, enabling a comprehensive understanding of the subject matter.
4. Reductionist approach: Analysis often adopts a reductionist approach, breaking down complex systems into simpler components. This reductionist perspective allows for a detailed examination of each part, facilitating a more in-depth understanding of the subject matter. However, it may sometimes overlook the holistic view or emergent properties of the system.
5. Diagnostic tool: Analysis is commonly used as a diagnostic tool to identify problems, errors, or inefficiencies within a system. By examining individual components and their interactions, analysis helps in pinpointing the root causes of issues, enabling effective problem-solving and optimization.
Attributes of Synthesis
1. Integration of ideas: Synthesis involves combining separate ideas, concepts, or elements to create a new whole or understanding. It aims to generate novel insights, solutions, or perspectives by integrating diverse information or viewpoints. Through synthesis, complex systems or ideas can be approached holistically, considering the interconnections and interdependencies between various components.
2. Creative thinking: Synthesis requires creative thinking skills to generate new ideas, concepts, or solutions. It involves making connections, recognizing patterns, and thinking beyond traditional boundaries. By embracing divergent thinking, synthesis enables innovation and the development of unique perspectives.
3. Systems thinking: Synthesis often adopts a systems thinking approach, considering the interactions and interdependencies between various components. It recognizes that the whole is more than the sum of its parts and aims to understand emergent properties or behaviors that arise from the integration of these parts. Systems thinking allows for a comprehensive understanding of complex phenomena.
4. Constructive approach: Synthesis is a constructive process that builds upon existing knowledge or ideas. It involves organizing, reorganizing, or restructuring information to create a new framework or understanding. By integrating diverse perspectives or concepts, synthesis helps in generating comprehensive and innovative solutions.
5. Design tool: Synthesis is often used as a design tool to create new products, systems, or theories. By combining different elements or ideas, synthesis enables the development of innovative and functional solutions. It allows for the exploration of multiple possibilities and the creation of something new and valuable.
Interplay between Analysis and Synthesis
While analysis and synthesis are distinct processes, they are not mutually exclusive. In fact, they often complement each other and are interconnected in various ways. Analysis provides the foundation for synthesis by breaking down complex ideas or systems into manageable components. It helps in understanding the individual parts and their relationships, which is essential for effective synthesis.
On the other hand, synthesis builds upon the insights gained from analysis by integrating separate elements or ideas to create a new whole. It allows for a holistic understanding of complex phenomena, considering the interconnections and emergent properties that analysis alone may overlook. Synthesis also helps in identifying gaps or limitations in existing knowledge, which can then be further analyzed to gain a deeper understanding.
Furthermore, analysis and synthesis often involve an iterative process. Initial analysis may lead to the identification of patterns or relationships that can inform the synthesis process. Synthesis, in turn, may generate new insights or questions that require further analysis. This iterative cycle allows for continuous refinement and improvement of understanding.
Analysis and synthesis are two essential processes that play a crucial role in various fields of study. While analysis focuses on breaking down complex ideas into smaller components to understand their individual parts and relationships, synthesis involves integrating separate elements or ideas to create a new whole or understanding. Both approaches have their unique attributes and strengths, and they often complement each other in a cyclical and iterative process. By employing analysis and synthesis effectively, we can gain a comprehensive understanding of complex phenomena, generate innovative solutions, and make informed decisions.
Comparisons may contain inaccurate information about people, places, or facts. Please report any issues.
- Open access
- Published: 09 March 2023
Needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions: a systematic review and narrative synthesis
- Arisa Kawashima 1 , 2 &
- Catherine J. Evans 2 , 3
BMC Palliative Care volume 22 , Article number: 20 ( 2023 ) Cite this article
3675 Accesses
1 Citations
29 Altmetric
Metrics details
Older people with noncancer conditions are less likely to be referred to palliative care services due to the inherent uncertain disease trajectory and a lack of standardised referral criteria. For older adults with noncancer conditions where prognostic estimation is unpredictable, needs-based criteria are likely more suitable. Eligibility criteria for participation in clinical trials on palliative care could inform a needs-based criteria. This review aimed to identify and synthesise eligibility criteria for trials in palliative care to construct a needs-based set of triggers for timely referral to palliative care for older adults severely affected by noncancer conditions.
A systematic narrative review of published trials of palliative care service level interventions for older adults with noncancer conditions. Electronic databases Medline, Embase, CINAHL, PsycINFO, CENTRAL, and ClinicalTrials.gov. were searched from inception to June 2022. We included all types of randomised controlled trials. We selected trials that reported eligibility criteria for palliative care involvement for older adults with noncancer conditions, where > 50% of the population was aged ≥ 65 years. The methodological quality of the included studies was assessed using a revised Cochrane risk-of-bias tool for randomized trials. Descriptive analysis and narrative synthesis provided descriptions of the patterns and appraised the applicability of included trial eligibility criteria to identify patients likely to benefit from receiving palliative care.
27 randomised controlled trials met eligibility out of 9,584 papers. We identified six major domains of trial eligibility criteria in three categories, needs-based, time-based and medical history-based criteria. Needs-based criteria were composed of symptoms, functional status, and quality of life criteria. The major trial eligibility criteria were diagnostic criteria ( n = 26, 96%), followed by medical history-based criteria ( n = 15, 56%), and physical and psychological symptom criteria ( n = 14, 52%).
For older adults severely affected by noncancer conditions, decisions about providing palliative care should be based on the present needs related to symptoms, functional status, and quality of life. Further research is needed to examine how the needs-based triggers can be operationalized as referral criteria in clinical settings and develop international consensus on referral criteria for older adults with noncancer conditions.
Peer Review reports
Inequities in the provision of palliative care remain globally, whilst palliative care should be available to all who need it regardless of their diagnosis [ 1 ]. Global ageing and the changes in the prevalence of diseases imply that most needing palliative care worldwide are older people living with noncancer conditions [ 1 , 2 , 3 , 4 ]. However, there is consistent evidence that older people with noncancer conditions experience inequitable access to palliative care with low rates of referral or late referral in the last days or weeks of life [ 5 , 6 ], such as in dementia [ 7 ] and heart failure [ 8 ].
There are several major barriers to referral in patients with noncancer conditions. A systematic review reported that one of the barriers to access and referral to palliative care is ‘a lack of national standardised referral criteria’ for screening patients with chronic noncancer disease regarding their need for palliative care [ 9 ]. A questionnaire survey showed that the highest barrier perceived by specialist palliative care service providers was ‘the unpredictable noncancer disease trajectory’ [ 10 ]. In primary care settings, the uncertainty of the illness trajectory was also identified as a barrier to effective primary palliative care provision for noncancer patients [ 11 ]. As a result, in clinical practice, key triggers or prompts for older adults with noncancer conditions to access palliative care are based on variable professional opinions or experiences [ 12 , 13 ]. This means referral triggers are typically informed by differences in education, interest, and understanding on the intended outcomes of palliative care.
Referral criteria are needed to address the inequity of access to palliative care for older adults with noncancer conditions. Systematic review on referral criteria for noncancer patients aged over 65 years identified predictor variables to aid clinicians’ prognostic estimation [ 5 ]. However, the inherent uncertain disease trajectory for older adults with noncancer conditions requires the provision of palliative care to be based upon need, rather than time-based criteria, such as disease trajectory and prognostic criteria [ 13 , 14 ]. Although systematic reviews identified referral criteria for palliative care among patients with heart failure [ 15 , 16 ], dementia [ 17 ], and Parkinson’s disease [ 18 ], there has been no referral criteria based on palliative care needs for older general noncancer populations and the reviews assert the lack of consensus on palliative care referral criteria for adults with noncancer conditions.
Eligibility criteria for participation in clinical trials on palliative care can inform a needs-based criteria for palliative care. This approach was used and advocated by Hui et al. [ 12 ] and others [ 15 , 17 , 18 ] in systematic reviews investigating eligibility criteria for trials to inform triggers for outpatient palliative cancer care. Hui et al.’s review identified six themes for referral, two time-based, including cancer trajectory and prognosis, and four needs-based, including physical symptoms, performance status, psychosocial distress, and end-of-life care planning [ 12 ]. As appropriate trial eligibility criteria are designed to measure efficacy of an intervention, the criteria include populations considered likely to benefit from the intervention compared with the control. Eligibility criteria in palliative care trials seeks to identify patients with palliative care needs and considered likely to benefit from palliative care. Although eligibility criteria for randomised controlled trials (RCTs) may not be specifically designed for referral, they can inform a needs-based set of triggers for timely referral to palliative care.
This systematic review aimed to identify, appraise the applicability, and synthesise patient eligibility criteria in published trials on palliative care service level interventions for older adults severely affected by noncancer conditions. The findings intended to construct a needs-based set of triggers for timely referral to palliative care.
Study design
A systematic narrative review of the published literature on palliative care interventions for older adults with noncancer conditions to identify and synthesise the criteria used to indicate eligibility for palliative care provision [ 19 ]. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines [ 20 ]. The PRISMA 2020 checklist is shown in Additional file 1 . The methodological quality of the included studies was assessed using a revised Cochrane risk of bias tool for randomized trials [ 21 ]. The protocol was registered in PROSPERO in November 2018 (CRD42018095845, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018095845845 ). We were not required to seek an institutional ethics approval because we only used publicly accessible documents.
Data sources and searches
Relevant articles were identified from six electronic searches: MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCOhost), PsycINFO (Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov. Search strategies were informed by previous systematic reviews related to palliative care and older adults [ 12 , 13 , 22 ]. A full search strategy can be seen in Additional file 2 . All searches were conducted from database inception to September 2018 and updated on June 2022. We supplemented the electronic searches with reference chaining and citation tracking, and handsearching two palliative medicine textbooks [ 23 , 24 ] and conference abstracts [Research Congress of the European Association for Palliative Care (EAPC), 2018]. All identified studies were managed in EndNote. There was no language restriction in the selection of studies.
Eligibility criteria
Types of studies.
We included RCTs, including cluster randomised trials, pilot, and feasibility trials. We sought to identify and collate trial eligibility criteria for patient participants and appraise what patterns of eligibility criteria were successful in terms of recruitment, attrition, attaining sample size, and effect on the primary outcome. Feasibility and pilot trials were included, as intention is to evaluate if they can recruit patients to the trial using the stated eligibility criteria. We excluded trials that focused exclusively on the economic evaluation of palliative care as not evaluating the effect of palliative care on patient outcomes, and non-experimental studies (observational studies) as our interest was patient eligibility criteria for palliative care intervention trials. We excluded opinion pieces including editorials, commentaries, letters, and dissertations.
Types of participants
We included adults (aged ≥ 65 years) severely affected by chronic noncancer illness, including chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic kidney failure, cirrhosis of the liver, stroke and long-term neurological conditions, including dementia and Parkinson’s disease. These conditions can cause considerable distressing symptoms and concerns [ 25 ]. Receipt of palliative care is shown to relive suffering [ 26 , 27 ]. We included studies where > 50% of the population was aged ≥ 65 years and > 50% of the population were people with noncancer conditions.
Types of interventions
We included palliative care trials to identify individuals who could benefit from palliative care. Specialist and general palliative care interventions were included that aimed to promote quality of life for adults aged older adults ≥ 65 years severely affected by noncancer conditions. We defined a palliative care intervention as a model or service of palliative care, not a discrete aspect of palliative care, such as oxygen therapy. We defined a palliative care model or service as comprising four key elements [ 28 , 29 , 30 , 31 ], namely:
All levels of palliative care in any setting
We referred to the model of a three-level structure: palliative care approach in all settings, general basic palliative care, and specialist palliative care with adequate skills for each level [ 32 ]. All study settings were included: community health services, including clinics and health centres, outpatient and ambulatory care settings, and inpatient units.
An intervention providing direct palliative care to older adults
Interventions that did not directly deliver care to patients were excluded (e.g., interventions to caregivers, education programs to healthcare professionals, or evaluations of assessment tools). We considered a palliative care service intervention if the authors described it as 'palliative' anywhere in the manuscript.
An intervention had multi-component services
A palliative care service is a multidimensional and holistic approach to meet the physical, psychological, social, and spiritual needs of patients. Interventions that delivered only one component of palliative care (e.g., medication, psychotherapy, complementary therapy, decision aid) were not considered as palliative care service.
An intervention was provided by a multidisciplinary team
We defined 'palliative care services' as multidisciplinary services providing comprehensive care aiming at different physical and psychosocial components of palliative care. We excluded interventions provided by only one professional (e.g., nursing intervention).
Types of outcome measures were not restricted.
Study selection and data extraction
The review author A.K. screened and assessed the identified titles and abstracts according to the inclusion criteria, followed by assessing all relevant full-articles by A.K. and E.A.D.P., independently. For the update search, A.K. and R.T. assessed full-articles, independently. Disagreements were resolved by consensus and discussed with C.J.E. The inter-rater reliability between the first author A.K. and E.A.D.P. and between A.K. and R.T. were assessed with a percentage of agreement. The selection process was presented in a PRISMA 2020 flow diagram (Fig. 1 ) [ 20 ]. Data were extracted by A.K. The PRISMA guideline [ 20 ] informed the data extraction detailing trial eligibility criteria, target population, impact on clinical outcome, and stated limitations, study design, study aim, including intervention, participant eligibility criteria, participant characteristics, screening to recruitment rate and main outcomes.
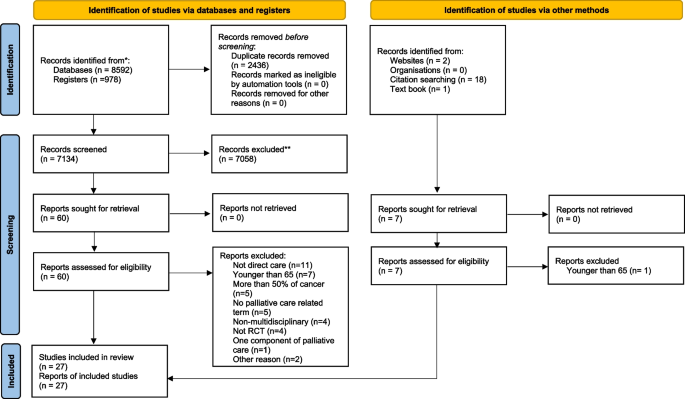
PRISMA 2020 flow diagram
Quality appraisal of included studies
The author A.K. assessed the risk of bias in all included RCTs, as described in the Cochrane Handbook for Systematic Reviews of Interventions [ 33 ]. A revised Cochrane risk-of-bias tool for randomized trials is composed of the following five domains of bias: risk of bias arising from the randomization process; risk of bias due to deviations from intended interventions; risk of bias due to missing outcome data; risk of bias in measurement of the outcome; and bias in selection of the reported result. The summary judgements of the level of risk of bias for each domain are presented in Table 1 . We used the robvis which is a visualization tool for tabulating a table of risk of bias and categorised them as, ‘low risk’, ‘high risk’, ‘some concerns’, or ‘no information’ [ 34 ].
Data analysis and synthesis
Descriptive analysis and narrative synthesis provided descriptions of the patterns and appraised the applicability of included trial eligibility criteria to identify patients likely to benefit from receiving palliative care. We sought to identify and collate eligibility criteria and appraise what patterns of eligibility criteria were successful in terms of recruitment, attrition, attaining sample size, and effect on the primary outcome. Eligibility criteria were summarized by frequency counts of domains and synthesized by intended sample size, attrition rate, causes of attrition, and limitation that reflected on the eligibility.
To assess the recruitment, we appraised whether: 1) the study recruited the relevant population to answer the study aim, 2) the actual sample size was larger than the intended sample size determined by sample size calculation, and 3) the explanation for revision of the target sample size was given.
We assessed the attrition rate in accordance with the implementation of sample size estimation, cause of attrition, and anticipated attrition rate. Rates of attrition in the included trials were assessed to explore levels of attrition and if high attrition, to consider the appropriateness of the trial eligibility criteria to identify patients for palliative care (or not). To describe causes of attrition, we used the MORECare classification of attrition to describe causes of attrition: attrition due to death (ADD), attrition due to illness (ADI), and attrition at random (AaR) [ 62 , 63 ]. While there is no standardised level of loss to follow-up which attrition related bias was identified as a problem, Schulz and Grimes noted that the readers should be concerned about the possibility of bias when the attrition rate was 20% or greater [ 64 ]. However, the weighted average attrition across palliative care trials involving adults with serious illness and increasing nearness to end of life in a systematic review was 29% [ 63 ]. For example, a review of interventional palliative oncology trials stated that the attrition rate was 26% for the primary endpoint and 44% for the end of the study [ 65 ]. Therefore, we considered high attrition rate when the rate was more than 25%. Although dropouts due to symptom progression or death were not considered as protocol failures in palliative care trials, we sought to appraise what patterns of eligibility criteria were successful in terms of recruitment and the effect on the primary outcome.
We identified for each trial the level of statistically difference on the primary outcome between the intervention and control groups and the effect size. We then explored the respective eligibility criteria and target population to map and identify criteria associated with effect on the primary patient outcome. This intended to explore further the appropriateness (or not) of the patient eligibility criteria used. Because the timing of outcome measurement could influence the attrition rate and the effect on the primary outcome, we also considered the impact of length of intervention and time points of data collection.
Study selection
The electronic search strategy identified total 9,584 papers (6,720 in 2018 and 2,850 in the update search). An additional 21 papers were identified by hand searching and citation tracking. After removing duplicates, 7,134 studies were screened at title and abstract, and 67 were assessed as full-text articles. 27 met eligibility (20 studies identified by electronic searches and seven from hand search) (see Fig. 1 ) [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ]. All included studies were written in English. The reasons for study exclusion are reported in Fig. 1 [ 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 ]. The inter-rater agreement between independent reviewers of full text screening were 89% in the initial search and 86% for the update search.
Quality appraisal
Only five studies were considered low risks of bias [ 36 , 37 , 50 , 52 , 53 ]. 20 studies had high risks of bias, mainly due to incomplete outcome data. Two study were assessed at some concerns because of unstated data and lack of information [ 46 , 56 ]. The risk of bias plots are presented in Additional files 3 and 4 . The average attrition rate was 23%, ranging from 0 to 52%. The major cause of attrition was death. Eleven papers stated that small sample size was one of the limitations. What we valued more than attrition rate when assessing incomplete outcome data was advance estimations of attrition, descriptions of reasons for missing data, and whether they integrated these into sample size calculation.
Study characteristics
This review included 27 RCTs written in English. Most studies were conducted in US. This is important to understand the context of the work and the applicability of the proposed triggers for which settings. The first study was conducted in US in 2000 [ 35 ]. (Table 1 ).
We included 18 phase III RCTs and nine feasibility trials. The 15 parallel RCTs compared the palliative care intervention with usual care. Five studies used fast-track design and compared a fast-track group with a waiting list group. Higginson et al. [ 57 ] used a parallel group fast-track trial design. Finally, five studies used a mixed methods trial design [ 49 , 50 , 53 , 54 , 57 ].
Participants
The studies included 3,663 participants ranging from 14 to 517 per study [ 54 , 55 ]. The mean age ranged from 65.5 years with heart failure [ 36 ] to 85.7 years with chronic noncancer conditions and frailty [ 53 ]. The female percentage ranged from 9.1% [ 41 ] to 81.8% [ 35 ]. Eleven studies described the ethnicity of the participants; the majority of participants were White, followed by African Americans.
Of the included 27 papers, eight were conducted with patients with heart failure (HF) [ 36 , 43 , 44 , 45 , 47 , 50 , 60 , 61 ]. Six included participants with respiratory disease, two with COPD [ 58 , 59 ], two with interstitial lung disease (ILD) [ 37 , 49 ], one with idiopathic pulmonary fibrosis (IPF) [ 41 ], and one with COPD/chronic obstructive airway disease (COAD) [ 54 ]. Other diagnoses were neurological diseases, two with Parkinson’s disease (PD) [ 42 , 52 ], one with dementia [ 35 ], and one stated long-term neurological conditions. Four studies [ 38 , 48 , 51 , 57 ] included multiple diseases, three included both cancer and noncancer conditions [ 38 , 51 , 57 ], and one included both CHF and COPD [ 48 ]. Three studies stated general chronic/advanced noncancer populations [ 46 , 53 , 55 ], and one included intensive care unit (ICU) populations [ 40 ].
Intervention and control
Eleven different models of home palliative care in 14 studies were identified [ 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ]. All eleven models were composed of multi-components of palliative care intervention, such as symptom management, self-management education of disease, end-of-life discussions, case conferences, documentation, regular home visits, or a telephone hotline. Seven studies [ 35 , 38 , 40 , 43 , 44 , 45 , 47 ] provided inpatient care and six [ 36 , 37 , 39 , 41 , 42 , 46 ] implemented outpatient palliative care services. Inpatient palliative care services in seven studies were developed based on the standard referral process of the hospital palliative care team or developed for the trial. As for control group, usual care differed across studies due to the wide variety of health systems and local service provisions. Several studies followed national or government guidelines.
Primary outcomes
20 studies [ 36 , 37 , 38 , 41 , 42 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 59 , 60 ] set quality of life (QOL) or symptom burden as a primary outcome, with marked heterogeneity in the measurements used. (Table 1 ). Eight used disease-specific QOL measurements, two used HF-specific QOL measures; The Kansas City Cardiomyopathy Questionnaire (KCCQ), four used respiratory diseases specific measures; a QOL domain in the Chronic Respiratory Disease Questionnaire (CRQ), the St. George’s Respiratory Questionnaire (SGRQ), or Maugeri Respiratory Questionnaire (MRQr), and two used measures for neurological conditions; the 39-Item Parkinson's Disease Questionnaire (PDQ-39) or the Quality of Life in Alzheimer’s Disease (QoL-AD). Other primary outcomes were medical service use, documentation of care preferences, and patient satisfaction.
Summary of eligibility criteria of included studies
Six main domains for eligibility criteria were identified, including diagnostic criteria ( n = 26 out of 27 included studies, 96%), medical history-based ( n = 15, 56%), symptoms (physical and psychological) ( n = 14, 52%), prognostic criteria ( n = 9, 33%), functional status (n = 8, 30%]), QOL ( n = 2 [7%]), and other criteria ( n = 4, 15%). We categorised these domains into three major criteria themes: needs-based, time-based, and medical history-based.
In Table 2 , we used the initial letter of each domain to show which category the eligibility trials were categorised. The letter D stands for ‘Diagnostic Criteria’, P for ‘Prognostic’, S for ‘Symptoms’, Q for ‘QOL’, F for ‘Functional Status’, M for ‘Medical History and Treatment’, and O for ‘Other’. The number of domains was calculated by adding the number of domains used in the trial as eligibility criteria. Since some standardised measures covered several domains, we analysed the domain in the measurements and counted the number of domains. For example, Bekelman et al. [ 36 ] used a score of HF-specific health status (KCCQ) to assess eligibility. As KCCQ is a reliable and valid measure of symptoms, functional status, and QOL, the number of domains counted was three [ 104 ]. Table 3 gives an overview of the different criteria and use by respective disease groups. Table 4 gives a systematization of the eligibility criteria which shows major categories for referral criteria.
Need-based criteria
Focused on three main areas of symptoms, function, and quality of life, including:
Around half of the 27 included studies set an existence of physical/psychological symptoms as eligibility criteria [ 36 , 42 , 45 , 46 , 48 , 50 , 53 , 54 , 55 , 56 , 57 , 59 , 60 , 61 ]. As for physical symptoms, Aiken et al. [ 48 ] included HF or COPD patients suffering from fatigue, palpitation, dyspnoea, or angina with any activity. For participants with HF, Brännström et al. [ 50 ] checked the presence of cardiac cachexia with involuntary non-oedematous weight loss ≥ 6% of total body weight within the preceding 6–12 months, and Bekelman et al. [ 36 ] confirmed reporting at least one of the target symptoms of fatigue, shortness of breath, pain, and/or depression. Only six studies [ 36 , 42 , 53 , 56 , 60 , 61 ] contained psychological symptoms as eligibility criteria. Four studies that provided breathlessness intervention/support service examined whether breathlessness existed in spite of optimisation of the underlying illness [ 46 , 50 , 54 , 57 ]. Among them, Higginson et al. [ 57 ] used the Medical Research Council (MRC) dyspnoea scale to assess the degree of refractory breathlessness. Four studies [ 42 , 53 , 56 , 59 ] conducted comprehensive screening of complex symptoms in palliative population.
Functional status
Eight studies included criteria that assess functional or performance status in their eligibility [ 35 , 36 , 40 , 42 , 46 , 51 , 53 , 59 ]. In trials of patients with HF or COPD, the Palliative Performance Scale (PPS) and KCCQ were employed to assess functional status alongside prognosis, symptoms, or QOL [ 36 , 51 ]. Regarding neurological conditions, Ahronheim et al. [ 35 ] used the Functional Assessment Staging Test (FAST) for systematic examination of the functional changes occurring in patients with dementia. Helgeson et al. [ 40 ] considered admission of patients with dementia from nursing care facilities as a pre-existing functional dependency. The trial eligibility criteria of Kluger et al. [ 42 ] on Parkinson's disease contain the Palliative Care Needs Assessment Tool (PC-NAT) and their criteria were based on a broad range of potential palliative care needs rather than time-based criteria.
Evans et al. [ 53 ] assessed the existence of frailty with the clinical frailty scale score. Shunk et al. [ 46 ] set the capability to participate in physiotherapy as a functional criterion because of the nature of the intervention programme.
Quality of life
Only two trials used QOL for trial eligibility [ 36 , 50 ]. Bekelman et al. [ 36 ] used KCCQ in their trial as a measurement of the patient’s perception of their health status which includes how their heart failure impacts their QOL within a 2-week recall period. Brännström et al. [ 50 ] measured QOL using a Visual Analogue Scale (VAS). VAS is commonly used to rate subjective experiences [ 105 ].
Time-based criteria
Diagnostic criteria.
Diagnostic criteria were a set of signs and tests for use in routine clinical care to guide the care of individual patients. In the 12 studies that included HF, six studies [ 43 , 44 , 48 , 50 , 60 , 61 ] used the New York Heart Association (NYHA) classification of II-IV [ 43 , 44 ], or NYHA III-IV [ 47 , 48 , 50 , 60 ]. Brumley et al. [ 51 ] included not only participants with HF, but also COPD and cancer, and used the Palliative Performance Scale to assess disease severity. Rogers et al. [ 45 ] measured signs of volume overload in accordance with the HF diagnosis. But, three HF studies [ 38 , 47 , 57 ] used no diagnostic criteria.
Similarly, diagnostic eligibility criteria were used in studies on lung disease and neurological conditions. Aiken et al. [ 48 ] in a study on COPD used measures of hypoxemia, oxygen saturation, pO2, and oxygen requirements. Three studies [ 37 , 41 , 49 ] on ILD or IPF used high-resolution computed tomography of lung or a composite physiologic index. Janssen et al. (2019) [ 58 ] and Scheerens et al. [ 59 ] used the Global Initiative for Chronic Obstructive Lung Disease (GOLD) system to categorize airflow limitation into stages of COPD.
Regarding neurological conditions, Gao et al. [ 56 ] employed the Hoehn and Yahr scale and the Expanded Disability Status Scale (EDSS) to describe the progression of each neurological disease. Hanson et al. [ 39 ] used the Global Deterioration Scale (GDS) to assess the severity of dementia. The other seven studies did not clearly state the measurements of diagnostic eligibility criteria [ 40 , 46 , 51 , 53 , 54 , 55 , 57 ].
Prognostic criteria
Nine studies included prognostic eligibility criteria [ 37 , 38 , 40 , 45 , 48 , 50 , 51 , 60 , 61 ]. The ‘surprise question’ was used in four studies [ 38 , 51 , 60 , 61 ]. The question is, “Would I be surprised if this patient died in the next 12 months?”, which has been used to identify patients at a high risk of death who might benefit from palliative care services [ 106 ]. Three [ 45 , 60 , 61 ] used HF-specific standardised prognostic measures. Ng et al. [ 60 ] and Wong et al. [ 61 ] and used multi-components of the prognostic indicator guidance to identify end-stage heart failure (ESHF) [ 107 ]. The indicators are constituted by three steps, which initiate intuitive surprise questions, followed by general and specific clinical indicators. In the three steps, they used only the last step, heart disease-specific clinical indicators. Rogers et al. [ 45 ] used the North American Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) risk score which uses clinical information to derive a discharge model for six months risk of rehospitalisation and mortality [ 108 ]. Bassi et al. [ 37 ] used the gender-age-physiology (GAP) index to estimate prognosis and enrol participants with advanced ILD [ 109 ]. Helgeson et al. [ 40 ] used APACHE and SOFA scoring models to measure severity of critically ill patients admitted to ICU and to predict their mortality [ 110 ].
Medical history-based criteria
We identified 15 articles that included medical history and treatment criteria. Among 15, eleven trials included criteria related to repeated unplanned hospital admissions due to the deterioration of illness [ 35 , 39 , 40 , 43 , 44 , 45 , 50 , 58 , 59 , 60 , 61 ]. The study of dementia by Ahronheim et al. [ 35 ] used medical history-based eligibility criteria for hospitalization for acute illness occurred with advanced dementia. However, as many participants died when they were admitted to a hospital, the authors assert these criteria limited study recruitment and attainment of sample size. Treatment-based criteria included previous/current administrative data, such as intravenous therapy support (e.g., diuretics), intubation or non-invasive ventilation, required diuretic dosing, required long-term oxygen therapy, post-cardiac arrest, and results of heart function [ 36 , 48 , 51 ]. Evans et al. [ 53 ] considered increasing health service use as a concern caused by severely affected non-malignant chronic conditions.
Other criteria
We found four other criteria which can be considered as psychosocial eligibility and needs perceived by healthcare professionals. Higginson et al. [ 57 ] asked patients their willingness to engage with a breathlessness support service. Farquhar et al. [ 54 , 55 ] assessed whether patients might benefit from a self-management programme. Although Helgeson et al. [ 40 ] considered the medical ICU perceived need, they did not clearly state the measurements of the criteria.
Main findings of the study
We systematically reviewed and narratively synthesised the eligibility criteria of 27 RCTs in palliative care. The findings of our review inform development of needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions. The results showed the list of potential needs-based triggers which were composed of three criteria; symptoms, functional status, and QOL. Eligibility criteria that were ‘successful’ tended to utilize at least one domain of needs-based criteria. Six studies used successful eligibility criteria according to the recruitment, attrition rate, and effect on primary outcomes in each study [ 50 , 51 , 53 , 54 , 57 , 61 ].
What this study adds
Decisions about informing palliative care should be based on individual needs related to symptoms, functional status, and QOL. Few studies used standardized measurements with specific cut-offs for symptoms, functional decline, or QOL assessment.
Comprehensive assessment aligned to philosophy of palliative care is essential to identify patients likely to benefit from receiving palliative care. In this review, trial eligibility criteria are limited to mainly physical symptoms with little consideration of psychological symptoms e.g. anxiety, depression. Outcome measures of specific physical symptoms such as pain are well developed, but psychosocial symptoms are liable to be considered less serious than physical symptoms. Furthermore, only one study used standardised measures to assess symptom control: MRC dyspnoea scale [ 57 ]. Four studies [ 54 , 55 , 60 , 61 ] measured physical symptoms such as breathlessness in spite of optimisation of underlying illness, though the measurements are not standardised tools. It implies that using a validated measure for palliative care referral is uncommon.
The best practice of symptom assessment is patient self-report outcomes for example using patient-reported outcome measures (PROMs/PROs) rather than clinician assessment due to the subjective nature of symptoms [ 111 ]. However, considering the illness trajectory and deterioration in physical/cognitive abilities in palliative care populations and the potential burden of completing PROMs, reporting by proxies such as relatives or healthcare professionals is important, especially for older adults [ 112 , 113 , 114 ]. For older adults with noncancer conditions, loss of mental capacity is common with advancing age associated with for example severe dementia and nearness to end of life. Therefore, measures used in palliative care need to be validated for the population and clinical practice, and for both self and proxy reporting [ 115 ]. Some outcome measures include a proxy version, for example the Integrated Palliative Care Outcome Scale (IPOS) [ 116 ]. This allows for the adjusting of proxy ratings if the patient is not able to complete the measure as their disease progresses.
Functional status and quality of life
Functional status and QOL are important needs-based triggers for older adults with noncancer conditions. Functional status is defined as the level of ability to do “activities performed by an individual to realize the needs of daily living in many aspects of life including physical, psychological, spiritual, intellectual, and roles [ 117 ]. Three of the included trials indicate that using standardised measures to assess functional status of patients is important to identify individuals likely to benefit from palliative care [ 35 , 36 , 51 ]. Most older adults severely affected by noncancer conditions experience progressive functional disability and subsequent health decline during the course of their disease. Moreover, some studies reported that functional status is significantly associated with health-related QOL (HRQOL) in people with noncancer conditions [ 118 , 119 ]. Although disease-specific functional assessment measures can be available in some noncancer diseases, the Australia-modified Karnofsky Performance Status (AKPS) is a modified version for palliative care that is widely used and appropriate for multiple care settings in palliative care populations [ 120 ]. Collecting and evaluating data on functional status during routine care could inform the need for palliative care for timely referral.
The primary goal of palliative care for older people is to improve QOL with provision based on their needs [ 13 , 29 ]. Quality of life can be defined as a complex, multifaceted construct that requires multiple approaches from different theoretical angles [ 121 ]. Although physical and psychological symptoms and functional impairment can be related to decline in QOL, QOL can be a trigger for referral as it can reflect an unmet need. In this review, eligibility criteria related to QOL were uncommon. As QOL that has a broad multidimensional concept can be difficult to be used as a single referral criterion, it could be operationalized as referral criteria in conjunction with other needs-based criteria. There are few relevant assessment tools addressing functional status and HRQOL for populations with multiple chronic conditions [ 122 ].
Willingness to engage with intervention
Psychosocial eligibility criteria were uncommon, mostly limited to views on willingness to engage with the intervention [ 54 , 55 , 57 ]. One of the major differences between palliative care and other fields of healthcare is the holistic approach it takes, including psychosocial and spiritual dimensions in addition to physical suffering. Willingness to receive palliative care may reflect the patient's preference and could form a needs-based trigger for a referral on preference for palliative care. However, patients who have preferences for palliative care may differ in characteristics compared to those with reluctance to refer to palliative care. For example, low level of health literacy of illness may preclude understanding on benefit of receiving palliative care service and impede individuals’ access to palliative care services. The Health Literacy Skills conceptual framework introduced by Squires et al. [ 123 ] illustrates mediators between health literacy and health outcomes. According to the framework, lack of knowledge about available palliative care services means patients do not request or access these services [ 124 , 125 ]. Educating individuals about the role and function of palliative care, and confirming the willingness to engage with the intervention, may be one of the simplest ways to assess needs-based triggers for a referral on preference for palliative care.
Limited availability of validated assessment tools
A barrier to using individual needs-based triggers for referral criteria is the limited availability of validated and brief standardised assessment tools encompassing symptom severity, functional status, and QOL for older people with noncancer conditions. Whilst generic measures are able to be used on a large range of health and in various health conditions and populations, specific measures specifically developed to measure outcomes in palliative care are more responsive to needs-based triggers than generic outcome measures. As palliative care focuses on providing holistic care, the outcome measure used to assess palliative care needs for people with noncancer diseases should be comprehensive and encompass multiple health domains [ 126 , 127 ]. Validated comprehensive measures for palliative care are available and used in clinical care, for example Edmonton Symptom Assessment Scale (ESAS) [ 128 ], and the IPOS [ 116 ] with condition specific measure for dementia and multi-morbidity (IPOS-Dem) [ 129 ]. They encompass holistic domains including, physical, psychological, social and spiritual dimensions.
Implications for further research and practice
Our review produced the initial step toward developing standardized referral criteria for clinical practice for older adults severely affected by noncancer conditions. Although the results inform a needs-based set of triggers for timely referral to palliative care, further research is needed to examine the feasibility, outcome and processes to operationalise the needs-based triggers as referral criteria in clinical settings.
Future research is needed to develop an international consensus on referral criteria for older adults with noncancer conditions and investigate if the developed referral criteria can be used in clinical settings to identify patients likely to benefit from receiving palliative care. The provision of palliative care should be based on needs assessment [ 32 ]. Our findings indicate that needs-based criteria are more likely to suit older people with noncancer conditions. Suitable needs-based referral criteria should meet the varied needs of people with different illness trajectories and different complexities of need for palliative care [ 32 ]. We recommend using measurements that encompass symptoms, QOL and functional status. Simple comprehensive measures developed and validated for palliative care population are practical for quick assessment of the palliative care needs, for example IPOS [ 116 ] or ESAS [ 128 ]. As palliative care needs vary widely, continued assessment of needs-based triggers are advocated. Standardised measures that can aid clinicians to assess palliative needs and concerns should be easy to use and interpret for all healthcare professionals and short to accommodate time constraints in clinical settings [ 130 ].
Strengths and limitations of the study
A key strength of this review is the identification and analysis of trial eligibility criteria for noncancer conditions without restricting by diagnosis. This intended to identify referral criteria applicable across noncancer conditions and multimorbidity. However, most participants in the included studies had heart failure or chronic respiratory disease. This strengthens the applicability of the identified needs-based set of triggers for these population groups, but may limit wider application to all older patients with other noncancer conditions. There is great heterogeneity among older people aged over 65 driven by for example variable diagnosis and multimorbidity, compared to any other age group. The impact this of heterogeneity on the recommendations for palliative care referral should be carefully considered. Though some studies that included mixed diagnosis attempted to reduce the heterogeneity of multimorbidity by identifying disease combinations, future research should consider how to manage heterogeneity, including stratification by age, diagnostic group, and number of co-morbidities. In the study selection process, although we assessed all relevant full-articles by two reviewers independently, the titles and abstracts of studies retrieved in bibliographic searches were assessed by one reviewer. Single screening of the titles and abstracts can influence the number of studies missed. Finally, the included studies were predominantly conducted in high-income countries in Europe and the US. This limits generalisability to non-Western regions and low-middle income countries.
The findings of this systematic review and narrative synthesis inform development of needs-based triggers for timely referral to palliative care for older people severely affected by noncancer conditions. For older people severely affected by noncancer conditions, decisions about providing palliative care should be based on the present needs related to symptoms, functional status, and quality of life. Further research is needed to examine the feasibility, outcome and processes to operationalise the needs-based triggers as referral criteria in clinical settings and develop international consensus on referral criteria for older adults with noncancer conditions.
Availability of data and materials
The PRISMA 2020 checklist, the full search strategy, and the risk of bias plots have been presented in Additional files. Any further data analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
The Australia-modified Karnofsky Performance Status
The Acute Physiology and Chronic Health Evaluation
Chronic Heart Failure
Chronic Obstructive Airways Disease
Chronic Obstructive Pulmonary Disease
Centre for Reviews and Dissemination
The Chronic Respiratory Disease Questionnaire
European Association for Palliative Care
Emergency Department
Edmonton Symptom Assessment System
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness risk score
End-Stage Heart Failure
Functional Assessment Staging Tool
The Gender-Age-Physiology index
The Global Deterioration Scale
The Global Initiative for Chronic Obstructive Lung Disease
Heart Failure
Health-related quality of life
Intensive Care Unit
Interstitial Lung Disease
Idiopathic pulmonary fibrosis
The Integrated Palliative care Outcome Scale
Kansas City Cardiomyopathy Questionnaire Short Version
Medical Research Council
Maugeri Respiratory Questionnaire
New York Heart Association
Parkinson’s Disease
The Palliative Care Needs Assessment Tool
The Parkinson's Disease Questionnaire
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Patient Reported Outcome Measures
The International Prospective Register of Systematic Reviews
The Quality of Life in Alzheimer’s Disease
Randomised Controlled Trial
The St. George’s Respiratory Questionnaire
The Sequential Organ Failure Assessment
Visual Analogue Scale
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90.
Article PubMed PubMed Central Google Scholar
Hall S, Petkova H, Tsouros AD, Costantini M and Higginson IJ. Palliative care for older people: better practices. WHO Regional Office for Europe. 2011. [ https://www.euro.who.int/__data/assets/pdf_file/0017/143153/e95052.pdf . ] Accessed 24 Apr 2022.
Addington-Hall J, Higginson IJ. Palliative care for non-cancer patients. Oxford: Oxford University Press; 2001.
Book Google Scholar
Sleeman KE, de Brito M, Etkind S, Nkhoma K, Guo P, Higginson IJ, Gomes B, Harding R. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health. 2019;7(7):e883–92.
Coventry PA, Grande GE, Richards DA, Todd CJ. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: a systematic review. Age Ageing. 2005;34(3):218–27.
Article PubMed Google Scholar
Burt J, Raine R. The effect of age on referral to and use of specialist palliative care services in adult cancer patients: a systematic review. Age Ageing. 2006;35(5):469–76.
Sampson EL, Candy B, Davis S, Gola AB, Harrington J, King M, Kupeli N, Leavey G, Moore K, Nazareth I, et al. Living and dying with advanced dementia: a prospective cohort study of symptoms, service use and care at the end of life. Palliat Med. 2018;32(3):668–81.
Kavalieratos D, Kamal AH, Abernethy AP, Biddle AK, Carey TS, Dev S, Reeve BB, Weinberger M. Comparing unmet needs between community-based palliative care patients with heart failure and patients with cancer. J Palliat Med. 2014;17(4):475–81.
Ahmed N, Bestall JC, Ahmedzai SH, Payne SA, Clark D, Noble B. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. Palliat Med. 2004;18(6):525–42.
Article CAS PubMed Google Scholar
O’Leary N, Tiernan E. Survey of specialist palliative care services for noncancer patients in Ireland and perceived barriers. Palliat Med. 2008;22(1):77–83.
Oishi A, Murtagh FE. The challenges of uncertainty and interprofessional collaboration in palliative care for non-cancer patients in the community: a systematic review of views from patients, carers and health-care professionals. Palliat Med. 2014;28(9):1081–98.
Hui D, Meng YC, Bruera S, Geng Y, Hutchins R, Mori M, Strasser F, Bruera E. Referral criteria for outpatient palliative cancer care: a systematic review. Oncologist. 2016;21(7):895–901.
Evans CJ, Ison L, Ellis-Smith C, Nicholson C, Costa A, Oluyase AO, Namisango E, Bone AE, Brighton LJ, Yi D, et al. Service delivery models to maximize quality of life for older people at the end of life: a rapid review. Milbank Q. 2019;97(1):113–75.
Connor SR. Global Atlas of Palliative Care, 2nd Ed 2020. Worldwide Palliative Care Alliance (WPCA). 2020. [ https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/csy/palliative-care/whpca_global_atlas_p5_digital_final.pdf?sfvrsn=1b54423a_3 .] Accessed 28 Oct 2022.
Chang YK, Kaplan H, Geng Y, Mo L, Philip J, Collins A, Allen LA, McClung JA, Denvir MA, Hui D. Referral criteria to palliative care for patients with heart failure: a systematic review. Circ Heart Fail. 2020;13(9): e006881.
Chang YK, Allen LA, McClung JA, Denvir MA, Philip J, Mori M, Perez-Cruz P, Cheng S-Y, Collins A, Hui D. Criteria for referral of patients with advanced heart failure for specialized palliative care. J Am Coll Cardiol. 2022;80(4):332–44.
Mo L, Geng Y, Chang YK, Philip J, Collins A, Hui D. Referral criteria to specialist palliative care for patients with dementia: a systematic review. J Am Geriatr Soc. 2021;69(6):1659–69.
Chen Y, Hou L, Li W, Wang Q, Zhou W, Yang H: Referral criteria to palliative care for patients with Parkinson’s disease: a systematic review. Curr Med Res Opin. 2022:1–36.
Pope C, Mays N, Popay J. Synthesizing qualitative and quantitative health evidence: a guide to methods. Maidenhead and New York: Open University Press, McGraw Hill Education; 2007.
Google Scholar
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;2013(6):CD007760.
PubMed PubMed Central Google Scholar
Bruera E Higginson IJ, von Gunten CF, Morita T: Textbook of Palliative Medicine and Supportive Care. 3rd edition.: CRC Press; 2021.
Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC. Oxford Textbook of Palliative Medicine. 6th ed. Oxford: Oxford University Press; 2021.
Chaudhry SI, Murphy TE, Gahbauer E, Sussman LS, Allore HG, Gill TM. Restricting symptoms in the last year of life: a prospective cohort study. JAMA Intern Med. 2013;173(16):1534–40.
Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR, Stall NM, Stukel TA, Goldman R, Horn D, Cram P, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA. 2020;324(14):1439–50.
Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, Jiang Kwete X, Arreola-Ornelas H, Gomez-Dantes O, Rodriguez NM, Alleyne GAO, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet. 2018;391(10128):1391–454.
Luckett T, Phillips J, Agar M, Virdun C, Green A, Davidson PM. Elements of effective palliative care models: a rapid review. BMC Health Serv Res. 2014;14:136.
Gómez Batiste X, Connor S: Building integrated palliative care programs and services. WHO Collaborating Centre Public Health Palliative Care Programmes; 2017. [ https://www.fnh.cl/publicaciones/BuildingIntegratedPalliativeCareProgramsandServices.pdf .] Accessed 24 Apr 2022.
Quill TE, Abernethy AP. Generalist plus specialist palliative care–creating a more sustainable model. N Engl J Med. 2013;368(13):1173–5.
Davidson P, Halcomb E, Hickman L, Phillips J, Graham B. Beyond the rhetoric: what do we mean by a “model of care”? Aust J Adv Nurs. 2006;23(3):47–55.
PubMed Google Scholar
Palliative Care Australia: National Palliative Care Standards. 5th edition.; 2018. [ https://palliativecare.org.au/wp-content/uploads/dlm_uploads/2018/11/PalliativeCare-National-Standards-2018_Nov-web.pdf .] Accessed 24 Apr 2022.
Training.cochrane.org. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. [ https://training.cochrane.org/handbook .] Accessed 16 Nov 2022.
Cochrane Handbook for Systematic Reviews of Interventions. Table 8.2.b: Reaching an overall risk-of-bias judgement for a specific outcome. [ https://training.cochrane.org/handbook/current/chapter-08 .] Accessed 16 Nov 2022
Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis. J Palliat Med. 2000;3(3):265–73.
Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, Turvey C, Meek PM. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA Randomized clinical trial. JAMA Intern Med. 2018;178(4):511–9.
Bassi I, Guerrieri A, Carpano M, Gardini A, Prediletto I, Polastri M, Curtis JR, Nava S. Feasibility and efficacy of a multidisciplinary palliative approach in patients with advanced interstitial lung disease. A pilot randomised controlled trial Pulmonology. 2021;27:27.
Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, Williams MP, Liberson M, Blum M, Della Penna R. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–90.
Hanson LC, Kistler CE, Lavin K, Gabriel SL, Ernecoff NC, Lin FC, Sachs GA, Mitchell SL. Triggered palliative care for late-stage dementia: a pilot randomized trial. J Pain Symptom Manage. 2019;57(1):10–9.
Helgeson SA, Burnside RC, Robinson MT, Mack RC, Ball CT, Guru PK, Moss JE: Early versus usual palliative care consultation in the intensive care unit. Am J Hosp Palliat Care® 2022:104990912211157.
Janssen K, Rosielle D, Wang Q, Kim HJ. The impact of palliative care on quality of life, anxiety, and depression in idiopathic pulmonary fibrosis: a randomized controlled pilot study. Respir Res. 2020;21(1):2.
Kluger BM, Miyasaki J, Katz M, Galifianakis N, Hall K, Pantilat S, Khan R, Friedman C, Cernik W, Goto Y, et al. Comparison of integrated outpatient palliative care with standard care in patients with parkinson disease and related disorders: a randomized clinical trial. JAMA Neurol. 2020;77(5):551–60.
O’Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, Desai AS. Social Worker-Aided Palliative Care Intervention in High-risk Patients With Heart Failure (SWAP-HF): a pilot randomized clinical trial. JAMA Cardiol. 2018;3(6):516–9.
O’Riordan DL, Rathfon MA, Joseph DM, Hawgood J, Rabow MW, Dracup KA, De Marco T, Pantilat SZ. Feasibility of implementing a palliative care intervention for people with heart failure: learnings from a pilot randomized clinical trial. J Palliat Med. 2019;22(12):1583–8.
Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical Trial. J Am Coll Cardiol. 2017;70(3):331–41.
Schunk M, Le L, Syunyaeva Z, Haberland B, Tänzler S, Mansmann U, Schwarzkopf L, Seidl H, Streitwieser S, Hofmann M, et al. Effectiveness of a specialised breathlessness service for patients with advanced disease in Germany: a pragmatic fast-track randomised controlled trial (BreathEase). Eur Respir J. 2021;58(2):2002139.
Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134–42.
Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med. 2006;9(1):111–26.
Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, Patel AS, Koffman J, Higginson IJ, Riley J. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax. 2015;70(9):830–9.
Brännström M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014;16(10):1142–51.
Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, McIlwane J, Hillary K, Gonzalez J. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55(7):993–1000.
Eggers C, Dano R, Schill J, Fink GR, Timmermann L, Voltz R, Golla H, Lorenzl S. Access to end-of life parkinson’s disease patients through patient-centered integrated healthcare. Front Neurol. 2018;9:627.
Evans CJ, Bone AE, Yi D, Gao W, Morgan M, Taherzadeh S, Maddocks M, Wright J, Lindsay F, Bruni C, et al. Community-based short-term integrated palliative and supportive care reduces symptom distress for older people with chronic noncancer conditions compared with usual care: A randomised controlled single-blind mixed method trial. Int J Nurs Stud. 2021;120: 103978.
Farquhar MC, Higginson IJ, Fagan P, Booth S. The feasibility of a single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. BMC Palliat Care. 2009;8:9.
Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, Todd CJ, Booth S. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials. 2016;17:185.
Gao W, Wilson R, Hepgul N, Yi D, Evans C, Bajwah S, Crosby V, Wilcock A, Lindsay F, Byrne A, et al. Effect of short-term integrated palliative care on patient-reported outcomes among patients severely affected with long-term neurological conditions: a randomized clinical trial. JAMA Netw Open. 2020;3(8): e2015061.
Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, McCrone P, Booth S, Jolley CJ, Moxham J. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2(12):979–87.
Janssens J-P, Weber C, Herrmann François R, Cantero C, Pessina A, Matis C, Merlet Viollet R, Boiche-Brouillard L, Stirnemann J, Pautex S. Can early introduction of palliative care limit intensive care, emergency and hospital admissions in patients with severe chronic obstructive pulmonary disease? A Pilot Randomized Study Respiration. 2019;97(5):406–15.
Scheerens C, Pype P, Van Cauwenberg J, Vanbutsele G, Eecloo K, Derom E, Van Belle S, Joos G, Deliens L, Chambaere K. Early integrated palliative home care and standard care for end-stage COPD (EPIC): a phase II Pilot RCT testing feasibility, acceptability, and effectiveness. J Pain Symptom Manage. 2020;59(2):206-224.e207.
Ng AYM, Wong FKY. Effects of a home-based palliative heart failure program on quality of life, symptom burden, satisfaction and caregiver burden: a randomized controlled trial. J Pain Symptom Manage. 2018;55(1):1–11.
Wong FK, Ng AY, Lee PH, Lam PT, Ng JS, Ng NH, Sham MM. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart. 2016;102(14):1100–8.
Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, Lewis P, Fayers P, Harding R, Hotopf M, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med. 2013;11:111.
Oriani A, Dunleavy L, Sharples P, Perez Algorta G, Preston NJ. Are the MORECare guidelines on reporting of attrition in palliative care research populations appropriate? a systematic review and meta-analysis of randomised controlled trials. BMC Palliat Care. 2020;19(1):6.
Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652–4.
Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119(5):1098–105.
Beernaert K, Smets T, Cohen J, Verhofstede R, Costantini M, Eecloo K, Van Den Noortgate N, Deliens L. Improving comfort around dying in elderly people: a cluster randomised controlled trial. Lancet. 2017;390(10090):125–34.
Buckingham S, Kendall M, Ferguson S, MacNee W, Sheikh A, White P, Worth A, Boyd K, Murray SA, Pinnock H. HELPing older people with very severe chronic obstructive pulmonary disease (HELP-COPD): mixed-method feasibility pilot randomised controlled trial of a novel intervention. NPJ Prim Care Respir Med. 2015;25:15020.
Chapman DG, Toseland RW. Effectiveness of advanced illness care teams for nursing home residents with dementia. Soc Work. 2007;52(4):321–9.
Di Pollina L, Guessous I, Petoud V, Combescure C, Buchs B, Schaller P, Kossovsky M, Gaspoz JM. Integrated care at home reduces unnecessary hospitalizations of community-dwelling frail older adults: a prospective controlled trial. BMC Geriatr. 2017;17(1):53.
Engelhardt JB, McClive-Reed KP, Toseland RW, Smith TL, Larson DG, Tobin DR. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care. 2006;12(2):93–100.
Fischer SM, Cervantes L, Fink RM, Kutner JS. Apoyo con Cariño: a pilot randomized controlled trial of a patient navigator intervention to improve palliative care outcomes for Latinos with serious illness. J Pain Symptom Manage. 2015;49(4):657–65.
Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial BMJ. 1999;319(7223):1472–5.
CAS PubMed Google Scholar
Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, Morgan H, Sykes J, Baxter K, Corfe F, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer. 2002;87(7):733–9.
Article CAS PubMed PubMed Central Google Scholar
Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? a randomized phase II trial. J Pain Symptom Manage. 2009;38(6):816–26.
Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost effectiveness of VA hospital-based home care for the terminally ill. Health Serv Res. 1992;26(6):801–17.
CAS PubMed PubMed Central Google Scholar
Kimbell B, Murray SA, Byrne H, Baird A, Hayes PC, MacGilchrist A, Finucane A, Brookes Young P, O’Carroll RE, Weir CJ, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med. 2018;32(5):919–29.
Kinley J, Hockley J, Stone L, Dewey M, Hansford P, Stewart R, McCrone P, Begum A, Sykes N. The provision of care for residents dying in U.K. nursing care homes. Age Ageing. 2014;43(3):375–9.
Kinley J, Stone L, Dewey M, Levy J, Stewart R, McCrone P, Sykes N, Hansford P, Begum A, Hockley J. The effect of using high facilitation when implementing the Gold standards framework in care homes programme: a cluster randomised controlled trial. Palliat Med. 2014;28(9):1099–109.
Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–84.
Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Hospital-based palliative medicine consultation: a randomized controlled trial. Arch Intern Med. 2010;170(22):2038–40.
Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164(1):83–91.
Taube E, Kristensson J, Midlöv P, Jakobsson U. The use of case management for community-dwelling older people: the effects on loneliness, symptoms of depression and life satisfaction in a randomised controlled trial. Scand J Caring Sci. 2018;32(2):889–901.
Temkin-Greener H, Mukamel DB, Ladd H, Ladwig S, Caprio TV, Norton SA, Quill TE, Olsan TH, Cai X. Impact of nursing home palliative care teams on end-of-life outcomes: a randomized controlled trial. Med Care. 2018;56(1):11–8.
Thoonsen B, Groot M, Engels Y, Prins J, Verhagen S, Galesloot C, van Weel C, Vissers K. Early identification of and proactive palliative care for patients in general practice, incentive and methods of a randomized controlled trial. BMC Fam Pract. 2011;12:123.
Thoonsen B, Vissers K, Verhagen S, Prins J, Bor H, van Weel C, Groot M, Engels Y. Training general practitioners in early identification and anticipatory palliative care planning: a randomized controlled trial. BMC Fam Pract. 2015;16:126.
Zimmer JG, Groth-Juncker A, McCusker J. Effects of a physician-led home care team on terminal care. J Am Geriatr Soc. 1984;32(4):288–92.
Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health. 1985;75(2):134–41.
Amado J, Vasquez R, Huari R, Rimache L, Lizonde R. Impact of applying palliative care on symptoms and survival of patients with advanced chronic disease admitted to the emergency department. Indian J. 2020;26(3):332–7.
Bakitas MA, Dionne-Odom JN, Ejem DB, Wells R, Azuero A, Stockdill ML, Keebler K, Sockwell E, Tims S, Engler S, et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med. 2020;180(9):1203–13.
Boogaard JA, de Vet HC, van Soest-Poortvliet MC, Anema JR, Achterberg WP, van der Steen JT. Effects of two feedback interventions on end-of-life outcomes in nursing home residents with dementia: a cluster-randomized controlled three-armed trial. Palliat Med. 2018;32(3):693–702.
Cox CE, Riley IL, Ashana DC, Haines K, Olsen MK, Gu J, Pratt EH, Al-Hegelan M, Harrison RW, Naglee C et al: Improving racial disparities in unmet palliative care needs among intensive care unit family members with a needs-targeted app intervention: The ICUconnect randomized clinical trial. Contemp Clin Trials 2021, 103 (no pagination).
Duenk R, Verhagen S, Bronkhorst E, Van Mierlo P, Broeders M, Collard S, Dekhuijzen R, Vissers K, Heijdra Y, Engels Y. Proactive palliative care for patients with COPD (PROLONG): a pragmatic cluster controlled trial. Palliat Med. 2017;28(12):2795–806.
Forbat L, Liu W-M, Koerner J, Lam L, Samara J, Chapman M, Johnston N. Reducing time in acute hospitals: a stepped-wedge randomised control trial of a specialist palliative care intervention in residential care homes. Palliat Med. 2020;34(5):571–9.
Kavalieratos D, Harinstein ME, Rose B, Lowers J, Hoydich ZP, Bekelman DB, Allen LA, Rollman BL, Ernecoff NC, Moreines LT, et al. Primary palliative care for heart failure provided within ambulatory cardiology: a randomized pilot trial. Heart Lung. 2022;56:125–32.
Koffman J, Yorganci E, Murtagh F, Yi D, Gao W, Barclay S, Pickles A, Higginson I, Johnson H, Wilson R, et al. The AMBER care bundle for hospital inpatients with uncertain recovery nearing the end of life: the improvecare feasibility cluster RCT. Health Technol Assess. 2019;23(55):1–150.
Koffman J, Yorganci E, Yi D, Gao W, Murtagh F, Pickles A, Barclay S, Johnson H, Wilson R, Sampson L, et al. Managing uncertain recovery for patients nearing the end of life in hospital: a mixed-methods feasibility cluster randomised controlled trial of the AMBER care bundle. Trials [Electronic Resource]. 2019;20(1):506.
Ma J, Chi S, Buettner B, Pollard K, Muir M, Kolekar C, Al-Hammadi N, Chen L, Kollef M, Dans M. Early palliative care consultation in the medical ICU: a cluster randomized crossover trial. Crit Care Med. 2019;47(12):1707–15.
Lindell KO, Klein S, Veatch MJ, Gibson KF, Kass D, Nouraie S, Rosenzweig MQ. Nurse-led palliative care clinical trial improves knowledge and preparedness in caregivers of patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(11):1811–21.
Liu WM, Koerner J, Lam L, Johnston N, Samara J, Chapman M, Forbat L. Improved quality of death and dying in care homes: a palliative care stepped wedge randomized control trial in australia. J Am Geriatr Soc. 2020;68(2):305–12.
Schmucker AM, Flannery M, Cho J, Goldfeld KS, Grudzen C, The EI, Blaum C, Bischof J, Ouchi K, Elie M-C, et al. Data from emergency medicine palliative care access (EMPallA): a randomized controlled trial comparing the effectiveness of specialty outpatient versus telephonic palliative care of older adults with advanced illness presenting to the emergency department. BMC emerg. 2021;21(1):1–11.
Shinall MC, Karlekar M, Martin S, Gatto CL, Misra S, Chung CY, Porayko MK, Scanga AE, Schneider NJ, Ely EW, et al. COMPASS: A pilot trial of an early palliative care intervention for patients with end-stage liver disease. J Pain Symptom Manage. 2019;58(4):614–614.
Solari A, Giordano A, Patti F, Grasso MG, Confalonieri P, Palmisano L, Ponzio M, Borreani C, Rosato R, Veronese S, et al. Randomized controlled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler. 2018;24(5):663–74.
Van den Block L, Honinx E, Pivodic L, Miranda R, Onwuteaka-Philipsen BD, van Hout H, Pasman HRW, Oosterveld-Vlug M, Ten Koppel M, Piers R, et al. Evaluation of a palliative care program for nursing homes in 7 Countries: the PACE cluster-randomized clinical trial. JAMA Intern Med. 2020;180(2):233–42.
Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, et al. Use of the Kansas City Cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–7.
Hauser K, Walsh D. Visual analogue scales and assessment of quality of life in cancer. J Support Oncol. 2008;6(6):277–82.
Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484–93.
Thomas K, Watson M, Armstrong J, Wilson and the GSF team. : The Gold Standards Framework Proactive Identification Guidance (PIG) (7th edition). The Gold Standards Framework Centre In End of Life Care. Edited by CIC; 2022. [ https://goldstandardsframework.org.uk/cd-content/uploads/files/PIG/Proactive%20Identification%20Guidance%20v7%20(2022).pdf .] Accessed 17 Nov 2022.
O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872–8.
Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, Elicker BM, Wolters PJ, Koth LL, King TE, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP Model. Chest. 2014;145(4):723–8.
Naqvi IH, Mahmood K, Ziaullaha S, Kashif SM, Sharif A. Better prognostic marker in ICU - APACHE II, SOFA or SAP II! Pak J Med Sci. 2016;32(5):1146–51.
McColl E: Best practice in symptom assessment: a review. Gut 2004, 53 Suppl 4(Suppl 4):iv49–54.
Herr KA, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med. 2001;17(3):457–78, vi.
Aiyegbusi OL, Roydhouse J, Rivera SC, Kamudoni P, Schache P, Wilson R, Stephens R, Calvert M. Key considerations to reduce or address respondent burden in patient-reported outcome (PRO) data collection. Nat Commun. 2022;13(1):6026.
Kroenke K, Stump TE, Monahan PO. Agreement between older adult patient and caregiver proxy symptom reports. J Patient Rep Outcomes. 2022;6(1):50.
Evans CJ, Benalia H, Preston NJ, Grande G, Gysels M, Short V, Daveson BA, Bausewein C, Todd C, Higginson IJ, et al. The selection and use of outcome measures in palliative and end-of-life care research: the MORECare International Consensus Workshop. J Pain Symptom Manage. 2013;46(6):925–37.
Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, Denzel J, Guo P, Bernhardt F, Schildmann E, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med. 2019;33(8):1045–57.
Wang TJ. Concept analysis of functional status. Int J Nurs Stud. 2004;41(4):457–62.
Habraken JM, van der Wal WM, Ter Riet G, Weersink EJ, Toben F, Bindels PJ. Health-related quality of life and functional status in end-stage COPD: a longitudinal study. Eur Respir J. 2011;37(2):280–8.
Jang H, Lee K, Kim S, Kim S. Unmet needs in palliative care for patients with common non-cancer diseases: a cross-sectional study. BMC Palliat Care. 2022;21(1):151.
Abernethy AP, Shelby-James T, Fazekas BS, Woods D, Currow DC. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care. 2005;4:7.
Theofilou P. Quality of life: definition and measurement. Eur J Psychol. 2013;9(1):p150-162.
Article Google Scholar
Dy SM, Pfoh ER, Salive ME, Boyd CM. Health-related quality of life and functional status quality indicators for older persons with multiple chronic conditions. J Am Geriatr Soc. 2013;61(12):2120–7.
Squiers L, Peinado S, Berkman N, Boudewyns V, McCormack L. The health literacy skills framework. J Health Commun. 2012;17(Suppl 3):30–54.
Lewis JM, DiGiacomo M, Currow DC, Davidson PM. Dying in the margins: understanding palliative care and socioeconomic deprivation in the developed world. J Pain Symptom Manage. 2011;42(1):105–18.
Matsuyama RKBWIK, Lyckholm L, Wilson-Genderson M, Smith TJ. Will patients want hospice or palliative care if they do not know what it is? J Hosp Palliat Nurs. 2011;13:p41-46.
Bausewein C, Daveson B, Benalia H, Simon ST, Higginson IJ. : Outcome Measurement in Palliative Care The Essentials. PRISMA. 2014. [ https://www.eapcnet.eu/wp-content/uploads/2021/03/Outcome-Measurement-in-Palliative-Care-The-Essentials-.pdf .] Accessed 24 Apr 2022.
Bausewein C, Daveson BA, Currow DC, Downing J, Deliens L, Radbruch L, Defilippi K, Lopes Ferreira P, Costantini M, Harding R, et al. EAPC White Paper on outcome measurement in palliative care: improving practice, attaining outcomes and delivering quality services - recommendations from the European Association for Palliative Care (EAPC) task force on outcome measurement. Palliat Med. 2016;30(1):6–22.
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9.
Ellis-Smith C, Higginson IJ, Daveson BA, Henson LA, Evans CJ. BuildCare: How can a measure improve assessment and management of symptoms and concerns for people with dementia in care homes? a mixed-methods feasibility and process evaluation of IPOS-Dem. PLoS ONE. 2018;13(7): e0200240.
Bradshaw A, Santarelli M, Mulderrig M, Khamis A, Sartain K, Boland JW, Bennett MI, Johnson M, Pearson M, Murtagh FEM. Implementing person-centred outcome measures in palliative care: an exploratory qualitative study using normalisation process theory to understand processes and context. Palliat Med. 2021;35(2):397–407.
Download references
Acknowledgements
We thank all the authors who participated in this research; Dr. Catherine J Evans for providing utmost support, Dr. Matthew Maddocks for checking the protocol, Ms. Elizabeth Alejandra Dominguez Palomerae and Ms. Rebeka Torlay for the support of an independent full paper review. This work was financially supported by JST SPRING. The author A.K. would like to take this opportunity to thank the ‘Interdisciplinary Frontier Next-Generation Researcher Programme of the Tokai Higher Education and Research System’.
The author A.K. is funded by JST SPRING (Grant Number JPMJSP2125) and C.J.E. by Health Education England/ National Institute of Health and Care Research (HEE/NIHR), Senior Clinical Lectureship (Grant Number ICA-SCL-2015–01-001). The views expressed in this publication are those of the authors and not necessarily those of the JST, the NHS, the NIHR or the Department of Health and Social Care. The funding agencies are not directly involved in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Author information
Authors and affiliations.
Department of Nursing for Advanced Practice, Division of Integrated Health Sciences, Nagoya University Graduate School of Medicine, Nagoya, Japan
Arisa Kawashima
King’s College London, Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation, Faculty of Nursing, Midwifery and Palliative Care, London, UK
Arisa Kawashima & Catherine J. Evans
Sussex Community NHS Foundation Trust, Brighton, UK
Catherine J. Evans
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation: C.J.E.; Methodology: A.K., C.J.E.; Data analysis and Writing: A.K.; Supervision: C.J.E.. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Catherine J. Evans .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2., additional file 3., additional file 4., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kawashima, A., Evans, C.J. Needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions: a systematic review and narrative synthesis. BMC Palliat Care 22 , 20 (2023). https://doi.org/10.1186/s12904-023-01131-6
Download citation
Received : 29 April 2022
Accepted : 01 February 2023
Published : 09 March 2023
DOI : https://doi.org/10.1186/s12904-023-01131-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Palliative care
- Systematic review
- Referral and consultation
BMC Palliative Care
ISSN: 1472-684X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Organizations
- Planning & Activities
- Product & Services
- Structure & Systems
- Career & Education
- Entertainment
- Fashion & Beauty
- Political Institutions
- SmartPhones
- Protocols & Formats
- Communication
- Web Applications
- Household Equipments
- Career and Certifications
- Diet & Fitness
- Mathematics & Statistics
- Processed Foods
- Vegetables & Fruits
Difference Between Analysis and Synthesis
• Categorized under Science | Difference Between Analysis and Synthesis

Analysis Vs Synthesis
Analysis is like the process of deduction wherein you cut down a bigger concept into smaller ones. As such, analysis breaks down complex ideas into smaller fragmented concepts so as to come up with an improved understanding. Synthesis, on the other hand, resolves a conflict set between an antithesis and a thesis by settling what truths they have in common. In the end, the synthesis aims to make a new proposal or proposition.
Derived from the Greek word ‘analusis’ which literally means ‘a breaking up,’ analysis is, by far, mostly used in the realm of logic and mathematics even before the time of the great philosopher Aristotle. When learners are asked to analyze a certain concept or subject matter, they are encouraged to connect different ideas or examine how each idea was composed. The relation of each idea that connects to the bigger picture is studied. They are also tasked to spot for any evidences that will help them lead into a concrete conclusion. These evidences are found by discovering the presence of biases and assumptions.
Synthesizing is different because when the learners are asked to synthesize, they already try to put together the separate parts that have already been analyzed with other ideas or concepts to form something new or original. It’s like they look into varied resource materials to get insights and bright ideas and from there, they form their own concepts.
Similar definitions of synthesis (from other sources) state that it is combining two (or even more) concepts that form something fresh. This may be the reason why synthesis in chemistry means starting a series of chemical reactions in order to form a complex molecule out of simpler chemical precursors. In botany, plants perform their basic function of photosynthesis wherein they use the sunlight’s energy as catalyst to make an organic molecule from a simple carbon molecule. In addition, science professors use this term like bread and butter to denote that something is being made. When they mention about amino acid (the building blocks of proteins) synthesis, then it is the process of making amino acids out of its many basic elements or constituents. But in the field of Humanities, synthesis (in the case of philosophy) is the end product of dialectic (i.e. a thesis) and is considered as a higher process compared to analysis.
When one uses analysis in Chemistry, he will perform any of the following: (quantitative analysis) search for the proportionate components of a mixture, (qualitative analysis) search for the components of a specific chemical, and last is to split chemical processes and observe any reactions that occur between the individual elements of matter.
1. Synthesis is a higher process that creates something new. It is usually done at the end of an entire study or scientific inquiry. 2. Analysis is like the process of deduction wherein a bigger concept is broken down into simpler ideas to gain a better understanding of the entire thing.
- Recent Posts
- Difference Between Plant Protein and Animal Protein - March 7, 2024
- Difference Between Crohn’s and Colitis - March 7, 2024
- Difference Between Expression and Equation - March 7, 2024
Sharing is caring!
Search DifferenceBetween.net :
- Difference Between Hydrolysis and Dehydration Synthesis
- Difference Between Idea and Concept
- Difference Between Anticodon and Codon
- Difference Between Compound and Mixture
- Difference Between Deep Learning and Surface Learning
Cite APA 7 , . (2011, March 19). Difference Between Analysis and Synthesis. Difference Between Similar Terms and Objects. http://www.differencebetween.net/science/difference-between-analysis-and-synthesis/. MLA 8 , . "Difference Between Analysis and Synthesis." Difference Between Similar Terms and Objects, 19 March, 2011, http://www.differencebetween.net/science/difference-between-analysis-and-synthesis/.
It’s very useful to understand the science and other subjects. Thanks
It was insightful
Thanks so much…. You explained so beautifully and simply….. Thanks again a lot
Thank you sir for your good explanation
Leave a Response
Name ( required )
Email ( required )
Please note: comment moderation is enabled and may delay your comment. There is no need to resubmit your comment.
Notify me of followup comments via e-mail
Written by : Julita. and updated on 2011, March 19 Articles on DifferenceBetween.net are general information, and are not intended to substitute for professional advice. The information is "AS IS", "WITH ALL FAULTS". User assumes all risk of use, damage, or injury. You agree that we have no liability for any damages.
Advertisments
More in 'science'.
- Difference Between El Nino and La Nina
- Difference Between Constipation and Bowel Obstruction
- Difference Between Psychopath and Sociopath
- Difference Between Climate Change and Global Warming
- Difference Between IQ and EQ
Top Difference Betweens
Get new comparisons in your inbox:, most emailed comparisons, editor's picks.
- Difference Between MAC and IP Address
- Difference Between Platinum and White Gold
- Difference Between Civil and Criminal Law
- Difference Between GRE and GMAT
- Difference Between Immigrants and Refugees
- Difference Between DNS and DHCP
- Difference Between Computer Engineering and Computer Science
- Difference Between Men and Women
- Difference Between Book value and Market value
- Difference Between Red and White wine
- Difference Between Depreciation and Amortization
- Difference Between Bank and Credit Union
- Difference Between White Eggs and Brown Eggs
- Open access
- Published: 16 April 2024
Synthesis, characterization, and antibacterial activity studies of two Co(II) complexes with 2-[( E )-(3-acetyl-4-hydroxyphenyl)diazenyl]-4-(2-hydroxyphenyl)thiophene-3-carboxylic acid as a ligand
- Emmanuel Sopbué Fondjo ORCID: orcid.org/0000-0002-1077-673X 1 ,
- Sorelle Songmi Feuze ORCID: orcid.org/0009-0003-2223-0413 1 ,
- Jean-de-Dieu Tamokou ORCID: orcid.org/0000-0002-8088-463X 2 ,
- Apollinaire Tsopmo 3 ,
- Giscard Doungmo 4 ,
- Peter Simon Friedrich Wilhelm ORCID: orcid.org/0000-0002-0297-8331 5 ,
- Donald Léonel Feugap Tsamo 1 ,
- Bruno Lenta Ndjakou 6 &
- Jules Roger Kuiate ORCID: orcid.org/0000-0002-5134-9427 2
BMC Chemistry volume 18 , Article number: 75 ( 2024 ) Cite this article
70 Accesses
Metrics details
Two new Cobalt(II) complexes 12 and 13 have been synthesized from 2-[( E )-(3-acetyl-4-hydroxyphenyl)diazenyl]-4-(2-hydroxyphenyl)thiophene-3-carboxylic acid ( 11 ) as a novel ligand. These three new compounds were characterized on the basis of their powder X-Ray Diffraction, UV–Vis, IR, NMR, elemental analysis and MS spectral data. DFT/B3LYP mode of calculations were carried out to determine some theorical parameters of the molecular structure of the ligand. The purity of the azoic ligand and the metal complexes were ascertained by TLC and melting points. The analysis of the IR spectra of the polyfunctionalized azo compound 11 and its metal complexes 12 and 13 , reveals that the coordination patterns of the ligand are hexadentate and tetradentate respectively. Based on the UV–Vis electronic spectral data and relevant literature reports , the ligand and derived complexes were assigned the E ( trans ) isomer form. Likewise, octahedral and square-planar geometries were respectively assigned to the cobalt(II) complexes. The broth microdilution method was used for antibacterial assays through the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The ligand 11 displayed moderate antibacterial activity (MIC = 32–128 μg/mL) against Staphylococcus aureus ATCC25923 , Escherichia coli ATCC25922, Pseudomonas aeruginosa and Klebsiella pneumoniae 22. The octahedral cobalt(II) complex 12 showed moderate activity against Pseudomonas aeruginosa (MIC = 128 μg/mL) and Klebsiella pneumoniae 22 (MIC = 64 μg/mL) and none against Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922, whereas the square-planar complex 13 displayed moderate activity only on Klebsiella pneumoniae 22 (MIC = 64 μg/mL) .
Graphical abstract
Peer Review reports
Introduction
Antimicrobial resistance (AMR) appears over time as a phenomenon mainly linked to the genetic evolution of pathogens. The direct consequence of this AMR is that it makes infections more difficult to treat [ 1 , 2 , 3 ]. There is therefore a constant search for new antimicrobial compounds from natural sources [ 4 , 5 , 6 , 7 ] or via synthetic routes [ 8 , 9 , 10 ] as possible solutions. Thus, among the broad range of bioactive molecules of synthetic origin, coordination compounds in general and those based on hybrid heterocyclic ligands such as azo thiophenes scaffolds such as 1 , 2 and 3 (Scheme 1 ) in particular [ 11 , 12 ], represent the most promising molecules for the discovery of novel antimicrobial drugs [ 13 , 14 ].
Structures of some azo thiophenes compounds
Such hybrid molecules are expected to combine the properties of the chelating heterocyclic ligands with those of the central metal ions and to exhibit much better biological profiles [ 15 , 16 , 17 , 18 ].
Azo compounds have a long history and are important part of our daily life. They are mainly used as dyes and pigments in various fields, such as: textile dyeing (mordant yellow 10 ( 4 ) [ 19 ]); the food (tartrazine ( 5 ) [ 20 ]) and cosmetics (red 6 ( 6 ) [ 21 ]) industries (Scheme 2 ).
Structures of some industrial azo dyes
They also have many other applications in physicochemistry, analysis, catalysis [ 22 , 23 ] and pharmacy [ 24 ] because of their special complexing abilities, sensitivity as chromogenic reagents, usage in spectrophotometry and ability to detect a variety of metal ions. In addition, they have been of major importance in drug development due to their antioxidant, anti-inflammatory, fungicidal, antidiabetic, bacteriostatic, and antiseptic activities [ 25 , 26 , 27 ]. These compounds and their derivatives have some potential applications in different fields, including industrial and biological research [ 28 , 29 ]. For instance, in the dyeing of wool and synthetic polyamides, the azo complexes of Cr(III) and Co(III) are extensively utilized in industry [ 30 ], as well as the azo complexes of Ni(II) and Cu(II), which are utilized in biology as antibacterial and anticancer medications [ 31 , 32 ]. Azo compounds having in their structures both the thiophenic and phenolic fragments (which separately have each amazing antiviral, antibacterial, antifungal, cytotoxic [ 33 ], antioxidant and antiradical [ 34 ] properties), are expected to combine the different properties of the latter in the hybrid structures [ 35 ].
Cobalt is the chemical element with atomic number 27, symbol Co and electronic structure [Ar]4s 2 3d 7 belonging to block “d” of the periodic table of elements. It is relatively rare, gray in color, ductile, fragile and magnetic. Relatively unreactive, it does not oxidize in humid or dry air at normal environmental temperatures. The two valence states, cobaltous(II) and cobaltic(III), melt at 1493 °C with limited water solubility. These properties are similar to those of iron and nickel, which are neighbors in the periodic table [ 36 ]. It is one of the most important transition metals from a biological point of view. Its ions act in the activation of cholinesterase and provide protection against excessive oxygen pressure in the lungs during respiration. They also act as bacteriostatic agents comparable with antibiotics [ 37 ]. The cobalt ion is an integral part of the vitamin B12 molecule [ 38 ] which has a key role in the maturation of red blood cells, the chemical name of this vitamin, cobalamin, also evokes the importance of the cobalt which is present in it at 4% [ 39 , 40 ]. Cobalt complexes have also been suggested to possess antirheumatic, antihistamine [ 41 , 42 ] antifungals and antivirals properties [ 43 ]. Cobalt coordination compounds are the earliest known metal complexes and coordination chemistry was founded with the study of these promising compounds [ 44 ].
As part of a continuing interest in the chemistry and biological properties of azo compounds having in their structures thiophenic and phenolic fragments, we have undertaken in this study to determine how they coordinate with cobalt(II) in order to evaluate the antimicrobial activities of the synthesized product and those of their cobalt(II) complexes as well, on certain resistant bacterial strains.
Results and discussion
The new azoic ligand 11 was prepared using the thienocoumarin 7 as starting material. Procedure for the preparation of 7 has been reported earlier [ 45 , 46 ]. The general preparation process of 11 is displayed in Scheme 3 [ 46 , 47 ].
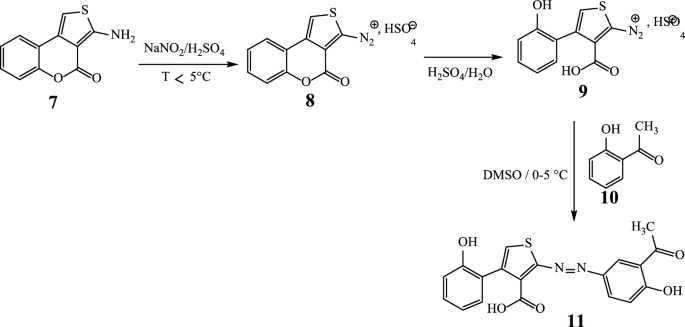
Reaction sequences for the preparation of compound 11
The structure of substrate ligand 11 (C 19 H 14 N 2 O 5 S) was confirmed with its physical and spectroscopic data. Reaction of compound 11 (previously dissolved in 4 mL of DMSO) with Co(C 2 O 4 )‧2H 2 O (dissolves in EtOH/MeOH 2:1) with constant stirring at room temperature for 48 h gave compounds 12 and 13 (Scheme 4 ).
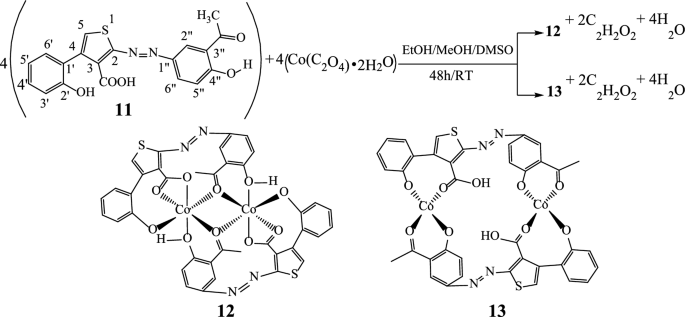
Reaction sequences to the complexes 12 and 13 ([Co 2 (C 19 H 12 N 2 O 5 S) 2 ])
The ligand and the complexes were obtained as dark green, black and green powders respectively, air stable and soluble in DMSO and acetone. The elemental analysis (C, H, N, and S) and melting points data of these compounds are recorded in Table 1 .
The UV–VIS spectrum of ligand 11 showed a strong band in the ultraviolet range at 332 nm and moderate bands above 350 nm, attributed to the π → π* and n → π* transitions (due to the azo bridge), respectively (Fig. 1 ). The maximum absorption peak of azo compounds in general is around 330 nm in the UV–visible absorption spectrum due to the π → π* electronic transition of trans isomers [ 48 ]. In the context of this study, these absorption maxima observed in the ultraviolet region of the UV–Vis spectra of ligand 11 and of the synthesized complexes, 12 and 13 , are found at 332 nm, close to that reported in the literature. Furthermore, the electronic spectral data were very useful for the assignments of the stereochemistry of the metal complexes based on the positions and number of d → d transition peaks.
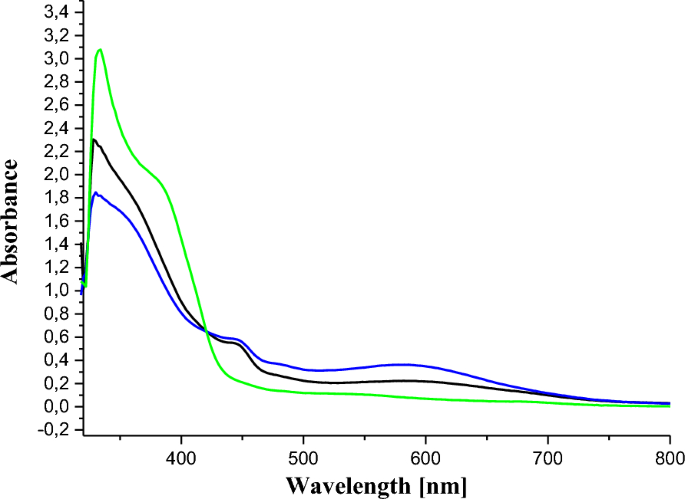
Electronic spectra of ligand 11 (black) and cobalt(II) complexes 12 (blue) and 13 (green)
For the cobalt (II) complexes, the only possible configurations found in the literature are square-plane, tetrahedral and octahedral. Therefore, for complex of cobalt(II) hexadentate 12 , the only possible geometry that could be envisaged is octahedral. In fact, its electronic spectrum shows two bands of low intensities in the visible range. The first at 490 nm is attributed to the 4 T1g(F) → 4 A2g(F) transition and the second around 600 nm is due to the 4 T1g(F) → 4 T1g(P) transition [ 49 ]. These 2 absorptions are characteristic of an octahedral environment around the cobalt(II) ion complexes [ 50 ].
For the tetradentate complex 13 , a square-planar or tetrahedral configuration could be envisaged. Based on its electronic spectrum, it was possible to differentiate between these two alternative configurations as follows. The absence of absorptions between 600 and 700 nm which are characteristic for tetrahedral cobalt(II) complexes [ 51 , 52 ], ruled out the hypothesis of a tetrahedral geometry for 13 . Moreover, the presence of an absorption (of very low intensity) above 500 nm (Fig. 1 ) makes more plausible the hypothesis of a low-spin square-planar geometry for this complex [ 53 , 54 ]. As a consequence, on the basis of the LCAO approach, the central Co(II) ions should be hybridized sp 3 d 2 and dsp 2 respectively to comply with the octahedral and square-planar geometries of the coordination spheres in compounds 12 and 13 respectively. The absorption spectra of the ligand and complexes are represented in Fig. 1 .
In the IR spectrum, the free ligand C 19 H 14 N 2 O 5 S shows a very strong and sharp band with well-structured peaks at 1726 and 1668 cm −1 due to the ν(C=O) (ketone and acid respectively) present in the molecule (Fig. 2 ). In the IR spectra of the complexes [Co 2 (C 19 H 12 N 2 O 5 S) 2 ] (Figs. 3 a and 4 a), these bands appear but with a pronounced shift towards higher frequencies at 1748 cm −1 and 1695 cm −1 respectively (for 13 ), and towards lower frequencies around 1713 cm −1 (for 12 ), indicating the involvement of the corresponding oxygen in the coordination with the central Co 2+ ion. In these complexes, the values of ν(N=N) observed at 1446 cm −1 in the ligand remain constant, meaning that the azo function does not participate in the coordination. The other atoms involved in the coordination bonds in these molecules are the oxygen atoms of the two phenolic hydroxyl groups and that of the carboxylic acid function present in ligand 11 . The absence of the ν(OH) frequencies in the IR spectrum of the complex 13 , observed at 3541 cm −1 and 3248 cm −1 (Fig. 4 b) in the starting ligand and assigned to free (2'-OH) and chelated (4''-OH) phenolic hydroxyls respectively, suggests the participation of the corresponding OH groups in the coordination with deprotonation (Fig. 4 a). In the IR spectrum of the complex 12 , the signal of the hydroxyl (2'-OH) appears in the higher frequency region at 3258 cm −1 and one can notice the disappearance of the signal of the carboxylic acid hydroxyl around 2575 cm −1 . These observations are suggestive of the participation of the corresponding oxygen atoms in the coordination without and with deprotonation respectively. The new bands that appeared in the IR spectrum of the complex in the region 521–570 cm −1 at 548 and 528 cm −1 (complex 12 ) and at 530 cm −1 (complex 13 ) were attributed to the Co–O bonds [ 55 , 56 ] between the central cobalt ion and all the oxygen atoms involved in coordination. The relative intensities as well as the provisional assignments of the various bands mentioned above are given in the Table 2 .
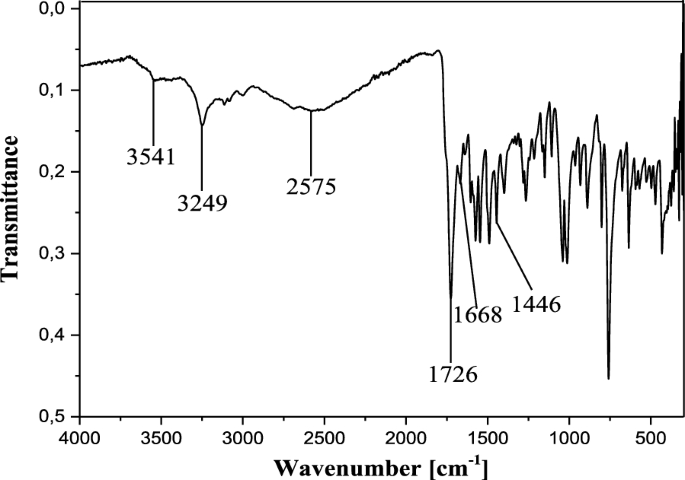
Infrared spectrum of compound 11
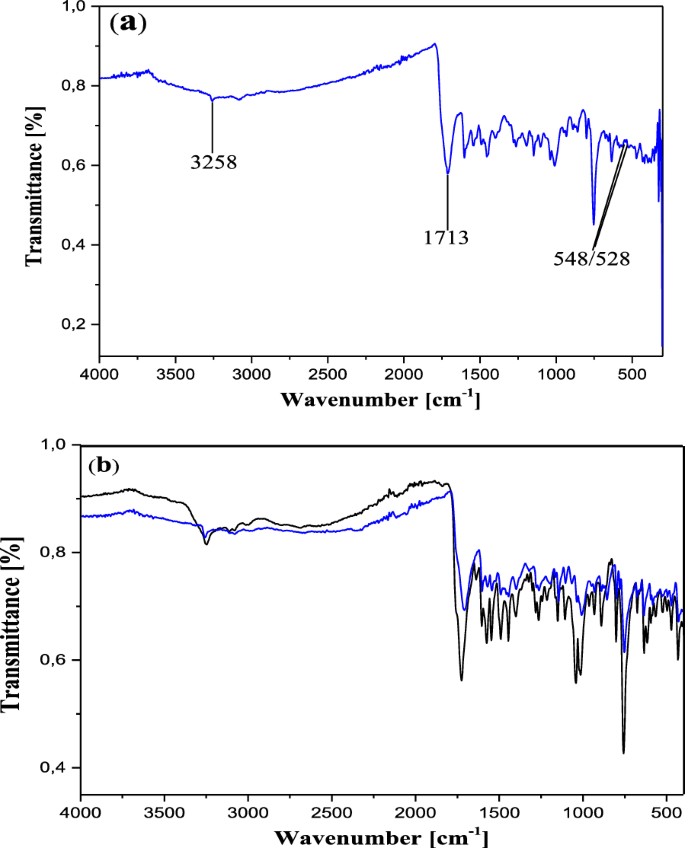
a Infrared spectrum of compound 12 . b Superposed infrared spectra of compounds 11 (black) and 12 (blue)
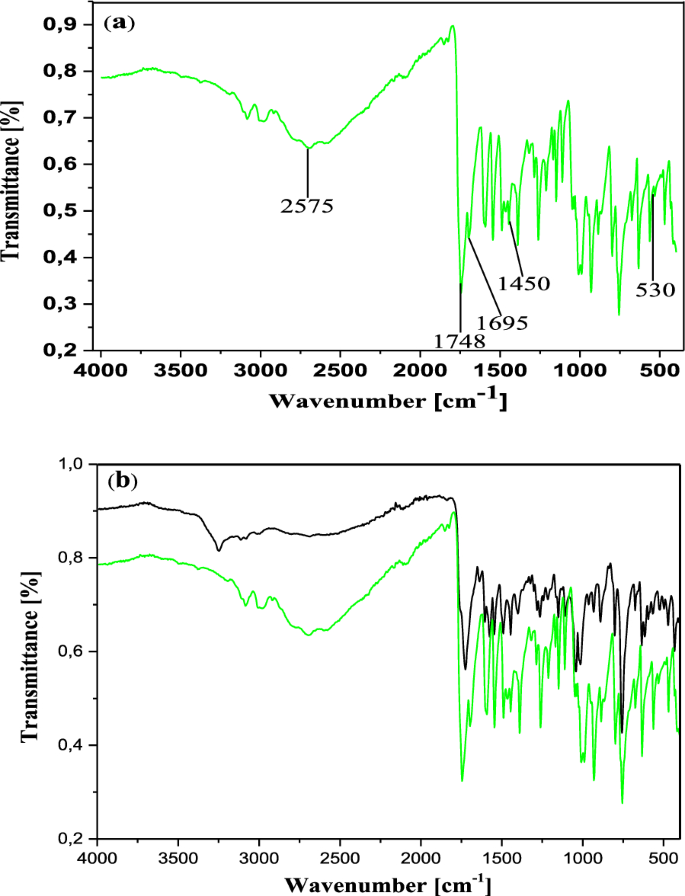
a Infrared spectrum of compound 13. b Superposed infrared spectra of compounds 11 (black) and 13 (green)
The suggested structures were supported by the mass spectral data of the free azo dye ligand and its Co(II) complexes, which were compatible with the molecular ion fragments (Fig. 5 ). Some of the fragments observed in the mass spectra of the ligand 11 and its Co(II) complexes 12 and 13 are rationalized in the fragmentation Schemes 5 , 6 and 7 .
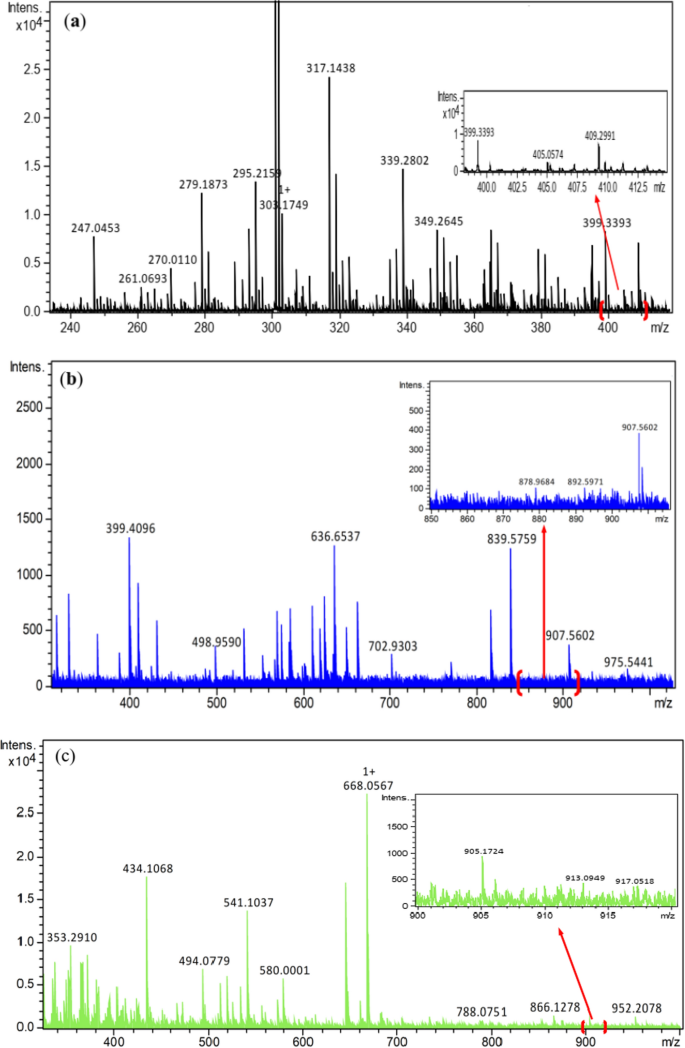
a HRESI + mass spectrum of azo ligand 11 . b HRESI + mass spectrum of complex 12 . c HRESI + mass spectrum of complex 13
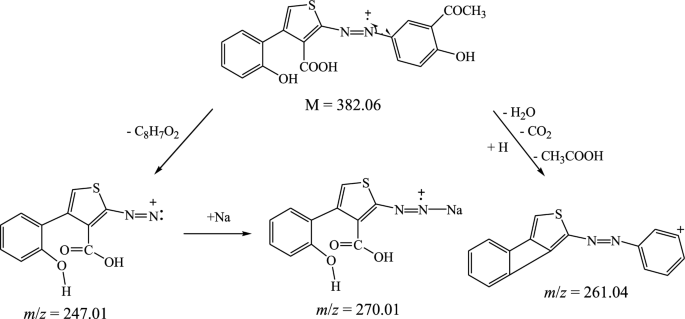
Suggested fragmentation pattern of azo ligand 11
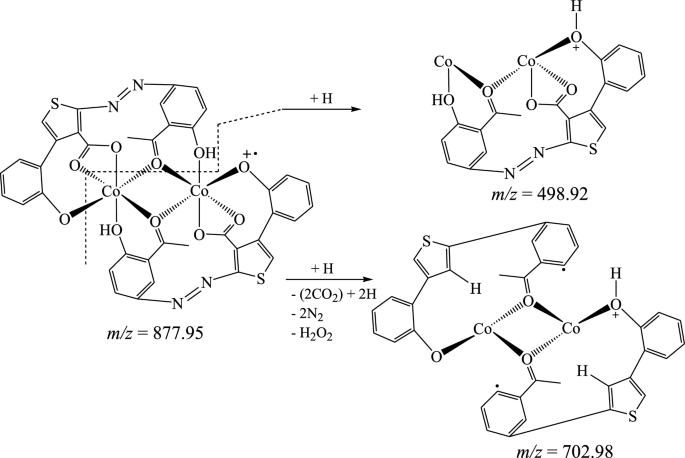
Suggested fragmentation pattern of Co(II) complex 12
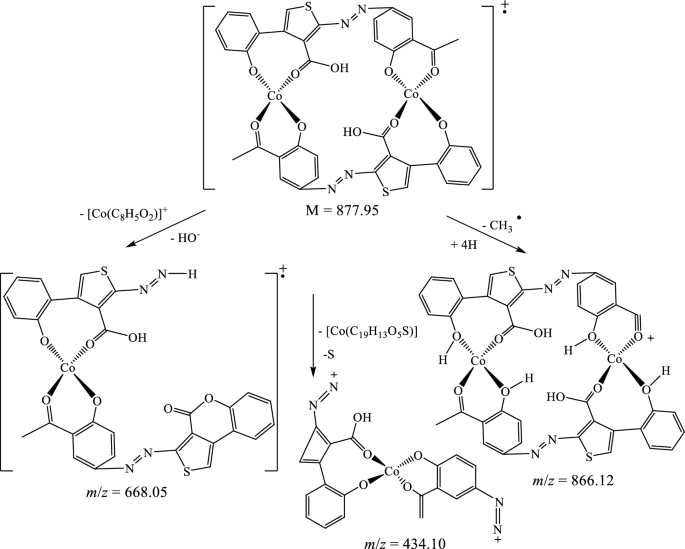
Suggested mass fragmentation pattern of Co(II) complex 13
Comparative 1 H NMR spectra of the ligand (Fig. 6 a) and the complexes (Fig. 6 b, c) clearly show that the ligand undergoes deprotonation with complexation. Indeed, in the spectra of complexes 12 (Fig. 6 b) and 13 (Fig. 6 c), the 2′-OH at 2.99 ppm in the ligand was not seen in Fig. 6 b, whereas, the 2′-OH at 2.99 ppm and 4″-OH at 11.93 ppm in the ligand were not seen in Fig. 6 c. These observations confirm the formation of the Co–O bonds with the corresponding oxygen atoms. Moreover the same signals with almost the same multiplicities are observed in the spectra of the ligand and the complexes with respect to the aromatic protons.
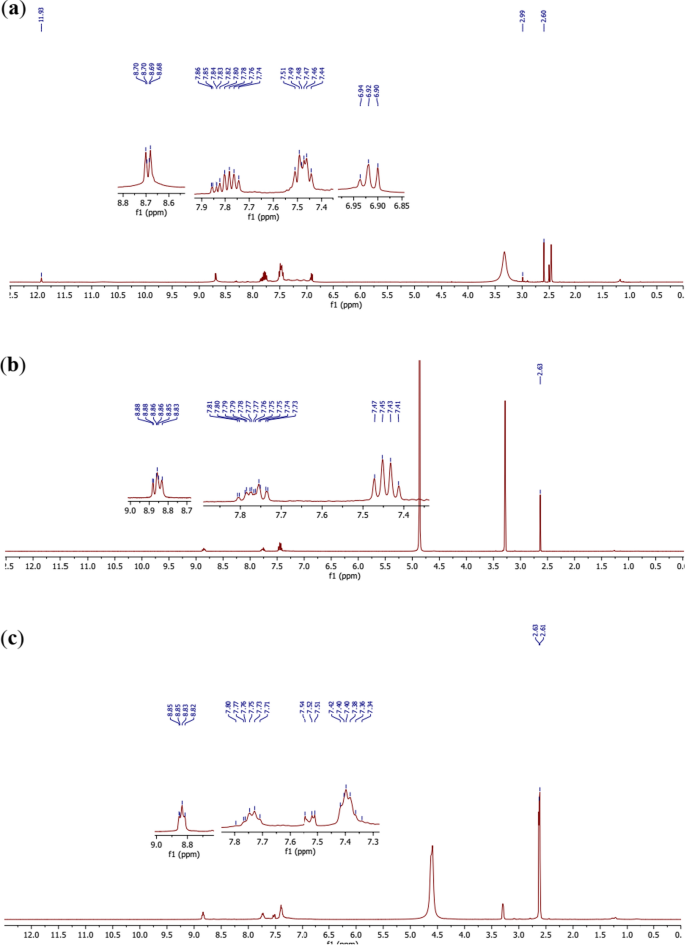
a 1 H-NMR spectrum of the ligand 11 . b 1H-NMR spectrum of the complex 12. c . 1 H-NMR spectrum of the complex 13
The 13 C NMR spectrum of the ligand 11 (C 19 H 14 N 2 O 5 S) (Fig. 7 a) displays 19 signals due to the 19 carbon atoms present in this molecule. The most important being the carbon atoms bearing the coordinating oxygen atoms, found at 205.2 ppm, 184.8 ppm, 161.3 ppm and 155.5 ppm for the carbons 3″– CO CH 3 , 3– CO OH, C-4″ and C-2′, respectively.
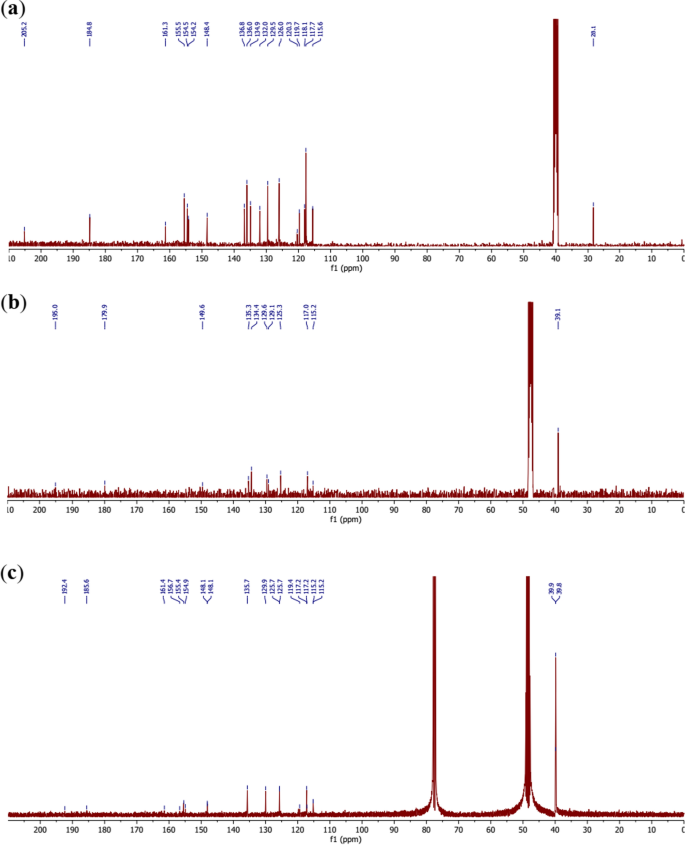
a 13 C-NMR spectrum of the ligand 11. b 13 C-NMR spectrum of the complex 12. c . 13 C-NMR spectrum of the complex 13
Thus, the comparison of this spectrum with those of the [Co 2 (C 19 H 12 N 2 O 5 S) 2 ] complexes 12 and 13 (Fig. 7 b, c) made it possible to assign the carbonyls 3″- CO CH 3 and 3- CO OH the chemical shift values 195.0 ppm and 179.9 ppm, respectively, and the phenolic carbons C-4″ and C-2′ the values 156.5 ppm and 156.0 ppm, respectively in the complex 12 whereas the values 192.4 ppm, 185.6 ppm, 161.4 ppm and 156.7 ppm could comparatively be assigned in the complex 13 , respectively for the above-mentioned atoms. The chemical shifts of the ligand and the complexes are summarized in Table 3 .
Figure 8 summarizes the two most significant interactions that were seen in the HSQC spectra of the ligand and compounds 12 and 13 . The first of these is the correlation spots between the methyl protons at 2.63 ppm (in 11 ) (one signal), and at 2.63 and 2.61 ppm (in 13 ) (two signals) and their carbons at 39.1 ppm and at 39.9 and 39.8 ppm respectively. On the other hand, correlation spots between protons H-6' at 8.87 ppm (in 12 ) and at 8.83 ppm (in 13 ) and their carbons at 129.6 ppm (in 12 ) and at 129.9 ppm (in 13 ) indicate the presence of the acetophenone fragment and that of the thiophenic moieties on each side of the N = N bridge.
HSQC spectra of complexes 12 ( a ) and 13 ( b ) with some correlations
The long-distance couplings ( 2 J and 3 J ) between the protons and the carbons of the chelating ligand moieties were highlighted by the HMBC experiment (Fig. 9 ). Indeed, it allowed to reconstruct the carbon skeleton of the coupling fragment through correlation spots between the 4″-OH proton (11.93 ppm) and the C-4″ carbons (161.3 ppm) and C-5″ (118.1 ppm), the proton H-6″ (around 7.85 ppm) and the carbons C-2″ (136.8 ppm) and C-4″ (161.3 ppm), the H-5″ proton (6.91 ppm) and the C-3″ carbons (120.0 ppm) and finally between the methyl CH 3 (2.60 ppm) and the carbonyl C=O (205.1 ppm), thus eliminating the hypothesis of multiple couplings on the aromatic ring of the coupler. Some of these correlations were also found in the HMBC spectra of the complexes despite their high complexity due to the overlapping of homologous proton systems of the chelating ligand moieties.
HMBC spectra of complexes 12 ( a ) and 13 ( b ) with some correlations
The COSY 1 H- 1 H experiment of the ligand (Fig. 10 ) clearly showed the correlation squares between the aromatic protons belonging to the molecular fragments on either side of the azo bridge. For the complexes, the most visible correlations are those of the benzene ring for the above mentioned similar reasons (Fig. 11 ).
COSY 1 H- 1 H spectrum of ligand 11 with some correlations
COSY 1 H- 1 H spectra of complexes 12 ( a ) and 13 ( b ) with some correlations
Theoretical calculations were performed on the ligand to determine the most reactive sites of the unsaturated system. The energies and electronic densities of the frontier molecular orbitals (FMO), HOMO and LUMO, as well as the molecular electrostatic potential (MEP) are important electronic parameters for this purpose [ 57 , 58 ]. The structures of the FMO and the MEP obtained from a B3LYP/6-311G mode of calculations are given in Fig. 12 . The E HOMO and E LUMO values are—6.114 eV and—2.960 eV respectively, resulting in an energy gap of 3.15 eV.
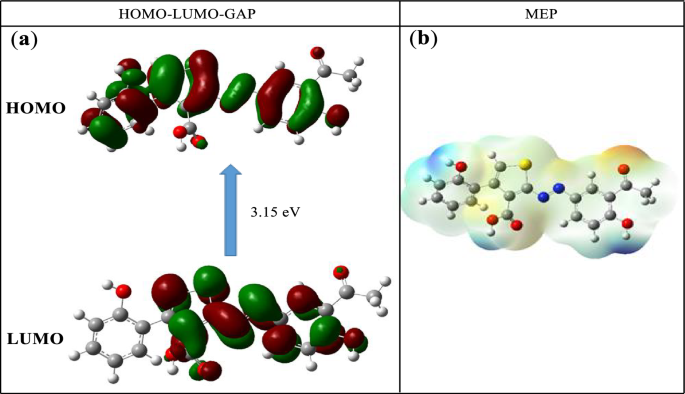
Structures of the FMO (HOMO and LUMO) ( a ) and MEP ( b ) of compound 11
XDR analysis
The powder X-ray diffraction of ligand 11 and complex 13 are different from each other (Fig. 13 ) and indicates a good crystalline structure and a good purity of these compounds. The spectra of compound 13 shows a significant number of sharp bands or peaks. This suggests that it is made up of well-organized particles. All the new peaks exhibited in the diffractogram of the complex 13 are in agreement with the fact that it is different from the ligand 11 . The optimized 3D view of compound 11, 12 and 13 are clearly presented in Fig. 14 .
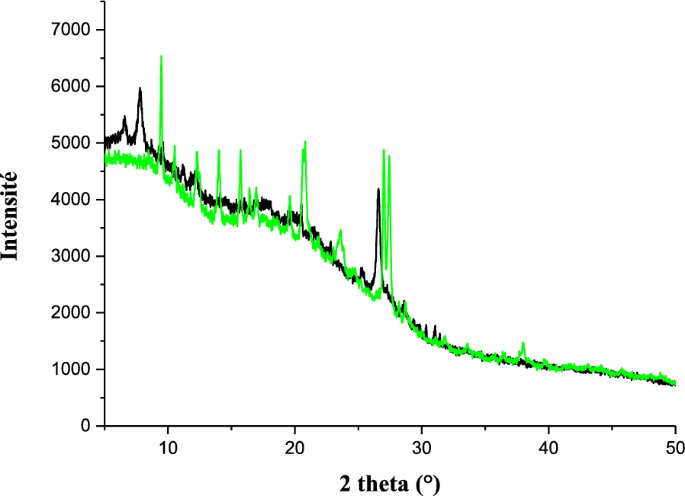
Ex situ PXRD pattern (Cu Kα1 radiation) of XRD of compounds 11 (black) and 13 (green)
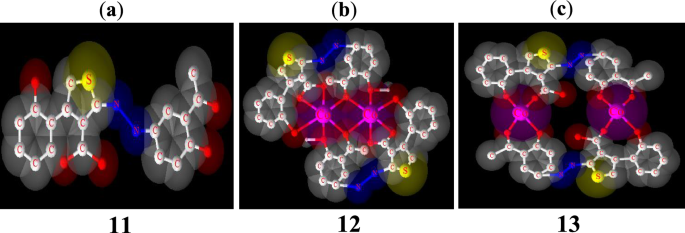
Optimized 3D view of compounds 11, 12 and 13 . The structures were drawn with the program ACD/3D viewer (freeware) of ACD/Labs
Antibacterial activity
The comparative study of the activity of the starting 2-aminothiophen ( 7) and the tree new compounds ( 11 , 12 and 13 ) was carried out on bacteria strains such as Staphylococcus aureus ATCC25923, Pseudomonas aeruginosa , Escherichia coli ATCC25922 and Klebsiella pneumoniae 22. Screening results showed that compound 7 had a moderate activity (CMI = 128 µg/mL) and (CMI = 64 µg/mL) on Escherichia coli ATCC25922 and Klebsiella pneumoniae 22 respectively, but its highest activity (CMI = 32 µg/mL) was found on Pseudomonas aeruginosa and Staphylococcus aureus ATCC25923 strains. These activities decrease in the azoic ligand 11 on Pseudomonas aeruginosa (CMI = 64 µg/mL) and Staphylococcus aureus ATCC25923 (CMI = 128 µg/mL); increase on Escherichia coli ATCC25922 (CMI = 32 µg/mL) and remains constant on Klebsiella pneumoniae 22 (CMI = 64 µg/mL). Complex 12 had no activity on two strains Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922, but had a moderate activity (CMI = 128 µg/mL) and (CMI = 64 µg/mL) on Pseudomonas aeruginosa and Klebsiella pneumoniae 22 respectively, while complex 13 had no activity on all strains except on Klebsiella pneumoniae 22 (CMI = 64 µg/mL) where the activity remains constant with respect to precursor 7 . All data are summarized in Table 4 .
Cytotoxic activity
To investigate the potential use of compounds 7 , 11, 12 and 13 , their cytotoxicity was evaluated. None of the tested samples showed hemolytic activities against red blood cells at concentrations up to 128 µg/mL (Table 5 ). However, at the highest concentration tested in this study (256 μg/mL), complexes caused less than 4% of hemolysis. This finding highlights that complexes are slightly hemolytic at 256 μg/mL.
In summary, two novel binuclear complexes of Co(II) with a novel multifunctional azo ligand incorporating a thiophenic and a phenolic moiety have been prepared, and their structures fully assigned on the basis of the available elemental, powder XRD and spectroscopic data. IR spectral data show that ligand 11 behaves as a hexadentate ligand in 12 , coordinating via all electron-donating oxygen atoms, and as a tetradentate ligand in 13 , coordinating via all oxygen atoms except that of carboxylic acid functions. It was established that in both complexes the central Co(II) ions were sp 3 d 2 and dsp 2 hybridized in 12 and 13, respectively. The models of metal ion binding to the coordination sites of chelating ligands display octahedral and planar-square geometries in complexes 12 and 13 respectively in agreement with their UV–Vis data. From the biological screenings carried out on selected strains of multiresistant bacteria, it was found that compared to the free ligand, the coordination compounds have relatively very low activity on most of the tested strains. Nevertheless, further similar studies need to be carried out on a more large number of pathogens before a rational conclusion could be drawn on the structure–activity relationship linked with the coordination process.
Materials and methods
Instrumental method.
All the reagents mentioned in this work were purchased from Aldrich and Fluka and were used without further purification. Melting points are corrected and were determined with a STUART SCIENTIFIC Melting Point Apparatus Model SMP3 at a heating rate of 2 °C/min. TLCs were performed on prefabricated silica gel plates, consisting of silica gel 60 F 254 on aluminum foil with a fluorescent indicator. A mixture of ethyl acetate and hexane (1:1) was used as eluent to develop the TLC plates and the spots were visualized using iodine vapor or by spraying with 10% H 2 SO 4 and heating at 100 ℃ for 2 min. The IR spectra were recorded with a Bruker Alpha spectrophotometer using the ATR (Attenuated Total Reflectance) technique on a diamond crystal. The HRESI-MS spectra were recorded on a Compact BRUKER brand spectrometer with a DIONEX Ultimate 3000 brand LC chain. Nuclear magnetic resonance (NMR) experiments (1D and 2D) were performed in DMSO- d 6 and MeOH- d 4 /CCl 4 on a 400 MHz JEOL ECZ spectrophotometer equipped with 5-mm digital auto tune Royal probe (JEOL USA, Peabody, MA). 1 H-NMR spectral data were recorded at 400 MHz, while 13 C-NMR data were measured at 100 MHz both with TMS used as internal reference. Powder XRD data was collected on a STOE Stadi-p X-ray powder diffractometer (STOE & Cie GmbH, Darmstadt, Germany) with Cu K α1 radiation (λ = 1.54056 Å; Ge monochromator; flat samples) in transmission geometry with a DECTRIS® MYTHEN 1 K detector (DECTRIS, Baden-Daettwil, Switzerland). Elemental analyses were performed with a Euro Vector CHNS-O element analyzer (Euro EA 3000) or a vario MICRO Cube (Co. Elementar Analysen Systeme). Theorical calculations were performed with Gaussian 9 software in a B3LYP/6-311G mode.
Synthesis of 2-[(E)-(3-acetyl-4-hydroxyphenyl)diazenyl]-4-(2-hydroxyphenyl)thio-phene-3-carboxylic acid (11)
To the thienyldiazonium ion in solution, 1.35 g (9.93 mmol) of 10 was added dropwise with stirring over 30 min and the mixture was further stirred for an additional 30 min to complete the reaction. At the end of this, 5 mL of a potassium bicarbonate solution (10% KHCO 3 ) was added in small portions to the mixture to neutralize the excess acid. 10 min later, a volume of 50 mL of ice water was added to the mixture and the latter was left to stand for 24 h before being filtered. The product obtained is then washed cold and then hot with water to remove any impurities in order to give 1.97 g of 11 (from 2 g of 7 ) as dark green powder; R f : 0.6, mp: 296–298 °C, yield 55.97%; HRESI-MS: 405.0574 (M + Na, 0.54%). UV–Vis: λ max (acetone): 332, 445, 586 nm. IR (ATR): 1450 cm −1 (N=N), 1667 (C=O) acid , 1729 (C=O) ketone , 1166 (C–O), 3541 (2′-OH), 3248 (4″-OH), 2579 (3-CO OH ) cm −1 . 1 H NMR (DMSO- d 6 ) δ ppm: 8.69 (dd, 1H, J = 8.1 and 1.2 Hz, H-6′), 7.80 (m, 1H, H-4′), 7.46 (m, 1H, H-5’), 7.21 (d, 1H; J = 7.1 Hz, H-3′), 6.94 (s,1H, H-5), 7.74 (d,1H, J = 1.9 Hz; H-2″), 6.91 (d, 1H, J = 8.1 Hz; H-5″), 7.85 (dd, 1H, J = 8.1 and 1.9 Hz; H-6″). 13 C NMR (DMSO- d 6 ) δ ppm: 154.5 (C-2), 154.2 (C-3), 184.8 (3- CO OH), 148.4 (C-4), 119.7 (C-5), 115.6 (C-1′), 155.5 (C-2′), 117.7 (C-3′), 136.0 (C-4′), 126.0 (C-5′), 129.5 (C-6′), 134.9 (C-1″), 136.8 (C-2″), 120.3 (C-3″), 205.1 (3″- CO CH 3 ), 161.3 (C-4″), 118.1 (C-5″) and 132.0 (C-6″). Anal. Calcd. for C 19 H 14 N 2 O 5 S (382.0623): C, 59.68; H, 3.69; N, 7.33; S, 8.38; found: C, 59.70; H, 3.68; N, 7.31; S, 8.37.
Synthesis of complexes 12 and 13 ([Co 2 (C 19 H 12 N 2 O 5 S) 2 ])
To a magnetically stirred solution of the ligand 11 (300 mg; 0.79 mmol) in DMSO (4 mL) a solution of Co(C 2 O 4 )‧2H 2 O (140 mg; 0.77 mmol) in 3 mL EtOH/MeOH (2:1) was gradually added and the reaction volume made up to 20 mL with ethanol. After 48 h, the product formed was collected by simple filtration then washed with ethanol after 10 days to give 53 mg of 12 as a black precipitate. From the resulted filtrate, 45 mg of 13 was collected after 30 days as a green precipitate. After washing, the complexes were left to stand and the solvent evaporated after 24 h. Compound 12 : R f : 0.66, mp: 288–290 °C, yield 31.18%; HRESI-MS: 878.9684 (M + H, 0.10%). UV–Vis: λ max (acetone): 332, 436, 490, 590 nm. IR (ATR): 3258 cm −1 (OH) chelated , 1713 (C=O ketone ), 1713 (C=O acid ), 1446 (N=N), 528/548 (Co–O) cm −1 . 1 H NMR (MeOH- d 4 /CHCl 3 - d 1 ) δ ppm: 8.83 (s, 2H, H-5), 7.41–7.47 (m, 2H, H-3′), 7.73–7.81 (m, 2H, H-4′), 7.41–7.47 (m, 2H, H-5′), 8.87 (m, 2H, H-6′), 7.73–7.81 (m, 2H, H-2″), 7.41–7.47 (m, 2H, H-5’’), 7.73–7.81 (m, 2H, H-6’’). 13 C NMR (DMSO- d 6 ) δ ppm: 179.9 (3- CO OH), 148.4 (C-4), 129.1 (C-5), 115.2 (C-1′), 156.0 (C-2′), 117.0 (C-3′), 135.3 (C-4′), 125.3 (C-5′), 129.6 (C-6′), 134.4 (C-1’’), 195.0 (3’’- CO CH 3 ) and 156.5 (C-4’’). Anal. Calcd. for [Co 2 (C 19 H 12 N 2 O 5 S) 2 ] (877.9592): C, 51.95; H, 2.75; N, 6.38; S, 7.30; found: C, 51.98; H, 2.78; N, 6.36; S, 7.27. Compound 13 : R f : 0.63, mp: 214–216 °C, yield 26.47%; HRESI-MS: 917.0518 (M + K, 0.18%). UV–Vis: λ max (acetone): 332, 382, 538 nm. IR (ATR): 2573 cm −1 (OH acid ), 1748 (C=O ketone ), 1695 (C=O acid ), 1446 (N=N), 530 (Co–O) cm −1 . 1 H-NMR (MeOH- d 4 /CHCl 3 - d 1 ) δ ppm: 7.54 (s, 2H, H-5), 7.34–7.42 (m, 2H, H-3′), 7.71–7.80 (m, 2H, H-4′), 7.34–7.42 (m, 2H, H-5′), 8.83 (m, 2H, H-6′), 7.51 (m, 2H, H-5″), 7.71–7.80 (m, 2H, H-6″). 13 C NMR (MeOH- d 4 /CHCl 3 - d 1 ) δ ppm: 155.4 (C-2), 154.9 (C-3), 185.6 (3- CO OH), 148.1 (C-4), 115.2 (C-1’), 156.7 (C-2’), 117.2 (C-3′), 135.7 (C-4′), 125.7 (C-5′), 129.9 (C-6′), 192.4 (3″- CO CH 3 ) and 161.4 (C-4″). Anal. Calcd. for [Co 2 (C 19 H 12 N 2 O 5 S) 2 ] (877.9598): C, 51.95; H, 2.75; N, 6.38; S, 7.30; found: C, 51.92; H, (2.77); N, 6.41; S, 7.33.
Antimicrobial evaluation
Tested microorganisms.
Against four different bacterial species, the antibacterial activity was conducted. One Gram-positive Staphylococcus aureus ATCC25923 and three Gram-negative Pseudomonas aeruginosa , Escherichia coli ATCC25922 , and Klebsiella pneumoniae 22 were the chosen bacteria. These microorganisms were collected from our laboratory collection. The different bacterial species were maintained at + 4 °C and activated on BBL ® nutrient agar (NA, Conda, Madrid, Spain) for 24 h before any antibacterial test.
Determination of minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC)
The MICs were determined by the method of microdilution in a liquid medium [ 59 ]. Stock solutions of samples were prepared in an aqueous solution of Dimethyl Sulphoxide 10% (DMSO, Fisher Chemicals, Strasbourg, France) at a concentration of 512 µg/mL. From these stock solutions, successive dilutions in series of 2 were carried out in Mueller–Hinton broth (MHB). For each test, the sterility test (aqueous solution of DMSO at 10% + culture medium), the negative control (aqueous solution of DMSO at 10% + culture medium + inoculum) and the positive control (aqueous solution of DMSO at 10% + culture medium + inoculum + reference drug) were included. 100 μL of each concentration were introduced into a well of a 96-well (200 μL per well) microtiter plate containing 90 μL of MHB and 10 μL of inoculum were added to obtain a range of concentrations varying from 256 to 0.125 μg/mL. Plates were covered and incubated at 37 °C for 24 h on a shaker (Flow Laboratories) at 300 rpm. At the end of the various incubation times, the minimum inhibitory concentrations (MIC) were considered to be the lowest concentrations of substances for which we did not have any macroscopic growth materialized by the cloudy appearance of the well. Minimum bactericidal concentrations (MBCs) were determined by subculturing 10 μL (using 90 mm Petrie dishes) of the contents of wells where growth was not visible to the naked eye with Mueller–Hinton Agar (MHA) medium. The MBCs were defined as the lowest concentration that produced no growth following subculturing. Each test was run three times.
Cytotoxicity assay
The animals were bred in the animal house of the University of Dschang, Cameroon. The study was conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration number 173/CPCSEA, issued January 28, 2000), Government of India, on the use of animals for scientific research. Euthanasia was done using noninhaled agents. Hence, all the rats were anaesthesized via intraperitoneal injection of the mixture of ketamine (50 mg/ kg body weight, BW) and xylazine (10 mg/kg BW), in a dose that is commonly used for operation purposes. A conical tube containing EDTA as an anticoagulant was used to collect 10 mL of whole blood from albino rats using a heart puncture. Centrifugation at room temperature for 10 min at 1000 × g was used to collect erythrocytes, which were then washed three times in PBS buffer [ 60 ]. The cytotoxicity was evaluated as previously reported [ 60 ]. Death was confirmed using a combination of criteria including lack of pulse, breathing, corneal reflex, response to firm toe pinch; inability to hear respiratory sounds and; graying of the mucous membranes and rigor mortis before disposal of any animal remains.
Availability of data and materials
All spectra for the compounds’ characterization are provided as Additional material. Similarly, the raw data for all biological evaluations are available from the corresponding author upon reasonable request.
Abbreviations
Ultra-Violet-visible
Fourier-transform infrared spectroscopy
High resolution electrospray ionization‐mass spectrometry
Proton nuclear magnetic resonance
Carbon nuclear magnetic resonance
Density functional theory
Linear combinations of atomic orbitals
Frontier molecular orbitals
Highest occupied molecular orbital
Lowest unoccupied molecular orbital
Molecular electrostatic potential
Degree centigrade
Minimum inhibitory concentration
Minimum microbicidal concentration
Minimum bactericidal concentration
Dimethylsulfoxide
OMS. Plan d’action mondial pour combattre la résistance aux antimicrobiens. Avenue Appia 20. 2016; Genève:Suisse.
Yuhan Z, Mengnan Z, Benke L, Beibei Z, Bing C, Yuanyuan W, Shan Y, Ruiqi X, Xiaoke Z, Weisheng F. Ephedra Herb extract ameliorates adriamycin-induced nephrotic syndrome in rats via the CAMKK2/AMPK/Mtor signaling pathway. Chin J Nat Med. 2023;21(5):371–82.
Google Scholar
Yi W, Weijie Z, Shujie C, Jinghua L, Hongyu Z. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 2023;11(8):1371–94.
Article Google Scholar
Fatima I, Safdar N, Akhtar W, Munir A, Saqib S, Ayaz A, Bahadur S, Alrefaei AF, Ullah F, Zaman W. Evaluation of potential inhibitory effects on acetylcholinesterase, pancreatic lipase, and cancer cell lines using raw leaves extracts of three fabaceae species. Heliyon. 2023;9:15909.
Tchinda MA, Nanfack DAR, Tamokou JDD, Matsuete-Takongmo G, Tsopmo A, Shaiq AM, Tene M. Echinograciolide, a new antibacterial nor-triterpenoid and other constituents from Echinops gracilis O. Hoffm. (Asteraceae). Nat Prod Res. 2022;10:1–12.
Metiave SAA, Tedonkeu AT, Tamokou JDD, Nanfack ARD, Matsuete-Takongmo G, Wetadieu KD, Tsopmo A, Tene M. Antibacterial stigmastane-type steroids and other constituents from the leaves of Vernonia glabra (Steetz) Vatke (Asteraceae). Nat Prod Res. 2023;13:1–15.
Jouogo NDC, Eckhardt P, Tamokou JDD, Takongmo MG, Voutquenne-Nazabadioko L, Opatz T, Tapondjou AL, Ngnokam D, Teponno RB. A new phenolic glycoside from the leaves of Flacourtia flavescens Willd. Nat Prod Res. 2023;5:1–11.
Shen B, Sun S, Zhu L, Yu J, Jiang L. Intelligent bio-FeS-loaded chitosan films with H 2 O 2 rapid response for advanced waterproof and antibacterial food packaging. Food Packag Shelf Life. 2023;37: 101083.
Article CAS Google Scholar
Saqib S, Faryad S, Afridi MI, Arshad B, Younas M, Naeem M, Zaman W, Ullah F, Nisar M, Ali S, Elgorban AM, Syed A, Elansary HO, El-Abedin TKZ. Bimetallic assembled silver nanoparticles impregnated in aspergillus fumigatus extract damage the bacterial membrane surface and release cellular contents. Coatings. 2022;12:1505.
Cui G, Li Y, Shi T, Gao Z, Qiu N, Satoh T, Kakuchi T, Duan Q. Synthesis and characterization of Eu(III) complexes of modified cellulose and poly(N-isopropylacrylamide). Carbohydr Polym. 2013;94:77–81.
Article CAS PubMed Google Scholar
Tsemeugne J, Emmanuel SF, Jean-De-Dieu T, Ignas KT, Irene CK, Arnaud DN, Stephen TL, Taoufik R, Jules RK, Beibam LS. Electrochemical behavior and in-vitro antimicrobial screening of some thienylazoaryls dyes. Chem Cent J. 2017;11(119):1–13.
Al-Mousawi SM, El-Apasery MA. Synthesis of some monoazo disperse dyes derived from minothienochromene. Molecules. 2013;18:8837–44.
Article CAS PubMed PubMed Central Google Scholar
Fondjo ES, Roosvelt KT, Jean-De-Dieu T, Désiré SA, Sorel DDK, Giscard D, Appolinaire T, Simon PFW, Roger KJ. Synthesis, characterization and antimicrobial activities of novel Hg(II) complex with 3-amino-1-[2-phenyldiazenyl]-4Hthieno [3,4-c]chromen-4-one. J App Chem. 2020;13:8–15.
Roosvelt KT, Emmanuel SF, Jean De-Dieu T, Chinda KI, Giscard D, Sorel DDK, Simon PFW, Apollinaire T, Roger JK. Sonochemical synthesis, characterization and antimicrobial properties of novel lanthanum(III) complex of 3-amino-1-[2-phenyldiazenyl]-4H-thieno[3,4-c]chromen-4-one. J Drug Design Res. 2020;7(1):1076.
Navarro M, Gabbiani C, Messori L, Gambino D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: recent achievements and perspectives. Drug Discov Today. 2010;15(23/24):1070–8.
Vieites M, Smircich P, Guggeri L, Marchàn E, Gόmez-Barrio A, Navarro M, Garat B, Gambino D. Synthesis and characterization of a pyridine-2-thiol N-oxide gold(I) complex with potent antiproliferative effect against Trypanosoma cruzi and Leishmania sp. insight into its mechanism of action. J Inorg Biochem. 2009;103(10):1300–6.
Kowalik M, Masternak J, Lakomska I, Kazimierczuk K, Zawilak-Pawlik A, Szczepanowski P, Khavryuchenko OV, Barszcz B. Structural insights into new Bi(III) coordination polymers with pyridine-2,3-dicarboxylic acid: photoluminescence properties and anti- helicobacter pylori activity. Int J Mol Sci. 2020;21(22):8696–721.
Abdalla KM, El-Zaher GMA, Eman H. Synthesis, structural characterization, and antimicrobial activities of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of triazole-based azodyes. Chin J Chem. 2011;29:1124–32.
Mansour HB, Boughzala O, Dridi D, Barillier D, Chekir-Ghedira L, Mosrati R. Textiles dyes as a source of wastewater contamination: screening of the toxicity and treatment methods. J Water Sci. 2011;24(3):209–38.
Kaya SI, Cetinkaya A, Ozkan SA. Latest advances on the nanomaterials-based electrochemical analysis of azo toxic dyes sunset yellow and tartrazine in food samples. Food Chem Toxicol. 2021;156: 112524.
Guerra E, Llompart M, Garcia-Jares C. Analysis of dyes in cosmetics: challenges and recent developments. Cosmetics. 2018;5:47.
Eltaboni F, Bader N, El-Kailany R, Elsharif N, Ahmida A. Chemistry and applications of azo dyes: a comprehensive review. J Chem Rev. 2022;4(4):313–30.
CAS Google Scholar
Purwanto A, Chen A, Shien K, Huebschmann HJ. Detection, identification, and quantitation of azo dyes in leather and textiles by GC/MS. Thermo Fisher Scientific, Singapore. 2012; 1–6.
Aljamali NM. Review in azo compounds and its biological activity. Anal biochem. 2015;4(2):100–69.
Kyhoiesh HAK, Al-Adilee KJ. Synthesis, spectral characterization, antimicrobial evaluation studies and cytotoxic activity of some transition metal complexes with tridentate (N, N, O) donor azo dye ligand. Results in Chem. 2021;3:100245–71.
Martino MD, Sessa L, Matteo MD, Panunzi B, Piotto S, Concilio S. Azobenzene as antimicrobial molecules. Molecules. 2022;27:5643–72.
Article PubMed PubMed Central Google Scholar
Abdallah SM. Metal complexes of azo compounds derived from 4-acetamidophenol and substituted aniline. Arab J Chem. 2012;5(2):251–6.
Liu Z, Fan B, Zhao J, Yang B, Zheng X. Benzothiazole derivatives-based supramolecular assemblies as efficient corrosion inhibitors for copper in artificial seawater: formation, interfacial release and protective mechanisms. Corros Sci. 2023;212: 110957.
Chen D, Wang Q, Li Y, Li Y, Zhou H, Fan Y. A general linear free energy relationship for predicting partition coefficients of neutral organic compounds. Chemosphere. 2020;247: 125869.
Kocaokutgen H, Erdem E, Gümrükçüoglu IE. Synthesis of HMFAN and its chromium and cobalt complexes and their application on nylon 6 and wool. J Stud Dyn Change. 1998;114:93–5.
Deghadil RG, Mahmoud WH, Mohamed GG. Metal complexes of tetradentate azo-dye ligand derived from 4,40-oxydianiline: preparation, structural investigation, biological evaluation and MOE studies. Appl Organomet Chem. 2020;34:5883–5802.
Mohammed HS. Synthesis, characterization, structure determination from powder x-ray diffraction data, and biological activity of azo dye of 3-aminopyridine and its complexes of Ni(II) and Cu(II). Bull Chem Soc Ethiop. 2020;34(3):523–32.
Bozorov K, Zhao J-Y, Nie LF, Ma H-R, Bobakulov K, Hu RN, Rustamova G, Huang T, Efferth HA. Synthesis and in vitro biological evaluation of novel diaminothiophene scaffolds as antitumor and anti-influenza virus agents. Part 2. RSC Adv. 2017;7(50):31417–27.
Ojeil A, El-Darra N, El-Hajj Y, Mouncef PB, Rizk TJ, Maroun RG. Identification and characterization of phenolic compounds extracted from chateau ksara grapes. Leban Sci J. 2010;11(2):117–31.
Fondjo ES, Tsemeugne J, Tamokou JDD, Djintchui AN, Kuiate JR, Sondengam BL. Synthesis and antimicrobial activities of some novel thiophene containing azo compounds. Heterocycl Commun. 2013;19(4):253–9.
Barceloux DG. Cobalt. Clin Toxicol. 1999;37(2):201–16.
Chang EL, Simmers C, Knight DA. Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals. 2010;3:1711–28.
Djebbar S, Benali-Baitich O, Deloume JP. Synthesis, characterization and electrochemical behaviour of cobalt(II) and cobalt(III): O 2 − complexes, respectively, with linear and tripodal tetradentate ligands derived from Schiff bases. J Mol Struct. 2001;569(1):121–8.
Nielsen I, Andersen AH, Bjergbæk L. Studying repair of a single protein-bound nick in vivo using the Flp-Nick system. Methods Mol Biol. 2012;920:393–415.
Chamlagain B, Edelmann M, Kariluoto S, Ollilainen V, Piironen V. Ultra- high performance liquid chromatographic and mass spectrometric analysis of active vitamin B 12 in cells of propionibacterium and fermented cereal matrices. Food Chem. 2015;166:630–8.
Ali HA, Shamma AA, Kamel S. New mixed ligand cobalt(II/III) complexes based on the drug sodium valproate and bioactive nitrogen-donor ligands. Synthesis, structure and biological properties. J Mol Struct. 2017;1142:40–7.
Kuckova L, Jomova K, Svorcova A, Valko M, Segla P, Moncol J, Kozisek J. Synthesis, crystal structure, spectroscopic properties and potential biological activities of salicylate neocuproine ternary copper(II) complexes. Mol. 2015;20(2):2115–37.
Jaman Z, Karim MR, Siddiquee TA, Mirza AH, Ali MA. Synthesis of 5-substituted 2,9-dimethyl-1,10-phenanthroline dialdehydes and their schiff bases with sulfur-containing amines. Int J Org Chem. 2013;3(3):214–9.
Schwarzenbach G. Alfred werner and his accomplishments. Helv Chim Acta. 1967;50:38–63.
Fondjo ES, Doepp D, Henkel G. Reactions of some anellated 2-aminothiophenes with electron poor acetylenes. Tetrahedron. 2006;62:7121–31.
Al-Mousawi SM, El-Apasery MA. Synthesis of some monoazo disperse dyes derived from aminothienochromene. Mol. 2013;18(8):8837–44.
Djeukoua KSD, Emmanuel SF, Tamokou JDD, Tsemeugne J, Simon PFW, Topmo A, Tchieno FMM, Ekom SE, Pecheu CN, Tonle IK, Kuiate JR. Synthesis, characterization, antimicrobial activities and electrochemical behavior of new phenolic azo dyes from two thienocoumarin amines. ARKIVOC. 2019;6:416–30.
Zhao J, Zhang Y, Gan L, Wang G. Experimental and DFT study of UV–Vis absorption spectra of azobenzene containing ester groups. Comput Theor Chem. 2021;1200: 113244.
Ahmad HI, Bayader FA, Alaa ES, Salah MA, Nadir FH. Synthesis, characterization and photo-kinetic study of diphenol schiff base and its metal complexes with (Co +2 , Ni +2 , Cu +2 ) ions. Ind J Med Forensic Med Toxicol. 2019;13(4):1246–53.
Salavati-Niasari M. Template synthesis and characterization of host (nanopores of zeolite Y)/guest (Co(II)-tetraoxodithiatetraaza macrocyclic complexes) nanocomposite materials. Polyhedron. 2008;27(15):3207–14.
Ghisolfi A, Fliedel C, Rosa V, Pattacini R, Thibon A, Monakhov KY, Braunstein P. Solvent-dependent reversible ligand exchange in nickel complexes of a monosulfide bis(diphenylphosphino)(n-thioether)-amine. Med Toxicol. 2013;8(8):1795–805.
Ebnou F, Ebeid K, M’Haiham M, Dhia MTB, Sanhoury MA. Synthèse et caractérisation des complexes de cobalt(II) et de nickel(II) avec des ligands du type (R 2 N) 3 P(E) (R 2 N = pipéridinyl ou morpholinyl; E = S ou Se). J Maur Chem Soc. 2020;2:1–7.
Loginova NV, Koval’chuk TV, Osipovich NP, Polozov GI, Sorokin VL, Chernyavskaya AA, Shadyro OI. Redox-active antifungal cobalt(II) and copper(II) complexes with sterically hindered o -aminophenol derivatives. Polyhedron. 2008;27(3):991–985.
Nejo AA, Kolawole GA, Nejo AO. Synthesis, characterization, antibacterial, and thermal studies of unsymmetrical schiff-base complexes of cobalt(II). J Coord Chem. 2010;63(24):4398–410.
Alaghaz A, Ammar YA, Bayoumi HA, Aldhlmani SA. Synthesis, spectral characterization, thermal analysis, molecular modeling and antimicrobial activity of new potentially N 2 O 2 azo-dye schiff base complexes. J Mol Struct. 2014;1074:359–75.
Mahmoud WH, Omar MM, Sayed FN. Synthesis, spectral characterization, thermal, anticancer and antimicrobial studies of bidentate azo dye metal complexes. J Therm Anal Calorim. 2016;124:1071–89.
Mabkhot YN, Aldawsari FD, Al-Showiman SS, Barakat A, Soliman SMS, Choudhary MI, Yousuf S, Mubarak MS, Hadda TB. Novel enaminone derived from thieno [2,3-b] thiene: Synthesis, x-ray crystal structure, HOMO, LUMO, NBO analyses and biological activity. Chem Cent J. 2015;9(1):24–34.
Mali SN, Anand A, Zaki MEA, Al-Hussain SA, Jawarkar RD, Pandey A, Kuznetsov A. Theoretical and anti- Klebsiella pneumoniae evaluations of substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and imidazopyridine hydrazide derivatives. Molecules. 2023;28:2801–16.
Tamokou JD, Tala FM, Wabo KH, Kuiate JR, Tane P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismiarubescens . J Ethnopharmacol. 2009;124:571–5.
Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000;44:1485–93.
Download references
Acknowledgements
Emmanuel Sopbué Fondjo warmly acknowledges DAAD funding (grant N° 91691265). The University of Dschang's research grant committee and the Cameroonian Ministry of Higher Education provided further financial support for the project. They also thank the German Academic Exchange Service (DAAD) for funding the Yaoundé-Bielefeld Graduate School of Natural Products with Antiparasite and Antibacterial Activities (YaBiNaPA, project number 57316173).
DAAD (Grant N° 91691265) for SFE and DAAD (YaBiNaPA, project No. 57316173) for BNL.
Author information
Authors and affiliations.
Laboratory of Applied Synthetic Organic Chemistry, Department of Chemistry, Faculty of Science, University of Dschang, P.O. Box 67, Dschang, Republic of Cameroon
Emmanuel Sopbué Fondjo, Sorelle Songmi Feuze & Donald Léonel Feugap Tsamo
Research Unit of Microbiology and Antimicrobial Substances, Department of Biochemistry, Faculty of Science, University of Dschang, PO Box 067, Dschang, Republic of Cameroon
Jean-de-Dieu Tamokou & Jules Roger Kuiate
Department of Chemistry, Carleton University, 1125 Colonel By Drive, Ottawa, K1S 5B6, Canada
Apollinaire Tsopmo
Institut für Anorganische Chemie, Christian-Albrechts-Universität zu Kiel, Max-Eyth-Str. 2, 24118, Kiel, Germany
Giscard Doungmo
Polymer Chemistry Laboratory, Faculty of Live Sciences, Rhine-Waal University of Applied Sciences, Campus Kleve, Marie-Curie Strasse 1, 47533, Kleve, Germany
Peter Simon Friedrich Wilhelm
Higher Teacher’s Training College, University of Yaounde I, P. O. Box 47, Yaounde, Cameroon
Bruno Lenta Ndjakou
You can also search for this author in PubMed Google Scholar
Contributions
ESF created the study idea, supplied the reagents, and oversaw the chemistry experimental work, as well as the compilation of the chemical sections of the publication; SFS performed the chemical portion of the experimental work in the lab, as well as contributed to data analysis and article writing; JDDT carried out the antibacterial and antifungal experiments and helped to compile the biological sections of the report; GD performed the powder diffraction XRD measurements, assisted in the interpretation of the XRD data, and reviewed the entire manuscript draft; DLFT assisted in the NMR measurements, assisted in the interpretation of the spectra, and reviewed the entire manuscript draft; PFWS performed the GC–MS measurements, assisted in the interpretation of the spectra, and reviewed the entire paper draft; BNL performed the LC–MS measurements, assisted in the interpretation of the spectra, and reviewed the entire manuscript draft; AT performed the NMR measurements, assisted in the interpretation of the spectra, and reviewed the entire manuscript draft; JRK directed the biological screening studies and reviewed the entire manuscript draft.
The final version of the manuscript has been approved by all authors. Final manuscript was read and approved by all writers.
Corresponding author
Correspondence to Emmanuel Sopbué Fondjo .
Ethics declarations
Ethics approval and consent to participate.
All the procedures and protocols involving the treatment of animals were carried out in accordance with institutional policies, with the Cameroon National Ethical Committee's approval (Reg. No. FWA-IRB00001954), and in accordance with the ARRIVE standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: figure s1..
Powder X-ray diffractogram of compound 11 . Figure S2 . UV-VIS spectrum of compound 11 . Figure S3 . IR spectrum of compound 11 with some assignments. Figure S4 . HRESI+ mass spectrum of compound 11 . Figure S5 . 1 H-NMR spectrum of compound 11 . Figure S6 . 13 C-NMR spectrum of compound 11 . Figure S7 . HSQC spectrum of compound 11 . Figure S8 . COSY 1 H- 1 H spectrum of compound 11 with some correlations. Figure S9 . HMBC spectrum of compound 11 . Figure S10 . UV-VIS spectrum of complex 12 . Figure S11 . IR spectrum of complex 12 . Figure S12 . HRESI+ mass spectrum of complex 12 . Figure S13 . 1 H-NMR spectrum of complex 12 . Figure S14 . 13 C-NMR spectrum of complex 12 . Figure S15 . HSQC spectrum of complex 12 with some correlations. Figure S16 . COSY 1 H- 1 H spectrum of complex 12 . Figure S17 . HMBC spectrum of complex 12 with some correlations. Figure S18 . Comparison of the UV-VIS spectra of compound 11 (black) and 12 (blue). Figure S19 . Comparison of the IR spectra of compounds 11 (black) and 12 (blue). Figure S20 . Comparison of the 1 H-NMR spectra of compounds 11 (a) and 12 (b). Figure S21 . Comparison of the 13 C-NMR spectra of compounds 11 (a) and 12 (b). Figure S22 . Powder X-ray diffractogram of compound 13 . Figure S23 . UV-VIS spectrum of compound 13 . Figure S24 . IR spectrum of compound 13 . Figure S25 . HRESI+ mass spectrum of compound 13 . Figure S26 . 1 H-NMR spectrum of compound 13 . Figure S27 . 13 C-NMR spectrum of compound 13 . Figure S28 . HSQC spectrum of compound 13 with some correlations. Figure S29 . COSY 1 H- 1 H spectrum of compound 13 . Figure S30 . HMBC spectrum of compound 13 with some correlations. Figure S31 . Comparison of the powder X-ray diffractograms of compounds 11 (black) and 13 (green). Figure S32 . Comparison of the UV-VIS spectra of compound 11 (black) and 13 (green). Figure S33 . Comparison of the IR spectra of compounds 11 (black) and 13 (green). Figure S34 . Comparison of the 1 H-NMR spectra of compound 11 (a) and 13 (b). Figure S35 . Comparison of the 13 C-NMR spectra of compound 11 (a) and 13 (b).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sopbué Fondjo, E., Songmi Feuze, S., Tamokou, JdD. et al. Synthesis, characterization, and antibacterial activity studies of two Co(II) complexes with 2-[( E )-(3-acetyl-4-hydroxyphenyl)diazenyl]-4-(2-hydroxyphenyl)thiophene-3-carboxylic acid as a ligand. BMC Chemistry 18 , 75 (2024). https://doi.org/10.1186/s13065-024-01179-2
Download citation
Received : 10 August 2023
Accepted : 02 April 2024
Published : 16 April 2024
DOI : https://doi.org/10.1186/s13065-024-01179-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Azoic ligand
- Cobalt(II) complexes
- Anti-bacterial activity
BMC Chemistry
ISSN: 2661-801X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Unlocking the magnetic potential of Fe 2 O 3 nanoparticles by single-step synthesis of cobalt-infused nanomaterials for chromium removal
- Original Paper
- Open access
- Published: 20 April 2024
Cite this article
You have full access to this open access article
- Bachir Yaou Balarabe 1 , 2 ,
- Primerose Bomokayi 2 ,
- Irédon Adjama 3 ,
- Abdoulkadri Ayouba Mahamane 4 ,
- Michael Olawale Daramola 5 &
- Samuel Ayodele Iwarere ORCID: orcid.org/0000-0001-8566-6773 5
The study optimized the chromium removal capacity of Fe 2 O 3 nanoparticles through the infusion of cobalt using a single-step synthesis method. This approach not only enhanced their magnetic properties but also employs less-chemical synthesis techniques, ultimately yielding highly magnetic CoFe 2 O 4 nanoparticles and less impurities. The prepared materials underwent comprehensive testing, encompassing examinations of their optical properties, structure, chemical composition, and surface characteristics using various analyticals methods. In a span of 90 min under visible light exposure, CoFe 2 O 4 nanoparticles exhibit the ability to remove more that 90% of chromium. This was corroborated through analysis using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). Moreover, the study illustrates that increased temperatures amplify the endothermic process of chromium adsorption. Positive ΔH°, negative ΔS°, and heightened Cr(IV) adsorption are linked to the temperature effects on solubility, mobility, and dissolved oxygen. Both Langmuir (R 2 = 0.95, R L = 0.055) and Freundlich models (R 2 = 0.98, n = 0.69) suggest favorable adsorption. The efficient Cr(IV) adsorption by CoFe 2 O 4 nanocomposite is attributed to a rapid reaction rate and substantial capacity, following pseudo-second order kinetics (rate constant 0.01 g mg −1 min −1 , R 2 = 0.99).
Graphical abstract
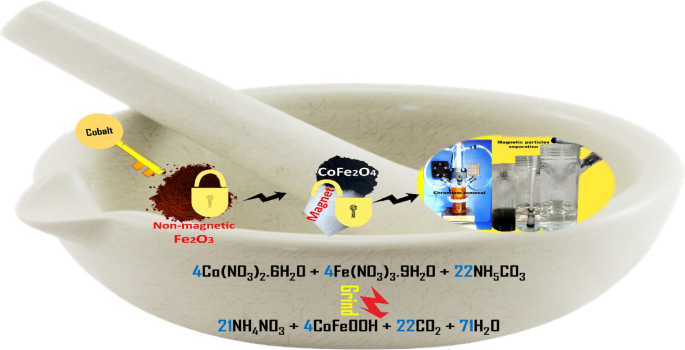
Avoid common mistakes on your manuscript.
Introduction
Heavy metals, including chromium, found in industrial effluents have a significant impact on water pollution and adverse health effects. Chromium is mainly produced by industrial processes, such as metallurgy, electroplating, tanning, wood preservation and petroleum refining [ 1 , 2 , 3 ]. In the aquatic environment, chromium is mainly present in Cr 3+ and Cr 6+ oxidation states. In addition to being carcinogenic, Cr 6+ species exhibit toxicity levels approximately one hundred times greater than those of Cr 3+ . Furthermore, Cr 6+ exhibits a greater degree of mobility as a result of its low adsorption on inorganic surfaces [ 4 , 5 , 6 ]. Excessive inhalation or absorption of Cr 6+ water may cause cancer, tissue damage, and dermatitis. Cr 3+ and Cr 6+ are permissible levels in the environment at 5 ppm and 0.05 ppm, respectively; however, water concentrations range from 10–100 ppm due to their increased use [ 7 ]. The presence of this pollutant in water necessitates treatment before use. Various methods, such as reduction, precipitation, ion exchange, reverse osmosis, electrodialysis, and adsorption processes, have been employed to treat water containing chromium [ 8 , 9 , 10 , 11 ]. Although many of these methods are effective in removing Cr 6+ , they are often costly or inefficient. Due to the ability to remove a wide variety of chemical pollutants, advanced oxidation technologies such as photocatalysis have received considerable attention [ 6 ]. A number of significant advantages of photocatalysis have recently attracted increasing attention due to their environmental friendliness, high efficiency, and cost-effectiveness. Photocatalysis occurs when semiconductor catalysts are exposed to light and facilitate the acceleration of photoreactions [ 12 , 13 ]. It is believed that both photo-oxidation and photo-reduction occur due to electron–hole pairs forming on the photocatalyst’s surface during photon absorption. Free radicals result from this process and are essential for detoxifying pollutants [ 14 , 15 ]. Metal oxide nanoparticles have been successfully used as photocatalysts to remove pollutants from wastewater but due to their primary activation occurring in the ultraviolet (UV) region, which is characterized by a wide bandgap, these semiconductors can only absorb a 4% fraction of sunlight [ 16 , 17 , 18 ]. As a result of this need, several approaches have been explored for highly active photocatalytic materials driven by visible light. These methods include embedding metal ions within a semiconductor and coupling semiconductors with narrow band gaps [ 19 , 20 , 21 ]. Recent advancements have introduced a range of advanced materials, including metal sulphides and their complex heterojunctions. Simultaneously, the field has experienced the introduction of Metal–Organic Frameworks (MOFs), biochar materials and MXene, expanding the diversity of innovative materials [ 22 , 23 , 24 , 25 ]. Notably, cobalt ferrite (CoFe 2 O 4 ) has gained recognition as a highly versatile material, proving effective in various applications such as photocatalysis, drug delivery, wastewater treatment, cancer treatment, and sensor technologies. CoFe 2 O 4 has excellent mechanical hardness and cost-effectiveness, as well as exceptional strength and magnetic properties [ 26 , 27 , 28 , 29 ]. The nanoparticles possess a narrow band gap, which makes them sensitive to visible light exposure and allows them to align with the solar spectrum [ 30 ]. The unique properties of CoFe 2 O 4 have been investigated in several studies to reduce hexavalent chromium through photocatalysis. Using CoFe 2 O 4 /BiOBr/Graphene composites, Li et al . developed a Z-scheme photocatalyst capable of reducing hexavalent chromium and degradation of organic dyes in visible light [ 31 ]. Emadian et al . investigated the potential of a magnetically separable CoFe 2 O 4 /ZrO 2 nanocomposite for the photocatalytic reduction of hexavalent chromium under visible light irradiation [ 32 ]. For the same application, Ibrahim et al . studied the magnetically separable TiO 2 /CoFe 2 O 4 /Ag nanocomposites under UV and artificial solar light [ 33 ].
Examining alternative approaches, this study aims to address the prevalent challenges of cost and inefficiency associated with current methods in treating chromium-containing effluents. The focus is on the unique synthesis method and resulting properties of CoFe 2 O 4 nanoparticles, offering a promising solution for efficient reduction of hexavalent chromium. The magnetic nature of the photocatalyst facilitates easy separation from the solution, addressing a key challenge in practical applications. The synthesis process involves a straightforward single-step grinding procedure, transforming non-magnetic Fe 2 O 3 nanoparticles into highly magnetic ones by incorporating cobalt. The resulting CoFe 2 O 4 nanoparticles are then assessed for their ability to reduce concentrated Cr(IV).
Experimental
All materials and solvents were obtained from commercial sources: Sisco Research Laboratories Pvt. Ltd, India, Sigma Aldrich, and Abhishek Enterprise Pvt. Ltd. Materials were used without any modification. In particular, the chemicals used included Iron (III) nitrate nonahydrate, (Fe(NO 3 ) 3 .9H 2 O) (extra pure, 99.9%), ammonium bicarbonate, NH 4 HCO 3 (extra pure AR, 99.9%), cobalt(II) nitrate hexahydrate, Co(NO 3 ) 2 xH 2 O (ACS reagent, 98%), and potassium dichromate (ACS reagent, 99.0%). All measurements were performed with milli-Q water and spectroscopic-grade solvents.
Characterization instruments
To record optical absorbance spectra, a JASCO-670 UV/VIS/NIR spectrometer (made in Japan). was used. For FT-IR spectra, a JASCO FT-4700 spectrometer (made in Japan) was used. Images of transmission electrons were captured with the Talos F200i S/TEM electron microscope (HRTEM-200 kV). As part of the quantitative elemental analysis and elemental mapping process, this device was equipped with an EDAX Bruker X Flash 6 30 EDS detector. The Raman spectra were recorded by a RENISHAW InVia Raman Microscope. The X-ray diffraction pattern of the samples was measured using a Cu–K light source X-ray diffractometer (GNR APD 2000 PRO). At room temperature, the VSM Lake Shore Model-7410 Series was used to study magnetic properties. In order to assess the effectiveness of photocatalytic chromium removal, both inductively coupled plasma optical emission spectroscopy (ICP-OES) using the Perkin Elmer Optima 5300 DV ICP-OES and a UV–Vis spectrometer were used. Utilizing a Micromeritics ASAP2020, N 2 adsorption on the solid was measured, and this data was employed to determine the (BET) specific surface area.
Synthesis of CoFe 2 O 4 nanoparticles
Fe 2 O 3 nanoparticles were synthesized by mechanically grinding 0.6 g ammonium bicarbonate with 1 g iron nitrate nonahydrate using a mortar and pestle. This was done in accordance with Eq. 1 . The FeOOH residue obtained was washed successively with ethanol and distilled water and dried at 100 °C before being calcined at 300 °C for two hours [ 34 ]. Similarly, the preparation of magnetic CoFe 2 O 4 nanoparticles was achieved by grinding simultaneously 1 g iron nitrate nonahydrate, 1.08 g ammonium bicarbonate, and 0.72 g of cobalt (II) nitrate hexahydrate simultaneously (Eq. 2 ), followed by washing and calcination at 300 °C.
Results and discussion
UV–vis diffuse reflectance spectroscopy (DRS) was employed to analyse the light absorption properties of the synthesized photocatalyst, as depicted in Fig. 1 a. Displaying enhanced absorption characteristics, the CoFe 2 O 4 nanocomposite shows maximum absorbance at approximately 500 nm in the visible region. This contrasts with the pristine Fe 2 O 3 nanoparticles, highlighting the nanocomposite's exceptional efficiency in capturing visible light. Efficiently converting solar energy into chemical energy is facilitated by the heightened light absorption capacity of CoFe 2 O 4 nanocomposites. To determine the optical band gap of the samples, Tauc's equation is used. Extrapolation lines for Fe 2 O 3 and CoFe 2 O 4 samples are shown in Fig. 1 b. In CoFe 2 O 4 nanoparticles, cobalt significantly decreased the band gap. As a result of cobalt incorporation into magnetic CoFe 2 O 4 nanoparticles, Fe 2 O 3 bandgaps were reduced from 1.5 eV to 1.32 eV. This provides strong evidence that CoFe 2 O 4 nanoparticles have been successfully formed. In line with previous research, this combination leads to nanocomposites with enhanced photon absorption capacity in visible light [ 15 ].
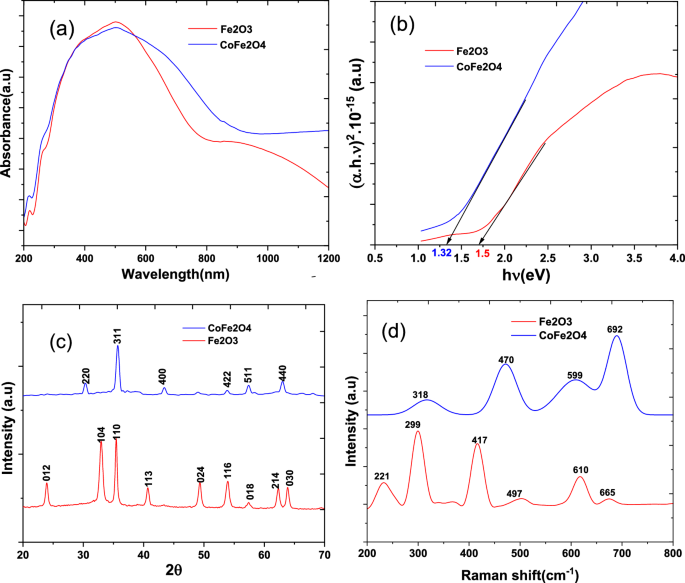
a UV–vis spectra, b direct bandgap, c XRD pattern and d Raman spectra of Fe 2 O 3 and CoFe 2 O 4 nanoparticles
X-ray diffraction (XRD) was used to analyse the crystal structure, phase, and purity of the synthesized material. Figure 1 c illustrates the X-ray diffraction pattern, which exhibits distinct peaks. There are perfectly aligned peaks at (012), (104), (110), (113), (124), (116), (214), (300), and (208) which indicate that the material is composed of a pure hematite α-Fe 2 O 3 ) structure. Alternatively, the XRD pattern also exhibits peaks at (220), (311), (400), (422), (511), and (440) corresponding to spinel-like CoFe 2 O 4 nanoparticles (JCPDF 22–1086).
Raman spectroscopy is an effective method for understanding nanoparticle atomic structure. Figure 1 d displays an analysis of the Raman spectrum obtained from synthesized composites. Raman peaks were observed at 221 cm −1 , 287 cm −1 , 405, 493 cm −1 , 608 cm −1 , and 610 cm −1 . These peaks can be attributed to hematite α-Fe 2 O 3 reported in a previous study [ 34 ]. Additionally, a Raman peak at 665 cm −1 indicates ferric oxide traces. These Raman peaks are similar to those reported by another researcher [ 35 ]. However, the Raman spectrum of the CoFe 2 O 4 sample indicates a cubic spinel structure composed of cobalt ferrite. Approximately 318 cm −1 , 470 cm −1 , 599 cm −1 and 692 cm −1 of the spectrum correspond to active Raman vibrational modes (A1g + Eg + 3T2g), respectively [ 36 ]. In addition, the Raman spectra recorded for the cobalt ferrite compound indicate that it is free of impurities such as α-Fe 2 O 3 .
To measure the synthetic specimens using FTIR spectroscopy, a range of 1200 to 400 spectral ranges were employed. Figure 2 a illustrates the FTIR spectra of synthesized Fe 2 O 3 and CoFe 2 O 3 . A distinct peak at 426 cm −1 can be attributed to the stretching vibrations of Co–O bonds in the CoFe 2 O 4 sample. The broad peak in the spectrum in each sample ranges from 1000 to 510 cm −1 with a maximum of 680 cm −1 [ 32 ]. It indicates stretching vibrations of Fe–O bonds. It is clear from the spectral features observed that the synthesized materials are bonded and that they contain Co–O and Fe–O bonds.
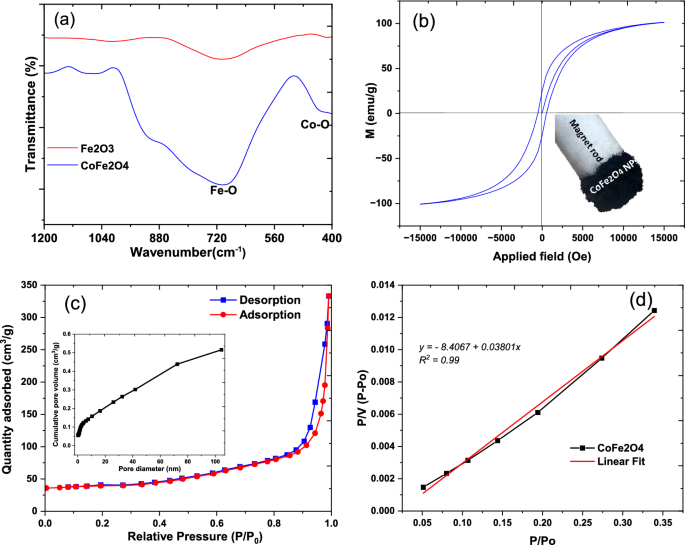
a Fourier transform infrared analysis (FT-IR) spectra of of Fe 2 O 3 and CoFe 2 O 4 nanoparticles, b Hysteresis loop of CoFe 2 O 4 nanoparticles, c N 2 adsorption–desorption curve for BET analysis and the BJH pore distribution curve (inset), d BET plot of N 2 adsorption
The field-dependent behavior (M-H curve) of CoFe 2 O 4 was studied using a vibrating sample magnetometer (VSM). As shown in Fig. 2 b and Table 1 , the synthesized samples were characterized by their coercivity, saturation, remanence, and squareness ratios. Interestingly, the cobalt-doped nanocomposite, CoFe 2 O 4 , exhibits a saturation point of 101.11 emu/g, exceeding the value reported by Emadian in a previous study of 49.17 emu/g [ 32 ]. Furthermore, CoFe 2 O 4 has a widening hysteresis loop, which suggests that this material is a hard magnetic material [ 37 ].
The N 2 adsorption–desorption curve, presented in Fig. 2 c, reveals a hysteresis curve indicative of a type IV Brunauer–Emmett–Teller (BET) adsorption isotherm. This BET isotherm pattern signifies an initial monolayer adsorption, succeeded by multilayer adsorption. Following the creation of the initial monolayer, the adsorption gradually increases, indicating the formation of multilayers of dye molecules. Such behaviour is typical in porous materials with diverse pore diameters. The accompanying hysteresis loop further underscores the mesoporous structure of the nanocomposite [ 38 ]. The CoFe 2 O 4 nanocomposite’s specific surface area was calculated to be 75 m 2 /g, while its pore volume and diameter were found to be 0.05548 cm 3 /g. The determined pore diameter of 12 nm, as analysed by Barrett-Joyner-Halenda (BJH), indicates the presence of mesopores on the catalyst’s surface, as illustrated in the inset of Fig. 2 c. This is consistent with the typical range of mesopores, which falls between 2 and 50 nm [ 39 ]. The correlation coefficient of 0.99 for the BET isotherm highlights the model’s robustness and accuracy in describing the observed adsorption behaviour. This strong correlation indicates a positive relationship between the experimental data and the theoretical predictions, confirming the BET model’s suitability for explaining the adsorption process under the investigated conditions (Fig. 2 d).
The chemical composition and oxidation state of CoFe 2 O 4 were determined using XPS analysis. Figure 3 illustrates high-resolution spectral data for Fe2 p , Co2 p , O1 s , and C1 s .
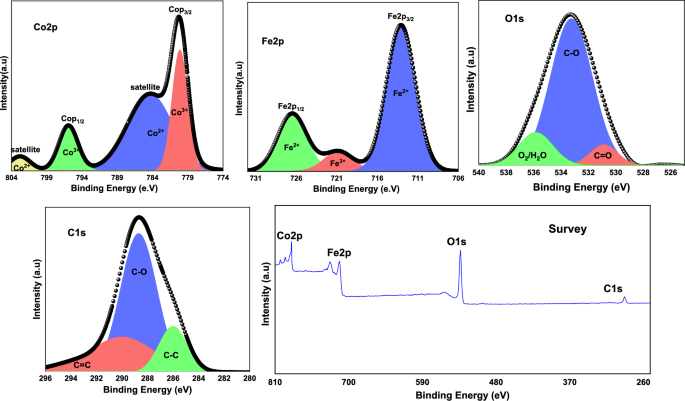
XPS spectra of CoFe 2 O 4 nanoparticles
Fe2 p spectra show two peaks: Fe2 p 1/2 at 712.95 eV and Fe2 p 3/2 at 726.40 eV, indicating Fe 2+ . An additional peak at 721 eV indicates Fe 3+ chemical structure [ 37 , 40 ]. The Co spectrum clearly displayed emerging peaks at 780 and 795 eV, corresponding to Co2 p 3/2 and Co2 p 1/2 , respectively, thus verifying the presence of Co 3+ . The presence of two satellite peaks indicates that the cobalt is also present in a 2+ oxidized state [ 41 ]. Furthermore, the O1 s spectrum showed three distinct peaks: one at 530 eV resulting from C=O, another at 533 eV resulting from C–O, and a third peak at 535 eV due to physically adsorbed O 2 /H 2 O [ 42 ]. It can be seen that the C1 s spectrum in the composite exhibits a distinct peak at 289 eV, which is consistent with the C binding energy seen in hydrocarbons. This peak is likely to be the result of surface contamination from air exposure [ 43 ].
As shown in Fig. 4 a, c, high-resolution transmission electron microscopy was used to examine the surface morphology and size of the nanoparticles. Cobalt and iron nanoparticles are bonded together in a spherical shape, with an average size of 150 nm. CoFe 2 O 4 nanoparticles are also shown to be composed of several crystallographic phases as shown in Fig. 4 b, indicating that these nanoparticles are composed of various crystal structures or polymorphs. Several factors have contributed to this phase variation, including synthesis conditions, postprocessing methods, and impurities in the sample [ 44 ]. As shown by Yuan et al., the presence of multiple crystallographic phases indicates that a nanoparticle has a high crystallinity level and is well-formed. The presence of several crystallographic phases can also significantly influence nanoparticle properties, such as electronic, optical, and catalytic properties. As a result, these nanoparticles have significant potential for numerous applications [ 45 ].
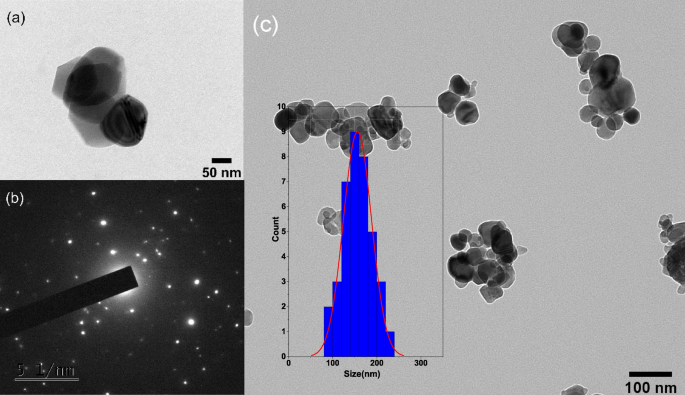
a , c TEM images (insert particles size), b SAED image of CoFe 2 O 4 nanoparticles
The mapping images in Fig. 5 a–d as well as the EDS analysis illustrated in Fig. 5 e confirmed the purity of the hybrid CoFe 2 O 4 nanoparticles. The sample contained both Fe and Co elements, indicating high purity. CoFe 2 O 4 nanoparticles contained 58.58% Fe, 13.88% Co, and 27.54% O.
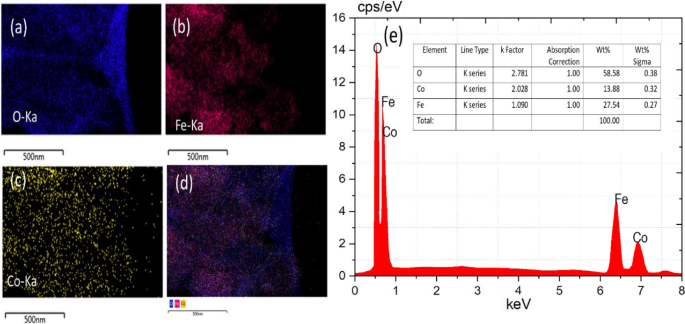
a–d Mapping images and e EDX spectrum of CoFe 2 O 4 nanoparticles
Photocatalytic activity measurements
As reported in a previous work, the photocatalytic performance of the nanoparticles was investigated to remove potassium dichromate (K 2 Cr 2 O 7 ) in a photocatalytic reactor with visible light irradiation (High-pressure mercury lamp, 125 W) at room temperature. The photocatalytic reaction was carried out in 500 ml closed flasks containing 200 ml of a chromium solution. Before irradiation, the suspension was magnetically stirred for 30 min in the dark to achieve an adsorption–desorption equilibrium. The reaction was monitored by taking 1 mL aliquots from the flask at regular intervals and measuring the absorbance [ 37 ]. As time progressed, the absorbance peak was progressively reduced. A photocatalyst’s removal efficiency is expressed in Eqs. 3 and 4 .
where Ci, Ct, and Ce represent the concentrations of the dye solution at the beginning, at time t, and at equilibrium, respectively, measured in milligrams per liter (mg/L), Qe (mg/g) signifies the maximum adsorption capacity of the nanocomposite, V and m the volume and the mass of the Cr(IV) and the catalyst respectively [ 46 ].
Effect of pH
A significant factor influencing pollutant removal is the solution pH. Cr(VI) photoreduction efficiency was evaluated at different pH levels (3, 5, 7, 9, and 12) using a photocatalyst dose of 50 mg and a Cr(VI) concentration of 40 ppm (Fig. 6 a). As the pH of the solution decreased, the reduction in Cr(VI) increased. At pH 3, the highest removal efficiency was observed. Specifically, CoFe 2 O 4 had 72% photoreduction efficiency at pH 3, while Fe 2 O 3 was 68%. These results indicate that Cr(VI) photoreduction occurs better under acidic conditions. This hypothesis was also confirmed by Emadian's study, as the zeta potential analysis demonstrated that at pH > 6, the surface of the CoFe 2 O 4 nanoparticles become positively charged due to the presence of H + ions. It is therefore believed that more Cr(VI) is reduced to Cr(III) at pH > 6 than at pH < 6 [ 31 , 32 ]. In the presence of acidic conditions, chromium reduction is more efficient due to increased surface protonation and electrostatic interactions between the charged photocatalyst surface and the Cr(VI) present [ 49 , 50 , 51 ].
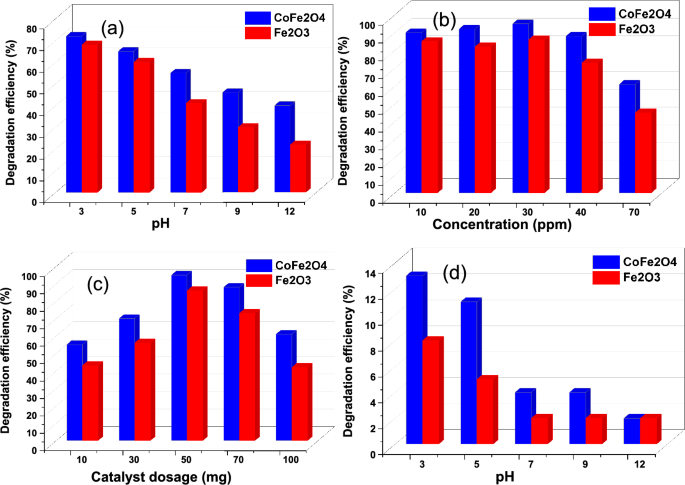
Effect of a pH, b concentration of chromium, c catalyst dosage and d pH study at different pH using CoFe 2 O 4 and Fe 2 O 3 nanoparticles for the adsorption of Cr(IV)
Effect of chromium concentration
The study included tests with different concentrations of heavy metals (10, 20, 30, 40, and 70 ppm) to examine their effect on photocatalytic removal for 90 min. All experiments were conducted with a constant catalyst mass of 50 mg at pH 3. As the concentration of heavy metal solution increased (Fig. 6 b), the rate of photocatalytic removal decreased. Accordingly, this phenomenon may be attributed to the increased adsorption of heavy metal molecules on the surface of the composites, which attenuates the particle activity. This reduced the efficiency of the photocatalyst removal response. Furthermore, increasing the initial heavy metals concentration led to an increased likelihood of free radical interactions with heavy metals atoms. However, a higher heavy metal concentration also resulted in significant radiation absorption by the heavy metal molecules. This led to a decrease in the penetration of irradiation into the semiconductor. As a result, free radical concentrations decreased, resulting in a reduction in photocatalytic removal efficiency [ 47 , 48 ]. In this study, the highest percentage of removal was observed at 30 ppm.
Effect of catalyst dosage
The impact of composite dosage on the removal of heavy metals under visible light conditions was investigated, as illustrated in Fig. 6 c. The photoreduction process was accelerated when the number of particles increased. A dosage of 50 mg of nanomaterials and 30 ppm heavy metals yielded the highest elimination rate in 90 min time at pH 3. In contrast, when the dosage of the catalyst is greater than 50 mg, the removal rate begins to decrease, which emphasizes the critical role that catalyst dosage plays in the photocatalytic removal of heavy metals. The reduction in removal rate with higher catalyst dosage can be attributed to the increased opacity of the suspension caused by an abundance of nanoparticles. In turn, this high degree of opacity hinders light penetration and the effective interaction between photons and heavy metal contaminants. In addition, exceeding the ideal concentration of photocatalysts can cause nanoparticles to coagulate and reduce their surface area. As a result, fewer photons are retained, resulting in slower removal of the photocatalyst [ 47 , 49 , 50 , 51 ].
Adsorption test at different pH
Under dark conditions, the chromium adsorption capacity of the catalysts shown in Fig. 6 d was evaluated at different pH levels (3, 6, 7, 9 and 12). As the pH increased to 12, the dark adsorption of chromium on the surface decreased to approximately 2%. The higher concentration of H + ions in an acidic environment is attributed to the higher adsorption rate in an acidic environment during the photocatalytic reduction process, which accelerates the photoreaction, as indicated by Eqs. ( 5 ) and ( 6 ). According to Eq. ( 6 ), a basic medium has a low concentration of H + ions, which slows down the reaction. In addition, the formation of Cr(OH) 3 can contribute to reduction efficiency by precipitating on the surface of the photocatalyst, thereby covering the active sites and preventing light penetration, as described in Eq. ( 7 ). Based on our study, pH = 3 was found to be the most effective pH for Cr(VI) photoreduction [ 32 ].
Effect of time
The removal efficiency of hexavalent chromium by CoFe 2 O 4 nanoparticles was found to be notably higher than that of Fe 2 O 3 nanoparticles, based on UV absorbance readings tracked over time. Specifically, after 70 min, CoFe 2 O 4 nanoparticles achieved an 90% removal efficiency, whereas Fe 2 O 3 nanoparticles showed a lower efficiency of 76.65% (Fig. 7 a–c). Notably, CoFe 2 O 4 nanoparticles exhibited high catalytic activity under visible light. When observing Cr(IV) adsorption over time for a constant adsorbent quantity of 50 mg, both CoFe 2 O 4 and Fe 2 O 3 nanoparticles demonstrated similar chromium removal, as depicted in Fig. 7 d.
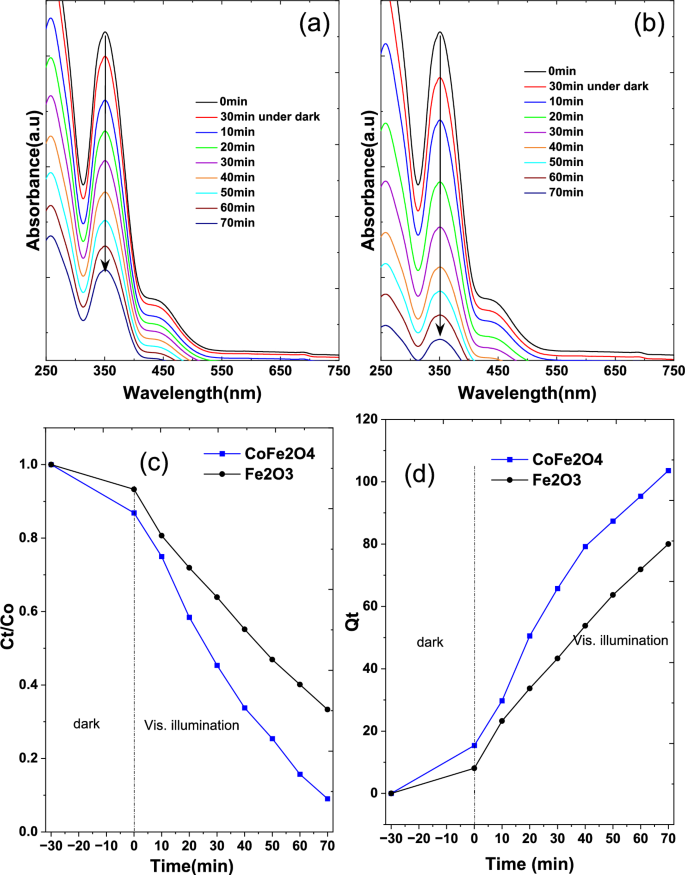
a , b Chromium removal efficiency under visible light, c photocatalytic removal curves (C t /C 0 vs. time) and d influence on qt with increasing time of Fe 2 O 3 and CoFe 2 O 4 nanoparticles
Adsorption thermodynamics
Thermodynamic factors crucial for assessing spontaneity in sorption techniques include Gibbs free energy (G°), enthalpy (H°), and entropy (ΔS°). The mathematical expressions for G° and the change in Gibbs free energy (ΔG°) are outlined in Eqs. 8 and 9 , respectively. Subsequently, Vant Hoff's equation is derived from these equations, as given in Eq. 10 , with Kad provided in Eq. 11 [ 38 , 52 ].
where enthalpy (ΔH°) and entropy (ΔS°), represented by R, T, and Kad as the gas constants, reaction temperature, and adsorption equilibrium constant. Furthermore, Ce (mg/L) and Cad (mg L −1 ) is the equilibrium solute concentration, and the concentration of the adsorbed solute.
Figure 8 a illustrates the relationship between ln Kad and 1/T, providing the thermodynamic parameters for adsorption. The slope and intercept of this plot, as shown in Table 2 , determine ΔH° and ΔS°, respectively. In the photocatalytic experiment, a composite of 50 mg was mixed with a 30 ppm chromium concentration, and the experiment ran for 70 min with temperatures varying from 298 to 333 K. The positive ΔH° value observed indicates endothermic adsorption, while the negative ΔS° implies a reduction in the degree of freedom of the adsorbed substance. The increasing G° with temperature suggests that adsorption becomes more favourable at higher temperatures. These findings align with similar results obtained by AL-Othman et al. [ 53 ].
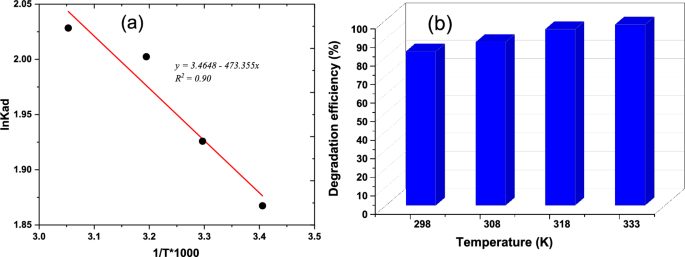
a Vant Hoff’s plot for the removal of Cr(IV), b effect of temperature variation on the adsorption of Cr(IV)
The rise in temperature is associated with an increase in adsorption, as depicted in Fig. 8 b, leading to a higher amount of Cr(IV) adsorption. This heightened adsorption can be attributed to an increased affinity or attraction between the adsorbent material and the molecules being absorbed. As per Balarabe et al., the solubility and mobility of Cr(IV) molecules experience an increase with rising temperatures, facilitating more effective interaction with the active sites on the adsorbents’ surfaces. Simultaneously, the increase in temperature results in a decrease in dissolved oxygen in the solution, preventing the nanoparticles from undergoing oxidation [ 34 ].
Adsorption isotherm
Widely utilized for data fitting, adsorption isotherm models are employed to examine the correlation between adsorbed quantity (Qe) and equilibrium aqueous concentration (Ce). Among these models, the Langmuir and Freundlich equations are the most commonly used (Eqs. 12 , 13 ). The Langmuir model suggests that adsorbate molecules are taken up on a homogeneous surface through monolayer adsorption without interactions between the adsorbed molecules. In contrast, the Freundlich model is suitable for non-ideal adsorption on heterogeneous surfaces, where heterogeneity arises from the presence of different functional groups on the surface and various interactions between the adsorbent and adsorbate [ 54 ]. In this study, isotherm evaluations were carried out by employing variable initial concentrations of Cr(IV) ranging from 10 to 100 mg L −1 at a temperature of 318 K, utilizing 50 mg of the synthesized nanocomposite. Experimental data were used to fit the isotherm models of Langmuir and Freundlich. Linear adjustments of these models are depicted in Fig. 9 a&b, with corresponding parameter values detailed in Table 3 .
where Ce represents the equilibrium concentration of the solute in the liquid phase (mg/L). Qe is the amount of solute adsorbed per unit weight of adsorbent at equilibrium (mg/g). Qmax is the maximum adsorption capacity (mg/g). K is the Langmuir adsorption equilibrium constant (L/mg). Kf is the Freundlich adsorption equilibrium constant (mg^(1–1/n) L^(1/n)/g). n is the Freundlich exponent, which is an empirical parameter characterizing the adsorption intensity.
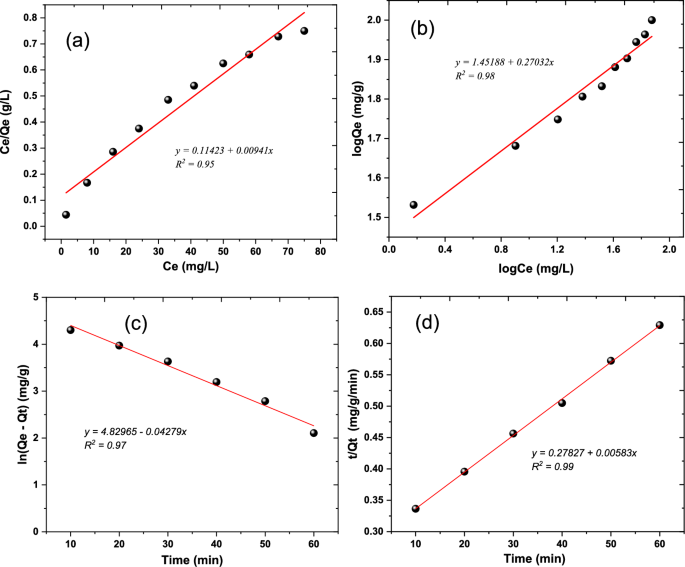
Adsorption isotherm models: a Langmuir and b Freundlich, kinetic models: c PFO and d PSO
As per Table 3 , the Langmuir model exhibited a determination coefficient (R 2 ) of 0.95. The separation factor (R L ), used to assess the favourability of an adsorption process (0 < R L < 1 for favourable, R L > 1 for unfavourable, R L = 0 for irreversible), yielded values ranging from 0.055. This suggests that the adsorption process is favourable within the studied concentration range [ 55 ]. The same Table 3 reveals that the Freundlich model yielded an R 2 of 0.98. The heterogeneity factor, represented by the parameter n, is utilized to assess the nature of the adsorption process—physical (n > 1), chemical (n < 1), or linear (n = 1). With an n value of 0.69, it indicates that the chemical process is favourable [ 56 ].
Adsorption kinetics
Efficient adsorption relies on a rapid reaction rate, a brief contact time, and a substantial adsorption capacity. The kinetics of Cr(IV) adsorption by the CoFe 2 O 4 nanocomposite, depicted in Fig. 9 c&d, were examined using pseudo-first order (PFO) and pseudo-second order (PSO) kinetic equations, specifically Eqs. 14 & 15 [ 53 ]. The investigation revealed that the adsorption adhered to PSO kinetics, with a rate constant of 0.01 g mg −1 min −1 , as evidenced by the highest correlation coefficient (R 2 ) of 0.99.
where Qe and Qt represent the quantities of Cr(VI) at equilibrium and time t, respectively, measured in mg/g. The pseudo-first-order and pseudo-second-order adsorption rate constants are denoted as k 1 (min −1 ) and k 2 (g mg −1 min −1 ), respectively.
Catalyst reusability
The efficiency of photocatalytic removal of chromium by CoFe 2 O 4 nanoparticles and their reusability were confirmed through a four-cycle study, each lasting 90 min. The removal percentage of potassium dichromate solution was analyzed using ICP-OES after each cycle. Remarkably, approximately 99% removal was achieved for Cr 119, Cr 126, and K 44, with a 10% removal for K 83 after 90 min (Fig. 10 a). The ICP results confirm the effective removal of chromium from the solution throughout the treatment process, and the nanoparticles displayed recyclability over four treatment cycles without significant alterations. The successful removal of chromium showcased the efficient magnetic collection of CoFe 2 O 4 nanoparticles, evident from their inherent magnetic properties as depicted in Fig. 10 b (2–4). Within just five minutes of immersing a bar magnet into the treated solution, the CoFe 2 O 4 nanoparticles were promptly gathered. Contrastingly, Fig. 10 b (5) emphasizes the nonmagnetic nature of Fe 2 O 3 nanoparticles, rendering them resistant to collection using a bar magnet due to their lack of magnetic responsiveness. Building on this, the magnetic properties of spinel CoFe 2 O 4 , in conjunction with its high surface area, make it an ideal nanocatalyst for efficient contaminant removal. The oxidation–reduction activity of Co and Fe ions in this magnetic nanoparticle enables the breakdown of contaminants through their oxidation–reduction reactions. The specific arrangement of ions in the crystal lattice provides catalytic sites, facilitating processes such as organic pollutant removal. Additionally, depending on the conditions, spinel CoFe 2 O 4 may exhibit photocatalytic properties, further enhancing its ability to remove contaminants. This dual functionality positions spinel CoFe 2 O 4 as a versatile and effective material for environmental remediation applications [ 26 , 27 , 28 , 29 , 57 ].
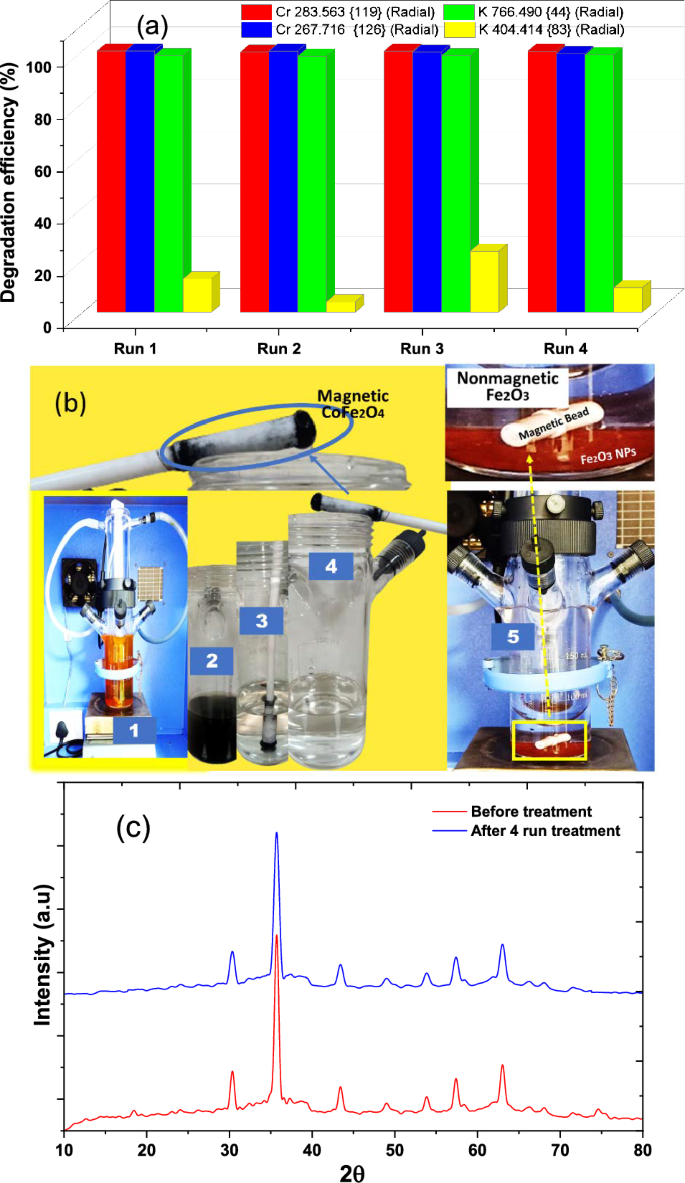
a Reussibility of CoFe 2 O 4 nanoparticles for 4 cycles, b 1-Photocatalytic reactor containing a mixture of dichromate potassium solution and nanoparticles, 2–4-magnetic separation of treated solution and magnetic nanoparticles of CoFe 2 O 4 , 5-nonmagnetic Fe 2 O 3 nanoparticles settle in to the treated sample and c XRD analysis after 4 cycles of treatment for CoFe 2 O 4 nanoparticles
The X-ray diffraction analysis conducted on the nanomaterials following four cycles of treatment closely resembles the characteristics of the fresh sample, as illustrated in Fig. 10 c. Moreover, the photocatalytic reduction of Cr(IV), as indicated in Table 4 , showcases a remarkably efficient and noteworthy removal response when compared to analogous studies utilizing diverse nanocomposites.
In conclusion, this comprehensive study underscores the successful single-step and facile synthesis of CoFe 2 O 4 magnetic nanoparticles, showcasing their outstanding photocatalytic chromium removal capabilities under visible light. Spectroscopic and microscopic analyses reveal these nanoparticles’ uniform distribution, emphasizing their practical applicability. The exceptional efficiency of CoFe 2 O 4 nanoparticles in chromium effluent removal underscores the crucial role of magnetic properties in nanoparticle separation techniques, facilitating their practical collection post-remediation. Furthermore, the comparison with non-magnetic Fe 2 O 3 nanoparticles emphasizes the importance of tailoring separation methods to nanoparticle characteristics. By effectively addressing the challenge of isolating different types of nanoparticles, this study contributes to advancing environmentally friendly technologies and practices. The optimized chromium removal capacity and the enhanced understanding of temperature effects on chromium adsorption further strengthen the potential applications of CoFe 2 O 4 nanocomposites in water treatment processes.
Data availability
All data are included in the article.
Sodhi V, Sohpal VK (2011) Treatment of chromium(VI) containing aqueous effluent of tanneries and electroplating units by membrane process
Gheju M, Iovi A, Balcu I (2008) Hexavalent chromium reduction with scrap iron in continuous-flow system: Part 1: effect of feed solution pH. J Hazard Mater 153:655–662. https://doi.org/10.1016/J.JHAZMAT.2007.09.009
Article CAS Google Scholar
Gong Y, Liu X, Huang L, Chen W (2010) Stabilization of chromium: an alternative to make safe leathers. J Hazard Mater 179:540–544. https://doi.org/10.1016/J.JHAZMAT.2010.03.037
Tafese Bezuneh T, Haile Fereja T, Li H, Jin Y (2023) Solid-phase pyrolysis synthesis of highly fluorescent nitrogen/sulfur codoped graphene quantum dots for selective and sensitive diversity detection of Cr(VI). Langmuir 39:1538–1547. https://doi.org/10.1021/acs.langmuir.2c02966
Costa M (2003) Potential hazards of hexavalent chromate in our drinking water. Toxicol Appl Pharmacol 188:1–5. https://doi.org/10.1016/S0041-008X(03)00011-5
Losi ME, Amrhein C, Frankenberger WT (1994) Environmental biochemistry of chromium
Poornima K, Karthik L, Swadhini SP et al (2010) Degradation of chromium by using a novel strains of Pseudomonas species. J Microb Biochem Technol 2:95–99. https://doi.org/10.4172/1948-5948.1000031
Djellabi R, Su P, Elimian EA et al (2022) Advances in photocatalytic reduction of hexavalent chromium: from fundamental concepts to materials design and technology challenges. J Water Process Eng 50:103301. https://doi.org/10.1016/J.JWPE.2022.103301
Article Google Scholar
Liu Z, Yu Y, Zhu X et al (2022) Semiconductor heterojunctions for photocatalytic hydrogen production and Cr(VI) reduction: a review. Mater Res Bull 147:111636. https://doi.org/10.1016/J.MATERRESBULL.2021.111636
Lu S, Shen L, Li X et al (2022) Advances in the photocatalytic reduction functions of graphitic carbon nitride-based photocatalysts in environmental applications: a review. J Clean Prod 378:134589. https://doi.org/10.1016/J.JCLEPRO.2022.134589
Wang X, Pehkonen SO, Ray AK (2004) Photocatalytic reduction of Hg(II) on two commercial TiO 2 catalysts. Electrochim Acta 49:1435–1444. https://doi.org/10.1016/J.ELECTACTA.2003.10.030
Pandikumar A, Jothivenkatachalam K (2019) Photocatalytic functional materials for environmental remediation, (1st ed)
Singh P, Shandilya P, Raizada P et al (2020) Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab J Chem 13:3498–3520. https://doi.org/10.1016/J.ARABJC.2018.12.001
Mohamed RM, McKinney DL, Sigmund WM (2012) Enhanced nanocatalysts. Mater Sci Eng R Rep 73:1–13. https://doi.org/10.1016/J.MSER.2011.09.001
Balarabe BY, Maity P (2022) Visible light-driven complete photocatalytic oxidation of organic dye by plasmonic Au-TiO 2 nanocatalyst under batch and continuous flow condition. Colloids Surf A Physicochem Eng Asp 655:130247. https://doi.org/10.1016/J.COLSURFA.2022.130247
Yaou Balarabe B, Maity P (2024) A polymer-Au/TiO 2 nano-composite based floating catalyst for photocatalytic dye degradation under natural sunlight. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2023.115405
Yaou Balarabe B (2023) Green synthesis of gold-titania nanoparticles for sustainable ciprofloxacin removal and phytotoxicity evaluation on aquatic plant growth. Hybrid Advances 4:100107. https://doi.org/10.1016/j.hybadv.2023.100107
Balarabe BY, Maity P (2022) Visible light-driven complete photocatalytic oxidation of organic dye by plasmonic Au-TiO 2 nanocatalyst under batch and continuous flow condition. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2022.130247
Balarabe BY, Maity P, Teixeira ACSC, Iwarere SA (2023) h-BN nanosheet-modified Ag 2 WO 4 nanocomposite for improved photocatalytic dye removal: insights into catalyst stability and reusability. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.111560
Yaou Balarabe B, Paria S, Sekou Keita D et al (2022) Enhanced UV-light active α-Bi 2 O 3 nanoparticles for the removal of methyl orange and ciprofloxacin. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2022.110204
Yaou Balarabe B, Irédon A, Hassimi M et al (2023) Effective removal of emerging organic pollutants using hybrid Ag@ZnO supported reduced-graphene oxide nanocomposite under visible light. Hybrid Advances 4:100114. https://doi.org/10.1016/j.hybadv.2023.100114
Sajid MM, Khan SB, Javed Y et al (2021) Bismuth vanadate/MXene (BiVO 4 /Ti 3 C 2 ) heterojunction composite: enhanced interfacial control charge transfer for highly efficient visible light photocatalytic activity. Environ Sci Pollut Res 28(29):35911–35923. https://doi.org/10.1007/s11356-021-13315-9
Gadore V, Mishra SR, Ahmaruzzaman M (2023) Metal sulphides and their heterojunctions for photocatalytic degradation of organic dyes—a comprehensive review. Environ Sci Pollut Res 30(35):90410–90457. https://doi.org/10.1007/s11356-023-28753-w
Dey AK, Mishra SR, Ahmaruzzaman M (2023) Solar light–based advanced oxidation processes for degradation of methylene blue dye using novel Zn-modified CeO 2 @biochar. Environ Sci Pollut Res 30(31):53887–53903. https://doi.org/10.1007/s11356-023-26183-2
Mishra SR, Gadore V, Ahmaruzzaman M (2023) A critical review on In 2 S 3 -based nanomaterial for emerging contaminants elimination through integrated adsorption-degradation technique: effect of reaction parameters and co-existing species. J Hazard Mater Lett. https://doi.org/10.1016/j.hazl.2023.100087
Kalam A, Al-Sehemi AG, Assiri M et al (2018) Modified solvothermal synthesis of cobalt ferrite (CoFe 2 O 4 ) magnetic nanoparticles photocatalysts for degradation of methylene blue with H 2 O 2 /visible light. Results Phys 8:1046–1053. https://doi.org/10.1016/J.RINP.2018.01.045
Liu Z, Feng H, Xue S et al (2018) The triple-component Ag 3 PO 4 -CoFe 2 O 4 -GO synthesis and visible light photocatalytic performance. Appl Surf Sci 458:880–892. https://doi.org/10.1016/J.APSUSC.2018.07.166
Melo RS, Banerjee P, Franco A (2018) Hydrothermal synthesis of nickel doped cobalt ferrite nanoparticles: optical and magnetic properties. J Mater Sci Mater Electron 29:14657–14667. https://doi.org/10.1007/s10854-018-9602-2
Zeng Y, Guo N, Song Y et al (2018) Fabrication of Z-scheme magnetic MoS 2 /CoFe 2 O 4 nanocomposites with highly efficient photocatalytic activity. J Colloid Interface Sci 514:664–674. https://doi.org/10.1016/J.JCIS.2017.12.079
Ghobadifard M, Mohebbi S (2018) Novel nanomagnetic Ag/β-Ag 2 WO 4 /CoFe 2 O 4 as a highly efficient photocatalyst under visible light irradiation. New J Chem 42:9530–9542. https://doi.org/10.1039/c8nj00834e
Li M, Song C, Wu Y et al (2019) Novel Z-scheme visible-light photocatalyst based on CoFe 2 O 4 /BiOBr/Graphene composites for organic dye degradation and Cr(VI) reduction. Appl Surf Sci 478:744–753. https://doi.org/10.1016/j.apsusc.2019.02.017
Emadian SS, Ghorbani M, Bakeri G (2020) Magnetically separable CoFe 2 O 4 /ZrO 2 nanocomposite for the photocatalytic reduction of hexavalent chromium under visible light irradiation. Synth Met. https://doi.org/10.1016/j.synthmet.2020.116470
Ibrahim I, Kaltzoglou A, Athanasekou C et al (2020) Magnetically separable TiO 2 /CoFe 2 O 4 /Ag nanocomposites for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122730
Yaou Balarabe B, Illiassou Oumarou MN, Koroney AS et al (2023) Photo-oxidation of organic dye by Fe 2 O 3 nanoparticles: catalyst, electron acceptor, and polyurethane membrane (PU-Fe 2 O 3 ) effects. J Nanotechnol. https://doi.org/10.1155/2023/1292762
Rackauskas S, Nasibulin AG, Jiang H et al (2009) A novel method for metal oxide nanowire synthesis. Nanotechnology. https://doi.org/10.1088/0957-4484/20/16/165603
Kharat PB, Somvanshi SB, Kounsalye JS, et al (2018) Temperature dependent viscosity of cobalt ferrite/ethylene glycol ferrofluids. In: AIP conference proceedings. American Institute of Physics Inc.
Balarabe BY, Bowmik S, Ghosh A, Maity P (2022) Photocatalytic dye degradation by magnetic XFe 2 O 3 (X: Co, Zn, Cr, Sr, Ni, Cu, Ba, Bi, and Mn) nanocomposites under visible light: a cost efficiency comparison. J Magn Magn Mater 562:169823. https://doi.org/10.1016/J.JMMM.2022.169823
Mishra SR, Roy P, Gadore V, Ahmaruzzaman M (2023) A combined experimental and modeling approach to elucidate the adsorption mechanism for sustainable water treatment via In2S3-anchored chitosan. Sci Rep. https://doi.org/10.1038/s41598-023-45506-4
Yadav N, Yadav G, Ahmaruzzaman M (2023) Fabrication of surface-modified dual waste-derived biochar for biodiesel production by microwave-assisted esterification of oleic acid: optimization, kinetics, and mechanistic studies. Renew Energy 218:119308. https://doi.org/10.1016/J.RENENE.2023.119308
Narsimulu D, Rao BN, Nagaraju G et al (2020) Enhanced energy storage performance of nanocrystalline Sm-doped CoFe 2 O 4 as an effective anode material for Li-ion battery applications. J Solid State Electrochem 24:225–236. https://doi.org/10.1007/s10008-019-04484-2
Singh H, Kumar Sinha A, Gupta SM, et al (2015) Two step solid state synthesis and Synchrotron X-ray characterizations of ceramic Co 3 TeO 6 ; an improper multiferroic two step solid state synthesis and synchrotron X-ray characterizations of ceramic Co 3 TeO 6 ; an improper multiferroic*
Zhang S, Zeng M, Li J et al (2014) Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J Mater Chem A Mater 2:4391–4397. https://doi.org/10.1039/c3ta14604a
Mao J, Zhao B, Zhou J et al (2019) Identification and characteristics of catalytic quad-functions on Au/Anatase TiO 2 . ACS Catal 9:7900–7911. https://doi.org/10.1021/acscatal.9b02090
Rosado G, Valenzuela-Muñiz AM, Miki-Yoshida M, Gómez YV (2020) Facile method to obtain anatase and anatase-brookite nanoparticles (TiO 2 ) with MWCNT towards reducing the bandgap. Diam Relat Mater 109:108015. https://doi.org/10.1016/J.DIAMOND.2020.108015
Yuan Y, Wood SM, He K et al (2015) Atomistic insights into the oriented attachment of tunnel-based oxide nanostructures. ACS Nano 10:539–548. https://doi.org/10.1021/acsnano.5b05535
Samrot AV, Sahithya CS, Jenifer Selvarani A et al (2019) Surface-engineered super-paramagnetic iron oxide nanoparticles for chromium removal. Int J Nanomedicine 14:8105–8119. https://doi.org/10.2147/IJN.S214236
Jegatha Christy A, Umadevi M (2013) Novel combustion method to prepare octahedral NiO nanoparticles and its photocatalytic activity. Mater Res Bull 48:4248–4254. https://doi.org/10.1016/j.materresbull.2013.06.072
Gondal MA, Saleh TA, Drmosh QA (2012) Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl Surf Sci 258:6982–6986. https://doi.org/10.1016/j.apsusc.2012.03.147
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis
Duraiswami D, Revathi S (2007) Photocatalytic degradation of tatrazine dye using TiO 2 catalyst: salt effect and kinetic studies oxidation reaction view project conversion of bio-glycerol into other value added products view project
Torki F, Faghihian H (2017) Photocatalytic activity of NiS, NiO and coupled NiS–NiO for degradation of pharmaceutical pollutant cephalexin under visible light. RSC Adv 7:54651–54661. https://doi.org/10.1039/c7ra09461b
Salman M, Athar M, Farooq U (2015) Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Rev Environ Sci Biotechnol 14:211–228
AL-Othman ZA, Ali R, Naushad M, (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247. https://doi.org/10.1016/J.CEJ.2012.01.048
Deng H, Yang L, Tao G, Dai J (2009) Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—application in methylene blue adsorption from aqueous solution. J Hazard Mater 166:1514–1521. https://doi.org/10.1016/J.JHAZMAT.2008.12.080
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers
Pezoti O, Cazetta AL, Souza IPAF et al (2014) Adsorption studies of methylene blue onto ZnCl 2 -activated carbon produced from buriti shells ( Mauritia flexuosa L.). J Ind Eng Chem 20:4401–4407. https://doi.org/10.1016/J.JIEC.2014.02.007
Mishra SR, Gadore V, Ahmaruzzaman M (2023) Inorganic–organic hybrid quantum dots for AOP-mediated photodegradation of ofloxacin and para-nitrophenol in diverse water matrices. NPJ Clean Water. https://doi.org/10.1038/s41545-023-00291-5
Lv Z, Tan X, Wang C et al (2020) Metal-organic frameworks-derived 3D yolk shell-like structure Ni@carbon as a recyclable catalyst for Cr(VI) reduction. Chem Eng J 389:123428. https://doi.org/10.1016/J.CEJ.2019.123428
Zhang G, Chen D, Li N et al (2018) Preparation of ZnIn 2 S 4 nanosheet-coated CdS nanorod heterostructures for efficient photocatalytic reduction of Cr(VI). Appl Catal B 232:164–174. https://doi.org/10.1016/J.APCATB.2018.03.017
Lara MA, Jaramillo-Páez C, Navío JA et al (2019) Coupling of WO 3 with anatase TiO 2 sample with high 001 facet exposition: effect on the photocatalytic properties. Catal Today 328:142–148. https://doi.org/10.1016/J.CATTOD.2018.11.012
Othman Ali I, Mostafa AG (2015) Photocatalytic reduction of chromate oxyanions on MMnFe 2 O 4 (M=Zn, Cd) nanoparticles. Mater Sci Semicond Process 33:189–198. https://doi.org/10.1016/J.MSSP.2015.01.030
Zhang G, Chen D, Li N et al (2018) SnS 2 /SnO 2 heterostructured nanosheet arrays grown on carbon cloth for efficient photocatalytic reduction of Cr(VI). J Colloid Interface Sci 514:306–315. https://doi.org/10.1016/J.JCIS.2017.12.045
Mishra SR, Gadore V, Ahmaruzzaman M (2023) Insights into persulfate-activated photodegradation of tinidazole and photoreduction of hexavalent chromium through β-In 2 S 3 anchored on Ag-doped fish scale-derived HAp composite quantum dots. J Clean Prod. https://doi.org/10.1016/j.jclepro.2023.139221
Download references
Acknowledgements
We are grateful for the assistance provided by the National Forensic Sciences University. The authors would also like to thank Ms Manka Marycleopha from the School of Forensic Sciences, National Forensic Sciences University, India for the necessary assistance on some of the analysis during the revision of the manuscript.
Open access funding provided by University of Pretoria.
Author information
Authors and affiliations.
Department of Chemistry, School of Science and Technology, Nazarbayev University, 010000, Astana, Kazakhstan
Bachir Yaou Balarabe
School of Engineering and Technology, National Forensic Sciences University, Sector-09, Gandhinagar, 382007, India
Bachir Yaou Balarabe & Primerose Bomokayi
School of Pharmacy, National Forensic Sciences University, Sector-09, Gandhinagar, 382007, India
Irédon Adjama
Department of Chemistry, Faculty of Science and Technology, Abdou Moumouni University, BP 237/10896, Niamey, Niger
Abdoulkadri Ayouba Mahamane
Department of Chemical Engineering, Faculty of Engineering, Built Environment and Information Technology, University of Pretoria, Hatfield, Private Bag X20, Pretoria, 0028, South Africa
Michael Olawale Daramola & Samuel Ayodele Iwarere
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Samuel Ayodele Iwarere .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical approval
There are no human or animal subjects included in this article.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 680 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Yaou Balarabe, B., Bomokayi, P., Adjama, I. et al. Unlocking the magnetic potential of Fe 2 O 3 nanoparticles by single-step synthesis of cobalt-infused nanomaterials for chromium removal. Nanotechnol. Environ. Eng. (2024). https://doi.org/10.1007/s41204-024-00366-9
Download citation
Received : 18 October 2023
Accepted : 15 March 2024
Published : 20 April 2024
DOI : https://doi.org/10.1007/s41204-024-00366-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanoparticles
- Visible light
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
This preliminary synthesis is the first step in systematically analysing the results—but it is only a preliminary analysis (not the endpoint). Possible examples of ways to approach this step are: Describe each of the included studies: summarising the same features for each study and in the same order).
The first is a well-developed research question that gives direction to the synthesis (e.g., meta-analysis, systematic review, meta-study, concept analysis, rapid review, realist synthesis). The second begins as a broad general question that evolves and becomes more refined over the course of the synthesis (e.g., meta-ethnography, scoping ...
Quantitative data synthesis (i.e. meta-analysis) The way the data is extracted from your studies, then synthesised and presented, depends on the type of data being handled. Qualitative data synthesis. In a qualitative systematic review, data can be presented in a number of different ways. A typical procedure in the health sciences is thematic ...
This article is a practical guide to conducting data analysis in general literature reviews. The general literature review is a synthesis and analysis of published research on a relevant clinical issue, and is a common format for academic theses at the bachelor's and master's levels in nursing, physiotherapy, occupational therapy, public health and other related fields.
Abstract. Data analysis and synthesis can be highly engaging because reviewers are constructing answers to their questions. They can see their contribution to knowledge materializing before their eyes. To adhere to the values associated with systematic reviewing, however, authors may need to temper their passion to ensure the analysis is ...
12.2 Statistical synthesis when meta-analysis of effect estimates is not possible. A range of statistical synthesis methods are available, and these may be divided into three categories based on their preferability (Table 12.2.a).Preferable methods are the meta-analysis methods outlined in Chapter 10 and Chapter 11, and are not discussed in detail here.
The chapter concludes with practical tips for checking data before synthesis (Section 9.4). Steps 2.1, 2.2 and 2.5 involve analysis and synthesis of mainly qualitative information about study characteristics. The process used to undertake these steps is rarely described in reviews, yet can require many subjective decisions about the nature and ...
Data analysis and synthesis are a challenging stage of the integrative review process. The description of explicit approaches to guide reviewers through the data analysis stage of an integrative review (IR) is underdeveloped (Whittemore and Knafl 2005).Furthermore, when reviewers look to published IRs for assistance, they often find the data analysis stage is only briefly and/or superficial ...
Quantitative synthesis, or meta-analysis, is often essential for Comparative Effective Reviews (CERs) to provide scientifically rigorous summary information. Quantitative synthesis should be conducted in a transparent and consistent way with methodologies reported explicitly. This guide provides practical recommendations on conducting synthesis. The guide is not meant to be a textbook on meta ...
Qualitative research synthesis is a diverse set of methods for combining the data or the results of multiple studies on a topic to generate new knowledge, theory and applications. Use of qualitative research synthesis is rapidly expanding across disciplines. Aggregative and interpretive models of qualitative research synthesis are defined and ...
Synthesis Methods. Narrative summary: is a summary of the review results when meta-analysis is not possible.Narrative summaries describe the results of the review, but some can take a more interpretive approach in summarising the results. [8] These are known as "evidence statements" and can include the results of quality appraisal and weighting processes and provide the ratings of the studies.
Analysis, Plus Synthesis: Turning Data into Insights. Research outputs that we build around a core insight or truth compel design teams to empathize with users, and thus, to design truly meaningful products and services. Conducting primary user research such as in-depth interviews or field studies can be fairly straightforward, when compared ...
This publication details the data analysis and synthesis process used within two realist evaluation studies of community health interventions taking place across Uganda, Tanzania, and Kenya. Using data from several case studies across all three countries and the data analysis software NVivo, we describe in detail how data were analyzed and ...
Analysis is the first step towards synthesis, which requires not only thinking critically and investigating a topic or source, but combining thoughts and ideas to create new ones. As you synthesize, you will draw inferences and make connections to broader themes and concepts. It's this step that will really help add substance, complexity, and ...
That's where data synthesis comes in. Synthesizing data requires: Bringing various sources and various formats into one platform. Creating a UX research taxonomy to make the data uniform. Drawing insights for action across the business. Deep analysis can take time, but it's a critical aspect of user-centered design and development.
Step 1: Review your research goals and use them as a North Star to focus your analysis. ... After you've conducted research and gleaned rich qualitative and/or quantitative data, you move on to the synthesis phase. You immerse yourself in the data and begin to make sense of it. Similarities turn into clusters, clusters turn into patterns, and ...
This tip sheet will help you complete the Data Analysis, Synthesis, and Interpretation section of the Evaluation Plan and Step 5 of the evaluation process. When justifying the conclusions or results of your project, you need to establish what you measured them against. Setting the level for success (e.g., benchmark) helps your stakeholders ...
U.S. Geological Survey Data Citation Analysis, 2016-2022. In 2022, publication and data linkages were evaluated using two methods in an effort to understand how a data citation workflow has been implemented by the U.S. Geological Survey (USGS) since the 2016 USGS instructional memorandum, Public Access to Results of Federally Funded Research at ...
Technology for Synthesis, Humans for Analysis Another helpful way to think about the distinction between synthesis and analysis is the way in which the work is completed. Today, the level of data that M/CI teams have access to continues to grow rapidly, and shows no signs of letting up.
Data analysis and synthesis can be highly engaging because reviewers are constructing answers to their questions. They can see their contribution to knowledge materializing before their eyes. To ...
On the other hand, synthesis involves combining different elements or ideas to create a new whole or solution. It involves integrating information from various sources, identifying commonalities and differences, and generating new insights or solutions. While analysis is more focused on understanding and deconstructing a problem, synthesis is ...
A qualitative evidence synthesis, or QES, is a type of systematic review that brings together the findings from primary qualitative research in a systematic way. A primary qualitative research study is one that uses a qualitative method of data collection and analysis.
Data analysis and synthesis. Descriptive analysis and narrative synthesis provided descriptions of the patterns and appraised the applicability of included trial eligibility criteria to identify patients likely to benefit from receiving palliative care. We sought to identify and collate eligibility criteria and appraise what patterns of ...
Synthesis is a higher process that creates something new. It is usually done at the end of an entire study or scientific inquiry. 2. Analysis is like the process of deduction wherein a bigger concept is broken down into simpler ideas to gain a better understanding of the entire thing. Author.
Business processes have undergone a significant transformation with the advent of the process-oriented view in organizations. The increasing complexity of business processes and the abundance of event data have driven the development and widespread adoption of process mining techniques. However, the size and noise of event logs pose challenges that require careful analysis.
Evidence on global development programs often remains fragmented by thematic areas of study or regions and populations. Evidence gap maps (EGMs) are the tools that visually highlight where evidence concentrations and gaps exist in a sector or topic area and, in doing so, consolidate knowledge of these programs to inform future investments in research and programming.In the field of health ...
Abstract Jumbo phages, phages with genomes >200 kbp, contain some unique genes for successful reproduction in their bacterial hosts. Due to complex and massive genomes analogous to those of small-celled bacteria, how do jumbo phages complete their life cycle remain largely undefined. In this study, we assembled 668 high-quality jumbo phage genomes from over 15 TB of intestinal metagenomic data ...
Two new Cobalt(II) complexes 12 and 13 have been synthesized from 2-[(E)-(3-acetyl-4-hydroxyphenyl)diazenyl]-4-(2-hydroxyphenyl)thiophene-3-carboxylic acid (11) as a novel ligand. These three new compounds were characterized on the basis of their powder X-Ray Diffraction, UV-Vis, IR, NMR, elemental analysis and MS spectral data. DFT/B3LYP mode of calculations were carried out to determine ...
The global market for peptide synthesis is expected to grow from $90.1 billion in 2023 and projected to reach $157.5 billion by the end of 2028, at a compound annual growth rate (CAGR) of 11.8% ...
The study optimized the chromium removal capacity of Fe 2 O 3 nanoparticles through the infusion of cobalt using a single-step synthesis method. This approach not only enhanced their magnetic properties but also employs less-chemical synthesis techniques, ultimately yielding highly magnetic CoFe 2 O 4 nanoparticles and less impurities. The prepared materials underwent comprehensive testing ...