
Psoriasis News
Top headlines, latest headlines.
- Pain Sensitivity in Psoriasis Plaques: Diet
- Alarm Molecule Drives Inflammation
- Thirdhand Smoke: Triggering Skin Diseases
- Uncovering the Skin's Secrets
- Females Itch Less Than Males
- Suffering from Psoriasis? Blame Trio of Proteins
- Controlling Inflammation in Psoriasis
- Balanced Diet May Reduce Skin, Joint ...
- Detecting Skin Disorders Based On Tissue ...
Earlier Headlines
Wednesday, july 6, 2022.
- Psoriasis: Study Lays Foundation for New Treatment Strategy
Friday, October 22, 2021
- Mechanism Behind Ineffective Psoriasis Drugs Identified
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Simple Brain-Computer Link: Gaming With Thoughts
- Clinical Reasoning: Chatbot Vs Physicians
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
Trending Topics
Strange & offbeat.
Advertisement
Phototherapy for Psoriasis: New Research and Insights
- Psoriasis (J Wu, Section Editor)
- Published: 04 January 2021
- Volume 10 , pages 16–20, ( 2021 )
Cite this article
- Nicholas Brownstone ORCID: orcid.org/0000-0002-1187-1712 1 ,
- Megan Mosca 1 ,
- Julie Hong 1 ,
- Edward Hadeler 1 &
- Tina Bhutani 1
343 Accesses
2 Citations
Explore all metrics
Purpose of Review
The purpose of the review is to highlight some of the new findings on phototherapy treatment for psoriasis and present clinical pearls for the practitioner.
Recent Findings
Recent research has helped to further elucidate the molecular mechanisms of UV light in the treatment of psoriasis. New evidence regarding the combination of phototherapy with biologic agents and phototherapy’s effect on quality of life is also highlighted.
Phototherapy remains an effective and viable option for patients seeking non-invasive and safe treatment of psoriasis. Further research is still needed to understand the exact immunological effects and molecular pathways regarding the response of the skin to UV light. Further research is also needed with combination treatment phototherapy and the newest biologic agents.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
A review and update of phototherapy treatment options for psoriasis.
Seth T. Howell, Leah A. Cardwell & Steven R. Feldman
Emerging trends in combination strategies with phototherapy in advanced psoriasis management
Ratnam Sreya, Shweta Nene, … Saurabh Srivastava
Phototherapy: a Review and Update of Treatment Options in Dermatology
Amanda Krenitsky, Rima I. Ghamrawi & Steven R. Feldman
Papers of particular interest, published recently, have been highlighted as: • Of importance
Racz E, Prens EP. Phototherapy and photochemotherapy for psoriasis. Dermatol Clin. 2015;33(1):79–89. https://doi.org/10.1016/j.det.2014.09.007 .
Article CAS PubMed Google Scholar
Joy Wan, Katrina Abuabara, Andrea B. Troxel, Daniel B. Shin, Abby S. Van Voorhees, Bruce F. Bebo Jr., et al. Dermatologist preferences for first-line therapy of moderate to severe psoriasis in healthy adult patients. Journal of the American Academy of Dermatology, Volume 66, Issue 3, 376 - 386. https://doi.org/10.1016/j.jaad.2011.03.012
Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. 2018;33(1):173–80. https://doi.org/10.1007/s10103-017-2360-1 .
Väkevä L, Niemelä S, Lauha M, Pasternack R, Hannuksela-Svahn A, Hjerppe A, et al. Narrowband ultraviolet B phototherapy improves quality of life of psoriasis and atopic dermatitis patients up to 3 months: results from an observational multicenter study. Photodermatol Photoimmunol Photomed. 2019;35(5):332–8. https://doi.org/10.1111/phpp.12479 .
Article PubMed Google Scholar
Hönigsmann H. Phototherapy for psoriasis. Clin Exp Dermatol. 2001;26(4):343–50. https://doi.org/10.1046/j.1365-2230.2001.00828.x .
Stern RS. Psoralen and ultraviolet a light therapy for psoriasis. N Engl J Med. 2007;357(7):682–90. https://doi.org/10.1056/NEJMct072317 .
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. https://doi.org/10.1056/NEJMra0804595 .
Enk CD, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154(9):4851–6.
CAS PubMed Google Scholar
Johnson-Huang LM, Suárez-Fariñas M, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Lowes MA. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol. 2010;130(11):2654–63. https://doi.org/10.1038/jid.2010.166 .
Article CAS PubMed PubMed Central Google Scholar
DeSilva B, McKenzie RC, Hunter JAA, Norval M. Local effects of TL01 phototherapy in psoriasis. Photodermatol Photoimmunol Photomed. 2008;24(5):268–9. https://doi.org/10.1111/j.1600-0781.2008.00366.x .
Weatherhead SC, Farr PM, Jamieson D, Hallinan JS, Lloyd JJ, Wipat A, et al. Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation. J Invest Dermatol. 2011;131(9):1916–26. https://doi.org/10.1038/jid.2011.134 .
Danno K, Toda K, Horio T. Ultraviolet-B radiation suppresses mast cell degranulation induced by compound 48/80. J Invest Dermatol. 1986;87(6):775–8. https://doi.org/10.1111/1523-1747.ep12458843 .
• Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. British Journal of Dermatology . 2020. https://doi.org/10.1111/bjd.19380 In this review, the authors discuss recent insights into Tregs in the setting of psoriasis with an emphasis on the effect of current treatments on Tregs and how already available therapeutics that modulate Treg frequency or functionality could be exploited for treatment of psoriasis.
Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJPM. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in Lesional skin. J Invest Dermatol. 2011;131(9):1853–60. https://doi.org/10.1038/jid.2011.139 .
Zhang D, Chen Y, Chen L, Yang R, Wang L, Liu W, et al. Ultraviolet irradiation promotes FOXP3 transcription via p53 in psoriasis. Exp Dermatol. 2016;25(7):513–8. https://doi.org/10.1111/exd.12942 .
• Martini S, Pozzi G, Carubbi C, et al. PKCε promotes human Th17 differentiation: Implications in the pathophysiology of psoriasis. European Journal of Immunology . 2018;48(4):644–54. https://doi.org/10.1002/eji.201747102 In this study, the authors provide a novel insight into the molecular mechanisms of Th17 cell polarization that is known to play a crucial role in autoimmunity, pinpointing PKCε as a potential target in Th17-mediated diseases .
Lüftl M, Röcken M, Plewig G, Degitz K. PUVA inhibits DNA replication, but not gene transcription at nonlethal dosages. J Investig Dermatol. 1998;111(3):399–405. https://doi.org/10.1046/j.1523-1747.1998.00316.x .
El-Domyati M, Moftah NH, Nasif GA, Abdel-Wahab HM, Barakat MT, Abdel-Aziz RT. Evaluation of apoptosis regulatory proteins in response to PUVA therapy for psoriasis. Photodermatol Photoimmunol Photomed. 2013;29(1):18–26. https://doi.org/10.1111/phpp.12012 .
• Chowdhari S, Saini N. Gene expression profiling reveals the role of RIG1 like receptor signaling in p53 dependent apoptosis induced by PUVA in keratinocytes. Cellular Signalling . 2016;28(1):25–33. https://doi.org/10.1016/j.cellsig.2015.10.015 This study highlights a potential novel mechanism of action of PUVA photochemotherapy treatment providing a possible new strategy for targeting proapoptotic function of RIG-1, a regulator of innate immune response or p53 for psoriasis therapy.
Rajpara AN, O’Neill JL, Nolan BV, Yentzer BA, Feldman SR. Review of home phototherapy. Dermatol Online J. 2010;16(12):2.
PubMed Google Scholar
Larkö O, Swanbeck G. Home solarium treatment of psoriasis. Br J Dermatol. 1979;101(1):13–6. https://doi.org/10.1111/j.1365-2133.1979.tb15286.x .
Koek MBG, Buskens E, van Weelden H, Steegmans PHA, Bruijnzeel-Koomen CAFM, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542. https://doi.org/10.1136/bmj.b1542 .
Article PubMed PubMed Central Google Scholar
Cline A, Unrue EL, Collins A, Feldman SR. Adherence to a novel home phototherapy system with integrated features. Dermatology Online Journal . 2019;25(3) https://escholarship.org/uc/item/1rw9f75h .
Kircik L, Bagel J, Korman N, Menter A, Elmets CA, Koo J, et al. Utilization of narrow-band ultraviolet light B therapy and etanercept for the treatment of psoriasis (UNITE): efficacy, safety, and patient-reported outcomes. J Drugs Dermatol. 2008;7(3):245–53.
De Simone C, D’Agostino M, Capizzi R, Capponi A, Venier A, Caldarola G. Combined treatment with etanercept 50 mg once weekly and narrow-band ultraviolet B phototherapy in chronic plaque psoriasis. Eur J Dermatol. 2011;21(4):568–72. https://doi.org/10.1684/ejd.2011.1330 .
Armstrong AW, Bagel J, Voorhees ASV, Robertson AD, Yamauchi PS. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151(4):432–8. https://doi.org/10.1001/jamadermatol.2014.3456 .
Farahnik B, Patel V, Beroukhim K, Hao Zhu T, Abrouk M, Nakamura M, et al. Combining biologic and phototherapy treatments for psoriasis: safety, efficacy, and patient acceptability. Psoriasis (Auckl). 2016;6:105–11. https://doi.org/10.2147/PTT.S98952 .
Article CAS Google Scholar
Busard CI, Cohen AD, Wolf P, Gkalpakiotis S, Cazzaniga S, Stern RS, et al. Biologics combined with conventional systemic agents or phototherapy for the treatment of psoriasis: real-life data from PSONET registries. J Eur Acad Dermatol Venereol. 2018;32(2):245–53. https://doi.org/10.1111/jdv.14583 .
Cutaneous Carcinogenic Risk of Phototherapy: An Updated Comprehensive Review - Erica Wang, Jodie Sasaki, Mio Nakamura, John Koo, 2015. . https://doi.org/10.1177/247553031500100107?journalCode=jpsa
Osmancevic A, Gillstedt M, Wennberg A-M, Larkö O. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Derm Venereol. 2014;94(4):425–30. https://doi.org/10.2340/00015555-175313 .
Download references
Author information
Authors and affiliations.
Department of Dermatology, Psoriasis and Skin Treatment Center, University of California San Francisco, 515 Spruce Street, San Francisco, CA, 94118, USA
Nicholas Brownstone, Megan Mosca, Julie Hong, Edward Hadeler & Tina Bhutani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nicholas Brownstone .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Psoriasis
Rights and permissions
Reprints and permissions
About this article
Brownstone, N., Mosca, M., Hong, J. et al. Phototherapy for Psoriasis: New Research and Insights. Curr Derm Rep 10 , 16–20 (2021). https://doi.org/10.1007/s13671-020-00324-z
Download citation
Accepted : 21 December 2020
Published : 04 January 2021
Issue Date : March 2021
DOI : https://doi.org/10.1007/s13671-020-00324-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phototherapy
- Narrowband-UVB
- Non-invasive therapy
- Photomedicine
- Find a journal
- Publish with us
- Track your research

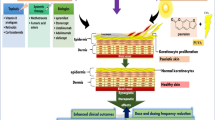
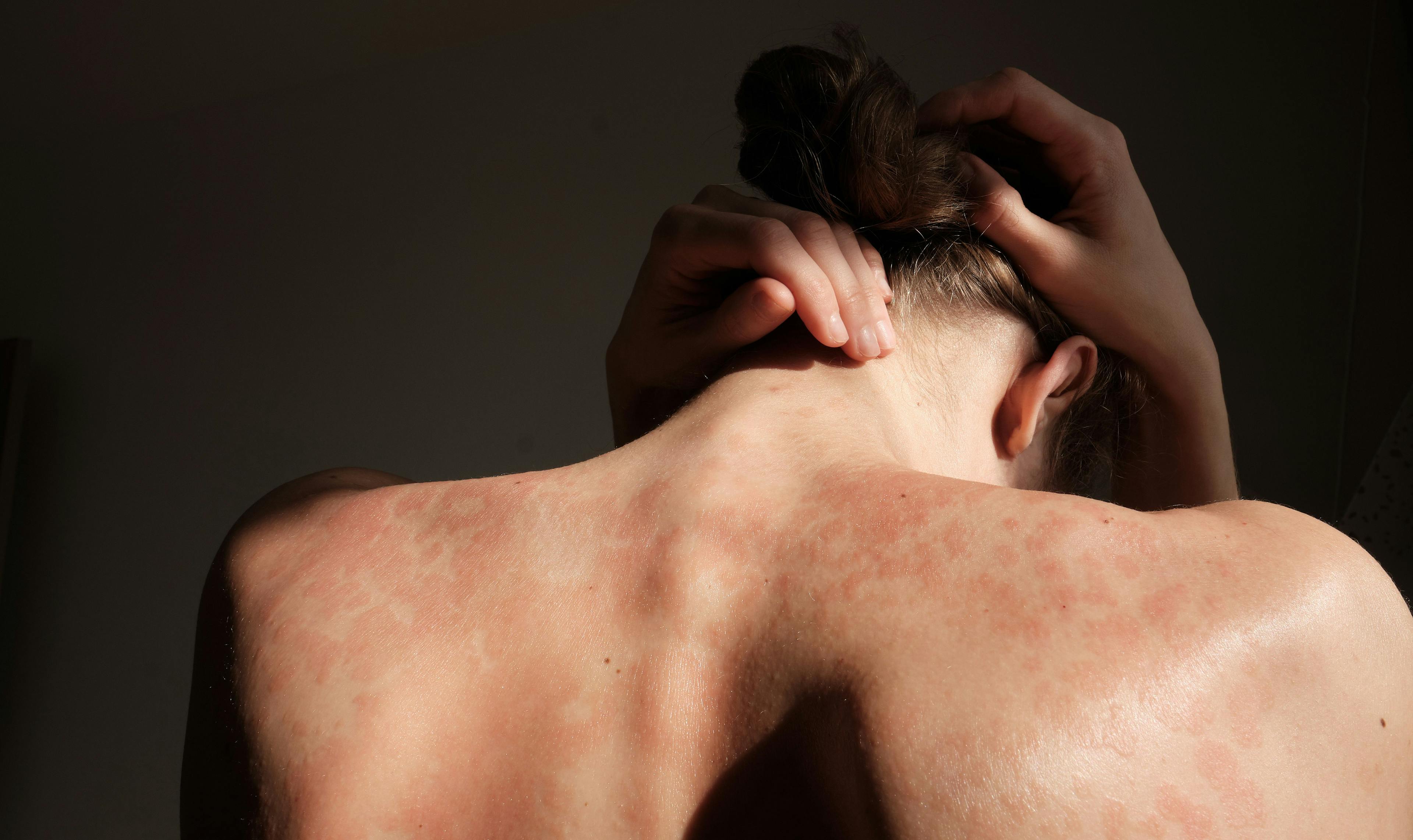
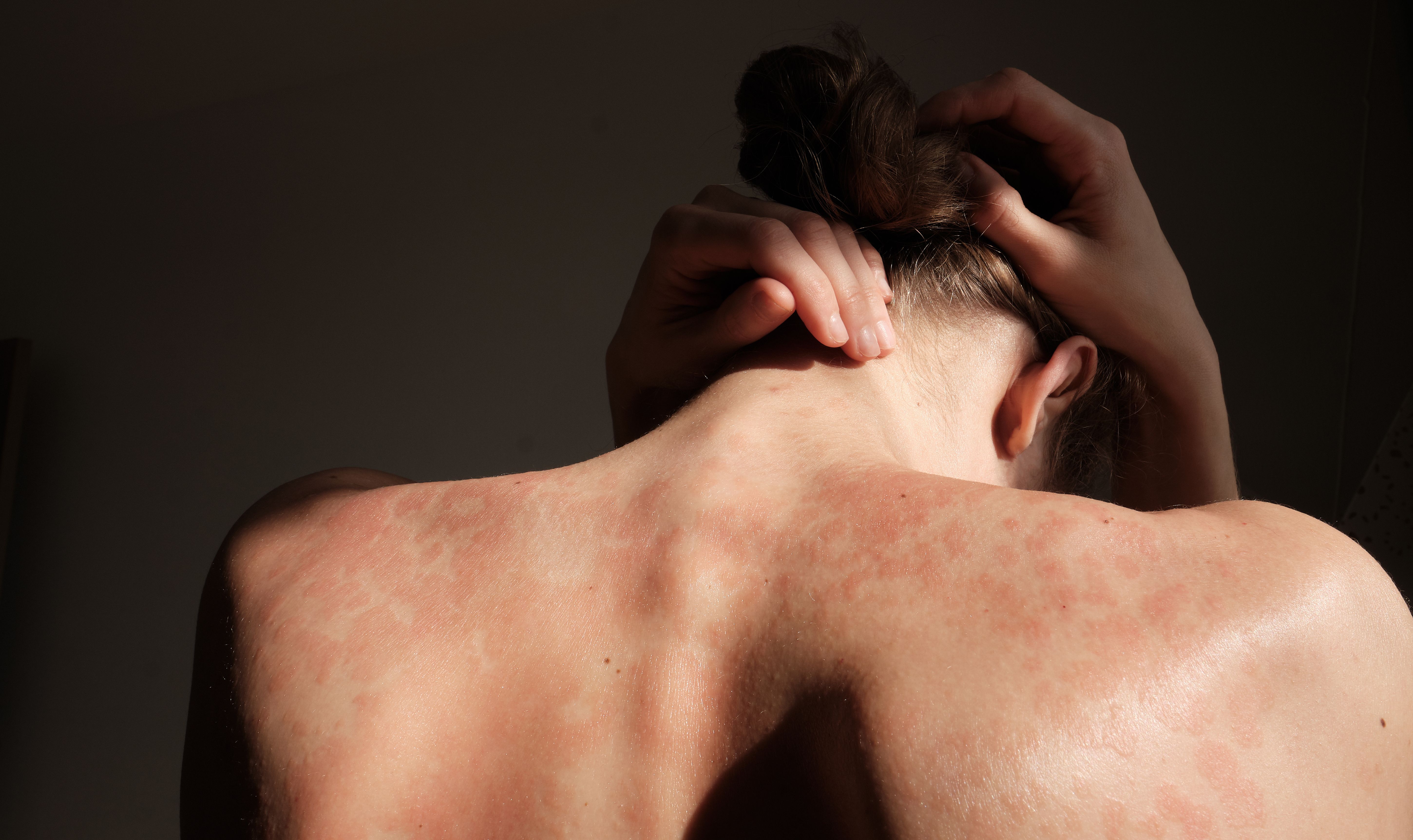
Psoriasis is a chronic skin condition characterized by itchy red patches and silvery scales, usually on the elbows, knees or scalp. It affects about 2 percent of Americans, and is sometimes associated with other health problems, such as arthritis, diabetes and heart disease. The causes are not fully understood, but the condition is related to an abnormal immune assault on skin cells that triggers inflammation.
Scientists have been trying to understand the molecular details of what causes psoriasis. Now, two studies funded in part by the NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and published in Nature and the Journal of Clinical Investigation have uncovered some contributing factors. The findings shed light on how psoriasis arises and how the body works to repair the damage, offering potential new strategies for treating the condition.
Nervous System Connection
Scientists are increasingly uncovering evidence of cross-talk between the nervous system and the immune system in many diseases, including psoriasis. Emotional stress can exacerbate psoriatic symptoms, while local anesthetics or nerve injury often reduce inflammation and induce remission.
Curious about the nervous system connection to psoriasis, scientists led by Ulrich von Andrian, M.D., Ph.D., of Harvard Medical School, focused their attention on a subset of pain-sensing neurons abundant in skin that confer the sensations of uncomfortable heat, cold and inflammatory pain. Working with a mouse model, they reasoned that if these neurons were involved in promoting psoriasis, removing them would ease symptoms of the condition.
They found that after removing these neurons, mice were less responsive to a compound called imiquimod, which normally triggers a psoriasis-like condition. The imiquimod-treated site was less swollen and inflamed than in control mice, and it contained less IL-23, a molecule known to mediate inflammation.
"From these results we knew that pain-sensing neurons, when exposed to imiquimod, trigger inflammation by stimulating the production of IL-23," said Jose Ordovas-Montanes, a NIAMS-supported graduate student at Harvard Medical School and co-first author of the study. "But we didn’t know where the IL-23 was coming from."
Further experiments identified the source of IL-23 to be immune cells called dermal dendritic cells (DDCs), and revealed that these cells are located in close proximity to pain-sensing neurons in mouse skin.
The researchers concluded that when mice are treated with imiquimod, pain-sensing nerve cells in their skin stimulate DDCs to make IL-23. IL-23, in turn, elicits an inflammatory cascade that ultimately produces the lesions that characterize psoriasis.
"By uncovering a role for the nervous system in psoriasis, we’ve discovered an entirely new pathway that we can explore for therapeutic purposes," said Dr. von Andrian. "By targeting these pain-sensing neurons, we may be able to develop new medicines for treating psoriasis and possibly other inflammatory skin diseases."
Repairing the Damage
While many scientists study the molecular events that trigger psoriasis, Bogi Andersen, M.D., and his team at the University of California, Irvine, uncovered a mechanism that the body uses to repair the skin damage caused by the condition.
Andersen’s team focused on a protein called GRHL3, a gene activator known to be involved in skin development in the embryo and in repair of the outer barrier layer of skin in adults. Recent findings have also found the GRHL3 gene to be unusually active in skin af+F93fected by psoriasis, and other studies suggested that alterations in the gene’s activity could increase susceptibility to the condition.
To better understand GRHL3’s role in psoriasis, the researchers deleted the gene in mice, then tested their response to imiquimod. If GRHL3 played a part in repairing damaged skin, the GRHL3-deficient mice would be expected to show greater sensitivity to the psoriasis-inducing compound compared to mice who possess the gene.
The results bore this out—the mice lacking GRHL3 responded to lower levels of imiquimod than control mice, and they healed more slowly. Twelve days after imiquimod treatment, the skin of GRHL3-deficient mice continued to show signs of inflammation, while the skin of control mice had healed completely.
Further experiments showed that GRHL3 suppresses the production of certain inflammatory molecules, indicating that it works in part by tamping down the inflammation that causes the psoriatic lesions. The results suggest that enhancing the GRHL3-dependent pathway could be an effective strategy for treating psoriasis.
"Our findings are consistent with a model in which psoriasis activates the GRHL3 pathway, which helps to heal the lesions," said Dr. Andersen. "In the future, we may be able to develop ways to stimulate this repair mechanism, leading to less severe disease and quicker resolution of symptoms."
The research reported in this article was supported in part by the NIAMS [grant numbers F31-AR063546 (Ordovas-Montanes/von Andrian study); R01-AR044882, F32-AR065356 and K08-AR060802 (Andersen study)]. The research was also supported by the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number T32-HD060555], National Library of Medicine [grant numbers R01-LM010235 and T15-LM007443] and National Institute of Allergy and Infectious Diseases [grant numbers R01-AI069259, P01-AI078897, U19-AI095261 and R01-AI111595]. The National Psoriasis Foundation, the A. Alfred Taubman Medical Research Institute, the Doris Duke Foundation, the American Skin Association, the University of California, Irvine, the National Science Foundation, the Human Frontiers Science Program and the Charles A. King Trust also contributed support.
Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nature. 2014 Jun 5;510(7503):157-61. doi: 10.1038/nature13199. Epub 2014 Apr 23. PMID: 24759321
A GRHL3-regulated repair pathway suppresses immune-mediated epidermal hyperplasia. Gordon WM, Zeller MD, Klein RH, Swindell WR, Ho H, Espetia F, Gudjonsson JE, Baldi PF, Andersen B. J Clin Invest. 2014 Oct 27. pii: 77138. [Epub ahead of print] doi: 10.1172/JCI77138. PMID: 25347468
The mission of the NIAMS , a part of the U.S. Department of Health and Human Services' National Institutes of Health, is to support research into the causes, treatment and prevention of arthritis and musculoskeletal and skin diseases; the training of basic and clinical scientists to carry out this research; and the dissemination of information on research progress in these diseases. For more information about the NIAMS, call the information clearinghouse at (301) 495-4484 or (877) 22-NIAMS (free call) or visit the NIAMS website at https://www.niams.nih.gov .
01604 251 620
07387 716 439
- Our Research Strategy
Current Research
- Research Collaborations
- Meet our Researchers
- Research Glossary
- Research Results
- Take Part in Research
- Research Network
Experts by Experience Committee
- Apply for a Research Grant
- Capturing Research Impact
- Supporting Researchers
The Psoriasis Association is currently funding a number of psoriasis research projects, from improving UVB treatment to investigating the impact of flare-ups.
To find out more about an individual project, please click on the title to read the project summary and most recent update provided by the researchers. Please see the glossary for an explanation of scientific terms used in the summaries. If you see a scientific term in the summaries below which you think needs adding to our glossary please contact [email protected]
Testing a diagnostic criteria questionnaire for psoriasis in children (DIPSOC-QC)” (Cecil King Memorial Fund Award)
Dr Esther Burden-Teh, University of Nottingham
“They couldn’t decide whether it was a fungal infection.”
“I had psoriasis for about six and a half months before someone correctly diagnosed me.”
These are the experiences of some children and young people who have psoriasis who took part in
the healthtalk.org project. They found the delay in getting a diagnosis to be frustrating and
Psoriasis is a skin condition that can cause red, flaky patches on any part of the body including the
face, scalp, hands and genitals. Psoriasis can affect adults and children, but psoriasis in children is
often confused with other common skin diseases.
Diagnostic criteria can help doctors make a diagnosis. Criteria are a list of skin changes to look for
and questions to ask when the patient is seen in clinic. We have developed a version that patients
can complete themselves but we don’t know how well it works so we need to test it.
Before starting a large study across several hospitals, it is important to test the design. Therefore, a
practice study, called a pilot study, will take place first.
In this practice study, one hundred children and young people will be invited to complete an online
diagnostic criteria questionnaire before they have their dermatology appointment. We will compare
their responses to the diagnosis given by the doctor. We will also collect information on what makes
them more, or less, likely to fill in the questionnaire.
Developing a social media intervention to increase awareness and understanding of psoriasis and reduce misconceptions: A mixed-methods project using co-production with adults with psoriasis (PhD Studentship)
Dr Ella Guest, University of the West of England
Individuals with psoriasis can experience negative attitudes and discrimination from the general population, which can affect their psychological wellbeing (e.g., low mood, anxiety, depression, appearance concerns) and life engagement (e.g., avoidance of activities that draw attention to their skin, romantic relationships) and make it more difficult to manage their condition. Given that most of these challenges stem directly from the attitudes and behaviours of society, which are influenced by the (mis)representation of psoriasis in the media, it is important that charitable organisations have tools to raise awareness of psoriasis, increase knowledge of the condition, and reduce misconceptions towards it. Social media provides a cost-effective and wide-reaching platform for population-level campaigns and is regularly used by the Psoriasis Association; however, the effectiveness of current campaigns has not been assessed. Limited current research suggests including personal stories, educational information, and presenting psoriasis in a positive light may effectively reduce negative attitudes; however, this research is in its infancy. Therefore, further evidence-based research is needed to target negative attitudes towards psoriasis and develop an effective social media campaign intervention. Therefore, in collaboration with adults with psoriasis, this PhD would use a mixed-methods approach to understand experiences of misconceptions and negative attitudes using qualitative interviews and co-produce and evaluate a social media intervention that the Psoriasis Association can use to improve the lives of people with psoriasis by targeting the general public.
Studying biological variation in the environmental sensor and novel psoriasis drug target Aryl Hydrocarbon Receptor (AHR): expression, regulation and biomarker potential (PhD Studentship)
Dr Paola Di Meglio, King’s College London
The focus of this proposal is a cellular protein called Aryl Hydrocarbon Receptor (AHR) which responds to dietary, light and microbial stimulation by switching off inflammation in the skin. Importantly, a compound called tapinarof, which works by instructing AHR to switch off inflammation, has been tested as topical medication in people with psoriasis, with good efficacy in around 50% of them. However, it is currently unclear how much AHR there is in the skin of people with psoriasis, what determines the amount, and whether that amount is enough for tapinarof to work as an anti-inflammatory drug. Moreover, some evidence suggests that the amount of AHR in the skin may differin people of different ethnicities. We aim to study the skin of people with and without psoriasis who define their ethnicity according to the UK Census 2021 categories as Asian or White to find out:1)What affects the amount of AHR present in skin cells and whether it varies according toethnicity2)How much AHR is present in their skin3)If the anti-inflammatory effect of tapinarof depends on the amount of AHR present in the skin and whether it varies according to ethnicity. Taken together, these experiments will enhance our understanding of how environmental factors influence psoriasis and treatment in people of different ethnic groups. Moreover, they can provide further opportunities to find novel effective medications or lifestyle intervention to improve the lives of people living with psoriasis.
Risk of serious infection associated with Interleukin 17 and 23 Inhibitors compared with other Biologics in people with psoriasis (PhD Studentship)
Dr Zenas Yiu, the University of Manchester
The newer injectable biologic therapies that target interleukin(IL)-17 and 23 are more effective for the treatment of psoriasis compared with the older biologics such as ustekinumab, a IL-12/23blocker, and TNF inhibitors. However, we do not yet know whether these newer treatments lead to more, less, or different types of serious infections in people with psoriasis in the routine clinical setting.
We will perform two studies to investigate this. Our first study will be a review of existing data on this topic, where we will look for all the relevant available evidence comparing the infection risk of IL-17 and IL-23 inhibitors with the older biologics. This will help us understand the strengths and flaws of current research and focus our further research on any gaps in knowledge that exist. If these studies are similar enough, we will combine this data together. We anticipate that the current evidence for the newer biologics will not be as robust.
Our second study will use a large national, well-established, long-term ongoing database of people with psoriasis on biologic therapies. We will test whether people on the newer IL-17 and IL-23inhibitorshave any difference in risk of serious infection, which are those that lead to hospital admission and require intravenous antibiotic treatment, or death, compared with the older biologics, and analyse whether there is any specific type of infection that is more common inpatients on individual biologic therapies.
These studies will provide both psoriasis patients and their doctors with an accurate estimate of the risk of infection while on the newer biologic therapies which will allow them to make well-informed decisions to choose a particular biologic treatment
Self-compassion and supporting adherence to topical therapies in people living with psoriasis (Cecil King Memorial Fund Award)
Dr Elaine Clarke, Sheffield Hallam University
Many people living with psoriasis can find it difficult to use topical treatments as prescribed. People often dislike their topical psoriasis treatments and receive little practical support to use them, so may not use them exactly as their doctor instructed. This can mean that the topical treatments do not relieve symptoms as well as they should, which can be distressing and make self-management of psoriasis difficult. It is therefore important that we work with people living with psoriasis to develop new ways of supporting people to use their topical treatments.
Self-compassion—being sensitive to your own distress and wanting to reduce it—may be helpful for this. We know that in people living with some long-term conditions, self-compassion is linked with people using their medication as prescribed. Furthermore, when people are helped to become more self-compassionate, they tend to act in healthier ways, including taking better care of themselves. However, we do not yet know whether self-compassion is linked with use of topical treatments in people with psoriasis. We also do not know whether people with psoriasis would consider help to become more self-compassionate as a relevant and acceptable way of supporting them to use their topical treatments. We propose to investigate the link between self-compassion and use of topical treatments in people living with psoriasis, and to use the findings from this work to co-develop new self-help materials to support people to use their topical treatments as prescribed.
We propose to carry out two pieces of work. In the first, we will conduct a survey of people living with psoriasis to investigate the link between self-compassion and use of topical psoriasis treatments. In the second piece of work, we will invite people living with psoriasis who are prescribed topical treatments to take part in co-production workshops. We will work together as a group, incorporating the survey findings, to co-design new self-help materials (e.g. information sheets or a self-help booklet) to support people to use their topical treatments as prescribed. These new co-produced materials will be given to the Psoriasis Association to share with the public.
Evaluation of Tumour Necrosis Factor Inhibitor Biosimilar Use in the UK: a study from the British Association of Dermatologists Biologics and Immunomodulator Register (PhD Studentship)
Biologic therapies for psoriasis, which are targeted injection treatments made of manufactured antibodies, are very effective and have transformed the level of success patients with psoriasis can achieve in clearing the skin. These medicines are made using living cell lines and are costly.
Biosimilar is a term used to describe a me-too medicine designed to work in the body in the same way as a biologic medicine already available for use by patients, also called originators. Biosimilars do not need to go through as much testing; makers of biosimilars only need to show there are no major differences between their product and the originator. The costs of biosimilars are usually much lower, offering the NHS cost savings whilst opening opportunities to treat more people in the future.
However, biologics have complex chemical designs, and it is difficult to make a biosimilar that is exactly the same as the originator. Although trials have not shown any difference between biosimilars and originators, we do not yet know whether this is the same in routine clinical practice.
We designed a study using data from the British Association of Dermatologists Biologics and Immunomodulator Register, a large multicentre registry of patients with severe psoriasis based in the UK and the Republic of Ireland, with the objectives to firstly describe the current use of biosimilars for the treatment of psoriasis in the UK; and secondly to generate evidence to show whether biosimilars have the same effectiveness and safety compared to originators under routine clinical practice settings.
Latest results summary
Tumour necrosis factor-alpha inhibitors (TNFi) are injectable treatments that are very effective for the treatment of moderate to severe psoriasis. These medicines were previously costly. Biosimilars, which are treatments that are manufactured so that they are like the original drug (originators),are much cheaper. However, the development process and regulatory pathway of biosimilars are not the same as orginators. Some people are therefore concerned that small differences between biosimilars and originators might have unwanted poorer treatment effects or safety problems when used with patients in clinic.
We performed a systematic review, which is a reproducible, rigorous, and transparent search of the evidence, and found reassuring data to suggest that the treatment effect and safety of biosimilars were no different to originators in people starting these drugs or in people switching to biosimilar drugs. However, we also found that this data was limited because most of the evidence was based on clinical trials, which are carried out over short periods of time in selected groups of patients. We did not find any high-quality studies investigating the use of biosimilars in routine settings.
We conducted a second study looking at the use of biosimilars using data from the British Association of Dermatologists Biologics and Immunomodulator Register (BADBIR), a large multicentre registry of patients with severe psoriasis based in the United Kingdom (UK) and the Republic of Ireland (RoI) and showed that the rate of switching to or starting TNFi biosimilars increased over time and varied across the UK and the RoI. Biosimilar use was found to be more common in male patients or patients with low disease severity.
For the next phase of the project, we will conduct a multi-centre international study in collaboration with research teams across the world to compare the effectiveness and safety of biosimilars to originators for the treatment of psoriasis in real-world settings.
Identifying biomarkers of disease remission in psoriasis (PhD Studentship)
Dr Satveer Mahil, King’s College London
Psoriasis research has delivered powerful drugs (called biologics) which act by influencing the immune system. ‘Remission’ (achieving clear skin with no/low risk of psoriasis recurrence upon stopping therapy) is now achieved more frequently than ever before. However, biologics are still continued lifelong at standard doses. This is costly and burdensome, with regular injections, blood tests, risk of infections and side effects.
We therefore need to identify patients in remission, in whom clear skin could be maintained with less frequent dosing (‘dose tapering’) or by stopping treatment.
1. Identify the immune cell types driving remission.
- We obtained skin biopsies before and after biologic treatment in patients in remission. We will examine individual immune cells present in these samples, using an innovative technique called single-cell sequencing.
2. Confirm that these immune cells are also observed in publicly available studies.
- We will validate the above findings by comparing our individual cell data with results of traditional studies, comprising information from a mixture of cells.
3. Demonstrate the presence of these immune cells in skin and blood collected from a new group of patients receiving biologics.
- We will use experimental techniques to detect the immune cells defined in the previous stages of the study.
The above steps will define the cells driving remission in people receiving biologics. This will identify patients suitable for dose tapering, which may reduce the burden of long-term biologics use and improve outcomes in psoriasis.
Biologic drugs have revolutionised outcomes in psoriasis, with a growing number of patients achieving clear skin (‘remission’). However, the cells and molecules in skin that drive remission to biologics remain poorly understood.
We used skin samples donated by people with psoriasis to investigate changes in the skin before and during early biologic treatment. We performed a technique called single cell sequencing. By studying individual cells, this powerful technology helped to identify a new cell type that may coordinate the early effects of biologic treatment. We followed up our findings using skin samples from independent donors with psoriasis. We used a method called RNA scope to find out where these cells are located in skin. We found that the new cell type was located near to the upper layer of the skin and decreased in abundance by day 14 of biologic treatment.
Our study has uncovered a new cell type that are ‘early responders’ to biologic drugs, potentially driving early skin clearance in response to treatment. This information may help us to select individuals most likely to respond well to treatments available already, and design better treatments for the future.
The APPLE Study – A Cross-sectional Observational Study Examining the Influence of Diet and Fasting on Psoriasis (PhD Studentship)
Dr Thiviyani Maruthappu, Queen Mary University of London
The APPLE Study (Asking People with Psoriasis about their Lifestyle and Eating) aims to help address one of the commonest questions that people living with psoriasis ask, whether changes in diet may or may not be helpful for their skin. Therefore, developing evidence-based dietary guidance is a key priority. This study aims to address this unmet need by firstly, examining the current diet patterns of people living with psoriasis around the UK, in addition to lifestyle factors such as sleep and exercise. This questionnaire survey will enquire whether people with psoriasis have observed whether particular foods trigger their psoriasis or whether any dietary changes may have helped. Secondly, a small trial will compare two popular evidence-based diets – the Mediterranean diet and “Intermittent Fasting” to observe whether either of these are helpful in improving psoriasis and potentially associated risk factors for heart disease such as high blood pressure and cholesterol levels. By examining the amount of inflammation in the blood as well as the extent of skin involvement at the beginning and end of the study, we will be able to see if either of these diet affects inflammation. The study brings together a unique group of UK experts for the first time, dermatologists, scientists and nutritionists to provide a robust approach in exploring the relationship between diet and psoriasis. The PhD candidate will be a Registered dietician who, at the end of the study, will have developed expertise in the specific needs and challenges faced by people living with psoriasis.
Predicting therapy response in Psoriasis (PhD Studentship)
Professor Miriam Wittmann, University of Leeds
We now have a range of medicines available to treat psoriasis. These therapies work in many but not all patients and some patients have to stop medication due to side effects. Unfortunately, we still do not have tests available to tell us which therapy works best for which patients. The answer to this question is of high importance. At present, many patients experience a phase of “trial and error” before a good therapy is identified. Failure to respond to treatment leads to frustration, depression and potential side effects from ineffective drugs.
Our project aims to predict therapy response to the commonly used drugs methotrexate and adalimumab. Our approach is different from already existing ones. Along with clinical data we also will collect information from affected skin but will not need biopsies. Instead we use a non-invasive tape stripping. We have used this method before and have optimised it so we can now detect thousands of proteins from each sample.
Due to the large amount of data collected it is impossible to analyse this information manually. We will therefore input all of the different and complex data into a “machine learning” computer program. Machine learning can be very powerful. Through many millions of calculations the computer is able to find “patterns” within very complex data. In our case we will look for the best “pattern” to predict response to methotrexate and adalimumab. We will share the program that we develop so that other researchers can use it to predict response to other drugs.
Choice of therapy for moderate-to-severe plaque psoriasis is currently dominated by a trial and error. This project aims to identify means of predicting, before treatment, whether a patient will respond to a given systemic treatment by clinical data and skin samples. Direct sampling of a lesion classically involves an invasive skin biopsy but this project will use adhesive tape strips as a painless means of sampling. This will allow for many more patients to be recruited, allowing for the reasonable application of Artificial Intelligence (AI) analysis methods.
Recruitment has been delayed due to the pandemic, but is now proceeding at a rate of approximately 12 patients per month with a total of 35 so far. In the meantime, data from another project – focused on psoriatic arthritis – has been used to develop relevant methods and skills. Data gathered in these two trials is similar in structure and will likely face the same challenges in terms of format and missing data. Currently, recruitment is ongoing and processing of samples already collected is in progress. In addition, proteins of interest for measurement in the samples are being identified.
Mast cell-CD8T cell interactions as drivers of psoriasis immune-pathogenesis (PhD Studentship)
Professor Silvia Bulfone-Paus, University of Manchester
Psoriasis is a common, chronic, and as yet incurable inflammatory skin disease. Our project seeks to investigate the contribution of specific skin immune cells, namely mast cells and CD8 T lymphocytes (CD8 T cells), to the development of psoriasis. The latter have long been known to play a critical role in psoriasis, while the former cells have mainly been studied in the context of psoriasis-associated itch. Instead, here we explore whether mast cell-CD8 T cells interactions actually can drive the disease and are thus an important, new therapeutic target.
In psoriasis plaques, both mast cells and CD8 T cells are higher in number than in normal, healthy skin and appear to interact closely with each other. Furthermore, by isolating mast cells from skin biopsies and comparing their gene expression in healthy skin and in psoriasis plaques we have identified a number of secreted products or mediators of mast cells whose expression is increased in psoriasis and can regulate CD8 T cell activities.
Therefore, the overall goal of our project is to characterize the biological significance of mast cells-CD8 T cell interactions and how these may contribute to the development of psoriasis and their response to treatment. We also study how their activities are affected by biologic therapy targeting a key immune system molecule – interleukin (IL) 17. This knowledge will suggest novel strategies for therapeutic intervention, e.g. by manipulating mast cell mediators in psoriatic plaques so as to block the activation of CD8 T cell and thus reduce skin inflammation.
The focus of this project is on mast cells and CD8 T cells, two immune cells that are enriched in psoriasis lesions. The hypothesis driving our study is that mast cells are critical modulators of pathogenic CD8 Tcell activities in psoriasis.This builds on work that has already been done in our lab that showed the increased gene expression of MC mediators that are capable of modulating CD8+ T cell activity in psoriasis skin. Our first aim was to identify the subpopulation of CD8+T cells that are dysregulated in psoriasis. To demonstrate that, we have designed a panelwith23 cell surface and intracellular markers and have optimized it. To establish a baseline, we analysed the blood samples from 8 healthy donors. In parallel, we have confirmed the spatial co-localization of mast cells and CD8 T cells in both healthy and psoriasis skin samples. In future, we will apply the established system to samples from psoriasis patients. We would like to investigate the alternation in mast cells and CD8 T cells as well as their connection with disease severity. We will also set up in vitro cell culture system to explore the mechanism and the factors that regulate the bidirectional interactions between these two cells.
Demonstrating the benefits of smoking cessation in psoriasis, a molecular approach (PhD Studentship)
Dr Francesca Capon, King’s College London
Several studies have demonstrated that smokers are at increased risk of psoriasis. At the same time, it is not clear whether giving up cigarettes can improve disease symptoms. Our study will address this important question by:
- Identifying the changes that occur in the skin of smokers affected by psoriasis
- Demonstrating that these alterations can be reversed by quitting smoking
Thus, our specific objectives will be:
- To identify changes in chromosome conformation in psoriatic skin. Smoke can affect the way that genes and proteins are packaged together into chromosomes. As this can influence the activity of genes that contribute to inflammation, we will compare chromosome conformation in individuals with psoriasis and healthy volunteers.
- To demonstrate that the chromosome changes observed in psoriatic skin are linked to smoke. We will grow skin cells in a dish, in the presence of chemical substances that are found in cigarette smoke. We will then determine whether these chemicals can induce the same changes we observed in the skin of affected individuals.
- To show that giving up cigarettes can reverse the chromosome changes induced by tobacco. We will obtain skin biopsies from psoriasis sufferers who have agreed to stop smoking. We will determine whether their chromosomes are reverting to a normal conformation and whether this correlates with an improvement in disease symptoms.
Taken together, these experiments are expected to provide a scientific rationale for introducing stop-smoking programmes in the treatment of psoriasis.
We initially focused our attention on palmoplantar pustulosis, as this is a severe disease variant that mostly affects smokers. While the Covid-19 epidemic has disrupted our research activity and limited access to our laboratories, we have been able to investigate whether genetic factors synergise with cigarette smoking in causing PPP. We initially examined a gene called CARD14 which is known to malfunction in many diseases that affect the skin. We have demonstrated that individuals who carry genetic defects in CARD14 are at increased risk of developing PPP, whether they smoke or not. We are now expanding our analysis to all the genes that are found in our DNA. Our preliminary results indicate the existence of additional genetic defects predisposing to the onset of PPP. Once we have characterised them, we will investigate whether they have a stronger effect in smokers.
Optimisation of NbUVB for psoriasis using a precision medicine approach (PHOTO-OPP study (PHOTOtherapy Optimisation Protocol in Psoriasis)) (Cecil King Memorial Fund Award)
Dr Alison Havelin, Royal Victoria Infirmary, Newcastle
Psoriasis is a chronic skin disease affecting 2% of the UK population. It is a visible, often stigmatising disease and can have a significant impact on patients’ quality of life. UVB (ultraviolet B light) is one of the few psoriatic treatments that can lead to complete psoriasis clearance and a period completely free of psoriasis (remission) after treatment has stopped. For patients, this means living a life without the burden of applying messy topical therapies or taking potentially harmful medications to control their disease. The achievement of improved clinical remission with UVB is therefore highly attractive.
UVB is more effective in some patients than others, with 2/3 of patients achieving good clearance. The response to UVB is variable and currently there is no accurate way of predicting which patients will do better than others.
A typical UVB treatment course requires patients to attend hospital 3 times weekly for 6-10 weeks. Our recent study identified a subgroup of patients who were less likely to achieve clearance of their psoriasis, by analysing their response after just 3 weeks of treatment.
We hypothesis that by identifying these “slow-responders” early and changing their phototherapy regimens from three times per week to five times per week, they will have a better chance of clearing. To the best of our knowledge, this has never been studied before.
The optimisation of UVB treatments based on individual responses allows us to develop personalised treatment plans. This would benefit patients by reducing the need for potentially toxic systemic treatments and would result in better utilisation of NHS resources.
This study was delayed due to Covid-19. All participants have now completed their UVB courses and follow ups and the study is at the write up stage.
Evaluating the effect of cannabinoid-induced inhibition of FABP5 for the treatment of psoriasis (Small Grant Award)
Dr David Hill, the University of Sunderland
Psoriasis is a chronic skin complaint characterised by raised red patches of skin called plaques. These plaques are caused by the over-production of skin cells, which in contrast to normal skin cells that die and become replaced by healthy cells from below, fail to die off correctly leading to thickened skin and impaired skin barrier function. As a result, psoriatic plaques can often become inflamed and painful. Unfortunately, despite the development of new immuno-therapies there remains no cure. Therefore, to develop more effective therapies for psoriasis we need a better understanding of the underlying causes of the disease.
Our preliminary data and results from previous studies suggest that psoriasis skin has increased expression of a protein called fatty acid binding protein 5 (FABP5), which is responsible for controlling the breakdown of a class of growth-regulators called endocannabinoids, and has been linked to increased growth of several cancers. We propose that endocannabinoids, which suppress the growth of normal skin cells, are degraded in the skin of psoriasis patients leading to increased cell production and defective skin barrier formation. Our research aims to investigate how frequently levels of FABP5 are increased in psoriasis by staining a small cohort of affected and unaffected skin biopsies with antibodies that detect FABP5. We will also reconstruct full-thickness skin in the lab from normal skin cells that we have genetically modified to increase levels of FABP5, which will tell us whether high FABP5 is sufficient to cause psoriasis. Finally, because psoriasis skin likely has reduced levels of endocannabinoids, we will investigate the effect of cannabinoid treatment (and specific FABP inhibition) on the growth and behaviour of FABP5-expressing skin cells.
Patients and health practitioners are increasingly looking at cannabis and cannabis-derived compounds as realistic and viable sources of medicine due to their excellent safety profile and changing legal status. However, the lack of preclinical evidence regarding disease-specific mechanisms and efficacy is a cause for concern. This study will allow us to better understand the role of cannabinoid signalling in psoriasis, which will form a rational basis for conducting future in-patient clinical trials.
This study was delayed due to Covid-19. The study is now at the write up stage.
Identifying immune determinants of clinical response to ustekinumab in psoriasis (PhD Studentship)
Biological drugs, such as ustekinumab (Stelara®), have a significant positive impact on the lives of people with psoriasis. Nevertheless, these expensive drugs do not work in every individual, and are still prescribed by trial-and-error. This process can be very frustrating for patients, and is not cost-effective for the NHS.
In order to prescribe the best possible drug to each individual with psoriasis, doctors need to be able to categorise people according to specific biological markers (“biomarkers”) that predict the likelihood that the drug will work. The Psoriasis Association-endorsed Psoriasis Stratification to Optimise Relevant Therapy (PSORT) is a multicentre study aimed at identifying biomarkers predictive of response to biologic drugs.
As part of PSORT, we are looking specifically at the white blood cells as potential predictive biomarkers. We aim to analyse cells already obtained from the blood of patients receiving ustekinumab, and measure a number of biological markers associated with them. Moreover, we will apply mathematical and statistical techniques to understand whether any of the biological markers measured can predict whether or not each individual patient will do well on ustekinumab.
Our findings will eventually be integrated with other datasets currently produced by PSORT (e.g. genetic data) to produce a clinically useful tool (“stratifier”) to guide psoriasis management, for the benefit of people with psoriasis, and to reduce costs for the NHS.
Biological drugs, such as ustekinumab (Stelara®), have a significant positive impact on the lives of people with moderate-to-severe psoriasis. Nevertheless, ustekinumab does not work in every individual. To prescribe the best possible drug to each individual with psoriasis, doctors need to be able to classify people according to specific biological markers (“biomarkers”) that predict the likelihood that a specific drug will work. In this PhD project, we are analysing white blood cells in the blood of people with psoriasis receiving ustekinumab and measuring a number of biological markers associated with them. In the first year of the project, we have developed a laboratory test to measure the effect of ustekinumab in specific white blood cells and applied this test to the first 10 samples from people with psoriasis. Moreover, we have applied mathematical and statistical techniques to understand whether any of the biological markers measured can predict whether each individual will do well on ustekinumab. Preliminary data suggest that some types of white blood cells may be more sensitive to the effect of ustekinumab and this may be associated with whether the drug works or not. Next steps will involve increasing the number of samples and analysing more biological markers with the aim to further verify and expand our findings. Taken together, our project has the potential for identifying biomarkers in the blood of people with psoriasis to predict response to ustekinumab, for the benefit of people with psoriasis, and to reduce costs for the NHS.
Impact of autophagy and nucleophagy deregulation in psoriasis (PhD Studentship)
Dr Daniele Bergamaschi, Queen Mary University of London
Autophagy is a detox process naturally supporting the epidermis in cleansing and replacing damaged cells in exchange of energy. This is a crucial mechanism as one of the main skin functions is to protect the organism from UV exposure and infections.
When keratinocytes are surrounded by inflammatory cells for a prolonged time, they lose their ability to detox and replace damaged cells. Inflamed skin cells release toxic substances (Reactive Oxygen Species) which reduce their healthy ability to purify themselves thus preventing them to transform their shape and size. We have recently shown that when skin cells detox, they also gradually lose their nucleus with a process called Nucleophagy. This mechanism is not correctly functioning in the few skin diseases including psoriasis.
In this project we will further determine the effects of inflammation on the autophagy machinery in healthy human and psoriatic epidermis and will establish the consequences of having a deregulated form of this metabolic process. This will be achieved by measuring where the protein involved in the autophagy process of the skin are expressed and whether they are correctly functioning. As a model we will use cell lines isolated from normal and psoriatic skin and with them we will also reconstruct in 3D psoriatic-like artificial skin to perform experiments of drug treatment.
This research project will significantly improve our understanding of how this detox metabolic mechanism can impact on development of psoriasis and may lead to the identification of novel therapeutic and preventative targets for this common skin condition.
In this part of the project, we have been trying to study and characterize the differences between human normal and psoriatic skin keratinocytes. These cells were isolated from either normal or psoriatic skin samples and immortalized in the lab, allowing us to measure important parameters in a simple live model. For example, we have been growing them in the lab and tested their ability to detox. Our data shows that psoriatic skin cells have a reduced capacity to eliminate their damaged or surplus cytoplasmic material. We are now investigating when exactly this defect is picked up by psoriatic cells and whether we can reproduce the same defect if we chronically expose normal skin cells with inflammatory stimulants. We are also imaging human skin samples from both healthy and psoriatic patients. We stain our samples with immunofluorescent protein tags which show us where in the skin certain detox pathway proteins are expressed. When we compared the amount and location of these detox proteins between healthy and psoriatic skin samples, we have noticed few differences about where these molecules shift or their expression changes within the separate layers of the skin upon chronic inflammation. Following this information, we have developed a 3D psoriatic-like organotypic skin model, to further investigate how inflammation can affect the skin detox ability.
Investigation of the prevalence of liver fibrosis in patients with psoriasis using Transient Elestography and evaluation of the relationship between liver fibrosis and methotrexate (Cecil King Memorial Fund Award)
Dr Parastoo Babakinejad, Royal Victoria Hospital, Newcastle
Patients with psoriasis appear to have higher rates of liver fibrosis in comparison to the general population. The prevalence of liver fibrosis in the psoriasis population in the UK has not been defined. The higher rates of risk factors for liver fibrosis such as obesity, alcohol and diabetes are important; however there have been concerns that methotrexate can contribute to liver fibrosis. Despite the increasing importance of biologic therapies, methotrexate remains the most commonly used systemic agent in the UK. The majority of patients needing systemic therapy will try methotrexate first as per NICE guidance.
This study aims to investigate the prevalence of liver fibrosis in a group of patients with psoriasis by measuring liver stiffness measurement (LSM) using Transient Elastography. The cumulative methotrexate dose in addition to other important factors including BMI, waist circumference and alcohol intake will be recorded. A univariate analysis will be performed to investigate the relationship between all measured factors and LSM. The relationship between the cumulative dose of Methotrexate and liver fibrosis will be addressed.
The ultimate goal is to use the prevalence data to perform a power calculation to determine the number of participants required to conduct a study to determine which factors can predict the risk of liver fibrosis and whether or not methotrexate is an independent risk factor for liver fibrosis in patients with psoriasis. Using this data a risk prediction model can be built to allow optimal and safe prescribing of methotrexate.
This study was delayed due to Covid-19 and has been extended.
Investigating the therapeutic benefits of exercise in patients with psoriasis. (PhD Studentship)
Dr Helen Young, University of Manchester
Psoriasis, a common skin disease which confers immense suffering on those it afflicts, is associated with an increased risk of developing cardiovascular disease (CVD). The severity of psoriasis and the number of individuals affected by the disease are increased by obesity. Patients with psoriasis are often embarrassed about exposing their skin in front of others, which leads to exercise avoidance. Lack of physical exercise and obesity are risk factors for the development of CVD.
Individuals with psoriasis have much to gain by regular exercise including an improvement in psoriasis itself, a reduced risk of CVD, weight management and enhanced wellbeing. Based on our research in this field, which was supported by a Psoriasis Association PhD studentship, we worked with individuals with psoriasis to develop an exercise programme that can be followed by sufferers – even on their worst day. This project will test our exercise programme in clinical practice and measure the improvement in psoriasis, CV health and overall well-being in patients. We will also use a laboratory-based technique called transcriptomic analysis to investigate how exercise exerts its beneficial effects in the body. We will learn more about psoriasis and how to treat it effectively.
This study was delayed due to Covid and has been extended.
We know that living with psoriasis can make it difficult to exercise and stay fit. In this PhD studentship we want to find out if exercise can help psoriasis or make psoriasis easier to live with or improve heart health in people who have psoriasis. To do this we have created a 10-week group walking exercise programme, during the first year of the project. Other people who have psoriasis have helped us do this. Despite some delays due to lockdown and HM Government restrictions volunteer participants have been recruited.
The risk of cancer in psoriasis patients treated with biologic therapies compared with conventional systemic therapies: results from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) (PhD Studentship)
Professor Richard Warren, University of Manchester
Patients with severe psoriasis (more than 10% of the body surface affected) are often offered two broad types of long-term systemic treatment: conventional systemic treatments, such as methotrexate, ciclosporin and acitretin; and biologics, such as Humira, Stelara, and Enbrel. Biologic therapies target the immune system and may affect the body’s ability to fight cancer. It is not known whether being treated with biologic therapies carries an increased risk of cancer in psoriasis patients compared to treatment with conventional systemic treatments. Very few studies have compared the safety of these two treatment options and have been limited by short duration with only small numbers of patients. As cancers develop relatively rarely, we need to compare large groups of psoriasis patients receiving biologic and conventional systemic treatments respectively.
Biologic therapies are injectable treatments for people with severe psoriasis who no longer respond to treatment with traditional oral systemic agents. There are some fears that people with psoriasis treated with biological therapies might have an increased risk of developing cancer compared with people treated with the older systemic therapies.
The first study of this project reviewed all the published evidence to date pertaining to the risk of developing the skin cancer, known as melanoma, after receiving treatment with biologic therapy for either psoriasis or conditions treated with the same biologics. Due to the small number of studies in psoriasis, we were unable rule out an increased risk of melanoma in patients treated with biologic therapy. These findings have only highlighted the urgent need to clarify if treatment with biologic therapy is a risk factor for cancer.
The next step of the project will aim to address this research question by exploring if the people with psoriasis, in the UK and the Republic of Ireland, treated with biologic therapy are at an increased risk of developing common cancers (breast, prostate, lung, colorectal and melanoma) compared with people treated with only non-biologic systemic therapies. The study will utilise data from The British Association of Dermatologists Biologics and Immunomodulators Register; known as BADBIR.
A pilot study to compare the response of psoriasis to narrow-band UVB phototherapy in the morning and afternoon (Small Grant Award)
Dr Henry Grantham, Royal Victoria Infirmary, Newcastle Upon Tyne
Psoriasis is a very common skin disease. We often think of psoriasis as a disease of just the skin, but research shows that people who have psoriasis may have different body clock rhythms from the general population. We know that people without psoriasis are more sensitive to phototherapy with ultraviolet B light at different times in the day. What we don’t know is if this is also true for people who have psoriasis.
The main aim of this research question is to see if patients' psoriasis is more sensitive to ultraviolet light at different times of the day.
We also aim to see whether the participants in our study have different body clock (circadian) rhythms to the general population. We will do this in multiple ways, such as looking at the levels of a few chemicals in the body that respond to body clock rhythms (melatonin and cortisol), asking questions about participants' sleep (by questionnaire), measuring sleep (with a wrist-worn motion sensor), and asking the patients to fill in questionnaires about depression.
We will recruit 15 patients who have been prescribed phototherapy for the treatment of their psoriasis. The study will last nine days per patient and after this they will proceed to their prescribed course of phototherapy.
Latest Results Summary
Humans, like in all living creatures, have an internal clock, or circadian rhythm. This means that expression of our genes and proteins changes throughout the day. Over the past few years, research into this has grown to become a huge discipline that aims to streamline and personalise medical treatment for people. In the same year the Nobel Prize was awarded for circadian rhythms, Professor Reynolds and I were awarded a Small Project Grant by The Psoriasis Association to set up, to our knowledge, the first research project looking into circadian rhythms in the skin of people with psoriasis. We also aimed to look into whether ultraviolet light affects psoriatic skin differently in the morning and afternoon, to see whether phototherapy – which is an ultraviolet light treatment given to people with psoriasis in hospitals – is better at a certain time of the day.
To this end, we have used the money to train a cohort of dermatology research nurses in how to administer UV light, how to read the results and how to take skin samples (our main source of research data). It hasn’t always been smooth, however! The field is always evolving, and so we have had to adapt our research to take into account new information. The Covid-19 pandemic has unfortunately been a big set-back for our study, initially halting all recruitment for six months. Once again, we had to modify our study, to make it safer for participants so we can continue to recruit. The pandemic has also changed our focus to analysing the data we have so far. Although it is from only five patients, we have forty skin samples – half of which are being analysed for gene changes and half for protein changes.
Ultimately, this is a pilot study, which means it aims to inform future research, but so far it has generated quite a lot of exciting data which we will share with you when we can. We are very grateful to you all for making this possible, and still look forward to a future where doctors personalise your treatment based upon your internal clock.
An innovative mixed methods study investigating altered emotional processing in psoriasis patients (Small Grant Award)
Dr C. Elise Kleyn, University of Manchester
Psoriasis is a chronic, inflammatory skin disease which affects 2-3% of the UK population. As a result of the appearance of their skin, patients with psoriasis commonly experience negative social interactions and reactions from others including facial expressions of disgust.
Our group were the first to use brain scanning (magnetic resonance imaging) to demonstrate that patients with psoriasis process facial expressions of disgust differently to individuals without skin disease. It was demonstrated that patients with psoriasis have a diminished signal in the insula, an area of the brain known to be important in disgust processing. This differential response may reflect a coping mechanism, adopted by patients to ‘block out’ the aversive reactions of others and protect themselves from stressful emotional responses.
More recent, (unpublished) work, by the group suggests that an individual’s response to disgust may vary depending on the length of time a patient has had psoriasis and the age at which they were diagnosed. However, it is not known when this mechanism develops, or its wider implications on patients’ quality of life.
This novel study will pilot the integration of different techniques, including in-depth patient interviews and state-of-the art brain scanning, to further understanding of this proposed coping mechanism in psoriasis. Addressing this current gap in knowledge will inform the development of personalised clinical and psychological interventions to support people living with psoriasis.
Neuropsychological morbidity in psoriasis (PhD Studentship)
Dr Elise Kleyn, University of Manchester
Psoriasis is a chronic skin disease for which there is currently no cure. It afflicts far more than the skin and there is limited knowledge of the mechanisms or way in which psychological effects as well as other brain effects are caused.
We were the first to use brain scanning techniques to show that patients with psoriasis process facial expressions of disgust differently to individuals without skin disease.
This PhD project will use state-of-the-art brain scanning, questionnaires and laptop-based tasks to investigate whether treating psoriasis lesions effectively will change altered processing of disgust in patients. Patients’ reactions to pictures of their own psoriasis lesions and those of others will also be studied in a separate group of patients who have had psoriasis for varying lengths of time.
Understanding the brain-skin connection is key to developing new approaches to help treat the psychological and other brain effects of psoriasis.
We use cookies to help us provide you with a better service, but do not track anything that can be used to personally identify you. If you prefer us not to set these cookies, please visit our Cookie Settings page or continue browsing our site to accept them. Close
COVID-19 Information
About psoriasis
Types of psoriasis
Living with psoriasis
Treatments for psoriasis
Psoriasis FAQs
Children and psoriasis
Good quality information
Psoriatic Arthritis
About psoriatic arthritis
Treatments for psoriatic arthritis
Additional information
Leaflets & information sheets
Unavailable treatments
Peer to peer support
Your stories
Support our work
Make a donation
Become a member
Leave a gift in your will
Support us while you shop
Fundraising
Fundraise for us
Fundraising events
Fundraising ideas
Volunteer/Awareness opportunities
Take part in research

Raising awareness
Psoriasis Awareness Week
Your Stories
Itching to Talk?
Psoriasis Awareness Week 2023
Sunday 29th October to Friday 4th November 2023.
Read the real life stories of people living with psoriasis and psoriatic arthritis. Find out how you can share your own story.
Our Research
Our research strategy
Our research
Research results
Getting Involved
Research network
For researchers
Apply for a grant
Capturing research impact
Supporting researchers
We are the leading national charity and membership organisation for people affected by psoriasis in the UK.
Through our work, we help people whose lives are affected by psoriasis and psoriatic arthritis, providing information and raising awareness.
Find out more
Work with us
Get in touch
Send us an email
Phone: 01604 251 620
WhatsApp: 07387716439
Psoriasis Experiences
Talk About Treatments
General Chat
Join our community
Register with us to connect and share with others around the world.
Create your free account
Existing members
Forgotten your password?
Browse news
All stories
Latest articles
Unavailable treatments - neutrogena® t gel therapeutic shampoo, betnovate & eumovate.
In March 2024 we have received updates regarding Neutrogena® T Gel Therapeutic Shampoo. In late 2023, we were made aware of a possible stock issue with Betnovate and Eumovate cream.
Navigating Tattoos with Psoriasis
Discover helpful advice and tips for people with psoriasis who want tattoos. From talking to your doctor before getting inked to taking care of your skin afterward, check out the article for handy pointers.

- General Dermatology
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Psoriatic Arthritis
- Atopic Dermatitis
- Skin Cancer
- Hidradenitis Suppurativa
- Pigmentary Disorders
- Pediatric Dermatology
- Practice Management
Psoriasis Therapies in 2024 and Beyond
This article will highlight recently approved psoriasis therapies that will shape the 2024 treatment landscape and provide some exciting updates in the psoriasis management market this coming year and beyond.
Decades ago, psoriasis was still primarily considered a problem with hyperproliferation of the epidermis. Given the antiquated understanding of this disease pathophysiology, traditional oral immunosuppressive agents were used for moderate to severe presentations. Recent research into the pathophysiology of psoriasis has highlighted the importance of the immune-mediated nature of this very common inflammatory skin disease. There now exists a clear mechanism down to the molecular level regarding which cytokines are implicated in the pathophysiology of psoriatic disease. When considering these different molecular signaling pathways, IL-23–mediated activation of the Th17 pathway is hypothesized to be the main contributor to the inflammation seen in psoriasis. 1 Due to its important role in the pathophysiology of psoriasis, IL-23 has been referred to as the master cytokine in psoriatic disease by many clinicians and researchers. Other important cytokines include TNF-α and IL-17. The fact that biologic agents interact with a specific cytokine (such as TNF-α, IL-17, or IL-23) in a targeted manner has revolutionized the capacity to manage psoriasis compared with the era of a more generalized immunosuppression reflected by the traditional oral medications (eg, methotrexate, cyclosporine, and acitretin). This represents an improved treatment paradigm where targeted immunomodulation has resulted in a great enhancement in both safety and efficacy for the biologic agents.
stockmaster /Adobe Stock
There are now more than 13 FDA-approved biologic options for moderate to severe psoriasis. Additionally, there have been new approvals for oral and topical therapies for psoriasis, including a topical first-in-class mechanism of action for psoriatic skin lesions. Given that psoriasis affects more than 7 million adults in the US, the therapeutic landscape is constantly evolving. 2 It is estimated that the psoriasis management market will be worth nearly $121 billion by the end of 2024. 3 This article will highlight recently approved psoriasis therapies that will shape the 2024 treatment landscape and provide some exciting updates in the psoriasis management market this coming year and beyond. It will also highlight how biosimilar medications will affect the field for years to come and cover new updates in the management of pediatric psoriasis.
The newest biologic agent for psoriasis, bimekizumab (Bimzelx), was approved by the FDA on October 18, 2023, for the management of moderate to severe psoriasis. Bimekizumab is unique in that it blocks both the IL-17A and IL-17F cytokines. The other IL-17 antagonists approved to manage psoriasis either only block IL-17A (ixekizumab and secukinumab) or block the IL-17 receptor (brodalumab). In findings from the phase 3 BE READY trial (NCT03410992), which studied bimekizumab in the management of moderate to severe psoriasis, 91% of 349 patients receiving this medication at 320 mg every 4 weeks achieved a Psoriasis Area and Severity Index (PASI) score of 90 compared with 1% of 86 patients receiving placebo. 4 In findings from the phase 3 BE OPTIMAL trial (NCT03895203), which studied bimekizumab in the management of psoriatic arthritis (for biologic-naive patients), significantly more patients receiving bimekizumab (44%) reached American College of Rheumatology 50% response vs those receiving placebo (10%). 5 “Head-to-head studies with bimekizumab vs currently FDA-approved biologic agents, including adalimumab, secukinumab, and ustekinumab, have highlighted the rapid onset of action of this new biologic medication. Even before the second dose, this is a significant PASI response, making this a great option for a patient whose main concern is rapid improvement with infrequent dosing compared with some competitors. Bimekizumab’s unique mechanism of action [IL-17A/F] is a novel and exciting addition to the current biologic landscape for psoriasis,” G. Michael Lewitt, MD, FAAD, a board-certified dermatologist at Rosealind Franklin-Chicago Medical School in Chicago, Illinois, said.
Pooled safety analysis from phase 2 and phase 3 showed that nasopharyngitis, oral candidiasis, and upper respiratory tract infection were the most common treatment-emergent adverse events reported with bimekizumab. IL-17 is involved in mucosal host defenses against fungal infections; therefore, anti–IL-17 biologics can be associated with an increased risk of oral mucocutaneous candidiasis. 6 Of the patients in the phase 2/3 trials, 15.4% reported an oral candidiasis event in the first year and 9.1% reported it during the second year. These rates are higher when compared with the other IL-17 antagonists.
Although not considered a new biologic, adalimumab will greatly affect the 2024 psoriasis treatment landscape because generic versions are approved for the management of adult plaque psoriasis. These include the 9 following FDA-approved agents: adalimumab-aacf (Idacio), adalimumab-fkjp (Hulio), adalimumab-adbm (Cyltezo), adalimumab-bwwd (Hadlima), adalimumab-adaz (Hyrimoz), adalimumab-aaty (Yuflyma), adalimumab-aqvh (Yusimry), adalimumab-afzb (Abrilada), and adalimumab-atto (Amjevita). 7 According to the FDA, biosimilars must prove bioequivalence and be tested by way of clinical trials to ensure they will exhibit no significant differences to their parent product. 8

Incyte and CMS Collaborate to Develop Povorcitinib in Numerous Chinese Regions

ReV Up Your Vitiligo Treatment Strategies
J-Code for Ycanth Officially Launches Today

The Cutaneous Connection: Splish, Splash, AD Management Starts in the Bath

Dermatology Conferences and Meetings Calendar 2024: April

Derm In The News: March 24-30
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Psoriasis in Women
— psoriasis takes a higher toll on female patients and may impact pregnancy outcomes.
by Diana Swift , Contributing Writer, MedPage Today March 29, 2024

Generally speaking, the incidence, prevalence, and manifestation of psoriasis of the skin are similar between the sexes, but the burden of psoriasis is somewhat heavier for women than men, and there are some sex-dependent differences in disease manifestation, severity, subjective disease perception, and treatment choices.
"Overall, women have lower disease severity, as measured by the Psoriasis Area Severity Index, but they experience higher impairment of their quality of life, as measured by the Dermatology Life Quality Index," said Chris G. Adigun, MD, of the Dermatology & Laser Center of Chapel Hill, North Carolina. "Women with psoriasis also have higher rates of depression compared to men."
However, Sonya Kenkare, MD, of the Illinois Dermatology Institute in Hinsdale, said that she "commonly see[s] depression in both sexes."
As for sex-specific lesion sites, "scalp psoriasis is more common in women, but men can get it there, too," Kenkare added. "In terms of age, there can be a peak in psoriasis frequency at puberty and a small peak at menopause."
In terms of treatment, data suggest women respond better to systemic therapy than men, but they also experience more adverse events related to treatment, said Adigun.
Female Hormones, Pregnancy
Hormones set off immune changes in the skin, and psoriasis symptoms can flare during puberty, after childbirth, and at menopause. Because this widespread dermatitis affects women in their reproductive years, female patients need special attention and more nuanced treatment.
The usual hormonal fluctuations of the monthly menstrual cycle do not have much impact, said Kenkare, but the high hormonal levels of pregnancy can correlate with symptom improvement. "I generally see improvement in about a third of pregnant women, but the condition quickly returns to pre-delivery status after delivery," she said.
"It is estimated that 30% to 40% of women have improvement in their psoriasis during pregnancy, with brisk worsening of their disease at 4 to 6 weeks postpartum," Adigun said. "And 40% to 90% of women experience a flare in their psoriasis in this immediate postpartum period."
One recent review reported that about 50% of women improve during gestation, while 25% worsen and 25% have no change.
There seems to be a correlation between high estrogen levels and improvements, which is supported by estrogen's dual effect: it can have both immunosuppressive and immunostimulatory functions . Gestational improvement may also be due in part to immune system alterations that occur to prevent rejection of the fetus.
According to one study , proinflammatory T helper (Th)-1 cytokines are upregulated in psoriasis and play a key role in the inflammatory cascades. "It is likely that during pregnancy the Th-2 cytokine-mediated downregulation of the immune response by virtue of its anti-inflammatory and antagonizing effects on the Th-1 cytokines improves psoriasis," these study authors wrote.
The effect on psoriasis of hormonal injections for pre-in vitro fertilization (IVF) egg stimulation and retrieval in IVF treatment has not really been studied, Kenkare said.
Since studies are few and results are mixed, there is no convincing evidence that psoriasis impairs fertility in either sex, according to Tina Bhutani, MD, MAS, of the Psoriasis and Skin Treatment Center at the University of California San Francisco. But based on proxy subfertility data from other systemic inflammatory diseases such as rheumatoid arthritis , "there may be an inflammatory effect that perhaps makes it harder for some to conceive," she said.
Most women with psoriasis can readily get pregnant, added Kenkare. "But there may be an indirect correlation with reduced fertility through comorbidities. Psoriasis is associated with metabolic syndrome, obesity, and type 2 diabetes, and these can be associated with ovarian suppression," she said.
Because some psoriasis treatments are teratogenic, therapy must be carefully managed in the pre-conception, pregnancy, and lactation stages, but treatment should continue -- especially for severe psoriasis. "There could be bad outcomes in the case of uncontrolled disease, although again the study results are mixed," said Bhutani.
Kenkare added that she "reassure[s] women that even if they are pregnant or nursing, we can still treat their psoriasis and we should." Options for safe treatment during pregnancy or lactation are limited, however, owing to lack of safety data for the developing fetus or secretion into breast milk.
Fortunately, there are safe therapies for pregnant women, including steroids, biologics, topicals, and photo therapy, and also a few that should categorically be avoided such as birth defect-linked retinol and isotretinoin. "Undertreating severe disease can negatively impact the pregnancy, so we need to evaluate what's happening in an individual very carefully," Kenkare said.
She recommended a consultation with a dermatologist for affected women who are pregnant or planning to be. "I've never known a woman with psoriasis who decided not to get pregnant because of her condition. Usually we can figure something out, but treatment during pregnancy is more complex and needs to be really nuanced."
Adigun noted that, "typically, topical therapies during pregnancy and/or lactation are limited to topical steroids, and systemic therapies to the tumor necrosis factor (TNF)-alpha inhibitors."
As for pregnancy outcomes, does the inflammatory milieu bode ill for maternal and fetal events? "Adverse events do not differ much from those in the general pregnant population, but there may be a trend in that direction, so we recommend that psoriasis patients see a dermatologist in conjunction with their obstetrician to make sure their inflammation is kept under control," said Bhutani.
A 2023 Iranian meta-analysis showed modest but significant associations between psoriasis and maternal outcomes, including cesarean delivery, pre-eclampsia/eclampsia, gestational diabetes and hypertension, and preterm birth. A significant association was also observed for adverse neonatal outcomes, including small for gestational age, low birth weight, and stillbirth.
In a large Danish case-control study , there was a 2.48% higher absolute risk of ectopic pregnancy for women with moderate-to-severe psoriasis compared with unaffected women, but no other adverse outcomes. In addition, a large British cohort study found slightly higher fertility rates in psoriasis-affected women versus unaffected women generally, but slightly lower rates in those with moderate to severe disease, as well as a slightly higher risk of pregnancy loss in all women with psoriasis, which was perhaps related to comorbidities.
Though mixed, such findings call for greater emphasis on managing comorbidities as part of routine obstetrical care. Also warranted is more research on the underlying causes of adverse pregnancy outcomes in affected women. All experts agree that the care of pregnant women with psoriasis should involve consultation with a dermatologist.
![latest research into psoriasis author['full_name']](https://clf1.medpagetoday.com/media/images/author/DSwift_188.jpg)
Diana Swift is a freelance medical journalist based in Toronto.
Disclosures
Adigun, Kenkare, and Bhutani reported no relevant conflicts of interest with regard to their comments.
Ted Danson felt like a liar on 'Cheers' because of plaque psoriasis. Now he's speaking out.

BEVERY HILLS, Calif. − When you think of Ted Danson , you probably picture the charming Sam Malone behind the bar on "Cheers." Or the conniving, supernatural architect on "The Good Place."
But life for the sitcom star hasn't been all laughs behind-the-scenes. For much of his career, Danson says, he's struggled with moderate-to-severe plaque psoriasis − a chronic skin condition that had a debilitating impact on his self-esteem and made him feel like a fraud, even as his star was on the rise.
"People would come up and compliment me or think of me as Sam Malone or whatever, and I was always lying, because part of my brain was going, 'If only you knew,' " Danson says. "This is not a boohoo moment, meaning life has been very kind to me. I'm so blessed. But it does make you feel like you got to hide something, and that's not a good way to go through life."
Looking back, the actor says, he wonders if his penchant for comedic roles can be traced back to his condition.
"I sometimes wonder whether or not my self-deprecating humor came as a defense: 'I'll make fun of myself before someone else does' kind of thing," he says. "I was obviously able to navigate it, but it takes a toll."
Now, the actor is teaming up with Bristol Myers Squibb − a pharmaceutical company that makes SOTYKTU, a drug aimed at reducing psoriasis symptoms − for the “SO, Have You Found It?” campaign, which encourages people with plaque psoriasis to explore treatment options with their dermatologists.
What is plaque psoriasis? And how is it treated?
Mayo Clinic describes plaque psoriasis as a skin disease that causes itchy, scaly rashes, usually on the knees, elbows and scalp. According to the American Academy of Dermatology , about 7.5 million people in the United States have psoriasis, which is not contagious and can be exacerbated by stress. Though there are treatment options to manage symptoms, there is no cure.
Danson says his psoriasis has been up and down since he was diagnosed at 25, a common age people learn they have the disease. At its worst, he says, plaque psoriasis caused painful, itchy, red sores all over his body, usually accompanying stress or tiredness. "You can just feel not good in your skin − literally, not just emotionally," he says.
Over the next three decades, Danson tried nearly everything to fix it, including light boxes, coal tar and various ointments and diets. The process of trying to get control over his psoriasis proved "very humbling," he says, adding nothing quite worked. Mayo Clinic says people with plaque psoriasis often try different drugs or treatment combinations before they find an effective approach.
Dr. Jennifer Soung, a dermatologist partnering with the campaign, encourages psoriasis patients to take a holistic approach to treatment, of which medication is but one component.
"It is very much true that in the last 10 to 20 years, there's really been a revolution of new treatments for psoriasis, because we now understand how to target the inflammation in a more specific way," Soung says. "Sometimes I'll joke with patients that this has never been a better time to have psoriasis, because you have treatment options."
Nate Berkus talks psoriasis struggles: 'Absolutely out of the blue'
For Ted Danson and others, plaque psoriasis takes a mental health toll
For many who struggle with plaque psoriasis, the mental health toll can be just as, if not more, debilitating than the physical symptoms. Danson says he avoided swimming or wearing shorts and stuck to long-sleeve shirts out of shame of having the condition.
"You feel victimized and betrayed by your body," he says. "You can rise above it, you can deal with it and all of that, but it's hard. It's like you're playing at a disadvantage in life."
Soung says depression often follows a diagnosis.
"It's clearly established in research that patients with psoriasis are depressed too," she says. "When they first learn, they're just overwhelmed and kind of a little bit in denial too."
More: LeAnn Rimes is 'tired of hiding' her psoriasis, shows off skin in powerful photos
Danson says he's grateful to have had his loving and supportive wife, actress Mary Steenburgen, whom he wed in 1995, to lean on. About 15 years ago, a dermatologist introduced him to a new medication developed to treat the disease. It worked well, the actor says, adding his psoriasis has now developed into psoriatic arthritis, which causes pain to his joints but at least doesn't come with severe skin irritation. The actors regards this as an improvement. "I feel I have other issues at 76, but I'm not worried about whether I have plaque psoriasis anymore," he says, chuckling.
Danson wants others diagnosed with psoriasis to know they're not alone.
"A young man called me who I knew," Danson says, "and he was devastated. He discovered he had psoriasis. And I was able to go, 'I totally get it. I know it's embarrassing … You're lucky. You've been born at the right time that medicine has come up with something that will really help you, so don't worry too much.' … It's about empowering people to not feel victimized by this disease."
'It’s psoriasis': Kim Kardashian West fires back at report about her 'bad skin day'
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Psoriasis Pathogenesis and Treatment
Research on psoriasis pathogenesis has largely increased knowledge on skin biology in general. In the past 15 years, breakthroughs in the understanding of the pathogenesis of psoriasis have been translated into targeted and highly effective therapies providing fundamental insights into the pathogenesis of chronic inflammatory diseases with a dominant IL-23/Th17 axis. This review discusses the mechanisms involved in the initiation and development of the disease, as well as the therapeutic options that have arisen from the dissection of the inflammatory psoriatic pathways. Our discussion begins by addressing the inflammatory pathways and key cell types initiating and perpetuating psoriatic inflammation. Next, we describe the role of genetics, associated epigenetic mechanisms, and the interaction of the skin flora in the pathophysiology of psoriasis. Finally, we include a comprehensive review of well-established widely available therapies and novel targeted drugs.
1. Definition and Epidemiology
Psoriasis is a chronic inflammatory skin disease with a strong genetic predisposition and autoimmune pathogenic traits. The worldwide prevalence is about 2%, but varies according to regions [ 1 ]. It shows a lower prevalence in Asian and some African populations, and up to 11% in Caucasian and Scandinavian populations [ 2 , 3 , 4 , 5 ].
1.1. Clinical Classification
The dermatologic manifestations of psoriasis are varied; psoriasis vulgaris is also called plaque-type psoriasis, and is the most prevalent type. The terms psoriasis and psoriasis vulgaris are used interchangeably in the scientific literature; nonetheless, there are important distinctions among the different clinical subtypes (See Figure 1 ).

Clinical manifestations of psoriasis. ( A , B ) Psoriasis vulgaris presents with erythematous scaly plaques on the trunk and extensor surfaces of the limbs. ( C ) Generalized pustular psoriasis. ( D ) Pustular psoriasis localized to the soles of the feet. This variant typically affects the palms of the hands as well; hence, psoriasis pustulosa palmoplantaris. ( E , F ) Inverse psoriasis affects the folds of the skin (i.e., axillary, intergluteal, inframammary, and genital involvement).
1.2. Psoriasis Vulgaris
About 90% of psoriasis cases correspond to chronic plaque-type psoriasis. The classical clinical manifestations are sharply demarcated, erythematous, pruritic plaques covered in silvery scales. The plaques can coalesce and cover large areas of skin. Common locations include the trunk, the extensor surfaces of the limbs, and the scalp [ 6 , 7 ].
1.3. Inverse Psoriasis
Also called flexural psoriasis, inverse psoriasis affects intertriginous locations, and is characterized clinically by slightly erosive erythematous plaques and patches.
1.4. Guttate Psoriasis
Guttate psoriasis is a variant with an acute onset of small erythematous plaques. It usually affects children or adolescents, and is often triggered by group-A streptococcal infections of tonsils. About one-third of patients with guttate psoriasis will develop plaque psoriasis throughout their adult life [ 8 , 9 ].
1.5. Pustular psoriasis
Pustular psoriasis is characterized by multiple, coalescing sterile pustules. Pustular psoriasis can be localized or generalized. Two distinct localized phenotypes have been described: psoriasis pustulosa palmoplantaris (PPP) and acrodermatitis continua of Hallopeau. Both of them affect the hands and feet; PPP is restricted to the palms and soles, and ACS is more distally located at the tips of fingers and toes, and affects the nail apparatus. Generalized pustular psoriasis presents with an acute and rapidly progressive course characterized by diffuse redness and subcorneal pustules, and is often accompanied by systemic symptoms [ 10 ].
Erythrodermic psoriasis is an acute condition in which over 90% of the total body surface is erythematous and inflamed. Erythroderma can develop on any kind of psoriasis type, and requires emergency treatment ( Figure 2 ).

Erythrodermic psoriasis.
1.6. Comorbidities in Psoriasis
Psoriasis typically affects the skin, but may also affect the joints, and has been associated with a number of diseases. Inflammation is not limited to the psoriatic skin, and has been shown to affect different organ systems. Thus, it has been postulated that psoriasis is a systemic entity rather than a solely dermatological disease. When compared to control subjects, psoriasis patients exhibit increased hyperlipidemia, hypertension, coronary artery disease, type 2 diabetes, and increased body mass index. The metabolic syndrome, which comprises the aforementioned conditions in a single patient, was two times more frequent in psoriasis patients [ 11 , 12 ]. Coronary plaques are also twice as common in psoriasis patients when compared to control subjects [ 13 ]. Several large studies have shown a higher prevalence of diabetes and cardiovascular disease correlating with the severity of psoriasis [ 14 , 15 , 16 , 17 , 18 ]. There are divided opinions regarding the contribution of psoriasis as an independent cardiovascular risk factor [ 19 , 20 ]; however, the collective evidence supports that psoriasis independently increases risk for myocardial infarction, stroke, and death due to cardiovascular disease (CVD) [ 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. In addition, the risk was found to apply also to patients with mild psoriasis to a lower extent [ 21 , 27 ].
Vascular inflammation assessed via 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) found psoriasis duration to be a negative predicting factor. It was suggested that the cumulative effects of low-grade chronic inflammation might accelerate vascular disease development [ 29 ]. In a study by Metha et al., systemic and vascular inflammation in six patients with moderate to severe psoriasis was quantified by FDG-PET/CT. Inflammation foci were registered as expected in the skin, joints, and tendons. In addition, FDG uptake in the liver and aorta revealed subclinical systemic inflammation [ 30 ]. Furthermore, standardized uptake values were reduced in the liver, spleen, and aorta following treatment with ustekinumab {Kim, 2018 #359}. A new biomarker to assess CVD risk in psoriasis patients was proposed by nuclear magnetic resonance spectroscopy [ 31 ]. The signal originating from glycan N-acetylglucosamine residues called GlycA in psoriasis patients was associated with psoriasis severity and subclinical CVD, and was shown to be reduced in response to the effective treatment of psoriasis.
Psoriatic inflammation of the joints results in psoriatic arthritis (PsA). The skin manifestations generally precede PsA, which shares the inflammatory chronicity of psoriasis and requires systemic therapies due to a potential destructive progression. Psoriatic arthritis develops in up to 40% of psoriasis patients [ 32 , 33 , 34 , 35 , 36 , 37 , 38 ]; around 15% of psoriasis patients are thought to have undiagnosed PsA [ 39 ]. It presents clinically with dactylitis and enthesitis in oligoarticular or polyarticular patterns. The polyarticular variant is frequently associated with nail involvement [ 40 ]. Nails are specialized dermal appendages that can also be affected by psoriatic inflammation. Nail psoriasis is reported to affect more than half of psoriasis patients, and can present as the only psoriasis manifestation in 5–10% of patients [ 41 ]. The clinical presentation of nail psoriasis depends on the structure affected by the inflammatory process. Nail matrix involvement presents as pitting, leukonychia, and onychodystrophy, whereas inflammation of the nail bed presents as oil-drop discoloration, splinter hemorrhages, and onycholysis ( Figure 3 ) [ 42 ]. Psoriatic nail involvement is associated with joint involvement, and up to 80% of patients with PsA have nail manifestations [ 43 , 44 ].

Onycholysis and oil drop changes on psoriatic nail involvement.
In addition to an increased risk for cardiometabolic disease, psoriasis has been associated with a higher prevalence of gastrointestinal and chronic kidney disease. Susceptibility loci shared between psoriasis and inflammatory bowel disease support this association in particular with regard to Crohn’s disease [ 45 , 46 ]. An association with mild liver disease, which correlates with imaging studies, has been reported [ 30 , 47 ]. Psoriasis might be a risk factor for chronic kidney disease and end-stage renal disease, independent of traditional risk factors (demographic, cardiovascular, or drug-related) [ 48 ].
Taken together, the different factors contributing to psoriasis as a systemic disease can have a dramatic effect on the quality of life of patients and their burden of disease. Psoriasis impairment to psychological quality of life is comparable to cancer, myocardial infarction, and depression [ 49 ]. The high burden of disease is thought to be owed to the symptoms of the disease, which include pain, pruritus, and bleeding, in addition to the aforementioned associated diseases [ 50 ]. The impact of psoriasis on psychological and mental health is currently an important consideration due to the implications of the disease on social well-being and treatment. Patients with psoriasis have an increased prevalence of depression and anxiety and suicidal ideation. Interestingly, psoriasis treatment leads to improvement in anxiety symptoms [ 51 , 52 ].
2. Pathogenesis
The hallmark of psoriasis is sustained inflammation that leads to uncontrolled keratinocyte proliferation and dysfunctional differentiation. The histology of the psoriatic plaque shows acanthosis (epidermal hyperplasia), which overlies inflammatory infiltrates composed of dermal dendritic cells, macrophages, T cells, and neutrophils ( Figure 4 ). Neovascularization is also a prominent feature. The inflammatory pathways active in plaque psoriasis and the rest of the clinical variants overlap, but also display discrete differences that account for the different phenotype and treatment outcomes.

Histopathology of psoriasis. ( A ) Psoriasis vulgaris characteristically shows acanthosis, parakeratosis, and dermal inflammatory infiltrates. ( B ) In pustular psoriasis, acanthotic changes are accompanied by epidermal predominantly neutrophilic infiltrates, which cause pustule formation.
2.1. Main Cytokines and Cell Types in Plaque Psoriasis
Disturbances in the innate and adaptive cutaneous immune responses are responsible for the development and sustainment of psoriatic inflammation [ 53 , 54 ]. An activation of the innate immune system driven by endogenous danger signals and cytokines characteristically coexists with an autoinflammatory perpetuation in some patients, and T cell-driven autoimmune reactions in others. Thus, psoriasis shows traits of an autoimmune disease on an (auto)inflammatory background [ 55 ], with both mechanisms overlapping and even potentiating one another.
The main clinical findings in psoriasis are evident at the outermost layer of the skin, which is made up of keratinocytes. However, the development of the psoriatic plaque is not restricted to inflammation in the epidermal layer, but rather is shaped by the interaction of keratinocytes with many different cell types (innate and adaptive immune cells, vasculature) spanning the dermal layer of the skin. The pathogenesis of psoriasis can be conceptualized into an initiation phase possibly triggered by trauma (Koebner phenomenon), infection, or drugs [ 53 ] and a maintenance phase characterized by a chronic clinical progression (see Figure 5 ).

The pathogenesis of psoriasis.
It is well known that dendritic cells play a major role in the initial stages of disease. Dendritic cells are professional antigen-presenting cells. However, their activation in psoriasis is not entirely clear. One of the proposed mechanisms involves the recognition of antimicrobial peptides (AMPs), which are secreted by keratinocytes in response to injury and are characteristically overexpressed in psoriatic skin. Among the most studied psoriasis-associated AMPs are LL37, β-defensins, and S100 proteins [ 56 ]. LL37 or cathelicidin has been attributed a pathogenic role in psoriasis. It is released by damaged keratinocytes, and subsequently forms complexes with self-genetic material from other damaged cells. LL37 bound to DNA stimulates toll-like receptor (TLR) 9 in plasmacytoid dendritic cells (pDCs) [ 57 ]. The activation of pDC is key in starting the development of the psoriatic plaque, and is characterized by the production of type I IFN (IFN-α and IFN-β). Type I IFN signaling promotes myeloid dendritic cells (mDC) phenotypic maturation, and has been implicated in Th1 and Th17 differentiation and function, including IFN-γ and interleukin (IL)-17 production, respectively [ 58 , 59 , 60 ].
Whilst LL37–DNA complexes stimulate pDCs through TLR9, LL37 bound to RNA stimulates pDCs through TLR7. In addition, LL37–RNA complexes act on mDCs via TLR8 [ 56 , 57 ]. Activated mDCs migrate into draining lymph nodes and secrete tumor necrosis factor (TNF)-α, IL-23, and IL-12, with the latter two modulating the differentiation and proliferation of Th17 and Th1 cell subsets, respectively. Furthermore, slan + monocytes, which are important pro-inflammatory cells found in psoriasis skin lesions, respond to LL37–RNA activation by secreting high amounts of TNF-α, IL-12, and IL-23 [ 61 ].
The activation of the adaptive immune response via the distinct T cell subsets drives the maintenance phase of psoriatic inflammation [ 62 ]. Th17 cytokines, namely IL-17, IL-21, and IL-22 activate keratinocyte proliferation in the epidermis.
The inflammatory milieu activates keratinocyte proliferation via TNF-α, IL-17, and IFN-γ. Keratinocytes are also activated by LL37 and DNA, and greatly increase the production of type I IFNs [ 57 ]. Furthermore, they participate actively in the inflammatory cascade through cytokine (IL-1, IL-6, and TNF-α), chemokine, and AMP secretion.
A widely used psoriasis-like inflammation mouse model relies on the effect of the TLR7/8 agonist imiquimod, and is thus in support of the TLR7/8 disease initiation model. In addition, the response to imiquimod was blocked in mice deficient of IL-23 or IL-17R, which highlights the involvement of the IL-23/IL-17 axis in skin inflammation and psoriasis-like pathology [ 63 ].
The TNFα–IL-23–Th17 inflammatory pathway characterizes plaque-type psoriasis. The IL-17 cytokine family is composed of six members: IL-17A–F. They are produced by different cell types, and are important regulators of inflammatory responses [ 64 ]. So far, the clinically relevant signaling in psoriasis is mediated mostly by IL-17A and IL-17F; both act through the same receptor, but have different potencies. IL-17A exerts a stronger effect than IL-17F, and the IL-17A/IL-17F heterodimer has an intermediate effect. IL-17A binds to its trimeric receptor complex composed of two IL-17RA subunits and one IL-17RC subunit, resulting in the recruitment of the ACT1 adaptor protein. The interaction between ACT1 and the IL-17 receptor complex leads to the activation of a series of intracellular kinases including: extracellular signal-regulated kinase (ERK), p38 MAPK, TGF-beta-activated Kinase 1 (TAK1), I-kappa B kinase (IKK), and glycogen synthase kinase 3 beta (GSK-3 beta). These kinases enable NFκB, AP-1, and C/EBP transcription of pro-inflammatory cytokines, chemokines, and antimicrobial peptides. Th1 and Th2 cytokines act through Janus kinase (JAK)-STAT signaling pathways, whereas Th17 responses are mediated by ACT1 and NFκB [ 65 ]. Alternatively, γδ T cells are able to produce IL-17A independently of the IL-23 stimulus [ 66 ].
Drugs targeting TNFα, IL-23, and IL-17 and signaling pathways such as JAK/STAT are effective in the clinical management of plaque psoriasis. However, alternate inflammatory pathways may be valid for distinct psoriatic variants.
2.2. Pathophysiology in Variants
Whereas the TNFα–IL23–Th17 axis plays a central role in T cell-mediated plaque psoriasis, the innate immune system appears to play a more prominent role in the pustular variants of psoriasis [ 55 ]. Different pathomechanisms are associated with distinct psoriasis subtypes.
In guttate psoriasis, streptococcal superantigens are thought to stimulate the expansion of T cells in the skin [ 67 ]. It was shown that there is a considerable sequence homology between streptococcal M proteins and human keratin 17 proteins. Molecular mimicry may play a role in patients with the major histocompatibility HLA-Cw6 allele, since CD8(+) T cell IFN-γ responses were elicited by K17 and M6 peptides in said patients [ 68 , 69 ].
Pustular psoriasis is characterized by the increased expression of IL-1β, IL-36α, and IL-36γ transcripts, which have been found in pustular psoriasis compared to psoriasis vulgaris [ 70 ]. Nevertheless, IL-17 signaling is also involved in pustular psoriasis and patients with generalized pustular psoriasis without IL-36R mutations responded to anti-IL-17 treatments [ 71 , 72 ].
In nail psoriasis and psoriatic arthritis (PsA), an increased expression of TNF-α, NFκB, IL-6, and IL-8 in psoriasis-affected nails is consistent with the inflammatory markers found on lesional psoriatic skin [ 73 ]. The pathophysiology of PsA and psoriasis is shared as synovial tissue in psoriatic arthritis expresses pro-inflammatory cytokines: IL-1, IFN-γ, and TNFα [ 74 , 75 ]. Infiltrating cells in psoriasis arthritis, tissues, and synovial fluid revealed large clonal expansions of CD8 + T cells. Joint pathology, specifically bone destruction, is partly mediated via IL-17A signaling, which induces the receptor activator of nuclear factor kappa b ligand (RANKL), and in turn activating osteoclasts. Pro-inflammatory cytokines IL-1β and TNF-α act in synergy with the local milleu [ 76 ].
2.3. Autoimmunity in Psoriasis
Psoriasis shows clear autoimmune-related pathomechanisms. This very important area of research will allow for a deeper understanding of to which extent autoantigen-specific T cells contribute to the development, chronification, and overall course of the disease.
LL37 is one of two well-studied T cell autoantigens in psoriasis. CD4 + and CD8 + T cells specific for LL37 were found in two-thirds of patients with moderate to severe plaque psoriasis in a study. LL37-specific T cells produce IFN-γ, and CD4 + T cells produce IL-17, IL-21, and IL-22 as well. LL37-specific T cells can be found in lesional skin or in the blood, where they correlate with disease activity [ 77 ]. CD8 + T cells activated through LL37 engage in epidermotropism, autoantigen recognition, and the further secretion of Th17 cytokines. The melanocytic protein ADAMTSL5 was found to be an HLA-C*06:02-restricted autoantigen recognized by an autoreactive CD8 + T cell TCR. This finding establishes melanocytes as autoimmune target cells, but does not exclude other cellular targets [ 78 ].
Other autoantigen candidates include lipid antigens generated by phospholipase A2 (PLA2) group IVD (PLA2G4D) and hair follicle-derived keratin 17 [ 79 , 80 ]. Interestingly, keratin 17 exposure only lead to CD8+ T cell proliferation in patients with the HLA-Cw*0602 allele (see above) [ 81 ].
2.4. Genetics
Psoriasis has a genetic component that is supported by patterns of familial aggregation. First and second-degree relatives of psoriasis patients have an increased incidence of developing psoriasis, while monozygotic twins have a two to threefold increased risk compared to dizygotic twins [ 82 , 83 ]. Determining the precise effect of genetics in shaping innate and adaptive immune responses has proven problematic for psoriasis and other numerous immune-mediated diseases [ 84 , 85 ]. The genetic variants associated with psoriasis are involved in different biological processes, including immune functions such as antigen presentation, inflammation, and keratinocyte biology [ 55 ].
2.4.1. Antigen Presentation
Genome-wide linkage studies of psoriasis-affected families have so far detected at least 60 chromosomal loci linked to psoriatic susceptibility [ 86 , 87 , 88 ]; the most prominent locus is PSORS1, which has been attributed up to 50% of the heritability of the disease [ 89 ]. PSORS1 is located on chromosome 6p21 within the major histocompatibility complex (MHC), which is specifically in the class I telomeric region of HLA-B, and spans an approximately 220 kb-long segment and corresponds to HLA-Cw6 (C*06:02). HLA-Cw6 is strongly linked to early and acute onset psoriasis [ 90 , 91 ]. The HLA-C*06:02 allele is present in more than 60% of patients, and increases the risk for psoriasis nine to 23-fold [ 92 ]. Nevertheless, no link between late-onset psoriasis or pustular psoriasis and PSORS1 could be established, possibly reflecting a genetically heterogenic background associated with different clinical phenotypes [ 93 ]. PSORS2 spans the CARD14 gene, while PSORS4 is located in the epidermal differentiation complex [ 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ].
The results of numerous genome-wide association studies (GWAS) in psoriasis are consistent with the prominent role of PSORS1 as a risk factor, but have also revealed over 50 single-nucleotide polymorphisms (SNPs) to be associated to psoriasis [ 102 , 103 , 104 ]. Variants involving the adaptive and immune system are a constant result in these studies [ 53 , 103 , 105 ].
2.4.2. Genetic Variants Implicated in Aberrant Keratinocyte Proliferation and Differentiation
The immunogenetics of IL-23 are strongly associated with psoriasis. IL-23 is a dimer composed of a specific subunit, p19, and a p40 subunit, which is shared with IL-12. IL-23 signals through a heterodimeric receptor expressed by both innate and adaptive immune cells, which include Th17, natural killer T, γδ T cells, and RORγt + innate lymphoid cells. The IL-23R signals through JAK2/TYK2 and STAT3 [ 106 ]. SNPs in the regions coding for the IL-23 cytokine (both the p40 and p19 subunit) as well as the IL-23R have been identified to convey psoriasis risk [ 107 , 108 , 109 ]. Furthermore, these variants have been found to be associated with Crohn’s disease, psoriatic arthritis, and ankylosing spondylitis [ 110 ] [ 74 , 75 ]. IL-23 drives the expansion of Th17 T cells that produce IL-17A/F, which is another set of cytokines whose role is pivotal in the pathogenesis of psoriasis. Monoclonal antibodies targeting both the common p40 and the specific p19 subunit of IL-23 have proven to have high clinical efficacy [ 109 ].
As mentioned above, STAT3 is found in downstream signaling by IL-23, and is therefore essential in T cell development and Th17 polarization. STAT3 has also been detected in psoriasis GWAS, and its variants are associated with psoriasis risk [ 107 , 111 ]. Furthermore, transcription factor Runx1 induces Th17 differentiation by interacting with RORγt. Interestingly, the interaction of Runx1 with Foxp3 results in reduced IL-17 expression [ 112 ].
CARD14 mapping was shown to correspond to PSORS2. The CARD family encompasses scaffolding proteins that activate NF-kB. It was suggested that in psoriasis patients with respective CARD14 mutations, a triggering event can result in an aberrant NF-kB over activation [ 96 ]. CARD14 is expressed in keratinocytes and in psoriatic skin; it is upregulated in the suprabasal epidermal layers and downregulated in the basal layers. In healthy skin, CARD14 is mainly localized in the basal layer. Mutations in CARD14 have been shown to be associated with psoriasis, as well as with familial pityriasis rubra pilaris (PRP) [ 113 ].
The NF-kB signaling pathway is involved in the production of both IL-17 and TNF-α, and thus participates in adaptive and innate immune responses [ 73 ]; it is upregulated in psoriatic lesions and is responsive to treatment [ 114 ]. Gene variations in NFKBIA, TNIP1 , and TRAF3PI2 affecting NF-kB regulatory proteins have been linked to psoriasis via GWAS [ 102 , 115 , 116 , 117 ]. TRAF3PI2 codes for the ACT1 adaptor protein and the specific variant TRAF3IP2 p. Asp10Asn was associated to both psoriasis and psoriatic arthritis [ 117 ].
The different clinical psoriasis variants may have additional genetic modifiers. For instance, mutations in the antagonist to the IL-36 receptor (IL-36RN), belonging to the IL-1 pro-inflammatory cytokine family, have been linked to pustular psoriasis [ 118 , 119 ]. Recessive mutations in IL36RN , coding for the IL-36 receptor antagonist, have been associated with generalized pustular psoriasis (GPP). This mutation is also found in palmar plantar pustulosis and acrodermatitis continua of Hallopeau. Furthermore, in patients with pre-existing plaque-type psoriasis, the gain of function mutation in CARD14 , p.Asp176His, was found to be a predisposing factor for developing GPP [ 120 ].
In addition to studies of genetic variants, the profiling of gene expression in psoriasis has aided in the understanding of the relevant pathophysiological pathways. Transcriptomic studies of psoriatic skin have revealed differentially expressed genes (DEGs) when compared to healthy skin, and also between lesional and nonlesional psoriatic skin [ 121 , 122 ]. Further underscoring their relevance in psoriasis pathogenesis, IL-17A genes were found to be upregulated in nonlesional psoriatic skin compared to healthy skin. This finding suggests that nonlesional psoriatic skin is also subclinically affected, and supports the concept of the widespread inflammation that is present in psoriasis [ 123 ]. In addition, data showing the upregulation of Th2 genes in nonlesional psoriatic skin may reflect the activation of T cell regulatory compensation mechanisms in an effort to override the inflammatory cascade [ 123 ]. ‘Cross-disease’ transcriptomics have aided in differentiating nonspecific DEGs present in inflammatory skin conditions (such as atopic dermatitis and squamous cell carcinoma) from DEGs specific to psoriasis. The latter are induced by IL-17A and are expressed by keratinocytes [ 124 ].
Despite solid evidence of genetic relevance in the pathogenesis of psoriasis, no single genetic variant seems to be sufficient to account on its own for the development of disease. Hence, a multifactorial setting including multiple genetic mutations and environmental factors, which have been attributed up to 30% of disease risk, ought to be considered [ 125 ].
2.5. Epigenetics
The quest for the missing heritability associated with psoriasis candidate genes has fueled the search for epigenetic modifications. Epigenetic mechanisms modify gene expression without changing the genomic sequence; some examples include: long noncoding RNA (lncRNA), microRNA (miRNA) silencing, and cytosine and guanine (CpG) methylation.
lncRNA are at least 200 nucleotides long, and are not transcribed to protein. At least 971 lncRNAs have been found to be differentially expressed in psoriatic plaques compared to normal skin [ 126 , 127 , 128 , 129 , 130 , 131 ]. Thereof, three differentially expressed lncRNAs in proximity to known psoriasis susceptibility loci at CARD14, LCE3B/LCE3C , and IL-23R , and are thought to modulate their function [ 127 ].
miRNAs are small, evolutionarily conserved, noncoding RNAs that base pair with complementary sequences within mRNA molecules, and regulate gene expression at the posttranscriptional level, usually downregulating expression. Most of the studies of miRNAs in association with psoriasis address the plaque-type variant (see Table 1 ), and so far, more than 250 miRNAs are aberrantly expressed in psoriatic skin [ 132 , 133 , 134 , 135 ]. A prominent role has been attributed to miR-31, which is upregulated in psoriatic skin and regulates NF-κB signaling as well as the leukocyte-attracting and endothelial cell-activating signals produced by keratinocytes [ 135 ]. miR-21 is an oncomiR with a role in inflammation, and has been found to be elevated in psoriatic skin. Increased miR-21 has been localized not only to the epidermis, but is also found in the dermal inflammatory infiltrates, and correlates with elevated TNF-α mRNA expression [ 136 ]. miR-221 and miR-222 are among other upregulated miRNAs in psoriatic skin [ 132 ]. The aberrant expression of miR-21, miR-221, and miR-222 correlates with a downregulation of the tissue inhibitor of metalloprotease 3 (TIMP3) [ 137 , 138 ]. TIMP3 is a member of the matrix metalloprotease family with a wide range of functions. Increased levels of said miRs are thought to result in unopposed matrix metalloprotease activity, leading to inflammation (partly via TNF-α-mediated signaling) and epidermal proliferation [ 138 ]. miR-210 was found to be highly expressed in psoriasis patients, and induced Th17 and Th1 differentiation while inhibiting Th2 differentiation through STAT6 and LYN repression [ 139 ].
MicroRNAs (miRNAs) increased in psoriasis.
Serum levels of miR-33, miR-126, and miR-143, among others, have been proposed as potential biomarkers of disease [ 140 , 141 ]. However, the studies have so far failed to consistently present elevations of a single miRNA in psoriatic patients. Thus, alterations of miRNA expression are better interpreted in the context of miRNA profiles, which have been reported to shift following psoriasis treatments [ 132 ]. Thus, miRNA expression profiles could potentially be used to predict response to treatment and personalize therapies.
DNA methylation is another epigenetic mechanism that can alter gene expression in a transient or heritable fashion, and primarily involves the covalent modification of cytosine and guanine (CpG) sequences. CpG methylation is usually repressive unless it inhibits transcriptional repressors, in which case it results in gene activation. Around 1100 differentially methylated CpG sites were detected between psoriatic and control skin. Of these sites, 12 corresponded to genes regulating epidermal differentiation, and were upregulated due to a lower methylation pattern. Said changes in DNA methylation reverted to baseline under anti-TNF-α treatment, indicating that CpG methylation in psoriasis is dynamic [ 148 , 149 ]. Further research will shed light on the functional relevance of epigenetic regulation in psoriasis.
2.6. Microbiome
The skin microbiome exerts an active role in immune regulation and pathogen defense by stimulating the production of antibacterial peptides and through biofilm formation. A differential colonizing microbiota in comparison to healthy skin has been found in several dermatologic diseases, including atopic dermatitis, psoriasis, and acne vulgaris [ 150 , 151 ]. It is hypothesized that an aberrant immune activation triggered by skin microbiota is involved in the pathogenesis of autoimmune diseases. For instance, there is growing evidence that the steady-state microbiome plays a role in autoimmune diseases such as in inflammatory bowel disease [ 152 ].
The overall microbial diversity is increased in the psoriatic plaque [ 151 ]. However, an increase in Firmicutes and Actinobacteria phyla were found in psoriatic plaques ( Table 2 ) [ 153 ]. Proteobacteria were found to be higher in healthy skin when compared to psoriatic patients [ 153 , 154 ]. Nevertheless, Proteobacteria were found to be increased in the trunk skin biopsies of psoriatic lesions [ 151 ]. A combined increase in Corynebacterium, Propionibacterium, Staphylococcus, and Streptococcus was found in psoriatic skin; however, in another study, Staphylococci were significantly lower in psoriatic skin compared to healthy controls [ 151 , 154 ].
Psoriasis microbiome. ↑ increased. > higher than.
Certain fungi such as Malassezia and Candida albicans, and viruses such as the human papilloma virus have been associated with psoriasis [ 155 ]. So far, Malassezia proved to be the most abundant fungus in psoriatic and healthy skin. Nevertheless, the colonization level of Malassezia in psoriasis patients was lower than that in healthy controls [ 156 ]. Further studies are required to explain the role of the microbiome signature and the dynamics among different commensal and pathogenic phyla [ 157 ].
Psoriasis is a chronic relapsing disease, which often necessitates a long-term therapy. The choice of therapy for psoriasis is determined by disease severity, comorbidities, and access to health care. Psoriatic patients are frequently categorized into two groups: mild or moderate to severe psoriasis, depending on the clinical severity of the lesions, the percentage of affected body surface area, and patient quality of life [ 159 ]. Clinical disease severity and response to treatment can be graded through a number of different scores. The PASI score has been extensively used in clinical trials, especially those pertaining to the development of the biologic drugs, and will be used throughout this review.
Mild to moderate psoriasis can be treated topically with a combination of glucocorticoids, vitamin D analogues, and phototherapy. Moderate to severe psoriasis often requires systemic treatment. The presence of comorbidities such as psoriasis arthritis is also highly relevant in treatment selection. In this review, we will address the systemic therapies as small-molecule (traditional and new) and biologic drugs.
A number of case reports and case series have suggested that tonsillectomy has a therapeutic effect in patients with guttate psoriasis and plaque psoriasis [ 69 , 160 , 161 ]. A systematic review concluded that the evidence is insufficient to make general therapeutic recommendations for tonsillectomy, except for selected patients with recalcitrant psoriasis, which is clearly associated to tonsillitis [ 162 ]. A recent study stated that HLA-Cw*0602 homozygosity in patients with plaque psoriasis may predict a favorable outcome to tonsillectomy [ 163 ]. To date, a single randomized, controlled clinical trial showed that tonsillectomy produced a significant improvement in patients with plaque psoriasis in a two-year follow-up timespan [ 164 ]. Furthermore, the same cohort was evaluated to assess the impact of the clinical improvement after tonsillectomy on quality of life. The study reported a 50% improvement in health-related quality of life, and a mean 59% improvement in psoriasis-induced stress. Tonsillectomy was considered worthwhile by 87% of patients who underwent the procedure [ 165 ].
3.1. Small-Molecule Therapies
In the past years, an accelerated development in psoriasis therapies has resulted in advanced targeted biological drugs. Methotrexate (MTX), cyclosporin A, and retinoids are traditional systemic treatment options for psoriasis. All of the former are oral drugs with the exception of MTX, which is also available for subcutaneous administration. They will be briefly discussed in this review (see Table 3 ). The section ends with an overview on dimethyl fumarate and apremilast, which are newer drugs that have been approved for psoriasis.
Drugs available for psoriasis therapy.
MTX is a folic acid analogue that inhibits DNA synthesis by blocking thymidine and purine biosynthesis. The initial recommended dose of 7.5–10 mg/weekly may be increased to a maximum of 25 mg/weekly [ 166 , 167 ]. A recent retrospective study reported successful treatment response (defined by PASI decrease of 50% to 75% and absolute DLQI value) was reached by 33%, 47%, and 64% of patients at three, six, and 12 months, respectively [ 168 ]. There is conflicting evidence regarding MTX effectiveness on psoriatic arthritis. A recent publication reported 22.4% of patients achieved minimal arthritic disease activity, and 27.2% reached a PASI 75 at week 12 [ 169 ]. Furthermore, HLA-Cw6 has been suggested as a potential marker for patients who may benefit from MTX treatment [ 170 ]. The most common side effects include nausea, leucopenia, and liver transaminase elevation. Despite the potential side effects and its teratotoxicity, it remains a frequently used cost-effective first-line drug, and the close monitoring of liver function and full blood count make a long-term administration feasible.
Cyclosporine is a T cell-inhibiting immunosuppressant from the group of the calcineurin inhibitors. Cyclosporine is effective as a remission inducer in psoriasis and as maintenance therapy for up to two years [ 171 ]. Hypertension, renal toxicity, and non-melanoma skin cancer are significant potential side effects. Nephrotoxicity is related to the duration of treatment and the dose. Cyclosporine is employed as an intermittent short-term therapy. The dosage is 2.5 to 5.0 mg/kg of body weight for up to 10 to 16 weeks. Tapering of the drug is recommended to prevent relapse [ 171 ].
Retinoids are natural or synthetic vitamin A-related molecules. Acitretin is the retinoid used in the treatment of psoriasis. It affects transcriptional processes by acting through nuclear receptors and normalizes keratinocyte proliferation and differentiation [ 172 , 173 ]. A multicenter, randomized study reported 22.2% and 44.4% of patients reaching PASI 75 and PASI 50 at 24 weeks [ 174 ]. Acitretin is initially administered at 0.3–0.5 mg/kg of body weight per day. The maximum dosage is 1 mg/kg body weight/daily. Cheilitis is the most common side effect appearing dose dependently in all patients. Other adverse effects include conjunctivitis, effluvium, hepatitis, and teratogenicity.
Fumaric acid esters (FAEs) are small molecules with immunomodulatory and anti-inflammatory properties [ 175 , 176 ]. The exact mechanism of action has not been cleared, but is thought to involve an interaction with glutathione, which among other mechanisms, inhibits the transcriptional activity of NF-κB [ 177 , 178 ]. FAEs were initially available as a mix of dimethyl fumarate and monoethyl fumarate (DMF/MEF), the former being the main active compound in the formulation. DMF has been reported to decrease the migratory capacity of slan+ monocytes, and also inhibited the induction of Th1/Th17 responses [ 178 ]. DMF/MEF was approved in 1994 in Germany for the treatment of severe plaque psoriasis, and in 2008, the indication was expanded for moderate psoriasis [ 179 ]. This licensing was exclusive to Germany, where it remains a first-line drug; nevertheless, DMF/MEF was used as off-label treatment in other European countries [ 180 , 181 , 182 , 183 ]. A new FAE formulation containing exclusively the main active metabolite DMF became available in 2017, and was approved for psoriasis treatment in the European Union, Iceland, and Norway [ 184 ]. Although there are no studies comparing DMF/MEF directly to biologics, several studies document its efficacy [ 185 , 186 , 187 , 188 , 189 ]. A marked improvement is also seen in patients with psoriatic arthritis and nail psoriasis. The most common side effects are gastrointestinal symptoms and flushing, which are generally mild in severity, resolve over time, and are dose related [ 184 ]. In addition, FAEs may decrease lymphocyte and leukocyte counts. Therefore, it is recommended to perform a complete blood count before treatment initiation and monthly for DMF/MEF or every three months for DMF [ 184 ].
Apremilast, a phosphodiesterase-4 inhibitor, inhibits the hydrolyzation of the second messenger cAMP. This leads to the reduced expression of pro-inflammatory cytokines TNF-α, IFN0γ, and IL-12, and increased levels of IL-10. Apremilast was shown to have broad anti-inflammatory effects on keratinocytes, fibroblasts, and endothelial cells [ 190 ]. We studied apremilast in the context of slan + cells, which is a frequent dermal inflammatory dendritic cell type derived from blood circulating slan + nonclassical monocytes. Here, apremilast strongly reduced TNF-α and IL-12 production, but increased IL-23 secretion and IL-17 production in T cells stimulated by apremilast-treated slan + monocytes [ 191 ]. These dual effects on slan + antigen-presenting cells may constrain therapeutic responses. No routine monitoring of hematologic parameters is required for apremilast, which is a major advantage compared to the other small molecule drugs. Apremilast showed a 33.1% PASI 75 response at week 16. It is also effective for palmoplantar, scalp psoriasis, and nail psoriasis in addition to psoriatic arthritis [ 192 , 193 , 194 ]. The most common adverse events affected the gastrointestinal tract (nausea and diarrhea) and the upper respiratory tract (infections and nasopharyngitis). These effects were mild in nature and self-resolving over time.
The traditional systemic drugs are immunomodulators, which except for apremilast require close clinical monitoring due to the common side effects involving mainly the kidney and the liver. Methotrexate and cyclosporine are the only systemic therapies for psoriasis included in the World Health Organization (WHO) Model List of Essential Medicines, albeit for the indications of joint disease for the former and immunosuppression for the latter. The potential side effects of FAE and apremilast are usually not life-threatening, but might be sufficient to warrant discontinuation.
3.2. Biologics
In the context of psoriasis treatment, current use of the term biologics refers to complex engineered molecules including monoclonal antibodies and receptor fusion proteins. Biologics are different from the above-described systemic therapies in that they target specific inflammatory pathways and are administered subcutaneously (s.c.) (or intravenously i.e., infliximab) on different weekly schedules. Biologics presently target two pathways crucial in the development and chronicity of the psoriatic plaque: the IL-23/Th17 axis and TNF-α-signaling (see Table 3 ).
3.2.1. TNF-α
TNF-α inhibitors have been available for over a decade. They are considered the first-generation biologics, and are effective for plaque psoriasis and psoriatic arthritis. TNF-α inhibitors are still the standard used to evaluate drug efficacy in psoriasis clinical research. There are currently four drugs in this category: etanercept, infliximab, adalimumab, and certolizumab.
Etanercept is unique in the biologics category in that it is not a monoclonal antibody, but rather a recombinant human fusion protein. The receptor portion for the TNF-α ligand is fused to the Fc portion of an IgG1 antibody. It was the first TNF-α inhibitor approved by the United States Food and Drug Administration (FDA) for psoriasis. Infliximab is a chimeric monoclonal IgG1 antibody, and adalimumab is a fully human monoclonal IgG1 antibody. They neutralize TNF-α activity by binding to its soluble and membrane-bound form. These drugs are particularly employed to treat psoriatic arthritis, and show a similar efficacy. In the treatment of psoriasis, they show different PASI 75 response rates: 52% for etanercept, 59% for adalimumab, and 80% for infliximab. Infliximab shows superiority in terms of efficacy when compared to the other TNF-α inhibitors, and when compared with ustekinumab, it showed a similar performance [ 195 ]. The chimeric nature of infliximab might contribute to a higher immunogenic potential of the drug, which in turn might influence drug survival. Certolizumab pegol is a pegylated Fab’ fragment of a humanized monoclonal antibody against TNF-α. PEGylation is the covalent conjugation of proteins with polyethylene glycol (PEG), and is attributed a number of biopharmaceutical improvements, including increased half-life and reduced immunogenicity [ 196 ]. The initial indication for treating Crohn’s disease was extended to psoriatic arthritis and recently to plaque psoriasis. Certolizumab has shown an 83% PASI 75 response. Unlike other anti-TNF-α agents, it has no Fc domain, and is thus not actively transported across the placenta. Thus, certolizumab pegol is approved for use during pregnancy and breastfeeding.
3.2.2. IL23/Th17 axis
As previously mentioned, IL-23 drives the expansion of Th17 cells whose inflammatory effects are in turn mediated by IL-17A, IL-17F, and IL-22.
IL-23 is a dimer composed of p40 and p19. The first biologic to be approved for psoriasis vulgaris after the TNF-α inhibitors was ustekinumab, which is a monoclonal antibody directed against the p40 subunit. P40 is not exclusive to IL-23, but rather is shared with IL-12. IL-12 is a dimer consisting of p40 and p35, and is involved in the differentiation of naïve T cells into Th1 cells. By targeting p40, ustekinumab blocks two different T-cell activating mechanisms, namely Th1 and Th17 selection. Ustekinumab is also effective for the treatment of PsA and Chron’s disease. It is available in two dosages, 45 mg and 90 mg, depending on a threshold body weight of 100 kg. Ustekinumab has extensive safety data, few side effects, good clinical efficacy, and long treatment drug survival was reported. At 90 mg, ustekinumab showed a PASI 75 response in 72.4% and in 61.2% at 45 mg [ 197 ]. Studies using real-life data compared ustekinumab with the anti-TNF-α drugs, and ustekinumab was found to have a significant longer drug survival [ 198 , 199 , 200 ]. Frequent adverse events include nasopharyngitis, upper respiratory tract infections, fatigue, and headache. Among the serious adverse events listed in the label of ustekinumab are infections. Tuberculosis (TB) has only been reported in two psoriasis patients receiving ustekinumab [ 201 , 202 ]. The clinical efficacy of ustekinumab and the further clarification of its mechanism of action highlighted the crucial role of IL-23 in shaping the Th17 response. On the other hand, Th1 signaling is important for the response against bacterial and viral pathogens, and a study showed IL-12 signaling to have a protective effect in a model of imiquimod psoriasis-like inflammation [ 203 ]. This rationale fueled the development of drugs targeting p19, which is the IL-23-exclusive subunit. This more specific molecular targeting approach has also achieved successful clinical outcomes. Three fully human monoclonal antibodies with p19 specificity are available: guselkumab, tildrakizumab, and risankizumab. Guselkumab is licensed for psoriasis, and showed clinical superiority when compared to adalimumab, with 85.1% of patients reaching a PASI 75, and 73.3% receiving a PASI 90 response at week 16 [ 204 , 205 ]. Patients receiving tildrakizumab showed a 74% PASI 75, and 52% PASI 90 at week 16. Tildrakizumab was compared to etanercept, and was more likely to reach PASI 75 at weeks 16 and 28 [ 206 , 207 ]. Risankizumab showed the following PASI responses at week 12: 88% PASI 75, 81% PASI 90, and 48% PASI 100. Patients were followed for 48 weeks after the last injection at week 16, and one-fourth of them showed a maintained PASI 100 [ 208 ]. Whether IL-23 inhibition has the potential to modify the course of the disease after subsequent drug retrieval is currently under study.
So far, three human monoclonal antibodies targeting IL-17 are available. Secukinumab and ixekizumab block IL-17A; whereas brodalumab is directed against the IL-17 receptor A. IL-17-targeted biologics are fast acting, showing significant differences from placebo within the first week of treatment. Secukinumab was the first IL-17A inhibitor approved for psoriasis in 2015. A year later, the approval extended to include PsA and ankylosing spondylitis. At week 12, 81.6% of patients on secukinumab reached a PASI 75 response, and 28.6% reached a PASI 100 response [ 209 ]. At week 52, over 80% maintained PASI 75. Secukinumab showed a rapid onset of action, reflecting a significant likelihood of achieving PASI 75 as early as the first week of treatment when compared to ustekinumab, and surpassed the latter in clinical superiority at week 16 and 52 [ 210 , 211 ].
Ixekizumab also showed a significantly rapid onset of action in the first week when compared to placebo: a 50% PASI 75 response at week four, and 50% PASI 90 by week eight. At week 12, response rates were 89.1% for PASI 75 and 35.3% for PASI 100 [ 212 ]. Secukinumab and ixekizumab have proven effective for scalp and nail psoriasis, which are two clinical variants that are resistant to conventional topical therapies.
Brodalumab is a human monoclonal antibody that targets the IL-17 receptor type A, thus inhibiting the biological activity of IL-17A, IL-17F, interleukin-17A/F, and interleukin-17E (also called interleukin-25). Brodalumab showed an 83.3% PASI 75, 70.3% PASI 90, and 41.9% PASI 100 response rate at week 12, and a satisfactory safety profile [ 213 , 214 ]. After the discontinuation of treatment with secukinumab, 21% of patients maintained their response after one year and 10% after two years [ 215 ]. This finding suggests that targeting IL-17 signaling exerts some disease-modifying effect that might reestablish the homeostasis of the inflammatory pathways in a subset of psoriasis patients. Frequent adverse effects under IL-17 blockade include nasopharyngitis, headache, upper respiratory tract infection, and arthralgia. Furthermore, IL-17 signaling is critical for the acute defense against extracellular bacterial and fungal infections. Candida infections are more frequent in patients receiving anti-IL17 biologics secukinumab and ixekizumab compared to etanercept [ 209 ]. Nonetheless, candida infections were not severe, and did not warrant treatment interruption. The risk of tuberculosis reactivation is considered small under biologic therapies other than anti-TNF-α [ 216 ]. Anti-IL-17 biologics should not be used in psoriasis patients also suffering from Chron’s disease.
3.2.3. Biosimilars in Psoriasis
The introduction of biosimilars for different diseases is revolutionizing the pharmaceutical arsenal at hand. As patents for many biologics face expiration, biosimilar versions of these drugs are being developed, or are already entering the market. A biosimilar is a biological product that must fulfill two requirements: it must be highly similar to an approved biologic product and have no clinically meaningful differences in safety, purity, or potency when compared with the reference product. Guidelines for the development and approval of biosimilars have been issued by the European Medicines Agency, the FDA, and the World Health Organization. There are currently eight adalimumab biosimilars, four infliximab biosimilars, and two etanercept biosimilars approved in Europe. By lowering the costs of systemic treatment for psoriasis patients, biosimilars may also increase access to biologics.
3.2.4. Drugs in the Research Pipeline
Tofacitinib is an oral Janus kinase (JAK) inhibitor currently approved for the treatment of rheumatoid arthritis (RA) and PsA. Tofacitinib showed a 59% PASI 75 and 39% PASI 90 response rate at week 16, and was also effective for nail psoriasis; however, its development for psoriasis was halted for reasons unrelated to safety. Upadacitinib is another JAK inhibitor currently undergoing phase III clinical trials for the treatment of psoriatic arthritis. Piclidenoson, an adenosine A3 receptor inhibitor, serlopitant, a neurokinin-1 receptor antagonist, and RORγt inhibitors are each being tested as oral treatments for psoriasis [ 217 ]. Two different biologics targeting IL-17 and one targeting IL-23 are being currently tested. In addition, there are currently 13 registered phase III clinical trials testing biosimilars for adalimumab (eight), infliximab (three), and etanercept (two).
Psoriasis is a complex multifactorial disease for which various novel therapies have arisen in the past years. In spite of the refinement of the targeted therapies, psoriasis remains a treatable but so far not curable disease. The targeted therapies show high clinical efficacy for the inhibition of IL-23 and IL-17. Some degree of a persistent antipsoriatic effect by these therapies could be demonstrated after drug discontinuation, and argue for disease modification concept [ 208 , 215 ]. This important finding will be followed up in ongoing and future studies. However, in other cases, an initial clinical response is only short lived, requiring treatment with a different biologic. Clearly, more research is required to answer the question of why the drug survival of some biologics is limited. The therapeutic arsenal for psoriasis is likely to increase in the near future, with studies on orally applied new small molecules such as inhibitors targeting RORγt. In spite of the safety and efficacy of targeted therapies, due to economic factors, dosage regimes, and adverse effect profiles, broader-acting drugs remain the mainstay of psoriasis systemic therapy in many clinical scenarios around the world. The role of genetics remains to be elucidated not only in the context of predisposition to disease, but also in the profiling of distinct psoriatic types based on cytokine signatures, and in identifying therapy response markers. Clearly, psoriasis is currently the best understood and the best treatable Th17-biased chronic inflammatory disease. After achieving excellent clinical responses for the majority of patients with available therapeutic approaches, the stratification of psoriasis patients to the optimal drug and ensuring the sustainability of our treatments are the major tasks to be resolved.
Acknowledgments
We kindly thank Lukas Freund for his comments on the manuscript, Galina Grabe for providing the histology images, Anja Heid and Christine Dorschel for their technical support in gathering the clinical pictures.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) to KS – SFB TRR 156, SCHA 1693/1-1 and project number 259332240/RTG 2099.
Conflicts of Interest
The authors declare no conflict of interest.

IMAGES
VIDEO
COMMENTS
A protective role for epidermal S100A9 in PsA. A new study uncovers a protective role for epidermal S100A9 in psoriasis and psoriatic arthritis, and highlights this alarmin as a potential ...
Targeted therapies for psoriasis. Psoriasis treatment aims to reduce inflammation, remove skin lesions, enhance quality of life, and avoid complications by eliminating lesions, lowering itching ...
Current and future research is looking at new treatments, different triggers, and how the gut relates to the condition. Psoriasis affects about 7.5 million adults in the United States.
Psoriasis is a common, chronic, and inflammatory skin disease affecting approximately 2-3% of the global population. It has been reported that the incidence of psoriasis varies from 30.3 per ...
ADX-629 (Aldeyra Therapeutics, Inc.) is a reactive aldehyde species inhibitor that recently completed a phase II multi-center, open-label trial for psoriasis ( NCT04908514) [ 82 ]. The trial enrolled 10 participants with moderate-to-severe disease, all of whom received 250 mg oral ADX-629 twice daily for 12 weeks.
Psoriasis is a common, chronic papulosquamous skin disease occurring worldwide, presenting at any age, and leading to a substantial burden for individuals and society. It is associated with several important medical conditions, including depression, psoriatic arthritis, and cardiometabolic syndrome. Its most common form, chronic plaque or psoriasis vulgaris, is a consequence of genetic ...
Psoriasis is a chronic inflammatory skin disorder, and current treatments include topical therapies, phototherapy, systemic immune modulators, and biologics, aiming to alleviate symptoms and improve quality of life. However, challenges persist, such as adverse effects, treatment resistance, high costs, and variability in response among individuals. The future of psoriasis treatment shows ...
Identifying dysregulated lncRNAs and the related networks involved in psoriasis will be an important area of research and would provide potential new clues for future diagnosis and treatment of ...
In recent years, extensive research into the underlying mechanisms of psoriasis has led to significant advancement in treatment options from small molecules to biologics. Area covered This review focuses on intracellular and molecular mechanisms such as AhR, A3AR, RIP1, CGRP, and S1P that serve as novel pharmacological targets for psoriasis.
This new treatment paradigm was made possible by the continually advancing knowledge of the pathophysiology of psoriasis. Thirty years ago, psoriasis was still primarily considered a problem with the hyperproliferation of the epidermis. ... Recent research into the pathophysiology of psoriasis has highlighted the importance of the immune system ...
Psoriasis is a chronic inflammatory skin disease that affects millions of people worldwide. This recent review published in BMJ provides a comprehensive and updated analysis of the national, regional, and global epidemiology of psoriasis, based on systematic literature search and modelling methods. The review also discusses the challenges and limitations of psoriasis data collection and ...
A research team has now discovered a key starting point for inhibiting inflammation in both psoriasis and psoriatic arthritis. The researchers' findings may form the basis for developing new ...
Psoriasis research. Learn about psoriasis medication as well as the effect of smoking, diet and genetics on psoriasis symptoms. Read about new treatment strategies for skin disorders.
Purpose of Review The purpose of the review is to highlight some of the new findings on phototherapy treatment for psoriasis and present clinical pearls for the practitioner. Recent Findings Recent research has helped to further elucidate the molecular mechanisms of UV light in the treatment of psoriasis. New evidence regarding the combination of phototherapy with biologic agents and ...
Psoriasis is a chronic skin condition characterized by itchy red patches and silvery scales, usually on the elbows, knees or scalp. It affects about 2 percent of Americans, and is sometimes associated with other health problems, such as arthritis, diabetes and heart disease. The causes are not fully understood, but the condition is related to ...
Please use one of the following formats to cite this article in your essay, paper or report: APA. Francisco de Souza, Hugo. (2023, September 14). Multi-omics study offers insights into psoriasis ...
Bimekizumab, a new treatment, targets certain parts of the immune system involved in psoriasis. Studies testing bimekizumab in different phases showed it is safe and effective in treating psoriasis. Many patients taking bimekizumab had good results with mild side effects, and higher doses were well tolerated.
Latest Results Summary. Humans, like in all living creatures, have an internal clock, or circadian rhythm. This means that expression of our genes and proteins changes throughout the day. Over the past few years, research into this has grown to become a huge discipline that aims to streamline and personalise medical treatment for people.
(This article belongs to the Special Issue New Insights into Psoriasis) Research. Jump to: Editorial, Review. attachment. Supplementary material: Supplementary File 1 (ZIP, 714 KiB) ... Our aim was to clarify the field of NET research in psoriasis and highlight the main factors required for NET generation, which may be a target of new therapies
The Stockholm Psoriasis Cohort was a noninterventional inception cohort study enrolling patients between 2001 and 2005. The present study was conducted from January 15, 2019, to February 5, 2021. At enrollment and 10 years, patients were examined by dermatologists and rheumatologists. Data from examinations were complemented by questionnaires ...
Recent research into the pathophysiology of psoriasis has highlighted the importance of the immune-mediated nature of this very common inflammatory skin disease. ... There are now more than 13 FDA-approved biologic options for moderate to severe psoriasis. Additionally, there have been new approvals for oral and topical therapies for psoriasis ...
psoriasis (Fig. 1).12,16-19 A growing body of research has indicated that psoriasis is a systemic disease with a variety of comorbidities, such as cardiovascular diseases, metabolic syndrome ...
Australian researchers say they have identified a gene mutation that causes the skin disease psoriasis. A chronic inflammatory condition, psoriasis causes red, scaly, itchy patches on the skin.
Share on Facebook. Opens in a new tab or window Share on X. Opens in a new tab or window Share on LinkedIn. Opens in a new tab or window Generally speaking, the incidence, prevalence, and ...
It worked well, the actor says, adding his psoriasis has now developed into psoriatic arthritis, which causes pain to his joints but at least doesn't come with severe skin irritation. The actors ...
Research on psoriasis pathogenesis has largely increased knowledge on skin biology in general. In the past 15 years, breakthroughs in the understanding of the pathogenesis of psoriasis have been translated into targeted and highly effective therapies providing fundamental insights into the pathogenesis of chronic inflammatory diseases with a dominant IL-23/Th17 axis.