Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 September 2019

Improving grain yield, stress resilience and quality of bread wheat using large-scale genomics
- Philomin Juliana 1 ,
- Jesse Poland ORCID: orcid.org/0000-0002-7856-1399 2 ,
- Julio Huerta-Espino 3 ,
- Sandesh Shrestha 2 ,
- José Crossa ORCID: orcid.org/0000-0001-9429-5855 1 ,
- Leonardo Crespo-Herrera 1 ,
- Fernando Henrique Toledo 1 ,
- Velu Govindan 1 ,
- Suchismita Mondal ORCID: orcid.org/0000-0002-8582-8899 1 ,
- Uttam Kumar 4 ,
- Sridhar Bhavani 1 ,
- Pawan K. Singh 1 ,
- Mandeep S. Randhawa 5 ,
- Xinyao He 1 ,
- Carlos Guzman 1 , 6 ,
- Susanne Dreisigacker ORCID: orcid.org/0000-0002-3546-5989 1 ,
- Matthew N. Rouse 7 ,
- Yue Jin 7 ,
- Paulino Pérez-Rodríguez 8 ,
- Osval A. Montesinos-López 9 ,
- Daljit Singh 2 ,
- Mohammad Mokhlesur Rahman ORCID: orcid.org/0000-0003-1831-3198 2 , 10 ,
- Felix Marza 11 &
- Ravi Prakash Singh ORCID: orcid.org/0000-0002-4676-5071 1
Nature Genetics volume 51 , pages 1530–1539 ( 2019 ) Cite this article
12k Accesses
172 Citations
83 Altmetric
Metrics details
- Genome-wide association studies
- Plant breeding
Bread wheat improvement using genomic tools is essential for accelerating trait genetic gains. Here we report the genomic predictabilities of 35 key traits and demonstrate the potential of genomic selection for wheat end-use quality. We also performed a large genome-wide association study that identified several significant marker–trait associations for 50 traits evaluated in South Asia, Africa and the Americas. Furthermore, we built a reference wheat genotype–phenotype map, explored allele frequency dynamics over time and fingerprinted 44,624 wheat lines for trait-associated markers, generating over 7.6 million data points, which together will provide a valuable resource to the wheat community for enhancing productivity and stress resilience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Elucidating the genetics of grain yield and stress-resilience in bread wheat using a large-scale genome-wide association mapping study with 55,568 lines

Harnessing landrace diversity empowers wheat breeding
Applying genomic resources to accelerate wheat biofortification
Data availability.
The phenotyping data for the lines used in this study are available in Supplementary Data 1 . The marker P values, additive effects and percentage variation explained by each marker are available in Supplementary Table 2 . The genomic fingerprints of 44,624 wheat lines for 195 trait-associated markers are available in Supplementary Table 4a–d . The raw genotyping data for the lines are available in FigShare ( https://doi.org/10.6084/m9.figshare.8940257.v1 ).
CGIAR Research Program on Wheat. Wheat in the World https://wheat.org/wheat-in-the-world/ (CRP, 2018).
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4 , 178–202 (2015).
PubMed PubMed Central Google Scholar
Shiferaw, B. et al. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 5 , 291–317 (2013).
Google Scholar
Curtis, T. & Halford, N. G. Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 164 , 354–372 (2014).
CAS PubMed PubMed Central Google Scholar
FAOSTAT http://www.fao.org/faostat/ (FAO, 2018).
Ray, D. K., Mueller, N. D., West, P. C. & Foley, J. A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8 , e66428 (2013).
Ray, D. K., Ramankutty, N., Mueller, N. D., West, P. C. & Foley, J. A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 3 , 1293 (2012).
PubMed Google Scholar
Singh, R. P. et al. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54 , 303–322 (2016).
CAS PubMed Google Scholar
Wheeler, T. & von Braun, J. Climate change impacts on global food security. Science 341 , 508–513 (2013).
Zampieri, M., Ceglar, A., Dentener, F. & Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 12 , 064008 (2017).
Meuwissen, T. H. E., Hayes, B. J. & Goddard, M. E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 157 , 1819–1829 (2001).
Meuwissen, T., Hayes, B. & Goddard, M. Genomic selection: a paradigm shift in animal breeding. Anim. Front. 6 , 6–14 (2016).
Heffner, E. L., Sorrells, M. E. & Jannink, J.-L. Genomic selection for crop improvement. Crop Sci. 49 , 1–12 (2009).
CAS Google Scholar
Crossa, J. et al. Genomic selection in plant breeding: methods, models, and perspectives. Trends Plant Sci. 22 , 961–975 (2017).
Yu, J. & Buckler, E. S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 17 , 155–160 (2006).
Thornsberry, J. M. et al. Dwarf8 polymorphisms associate with variation in flowering time. Nat. Genet. 28 , 286–289 (2001).
Quarrie, S. A. A. et al. A high-density genetic map of hexaploid wheat ( Triticum aestivum L.) from the cross Chinese Spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 110 , 865–880 (2005).
Snape, J. W. et al. Dissecting gene × environmental effects on wheat yields via QTL and physiological analysis. Euphytica 154 , 401–408 (2007).
International Wheat Genome Sequencing Consortium (IWGSC) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361 , eaar7191 (2018).
Lantican, M. A. et al. Impacts of International Wheat Improvement Research, 1994–2014 (CIMMYT, 2016).
Poland, J. et al. Genomic selection in wheat breeding using genotyping-by-sequencing. Plant Genome 5 , 103–113 (2012).
Poland, J. A. & Rife, T. W. Genotyping-by-sequencing for plant breeding and genetics. Plant Genome 5 , 92–102 (2012).
Elshire, R. J. et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6 , e19379 (2011).
Helguera, M., Khan, I. A., Kolmer, J., Lijavetzky, D. & Dubcovsky, J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use. Crop Sci. 43 , 1839–1847 (2003).
Ma, L. et al. TaGS5-3A , a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnol. J. 14 , 1269–1280 (2016).
Rustgi, S. et al. Genetic dissection of yield and its component traits using high-density composite map of wheat chromosome 3A: bridging gaps between QTLs and underlying genes. PLoS ONE 8 , e70526 (2013).
Mason, R. E., Mondal, S., Beecher, F. W. & Hays, D. B. Genetic loci linking improved heat tolerance in wheat ( Triticum aestivum L.) to lower leaf and spike temperatures under controlled conditions. Euphytica 180 , 181–194 (2011).
Zang, X. et al. Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol. 17 , 14 (2017).
Hou, J. et al. Global selection on sucrose synthase haplotypes during a century of wheat breeding. Plant Physiol. 164 , 1918–1929 (2014).
Díaz, A., Zikhali, M., Turner, A. S., Isaac, P. & Laurie, D. A. Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat ( Triticum aestivum ). PLoS ONE 7 , e33234 (2012).
Griffiths, S. et al. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor. Appl. Genet. 119 , 383–395 (2009).
Yan, L. et al. Positional cloning of the wheat vernalization gene VRN1 . Proc. Natl Acad. Sci. USA 100 , 6263–6268 (2003).
Yan, L. et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT . Proc. Natl Acad. Sci. USA 103 , 19581–19586 (2006).
Himi, E. & Noda, K. Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 143 , 239–242 (2005).
Sun, X., Bai, G., Carver, B. F. & Bowden, R. Molecular mapping of wheat leaf rust resistance gene Lr42 . Crop Sci. 50 , 59 (2010).
Kassa, M. T. et al. Highly predictive SNP markers for efficient selection of the wheat leaf rust resistance gene Lr16 . BMC Plant Biol. 17 , 45 (2017).
Edae, E. A., Pumphrey, M. O. & Rouse, M. N. A genome-wide association study of field and seedling response to individual stem rust pathogen races reveals combinations of race-specific genes in North American spring wheat. Front. Plant Sci. 9 , 52 (2018).
Nirmala, J. et al. Markers linked to wheat stem rust resistance gene Sr11 effective to Puccinia graminis f. sp. tritici race TKTTF. Phytopathology 106 , 1352–1358 (2016).
Turner, M. K., Jin, Y., Rouse, M. N. & Anderson, J. A. Stem rust resistance in ‘Jagger’ winter wheat. Crop Sci. 56 , 1719–1725 (2016).
Gao, L. et al. Genetic characterization of stem rust resistance in a global spring wheat germplasm collection. Crop Sci. 57 , 2575–2589 (2017).
Hiebert, C. W., Rouse, M. N., Nirmala, J. & Fetch, T. Genetic mapping of stem rust resistance to Puccinia graminis f. sp. tritici race TRTTF in the Canadian wheat cultivar harvest. Phytopathology 107 , 192–197 (2017).
Mago, R. et al. An accurate DNA marker assay for stem rust resistance gene Sr2 in wheat. Theor. Appl. Genet . 122 , 735–744 (2011).
Krattinger, S. G. et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323 , 1360–1363 (2009).
Rouse, M. N., Talbert, L. E., Singh, D. & Sherman, J. D. Complementary epistasis involving Sr12 explains adult plant resistance to stem rust in Thatcher wheat ( Triticum aestivum L.). Theor. Appl. Genet. 127 , 1549–1559 (2014).
Hiebert, C. W. et al. Major gene for field stem rust resistance co-locates with resistance gene Sr12 in ‘Thatcher’ wheat. PLoS ONE 11 , e0157029 (2016).
Yang, E. N. et al. QTL analysis of the spring wheat ‘Chapio’ identifies stable stripe rust resistance despite inter-continental genotype × environment interactions. Theor. Appl. Genet. 126 , 1721–1732 (2013).
McDonald, D. B., McIntosh, R. A., Wellings, C. R., Singh, R. P. & Nelson, J. C. Cytogenetical studies in wheat XIX. Location and linkage studies on gene Yr27 for resistance to stripe (yellow) rust. Euphytica 136 , 239–248 (2004).
Singh, R. P., William, H. M., Huerta-Espino, J. & Crosby, M. Identification and mapping of gene Yr31 for resistance to stripe rust in Triticum aestivum cultivar Pastor. In Proc. 10th International Wheat Genetics Symposium . (eds Pogna N. E. et al.) 411–413 (Instituto Sperimentale per la Cerealicoltura, 2003).
Lu, P. et al. Fine genetic mapping of spot blotch resistance gene Sb3 in wheat ( Triticum aestivum ). Theor. Appl. Genet. 129 , 577–589 (2016).
Morris, C. F. Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol. Biol . 48 , 633–647 (2002).
Færgestad, E. M. et al. Relationships between storage protein composition, protein content, growing season and flour quality of bread wheat. J. Sci. Food Agric . 84 , 877–886 (2004).
Zhen, S. et al. Deletion of the low-molecular-weight glutenin subunit allele Glu-A3a of wheat ( Triticum aestivum L.) significantly reduces dough strength and breadmaking quality. BMC Plant Biol. 14 , 367– (2014).
Bonafede, M. D., Tranquilli, G., Pflüger, L. A., Peña, R. J. & Dubcovsky, J. Effect of allelic variation at the Glu-3/Gli-1 loci on breadmaking quality parameters in hexaploid wheat ( Triticum aestivum L.). J. Cereal Sci. 62 , 143–150 (2015).
Wang, Y. et al. Low molecular weight glutenin subunit gene Glu-B3h confers superior dough strength and breadmaking quality in wheat ( Triticum aestivum L.). Sci. Rep. 6 , 27182 (2016).
Cooper, J. K., Stromberger, J. A., Morris, C. F., Bai, G. & Haley, S. D. End-use quality and agronomic characteristics associated with the Glu-B1al high-molecular-weight glutenin allele in U.S. hard winter wheat. Crop Sci. 56 , 2348–2353 (2016).
Maucher, T., Figueroa, J. D. C., Reule, W. & Peņa, R. J. Influence of low molecular weight glutenins on viscoelastic properties of intact wheat kernels and their relation to functional properties of wheat dough. Cereal Chem . 86 , 372–375 (2009).
Guzmán, C. et al. Sources of the highly expressed wheat bread making ( wbm ) gene in CIMMYT spring wheat germplasm and its effect on processing and bread-making quality. Euphytica 209 , 689–692 (2016).
Uauy, C., Distelfeld, A., Fahima, T., Blechl, A. & Dubcovsky, J. A. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314 , 1298–1301 (2006).
Avni, R. et al. Functional characterization of GPC-1 genes in hexaploid wheat. Planta 239 , 313–324 (2014).
Assanga, S. O. et al. Mapping of quantitative trait loci for grain yield and its components in a US popular winter wheat TAM 111 using 90K SNPs. PLoS ONE 12 , e0189669 (2017).
Ma, D., Yan, J., He, Z., Wu, L. & Xia, X. Characterization of a cell wall invertase gene TaCwi-A1 on common wheat chromosome 2A and development of functional markers. Mol. Breed. 29 , 43–52 (2012).
Hanif, M. et al. TaTGW6-A1 , an ortholog of rice TGW6 , is associated with grain weight and yield in bread wheat. Mol. Breed. 36 , 1 (2016).
Qin, L. et al. TaGW2 , a good reflection of wheat polyploidization and evolution. Front. Plant Sci . 8 , 318 (2017).
Juliana, P. et al. Prospects and challenges of applied genomic selection—a new paradigm in breeding for grain yield in bread wheat. Plant Genome 11 , 180017 (2018).
Crossa, J. et al. Genomic prediction in CIMMYT maize and wheat breeding programs. Heredity 112 , 48–60 (2014).
Reif, J. C., Zhao, Y., Würschum, T., Gowda, M. & Hahn, V. Genomic prediction of sunflower hybrid performance. Plant Breed. 132 , 107–114 (2013).
Voss-Fels, K. P., Cooper, M. & Hayes, B. J. Accelerating crop genetic gains with genomic selection. Theor. Appl. Genet . 132 , 669–686 (2019).
Pryce, J. E. & Daetwyler, H. D. Designing dairy cattle breeding schemes under genomic selection: a review of international research. Anim. Prod. Sci. 52 , 107–114 (2012).
Hayes, B. J., Bowman, P. J., Chamberlain, A. J. & Goddard, M. E. Invited review: Genomic selection in dairy cattle: progress and challenges. J. Dairy Sci. 92 , 433–443 (2009).
García-Ruiz, A. et al. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl Acad. Sci . USA 113 , E3995–E4004 (2016).
Luan, T. et al. The accuracy of genomic selection in Norwegian red cattle assessed by cross-validation. Genetics 183 , 1119–1126 (2009).
Lenz, P. R. N. et al. Factors affecting the accuracy of genomic selection for growth and wood quality traits in an advanced-breeding population of black spruce ( Picea mariana ). BMC Genom. 18 , 335 (2017).
Moser, G., Khatkar, M. S., Hayes, B. J. & Raadsma, H. W. Accuracy of direct genomic values in Holstein bulls and cows using subsets of SNP markers. Genet. Sel. Evol. 42 , 37 (2010).
Bariana, H. S. & Mcintosh, R. A. Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome 36 , 476–482 (1993).
Cruz, C. D. et al. The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae . Crop Sci. 56 , 990–1000 (2016).
Zhang, X., Rouse, M. N., Nava, I. C., Jin, Y. & Anderson, J. A. Development and verification of wheat germplasm containing both Sr2 and Fhb1 . Mol. Breed . 36 , 85 (2016).
Zhao, Y. et al. Characterization of wheat MYB genes responsive to high temperatures. BMC Plant Biol. 17 , 208 (2017).
Zhang, Y. et al. OsMPH1 regulates plant height and improves grain yield in rice. PLoS ONE 12 , e0180825 (2017).
Brevis, J. C. & Dubcovsky, J. Effects of the chromosome region including the Gpc-B1 locus on wheat grain and protein yield. Crop Sci . 50 , 93–104 (2010).
Su, Z., Hao, C., Wang, L., Dong, Y. & Zhang, X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat ( Triticum aestivum L.). Theor. Appl. Genet . 122 , 211–223 (2011).
Singh, R. P. et al. Emergence and Spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology 105 , 872–884 (2015).
Cruz, C. D. & Valent, B. Wheat blast disease: danger on the move. Trop. Plant Pathol. 42 , 210–222 (2017).
Torriani, S. F. F. et al. Zymoseptoria tritici : a major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 79 , 8–12 (2015).
Tack, J., Barkley, A. & Nalley, L. L. Effect of warming temperatures on US wheat yields. Proc. Natl Acad. Sci. USA 112 , 6931–6936 (2015).
Trnka, M. et al. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Change 4 , 637–643 (2014).
Herrera-Foessel, S. A. et al. Lr68: A new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 124 , 1475–1486 (2012).
Juliana, P. et al. Genomic and pedigree-based prediction for leaf, stem, and stripe rust resistance in wheat. Theor. Appl. Genet . 130 , 1415–1430 (2017).
Roelfs, A. P., Singh, R. P. & Saari, E. E. Rust Diseases of Wheat: Concepts and Methods of Disease Management (CIMMYT, 1992).
Chen, S. et al. Fine mapping and characterization of Sr21 , a temperature-sensitive diploid wheat resistance gene effective against the Puccinia graminis f. sp. tritici Ug99 race group. Theor. Appl. Genet. 128 , 645–656 (2015).
Jin, Y. et al. Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici . Plant Dis. 91 , 1096–1099 (2007).
Rouse, M. N. & Jin, Y. Stem rust resistance in A-genome diploid relatives of wheat. Plant Dis . 95 , 941–944 (2011).
Stakman, E. C., Stewart, D. M. & Loegering, W. Q. Identification of Physiologic Races of Puccinia graminis var. tritici USDA-ARS E-617 (USDA, 1962).
Juliana, P. et al. Genome-wide association mapping for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor. Appl. Genet. 131 , 1405–1422 (2018).
Randhawa, M. S. et al. Identification and validation of a common stem rust resistance locus in two bi-parental populations. Front. Plant Sci. 9 , 1788 (2018).
Peterson, R. F., Campbell, A. B. & Hannah, A. E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 26c , 496–500 (1948).
Juliana, P. et al. Comparison of models and whole-genome profiling approaches for genomic-enabled prediction of Septoria tritici blotch, Stagonospora nodorum blotch, and tan spot resistance in wheat. Plant Genome https://doi.org/10.3835/plantgenome2016.08.0082 (2017).
Saari, E. E. & Prescott, J. A scale for appraising the foliar intensity of wheat diseases. Plant Dis. Rep. 59 , 376–381 (1975).
Eyal, Z., Scharen, A. L., Prescott, J. M. & van Ginkel, M. The Septoria Diseases of Wheat: Concepts and Methods of Disease Management (CIMMYT, 1987).
Simko, I. & Piepho, H.-P. The Area under the disease progress stairs: calculation, advantage, and application. Phytopathology 102 , 381–389 (2012).
Singh, P. et al. Resistance to spot blotch in two mapping populations of common wheat is controlled by multiple QTL of minor effects. Int. J. Mol. Sci. 19 , 4054 (2018).
PubMed Central Google Scholar
AACC. Approved Methods of Analysis 11th edn (American Association of Cereal Chemists, 2000); https://doi.org/10.1094/AACCIntMethod-10-05.01
Pena, R. J., Amaya, A., Rajaram, S. & Mujeeb-Kazi, A. Variation in quality characteristics associated with some spring 1B/1R translocation wheats. J. Cereal Sci . 12 , 105–112 (1990).
Guzmán, C., Posadas-Romano, G., Hernández-Espinosa, N., Morales-Dorantes, A. & Peña, R. J. A new standard water absorption criteria based on solvent retention capacity (SRC) to determine dough mixing properties, viscoelasticity, and bread-making quality. J. Cereal Sci . 66 , 59–65 (2015).
Huber P. J. & Ronchetti, E. M. Robust Statistics 2nd edn (John Wiley & Sons, 2009).
Gilmour, A. R. ASREML for testing fixed effects and estimating multiple trait variance components. Proc. Assoc. Adv. Anim. Breed. Genet. 12 , 386–390 (1997).
Glaubitz, J. C. et al. TASSEL-GBS: a high capacity genotyping by sequencing analysis pipeline. PLoS ONE 9 , e90346 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 , 357–359 (2012).
Money, D. et al. LinkImpute: fast and accurate genotype imputation for nonmodel organisms. G3 (Bethesda) 5 , 2383–2390 (2015).
Bradbury, P. J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23 , 2633–2635 (2007).
Heslot, N., Yang, H., Sorrells, M. E. & Jannink, J.-L. Genomic selection in plant breeding: a comparison of models. Crop Sci. 52 , 146–160 (2012).
Rutkoski, J. et al. Evaluation of genomic prediction methods for fusarium head blight resistance in wheat. Plant Genome 5 , 51–61 (2012).
VanRaden, P. M. Efficient methods to compute genomic predictions. J. Dairy Sci. 91 , 4414–4423 (2008).
Habier, D., Fernando, R. L., Kizilkaya, K. & Garrick, D. J. Extension of the Bayesian alphabet for genomic selection. BMC Bioinformatics 12 , 186 (2011).
Pérez, P. & de los Campos, G. Genome-wide regression and prediction with the BGLR statistical package. Genetics 198 , 483–495 (2014).
Yu, J. et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38 , 203–208 (2006).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38 , 904–909 (2006).
Frichot, E., Mathieu, F., Trouillon, T., Bouchard, G. & François, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 196 , 973–983 (2014).
Zhang, Z. et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42 , 355–360 (2010).
Wu, Y., Bhat, P. R., Close, T. J. & Lonardi, S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet . 4 , e1000212 (2008).
Taylor, J. & Butler, D. ASMap: Linkage Map Construction using the MSTmap Algorithm. R version 0.4-4 (2015).
Barton, N. H., Briggs, D. E. G., Eisen, J. A., Goldstein, D. B. & Patel, N. H. Evolution (Cold Spring Harbor Laboratory Press, 2007).
Download references
Acknowledgements
This research was supported by the Feed the Future project through the US Agency for International Development (USAID), under the terms of contract no. AID-OAA-A-13-00051 (J.P. and R.P.S.). The opinions expressed herein are those of the authors and do not necessarily reflect the views of the USAID. We thank the innovation laboratory at Kansas State University, the CGIAR Research Program on Wheat, the Indian Council of Agricultural Research (ICAR), the Australian Centre for International Agricultural Research (ACIAR), several national partners (Afghanistan, Bangladesh, Canada, Egypt, India, Morocco, Pakistan and Sudan) and field technicians for their support in generating the genotyping and phenotyping data.
Author information
Authors and affiliations.
International Maize And Wheat Improvement Center (CIMMYT), Texcoco, Mexico
Philomin Juliana, José Crossa, Leonardo Crespo-Herrera, Fernando Henrique Toledo, Velu Govindan, Suchismita Mondal, Sridhar Bhavani, Pawan K. Singh, Xinyao He, Carlos Guzman, Susanne Dreisigacker & Ravi Prakash Singh
Wheat Genetics Resource Center, Department of Plant Pathology, Kansas State University, Manhattan, KS, USA
Jesse Poland, Sandesh Shrestha, Daljit Singh & Mohammad Mokhlesur Rahman
Campo Experimental Valle de Mexico, Instituto Nacional de Investigaciones Forestales, Agricolas y Pecuarias (INIFAP), Chapingo, Mexico
Julio Huerta-Espino
Borlaug Institute for South Asia (BISA), New Delhi, India
Uttam Kumar
International Maize and Wheat Improvement Center (CIMMYT), Nairobi, Kenya
Mandeep S. Randhawa
Departamento de Genética, Escuela Técnica Superior de Ingeniería Agronómica y de Montes, Campus de Rabanales, Universidad de Córdoba, Cordoba, Spain
Carlos Guzman
United States Department of Agriculture, Agricultural Research Service (USDA-ARS), Cereal Disease Laboratory and Department of Plant Pathology, University of Minnesota, St Paul, MN, USA
Matthew N. Rouse & Yue Jin
Colegio de Post graduados, Montecillos, Mexico
Paulino Pérez-Rodríguez
Facultad de Telematica, Universidad de Colima, Colima, Mexico
Osval A. Montesinos-López
Regional Agricultural Research Station, Bangladesh Agricultural Research Institute (BARI), Jamalpur, Bangladesh
Mohammad Mokhlesur Rahman
Instituto Nacional de Innovación Agropecuaria y Forestal (INIAF), La Paz, Bolivia
Felix Marza
You can also search for this author in PubMed Google Scholar
Contributions
P.J. drafted the manuscript and performed the analyses. R.P.S., J.P., J.H.-E. and J.C. planned the study and supervised the analysis. F.H.T., P.P.-R. and O.A.M.-L. performed some of the analyses. L.C.-H., V.G., S.M., U.K., S.B., P.K.S., M.S.R., X.H., C.G., M.N.R., Y.J., D.S., M.M.R. and F.M. generated the phenotyping data. S.D. performed the DNA extraction and S.S. called the marker polymorphisms.
Corresponding author
Correspondence to Ravi Prakash Singh .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Figs. 1–6
Reporting Summary
Supplementary tables.
Supplementary Tables 1–7
Supplementary Data 1
Phenotyping data for the lines in the YT, EYTs, SABWYTs, IBWSNs, SRRSNs and ESWYTs
Supplementary Data 2
Manhattan plots of the absolute marker effects for all the traits estimated using Bayes B approach in the combined EYT panel of 3,485 lines and the genomic prediction accuracies within and across panels using the Bayes B approach
Supplementary Data 3
Chromosome-wise linkage disequilibrium between the significant markers, shown by the marker R 2 vales (the correlation between the alleles at two loci) and the P values for the test of linkage disequilibrium using a two-sided Fisher’s exact test
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Juliana, P., Poland, J., Huerta-Espino, J. et al. Improving grain yield, stress resilience and quality of bread wheat using large-scale genomics. Nat Genet 51 , 1530–1539 (2019). https://doi.org/10.1038/s41588-019-0496-6
Download citation
Received : 11 March 2019
Accepted : 13 August 2019
Published : 23 September 2019
Issue Date : October 2019
DOI : https://doi.org/10.1038/s41588-019-0496-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Prediction accuracy of genomic estimated breeding values for fruit traits in cultivated tomato (solanum lycopersicum l.).
- Thuy Tien Phan Nguyen
- Sung-Chur Sim
BMC Plant Biology (2024)
Are cereal grasses a single genetic system?
- Martin Mascher
- Marina Püpke Marone
Nature Plants (2024)
Toxic effects of lead on plants: integrating multi-omics with bioinformatics to develop Pb-tolerant crops
- Muhammad Zahaib Ilyas
- Ju Kyong Lee
Planta (2024)
The wheat powdery mildew resistance gene Pm4 also confers resistance to wheat blast
- Andrew Steed
- Paul Nicholson
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Meeting the challenges facing wheat production: the strategic research agenda of the global wheat initiative.

1. Introduction
2. background, 2.1. why wheat, 2.2. impact of climate change, 2.3. the wheat initiative, 2.4. global wheat research, 3. existing strategic research agenda—work in progress.
| A fully assembled and aligned wheat genome sequence | Complete and pan genome also developed | Transcript databases and germplasm collection sequenced |
| Wheat data availability via an open information exchange framework | WheatIS developed | Expand databases linked to WheatIS and increase functionality |
| The ability to build new combinations of alleles | Continuing work | Improve access to germplasm with complex allele combinations |
3.1. Objective 1: To Increase Yield Potential
3.2. objective 2: to protect ‘on farm’ yield, 3.3. objective 3: ensuring the supply of high-quality safe wheat, 3.4. objective 4: enabling technologies and the sharing of resources, 3.5. objective 5: germplasm accessibility, 3.6. objective 6: knowledge exchange, education and training, 4. major issues and challenges facing wheat production and research, 4.1. inconsistencies in regulatory environment, 4.2. access to staff with the necessary skills in both new and old technologies, 4.3. data access and standards, 4.4. support for multinational research and public–private partnerships, 5. research priorities, 5.1. strengthen existing research activities, 5.2. enhance agronomy in its broadest definition (crop production and soil management), 5.3. increase genetic diversity.
- A broad series of activities can be undertaken to address this research priority:
- Revise and update the Global Wheat Conservation Strategy prepared in 2007 [ 26 ].
- Encourage the large-scale genotyping and phenotypic characterisation of germplasm held in the major genebanks.
- Advocate for the free and open exchange of germplasm and associated data.
- Encourage the utilisation of existing specialist germplasm collections collated by EWGs and share the outcomes: ◦ Tetraploid collections developed by the Durum EWG ▪ Durum elite and landrace collection in conjunction with a tetraploid core collection (GDP: Global Durum wheat Panel) capturing about 80% of the AABB haplotypes [ 27 ] of the collection (TGC: Tetraploid wheat Global Collection) described in [ 28 ]. ◦ Heat and drought tolerant germplasm collections developed by HeDWIC. ◦ Wheat quality assessment panels developed by the Quality EWG.
- Support research aimed at the enhanced utilisation of unadapted germplasm: ◦ Development of introgression populations. ◦ Re-domestication. ◦ Exploration of novel germplasm evaluation strategies. ◦ Development of efficient methods for gene editing.
5.4. Understanding Root and Soil Biology
- Continuing improvement of root phenotyping techniques, particularly in the field.
- Expand information of soil–microbe–plant interactions.
- Integration of data and information on roots and the microbiome in the analysis of wheat production with the full cropping system. It will also be important to emphasise the differences between low and high input systems and organic farming.
6. Wheat Initiative Structure and Organisation
6.1. develop educational and training programs.
- Ensure the full and rapid implementation of the postgraduate and ECR plan for involvement in the EWGs.
- Establish an exchange program that provides partial funding for students to work in other laboratories.
- Encourage EWGs to deliver training workshops and courses, and link to existing options offered by other organisations, such as universities, CIMMYT and ICARDA.
- Develop an online Wheat Initiative seminar program.
- Develop mentoring programs to support students and link to industry.
6.2. The Wheat Initiative as an Advocacy and Lobby Organisation
- Produce public explanatory documents and videos covering the Wheat Initiative activities, major topics and issues affecting wheat production, such as the role of germplasm exchange, gene editing, hybrid wheat, and crop protection.
- Participate in relevant G20 workshops and meetings and develop links to government agencies and international organisations.
- Advocate and lobby for the support of transnational research.
- Develop links to the wheat grower and processing industry organisations.
- Promote wheat resources such as WheatIS and WheatVIVO.
6.3. Expand Engagement
- The Institutions’ Coordination Committee has established a sub-committee to work through the options to build membership.
- Develop and distribute documentation explaining the value to industry from joining the WI—Industry.
- Increase industry participation in WI activities, particularly in training and mentorship: a component would be to identify platforms and capabilities that could be used by industry.
- Identify and target government and institutional organisations in major wheat producing and wheat-importing countries to seek greater engagement in the WI.
- Target early career researchers in under-represented countries to encourage the membership of EWGs. In addition, provide support to allow key people from these regions to participate in WI activities.
6.4. Supporting Multinational Research
- Stage 1 —Coordination across existing research to capture synergies, prevent duplication and identify gaps—low incremental costs but a proactive coordination is instrumental and essential.
- Stage 2 —Project alignment and leverage of existing investments: initially focus on the twinning of existing projects or building on a call(s) for proposals by one or more national funders joining (e.g., recent AAFC (Canada)/BBSRC (UK) IWYP-aligned call-linked consecutive calls for proposals in each country).
- Stage 3 —Scaling-up joint investment: under the key areas of interest to all funders, funding can be allocated to a common/centrally managed pot/program or managed nationally by a lead funder, still aligned under a broad umbrella theme.
7. Conclusions
- Boost research and technology delivery capabilities by investing in staff and student training and encourage and support the exchange of personnel between research organisations and building research infrastructure. This can be achieved if national research programmes place priority on activities with strong international linkages. Financial or organisational support from national agencies to research groups seeking participation in international partnerships would be beneficial.
- Provide support, both financial and organisational, to international activities aiming to facilitate the exchange of resources, particularly germplasm, and support the evaluation and delivery of research outcomes.
- Actively participate in Wheat Initiative research alliances that gather the capabilities and resources targeting global research challenges. These include the work of the Expert Working Groups and the three current alliances: The International Wheat Yield Partnership (boosting wheat yield potential), the Alliance for Wheat Adaptation to Heat and Drought (producing heat- and drought-tolerant germplasm) and the Wheat Initiative Crop Health Alliance (diagnosis and monitoring of wheat diseases).
Author Contributions
Data availability statement, acknowledgments, conflicts of interest, abbreviations.
| AAFC | Agriculture and Agri-Food Canada |
| AHEAD | Alliance for Wheat Adaptation to Heat and Drought |
| BBSRC | Biotechnology and Biological Sciences Research Council |
| CIMMYT | International Maize and Wheat Improvement Centre |
| EWG | Expert Working Group(s) |
| FEWG | Funding Expert Working Group |
| HeDWIC | Heat and Drought Wheat Improvement Consortium |
| ICARDA | International Centre for Agricultural Research in the Dry Areas |
| IWGSC | International Wheat Genome Sequencing Consortium |
| IWYP | International Wheat Yield Partnership |
| SRA | Strategic Research Agenda |
| UK | United Kingdom |
| WATCH-A | Wheat Initiative Crop Health Alliance |
| WheatIS | Wheat Information System |
| WI | Wheat Initiative |
- FAO. Land Use in Agriculture by the Numbers. 2022. Available online: https://www.fao.org/sustainability/news/detail/en/c/1274219/#:~:text=Global%20trends,and%20pastures)%20for%20grazing%20livestock (accessed on 19 September 2022).
- World Bank. Arable Land (Hectares per Person). 2022. Available online: https://data.worldbank.org/indicator/AG.LND.ARBL.HA.PC (accessed on 19 September 2022).
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data (accessed on 19 September 2022).
- Braun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. In Climate Change & Crop Production ; Reynolds, M.P., Ed.; CABI: Oxfordshire, UK, 2010; pp. 115–138. [ Google Scholar ] [ CrossRef ]
- Cossani, C.M.; Reynolds, M.P. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012 , 160 , 1710–1718. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Biol. 2021 , 72 , 2822–2844. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sarhadi, A.; Ausín, M.C.; Wiper, M.P.; Touma, D.; Diffenbaugh, N.S. Multidimensional risk in a nonstationary climate: Joint probability of increasingly severe warm and dry conditions. Sci. Adv. 2018 , 4 , eaau3487. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Gaupp, F.; Hall, J.; Hochrainer-Stigler, S.; Dadson, S. Changing risks of simultaneous global breadbasket failure. Nat. Clim. Chang. 2020 , 10 , 54–57. [ Google Scholar ] [ CrossRef ]
- Kornhuber, K.; Coumou, D.; Vogel, E.; Lesk, C.; Donges, J.F.; Lehmann, J.; Horton, R.M. Amplified Rossby waves enhance risk of concurrent heatwaves in major breadbasket regions. Nat. Clim. Chang. 2020 , 10 , 48–53. [ Google Scholar ] [ CrossRef ]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017 , 12 , 064008. [ Google Scholar ] [ CrossRef ]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019 , 5 , eaau2406. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Chang. 2016 , 6 , 1130–1136. [ Google Scholar ] [ CrossRef ]
- Zaoh, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017 , 114 , 9326–9331. [ Google Scholar ] [ CrossRef ]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014 , 4 , 287–291. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2020 , 27 , 27–49. [ Google Scholar ] [ CrossRef ]
- Russell, K.; Van Sanford, D.A. Breeding wheat for resilience to increasing nighttime temperatures. Agronomy 2020 , 10 , 531. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Beres, B.L.; Hatfield, J.L.; Kirkegaard, J.A.; Eigenbrode, S.D.; Pan, W.L.; Lollato, R.P.; Hunt, J.R.; Strydhorst, S.; Porker, K.; Lyon, D.; et al. Towards a better understanding of genotype × environment × management interactions—A global wheat initiative agronomic research strategy. Front. Plant Sci. 2020 , 11 , 828. [ Google Scholar ] [ CrossRef ]
- Pardey, P.G.; Chan-Kang, C.; Dehmer, S.P.; Beddow, J.M. Agriculture R&D is on the move. Nature 2016 , 537 , 301–303. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pardey, P.G.; Chan-Kang, C.; Beddow, J.M.; Dehmer, S.P. Long-run and Global R&D Funding Trajectories: The US Farm Bill in a Changing Context. Am. J. Agric. Econ. 2015 , 97 , 1312–1323. [ Google Scholar ] [ CrossRef ]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 2020 , 588 , 277–283. [ Google Scholar ] [ CrossRef ]
- McCouch, S.; Baute, G.J.; Bradeen, J.; Bramel, P.; Bretting, P.K.; Buckler, E.; Burke, J.M.; Charest, D.; Cloutier, S.; Cole, G.; et al. Agriculture: Feeding the future. Nature 2013 , 499 , 23–24. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Feuillet, C.; Langridge, P.; Waugh, R. Cereal breeding takes a walk on the wild side. Trends Genet. 2008 , 24 , 24–32. [ Google Scholar ] [ CrossRef ]
- Dreisigacker, S.; Kishee, M.; Lahe, J.; Warburton, M. Use of synthetic hexaploid wheat to increase diversity for CIMMYT bread wheat improvement. Aust. J. Agric. Res. 2008 , 59 , 413–420. [ Google Scholar ] [ CrossRef ]
- Miller, T.E. Systematics and evolution. In Wheat Breeding: Its Scientific Basis ; Lupton, F.G.H., Ed.; Chapman & Hall: London, UK, 1987; pp. 1–30. [ Google Scholar ]
- Hatta, M.A.M.; Steuernagel, B.; Wulff, B.B.H. Rapid gene cloning in wheat. In Applications of Genetics and Genomic Research in Cereals ; Miedaner, T., Korzun, V., Eds.; Woodhead Publishing: Swaston, UK, 2019; pp. 65–95. [ Google Scholar ] [ CrossRef ]
- CIMMYT. Global Strategy for the Ex Situ Conservation with Enhanced Access to Wheat, Rye and Triticale Genetic Resources. 2007. Available online: https://www.croptrust.org/fileadmin/uploads/croptrust/Documents/Ex_Situ_Crop_Conservation_Strategies/Wheat-Strategy-FINAL-20Sep07.pdf (accessed on 19 September 2022).
- Mazzucotelli, E.; Sciara, G.; Mastrangelo, A.M.; Desiderio, F.; Xu, S.S.; Faris, J.; Hayden, M.J.; Tricker, P.J.; Ozkan, H.; Echenique, V.; et al. The Global Durum Wheat Panel (GDP): An International Platform to Identify and Exchange Beneficial Alleles. Front. Plant Sci. 2020 , 11 , 569905. [ Google Scholar ] [ CrossRef ]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Gen. 2019 , 51 , 885–895. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat root systems as a breeding target for climate resilience. Theor. Appl. Genet. 2021 , 134 , 1645–1662. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maccaferri, M.; El-Feki, W.; Nazemi, G.; Salvi, S.; Canè, M.A.; Cholalongo, M.C.; Stefanelli, S.; Tuberosa, R. Prioritizing quantitative trait loci for root system architecture in tetraploid wheat. J. Exp. Bot. 2016 , 67 , 1161–1178. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alahmad, S.; El Hassouni, K.; Bassi, F.M.; Dinglasan, E.; Youssef, C.; Quarry, G.; Aksoy, A.; Mazzucotelli, E.; Juhász, A.; Able, J.A.; et al. A major root architecture QTL responding to water limitation in durum wheat. Front. Plant Sci. 2019 , 10 , 436. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Annual Average for 2011–2020 Data | Maize | Rice | Wheat | |
|---|---|---|---|---|
| Area sown | Million hectares | 191 | 162 | 219 |
| Production | Million tonnes | 1057 | 739 | 733 |
| Import | Million tonnes | 149 | 42 | 189 |
| Value (USD billion) | 3.8 | 2.5 | 5.3 | |
| Export | Million tonnes | 153 | 43 | 192 |
| Value (USD billion) | 3.4 | 2.4 | 4.9 | |
| % Production traded | 14 | 6 | 26 | |
| Annual average for 2010–2019 data | ||||
| Food quantity | Million tonnes | 139 | 584 | 499 |
| kg/capita/year | 19 | 80 | 66 | |
| Calories | Kcal/capita/day | 159 | 542 | 540 |
| Protein | g/capita/day | 3.8 | 9.9 | 16.4 |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Langridge, P.; Alaux, M.; Almeida, N.F.; Ammar, K.; Baum, M.; Bekkaoui, F.; Bentley, A.R.; Beres, B.L.; Berger, B.; Braun, H.-J.; et al. Meeting the Challenges Facing Wheat Production: The Strategic Research Agenda of the Global Wheat Initiative. Agronomy 2022 , 12 , 2767. https://doi.org/10.3390/agronomy12112767
Langridge P, Alaux M, Almeida NF, Ammar K, Baum M, Bekkaoui F, Bentley AR, Beres BL, Berger B, Braun H-J, et al. Meeting the Challenges Facing Wheat Production: The Strategic Research Agenda of the Global Wheat Initiative. Agronomy . 2022; 12(11):2767. https://doi.org/10.3390/agronomy12112767
Langridge, Peter, Michael Alaux, Nuno Felipe Almeida, Karim Ammar, Michael Baum, Faouzi Bekkaoui, Alison R. Bentley, Brian L. Beres, Bettina Berger, Hans-Joachim Braun, and et al. 2022. "Meeting the Challenges Facing Wheat Production: The Strategic Research Agenda of the Global Wheat Initiative" Agronomy 12, no. 11: 2767. https://doi.org/10.3390/agronomy12112767
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
REVIEW article
Salinity stress in wheat ( triticum aestivum l.) in the changing climate: adaptation and management strategies.

- 1 Department of Agronomy, Faculty of Agriculture, University of Kafrelsheikh, Kafr El-Shaikh, Egypt
- 2 Department of Field Crops, Faculty of Agriculture, Siirt University, Siirt, Turkey
- 3 Department of Agronomy, Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh
- 4 Department of Botany and Plant Physiology, Faculty of Agrobiology, Food, and Natural Resources, Czech University of Life Sciences Prague, Prague, Czechia
- 5 College of Agronomy, Sichuan Agricultural University, Chengdu, China
- 6 Principal Scientist (Agronomy), Punjab Agricultural University, Regional Research Station, Faridkot, India
- 7 Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 8 Department of Agronomy, Bangladesh Wheat and Maize Research Institute, Dinajpur, Bangladesh
- 9 College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, China
- 10 Plant Physiology Division, Nuclear Institute of Agriculture (NIA), Tandojam, Pakistan
- 11 Department of Agronomy, Faculty of Agriculture, University of Poonch Rawalakot (AJK), Rawalakot, Pakistan
- 12 Department of Agricultural Biology, Faculty of Agriculture, University of Ruhuna, Matara, Sri Lanka
- 13 ICAR-Indian Grassland and Fodder Research Institute, Jhansi, India
- 14 International Rice Research Institute, Bangladesh Office, Dhaka, Bangladesh
- 15 Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, India
- 16 College of Agriculture, Bahauddin Zakariya University, Bahadur Sub-Campus Layyah-Pakistan, Layyah, Pakistan
- 17 Department of Plant Physiology, Slovak University of Agriculture, Nitra, Slovakia
- 18 Institute of Crop Science and Resource Conservation (INRES), Crop Science Group, University of Bonn, Bonn, Germany
- 19 Faculty of Science, Plant Breeding Institute, Sydney Institute of Agriculture, School of Life and Environmental Sciences, University of Sydney, Sydney, NSW, Australia
- 20 College of Resources and Environmental Sciences, China Agricultural University, Beijing, China
Wheat constitutes pivotal position for ensuring food and nutritional security; however, rapidly rising soil and water salinity pose a serious threat to its production globally. Salinity stress negatively affects the growth and development of wheat leading to diminished grain yield and quality. Wheat plants utilize a range of physiological biochemical and molecular mechanisms to adapt under salinity stress at the cell, tissue as well as whole plant levels to optimize the growth, and yield by off-setting the adverse effects of saline environment. Recently, various adaptation and management strategies have been developed to reduce the deleterious effects of salinity stress to maximize the production and nutritional quality of wheat. This review emphasizes and synthesizes the deleterious effects of salinity stress on wheat yield and quality along with highlighting the adaptation and mitigation strategies for sustainable wheat production to ensure food security of skyrocketing population under changing climate.
Introduction
Recently, climate change and global warming have directly affected the crops yield and quality by intensifying the frequency and extent of numerous stresses. Wheat, rice, and maize are the most important staple crops globally and contribute a significant part of daily calories and protein intake ( Kizilgeci et al., 2021 ). Among these major cereals, wheat is ranked at the first position due to its domestication and contribution as the primary staple food crop globally ( Iqbal et al., 2021 ). Currently, it is dominating the most of arable land (38.8%), with relatively higher grain protein (12–15%) than other cereals, but the productivity remains low [ Food and Agriculture Organization (FAO) of the United Nations, 2016 ]. It can further decrease owing to climate change that has given to rise a variety of abiotic stresses. Various climate models projected that wheat production could decrease by 6% due to stressful environments ( Asseng et al., 2015 ).
Salt stress affects 20% of global cultivable land and is increasing continuously owing to the change in climate and anthropogenic activities ( Arora, 2019 ). Environmental stress including salinity can cause about 50% of production losses ( Acquaah, 2007 ). Furthermore, the continuous increase in the human population put pressure on global food security as the world's food supply needs to be increased by up to 70% by 2050 ( FAO, 2009 ). Wheat ( Triticum aestivum ) is considered the most significant grain crop among all the cereals and ranked 1st globally among grain-producing crops, especially for human consumption ( Giraldo et al., 2019 ). About 36% of the world's population is dependent on wheat as a staple food. About 20% of calories and 55% of carbohydrates are being provided by wheat across the globe. Both growth and yield of wheat are negatively influenced by salinity ( Royo and Abió, 2003 ). Salinity stress causes osmotic stress and ion toxicity, through increasing the assimilation of Na + ion and decreasing the Na + /K + ratio due to lower osmotic potential within the plant roots. Further, these ionic imbalance affects the uptake, and transport of other important essential ions in target cells and hamper the crucial plant processes and functions ( Arif et al., 2020 ). Salinity impairs the seedling establishment, stunted plant growth, poor reproductive development, and ultimately declines the crop yield ( Turan et al., 2009 ). Salinity also alters the ultrastructural cell components, disturbs the photosynthesis machinery, damages the membranous structure, increases the reactive oxygen species production, reduces the enzymatic activity, which limit the growth and yield of crops ( Hasanuzzaman et al., 2014 ). Tolerancey of plants to salinity is a polygenic character that is governed by many genetic factors ( Arzani and Ashraf, 2016 ). Crop growth is improved under salinity by the increase in K + , elimination of Na + or by optimizing the ratio of both Na + and K + ions, improving transpiration efficiency, regulation of osmotic potential, and by the antioxidant, immune system of plants ( Rahman et al., 2005 ).
Among various field crops, generally, wheat is more sensitive to salinity that hampers the growth and development of plant, leads to low productivity or even complete crop failure under extreme severity of salinity. The knowledge of stress tolerance in plants regarding the physiological basis is important for selection and breeding programs ( Chaves et al., 2003 ). Thus, understanding of morphoanatomical, physiological, biochemical, and molecular mechanisms of wheat responses to salinity stress at each phase of growth is essential to improve breeding techniques and to develop salt-tolerant varieties with genetic modifications. The recent findings indicate that change in leaf and stem anatomical features in different genotypes of wheat are crucial traits to adaptation under salinity stress ( Nassar et al., 2020 ). The research on the physiological changes that occur during leaf senescence due to some stresses has been primarily focused on the loss of photosynthetic pigments, protein degradation, and re-absorption of mineral nutrients ( Zheng et al., 2008 ). At the same time accumulation of secondary metabolites (anthocyanins, flavones, phenolics, and specific phenolic acids) often occurs in plants subjected to stresses including various elicitors or signal molecules ( Sytar et al., 2018 ). It was demonstrated that pigmented wheat genotypes with high anthocyanin content can maintain significantly higher dry matter production under salt stress conditions ( Mbarki et al., 2018 ), which shown the role of phenolic compounds in salinity tolerance together with new breaded pigmented wheat genotypes.
Determination of physiological traits related to stress tolerance could be used as a selection criterion to enhance wheat adaptation to stress conditions. According to the previous investigations, there is a link between different physiological responses of crops to stress and their tolerance mechanisms, such as high relative water content and water potential ( Datta et al., 2011 ). Moreover, it has been observed that anthesis and grain filling period are very sensitive stages under multiple environmental stresses including salinity and have been identified as major constraints to wheat production worldwide ( Ghosh et al., 2016 ). Therefore, it is crucial to understand the effects of salt stress on wheat yield improvement while maintaining superior productivity and adopting mitigation strategies toward the long-term goal of sustainable food security. This study aimed to synthesize salinity effects on wheat germination, seedling growth, reproductive development, grain yield, and quality. Additionally, deleterious effects of salinity in relations to nutrient imbalance and water relations have been objectively described. Moreover, integrated approach for salinity mitigation through osmoprotectants, plant hormones, mineral nutrients, and signaling molecules has been elucidated.
The current review overviews the adverse effects of salinity stress on wheat and its adaptation and mitigation strategies for the sustainability of wheat productivity under the changing climate.
Adverse Effects of Salinity Stress on Wheat
Most of the agricultural lands, which are affected by different degrees of salinity, are located in semi-arid or arid regions ( Liu et al., 2020 ). Huang et al. (2019) concluded that the damage of crops is mostly intensified by the synchronized action of xerothermic aspects, such as aridity and high temperature. Salinity is the most adversely affecting factor on productivity and quality of wheat through altering the physiological as well as biochemical activities in plants. Generation of ROS due to Na + toxicity, which damage biomolecules (e.g., lipids, proteins, and nucleic acids) ( Apel and Hirt, 2004 ) on the cellular level and alters redox homeostasis, is a common phenomenon under salt stress ( Kundu et al., 2018 ). However, salt impacted soils are difficult to remediate due to the circumstances outlined by Arzani and Ashraf (2016) . First, Na + and Cl − ions are highly mobile in soils. Second, it is often an expensive and short-term solution for the chronic problem. Third, soil salinity has a dynamic nature and spatial variation in salinity is generated by the interactions among different variables of edaphic effects (soil pH, bulk density, permeability, topography, geohydrology, water table depth, and groundwater salt content), geographic factors (elevation, slope, and aspect), agronomic practices (irrigation, drainage, tillage, crop rotation, and fertilization), and climatic effects (temperature, humidity precipitation, wind, and evaporation) ( Bui, 2013 ). Therefore, the integrated agronomical, physiological, and soil management approaches and targeting multiple traits at the same time are a crucial step to achieve salinity tolerance. Therefore, it is important to substitute Na + with the Ca 2+ followed by removal/leaching of salts derived by the reaction of the amendment from sodic soil for sustainable crop production ( Sorour et al., 2019 ).
Salinity delays the onset of seedlings germination, decreases the seedling growth and the dispersion of germination events, seedling metabolism, causing a reduction in plant growth and crop productivity ( El Sabagh et al., 2019a , b , c , 2020 ). One important approach is to develop an understanding of the plant response toward salinity stress. The response of plants to salinity can be described in two subsequent phases ( Arzani and Ashraf, 2016 ), during the first phase, salinity causes osmotic stress because of a decrease in the soil water potential ( James et al., 2006 ). The second phase develops within a few days or weeks (depend on the severity of salinity) and accumulates Na + ions in different plant tissues, causing reduced yield and even plant death ( Munns and Tester, 2008 ). Under salinity, Na + is the principle of toxic ion imposing both osmotic stress and ionic toxicity ( Munns and Tester, 2008 ). Salinity also negatively affects wheat phenological developments such as leaf number, leaf expansion rate, and root/shoot ratio ( El-Hendawy et al., 2005 ), and biomass production ( Sorour et al., 2019 ). The saline environment disturbs plant water relations including relative water content, leaf water potential, water uptake, transpiration rate, water retention, and water use efficiency ( Nishida et al., 2009 ).
Salinity adversely affects the growth and yield of crop plants by decreasing the availability of soil moisture, and due to the toxicity effects of sodium and chloride ions at high concentrations to the plant ( Munns and Tester, 2008 ). Salinity stress accelerates all phenological phases of wheat ( Grieve et al., 1994 ), reduces the number of fertile tillers ( Abbas et al., 2013 ), decreases the number of spikelet number spike −1 ( Frank et al., 1987 ), kernel weight ( Abbas et al., 2013 ), and affects grain yield adversely ( Sorour et al., 2019 ). For instance, yield losses up to 45% have been recorded in salt-stressed wheat ( Ali et al., 2009 ). Hasan et al. (2015) observed that saline stress (15 dSm −1 ) significantly decreases grains per spike, 1,000-grain weight, and seed yield in tolerant and sensitive wheat cultivars. The effect of salinity stress on root activity, germination, morphological traits, crucial plant processes, yield, and yield attributes in wheat are illustrated in Figure 1 .
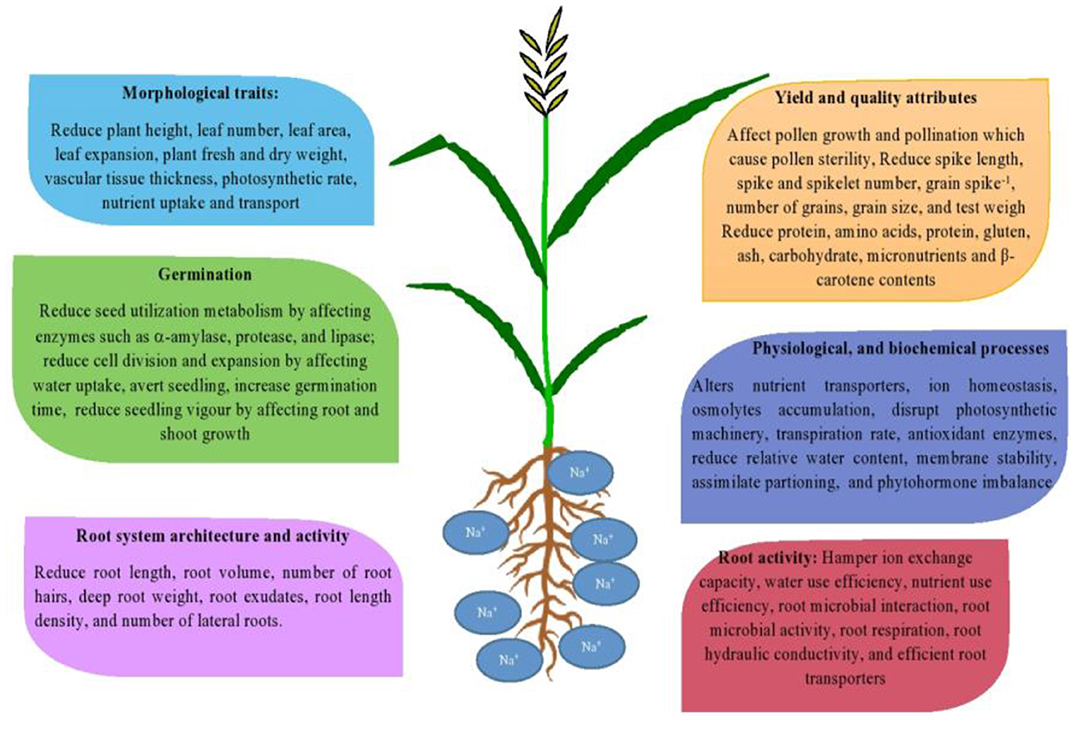
Figure 1 . Illustrates the effects of salinity stress on different growth stages and crucial plant processes.
Germination and Plant Growth
Soil salinity is the second major factor responsible for land degradation after soil erosion, causing a decline in agricultural economic outputs for 10,000 years ( Shahid et al., 2018 ). Poor salinity management can cause soil sodicity of farming soils, where sodium (Na) binds to negatively charged clay, causing clay swelling and dispersal, subsequently decreasing the crop yield. Higher levels of salinity confiscate 1.5 million hectares of land globally every year, and hence ~50% of cultivable land could be deteriorated by the mid of 21st century. During salinity, the exaggeration of most plants is apparent in the early stages, especially during seedling establishment, as it is the most responsive and critical stage that is reported to be strongly associated with successful germination and seedling development. Various factors hamper the crop yield under salinity stress, but osmotic stress, ionic imbalance, and oxidative stress are the major ones among them. In brief, the osmotic stress leads to a higher accumulation of salts in cell sap and tissues which become observable as leaf burn and wilting. These symptoms are reported to be associated directly with the accumulation of Na + and Cl − . Thus, this ionic imbalance causes disequilibrium of nutrients that declines germination, and adversely affects the subsequent metabolic processes ( Hussain et al., 2019 ). Furthermore, the oxidative stress exerted via accelerated ROS generation induces lipid peroxidation, disrupt nucleic acids that ultimately decreases the consistency and overall yield of the affected seed ( Dehnavi et al., 2020 ; Kumari and Kaur, 2020 ).
Germination is a dynamic and critical phase in the lifecycle of a plant that pledges via the imbibition of water ( Kumari and Kaur, 2018 ). It is a triphasic process, and during phase 1, the seeds absorb water, followed by the second phase, i.e. , the “ plateau phase” (stable water content), and characterized by test rupturing. In the third phase, endosperm ruptures and radicle protrusion take place, and it is also referred to as the post-germination phase ( Chamorro et al., 2017 ). In 2007, Läuchli and Grattan proposed a general scheme for depicting the relationship of germination percentage with the time of germination under low, moderate, and high salinity levels ( Figure 2 ).
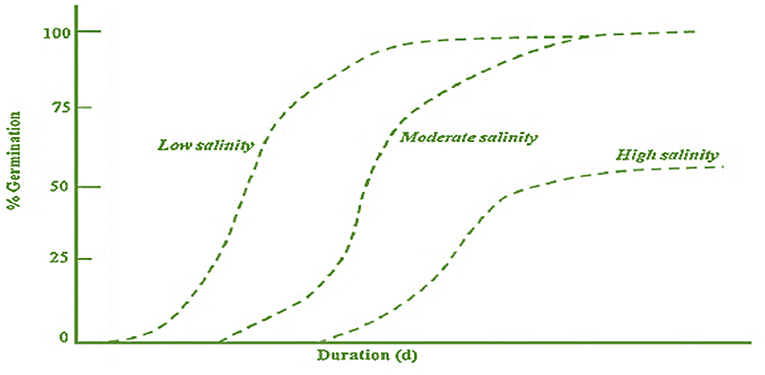
Figure 2 . Association between germination rate and exposure duration after sowing at different salinity levels (low, moderate, and high) (Source: Läuchli and Grattan, 2007 ).
Salinity inhibits seed germination by either exerting osmotic stress that thwarts water uptake or causes ionic toxicity. These consequences collectively inhibit cell division and expansion, as well as modulates the activity of some key enzymes, thus lastly reduces the seed reserves utilization ( El-Hendawy et al., 2019 ). Thus, it can be said that salinity negatively affected the process of germination by altering the normal germination mechanism that resulted in reduced growth and development, ultimately declined the economic yield. Moreover, the increased incidence of salt stress in the arable land indicates that there is an urgent need for a deeper understanding of the mechanisms for plant tolerance to preserve crop productivity through the optimal regulation of growing conditions of cereal crops. There is dire need to develop strategies such seed priming with natural or synthetic growth regulators etc. for boosting cereals germination in saline environment, otherwise significant decline in germination could lead food crisis in future.
Adverse Effects of Salinity Stress on Morphological Processes of Plants
Soil salinity detrimentally affects the various morphological characteristics of wheat plants including seedling growth, plant height, shoot, and root length, the number of roots, leaves, leaf area, fresh and dry weight, root/shoot ratio, and chlorophyll content. Ahmad et al. (2013a) observed that the early maturity of wheat due to salinity stress reduced the crop height and leaf area and found that plumule length was the most sensitive during early growth stages. Bacilio et al. (2004) found that root colonization pattern, leaf expansion, and flag leaf area in wheat was significantly lower under salinity stress. Furthermore, the number of leaves plant −1 and leaf size, along with leaf longevity, were shriveled because of salinity stress ( El-Hendawy et al., 2009 ). Otu et al. (2018) reported that wheat facing salinity stress exhibited reduced stem length, stem weight, and shoot dry matter. Such detrimental effects of salinity stress on the dry weight of shoot may be due to the direct impact on photosynthesis ( Parida et al., 2005 ). Maas (1990) stated that the salinity changes the final size of a spike and a significant reduction occurs in spike length and number, and grains spike −1 . Shafi et al. (2010) found that salinity stress inhibited the shoot and root dry weight, number of root tips (lateral roots), total root length, average root diameter, and total root volume. The root is the first important organ, and a well-developed root system can contribute advantages to wheat plant to maintain plant growth for the period of early growth phases, and extracts water and micro-nutrients through the soil ( Egamberdieva, 2009 ). The production of the well-developed root system under stress conditions is vital for above-ground biomass production ( Iqbal et al., 2018 ). Saboora et al. (2006) observed that the root development inclined severely due to salinity stress in wheat besides reduced root length as well as area. Ammonium inhibits the root growth of many plants, including wheat, whereas NaCl stimulates the accumulation of ammonium in roots which restrains the root growth. New cultivars having improved agro-morphological traits and potential to ameliorate the adverse effect of salinity must be developed through a targeted breeding program.
Adverse Effects of Salinity Stress at the Reproductive Stage on Plant Growth and Yield
Several earlier studies illustrated that the reproductive phase of any crop is the most sensitive stage to the abiotic stresses, including salinity ( Ehtaiwesh and Rashed, 2020 ), and cause massive yield penalty in important crops, including wheat ( Kalhoro et al., 2016 ). However, it is well-postulated that salinity influences plant growth and yield attributes primarily due to ion toxicity and osmotic stress. Although, the intrinsic pathways and molecular mechanisms are so far not clear. Salt stress influences cell ion homeostasis by altering ion balance, such as increased Na + and a simultaneous decreased Ca 2+ and K + content. A recent study suggested that the class I high-affinity K + transporter (HKT) family is involved in the exclusion of Na + from leaf blades at the reproductive stage, and this significantly influences sodium ion homeostasis under salinity stress ( Suzuki et al., 2016 ). Likewise, the grain dry matter and K + /Na + ratio showed a significant correlation and regulated the grain filling rate and duration under salt stress ( Poustini and Siosemardeh, 2004 ). Similarly, an isotopic study (δ 13 C, δ 18 O, and δ 15 N) in wheat crop under different salinity regimes depicted that nitrogen metabolism and stomatal limitations at the anthesis stage are among the major cause of biomass reduction ( Yousfi et al., 2013 ). Furthermore, transcriptomics study in wheat (Arg cultivar) genotype revealed that 109 genes are unique in treated plants, which are mainly associated with transcription factors gene (late embryogenic abundant [ LEA ] protein, dehydrin, MYB , and ERF ), ion transporter ( SOS1 ), antioxidant defense (peroxidase, glutathione S -transferase, GST) and secondary metabolites (caffeic acid 3-O-methyltransferases), and have a role in the regulation of ion homeostasis, cell redox balance, and oxidative stress ( Amirbakhtiar et al., 2019 ).
Besides ionic imbalance, salinity stress influences available soil water, tissue water content, water use efficiency, water potential, transpiration rate, rooting depth, root respiration, root biomass, root hydraulic conductance, cell turgidity, and osmolytes accumulations ( Zheng et al., 2008 ). Besides, it also reduces the photosynthetic rate, biomass accumulation, and source-sink activity, which hastens the reproductive organ's senescence and negatively affects the yield response factors ( Khataar et al., 2018 ). Similarly, at the reproductive phase, alteration in water potential reduces the cell elongation, flag leaf thickness, vascular tissue thickness, mesophyll, and epidermal cell size, which are responsible for the reduction of flag leaf turgidity and leaf area, assimilates synthesis, and finally yield potential ( Farouk, 2011 ).
Although, Ashraf and Ashraf (2016) suggested that these physiological and biochemical trait alterations are stage-specific and attribute to final yield potential. For example, salinity at different stages such as anthesis, early booting, and mid grain filling causes a reduction of grain yield by 39.1, 24.3, and 13.4%, respectively. Salt stress caused the acceleration of shoot apex development but decreased the number of spikelet primordia and also resulted in early terminal spikelet stage and anthesis. This decreased the number of spikes and kernels per spikes, which ultimately reduced the yield potential in wheat ( Maas and Grieve, 1990 ). Likewise, use of 200 mM NaCl stress at pre-anthesis and post-anthesis stage caused reduction in aboveground biomass, ears plant −1 , ear weight, number of grains plant −1 , C, N, and C/N ratio in grains, and carbon use efficiency at both stages, although the reductions were higher due to imposition of stress at both stages as compared to single-stage ( Eroglu et al., 2020 ). It has also been observed that the unavailability of sufficient photo-assimilates during the reproductive stage is the leading cause for losing yield potential in wheat, and this might be due to the changes in gene expression caused by salt stress during the pre-anthesis and grain filling stage. For example, alteration of sucrose 1-fructosyltransferase, sucrose: fructan6-fructosyltransferase, and fructanexohydrolase hampers the fructan accumulation, and remobilization of carbohydrate to grains ( Sharbatkhari et al., 2016 ). Similarly, yield component traits such as spike length, spike weight, filled spikelet plant −1 , total spikelet plant −1 , and test weight were reduced to 8, 3, 37, 20, and 10%, respectively under stress condition, and resulted in 16% low total grain weight plant −1 ( Tareq et al., 2011 ). Further, losses of grain weight under saline stress occurs due to pollen sterility, less production of assimilates, and reduced partitioning toward economic parts (grains) of plants. Likewise, the study on 151 synthetic wheat-breeding lines suggested that salt stress to be associated with Na + toxicity, which reduced the total kernel weight, and starch content by 20 and 6%, respectively ( Dadshani et al., 2019 ).
In spite of these changes, salinity stress has substantial impacts on grain quality traits. For example, the use of 200 mM of NaCl in wheat (cv. Shatabdi) showed an increase in Na + , K + , and Ca +2 content in grains by 155, 10, and 20%, respectively under stress ( Tareq et al., 2011 ). Similarly, the use 0.75% NaCl salt increased the contents of high molecular weight glutenin subunit, glutenin macro-polymers (36.14%), and amino acids and improved wheat quality at certain levels but reduce grain yield ( Zhang et al., 2016 ). Similarly, Nadeem et al. (2020) reported that salinity negatively influenced the yield (grain length, test weight, and grain yield), nutritional quality traits (moisture, fat, ash, fiber, and gluten content), and mineral nutrient content (K, Ca, Fe, P, Zn, and Mg) in wheat crop. Therefore, it can be concluded that salinity stress is a major factor for limiting yield and yield quality traits, and it affects the reproductive phage severely by altering ion homeostasis, water status, and assimilate partitioning.
Adverse Effects of Salinity on Grain Quality
Soil salinity imparts detrimental impacts on vital metabolic, biochemical, and physiological processes occurring within the plants leading to the deterioration of grain quality. The extent of changes in grain quality caused by salinity depends on the sternness of the stress. From physiological perspectives, grain quality is affected owing to the accumulation of salts in the root zone leading to osmotic stress induction, which vigorously disrupted cell ion homeostasis. Salt exposure causes osmotic stress at the beginning, while subsequently, ion toxicity hampers growth, grain development, and quality, especially if the exposure periods get prolonged. The deterioration of grain quality of cereals has also been explained in agronomic perspectives as well. The reduction in the capability of roots for water uptake owing to osmotic stress contributes to growth inhibition, declined crop productivity, and inferior grain quality ( Netondo et al., 2004 ). Thus, grain quality is drastically affected by the devastating effects of osmotic stress, while the subsequent slower ion toxicity phase is even more detrimental.
Winter wheat constitutes a vital source of carbohydrates and protein for humans across the globe ( Siddiqui et al., 2019 ). There have been significant achievements pertaining to boost wheat yield over the decades. However, the demand for higher-quality grain has also increased with the improvement in human lifestyle ( Park et al., 2009 ). Plant variety, in conjunction with the prevalent environment, has also been reported to determine the wheat quality to a certain extent ( Sairam et al., 2002 ). Previously, most researchers focused on the impacts of salinity on wheat grain yield ( Zheng et al., 2009 ), but little is known about the relationships between salt-tolerance and grain quality. There are diverse effects of salinity levels on the grain quality of cereals. It has been inferred that salinity levels, especially beyond 150 mM of NaCl, significantly reduced the grain yield, whereby grain quality deterioration remained significant at 100 mM ( Farooq and Azam, 2005 ). There is a varying impact of salinity on the nutritional value of grains depending upon the plant growth stage at which stress occurs. Breeding approaches to improve grain yield, especially in the salt-tolerant varieties have negatively affected the grain quality as these traits are often inversely related.
As far as grain quality of cereals under abiotic stresses especially salinity is concerned, there have been relatively limited investigations. Protein, fat, and fibers contents in grain decreased significantly due to salinity. In response to imposed salinity, protein content improved in the sensitive wheat genotypes, while it decreased in tolerant genotypes. The ash and beta-carotene contents were enhanced, while the gluten index got declined considerably ( Katerji et al., 2005 ). At the same time, Maqsood et al. (2008) inferred that the decrease in protein and fiber contents of cereals was due to the accumulation of salts in the root zone, which deteriorated the grain quality. Additionally, higher concentration of Na + in the external environment interferes with the absorption of nitrogen, which leads to lower protein content in wheat grains. Thus, the nutritional imbalance is reported to be the major factor behind the deteriorated grain quality of wheat under salt stress. Furthermore, salt stress interfered with prime processes, including photosynthesis, energy production, lipid metabolism, and protein synthesis. Lastly, salinity reduced phosphorus (P) concentration in plant shoots, which interfered with grain development and nutritional quality. Optimization of P foliar doses might be developed as a potent approach to alleviate the salinity effects on grain size and weights which are the vital yield attributes of cereals.
Protein Content
Protein content is the most important indicator of wheat grain quality and hence it governs and determines the end-use quality. The grain quality of wheat, especially the quality of protein as well as its quantity, is vital for dough properties and the bread-making quality of wheat flour. Under the saline condition, the protein quantity is increased, but the protein quality is decreased in wheat and triticale. The protein content is controlled by the genetic makeup of a particular cultivar or line, environmental factors, especially temperature and soil fertility status predominantly concerning N concentration in soil solution. It is significantly affected by environmental factors and their interactions. Positive correlations between environmental factors and wheat grain protein content have been reported during grain filling ( Huebner et al., 1997 ). Protein content, along with composition, significantly modifies wheat flour quality for bread-making ( Branlard et al., 2001 ). Nevertheless, the high protein content of the wheat grain is privileged because flour protein content has a linear relationship with bread-making quality ( Schofield, 1994 ). Conversely, protein quality signifies the quality of bread-making. It has been established that the composition of grain protein primarily depends on wheat genotypes; however, environmental factors and their association significantly affect protein composition ( Zhu and Khan, 2001 ). Among environmental factors, soil fertility status, especially nitrogen concentration, temperature, and moisture, influence cereals grain protein content ( Rao et al., 1993 ). Environmental stresses also impart their influence on milling-quality as indicated by test weight. Test weight is a highly heritable character and has a direct relationship with grain quality, which can find its use for early selection of wheat lines in breeding programs ( Troccoli et al., 2000 ). The crude protein content of cereal declined from 17.21 to 8.51% under salt stress ( Fernandez-Figares et al., 2000 ). Similarly, Darvey et al. (2000) recorded a higher reduction of protein content in wheat compared to triticale. Contrarily, Francois et al. (1986) observed the increment of protein content in durum wheat under salt stress. Environmental factors, especially heat and salt-stressed to promote leaf senescence at grain filling, which promotes protein deposition over accumulation of starch in the grain. It is due to the fact of carbohydrate synthesis, as well as translocation to the grain, is highly prone to adverse and harsh growing conditions compared to protein synthesis and translocation ( Fernandez-Figares et al., 2000 ). Furthermore, it is also established a fact that the protein and starch deposition rate and duration in the cereals grain are entirely independent events, which are influenced and governed by a group of environmental factors ( Jenner et al., 1991 ). Likewise, starch deposition seemingly remains more sensitive under salt stress for shortening of grain filling period in comparison to the deposition of protein. Moreover, optimum environmental factors cause a delay in leaf senescence, which promote nitrogen absorption from the soil and subsequently translocate it to leaves. In this way, a higher grain yield with lower protein content is produced by wheat. The existence of a negative relationship between protein content and grain yield has been reported in wheat, barley, and triticale (manmade cereal developed by crossing wheat and rye) crops ( Garcia del Moral et al., 1995 ). In response to imposed salinity, photosynthetic rate declines as feedback response due to several internal changes leading to higher demand for assimilates; triggering the rate of carbohydrate accumulation and cause a delay in leaf senescence and consequently promoting the onset of Rubisco hydrolysis. The net result is limited N availability, which tends to remobilize toward the grains. Moreover, salinity exposure results in rapid degradation of the RuBisCo enzyme, leading to the higher redistribution of N to the developing grains ( Fernandez-Figares et al., 2000 ).
Gluten Content
The proteins for gluten storage are divided into gliadins (confers extensibility) and glutenins (causes elasticity). Similar to the protein content of the wheat grain, salinity tends to boost wet and dry gluten content in salt-tolerant wheat cultivars, while the opposite has been observed for salt-sensitive cultivars ( Khan et al., 2008 ). Regarding the salt stress effect on the gluten content of wheat grains, it multiplied with increasing salt content in the soil ( Shen et al., 2007 ). In contrast, Kahrizi and Sedghi (2013) inferred that salinity has a very minute impact on the gluten content of cereals grains, while Houshmand et al. (2014) observed that abiotic stresses (salt and drought) result in a significant increment of wet and dry gluten contents. Zheng et al. (2009) also found that the gluten content of wheat cultivars has a direct relationship with the salt concentration of soil. There is a dire need to conduct further in-depth studies to determine the impact of salinity levels on gluten concentration and composition in wheat grain under varying pedo-environmental conditions.
Ash Content
The Ash content of wheat grain represents the mineral constituents of grain. In comparison to saline conditions, optimal growing conditions give rise to the higher ash content of whole-grain owing to improved minerals uptake from the soil solution ( Troccoli et al., 2000 ). Contrarily, Katerji et al. (2005) found an inverse relationship between salinity and ash content of durum wheat. Likewise, Francois et al. (1986) reported that soil salinity reduces ash content of durum as well as semi-dwarf bread wheat; thus, it may be inferred that there are disturbing effects of soil salinity pertaining to the minerals uptake, translocation as well as accumulation processes which result in a severe decline of ash content in wheat grain. Reddy et al. (2003) examined that salinity significantly reduced the grain yield as well as quality along with crop residue quality. Francois et al. (1986) concluded that soil salinity reduced the ash content and improved the flour color as well as protein content. Katerji et al. (2005) reported that excessive salt decreases ash content of wheat, and in this way, wheat grain quality gets improved. Moreover, the direct relationship between water use efficiency (WUE) and ash content of wheat grain can be a useful indicator to select wheat varieties having superior WUE under saline conditions.
Carbohydrate Content
Carbohydrate content is an important indicator of wheat grain quality, which is influenced by salinity stress, especially when wheat plants are exposed to saline environment at the grain filling stage. The synthesis and translocation of carbohydrates are more sensitive to suboptimal growing conditions compared to protein production ( Rao et al., 1993 ). Salinity stress at the post-anthesis stage seriously shortens the accumulation duration of storage proteins leading to modification in gliadins and glutenins accumulation pathways. Moreover, the disruption effects of salinity on photosynthesis rate reduces carbohydrates synthesis at the vegetative growth stage, while it also disrupts or halts the translocation of carbohydrates toward grains at the initiation of grain filling stage, and thus it results in a significant reduction of carbohydrates concentration in wheat grain ( Fernandez-Figares et al., 2000 ).
Beta-Carotene Content
There are rare research investigations that have focused on wheat grain quality, especially beta-carotene content under salinity stress. The beta-carotene contents varied significantly in salt-tolerant and sensitive cultivars of durum wheat under saline conditions. The beta-carotene content of grains recorded a sharp decline in wheat cultivars under salt stress environments ( Katerji et al., 2005 ). Likewise, Francois et al. (1986) reported that both beta-carotene and ash content witnessed a sharp decline owing to salinity. Future studies need to explore the detrimental effect of saline environment on carotene contents with respect to interactive effect of varying genotypes, agro-environmental factors and agronomic management practices along with frequency and intensity of salinity.
Salinity and Nutrient Imbalance
One of the adverse effects of a saline environment, especially high salt concentration in soil solution, causes a severe reduction in the uptake of nutrients and water. Resultantly, osmotic stress intensifies ion toxicity, imbalance of nutrients under water-deficit conditions. Salinity leads to injury of photo-synthetically active leaves by causing chlorosis and triggering leaf senescence in cereals ( Hanin et al., 2016 ). Ion imbalance is one of the most severe impacts caused by a saline environment dominated by NaCl presence, while numerous other ions also multiply the severity of the stress. The combinations of ions in the saline environment determine the types of nutrients which become either deficient or excessive ( Hawkins and Lewis, 1993 ). The underlying mechanism of ionic imbalance has recently been elaborated. Under low-moderate salinity, the nutrient imbalance is prevented by salt ions transportation, which occurs within the vacuole that tends to off-set the ion flux into the cell from the plasma membrane ( Blumwald et al., 2000 ). When the influx rate is higher, ionic homeostasis in cells gets compromised, and anions (Cl − ) and cations (Na + , Mg 2+ , Ca 2+ ) accumulate in the plasmatic compartments (cytosol, matrix, and stroma) of the cell instead of the vacuole, which results in nutrient imbalance especially K and P. To avoid ion imbalance in the short term, accumulation of ions in the apoplast continues, and resultantly no transportation of ions from the apoplast to the symplast occurs. Such ion accumulation in apoplast is usually regarded as the causal mechanism behind salinity-induced ionic imbalance ( Speer and Kaiser, 1991 ). Accumulation of salt in the leaf apoplast disrupts cell water relations leading to wilting ( Flowers et al., 1991 ).
Inorganic ions often perform to be competitive enzyme inhibitors, which host ionic substrates but also interfere with protein surface charges besides destabilizing molecular level interactions. The nutrient imbalance under saline conditions leads Na + to substitute K + from the essential binding sites. In biochemistry, the typical instances for K + dependency are ribosomes and pyruvate kinase. It has been reported that K + presence in optimal concentration boosts pyruvate kinase activity (Vmax) as much as 400 times ( Oria-Hernández et al., 2005 ), while K + substitution by Na + causes inhibition up to 92%. In addition, peptidyl transferase activity in eukaryotic ribosomes gets regulated by K + concentration and might reach upto 20 s −1 under optimal conditions ( Ioannou and Coutsogeorgopoulos, 1997 ). However, metabolic imbalances occur in the case of drop-in K + concentration and an increase in Na + concentration, and such ionic imbalance leads to the initiation of numerous inhibitory processes that cause redox and energy metabolism.
Under normalized conditions, nutrient ion net fluxes of different cereals (maize, wheat, and barley) and legumes such as broad beans get adjusted in accordance with cellular requirements and crop development phases, leading to the establishment of ionic homeostasis ( Niu et al., 1995 ). However, salinity severely disturbs ionic harmony as excessive salt ions (e.g., Na + , Cl − or Mg 2+ and SO 4 2 - ) accumulation alters the composition of the soil solution. Excessive accumulation of the Na + cation disturbs uptake of many cationic nutrients such as K + ( Wakeel et al., 2011 ) or Ca 2+ ( Gardner, 2016 ); resulting in nutrient imbalance. For instance, excessive Na + induces Ca 2+ deficiency having an appearance like lesions on aerial plant parts along with a reduction in leaf blade dry weight ( Maas and Grieve, 1990 ). Furthermore, Na + induces K + uptake reduction leading to declined shoot growth. In contrast, Cl − presence in excessive concentration leads to impairment of nutrient uptake by disturbing anions uptake ( Geilfus, 2018a , b ). The uptake and anion-anion interactions are antagonistic in nature as the Cl − concentration multiplies by many folds in the soil solution under NaCl salt stress. It has been established that Cl − is greatly mobile in the soil, and negatively charged kaolin and clay minerals significantly repel Cl − leading to its accumulation in the soil macro-pores and soil solution ( Thomas and Swoboda, 1970 ). On the other hand, cations, including Na + , get absorbed in very minute concentration by the negatively charged soil surface under the soil environment ( Borggaard, 1984 ). Moreover, antagonism has been reported among Cl − and nitrate (NO 3 − ) when external Cl − concentrations become too higher ( Abdelgadir et al., 2005 ), which causes a reduction in the growth and yield of wheat ( Hu and Schmidhalter, 1998 ). However, this has not been similar for corn crop ( Hütsch et al., 2016 ). Interestingly, severe competition for anion-anion uptake has also been described for Cl − and phosphate (PO 4 3− ). In addition to cereals, such competition has also been reported for tomatoes as well as rose plants ( Massa et al., 2009 ).
Under NaCl salinity, it seems that Cl − tends to hinder the growth and development of crops by inducing the deficiency of phosphorous and sulfur through inhibiting PO 4 3− and SO 4 2− uptake. However, facts remain that generalizable conclusions may not be drawn from the published research and relevant findings. For concise conclusiveness, it becomes pertinent to distinguish between Cl − and counter-cations effect under saline environment. There is a dire need to perform further experiments regarding the behavior of membrane-impermeable counter-cations of a specific salt. Besides, the underlying molecular mechanism of nutrient-nutrient antagonistic uptake remains unclear. However, one of the possible justifications can be attributed to antagonistic competition for a binding site at transport proteins of salts ions. Another justification can be leaking of Cl − from protein pores, which quantitatively displace PO 4 3− or SO 4 2− leading to a sharp decline in their take up. Both above-stated scenarios rely on transmembrane pores, physicochemical attributes such as their charge and size. For instance, hydrated Cl − ion radius is similar to SO 4 2− . It seems that under salinity stress, glycophytic crops did not encounter the necessity to escape Na + and Cl − uptake during the breeding and evolution process, which hampered the development of adaptive mechanism at the transporter site for differentiating among the requisite nutrients and undesired ions of salts. Thus, it might be inferred that two pronged strategy encompassing reduction in the uptake of salt ions and their replacement in the soil solution as well as plant need further investigations to cope with the serious challenge of salinity and impart sustainability to cereals production.
Water Relation to Salinity Stress
Wheat plants exposed to salt stress change their environmental condition. The capability of plants to tolerate salt is determined by several biochemical ways that facilitate the acquisition or maintenance of water relations, ionic homeostasis, and protect chloroplast functioning. In agriculture, salinity has been the most distressing abiotic stress having pronounced damaging effect on physiological, morphological, and biochemical characteristics of the crop plants, including uptake of water and nutrients, germination, growth, photosynthesis, enzyme actions, and yield ( Cisse et al., 2019 ). Plant water status is hard to measure as it keeps on changing every minute-to-minute. Since stomatal conductance on which it is entirely dependent in the short term ( Gil-Muñoz et al., 2020 ), and psychometric or pressure chamber measurements are often tricky to deliver accurate values ( El-Hendawy et al., 2005 ). In areas where the rainfall is low and the salt remains in the subsoil, increased salt tolerance would allow plants to extract more water ( Munns et al., 2006 ). Mostly wheat productivity is reduced when water stress occurs at heading time, and the reduction is severe when it occurs after anthesis ( Barutcular et al., 2016a , b ).
Accessibility of water in plants is a crucial factor for all physiological and metabolic processes of plants ( Sreenivasulu et al., 2007 ). The higher concentration of salts causes osmotic stress to plants, which results in low water potential in wheat crop ( Qamar et al., 2020 ). The rate at which new leaves are produced depends basically on the water potential of the soil water, in the same way as for a drought-stressed plant. According to the previous investigations, there is a link between the different physiological reaction of crops to stress and their tolerance mechanisms, viz. high relative water content and water potential ( Datta et al., 2011 ). Singh et al. (2015) reported that salinity prevents seed germination by either exerting osmotic stress that thwarts uptake of water. Such stresses cooperatively inhibit cell expansion and division, as well as modulates the activity of some key enzymes, thus reduces the seed reserves utilization ( El-Hendawy et al., 2019 ).
Water potential of plants at reproductive phases also reduces the cell extension, vascular tissue thickness, flag leaf thickness, mesophyll, and epidermal cell size, which are responsible for the reduction of flag leaf turgidity, flag leaf area, assimilates synthesis, and yield potential ( Nassar et al., 2020 ). Zhang S. et al. (2016) reported that relative water content (RWC) declined by 3.5 and 6.7%, compared to their controls in the salt-tolerant, and salt-sensitive cultivars, respectively, after 6 d of 100 mM NaCl exposure. Further research resulted in a significant reduction in water use efficiency (WUE) of both tolerant and sensitive cultivars under saline field conditions ( Gil-Muñoz et al., 2020 ). The ratio of water content decreased in the root but increased in shoot and spike of other cultivars of wheat ( Tammam et al., 2008 ). Moreover, a direct relationship between WUE and an ash content of wheat grain can be a useful indicator to select wheat varieties having superior WUE under salinity ( Zhang et al., 2020 ).
Consequently, from these findings, we conclude that salinity stress is a major factor for limiting yield and grain quality traits, and it affects the reproductive phase severely by altering ionic homeostasis, water status, and assimilate partitioning. Foliar application of antioxidants and growth regulators that maintain an appropriate water level in the leaves to facilitate adjustment of osmotic and stomatal activity ( Arshad et al., 2020 ). The introduction of deep-rooted crop species is necessary to lower the water table, but salt tolerance will be required not only for the “de-watering” species but also for the annual crops that follow, as salt will be left in the soil when the water table is lowered.
Approaches to Improve Salt Stress Tolerance in Wheat
Improving the crop performance by conventional breeding methods, introducing gene markers, and selection of genetically modified genotypes are the basic approaches to produce tolerance against salinity stress in plants ( Hasanuzzaman et al., 2011 ). In recent year development of transgenics (transfer of genes from tolerant sources) are a promising approach to developing stress-tolerant varieties. Several transgenics are also developed in wheat crop to ameliorate the adverse effects of salinity. Table 1 represents the transgenic developed in wheat crop using the osmoprotectant, ion transporters genes and their source and influenced traits to make salinity tolerant wheat.
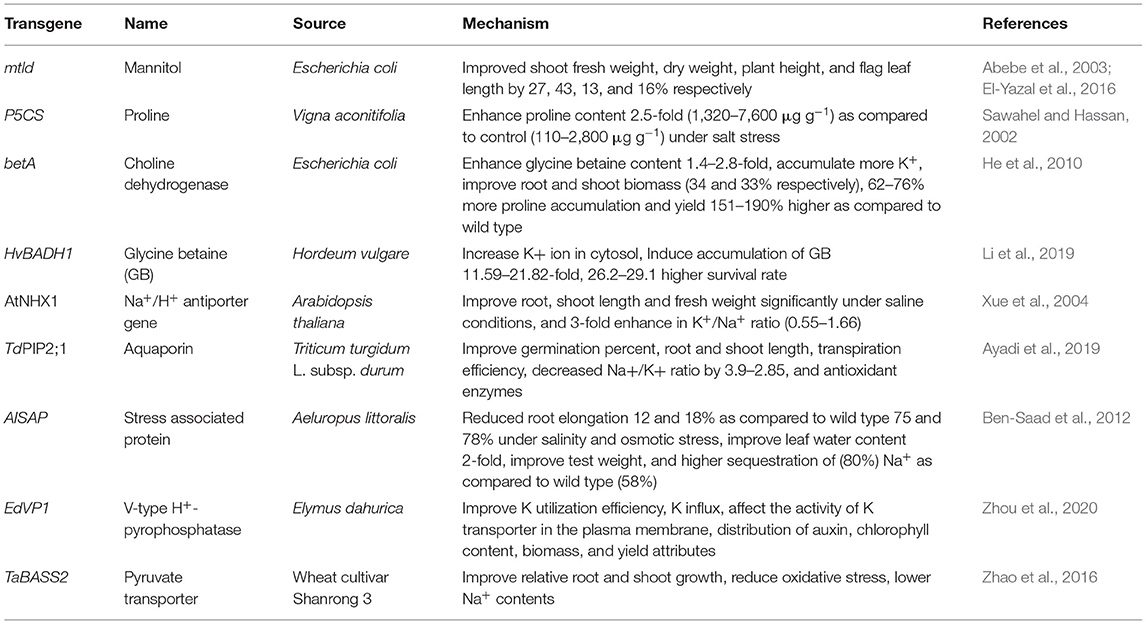
Table 1 . Highlights the some transgenic developed in wheat crop and influenced traits for salinity tolerance.

Salt Tolerance Through Osmoprotectants
Various mechanisms are adopted by plants under salinity stress at the organism and tissue level to avoid adverse effects of salinity. Plants produce osmolytes and some beneficial solutes that prevent them from the impact of salinity stress by maintaining osmotic and ionic balance ( Ashraf and Foolad, 2007 ). These compatible solutes and osmoprotectants are uncharged, less toxic soluble organic solutes that provide a -optimal environment to plants under salt stress. Proline, glycine betaine, salicylic acid (SA), and sugar alcohols like trehalose, sorbitol, and mannitol are examples of osmolytes. Membrane stabilization, protein synthesis, and maintenance of osmotic pressure are the important functions performed by these osmolytes and exogenous application of these compounds are helpful for improving salt tolerance in wheat. Plant's exposure to salt stress improves their ability to synthesize osmolytes like glycine betaine, sugar alcohols, and sorbitol that helps them to survive under salinity ( Chen and Jiang, 2010 ). The use of osmolytes was found very vital against salt tolerance induction ( Alam et al., 2014 ). Application of trehalose (10 mM) reduces H 2 O 2 level, lipid peroxidation, free amino acids, proline and LOX enzyme activity, while improves photosynthetic pigments, accumulation of organic solutes and elevating the expression of AOX, NHX1, SOS1 to ion homeostasis under salinity stress ( Alla et al., 2019 ). Further, seed priming and foliar application with plant growth regulators (PGRs), inorganic and organic ion found effective in salinity tolerance as they improve osmolytes production and strength the antioxidant system of plants ( Wajid et al., 2019 ). Recently, the metabolic engineering of osmoprotectants elucidates the mechanisms of salinity tolerance in crops. Further, they also suggested that engineering of these metabolic compounds open a way for understanding the complex nature of salt stress and its relation with other crucial plant processes ( Alzahrani, 2021 ). However, thorough investigations are needed to identify crop-specific osmoprotectants, their dose optimization for exogenous application, time of foliar sprays and stage of crops.
Salinity Tolerance Through Plant Hormones
Salt stress tolerance and plant growth regulation are associated with the biosynthesis of a variety of compounds in minute concentrations, which are termed asplant hormones ( Ryu and Cho, 2015 ). Salt stress was mitigated by several types of plant growth hormones, abscisic acid (ABA), auxins (AUX), cytokinin (CK), and ethylene (ET). Auxin is an important growth regulator that enhances seedling establishment, shoot dry weight, and also balanced the ionic pressure in plants under salinity stress ( Iqbal and Ashraf, 2007 ). Primed seeds with growth regulator auxin mitigated the salinity by improving the hormone balance, nutrients uptake leading to better yield and productivity of both salt resistant and susceptible genotypes. Crop growth rate, leaf area index, photosynthetic efficiency, grain yield, and its quality were improved by seed priming with gibberellic acid (GA) ( Shaddad et al., 2013 ). Foliar applied -GA also enhances salinity tolerance by increasing seedling establishment, plant productivity, and antioxidant enzymatic efficiency under salt stress ( Tabatabaei, 2013 ). Salinity stress could be mitigated by priming with cytokinin that improved seedling establishment, tillers formation, and grain weight under salt-affected soils ( Iqbal et al., 2006 ).
Plant Nutrients
The availability of nutrients is also responsible for mitigating the salt stress effect by various physiochemical and biological mechanisms. Foliar application of potassium improves photosynthesizing efficiency, antioxidant enzymatic efficiency, potassium intake by plants, and sodium absorption and salt stress environments ( El-Lethy et al., 2013 ). Sodium absorption, leaf area index, and biological yield were found to be improved under stressed environments by external application of phosphorous ( Khan et al., 2013 ). Calcium sulfate is also an important nutrient that alleviates the ill effect of salt stress by improving the intake of calcium and potassium and improves crop growth in salinity stress ( Zaman et al., 2005 ). The application of calcium nitrate decreased the peroxidation of lipids and excretion of electrolytes that enhanced the salt tolerance in plants ( Tian et al., 2015 ). Recently the application of inorganic nutrients (Si, K, Zn), organic extract (moringa leaf extract, biochar), as seed or foliar treatments improve the salinity stress tolerance ( Jan et al., 2017 ). Likewise, the application of nanoparticles (Zn, Si) ( Mushtaq et al., 2017 ) showed promising results. Although, the molecular understanding regarding the application of different nutrients and transporters response can accelerate the salinity stress tolerance program in plants.
Salt Tolerance Through Various Signaling Molecules
Various signaling molecules can crosstalk with phytohormones and antioxidants within the plants that help plants to survive under salt stress. Nitric oxide (NO) is the most widely used signaling molecule to mitigate various abiotic stresses, particularly salt stress. It has interaction with other molecules by several pathways due to its signaling role besides improved crop productivity under salt-stressed environments ( Hasanuzzaman et al., 2013 ). A valuable improvement was noticed in the wheat seedling establishment by exogenous application of NO under salt stress. Other than the NO, hydrogen sulfide (H 2 S), H 2 O 2 , and ROS have a significant role in salinity stress tolerance ( Khan et al., 2017 ). These compounds have crucial roles in the regulation of salinity stress tolerance via ion, hormonal, and redox homeostasis in salt-affected cells and reduce the drastic effect by strengthening the antioxidant defense ( Bhuyan et al., 2020 ). Moreover, these compounds have the ability to crosstalk with other signaling compounds and plant growth regulators such as melatonin, ET, ABA, and salicylic acid ( Kolbert et al., 2019 ). Although, the last few years study of gas transmitters (gaseous signaling molecules) under plant abiotic stresses opens gate for further understanding the abiotic stress signaling mechanisms in depth. However, depth study at molecular level still required to complete understanding of abiotic stresses signaling mechanisms.
Use of Plant Growth Hormones in Mitigating Salinity-Induced Damages
Salt stress adversely affects plant growth and related metabolites, and consequently reduces crop yield ( Islam et al., 2011 ). The reduction of plant growth and alteration of related metabolites is evident due to the decline of natural growth hormones in plant tissues ( Yurekli et al., 2004 ). Wang et al. (2001) stated that salinity stress decreased the indole-3-acetic acid (IAA) and salicylic acid (SA), whereas increased the abscisic acid (ABA) and jasmonic acid (JA). Exogenous application of hormones, i.e., plant growth regulators (PGRs), ameliorated the negative effect of salt-induced stress and enhanced the crop yield. PGRs under the saline environment can alleviate the adverse effects of salt stress by enhancing the germination and seedling growth of wheat ( Samad and Karmoker, 2012 ).
Gibberellic Acid (GA 3 )
Gibberellic acid improved the growth criteria, photosynthetic pigments, and consequently the crop yield of wheat cultivars due to better osmoregulation resulting in increased water flow and water status using the organic solutes (saccharides and proteins), which in turn increased the photosynthetic area and yield ( Shaddad et al., 2013 ). Under saline stress conditions, GA 3 stimulated the growth of wheat ( Afzal et al., 2005 ), maize ( Hassanein et al., 2009 ), and sorghum ( Azooz et al., 2004 ). At the same time, GA 3 modulation could support abiotic stress tolerance in wheat crop ( Abhinandan et al., 2018 ). GA 3 signaling mechanism involved in various developmental and abiotic stress conditions is mediated through an important GA 3 signaling molecule called DELLA protein. DELLA protein regulates a complex network of transcription factors and genes ( Schwechheimer, 2012 ). GA 3 signaling has shown the targeted deterioration of a group of GA 3 -response through transcriptional repressors ( Golldack et al., 2014 ). Such signal pathway has observed to be dominant to the success of wheat crop seedlings in the Mediterranean soil under water deficit conditions ( Amram et al., 2015 ). It was found that the endogenous level of GA 3 is inhibited under drought and salt stress ( Llanes et al., 2016 ). Uses of GA-inhibiting compounds drought-stressed plants alleviate the negative effects of stress and increase bio weight and yield ( Plaza-Wüthrich et al., 2016 ). It has been also found that GA and auxin (IAA) treatment in wheat reduces the negative effects of ethylene and regulate salinity tolerance in wheat crop ( Abd El-Samad, 2013 ).
Abscisic Acid (ABA)
Abscisic acid has been found to be the main regulator of abiotic stress tolerance in wheat via regulation of protein kinases activities, which is important for phosphorylation processes ( Umezawa et al., 2009 ). The contribution of Snf1-related protein kinases (SnRKs) has been studied extensively in Arabidopsis. Some studies of SnRKs in wheat has shown the presence of other SnRK2 homologs that appear to play a role in ABA-mediated abiotic stress signaling ( Mao et al., 2010 ). The expression of wheat SnRK homologs is stimulated by salt stress, cold stress, drought (induced by PEG), and the application of exogenous ABA ( Tian et al., 2013 ). Likewise, Dong et al. (2013) reported that 12-oxo-phytodienoic acid reductases (OPRs) gene associated with the synthesis of ABA and promotion of ABA-dependent and ROS related stress signaling mechanisms and confer salinity tolerance in wheat. Recently, an ABA functional analog B2 has been shown a positive response under salinity stress by enhancing the expression of ABA-responsive genes and antioxidant activity ( Duan, 2020 ). Moreover, ABA is a crucial stress hormone that regulates the multiple abiotic stresses in the plant including salinity via crosstalk with other signaling molecules and PGRs. To date, numerous genes/transcription factor are identified (TaASN1, TabHLH1) ( Yang et al., 2016 ), which associated with ABA signaling and stress tolerance.
Auxin and Cytokinin
The exogenous application of auxins, cytokinins mitigate the adverse effects of salt stress and consequently improved seed germination and growth ( Naidu, 2001 ; Khan et al., 2004 ). Auxin and targeted cytokinin signaling is observed to be suppressed during abiotic stress signaling in wheat plants ( Abhinandan et al., 2018 ). Furthermore, the cytokinin and auxin pathways regulate a plethora of developmental processes through their dynamic and complementary actions and their ability to crosstalk support as perfect candidates for mediating stress-adaptation responses ( Bielach et al., 2017 ). Tryptophan derivative auxin is mostly present in the form of indole-3-acetic acid (IAA), and it was decreased in the leaves of wheat under the cold or frost stress ( Kosová et al., 2012 ). It was shown that the cold-sensitive wheat variety was characterized by a high concentration of auxin followed by an acclimatization period connected with reduced fitness. The cytokinin level is reduced early in cold-tolerant wheat varieties as compared to the cold-sensitive ones ( Kosová et al., 2012 ). The imposition of drought and salt stress during the reproductive stages of wheat has been shown to reduce the grain filling, which allied with reduced auxin concentration ( Abid et al., 2017 ). Nishiyama et al. (2011) observed that drought stress decreased the cytokinin biosynthetic enzymes along with suppressed positive cytokinin signaling components, which might improve the drought-tolerance of wheat plants. The reproductive phase of wheat is more sensitive to stress, and the imposition of drought stress at this stage reduced grain filling, which is linked with low levels of auxin and cytokinin, while external cytokinin application rescued this problem ( Abid et al., 2017 ). It has been reported that cytokinin increases the activities of antioxidant enzymes catalase (CAT) and ascorbate peroxidase (APX), prevent cellular damage by ROSs, and shore up antioxidant molecules, xanthophyll ( Wilkinson et al., 2012 ).
Salicylic Acid
The application of salicylic acid (SA) mitigates the harmful effect of abiotic stresses ( Abhinandan et al., 2018 ). External application of SA in wheat has been shown to be a signaling molecule to stimulate the internal radical detoxification system ( Noreen et al., 2017 ; Fardus et al., 2018 ). Exogenous application of SA in wheat supports antioxidants production via utilizing ROS to be a second messenger ( Agarwal et al., 2005a , b ). Such responses can reduce oxidative damage under drought, heat, and saline conditions ( Fardus et al., 2018 ). Salicylic acid also induces the accumulation of auxin and ABA, which provides tolerance against salinity stress ( Shakirova et al., 2003 ). Likewise, the seed priming with SA, ABA and ascorbic acid improved the seedling performance by enhancing seedling fresh weight, length, and decrease electrolyte leakage. Although, the authors suggested that the priming with ABA is not so effective ( Afzal et al., 2006 ).
The promotion of abiotic stress tolerance in wheat has been shown by ethylene inhibitors ( Abhinandan et al., 2018 ). Ethylene modulates salinity stress responses largely via maintaining the homeostasis of Na+/K+, nutrients, and ROS by inducing antioxidant defense in addition to elevating the assimilation of nitrates and sulfates. Moreover, a cross-talk of ethylene signaling with other phytohormones has also been observed, which collectively regulate the salinity stress responses in plants ( Riyazuddin et al., 2020 ). Ethylene participation in the later phase of plant growth and development relates to leaf abscission, ripening of fruit, maturation of seeds, and plant drying ( Wilkinson et al., 2012 ). It was shown the role of glycine betaine (GB) and ethylene in salt tolerance variation of different wheat cultivars. The salt-tolerant cultivar exhibited GB content, which was found correlative with ethylene ( Khan et al., 2012 ).
The activation and expression of some ethylene-responsive factors, proteins like TaERF1 and TaERF3, which support resistance to multiple stresses, can be induced by the perception of abscisic acid or ethylene ( Rong et al., 2014 ). The study with isolation and molecular characterization of TdERF1, an ERF gene from durum wheat (Triticum turgidum L. subsp. durum) different varieties showed that TdERF1 gene may provide a discriminating marker between tolerant and sensitive wheat varieties ( Makhloufi et al., 2014 ). The severity as well as stress period dictates the level of ethylene evolution and its response levels.
Salt stress declines the metabolic activity of plant cells, which inevitably reflects the inhibition of plant growth ( Çiçek and Çakirlar, 2002 ). Application of ethephon and zeatin reverse the growth-inhibiting effect of salt stress and increase the shoot and root growth of wheat ( Egamberdieva, 2009 ). Gul et al. (2000) stated that the plant growth stimulating compounds such zeatin, and ethephon alleviate the negative effect of salt stress and increase the germination and growth of Ceratoideslanata, Halogetonglomeratus ( Khan et al., 2004 ).
Brassinosteroids (BRs)
The application of brassinosteroids (BRs) exogenously increases plant tolerance against abiotic stress ( Abhinandan et al., 2018 ). Though the effect of BRs on growth and development of wheat has been studied extensively but has not been much studied at the molecular level. Although a detailed study regarding the BR receptor and other signaling components has been performed in the case of Arabidopsis , there are only limited studies of BRI1 orthologs availability in other taxa. Navarro et al. (2015) reported the presence of a single copy of BRI1 ( TaBRI1 ) in each of three wheat genomes on the long arm of chromosome 3 with in silico analysis. It is very difficult to prove directly that BRs can improve abiotic stress tolerance due to lacking in vivo genetic data. But the exogenous application of BRsis significantly able to improve abiotic stress tolerance in wheat crop. The growing of wheat seedlings under 0.4 μM 24-epibrassinolide treatment has shown high levels of cytokinin in roots and shoots as compared to control plants ( Yuldashev et al., 2012 ). The BRs acts as a defense agent under cadmium (Cd), lead (Pb), and salinity stress-induced Triticum aestivum . Wheat plants grown in cadmium and saline conditions normally have decreased photosynthetic activity and growth, but simulated antioxidant enzymatic activity and increased Pro concentration ( Hayat et al., 2014 ). The manipulation with strigolactone, which is engaged in symbiotic relations in the rhizosphere, provides new ways for generating stress tolerant wheat germplasm ( Abhinandan et al., 2018 ). Plants treated with synthetic SL analog GR24 exogenously under drought stress conditions significantly increased the thousand-grain weight as compare to non-treated drought-stressed two winter wheat cultivars ( Sedaghat et al., 2017 ). Future research need to focus the vital role of synthetic and natural hormones for alleviating salinity stress as various hormones hold potential to modulate and regulate vital metabolic processes under stressful environment.
Plant Growth-Promoting Bacteria (PGPB)
Plant growth-promoting bacteria directly or indirectly enhanced the growth of plants ( Ahmad et al., 2013b ). It has been reported by Kotuby-Amazher et al. (2000) that beneficial microorganisms could reduce salt stress by ~50% in wheat. PGPB produce some beneficial compounds like indole acetic acid, siderophore, HCN, etc., solubilize minerals and break down organic matters for easy uptake by plants, fix atmospheric nitrogen, and produce siderophores that enhance the bioavailability of iron, and synthesize phytohormones such as cytokinin's, auxins and gibberellins which have beneficial roles in various stages of plant growth ( Richardson and Simpson, 2011 ). PGPB also decreases or inhibits the detrimental effects of pathogenic organisms by enhancing the host resistance to pathogenic organisms ( Van Loon, 2007 ). Orhan (2016) reported that halotolerant and halophilic bacterial strains significantly increased the root and shoot length, and total fresh weight of wheat under severe salt stress (200 mM NaCl). IAA is declined due to the negative effect of salt stress ( Wang et al., 2001 ), and bacterial strains P. aureantiaca TSAU22, P. extremorientalis TSAU6, and P. extremorientalis TSAU20 produce IAA, which alleviate the reductive effect of salt stress and increase the germination percentage (up to 79%) of wheat ( Egamberdieva, 2009 ).
The cross talks of signaling molecules with other PGRs compounds in wheat crop under salinity are highlighted in Table 2 . In this table different signaling molecules and PGRs are participating in common signaling pathways and regulates the multiple stresses tolerance.
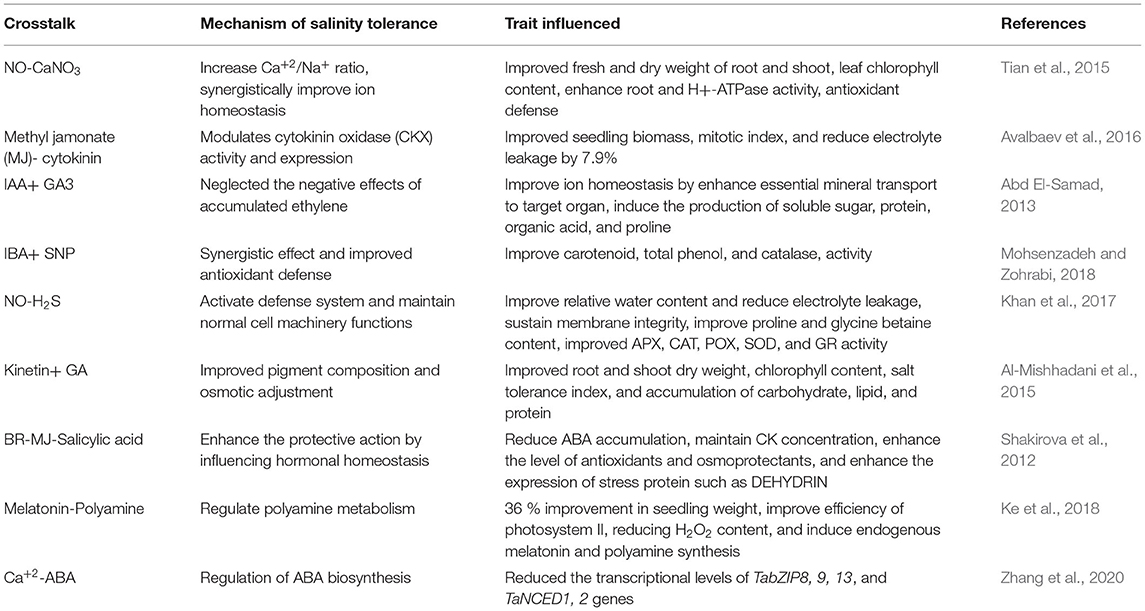
Table 2 . Highlights the crosstalk between plant growth regulators and signaling compounds to ameliorate the salinity stress in wheat crop.
Antioxidant Defense in Response to Salinity-Induced Oxidative Stress
Salinity stress hampers the antioxidant strength via altering antioxidant enzyme activity viz. biosynthesis of ascorbate peroxidase (APX), sodium dismutase (SOD), and glutathione reductase (GR), and non-enzyme antioxidant (ascorbic acid, glycine betaine, and proline). These changes in antioxidants cause the accumulation of harmful ROS, malondialdehyde (MDA), and elevation of lipid peroxidation, ion leakage, membrane stability, and ultimately weakening of antioxidant system ( Sairam et al., 2002 ).
Oxidative stress is caused by production and accumulation of ROS in cells and tissues owing to irregularities in the electron transport chain (ETC) that cause lipid peroxidation, protein oxidation, nucleic acid damage, enzyme inhibition, activation of programmed cell death pathway, and ultimately causing cell death ( Hossain et al., 2021 ). In the biological system, the plants can generally detoxify these reactive products. Under normal growth conditions of the plant, toxic oxygen metabolites are produced in low volume, and there is desired balance between production and ROS quenching; however, this balance may be disturbed by several adverse conditions, including salinity stress. The common ROS are superoxide radicals (O 2 ∙−), hydrogen peroxide (H 2 O 2 ), hydroxyl radicals (∙OH), and singlet oxygen ( 1 O 2 ) ( Navarro-Yepes et al., 2014 ). All ROS are extremely deleterious to plants at higher concentration. To understand salt adaptation strategies of plants, it is an important task of plant biologists to find out suitable mechanisms for developing salt-tolerant wheat that can produce sufficient yield under stress ( Kaur et al., 2017 ). Priming and exogenous use of different eco-friendly compounds had been exploited to tolerate the salt stress-induced oxidative stress in different crops, including wheat ( Kaur et al., 2017 ).
Several antioxidants have been exploited in recent years, which have a beneficial effect against oxidative stress, and to avoid oxidative damage higher plants usually raise the concentration of the endogenous antioxidant system comprising of enzymatic and non-enzymatic components to scavenge ROS ( Sharma et al., 2012 ). In-plant cells, specific ROS producing, and scavenging systems have been established in different organelles viz., mitochondria, chloroplast, and peroxisomes etc. The enzymatic components of the antioxidative defense system (ADS) are comprised of several antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) ( Hossain et al., 2021 ). The non-enzymic components of the ADS include the major cellular redox buffers, ascorbate (AsA) and glutathione (GSH), as well as tocopherol, carotenoids, and phenolic compounds ( Mandhania et al., 2006 ). They interact with numerous cellular components and, in addition to crucial roles in defense and as enzyme cofactors ( Rakhmankulova et al., 2015 ). Ascorbate has remained the most abundant, low molecular weight antioxidant playing a vital role in defense against oxidative stress caused by increased ROS level by scavenging O 2 - and H 2 O 2 ( Karuppanapandian et al., 2011 ). Mandhania et al. (2006) found the NaCl induced enhancement of peroxidase activity in salinized cells of wheat by decomposition of H 2 O 2 generated by antioxidant SOD. Catalase is another enzyme involved in H 2 O 2 detoxification and its conversion into water and oxygen. Similar results were also found by Sairam et al. (2002) , who reported enhancement in CAT activity in both salt-sensitive as well as salt-tolerant wheat cultivars. Mandhania et al. (2006) also reported that stimulation of APX and GR activity under salt stress was much higher in the salt-tolerant cultivar. Kaur et al. (2017) found that exogenous application of phenolic acids (caffeic and sinapic acids) upregulated the stressed shoots of a salt-tolerant wheat cultivar by increasing the activity of CAT and peroxidase. In comparison to stress plants, without the application of phenolic acids, APX activity was upregulated in stressed seedlings, and GB was increased in the stressed roots. Phenolic acids being major non-enzymatic components of the defense system can be used to enhance endogenous levels of these antioxidants in wheat seedlings grown under salt stress conditions. In contrast, wheat crop crucial plant processes are affected by the salinity stress. Therefore, soil and agronomical (drainage system management, leaching of salt, nutrient managements), physiological (osmotic adjustment, seed priming, improve photosynthesis efficiency, and water relation), biochemical (redox, ion, and hormonal homeostasis), and molecular (development of transgenic, genetical engineering, identification of gene) are the crucial step in wheat salinity stress tolerant program ( Figure 3 ).
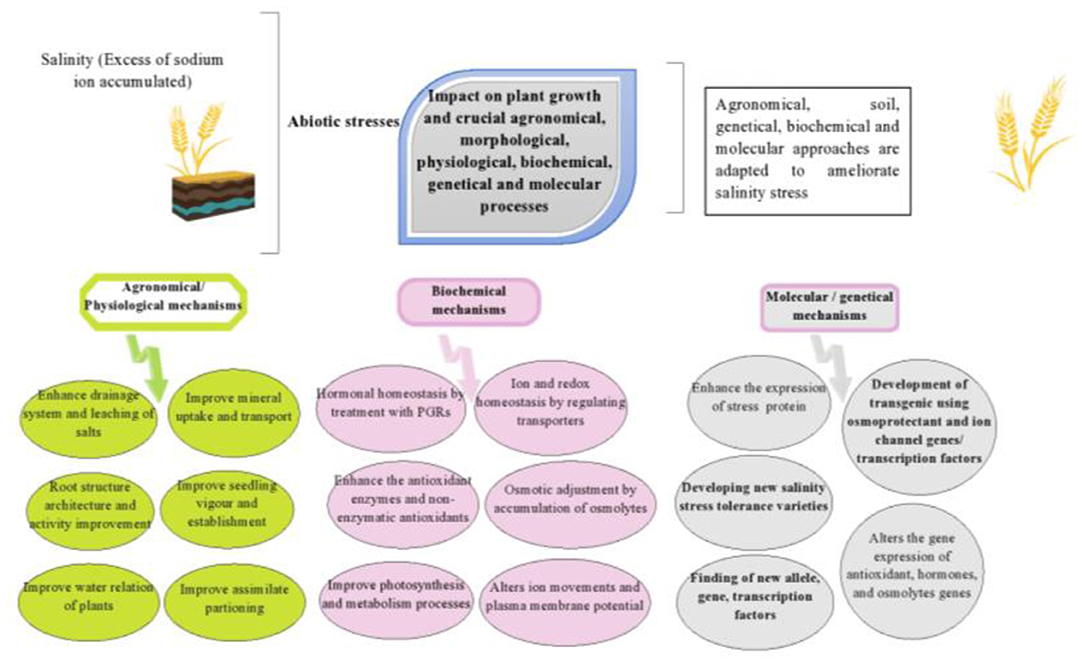
Figure 3 . Illustrate the integrated approaches (physiological, biochemical and molecular) of salinity stress tolerance in wheat crop.
Conclusions
Among abiotic stresses, salinity stress especially in the arid and semi-arid regions of the world is one of has emerged as one of the most important threats to the sustainability of wheat production. It reduces germination, seedling growth as well as reproductive growth by disrupting numerous vital physiological and metabolic processes which lead to sharp decline in yield and quality depending on frequency and extent of saline environment. Although salinity tolerant plants employ several physiological and biochemical mechanisms to adapt under salinity stress, there is a lack of robust salinity tolerant wheat cultivars globally. Therefore, plant physiologists, breeders, and agronomists need to develop an integrated and sustainable strategy to enhance salt tolerance in wheat. Among these mitigation strategies, soil management practices, crop establishment, as well as the foliar application of antioxidants and growth regulators through maintaining an appropriate water level in the leaves to facilitate adjustment of osmotic and stomatal performance could be explored further to mitigate the adverse effect of salinity on wheat yield and grain quality. However, breeding strategies especially gene pyramiding should be undertaken to develop salt-tolerant varieties by exploring halotolerant gene homologs from wheat germplasm. However, an integrated approach involving soil and agronomic practices (drainage system management, salt leaching, nutrients managements for salt ions replacement), physiological strategies (osmotic adjustment, seed priming, improve photosynthesis efficiency, and water relation), biochemical (redox, ion, and hormonal homeostasis), and molecular tools (development of transgenic, genetic engineering, identification of gene, genes insertion, editing, or slicing) needs to be developed to ameliorate salinity effects and boost cereal production on sustainable basis.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbas, G., Saqib, M., Rafique, Qur-Rahman, M. A., Akhtar, J., ul- Haq, M. A., and Nasim, M. (2013). Effect of salinity on grain yield and grain quality of wheat ( Triticum aestivum L.). Pakistan J. Agric. Res .. 50, 185–189.
Google Scholar
Abd El-Samad, H. M. (2013). The physiological response of wheat plants to exogenous application of gibberellic acid (GA3) or indole-3-acetic acid (IAA) with endogenous ethylene under salt stress conditions. Int. J. Plant Physiol. Biochem. 5, 58–64. doi: 10.5897/IJPPB12.016
CrossRef Full Text | Google Scholar
Abdelgadir, E. M., Oka, M., and Fujiyama, H. (2005). Characteristics of nitrate uptake by plants under salinity. J. Plant Nutr. 28, 33–46. doi: 10.1081/PLN-200042156
Abebe, T., Guenzi, A. C., Martin, B., and Cushman, J. C. (2003). Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol. 131, 1748–1755. doi: 10.1104/pp.102.003616
PubMed Abstract | CrossRef Full Text | Google Scholar
Abhinandan, K., Skori, L., Stanic, M., Hickerson, N., Jamshed, M., and Samuel, M. A. (2018). Abiotic stress signaling in wheat–an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci . 9:734. doi: 10.3389/fpls.2018.00734
Abid, M., Shao, Y., Liu, S., Wang, F., Gao, J., and Jiang, D. (2017). Pre-drought priming sustains grain development under post-anthesis drought stress by regulating the growth hormones in winter wheat ( Triticum aestivum L.). Planta 246, 509–524. doi: 10.1007/s00425-017-2698-4
Acquaah, G. (2007). Principles of Plant Genetics and Breeding . 2nd Edn. Oxford: Blackwell, 740.
Afzal, I., Basra, S. A., and Iqbal, A. (2005). The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 1, 6–14.
Afzal, I., Basra, S. M., Farooq, M., and Nawaz, A. (2006). Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int. J. Agric. Biol. 8, 23–28.
Agarwal, S., Sairam, R. K., Srivastava, G. C., and Meena, R. C. (2005a). Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol. Plant. 49, 541–550. doi: 10.1007/s10535-005-0048-z
Agarwal, S., Sairam, R. K., Srivastava, G. C., Tyagi, A., and Meena, R. C. (2005b). Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 169, 559–570. doi: 10.1016/j.plantsci.2005.05.004
Ahmad, M., Shahzad, A., Iqbal, M., Asif, M., and Hirani, A. H. (2013a). Morphological and molecular genetic variation in wheat for salinity tolerance at germination and early seedling stage. Austral. J. Crop Sci. 7:66.
Ahmad, M., Zahir, Z. A., Nazli, F., Akram, F., Arshad, M., and Khalid, M. (2013b). Effectiveness of halo-tolerant, auxin producing Pseudomonas and Rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.). Brazil. J. Microbiol. 44, 1341–1348. doi: 10.1590/S1517-83822013000400045
Alam, M. M., Nahar, K., Hasanuzzaman, M., and Fujita, M. (2014). Trehalose-induced drought stress tolerance: a comparative study among different Brassica species. Plant Omics 7:271.
Ali, A., Basra, S. M. A., Ahmad, R., and Wahid, A. (2009). Optimizing silicon application to improve salinity tolerance in wheat. Soil Environ. 28, 136–144.
Alla, M. N., Badran, E., and Mohammed, F. (2019). Exogenous trehalose alleviates the adverse effects of salinity stress in wheat. Turkish J. Bot. 43, 48–57. doi: 10.3906/bot-1803-36
Al-Mishhadani, I. I., Ismail, E. N., Jaddoa, K. A., Majeed, D. M., and Mohammed, O. A. (2015). Estimation of the interaction effect between salinity and growth regulators on salt tolerance of two bread wheat cultivars. Int. J. Appl. Agric. Sci. 1, 95–101. doi: 10.11648/j.ijaas.20150104.12
Alzahrani, F. O. (2021). Metabolic engineering of osmoprotectants to elucidate the mechanism (s) of salt stress tolerance in crop plants. Planta 253, 1–17. doi: 10.1007/s00425-020-03550-8
Amirbakhtiar, N., Ismaili, A., Ghaffari, M. R., Nazarian Firouzabadi, F., and Shobbar, Z. S. (2019). Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS ONE 14:e0213305. doi: 10.1371/journal.pone.0213305
Amram, A., Fadida-Myers, A., Golan, G., Nashef, K., Ben-David, R., and Peleg, Z. (2015). Effect of GA-sensitivity on wheat early vigor and yield components under deep sowing. Front. Plant Sci. 6:487. doi: 10.3389/fpls.2015.00487
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A., and Hayat, S. (2020). Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol. Biochem. 156, 64–77. doi: 10.1016/j.plaphy.2020.08.042
Arora, N. K. (2019). Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2, 95–96. doi: 10.1007/s42398-019-00078-w
Arshad, A., Qamar, H., Siti-Sundari, R., Zhang, Y., Zubair, M., Raza, M. A., et al. (2020). Phenotypic plasticity of spineless safflower (Carthamus tinctorius L.) cultivars in response to exogenous application of salicylic acid under rainfed climate conditions. Pakistan J. Agric. Res. 33, 729–743. doi: 10.17582/journal.pjar/2020/33.4.729.743
Arzani, A., and Ashraf, M. (2016). Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Critic. Rev. Plant Sci. 35, 146–189. doi: 10.1080/07352689.2016.1245056
Ashraf, M., and Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. doi: 10.1016/j.envexpbot.2005.12.006
Ashraf, M. A., and Ashraf, M. (2016). Growth stage-based modulation in physiological and biochemical attributes of two genetically diverse wheat ( Triticum aestivum L.) cultivars grown in salinized hydroponic culture. Environ. Sci. Pollut. Res. 23, 6227–6243. doi: 10.1007/s11356-015-5840-5
Asseng, S., Ewert, F., Martre, P., Rötter, R. P., Lobell, D. B., Cammarano, D., et al. (2015). Rising temperatures reduce global wheat production. Nat. Clim. Change 5, 143–147. doi: 10.1038/nclimate2470
Avalbaev, A., Yuldashev, R., Fedorova, K., Somov, K., Vysotskaya, L., Allagulova, C., et al. (2016). Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J. Plant Physiol. 191, 101–110. doi: 10.1016/j.jplph.2015.11.013
Ayadi, M., Brini, F., and Masmoudi, K. (2019). Overexpression of a wheat aquaporin gene, TdPIP2; 1, enhances salt and drought tolerance in transgenic durum wheat cv. maali. Int. J. Mol. Sci. 20:2389. doi: 10.3390/ijms20102389
Azooz, M. M., Shaddad, M. A., and Abdel-Latef, A. A. (2004). Leaf growth and K + /Na + ratio as an indication of the salt tolerance of three sorghum cultivars grown under salinity stress and IAA treatment. Acta Agron. Hungarica 52, 287–296. doi: 10.1556/AAgr.52.2004.3.10
Bacilio, M., Rodriguez, H., Moreno, M., Hernandez, J. P., and Bashan, Y. (2004). Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol. Fert. Soils 40, 188–193. doi: 10.1007/s00374-004-0757-z
Barutcular, C., Yildirim, M., Koc, M., Akinc,i, C., Tanrikulu, A., El Sabagh, A., et al. (2016b). Quality traits performance of bread wheat genotypes under drought and heat stress conditions. Fresenius Environ. Bull. 25, 6159–6165.
Barutcular, C., Yildirim, M., Koc, M., Akinci, C., Toptaş, I., Albayrak, O., et al. (2016a). Evaluation of SPAD chlorophyll in spring wheat genotypes under different environments. Fresenius Environ. Bull. 25, 1258–1266.
Ben-Saad, R., Ben-Ramdhan, W., Zouari, N., Azaza, J., Mieulet, D., Guiderdoni, E., et al. (2012). Marker-free transgenic durum wheat cv. Karim expressing the AlSAP gene exhibits a high level of tolerance to salinity and dehydration stresses. Mol. Breeding 30, 521–533. doi: 10.1007/s11032-011-9641-3
Bhuyan, M. B., Hasanuzzaman, M., Parvin, K., Mohsin, S. M., Al Mahmud, J., Nahar, K., et al. (2020). Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 90, 409–424. doi: 10.1007/s10725-020-00594-4
Bielach, A., Hrtyan, M., and Tognetti, V. B. (2017). Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 18:1427. doi: 10.3390/ijms18071427
Blumwald, E., Aharon, G. S., and Apse, M. P. (2000). Sodium transport in plant cells. Biochim. Biophys. Acta 1465, 140–151. doi: 10.1016/S0005-2736(00)00135-8
Borggaard, O. K. (1984). Influence of iron oxides on the non-specific anion (chloride) adsorption by soil. J. Soil Sci. 35, 71–78. doi: 10.1111/j.1365-2389.1984.tb00261.x
Branlard, G., Dardevet, M., Saccomano, R., Lagoutte, F., and Gourdon, J. (2001). Genetic diversity of wheat storage proteins and bread wheat quality. Euphytica 119, 59–67. doi: 10.1007/978-94-017-3674-9_18
Bui, E. N. (2013). Soil salinity: a neglected factor in plant ecology and biogeography. J. Arid Environ. 92, 14–25. doi: 10.1016/j.jaridenv.2012.12.014
Chamorro, D., Luna, B., Ourcival, J. M., Kavgac,i, A., Sirca, C., Mouillot, F., et al. (2017). Germination sensitivity to water stress in four shrubby species across the Mediterranean Basin. Plant Biol. 19, 23–31. doi: 10.1111/plb.12450
Chaves, M. M., Maroco, J. P., and Pereira, J. S. (2003). Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 30, 239–264 doi: 10.1071/FP02076
Chen, H., and Jiang, J. G. (2010). Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 18, 309–319. doi: 10.1139/A10-014
Çiçek, N., and Çakirlar, H. (2002). The effect of salinity on some physiological parameters in two maize cultivars. Bulgarian J. Plant Physiol. 28, 66–74.
Cisse, A., Arshad, A., Wang, X., Yattara, F., and Hu, Y. (2019). Contrasting impacts of long-term application of biofertilizers and organic manure on grain yield of winter wheat in North China Plain. Agronomy 9:312. doi: 10.3390/agronomy9060312
Dadshani, S., Sharma, R. C., Baum, M., Ogbonnaya, F. C., Léon, J., and Ballvora, A. (2019). Multi-dimensional evaluation of response to salt stress in wheat. PLoS ONE 14:e0222659. doi: 10.1371/journal.pone.0222659
Darvey, N. L., Naeem, H., and Gustafson, J. P. (2000). “Triticale: production and utilization,” in Handbook of Cereal Science and Technology , eds K. Klup and J. Ponte J. 2nd edn (New York, NY: Marcel Dekker).
Datta, J. K., Mondal, T., Banerjee, A., and Mondal, N. K. (2011). Assessment of drought tolerance of selected wheat cultivars under laboratory condition. J. Agric. Technol. 7, 383–393.
Dehnavi, A. R., Zahedi, M., Ludwiczak, A., Cardenas Perez, S., and Piernik, A. (2020). Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 10:859. doi: 10.3390/agronomy10060859
Dong, W., Wang, M., Xu, F., Quan, T., Peng, K., Xiao, L., et al. (2013). Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging. Plant Physiol. 161, 1217–1228. doi: 10.1104/pp.112.211854
Duan, l. (2020). A novel aba functional analogue b2 enhances salinity tolerance in wheat. Appl. Ecol. Environ. Res. 18, 7139–7157. doi: 10.15666/aeer/1805_71397157
Egamberdieva, D. (2009). Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 31, 861–864. doi: 10.1007/s11738-009-0297-0
Ehtaiwesh, F. A., and Rashed, H. F. (2020). Growth and yield responses of libyan hard wheat (Triticum durum Desf) genotypes to salinity stress. Zawia Univ. Bull. 22, 33–58.
El Sabagh, A., Hossain, A., Barutçular, C., Iqbal, M. A., Islam, M. S., Fahad, S., et al. (2020). “Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: an outlook of arid and semi-arid regions,” in Environment, Climate, Plant and Vegetation Growth , eds A. Fahad, M. Hasanuzzaman, M. Alam, H. Ullah, M. Saeed, I. A. Khan, and M. Adnan (Cham: Springer), 503–533. doi: 10.1007/978-3-030-49732-3_20
El Sabagh, A., Hossain, A., Barutçular, C., Islam, M. S., Ratnasekera, D., Kumar, N., et al. (2019b). Drought and salinity stress management for higher and sustainable canola (Brassica napus L.) production: a critical review. Austral. J. Crop Sci. 13, 88–97. doi: 10.21475/ajcs.19.13.01.p1284
El Sabagh, A., Hossain, A., Islam, M. S., Barutcular, C., Hussain, S., Hasanuzzaman, M., et al. (2019a). Drought and salinity stresses in barley: consequences and mitigation strategies. Austral. J. Crop Sci. 13:810. doi: 10.21475/ajcs.19.13.06.p1286
El Sabagh, A., Hossain, A., Islam, M. S., Barutçular, C., Ratnasekera, D., Kumar, N., et al. (2019c). Sustainable soybean production and abiotic stress management in saline environments: a critical review. Austr. J. Crop Sci. 13, 228–236. doi: 10.21475/ajcs.19.13.02.p1285
El-Hendawy, S., Elshafei, A., Al-Suhaibani, N., Alotabi, M., Hassan, W., Dewir, Y. H., et al. (2019). Assessment of the salt tolerance of wheat genotypes during the germination stage based on germination ability parameters and associated SSR markers. J. Plant Interact. 14, 151–163. doi: 10.1080/17429145.2019.1603406
El-Hendawy, S. E., Hu, Y., and Schmidhalter, U. (2005). Growth, ion content, gas exchange, and water relations of wheat genotypes differing in salt tolerances. Austral. J. Agric. Res. 56, 123–134. doi: 10.1071/AR04019
El-Hendawy, S. E., Ruan, Y., Hu, Y., and Schmidhalter, U. (2009). A comparison of screening criteria for salt tolerance in wheat under field and controlled environmental conditions. J. Agron. Crop Sci. 195, 356–367. doi: 10.1111/j.1439-037X.2009.00372.x
El-Lethy, S. R., Abdelhamid, M. T., and Reda, F. (2013). Effect of potassium application on wheat ( Triticum aestivum L.) cultivars grown under salinity stress. World Appl. Sci. J. 26, 840–850. doi: 10.5829/idosi.wasj.2013.26.07.13527
El-Yazal, M. A. S., Eissa, H. F., Ahmed, S. M. A. E., Howladar, S. M., Zaki, S. N. S., and Rady, M. M. (2016). The mtlD gene-overexpressed transgenic wheat tolerates salt stress through accumulation of mannitol and sugars. Plant 4, 78–90. doi: 10.11648/j.plant.20160406.15
Eroglu, Ç., Cabral, C., Ravnskov, S., Topbjerg, H., and Wollenweber, B. (2020). Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol. 22, 863–871. doi: 10.1111/plb.13123
FAO (2009). High Level Expert Forum-How to Feed the World in 2050. Economic and Social Development . Rome: Food and Agricultural Organization of the United Nations.
Fardus, J., Matin, M. A., Hasanuzzaman, M., Hossain, M. A., and Hasanuzzaman, M. (2018). Salicylic acid-induced improvement in germination and growth parameters of wheat under salinity stress. J. Anim. Plant Sci. 28, 197–207.
Farooq, S., and Azam, F. (2005). The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant Physiol. 163, 629–637. doi: 10.1016/j.jplph.2005.06.006
Farouk, S. (2011). Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J. Stress Physiol. Biochem. 7, 58–79.
Fernandez-Figares, I., Marinetto, J., Royo, C., Ramos, J. M., and Del Moral, L. G. (2000). Amino-acid composition and protein and carbohydrate accumulation in the grain of triticale grown under terminal water stress simulated by a senescing agent. J. Cereal Sci. 32, 249–258. doi: 10.1006/jcrs.2000.0329
Flowers, T. J., Hajibagherp, M. A., and Yeo, A. R. (1991). Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant Cell Environ. 14, 319–325. doi: 10.1111/j.1365-3040.1991.tb01507.x
Food and Agriculture Organization (FAO) of the United Nations (2016). Pulses: Nutritious Seeds for a Sustainable Future . Rome: Food and Agriculture Organization of the United Nations.
Francois, L. E., Maas, E. V., Donovan, T. J., and Youngs, V. L. (1986). Effect of salinity on grain yield and quality, vegetative growth, and germination of semi-dwarf and durum wheat. Agron. J. 78, 1053–1058. doi: 10.2134/agronj1986.00021962007800060023x
Frank, A. B., Bauer, A., and Black, A. I. (1987). Effects of air temperature and water stress on apex development in spring wheat. Crop Sci. 27, 113–116. doi: 10.2135/cropsci1987.0011183X002700010028x
Garcia del Moral, L. F., Boujenna, A., Yanez, J. A., and Ramos, J. M. (1995). Forage production, grain yield, and protein content in dual-purpose triticale grown for both grain and forage. Agron. J. 87, 902–908. doi: 10.2134/agronj1995.00021962008700050021x
Gardner, W. K. (2016). Sodium, calcium and magnesium ratios in soils of NW Victoria, Australia may restrict root growth and crop production. J. Plant Nutr. 39, 1205–1215. doi: 10.1080/01904167.2014.999946
Geilfus, C. M. (2018a). Chloride from nutrient to toxicant. Plant Cell Physiol. 59, 877–886. doi: 10.1093/pcp/pcy071
Geilfus, C. M. (2018b). Review on the significance of chlorine for crop yield and quality. Plant Sci. 270, 114–122. doi: 10.1016/j.plantsci.2018.02.014
Ghosh, B., Md, N. A., and Gantait, S. (2016). Response of rice under salinity stress: a review update. Rice Res. 4:167. doi: 10.4172/2375-4338.1000167
Gil-Muñoz, F., Pérez-Pérez, J. G., Quiñones, A., Primo-Capella, A., Cebolla, J., Forner-Giner, M. Á., et al. (2020). A cross population between D. kaki and D. virginiana shows high variability for saline tolerance and improved salt stress tolerance. PLoS ONE 15:e0229023. doi: 10.1371/journal.pone.0229023
Giraldo, P., Benavente, E., Manzano-Agugliaro, F., and Gimenez, E. (2019). Worldwide research trends on wheat and barley: a bibliometric comparative analysis. Agronomy 9:352. doi: 10.3390/agronomy9070352
Golldack, D., Li, C., Mohan, H., and Probst, N. (2014). Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front. Plant Sci. 5:151. doi: 10.3389/fpls.2014.00151
Grieve, C. M., Francois, L. E., and Maas, E. V. (1994). Salinity affects the timing of phasic development in spring wheat. Crop Sci. 34, 1544–1549. doi: 10.2135/cropsci1994.0011183X003400060024x
Gul, B., Khan, M. A., and Weber, D. J. (2000). Alleviation of salinity and dark-enforced dormancy in Allenrolfea occidentalis seeds under various thermoperiods. Austral. J. Bot. 48, 745–752. doi: 10.1071/BT99069
Hanin, M., Ebel, C., Ngom, M., Laplaze, L., and Masmoudi, K. (2016). New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 7:1787. doi: 10.3389/fpls.2016.01787
Hasan, A., Hafiz, H. R., Siddiqui, N., Khatun, M., Islam, R., and Mamun, A. A. (2015). Evaluation of wheat genotypes for salt tolerance based on some physiological traits. J. Crop Sci. Biotechnol. 18, 333–340. doi: 10.1007/s12892-015-0064-2
Hasanuzzaman, M., Alam, M., Rahman, A., Hasanuzzaman, M., Nahar, K., and Fujita, M. (2014). Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed. Res. Int. 2014:757219. doi: 10.1155/2014/757219
Hasanuzzaman, M., Hossain, M. A., and Fujita, M. (2011). Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Element Res. 143, 1704–1721. doi: 10.1007/s12011-011-8958-4
Hasanuzzaman, M., Nahar, K., and Fujita, M. (2013). “Plant responses to salt stress and role of exogenous protectants to mitigate salt-induced damages,” in Ecophysiology and Responses of Plants Under Salt Stress , eds P. Ahmad P, M. M. Azooz, and M. N. V. Prasad MNV (New York, NY: Springer), 25–87. doi: 10.1007/978-1-4614-4747-4_2
Hassanein, R. A., Hassanein, A. A., Haider, A. S., and Hashem, H. A. (2009). Improving salt tolerance of Zea mays L. plants by presoaking their grains in glycine betaine. Austral. J. Basic Appl. Sci. 3, 928–942.
Hawkins, H. J., and Lewis, O. A. M. (1993). Combination effect of NaCl salinity, nitrogen form and calcium concentration on the growth, ionic content and gaseous exchange properties of Triticum aestivum L. cv. Gamtoos. New Phytol. 124, 161–170. doi: 10.1111/j.1469-8137.1993.tb03806.x
Hayat, S., Khalique, G., Wani, A. S., Alyemeni, M. N., and Ahmad, A. (2014). Protection of growth in response to 28-homobrassinolide under the stress of cadmium and salinity in wheat. Int. J. Biol. Macromol. 64, 130–136. doi: 10.1016/j.ijbiomac.2013.11.021
He, C., Yang, A., Zhang, W., Gao, Q., and Zhang, J. (2010). Improved salt tolerance of transgenic wheat by introducing betA gene for glycine betaine synthesis. Plant Cell Tissue Organ Cult. 101, 65–78. doi: 10.1007/s11240-009-9665-0
Hossain, M. A., Hoque, T. S., Zaid, A., Wani, S. H., Mostofa, M. G., and Henry, R. (2021). “Targeting the ascorbate-glutathione pathway and the glyoxalase pathway for genetic engineering of abiotic stress-tolerance in rice,” in Molecular Breeding for Rice Abiotic Stress Tolerance and Nutritional Quality , eds M. A. Hossain, L. Hassan, K. M. Iftekharuddaula, A. Kumar A, and R. Henry R (Wiley-Blackwell), 398–427. doi: 10.1002/9781119633174.ch21
Houshmand, S., Arzani, A., and Mirmohammadi-Maibody, S. A. M. (2014). Effects of salinity and drought stress on grain quality of durum wheat. Commun. Soil Sci. Plant Anal. 45, 297–308. doi: 10.1080/00103624.2013.861911
Hu, Y., and Schmidhalter, U. (1998). Spatial distribution sand net deposition rates of mineral elements in the elongating wheat ( Triticum aestivum L.) leaf under saline soil conditions. Planta 204, 212–219. doi: 10.1007/s004250050249
Huang, M., Chai, L., Jiang, D., Zhang, M., Zhao, Y., and Huang, Y. (2019). Increasing aridity affects soil archaeal communities by mediating soil niches in semi-arid regions. Sci. Total Environ. 647, 699–707. doi: 10.1016/j.scitotenv.2018.07.305
Huebner, F. R., Nelsen, T. C., Chung, O. K., and Bietz, J. A. (1997). Protein distributions among hard red winter wheat varieties as related to environment and baking quality. Cereal Chem. 74, 123–128. doi: 10.1094/CCHEM.1997.74.2.123
Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., and Zhang, J. (2019). Salinity stress in arid and semi-arid climates: Effects and management in field crops. Clim. Change Agric 123–145. doi: 10.5772/intechopen.87982
Hütsch, B. W., He, W., and Schubert, S. (2016). Nitrogen nutritional status of young maize plants (Zea mays) is not limited by NaCl stress. J. Plant Nutr. Soil Sci. 179, 775–783. doi: 10.1002/jpln.201500565
Ioannou, M., and Coutsogeorgopoulos, C. (1997). Kinetic studies on the activation of eukaryotic peptidyltransferase by potassium. Arch. Biochem. Biophys. 345, 325–331. doi: 10.1006/abbi.1997.0256
Iqbal, M., and Ashraf, M. (2007). Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integrat. Plant Biol. 49, 1003–1015. doi: 10.1111/j.1672-9072.2007.00488.x
Iqbal, M., Ashraf, M., and Jamil, A. (2006). Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regulat. 50, 29–39. doi: 10.1007/s10725-006-9123-5
Iqbal, M., Irshad, S., Nadeem, M., Fatima, T., and Itrat, A. B. (2018). Salinity effects on wheat ( Triticum aestivum L.) characteristics: a review article. Int. J Agric. Biol. 12, 1–15. doi: 10.12692/ijb/12.3.131-146
Iqbal, M. A., Junaid, R., Wajid, N., Sabry, H., Yassir, K., and Ayman, S. (2021). Rainfed winter wheat ( Triticum aestivum L.) cultivars respond differently to integrated fertilization in Pakistan. Fresenius Environ. Bull. 30, 3115–3121.
Islam, M. S., Akhter, M. M., El Sabagh, A., Liu, L. Y., Nguyen, N. T., Ueda, A., et al. (2011). Comparative studies on growth and physiological responses to saline and alkaline stresses of Foxtail millet ( Setaria italica L.) and Proso millet ( Panicum miliaceum L.). Austral. J. Crop Sci. 5:1269.
James, R. A., Munns, R., Von Caemmerer, S., Trejo, C., Miller, C., and Condon, T. (2006). Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl-in salt-affected barley and durum wheat. Plant Cell Environ. 29, 2185–2197. doi: 10.1111/j.1365-3040.2006.01592.x
Jan, A. U., Hadi, F., Nawaz, M. A., and Rahman, K. (2017). Potassium and zinc increase tolerance to salt stress in wheat ( Triticum aestivum L.). Plant Physiol. Biochem. 116, 139–149. doi: 10.1016/j.plaphy.2017.05.008
Jenner, C. F., Ugalde, T. D., and Aspinall, D. (1991). The physiology of starch and protein deposition in the endosperm of wheat. Funct. Plant Biol. 18, 211–226. doi: 10.1071/PP9910211
Kahrizi, S., and Sedghi, M. (2013). Effect of salt stress on grain reserve composition in ten durum wheat cultivars. J. Stress Physiol. Biochem. 3, 113–121.
Kalhoro, N. A., Rajpar, I., Kalhoro, S. A., Ali, A., Raza, S., Ahmed, M., et al. (2016). Effect of salts stress on the growth and yield of wheat ( Triticum aestivum L.). Am. J. Plant Sci. 7:2257. doi: 10.4236/ajps.2016.715199
Karuppanapandian, T., Moon, J. C., Kim, C., Manoharan, K., and Kim, W. (2011). Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Austral. J. Crop Sci. 5, 709–725.
Katerji, N., Van Hoorn, J. W., Fares, C., Hamdy, A., Mastrorilli, M., and Oweis, T. (2005). Salinity effect on grain quality of two durum wheat varieties differing in salt tolerance. Agric. Water Manage. 75, 85–91. doi: 10.1016/j.agwat.2004.12.005
Kaur, H., Bhardwaj, R. D., and Grewal, S. K. (2017). Mitigation of salinity-induced oxidative damage in wheat ( Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol. Plant . 39:221. doi: 10.1007/s11738-017-2521-7
Ke, Q., Ye, J., Wang, B., Ren, J., Yin, L., Deng, X., et al. (2018). Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 9:914. doi: 10.3389/fpls.2018.00914
Khan, A., Ahmad, I., Shah, A., Ahmad, F., Ghani, A., Nawaz, M., et al. (2013). Amelioration of salinity stress in wheat ( Triticum aestivum L.) by foliar application of phosphorus. Phyton 82, 281–287. doi: 10.32604/phyton.2013.82.281
Khan, A. L., Waqas, M., Asaf, S., Kamran, M., Shahzad, R., Bilal, S., et al. (2017). Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum pimpinellifolium. Environ. Exp. Bot. 133, 58–69. doi: 10.1016/j.envexpbot.2016.09.009
Khan, M. A., Gul, B., and Weber, D. J. (2004). Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Canad. J. Bot. 82, 37–42. doi: 10.1139/b03-140
Khan, M. I. R., Iqbal, N., Masood, A., and Khan, N. A. (2012). Variation in salt tolerance of wheat cultivars: role of glycinebetaine and ethylene. Pedosphere 22, 746–754. doi: 10.1016/S1002-0160(12)60060-5
Khan, N. A., Shamim, M., and Shambhoo, P. (2008). Biochemical changes in wheat plants in response to salinity. Int. J. Plant Sci. 3, 11–15.
Khataar, M., Mohammadi, M. H., and Shabani, F. (2018). Soil salinity and matric potential interaction on water use, water use efficiency and yield response factor of bean and wheat. Sci. Rep. 8:2679. doi: 10.1038/s41598-018-20968-z
Kizilgeci, F., Yildirim, M., Islam, M. S., Ratnasekera, D., Iqbal, M. A., and Sabagh, A. E. (2021). Normalized difference vegetation index and chlorophyll content for precision nitrogen management in durum wheat cultivars under semi-arid conditions. Sustainability 13:3725. doi: 10.3390/su13073725
Kolbert, Z., Feigl, G., Freschi, L., and Poór, P. (2019). Gasotransmitters in action: nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants 8:167. doi: 10.3390/antiox8060167
Kosová, K., Prášil, I. T., Vítámvás, P., Dobrev, P., Motyka, V., Floková, K., et al. (2012). Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 169, 567–576. doi: 10.1016/j.jplph.2011.12.013
Kotuby-Amazher, J., Koenig, K., and Kitchen, B. (2000). Salinity and Plant Tolerance . Available online at: https://extension.usu.edu/files/publications/publication/AG-SO-03.pdf (accessed June 20, 2020).
Kumari, A., and Kaur, R. (2018). Evaluation of benzyl-butyl phthalate induced germination and early growth vulnerability to barley seedlings ( Hordeum vulgare L.). Indian J. Ecol. 45, 174–177.
Kumari, A., and Kaur, R. (2020). A review on morpho-physiological traits of plants under phthalates stress and insights into their uptake and translocation. Plant Growth Regul. 91, 327–347. doi: 10.1007/s10725-020-00625-0
Kundu, P., Gill, R., Ahlawat, S., Anjum, N. A., Sharma, K. K., Ansari, A. A., et al. (2018). “Targeting the redox regulatory mechanisms for abiotic stress tolerance in crops” in Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants , ed S. H. Wani (Elsevier Academic Press), 151–220. doi: 10.1016/B978-0-12-813066-7.00010-3
Läuchli A, A., and Grattan, S. R. (2007). “Plant growth and development under salinity stress,” in Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops , eds M. A. Jenks, P. M. Hasegawa, and S. M. Jain (Dordrecht: Springer), 1–32. doi: 10.1007/978-1-4020-5578-2_1
Li, P., Cai, J., Luo, X., Chang, T., Li, J., Zhao, Y., et al. (2019). Transformation of wheat Triticum aestivum with the HvBADH1 transgene from hulless barley improves salinity-stress tolerance. Acta. Physiol. Plant 41:155. doi: 10.1007/s11738-019-2940-8
Liu, X., Chen, D., Yang, T., Huang, F., Fu, S., and Li, L. (2020). Changes in soil labile and recalcitrant carbon pools after land-use change in a semi-arid agro-pastoral ecotone in Central Asia. Ecol. Indic. 110:105925. doi: 10.1016/j.ecolind.2019.105925
Llanes, A., Andrade, A., Alemano, S., and Luna, V. (2016). Alterations of endogenous hormonal levels in plants under drought and salinity. Am. J. Plant Sci. 7:1357. doi: 10.4236/ajps.2016.79129
Maas, E. V. (1990). “Crop salt tolerance,” in Agricultural Salinity Assessment and Management. ASCE Publications , ed K. K. Tanji (Reston, VA), 262–303.
Maas, E. V., and Grieve, C. M. (1990). Spike and leaf development of sal-stressed wheat. Crop Sci. 30, 1309–1313. doi: 10.2135/cropsci1990.0011183X003000060031x
Makhloufi, E., Yousfi, F. E., Marande, W., Mila, I., Hanana, M., Bergès, H., et al. (2014). Isolation and molecular characterization of ERF1, an ethylene response factor gene from durum wheat (Triticum turgidum L. subsp. durum), potentially involved in salt-stress responses. J. Exp. Bot . 5, 6359–6371. doi: 10.1093/jxb/eru352
Mandhania, S., Madan, S., and Sawhney, V. (2006). Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 50, 227–231. doi: 10.1007/s10535-006-0011-7
Mao, X., Zhang, H., Tian, S., Chang, X., and Jing, R. (2010). TaSnRK2. 4, an SNF1-type serine/threonine protein kinase of wheat ( Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 61, 683–696. doi: 10.1093/jxb/erp331
Maqsood, T., Akhtar, J., Farooq, M. R., Haq, M. A., and Saqib, Z. A. (2008). Biochemical attributes of salt tolerant and salt sensitive maize cultivars to salinity and potassium nutrition. Pakistan J. Agric. Sci. 45, 1–5.
Massa, D., Mattson, N. S., and Lieth, H. J. (2009). Effects of saline root environment (NaCl) on nitrate and potassium uptake kinetics for rose plants: a Michaelis–Menten modelling approach. Plant Soil 318, 101–115. doi: 10.1007/s11104-008-9821-z
Mbarki, S., Sytar, O., Zivcak, M., Abdelly, C., Cerda, A., and Brestic, M. (2018). Anthocyanins of coloured wheat genotypes in specific response to salstress. Molecules 23:1518. doi: 10.3390/molecules23071518
Mohsenzadeh, S., and Zohrabi, M. (2018). Auxin and sodium nitroprusside effects on wheat antioxidants in salinity. Russian J. Plant Physiol. 65, 651–657. doi: 10.1134/S1021443718050138
Munns, R., James, R. A., and Läuchli, A. (2006). Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 57, 1025–1043. doi: 10.1093/jxb/erj100
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Mushtaq, A., Jamil, N., Riaz, M., Hornyak, G. L., Ahmed, N., Ahmed, S. S., et al. (2017). Synthesis of silica nanoparticles and their effect on priming of wheat ( Triticum aestivum L.) under salinity stress. Biol. Forum 9, 150–157.
Nadeem, M., Tariq, M. N., Amjad, M., Sajjad, M., Akram, M., Imran, M., et al. (2020). Salinity-induced changes in the nutritional quality of bread wheat ( Triticum aestivum L.) genotypes. Agrivita 42, 1–12. doi: 10.17503/agrivita.v42i1.2273
Naidu, C. V. (2001). Improvement of seed germination in red sanders (Pterocarpus santalinus Linn. F.) by plant growth regulators. Indian J. Plant Physiol. 6, 205–207.
Nassar, R., Kamel, H. A., Ghoniem, A. E., Alarcón, J. J., Sekara, A., Ulrichs, C., et al. (2020). Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 9:237. doi: 10.3390/plants9020237
Navarro, C., Moore, J., Ott, A., Baumert, E., Mohan, A., Gill, K. S., et al. (2015). Evolutionary, comparative and functional analyses of the brassinosteroid receptor gene, BRI1, in wheat and its relation to other plant genomes. PLoS ONE 10:e0127544. doi: 10.1371/journal.pone.0127544
Navarro-Yepes, J., Burns, M., Anandhan, A., Khalimonchuk, O., Del Razo, L. M., Quintanilla-Vega, B., et al. (2014). Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxidants Redox Signal. 21, 66–85. doi: 10.1089/ars.2014.5837
Netondo, G. W., Onyango, J. C., and Beck, E. (2004). Sorghum and salinity: II. gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 44, 806–811. doi: 10.2135/cropsci2004.8060
Nishida, K., Khan, N. M., and Shiozawa, S. (2009). Effects of salt accumulation on the leaf water potential and transpiration rate of pot-grown wheat with a controlled saline groundwater table. Soil Sci. Plant Nutr. 55, 375–384. doi: 10.1111/j.1747-0765.2009.00368.x
Nishiyama, R., Watanabe, Y., Fujita, Y., Le, D. T., Kojima, M., Werner, T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23, 2169–2183. doi: 10.1105/tpc.111.087395
Niu, X., Bressan, R. A., Hasegawa, P. M., and Pardo, J. M. (1995). Ion homeostasis in NaCl stress environments. Plant Physiol. 109:735. doi: 10.1104/pp.109.3.735
Noreen, S., Fatima, K., Athar, H. U. R., Ahmad, S., and Hussain, K. (2017). Enhancement of physio-biochemical parameters of wheat through exogenous application of salicylic acid under drought stress. J. Anim. Plant Sci. 27, 153–163.
Orhan, F. (2016). Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat ( Triticum aestivum ). Brazil. J. Microbiol. 47, 621–627. doi: 10.1016/j.bjm.2016.04.001
Oria-Hernández, J., Cabrera, N., Pérez-Montfort, R., and Ramírez-Silva, L. (2005). Pyruvate kinase revisited the activating effect of k+. J. Biol. Chem. 280, 37924–37929. doi: 10.1074/jbc.M508490200
Otu, H., Celiktas, V., Duzenli, S., Hossain, A., and El Sabagh, A. (2018). Germination and early seedling growth of five durum wheat cultivars (Triticum durum Desf.) is affected by different levels of salinity. Fresenius Environ. Bull. 27, 7746–7757.
Parida, A. K., Mittra, B., Das, A. B., Das, T. K., and Mohanty, P. (2005). High salinity reduces the content of a highly abundant 23-kDa protein of the mangrove Bruguiera parviflora. Planta 221, 135–140. doi: 10.1007/s00425-004-1415-2
Park, S. H., Wilson, J. D., and Seabourn, B. W. (2009). Starch granule size distribution of hard red winter and hard red spring wheat: its effects on mixing and breadmaking quality. J. Cereal Sci. 49, 98–105. doi: 10.1016/j.jcs.2008.07.011
Plaza-Wüthrich, S., Blösch, R., Rindisbacher, A., Cannarozzi, G., and Tadele, Z. (2016). Gibberellin deficiency confers both lodging and drought tolerance in small cereals. Front. Plant Sci. 7:643. doi: 10.3389/fpls.2016.00643
Poustini, K., and Siosemardeh, A. (2004). Ion distribution in wheat cultivars in response to salinity stress. Field Crops Res. 85, 125–133. doi: 10.1016/S0378-4290(03)00157-6
Qamar, H., Ilyas, M., Jan, S. A., Mustafa, H. S. B., Arshad, A., Yar, M. S., et al. (2020). Recent trends in molecular breeding and biotechnology for the genetic improvement of Brassica species against drought stress. Fresenius Environ. Bull. 29, 19–25.
Rahman, M. A., Chikushi, J., Yoshida, S., Yahata, H., and Yasunaga, E. (2005). Effect of high air temperature on grain growth and yields of wheat genotypes differing in heat tolerance. J. Agric. Meteorol. 60, 605–608. doi: 10.2480/agrmet.605
Rakhmankulova, Z. F., Shuyskaya, E. V., Shcherbakov, A. V., Fedyaev, V. V., Biktimerova, G. Y., Khafisova, R. R., et al. (2015). Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russian J. Plant Physiol. 62, 71–79. doi: 10.1134/S1021443715010112
Rao, A. C. S., Smith, J. L., Jandhyala, V. K., Papendick, R. I., and Parr, J. F. (1993). Cultivar and climatic effects on the protein content of soft white winter wheat. Agron. J. 85, 1023–1028. doi: 10.2134/agronj1993.00021962008500050013x
Reddy, B. V. S., Reddy, P. S., Bidinger, F., and Blümmel, M. (2003). Crop management factors influencing yield and quality of crop residues. Field Crops Res. 84, 57–77. doi: 10.1016/S0378-4290(03)00141-2
Richardson, A. E., and Simpson, R. J. (2011). Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 156, 989–996. doi: 10.1104/pp.111.175448
Riyazuddin, R., Verma, R., Singh, K., Nisha, N., Keisham, M., Bhati, K. K., et al. (2020). Ethylene: a master regulator of salinity stress tolerance in plants. Biomolecules 10:959. doi: 10.3390/biom10060959
Rong, W., Qi, L., Wang, A., Ye, X., Du, L., Liang, H., et al. (2014). The ERF transcription factor Ta ERF 3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 12, 468–479. doi: 10.1111/pbi.12153
Royo, A., and Abió, D. (2003). Salt tolerance in durum wheat cultivars. Spanish J. Agric. Res. 1, 27–36. doi: 10.5424/sjar/2003013-32
Ryu, H., and Cho, Y. G. (2015). Plant hormones in salt stress tolerance. J. Plant Biol. 58, 147–155. doi: 10.1007/s12374-015-0103-z
Saboora, A., Kiarostami, K., Behroozbayati, F., and Hajihashemi, S. (2006). Salinity (NaCl) tolerance of wheat genotypes at germination and early seedling growth. Pakistan J. Biol. Sci. 9, 2009–2021. doi: 10.3923/pjbs.2006.2009.2021
Sairam, R. K., Rao, K. V., and Srivastava, G. C. (2002). Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163, 1037–1046. doi: 10.1016/S0168-9452(02)00278-9
Samad, R., and Karmoker, J. L. (2012). Effects of gibberellic acid and Kn on seed germination and accumulation of Na + and K + in the seedlings of triticale-I under salinity stress. Bangladesh J. Bot. 41, 123–129. doi: 10.3329/bjb.v41i2.13435
Sawahel, W. A., and Hassan, A. H. (2002). Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotechnol. Lett. 24, 721–725. doi: 10.1023/A:1015294319114
Schofield, J. D. (1994). “Wheat proteins: structure and functionality in milling and breadmaking,” in Wheat , eds W. Bushuk and V. F. Rasper (Boston, MA: Springer), 73–106. doi: 10.1007/978-1-4615-2672-8_7
Schwechheimer, C. (2012). Gibberellin signaling in plants–the extended version. Front. Plant Sci. 2:107. doi: 10.3389/fpls.2011.00107
Sedaghat, M., Sarvestani, Z. T., Emam, Y., and Bidgoli, A. M. (2017). Do phytohormones influence the grain quality and yield of winter wheat under drought conditions? J. Adv. Agric. Technol. 4, 151–158. doi: 10.18178/joaat.4.2.151-158
Shaddad, M. A. K., HM, A. E. S., and Mostafa, D. (2013). Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int. J. Plant Physiol. Biochem. 5, 50–57. doi: 10.5897/IJPPB2011.055
Shafi, M., Zhang, G., Bakht, J., Khan, M. A., Islam, U. E., Khan, M. D., et al. (2010). Effect of cadmium and salinity stresses on root morphology of wheat. Pakistan J. Bot. 42, 2747–2754.
Shahid, S. A., Zaman, M., and Heng, L. (2018). “Introduction to soil salinity, sodicity and diagnostics techniques,” in Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques , eds M. Zaman, S. A. Shahid, and L. Heng (Cham: Springer), 1–42. doi: 10.1007/978-3-319-96190-3_1
Shakirova, F. M., Avalbaev, A. M., Bezrukova, M. V., Fatkhutdinova, R. A., Maslennikova, D. R., and Yuldashev, R. A. (2012). “Hormonal intermediates in the protective action of exogenous phytohormones in wheat plants under salinity,” in Phytohormones and Abiotic Stress Tolerance in Plants , eds N. Khan, R. Nazar, N. Iqbal, and N. Anjum (Berlin; Heidelberg: Springer), 185–228. doi: 10.1007/978-3-642-25829-9_9
Shakirova, F. M., Sakhabutdinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A., and Fatkhutdinova, D. R. (2003). Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 164, 317–322. doi: 10.1016/S0168-9452(02)00415-6
Sharbatkhari, M., Shobbar, Z. S., Galeshi, S., and Nakhoda, B. (2016). Wheat stem reserves and salinity tolerance: molecular dissection of fructan biosynthesis and remobilization to grains. Planta 244, 191–202. doi: 10.1007/s00425-016-2497-3
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012. doi: 10.1155/2012/217037
Shen, Y., Guo, W., Zhou, Y., Zhu, X., Feng, C., and Peng, Y. (2007). Effects of salinity stress on the dynamic changes in the accumulation of grain protein and its components in wheat. J. Triticeae Crops 26, 100–103.
Siddiqui, M. H., Iqbal, M. A., Wajid, N., Imtiaz, H., and Khaliq, A. (2019). Bio-economic viability of rainfed wheat ( Triticum aestivum L.) cultivars under integrated fertilization regimes in Pakistan. Custos e Agronegocio 15, 81–96.
Singh, R. P., Jha, P., and Jha, P. N. (2015). The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat ( Triticum aestivum ) under salt stress. J. Plant Physiol. 184, 57–67. doi: 10.1016/j.jplph.2015.07.002
Sorour, S. G., Aiad, M. A., Ahmed, A. A., Henash, M. I. A., Metwaly, E. M., Alharby, H., et al. (2019). Yield of wheat is increased through improving the chemical properties, nutrient availability and water productivity of salt affected soils in the north delta of Egypt. Appl. Ecol. Environ. Res. 17, 8291–8306. doi: 10.15666/aeer/1704_82918306
Speer, M., and Kaiser, W. M. (1991). Ion relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L . under salinity. Plant Physiol. 97, 990–997. doi: 10.1104/pp.97.3.990
Sreenivasulu, N., Sopory, S. K., and Kishor, P. K. (2007). Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 388, 1–13. doi: 10.1016/j.gene.2006.10.009
Suzuki, K., Yamaji, N., Costa, A., Okuma, E., Kobayashi, N. I., Kashiwagi, T., et al. (2016). OsHKT1; 4-mediated Na + transport in stems contributes to Na + exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol. 16:22. doi: 10.1186/s12870-016-0709-4
Sytar, O., Mbarki, S., Zivcak, M., and Brestic, M. (2018). “The involvement of different secon dary metabolites in salinity tolerance of crops,” in Salinity Responses and Tolerance in Plants , eds V. Kumar, S. Wani, P. Suprasanna, and L. S. Tran. Vol. 2. (Cham: Springer), 21–48. doi: 10.1007/978-3-319-90318-7_2
Tabatabaei, S. A. (2013). The effect of salicylic acid and gibberellin on enzyme activity and germination characteristics of wheat seeds under salinity stress conditions. Int. J. Agric. Crop Sci. 6, 236–240.
Tammam, A. A., Alhamd, M. F. A., and Hemeda, M. M. (2008). Study of salt tolerance in wheat (Triticum aestium L.) cultivar Banysoif 1. Austral. J. Crop Sci. 1, 115–125.
Tareq, M. Z., Hossain, M. A., Mojakkir, M. A., Ahmed, R., and Fakir, M. S. A. (2011). Effect of salinity on reproductive growth of wheat. Bangladesh J. Seed Sci. Technol. 15, 111–116.
PubMed Abstract | Google Scholar
Thomas, G. W., and Swoboda, A. R. (1970). Anion exclusion effects on chloride movement in soils. Soil Sci. 110, 163–166. doi: 10.1097/00010694-197009000-00003
Tian, S., Mao, X., Zhang, H., Chen, S., Zhai, C., Yang, S., et al. (2013). Cloning and characterization of TaSnRK2. 3, a novel SnRK2 gene in common wheat. J. Exp. Bot. 64, 2063–2080. doi: 10.1093/jxb/ert072
Tian, X., He, M., Wang, Z., Zhang, J., Song, Y., He, Z., et al. (2015). Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regulat. 77, 343–356. doi: 10.1007/s10725-015-0069-3
Troccoli, A., Borrelli, G. M., De Vita, P., Fares, C., and Di Fonzo, N. (2000). Mini review: durum wheat quality: a multidisciplinary concept. J. Cereal Sci. 32, 99–113. doi: 10.1006/jcrs.2000.0322
Turan, M. A., Elkarim, A. H. A., Taban, N., and Taban, S. (2009). Effect of salt stress on growth, stomatal resistance, proline and chlorophyll concentrations on maize plant. Afr. J. Agric. Res. 4, 893–897. doi: 10.5897/AJAR.9000223
Umezawa, T., Sugiyama, N., Mizoguchi, M., Hayashi, S., Myouga, F., Yamaguchi-Shinozaki, K., et al. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 17588–17593. doi: 10.1073/pnas.0907095106
Van Loon, L. C. (2007). “Plant responses to plant growth-promoting rhizobacteria,” in New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research , eds P. A. H. M. Bakker, J. M. Raaijmakers, G. Bloemberg, M. Höfte, P. Lemanceau, and B. M. Cooke (Dordrecht: Springer), 243–54. doi: 10.1007/978-1-4020-6776-1_2
Wajid, M., Khan, M. A., Shirazi, M. U., and Summiya, F. (2019). Seed priming modulates germination potential, osmoprotectants accumulation and ionic uptake in wheat seedlings under salt stress. Int. J. Agric. Biol. 22, 594–600.
Wakeel, A., Farooq, M., Qadir, M., and Schubert, S. (2011). Potassium substitution by sodium in plants. Critic. Rev. Plant Sci. 30, 401–413. doi: 10.1080/07352689.2011.587728
Wang, Y., Mopper, S., and Hasenstein, K. H. (2001). Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 27, 327–342. doi: 10.1023/A:1005632506230
Wilkinson, S., Kudoyarova, G. R., Veselov, D. S., Arkhipova, T. N., and Davies, W. J. (2012). Plant hormone interactions: innovative targets for crop breeding and management. J. Exp. Bot. 63, 3499–3509. 10.1093/jxb/ers148 doi: 10.1093/jxb/ers148
Xue, Z. Y., Zhi, D. Y., Xue, G. P., Zhang, H., Zhao, Y. X., and Xia, G. M. (2004). Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci. 167, 849–859. doi: 10.1016/j.plantsci.2004.05.034
Yang, T., Yao, S., Hao, L., Zhao, Y., Lu, W., and Xiao, K. (2016). Wheat bHLH-type transcription factor gene TabHLH1 is crucial in mediating osmotic stresses tolerance through modulating largely the ABA-associated pathway. Plant Cell Rep. 35, 2309–2323. doi: 10.1007/s00299-016-2036-5
Yousfi, S., Serret, M. D., and Araus, J. L. (2013). Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell Environ. 36, 1214–1227. doi: 10.1111/pce.12055
Yuldashev, R., Avalbaev, A., Bezrukova, M., Vysotskaya, L., Khripach, V., and Shakirova, F. (2012). Cytokinin oxidase is involved in the regulation of cytokinin content by 24-epibrassinolide in wheat seedlings. Plant Physiol. Biochem. 55, 1–6. doi: 10.1016/j.plaphy.2012.03.004
Yurekli, F., Porgali, Z. B., and Turkan, I. (2004). Variations in abscisic acid, indole-3-acetic acid, gibberellic acid and zeatin concentrations in two bean species subjected to salt stress. Acta Biol. Cracoviensia Series Botanica , 46, 201–212.
Zaman, B., Niazi, B. H., Athar, M., and Ahmad, M. (2005). Response of wheat plants to sodium and calcium ion interaction under saline environment. Int. J. Environ. Sci. Technol. 2, 7–12. doi: 10.1007/BF03325852
Zhang, L., Xie, J., Wang, L., Si, L., Zheng, S., Yang, Y., et al. (2020). Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. Biochem. 151, 650–658. doi: 10.1016/j.plaphy.2020.03.039
Zhang, S., Gan, Y., and Xu, B. (2016). Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 7:1405. doi: 10.3389/fpls.2016.01405
Zhang, X., Shi, Z., Tian, Y., Zhou, Q., Cai, J., Dai, T., et al. (2016). Salt stress increases content and size of glutenin macropolymers in wheat grain. Food Chem. 197, 516–521. doi: 10.1016/j.foodchem.2015.11.008
Zhao, Y., Ai, X., Wang, M., Xiao, L., and Xia, G. (2016). A putative pyruvate transporter TaBASS2 positively regulates salinity tolerance in wheat via modulation of ABI4 expression. BMC Plant Biol. 16:109. doi: 10.1186/s12870-016-0795-3
Zheng, Y., Wang, Z., Sun, X., Jia, A., Jiang, G., and Li, Z. (2008). Higher salinity tolerance cultivars of winter wheat relieved senescence at reproductive stage. Environ. Exp. Bot. 62, 129–138. doi: 10.1016/j.envexpbot.2007.07.011
Zheng, Y., Xu, X., Li, Z., Yang, X., Zhang, C., Li, F., et al. (2009). Differential responses of grain yield and quality to salinity between contrasting winter wheat cultivars. Seed Sci. Biotechnol. 3, 40–43.
Zhou, Y., Li, Y., Qi, X., Liu, R., Dong, J., Jing, W., et al. (2020). Overexpression of V-type H+ pyrophosphatase gene EdVP1 from Elymus dahuricus increases yield and potassium uptake of transgenic wheat under low potassium conditions. Sci. Rep. 10:5020. doi: 10.1038/s41598-020-62052-5
Zhu, J., and Khan, K. (2001). Effects of genotype and environment on glutenin polymers and breadmaking quality. Cereal Chem. 78, 125–130. doi: 10.1094/CCHEM.2001.78.2.125
Keywords: salinity stress, antioxidant defense, wheat, stress tolerance, physiological and biochemical mechanisms
Citation: EL Sabagh A, Islam MS, Skalicky M, Ali Raza M, Singh K, Anwar Hossain M, Hossain A, Mahboob W, Iqbal MA, Ratnasekera D, Singhal RK, Ahmed S, Kumari A, Wasaya A, Sytar O, Brestic M, ÇIG F, Erman M, Habib Ur Rahman M, Ullah N and Arshad A (2021) Salinity Stress in Wheat ( Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 3:661932. doi: 10.3389/fagro.2021.661932
Received: 31 January 2021; Accepted: 27 May 2021; Published: 08 July 2021.
Reviewed by:
Copyright © 2021 EL Sabagh, Islam, Skalicky, Ali Raza, Singh, Anwar Hossain, Hossain, Mahboob, Iqbal, Ratnasekera, Singhal, Ahmed, Kumari, Wasaya, Sytar, Brestic, ÇIG, Erman, Habib Ur Rahman, Ullah and Arshad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayman EL Sabagh, ayman.elsabagh@agr.kfs.edu.eg
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Current Trends in Wheat Research
Introductory Chapter: Current Trends in Wheat Research
Published: 11 May 2022
DOI: 10.5772/intechopen.103763
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Current Trends in Wheat Research
Edited by Mahmood-ur-Rahman Ansari
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
273 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Author Information
Nazia nahid.
- Department of Bioinformatics and Biotechnology, GC University–Faisalabad, Pakistan
Parwsha Zaib
Tayyaba shaheen *, kanval shaukat.
- Department of Botany, University of Balochistan, Pakistan
Akmaral U. Issayeva
- M. Auezov South Kazakhstan University, Kazakhstan
Mahmood-ur-Rahman Ansari *
*Address all correspondence to: [email protected] and [email protected]
1. Introduction
Wheat ( Triticum aestivum ) is known as one of the most important cereal crops and is extensively grown worldwide [ 1 ]. Wheat contributes to 50% and 30% of the global grain trade and production respectively [ 2 ]. Wheat is also known as a staple food in more than 40 countries of the world. Wheat provides 82% of basic calories and 85% of proteins to the world population [ 3 , 4 ]. Wheat-based food is rich in fiber contents than meat-based food. Dough produced from bread wheat flour has different viscoelastic properties than other cereals. It is considered a higher fiber food. Therefore, its positive effects on controlling cholesterol, glucose, and intestinal functions in the body were observed [ 5 ]. Primarily, wheat is being used to make Chapatti (Bread) but it also contributes to other bakery products. Wheat utility and high nutritional value made it the staple food for more than 1/3rd population of the world. Wheat grain is separated from the chaff and stalks after the harvesting of wheat. Stalks of wheat are further used in animal bedding and construction material. Globally, the need for wheat production is enhancing even in countries having unfavorable climates for its production. Global climate changes are badly affecting the production of wheat and it raised the concern for food security.
It is estimated that annual cereal production should be increased by 1 billion tons to feed the expected population of 9.1 billion by 2050 [ 1 ]. The current scenario demands an increase in crop productivity to meet the increased requirements of food supply [ 6 ]. Wheat is grown in tropical and subtropical regions which experiences a lot of stress. These stresses result in a reduction of yield [ 7 ]. Major environmental stresses include cold, salinity, heat, and drought which are drastically affecting its yield. However, water and heat are considered as the key environmental stresses which caused in reduction of the wheat yield globally [ 8 , 9 ]. So, genetic improvements related to yield and stress tolerance are mandatory to enhance the production of wheat [ 10 , 11 ].
2. Genetically modified wheat plants
Genetically modified wheat plants have been produced by the use of bacteria. Wheat plants were inoculated with the plant-growth-promoting bacteria (PGPB) which resulted in the higher expression of abiotic stress (mainly drought and salinity) tolerant genes [ 12 ]. PGPB inoculated wheat cultivars also showed the higher expression of genes encoding antioxidant-enzymes, such as catalase (CAT), peroxidase , ascorbate peroxidase (APX), and glutathione peroxidase (GPX). So, it was concluded that PGPB used in wheat plants resulted in increased tolerance to abiotic stresses [ 12 ]. Cold shock proteins increase the survival of bacteria in severe environmental conditions. CspA and CspB genes from bacteria were transformed into wheat. Transgenic wheat plants expressing SeCspA and SeCspB were observed to have decreased water loss rate, increased proline and chlorophyll contents under salinity, and less water-stress conditions [ 13 ]. It was further investigated that SeCsp transgenic wheat plants resulted in enhanced weight and yield of grain than the control plants. SeCspA transgenic wheat plants were observed to have an improved water-stress tolerance than the control plants ( Table 1 , [ 13 ]).
| S. No. | Gene Name | Trait/Phenotype | Reference |
|---|---|---|---|
| 1. | Increased yield | [ ] | |
| 2. | Increased Nitrogen and Phosphorus uptake | [ ] | |
| 3. | More grain yield | [ ] | |
| 4. | More root growth | [ ] | |
| 5. | Increased heat tolerance | [ ] | |
| 6. | More yield | [ ] | |
| 7. | More yield | [ ] | |
| 8. | More yield, More seed protein contents | [ ] | |
| 9. | Iron biofortification | [ ] | |
| 10. | Drought and frost tolerance | [ ] | |
| 11. | Drought tolerant | [ ] | |
| 12. | Drought-stress tolerance | [ ] | |
| 13. | Abiotic-stress tolerance | [ ] | |
| 14. | Drought-stress tolerance | [ ] |
Development of transgenic wheat having various traits/phenotypes.
Gluten is a protein comprised of gliadins found in wheat. Gluten is the main cause of coeliac disease in individuals. Bread-making quality of wheat is determined by the gluten proteins. Wheat varieties with less gliadin contents were produced using gene-editing technologies and RNAi (RNA interference). Wheat lines lacking immunogenic gluten were produced. Low immunogenic gluten and more nutritional values were added in one wheat line named E82. A better microbiota profile (protection microorganisms available in the gut) was observed in the NCWS patients using the bread made with E82 [ 28 ]. Plant cuticle has a positive role in the protection of plant against biotic and abiotic stresses. Wheat plants transformed with TaSHN1 resulted in increased water-stress tolerance by reducing the leaf stomatal density and changing the composition of the cuticle [ 29 ].
3. Biotic stress tolerance in wheat
Wheat is considered an excessive contributor toward the human calorie intake [ 30 ]. Pests and pathogens cause yield losses in wheat up to 21.5% of the total losses and could be reached to 28.1% [ 31 ]. Wheat is affected by the fungal disease, powdery mildew caused by Blumeria graminis f. sp. tritici (Bgt). Powdery mildew is a damaging disease that resulted in greater loss of wheat [ 32 ]. Broad-spectrum resistant genes (BSR) are considered to have the most significant role to control powdery mildew. CMPG1-V gene was cloned from the Hynaldia villosa and it was observed that higher expression of CMPG1-V gene resulted in the Broad-spectrum resistance against powdery mildew [ 33 , 34 ]. Barley chi26 gene could also be used to enhance the resistance against powdery mildew and rust through genetic modification [ 35 ]. Some epigenetic regulators were determined to have a role in wheat powdery mildew resistance. TaHDT701 is a histone deacetylase that was found as a negative regulator of wheat defense against powdery mildew. TaHDT701 was observed to be associated with the one repeat protein (TaHOS15) and RPD3 type histone deacetylase TaHDA6. Knockdown of this histone deacetylase complex ( TaHDT701 , TaHDA6 , TaHOS15 ) in wheat resulted in increased powdery mildew tolerance [ 36 ].
Fusarium graminearum is a plant fungal pathogen that causes a devastating disease called Fusarium head blight in wheat. It results in the reduction of wheat production. Genetic techniques were used to increase the FHB (Fusarium head blight) resistance in wheat. Transgenic wheat plants expressing barley class II chitinase gene 2 were observed to have a higher resistance against Fusarium graminearum [ 37 ]. Lr10 and Lr21 were cloned and transformed into wheat. The transgenic plants were reported to be resistant to leaf rust disease. Evolution and diversification of HIPPs (heavy metal-associated isoprenylated plant proteins) genes were studied in Triticeae [ 38 ]. HIPPs genes of Hynaldia villosa were cloned through homology-based cloning. Transgenic wheat having HIPP1-V was developed and the role of HIPP1-V in cadmium stress was characterized. It was observed that higher expression of this gene resulted in increased tolerance to cadmium stress. Therefore, HIPP1-V could be used to increase the tolerance in wheat against cadmium [ 39 ].
4. Abiotic stress tolerance in wheat
Grain number, weight, and size are greatly reduced under the negative effects of environmental stresses. However, the timing, duration, and intensity of stress determine the severity of the negative effects [ 40 , 41 ]. Wheat is a major source of protein and calories for the human diet. High temperature is badly affecting the yield of wheat which is a main concern worldwide. Drought and heat stresses are the two main abiotic stresses which are playing a greater role in the reduction of wheat yield. Reduction in starch contents, photosynthetic activity, grain number, and chlorophyll contents in the endosperm is caused due to rise in temperature. Heat stress results in the accumulation of reactive oxygen species (ROS) which is the main reason for higher oxidative damage to the plant. Heat stress also results in the variation of wheat biochemistry, morphology, and physiology. Tolerance, avoidance, and escape are known as the three major mechanisms that support the plant to grow in a heat-stress environment. Major heat tolerance mechanisms in wheat are known as stay green, heat shock proteins, and antioxidant defense [ 42 ]. Protein synthesis and folding were observed to be interrupted during heat stress. Heat stress also resulted in the production of several stress agents badly affecting transcription, translation, and DNA replication in plants [ 43 ]. Plants speed up the production of heat shock proteins as a defense mechanism [ 44 ]. Higher activity of antioxidants, such as peroxidases, catalase, and superoxide dismutase, was observed under heat stress. Wheat cultivar showing greater tolerance to heat stress was observed to have higher activity of catalase, ascorbate peroxidase, and S-transferase [ 45 ].
Salt stress greatly affects the growth of wheat plants. Salinity stress has a higher impact on the morphology and physiology of wheat plants. Plants having less tolerance to salinity are not suitable for cropping. Potassium transporter ( HKT ) genes have a greater role in achieving salinity tolerance in wheat. Sodium (Na + ) exclusion through HKT genes is a major mechanism in wheat to have a salinity tolerance. OsMYBSs and AtAB14 are the transcription factors having a role in regulating HKT genes, which are considered as the candidate targets for increasing salinity tolerance in wheat [ 46 ]. Wheat transformed with a mutated transcription factor, HaHB4 showed higher water-use efficiency and was more yielding under drought stress [ 26 ]. Transgenic wheat expressing GmDREB1 gene from soybean was also observed to have higher drought tolerance under water-stress conditions [ 47 ]. DREB1A gene from Arabidopsis thaliana was introduced to bread wheat and increased tolerance against water stress in the transgenic wheat was observed. Bread wheat under drought stress was observed to have a higher level of WRKY proteins [ 48 ]. Higher expression of AtHDG11 gene in transgenic wheat resulted in increased water-stress tolerance during drought-stress conditions. Enhanced TaNAC69 expression in root and leaf of wheat during drought stress was observed [ 49 ]. Researchers are working to develop transgenic wheat having various traits/phenotypes by using advanced approaches of biotechnology for the last several decades ( Table 1 ). Numbers of transgenic wheat cultivars are being grown in the fields and several more are under trial.
5. CRISPR/Cas9 system in wheat
Gliadins and glutenins are known as the gluten proteins and ingestion of these proteins from barley, rye, and wheat could cause the disease called coeliac disease in humans. The only remedy is to develop gluten-free food. Transgenic wheat which retains baking quality and is safe for coeliac could not be produced using conventional methods because of the complexity of the wheat genome. Coeliac disease (CD) is activated by the immunogenic isotopes mainly gliadins. Gliadin families were downregulated by the use of RNA interference. CRISPR/Cas9 is a targeted gene manipulation tool considered to have a potential role in genetic modification ( Table 2 , [ 60 , 61 ]). CRISPR/Cas9 system was recently used for gene editing of gliadins. Offsprings with deleted, edited, or silenced gliadins were produced by CRISPR/Cas9. They helped to decrease the exposure of the patient to the CD epitopes [ 62 ]. This technology has been used to develop wheat cultivars having gluten genes with inactivated CD epitopes [ 62 , 63 ].
| S. No. | Gene Name | Trait/Phenotype | Reference |
|---|---|---|---|
| 1. | Powdery mildew resistance | [ ] | |
| 2. | Improved Phosphorus uptake | [ ] | |
| 3. | Improved yield | [ ] | |
| 4. | Powdery mildew resistance | [ ] | |
| 5. | Improved yield | [ ] | |
| 6. | Male sterility | [ ] | |
| 7. | High amylase contents | [ ] | |
| 8. | Improved quality | [ ] | |
| 9. | Herbicide tolerance | [ ] | |
| 10. | Herbicide tolerance | [ ] |
Genome edited wheat developed by CRISPR/Cas9 system.
CRISPR/Cas9 system and TALENS (transcription activator-like effector nuclease) were used in the bread wheat to generate the mutations in three homoeoalleles that encode MLO locus proteins against mildew. Mutations in all three TaMLO were generated by using TALENS which resulted in resistance against powdery mildew. The MLO homoeoalleles ( TaMLOA1 , TaMLOB1, and TaMLOD1 ) of bread wheat contributed to the mildew infection. Mutation of MLO alleles resulted in powdery mildew tolerance in wheat [ 50 ]. Genome editing was reported in which pds (phytoene desaturase) and inox (inositol oxygenase) genes in the cell suspension-culture of wheat were targeted. It was demonstrated that the genome-editing technique could also be applied in the cell suspension of wheat [ 64 ]. Very recently, various research groups are involved to develop transgenic wheat by using genome-editing technology. Some of the experiments are listed in Table 2 .
6. Wheat computational analysis
A comprehensive resource for wheat reference genome was developed by International Wheat Genome Sequencing Consortium. The URGI portal ( https://wheat-urgi.versailles.inra.fr/ ) was developed for the breeders and researchers to access the genome sequence data of bread-wheat. InterMine tools, genome browser, and BLAST were established for the exploration of genome sequences together with the additional linked datasets, including gene expression, physical maps, and sequence variation. Portal provided the higher browser and search features that facilitated the use of the latest genomic resources required for the upgradation of wheat [ 65 ].
DNA binding with one finger (Dof) transcription factors is known to have an important role in abiotic stress tolerance as well as the growth of plants. Ninety-six TaDof members of the gene family have been studied using computational approaches. By qPCR analysis, it was revealed that TaDof genes were upregulated under heavy metal and heat stress in wheat. Consequently, it could be concluded that detection of amino acid sites, genome-wide analysis, and identification of the Dof transcription factor family could provide us the new insight into the function, structure, and evolution of the Dof gene family [ 66 ].
Acknowledgments
This work was supported by funds from the Higher Education Commission of Pakistan.
- 1. FAO. World Food and Agriculture-FAO Statistical Pocketbook. Rome, Italy: FAO; 2015
- 2. Akter N, Islam MR. Heat stress effects and management in wheat: A review. Agronomy for Sustainable Development. 2017; 37 (5):1-17
- 3. Chaves MS, Martinelli JA, Wesp-Guterres C, Graichen FAS, Brammer SP, Scagliusi SM, et al. The importance for food security of maintaining rust resistance in wheat. Food Security. 2013; 5 (2):157-176
- 4. Sharma D, Singh R, Tiwari R, Kumar R, Gupta VK. Wheat responses and tolerance to terminal heat stress: A review. In: Hasanuzzaman M, Nahar K, Hossain M, editors. Wheat Production in Changing Environments. Singapore: Springer; 2019. pp. 149-173
- 5. Giraldo P, Benavente E, Manzano-Agugliaro F, Gimenez E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomie. 2019; 9 (7):352
- 6. Iqbal M, Raja NI, Yasmeen F, Hussain M, Ejaz M, Shah MA. Impacts of heat stress on wheat: A critical review. Advances in Crop Science and Technology. 2017; 5 (1):01-09
- 7. Rahaie M, Xue GP, Schenk PM. The role of transcription factors in wheat under different abiotic stresses. Abiotic Stress - Plant Responses and Applications in Agriculture. 2013; 2 :367-385
- 8. Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016; 529 (7584):84-87
- 9. Liu B, Asseng S, Müller C, Ewert F, Elliott J, Lobell DB, et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nature Climate Change. 2016; 6 (12):1130-1136
- 10. Tester M, Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010; 327 (5967):818-822
- 11. He Z, Joshi AK, Zhang W. Climate vulnerabilities and wheat production. In: Pielke RA, editor. Climate Vulnerability: Understanding and Addressing Threats to Essential Resources. Waltham: Academic Press; 2013. pp. 57-67
- 12. Sudan J, Sharma D, Mustafiz A, Kumari S. Signaling peptides: Hidden molecular messengers of abiotic stress perception and response in plants. In: Zargar S, Zargar M, editors. Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective. Singapore: Springer; 2018. pp. 95-125
- 13. Yu TF, Xu ZS, Guo JK, Wang YX, Abernathy B, Fu JD, et al. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Scientific Reports. 2017; 7 (1):1-4
- 14. Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Changa T, et al. Expression of the maize Dof1 transcription factor in wheat and sorghum. Frontiers in Plant Science. 2017; 8 :434
- 15. Qu B, He X, Wang J, Zhao Y, Teng W, Shao A, et al. A wheat CCAAT box-binding transcription factor increases the grain yield of wheat with less fertilizer input. Plant Physiology. 2015; 167 (2):411-423
- 16. Yadav D, Shavrukov Y, Bazanova N, Chirkova L, Borisjuk N, Kovalchuk N, et al. Constitutive overexpression of the TaNF-YB4 gene in transgenic wheat significantly improves grain yield. Journal of Experimental Botany. 2015; 66 (21):6635-6650
- 17. He X, Qu B, Li W, Zhao X, Teng W, Ma W, et al. The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiology. 2015; 169 (3):1991-2005
- 18. Tian B, Talukder SK, Fu J, Fritz AK, Trick HN. Expression of a rice soluble starch synthase gene in transgenic wheat improves the grain yield under heat stress conditions. In Vitro Cellular & Developmental Biology. Plant. 2018; 54 (3):216-227
- 19. Hu M, Zhao X, Liu Q , Hong X, Zhang W, Zhang Y, et al. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnology Journal. 2018; 16 (11):1858-1867
- 20. Smidansky ED, Meyer FD, Blakeslee B, Weglarz TE, Greene TW, Giroux MJ. Expression of a modified ADP-glucose pyrophosphorylase large subunit in wheat seeds stimulates photosynthesis and carbon metabolism. Planta. 2007; 225 (4):965-976
- 21. Zhao XQ , Nie XL, Xiao XG. Over-expression of a tobacco nitrate reductase gene in wheat (Triticum aestivum L.) increases seed protein content and weight without augmenting nitrogen supplying. PLoS One. 2013; 8 (9):e74678
- 22. Connorton JM, Jones ER, Rodríguez-Ramiro I, Fairweather-Tait S, Uauy C, Balk J. Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiology. 2017; 174 (4):2434-2444
- 23. Yang Y, Luang S, Harris J, Riboni M, Li Y, Bazanova N, et al. Overexpression of the class I homeodomain transcription factor Ta HDZ ipI-5 increases drought and frost tolerance in transgenic wheat. Plant Biotechnology Journal. 2018; 16 (6):1227-1240
- 24. Saint Pierre C, Crossa JL, Bonnett D, Yamaguchi-Shinozaki K, Reynolds MP. Phenotyping transgenic wheat for drought resistance. Journal of Experimental Botany. 2012; 63 (5):1799-1808
- 25. Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology. 2008; 147 (2):446-455
- 26. González FG, Capella M, Ribichich KF, Curín F, Giacomelli JI, Ayala F, et al. Field-grown transgenic wheat expressing the sunflower gene HaHB4 significantly outyields the wild type. Journal of Experimental Botany. 2019; 70 (5):1669-1681
- 27. Shavrukov Y, Baho M, Lopato S, Langridge P. The TaDREB3 transgene transferred by conventional crossings to different genetic backgrounds of bread wheat improves drought tolerance. Plant Biotechnology Journal. 2016; 14 :313-322
- 28. García-Molina MD, Giménez MJ, Sánchez-León S, Barro F. Gluten free wheat: Are we there? Nutrients. 2019; 11 (3):487
- 29. Bi H, Shi J, Kovalchuk N, Luang S, Bazanova N, Chirkova L, et al. Overexpression of the TaSHN1 transcription factor in bread wheat leads to leaf surface modifications, improved drought tolerance, and no yield penalty under controlled growth conditions. Plant, Cell and Environment. 2018; 41 (11):2549-2566
- 30. Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, et al. Food security: The challenge of feeding 9 billion people. Science. 2010; 327 (5967):812-818
- 31. Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nature Ecology & Evolution. 2019; 3 (3):430-439
- 32. Menardo F, Praz CR, Wyder S, Ben-David R, Bourras S, Matsumae H, et al. Hybridization of powdery mildew strains gives rise to pathogens on novel agricultural crop species. Nature Genetics. 2016; 48 (2):201-205
- 33. Zhu Y, Li Y, Fei F, Wang Z, Wang W, Cao A, et al. E3 ubiquitin ligase gene CMPG 1–V from Haynaldia villosa L. contributes to powdery mildew resistance in common wheat (Triticum aestivum L.). The Plant Journal. 2015; 84 (1):154-168
- 34. Liu J, Sun L, Chen Y, Wei L, Hao Y, Yu Z, et al. The regulatory network of CMPG1-V in wheat–Blumeria graminis f. sp. tritici interaction revealed by temporal profiling using RNA-Seq. International Journal of Molecular Sciences. 2020; 21 (17):5967
- 35. Eissa HF, Hassanien SE, Ramadan AM, El-Shamy MM, Saleh OM, Shokry AM, et al. Developing transgenic wheat to encounter rusts and powdery mildew by overexpressing barley chi26 gene for fungal resistance. Plant Methods. 2017; 13 (1):1-3
- 36. Zhi P, Kong L, Liu J, Zhang X, Wang X, Li H, et al. Histone deacetylase TaHDT701 functions in TaHDA6-TaHOS15 complex to regulate wheat defense responses to Blumeria graminis f. sp. tritici. International Journal of Molecular Sciences. 2020; 21 (7):2640
- 37. Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, et al. Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. Journal of Experimental Botany. 2008; 59 (9):2371-2378
- 38. Zhang H, Zhang X, Liu J, Niu Y, Chen Y, Hao Y, et al. Characterization of the heavy-metal-associated Isoprenylated plant protein (HIPP) gene family from Triticeae species. International Journal of Molecular Sciences. 2020; 21 (17):6191
- 39. Hura T. Wheat and barley: Acclimatization to abiotic and biotic stress. International Journal of Molecular Sciences. 2020; 21 (19):7423
- 40. Foulkes MJ, Scott RK, Sylvester-Bradley R. The ability of wheat cultivars to withstand drought in UK conditions: Formation of grain yield. The Journal of Agricultural Science. 2002; 138 (2):153-169
- 41. Weldearegay DF, Yan F, Jiang D, Liu F. Independent and combined effects of soil warming and drought stress during anthesis on seed set and grain yield in two spring wheat varieties. Journal of Agronomy and Crop Science. 2012; 198 (4):245-253
- 42. Poudel PB, Poudel MR. Heat stress effects and tolerance in wheat: A review. Journal of Biology and Today's World. 2020; 9 (3):1-6
- 43. Biamonti G, Caceres JF. Cellular stress and RNA splicing. Trends in Biochemical Sciences. 2009; 34 (3):146-153
- 44. Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: How close and how far? Life Sciences. 2010; 86 (11-12):377-384
- 45. Balla K, Bencze S, Janda T, Veisz O. Analysis of heat stress tolerance in winter wheat. Acta Agronomica Hungarica. 2009; 57 (4):437-444
- 46. Kunika BK, Singh PK, Rani V, Pandey GC. Salinity tolerance in wheat: An overview. International Journal of Chemical Studies. 2019; 6 :815-820
- 47. Shiqing GA, Huijun XU, Xianguo C, Ming C, Zhaoshi XU, Liancheng L, et al. Improvement of wheat drought and salt tolerance by expression of a stress-inducible transcription factor GmDREB of soybean (Glycine max). Chinese Science Bulletin. 2005; 50 :2714-2723, 2723
- 48. Okay S, Derelli E, Unver T. Transcriptome-wide identification of bread wheat WRKY transcription factors in response to drought stress. Molecular Genetics and Genomics. 2014; 289 (5):765-781
- 49. Xue GP, Bower NI, McIntyre CL, Riding GA, Kazan K, Shorter R. TaNAC69 from the NAC superfamily of transcription factors is up-regulated by abiotic stresses in wheat and recognises two consensus DNA-binding sequences. Functional Plant Biology. 2006; 33 (1):43-57
- 50. Wang Y, Cheng X, Shan Q , Zhang Y, Liu J, Gao C, et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nature Biotechnology. 2014; 32 (9):947-951
- 51. Ouyang X, Hong X, Zhao X, Zhang W, He X, Ma W, et al. Knock out of the PHOSPHATE 2 gene TaPHO2-A1 improves phosphorus uptake and grain yield under low phosphorus conditions in common wheat. Scientific Reports. 2016; 6 :29850
- 52. Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, et al. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nature Communications. 2016; 7 :12617
- 53. Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, et al. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. The Plant Journal. 2017; 91 :714-724
- 54. Wang W, Mauleon R, Hu Z, Chebotarov D, Tai S, Wu Z, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018; 557 (7703):43-49
- 55. Okada A, Arndell T, Borisjuk N, Sharma N, Watson-Haigh NS, Tucker EJ, et al. CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnology Journal. 2019; 17 :1905-1913
- 56. Li J, Jiao G, Sun Y, Chen J, Zhong Y, Yan L, et al. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnology Journal. 2020; 19 :937-951
- 57. Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, et al. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nature Biotechnology. 2017; 35 :438-440
- 58. Zhang R, Liu J, Chai Z, Chen S, Bai Y, Zong Y, et al. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nature Plants. 2019; 5 :480-485
- 59. Zhang Y, Malzahn AA, Sretenovic S, Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nature Plants. 2019; 5 :778-794
- 60. Schaart JG, van de Wiel CC, Lotz LA, Smulders MJ. Opportunities for products of new plant breeding techniques. Trends in Plant Science. 2016; 21 (5):438-449
- 61. Van de Wiel CC, Schaart JG, Lotz LA, Smulders MJ. New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnology Reports. 2017; 11 (1):1-8
- 62. Jouanin A, Gilissen LJ, Schaart JG, Leigh FJ, Cockram J, Wallington EJ, et al. CRISPR/Cas9 gene editing of gluten in wheat to reduce gluten content and exposure—Reviewing methods to screen for coeliac safety. Frontiers in Nutrition. 2020; 7 :51
- 63. Jouanin A, Schaart JG, Boyd LA, Cockram J, Leigh FJ, Bates R, et al. Outlook for coeliac disease patients: Towards bread wheat with hypoimmunogenic gluten by gene editing of α-and γ-gliadin gene families. BMC Plant Biology. 2019; 19 (1):1-6
- 64. Upadhyay SK, Kumar J, Alok A, Tuli R. RNA-guided genome editing for target gene mutations in wheat. G3: Genes, Genomes, Genetics. 2013; 3 (12):2233-2238
- 65. Alaux M, Rogers J, Letellier T, Flores R, Alfama F, Pommier C, et al. Linking the international wheat genome sequencing consortium bread wheat reference genome sequence to wheat genetic and phenomic data. Genome Biology. 2018; 19 (1):1
- 66. Liu Y, Liu N, Deng X, Liu D, Li M, Cui D, et al. Genome-wide analysis of wheat DNA-binding with one finger (Dof) transcription factor genes: Evolutionary characteristics and diverse abiotic stress responses. BMC Genomics. 2020; 21 (1):1-8
© 2022 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
By Harmeet Singh Bakala, Kamalpreet Singh Mandahal, ...
708 downloads
By Mirza Abdul Qayyum, Shafqat Saeed, Unsar Naeem-Ull...
585 downloads
By Juan Manuel Sánchez-Yañez
357 downloads
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Measuring the Effects of Climate Change on Wheat Production: Evidence from Northern China
Associated data.
The data will be available on request.
The current study examines the long-run effects of climatic factors on wheat production in China’s top three wheat-producing provinces (Hebei, Henan, and Shandong). The data set consists of observations from 1992 to 2020 on which several techniques, namely, fully modified OLS (FMOLS), dynamic OLS (DOLS), and canonical co-integrating regression (CCR) estimators, and Granger causality, are applied. The results reveal that climatic factors, such as temperature and rainfall, negatively influenced wheat production in Henan Province. This means that Henan Province is more vulnerable to climate change. In contrast, it is observed that climatic conditions (via temperature and rainfall) positively contributed to wheat production in Hebei Province. Moreover, temperature negatively influenced wheat production in Shandong Province, while rainfall contributed positively to wheat production. Further, the results of Granger causality reveal that climatic factors and other determinants significantly influenced wheat production in the selected provinces.
1. Introduction
China cultivates only eight percent of the world’s arable land to feed eighteen percent of the global population. It is expected that China’s population will peak around 2030 [ 1 ]; hence, the food security problem in China has always been a concern. In the “Outline of the 14th Five-Year Plan for National Economic and Social Development of the People’s Republic of China and the Long-term Goals in 2035,” the Chinese government, for the first time, incorporated the food security strategy into the planning system and set the goal of ensuring that grain output will remain stable at over 650 million tons over the 14th Five-Year Plan period. Climate has a strong influence on food production. Climate change and more frequent bad weather events around the world exacerbate the insecurity of China’s grain production [ 2 , 3 ]. As a result, understanding the impact of climate change on China’s grain crop productivity and devising countermeasures to implement China’s food security strategy are critical.
The impact of climate change on grain crop production has both advantages and disadvantages. Nevertheless, the disadvantages outweigh the advantages overall, and different climate variables have different impacts on different crops and regions [ 4 , 5 , 6 ]. In recent years, the increased heat caused by climate change has been conducive to expanding the grain sown area and producing more grain [ 7 ]. Increasing rainfall and CO 2 concentrations are beneficial for crop production to some extent, but high temperatures may negate this effect in some areas [ 8 , 9 ]. Similarly, climate change had a negative impact on grain production by expanding pest and disease occurrence areas, shortening crop growth cycles, and increasing the frequency of extreme weather events [ 10 , 11 ].
The global climatic variations are a sensitive topic being discussed in China. According to the National Meteorological Administration, China’s temperature has increased by 0.3 °C every 10 years (higher than the global average during the same period), and its annual precipitation has increased by 5.1 mm every 10 years between 1961 and 2020 [ 12 ]. Climate change is causing a “double increase in water and heat.” By evaluating the expected impact of global warming on the yields of China’s main crops (wheat, rice, and corn), it was discovered that the crop yield effect emanating from climate change is primarily due to an increase in air temperature [ 13 ]. Grain production has been impacted by significant deviations in China’s agricultural climate resources. The regional space of grain production is also changing, with the emphasis shifting to the main production areas in the north [ 14 ]. Researchers incorporate technological progress factors into the research system of food production under climate change [ 15 , 16 , 17 , 18 ]. Food security is a technical issue in terms of production mode, and the level of technology determines the ability to ensure food security. As a result, technological advancement can be used as an effective tool for grain production to cope with climate change [ 19 , 20 ].
Many studies have found that technological progress significantly impacts grain production, which is primarily reflected in the two factors listed below. First, technological advancement has improved the crops’ ability to withstand disasters. The advancement of bio-pesticides due to technological advancement has increased the agricultural departments’ ability to prevent and control major agricultural pests and diseases while causing less environmental impact [ 21 , 22 ]. Simultaneously, the monitoring technology system of major sudden agricultural meteorological disasters constructed by the agricultural sector is also conducive to the agricultural sector’s better response to extreme weather events [ 23 ]. Second, technological advancement is a driving force in changing the mode of production. Modern agricultural mechanization can promote rapid agricultural production growth while producing good environmental results [ 24 , 25 ]. Fertilizer use can improve crop yield, but excessive use reduces crop yield [ 26 ]. Improved seed varieties, fertilizers, pesticides, technical equipment, and infrastructure are used as proxies for technological progress in agricultural production [ 18 , 27 , 28 , 29 ]. Hence, this study also considers fertilizer use a proxy for technological advancement, as it is a crucial factor in crop production.
Wheat is China’s second most important food crop, after rice, and its planting area accounts for 22~30% of the total cultivated land [ 30 ]. Winter wheat is the predominant crop in China, accounting for more than 90 percent of the total output [ 31 ]. The regions of China that produce winter wheat can be divided into Southern and Northern cultivation areas. The northern winter wheat producing region is the most concentrated wheat growing and consuming region in China, and its sown area and wheat output account for around two-thirds of the country. In recent years, the top three provinces in northern China for winter wheat production were Henan, Shandong, and Hebei. Henan Province has an alternating temperate monsoon and subtropical climate, whereas Shandong and Hebei Provinces have a temperate monsoon climate. Figure 1 and Figure 2 show their geographical location, wheat yield, temperature, and rainfall.

The distribution of wheat production (10,000 tons) in Hebei, Henan, and Shandong Provinces.

The distribution of temperature (°C) and rainfall (mm) in Hebei, Henan, and Shandong Provinces.
Previous studies in various parts of the world have extensively documented the impact of changing climate on wheat crop yield. Most existing research on China discussed the relationship between the two at the national and regional levels [ 31 , 32 , 33 ] or only focused on a specific province, such as Henan Province, which has the highest wheat yield [ 30 , 34 ]. However, climate change causes food production variability in regions with varying climate resources. The current paper assesses the long-term effects of changing climate on wheat production in the top three provinces in northern China to further analyze the heterogeneous influence of changing climate on wheat production, propose targeted measures to deal with climate change for the main grain-producing areas in China, and contribute to China’s food security strategy. Figure 3 shows the dynamic nexus between climatic factors, other determinants, and wheat production.

The study’s conceptual framework.
2. Literature Review
Wheat may be one of the crops most susceptible to the effects of climate change, but its substantial impact on crop production is profound. Numerous research works have investigated the impacts of climate change on wheat development and harvest in major wheat-producing regions in Asia, Europe, and northern Africa. However, it is important to note that varied temperature conditions and precipitation patterns affect wheat growth and yield differently in different regions.
Several researchers, for example, Zhai et al. [ 18 ], Abbas [ 27 ], Gul et al. [ 28 ], Ali et al. [ 35 ], and Warsame et al. [ 36 ], have explored the short-term and long-term climate change effects on food crop production by applying the autoregressive distributed lag (ARDL) methodology and reported mix outcomes related to climate variables. While, a study by You et al. [ 37 ] revealed that climate warming lowered wheat yield growth, a 1 °C rise in wheat growing temperature reduced wheat production by 3–10% in China. However, the findings of Zhai et al. [ 18 ] from 1970 to 2014 in China evaluated that temperature did not significantly influence the amount of wheat produced per unit of land in the short run and long run, while farm mechanization and fertilizer usage increased wheat output in the long run.
More recently, the research by Gul et al. [ 5 ] from 1985 to 2016 in Pakistan established the long-term link between climate variables and main food crop production. The findings showed that temperature negatively affects key food crop production, while rainfall improves food production. Similarly, the study of Chandio et al. [ 38 ] from 1977 to 2014 in Pakistan revealed that climate change and CO 2 have a detrimental short- and long-term influence on grain productivity, reducing cereal production and causing food security issues in the country.
Another similar study by Warsame et al. [ 39 ] for the period of 1980–2017 in Somalia examined the effects of climate change along with political instability on the productivity of sorghum by using various estimation techniques (i.e., FMOLS, DOLS, and CCR). The findings revealed that political instability and temperature significantly declined the productivity of sorghum, while rainfall and cultivated area enhanced the production in the long term. The long-term findings are also verified by the CCR approach. In the case of India, Bhardwaj et al. [ 40 ] reported that climate variables negatively contributed to wheat and paddy production; moreover, excessive rainfall had a detrimental influence on wheat and rice yields.
In the case of Ghana, Ntiamoah et al. [ 41 ] used a novel dynamic simulated autoregressive distributed lag (ARDL) model to examine the impact of CO 2 emissions, rainfall, credit supply, and fertilizer on the productivity of maize and soybean, covering the period from 1990 to 2020. The findings revealed that CO 2 emissions, as well as rainfall, have a significant and positive impact on crop production, while the supply of credit and fertilizer negatively influenced maize production. In the context of Asia, Ozdemir [ 42 ] studied the effects of climate change on agricultural output by using various estimation techniques (i.e., PMG and CCEMG). The outcomes showed that temperature and CO 2 affected agricultural output negatively and significantly in the long term, while precipitation improved productivity. In addition, other factors, such as power consumption for agricultural machinery and fertilizer, significantly enhanced agricultural output in the same period.
According to research by Akhtar and Masud [ 4 ] on the influence of climatic factors on rice and cereal production from the period 1985–2016, it was found that temperature and energy usage severely influence rice and vegetable output, although their effect on cereal productivity is minor. However, CO 2 emissions negatively influenced coffee production, and the temperature, energy use, and fossil fuel usage induced climate change, which had a negative impact on Malaysian agriculture. The empirical study of Kumar et al. [ 43 ] in selected lower-middle-income nations from 1971 to 2016 assessed the climate change–cereal crops production association. The authors’ findings revealed that rising temperatures diminish crop productivity. Rainfall and CO 2 emissions boosted crop yields, and a bidirectional causation between grain output, temperature, and CO 2 emissions was discovered. Rainfall and temperature affect grain production uni-directionally and might threaten the food security of the rural populations.
In particular, a study in China by Pickson et al. [ 44 ] from 1998 to 2017 examined the effects of climate change on rice cultivation. The findings showed that the climate variable, such as the temperature, adversely influences rice cultivation, while average rainfall influences the rice output but is insignificant. The farmed area positively affected short-term crop output. At the same time, fertilizer use had little effect and bidirectional causation between rice production and the cultivated area. Similarly, the investigation of Pickson et al. [ 6 ] in China over the period 1990Q1–2013Q4 found that the average temperature and its variability associated with cereal production were negative but significant in the long run. Additionally, rainfall variability and cereal production linkage showed no significant effect in the long run, but two variables (CO 2 and temperature variability) had a negatively significant association in the short run.
Likewise, Pickson et al. [ 45 ] investigated the impact of global warming on the main food crops (i.e., rice and maize) production in the case of China over the periods 1978Q1–2015Q4. The outcomes revealed a significantly positive trend in average temperature and seasonal temperature increases during the spring, summer, and fall with the insignificant change in the monthly, seasonal, and annual precipitation. The impact of temperature decreases maize and rice production at higher quantiles.
Due to the diverse time scales, geographic locations, and techniques, the following research work has not yet formed consistent findings on climate change’s influence on wheat growth and yield. These research works failed to combine the climatic conditions and agricultural progress elements into crop yield–climate functions to investigate their influence, and the long-run impacts on wheat must be studied. In this work, we employed the FMOLS method to assess the long-run climate variations’ influence on wheat yield in the northern region of China. The findings of the FMOLS approach are verified by the DOLS and CCR estimators.
3. Materials and Methods
The present study intends to investigate the long-run impact of temperature, rainfall, fertilizer usage, power usage, farming area, and labor on wheat production in the context of top 3 wheat-producing provinces of northern China. Wheat production is used as the dependent variable, and it is measured in 10,000 tons, while climatic factors, namely, temperature measured in degrees Celsius, rainfall measured in millimeters, fertilizer usage measured in 10,000 tons, power consumption measured in 1000 kWh, the cultivated area measured in 1000 hectares, and labor measured in 10,000 people, are used as the independent variables. The data were extracted from the China Rural Statistical Yearbook ( https://data.cnki.net/Yearbook/Navi?type=type&code=A# (accessed on 1 June 2022)) and the National Weather Science Data Center ( http://data.cma.cn/ (accessed on 1 June 2022)).
3.2. Econometric Modeling
This study examines the long-run impact and the causal relationship between temperature, rainfall, fertilizer usage, power consumption, cultivated area, labor, and wheat production in the selected provinces of China. Several investigation tests were carried out to achieve the research objective, including the co-integration test, FMOLS, DOLS, and CCR estimators, and the Granger causality test. The basic model is constructed as shown below:
The logarithmic arrangement of Equation (1) can be developed as follows:
where WP indicates wheat production, TEMP denotes average annual temperature, RF represents average annual rainfall, FER indicates fertilizer usage, PC shows the power consumption, WA stands for wheat cultivated area, LF indicates rural labor force.
3.3. FMOLS Long-Run Estimator
This paper uses the fully modified OLS (FMOLS) proposed by Phillips and Hansen [ 46 ] to estimate the co-integration coefficient. Based on the OLS, this method uses the semi-parametric two-stage estimation method to correct the equalization error and the explained variable, which can effectively eliminate the endogeneity caused by the co-integration relationship and the sequence correlation of error terms, thus obtaining the consistent estimator of co-integration parameter estimator and the asymptotic normal distribution of FMOLS estimator. Suppose the model is
Let μ t ′ = μ 1 t ′ + μ 2 t ′ . First, perform OLS estimation on Equation (3) to obtain the θ ^ and μ t ^ of OLS estimators. Second, estimate the long-term variance of μ t . Let Ω and ∆ represent long-term variance and one-sided long-term variance, respectively, and the estimates are shown in Formulae (5) and (6).
By adjusting the endogeneity, we can obtain Equation (7).
By adjusting the sequence correlation, we can obtain Equation (8).
The final FMOLS estimator is obtained as shown in Equation (9).
Furthermore, we use the dynamic OLS (DOLS) proposed by Stock and Watson [ 47 ] and the canonical co-integrating regressions (CCR) proposed by Park [ 48 ] to verify the robustness of FMOLS estimation results. The DOLS estimation model contains the lag term of explanatory variables, and the standard deviation of its estimator has a normal asymptotic distribution, which is also better than the OLS estimation [ 40 ]. The idea of the CCR model is the same as the FMOLS model, but the difference is that it transforms the data stationarity to obtain the least-square estimation and then eliminates the dependency between the co-integration equation and the random correction equation of the explanatory variables.
4. Results and Discussion
We use the FMOLS, DOLS, and CCR estimators to examine the long-term influence of temperature, rainfall, fertilizer use, power consumption, wheat farming area, and labor on wheat production in the selected three provinces of China. Table 1 reports the statistical summary of the dependent and independent variables for the Hebei, Henan, and Shandong Provinces. The J-B test confirms that all the studied variables are normally distributed. Figure 4 shows the trend of wheat production and climatic factors in the selected provinces of China.

Trend of wheat production, temperature, and rainfall in Hebei, Henan, and Shandong Provinces of China.
Statistical summary for Hebei, Henan, and Shandong Provinces.
| Hebei Province | |||||||
|---|---|---|---|---|---|---|---|
| LWP | LTEMP | LRF | LFER | LPC | LWA | LLF | |
| Mean | 3.1027 | 1.0793 | 2.6475 | 2.4727 | 3.9040 | 3.3885 | 3.4696 |
| Median | 3.0969 | 1.0779 | 2.6492 | 2.4830 | 3.8975 | 3.3859 | 3.4767 |
| Maximum | 3.1772 | 1.1205 | 2.7955 | 2.5258 | 4.0454 | 3.4415 | 3.5288 |
| Minimum | 3.0081 | 1.0338 | 2.4567 | 2.3438 | 3.6371 | 3.3347 | 3.4105 |
| Std. Dev. | 0.0515 | 0.0235 | 0.0839 | 0.0444 | 0.1032 | 0.0272 | 0.0387 |
| Skewness | −0.1277 | −0.0406 | −0.3541 | −0.9106 | −0.6792 | 0.1607 | −0.1323 |
| Kurtosis | 1.8645 | 2.1997 | 2.6445 | 3.6768 | 3.1674 | 2.8598 | 1.5297 |
| J-B | 1.4673 | 0.7008 | 0.6804 | 4.0902 | 2.0299 | 0.1332 | 2.4176 |
| Prob. | 0.4801 | 0.7043 | 0.7115 | 0.1293 | 0.3624 | 0.9355 | 0.2985 |
| Obs. | 26 | 26 | 26 | 26 | 26 | 26 | 26 |
| Mean | 3.4487 | 1.1764 | 2.8157 | 2.7334 | 3.8913 | 3.7184 | 3.6723 |
| Median | 3.4766 | 1.1752 | 2.8199 | 2.7675 | 3.9574 | 3.7216 | 3.6727 |
| Maximum | 3.5743 | 1.2103 | 2.9511 | 2.8549 | 4.0685 | 3.7589 | 3.7319 |
| Minimum | 3.2440 | 1.1439 | 2.6183 | 2.5081 | 3.4935 | 3.6813 | 3.5766 |
| Std. Dev. | 0.0966 | 0.0158 | 0.0917 | 0.1129 | 0.1639 | 0.0288 | 0.0441 |
| Skewness | −0.3490 | −0.0922 | −0.4660 | −0.5676 | −0.9815 | 0.0464 | −0.5964 |
| Kurtosis | 1.9237 | 2.6353 | 2.3330 | 1.9491 | 2.8955 | 1.4106 | 2.5309 |
| J-B | 1.7827 | 0.1809 | 1.4228 | 2.5924 | 4.1870 | 2.7458 | 1.7800 |
| Prob. | 0.4100 | 0.9135 | 0.4909 | 0.2735 | 0.1232 | 0.2533 | 0.4106 |
| Obs. | 26 | 26 | 26 | 26 | 26 | 26 | 26 |
| Mean | 3.3183 | 1.1379 | 2.7570 | 2.6420 | 3.9461 | 3.5722 | 3.5979 |
| Median | 3.3215 | 1.1386 | 2.7671 | 2.6487 | 3.9937 | 3.5792 | 3.6005 |
| Maximum | 3.4097 | 1.1643 | 2.9106 | 2.6992 | 4.1255 | 3.6110 | 3.6511 |
| Minimum | 3.1895 | 1.1093 | 2.5093 | 2.5590 | 3.6038 | 3.4724 | 3.5520 |
| Std. Dev. | 0.0652 | 0.0163 | 0.0922 | 0.0389 | 0.1504 | 0.0378 | 0.0352 |
| Skewness | −0.5563 | −0.0943 | −0.6423 | −0.5870 | −0.9727 | −1.0561 | 0.1103 |
| Kurtosis | 2.4971 | 2.0359 | 3.4293 | 2.2740 | 2.8985 | 3.4102 | 1.4679 |
| J-B | 1.6152 | 1.0454 | 1.9874 | 2.0641 | 4.1115 | 5.0157 | 2.5956 |
| Prob. | 0.4459 | 0.5929 | 0.3701 | 0.3562 | 0.1279 | 0.0814 | 0.2731 |
| Obs. | 26 | 26 | 26 | 26 | 26 | 26 | 26 |
Note: LWP, LTEMP, LRF, LFER, LPC, LWA, LLF signify the natural log of wheat production, average annual temperature, average annual rainfall, fertilizer usage, power consumption, wheat cultivated area, and rural labor force, while J-B denotes the Jarque–Bera test.
The outcomes of the correlation matrix for Hebei, Henan, and Shandong Provinces are presented in Table 2 . The findings for Hebei Province reveal that temperature, rainfall, fertilizer usage, power consumption, and labor are significantly and positively associated with wheat production, while the cultivated area is negatively associated. Further, the findings for Henan Province show that all the studied variables are significantly linked with wheat production, except rainfall. In addition, the outcomes for Shandong Province indicate that temperature, cultivated area, and labor are significant and interrelated with wheat production, whereas fertilizer is negatively associated.
Results of the correlation matrix for Hebei, Henan, and Shandong Provinces.
| Hebei Province | |||||||
|---|---|---|---|---|---|---|---|
| LWP | LTEMP | LRF | LFER | LPC | LWA | LLF | |
| LWP | 1.0000 | ||||||
| LTEMP | 0.6897 *** | 1.0000 | |||||
| LRF | 0.3649 * | 0.1626 | 1.0000 | ||||
| LFER | 0.5910 *** | 0.4553 ** | 0.2603 | 1.0000 | |||
| LPC | 0.3733 * | 0.3439 * | 0.1293 | 0.9014 | 1.0000 | ||
| LWA | −0.0362 | −0.1602 | −0.3598 * | −0.3973 ** | −0.4116 ** | 1.0000 | |
| LLF | 0.6651 ** | 0.3895 ** | 0.4921 ** | 0.8690 *** | 0.7928 *** | −0.4726 ** | 1.0000 |
| LWP | 1.0000 | ||||||
| LTEMP | 0.5545 *** | 1.0000 | |||||
| LRF | 0.1796 | −0.1576 | 1.0000 | ||||
| LFER | 0.9509 *** | 0.4881 ** | 0.2164 | 1.0000 | |||
| LPC | 0.9201 *** | 0.4610 ** | 0.1996 | 0.9793 *** | 1.0000 | ||
| LWA | 0.9520 *** | 0.6116 *** | 0.2225 | 0.8954 *** | 0.8180 *** | 1.0000 | |
| LLF | 0.8656 *** | 0.5235 *** | 0.1868 | 0.8916 *** | 0.8873 *** | 0.7996 *** | 1.0000 |
| LWP | 1.0000 | ||||||
| LTEMP | 0.5323 *** | 1.0000 | |||||
| LRF | 0.3144 | 0.0439 | 1.0000 | ||||
| LFER | −0.1036 | 0.1261 | 0.0995 | 1.0000 | |||
| LPC | 0.2986 | 0.4270 ** | 0.3541 * | 0.7304 *** | 1.0000 | ||
| LWA | 0.8281 *** | 0.3793 ** | −0.048 | −0.4079 ** | −0.144 | 1.0000 | |
| LLF | 0.7342 *** | 0.5986 *** | 0.4947 ** | 0.3109 | 0.8066 *** | 0.3404 * | 1.0000 |
Note: *** p value < 0.01, ** p value < 0.05, and * p value < 0.1.
We employ the Johansen and Juselius co-integration procedure to explore the long-term association between the explained variables, such as wheat production and its explanatory variables. We test the research hypothesis, as the null hypothesis states that the explained variables, wheat production and its explanatory variables, are not co-integrated in the long term. In contrast, the alternative hypothesis mentions that the considered variables are co-integrated in the long term. To reach a decision about the hypothesis, we use the Trace t-statistic test, and the findings for Hebei, Henan, and Shandong Provinces are reported in Table 3 . The findings reveal that wheat production, temperature, rainfall, fertilizer usage, power consumption, cultivated area, and labor force are co-integrated in the long term in China’s selected wheat-producing provinces.
Co-integration outcomes for Hebei, Henan, and Shandong Provinces.
| Hebei Province | Henan Province | Shandong Province | |||
|---|---|---|---|---|---|
| Rank | TS | Rank | TS | Rank | TS |
| None * | 242.8063 (0.0000) | None * | 250.6836 (0.0000) | None * | 245.8051 (0.0000) |
| At most 1 * | 151.8709 (0.0000) | At most 1 * | 143.9006 (0.0000) | At most 1 * | 165.5126 (0.0000) |
| At most 2 * | 91.0448 (0.0004) | At most 2 * | 98.4322 (0.0001) | At most 2 * | 95.6523 (0.0001) |
| At most 3 * | 54.7745 (0.0098) | At most 3 * | 59.4932 (0.0028) | At most 3 * | 60.6158 (0.0020) |
| At most 4 | 24.8771 (0.1659) | At most 4 * | 34.6498 (0.0128) | At most 4 | 29.5732 (0.0531) |
| At most 5 | 6.4268 (0.6451) | At most 5 | 13.2864 (0.1047) | At most 5 | 14.3444 (0.0740) |
| At most 6 | 0.0618 (0.8036) | At most 6 | 3.6392 (0.0564) | At most 6 | 3.8247 (0.0505) |
Note: TS indicates the trace statistic, * signifies rejection of the hypothesis at the 0.05 level.
Table 4 reports the estimated results of long-term effect of climate variables and other control variables on wheat yield in Hebei, Henan, and Shandong Provinces, respectively.
Results of FMOLS estimator for top three provinces in northern China.
| Variables | Hebei Province | Henan Province | Shandong Province | |||
|---|---|---|---|---|---|---|
| Coefficient | Prob. | Coefficient | Prob. | Coefficient | Prob. | |
| LTEMP | 1.1600 *** | 0.0000 | −0.5129 * | 0.0929 | −0.0701 | 0.7446 |
| LRF | 0.0136 | 0.8277 | −0.0576 | 0.1387 | 0.0823 * | 0.0522 |
| LFER | 0.1726 | 0.5623 | −0.6117 ** | 0.0325 | 0.2917 * | 0.0695 |
| LPC | −0.3626 *** | 0.0009 | 0.4885 *** | 0.0037 | −0.1421 * | 0.0980 |
| LWA | 0.5805 *** | 0.0019 | 2.9805 *** | 0.0000 | 1.1690 *** | 0.0000 |
| LLF | 1.3669 *** | 0.0000 | 0.2161 | 0.1962 | 0.2351 | 0.7447 |
| C | −3.9019 *** | 0.0001 | −7.8904 *** | 0.0000 | −2.1196 | 0.3335 |
| R | 0.8061 | 0.9722 | 0.9332 | |||
| Adj-R | 0.7415 | 0.9630 | 0.9058 | |||
In the case of Hebei Province, the climate variables (i.e., temperature and rainfall) have a positive, significant impact on wheat production. This means the climate conditions are more favorable for wheat cultivation in Hebei Province. Specific to the North China Plain, where this study area is located, some research evidence shows that in the north of this plain, the impact of rainfall on wheat production is positive, while in the south of this plain, the impact of rainfall turns negative [ 49 ]. Similarly, for temperature, the increase in temperature increases the winter wheat yield in the northern part of the North China Plain but decreases the wheat yield produced in winter in the south of the North China Plain [ 50 ]. The top three wheat-producing provinces are selected for this investigation. Hebei, Shandong, and Henan Provinces are distributed in the North China Plain. The three provinces’ yearly mean temperature and yearly mean precipitation are ranked from low to high in Hebei, Shandong, and Henan (see Figure 2 ). The temperature and rainfall in Hebei Province are low, and the impacts of temperature and precipitation on wheat yield are positive.
Further results reveal that fertilizer use, cultivated area, and labor force also have a positive, significant influence on wheat production. The long-run coefficients of fertilizer use, cultivated area, and labor force indicate that a 1% increase in fertilizer treatment use, cultivated area, and labor force wheat production improved by 0.17%, 0.58%, and 1.36%, respectively.
In the case of Henan Province, the climatic factors (i.e., temperature and rainfall) and wheat production relationship was significant and negative. This means that climatic factors severely impact wheat production in Henan Province. The long-run coefficient of both climate variables, temperature and rainfall, indicates that with a 1% increase in both climate variables (i.e., temperature and rainfall), wheat production decreases by 0.51%, and 0.05%. Geng et al. [ 51 ] reported that high temperatures will be detrimental to wheat production by shortening the growth cycle of the wheat crop. Further, Song et al. [ 33 ] stated that excessive rainfall causes excessive water accumulation, which will aggravate the wet damage of wheat and negatively affect wheat production.
Moreover, the results show that fertilizer usage also significantly negatively impacts wheat production. The long-term coefficient of fertilizer usage reveals that if a farmer overuses the fertilizer by 1%, wheat production declines by 0.61%. Fertilization can not only supplement the nutrients needed by wheat but also improve the utilization rate of water, thus increasing the yield of wheat [ 52 , 53 ]. However, unreasonable and excessive use of chemical fertilizers will cause soil degradation and adversely affect wheat yield. This shows that the rational use of chemical fertilizers is very important for wheat production, and Henan Province should pay more attention to improving chemical fertilizer use efficiency.
In contrast, these variables (power usage, wheat farming area, and labor force) and the wheat production relationship were significant and positive. The long-run coefficient of power usage, wheat farming area, and labor force reveals that a 1% increase in power usage, wheat farming area, and labor force increases wheat production by 0.48%, 2.98%, and 0.21%, respectively.
In the case of Shandong Province, the climate variables, temperature, and wheat production displayed a diverse relationship. At the same time, rainfall had a significant and positive influence, suggesting that with a 1% increase in temperature and rainfall, wheat production decreased by 0.07% and improved by 0.08%. The heterogeneous effect of the climate variables on regional wheat yield is verified by some existing studies. For example, the evidence from Mexico and China verified that the sensitivity of wheat yield to climate variables is uneven in space [ 54 , 55 ]. Tao et al. [ 56 ] studied climate change’s influence on wheat productivity and found the prospective consequences of climate change on winter wheat output in northern China under 10 climatic scenarios and concluded that environmental variability might enhance wheat yield by 37.7% (18.6%), 67.8% (23.1%), and 87.2% (34.4%), with (without) CO 2 fertilization effects in the 2020s, 2050s, and 2080s, respectively, in the future. The temperature and rainfall in Shandong Province are in the middle of the three provinces, and the impact of temperature on wheat yield is negative, but the impact of rainfall is positive. In Henan Province, it is observed that the temperature is higher, and the rainfall is higher; the influence of temperature and rainfall on wheat is negative. Moreover, the results show that these variables (fertilizer use, cultivated area, and labor force) and wheat production association was significant and positive, suggesting that a 1% increase in fertilizer usage, cultivated area, and labor force enhanced wheat production by 0.29%, 1.16%, and 0.23%, respectively.
This study applied the DOLS and CCR long-run estimators as a robust check approach for the FMOLS findings. Table 5 shows that climate variables positively affect wheat production in the context of Hebei Province. The estimated coefficients of DOLS and CCR are consistent with the findings of the FMOLS model. Likewise, in Henan Province’s case, climatic factors negatively influence wheat production. These outcomes are also consistent with the outcomes of the FMOLS model. In addition, climatic factors, such as temperature, only have a negative impact on wheat production. Meanwhile, rainfall has a significant and positive linkage with wheat production. Hence, the results of both techniques, such as DOLS and CCR, are similar to the results of the FMOLS method.
Robustness check.
| Hebei Province | Henan Province | Shandong Province | ||||
|---|---|---|---|---|---|---|
| DOLS | CCR | DOLS | CCR | DOLS | CCR | |
| Variables | Coefficient | Coefficient | Coefficient | Coefficient | Coefficient | Coefficient |
| LTEMP | 1.3956 *** (0.0003) | 1.3629 *** (0.0000) | −0.2405 (0.4173) | −0.6182 (0.1652) | −0.1411 (0.7269) | −0.0961 (0.4601) |
| LRF | 0.0837 (0.4661) | 0.0293 (0.6728) | −0.0107 (0.8369) | −0.0187 (0.7913) | 0.4315 * (0.0791) | 0.1276 *** (0.0054) |
| LFER | 0.2372 (0.5224) | 0.1416 (0.5718) | −0.5957 * (0.0900) | −0.7901 ** (0.0292) | 0.1267 (0.7971) | 0.3789 *** (0.0033) |
| LPC | −0.2898 ** (0.0213) | −0.3205 *** (0.0002) | 0.1385 (0.4038) | 0.5703 *** (0.0023) | 0.0943 (0.7438) | −0.1125 * (0.0580) |
| LWA | 0.5525 *** (0.0038) | 0.5663 *** (0.0000) | 2.2683 ** (0.0167) | 3.1978 *** (0.0000) | 1.6290 ** (0.0153) | 1.4074 *** (0.0000) |
| LLF | 1.0557 *** (0.0081) | 1.2416 *** (0.0000) | −0.2059 (0.4506) | 0.2741 (0.1504) | −0.4386 (0.7924) | −0.8140 * (0.0798) |
| C | −3.6129 *** (0.0016) | −3.7710 *** (0.0000) | −2.9953 (0.3278) | −8.7276 *** (0.0000) | −2.6588 (0.5218) | 0.3107 (0.8046) |
| R | 0.9402 | 0.7980 | 0.9922 | 0.9662 | 0.9881 | 0.9251 |
| Adj-R | 0.8805 | 0.7307 | 0.9814 | 0.9549 | 0.9454 | 0.8943 |
Although the long-run impact of the variables concerned was explored through the FMOLS, DOLS, and CCR estimators, the causal connection between the underlying variables is still in question. Therefore, we further apply the Granger causality method. The findings for Hebei, Henan, and Shandong Provinces are presented in Table 6 . A bidirectional causality between precipitation and fertilizer usage with wheat production in the context of Hebei Province can be observed. This means that rainfall and fertilizer usage significantly contributed to Hebei Province’s wheat production.
Granger causality test outcomes for Hebei, Henan, and Shandong Provinces.
| Null Hypothesis: | Hebei Province | Henan Province | Shandong Province | |||
|---|---|---|---|---|---|---|
| F-Statistic | Prob. | F-Statistic | Prob. | F-Statistic | Prob. | |
| LTEMP LWP | 0.32014 | 0.7299 | 0.48642 | 0.4928 | 6.9 × 10 | 0.9934 |
| LOGWP LTEMP | 3.85467 ** | 0.0393 | 7.70193 ** | 0.0110 | 6.21589 ** | 0.0207 |
| LRF LOGWP | 14.5460 *** | 0.0001 | 11.2664 *** | 0.0029 | 12.7836 *** | 0.0017 |
| LWP LRF | 5.86390 ** | 0.0104 | 2.30976 | 0.1428 | 1.31051 | 0.2646 |
| LFER LWP | 14.0077 *** | 0.0002 | 5.55914 ** | 0.0277 | 1.38081 | 0.2525 |
| LWP LFER | 11.1690 *** | 0.0006 | 1.33921 | 0.2596 | 14.7157 *** | 0.0009 |
| LPC LWP | 0.34197 | 0.7147 | 0.68055 | 0.4183 | 2.40316 | 0.1354 |
| LWP LPC | 1.18261 | 0.3280 | 0.09899 | 0.7560 | 0.30692 | 0.5852 |
| LWA LWP | 2.59154 | 0.1011 | 3.89070 * | 0.0613 | 7.44369 ** | 0.0123 |
| LOGWP LWA | 1.66343 | 0.2159 | 4.21314 * | 0.0522 | 11.3607 *** | 0.0028 |
| LLF LWP | 1.83122 | 0.1874 | 0.00149 | 0.9695 | 4.15717 * | 0.0537 |
| LWP LLF | 0.25914 | 0.7744 | 2.30602 | 0.1431 | 0.15467 | 0.6979 |
Note: ⇏ indicates “does not cause Granger”, *** p value < 0.01, ** p value < 0.05, and * p value < 0.1.
Further, the results only discover a unidirectional causality association between wheat production and temperature. In the context of Henan Province, it is revealed that a unidirectional causality association runs from precipitation and fertilizer usage to wheat production. In contrast, a bidirectional causality exists between power consumption and wheat production. This depicts that climate change factors, such as rainfall, and other inputs also positively influence wheat production. In addition, a bidirectional causality is established between the farming area and wheat production, while a unidirectional causality is detected from precipitation and labor to wheat production. These results imply that the cultivated area, rainfall, and labor significantly improve wheat production in the context of Shandong Province.
5. Conclusions
The current study assesses the climate variables’ long-run impact on wheat production in China’s top three wheat-producing provinces. The other important factors considered in this paper include fertilizer usage, cultivated area, power consumption, and labor. The data set consists of observations from 1992 to 2020 on which several time-series techniques, namely, the DOLS, FMOLS, CCR, and Granger causality, were applied. Based on the estimations, the findings revealed that wheat production is negatively affected by climate change in Henan Province. In contrast, climate change is more favorable for wheat production in Hebei Province.
On the other hand, temperature negatively influenced wheat production but was not significant, while rainfall significantly contributed positively to wheat production in Shandong Province. Further findings showed that fertilizer usage, cultivated area, and labor positively and significantly improved wheat production in Hebei and Shandong Provinces. In contrast, power usage, wheat farming area, and labor force significantly and positively enhanced wheat production in Henan Province. In addition, the findings of the Granger causality test reported a bidirectional causality between rainfall and fertilizer use with wheat production in Hebei Province, while a unidirectional causality connection was revealed between wheat production and temperature. In the context of Henan Province, it was discovered that a unidirectional causality link was observed from rainfall and fertilizer use to wheat production. In contrast, a bidirectional causality existed between power consumption and wheat production. Moreover, a bidirectional causality was established between the cultivated area and wheat production, while a unidirectional causality was detected from the rainfall and labor to wheat production in Shandong Province.
Based on the estimated outcomes, the current paper offers several policy implications:
With both advantages and disadvantages, China’s wheat production is affected by global warming. To mitigate the effects of a changing climate on China’s wheat yield, it is vital to increase the adaptability of wheat production. First, modify wheat’s sowing date and area in a reasonable manner. Adjust the sowing date of crops, rationally plan the planting areas, fully utilize the additional heat resources brought about by climate change, decrease the impact of meteorological disasters, and increase the stability of wheat production based on the climatic conditions of various regions.
Second, agricultural technology advancement will continue to be important in ensuring wheat yield stability. On the one hand, the Chinese government must prioritize research and develop seed resources resistant to extreme weather conditions. It is crucial to develop and store wheat germplasm resources that can respond to adverse weather conditions, given the prevalence of extreme weather events (high-temperature resistance, waterlogging resistance, low-temperature resistance, etc.). On the other hand, it is essential to continue using advanced agricultural technologies to produce wheat. For instance, more fertilizer use techniques should be implemented to increase the input effectiveness of chemical fertilizers and ensure the sustainability of agricultural production.
Furthermore, there are regional differences in wheat planting varieties and methods in China, making it difficult to continuously improve wheat production levels by relying solely on a single technology. As a result, it is necessary to promote improved varieties in conjunction with good methods, agricultural machinery, and agronomy, as well as to further tap the potential of science and technology to increase production.
Funding Statement
This research was funded by the National Social Science Fund of China (Grant number: 19CSH029).
Author Contributions
Conceptualization, A.A.C. and H.Z.; methodology, A.A.C.; software, A.A.C. and Y.T.; validation, G.R.S.; formal analysis, A.A.C. and Y.T.; investigation, A.A.C. and Y.T.; resources, H.Z.; data curation, Y.T.; writing—original draft preparation, A.A.C. and Y.T.; writing—review and editing, M.A.T. and G.R.S.; visualization, H.Z.; supervision, A.A.C.; project administration, H.Z.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

- Our work >
Demand for wheat by 2050 is predicted to increase by 50 percent from today’s levels. Meanwhile, the crop is at risk from new and more aggressive pests and diseases, diminishing water resources, limited available land and unstable weather conditions—heat in particular.

Through this program, CIMMYT works with more than 200 research and breeding institutions, including the International Center for Agriculture Research in the Dry Areas ( ICARDA ), sharing elite breeding lines and associated data through its system of international nurseries.
The Wheat Molecular Breeding laboratory develops tools and information for breeders around the world. The Wheat Quality laboratory ensures that CIMMYT varieties meet market demands for flour and bread quality.
CIMMYT’s wheat research aims to
- Develop climate resilient, nutritious, high yielding disease and pest tolerant wheat lines.
- Use the latest molecular breeding tools, bioinformatics and selection methods.
- Ensure that national agricultural research system partners are active participants in breeding.
- Apply more precise phenotyping approaches—phenotyping platforms—and other tools, like remote sensing, to develop genetically diverse wheat varieties so that globally, annual genetic yield gains of at least 0.7 percent are achieved.
- Provide diverse, high-yielding wheat varieties that withstand infertile soils, drought, pests and diseases.
- Conduct research to help farmers exploit the full potential of improved seed while conserving soil and water resources.
- Explore new market opportunities for smallholder farmers.
- Provide training opportunities in wheat breeding and crop management research.
- Exploit genetic variation in wheat wild relatives.
CIMMYT led the CGIAR Research Program on Wheat ( WHEAT ) from January 2012 through December 2021.
To order seeds, please click here .

Kevin Pixley
Dryland Crops Program Director (DCP) and Wheat Program Director a.i. (GWP)

Hamish Dunsford
Program Manager, Wheat
More stories
Climate-proofing India’s daily bread: The race for...
Cimmyt scientist recognized with research leader award, wheat cultivation in africa at risk of..., building global capacity to combat wheat blast, new heat-tolerant wheat varieties prove fruitful for..., re-imagining heat tolerance traits in wheat –..., wheat projects, protected: csp2301-008rtx: acrcp phase 5: optimising genetic..., protected: gruma’s sustainability plan: promoting sustainable agrifood..., protected: training program for chinese young scientists..., protected: china 2023 contribution (to be applied..., protected: fusarium head blight (fhb) of wheat, protected: bni- wheat future: towards reducing global..., wheat disease early warning advisory system (dewas), institutionalizing monitoring of crop variety adoption using..., nitrogen-efficient wheat production systems in the indo-gangetic..., rapid point-of-care diagnostics for wheat rusts (marple), managing wheat blast in bangladesh, adaptation, demonstration and piloting of wheat technologies....
Advertisement
Wheat improvement in India: present status, emerging challenges and future prospects
- Original Paper
- Published: 04 April 2007
- Volume 157 , pages 431–446, ( 2007 )
Cite this article

- A. K. Joshi 1 ,
- B. Mishra 2 ,
- R. Chatrath 2 ,
- G. Ortiz Ferrara 3 &
- Ravi P. Singh 4
4265 Accesses
218 Citations
3 Altmetric
Explore all metrics
India is the second largest producer of wheat in the world, with production hovering around 68–75 million tons for past few years. The latest estimated demand for wheat production for the year 2020 is approximately 87.5 million tons, or about 13 million tons more than the record production of 75 million tons harvested in crop season 1999–2000. Since 2000, India has struggled to match that record production figure and thus faces a critical challenge in maintaining food security in the face of its growing population. The current major challenges facing future wheat production in India are increasing heat stress; dwindling water supplies for irrigation; a growing threat of new virulence of diseases such as wheat rusts (yellow, brown, and black) and leaf blight; continuous adoption of rice-wheat systems on around 11 million hectares; changes in urbanization patterns, and demand for better quality wheat. In addition, the threat posed by the new stem rust race Ug99 can not be underestimated. The wide gap (around 2.5 t/ha) between the potential and harvested yield in the eastern Gangetic Plains also cries out for solutions. Addressing issues related to different stresses will require harnessing genes discovered in landraces and wild relatives following conventional as well as non-conventional approaches. For effective technology delivery in areas that suffer from poor linkages with farmers, participatory research needs to be strengthened. The future germplasm requirements from a dependable collaborator such as CIMMYT are largely being dictated by the above factors.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Wheat Improvement

Global Crop Improvement Networks to Bridge Technology Gaps
Abbreviations.
Eastern Gangetic Plains
Eastern Gangetic Plains Yield Trial
Eastern Gangetic Plains Screening Nursery
Anonymous (2003) Salient features of Lok1. www.lokbharti.org. Verified 27 January, 2007
Anonymous (2007) CIMMYT. 2007. Dangerous wheat disease jumps red sea. Mexico, DF: CIMMYT. Available on-line at: http://www.globalrust.org (verified 27/1/07)
Araus JL, Reynolds MP, Acevedo E (1993) Leaf posture, grain yield, growth, leaf structure and carbon isotope discrimination in wheat. Crop Sci 33:1273–1279
Article Google Scholar
Arun B, Joshi AK, Chand R, Singh D (2003) Wheat somaclonal variants showing, earliness, improved spot blotch resistance and higher yield. Euphytica 132:235–241
Bhalla GS, Hazell P, Kerr J (1999) Prospects for India’s cereal supply and demand to 2020. International Food Policy Research Institute, USA
Google Scholar
Chatrath R (2004) Breeding strategies for developing wheat varieties targeted for rice-wheat cropping system of Indo-Gangetic Plains of Eastern India. In: Joshi AK, Chand R, Arun B, Singh G (eds) A compendium of the training program (26–30 December, 2003) on wheat improvement in eastern and warmer regions of India: conventional and non-conventional approaches. NATP project, (ICAR), BHU, Varanasi, India
Chatrath R, Mishra B, Shoran J (2006a) Yield potential survey–India. In: Reynolds MP, Godinez D (eds) Extended abstracts of the international symposium on wheat yield potential “Challenges to International Wheat Breeding”, March 20–24, 2006, Cd. Obregon, Mexico. CIMMYT, Mexico, D.F., p 53
Chatrath R, Mishra B, Joshi AK, Ortiz-Ferrara G (2006b) Challenges to wheat production in South Asia. In: Reynolds MP, Godinez D (eds) Extended abstracts of the international symposium on wheat yield potential “Challenges to International Wheat Breeding”, March 20–24, 2006, Cd. Obregon, Mexico. CIMMYT, Mexico, D.F., p 6
Directorate of Wheat Research (2006) Project Director’s Report: 2005–2006. B Mishra, Project Director, Directorate of Wheat Research, Karnal 132001, pp 29
Evenson RE, Pray CE, Rosegrant MV (1999) Agricultural research and productivity growth in India. IFPRI, Washington, DC
FAO (2004) Statistical database. www.fao.org. Verified 4 January, 2007
Fischer RA (1985) Number of kernels in wheat crops and the influence of solar radiation and temperature. J Agric Sci (Camb) 105:447–461
Fischer RA, Byerlee DB (1991) Trends of wheat production in the warmer areas: major issues and economic considerations. In: Wheat for the Non-traditional Warm Areas. Proc. of Conf., Iguazu, Brazil, 29 Jul.–3 Aug. 1990. CIMMYT, Mexico, DF. pp 3–27
Fischer RA (1996) Wheat physiology at CIMMYT and raising the yield plateau. In: Reynolds MP, Rajaram S, McNab A (eds) Increasing yield potential in wheat: breaking the barriers. Proc. Workshop, Cd. Obregon, Mexico, 28–30 March 1996. CIMMYT, Mexico, DF, pp 150–166
Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Larqué-Saavedra A (1998) Wheat yield progress is associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 38:1467–1475
Government of India (1996) Population projections for India and states 1996–2016, Report of Technical Group on Population Projections. New Delhi, Registrar General
Gupta RK (2004) Quality of Indian wheat and infrastructure for analysis. In: Joshi AK, Chand R, Arun B, Singh G (eds) A compendium of the training program (26–30 December, 2003) on wheat improvement in eastern and warmer regions of India: Conventional and non-conventional approaches. NATP project, (ICAR), BHU, Varanasi, India
Harrington LW, Fujisaka S, Morris ML, Hobbs PR, Sharma HC, Singh RP, Chaudhary MK, Dhiman SD (1993) Wheat and Rice in Karnal and Kurukshetra Districts, Haryana, India: Farmers’ Practices, Problems and an Agenda for Action. Mexico, DF: Haryana Agricultural University (HAU), Indian Council for Agricultural Research (ICAR), CIMMYT, and the International Rice Research Institute (IRRI)
Hobbs PR (2001) Tillage and crop establishment in South Asian rice-wheat systems: present and future options. In: Kataki PK (ed) The Rice-Wheat Cropping System of South Asia: Efficient Production Management. J Crop Prod 4:1–23
Hobbs P, Morris M (1996) Meeting South Asia’s Future Food Requirements from Rice-Wheat Cropping Systems: Priority Issues Facing Researchers in the Post-Green Revolution Era. NRG paper 96–01. Mexico, DF: CIMMYT. pp 46
Howard A (1924) Crop production in India: a critical survey of its problems. Oxford University Press, Oxford, UK, p 156
Innes P, Blackwell RD (1983) Some effects of leaf posture on the yield and water economy of winter wheat. J Agric Sci (Camb) 101:367–376
Johnson R (1988) Durable resistance to yellow (stripe) rust in wheat and its implications in plant breeding. In: Simmonds NW, Rajaram S (eds) Breeeding strategies for resistance to the rusts of wheat. CIMMYT, Mexico, DF, pp 63–75
Joshi AK, Chand R (2002) Variation and inheritance of leaf angle, and its association with spot blotch ( Bipolaris sorokiniana ) severity in wheat ( Triticum aestivum ). Euphytica 124:283–291
Joshi AK, Chand R, Arun B (2002) Relationship of plant height and days to maturity with resistance to spot blotch in wheat. Euphytica 123:221–228
Joshi AK, Chand R, Chandola VK (2003) 1st Annual Report of CIMMYT collaborated, DFID funded project, Participatory Research to Increase the Productivity and Sustainability of Wheat Cropping Systems in the Eastern Subcontinent of South Asia. CIMMYT, BHU, Varanasi, India
Joshi AK, Chand R, Kumar S, Singh RP (2004a) Leaf tip necrosis: a phenotypic marker associated with resistance to spot blotch disease in wheat. Crop Sci 44:792–796
Joshi AK, Kumar S, Chand R, Ortiz-Ferrara G (2004b) Inheritance of resistance to spot blotch caused by Bipolaris sorokiniana in spring wheat. Plant Breed 123:213–219
Joshi AK, Chand R, Chandola VK, Prasad LC, Arun B, Tripathi R, Ortiz Ferrara G (2005) Approaches to Germplasm Dissemination and Adoption - Reaching Farmers in the Eastern Gangetic Plains. Proceedings of 7th International Wheat Symposium, Nov 27–Dec 2, 2005, Mar del Plata, Argentina
Joshi AK, Chand R, Arun B, Singh RP, Ortiz R (2007a) Breeding crops for reduced-tillage management in the intensive, rice-wheat systems of South Asia. Euphytica 153:135–151
Joshi AK, Kumari M, Singh VP, Reddy CM, Kumar S, Rane J, Chand R (2007b) Stay green trait: variation, inheritance and its association with spot blotch resistance in spring wheat ( Triticum aestivum L.). Euphytica 153:59–71
Joshi AK, Ortiz-Ferrara G, Crossa J, Singh G, Alvarado G, Bhatta MR, Duveiller E, Sharma RC, Pandit DB, Siddique AB, Das SY, Sharma RN, Chand R (2007c) Associations of environments in South Asia based on spot blotch disease of wheat caused by Cochliobolus sativus . Crop Sci (In press)
Kulshrestha VP, Jain HK (1982) Eighty years of wheat breeding in India: Past selection pressures and future prospects. Z Pflanzenzuchtg 89:19–30
Kronstad WE, McCuistion WL, Swearingen ML, Qualset CO (1978) Crop selection for specific residue management systems. In: Oschwald WR (ed) Crop residue management systems. American Society of Agronomy, Madison, WI, pp 207–217 Spec Pub 31
Lal R, Hansen DO, Hobbs P, Uphoff N (2004) Reconciling food security with environment quality through no-till farming. In: Lal R, Hobbs P, Uphoff N, Hansen DO (eds) Sustainable agriculture and the rice-wheat system. Marcel Dekker, Inc., New York, pp 495–512
Ladha JK, Fischer KS, Hossain M, Hobbs PR, Hardy B (2000) Improving the productivity of rice-wheat systems of Indo-Gangetic Plains: A synthesis of NARS-IRRI partnership research. IRRI Discussion Paper No. 40. Los Banos, Philippines: IRRI
McIntosh RA, Devos KM, Dubcovsky J, Rogers WJ, Morris CF, Apples R, Anderson OD (2005) Catalogue of gene symbols for wheat: 2005 supplement. http://www.shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2005
Nagarajan S (2005) Can India produce enough wheat even by 2020. Curr Sci 89:1467–1471
Ortiz-Ferrara G, Bhatta MR, Pokharel T, Mudwari A, Thapa DB, Joshi AK, Chand R, Muhammad D, Duveiller E, Rajaram S (2001) Farmers’ participatory variety selection in South Asia. Research Highlights of the CIMMYT Wheat Program, 1999–2000. CIMMYT, Mexico D.F., pp 33–37
Pandey SP, Kumar S, Kumar U, Chand R, Joshi AK (2005) Sources of inoculum and reappearance of spot blotch of wheat in rice-wheat cropping system in eastern India. Eur J Plant Pathol 111:47–55
Peña RJ, Trethowan R, Pfeiffer WH, van Ginkel M (2002) Quality (End-Use) Improvement in wheat: Compositional, Genetic and Environmental Factors. In: Basra AS, Randhawa LS (eds) Quality improvement in field crops. The Howarth Press, New York, pp 1–38
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84:203
Rajaram S (2001) Prospects and promise of wheat breeding in the 21st century. Euphytica 119:3–15
Rane J, Shoran J, Nagarajan S (2000) Heat stress environments and impact on wheat productivity in India: Guestimate of losses. Indian Wheat News Lett 6(1):5–6
Reynolds MP, Acevedo E, Sayre KD, Fischer RA (1994) Yield potential in modern wheat varieties: its association with a less competitive ideotype. Field Crops Res 37:149–160
Reynolds MP, Rajaram S, Sayre KD (1999) Physiological and genetic changes of irrigated wheat in the post-Green Revolution period and approaches for meeting projected global demand. Crop Sci 39:1611–1621
Reynolds MP, van Ginkel M, Ribaut J-M (2000) Avenues for genetic modification of radiation use efficiency in wheat. J Exp Bot 51:459–473
Article PubMed CAS Google Scholar
Reynolds MP, Calderini DF, Condon AG, Rajaram S (2001) Physiological basis of yield gains in wheat associated with the Lr19 translocation from Agropyron elongatum . In: Bedo Z, Lang L (eds) Wheat in a global environment. Kluwer Academic Publishers, the Netherlands, pp 345–351
Reynolds MP, Trethowan R, Crossa J, Vargas M, Sayre KD (2004) Erratum to “Physiological factors associated with genotype by environment interaction in wheat”. Field Crops Res 85:253–274
Reynolds MP, Borlaug NE (2006) Applying innovations and new technologies for international collaborative wheat improvement. J Agric Sci 144:99–110
Richards RA, Rebetzke GJ, Condon AG, Mickelson BJ (1996) Targeting traits to increase the grain yield of wheat. In: Richards RA, Wrigley CW, Rawson HM, Rebetzke GJ, Davidson JL, Brettell RIS (eds) Proc. 8th assembly, wheat breeding society of Australia. Wheat Breeding Society of Australia, Sydney, Australia, pp 054–057
Saari EE (1998) Leaf blight disease and associated soil-borne fungal pathogens of wheat in south and southeast Asia. In Duvellier E, Dubin HJ, Reeves J, McNab A (eds) Helminthosporium blights of wheat: spot blotch and tan spot. CIMMYT, Mexico, DF, pp 37–51
Sharma SN, Bhatnagar VK, Mann MS, Shekhawat US, Sain RS (2002) Maximization of wheat yields with a unique variety in warmer areas. Wheat Inform Ser 95:11–16
Singh RP, Huerta-Espino J, Rajaram S, Crossa J (1998a) Agronomic effects from chromosome translocations 7DL.7Ag and 1BL.1RS in spring wheat. Crop Sci 38:27–33
Singh RP, Rajaram S, Miranda A, Huerto-Espino J, Autroque E (1998b) Comparison of two crossing and four selection schemes for yield, yield traits, and slow rusting resistance to leaf rust in wheat. Euphytica 100:25–43
Singh RP, Huerta-Espino J, William M (2004) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. In: Joshi AK, Chand R, Arun B, Singh G (eds) A compendium of training program (26–30 December, 2003) on wheat improvement in eastern and warmer regions of India: conventional and non-conventional approaches. NATP project (ICAR), BHU, Varanasi, India
Singh RP, Huerta-Espino J, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turkish J Agric Forestry 29:121–27
CAS Google Scholar
Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 1, No. 054: 1–13 ( http://www.cababstractsplus.org/cabreviews )
Singh RP, Huerta-Espino J, Sharma R, Joshi AK, Trethowan R (2007) High Yielding Spring Bread Wheat Germplasm for Global Irrigated and Rainfed Production Systems. Euphytica (In this issue)
Stone PJ, Nicolas ME (1995) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Aust J Plant Physiol 22: 927–934
Tandon JP (1994) Wheat cultivation, research organization and production technology in the hot dry regions of India. In: Saunders DA, Hettel GP (eds) Wheat in heat-stressed environments: irrigated, dry areas and rice-wheat farming systems. CIMMYT, Mexico, DF, pp 17–23
Trethowan RM, Singh RP, Huerta-Espino J, Crossa J, van Ginkel M (2001) Coleoptile length variation of near-isogenic Rht lines of modern CIMMYT bread and durum wheats. Field Crops Res 70:167–176
Trethowan RM, Borja J, Kazi-Mujeeb (2003) The impact of synthetic wheat on breeding for stress tolearance at CIMMYT. Ann Wheat News Lett 49:67–69
Trethowan RM, Reynolds M (2005) Drought resistance: genetic approaches for improving productivity under stress. In: Proc 7 th Intl. Wheat Conf, 27 Nov-2 Dec 2005, Mar del Plata, Argentina
Trethowan RM, Reynolds MP, Sayre KD, Ortiz-Monasterio I (2005) Adapting wheat cultivars to resource conserving farming practices and human nutritional needs. Ann Appl Biol 146: 404–413
United Nations (1995) World urbanization prospects, the 1994 revision. United Nations, New York
Villareal RL, Banuelos O, Borja J, Mujeeb-Kazi A (1998) Drought tolerance of synthetic wheats ( Triticum turgidum x Aegilops tauschii ). Ann Wheat Newslett 44:142–144
Wardlaw IF (1994) The effect of high temperature on kernel development in wheat: Variability related to pre-heading post-anthesis conditions. Aust J Plant Physiol 21:731–739
Witcombe JR, Joshi A, Goyal SN (2003) Participatory plant breeding in maize: A case study from Gujarat, India. Euphytica 130:413–22
Witcombe JR, Joshi KD, Rana RB, Virk DS (2001) Increasing genetic diversity by participatory varietal selection in high potential production systems in Nepal and India. Euphytica 122:575–88
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weeds Res 14:415–421
Download references
Author information
Authors and affiliations.
Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, 221005, India
A. K. Joshi
Directorate of Wheat Research, Indian Council of Agricultural Research, Karnal, 132 001, Haryana, India
B. Mishra & R. Chatrath
CIMMYT South Asia Office, Kathmandu, Nepal
G. Ortiz Ferrara
Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT), Apdo. Postal 6-641, C.P. 06600, Mexico, DF, Mexico
Ravi P. Singh
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to A. K. Joshi .
Rights and permissions
Reprints and permissions
About this article
Joshi, A.K., Mishra, B., Chatrath, R. et al. Wheat improvement in India: present status, emerging challenges and future prospects. Euphytica 157 , 431–446 (2007). https://doi.org/10.1007/s10681-007-9385-7
Download citation
Received : 12 September 2006
Accepted : 20 February 2007
Published : 04 April 2007
Issue Date : October 2007
DOI : https://doi.org/10.1007/s10681-007-9385-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Triticum aestivum
- Wheat improvement
- Present status
- Future prospects
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Adamski, N. M. et al. A roadmap for gene functional characterisation in crops with large genomes: lessons from polyploid wheat. eLife 9, e55646 (2020). Uauy, C. Wheat genomics comes of age. Curr ...
Wheat (Triticum aestivum L.) belonging to one of the most diverse and substantial families, Poaceae, is the principal cereal crop for the majority of the world's population.This cereal is polyploidy in nature and domestically grown worldwide. Wheat is the source of approximately half of the food calories consumed worldwide and is rich in proteins (gluten), minerals (Cu, Mg, Zn, P, and Fe ...
Wheat (Triticum aestivum L.) is the most widely cultivated crop on Earth, contributing about a fifth of the total calories consumed by humans.Consequently, wheat yields and production affect the global economy, and failed harvests can lead to social unrest. Breeders continuously strive to develop improved varieties by fine-tuning genetically complex yield and end-use quality parameters while ...
The paper summarized the state of wheat production, consumption, and international trade at the global and regional levels. ... Dixon J (2007) The economics of wheat: research challenges from field to fork. In: Buck H, Nisi J, Salomon N (eds) Wheat production in stressed environments. ... (2020) Canada markets: a look at USDA's growing global ...
Article 11 June 2020. Main. ... We thank the innovation laboratory at Kansas State University, the CGIAR Research Program on Wheat, the Indian Council of Agricultural Research (ICAR), the ...
The importance of wheat research is also apparent through the strong public investment; for example, a survey in 2020 identified 771 funded research projects on different aspects of wheat improvement and agronomy in just five countries (Australia, Canada, China, Spain and the USA) . An international survey in 2018 of wheat research projects ...
Therefore, the present research aims to assess the effect of sowing at different thermal environments and foliar spray of bio-regulators on productivity and nutritional composition of wheat under the era of climate ... Int.J.Curr.Microbiol.App.Sci (2020) 9(10): 2609-2615 . ) (2020) et wheat (Triticum aestivum. L.)
According to estimation wheat production exceeded as 761.7 million tons in (2017/2018), but its demand exceeded 762.4 million tons due to enhanced world population in the survey of (2019/2020) [12 ...
Wheat constitutes pivotal position for ensuring food and nutritional security; however, rapidly rising soil and water salinity pose a serious threat to its production globally. Salinity stress negatively affects the growth and development of wheat leading to diminished grain yield and quality. Wheat plants utilize a range of physiological biochemical and molecular mechanisms to adapt under ...
Purpose and Scope of the CRP 2020 Review. The review's purposes are to assess to what extent WHEAT is (1) delivering quality of science, and (2) demonstrating effectiveness in relation to its own Theories of Change (ToC). The third purpose is to provide insights and lessons to inform the program's future.
High temperature stress (HTS) inhibits almost all physiological processes in wheat causing extensive cellular damage ( Mishra et al., 2021a ). Increased temperature, even for short duration, significantly reduces grain production and quality. The effects of HTS in wheat have been discussed by several researchers based on the comprehensive ...
expected to increase from nearly 600 million tons to around 760 million tons in 2020, ... very high, partly because of the wide adaptability of many new wheat cultivars. The paper distinguishes returns to productivity and maintenance research, as well as socio- ... During the late 1950s and 1960s, wheat research in Mexico and South Asia ...
Wheat contributes to 50% and 30% of the global grain trade and production respectively [ 2 ]. Wheat is also known as a staple food in more than 40 countries of the world. Wheat provides 82% of basic calories and 85% of proteins to the world population [ 3, 4 ]. Wheat-based food is rich in fiber contents than meat-based food.
Wheat species. The major wheat species grown throughout the world is Triticum aestivum, a hexaploid species usually called "common" or "bread" wheat.However, the total world production includes about 35-40 mt of T. turgidum var. durum, a tetraploid species which is adapted to the hot dry conditions surrounding the Mediterranean Sea and similar climates in other regions.
Increasing temperature and consequent changes in climate adversely affect plant growth and development, resulting in catastrophic loss of wheat productivity. For each degree rise in temperature, wheat production is estimated to reduce by 6%. A detailed overview of morpho-physiological responses of wheat to heat stress may help formulating appropriate strategies for heat-stressed wheat yield ...
The current study examines the long-run effects of climatic factors on wheat production in China's top three wheat-producing provinces (Hebei, Henan, and Shandong). The data set consists of observations from 1992 to 2020 on which several techniques, namely, fully modified OLS (FMOLS), dynamic OLS (DOLS), and canonical co-integrating ...
Abstract. Wheat (Triticum aestivum L) is the most extensively grown cereal crop in the world, covering about 237 million hectares annually, accounting for a total of 420 million tonnes (Isitor et ...
Soybean-wheat is one of the predominant cropping systems in India where wheat is the second most important cereal crop and widely grown by the farmers as rabi crop, covering 29.58 million hectare ...
The Wheat Molecular Breeding laboratory develops tools and information for breeders around the world. The Wheat Quality laboratory ensures that CIMMYT varieties meet market demands for flour and bread quality. CIMMYT's wheat research aims to. Develop climate resilient, nutritious, high yielding disease and pest tolerant wheat lines.
people by 2020 requiring 5-6 million tons (henceforth 'mt') ... ha-1 between research farm and farmers field. Wheat is a staple crop in many countries and hence its ... own country. In the milieu, the present paper analyse the performance of wheat production in India. J. Wheat Res. 4(2): 37-44. J. Wheat Res. 4 (2) 38 Material and methods
In India, during 2018-19 Rabi season, wheat was cultivated in 29.55 mha and barley in 0.66 mha, constituting 24.35 per cent of the total crop acreage. Indian wheat production in 2018-19 has made a ...
The latest estimated demand for wheat production for the year 2020 is approximately 87.5million tons, or about 13million tons more than the record production of 75million tons harvested in crop ...
India is the second largest producer of wheat in the world, with production hovering around 68-75 million tons for past few years. The latest estimated demand for wheat production for the year 2020 is approximately 87.5 million tons, or about 13 million tons more than the record production of 75 million tons harvested in crop season 1999-2000. Since 2000, India has struggled to match that ...