
- Book Solutions
- State Boards

Case Study Questions Class 11 Chemistry Hydrocarbon
Case study questions class 11 chemistry chapter 13 hydrocarbon.
CBSE Class 11 Case Study Questions Chemistry Hydrocarbon. Important Case Study Questions for Class 11 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Hydrocarbon.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 11 Chemistry Hydrocarbon
Case study – 1.
The term ‘hydrocarbon’ is self-explanatory which means compounds of carbon and hydrogen only. Hydrocarbons play a key role in our daily life. You must be familiar with the terms ‘LPG’ and ‘CNG’ used as fuels. LPG is the abbreviated form of liquified petroleum gas whereas CNG stands for compressed natural gas. Another term ‘LNG’ (liquified natural gas) is also in news these days. This is also a fuel and is obtained by liquifaction of natural gas. Petrol, diesel and kerosene oil are obtained by the fractional distillation of petroleum found under the earth’s crust. Coal gas is obtained by the destructive distillation of coal. Natural gas is found in upper strata during drilling of oil wells. The gas after compression is known as compressed natural gas. LPG is used as a domestic fuel with the least pollution. Kerosene oil is also used as a domestic fuel but it causes some pollution. Automobiles need fuels like petrol, diesel and CNG. Petrol and CNG operated automobiles cause less pollution. All these fuels contain mixture of hydrocarbons, which are sources of energy. Hydrocarbons are also used for the manufacture of polymers like polythene, polypropene, polystyrene etc. Higher hydrocarbons are used as solvents for paints. They are also used as the starting materials for manufacture of many dyes and drugs. Thus, you can well understand the importance of hydrocarbons in your daily life. In this unit, you will learn more about hydrocarbons.
Classification Hydrocarbons are of different types. Depending upon the types of carbon-carbon bonds present, they can be classified into three main categories – (i) saturated
(ii) unsaturated and
(iii) aromatic hydrocarbons. Saturated hydrocarbons contain carbon-carbon and carbon-hydrogen single bonds. If different carbon atoms are joined together to form open chain of carbon atoms with single bonds, they are termed as alkanes. On the other hand, if carbon atoms form a closed chain or a ring, they are termed as cycloalkanes. Unsaturated hydrocarbons contain carbon-carbon multiple bonds – double bonds, triple bonds or both. Aromatic hydrocarbons are a special type of cyclic compounds. You can construct a large number of models of such molecules of both types (open chain and close chain) keeping in mind that carbon is tetravalent and hydrogen is monovalent. For making models of alkanes, you can use toothpicks for bonds and plasticine balls for atoms. For alkenes, alkynes and aromatic hydrocarbons, spring models can be constructed.
Alkanes As already mentioned, alkanes are saturated open chain hydrocarbons containing carbon – carbon single bonds. Methane (CH 4 ) is the first member of this family. Methane is a gas found in coal mines and marshy places. If replace one hydrogen atom of methane by carbon and join the required number of hydrogens to satisfy the tetravalence of the other carbon atom,it get C2H6. This hydrocarbon with molecular formula C2H6 is known as ethane. Thus it can consider C2H6 as derived from CH4 by replacing one hydrogen atom by -CH3 group. Go on constructing alkanes by doing this theoretical exercise i.e., replacing hydrogen atom by –CH3 group. The next molecules will be C 3 H 8 , C 4 H 10 …

These hydrocarbons are inert under normal conditions as they do not react with acids, bases and other reagents. Hence, they were earlier known as paraffins (latin : parum, little; affinis, affinity). the general formula for alkanes is C n H 2n+2 . It represents any particular homologue when n is given appropriate value. methane has a tetrahedral structure , in which carbon atom lies at the centre and the four hydrogen atoms lie at the four corners of a regular tetrahedron. All H-C-H bond angles are of 109.5°.
In alkanes, tetrahedra are joined together in which C-C and C-H bond lengths are 154 pm and 112 pm respectively. We have already read that C–C and C–H σ bonds are formed by head-on overlapping of sp 3 hybrid orbitals of carbon and 1s orbitals of hydrogen atoms.
Difference in properties is due to difference in their structures, they are known as structural isomers. structural isomers which differ in chain of carbon atoms are known as chain isomers.
Preparation- Petroleum and natural gas are the main sources of alkanes. However, alkanes can be prepared by following methods :

1) From unsaturated hydrocarbons Dihydrogen gas adds to alkenes and alkynes in the presence of finely divided catalysts like platinum, palladium or nickel to form alkanes. This process is called hydrogenation. These metals adsorb dihydrogen gas on their surfaces and activate the hydrogen – hydrogen bond. Platinum and palladium catalyse the reaction at room temperature but relatively higher temperature and pressure are required with nickel catalysts.
2) From alkyl halides
i) Alkyl halides (except fluorides) on reduction with zinc and dilute hydrochloric acid give alkanes.
ii) Alkyl halides on treatment with sodium metal in dry ethereal (free from moisture) solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing even number of carbon atoms.
1) Which of the following catalyst is used for hydrogenation…
a) platinum
b) palladium
d) All the above
Ans- d) All the above
2) LPG stands for ….
a) Liquid Pressure Gas
b) Liquefied Petroleum Gas
c) Liquid Platinum Gas
d) Liquid Processed Gas
Ans- b) Liquefied Petroleum Gas
3) Difference in properties is due to difference in their structures, they are known as …. isomers.
a) Functional
b) Positional
c) Structural
Ans- c) Structural
4) Structural isomers which differ in chain of carbon atoms are known as… isomers.
Ans- d) chain
5) CNG Stands for ..
a) Compressed Natural Gas
b) Condensed Natural Gas
c) Compressed Neutral Gas
d) Compressed Neutral Gallium
Ans – a) compressed natural gas
[B]Short Answers
1) Give classification of hydrocarbons.
Ans- Hydrocarbons are of different types. Depending upon the types of carbon-carbon bonds present, they can be classified into three main categories – (i) saturated
2) Write short note on alkanes .
Ans- alkanes are saturated open chain hydrocarbons containing carbon – carbon single bonds. Methane (CH4) is the first member of this family. Methane is a gas found in coal mines and marshy places. If replace one hydrogen atom of methane by carbon and join the required number of hydrogens to satisfy the tetravalence of the other carbon atom,it get C2H6. This hydrocarbon with molecular formula C2H6 is known as ethane. Thus it can consider C2H6 as derived from CH4 by replacing one hydrogen atom by -CH3 group. Go on constructing alkanes by doing this theoretical exercise i.e., replacing hydrogen atom by –CH3 group. The next molecules will be C 3 H 8 , C 4 H 10 …

3) How to prepare alkanes from unsaturated hydrocarbons.

Ans- 1) From unsaturated hydrocarbons Dihydrogen gas adds to alkenes and alkynes in the presence of finely divided catalysts like platinum, palladium or nickel to form alkanes. This process is called hydrogenation. These metals adsorb dihydrogen gas on their surfaces and activate the hydrogen – hydrogen bond. Platinum and palladium catalyse the reaction at room temperature but relatively higher temperature and pressure are required with nickel catalysts.
[C] Long Answers
1) Write short note on hydrocarbons.
Ans- The term ‘hydrocarbon’ is self-explanatory which means compounds of carbon and hydrogen only. Hydrocarbons play a key role in our daily life. You must be familiar with the terms ‘LPG’ and ‘CNG’ used as fuels. LPG is the abbreviated form of liquified petroleum gas whereas CNG stands for compressed natural gas. Another term ‘LNG’ (liquified natural gas) is also in news these days. This is also a fuel and is obtained by liquifaction of natural gas. Petrol, diesel and kerosene oil are obtained by the fractional distillation of petroleum found under the earth’s crust. Coal gas is obtained by the destructive distillation of coal. Natural gas is found in upper strata during drilling of oil wells. The gas after compression is known as compressed natural gas. LPG is used as a domestic fuel with the least pollution. Kerosene oil is also used as a domestic fuel but it causes some pollution. Automobiles need fuels like petrol, diesel and CNG. Petrol and CNG operated automobiles cause less pollution. All these fuels contain mixture of hydrocarbons, which are sources of energy. Hydrocarbons are also used for the manufacture of polymers like polythene, polypropene, polystyrene etc. Higher hydrocarbons are used as solvents for paints. They are also used as the starting materials for manufacture of many dyes and drugs. Thus, you can well understand the importance of hydrocarbons in your daily life. In this unit, you will learn more about hydrocarbons.
2) How to prepare alkanes from alkyl halides?
Ans- From alkyl halides

Case study – 2

Alkanes are generally inert towards acids, bases, oxidising and reducing agents. However, they undergo the following reactions under certain conditions.
1) Substitution reactions One or more hydrogen atoms of alkanes can be replaced by halogens, nitro group and sulphonic acid group. Halogenation takes place either at higher temperature (573-773 K) or in the presence of diffused sunlight or ultraviolet light. Lower alkanes do not undergo nitration and sulphonation reactions. These reactions in which hydrogen atoms of alkanes are substituted are known as substitution reactions. As an example, chlorination of methane is given below: Halogenation

It is found that the rate of reaction of alkanes with halogens is F2 > Cl2 > Br2 > I2. Rate of replacement of hydrogens of alkanes is : 3° > 2° > 1°. Fluorination is too violent to be controlled. Iodination is very slow and a reversible reaction. It can be carried out in the presence of oxidizing agents like HIO3 or HNO3.

Halogenation is supposed to proceed via free radical chain mechanism involving three steps namely initiation, propagation and termination

Combustion Alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of large amount of heat.
Due to the evolution of large amount of heat during combustion, alkanes are used as fuels. During incomplete combustion of alkanes with insufficient amount of air or dioxygen, carbon black is formed which is used in the manufacture of ink, printer ink, black pigments and as filters.

Controlled oxidation Alkanes on heating with a regulated supply of dioxygen or air at high pressure and in the presence of suitable catalysts give a variety of oxidation products.
Ordinarily alkanes resist oxidation but alkanes having tertiary H atom can be oxidized to corresponding alcohols by potassium permanganate .
Pyrolysis Higher alkanes on heating to higher temperature decompose into lower alkanes, alkenes etc. Such a decomposition reaction into smaller fragments by the application of heat is called pyrolysis or cracking.

Pyrolysis of alkanes is believed to be a free radical reaction. Preparation of oil gas or petrol gas from kerosene oil or petrol involves the principle of pyrolysis. For example, dodecane, a constituent of kerosene oil on heating to 973K in the presence of platinum, palladium or nickel gives a mixture of heptane and pentene.

Conformations- Alkanes contain carbon-carbon sigma (σ) bonds. Electron distribution of the sigma molecular orbital is symmetrical around the internuclear axis of the C–C bond which is not disturbed due to rotation about its axis. This permits free rotation about C–C single bond. This rotation results into different spatial arrangements of atoms in space which can change into one another. Such spatial arrangements of atoms which can be converted into one another by rotation around a C-C single bond are called conformations or conformers or rotamers. Alkanes can thus have infinite number of conformations by rotation around C-C single bonds. However, it may be remembered that rotation around a C-C single bond is not completely free. It is hindered by a small energy barrier of 1-20 kJ mol–1 due to weak repulsive interaction between the adjacent bonds. Such a type of repulsive interaction is called torsional strain. Conformations of ethane : Ethane molecule (C2H6) contains a carbon – carbon single bond with each carbon atom attached to three hydrogen atoms. Considering the ball and stick model of ethane, keep one carbon atom stationary and rotate the other carbon atom around the C-C axis. This rotation results into infinite number of spatial arrangements of hydrogen atoms attached to one carbon atom with respect to the hydrogen atoms attached to the other carbon atom. These are called conformational isomers (conformers). Thus there are infinite number of conformations of ethane. However, there are two extreme cases. One such conformation in which hydrogen atoms attached to two carbons are as closed together as possible is called eclipsed conformation and the other in which hydrogens are as far apart as possible is known as the staggered conformation. Any other intermediate conformation is called a skew conformation. It may be remembered that in all the conformations, the bond angles and the bond lengths remain the same. Eclipsed and the staggered conformations can be represented by Sawhorse and Newman projections.
1) Alkanes contain carbon-carbon … bonds.
b) pi bond π
Ans- a) sigma σ
2) C-C single bond is
hindered by a small energy barrier of
…. kJ mol –1
c) 100- 427
d) 342- 786
Ans- b) 1-20
3) A decomposition reaction into smaller fragments by the application of heat is called as ….
a) pyrolysis
b) cracking
c) both a) & b)
d) combustion
Ans- c) both a) and b)
4) Which of the following steps are involving in free radical chain mechanism
a) initiation
b) propagation
c) termination
5) The … reaction in which alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of large amount of heat .
Ans- d) combustion
1) What is combustion reaction ?

Ans- Alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of large amount of heat.
2) What is pyrolysis ?
Ans- Higher alkanes on heating to higher temperature decompose into lower alkanes, alkenes etc. Such a decomposition reaction into smaller fragments by the application of heat is called pyrolysis or cracking.

3) Explain controlled oxidation reaction .
Ans- Alkanes on heating with a regulated supply of dioxygen or air at high pressure and in the presence of suitable catalysts give a variety of oxidation products.

Ordinarily alkanes resist oxidation but alkanes having tertiary H atom can be oxidized to corresponding alcohols by potassium permanganate
[C] Long Answers
1) Explain in brief – substitution reaction .

Ans- Alkanes are generally inert towards acids, bases, oxidising and reducing agents. However, they undergo the following reactions under certain conditions.

2) Write short note on Conformation.
Ans- Alkanes contain carbon-carbon sigma (σ) bonds. Electron distribution of the sigma molecular orbital is symmetrical around the internuclear axis of the C–C bond which is not disturbed due to rotation about its axis. This permits free rotation about C–C single bond. This rotation results into different spatial arrangements of atoms in space which can change into one another. Such spatial arrangements of atoms which can be converted into one another by rotation around a C-C single bond are called conformations or conformers or rotamers. Alkanes can thus have infinite number of conformations by rotation around C-C single bonds. However, it may be remembered that rotation around a C-C single bond is not completely free. It is hindered by a small energy barrier of 1-20 kJ mol–1 due to weak repulsive interaction between the adjacent bonds. Such a type of repulsive interaction is called torsional strain. Conformations of ethane : Ethane molecule (C2H6) contains a carbon – carbon single bond with each carbon atom attached to three hydrogen atoms. Considering the ball and stick model of ethane, keep one carbon atom stationary and rotate the other carbon atom around the C-C axis. This rotation results into infinite number of spatial arrangements of hydrogen atoms attached to one carbon atom with respect to the hydrogen atoms attached to the other carbon atom. These are called conformational isomers (conformers). Thus there are infinite number of conformations of ethane. However, there are two extreme cases. One such conformation in which hydrogen atoms attached to two carbons are as closed together as possible is called eclipsed conformation and the other in which hydrogens are as far apart as possible is known as the staggered conformation. Any other intermediate conformation is called a skew conformation. It may be remembered that in all the conformations, the bond angles and the bond lengths remain the same. Eclipsed and the staggered conformations can be represented by Sawhorse and Newman projections.
Case study – 3
Alkenes are unsaturated hydrocarbons containing at least one double bond. What should be the general formula of alkenes? If there is one double bond between two carbon atoms in alkenes, they must possess two hydrogen atoms less than alkanes. Hence, general formula for alkenes is C n H 2n . Alkenes are also known as olefins (oil forming) since the first member, ethylene or ethene (C2H4) was found to form an oily liquid on reaction with chlorine.
Structure of Double Bond Carbon-carbon double bond in alkenes consists of one strong sigma (σ) bond (bond enthalpy about 397 kJ mol–1) due to head-on overlapping of sp 2 hybridised orbitals and one weak pi (π) bond (bond enthalpy about 284 kJ mol–1) obtained by lateral or sideways overlapping of the two 2p orbitals of the two carbon atoms. The double bond is shorter in bond length (134 pm) than the C–C single bond (154 pm). You have already read that the pi (π) bond is a weaker bond due to poor sideways overlapping between the two 2p orbitals. Thus, the presence of the pi (π) bond makes alkenes behave as sources of loosely held mobile electrons. Therefore, alkenes are easily attacked by reagents or compounds which are in search of electrons. Such reagents are called electrophilic reagents. The presence of weaker π-bond makes alkenes unstable molecules in comparison to alkanes and thus, alkenes can be changed into single bond compounds by combining with the electrophilic reagents. Strength of the double bond (bond enthalpy, 681 kJ mol–1) is greater than that of a carbon-carbon single bond in ethane (bond enthalpy, 348 kJ mol–1). Orbital diagrams of ethene molecule are shown in Figure.

Geometrical isomerism: Doubly bonded Carbon atoms have to satisfy the remaining two Valences by joining with two atoms or groups. If the two atoms or groups attached to each Carbon atom are different, they can be Represented by YX C = C XY like structure. YX C = C XY can be represented in space in the Following two ways :

In (a), the two identical atoms i.e., both the X or both the Y lie on the same side of the Double bond but in (b) the two X or two Y lie Across the double bond or on the opposite Sides of the double bond. This results in Different geometry of (a) and (b) i.e. disposition Of atoms or groups in space in the two Arrangements is different. Therefore, they are Stereoisomers. They would have the same Geometry if atoms or groups around C=C bond Can be rotated but rotation around C=C bond Is not free. It is restricted. For understanding This concept, take two pieces of strong Cardboards and join them with the help of two Nails. Hold one cardboard in your one hand And try to rotate the other. Can you really rotate The other cardboard ? The answer is no. The Rotation is restricted. This illustrates that the Restricted rotation of atoms or groups around The doubly bonded carbon atoms gives rise to Different geometries of such compounds. The Stereoisomers of this type are called Geometrical isomers. The isomer of the type (a), in which two identical atoms or groups lie On the same side of the double bond is called Cis isomer and the other isomer of the type (b), in which identical atoms or groups lie on The opposite sides of the double bond is called Trans isomer . Thus cis and trans isomers Have the same structure but have different Configuration (arrangement of atoms or groups In space). Due to different arrangement of Atoms or groups in space, these isomers differ In their properties like melting point, boiling Point, dipole moment, solubility etc. Geometrical or cis-trans isomers of but-2-ene Are represented below :

Cis form of alkene is found to be more polar Than the trans form. For example, dipole Moment of cis-but-2-ene is 0.33 Debye, Whereas, dipole moment of the trans form Is almost zero or it can be said that trans-but-2-ene is non-polar. This can be understood by drawing geometries of the two forms as given below from which it is clear that in the trans-but-2-ene, the two methyl groups are in opposite directions, Therefore, dipole moments of C-CH3 bonds cancel, thus making the trans form non-polar.

In the case of solids, it is observed that the trans isomer has higher melting point than the cis form. Geometrical or cis-trans isomerism is also shown by alkenes of the types XYC = CXZ and XYC = CZW
Preparation – From alkynes: Alkynes on partial reduction with calculated amount of dihydrogen in the presence of palladised charcoal partially deactivated with poisons like sulphur
compounds or quinoline give alkenes. Partially deactivated palladised charcoal is known as Lindlar’s catalyst. Alkenes thus obtained are having cis geometry. However, alkynes on reduction with sodium in liquid ammonia form trans alkenes.

From alkyl halides: Alkyl halides (R-X) on heating with alcoholic potash (potassium hydroxide dissolved in alcohol, say, ethanol) eliminate one molecule of halogen acid to form alkenes. This reaction is known as dehydrohalogenation i.e., removal of halogen acid. This is example of β-elimination reaction, since hydrogen atom is eliminated from the β carbon atom (carbon atom next to the carbon to which halogen is attached).

Nature of halogen atom and the alkyl group determine rate of the reaction. It is observed that for halogens, the rate is: iodine > bromine > chlorine, while for alkyl groups it is : tert > secondary > primary.
Physical properties Alkenes as a class resemble alkanes in physical properties, except in types of isomerism and difference in polar nature. The first three members are gases, the next fourteen are liquids and the higher ones are solids. Ethene is a colourless gas with a faint sweet smell. All other alkenes are colourless and odourless, insoluble in water but fairly soluble in non- polar solvents like benzene, petroleum ether. They show a regular increase in boiling point with increase in size i.e., every – CH2 group added increases boiling point by 20–30 K. Like alkanes, straight chain alkenes have higher boiling point than isomeric branched chain compounds.
1) The first three members of alkenes are …
d) None of above
Ans- a) gases
2) General formula for alkenes is ….
a) C n H 2n+1
b) C n H 2n
c) C n H 2n-1
d) C n H 2n+2
Ans- b) C n H 2n
3) The colour of ethene gas is …
c) Pale Green
Ans- d) None of above
4) The bond length of carbon carbon double bond is … pm
Ans- c) 134
5) Alkenes are also knows as …
a) olefines
b) paraffines
c) oleofines
d) paracetofines
Ans- a) Olefines
1) Give the preparation of alkenes from alkynes.
Ans- Alkynes on partial reduction with calculated amount of dihydrogen in the presence of palladised charcoal partially deactivated with poisons like sulphur

2) Give the preparation of alkenes from alkyl halides.
Ans- Alkyl halides (R-X) on heating with alcoholic potash (potassium hydroxide dissolved in alcohol, say, ethanol) eliminate one molecule of halogen acid to form alkenes. This reaction is known as dehydrohalogenation i.e., removal of halogen acid. This is example of β-elimination reaction, since hydrogen atom is eliminated from the β carbon atom (carbon atom next to the carbon to which halogen is attached).

3) Write the physical properties of alkenes .
Ans- Alkenes as a class resemble alkanes in physical properties, except in types of isomerism and difference in polar nature. The first three members are gases, the next fourteen are liquids and the higher ones are solids. Ethene is a colourless gas with a faint sweet smell. All other alkenes are colourless and odourless, insoluble in water but fairly soluble in non- polar solvents like benzene, petroleum ether. They show a regular increase in boiling point with increase in size i.e., every – CH2 group added increases boiling point by 20–30 K. Like alkanes, straight chain alkenes have higher boiling point than isomeric branched chain compounds.
1) Explain the structure of carbon carbon double bond .
Ans- Carbon-carbon double bond in alkenes consists of one strong sigma (σ) bond (bond enthalpy about 397 kJ mol–1) due to head-on overlapping of sp 2 hybridised orbitals and one weak pi (π) bond (bond enthalpy about 284 kJ mol–1) obtained by lateral or sideways overlapping of the two 2p orbitals of the two carbon atoms. The double bond is shorter in bond length (134 pm) than the C–C single bond (154 pm). You have already read that the pi (π) bond is a weaker bond due to poor sideways overlapping between the two 2p orbitals. Thus, the presence of the pi (π) bond makes alkenes behave as sources of loosely held mobile electrons. Therefore, alkenes are easily attacked by reagents or compounds which are in search of electrons. Such reagents are called electrophilic reagents. The presence of weaker π-bond makes alkenes unstable molecules in comparison to alkanes and thus, alkenes can be changed into single bond compounds by combining with the electrophilic reagents. Strength of the double bond (bond enthalpy, 681 kJ mol–1) is greater than that of a carbon-carbon single bond in ethane (bond enthalpy, 348 kJ mol–1). Orbital diagrams of ethene molecule are shown in Figure.

2) Explain the geometrical isomerism in ethene.

Ans- Doubly bonded Carbon atoms have to satisfy the remaining two Valences by joining with two atoms or groups. If the two atoms or groups attached to each Carbon atom are different, they can be Represented by YX C = C XY like structure. YX C = C XY can be represented in space in the Following two ways :

Cis form of alkene is found to be more polar Than the trans form. For example, dipole Moment of cis-but-2-ene is 0.33 Debye, Whereas, dipole moment of the trans form Is almost zero or it can be said that trans-but-2-ene is non-polar. This can be understood by drawing geometries of the two forms as given below from which it is clear that in the trans-but-2-ene, the two methyl groups are in opposite directions, Threfore, dipole moments of C-CH3 bonds cancel, thus making the trans form non-polar.

Case study – 4
Alkynes – Like alkenes, alkynes are also unsaturated hydrocarbons. They contain at least one triple bond between two carbon atoms. The number of hydrogen atoms is still less in alkynes as compared to alkenes or alkanes. Their general formula is C n H 2n–2 . The first stable member of alkyne series is ethyne which is popularly known as acetylene. Acetylene is used for arc welding purposes in the form of oxyacetylene flame obtained by mixing acetylene with oxygen gas. Alkynes are starting materials for a large number of organic compounds. Hence, it is interesting to study this class of organic compounds.
Structure of Triple Bond Ethyne is the simplest molecule of alkyne series. Structure of ethyne is shown in Figure. Each carbon atom of ethyne has two sp hybridised orbitals. Carbon-carbon sigma (σ) bond is obtained by the head-on overlapping of the two sp hybridised orbitals of the two carbon atoms. The remaining sp hybridised orbital of each carbon atom undergoes overlapping along the internuclear axis with the 1s orbital of each of the two hydrogen atoms forming two C-H sigma bonds. H-C-C bond angle is of 180°.

Each carbon has two unhybridised p orbitals which are perpendicular to each other as well as to the plane of the C-C sigma bond. The 2p orbitals of one carbon atom are parallel to the 2p orbitals of the other carbon atom, which undergo lateral or sideways overlapping to form two pi (π) bonds between two carbon atoms. Thus ethyne molecule consists of one C–C σ bond, two C–H σ bonds and two C–C π bonds. The strength of C≡C bond (bond enthalpy 823 kJ mol-1) is more than those of C=C bond (bond enthalpy 681 kJ mol–1) and C–C bond (bond enthalpy 348 kJ mol–1). The C≡C bond length is shorter (120 pm) than those of C=C (133 pm) and C–C (154 pm). Electron cloud between two carbon atoms is cylindrically symmetrical about the internuclear axis. Thus, ethyne is a linear molecule.
Preparation – From calcium carbide: On industrial scale, ethyne is prepared by treating calcium carbide with water. Calcium carbide is prepared by heating quick lime with coke. Quick lime can be obtained by heating limestone as shown in the following reactions:

From vicinal dihalides : Vicinal dihalides on treatment with alcoholic potassium hydroxide undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with sodamide gives alkyne.

Aromatic hydrocarbon- These hydrocarbons are also known as ‘arenes’. Since most of them possess pleasant odour , the class of compounds was named as ‘aromatic compounds’. Most of such compounds were found to contain benzene ring. Benzene ring is highly unsaturated but in a majority of reactions of aromatic compounds, the unsaturation of benzene ring is retained. However, there are examples of aromatic hydrocarbons which do not contain a benzene ring but instead contain other highly unsaturated ring. Aromatic compounds containing benzene ring are known as benzenoids and those not containing a benzene ring are known as non-benzenoids. Some examples of arenes are given below:

Friedel-Crafts alkylation reaction: When benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkylbenene is formed
Friedel-Crafts acylation reaction: The reaction of benzene with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl3) yields acyl benzene.

If excess of electrophilic reagent is used, further substitution reaction may take place in which other hydrogen atoms of benzene ring may also be successively replaced by the electrophile. For example, benzene on treatment with excess of chlorine in the presence of anhydrous AlCl3 can be chlorinated to hexachlorobenzene (C6Cl6)

1) The general formula of Alkynes is …
a) C n H 2n–2
b) C n H 2n–2
c) C n H 2n–2
d) C n H 2n–2
Ans- a) C n H 2n–2
2) Calcium carbide is prepared by heating quick lime with …
a) backing soda
Ans- b) coke
3) Vicinal dihalides on treatment with alcoholic potassium hydroxide undergo …
a) dehydrogenation.
b) hydrohalogenation.
c) dehydrohalogenation
d) dehalogenation.
Ans- c) dehydrohalogenation.
4) The bond enthalpy of C≡C is …
a) 523 kJ mol
b) 623 kJ mol
c) 723 kJ mol
d) 823 kJ mol
Ans- d) 823 kJ mol
5) The C≡C bond length is …
Ans- d) 120 pm
1) What are alkynes ?
Ans- Like alkenes, alkynes are also unsaturated hydrocarbons. They contain at least one triple bond between two carbon atoms. The number of hydrogen atoms is still less in alkynes as compared to alkenes or alkanes. Their general formula is C n H 2n–2 . The first stable member of alkyne series is ethyne which is popularly known as acetylene. Acetylene is used for arc welding purposes in the form of oxyacetylene flame obtained by mixing acetylene with oxygen gas. Alkynes are starting materials for a large number of organic compounds. Hence, it is interesting to study this class of organic compounds.
2) Give the preparation of alkynes from
i) From calcium carbide
ii) From vicinal dihalides

Ans- From calcium carbide: On industrial scale, ethyne is prepared by treating calcium carbide with water. Calcium carbide is prepared by heating quick lime with coke. Quick lime can be obtained by heating limestone as shown in the following reactions:
3) What are aromatic hydrocarbon ?

Ans- Aromatic hydrocarbon- These hydrocarbons are also known as ‘arenes’. Since most of them possess pleasant odour , the class of compounds was named as ‘aromatic compounds’. Most of such compounds were found to contain benzene ring. Benzene ring is highly unsaturated but in a majority of reactions of aromatic compounds, the unsaturation of benzene ring is retained. However, there are examples of aromatic hydrocarbons which do not contain a benzene ring but instead contain other highly unsaturated ring. Aromatic compounds containing benzene ring are known as benzenoids and those not containing a benzene ring are known as non-benzenoids. Some examples of arenes are given below:
1) Explain the following reactions –
i) Friedel-Crafts alkylation reaction
ii) Friedel-Crafts acylation reaction

Ans- Friedel-Crafts alkylation reaction: When benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkylbenene is formed

2) Explain the structure of Triple Bond Ethyne.

Ans- Ethyne is the simplest molecule of alkyne series. Structure of ethyne is shown in Figure. Each carbon atom of ethyne has two sp hybridised orbitals. Carbon-carbon sigma (σ) bond is obtained by the head-on overlapping of the two sp hybridised orbitals of the two carbon atoms. The remaining sp hybridised orbital of each carbon atom undergoes overlapping along the internuclear axis with the 1s orbital of each of the two hydrogen atoms forming two C-H sigma bonds. H-C-C bond angle is of 180°.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Sikkim scert class 4 evs chapter 8 fields to market to home solution, the swami and mother-worship by sister nivedita mcq question answers, tripura board class 6 bengali solutions chapter 6 খোকার আকাঙ্ক্ষা, the bangle sellers class 11 mcq questions and answers for semester 1.
Sign in to your account
Username or Email Address
Remember Me
- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 1st Standard - CVBHSS Materials
- 2nd Standard - CVBHSS Materials
- 3rd Standard - CVBHSS Materials
- 4th Standard - CVBHSS Materials
- 5th Standard - CVBHSS Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

Class 11th Chemistry - Hydrocarbons Case Study Questions and Answers 2022 - 2023
By QB365 on 09 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 11 Chemistry Subject - Hydrocarbons, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Hydrocarbons case study questions with answer key.
11th Standard CBSE
Final Semester - June 2015
Organic reactions can be classified into four main categories. Substitution reactions, addition reactions, elimination reactions and rearrangement reactions. Substitution reactions can be further classified into free radical, nucleophilic and electrophilic substitution reactions. Addition reactions can be nucleophilic as well as electrophilic addition reactions. Dehydration, dehydrohalogenation, dehalogenation are examples of elimination reactions. Conversion by ammonium cyanate to urea is an example of rearrangement reactions. Reactions are classified on the basis to nature of intermediate species formed. Mechanism of reaction is exact path followed by the reaction involving all steps showing intermediates and slowest steps of the reaction which is rate determining step. Oxidation, reduction, combustion reactions are also important in hydrocarbons. (a) Halogenation of alkane is an example of which type of reaction? (b) What happens when 2-methyl propane is heated with KMnO 4 ? (c) What type of reaction takes place when n-hexane is heated in presence of AICI 3 (anhy.) and HCI?CH2 =CH2 + KCI + HzO (d) What happens when but-2-yne reacts with H 2 in presence of Lindlar's catalyst. (e) CH 3 CH 2 C1 + KOH(alc) \(\longrightarrow\) CH 2 =CH 2 + KCI + H 2 O What is type of reaction? (f) Why do aromatic hydrocarbons undergo electrophilic substitution reaction?
Observe the table of variation of melting and boiling point in Alkanes. Study the table and answer the questions that follow based on table and related studied concepts. Variation of Melting Point and Boiling Point in Alkanes
(a) How does boiling point varies with the increase in carbon chain? Why? (b) Why does 2, 2-dimethyl propane has lower boiling point than 2-methyl butane and n-pentane? (c) Why is melting point of 2, 2-dimethyl propane is higher than n-pentane and 2-methyl butane? (d) What is physical state of heptane? (e) How does density vary with the increase in molar mass in hydrocarbons? (f) What is physical state of C 20 H 42 (Eicosane)?
Hydrocarbons are compounds of carbon and hydrogen only, obtained from coal and petroleum mainly which are major sources of energy. Hydrocarbons are classified as open chain, saturated (alkanes), unsaturated (alkenes and alkynes), cyclic (alicyclic) and aromatic based on structure. Alkanes show conformational isomerism due to free rotation along C-C bond leading to staggered and eclipsed conformations of ethane. Staggered conformation is more stable. Alkenes show geometrical (Cis-trans) isomerism due to restricted rotation around carbon-carbon double bond. Benzene and benzenoid show aromatic character. They follow Huckel rule \((4 n+2) \pi\) electrons which must be delocalised. The presence of activating and deactivating groups decide the position of electrophile after electrophilic substitution. Polynuclear fused aromatic hydrocarbons have carcinogenic property. Benzene is prepared by polymerisation of ethyne and by heating sodium benzoate with soda lime. (a) Why is staggered form of ethane more stable than eclipsed form? (b) Out of T-butene and 2-butene which will show geometrical isomerism. (c) Why is cis-2.-butene has higher boiling point than trans-2-butene? (d) Why is cyclopentadienyl anion is aromatic? (e) Why is -NO 2 group m-directing towards electrophilic substitution? (f) Convert acetylene to benzene . (g) Sodium benzoate, on heating with soda lime gives benzene, name the reaction.
*****************************************
Hydrocarbons case study questions with answer key answer keys.
(a) Boiling point increases with increase in carbon chain because surface area increases, van der Waals' forces of attraction increases, hence boiling point increases. (b) It is because boiling point decreases with increase in branching because surface area decreases, van der Waals' forces of attraction decreases, hence it has lower boiling point. (c) It is because molecules are closely packed in 2, 2-dimethyl propane as compared to 2-methyl butane and n-pentane (d) It is liquid. (e) The density goes on increasing with the increase in molar mass. (f) It exist in solid state.
Related 11th Standard CBSE Chemistry Materials
11th standard cbse syllabus & materials, cbse 11th physics motion in a straight line chapter case study questions with answers, cbse 11th physics units and measurements chapter case study questions with answers, 11th biology biological classification chapter case study question with answers cbse, 11th biology the living world chapter case study question with answers cbse, class 11th business studies - internal trade case study questions and answers 2022 - 2023, class 11th business studies - social responsibilities of business and business ethics case study questions and answers 2022 - 2023, class 11th business studies - emerging modes of business case study questions and answers 2022 - 2023, class 11th business studies - business service case study questions and answers 2022 - 2023, class 11th business studies - private, public and global enterprises case study questions and answers 2022 - 2023, class 11th business studies - business, trade and commerce case study questions and answers 2022 - 2023.

Class 11th Applied Mathematics - Coordinate Geometry Case Study Questions and Answers 2022 - 2023
Class 11th applied mathematics - basics of financial mathematics case study questions and answers 2022 - 2023, class 11th applied mathematics - descriptive statistics case study questions and answers 2022 - 2023, class 11th applied mathematics - probability case study questions and answers 2022 - 2023, class 11th applied mathematics - calculus case study questions and answers 2022 - 2023.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

11th Standard CBSE Study Materials

11th Standard CBSE Subjects

CBSE Expert
Case Study Questions for Class 11 Chemistry PDF Download
We have provided here Case Study questions for the Class 11 Chemistry for final board exams. You can read these chapter-wise Case Study questions. These questions are prepared by subject experts and experienced teachers. The answer key is also provided so that you can check the correct answer for each question. Practice these questions to score well in your exams.

CBSE 11th Standard CBSE Chemistry question papers, important notes, study materials, Previous Year questions, Syllabus, and exam patterns. Free 11th Standard CBSE Chemistry books and syllabus online. Important keywords, Case Study Questions, and Solutions.
Class 11 Chemistry Case Study Questions
CBSE Class 11 Chemistry question paper will have case study questions too. These case-based questions will be objective type in nature. So, Class 11 Chemistry students must prepare themselves for such questions. First of all, you should study NCERT Textbooks line by line, and then you should practice as many questions as possible.
Chapter-wise Solved Case Study Questions for Class 11 Chemistry
- Case Study Based Questions on Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
- Case Study Based Questions on Class 11 Chemistry Chapter 2 Structure of Atom
- Case Study Based Questions on Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- Case Study Based Questions on Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- Case Study Based Questions on Class 11 Chemistry Chapter 6 Thermodynamics
- Case Study Based Questions on Class 11 Chemistry Chapter 7 Equilibrium
- Case Study Based Questions on Class 11 Chemistry Chapter 8 Redox Reactions
- Case Study Based Questions on Class 11 Chemistry Chapter 12 Organic Chemistry -Some Basic Principles and Techniques
- Case Study Based Questions on Class 11 Chemistry Chapter 13 Hydrocarbons
Class 11 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 11 Chemistry examinations. Our expert faculty for standard 11 Chemistry have designed these questions based on the trend of questions that have been asked in last year’s exams. The solutions have been designed in a manner to help the grade 11 students understand the concepts and also easy to learn solutions.
Class 11 Chemistry Books

Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
myCBSEguide
- Class 11 Chemistry Case...
Class 11 Chemistry Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Are you perplexed? Are you having trouble finding different Class 11 Chemistry Case Study Questions? If the answer is affirmative! You’ve arrived at the perfect place. The myCBSEguide App offers case study questions for CBSE class 11 Chemistry.
CBSE class 11 Chemistry students have always been under a lot of pressure to perform well in their board exams. This pressure has increased in recent years, with the inclusion of case study questions and other Higher Order Thinking Skills Questions. myCBSEguide provides with all the resources that class 11 Chemistry students need to prepare for their class 11 Chemistry exams. This includes sample papers, question banks, case study questions and revision notes prepared by experienced teachers.
The logical reason for Chemistry
Higher Secondary is the most important stage of schooling because it is at this point that specialized discipline-based, content-oriented courses are introduced. Students reach this stage after completing ten years of general education and choose Chemistry to pursue a career in basic sciences or professional courses such as medicine, engineering, or technology, as well as tertiary study courses in applied science and technology. As a result, learners must be provided with a sufficient conceptual background in Chemistry in order to be prepared to meet the challenges of academic and professional courses after high school.
Chemistry in Class 11
Chemistry studies the composition, structure, properties, and reactions of matter. Chemistry is included as one of the major disciplines in CBSE class 11 for a variety of reasons.
- First and foremost, chemistry is essential for understanding the basic structure and composition of matter.
- In addition, chemistry plays a critical role in many other disciplines, such as biology, environmental science, and engineering.
- Furthermore, chemistry is a fascinating subject in its own right, and studying it can provide a deeper understanding of the world around us.
- Finally, chemistry is a practical subject that can be used in everyday life, and it is therefore important for students to have a good understanding of it.
Integrating Case Study Questions in Chemistry for Class 11
Case study questions can be a great way to integrate real-world applications of chemistry into your class 11 curriculum. By posing questions that require students to analyze and apply their knowledge of chemical concepts, you can help them to see how those concepts are relevant to their lives outside of the classroom. Additionally, case study questions can be used to spark lively classroom discussion and debate.
Sample Class 11 Case Study Questions
The recent addition of Case Study Questions in Chemistry for Class 11 by CBSE has been welcomed by students and teachers alike. In Class 11 Chemistry, the case study approach is seen as an engaging and effective way to learn, and the questions included in Class 11 Chemistry is designed to challenge and stimulate students’ thinking. Class 11 Chemistry Case Study Questions cover a wide range of topics, from the basics of atomic structure to more complex concepts such as chemical reactions and equilibrium. The addition of this resource is sure to enhance the learning experience for Class 11 Chemistry students of all abilities while solving Class 11 Chemistry Case Study Questions.
myCBSEguide is the only app that provides a wide range of Class 11 Chemistry Case Study Questions curated by expert teachers. By providing access to this wealth of resources, myCBSEguide makes it easy for Class 11 Chemistry students to learn about and understand complex chemical concepts. In addition, the app’s comprehensive collection of Class 11 Chemistry Case Study Questions covers all major topics in the subject, making it an invaluable resource for students preparing for Class 11 Chemistry exams.
Have a look at few samples of Class 11 Chemistry Case Study Questions for better understanding.
Class 11 Chemistry Case Study Question 1
Read the passage and answer the following question: In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the liberation and deposition of matter at the electrodes. In mid-1850s Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode to the positive electrode. These were called cathode rays or cathode ray particles. J.J. Thomson measured the ratio of electrical charge (e) to the mass of the electron (m e ) by using a cathode ray tube and applying electrical and magnetic fields perpendicular to each other as well as to the path of electrons. A positively charged particle was characterised in 1919. Later, a need was felt for the presence of an electrically neutral particles as one of the constituents of the atom.
In this question, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices
- Assertion and reason both are correct statements and reason is the correct explanation for assertion.
- Assertion and reason both are correct statements and reason is not the correct explanation for assertion.
- Assertion is the correct statement but reason is wrong statement.
- Assertion is the wrong statement but reason is correct statement.
- Assertion: The cathode rays start from cathode and move towards the anode. Reason: In the absence of electrical or magnetic field, cathode rays travel in straight lines.
- Assertion: Thomas argued that the lighter the particle, greater the deflection. Reason: Deflection depends upon the mass of the particle.
- Assertion: Television picture tubes are anode ray tubes. Reason: Electrons are the basic constituent of all the atoms.
- Assertion: The charge to mass ratio of the particles depends on the gas from which these originate. Reason: The smallest and lightest positive ion was obtained from hydrogen and was called proton.
Assertion: A cathode ray tube is made of glass containing two thin pieces of metal electrodes. Reason: The value of e/m e is 2.758820 × 10 11 C kg -11 .
Answer Key:
- (b) Assertion and reason both are correct statements and reason is not the correct explanation for assertion.
- (a) Assertion and reason both are correct statements and reason is the correct explanation for assertion.
- (d) Assertion is the wrong statement but reason is correct statement.
- (c) Assertion is the correct statement but reason is wrong statement.
Class 11 Chemistry Case Study Question 2
Read the passage and answer the following question: The existing large number of organic compounds and their ever-increasing numbers has made it necessary to classify them on the basis of their structures. Organic compounds are broadly classified as open-chain compounds which are also called aliphatic compounds. Aliphatic compounds further classified as homocyclic and heterocyclic compounds. Aromatic compounds are special types of compounds. Alicyclic compounds, aromatic compounds may also have heteroatom in the ring. Such compounds are called heterocyclic aromatic compounds. Organic compounds can also be classified on the basis of functional groups, into families or homologous series. The members of a homologous series can be represented by general molecular formula and the successive members differ from each other in molecular formula by a –CH 2 unit.
In these questions, a statement of assertion followed by the statement of reason is given. Choose the correct answer out of the following choices
- Assertion: Tetrahydrofuran is aliphatic compounds Reason: Sometimes atoms other than carbon are also present in the ring known as heterocyclic.
- Assertion: Hydroxyl group (–OH) is a functional group. Reason: The functional group is defined as an atom or group of atoms joined in a specific manner with characteristic chemical properties of the organic compounds.
- Assertion: Non-benzenoid compound is a classification as the alicyclic compound. Reason: Aniline is a benzenoid compound.
- Assertion: H 2 C=CH 2 is a condensed structural formula. Reason: Condensed structural formula is represented by omitting some or all of the dashes representing covalent bonds.
Assertion: Cyclic compound is classified as a carbocyclic and heterocyclic compound. Reason: Thiophene is a homocyclic compound.
Class 11 Chemistry Case Study Question 3
Read the passage and answer the following questions: A large number of orbitals are possible in an atom. Qualitatively these orbitals can be distinguished by their size, shape and orientation. An orbital of smaller size means there is more chance of finding the electron near the nucleus. Similarly, shape and orientation mean that there is more probability of finding the electron along with certain directions than along others. The principal quantum number determines the size and to large extent the energy of the orbital. Azimuthal quantum number, ‘l’ is also known as orbital angular momentum or subsidiary quantum number. It defines the three-dimensional shape of the orbital. Each shell consists of one or more subshells or sub-levels. The number of sub-shells in a principal shell is equal to the value of n. Magnetic orbital quantum number. ‘ml ’ gives information about the spatial orientation of the orbital with respect to a standard set of co-ordinate axis. The fourth quantum number is known as the electron spin quantum number (m s ). An electron spins around its own axis, much in a similar way as the earth spins around its own axis while revolving around the sun. In these questions, a statement of assertion followed by the statement of reason is given. Choose the correct answer out of the following choices:
- Assertion: Each orbital is designated by three quantum numbers labelled as n, l and m l . Reason: ‘n’ is a positive integer with value of n = 1,2,3.
- Assertion: The principal quantum number identifies the shell. Reason: Size of an orbital decrease with the increase of principal quantum number ‘n’.
- Assertion: For n = 2, the possible value of l can be 0 and 1. Reason: For a given value of n, l can have n values ranging from 0 to n – 1.
- Assertion: Each orbital in an atom, is defined by a set of values for n, l and m l . Reason: m l designates the orientation of the orbital. OR Assertion: Spin quantum numbers m s can take the values of +½ or –½. Reason: Two spin states of the electron and are normally represented by two arrows, ↑ (spin down) and ↓ (spin up).
- (b) Assertion and reason both are correct statements and reason is not the correct explanation for assertion. OR (c) Assertion is the correct statement but reason is wrong statement.
Significance of Class 11 Chemistry case study questions
Case study questions in Class 11 Chemistry are important because:
- They help Class 11 Chemistry students understand basic chemistry facts and concepts while keeping the excitement alive.
- It prepares Class 11 Chemistry students to study chemistry in tertiary courses such as medicine, engineering, and technology. • introduce students to a variety of emerging new areas of chemistry and inform them of their importance in future studies as well as their applications in various fields of chemical science and technology.
- It prepares Class 11 Chemistry students to face a variety of health, nutrition, environmental, population, weather, industry, and agriculture challenges.
- It also improves Class 11 Chemistry students’ problem-solving abilities.
- It helps in familiarizing Class 11 Chemistry students with various industrial processes as well as their technological applications.
Glancing the Class 11 Chemistry syllabus
The revised and updated Class 11 Chemistry syllabus is built on a disciplinary approach with rigour and depth, while keeping the Class 11 Chemistry syllabus light and comparable to the worldwide level. Greater emphasis has been placed in the Class 11 Chemistry syllabus on the use of new nomenclature, symbols, and formulations, the teaching of fundamental concepts, the application of concepts in chemistry to industry/technology, logical sequencing of units, the removal of obsolete content and repetition, and so on.
CBSE Class 11 Chemistry (Code No. 043) Syllabus (2022-23)
myCBSEguide: Balanced equation for Class 11 Chemistry students
For Class 11 Chemistry students, myCBSEguide is always a balanced equation. It includes a plethora of class 11 chemistry case study questions to help students better understand the ideas in Class 11 Chemistry. Class 11 chemistry case study questions are intended to assess students’ comprehension while also providing a platform for them to strengthen their problem-solving abilities. myCBSEguide also provides a number of other tools that students can utilise to deepen their grasp of the subject. Additionally, the answer keys and explanations provided by myCBSEguide are always clear and concise, making it an excellent tool for students who want to improve their grades. So, what are you waiting for? Get myCBSEguide today and start studying for your future.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 13: Hydrocarbons
About this unit.
A hydrocarbon is an organic chemical compound composed exclusively of hydrogen and carbon atoms. Hydrocarbons occur naturally and form the basis of crude oil, natural gas, coal, and other important energy sources.
In this unit of class 11 chemistry, we will learn in detail about nomenclature, isomerism, structure, preparation, physical & chemical properties of alkanes, alkenes, alkynes, & aromatic hydrocarbons. We will learn to explain different conformations of ethane (staggered & eclipsed). In aromatic hydrocarbon, we will also learn resonance structures, we will also think about how those reactions are occurring on a molecular level with reaction mechanisms- electrophilic substitution reactions of benzene (nitration, sulphonation, halogenation, Friedel craft's alkylation & acylation.
Classification
- Alkanes, Alkenes, and Alkynes- General molecular formula (Opens a modal)
- Nomenclature of hydrocarbons: Alkanes, Alkenes, & Alkynes (Opens a modal)
Alkanes: Nomenclature and isomerism
- Naming simple alkanes (Opens a modal)
- Naming alkanes with alkyl groups (Opens a modal)
- Correction - 2-propylheptane should never be the name! (Opens a modal)
- Naming alkanes with ethyl groups (Opens a modal)
- Alkane with isopropyl group (Opens a modal)
- Representing structures of organic molecules (Opens a modal)
Alkanes: Properties
- Free radical reactions (Opens a modal)
Alkanes: Conformations
- Newman projections (Opens a modal)
- Conformations of ethane (Opens a modal)
- Conformational analysis of ethane (Opens a modal)
Alkenes: Structure, nomenclature, and isomerism
- Alkene structure and classification (Opens a modal)
- sp² hybridization (Opens a modal)
- Alkene nomenclature (Opens a modal)
- Naming alkenes examples (Opens a modal)
- cis-trans and E-Z naming scheme for alkenes (Opens a modal)
- Cis–trans isomerism (Opens a modal)
- E–Z system (Opens a modal)
- Alkene stability (Opens a modal)
Alkenes: Preparation
- Reduction of alkynes (Opens a modal)
Alkenes: Properties
- Introduction to reaction mechanisms (Opens a modal)
- Markovnikov's rule and carbocations (Opens a modal)
- Free radical addition of HBr to an alkene (peroxide effect) (Opens a modal)
- Addition of water (acid-catalyzed) mechanism (Opens a modal)
- Alkene halogenation (Opens a modal)
- Halohydrin formation (Opens a modal)
- Hydroboration-oxidation (Opens a modal)
- Hydroboration-oxidation: Mechanism (Opens a modal)
- Polymerization of alkenes with acid (Opens a modal)
- Ozonolysis (Opens a modal)
- Electrophilic addition of Hydrogen halide( HX) 4 questions Practice
- Peroxide effect 4 questions Practice
- Electrophilic addition of water 4 questions Practice
- Electrophilic addition of halogens to alkenes 4 questions Practice
- Electrophilic addition of halogens: Halohydrin formation 4 questions Practice
- Hydroboration of alkenes 4 questions Practice
Alkynes: Structure, nomenclature, isomerism, & preparation
- Alkyne nomenclature (Opens a modal)
- Preparation of alkynes (Opens a modal)
Alkynes: Properties
- Alkyne acidity and alkylation (Opens a modal)
- Hydrohalogenation of alkynes (Opens a modal)
- Hydrohalogenation of alkynes- Part 1 (Opens a modal)
- Hydrohalogenation of alkynes- Part 2 (Opens a modal)
- Hydration of alkynes (Opens a modal)
- Hydroboration-oxidation of alkynes (Opens a modal)
- Halogenation and ozonolysis of alkynes (Opens a modal)
- Synthesis using alkynes (Opens a modal)
- Hydrohalogenation of alkynes 4 questions Practice
- Acid-catalysed hydration of alkynes 4 questions Practice
- Hydroboration of alkynes 4 questions Practice
Aromatic hydrocarbon: Nomenclature and isomerism
- Naming benzene derivatives introduction (Opens a modal)
- Naming benzene derivatives (Opens a modal)
Resonance stability and Aromaticity of benzene
- Resonance structures for benzene and the phenoxide anion (Opens a modal)
- Aromatic stability I (Opens a modal)
- Aromatic stability II (Opens a modal)
- Aromatic stability III (Opens a modal)
- Aromatic stability IV (Opens a modal)
- Aromatic stability V (Opens a modal)
- Aromatic heterocycles I (Opens a modal)
- Aromatic heterocycles II (Opens a modal)
- Application of aromaticity 4 questions Practice
Properties of benzene
- Electrophilic aromatic substitution (Opens a modal)
- Bromination of benzene (Opens a modal)
- Friedel-Crafts acylation (Opens a modal)
- Friedel crafts acylation addendum (Opens a modal)
Mechanism of electrophilic substitution reactions
- Electrophilic aromatic substitution mechanism (Opens a modal)
- Aromatic halogenation (Opens a modal)
- Nitration (Opens a modal)
- Sulfonation (Opens a modal)
- Friedel-Crafts alkylation (Opens a modal)
- Reactivity of molecules towards EAS (BASIC) 4 questions Practice
- Where will EAS occur? 4 questions Practice
- Halogenation of benzene 4 questions Practice
- Nitration and Sulphonation of Benzene. 4 questions Practice
- Friedel-Crafts Reactions on Benzene 4 questions Practice
- Friedel-Crafts Alkylation reactions of benzene (INTERMEDIATE) 4 questions Practice
Directive influence of a functional group in a mono substituted benzene
- Ortho-para directors I (Opens a modal)
- Ortho-para directors II (Opens a modal)
- Ortho-para directors III (Opens a modal)
- Meta directors I (Opens a modal)
- Meta directors II (Opens a modal)
- Multiple substituents (Opens a modal)
- Nitration of Phenols (Opens a modal)
- Bromination of Phenols (Opens a modal)
- Kolbe's Reaction (Opens a modal)
- Reimer Tiemann Reaction (Opens a modal)
- Bromination of Aniline (Opens a modal)
- Nitration of Aniline (Opens a modal)
- Electrophilic Aromatic Substitution Reactions of substituted benzene 4 questions Practice
- Interesting behaviour of phenol towards some EAS reactions. 4 questions Practice
- Interesting behaviour of aniline towards EAS. 4 questions Practice
Elimination reactions
- No videos or articles available in this lesson
- Which is a base and which is a nucleophile? 4 questions Practice
CBSE Class 11 Chemistry – Chapter 13 Hydrocarbons- Study Materials
NCERT Solutions Class 11 All Subjects Sample Papers Past Years Papers
Hydrocarbons : Notes and Study Materials -pdf
- Concepts of Hydrocarbons
- Hydrocarbons Master File
- Hydrocarbons Revision Notes
- Hydrocarbons MindMap
- NCERT Solution Hydrocarbons
- NCERT Exemplar Solution Hydrocarbons
- Hydrocarbons : Solved Example 1
- Hydrocarbons: Solved Example 2
- Hydrocarbons : Practice Paper 1
Subtopics of Class 11 Chemistry Chapter 13 – Hydrocarbons
- Classification
- Nomenclature And Isomerism
- Preparation
- Conformations
- Structure Of Double Bond
- Nomenclature
- Structure Of Triple Bond
- Structure Of Benzene
- Aromaticity
- Preparation Of Benzene
- Directive Influence Of A Functional Group In Monosubstituted Benzene
- Carcinogenicity And Toxicity
Hydrocarbons Class 11 Notes Chemistry Chapter 13
• Hydrocarbon A compound of carbon and hydrogen is known as hydrocarbon. • Saturated Hydrocarbon A hydrocarbon is said to be saturated if it contains only C—C single bonds. For example: Ethane CH 3 —CH 3 • Unsaturated Hydrocarbon • Aromatic Hydrocarbon Benzene and its derivatives are called aromatic compounds. Example: • Alicyclic Compounds Cyclic compounds which consist only of carbon atoms are called alicyclic or carboeyclic compounds. • Heterocyclic Compounds Cyclic compounds in which the ring atoms are of carbon and some other element (For example, N, S, or O) are called heterocyclic compounds. • Alkanes Alkanes are the simplest organic compounds made of carbon and hydrogen only. They have the general formula C n HC 2n+2 (where n = 1, 2, 3, etc.) The carbon atoms in their molecules are bonded to each other by single covalent bonds. Since the carbon skeleton of alkanes is fully saturated’ with hydrogens, they are also called saturated hydrocarbons. Alkanes contain strong C —C and C —H bonds. Therefore, this class of hydrocarbons are relatively chemically inert. Hence they are sometimes referred to as paraffins (Latin parum affinis = little affinity). First three members of this class can be represented as Structure: In methane carbon forms single bonds with four hydrogen atoms. All H—G—H bond angles are of 109.5°. Methane has a tetrahedral structure. C—C and C—H bonds are formed by head-on overlapping of sp 3 hybrid orbitals of carbon and Is orbitals of hydrogen atoms. • Nomenclature Guidelines Use the following step-by-step procedure to write the IUPAC names from the structural formulas. Consider the following structural formula: Step 1. Identify the longest chain: In the given example, longest chain has seven carbons. The seven carbon chain is heptane. Step 2. Number the chain: The chain is numbered from left to right. This gives lowest numbers to the attached alkyl group. Step 3. Identify the alkyl group: There are two methyl groups at C-2 and C-3, there is one ethyl group of C-4. Step 4. Write the IUPAC name: In this case the IUPAC name is 4-Ethyl-2,3-dimethyl heptane. Always keep in mind (a) Numbers are separated from each other by commas. (b) Numbers are separated from names by hyphens, (c) Prefixes di, tri are not taken into account in alphabetising substituent names. • Newman Projections In this projection, the molecule is viewed at the C—C bond head on. • Relative Stability of Conformations In staggered form of ethane there are maximum repulsive forces, minimum energy and maximum stability of molecule. On the other hand, when the staggered form changes in the eclipsed form the electron clouds of the carbon hydrogen bonds come closer to each other resulting in increase in electron cloud repulsions, molecule have to possess more energy and thus has lower stability. Torsional Angle: Magnitude of torsional strain depends upon the angle of rotation about C—C bond. This angle is also called dihedral angle or torsional angle. • Alkenes Alkenes are hydrocarbons that contain a carbon-carbon double bond (C=C) in their molecule. They have the general formula Structure: Let us consider (H 2 C=CH 2 ) for illustrating the orbital make up of alkenes. In ethylene the carbon atoms are sp 2 hybridized- They are attached to each other by a a bond and a σ bond. The a bond results from the overlap of two sp 2 hybrid orbitals. The π bond is formed from overlap of the unhybridized p-orbitals. Ethylene is a planar molecule. Ppints to be noted (i) The carbon-carbon double bond in alkenes is made up of one σ and one π-bond. (ii) Alkenes are more reactive than Alkanes. This is due to the availability of n electrons. • Nomenclature In IUPAC system (i) The name of the hydrocarbon is based on the parent alkene having the longest ‘ carbon chain of which double bond is apart. (ii) This chain is numbered from the end near the double bond and its position is indicated by the number of the carbon atom not which the double bond originates, (iii) The name of the parent alkene with the position number of the double bond is written first and then the names of other substituents prefixed to it. (iv) When there are two or three double bonds in a molecule, the ending-one of the corresponding alkane is replaced by-a diene to get the name. • Isomerism Structural Isomerism: Ethene and propene have no structural isomers, but there are three structures of butenes. Of these, two are straight chain structures with the difference being in the position of double bond in the molecules. These are position isomers and third structure is a branched-chain isomer. Geometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond. This presentation of rotation about the carbon-carbon double bond gives rise to the phenomenon of geometrical isomerism. An alkene having a formula RCH=CHR can have two stereoisomers, depending upon whether the two alkyl groups are on the same or opposite sides of the double bond. If they are on the same side, then it is called cis-isomer. If they are on opposite sides, then it is called trans-isomer. Due to different arrangement of atoms or groups in space, these isomers differ in their properties like melting point, boiling point, dipole moment, solubility, etc. • Alkynes Alkynes are characterised by the presence of a triple bond in the molecule. Their general formula is C n H 2n-2 . The first and the most important member of this series of hydrocarbons is acetylene, HC=CH, and hence they are also called the Acetylenes. Structure: Let us consider ethyne (HC=CH) for illustrating the orbital make up of ethyne. In ethyne, the carbon atoms are sp hybridized. They are attached to each other by a σ-bond and two π-bonds. The σ -bond results from the overlap of two sp hybrid orbitals. The π bonds are formed from the separate overlap of the two p-orbitals from the two adjacent carbon atoms. The other sp hybrid orbital of each carbon atom forms a σ bond with another carbon or hydrogen atom. Ethyne is a linear molecule. Points to be noted: (i) The carbon-carbon triple bond in alkynes is made up of one σ and two π bonds. (ii) Like alkenes, alkynes undergo addition reaction. These reactions are due to the availability of more exposed π electrons. • Nomenclature IUPAC System: The IUPAC names of alkynes are obtained by dropping the ending-ane of the parent alkane and adding the suffix-yne. Carbon chain including the triple bond is – numbered from the end nearest this bond. The position of the triple bond is indicated by prefixing the number of carbon preceding it to the name of the alkyne. Preparation: From calcium carbide: Ethyne is prepared by treating calcium carbide with water. Calcium carbide is prepared as follows: From vicinal dihalides : When reacted with vicinal dihalides, alcoholic potassium hydroxide undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with sodamide gives alkyne. • Aromatic Hydrocarbons These hydrocarbons are also known as ‘arenes’. Most of such compounds were found to contain benzene ring. Aromatic compounds containing benzene ring are known as benzenoids and those not containing a benzene ring are known as non-benzenoids. Some examples of arenes are given below. Nomenclature and Isomerism: Benzene and its homologous are generally called by their common names which are accepted by the IUPAC system. The homologous of benzene having a single alkyl group are named as Alkyl benzenes. Structure of Benzene: By elemental analysis, it is found that molecular formula of benzene is C 6 H 6 . This indicates that benzene is a highly unsaturated compound. In 1865, Kekule gave the cyclic planar structure of benzene with six carbons with alternate double and single bonds. The Kekule structure indicates the possibility of two isomeric 1,2-dibromobenzenes. In one of the isomers, the bromine atoms are attached to the doubly bonded carbon atoms whereas in the other they are attached to the singly bonded carbon. In fact, only one ortho-dibromobenzene could be prepared. To overcome this problem Kekule suggested that benzene was a mixture of two forms. Failure of Kekule’s structure: Kekule structure of benzene failed to explain the unique stability and its preference to substitution reaction than addition reactions. Resonance Structure of Benzene: The phenomenon in which two or more structures can be written for a substance which involve identical positions of atoms is called resonance. In benzene’s Kekule’s structures (1) and (2) represent the resonance structures. Actual structure – of the molecule is represented by hybrid of the these two structures. Orbital structure of benzene: All six carbon atoms in benzene are sp 2 hybridized. The sp 2 hybrid orbitals overlap with each other and with s orbitals of the six hydrogen atoms forming C—C and C—H σ-bonds. X-Ray diffraction data indicates that benzene is a planar molecule. The data indicates that all the six C—C bond length are of the same order (139 pm) which is intermediate between (C—C) single bond (154 pm) and C—C double bond (133 pm). Thus the presence of pure double bond in benzene gives the idea of reductance of benzene to show addition reaction under normal condition. The is, It explains the unusual behaviour of benzene. Aromaticity: It is a property of the sp 2 hybridized planar rings in which the p orbitals allow cyclic delocalization of π electrons. Conditions for Aromaticity: (i) An aromatic compound is cyclic and planar. (ii) Each atom in an aromatic ring has a p orbital. These p orbitals must be parallel so that a continuous overlap is possible around the ring. (iii) The cyclic π molecular orbital (electron cloud) formed by overlap of p orbitals must contain (4n + 2) π electrons. Where n = integer (0, 1, 2, 3, etc.). This is known as Huckel rule. Some Examples of Atomic Compounds are given below: Preparation of Benzene: Benzene is commercially isolated from coaltar. However, there are some synthetic methods which is applied in the laboratory for the preparation of benzene. Physical Properties of Benzene: (i) Benzene is a colourless liquid. (ii) It is’ insoluble in water. It is soluble in alcohol, ether, chloroform etc. (iii) Benzene itself is a good solvent for many organic and inorganic substances e.g., fat, resins, sulphur and iodine. (iv) It bums with a luminous, sooty flame in contrast to alkanes and alkenes which usually bum with a bluish flame. Chemical Properties: Benzene undergeos following types of chemical reactions. (i) Electrophillic Substitution Reaction (ii) Addition Reaction Electrophillic Substitution Reactions: Benzene on treatment with excess of chlorine in the presence of anhydrous AlCl 3 can be chlorinated to hexachlorobenzene (C 6 Cl 6 ) Mechanism of electrophilic substitution reactions: All electrophilic substitution reactions follow the same three step mechanism. Setp 1. Formation of an electrophile: Step 2. The electrophile attacks the aromatic ring to form a carbonium ion. Step 3. Loss of proton gives the substitution product. Activating groups: These group activates the benzene ring for the attack by an electrophile. Example, —OH; —NH 2 , —NHR, —NHCOCH 3 , —OCH 3 —CH 3 —C 2 H 5 etc. Deactivating groups: Due to deactivating group because of strong —I effect, overall electron density on benzene ring decreases. It makes further substitution difficult. Metadirecting group: The groups which direct the incoming group to meta position are called meta directing groups. Some examples of meta directing groups are —N0 2 , —CN, —CHO, —COR, —COOH, —COOR, -S0 3 H etc. Let us consider the example of nitro group: Since Nitro group due to its strong -I effect reduces the electron density in benzene ring. Nitrobenzene is a resonance hybrid of following structures. Carcinogenicity and Toxicity: Some polynuclear hydrocarbons containing more than two benzene rings fused together become toxic and they are having cancer producing property. They are actually formed due to incomplete combustion of some organic materials like tobacco, coal and petroleum, etc. Some of the carcinogenic hydrocarbons are given below. • Hydrocarbons: They are compounds of carbon and hydrogen only. Open Chain saturated compound—Alkane Unsaturated Compound—Alkenes and Alkynes Aromatic Compound—Benzene and its derivatives Terminal alkynes are weakly acidic in nature. • Conformation: Spatial arrangements obtained by rotation around sigma bonds. • Eclipsed Conformation: Less stable because of more repulsion between bond pairs of electrons. • Staggered: It is more stable since there is less repulsion between bond pairs of electrons. • Geometrical isomerism: Observed only in compounds containing a double bond. • Stability of benzene. Is explained on the basis of resonance hybrid. • Arenes: Take part in electrophilic substitution reaction. Aromaticity is determined by Huckle’s rule (4n + 2) rule
CBSE Class 11 Chemistry Chapter-13 Important Questions
1 Marks Questions
1.Classify the hydrocarbons according to the carbon – carbon bond
Ans . Hydrocarbons are categorized into three categories according to the carbon – carbon bond that exists between then-
(a) saturated hydrocarbon
(b) Unsaturated hydrocarbon
(c) Aromatic hydrocarbon.
2.What are cycloalkanes?
Ans When carbon atoms form a closed chain or a ring, they are termed as cycloalkanes.
3. Why carbon does have a larger tendency of catenation than silicon although they have same number of electrons?
Ans . It is due to the smaller size C-C bond which is stronger (335 KJ mol -1 ) than in Si bond (225.7 KJ mol -1 ).
5.What is hydrogenation?
Ans . Dihydrogen gas gets added to alkenes and alkenes in the presence of finely divided catalysts like Pt, Pd or Ni to form alkanes. This process is called hydrogenation.
6. How would you convert ethene to ethane molecule?
7.Give the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon.
Ans 03. 2, 2-dimethyl propane.
8.Methane does not react with chlorine in dark. Why?
Ans .Chlorination of methane is a free radical substitution reaction. In dark, chlorine is unable to be converted into free radicals, hence the reaction does not occur.
9.Which conformation of ethane is more stable?
Ans .Staggered conformation.
10. State Le chatelier’s principle.
Ans . It states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change.
11.Can a catalyst change the position of equilibrium in a reaction?
Ans . No, a catalyst cannot change the position of equilibrium in a chemical reaction. A catalyst, however, affects the rate of reaction.
14.Can a catalyst change the position of equilibrium in a reaction?
Ans . No, a catalyst cannot change the position of equilibrium in a chemical reaction. A catalyst affects the rate of reaction.
15.If Qc < Kc, when we continuously remove the product, what would be the direction of the reaction?
Ans. Continuous removal of a product maintains Qc at a value less than Kc and reaction continues to move in the forward direction.
16.What is a Lindlars’ catalyst?
Ans. Partially deactivated palletized charcoal is known as Lindlar’s catalyst.
17.How is alkene produced by vicinal dihalide?
Ans . Vicinal dihalide on treatment with Zn metal lose a molecule of ZnX 2 to from an alkene. This reaction is known as dehalogenation.
CH 2 Br-CH 2 Br+Zn→ CH 2 =CH 2 +ZnBr 2 .
18.Arrange the following halogen atom to determine rate of the reaction. Iodine, chlorine. Bromine.
Ans . iodine > bromine > chlorine.
19.What is β-elimination reaction?
Ans. When hydrogen atom is eliminated from the β-carbon atom (carbon atom next to the carbon to which halogen is attached).
20.What is the number of σ and π bond in
N ≡ C – CH = CH – C ≡ N?
Ans .There are 7σ bonds and 5 π-bonds.

Class 11 Chemistry Case Study Questions Chapterwise PDF Download
- Post author: studyrate
- Post published:
- Post category: Chemistry / Class 11
- Post comments: 0 Comments
Case Study questions for the Class 11 Chemistry final exams are available here. For your Chemistry exam, you can read these chapter-by-chapter Case Study questions. Subject matter specialists and seasoned teachers created these quizzes. You can verify the right response to each question by referring to the answer key, which is also provided. To achieve success on your final exams, practice the following questions.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

Class 11 Chemistry is divided into intriguing chapters, each unfolding a different aspect of chemical science. From understanding atomic structures to diving into organic chemistry, it’s a vast field.
In this article, we’ll delve into various aspects of Class 11 Chemistry Case Study Questions, exploring everything from the basics to complex analytical techniques. By the time you finish reading, you’ll have gained insights and valuable information that can enhance your understanding and performance.
Table of Contents
CBSE Class 11th Chemistry: Chapterwise Case Study Question & Solution
CBSE will ask two Case Study Questions in the CBSE class 11 Chemistry questions paper. Question numbers 15 and 16 are case-based questions where 5 MCQs will be asked based on a paragraph. Each theme will have five questions and students will have a choice to attempt any four of them.
Case Study-Based Questions for Class 11 Chemistry
- Case Study Based Questions on Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
- Case Study Based Questions on Class 11 Chemistry Chapter 2 Structure of Atom
- Case Study Based Questions on Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- Case Study Based Questions on Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- Case Study Based Questions on Class 11 Chemistry Chapter 5 States of Matter
- Case Study Based Questions on Class 11 Chemistry Chapter 6 Thermodynamics
- Case Study Based Questions on Class 11 Chemistry Chapter 7 Equilibrium
- Case Study Based Questions on Class 11 Chemistry Chapter 8 Redox Reactions
- Case Study Based Questions on Class 11 Chemistry Chapter 10 The s-block elements
- Case Study Based Questions on Class 11 Chemistry Chapter 11 The p-block elements
- Case Study Based Questions on Class 11 Chemistry Chapter 12 Organic Chemistry -Some Basic Principles and Techniques
- Case Study Based Questions on Class 11 Chemistry Chapter 13 Hydrocarbons
Class 11 Chemistry M CQ Questions
Before the exams, students in class 11 should review crucial Chemistry Case Study issues. They will gain a better understanding of the kinds of Case Study questions that may be asked in Chemistry exams for Grade 11 as a result. These questions were created by our knowledgeable faculty for standard 11 Chemistry based on the questions that appeared most frequently in last year’s tests. The solutions have been written in a way that will make them simple to grasp and will aid students in grade 11 in understanding the topics.
Class 11 Chemistry Books


Importance Class 11 Chemistry Case Study Questions
Class 11 Chemistry Case Study Questions are pivotal in building a strong foundation for future studies in chemistry. They help in:
- Analyzing Problem-solving Skills: These questions test your ability to apply theoretical knowledge in practical situations.
- Developing Critical Thinking: Encouraging logical reasoning and systematic approach to complex problems.
- Enhancing Conceptual Understanding: Making connections between different areas of chemistry.
Study Strategies for Class 11 Chemistry
Time management.
Managing time effectively is key to success. Tips include:
- Creating a Study Schedule: Allocating time for each topic.
- Prioritizing Topics: Focusing on challenging areas first.
Effective Note-taking
Taking effective notes enhances understanding. Methods include:
- Summarizing Information: Writing concise summaries of key points.
- Using Visual Aids: Such as diagrams and charts.
Preparing for Class 11 Chemistry Case Study Questions
Question analysis.
Understanding the question is the first step to a correct answer. Techniques include:
- Identifying Keywords: Recognizing the main aspects of the question.
- Understanding the Context: Relating the question to real-world applications.
Solving Strategies
Strategies for solving Class 11 Chemistry Case Study Questions include:
- Breaking Down the Problem: Dividing the question into smaller, manageable parts.
- Applying Relevant Concepts: Utilizing the appropriate concepts and principles.
Class 11 Chemistry Syllabus 2024
Unit I: Some Basic Concepts of Chemistry ( 18 Periods )
General Introduction: Importance and scope of Chemistry. Nature of matter, laws of chemical combination, Dalton’s atomic theory: concept of elements, atoms and molecules. Atomic and molecular masses, mole concept and molar mass, percentage composition, empirical and molecular formula, chemical reactions, stoichiometry and calculations based on stoichiometry.
Unit II: Structure of Atom ( 20 Periods )
Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson’s model and its limitations. Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p and d orbitals, rules for filling electrons in orbitals – Aufbau principle, Pauli’s exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half-filled and completely filled orbitals.
Unit III: Classification of Elements and Periodicity in Properties ( 12 Periods )
Significance of classification, brief history of the development of periodic table, modern periodic law and the present form of periodic table, periodic trends in properties of elements -atomic radii, ionic radii, inert gas radii, Ionization enthalpy, electron gain enthalpy, electronegativity, valency. Nomenclature of elements with atomic number greater than 100.
Unit IV: Chemical Bonding and Molecular Structure ( 20 Periods )
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis’s structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization, involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only), Hydrogen bond.
Unit VI: Chemical Thermodynamics ( 23 Periods )
Concepts of System and types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. First law of thermodynamics -internal energy and enthalpy, heat capacity and specific heat, measurement of ΔU and ΔH, Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. Second law of Thermodynamics (brief introduction) Introduction of entropy as a state function, Gibb’s energy change for spontaneous and non- spontaneous processes, criteria for equilibrium. Third law of thermodynamics (brief introduction).
Unit VII: Equilibrium ( 20 Periods )
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle, ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of poly basic acids, acid strength, concept of pH, hydrolysis of salts (elementary idea), buffer solution, Henderson Equation, solubility product, common ion effect (with illustrative examples).
Unit VIII: Redox Reactions ( 09 Periods )
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, in terms of loss and gain of electrons and change in oxidation number, applications of redox reactions.
Unit XII: Organic Chemistry -Some Basic Principles and Techniques ( 20 Periods )
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions.
Unit XIII: Hydrocarbons ( 18 Periods )
Classification of Hydrocarbons
Aliphatic Hydrocarbons:
Alkanes – Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis.
Alkenes – Nomenclature, the structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition.
Alkynes – Nomenclature, the structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of – hydrogen, halogens, hydrogen halides and water.
Aromatic Hydrocarbons: Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft’s alkylation and acylation, directive influence of the functional group in monosubstituted benzene. Carcinogenicity and toxicity.
FAQs about Class 11 Chemistry Case Studies
What is the best website for a case study of chemistry class 11 .
studyrate.in is the best website for Class 11 Chemistry Case Study Questions for Board Exams. Here you can find various types of Study Materials, Ebooks, Notes, and much more free of cost.
What are Class 11 Chemistry Case Study Questions?
Class 11 Chemistry Case Study Questions are examination questions that test a student’s ability to apply theoretical concepts to practical scenarios. They often include real-world situations, requiring critical thinking and problem-solving skills.
Why are Class 11 Chemistry Case Study Questions important?
These questions help in evaluating a student’s understanding of key concepts and their ability to apply them in real-life situations. They encourage analytical thinking, enhance conceptual knowledge, and prepare students for advanced studies.
How can I prepare for Class 11 Chemistry Case Study Questions?
Preparation involves understanding core concepts, practicing regularly, utilizing resources like textbooks and online platforms, and developing problem-solving strategies. Time management and effective note-taking also play vital roles.

You Might Also Like
Class 11 biology: case study of chapter 8 cell: the unit of life pdf download, 50+ jee mains mcq questions haloalkanes and haloarenes with solutions, 50+ jee mains mcq questions aldehydes ketones and carboxylic acids with solutions, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Topper Combo Flashcards
- Contains the Latest NCERT in just 350 flashcards.
- Colourful and Interactive
- Summarised Important reactions according to the latest PYQs of NEET(UG) and JEE
No thanks, I’m not interested!
- School Solutions
- Star Program
- NCERT Solutions Class 12 Maths
- NCERT Solutions Class 12 Physics
- NCERT Solutions Class 12 Chemistry
- NCERT Solutions Class 12 Biology
- NCERT Solutions Class 12 Commerce
- NCERT Solutions Class 12 Economics
- NCERT Solutions Class 12 Accountancy
- NCERT Solutions Class 12 English
- NCERT Solutions Class 12 Hindi
- NCERT Solutions Class 11 Maths
- NCERT Solutions Class 11 Physics
- NCERT Solutions Class 11 Chemistry
- NCERT Solutions Class 11 Biology
- NCERT Solutions Class 11 Commerce
- NCERT Solutions Class 11 Accountancy
- NCERT Solutions Class 11 English
- NCERT Solutions Class 11 Hindi
- NCERT Solutions Class 11 Statistics
- NCERT Solutions Class 10 Maths
- NCERT Solutions Class 10 Science
- NCERT Solutions Class 10 English
- NCERT Solutions Class 10 Hindi
- NCERT Solutions Class 10 Social Science
- NCERT Solutions Class 9 Maths
- NCERT Solutions Class 9 Science
- NCERT Solutions Class 9 English
- NCERT Solutions Class 9 Hindi
- NCERT Solutions Class 9 Social Science
- NCERT Solutions Class 8 Maths
- NCERT Solutions Class 8 Science
- NCERT Solutions Class 8 English
- NCERT Solutions Class 8 Hindi
- NCERT Solutions Class 8 Social Science
- NCERT Solutions Class 7 Maths
- NCERT Solutions Class 7 Science
- NCERT Solutions Class 7 English
- NCERT Solutions Class 7 Hindi
- NCERT Solutions Class 7 Social Science
- NCERT Solutions Class 6 Maths
- NCERT Solutions Class 6 Science
- NCERT Solutions Class 6 English
- NCERT Solutions Class 6 Hindi
- NCERT Solutions Class 6 Social Science
- NCERT Solutions Class 5 Maths
- NCERT Solutions Class 5 English
- NCERT Solutions Class 5 EVS
- NCERT Solutions Class 4 Maths
- NCERT Solutions Class 4 English
- NCERT Solutions Class 4 EVS
- NCERT Solutions Class 4 Hindi
- NCERT Solutions Class 3 Maths
- NCERT Solutions Class 3 English
- NCERT Solutions Class 3 EVS
- NCERT Solutions Class 3 Hindi
- NCERT Solutions Class 2 Maths
- NCERT Solutions Class 2 English
- NCERT Solutions Class 2 Hindi
- NCERT Solutions Class 1 Maths
- NCERT Solutions Class 1 English
- NCERT Solutions Class 1 Hindi
- NCERT Books Class 12
- NCERT Books Class 11
- NCERT Books Class 10
- NCERT Books Class 9
- NCERT Books Class 8
- NCERT Books Class 7
- NCERT Books Class 6
- NCERT Books Class 5
- NCERT Books Class 4
- NCERT Books Class 3
- NCERT Books Class 2
- NCERT Books Class 1
- Important Questions Class 12
- Important Questions Class 11
- Important Questions Class 10
- Important Questions Class 9
- Important Questions Class 8
- Important Questions Class 7
- important questions class 6
- CBSE Class 12 Revision Notes
- CBSE Class 11 Revision Notes
- CBSE Class 10 Revision Notes
- CBSE Class 9 Revision Notes
- CBSE Class 8 Revision Notes
- CBSE Class 7 Revision Notes
- CBSE Class 6 Revision Notes
- CBSE Class 12 Syllabus
- CBSE Class 11 Syllabus
- CBSE Class 10 Syllabus
- CBSE Class 9 Syllabus
- CBSE Class 8 Syllabus
- CBSE Class 7 Syllabus
- CBSE Class 6 Syllabus
- CBSE Class 5 Syllabus
- CBSE Class 4 Syllabus
- CBSE Class 3 Syllabus
- CBSE Class 2 Syllabus
- CBSE Class 1 Syllabus
- CBSE Sample Question Papers For Class 12
- CBSE Sample Question Papers For Class 11
- CBSE Sample Question Papers For Class 10
- CBSE Sample Question Papers For Class 9
- CBSE Sample Question Papers For Class 8
- CBSE Sample Question Papers For Class 7
- CBSE Sample Question Papers For Class 6
- CBSE Sample Question Papers For Class 5
- CBSE Sample Question Papers For Class 4
- CBSE Sample Question Papers For Class 3
- CBSE Sample Question Papers For Class 2
- CBSE Sample Question Papers For Class 1
- CBSE Previous Year Question Papers Class 12
- CBSE Previous Year Question Papers Class 10
- Extra Questions For Class 8 Maths
- Extra Questions For Class 8 Science
- Extra Questions For Class 9 Maths
- Extra Questions For Class 9 Science
- Extra Questions For Class 10 Maths
- Extra Questions For Class 10 Science
- NEET 2021 Question Paper
- NEET 2020 Question Paper
- NEET 2019 Question Paper
- NEET 2018 Question Paper
- NEET 2017 Question Paper
- NEET 2016 Question Paper
- NEET 2015 Question Paper
- NEET Physics Questions
- NEET Chemistry Questions
- NEET Biology Questions
- NEET Sample Papers
- NEET Physics Syllabus
- NEET Chemistry Syllabus
- NEET Biology Syllabus
- NEET Mock Test
- NEET Eligibility Criteria
- JEE Main 2021 Question Paper
- JEE Main 2020 Question Paper
- JEE Main 2019 Question Paper
- JEE Main 2018 Question Paper
- JEE Main 2017 Question Paper
- JEE Main 2016 Question Paper
- JEE Main 2015 Question Paper
- JEE Main Sample Papers
- JEE Main Physics Syllabus
- JEE Main Chemistry Syllabus
- JEE Main Maths Syllabus
- JEE Main Physics Questions
- JEE Main Chemistry Questions
- JEE Main Maths Questions
- JEE main revision notes
- JEE Main Mock Test
- JEE Advanced Physics Questions
- JEE Advanced Chemistry Questions
- JEE Advanced Maths Questions
- JEE Advanced 2021 Question Paper
- JEE Advanced 2020 Question Paper
- JEE Advanced 2019 Question Paper
- JEE Advanced 2018 Question Paper
- JEE Advanced 2017 Question Paper
- JEE Advanced 2016 Question Paper
- JEE Advanced 2015 Question Paper
- JEE Advanced Physics Syllabus
- JEE Advanced Chemistry Syllabus
- JEE Advanced Maths Syllabus
- JEE Advanced Mock Test
- ISC Class 12 Syllabus
- ISC Class 11 Syllabus
- ICSE Class 10 Syllabus
- ICSE Class 9 Syllabus
- ICSE Class 8 Syllabus
- ICSE Class 7 Syllabus
- ICSE Class 6 Syllabus
- ISC Sample Question Papers for Class 12
- ISC Sample Question Papers for Class 11
- ICSE Sample Question Papers for Class 10
- ICSE Sample Question Papers for Class 9
- ICSE Sample Question Papers for Class 8
- ICSE Sample Question Papers for Class 7
- ICSE Sample Question Papers for Class 6
- ICSE Class 10 Revision Notes
- ICSE Class 9 Revision Notes
- ISC Important Questions for Class 12
- ISC Important Questions for Class 11
- ICSE Important Questions for Class 10
- ICSE Important Questions for Class 9
- ICSE Important Questions for Class 8
- ICSE Important Questions for Class 7
- ICSE Important Questions for Class 6
- ISC Class 12 Question Paper
- ICSE Class 10 Question Paper
- Maharashtra Board Syllabus
- Maharashtra Board Sample Question Paper
- Maharashtra Board Previous Year Question Paper
- AP Board Syllabus
- AP Board Sample Question Paper
- AP Board Previous Year Question Paper
- Tamilnadu Board Syllabus
- Tamilnadu Board Sample Question Paper
- Tamilnadu Board Previous Year Question Paper
- Telangana Board Syllabus
- Telangana Board Sample Question Paper
- Telangana Board Previous Year Question Paper
- Karnataka Board Syllabus
- Karnataka Board Sample Question Paper
- Karnataka Board Previous Year Question Paper
- Examination Full Forms
- Physics Full Forms
- Chemistry Full Forms
- Biology Full Forms
- Educational Full Form
- CUET Eligibility Criteria
- CUET Exam Pattern
- CUET Cutoff
- CUET Syllabus
- CUET Admit Card
- CUET Counselling
- CUET Previous Year Question Papers
- CUET Application Form
- CUET Sample Papers
- CUET Exam Centers
- CUET Exam Dates
- CUET Results
- Physics Formulas
- Chemistry Formulas
- Math Formulas
- Algebra Formulas
- Geometry Formulas
- Trigonometry Formulas
- Subscription
Important Questions Class 11 Chemistry Chapter 13
Home » CBSE » Important Questions Class 11 Chemistry Chapter 13

- CBSE Important Questions
- Important Questions Class 6
- CBSE Previous Year Question Papers
- CBSE Revision Notes
- CBSE Syllabus
- CBSE Extra Questions
- CBSE Sample Papers
- ISC & ICSE Syllabus
- ICSE Syllabus Class 9
- ICSE Syllabus Class 8
- ICSE Syllabus Class 7
- ICSE Syllabus Class 6
- ICSE Syllabus Class 10
- ICSE Question Paper
- ICSE Sample Question Papers
- ISC Sample Question Papers For Class 12
- ISC Sample Question Papers For Class 11
- ICSE Sample Question Papers For Class 10
- ICSE Sample Question Papers For Class 9
- ICSE Sample Question Papers For Class 8
- ICSE Sample Question Papers For Class 7
- ICSE Sample Question Papers For Class 6
- ICSE Revision Notes
- ICSE Important Questions
- ISC Important Questions For Class 12
- ISC Important Questions For Class 11
- ICSE Important Questions For Class 10
- ICSE Important Questions For Class 9
- ICSE Important Questions For Class 8
- ICSE Important Questions For Class 7
- ICSE Important Questions For Class 6
- Maharashtra board
- Rajasthan-Board
- Andhrapradesh Board
- AP Board syllabus
- Telangana Board
- Tamilnadu Board
- Tamilnadu Sample Question Paper
- Tamilnadu Syllabus
- Tamilnadu Previous Year Question Paper
- NCERT Solutions Class 12
- NCERT Solutions Class 10
- NCERT Solutions Class 11
- NCERT Solutions Class 9
- NCERT Solutions Class 8
- NCERT Solutions Class 7
- NCERT Solutions Class 6
- NCERT Solutions Class 5
- NCERT Solutions Class 4
- NCERT Solutions Class 3
- NCERT Solutions Class 2
- NCERT Solutions Class 1
- JEE Main Question Papers
- JEE Main Syllabus
- JEE Main Questions
- JEE Main Revision Notes
- JEE Advanced Question Papers
- JEE Advanced Syllabus
- JEE Advanced Questions
- JEE Advanced Sample Papers
- NEET Question Papers
- Neet 2021 Question Paper
- Neet 2020 Question Paper
- Neet 2019 Question Paper
- Neet 2018 Question Paper
- Neet 2017 Question Paper
- Neet 2016 Question Paper
- Neet 2015 Question Paper
- NEET Syllabus

Important Questions Class 11 Chemistry Chapter 13 – Hydrocarbons
Chemistry helps you to comprehend changes in the natural and physical world. The field of Chemistry is interesting and students are intrigued to learn more. Understanding the characteristics of materials and converting them into new, and useful chemicals is made possible by chemistry but certain concepts are challenging to comprehend. Therefore, students need to make extra effort to understand its reactions and chemical formulas. Chapter 13 of Class 11 Chemistry is about ‘Hydrocarbons’.
Extramarks is one of the leading educational platforms providing the best study materials for students from Class 1 to Class 12. Students can find comprehensive study resources, including NCERT solutions, CBSE revision notes, CBSE sample papers, CBSE past years’ question papers, and more on our website. To enjoy the maximum benefit of these resources, students just need to register themselves at Extramarks’ official website and stay ahead of the competition.
We at Extramarks take our role seriously and try to aid students with valuable guidance and support to help them excel in the board exams. The in-house subject experts highlight the key concepts and questions from each chapter to help students with their studies. The students will become more familiar with final exam questions by solving questions from our question bank of Important Questions Class 11 Chemistry Chapter 13. Chemistry requires deep conceptual understanding so cramming answers won’t help students in the long run. The only way is to solve regular questions and get their doubts cleared. The old adage says the more you practice, the better you’ll get. Extramarks experts believe that students must study regularly with concentration and focus to get 100% marks in Chemistry.
Students must therefore know how to answer exam-oriented questions. NCERT textbook questions, NCERT exemplar, and other reference books have been collated in our question bank Important Questions Class 11 Chemistry Chapter 13. To create a solid base for the chapter, students are advised to study regularly, revise the chapter and practise questions from our Important Questions Class 11 Chemistry Chapter 13 to check their preparedness for the exam.
Students should make an effort to comprehend the question paper pattern, write quick responses and how minimise those silly mistakes. . Extramarks experts have all the answers to your problems. Students can access the entire collection of important questions for all Chemistry chapters by registering on the Extramarks website. Additionally, students can refer to other study materials such as CBSE past years’ question papers and NCERT solutions.
Important Questions Class 11 Chemistry Chapter 13 – With Solutions
Students can refer to Extramarks NCERT solutions which comprise chapter notes, revision notes, and solved exam-related questions. To regularly practise questions, students can rely on our question bank of Important Questions Chapter 13 Class 11 Chemistry. The questions cover all aspects of the chapter and will help students to revise all concepts from the hydrocarbons, important reactions of alkanes and alkenes and their characteristics.
Here is a list of questionnaires and their answers from our Chapter 13 Class 11 Chemistry Important Questions.
Question 1: Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
(i) HCl > HBr > HI
(ii) HBr > HI > HCl
(iii) HI > HBr > HCl
(iv) HCl > HI > HBr
Answer 1: (iii) HI > HBr > HCl
Explanation: Bond strength and bond dissociation energy are two factors that impact the reactivity of hydrogen halides. We are aware that the size of the halogen atom increases while the bond dissociation energy and bond strength decreases in halogen halides. Hence, the reactivity increases.
Question 2: Arrange the following carbanions in order of their decreasing stability.
(i) A > B > C
(ii) B > A > C
(iii) C > B > A
(iv) C > A > B
Answer 2 : (ii) B > A > C
Explanation: In C, the +I effect of the CH 3 group directly affects the negatively charged carbon, reducing the stability of the carbon anion. Additionally, the +I effect is present in A, but it is further away from the negatively charged carbon, while it is absent in B.
Question 3: Arrange the following alkyl halides in decreasing order of the rate of – elimination reaction with alcoholic KOH.
(B) CH 3 -CH 2 -Br
(C) CH 3 -CH 2 -CH 2 -Br
(ii) C > B > A
(iii) B > C > A
(iv) A > C > B
Answer 3: (iv) A > C > B
Explanation: KOH, an alkene, is created when alkyl halides are heated with alcohol by getting rid of one molecule of halogen acid. The beta carbon atom 3 0 > 2 0 > 1 0 loses its hydrogen atom. The nature of alkyl groups determines the rate of reaction.
Question 4: Which of the following reactions of methane is incomplete combustion:
(iii) CH 4 + O 2 → C(s) + 2H 2 O(l)
(iv) CH 4 + 2O 2 → CO 2 (g) + 2H 2 O(l)
Answer 4: (iii) CH 4 + O 2 → C(s) + 2H 2 O(l)
Explanation: Carbon black is produced when there is not enough oxygen or air to complete the combustion process.
Hence, CH 4 + O 2 → C(s) + 2H 2 O(l)
Question 5: Arrange the following in decreasing order of their boiling points.
(A) n-butane
(B) 2–methylbutane
(C) n-pentane
(D) 2,2–dimethylpropane
(i) A > B > C > D
(ii) B > C > D > A
(iii) D > C > B > A
(iv) C > B > D > A
Answer 5: (iv) C > B > D > A
Explanation: We are aware that the boiling point depends on both the molar mass and surface area.
It implies that branching will cause the boiling point to drop (surface area decreases on branching). As a result, n-butane has the lowest boiling point while n-pentane has the highest. Since the other two alternatives have branches, 2-methyl butane boils at a higher temperature than 2,2-dimethylpropane.
Question 6: The increasing order of reduction of alkyl halides with zinc and dilute HCl is
(i) R–Cl < R–I < R–Br
(ii) R–Cl < R–Br < R–I
(iii) R–I < R–Br < R–Cl
(iv) R–Br < R–I < R–Cl
Answer 6: (ii) R-Cl < R-Br < R-I
Explanation: Since we are aware that halogen reactivity diminishes as the group size increases, the reduction of alkyl halides with ZnCl/HCl occurs in the opposite order.
Reactivity of reduction 1bond strength of C-X
Reactivity of reduction 1 bond strength C-X
It also depends on the size of the halogen. As a result, we can conclude that reducing binding strength is necessary to increase reactivity.
Question 7:
The correct IUPAC name of the following alkane is
(i) 3,6 – Diethyl – 2 – methyloctane
(ii) 5 – Isopropyl – 3 – ethyloctane
(iii) 3 – Ethyl – 5 – isopropyloctane
(iv) 3 – Isopropyl – 6 – ethyloctane
Answer 7: (i) 3,6-Diethyl-2-methyloctane
Explanation: The alkane is octane because it has 8 carbon atoms, making it the longest chain. Carbon 2 has a methyl group, and carbon 3 and carbon 6 have ethyl groups. Since there are two ethyl groups, it will be diethyl, which is alphabetically before methyl. The side chains on carbon atoms 2, 3, and 6 adhere to the lowest sum rule.
Question 8:
The addition of HBr to 1-butene gives a mixture of products A, B and C
(C) CH 3 – CH 2 -CH 2 – CH 2 -Br
The mixture consists of
(i) A and B as major and C as minor products
(ii) B as major, A and C as minor products
(iii) B as minor, A and C as major products
(iv) A and B as minor and C as major products
Answer 8: (i) A and B as major and C as minor products.
Explanation: The major product is 2-Bromobutane, and the minor product is I-Bromobutane, according to Markovnikov’s rule. It is asymmetrical, butane-1. Due to the chiral nature of the carbon in 2-bromobutane, there are two enantiomers.
Question 10: What are cycloalkanes?
Answer 10: When carbon atoms form closed chains or ring compounds, w hich are similar to alkanes, all carbon is connected with single bonds in such a way that it gives a closed structure, and cycloalkanes are formed.
Some common examples of cycloalkanes are cyclopentane, Cyclobutane, cyclohexane, cycloheptane, cyclooctane, etc
Question 11: What is hydrogenation?
Answer 11: Dihydrogen gas is added to gas and alkenes in the presence of finely divided catalysts like Pt, Pd, or Ni to create alkanes. This process is known as hydrogenation.
Question 12: Methane does not react with chlorine in the dark. Why?
Answer 12: Chlorination of Methane is a substitution process that uses free radicals. The reaction does not occur because chlorine cannot be converted into free radicals in the dark.
Question 13: State Le Chatelier’s principle.
Answer 13: It states that when one of the factors defining a system’s equilibrium conditions changes, the system will adjust in a way that lessens or cancels out the effect of the change.
Question 14: If Q c < K c , when we continuously remove the product, what would be the direction of the reaction?
Answer 14: When a product is continuously removed, Q c remains smaller than K c , and the reaction moves in the direction of the reactant.
Question 15: What is -elimination reaction?
Answer 15: In the -elimination reaction, the hydrogen atom is removed from the -carbon atom next to the carbon atom to which the halogen is attached.
Question 16: Why do alkynes not show geometrical isomerism?
Answer 16: The structure of alkynes is linear. Therefore, they are unable to show geometric isomerism.
Question 17: Although benzene is highly saturated, it does not undergo addition reactions. Give reasons.
Answer 17: In contrast to olefins, benzene -electrons are moved via resonance, making them less reactive for subsequent reactions.
Question 18: Unsaturated compounds undergo addition reactions. Justify.?
Answer 18: Two- or three-fold carbon bonds can be found in unsaturated hydrocarbon molecules. The -bond is a multiple bond that adds across several bonds when it becomes unstable.
Question 19: Cyclobutane is less reactive than cyclopropane. Justify.
Answer 19: Cyclobutane has a C-C-C bond angle of 90 0 , while cyclopropane has a C-C-C bond angle of 60 0 . It proves that there is a lesser variation in bond angles between cyclobutane (109 0 28’) and cyclopropane. In other words, cyclopropane is more reactive than cyclobutane because it is under far greater stress.
Question 20: When alkanes are heated, the C-C bonds rather than the C-H bonds break. Give reasons.
Answer 20: When the alkanes are heated, the C-C bond instead of the C-H bond breaks because the C-C bond ( H=83 KCal/mole) has a lower energy bond than the C-H bond ( H=90 KCal/mole) .
Question 21: State Markovnikov’s Rule.
Answer 21: According to Markovnikov’s Rule, when a polar compound is added to an unsymmetrical alkene or alkyne, the positive part goes to the carbon atom with the most substitutes, and the negative part goes to the carbon atom with the least substitutes.
Question 22: How will you distinguish between butene-1 and butene-2?
Answer 22: By ozonolysis or oxidation with an acidic KMnO 4 solution, which yields unique carbonyl compounds, butenes 1 and 2 can be distinguished from one another.
The chemical representation is as follows:
CH 3 -CH 2 -CH=CH 2 → CH 3 CH 2 CHO + HCHO
CH 3 -CH=CH-CH 3 → CH 3 CHO + CH 3 CHO
Question 23: State Kharasch effect.
Answer 23: It asserts that the addition of HBr (but not HCl or HI) to unsymmetrical alkenes breaks Markownikov’s rule when peroxides, such as benzoyl peroxide, are present.
The reaction is as follows:
CH 3 CH=CH 2 + HBr (peroxide) → CH 3 -CH 2 -CH 3
Question 24: What do you understand by resonance energy?
Answer 24: The difference in energy between the most stable contributing structure and the resonance hybrid is known as resonance energy. Benzene has resonance energy of 147 KJ/mole.
Question 25: How is the aromaticity of a compound judged?
Answer 25: The following characteristics determine a compound’s aromaticity:
- Delocalisation of the -electrons in the ring completely.
- 4n+2 electrons are present in the ring, where n is an integer (0, 1, 2, etc.).
This is generally known as the Huckel Rule.
Question 26: Explain the term polymerisation with two examples.
Answer 26: In the right conditions, polymerisation is the process of joining two or more molecules of unsaturated chemicals to produce a larger complex. The process is called polymerisation, and the end result is referred to as a polymer.
Addition polymerisation: Nothing is lost during addition polymerisation since the larger molecule (polymer) is an exact multiple of the smaller molecule.
Condensation polymerisation: Water, hydrochloric acid, and other molecules are frequently lost during the condensation polymerisation process. During this type of polymerisation, the polymer is not a precise multiple of the smaller molecule.
Question 27: Ethyne is acidic in nature in comparison to ethene and ethane. Why is it so?
Answer 27: Hydrogen atoms are joined to sp hybridised carbon atoms in ethyne, whereas they are joined to sp 2 hybridised carbon atoms in ethene and sp 3 hybridised carbons in ethane.
Due to the largest percentage of s-character (50%) in ethyne molecules, the carbon atoms’ sp hybridised orbitals have the highest electronegativity (50%) of any orbital.
In comparison to the sp 2 hybridised orbitals of carbon in ethene and the sp 3 hybridised orbitals of carbon in ethane, this attracts the shared pair of the C-H bond of ethyne more strongly. Hydrogen atoms can be released as protons more readily in the ethyne molecule as compared to ethene and ethane.
Question 28: Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Answer 28: The maximum distance between the hydrogen atoms and the electron bonding pairs, which results in the least amount of repulsion, makes the staggered conformation the most stable. The other intermediate conformation (Gauche or skew form), which is between them, is less stable than the eclipsed shape.
Question 29: Despite their – I effect, halogens are o- and p-directing in haloarenes. Explain.
Answer 29: The effects of halogens on benzene rings are -I and +R. The +R effect raises the electron density on ortho and para locations, while the -I effect deactivates the ring. Halogens are hence ortho- and para-directing.
Question 30: Why does the presence of a nitro group make the benzene ring less reactive than the unsubstituted benzene ring? Explain.
Answer 30: An electron-withdrawing group is the Nitro group (-R and -I effects). By reducing the nucleophilicity for subsequent substitution, it deactivates the ring.
Question 31: What products are formed when zinc reacts with
(i) vicinal C 2 H 4 Br 2 and
Answer 31: CH 2 Br – CH 2 Br + Zn → CH 2 = CH 2 + ZnBr 2 .
(ii) CH 3 CHBr – CH 2 Br.
Answer: CH 3 – CHBr – CH 2 Br + Zn → CH 3 — CH = CH 2 + ZnBr 2 .
Question 32: How will you prepare Alkanes by
(i) Wurtz reaction
Answer 32:
Methods of preparation of Alkanes
Wurtz reaction. After being treated with sodium in dry ether, alkyl halides produce higher alkanes, ideally with an even number of carbon atoms.
(ii) Decarboxylation of sodium salts of fatty acids
When heated with soda lime (a solution of NaOH and CaO), sodium salts of fatty acids produce alkanes with one fewer carbon atom than the carboxylic acid. The procedure of decarboxylation involves eliminating carbon dioxide from a carbonyl group.
iii) Kolbe’s electrolytic method.
Kolbe’s electrolytic method: Alkanes with an even number of carbon atoms are produced at the anode by electrolysing an aqueous solution of sodium or potassium salt of a carboxylic acid.
The probable mechanism for the reaction is
Question 34: What effect does branching of an alkane chain have on its boiling point?
Answer 34: Intermolecular Van der Waals forces are present in alkanes. The boiling point of the alkane will increase with increasing force. A smaller area of contact occurs from the molecule’s surface area, decreasing as branching increases. This results in a decreased Van der Waals force that can be resisted at a substantially lower temperature. As a result, the boiling point of an alkane chain drops as branching increases.
Question 35: Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer 35: Planar molecules like benzene have electrons that are delocalised above and below the plane of the ring. It is so rich in electrons. It is therefore extremely alluring to organisms lacking in electrons or electrophiles. It is hence particularly susceptible to electrophilic substitution reactions. Electrophiles have a lot of electrons. Benzene repels them as a result. As a result, nucleophilic replacements of benzene are challenging.
Question 36: Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer 36: An ethyl group is added to the benzene ring as part of the methylation reaction. A Friedel-Craft alkylation reaction is what is known as such a reaction. A Lewis acid is necessary for this reaction to occur. Any Lewis acid, including anhydrous FeCl 3 , SnCl 4 , BF 3 , and others, may be used to methylate benzene.
Question 37: Why is Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms? Illustrate your answer with one example.
Answer 37: The synthesis of symmetrical alkanes (alkanes with an even number of carbon atoms) is only possible by the Wurtz reaction, which uses two identical alkyl halides as reactants to create an alkane with twice as many carbon atoms.
Because the Ethane Wurtz reaction produces a mixture of alkanes when two different alkyl halides are utilised as the reactants, it cannot be used to create unsymmetrical alkanes. Free radical species are involved in the reaction. Therefore a side reaction that results in an alkene also takes place. For instance, a mixture of alkanes is produced when bromoethane and iodoethane combine.
Question 38: Arrange the following set of compounds in order of their decreasing
relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p-H 3 C – C 6 H 4 – NO 2 , p-O 2 N – C 6 H 4 – NO 2
Reagents called electrophiles to take part in reactions by attaching themselves to nucleophiles by accepting two electrons.
The more electrons there are on a benzene ring, the more electrophilic (E+) the molecule is (Electrophilic reaction).
(a) Due to the presence of an electron-withdrawing group (NO 2 – and Cl – ), which deactivates the aromatic ring, the aromatic ring’s electron density lowers.
The increasing sequence of reactivity is as follows because the Cl – group is less electron-withdrawing (due to the inductive effect) than the NO 2 – group (due to the resonance effect):
2, 4 – dinitrochlorobenzene < p – nitrochlorobenzene < Chlorobenzene
(b) CH 3 – is an electron-donating group, whereas NO 2- is an electron-withdrawing group.
As a result, toluene has the highest electron density and is most susceptible to E+ attack. NO 2- is an electron-removing group, hence. As a result, when there are more NO 2 alternatives, the order is as follows:
p -O 2 N – C 6 H 4 – NO 2 < p – H 3 C – C 6 H 4 – NO 2 < Toluene.
Question 39: List the names of some Lewis acids which can be used during the ethylation of benzene in a Friedel-Craft alkylation reaction.
Answer 39: Acyl benzene is produced when benzene reacts with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl 3 ) (or benzene ring). Such a process is referred to as a Friedel-Craft alkylation reaction. In the presence of a Lewis acid, the reaction takes place.
Any Lewis acid, including anhydrous AlCl 3 , FeCl 3 , SnCl 4 , BF 3 , etc., may be used to ethylate benzene in the Friedel-Craft alkylation reaction.
Question 40: Why is benzene extraordinarily stable though it contains three double bonds?
Answer 40: The resonating structure of benzene can be represented as:
The benzene molecule contains six sp2 hybridised carbon atoms. Six sigma bonds are created in the hexagonal plane by the two sp2 hybrid orbitals of each carbon atom overlapping with the sp2 hybrid orbitals of nearby carbon atoms. Six sigma bonds are created when the remaining sp2 hybrid orbitals on each carbon atom overlap with the hydrogen s-orbital. The option exists for the remaining unhybridised carbon atoms to create three pi bonds via the lateral overlap of C1-C2, C3-C4, C5-C6, or C2-C3, C4-C5, C6-C1.
Due to their delocalisation, the six pi can freely migrate among the six carbon nuclei. The benzene is stabilised by these delocalised pi- electrons even when there are three double bonds present.
Question 41: Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Answer 41: According to the information provided, an alkene’s ozonolysis produces propanal and pentan-3-one. Please use the alkene supplied, “A.” When we write the ozonolysis reaction backwards, we get:
On the cleavage of ozonide “X,” the products are produced. As a result, both products are present in ‘X’ in cyclic form. The following is a possible representation of ozonide’s structure::
Alkene “A” and ozone now produce “X,” a byproduct. The following is an example of an alkene’s potential structure:
Question 42: Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Answer 42:
Hex-2-ene is represented as
CH 3 – CH=CH-CH 2 -CH 2 -CH 3
Geometrical isomers of hex-2-ene are:
The total of the dipole moments of the C-CH 3 and C-CH 2 CH 2 CH 3 bonds acting in the same direction makes up the dipole moment of the cis-compound.
The dipole moments of the C-CH 3 and C-CH 2 CH 2 CH 3 bonds operating in opposition to one another create the trans-dipole compound’s moment.
The cis-isomer is, therefore, more polar than the trans-isomer. The intermolecular dipole-dipole interaction is stronger, and the boiling point rises with increasing polarity. As a result, the boiling point of the cis-isomer will be greater than the trans-isomer.
Question 43: How will you convert benzene into
(i) p-nitrobromobenzene
(ii) m-nitrochlorobenzene
(iii) p -nitrotoluene
(iv) acetophenone
Answer 43:
(i)Benzene can be converted into p-nitrobromobenzene as:
(ii)Benzene can be converted into m-nitrochlorobenzene as:
(iii)Benzene can be converted into p-nitrotoulene as:
(iv)Benzene can be converted into acetophenone as:
Question 44: In the alkane H 3 C– CH 2 – C(CH 3 ) 2 – CH 2 – CH(CH 3 ) 2 , identify 1°,2°, and 3° carbon atoms and give the number of H atoms bonded to each one of these.
Answer 44:
One-carbon-atom bonds, or having just one carbon atom as a neighbour, are referred to as 1° carbon atoms. The provided structure is made up of fifteen hydrogen atoms and five carbon atoms at 1°.
Carbon atoms with a 2° link to another carbon atom have another two carbon atoms as their neighbours. Four hydrogen atoms are
joined to the two 2° carbon atoms in the provided structure.
Three carbon atoms are connected to three carbon atoms, making them the neighbours of three carbon atoms. Only one hydrogen atom and one 3° carbon atom make up the given structure.
Question 45: Addition of HBr to propene yields 2-bromopropane, while in benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give a mechanism.
Answer 45: An example of an electrophilic substitution reaction is the addition of HBr to propene.
H+, an electrophile, is produced by hydrogen bromide. As demonstrated, this electrophile attacks the double bond to produce the 1° and 2° carbocations.
Compared to primary carbocations, secondary carbocations are more stable. As a result, the former prevails since it will form more quickly. As a result, in the following step, Br- attacks the carbocation to produce the main product, 2 – bromopropane.
This reaction adheres to Markovnikov’s rule, according to which the carbon atom with the fewest hydrogen atoms receives the addendum’s negative portion.
Benzoyl peroxide causes an additional reaction that goes against Markovnikov’s law. The process of the reaction is a free radical chain as follows:
Compared to primary radicals, secondary free radicals are more stable. As a result, the former prevails since it forms more quickly. As a result, the main product is 1 – bromopropane.
Br free radical behaves like an electrophile in the presence of peroxide. As a result, when HBr is added to propene, two distinct compounds are produced in both the presence and absence of peroxide.
Question 46: Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekulé structure for benzene?
Answer 46:
O-xylene has two resonance structures:
Two Kekule structures are used to produce all three products, methyl glyoxal, 1, 2-dimethylglyoxal, and glyoxal. This demonstrates that o-xylene is a resonance hybrid of two Kekule structures because none of the three products can be produced by either of the two structures alone (I and II).
Question 47: Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also, give the reason for this behaviour.
Answer 47:
A species’ acidic nature is determined by how readily it may shed its H-atoms.
The hybridisation state of carbon in the given compound is:
The electronegativity of carbon rises with increasing s-character as the C-H bond pair’s electrons move in closer to the carbon atom. As a result, the H-partial atom’s positive charge rises and H+ ions are liberated.
The s–character increases in the order:
sp 3 < sp 2 < sp
Hence, the decreasing order of acidic behaviour is Ethyne > Benzene > Hexane.
Question 48: Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer 48: Planar molecules like benzene have electrons that are delocalised above and below the plane of the ring. It is so rich in electrons. It is therefore extremely alluring to organisms lacking in electrons or electrophiles.
It is hence particularly susceptible to electrophilic substitution reactions. Electrophiles have a lot of electrons. Benzene repels them as a result. As a result, nucleophilic replacements of benzene are challenging.
Question 49: How would you convert the following compounds into benzene?
(ii) Ethene
(iii) Hexane
Answer 49:
(i)Benzene from Ethyne:
(ii)Benzene from Ethene:
(iii)Hexane to Benzene
Question 50: Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer 50:
The skeleton of 2-methylbutane is shown below:
On the basis of this structure, alkenes that will give 2-methylbutane on hydrogenation are:
Question 51: Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(b) Toluene, p-H 3 C – C 6 H 4 – NO 2 , p-O 2 N – C 6 H 4 – NO 2 .
Answer 5511: Reagents called electrophiles take part in reactions by bonding with nucleophiles by accepting an electron pair.
The benzene ring’s electron density determines how reactive the molecule is to an electrophile, E+ (Electrophilic reaction).
(a) By lowering the electron density, electron-withdrawing groups like NO 2- and Cl – deactivate the aromatic ring.
The decreasing sequence of reactivity is as follows because the NO 2 – group is more electron withdrawing (due to resonance effect) than the Cl- group (due to inductive action):
Chlorobenzene > p – nitrochlorobenzene > 2, 4 – dinitrochlorobenzene
(b) The electron-withdrawing NO 2- group contrasts with the electron-donating CH 3 – group. Toluene will have the highest electron density and be susceptible to E+ attack.
An electron-withdrawing group is NO 2 . Consequently, the sequence is as follows when there are more NO2- substituents:
Toluene > p– CH 3 –C 6 H 4 – NO 2 , p –O 2 N– C 6 H 4 – NO 2
Question 52: Out of benzene, m–dinitrobenzene and toluene which will undergo nitration most easily and why?
Answer 52: The compound’s ability to generate nitrates depends on the amount of electron density there is. Examples of nitration reactions include electrophilic substitution reactions in which a nitronium ion attacks an electron-rich species (NO 2– ).
Now, the CH 3 – group donates electrons while the NO 2- group withdraws them. Thus, of the three chemicals, toluene will have the highest electron density, followed by benzene. m- Dinitrobenzene, on the other hand, will have the lowest electron density. As a result, nitration will be challenging. Consequently, the nitration is in increasing sequence as follows:
Question 53: Why is Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms? Illustrate your answer with one example.
Answer 53:
Wurtz reaction can only be used to create symmetrical alkanes (alkanes with an even number of carbon atoms). Two identical alkyl halides are used as reactants in the reaction, and an alkane with twice as many carbon atoms is produced.
Because the Wurtz reaction produces a mixture of alkanes when two different alkyl halides are utilised as the reactants, it cannot be used to create unsymmetrical alkanes. Free radical species are involved in the reaction, therefore a side reaction that results in an alkene also takes place. For instance, a mixture of alkanes is produced when bromoethane and iodoethane combine.
Alkanes produced from the mixture have extremely similar boiling temperatures. Consequently, it becomes challenging to distinguish them.
Question 54: Which of the following alkenes on ozonolysis give a mixture of ketones only?
Answer 54: (iii) and (iv)
Explanation: Alkenes produce two molecules of carbonyl compounds when subjected to ozonolysis, depending on the groups or atoms attached. If the alkyl groups on the double-bonded carbon atoms are present, ketones are produced.
Question 55: Alkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism?
Answer 55:
On the reduction of 2-Butyne, butene-2 is produced, with the methyl groups either on the same side or the opposite side to display geometric isomers.
Question 56: Rotation around the carbon-carbon single bond of ethane is not completely free. Justify the statement.
Answer 56:
(i)Alkanes feature a carbon-carbon sigma bond in which the distribution of the sigma molecular orbit’s electrons is symmetrical around the bond’s internuclear axis, i.e., not distributed as a result of rotation about its axis.
(ii) As a result, the C-C single bond is allowed to rotate freely, resulting in various spatial configurations of atoms that are interchangeable.
These atomic configurations are known as conformations, rotamers, or conformers.
(iii) Alkenes have an endless number of conformations because of rotation around C-C bonds. However, due to a mild repulsive contact between nearby bonds, rotation around a C-C single bond is not entirely free and is hampered by a tiny energy barrier of 1–20 kJ mol-1. The strain is referred to as a torsion.
Question 57: The intermediate carbocation formed in the reactions of HI, HBr and HCl with propene is the same, and the bond energy of HCl, HBr and HI is 430.5 kJ mol-1, 363.7 kJ mol-1 and 296.8 kJ mol-1 respectively. What will be the order of reactivity of these halogen acids?
Answer 57:
The dissociation enthalpy of H-X determines how reactive hydrogen halides are. Hydrogen halides are more reactive in the following order: HI > HBr > HCl. To create alkyl halides, they combine with alkanes.
Due to the bond enthalpy of HI, HBr, and HCl, the order of their reactivity is
HCl < HBr < HI.
Question 58: Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile. Give a reason.
Answer 58:
The methoxy group makes anisole more reactive than benzene because it is an electron releasing group that increases the benzene ring’s electron density due to the +R effect.
Since the Cl group produces the +R and -I effects, it is less reactive than methoxybenzene. Additionally, because it has the -I and -R effect, chlorobenzene is more reactive than the group .
Question 59: Despite their – I effect, halogens are o- and p-directing in haloarenes. Explain.
Answer 59:
Halogens are ortho and para directing because they have a +R and -I effect. Now, halogens present on the benzene ring have these effects: the -I effect deactivates the ring, and the +R effect increases the electron density on ortho and para positions.
Question 60: Why does the presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
Answer 60:
Due to the presence of the nitro group, which possesses -I and -R effects, the benzene ring deactivated and the electron density decreased in the ortho and para positions relative to the meta locations.
Question 61: Suggest a route for the preparation of nitrobenzene starting from acetylene.
Answer 61:
When ethanol is pushed through an extremely hot iron tube at 873 K, it undergoes cyclic polymerization and produces benzene, from which nitrobenzene can be made through nitration.
Question 62: Write hydrocarbon radicals that can be formed as intermediates during monochlorination of 2-methylpropane? Which of them is more stable? Give reasons.
Answer 62:
Tertiary free radicals are more stable and sit stabilised due to hyperconjugation and nine hydrogen, whereas free radicals with one hydrogen and one hyper conjugative structure are less stable.
Question 63: Suggest a route to prepare ethyl hydrogen sulphate (CH 3 – CH 2 – OSO 2 -OH) starting from ethanol (C 2 H 5 OH).
Answer 63:
Preparing ethyl hydrogen sulphate starting from ethanol.
Protonation of alcohol and formation of a carbocation.
H 2 SO 4 → H + + – OSO 2 OH
CH 3 – CH 2 – O – H + H + → CH 3 – CH 2 – + OH 2
CH 3 – CH 2 – + OH 2 → CH 3 – + CH 2 + H 2 O
Attack of nucleophile
HO – SO 2 – O – + + CH 2 – CH 3 → CH 3 – CH 2 – O – SO 2 – OH
Question 64: An alkyl halide C 5 H 11 Br (A) reacts with ethanolic KOH to give an alkene ‘B’, which reacts with Br 2 to give a compound ‘C’, which on dehydrobromination gives an alkyne ‘D’. On treatment with sodium metal in liquid ammonia one mole of ‘D’ gives one mole of the sodium salt of ‘D’ and half a mole of hydrogen gas. Complete hydrogenation of ‘D’ yields a straight chain alkane. Identify A, B, C and D. Give the reactions involved.
Answer 64:
To identify A, B, C and D, the reactions involved are-
C 5 H 11 Br(A) + alc. KOH → C 5 H 10 (B)
C 5 H 10 (B) + Br /CS 2 → C 5 H 10 Br 2 (C)
C 5 H 10 Br 2 (C) + alc. KOH → C 5 H 8 (D) Alkyne
2C 5 H 8 + 2 Na → 2 C 5 H 7 Na + H 2
All the compounds above must be straight-chain as hydrogenation of alkyne (D) gives straight-chain alkane. It is clear that D is terminal alkyne as alkyne gives sodium alkenyde.
Question 65: An unsaturated hydrocarbon ‘A’ adds two molecules of H 2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
Answer 65:
Two molecules of hydrogen add on ‘A’; this shows that ‘A’ is either an alkadiene or an alkyne.
On ozonolysis, compound A gives
CH 3 CHO + O = CH – CH 2 – CH 2 – CHO + O = C ( CH 3 ) 2
Hence, the structure of A (Its IUPAC name will be 2-methyl octa 2,6 diene) is,
Question 66: In the presence of peroxide, addition of HBr to propene takes place according to anti-Markovnikov’s rule, but peroxide effect is not seen in the case of HCl and HI. Explain.
Answer 66:
The peroxide effect only occurs in the case of HBr because the H-Cl link is stronger than the H-Br bond. Additionally, in the case of HI, it is not seen.
While the H-I bond is weaker and iodine free radicals unite to produce dimer iodine molecules because the H-Br link has less bond energy than the H-Cl bond, the H-Cl bond is not cleaved by the free radical.
Benefits of Solving Important Questions Class 11 Chemistry Chapter 13
Students can face difficulty understanding the complex concepts in Chemistry. One way to tackle this is to practice questions given in our Chemistry Class 11 Chapter 13 important questions. The questions given in Class 11 Chemistry Chapter 13 important questions cover all the main topics, and these questions are created from an exam perspective and are likely to come in the exam. Solving important questions gives students a competitive edge.
Here are the benefits of regularly solving our important questions Class 11 Chemistry Chapter 13:
- Exam questions are created exclusively by the subject experts for the board and entrance exams, and students will revise everything and cover the entire syllabus.
- Some of these questions are more likely to be asked during the exam. It helps students to be prepared by giving them a general concept of how the questions might be expected in their exams.
- All types of questions, including MCQs, objective-type, short-answer, and long-answer questions, are included in the list of Class 11 Chemistry Chapter 13 important questions. It gives them a thorough comprehension of the concepts and supports their balanced exam preparation.
Students from grade 1 through grade 12 can access comprehensive learning solutions through Extramarks, a top online learning platform. In addition, we provide additional reading and course materials. Students may click the links below to get access to multiple resources:
- NCERT books
- CBSE syllabus
- CBSE sample papers
- CBSE previous year’s question papers
- Important formulas
- CBSE extra questions
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
Q.1 Write the names and draw the structures of any 3 isomers of dichloropropane.
Q.2 Answer the following:
(a) Out of CH3-CH=CH- and CH3-C?C- which is more basic?
(b) A cyclic hydrocarbon (A) has a molar mass of 78 gmol-1. It reacts with acyl halide in presence of anhydrous aluminium chloride forms acetophenone. (A) can be formed by a saturated open chain hydrocarbon (B) containing six carbon atoms. Identify (A) and (B) and write the reactions involved.
(a) CH3-CH=CH- is more basic. CH3-CH=CH- is the conjugate base of the alkene CH3-CH=CH2 while CH3-C¡C- is the conjugate base of the alkyne CH3-C¡CH. Since an alkyne is a stronger acid than an alkene, its conjugate base will be a weaker base than the conjugate base of an alkene.
(b) The molar mass of hydrocarbon (A) is 78gmol-1 and it forms acetophenone with acyl halide in presence of anhydrous AlCl3. The hydrocarbon (A) is benzene, and its chemical formula is C6H6.
Benzene can also be prepared by the aromatisation of a saturated hydrocarbon containing six carbon atoms.
The compound (B) is n-hexane.
Q.3 Answer the following questions:
(a) Describe the action of Lindlar?s catalyst.
(b) Just like alkenes, do alkynes also exhibit geometrical isomerism?
(c) Using chemical reaction, explain what happens when ethanol is heated to 443K.
(a) Lindlars catalyst is palladised charcoal partially deactivated with poisons like quinoline or sulphur compounds. It is used to catalyse the conversion of alkynes to yield alkenes having cis geometry.
(b) Geometric isomerism is shown by compounds which contain the same number and types of atoms, but which have different spatial arrangements of the atoms. Geometrical isomerism is possible in alkenes but not possible in alkynes because alkynes are linear molecules which contain triple bond around which rotation is not possible.
(c) Ethanol when heated with concentrated sulphuric acid at 443K forms ethene. The reaction is called acidic dehydration of alcohols and involves the elimination of one water molecule from the ethanol molecule in presence of an acid. This reaction is also an example of β-elimination reaction since there occurs the loss of one hydrogen atom from the β-carbon atom.
Q.4Given below are two statements labelled as Assertion (A) and Reason (R)
Assertion (A): In nitrobenzene, the transfer of electrons is towards the nitro group.
Reason (R): Nitrobenzene is an electron withdrawing group and it shows -R effect.
Select the most appropriate answer from the options given below:
Both A and R are true, and R is the correct explanation of A.
Both A and R are true but R is not correct explanation of A.
A is true but R is false.
A is false bur R is true.
Both A and R are true, but R is not correct explanation of A.
Explanation:
Nitrobenzene is an electron withdrawing group it shows ?R effect.
Q.5 Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Assertion: Acetylene on reaction with NaNH2 gives ammonia only.
Reason: In acetylene both the carbon atoms are sp hybridised.
HC¡CH†NaNH2HC¡CNa++NH3
Please register to view this section
Faqs (frequently asked questions), 1. what are hydrocarbons in chemistry.
Any class of organic compounds with only the atoms carbon (C) and hydrogen are known as hydrocarbons (H). The hydrogen atoms bind to the carbon atoms in a variety of ways to create the compound’s structural framework.
2. From where should students prepare for important questions of Class 11 Chemistry Chapter 13?
Students can prepare important questions Class 11 Chemistry Chapter 13 from the Extramarks website to better comprehend the concepts and do well in exams. It provides NCERT solutions chapter-wise to enhance the understanding of the students.
3. How do aromatic hydrocarbons affect humans?
Humans are often only mildly acutely toxic to PAHs. Cancer is the most important outcome of PAH poisoning. Incidences of bladder, skin, and lung cancer are rising due to occupational exposure to PAHs.
CBSE Related Links

Fill this form to view question paper
Otp verification.
- NCERT Solutions
- NCERT Class 11
- NCERT 11 Chemistry
- Chapter 13: Hydrocarbons
NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
Ncert solutions for class 11 chemistry chapter 13 – free pdf download.
* According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 9.
NCERT Solutions for Class 11 Chapter 13 Chemistry Hydrocarbons is a premier study material that helps you score excellent marks in the Class 11 examination. These solutions comprise answers to the textbook questions along with extra questions and important questions from previous years’ question papers. Studying the NCERT Solutions for Class 11 Chemistry will help you to understand the chapters in an effective way.
To kickstart your preparations in this chapter, access the NCERT Solutions for Class 11 Chemistry online or download it as a PDF from the link below for offline viewing. The NCERT Solutions for Class 11 are created by subject experts according to the latest update on the CBSE Syllabus 2023-24 and its guidelines.
Download NCERT Solutions Class 11 Chemistry Chapter 13 PDF

Organic chemistry is the scientific study of the structure, properties, composition, reactions and synthesis of organic compounds. To understand the chapter hydrocarbons, you have to study the chapter thoroughly and solve NCERT questions. Further, students can access NCERT Solutions while solving the questions.
NCERT Solutions for Chemistry – Class 11, Chapter 13: Hydrocarbons
The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbon is one of the important chapters of organic chemistry. Class 11 is an important phase of a student’s life because it sheds light on important topics such as hydrocarbons. CBSE Class 11 Hydrocarbons of the syllabus teaches about alkanes, the preparation of alkanes from unsaturated hydrocarbons, alkyl halides, carboxylic acids, and the physical and chemical properties of alkanes.
Hydrocarbons topic gives knowledge about Alkenes, the nomenclature of alkenes, isomers, and the preparation of alkene using alkyne. The NCERT Class 11 Chemistry textbook is one of the best study materials to study chemistry. The questions provided at the end of the chapter are very much important from the examination point of view. Sometimes the same questions are directly asked in the examinations, so it is important that students should solve all the NCERT questions.
Subtopics of Class 11 Chemistry Chapter 13 – Hydrocarbons
- Classification
- Nomenclature and Isomerism
- Preparation
- Conformations
- Structure of Double Bond
- Nomenclature
- Structure of Triple Bond
- Structure of Benzene
- Aromaticity
- Preparation of Benzene
- Directive Influence of a Functional Group in Monosubstituted Benzene
- Carcinogenicity And Toxicity
Class 11 Chemistry NCERT Solutions (Hydrocarbons) – Important Questions
Question 13.1: How do you account for the formation of ethane during chlorination of methane?
Answer 13.1:
The methane chlorination process works through a free radical chain mechanism.
Step 1: Initiation:

Step 2: Propagation:

When these methyl radicals react with other chlorine-free radicals, methyl chloride is formed next to the liberation of a chlorine-free radical.

Step 3: Termination:

Through this process, ethane is thus obtained as a by-product of methane chlorination.
Question 13.2:
Write the IUPAC names of the following compounds :
(i) CH 3 CH = C (CH 3 ) 2
(ii) CH 2 = CH – C C – CH 3

Answer 13.2:

2-Methylbut-2-ene is the required IUPAC name

Pen-1-ene-3-yne is the required IUPAC name
1, 3-Butadiene or Buta-1,3-diene is the required IUPAC name

4-Phenyl but-1-ene is the required IUPAC name

2-Methyl phenol is the required IUPAC name

5-(2-Methylpropyl)-decane is the required IUPAC name

4-Ethyldeca-1, 5, 8-triene is the required IUPAC name
Question 13.3:
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of the double or triple bond as indicated :
(a) C 4 H 8 (one double bond)
(b) C 5 H 8 (one triple bond)
Answer 13.3:
(a) The resulting structural isomers with one double bond are probable for C 4 H 8 :

The IUPAC name of
Compound (I) is But-1-ene,
Compound (II) is But-2-ene, and
Compound (III) is 2-Methylprop-1-ene.
(b) The subsequent structural isomers are probable for C 5 H 8 with one triple bond:

Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-ene.
Question 13.4:
Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
(i) Pent-2-ene
(ii) 3, 4 – Dimethyl-hept-3-ene
(iii) 2-Ethylbut-1-ene
(iv) 1 – Phenylbut-1-ene
Answer 13.4:
(i) Ozonolysis of Pent-2-ene is shown as:

The following Product IUPAC names are:
Product (I) ethanal, and
Product (II) propanal.
(ii) Ozonolysis of 3, 4-Dimethylhept-3-ene is shown as:

The product names subsequent to IUPAC are:
Product (I) butan-2-one, and
Product (II) Pentan-2-one
(iii) Ozonolysis of 2-Ethylbut-1-ene is shown as:

The subsequent IUPAC names of the products are:
Product (I) pentan-3-one, and
Product (II) methanal
(iv) Ozonolysis of 1 – Phenylbut-1-ene is shown as

The subsequent IUPAC names of the products are :
Product (I) benzaldehyde, and
Product (II) propanal
Question 13.5:
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
Answer 13.5:

In the course of ozonolysis, an ozonide is produced as an intermediate that has a cyclic structure; it undergoes cleavage to provide the final products. Ethanal and pentan-3-one are obtained from the ozonide intermediates. So, the possible ozonide structure will be:

This ozonide is produced by adding ozone to ‘ A ‘ as a transitional product that forms final products undergoing cleavage. The preferred ‘ A ‘ arrangement may be obtained by removing ozone from the ozonide. Therefore ‘ A ”s structural formula is:

As an outcome, ‘A’ is 3-Ethylpent-2-ene is the IUPAC name of alkene.
Question 13.6:
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Answer 13.6:
From the information given, it gives two moles of an aldehyde of molar mass 44 u when ‘ A ‘ undergoes ozonolysis. The formation of an aldehyde’s two moles suggests that the presence of similar structural units on both sides of the C-C ÿ bond is present. Consequently, the ‘ A ‘ structure can be represented as shown below:
We know XC = CX
Because there are eight C – H σ bonds, 8 hydrogen atoms are present in ‘ A. ‘ There are also three C – C bonds, so the ‘ A ‘ structure contains four carbon atoms.
The combination of all these inferences can represent the structure of ‘ A ‘ as shown below:

Therefore, the IUPAC name of ‘A’ is But-2-ene.
After Ozonolysis of ‘A’ takes place,
The end product is ethanol with molecular weight = 44 u

Question 13.7:
Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Answer 13.7:
From the given information, the two ozonolysis products of an alkene are pentan-3-one and propanal.
Suppose the alkene given is A. The reverse of the ozonolysis reaction is what we get,

Because ‘ X ‘ is an additional component of the ozone alkene ‘ A. ‘ Therefore, the potential structure of the given ‘ A ‘ alkene will be:

Question 13.8:
Write chemical equations for the combustion reaction of the following hydrocarbons:
(i) Butane, (ii) Pentene, (iii) Hexyne, (iv) Toluene
Answer 13.8:
Combustion reactions may be defined as an oxygen or oxygen reaction of a compound.

Question 13.9:
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Answer 13.9:
Hex-2-ene is represented as shown below:

On the other hand, a trans-compound dipole moment is the result of the dipole moments of C – CH 3 bonds and C – CH 2 CH 3 bonds both acting in opposite directions
As cis-isomer is more polar than trans-isomer. Thus, the higher the polarity, the greater the intermolecular dipole-dipole interaction and the need for more heat to break the bonds. The boiling will be higher, therefore.
Therefore, cis-isomer of a compound will have a higher boiling point than trans-isomer of that compound.
Question 13.10:
Why is benzene extraordinarily stable though it contains three double bonds?
Answer 13.10:

All six carbon atoms in benzene are hybridized to sp 2 .In benzene, each carbon atom’s two sp 2 hybrid orbital overlaps with its adjacent carbon atoms ‘ sp 2 hybrid orbital, forming a six-sigma bond in the hexagonal plane. The remaining hybrid orbital sp 2 on each carbon atom overlaps with the hydrogen atom’s s-orbital to form six sigma C – H bonds. Now, the rest are unhybridized p -orbital of carbon atoms that will have the possibility of forming three C-C π bonds by the lateral overlap of

These six π-bonds are delocalized and can move about the six-carbon nuclei freely. Therefore, due to the delocalization of these π-bonds, benzene is stabilized even after the presence of three C-C π -bonds.
Question 13.11:
What are the necessary conditions for any system to be aromatic?
Answer 13.11:
The essential conditions for any aromatic system are as follows:
(i) Firstly, the compound arrangement or structure should be planar.
(ii) The n-electrons are completely delocalized in the ring of a compound
(iii) The sum of n-electrons in the ring of a compound must be equal to (4n + 2)π,
Where n = 0, 1, 2, ….. and so on. This rule is called Huckel’s rule.
Question 13.12: Explain why the following systems are not aromatic?

Answer 13.12:

Due to the presence of a sp 3 -hybridized carbon, the system is not planar. It does contain six n-electrons but the system is not fully conjugated since all the six n-electrons do not form a single cyclic electron cloud that surrounds all the atoms of the ring. Therefore, it is not an aromatic compound.

Due to the presence of sp 3 – hybridized carbon, the system is not planar. Further, it contains only four n-electrons, therefore, the system is not aromatic because it does not contain planar cyclic cloud having (4n+2) n-electrons.

Cyclo-octatetraene is not planar but is tub-shaped. It is, therefore, a non-planar system having 8 n-electrons. Therefore, the molecule is not aromatic as it does not contain a planar cyclic cloud having (4n + 2) n-electrons.
Question 13.13:
How will you convert benzene into:
(i) p – nitrobromobenzene
(ii) m-nitrochlorobenzene
(iii) p -nitrotoluene
(iv) acetophenone
Answer 13.13:
(i) Benzene converted to p – nitrobromobenzene

(ii) Benzene converted to m-nitrochlorobenzene

(iii) Benzene converted to p –nitrotoluene

(iv) Benzene converted to acetophenone

Question 13.14:
In the alkane H 3 C– CH 2 – C(CH 3 ) 2 – CH 2 – CH(CH 3 ) 2 , identify 1°, 2°, 3° carbon atoms and give the number of H atoms bonded to each one of these.
Answer 13.14:

Primary carbon atoms (1 o ): Carbon atoms bonded to a single atom of carbon are called primary atoms of carbon. The arrangement given has corresponding five 1o carbon atoms and fifteen H atoms.
Secondary carbon atoms (2 o ): The secondary carbon atom is called a carbon atom bonded to a double carbon atom. The arrangement given has two corresponding 2o carbon atoms and four H atoms.
Tertiary carbon atoms (3 o ): The term tertiary carbon atom is used to refer to carbon atoms bonded to three carbon atoms. The arrangement given has one corresponding 3 degree carbon atom and a single H atom.
Question 13.15:
What effect does branching of an alkane chain has on its boiling point?
Answer 13.15:
Alkanes encounter Van-der Waals forces between molecules. The higher the alkane’s power, the greater is the boiling point.
As the molecule branching increases, the surface area decreases, which leads to a small contact area. As a result, the force of the Van-der Waals (or intermolecular force) decreases too. Those forces can be overcome very easily at a relatively lower temperature. Thus, the boiling point of an alkane chain decreases as branching increases.
Question 13.16:
Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer 13.16:
The addition of HBr to propene is an example of an electrophilic substitution reaction.
Being an acid, the hydrogen bromide provides an electrophile, H+. This electrophile attacks the propene double bond to form carbocations of 1 ° and 2 °, as shown below:

Secondary carbocations are stable in comparison with primary carbocations. The secondary carbocations therefore predominate, as they form at a faster rate than primary carbocations. Thus, Br– attacks the primary carbocation to form 2-bromopropane as the main product in the next step.

This reaction follows the rule of Markovnikov, in which the negative part of the addendum is attached to the carbon atom with fewer hydrogen atoms than other carbon atoms present in the compound.
While, in the presence of benzoyl peroxide, an addition reaction takes place anti to Markovnikov’s rule. The reaction follows a free radical chain mechanism as shown below:

Secondary free radicals are stable in comparison with primary radicals. The secondary radical therefore predominates, for it forms at a faster rate than the primary radical. Therefore 1 – bromopropane is obtained as the main product.

Br acts as an electrophile in the presence of peroxide, as a free radical. Thus, in the presence and absence of peroxide, two different products are obtained on addition of HBr to propene.
Question 13.17:
Write down the products of ozonolysis of 1,2-dimethylbenzene (o-xylene). How does the result support Kekulé structure for benzene?
Answer 13.17:
o -xylene has two resonance structures, which are as follows:

All three products are obtained from two Kekule structures of o-xylene, i.e., methyl glyoxal, 1, 2-demethylglyoxal, and glyoxal. As all three products can not be obtained from either of the two structures, this proves that o-xylene is a resonance hybrid of two Kekule (I and II) structures.
Question 13.18:
Arrange benzene, n -hexane and ethyne in increasing order of their acidic behavior. Also, give a reason for this behavior.
Answer 13.18:
Acidic character of a species is defined on the basis of the ease with which it can lose its H– atoms.
The hybridization state of carbon in the given compound is:

As the s – character decreases, carbon electronegativity decreases and C – H bond pair electrons lie away from the carbon atom. As a result, H– atom partially positive charge increases, and H+ ions are set free.
The s –character decreases in the order:
sp > sp 2 > sp 3
Hence, the increasing order of acidic behavior is Hexane < Benzene < Ethyne.
Question 13.19:
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer 13.19:
Benzene is a planar molecule with electrons delocalized under and above the ring plane. Hence, it is a material rich in electrons. As a consequence, electron-deficient species, i.e., electrophiles, are extremely attractive.
Benzene, therefore, very easily undergoes electrophilic substitution reactions. Nucleophiles, on the other hand, are also species that are rich in electrons. Therefore, benzene is repelled as compared to electrophiles. Thus, benzene suffers from difficulty with nucleophilic substitutions.
Question 13.20:
How would you convert the following compounds into benzene?
(i) Ethyne (ii) Ethene (iii) Hexane
Answer 13.20:
(i)Benzene from Ethyne:

(ii)Benzene from Ethene:

(iii) Hexane to Benzene

Question 13.21:
Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer 13.21:
The basic structure of 2-methylbutane is shown below:
On the basis of the above structure, various alkenes that will give 2-methylbutane on hydrogenation are shown below:

Question 13.22:
Arrange the following set of compounds in order of their increasing relative reactivity with an electrophile, E +
(a) p -nitrochlorobenzene, Chlorobenzene, 2,4-dinitrochlorobenzene,
(b) p – H 3 C – C 6 H 4 – NO 2 , Toluene, p -O 2 N – C 6 H 4 – NO 2 .
Answer 13.22:
Electrophiles are reagents that participate in a reaction by accepting a pair of electrons to bind to nucleophiles.
The higher the density of electrons on a benzene ring, the more reactive the compound is to an electrophile, E + (Electrophilic reaction).
(a) The electron density of the aromatic ring decreases due to the presence of an electron-withdrawing group (i.e., NO 2 – and Cl –) which deactivates the aromatic ring.
Since Cl – group is less electron-withdrawing (due to the inductive effect) than NO 2 – group (due to the resonance effect), the increasing order of reactivity is as follows:
2, 4 – dinitrochlorobenzene < p – nitrochlorobenzene < Chlorobenzene
(b) While NO 2 – group is electron-withdrawing, CH 3 – is an electron-donating group.
Toluene, therefore, has the maximum density of electrons and is most easily attacked by E + . Since NO 2 – is an electron-removing group. Therefore, when the number of NO 2 substitutes is higher, the order is the following.:
p -O 2 N – C 6 H 4 – NO 2 < p – H 3 C – C 6 H 4 – NO 2 < Toluene.
Question 13.23:
Out of benzene, m –dinitrobenzene and toluene, state the increasing order of nitration. Justify your answer?
Answer 13.23:
The ease of nitration depends on the presence of electron density on the compound to form nitrates. Nitration reactions are examples of electrophilic substitution reactions where a nitronium ion (NO 2 –) attacks an electron-rich species.
Now NO 2 – is electron-withdrawing and CH 3 – group is electron-donating. Since, m– Dinitrobenzene will have the least electron density. Hence, it will undergo nitration with difficulty. Therefore, toluene will have the maximum electron density among the three compounds, followed by benzene. Hence, the increasing order of nitration is as follows:

Question 13.24:
List the names of some Lewis acid which can be used during ethylation of benzene in a Friedel-Craft alkylation reaction.
Answer 13.24:
The reaction of benzene to the presence of Lewis acids (AlCl 3 ) with an acyl halide or acid anhydride yields acyl benzene (or benzene ring). A Friedel-Craft alkylation reaction is called such a reaction. The reaction occurs in the presence of a Lewis acid.
In the Friedel-Craft alkylation reaction, any Lewis acid, such as anhydrous AlCl 3 , FeCl 3 , SnCl 4 , BF 3 etc., may be used during the ethylation of benzene.
Question 13.25:
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example
Answer 13.25
For the synthesis of symmetrical alkanes (i.e. alkanes with an even number of carbon atoms), the Wurtz reaction is limited. Two similar alkyl halides are taken as reactants in the reaction, then an alkane is formed, which contains double the number of carbon atoms.

Wurtz reaction can not be used to produce unsymmetric alkanes (i.e. alkanes with an odd number of carbon atoms), because if two dissimilar alkyl halides are used as reactants, a mixture of alkanes is obtained as the items. Since the reaction involves free radical species, there is also a side reaction to creating an alkene.

The boiling points of alkanes (obtained in the mixture) are very close. Hence, it becomes difficult to separate them.
The important topics covered in NCERT Solutions for Class 11 Chemistry Chapter 13 are:
- Classification of hydrocarbons
- Nomenclature, preparation, properties and isomerism of:
- Aromatic hydrocarbons
- Carcinogenicity and toxicity
The topics given in the Class 11 CBSE Syllabus are the basics of the topics to be taught in Class 12. Also, students are advised to solve and go through this NCERT Solutions for Class 11 Chapter 13 to determine their strengths and weaknesses and plan their studies accordingly. Along with NCERT questions, students should try to solve the previous years’ questions as well as the CBSE Sample Papers to get acquainted with the latest exam pattern and marking scheme.
Students are advised to visit the website for the latest NCERT Solutions for Classes 6 to 12 and get full assistance for the exam. Students can also download BYJU’S – The Learning App to study using interactive and engaging videos.

Frequently Asked Questions on NCERT Solutions for Class 11 Chemistry Chapter 13
Why should we follow ncert solutions for class 11 chemistry chapter 13, how do byju’s ncert solutions for class 11 chemistry chapter 13 help the students in preparing for the exams, what are the topics covered under ncert solutions for class 11 chemistry chapter 13, leave a comment cancel reply.
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Important Questions for CBSE Class 11 Chemistry Chapter 13 – Hydrocarbons
Important questions for cbse class 11 chemistry chapter 13 - hydrocarbons, cbse class 11 chemistry chapter-13 important questions - free pdf download.
Free PDF download of Important Questions for CBSE Class 11 Chemistry Chapter 13 - Hydrocarbons prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books. Register online for Chemistry tuition on CoolGyan.Org to score more marks in your examination.
1 Marks Questions
1.Classify the hydrocarbons according to the carbon – carbon bond
Ans . Hydrocarbons are categorized into three categories according to the carbon – carbon bond that exists between then-
(a) saturated hydrocarbon
(b) Unsaturated hydrocarbon
(c) Aromatic hydrocarbon.
2.What are cycloalkanes?
Ans When carbon atoms form a closed chain or a ring, they are termed as cycloalkanes.
3. Why carbon does have a larger tendency of catenation than silicon although they have same number of electrons?
Ans . It is due to the smaller size C-C bond which is stronger (335 KJ mol -1 ) than in Si bond (225.7 KJ mol -1 ).
4. Write IVPAC names of the following

CH 2 – CH (CH 3 ) 2 .
Ans 09. 5-(2 – Methyl propyl) – decane.
5.What is hydrogenation?
Ans . Dihydrogen gas gets added to alkenes and alkenes in the presence of finely divided catalysts like Pt, Pd or Ni to form alkanes. This process is called hydrogenation.
6. How would you convert ethene to ethane molecule?

7.Give the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon.
Ans 03. 2, 2-dimethyl propane.
8.Methane does not react with chlorine in dark. Why?
Ans .Chlorination of methane is a free radical substitution reaction. In dark, chlorine is unable to be converted into free radicals, hence the reaction does not occur.
9.Which conformation of ethane is more stable?
Ans .Staggered conformation.
10. State Le chatelier’s principle.
Ans . It states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change.
11.Can a catalyst change the position of equilibrium in a reaction?
Ans . No, a catalyst cannot change the position of equilibrium in a chemical reaction. A catalyst, however, affects the rate of reaction.
12.What is the effect of reducing the volume on the system described below?

Ans . The forward reaction is accompanied by increase in volume. Hence according to Chatelier’s principle, reducing the volume will shift the equilibrium in the forward direction.
13.What happens when temperature increases for a reaction?

14.Can a catalyst change the position of equilibrium in a reaction?
Ans . No, a catalyst cannot change the position of equilibrium in a chemical reaction. A catalyst affects the rate of reaction.
15.If Qc < Kc, when we continuously remove the product, what would be the direction of the reaction?
Ans. Continuous removal of a product maintains Qc at a value less than Kc and reaction continues to move in the forward direction.
16.What is a Lindlars’ catalyst?
Ans. Partially deactivated palletized charcoal is known as Lindlar’s catalyst.
17.How is alkene produced by vicinal dihalide?
Ans . Vicinal dihalide on treatment with Zn metal lose a molecule of ZnX 2 to from an alkene. This reaction is known as dehalogenation.
CH 2 Br-CH 2 Br+Zn→ CH 2 =CH 2 +ZnBr 2 .
18.Arrange the following halogen atom to determine rate of the reaction. Iodine, chlorine. Bromine.
Ans . iodine > bromine > chlorine.
19.What is β-elimination reaction?
Ans. When hydrogen atom is eliminated from the β-carbon atom (carbon atom next to the carbon to which halogen is attached).
20.What is the number of σ and π bond in
N ≡ C – CH = CH – C ≡ N?
Ans .There are 7σ bonds and 5 π-bonds.
21.Name the type of hybridization in C (2) and C (3) in the following molecule

Ans. .C(2) is sp-hybridized and C(3) is sp 2 hybridized.
22.Why do alkynes not show geometrical isomerism?
Ans. Alkynes have linear structure. So they cannot show geometrical isomerism.
23.Write the general formula for alkynes.
Ans .C n H 2n −2.
24.Name the simplest alkyne
Ans . Ethyne is the simplest alkyne.
25.Write combustion reaction for hexyne.
Ans . Combustion reaction for hexyne.

26.How will you convert ethyne to benzene?

27.What are benzenoids?
Ans . Aromatic hydrocarbon compound containing benzene ring are known as benzenoids.
28.Although benzene is highly unsaturated; it does not undergo addition reactions. Give reason.
Ans . Unlike olefins, π-electrons of benzene are delocalized (resonance) and hence these are uncreative towards addition reactions.
29.How will you convert the following compounds into benzene?
(i) ethene (ii) hexane.

2 Marks Questions
1.The boiling point of hydrocarbons decreases with increase in branching. Give reason.
Ans. Branching result into a more compact (nearly spherical) structure. This reduces the effective surface area and hence the strength of the Vander wall’s forces, thereby leading to a decrease in the boiling point.
2.Unsaturated compounds undergo addition reactions. Why?
Ans. Unsaturated hydrocarbon compounds contain carbon – carbon double or triple bonds. The π-bond is multiple bond is unstable and therefore addition takes place across the multiple bonds.
3.To which category of compounds does cyclohexane belong?
Ans. Saturated alicyclic hydrocarbons.
4.Draw the structure of the following compounds all showing C and H atoms.
(a) 2-methyl -3-iso propyl heptanes
(b) Dicyclopropyl methane.

(dicyclopropyle methane)
5.Draw all the possible structural isomers with the molecular formula C 6 H 14 , Name them.[2.5]
Ans . (i) CH 3 – CH 2 – CH 2 – CH 2 – CH 2 – CH 3 (n– hexane)

6.Sodium salt of which acid will be needed for the preparation of propane? Write chemical equation for the reaction.
Ans. Butanoic acid,

7.Cyclobutane is less reactive than cyclopropane. Justify.

8.How will you prepare isobutane?
Ans . Isobutane is obtained by decarboxylation of 3-methyl butanoic acid with soda lime at 630K.

9.The boiling point of alkanes shows a steady increase with increase in molecular mass. Why?
Ans . This is due to the fact that the intermolecular van der walls forces increase with increase of the molecular size or the surface area of the molecule.
10.Pentane has three isomers i.e; pentane, 2-methyl butane and 2,2-dimethyl propane . The b.p of pentane is 309.1K whereas 2,2-dimethyl propane shows a b.p of 282.5k. Why?
Ans . With the increase in number of branched chains, the molecule attains the shape of a sphere. This results in smaller area of contact and therefore weak inter molecular forest between spherical molecules, which are overcome a relatively lower temperatures.
11.Draw the New man’s projection formula of the staggered form of 1,2-dichloro ethane.

12.All the four C-H bonds in methane are identical. Give reasons.
Ans . The four C-H bonds of methane are identical because all of these are formed by the overlapping of the same type of orbital’s i.e; hybrid orbital’s of carbon and s-orbital of hydrogen.
13.When alkanes are heated, the C-C bonds rather than the C-H bonds break. Give reason.
Ans. When alkanes are heated, the C-C bonds rather than the C-H bonds breaks because the C-C bond has a lower bond energy (∆H=83K Cal/mole) than the C-H bond (∆H=99 K Cal / mole).
14.How would you convert cyclohexane to benzene?
Ans . Cyclohexane when treated with iron or quartz in a red hot tube undergoes oxidation to form benzene.

15.OEHow is iso-butane prepared?
Ans . By decarboxylation of 3 – methyl butanoic acid with soda lime at 630 K.

16.Why the addition of inert gas does does not change the equilibrium?
Ans. It is because the addition of an inert gas at constant volume does not change the partial pressures or the molar concentrations of the substance involved in the reaction.
17.The equilibrium constant of a reaction increases with rise in temperature. Is the reaction exo – or endothermic?
Ans. The equilibrium constant increases with a rise in temperature. Therefore, the reaction is endothermic.
18.Using Le – chatelier principle, predict the effect of
(a) decreasing the temperature
(b) increasing the temperature
in each of the following equilibrium systems:

Ans.(i) For an exothermic reaction increase in temperature shifts the equilibrium to the left and decrease in temperature shifts it to the left.
(ii) For an endothermic reaction increase in temperature shifts the equilibrium to the right and decrease in temperature shifts it to the right.
19.(i) In the reaction equilibrium
What will happen to the concentrations of A, B and D if concentration of C is increased.
(ii) what will happen if concentration of A is increased?
Ans. (i) For an equilibrium reaction

If the concentration of a product is increased, the concentration of other components changes in such a way that the conc of C decreases and vice – versa.
If the conc of C is increased the conc of D will decrease and those of A and B will increase simultaneously so that the numerical value of Kc is the same and vice – versa. The equilibrium shifts to the left.
(ii) If the conc of A is increase, conc of B will decrease and those of C and D will increase simultaneously so that the numerical value of Kc is the same and vice – versa. The equilibrium shifts to the right
20.How is alkene produced by Kolbe’s electrolytic method?

21.How is alkene prepared from alcohol by acidic dehydration?
Ans . Alcohols on heating with concentrated sulphuric acid form alkenes with the elimination of one water molecule.

22.How are trans alkenes formed by alkynes?
Ans. Alkynes on reduction with sodium in liquid ammonia form trans alkenes.

23.How are cis – alkenes formed by alkynes?
Ans. Alknes on partial reduction with calculated amount of dihydrogen in the presence of palladised charcoal partially deactivated with poisons like sulphur compounds or quinoline give cis-alkene.

24.Stale Markownikov’s Rule.
Ans. It states that when a polar compound is added to an unsymmetrical alkenes, or alkynes positive part goes to the most substituted carbon atom and negative part goes to the least substituted carbon atom.
25.Write the chemical equations of reactions involved in ozonolysis of alkenes.
Ans. It is a process in which alkenes react with ozone to form ozonide which on reduction in presence of Zn give aldehyde and ketones. E.g;

26.How will you distinguish between butene – 1 and butene – 2?
Ans. Butene – 1 and butene – 2 can be distinguished either by ozonolysis or by oxidation with acidic KMnO 4 solution which they give different carbonyl compounds.

27.State kharasch effect.
Ans. It states that in presence of peroxides such as benzoyl peroxide, addition of HBr (but not of HCl or HI) to unsymmetrical alkenes occurs contrary to Markontkov’s rule.

28.How is alkyne prepared from calcium carbide?
Ans. Calcium carbide is treated with water to get ethyne.
CaC 2 + 2H 2 O → Ca(OH) 2 + C 2 H 2
29.How is alkyne prepared by Kolbe’s method?

30.How is alkyne prepared from vicinal dihalides?
Ans. Vicinal dihalides on treatment with alcoholic potassium hydroxide undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with sodiumamide gives alkynes.

31.How will you distinguish between ethylene and methane?
Ans. Ethylene discharges bromine water colour and Baeyer’s reagent colour while methane does not.
32.Although acetylene is acidic in nature, it does not react with NaOH or KOH. Give reason?
Ans. Acetylene is a very weak acid (pKa=25) and hence only an extremely strong base like amide ion (NH 2 - ) can successfully remove a proton.
33.Write the conversion of ethene to ethyne.

34.How would you distinguish between butyne – 1 and butyne – 2?
Ans. Butyne – 1 (CH 3 CH 2 C ≡ CH), having an acetylene hydrogen atom will give white precipitate with ammonical silver nitrate and red precipitate with ammonical cuprous chloride. On the other hand, butyne – 2 (CH 3 C ≡ C CH 3 ) having no acetylene hydrogen atom does not respond to either of the two reagent.
35.How would you carry out the following conversion propene to ethyne.

36.How will you convert propyne to propanone?

37.How will you convert ethyne to ethane?

38.Convert 2- butyne to trans – 2- butane.

39.How will you prepare 3-methyl but -1 – yne by starting with ethyne?

40.Write the IUPAC name of the following compound-

Ans.(i) 4 – phenyl – but – 1 – ene.
(ii) 2 – Methyl phenol.
41.What do you mean by delocalization?
Ans. Delocalisation – Delocalisation implies that pairs of bonding electrons extend over three or more atoms and belong to the whole molecule. Delocalized π-orbitals are much larger than the localized π-orbitals and are therefore more stable.
42. What do you understated by Resonance energy?
Ans.The difference between the energy of the most stable contributing structure and the energy of the resonance hybrid is known as resonance energy. In case of benzene, the resonance hybrid has (147KJ/mol -1 ) less energy than either A to B. Thus resonance energy of benzene is 147KJ/mole.
43.How is phenol reduced to benzene?

44.How is aromaticity of a compound judged?
Ans. The following characteristics decides aromaticity of a compound-:
(i) Planarity
(ii) Complete delocalization of the π-electrons in the ring.
(iii) Presence of (4n+2) π electrons in the ring where n is an integer (n=0, 1, 2 ----)
This is often referred to as Huckel Rule.
45.Give some examples of aromatic compounds.

46.How will you account for the structure of benzene?
Ans. All the six carbon atoms in benzene are sp2 hydridised. Two sp2 hydrid orbitals of each carbon atom overlap with sp2 hydrid orbitals of adjacent carbon atoms to form six C-C sigma bonds with are in the hexagonal plane. The remaining sp2 hybrid orbital of each carbon atom overlaps with s-orbital of a hydrogen atom to form six C-H sigma bonds. Each carbon atom is now left with one hybridized p-orbital perpendicular to the plane of the ring.

47.How is benzene prepared from aromatic acids?
Ans. Sodium salt of benzoic acid on heating with soda lime gives benzene.

48.How is phenol reduced to benzene?
Ans. Phenol is reduced to benzene by passing its vapours over heated zinc dust.

49.Why is benzene extra ordinarily stable though it contains three double bounds?
Ans. Due to resonance.
50.What is friedel craft’s reaction? Give an example.
Ans. When benzene or its derivative reacts with alkyl halide in presence of AlCl 3 , we get alkyl benzene.

51.What happens when benzene is oxidized at 770K in presence of V 2 O 5 ? Give chemical equation.

52.How will you convert benzene to iodobenzene? Give chemical equation.

53.What are electrophilic substitution reactions?
Ans. Those reactions in which weaker electrophile are replaced by a stronger electrophile are called electrophilic substitution reactions.
54.How will you distinguish between Ethene and benzene
Ans. Ethene discharges bromine water colour and Baeyer’s reagent colour while benzene does not.
55.How is benzene converted to benzene hexachloride? Ans. Under ultra-violet light, three chlorine molecules add to benzene to produce benzene hexachloride, C 6 H 6 Cl 6 which is also called gammaxane.

56.How will you convert benzene to hexachlorobenzene? Ans. Benzene on treatment with of chlorine in the presence of anhydrous AlCl 3 in dark yields hexachloroben - zene (C 6 Cl 6 )
3 Marks Questions
1. N – pentane has higher boiling point than neopentane but the melting point of neopentane is higher than that of n – pentane.
Ans .Because of the presence of branches in neo-pentane the surface area and van der walls forces of attraction are very weak in neopentane than in n-pentane. Therefore the b.p of neopentane is lower than that of n-pentane.
M.P depends upon the packing of the molecules in the crystal lattice. Since neopentane are more symmetrical than n-pentane therefore, it packs much more closely in the crystal lattice than n-pentane and hence neopentane has much higher m.p than n-pentane.
2.The dipole moment of trans 1,2-dichloroethane is less than the cis – isomer. Explain.
Ans. The structure of trans isomer is more symmetrical as compared to the cis – isomer. In the trans – isomer, the dipole moments of the polar C-Cl bonds are likely to cancel effect of each other and the resultant dipole moment of the molecule is nearly zero. But in the cis – isomer, these do not cancel. Therefore, the cis isomer has a specific moment but is zero in case of trans isomer.

3.Explain wurtz reaction with an example.
Ans. Wurtz reaction – This reaction is employed to obtain higher alkanes from the halides of lower alkanes. The halides of lower alkanes are treated with sodium metal in ether:

4.Discuss the hybridization of carbon atoms in alkene C 3 H 4 and show the π-orbital overlaps.
Ans. The structure of alkene (C 3 H 4 ) is given here.

The carbon atom 1 and 3 are sp 2 hybridised since each one of them is joined by a double bond. In contrast, carbon atom 2 is sp hydridiesed since it has two double bonds thus the two double bonds in

alkenes are perpendicular to each other.
5.Write IUPAC name of the products obtained by addition reactions of HBr to hex – 1 – ene.
(i) in the absence of peroxide, and
(ii) in the presence of peroxide.

6.Explain the term polymerization with two examples.
Ans . Polymerization – when two or more molecules of unsaturated compounds are made to combine under suitable conditions to form a bigger compound, the compound formed is known as the polymer and the process is known as polymerization.
(a) Addition polymerization –
The bigger molecule i.e; polymer is an exact multiple of the smaller molecule and nothing is lost during the reaction

(b) Condensation polymerization : There is generally the loss of molecules such as water, hydrochloric acid etc. During the polymerization, the polymer is not an exact multiple of the smaller molecule.
7.Draw the orbital picture of ethyne showing.
(a) sigma overlaps
(b) pi – overlaps.

8.Give the different isomers formed by C 5 H 8 along with their IUPAC name.

Structures I and II are position isomers and structures I and III or II and III are chain isomers.
9.Write structures of different isomers formed by C 6 H 10 . Also write IUPAC names of the all the isomers
Ans. The possible isomers are
(a) HC ≡ C – CH 2 – CH 2 – CH 2 – CH 3 (Hex – 1- yne)
(b) CH 3 – C ≡ C – CH 2 – CH 2 – CH 3 (Hex – 2- yne)
(c) CH 3 – CH 2 – C ≡ C - CH 2 - CH 3 (Hex-3- yne)

10.Ethyne is acidic in nature in comparison to ethene and ethane. Why is it so?
Ans . Hydrogen atoms in ethyne are attached to the sp hybirdised carbon atoms whereas they are attached to sp 2 hybridized carbon atoms in ethene and sp 3 hydridised carbons in ethane. Due to the maximum percentage of s – character (50%), the sp hybridized orbital’s of carbon atoms in ethyne molecules have highest etcetronegativity : Which attracts the shared pair of the C-H bond of ethyne to a greater extent than that of the sp 2 hybridized orbital’s of carbon in ethene and the sp3 hybridized orbital of carbon in ethane. Thus in ethyne molecule, hydrogen atoms can be liberated as protons more easily as compared to ethene and ethane.
11.Butanone is formed when an alkyne is passed through a dil sol of H 2 SO 4 at 330K in presence of mercuric sulphate. Write the possible structure of the alkyne.
Ans. Since Butanone is a four carbon atom, therefore both but – 1- yne and but – 2 – yne on hydration will produce butanone.

12.How would you convert ethanoic acid into benzene?

13..Name some carcinogenic hydrocarbons.

4 Marks Questions
1. How would you prepare benzene from lime?
Ans. Benzene can be prepared from lime by the following methods:

2. p-chloro nitro benzene has less dipole moment (2.4 D) than p-nitro toluene (4.4 D). Why?
Ans. In p-chloral nitro benzene the individual moments are in opposite directions and hencepartially cancel. When in p-nitro toluene, both moments are in the same direction and hence add each

8 Marks Questions
1. How will you convert the following compounds to benzene?
(i) Acetylene (ii) Benzoic acid
(iii) Cyclohexane (iv) Benzene diazonium chloride.
Ans. (i) When ethyne is heated at a higher temperature it polymerizes to give bnzene.

(ii) Benzoic acid when treated with NH 3 and heat changes to amide which on treatment with Br 2 / KOH gives aniline which converts to diazonium salt which on acid hydrolysis gives benzene.

(iii) Cyclohexane when treated with iron or quartz in a red hot tube under goes oxidation to form benzene.

(iv) In the presence of hypoposphorus acid benzene diazonium chloride is converted into benzene. (diazo group is replaced by H)

2. How will you convert benzene into
(i) p – Nitro bromo benzene (ii) m – Nitrochloro benzene (iii) p – Nitro toluene (iv) Aceto phenone?
Ans . Under ultra-violet light, three chlorine molecules add to benzene to produce benzene hexachloride, C 6 H 6 Cl 6 which is also called gammaxane.

Talk to our experts
1800-120-456-456
NCERT Solutions for Class 11 Chemistry Chapter 13 - Hydrocarbons
- NCERT Solutions
- Chapter 13 Hydrocarbons

The interaction of hydrogen with carbon becomes very easy to understand with reliable NCERT solutions for Class 11 Chemistry Chapter 13. The explanation of different hydrocarbons will let you do deeper into the organic world of chemistry. The expert teachers of Vedantu provide clear and easy notes for the Hydrocarbons Class 11 NCERT. You can just download them and get access to the PDF notes of hydrocarbons and quickly learn chemistry. The notes are suitable as per Class 11 chemistry, chapter 13.
Note: ➤ Unlock your dream college possibilities with our NEET College Predictor !
Topics and Subtopics
Let us look at the Topics and Subtopics of Class 11 Chemistry Chapter 13 - Hydrocarbons
Classification
Nomenclature And Isomerism
Preparation
Conformations
Alkenes
Structure Of Double Bond
Nomenclature
Structure Of Triple Bond
Aromatic Hydrocarbon
Structure Of Benzene
Aromaticity
Preparation Of Benzene
Directive Influence Of A Functional Group In Monosubstituted Benzene
Carcinogenicity And Toxicity
Important points
The important points that students can learn from NCERT Class 11 Chemistry Chapter 13 are given below:
A hydrocarbon is a carbon and hydrogen-based molecule.
Aromatic chemicals include benzene and its derivatives.
Alicyclic or carbocyclic chemicals are cyclic molecules that contain just carbon atoms.
Heterocyclic compounds are cyclic compounds in which the ring atoms are carbon and another element (for example, N, S, or O).
Alkanes are the most basic organic molecules, consisting just of carbon and hydrogen.
Hydrocarbons Chapter at a Glance - Class 11 NCERT Solutions
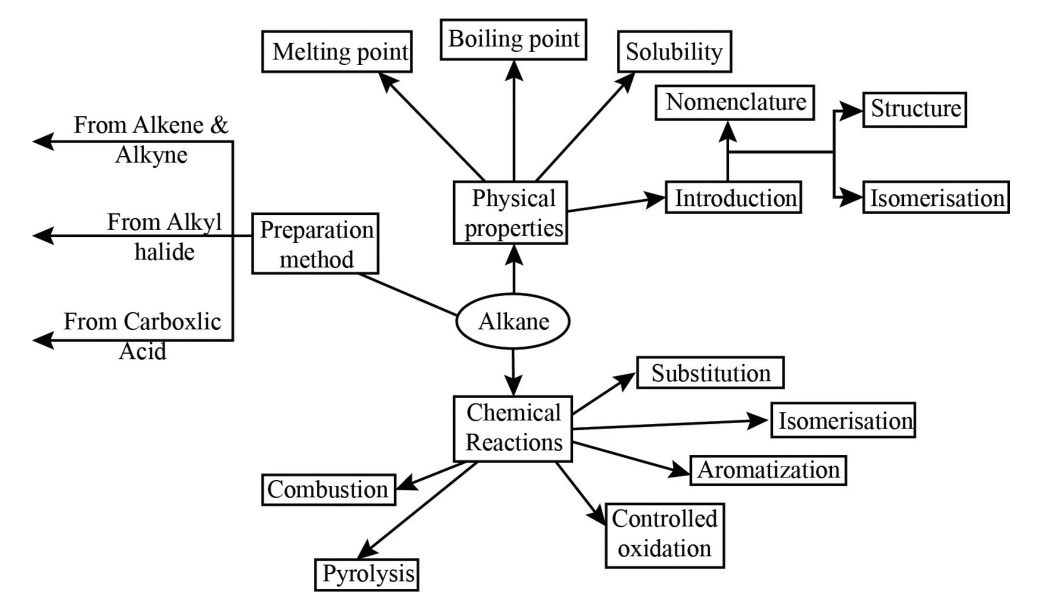
Access NCERT solutions for Class 11 Chapter 13- Hydrocarbons
NCERT Exercise
1. How do you account for the formation of ethane during chlorination of methane?
Chlorination of methane proceeds by free radical chain mechanism which involves three steps as follows:
Initiation:
The reaction begins with the homolytic bond cleavage within Cl – Cl bond that results in the formation of chlorine free radicals as;
(Image to be added soon)
Propagation:
In this step, chlorine free radicals formed in the prior step abstracts a hydrogen atom from methane to generate methyl radicals as;
The above methyl radicals then react with chlorine molecules to form methyl chloride along with the liberation of a chlorine free radical.
Thus, methyl and chlorine free radicals set up a chain reaction. When HCl and $C{{H}_{3}}Cl$ are formed as major products, other higher halogenated compounds are also formed as follows;
Termination:
When all the reactants are consumed, the reaction stops and the chain gets to the point of termination. This happens by the combination of different free radicals.
Chlorine free radicals combine to form a chlorine molecule.
Methyl free radicals combine to form ethane.
Hence, by the process of chlorination of methane, ethane is obtained as a by-product.
2. Write IUPAC names of the following compounds:
The IUPAC name of the above compound is 2-Methylbut-2-ene.
The IUPAC name of the above compound is Pen-1-en-3-yne.
The IUPAC name of the above compound is Buta-1,3-diene or 1,3-Butadiene.
The IUPAC name of the above compound is 4-Phenylbut-1-ene.
The IUPAC name of the above compound is 2-Methylphenol.
The above compound can be easily formulated as;
The IUPAC name of the above compound is 5-(2-Methylpropyl) decane.
The IUPAC name of the above compound is 4-Ethyldeca-1,5,8-triene.
3. For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
a) ${{C}_{4}}{{H}_{8}}$ (one double bond)
The structures with their IUPAC names are given as;
IUPAC name: But-1-ene
IUPAC name: But-2-ene
IUPAC name: 2-Methylprop-1-ene
b) ${{C}_{5}}{{H}_{8}}$ (one triple bond)
IUPAC name: Pent-1-yne
IUPAC name: Pent-2-yne
IUPAC name: 3-Methylbut-1-yne
4. Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
i) Pent-2-ene
The above compound undergo ozonolysis as follows;
The products of the reaction are ethanal and propanal.
ii) 3,4-Dimethyl-hept-3-ene
The products of reaction are Butan-2-one and Pentan-2-one.
iii) 2-Ethylbut-1-ene
The products of the reaction are Pentan-3-one and methanal.
iv) 1-Phenylbut-1-ene
The products of the reaction are benzaldehyde and propanal.
5. An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of ‘A’.
According to the given data the following reaction format can be formed as:
While ozonolysis, an ozonide having a cyclic structure is formed as an intermediate which then undergoes cleavage to give the final products. Ethanal and pentan-3-one are obtained from the same intermediate ozonide. Thus, the expected structure of the ozonide is:
This ozonide is formed as an addition of ozone to reactant ‘A’. Thus, the desired structure of ‘A’ can be obtained by the removal of ozone from the above ozonide.
The structural formula of ‘A’ is as follows;
The IUPAC name of the above compound is 3-Ethylpent-2-ene.
6. An alkene ‘A’ contains three C – C, eight C – H $\sigma $ bonds and one C – C $\pi $ bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
According to the given information, ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. The formation of two moles of an aldehyde indicates the presence of identical structural units on both sides of the double bond containing carbon atoms. Hence, the structure of ‘A’ can be represented as:
There are 8 C–H $\sigma $ bonds which state that there are 8 hydrogen atoms in ‘A’. Also, there are 3 C–C bonds which state that there are 4 carbon atoms present in the same.
Combining the information given, the structure of ‘A’ can be represented as:
‘A’ has 3 C-C bonds, 8 C-H $\sigma $bonds along with a C-C$\pi $ bond which states that the IUPAC name of the same is But-2-ene.
Ozonolysis of ‘A’ takes place as;
Here, we can say that the final product is being proved as ethanal with molar mass 44u.
7. Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
According to the given information, propanal and pentan-3-one are the ozonolysis products of an alkene. Consider the given alkene as ‘A’.
Now, writing the reverse of the ozonolysis reaction, we get;
The products are obtained on the cleavage of ozonide ‘X’. Thus, ‘X’ contains both products in cyclic form. The possible structure of ozonide can be represented as follows;
We know that, ‘X’ is an addition product of alkene ‘A’ with ozone. Thus, the possible structure of alkene ‘A’ is:
8. Write chemical equations for combustion reaction of the following hydrocarbons:
The combustion reaction is given as;
\[2{{C}_{4}}{{H}_{10\left( g \right)}}+13{{O}_{2\left( g \right)}}\to 8C{{O}_{2\left( g \right)}}+10{{H}_{2}}{{O}_{\left( g \right)}}+Heat\]
ii) Pentene
\[2{{C}_{5}}{{H}_{10\left( g \right)}}+15{{O}_{2\left( g \right)}}\to 10C{{O}_{2\left( g \right)}}+10{{H}_{2}}{{O}_{\left( g \right)}}+Heat\]
iii) Hexyne
\[2{{C}_{6}}{{H}_{10\left( g \right)}}+17{{O}_{2\left( g \right)}}\to 12C{{O}_{2\left( g \right)}}+10{{H}_{2}}{{O}_{\left( g \right)}}+Heat\]
iv) Toluene
9. Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Hex-2-ene is represented as $C{{H}_{3}}-CH=CH-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$. Geometrical isomers of hex-2-ene are:
The dipole moment of cis-compound is a sum of the dipole moments of$C-C{{H}_{3}}$ and$C-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$ bonds acting in the same direction. The dipole moment of a trans-compound is the resultant of the dipole moments of the same bonds acting in opposite directions.
Thus, cis-isomer is more polar than trans-isomer. The higher the polarity, the greater is the intermolecular dipole-dipole interaction and the higher will be the boiling point.
Therefore, cis-isomer will have a higher boiling point than trans-isomer.
10. Why is benzene extraordinarily stable though it contains three double bonds?
Benzene has resonating structures which define its stability perfectly. They can be represented as;
All 6 carbon atoms in benzene are $s{{p}^{2}}$ hybridized. The 2 $s{{p}^{2}}$ hybrid orbitals of each carbon atom overlaps with the $s{{p}^{2}}$ hybrid orbitals of adjacent carbon atoms to form 6 $\sigma $ bonds in the hexagonal plane. The remaining $s{{p}^{2}}$ hybrid orbital on each carbon atom overlaps with the s -orbital of hydrogen to form 6 sigma C–H bonds. The remaining unhybridized p -orbital of carbon atoms has the possibility of forming 3 $\pi $ bonds by the lateral overlap of adjacent C atoms.
The 6 $\pi $ electrons are delocalized and can move freely about the 6 carbon nuclei. Even after the presence of 3 double bonds, these delocalized $\pi $-electrons stabilize benzene.
11. What are the necessary conditions for any system to be aromatic?
A compound is only said to be aromatic if it completely satisfies the following conditions;
It should have a planar structure and should be cyclic.
The $\pi $-electrons of the compound must be completely delocalized in the ring.
The total number of $\pi $-electrons present in the ring should be equal to (4n+ 2), where n = 0, 1, 2 … etc. {Huckel’s rule}.
12. Explain why the following systems are not aromatic?
In the given compound, one carbon atom is $s{{p}^{3}}$ hybridized which signifies that it is tetrahedral (not planar). As for the compound to be aromatic, it should be planar. Thus, the given compound is not aromatic in nature.
In the given compound, one carbon atom is $s{{p}^{3}}$ hybridized which signifies that it is tetrahedral (not planar). As for the compound to be aromatic, it should be planar.
Also, for the given compound, the number of $\pi -$ electrons is 4 so, by Huckel’s rule;
\[n=\frac{1}{2}\]
For a compound to be aromatic, the value of n must be an integer i.e. 0, 1, 2… etc. which is not satisfied for the given compound. Therefore, it is not aromatic in nature.
For the given compound, the number of $\pi -$electrons is 8 so, by Huckel’s rule;
\[4n+2=8\]
\[n=\frac{2}{3}\]
13. How will you convert benzene into
p-nitrobromobenzene
The reactions are given as;
m-nitrochlorobenzene
p -nitrotoluene
acetophenone
14. In the alkane $C{{H}_{3}}-C{{H}_{2}}-C{{(C{{H}_{3}})}_{2}}-C{{H}_{2}}-CH{{(C{{H}_{3}})}_{2}}$, identify $1{}^\circ ,2{}^\circ ,3{}^\circ $ carbon atoms and give the number of H atoms bonded to each one of these.
The given alkane can be represented as;
Primary carbon atoms are those which are bonded to only one carbon atom or none. i.e., they have only 1 carbon atom as their neighbor or none (in case of methane). The given structure has 5 primary carbon atoms and 15 hydrogen atoms attached to it.
Secondary carbon atoms are those which are bonded to 2 carbon atoms i.e., they have 2 carbon atoms as their neighbors. The given structure has 2 secondary carbon atoms and 4 hydrogen atoms attached to it.
Tertiary carbon atoms are those which are bonded to 3 carbon atoms i.e., they have 3 carbon atoms as their neighbors. The given structure has 1 tertiary carbon atom and only 1 hydrogen atom is attached to it.
15. What effect does branching of an alkane chain have on its boiling point?
Alkanes mainly experience intermolecular Van der Waals forces. The stronger the force, the greater the boiling point of the alkane.
As branching increases further, the surface area of the molecule decreases which eventually results in a small area of contact. Thus, the Van der Waals force also decreases which can be overcome at a relatively lower temperature. Therefore, the boiling point of an alkane chain decreases with an increase in branching.
16. Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give a mechanism.
Addition of HBr to propene is an example of an electrophilic addition reaction. Hydrogen bromide provides an electrophile, ${{H}^{+}}$ . This electrophile attacks the double bond to form primary and secondary carbocations as shown:
Secondary carbocations are comparatively more stable than primary carbocations. Thus, the former predominates since it will form at a faster rate. Thus, now $B{{r}^{-}}$ attacks the carbocation to form 2 – bromopropane as the major product.
This reaction follows Markovnikov’s rule.
In the presence of benzoyl peroxide, an additional reaction takes place by anti-Markovnikov’s rule. The reaction follows a free radical chain mechanism as;
Here, 1 – bromopropane is obtained as the major product.
In the presence of peroxide, Br free radical acts as an electrophile. Therefore, two different products are obtained in addition of HBr to propene according to the absence and presence of peroxide.
17. Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
o-xylene has two resonating structures showing different reactions as follows;
The three products are formed i.e., methyl glyoxal, 1,2-demethylglyoxal, and glyoxal from two Kekule structures. Since all three products cannot be obtained from any one of the two structures, this proves that o-xylene is a resonance hybrid of two Kekule structures.
18. Arrange benzene, n-hexane and ethyne in decreasing order of acidic behavior. Also give reason for this behavior.
Acidic character of any species is defined on the basis of its ease with which it can lose the H– atoms. The hybridization state of carbon in the given compound is given as;
According to the hybridization criterion, as the s–character increases the electronegativity of carbon increases and the electrons of C–H bond pair lie closer to the C atom.
The s–character increases in the order:
\[s{{p}^{3}}<s{{p}^{2}}<sp\]
Thus, the decreasing order of acidic behavior is Ethyne > Benzene > Hexane.
19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Benzene is a planar molecule having delocalized electrons above and below the plane of the ring. Thus, this makes it electron-rich. As a result, it is highly attractive to electron deficient species i.e., electrophiles. This is the reason; benzene undergoes electrophilic substitution reactions very easily.
Whereas, nucleophiles are electron-rich. Hence, they are repelled by benzene. Therefore, benzene undergoes nucleophilic substitutions with much difficulty.
20. How would you convert the following compounds into benzene?
iii) Hexane
21. Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
The structure of 2-methylbutane can be stated as (skeleton);
On the basis of the above structure, various alkenes that will give 2-methylbutane on hydrogenation are given as;
22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, ${{E}^{+}}$
a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
Electrophiles are reagents that participate in a reaction by accepting an electron pair in order to bond to the corresponding nucleophiles.
The higher the electron density on a benzene ring, the more reactive is the compound towards an electrophile.
The presence of an EWG deactivates the aromatic ring by decreasing the electron density. Now, as the $N{{O}_{2}}^{-}$ group is more EWG that $C{{l}^{-}}$ group.
Thus, the decreasing order of EWG is given as;
Chlorobenzene > p-nitrochlorobenzene > 2,4-dinitrochlorobenzene
b) Toluene, $p-C{{H}_{3}}-{{C}_{6}}{{H}_{4}}-N{{O}_{2}}$ , $p-{{O}_{2}}N-{{C}_{6}}{{H}_{4}}-N{{O}_{2}}$ .
Here, $C{{H}_{3}}^{-}$ is an EDG and $N{{O}_{2}}^{-}$is an EWG.
Thus, toluene will have the maximum electron density and is most easily attacked by ${{E}^{+}}$ .
The number of $N{{O}_{2}}^{-}$substituents define the order as;
Toluene > $p-C{{H}_{3}}-{{C}_{6}}{{H}_{4}}-N{{O}_{2}}$ > $p-{{O}_{2}}N-{{C}_{6}}{{H}_{4}}-N{{O}_{2}}$
23. Out of benzene, m–dinitrobenzene and toluene which will undergo nitration most easily and why?
The ease of nitration depends on the presence of electron density on the compound to form nitrates. Nitration reactions are examples of electrophilic substitution reactions where an electron-rich species is attacked by a nitronium ion ($N{{O}_{2}}^{+}$ ).
Now as we know, $C{{H}_{3}}^{-}$group is electron donating and $N{{O}_{2}}^{-}$ is electron withdrawing. Therefore, toluene will have the maximum electron density among the 3 compounds followed by benzene. On the other hand, m– Dinitrobenzene will have the least electron density. Thus, it will undergo nitration with difficulty. Hence, the increasing order of nitration is as;
24. Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
The ethylation reaction of benzene involves the addition of an ethyl group on the benzene ring. This reaction is called Friedel-Craft alkylation reaction and takes place in the presence of a Lewis acid.
Any Lewis acid like anhydrous $FeC{{l}_{3}},SnC{{l}_{4}},B{{F}_{3}}$ etc. can be used during the ethylation of benzene.
25. Why is the Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms? Illustrate your answer by taking one example.
Wurtz reaction is limited for the synthesis of symmetrical alkanes (alkanes with an even number of carbon atoms). In the reaction, two similar alkyl halides are taken as reactants and an alkane, containing double the number of carbon atoms, are formed.
This reaction cannot be used for the preparation of unsymmetrical alkanes because if two dissimilar alkyl halides are taken as the reactants, then a mixture of alkanes is obtained as the products.
(Image to be added soon) (Image to be added soon)
The boiling points of the above alkanes are very close. Hence, it becomes difficult to separate them.
NCERT Solutions for Class 11 Chemistry – Free PDF Download
Vedantu provides Class 11 chemistry Ch 13 NCERT solutions and easy notes in the PDF form. You can register on the site and get more knowledge about hydrocarbons. NCERT Class 11 Chemistry Chapter 13 available on the main site for free download will allow students to gain more knowledge about the Hydrocarbons and score more in your examinations.
Chapter 13 – The Hydrocarbons
NCERT Solutions for Class 11 Chemistry Chapter 13
Hydrocarbon
A compound of hydrogen and carbon is called a hydrocarbon.
Saturated Hydrocarbon
If the hydrocarbon contains C—C single bonds only.
Example:
Ethane CH 3 —CH 3
Unsaturated Hydrocarbon
The Benzene and its derivatives are known as aromatic compounds.
Alicyclic Compounds
Cyclic compounds consisting of carbon atoms only are known as carbocyclic or alicyclic compounds.
Heterocyclic Compounds
Cyclic compounds where the ring atoms are of some element like N, S or O and carbon. They are known as heterocyclic compounds.
The simplest organic compounds created from only carbon and hydrogen only are called Alkanes.
The general formula of Alkanes is C n HC 2n+2 (where n donates = 1, 2, 3, etc.)
Single covalent bonds bond the C atoms in their molecules. As the carbon skeleton of alkanes is completely saturated with hydrogens, they are also known as saturated hydrocarbons. Alkanes have strong C —H and C —C bonds. Hence, this class of hydrocarbons are comparatively chemically inert. So, they are often called paraffin.
The carbon of Methane forms single bonds with four hydrogen atoms, so it is called Methane. It has a tetrahedral structure.
Nomenclature Guidelines
Use the following step-by-step process to write the IUPAC names from the structural formulas of the hydrocarbons. Consider the following structural formula:
Step 1. The first step is to identify the longest chain in the hydrocarbon: longest chain here has seven carbons. The seven carbon chain is heptane.
Step 2. Number the chain: The chain has to be numbered from left to right, which will give the lowest numbers to the alkyl group.
Step 3. Identify the alkyl group in the hydrocarbon. So, there are two methyl groups at C-2 and C-3, there is one ethyl group of C-4.
Step 4. So, the IUPAC name, in this case, is 4-Ethyl-2,3-dimethyl heptane. Points to note:
Always use commas to separate numbers.
Hyphens are used to separate names from numbers.
Prefixes di, tri is not taken into consideration in naming substituent names.
New Man Projections
The molecule is looked at the C—C bond head-on in this projection
Relative Stability of Conformations
When Ethane is in the staggered form, then there are maximum repulsive forces and stability of the molecule and minimum energy. On the other hand, the eclipsed way the electron clouds of the C-H bond come closer to each other, which will result in increasing the electron cloud repulsion. Hence, the molecule will have more energy and less stability.
Alkenes are hydrocarbons that have a double bond (C=C) in their C atoms
The general formula:
Let us consider (H 2 C=CH 2 ) for visualizing the orbital make up of alkenes.
In ethylene, the C atoms are sp 2 hybridized in the ethylene- The bond attaches them.
The bond derives from the overlap of two sp 2 hybrid orbitals.
The π bond results from the overlap of the unhybridized p-orbitals. Ethylene is a planar molecule.
Points To Be Noted
The carbon-carbon double bond in alkenes consists of one σ and one π-bond.
Alkanes are less reactive, and alkenes are more reactive due to the availability of n electrons.
In the IUPAC system
The longest ‘ C chain of which double bond is a part has to be identified.
This chain is seen from the end closer to the double bond, and its position is defined by the number of the C atom not which the double bond originates.
First, mention the name of the parent alkene along with the position number of the double bond and then the names of the other substitutes prefixed to it.
The last one of the corresponding alkane is replaced by-a diene to get the name if there is more than one double bond.
Structural Isomerism: Structural isomers are those isomers in which the atoms are organized in a different order but having the same molecular formula. Ethene and propene do not show any structural isomers; however, butene has three structural isomers.
Among them, two are straight-chain structures but having different positions of double bonds in the molecules.
Geometrical Isomerism: They are molecules that are locked into their spatial positions concerning one another because of the double bond or a ring structure. For example, consider the following two molecules.
This rotation about the carbon-carbon double bond results in geometrical isomerism. An alkene with a formula RCH=CHR can have two stereoisomers, based on if the two alkyl groups are on the opposite or same sides of the double bond.
But they are called cis-isomer if they are on the same side and they are called trans-isomer if they are on opposite sides.
Alkynes are defined by the occurrence of a triple bond in the molecule.
C n H 2n-2 is the general formula.
The primary member of this is acetylene, HC=CH, and so they are known as the Acetylenes.
Structure: Consider ethyne (HC=CH) for visualizing the orbital make up of ethyne. The c atoms are sp hybridized in the ethyne. They are bonded by a σ-bond and two π-bonds.
The σ -bond comes from the overlap of two sp hybrid orbitals.
The π bonds are made from the different overlap of the two p-orbitals from the two adjacent C atoms.
The other sp hybrid orbital makes a σ bond with another carbon or hydrogen atom.
Ethyne is a linear molecule.
The carbon-carbon triple bond consists of one σ and two π bonds in alkynes.
Like alkenes, more exposure of π electrons makes the alkylene undergo different reactions.
IUPAC System
It is obtained by omitting the suffix-ane of the parent alkane and an addition of a new suffix-yne.
Carbon chain having the triple bond is – numbered from the end closest to this bond. The position of the triple bond is defined by prefixing the number of carbon before the name of the alkyne.
From calcium carbide: Ethyne is prepared when calcium carbide is mixed with water.
The preparation of calcium carbide is as follows:
From Vicinal Dihalides: The alcoholic potassium hydroxide undergoes dehydrohalogenation when reacted with vicinal dihalides. One molecule of hydrogen halide is dropped to make alkyl halide which, when treated with sodamide results in the formation of alkyne.
Aromatic Hydrocarbons
These hydrocarbons are also called ‘arenes’. Mostly such compounds contain a benzene ring.
Benzenoids are the category of aromatic compounds having benzene rings. On the other hand, aromatic compounds not having the benzene rings are called non- benzenoids.
Structure of Benzene: C 6 H 6 is the molecular formula for Benzene. This shows that Benzene is too unsaturated.
The Kekule structure given by Kekule shows the chances of two isomeric 1,2-dibromobenzene. From one of them is When the bromine atoms are attached to the double bond C atoms. However, in the other one, they are attached to the single bond C.
Benzene Was An Amalgamation Of These Two Forms
However, this structure was not successful in explaining the unique stability and its inclination to substitution reaction rather than addition reactions.
Resonance Structure of Benzene: Resonance is a phenomenon where two or more structures can be used for a substance involving the same positions of atoms.
Benzene's Kekule’s structures (1) and (2) show the resonance structures. The original structure – of the molecule is represented by the mixture of these two structures.
Orbital structure of Benzene: All C atoms in Benzene are sp 2 hybridized. The hybrid sp2 orbitals overlap with each other and also with s orbitals of the six hydrogen atoms results in the formation C—C and C—H σ-bonds.
X-ray diffraction data show that Benzene is a planar molecule. The data indicate that all the six Carbon bonds length is of the same order (139 pm) which is intermediate between carbon-carbon single bond (154 pm) and a carbon-carbon double bond (133 pm). Hence, the presence of the double bond in Benzene proves the idea of the reluctance of Benzene to show additional reactions under average condition. Therefore, it justifies the peculiar behaviour of Benzene.
Aromaticity: The property of the sp2 hybridized planar rings where the p orbitals permit the cyclic delocalization of π electrons.
Conditions For Aromaticity:
An aromatic compound is planar and cyclic.
Every atom has a p orbital in an aromatic ring. These p orbitals should be parallel so that a consistent overlapping is made around the ring.
The cyclic π molecular orbital (electron cloud) which is formed by the overlap of p orbitals should contain (4n + 2) π electrons.
Where n donates any integer (0, 1, 2, 3, etc.).
This rule is called the Huckel rule.
Preparation of Benzene: There are some laboratory methods for preparing Benzene.
Physical Properties Of Benzene
It is a colourless liquid.
It is not soluble in water. However, it is soluble in alcohol, ether, chloroform etc.
It is a suitable solvent for many inorganic and organic substances, e.g., resins, fats, sulphur and iodine.
It produces a luminous flame while burning in contrast to alkanes and alkenes which usually have a bluish flame.
Chemical Properties
Benzene goes through the following chemical reactions.
Electrophilic Substitution Reaction
Addition Reaction
Electrophilic Substitution Reactions
When too much of chlorine is treated with Benzene in the presence of ALCL 3 , then Benzene can be chlorinated to hexachlorobenzene (C 6 Cl 6 )
Carcinogenicity and Toxicity: Some polynuclear hydrocarbons having more than two Benzene rings mix together and become more toxic, and they have cancer-producing properties. The incomplete combustion of some organic materials like tobacco, coal and petroleum, etc. leads to the formation of this.
Hydrocarbons: They are compounds of carbon and hydrogen only.
Alkane is an open chain saturated compound.
Unsaturated Compound - Alkynes Aromatic Compound—Benzene and its derivatives and Alkenes are unsaturated compounds, and they are weak acids.
Conformation: The Spatial arrangements made by rotation around sigma bonds.
Eclipsed Conformation: The more repulsion between bond pairs of electrons makes it less stable.
Staggered: The lesser repulsion between bond pairs of electrons makes it more stable.
Key Features Of NCERT Solutions For Class 11 Chemistry Chapter 13
Check NCERT Chemistry solutions for Class 11 Chapter 13 offered by Vedantu to know more about hydrocarbons The proper explanation of all types of hydrocarbons and their nomenclature in the notes will improve your knowledge about hydrocarbons. Some of the important features of downloading NCERT solutions Class 11 chemistry hydrocarbons notes are:
These notes are brought to you by the experienced faculty.
The notes will build a proper understanding of the concepts.
This will help the students to perform better.
NCERT Solutions for Class 11 Chemistry Other Chapter Wise Solutions PDFs
Chapter 1 - Some Basic Concepts of Chemistry
Chapter 2 - Structure of Atom
Chapter 3 - Classification of Elements and Periodicity in Properties
Chapter 4 - Chemical Bonding and Molecular Structure
Chapter 5 - States of Matter
Chapter 6 - Thermodynamics
Chapter 7 - Equilibrium
Chapter 8 - Redox Reactions
Chapter 9 - Hydrogen
Chapter 10 - The s-Block Elements
Chapter 11 - The p-Block Elements
Chapter 12 - Organic Chemistry - Some Basic Principles and Techniques
Chapter 14 - Environmental Chemistry

FAQs on NCERT Solutions for Class 11 Chemistry Chapter 13 - Hydrocarbons
Q1. Statement – 90-95% of Methane is produced from organic substances. True or False.
Ans: It's true, 90 - 95 % of the methane on Earth is produced from various organic sources, like petroleum. Coal, gas and oil are some of the end products of organic material conversion in anaerobic conditions.
Q2. What is the significance of Hydrocarbons?
Ans: Hydrocarbons are the major constituents of petroleum and natural gas. They are used as fuels and lubricants as well as raw materials for the production of plastics, fibres, rubbers, solvents, explosives, and industrial chemicals.
Q2. Why should we follow NCERT Solutions for Class 11 Chemistry Chapter 13?
Ans: Chemistry in class 11 is divided into Physical, Inorganic, and Organic Chemistry. The chapter Hydrocarbons forms a major part of Class 11 Organic Chemistry syllabus. The chapter is of utmost importance when it comes to any examination. If your basics are not clear, you will not be able to understand organic chemistry in higher classes either. This is why students should diligently follow Vedantu's NCERT Class 11 Chemistry Chapter 13 Solutions.
Q4. What are the topics covered under NCERT Solutions for Class 11 Chemistry Chapter 13?
Ans: A variety of topics are covered under NCERT Class 11 Chemistry Chapter 13. These topics include the classification of compounds, IUPAC Nomenclature guidelines, the relative stability of conformations, isomerism, and aromaticity. Primarily, one should know that depending upon the types of carbon-carbon bonds present; they can be classified into three main categories – saturated, unsaturated, and aromatic hydrocarbons. Saturated hydrocarbons generally form the main source of energy.
Q5. Write IUPAC names of the products obtained by the ozonolysis of the following compounds (i)Pent-2-ene (ii)3,4-Dimethylhept-3-ene (iii)2-Ethylbut-1-ene (iv) 1-Phenylbut-1-ene
Ans: When Pent-2-ene undergoes ozonolysis, two end products are obtained. The IUPAC name of Product (I) is ethanal, and Product (II) is propanal. As for 3,4-Dimenthylhept-3-ene, the IUPAC name of Product (I) is butan-2-one, and Product (II) is Pentan-2-one. In the case of 2-ethylbut-1-ene, the IUPAC name of Product (I) is pentan-3-one, and Product (II) is methanal while for 1-Phenylbut-1-ene benzaldehyde and propanal are formed.
Q6. An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
Ans: According to the solutions present in NCERT Class 11 Chemistry Chapter 13, the IUPAC name of this alkene is supposed to be 3-ethylpent-2-ene. Therefore, the answer is that 3-ethylpent-2-ene on ozonolysis gives a mixture of ethanal and pentan-3-one. You can find more answers to similar questions on that webpage. Pages like this have especially been curated by Vedantu to aid students in their preparation for all kinds of examinations that are held both nationally and internationally.
Q7. Do I need to practice all the questions provided in Chemistry Class 11 Chapter 13 NCERT Solutions?
Ans: We have clearly established that the chapter Hydrocarbons has to be studied thoroughly in order for students to score well. Organic chemistry can be quite complicated for beginners to understand without adequate guidance. Note that no topic in the chapter can be skipped. You will enhance your knowledge by practising as many questions as possible on this topic. Check out all the solutions given in NCERT Class 11 Chemistry Chapter 13. These are free of cost and also accessible on the Vedantu Mobile app.
NCERT Solutions for Class 11 Chemistry

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF Download
The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons contains a vast collection of questions to practise. By practising these questions of Chapter 13 Hydrocarbons, students can strengthen their problem solving skills as well as reasoning skills; these skills can be used in further chapters and real-life problems. These questions of Chapter 13 Hydrocarbons can also help students to build a strong foundation for the chapter.
NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF
The Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons can be a bit confusing if students are not able to understand the concepts properly. For that purpose students need to start practising Chapter 13 Hydrocarbons questions from the NCERT textbook. After practising those questions, students can prefer referring to the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF which is available in the Selfstudys website.
Exercise Wise NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
In the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons, a plethora of exercises are given so that students can understand the concepts as well as can solve confusions then and there. By practising exercise wise questions, students can learn to approach different questions of Chapter 13 Hydrocarbons in different and creative ways.
Formula wise NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
The questions in the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons are arranged according to each and every formula. By practising the questions of Chapter 13 Hydrocarbons formula wise, students can become more confident while applying the formulas. By applying the right formulas in the right questions of Chapter 13 Hydrocarbons, students can score good marks in those questions.
Where can Students Find the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
Students can find the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons, steps to attempt are clearly explained below:
- Visit the Selfstudys website.
- Bring the arrow towards NCERT Books & Solutionss which can be seen in the navigation bar.
- A drop down menu will appear, select NCERT Solutionss from the list.
- A new page will appear, select Class 11 from the list of classes.
- Now select the subject Chemistry from the list.
- Again a new page will appear, select the chapter Chapter 13 Hydrocarbons.
Features of NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
The features of NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons is considered to be distinctive trait, some important features are discussed below:
- Based on NCERT Syllabus: The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision are based on the NCERT syllabus so that by referring to it students can have an updated knowledge.
- Different Levels of Questions: In the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons theory, different levels of questions are asked; that is easy to difficult.
- Hints and Solutionss are Given: In the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF, hints and Solutionss are given so that students can easily solve all their doubts and confusions.
- All Exercises are Covered: Inside the Class 11 Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons, all the exercises are covered in the NCERT Solutions; accordingly students can learn to approach in different ways.
- Diagrams are Given: Diagrams are generally considered to be visual representation of the Class 11 Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons questions and concepts; the same is followed in NCERT Solutions.
- Available in the PDF: The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons is available in the PDF so that students can access answers whenever they want to.
What Are the Advantages of Using NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons provides plenty of advantages, some of the advantages are discussed below:
- Improves Performance: By solving questions in the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision, students can improve their problem solving skills and can increase the chances of getting good marks in the test. The Solutions helps students understand the concepts of Chapter 13 Hydrocarbons as well as questions effectively and efficiently.
- Builds Confidence: By solving questions from NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision, students can build their confidence as it provides a variety of questions. Being confident while attempting Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons questions can help one to get right and accurate answers.
- Enhances the Learning Process: By using the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons theory, students can enhance their learning process and can reinforce the learning strategies.
- Saves Time and Effort: Students don’t need to search for answers as it is already available in the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF as it saves both time and effort of students.
- Easy to Understand: As the answers of Class 11 Chemistry Chapter 13 Hydrocarbons are very simple this makes students understand all levels of questions easily.
- Helps to Maintain Accuracy: Accuracy is considered to be the freedom from making mistakes; students can maintain the accuracy level by solving Class 11 Chemistry Chapter 13 Hydrocarbons questions from NCERT Solutions.
Is NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Right for Students?
The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons is right for students as it is arranged according to the latest syllabus,some of the key factors are discussed below:
- To Understand Concepts: The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision is considered to be the right one as it allows students to understand the concepts easily.
- Comprehensive Coverage: The topics are covered in a comprehensive way in the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons theory so it is right for students to refer.
- Reliable: Students can be dependent on the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF as the questions and answers are accurate and reliable. Accordingly, students don’t need to check for the right answers of Chapter 13 Hydrocarbons questions from other reference materials.
- Improves Problem Solving Skills: It is a skill in which students tend to look up to questions of Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons in a logical way and solve those questions in a logical way. So to improve problem solving skills while attempting questions of Chapter 13 Hydrocarbons, students can refer to the NCERT Solutions of Class 11 Chemistry.
- Follows the Latest Syllabus: The Chapter 13 Hydrocarbons questions in the NCERT Solutions Class 11 Chemistry follows the latest syllabus so it is right for students to choose it.
- Can Solve Questions with Ease: Students can choose the NCERT Solutions for Class 11 Chemistry so that they can solve the Chapter 13 Hydrocarbons questions with ease.
When Is the Best Time to Use NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons can be used at any time while preparing; but is it better to follow the given tips:
- After Completing the Chapter: Students can prefer utilising the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision after completing the chapter thoroughly.
- To Identify Gaps in Knowledge: It is advisable for students to utilise the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons theory if they want to identify the gaps in their knowledge. After identification of gaps, students can also eliminate those gaps by solving the Chapter 13 Hydrocarbons questions on a regular basis.
- To Verify the Answers: Once students have attempted the questions of Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons then they can prefer utilising the NCERT Solutions so that they can match their own answers.
- To Practise More Questions: If students want to practise more questions on Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons then they can prefer using the NCERT Solutions for Class 11 Chemistry. By practising more Chapter 13 Hydrocarbons questions, students can easily enhance their problem solving skills.
- To Revise: The NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF can be utilised as good study material as through it, students can easily revise the topics and concepts.
- To Build Strong Foundation: If students want to build a strong foundation for the Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons, then they can prefer to utilise the NCERT Solutions for Class 11 Chemistry. By building a strong foundation, students can score good marks in questions related to Chapter 13 Hydrocarbons.
What are the Challenges Faced While Referring to the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
While referring to the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons, students may face challenges, those challenges are discussed below:
- Lack of Understanding: Students may struggle to understand the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons revision if they don’t have a clear understanding of the concepts and topics. In this case, students need to have a clear understanding of the Chapter 13 Hydrocarbons concepts.
- Lengthy Solutionss: Some answers in the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons theory can be lengthy so in this case students may face difficulty in understanding the Solutions. To overcome this, students can break the answers of Chapter 13 Hydrocarbons questions into smaller parts.
- Technical Language: If the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons PDF contains any kind of technical language then some students may face difficulty in understanding those words.
- Lack of Visual Aids: If there are not enough visual diagrams of Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons questions, then they may face difficulty in understanding the NCERT Class 11 Chemistry Solutionss. To overcome this, students may refer to the Selfstudys website as there are enough visual diagrams related to Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons.
- Limited Scope: The NCERT Solutionss for Class 11 Chemistry provides a limited number of Chapter 13 Hydrocarbons questions to solve; for this students need to refer to other study materials which can be available on the website.
- Inadequate Explanations: Some answers of Chapter 13 Hydrocarbons Chapter 13 Hydrocarbons may not provide adequate explanations in the NCERT Solutionss; so in this case students may face difficulty understanding the questions.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

One Last Step...

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Tag: hydrocarbons class 11 chemistry case study questions
Join our Telegram Channel for Free PDF Download
Case Study Questions for Class 11 Chemistry Chapter 13 Hydrocarbons
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs

IMAGES
VIDEO
COMMENTS
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks. CBSE Case Study Questions Class 11 Chemistry Hydrocarbon Case study - 1
Case Study Questions for Class 11 Chemistry Chapter 13 Hydrocarbons Case Study Questions Question 1: The rotation of carbon-carbon single bond (s-bond), due to cylindrical symmetry of s-MOs (molecular orbitals) long internuclear axis, in alkanes results into different spatial arrangements of atoms in space, that are interconvertible. These arrangements are called conformations. However, weak ...
By QB365 on 09 Sep, 2022 . QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 11 Chemistry Subject - Hydrocarbons, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
Case Study Based Questions on Class 11 Chemistry Chapter 13 Hydrocarbons Class 11 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 11 Chemistry examinations.
Significance of Class 11 Chemistry case study questions. Case study questions in Class 11 Chemistry are important because: They help Class 11 Chemistry students understand basic chemistry facts and concepts while keeping the excitement alive. ... 11: 9: Hydrocarbons: 18: 10: TOTAL: 160: 70:
A hydrocarbon is an organic chemical compound composed exclusively of hydrogen and carbon atoms. Hydrocarbons occur naturally and form the basis of crude oil, natural gas, coal, and other important energy sources. In this unit of class 11 chemistry, we will learn in detail about nomenclature, isomerism, structure, preparation, physical & chemical properties of alkanes, alkenes, alkynes ...
Q2. 896 mL vapour of a hydrocarbon 'A' having carbon 87.80% and hydrogen 12.19% weighs 3.28g at STP. Hydrogenation of "A' gives 2-methyl pentane. Also, "A' on hydration in the presence of H 2 SO 4 and HgSO 4 provides a ketone with 'B' having molecular formula C 6 H 12 O. The ketone 'B' gives a positive iodoform test.
Hydrocarbons Class 11 Notes Chemistry Chapter 13. A compound of carbon and hydrogen is known as hydrocarbon. A hydrocarbon is said to be saturated if it contains only C—C single bonds. Benzene and its derivatives are called aromatic compounds. Cyclic compounds which consist only of carbon atoms are called alicyclic or carboeyclic compounds.
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 13 - Hydrocarbons solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 13 - Hydrocarbons exercise questions with solutions to help you to revise complete syllabus and score more marks in your examinations.
When we speak about the hydrocarbons in chemistry, they can be explained as organic compounds composed of hydrogen and carbon elements. However, when we study this topic in class 11, which is found in the CBSE books of chapter 13, we need to learn about various things such as the classification of hydrocarbons, alkenes, alkanes, alkynes, toxicity, and carcinogenicity, and understand the ...
Competitive Exams after 12th Science. Study Important Questions for Class 11 Chemistry Chapter 13 - Hydrocarbons. 1 Mark Questions. 1. Classify the hydrocarbons according to the carbon - carbon bond. Ans: According to the carbon-carbon bond that occurs between them, hydrocarbons are divided into three categories:
Case Study questions for the Class 11 Chemistry final exams are available here. For your Chemistry exam, you can read these chapter-by-chapter Case Study questions. Subject matter specialists and seasoned teachers created these quizzes. You can verify the right response to each question by referring to the answer key, which is also provided.
Here is a list of questionnaires and their answers from our Chapter 13 Class 11 Chemistry Important Questions. Question 1: Arrange the following hydrogen halides in order of their decreasing reactivity with propene. (i) HCl > HBr > HI. (ii) HBr > HI > HCl. (iii) HI > HBr > HCl.
NCERT Solutions for Class 11 Chapter 13 Chemistry Hydrocarbons is a premier study material that helps you score excellent marks in the Class 11 examination. These solutions comprise answers to the textbook questions along with extra questions and important questions from previous years' question papers. Studying the NCERT Solutions for Class ...
January 28, 2023 September 21, 2023 Physics Gurukul Leave a Comment on Case Study Questions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties. ... Case Study Questions for Class 11 Chemistry Chapter 13 Hydrocarbons. June 14, 2022 September 21, ...
Write the structure of the products side by side with their oxygen atoms pointing towards each other. Step 2. Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene 'A' is. Question 6. An alkene 'A' contains three C—C, eight C—H, a-bonds, and one C—C n-bond.
1 Marks Questions. 1.Classify the hydrocarbons according to the carbon - carbon bond. Ans . Hydrocarbons are categorized into three categories according to the carbon - carbon bond that exists between then-. (a) saturated hydrocarbon. (b) Unsaturated hydrocarbon. (c) Aromatic hydrocarbon.
Important points. The important points that students can learn from NCERT Class 11 Chemistry Chapter 13 are given below: A hydrocarbon is a carbon and hydrogen-based molecule. Aromatic chemicals include benzene and its derivatives. Alicyclic or carbocyclic chemicals are cyclic molecules that contain just carbon atoms.
NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons. Multiple Choice Questions. Single Correct Answer Type. Q1. Arrange the following ia decreasing order of their boiling points. (A) n-Butane. (B) 2-Methylbutane. (C) n-Pentane. (D) 2,2-Dimethylpropane.
CBSE Chapter 13 Hydrocarbons HOTs Questions for Class 11. CBSE Board introduced questions based on - Higher Order Thinking Skills (HOTs). The primary objective here was to improve the evaluating and analytical skills of a student. A lot of students tend to only focus on memorizing information and relying on cramming learning.
To be able to cover all the concepts and topics of the Hydrocarbons, students can download the Hydrocarbons Class 11 notes from the Selfstudys website. Steps to download are-. Open the Selfstudys website. Bring the arrow towards the NCERT Books & Solutions which can be seen in the navigation bar.
In the NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons, a plethora of exercises are given so that students can understand the concepts as well as can solve confusions then and there. By practising exercise wise questions, students can learn to approach different questions of Chapter 13 Hydrocarbons in different and creative ways.
October 16, 2022 September 21, 2023 Manju Kaushik Leave a Comment on Case Study Questions for Class 11 Chemistry Chapter 13 Hydrocarbons. Case Study Questions for Class 11 Chemistry Chapter 13 Hydrocarbons. Join our Telegram Channel for Free PDF Download. Join Now! NCERT Books & Solutions. NCERT Books 2022-23. NCERT Solutions ...