- Scoping Review
- Open access
- Published: 14 November 2021

Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
58k Accesses
214 Citations
369 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
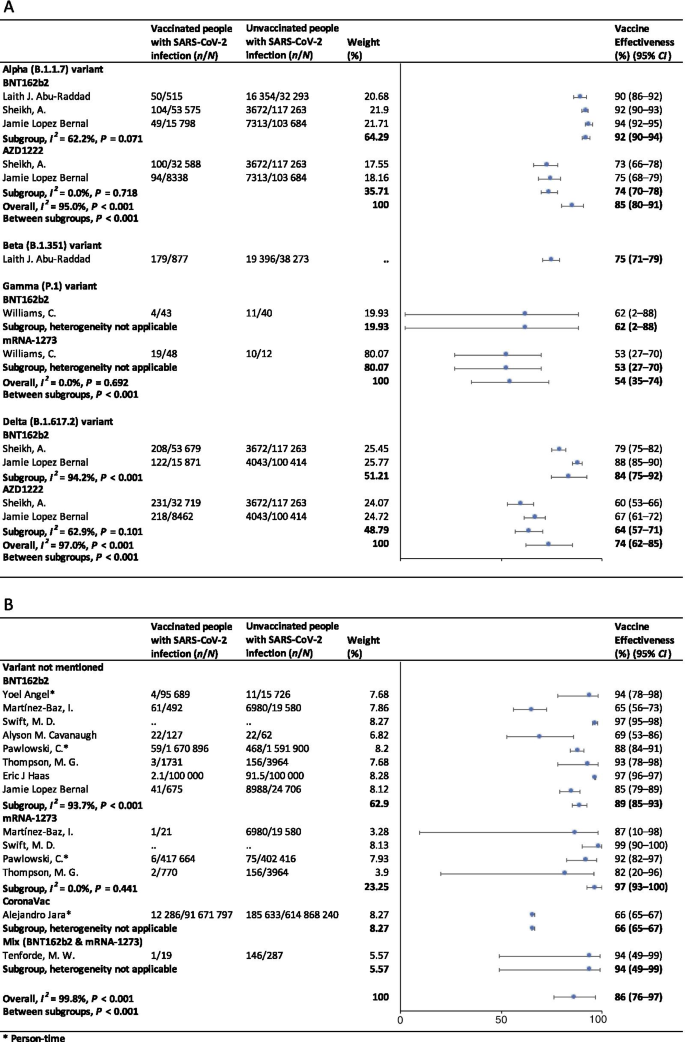
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 June 2024
Effectiveness of COVID-19 vaccines against severe COVID-19 among patients with cancer in Catalonia, Spain
- Felippe Lazar Neto ORCID: orcid.org/0000-0002-0051-9537 1 , 2 ,
- Núria Mercadé-Besora 3 ,
- Berta Raventós 3 , 4 ,
- Laura Pérez-Crespo 3 ,
- Gilberto Castro Junior ORCID: orcid.org/0000-0001-8765-3044 2 ,
- Otavio T. Ranzani ORCID: orcid.org/0000-0002-4677-6862 1 , 5 na1 &
- Talita Duarte-Salles ORCID: orcid.org/0000-0002-8274-0357 3 , 6 na1
Nature Communications volume 15 , Article number: 5088 ( 2024 ) Cite this article
139 Accesses
49 Altmetric
Metrics details
- Epidemiology
Patients with cancer were excluded from pivotal randomized clinical trials of COVID-19 vaccine products, and available observational evidence on vaccine effectiveness (VE) focused mostly on mild, and not severe COVID-19, which is the ultimate goal of vaccination for high-risk groups. Here, using primary care electronic health records from Catalonia, Spain (SIDIAP), we built two large cohorts of vaccinated and matched control cancer patients with a primary vaccination scheme ( n = 184,744) and a booster ( n = 108,534). Most patients received a mRNA-based product in primary (76.2%) and booster vaccination (99.9%). Patients had 51.8% (95% CI 40.3%−61.1%) and 58.4% (95% CI 29.3%−75.5%) protection against COVID-19 hospitalization and COVID-19 death respectively after full vaccination (two-doses) and 77.9% (95% CI 69.2%−84.2%) and 80.2% (95% CI 63.0%−89.4%) after booster. Compared to primary vaccination, the booster dose provided higher peak protection during follow-up. Calibration of VE estimates with negative outcomes, and sensitivity analyses with slight different population and COVID-19 outcomes definitions provided similar results. Our results confirm the role of primary and booster COVID-19 vaccination in preventing COVID-19 severe events in patients with cancer and highlight the need for the additional dose in this population.
Similar content being viewed by others

Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes
Effectiveness of inactivated COVID-19 vaccines among older adults in Shanghai: retrospective cohort study

COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials
Introduction.
The coronavirus disease 2019 (COVID-19) has caused millions of deaths worldwide since the first reported suspected case in Wuhan, China, in late December 2019 1 . Even though most patients will report mild symptoms, nearly 5% will present the severe form of the disease, requiring intensive support 2 . Vulnerable groups at increased risk of severe illness include patients of older age, nursing home facility residents, and those with severe comorbidities, particularly cancer 3 . Compared to healthier individuals, patients with cancer have an increased risk of death following infection, with an additional incremental risk among those with lung cancer, hematological cancer, or under systemic oncological treatment 4 , 5 , 6 . For instance, in patients with lung cancer, COVID-19-associated mortality was two times higher than in patients without cancer 7 .
Randomized clinical trials of COVID-19 vaccines have shown high efficacy and safety in preventing severe outcomes 8 , 9 , 10 ; however, these trials targeted the general population and included only patients with pre-existing, stable cancers 11 , limiting the generalizability of these results to patients with active cancer. Prospective data on immunogenicity following initial vaccination have shown that patients with cancer develop protective antibodies, but in a lower proportion when compared to the general population. This has been observed particularly after the administration of only one vaccine dose among patients with hematological neoplasms, and those undergoing cytotoxic treatments 12 , 13 , 14 , 15 , 16 , 17 . Booster dose administration can elicit strong and durable immune responses in approximately 50% of the patients who were seronegative after the first dose 18 , 19 , 20 .
Small retrospective, real-world studies focused on patients with cancer have found low rates of COVID-19 infection following vaccination, and decreased risk of severe disease after infection 21 , 22 , 23 , 24 , 25 , 26 . Cohorts including only vaccinated people have shown that patients with cancer are more susceptible to breakthrough infections compared to healthy individuals 5 , 27 , 28 , particularly those with only one dose 27 , 28 . A UK population-level study indicates a reduced temporal cancer COVID-19 fatality rate after vaccination, though still higher than in healthy individuals 29 . Few studies estimated vaccine effectiveness among patients with cancer 30 , 31 , 32 , 33 , 34 and were either focused on COVID-19 infection 31 , 32 , 35 or included cancer as a subgroup of comorbidities 33 , 34 . Only a minority of them estimated booster vaccine effectiveness 32 , 33 . Although there is promising evidence on COVID-19 vaccine effectiveness in the general population, we still lack evidence for cancer patients, particularly for booster doses and severe disease.
The combination of large amounts of population-level data and the causal inference framework of target trials 36 can provide valuable opportunities to assess the real-world effectiveness of medical interventions 37 when randomized data is not available. We aim to investigate COVID-19 vaccine effectiveness against severe COVID-19 outcomes among adults with cancer living in Catalonia, evaluating the vaccine effectiveness (VE) of primary schemes against unvaccinated individuals and the relative VE (rVE) of the booster dose compared with two doses.
Vaccine uptake
Of 171,284 patients with cancer diagnosis excluding non-melanoma skin cancer between 27th December 2015 and 27th December 2020, 111,576 patients remained after excluding those that died (any cause, N = 41461), moved out from SIDIAP area (N = 3575), had previous COVID-19 (N = 11992), or were nursing home residents (N = 2680) before the beginning of the vaccination campaign. The proportion that received one, two, and three (booster) doses of COVID-19 vaccines were 87.2%, 84.9%, and 68.2%, respectively. Among vaccinated patients, nearly 76% received an initial two-dose mRNA-based vaccination scheme compared to 15% that received two doses of ChAdOX1 and 3.2% that received Ad26.COV2.S as the first dose. The booster dose was composed of almost only mRNA vaccines (76% mRNA-1273 and 24% mRNA-BNT-162b). Suppl. Figure 1 shows the number of COVID-19 cases (Suppl. Figure 1A ), the predominant variant of concern (VoC) during each period (Suppl. Figure 1B ), and the cumulative vaccine rollout (Suppl. Figure 1C ). Suppl. Figure 2 shows the product types and doses of vaccines administered by age groups. The ChAdOX1 vaccine scheme was predominantly administered in adult patients aged 69 years or lower.
Baseline cohort characteristics
We built two matched cohorts: 184,744 patients (92,372 matched pairs) were included in the first and second dose (primary) vaccination cohort (Cohort A, Suppl. Figure 3 ) and 108,534 (54,267 matched pairs) in the booster vaccination cohort (Cohort B, Suppl. Figure 4 ). Compared to un-matched but eligible patients, matched patients had lower proportions of very old (>80 years) and younger (<50 years) patients, a higher proportion of recently diagnosed patients, fewer comorbidities as per the Charlson Comorbidity Index, fewer diagnoses of metastatic disease, and a higher number of outpatient visits (Suppl. Table 1 and Supp. Table 2 ).
Matched patients in both cohorts (A and B) had well-balanced characteristics between vaccinated and control groups (Table 1 ). The majority of patients were older (greater than 60 years old), with a similar proportion of males and females. As expected, the majority of vaccinated individuals in Cohort A received a mRNA-based combination scheme. Among those in Cohort B, approximately 24% previously received the ChAdOx1 combination scheme. The most prevalent cancer diagnosis was breast, followed by prostate and colorectal cancers. 14% and 11% of patients had metastatic disease in Cohort A and B, respectively.
Vaccine effectiveness
Figure 1 shows the cumulative incidence for the primary outcome of COVID-19-associated hospitalization, and Fig. 2 and Fig. 3 shows the estimated VE for each time period for the primary vaccination (Cohort A) and booster vaccination (Cohort B) cohorts respectively. For the primary vaccination, the estimated VE against COVID-19 hospitalization for the first (partially vaccinated) and second dose (fully vaccinated) was 42.0% (95%CI 22.3 - 56.7) and 51.8% (95%CI 40.3 - 61.1) respectively. When expanding the time periods, we observed an increase in VEs, particularly after the second dose, which peaked after 60 days (58.4%, 95%CI 34.5 - 73.6) but waned after 120 days (-19.7%, 95%CI -52.7 - 26.6). For the booster vaccine, we found high rVE (> 75%) already in the immediate period post-vaccination, which remained high until 120 days, when a decrease in rVE was observed. Visual inspection of cumulative incidence graphs during the initial period of 0 to 14 days after vaccination (Suppl. Figure 5 ) shows low residual confounding for both cohorts. Results for the secondary outcomes COVID-19 severe hospitalization, and COVID-19 deaths showed similar results (Fig. 2 and Fig. 3 ). Competing hazards model (all-cause death as competing risk) showed comparable results (Suppl. Table 3 ).

Cumulative incidence of COVID-19 hospitalizations (primary outcome) for the primary vaccination (Figure A ) and booster vaccination (Figure B ) cohorts between vaccinated and control groups. The solid lines represent the estimated cumulative hazards, while the shaded areas indicate the 95% confidence intervals.

Forest plot of estimated vaccine effectiveness (point estimate) and its 95% confidence interval (error bars) since the time from vaccination for the initial vaccination scheme (Cohort A). Here we show vaccine effectiveness for the primary (hospitalization) and secondary endpoints (severe hospitalization, and death) separately. VE = Vaccine Effectiveness.

Forest plot of estimated COVID-19 relative vaccine effectiveness (point estimate) and its 95% confidence interval (error bars) since the time from vaccination for the booster vaccination (Cohort B). Here we show vaccine effectiveness for the primary (hospitalization) and secondary endpoints (severe hospitalization, and death) separately. rVE = Relative Vaccine Effectiveness. Counts below five have been masked to protect patients’ privacy.
Subgroup analysis
Figure 4 shows the results for subgroup effect modification analysis for both cohorts. For the primary vaccination scheme (Cohort A), subgroup analysis has found lower VE after full vaccination for the elderly (33.2% vs 74.7%, p < 0.001, Suppl. Figure 6 ) and metastatic patients (24.0% vs 59.6%, p = 0.025), and no effectiveness during the Delta VoC. For the booster (Cohort B), we found higher VE after 14 days for older (82.2% vs 49.3%, p = 0.028, Suppl. Figure 7 ), and male patients (81.4% vs 69.8%, p = 0.006). We found a numerical increase in rVE for those who previously did not receive the mRNA-based vaccination scheme (between 14 and 60 days 80.5% vs 77.4%, after 60 days 73.7% vs 39.4%, p = 0.155). We did not find any effect modification by a diagnosis of hematological malignancy and a lower vaccine effectiveness during the Omicron period for the booster dose compared with the Delta period.

Forest plot of estimated COVID-19 vaccine effectiveness (point estimate) and its 95% confidence interval (error-bars) among subgroups for the primary vaccination (Cohort A) and booster vaccination (Cohort B). The detailed number of events, observations and precise confidence interval estimates for each sub-group are shown in Suppl. Figures 6 and 7 .
Negative Outcomes Calibration
Negative outcomes estimands for each cohort and period are shown in Suppl. Figures 8 and 9. After adjustment for negative outcomes, VE against COVID-19 hospitalization for the primary vaccination scheme was 42.7% (95% CI 11.2 - 63.0) for partially vaccinated and 58.2% (95% CI 43.8 - 68.9) for fully vaccinated individuals. For the booster dose, calibrated VE was 73.5% (95%CI 61.3 - 81.8) during the 14 - 60 days period and 50.8% (95% CI -1.2 - 76.0) after 60 days (Suppl. Table 4 ). Sensitivity analysis with an expanded set of negative outcomes provided similar results (Suppl. Table 4 ).
Non-COVID outcomes
Vaccination was associated with decreased hazards of all-cause hospitalizations and non-COVID deaths in the immediate period post-vaccination for both cohorts (Suppl. Tables 5 and 6 ). For Cohort A, primary vaccination was associated with a non-significant decrease in all-cause hospitalizations during follow-up but a sustained decreased hazard for non-COVID death (Suppl. Table 5 ). For Cohort B, booster vaccination was associated with a lower risk of all-cause hospitalizations and non-COVID death in all time periods (Suppl. Table 6 ). We observed a higher proportion of non-COVID-19 deaths without preceding hospitalization than COVID-19 deaths (Suppl. Table 7 ). Analysis of health services usage (outpatient, telehealth, home, inpatient, and ICU visits) by vaccination status showed lower likelihood in the vaccination group for most outcomes in cohort B, but not for Cohort A; which showed increased hazards for tele-health, home and outpatient visits and lower hazards for inpatient and ICU visits (Suppl. Table 8 ).
Sensitivity analysis
Sensitivity analysis including previous influenza vaccine receipt in matching and excluding those hospitalized a month prior to vaccination in the primary and booster cohorts (restricted matching cohort, Suppl. Tables 9 and 10 ) reduced the vaccine protection for all-cause hospitalizations, but not non-COVID death, which remained lower in the vaccinated group (Suppl.Tables 11 and 12 , Suppl. Figures 10 and 11 ). The COVID-19 primary outcome had comparable results (Suppl. Table 13 ).
Additional sensitivity analyses with different definitions for the cancer cohort and COVID-19 outcomes showed little deviations from the original results (Suppl. Figures 12 and 13 ).
In this matched cohort study, we found that the two-dose primary vaccination scheme effectively reduced COVID-19 hospitalization and mortality outcomes in individuals with cancer, and that administering a booster dose provided substantial and meaningful additional protection for those who had already received the initial two-dose vaccination scheme. We found that the booster dose provided higher peak effectiveness over time.
Two test-negative case-control studies using a network of hospitals across nine states in the United States 34 and linked cancer registry with surveillance data in the United Kingdom (UK) 31 have estimated the initial two-dose VE for hospitalization among patients with cancer as 79% (95% CI, 73-84%) and 84.5% (95% CI, 83.6-85.4) respectively, which is slightly higher than what we found in our study: 52% after the second dose. Regarding the first booster, another study from the same group in the UK 32 showed a VE of 80.5% (95% CI, 77.3-83.2) against hospitalization when comparing booster vs. unvaccinated population, which is not straightforward to compare with our estimate of relative effectiveness (i.e., booster compared with primary vaccination scheme protection). There are several possible explanations for the observed differences, including characteristics both at the individual level, such as different population distribution on age, sex, income, cancer staging, healthy-seeking behavior, vaccine type, and time of follow-up, and at the local level, such as pandemic period and predominant VoC during effectiveness evaluation.
Although previous investigations 30 , 31 , 32 have indicated decreased VE for COVID-19 infection among patients with a hematological neoplasm or within one year of initial diagnosis, we did not find decreased effectiveness for severe COVID-19 for either of these subgroups. This is likely explained by the different outcomes we investigated: severe disease (COVID-19 hospitalization or death), instead of mild COVID-19 infection. For example, patients with lymphoma in the UK cohort had a 10.5% reduction in breakthrough infections following booster dose but a 80% reduction in COVID-19 death 32 . The lack of subgroup analysis for severe outcomes in previous studies limits further comparisons 30 , 31 , 32 .
Older patients (≥ 65 years) had lower VE following the initial scheme, as previously described for the general population 38 , 39 . Interestingly, booster vaccination among this subgroup provided additional significant protection, better explained by the probable different baseline risk following initial vaccination and the higher relative VE estimated. Serological studies have shown that approximately half of seronegative oncological patients can have seroconversion following a booster administration. We found a similar effect for sex: male patients had possibly lower VE following the initial scheme and higher after the booster. Past studies have shown that among vaccinated individuals, male patients may still have a higher risk of severe COVID-19 outcomes 27 , 40 , and serological studies suggest a higher waning effect compared to females 27 , 41 , which can help explain baseline risk differences and different relative effectiveness after booster vaccination.
We found a waning effect for the initial two-dose vaccination scheme but a higher peak effectiveness and more sustained protection against COVID-19 death for the booster dose (> 70% two months after booster). In addition, we have shown that the booster dose was likely effective during the Omicron period (rVE 29.3% 95%CI -1.4 - 50.7), in line with previous serological studies in the general population showing maintained protection against Omicron for mRNA vaccines 42 . This is one of the first studies to assess waning in both schemes (two-dose initial scheme and booster) and evaluate the impact of the VoC period on effectiveness in patients with cancer. Although results are promising, they should be interpreted having in mind the potential bias introduced by susceptibles depletion 43 and undocumented infections. We found an increased numerical benefit for those who received a mRNA booster after a two-dose ChAdOX1 homologous initial scheme. Previous evidence suggests that heterologous schemes may provide additional protection compared to homologous schemes 44 . Because of the restricted range of ages (mostly ≤ 69 years) that received initial ChAdOX1 vaccination, results might be not generalizable to all adults with cancer, particularly the older ones that did not receive ChAdOX1.
Estimation of VE from observational studies 45 is challenging as vaccinated patients are often healthier (healthy bias) and more health conscious (health-seeking bias) than unvaccinated individuals. Although proper matching on relevant covariates may provide well-balanced characteristics between groups, it is often insufficient to adjust for unmeasured confounders. Previous research on COVID-19 vaccine effectiveness has shown that, despite proper matching, vaccinated patients had a lower risk of non-COVID death compared to control 45 , 46 , 47 , partially explained by healthier conditions of vaccinated patients. In our study, we showed a lower risk of non-COVID deaths among vaccinated patients but a much lower magnitude for all-cause hospitalizations. Further adjustment for previous influenza receipt and exclusion of recently hospitalized patients, variables that would capture part of the healthy and health-seeking biases, improved all cause-hospitalization differences between groups, but not non-COVID death. We hypothesize two other reasons for this finding. First, the principal difference between vaccinated and control ones occurred in the immediate period after the vaccine (Suppl. Table 5 and 6 ), a finding observed by other studies and improbable to be related to the biological action of vaccines. This is likely because the most ill or frail individuals ended up dying early on after matching, and in a clinical trial, they would likely not be eligible to be randomized. Second, part of the non-COVID-19 deaths could be actually COVID-19 deaths, leading to misclassification of the outcome. In the analyzed population, there is a high proportion of non-COVID-19 deaths that occurred without hospitalization (60% for Cohort A and 71% for Cohort B), which is associated with a great network of home and palliative care in the region, making us hypothesize that these individuals are unlikely to get tested (and consequently being diagnosed) 48 . Finally, socio-economic variables in large databases can not capture all socio-economic nuances and wealthy bias is a concern. The comparable risk in all-cause hospitalizations and similar effectiveness after negative outcomes calibration and across the sensitivity analyses result in confidence in the vaccine protection observed. However, the magnitude and direction of the potential bias is uncertain.
This study has many strengths. We included a large number of patients in each assessment - 184,744 patients for the two-dose initial vaccination and 108,534 patients for the booster analysis. In addition, cancer diagnosis had been previously validated in the SIDIAP database with good agreement with population-based cancer registries in Catalonia 49 providing a quality assessment of our population of interest. We designed an observational study with robust methodology including a target-trial framework with rolling entry matching on a daily basis with adjustment for all potential observable confounders and similar baseline characteristics between groups. This methodology has already been validated in similar scenarios of vaccine effectiveness 38 , 50 with valid estimates of effectiveness. We calibrated our estimates with negative control outcomes that are highly improbable to be associated with our exposure of interest, addressing unobservable residual confounding which is a major issue in large population studies. Finally, we provided a comprehensive analysis of our findings, presenting sensitivity analyses with different outcomes and cohort definitions, including non-COVID outcomes, and creating additional cohorts matched on previous influenza receipt to investigate for potential healthy-vaccine bias.
The main limitation of this study is its observational design. Although large observational databases with adequate methodology may duplicate the results of randomized clinical trials 37 , residual confounding cannot be excluded. However, we tried to minimize the chances of confounding by including variables associated with health-seeking behaviors (number of outpatient visits) and socioeconomic factors (the MEDEA deprivation index) in addition to calibrating for negative control outcomes. Visual inspection of bias indicators (i.e., during the initial days following vaccination) 51 , 52 showed a low risk of bias, and negative control adjustment showed similar results. Although the selection of negative outcomes might be debatable, we have chosen negative outcomes previously validated in very similar settings 53 . However, the decreased hazards of non-COVID death, particularly in the immediate period post-vaccination, indicates residual healthy bias. We attempt to reduce this bias by matching new cohorts on previous influenza vaccine receipt, excluding frail patients (patients hospitalized a month prior), and adjusting variables that could capture healthy-seeking behavior, but lower hazards for non-COVID death persisted, and our results should be interpreted in light of these findings. It is possible that the VE and rVE estimates are overestimated because of this residual healthy bias, particularly in the immediate period post-vaccination when most non-COVID deaths were observed. We defined COVID-19 outcomes based on the temporal association of a positive diagnosis and the outcome (hospitalization and death) as causes for hospitalization and death were unavailable; however, sensitivity analysis with different definitions provided similar results. Another limitation is the lack of data granularity regarding cancer staging and treatment (including chemotherapy and radiation therapy, among others), which limits our capability of answering questions regarding the timing and type of treatment provided. To overcome this limitation, we used time from cancer diagnosis as a surrogate for cancer treatment receipt, as patients with recent diagnoses may have higher chances of being under treatment. Additionally, during the COVID-19 pandemic, patients at higher risk of severe outcomes might have taken additional measures to prevent infection, such as avoiding gatherings or using face shields which are not captured by data and may influence results 54 . Lastly, during the Omicron wave, we could not differentiate between patients who have been hospitalized with COVID-19 and not because of COVID-19. However, this is even harder to ascertain in patients with cancer. We included a sensitivity analysis with COVID-19 diagnosis up to 3 days after admission with similar results, showing that the time of COVID-19 infection (pre-admission vs during admission) did not change outcomes.
In conclusion, we found a significant protective effect for both the primary and booster vaccination schemes on hospitalization and mortality outcomes among patients with cancer. The booster protective effect was high and more durable, particularly against COVID-19 death. Patients should be encouraged to get vaccinated if not and boosted if they have had only two doses. Because of the higher risk of breakthrough infections, hospitalizations, and death compared to healthy individuals, patients with cancer should be prioritized in future additional dose studies and vaccination campaigns.
Study design, settings, and data source
We conducted a matched population-based cohort study using the Information System for Research in Primary Care (SIDIAP; www.sidiap.org ) database 55 . SIDIAP is a primary care longitudinal database from Catalonia, Spain, which contains pseudo-anonymized individual-level patient data since 2006, with 5.8 million people active in June 2021 (75% of the Catalan population). The present study was conducted using data from December 27th, 2020 to June 30th, 2022. The SIDIAP database includes clinical diagnosis, lifestyle information, and dispensed medications in primary care, including COVID-19 vaccine products, linked to both the SARS-CoV-2 polymerase chain reaction (RT-PCR) and rapid antigen tests results database and hospital records database. The SIDIAP has been mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM), allowing the reproducibility of study definitions across a wide range of mapped databases 56 , 57 . The current work was approved by the Clinical Research Ethics Committee of IDIAPJGol (project code 23/023-EOm).
The Spanish national COVID-19 vaccination campaign was launched on 27th December 2020. Because of the initially limited availability of vaccines, groups considered at higher risk were prioritized, including healthcare workers, nursing home residents, and older subjects 58 . According to national guidelines, patients with cancer under active treatments or other serious immunosuppressive comorbidities were prioritized before the general population but only after older patients. Vaccine products used for the national COVID-19 vaccination campaign were progressively extended as new authorizations were granted and included: BNT162b2 ( Pfizer , mRNA, two-dose, 21-day interval), ChAdOx1 ( AstraZeneca , adenovirus, two-dose, 3-month interval), mRNA-1273 ( Moderna , mRNA, two-dose, 28-days interval), and Ad26.COV2.S ( Janssen , adenovirus, single-dose). People who were vaccinated with Ad26.COV2.S were later recommended for an additional second dose because of lower expected vaccine effectiveness at that time 59 .
Study population, exposure definitions, and outcomes
Eligible individuals were adults aged 18 or older with at least one year of prior observation, with a record of cancer diagnosis (excluding non-melanoma skin cancer) within the last 5 years prior to the index date, which represents the date at which individuals were eligible to enter the matching pool. We excluded patients with any previous diagnosis of COVID-19, defined as a combination of either clinical or laboratory diagnoses of COVID-19 prior to the index date. Patients who were transferred out of SIDIAP before matching and those living in nursing homes were also excluded.
The exposure of interest in this study was COVID-19 vaccination defined as the receipt of any COVID-19 vaccine among all available vaccine products at the time (BNT162b2, mRNA-1273, ChAdOx1, and Ad26.COV2.S). Overall vaccine uptake was described including all eligible patients at the beginning of the vaccination campaign on 27th December 2020.
We built two matched cohorts: Cohort A to evaluate the VE of the first and second doses (primary vaccination) compared with unvaccinated individuals; and Cohort B to evaluate the relative VE of the booster compared with two doses. Cohort A included all adults eligible for primary vaccination with a cancer diagnosis up to five years before the first dose vaccination date. Cohort B, a subset of Cohort A, included only patients that previously received homologous vaccine schemes with BNT162b2, mRNA-1273, and ChAdOx1, with adequate interval between the second and third dose (a minimum of 90 days intervals for ChAdOx1 and 180 days for BNT162b2 and mRNA-1273). For Cohort B, patients had to have a cancer diagnosis before the first dose vaccination date up to five years from the booster vaccination date to ensure the patient had a cancer diagnosis at the time of the primary vaccination.
The index date was set to the first dose date for vaccinated individuals in Cohort A and the booster date for exposed individuals in Cohort B. The Index date for patients in the control groups was set as the index date of their matched counterparts. The study end date for Cohort A was on 20 th November 2021 (one month before the Omicron VoC predominance) and for Cohort B at the last available information date (30 th June 2022)
Predominant variants of concern (VoC) (Delta, Omicron, and others) at each time period were defined as ≥ 50% of weekly tested samples in Catalonia, extracted from the Global Initiative on Sharing All Influenza Data (GISAID) 60 .
The primary outcome of this study was COVID-19 hospitalization, and secondary outcomes included COVID-19 severe hospitalization - defined as a COVID-19 hospitalization with the need for invasive oxygen supplementation, and COVID-19 death. Consistent with prior research on severe COVID-19 61 , 62 , we defined COVID-19 hospitalization as any hospital admission within 21 days from the COVID-19 diagnosis up to the entire duration of hospitalization. Likewise, death was classified as any cause of death occurring within 28 days from the date of COVID-19 diagnosis. In addition to COVID-19-associated outcomes, we report all-cause hospitalizations and non-COVID deaths. A complete list of variables, exposures and outcome definitions can be found in Suppl. Tables 14 , 15 and 16 . The decision to set COVID-19 hospitalization as primary outcome (in contrast with the composite outcome of COVID-19 hospitalization and/or death) and evaluate COVID-19 severe hospitalization, all-cause hospitalizations and non-COVID-19 deaths was made post-hoc during peer review.
Statistical analysis
We emulated a pragmatic target trial of COVID-19 vaccination among patients with cancer within the SIDIAP database using rolling entry matching (REM) 45 , 63 , on a daily basis ( Suppl. Figure 14 ) . For Cohort A, eligible patients upon their first dose date were matched in a 1:1 ratio to eligible un-vaccinated individuals. For Cohort B, individuals with a completed primary scheme upon their booster dose date were matched in a 1:1 ratio to individuals with a completed primary scheme and eligible to receive a booster dose. Matching was performed by combining exact and caliper matching. Age (bins of five years), sex, cancer diagnosis time (categories zero to five years), the municipality of residence, the MEDEA deprivation index 64 (a proxy for deprivation based on place of residence from Q1 [least deprived] to Q5 [most deprived]), the number of outpatient visits in the previous year (as a proxy for healthy seeking behavior), the Charlson Comorbidity Index and metastatic disease were used to build a propensity score with a logistic regression model, which was used for matching in a caliper of 0.01 while ensuring exact matching for age, sex, cancer diagnosis time and the municipality of residence. For Cohort B, the exact matching also included the previous vaccination scheme (i.e., first and second dose product). Matching variables were chosen based on their potential association with receiving the vaccine (exposure of interest) and the risk of severe COVID-19 (outcome of interest).
After matching, patients were followed until an outcome event of interest occurred, death, or were censored at the last follow-up date. In case the control-matched patient was vaccinated, the matched-pair was censored at the date of control vaccination. Patients who had COVID-19 infection but were not hospitalized nor died were censored 28 days after the date of infection to account for a window of susceptibility to events of interest. The censoring of those who had COVID-19 without an event of interest was based on the following rationale: we are evaluating the first COVID-19 infection-associated event, and those with a previous infection would have low susceptibility to subsequent infections and consequently low risk of the outcome (i.e., an individual is still at risk during its first COVID-19 infection and subsequent window of susceptibility to the event, but this risk decreases if no event occurs and immunity is created).
Baseline covariable balance was evaluated by standardized mean differences (SMD) and descriptive characteristics between groups. Continuous variables were described with mean, median, standard deviation (SD), or interquartile range depending on variable distribution, and categorical variables with absolute numbers and relative proportions.
We calculated and plotted the cumulative incidence between groups with the Kaplan-Meyer estimator. VE was estimated using a Cox Proportional hazards model in the matched cohorts as 1 minus the hazard ratio (HR) between groups. HR and 95% confidence intervals (CI) were calculated in different periods of time (time-stratified Cox model) since vaccination (day zero) to investigate the long-term effectiveness and waning effect. Our primary analysis estimated the VE for the period after 14 days of the first dose (partially vaccinated), after 7 days of the second dose (fully vaccinated), and relative VE 14 days and 60 days after the third dose. Potential vaccine waning was evaluated with a larger number of period breaks from vaccination until 120 days or more thereafter (all periods). All Cox models accounted for the competing risk of death under the framework of cause-specific competing risks, more suitable for etiological research questions 65 , 66 , 67 , 68 . In a post-hoc decision during the peer review, we also estimated the VE using the Fine-Gray models considering the competing risk of death, deriving sub-distribution HRs for the main analysis, an approach more suitable for prediction and prognostic, to complement the cause-specific evaluation 65 , 69 . The competing event was all-cause death for the COVID-19 hospitalization outcome.
We investigated residual confounding by visually inspecting the cumulative incidence and estimated VE differences in the immediate (0 - 14 days) post-vaccination period when no protection is expected 52 . In addition, residual confounding could remain after matching due to unobserved confounding variables, particularly ones associated with wealthy-healthy bias that may affect results. Thus, we performed a negative control outcomes (NCO) analysis 70 with a previously published list of 43 validated outcomes that were highly improbable to be associated with our exposure of interest (COVID-19 vaccination) 53 . In addition to these previous validated negative outcomes, we performed an additional analysis including 11 additional outcomes in the negative outcomes set ( Suppl. Table 17 ) . Results from NCO were used to empirically calibrate our estimates with the EmpiricalCalibration package in R 53 , 71 . Additionally, we report estimates of health-services-seeking behavior, including outpatient visits, telehealth visits, home visits, and inpatient and ICU visits after vaccination. The decision to evaluate these other negative control outcomes (expanded set of negative outcomes and health-services-seeking behavior) was made post-hoc during peer review.
Subgroup effect modification of the primary outcome was defined based on previous knowledge of possible effect modifiers in this population and included: age (<65 years old vs ≥ 65 years old), sex (male vs female), cancer diagnosis time (1 year vs 1-5 years), metastatic disease (yes vs no), lung cancer diagnosis (yes vs no), hematological cancer diagnosis (yes vs no), and COVID-19 VoC period (other vs delta vs omicron). For Cohort B (booster vaccine), we also investigate the effect of a previous mRNA vaccine scheme (yes vs no) as a subgroup. Subgroup analyses were evaluated with an interaction term between the vaccine and the subgroup of interest.
Sensitivity analyses were performed and included: stricter cancer definition, only tested patients (any test during the whole period), only RT-PCR COVID-19 diagnosis, and modified COVID-19 hospitalization outcome to any COVID-19 diagnosis from 21 days before admission to 3 days after, and from 14 days before admission to 3 days after, to exclude potential hospital-acquired COVID-19 infections and non-COVID-19 directly related hospitalizations respectively. Finally, to better address health bias, we built two additional matched cohorts for both exposures (primary vaccination and booster vaccination), including previous influenza vaccine receipt as exact matching, excluding patients hospitalized a month prior to the matching date, and propensity matching on a number of outpatient visits as numerical and not categorical variable ( restricted matching cohorts). We compared COVID-19 and non-COVID-19 outcomes between matched cohorts (original versus restricted matching cohorts). The decision to evaluate different matching was made post-hoc during peer review.
We report 95% CI for all estimates. A p-value less than 5% was considered statistically significant. We performed all analyses in R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
In accordance with the current European and national law, the data used in this study are only available for the researchers participating in this study. Thus, we are not allowed to distribute or make publicly available the data to other parties. However, researchers from public institutions can request data from SIDIAP if they comply with certain requirements. Further information is available online ( https://www.sidiap.org/index.php/menu-solicitudesen/application-proccedure ) or by contacting SIDIAP ( [email protected] ).
Code availability
R scripts were made available to ensure the reproducibility of results and in accordance with good research practice ( https://github.com/felippelazar/SIDIAP-CovidVaccineCancer/ ) 72 .
Zhu, N. et al. A novel coronavirus from patients with pneumonia in china, 2019. N. Engl. J. Med. 382 , 727–733 (2020).
Article CAS PubMed PubMed Central Google Scholar
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323 , 1239–1242 (2020).
Article CAS PubMed Google Scholar
Booth, A. et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One 16 , e0247461 (2021).
Khoury, E. et al. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 5 , e2210880 (2022).
Article PubMed PubMed Central Google Scholar
Salvatore, M. et al. COVID-19 Outcomes by cancer status, site, treatment, and vaccination. Cancer Epidemiol. Biomark. Prev. 32 , 748–759 (2023).
Article Google Scholar
Roel, E. et al. Cancer and the risk of coronavirus disease 2019 diagnosis, hospitalisation and death: a population-based multistate cohort study including 4 618 377 adults in Catalonia, Spain. Int. J. Cancer 150 , 782–794 (2022).
Oldani, S. et al. COVID-19 and lung cancer survival: an updated systematic review and meta-analysis. Cancers 14 , 5706 (2022).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384 , 403–416 (2021).
Falsey, A. R. et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 385 , 2348–2360 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Thomas, S. J. et al. Efficacy and safety of the BNT162b2 mRNA COVID-19 vaccine in participants with a history of cancer: subgroup analysis of a global phase 3 randomized clinical trial. Vaccine 40 , 1483–1492 (2022).
Tran, S., Truong, T. H. & Narendran, A. Evaluation of COVID-19 vaccine response in patients with cancer: An interim analysis. Eur. J. Cancer 159 , 259–274 (2021).
Zeng, C. et al . Impaired neutralizing antibody response to COVID-19 mRNA vaccines in cancer patients. medRxiv (2021) https://doi.org/10.1101/2021.10.20.21265273 .
Peeters, M. et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 6 , 100274 (2021).
Lasagna, A. et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open 6 , 100272 (2021).
Martins-Branco, D. et al. Immune response to anti-SARS-CoV-2 prime-vaccination in patients with cancer: a systematic review and meta-analysis. J. Cancer Res. Clin. Oncol . 149 , 1–6 (2022).
Palich, R. et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated with chemotherapy for solid cancers. Ann. Oncol. 32 , 1294–1295 (2021).
Shroff, R. T. et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 27 , 2002–2011 (2021).
Thakkar, A. et al. Study of efficacy and longevity of immune response to third and fourth doses of COVID-19 vaccines in patients with cancer: a single arm clinical trial. Elife 12 , e83694 (2023).
Shapiro, L. C. et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 40 , 3–5 (2022).
Waldhorn, I. et al. Six-month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 11 , 2430–2435 (2021).
Macrae, K., Martinez-Cajas, J., Bessai, K., Abdulhamed, A. & Gong, Y. Quantitative analysis of SARS-CoV-2 antibody levels in cancer patients post three doses of immunization and prior to breakthrough COVID-19 infections. Curr. Oncol. 29 , 7059–7071 (2022).
Di Lorenzo, G. et al. The effect of vaccination against COVID-19 in cancer patients: final results of the COICA trial. Oncology 100 , 512–518 (2022).
Article PubMed Google Scholar
Pinato, D. J. et al. Vaccination against SARS-CoV-2 protects from morbidity, mortality and sequelae from COVID19 in patients with cancer. Eur. J. Cancer 171 , 64–74 (2022).
Rooney, A. et al. Risk of SARS-CoV-2 breakthrough infection in vaccinated cancer patients: A retrospective cohort study. J. Hematol. Oncol. 15 , 67 (2022).
Simsek, M. et al. The efficacy of BNT162b2 (Pfizer-BioNTech) and CoronaVac vaccines in patients with cancer. J. Med. Virol. 94 , 4138–4143 (2022).
Hippisley-Cox, J. et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ 374 , n2244 (2021).
Mittelman, M. et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 139 , 1439–1451 (2022).
Starkey, T. et al. A population-scale temporal case–control evaluation of COVID-19 disease phenotype and related outcome rates in patients with cancer in England (UKCCP). Sci. Rep. 13 , 11327 (2023).
Wu, J. T.-Y. et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide veterans affairs study. JAMA Oncol. 8 , 281–286 (2022).
Lee, L. Y. W. et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 23 , 748–757 (2022).
Lee, L. Y. W. et al. COVID-19: Third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: A population-based study. Eur. J. Cancer 175 , 1–10 (2022).
Mallah, N. et al. Effectiveness of COVID-19 vaccine booster in the general population and in subjects with comorbidities. A population-based study in Spain. Environ. Res. 215 , 114252 (2022).
Embi, P. J. et al. Effectiveness of 2-Dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. MMWR Morb. Mortal. Wkly. Rep. 70 , 1553–1559 (2021).
Leuva, H. et al. Influence of cancer on COVID-19 incidence, outcomes, and vaccine effectiveness: a prospective cohort study of U.S. veterans. Semin. Oncol. 49 , 363–370 (2022).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183 , 758–764 (2016).
Wang, S. V. et al. Emulation of randomized clinical trials with nonrandomized database analyses: results of 32 clinical trials. JAMA 329 , 1376–1385 (2023).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384 , 1412–1423 (2021).
Lopez Bernal, J. et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 373 , n1088 (2021).
Bignucolo, A. et al. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines (Basel) 9 , 825 (2021).
Levin, E. G. et al. Waning immune humoral response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 385 , e84 (2021).
Zeng, C. et al. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell 40 , 117–119 (2022).
Kahn, R., Schrag, S. J., Verani, J. R. & Lipsitch, M. Identifying and alleviating bias due to differential depletion of susceptible people in postmarketing evaluations of COVID-19 Vaccines. Am. J. Epidemiol. 191 , 800–811 (2022).
Atmar, R. L. et al. Homologous and heterologous Covid-19 booster vaccinations. N. Engl. J. Med 386 , 1046–1057 (2022).
Hulme, W. J. et al. Challenges in estimating the effectiveness of COVID-19 vaccination using observational data. Ann. Intern Med 176 , 685–693 (2023).
Høeg, T., Duriseti, R. & Prasad, V. Potential “Healthy Vaccinee Bias” in a study of BNT162b2 vaccine against Covid-19. N. Engl. J. Med 389 , 284–286 (2023).
Xu, S. et al. A safety study evaluating non-COVID-19 mortality risk following COVID-19 vaccination. Vaccine 41 , 844–854 (2023).
Benn, C. S., Schaltz-Buchholzer, F., Nielsen, S., Netea, M. G. & Aaby, P. Randomized clinical trials of COVID-19 vaccines: do adenovirus-vector vaccines have beneficial non-specific effects? iScience 26 , 106733 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Recalde, M. et al. Validation of cancer diagnoses in electronic health records: results from the information system for research in primary care (SIDIAP) In Northeast Spain. Clin. Epidemiol. 11 , 1015–1024 (2019).
Dickerman, B. A. et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N. Engl. J. Med. 386 , 105–115 (2022).
Magen, O. et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med 386 , 1603–1614 (2022).
Hitchings, M. D. T. et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. Epidemiology 33 , 450–456 (2022).
Català, M. et al. Observational methods for COVID-19 vaccine effectiveness research: an empirical evaluation and target trial emulation. Int. J. Epidemiol. 53 , dyad138 (2024).
Kaslow, D. C. Force of infection: a determinant of vaccine efficacy? npj Vaccines 6 , 51 (2021).
Recalde, M. et al. Data Resource Profile: The Information System for Research in Primary Care (SIDIAP). Int. J. Epidemiol. 51 , e324–e336 (2022).
Burn, E. et al. Establishing and characterising large COVID-19 cohorts after mapping the Information System for Research in Primary Care in Catalonia to the OMOP Common Data Model. medRxiv https://doi.org/10.1101/2021.11.23.21266734 (2021).
OHDSI. The Book of OHDSI: Observational Health Data Sciences and Informatics . (OHDSI, 2019).
Grupo de Trabajo Técnico de Vacunación COVID-19, de la Ponencia de Programa y Registro de Vacunaciones. Estrategia de vacunación frente a COVID-19 en España. (2021).
European Medicines Agency (EMA). COVID-19 Vaccine Janssen: EMA recommendation on booster dose. https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-recommendation-booster-dose . Accessed Sep 21th 2023.
Elbe, S. & Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. 1 , 33–46 (2017).
Hitchings, M. D. T. et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat. Commun. 12 , 1–8 (2021).
Roel, E. et al. Characteristics and outcomes of over 300,000 patients with COVID-19 and history of cancer in the United States and Spain. Cancer Epidemiol. Biomark. Prev. 30 , 1884–1894 (2021).
Article CAS Google Scholar
Kerr, S. et al . Waning of first- and second-dose ChAdOx1 and BNT162b2 COVID-19 vaccinations: a pooled target trial study of 12.9 million individuals in England, Northern Ireland, Scotland and Wales. Int. J. Epidemiol . 52 , 22–31 (2023).
Domínguez-Berjón, M. F. et al. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas: (Proyecto MEDEA). Gac. Sanit. 22 , 179–187 (2008).
Wolbers, M. et al. Competing risks analyses: objectives and approaches. Eur. Heart J. 35 , 2936–2941 (2014).
Wolbers, M., Koller, M. T., Witteman, J. C. M. & Steyerberg, E. W. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 20 , 555–561 (2009).
Andersen, P. K., Geskus, R. B., De Witte, T. & Putter, H. Competing risks in epidemiology: possibilities and pitfalls. Int. J. Epidemiol. 41 , 861–870 (2012).
Latouche, A., Allignol, A., Beyersmann, J., Labopin, M. & Fine, J. P. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J. Clin. Epidemiol. 66 , 648–653 (2013).
Austin, P. C. & Fine, J. P. Practical recommendations for reporting F ine‐ G ray model analyses for competing risk data. Stat. Med. 36 , 4391–4400 (2017).
Article MathSciNet PubMed PubMed Central Google Scholar
Arnold, B. F. & Ercumen, A. Negative control outcomes: a tool to detect bias in randomized trials. JAMA 316 , 2597–2598 (2016).
Schuemie, M. J., Hripcsak, G., Ryan, P. B., Madigan, D. & Suchard, M. A. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc. Natl Acad. Sci. Usa. 115 , 2571–2577 (2018).
FELIPPE LAZAR. felippelazar/SIDIAP-CovidVaccineCancer: SIDIAP COVID-19 Vaccine Effectiveness Catalonia. Zenodo https://doi.org/10.5281/zenodo.11237690 (2024).
Download references
Acknowledgements
We thank all healthcare professionals in Catalonia who daily register information in the populations’ electronic health records; the Institut Català de la Salut and the Programa d’analítica de dades per a la recerca i la innovació en salut for providing access to the different data sources accessible through SIDIAP. OTR acknowledges support from the END-VOC Project (Horizon 2021-2024), funded by the European Union under grant agreement no. 101046314. This manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted. The funding source had no role during the design, analysis or writing of the current study.
Author information
These authors jointly supervised this work: Ranzani Otavio T, Duarte-Salles Talita.
Authors and Affiliations
Pulmonary Division, Heart Institute (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
Felippe Lazar Neto & Otavio T. Ranzani
Serviço de Oncologia Clínica, Instituto do Câncer do Estado de São Paulo (ICESP), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
Felippe Lazar Neto & Gilberto Castro Junior
Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain
Núria Mercadé-Besora, Berta Raventós, Laura Pérez-Crespo & Talita Duarte-Salles
Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès), Spain
Berta Raventós
ISGlobal, Hospital Clínic-Universitat de Barcelona, Barcelona, Spain
Otavio T. Ranzani
Department of Medical Informatics, Erasmus University Medical Center, Rotterdam, The Netherlands
Talita Duarte-Salles
You can also search for this author in PubMed Google Scholar
Contributions
F.L.N., O.T.R., and T.D.S. were involved in the conceptualization, methodology, and data curation. F.L.N., O.T.R., G.C.J., N.M.B., B.R., and L.P.C. in formal analysis and investigation. F.L.N and O.T.R were involved in writing—the original draft. All authors were involved in writing—review, and editing.
Corresponding authors
Correspondence to Otavio T. Ranzani or Talita Duarte-Salles .
Ethics declarations
Competing interests.
GCJ reports outside the scope of this paper to have received honorariums from AstraZeneca, Pfizer, Merck, Bristol-Myers, Novartis, Roche, Amgem, Janssen, Lilly, Takeda, Daiichi Sankyo, in addition to playing advisory roles to Boehringer, Pfizer, Bayer, Roche, Merck, Bristol-Meyers, AstraZeneca, Yuhan, Janssen, Libbs, Sanofi, Novartis, Lilly, Takeda, and Daiichi Sankyo. The remaining authors have no conflicts of interest to disclose.
Peer review
Peer review information.
Nature Communications thanks Lone Graff Stensballe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lazar Neto, F., Mercadé-Besora, N., Raventós, B. et al. Effectiveness of COVID-19 vaccines against severe COVID-19 among patients with cancer in Catalonia, Spain. Nat Commun 15 , 5088 (2024). https://doi.org/10.1038/s41467-024-49285-y
Download citation
Received : 02 November 2023
Accepted : 28 May 2024
Published : 19 June 2024
DOI : https://doi.org/10.1038/s41467-024-49285-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
Affiliations.
- 1 Thibodaux Regional Health System, Thibodaux, LA, USA. Electronic address: [email protected].
- 2 Unit of Innovation and Organization, Navarre Health Service, Spain. Electronic address: [email protected].
- 3 Institute of Evidence-Based Healthcare, Bond University, Gold Coast, QLD, Australia. Electronic address: [email protected].
- 4 Fielding School of Public Health and College of Letters and Science, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 5 Geffen School of Medicine, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected].
- 7 School of Pharmacy, University of Maryland, Baltimore, MD, USA. Electronic address: [email protected].
- PMID: 36055877
- PMCID: PMC9428332
- DOI: 10.1016/j.vaccine.2022.08.036
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
Methods: Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults ( NCT04368728 and NCT04470427 ), focusing analysis on Brighton Collaboration adverse events of special interest.
Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2 (95 % CI -0.4 to 20.6 and -3.6 to 33.8), respectively. Combined, the mRNA vaccines were associated with an excess risk of serious adverse events of special interest of 12.5 per 10,000 vaccinated (95 % CI 2.1 to 22.9); risk ratio 1.43 (95 % CI 1.07 to 1.92). The Pfizer trial exhibited a 36 % higher risk of serious adverse events in the vaccine group; risk difference 18.0 per 10,000 vaccinated (95 % CI 1.2 to 34.9); risk ratio 1.36 (95 % CI 1.02 to 1.83). The Moderna trial exhibited a 6 % higher risk of serious adverse events in the vaccine group: risk difference 7.1 per 10,000 (95 % CI -23.2 to 37.4); risk ratio 1.06 (95 % CI 0.84 to 1.33). Combined, there was a 16 % higher risk of serious adverse events in mRNA vaccine recipients: risk difference 13.2 (95 % CI -3.2 to 29.6); risk ratio 1.16 (95 % CI 0.97 to 1.39).
Discussion: The excess risk of serious adverse events found in our study points to the need for formal harm-benefit analyses, particularly those that are stratified according to risk of serious COVID-19 outcomes. These analyses will require public release of participant level datasets.
Keywords: Adverse events of special interest; Brighton Collaboration; COVID-19; COVID-19 vaccines; Coalition for Epidemic Preparedness Innovations; Moderna COVID-19 vaccine mRNA-1273; NCT04368728 ; NCT04470427 ; Pfizer-BioNTech COVID-19 vaccine BNT162b2; SARS-CoV-2; Safety Platform for Emergency vACcines; Serious adverse events; Vaccines; mRNA vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Serious adverse events following mRNA vaccination in randomized trials in adults. Black S, Evans S. Black S, et al. Vaccine. 2023 May 26;41(23):3473-3474. doi: 10.1016/j.vaccine.2023.04.040. Epub 2023 Apr 28. Vaccine. 2023. PMID: 37121802 No abstract available.
Similar articles
- Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Pillay J, et al. BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
- Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Buchan SA, et al. JAMA Netw Open. 2022 Jun 1;5(6):e2218505. doi: 10.1001/jamanetworkopen.2022.18505. JAMA Netw Open. 2022. PMID: 35749115 Free PMC article.
- Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 2021. Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, Nair N, Klein NP, Hanson KE, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Mbaeyi SA, Oliver SE. Rosenblum HG, et al. MMWR Morb Mortal Wkly Rep. 2021 Aug 13;70(32):1094-1099. doi: 10.15585/mmwr.mm7032e4. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34383735 Free PMC article.
- A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial. Akova M, Unal S. Akova M, et al. Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
- COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. Meo SA, et al. Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
- Decoding trends in mRNA vaccine research: A comprehensive bibliometric study. Zhang C, Wang Y, Peng J, Wen X, Zhang Y, Li K, Du H, Hu X. Zhang C, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2355037. doi: 10.1080/21645515.2024.2355037. Epub 2024 May 30. Hum Vaccin Immunother. 2024. PMID: 38813652 Free PMC article.
- From Detection to Protection: Antibodies and Their Crucial Role in Diagnosing and Combatting SARS-CoV-2. Kumar A, Tripathi P, Kumar P, Shekhar R, Pathak R. Kumar A, et al. Vaccines (Basel). 2024 Apr 25;12(5):459. doi: 10.3390/vaccines12050459. Vaccines (Basel). 2024. PMID: 38793710 Free PMC article. Review.
- mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Bakos T, Mészáros T, Kozma GT, Berényi P, Facskó R, Farkas H, Dézsi L, Heirman C, de Koker S, Schiffelers R, Glatter KA, Radovits T, Szénási G, Szebeni J. Bakos T, et al. Int J Mol Sci. 2024 Mar 22;25(7):3595. doi: 10.3390/ijms25073595. Int J Mol Sci. 2024. PMID: 38612407 Free PMC article.
- Ethics of Nudging in the COVID-19 Crisis and the Necessary Return to the Principles of Shared Decision Making: A Critical Review. Junger N, Hirsch O. Junger N, et al. Cureus. 2024 Apr 10;16(4):e57960. doi: 10.7759/cureus.57960. eCollection 2024 Apr. Cureus. 2024. PMID: 38601812 Free PMC article. Review.
- Who is "anti-science"? Paul E, Brown GW, Ridde V, Sturmberg JP. Paul E, et al. Public Health Pract (Oxf). 2024 Mar 29;7:100493. doi: 10.1016/j.puhip.2024.100493. eCollection 2024 Jun. Public Health Pract (Oxf). 2024. PMID: 38601178 Free PMC article.
- Law B, Pim C. SO2-D2.1.3 Priority List of COVID-19 Adverse events of special interest [Internet]. 2021 Oct [cited 2022 Feb 17]. Available from: https://brightoncollaboration.us/wp-content/uploads/2021/11/SO2_D2.1.3_C... .
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. - PMC - PubMed
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. - PMC - PubMed
- Sadoff J., Gray G., Vandebosch A.n., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. - PMC - PubMed
- Health Canada. Search for clinical information on drugs and medical devices [Internet]. 2019 [cited 2021 Nov 9]. Available from: https://clinical-information.canada.ca/ .
Publication types
- Search in MeSH
Associated data
- Search in ClinicalTrials.gov
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Gender, immunological response, and covid-19: an assessment of vaccine strategies in a pandemic region of oaxaca, méxico.

1. Introduction
2. materials and methods, 2.1. study population, inclusion criteria, and vaccines, 2.2. sample collection, 2.3. the enzyme-linked immunosorbent assay (elisa), 2.4. statistical analysis, 4. discussion, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021 , 19 , 409–424. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Noureddine, F.Y.; Chakkour, M.; El Roz, A.; Reda, J.; Al Sahily, R.; Assi, A.; Joma, M.; Salami, H.; Hashem, S.J.; Harb, B.; et al. The Emergence of SARS-CoV-2 Variant(s) and Its Impact on the Prevalence of COVID-19 Cases in the Nabatieh Region, Lebanon. Med. Sci. 2021 , 9 , 40. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, L.; Xu, F.; Han, Z.; Tang, K.; Hui, P.; Evans, J.; Li, Y. Strategic COVID-19 vaccine distribution can simultaneously elevate social utility and equity. Nat. Hum. Behav. 2022 , 6 , 1503–1514. [ Google Scholar ] [ CrossRef ]
- Paltiel, A.D.; Schwartz, J.L.; Zheng, A.; Walensky, R.P. Clinical Outcomes of a COVID-19 Vaccine: Implementation over Efficacy. Health Aff. 2021 , 40 , 42. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fajar, J.K.; Sallam, M.; Soegiarto, G.; Sugiri, Y.J.; Anshory, M.; Wulandari, L.; Kosasih, S.A.P.; Ilmawan, M.; Kusnaeni, K.; Fikri, M.; et al. Global Prevalence and Potential Influencing Factors of COVID-19 Vaccination Hesitancy: A Meta-Analysis. Vaccines 2022 , 10 , 1356. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fergie, J.; Moran, M.M.; Cane, A.; Pather, S.; Türeci, O.; Srivastava, A. COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review. Vaccines 2022 , 10 , 2039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tian, W.; Ren, X.; Han, M.; Zhang, Y.; Gao, X.; Chen, Z.; Zhang, W. Epidemiological and clinical characteristics of vaccinated COVID-19 patients: A meta-analysis and systematic review. Int. J. Immunopathol. Pharmacol. 2022 , 36 . [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Makhoul, M.; Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Al-Omari, S.; Abu-Raddad, L.J. Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses. Vaccines 2020 , 8 , 668. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alhinai, Z.; Park, S.; Choe, Y.J.; Michelow, I.C. A global epidemiological analysis of COVID-19 vaccine types and clinical outcomes. Int. J. Infect. Dis. 2022 , 124 , 206–211. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saad-Roy, C.M.; Morris, S.E.; Metcalf, C.J.E.; Mina, M.J.; Baker, R.E.; Farrar, J.; Holmes, E.C.; Pybus, O.G.; Graham, A.L.; Levin, S.A.; et al. Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. Science 2021 , 372 , 363–370. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Corey, K.B.; Koo, G.; Phillips, E.J. Adverse Events and Safety of SARS-CoV-2 Vaccines: What’s New and What’s Next. J. Allergy Clin. Immunol. Pract. 2022 , 10 , 2254. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Firouzabadi, N.; Ghasemiyeh, P.; Moradishooli, F.; Mohammadi-Samani, S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int. Immunopharmacol. 2023 , 117 , 109968. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Apio, C.; Han, K.; Heo, G.; Park, T. A statistical look at the COVID-19 vaccine development and vaccine policies. Front. Public Health 2022 , 10 , 1048062. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stefanelli, P.; Rezza, G. COVID-19 Vaccination Strategies and Their Adaptation to the Emergence of SARS-CoV-2 Variants. Vaccines 2022 , 10 , 905. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huespe, I.A.; Ferraris, A.; Lalueza, A.; Valdez, P.R.; Peroni, M.L.; Cayetti, L.A.; Mirofsky, M.A.; Boietti, B.; Gómez-Huelgas, R.; Casas-Rojo, J.M.; et al. COVID-19 vaccines reduce mortality in hospitalized patients with oxygen requirements: Differences between vaccine subtypes. A multicontinental cohort study. J. Med. Virol. 2023 , 95 , e28786. [ Google Scholar ] [ CrossRef ]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 2022 , 10 , 873596. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Comisión Federal para la Protección contra Riesgos Sanitario. Vacunas COVID 19 Autorizadas|Comisión Federal para la Protección Contra Riesgos Sanitarios|Gobierno|gob.mx. Vacunas COVID 19 Autorizadas. 2022. Available online: https://www.gob.mx/cofepris/acciones-y-programas/vacunas-covid-19-autorizadas (accessed on 10th June 2024).
- Camacho-Sandoval, R.; Nieto-Patlán, A.; Carballo-Uicab, G.; Montes-Luna, A.; Jiménez-Martínez, M.C.; Vallejo-Castillo, L.; González-González, E.; Arrieta-Oliva, H.I.; Gómez-Castellano, K.; Guzmán-Bringas, O.U.; et al. Development and evaluation of a set of spike and receptor binding domain-based enzyme-linked immunosorbent assays for SARS-CoV-2 serological testing. Diagnostics 2021 , 11 , 1506. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bonett, D.G.; Price, R.M. Adjusted Wald Confidence Interval for a Difference of Binomial Proportions Based on Paired Data. J. Educ. Behav. Stat. 2012 , 37 , 479–488. [ Google Scholar ] [ CrossRef ]
- Ingersoll, M.A. Sex differences shape the response to infectious diseases. PLoS Pathog. 2017 , 13 , e1006688. [ Google Scholar ] [ CrossRef ]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016 , 16 , 626–638. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klein, S.L. Immune Cells Have Sex and So Should Journal Articles. Endocrinology 2012 , 153 , 2544–2550. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Women’s Health 2021 , 2 , 761511. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alcorta-Nuñez, F.; Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; Garza, M.Á.; González-Escamilla, M.; Rodríguez-Niño, P.; González-Guerrero, J.F.; Alcorta-Garza, A.; Vidal-Gutiérrez, O.; Ramírez-Correa, G.A.; et al. SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison. Diagnostics 2023 , 13 , 1194. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021 , 384 , 1412–1423. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fernandes-Matano, L.; Salas-Lais, A.G.; Grajales-Muñiz, C.; Hernández-Ávila, M.; Garfias-Becerra, Y.O.; Rodríguez-Sepúlveda, M.C.; Segura-Sánchez, C.; Montes-Herrera, D.; Mendoza-Sánchez, D.; Angeles-Martínez, J.; et al. Longevity and Neutralizing Capacity of IgG Antibodies against SARS-CoV-2 Generated by the Application of BNT162b2, AZD1222, Convidecia, Sputnik V, and CoronaVac Vaccines: A Cohort Study in the Mexican Population. Microbiol. Spectr. 2023 , 11 , e02376-22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abufares, H.I.; Oyoun Alsoud, L.; Alqudah, M.A.Y.; Shara, M.; Soares, N.C.; Alzoubi, K.H.; El-Huneidi, W.; Bustanji, Y.; Soliman, S.S.M.; Semreen, M.H. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022 , 23 , 15415. [ Google Scholar ] [ CrossRef ]
- Sadarangani, M.; Abu Raya, B.; Conway, J.M.; Iyaniwura, S.A.; Falcao, R.C.; Colijn, C.; Coombs, D.; Gantt, S. Importance of COVID-19 vaccine efficacy in older age groups. Vaccine 2021 , 39 , 2020–2023. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, Z.; Liu, S.; Li, F.; Li, Y.; Li, Y.; Peng, P.; Li, S.; He, L.; Liu, T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis. Front. Immunol. 2022 , 13 , 965971. [ Google Scholar ] [ CrossRef ]
- Mwimanzi, F.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Datwani, S.; Omondi, F.H.; Burns, L.; et al. Older Adults Mount Less Durable Humoral Responses to Two Doses of COVID-19 mRNA Vaccine but Strong Initial Responses to a Third Dose. J. Infect. Dis. 2022 , 226 , 983–994. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021 , 73 , 2065–2072. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019 , 32 , e00084-18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Diallo, A.; Carlos-Bolumbu, M.; Diallo, M.H.; Makinson, A.; Galtier, F. Efficacy of approved vaccines to prevent COVID-19: A systematic review and network meta-analysis of reconstructed individual patient data from randomized trials. Z. Gesundh. Wiss. 2022 , 31 , 1463–1472. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ashrafian, F.; Bagheri Amiri, F.; Bavand, A.; Zali, M.; Sadat Larijani, M.; Ramezani, A. A Comparative Study of Immunogenicity, Antibody Persistence, and Safety of Three Different COVID-19 Boosters between Individuals with Comorbidities and the Normal Population. Vaccines 2023 , 11 , 1376. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Farid, E.; Herrera-Uribe, J.; Stevenson, N.J. The Effect of Age, Gender and Comorbidities upon SARS-CoV-2 Spike Antibody Induction after Two Doses of Sinopharm Vaccine and the Effect of a Pfizer/BioNtech Booster Vaccine. Front. Immunol. 2022 , 13 , 817597. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, R.; Kang, A.; Luo, X.; Jeyanathan, M.; Gillgrass, A.; Afkhami, S.; Xing, Z. COVID-19: Current knowledge in clinical features, immunological responses, and vaccine development. FASEB J. 2021 , 35 , e21409. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Falahi, S.; Kenarkoohi, A. Host factors and vaccine efficacy: Implications for COVID-19 vaccines. J. Med. Virol. 2022 , 94 , 1330–1335. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Variable | Total Samples | Positive Samples | ||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Females | 88 | 58.7 | 83 | 94.3 |
| Males | 62 | 41.3 | 56 | 90.3 |
| Type of vaccine | ||||
| CanSino | 118 | 78.6 | 107 | 90.6 |
| AstraZeneca | 16 | 10.6 | 16 | 100 |
| Others | 16 | 10.6 | 16 | 100 |
| Age group | ||||
| 18–30 years old | 30 | 20.0 | 30 | 100 |
| 31–45 years old | 29 | 19.3 | 25 | 86.0 |
| 46–59 years old | 27 | 18.0 | 25 | 92.5 |
| 60–69 years old | 31 | 20.7 | 28 | 90.3 |
| 70 years and older | 33 | 22.0 | 31 | 93.9 |
| Comorbidity | ||||
| Absence | 104 | 69.4 | 97 | 93.2 |
| ≥1 | 46 | 30.6 | 42 | 91.3 |
| BMI | ||||
| Healthy weight | 26 | 29.9 | 24 | 92.3 |
| Overweight | 35 | 40.2 | 35 | 100 |
| Obesity | 26 | 29.9 | 23 | 88.5 |
| Variable | No. of Positive Individuals | Antibody Rate % | 95% ICs |
|---|---|---|---|
| Gender | |||
| Female | 83 | 60 | 51–67 |
| Male | 56 | 40 | 32–48 |
| Age group | |||
| 18–30 years old | 30 | 22 | 15–29 |
| 31–45 years old | 25 | 18 | 12–25 |
| 46–59 years old | 25 | 18 | 12–25 |
| 60–69 years old | 28 | 20 | 14–27 |
| 70 years and older | 31 | 22 | 16–29 |
| BMI | |||
| Healthy weight | 24 | 29 | 20–39 |
| Overweight | 35 | 42 | 32–53 |
| Obesity | 23 | 28 | 19–38 |
| -value | ||
| Gender | −2.21 | 0.028 |
| Comorbidity | 1.23 | 0.220 |
| -value | ||
| Type of vaccine | 0.73 | 0.483 |
| Age group | 0.36 | 0.839 |
| Body mass index * | 1.65 | 0.198 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Rodríguez-Martínez, L.M.; Chavelas-Reyes, J.L.; Medina-Ramírez, C.F.; Cabrera-Santos, F.J.; Fernández-Santos, N.A.; Aguilar-Durán, J.A.; Pérez-Tapia, S.M.; Rodríguez-González, J.G.; Rodríguez Pérez, M.A. Gender, Immunological Response, and COVID-19: An Assessment of Vaccine Strategies in a Pandemic Region of Oaxaca, México. Microbiol. Res. 2024 , 15 , 1007-1015. https://doi.org/10.3390/microbiolres15020066
Rodríguez-Martínez LM, Chavelas-Reyes JL, Medina-Ramírez CF, Cabrera-Santos FJ, Fernández-Santos NA, Aguilar-Durán JA, Pérez-Tapia SM, Rodríguez-González JG, Rodríguez Pérez MA. Gender, Immunological Response, and COVID-19: An Assessment of Vaccine Strategies in a Pandemic Region of Oaxaca, México. Microbiology Research . 2024; 15(2):1007-1015. https://doi.org/10.3390/microbiolres15020066
Rodríguez-Martínez, Luis M., José L. Chavelas-Reyes, Carlo F. Medina-Ramírez, Francisco J. Cabrera-Santos, Nadia A. Fernández-Santos, Jesús A. Aguilar-Durán, Sonia M. Pérez-Tapia, Josefina G. Rodríguez-González, and Mario A. Rodríguez Pérez. 2024. "Gender, Immunological Response, and COVID-19: An Assessment of Vaccine Strategies in a Pandemic Region of Oaxaca, México" Microbiology Research 15, no. 2: 1007-1015. https://doi.org/10.3390/microbiolres15020066
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Exp Vaccine Res
- v.10(2); 2021 May
Inside the story about the research and development of COVID-19 vaccines
Shrina p. patel.
Ramanbhai Patel College of Pharmacy, Charusat University, Anand, India.
Gayatri S. Patel
Jalpa v. suthar.
The ongoing coronavirus threat from China has spread rapidly to other nations and has been declared a global health emergency by the World Health Organization (WHO). The pandemic has resulted in over half of the world's population living under conditions of lockdown. Several academic institutions and pharmaceutical companies that are in different stages of development have plunged into the vaccine development race against coronavirus disease 2019 (COVID-19). The demand for immediate therapy and potential prevention of COVID-19 is growing with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics. The challenging part is a need for vigorous testing for immunogenicity, safety, efficacy, and level of protection conferred in the hosts for the vaccines. As the world responds to the COVID-19 pandemic, we face the challenge of an overabundance of information related to the virus. Inaccurate information and myths spread widely and at speed, making it more difficult for the public to identify verified facts and advice from trusted sources, such as their local health authority or WHO. This review focuses on types of vaccine candidates against COVID-19 in clinical as well as in the preclinical development platform.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originated in Hubei Province, China, in December 2019 (and possibly earlier, though unrecognized), as a pneumonia-causing disorder [ 1 ], most likely the result of natural selection in animal hosts (bats, pangolins) before the zoonotic transition [ 2 ]. Seven members of this viral family are now known to infect humans, three of whom have the potential to cause severe respiratory diseases [ 3 ]. Coronaviruses (CoVs) are positive-sense, single-stranded Coronaviridae family (subfamily Coronavirinae) RNA viruses that infect a broad range of hosts to produce diseases ranging from the common cold to severe/fatal diseases [ 4 ]. The novel virus was initially named “2019-nCoV” by the International Committee on Virus Taxonomy. It was changed to “SARS-CoV-2” since it was found to be the sister virus of an extreme acute respiratory syndrome (SARS-CoV) [ 5 ]. The ongoing threat of coronavirus emerging in China has spread rapidly to other countries and has been declared by the World Health Organization (WHO) as a global health emergency [ 6 ].
Virus genetic sequencing shows that it is a beta coronavirus that is closely related to the SARS virus [ 7 ]. Currently, immunization prevents 2–3 million deaths from more than 20 life-threatening diseases that are now being controlled by vaccinations, and work is underway at an unprecedented pace to make coronavirus disease 2019 (COVID-19) a vaccine-preventable illness [ 8 ]. To accelerate the research and development process and to establish new standards and standards to prevent the spread of the coronavirus pandemic and care for those affected, WHO brings together the world's scientists and public health practitioners [ 7 ]. In human medical intervention, vaccines are one of the monumental achievements in mitigating the dispersion and effects of infectious diseases [ 9 ]. Vaccines are the most useful method for contagious disease prevention because they are more cost-effective than treatment and reduce morbidity and mortality without long-lasting effects [ 10 ]. Preventive and therapeutic vaccines will be of fundamental significance as the most obvious way to safeguard public health [ 11 ]. Since the coronavirus shares substantial sequence homology with two other lethal coronaviruses, SARS and Middle East respiratory syndrome (MERS), the vaccines identified could potentially promote the design of anti-SARS-CoV-2 vaccines. It is essential to establish safe and effective vaccines to contain the COVID-19 pandemic, eradicate its spread, and eventually prevent its future recurrence [ 12 ]. By exposing individuals to antigens, vaccination can produce long-lasting immunity to drive the production of immunological memory before meeting live pathogens. Thus the resulting immunity can be mediated by the activation of humoral antibodies and the effector function of cellular T-cells [ 13 ]. The full development path for an effective SARS-CoV-2 vaccine will involve th e cooperation of industry, government, and academia in unprecedented ways, each contributing its strengths [ 14 ].
It is a difficult task to develop a SARS-CoV-2 vaccine to control its spread and help remove it from the human population since there is a lack of knowledge on its biological properties, epidemiology, individual immune responses to it, and so forth [ 15 ]. The S protein is the critical target of vaccine production since it includes a receptor-binding domain (RBD) and viral functions. It will be essential to confirm the clinical significance of the SARS-CoV-2 binding and neutralizing antibody titers and their ability to predict efficacy [ 16 ]. Only in a significant clinical efficacy study would it be possible to confirm the association between antibody titers and defense against COVID-19 [ 17 ]. For any frequently used vaccine, there is a theoretical risk that vaccination could cause subsequent infection with SARS-CoV-2 more severe. This has been confirmed in feline coronaviruses and has been observed in some SARS-CoV-1 animal vaccine challenge models [ 18 ].
The key benefit of next-generation vaccines is that they can be produced based on sequence data alone [ 19 ]. If the viral protein(s) that are essential for the defense against infection or disease and therefore for inclusion in the vaccine is established, the availability of coding sequences for the viral protein(s) is sufficient to start the production of the vaccine rather than to rely on the ability to grow the virus [ 20 ]. This makes these platforms extremely adaptable and dramatically accelerates the production of vaccines, as is evident from the fact that the majority of currently underway clinical trials of COVID-19 vaccines include a next-generation platform [ 19 ]. A prospective pharmaceutical manufacturer must send an application to a regulatory authority such as the Food and Drug Administration (FDA) to examine the new vaccine after a possible vaccine has been announced by a researcher [ 21 ].
The demand for immediate therapy and potential prevention of COVID-19 is growing [ 22 ] with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics [ 23 ]. Attempts are being made to establish secure and successful methods for prophylactics [ 24 , 25 ]. Several vaccines are in different phases of clinical trials [ 6 ], but there is a lack of prophylactics in the present scenario [ 26 ]. Several attempts have been made to create COVID-19 vaccines to avoid the pandemic condition as well as the S-protein SARS-CoV-2 has been used for most of the emerging vaccine candidates. In Fig. 1 , the overview of vaccine candidates in their respective ongoing clinical phases depicts the percentage of vaccine candidates amongst which the majority of developing vaccines is in phase 1/2. The data shown below in the graph is assessed until 15 October 2020, in the pipeline of vaccine development and registered clinical trials globally.

In Fig. 2 , the overview of the global COVID-19 vaccine landscape in clinical development depicts that there are seven major types of vaccine candidates for COVID-19 is illustrated as (inactivated, non-replicating viral vectors, replicating viral vectors, protein subunit, nucleic acid-based, and virus-like particles [VLP]), showing the percentage of candidate vaccines that are currently under clinical development. The nucleic acid-based platform includes both RNA vaccines and DNA vaccines. Among the seven types of vaccine candidates, protein subunit-based vaccines constitute the highest 31% in clinical development. In contrast, VLP-based vaccine and replicating viral vectors comprises the lowest as 5% in the clinical development.

In Fig. 3 , the overview of global COVID-19 vaccine landscape in preclinical development depicts that there are 10 significant types of vaccine candidates for COVID-19 is illustrated as (inactivated, replicating bacteria vector, DNA, live attenuated virus, non-replicating viral vectors, protein subunit, t-cell based, replicating viral vectors, RNA, and VLP), showing the percentage of candidate vaccines that are currently under preclinical development. Among the 10 types of vaccine candidates, protein subunit-based vaccines constitute the highest 36% in clinical development whereas T-cell based vaccine and replicating bacteria vector comprises the lowest at 1% in the preclinical development globally.

RNA-Based Vaccine
As a result of considerable developments in biotechnology, due to their higher potency, short development cycles, low-cost product, and safe administration, mRNA vaccines represent a substantial improvement over traditional vaccine strategies [ 27 ]. The mRNA is an evolving platform that is non-infectious and non-integrated and has almost no possible risk of insertional mutagenesis. Antigen discovery, sequence analysis, and optimization, screening of modified nucleotides, delivery system discovery, and immune response and safety assessment tests are the sequential events in the mRNA vaccine production process [ 28 ]. In vaccines, two primary forms of RNA are investigated: virally derived, RNA self-replicating, and mRNA non-replicating. The antigen and the necessary viral replication machinery are typically self-replicating RNAs, whereas conventional mRNA-based vaccines encode only the antigen of interest with 50 and 30 untranslated regions (UTRs) [ 27 ].
The immunogenicity of mRNA can be decreased, and changes can be made to enhance the stability of these vaccines [ 29 ]. Furthermore, anti-vector immunity is also resisted as mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine [ 30 ]. This platform has empowered the rapid vaccine development program due to its flexibility and ability to reproduce the structure and expression of the antigen as seen in the course of natural infection [ 31 ]. A possible benefit of mRNA vaccines is the convenient availability of a portable mRNA “printing” facility for large-scale production of mRNA [ 32 ].
mRNA-1273 (Moderna TX Inc.)
It is a vaccine composed of lipid nanoparticle (LNP) encapsulated synthetic mRNA that codes for SARS-CoV-2 full-length, pre-fusion stabilized spike protein (S) [ 33 ]. It has the potential to induce an antiviral response that is highly S-protein specific. Also, it is known to be relatively harmless since it is neither composed of the inactivated pathogen nor of the live pathogen sub-units [ 34 ]. To perform the phase II trials, the vaccine has received FDA fast-track approval. The company published the interim antibody data for phase I of eight participants who received different levels of dose [ 33 ]. For the participants receiving 100 µg dose, neutralizing antibody levels were significantly higher than those observed in convalescent sera. In the 25 µg and 100 µg dose cohorts, the vaccine was found to be primarily safe and well-tolerated. In comparison, three participants reported systemic symptoms of grade 3 following administration of the second 250 µg dose level [ 26 ]. The possible benefits of a prophylactic vaccine mRNA strategy include the ability to replicate natural infection to induce a more effective immune response and the ability to incorporate multiple mRNAs into a single vaccine [ 12 ].
On 24 February 2020, Moderna declared that it had released the first batch of mRNA-1273 against SARS-CoV-2 for human use, prepared using the methods and strategies outlined in its previous patents. mRNA-1273 vials have been shipped to the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH), to be used in the United States in the proposed phase 1 study [ 35 ]. In collaboration with researchers at the NIAID Vaccine Research Centre, Moderna reports that mRNA-1273 is an mRNA vaccine targeting a prefusion stabilized form of the S protein associated with SARS-CoV-2, which was chosen by Moderna [ 32 ]. Patent application WO2018115527 describes vaccines consisting of mRNA encoding at least one MERS coronavirus antigen, preferably an S protein or an S protein fragment (S1), an envelope protein (E), a membrane protein (M), or a nucleocapsid protein (N), all of which have been successful in inducing an immune response unique to the antigen [ 33 ]. Intradermal administration of a LNP-encapsulated mRNA mixture encoding MERS-CoV S proteins into mice has been shown to result in vivo translation and humoral immune response induction [ 12 ].
BNT162b1 (BioNTech, Fosun Pharma, Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that codes for the essential target of the neutralizing antibody virus, trimerized SARS-CoV-2 RBD [ 29 ]. The vaccine shows improved immunogenicity due to the addition of the foldon trimerization domain of T4 fibrin-derived to the RBD antigen. In 80 nm ionizable cationic LNPs, the mRNA is encapsulated, which guarantees its efficient delivery [ 31 ]. In phase 1/2 clinical trials, elevated levels of RBD-specific immunoglobulin G (IgG) antibodies with a geometric mean concentration were found to be 8 to 46.3 times the convalescent serum titer. Whereas, the SARS-CoV-2 neutralizing antibody geometric mean titers were found to be 1.8 to 2.8 times the convalescent serum panel [ 29 ]. With no adverse effects, mild and transient local reactions and systemic events were observed. The data review did not, however, assess the protection and immune response beyond 2 weeks after the second dose administration [ 31 ].
Report of available effectiveness, tolerability, and immunogenicity results from an ongoing placebo-controlled, observer-blinded dose-escalation study in healthy adults 18–55 years of age, randomized to receive two 21-day separate doses of 10 µg, 30 µg, or 100 µg of BNT162b1, a nucleoside-modified LNP mRNA vaccine encoding trimerized SARS-CoV-2 spike glycoprotein dose-dependent, usually mild to moderate, and temporary, was the local reactions and systemic events [ 29 ]. The BNT162b1 vaccine candidate now being clinically studied integrates such nucleoside modified RNA and encodes the SARS-CoV-2 spike protein RBD, a primary target of virus-neutralizing antibodies [ 31 ]. Sera's RBD-binding IgG and SARS-CoV-2 neutralizing titers increased both at the dose level and after the second dose. Geometric mean neutralizing titers were 1.8 to 2.8 times those of a panel of human sera convalescent COVID-19. These findings help further evaluation of this candidate for the mRNA vaccine [ 33 ]. By adding a T4 fibritin-derived “foldon” trimerization domain, the RBD antigen expressed by BNT162b1 is modified to improve its immunogenicity by a multivalent display. The RNA vaccine is formulated in LNPs for more effective delivery to cells after intramuscular injection [ 31 ]. In Table 1 , potential RNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) | This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2. | LNP-encapsulated mRNA | Phase 1 | NIAID | NCT04283461 [ ] |
| 2 | Dose-confirmation study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1273 COVID-19 vaccine in adults aged 18 years and older | This clinical study will assess the safety, reactogenicity, and immunogenicity of 2 dose levels of mRNA-1273 SARS-CoV-2 vaccine. | LNP-encapsulated mRNA | Phase 2 | Sponsor: Moderna TX Inc. | NCT04405076 [ ] |
| Collaborator: Biomedical Advanced Research and Development Authority | ||||||
| 3 | A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19 | The study was designed to primarily evaluate the efficacy, safety, and immunogenicity of mRNA-1273 to prevent COVID-19 for up to 2 years after the second dose of mRNA-1273. | LNP-encapsulated mRNA | Phase 3 | Sponsor: Moderna TX Inc. | NCT04470427 [ ] |
| Collaborator: Biomedical Advanced Research and Development Authority & NIAID | ||||||
| 4 | A phase I clinical trial of novel coronavirus pneumonia (COVID-19) mRNA vaccine (BNT162b1) in China | To evaluate the safety and tolerability profiles of BNT162b1 P/B immunization given 21 days apart on healthy Chinese subjects through 28 days after boost vaccination. | 3 LNP-mRNAs | Phase 1 | Jiangsu Provincial Center for Disease Prevention and Control | ChiCTR2000034825 [ ] |
| 5 | A trial investigating the safety and effects of one BNT162 vaccine against COVID-19 in healthy adults | The vaccine BNT162b3 will be administered using a P/B regimen. This trial has been divided into two parts for dose-escalation cohorts in older subjects. | 3 LNP-mRNAs | Phase 1/2 | BioNTech RNA Pharmaceuticals GmbH | NCT04537949 [ ] |
| 6 | Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals | This study is a phase 1/2/3, randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in healthy individuals. | 3 LNP-mRNAs | Phase 3 | Sponsor: BioNTech SE | NCT04368728 [ ] |
| Collaborator: Pfizer | ||||||
| 7 | A study to evaluate the safety, reactogenicity, and immunogenicity of vaccine CVnCoV in healthy adults | This study aims to evaluate the safety and reactogenicity profile after 1 and 2 dose administrations of CVnCoV at different dose levels. | mRNA | Phase 1 | Sponsor: CureVac AG | NCT04449276 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations (CEPI) | ||||||
| 8 | Ascending dose study of investigational SARS-CoV-2 vaccine ARCT-021 in healthy adult | To determine safety and tolerability and immunogenicity of investigational vaccine ARCT-021 in healthy adult volunteers. | mRNA | Phase 1/2 | Arcturus Therapeutics Inc. | NCT04480957 [ ] |
| 9 | A clinical trial to assess the safety of a coronavirus vaccine in healthy men and women | The main aim of the study is to assess the safety of the vaccine and its effects on the immune system. | LNP-nCoVsaRNA | Phase 1 | Imperial College London | ISRCTN17072692 [ ] |
| 10 | A phase I clinical trial to evaluate the safety, tolerance, and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18–59 years and 60 years and above | To explore the immune persistence of the investigational vaccine at the recommended dose and the specific cellular immune response to the RBD of S protein. | mRNA | Phase 1 | People's Liberation Army (PLA) Academy of Military Sciences, Walvax Biotech. | ChiCTR2000034112 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LNP, lipid nanoparticle; NIAID, National Institute of Allergy and Infectious Diseases; P/B, prime/boost; RBD, receptor-binding domain.
Viral Vector-Based Vaccines
Viral vector-based vaccines have a high degree of protein expression and long-term stability, inducing strong immune responses [ 46 ]. These include vaccines focused on chemically weakened viruses used to bear antigens or pathogens of concern for immune response induction [ 47 ]. A possible prophylactic strategy against a pathogen is a viral vector-based vaccine. These vaccines are highly selective in transmitting genes to the target cells, are highly effective in gene transduction, and are useful in inducing immune responses [ 48 ]. They have a long-term and high level of antigenic protein expression and thus have an excellent potential for prophylactic use as these vaccines activate and facilitate cytotoxic T cells, eventually contributing to the elimination of infected virus cells [ 46 ]. The generation of immunity to the vector is an essential consideration for the development of virus vectored vaccines, which could impede the antigen-specific response to boost vaccination [ 49 ]. Reports from preclinical and clinical trials suggested that adequate safety can be obtained from a single dose [ 50 ].
Ad5-nCoV (CanSino Biologics Inc., Beijing Institute of Biotechnology)
A four-fold increase in RBD and S protein-specific neutralizing antibodies was observed within 14 days [ 51 ]. Ad5-nCoV is a recombinant type-5 adenovirus (Ad5) replication-defective vector expressing the recombinant SARS-CoV-2 spike protein. It was prepared by cloning, together with the plasminogen activator signal peptide gene, an optimized full-length gene of the S protein in the Ad5 vector devoid of genes E1 and E3 [ 29 ]. The vaccine was constructed from the Microbix Biosystem using the Admax system. A positive antibody reaction or seroconversion of immunization was identified in phase I clinical trials and peaked at day 28, post-vaccination. Also, the response of CD4+T cells and CD8+T cells peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity has partially restricted the reaction of both the antibody and the T cell [ 51 ]. The study would further assess the antibody response in recipients between 18 and 60 years of age who received one of three doses in the study, with follow-up at 3- and 6-months post-vaccination [ 29 ].

Coroflu (University of Wisconsin-Madison, FluGen, Bharat Biotech)
M2SR, a self-limiting variant of the influenza virus that is modified by spike protein sequence insertion of the SARS-CoV-2 gene. Besides, the vaccine expresses the influenza virus' hemagglutinin protein, thereby triggering an immune response to both viruses [ 52 ]. The M2SR is self-limiting and, since it lacks the M2 gene, does not undergo replication. It is capable of entering the cell, thereby causing immunity to the virus [ 32 ]. It is delivered intra-nasally, mimicking the normal viral infection pathway. Compared to intramuscular injections, this route stimulates many immune system modes and has higher immunogenicity [ 52 ].
LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
By using SMENP minigenes to engineer dendritic cells (DC) with a lentiviral vector expressing the conserved domains of the structural proteins SARS-CoV-2 and protease [ 29 ], the LV-SMENP-DC vaccine is prepared. Subcutaneous vaccine inoculation introduces antigen-presenting cell antigens, which eventually cause cytotoxic T cells and produce an immune response [ 48 ].
ChAdOx1 (University of Oxford)
The recombinant adenovirus vaccine ChAdOx1 was developed using codon-optimized S glycoprotein and synthesized at the 5 ends with the leading tissue plasminogen activator (tPA) sequence [ 50 ]. The SARS-CoV-2 amino acid coding sequence (2 to 1273) and the tPA leader have been propagated in the shuttle plasmid. This shuttle plasmid is responsible for the coding between the Gateway recombination cloning site of the main immediate-early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator sites and polyadenylation signal from bovine growth hormone (BGH) [ 29 ]. In the bacterial artificial chromosome, the adenovirus vector genome is built by inserting the SARS-CoV-2 S gene into the ChAdOx1 adenovirus genome's E1 locus. In the T-Rex human embryonic kidney 293 (HEK-293) cell lines, the virus was then allowed to replicate and purified by ultracentrifugation of the CsCl gradient [ 53 ]. The absence of any subgenomic RNA from preclinical trials in intra-muscularly vaccinated animals is suggestive of improved immunity to the virus [ 50 ]. Previous studies have proposed that the immune response should be marshalled by a single shot [ 53 ]. In Table 2 , potential viral vector-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Clinical trial to evaluate the safety and immunogenicity of the COVID-19 vaccine (COVID-19-101) | This is a randomized, placebo-controlled, two-center, trial in healthy adult volunteers to investigate the safety, tolerability, and immunogenicity of a novel measles-vector-based vaccine candidate against SARS-CoV-2 infection (TMV-083). | Measles-vector based | Phase 1 | Sponsor: Institute Pasteur | NCT04497298 [ ] |
| Collaborator: Themis Bioscience GmbH, Coalition for Epidemic Preparedness Innovations | ||||||
| 2 | A phase I clinical trial of influenza virus vector COVID-19 vaccine for intranasal spray (DelNS1-2019-nCoV-RBD-OPT1) | The effect of pre-existing antibodies against influenza A (H1N1) virus on the immunogenicity of Influenza virus vector COVID-19 vaccine for intranasal spray (DelNS1-2019-nCoVRBD-OPT1) in a healthy population for safety. | Intranasal flu-based-RBD | Phase 1 | Sponsor: Beijing Wantai Biological Pharmacy | ChiCTR2000037782 [ ] |
| Collaborator: Xiamen University | ||||||
| 3 | A phase I/II study to determine efficacy, safety, and immunogenicity of the candidate coronavirus disease (COVID-19) vaccine ChAdOx1 nCoV-19 in UK healthy adult volunteers | To assess the efficacy of ChAdOx1 nCoV-19 against COVID-19. To assess the safety of the candidate vaccine ChAdOx1 nCoV. | ChAdOx1-S | Phase 1/2 | Sponsor: University of Oxford | 2020-001072-15 [ ] |
| Collaborator: AstraZeneca | ||||||
| 4 | A phase III study to investigate a vaccine against COVID-19 | This study aims to assess whether healthy people in Brazil can be protected from COVID-19 with a new vaccine called ChAdOx1 nCoV-19. | ChAdOx1-S | Phase 3 | Sponsor: University of Oxford | ISRCTN89951424 [ ] |
| Collaborator: AstraZeneca | ||||||
| 5 | Study of AZD1222 for the prevention of COVID-19 in Japan | A safe and effective vaccine for COVID-19 prevention would have a significant global public health impact because currently, there are no licensed preventions available against COVID-19. | AZD1222 | Phase 1/2 | Sponsor: AstraZeneca | NCT04568031 [ ] |
| Collaborator: Iqvia Pty. Ltd. | ||||||
| 6 | Phase III double-blind, placebo-controlled study of AZD1222 for the prevention of COVID-19 in adults | The study aims to assess the safety, efficacy, and immunogenicity of AZD1222 for the prevention of COVID-19. | AZD1222 | Phase 3 | Sponsor: AstraZeneca | NCT04516746 [ ] |
| Collaborator: Iqvia Pty. Ltd. | ||||||
| 7 | Replication defective simian adenovirus (GRAd) encoding S | RT-CoV-2 is an open-label, dose-escalation multicenter clinical trial to assess the safety and immunogenicity of the candidate COVID-19 vaccine GRAd-CoV-2 in healthy Italian volunteers aged 18–55 years and 65–85 years inclusive. | Replication defective simian adenovirus (GRAd) encoding S | Phase 1 | Sponsor: ReiThera Srl | NCT04528641 [ ] |
| Collaborator: Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani | ||||||
| 8 | A clinical trial of a recombinant adenovirus 5 vectored COVID-19 vaccine (Ad5-nCoV) with two doses in healthy adults | This is a clinical trial to evaluate the safety and immunogenicity of a recombinant Ad5-nCoV with two doses and with different administration routes in healthy adults aged 18 years and older. | Ad5-nCoV | Phase 1 | Sponsor: Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | NCT04552366 [ ] |
| Collaborator: Zhongnan Hospital | ||||||
| 9 | Safety and immunogenicity trial of an oral SARS-CoV-2 vaccine (VXA-CoV2-1) for prevention of COVID-19 in healthy adults | VXA-CoV2-1 is a non-replicating Ad5 vector adjuvanted oral tableted vaccine being developed to prevent COVID-19. | Ad5 adjuvanted oral vaccine platform | Phase 1 | Vaxart | NCT04563702 [ ] |
| 10 | Safety, tolerability, and immunogenicity of the candidate vaccine MVA-SARS-2-S against COVID-19 | In this clinical trial, healthy volunteers in two different dose cohorts will be vaccinated twice with the candidate vaccine MVA-SARS-2-S. | MVA-SARS-2-S | Phase 1 | Sponsor: Universitätsklinikum Hamburg-Eppendorf | NCT04569383 [ ] |
| Collaborator: German Center for Infection Research, Philipps University Marburg Medical Center, Ludwig-Maximilians–University of Munich |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain; MVA, modified vaccinia Ankara.
Protein Subunit-Based Vaccines
Subunit vaccines, safer and more straightforward to manufacture, present a host with high immunogenicity with one or few antigens, but need adjuvants to evoke a strong defensive immune response [ 62 ]. A subunit vaccine is a synthetic peptide or recombinant antigenic protein-dependent vaccine which is essential for long-term protection and a therapeutic invigoration of the immune response [ 63 ]. The subunit vaccine exhibits low immunogenicity and requires an adjuvant's additional assistance to potentiate the vaccine-induced immune responses. An adjuvant may improve the biological half-life of the antigenic material, or the immunomodulatory cytokine response may be improved. The use of an adjuvant, therefore, helps to overcome the shortcomings of the protein subunit vaccines [ 64 ]. Subunit vaccines may be designed to concentrate the immune response on the neutralization of epitopes, thus preventing the development of non-neutralizing antibodies that may encourage disease-related antibody-dependent enhancement [ 65 ]. Antigenic proteins thought to cause a defensive immune response are used in protein subunit vaccines. The S protein of SARS-CoV-2 is the most appropriate antigen to induce neutralizing antibodies against the pathogen [ 13 ]. The S protein is comprised of two subunits. In the S1 subunit, the N-terminal domain, RBD, and receptor-binding motif (RBM) domains are found, while the S2 subunit consists of FP, HR 1, and 2 [ 62 ]. The virus reaches the cell by endocytosis using S-protein mediated binding to the human angiotensin-converting enzyme 2 (hACE2) receptor. Therefore, S-protein and its antigenic fragments are key objectives for the establishment of a subunit vaccine [ 63 ]. A complex protein with two conformation states, i.e., a pre-fusion and post-fusion state, is the S glycoprotein [ 62 ]. Therefore, the antigen must maintain its surface chemistry and the profile of the initial pre-fusion spike protein to retain the epitopes for igniting good quality antibody responses. Also, targeting the masked RBM as an antigen, it will increase the neutralizing antibody response and enhance the overall efficacy of the vaccine [ 66 ].
NVX-CoV2373 (Novavax Inc., Emergent BioSolutions)
NVX-CoV2373 is a nano-particle-mediated immunogenic vaccine-mediated on coronavirus S-protein, the recombinant expression of stable pre-fusion [ 67 ]. In the baculovirus system, the protein has been stably expressed. By inducing high levels of neutralizing antibodies, the company aims to use the matrix-M adjuvant to strengthen the immune response against the SARS-CoV-2 spike protein [ 35 ]. A single immunization in animal models resulted in a high level of anti-spike protein antibodies that blocked the binding domain of the hACE2 receptor and could elicit SARS-CoV-2 wild-type virus-neutralizing antibodies [ 68 ].
Molecular clamp stabilized spike protein vaccine candidate
It is being developed in partnership with GSK and Dynavax by the University of Queensland [ 29 ]. The University will have access to the vaccine adjuvant (AS03 Adjuvant) platform technology, which is believed to enhance the response of the vaccine and reduce the amount of vaccine needed per dose [ 69 ]. The University is developing a stabilized pre-fusion, recombinant viral protein subunit vaccine based on the molecular clamp technology. It has been established that this technology induces the development of neutralizing antibodies [ 34 ].
PittCoVacc (University of Pittsburgh)
It is a recombinant SARS-CoV-2 vaccine based on the micro-needle array (MNA) that involves administering rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens) [ 70 ]. A significant increase in statistically significant antigen-specific antibodies was found in the mice models in preclinical studies at the end of 2 weeks [ 29 ]. Furthermore, even after sterilization using gamma rays, the immunogenicity of the vaccine was maintained. Statistically, relevant antibody titers confirm the feasibility of the MNA-SARS-CoV-2 vaccine at the early stage and even before boosting [ 70 ].
Triple antigen vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype in which an engineered Saccharomyces cerevisiae expression platform (D-CryptTM) co-expresses the recombinant spike, membrane, and envelope protein of SARS-CoV-2 [ 71 ]. The proteins then, like the VLP, undergo self-assembly. The biophysical characterization of the VLP was simultaneously given by the transmission electron microscopy and allied analytical data [ 29 ]. After more research and development, this prototype has the potential to engage in preclinical trials as a vaccine candidate. Besides, cost-effectively, it is assumed to be safe and easy to produce on a mass scale [ 71 ]. In Table 3 , potential protein subunit-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Evaluation of the safety and immunogenicity of a SARS-CoV-2 rS nanoparticle vaccine with/without matrix-M adjuvant | The study is designed to evaluate the safety and immunogenicity in 131 healthy participants ≥18 to 59 (inclusive) years of age at two sites in Australia. | Full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with matrix M | Phase 1/2 | Sponsor: Novavax | NCT04368988 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations | ||||||
| 2 | A study looking at the effectiveness and safety of a COVID-19 vaccine in South African adults | This is a study to evaluate the effectiveness and safety of healthy HIV-negative (HIV−) adult participants and in medically stable HIV-positive (HIV+) adult participants in up to 10 sites across South Africa. | Full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with matrix M | Phase 2 | Novavax | NCT04533399 [ ] |
| 3 | Phase I clinical study of recombinant novel coronavirus vaccine | In this trial, a total of 50 subjects were recruited; the test vaccines were divided into three groups, low-dose, high-dose vaccine groups, and placebo groups. | Adjuvanted recombinant protein (RBD-Dimer) | Phase 1 | Sponsor: Anhui Zhifei Longcom Biologic Pharmacy Co. Ltd. | NCT04445194 [ ] |
| Collaborator: Beijing Chao Yang Hospital | ||||||
| 4 | Recombinant new coronavirus vaccine (CHO cells) to prevent SARS-CoV-2 phase i clinical trial (≥60 years old) | To evaluate the safety and tolerability of recombinant new coronavirus vaccine (CHO cells) to explore the immunogenicity and durability of different doses. | Adjuvanted recombinant protein (RBD-Dimer) | Phase 1/2 | Anhui Zhifei Longcom Biologic Pharmacy Co. Ltd. | NCT04550351 [ ] |
| 5 | KBP-201 COVID-19 vaccine trial in healthy volunteers | This is a FIH, observer-blinded, randomized, placebo-controlled, parallel-group study to evaluate the safety and immunogenicity of the KBP-COVID-19 vaccine. | RNA-based protein subunit | Phase 1/2 | Kentucky Bioprocessing Inc. | NCT04473690 [ ] |
| 6 | Study of recombinant protein vaccine formulations against COVID-19 in healthy adults 18 years of age and older | The objective of the study is to describe the neutralizing antibody profile and safety profile of all participants in each group up to 12 months post-last injection. | S protein (baculovirus production) | Phase 1/2 | Sponsor: Sanofi Pasteur, a Sanofi Company | NCT04537208 [ ] |
| Collaborator: GlaxoSmithKline | ||||||
| 7 | A study to evaluate the safety, tolerability, and immunogenicity of UB-612 COVID-19 vaccine | This is an open-label, dose-escalation clinical study of 3 ascending doses of UB-612 COVID-19 vaccine in healthy adults, aged from 20 to 55 years old. | S1-RBD-protein | Phase 1 | Sponsor: United Biomedical Inc., Asia | NCT04545749 [ ] |
| Collaborator: COVAXX | ||||||
| 8 | SCB-2019 as COVID-19 vaccine | This is a randomized, double-blind, placebo-controlled, FIH study to assess safety, reactogenicity, and immunogenicity of SCB-2019 at multiple dose levels. | Native like trimeric subunit spike protein vaccine | Phase 1 | Clover Biopharmaceuticals AUS Pty. Ltd. | NCT04405908 [ ] |
| 9 | Monovalent recombinant COVID19 vaccine (COVAX19) | This is a study to test a new vaccine (Covax-19) against COVID-19 | Recombinant spike protein with Advax adjuvant | Phase 1 | Sponsor: Vaxine Pty. Ltd. | NCT04453852 [ ] |
| Collaborator: Central Adelaide Local Health Network Incorporated | ||||||
| 10 | An interventional study to evaluate the safety and immune response of a vaccine against SARS-CoV-2, when given to healthy adult participants | To assess the safety and tolerability of SARS-CoV-2 Sclamp vaccine compared to placebo by evaluating solicited local adverse events will be evaluated by severity score, frequency, duration, and intensity by FDA toxicity scoring. | Molecular clamp stabilized spike protein with MF59 adjuvant | Phase 1 | University of Queensland, CSL, Seqirus | ACTRN12620000674932 [ ] |
| 11 | A study to evaluate the safety and immunogenicity of MVC-COV1901 against COVID-19 | This is a prospective, open-labelled, single-center study to evaluate the safety and immunogenicity of MVC-COV1901. | S-2P protein+CpG 1018 | Phase 1 | Medigen Vaccine Biologics Corp. | NCT04487210 [ ] |
| 12 | Study of the safety, reactogenicity, and immunogenicity of “EpiVacCorona” vaccine for the prevention of COVID-19 (EpiVacCorona) | The research tasks are to evaluate the safety, reactogenicity of the EpiVacCorona vaccine when administered twice intramuscularly and to identify the development of adverse. | Peptide | Phase 1 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | NCT04527575 [ ] |
| 13 | A randomized, double-blind, placebo-controlled phase I trial for anti-novel coronavirus pneumonia (COVID-19) recombinant vaccine (Sf9) | The aim is to evaluate the safety, tolerability, and immunogenicity of a recombinant SARS-CoV-2 vaccine (Sf9 cell) in a healthy Chinese population aged 18 years and older. | RBD (baculovirus production expressed in Sf9 cells) | Phase 1 | West China Hospital, Sichuan University | ChiCTR2000037518[ ] |
| 14 | Safety and immunogenicity trial of multi-peptide vaccination to prevent COVID-19 infection in adults (pVAC) | To evaluate the safety and immunogenicity of a single use of a SARS-CoV-2-derived multi-peptide vaccine in combination with the toll-like receptor 1/2 ligand XS15 in adults. | SARS-CoV-2 HLA-DR peptides | Phase 1 | University Hospital Tuebingen | NCT04546841 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HIV, human immunodeficiency virus; RBD, receptor-binding domain; FIH, first-in-human; FDA, U.S. Food and Drug Administration.
DNA-Based Vaccines
A typical DNA vaccine is a plasmid DNA molecule that codes for the host immune system to be presented with one or more antigens [ 62 ]. They have the advantages of stability and successful delivery over mRNA vaccines [ 84 ]. Still, since they are needed to reach the nucleus, they have the risk of vector mutations and incorporation into the host genome [ 85 ]. DNA vaccines reflect a revolutionary approach, followed by a wide variety of immune responses, by the direct injection of plasmids encoding antigens [ 86 ]. The most groundbreaking approach to vaccination is the introduction of the DNA vaccine that codes for the antigen and an adjuvant that stimulates the adaptive immune response [ 87 ]. The transfected cells express the transgene, which gives a steady supply of transgene-specific proteins very similar to the live virus [ 84 ]. Also, immature DCs, which eventually present the antigen on the cell surface to the CD4 + and CD8 + T cells in combination with the major histocompatibility complex (MHC) 2 and MHC 1 antigens, endocytose the antigen material, thereby stimulating both successful humoral and cell-mediated immune systems [ 87 ]. DNA vaccines are considered safe and stable and can be developed easily, but their immunogenicity and immune response efficiency in humans have not yet been demonstrated [ 21 ].
INO-4800 (Inovio Pharmaceuticals)
It is an anti-SARS-CoV-2 prophylactic DNA vaccine. It uses the SARS-CoV-2 codon-optimized S protein sequence to which an immunoglobulin E (IgE) leader sequence is attached [ 29 ]. Using BamHI and XhoI, the SARS-CoV-2 IgE-spike sequence was synthesized and digested. Under the management of IE CMV, and BGH polyadenylation signal, the digested DNA was incorporated into the expression plasmid pGX0001 [ 85 ]. In preclinical studies, the existence of functional antibodies and the response of T cells indicate that the vaccine will produce an efficient immune response within seven days after vaccination [ 88 ]. The vaccine has entered phase I clinical trials (phase I: {"type":"clinical-trial","attrs":{"text":"NCT04336410","term_id":"NCT04336410"}} NCT04336410 ) and it is anticipated that this phase of clinical trials will be completed by July, with participants receiving 1.0 mg of INO-4800 by electroporation with CELLECTRA 2000 per dosing visit. The research will assess the immunological profile, efficacy, and tolerability of the candidate vaccine in healthy human adults upon intradermal injection and electroporation [ 29 ]. INO-4800 and the previous Inovio vaccine INO-4700 express either SARS-CoV-2-S or MERS-CoV-S inside the same DNA vector, respectively [ 85 ]. The vaccine is delivered by intramuscular injection, accompanied by injection site electroporation. The need for electroporation could restrict INO-4800's ability to be expanded to the scales necessary for a global pandemic and may be difficult to handle globally [ 13 ].
bacTRL (Symvivo Corporation)
Symvivo Corporation's bacTRL platform uses the engineered probiotic Bifidobacterium longum to deliver a SARS-CoV-2-S expressing DNA vaccine into intestinal cells. The first-in-man study of the bacTRL platform will also be a phase I study of the COVID-19 vaccine, so no prior immunological results are available [ 13 ]. In Table 4 , DNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 89 , 90 , 91 , 92 , 93 , 94 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Safety, tolerability, and immunogenicity of INO-4800 followed by electroporation in healthy volunteers for COVID-19 | INO-4800 was administered by intradermal injection followed by electroporation using the CELLECTRA 2000 device in healthy adults aged 19 to 64 years of Korea. | DNA plasmid vaccine with electroporation | Phase 1/2 | Sponsor: International Vaccine Institute | NCT04447781 [ ] |
| Collaborator: Inovio Pharmaceuticals | ||||||
| 2 | Safety, tolerability, and immunogenicity of INO-4800 for COVID-19 in healthy volunteers | This is an open-label trial of INO-4800 which contains the plasmid pGX9501, which encodes for the full length of the Spike glycoprotein of SARS-CoV-2. | DNA plasmid vaccine with electroporation | Phase 1 | Sponsor: Inovio Pharmaceuticals | NCT04336410 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations | ||||||
| 3 | Study of COVID-19 DNA vaccine (AG0301-COVID19) | This is a single-center, non-randomized, open-label, non-controlled trial. 30 healthy volunteers aged 20–65, will be enrolled for low and high dose group. | DNA plasmid vaccine+adjuvant | Phase 1/2 | Sponsor: AnGes Inc. | NCT04463472 [ ] |
| Collaborator: Japan Agency for Medical Research and Development | ||||||
| 4 | Study of COVID-19 DNA vaccine (AG0302-COVID19) | This study will assess the safety and immunogenicity of AG0302-COVID19 in healthy adult volunteers. | DNA plasmid vaccine+adjuvant 2 | Phase 1/2 | AnGes Inc. | NCT04527081 [ ] |
| 5 | Novel corona virus-2019-nCov vaccine by intradermal route in healthy subjects | A prospective, randomized, adaptive clinical study to evaluate the safety and immunogenicity of novel corona virus-2019-nCov vaccine candidate. | DNA plasmid vaccine | Phase 1/2 | Cadila Healthcare Limited | CTRI/2020/07/026352 [ ] |
| 6 | Safety and immunogenicity study of GX-19, a COVID-19 preventive DNA vaccine in healthy adults | This clinical study is to evaluate the safety, tolerability, and immunogenicity of the COVID-19 preventive vaccine by intramuscular administration in healthy volunteers. | DNA Vaccine (GX-19) | Phase 1/2 | Genexine Inc. | NCT04445389 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Virus-Like Particles Vaccine
VLPs are particles that form spontaneously, consisting of many co-expressed or mixed structural viral proteins. Several commercial vaccines are based on VLPs, such as hepatitis B and human papillomavirus vaccines [ 95 ]. Without the need for adjuvants, these vaccines can be constructed and used. Only when antigens with neutralizing epitopes are extensively investigated is the production of such vaccines possible [ 22 ]. A VLP is a self-assembled nanostructure incorporating essential viral structural proteins. VLP is similar to true viruses' molecular and morphological features but is non-infectious and non-replicating due to the absence of genetic materials [ 26 ]. Successful applications of VLP have been proved by vaccinological and virological study [ 95 ]. In the ongoing battle against the COVID-19 pandemic, the development of SARS-CoV-2 VLPs is highly in demand as an accessibly safe and relevant substitute for naturally pathogenic viruses [ 26 ]. A study suggested the possible use of plant biotechnology for the development of low-cost COVID-19 vaccines and plant-made antibodies for diagnosis, prophylaxis, and therapy [ 22 ].
In the current research, we have established SARS-CoV-2 VLPs effectively, using the mammalian expression system [ 47 ], which helps maintain specific patterns of protein glycosylation [ 22 ]. For the efficient formation and release of SARS-CoV2 VLPs among the four SARS-CoV-2 structural proteins, we have shown that membrane protein (M) expression and small envelope protein (E) are essential [ 47 ]. Also, the corona-like structure presented in SARS-CoV-2 VLPs from Vero E6 cells is more stable and unified in comparison with those from HEK-293 T cells. Our data show that the molecular and morphological characteristics of native virion particles in SARS-CoV-2 VLPs make SARS-CoV-2 VLPs a promising candidate vaccine and a powerful tool for research into SARS-CoV-2 [ 96 ]. The immunogenic composition composed of MERS-CoV nanoparticle VLPs containing at least one trimer of S protein formed by baculovirus overexpression in Sf9 cells was disclosed in patent application WO2015042373 by Novavax in 2015 [ 35 ]. When administered along with their patented adjuvant Matrix M, this VLP preparation induced a neutralizing antibody response in mice and transgenic cattle. Sera preparations from vaccinated cattle (SAB-300 or SAB-301) were also injected into Ad5-hDPP4 transduced BALB/c mice before the MERS-CoV challenge [ 22 ]. With a single prophylactic injection, both SAB-300 and SAB-301 were able to protect these mice from MERS-CoV infection [ 96 ]. On 26 February, Novavax announced that due to their prior experience dealing with other coronaviruses, including both MERS and SARS, animal testing of possible COVID-19 vaccine candidates had begun. Using their recombinant nanoparticle vaccine technology along with their proprietary adjuvant matrix-M, their COVID-19 candidate vaccines targeting the S protein of SARS-CoV-2 were created [ 35 ].
UMass Medical School researchers have developed a framework to create vaccines using VLPs, which one scientist claims may be a successful and safer alternative to a COVID-19 vaccine. Trudy Morrison, Ph.D., professor of Microbiology & Physiological Systems, said her work on a VLP-based respiratory syncytial virus vaccine that can be modified to COVID-19 causes severe lower respiratory tract disease in young children and the elderly. And some of the problems inherent in the production of vaccines from inactivated or live viruses will be avoided [ 97 ].
Medicago, a biopharmaceutical company, headquartered in Quebec City, announced the successful development of a coronavirus VLP only 20 days after the SARS-CoV-2 (COVID-19 disease virus) gene was obtained [ 29 ]. The manufacturing of VLP is the first step in the development of the COVID-19 vaccine, which will now undergo preclinical protection and efficacy testing. They plan to negotiate clinical testing of the vaccine with the relevant health authorities by summer (July/August) 2020 once this is done. Medicago uses its technology platform to create antibodies against SARS-CoV-2. These antibodies to SARS-CoV-2 might theoretically be used to treat people who are infected by the virus. In part, this study is sponsored by the Canadian Institutes for Health Research [ 98 ]. In Table 5 , potential VLPs-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 81 , 99 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults | RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. | RBD-HBsAg VLPs | Phase 1/2 | Sponsor: SpyBiotech | ACTRN12620000817943 [ ] |
| Collaborator: Serum Institute of India | ||||||
| 2 | Safety, tolerability, and immunogenicity of a coronavirus-like particle COVID-19 vaccine in adults aged 18–55 years | The study will be a randomized, partially-blinded, prime-boost, staggered dose-escalation study at three dose levels (3.75 µg, 7.5 µg, and 15 µg VLP). | Plant-derived VLP was adjuvanted with GSK or Dynavax adjs. | Phase 1 | Medicago | NCT04450004 [ ] |
VLP, virus-like particle; COVID-19, coronavirus disease 2019; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HBsAg, Hepatitis B surface antigen.
Current Updates
To bring this pandemic to an end, a large share of the world needs to be immune to the virus. The safest way to achieve this is with a vaccine. Vaccines are a technology that humanity has often relied on in the past to bring down the death toll of infectious diseases. Within less than 12 months after the beginning of the COVID-19 pandemic, several research teams rose to the challenge and developed vaccines that protect from SARS-CoV-2, the virus that causes COVID-19. Now the challenge is to make these vaccines available to people around the world.
To resume a normal lifestyle, free from government lockdowns, and fear of continuing pandemic waves over the coming months, the world is anxiously awaiting a safe, successful vaccine to protect against COVID-19. Innovative ties with both pharmaceutical companies and medical start-ups are joining hands with scientists across the continents to repurpose medications, create vaccines, and devices to hinder the progress of this overwhelming pandemic. A large number of vaccine candidates for COVID-19 based on different platforms have already been identified. Current review shows preclinical as well as in clinical development of vaccine candidates, wherein, five major vaccine platforms for COVID-19 namely RNA, DNA, viral vector, protein subunit, and VLP which constitutes 10, 2, 10, 14, and 2 vaccine candidates globally in clinical development as of 15 October 2020. Among all the vaccine platforms, extensive research and development are going on protein subunit-based vaccine which has the maximum candidates in the clinical development.
A significant amount of hindrance to the rapid production of vaccines is the length of clinical trials. With several phases, including the preclinical stage and clinical development, which is a three-phase process, the vaccine development process is very laborious. However, if adequate data is already available, it has been proposed that a few stages be skipped to accelerate the achievement of a vaccine faster with a rapid regulatory review, approval, development, and quality control. By looking towards pandemic conditions, the scientific fraternity will be ready for any harmful situation to overwhelmed opportunities. Therefore, the current situation has given the world a new perspective to facilitate research in the worst circumstances and hasten the drug development process.
No potential conflict of interest relevant to this article was reported.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Medicinal Chemistry and Pharmacognosy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Family and Community Medicine, Faculty of Medicine, University of Jordan, Amman, Jordan
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Roles Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing
Roles Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing
Affiliations Faculty of Pharmacy, QU Health, Qatar University, Doha, Qatar, Biomedical and Pharmaceutical Research Unit, QU Health, Qatar University, Doha, Qatar
- Tamam El-Elimat,
- Mahmoud M. AbuAlSamen,
- Basima A. Almomani,
- Nour A. Al-Sawalha,
- Feras Q. Alali

- Published: April 23, 2021
- https://doi.org/10.1371/journal.pone.0250555
- Reader Comments
Vaccines are effective interventions that can reduce the high burden of diseases globally. However, public vaccine hesitancy is a pressing problem for public health authorities. With the availability of COVID-19 vaccines, little information is available on the public acceptability and attitudes towards the COVID-19 vaccines in Jordan. This study aimed to investigate the acceptability of COVID-19 vaccines and its predictors in addition to the attitudes towards these vaccines among public in Jordan. An online, cross-sectional, and self-administered questionnaire was instrumentalized to survey adult participants from Jordan on the acceptability of COVID-19 vaccines. Logistic regression analysis was used to find the predictors of COVID-19 vaccines’ acceptability. A total of 3,100 participants completed the survey. The public acceptability of COVID-19 vaccines was fairly low (37.4%) in Jordan. Males (OR = 2.488, 95CI% = 1.834–3.375, p < .001) and those who took the seasonal influenza vaccine (OR = 2.036, 95CI% = 1.306–3.174, p = .002) were more likely to accept COVID-19 vaccines. Similarly, participants who believed that vaccines are generally safe (OR = 9.258, 95CI% = 6.020–14.237, p < .001) and those who were willing to pay for vaccines (OR = 19.223, 95CI% = 13.665–27.042, p < .001), once available, were more likely to accept the COVID-19 vaccines. However, those above 35 years old (OR = 0.376, 95CI% = 0.233–0.607, p < .001) and employed participants (OR = 0.542, 95CI% = 0.405–0.725, p < .001) were less likely to accept the COVID-19 vaccines. Moreover, participants who believed that there was a conspiracy behind COVID-19 (OR = 0.502, 95CI% = 0.356–0.709, p < .001) and those who do not trust any source of information on COVID-19 vaccines (OR = 0.271, 95CI% = 0.183–0.400, p < .001), were less likely to have acceptance towards them. The most trusted sources of information on COVID-19 vaccines were healthcare providers. Systematic interventions are required by public health authorities to reduce the levels of vaccines’ hesitancy and improve their acceptance. We believe these results and specifically the low rate of acceptability is alarming to Jordanian health authorities and should stir further studies on the root causes and the need of awareness campaigns. These interventions should take the form of reviving the trust in national health authorities and structured awareness campaigns that offer transparent information about the safety and efficacy of the vaccines and the technology that was utilized in their production.
Citation: El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ (2021) Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PLoS ONE 16(4): e0250555. https://doi.org/10.1371/journal.pone.0250555
Editor: Francesco Di Gennaro, National Institute for Infectious Diseases Lazzaro Spallanzani-IRCCS, ITALY
Received: December 23, 2020; Accepted: April 11, 2021; Published: April 23, 2021
Copyright: © 2021 El-Elimat et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: TE received the fund. This research was supported by the Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan [Grant number 816/2020]. https://www.just.edu.jo/Deanships/DeanshipofResearch/Pages/Default.aspx The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative virus for the coronavirus disease 2019 (COVID-19) ongoing pandemic [ 1 – 4 ]. SARS-CoV-2 first emerged in late 2019 in Wuhan (Hubei, China) and hastily become a global threat affecting 220 countries [ 1 , 2 ]. As of 22 December, the COVID-19 pandemic has resulted in more than 76.2 M cases and more than 1.6 M deaths worldwide [ 1 ]. The pandemic has resulted in a devastating impact worldwide, which prompted the need for mitigation policies to contain the pandemic [ 5 ]. The ground strategy followed by most countries around the world was to reduce the transmissibility of the disease, often by non-pharmaceutical interventions (NPIs), including enforcing masks policy, hands sanitization, social distancing, travel restrictions, schools’ closures, and partial or complete lockdowns. [ 6 ]. So far, NPIs were able to slow down the progression of the disease, but the most promising strategy to confine the pandemic and providing hope to reduce the mortality and morbidity rates remains within the capacity of medical technology. Such medical technology includes effective, safe, and affordable antiviral agents and vaccines. As of December 2020, no antiviral drugs have been approved that were specifically developed against SARS-CoV-2 [ 7 ]. The US Food and Drug Administration (FDA) has granted remdesivir an Emergency Use Authorization for severely ill hospitalized patients with COVID-19 [ 8 , 9 ]. However, the WHO recommended against its use in November 2020 [ 10 ].
Vaccines are one of the most reliable and cost-effective public health interventions ever implemented that are saving millions of lives each year [ 11 – 13 ]. Following the deciphering of the genome sequence of SARS-CoV-2 in early 2020 [ 3 ] and the declaration of the pandemic by WHO in March 2020 [ 4 ], scientists and pharmaceutical companies are racing against time in efforts to develop vaccines [ 14 , 15 ]. As of December 22, 2020, at least 85 vaccines are in preclinical development in animals and 63 are in clinical development in humans, of which 43 in phase I, 21 in phase II, 18 in phase III, 6 have been approved for early or limited use, 2 have been approved for full use, and one vaccine has been abandoned [ 14 ]. Pfizer-BioNTech’s (BNT162b2) and Moderna (mRNA-1273) mRNA vaccines have been approved for emergency use in the US [ 14 ].
With the uplifting news about SARS-CoV-2 vaccines approval, optimism is raising to see an end to the pandemic through herd immunity [ 16 , 17 ]. The threshold for SARS-CoV-2 herd immunity is estimated to range between 50% and 67% [ 16 ]. One major obstacle facing the achievement of such a goal is believed to be vaccine hesitancy and skepticism among the population worldwide [ 15 , 18 – 20 ]. Vaccine hesitancy was defined by the WHO Strategic Advisory Group of Experts (SAGE) as “ delay in acceptance or refusal of vaccination despite availability of vaccination services ” [ 19 ]. Vaccine acceptability is determined by three factors: confidence, convenience, and complacency [ 21 ]. Confidence is defined as the trust in the safety and effectiveness of the vaccine, trust in the delivery system as the healthcare system, and the trust in the policymakers [ 22 ]. Many people have doubts about vaccine safety, and this is going to be a major challenge to be resolved by health care providers, policymakers, community leaders, and governments to increase the widespread acceptance of the vaccines [ 15 , 18 , 19 ]. Moreover, vaccination convenience refers to the relative ease of access to the vaccine that includes physical availability, affordability, and accessibility [ 19 ]. Vaccine complacency is associated with a low realized risk of the vaccine-preventable disease and hence more negative attitudes towards the vaccines [ 22 ]. Such skepticism was demonstrated in a poll that was conducted in the US, where 50% of the Americans said they are willing to take the vaccine, 30% are unsure, while 20% are refusing the vaccine [ 23 ]. In another survey of adult Americans, 58% intended to be vaccinated, 32% were not sure, and 11% did not intend to be vaccinated [ 24 ]. However, one more study reported 67% of the Americans would accept a COVID-19 vaccine if it is recommended to them, although there were significant demographic differences in vaccine acceptance [ 25 ].
Jordan, with a population of 10 M, has one of the highest per-capita rates of COVID-19 infection in the world [ 1 , 26 ]. As of December 2020, Jordan has reported more than 271,000 COVID-19 confirmed cases and over 3,500 deaths [ 1 , 26 ]. This drastic increase, which started in September 2020 was embarked upon after a few months of the control of the pandemic in the country by implementing a very strict preventive lockdown that had painful societal and economic impacts [ 27 ]. The Jordanian government has announced that the first doses of Pfizer-BioNTech’s vaccine are going to be available by the end of January 2021 [ 28 ]. Enough vaccine doses to achieve the herd immunity threshold will not be available till 2022. Hence, it is crucial to explore the acceptance of COVID-19 vaccines and its predictors as well as the attitudes towards COVID-19 vaccines among Jordanian population. The results of current study could assist the policymakers to undertake proactive campaigns and well-designed strategies by highlighting the importance of vaccination to the community and encouraging vaccine uptake and acceptance, especially by vulnerable patients to stop further deaths and to confine the spread of the pandemic.
Materials and methods
Study design.
The study was approved by the Institutional Review Board at Jordan University of Science and Technology (Ref. 110/136/2020). No consent obtained as the data were collected and analyzed anonymously. A cross-sectional survey-based study was conducted in November 2020. A convenience sample approach was adopted in this study where people from the different Jordanian regions (Northern, Central, and Southern) were invited to participate. Amid the global pandemic, researchers utilized social media platforms to collect data. In this study, online social media platforms (Facebook, WhatsApp) were used to recruit participants [ 21 , 22 , 29 ]. National internet use in Jordan stands at 66.8% while social media penetration is 61.5% [ 30 ]. Participants were encouraged to pass on the questionnaire to their contacts or acquaintances. The main outcome of the study was the public acceptance of the COVID-19 vaccine.
Instrument development and measures
The questionnaire used in this study was developed based on literature review [ 22 , 31 ] and discussion within the research team. To reduce potential bias introduced by self-reported data, participants were ensured on the confidentiality and privacy of their responses. The questionnaire was designed to reduce survey fatigue and was reviewed by experts in survey research for face validity. The questionnaire was structured into 4 sections. A pilot sample (n = 26) was used to improve the wording and clarity of expression of the survey items. Data from the pilot sample was not used in any further analysis. The final version of the questionnaire required an estimated time of 5–10 minutes to complete. The questionnaire was originally developed in English and forward translated into Arabic language. The questionnaire was piloted and distributed in Arabic. A copy of the administered questionnaire both in Arabic and English can be found in S1 Table .
Sociodemographic characteristics and medical history
The sociodemographic characteristics of the participants were obtained as described below. Data collected were age, gender, marital status, smoking, employment status, academic level, and medical insurance. Additionally, participants were asked to report their history with chronic conditions and whether they took a seasonal flu vaccine this year or not.
COVID-19 pandemic related information
Participants were asked to indicate if they were infected with COVID-19 or knew anyone who was infected with confirmation of diagnosis using standard laboratory testing protocols. Another question item was dedicated to surveying participants who believe they may have contracted the virus but without a confirming test.
Participants were asked to indicate their most trusted sources when seeking knowledge of COVID-19 vaccines. Besides, participants were asked about their concerns during the COVID-19 pandemic.
Acceptance of COVID-19 vaccines
Participants were asked whether they accept to receive COVID-19 vaccines when they are approved and available in Jordan with 3 response levels (non-acceptance, neutral, acceptance). Variables that were investigated as potential predictors of COVID-19 vaccines acceptance were: age, gender, marital status, having children, academic area, employment, smoking status, whether the person received a seasonal flu vaccine this year, stating that vaccines are safe, concerned that there is a conspiracy behind COVID-19 pandemic, not having any trust in any source of information on vaccines, and willingness to pay for COVID-19 vaccines.
Attitudes toward COVID-19 vaccines
The attitudes towards COVID-19 vaccines’ section consists of 6 statements with a 5-point Likert scale (5 = strongly agree, 4 = agree, 3 = neutral, 2 = disagree, 1 = strongly disagree), with questions about hesitancy and concerns regarding COVID-19 vaccines. Items in this section had a Cronbach’s alpha of 0.6 which indicate good reliability.
Statistical analysis
Categorical variables were presented as numbers and percentages, while continuous variables were presented as median [interquartile range]. The univariate analysis was performed using an independent Mann–Whitney U test for continuous variables and Chi-square test for categorical variables as appropriate. For analysis, responses to the attitudes section were combined. For example, both responses "strongly agree" and "agree" were combined in one category and both responses "strongly disagree" and "disagree" in one category. Prior to analysis, independence of variables was analysed using a correlation matrix. No multicollinearity was detected among predictor variables.
The main outcome of the study was the public acceptance of COVID-19 vaccines. To determine the factors that affect the acceptance of the Jordanian population to receive COVID-19 vaccines, both multinomial and binary logistic regressions were performed. At first, potential predictors for COVID-19 vaccines were screened using univariable analysis, and variables with p < .05 were considered in both multinomial and binary logistic regression. When the multinomial logistic regression was conducted, the acceptance outcome was trichotomized as (non-acceptance, neutral, and acceptance). For a simpler interpretation of the analysis, the participants who answered ‘neutral’ were then removed and a binary logistic regression was performed. In the binary logistic regression model, the participants were dichotomized as acceptable or not acceptable. In both models, the odds ratio (OR) values and their 95% confidence intervals (95% CI) were calculated. A p -value of less than .05 was considered statistically significant. The analysis was carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) version 23.
Demographics
The study received 3180 submissions of which 3100 were complete and included in the final analysis. The median age of participants was 29 years old and more than half of them (67.4%) were females. Half of the respondents (49.8%) were married and had kids (46.1%). About 70% had an undergraduate degree and more than half (53.8%) with health-related educational backgrounds. Besides, 46.4% of the participants were employed and only 13.4% had chronic diseases. Detailed demographics are presented in Table 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0250555.t001
Less than 10% of the participants received the influenza vaccine this year. About 10% of the respondents reported that they had tested positive for COVID-19. However, about one-third of the participants (37.1%) stated that they might have been infected with COVID-19, but they did not confirm it by any laboratory testing.
As shown in Fig 1 , almost half (45.4%) of the participants trusted healthcare providers as a source of information about COVID-19 vaccines. About 30%, 17%, and 16% of the participants trusted pharmaceutical companies, the internet, and media as sources of information, respectively. However, 18.1% of the participants did not trust any source.
https://doi.org/10.1371/journal.pone.0250555.g001
The participants were concerned about different issues during the COVID-19 pandemic ( Fig 2 ). The most-reported concern by participants was a fear of family members being infected with COVID-19 (73.1%), which is higher than a concern about themselves being infected (27.3%). Almost one-third of the participants (30%) were concerned about death and 17.5% about the unavailability of vaccines. Approximately, a tenth of the participants was not worried about any issue.
https://doi.org/10.1371/journal.pone.0250555.g002
Acceptance for COVID-19 vaccines
In the present study, 37.4% of the public were acceptable, 36.3% were not acceptable and 26.3% were neutral to receive COVID-19 vaccines. As shown in Table 2 , the results of multivariate analysis (binary logistic regression) identified the independent factors that predicted the level of acceptance. The result indicated that the older age groups (>35 years old) were less likely to accept for COVID-19 vaccines compared to younger age groups (OR = 0.376, 95CI% = 0.233–0.607, p < .001). In addition, employed participants (OR = 0.542, 95CI% = 0.405–0.725, p < .001) were less likely to accept COVID-19 vaccines compared to unemployed participants. Furthermore, participants who believed that the COVID-19 pandemic is a conspiracy (OR = 0.502, 95CI% = 0.356–0.709, p < .001) and those who did not trust any information (OR = 0.271, 95CI% = 0.183–0.400, p < .001) were less acceptable for the vaccine.
https://doi.org/10.1371/journal.pone.0250555.t002
On the other hand, males were more likely to have acceptance for COVID-19 vaccines (OR = 2.488, 95CI% = 1.834–3.375, p < .001) compared to females. In addition, participants who took the influenza vaccine this year were more likely to accept COVID-19 vaccines compared to those who did not take the influenza vaccine (OR = 2.036, 95CI% = 1.306–3.174, p = .002). Furthermore, participants who stated that vaccines are safe in general were 9 times more likely to accept taking COVID-19 vaccines compared to those who stated that vaccines are not safe (OR = 9.258, 95CI% = 6.020–14.237, p < .001). Moreover, participants who expressed their willingness to pay for COVID-19 vaccines were 19 times more likely to accept taking them compared to those who did not show their willingness to pay (OR = 19.223, 95CI% = 13.665–27.042, p < .001).
Similarly, results from multinomial logistic regression including the ‘neutral’ group, suggested that the predictors that make participants more likely to be in the acceptance group compared to the neutral group were being male (OR = 1.522, 95% CI = 1.226–1.890, p < .0001) and among the age group of 18–25 years (OR = 1.496, 95% CI = 1.083–2.067, p = .015). Additionally, people who did not believe that there is a conspiracy behind COVID-19 were also 1.4 times more likely to be in the acceptance group (OR = 1.392, 95% CI = 1.022–1.898, p = .036). However, predictors that make people less likely to be in the acceptance group compared to the neutral group were people who did not take the seasonal flu vaccine this year (OR = 0.675, 95% CI = 0.485–0.941, p = .02), those who believe that vaccines are unsafe (OR = 0.333, 95% CI = 0.223–0.498, p < .0001), and those who are not willing to pay for the vaccine once available (OR = 0.321, 95% CI = 0.258–0.399, p < .0001).
Almost two-thirds (66.5%) of the participants were strongly agreed/agreed that it is important to get a vaccine to protect people from COVID-19. Besides, less than 60% of the participants agreed that pharmaceutical companies will be able to develop safe and effective COVID-19 vaccines. Moreover, about half of the respondents (49.6%) reported that side effects will prevent them from taking a COVID-19 vaccine and that 49.3% will refuse to take COVID-19 vaccines once licensed. Importantly, around a quarter of all respondents were neutral regarding most attitudes as shown in Table 3 .
https://doi.org/10.1371/journal.pone.0250555.t003
This study sought to examine, for the first time, the Jordanians’ population acceptance of COVID-19 vaccines. Vaccine hesitancy could threaten the efficiency of COVID-19 vaccines once they become commercially available worldwide [ 22 ]. Compared to reports from studies conducted on public acceptance and willingness to receive the COVID-19 vaccines worldwide, Jordan stands among the lowest countries as 37.4% of our public were acceptable. A study based on a sample from 19 countries involving 13,426 participants showed that the global acceptance of COVID-19 vaccines ranges between as low as 54.8% from Russia to as high as 88.6% from China [ 32 ]. Moreover, most western countries report relatively higher public acceptance (59–75%) [ 32 ]. Similarly, Saudi Arabia, a country with similar demographic distributions as Jordan, reported a higher acceptance level (64.7%) [ 21 ]. The acceptability level of vaccinations in Jordan was often lower than global averages, including seasonal influenza vaccines in 2016–2018 [ 33 , 34 ].
In the current study, predictors of COVID-19 vaccines from both multinomial and binary logistic regression were similar. Younger participants were more likely to accept COVID-19 vaccines in the current study, contrary to studies reporting higher acceptance among older age groups [ 25 , 21 , 32 ]. This can be explained by the different age distribution among countries, given Jordan as a country with mostly a young population and high literacy levels. Another reason could be related to the nature of the study design, this study could have been more biased towards the young, as the elderly are less likely to engage with online-based surveys. Moreover, there are contrasting reports of gender effects in the literature, wherein some males were more likely to accept the vaccine [ 25 , 35 ], compared to others reporting higher acceptance among females [ 21 , 32 ]. In our study, Jordanian males were more likely to take the vaccine, in agreement with studies reported elsewhere [ 25 ]. Interestingly, Jordanian males were more likely to participate in COVID-19 vaccine clinical trials compared to females in 2020 [ 29 ]. Moreover, employed participants were less likely to accept taking the COVID-19 vaccines, in a contrasting result to available studies in the literature suggesting that employed individuals were more likely to accept COVID-19 vaccines. In our study, the employed participants were older than the unemployed ones, which were found to be less acceptable to get COVID-19 vaccines.
The low acceptance level of COVID-19 vaccines among Jordanians can be attributed to multi factors, some of which are shared with the wide global community. There is an evident uncertainty clouding the COVID-19 vaccines. Firstly, the new mRNA-based vaccines as a novel technology could be received with some skepticism since no prior experience or successes with such approach have been reported in the past. Also, the speed of vaccine development and registration in less than a year may have mediated a role in lowering the acceptance level. The current study revealed that half of the participants had safety concerns about the vaccine once it being available as indicated by their concerns about related side effects. This is consistent with Pogue and colleagues finding where the majority of participants (~63%) in the USA stated that they were worried about the side effects of the COVID-19 vaccines [ 36 ]. Also, most of the participants (66.5%) in the current study stated that receiving the vaccine is important to protect against COVID-19. However, almost half of them (49%) agreed that most people would refuse to take the vaccine. This discrepancy could be due to their concerns about the vaccine’s side effects. Another global phenomenon that negatively contributed to such a low level is the numerous campaigns launched by anti-vaccinationists fueled by the new technology and short span of vaccine development. Such campaigns on social media with fabricated, false, and sometimes misleading Arabic translations feed the conspiracy beliefs of some people. Our results supported such perceived viewpoints, where those who did not believe in a conspiracy behind COVID-19 were more likely to accept COVID-19 vaccines. Some factors that are specific to the country and the region could also play a role in this. For example, there is a sector of the public who had their trust shaken in local Jordanian authorities and/or disapprove the overall handling of the pandemic. Some people expressed their frustration as many decisions were unwelcomed, disproportional with the pandemic status, not justified or backed with science. During August-October 2020, an increasingly larger sector of the public expressed their belief that Jordan was moving in the negative direction, amid rising daily confirmed cases and high death rate, reaching 80.0% disapproval rate in late October 2020 [ 37 ]. These indications were in agreement with reports associating lower acceptance levels of COVID-19 vaccines with lower trust in government handling of the pandemic [ 32 ]. Such low acceptance levels should prompt the government to offer commensurate efforts in offering vaccine awareness campaigns that can regain people’s trust in their government handling of the ongoing crisis. Future research may capture the evidence for COVID19 vaccines hesitancy and governance in Jordan.
An important factor to consider when exploring vaccine acceptability is vaccine convenience in terms of its availability and affordability [ 19 ]. In the current study, the willingness to pay for the vaccine was a predictor of vaccine acceptance. According to the Jordanian Ministry of Health (MOH), the government will only be able to provide free COVID-19 vaccines for 20.0% of the population, while the rest will have to purchase it, without information whether it will be available at a subsidized price or not [ 38 ]. This should be factored in the government’s planning for vaccination programs and how acceptance level may change depending on the prices ascribed to the vaccines. In the current study, only 36.2% believed that the government will be able to provide the vaccine for free, indicating that the economic challenges faced by the Jordanian government may have played a role in shrinking the acceptance level. Nevertheless, this study did not investigate the issue of the participants economic capacity and ability to buy the vaccine if available, which should be investigated in the future. Further, the trust in the manufacturer that provides effective and noncontaminated products is another important determinant of confidence. About two-thirds of participants (59%) in the current study had confidence in pharmaceutical companies to develop safe and effective COVID-19 vaccines. However, the source of the vaccine affects the perceived safety, as only one-third of the participants in the current study perceived that COVID-19 vaccines that were manufactured in Europe or America were safer than those made in other countries. This is rather lower than the reported percentage by Pogue and colleagues where ~55% and 36% of participants stated that they were more comfortable with vaccines made in the USA and Europe, respectively [ 36 ].
COVID-19 pandemic as with other previous pandemics is associated with feelings of fears, anxiety, and worries [ 39 , 40 ]. However, it is unique in terms that people are not worried only about getting infected or transmit the disease to others [ 41 ], but they suffered societal and economic concerns due to the measures that were undertaken by the governments to confine the pandemic and stopping the human-human transmission of the disease [ 6 ]. These measures include enforcement of curfews and lockdowns (the largest throughout history), social distancing and self-isolation, schools and universities closures, borders’ shutdowns, travel restrictions, and quarantine [ 6 ]. In the current study, family members being infected (73.1%), topped the list of Jordan population worries during the COVID-19 pandemic followed by fears of death (30.0%), and then anxieties of becoming infected themselves (27.3%). The least-worries were financial related worries and being forced to take medication, respectively. Our findings were in alignment with the findings of Mertens et al, where increased fear during the COVID-19 pandemic was found to be related to perceived risks for family members and health anxiety [ 42 ]. Such a high percentage of fear over loved ones get infected could be attributed to the reports identifying elderly people with chronic diseases such as hypertension, diabetes, chronic respiratory disease, and weakened immune systems as a high-risk group to get infected with COVID-19 [ 43 ].
One of the themes of worries during the COVID-19 pandemic that were identified in a study from the Philippines is the worry of acquiring the diseases for self, family, and others [ 44 ]. Interestingly, the self-worry is focused mainly on preventing the transmission of the disease to family members especially older ones who were identified as vulnerable to COVID-19 [ 44 ].
Holingue et al showed in a population-based study of US adults that the fears and anxiety of getting infected with and die from COVID-19 were associated with increased mental distress [ 41 ]. Moreover, the personal hygienic precautions that were undertaken by individuals to avoid infecting others had increased the probability of becoming mentally distressed [ 41 ]. A systematic review and meta-analysis of the psychological and mental impact of COVID-19 showed that the prevalence of anxiety and depression was 33% and 28%, respectively [ 45 ].
During the COVID-19 pandemic, people used multiple information resources to gain knowledge and health information about the disease, including television, radio, newspapers, social media, friends, co-workers, healthcare providers, scientists, governments, etc [ 46 ]. Since such information sources can shape peoples’ acceptance or refusal of COVID-19 vaccines [ 47 ], it is crucial to disseminate transparent and accurate information about vaccines’ safety and efficacy to gain the trust of the population especially the hesitant and skeptical ones [ 48 ]. Hence, gaining an understanding of the resources that people trust the most to get information about COVID-19 vaccines is critical for the success of any future national vaccination campaign.
When Jordanians were asked about the most-trusted information sources about COVID-19 vaccines, health care providers topped the list, followed by pharmaceutical companies reports, and the national Jordanian government. The least-trusted information sources were social media and family members, respectively. Our findings are consistent with the KFF COVID-19 Vaccine Monitor: December 2020; where about 85% of the U.S. adults said they trust the most their doctors or healthcare providers for information related to COVID-19 vaccines, followed by national messengers (73%), and the FDA (70%) [ 49 ]. Our data were also in agreement with another study where the participants reported that they trust health care professionals the most, followed by their physicians [ 25 ]. In a further study, COVID-19 vaccine acceptance among college students in South Carolina was found to be affected by the information resources. Students largely trusted scientists (83%), followed by healthcare providers (74%), and then health agencies (70%) [ 50 ]. However, contrary to our study, college students do not trust information disseminated by pharmaceutical companies [ 50 ]. In a study from France, vaccination practices and acceptance toward MMR and HBV vaccines were better when parents had reported getting the information from their healthcare providers compared with parents getting information from the internet or their relatives [ 51 ]. The trust of the Jordanian population in the government as a source of information for COVID-19 vaccines is indicative of the peoples’ trust in the health system and the registration procedure of vaccines in Jordan. However, it should be stressed that the trust in most sources among the participants of this study are not as high compared to other studies in the US, which could be attributed to different demographics, health systems, governance and scientific communication.
The nature of this research utilized an unrestricted self-administered survey that was dependent on the online reachability and shareability among participants. This study design also measured COVD-19 vaccines acceptance at a certain point in time that is potentially prone to change with the vaccine roll out in Jordan. The limitations in study design may have introduced selection bias and may have limited generalizability to the general population. Future research is recommended to employ a mixed methods research involving both qualitative and quantitative approaches to fully capture the COVID-19 vaccines hesitancy in Jordan. Future research may also give further insights on COVID-19 vaccines hesitancy variance over time and what factors are associated with such changes. So far, COVID-19 vaccines’ registration and uptake in Jordan are not up to the expected level, and echo the results of this study, as only 360,000 people have registered to be vaccinated after two months of the call to register. Research to be done at this stage may provide an opportunity to study the shifting behaviors of Jordanians in regard to the vaccines.
Conclusions
In conclusion, we identified Jordan as one of the lowest countries in the acceptance of COVID-19 vaccines, where a considerable percentage of the population of Jordan (36.3%) indicated a refusal to get vaccinated, while 26.3% were not sure. Vaccines perceived safety concerns and cost were associated with this refusal. Hence, the health authorities via health care providers, who were identified by the people as the most trust source of information regarding information about COVID-19 vaccines, should design interventions in terms of awareness campaigns via all types of multimedia to spread more transparent information about the safety and efficacy of the vaccines. The awareness campaigns should also shed the light over the new technology that was utilized in the production of few of them in order to boost COVID-19 vaccines acceptance. Making the vaccine available for free or at subsidized prices by the government could as well enhance vaccines acceptance among the population.
Supporting information
S1 table. the final version of the questionnaire in arabic and english..
https://doi.org/10.1371/journal.pone.0250555.s001
- 1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard: World Health Organization; 2020 [cited 2020 13 December]. Available from: https://covid19.who.int/ .
- View Article
- PubMed/NCBI
- Google Scholar
- 9. FDA Approves First Treatment for COVID-19 [Internet]. 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 .
- 23. Neergaard L, Fingerhut H. AP-NORC poll: Half of Americans would get a COVID-19 vaccine: Associated Press; May 28, 2020 [cited 2020 December 14]. Available from: https://apnews.com/article/dacdc8bc428dd4df6511bfa259cfec44 .
- 26. Ministry of Health. COVID-19 Updates in Jordan: Ministry of Health; 2020 [cited 2020 13/12/2020]. Available from: https://corona.moh.gov.jo/en/MediaCenter/1491 .
- 27. Jensehaugen J. Jordan and COVID-19: Effective Response at a High Cost. Oslo: PRIO, 2020.
- 30. Kemp S. Digital 2021: Jordan, retrieved from https://datareportal.com/reports/digital-2021-jordan . 2021.
- 31. World Health Organization. Report of the SAGE Working Group on Vaccine Hesitancy. 2014 [accessed November, 2020]. Available from: http://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf .
- 37. Center for Strategic Studies. Jordanian Index Surveys Series: Jordanian Street Pulse—24 Amman: University of Jordan; 2020 [updated October 6; cited 2020 December 20]. Available from: http://jcss.org/ShowNewsAr.aspx?NewsId=862 .
- Introduction
- Conclusions
- Article Information
County-level death counts for Florida and Ohio based on Datavant data that link mortality records to voter registration files against the CDC data. Each observation represents a single county. Additional details on the data are provided in the eMethods in Supplement 1 .
Weekly excess deaths for Florida and Ohio based on mortality records linked to voter registration files. A, Overall excess death rates in Florida and Ohio. B, Excess death rates by registered party. C, The percentage-point difference between the registered parties, after adjusting for age and state-level differences; the smooth brown curve was fit with locally estimated scatterplot smoothing. A-C, Excess death rates were calculated for each week by comparing the observed deaths in that week with expected deaths based on a Poisson model. The 95% prediction intervals (shaded areas) were determined using simulations from the Poisson coefficient and outcome distribution, with SEs clustered at the county level. Additional details on the excess death methods and statistical analyses are presented in the eMethods in Supplement 1 .
The analyses were additionally adjusted for age and state-level differences in subgroup analyses where these covariates were not used for stratification. The 95% prediction intervals (horizontal lines) were determined using simulations from the Poisson coefficient and outcome distribution, with SEs clustered at the county level. Additional details on the excess death methodology and statistical analyses are presented in the eMethods in Supplement 1 .
The diamonds are binned means; counties with similar vaccination rates were binned to form 8 equally sized bins. The curves were fit to the underlying data using locally estimated scatterplot smoothing. In the pre–COVID-19 period (before April 2020), excess death rates for both Republican and Democratic voters hover around 0. During the beginning pandemic but before open vaccine eligibility (April 2020 to March 2021), the association between excess death rates and county-level vaccination rates were generally negative and nearly identical for Republican and Democratic voters. However, in the period after open vaccine eligibility (April 2021 to December 2021), there was a clear difference between Republican and Democratic voters, with higher excess death rates for Republicans concentrated in counties with lower overall vaccination rates and minimal differences in counties with the highest vaccination rates.
eMethods . Supplemental Description of Methods
eFigure 1. Excess Death Rates by Age in Florida and Ohio: 2018-2021
eFigure 2. Excess Death Rates in Florida: 2018-2021
eFigure 3. Excess Death Rates in Ohio: 2018-2021
eFigure 4. Excess Death Rates and Vaccination Rates in Florida and Ohio During the COVID-19 Pandemic Using October 1, 2021, Vaccination Rates
eFigure 5. Excess Death Rates and Vaccination Rates in Florida and Ohio During the COVID-19 Pandemic Using March 1, 2021, Vaccination Rates
eTable 1. Summary Statistics
eTable 2. Sensitivity of Estimated Difference in Excess Death Rates Between Republican and Democratic Voters to Alterations in Excess Death Methodology and Statistical Model
Data Sharing Statement
- Discrepancies in Estimating Excess Death by Political Party Affiliation—Reply JAMA Internal Medicine Comment & Response January 1, 2024 Jacob Wallace, PhD; Paul Goldsmith-Pinkham, PhD; Jason L. Schwartz, PhD
- Discrepancies in Estimating Excess Death by Political Party Affiliation JAMA Internal Medicine Comment & Response January 1, 2024 Christopher Dasaro, BS; Alyson Haslam, PhD; Vinay Prasad, MD, MPH
- Discrepancies in Estimating Excess Death by Political Party Affiliation JAMA Internal Medicine Comment & Response January 1, 2024 Patrick O’Mahen, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
For this paper to be accurate, voting records must be accurate. Ohio has historically had inaccurate voting records, so much so that a recent Supreme Court Decision recently went against the state of Ohio, see https://www.judicialwatch.org/wp-content/uploads/2018/06/Jon-Husted-Ohio-Secretary-of-State-v.-Philip-Randolph-Institute-et-al.-decision-16-980.pdf.
Also this paper contradicts more recent studies that have showed that mRNA vaccination decreases death rates from COVID, but increases deaths from other causes, so that all cause mortality is unchanged, with a relative risk of dying of 1.03 in the vaccinated group vas the unvaccinated group. https://www.cell.com/iscience/fulltext/S2589-0042(23)00810-6
This is a very interesting and informative study of public health value, but the findings are not unexpected.
It demonstrates the value of preventing disruption of health, and perhaps equally importantly emphasizes the direct and indirect economic loss to the society and the state when public health is compromised.
Political affiliation should not influence health care. Medicine is beyond politics!
- Download PDF
- X Facebook More LinkedIn
Wallace J , Goldsmith-Pinkham P , Schwartz JL. Excess Death Rates for Republican and Democratic Registered Voters in Florida and Ohio During the COVID-19 Pandemic. JAMA Intern Med. 2023;183(9):916–923. doi:10.1001/jamainternmed.2023.1154
Manage citations:
© 2024
- Permissions
Excess Death Rates for Republican and Democratic Registered Voters in Florida and Ohio During the COVID-19 Pandemic
- 1 Yale School of Public Health, New Haven, Connecticut
- 2 Yale School of Management, New Haven, Connecticut
- Comment & Response Discrepancies in Estimating Excess Death by Political Party Affiliation—Reply Jacob Wallace, PhD; Paul Goldsmith-Pinkham, PhD; Jason L. Schwartz, PhD JAMA Internal Medicine
- Comment & Response Discrepancies in Estimating Excess Death by Political Party Affiliation Christopher Dasaro, BS; Alyson Haslam, PhD; Vinay Prasad, MD, MPH JAMA Internal Medicine
- Comment & Response Discrepancies in Estimating Excess Death by Political Party Affiliation Patrick O’Mahen, PhD JAMA Internal Medicine
Question Was political party affiliation a risk factor associated with excess mortality during the COVID-19 pandemic in Florida and Ohio?
Findings In this cohort study evaluating 538 159 deaths in individuals aged 25 years and older in Florida and Ohio between March 2020 and December 2021, excess mortality was significantly higher for Republican voters than Democratic voters after COVID-19 vaccines were available to all adults, but not before. These differences were concentrated in counties with lower vaccination rates, and primarily noted in voters residing in Ohio.
Meaning The differences in excess mortality by political party affiliation after COVID-19 vaccines were available to all adults suggest that differences in vaccination attitudes and reported uptake between Republican and Democratic voters may have been a factor in the severity and trajectory of the pandemic in the US.
Importance There is evidence that Republican-leaning counties have had higher COVID-19 death rates than Democratic-leaning counties and similar evidence of an association between political party affiliation and attitudes regarding COVID-19 vaccination; further data on these rates may be useful.
Objective To assess political party affiliation and mortality rates for individuals during the initial 22 months of the COVID-19 pandemic.
Design, Setting, and Participants A cross-sectional comparison of excess mortality between registered Republican and Democratic voters between March 2020 and December 2021 adjusted for age and state of voter registration was conducted. Voter and mortality data from Florida and Ohio in 2017 linked to mortality records for January 1, 2018, to December 31, 2021, were used in data analysis.
Exposures Political party affiliation.
Main Outcomes and Measures Excess weekly deaths during the COVID-19 pandemic adjusted for age, county, party affiliation, and seasonality.
Results Between January 1, 2018, and December 31, 2021, there were 538 159 individuals in Ohio and Florida who died at age 25 years or older in the study sample. The median age at death was 78 years (IQR, 71-89 years). Overall, the excess death rate for Republican voters was 2.8 percentage points, or 15%, higher than the excess death rate for Democratic voters (95% prediction interval [PI], 1.6-3.7 percentage points). After May 1, 2021, when vaccines were available to all adults, the excess death rate gap between Republican and Democratic voters widened from −0.9 percentage point (95% PI, −2.5 to 0.3 percentage points) to 7.7 percentage points (95% PI, 6.0-9.3 percentage points) in the adjusted analysis; the excess death rate among Republican voters was 43% higher than the excess death rate among Democratic voters. The gap in excess death rates between Republican and Democratic voters was larger in counties with lower vaccination rates and was primarily noted in voters residing in Ohio.
Conclusions and Relevance In this cross-sectional study, an association was observed between political party affiliation and excess deaths in Ohio and Florida after COVID-19 vaccines were available to all adults. These findings suggest that differences in vaccination attitudes and reported uptake between Republican and Democratic voters may have been factors in the severity and trajectory of the pandemic in the US.
As of May 2023, there had been approximately 1.1 million deaths from COVID-19 in the US. 1 There is evidence that Republican-leaning counties have had higher COVID-19 death rates than Democratic-leaning counties and similar evidence of an association between political party affiliation and attitudes regarding COVID-19 vaccination, social distancing, and other mitigation strategies based on political party affiliation. 2 - 6
Prior studies 7 , 8 have found that Republican-leaning counties have had higher COVID-19 death rates than Democratic-leaning counties. It is unknown whether this county-level association persists at the individual level and whether it may be subject to the ecologic fallacy. 9 The ecologic fallacy is the incorrect assumption that associations observed at an aggregated level (eg, a county) will be the same at the individual level. Republican-leaning and Democratic-leaning counties differ in ways other than political party affiliation, 10 , 11 such as racial and ethnic composition, rurality, and educational levels, making it difficult to establish whether the differences in COVID-19 death rates are associated with political party affiliation or other differences in county-level characteristics. Research before the COVID-19 pandemic has also found evidence of higher death rates in Republican-leaning counties than Democratic-leaning counties. 12
To assess the association between political party affiliation and excess mortality for individuals during the COVID-19 pandemic, we linked voter registration data in Florida and Ohio to mortality data at the individual level to calculate excess death rates for Republican and Democratic voters and compare excess death rates before and after vaccines became available to the full adult population. 13 , 14 Because individual-level vaccination status was not included in the available data, we were able to assess excess death rates and vaccination rates only at the county level.
The eMethods in Supplement 1 provides additional details of all the methods. We obtained detailed US weekly mortality data from January 1, 2018, to December 31, 2021, from Datavant, an organization that augments the Social Security Administration Death Master File with information from newspapers, funeral homes, and other sources to construct an individual-level database containing 10 325 730 deaths in the US to individuals aged 25 or older during this period. This data set, which includes deaths reported to Datavant through March 31, 2023, covers approximately 83.5% of the Centers for Disease Control and Prevention death count for individuals who died at age 25 or older during the period from January 1, 2018, to December 31, 2021. Because the Datavant mortality data do not contain state identifiers, we are unable to assess data completeness in our individual study states of Florida and Ohio. During the COVID-19 pandemic, Datavant mortality data have been used in other peer-reviewed 15 and publicly available 16 research on excess mortality. The Yale University Institutional Review Board exempted the study from review because the data were deidentified, and reporting adheres to the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
We linked the mortality data at the individual level to 2017 Florida and Ohio voter registration files; these were the only states for which historical publicly available voter registration data were readily available. The linkage was performed from April 11 to 14, 2023. For each record, the linked data included week of death, age of deceased, county of residence, and 2017 political party affiliation. Political party affiliation in Ohio was defined by whether an individual voted in a party’s primary election within the preceding 2 calendar years; in Florida, political party affiliation was based on party registration. We excluded individuals whose political party affiliation was independent and those who were affiliated with third parties. Because COVID-19 deaths are most common at older ages, 17 we included only death records for individuals who died at age 25 years or older.
We also obtained death counts for the study period from the National Center for Health Statistics 18 and county-level vaccination rates from the Centers for Disease Control and Prevention. 19 We selected May 1, 2021, as the date for the county-level vaccination rate—1 month after eligibility for vaccines opened to all adults in the study states—because it represented the approximate date when all adults would have had the opportunity to receive at least 1 dose of a COVID-19 vaccine if they so desired, taking into account the time that states required during April 2021 to schedule and administer vaccines to newly eligible adults seeking them. As a robustness check, we assessed the sensitivity of our findings to using county-level vaccination rates on alternative dates before (March 1, 2021) and after (October 1, 2021) May 1, 2021.
We aggregated weekly death counts from January 1, 2018, to December 31, 2021, at the county-by-party-by-age level. The age ranges used were 25 to 64, 65 to 74, 75 to 84, and 85 years or older. The observed death counts included all the deaths from our mortality data that linked to Republican or Democratic voters who were registered in Florida and Ohio as of 2017.
To calculate the number of excess deaths, we estimated the number of deaths we would expect in the absence of the COVID-19 pandemic. First, we estimated expected weekly deaths at the county-by-party-by-age level by fitting a Poisson regression model to observed weekly death counts at the county-by-party-by-age-level for January 1, 2018, through December 31, 2019. 20 , 21 We then predicted expected deaths over our full sample. Excess deaths were defined as the difference between observed and expected deaths for January 1, 2018, to December 31, 2021. As a check on the model, we used predictions from the model in the weeks before the onset of COVID-19 (January 1, 2018, to March 31, 2020) to estimate excess deaths during this period.
We calculated excess death rates (the primary outcome) as the ratio of observed deaths (the numerator) to expected deaths (the denominator). To obtain estimates of excess death rates at aggregated levels, we used a weighted average of estimated excess death rates in each of the underlying cells (eg, county-by-party-by-age), weighted by their expected death counts. We estimated Poisson 95% prediction intervals (PIs), simulating from the coefficient distribution and outcome distribution, with SEs clustered by county. 22 We additionally adjusted estimated differences in excess death rates between Republican and Democratic voters—the primary estimate of interest—for differences in excess death rates by age group and state during the COVID-19 pandemic. Intuitively, this approach compared excess death rates between Democratic and Republican voters of the same age residing in the same states during the same week of the pandemic and then weighted those differences in excess death rates to either the weekly level, when plotting weekly differences in excess death rates, or to 3 broader time periods: (1) April 1, 2020, to December 31, 2021 (the part of the study period overlapping the COVID-19 pandemic); (2) April 1, 2020, to March 31, 2021 (the period during the pandemic before open vaccine eligibility for all adults); and (3) April 1, 2021, to December 31, 2021 (the period during the pandemic after open vaccine eligibility for all adults).
We also assessed county-level vaccination rates (as of May 1, 2021) and excess death rates by plotting average excess death rates for Republican and Democratic voters against the county-level vaccination rate during (1) the pre–COVID-19 pandemic period, (2) the period during the pandemic before open vaccine eligibility for all adults, and (3) the period during the pandemic after open vaccine eligibility for all adults.
In sensitivity analyses, we altered the Poisson model used to predict baseline death counts by including a linear time trend (and in one analysis allowing it to vary by state) and additional seasonality terms to capture higher frequency season-of-the-year trends. 23 For transparency, we calculated differences in the excess death rates between Republican and Democratic voters with no adjustments (removing our state and age group adjustments) and, separately, with a model that included our primary adjustments (state and age group) and additional adjustments for county-by-age differences in excess death rates during the pandemic.
We performed all calculations using R, version 4.1.3 (R Foundation for Statistical Computing). Statistical analyses report 95% PIs using simulations from the coefficient distribution and outcome distribution, with SEs clustered by county. Significance testing was 2-sided, and a P < .05 was considered statistically significant.
Our study included 538 159 deaths for individuals aged 25 years and older in Florida and Ohio between January 2018 and December 2021 linked to their 2017 voter data (eTable 1 in Supplement 1 ). The median age at death was 78 years (IQR, 71-89 years). The pattern of death counts in our linked data and in the National Center for Health Statistics data was similar ( Figure 1 ).
Using these data, we found a 20.5 percentage-point (95% PI, 15.6-25.6 percentage points) increase in weekly death counts in Florida and Ohio in the March 2020 to December 2021 period relative to the expected death counts for those weeks ( Figure 2 A and Table ). By comparison, for the time periods before the pandemic, we found only small fluctuations in excess death rates around 0.
Before the pandemic, excess death rates for Republican and Democratic voters were centered around 0 ( Figure 2 B). In the winter of 2021, both groups experienced sharp increases of similar magnitude in excess death rates. However, in the summer of 2021, after vaccines were available to all adults, the excess death rate among Republican voters began to increase relative to the excess death rate among Democratic voters; in the fall of 2021, the gap widened further. Between March 2020 and December 2021, excess death rates were 2.8 percentage points (15%) higher for Republican voters compared with Democratic voters (95% PI, 1.6-3.7 percentage points) ( Table ). After April 1, 2021, when all adults were eligible for vaccines in Florida and Ohio, this gap widened from −0.9 percentage point (95% PI, −2.5 to 0.3 percentage points) between March 2020 and March 2021, to 7.7 percentage points (95% PI, 6.0-9.3 percentage points) in the adjusted analysis, or a 43% difference ( Table ).
The estimates of differences in excess death rates between Republican and Democratic voters (adjusted for age, time, and state) were small until the summer of 2021, when excess death rates among Republican voters began to increase compared with excess death rates among Democratic voters ( Figure 2 C). The analyses stratified by age showed that Republican voters had significantly higher excess death rates compared with Democratic voters for 2 of the 4 age groups in the study, the differences for the age group 25 to 64 years were not significant ( Figure 3 ; eFigure 1 in Supplement 1 ). Democratic voters had significantly higher excess death rates compared with Republican voters for the age group 65 to 74 years. The analyses stratified by state showed that differences in excess death rates between Republican and Democratic voters were primarily seen in voters residing in Ohio, with smaller, and generally nonsignificant, differences in weekly excess death rates between Republican and Democratic voters in Florida (eFigure 2 and eFigure 3 in Supplement 1 ). In analyses that pooled data from March 2020 to December 2021, Republican voters in Florida did not have a statistically significantly higher excess death rate than Democratic voters in Florida ( Figure 3 ). Additional sensitivity analyses supported our main conclusions (eTable 2 in Supplement 1 ).
Before the COVID-19 pandemic, there was no association between county-level excess death rates, which hovered around 0, and the county-level vaccination rates ( Figure 4 A). During the pandemic, there was generally a negative association between county-level excess death rates and the share of the county population administered at least 1 dose of the vaccine as of May 1, 2021 ( Figure 4 B and C). In the period before open vaccine eligibility for adults (April 2020 to March 2021), the association between excess death rates and county-level vaccination rates was nearly identical for Republican and Democratic voters ( Figure 4 B). In the period after open vaccine eligibility (April to December 2021), there was a clear difference between Republican and Democratic voters, with higher excess death rates for Republicans in counties with lower overall vaccination rates ( Figure 4 C). Sensitivity analyses supported our main conclusions (eFigure 4 and eFigure 5 in Supplement 1 ).
During the initial years of the COVID-19 pandemic, political party affiliation in the US was associated with excess death rates in Florida and Ohio at the individual level. Republican voters had higher excess death rates than Democratic voters, as noted in a large mortality gap in the period after, but not before, all adults were eligible for vaccines in Florida and Ohio. With adjustments for differences in age and state of residence between Republican and Democratic voters, our findings suggest that, among individuals in the same age groups living in the same states, there were significant differences in excess death rates during the COVID-19 pandemic associated with political party affiliation. The results were robust to alterations in the methods used to estimate excess mortality as well as the statistical model used to estimate the difference in excess death rates between Republican and Democratic voters.
Our findings suggest that political party affiliation became a substantial factor only after COVID-19 vaccines were available to all adults in the US. Although the lack of individual-level vaccination status limited our ability to note further associations, the results suggest that well-documented differences in vaccination attitudes and reported uptake between Republican and Democratic voters 24 , 25 may have been factors in the severity and trajectory of the pandemic. However, one alternative explanation is that political party affiliation is a proxy for other risk factors (beyond age, which we adjusted for) for excess mortality during the COVID-19 pandemic, such as rates of underlying medical conditions, race and ethnicity, socioeconomic status, or health insurance coverage, 26 - 29 and these risk factors may be associated with differences in excess mortality by political party, even though we only observed differences in excess mortality after vaccines were available to all adults. It is also possible that specific risk factors for excess mortality interact with the emergence of COVID-19 variants (eg, Delta) or changes in vaccine-associated protection over time to be more consequential at different stages of the pandemic. Because data limitations prevented us from directly adjusting for these factors, their potential influence remains an important question for future research.
In addition to vaccines, nonpharmaceutical interventions, including facial masks and restrictions on large gatherings, have been reported to contribute to reductions in transmission of COVID-19 or its severe outcomes, including death, in experimental, quasi-experimental, and modeling studies. 30 - 33 However, differences in support for these measures by political party affiliation emerged early in the pandemic, 34 and the gradual loosening of the strictest government policies regarding the use of facial masks and restrictions on large gatherings predated April 2021, when vaccines became available to all adults in the study states. The extent of public adherence to these and other interventions at various stages of the pandemic, associations between individual political party affiliation and the adoption over time of these interventions in specific geographic areas, and their relative contribution to trends in individual and community COVID-19 mortality over time are also worthwhile areas for further investigation.
Since the fall of 2022, the focus of the US COVID-19 vaccination program has turned to the administration of updated, bivalent booster doses to those who have already received a primary vaccine series and, in many cases, 1 or more prior booster dose. Federal health officials have also begun considering future strategies for COVID-19 vaccination, including annual revaccination campaigns using vaccines reformulated to match circulating variants. 35 Yet more than 2 years into the vaccination effort, more than 50 million adults in the US have not completed a primary series, and these individuals remain at a substantially increased risk of hospitalization and death. 36 The causes of this vaccine hesitancy and refusal are varied and extend beyond political beliefs or party affiliation alone. 37 It therefore remains imperative for public health officials to continue and enhance activities intended to improve initial vaccination coverage, in tandem with current or future booster campaigns. To be most effective, these efforts—and corresponding messages—should be tailored to their intended audiences, address the particular sources of vaccine hesitancy among those groups, and seek to include direct participation from members of those communities as trusted ambassadors of provaccine messages. 38 As part of this work, engagement with conservative and Republican leaders, in particular, has been identified as a promising approach to promoting COVID-19 vaccine acceptance. 38
Our study has several limitations. First, there are plausible alternative explanations for the difference in excess death rates by political party affiliation beyond the explanatory role of vaccines discussed herein. Second, our mortality data, although detailed and recent, only included approximately 83.5% of deaths in the US and did not include cause of death. Although overall excess death patterns in our data are similar to those in other reliable sources, such as the Centers for Disease Control and Prevention National Center for Health Statistics data, it is possible that the deaths that our study data did not include may disproportionately occur among individuals registered with a particular political party, potentially biasing our results. In addition, the completeness of our mortality data may vary across states or time, potentially biasing our estimates of excess death rates. Third, all excess death models rely on fundamentally untestable assumptions to construct the baseline number of deaths we would expect in the absence of the COVID-19 pandemic. Fourth, because we did not have information on individual vaccination status, analyses of the association between vaccination rates and excess deaths relied on county-level vaccination rates. Fifth, our study was based on data from 2 states with readily obtainable historical voter registration information (Florida and Ohio); hence, our results may not generalize to other states.
Our study found evidence of higher excess mortality for Republican voters compared with Democratic voters in Florida and Ohio after, but not before, COVID-19 vaccines were available to all adults in the US. These differences in excess death rates were larger in counties with lower vaccination rates. If differences in COVID-19 vaccination by political party affiliation persist, particularly in the absence of other pandemic mitigation strategies, the higher excess death rate observed among Republican voters may continue through subsequent stages of the pandemic.
Accepted for Publication: March 4, 2023.
Published Online: July 24, 2023. doi:10.1001/jamainternmed.2023.1154
Corresponding Author: Jacob Wallace, PhD, Department of Health Policy and Management, Yale School of Public Health, 60 College St, New Haven, CT 06510 ( [email protected] ).
Author Contributions: Drs Wallace and Goldsmith-Pinkham had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Goldsmith-Pinkham.
Obtained funding: Wallace, Schwartz.
Supervision: Wallace.
Conflict of Interest Disclosures: None reported.
Funding/Support: The Tobin Center for Economic Policy at Yale University and the Yale School of Public Health COVID-19 Rapid Response Research Fund funded this study.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Disclaimer: The content is solely the responsibility of the authors and does not necessary reflect the official views of the supporting organizations.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
COMMENTARY: Misleading BMJ Public Health paper on COVID-19 excess mortality needs to be retracted

US Navy, Tristan B. Lotz / Flickr cc
In a publication in BMJ Public Health on June 3, Saskia Mostert, MD, PhD, and colleagues discuss excess mortality during the COVID-19 pandemic, and this paper has already led to much debate and confusion on both traditional and social media and has been used as fodder for anti-vaccine advocates. The paper's results have been taken to mean that vaccines are dangerous, and this has led to critical commentaries from other researchers as well as some of the authors who felt their work was not cited correctly.
We give a brief summary of some of this criticism, add some additional concerns about the paper, and make the case for retraction of the paper.
Multiple concerns
Mostert et al discuss estimates of excess mortality—the increase above an expected pre-pandemic baseline—during the COVID-19 pandemic period of 2020 to 2022 for 47 countries of the Western world. They conclude that the excess mortality was high during these years, despite the implementation of containment measures and COVID-19 vaccines and that this raises serious concern. They write, "Government leaders and policymakers need to thoroughly investigate the underlying causes of persistent excess mortality."
It is not immediately clear from the abstract of the paper what the authors saw in the excess mortality data that concerns them so. However, as major sections in the paper are dedicated to the discussion of perceived problems of serious adverse effects of vaccines and indirect mortality caused by non-pharmaceutical interventions, the public response to the article has been to take the article as evidence for vaccination and mitigation being the main causes of excess mortality—rather than the far more plausible explanation that widespread COVID-19 disease was the main cause of excess mortality.
It is not immediately clear from the abstract of the paper what the authors saw in the excess mortality data that concerns them so.
The work of Mostert and colleagues has been called into question by others, as cataloged on pubpeer and by Retraction Watch . Stuart McDonald, MBE, has a thorough discussion in a blog post detailing many of the concerns with the paper. A commentary co-authored by one of the plagiarized authors, Ariel Karlinsky, is also due to appear shortly. Finally, the research institutions of three of the four authors have distanced themselves from the paper. The cited funding agency has said it has been incorrectly listed as a sponsor of the publication.
Just 3 days after publication, the journal that published the work, BMJ Public Health , issued a statement emphasizing that the news coverage of the publication has misrepresented the contents of the study. The statement, however, does not respond to claims of plagiarism or whether the article is under consideration for retraction. In a June 13 BMJ press release , the journal announced its intentions to publish an expression of concern on the paper and to investigate the quality of the research.
Here are some concerns that such an investigation needs to address.
Misquotes and omissions on vaccine effects
Mostert et al make a lengthy argument that COVID-19 vaccines are associated with a high risk of severe adverse events. They write, "Numerous studies reported that COVID-19 vaccination may induce myocarditis, pericarditis and autoimmune disease." For this they misquote a review by Dotan et al on the risk of autoimmunity following SARS-CoV-2 infection. Dotan et al had in fact concluded vaccination can overcome this problem.
They quote one study ( Fraiman et al ) that calculated as many as 1 to 2 severe adverse event per 1,000 vaccines, something that is in stark contrast to the conclusions of no evidence of severe adverse events in the original Pfizer and Moderna clinical trial publications. More helpful would have been to cite real-world evidence from a cohort study of 23 million Nordic residents—what actually happened when millions of people used COVID-19 vaccines. This study found far lower levels of myocarditis and pericarditis associated with vaccination among young adults and no deaths. Therefore, these rare events in young adults that were not deadly could never explain the excess mortality during the COVID-19 pandemic which was largely in the elderly.
Mostert et al do not dwell on the fact that COVID-19 vaccines have been shown repeatedly to be highly effective: Both clinical trials and observational studies have found that they prevented about 9 out of 10 (~90%) of severe COVID-19 outcomes (severe disease and death). A new WHO study estimates that COVID-19 vaccine saved 1.4 million lives in Europe and more than halved the number of COVID-19 death toll that could have happened. The vaccinations attenuated the mortality potential, and the remaining excess mortality of 2.5 million is what could not be prevented, either before the vaccines were available by mid-2021, or because of low vaccination coverage in some settings, especially in Eastern Europe. Knowing this and writing about this is, of course, important if one wishes to seriously evaluate the observed excess mortality during COVID-19.
Misrepresentation of the severity of the pandemic
Mostert et al also have a lengthy section that suggests the pandemic wasn't very severe. They write that the infection-fatality rate (IFR) of COVID-19 before vaccines was 0.23% globally, and as low as 0.03% in adults under 60 years of age. This can erroneously be interpreted to mean that mortality caused by COVID-19 in Western countries was negligible.
The authors did not cite the relevant studies of IFR as it has played out in aging Western populations. A November 2020 Nature paper by O'Driscoll et al computes an IFR of ~0.8% in Western countries. And this may in fact be a low estimate, as this analysis was published before the emergence of the deadlier Alpha and Delta variants in 2021 ( Davies et al [Nature March 2021] and Twohig et al [Lancet Inf Dis August 2021]). When Mostert et al ignore the higher IFRs for Western populations, they mislead the reader to think that the COVID-19 pandemic was not serious. This is simply untrue, COVID-19 had a great severe disease and mortality potential especially in aging western populations and was a real 100-year event and a serious societal threat that required a forceful response.
The authors did not cite the relevant studies of IFR as it has played out in aging Western populations
The disaster in the Lombardy region of northern Italy early in the pandemic (caught unaware, and before vaccines) clearly demonstrates what could have happened ( Modi et al [Nature May 2021]).
But Mostert et al state, "Although COVID-19 containment measures and COVID-19 vaccines were thus implemented to protect citizens from suffering morbidity and mortality by the COVID-19 virus, they may have detrimental effects that cause inferior outcomes as well," and this can be erroneously taken to mean that the cure was worse than the disease.
On top of all of this, serious concerns about plagiarism have also been raised, as the excess mortality data presented are taken from previously published work by Karlinsky and Kobak (2021) and their World Mortality website , where the two scientists continuously provided excess mortality data throughout the pandemic.
Why Mostert et al copy Karlinsky and Kobak's prose and equations from their June 2021 eLIFE paper verbatim is unclear, but it is certainly not following good practices for citations. As Mostert et al did not further analyze these excess mortality estimates, the BMJ Public Health paper is not truly an original research contribution.
A retraction is warranted
Mostert et al should not disregard the most likely explanation for excess mortality: namely that the emerging COVID-19 virus explains most excess deaths during the pandemic. Lee et al in February 2023 computed that 85% of excess deaths in the United States were explained directly by the COVID-19 virus. Thus, there is no need to invoke other and unlikely explanations—such as vaccine adverse events—to explain excess mortality in Western countries.
A retraction is appropriate for this misleading paper that is not an original contribution.
In our opinion, a retraction is appropriate for this misleading paper that is not an original contribution. The publication of such work in a journal like BMJ Public Health can, to use the words of one commenter , be used as a figurative Trojan horse, seemingly giving unwarranted credibility to vaccine misinformation under the guise of statistical estimates of excess mortality. It is so important that scientific journals like BMJ take action and responsibility in an unfortunate situation like this where vaccine and pandemic misinformation appears credible by appearing in a top line peer reviewed medical journal.
_________________________
Dr Simonsen, a professor of epidemiology, is director of PandemiX, a Center of Excellence at Roskilde University in Denmark. Dr. Pedersen is a mathematical modeler and postdoc at PandemiX.
Related news
Cannabis use linked to worse covid-19 outcomes.

Study: Babies whose moms had high pandemic stress had altered brain growth

Study suggests cancer patients should stay current on COVID-19 boosters

Women twice as likely as men to report local flu, COVID vaccine side effects, data suggest

Study identifies female sex, heart disease as long-COVID risk factors, vaccination as protective

US poll shows fair amount of common ground on preventive COVID-19 steps

FDA modifies strain recommendation for fall COVID vaccine amid variant shifts, uptick in cases
More COVID-19 patients died in understaffed hospitals, new data show

This week's top reads
Usda reports reveal biosecurity risks at h5n1-affected dairy farms.
Shared equipment, shared staff, and animal movements were among key findings for virus spread among different farms.

The CDC estimates that cases are growing or likely growing in 34 states and territories.
Studies find little to no immunity to H5N1 avian flu virus in Americans
The CDC said the findings aren't surprising, given that the virus hasn't spread widely in people and is very different from seasonal flu strains.
The paper has already led to much confusion and has been used as fodder for anti-vaccine advocates.

Study details reduced Tamiflu susceptibility in H1N1 flu cases
The Netherlands had the most detections.
The researchers also show that more than 1 in 5 adults had protracted recoveries.

NIAID experiments show H5N1 levels plummet after heat treatment, but not to zero in some cases
Researchers emphasized that the findings reflect experimental lab conditions and don't reflect large-scale industrial pasteurization of raw milk.
Low-cost, at-home antibody test can flag low immunity against COVID-19, researchers say
A negative result indicates low immunity, but a positive result doesn't necessarily indicate sufficient immunity.
Study shows 12% long-COVID prevalence following Omicron infection
The odds ratio of any persistent symptoms was more than double in COVID-19 patients.
China reports another fatal H5N6 avian flu case
Officials didn't say how the man contracted the virus, but most past cases involved contact with poultry or poultry environments.
Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Understanding How COVID-19 Vaccines Work
What you need to know.
COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness.
- Different COVID-19 vaccines may work in our bodies differently but all provide protection against the virus that causes COVID-19.
- None of the COVID-19 vaccines can give you COVID-19.
- Bringing new vaccines to the public involves various steps, all which must be followed to ensure they are safe and effective before they are made available for use.
How COVID-19 Vaccines Work

Different types of vaccines work in different ways to offer protection. But with all types of vaccines, the body is left with a supply of “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus in the future.
It typically takes a few weeks after vaccination for the body to produce T-lymphocytes and B-lymphocytes.
Sometimes after vaccination, the process of building immunity can cause symptoms, such as fever. These symptoms are normal signs the body is building immunity.
Types of Vaccines: mRNA, and Protein Subunit
There are different types of vaccines.
- All COVID-19 vaccines prompt our bodies to recognize and help protect us from the virus that causes COVID-19.
- Currently, there are two types of COVID-19 vaccines for use in the United States: mRNA , and protein subunit vaccines.
None of these vaccines can give you COVID-19.
- Vaccines do not use any live virus.
- Vaccines cannot cause infection with the virus that causes COVID-19 or other viruses.
They do not affect or interact with our DNA.
- These vaccines do not enter the nucleus of the cell where our DNA (genetic material) is located, so it cannot change or influence our genes.
mRNA vaccines (Pfizer-BioNTech or Moderna)
To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Not mRNA vaccines. Instead, mRNA vaccines use mRNA created in a laboratory to teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. This immune response, which produces antibodies, is what helps protect us from getting sick from that germ in the future.
Research for mRNA technology
Researchers have been studying and working with mRNA vaccines for decades .
- In fact, mRNA vaccines have been studied before for flu, Zika, rabies, and cytomegalovirus (CMV).
- Beyond vaccines, cancer research has also used mRNA to trigger the immune system to target specific cancer cells.
- First, mRNA COVID-19 vaccines are given in the upper arm muscle or upper thigh, depending on the age of who is getting vaccinated.
- After vaccination, the mRNA will enter the muscle cells. Once inside, they use the cells’ machinery to produce a harmless piece of what is called the spike protein. The spike protein is found on the surface of the virus that causes COVID-19. After the protein piece is made, our cells break down the mRNA and remove it, leaving the body as waste.
- Next, our cells display the spike protein piece on their surface. Our immune system recognizes that the protein does not belong there. This triggers our immune system to produce antibodies and activate other immune cells to fight off what it thinks is an infection. This is what your body might do if you got sick with COVID-19.
- At the end of the process, our bodies have learned how to help protect against future infection with the virus that causes COVID-19. The benefit is that people get this protection from a vaccine, without ever having to risk the potentially serious consequences of getting sick with COVID-19. Any side effects from getting the vaccine are normal signs the body is building protection.
How mRNA COVID-19 Vaccines Work
PDF infographic explaining how mRNA COVID-19 vaccines work.
- English [128 KB, 1 page]
- Other Languages
Protein subunit vaccines (Novavax)
Protein subunit vaccines contain pieces (proteins) of the virus that causes COVID-19. These virus pieces are the spike protein. The vaccine also contains another ingredient called an adjuvant that helps the immune system respond to that spike protein in the future. Once the immune system knows how to respond to the spike protein, the immune system will be able to respond quickly to the actual virus spike protein and protect you against COVID-19.
Research for protein subunit technology
Protein subunit vaccines have been used for years.
- More than 30 years ago, a hepatitis B vaccine became the first protein subunit vaccine to be approved for use in people in the United States.
- Another example of other protein subunit vaccines used today include whooping cough vaccines.
- Protein subunit COVID-19 vaccines are given in the upper arm muscle. After vaccination, nearby cells pick up these proteins.
- Next, our immune system recognizes that these proteins do not belong there. Another ingredient in the vaccine, the adjuvant, helps our immune system to produce antibodies and activate other immune cells to fight off what it thinks is an infection. This is what your body might do if you got sick with COVID-19.
- At the end of the process, our bodies have learned how to help protect against future infection with the virus that causes COVID-19. The benefit is that people get this protection from a vaccine, without ever having to risk the potentially serious consequences of getting sick with COVID-19. Many side effects from getting the vaccine are normal signs the body is building protection.
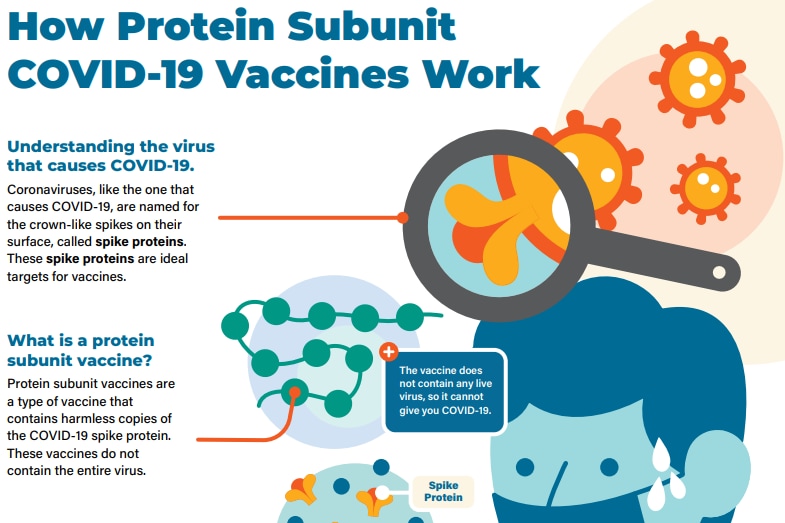
How Protein Subunit COVID-19 Vaccines Work
PDF infographic explaining how Protein Subunit COVID-19 vaccines work.
- English [953 KB, 1 page]
Developing COVID-19 Vaccines
While COVID-19 vaccines were developed rapidly, all steps have been taken to ensure their safety and effectiveness. Bringing a new vaccine to the public involves many steps including:
- vaccine development,
- clinical trials,
- U.S. Food and Drug Administration (FDA) authorization or approval,
- and development and approval of vaccine recommendations through the Advisory Committee on Immunization Practices (ACIP) and CDC.
As vaccines are distributed outside of clinical trials, monitoring systems are used to make sure that COVID-19 vaccines are safe.
New vaccines are first developed in laboratories. Scientists have been working for many years to develop vaccines against coronaviruses, such as those that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS-CoV-2, the virus that causes COVID-19, is related to these other coronaviruses. The knowledge that was gained through past research on coronavirus vaccines helped speed up the initial development of the current COVID-19 vaccines.
After initial laboratory development, vaccines go through three phases of clinical trials to make sure they are safe and effective. No trial phases have been skipped.
The clinical trials for COVID-19 vaccines have involved tens of thousands of volunteers of different ages, races, and ethnicities.
Clinical trials for vaccines compare outcomes (such as how many people get sick) between people who are vaccinated and people who are not. Results from these trials have shown that COVID-19 vaccines are safe and effective , especially against severe illness, hospitalization, and death.
Before vaccines are made available to people in real-world settings, FDA assesses the findings from clinical trials. Initially, they determined that COVID-19 vaccines met FDA’s safety and effectiveness standards and granted those vaccines Emergency Use Authorizations (EUAs) . The EUAs allowed the vaccines to be quickly distributed for use while maintaining the same high safety standards required for all vaccines. Learn more in this video about EUAs .
FDA has granted full approval for some COVID-19 vaccines. Before granting approval, FDA reviewed evidence that built on the data and information submitted to support the EUA. This included:
- preclinical and clinical trial data and information,
- as well as details of the manufacturing process,
- vaccine testing results to ensure vaccine quality, and
- inspections of the sites where the vaccine is made.
These vaccines were found to meet the high standards for safety, effectiveness, and manufacturing quality FDA requires of an approved product. Learn more about the process for FDA approval .
When FDA authorizes or approves a COVID-19 vaccine, ACIP reviews all available data about that vaccine to determine whether to recommend it and who should receive it. These vaccine recommendations then go through an approval process that involves both ACIP and CDC.
Watch Video: Understanding ACIP and How Vaccine Recommendations are Made [00:05:02]
Hundreds of millions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in U.S. history.
Several monitoring systems continue to track outcomes from COVID-19 vaccines to ensure their safety. Some people have no side effects. Many people have reported common side effects after COVID-19 vaccination , like pain or swelling at the injection site, a headache, chills, or fever. These reactions are common and are normal signs that your body is building protection.
Reports of serious adverse events after vaccination are rare .
- How can you prepare for vaccination?
- What can you expect during and after your vaccination?
- Uninsured? You can still get a free COVID-19 vaccine. Learn more about CDC’s Bridge Access program .
COVID-19 Clinical and Professional Resources
- Coronaviruses
- Vaccine Development Process: How Was Time Saved [779 KB, 1 Page]
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
This article has a correction
Expression of concern: Excess mortality across countries in the western world since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022 - 14 June 2024
Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022

Marcel Hoogland ,
Minke Huibers ,
Gertjan Kaspers .
https://doi.org/ 10.1136/bmjph-2023-000282
Introduction Excess mortality during the COVID-19 pandemic has been substantial. Insight into excess death rates in years following WHO’s pandemic declaration is crucial for government leaders and policymakers to evaluate their health crisis policies. This study explores excess mortality in the Western World from 2020 until 2022.
Methods All-cause mortality reports were abstracted for countries using the ‘Our World in Data’ database. Excess mortality is assessed as a deviation between the reported number of deaths in a country during a certain week or month in 2020 until 2022 and the expected number of deaths in a country for that period under normal conditions. For the baseline of expected deaths, Karlinsky and Kobak’s estimate model was used. This model uses historical death data in a country from 2015 until 2019 and accounts for seasonal variation and year-to-year trends in mortality.
Results The total number of excess deaths in 47 countries of the Western World was 3 098 456 from 1 January 2020 until 31 December 2022. Excess mortality was documented in 41 countries (87%) in 2020, 42 countries (89%) in 2021 and 43 countries (91%) in 2022. In 2020, the year of the COVID-19 pandemic onset and implementation of containment measures, records present 1 033 122 excess deaths (P-score 11.4%). In 2021, the year in which both containment measures and COVID-19 vaccines were used to address virus spread and infection, the highest number of excess deaths was reported: 1 256 942 excess deaths (P-score 13.8%). In 2022, when most containment measures were lifted and COVID-19 vaccines were continued, preliminary data present 808 392 excess deaths (P-score 8.8%).
Conclusions Excess mortality has remained high in the Western World for three consecutive years, despite the implementation of containment measures and COVID-19 vaccines. This raises serious concerns. Government leaders and policymakers need to thoroughly investigate underlying causes of persistent excess mortality.
What is already known on this topic
Excess mortality during the COVID-19 pandemic has been substantial. Insight into excess death rates in years following WHO’s pandemic declaration is crucial for government leaders and policymakers to evaluate their health crisis policies.
What this study adds
Excess mortality has remained high in the Western World for three consecutive years, despite the implementation of containment measures and COVID-19 vaccines. This raises serious concerns.
How this study might affect research, practice or policy
Government leaders and policymakers need to thoroughly investigate the underlying causes of persistent excess mortality.
- Introduction
Excess mortality is internationally recognised as an accurate measure for monitoring and comparing health crisis policies across geographic regions. 1–4 Excess mortality concerns the number of deaths from all causes during a humanitarian emergency, such as the COVID-19 pandemic, above the expected number of deaths under normal circumstances. 5–7 Since the outbreak of the COVID-19 pandemic, excess mortality thus includes not only deaths from SARS-CoV-2 infection but also deaths related to the indirect effects of the health strategies to address the virus spread and infection. 1–4 The burden of the COVID-19 pandemic on disease and death has been investigated from its beginning. Numerous studies expressed that SARS-CoV-2 infection was likely a leading cause of death among older patients with pre-existing comorbidities and obesity in the early phase of the pandemic, that various containment measures were effective in reducing viral transmission and that COVID-19 vaccines prevented severe disease, especially among the elderly population. 1 8–14 Although COVID-19 containment measures and COVID-19 vaccines were thus implemented to protect citizens from suffering morbidity and mortality by the COVID-19 virus, they may have detrimental effects that cause inferior outcomes as well. 1 2 15 It is noteworthy that excess mortality during a crisis points to a more extensive underlying burden of disease, disablement and human suffering. 16
On 11 March 2020, WHO declared the COVID-19 pandemic. 17 Countries in the Western World promptly implemented COVID-19 containment measures (such as lockdowns, school closures, physical distancing, travel restrictions, business closures, stay-at-home orders, curfews and quarantine measures with contact tracing) to limit virus spread and shield its residents from morbidity and mortality. 18 These non-pharmaceutical interventions however had adverse indirect effects (such as economic damage, limited access to education, food insecurity, child abuse, limited access to healthcare, disrupted health programmes and mental health challenges) that increased morbidity and mortality from other causes. 19 Vulnerable populations in need of acute or complex medical treatment, such as patients with cardiovascular disease, cerebrovascular conditions, diabetes and cancer, were hurt by these interventions due to the limited access to and delivery of medical services. Shortage of staff, reduced screening, delayed diagnostics, disrupted imaging, limited availability of medicines, postponed surgery, modified radiotherapy and restricted supportive care hindered protocol adherence and worsened the condition and prognosis of patients. 19–26 A recent study investigated excess mortality from some major non-COVID causes across 30 countries in 2020. Significant excess deaths were reported from ischaemic heart diseases (in 10 countries), cerebrovascular diseases (in 10 countries) and diabetes (in 19 countries). 27 On 14 October 2020, Professor Ioannidis from Stanford University published an overall Infection Fatality Rate of COVID-19 of 0.23%, and for people aged <70 years, the Infection Fatality Rate was 0.05%. 28 Governments in the Western World continued to impose lockdowns until the end of 2021.
In December 2020, the UK, the USA and Canada were the first countries in the Western World that started with the roll-out of the COVID-19 vaccines under emergency authorisation. 29–31 At the end of December 2020, a large randomised and placebo-controlled trial with 43 548 participants was published in the New England Journal of Medicine , which showed that a two-dose mRNA COVID-19 vaccine regimen provided an absolute risk reduction of 0.88% and relative risk reduction of 95% against laboratory-confirmed COVID-19 in the vaccinated group (8 COVID-19 cases/17 411 vaccine recipients) versus the placebo group (162 COVID-19 cases/17 511 placebo recipients). 32 33 At the beginning of 2021, most other Western countries followed with rolling out massive vaccination campaigns. 34–36 On 9 April 2021, the overall COVID-19 Infection Fatality Rate was reduced to 0.15% and expected to further decline with the widespread use of vaccinations, prior infections and the evolution of new and milder variants. 37 38
Although COVID-19 vaccines were provided to guard civilians from suffering morbidity and mortality by the COVID-19 virus, suspected adverse events have been documented as well. 15 The secondary analysis of the placebo-controlled, phase III randomised clinical trials of mRNA COVID-19 vaccines showed that the Pfizer trial had a 36% higher risk of serious adverse events in the vaccine group. The risk difference was 18.0 per 10 000 vaccinated (95% CI 1.2 to 34.9), and the risk ratio was 1.36 (95% CI 1.02 to 1.83). The Moderna trial had a 6% higher risk of serious adverse events among vaccine recipients. The risk difference was 7.1 per 10 000 vaccinated (95% CI −23.2 to 37.4), and the risk ratio was 1.06 (95% CI 0.84 to 1.33). 39 By definition, these serious adverse events lead to either death, are life-threatening, require inpatient (prolongation of) hospitalisation, cause persistent/significant disability/incapacity, concern a congenital anomaly/birth defect or include a medically important event according to medical judgement. 39–41 The authors of the secondary analysis point out that most of these serious adverse events concern common clinical conditions, for example, ischaemic stroke, acute coronary syndrome and brain haemorrhage. This commonality hinders clinical suspicion and consequently its detection as adverse vaccine reactions. 39 Both medical professionals and citizens have reported serious injuries and deaths following vaccination to various official databases in the Western World, such as VAERS in the USA, EudraVigilance in the European Union and Yellow Card Scheme in the UK. 42–48 A study comparing adverse event reports to VAERS and EudraVigilance following mRNA COVID-19 vaccines versus influenza vaccines observed a higher risk of serious adverse reactions for COVID-19 vaccines. These reactions included cardiovascular diseases, coagulation, haemorrhages, gastrointestinal events and thromboses. 39 49 Numerous studies reported that COVID-19 vaccination may induce myocarditis, pericarditis and autoimmune diseases. 50–57 Postmortem examinations have also ascribed myocarditis, encephalitis, immune thrombotic thrombocytopenia, intracranial haemorrhage and diffuse thrombosis to COVID-19 vaccinations. 58–67 The Food and Drug Administration noted in July 2021 that the following potentially serious adverse events of Pfizer vaccines deserve further monitoring and investigation: pulmonary embolism, acute myocardial infarction, immune thrombocytopenia and disseminated intravascular coagulation. 39 68
Insight into the excess death rates in the years following the declaration of the pandemic by WHO is crucial for government leaders and policymakers to evaluate their health crisis policies. 1–4 This study therefore explores excess mortality in the Western World from 1 January 2020 until 31 December 2022.
- Materials and methods
The Western World is primarily defined by culture rather than geography. It refers to various countries in Europe and to countries in Australasia (Australia, New Zealand) and North America (the USA, Canada) that are based on European cultural heritage. The latter countries were once British colonies that acquired Christianity and the Latin alphabet and whose populations comprised numerous descendants from European colonists or migrants. 69
Study design
All-cause mortality reports were abstracted for countries of the Western World using the ‘Our World in Data’ database. 12 Only countries that had all-cause mortality reports available for all three consecutive years (2020–2022) were included. If coverage of one of these years was missing, the country was excluded from the analysis.
The ‘Our World in Data’ database retrieves their reported number of deaths from both the Human Mortality Database (HMD) and the World Mortality Dataset (WMD). 5 HMD is sustained by research teams of both the University of California in the USA and the Max Planck Institute for Demographic Research in Germany. HMD recovers its data from Eurostat and national statistical agencies on a weekly basis. 5 70 The ‘Our World in Data’ database used HMD as their only data source until February 2021. 5 WMD is sustained by the researchers Karlinsky and Kobak. WMD recovers its data from HMD, Eurostat and national statistical agencies on a weekly basis. 5 71 The ‘Our World in Data’ database started to use WMD as a data source next to HMD since February 2021. 5
‘Excess mortality’ is assessed as the deviation between the reported number of deaths in a country during a certain week or month in 2020 until 2022 and the expected or projected number of deaths in a country for that period under normal conditions. 5 For the baseline of expected deaths, the estimate model of Karlinsky and Kobak was used. This linear regression model uses historical death data in a country from 2015 until 2019 and accounts for seasonal variation in mortality and year-to-year trends due to changing population structure or socioeconomic factors. 5 7
‘Excess mortality P-score’ concerns the percentage difference between the reported number of deaths and the projected number of deaths in a country. 5 This measure permits comparisons between various countries. Although presenting the raw number of excess deaths provides insight into the scale, it is less useful to compare countries because of their large population size variations. 5 The ‘Our World in Data’ database presents P-scores in a country during a certain week or month in 2020 until 2022. 5 These P-scores are calculated from both the reported number of deaths in HMD and WMD and the projected number of deaths using the estimate model of Karlinsky and Kobak in WMD. 5 7 70 71
For correct interpretation of excess mortality provided by the ‘Our World in Data’ database, the following needs to be taken into consideration: the reported number of deaths may not represent all deaths, as countries may lack the infrastructure and capacity to document and account for all deaths. 5 In addition, death reports may be incomplete due to delays. It may take weeks, months or years before a death is actually reported. The date of a reported death may refer to the actual death date or to its registration date. Sometimes, a death may be recorded but not the date of death. Countries that provide weekly death reports may use different start and end dates of the week. Most countries define the week from Monday until Sunday, but not all countries do. Weekly and monthly reported deaths may not be completely comparable, as excess mortality derived from monthly calculations inclines to be lower. 5 7
For our analysis, weekly all-cause mortality reports from the ‘Our World in Data’ database were converted to monthly reports. Subsequently, the monthly reports were converted to annual reports.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
The ‘Our World in Data’ database contained all-cause mortality reports of 47 countries (96%) in the Western World for the years 2020, 2021 and 2022. Only Andorra and Gibraltar were excluded. Both countries lacked all-cause mortality reports for the year 2022. Most countries (n=36, 77%) present weekly all-cause mortality reports, whereas 11 countries (23%) report monthly. The latter countries include the following: Albania, Bosnia Herzegovina, Faeroe Islands, Greenland, Kosovo, Liechtenstein, Moldova, Monaco, North Macedonia, San Marino and Serbia.
The all-cause mortality reports were abstracted from the ‘Our World in Data’ database on 20 May 2023. At this date, four countries (9%) still lacked all-cause mortality reports for various periods: Canada (1 month), Liechtenstein (3 months), Monaco (3 months) and Montenegro (4 months). It is noteworthy that all-cause mortality reports are also still being updated for the other countries due to registration delays which may take weeks, months or even years.
Excess mortality
Online supplemental table 1 illustrates that the total number of excess deaths in the 47 countries of the Western World was 3 098 456 from 1 January 2020 until 31 December 2022. Excess mortality was documented in 41 countries (87%) in 2020, in 42 countries (89%) in 2021 and in 43 countries (91%) in 2022.
In 2020, the year of the COVID-19 pandemic and implementation of the containment measures, 1 033 122 excess deaths (P-score 11.4%) were recorded. In 2021, the year in which both COVID-19 containment measures and COVID-19 vaccines were used to address virus spread and infection, a total of 1 256 942 excess deaths (P-score 13.8%) were reported. In 2022, the year in which most containment measures were lifted and COVID-19 vaccines were continued, preliminary available data counts 808 392 excess deaths (P-score 8.8%).
Figure 1 presents the excess mortality and cumulative excess mortality in 47 countries of the Western World over the years 2020, 2021 and 2022. The linear excess mortality trendline is almost horizontal.
Excess mortality and cumulative excess mortality in the Western World (n=47 countries). Preliminary and incomplete all-cause mortality reports are available for 2022.
Excess mortality P-scores
Figure 2 shows the excess mortality P-scores per country in the Western World. Only Greenland had no excess deaths between 2020 and 2022. Among the other 46 countries with reported excess mortality, the percentage difference between the reported and projected number of deaths was highest in 13 countries (28%) during 2020, in 21 countries (46%) during 2021 and in 12 countries (26%) during 2022. Figure 3 exemplifies excess mortality P-score curves of the highest-populated country of North America (the USA), the four highest-populated countries of Europe (Germany, France, the UK and Italy) and the highest-populated country of Australasia (Australia).
Excess mortality P-scores per country in the Western World (n=47 countries). Preliminary and incomplete all-cause mortality reports are available for 2022.
Excess mortality P-score curves of six countries in the Western World. Preliminary and incomplete all-cause mortality reports are available for 2022.
Figure 4 highlights a map of excess mortality P-scores in the Western World over the years 2020, 2021 and 2022. 74 Table 1 presents a classification of excess mortality P-scores in the Western World.
Map of excess mortality P-scores in the Western World (n=47 countries). 74 Preliminary and incomplete all-cause mortality reports are available for 2022.
This study explored the excess all-cause mortality in 47 countries of the Western World from 2020 until 2022. The overall number of excess deaths was 3 098 456. Excess mortality was registered in 87% of countries in 2020, in 89% of countries in 2021 and in 91% of countries in 2022. During 2020, which was marked by the COVID-19 pandemic and the onset of mitigation measures, 1 033 122 excess deaths (P-score 11.4%) were to be regretted. 17 18 A recent analysis of seroprevalence studies in this prevaccination era illustrates that the Infection Fatality Rate estimates in non-elderly populations were even lower than prior calculations suggested. 37 At a global level, the prevaccination Infection Fatality Rate was 0.03% for people aged <60 years and 0.07% for people aged <70 years. 38 For children aged 0–19 years, the Infection Fatality Rate was set at 0.0003%. 38 This implies that children are rarely harmed by the COVID-19 virus. 19 38 During 2021, when not only containment measures but also COVID-19 vaccines were used to tackle virus spread and infection, the highest number of excess deaths was recorded: 1 256 942 excess deaths (P-score 13.8%). 26 37 Scientific consensus regarding the effectiveness of non-pharmaceutical interventions in reducing viral transmission is currently lacking. 75 76 During 2022, when most mitigation measures were negated and COVID-19 vaccines were sustained, preliminary available data count 808 392 excess deaths (P-score 8.8%). 39 The percentage difference between the documented and projected number of deaths was highest in 28% of countries during 2020, in 46% of countries during 2021, and in 26% of countries during 2022.
This insight into the overall all-cause excess mortality since the start of the COVID-19 pandemic is an important first step for future health crisis policy decision-making. 1–4 The next step concerns distinguishing between the various potential contributors to excess mortality, including COVID-19 infection, indirect effects of containment measures and COVID-19 vaccination programmes. Differentiating between the various causes is challenging. 16 National mortality registries not only vary in quality and thoroughness but may also not accurately document the cause of death. 1 19 The usage of different models to investigate cause-specific excess mortality within certain countries or subregions during variable phases of the pandemic complicates elaborate cross-country comparative analysis. 1 2 16 Not all countries provide mortality reports categorised per age group. 2 12 Also testing policies for COVID-19 infection differ between countries. 1 2 Interpretation of a positive COVID-19 test can be intricate. 77 Consensus is lacking in the medical community regarding when a deceased infected with COVID-19 should be registered as a COVID-19 death. 1 77 Indirect effects of containment measures have likely altered the scale and nature of disease burden for numerous causes of death since the pandemic. However, deaths caused by restricted healthcare utilisation and socioeconomic turmoil are difficult to prove. 1 78–81 A study assessing excess mortality in the USA observed a substantial increase in excess mortality attributed to non-COVID causes during the first 2 years of the pandemic. The highest number of excess deaths was caused by heart disease, 6% above baseline during both years. Diabetes mortality was 17% over baseline during the first year and 13% above it during the second year. Alzheimer’s disease mortality was 19% higher in year 1 and 15% higher in year 2. In terms of percentage, large increases were recorded for alcohol-related fatalities (28% over baseline during the first year and 33% during the second year) and drug-related fatalities (33% above baseline in year 1 and 54% in year 2). 82 Previous research confirmed profound under-reporting of adverse events, including deaths, after immunisation. 83 84 Consensus is also lacking in the medical community regarding concerns that mRNA vaccines might cause more harm than initially forecasted. 85 French studies suggest that COVID-19 mRNA vaccines are gene therapy products requiring long-term stringent adverse events monitoring. 85 86 Although the desired immunisation through vaccination occurs in immune cells, some studies report a broad biodistribution and persistence of mRNA in many organs for weeks. 85 87–90 Batch-dependent heterogeneity in the toxicity of mRNA vaccines was found in Denmark. 48 Simultaneous onset of excess mortality and COVID-19 vaccination in Germany provides a safety signal warranting further investigation. 91 Despite these concerns, clinical trial data required to further investigate these associations are not shared with the public. 92 Autopsies to confirm actual death causes are seldom done. 58 60 90 93–95 Governments may be unable to release their death data with detailed stratification by cause, although this information could help indicate whether COVID-19 infection, indirect effects of containment measures, COVID-19 vaccines or other overlooked factors play an underpinning role. 1 8–14 20–25 39–60 68 90 This absence of detailed cause-of-death data for certain Western nations derives from the time-consuming procedure involved, which entails assembling death certificates, coding diagnoses and adjudicating the underlying origin of death. Consequently, some nations with restricted resources assigned to this procedure may encounter delays in rendering prompt and punctual cause-of-death data. This situation existed even prior to the outbreak of the pandemic. 1 5
A critical challenge in excess mortality research is choosing an appropriate statistical method for calculating the projected baseline of expected deaths to which the observed deaths are compared. 96 Although the analyses and estimates in general are similar, the method can vary, for instance, per length of the investigated period, nature of available data, scale of geographic area, inclusion or exclusion of past influenza outbreaks, accounting for changes in population ageing and size and modelling trend over years or not. 7 96 Our analysis of excess mortality using the linear regression model of Karlinsky and Kobak varies thus to some extent from previous attempts to estimate excess deaths. For example, Islam et al conducted an age- and sex-disaggregated time series analysis of weekly mortality data in 29 high-income countries during 2020. 97 They used a more elaborate statistical approach, an overdispersed Poisson regression model, for estimating the baseline of expected deaths on historical death data from 2016 to 2019. In contrast to the model of Karlinsky and Kobak, their baseline is weighing down prior influenza outbreaks so that every novel outbreak evolves in positive excess mortality. 7 97 Islam’s study found that age-standardised excess death rates were higher in men than in women in nearly all nations. 97 Alicandro et al investigated sex- and age-specific excess total mortality in Italy during 2020 and 2021, using an overdispersed Poisson regression model that accounts for temporal trends and seasonal variability. Historical death data from 2011 to 2019 were used for the projected baseline. When comparing 2020 and 2021, an increased share of the total excess mortality was attributed to the working-age population in 2021. Excess deaths were higher in men than in women during both periods. 98 Msemburi et al provided WHO estimates of the global excess mortality for its 194 member states during 2020 and 2021. For most countries, the historical period 2015–2019 was used to determine the expected baseline of excess deaths. In locations missing comprehensive data, the all-cause deaths were forecasted employing an overdispersed Poisson framework that uses Bayesian inference techniques to measure incertitude. This study describes huge differences in excess mortality between the six WHO regions. 99 Paglino et al used a Bayesian hierarchical model trained on historical death data from 2015 to 2019 and provided spatially and temporally granular estimates of monthly excess mortality across counties in the USA during the first 2 years of the pandemic. The authors found that excess mortality decreased in large metropolitan counties but increased in non-metropolitan counties. 100 Ruhm examined the appropriateness of reported excess death estimates in the USA by four previous studies and concluded that these investigations have likely understated the projected baseline of excess deaths and therewith overestimated excess mortality and its attribution to non-COVID causes. Ruhm explains that the overstatement of excess deaths may partially be explained by the fact that the studies did not adequately take population growth and age structure into account. 96 101–104 Although all the above-mentioned studies used more elaborate statistical approaches for estimating baseline mortality, Karlinsky and Kobak argue that their method is a trade-off between suppleness and chasteness. 7 It is the simplest method to captivate seasonal fluctuation and annual trends and more transparent than extensive approaches. 7
This study has various significant limitations. Death reports may be incomplete due to delays. It may take weeks, months or years before a death is registered. 5 Four nations still lack all-cause mortality reports for 1–4 months. Some nations issue complete data with profound arrears, whereas other nations publish prompt, yet incomplete data. 5 7 The presented data, especially for 2022, are thus preliminary and subject to backward revisions. The more recent data are usually more incomplete and therefore can undergo upward revisions over time. This implies that several of the reported excess mortality estimates can be underestimations. 7 The completeness and reliability of death registration data can also differ per nation for other reasons. The recorded number of deaths may not depict all deaths accurately, as the resources, infrastructure and registration capacity may be limited in some nations. 5 7 Most countries report per week, but some per month. Weekly reports generally provide the date of death, whereas monthly reports often provide the date of registration. Weekly and monthly reports may not be entirely comparable. 5 7 Our data are collected at a country level and provide no detailed stratification for sociodemographic characteristics, such as age or gender. 5 7
In conclusion, excess mortality has remained high in the Western World for three consecutive years, despite the implementation of COVID-19 containment measures and COVID-19 vaccines. This is unprecedented and raises serious concerns. During the pandemic, it was emphasised by politicians and the media on a daily basis that every COVID-19 death mattered and every life deserved protection through containment measures and COVID-19 vaccines. In the aftermath of the pandemic, the same morale should apply. Every death needs to be acknowledged and accounted for, irrespective of its origin. Transparency towards potential lethal drivers is warranted. Cause-specific mortality data therefore need to be made available to allow more detailed, direct and robust analyses to determine the underlying contributors. Postmortem examinations need to be facilitated to allot the exact reason for death. Government leaders and policymakers need to thoroughly investigate underlying causes of persistent excess mortality and evaluate their health crisis policies.
Dissemination to participants and related patient and public communities
We will disseminate findings through a press release on publication and contact government leaders and policymakers to raise awareness about the need to investigate the underlying causes of persistent excess mortality.
- Supplementary files
- Publication history
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment case
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Coronavirus disease (COVID-19) /
Global research on coronavirus disease (COVID-19)
Section navigation.
- WHO COVID-19 Solidarity Therapeutics Trial
- "Solidarity II" global serologic study for COVID-19
- Accelerating a safe and effective COVID-19 vaccine
- Solidarity Trial Vaccines
WHO is bringing the world’s scientists and global health professionals together to accelerate the research and development process, and develop new norms and standards to contain the spread of the coronavirus pandemic and help care for those affected.
The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus.
The solidarity of all countries will be essential to ensure equitable access to COVID-19 health products.
WHO COVID-19 Research Database
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO Library & Digital Information Networks and was funded by COVID-19 emergency funds. The database was built by BIREME, the Specialized Center of PAHO/AMRO. Its content spanned the time period March 2020 to June 2023. It has now been archived, and no longer searchable since January 2024.
Choisir votre langue

BMJ study did not prove Covid-19 vaccines caused excess deaths
- Published on June 20, 2024 at 21:54
- By Rossen BOSSEV , AFP Bulgaria , AFP Canada
- Translation and adaptation Gwen ROLEY
Copyright © AFP 2017-2024. Any commercial use of this content requires a subscription. Click here to find out more.
"And right when the shots start the deaths happen and then continues on. And the countries with the highest uptake of the shots have the highest death numbers," says conspiracy theorist Alex Jones in a video posted to X on June 6, 2024.
In the clip, Jones discusses a June 3 study published in the BMJ titled: "Excess mortality across countries in the Western World since the Covid-19 pandemic: 'Our World in Data' estimates of January 2020 to December 2022" (archived here ).
Similar claims about the paper spread across X while jumping to Facebook and Instagram -- often referencing an article by British daily The Telegraph covering the study (archived here ). The posts also circulated in other languages, including French and Bulgarian .

The posts come amid a wave of vaccine misinformation , i ncluding false claims about adverse effects, which has undercut confidence in public health efforts to fight Covid-19 and other diseases.
The New York Post also shared a link to an article on X on June 6, 2024 with the headline: "Covid vaccines may have helped fuel rise in excess deaths since pandemic: study." However, an editor's note attached to the story on its website said the text had been updated to reflect that the study did not analyze the impact of vaccination (archived here )
On that same day, the BMJ stated on its X account that the paper had been misreported and added that various news outlets "have claimed that this research implies a direct causal link between Covid-19 vaccination and mortality. This study does not establish any such link."
Statement in response to misreporting of BMJ Public Health @BMJPublicHealth research on excess deaths since the COVID-19 pandemic. Link: https://t.co/oe8tlcMi9M pic.twitter.com/y2J2g3wSyu — BMJ Group (@bmj_company) June 6, 2024
What did the study find?
According to the paper's abstract, the Dutch researchers used figures from 47 countries a s found in aggregator Our World in Data to assess excess mortality -- or the number of deaths above the anticipated amount -- between 2020 and 2022.
The study concluded that the rate of excess mortality was high in the observed countries, which included the United States, United Kingdom and Canada , during that time, despite the pandemic mitigation measures that were in place.
"This raises serious concerns. Government leaders and policymakers need to thoroughly investigate underlying causes of persistent excess mortality," the researchers wrote in their conclusion.
While the paper comments on the possibility of suspected adverse events of Covid-19 vaccines contributing to excess deaths, the text also points to infection from the virus and indirect effects of containment measures as potential underpinning factors driving the trend.
The research does not present evidence for a link between vaccination and excess mortality, with the authors pointing out that governments "may be unable to release their death data with detailed stratification by cause."
Experts criticize the paper
After the study was published, it received criticism online, including an X thread (archived here ) by Jeffrey Morris , a professor of public health and preventative medicine and director of the Division of Biostatistics at the University of Pennsylvania's Perelman School of Medicine (archived here ).
"This paper provides no evidence whatsoever that Covid-19 vaccines have increased mortality," he told AFP in a June 13 email. "All they do is demonstrate excess deaths did not stop in 2020 but continued in 2021-2022 'in spite of containment measures and vaccines.'"
John Ioannidis , a professor of medicine at the Stanford Prevention Research Center, said placing the burden of excess deaths on Covid-19 vaccines was "a long stretch"(archived here ).
"I think that overall vaccines saved many lives in the balance: not as many as some claim, trying to paint an all-perfect story around them, but I definitely don't think that they killed more people than they saved!" he said in a June 13 email.
Publisher and hospital pull back from research
Following criticism of the study BMJ said in a June 13 press release (archived here ) that an expression of concern would be placed on the paper.
On June 11, the Utrecht-based Princess Máxima Center for Pediatric Oncology , listed as the affiliation for three of the study's four authors, also published a statement distancing itself from the paper (archived here ).
According to the statement, the original idea of the study "was to look at the effect of Covid measures on, among other things, the mortality rate of children with cancer in low-income countries".
However, during the course of the study, the hospital said: "The focus shifted and diverted in a direction that we felt was too far from our expertise: pediatric oncology. We are not experts in epidemiology, nor do we want to give that impression."
Its statement said: "The study in no way demonstrates a link between vaccinations and excess mortality; that is explicitly not the researchers' finding. We therefore regret that this impression has been created."
Benefits outweigh risks
Some studies have estimated that Covid-19 vaccines saved millions of lives ( archived here and here ). Physicians have continually told AFP the shots are effective at preventing severe illness and death , outweighing the risks of possible side effects (archived here ).
Researchers estimate the Covid-19 virus itself directly led to more than 7 million deaths worldwide, according to the Kaiser Family Foundation (archived here ).
In Canada, the most recent government data reports that out of more than 100 million doses of the shot, 488 deaths were reported after vaccination with four of these being consistent with causal association to immunization (archived here ).
"The BMJ paper gives an overall balanced discussion of many possibilities of contributing factors that are very difficult or even impossible to disentangle as to their relative contribution with these types of data," Ioannidis said. "Many of the contributing factors tend to co-exist, making their disentanglement even more difficult."
Read more of AFP's reporting on vaccine and health misinformation here .
Is there content that you would like AFP to fact-check? Get in touch.


IMAGES
VIDEO
COMMENTS
Discussion. A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose.
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement ...
Safety and adverse effects of current COVID-19 vaccines. As shown in Table I, current vaccines have demonstrated considerable efficacy in diminishing mild, moderate and severe cases with a low risk of adverse events 21.For some of these vaccines [such as Convidicea (AD5-nCoV), Janssen (Ad26.COV2.S), Sinopharm (BBIBP-CorV), Covaxin (BBV152) and Sinovac (CoronaVac)], there is the information ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
The 3 × 3 × 3 factorial design requires judgments of 27 cases. The effectiveness factor varied level of vaccine effectiveness for preventing COVID-19: 50%, 70%, or 90% protection. The three levels were chosen to reflect the effectiveness of common vaccines, with particular emphasis on influenza vaccines.
Participants were enrolled from December 28, 2020 (2 weeks after the introduction of a Covid-19 vaccine), through May 19, 2021, at 33 sites across 25 U.S. states, representing more than 500,000 ...
Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance Robert M. Kaplana,1 and Arnold Milsteina aClinical Excellence Research Center, Stanford University School of Medicine, Stanford, CA 94305 Edited by Douglas S. Massey, Princeton University, Princeton, NJ, and approved January 21, 2021 (received for review October 16, 2020)
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
Over the course of the first year of COVID-19 vaccination, between Dec 8, 2020, and Dec 8, 2021, 8·33 billion doses were administered among 4·36 billion people globally. 1 In their study in The Lancet Infectious Diseases, by fitting a mathematical model to excess mortality, Oliver J Watson and colleagues 2 estimated that in 185 countries and ...
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
The coronavirus disease 2019 (COVID-19) has caused millions of deaths worldwide since the first reported suspected case in Wuhan, China, in late December 2019 1.Even though most patients will ...
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
During COVID-19, research on vaccine attitudes and behaviours largely delivered on its promises. The field of research on vaccine attitudes and behaviors was delivering on its promises in the years before the pandemic, but this has accelerated since 2020. We saw an explosion of the volume of papers, especially questionnaire-based studies.
COVID-19 is no longer a public health emergency of international concern, but long COVID's effects are yet to be fully understood. Hence, globally, SARS-CoV-2 is still a profound threat to public health and of perilous nature as a zoonotic disease. Timely vaccination provided to individuals worldwide during the pandemic phase was under a certain degree of control; however, few studies have ...
In Fig. 2, the overview of the global COVID-19 vaccine landscape in clinical development depicts that there are seven major types of vaccine candidates for COVID-19 is illustrated as (inactivated, non-replicating viral vectors, replicating viral vectors, protein subunit, nucleic acid-based, and virus-like particles [VLP]), showing the percentage of candidate vaccines that are currently under ...
Vaccines are effective interventions that can reduce the high burden of diseases globally. However, public vaccine hesitancy is a pressing problem for public health authorities. With the availability of COVID-19 vaccines, little information is available on the public acceptability and attitudes towards the COVID-19 vaccines in Jordan. This study aimed to investigate the acceptability of COVID ...
COVID-19 was—and to a large extent remains—the most meaningful health event in recent global history ().Unlike the 2003 Severe Acute Respiratory Syndrome (SARS) epidemic, it spread globally; unlike Zika, everyone is at risk of infection with COVID-19; and unlike recent swine flu pandemics, the disease severity and mortality from COVID-19 were so high it led to life expectancy reversals in ...
Also this paper contradicts more recent studies that have showed that mRNA vaccination decreases death rates from COVID, but increases deaths from other causes, so that all cause mortality is unchanged, with a relative risk of dying of 1.03 in the vaccinated group vas the unvaccinated group. ... Research before the COVID-19 pandemic has also ...
This study evaluates the effectiveness of the novel BNT162b2 mRNA vaccine 1 against Covid-19 in a nationwide mass vaccination setting. Estimated vaccine effectiveness during the follow-up period ...
In a publication in BMJ Public Health on June 3, Saskia Mostert, MD, PhD, and colleagues discuss excess mortality during the COVID-19 pandemic, and this paper has already led to much debate and confusion on both traditional and social media and has been used as fodder for anti-vaccine advocates. The paper's results have been taken to mean that vaccines are dangerous, and this has led to ...
The effectiveness against infection of COVID-19 vaccines waned considerably 5-8 months after primary vaccination, although it remained high, particularly among people younger than 55 years. Vaccine boosters were effective in restoring protection against infection and had a good safety profile in the community.
Different COVID-19 vaccines may work in our bodies differently but all provide protection against the virus that causes COVID-19. None of the COVID-19 vaccines can give you COVID-19. Bringing new vaccines to the public involves various steps, all which must be followed to ensure they are safe and effective before they are made available for use.
Introduction. Excess mortality is internationally recognised as an accurate measure for monitoring and comparing health crisis policies across geographic regions.1-4 Excess mortality concerns the number of deaths from all causes during a humanitarian emergency, such as the COVID-19 pandemic, above the expected number of deaths under normal circumstances.5-7 Since the outbreak of the COVID ...
The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus. ... The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints ...
Articles and social media posts claimed that research published in the British Medical Journal (BMJ) proves Covid-19 vaccines caused global excess mortality. This is misleading; the researchers theorize vaccination may have been a contributing factor, but the study's publisher and medical experts point out that the paper does not establish a link between mortality and the shots.