- Analytical Chemistry

Paper Chromatography
What is paper chromatography.
Chromatography technique that uses paper sheets or strips as the adsorbent being the stationary phase through which a solution is made to pass is called paper chromatography. It is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper. It is a powerful analytical tool that uses very small quantities of material. Paper chromatography was discovered by Synge and Martin in the year 1943.
Table of Contents
Paper chromatography principle, paper chromatography diagram, paper chromatography procedure, paper chromatography applications.
- Types of Paper Chromatography
- Frequently Asked Questions – FAQs
The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper. When the mobile phase moves, the separation of the mixture takes place. The compounds in the mixture separate themselves based on the differences in their affinity towards stationary and mobile phase solvents under the capillary action of pores in the paper. Adsorption chromatography between solid and liquid phases, wherein the solid surface of the paper is the stationary phase and the liquid phase is the mobile phase.
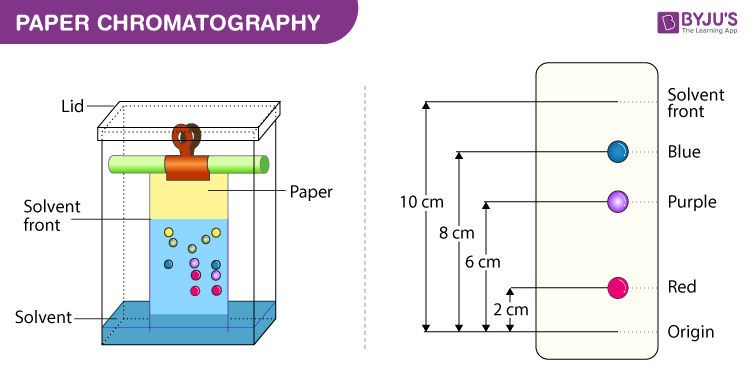
Below we have explained the procedure to conduct Paper Chromatography Experiment for easy understanding of students.
- Selecting a suitable type of development: It is decided based on the complexity of the solvent, paper, mixture, etc. Usually ascending type or radial paper chromatography is used as they are easy to perform. Also, it is easy to handle, the chromatogram obtained is faster and the process is less time-consuming.
- Selecting a suitable filter paper : Selection of filter paper is done based on the size of the pores and the sample quality.
- Prepare the sample: Sample preparation includes the dissolution of the sample in a suitable solvent (inert with the sample under analysis) used in making the mobile phase.
- Spot the sample on the paper: Samples should be spotted at a proper position on the paper by using a capillary tube.
- Chromatogram development: Chromatogram development is spotted by immersing the paper in the mobile phase. Due to the capillary action of paper, the mobile phase moves over the sample on the paper.
- Paper drying and compound detection : Once the chromatogram is developed, the paper is dried using an air drier. Also, detecting solution can be sprayed on the chromatogram developed paper and dried to identify the sample chromatogram spots.
There are various applications of paper chromatography . Some of the uses of Paper Chromatography in different fields are discussed below:
- To study the process of fermentation and ripening.
- To check the purity of pharmaceuticals.
- To inspect cosmetics.
- To detect the adulterants.
- To detect the contaminants in drinks and foods.
- To examine the reaction mixtures in biochemical laboratories.
- To determine dopes and drugs in humans and animals.
Types of paper chromatography:
- Ascending Paper Chromatography – The techniques goes with its name as the solvent moves in an upward direction.
- Descending Paper Chromatography – The movement of the flow of solvent due to gravitational pull and capillary action is downwards, hence the name descending paper chromatography.
- Ascending – Descending Paper Chromatography – In this version of paper chromatography, movement of solvent occurs in two directions after a particular point. Initially, the solvent travels upwards on the paper which is folded over a rod and after crossing the rod it continues with its travel in the downward direction.
- Radial or Circular Paper Chromatography – The sample is deposited at the centre of the circular filter paper. Once the spot is dried, the filter paper is tied horizontally on a Petri dish which contains the solvent.
- Two Dimensional Paper Chromatography – Substances which have the same r f values can be resolved with the help of two-dimensional paper chromatography.
Frequently Asked Questions – FAQs
What are the advantages of paper chromatography.
Paper Chromatography Has Many Benefits Simple and rapid Paper chromatography necessitates a minimal amount of quantitative material. Paper chromatography is less expensive than other chromatography methods. The paper chromatography method can identify both unknown inorganic and organic compounds. Paper chromatography takes up little space when compared to other analytical methods or equipment. Outstanding resolving power
Why water is not used in paper chromatography?
It is preferable to use a less polar solvent, such as ethanol, so that the non-polar compounds will travel up the paper while the polar compounds will stick to the paper, separating them.
What are the limitations of Paper Chromatography?
Limitations of Paper Chromatography are as follows- Paper chromatography cannot handle large amounts of sample. Paper chromatography is ineffective in quantitative analysis. Paper chromatography cannot separate complex mixtures. Less Accurate than HPLC or HPTLC
What is the importance of paper chromatography?
Paper chromatography has traditionally been used to analyse food colours in ice creams, sweets, drinks and beverages, jams and jellies. Only edible colours are permitted for use to ensure that no non-permitted colouring agents are added to the foods. This is where quantification and identification come into play.
Is paper chromatography partition or adsorption?
A type of partition chromatography is paper chromatography.
To learn more about the different types of paper chromatography from the experts, register with BYJU’S now!
Other important links:

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
It is so easy to understand by students Explained with applications also.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Microbe Notes
Paper Chromatography- Definition, Types, Principle, Steps, Uses
Table of Contents
Interesting Science Videos
What is Paper Chromatography?
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper.
PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
It was first introduced by German scientist Christian Friedrich Schonbein (1865).
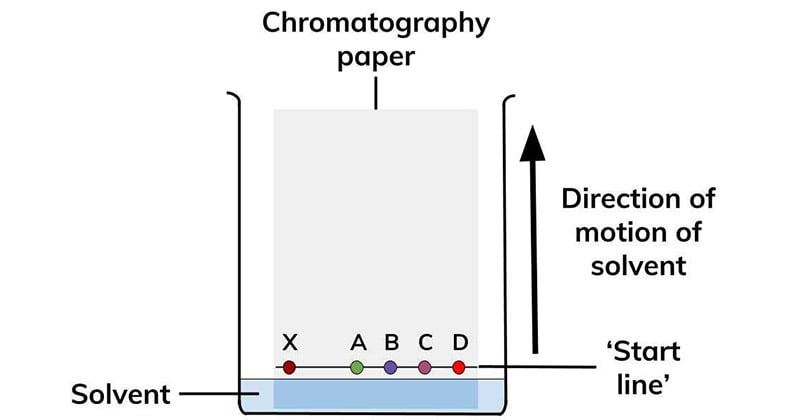
Types of Paper chromatography
Paper adsorption chromatography.
Paper impregnated with silica or alumina acts as adsorbent (stationary phase) and solvent as mobile phase.
Paper Partition Chromatography
Moisture / Water present in the pores of cellulose fibers present in filter paper acts as stationary phase & another mobile phase is used as solvent In general paper chromatography mostly refers to paper partition chromatography.
Principle of Paper chromatography
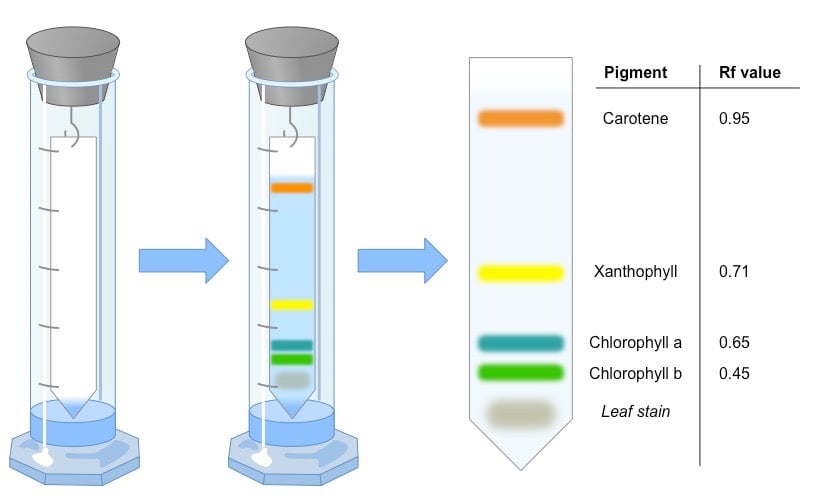
The principle of separation is mainly partition rather than adsorption. Substances are distributed between a stationary phase and a mobile phase. Cellulose layers in filter paper contain moisture which acts as a stationary phase. Organic solvents/buffers are used as mobile phase. The developing solution travels up the stationary phase carrying the sample with it. Components of the sample will separate readily according to how strongly they adsorb onto the stationary phase versus how readily they dissolve in the mobile phase.
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
1. STATIONARY PHASE AND PAPERS
- Whatman filter papers of different grades like No.1, No.2, No.3, No.4, No.20, No.40, No.42 etc
- In general the paper contains 98-99% of α-cellulose, 0.3 – 1% β -cellulose.
Other modified papers
- Acid or base washed filter paper
- Glass fiber type paper.
- Hydrophilic Papers – Papers modified with methanol, formamide, glycol, glycerol etc.
- Hydrophobic papers – acetylation of OH groups leads to hydrophobic nature, hence can be used for reverse phase chromatography.
- Impregnation of silica, alumna, or ion exchange resins can also be made.
2. PAPER CHROMATOGRAPHY MOBILE PHASE
- Pure solvents, buffer solutions or mixture of solvents can be used.
Hydrophilic mobile phase
- Isopropanol: ammonia:water 9:1:2
- Methanol : water 4:1
- N-butanol : glacial acetic acid : water 4:1:5
Hydrophobic mobile phases
- dimethyl ether: cyclohexane kerosene : 70% isopropanol
- The commonly employed solvents are the polar solvents, but the choice depends on the nature of the substance to be separated.
- If pure solvents do not give satisfactory separation, a mixture of solvents of suitable polarity may be applied.
3. CHROMATOGRAPHIC CHAMBER
- The chromatographic chambers are made up of many materials like glass, plastic or stainless steel . Glass tanks are preferred most.
- They are available invarious dimensional size depending upon paper length and development type.
- The chamber atmosphere should be saturated with solvent vapor.
Steps in Paper Chromatography
In paper chromatography, the sample mixture is applied to a piece of filter paper, the edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action. The basic steps include:
Selection of Solid Support
Fine quality cellulose paper with defined porosity, high resolution, negligible diffusion of the sample, and favoring good rate of movement of solvent.
Selection of Mobile Phase
Different combinations of organic and inorganic solvents may be used depending on the analyte.
Example. Butanol: Acetic acid: Water (12:3:5) is a suitable solvent for separating amino acids.
Saturation of Tank
The inner wall of the tank is wrapped with filter paper before the solvent is placed in the tank to achieve better resolution.
Sample Preparation and Loading
If the solid sample is used, it is dissolved in a suitable solvent. Sample (2-20ul) is added on the baseline as a spot using a micropipette and air dried to prevent the diffusion.
Development of the Chromatogram
Different types of development techniques can be used:
ASCENDING DEVELOPMENT
- Like conventional type, the solvent flows against gravity.
- The spots are kept at the bottom portion of paper and kept in a chamber with mobile phase solvent at the bottom.
DESCENDING TYPE
- This is carried out in a special chamber where the solvent holder is at the top.
- The spot is kept at the top and the solvent flows down the paper.
- In this method solvent moves from top to bottom so it is called descending chromatography.
ASCENDING – DESCENDING DEVELOPMENT
- A hybrid of above two techniques is called ascending-descending chromatography.
- Only length of separation increased, first ascending takes place followed by descending.
CIRCULAR / RADIAL DEVELOPMENT
- Spot is kept at the centre of a circular paper.
- The solvent flows through a wick at the centre & spreads in all directions uniformly.
Drying of Chromatogram
After the development, the solvent front is marked and left to dry in a dry cabinet or oven.
Colorless analytes were detected by staining with reagents such as iodine vapor, ninhydrin, etc.
Radiolabeled and fluorescently labeled analytes were detected by measuring radioactivity and fluorescence respectively.
Some compounds in a mixture travel almost as far as the solvent does; some stay much closer to the baseline . The distance traveled relative to the solvent is a constant for a particular compound as long as other parameters such as the type of paper and the exact composition of the solvent are constant. The distance traveled relative to the solvent is called the Rf value.
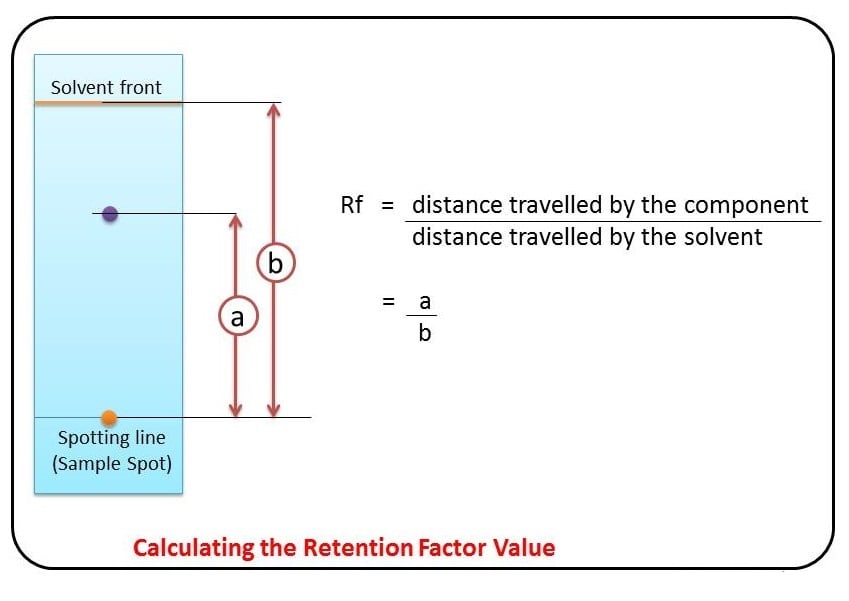
Thus, in order to obtain a measure of the extent of movement of a component in a paper chromatography experiment, “Rf value” is calculated for each separated component in the developed chromatogram. An Rf value is a number that is defined as the distance traveled by the component from the application point.
Applications of Paper Chromatography
- To check the control of purity of pharmaceuticals,
- For detection of adulterants,
- Detect the contaminants in foods and drinks,
- In the study of ripening and fermentation,
- For the detection of drugs and dopes in animals & humans
- In analysis of cosmetics
- Analysis of the reaction mixtures in biochemical labs.
Advantages of Paper Chromatography
- Paper Chromatography requires very less quantitative material.
- Paper Chromatography is cheaper compared to other chromatography methods.
- Both unknown inorganic as well as organic compounds can be identified by paper chromatography method.
- Paper chromatography does not occupy much space compared to other analytical methods or equipments.
- Excellent resolving power
Limitations of Paper Chromatography
- Large quantity of sample cannot be applied on paper chromatography.
- In quantitative analysis paper chromatography is not effective.
- Complex mixture cannot be separated by paper chromatography.
- Less Accurate compared to HPLC or HPTLC
- http://frndzzz.com/Advantages-and-Disadvantages-of-Paper-Chromatography
- https://www.slideshare.net/shaisejacob/paper-chromatography-pptnew?next_slideshow=1
- https://www.slideshare.net/shaisejacob/paper-chromatography-ppt-new
- https://www.biochemden.com/paper-chromatography/
- http://web.engr.oregonstate.edu/~rochefow/K-12%20Outreach%20Activities/Microfluidics%20&%20Pregnancy%20Test%20Kit%20Lab/paper%20chromatography_Chemguide.pdf
- https://pubs.acs.org/doi/abs/10.1021/ac60051a002
About Author
Sagar Aryal
6 thoughts on “Paper Chromatography- Definition, Types, Principle, Steps, Uses”
Examples of substances that can be effectively separated by paper chromatography is necessary.
I enjoy this write up, but the definition of Rf Values just mention distance traveled by the solute from the point from the point of application of the sample, how about the total distance traveled by the solvent? Some examples of how Rf value can be calculated is necessary.
Pls how is charging a disadvantage of filter paper in chromatography
Hi! I enjoyed reading it. Hmm just wanna know somepoints.
Why it is best to use the farthest distance traveled by a sugar if and when the solvent went over the paper and what is the purpose of heating the chromatographic paper after running the procedure?
This links are so much useful and it’s very helpful also….
Thanks for sharing important details like this. I enjoyed reading your site, and love to know the latest updates.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Practical videos | 14–16 years
- 1 Access free videos to support your teaching
- 2 Paper chromatography
- 3 Rates of reaction
- 4 Simple distillation
- 5 Enthalpy change of combustion
- 6 Conservation of mass
- 7 Electrolysis of aqueous solutions
- 8 Halogen displacement reactions
- 9 Identifying ions
- 10 Preparing a soluble salt
- 11 Reactivity series of metals
- 12 Simple titration
- 13 Temperature change (neutralisation)
- 14 Potable water
Paper chromatography

- No comments
Investigate the separation of inks using paper chromatography
The value of experiencing live practical work cannot be overstated. Numerous studies provide evidence of its value in terms of learner engagement, understanding, results and the likelihood of continuing to study chemistry or work in a related field.
Use this video to complement live practical work, or to help learners understand the methods, equipment and skills when they cannot access the lab.
Source: © Royal Society of Chemistry
Investigate the separation of inks using this paper chromatography video, including a step-by-step method, animation and calculation support for learners
Chapter titles: 00:10 Introduction to chromatography; 00:44 Carrying out the experiment; 03:59 Results; 04:43 Animation; 05:11 Alternative phases; 06:05 Calculating R f values.
- Teacher notes
Full teacher notes are available in the supporting resources booklet (also available in MS Word ), including ideas for how to use this video and the accompanying activites and answers to use as part of your teaching.
Get the resources

Supporting resources
Detailed teacher notes, learner activities and answers
Learner slides
Integrated instructions, Frayer models and Johnstone's triangle

- Technician notes
Equipment, chemicals, hazards and disposal information
Notes on running the practical experiments
This experiment is designed to cover most of the questions asked in a variety of syllabuses. You or your school’s technician will have to do some trial and error to get the ink composition correct to get the desired results. The suggestion is that one ink is not soluble in the water (solvent) and so doesn’t move, one colour moves to the top of the paper (learners can identify this as the most soluble) and there is a colour in common between two of the samples. Although the video and worksheets refer to particular colours seen in the video, the actual colours don’t matter and it is their characteristics that are key to the success of the practical.
Use the video’s animation and Johnstone’s triangle , available in the PowerPoint slides , to help learners link their observations to what’s going on at the submicroscopic level.

Health, safety and technical notes
You may want to demonstrate the experiment using a different solvent, such as ethanol ( CLEAPSS student safety sheet 60 ). Use a lid and wear eye protection (safety glasses to EN166 F) when using the alcohol. To show a different stationary phase, use a TLC plate.
Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Refer to SSERC/CLEAPSS Hazcards, recipe books and student safety sheets. Hazard classification may vary depending on supplier. Download the technician notes for the full equipment list, safety notes and tips.
- Cut a piece of filter paper to fit inside a beaker (size of the beaker is not relevant) so that it does not curve and lies flat, not touching the edges of the beaker.
- Using a pencil, draw an origin line on the paper and label 1, 2 and 3 at equal distances below the line.
- Using a separate capillary tube for each ink, transfer a small spot onto the correct labelled position on the origin line.
- Add a small amount of the solvent to just cover the bottom of the beaker.
- Check the paper is the right length by lining it up on the outside of the beaker so that the water is below the origin line. Roll the paper round a splint and hold it with a paper clip.
- Place the paper inside the beaker. Make sure it just touches the water and it is vertical.
- Remove the filter paper from the beaker when the solvent has nearly reached the top of the filter paper.
- Leave the filter paper to dry or use a hairdryer.
- Measure the distances from the origin line to the middle of the colours and from the origin line to the solvent front to find the R f values.
Find the integrated instructions for this experiment in the PowerPoint slides .
Real-world contexts
- Highlight real-world uses of chromatography with the article Poisoned by milk .
- Complete this project on the chemistry of food in a sequence of timetabled chemistry lessons, STEM clubs or during an activity day to provide context to analytical techniques.
- Engage younger learners with this investigation to solve who stole a famous painting.
- Watch this video to see how senior science manager Paul uses liquid chromatography in his job at British Sugar.
Learners will need to have a clear understanding of the following scientific terminology:
- Chromatography – a technique for separating mixtures of soluble substances.
- Chromatogram – the results of separating mixtures by chromatography.
- Mixture – two or more different substances, not chemically joined together.
- Solvent – the substance a solute dissolves in to form a solution.
- Solute – a substance that will dissolve in a solvent.
- Dissolve – when a solute mixes completely with a solvent to produce a solution.
- Solution – a mixture formed by a solute and a solvent.
- Soluble – a substance that will dissolve.
- Insoluble – a substance that will not dissolve.
- Pure – a substance made of only one element or compound.
- Impure – a substance made of more than one element or compound.
- R f value – the ratio of the solute’s distance travelled to the solvent’s distance travelled.
- Stationary phase – the substance that does not move, eg paper.
- Mobile phase – the solvent that moves up the stationary phase, eg water.
- Origin line – the mark you add the samples to at the start of chromatography.
- Solvent front – the distance travelled by the solvent.
- Volatile – evaporates easily.
You will find a template, example Frayer model and suggested answers for the terms: ‘chromatography’, ‘mobile phase’, ‘stationary phase’, ‘origin line’ and ‘solvent front’ in the PowerPoint slides . Find more examples and tips on how to use Frayer models in your teaching.
Cross-curriculum links and skills
Learners will use key maths skills in the practical including:
- Measuring distances accurately.
- Giving answers to a specified decimal place.
Common misconceptions
Using pen to draw the origin line. Bring attention to using pencil and encourage learners to think why pens might interfere with the separation.
Putting too much solvent so learners submerge the origin line. Ensure the solvent is below the origin line and learners understand why.
Allowing the solvent to travel too far up the paper. The solvent front will be lost meaning the R f value cannot be calculated.
All mixtures are separated using only one separating technique . Practise a range of techniques with learners and highlight that chromatography is a method of separation for analysing a mixture but does not achieve separation of the entire test mixture. Carry out further methods once purity has been determined and/or the impurities identified.
The solvent/mobile phase can only be water . Use different solvents, such as sodium chloride solution and ethanol.
The stationary phase in chromatography can only be paper . Introduce alternatives, such as thin layer chromatography using silica on a plastic backing or aluminium oxide coated plates.
Dyes have a preference or ‘like’ to be in the stationary or the mobile phase . Avoid anthropomorphising as analogies like this can be a barrier to deep learning. Use ‘is attracted to’ or ‘hydrophobic/philic’ instead.
More resources
- Do this chromatography practical using dye from different coloured sweets with your 11–16 year-old learners and read the accompanying article to find out more about baking with sprinkles.
- Make cross-curricular links to biology and investigate the pigments in a leaf .
- Practical planning: spot the mistakes includes an exam-style answer on chromatography and is part of the Teaching science skills series.
Paper chromatography supporting resources
Paper chromatography technician notes, paper chromatography slides, additional information.
The original video script, supporting resources and slides were written by Karen Marshall. The technician notes were adapted by Sandrine Bouchelkia.

More Sandrine Bouchelkia

Enthalpy change of combustion of ethanol | practical videos | 14–16 years

Simple distillation | practical videos | 14–16 years

Rates of reaction | practical videos | 14–16 years

Access free videos to support your teaching

Rates of reaction

Simple distillation

Enthalpy change of combustion

Conservation of mass

Electrolysis of aqueous solutions

Halogen displacement reactions

Identifying ions

Preparing a soluble salt

Reactivity series of metals

Simple titration

Temperature change (neutralisation)

Potable water
- 11-14 years
- 14-16 years
- Practical experiments
- Practical skills and safety
- Observing and measuring
- Recording data
- Applications of chemistry
- Chromatography
Specification
- the distance travelled by the substance divided by the distance travelled by the solvent
- the Rf value should be the same if it is the same substance (under the same conditions)
Related articles

Alkali sponge fights climate change
2024-08-16T07:30:00Z By Nina Notman
Rechargeable hydrolysis produces a sustainable method for carbon capture

What’s the world’s strongest glue?
2024-07-19T05:32:00Z By Kit Chapman
Find out about the world’s stickiest glue and how adhesives bond things together

How to teach titration post-16
2024-07-08T05:32:00Z By Jo Haywood
Tips for teaching practical titration techniques and the underlying theory
No comments yet
Only registered users can comment on this article., more practical.
By Karen Marshall and Sandrine Bouchelkia
Video and resources investigating the heat energy change of combustion of ethanol
By Dorothy Warren and Sandrine Bouchelkia
Video and resources showing how to separate water from a coloured solution
Video and resources showing how the concentration of sodium thiosulfate solution affects its rate of reaction with hydrochloric acid
- Contributors
- Email alerts
Site powered by Webvision Cloud

Paper Chromatography
- Explain how chromatography works.
- Explain how intermolecular forces apply to chromatography.
- Calculate the R f factor for chemicals tested using paper chromatography.
- Identify errors and explain their effect on experimental data.
- Draw conclusions based on experimental data.
Related Textbook
Please read section 14.7 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information that will help you succeed on this assignment.

Introduction
Paper chromatography is a process that uses intermolecular forces to separate the components of a sample. The more soluble a component of a sample is in the mobile phase, the further up the paper the component will travel. Figure 1.o explains this concept, provides an example of setting up this week’s experiment, and defines some key terms that apply to paper chromatography. When the samples on a piece of chromatography paper are developed, it’s called a chromatogram. An example of a chromatogram is provided at the top right of this page.
We are using two different solvents, or mobile phases , during this experiment. Water will be used in the first trial and isopropyl alcohol (also known as 2-propanol) will be used in the second trial. These two chemicals have different degrees of polarity. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Water is very polar. The two pairs of non-bonding electrons on the oxygen and the small structure of this molecule contribute to its strong polarity. Isopropyl alcohol also has two pairs of nonbonding electrons on the oxygen; however, it also has several carbon atoms and many more hydrogens present that increase the structure’s size and diminish the molecule’s polarity. This molecule is slightly polar, significantly less polar than the water. Figure 2.0 illustrates the structures of water and isopropyl alcohol.
Be sure to define the following terms before beginning this experiment. The video in Figure 1.0 discusses these terms. You will need to define these terms in the lab report you will complete on this lab later in this class.
- Stationary Phase
- Mobile Phase
Figure 1.0 – Paper and thin layer chromatography. (2)
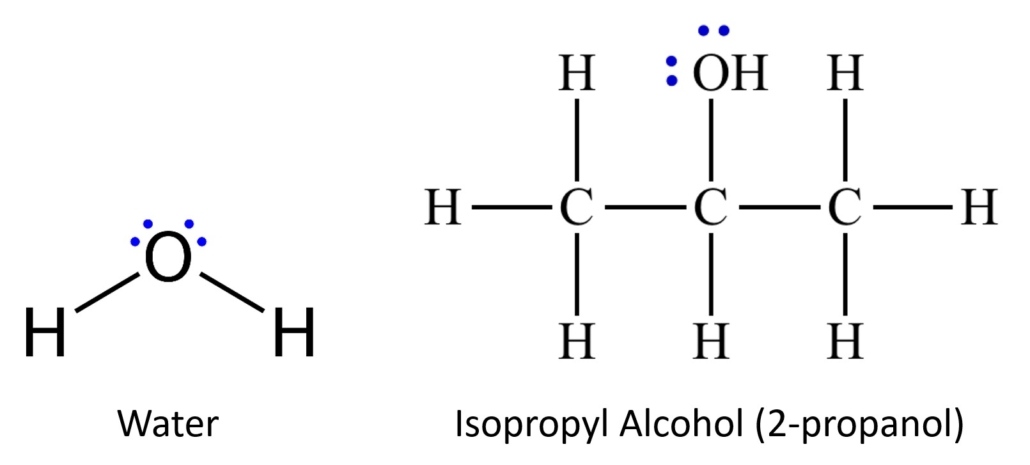
Figure 2.0 – Structures of water and isopropyl alcohol. Notice the nonbonding electrons in blue.
In this experiment, we will test several different markers to see what pigments are present in each. Based on the experimental results, we will also determine if those pigments are polar or nonpolar.
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled. ALL values in this lab MUST be measured in metric units.
Paper Chromatography Data Table
Safety Concerns
Isoproply alcohol is highly flammable and can cause eye irritation. Please see the SDS sheet on isopropyl alcohol for more information. (1)
Experimental Procedure
Chemicals and Supplies
90% Isopropyl Alcohol
500mL beaker
White, matte printer paper (do not use any paper that has a sheen)
Crayola Washable Markers or similar washable marker
Sharpe Marker
Metric Ruler
Plastic Wrap or Aluminum Foil
Rubber Band
- Cut two rectangular pieces of paper approximately 9cm long by 5 cm wide. These are your chromatography paper for this experiment.
- Draw a pencil line approximately 2cm from the bottom on each piece of paper (on the 5cm/shorter side).
- Using the Sharpe Marker, place a dot approximately 1cm from the edge of the chromatography paper on the pencil line. Place a dot on the paper approximately 1cm from the Sharpe dot using the green marker. Using the black marker (NOT the Sharpe), place a dot on the paper approximately 1cm from the green dot. Pick another color from the pack of markers and place a dot on the chromatography paper approximately 1cm from the black dot. See Figure 3.0 for an example of the setup for this step.
- Attach the marked chromatography paper to the glass rod using a piece of tape. The marker spots should be at the bottom of the chromatography paper and the glass rod at the top.
- Place the paper in the empty 500mL beaker, using the glass rod to suspend the chromatography paper in it (See Figure 4.o).
- Determine (eyeball) how much DI water should be added to the beaker without allowing the water to touch the marker dots.
- Remove the chromatography paper and add the appropriate amount of DI water to the beaker as determined in Step #5.
- Carefully add the chromatography paper to the beaker as in Step #5, being careful to allow the full end of the paper to touch the water without allowing the water level in the beaker to rise above the marker dots. If the water level in the beaker appears that it will be above the marker dots, remove some of the water from the beaker and try again.
NOTE: If the dot touches the water and start to run, you will need to prepare a new piece of chromatography paper and start again. Water will travel up the chromatography paper; however, the dots should not be submerged below the water level in the beaker.
- Cover the top of the beaker with aluminum foil or plastic wrap. Secure the foil/plastic wrap with the rubber band. The glass rod will probably stick out of the sides of the covering on the top of the beaker. This is ok.
- Allow the DI water to travel up the chromatography paper until the water level is either approximately 2cm from the top or the water level does not move for 3 minutes.
NOTE: The travel times for each solvent (water vs alcohol) will likely vary.
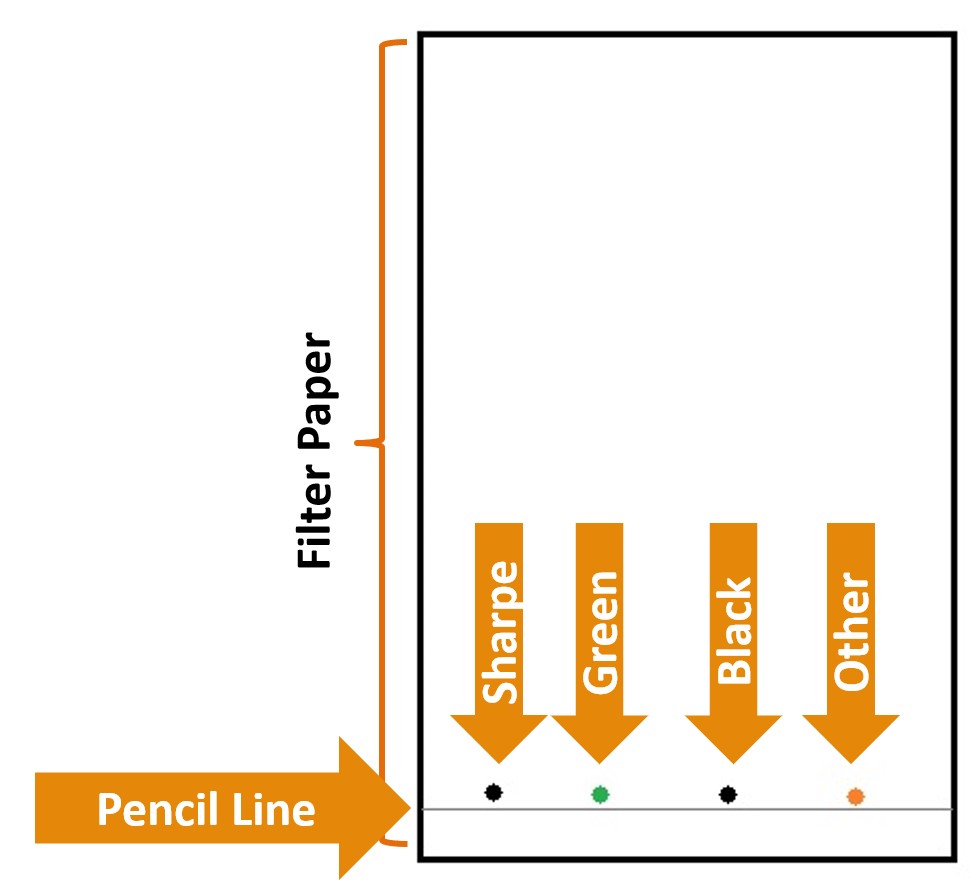
Figure 3.0 – Spotting the paper.
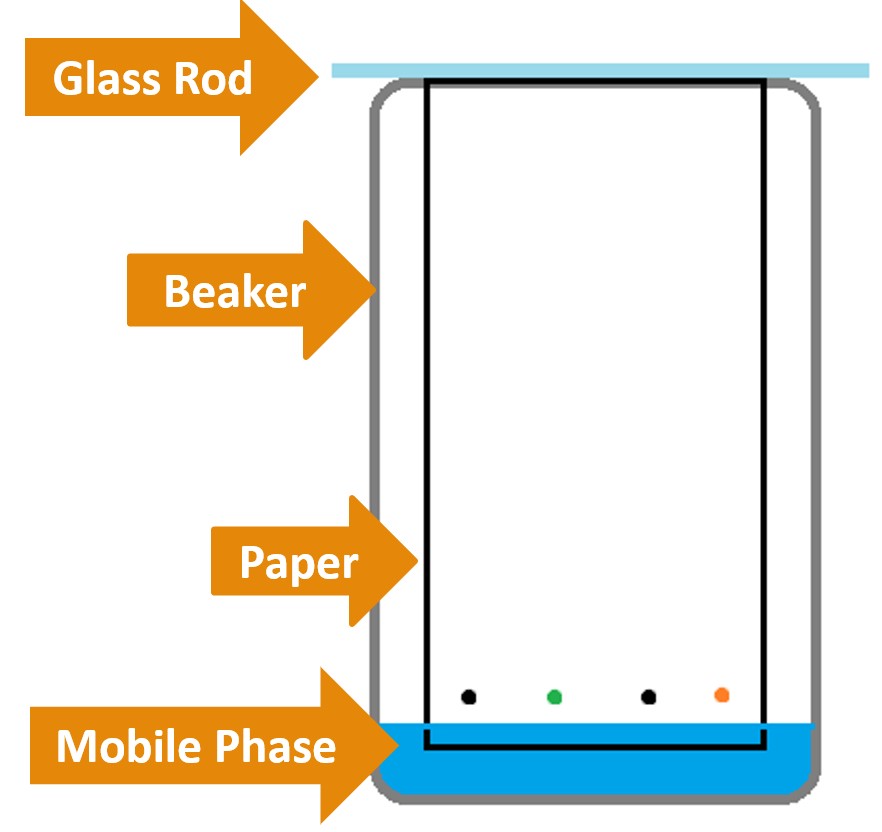
Figure 4.0 – Paper chromatography experimental setup.
- Remove the chromatography paper from the beaker. Immediately mark the solvent front. Allow the chromatography paper to dry.
- Measure the distances the colors have traveled. BE SURE TO USE CM. This was outlined in the video you viewed at the start of this experiment. Record your data on the data table provided. Do not dispose of the chromatographypaper yet.
- Repeat Steps #3 through #13 using 90% Isopropyl Alcohol instead of DI water.
- Take the picture described below after both spotted chromatography papers have been developed.
Take a photo of both chromatography papers at this experiment’s end. The pieces of paper can either be included in the same photo or two photos can be taken, each showing only one of the dry chromatography papers. A piece of paper that includes your name, CHEM 1000, and the semester you are taking this class (for example, if you take this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in this photo. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. The assignment tied to this laboratory will not be graded unless all of these photos have been submitted to the assignment folder on Brightspace.
- Record your data on the data table provided.
- Do not dispose of the dry pieces of chromatography paper until after you have completed the attached assignment. You will need them to answer some of the post-lab questions.
Waste Disposal
- Place the used paper and any scraps in the trash.
- Dispose of the chemicals down the sink. Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
Now that you have completed the experiment and recorded your data:
- Complete and submit the data table and the following questions for this experiment to the assignment folder on Brightspace. You must complete and submit the data table and the rest of your assignment or you will receive an automatic zero for this experiment. Your submission should be handwritten. Typed submissions will not be accepted.
- Submit the photograph(s) of the dried chromatography papers as outlined in the box under Step #14. You will receive a zero for this assignment if you fail to submit the photo.
- This assignment is worth 10 points.
Paper Chromatography Questions
- What is/are the mobile phase(s) in this experiment? Identify the mobile phase for both trials. (1 point)
- What is/are the stationary phase(s) in this experiment? Identify the stationary phase for both trials. (1 point)
- Calculate the R f factor for each color you tested. Be sure to calculate this for each of the different colors that appeared for each of the four marker colors tested. For instance, if a color is separated into multiple spots, you need to figure the R f factor for each spot. Show all of your work. No points will be awarded if work is missing. (4 points)
- Of the markers you tested, which are washable in water? Explain your reasoning using your experimental data. (1 point)
- Of the markers you tested, which would you predict are soluble in nonpolar solvents? Explain your reasoning using your experimental data. (1 point)
- What are two sources of error for this experiment? How would each affect your scientific data? (2 points)
(1) Finn Scientific. Isopropyl Alcohol 40-90% Safety Data Sheet. https://www.flinnsci.com/sds_420.5-isopropyl-alcohol-40-90/sds_420.5/ (accessed July 31, 2023).
(2) Fuse School. Tutorial: Paper and Thin Layer Chromatography [Video file]. YouTube, June 6, 2013. https://youtu.be/J8r8hN05xXk (July 31, 2023).
This page was created on July 31, 2023, and was last updated on August 3, 2023.
©2023 Catherine Haslag. All Rights Reserved.

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center


COMMENTS
Paper Chromatography is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper. Learn the principle, procedure of Paper Chromatography along with its types and applications.
The process has two distinct phases before it gets completed; these phases are the stationary phase and the mobile phase. The stationary phase (paper chromatography) is when the paper absorbs the pigment, and the mobile phase is when the solvent is used in the process moves through the paper. The moving solvent distinguishes the proportion of the different component of the mixture dissolved.
Paper Chromatography- Introduction, Types, Principle, Instrumentation, Steps, Rf values, Applications, Advantages and Limitations. Paper Chromatography.
Paper Chromatography In paper chromatography, the stationary phase is a very uniform absorbent paper. The mobile phase is a suitable liquid solvent or mixture of solvents.
What Is Paper Chromatography? Paper chromatography is one of the types of chromatography procedures that runs on a piece of specialized paper. It is a planar chromatography system wherein a cellulose filter paper acts as a stationary phase where compounds are separated. It is a very inexpensive way to separate different dissolved chemical substances and, thus, a powerful analytical tool ...
Investigate the separation of inks using paper chromatography The value of experiencing live practical work cannot be overstated. Numerous studies provide evidence of its value in terms of learner engagement, understanding, results and the likelihood of continuing to study chemistry or work in a related field.
Paper chromatography is a process that uses intermolecular forces to separate the components of a sample. The more soluble a component of a sample is in the mobile phase, the further up the paper the component will travel. Figure 1.o explains this concept, provides an example of setting up this week's experiment, and defines some key terms ...
Paper chromatography, in analytical chemistry, a technique for separating dissolved chemical substances by taking advantage of their different rates of migration across sheets of paper. It is an inexpensive but powerful analytical tool that requires very small quantities of material.
Paper Chromatography Prelaboratory Assignment 1. The mobile phase. a. What is the function of the mobile phase for developing a chromatogram?
Study with Quizlet and memorize flashcards containing terms like chromatography, stationary phase, mobile phase and more.
PAPER CHROMATOGRAPHY. This page is an introduction to paper chromatography - including two way chromatography. Chromatography is used to separate mixtures of substances into their components. All forms of chromatography work on the same principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a ...
Thin- Layer chromatography (TLC) is a technique in which compounds are separated on a thin layer of adsorbent material, typically a coating of silica gel on a glass plate or plastic sheet. Paper chromatography is an analytical method used to separate
The students separated food dyes using paper chromatography. They identified multiple color spots from each dye. Blue dye produced blue and red spots, green produced yellow and blue, and yellow produced yellow and red. Red dye only produced red spots. Rf values were calculated and showed red from yellow dye had the lowest polarity while blue spots had the highest. This experiment demonstrated ...
Chemists and biologists also use chromatography to identify the compounds present in a sample, such as plants. In this science project, you will explore how the use of different stationary and mobile phases can affect the separation of marker ink. You will use chalk, chromatography paper, isopropyl alcohol, acetone, turpentine, and water.
The difference between paper and thin layer chromatography is that in paper chromatography the ink separates when the ink moves towards the top, the stationary phase is the absorbent paper, and the mobile phase is the solvent which is a liquid.
Paper Chromatography Background Information: Paper Chromatography is used for separating chemicals based on their different properties (ex: solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and inorganic materials.
Chromatography is a process that involves passing a liquid or gas through a filter material. It also has many factors in paper chromatography, such as stationary phase, mobile phase and Rf values (retention factor).
Unit 2, Biology, Chromatography assignment, complete to a distinction This is my effort of doing the unit 2 chromatography practical, I look...