Alzheimer’s Disease: Epidemiology and Clinical Progression
- Open access
- Published: 14 March 2022
- Volume 11 , pages 553–569, ( 2022 )

Cite this article
You have full access to this open access article
- Amir Abbas Tahami Monfared 1 , 2 ,
- Michael J. Byrnes ORCID: orcid.org/0000-0002-2681-5510 3 ,
- Leigh Ann White 3 &
- Quanwu Zhang 1
23k Accesses
103 Citations
25 Altmetric
Explore all metrics
Alzheimer’s disease (AD) is prevalent throughout the world and is the leading cause of dementia in older individuals (aged ≥ 65 years). To gain a deeper understanding of the recent literature on the epidemiology of AD and its progression, we conducted a review of the PubMed-indexed literature (2014–2021) in North America, Europe, and Asia. The worldwide toll of AD is evidenced by rising prevalence, incidence, and mortality due to AD—estimates which are low because of underdiagnosis of AD. Mild cognitive impairment (MCI) due to AD can ultimately progress to AD dementia; estimates of AD dementia etiology among patients with MCI range from 40% to 75% depending on the populations studied and whether the MCI diagnosis was made clinically or in combination with biomarkers. The risk of AD dementia increases with progression from normal cognition with no amyloid-beta (Aβ) accumulation to early neurodegeneration and subsequently to MCI. For patients with Aβ accumulation and neurodegeneration, lifetime risk of AD dementia has been estimated to be 41.9% among women and 33.6% among men. Data on progression from preclinical AD to MCI are sparse, but an analysis of progression across the three preclinical National Institute on Aging and Alzheimer’s Association (NIA-AA) stages suggests that NIA-AA stage 3 (subtle cognitive decline with AD biomarker positivity) could be useful in combination with other tools for treatment decision-making. Factors shown to increase risk include lower Mini-Mental State Examination (MMSE) score, higher Alzheimer’s Disease Assessment Scale (ADAS-cog) score, positive APOE4 status, white matter hyperintensities volume, entorhinal cortex atrophy, cerebrospinal fluid (CSF) total tau, CSF neurogranin levels, dependency in instrumental activities of daily living (IADL), and being female. Results suggest that use of biomarkers alongside neurocognitive tests will become an important part of clinical practice as new disease-modifying therapies are introduced.
Similar content being viewed by others
Prevalence and risk of progression of preclinical Alzheimer’s disease stages: a systematic review and meta-analysis
Lucilla Parnetti, Elena Chipi, … Paolo Eusebi
Research diagnostic criteria for Alzheimer’s disease: findings from the LipiDiDiet randomized controlled trial
Anna Rosenberg, Alina Solomon, … on behalf of the LipiDiDiet clinical study group

Impact of Differential Rates of Disease Progression in Amyloid-Positive Early Alzheimer’s Disease: Findings from a Longitudinal Cohort Analysis
J. Chandler, M. Georgieva, … T. Schilling
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the leading cause of cognitive impairment and dementia in older individuals (aged ≥ 65 years) throughout the world [ 1 ]. AD follows a prolonged, progressive disease course that begins with pathophysiological changes in the brains of affected individuals years before any clinical manifestations are observed [ 2 ]. These pathophysiological changes include the accumulation of toxic species of amyloid-β (Aβ), the development of neurofibrillary tangles of hyperphosphorylated tau protein, and neurodegeneration that may result from uncontrolled activation of microglia in the brain leading to secretion of neurotoxins and inflammatory factors [ 3 , 4 , 5 ]. Individuals harboring such changes may be asymptomatic or may exhibit clinical manifestations varying from memory lapses to severe and debilitating loss of memory and cognitive function [ 2 ]. As AD progresses, additional neuropsychiatric symptoms may manifest, including periods of confusion, disorientation, mood change, aggression/agitation, and eventually delusion/hallucination in later stages.
Because normal aging involves subtle cognitive deterioration, it can often be difficult to distinguish cognitive decline due to AD from the declines associated with normal aging (e.g., declines in processing speed, and certain abilities related to memory, language, visuospatial, and executive function) [ 6 , 7 ]. As a result of the challenges of diagnosis and of a historical perceived lack of benefit from diagnosis (due to the lack of an effective disease-modifying therapy, DMT), underdiagnosis is common, with some evidence suggesting that more than half of those who develop AD dementia may never be formally diagnosed [ 8 , 9 , 10 ]. However, because a novel DMT may be most effective before years of potentially irreversible pathologic changes have occurred, identifying individuals destined to develop AD dementia is vital [ 11 ]. The 2011 National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines defined three phases of AD: preclinical AD (early pathologic changes in the brains of cognitively normal individuals), MCI (symptomatic predementia), and dementia [ 12 , 13 , 14 ].
Preclinical AD, the earliest phase of the continuum from normal cognition to AD dementia, is characterized by the occurrence of the aforementioned pathophysiological changes including accumulation of toxic Aβ species and hyperphosphorylated tau protein [ 2 , 5 , 15 ]. Importantly, toxic Aβ species and hyperphosphorylated tau can be identified in cerebrospinal fluid (CSF) and blood and can therefore be employed as diagnostic biomarkers [ 16 , 17 ]. Definitions for preclinical AD have been proposed by four different groups (NIA-AA criteria, Dubois criteria, International Working Group-2 criteria, and the NIA-AA Research Framework criteria) and based on various combinations of biomarkers [ 18 ]. There is some correspondence among categories in the four systems [ 18 ].
MCI identifies individuals who do not have dementia, but who do have some deficits in cognition. The definition of MCI has evolved over the past 50 years, and there are several clinical definitions [ 11 , 19 ]. The Peterson criteria define MCI as individuals performing 1.5 standard deviations below normal on memory tasks, with preservation of activities of daily living (ADL) [ 19 ]. The Winblad criteria expand the definition to include and distinguish individuals with cognitive impairment in domains beyond memory impairment, resulting in amnestic MCI (aMCI; subjective and objective memory impairment) and non-amnestic MCI (naMCI; impairment in a cognitive domain other than memory) [ 11 , 19 , 20 ]. In addition, the range of cognitive deficit is further categorized as single-domain (sd) or multiple-domain (md) aMCI and naMCI [ 11 , 19 , 20 ]. However, clinical definitions of MCI that do not include neuropathologic changes in the brain as a criterion for diagnosis lack specificity for AD as the underlying cause of impaired cognition [ 15 , 21 ].
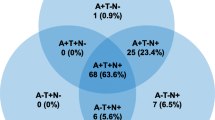
In 2018, the NIA-AA published a research framework that developed a biological rather than clinical definition of AD for use in clinical research [ 15 ]. The NIA framework defined AD on the basis of the AT(N) classification, which assessed the presence of amyloid pathology (A), tau pathology (T), and neuronal injury (N) to categorize the presence and extent of AD [ 15 ]. In the framework, the presence of amyloid pathology was indicative of AD pathologic change and amyloid pathology in combination with tau pathology and/or neuronal injury was indicative of AD [ 15 ]. In response, an International Working Group provided recommendations for a clinical-biological diagnosis of AD that incorporated both clinical (e.g., impaired cognition) and biomarker (amyloid and tau pathology) evidence to support a diagnosis. However, the International Working Group recommended against widespread biomarker testing of cognitively unimpaired individuals [ 21 ].
As new DMTs are introduced into practice, it is useful to understand what is currently known about the clinical burden of AD across the spectrum of the disease. To gain a deeper understanding of the clinical burden of AD in terms of prevalence, incidence, mortality, and progression (including preclinical AD and that of MCI due to AD), we reviewed the epidemiologic and clinical literature on AD with a focus on recent English-language literature published in North America, Europe, and Asia.
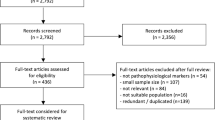
A review of the recent literature on the epidemiology and clinical burden of AD in North America, Europe, and Asia was conducted. Our primary literature search covered the PubMed-indexed literature published between 2014 and January 2021 and included search terms related to AD (including MCI) and epidemiology (e.g., “incidence”, “prevalence”, “epidemiology”, “risk factors”) or clinical burden (e.g., “morality”, “progression”, “stages”) as well as relevant Medical Subject Heading (MeSH) terms for AD epidemiology and clinical burden. Supplemental searches of indexed and non-indexed literature were also conducted. The year 2014 was selected as a cutoff date in an effort to focus our review on studies published after the publication of the 2011 NIA-AA; additionally, we did not want to look further back than 2014 for studies of epidemiologic data as such data may be outdated.
While this research is not based on a systematic literature review, articles identified in the literature search were considered for inclusion if they reported relevant outcomes and were conducted globally or in the United States (USA), France, Germany, Italy, Spain, the United Kingdom (UK), China, and Japan. Articles reporting on patients with AD were prioritized, whereas articles reporting on related populations (e.g., patients with dementia, AD and related dementia [ADRD], or AD and other dementia [ADOD]) were not prioritized unless they reported specific information on patients with AD. Where many articles on a specific topic were identified, those publishing unique information were prioritized for inclusion. Additional priorities included more recently published articles and, where relevant, studies of larger patient populations and studies reporting on longer periods of follow-up. In total, 53 articles were selected for inclusion in the literature review. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Epidemiology of AD and MCI
Prevalence of clinical ad dementia.
The prevalence of clinically diagnosed AD dementia is high and expected to rise over time consistent with the aging of the population [ 8 , 22 , 23 ]. According to a range of estimates from different studies (Table 1 ), AD dementia affects 3–4% of adults in their late working or retirement years [ 24 , 25 , 26 , 27 , 28 ]. These estimates may reflect regional differences or differences in study design (e.g., varying ages of study populations, diagnostic criteria for AD dementia) [ 24 , 26 ].
The prevalence of clinically diagnosed AD dementia rises with increasing age [ 25 , 26 ]. In China, 2010 estimates of prevalence from a systematic review increased with advancing age from 0.2% among persons aged 55–59 to 48.2% among persons aged 95–99 [ 29 ]. AD dementia is also more common among women than among men, as evidenced by a systematic review in which all five studies that included prevalence in men and women reported a higher prevalence in women, and by a meta-analysis that found AD affected 3.31% (95% confidence interval [CI] 2.85, 3.80) of men and 7.13% (95% CI 6.56, 7.72) of women [ 25 ]. A separate study conducted in China similarly found that women were more than twice as likely to have AD dementia than men (prevalence ratio, 2.37, 95% CI 1.90, 2.96, p < 0.0001) after adjustment for age, period of study, and region (urban or rural) [ 29 ].
Incidence of Clinical AD
The incidence of clinically diagnosed AD dementia reported in studies (Table 2 ) varies from 2.0 to 16.8 new cases of AD per 1000 person-years across studies in the USA, Europe, Japan, and China [ 23 , 24 , 25 , 26 , 29 , 30 ]. Some of the wide variation in incidence may be due to variations in age ranges of included study populations, country population characteristics, time periods studied, and operational diagnosis of AD dementia. For example, a meta-analysis investigating the incidence of clinical AD in Europe found that incidence rose with increasing age strata (cases of clinical AD per 1000 person-years: 65–74 years, 3.4; 75–84 years, 13.8; ≥ 85 years, 35.8) [ 25 ]. As with prevalence, the incidence of AD dementia rises with age: a recent systematic review found that seven of nine studies reported a positive association with age [ 26 ]. A meta-analysis of studies conducted in Europe also observed a higher incidence of AD dementia in women than in men (women, 13.3 cases per 1000 person-years [95% CI 12.1, 14.5]; men, 7.02 cases per 1000 person-years [95% CI 6.1, 8.1]), although study authors did not report on the relationship between patient age and sex [ 25 ].
Globally, dementia (all-cause) is the fifth leading cause of death, with 4.4% of all deaths attributable to dementia in 2016 [ 31 ]. Deaths due to dementia have steadily increased over time, partly due to population growth and population aging, more than doubling from 1990 to 2016 [ 31 ]. AD dementia-related deaths have also risen. An analysis of AD dementia mortality in Europe found that deaths from AD among patients aged ≥ 50 years (as identified by diagnostic codes) more than doubled from 1994 [41,255 deaths] to 2013 [86,822 deaths] [ 32 ]. The age-standardized mortality rate for deaths caused by AD dementia in Europe was 45.2 per 100,000 in 2013 [ 32 ].
Recording of cause of death is variable and AD dementia may be either a contributing cause or an underlying cause [ 33 , 34 ]. In the USA in 2017, 46.4% of dementia-related deaths were attributable to AD [ 34 ]. One study of AD dementia in Canada investigated multiple causes of death when AD was listed on death certificates [ 33 ]. In this analysis, 4.3% of all deaths occurring from 2004 to 2011 were AD dementia-related, but 2.6% of deaths had AD dementia as an underlying cause, in contrast to 1.7% of deaths having AD dementia as a contributing cause [ 33 ]. Usually, when AD dementia was listed as an underlying cause, cardiovascular disease was the most common contributing cause (46%); when AD dementia was listed as a contributing cause, cardiovascular disease was the most common underlying cause (41%) [ 33 ]. The crude mortality rate in Canada for deaths from AD dementia as an underlying cause increased over the period 2004 to 2011, from 10.1 to 11.5 per 100,000 for men and from 24.4 to 25.4 per 100,000 for women [ 33 ].
Data on survival from early stages of AD dementia are scarce. However, median survival among persons with AD dementia has been estimated to be approximately 7 years from presentation with cognitive decline (according to an analysis of data from one geographic area in England) [ 35 ].
MCI due to AD
Patients with MCI due to AD are more likely to progress to dementia than those with MCI that is not associated with toxic species of Aβ and/or the development of neurofibrillary tangles of hyperphosphorylated tau protein [ 5 , 15 , 21 , 36 , 37 , 38 ]. Data from 13 cohort studies representative of multiple countries has demonstrated a high prevalence of AD at the MCI stage according to the IWG-1, IWG-2, and NIA-AA criteria, which identifies patients at higher risk of dementia based on the presence of AD-related biomarkers (abnormal Aβ and/or tau) and or neuronal injury [ 38 ]. On the basis of the IWG criteria, 53% (IWG-1) and 40% (IWG-2) of subjects with MCI were identified as having AD; 3-year progression rates were 50% (IWG-1 criteria) in persons with prodromal AD versus 21% without prodromal AD, and 61% (IWG-2) with prodromal AD versus 22% without prodromal AD. On the basis of NIA-AA criteria, 46% of subjects with MCI were classified as being in the “high likelihood AD” group [ 38 ]; and the 3-year progression rate was 59% (subjects in lower risk groups had 3-year progression rates of 5–24%).
The incidence of clinically diagnosed MCI has been examined in a systematic review of studies in Europe, the Americas, and Australia [ 39 ]. Incidence rates ranged from 22.5 to 60.1 per 1000 person-years depending on age, but meta-analysis suggests substantial heterogeneity in incidence estimates due to methodological and population sample characteristics in the included studies. Analyses resulted in MCI incidence of 22.5 (95% CI 5.1, 51.4) per 1000 person-years for ages 75–79, 40.9 (95% CI 7.7, 97.5) for ages 80–84, and 60.1 (95% CI 6.7, 159.0) for ages 85 and older [ 39 ]. Other research has examined etiologic diagnoses of AD in MCI (as evidenced by NIA-AA clinical criteria), finding that 75% of subjects with MCI had an etiology of AD, while the remainder were classified with etiologies such as CVD and Lewy body dementia [ 40 ].
Several recent studies in China have documented population-based prevalence of clinically diagnosed MCI [ 37 , 41 , 42 ]. In a meta-analysis of 41 studies of Chinese community-dwelling populations over 55 years of age, the pooled prevalence of MCI was 12.2% (95% CI 10.6, 14.2) for MCI and 10.9% (95% CI 7.7, 15.4) for amnestic MCI. There were slight differences in prevalence according to diagnostic criteria, with rates of MCI of 13.5% using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria, 12.9% using the Petersen criteria, and 10.3% using NIA-AA [ 42 ]. There was a higher prevalence among women than men (13.8% [95% CI 9.7, 13.6] vs. 11.5% [95% CI 11.7, 16.3]), and people living alone versus in families (18.2% [95% CI 13.6, 24.4] vs. 14.1% [95% CI 11.0, 18.2]); those that had education levels lower than primary school had much higher prevalence of MCI (17.2% [95% CI 12.2, 24.3]) when compared to persons with higher levels of education. Studies in this meta-analysis based on more recent observation periods resulted in higher prevalence estimates (before 2005, 3.7% [95% CI 1.6, 8.7]; in 2005 or later, 14.1 [95% CI 12.4, 16.0]) [ 42 ]. One study of a rural area in Northern China in 2015 reported prevalence of MCI and AD, finding rates of 27.8% (overall MCI), 18.4% (MCI due to AD), and 6.5% (AD) [ 41 ]. Another national cross-sectional study of adults over the age of 60 in China reported age- and sex-adjusted overall MCI prevalence of 15.5% (95% CI 15.2, 15.9) [ 37 ].
Preclinical Stages of AD
AD dementia develops by progression through several stages, from no accumulation of toxic Aβ species to normal cognition with Aβ deposition and then neurodegeneration (as evidenced by elevated CSF tau protein and fluorodeoxyglucose (FDG) positron electron tomography (PET) imaging), and subsequently to MCI. According to a multistate disease model, the lifetime risk of AD for a 60-year-old person with normal cognition and no toxic Aβ accumulation has been estimated to be 20.1% (range 10.6–34.0%) among women and 13.9% (range 6.9–25.1%) among men [ 43 ]. For patients with Aβ deposition and neurodegeneration, estimates of lifetime risk are 41.9% among women and 33.6% among men, and for patients with MCI, lifetime risk is more than twice that of the earlier stage. By contrast, a much higher percentage of patients with MCI due to AD were estimated to progress to dementia (men 92.9%; women 95.6%).
Prevalence estimates studied in a systematic literature review that measured amyloid PET or CSF in subjects with normal pathology showed rates of 22% (CI 18–27%) and 21% (CI 15–29%) for PET and CSF, respectively; however, prevalence rates across the studies including biomarker data ranged widely (7–48% for PET studies, and 4–52% for CSF studies) [ 44 ]. Nevertheless, results suggest that measuring biomarkers is an important component of identifying risk of progression from preclinical AD to MCI and ultimately to AD dementia. In the same systematic literature review, an analysis of the studies that provided data across NIA-AA preclinical stages resulted in prevalence estimates of 13% (CI 9–18%), 16% (CI 9–25%), and 5% (CI 3–9%) for stages 1, 2, and 3, respectively.
Other research using the NIA-AA criteria has estimated the prevalence of preclinical and clinical AD by stage [ 36 ]. Rates were calculated on the basis of a multistate Markov model with data on incidence from longitudinal studies, as well as mortality rates and population projections. Most cases with AD pathology (i.e., Aβ deposition, CSF tau, and neurodegeneration based on FDG-PET imaging) were preclinical, with rates from this model declining from early to later stages of AD owing primarily to attrition from death (from age, other conditions, or dementia). Projected 2060 prevalence estimates increased across all stages from 2015, more than doubling for each of the later stages: MCI due to AD (2.4 million in 2017; 5.7 million in 2060), early-stage AD dementia (2.1 million in 2017; 5.3 million in 2060), and late-stage AD dementia (1.5 million in 2017; 4.0 million in 2060). Prevalence estimates for preclinical AD also increased in patients with Aβ deposition only (22.1 million in 2017; 31.9 million in 2060) and in those with Aβ deposition and neurodegeneration (16.2 million in 2017; 30.2 million in 2060).
This multistate model was also used to assess the impact of hypothetical disease-modifying health interventions on forecasts. In one scenario, an intervention delaying the annual risk of progression to MCI by 50% resulted in a decrease in the prevalence of MCI (from 5.70 million to 5.01 million) as well as a decrease in the prevalence of AD dementia (from 9.3 million to 6.95 million) [ 36 ].
Disease Progression
Progressive deterioration of cognitive function in persons with AD varies according to the affected elements of cognition. In an analysis of data from 1495 adults with clinically diagnosed AD dementia from the Geriatric Education and Research in Aging Sciences (GERAS) study, deterioration on both the Mini Mental State Exam (MMSE) and the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) showed a similar pattern of progressive decline in which word recall and orientation were affected first, with declines in attention and concentration, language, constructional praxis, and executive function occurring next, and immediate memory deteriorating in severe AD dementia [ 45 ]. A systematic literature review of studies examining acceleration points in cognitive decline showed that verbal memory deficits appeared first, followed by deficits in visuospatial ability, executive functions, fluency, and verbal IQ [ 46 ].
Data on the risk of progression from preclinical AD to MCI are sparse, but a systematic literature review and meta-analysis (2011–2018) included studies that provided progression data across the three preclinical NIA-AA stages [ 44 ]. Rates of progression increased across stages: 73% (95% CI 40–92%) at the preclinical AD stage 3 (subtle cognitive decline with AD biomarker positivity), 38% (95% CI 21–59%) at stage 2 (toxic Aβ accumulation and neurodegeneration), and 20% (95% CI 10–34%) at stage 1 (toxic Aβ accumulation but asymptomatic) [ 44 ]. The relative risk of progression increased across stage and was over six times higher at stage 3 (RR = 6.38; 95% CI 3.33–12.24) compared to individuals with normal biomarkers [ 44 ]. Authors suggested that on the basis of these data, NIA-AA stage 3 should be used along with MCI due to AD in treatment decision-making [ 44 ].
Data from studies of progression from clinically diagnosed MCI to AD dementia have shown that the majority of patients with MCI either remain cognitively stable or revert to normal values, versus progressing to AD [ 11 , 20 , 47 , 48 ]. However, comparing results across studies can be challenging because of inconsistent study designs, differing clinical definitions of MCI, and differences in patient populations [ 20 ]. For example, in a meta-analysis of 28 studies (through 2014) including 2365 patients with MCI, 38.7% progressed to AD dementia over a mean follow-up of 31 months; however, progression rates in the individual studies varied from 6% to 39% per year.
One factor that has implications for estimating progression rates is the subtype of MCI. A meta-analysis of 33 studies (1999–2017) of 4907 patients with clinically diagnosed MCI showed that approximately half of patients with sd-aMCI or md-aMCI progressed (sd-aMCI odds of progression to AD, 0.47; 95% CI 0.33, 0.66; p < 0.001; md-aMCI odds of progression to AD dementia, 0.52; 95% CI 0.36, 0.75; p < 0.001), but that risk of progression was substantially lower for patients with naMCI (sd-naMCI odds of progression to AD dementia, 0.11; 95% CI 0.07, 0.16; p < 0.001; md-naMCI odds of progression to AD dementia, 0.18; 95% CI 0.11, 0.27; p < 0.001) [ 20 ]. This was also illustrated in the prospective Australian Imaging, Biomarkers and Lifestyle (AIBL) study in which patients ( n = 866) were classified by subtype of clinically diagnosed MCI and then also by the severity of their memory impairment [ 11 ]. After 3 years, patients with aMCI were more likely than the healthy controls to develop AD dementia (positive predictive value [PPV] 24.1%; 95% CI 18.4, 30.6), and risk increased with the severity of memory impairment (grade 1 PPV = 10.0%; 95% CI 5.1, 17.2; grade 2 PPV = 43.0%; 95% CI 32.8, 53.7) [ 11 ]. Patients with md-aMCI had a higher risk of progression vs. sd-aMCI (PPV of 47.3%; 95% CI 33.7, 61.2 vs. PPV of 15.5%; 95% CI 10.1, 22.4), and risk also increased with increasing severity of memory loss [ 11 ]. The annual progression rates were 8.0% for aMCI, 5.2% for sd-aMCI, and 15.8% for md-aMCI [ 11 ]. Other studies have reported rates of progression from clinically diagnosed MCI to AD dementia varying from 18.3% (9 of 51 people) to 48.3% (86 of 178 people) within 2 years [ 49 , 50 ]. A larger study found that 33.3% (181 of 544 patients) progressed from clinically diagnosed MCI to AD dementia within a median of 46 months [ 51 ].
The progression rates reported in studies can also be influenced by the source of recruitment of patients: the aforementioned meta-analysis of 33 studies also classified patients as community-dwelling or from specialist clinics and showed that rates of progression were higher for patients from specialist clinics vs. community samples (sd-aMCI, 40% vs. 18%; md-aMCI, 35% vs. 21%) [ 20 ]. This concept was also demonstrated in a systematic literature review and meta-analysis of 59 studies (1999–2016) [ 52 ]. The overall progression rate (timeframe not reported) from clinically diagnosed MCI (subtype not stated) to AD dementia (adjusted for prevalence and weighted by follow-up time) was 28% (95% CI 22, 33) and to dementia was 34% (95% CI 27, 40), with 45% (95% CI 34, 55) remaining stable and 15% (95% CI 10, 19) improving their status to normal [ 52 ]. When the data were grouped according to community vs. specialist clinic patients, progression to AD was higher in the clinic sample compared to the community sample (31% vs. 13%), which may be due differences between clinic and community populations [ 52 ].
Another confounding factor is that a sizable percentage of healthy elderly adults score below normal on at least one cognitive test when given a battery of tests [ 47 ]. In order to resolve this issue, some researchers used the concept of “base rate of low scores” (BRLS) to classify patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database [ 47 ]. According to this approach, a person was deemed to have MCI if their number of low scores on a panel of cognitive tests was greater than or equal to the number of the 10% of worst-performing patients [ 47 ]. According to these criteria, 34% of patients defined as MCI progressed to AD dementia, compared to 22% defined by the Petersen criteria, and 11% of sd-aMCI and 29% of md-aMCI defined by the Winblad criteria [ 47 ].
Several modeling studies have estimated the progression of dementia severity in patients with AD. An analysis based on patients from the National Alzheimer’s Coordinating Center (NACC) ( n = 3009) estimated that a starting population of patients with mild (69.5%) or moderate (30.5%) dementia would progress to mild (38.6%), moderate (44.1%), or severe (7.3%) dementia or death (10.0%) after 1 year [ 53 ]. Another analysis based on a larger NACC population ( n = 18,103) estimated that patients aged 65 years with mild AD dementia had a 25% chance of progression to moderate (19%) or severe (1%) dementia or death (4%) within 1 year, whereas those with moderate AD dementia would have a 36% chance of progression to severe dementia (27%) or deal (9%) [ 54 ]. Slightly higher progression rates were estimated for patients aged 75 years. Unfortunately, neither of these studies took patient amyloid status into consideration. A more recent analysis limited to Aβ-positive patients estimated higher annual rates of progression. Among Aβ-positive patients with mild dementia, 45.1% of patients with progressed to moderate (31.6%) or severe (4.3%) dementia or death (9.2%) within 1 year. Among those with moderate dementia, 59.8% progressed to severe dementia (28.6%) or death (31.2%) [ 55 ]. These analyses highlight the limitation of studying AD progression without incorporating biomarker estimates including amyloid status.
Risk Factors for Disease Progression
Understanding risk factors associated with disease progression can help individual patients make decisions regarding their future as well as guide clinical development and use of treatments for slowing progression [ 56 , 57 ]. However, the factors noted previously that influence data on reported rates of progression also are likely to influence data on risk factors, sometimes resulting in conflicting results.
Some researchers have examined which tests (neurocognitive or biomarkers) might be the most valuable in identifying patients who are likely to progress from clinically diagnosed MCI to AD dementia or from mild to more severe AD dementia. In the meta-analysis of 28 studies (through 2014) that included 2365 patients with MCI, described previously, the objective was to evaluate the predictive value of neurocognitive tests for progression to dementia [ 48 ]. Five tests were shown to have excellent (≥ 0.90) sensitivity and specificity: Addenbrooke’s Cognitive Examination (a global measure), Visual Object and Space Perception Silhouettes (visuospatial), Object Function Recognition (language), and three tests of verbal episodic memory (Face–Name Association Task; Rey Auditory Verbal Learning Test, and Guild Paragraph) [ 48 ]. The meta-analysis of 24 studies (through 2018) that included 2689 patients with clinically diagnosed MCI (primarily aMCI) examined the ability of neuropsychological testing to predict progression to AD dementia [ 58 ]. A mean of 37% of these patients progressed to AD dementia [ 58 ]. Overall, patients who progressed did worse on a variety of measures than did non-progressors, affirming the relevance of neuropsychiatric testing [ 58 ]. In a systematic analysis of 48 studies that examined biomarkers in patients with AD dementia, little information was available from studies with follow-up of more than 2.5 years or with repeated testing [ 59 ]. Although magnetic resonance imaging (MRI) scan of whole brain or hippocampal atrophy was the most common testing measure, the investigators noted it is not specific to AD [ 59 ]. In most studies, evaluation of progression was based on cognitive testing, but there was no consensus on which tests or combinations of tests were most valuable [ 59 ].
Risk factors for progressing from unimpaired cognition to aMCI were evaluated on the basis of data from approximately 1500 individuals in the ADNI database [ 60 ]. Over 4 years, 17% of unimpaired participants converted to aMCI [ 60 ]. Significant risk factors for this conversion were low hippocampal volume, a high CSF tau/Aβ ratio, and a low memory score; neither increasing age nor family history of AD was significant [ 60 ]. The risk of conversion from no impairment to aMCI increased with the number of risk factors; participants with three risk factors had an estimated probability of developing aMCI of 0.35 over 4 years of follow-up [ 60 ].
Multiple risk factors for progressing from MCI to AD dementia have been proposed. A meta-analysis of 53 studies with 12,396 patients with AD dementia and 1934 with MCI examined progression risk [ 61 ]. For patients with MCI, rapid progression to AD dementia was associated with positive APOE4 , greater white matter hyperintensity volume, entorhinal cortex atrophy, high CSF neurogranin levels, and high dependency in instrumental activities of daily living (IADLs) [ 61 ]. More engagement in social activities and a higher body mass index (BMI) were associated with slower progression to AD dementia [ 61 ]. Evaluation of the ADNI database showed that of patients with aMCI at baseline, 35% had progressed to AD dementia by 4 years [ 60 ]. Significant risk factors for progression were APOE4 positive, high FAQ score, a low memory score, hippocampal atrophy, and a high tau/Aβ ratio [ 60 ]. Risk increased with the number of risk factors; participants with five risk factors had an estimated probability of developing aMCI of 0.90 over 4 years of follow-up [ 60 ]. In a meta-analysis of 60 studies (1966–2015) of patients with MCI, the greatest risk factors for progression to AD dementia were abnormal CSF tau, abnormal CSF tau/Aβ ratio, hippocampal atrophy, medial temporal lobe atrophy, and entorhinal atrophy [ 62 ]. Other risk factors were APOE4 positive, CSF total tau, white matter hyperintensity volume, depression, diabetes, hypertension, older age, female gender, lower MMSE score, and higher ADAS-cog score [ 62 ]. Protective factors were high BMI and higher auditory verbal learning test delay score. Individuals with a combination of risk factors had an even greater risk for progression [ 62 ]. It has been demonstrated that declines in both cognitive and financial skills in patients with clinically diagnosed MCI are significant predictors of clinical progression [ 50 ].
Many studies have attempted to identify risk factors for progression from mild to more severe AD dementia. Several factors increasing the risk of AD dementia have been identified in this review (Table 3 ). There are conflicting results for some factors (e.g., age, comorbidities, education level), but many studies agree that atrophy in various brain regions, poor baseline memory and ADAS-cog scores, and abnormal CSF biomarkers are risk factors [ 49 ]. Other potential factors that vary by AD dementia severity include hair cortisol levels, which have been observed to be higher in patients with more severe dementia compared to those with mild dementia, and salivary IgA levels, which exhibit the inverse pattern [ 63 ]. Systemic inflammation, as evidenced by consistently elevated concentrations of inflammatory biomarkers and cytokines, has been observed in patients with AD dementia [ 64 ]. However, inflammatory biomarkers have not always been associated with measures of clinical disease progression in patients with clinically diagnosed MCI or AD dementia [ 65 ]. A recent study in a small number of patients ( n = 39 patients with AD dementia and n = 21 healthy controls) found that the levels of oligomeric Aβ in nasal discharge were higher in patients with AD dementia and that the presence of specific Aβ oligomers could distinguish between patients with mild and moderate cognitive dysfunction [ 66 ].
Some analyses have focused on factors associated with rapid or slow decline. The previously described systematic literature review of 53 studies of patients with MCI and AD dementia reported that risk factors for rapid progression in patients with dementia were APOE4 positive, early age at onset, higher level of education, early appearance of extrapyramidal signs, and neuropsychiatric conditions [ 61 ]. Factors such as age ≥ 75 years, diabetes, and multidrug therapy lowered the risk of rapid progression [ 61 ]. In the Impact of Cholinergic Use (ICTUS) study, a prospective study in 12 European countries that followed 1005 patients with AD dementia, a worse baseline ADAS-cog score was significantly associated with rapid decline, while increased age at baseline was a protective factor [ 57 ]. The Progression of Alzheimer's Disease and Resource use (PADR) study evaluated 282 Norwegian patients with AD dementia for a mean of 2 years [ 67 ]. Over 40% of patients progressed slowly (47% by change in Clinical Dementia Rating Scale-Sum of Boxes [CDR-SB] and 44% by change in MMSE) [ 67 ]. Baseline factors associated with slower progression on the CDR-SB were younger age at diagnosis, higher education, better IADL function, better scores on cognitive tests, used fewer drugs, and 1-point lower CSR-SB score, while better scores on cognitive tests and better CDR-SB score were associated with slower progression on the MMSE and IADL [ 67 ].
The Canadian Outcomes Study in Dementia (COSID) evaluated overall progression using data from 488 patients with AD dementia [ 68 ]. After adjustment for age, patients who progressed were significantly more likely at baseline to have poorer cognition, greater dependence, and more neuropsychiatric symptoms [ 68 ]. Male sex and having a worse baseline Global Deterioration Scale (GDS) score were significant protective factors [ 68 ]. A systematic literature review of 11 studies (to 2016) evaluating the influence of comorbid conditions on progression of late-onset AD dementia [ 56 ] found associations between comorbidities and declines in cognitive function and functional abilities, as well as increases in neuropsychiatric symptoms [ 56 ]. Other studies have examined specific factors protective or predictive of clinical progression. Blood pressure variability [ 69 ] has been proposed as a risk factor for progression, and cognitive reserve (e.g., IQ prior to disease onset) [ 70 ] has been proposed as a protective factor. A recent analysis of healthy controls ( n = 318) and individuals with MCI ( n = 168) or AD dementia ( n = 269) found that neuroinflammation (as evidenced by translocator protein (TSPO) identified during PET) increases with more severe AD dementia [ 71 ]. Compared with healthy controls, individuals with MCI had increased TSPO levels in the neocortex and those with AD had even greater levels of TSPO present throughout the brain [ 71 ]. There was a significant inverse association between TSPO levels in the parietal region of the brain and MMSE scores ( p = 0.024) [ 71 ].
This review of the literature on epidemiology and clinical progression of AD underscores the importance of identifying patients who are most at risk of developing AD dementia. The worldwide toll of AD is evidenced by rising prevalence and mortality due to AD dementia. With many countries experiencing population aging, the prevalence of AD dementia continues to rise, thereby increasing the already substantial clinical and socioeconomic burden of this disease. The state of knowledge regarding AD epidemiology suggests that clinicians will face significant and increasing numbers of patients presenting with potential risk factors for progression to clinical AD, and thus will need to have the capability to identify patients early in the disease process who are most likely to benefit from treatment. However, studies of AD prevalence, incidence and mortality face challenges in diagnosis as well as classification of disease stage.
The criteria developed to classify patients into categories of preclinical AD, MCI due to AD, and AD dementia, while aligned in some ways, vary enough to prevent accurate comparisons across studies of epidemiology and progression risk. It is particularly difficult to distinguish MCI with AD etiology from MCI due to other etiologies such as CVD and Lewy body dementia.
The risk of dementia increases with stages of progression, from normal cognition with no Aβ deposition to Aβ deposition, hyperphosphorylated tau, and neuronal injury, and subsequently to MCI due to AD. More research is needed to understand the spectrum of disease from preclinical AD to clinical dementia. While data on progression of preclinical AD to MCI are sparse, evidence is available on the progression from MCI to dementia [ 11 , 20 , 44 , 47 , 48 ]. Current research suggests that almost 40% of patients with clinically diagnosed MCI progress to AD dementia over an average of 18 months [ 48 ]. Key risk or protective factors for progression to AD dementia from clinically diagnosed MCI include MCI subtype (e.g., aMCI, naMCI), poor performance on various neurocognitive tests, and biomarkers such as abnormal CSF tau or tau/Aβ ratio, APOE4 positive status, white matter hyperintensities, and atrophy in the hippocampal, medial temporal, or entorhinal regions [ 20 , 58 , 61 ]. Newer emerging treatments are more likely to prevent or delay further progression of AD dementia than to reverse it [ 11 ].
Unfortunately, most of the literature on AD reports on patients diagnosed on the basis of clinical criteria such as the DSM-IV, the 1984 National Institute of Neurological and Communicative Disorders and Stroke (NINCDS)/Alzheimer’s Disease and Related Disorders Association (ADRDA), or the 2011 NIA-AA [ 12 , 13 , 14 , 72 , 73 ]. To treat individuals most likely to benefit from a DMT, it is important for clinicians to have the ability to identify patients at risk of AD in early stages, prior to progressive deterioration. Biomarker identification added to neurocognitive testing will be an increasingly important component of diagnosis and measurement of progression and is likely to improve the ability of practicing clinicians to identify patients who will experience a treatment benefit.
AD dementia incidence and prevalence are high worldwide and likely underestimated. Recent studies show that the risk of AD dementia is significant among older people with normal pathology and increases with progression from normal pathology to neurodegeneration and MCI. Studies of AD remain challenged by disease classification due to differential diagnosis, multiple biomarkers and neuropsychiatric tests, and variation in consensus criteria. As new DMTs are introduced, clinicians will require combinations of diagnostic approaches to identify patients with preclinical AD and MCI due to AD and to assess the impact of treatment on slowing progression.
Atri A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103(2):263–93.
Article PubMed Google Scholar
Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16.
Article CAS PubMed PubMed Central Google Scholar
Li Q, Wu Y, Chen J, et al. Microglia and immunotherapy in Alzheimer’s disease. Acta Neurol Scand. 2022;145(3):273–8.
Article CAS PubMed Google Scholar
Spanic E, Langer Horvat L, Hof PR, et al. Role of microglial cells in Alzheimer’s disease tau propagation. Front Aging Neurosci. 2019;11:271.
Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–17.
Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52.
Article PubMed PubMed Central Google Scholar
Steiner ABQ, Jacinto AF, Mayoral VFS, et al. Mild cognitive impairment and progression to dementia of Alzheimer's disease. Rev Assoc Med Bras (1992). 2017;63(7):651–5.
Alzheimer's Association. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327–406.
Marasco RA. Current and evolving treatment strategies for the Alzheimer disease continuum. Am J Manag Care. 2020;26(8 Suppl):S167–76.
PubMed Google Scholar
Wong W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care. 2020;26(8 Suppl):S177–83.
Bradfield NI, Ellis KA, Savage G, et al. Baseline amnestic severity predicts progression from amnestic mild cognitive impairment to Alzheimer disease dementia at 3 years. Alzheimer Dis Assoc Disord. 2018;32(3):190–6.
Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92.
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Babic M, Svob Strac D, Muck-Seler D, et al. Update on the core and developing cerebrospinal fluid biomarkers for Alzheimer disease. Croat Med J. 2014;55(4):347–65.
Qu Y, Ma YH, Huang YY, et al. Blood biomarkers for the diagnosis of amnestic mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;128:479–86.
Kern S, Zetterberg H, Kern J, et al. Prevalence of preclinical Alzheimer disease: comparison of current classification systems. Neurology. 2018;90(19):e1682–91.
Bradfield NI, Ames D. Mild cognitive impairment: narrative review of taxonomies and systematic review of their prediction of incident Alzheimer’s disease dementia. BJPsych Bull. 2020;44(2):67–74.
Oltra-Cucarella J, Ferrer-Cascales R, Alegret M, et al. Risk of progression to Alzheimer’s disease for different neuropsychological mild cognitive impairment subtypes: a hierarchical meta-analysis of longitudinal studies. Psychol Aging. 2018;33(7):1007–21.
Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484–96.
Asada T. Epidemiology of dementia in Japan. In: Matsuda H, Adada T, Tokumaru AM, editors. Neuroimaging diagnosis for Alzheimer’s disease and other dementias. Tokyo: Springer; 2017.
Google Scholar
Montgomery W, Ueda K, Jorgensen M, et al. Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer’s disease in Japan. ClinicoEcon Outcomes Res. 2018;10:13–28.
Fiest KM, Roberts JI, Maxwell CJ, et al. The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(Suppl 1):S51-82.
Niu H, Alvarez-Alvarez I, Guillen-Grima F, et al. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia. 2017;32(8):523–32.
Takizawa C, Thompson PL, van Walsem A, et al. Epidemiological and economic burden of Alzheimer’s disease: a systematic literature review of data across Europe and the United States of America. J Alzheimers Dis. 2015;43(4):1271–84.
Cui L, Hou NN, Wu HM, et al. Prevalence of Alzheimer’s disease and Parkinson’s disease in China: an updated systematical analysis. Front Aging Neurosci. 2020;12: 603854.
Zhao X, Li X. The prevalence of Alzheimer’s disease in the Chinese Han population: a meta-analysis. Neurol Res. 2020;42(4):291–8.
Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381(9882):2016–23.
Rajan KB, Weuve J, Barnes LL, et al. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15(1):1–7.
Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):88–106.
Article Google Scholar
Niu H, Alvarez-Alvarez I, Guillen-Grima F, et al. Trends of mortality from Alzheimer’s disease in the European Union, 1994–2013. Eur J Neurol. 2017;24(6):858–66.
Park J. Mortality from Alzheimer’s disease in Canada: a multiple-cause-of-death analysis, 2004 to 2011. Health Rep. 2016;27(5):17–21.
Kramarow EA, Tejada-Vera B. Dementia mortality in the United States, 2000–2017. Natl Vital Stat Rep. 2019;68(2):1–29.
Price A, Farooq R, Yuan JM, et al. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open. 2017;7(11): e017504.
Brookmeyer R, Abdalla N, Kawas CH, et al. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121–9.
Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661–71.
Vos SJ, Verhey F, Frölich L, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(Pt 5):1327–38.
Gillis C, Mirzaei F, Potashman M, et al. The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement (Amst). 2019;11:248–56.
Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11.
Lu H, Wang XD, Shi Z, et al. Comparative analysis of cognitive impairment prevalence and its etiological subtypes in a rural area of northern China between 2010 and 2015. Sci Rep. 2019;9(1):851.
Article PubMed PubMed Central CAS Google Scholar
Lu Y, Liu C, Yu D, et al. Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: a meta-analysis and systematic review. BMC Geriatr. 2021;21(1):10.
Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018;14(8):981–8.
Parnetti L, Chipi E, Salvadori N, et al. Prevalence and risk of progression of preclinical Alzheimer’s disease stages: a systematic review and meta-analysis. Alzheimers Res Ther. 2019;11(1):7.
Henneges C, Reed C, Chen YF, et al. Describing the sequence of cognitive decline in Alzheimer’s disease patients: results from an observational study. J Alzheimers Dis. 2016;52(3):1065–80.
Karr JE, Graham RB, Hofer SM, et al. When does cognitive decline begin? A systematic review of change point studies on accelerated decline in cognitive and neurological outcomes preceding mild cognitive impairment, dementia, and death. Psychol Aging. 2018;33(2):195–218.
Oltra-Cucarella J, Sanchez-SanSegundo M, Lipnicki DM, et al. Using base rate of low scores to identify progression from amnestic mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. 2018;66(7):1360–6.
Belleville S, Fouquet C, Hudon C, et al. Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer’s type dementia in older adults: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(4):328–53.
Fang Y, Du N, Xing L, et al. Evaluation of hippocampal volume and serum brain-derived neurotrophic factor as potential diagnostic markers of conversion from amnestic mild cognitive impairment to Alzheimer disease: a STROBE-compliant article. Medicine (Baltimore). 2019;98(30): e16604.
Article CAS Google Scholar
Gerstenecker A, Triebel KL, Martin R, et al. Both financial and cognitive decline predict clinical progression in MCI. Alzheimer Dis Assoc Disord. 2016;30(1):27–34.
Sörensen A, Blazhenets G, Rücker G, et al. Prognosis of conversion of mild cognitive impairment to Alzheimer’s dementia by voxel-wise Cox regression based on FDG PET data. Neuroimage Clin. 2019;21: 101637.
Hu C, Yu D, Sun X, et al. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(10):1595–608.
Green C, Zhang S. Predicting the progression of Alzheimer’s disease dementia: a multidomain health policy model. Alzheimers Dement. 2016;12(7):776–85.
Davis M, O’Connell T, Johnson S, et al. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15(8):777–88.
Potashman M, Buessing M, Levitchi Benea M, et al. Estimating progression rates across the spectrum of Alzheimer’s disease for amyloid-positive individuals using national Alzheimer’s coordinating center data. Neurol Ther. 2021;10(2):941–53.
Haaksma ML, Vilela LR, Marengoni A, et al. Comorbidity and progression of late onset Alzheimer’s disease: a systematic review. PLoS ONE. 2017;12(5): e0177044.
Canevelli M, Kelaiditi E, Del Campo N, et al. Predicting the rate of cognitive decline in Alzheimer disease: data from the ICTUS study. Alzheimer Dis Assoc Disord. 2016;30(3):237–42.
Prado CE, Watt S, Treeby MS, et al. Performance on neuropsychological assessment and progression to dementia: a meta-analysis. Psychol Aging. 2019;34(7):954–77.
Lawrence E, Vegvari C, Ower A, et al. A systematic review of longitudinal studies which measure Alzheimer’s disease biomarkers. J Alzheimers Dis. 2017;59(4):1359–79.
Steenland K, Zhao L, John SE, et al. A “Framingham-like” algorithm for predicting 4-year risk of progression to amnestic mild cognitive impairment or Alzheimer’s disease using multidomain information. J Alzheimers Dis. 2018;63(4):1383–93.
Song YN, Wang P, Xu W, et al. Risk factors of rapid cognitive decline in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 2018;66(2):497–515.
Li JQ, Tan L, Wang HF, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87(5):476–84.
de la Rubia Ortí JE, Prado-Gascó V, Sancho Castillo S, et al. Cortisol and IgA are involved in the progression of Alzheimer’s disease. A pilot study. Cell Mol Neurobiol. 2019;39(7):1061–5.
Su C, Zhao K, Xia H, et al. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2019;19(4):300–9.
Hazen J, Vistnes M, Barca ML, et al. The Association between circulating inflammatory markers and the progression of Alzheimer disease in Norwegian memory clinic patients with mild cognitive impairment or dementia. Alzheimer Dis Assoc Disord. 2020;34(1):47–53.
Yoo SJ, Son G, Bae J, et al. Longitudinal profiling of oligomeric Aβ in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci Rep. 2020;10(1):11234.
Eldholm RS, Barca ML, Persson K, et al. Progression of Alzheimer’s disease: a longitudinal study in Norwegian memory clinics. J Alzheimers Dis. 2018;61(3):1221–32.
Herrmann N, Harimoto T, Balshaw R, et al. Risk factors for progression of Alzheimer disease in a Canadian population: the Canadian outcomes study in dementia (COSID). Can J Psychiatry. 2015;60(4):189–99.
de Heus RAA, Olde Rikkert MGM, Tully PJ, et al. Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74(5):1172–80.
Article PubMed CAS Google Scholar
Osone A, Arai R, Hakamada R, et al. Impact of cognitive reserve on the progression of mild cognitive impairment to Alzheimer’s disease in Japan. Geriatr Gerontol Int. 2015;15(4):428–34.
Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev. 2019;50:1–8.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. Washington: American Psychiatric Association; 1994.
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–44.
Download references
Acknowledgements
Eisai provided the funding for the literature review and the manuscript, including the journal’s Rapid Service Fees.
All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception and design of the manuscript. Amir Abbas Tahami Monfared and Quanwu Zhang provided the idea behind the article, Michael Byrnes conducted the literature review, Michael Byrnes and Leigh Ann White designed and drafted the manuscript, and Amir Abbas Tahami Monfared and Quanwu Zhang provided feedback and critical analysis of literature review methods, manuscript outlines, and manuscript text. Karin Hawkinson, a former employee of Evidera, contributed to the development of the sections of the manuscript focused on humanistic burden.
Disclosures
Amir Abbas Tahami Monfared and Quanwu Zhang are employees of Eisai; Michael Byrnes and Leigh Ann White are employees of Evidera, Inc.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and affiliations.
Eisai, 200 Metro Blvd, Nutley, NJ, 07110, USA
Amir Abbas Tahami Monfared & Quanwu Zhang
Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, QC, Canada
Amir Abbas Tahami Monfared
Evidera, Waltham, MA, USA
Michael J. Byrnes & Leigh Ann White
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Amir Abbas Tahami Monfared .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Tahami Monfared, A.A., Byrnes, M.J., White, L.A. et al. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol Ther 11 , 553–569 (2022). https://doi.org/10.1007/s40120-022-00338-8
Download citation
Received : 10 December 2021
Accepted : 23 February 2022
Published : 14 March 2022
Issue Date : June 2022
DOI : https://doi.org/10.1007/s40120-022-00338-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alzheimer’s Disease
- Clinical Progression
- Epidemiology
- Mild Cognitive Impairment
- Preclinical Alzheimer’s Disease
- Find a journal
- Publish with us
- Track your research
2021 Alzheimer's disease facts and figures
- PMID: 33756057
- DOI: 10.1002/alz.12328
This article describes the public health impact of Alzheimer's disease (AD), including incidence and prevalence, mortality and morbidity, use and costs of care, and the overall impact on caregivers and society. The Special Report discusses the challenges of providing equitable health care for people with dementia in the United States. An estimated 6.2 million Americans age 65 and older are living with Alzheimer's dementia today. This number could grow to 13.8 million by 2060 barring the development of medical breakthroughs to prevent, slow or cure AD. Official death certificates recorded 121,499 deaths from AD in 2019, the latest year for which data are available, making Alzheimer's the sixth-leading cause of death in the United States and the fifth-leading cause of death among Americans age 65 and older. Between 2000 and 2019, deaths from stroke, heart disease and HIV decreased, whereas reported deaths from AD increased more than 145%. This trajectory of deaths from AD was likely exacerbated in 2020 by the COVID-19 pandemic. More than 11 million family members and other unpaid caregivers provided an estimated 15.3 billion hours of care to people with Alzheimer's or other dementias in 2020. These figures reflect a decline in the number of caregivers compared with a decade earlier, as well as an increase in the amount of care provided by each remaining caregiver. Unpaid dementia caregiving was valued at $256.7 billion in 2020. Its costs, however, extend to family caregivers' increased risk for emotional distress and negative mental and physical health outcomes - costs that have been aggravated by COVID-19. Average per-person Medicare payments for services to beneficiaries age 65 and older with AD or other dementias are more than three times as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 23 times as great. Total payments in 2021 for health care, long-term care and hospice services for people age 65 and older with dementia are estimated to be $355 billion. Despite years of efforts to make health care more equitable in the United States, racial and ethnic disparities remain - both in terms of health disparities, which involve differences in the burden of illness, and health care disparities, which involve differences in the ability to use health care services. Blacks, Hispanics, Asian Americans and Native Americans continue to have a higher burden of illness and lower access to health care compared with Whites. Such disparities, which have become more apparent during COVID-19, extend to dementia care. Surveys commissioned by the Alzheimer's Association recently shed new light on the role of discrimination in dementia care, the varying levels of trust between racial and ethnic groups in medical research, and the differences between groups in their levels of concern about and awareness of Alzheimer's disease. These findings emphasize the need to increase racial and ethnic diversity in both the dementia care workforce and in Alzheimer's clinical trials.
Keywords: Alzheimer's dementia; Alzheimer's disease; Biomarkers; COVID-19; Caregivers; Dementia; Diagnostic criteria; Discrimination; Diversity; Equity; Ethnicity; Family caregiver; Health care costs; Health care disparities; Health care expenditures; Health care professional; Health disparities; Implicit bias; Incidence; Long-term care costs; Medicaid spending; Medicare spending; Morbidity; Mortality; Prevalence; Race; Risk factors; Spouse caregiver.
© 2021 the Alzheimer's Association.
- Aged, 80 and over
- Alzheimer Disease / economics
- Alzheimer Disease / epidemiology*
- Alzheimer Disease / mortality
- Alzheimer Disease / therapy
- COVID-19 / epidemiology
- COVID-19 / mortality
- Cause of Death
- Comorbidity
- Cost of Illness
- Ethnicity / statistics & numerical data
- Health Care Costs / statistics & numerical data
- Health Services Needs and Demand / statistics & numerical data
- Healthcare Disparities / statistics & numerical data
- Public Health / statistics & numerical data*
- Risk Factors
- Sex Factors
- Survival Analysis
- United States
Alzheimer's Research & Therapy
Announcing the launch of mini reviews.

Concise overview articles of key topics in neurodegeneration, which can be read wherever you are, whenever suits you.
Find out more here .
New Thematic Series - AI in Dementia

Alzheimer's Research & Therapy presents a thematic series focusing on the use of artificial intelligence, machine learning and related techniques in dementia research.
Find out more about the series here .
- Most accessed
White matter hyperintensity patterns: associations with comorbidities, amyloid, and cognition
Authors: Dario Bachmann, Bettina von Rickenbach, Andreas Buchmann, Martin Hüllner, Isabelle Zuber, Sandro Studer, Antje Saake, Katrin Rauen, Esmeralda Gruber, Roger M. Nitsch, Christoph Hock, Valerie Treyer and Anton Gietl
A systematic review of progranulin concentrations in biofluids in over 7,000 people—assessing the pathogenicity of GRN mutations and other influencing factors
Authors: Imogen J. Swift, Rosa Rademakers, NiCole Finch, Matt Baker, Roberta Ghidoni, Luisa Benussi, Giuliano Binetti, Giacomina Rossi, Matthis Synofzik, Carlo Wilke, David Mengel, Caroline Graff, Leonel T. Takada, Raquel Sánchez-Valle, Anna Antonell, Daniela Galimberti…
ANU-ADRI scores, tau pathology, and cognition in non-demented adults: the CABLE study
Authors: Shan Yin, Pei-Yang Gao, Ya-Nan Ou, Yan Fu, Ying Liu, Zuo-Teng Wang, Bao-Lin Han and Lan Tan
Potential ocular indicators to distinguish posterior cortical atrophy and typical Alzheimer’s disease: a cross-section study using optical coherence tomography angiography
Authors: Yan Sun, Lumi Zhang, Hui Ye, Lumin Leng, Yi Chen, Yujie Su, Peifang Ren, Hong Lu and Guoping Peng
Navigating the metabolic maze: anomalies in fatty acid and cholesterol processes in Alzheimer’s astrocytes
Authors: Xiaoyu Zhang, Chuanying Chen and Yi Liu
Most recent articles RSS
View all articles
Stem cell factor and granulocyte colony-stimulating factor reduce β-amyloid deposits in the brains of APP/PS1 transgenic mice
Authors: Bin Li, Maria E Gonzalez-Toledo, Chun-Shu Piao, Allen Gu, Roger E Kelley and Li-Ru Zhao
Cerebral microbleeds: overview and implications in cognitive impairment
Authors: Sergi Martinez-Ramirez, Steven M Greenberg and Anand Viswanathan
BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease
Authors: Robert Vassar
A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody
Authors: Chad J. Swanson, Yong Zhang, Shobha Dhadda, Jinping Wang, June Kaplow, Robert Y. K. Lai, Lars Lannfelt, Heather Bradley, Martin Rabe, Akihiko Koyama, Larisa Reyderman, Donald A. Berry, Scott Berry, Robert Gordon, Lynn D. Kramer and Jeffrey L. Cummings
The Correction to this article has been published in Alzheimer's Research & Therapy 2022 14 :70
Alzheimer’s disease drug-development pipeline: few candidates, frequent failures
Authors: Jeffrey L Cummings, Travis Morstorf and Kate Zhong
Most accessed articles RSS
Early Career Researcher Reviewer Panel

Interested in an introduction to peer reviewing articles for Alzheimer's Research & Therapy?
Find out more about our ECR Peer Reviewer Panel!
Aims and scope
Alzheimer's Research & Therapy is the major forum for translational research into Alzheimer's disease. An international peer-reviewed journal, it publishes open access basic research with a translational focus, as well as clinical trials, research into drug discovery and development, and epidemiologic studies. The journal also provides reviews, viewpoints, commentaries, debates and reports.
Although the primary focus is Alzheimer's disease, the scope encompasses translational research into other neurodegenerative diseases.
Published Thematic Series

10 Years of Alzheimer's Research & Therapy
Subject Cognitive Decline
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Alzheimer's blogs from BMC
Failed to load RSS feed.
- Editorial Board
- Instructions for Editors
- Sign up for article alerts and news from this journal
Affiliated with

Annual Journal Metrics
2022 Citation Impact 9.0 - 2-year Impact Factor 9.2 - 5-year Impact Factor 1.849 - SNIP (Source Normalized Impact per Paper) 2.650 - SJR (SCImago Journal Rank)
2023 Speed 15 days submission to first editorial decision for all manuscripts (Median) 152 days submission to accept (Median)
2023 Usage 1,914,774 downloads 2,982 Altmetric mentions
- More about our metrics
ISSN: 1758-9193
- General enquiries: [email protected]
Journal Articles by Date
Professional and scientific articles from the Alzheimer’s Disease Program authors whose names are in bold face . Bibliographies are listed by year.
Suchsland, M.Z., Gaster, B., Raetz, J., Belza, B., McGuire, L. , Olivari, B. , Tracy, K., & Fitzpatrick, A.L. Developing a cognitive assessment toolkit for primary care: qualitative assessment of providers’ needs and perceptions of usability in clinical practice. BMC Health Serv Res 23, 1006 (2023). https://doi.org/10.1186/s12913-023-09991-7
Eustaquio PC, Salmon-Trejo LA, McGuire LC , Ellington SR. Epidemiologic and Clinical Features of Mpox in Adults Aged >50 Years — United States, May 2022–May 2023. MMWR Morb Mortal Wkly Rep 2023; 72:893–896. DOI: http://dx.doi.org/10.15585/mmwr.mm7233a3 .
Jackson EMJ , O’Brien K, McGuire LC , Baumgart M, Gore J , Brandt K, Levey AI, Lamont H. Promoting Healthy Aging: Public Health as a Leader for Reducing Dementia Risk, Public Policy & Aging Report , 2023;, prad011, https://doi.org/10.1093/ppar/prad011
Gore J, Denno B, Omura JD, Baumgart M, McGuire LC, O’Brien K. Promoting Healthy Aging to Reduce the Risk of Dementia: A Public Health Imperative. Generations Journal, Vol. 47, No. 1 (Spring 2023). DOI: https://generations.asaging.org/promoting-healthy-aging-reduce-risk-dementia
Miyawaki, C. E., Bouldin, E. D., Taylor, C. A., McGuire, L. C. , & Markides, K. S. (2023). Characteristics of Asian American Family Caregivers of Older Adults Compared to Caregivers of Other Racial/Ethnic Groups: Behavioral Risk Factor Surveillance System 2015–2020. Journal of Applied Gerontology, 42(5), 1101–1107. https://doi.org/10.1177/07334648221146257
Wooten KG, McGuire LC, Olivari BS, Jackson EM, Croft JB . Racial and Ethnic Differences in Subjective Cognitive Decline — United States, 2015–2020. MMWR Morb Mortal Wkly Rep 2023; 72: 250-255. DOI: https://www.cdc.gov/mmwr/volumes/72/wr/mm7210a1.htm
Jackson EM , Omura JD , Boring MA, Odom EL, Foster AL, Olivari BS , McGuire LC , Croft JB . Prevalence and Characteristics of Arthritis Among Caregivers — 17 States, 2017 and 2019. MMWR Morb Mortal Wkly Rep 2022;71:1389–1395. DOI: http://dx.doi.org/10.15585/mmwr.mm7144a1 .
Omura JD, McGuire LC, Patel R , Baumgart M, Lamb, R, Jeffers EM, Olivari BS, Croft JB, Thomas CW, Hacker K . Modifiable Risk Factors for Alzheimer Disease and Related Dementias Among Adults Aged ≥45 Years — United States, 2019. MMWR Morb Mortal Wkly Rep 2022;71:680–685. DOI: http://dx.doi.org/10.15585/mmwr.mm7120a2external icon .
Downing KF, Oster ME, Olivari BS , Farr SL. Early-onset dementia among privately-insured adults with and without congenital heart defects in the United States, 2015-2017. Int J Cardiol. 2022 Jul 1;358:34-38. doi: 10.1016/j.ijcard.2022.04.019. Epub 2022 Apr 11. PMID: 35417738. https://pubmed.ncbi.nlm.nih.gov/35417738/
Samson ME , Yeung LF, Rose CE, Qi YP, Taylor CA , Crider KS, Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011–2014, The American Journal of Clinical Nutrition , Volume 116, Issue 1, July 2022, Pages 74–85, https://doi.org/10.1093/ajcn/nqac065
Olivari BS, Jeffers EM, Tang KW, McGuire LC . Improving Brain Health for Populations Disproportionately Affected by Alzheimer’s Disease and Related Dementias. Clin Gerontol. 2022;1-5. doi: 10.1080/07317115.2022.2043977
Jeffers EM , Bouldin ED, McGuire LC , Knapp KA, Patel R, Guglielmo D, Taylor CA, Croft JB . Prevalence and Characteristics of Subjective Cognitive Decline Among Unpaid Caregivers Aged ≥45 Years – 22 States, 2015-2019. MMWR Morb Mortal Wkly Rep. 2021 Nov 19;70(46):1591-1596. doi: 10.15585/mmwr.mm7046a1.PMID: 34793418
Miyawaki CE, Bouldin ED, Taylor CA, McGuire LC. Baby Boomers Who Provide Informal Care for People Living with Dementia in the Community. Int J Environ Res Public Health. 2021 Sep 15;18(18):9694. doi: 10.3390/ijerph18189694.PMID: 34574619
Yang Q, Tong X, Coleman King S, Olivari BS , Merritt RK. Stroke Hospitalizations Before and During COVID-19 Pandemic Among Medicare Beneficiaries in the United States. Stroke. 2021 Jul 29:STROKEAHA121034562. doi: 10.1161/STROKEAHA.121.034562. Online ahead of print. PMID: 34320816
Havers FP, Whitaker M, Self JL, … Taylor CA ; COVID-NET Surveillance Team. Hospitalization of Adolescents Aged 12-17 Years with Laboratory-Confirmed COVID-19 – COVID-NET, 14 States, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021 Jun 11;70(23):851-857. doi: 10.15585/mmwr.mm7023e1. PMID: 34111061
Flatt JD, Cicero EC, Lambrou NH, Wharton W, Anderson JG, Bouldin ED, McGuire LC, Taylor CA . Subjective cognitive decline higher among sexual and gender minorities in the United States, 2015—2018. https://doi.org/10.1002/trc2.12197
Barrett J, Olivari BS , Price A, Taylor CA . Cognitive Decline and Dementia Risk Reduction: Promoting Healthy Lifestyles and Blood Pressure Control. 2021. Am J Prev Med 2021;1−4. https://doi.org/10.1016/j.amepre.2021.03.005
Olivari BS, Baumgart M , Taylor CA, McGuire, LC . Population measures of subjective cognitive decline: A means of advancing public health policy to address cognitive health. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2021; 7:e12142. https://doi.org/10.1002/trc2.12142
Bouldin ED, Taylor CA, Knapp KA, Miyawaki CE, Mercado NR, Wooten KG, McGuire LC. Unmet needs for assistance related to subjective cognitive decline among community-dwelling middle-aged and older adults in the US: prevalence and impact on health-related quality of life. Int Psychogeriatr. 2021 Jul;33(7):689-702. doi: 10.1017/S1041610220001635. Epub 2020 Sep 4.PMID: 32883384
Matthews KA, Gaglioti AH, Holt JB, McGuire LC , Greenlund KJ. County-Level Concentration of Selected Chronic Conditions Among Medicare Fee-for-Service Beneficiaries and Its Association with Medicare Spending in the United States, 2017. Popul Health Manag. 2021 Apr;24(2):214-221. doi: 10.1089/pop.2019.0231. Epub 2020 Apr 1.PMID: 32233970
Quinn K, Miyawaki CE, Croff R, Vogel MT, Belza B, Souza AM, Liu M, Edwards VJ , Friedman DB. Terms and Measures of Cognitive Health Associated With Dementia and Alzheimer’s Disease: A Scoping Review. Res Aging. 2020 Jun-Jul;42(5-6):174-185. doi: 10.1177/0164027520911284.
Omura JD, Brown DR, McGuire LC, Taylor CA , Fulton JE, Carlson SA. Cross-sectional association between physical activity level and subjective cognitive decline among US adults aged ≥45 years, 2015. Prev Med. 2020 Oct 6;141:106279. doi: 10.1016/j.ypmed.2020.106279 .
Bouldin ED , Taylor CA, Knapp KA, Miyawaki CE, Mercado NR, Wooten KG , McGuire LC . Unmet needs for assistance related to subjective cognitive decline among community-dwelling middle-aged and older adults in the US: prevalence and impact on health-related quality of life. Int Psychogeriatr . 2020;1-14. doi:10.1017/S1041610220001635
Miyawaki CD, Bouldin ED , Taylor CA, McGuire LC. Baby Boomers as Caregivers: Results From the Behavioral Risk Factor Surveillance System in 44 States, the District of Columbia, and Puerto Rico, 2015–2017. Prev Chronic Dis 2020;17:200010.
Edwards VJ, Bouldin ED , Taylor CA , Olivari BS , McGuire LC . Characteristics and Health Status of Informal Unpaid Caregivers—44 States, District of Columbia, and Puerto Rico, 2015–2017. MMWR Morb Mortal Wkly Rep 2020;69:183-188. DOI: http://dx.doi.org/10.15585/mmwr.mm6907a2
Taylor, C.A ., Bouldin, E.D. , Greenlund, K.J., McGuire, L.C . Comorbid Chronic Conditions Among Older Adults with Subjective Cognitive Decline, United States, 2015–2017. Innovation in Aging, Volume 4(1).
Olivari BS , French ME, McGuire LC. The Public Health Road Map to Respond to the Growing Dementia Crisis. Innovation in Aging, Volume 4(1) .
Brody DJ, Kramarow EA, Taylor CA , McGuire LC (2019). Cognitive Performance in Adults Aged 60 and Over: National Health and Nutrition Examination Survey, 2011–2014. National Health Statistics Reports ; no. 126. Hyattsville, MD: National Center for Health Statistics. https://www.cdc.gov/nchs/data/nhsr/nhsr126-508.pdf [PDF 659 KB]
Saydah S, Gerzoff RB, Taylor CA , Ehrlich JR, Saaddine J (2019). Vision Impairment and Subjective Cognitive Decline–Related Functional Limitations — United States, 2015–2017. MMWR Morb Mortal Wkly Rep , 68(20): 453–457. https://www.cdc.gov/mmwr/volumes/68/wr/mm6820a2.htm?s_cid=mm6820a2_w
Peterson RL, Carvajal SC, McGuire LC , Fain MJ, Bell ML (2019). State Inequality, Socioeconomic Position, and Subjective Cognitive Decline in the United States. SSM Popul Health , 7: 100357. https://www.ncbi.nlm.nih.gov/pubmed/30886886
Matthews KA, Wei X, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019). Racial and Ethnic Estimates of Alzheimer’s Disease and Related Dementias in the United States (2015 – 2060) in Adults Aged ≥65 Years. Alzheimer’s & Dementia , 15(1): 17–24. https://www.ncbi.nlm.nih.gov/pubmed/30243772
Bouldin, E. D. , Shaull, L., Andresen, E. M., Edwards, V. J. , & McGuire, L. C. (2018). Financial and Health Barriers and Caregiving-Related Difficulties Among Rural and Urban Caregivers. The Journal of Rural Health , 34(3), 263-274. http://dx.doi.org/10.1111/jrh.12273
Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C . (2018). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia . https://doi.org/10.1016/j.jalz.2018.06.3063 external icon
Kelley, M., Ulin, B., & McGuire, L. C. (2018). Reducing the Risk of Alzheimer’s Disease and Maintaining Brain Health in an Aging Society. Public Health Reports , 133(3), 225-229. http://dx.doi.org/10.1177/0033354918763599
Lee-Kwan, S. H., Park, S., Maynard, L. M., Blanck, H. M., McGuire, L. C. , & Collins, J. L. (2018). Parental Characteristics and Reasons Associated With Purchasing Kids’ Meals for Their Children. American Journal of Health Promotion , 32(2), 264-270. http://dx.doi.org/10.1177/0890117116683797
Olivari, B. S. , Baumgart, M., Lock, S. L., Whiting, C. G., Taylor, C. A. , Iskander, J., . . . McGuire, L. C. (2018). CDC Grand Rounds: Promoting Well-Being and Independence in Older Adults. MMWR. Morbidity and Mortality Weekly Report , 67(37), 1036-1039. http://dx.doi.org/10.15585/mmwr.mm6737a4
Rabarison, K. M., Bouldin, E. D. , Bish, C. L., McGuire, L. C. , Taylor, C. A. , & Greenlund, K. J. (2018). The Economic Value of Informal Caregiving for Persons With Dementia: Results From 38 States, the District of Columbia, and Puerto Rico, 2015 and 2016 BRFSS. American Journal of Public Health , 108(10), 1370-1377. http://dx.doi.org/10.2105/ajph.2018.304573
Taylor, C. A. , Bouldin, E. D. , & McGuire, L. C. (2018). Subjective Cognitive Decline Among Adults Aged ≥45 Years — United States, 2015–2016. MMWR. Morbidity and Mortality Weekly Report , 67(27), 753-757. http://dx.doi.org/10.15585/mmwr.mm6727a1
Bouldin, E. , Trivedi, R., Reiber, G., Rosland, A., Silverman, J., Krieger, J., & Nelson, K. (2017). Associations between having an informal caregiver, social support, and self-care among low-income adults with poorly controlled diabetes. Chronic Illness , 13(4), 239-250. http://dx.doi.org/10.1177/1742395317690032
Bouldin, E. , Wong, E., Liu, C., Littman, A., Taylor, L., Rice, K., & Reiber, G. (2017). Chronic wound care utilization among Veterans using VHA and Medicare. Wound Medicine , 17, 1-6. http://dx.doi.org/10.1016/j.wndm.2017.01.003
Bouldin ED , Shaull L, Andresen EM, Edwards VJ , McGuire LC . Financial and Health Barriers and Caregiving-Related Difficulties Among Rural and Urban Caregivers. J Rural Health . 2017 Sep 23. http://dx.doi.org/10.1111/jrh.12273 .
Cancelliere C, Coronado VG, Taylor CA , Xu L (2017). Epidemiology of Isolated vs. Non-Isolated Mild Traumatic Brain Injury Treated in Emergency Departments in the United States, 2006–2012: Sociodemographic Characteristics. J Head Trauma Rehabil , 32(4):E37–E46 [ http://dx.doi.org/10.1097/HTR.0000000000000260 ].
Edwards VJ , Anderson LA , Thompson WW, Deokar AJ . Mental health differences between men and women caregivers, BRFSS 2009. J Women Aging . 2017;29(5):385-391. http://dx.doi.org/10.1080/08952841.2016.1223916 .
Eisenberg, Y., Bouldin, E . D., Gell, N., & Rosenberg, D. (2017). Planning Walking Environments for People with Disabilities and Older Adults. In Walking: Connecting Sustainable Transport with Health (pp. 187-209). Emerald Publishing Limited.
Haarbauer-Krupa J, Taylor CA , Yue JK, Winkler EA, Pirracchio R, Cooper SR, Burke JF, Stein MB, Manley GT, The TRACK-TBI Investigators (2017). Screening for Post-Traumatic Stress Disorder in a Civilian Emergency Department Population with Traumatic Brain Injury. J Neurotrauma , 34(1): 50–58 [ http://dx.doi.org/10.1089/neu.2015.4158 ].
Littman AJ, Bouldin ED , Haselkorn JK. This is your new normal: A qualitative study of barriers and facilitators to physical activity in Veterans with lower extremity loss. Disabil Health J . 2017 Oct;10(4):600-606. http://dx.doi.org/10.1016/j.dhjo.2017.03.004 .
Lock, S. L., Baumgart, M., Whiting, C. G., McGuire, L.C., Iskander, J. K., Thorpe, P., & Laird, S. (2017). Healthy aging: promoting well-being in older adults. CDC Grand Rounds.
Miyawaki CE, Bouldin ED , Kumar GS, McGuire LC . Associations between Physical Activity and Cognitive Functioning among Middle-Aged and Older Adults. J Nutr Health Aging . 2017;21(6):637-647. http://dx.doi.org/10.1007/s12603-016-0835-6 .
Snowden MB, Steinman LE, Bryant LL, Cherrier MM, Greenlund KJ, Leith KH, Levy C, Logsdon RG, Copeland C, Vogel M, Anderson LA , Atkins DC, Bell JF, Fitzpatrick AL. Dementia and co-occurring chronic conditions: a systematic literature review to identify what is known and where are the gaps in the evidence? Int J Geriatr Psychiatry . 2017 Apr;32(4):357-371. http://dx.doi.org/10.1002/gps.4652 .
Tang W, Kannaley K, Friedman DB, Edwards VJ , Wilcox S, Levkoff SE, Hunter RH, Irmiter C, Belza B. Concern about developing Alzheimer’s disease or dementia and intention to be screened: An analysis of national survey data. Arch Gerontol Geriatr . 2017 Jul;71:43-49. http://dx.doi.org/10.1016/j.archger.2017.02.013 .
Taylor CA , Greenlund SF, McGuire LC , Lu H, Croft JB. Deaths from Alzheimer’s Disease – United States, 1999-2014. MMWR Morb Mortal Wkly Rep . 2017 May 26;66(20):521-526. http://dx.doi.org/10.15585/mmwr.mm6620a1 .
Taylor CA , Bell JM, Breiding MB, Xu L (2017). Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ , 66(No. SS-9): 1–16 [ http://dx.doi.org/10.15585/mmwr.ss6609a1 ].
Wiltz, J. L., Blanck, H. M., Lee, B., Kocot, S. L., Seeff, L., McGuire, L. C ., & Collins, J. (2017). Electronic Information Standards to Support Obesity Prevention and Bridge Services Across Systems, 2010–2015. Preventing Chronic Disease , 14, E103. http://doi.org/10.5888/pcd14.160299
Alzheimer’s Disease and Healthy Aging Program authors are in bold face .
Matthews, K. A., Holt, J., Gaglioti, A. H., Lochner, K. A., Shoff, C., McGuire, L. C. , & Greenlund, K. J. (2016). Peer Reviewed: County-Level Variation in Per Capita Spending for Multiple Chronic Conditions Among Fee-for-Service Medicare Beneficiaries, United States, 2014. Preventing Chronic Disease , 13 . DOI: 10.5888/pcd13.160240.
Eke, P. I., Wei, L., Borgnakke, W. S., Thornton-Evans, G., Zhang, X., Lu, H., McGuire, L. C. and Genco, R. J. (2016). Periodontitis prevalence in adults≥ 65 years of age, in the USA. Periodontology 2000 , 72 (1), 76-95. DOI: 10.1111/prd.12145.
VanFrank B.K., Park S., Foltz J.L., McGuire L.C. , Harris D.M. (2016). Physician Characteristics Associated With Sugar-Sweetened Beverage Counseling Practices. American Journal of Health Promotion , 0890117116680472. DOI: 10.1177/0890117116680472.
Park S., Akinbami L.J., McGuire L.C. , Blanck H.M. Association of sugar-sweetened beverage intake frequency and asthma among US adults, 2013. Preventive medicine , 91 , 58-61. DOI: 10.1016/j.ypmed.2016.08.004.
Park S., Thompson F.E., McGuire L.C. , Pan L., Galuska D.A., Blanck H.M. (2016). Sociodemographic and Behavioral Factors Associated with Added Sugars Intake among US Adults. Journal of the Academy of Nutrition and Dietetics, 116 (10), 1589-98. DOI: 10.1016/j.jand.2016.04.012.
Freedman D.S., Lawman H.G., Pan L., Skinner A.C., Allison D.B., McGuire L.C. , Blanck H.M. (2016). The prevalence and validity of high, biologically implausible values of weight, height, and BMI among 8.8 million children. Obesity , 24 (5), 1132-1139. DOI: 10.1002/oby.21446.
Lee-Kwan S.H., Pan L., Maynard L.M., McGuire L.C. , Park S. (2016). Factors Associated with Self-Reported Menu-Labeling Usage among US Adults. Journal of the Academy of Nutrition and Dietetics, 116 (7), 1127-35. DOI: 10.1016/j.jand.2015.12.015.
VanFrank, B. K., Park, S., Foltz, J., McGuire, L. C., & Harris, D. M. (2016). Physician Counseling on Sugar-Sweetened Beverages: Areas for Improvement. Pediatrics , 137 (Supplement 3), 121A-121A. DOI: 10.1542/peds.137.Supplement_3.121A.
Lee-Kwan, S. H., Park, S., Maynard, L. M., Blanck, H. M., McGuire, L. C. , & Collins, J. L. (2016). Parental Characteristics and Reasons Associated With Purchasing Kids’ Meals for Their Children. American Journal of Health Promotion , 0890117116683797. DOI: 10.1177/0890117116683797.
Jewett, A., Beck, L. F., Taylor, C ., & Baldwin, G. (2016). Bicycle helmet use among persons 5years and older in the United States, 2012. Journal of safety research , 59 , 1-7. DOI: 10.1016/j.jsr.2016.09.001.
Haarbauer-Krupa, J., Taylor, C. A ., Yue, J. K., Winkler, E. A., Pirracchio, R., Cooper, S. R., … & Manley, G. T. (2016). Screening for post-traumatic stress disorder in a civilian emergency department population with traumatic brain injury. Journal of neurotrauma , (ja). DOI: 10.1089/neu.2015.4158.
Edwards, V. J. , Anderson, L. A. , Thompson, W. W., & Deokar, A. J. (2016). Mental health differences between men and women caregivers, BRFSS 2009. Journal of Women & Aging , 1-7. DOI: 10.1080/08952841.2016.1223916.
Nicklett, E. J., Anderson, L. A., & Yen, I. H. (2016). Gardening Activities and Physical Health Among Older Adults A Review of the Evidence. Journal of Applied Gerontology , 35 (6), 678-690. DOI: 10.1177/0733464814563608.
Vandenberg, A. E., Hunter, R. H., Anderson, L. A., Bryant, L. L., Hooker, S. P., & Satariano, W. A. (2016). Walking and Walkability: Is Wayfinding a Missing Link? Implications for Public Health Practice. Journal of physical activity & health , 13 (2). DOI: 10.1123/jpah.2014-0577.
Anderson, L. A., & Slonim, A. (2016). Perspectives on the strategic uses of concept mapping to address public health challenges. Evaluation and Program Planning, 60, 194-201. DOI: 10.1016/j.evalprogplan.2016.08.011
Hunter, R. H., Anderson, L. A., & Belza, B. L. (Eds.). (2016). Community Wayfinding: Pathways to Understanding . Springer. ISBN 978-3-319-31072-5.
Greenlund, K. J., Liu, Y., Deokar, A. J., Wheaton, A. G., & Croft, J. B. (2016). Association of Chronic Obstructive Pulmonary Disease With Increased Confusion or Memory Loss and Functional Limitations Among Adults in 21 States, 2011 Behavioral Risk Factor Surveillance System. Preventing chronic disease , 13 . DOI: 10.5888/pcd13.150428.
Faul, M., Stevens, J. A., Sasser, S. M., Alee, L., Deokar, A. J., Kuhls, D. A., & Burke, P. A. (2016). Older adult falls seen by emergency medical service providers: a prevention opportunity. American journal of preventive medicine , 50 (6), 719-726. DOI: 10.1016/j.amepre.2015.12.011.
Bouldin, E. D., Thompson, M. L., Boyko, E. J., Morgenroth, D. C., & Littman, A. J. (2016). Weight change trajectories after incident lower-limb amputation. Archives of physical medicine and rehabilitation , 97 (1), 1-7. DOI: 10.1016/j.apmr.2015.09.017
Bouldin, E. D., Littman, A. J., Wong, E., Liu, C. F., Taylor, L., Rice, K., & Reiber, G. E. (2016). Medicare‐VHA dual use is associated with poorer chronic wound healing. Wound Repair and Regeneration , 24 (5), 913-922. DOI: 10.1111/wrr.12454
Cannell, M. B., Bouldin, E. D., Teigen, K., Akhtar, W. Z., & Andresen, E. M. (2016). The cross-sectional association between severity of non-cognitive disability and self-reported worsening memory. Disability and health journal , 9 (2), 289-297. DOI: 10.1016/j.dhjo.2015.09.001
CDC Healthy Aging Program authors are in bold face .
Anderson, L. (In Press). Population Aging. In Oxford Bibliographies in Public Health. Ed. David McQueen. New York: Oxford University Press.
Anderson, L.A. , Prohaska, T.R., & Satariano, W.A. (In Press). Prevention. Whitbourne, S.K. (ed.), The Encyclopedia of Adulthood and Aging. Hoboken, NJ: John Wiley & Sons, Inc.
Anderson, L.A., Deokar, A., Edwards, V.J. , Bouldin. E.D., & Greenlund, K.J. (2015). Demographic and Health Status Differences Among People Aged 45 or Older With and Without Functional Difficulties Related to Increased Confusion or Memory Loss, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140429. DOI: http://dx.doi.org/10.5888/pcd12.140429 .
Brady, T.J., Anderson, L.A. , & Kobau, R. (2015). Chronic Disease Self-Management Support: Public Health Perspectives. Frontiers in Public Health , http://dx.doi.org/10.3389/fpubh.2014.00234 .
Deokar, A.J. , Bouldin, E.D., Edwards, V.J., & Anderson, L.A. (2015). Increased Confusion and Memory Loss in Households, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140430. DOI: http://dx.doi.org/10.5888/pcd12.140430 .
Edwards, V.J., Anderson, L.A., & Deokar, A.J. (2015). Proxy Reports About Household Members With Increased Confusion or Memory Loss, 2011 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease , 12:140427. DOI: http://dx.doi.org/10.5888/pcd12.140427 .
Friedman, D.B., Becofsky, K., Anderson, L.A. , et al. (2015). Public perceptions about risk and protective factors for cognitive health and impairment: A review of the literature. International Psychogeriatrics . Jan 16:1-13. doi:10.1017/S1041610214002877. (attached PDF for personal reading)
Phelan, E., Debman, K., Anderson, L.A. , & Owen, S. (2015). A Systematic Review of Intervention Studies to Prevent Hospitalizations of Community-dwelling Older Adults with Dementia. Medical Care . 53(2), 207–213. doi: 10.1097/MLR.0000000000000294
Petrescu-Prahova, M., Belza, B., Leith, K., Allen, P., Coe, N., & Anderson, L. (In Press). Using Social Network Analysis to Assess Mentorship and Collaboration in a Public Health Network. Preventing Chronic Disease .
Shenson, D., Moore, R.T., Benson, W., & Anderson, L.A. (2015). Polling Places, Pharmacies, and Public Health: Vote & Vax 2012. American Journal of Public Health . doi:10.2105/AJPH.2015.302628
Snowden, M., Steinman, L., Carlson, W.L., Mochan, K.N., Abraido-Lanza, A.F., Bryant , L. … & Anderson, L.A. (2015). Effect of Physical Activity, Social Support and Skills Training on Late-Life Emotional Health: A Systematic Literature Review and Implications for Public Health Research” Frontiers in Public Health .
Anderson, L.A. , & Egge, R. (2014). Expanding efforts to address Alzheimer’s disease: The Healthy Brain Initiative. Alzheimer’s & Dementia . 10:S453–S456. [doi:10.1016/j.jalz.2014.05.1748]
Anderson, L.A. , & Prohaska, T.R. (2014). Fostering Engagement and Independence: Opportunities and Challenges for an Aging Society, Health Education & Behavior , 41:5S-9S. [doi:10.1177/1090198114547818]
Anderson, L.A. , Slonim, A., Yen, I.H., Jones, D.L., Allen, P., Hunter, R.H., Goins, R.T., Leith, K.H., Rosenberg, D., Satariano, W.A., & McPhillips-Tangum, C. (2014). Developing a Framework and Priorities to Promote Mobility Among Older Adults. Health Education & Behavior . 41:10S-18S. [doi:10.1177/1090198114537492]
Barbour, K.E., Stevens, J.A., Helmick, C.G., Lou, Y-H, Murphy, L.B., Hootman, .J.M., Theis K., Anderson, L.A. , Baker, N.A., & Sugerman, D.E. (2014). Falls and Fall Injuries Among Adults with Arthritis — United States, 2012. MMWR . 63 (17):379–383.
Barile, J.P., Edwards, V.J. , Dhingra, S.S., & Thompson, W.W. (2014). Associations Among County-Level Social Determinants of Health, Child Maltreatment, and Emotional Support on Health-Related Quality of Life in Adulthood. Psychology of Violence . doi:10.1037/a0038202
Liu, Y., Croft, J.B., Anderson, L.A. , Wheaton, A.G., Presley-Cantrell, L.R., & Ford, E.S. (2014). The Association of Chronic Obstructive Pulmonary Disease, Disability, Engagement in Social Activities, and Mortality Among Older Adults: United States, 1994-2006. International Journal of Chronic Obstructive Pulmonary Disease . 9:75 – 83. [doi:dx.doi.org/10.2147/COPD.S53676]
Nicklett, E., Anderson, L.A. , & Yen, I. (2014). Gardening Activities and Physical Health among Older Adults: A Review of the Evidence. Journal of Applied Gerontology .DOI: 10.1177/0733464814563608
Ory, M.G., Anderson, L.A. , Friedman, D.B., Pulczinskia, J.C., Eugene, N., & Satariano, W.A., (2014). Setting the Stage: Cancer Prevention among Adults Aged 45 to 64. American Journal of Preventive Medicine . 46(3), Supplement 1: S1–S6.
Rao, J., Anderson, L.A. , Lin, F.C., & Laux, J.P. (2014). Completion of Advance Directives Among U.S. Consumers, American Journal of Preventive Medicine . 46(1):65–70.
Yen, I.H., Flood, J.F., Thompson, H., Anderson, L.A. , & Wong, G. (2014). How Design of Places Promotes or Inhibits Mobility of Older Adults: Realist Synthesis of 20 Years of Research. Journal of Aging and Health . Published online 30 April 2014 [doi:10.1177/0898264314527610]
Krist, A.H., Shenson, D., Woolf, S.H., Bradley, C., Liaw, W., Rothemich, S., Slonim, A., Benson, W. & Anderson, L.A. (2013). Clinical and Community Delivery Systems for Preventive Care: An Integration Framework. American Journal of Preventive Medicine . 45(4):508–516.
Liu Y, Croft JB, Chapman DP, Perry GS, Greenlund KJ, Zhao G, Edwards VJ . (2013). Relationship between adverse childhood experiences and unemployment among adults from five US states. Social Psychiatry and Psychiatric Epidemiology , 48, 357-369. doi:10.1007/s00127-012-0554-1.
Slonim, A., Benson, W., Anderson, L.A. , Jones, E. (2013). Strategic Priorities to Increase Use of Clinical Preventive Services Among Older US Adults. Preventing Chronic Disease . 10:120231. [doi:http://dx.doi.org/10.5888/pcd10.120231]
Spencer, L.M., Schooley, M.W., Anderson, L.A. , Kochtitzky, C.S., DeGroff, A..S, Devlin, H.M., & Mercer, S.L. (2013) Seeking Best Practices: A Conceptual Framework for Planning and Improving Evidence-Based Practices. Preventing Chronic Disease . 10:130186. [doi: http://dx.doi.org/10.5888/pcd10.130186]
Wilcox, S., Altpeter, M., Anderson, L.A. , Belza, B., Bryant, L., Jones, D.L., Leith, K.H., Phelan, E.A., & Satariano, W.A. (2013). The Healthy Aging Research Network: Resources for Building Capacity for Public Health and Aging Practice. American Journal of Health Promotion. 28(1):2-6. [doi: 10.4278/ajhp.121116-CIT-564]
Anderson, L.A. , Goodman, R., Holtzman R, Posner, S, & Northridge, M. (2012). Aging in the United States: Opportunities and Challenges for Public Health. American Journal of Public Health , 102(3):393–5.
Furner, S.E., & Anderson, L.A. (2012). Assessing the Health and Quality of Life of Older Populations: Concepts, Resources, and Systems. In Prohaska T, Anderson LA, Binstock R. editors. Public Health for an Aging Society. Baltimore, Maryland: Johns Hopkins University Press.
Holtzman, D., & Anderson, L.A. (2012). Aging and health in America: a tale from two boomers. American Journal of Public Health, 102(3):392.
Prohaska, T., Anderson, L.A. , Binstock. R., editors. (2012). Public Health for an Aging Society. Baltimore, Maryland: Johns Hopkins University Press.
Shenson, D., Adams, M., Bolen, J., Wooten K., Clough, J. Giles, W., & Anderson, L.A. (2012). Developing an Integrated Strategy to Reduce Ethnic and Racial Disparities in the Delivery of Clinical Preventive Services for Older Americans. American Journal of Public Health , On-line, Jun 14, 2012.
Yen, I.H., & Anderson, L.A. (2012). Built Environment and Mobility of Older Adults: Key Policy and Practice Efforts, Journal of the American Geriatrics Society. Article first published online: 9 MAY 2012. DOI: 10.1111/j.1532-5415.2012.03949
Anderson, L.A. Population Aging. Oxford Bibliographies Online, 2011. (Chapter for the Public Health section of Oxford Bibliographics Online. This chapter includes over 100 citations on aging related issues.
Anderson, L.A. , Day, K.L. , & Vandenberg A.E. (2011). Using a concept map as a tool for strategic planning: The Healthy Brain Initiative. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 8(5):A117. http://www.cdc.gov/pcd/issues/2011/sep/10_0255.htm .
Day, K.L. , Friedman, D.B., Laditka, J.N., Anderson, L.A. , Hunter, R., Laditka, S.B., Wu, B., McGuire, L.C., & Coy, M.C. (2011). Prevention of Cognitive Impairment: Physician Perceptions and Practices. Journal of Applied Gerontology. DOI: 10.1177/0733464811401354
Kusano, C.T., Bouldin, E.D., Anderson, L.A. , McGuire, L.C. , Salvail, F.R., Wynkoop Simmons, K., Andresen, E.M. (2011). Adult informal caregivers reporting financial burden in Hawaii, Kansas, and Washington: Results from the 2007 Behavioral Risk Factor Surveillance System. Disability and Health Journal. 4(4):229–237.
Prohaska, T., Anderson, L.A. , Hooker, S.P., Hughes, S.L., & Belza, B. (2011). Editorial: Mobility and Aging: Transference to Transportation. Journal of Aging Research . Article ID 392751, doi:10.4061/2011/392751.
Shenson, D., Adams, M., Bolen, J., & Anderson, L.A. (2011). Routine checkups don’t ensure that seniors get preventive services. The Journal of Family Practice . 60(1): E1–10.
Snowden, M., Steinman, L., Mochan, K., Grodstein, F., Thurman, D., Brown, D., … & Anderson, L. A. (2011). Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. Journal of the American Geriatrics Society . 59:704–716.
Vandenberg, A.E., Price, A.E., Friedman, D.B., Marchman, G, & Anderson, L.A. , (2011). How Do Top Cable News Websites Portray Cognition as an Aging Issue? The Gerontologist . 52 (3): 367–382.
McGuire, L.C., Bouldin, E.L., Andresen, E.M., & Anderson, L.A. (2010). Examining modifiable health behaviors, body weight, and use of preventive health services among caregivers and non-caregivers aged 65 years and older using in Hawaii, Kansas, and Washington using BRFSS, 2007. Journal of Nutrition, Health and Aging . 14(5):373-9.
Rao, J.K. , Anderson, L.A. , Sukumar, B., Beauchesne, D.A., Stein, T., & Frankel, R.M. Engaging communication experts in a Delphi process to identify patient behaviors that could impact communication in medical encounters.BMC Health Services Research 2010, 10:97 (19 April 2010).
Snowden, M., Dhingra, S.S., Keyes, C.L.M., & Anderson, L.A. (2010). Changes in Mental Well-Being in the Transition to Late Life: Findings From MIDUS I and II. American Journal of Public Health . 100(12):2385-2388.
Anderson LA , Logsdon RG, Hochhalter AK, Sharkey JR. Introduction. The Gerontologist .2009; 49(S1):S1-S2.
Anderson LA , Day KL , Beard RL, Reed PS, Wu B. The public’s perceptions about cognitive health and Alzheimer’s disease among the U.S. population: A national review. The Gerontologist . 2009; 49(S1):S3-S11.
Day KL , McGuire LC , Anderson LA .The Centers for Disease Control and Prevention’s Healthy Brain Initiative: emerging implications of cognitive impairment. Generations . 2009; 33(1):11-17.
DeFries EL, McGuire LC , Andresen EM, Brumback BA, Anderson LA. Caregivers of older adults with cognitive impairment. Prev Chronic Dis . 2009 Apr;6(2):A46.
McGuire LC , Strine T, Vachirasudlekha S, Anderson LA ,Berry JT, Mokdad AH. Modifiable characteristics of a healthy lifestyle in US older adults with or without serious psychological distress, 2007 Behavioral Risk Factor Surveillance System. Int J Public Health . 2009;54:S84-S93.
McGuire LC, Strine T, Allen RS, Anderson LA , Mokdad AH. The Patient Health Questionnaire 8: current depressive symptoms among U.S. older adults, 2006 Behavioral Risk Factor Surveillance System. y . 2009 Apr;17(4):324-34.
Rao JK, Abraham L, Anderson LA . Novel approach, using end-of-life issues, for identifying items for public health surveillance. Preventing Chronic Disease . 2009;6(2):A57.
White-Cooper S, Dawkins NU, Kamin SL, Anderson LA. Community-Institutional Partnerships: Understanding Trust Among Partners. Health Educ Behav 2009 36: 334-347.
Ford ES, Mokdad AH, Li C, McGuire LC , Strine TW, Okoro CA, et al. Gender differences in coronary heart disease and health-related quality of life: Findings from 10 states from the 2004 Behavioral Risk Factor Surveillance System. Journal of Women’s Health 2008;17(5):757-768.
Frank E, Carrera JS, Rao JK , Anderson LA . Satisfaction with career choice among U.S. medical students. Archives of Internal Medicine 2008;168(15):1712-1716.
Garrett MD, Baldridge D, Benson WF , McGuire LC . Missing cohorts of caregivers among American Indian and Alaska Native communities. The IHS Primary Care Provider 2008;33:105-111.
Mark H, Irwin K, Sternberg M, Anderson LA , Magid DJ, Stiffman M. Providers’ perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sexually Transmitted Diseases 2008;35(2):184-189.
McGuire LC , Strine TW, Vachirasudlekha S , Mokdad AH, Anderson, LA . The prevalence of depression in older U.S. women, 2006 Behaviorial Risk Factor Surveillance System. Journal of Women’s Health 2008;17:501-507.
Rao JK . Applying the findings of public health research to communities: Balancing ideal conditions with real-world circumstances . Preventing Chronic Disease 2008;5(2):[4 pages].
Shenson D, Benson W , Harris AC . Expanding the delivery of clinical preventive services through community collaboration: The SPARC model . Preventing Chronic Disease 2008;5(1):[8 pages].
Thurman DJ, Stevens JA, Rao JK . Practice parameter: Assessing patients in a neurology practice for risk of falls (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008;70(6):473-479.
Wright DS, Anderson LA , Brownson RC, Gwaltney MK, Scherer J, Cross AW, et al. Engaging partners to initiate evaluation efforts: Tactics used and lessons learned from the Prevention Research Centers Program . Preventing Chronic Disease 2008;5(1):[7 pages].
Anderson LA , McConnell SR. The healthy brain and our aging population: translating science to public health practice. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S1-S2.
Anderson LA , McConnell SR. Cognitive health: an emerging public health issue. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S70-S73.
Cunningham-Sabo L, Carpenter WR, Peterson JC, Anderson LA , Helfrich CD, Davis SM. Utilization of prevention research: searching for evidence. American Journal of Preventive Medicine 2007;33(1S):S9–S20.
Davis SM, Anderson LA (Guest Editors). The dissemination and utilization of prevention research: increasing our knowledge and understanding. American Journal of Preventive Medicine 2007;33(1S).
Ford ES, Ajani UA, McGuire LC , Liu S. Dietary magnesium and the metabolic syndrome among U.S. adults. Obesity 2007;15(5):1139-1146.
Li C, Ford ES, McGuire LC , Mokdad AH. Association of metabolic syndrome and insulin resistance with congestive heart failure: findings from National Health and Nutrition Examination Survey III”. Journal of Epidemiology and Community Health 2007;61(1):67-73.
Li C, Ford E, McGuire LC , Mokdad A. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity 2007;15:217-223.
McGuire LC , Ajani UA, Ford ES. Cognitive functioning in late life: the impact of moderate alcohol consumption. Annals of Epidemiology 2007;17:93-99.
McGuire LC , Anderson LA , Talley RC, Crews JE. Supportive care needs of Americans: a major issue for women as both recipients and providers of such care. Journal of Women’s Health 2007:16(6):784-789.
McGuire LC , Ford ES, Okoro CA. U.S. older adults with disabilities in the face of natural disasters: implications for evacuation. Disasters 2007;31:49-56.
McGuire LC , Strine TW, Okoro CA, Ahluwalia IB, Ford ES. Healthy lifestyle behaviors in U.S. older adults with and without disabilities, Behavioral Risk Factor Surveillance System 2003 . Preventing Chronic Disease 2007:4(1):[11 pages].
McGuire LC , Strine TW, Okoro CA, Ahluwalia, IB, Ford ES. Modifiable characteristics of a healthy lifestyle in U.S. older adults with or without frequent mental distress, Behavioral Risk Factor Surveillance System. American Journal of Geriatric Psychiatry 2007;15:754-761.
McGuire LC , Rao JK, Anderson LA , Ford ES. Completion of a durable power of attorney for health care: what does cognition have to do with it? The Gerontologist :47(4),457-467.
Okoro CA, Strine TW, McGuire LC , Balluz LS, Mokdad AH. Employment status and frequent mental distress among adults with disabilities, Occupational Medicine 2007;57(3):217-220. [Epub ahead of print].
Okoro CA, Denny CH, McGuire LC , Balluz L.S, Goins RT, Mokdad AH. Disability among older American Indians and Alaska Natives: Disparities in prevalence, health-risk behaviors, obesity, and chronic conditions. Ethnicity and Disease 2007;17(4):686-692.
Ory MG, Mier N, Sharkey JR, Anderson LA . Translating science into public health practice: lessons from physical activity interventions. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2007;3(S1):S52-S57.
Rao JK . Complementary and alternative medicine for arthritis. North Carolina Medical Journal 2007;68(6):453-456.
Rao JK . Looking back and looking forward [editorial] . Preventing Chronic Disease 2007;5(1):[3 pages].
Rao JK , Anderson LA , Frankel RM, Inui TS. Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Medical Care 2007;45:340-349.
Theis KA, Rao JK , Anderson LA , Thompson PM. End-of-life content in Comprehensive Cancer Control Plans: A systematic review. American Journal of Hospice Palliative Care 2007;24(5):390-398.
Anderson LA , Gwaltney MK, Sundra DL, Brownson RC, Kane M, Cross AW, Mack R, Schwartz R, Sims T, White CR. Using concept mapping to develop a logic model for the Prevention Research Centers Program . Preventing Chronic Disease . [serial online] 2006 Jan [date cited].
Chapman DP, Williams SM, Strine TW, Anda RF, Moore MJ . Dementia and its implications for public health . Preventing Chronic Disease . [serial online] 2006 Apr [date cited].
Ford ES, Mokdad AH, Link MW, Garvin WS, McGuire LC , Jiles RB, Balluz LS. Chronic disease in health emergencies: in the eye of the hurricane . Preventing Chronic Disease . [serial online] 2006 Apr [date cited].
Healthy Aging Research Network Writing Group. The prevention research centers healthy aging research network . Preventing Chronic Disease . [serial online] 2006 Jan [date cited].
Kobau R, DiIorio CA, Anderson LA , Price PH. Further validation and reliability testing of the Attitudes and Beliefs about Living with Epilepsy (ABLE) components of the CDC Epilepsy Program Instrument on Stigma . Epilepsy & Behavior . 2006; 8(3): 552–9.
McGuire LC , Ahluwalia IB, Strine TW. Chronic disease-related behaviors in U.S. older women: behavioral risk factor surveillance system, 2003 . Journal of Women’s Health . 2006; 15(1): 3–7.
McGuire LC , Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life . American Journal of Geriatric Psychiatry . 2006; 14(1): 36–42.
McGuire LC , Ford ES, Ajani UA. The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging [PDF–295 KB] . BMC Geriatrics . 2006; 6:8.
Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK . Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate . Medical Decision Making . 2006; 26(2): 194–206.
Simoes EJ, Kobau R, Kapp J, Waterman B, Mokdad A, Anderson LA . Associations of physical activity and body mass index with activities of daily living in older adults. Journal of Community Health . 2006; 31(6): 453–467.
Lang JE, Benson WF, Anderson LA . Aging and public health: partnerships that can impact cardiovascular health programs . American Journal of Preventive Medicine . 2005; 29(5S1): 158–163.
Lang JE, Moore MJ, Harris AC, Anderson LA . Healthy aging: priorities and programs of the Centers for Disease Control and Prevention. Generations . 2005; 29(2): 24–29.
Maylahn C, Alongi S, Alongi J, Moore MJ, Anderson LA . Data needs and uses for older adult health surveillance: perspectives from state health agencies . Preventing Chronic Disease [serial online] 2005 Jul [date cited].
Mokdad AH, Mensah GA, Posner SF, Reed E, Simoes EJ, Engelgau MM, and the Chronic Diseases and Vulnerable Populations in Natural Disasters Working Group. When chronic conditions become acute: prevention and control of chronic diseases and adverse health outcomes during natural disasters . Preventing Chronic Disease [serial online] 2005 Nov [date cited].
Rao JK, Alongi J, Anderson LA , Jenkins L, Stokes GA, Kane M. Development of public health priorities for end-of-life initiatives . American Journal of Preventive Medicine . 2005; 29(5): 453–460.
Shenson, D, Bolen, J, Adams, M, Seeff, L, Blackman, D. Are older adults up-to-date with cancer screening and vaccinations? Preventing Chronic Disease . [serial online] 2005 Jul [date cited].
Wheeler FC, Anderson LA , Boddie-Willis C, Price PH, Kane M. The role of state public health agencies in addressing less prevalent chronic conditions . Preventing Chronic Disease . [serial online] 2005 Jul [date cited].
Wilson RS, Scherr PA, Bienias JL, Mendes de Leon CF, Everson-Rose SA, Bennett DA, Evans DA. Socioeconomic characteristics of the community in childhood and cognition in old age . Experimental Aging Research . 2005; 31(4): 393–407.
Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease . Neuroepidemiology . 2005; 25(1): 8–14.
Anderson LA , Cornell CE. Prevention research in women’s health: studies from the field-introduction . Health Education & Behavior . 2004; 31(4 Suppl): 12S–17S.
Mack KA, Anderson LA , Galuska D, Zablotsky D, Holtzman D, Ahluwalia I. Health and sociodemographic factors associated with body weight and weight objectives for women: 2000 Behavioral Risk factor Surveillance System. Journal of Women’s Health . 2004; 13(9): 1019–1032.
Phelan EA, Anderson LA , LaCroix AZ, Larson EB. Older adults’ views of “successful aging”–how do they compare with researchers’ definitions . Journal of the American Geriatrics Society . 2004; 52(2): 211–216.
Rao JK , Hootman JM. Prevention research and rheumatic disease . Current Opinion in Rheumatology . 2004; 16(2): 119-124.
Rao JK , Weinberger M, Anderson LA , Kroenke K. Predicting reports of unmet expectations among rheumatology patients . Arthritis and Rheumatism . 2004; 51(3): 215–221.
Rein DB, Anderson LA , Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population . American Journal of Managed Care . 2004; 10(12): 917–924.
Caplan LS, Mandelson MT, Anderson LA . Validity of self-reported mammography: examining recall and covariates among older women in a health maintenance organization . American Journal of Epidemiology . 2003; 157(3): 267–272.
Currey SS, Rao JK , Winfield JB, Callahan LF. Performance of a generic health-related quality of life measure in a clinic population with rheumatic disease . Arthritis and Rheumatism . 2003; 49(5): 658–664.
Damush TM, Weinberger M, Perkins SM, Rao JK , Tierney WM, Qi R, Clark DO. Randomized trial of a self-management program for primary care patients with acute low back pain: short-term effects . Arthritis and Rheumatism . 2003; 49(2): 179–186.
Damush TM, Weinberger M, Perkins SM, Rao JK , Tierney WM, Qi R, Clark DO. The long term effects of a self-management program for inner-city primary care patients with acute low back pain . Archives of Internal Medicine . 2003; 163(21): 2632–2638.
Freburger JK, Callahan LF, Currey SS, Anderson LA . Use of the Trust in Physician Scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist . Arthritis & Rheumatism . 2003; 49(1): 51–58.
Rao JK , Kroenke K, Mihaliak KA, Grambow SC, Weinberger M. Rheumatology patients’ use of complementary therapies: results from a one-year longitudinal study . Arthritis and Rheumatism . 2003; 49(5): 619-625.
Swindle RW, Rao JK , Helmy A, Plue L, Zhou XH, Eckert GJ, Weinberger M. Integrating clinical nurse specialists into the treatment of primary care patients with depression . International Journal of Psychiatry in Medicine . 2003; 33(1): 17–37.
Tao G, Branson B, Anderson LA , Irwin KL. Do physicians provide counseling with HIV and STD testing at physician offices or hospital outpatient departments . AIDS. 2003; 17(8): 1243–1247.
Zhang P, Tao G, Anderson LA . Differences in access to health care services among adults in rural America by rural classification categories and age . Australian Journal of Rural Health . 2003; 11(2): 64–72.
Ziemer DC, Berkowitz KJ, Panayioto RM, El-Kebbi IM, Musey VC, Anderson LA, Wanko NS, Fowke ML, Brazier CW, Dunbar VG, Slocum W, Bacha GM, Gallina DL, Cook CB, Phillips LS. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes . Diabetes Care . 2003; 26(6): 1719–24.
Anderson LA , Eyler AA, Galuska D, Brown DL, Brownson RC. Relationship of satisfaction with body size and trying to lose weight in a national survey of overweight and obese women aged 40 and older, United States . Preventive Medicine . 2002; 35(4): 390–396.
Damush TM, Weinberger M, Clark DO, Tierney WM, Rao JK , Perkins SM, Verel K. Acute low back pain self-management intervention for urban primary care patients: rationale, design, and predictors of participation . Arthritis and Rheumatism . 2002; 47(4): 372–379.
Newton KM, Buist DSM, Keenan N, Anderson LA , LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey . Obstetrics & Gynecology . 2002; 100(1): 18–25.
Rao JK , Anderson LA , Smith SM. End of life is a public health priority . American Journal of Preventive Medicine . 2002; 23(3): 215–220.
Rao JK , Koppaka VR. Şanti . Annals of Internal Medicine . 2002; 137(10): 852–854.
Rao JK , Kroenke K, Mihaliak KA, Eckert GJ, Weinberger M. Can guidelines impact the ordering of magnetic resonance imaging studies by primary care providers for low back pain . American Journal of Managed Care . 2002; 8(1): 27–35.
Kennet J, McGuire LC , Willis SL, Schaie KW. Memorability functions in verbal memory: a longitudinal approach . Experimental Aging Research . 2000; 26(2): 121–137.
McGuire LC, Morian A, Codding R, Smyer MA. Older adults’ memory for medical information: influence of elderspeak and note taking. International Journal of Rehabilitation and Health . 2000; 5(2): 117–127.
Rao JK , Weinberger M, Kroenke K. Visit-specific expectations and patient-centered outcomes: a literature review . Archives of Family Medicine . 2000; 9(10): 1148–1155.
To receive email updates about Alzheimer's Disease and Healthy Aging, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
New treatment target identified for Alzheimer's disease
Researchers at the University of Leeds and Lancaster University in the UK have identified a new potential target for the treatment of Alzheimer's disease -- PDE4B.
Alzheimer's disease is the leading cause of dementia and disability in old age. As the number of people diagnosed with Alzheimer's disease is on the increase, new treatments are urgently needed to improve the quality of life for people living with the disease.
PDE4B is an enzyme inside cells that breaks down a molecule known as cyclic AMP, which regulates a range of cellular processes. Based on an Australian study that identified the PDE4B gene as a risk factor for developing Alzheimer's disease, the UK team investigated whether reducing PDE4B activity might protect against Alzheimer's disease pathology and be a useful treatment approach. To this end, they introduced a gene for reduced PDE4B activity into an Alzheimer's disease (AD) mouse model that develops amyloid plaques in the brain, a key pathological feature of the disease.
The researchers observed that AD mice showed memory deficits in maze tests, but memory was unimpaired in AD mice with genetically reduced PDE4B activity. Using functional brain imaging, the team found the metabolism of glucose, the main source of energy in the brain, was impaired in AD mice, like that seen in patients with the disease. However, AD mice with genetically reduced PDE4B activity showed healthy levels of glucose metabolism in the brain.
To understand the mechanisms involved, the researchers next looked at gene and protein expression levels in the brain. This identified increased inflammation in the brains of AD mice, like that seen in Alzheimer's disease patients, but inflammation was lower in AD mice with genetically reduced PDE4B activity. Similar effects were seen for a range of other proteins involved in Alzheimer's disease pathology. Overall, these data suggest that reducing PDE4B activity might be a useful approach for the treatment of Alzheimer's disease, although more research is needed to validate the use of drugs that target the enzyme.
Dr Steven Clapcote, the lead researcher, from the University of Leeds, said, "Reducing the activity of the PDE4B enzyme had a profound protective effect on memory and glucose metabolism in the AD mouse model, despite these mice showing no decrease in the number of amyloid plaques in the brain. This raises the prospect that reducing PDE4B activity may protect against cognitive impairment not only in Alzheimer's disease but also in other forms of dementia, such as Huntington's disease."
Dr Neil Dawson, a co-author of the paper, from Lancaster University, echoed these sentiments: "These results offer real hope for the development of new treatments that will benefit patients with Alzheimer's disease in the future. It was intriguing to find that reducing PDE4B activity by just 27% could dramatically rescue memory, brain function and inflammation in the AD mice. The next stage is to test whether PDE4B inhibiting drugs have similar beneficial effects in the AD mouse model, to test their potential efficacy in Alzheimer's disease."
The research was published in the Nature Portfolio journal Neuropsychopharmacology and was supported by the Dunhill Medical Trust, BBSRC, Alzheimer's Research UK, and the Scientific and Technological Research Council of Turkey.
- Alzheimer's Research
- Healthy Aging
- Diseases and Conditions
- Alzheimer's
- Disorders and Syndromes
- Alzheimer's disease
- House mouse
- Stem cell treatments
- Memory-prediction framework
- Memory bias
- Dementia with Lewy bodies
Story Source:
Materials provided by Lancaster University . Note: Content may be edited for style and length.
Journal Reference :
- Paul Armstrong, Hüseyin Güngör, Pariya Anongjanya, Clare Tweedy, Edward Parkin, Jamie Johnston, Ian M. Carr, Neil Dawson, Steven J. Clapcote. Protective effect of PDE4B subtype-specific inhibition in an App knock-in mouse model for Alzheimer’s disease . Neuropsychopharmacology , 2024; DOI: 10.1038/s41386-024-01852-z
Cite This Page :
Explore More
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
- Robot, Can You Say 'Cheese'?
- Researchers Turn Back the Clock On Cancer Cells
Trending Topics
Strange & offbeat.
6.9 million Americans have Alzheimer's disease: How to reduce your risk

A new report estimates 6.9 million older Americans are living with Alzheimer’s disease in 2024, an increase of about 200,000 cases of the mind-robbing disease from 2023 and "a significant public health crisis," according to an expert.
Another 5 million to 7 million adults have mild cognitive impairment, a set of early changes to memory and thinking linked to Alzheimer's, according to an Alzheimer's Association's annual facts and figures report released Wednesday.
The report also highlights good news. Other studies indicate that dementia rates have declined over the past 25 years as more adults are achieving higher levels of education, staying active and exercising, reducing their blood pressure, avoiding cigarettes and staying socially engaged.
Adults face a higher risk of Alzheimer's and other types of dementia as they age, and the number of Americans 65 and older is projected to swell from 58 million in 2022 to 82 million in 2050. In just six years, the youngest baby boomers will be 65.
The nation's aging population will create profound economic and social challenges. The annual cost of caring for people with Alzheimer’s or other types of dementia will be $360 billion in 2024, up $15 billion from a year ago, the report said.
Medicare and Medicaid will cover the bulk of that, spending $231 billion this year to care for people with Alzheimer’s and dementia. Public and private spending to take care of Alzheimer's and dementia patients will skyrocket to nearly $1 trillion in 2050, the report projects.
"Our population is aging, so we really need to address these issues," said Sam Fazio, the Alzheimer Association's senior director of quality care and psychosocial research. "Alzheimer's disease remains a significant public health crisis."
Lifestyle changes reduce risk
Other Alzheimer's experts not involved with the report said more Americans are taking steps to reduce their risk for Alzheimer's or dementia.
Research suggests up to 40% of dementia cases can be prevented through lifestyle changes, said Dr. Keith Vossel, a neurologist and director of the Mary S. Easton Center for Alzheimer’s Research and Care at the University of California, Los Angeles.
Vossel said people who exercise regularly, do not smoke and achieve higher levels of education tend to have lower risk. Reducing blood pressure in midlife, in particular, is linked to lower risk, he said.
Paying close attention to elevated blood pressure is especially important, Vossel said. "We know that lowering blood pressure among people with elevated blood pressure in middle life can lower risk of dementia or (mild cognitive impairment) later on."
Caregivers spend 31 hours a week on Alzheimer's, dementia patients
Families and other caregivers take on an array of tasks, scheduling appointments and feeding and caring for people with Alzheimer's or dementia. The report said 11.5 million relatives and caregivers provided more than 18 million hours of unpaid care last year.
That amounted to a full-time job for caregivers who spent an average of nearly 31 hours a week caring for a person with Alzheimer's or dementia.
In July, the Centers for Medicare & Medicaid Services will launch an initiative to improve the quality of life for people with dementia, allowing them to remain at home and reduce the strain on unpaid caregivers. The model, called Guiding an Improved Dementia Experience , will coordinate care and provide a 24/7 support line. Families also can access care navigators who can connect patients and caregivers to services and support. Doctors and clinics who participate will receive a monthly per-patient fee from Medicare.
Fazio said access to navigators is crucial because the report showed that families live through a great deal of stress and that workers in the field believe the health care system is not equipped to help people living with dementia. President Joe Biden recently expanded a similar navigator plan for cancer patients in which private health insurers will cover such services.
Families "really want help and need help to navigate the system," Fazio said.
New drugs, old target
Of the eight drugs approved for Alzheimer's patients, only two attempt to attack the disease and slow memory and cognitive decline. Biogen has discontinued one of those drugs, aducanumab, sold under the brand Aduhelm. The Food and Drug Administration approved the drug despite mixed clinical trial results . Biogen also faced withering criticism when it launched Aduhelm, initially priced at $56,000 a year.
In January 2023, Eisai won FDA approval for its amyloid beta-busting drug , lecanemab. Sold under the brand name Leqembi, the drug is intended for patients in the early stages of the disease, the population studied in clinical trials.
The Alzheimer's Association report notes that the benefits of lecanemab "in the short term may be imperceptible" because it's designed to slow the disease, not reverse cognitive decline. The report said the long-term results of the drug are not clear.
Earlier this month, the FDA delayed action for Eli Lilly's drug donanemab , the drug manufacturer said. The FDA expects to convene an advisory committee to discuss the treatment.
Clinical trials of all three amyloid-removing drugs have side effects visible on brain scans, such as brain swelling and bleeding. Some patients don't notice symptoms. Others have experienced headaches, dizziness, nausea, confusion and vision changes.
Though drugmakers largely have focused on drugs to target and clear amyloid from the brains of Alzheimer's patients, the report says, other studies are examining different methods of attacking the disease. Other potential drugs are being studied to limit the accumulation of tau protein, inflammation, altered cell metabolism and damage from toxic oxygen molecules, the report said.
Ken Alltucker is on Twitter at @kalltucker, or can be emailed at [email protected].
Study finds 3 big risk factors for dementia

Diabetes, air pollution and alcohol consumption could be the biggest risk factors for dementia, a study has found.
Researchers compared modifiable risk factors for dementia — which is characterized by the impairment of memory, thinking and reasoning — and studied how these factors appear to affect certain brain regions that are already particularly vulnerable to Alzheimer’s disease and schizophrenia.
The research, based on brain scans of nearly 40,000 adults, between ages 44 and 82, in Britain was published Wednesday in Nature Communications.
These vulnerable regions of the brain develop during adolescence and help the brain process and integrate “bits of information across different modalities, across different senses,” said Gwenaëlle Douaud , an associate professor at the University of Oxford and co-author of the study. But “they’re the first ones to go when we start aging.”
“What we’re trying to do is say: What are the common risk factors for dementia that are affecting these regions?” Douaud said. “These are the three most harmful but then, obviously, the others, they have an effect.”
- Researchers investigated the genetic and modifiable risk factors that contribute to the vulnerability of the “most fragile parts of the brain” by studying the brain scans of nearly 40,000 relatively healthy participants from the U.K. Biobank.
- The study examined 161 modifiable risk factors, including blood pressure, cholesterol, diabetes, weight, alcohol consumption, smoking, mood, inflammation, pollution, hearing, sleep, socialization, diet, physical activity and education.
- A diagnosis of diabetes, the amount of nitrogen dioxide in the air and how often someone drinks alcohol — from never to daily, or nearly every day — were found to be the three most detrimental risk factors to these regions of the brain, Douaud said.
- Diabetes, air pollution and alcohol consumption each has an effect that is about twice as much as the other leading risk factors, Douaud said. The next major risk factors are sleep, weight, smoking and blood pressure.
- Researchers identified seven genetic clusters that affect these vulnerable parts of the brain, some of which are also associated with Parkinson’s and Alzheimer’s diseases. Douaud said the genetic and modifiable risk factors are not comparable.
More than 55 million people live with dementia around the world, and that figure is expected to increase to 153 million by 2050, according to the World Health Organization.
Get Well+Being tips straight to your inbox

Dementia is the loss of cognitive function, and symptoms result from brain neurons losing their connection to other brain cells and eventually dying, according to the National Institute on Aging . Everyone loses neurons over time, but the loss is more significant in dementia patients.
Diabetes and alcohol consumption “have been consistently shown to be associated with both cerebral and cognitive decline,” the researchers wrote in the Nature Communications study. And there is growing evidence that exposure to air pollution is a risk factor for cognitive decline and dementia.
A 2020 Lancet report on dementia found that a dozen modifiable risk factors such as hypertension, hearing impairment, smoking and obesity together account for up to 40 percent of dementia cases worldwide.
A person’s age, genes, family history, a traumatic brain injury or a stroke also are potential risk factors .
What other experts say
Gill Livingston , a professor of psychiatry of older people at University College London and the lead author of the 2020 Lancet report, said that the new study was “very interesting” but that the participants in the U.K. Biobank are a “much healthier” and highly motivated group. The findings may not be applicable to a broader population.
Still, they show that people can make decisions to reduce their risk of cognitive decline as they age, Livingston said.
“There’s quite a lot people can do in their everyday life to maintain cognitive health ,” she said. “This just reinforces it.”
What you can do about it
Try to eat a healthy, varied diet to help lower your blood sugar, take measures to protect against “traffic-related pollution” and drink alcohol in moderation, Douaud said in an email.
“Of course, some of these should not only be down to individuals, and the burden should also be shared with (local) governments devising helpful policies,” she said.
Livingston said social and physical activity — talking with friends and exercising — “make a huge difference.” And stimulating experiences, walking outside , “seeing different things” can be beneficial, she said.
Hearing loss , which is more likely as a person ages, can take away opportunities for conversations that lead to “rapid brain stimulation,” Livingston said.
“And if you smoke, stop,” she said.
Marlene Cimons contributed to this report.
Read more from Well+Being
Well+Being shares news and advice for living well every day. Sign up for our newsletter to get tips directly in your inbox.
Vitamin B12 for fatigue has no proven benefit unless you have a deficiency that causes anemia.
Flashes, shimmers and blind spots: Here’s what migraine aura looks like.
Sparkling water is a better choice for your teeth than most popular beverages.
You can help your brain form healthy habits and break the bad ones.
Our 7 best tips to build an exercise habit .

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 March 2024
The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease
- Jordi Manuello ORCID: orcid.org/0000-0002-9928-0924 1 , 2 ,
- Joosung Min ORCID: orcid.org/0000-0002-5541-5014 3 ,
- Paul McCarthy 1 ,
- Fidel Alfaro-Almagro 1 ,
- Soojin Lee 1 , 4 ,
- Stephen Smith 1 ,
- Lloyd T. Elliott 3 na1 ,
- Anderson M. Winkler 5 , 6 na1 &
- Gwenaëlle Douaud ORCID: orcid.org/0000-0003-1981-391X 1
Nature Communications volume 15 , Article number: 2576 ( 2024 ) Cite this article
10k Accesses
2056 Altmetric
Metrics details
- Genetics research
- Neuroscience
- Risk factors
We have previously identified a network of higher-order brain regions particularly vulnerable to the ageing process, schizophrenia and Alzheimer’s disease. However, it remains unknown what the genetic influences on this fragile brain network are, and whether it can be altered by the most common modifiable risk factors for dementia. Here, in ~40,000 UK Biobank participants, we first show significant genome-wide associations between this brain network and seven genetic clusters implicated in cardiovascular deaths, schizophrenia, Alzheimer’s and Parkinson’s disease, and with the two antigens of the XG blood group located in the pseudoautosomal region of the sex chromosomes. We further reveal that the most deleterious modifiable risk factors for this vulnerable brain network are diabetes, nitrogen dioxide – a proxy for traffic-related air pollution – and alcohol intake frequency. The extent of these associations was uncovered by examining these modifiable risk factors in a single model to assess the unique contribution of each on the vulnerable brain network, above and beyond the dominating effects of age and sex. These results provide a comprehensive picture of the role played by genetic and modifiable risk factors on these fragile parts of the brain.
Similar content being viewed by others

Single-cell multiplex chromatin and RNA interactions in ageing human brain
Xingzhao Wen, Zhifei Luo, … Sheng Zhong

A concerted neuron–astrocyte program declines in ageing and schizophrenia
Emi Ling, James Nemesh, … Steven A. McCarroll

Cell subtype-specific effects of genetic variation in the Alzheimer’s disease brain
Masashi Fujita, Zongmei Gao, … Philip L. De Jager
Introduction
The development of preventative strategies based on modifying risk factors might prove to be a successful approach in ensuring healthy ageing. Factors particularly scrutinised in dementia and unhealthy ageing have included cerebrovascular factors such as high blood pressure, diabetes and obesity, but also lifestyle ones such as alcohol consumption, and protective factors such as exercise 1 . Assessing these modifiable risk factors together makes it possible to identify the unique contribution of each of these factors on the brain or on cognitive decline. A Lancet commission, updated in 2020 to include, e.g., pollution for its possible role in the incidence of dementia 2 , examined the relative impact of 12 modifiable risk factors for dementia, and showed that these 12 factors may account for 40% of the cases worldwide 3 . Conversely, genetic factors are non-modifiable in nature, but can inform us about the mechanisms underlying the phenotypes of interest. These mechanisms sometimes can be shared across these phenotypes. For instance, genetic overlap has been found for Alzheimer’s and Parkinson’s diseases at a locus in the MAPT region 4 . Likewise, one of the most pleiotropic variants, in the SLC39A8 / ZIP8 gene, shows genome-wide associations with both schizophrenia and fluid intelligence, amongst many other phenotypes 5 , 6 .
One way to objectively and robustly assess susceptibility for unhealthy ageing is to look non-invasively at brain imaging markers 7 . Using a data-driven approach on a lifespan cohort, we previously identified an ensemble of higher-order, ‘transmodal’ brain regions that degenerates earlier and faster than the rest of the brain 8 . The very same areas also develop relatively late during adolescence, thus supporting the ‘last in, first out’ (LIFO) hypothesis, which posits that the process of age-related brain decline mirrors developmental maturation. Importantly, this network of brain regions further demonstrated heightened vulnerability to schizophrenia and Alzheimer’s disease, two disorders that impact on brain structure during adolescence and ageing respectively. Accordingly, this LIFO network was strongly associated with cognitive traits whose impairment is specifically related to these two disorders, namely fluid intelligence and long-term memory 8 .
Here, our main objective was to assess both the genetic and modifiable risk factors’ contributions to the vulnerability of these most fragile parts of the brain. We conducted a genome-wide association study on a prospective cohort of nearly 40,000 participants of the UK Biobank study who had received brain imaging, and in total evaluated the association between the LIFO brain network and 161 modifiable risk factors, classified according to 15 broad categories: blood pressure, cholesterol, diabetes, weight, alcohol consumption, smoking, depressive mood, inflammation, pollution, hearing, sleep, socialisation, diet, physical activity and education.
The vulnerable LIFO brain network in UK Biobank
Similar to our previously observed results 8 , the loadings of the LIFO brain network, i.e., the normalised grey matter volume in the network after regressing out the effects of all the other brain maps (see Methods), demonstrated a strong quadratic association with age in the UK Biobank cohort of 39,676 participants ( R 2 = 0.30, P < 2.23 × 10 −308 , Fig. 1 ). These higher-order regions thus show an accelerated decrease of grey matter volume compared with the rest of the brain. Furthermore, these areas define a network mainly involved in behavioural tasks related to execution, working memory, and attention (Fig. 1 , Supplementary Information ).
Top left, spatial map of the LIFO network (in red-yellow, thresholded at Z > 4 for visualisation) used to extract the loadings from every scanned participant from UK Biobank ( n = 39,676). Top right, these LIFO loadings (in arbitrary units) show a strong quadratic association with age in the UK Biobank cohort, i.e. grey matter volume decreases quadratically with older age in these specific regions ( R 2 = 0.30, P < 2.23 × 10 −308 ; inset: residual scatterplot). Bottom, the vulnerable network appears to encompass areas mainly involved in execution, working memory, and attention (using the BrainMap taxonomy 60 , and with the LIFO brain network thresholded at both Z = 4 and Z = 10, see Supplementary Information ).
Genetic influences over the vulnerable LIFO brain network
Using a minor allele frequency filter of 1% and a –log 10 (P) threshold of 7.5, we found, in the 39,676 participants, genome-wide associations between the LIFO brain network and seven genetic clusters whose top variants were all replicated (Table 1 /Supplementary Data 1 , Fig. 2 ).
Top row, Manhattan plot showing the 7 significant genetic clusters associated with the LIFO brain network (–log 10 ( P ) > 7.5). Second and third rows, regional association plots of the top variants for each of the 5 autosomal genetic clusters: rs6540873 on chromosome (Chr) 1 ( KCNK2 ), rs13107325 on Chr4 ( SLC39A8 ), rs2677109 on Chr6 ( RUNX2 ) (as a proxy in high LD R 2 = 0.86 with indel 6:45442860_TA_T), rs12146713 on Chr12 ( NUAK1 ), and rs2532395 on Chr17 ( MAPT , KANSL1 )(highest variant after tri-allelic rs2693333; see Supplementary Data 4 for a complete list of significant variants in this 5th MAPT genetic cluster). Bottom row, regional association plots of the top variants for the two genetic clusters in the pseudo-autosomal region PAR1 of the X chromosome: rs312238 ( XG , CD99 ) and rs2857316 ( XG )(UK Biobank has no genotyped variants on the 3’ side). Based on Human Genome build hg19. P -values are derived from a two-sided linear association test.
The first autosomal genetic cluster, on chromosome 1, included two variants (lead variant: rs6540873, β = 0.06, P = 1.71 × 10 −8 , and rs1452628, with posterior probabilities of inclusion in the causal variant set of 0.56 and 0.45, respectively) close to, and eQTL of, KCNK2 ( TREK1 ). This gene regulates immune-cell trafficking into the central nervous system, controls inflammation, and plays a major role in the neuroprotection against ischemia. Of relevance, these two loci are in particular related in UK Biobank participants with the amount of alcohol consumed, insulin levels, inflammation with interleukin-8 levels, as well as, crucially, with late-onset Alzheimer’s disease (Table 1 /Supplementary Data 1 ).
The second autosomal genetic cluster on chromosome 4 was made of 7 loci, with the lead variant rs13107325 in an exon of SLC39A8/ZIP8 ( β = 0.14, P = 2.82 × 10 −13 , posterior probability: 0.99). This locus is one of the most pleiotropic SNPs identified in GWAS, and is, amongst many other associations, related in UK Biobank with cholesterol, blood pressure, weight, inflammation with C-reactive proteins levels, diabetes with insuline-like growth factor 1 levels, alcohol intake, sleep duration, and cognitive performance/impairment, including prospective memory (Table 1 /Supplementary Data 1 ).
The third locus was an indel in chromosome 6 in an intron, and eQTL, of RUNX2 (rs35187443, β = 0.06, P = 9.03 × 10 −9 ), which plays a key role in differentiating osteoblasts, and has been very recently shown to limit neurogenesis and oligodendrogenesis in a cellular model of Alzheimer’s disease 9 .
The fourth locus was a SNP in chromosome 12, in an intron of NUAK1 (rs12146713, β = −0.10, P = 1.26 × 10 −9 ), and remarkably its top association in UK Biobank was with the contrast between schizophrenia and major depressive disorder 10 , and it was also associated with insulin-like growth factor 1 levels (Table 1 /Supplementary Data 1 ).
The final genetic autosomal genetic cluster was made of 3,906 variants in the MAPT region. Its lead non-triallelic variant, rs2532395 ( β = −0.09, P = 3.56 × 10 −15 ) was more specifically <10 kb from KANSL1 and an eQTL of KANSL1 , MAPT and other genes in brain tissues (Table 1 /Supplementary Data 1 , Supplementary Data 4 ). This locus was also associated in UK Biobank with tiredness and alcohol intake. MAPT is in 17q21.31, a chromosomal band involved with a common chromosome 17 inversion 11 . Adding chromosome 17 inversion status as a confounder reduced the significance of the association ( β = −0.15, P = 8.45 × 10 −3 ). Since the genotype for rs2532395 was also strongly correlated with chromosome 17 inversion in our dataset (Pearson correlation r = 0.98, P < 2 × 10 −16 ), this would suggest that the association between MAPT and the LIFO network is not independent from chromosome 17 inversion. As this extended genetic region is known for its pathological association with many neurodegenerative disorders including Alzheimer’s disease, we investigated whether the LIFO brain regions mediated the effect of the MAPT genetic cluster (using the lead bi-allelic variant rs2532395) on Alzheimer’s disease (see Methods). Despite small average causal mediated effect (ACME) sizes, we found a significant effect for both the dominant model (ACME β = 1.16 × 10 −4 ; 95% CI = [5.19 × 10 −5 , 1.99 × 10 −4 ]; P = 4 × 10 −5 ) and the recessive model (ACME β = 1.55 × 10 −4 ; 95% CI = [3.96 × 10 −5 , 3.74 × 10 −4 ]; P = 4 × 10 −5 ; full output of the mediation package on the dominant and recessive models in Supplementary Information ).
The two last genetic clusters of 8 and 9 variants respectively were found on the X chromosome, notably in a pseudo-autosomal region (PAR1), which is interestingly hit at a higher rate than the rest of the genome ( P = 1.56 × 10 −5 , see Supplementary Information ). The top variants for these clusters were related to two homologous genes coding for the two antigens of the XG blood group: rs312238 ( β = −0.05, P = 1.77 × 10 −10 ) ~ 10 kb from, and an eQTL of, CD99/MIC2 , and rs2857316 ( β = −0.08, P = 2.27 × 10 −29 ) in an intron and eQTL of XG (Table 1 /Supplementary Data 1 ). Since chromosome X has hardly been explored, we carried out our own association analyses between these two top variants and non-imaging variables in UK Biobank. Intriguingly, the first of these two PAR1 loci, rs312238, was found to be significantly associated in the genotyped participants who had not been scanned (out-of-sample analysis in n = 374,230 UK Biobank participants) with nitrogen dioxide air pollution, our ‘best’ MRF for pollution (see below), and many other environmental, socioeconomic, and early life factors (such as urban or rural setting, distance from the coast, place of birth, number of siblings, breastfed as a baby, maternal smoking around birth), as well as health outcomes (Supplementary Data 2 ). In particular, amongst the more easily interpretable findings of the most associated variables with rs312238, the T allele of this locus was associated with two increased measures of deprivation and/or disability (worse socioeconomic status), the ‘Townsend deprivation index’ and the ‘Health score’, but also with ‘Nitrogen dioxide air pollution’, ‘Maternal smoking around birth’, as well as ‘Number of full brothers’ and ‘Number of full sisters’, thus showing consistent signs of association between this variant and these phenotypes.
We found that the heritability of the LIFO network was significant, with h 2 = 0.15 (se = 0.01). The genetic co-heritability between the LIFO network and Alzheimer’s disease or schizophrenia was not statistically significant (coefficient of co-heritability = −0.12, se = 0.10; P = 0.23; coefficient of co-heritability = −0.16, se = 0.04, P = 0.07, respectively).
Modifiable risk factors’ associations with the vulnerable LIFO brain network
Including the modifiable risk factors (MRFs) in a single general linear model allows us to assess the unique contribution of each factor on the LIFO brain network. Not all UK Biobank participants have data available for all of the MRF variables however. An analysis limited to those with complete data for all MRFs would be biased, and based on a relatively small, low-powered sample. We addressed this issue via a two-stage analysis in which: (i) we first identified which variable within each of the 15 MRF categories best represented associations of that category with the LIFO brain network loadings (based on two criteria: significance and <5% missing values), (ii) we investigated the unique contribution of that MRF category, over and above all other categories and the dominating effects of age and sex, to the LIFO loadings.
From the first stage of our analysis, 12 of the 15 categories of MRFs had at least one ‘best’ MRF, i.e., with a significant effect on the LIFO brain network and enough non-missing values across all scanned participants to be investigated further (Table 2 /Supplementary Data 3 ). The contribution of the MRFs on the vulnerable brain network differed vastly depending on whether confounding effects of age, sex and head size were taken into account. The effect size and significance of some MRFs diminished because of some clear collinearity with the confounders. For instance, for the category of blood pressure, the most significant MRF was first “systolic blood pressure, automatic (second) reading” ( r = −0.20, P < 2.23 × 10 −308 ), but after regressing out the confounders, the ‘best’ MRF for this category was “medication for blood pressure” ( r = −0.05, P = 7.55 × 10 −22 ). Conversely, regressing out the effects of age served to unmask the significant deleterious effects of pollution on the vulnerable brain regions, such as nitrogen dioxide air pollution or particulate matter air pollution (Table 2 /Supplementary Data 3 ).
When considered together in a single model in the second stage of the analysis, 3 best MRFs had an effect on the LIFO brain network that remained significant beyond the dominating effects of age and sex, and of the 9 other best MRFs: diabetes (“diabetes diagnosed by doctor”, r = −0.05, P = 1.13 × 10 −24 ), pollution (“nitrogen dioxide air pollution in 2005”, r = −0.05, P = 5.39 × 10 −20 ) and alcohol (“alcohol intake frequency”, r = −0.04, P = 3.81 × 10 −17 ) (Table 3 ). No MRFs showed any bias in their sub-sampling distribution, i.e., any significant difference between the original sample and the reduced sample of 35,527 participants who had values for all 18 variables considered (the 12 best MRFs and 6 confounders: age, sex, age 2 , age × sex, age 2 × sex, head size; Supplementary Information ). In total, the 12 best MRFs explained 1.5% of the effect on the vulnerable brain network ( F 12;35509 = 43.5).
While 6 out of the 7 genetic clusters associated with the LIFO network were correlated with many variables related to each of the 15 MRF categories, including diabetes, alcohol consumption and traffic pollution (Supplementary Data 1 ), we also found some genetic overlap between the very specific best MRF of “alcohol intake frequency” and the LIFO network in the pleiotropic rs13107325 variant (cluster 2), as well as rs17690703, part of the large genetic cluster 5 in MAPT (Supplementary Data 4 ). No genetic overlap was found for the precise “nitrogen dioxide air pollution in 2005” or “diabetes diagnosed by doctor”, nor for approximate variables.
This study reveals, in a cohort of nearly 40,000 UK Biobank participants, the genetic and modifiable risk factors’ associations with brain regions in a ‘last in, first out’ (LIFO) network that show earlier and accelerated ageing and are particularly vulnerable to disease processes such as that of Alzheimer’s disease 8 . Seven genetic clusters, two of which in the pseudo-autosomal region of the sex chromosomes coding for two antigens of the XG blood system, were found significantly associated and replicated genome-wide. In addition, after accounting for age and sex effects, diabetes, traffic-related pollution and alcohol were the most deleterious modifiable risk factors (MRFs) on these particularly vulnerable brain regions.
Three lead variants for our significant genetic clusters have been previously associated with ageing-related brain imaging measures in recent studies: one, in cluster 1, an eQTL of KCNK2 ( TREK1 ) 12 , 13 , whose increase in expression mediates neuroprotection during ischemia 14 , the ubiquitous rs13107325 (cluster 2), and one, in cluster 4, in an intron of NUAK1 ( ARK5 ) 15 , 16 , 17 , which has been associated with tau pathology 18 (Table 1 /Supplementary Data 1 ). On the other hand, of the seven genetic clusters, three were entirely novel (clusters 3, 6 and 7), and not found in other brain imaging studies, including our most recent work that expanded on our previous GWAS of all of the brain IDPs available in UK Biobank 19 by including more participants—in fact, the same number of participants as analysed in this present work—and, crucially, by also including the X chromosome 20 (Table 1 /Supplementary Data 1 ). This suggests that, beyond the genetic hits that were meaningfully associated with the LIFO brain network and an array of relevant risk factors, lifestyle variables and brain disorders, and found in a few other imaging GWAS, some of the genetic underpinnings of the LIFO network are intrinsically specific to it and to no other pre-existing imaging phenotype.
All five autosomal genetic clusters identified through the GWAS of the LIFO phenotype had relevant associations with risk factors for dementia (Results; Supplementary Data 1 ), including precisely two of the best MRFs (for clusters 2 and 5), and three of them directly related in UK Biobank to the two diseases showing a pattern of brain abnormalities following the LIFO network: schizophrenia (clusters 2 and 4) and Alzheimer’s disease (cluster 1) (Supplementary Data 1 ). In particular, cluster 2 has its lead variant rs13107325 in an exon of one of the most pleiotropic genes ZIP8 , which codes for a zinc and metal transporter. Considering the vulnerability of the LIFO brain network to adolescent-onset schizophrenia and its significant association with fluid intelligence that we previously demonstrated 8 , it is notable that this variant has been associated genome-wide with schizophrenia 6 , as well as intelligence, educational attainment and mathematics ability 5 , 21 . In line with the LIFO brain network being both prone to accelerated ageing and susceptible to Alzheimer’s disease, this genetic locus has also been associated genome-wide with well-known risk factors for dementia. These comprise alcohol—including the exact same variable of “alcohol intake frequency” as identified as one of the best MRFs—cholesterol, weight, sleep—including “sleep duration”—and blood pressure 22 , 23 , 24 , 25 , 26 , all of which significantly contribute to modulating the LIFO brain network when considered separately (Table 2 /Supplementary Data 3 ). Of relevance, this genetic locus is also associated to an increased risk of cardiovascular death 27 . Cluster 5, a large genetic cluster in the MAPT region (Microtubule-Associated Protein Tau), comprised in total 3906 significant variants (Supplementary Data 4 ). This genetic region plays a role in various neurodegenerative disorders related to mutations of the protein tau, such as frontotemporal dementia 28 and progressive supranuclear palsy 29 , but also, of particular pertinence to the LIFO brain network, Alzheimer’s and Parkinson’s disease, with a genetic overlap between these two diseases in a locus included in our significant cluster 5 (rs393152, β = −0.09, P = 6.35 × 10 −14 ) 4 . Despite the relatively low number of people with diagnosed Alzheimer’s disease in the genetic discovery cohort, we were able to establish—albeit with small effect sizes—a significant mediation role for the LIFO brain regions between the lead bi-allelic variant for cluster 5 and this Alzheimer’s diagnosis, suggesting once more the importance played by these vulnerable brain areas in unhealthy ageing.
Finally, of the seven clusters, two were located in the pseudo-autosomal region (PAR1) of the sex chromosomes corresponding to the genes XG and CD99 , coding for the two antigens of the XG blood group. This blood group system has been largely neglected, its main contribution related to the mapping of the X chromosome itself, and its clinical role remains elusive 30 . In order to investigate further the possible role of these two variants of the XG blood group, we examined out-of-sample their associations with thousands of non-imaging phenotypes. This analysis revealed that the first of these two loci was significantly and consistently associated with early life factors, environmental factors and health outcomes, including particulate matter and nitrogen dioxide air pollution, the second most deleterious MRF to the LIFO brain network (Supplementary Data 2 ). Whether these associations are due to stratification or genotyping artefacts, or to the fact that this specific variant, which is inherited from a parent, has a parental impact that modulates the effect of early life environment of the UK Biobank participants, the so-called “nature of nurture”, will need further investigation 31 .
Intriguingly, an analysis revealed that the genes involved in the loci associated with the LIFO network (Table 1 /Supplementary Data 1 ) are enriched for the gene ontology terms of leucocyte extravasation, namely “positive regulation of neutrophil extravasation” ( P = 4.75 × 10 −6 ) and “T cell extravasation” ( P = 4.75 × 10 −6 ). This result held when removing the genes included in the MAPT extended region (with P = 2.54 × 10 −6 and P = 2.54 × 10 −6 , respectively). Leucocyte extravasation facilitates the immune and inflammatory response, and there has been renewed focus on the fact that a breakdown of the blood-brain barrier together with leukocyte extravasation might contribute to both Alzheimer’s disease and schizophrenia 32 , 33 . In line with the enrichment findings, 4 out of the 7 genetic clusters associated with the LIFO network are correlated in UK Biobank blood assays with percentage or count of immune cells (neutrophil, lymphocyte, platelet, monocyte, etc.; Supplementary Data 1 ).
Regarding MRFs’ effects on the LIFO brain network, diabetes and alcohol consumption have been consistently shown to be associated with both cerebral and cognitive decline 34 , 35 . On the other hand, pollution—and notably that of nitrogen oxides—has emerged more recently as a potential MRF for dementia 2 , 36 . In particular, the increase of dementia risk due to nitrogen oxide pollution, a proxy for traffic-related air pollution, seems to be enhanced by cardiovascular disease 37 . In this study, we found that nitrogen dioxide pollution has one of the most deleterious effects onto the fragile LIFO brain regions. This effect could only be unmasked by regressing out the effects of age and sex, as traffic-related air pollution is modestly inversely-correlated with age (Supplementary Data 5 ). It is also worth noting that including age and sex as confounding variables in the first stage of our analysis reduced considerably the contribution of what had appeared at first—before regression—as the most harmful risk factors: blood pressure, cholesterol and weight (Table 2 /Supplementary Data 3 ). Furthermore, the benefit of examining these MRFs in a single model in the second stage of our analysis is that we can assess the unique contribution of each of these factors on the LIFO brain network; in doing so, blood pressure, cholesterol and weight were no longer significant (Table 3 ).
One defining characteristic of the LIFO brain network is how much age explains its variance. Indeed, in the dataset covering most of the lifespan that was initially used to identify the LIFO and spatially define it 8 , age explained 50%. In the UK Biobank imaging project, where imaged participants are over 45 years old, age explained 30% (Fig. 1 ). It is thus perhaps unsurprising that, while the explained variance by each of the MRFs varies widely (Table 2 /Supplementary Data 3 ), it reduces notably once the effect of age and other confounders has been regressed out (without confounders included in the model: maximum 8.4%; with confounders: maximum 0.5%). Combined, the 12 best MRFs explained a significant 1.5% of the effect on the vulnerable brain network after regressing out age, head size and sex effects. Regarding the genetic hits, we found a significant heritability with h 2 = 0.15, in keeping with our results for structural brain phenotypes (except for subcortical and global brain volumes, which demonstrate higher heritability 19 ).
The uniqueness of this study relies on the fact that we combined the strengths of two different cohorts: the first, which revealed the LIFO grey matter network, is lifespan, demonstrating the mirroring of developmental and ageing processes in the LIFO brain areas, something that could never be achieved with UK Biobank because of its limited age range. Of note, for this initial work with the lifespan cohort 8 , we not only included grey matter partial volume images, as done in this current study, but also Freesurfer information of cortical thickness and surface area. The LIFO network showed no contribution from Freesurfer cortical thickness or area. This might hint at processes that only partial volume maps are able to detect due to the LIFO network’s specific localisation, including in the cerebellum and subcortical structures, which are not included in the area and thickness surface methods from Freesurfer.
Limitations of our study pertain to the nature of the data itself and the way each variable is encoded in the UK Biobank (binary, ordinal, categorical, continuous), the number of missing values, what is offered as variables for each modifiable risk factor category (e.g. we chose not to create any compound variables, such as the ratio of cholesterol levels or systolic and diastolic blood pressures), and the curation of each of these variables. Some of the factors might be proxies for another category, but including the ‘best’ ones in a single model alleviate these issues to some extent. Another limitation is the assumption in our models that each risk factor has a linear, additive effect on the vulnerable LIFO brain network. It is also important to note that cross-sectional and longitudinal patterns of brain ageing can differ, as has been shown for instance for adult span trajectories of episodic and semantic memory, especially in younger adults 38 . A recent study has also demonstrated a specific ‘brain age’ imaging measure to be more related to early life influences on brain structure than within-person rates of change in the ageing brain 39 . Further work will be needed to establish how the LIFO network data changes in terms of within-person trends, for instance by investigating the growing UK Biobank longitudinal imaging database. While we took care of assessing the replicability of our genetic results by randomly assigning a third of our dataset for such purposes (all our significant genetic hits were replicated), this was performed within the UK Biobank cohort that exhibits well-documented biases, being well-educated, less deprived, and healthier than the general population, especially for its imaging arm 40 . Independent replications will be needed to confirm the existence of the LIFO-associated genetic loci.
In conclusion, our study reveals the modifiable and non-modifiable factors associated with some of the most fragile parts of the brain particularly vulnerable to ageing and disease process. It shows that, above and beyond the effect of age and sex, the most deleterious modifiable risk factors to this brain network of higher-order regions are diabetes, pollution and alcohol intake. Genetic factors are related to immune and inflammatory response, tau pathology, metal transport and vascular dysfunction, as well as to the XG blood group system from the pseudo-autosomal region of the sex chromosomes, and meaningfully associated with relevant modifiable risk factors for dementia. The unprecedented genome-wide discovery of the two variants on the sex chromosomes in this relatively unexplored blood group opens the way for further investigation into its possible role in underlying unhealthy ageing.
Supplementary Information is available for this paper.
For the present work the imaging cohort of UK Biobank was used and we included 39,676 subjects who had been scanned and for whom the brain scans had been preprocessed at the time of the final set of analyses (M/F 47–53%; 44–82 years, mean age 64 ± 7 years; as of October 2020) 41 , 42 . Structural T1-weighted scans for each participant were processed using the FSL-VBM automated tool to extract their grey matter map 43 , 44 . The ‘last in, first out’ (LIFO) network of mainly higher-order brain regions was initially identified by performing a linked independent component analysis on the grey matter images of another, lifespan observational cohort of 484 subjects 8 , 45 , 46 . This map of interest, along with the other 69 generated by the analysis, was first realigned to the UK Biobank ‘standard’ space defined by the grey matter average across the first 15,000 participants, then regressed into the UK Biobank participants’ grey matter data, to extract weighted average values of grey matter normalised volume inside each of the z-maps, using the z-score as weighting factor. This made it possible to assess the unique contribution of this specific LIFO map, above and beyond all the rest of the brain represented in the other 69 maps. At the end of this process, we obtained a single imaging measure for each of the 39,676 participants, i.e. a ‘loading’ corresponding to their amount of grey matter normalised volume in the LIFO brain network.
Human participants: UK Biobank has approval from the North West Multi-Centre Research Ethics Committee (MREC) to obtain and disseminate data and samples from the participants ( http://www.ukbiobank.ac.uk/ethics/ ), and these ethical regulations cover the work in this study. Written informed consent was obtained from all of the participants.
Modifiable risk factors selection
The following 15 categories of modifiable risk factors (MRFs) for dementia were investigated based on previous literature: blood pressure, diabetes, cholesterol, weight, alcohol, smoking, depression, hearing, inflammation, pollution, sleep, exercise, diet/supplementation, socialisation, and education. These included well-documented cerebrovascular risk factors, and in particular included all of the 12 modifiable risk factors considered in the updated Lancet commission on dementia, with the sole exception of traumatic brain injury 3 . For each category, several MRF variables from UK Biobank were very minimally pre-processed ( Supplementary Information ). In total, 161 MRF variables were obtained. To optimise the interpretability of the results, and to be able to relate them to previous findings, we did not carry out any data reduction, which would have prevented us from identifying exactly which variable—and subsequently, which genetic component for this specific variable—contribute to the effect. For these same reasons, we did not create any compound variable.
Statistical analyses
Genome-wide association study.
We followed the same protocol we had developed for the first genome-wide association study (GWAS) with imaging carried out on UK Biobank 19 . Briefly, we examined imputed UK Biobank genotype data 47 , and restricted the analysis to samples that were unrelated (thereby setting aside only ~450 participants), without aneuploidy and with recent UK ancestry. To account for population stratification, 40 genetic principal components were used in the genetic association tests as is recommended for UK Biobank genetic studies 19 , 20 , 47 . We excluded genetic variants with minor allele frequency <0.01 or INFO score <0.03 or Hardy-Weinberg equilibrium –log 10 ( P ) > 7. We then randomly split the samples into a discovery set with 2/3 of the samples ( n = 22,128) and a replication set with 1/3 of the samples ( n = 11,083). We also examined the X chromosome with the same filters, additionally excluding participants with sex chromosome aneuploidy: 12 in non-pseudoautosomal region (PAR) and 9 in PAR for the discovery set, 3 in non-PAR and 6 in PAR for the replication set. Variants were considered significant at –log 10 ( P ) > 7.5, and replicated at P < 0.05.
Modifiable risk factor study
In the first stage, the general linear model was used to investigate, separately, the association between each of these 161 MRFs and the LIFO network loadings in all the scanned UK Biobank participants ( n = 39,676). We ran each model twice: once as is, and once adding 6 confounders: age, age 2 , sex, age × sex, age 2 × sex, and head size, to estimate the contribution of these MRFs on the LIFO network above and beyond the dominating effects of age and sex. Sex was based on the population characteristics entry of UK Biobank. This is a mixture of the sex the NHS had recorded for the participant at recruitment, and updated self-reported sex. For the GWAS, both sex and genetic sex were used (the sample was excluded in case of a mismatch). In total, 32 variables tailored to structural imaging had been considered as possible confounders, and we retained those with the strongest association ( R 2 ≥ 0.01; see Supplementary Information ). Socioeconomic status via the Townsend deprivation index was also considered as a possible confounding variable but explained little variance ( R 2 < 0.001) and thus was not included as a confounder.
MRFs were not considered further if they were not significant—not surviving Bonferroni-correction, i.e., P > 1.55 × 10 −4 —and if more than 5% of the subjects had their MRF values missing. For each category, a single ‘best’ MRF was then selected as the variable with the highest R 2 among those remaining, after regressing out the confounding effects of age and sex.
In the second stage, all these best MRFs were then included in a single general linear model, together with the same 6 confounders used in the first stage, to assess the unique contribution of each factor on the LIFO brain network loadings. A prerequisite to carry out this single general linear model analysis was to only include participants who would have values for all best MRFs and confounders. This explains the additional criterion of only including MRFs that had no more than 5% of values missing, to ensure that the final sample of participants who had values for all these best and confounding factors would not be biased compared with the original sample—something we formally tested (see Supplementary Information )—especially as data are not missing at random in UK Biobank, and exhibit some genetic structure 48 . The sample was therefore reduced to a total of 35,527 participants for this second stage analysis (M/F 17,290–18,237; 45–82 years, mean 64 ± 7 years). The effect of these best MRFs taken altogether was considered significant with a very conservative Bonferroni correction for multiple comparisons across all combinations of every possible MRF from each of the initial 15 MRF categories ( P < 4.62 × 10 −17 , see Supplementary Information for more details). In addition, both full and partial correlations were computed for the same set of best MRFs and confounders, in order to assess possible relationships between variables.
Post hoc genetic analyses
Chromosome 17 inversion.
We investigated chromosome 17 inversion status of the participants in the discovery cohort by considering their genotype on 32 variants that tag chromosome 17 inversion according to Steinberg et al. 11 . Of these 32 variants, 24 were present in our genetic data. We labelled the participants homozygous inverted, heterozygous, or homozygous direct (not inverted) when all 24 of these alleles indicated the same zygosity. This yielded an unambiguous inversion status for 21,969 participants (99% of the discovery cohort). To examine if the association between the non-triallelic lead variant of the MAPT genetic cluster (rs2532395, Table 1 /Supplementary Data 1 ) and the LIFO network was independent from this common inversion, we determined inversion/direct status of the discovery cohort and: 1. repeated the association test between rs2532395 and the LIFO phenotype, with chromosome 17 inversion status added as a confounder; and 2. correlated the genotype for rs2532395 with chromosome 17 inversion.
Causality within each genetic cluster
We used CAVIAR (Causal Variants Identification in Associated Regions 49 ) to assess causality of variants that passed the genome-wide significance threshold in each of the genetic clusters we report. CAVIAR uses a Bayesian model and the local linkage disequilibrium structure to assign posterior probabilities of causality to each variant in a region, given summary statistics for an association. We did not perform CAVIAR analysis on the genetic cluster on chromosome 17, as its non-triallelic lead variant (rs2532395) was strongly correlated with chromosome 17 inversion, and the LD matrix was large and low rank. We excluded the X chromosome loci from this analysis due to the difficulty in assessing LD in this chromosome.
Enrichment analysis
Based on the genes listed in the ‘Genes’ column of Table 1 /Supplementary Data 1 , we performed an enrichment analysis for the genes associated with the LIFO brain network using PANTHER 50 . PANTHER determines whether a gene function is overrepresented in a set of genes, according to the gene ontology consortium 51 , 52 .
Mediation analysis between MAPT top variant and Alzheimer’s disease, via the LIFO brain network
As the gene MAPT is associated with Alzheimer’s disease, and as we found a significant association between MAPT and the LIFO brain network, we examined to what extent the effect of MAPT is mediated by the LIFO brain regions. We conducted a mediation analysis using the counterfactual framework in which the average indirect effect of the treatment on the outcome through the mediator is nonparametrically identified (version 4.5.0 of the R package ‘mediation' 53 ). This is a general approach that encompasses the classical linear structural equation modelling framework for causal mediation, allowing both linear and non-linear relationships. In this analysis, the genotype for the lead bi-allelic variant of the MAPT association was used as the treatment, the LIFO loadings as the mediator, and Alzheimer’s disease diagnosis as the outcome.
From the ~43 K UK Biobank participants who had been scanned, we searched for those who had been diagnosed with Alzheimer’s disease specifically, regardless of whether this diagnosis occurred before, or after their brain scans. Based on hospital inpatient records (ICD10: F000, F001, F002, F009, G300, G301, G308, and G309 and ICD9: 3310) and primary care (GP) data (Eu00., Eu000, Eu001, Eu002, Eu00z, F110., F1100, F1101, Fyu30, X002x, X002y, X002z, X0030, X0031, X0032, X0033, XaIKB, XaIKC, and XE17j), we identified 65 such cases— UK Biobank being healthier than the general population, and those scanned showing an even stronger healthy bias—of which 34 were included in the discovery set after QC.
We considered two conditions for the effect of the treatment on the outcome. First, a dominant condition in which the minor allele is assumed to be dominant and for which at least one copy of the minor allele is considered treated. Second, a recessive condition in which the minor allele is assumed to be recessive. We considered that either condition was nominally significant if the confidence interval of the average causal mediated effect did not intersect zero, and had an associated P < 0.05 ÷ 2 (correcting for the two conditions). We assessed confidence intervals and P -values using 50,000 bootstrapped samples.
Associations between the LIFO brain network’s genetic hits and the MRFs
First, we reported in Table 1 / Supplementary Data 1 the significant associations between the LIFO genetic hits and UK Biobank variables related to the 15 categories listed for the MRFs. For this, we used the Open Targets Genetics website, which reports the GWAS carried out in UK Biobank ( https://genetics .opentargets.org/ ). Second, we assessed whether there was any genetic overlap between the known genetic components of the 3 best MRFs and the LIFO phenotype. Again, we used the Open Targets Genetics website outputs for these 3 very specific UK Biobank variables, and compared the significant hits for these 3 best MRFs within ±250 kbp of, or in high LD (>0.8) with, our own LIFO variants. If reported hits were limited, we also searched online for GWAS done on similar variables. Finally, we also included the list of significant hits for diabetes 54 , which focused on a potential genetic overlap between diabetes and Alzheimer’s disease.
Post hoc association for the sex chromosomes variants
The allele counts of each participant for two specific significant variants of the sex chromosomes not—or hardly—available in open databases such as https://genetics.opentargets.org/ 55 were further associated out-of-sample with all non-imaging phenotypes of UK Biobank ( n = 16,924). This analysis was carried out in the entire genotyped, quality-controlled sample where participants who had been scanned were removed (final sample: 374,230 participants), taking into account the population structure (40 genetic principal components), as well as the confounding effects of age, sex, age x sex, age 2 and age 2 x sex. Results were corrected for multiple comparisons across all non-imaging phenotypes and the two variants.
Heritability
We examined the heritability of the LIFO phenotype, and the coheritability between the LIFO network and Alzheimer’s disease or schizophrenia using LDSC 56 . This method uses regression on summary statistics to determine narrow sense heritability h 2 of a trait, or the shared genetic architecture between two traits. LDSC corrects for bias LD structure using LD calculated from a reference panel (we used LD from the Thousand Genomes Project Phase 1 57 ). We obtained summary statistics for a meta-analysis of Alzheimer’s disease involving 71,880 cases and 383,378 controls 58 . The number of genetic variants in the intersection between the summary statistics was 1,122,435. For schizophrenia, the summary statistics were obtained from a meta-analysis involving 53,386 cases and 77,258 controls 59 . A total of 1,171,319 genetic variants were in the intersection with the summary statistics for LIFO. For both Alzheimer’s and schizophrenia, the X chromosome was not included in the heritability calculation, as it was excluded from the meta-analysis that we sourced the summary statistics from.
Reproducibility
No data was excluded for the MRF analyses. For the genetic analyses, these were restricted to samples that were unrelated, without aneuploidy and with recent UK ancestry (see above).
No statistical method was used to predetermine sample size. The experiments were not randomised. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the FLICA decomposition maps − including the LIFO grey matter network − in UK Biobank standard space, the UK Biobank grey matter template, scripts, and the LIFO loadings for all of the participants are freely available on a dedicated webpage: open.win.ox.ac.uk/pages/douaud/ukb-lifo-flica/ .
Baumgart, M. et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11 , 718–726 (2015).
Article PubMed Google Scholar
Chen, H. et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ. Int. 108 , 271–277 (2017).
Article CAS PubMed Google Scholar
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396 , 413–446 (2020).
Article PubMed PubMed Central Google Scholar
Desikan, R. S. et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol. Psychiatry 20 , 1588–1595 (2015).
Article CAS PubMed PubMed Central Google Scholar
Hill, W. D. et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 24 , 169–181 (2019).
Pardinas, A. F. et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 50 , 381–389 (2018).
Douaud, G. et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl Acad. Sci. USA 110 , 9523–9528 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Douaud, G. et al. A common brain network links development, aging, and vulnerability to disease. Proc. Natl Acad. Sci. USA 111 , 17648–17653 (2014).
Nakatsu, D. et al. BMP4-SMAD1/5/9-RUNX2 pathway activation inhibits neurogenesis and oligodendrogenesis in Alzheimer’s patients’ iPSCs in senescence-related conditions. Stem Cell Rep. 18 , 1246 (2023).
Article CAS Google Scholar
Peyrot, W. J. & Price, A. L. Identifying loci with different allele frequencies among cases of eight psychiatric disorders using CC-GWAS. Nat. Genet. 53 , 445–454 (2021).
Steinberg, K. M. et al. Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat. Genet. 44 , 872–880 (2012).
Le Guen, Y. et al. eQTL of KCNK2 regionally influences the brain sulcal widening: evidence from 15,597 UK Biobank participants with neuroimaging data. Brain Struct. Funct. 224 , 847–857 (2019).
Jonsson, B. A. et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 10 , 5409 (2019).
Heurteaux, C. et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 23 , 2684–2695 (2004).
Vojinovic, D. et al. Genome-wide association study of 23,500 individuals identifies 7 loci associated with brain ventricular volume. Nat. Commun. 9 , 3945 (2018).
Article ADS PubMed PubMed Central Google Scholar
Zhao, B. et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat. Genet. 51 , 1637–1644 (2019).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits ( n = 17,706). Mol. Psychiatry https://doi.org/10.1038/s41380-019-0569-z (2019).
Lasagna-Reeves, C. A. et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92 , 407–418 (2016).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562 , 210–216 (2018).
Smith, S. M. et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci. 24 , 737–745 (2021).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50 , 1112–1121 (2018).
Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 , 707–713 (2010).
Sanchez-Roige, S. et al. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. Am. J. Psychiatry 176 , 107–118 (2019).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10 , 1100 (2019).
International Consortium for Blood Pressure Genome-Wide Association, S. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 , 103–109 (2011).
Article ADS Google Scholar
Johansson, A. et al. Genome-wide association and Mendelian randomization study of NT-proBNP in patients with acute coronary syndrome. Hum. Mol. Genet. 25 , 1447–1456 (2016).
Poorkaj, P. et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43 , 815–825 (1998).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8 , 711–715 (1999).
Johnson, N. C. XG: the forgotten blood group system. Immunohematology 27 , 68–71 (2011).
Kong, A. et al. The nature of nurture: Effects of parental genotypes. Science 359 , 424–428 (2018).
Zenaro, E., Piacentino, G. & Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 107 , 41–56 (2017).
Pong, S., Karmacharya, R., Sofman, M., Bishop, J. R. & Lizano, P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry 6 , 30–46 (2020).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Veldsman, M. et al. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat. Commun. 11 , 4340 (2020).
Power, M. C., Adar, S. D., Yanosky, J. D. & Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56 , 235–253 (2016).
Grande, G., Ljungman, P. L. S., Eneroth, K., Bellander, T. & Rizzuto, D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 77 , 801–809, (2020).
Ronnlund, M., Nyberg, L., Backman, L. & Nilsson, L. G. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol. Aging 20 , 3–18 (2005).
Vidal-Pineiro, D. et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. Elife https://doi.org/10.7554/eLife.69995 (2021).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186 , 1026–1034 (2017).
Alfaro-Almagro, F. et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166 , 400–424 (2018).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19 , 1523–1536 (2016).
Good, C. D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14 , 21–36 (2001).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130 , 2375–2386 (2007).
Groves, A. R. et al. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. NeuroImage 63 , 365–380 (2012).
Smith, S. et al. Structural variability in the human brain reflects fine-grained functional architecture at the population level. J. Neurosci. 39 , 6136–6149 (2019).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562 , 203–209 (2018).
Mignogna, G. et al. Patterns of item nonresponse behaviour to survey questionnaires are systematic and associated with genetic loci. Nat. Hum. Behav . https://doi.org/10.1038/s41562-023-01632-7 (2023).
Hormozdiari, F., Kostem, E., Kang, E. Y., Pasaniuc, B. & Eskin, E. Identifying causal variants at loci with multiple signals of association. Genetics 198 , 497–508 (2014).
Thomas, P. D. et al. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 31 , 8–22 (2022).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25 , 25–29 (2000).
Gene Ontology, C. et al. The Gene Ontology knowledgebase in 2023. Genetics https://doi.org/10.1093/genetics/iyad031 (2023).
Imai, K., Keele, L. & Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 15 , 309–334 (2010).
Hardy, J., de Strooper, B. & Escott-Price, V. Diabetes and Alzheimer’s disease: shared genetic susceptibility? Lancet Neurol. 21 , 962–964 (2022).
Carvalho-Silva, D. et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 47 , D1056–D1065 (2019).
Gazal, S., Marquez-Luna, C., Finucane, H. K. & Price, A. L. Reconciling S-LDSC and LDAK functional enrichment estimates. Nat. Genet. 51 , 1202–1204 (2019).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Jansen, I. E. et al. Author Correction: Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 52 , 354 (2020).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604 , 502–508 (2022).
Laird, A. R. et al. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinform. 3 , 23 (2009).
Download references
Acknowledgements
We are grateful to Profs Christian K. Tamnes, Lars T. Westlye, Kristine B. Walhovd and Anders M. Fjell, and Dr Andreas Engvig for providing the lifespan cohort which was used to initially derive the original ‘last in, first out’ brain network map, and to Prof Augustine Kong for helpful discussion on the associations between the PAR hit and early life and environmental factors. G.D. was supported by a UK MRC Career Development Fellowship (MR/K006673/1) and a Wellcome Collaborative Award (215573/Z/19/Z). S.S. was supported by Wellcome (203139/Z/16/Z; 215573/Z/19/Z). L.E. was funded by NSERC grants (RGPIN/05484-2019; DGECR/00118-2019) and a Michael Smith Health Research BC Scholar Award. A.M.W. received support through the NIH Intramural Research Program (ZIA-MH002781; ZIA-MH002782). This research was funded in whole, or in part, by the Wellcome Trust (215573/Z/19/Z; 203139/Z/16/Z; 203139/A/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This research was also supported by the NIHR Oxford Health Biomedical Research Centre (NIHR203316). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z and 203139/A/16/Z).
Author information
These authors contributed equally: Lloyd T. Elliott, Anderson M. Winkler.
Authors and Affiliations
FMRIB Centre, Wellcome Centre for Integrative Neuroimaging (WIN), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
Jordi Manuello, Paul McCarthy, Fidel Alfaro-Almagro, Soojin Lee, Stephen Smith & Gwenaëlle Douaud
FOCUS Lab, Department of Psychology, University of Turin, Turin, Italy
Jordi Manuello
Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby, Canada
Joosung Min & Lloyd T. Elliott
Pacific Parkinson’s Research Centre, The University of British Columbia, Vancouver, BC, Canada
National Institutes of Mental Health, National Institutes of Health, Bethesda, MD, USA
Anderson M. Winkler
Department of Human Genetics, University of Texas Rio Grande Valley, Brownsville, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
G.D. conceived and supervised the work, and carried out some of the genetic and modifiable risk factors analyses. J.Ma. carried out most of the genetic and modifiable risk factors analyses. J.Mi., S.L., A.M.W., and L.T.E. carried out additional genetics analyses. G.D., P. McC., F.A.-A., S.S., and L.T.E. created/extracted the imaging and genetics data, and organised the non-imaging data and confound variables. L.T.E. co-supervised the genetic analyses. A.M.W. co-supervised the modifiable risk factor analyses. G.D. interpreted the results and wrote the paper. J.Ma., S.S., L.T.E., and A.M.W. revised the paper.

Corresponding author
Correspondence to Gwenaëlle Douaud .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Xavier Caseras and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1-5, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Manuello, J., Min, J., McCarthy, P. et al. The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease. Nat Commun 15 , 2576 (2024). https://doi.org/10.1038/s41467-024-46344-2
Download citation
Received : 15 February 2023
Accepted : 22 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1038/s41467-024-46344-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Dementia prevention, intervention, and care: 2020 report of the Lancet Commission
Gill livingston.
a Division of Psychiatry, University College London, London, UK
d Camden and Islington NHS Foundation Trust, London, UK
Jonathan Huntley
Andrew sommerlad.
f National Ageing Research Institute and Academic Unit for Psychiatry of Old Age, University of Melbourne, Royal Melbourne Hospital, Parkville, VIC, Australia
Clive Ballard
g University of Exeter, Exeter, UK
Sube Banerjee
h Faculty of Health: Medicine, Dentistry and Human Sciences, University of Plymouth, Plymouth, UK
Carol Brayne
i Cambridge Institute of Public Health, University of Cambridge, Cambridge, UK
Alistair Burns
j Department of Old Age Psychiatry, University of Manchester, Manchester, UK
Jiska Cohen-Mansfield
k Department of Health Promotion, School of Public Health, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
l Heczeg Institute on Aging, Tel Aviv University, Tel Aviv, Israel
m Minerva Center for Interdisciplinary Study of End of Life, Tel Aviv University, Tel Aviv, Israel
Claudia Cooper
Sergi g costafreda.
n Department of Preventive and Social Medicine, Goa Medical College, Goa, India
b Dementia Research Centre, UK Dementia Research Institute, University College London, London, UK
o Institute of Neurology, National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, UK
Laura N Gitlin
p Center for Innovative Care in Aging, Johns Hopkins University, Baltimore, MA, USA
Robert Howard
Helen c kales.
r Department of Psychiatry and Behavioral Sciences, UC Davis School of Medicine, University of California, Sacramento, CA, USA
Mika Kivimäki
c Department of Epidemiology and Public Health, University College London, London, UK
Eric B Larson
s Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA
Adesola Ogunniyi
t University College Hospital, Ibadan, Nigeria
Vasiliki Orgeta
Karen ritchie.
u Inserm, Unit 1061, Neuropsychiatry: Epidemiological and Clinical Research, La Colombière Hospital, University of Montpellier, Montpellier, France
v Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK
Kenneth Rockwood
w Centre for the Health Care of Elderly People, Geriatric Medicine Dalhousie University, Halifax, NS, Canada
Elizabeth L Sampson
e Barnet, Enfield, and Haringey Mental Health Trust, London, UK
Quincy Samus
q Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MA, USA
Lon S Schneider
x Department of Psychiatry and the Behavioural Sciences and Department of Neurology, Keck School of Medicine, Leonard Davis School of Gerontology of the University of Southern California, Los Angeles, CA, USA
Geir Selbæk
y Norwegian National Advisory Unit on Ageing and Health, Vestfold Hospital Trust, Tønsberg, Norway
z Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
aa Geriatric Department, Oslo University Hospital, Oslo, Norway
ab Department Psychosocial and Community Health, School of Nursing, University of Washington, Seattle, WA, USA
Naaheed Mukadam
Associated data, executive summary.
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled by the 2017 Lancet Commission on dementia prevention, intervention, and care: less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and low social contact. We now add three more risk factors for dementia with newer, convincing evidence. These factors are excessive alcohol consumption, traumatic brain injury, and air pollution. We have completed new reviews and meta-analyses and incorporated these into an updated 12 risk factor life-course model of dementia prevention. Together the 12 modifiable risk factors account for around 40% of worldwide dementias, which consequently could theoretically be prevented or delayed. The potential for prevention is high and might be higher in low-income and middle-income countries (LMIC) where more dementias occur.
Our new life-course model and evidence synthesis has paramount worldwide policy implications. It is never too early and never too late in the life course for dementia prevention. Early-life (younger than 45 years) risks, such as less education, affect cognitive reserve; midlife (45–65 years), and later-life (older than 65 years) risk factors influence reserve and triggering of neuropathological developments. Culture, poverty, and inequality are key drivers of the need for change. Individuals who are most deprived need these changes the most and will derive the highest benefit.
Policy should prioritise childhood education for all. Public health initiatives minimising head injury and decreasing harmful alcohol drinking could potentially reduce young-onset and later-life dementia. Midlife systolic blood pressure control should aim for 130 mm Hg or lower to delay or prevent dementia. Stopping smoking, even in later life, ameliorates this risk. Passive smoking is a less considered modifiable risk factor for dementia. Many countries have restricted this exposure. Policy makers should expedite improvements in air quality, particularly in areas with high air pollution.
We recommend keeping cognitively, physically, and socially active in midlife and later life although little evidence exists for any single specific activity protecting against dementia. Using hearing aids appears to reduce the excess risk from hearing loss. Sustained exercise in midlife, and possibly later life, protects from dementia, perhaps through decreasing obesity, diabetes, and cardiovascular risk. Depression might be a risk for dementia, but in later life dementia might cause depression. Although behaviour change is difficult and some associations might not be purely causal, individuals have a huge potential to reduce their dementia risk.
In LMIC, not everyone has access to secondary education; high rates of hypertension, obesity, and hearing loss exist, and the prevalence of diabetes and smoking are growing, thus an even greater proportion of dementia is potentially preventable.
Amyloid-β and tau biomarkers indicate risk of progression to Alzheimer's dementia but most people with normal cognition with only these biomarkers never develop the disease. Although accurate diagnosis is important for patients who have impairments and functional concerns and their families, no evidence exists to support pre-symptomatic diagnosis in everyday practice.
Our understanding of dementia aetiology is shifting, with latest description of new pathological causes. In the oldest adults (older than 90 years), in particular, mixed dementia is more common. Blood biomarkers might hold promise for future diagnostic approaches and are more scalable than CSF and brain imaging markers.
Wellbeing is the goal of much of dementia care. People with dementia have complex problems and symptoms in many domains. Interventions should be individualised and consider the person as a whole, as well as their family carers. Evidence is accumulating for the effectiveness, at least in the short term, of psychosocial interventions tailored to the patient's needs, to manage neuropsychiatric symptoms. Evidence-based interventions for carers can reduce depressive and anxiety symptoms over years and be cost-effective.
Keeping people with dementia physically healthy is important for their cognition. People with dementia have more physical health problems than others of the same age but often receive less community health care and find it particularly difficult to access and organise care. People with dementia have more hospital admissions than other older people, including for illnesses that are potentially manageable at home. They have died disproportionately in the COVID-19 epidemic. Hospitalisations are distressing and are associated with poor outcomes and high costs. Health-care professionals should consider dementia in older people without known dementia who have frequent admissions or who develop delirium. Delirium is common in people with dementia and contributes to cognitive decline. In hospital, care including appropriate sensory stimulation, ensuring fluid intake, and avoiding infections might reduce delirium incidence.
Key messages
- • New evidence supports adding three modifiable risk factors—excessive alcohol consumption, head injury, and air pollution—to our 2017 Lancet Commission on dementia prevention, intervention, and care life-course model of nine factors (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and infrequent social contact).
- • Modifying 12 risk factors might prevent or delay up to 40% of dementias.
- • Prevention is about policy and individuals. Contributions to the risk and mitigation of dementia begin early and continue throughout life, so it is never too early or too late. These actions require both public health programmes and individually tailored interventions. In addition to population strategies, policy should address high-risk groups to increase social, cognitive, and physical activity; and vascular health.
- • Aim to maintain systolic BP of 130 mm Hg or less in midlife from around age 40 years (antihypertensive treatment for hypertension is the only known effective preventive medication for dementia).
- • Encourage use of hearing aids for hearing loss and reduce hearing loss by protection of ears from excessive noise exposure.
- • Reduce exposure to air pollution and second-hand tobacco smoke.
- • Prevent head injury.
- • Limit alcohol use, as alcohol misuse and drinking more than 21 units weekly increase the risk of dementia.
- • Avoid smoking uptake and support smoking cessation to stop smoking, as this reduces the risk of dementia even in later life.
- • Provide all children with primary and secondary education.
- • Reduce obesity and the linked condition of diabetes. Sustain midlife, and possibly later life physical activity.
- • Addressing other putative risk factors for dementia, like sleep, through lifestyle interventions, will improve general health.
- • Many risk factors cluster around inequalities, which occur particularly in Black, Asian, and minority ethnic groups and in vulnerable populations. Tackling these factors will involve not only health promotion but also societal action to improve the circumstances in which people live their lives. Examples include creating environments that have physical activity as a norm, reducing the population profile of blood pressure rising with age through better patterns of nutrition, and reducing potential excessive noise exposure.
- • Dementia is rising more in low-income and middle-income countries (LMIC) than in high-income countries, because of population ageing and higher frequency of potentially modifiable risk factors. Preventative interventions might yield the largest dementia reductions in LMIC.
For those with dementia, recommendations are:
- • Post-diagnostic care for people with dementia should address physical and mental health, social care, and support. Most people with dementia have other illnesses and might struggle to look after their health and this might result in potentially preventable hospitalisations.
- • Specific multicomponent interventions decrease neuropsychiatric symptoms in people with dementia and are the treatments of choice. Psychotropic drugs are often ineffective and might have severe adverse effects.
- • Specific interventions for family carers have long-lasting effects on depression and anxiety symptoms, increase quality of life, are cost-effective and might save money.
Acting now on dementia prevention, intervention, and care will vastly improve living and dying for individuals with dementia and their families, and thus society.
Introduction
Worldwide around 50 million people live with dementia, and this number is projected to increase to 152 million by 2050, 1 rising particularly in low-income and middle-income countries (LMIC) where around two-thirds of people with dementia live. 1 Dementia affects individuals, their families, and the economy, with global costs estimated at about US$1 trillion annually. 1
We reconvened the 2017 Lancet Commission on dementia prevention, intervention, and care 2 to identify the evidence for advances likely to have the greatest impact since our 2017 paper and build on its work. Our interdisciplinary, international group of experts presented, debated, and agreed on the best available evidence. We adopted a triangulation framework evaluating the consistency of evidence from different lines of research and used that as the basis to evaluate evidence. We have summarised best evidence using, where possible, good- quality systematic reviews, meta-analyses, or individual studies, where these add important knowledge to the field. We performed systematic literature reviews and meta-analyses where needed to generate new evidence for our analysis of potentially modifiable risk factors for dementia. Within this framework, we present a narrative synthesis of evidence including systematic reviews and meta-analyses and explain its balance, strengths, and limitations. We evaluated new evidence on dementia risk in LMIC; risks and protective factors for dementia; detection of Alzheimer's disease; multimorbidity in dementia; and interventions for people affected by dementia.
Nearly all the evidence is from studies in high-income countries (HIC), so risks might differ in other countries and interventions might require modification for different cultures and environments. This notion also underpins the critical need to understand the dementias related to life-course disadvantage—whether in HICs or LMICs.
Our understanding of dementia aetiology is shifting. A consensus group, for example, has described hippocampal sclerosis associated with TDP-43 proteinopathy, as limbic-predominant age-related TDP-43 encephalopathy (LATE) dementia, usually found in people older than 80 years, progressing more slowly than Alzheimer's disease, detectable at post-mortem, often mimicking or comorbid with Alzheimer's disease. 3 This situation reflects increasing attention as to how clinical syndromes are and are not related to particular underlying pathologies and how this might change across age. More work is needed, however, before LATE can be used as a valid clinical diagnosis.
The fastest growing demographic group in HIC is the oldest adults, those aged over 90 years. Thus a unique opportunity exists to focus on both human biology, in this previously rare population, as well as on meeting their needs and promoting their wellbeing.
Prevention of dementia
The number of people with dementia is rising. Predictions about future trends in dementia prevalence vary depending on the underlying assumptions and geographical region, but generally suggest substantial increases in overall prevalence related to an ageing population. For example, according to the Global Burden of Diseases, Injuries, and Risk Factors Study, the global age-standardised prevalence of dementia between 1990 and 2016 was relatively stable, but with an ageing and bigger population the number of people with dementia has more than doubled since 1990. 4
However, in many HIC such as the USA, the UK, and France, age-specific incidence rates are lower in more recent cohorts compared with cohorts from previous decades collected using similar methods and target populations 5 ( figure 1 ) and the age-specific incidence of dementia appears to decrease. 6 All-cause dementia incidence is lower in people born more recently, 7 probably due to educational, socio-economic, health care, and lifestyle changes. 2 , 5 However, in these countries increasing obesity and diabetes and declining physical activity might reverse this trajectory. 8 , 9 In contrast, age-specific dementia prevalence in Japan, South Korea, Hong Kong, and Taiwan looks as if it is increasing, as is Alzheimer's in LMIC, although whether diagnostic methods are always the same in comparison studies is unclear. 5 , 6 , 7

Incidence rate ratio comparing new cohorts to old cohorts from five studies of dementia incidence 5
IIDP Project in USA and Nigeria, Bordeaux study in France, and Rotterdam study in the Netherlands adjusted for age. Framingham Heart Study, USA, adjusted for age and sex. CFAS in the UK adjusted for age, sex, area, and deprivation. However, age-specific dementia prevalence is increasing in some other countries. IID=Indianapolis–Ibadan Dementia. CFAS=Cognitive Function and Ageing Study. Adapted from Wu et al, 5 by permission of Springer Nature.
Modelling of the UK change suggests a 57% increase in the number of people with dementia from 2016 to 2040, 70% of that expected if age-specific incidence rates remained steady, 10 such that by 2040 there will be 1·2 million UK people with dementia. Models also suggest that there will be future increases both in the number of individuals who are independent and those with complex care needs. 6
In our first report, the 2017 Commission described a life-course model for potentially modifiable risks for dementia. 2 Life course is important when considering risk, for example, obesity and hypertension in midlife predict future dementia, but both weight and blood pressure usually fall in later life in those with or developing dementia, 9 so lower weight and blood pressure in later life might signify illness, not an absence of risk. 11 , 12 , 13 , 14 We consider evidence on other potential risk factors and incorporate those with good quality evidence in our model.
Figure 2 summarises possible mechanisms of protection from dementia, some of which involve increasing or maintaining cognitive reserve despite pathology and neuropathological damage. There are different terms describing the observed differential susceptibility to age-related and disease-related changes and these are not used consistently. 15 , 16 A consensus paper defines reserve as a concept accounting for the difference between an individual's clinical picture and their neuropathology. It, divides the concept further into neurobiological brain reserve (eg, numbers of neurones and synapses at a given timepoint), brain maintenance (as neurobiological capital at any timepoint, based on genetics or lifestyle reducing brain changes and pathology development over time) and cognitive reserve as adaptability enabling preservation of cognition or everyday functioning in spite of brain pathology. 15 Cognitive reserve is changeable and quantifying it uses proxy measures such as education, occupational complexity, leisure activity, residual approaches (the variance of cognition not explained by demographic variables and brain measures), or identification of functional networks that might underlie such reserve. 15 , 16 , 17 , 18 , 19 , 20

Possible brain mechanisms for enhancing or maintaining cognitive reserve and risk reduction of potentially modifiable risk factors in dementia
Early-life factors, such as less education, affect the resulting cognitive reserve. Midlife and old-age risk factors influence age-related cognitive decline and triggering of neuropathological developments. Consistent with the hypothesis of cognitive reserve is that older women are more likely to develop dementia than men of the same age, probably partly because on average older women have had less education than older men. Cognitive reserve mechanisms might include preserved metabolism or increased connectivity in temporal and frontal brain areas. 17 , 18 , 19 , 20 , 21 People in otherwise good physical health can sustain a higher burden of neuropathology without cognitive impairment. 22 Culture, poverty, and inequality are important obstacles to, and drivers of, the need for change to cognitive reserve. Those who are most deprived need these changes the most and will derive the highest benefit from them.
Smoking increases air particulate matter, and has vascular and toxic effects. 23 Similarly air pollution might act via vascular mechanisms. 24 Exercise might reduce weight and diabetes risk, improve cardiovascular function, decrease glutamine, or enhance hippocampal neurogenesis. 25 Higher HDL cholesterol might protect against vascular risk and inflammation accompanying amyloid-β (Aβ) pathology in mild cognitive impairment. 26
Dementia in LMIC
Numbers of people with dementia in LMIC are rising faster than in HIC because of increases in life expectancy and greater risk factor burden. We previously calculated that nine potentially modifiable risk factors together are associated with 35% of the population attributable fraction (PAFs) of dementia worldwide: less education, high blood pressure, obesity, hearing loss, depression, diabetes, physical inactivity, smoking, and social isolation, assuming causation. 2 Most research data for this calculation came from HIC and there is a relative absence of specific evidence of the impact of risk factors on dementia risk in LMIC, particularly from Africa and Latin America. 27
Calculations considering country-specific prevalence of the nine potentially modifiable risk factors indicate PAF of 40% in China, 41% in India and 56% in Latin America with the potential for these numbers to be even higher depending on which estimates of risk factor frequency are used. 28 , 29 Therefore a higher potential for dementia prevention exists in these countries than in global estimates that use data predominantly from HIC. If not currently in place, national policies addressing access to education, causes and management of high blood pressure, causes and treatment of hearing loss, socio-economic and commercial drivers of obesity, could be implemented to reduce risk in many countries. The higher social contact observed in the three LMIC regions provides potential insights for HIC on how to influence this risk factor for dementia. 30 We could not consider other risk factors such as poor health in pregnancy of malnourished mothers, difficult births, early life malnutrition, survival with heavy infection burdens alongside malaria and HIV, all of which might add to the risks in LMIC.
Diabetes is very common and cigarette smoking is rising in China while falling in most HIC. 31 A meta-analysis found variation of the rates of dementia within China, with a higher prevalence in the north and lower prevalence in central China, estimating 9·5 million people are living with dementia, whereas a slightly later synthesis estimated a higher prevalence of around 11 million. 30 , 32 These data highlight the need for more focused work in LMIC for more accurate estimates of risk and interventions tailored to each setting.
Specific potentially modifiable risk factors for dementia
Risk factors in early life (education), midlife (hypertension, obesity, hearing loss, traumatic brain injury, and alcohol misuse) and later life (smoking, depression, physical inactivity, social isolation, diabetes, and air pollution) can contribute to increased dementia risk ( table 1 ). Good evidence exists for all these risk factors although some late-life factors, such as depression, possibly have a bidirectional impact and are also part of the dementia prodrome. 33 , 34
PAF for 12 dementia risk factors
Data are relative risk (95% CI) or %. Overall weighted PAF=39·7%. PAF=population attributable fraction.
In the next section, we briefly describe relevant newly published and illustrative research studies that add to the 2017 Commission's evidence base, including risks and, for some, mitigation. We have chosen studies that are large and representative of the populations, or smaller studies in areas where very little evidence exists. We discuss them in life-course order and within the life course in the order of magnitude of population attributable factor.
Education and midlife and late-life cognitive stimulation
Education level reached.
Higher childhood education levels and lifelong higher educational attainment reduce dementia risk. 2 , 35 , 36 , 37 New work suggests overall cognitive ability increases, with education, before reaching a plateau in late adolescence, when brain reaches greatest plasticity; with relatively few further gains with education after age 20 years. 38 This suggests cognitive stimulation is more important in early life; much of the apparent later effect might be due to people of higher cognitive function seeking out cognitively stimulating activities and education. 38 It is difficult to separate out the specific impact of education from the effect of overall cognitive ability, 38 , 39 and the specific impact of later-life cognitive activity from lifelong cognitive function and activity. 39 , 40
Cognitive maintenance
One large study in China tried to separate cognitive activity in adulthood from activities for those with more education, by considering activities judged to appeal to people of different levels of education. 40 It found people older than 65 years who read, played games, or bet more frequently had reduced risk of dementia (n=15 882, odds ratio [OR]=0·7, 95% CI 0·6–0·8). The study excluded people developing dementia less than 3 years after baseline to reduce reverse causation.
This finding is consistent with small studies of midlife activities which find them associated with better late-life cognition; so for example, in 205 people aged 30–64 years, followed up until 66–88 years, travel, social outings, playing music, art, physical activity, reading, and speaking a second language, were associated with maintaining cognition, independent of education, occupation, late-life activities, and current structural brain health. 41 Similarly, engaging in intellectual activity as adults, particularly problem solving, for 498 people born in 1936, was associated with cognitive ability acquisition, although not the speed of decline. 42
Cognitive decline
The use it or lose it hypothesis suggests that mental activity, in general, might improve cognitive function. People in more cognitively demanding jobs tend to show less cognitive deterioration before, and sometimes after retirement than those in less demanding jobs. 43 , 44 One systematic review of retirement and cognitive decline found conflicting evidence. 45 Subsequently, a 12-year study of 1658 people found older retirement age but not number of years working, was associated with lower dementia risk. 46 Those retiring because of ill health had lower verbal memory and fluency scores than those retiring for other reasons. 47 Another study found a two-fold increase in episodic memory loss attributable to retirement (n=18 575, mean age 66 years), compared to non-retirees, adjusting for health, age, sex, and wealth. 48 Similarly, in a cohort of 3433 people retiring at a mean age of 61 years, verbal memory declined 38% (95% CI 22–60) faster than before retirement. 44 In countries with younger compared to higher retirement ages, average cognitive performance drops more. 49
Cognitive interventions in normal cognition and mild cognitive impairment
A cognitive intervention or cognition-orientated treatment comprises strategies or skills to improve general or specific areas of cognition. 50 Computerised cognitive training programmes have increasingly replaced tasks that were originally paper-and-pencil format with computer-based tasks for practice and training. 51
Three systematic reviews in the general population found no evidence of generalised cognition improvement from specific cognitive interventions, including computerised cognitive training, although the domain trained might improve. 52 , 53 , 54
A meta-analysis of 17 controlled trials of at least 4 hours of computerised cognitive training, (n=351, control n=335) for mild cognitive impairment, found a moderate effect on general cognition post-training (Hedges' g=0·4, 0·2–0·5); 55 however few high quality studies and no long-term high quality evidence about prevention of dementia currently exists. A meta-analysis of 30 trials of computerised, therapy-based and multimodal interventions for mild cognitive impairment found an effect on activities of daily living (d=0·23) and metacognitive outcomes (d=0·30) compared to control. 56 A third systematic review identified five high quality studies, four group-delivered and one by computer, and concluded the evidence for the effects of cognitive training in mild cognitive impairment was insufficient to draw conclusions. 53 A comprehensive, high quality, systematic overview of meta-analyses of cognitive training in healthy older people, those with mild cognitive impairment and those with dementia, found that most were of low standard, were positive and most reached statistical significance but it was unclear whether results were of clinical value because of the poor standard of the studies and heterogeneity of results ( figure 3 ). 51

Pooled results of meta-analyses investigating objective cognitive outcomes of cognition-oriented treatment in older adults with and without cognitive impairment
K represents the number of primary trials included in the analysis. If a review reported several effect sizes within each outcome domain, a composite was created and k denotes the range of the number of primary trials that contributed to the effect estimate. AMSTAR=A MeaSurement Tool to Assess systematic Reviews (max score 16). Adapted from Gavelin et al, 51 by permission of Springer Nature.
In the only randomised controlled trial (RCT) of behavioural activation (221 people) for cognition in amnestic mild cognitive impairment, behavioural activation versus supportive therapy was associated with a decreased 2-year incidence of memory decline (relative risk [RR] 0·12, 0·02–0·74). 57
Hearing impairment
Hearing loss had the highest PAF for dementia in our first report, using a meta-analysis of studies of people with normal baseline cognition and hearing loss present at a threshold of 25 dB, which is the WHO threshold for hearing loss. In the 2017 Commission, we found an RR of 1·9 for dementia in populations followed up over 9–17 years, with the long follow-up times making reverse causation bias unlikely. 2 A subsequent meta-analysis using the same three prospective studies measuring hearing using audiometry at baseline, found an increased risk of dementia (OR 1·3, 95% CI 1·0–1·6) per 10 dB of worsening of hearing loss. 58 A cross-sectional study of 6451 individuals designed to be representative of the US population, with a mean age of 59·4 years, found a decrease in cognition with every 10 dB reduction in hearing, which continued to below the clinical threshold so that subclinical levels of hearing impairment (below 25 dB) were significantly related to lower cognition. 59
Although the aetiology still needs further clarification, a small US prospective cohort study of 194 adults without baseline cognitive impairment, (baseline mean age 54·5 years), and at least two brain MRIs, with a mean of 19 years follow-up, found that midlife hearing impairment measured by audiometry, is associated with steeper temporal lobe volume loss, including in the hippocampus and entorhinal cortex. 60
Hearing aids
A 25-year prospective study of 3777 people aged 65 years or older found increased dementia incidence in those with self-reported hearing problems except in those using hearing aids. 61 Similarly, a cross–sectional study found hearing loss was only associated with worse cognition in those not using hearing aids. 62 A US nationally representative survey of 2040 people older than 50 years, tested every two years for 18 years, found immediate and delayed recall deteriorated less after initiation of hearing aid use, adjusting for other risk factors. 63 Hearing aid use was the largest factor protecting from decline (regression coefficient β for higher episodic memory 1·53; p<0·001) adjusting for protective and harmful factors. The long follow-up times in these prospective studies suggest hearing aid use is protective, rather than the possibility that those developing dementia are less likely to use hearing aids. Hearing loss might result in cognitive decline through reduced cognitive stimulation.
Traumatic brain injury (TBI)
The International Classification of Disease (ICD) defines mild TBI as concussion and severe TBI as skull fracture, oedema, brain injury or bleed. Single, severe TBI is associated in humans, and mouse models, with widespread hyperphosphorylated tau pathology, and mice with APOE ε4 compared to APOE ε3 allele have more hippocampal hyper-phosphorylated tau after TBI. 64 , 65 TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. 66 A nationwide Danish cohort study of nearly 3 million people aged 50 years or older, followed for a mean of 10 years, found an increased dementia (HR 1·2, 95% CI 1·2–1·3) and Alzheimer's disease risk (1·2, 1·1–1·2). 67 Dementia risk was highest in the 6 months after TBI (4·1, 3·8–4·3) and increased with number of injuries in people with TBI (one TBI 1·2, 1·2–1·3; ≥5 TBIs 2·8, 2·1–3·8). Risk was higher for TBI than fractures in other body areas (1·3, 1·3–1·3) and remained elevated after excluding those who developed dementia within 2 years after TBI, to reduce reverse causation bias. 67
Similarly, a Swedish cohort of over 3 million people aged 50 years or older, found TBI increased 1-year dementia risk (OR 3·5, 95% CI 3·2–3·8); and risk remained elevated, albeit attenuated over 30 years (1·3, 1·1–1·4). 68 ICD defined single mild TBI increased the risk of dementia less than severe TBI and multiple TBIs increased the risk further (OR 1·6, 95% CI 1·6–1·7 for single TBI; 2·1, 2·0–2·2 for more severe TBI; and 2·8, 2·5–3·2 for multiple TBI). A nested case control study of early onset clinically diagnosed Alzheimer's disease within an established cohort also found TBI was a risk factor, increasing with number and severity. 69 A stronger risk of dementia was found nearer the time of the TBI, leading to some people with early-onset Alzheimer's disease.
Military veterans have a high risk of occupational TBI, and formal record keeping allows long-term follow-up. A study of 178 779 veterans with TBI with propensity-matched veterans without TBI found dementia risk was associated with TBI severity (HR 2·4, 95% CI 2·1–2·7 for mild TBI without loss of consciousness; 2·5, 2·3–2·8 for mild TBI with loss of consciousness; and 3·8, 3·6–3·9 for moderate to severe TBI). 70 Similarly women veterans with TBI had increased risk of dementia compared to those without TBI (1·5, 1·0–2·2). 71
A cohort study of 28 815 older adults with concussion, found the risk of dementia doubled, with 1 in 6 developing dementia over a mean follow-up of 3·9 years, although those taking statins had a 13% reduced risk of dementia compared to those who were statin-free. They suggest future RCTs as statins might mitigate injury-related brain oedema, oxidative stress, amyloid protein aggregation, and neuroinflammation. 72
The term chronic traumatic encephalopathy describes sports head injury, which is not yet fully characterised and covers a broad range of neuropathologies and outcomes, with current views largely conjecture. 73 The evidence has subsequently been strengthened by a study on Scottish former soccer players reporting that they are more likely than controls to have Alzheimer's disease specified on their death certificates (HR 5·1, 95% CI 2·9–8·8) and to have been prescribed any dementia-related medications (OR 4·9, 95% CI 3·8–6·3) but not on medical records. 74 The study controlled for socio-economic class based on residential address, which in footballers might be less linked to level of education.
Hypertension
Persistent midlife hypertension is associated with increased risk of a late life dementia. In the Framingham Offspring cohort comprising 1440 people, elevated systolic blood pressure (≥140 mm Hg in midlife; mean age 55 years) was associated with an increased risk of developing dementia (HR 1·6, 95% CI 1·1–2·4) over an 18 year follow-up period. 12 In this study risk increased further if hypertension persisted into later life (mean age 69 years; HR 2·0, 95% CI 1·3–3·1). In the same cohort, people in late midlife (mean age 62 years) with ideal cardiovascular parameters (current non-smoker, body mass index [BMI] 18·5–25 kg/m 2 , regular physical activity, healthy diet, optimum blood pressure <120/<80 mm Hg, cholesterol, and normal fasting blood glucose) were compared to people with at least one of these risks. 75 Those with ideal cardiovascular parameters had a lower 10-year risk of all-cause dementia (HR 0·8, 95% CI 0·1–1·0), vascular dementia (0·5, 0·3–0·8) and clinically diagnosed Alzheimer's disease (0·8, 0·6–1·0). In a UK cohort study of 8639 civil servants, a single measure of systolic blood pressure of 130 mm Hg or higher at age 50 years but not at age 60 or 70 years was associated with increased risk of dementia (1·4, 1·1–1·7). 13 In those with persistent systolic blood pressure of 130 mm Hg or higher, from mean age 45 to 61 years, dementia risk is increased even if free of cardiovascular disease relative to those without hypertension (1·3, 1·0–1·7).
A further cohort study has provided potential insights into mechanisms, reporting that midlife hypertension, defined as from age 40 years, was associated with reduced brain volumes and increased white matter hyperintensity volume but not amyloid deposition. 76 Of note, blood pressure declines in later life and this decline is associated with and, potentially caused by, dementia development (HR 2·4, 95% CI 1·4–4·2). 12 , 13 , 77
Antihypertensive drugs, aspirin, and statins
The US and Puerto Rico Systolic Blood Pressure Intervention Trial (SPRINT) in 9361 hypertensive adults aged 50 years and older, was stopped early because of significantly fewer cardiovascular events and deaths occurring in the intensive treatment arm (aiming for systolic <120 mm Hg, n=4678) in comparison with standard treatment (systolic <140 mm Hg, n=4683). 78 Cognitive assessment continued after stopping the trial intervention in SPRINT MIND. 79 In the intensive compared with the standard treatment group, there were 7·2 dementia cases as opposed to 8·6 cases/1000 person-years (HR 0·8; 95% CI 0·7–1·0) within on average 2 years from the end of the intervention period and 5 years after baseline. Pre-specified secondary outcomes were also reduced in the intensive arm for mild cognitive impairment (14·6 vs 18·3 cases/1000 person-years; HR 0·8, 95% CI 0·7–1·0), combined mild cognitive impairment or dementia (20·2 vs 24·1 cases/1000 person-years; HR 0·9, 95% CI 0·7–1·0) 79 making this the first trial to suggest reduction of risk for mild cognitive impairment. Those who were lost to follow-up were at greater risk of dementia than those who continued but follow-up rates did not differ according to intervention group. 80
Four meta-analyses of blood pressure medications to lower high blood pressure with six studies overlap have provided combined estimates of effects. All meta-analyses suggest reduced dementia in those in the interventions arms for outcomes of any dementia as well as clinically diagnosed Alzheimer's disease. The first included randomised controlled trials (RCTs) of any drug to lower blood pressure and reported a reduction in risk of around 10% at marginal significance (RR 0·9, 95% CI 0·9–1·0). 81 Meta-regression showed risk lowered more if the achieved systolic pressure differential was larger between the intervention and control group. The second included 15 trials and observational studies of diuretics involving 52 599 people (median age 76 years) with 6·1 years median follow-up (dementia HR 0·8, 95% CI 0·8–0·9 and Alzheimer's disease 0·8, 0·7–0·9). 82 The third included used individual participant data from six observational studies; (dementia 0·9, 0·8–1·0 and Alzheimer's disease 0·8, 0·7–1·0; figure 4 ). 83 The fourth focused on people prescribed calcium channel blocker only, included 10 RCTs and observational studies comprising 75 239 hypertensive older adults (median age 72 years, median follow-up 8·2 years) found lowered dementia risk (RR 0·7, 95% CI 0·6–0·9). 84 A 2019 meta-analysis addressing which class of anti-hypertensive drug to use to lower risk of either incident dementia or cognitive decline, found over 50 000 participants in 27 studies and reported no consistent difference in effect according to which class of drug was used. 85

Associations of antihypertensive medication use with incident dementia in those with high blood pressure
Adapted from Ding et al, 83 by permission of Elsevier.
A Cochrane review reported good evidence that statins given to older people at risk of vascular disease do not prevent cognitive decline or dementia. 86 One RCT found 100 mg aspirin versus placebo in 19 114 healthy adults older than 65 years did not reduce dementia (HR 1·0, 95% CI 0·8–1·2), death, physical disability, or cardiovascular disease over a period of 4·7 years. 87
Physical inactivity, exercise, and fitness
Studies of physical activity are complex. Patterns of physical activity change with age, generation, and morbidity and are different across sex, social class, and cultures. The studies suggest a complicated relationship with the potential for both risk reduction and reverse causation.
Meta-analyses of longitudinal observational studies of 1–21 years duration showed exercise to be associated with reduced risk of dementia. 2 A further overview of systematic reviews concluded that there is convincing evidence for physical activity protecting against clinically diagnosed Alzheimer's disease. 88
Since the 2017 Commission, the HUNT study of 28 916 participants aged 30–60 years has been published, reinforcing the previous literature in this area. At least weekly midlife moderate-to-vigorous physical activity (breaking into a sweat) was associated with reduced dementia risk over a 25-year period of follow-up (HR 0·8, 95% CI 0·6–1·1) but the confidence intervals were wide. 89 In contrast the Whitehall Study reporting on the 28-year follow-up of 10 308 people, found that more than 2·5 hours of self-reported moderate-to-vigorous physical activity per week, lowered dementia risk over 10, but not 28 years. 33 Very long-term studies are unusual; however, one 44-year study recruited 191 women (mean age 50) purposively to be representative of the Swedish population and reported that 32% of the participants with low baseline peak fitness, 25% with medium, and 5% with high fitness developed dementia (high vs medium HR 0·1, 95% CI 0·03–0·5, low vs medium 1·4, 0·7–2·8). 90
An individual-level meta-analysis of 19 observational studies of relatively younger adults included 404 840 participants' data (mean baseline age 45·5 years; mean follow-up duration 14·9 years), reporting an increased incidence of all-cause dementia (HR 1·4, 95% CI 1·2–1·7) and clinically diagnosed Alzheimer's disease (1·4, 1·1–1·7) in those who were physically inactive in the 10-year period before diagnosis. 91 Notably, however, no difference in dementia risk measured 10–15 years before time of dementia incidence was found except in those with comorbid cardio-metabolic disease (RR 1·3, 95% CI 0·8–2·1).
People might stop exercising due to prodromal dementia so inactivity might be either a consequence or a cause or both in dementia and might be more of a risk in those with cardiovascular morbidity. As with other outcomes, exercise might be required to be sustained and continue nearer the time of risk. 92
Trials of exercise
Since the 2017 Commission several meta-analyses and systematic reviews have been published with three high quality meta-analyses which we include. The first included 39 RCTs with an unclear total number of participants examining moderate or vigorous exercise of any frequency lasting 45–60 min per session in cognitively normal adults aged older than 50 years. This analysis reported global cognitive improvements (standard mean difference [SMD]=0·3, 95% CI 0·2–0·4) for moderate or vigorous resistance (13 studies) or aerobic exercise (18 studies) lasting 45–60 min per session with no difference between them but no effect found for yoga. 93 A second meta-analysis of RCTs in people with mild cognitive impairment found global cognition improved in the intervention group (0·3, 0·1–0·5) with aerobic exercise having a higher effect (0·6, 0·5–0·6). 94 This study did not have dementia as an outcome measure. A third meta-analysis of RCTs of longer term exercise found five studies (four lasting 12 months and one 24 months) with 2878 participants with normal baseline cognition. 95 The incidence of dementia was 3·7% (n=949) for exercisers and 6·1% (n=1017) for controls (random effect RR 0·6, 95% CI 0·3–1·1; fixed effect as no evidence of heterogeneity 0·7, 0·4–1·0). The authors concluded that the study showed no significant effect of exercise for reducing dementia, mild cognitive impairment, or clinically significant cognitive decline but was underpowered. WHO guidelines have been published since the 2017 Commission, suggesting specific activity levels drawing on these, and one further systematic review which considered sex differences on the effect of exercise. 96 , 97 It concluded the evidence points towards physical activity having a small, beneficial effect on normal cognition, with a possible effect in mild cognitive impairment, mostly due to aerobic exercise. 97 Evidence about the effect of specific types of exercise, such as progressive muscle resistance training, on dementia risk is scarce.
In the 2017 Commission we reported on diabetes as a risk factor for dementia. Distinguishing between treated and untreated diabetes as a risk factor for dementia is challenging in observational studies. In a pooled meta-analysis from over 2·3 million individuals with type 2 diabetes across 14 cohort studies, including 102 174 with dementia, diabetes was associated with an increased risk of any dementia (RR 1·6, 95% CI 1·5–1·8 for women and 1·6, 1·4–1·8 for men). 98 The risk of dementia increased with the duration and severity of diabetes. The effect of different diabetic medications on cognition or dementia outcomes remains unclear as few studies have investigated this area. 99 However, one meta-analysis of cohort studies of diabetes reported that, cross sectionally, people with diabetes taking metformin had lower prevalence of cognitive impairment (three studies OR 0·6, 95% CI 0·4–0·8) and, longitudinally, reduced dementia incidence (six studies HR 0·8, 95% CI 0·4–0·9) compared with those taking other medications or no medication. 100 However another analysis did not find a protective effect of metformin for incident dementia (three studies, RR 1·1, 95% CI 0·5–2·4) with possible harm with insulin therapy (1·2, 1·1–1·4); but this did not account for severity of diabetes of those with type 2 diabetes on insulin. 99 A Cochrane review reported intensive compared to standard diabetes control trials with 5 year follow up (n=11 140), showing no impact on cognitive decline (1·0, 95% CI 0·9–1·1) or dementia (1·3, 0·9–1·9). 101
Overall type 2 diabetes is a clear risk factor for development of future dementia; however, whether any particular medication ameliorates this risk is unclear. Intensive diabetic control does not decrease the risk of dementia.
Combined cardiovascular risk factors
Studies of individual cardiovascular risk factors usually control for other cardiovascular risks, which cluster in individual people. This does not take into account the combinations and contexts in which risk occurs. A UK study of 7899 people aged 50 years followed up for 25 years, calculated a cardiovascular health score based on four behaviour-related (smoking, diet, physical activity, BMI) and three biological (fasting glucose, blood cholesterol, blood pressure) metrics each coded on a three-point scale (0, 1, 2). 100 A better score was associated with a lower risk of dementia (HR 0·9, 95% CI 0·9–1·0 per 1 point scale increment), for both behaviour-related (HR/1 point increment in subscales 0·9, 95% CI 0·8–0·9) and biological subscales (0·9, 0·8–1·0), maintained in people free of cardiovascular disease over the follow-up (0·9, 95% CI 0·8–1·0). These authors also reported an association of the score on the scale with hippocampal atrophy and total brain volume but not white matter hyperintensities. This finding underlines the importance of clustering of cardiovascular risk factors in midlife, as studies of individual risk factors in this sample had not shown a significant association, when controlling for other individual risks. 33
Excessive alcohol consumption
Heavy drinking is associated with brain changes, cognitive impairment, and dementia, a risk known for centuries. 102 An increasing body of evidence is emerging on alcohol's complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record based studies. Alcohol is strongly associated with cultural patterns and other sociocultural and health-related factors, making it particularly challenging to understand the evidence base.
A French 5-year longitudinal study of over 31 million people admitted to hospital, found alcohol use disorders (harmful use or dependence as defined in ICD) were associated with increased dementia risk, calculated separately for men and women (women HR 3·3, 95% CI 3·3–3·4, men 3·4, 3·3–3·4). 103 The relationship of dementia with alcohol use disorders was particularly clear in the earlier onset dementias (age less than 65 years) in which 56·6% had an alcohol use disorder noted in their records (n=57 353; 5·2% all dementias).
A systematic review incorporating 45 studies of light to moderate drinking using a variety of definitions reported a reduced risk of dementia compared with not drinking (RR 0·7; 95% CI 0·6–0·91). 104 Risk was not reported separately for men and women. Drinking less than 21 units of alcohol per week (1 unit of alcohol=10 mL or 8 g pure alcohol) might be associated with a lower risk of dementia. 105 , 106 A 5-year follow-up study of 13 342 men and women volunteers from UK biobank aged 40–73 years who drank, included few heavy drinkers and did not analyse abstainers. 106 The study reported that those who drank more than 12 units per week declined slightly more in reaction time in a perceptual matching task than those who drank less (β2=−0·07, 95% CI −0·09 to −0·04). 106 The UK Whitehall study with 23 years follow-up, included 9087 participants aged 35–55 years at baseline. 107 Drinking more than 21 units per week and long-term abstinence were both associated with a 17% (95% CI 4–32 and 13–23 respectively) increase in dementia compared to drinking less than 14 units. Drinking more than 14 units was also associated with right sided hippocampal atrophy on MRI. 108
Weight control and obesity
Overweight is an emerging concern, given the changing BMI across the world's ageing population. New evidence supports the relationship between increased BMI and dementia from a review of 19 longitudinal studies including 589 649 people aged 35 to 65 years, followed up for up to 42 years. It reported obesity (BMI ≥30; RR 1·3, 95% CI 1·1–1·6) but not being overweight (BMI 25–30; 1·1, 1·0–1·2) was associated with late-life dementia. 109 In a further meta-analysis of individual level data from 1·3 million adults (aged ≥18 years), which included two studies from the meta-analysis cited above, 109 higher body mass measured before probable preclinical and prodromal dementia was associated with increased dementia risk (RR 1·3, 1·1–1·7/5-unit increase in BMI). 11
Weight loss in midlife and dementia risk
A meta-analysis of seven RCTs (468 participants) and 13 longitudinal studies (551 participants) of overweight and obese adults without dementia, mean age 50 years, found weight loss of 2 kg or more in people with BMI greater than 25 was associated with a significant improvement in attention and memory. All but one of the studies included participants aged younger than 65 years. The RCTs reported memory improvement over 8–48 weeks (SMD=0·4, 95% CI 0·2–0·6) and short-term longitudinal studies found improvement over a median of 24 weeks (SMD=0·7, 95% CI 0·5–0·8); however, data about the long-term effects or the effect of weight loss in preventing dementia are absent. 110
Smokers are at higher risk of dementia than non-smokers, 2 and at a higher risk of premature death before the age at which they might have developed dementia, introducing some bias and uncertainty in the association between smoking and risk of dementia. 111 , 112 Stopping smoking, even when older, reduces this risk. Among 50 000 men aged older than 60 years, stopping smoking for more than 4 years, compared to continuing, substantially reduced dementia risk over the subsequent 8 years (HR 0·9; 95% CI 0·7–1·0). 113 Worldwide, 35% of non-smoking adults and 40% of children are estimated to be exposed to second-hand smoke; 114 although literature on the impact of this exposure and dementia risk is scarce. One study indicated that in women aged 55–64 years, second-hand smoke exposure was associated with more memory deterioration and the risk increased with exposure duration even after controlling for other confounding factors. 115
Depression is associated with dementia incidence, with a variety of possible psychological or physiological mechanisms. It is also part of the prodrome and early stages of dementia. Reverse causation is possible whereby depressive symptoms result from dementia neuropathology that occurs years before clinical dementia onset. These explanations are not mutually exclusive. As in diabetes, few studies considering depression as a risk factor for dementia have distinguished between treated and untreated depression. In a meta-analysis of 32 studies, with 62 598 participants, with follow-up from 2 to 17 years, a depressive episode was a risk factor for dementia (pooled effect size 2·0, 95% CI 1·7–2·3). 116 Meta-regression analysis revealed a non-significant trend for the association between depression and incident dementia to be weaker when the length of follow-up was longer. The Norwegian HUNT study, suggested that symptoms of psychological distress predicted dementia 25 years later however with wide bounds of uncertainty (HR 1·3, 95% CI 1·0–1·7). 89 Two further studies differentiate between late-life and earlier life depressive symptoms. The UK Whitehall study, in a follow-up of 10 189 people, reports that in late life these symptoms increase dementia risk but not at younger ages (follow-up 11 years HR 1·7; 95% CI 1·2–2·4; follow-up 22 years 1·0, 0·7–1·4). 34 , 117 A 14-year longitudinal study of 4922 initially cognitively healthy men, aged 71–89 years, found depression was associated with 1·5 (95% CI 1·2- 2·0) times the incidence of dementia but this association was accounted for by people developing dementia within 5 years of depression. 118 The use of antidepressants did not decrease this risk.
A study of 755 people with mild cognitive impairment and with a history of depression from the Australian longitudinal Alzheimer's Disease Neuroimaging Initiative, considered the effect of selective serotonin-reuptake inhibitor (SSRI) treatment, such as citalopram, known to reduce amyloid plaque generation and plaque formation in animal models. 119 The study found that more than 4 years of such treatment was associated with delayed progression to clinically diagnosed Alzheimer's disease. People treated with antidepressants seem likely to differ from those who are not treated. Thus, the question of whether antidepressant treatment mitigates dementia risk remains open.
Social contact
Social contact, now an accepted protective factor, enhances cognitive reserve or encourages beneficial behaviours, although isolation might also occur as part of the dementia prodrome. Several studies suggest that less social contact increases the risk of dementia. Although most people in mid and later life are married, by the time they reach older age, disproportionate numbers of women are widowed as they outlive their husbands, thus reducing their social contact. In these generations, marital status is therefore an important contributor to social engagement. Additionally, most marriages are in the relatively young, and married people usually have more interpersonal contact than do single people—this gives a long-term estimate of the effect of social contact. A systematic review and meta-analysis including 812 047 people worldwide found dementia risk to be elevated in lifelong single (RR 1·4, 95% CI 1·1–1·9) and widowed people (1·2, 1·0–1·4), compared with married people and the association was consistent in different sociocultural settings. 120 Studies adjusted for sex and we do not know if a differential risk between men and women exists. Differences persisted in studies that adjusted for education and physical health so might be attributable to married people having more social contact, rather than solely because they tend to have better physical health and more education, although residual confounding is possible. A systematic review and meta-analysis of 51 longitudinal cohort studies of social isolation and cognition included 102 035 participants aged 50 or more years at baseline, with follow-up of 2–21 years. 121 High social contact (measured through either or both of social activity and social network) was associated with better late-life cognitive function (r=0·05, 95% CI: 0·04–0·065) and no differences according to sex or length of time followed up.
A new meta-analysis found that in long-term studies (≥10 years), good social engagement was modestly protective (n=8876, RR=0·9, 95% CI 0·8–1·0); but loneliness was not associated with dementia risk. 122 No long term (>10 years) studies of loneliness and dementia outcomes have been done.
A UK 28-year follow-up study of 10 308 people found that more frequent social contact at age 60 years was associated with lower dementia risk over 15 years of follow-up (HR for one standard deviation social contact frequency 0·9, 95% CI 0·8–1·0). This finding suggests more frequent social contact during late middle age is associated with a modest reduction in dementia risk, independent of socio-economic and other lifestyle factors. 123 A Japanese longitudinal cohort study of 13 984 adults aged older than 65 years with a mean of 10 years follow-up calculated a five-point social contact scale based on: marital status; exchanging support with family members; having contact with friends; participating in community groups; and engaging in paid work. It found the score to be linearly associated with reduced dementia risk; those who scored highest on the five-point scale were 46% less likely to develop incident dementia compared with those in the lowest category. 124
Despite clear cultural variation in the meaning and perception of social isolation, findings of protective effect of more social contact are largely consistent in different settings and for either sex across the studies and meta-analyses. 118 , 120 , 121
Social interventions
Little evidence of the effects of social interventions on dementia exists but a systematic review of low quality RCTs of 576 adults aged 60 or more years with normal cognition found facilitated meeting and discussion groups were associated with improved global cognition and increased brain volume at follow-up. 118
Air pollutants
Air pollution and particulate pollutants are associated with poor health outcomes, including those related to non-communicable diseases. Attention has turned to their potential effect on the brain. Animal models suggest airborne particulate pollutants accelerate neurodegenerative processes through cerebrovascular and cardiovascular disease, Aβ deposition, and amyloid precursor protein processing. 125 , 126 Although the higher levels of dementia from air pollutants are still subject to the potential for residual confounding, the effects on animal models are evidence of physiological effects over and above those driven by life-course deprivation.
High nitrogen dioxide (NO 2 ) concentration (>41·5 μg/m 3 ; adjusted HR 1·2, 95% CI 1·0–1·3), fine ambient particulate matter (PM) 2·5 from traffic exhaust (1·1, 1·0–1·2) 127 , 128 , 129 and PM 2·5 from residential wood burning (HR=1·6, 95% CI 1·0–2·4 for a 1 μg/m 3 increase) are associated with increased dementia incidence. Traffic often produces NO 2 and PM 2·5 and it is hard to separate their effects, although evidence for additive effects of different pollutants exists. 127 , 128 , 129 A systematic review of studies until 2018 including 13 longitudinal studies with 1–15 years follow-up of air pollutants exposure and incident dementia, found exposure to PM 2·5, NO 2 , and carbon monoxide were all associated with increased dementia risk. 24 The attributable burden of dementia and excess death from PM 2·5 in one large 10-year US study was particularly high in Black or African American individuals and socio-economically disadvantaged communities and related to particulate PM 2·5 concentrations above the US guidelines. 130
Mechanisms by which sleep might affect dementia remain unclear, but sleep disturbance has been linked with β-amyloid (Aβ) deposition, 131 , 132 reduced glymphatic clearance pathways activation, 133 low grade inflammation, increased Tau, hypoxia 132 , 134 and cardiovascular disease. 135 Sleep disturbance is hypothesised to increase inflammation which raises Aβ burden, leading to Alzheimer's disease and further sleep disturbance. 136
Two meta-analyses showed similar findings. The first was a synthesis of longitudinal studies with an average of 9·5 years follow-up and the second reported cross-sectional and prospective cohort studies of mixed quality with different methods of measuring sleep. Sleep disturbances were defined broadly, often self-reported and including short and long sleep duration, poor sleep quality, circadian rhythm abnormality, insomnia, and obstructive sleep apnoea. All these disturbances were associated with a higher risk of all-cause dementia (RR 1·2; 95% CI 1·1–1·3) 137 and clinically diagnosed Alzheimer's disease (1·6, 1·3–1·9) compared with no sleep disturbance, although not all cohort studies excluded those with cognitive impairment or dementia at baseline from their analyses. 138 A U-shaped association has been reported between sleep duration and risk of mild cognitive impairment or dementia with higher risks of dementia with less than 5 hours (HR=2·6; 95% CI 1·4–5·1) compared with more than 5 and less than 7 and more than 10 hours sleep (2·2, 1·4–3·5) and risks for all-cause dementia and clinically diagnosed Alzheimer's disease being similar. 135 , 139 , 140 , 141
The postulated mechanisms of reduced sleep leading to accumulation of Alzheimer's type pathology is inconsistent with the evidence that both more sleep and less sleep are associated with increased risk of dementia. New onset late-life sleep disturbance, a few years before clinical dementia, might be part of the natural history of the dementia syndrome, appearing to be a risk factor, or reflect other disorders, for example, mood disturbances or cardiovascular disease. 135 , 142 Hypnotic use might increase risks although this is unclear and a 2018 study 139 suggests that findings of a connection were related to reverse causality and confounders. 143 When benzodiazepine use was considered, in one study, sleep length was no longer significant 139 but not in all studies. 135 Those taking hypnotics were at greater risk of dementia than those who did not regardless of sleep duration. 139 Medication for sleep disturbance might be harmful and benzodiazepines are associated with falls, hospital admissions, and possibly dementia. 139 , 144
Nutrition and dietary components are challenging to research with controversies still raging around the role of many micronutrients and health outcomes in dementia. Observational studies have focused on individual components ranging from folate and B vitamins, Vitamin C, D, E, and selenium amongst others as potential protective factors. 88 There has been a move towards considering the evidence base for whole diets in the last 5 years, particularly high plant intake such as in the Mediterranean diet (high intake of vegetables, legumes, fruits, nuts, cereals, and olive oil; low intake of saturated lipids and meat) or the similar Nordic diet, rather than individual nutrients, which might reduce cognitive decline and dementia. 145 One example is a longitudinal cohort study of 960 participants, ages 58–99 years, in which those reporting the highest intake of green leafy vegetables, equivalent to 1·3 servings per day, had less cognitive decline over 4·7 years than those reporting the lowest intake (β=0·05 standardised units 95% CI 0·02–0·07). 146 The authors report this difference as being equivalent to being 11 years younger. A further prospective cohort study with three midlife dietary assessments in 8255 people, followed up for a mean of nearly 25 years, found neither healthy dietary pattern nor Mediterranean diet protected from dementia, except in those with cardiovascular disease, suggesting that diet might influence dementia risk by protecting from the excess risk of cardiovascular risk factors. 147
Dietary interventions
As well as whole diets, there has been some interest in multi-nutrient interventions. A systematic review and a Cochrane review including RCTs of supplements (A, B, C, D, and E; calcium, zinc, copper, and multivitamins trials, n-3 fatty acids, antioxidant vitamins, and herbs) found a lack of evidence for supplement use to preserve cognitive function or prevent dementia in middle-aged (45–64 years) or older people (aged 65 years and older). 148 , 149 Cochrane reviews found no evidence for beneficial effects on cognition of those with mild cognitive impairment of supplementation with B vitamins for 6 to 24 months 150 or with vitamin E in preventing progression from mild cognitive impairment to dementia. 151 A 24-month RCT of 311 people of a multi-nutrient drink containing docosahexaenoic acid, vitamins B12, B6, folic acid, and other nutrients; found no significant effect on preventing cognitive deterioration in prodromal Alzheimer's disease. 152 The authors comment that the control group's cognitive decline was much lower than expected, leading to an inadequately powered trial.
Meta-analysis of two RCTs with 471 participants with normal cognition found the Mediterranean diet improved global cognition compared to controls (SMD 0·2, 95% CI 0·0–0·4). 153 A further meta-analysis identified five RCTs (n=1888) with a weak effect on global cognition (SMD 0·2, 95% Cl 0·0–0·5) 154 but no benefit of Mediterranean diet for incident cognitive impairment or dementia.
The WHO guidelines recommend a Mediterranean diet to reduce the risk of cognitive decline or dementia, as it might help and does not harm, but conclude Vitamins B and E, polyunsaturated fatty acid, and multicomplex supplementation should not be recommended. 97
Trials of combination strategies to prevent dementia
The FINGER RCT was a 2-year multidomain intervention to prevent cognitive decline and dementia in 1260 people with cardiovascular risk factors aged 60–77 years, recruited from a Finnish national survey. Similar multidomain studies were discussed in the 2017 Commission. 2 FINGER found a small group reduction in cognitive decline in the intervention group compared with control (comprehensive neuropsychological test battery Z score 0·02, 95% Cl 0·00–0·04) regardless of baseline sociodemographic, socio-economic, cognitive, or cardiovascular status. 155 However, in a subgroup analysis, greater beneficial effects were observed on processing speed in individuals with higher baseline cortical thickness in Alzheimer's disease areas. 156
The Healthy Ageing Through Internet Counselling in the Elderly (HATICE) study recruited 2724 older people (≥65 years) in the Netherlands, Finland, and France with two or more cardiovascular risk factors. 157 , 158 It compared an interactive internet platform plus remote support by a coach, aiming to improve self-management of vascular risk factors, with a non-interactive control platform with basic health information. A small improvement in the cardiovascular risk composite primary outcome was observed in the intervention group compared with the control group at 18 months, mainly through weight loss, and the dementia risk score was slightly lower in those who received the intervention (mean difference −0·15, 95% CI −0·3 to −0·0). A larger effect was observed in the younger age group (65–70 years) and those with the lowest level of education, who had a higher baseline risk, suggesting that targeting high-risk populations might be more effective. Several multidomain preventive trials are ongoing—for example, World Wide FINGERS .
Total PAF calculation
We incorporated excessive alcohol consumption, TBI, and air pollution into our life-course model of dementia, as well as the original nine risk factors, because of the updated evidence. To calculate new RRs for excessive alcohol consumption, TBI and air pollution, we systematically reviewed the literature and did new meta-analyses for excessive alcohol consumption and TBI. For the other nine factors, we used values for RR and risk factors prevalence from our previous analysis and calculated communality using the same method as in the 2017 Commission. 2
PAF calculation
We used a representative sample of over 10 000 UK community-dwelling adults, to calculate communality (clustering of risk factors) of 11 risk factors for which data existed, 159 to allow calculation of each factor's unique risk. As we could find no datasets measuring TBI, with the other 11 risk factors of interest, we could not calculate its communality. We therefore used the mean of the other 11 communalities to calculate a weighted PAF, so we could include TBI. We used cohabitation as a proxy measure for social contact, and urbanicity for air pollution exposure. Our analysis found four principal components, explaining 55% of the total variance between the eleven risk factors, suggesting substantial overlap. The appendix (p 2) shows the PAF formula and the steps in calculating communality and we detail our new meta-analyses next, which we used to update the figure and perform our new calculations.
Incorporation of the new chosen risks in new systematic reviews
We searched, from inception to Oct 29, 2019, Embase, Allied, and Complementary Medicine, MEDLINE, and PsycINFO terms “dementia” OR “dement*” OR “AD” OR “VaD”, “Alzheimer*” AND “alcohol” OR “ethanol” OR “alcohol*” OR “drink*” OR “drunk*” to update an earlier review. 160 We used inclusion criteria: original population-based cohort studies measuring drinking during midlife, as alcohol intake tends to fall with age; 161 alcohol consumption quantified at baseline by units or number of drinks (one drink, 1·5 units) per week; and all-cause dementia ascertained at follow-up using validated clinical measures. We contacted authors for additional data. 162 Three studies met our inclusion criteria. 107 , 162 , 163 We converted HRs to RRs 164 and used raw data 162 to calculate RR, 165 for our random effects meta-analysis using Generic Inverse Variance Methods. The RR associated with drinking—more than 21 units (168 g) of alcohol weekly—compared with lighter drinking was 1·18 (95% Cl 1·06–1·31; figure 5 ). We used Health Survey England figures for heavier drinking prevalence to calculate PAF as we could not find a worldwide estimate. The weighted PAF was 0·8.

Meta-analysis of relative risk of dementia associated with drinking more than 21 units of alcohol per week in midlife compared to lighter consumption of alcohol
To estimate the RR of TBI of all severities for all cause dementia, we searched Embase, Medline, and PsycINFO from Jan 1, 2016, to Oct 21, 2019, updating an earlier search, 166 using terms (“traumatic brain injury” or “head injury” or “brain injury” or TBI) AND (neurodegeneration or “cognitive dysfunction” or dementia or “Alzheimer's disease” or “Parkinson's disease” or “frontotemporal dementia”). We converted HR figures to RR. 164 , 167 We used inclusion criteria: original population-based cohort studies, baseline TBI of all severities reported, and all-cause dementia ascertained at follow-up using validated clinical measures. We combined four new studies meeting inclusion criteria 67 , 68 , 71 , 168 with the four studies meeting criteria from the original review in a random effects meta-analysis. 166 The pooled RR was 1·84 (95% CI 1·54–2·20) for all cause dementia from all severities of TBI ( figure 6 ) although there was heterogeneity in study-specific estimates, possibly because of different populations. We used the TBI adult population prevalence of 12·1% from a meta-analysis to calculate PAF. 173 The weighted PAF was 3·4.

Meta-analysis of relative risk of all-cause dementia associated with all severity midlife traumatic brain injury
A 2019 systematic review synthesised observational studies, finding consistently increased risk of dementia from air pollution, but heterogeneous comparator groups precluded meta-analysis. 24 We updated the search, using the same search terms and searching MEDLINE, Embase, and PsycINFO from Sept 20, 2018, (the end date of the last search) to Oct 22, 2019. We included longitudinal studies with assessment of all cause air pollution exposure; use of formal assessment of cognitive function at baseline; report of incident all-cause dementia, data from adults (age ≥18 years); and a minimum follow-up of 6 months. As meta-analysis was not possible, we used data from the only study of all-cause air pollution with the outcome of all-cause dementia, with low-moderate risk of bias. This population-based, observational cohort was from Canada, where pollutant concentrations are among the lowest in the world and examined 2 066 639 people, with a mean baseline age of 67 years. 174 We calculated the RR of dementia for those in the three highest quartiles compared to the lowest was 1·09 (1·07–1·11). The attributable fraction for exposure to the highest three quartiles versus the lowest quartile of PM 2·5 and NO 2 was 6·1% (4·8–7·5). The weighted PAF was 2·3.
Table 1 displays the prevalence, communality, relative risk, unweighted and weighted PAFs adjusted for communality. Figure 7 shows the updated life-course model of potentially modifiable risk factors for dementia, including the three new risk factors.

Population attributable fraction of potentially modifiable risk factors for dementia
Strengths and limitations
This Commission is the most comprehensive analysis to date and updates the 2017 Commission with emerging risk factor evidence convincing enough to calculate PAF for potentially reversible risk factors. We reviewed the literature systematically for the chosen risk factors and provided illustrative new literature to update our synthesis and identify data to calculate communality. We find a hopeful picture with an estimate of around 40% of all cases of dementia being associated with 12 potentially modifiable risk factors.
We have made assumptions to calculate this new model. We used global figures for dementia risk although we know the risk factors prevalence varies between countries and most global research is from HIC, so LMIC are under-represented because of lack of data. We have assumed a causal relationship between risk factors and dementia, although we have been cautious and not included risk factors with less good evidence. No single database exists with all 12 risk factors together, but we found 11 of the factors in a UK database and used the mean figure for communality calculations for TBI. We calculated communality for the other 11. We do not know how far findings of communality in other geographical populations might differ, or in those with a differing distribution of age groups or sex. We found that social isolation was not explicitly measured and had to use proxies, such as cohabitation when considering prevalence, which are approximate.
Specifically, evidence for the association of alcohol misuse with dementia comes from HIC and future studies from LMIC are needed to complete the picture. Exposure to air pollution changes over a lifetime and is inextricably linked to poverty and deprivation. However, the effects on animal models suggests specific physiological effects over and above those driven by life-course deprivation. We also considered the overlap with education for this and other risk factors and the correction for education, strongly inversely linked to deprivation, will address at least some of the confounding. However, the results in one study which reported the effect of air pollution on incident dementia showed very little difference in estimates before and after adjustment for education and other risk factors, suggesting little residual confounding exists. 174 We were also unable to meta-analyse data on pollution and thus unlike the other relative risks, the figure comes from only one study, from an area of low pollution so is likely to be an underestimate.
The longitudinal evidence linking potentially modifiable risk factors to dementia generally fulfils causality criteria in observational data (strength, consistency, biological plausibility, temporality, dose–response, coherence, and quasi-experimental studies, for example, more education or using hearing aids). When measuring a risk nearer to the age of dementia onset, then it is more likely that prodromal change affects, or even causes it. Alternatively, a risk factor might act on preclinical pathology or even cause dementia near the time of exposure. Thus, excessive alcohol, and TBI are particularly important in young-onset dementia, although many early onset dementias relate to genetic risks. Risk factors might also matter more at a time of higher biological vulnerability, which the studies we have drawn on cannot establish. The length of exposure required for risk or protection effect, and their inter-relationships as they change across life is unclear—it seems probable that longer or more intense exposure has stronger effects. Additionally, as our communality figures show, risk factors overlap. We cannot establish from these data if having multiple risk factors has an additive or synergistic effect. Association does not prove causation, however, as already noted, the reductions in prevalence and incidence in several HIC suggests that at least some of the risk factors estimated here do have a causal relationship with the clinical expression of dementia.
Key points and recommendations
We judge that sufficient new evidence supports adding three additional modifiable risk factors for dementia to our 2017 Commission model (excessive alcohol, traumatic brain injury, and air pollution). We have been able to add updated evidence on the nine risk factors implicated in the 2017 Commission (education, hypertension, hearing impairment, smoking, obesity, depression, inactivity, diabetes, and social contact). Reduction of these risk factors might be protective for people with or without a genetic risk, although study findings have not been entirely consistent. 175 , 176 , 177 , 178 As we noted in the 2017 Commission, others have previously calculated an estimate of the risk associated with APOε4 at 7% taking into account some other risk factors and this estimate highlights how relatively important potentially modifiable risk factors are in dementia. 2 , 179
For some risk factors, the pattern of risk and the individual's other health, both physical and mental, might be especially important. Currently, the evidence suggests a Mediterranean or Scandinavian diet might have value in preventing cognitive decline in people with intact cognition, particularly as one component of a healthy lifestyle, although how long the exposure has to be or during which ages is unclear. We do not recommend taking additional vitamins, oils, or mixed dietary supplements as a means of preventing dementia as extensive testing in trials has not led to signals of beneficial effects.
Data from RCTs on interventions to prevent cognitive decline, all-cause dementia, or Alzheimer's disease are few. For some key life influences, only observational data, particularly related to natural experiments such as changing the statutory education age, are possible. These influences should be investigated systematically wherever possible. Others can theoretically be investigated but the long follow-up required for midlife risk and protective factors and non-random attrition in longer studies are challenging. Using intermediate endpoints, such as cognition, and dementia onset in research remains uncertain because no intermediate markers with such a close relationship to dementia outcomes exist that it would be possible to predict with certainty for any given individual, age, and sex. Overall, the evidence for treating hypertension is strongest and high blood pressure throughout midlife increases the risk of dementia even without stroke.
Although a need for more evidence is apparent, recommendations should not wait, as clear indications of ways to reduce the chances of developing dementia without causing harm will also lead to other health and wellbeing benefits.
Our recommended strategies for dementia risk reduction include both population-wide and targeted interventions ( panel ). It is important to remember that more socially disadvantaged groups, including Black, Asian, and minority ethnic groups, are particularly at risk.
Recommended strategies for dementia risk reduction
Risks are particularly high in more socially disadvantaged populations including in Black, Asian, and minority ethnic groups.
Population-wide
- • Prioritise childhood education for all, worldwide
- • Implement social public health policies that reduce hypertension risk in the entire population
- • Develop policies that encourage social, cognitive, and physical activity across the life course for all (with no evidence for any specific activities being more protective)
- • Scrutinise the risks for hearing loss throughout the life course, to reduce the risk of exposure to this risk factor
- • Reduce the risk of serious brain trauma in relevant settings, including occupational and transport
- • National and international policies to reduce population exposure to air pollution
- • Continue to strengthen national and international efforts to reduce exposure to smoking, both for children and adults, and to reduce uptake and encourage cessation
Targeted on individuals
- • Treat hypertension and aim for SBP <130 mm Hg in midlife
- • Use hearing aids for hearing loss; we need to help people wear hearing aids as many find them unacceptable, too difficult to use, or ineffective
- • Avoid or discourage drinking 21 or more units of alcohol per week
- • Prevent head trauma where an individual is at high risk
- • Stopping smoking is beneficial regardless of age
- • Reduce obesity and the linked condition of diabetes by healthy food availability and an environment to increase movement
- • Sustain midlife, and possibly late-life physical activity
Although we have more to learn about effectiveness, avoiding or delaying even a proportion of potentially modifiable dementias should be a national priority for all.
Interventions and care in dementia
Not all dementia will be preventable and we present the latest evidence on intervention and care for dementia. To date the emphasis has been on specific subtypes of dementia, most notably on Alzheimer's disease, which has been conceptualised over the years in a variety of changing diagnostic criteria—eg, DSM IV and DSM V. 180 , 181 Intense efforts have been put into biomarkers for early preclinical detection of the disease process before it becomes dementia. Biomarkers need to show reliability and validity, and for dementias they also need to be very closely and clearly related to clinical syndrome outcomes in the way that, for example, human papillomavirus is for cervical cancer, and hypertension has been for stroke.
Biomarkers and detection of Alzheimer's disease
Markers of neurodegeneration linked to clinical dementia include brain volume loss—ie, hippocampal volume loss and entorhinal cortex and medial temporal cortical thinning—seen in structural imaging. The most studied molecular markers are in Alzheimer's disease and are amyloid and tau, which PET and CSF detect clinically. The prevalence of particular pathologies at different ages is important in interpretation of such studies. So, for example, population derived studies show increases in plaques in the population from less than 3% at age 50–59 years to around 40% at age 80–89 years. 182
Amyloid imaging
Amyloid imaging detects amyloid in the brain with high sensitivity and specificity in both cognitively normal and people with Alzheimer's disease when the gold-standard comparison is either neuropathology or clinical diagnosis, distinguishing Alzheimer's disease from other neurodegenerative conditions. 183 Amyloid imaging is not a diagnostic test for dementia. A US study of randomly selected older people from the community recruited 1671 people (mean age of 71 years). 182 The prevalence of PET detected amyloid positivity increased from 2·7% (95% CI 0·5–4·9) of people without cognitive impairment aged 50–59 years to 41·3% (95% CI 33·4–49·2%) aged 80–89 years. 182 In 10-year follow-up PET positivity was associated with a higher probability of developing Alzheimer's disease compared with those who were amyloid negative (HR 2·6, 95% CI 1·4–4·9). In participants with mild cognitive impairment who were amyloid positive the probability (HR 1·9, 95% CI 0·9–3·9) was not very different to those who were amyloid negative (1·6, 0·8–3·4).
Similarly, an 8-year follow-up study of 599 volunteers (average age 70 years) in Australia found that cognitively normal PET amyloid-positive people had an elevated risk of developing Alzheimer's disease compared with amyloid negative (17·7% vs 8·1%; OR 2·4, 95% CI 1·5–4·0). 184 Over 80% of the 266 people who were PET amyloid-positive did not go onto develop a cognitive impairment within 8 years, showing positive status does not predict impairment for most people in a timeframe that might be a useful prognostic window. Follow-up at 5 years of amyloid-positive participants with normal cognition or mild cognitive impairment versus amyloid negative people found the same pattern of increased risk (2·6, 1·4–4·9). Risk also increases per 1 year of age (HR 1·05, 95% CI 0·55–2·0/year), and APOEε4 status (2·6, 1·4–5·0). 184
Most people who are amyloid positive with no other markers have not developed Alzheimer's disease dementia during their lifetime. A model of lifetime risks of people who are amyloid positive without any other biomarkers finds it to be 8·4% for a 90-year-old woman who is cognitively normal at baseline, 23·5% for a 75-year-old woman and 29·3% for a 65-year-old woman. 185 The 10-year risk is considerably less, so a 65-year-old woman with only amyloid biomarkers but who is cognitively normal and has no neurodegeneration has a 10-year Alzheimer's disease risk of 2·5% and a man 2·3%, but the risk is higher with accompanying neurodegeneration ( table 2 ). 185
Ten-year risks by age of developing Alzheimer's disease for women based on amyloidosis alone and in the presence of neurodegeneration and mild cognitive impairment
Data are relative risk (95% CI) or %. Reproduced from Brookmeyer and Abdalla 185 by permission of Elsevier.
Overall, the knowledge of PET-measured amyloid and tau status and MRI-derived cortical thickness in a general population derived sample, only adds a small improvement, which might not be clinically important for predicting memory decline over a model with clinical and genetic variables. 186
Using amyloid PET in patients with cognitive impairment of uncertain causes, results in changes to the clinical diagnosis of Alzheimer's disease 187 and sometimes to medication prescription. We do not know whether PET use improves patient care or decreases care costs. Many people have a mixed cause of dementia and a positive result does not indicate only Alzheimer's disease.
Fluid biomarkers
PET imaging is very costly (US$3000 in the USA) and although used in some clinical settings remains the topic of research to understand its usefulness in broader populations. Fluid biomarkers—ie, blood and cerebrospinal fluid tests—have become a more practical focus of interest since it has become possible to measure specific proteins linked to the proteins associated with the neuropathologies of Alzheimer's disease. 188 A composite blood biomarker for amyloid tested in a discovery dataset and then a validation cohort of participants aged 60–90 years who were already taking part in studies in Japan or Australia had areas under the receiver operating characteristic curves of 96·7% for discovery and 94·1% for validation. The blood biomarker had sensitivity and specificity above 80% against amyloid PET measurement 188 and correlated with CSF concentrations of Aβ1–42. These results are similar to other amyloid blood biomarkers 189 , 190 and harmonisation to a common reference standard is now vital. Although CSF Aβ1–42/1–40 ratio and amyloid PET are now considered interchangeable, 191 CSF tau biomarkers have only correlated weakly with brain tau as currently measured by radioligands. 192 Neurofilament light protein is measured in many cohorts; however, it is non-specific. People with Huntington's disease, multiple sclerosis, mild cognitive impairment, and Alzheimer's disease might have raised blood neurofilament light concentrations, which are a marker of neurodegeneration. 193 , 194 , 195
Key points and conclusions
To be useful in clinical practice biomarkers must be well understood in the populations to which they are going to be applied, including the effects of age and sex on results. There is now reasonable evidence that amyloid and tau measured by PET or in fluid indicate increased risk for development of cognitive impairment in older adults but at the individual level prognostication is not possible as most cognitively normal people with these markers do not develop dementia within a clinically relevant timeframe. Negative amyloid results can be useful for ruling out current Alzheimer's pathology in people with cognitive impairment when the cause is uncertain and show an individual is unlikely to develop Alzheimer's disease during the next few years. High neurofilament light concentrations indicate a neurodegenerative process but not its cause. The value of biomarkers, in terms of diagnostic value, has not been addressed in different representative populations and particularly not in those from LMIC. The potential advantages of blood biomarkers are their low cost and their wider acceptability and applicability in many settings. In many areas of medicine more reliable diagnostic tests have improved research, including epidemiological and public health research and trials, to help distinguish cause from symptom (tuberculosis from a fever) or assess risk factor and disease (hypercholesterolaemia and ischaemic heart disease). Those biomarkers developed for the underlying biology of the dementia syndrome are subject to the same assessment of value.
Principles of intervention in people with dementia
In the 2017 Commission, we discussed that when concerns are raised by patients or family, an accurate diagnosis is helpful. Such a diagnosis provides a gateway to intervention and services where available, for planning for possible futures, and support for family, as well as to research. Unfortunately, these services are not always available. National plans for dementia support timely diagnosis and offer help to individuals and their families.
We did not address screening of those not presenting with concerns but rigorous systematic reviews by the US Task Force on Prevention have found an absence of evidence of benefit and harm. 196 The first trial of population screening took place in the USA, screening 4005 primary care patients aged 65 years or older. No clear benefit or harm in terms of quality of life, mood, or increasing diagnostic rates was found. 197 Other strategies might become more valuable in time such as sensitive awareness of risk factors, when routine records suggest an individual might be deteriorating cognitively. 198
People with dementia have complex problems with symptoms in many domains. Those providing support and any interventions must consider the person as a whole, as well as their context and their close carers, whether family or friends. Individuals' medical, cognitive, psychological, environmental, cultural, and social needs must be given consideration. 2 In the context of under provision of services, this notion is and will continue to be a challenge. Dementia, as an illness which affects cognition by definition, affects the ability to organise activities and people with dementia often need help to do what they enjoy—for example, listen to music, or go to gardens and parks. Wellbeing is one of the goals of dementia care.
Interventions once a diagnosis has been made
Cholinesterase inhibitors have a useful, modest role in improving cognition and activities of daily living in patients with mild-to-moderate Alzheimer's disease and memantine can be prescribed in combination or each drug used separately for moderate and severe Alzheimer's disease. 2 , 199 , 200 However, although available in most countries these drugs are no longer remunerated in France because it is felt that they offer only a small benefit while shifting clinician's attention from other interventions. Whether non-prescribing of this drug will help patients by removing an intervention with known benefit or be detrimental to them is unknown. 201 No advances have been reported in Aβ therapeutics, with negative results from phase 3 trials of monoclonal antibodies (eg, solanezumab, crenezumab) and inhibitors of β-secretase, a protease involved in the production of Aβ peptides. 202 Aducanumab previously abandoned as futile now has further unpublished results. Three 5HT6 antagonists and the calcium channel blocker nilvadipine 203 , 204 have also been ineffective. These drugs also show substantial impact during treatments at so-called therapeutic concentrations on the leakiness of blood vessels. The long-term impact of such side-effects is unknown. Anti-tau, anti-amyloid, and anti-inflammatory drugs continue to be in focus and some argue that pre-symptomatic interventions are necessary, especially if targeting Aβ production, but no evidence of efficacy 205 and some evidence of worsening target symptoms currently exists. 206
Cognitive training in people with dementia
A meta-analysis of 12 controlled trials of 389 people with mild dementia, completing 4 or more hours of group-based computerised cognitive training (mean age 66–81 years, 63·5% female participants), found a small, statistically significant beneficial effect on overall cognition, driven by two trials of virtual reality or Video games (SMD=0·3, 95% CI 0·0–0·5), one with a low and one with a high risk of bias. 55
A Cochrane review 207 found 33 trials of cognitive training, only one of which overlapped with the study above, with around 2000 participants with mild-to-moderate dementia, most with a high or uncertain risk of bias. 207 People completing cognitive training, compared with usual treatment or non-specific activities, had small-to-moderate effects on overall cognition (SMD 0·4, 95% CI 0·2–0·6) and specific cognitive abilities such as verbal fluency and improvements lasted for a few months to 1 year. No direct evidence was observed to suggest that cognitive training was better than cognitive stimulation therapy.
Exercise and physical activity
The Dementia and Physical Activity RCT 208 found moderate-to-high intensity aerobic and strength exercise training did not slow cognitive impairment in people with mild-to-moderate dementia but improved physical fitness. The US Reducing Disability in Dementia study 209 implemented an at-home multicomponent intervention including exercise education, training to increase pleasant events, and activator-behaviour-consequence problem-solving approach over 6 weeks by case managers in 255 community dwelling people with dementia older than 60 years and their family carer and were able to follow up 140 (54·9%). The study found increased physical activity; days of taking 30 or more minutes of exercise (effect size 0·6, 95% CI 0·4–0·8 after the treatment and 0·3, 0·1–0·5 at 13 months) in a before and after intervention comparison.
Interventions for neuropsychiatric symptoms of dementia
Neuropsychiatric symptoms are common and often clustered in people with dementia. These symptoms might precede dementia and are associated with tau and amyloid neuropathology. 210 This suggests that underlying neurobiological mechanisms might underpin neuropsychiatric symptoms. However, other drivers relating to the personal history and the environment of the person with dementia are also likely to exist. Neurodegeneration could lead to increased vulnerability to stressors or triggers. Genetics, cognitive reserve, resilience, medical comorbidities, and environment including responses of carers might modify these relationships. Needs and responses will also be individual and relate to a person's own social, cultural, and historical context. First-line assessment and management of neuropsychiatric symptoms should focus on basic health: describe and diagnose symptoms; look for causes such as pain (using validated pain assessments might help), illness, discomfort, hunger, loneliness, boredom, lack of intimacy and worry that could cause the behaviours and alleviate these while considering risks of harm. 2
No new evidence of medication effectiveness for these symptoms exists; risperidone in low doses (0·5 mg daily) and some other antipsychotics are sometimes effective but often ineffective and have adverse effects. 2 Specific initiatives have led to a decrease in antipsychotic prescriptions for people with dementia, although often replaced with other psychotropics ( figure 8 ), such as benzodiazepines, antidepressants, and mood stabilisers. 211 These psychotropics lack evidence of efficacy for neuropsychiatric symptoms but show clear evidence of possible harm; for example, trazodone and benzodiazepines increase fall-related injuries. 144 Major policy changes should be assessed carefully, within and across countries for unintended consequences (and perhaps unexpected benefits) and their costs.

Proportion of patients with a diagnosis of dementia prescribed an antipsychotic drug (A) and those prescribed an anxiolytic, hypnotic, or antidepressant (B)
CPRD=Clinical Practice Research Datalink. Reproduced from Donegan et al, 211 by permission of Elsevier.
Evidence is slowly accumulating for the effectiveness, at least in the short term, of person-centred evidence-based psychosocial interventions. In Germany, a 6-month cluster RCT of nurse-delivered, supervised dementia care management used a computer-assisted nurse assessment to determine personalised intervention modules, then a multi-disciplinary team discussion and agreement with the physician for 634 people (mean age 80 years) with dementia living at home with a primary carer or alone. 212 The mean mini mental state examination (MMSE) was 23, only 38% had a formal diagnosis of dementia; the majority of participants (51%) had mild dementia but some had moderate and some severe dementia. The intervention consisted of psychosocial management of treatment and care, medication management and carer support, and education and discussion with a psychiatrist or neurologist. The intervention, compared with care as usual, was associated with better outcomes for neuropsychiatric symptoms (Neuropsychiatric Inventory [NPI] score −7·5, 95% CI −11·1 to −3·8), however this effect could be because of deterioration in care as usual (in the care as usual group NPI increased from 7·2 to 15·2; in the intervention group NPI increased from 7·6 to 8·2). This between-group reduction in neuropsychiatric symptoms was greater than that expected, extrapolating from other study results, with antipsychotic medication. Effects on quality of life were only apparent for those people living with a carer.
An eight-session home-based tailored activity programme RCT, tailored both to the person with dementia living at home and to a family member compared with eight telephone-based education sessions, recruited 160 participants with 64% follow-up, imputing values for the rest. 213 The study reported a large reduction in overall neuropsychiatric symptoms immediately after the intervention, which were better in the group receiving home-based tailored activity programme on the neuropsychiatric inventory (mean difference in score 24·3, 95% CI 3·1–45·6), and on functional dependence and pain but this was not sustained 4 months later. Non-completers had more severe neuropsychiatric symptoms.
Since the 2017 Commission two new systematic reviews of antidepressants to treat depression in dementia reported moderate quality evidence that antidepressant treatment for people with dementia does not lead to better control of symptomatology compared with placebo. 214 , 215
Agitation is distressing for people with dementia and those around them, and contributes substantially to the overall costs as the level of agitation increases. 216 The body of evidence on this key behaviour is growing, mostly focused on care-home settings. These findings are valuable as these populations are most affected; however, because many people with dementia reside at home a major gap in knowledge remains.
Care home residents with agitation often find sitting still difficult and therefore might not be included in activities. 217 , 218 Two new cluster RCTs of professionals delivering multicomponent, interdisciplinary, interventions in care homes successfully reduced agitation. The WHELD study 219 included participants with or without neuropsychiatric symptoms and provided person-centred care, aiming to improve communication with people with dementia. It implemented social, sensory experiences or other activities; educated about antipsychotic review; and addressed physical problems, finding lower Cohen Mansfield Agitation Inventory (CMAI) at 9 months (MD −4·3 points, 95% CI −7·3 to −1·2). 219 The TIME study 220 for people with moderate-to-high levels of agitation consisted of a manual-based comprehensive assessment of the resident and structured case conference for the staff and doctor, to create a tailored plan, and then implement it. This intervention led to reduced agitation at 8 weeks (NPI −1·1 points, 95% CI −0·1 to −2·1; CMAI −4·7 points, −0·6 to −8·8) and 12 weeks (NPI −1·6, −0·6 to −2·7; CMAI −5·9, −1·7 to −10·1). 220 These effect sizes are similar to those seen for medications, but without harmful side-effects. 2 , 221 A further RCT studied a six-session intervention with staff in groups, teaching staff to understand agitation as related to medical, psychological, or social unmet needs and to implement strategies to meet these needs, using the describe, investigate, create, and evaluate approach. 222 The intervention did not reduce agitation symptoms, although it was cost-effective, improving quality of life. 223 Overall, the current evidence for agitation in care homes favours multi-component interventions by clinical staff, including considering if drugs might harm, and not drug interventions. Thus a major gap remains in knowledge about people living at home who comprise the majority of those with dementia.
Psychotic symptoms in dementia
People with dementia might be wrongly thought to have delusions when they misremember, and new psychotic symptoms are often due to delirium, thus thorough assessment of symptoms is essential. 2 Management of psychosis in dementia should start with non-pharmacological interventions; however, evidence for effectiveness of these interventions for psychosis in dementia is weaker than for agitation. 224 Antipsychotics for psychosis in dementia should be prescribed in as low a dose and for the shortest duration possible. 2 However, a Cochrane review of antipsychotics withdrawal found two trials with participants with dementia who had responded to antipsychotic treatment. These reported that stopping antipsychotics was associated with symptomatic relapse 225 suggesting the need for caution in any medication withdrawal in this group. There was low-quality evidence that, in general, discontinuation might make little or no difference to overall neuropsychiatric symptoms, adverse events, quality of life or cognitive function. 226
Apathy might be conceptualised as the opposite of engagement, comprising reduced interest, initiative, and activity. Like people without dementia, those with dementia engage more in preferred activities, but require additional support to do so. 227 A study in care homes observed engagement increased during activities in those who attended the groups. 228 A Cochrane review of the few people who had been in drug RCTs of methylphenidate versus placebo for apathy in dementia found small improvements on the apathy evaluation scale (MD −5·0, 95% CI −9·6 to−0·4, n=145, three studies, low-quality evidence) but not on the NPI apathy subscale (MD −0·1, 95% CI −3·9 to 3·7, n=85, two studies). 229
There is no evidence that medication for sleep in dementia is effective 230 and considerable evidence for harm—ie, earlier death, increased hospitalisation, and falls—exists. 139 , 144 Testing of non-pharmacological interventions is ongoing. 231
Carer distress related to neuropsychiatric symptoms rather than the dementia symptoms was associated in one study with increased use and costs of health services, 232 highlighting the need for effectively identifying, educating, and supporting distressed carers. An RCT 233 reporting 6-year follow-up after the eight session STrAtegies for RelaTives intervention—manual-based coping intervention delivered by supervised psychology graduates—found continuing effectiveness for depressive symptoms in carers (adjusted MD −2·00; 95% CI −3·4 to −0·6) and risk of case-level depression, with patient-related cost being approximately 3 times lower than those who did not receive the intervention (median £5759 vs £16 964 in the final year; p=0·07). 233 Another US study 234 followed up 663 people, mean age 77 years, 55% women. Caregiver depression rather than symptoms of people with dementia predicted emergency department use for people with dementia, with a 73% (RR 1·73, 95% CI 1·3–2·3) increase. 234
Functioning
A UK RCT of 14 sessions of cognitive rehabilitation focused on individual goal attainment with therapy delivered at home by an occupational therapist or nurse to 475 participants with mild-to-moderate dementia (MMSE ≥18 for inclusion; mean 24) and a family carer. 235 Individuals had two or three goals; the most common was engaging in activities (21% of goals). The intervention group reported increased goal attainment over 3 and 9 months compared with usual treatment (effect size 0·8, 95% CI 0·6–1·0 at both 3 and 9 months). 235 The treatment did not improve participants' quality of life, mood, self-efficacy, cognition, carer stress, or health status and was not cost-effective. A systematic review 236 of RCTs without meta-analysis for overall effect size, concluded that all interventions which had improved functioning in people living with dementia in the community have been individual rather than group interventions. These were: in-home physiotherapist delivered aerobic exercise (two studies, larger one positive, 140 people with Alzheimer's disease; smaller study negative, 30 people with Alzheimer's disease), individualised cognitive rehabilitation (mild or moderate dementia; two studies; 257 cognitive reserve intervention groups and 255 controls), and in-home activities-focused occupational therapy (people with mild to moderate dementia, three studies, 201 intervention, 191 controls) reduced functional decline compared to controls but group-exercise and reminiscence therapies were ineffective. 236
People with dementia have other illnesses
Multimorbidity is a huge challenge in dementia, not only because people with dementia have increased rates of other illnesses, but also because they often find it particularly difficult to organise care. People with dementia might forget to tell their family or health professionals of symptoms, struggle to understand or follow agreed plans, and are more likely to forget to drink and eat, increasing falling and infection rates. 237 People with dementia consult primary care less often 238 and have fewer dental visits 239 than those without dementia and their family members, if involved, often feel they lack knowledge to assist. 240 Health-care professionals need education to be more comfortable, understanding, and positive in communicating with people with dementia. 241
Around 70–80% of people diagnosed with dementia in primary care have at least two other chronic illnesses. 242 , 243 People who are physically more frail are more likely to have dementia, but the relationship between pathology and symptoms in these people is comparatively weak suggesting that dementia might be from other causes. 22 Compared to the general older population, people with dementia have increased rates of cerebrovascular disease, 243 , 244 , 245 , 246 stroke, 247 Parkinson's disease, 243 , 245 diabetes, 245 , 247 skin ulcers, anxiety and depression, 243 , 245 pneumonia, incontinence, and electrolyte disturbance. 245 Multimorbidity in people with dementia is associated with faster functional decline 248 and worse quality of life for people with dementia and their family carers. 249
Dementia and COVID-19
Severe acute respiratory syndrome coronavirus 2, was first identified in patients with viral pneumonia in Hubei province, China. 250 Severity and mortality of the associated disease (COVID-19) worsen with increasing age 251 and with pre-existing illnesses such as hypertension and diabetes, 252 and thus many people with dementia are at particular risk. Death certificates from the UK indicate that dementia and Alzheimer's disease were the most common underlying conditions, specified in 11 950 deaths (25·6% of all deaths involving COVID-19) in March to May, 2020. 253 Many charities, practitioners, and academics supporting people with dementia have issued guidance based on current evidence and best practice, including advance consideration of whether people would wish to be hospitalised if they develop severe COVID-19. Concern has been expressed that the illness and consequent distancing might increase family carer stress, loneliness, neuropsychiatric symptoms and use of psychotropic medication, and lead to complications, including future dementia. Interventions delivered remotely through technology have also been implemented in some places. 254 , 255 , 256 , 257
People with dementia might struggle to adhere to measures to reduce virus transmission, as they might not understand or remember about required changes to behaviour, such as physical distancing and hygiene, leading to increased risk to themselves and their carers. 258 They might additionally be vulnerable if they depend on others for daily activities or personal care, as this necessitates close personal contact.
This situation is particularly concerning in those care homes, where many residents have dementia and where many COVID-19 deaths have occurred in many countries 259 , 260 , 261 with reports of more than half of residents being admitted to hospital. In US nursing homes, among 10 576 people with confirmed COVID-19, residents living with dementia made up 52% of COVID-19 cases; yet, accounted for 72% of all deaths (an increased risk of 1·7). 262 The number of people living together in care homes means that the infection of an individual, either staff or resident, could endanger more people than in traditional or family households. Although evidence exists that if staff are sufficiently and rigorously protected they are unlikely to develop COVID-19, many staff have become unwell and some have died. 263 , 264 Illness means that there are fewer people to care for residents at a time when they need particularly high levels of care. This situation is particularly relevant in the care of residents with dementia, if they are expected to remain in their own rooms, rather than eating and participating in activities with others. Staff or residents might also be moved between care homes and increase risk in other homes. 261 Restrictions on visitors to private homes, care homes, and hospitals might cause greater distress for people with dementia and they might not understand why people are wearing masks, recognise who is behind it, or understand speech when lips are covered. Lack of restrictions means that the visitors might also be at elevated risk. 261
The impacts of COVID-19 on people with dementia might be particularly severe in LMICs, due to smaller health budgets for testing and protective equipment, capacity of health-care systems, quality of care home provision and patterns of workforce mobility. 264
Thus, people with dementia are particularly vulnerable to COVID-19 because of their age, multimorbidity, and difficulties in maintaining physical distancing. 250 , 251 , 252
We recommend rigorous public health measures of protective equipment and hygiene, including not moving staff or residents between care homes or admitting new residents when their COVID-19 status is unknown, should mitigate impacts on people with dementia. It is also imperative that there is frequent and regular testing of staff in care homes for infection, ensuring staff have sick pay so that they do not come in when symptomatic and interim care is being set up for people discharged from hospital so that only those who are COVID-19 free come to live in care homes. Resident testing should encompass asymptomatic as well as symptomatic people, when there is exposure within the home to COVID-19. In the future, many homes might be able to start to provide oxygen therapy so that those who do not want to be admitted to hospital are still able to access oxygen therapy. In addition, it is also important to reduce isolation by providing the necessary equipment and a brief training to relatives on how to protect themselves and others from COVID-19; so that they can visit their relatives with dementia in nursing homes safely when it is allowed. Further evidence is needed to inform responses to this and future public health emergencies.
Hospital admissions
Hospitalisation in people with dementia is associated with adverse, unintended consequences, including distress, functional and cognitive decline, and high economic costs. 265 , 266 , 267 People with dementia have 1·4 to 4 times more hospital admissions than others with similar illnesses. 266 , 268 , 269 , 270
A systematic review and meta-analysis including 34 studies of 277 432 people with dementia found that in the six studies which compared the two groups, people with dementia had increased hospital admissions compared with those without dementia, after adjusting for age, sex, and physical comorbidity (RR 1·4, 95% CI 1·2–1·7; figure 9 ). 271 Hospitalisation rates in people with dementia ranged from 0·37 to 1·26 per person-year in high-quality studies. Admissions are often for conditions that might be manageable in the community (potentially preventable hospitalisations). 268 People with dementia experience longer and more frequent admissions and readmissions; health-care expenditure for people with moderate-severe dementia is around double that of people without dementia. 269 , 272 , 273 Early detection and management of physical ill-health in people with dementia, particularly of pain, falls, diabetes, incontinence, and sensory impairment, is important. 199 , 274 , 275 However, no intervention has successfully reduced number of hospital admissions of community-dwelling people with dementia, 276 although education, exercise, rehabilitation, and telemedicine have reduced admissions for older people without dementia. 277

Systematic review and meta-analysis of hospitalisation rates of people with dementia compared to those without dementia controlled for age and sex
Reproduced from Shepherd et al, 271 by permission of Springer Nature.
High-quality care for people with dementia takes longer than caring for others with the same condition. 278 Recognition of dementia in hospital inpatients is necessary for optimum care, 279 but dementia is often undetected or unrecorded. 280 In the UK however, detection rates have increased over the past 10 years. 281
Physical illness, delirium, and dementia
Dementia and delirium frequently occur together. In one hospital inpatients' survey nearly 35% of those older than 80 years experienced delirium; those with prior cognitive impairment had 15 times the risk of developing delirium than those without (OR 15·3, 95% CI 5·2–45·4). 282 People with delirium without known dementia are more likely to be diagnosed with dementia in the future than others, either because of pre-existing undiagnosed dementia or cognitive impairment, present in 20·7% (95% CI 11·9–29·5) and 37·8% (27·3–88·3) respectively of one cohort, or because delirium has neurotoxic effects and so precipitates dementia. 283 People with similar neuropathology show faster cognitive decline if they develop delirium than if they do not. 284 Additionally, older people without dementia declined cognitively more than twice as fast after an emergency hospital admission for any cause, compared with those not admitted, suggesting any severe illness is associated with cognitive decline. 285 Risk factors for delirium in dementia include sensory impairment, pain, polypharmacy, dehydration, intercurrent illnesses, such as urinary tract infections or faecal impaction, and an unfamiliar or changing environment. 286 Delirium in older people should prompt consideration of underlying dementia.
Most research on delirium prevention has been in people without dementia. It suggests targeting hydration, stopping medication predisposing to delirium, monitoring the depth of anaesthesia, and sleep promotion. However, no evidence for medication efficacy, including cholinesterase inhibitors, antipsychotic medication, or melatonin exists. 287 , 288 , 289 The Hospital Elder Life Program 290 —an intervention to prevent delirium in those admitted to hospital—reduces delirium incidence and includes people who are cognitively impaired. This multidisciplinary treatment consists of daily visits, orientation, therapeutic activities, sleep enhancement, early mobilisation, vision and hearing adaptation, fluid repletion, infection prevention and management of constipation, pain, and hypoxia, and feeding assistance. 290
A network meta-analysis of drugs for prevention and treatment of delirium did not include studies of people with dementia, thus we cannot use this to recommend drugs for people with dementia and delirium as this research might be inapplicable to them. 291
Little high-quality research exists on managing delirium in dementia. One RCT compared care at a specialist medical and mental health unit to usual care for 600 confused people older than 65 years, acutely admitted to hospital and found no difference in the primary outcome of days spent at home or in hospital, but increased family satisfaction. 292 A further RCT of cognitively stimulating activities for people with delirium in dementia did not improve the delirium. 293 No definitive evidence that any medication improves delirium in people with dementia exists: cholinesterase inhibitors, antipsychotics, and sedating benzodiazepines are ineffective and antipsychotics and benzodiazepines are associated with mortality and morbidity. 265 , 288 , 294 , 295 , 296 , 297 Given the risk of dementia in people who develop delirium, its prevention, and possibly advances in its management, might offer a means for dementia prevention. 298
Link between very old age, frailty, and dementia
The fastest growing demographic group in most advanced countries are people aged 90 years and older. One well characterised post-mortem cohort of the oldest old (n=1079; mean age 90 years) dying with dementia, found that neuropathological features of Alzheimer's disease account for about half of the cognitive decline seen as people diagnosed with Alzheimer's disease had mixed causes of dementia. 299 Although Alzheimer's disease neuropathology was the commonest cause of dementia, Alzheimer's disease changes rarely occurred on their own, so only 9% of people with dementia had pure Alzheimer's disease pathology. 300 People who have Alzheimer's disease pathology without developing dementia tend to have fewer age-related health deficits than those who develop it with even low concentrations of plaques and tangles. 301 A moderation analysis showed that the relationship between Alzheimer's disease pathology and dementia status differed according to level of frailty (adjusted for age, sex, and education) with increasing frailty weakening the relationship between Alzheimer's disease pathology and dementia ( figure 10 ). 22 As with delirium, some of this additional health risk might be modifiable. This approach suggests a new type of therapy focus on specific age-related processes that underpin many diseases of late life might reduce the incidence or severity of dementia.

Moderation analyses of the relationship between Alzheimer's disease pathology and clinical diagnosis of Alzheimer's dementia (adjusted for age, sex, and education)
As frailty increased, the odds of a neuropathological diagnosis of Alzheimer disease corresponding to a clinical diagnosis decreased. Reproduced from Wallece et al, 22 by permission of Elsevier.
End-of-life care in dementia
The numbers of people dying with dementia are increasing but the evidence for the best end-of-life care is scarce. Trends in age-standardised death rates (3·6%) for dementia increased slightly between 1990–2016, with pronounced increases in the USA and Japan and decreases in western Europe and central Latin America. 4 Dementia is more readily being included on death certificates, which accounts for some of the rise. The increase might be related to dementia manifesting at later ages, with higher physical frailty 22 leading to a faster decline.
Most people with dementia might die while still in the mild-to-moderate stages whereas only about a quarter of those dying with dementia have severe dementia. 302 , 303 The trajectory of dementia is often unpredictable 304 and palliative care initiation should reflect need not prognosis.
Decision making about end of life is complex and simple rules of thumb, co-designed with staff and carers, provided clarity in some small studies. 304 One RCT testing decision-aids about families' and doctors' goals of care for people with advanced dementia led to increased palliative care content in care plans. 305 , 306 In a 9-month UK prospective study, 85 care home residents with advanced dementia from 14 homes were likely to be living with distressing symptoms, specifically agitation (54%) or pain (61% on movement). 304
Capacity to make abstract decisions, including about the future, might be lost early in dementia. 307 Therefore, advance care planning, designed to empower people with dementia and improve quality of dying, might theoretically be something everyone should do before developing dementia. 308 However, people might not be able to predict their future wishes. This might explain why family carer proxies show only low-to-moderate agreement with stated end-of-life treatment preferences of people with dementia. 309 Advance care planning might, however, reduce carers' uncertainty in decision making and improve perceptions of quality of care. 310
Partners of people dying with dementia experience poorer mental health than those facing bereavement from other causes 311 possibly because of long and difficult caring responsibilities. This might be ameliorated through sensitive and timely information, particularly regarding the progression of dementia, 312 individually or through family and staff case-conferencing. 313 , 314
Conclusions
Knowledge about risk factors and potential prevention, detection, and diagnosis of dementia is improving although significant gaps remain. 315 In this Commission report, we have specified policy and individual changes to delay the onset of cognitive impairment and dementia and better ways to support and treat people with dementia and their families and to improve their quality of life.
Interventions, including organisation of the complex physical illness and social needs, to support people affected by dementia can have a huge effect when taken as a whole. Our ambition is for worldwide provision of resources for an adequate level of wellbeing to people with dementia and their carers with a better evidence base to guide individual care and policy making alike. With good quality care, people can live well with dementia and families can feel supported.
Acknowledgments
We are partnered by University College London (UCL), the Alzheimer's Society, UK, the Economic and Social Research Council, and Alzheimer's Research UK, and would like to thank them for financial help. These organisations funded the fares, accommodation, and food for the Commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication. We would like to thank Bernadette Courtney, Jacques Gianino, and Nuj Monowari, from UCL, London, UK, for their administrative help, including managing finances, booking rooms and food, and setting up a website supported by the University College London Hospitals National Institute for Health Research Biomedical Research Centre. We would like thank Henrik Zetterberg for advice on biomarkers and dementia.
Contributors
GL, JH, AS, and NM contributed to literature searches and quality assessments for systematic reviews. JH and NM performed meta-analyses. GL, JH, AS, and NM conceived the new PAF calculation and NM led the statistical analysis. GL, JH, AS, NM, DA, CLB, SB, AB, JC-M, CC, SGC, NF, RH, HCK, EBL, VO, KRi, KRo, ELS, QS, LSS, and GS attended the conference to discuss the content. GL, JH, EBL, AS, DA, and ELS wrote first drafts of sections of the paper. GL wrote the first draft of the whole paper and revisions of drafts. CBa reviewed and contributed to revision of the final drafts. All authors contributed to sections of the reports and all revised the paper for important intellectual content.
Declaration of interests
AS reports grants from Wellcome Trust (200163/Z/15/Z), outside the submitted work. DA reports grants from Eli Lilly, during the conduct of the study. CBa reports grants and personal fees from Aca-dia and Lundbeck; and personal fees from Roche, Otsuka, Biogen, Eli Lilly, and Pfizer, outside the sub-mitted work. SB reports grants and personal fees from AbbVie, personal fees and non-financial sup-port from Eli Lilly, and personal fees from Eleusis, Daval International, Boehringer Ingelheim, Axovant Sciences, Lundbeck, and Nutricia, outside the submitted work; and he has been employed by the Department of Health for England. NF reports non-financial support from Eli Lilly, outside the submitted work. LNG and her institutions (Johns Hopkins University, Baltimore, MD, USA, Drexel University, Philadelphia, PA, USA, and Thomas Jefferson University, Philadelphia, PA, USA) are entitled to receive royalties from fees associated with online training for the tailored activity program, which is an evidence-based program referenced in the Review. RH reports grants from Department of Health, NIHR HTA Programme, outside the submitted work; and he is a Scientific Trustee of the charity Alzheimer's Research UK. MK reports grants from the UK Medical Research Council (S011676, R024227), NordForsk (the Nordic Programme on Health and Welfare, 75021) and the Academy of Finland (311492), outside the submitted work. EBL reports other (royalties) from UpToDate, outside the submitted work. KRo reports personal fees from Clinical Cardio Day-Cape Breton University, Sydney, NS, Canada, CRUIGM-Montreal, Jackson Laboratory, Bar Harbor, MA, USA (speaker fees), MouseAge, Rome, Italy (speaker fees), Lundbeck, Frontemporal Dementia Study-Group, SunLife Insurance, Japan, outside the submitted work. He is a President and Chief Science Officer of DGI Clinical, which in the last 5 years has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, Otsuka) on individualised outcome measurement. In 2017, he attended an advisory board meeting with Lundbeck. He is also Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities, as well as, in its first phase (2013-2018), from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, the QEII Health Science Centre Foundation, the Capital Health Research Fund and the Fountain Family Innovation Fund of the QEII Health Science Centre Foundation. LSS reports grants and personal fees from Eli Lilly, Merck, and Roche/Genentech; personal fees from Avraham, Boehringer Ingelheim, Neurim, Neuronix, Cognition, Eisai, Takeda, vTv, and Abbott; and grants from Biogen, Novartis, Biohaven, and Washington University DIAN-TU, outside the submitted work. The remaining authors declare no conflict of interests.
Supplementary Material
Uncited references.

IMAGES
COMMENTS
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Memory declines slowly in normal aging (1). Alzheimer's disease is marked by more rapid cognitive decline, often starting earlier in life (2). Current therapies enhance cognition without changing the rate of decline in AD (3). The anticipated effect of novel therapies is reduction in the rate of decline (4). Go to:
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
Alzheimer's disease articles from across Nature Portfolio. Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by ...
Alzheimer's disease (AD) is the most common cause of dementia, and its underlying mechanisms have been a subject of great interest. The mainstream theory of AD pathology suggests that the disease is primarily... Xiaoyu Zhang, Chuanying Chen and Yi Liu. Alzheimer's Research & Therapy 2024 16 :63.
In studies of potential disease-modifying drugs for Alzheimer's disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says ...
Over the past two decades, the landscape of dementia research has changed drastically due to advances in knowledge at the molecular, cellular, animal, and human levels. Advances have not been limited to the Alzheimer's disease spectrum but include improved understanding of other disorders that can also lead to dementia. In this Anniversary Round-up, I discuss what I consider to be the most ...
Abstract. Alzheimer disease (AD) is the most common contributor to dementia in the world, but strategies that slow or prevent its clinical progression have largely remained elusive, until recently ...
More than 50% of people diagnosed with Alzheimer's dementia who were studied at Alzheimer's Disease Research Centers had mixed dementia. 22 In community-based studies, the percentage is considerably higher. 24 Mixed dementia is most common in people age 85 or older. 27, 28: Symptoms vary depending on the combination of brain changes present.
Indeed, in one study of 184 individuals who met research neuropathological criteria for AD 9, ... C. et al. Contribution to Alzheimer's disease risk of rare variants in TREM2, SORL1, and ABCA7 ...
Alzheimer's disease (AD) is prevalent throughout the world and is the leading cause of dementia in older individuals (aged ≥ 65 years). To gain a deeper understanding of the recent literature on the epidemiology of AD and its progression, we conducted a review of the PubMed-indexed literature (2014-2021) in North America, Europe, and Asia. The worldwide toll of AD is evidenced by rising ...
Allan I. Levey, M.D., Ph.D. An estimated 50 million people worldwide have dementia, mostly due to Alzheimer's disease. The inexorable progression of Alzheimer's disease exerts a huge toll on ...
An estimated 6.2 million Americans age 65 and older are living with Alzheimer's dementia today. This number could grow to 13.8 million by 2060 barring the development of medical breakthroughs to prevent, slow or cure AD. Official death certificates recorded 121,499 deaths from AD in 2019, the latest year for which data are available, making ...
Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer's Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD.
Seven recent AMP AD reports showcase research advances related to the discovery of new drug candidate targets, identification of molecular subtypes of the disease, and new potential biomarkers that can serve as the basis for a precision medicine approach to therapy development. Identifying ATP6VA1 gene as a candidate target for treatment ...
November 08, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
Aims and scope. Alzheimer's Research & Therapy is the major forum for translational research into Alzheimer's disease. An international peer-reviewed journal, it publishes open access basic research with a translational focus, as well as clinical trials, research into drug discovery and development, and epidemiologic studies.
As one of the most common neurodegenerative diseases and causes of dementia, Alzheimer disease (AD) is a critical topic for biomedical research. The main clinical manifestation of AD is the progressive decline of cognitive function and activities of daily living, and the pivotal pathological features of AD are amyloid beta (Aβ) deposition ...
The healthy brain and our aging population: translating science to public health practice. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 2007;3(S1):S1-S2. Anderson LA, McConnell SR. Cognitive health: an emerging public health issue. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 2007;3(S1):S70-S73.
The UC Davis Alzheimer's Disease Center is one of only 33 research centers designated by the National Institute on Aging. With locations in Sacramento and Walnut Creek, the center is focused on translating research findings into better tools to diagnose dementia and treatment for patients while focusing on the long-term goal of finding a way to ...
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
March 26, 2024. Source: Lancaster University. Summary: Researchers have identified a new potential target for the treatment of Alzheimer's disease -- PDE4B. The researchers observed that AD mice ...
A new report estimates 6.9 million older Americans are living with Alzheimer's disease in 2024, an increase of about 200,000 cases of the mind-robbing disease from 2023 and "a significant public ...
A randomized, blinded study of photobiomodulation in a mouse model of Alzheimer's disease showed no preventive effect. Mélanie Sipion. , Filipa M. Ferreira. & Aleksander Sobolewski. Article.
March 27, 2024 at 6:00 a.m. EDT. Diabetes is among three major risk factors for dementia, a study has found. (iStock) Diabetes, air pollution and alcohol consumption could be the biggest risk ...
Dementia is an acquired loss of cognition in multiple cognitive domains sufficiently severe to affect social or occupational function. In the US, Alzheimer's disease (AD) affects 5.8 million people. However, dementia is commonly associated with more than one neuropathology, usually AD with cerebrovascular pathology.
R. MayeuxN Engl J Med 2024;390:761-763. Alzheimer's disease typically evolves over a period of several years before an affected person seeks medical attention for a diagnosis. The pathologic ...
As this extended genetic region is known for its pathological association with many neurodegenerative disorders including Alzheimer's disease, we investigated whether the LIFO brain regions ...
Animal research suggests that a rare, familial form of Alzheimer's disease can be transmitted via bone marrow transplantation, but experts urge caution in interpreting this finding.
All these disturbances were associated with a higher risk of all-cause dementia (RR 1·2; 95% CI 1·1-1·3) 137 and clinically diagnosed Alzheimer's disease (1·6, 1·3-1·9) compared with no sleep disturbance, although not all cohort studies excluded those with cognitive impairment or dementia at baseline from their analyses. 138 A U ...